User login
Man, 26, With Sudden-Onset Right Lower Quadrant Pain
A 26-year-old man presented to the emergency department (ED) with a chief complaint of abdominal pain. After triage was complete, he was transported to an examination room, where the clinician obtained the history of presenting illness. The onset of pain was approximately 90 minutes prior to arrival at the ED and woke the patient from a “sound sleep.” He stated that the pain initially started as a “3 out of 10” but had progressed to a “12 out of 10,” and he described it as being in the right lower quadrant of his abdomen, with radiation to his right testicle. However, he was unsure where the pain started or if it was worse in either location. Nausea was the primary associated symptom, but he denied vomiting, diarrhea, fever, dysuria, or hematuria. Last, the patient denied history of trauma.
Medical history was noncontributory: He denied previous gastrointestinal diseases, and there was no history of renal stones, urinary tract infection, or any other genitourinary disease. He had no surgical history. The patient smoked less than a pack of cigarettes per day but denied alcohol or drug use.
Physical examination revealed a young man in moderate discomfort. Despite describing his pain as a “12 out of 10,” he had a blood pressure of 121/72 mm Hg; pulse, 59 beats/min; respiratory rate, 20 breaths/min; and temperature, 96.8°F. HEENT and cardiovascular, respiratory, musculoskeletal, and neurologic exam results were all within normal limits. Abdominal examination revealed a mildly tender right lower quadrant with deep palpation, but no rebound or guarding. Murphy sign was negative.
Because of the complaint of pain radiating to the testicles, a genitourinary examination was performed. The penis appeared unremarkable, with no lesions or discharge. There was no inguinal lymphadenopathy. The scrotum appeared appropriate in size and was also grossly unremarkable. The left testicle was nontender. However, palpation of the right testicle elicited moderate to severe pain. There was no visible swelling, and there were no palpable hernias or other masses. Cremasteric reflex was assessed bilaterally and deemed to be absent on the right side.
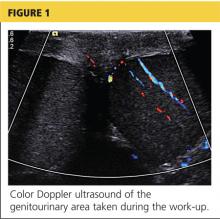
A workup was initiated that included a complete blood count, comprehensive metabolic panel, and urinalysis; the results of these tests were unremarkable. A differential diagnosis was formed, with emphasis on appendicitis and testicular torsion. Because of the specific nature and location of the pain, both ultrasound and CT of the abdomen/pelvis were considered. It was decided to order the ultrasound, with a plan to perform CT only if ultrasound was unremarkable. The patient was medicated for his pain and the ultrasound commenced. Halfway through the imaging, the clinician and attending physician were summoned to the examination room to review the image seen in Figure 1.
On the next page: Discussion and diagnosis >>
DISCUSSION
Testicular torsion may occur if the testicle twists or rotates on the spermatic cord. The twisting causes arterial ischemia and venous outflow obstruction, cutting off the testicle’s blood supply.1,2 Torsion may be extravaginal or intravaginal, depending on the extent of involvement of the surrounding structures.2
Extravaginal torsion is most commonly seen in neonates and occurs because the entire testicle may freely rotate prior to fixation to the scrotal wall via the tunica vaginalis.2Intravaginal torsion is more common in adolescents and often occurs as a result of a condition known as bell clapper deformity. This congenital abnormality enables the testicle to rotate within the tunica vaginalis and rest transversely in the scrotum instead of in a more vertical orientation.2,3 Torsion occurs if the testicle rotates 90° to 180°, with complete torsion occurring at 360° (torsion may extend to as much as 720°).2 Torsion may also occur as a result of trauma.1
Peak incidence of testicular torsion occurs at ages 13 to 14, but it can occur at any age; torsion affects approximately 1 in 4,000 males younger than 25.2-5 Ninety-five percent of all torsions are intravaginal.2 Torsion is the most common pathology for males who undergo surgical exploration for scrotal pain.3
The main goal in the diagnosis and treatment of torsion is testicular salvage. Torsion is considered a urologic emergency, making early diagnosis and treatment critical to prevent testicular loss. In fact, a review of the relevant literature reveals that the rate of testicular salvage is much higher if the diagnosis is made within 6 to 12 hours.1,2,5 Potential sequelae from delayed treatment include testicular infarction, loss of testicle, infertility problems, infections, cosmetic deformity, and increased risk for testicular malignancy.2
Because many men hesitate to seek medical attention for symptoms of testicular pain and swelling, the primary care clinician should openly discuss testicular disorders, especially with preadolescent males, during testicular examinations.6
Diagnosis
A testicular examination should be performed on any male presenting with a chief complaint of lower abdominal pain, back/flank pain, or any pain that radiates to the groin. The cremasteric reflex should be assessed because it can help differentiate among the causes of testicular pain.7 It is performed by gently stroking the upper inner thigh and observing for contraction of the ipsilateral testicle. One study found that, in cases of torsion, the absence of a cremasteric reflex had a sensitivity of 96% and a specificity of 88%.7 See the Table for the differential diagnosis for acute testicular pain.
While it is often possible to make the diagnosis of testicular torsion clinically, ultrasound with color Doppler is the diagnostic test of choice in cases for which the cause of acute scrotal pain is unclear.8 Ultrasound provides anatomic detail of the scrotum and its contents, and perfusion is assessed by adding the color Doppler images.8 It is important to note that, while the absence of blood flow is considered diagnostic for testicular torsion, the presence of flow does not necessarily exclude it.4
On the next page: Treatment >>
Treatment
Surgical exploration with intraoperative detorsion and orchiopexy (fixation of the testicle to the scrotal wall) is the mainstay of treatment for testicular torsion.1 Orchiopexy is often performed bilaterally in order to prevent future torsion of the unaffected testicle. In about 40% of males with the bell clapper deformity, the condition is present on both sides.2 Orchiectomy, the complete removal of the testicle, is necessary when the degree of torsion and subsequent ischemia have caused irreversible damage to the testicle.6 In one study in which 2,248 cases of torsion were reviewed, approximately 34% of males required orchiectomy.6
If surgery may be delayed, the clinician may attempt manual detorsion at the bedside. Despite the “open book” method described in many texts—which instructs the practitioner to rotate the testicle laterally—a review of the literature reveals that torsion takes place medially only 70% of the time.1,5 The clinician should always consider this when any attempts at manual detorsion are made and correlate his or her technique with physical examination and the patient’s response.5
Relief of pain and return of the testicle to its natural longitudinal lie are considered indicators of successful detorsion.1 Color Doppler ultrasound should be used to confirm the return of circulation. However, in one case review of pediatric patients who underwent surgical exploration after manual detorsion, some degree of residual torsion remained in 32%.5 Because of this risk, surgery is still indicated even in cases of successful bedside detorsion.5
On the next page: Case continuation >>
CASE CONTINUATION
The decision to perform bedside ultrasound was made because the diagnosis of testicular torsion is a surgical emergency, and the window of time to prevent complications can be extremely narrow. If the ultrasound had been normal, then a CT scan may have provided additional data on which to base the diagnosis.
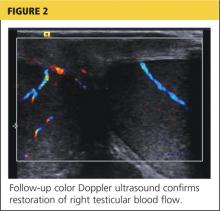
The patient was given adequate parenteral pain medication. After color Doppler ultrasound confirmed the torsion, the testicle was laterally rotated approximately 360°. The patient reported alleviation of his symptoms. Color Doppler was again performed to confirm the return of hyperemic blood flow to the affected testicle (Figure 2). The urologist arrived shortly thereafter and the patient was taken to the operating room, where he underwent scrotal exploration and bilateral orchiopexy.
On the next page: Conclusion >>
CONCLUSION
A testicular examination should be performed on any male presenting with a chief complaint of lower abdominal pain, back/flank pain, or any pain that radiates to the groin. Testicular torsion is most commonly seen in infants and adolescents but can occur at any age. The condition is a surgical emergency and the goal is testicular salvage, which is most likely to occur before 12 hours have elapsed since the onset of symptoms. An important component of the physical examination is attempting to elicit the cremasteric reflex, which is likely to be absent in the presence of torsion.
The primary care provider’s goal is to rapidly diagnose testicular torsion, then refer the patient immediately to a urologist or ED. The skilled clinician may attempt manual detorsion, based on his/her expertise and comfort level; however, this procedure should never delay prompt surgical intervention.
REFERENCES
1. Eyre RC. Evaluation of the acute scrotum in adults. www.uptodate.com/contents/evaluation-of-the-acute-scrotum-in-adults. Accessed May 16, 2014.
2. Ogunyemi OI, Weiker M, Abel EJ. Testicular torsion. http://emedicine.medscape.com/article/2036003-overview. Accessed May 16, 2014.
3. Khan F, Muoka O, Watson GM. Bell clapper testis, torsion, and detorsion: a case report. Case Rep Urol. 2011;2011:631970.
4. Molokwu CN, Somani BK, Goodman CM. Outcomes of scrotal exploration for acute scrotal pain suspicious of testicular torsion: a consecutive case series of 173 patients. BJU Int. 2011;107(6):990-993.
5. Sessions AE, Rabinowitz R, Hulbert WC, et al. Testicular torsion: direction, degree, duration and disinformation. J Urol. 2003;169(2):663-665.
6. Mansbach JM, Forbes P, Peters C. Testicular torsion and risk factors for orchiectomy. Arch Pediatr Adolesc Med. 2005;159:1167-1171.
7. Schmitz D, Safranek S. How useful is a physical exam in diagnosing testicular torsion? J Fam Pract. 2009;58(8):433-434.
8. D’Andrea A, Coppolino F, Cesarano E, et al. US in the assessment of acute scrotum. Crit Ultrasound J. 2013;5(suppl 1):S8. www.criticalultrasound journal.com/content/5/S1/S8/. Accessed May 16, 2014.
A 26-year-old man presented to the emergency department (ED) with a chief complaint of abdominal pain. After triage was complete, he was transported to an examination room, where the clinician obtained the history of presenting illness. The onset of pain was approximately 90 minutes prior to arrival at the ED and woke the patient from a “sound sleep.” He stated that the pain initially started as a “3 out of 10” but had progressed to a “12 out of 10,” and he described it as being in the right lower quadrant of his abdomen, with radiation to his right testicle. However, he was unsure where the pain started or if it was worse in either location. Nausea was the primary associated symptom, but he denied vomiting, diarrhea, fever, dysuria, or hematuria. Last, the patient denied history of trauma.
Medical history was noncontributory: He denied previous gastrointestinal diseases, and there was no history of renal stones, urinary tract infection, or any other genitourinary disease. He had no surgical history. The patient smoked less than a pack of cigarettes per day but denied alcohol or drug use.
Physical examination revealed a young man in moderate discomfort. Despite describing his pain as a “12 out of 10,” he had a blood pressure of 121/72 mm Hg; pulse, 59 beats/min; respiratory rate, 20 breaths/min; and temperature, 96.8°F. HEENT and cardiovascular, respiratory, musculoskeletal, and neurologic exam results were all within normal limits. Abdominal examination revealed a mildly tender right lower quadrant with deep palpation, but no rebound or guarding. Murphy sign was negative.
Because of the complaint of pain radiating to the testicles, a genitourinary examination was performed. The penis appeared unremarkable, with no lesions or discharge. There was no inguinal lymphadenopathy. The scrotum appeared appropriate in size and was also grossly unremarkable. The left testicle was nontender. However, palpation of the right testicle elicited moderate to severe pain. There was no visible swelling, and there were no palpable hernias or other masses. Cremasteric reflex was assessed bilaterally and deemed to be absent on the right side.
A workup was initiated that included a complete blood count, comprehensive metabolic panel, and urinalysis; the results of these tests were unremarkable. A differential diagnosis was formed, with emphasis on appendicitis and testicular torsion. Because of the specific nature and location of the pain, both ultrasound and CT of the abdomen/pelvis were considered. It was decided to order the ultrasound, with a plan to perform CT only if ultrasound was unremarkable. The patient was medicated for his pain and the ultrasound commenced. Halfway through the imaging, the clinician and attending physician were summoned to the examination room to review the image seen in Figure 1.
On the next page: Discussion and diagnosis >>
DISCUSSION
Testicular torsion may occur if the testicle twists or rotates on the spermatic cord. The twisting causes arterial ischemia and venous outflow obstruction, cutting off the testicle’s blood supply.1,2 Torsion may be extravaginal or intravaginal, depending on the extent of involvement of the surrounding structures.2
Extravaginal torsion is most commonly seen in neonates and occurs because the entire testicle may freely rotate prior to fixation to the scrotal wall via the tunica vaginalis.2Intravaginal torsion is more common in adolescents and often occurs as a result of a condition known as bell clapper deformity. This congenital abnormality enables the testicle to rotate within the tunica vaginalis and rest transversely in the scrotum instead of in a more vertical orientation.2,3 Torsion occurs if the testicle rotates 90° to 180°, with complete torsion occurring at 360° (torsion may extend to as much as 720°).2 Torsion may also occur as a result of trauma.1
Peak incidence of testicular torsion occurs at ages 13 to 14, but it can occur at any age; torsion affects approximately 1 in 4,000 males younger than 25.2-5 Ninety-five percent of all torsions are intravaginal.2 Torsion is the most common pathology for males who undergo surgical exploration for scrotal pain.3
The main goal in the diagnosis and treatment of torsion is testicular salvage. Torsion is considered a urologic emergency, making early diagnosis and treatment critical to prevent testicular loss. In fact, a review of the relevant literature reveals that the rate of testicular salvage is much higher if the diagnosis is made within 6 to 12 hours.1,2,5 Potential sequelae from delayed treatment include testicular infarction, loss of testicle, infertility problems, infections, cosmetic deformity, and increased risk for testicular malignancy.2
Because many men hesitate to seek medical attention for symptoms of testicular pain and swelling, the primary care clinician should openly discuss testicular disorders, especially with preadolescent males, during testicular examinations.6
Diagnosis
A testicular examination should be performed on any male presenting with a chief complaint of lower abdominal pain, back/flank pain, or any pain that radiates to the groin. The cremasteric reflex should be assessed because it can help differentiate among the causes of testicular pain.7 It is performed by gently stroking the upper inner thigh and observing for contraction of the ipsilateral testicle. One study found that, in cases of torsion, the absence of a cremasteric reflex had a sensitivity of 96% and a specificity of 88%.7 See the Table for the differential diagnosis for acute testicular pain.
While it is often possible to make the diagnosis of testicular torsion clinically, ultrasound with color Doppler is the diagnostic test of choice in cases for which the cause of acute scrotal pain is unclear.8 Ultrasound provides anatomic detail of the scrotum and its contents, and perfusion is assessed by adding the color Doppler images.8 It is important to note that, while the absence of blood flow is considered diagnostic for testicular torsion, the presence of flow does not necessarily exclude it.4
On the next page: Treatment >>
Treatment
Surgical exploration with intraoperative detorsion and orchiopexy (fixation of the testicle to the scrotal wall) is the mainstay of treatment for testicular torsion.1 Orchiopexy is often performed bilaterally in order to prevent future torsion of the unaffected testicle. In about 40% of males with the bell clapper deformity, the condition is present on both sides.2 Orchiectomy, the complete removal of the testicle, is necessary when the degree of torsion and subsequent ischemia have caused irreversible damage to the testicle.6 In one study in which 2,248 cases of torsion were reviewed, approximately 34% of males required orchiectomy.6
If surgery may be delayed, the clinician may attempt manual detorsion at the bedside. Despite the “open book” method described in many texts—which instructs the practitioner to rotate the testicle laterally—a review of the literature reveals that torsion takes place medially only 70% of the time.1,5 The clinician should always consider this when any attempts at manual detorsion are made and correlate his or her technique with physical examination and the patient’s response.5
Relief of pain and return of the testicle to its natural longitudinal lie are considered indicators of successful detorsion.1 Color Doppler ultrasound should be used to confirm the return of circulation. However, in one case review of pediatric patients who underwent surgical exploration after manual detorsion, some degree of residual torsion remained in 32%.5 Because of this risk, surgery is still indicated even in cases of successful bedside detorsion.5
On the next page: Case continuation >>
CASE CONTINUATION
The decision to perform bedside ultrasound was made because the diagnosis of testicular torsion is a surgical emergency, and the window of time to prevent complications can be extremely narrow. If the ultrasound had been normal, then a CT scan may have provided additional data on which to base the diagnosis.
The patient was given adequate parenteral pain medication. After color Doppler ultrasound confirmed the torsion, the testicle was laterally rotated approximately 360°. The patient reported alleviation of his symptoms. Color Doppler was again performed to confirm the return of hyperemic blood flow to the affected testicle (Figure 2). The urologist arrived shortly thereafter and the patient was taken to the operating room, where he underwent scrotal exploration and bilateral orchiopexy.
On the next page: Conclusion >>
CONCLUSION
A testicular examination should be performed on any male presenting with a chief complaint of lower abdominal pain, back/flank pain, or any pain that radiates to the groin. Testicular torsion is most commonly seen in infants and adolescents but can occur at any age. The condition is a surgical emergency and the goal is testicular salvage, which is most likely to occur before 12 hours have elapsed since the onset of symptoms. An important component of the physical examination is attempting to elicit the cremasteric reflex, which is likely to be absent in the presence of torsion.
The primary care provider’s goal is to rapidly diagnose testicular torsion, then refer the patient immediately to a urologist or ED. The skilled clinician may attempt manual detorsion, based on his/her expertise and comfort level; however, this procedure should never delay prompt surgical intervention.
REFERENCES
1. Eyre RC. Evaluation of the acute scrotum in adults. www.uptodate.com/contents/evaluation-of-the-acute-scrotum-in-adults. Accessed May 16, 2014.
2. Ogunyemi OI, Weiker M, Abel EJ. Testicular torsion. http://emedicine.medscape.com/article/2036003-overview. Accessed May 16, 2014.
3. Khan F, Muoka O, Watson GM. Bell clapper testis, torsion, and detorsion: a case report. Case Rep Urol. 2011;2011:631970.
4. Molokwu CN, Somani BK, Goodman CM. Outcomes of scrotal exploration for acute scrotal pain suspicious of testicular torsion: a consecutive case series of 173 patients. BJU Int. 2011;107(6):990-993.
5. Sessions AE, Rabinowitz R, Hulbert WC, et al. Testicular torsion: direction, degree, duration and disinformation. J Urol. 2003;169(2):663-665.
6. Mansbach JM, Forbes P, Peters C. Testicular torsion and risk factors for orchiectomy. Arch Pediatr Adolesc Med. 2005;159:1167-1171.
7. Schmitz D, Safranek S. How useful is a physical exam in diagnosing testicular torsion? J Fam Pract. 2009;58(8):433-434.
8. D’Andrea A, Coppolino F, Cesarano E, et al. US in the assessment of acute scrotum. Crit Ultrasound J. 2013;5(suppl 1):S8. www.criticalultrasound journal.com/content/5/S1/S8/. Accessed May 16, 2014.
A 26-year-old man presented to the emergency department (ED) with a chief complaint of abdominal pain. After triage was complete, he was transported to an examination room, where the clinician obtained the history of presenting illness. The onset of pain was approximately 90 minutes prior to arrival at the ED and woke the patient from a “sound sleep.” He stated that the pain initially started as a “3 out of 10” but had progressed to a “12 out of 10,” and he described it as being in the right lower quadrant of his abdomen, with radiation to his right testicle. However, he was unsure where the pain started or if it was worse in either location. Nausea was the primary associated symptom, but he denied vomiting, diarrhea, fever, dysuria, or hematuria. Last, the patient denied history of trauma.
Medical history was noncontributory: He denied previous gastrointestinal diseases, and there was no history of renal stones, urinary tract infection, or any other genitourinary disease. He had no surgical history. The patient smoked less than a pack of cigarettes per day but denied alcohol or drug use.
Physical examination revealed a young man in moderate discomfort. Despite describing his pain as a “12 out of 10,” he had a blood pressure of 121/72 mm Hg; pulse, 59 beats/min; respiratory rate, 20 breaths/min; and temperature, 96.8°F. HEENT and cardiovascular, respiratory, musculoskeletal, and neurologic exam results were all within normal limits. Abdominal examination revealed a mildly tender right lower quadrant with deep palpation, but no rebound or guarding. Murphy sign was negative.
Because of the complaint of pain radiating to the testicles, a genitourinary examination was performed. The penis appeared unremarkable, with no lesions or discharge. There was no inguinal lymphadenopathy. The scrotum appeared appropriate in size and was also grossly unremarkable. The left testicle was nontender. However, palpation of the right testicle elicited moderate to severe pain. There was no visible swelling, and there were no palpable hernias or other masses. Cremasteric reflex was assessed bilaterally and deemed to be absent on the right side.
A workup was initiated that included a complete blood count, comprehensive metabolic panel, and urinalysis; the results of these tests were unremarkable. A differential diagnosis was formed, with emphasis on appendicitis and testicular torsion. Because of the specific nature and location of the pain, both ultrasound and CT of the abdomen/pelvis were considered. It was decided to order the ultrasound, with a plan to perform CT only if ultrasound was unremarkable. The patient was medicated for his pain and the ultrasound commenced. Halfway through the imaging, the clinician and attending physician were summoned to the examination room to review the image seen in Figure 1.
On the next page: Discussion and diagnosis >>
DISCUSSION
Testicular torsion may occur if the testicle twists or rotates on the spermatic cord. The twisting causes arterial ischemia and venous outflow obstruction, cutting off the testicle’s blood supply.1,2 Torsion may be extravaginal or intravaginal, depending on the extent of involvement of the surrounding structures.2
Extravaginal torsion is most commonly seen in neonates and occurs because the entire testicle may freely rotate prior to fixation to the scrotal wall via the tunica vaginalis.2Intravaginal torsion is more common in adolescents and often occurs as a result of a condition known as bell clapper deformity. This congenital abnormality enables the testicle to rotate within the tunica vaginalis and rest transversely in the scrotum instead of in a more vertical orientation.2,3 Torsion occurs if the testicle rotates 90° to 180°, with complete torsion occurring at 360° (torsion may extend to as much as 720°).2 Torsion may also occur as a result of trauma.1
Peak incidence of testicular torsion occurs at ages 13 to 14, but it can occur at any age; torsion affects approximately 1 in 4,000 males younger than 25.2-5 Ninety-five percent of all torsions are intravaginal.2 Torsion is the most common pathology for males who undergo surgical exploration for scrotal pain.3
The main goal in the diagnosis and treatment of torsion is testicular salvage. Torsion is considered a urologic emergency, making early diagnosis and treatment critical to prevent testicular loss. In fact, a review of the relevant literature reveals that the rate of testicular salvage is much higher if the diagnosis is made within 6 to 12 hours.1,2,5 Potential sequelae from delayed treatment include testicular infarction, loss of testicle, infertility problems, infections, cosmetic deformity, and increased risk for testicular malignancy.2
Because many men hesitate to seek medical attention for symptoms of testicular pain and swelling, the primary care clinician should openly discuss testicular disorders, especially with preadolescent males, during testicular examinations.6
Diagnosis
A testicular examination should be performed on any male presenting with a chief complaint of lower abdominal pain, back/flank pain, or any pain that radiates to the groin. The cremasteric reflex should be assessed because it can help differentiate among the causes of testicular pain.7 It is performed by gently stroking the upper inner thigh and observing for contraction of the ipsilateral testicle. One study found that, in cases of torsion, the absence of a cremasteric reflex had a sensitivity of 96% and a specificity of 88%.7 See the Table for the differential diagnosis for acute testicular pain.
While it is often possible to make the diagnosis of testicular torsion clinically, ultrasound with color Doppler is the diagnostic test of choice in cases for which the cause of acute scrotal pain is unclear.8 Ultrasound provides anatomic detail of the scrotum and its contents, and perfusion is assessed by adding the color Doppler images.8 It is important to note that, while the absence of blood flow is considered diagnostic for testicular torsion, the presence of flow does not necessarily exclude it.4
On the next page: Treatment >>
Treatment
Surgical exploration with intraoperative detorsion and orchiopexy (fixation of the testicle to the scrotal wall) is the mainstay of treatment for testicular torsion.1 Orchiopexy is often performed bilaterally in order to prevent future torsion of the unaffected testicle. In about 40% of males with the bell clapper deformity, the condition is present on both sides.2 Orchiectomy, the complete removal of the testicle, is necessary when the degree of torsion and subsequent ischemia have caused irreversible damage to the testicle.6 In one study in which 2,248 cases of torsion were reviewed, approximately 34% of males required orchiectomy.6
If surgery may be delayed, the clinician may attempt manual detorsion at the bedside. Despite the “open book” method described in many texts—which instructs the practitioner to rotate the testicle laterally—a review of the literature reveals that torsion takes place medially only 70% of the time.1,5 The clinician should always consider this when any attempts at manual detorsion are made and correlate his or her technique with physical examination and the patient’s response.5
Relief of pain and return of the testicle to its natural longitudinal lie are considered indicators of successful detorsion.1 Color Doppler ultrasound should be used to confirm the return of circulation. However, in one case review of pediatric patients who underwent surgical exploration after manual detorsion, some degree of residual torsion remained in 32%.5 Because of this risk, surgery is still indicated even in cases of successful bedside detorsion.5
On the next page: Case continuation >>
CASE CONTINUATION
The decision to perform bedside ultrasound was made because the diagnosis of testicular torsion is a surgical emergency, and the window of time to prevent complications can be extremely narrow. If the ultrasound had been normal, then a CT scan may have provided additional data on which to base the diagnosis.
The patient was given adequate parenteral pain medication. After color Doppler ultrasound confirmed the torsion, the testicle was laterally rotated approximately 360°. The patient reported alleviation of his symptoms. Color Doppler was again performed to confirm the return of hyperemic blood flow to the affected testicle (Figure 2). The urologist arrived shortly thereafter and the patient was taken to the operating room, where he underwent scrotal exploration and bilateral orchiopexy.
On the next page: Conclusion >>
CONCLUSION
A testicular examination should be performed on any male presenting with a chief complaint of lower abdominal pain, back/flank pain, or any pain that radiates to the groin. Testicular torsion is most commonly seen in infants and adolescents but can occur at any age. The condition is a surgical emergency and the goal is testicular salvage, which is most likely to occur before 12 hours have elapsed since the onset of symptoms. An important component of the physical examination is attempting to elicit the cremasteric reflex, which is likely to be absent in the presence of torsion.
The primary care provider’s goal is to rapidly diagnose testicular torsion, then refer the patient immediately to a urologist or ED. The skilled clinician may attempt manual detorsion, based on his/her expertise and comfort level; however, this procedure should never delay prompt surgical intervention.
REFERENCES
1. Eyre RC. Evaluation of the acute scrotum in adults. www.uptodate.com/contents/evaluation-of-the-acute-scrotum-in-adults. Accessed May 16, 2014.
2. Ogunyemi OI, Weiker M, Abel EJ. Testicular torsion. http://emedicine.medscape.com/article/2036003-overview. Accessed May 16, 2014.
3. Khan F, Muoka O, Watson GM. Bell clapper testis, torsion, and detorsion: a case report. Case Rep Urol. 2011;2011:631970.
4. Molokwu CN, Somani BK, Goodman CM. Outcomes of scrotal exploration for acute scrotal pain suspicious of testicular torsion: a consecutive case series of 173 patients. BJU Int. 2011;107(6):990-993.
5. Sessions AE, Rabinowitz R, Hulbert WC, et al. Testicular torsion: direction, degree, duration and disinformation. J Urol. 2003;169(2):663-665.
6. Mansbach JM, Forbes P, Peters C. Testicular torsion and risk factors for orchiectomy. Arch Pediatr Adolesc Med. 2005;159:1167-1171.
7. Schmitz D, Safranek S. How useful is a physical exam in diagnosing testicular torsion? J Fam Pract. 2009;58(8):433-434.
8. D’Andrea A, Coppolino F, Cesarano E, et al. US in the assessment of acute scrotum. Crit Ultrasound J. 2013;5(suppl 1):S8. www.criticalultrasound journal.com/content/5/S1/S8/. Accessed May 16, 2014.
Chronic vulvar irritation, itching, and pain. What is the diagnosis?
Chronic irritation, itching, and pain are only rarely due to infection. These symptoms are more likely to be caused by dermatoses, vaginal abnormalities, and pain syndromes that may be difficult to diagnose. Careful evaluation should include a wet mount and culture to eliminate infection as a cause so that the correct diagnosis can be ascertained and treated.
In Part 2 of this two-part series, we focus on five cases of vulvar dermatologic disruptions:
- atrophic vagina
- irritant and allergic contact dermatitis
- complex vulvar aphthosis
- desquamative inflammatory vaginitis
- inverse psoriasis.
CASE 1. INTROITAL BURNING AND A FEAR OF BREAST CANCER
A 56-year-old woman visits your office for management of recent-onset introital burning during sexual activity. She reports that her commercial lubricant causes irritation. Topical and oral antifungal therapies have not been beneficial. She has a strong family history of breast cancer.
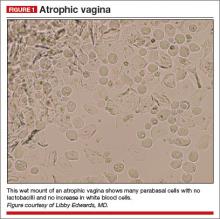
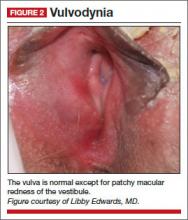
On examination, she exhibits small, smooth labia minora and experiences pain when a cotton swab is pressed against the vestibule. The vagina is also smooth, with scant secretions. Microscopically, these secretions are almost acellular, with no increase in white blood cells and no clue cells, yeast forms, or lactobacilli. The pH is greater than 6.5, and most epithelial cells are parabasal (FIGURE 1).
You prescribe topical estradiol cream for vaginal use three nights per week, but when the patient returns 1 month later, her condition is unchanged. She explains that she never used the cream after reading the package insert, which reports a risk of breast cancer.
Diagnosis: Atrophic vagina (not atrophic vaginitis, as there is no increase in white blood cells).
Treatment: Re-estrogenization should relieve her symptoms.
Several options for local estrogen replacement are available. Creams include estradiol (Estrace) and conjugated equine estrogen (Premarin), the latter of which is arguably slightly more irritating. These are prescribed at a starting dose of 1 g in the vagina three nights per week. After several weeks, they can be titrated to the lowest frequency that controls symptoms.
The risk of vaginal candidiasis is fairly high during the first 2 or 3 weeks of re-estrogenization, so patients should be warned of this possibility. Also consider prophylactic weekly fluconazole or an azole suppository two or three times a week for the first few weeks. Estradiol tablets (Vagifem) inserted in the vagina are effective, less messy, and more expensive, as is the estradiol ring (Estring), which is inserted and changed quarterly.
It is not unusual for a woman to avoid use of topical estrogen out of fear, or to use insufficient amounts only on the vulva, or to use it for only 1 or 2 weeks.1
Women should be scheduled for a return visit to ensure they have been using the estrogen, their wet mount has normalized, and discomfort has cleared.
Related article: Your menopausal patient's breast biopsy reveals atypical hyperplasia. JoAnn V. Pinkerton, MD (Cases in Menopause; May 2013)
When a woman is reluctant to use local estrogen
We counsel women that small doses of vaginal estrogen used for limited periods of time are unlikely to influence their breast cancer risk and are the most effective treatment for symptoms of atrophy. Usually, this explanation is sufficient to reassure a woman that topical estrogen is safe. Otherwise, use of commercial personal lubricants (silicone-based lubricants are well tolerated) and moisturizers such as Replens and RePhresh can be comforting.
The topical anesthetics lidocaine 2% jelly or lidocaine 5% ointment (which sometimes burns) can minimize pain with sexual activity for those requiring more than lubrication.
Ospemifene (Osphena) is used by some clinicians in this situation, but this medication is labeled as a risk for all of the same contraindications as systemic estrogen, and it is much more expensive than topical estrogen. Ospemifene is an estrogen agonist/antagonist. Although it is the only oral medication indicated for the treatment of menopause-related dyspareunia, the long-term effects on breast cancer risk are unknown. Also, it has an agonist effect on the endometrium and, again, the long-term risk is unknown.
Related article: New treatment option for vulvar and vaginal atrophy. Andrew M. Kaunitz, MD (News for your Practice; May 2013)
Fluconazole use is contraindicated with ospemifene, as is the use of any estrogen products.
CASE 2. RECALCITRANT ITCHING, BURNING, AND REDNESS
A 25-year-old woman reports anogenital itching, burning, and redness, which have been present for 3 months. She says she developed a yeast infection after antibiotic therapy for a dental infection; the yeast infection was treated with terconazole. She reports an allergic reaction to the terconazole, with immediate severe burning, redness, and swelling. The clobetasol cream she was given to use twice daily also caused burning, so she discontinued it. Her symptoms improved when she tried cool soaks and applied topical benzocaine gel as a local anesthetic. However,
2 weeks later, she experienced increasing redness, itching, and burning. Although the benzocaine relieved these symptoms, it required almost continual reapplication for comfort.
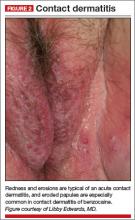
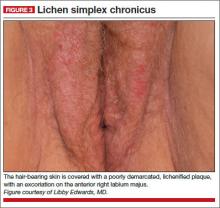
A physical examination of the vulva reveals generalized, poorly demarcated redness, edema, and superficial erosions (FIGURE 2).
Diagnosis: Irritant contact dermatitis (as opposed to allergic contact dermatitis) associated with the use of terconazole and clobetasol. This was followed by allergic contact dermatitis in association with benzocaine.
Treatment: Withdrawal of benzocaine, with reinitiation of cool soaks and a switch to clobetasol ointment rather than cream. Nighttime sedation allows the patient to sleep through the itching and gradually allows her skin to heal.
Contact dermatitis is a fairly common cause of vulvar irritation, with two main types:
- Irritant contact dermatitis—The most common form, it occurs in any individual exposed to an irritating substance in sufficient quantity or frequency. Irritant contact dermatitis is characterized mostly by sensations of rawness or burning and generally is caused by urine, feces, perspiration, friction, alcohols in topical creams, overwashing, and use of harsh soaps.
- Allergic contact dermatitis—This form is characterized by itching, although secondary pain and burning from scratching and blistering can occur as well. Common allergens in the genital area include benzocaine, diphenhydramine (Benadryl), neomycin in triple antibiotic ointment (Neosporin), and latex. Allergic contact dermatitis occurs after 1 or 2 weeks of initial exposure or 1 or 2 days after re-exposure.
The diagnosis of an irritant or allergic contact dermatitis can be based on a history of incontinence, application of high-risk substances, or inappropriate washing. Management generally involves discontinuation of all panty liners and topical agents except for water, with a topical steroid ointment used twice a day and pure petroleum jelly used as often as necessary for comfort. Nighttime sedation to allow a reprieve from rubbing and scratching may be helpful, and narcotic pain medications may be useful for the first 1 to 2 weeks of treatment.
Women who fail to respond to treatment should be referred for patch testing by a
dermatologist.
Related article: Vulvar pain syndromes: Making the correct diagnosis. Neal M. Lonky, MD, MPH; Libby Edwards, MD; Jennifer Gunter, MD; Hope K. Haefner, MD (Roundtable, part 1 of 3; September 2011)
CASE 3. TEENAGER WITH VULVAR PAIN AND SORES
A woman brings her 13-year-old daughter to your office for treatment of sudden-onset vulvar pain and sores. The child developed a sore throat and low-grade fever 3 days earlier, with vulvar pain and vulvar dysuria the next day. The pediatrician diagnosed a herpes simplex virus infection and prescribed oral acyclovir, but the girl’s condition has not improved, and the mother believes her daughter’s claims of sexual abstinence.
The girl is otherwise healthy, aside from a history of trivial oral canker sores without arthritis, headaches, abdominal pain, eye pain, or vision changes.
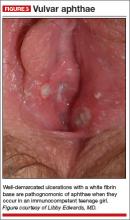
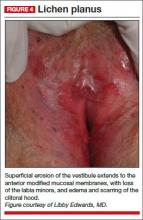
Physical examination of the vulva reveals soft, painful, well-demarcated ulcers with a white fibrin base (FIGURE 3).
Diagnosis: Complex aphthosis. Further testing is unnecessary.
Treatment: Prednisone 40 mg/day plus hydrocodone in usual doses of 5/325, one or two tablets every 4 to 6 hours, as needed; topical petroleum jelly (especially before urination); and sitz baths. When the patient returns 1 week later, she is much improved.
Aphthae are believed to be of hyperimmune origin, often precipitated by a viral syndrome. They are most common in girls aged 9 to 18 years. Vulvar aphthae are triggered by various viral infections, including Epstein-Barr.2 The offending virus is not located in the ulcer proper, however, but is identified serologically.
Aphthae are uncommon and under-recognized on the vulva, and genital aphthae are usually much larger than oral aphthae. Most patients initially are mistakenly evaluated and treated for sexually transmitted disease, but the large, well-demarcated, painful, nonindurated deep nature of the ulcer is pathognomonic for an aphthous ulcer.
The presence of oral and genital aphthae does not constitute a diagnosis of Behçet disease, an often-devastating systemic inflammatory condition occurring almost exclusively in men in the Middle and Far East. The diagnosis of Behçet disease requires the identification of objective inflammatory disease of the eyes, joints, gastrointestinal tract, or neurologic system. True Behçet disease is incredibly uncommon in the United States. When it is diagnosed in Western countries, it takes an attenuated form, most often occurring in women who experience multisystem discomfort rather than identifiable inflammatory disease. End-organ damage is uncommon. Evaluation for Behçet disease in women with vulvar aphthae generally is not indicated, although a directed review of systems is reasonable. The rare patient who experiences frequent recurrence and symptoms of systemic disease should be referred to an ophthalmologist and other relevant specialists to evaluate for inflammatory disease.
The treatment of vulvar aphthae consists of systemic corticosteroids such as prednisone 40 mg/day for smaller individuals and 60 mg/day for larger women, with follow-up to ensure a good response. Often, the prednisone can be discontinued when pain relents rather than continued through complete healing. Reassurance, without discussing Behçet disease, is paramount, as is pain control. The heavy application of petroleum jelly can decrease pain and prevent urine from touching the ulcer.
Some patients experience recurrent ulcers. A second prescription of prednisone can be provided for immediate reinstitution with onset of symptoms. However, frequent recurrences may require ongoing suppressive medication, with dapsone being the usual first choice. Colchicine often is used, and thalidomide and tumor necrosis factor-a blockers (adalimumab, etanercept, and infliximab) also are extremely beneficial.3,4
CASE 4. INCREASED NEUTROPHILS AND NO LACTOBACILLI
A 36-year-old woman visits your office reporting introital itching, vulvar dysuria, and superficial dyspareunia that have lasted 6 months. She has tried over-the-counter antifungal therapy, with only slight improvement while using the cream. Her health is normal otherwise, lacking pain syndromes or abnormalities suggestive of pelvic floor dysfunction. She experienced comfortable sexual activity until 6 months ago.
The only abnormalities apparent on physical examination are redness of the vestibule, medial labia minora, and vaginal walls, with edema of the surrounding skin and no oral lesions (FIGURE 4A). Copious vaginal secretions are visible at the introitus. A wet mount shows a marked increase in neutrophils with scattered parabasal cells (FIGURE 4B). There are no clue cells, lactobacilli, or yeast forms. The patient’s pH level is greater than 6.5. Routine and fungal cultures and molecular studies for chlamydia, trichomonas, and gonorrhea are returned as normal.
Diagnosis: Desquamative inflammatory vaginitis.
Treatment: Clindamycin vaginal cream, 1/2 to 1 full applicator nightly, with a weekly oral fluconazole tablet (200 mg is more easily covered by insurance) to prevent secondary candidiasis. You schedule a follow-up visit in 1 month.
Desquamative inflammatory vaginitis (DIV) is described as noninfectious inflammatory vaginitis in a setting of normal estrogen and absence of skin disease of the mucous membranes of the vagina. The condition is characterized by an increase in white blood cells and parabasal cells, and absent lactobacilli, with relatively high vaginal pH. DIV is thought to represent an inflammatory dermatosis of the vaginal epithelium.5 Although some clinicians believe that DIV is actually lichen planus, the latter exhibits erosions as well as redness, nearly always affects the mouth and the vulva, and produces remarkable scarring. DIV does not erode, affect any other skin surfaces, or scar.
Other rare skin diseases that produce erosions and scarring also can be ruled out by the presence of erosions, absence of oral disease, and absence of other mucosal involvement. These diseases include cicatricial pemphigoid, pemphigus vulgaris, Stevens-Johnson syndrome, and toxic epidermal necrolysis. Infectious diseases characterized by inflammation are excluded by culture or molecular studies, and atrophic vaginitis and retained foreign bodies (especially retained tampons) can produce a similar picture.
The vulvar itching and irritation that occur with DIV most likely represent an irritant contact dermatitis, with vaginal secretions serving as the irritant.
How to treat DIV
The management of DIV consists of either topical clindamycin cream (theoretically for its anti-inflammatory rather than antimicrobial properties) or intravaginal corticosteroids, especially hydrocortisone acetate.6 Hydrocortisone can be tried at the low commercially available dose of 25-mg rectal suppositories, which should be inserted into the vagina nightly, or it can be compounded at 100 or 200 mg, if needed. If the condition is recalcitrant, combination therapy can be used.
When signs and symptoms abate, the frequency of use can be decreased, or hydrocortisone can be discontinued and restarted again with any recurrence of discomfort. Many clinicians also prescribe weekly fluconazole to prevent intercurrent candidiasis.
Related article: Successful treatment of chronic vaginitis. Robert L. Barbieri, MD (Editorial, July 2013)
CASE 5. PLAQUES ON VULVA AND IN SKIN FOLDS
A 43-year-old woman reports a recalcitrant yeast infection of the vulva, with itching and irritation. She is overweight and diabetic, with mild stress incontinence.
Physical examination reveals a fairly well-demarcated plaque of redness of the vulva and labiocrural folds, with satellite red papules and peripheral peeling (FIGURE 5). An examination of other skin surfaces reveals similar plaques in the gluteal cleft, umbilicus, and axillae as well as under the breasts. A fungal preparation of the vagina and skin is negative. You obtain a fungal culture and prescribe topical and oral antifungal therapy and see the patient again 1 week later. Her condition is unchanged.
Diagnosis: You make a presumptive diagnosis of inverse psoriasis and do a confirmatory punch biopsy.
Treatment: Clobetasol ointment applied to the skin folds, along with continuation of the topical miconazole cream. A week later, the patient’s condition is remarkably improved, and her biopsy shows psoriasiform dermatitis. You reduce the potency of her corticosteroid, switching to desonide cream sparingly applied daily.
Psoriasis is a common skin disease of immunologic origin. The skin is classically red and thick, with heavy white scale produced by rapid turnover of epithelium. However, there are several morphologic types of psoriasis, and anogenital psoriasis is most often of the inverse pattern. Inverse psoriasis preferentially affects skin folds and is frequently mistaken for (and often initially superinfected with) candidiasis. Scale is thin and unapparent, and there often is a shiny, glazed appearance to the skin. Tiny satellite lesions often are visible as well. A skin biopsy of inverse psoriasis often is not diagnostic, showing only nonspecific psoriasiform dermatitis; this does not disprove psoriasis.
Psoriasis is a systemic condition and is associated with metabolic syndrome, carrying an increased risk of overweight, hypertension, diabetes, and cardiovascular disease. Management of these conditions is very important in the treatment of the patient overall.
Unlike lichen planus and lichen sclerosus, scarring is rare with psoriasis, and squamous cell carcinoma generally is unassociated.7,8
Anogenital psoriasis is treated with topical corticosteroids and, when needed, topical vitamin D preparations. Generally, inverse psoriasis is controlled with low-potency topical corticosteroids, with management of secondary infection and irritants. Otherwise, ultraviolet light is a time-honored therapy for psoriasis but not practical for skin folds. It also is difficult for many patients to manage with a busy life. Systemic therapy, including methotrexate and oral retinoids are often used, as are newer biologic agents such as etanercept, adalimumab, infliximab, and ustekinumab.
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article or on any topic relevant to ObGyns and women’s health practitioners. Tell us which topics you’d like to see covered in future issues, and what challenges you face in daily practice. We will consider publishing your letter and in a future issue. Send your letter to: [email protected] Please include the city and state in which you practice. Stay in touch! Your feedback is important to us!
- Kingsberg SA, Krychman ML. Resistance and barriers to local estrogen therapy in women with atrophic vaginitis.
J Sex Med. 2013;10(6):1567–1574. - Huppert JS, Gerber MA, Deitch HR, et al. Vulvar ulcers in young females: A manifestation of aphthosis. J Pediatr Adolesc Gynecol. 2006;19(3):195–204.
- O’Neill ID. Efficacy of tumour necrosis factor-a antagonists in aphthous ulceration: Review of published individual patient data. J Eur Acad Dermatol Venereol. 2012;26(2):231–235.
- Sanchez-Cano D, Callejas-Rubio JL, Ruiz-Villaverde R, Ortego-Centeno N. Recalcitrant, recurrent aphthous stomatitis successfully treated with adalimumab. J Eur Acad Dermatol Venereol. 2009;23(2):206.
- Stockdale CK. Clinical spectrum of desquamative inflammatory vaginitis. Curr Infect Dis Rep. 2010;12(6):479–483.
- Sobel JD, Reichman O, Misra D, Yoo W. Prognosis and treatment of desquamative inflammatory vaginitis. Obstet Gynecol. 2011;117(4):850–855.
- Albert S, Neill S, Derrick EK, Calonje E. Psoriasis associated with vulvar scarring. Clin Exp Dermatol. 2004;29(4):354–356.
- Boffetta P, Gridley G, Lindelöf B. Cancer risk in a population-based cohort of patients hospitalized for psoriasis in Sweden. J Invest Dermatol. 2001;117(6):1531–1537.
Chronic irritation, itching, and pain are only rarely due to infection. These symptoms are more likely to be caused by dermatoses, vaginal abnormalities, and pain syndromes that may be difficult to diagnose. Careful evaluation should include a wet mount and culture to eliminate infection as a cause so that the correct diagnosis can be ascertained and treated.
In Part 2 of this two-part series, we focus on five cases of vulvar dermatologic disruptions:
- atrophic vagina
- irritant and allergic contact dermatitis
- complex vulvar aphthosis
- desquamative inflammatory vaginitis
- inverse psoriasis.
CASE 1. INTROITAL BURNING AND A FEAR OF BREAST CANCER
A 56-year-old woman visits your office for management of recent-onset introital burning during sexual activity. She reports that her commercial lubricant causes irritation. Topical and oral antifungal therapies have not been beneficial. She has a strong family history of breast cancer.
On examination, she exhibits small, smooth labia minora and experiences pain when a cotton swab is pressed against the vestibule. The vagina is also smooth, with scant secretions. Microscopically, these secretions are almost acellular, with no increase in white blood cells and no clue cells, yeast forms, or lactobacilli. The pH is greater than 6.5, and most epithelial cells are parabasal (FIGURE 1).
You prescribe topical estradiol cream for vaginal use three nights per week, but when the patient returns 1 month later, her condition is unchanged. She explains that she never used the cream after reading the package insert, which reports a risk of breast cancer.
Diagnosis: Atrophic vagina (not atrophic vaginitis, as there is no increase in white blood cells).
Treatment: Re-estrogenization should relieve her symptoms.
Several options for local estrogen replacement are available. Creams include estradiol (Estrace) and conjugated equine estrogen (Premarin), the latter of which is arguably slightly more irritating. These are prescribed at a starting dose of 1 g in the vagina three nights per week. After several weeks, they can be titrated to the lowest frequency that controls symptoms.
The risk of vaginal candidiasis is fairly high during the first 2 or 3 weeks of re-estrogenization, so patients should be warned of this possibility. Also consider prophylactic weekly fluconazole or an azole suppository two or three times a week for the first few weeks. Estradiol tablets (Vagifem) inserted in the vagina are effective, less messy, and more expensive, as is the estradiol ring (Estring), which is inserted and changed quarterly.
It is not unusual for a woman to avoid use of topical estrogen out of fear, or to use insufficient amounts only on the vulva, or to use it for only 1 or 2 weeks.1
Women should be scheduled for a return visit to ensure they have been using the estrogen, their wet mount has normalized, and discomfort has cleared.
Related article: Your menopausal patient's breast biopsy reveals atypical hyperplasia. JoAnn V. Pinkerton, MD (Cases in Menopause; May 2013)
When a woman is reluctant to use local estrogen
We counsel women that small doses of vaginal estrogen used for limited periods of time are unlikely to influence their breast cancer risk and are the most effective treatment for symptoms of atrophy. Usually, this explanation is sufficient to reassure a woman that topical estrogen is safe. Otherwise, use of commercial personal lubricants (silicone-based lubricants are well tolerated) and moisturizers such as Replens and RePhresh can be comforting.
The topical anesthetics lidocaine 2% jelly or lidocaine 5% ointment (which sometimes burns) can minimize pain with sexual activity for those requiring more than lubrication.
Ospemifene (Osphena) is used by some clinicians in this situation, but this medication is labeled as a risk for all of the same contraindications as systemic estrogen, and it is much more expensive than topical estrogen. Ospemifene is an estrogen agonist/antagonist. Although it is the only oral medication indicated for the treatment of menopause-related dyspareunia, the long-term effects on breast cancer risk are unknown. Also, it has an agonist effect on the endometrium and, again, the long-term risk is unknown.
Related article: New treatment option for vulvar and vaginal atrophy. Andrew M. Kaunitz, MD (News for your Practice; May 2013)
Fluconazole use is contraindicated with ospemifene, as is the use of any estrogen products.
CASE 2. RECALCITRANT ITCHING, BURNING, AND REDNESS
A 25-year-old woman reports anogenital itching, burning, and redness, which have been present for 3 months. She says she developed a yeast infection after antibiotic therapy for a dental infection; the yeast infection was treated with terconazole. She reports an allergic reaction to the terconazole, with immediate severe burning, redness, and swelling. The clobetasol cream she was given to use twice daily also caused burning, so she discontinued it. Her symptoms improved when she tried cool soaks and applied topical benzocaine gel as a local anesthetic. However,
2 weeks later, she experienced increasing redness, itching, and burning. Although the benzocaine relieved these symptoms, it required almost continual reapplication for comfort.
A physical examination of the vulva reveals generalized, poorly demarcated redness, edema, and superficial erosions (FIGURE 2).
Diagnosis: Irritant contact dermatitis (as opposed to allergic contact dermatitis) associated with the use of terconazole and clobetasol. This was followed by allergic contact dermatitis in association with benzocaine.
Treatment: Withdrawal of benzocaine, with reinitiation of cool soaks and a switch to clobetasol ointment rather than cream. Nighttime sedation allows the patient to sleep through the itching and gradually allows her skin to heal.
Contact dermatitis is a fairly common cause of vulvar irritation, with two main types:
- Irritant contact dermatitis—The most common form, it occurs in any individual exposed to an irritating substance in sufficient quantity or frequency. Irritant contact dermatitis is characterized mostly by sensations of rawness or burning and generally is caused by urine, feces, perspiration, friction, alcohols in topical creams, overwashing, and use of harsh soaps.
- Allergic contact dermatitis—This form is characterized by itching, although secondary pain and burning from scratching and blistering can occur as well. Common allergens in the genital area include benzocaine, diphenhydramine (Benadryl), neomycin in triple antibiotic ointment (Neosporin), and latex. Allergic contact dermatitis occurs after 1 or 2 weeks of initial exposure or 1 or 2 days after re-exposure.
The diagnosis of an irritant or allergic contact dermatitis can be based on a history of incontinence, application of high-risk substances, or inappropriate washing. Management generally involves discontinuation of all panty liners and topical agents except for water, with a topical steroid ointment used twice a day and pure petroleum jelly used as often as necessary for comfort. Nighttime sedation to allow a reprieve from rubbing and scratching may be helpful, and narcotic pain medications may be useful for the first 1 to 2 weeks of treatment.
Women who fail to respond to treatment should be referred for patch testing by a
dermatologist.
Related article: Vulvar pain syndromes: Making the correct diagnosis. Neal M. Lonky, MD, MPH; Libby Edwards, MD; Jennifer Gunter, MD; Hope K. Haefner, MD (Roundtable, part 1 of 3; September 2011)
CASE 3. TEENAGER WITH VULVAR PAIN AND SORES
A woman brings her 13-year-old daughter to your office for treatment of sudden-onset vulvar pain and sores. The child developed a sore throat and low-grade fever 3 days earlier, with vulvar pain and vulvar dysuria the next day. The pediatrician diagnosed a herpes simplex virus infection and prescribed oral acyclovir, but the girl’s condition has not improved, and the mother believes her daughter’s claims of sexual abstinence.
The girl is otherwise healthy, aside from a history of trivial oral canker sores without arthritis, headaches, abdominal pain, eye pain, or vision changes.
Physical examination of the vulva reveals soft, painful, well-demarcated ulcers with a white fibrin base (FIGURE 3).
Diagnosis: Complex aphthosis. Further testing is unnecessary.
Treatment: Prednisone 40 mg/day plus hydrocodone in usual doses of 5/325, one or two tablets every 4 to 6 hours, as needed; topical petroleum jelly (especially before urination); and sitz baths. When the patient returns 1 week later, she is much improved.
Aphthae are believed to be of hyperimmune origin, often precipitated by a viral syndrome. They are most common in girls aged 9 to 18 years. Vulvar aphthae are triggered by various viral infections, including Epstein-Barr.2 The offending virus is not located in the ulcer proper, however, but is identified serologically.
Aphthae are uncommon and under-recognized on the vulva, and genital aphthae are usually much larger than oral aphthae. Most patients initially are mistakenly evaluated and treated for sexually transmitted disease, but the large, well-demarcated, painful, nonindurated deep nature of the ulcer is pathognomonic for an aphthous ulcer.
The presence of oral and genital aphthae does not constitute a diagnosis of Behçet disease, an often-devastating systemic inflammatory condition occurring almost exclusively in men in the Middle and Far East. The diagnosis of Behçet disease requires the identification of objective inflammatory disease of the eyes, joints, gastrointestinal tract, or neurologic system. True Behçet disease is incredibly uncommon in the United States. When it is diagnosed in Western countries, it takes an attenuated form, most often occurring in women who experience multisystem discomfort rather than identifiable inflammatory disease. End-organ damage is uncommon. Evaluation for Behçet disease in women with vulvar aphthae generally is not indicated, although a directed review of systems is reasonable. The rare patient who experiences frequent recurrence and symptoms of systemic disease should be referred to an ophthalmologist and other relevant specialists to evaluate for inflammatory disease.
The treatment of vulvar aphthae consists of systemic corticosteroids such as prednisone 40 mg/day for smaller individuals and 60 mg/day for larger women, with follow-up to ensure a good response. Often, the prednisone can be discontinued when pain relents rather than continued through complete healing. Reassurance, without discussing Behçet disease, is paramount, as is pain control. The heavy application of petroleum jelly can decrease pain and prevent urine from touching the ulcer.
Some patients experience recurrent ulcers. A second prescription of prednisone can be provided for immediate reinstitution with onset of symptoms. However, frequent recurrences may require ongoing suppressive medication, with dapsone being the usual first choice. Colchicine often is used, and thalidomide and tumor necrosis factor-a blockers (adalimumab, etanercept, and infliximab) also are extremely beneficial.3,4
CASE 4. INCREASED NEUTROPHILS AND NO LACTOBACILLI
A 36-year-old woman visits your office reporting introital itching, vulvar dysuria, and superficial dyspareunia that have lasted 6 months. She has tried over-the-counter antifungal therapy, with only slight improvement while using the cream. Her health is normal otherwise, lacking pain syndromes or abnormalities suggestive of pelvic floor dysfunction. She experienced comfortable sexual activity until 6 months ago.
The only abnormalities apparent on physical examination are redness of the vestibule, medial labia minora, and vaginal walls, with edema of the surrounding skin and no oral lesions (FIGURE 4A). Copious vaginal secretions are visible at the introitus. A wet mount shows a marked increase in neutrophils with scattered parabasal cells (FIGURE 4B). There are no clue cells, lactobacilli, or yeast forms. The patient’s pH level is greater than 6.5. Routine and fungal cultures and molecular studies for chlamydia, trichomonas, and gonorrhea are returned as normal.
Diagnosis: Desquamative inflammatory vaginitis.
Treatment: Clindamycin vaginal cream, 1/2 to 1 full applicator nightly, with a weekly oral fluconazole tablet (200 mg is more easily covered by insurance) to prevent secondary candidiasis. You schedule a follow-up visit in 1 month.
Desquamative inflammatory vaginitis (DIV) is described as noninfectious inflammatory vaginitis in a setting of normal estrogen and absence of skin disease of the mucous membranes of the vagina. The condition is characterized by an increase in white blood cells and parabasal cells, and absent lactobacilli, with relatively high vaginal pH. DIV is thought to represent an inflammatory dermatosis of the vaginal epithelium.5 Although some clinicians believe that DIV is actually lichen planus, the latter exhibits erosions as well as redness, nearly always affects the mouth and the vulva, and produces remarkable scarring. DIV does not erode, affect any other skin surfaces, or scar.
Other rare skin diseases that produce erosions and scarring also can be ruled out by the presence of erosions, absence of oral disease, and absence of other mucosal involvement. These diseases include cicatricial pemphigoid, pemphigus vulgaris, Stevens-Johnson syndrome, and toxic epidermal necrolysis. Infectious diseases characterized by inflammation are excluded by culture or molecular studies, and atrophic vaginitis and retained foreign bodies (especially retained tampons) can produce a similar picture.
The vulvar itching and irritation that occur with DIV most likely represent an irritant contact dermatitis, with vaginal secretions serving as the irritant.
How to treat DIV
The management of DIV consists of either topical clindamycin cream (theoretically for its anti-inflammatory rather than antimicrobial properties) or intravaginal corticosteroids, especially hydrocortisone acetate.6 Hydrocortisone can be tried at the low commercially available dose of 25-mg rectal suppositories, which should be inserted into the vagina nightly, or it can be compounded at 100 or 200 mg, if needed. If the condition is recalcitrant, combination therapy can be used.
When signs and symptoms abate, the frequency of use can be decreased, or hydrocortisone can be discontinued and restarted again with any recurrence of discomfort. Many clinicians also prescribe weekly fluconazole to prevent intercurrent candidiasis.
Related article: Successful treatment of chronic vaginitis. Robert L. Barbieri, MD (Editorial, July 2013)
CASE 5. PLAQUES ON VULVA AND IN SKIN FOLDS
A 43-year-old woman reports a recalcitrant yeast infection of the vulva, with itching and irritation. She is overweight and diabetic, with mild stress incontinence.
Physical examination reveals a fairly well-demarcated plaque of redness of the vulva and labiocrural folds, with satellite red papules and peripheral peeling (FIGURE 5). An examination of other skin surfaces reveals similar plaques in the gluteal cleft, umbilicus, and axillae as well as under the breasts. A fungal preparation of the vagina and skin is negative. You obtain a fungal culture and prescribe topical and oral antifungal therapy and see the patient again 1 week later. Her condition is unchanged.
Diagnosis: You make a presumptive diagnosis of inverse psoriasis and do a confirmatory punch biopsy.
Treatment: Clobetasol ointment applied to the skin folds, along with continuation of the topical miconazole cream. A week later, the patient’s condition is remarkably improved, and her biopsy shows psoriasiform dermatitis. You reduce the potency of her corticosteroid, switching to desonide cream sparingly applied daily.
Psoriasis is a common skin disease of immunologic origin. The skin is classically red and thick, with heavy white scale produced by rapid turnover of epithelium. However, there are several morphologic types of psoriasis, and anogenital psoriasis is most often of the inverse pattern. Inverse psoriasis preferentially affects skin folds and is frequently mistaken for (and often initially superinfected with) candidiasis. Scale is thin and unapparent, and there often is a shiny, glazed appearance to the skin. Tiny satellite lesions often are visible as well. A skin biopsy of inverse psoriasis often is not diagnostic, showing only nonspecific psoriasiform dermatitis; this does not disprove psoriasis.
Psoriasis is a systemic condition and is associated with metabolic syndrome, carrying an increased risk of overweight, hypertension, diabetes, and cardiovascular disease. Management of these conditions is very important in the treatment of the patient overall.
Unlike lichen planus and lichen sclerosus, scarring is rare with psoriasis, and squamous cell carcinoma generally is unassociated.7,8
Anogenital psoriasis is treated with topical corticosteroids and, when needed, topical vitamin D preparations. Generally, inverse psoriasis is controlled with low-potency topical corticosteroids, with management of secondary infection and irritants. Otherwise, ultraviolet light is a time-honored therapy for psoriasis but not practical for skin folds. It also is difficult for many patients to manage with a busy life. Systemic therapy, including methotrexate and oral retinoids are often used, as are newer biologic agents such as etanercept, adalimumab, infliximab, and ustekinumab.
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article or on any topic relevant to ObGyns and women’s health practitioners. Tell us which topics you’d like to see covered in future issues, and what challenges you face in daily practice. We will consider publishing your letter and in a future issue. Send your letter to: [email protected] Please include the city and state in which you practice. Stay in touch! Your feedback is important to us!
Chronic irritation, itching, and pain are only rarely due to infection. These symptoms are more likely to be caused by dermatoses, vaginal abnormalities, and pain syndromes that may be difficult to diagnose. Careful evaluation should include a wet mount and culture to eliminate infection as a cause so that the correct diagnosis can be ascertained and treated.
In Part 2 of this two-part series, we focus on five cases of vulvar dermatologic disruptions:
- atrophic vagina
- irritant and allergic contact dermatitis
- complex vulvar aphthosis
- desquamative inflammatory vaginitis
- inverse psoriasis.
CASE 1. INTROITAL BURNING AND A FEAR OF BREAST CANCER
A 56-year-old woman visits your office for management of recent-onset introital burning during sexual activity. She reports that her commercial lubricant causes irritation. Topical and oral antifungal therapies have not been beneficial. She has a strong family history of breast cancer.
On examination, she exhibits small, smooth labia minora and experiences pain when a cotton swab is pressed against the vestibule. The vagina is also smooth, with scant secretions. Microscopically, these secretions are almost acellular, with no increase in white blood cells and no clue cells, yeast forms, or lactobacilli. The pH is greater than 6.5, and most epithelial cells are parabasal (FIGURE 1).
You prescribe topical estradiol cream for vaginal use three nights per week, but when the patient returns 1 month later, her condition is unchanged. She explains that she never used the cream after reading the package insert, which reports a risk of breast cancer.
Diagnosis: Atrophic vagina (not atrophic vaginitis, as there is no increase in white blood cells).
Treatment: Re-estrogenization should relieve her symptoms.
Several options for local estrogen replacement are available. Creams include estradiol (Estrace) and conjugated equine estrogen (Premarin), the latter of which is arguably slightly more irritating. These are prescribed at a starting dose of 1 g in the vagina three nights per week. After several weeks, they can be titrated to the lowest frequency that controls symptoms.
The risk of vaginal candidiasis is fairly high during the first 2 or 3 weeks of re-estrogenization, so patients should be warned of this possibility. Also consider prophylactic weekly fluconazole or an azole suppository two or three times a week for the first few weeks. Estradiol tablets (Vagifem) inserted in the vagina are effective, less messy, and more expensive, as is the estradiol ring (Estring), which is inserted and changed quarterly.
It is not unusual for a woman to avoid use of topical estrogen out of fear, or to use insufficient amounts only on the vulva, or to use it for only 1 or 2 weeks.1
Women should be scheduled for a return visit to ensure they have been using the estrogen, their wet mount has normalized, and discomfort has cleared.
Related article: Your menopausal patient's breast biopsy reveals atypical hyperplasia. JoAnn V. Pinkerton, MD (Cases in Menopause; May 2013)
When a woman is reluctant to use local estrogen
We counsel women that small doses of vaginal estrogen used for limited periods of time are unlikely to influence their breast cancer risk and are the most effective treatment for symptoms of atrophy. Usually, this explanation is sufficient to reassure a woman that topical estrogen is safe. Otherwise, use of commercial personal lubricants (silicone-based lubricants are well tolerated) and moisturizers such as Replens and RePhresh can be comforting.
The topical anesthetics lidocaine 2% jelly or lidocaine 5% ointment (which sometimes burns) can minimize pain with sexual activity for those requiring more than lubrication.
Ospemifene (Osphena) is used by some clinicians in this situation, but this medication is labeled as a risk for all of the same contraindications as systemic estrogen, and it is much more expensive than topical estrogen. Ospemifene is an estrogen agonist/antagonist. Although it is the only oral medication indicated for the treatment of menopause-related dyspareunia, the long-term effects on breast cancer risk are unknown. Also, it has an agonist effect on the endometrium and, again, the long-term risk is unknown.
Related article: New treatment option for vulvar and vaginal atrophy. Andrew M. Kaunitz, MD (News for your Practice; May 2013)
Fluconazole use is contraindicated with ospemifene, as is the use of any estrogen products.
CASE 2. RECALCITRANT ITCHING, BURNING, AND REDNESS
A 25-year-old woman reports anogenital itching, burning, and redness, which have been present for 3 months. She says she developed a yeast infection after antibiotic therapy for a dental infection; the yeast infection was treated with terconazole. She reports an allergic reaction to the terconazole, with immediate severe burning, redness, and swelling. The clobetasol cream she was given to use twice daily also caused burning, so she discontinued it. Her symptoms improved when she tried cool soaks and applied topical benzocaine gel as a local anesthetic. However,
2 weeks later, she experienced increasing redness, itching, and burning. Although the benzocaine relieved these symptoms, it required almost continual reapplication for comfort.
A physical examination of the vulva reveals generalized, poorly demarcated redness, edema, and superficial erosions (FIGURE 2).
Diagnosis: Irritant contact dermatitis (as opposed to allergic contact dermatitis) associated with the use of terconazole and clobetasol. This was followed by allergic contact dermatitis in association with benzocaine.
Treatment: Withdrawal of benzocaine, with reinitiation of cool soaks and a switch to clobetasol ointment rather than cream. Nighttime sedation allows the patient to sleep through the itching and gradually allows her skin to heal.
Contact dermatitis is a fairly common cause of vulvar irritation, with two main types:
- Irritant contact dermatitis—The most common form, it occurs in any individual exposed to an irritating substance in sufficient quantity or frequency. Irritant contact dermatitis is characterized mostly by sensations of rawness or burning and generally is caused by urine, feces, perspiration, friction, alcohols in topical creams, overwashing, and use of harsh soaps.
- Allergic contact dermatitis—This form is characterized by itching, although secondary pain and burning from scratching and blistering can occur as well. Common allergens in the genital area include benzocaine, diphenhydramine (Benadryl), neomycin in triple antibiotic ointment (Neosporin), and latex. Allergic contact dermatitis occurs after 1 or 2 weeks of initial exposure or 1 or 2 days after re-exposure.
The diagnosis of an irritant or allergic contact dermatitis can be based on a history of incontinence, application of high-risk substances, or inappropriate washing. Management generally involves discontinuation of all panty liners and topical agents except for water, with a topical steroid ointment used twice a day and pure petroleum jelly used as often as necessary for comfort. Nighttime sedation to allow a reprieve from rubbing and scratching may be helpful, and narcotic pain medications may be useful for the first 1 to 2 weeks of treatment.
Women who fail to respond to treatment should be referred for patch testing by a
dermatologist.
Related article: Vulvar pain syndromes: Making the correct diagnosis. Neal M. Lonky, MD, MPH; Libby Edwards, MD; Jennifer Gunter, MD; Hope K. Haefner, MD (Roundtable, part 1 of 3; September 2011)
CASE 3. TEENAGER WITH VULVAR PAIN AND SORES
A woman brings her 13-year-old daughter to your office for treatment of sudden-onset vulvar pain and sores. The child developed a sore throat and low-grade fever 3 days earlier, with vulvar pain and vulvar dysuria the next day. The pediatrician diagnosed a herpes simplex virus infection and prescribed oral acyclovir, but the girl’s condition has not improved, and the mother believes her daughter’s claims of sexual abstinence.
The girl is otherwise healthy, aside from a history of trivial oral canker sores without arthritis, headaches, abdominal pain, eye pain, or vision changes.
Physical examination of the vulva reveals soft, painful, well-demarcated ulcers with a white fibrin base (FIGURE 3).
Diagnosis: Complex aphthosis. Further testing is unnecessary.
Treatment: Prednisone 40 mg/day plus hydrocodone in usual doses of 5/325, one or two tablets every 4 to 6 hours, as needed; topical petroleum jelly (especially before urination); and sitz baths. When the patient returns 1 week later, she is much improved.
Aphthae are believed to be of hyperimmune origin, often precipitated by a viral syndrome. They are most common in girls aged 9 to 18 years. Vulvar aphthae are triggered by various viral infections, including Epstein-Barr.2 The offending virus is not located in the ulcer proper, however, but is identified serologically.
Aphthae are uncommon and under-recognized on the vulva, and genital aphthae are usually much larger than oral aphthae. Most patients initially are mistakenly evaluated and treated for sexually transmitted disease, but the large, well-demarcated, painful, nonindurated deep nature of the ulcer is pathognomonic for an aphthous ulcer.
The presence of oral and genital aphthae does not constitute a diagnosis of Behçet disease, an often-devastating systemic inflammatory condition occurring almost exclusively in men in the Middle and Far East. The diagnosis of Behçet disease requires the identification of objective inflammatory disease of the eyes, joints, gastrointestinal tract, or neurologic system. True Behçet disease is incredibly uncommon in the United States. When it is diagnosed in Western countries, it takes an attenuated form, most often occurring in women who experience multisystem discomfort rather than identifiable inflammatory disease. End-organ damage is uncommon. Evaluation for Behçet disease in women with vulvar aphthae generally is not indicated, although a directed review of systems is reasonable. The rare patient who experiences frequent recurrence and symptoms of systemic disease should be referred to an ophthalmologist and other relevant specialists to evaluate for inflammatory disease.
The treatment of vulvar aphthae consists of systemic corticosteroids such as prednisone 40 mg/day for smaller individuals and 60 mg/day for larger women, with follow-up to ensure a good response. Often, the prednisone can be discontinued when pain relents rather than continued through complete healing. Reassurance, without discussing Behçet disease, is paramount, as is pain control. The heavy application of petroleum jelly can decrease pain and prevent urine from touching the ulcer.
Some patients experience recurrent ulcers. A second prescription of prednisone can be provided for immediate reinstitution with onset of symptoms. However, frequent recurrences may require ongoing suppressive medication, with dapsone being the usual first choice. Colchicine often is used, and thalidomide and tumor necrosis factor-a blockers (adalimumab, etanercept, and infliximab) also are extremely beneficial.3,4
CASE 4. INCREASED NEUTROPHILS AND NO LACTOBACILLI
A 36-year-old woman visits your office reporting introital itching, vulvar dysuria, and superficial dyspareunia that have lasted 6 months. She has tried over-the-counter antifungal therapy, with only slight improvement while using the cream. Her health is normal otherwise, lacking pain syndromes or abnormalities suggestive of pelvic floor dysfunction. She experienced comfortable sexual activity until 6 months ago.
The only abnormalities apparent on physical examination are redness of the vestibule, medial labia minora, and vaginal walls, with edema of the surrounding skin and no oral lesions (FIGURE 4A). Copious vaginal secretions are visible at the introitus. A wet mount shows a marked increase in neutrophils with scattered parabasal cells (FIGURE 4B). There are no clue cells, lactobacilli, or yeast forms. The patient’s pH level is greater than 6.5. Routine and fungal cultures and molecular studies for chlamydia, trichomonas, and gonorrhea are returned as normal.
Diagnosis: Desquamative inflammatory vaginitis.
Treatment: Clindamycin vaginal cream, 1/2 to 1 full applicator nightly, with a weekly oral fluconazole tablet (200 mg is more easily covered by insurance) to prevent secondary candidiasis. You schedule a follow-up visit in 1 month.
Desquamative inflammatory vaginitis (DIV) is described as noninfectious inflammatory vaginitis in a setting of normal estrogen and absence of skin disease of the mucous membranes of the vagina. The condition is characterized by an increase in white blood cells and parabasal cells, and absent lactobacilli, with relatively high vaginal pH. DIV is thought to represent an inflammatory dermatosis of the vaginal epithelium.5 Although some clinicians believe that DIV is actually lichen planus, the latter exhibits erosions as well as redness, nearly always affects the mouth and the vulva, and produces remarkable scarring. DIV does not erode, affect any other skin surfaces, or scar.
Other rare skin diseases that produce erosions and scarring also can be ruled out by the presence of erosions, absence of oral disease, and absence of other mucosal involvement. These diseases include cicatricial pemphigoid, pemphigus vulgaris, Stevens-Johnson syndrome, and toxic epidermal necrolysis. Infectious diseases characterized by inflammation are excluded by culture or molecular studies, and atrophic vaginitis and retained foreign bodies (especially retained tampons) can produce a similar picture.
The vulvar itching and irritation that occur with DIV most likely represent an irritant contact dermatitis, with vaginal secretions serving as the irritant.
How to treat DIV
The management of DIV consists of either topical clindamycin cream (theoretically for its anti-inflammatory rather than antimicrobial properties) or intravaginal corticosteroids, especially hydrocortisone acetate.6 Hydrocortisone can be tried at the low commercially available dose of 25-mg rectal suppositories, which should be inserted into the vagina nightly, or it can be compounded at 100 or 200 mg, if needed. If the condition is recalcitrant, combination therapy can be used.
When signs and symptoms abate, the frequency of use can be decreased, or hydrocortisone can be discontinued and restarted again with any recurrence of discomfort. Many clinicians also prescribe weekly fluconazole to prevent intercurrent candidiasis.
Related article: Successful treatment of chronic vaginitis. Robert L. Barbieri, MD (Editorial, July 2013)
CASE 5. PLAQUES ON VULVA AND IN SKIN FOLDS
A 43-year-old woman reports a recalcitrant yeast infection of the vulva, with itching and irritation. She is overweight and diabetic, with mild stress incontinence.
Physical examination reveals a fairly well-demarcated plaque of redness of the vulva and labiocrural folds, with satellite red papules and peripheral peeling (FIGURE 5). An examination of other skin surfaces reveals similar plaques in the gluteal cleft, umbilicus, and axillae as well as under the breasts. A fungal preparation of the vagina and skin is negative. You obtain a fungal culture and prescribe topical and oral antifungal therapy and see the patient again 1 week later. Her condition is unchanged.
Diagnosis: You make a presumptive diagnosis of inverse psoriasis and do a confirmatory punch biopsy.
Treatment: Clobetasol ointment applied to the skin folds, along with continuation of the topical miconazole cream. A week later, the patient’s condition is remarkably improved, and her biopsy shows psoriasiform dermatitis. You reduce the potency of her corticosteroid, switching to desonide cream sparingly applied daily.
Psoriasis is a common skin disease of immunologic origin. The skin is classically red and thick, with heavy white scale produced by rapid turnover of epithelium. However, there are several morphologic types of psoriasis, and anogenital psoriasis is most often of the inverse pattern. Inverse psoriasis preferentially affects skin folds and is frequently mistaken for (and often initially superinfected with) candidiasis. Scale is thin and unapparent, and there often is a shiny, glazed appearance to the skin. Tiny satellite lesions often are visible as well. A skin biopsy of inverse psoriasis often is not diagnostic, showing only nonspecific psoriasiform dermatitis; this does not disprove psoriasis.
Psoriasis is a systemic condition and is associated with metabolic syndrome, carrying an increased risk of overweight, hypertension, diabetes, and cardiovascular disease. Management of these conditions is very important in the treatment of the patient overall.
Unlike lichen planus and lichen sclerosus, scarring is rare with psoriasis, and squamous cell carcinoma generally is unassociated.7,8
Anogenital psoriasis is treated with topical corticosteroids and, when needed, topical vitamin D preparations. Generally, inverse psoriasis is controlled with low-potency topical corticosteroids, with management of secondary infection and irritants. Otherwise, ultraviolet light is a time-honored therapy for psoriasis but not practical for skin folds. It also is difficult for many patients to manage with a busy life. Systemic therapy, including methotrexate and oral retinoids are often used, as are newer biologic agents such as etanercept, adalimumab, infliximab, and ustekinumab.
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article or on any topic relevant to ObGyns and women’s health practitioners. Tell us which topics you’d like to see covered in future issues, and what challenges you face in daily practice. We will consider publishing your letter and in a future issue. Send your letter to: [email protected] Please include the city and state in which you practice. Stay in touch! Your feedback is important to us!
- Kingsberg SA, Krychman ML. Resistance and barriers to local estrogen therapy in women with atrophic vaginitis.
J Sex Med. 2013;10(6):1567–1574. - Huppert JS, Gerber MA, Deitch HR, et al. Vulvar ulcers in young females: A manifestation of aphthosis. J Pediatr Adolesc Gynecol. 2006;19(3):195–204.
- O’Neill ID. Efficacy of tumour necrosis factor-a antagonists in aphthous ulceration: Review of published individual patient data. J Eur Acad Dermatol Venereol. 2012;26(2):231–235.
- Sanchez-Cano D, Callejas-Rubio JL, Ruiz-Villaverde R, Ortego-Centeno N. Recalcitrant, recurrent aphthous stomatitis successfully treated with adalimumab. J Eur Acad Dermatol Venereol. 2009;23(2):206.
- Stockdale CK. Clinical spectrum of desquamative inflammatory vaginitis. Curr Infect Dis Rep. 2010;12(6):479–483.
- Sobel JD, Reichman O, Misra D, Yoo W. Prognosis and treatment of desquamative inflammatory vaginitis. Obstet Gynecol. 2011;117(4):850–855.
- Albert S, Neill S, Derrick EK, Calonje E. Psoriasis associated with vulvar scarring. Clin Exp Dermatol. 2004;29(4):354–356.
- Boffetta P, Gridley G, Lindelöf B. Cancer risk in a population-based cohort of patients hospitalized for psoriasis in Sweden. J Invest Dermatol. 2001;117(6):1531–1537.
- Kingsberg SA, Krychman ML. Resistance and barriers to local estrogen therapy in women with atrophic vaginitis.
J Sex Med. 2013;10(6):1567–1574. - Huppert JS, Gerber MA, Deitch HR, et al. Vulvar ulcers in young females: A manifestation of aphthosis. J Pediatr Adolesc Gynecol. 2006;19(3):195–204.
- O’Neill ID. Efficacy of tumour necrosis factor-a antagonists in aphthous ulceration: Review of published individual patient data. J Eur Acad Dermatol Venereol. 2012;26(2):231–235.
- Sanchez-Cano D, Callejas-Rubio JL, Ruiz-Villaverde R, Ortego-Centeno N. Recalcitrant, recurrent aphthous stomatitis successfully treated with adalimumab. J Eur Acad Dermatol Venereol. 2009;23(2):206.
- Stockdale CK. Clinical spectrum of desquamative inflammatory vaginitis. Curr Infect Dis Rep. 2010;12(6):479–483.
- Sobel JD, Reichman O, Misra D, Yoo W. Prognosis and treatment of desquamative inflammatory vaginitis. Obstet Gynecol. 2011;117(4):850–855.
- Albert S, Neill S, Derrick EK, Calonje E. Psoriasis associated with vulvar scarring. Clin Exp Dermatol. 2004;29(4):354–356.
- Boffetta P, Gridley G, Lindelöf B. Cancer risk in a population-based cohort of patients hospitalized for psoriasis in Sweden. J Invest Dermatol. 2001;117(6):1531–1537.
Read Part 1: Chronic vulvar symptoms and dermatologic disruptions: How to make the correct diagnosis (May 2014)
How to identify and manage cesarean-scar pregnancy
Few ObGyn clinicians have faced a patient with a cesarean-scar pregnancy (CSP). Those few were confronted with a management dilemma. Continue the gestation, which would expose the mother to an elevated risk of heavy bleeding? Or terminate the pregnancy? And if termination is the patient’s choice, what is the most effective method?
The literature contains more than 750 reports of CSP, ranging from a single sporadic case to a series of one to two dozen cases. It is impossible to make sense of the numerous treatments used in the past, which were “tested” on extremely small numbers of patients (sometimes as few as one). In this article, we formulate a management plan for the diagnosis and treatment of CSP based on an in-depth review of the published literature and our personal experience in treating more than four dozen patients with CSP.
We’re all familiar with the “epidemic” of cesarean deliveries in this country, including late consequences of cesarean such as placenta previa and morbidly adherent placenta. One of the long-term consequences of cesarean delivery—the first-trimester CSP—is less well known and documented.
Our in-depth review of 751 CSP cases found no less than 30 published therapeutic approaches.1 No consensus exists as to management guidelines. We have formulated this clinical guide, based on the literature and our experience managing CSP, for clinicians who encounter this dangerous form of pregnancy.2
DIAGNOSIS REQUIRES TRANSVAGINAL SONOGRAPHY
Transvaginal sonography (TVS) is thought to be the best and first-line diagnostic tool, with magnetic resonance imaging (MRI) reserved for cases in which there is a diagnostic problem.
In making a diagnosis, consider two main differential diagnoses:
- Cervical pregnancy—This type of gestation is more likely to occur in women with no history of cesarean delivery
- Spontaneous miscarriage in progress—In a number of cases, the miscarriage happened to be caught on imaging as it passed the area where the CSP usually resides. Because there is no live embryo or fetus in spontaneous miscarriage, a heartbeat cannot be documented.
Components of diagnosis by TVS
Accurate identification of CSP depends on the following sonographic criteria:
- empty uterine cavity and cervical canal (FIGURE 1A)
- close proximity of the gestational sac and the placenta to the anterior uterine surface within the scar or niche of the previous cesarean delivery (FIGURES 1B, 2A, and 2B)
- color flow signals between the posterior bladder wall and the gestation within the placenta (FIGURES 1B, 2B, and 3B)
- abundant blood flow around the gestational sac, at times morphing into an arteriovenous malformation with a high peak systolic velocity blood flow demonstrable on pulsed Doppler.
Our analysis of 751 cases of CSP found that almost a third—30%—were misdiagnosed, contributing to a large number of treatment complications. Most of these complications could have been avoided if diagnosis had been early and correct. The earlier the diagnosis, the better the outcome seemed to be. This was true even when treatment modalities with slightly higher complication rates were used in very early gestation.
Related articles:
• Is the hCG discriminatory zone a reliable indicator of intrauterine or ectopic pregnancy? Andrew M. Kaunitz, MD (Examining the Evidence; February 2012)
• Can a single progesterone test distinguish viable and nonviable pregnancies accurately in women with pain or bleeding? Linda R. Chambliss, MD, MPH (Examining the Evidence; March 2013)
THOROUGH COUNSELING OF THE PATIENT IS PARAMOUNT
Once a diagnosis of CSP has been established, the patient should be counseled about her options. The presence of a live CSP requires immediate and decisive action to prevent further growth of the embryo or fetus. Literature from the past decade, particularly from the past several years, makes evidence-based counseling possible.
In general, treatment should be individualized, based on the patient’s age, number of previous cesarean deliveries, number of children, and the expertise of the clinicians managing her care. Options include:
- termination of the pregnancy
- continuation of the pregnancy with the possibility of delivering a live offspring, provided the patient understands that a morbidly adherent placenta may occur, often necessitating emergency hysterectomy.3,4
MANAGEMENT APPROACHES
Most treatment regimens and combinations thereof can be classified as one of the following:
- Surgical—requiring general anesthesia and either laparotomy with excision or hysterectomy, or laparoscopic or hysteroscopic excision followed by dilation and curettage (D&C).
- Minimally invasive—involving local injection of methotrexate or potassium chloride or systemic intervention, involving a major procedure such as uterine artery embolization in combination with a less complicated one: intramuscular injection of methotrexate in a single or a multidose regimen.
A variety of simultaneous as well as sequential combination treatments also were used. More recently, an ingenious adjunct to treatment is gaining attention: insertion and inflation of a Foley balloon catheter to prevent or tamponade bleeding.
A large number of treatments described in the literature—and their different combinations—have been reported as relatively small case series. Gynecologic surgeons generally perform D&C, laparoscopy, and hysteroscopy or laparotomy as the first-line approach. Obstetricians, radiologists, and in vitro fertilization specialists usually prefer systemic, parenteral administration of methotrexate or ultrasound-guided local methotrexate (or potassium chloride) as an injection into the gestational sac. On occasion, the help of an interventional radiologist was requested to embolize the area of the CSP through the uterine arteries.
POTENTIAL COMPLICATIONS
In our analysis of 751 cases of CSP, we used a rigorous definition of complication, which included an immediate or delayed need for a secondary treatment for blood loss exceeding 200 mL or requiring blood transfusion. If general anesthesia or major surgery was required, we classified that need as a complication.
Utilizing these criteria, we observed an overall complication rate of 44.1% (331 of 751 cases).1
Complications occurred most often when the following treatment modalities were used alone:
- single systemic dose of methotrexate
- D&C
- uterine artery embolization.
Of the 751 cases reviewed, 21.8% resulted in major surgery or interventional radiology procedures (primary or emergency). The total planned primary (nonemergency) interventions performed were 66 (8.7%), which included 3 hysterectomies, 14 laparotomies, and 49 uterine artery embolizations or ligations. There were 98 (13.0%) emergency interventions, which included 36 hysterectomies, 40 laparotomies, and 22 uterine artery embolizations or ligations.1
Related article: Eight tools for improving obstetric patient safety and unit performance. Henry M. Lerner, MD (Professional Liability; March 2014)
NINE TREATMENTS AND THEIR COMPLICATIONS
1. Systemic, single-dose methotrexate
The usual protocols were 1 mg/kg of body weight or 50 mg/m2 of body surface area. This treatment was associated with a complication rate of 64.6%, mostly because it required a second treatment when the fetal heart beat did not cease after several days.1
We speculate that the high failure rate with this treatment may be caused by its slow action and questionable ability to stop cardiac activity and placental expansion. The expected result can take days, and all the while the gestational sac, the embryo or fetus, and its vascularity are growing. Secondary treatment has to address a larger gestation with more abundant vascularization.
2. Systemic, multidose, sequential methotrexate
In this regimen, the amounts of methotrexate injected are similar to the dose for the single-dose regimen. Two to three intramuscular injections (1 mg/kg of body weight or 50 mg/mm2 of surface area) are given at an interval of 2 or 3 days over the course of a week. Be aware of the cumulative adverse effects of this drug on the liver and bone marrow—and the fact that even multidose treatment can fail.1
We found it impossible to assess the complication rate associated with this approach because it was often used in conjunction with another “first-line” treatment or after it. However, it is clear that methotrexate can be combined with other, mostly nonsurgical treatments.
3. Suction aspiration or D&C, alone or in combination
This option requires general anesthesia. The 305 cases involving this treatment had a mean complication rate of about 62% (range, 29%–86%).1 This approach caused the greatest number of bleeding complications, necessitating a third-line treatment that almost always was surgical.
At delivery or the time of spontaneous abortion, the multilayered myometrial grid in the uterine body is able to contain bleeding vessels after placental separation. However, in CSP, the exposed vessels in the cervical scar tissue bleed because there is no muscle grid to contract and contain the profuse bleeding.
If you choose D&C or aspiration, have blood products available and a Foley balloon catheter handy! In several reports, a Foley balloon catheter was used as backup after significant bleeding occurred following curettage.5,6
In one of the series involving 45 cases treated by methotrexate followed by suction curettage, mean blood loss was significant at 707 mL (standard deviation, 642 mL; range, 100–2,000 mL), and treatment failed in three patients despite insertion of a Foley balloon catheter.
4. Uterine artery embolization, alone or in combination
This treatment requires general anesthesia. The complication rate was 47% among the 64 cases described in the literature.1 Uterine artery embolization appeared to work better when it was combined with other noninvasive treatments. It probably is not the best first-line treatment because the delay between treatment and effect allows the gestation to grow and vascularity to increase. And if uterine artery embolization fails, the clinician must contend with a larger gestation.
5. Excision by laparotomy, alone or in combination with hysteroscopy
General anesthesia is required. Of the 18 cases described in the literature, only five complications were reported—and only when used in an emergency situation.1
6. Laparoscopic excision
Again, general anesthesia is required. Fifteen of the 49 cases (30.6%) described in the literature involved complications, but only one of five cases (20%) experienced complications if hysteroscopy and laparoscopy were combined. Small numbers may not allow meaningful evaluation of the latter approach.1
7. Operative hysteroscopy, alone or in combination
General anesthesia is required. The overall complication rate for 108 cases was 13.8%. However, if hysteroscopy was combined with transabdominal ultrasound guidance (as it was in nine cases), no complications were noted. If hysteroscopy was combined with mifepristone, the complication rate was 17%.1 It appears that, when it is performed by an experienced clinician with ultrasound guidance, hysteroscopy may be a reasonable operative solution to CSP.
8. Intragestational-sac injection of methotrexate or potassium chloride, with ultrasound guidance
No anesthesia is required. This approach (FIGURE 4) had the fewest and least-involved complications. Of 83 cases, only 9 (10.8%) involved complications.
Cases performed with transabdominal sonography guidance had a slighter higher complication rate (15%) than those using TVS guidance.1
Because local injections are performed without general anesthesia and provide a final treatment by stopping heart activity, they appear to be the most effective intervention and may be especially useful when future fertility is desired.
9. Use of a Foley balloon catheter
Inserting a Foley balloon catheter and inflating it at the site of the CSP is an ingenious, relatively new approach.1,2,5–7 The catheters come with balloons of different capacity (FIGURE 5A). They can be used alone (usually in gestations of 5–7 weeks) in the hope of stopping the evolution of the pregnancy by placing pressure on a small gestational sac. Even so, this approach is almost always used in a planned fashion in conjunction with another treatment or as backup if bleeding occurs.
Our impression of the value of the balloon catheters is positive. We suggest the French-12 size 10-mL silicone balloon catheter (prices range from $2 to $20), although we used a French-14 catheter with a 30-mL balloon successfully in a case of an 8-week CSP.
Insert the catheter using real-time transabdominal sonographic guidance when the patient has a comfortably full bladder. One also can switch to TVS guidance to allow for more precise placement and assess the pressure, avoiding overinflation of the balloon (FIGURE 5B).
There is no information in the literature about how long such a catheter should be kept in place. In our experience, 24 to 48 hours is the preferred duration, with the outer end of the catheter fastened to the patient’s thigh. We also provide antibiotic coverage and reevaluate the effect in 2 days or as needed. General anesthesia is not required.
KEY TAKEAWAYS
Is there any single and effective treatment protocol? Probably not. Our management approach is presented as an algorithm (FIGURE 6).
We also offer the following guidelines:
- Do not confuse CSP with ectopic pregnancy. Such nomenclature has caused some referring physicians to simply use methotrexate protocols developed on “garden variety” tubal ectopic pregnancies, which not only failed but yielded disastrous results.
- Early diagnosis matters. TVS is the most effective and preferred diagnostic tool. Delay in the diagnosis delays treatment, increasing the possibility of complications.
- Cervical pregnancy is rare. In a patient who has had a cesarean delivery, a low chorionic sac is almost always a CSP.
- A key first step: Determine whether heart activity is present, and avoid methotrexate if no heart activity is observed.
- Counsel the patient. If heart activity is documented, provide evidence-based counseling about the patient’s options.
- Act fast. If continuation of the pregnancy is not desired, provide a reliable treatment that stops the embryonic or fetal heart beat without delay. Early treatment minimizes complications.
- Avoid single treatments unlikely to be effective, including D&C, suction curettage, single-dose intramuscular methotrexate, and uterine artery embolization applied alone.
- Keep a catheter at hand. Foley balloon tamponade to prevent or treat bleeding is a useful adjunct to have within easy reach.
- Consider combination treatments, as they may provide the best results.
- Fully inform the patient of the risks of pregnancy continuation. If a patient elects to continue the pregnancy, schedule an additional counseling session in which a more detailed overview of the anticipated clinical road is thoroughly explained.
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article. Send your letter to the Editor to: [email protected] Please include the city and state in which you practice.
- Timor-Tritsch IE, Monteagudo A. Unforeseen consequences of the increasing rate of cesarean deliveries: early placenta accreta and cesarean scar pregnancy. A review. [published correction appears in Am J Obstet Gynecol. 2014;210(4):371–374.] Am J Obstet Gynecol. 2012;207(1):14–29.
- Timor-Tritsch IE, Monteagudo A, Santos R, Tsymbal T, Pineda G, Arslan AA. The diagnosis, treatment, and follow-up of cesarean scar pregnancy. Am J Obstet Gynecol. 2012;207(1):44.e1–e13.
- Ballas J, Pretorius D, Hull AD, Resnik R, Ramos GA. Identifying sonographic markers for placenta accreta in the first trimester. J Ultrasound Med. 2012;31(11):1835–1841.
- Timor-Tritsch IE, Monteagudo A, Cali P, et al. Cesarean scar pregnancy and early placenta accreta share a common histology. Ultrasound Obstet Gynecol. 2014;43(4):383–395.
- Yu XL, Zhang N, Zuo WL. Cesarean scar pregnancy: An analysis of 100 cases [in Chinese]. Zhonghua Yi Xue Za Zhi. 2011;91(45):3186–3189.
- Jiang T, Liu G, Huang L, Ma H, Zhang S. Methotrexate therapy followed by suction curettage followed by Foley tamponade for cesarean scar pregnancy. Eur J Obstet Gynecol Reprod Biol. 2011;156(2):209–211.
- Hamilton BE, Martin JA, Ventura SJ. Births: Preliminary data for 2012. Natl Vital Stat Rep. 2013;62(3):1–20.
Few ObGyn clinicians have faced a patient with a cesarean-scar pregnancy (CSP). Those few were confronted with a management dilemma. Continue the gestation, which would expose the mother to an elevated risk of heavy bleeding? Or terminate the pregnancy? And if termination is the patient’s choice, what is the most effective method?
The literature contains more than 750 reports of CSP, ranging from a single sporadic case to a series of one to two dozen cases. It is impossible to make sense of the numerous treatments used in the past, which were “tested” on extremely small numbers of patients (sometimes as few as one). In this article, we formulate a management plan for the diagnosis and treatment of CSP based on an in-depth review of the published literature and our personal experience in treating more than four dozen patients with CSP.
We’re all familiar with the “epidemic” of cesarean deliveries in this country, including late consequences of cesarean such as placenta previa and morbidly adherent placenta. One of the long-term consequences of cesarean delivery—the first-trimester CSP—is less well known and documented.
Our in-depth review of 751 CSP cases found no less than 30 published therapeutic approaches.1 No consensus exists as to management guidelines. We have formulated this clinical guide, based on the literature and our experience managing CSP, for clinicians who encounter this dangerous form of pregnancy.2
DIAGNOSIS REQUIRES TRANSVAGINAL SONOGRAPHY
Transvaginal sonography (TVS) is thought to be the best and first-line diagnostic tool, with magnetic resonance imaging (MRI) reserved for cases in which there is a diagnostic problem.
In making a diagnosis, consider two main differential diagnoses:
- Cervical pregnancy—This type of gestation is more likely to occur in women with no history of cesarean delivery
- Spontaneous miscarriage in progress—In a number of cases, the miscarriage happened to be caught on imaging as it passed the area where the CSP usually resides. Because there is no live embryo or fetus in spontaneous miscarriage, a heartbeat cannot be documented.
Components of diagnosis by TVS
Accurate identification of CSP depends on the following sonographic criteria:
- empty uterine cavity and cervical canal (FIGURE 1A)
- close proximity of the gestational sac and the placenta to the anterior uterine surface within the scar or niche of the previous cesarean delivery (FIGURES 1B, 2A, and 2B)
- color flow signals between the posterior bladder wall and the gestation within the placenta (FIGURES 1B, 2B, and 3B)
- abundant blood flow around the gestational sac, at times morphing into an arteriovenous malformation with a high peak systolic velocity blood flow demonstrable on pulsed Doppler.
Our analysis of 751 cases of CSP found that almost a third—30%—were misdiagnosed, contributing to a large number of treatment complications. Most of these complications could have been avoided if diagnosis had been early and correct. The earlier the diagnosis, the better the outcome seemed to be. This was true even when treatment modalities with slightly higher complication rates were used in very early gestation.
Related articles:
• Is the hCG discriminatory zone a reliable indicator of intrauterine or ectopic pregnancy? Andrew M. Kaunitz, MD (Examining the Evidence; February 2012)
• Can a single progesterone test distinguish viable and nonviable pregnancies accurately in women with pain or bleeding? Linda R. Chambliss, MD, MPH (Examining the Evidence; March 2013)
THOROUGH COUNSELING OF THE PATIENT IS PARAMOUNT
Once a diagnosis of CSP has been established, the patient should be counseled about her options. The presence of a live CSP requires immediate and decisive action to prevent further growth of the embryo or fetus. Literature from the past decade, particularly from the past several years, makes evidence-based counseling possible.
In general, treatment should be individualized, based on the patient’s age, number of previous cesarean deliveries, number of children, and the expertise of the clinicians managing her care. Options include:
- termination of the pregnancy
- continuation of the pregnancy with the possibility of delivering a live offspring, provided the patient understands that a morbidly adherent placenta may occur, often necessitating emergency hysterectomy.3,4
MANAGEMENT APPROACHES
Most treatment regimens and combinations thereof can be classified as one of the following:
- Surgical—requiring general anesthesia and either laparotomy with excision or hysterectomy, or laparoscopic or hysteroscopic excision followed by dilation and curettage (D&C).
- Minimally invasive—involving local injection of methotrexate or potassium chloride or systemic intervention, involving a major procedure such as uterine artery embolization in combination with a less complicated one: intramuscular injection of methotrexate in a single or a multidose regimen.
A variety of simultaneous as well as sequential combination treatments also were used. More recently, an ingenious adjunct to treatment is gaining attention: insertion and inflation of a Foley balloon catheter to prevent or tamponade bleeding.
A large number of treatments described in the literature—and their different combinations—have been reported as relatively small case series. Gynecologic surgeons generally perform D&C, laparoscopy, and hysteroscopy or laparotomy as the first-line approach. Obstetricians, radiologists, and in vitro fertilization specialists usually prefer systemic, parenteral administration of methotrexate or ultrasound-guided local methotrexate (or potassium chloride) as an injection into the gestational sac. On occasion, the help of an interventional radiologist was requested to embolize the area of the CSP through the uterine arteries.
POTENTIAL COMPLICATIONS
In our analysis of 751 cases of CSP, we used a rigorous definition of complication, which included an immediate or delayed need for a secondary treatment for blood loss exceeding 200 mL or requiring blood transfusion. If general anesthesia or major surgery was required, we classified that need as a complication.
Utilizing these criteria, we observed an overall complication rate of 44.1% (331 of 751 cases).1
Complications occurred most often when the following treatment modalities were used alone:
- single systemic dose of methotrexate
- D&C
- uterine artery embolization.
Of the 751 cases reviewed, 21.8% resulted in major surgery or interventional radiology procedures (primary or emergency). The total planned primary (nonemergency) interventions performed were 66 (8.7%), which included 3 hysterectomies, 14 laparotomies, and 49 uterine artery embolizations or ligations. There were 98 (13.0%) emergency interventions, which included 36 hysterectomies, 40 laparotomies, and 22 uterine artery embolizations or ligations.1
Related article: Eight tools for improving obstetric patient safety and unit performance. Henry M. Lerner, MD (Professional Liability; March 2014)
NINE TREATMENTS AND THEIR COMPLICATIONS
1. Systemic, single-dose methotrexate
The usual protocols were 1 mg/kg of body weight or 50 mg/m2 of body surface area. This treatment was associated with a complication rate of 64.6%, mostly because it required a second treatment when the fetal heart beat did not cease after several days.1
We speculate that the high failure rate with this treatment may be caused by its slow action and questionable ability to stop cardiac activity and placental expansion. The expected result can take days, and all the while the gestational sac, the embryo or fetus, and its vascularity are growing. Secondary treatment has to address a larger gestation with more abundant vascularization.
2. Systemic, multidose, sequential methotrexate
In this regimen, the amounts of methotrexate injected are similar to the dose for the single-dose regimen. Two to three intramuscular injections (1 mg/kg of body weight or 50 mg/mm2 of surface area) are given at an interval of 2 or 3 days over the course of a week. Be aware of the cumulative adverse effects of this drug on the liver and bone marrow—and the fact that even multidose treatment can fail.1
We found it impossible to assess the complication rate associated with this approach because it was often used in conjunction with another “first-line” treatment or after it. However, it is clear that methotrexate can be combined with other, mostly nonsurgical treatments.
3. Suction aspiration or D&C, alone or in combination
This option requires general anesthesia. The 305 cases involving this treatment had a mean complication rate of about 62% (range, 29%–86%).1 This approach caused the greatest number of bleeding complications, necessitating a third-line treatment that almost always was surgical.
At delivery or the time of spontaneous abortion, the multilayered myometrial grid in the uterine body is able to contain bleeding vessels after placental separation. However, in CSP, the exposed vessels in the cervical scar tissue bleed because there is no muscle grid to contract and contain the profuse bleeding.
If you choose D&C or aspiration, have blood products available and a Foley balloon catheter handy! In several reports, a Foley balloon catheter was used as backup after significant bleeding occurred following curettage.5,6
In one of the series involving 45 cases treated by methotrexate followed by suction curettage, mean blood loss was significant at 707 mL (standard deviation, 642 mL; range, 100–2,000 mL), and treatment failed in three patients despite insertion of a Foley balloon catheter.
4. Uterine artery embolization, alone or in combination
This treatment requires general anesthesia. The complication rate was 47% among the 64 cases described in the literature.1 Uterine artery embolization appeared to work better when it was combined with other noninvasive treatments. It probably is not the best first-line treatment because the delay between treatment and effect allows the gestation to grow and vascularity to increase. And if uterine artery embolization fails, the clinician must contend with a larger gestation.
5. Excision by laparotomy, alone or in combination with hysteroscopy
General anesthesia is required. Of the 18 cases described in the literature, only five complications were reported—and only when used in an emergency situation.1
6. Laparoscopic excision
Again, general anesthesia is required. Fifteen of the 49 cases (30.6%) described in the literature involved complications, but only one of five cases (20%) experienced complications if hysteroscopy and laparoscopy were combined. Small numbers may not allow meaningful evaluation of the latter approach.1
7. Operative hysteroscopy, alone or in combination
General anesthesia is required. The overall complication rate for 108 cases was 13.8%. However, if hysteroscopy was combined with transabdominal ultrasound guidance (as it was in nine cases), no complications were noted. If hysteroscopy was combined with mifepristone, the complication rate was 17%.1 It appears that, when it is performed by an experienced clinician with ultrasound guidance, hysteroscopy may be a reasonable operative solution to CSP.
8. Intragestational-sac injection of methotrexate or potassium chloride, with ultrasound guidance
No anesthesia is required. This approach (FIGURE 4) had the fewest and least-involved complications. Of 83 cases, only 9 (10.8%) involved complications.
Cases performed with transabdominal sonography guidance had a slighter higher complication rate (15%) than those using TVS guidance.1
Because local injections are performed without general anesthesia and provide a final treatment by stopping heart activity, they appear to be the most effective intervention and may be especially useful when future fertility is desired.
9. Use of a Foley balloon catheter
Inserting a Foley balloon catheter and inflating it at the site of the CSP is an ingenious, relatively new approach.1,2,5–7 The catheters come with balloons of different capacity (FIGURE 5A). They can be used alone (usually in gestations of 5–7 weeks) in the hope of stopping the evolution of the pregnancy by placing pressure on a small gestational sac. Even so, this approach is almost always used in a planned fashion in conjunction with another treatment or as backup if bleeding occurs.
Our impression of the value of the balloon catheters is positive. We suggest the French-12 size 10-mL silicone balloon catheter (prices range from $2 to $20), although we used a French-14 catheter with a 30-mL balloon successfully in a case of an 8-week CSP.
Insert the catheter using real-time transabdominal sonographic guidance when the patient has a comfortably full bladder. One also can switch to TVS guidance to allow for more precise placement and assess the pressure, avoiding overinflation of the balloon (FIGURE 5B).
There is no information in the literature about how long such a catheter should be kept in place. In our experience, 24 to 48 hours is the preferred duration, with the outer end of the catheter fastened to the patient’s thigh. We also provide antibiotic coverage and reevaluate the effect in 2 days or as needed. General anesthesia is not required.
KEY TAKEAWAYS
Is there any single and effective treatment protocol? Probably not. Our management approach is presented as an algorithm (FIGURE 6).
We also offer the following guidelines:
- Do not confuse CSP with ectopic pregnancy. Such nomenclature has caused some referring physicians to simply use methotrexate protocols developed on “garden variety” tubal ectopic pregnancies, which not only failed but yielded disastrous results.
- Early diagnosis matters. TVS is the most effective and preferred diagnostic tool. Delay in the diagnosis delays treatment, increasing the possibility of complications.
- Cervical pregnancy is rare. In a patient who has had a cesarean delivery, a low chorionic sac is almost always a CSP.
- A key first step: Determine whether heart activity is present, and avoid methotrexate if no heart activity is observed.
- Counsel the patient. If heart activity is documented, provide evidence-based counseling about the patient’s options.
- Act fast. If continuation of the pregnancy is not desired, provide a reliable treatment that stops the embryonic or fetal heart beat without delay. Early treatment minimizes complications.
- Avoid single treatments unlikely to be effective, including D&C, suction curettage, single-dose intramuscular methotrexate, and uterine artery embolization applied alone.
- Keep a catheter at hand. Foley balloon tamponade to prevent or treat bleeding is a useful adjunct to have within easy reach.
- Consider combination treatments, as they may provide the best results.
- Fully inform the patient of the risks of pregnancy continuation. If a patient elects to continue the pregnancy, schedule an additional counseling session in which a more detailed overview of the anticipated clinical road is thoroughly explained.
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article. Send your letter to the Editor to: [email protected] Please include the city and state in which you practice.
Few ObGyn clinicians have faced a patient with a cesarean-scar pregnancy (CSP). Those few were confronted with a management dilemma. Continue the gestation, which would expose the mother to an elevated risk of heavy bleeding? Or terminate the pregnancy? And if termination is the patient’s choice, what is the most effective method?
The literature contains more than 750 reports of CSP, ranging from a single sporadic case to a series of one to two dozen cases. It is impossible to make sense of the numerous treatments used in the past, which were “tested” on extremely small numbers of patients (sometimes as few as one). In this article, we formulate a management plan for the diagnosis and treatment of CSP based on an in-depth review of the published literature and our personal experience in treating more than four dozen patients with CSP.
We’re all familiar with the “epidemic” of cesarean deliveries in this country, including late consequences of cesarean such as placenta previa and morbidly adherent placenta. One of the long-term consequences of cesarean delivery—the first-trimester CSP—is less well known and documented.
Our in-depth review of 751 CSP cases found no less than 30 published therapeutic approaches.1 No consensus exists as to management guidelines. We have formulated this clinical guide, based on the literature and our experience managing CSP, for clinicians who encounter this dangerous form of pregnancy.2
DIAGNOSIS REQUIRES TRANSVAGINAL SONOGRAPHY
Transvaginal sonography (TVS) is thought to be the best and first-line diagnostic tool, with magnetic resonance imaging (MRI) reserved for cases in which there is a diagnostic problem.
In making a diagnosis, consider two main differential diagnoses:
- Cervical pregnancy—This type of gestation is more likely to occur in women with no history of cesarean delivery
- Spontaneous miscarriage in progress—In a number of cases, the miscarriage happened to be caught on imaging as it passed the area where the CSP usually resides. Because there is no live embryo or fetus in spontaneous miscarriage, a heartbeat cannot be documented.
Components of diagnosis by TVS
Accurate identification of CSP depends on the following sonographic criteria:
- empty uterine cavity and cervical canal (FIGURE 1A)
- close proximity of the gestational sac and the placenta to the anterior uterine surface within the scar or niche of the previous cesarean delivery (FIGURES 1B, 2A, and 2B)
- color flow signals between the posterior bladder wall and the gestation within the placenta (FIGURES 1B, 2B, and 3B)
- abundant blood flow around the gestational sac, at times morphing into an arteriovenous malformation with a high peak systolic velocity blood flow demonstrable on pulsed Doppler.
Our analysis of 751 cases of CSP found that almost a third—30%—were misdiagnosed, contributing to a large number of treatment complications. Most of these complications could have been avoided if diagnosis had been early and correct. The earlier the diagnosis, the better the outcome seemed to be. This was true even when treatment modalities with slightly higher complication rates were used in very early gestation.
Related articles:
• Is the hCG discriminatory zone a reliable indicator of intrauterine or ectopic pregnancy? Andrew M. Kaunitz, MD (Examining the Evidence; February 2012)
• Can a single progesterone test distinguish viable and nonviable pregnancies accurately in women with pain or bleeding? Linda R. Chambliss, MD, MPH (Examining the Evidence; March 2013)
THOROUGH COUNSELING OF THE PATIENT IS PARAMOUNT
Once a diagnosis of CSP has been established, the patient should be counseled about her options. The presence of a live CSP requires immediate and decisive action to prevent further growth of the embryo or fetus. Literature from the past decade, particularly from the past several years, makes evidence-based counseling possible.
In general, treatment should be individualized, based on the patient’s age, number of previous cesarean deliveries, number of children, and the expertise of the clinicians managing her care. Options include:
- termination of the pregnancy
- continuation of the pregnancy with the possibility of delivering a live offspring, provided the patient understands that a morbidly adherent placenta may occur, often necessitating emergency hysterectomy.3,4
MANAGEMENT APPROACHES
Most treatment regimens and combinations thereof can be classified as one of the following:
- Surgical—requiring general anesthesia and either laparotomy with excision or hysterectomy, or laparoscopic or hysteroscopic excision followed by dilation and curettage (D&C).
- Minimally invasive—involving local injection of methotrexate or potassium chloride or systemic intervention, involving a major procedure such as uterine artery embolization in combination with a less complicated one: intramuscular injection of methotrexate in a single or a multidose regimen.
A variety of simultaneous as well as sequential combination treatments also were used. More recently, an ingenious adjunct to treatment is gaining attention: insertion and inflation of a Foley balloon catheter to prevent or tamponade bleeding.
A large number of treatments described in the literature—and their different combinations—have been reported as relatively small case series. Gynecologic surgeons generally perform D&C, laparoscopy, and hysteroscopy or laparotomy as the first-line approach. Obstetricians, radiologists, and in vitro fertilization specialists usually prefer systemic, parenteral administration of methotrexate or ultrasound-guided local methotrexate (or potassium chloride) as an injection into the gestational sac. On occasion, the help of an interventional radiologist was requested to embolize the area of the CSP through the uterine arteries.
POTENTIAL COMPLICATIONS
In our analysis of 751 cases of CSP, we used a rigorous definition of complication, which included an immediate or delayed need for a secondary treatment for blood loss exceeding 200 mL or requiring blood transfusion. If general anesthesia or major surgery was required, we classified that need as a complication.
Utilizing these criteria, we observed an overall complication rate of 44.1% (331 of 751 cases).1
Complications occurred most often when the following treatment modalities were used alone:
- single systemic dose of methotrexate
- D&C
- uterine artery embolization.
Of the 751 cases reviewed, 21.8% resulted in major surgery or interventional radiology procedures (primary or emergency). The total planned primary (nonemergency) interventions performed were 66 (8.7%), which included 3 hysterectomies, 14 laparotomies, and 49 uterine artery embolizations or ligations. There were 98 (13.0%) emergency interventions, which included 36 hysterectomies, 40 laparotomies, and 22 uterine artery embolizations or ligations.1
Related article: Eight tools for improving obstetric patient safety and unit performance. Henry M. Lerner, MD (Professional Liability; March 2014)
NINE TREATMENTS AND THEIR COMPLICATIONS
1. Systemic, single-dose methotrexate
The usual protocols were 1 mg/kg of body weight or 50 mg/m2 of body surface area. This treatment was associated with a complication rate of 64.6%, mostly because it required a second treatment when the fetal heart beat did not cease after several days.1
We speculate that the high failure rate with this treatment may be caused by its slow action and questionable ability to stop cardiac activity and placental expansion. The expected result can take days, and all the while the gestational sac, the embryo or fetus, and its vascularity are growing. Secondary treatment has to address a larger gestation with more abundant vascularization.
2. Systemic, multidose, sequential methotrexate
In this regimen, the amounts of methotrexate injected are similar to the dose for the single-dose regimen. Two to three intramuscular injections (1 mg/kg of body weight or 50 mg/mm2 of surface area) are given at an interval of 2 or 3 days over the course of a week. Be aware of the cumulative adverse effects of this drug on the liver and bone marrow—and the fact that even multidose treatment can fail.1
We found it impossible to assess the complication rate associated with this approach because it was often used in conjunction with another “first-line” treatment or after it. However, it is clear that methotrexate can be combined with other, mostly nonsurgical treatments.
3. Suction aspiration or D&C, alone or in combination
This option requires general anesthesia. The 305 cases involving this treatment had a mean complication rate of about 62% (range, 29%–86%).1 This approach caused the greatest number of bleeding complications, necessitating a third-line treatment that almost always was surgical.
At delivery or the time of spontaneous abortion, the multilayered myometrial grid in the uterine body is able to contain bleeding vessels after placental separation. However, in CSP, the exposed vessels in the cervical scar tissue bleed because there is no muscle grid to contract and contain the profuse bleeding.
If you choose D&C or aspiration, have blood products available and a Foley balloon catheter handy! In several reports, a Foley balloon catheter was used as backup after significant bleeding occurred following curettage.5,6
In one of the series involving 45 cases treated by methotrexate followed by suction curettage, mean blood loss was significant at 707 mL (standard deviation, 642 mL; range, 100–2,000 mL), and treatment failed in three patients despite insertion of a Foley balloon catheter.
4. Uterine artery embolization, alone or in combination
This treatment requires general anesthesia. The complication rate was 47% among the 64 cases described in the literature.1 Uterine artery embolization appeared to work better when it was combined with other noninvasive treatments. It probably is not the best first-line treatment because the delay between treatment and effect allows the gestation to grow and vascularity to increase. And if uterine artery embolization fails, the clinician must contend with a larger gestation.
5. Excision by laparotomy, alone or in combination with hysteroscopy
General anesthesia is required. Of the 18 cases described in the literature, only five complications were reported—and only when used in an emergency situation.1
6. Laparoscopic excision
Again, general anesthesia is required. Fifteen of the 49 cases (30.6%) described in the literature involved complications, but only one of five cases (20%) experienced complications if hysteroscopy and laparoscopy were combined. Small numbers may not allow meaningful evaluation of the latter approach.1
7. Operative hysteroscopy, alone or in combination
General anesthesia is required. The overall complication rate for 108 cases was 13.8%. However, if hysteroscopy was combined with transabdominal ultrasound guidance (as it was in nine cases), no complications were noted. If hysteroscopy was combined with mifepristone, the complication rate was 17%.1 It appears that, when it is performed by an experienced clinician with ultrasound guidance, hysteroscopy may be a reasonable operative solution to CSP.
8. Intragestational-sac injection of methotrexate or potassium chloride, with ultrasound guidance
No anesthesia is required. This approach (FIGURE 4) had the fewest and least-involved complications. Of 83 cases, only 9 (10.8%) involved complications.
Cases performed with transabdominal sonography guidance had a slighter higher complication rate (15%) than those using TVS guidance.1
Because local injections are performed without general anesthesia and provide a final treatment by stopping heart activity, they appear to be the most effective intervention and may be especially useful when future fertility is desired.
9. Use of a Foley balloon catheter
Inserting a Foley balloon catheter and inflating it at the site of the CSP is an ingenious, relatively new approach.1,2,5–7 The catheters come with balloons of different capacity (FIGURE 5A). They can be used alone (usually in gestations of 5–7 weeks) in the hope of stopping the evolution of the pregnancy by placing pressure on a small gestational sac. Even so, this approach is almost always used in a planned fashion in conjunction with another treatment or as backup if bleeding occurs.
Our impression of the value of the balloon catheters is positive. We suggest the French-12 size 10-mL silicone balloon catheter (prices range from $2 to $20), although we used a French-14 catheter with a 30-mL balloon successfully in a case of an 8-week CSP.
Insert the catheter using real-time transabdominal sonographic guidance when the patient has a comfortably full bladder. One also can switch to TVS guidance to allow for more precise placement and assess the pressure, avoiding overinflation of the balloon (FIGURE 5B).
There is no information in the literature about how long such a catheter should be kept in place. In our experience, 24 to 48 hours is the preferred duration, with the outer end of the catheter fastened to the patient’s thigh. We also provide antibiotic coverage and reevaluate the effect in 2 days or as needed. General anesthesia is not required.
KEY TAKEAWAYS
Is there any single and effective treatment protocol? Probably not. Our management approach is presented as an algorithm (FIGURE 6).
We also offer the following guidelines:
- Do not confuse CSP with ectopic pregnancy. Such nomenclature has caused some referring physicians to simply use methotrexate protocols developed on “garden variety” tubal ectopic pregnancies, which not only failed but yielded disastrous results.
- Early diagnosis matters. TVS is the most effective and preferred diagnostic tool. Delay in the diagnosis delays treatment, increasing the possibility of complications.
- Cervical pregnancy is rare. In a patient who has had a cesarean delivery, a low chorionic sac is almost always a CSP.
- A key first step: Determine whether heart activity is present, and avoid methotrexate if no heart activity is observed.
- Counsel the patient. If heart activity is documented, provide evidence-based counseling about the patient’s options.
- Act fast. If continuation of the pregnancy is not desired, provide a reliable treatment that stops the embryonic or fetal heart beat without delay. Early treatment minimizes complications.
- Avoid single treatments unlikely to be effective, including D&C, suction curettage, single-dose intramuscular methotrexate, and uterine artery embolization applied alone.
- Keep a catheter at hand. Foley balloon tamponade to prevent or treat bleeding is a useful adjunct to have within easy reach.
- Consider combination treatments, as they may provide the best results.
- Fully inform the patient of the risks of pregnancy continuation. If a patient elects to continue the pregnancy, schedule an additional counseling session in which a more detailed overview of the anticipated clinical road is thoroughly explained.
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article. Send your letter to the Editor to: [email protected] Please include the city and state in which you practice.
- Timor-Tritsch IE, Monteagudo A. Unforeseen consequences of the increasing rate of cesarean deliveries: early placenta accreta and cesarean scar pregnancy. A review. [published correction appears in Am J Obstet Gynecol. 2014;210(4):371–374.] Am J Obstet Gynecol. 2012;207(1):14–29.
- Timor-Tritsch IE, Monteagudo A, Santos R, Tsymbal T, Pineda G, Arslan AA. The diagnosis, treatment, and follow-up of cesarean scar pregnancy. Am J Obstet Gynecol. 2012;207(1):44.e1–e13.
- Ballas J, Pretorius D, Hull AD, Resnik R, Ramos GA. Identifying sonographic markers for placenta accreta in the first trimester. J Ultrasound Med. 2012;31(11):1835–1841.
- Timor-Tritsch IE, Monteagudo A, Cali P, et al. Cesarean scar pregnancy and early placenta accreta share a common histology. Ultrasound Obstet Gynecol. 2014;43(4):383–395.
- Yu XL, Zhang N, Zuo WL. Cesarean scar pregnancy: An analysis of 100 cases [in Chinese]. Zhonghua Yi Xue Za Zhi. 2011;91(45):3186–3189.
- Jiang T, Liu G, Huang L, Ma H, Zhang S. Methotrexate therapy followed by suction curettage followed by Foley tamponade for cesarean scar pregnancy. Eur J Obstet Gynecol Reprod Biol. 2011;156(2):209–211.
- Hamilton BE, Martin JA, Ventura SJ. Births: Preliminary data for 2012. Natl Vital Stat Rep. 2013;62(3):1–20.
- Timor-Tritsch IE, Monteagudo A. Unforeseen consequences of the increasing rate of cesarean deliveries: early placenta accreta and cesarean scar pregnancy. A review. [published correction appears in Am J Obstet Gynecol. 2014;210(4):371–374.] Am J Obstet Gynecol. 2012;207(1):14–29.
- Timor-Tritsch IE, Monteagudo A, Santos R, Tsymbal T, Pineda G, Arslan AA. The diagnosis, treatment, and follow-up of cesarean scar pregnancy. Am J Obstet Gynecol. 2012;207(1):44.e1–e13.
- Ballas J, Pretorius D, Hull AD, Resnik R, Ramos GA. Identifying sonographic markers for placenta accreta in the first trimester. J Ultrasound Med. 2012;31(11):1835–1841.
- Timor-Tritsch IE, Monteagudo A, Cali P, et al. Cesarean scar pregnancy and early placenta accreta share a common histology. Ultrasound Obstet Gynecol. 2014;43(4):383–395.
- Yu XL, Zhang N, Zuo WL. Cesarean scar pregnancy: An analysis of 100 cases [in Chinese]. Zhonghua Yi Xue Za Zhi. 2011;91(45):3186–3189.
- Jiang T, Liu G, Huang L, Ma H, Zhang S. Methotrexate therapy followed by suction curettage followed by Foley tamponade for cesarean scar pregnancy. Eur J Obstet Gynecol Reprod Biol. 2011;156(2):209–211.
- Hamilton BE, Martin JA, Ventura SJ. Births: Preliminary data for 2012. Natl Vital Stat Rep. 2013;62(3):1–20.
2014 Update on operative vaginal delivery
The past year has seen the publication of four studies with relevance for clinicians:
- a retrospective cohort study that examined the maternal risks of operative vaginal delivery using forceps, vacuum extraction (FIGURE 1), or a combination of forceps and vacuum
- a prospective cohort study that investigated the efficacy and safety of three different techniques for midcavity rotational delivery in the setting of transverse arrest, namely manual rotation, vacuum rotation, and rotational forceps
- another retrospective cohort study that compared maternal morbidity among operative vaginal deliveries performed by midwives and physician providers in the United Kingdom
- a description of a new technique for instrumental vaginal delivery that is low-cost, simple, and easy to perform.
FIGURE 1. In trained hands, operative vaginal delivery can be an extremely effective intervention to expedite delivery when nonreassuring fetal
testing is noted during the second stage of labor.
OBSTETRIC PRACTICE CHANGERS 2014
Hypertension and pregnancy and preventing the first cesarean delivery
John T. Repke, MD, author of the June Guest Editorial titled "Low-dose aspirin and preeclampsia prevention: Ready for prime time, but as a 're-run' or as a 'new series'?" recently sat down with Errol R. Norwitz, MD, PhD, fellow OBG Management Board of Editors Member and author of this month’s "Update on Operative Vaginal Delivery." Their discussion focused on individual takeaways from ACOG’s Hypertension in Pregnancy guidelines and the recent joint ACOG−Society of Maternal-Fetal Medicine report on emerging clinical and scientific advances in safe prevention of the primary cesarean delivery.
From their conversation:
Dr. Repke: About 60 recommendations came out of ACOG’s Hypertension in Pregnancy document; only six had high-quality supporting evidence, and I think most practitioners already did them. Many really were based on either moderate- or low-quality evidence, with qualified recommendations. I think this has led to confusion.
Dr. Norwitz, how do you answer when a clinician asks you, “Is this gestational hypertension or is this preeclampsia?”
Click here to access the audiocast with full transcript.
DO NOT SWITCH INSTRUMENTS
Fong A, Wu E, Pan D, Chung HJ, Ogunyemi DA. Temporal trends and morbidities of vacuum, forceps, and combined use of both [published online ahead of print April 9, 2014]. J Matern Fetal Neonatal Med. doi:10.3109/14767058.2014.904282.
In trained hands, operative vaginal delivery can be an extremely effective intervention to expedite delivery in the setting of nonreassuring fetal testing (“fetal distress”) in the second stage of labor. It takes just a few minutes to perform and can avert a frantic dash to the operating room for an emergent cesarean delivery. What to do then in a situation where the vacuum extractor keeps popping off, the vertex is at +3/+5 station, and the fetal heart rate has been at 80 bpm for 8 minutes? It is extremely tempting to discard the ventouse and grab the forceps. But would that be the right decision?
Related article: Is the rate of progress the same for induced and spontaneous labors? William F. Rayburn, MD, MBA (Examining the Evidence; November 2012)
Details of the study
Earlier studies suggested that the combination of vacuum and forceps is associated with an increased risk of fetal injury. Whether this is also true of injury to the mother was not known. To address this issue, Fong and colleagues performed a retrospective cohort study of all successful operative vaginal deliveries identified using ICD-9 procedure codes in the California Health Discharge Dataset from 2001 through 2007. Maternal outcomes were compared between the 202,439 fetuses delivered by vacuum extraction (reference group), 13,555 fetuses delivered by forceps, and 710 fetuses delivered using a combination of the two methods.
Using multivariate analysis modeling, Fong and colleagues demonstrated that, when compared with the vacuum alone, the combined use of vacuum and forceps was associated with significantly higher odds of:
- third- and fourth-degree perineal lacerations (adjusted odds ratio [aOR], 2.86; 95% confidence interval [CI], 2.43–3.36)
- postpartum hemorrhage (aOR, 1.81; 95% CI, 1.33–2.46)
- operative delivery failure (aOR, 2.81; 95% CI, 2.27–3.48).
Related articles:
• Develop and use a checklist for 3rd- and 4th-degree perineal lacerations. Robert L. Barbieri, MD (Editorial; August 2013)
• Postpartum hemorrhage: 11 critical questions, answered by an expert. Q&A with Haywood L. Brown, MD (January 2011)
Fortunately, combined vacuum/forceps deliveries are uncommon, comprising only 0.33% of operative deliveries in this cohort.
Despite the large dataset used, the study was underpowered to examine the effect of combined vacuum/forceps on the incidence of rare events, such as pelvic hematoma, cervical laceration, thromboembolism, and maternal death.
What this EVIDENCE means for practice
The message is clear: Avoid combined vacuum/forceps deliveries. Choose your initial instrument with care because a failed operative vaginal delivery means a cesarean. You don’t get to choose again. The American College of Obstetricians and Gynecologists also recommends against using multiple instruments “unless there is a compelling and justifiable reason.”1
LEARN TO PERFORM MIDCAVITY ROTATIONAL DELIVERIES
Bahl R, Van de Venne M, Macleod M, Strachan B, Murphy DJ. Maternal and neonatal morbidity in relation to the instrument used for midcavity rotational operative vaginal delivery: A prospective cohort study. BJOG. 2013;120(12):1526–1532.
Cesarean delivery during the second stage of labor used to be an uncommon event. It was said that if labor progressed adequately to achieve full cervical dilatation, a vaginal delivery should be achieved. Over the past few decades, however, the rate of cesarean delivery at full cervical dilatation has increased substantially, thereby contributing to the well-documented cesarean epidemic.
The most common indication for cesarean delivery during the second stage of labor is arrest of descent due to malposition of the fetal head, typically a transverse arrest. A number of alternatives to cesarean are available, all of which involve assisted rotation of the fetal head. Historical case series reporting increased neonatal morbidity have led to a reduction in the use of rotational forceps to facilitate this rotation. Attempted manual rotation and “rotational vacuum extraction” are now preferred, particularly by less experienced providers. Which of these three approaches is most effective is unknown.
Related article: You are the second responder to a shoulder dystocia emergency. What do you do first? Robert L. Barbieri, MD (Editorial; July 2011)
Details of the study
A prospective cohort study was carried out at two university hospitals in Scotland and England to compare maternal and neonatal morbidity associated with alternative techniques for midcavity rotational delivery. The choice of instrument was left to the provider.
Of the 381 nulliparous women who had an attempted midcavity rotational operative vaginal delivery, 163 (42.8%) underwent manual rotation followed by nonrotational forceps delivery, 73 (19.1%) had a rotational vacuum delivery, and 145 (38.1%) delivered with the assistance of rotational (Kielland) forceps.
Regardless of the instrument used, successful rotation and vaginal delivery were achieved in more than 90% of cases, with a cesarean rate of 4.2%, 6.8%, and 9.6% for manual rotation, vacuum, and rotational forceps, respectively (aOR, 0.39; 95% CI, 0.14–1.06). There were no significant differences in maternal complications (postpartum hemorrhage, third- and fourth-degree perineal lacerations) and neonatal morbidity (low cord pH, neonatal trauma, and neonatal intensive care unit admission) between the three instruments.
What this EVIDENCE means for practice
Midcavity rotational delivery can be achieved with a high degree of success and few adverse events in women who develop transverse arrest in the second stage of labor. Maternal and perinatal outcomes are comparable with rotational forceps, vacuum extraction, and manual rotation. With appropriate training, midcavity rotational delivery can be practiced safely, including the use of Kielland forceps.
SHOULD MIDWIVES PERFORM OPERATIVE VAGINAL DELIVERIES?
Black M, Mitchell E, Danielian P. Instrumental vaginal deliveries; are midwives safer practitioners? A retrospective cohort study. Acta Obstet Gynecol Scand. 2013;92(12):1383–1387.
In the United States, instrumental vaginal deliveries are performed only by physicians. In the United Kingdom, the opportunity to perform such deliveries has recently become available to midwives as well. Because midwives have less experience in performing surgical procedures, the question has arisen as to whether their complication rate is higher than that of physicians. Alternatively, because midwives typically are more patient than physicians and more reluctant to resort to obstetric interventions, it is possible that their complication rate may be lower.
Details of the study
To address this issue, Black and colleagues performed a retrospective cohort study of consecutive women who had a successful nonrotational instrumental vaginal delivery of a liveborn singleton infant outside of the operating room between June 2005 and June 2010 at Aberdeen Maternity Hospital in the United Kingdom.
Of the 2,540 women included in the final analysis, 330 (13%) were delivered by midwives and the remaining 2,210 (87%) by physicians—1,049 (41%) by junior doctors and 1,161 (46%) by more senior doctors. All midwives had undergone formal training at the University of Bradford. There were no differences between groups in demographic characteristics (maternal age, gestational age, parity, body mass index, or birth weight) or in the indications for instrumental delivery.
Major findings were that midwives were significantly less likely than junior and senior physicians to use forceps as the instrument of choice for delivery (OR, 0.6; 95% CI, 0.4–0.7). Mean blood loss was significantly lower in the midwife group (57 mL), although it is unlikely that this finding was clinically significant. There were no differences in severe perineal injury (third- or fourth-degree perineal lacerations), arterial cord pH, or postpartum hemorrhage.
A secondary analysis comparing the outcome of operative vaginal deliveries by trained midwives with the outcome by junior physicians alone produced almost identical results.
Strengths of the study include the fact that it was conducted at a single center and had a large sample number. Weaknesses include its retrospective design and the fact that one major outcome (namely, failed operative vaginal delivery leading to cesarean) was not examined. This study was not designed or powered to examine neonatal outcomes.
What this EVIDENCE means for practice
These data demonstrate that midwives can perform operative vaginal deliveries using either forceps or vacuum with a rate of maternal morbidity equivalent to those performed by physicians.
Are these findings truly revolutionary? Although midwives do not perform cesarean deliveries, they do perform and repair episiotomies when indicated. Restricting instrumental vaginal deliveries to physicians alone may be motivated more by tradition and logistics than concerns over patient safety. Indeed, the ability of a midwife working in a remote area to perform an instrumental vaginal delivery in an emergency situation may be highly beneficial to perinatal outcome, although it should be stressed that such an approach ought to be limited to practitioners who have undergone rigorous formal training.
Other benefits of midwives performing operative vaginal deliveries may include increased autonomy for the midwifery providers, improvements in physician-midwife interactions, and enhanced continuity of care for women.
IN THE PIPELINE: THE ODÓN DEVICE FOR OPERATIVE VAGINAL DELIVERY
World Health Organization Odón Device Research Group. Feasibility and safety study of a new device (Odón device) for assisted vaginal deliveries: Study protocol. Reprod Health. 2013;10:33.
Childbirth remains a risky venture. According to the World Health Organization (WHO), approximately 2.6 million babies are stillborn and 260,000 women die in childbirth each year, with developing countries disproportionately affected. Many of these adverse events result from complications at the time of delivery. Instrumental vaginal delivery is used to shorten the second stage of labor and improve perinatal and maternal outcomes.
Operative vaginal delivery likely does reduce the rate of stillbirth and early neonatal death and lower the cesarean delivery rate, but the instruments themselves do occasionally cause maternal and fetal injury, including cephalohematoma, retinal hemorrhage, facial nerve palsy, and skull fractures. Although numerous modifications to the design of forceps and the vacuum extractor have been made over the years, no new technology has been introduced for centuries.
In 2005, Mr. Jorge Odón, a car mechanic from Argentina with no formal training in medicine or obstetrics (aside from being the father of five), came up with an idea for a novel technique to assist in delivery. He was inspired by a simple trick he used to entertain his friends. It involved removing a loose cork from the inside of an empty bottle using a plastic bag. It occurred to him one day that this same scientific principle could be used to expedite delivery of the fetal head from the birth canal, and so he built the first prototype. The device has since been named in his honor.
Description of the Odón device
The Odón device consists of a tube containing a polyethylene bag. The tube is inserted into the birth canal and the bag is deployed and inflated to create a plastic sleeve that hugs the baby’s head. The applicator tube is then discarded and traction is applied to the plastic bag to move the head (and the entire fetus) down the birth canal (FIGURE 2).
![]()
The advantages of the Odón device are that it is:
- low-cost
- simple to use
- compact, easy to transport and store
- designed to minimize trauma to the mother and fetus.
Current stage of development
The Odón device already has been piloted in the United States and South America. The WHO plans to introduce it into the obstetric armamentarium in a three-phase clinical trial outlined in the Odón Device Research Project report. The first phase is under way and involves testing the device under normal delivery conditions in tertiary hospitals in Argentina and South Africa. The next two phases will 1) assess its efficacy in women with a prolonged second stage of labor but no “fetal distress” and 2) compare its performance head-to-head against the vacuum extractor and forceps.
What this EVIDENCE means for practice
Enthusiasm for the Odón device is fueled by its simplicity and the likelihood that midlevel providers working in remote obstetric units can be trained in its use, thereby increasing access to an important modality of emergency obstetric care. This is particularly important in centers that lack immediate access to cesarean delivery capabilities. Whether the device can be used in developing countries to more effectively manage the second stage of labor and thereby reduce infectious morbidity and pelvic floor injuries has yet to be confirmed but is a testable hypothesis.
WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: [email protected]
Reference
- American College of Obstetricians and Gynecologists. ACOG practice bulletin #17: Operative vaginal delivery. Washington, DC: ACOG; 2000.
The past year has seen the publication of four studies with relevance for clinicians:
- a retrospective cohort study that examined the maternal risks of operative vaginal delivery using forceps, vacuum extraction (FIGURE 1), or a combination of forceps and vacuum
- a prospective cohort study that investigated the efficacy and safety of three different techniques for midcavity rotational delivery in the setting of transverse arrest, namely manual rotation, vacuum rotation, and rotational forceps
- another retrospective cohort study that compared maternal morbidity among operative vaginal deliveries performed by midwives and physician providers in the United Kingdom
- a description of a new technique for instrumental vaginal delivery that is low-cost, simple, and easy to perform.
FIGURE 1. In trained hands, operative vaginal delivery can be an extremely effective intervention to expedite delivery when nonreassuring fetal
testing is noted during the second stage of labor.
OBSTETRIC PRACTICE CHANGERS 2014
Hypertension and pregnancy and preventing the first cesarean delivery
John T. Repke, MD, author of the June Guest Editorial titled "Low-dose aspirin and preeclampsia prevention: Ready for prime time, but as a 're-run' or as a 'new series'?" recently sat down with Errol R. Norwitz, MD, PhD, fellow OBG Management Board of Editors Member and author of this month’s "Update on Operative Vaginal Delivery." Their discussion focused on individual takeaways from ACOG’s Hypertension in Pregnancy guidelines and the recent joint ACOG−Society of Maternal-Fetal Medicine report on emerging clinical and scientific advances in safe prevention of the primary cesarean delivery.
From their conversation:
Dr. Repke: About 60 recommendations came out of ACOG’s Hypertension in Pregnancy document; only six had high-quality supporting evidence, and I think most practitioners already did them. Many really were based on either moderate- or low-quality evidence, with qualified recommendations. I think this has led to confusion.
Dr. Norwitz, how do you answer when a clinician asks you, “Is this gestational hypertension or is this preeclampsia?”
Click here to access the audiocast with full transcript.
DO NOT SWITCH INSTRUMENTS
Fong A, Wu E, Pan D, Chung HJ, Ogunyemi DA. Temporal trends and morbidities of vacuum, forceps, and combined use of both [published online ahead of print April 9, 2014]. J Matern Fetal Neonatal Med. doi:10.3109/14767058.2014.904282.
In trained hands, operative vaginal delivery can be an extremely effective intervention to expedite delivery in the setting of nonreassuring fetal testing (“fetal distress”) in the second stage of labor. It takes just a few minutes to perform and can avert a frantic dash to the operating room for an emergent cesarean delivery. What to do then in a situation where the vacuum extractor keeps popping off, the vertex is at +3/+5 station, and the fetal heart rate has been at 80 bpm for 8 minutes? It is extremely tempting to discard the ventouse and grab the forceps. But would that be the right decision?
Related article: Is the rate of progress the same for induced and spontaneous labors? William F. Rayburn, MD, MBA (Examining the Evidence; November 2012)
Details of the study
Earlier studies suggested that the combination of vacuum and forceps is associated with an increased risk of fetal injury. Whether this is also true of injury to the mother was not known. To address this issue, Fong and colleagues performed a retrospective cohort study of all successful operative vaginal deliveries identified using ICD-9 procedure codes in the California Health Discharge Dataset from 2001 through 2007. Maternal outcomes were compared between the 202,439 fetuses delivered by vacuum extraction (reference group), 13,555 fetuses delivered by forceps, and 710 fetuses delivered using a combination of the two methods.
Using multivariate analysis modeling, Fong and colleagues demonstrated that, when compared with the vacuum alone, the combined use of vacuum and forceps was associated with significantly higher odds of:
- third- and fourth-degree perineal lacerations (adjusted odds ratio [aOR], 2.86; 95% confidence interval [CI], 2.43–3.36)
- postpartum hemorrhage (aOR, 1.81; 95% CI, 1.33–2.46)
- operative delivery failure (aOR, 2.81; 95% CI, 2.27–3.48).
Related articles:
• Develop and use a checklist for 3rd- and 4th-degree perineal lacerations. Robert L. Barbieri, MD (Editorial; August 2013)
• Postpartum hemorrhage: 11 critical questions, answered by an expert. Q&A with Haywood L. Brown, MD (January 2011)
Fortunately, combined vacuum/forceps deliveries are uncommon, comprising only 0.33% of operative deliveries in this cohort.
Despite the large dataset used, the study was underpowered to examine the effect of combined vacuum/forceps on the incidence of rare events, such as pelvic hematoma, cervical laceration, thromboembolism, and maternal death.
What this EVIDENCE means for practice
The message is clear: Avoid combined vacuum/forceps deliveries. Choose your initial instrument with care because a failed operative vaginal delivery means a cesarean. You don’t get to choose again. The American College of Obstetricians and Gynecologists also recommends against using multiple instruments “unless there is a compelling and justifiable reason.”1
LEARN TO PERFORM MIDCAVITY ROTATIONAL DELIVERIES
Bahl R, Van de Venne M, Macleod M, Strachan B, Murphy DJ. Maternal and neonatal morbidity in relation to the instrument used for midcavity rotational operative vaginal delivery: A prospective cohort study. BJOG. 2013;120(12):1526–1532.
Cesarean delivery during the second stage of labor used to be an uncommon event. It was said that if labor progressed adequately to achieve full cervical dilatation, a vaginal delivery should be achieved. Over the past few decades, however, the rate of cesarean delivery at full cervical dilatation has increased substantially, thereby contributing to the well-documented cesarean epidemic.
The most common indication for cesarean delivery during the second stage of labor is arrest of descent due to malposition of the fetal head, typically a transverse arrest. A number of alternatives to cesarean are available, all of which involve assisted rotation of the fetal head. Historical case series reporting increased neonatal morbidity have led to a reduction in the use of rotational forceps to facilitate this rotation. Attempted manual rotation and “rotational vacuum extraction” are now preferred, particularly by less experienced providers. Which of these three approaches is most effective is unknown.
Related article: You are the second responder to a shoulder dystocia emergency. What do you do first? Robert L. Barbieri, MD (Editorial; July 2011)
Details of the study
A prospective cohort study was carried out at two university hospitals in Scotland and England to compare maternal and neonatal morbidity associated with alternative techniques for midcavity rotational delivery. The choice of instrument was left to the provider.
Of the 381 nulliparous women who had an attempted midcavity rotational operative vaginal delivery, 163 (42.8%) underwent manual rotation followed by nonrotational forceps delivery, 73 (19.1%) had a rotational vacuum delivery, and 145 (38.1%) delivered with the assistance of rotational (Kielland) forceps.
Regardless of the instrument used, successful rotation and vaginal delivery were achieved in more than 90% of cases, with a cesarean rate of 4.2%, 6.8%, and 9.6% for manual rotation, vacuum, and rotational forceps, respectively (aOR, 0.39; 95% CI, 0.14–1.06). There were no significant differences in maternal complications (postpartum hemorrhage, third- and fourth-degree perineal lacerations) and neonatal morbidity (low cord pH, neonatal trauma, and neonatal intensive care unit admission) between the three instruments.
What this EVIDENCE means for practice
Midcavity rotational delivery can be achieved with a high degree of success and few adverse events in women who develop transverse arrest in the second stage of labor. Maternal and perinatal outcomes are comparable with rotational forceps, vacuum extraction, and manual rotation. With appropriate training, midcavity rotational delivery can be practiced safely, including the use of Kielland forceps.
SHOULD MIDWIVES PERFORM OPERATIVE VAGINAL DELIVERIES?
Black M, Mitchell E, Danielian P. Instrumental vaginal deliveries; are midwives safer practitioners? A retrospective cohort study. Acta Obstet Gynecol Scand. 2013;92(12):1383–1387.
In the United States, instrumental vaginal deliveries are performed only by physicians. In the United Kingdom, the opportunity to perform such deliveries has recently become available to midwives as well. Because midwives have less experience in performing surgical procedures, the question has arisen as to whether their complication rate is higher than that of physicians. Alternatively, because midwives typically are more patient than physicians and more reluctant to resort to obstetric interventions, it is possible that their complication rate may be lower.
Details of the study
To address this issue, Black and colleagues performed a retrospective cohort study of consecutive women who had a successful nonrotational instrumental vaginal delivery of a liveborn singleton infant outside of the operating room between June 2005 and June 2010 at Aberdeen Maternity Hospital in the United Kingdom.
Of the 2,540 women included in the final analysis, 330 (13%) were delivered by midwives and the remaining 2,210 (87%) by physicians—1,049 (41%) by junior doctors and 1,161 (46%) by more senior doctors. All midwives had undergone formal training at the University of Bradford. There were no differences between groups in demographic characteristics (maternal age, gestational age, parity, body mass index, or birth weight) or in the indications for instrumental delivery.
Major findings were that midwives were significantly less likely than junior and senior physicians to use forceps as the instrument of choice for delivery (OR, 0.6; 95% CI, 0.4–0.7). Mean blood loss was significantly lower in the midwife group (57 mL), although it is unlikely that this finding was clinically significant. There were no differences in severe perineal injury (third- or fourth-degree perineal lacerations), arterial cord pH, or postpartum hemorrhage.
A secondary analysis comparing the outcome of operative vaginal deliveries by trained midwives with the outcome by junior physicians alone produced almost identical results.
Strengths of the study include the fact that it was conducted at a single center and had a large sample number. Weaknesses include its retrospective design and the fact that one major outcome (namely, failed operative vaginal delivery leading to cesarean) was not examined. This study was not designed or powered to examine neonatal outcomes.
What this EVIDENCE means for practice
These data demonstrate that midwives can perform operative vaginal deliveries using either forceps or vacuum with a rate of maternal morbidity equivalent to those performed by physicians.
Are these findings truly revolutionary? Although midwives do not perform cesarean deliveries, they do perform and repair episiotomies when indicated. Restricting instrumental vaginal deliveries to physicians alone may be motivated more by tradition and logistics than concerns over patient safety. Indeed, the ability of a midwife working in a remote area to perform an instrumental vaginal delivery in an emergency situation may be highly beneficial to perinatal outcome, although it should be stressed that such an approach ought to be limited to practitioners who have undergone rigorous formal training.
Other benefits of midwives performing operative vaginal deliveries may include increased autonomy for the midwifery providers, improvements in physician-midwife interactions, and enhanced continuity of care for women.
IN THE PIPELINE: THE ODÓN DEVICE FOR OPERATIVE VAGINAL DELIVERY
World Health Organization Odón Device Research Group. Feasibility and safety study of a new device (Odón device) for assisted vaginal deliveries: Study protocol. Reprod Health. 2013;10:33.
Childbirth remains a risky venture. According to the World Health Organization (WHO), approximately 2.6 million babies are stillborn and 260,000 women die in childbirth each year, with developing countries disproportionately affected. Many of these adverse events result from complications at the time of delivery. Instrumental vaginal delivery is used to shorten the second stage of labor and improve perinatal and maternal outcomes.
Operative vaginal delivery likely does reduce the rate of stillbirth and early neonatal death and lower the cesarean delivery rate, but the instruments themselves do occasionally cause maternal and fetal injury, including cephalohematoma, retinal hemorrhage, facial nerve palsy, and skull fractures. Although numerous modifications to the design of forceps and the vacuum extractor have been made over the years, no new technology has been introduced for centuries.
In 2005, Mr. Jorge Odón, a car mechanic from Argentina with no formal training in medicine or obstetrics (aside from being the father of five), came up with an idea for a novel technique to assist in delivery. He was inspired by a simple trick he used to entertain his friends. It involved removing a loose cork from the inside of an empty bottle using a plastic bag. It occurred to him one day that this same scientific principle could be used to expedite delivery of the fetal head from the birth canal, and so he built the first prototype. The device has since been named in his honor.
Description of the Odón device
The Odón device consists of a tube containing a polyethylene bag. The tube is inserted into the birth canal and the bag is deployed and inflated to create a plastic sleeve that hugs the baby’s head. The applicator tube is then discarded and traction is applied to the plastic bag to move the head (and the entire fetus) down the birth canal (FIGURE 2).
![]()
The advantages of the Odón device are that it is:
- low-cost
- simple to use
- compact, easy to transport and store
- designed to minimize trauma to the mother and fetus.
Current stage of development
The Odón device already has been piloted in the United States and South America. The WHO plans to introduce it into the obstetric armamentarium in a three-phase clinical trial outlined in the Odón Device Research Project report. The first phase is under way and involves testing the device under normal delivery conditions in tertiary hospitals in Argentina and South Africa. The next two phases will 1) assess its efficacy in women with a prolonged second stage of labor but no “fetal distress” and 2) compare its performance head-to-head against the vacuum extractor and forceps.
What this EVIDENCE means for practice
Enthusiasm for the Odón device is fueled by its simplicity and the likelihood that midlevel providers working in remote obstetric units can be trained in its use, thereby increasing access to an important modality of emergency obstetric care. This is particularly important in centers that lack immediate access to cesarean delivery capabilities. Whether the device can be used in developing countries to more effectively manage the second stage of labor and thereby reduce infectious morbidity and pelvic floor injuries has yet to be confirmed but is a testable hypothesis.
WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: [email protected]
The past year has seen the publication of four studies with relevance for clinicians:
- a retrospective cohort study that examined the maternal risks of operative vaginal delivery using forceps, vacuum extraction (FIGURE 1), or a combination of forceps and vacuum
- a prospective cohort study that investigated the efficacy and safety of three different techniques for midcavity rotational delivery in the setting of transverse arrest, namely manual rotation, vacuum rotation, and rotational forceps
- another retrospective cohort study that compared maternal morbidity among operative vaginal deliveries performed by midwives and physician providers in the United Kingdom
- a description of a new technique for instrumental vaginal delivery that is low-cost, simple, and easy to perform.
FIGURE 1. In trained hands, operative vaginal delivery can be an extremely effective intervention to expedite delivery when nonreassuring fetal
testing is noted during the second stage of labor.
OBSTETRIC PRACTICE CHANGERS 2014
Hypertension and pregnancy and preventing the first cesarean delivery
John T. Repke, MD, author of the June Guest Editorial titled "Low-dose aspirin and preeclampsia prevention: Ready for prime time, but as a 're-run' or as a 'new series'?" recently sat down with Errol R. Norwitz, MD, PhD, fellow OBG Management Board of Editors Member and author of this month’s "Update on Operative Vaginal Delivery." Their discussion focused on individual takeaways from ACOG’s Hypertension in Pregnancy guidelines and the recent joint ACOG−Society of Maternal-Fetal Medicine report on emerging clinical and scientific advances in safe prevention of the primary cesarean delivery.
From their conversation:
Dr. Repke: About 60 recommendations came out of ACOG’s Hypertension in Pregnancy document; only six had high-quality supporting evidence, and I think most practitioners already did them. Many really were based on either moderate- or low-quality evidence, with qualified recommendations. I think this has led to confusion.
Dr. Norwitz, how do you answer when a clinician asks you, “Is this gestational hypertension or is this preeclampsia?”
Click here to access the audiocast with full transcript.
DO NOT SWITCH INSTRUMENTS
Fong A, Wu E, Pan D, Chung HJ, Ogunyemi DA. Temporal trends and morbidities of vacuum, forceps, and combined use of both [published online ahead of print April 9, 2014]. J Matern Fetal Neonatal Med. doi:10.3109/14767058.2014.904282.
In trained hands, operative vaginal delivery can be an extremely effective intervention to expedite delivery in the setting of nonreassuring fetal testing (“fetal distress”) in the second stage of labor. It takes just a few minutes to perform and can avert a frantic dash to the operating room for an emergent cesarean delivery. What to do then in a situation where the vacuum extractor keeps popping off, the vertex is at +3/+5 station, and the fetal heart rate has been at 80 bpm for 8 minutes? It is extremely tempting to discard the ventouse and grab the forceps. But would that be the right decision?
Related article: Is the rate of progress the same for induced and spontaneous labors? William F. Rayburn, MD, MBA (Examining the Evidence; November 2012)
Details of the study
Earlier studies suggested that the combination of vacuum and forceps is associated with an increased risk of fetal injury. Whether this is also true of injury to the mother was not known. To address this issue, Fong and colleagues performed a retrospective cohort study of all successful operative vaginal deliveries identified using ICD-9 procedure codes in the California Health Discharge Dataset from 2001 through 2007. Maternal outcomes were compared between the 202,439 fetuses delivered by vacuum extraction (reference group), 13,555 fetuses delivered by forceps, and 710 fetuses delivered using a combination of the two methods.
Using multivariate analysis modeling, Fong and colleagues demonstrated that, when compared with the vacuum alone, the combined use of vacuum and forceps was associated with significantly higher odds of:
- third- and fourth-degree perineal lacerations (adjusted odds ratio [aOR], 2.86; 95% confidence interval [CI], 2.43–3.36)
- postpartum hemorrhage (aOR, 1.81; 95% CI, 1.33–2.46)
- operative delivery failure (aOR, 2.81; 95% CI, 2.27–3.48).
Related articles:
• Develop and use a checklist for 3rd- and 4th-degree perineal lacerations. Robert L. Barbieri, MD (Editorial; August 2013)
• Postpartum hemorrhage: 11 critical questions, answered by an expert. Q&A with Haywood L. Brown, MD (January 2011)
Fortunately, combined vacuum/forceps deliveries are uncommon, comprising only 0.33% of operative deliveries in this cohort.
Despite the large dataset used, the study was underpowered to examine the effect of combined vacuum/forceps on the incidence of rare events, such as pelvic hematoma, cervical laceration, thromboembolism, and maternal death.
What this EVIDENCE means for practice
The message is clear: Avoid combined vacuum/forceps deliveries. Choose your initial instrument with care because a failed operative vaginal delivery means a cesarean. You don’t get to choose again. The American College of Obstetricians and Gynecologists also recommends against using multiple instruments “unless there is a compelling and justifiable reason.”1
LEARN TO PERFORM MIDCAVITY ROTATIONAL DELIVERIES
Bahl R, Van de Venne M, Macleod M, Strachan B, Murphy DJ. Maternal and neonatal morbidity in relation to the instrument used for midcavity rotational operative vaginal delivery: A prospective cohort study. BJOG. 2013;120(12):1526–1532.
Cesarean delivery during the second stage of labor used to be an uncommon event. It was said that if labor progressed adequately to achieve full cervical dilatation, a vaginal delivery should be achieved. Over the past few decades, however, the rate of cesarean delivery at full cervical dilatation has increased substantially, thereby contributing to the well-documented cesarean epidemic.
The most common indication for cesarean delivery during the second stage of labor is arrest of descent due to malposition of the fetal head, typically a transverse arrest. A number of alternatives to cesarean are available, all of which involve assisted rotation of the fetal head. Historical case series reporting increased neonatal morbidity have led to a reduction in the use of rotational forceps to facilitate this rotation. Attempted manual rotation and “rotational vacuum extraction” are now preferred, particularly by less experienced providers. Which of these three approaches is most effective is unknown.
Related article: You are the second responder to a shoulder dystocia emergency. What do you do first? Robert L. Barbieri, MD (Editorial; July 2011)
Details of the study
A prospective cohort study was carried out at two university hospitals in Scotland and England to compare maternal and neonatal morbidity associated with alternative techniques for midcavity rotational delivery. The choice of instrument was left to the provider.
Of the 381 nulliparous women who had an attempted midcavity rotational operative vaginal delivery, 163 (42.8%) underwent manual rotation followed by nonrotational forceps delivery, 73 (19.1%) had a rotational vacuum delivery, and 145 (38.1%) delivered with the assistance of rotational (Kielland) forceps.
Regardless of the instrument used, successful rotation and vaginal delivery were achieved in more than 90% of cases, with a cesarean rate of 4.2%, 6.8%, and 9.6% for manual rotation, vacuum, and rotational forceps, respectively (aOR, 0.39; 95% CI, 0.14–1.06). There were no significant differences in maternal complications (postpartum hemorrhage, third- and fourth-degree perineal lacerations) and neonatal morbidity (low cord pH, neonatal trauma, and neonatal intensive care unit admission) between the three instruments.
What this EVIDENCE means for practice
Midcavity rotational delivery can be achieved with a high degree of success and few adverse events in women who develop transverse arrest in the second stage of labor. Maternal and perinatal outcomes are comparable with rotational forceps, vacuum extraction, and manual rotation. With appropriate training, midcavity rotational delivery can be practiced safely, including the use of Kielland forceps.
SHOULD MIDWIVES PERFORM OPERATIVE VAGINAL DELIVERIES?
Black M, Mitchell E, Danielian P. Instrumental vaginal deliveries; are midwives safer practitioners? A retrospective cohort study. Acta Obstet Gynecol Scand. 2013;92(12):1383–1387.
In the United States, instrumental vaginal deliveries are performed only by physicians. In the United Kingdom, the opportunity to perform such deliveries has recently become available to midwives as well. Because midwives have less experience in performing surgical procedures, the question has arisen as to whether their complication rate is higher than that of physicians. Alternatively, because midwives typically are more patient than physicians and more reluctant to resort to obstetric interventions, it is possible that their complication rate may be lower.
Details of the study
To address this issue, Black and colleagues performed a retrospective cohort study of consecutive women who had a successful nonrotational instrumental vaginal delivery of a liveborn singleton infant outside of the operating room between June 2005 and June 2010 at Aberdeen Maternity Hospital in the United Kingdom.
Of the 2,540 women included in the final analysis, 330 (13%) were delivered by midwives and the remaining 2,210 (87%) by physicians—1,049 (41%) by junior doctors and 1,161 (46%) by more senior doctors. All midwives had undergone formal training at the University of Bradford. There were no differences between groups in demographic characteristics (maternal age, gestational age, parity, body mass index, or birth weight) or in the indications for instrumental delivery.
Major findings were that midwives were significantly less likely than junior and senior physicians to use forceps as the instrument of choice for delivery (OR, 0.6; 95% CI, 0.4–0.7). Mean blood loss was significantly lower in the midwife group (57 mL), although it is unlikely that this finding was clinically significant. There were no differences in severe perineal injury (third- or fourth-degree perineal lacerations), arterial cord pH, or postpartum hemorrhage.
A secondary analysis comparing the outcome of operative vaginal deliveries by trained midwives with the outcome by junior physicians alone produced almost identical results.
Strengths of the study include the fact that it was conducted at a single center and had a large sample number. Weaknesses include its retrospective design and the fact that one major outcome (namely, failed operative vaginal delivery leading to cesarean) was not examined. This study was not designed or powered to examine neonatal outcomes.
What this EVIDENCE means for practice
These data demonstrate that midwives can perform operative vaginal deliveries using either forceps or vacuum with a rate of maternal morbidity equivalent to those performed by physicians.
Are these findings truly revolutionary? Although midwives do not perform cesarean deliveries, they do perform and repair episiotomies when indicated. Restricting instrumental vaginal deliveries to physicians alone may be motivated more by tradition and logistics than concerns over patient safety. Indeed, the ability of a midwife working in a remote area to perform an instrumental vaginal delivery in an emergency situation may be highly beneficial to perinatal outcome, although it should be stressed that such an approach ought to be limited to practitioners who have undergone rigorous formal training.
Other benefits of midwives performing operative vaginal deliveries may include increased autonomy for the midwifery providers, improvements in physician-midwife interactions, and enhanced continuity of care for women.
IN THE PIPELINE: THE ODÓN DEVICE FOR OPERATIVE VAGINAL DELIVERY
World Health Organization Odón Device Research Group. Feasibility and safety study of a new device (Odón device) for assisted vaginal deliveries: Study protocol. Reprod Health. 2013;10:33.
Childbirth remains a risky venture. According to the World Health Organization (WHO), approximately 2.6 million babies are stillborn and 260,000 women die in childbirth each year, with developing countries disproportionately affected. Many of these adverse events result from complications at the time of delivery. Instrumental vaginal delivery is used to shorten the second stage of labor and improve perinatal and maternal outcomes.
Operative vaginal delivery likely does reduce the rate of stillbirth and early neonatal death and lower the cesarean delivery rate, but the instruments themselves do occasionally cause maternal and fetal injury, including cephalohematoma, retinal hemorrhage, facial nerve palsy, and skull fractures. Although numerous modifications to the design of forceps and the vacuum extractor have been made over the years, no new technology has been introduced for centuries.
In 2005, Mr. Jorge Odón, a car mechanic from Argentina with no formal training in medicine or obstetrics (aside from being the father of five), came up with an idea for a novel technique to assist in delivery. He was inspired by a simple trick he used to entertain his friends. It involved removing a loose cork from the inside of an empty bottle using a plastic bag. It occurred to him one day that this same scientific principle could be used to expedite delivery of the fetal head from the birth canal, and so he built the first prototype. The device has since been named in his honor.
Description of the Odón device
The Odón device consists of a tube containing a polyethylene bag. The tube is inserted into the birth canal and the bag is deployed and inflated to create a plastic sleeve that hugs the baby’s head. The applicator tube is then discarded and traction is applied to the plastic bag to move the head (and the entire fetus) down the birth canal (FIGURE 2).
![]()
The advantages of the Odón device are that it is:
- low-cost
- simple to use
- compact, easy to transport and store
- designed to minimize trauma to the mother and fetus.
Current stage of development
The Odón device already has been piloted in the United States and South America. The WHO plans to introduce it into the obstetric armamentarium in a three-phase clinical trial outlined in the Odón Device Research Project report. The first phase is under way and involves testing the device under normal delivery conditions in tertiary hospitals in Argentina and South Africa. The next two phases will 1) assess its efficacy in women with a prolonged second stage of labor but no “fetal distress” and 2) compare its performance head-to-head against the vacuum extractor and forceps.
What this EVIDENCE means for practice
Enthusiasm for the Odón device is fueled by its simplicity and the likelihood that midlevel providers working in remote obstetric units can be trained in its use, thereby increasing access to an important modality of emergency obstetric care. This is particularly important in centers that lack immediate access to cesarean delivery capabilities. Whether the device can be used in developing countries to more effectively manage the second stage of labor and thereby reduce infectious morbidity and pelvic floor injuries has yet to be confirmed but is a testable hypothesis.
WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: [email protected]
Reference
- American College of Obstetricians and Gynecologists. ACOG practice bulletin #17: Operative vaginal delivery. Washington, DC: ACOG; 2000.
Reference
- American College of Obstetricians and Gynecologists. ACOG practice bulletin #17: Operative vaginal delivery. Washington, DC: ACOG; 2000.
Have you read the June Guest Editorial? Click here to access Dr. John T. Repke's Low-dose aspirin and preeclampsia prevention: Ready for prime time, but as a “re-run” or as a “new series”?
Computer Navigation Systems in Unicompartmental Knee Arthroplasty: A Systematic Review
Reducing Transmission of Methicillin-Resistant <em>Staphylococcus aureus</em> and Vancomycin-Resistant <em>Enterococcus</em> in the ICU—An Update on Prevention and Infection Control Practices
From the Department of Medicine, Infectious Disease Practice and Innovations, The Medical City, Pasig City, Philippines (Dr. Abad), the Division of Emergency Medicine, University of Wisconsin Medical School, Madison, WI (Dr. Pulia), University of Wisconsin Hospital and Clinics, Madison, WI (Ms. Krupp), and the Willam S. Middleton Memorial Veterans Affairs Hospital, Madison, WI (Dr. Safdar).
Patients in intensive care units (ICUs) are at greatly increased risk of developing health care-associated infections (HAIs) [1]. More than 70% of the bacteria that cause HAIs are resistant to at least one of the antimicrobials commonly used to treat these infections [2]. Two such pathogens, methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE) are responsible for a considerable proportion of ICU infections that are associated with increased morbidity, mortality, and costs [3–5]. In this review, we discuss the epidemiology of colonization and infection by MRSA and VRE and provide an update on practices for prevention of transmission and infection by MRSA and VRE in the ICU.
EPIDEMIOLOGY AND MECHANISMS OF RESISTANCE
MRSA is the major cause of HAIs worldwide [6]. Among ICUs in the United States, the proportion of methicillin resistance among S. aureus isolates increased from 35.9% in 1992 to 64.4% in 2003 [4]. Approximately 8% of patients are colonized with MRSA upon admission, and an average of 5% will acquire MRSA colonization in the ICU [7,8]. A comparison study of academic tertiary care facilities found medical ICUs had higher MRSA admission prevalence rates than surgical ICUs, whereas surgical ICUs had a higher incidence rate [7]. Enteroccoccus is the third most common pathogen associated with HAIs, with 33% resistant to vancomycin [9]. VRE infection is associated with increased ICU cost and increased length of stay [10]. Incidence of ICU-acquired VRE varies among regions and countries. For example, in the United States, Warren et al [11] reported a VRE incidence of 27 cases per 1000 patient ICU days, whereas Kohlenberg et al [12] reported a mean incidence of 0.29 cases per 1000 patient ICU days in Germany.
Understanding the mechanisms that allow development of resistant strains of S. aureus and Enterococcus species is important to devise preventive strategies. Methicillin resistance in MRSA is determined by the staphylococcal cassette chromosome mec (SCCmec), a mobile genetic element that carries the mecA gene. The mecA gene codes for an additional penicillin-binding protein (PBP) that has a reduced affinity towards methicillin (PBP2a/PBP2'). This results in a reduced ability to bind to the bacterial cell wall and inhibit synthesis [13,14]. Study of molecular epidemiology has identified MRSA as originating from 8 major variants of the mecA gene [15]. The majority of MRSA infections are caused by strains belonging to a few internationally disseminated clones [14]. The first identified strains were associated with infections in hospitalized patients (hospital-associated MRSA), but community-associated MRSA strains have since emerged and have become established globally, including in health care institutions [16].
Community-acquired MRSA can cause severe infections in health hosts [17]; possible explanations include increased CA-MRSA virulence due to the acquisition of mobile genetic elements, namely those containing Panton-Valentine leukocidin (PVL) [18] or increased expression of core genome-encoded virulence genes, such as phenol-soluble modulin (PSM) cytolysins, α-toxin, and other virulence determinants [19].
Enterococcus is intrinsically resistant to several antimicrobial drugs, with resistance to vancomycin encoded by several clusters of genes known as vancomycin resistance gene clusters (eg, vanA, vanB). The gene clusters generate resistance through multiple pathways which encode enzymes to determine the structure of peptidoglycan precursors [20,21]. Genetically diverse, hospital-associated VRE outbreaks have been associated with single clones, multiple clones, and changing molecular epidemiology over time [21]. While up to 25% of the VRE genome includes acquired elements, the majority of hospital-associated infections are traced to a few clonal complexes, which differ from community-associated VRE strains [22].
The evolution of these efficient mechanisms that promote drug resistance has made it extremely challenging to eradicate organisms such as MRSA and VRE. However, advances in recent years have furthered our understanding of the epidemiology, pathogenesis, and methods of prevention and containment.
RISK FACTORS FOR COLONIZATION AND INFECTION
MRSA
The risk factors underlying MRSA colonization and infection in the ICU setting can be categorized as either patient/host or environmental factors. A wide range of patient-level factors is associated with MRSA colonization upon admission. General principles regarding the transmission of MRSA in the community include close contact with colonized or infected individuals, breaks in the skin, crowded living conditions and poor hygiene. These factors, alone or in combination, are thought to underlie observed outbreaks among sports teams, military personnel, correction facilities, American Indian communities, and daycare centers [23–34].
Two recently published systematic reviews have summarized important patient-level factors associated with MRSA colonization at the time of hospital admission. Forster et al [35] examined 27 studies and identified previous admission to hospital, transfer from nursing home or long-term care facility, and previous antibiotic use as the top 3 factors associated with MRSA colonization. A similar review conducted by McKinnell and colleagues [36] found that prior hospitalization, nursing home contact, recent antibiotic use, and exposure to health care-associated pathogens (MRSA carriage, VRE carriage, or Clostridium difficile infection) were found to portend the highest risk. Specific comorbid conditions also conveyed an increased risk, including congestive heart failure, chronic wounds/bedsores, diabetes mellitus, pulmonary disease, immunosuppression, urinary catheter, and renal failure/dialysis. It is clear that health care contacts, especially recent hospitalization, residence in a long-term care facility, and antibiotic use, are significant risk factors for MRSA colonization [37–39].
 In contrast to those already colonized with MRSA, some patients acquire MRSA during hospitalization. In these cases, transmission via hands of health care workers is likely the most common mechanism for spread of MRSA [6,40–42]. An understaffed ICU has also been cited as a potential risk factor for ICU MRSA transmission, perhaps due to sacrifices in hand hygiene practices by overextended staff [6]. Additional factors associated with increased risk of nosocomial MRSA acquisition include duration of antibiotic therapy, exposure to quinolone or macrolide antibiotics, length of hospital stay, enteral feeding, post-surgical status, insertion of central line or urinary catheter during admission, ICU admission, and proximity to another patient with MRSA infection or colonization [43–45]. A summary of risk factors for MRSA acquisition is shown in Table 1.
In contrast to those already colonized with MRSA, some patients acquire MRSA during hospitalization. In these cases, transmission via hands of health care workers is likely the most common mechanism for spread of MRSA [6,40–42]. An understaffed ICU has also been cited as a potential risk factor for ICU MRSA transmission, perhaps due to sacrifices in hand hygiene practices by overextended staff [6]. Additional factors associated with increased risk of nosocomial MRSA acquisition include duration of antibiotic therapy, exposure to quinolone or macrolide antibiotics, length of hospital stay, enteral feeding, post-surgical status, insertion of central line or urinary catheter during admission, ICU admission, and proximity to another patient with MRSA infection or colonization [43–45]. A summary of risk factors for MRSA acquisition is shown in Table 1.
Regardless of whether MRSA colonization precedes admission or occurs due to nosocomial spread, it is associated with increased risk of developing a HAI [46–49]. In 2 large prospective observational cohort studies, the hazard ratios of MRSA colonization developing into S. aureus infections during the ICU stay were 3.84 and 4.70, respectively [50,51]. High levels of concordance between MRSA colonization strains and HAI strains have also been reported [52]. Nasal colonization with S. aureus has also been identified as an independent risk factor for developing ventilator-associated pneumonia (VAP) and bacteremia [53,54]. A case series of ICU patients with S. aureus nasal colonization who developed lower respiratory tract infections demonstrated genetically identical nasal and bronchial strains in 15/16 cases [55]. This finding strongly suggests that nasopharyngeal colonization with S. aureus contaminates oral secretions that are aspirated by critically ill patients, resulting in subsequent pneumonia. In a long-term outcomes study among a matched cohort of veterans, MRSA colonization was associated with an increased risk of infection-related readmission and mortality [56]. These findings reflect the critically important nature of measures designed to curb nosocomial transmission and acquisition of MRSA, especially among the vulnerable ICU population.
VRE
As with MRSA, risk factors associated with VRE colonization include both patient-level and ICU-level (or environmental) factors [57]. Examples of patient-level factors include previous antimicrobial exposure [58–62], underlying medical illnesses such as chronic renal failure requiring hemodialysis [11,63], length of hospital or ICU stay [11,59,64,65], and recent exposure to health care facilities. ICU-level factors of relevance are the prevalence of VRE in the unit, with high levels of endemicity leading to higher risk of colonization and transmission.
Antibiotic use is a major risk factor for VRE acquisition, although the type and class of antibiotic varies considerably across studies; the most frequently identified antibiotics are broad-spectrum cephalosporins, vancomycin, and anti-anaerobic agents [58,62,64]. Patients with chronic liver disease and post-transplantation are at exceedingly high risk for VRE acquisition [59]. In a recent study by Pan [66], for example, the authors found that the incidence of newly acquired VRE was 21.9 per 1000 patient-days in an ICU setting. On multivariate analysis, the authors found that, similar to other reports [11,59,67], length of stay in the ICU was associated with increased risk of VRE acquisition, with each additional day of stay increasing risk of VRE by 1.03 times. Warren et al undertook a prospective cohort study involving 519 patients admitted to the ICU for more than 48 hours [11]. Seventy-four (21%) of 352 patients were subsequently colonized with VRE. The median time to development of a positive VRE culture after ICU admission was 6 days. Increased mean APACHE II score on ICU admission (P = 0.002), sucralfate use (P = 0.003), vasopressor use (P = 0.01), tracheostomy in the ICU (P = 0.02), and C. difficile diarrhea (P = 0.002) appeared to be associated with VRE acquisition.
 It appears that VRE acquisition is often associated with the sick subgroup of patients, and risk factors generally associated with VRE colonization and infection co-relate with disease chronicity and severity of illness. Length of hospitalization, ICU stay, hemodialysis, or transplantation may all be markers of disease severity. A summary of risk factors for VRE acquisition is shown in Table 2.
It appears that VRE acquisition is often associated with the sick subgroup of patients, and risk factors generally associated with VRE colonization and infection co-relate with disease chronicity and severity of illness. Length of hospitalization, ICU stay, hemodialysis, or transplantation may all be markers of disease severity. A summary of risk factors for VRE acquisition is shown in Table 2.
REDUCING TRANSMISSION—MRSA AND VRE PREVENTION STRATEGIES
 Evidence-based guidelines developed by the Centers for Disease Control (CDC) Hospital Infection Control Practices Advisory Committee (HICPAC) for prevention of MRSA and VRE are available [68]. Several recently conducted well-designed clinical trials also provide additional insight that may be particularly helpful in the ICU setting [69]. A summary of the MRSA prevention guidelines issued by the CDC and included in its “MRSA toolkit” is provided in Table 3. A similar guideline on prevention of VRE [70], published more than a decade ago, has similar elements. Table 3 shows a side-by-side comparison of these elements. Unfortunately, despite these guidelines and extensive research regarding prevention and control, considerable controversy exists as to the most effective approaches. As such, these recommendations should be tailored to meet the needs of the specific ICU setting.
Evidence-based guidelines developed by the Centers for Disease Control (CDC) Hospital Infection Control Practices Advisory Committee (HICPAC) for prevention of MRSA and VRE are available [68]. Several recently conducted well-designed clinical trials also provide additional insight that may be particularly helpful in the ICU setting [69]. A summary of the MRSA prevention guidelines issued by the CDC and included in its “MRSA toolkit” is provided in Table 3. A similar guideline on prevention of VRE [70], published more than a decade ago, has similar elements. Table 3 shows a side-by-side comparison of these elements. Unfortunately, despite these guidelines and extensive research regarding prevention and control, considerable controversy exists as to the most effective approaches. As such, these recommendations should be tailored to meet the needs of the specific ICU setting.
Antimicrobial Stewardship
Antibiotic use is a major driver of antibiotic resistance. A meta-analysis by de Bruin and Riley [71] studied the effect of vancomycin usage on VRE colonization and infection. A total of 12 articles describing 13 studies were included; none were randomized controlled trials. All studies were quasi-experimental and lacked control groups. Among all studies, less than half (46%) implemented vancomycin reduction measures as the sole type of intervention [72–76]. The remaining studies implemented other infection control modalities and or restricted the use of other antimicrobials [77–83]. Although all studies that implemented vancomycin restriction alone as a single strategy showed a decline in vancomycin usage, only 2 of these [74,75] showed a relative risk reduction in VRE acquisition post-intervention. Also, studies that restricted vancomycin alone revealed a trend towards lower efficacy in reducing VRE colonization and infection (33%) when compared with those that used additional measures (71%). While judicious antibiotic use should always be practiced, the evidence for vancomycin restriction as a sole intervention to control VRE is scant. It may be that other antibiotics are as big or bigger drivers of resistance in enterococci than vancomycin. For example, a growing body of literature supports antibiotic restriction, especially fluoroquinolones, for reducing MRSA. In several time-series quasi-experimental studies, restriction of fluorquinolones was associated with reduced trends in MRSA infections in the acute care setting, and consideration should be given to monitor and optimize fluoroquinolone use in the ICU setting [84,85].
Antimicrobial stewardship programs are fundamental to optimizing antibiotic use in the ICU and the authors strongly recommend that all ICUs should have such a program in place.
Educational Interventions
Infection control and multidrug-resistant organism (MDRO)–specific education programs for health care workers is a core principle of the CDC’s prevention guidelines. The HICPAC VRE guideline also explicitly states “continuing education programs for hospital staff (including attending and consulting physicians, medical residents, and students; pharmacy, nursing, and laboratory personnel; and other direct patient-care providers) should include information concerning the epidemiology of VRE and the potential impact of this pathogen on the cost and outcome of patient care [70].” A systematic review published in 2008 [86] that included 26 studies showed that such interventions to prevent HCAIs are usually successful; in this review, 20 of 26 studies showed a statistically significant decrease in infection rates, with risk ratios ranging from 0 to 1.6. Education was usually part of a broader array of infection control interventions. While clearly essential, education alone is unlikely to have a sustained impact on reducing MRSA and VRE infections.
Infection Control Measures
Major infection control interventions include hand hygiene, the use of personal protective equipment (PPE), and cohorting. These measures can be grouped into “horizontal” (or global) vs. “vertical” (or targeted) strategies. Although not mutually exclusive, horizontal approaches are designed to have an impact on multiple pathogens (pathogen nonspecific), whereas vertical approaches are designed to reduce the impact of specific pathogens (such as VRE). For the purposes of this review, we will discuss both strategies for containment of MRSA and VRE. Horizontal strategies include hand hygiene, universal gloving and/or gowning, environmental cleaning, and daily bathing with chlorhexidine. Vertical strategies include screening for either MRSA or VRE followed by placement in contact precautions and decolonization with mupirocin.
Hand Hygiene
Hand washing is fundamental to reducing transmission of MDROs in health care institutions; however, optimal compliance is hard to achieve and sustain. Barriers to adherence may include unavailability of sinks or hand hygiene materials (eg, alcohol-based gels, gloves) time constraints, forgetfulness, or lack of knowledge [87–95]. Several monitoring strategies have been evaluated to increase compliance with hand hygiene. Most involve direct observation followed by performance assessment and feedback.
Trials examining the impact of improvements in hand hygiene compliance on HAIs in the ICU setting have largely found benefit, although not all studies showed a decline in HAI. In a prospective crossover trial, Rupp et al [96] found dramatic improvements in compliance with hand gel availability, but this did not translate to decreased nosocomial MRSA infections. Venkatesh et al [97] carried out a before-and-after interventional prospective study in a hematology unit in a tertiary level hospital to evaluate the use of an electronic method of surveillance to determine compliance with hand hygiene. The authors also used rates of horizontal transmission of VRE as a secondary end-point. Results of the study showed that hand hygiene compliance improved from 36.3% at baseline to 70.1%. This represented an OR of 4.1 (95% confidence interval, 3.7–4.5), which the authors attributed to the use of automated alerts. VRE transmission rates before and during intervention were not statistically different, but the rates of infection were lower at 1.0 per month in comparison with 4.7 infections per month in the preceding 6 months (P = 0.096).
While improved hand hygiene may result in significant reductions in HAIs [40], research indicates hand hygiene alone influences about 40% of infections in the ICU setting [98]. As such, hand hygiene should be viewed as a necessary component of a comprehensive infection control program [99]. Despite the success of hand hygiene in reducing HAIs in the ICU, effective strategies to improve compliance remain elusive even under study conditions and further research is needed in this area [100].
Personal Protective Equipment
Tenorio et al [101] conducted a study to assess the effectiveness of gloving in the prevention of hand carriage of VRE by health care workers. The study showed that among 50 health care workers who had contact with patients colonized with VRE, 6 carried a similar patient strain even prior to known contact, and 17 of 44 (69%) had a patient-related VRE strain on their gloves after contact. This suggests a relatively high rate of colonization after usual patient-care contact. Factors associated with acquisition of VRE on gloves included duration of contact, contact with a patient’s body fluids, presence of diarrhea in a patient, mean VRE colony counts on a patient’s skin, and number of body sites colonized with VRE. Although gloves reduced the risk of VRE acquisition of VRE by 71% (ie, 12/17 did not have VRE on their hands after de-gloving) the protection afforded by gloves was incomplete. As such, hand hygiene after glove removal is recommended.
Slaughter et al [102] compared the use of personal protective equipment in the acquisition of VRE. During this study, 93 patients in glove-and-gown rooms and 88 patients in glove-only rooms had similar rates of VRE at baseline entry into the ICU and after the intervention. Mean times to colonization among the patients who became colonized were 8.0 days in the glove-and-gown group and 7.1 days in the glove-only group. None of these comparisons were statistically significant and the authors concluded that the universal use of gown and gloves was no better than the use of gloves alone in preventing VRE colonization.
A recent cluster randomized trial compared the effect of universal PPE (ie, gowning and gloving) with usual care for reducing acquisition of MRSA or VRE as a composite outcome [103]. The study did not find that universal gowning and gloving reduced VRE or MRSA acquisition but found a 40% decline in MRSA acquisition in the intervention ICUs compared with baseline rates of MRSA. No major adverse effects of universal gowning and gloving were noted in this study. A thoughtful editorial commenting on this article proposes that several aspects of the study deserve consideration, including the possibility of false-negative screening tests for VRE, which may have partially accounted for the negative primary outcome [69].
Based on these studies, it appears that the use of barrier precautions may be of value more for MRSA than VRE but further studies are needed to examine its impact on other types of pathogens, including new and emerging MDROs. Until further evidence becomes available, routine gowning and gloving may be of value in units with a high prevalence of MRSA.
Environmental Cleaning
Accumulating data suggests that the environment may play a major role in transmission of pathogens. MRSA has the ability to survive for days to weeks on inanimate objects [104–107]. Environmental contamination results in contamination of staff clothing and gloves [107,108] and is highly correlated with colonization strains among inpatients [109]. Although some studies of enhanced cleaning techniques and increased environmental services staff time have demonstrated reductions in MRSA outbreaks [110–112], the results are not universally favorable [113,114] and further studies are needed to examine the impact of environmental cleaning on rates of MRSA colonization or infection.
Several studies have implicated contaminated equipment as vectors for transmission of VRE during outbreaks [115–117], but the direction of fomite transfer from patient to environment has been difficult to ascertain. VRE have been found frequently on a variety of inanimate objects and surfaces in different health care environments [118–123], including gloved or ungloved hands of health care workers [101,124,125]. Hayden et al [126] determined the effect of improved environmental cleaning on VRE acquisition rates. This study was a pre-and-post intervention study carried out in a 21-bed medical intensive care unit (MICU) in a tertiary hospital over several phases. The intervention included the creation of a unique and improved cleaning program, as well as in-services to housekeeper services, education of the MICU staff, and a hand hygiene campaign. The results of the study showed decreased acquisition of VRE from 33.47 cases per 1000 patient days at risk in period 1 to 10.40 cases per 1000 patient-days at risk by period 4 of the study. Increased environmental cleaning was also associated with reduced growth of VRE from environmental cultures. At baseline, weekly contamination rates were 0.15 and 0.1 for samples obtained before and after cleaning, respectively. Culture positivity decreased to 0.07 and 0.04 for before and after cleaning in period 2 and then remained at low levels during the remainder of the study. It is important to note that the method for disinfecting used in this study was the “bucket method” as promoted by Byers [127]. This study provides further support for the importance of an environmental reservoir and of environmental decontamination to prevent endemic cross-transmission of VRE [126].
Goodman et al [128] used similar interventions but added a feedback tool using a black-light monitoring system (ie, use of an invisible, nontoxic marker to delineate areas that are adequately or inadequately cleaned) to reduce the likelihood of isolating either MRSA or VRE from an ICU environment. This study also showed favorable results, and notably, the use of the black-light monitoring system identified specific areas that were typically inadequately disinfected. Results showed that flat, horizontal surfaces (eg, countertops, bedside tray tables, and hamper tops) were adequately cleaned more often than small, vertical surfaces (eg, doorknobs, toilet handles, light switches, and electronics).
Part of the controversy surrounding the impact of environmental cleaning is the difficulty in determining its individual value as part of an overall infection control bundle [129]. A proposed area of demonstrable impact for environmental cleaning are frequently touched sites which are more likely to be contaminated with pathogens. Focusing on these “hot-bed” areas of the care environment may offer a useful adjunct to other infection control measures [129].
Active Surveillance
Active surveillance refers to periodic screening for asymp-tomatic carriers followed by placement of colonized patients in contact isolation. This practice is highly variable across institutions, as the evidence supporting this practice is conflicting and there are concerns about the cost of implementing this approach without solid evidence [70,130,131]. Despite lack of randomized controlled trials to guide this practice for MRSA prevention, many hospitals utilize MRSA surveillance and it is mandated by law in 9 states [132,133].
A prospective, interventional cohort study of universal MRSA screening on admission to surgical wards failed to reduce nosocomial MRSA infections [134]. Most recently, a pragmatic, cluster-randomized ICU trial reported that universal decolonization with chlorhexidine wipes and mupirocin use was more effective than screening and isolation in reducing rates of MRSA clinical isolates [65]. However, concerns regarding the risk of mupirocin resistance have been expressed [135,136]. The only randomized trial that compared active surveillance for MRSA and VRE followed by contact precautions to usual care did not find a benefit to active surveillance.
Huskins et al [137], in a large, cluster-randomized trial of 19 ICUs from different hospitals, determined the utility of using a culture-based active surveillance and contact isolation, compared with usual care (contact isolation for patients colonized with MRSA or VRE) as identified by existing hospital protocols, to reduce the incidence of colonization or infection with MRSA or VRE. In this trial, which spanned 6 months and involved 3488 participants, the authors found no significant difference between the intervention and control ICUs in terms of MRSA and VRE colonization or infection rates.
Conflicting with these findings is an observational study comparing MRSA infection rates before and after institution of a universal screening protocol, which demonstrated a 69.6% (CI, –89.2% to –19.6%]; P = 0.03) reduction in hospital wide MRSA prevalence density with screening [138]. The “MRSA bundle” implemented in 2007 at VA hospitals nationwide, which included universal screening, produced a 62% (P < 0.001) reduction in MRSA ICU infections; the relative contribution of the various bundle components is uncertain [139,140].
A proposed cost-saving alternative to universal screening is selective screening based on risk factor assessment [141]. The effectiveness of this type of program depends on creating a clinical decision-making tool capable of accurately identifying high-risk individuals while also accounting for the different risk factor profiles between HA-MRSA and CA-MRSA [142]. It has been proposed that targeted screening protocols may be more cost-effective in settings with < 5% prevalence of MRSA colonization on admission [143].
Many studies [61,144–149] have shown that active surveillance against VRE is cost-effective. For example, Calfee et al [144] showed that an established active surveillance program results in control of endemic VRE in high-risk patients. The infection control program was established in response to a hospital-wide VRE outbreak, and was sustained after the outbreak was controlled. The study by Calfee et al spanned 5 years and was performed at a tertiary-level university hospital, where cultures from perirectal areas were used to identify high-risk patients who were asymptomatically colonized with VRE. During the latter 2 years, 768 new cases of VRE colonization were detected among 69,672 admissions (1.1% of admissions), of which 730 (95.1%) were identified by active surveillance methods. This implies that routine clinical cultures would probably have missed the majority of colonized patients. During this period, the incidence of VRE infection was likewise extremely low at 0.12/1000 patient days (ie, 90 nosocomial VRE infections were identified in 83 patients during 743,956 days of patient care). Sixty-nine of the 83 patients (83%) who developed nosocomial VRE infections were found to be colonized with VRE by surveillance culture before the onset of infection.
Patient Decolonization
Chlorhexidine gluconate has been used in several settings to control outbreaks and infections related to MRSA and VRE due to its broad-spectrum activity against these pathogens. Chlorhexidine-based solutions reduce the density of skin colonization with pathogens such as MRSA and VRE (skin asepsis), thus lowering the risk for horizontal transmission between health care workers and patients.
Decolonization with chlorhexidine as an MRSA infection reduction technique has demonstrated benefit in the ICU setting [150]. The previously mentioned large, cluster-randomized ICU trial by Huang and colleagues found universal decolonization with twice-daily intranasal mupirocin for 5 days and daily bathing with chlorhexidine-impregnated cloths for the entire ICU stay was superior to targeted decolonization of known MRSA carriers in preventing overall MRSA isolates. However, universal decolonization failed to show a reduction in MRSA bacteremia [151], and concerns about mupirocin resistance may limit the applicability of this approach.
There are now several studies [152–154] that show decreased acquisition of VRE with use of daily chlorhexidine bathing. In a study including 1787 ICU patients, Vernon et al found [154] that the reducing microbial density of VRE on patient’s skin by using chlorhexidine led to decreased transmission. In another study by Climo et al [153] that involved 6 ICUs at 4 academic centers and measured the incidence of MRSA and VRE colonization and blood stream infections (BSI) during a period of bathing with routine soap for 6 months compared with a 6-month period where all admitted patients received daily bathing with a chlorhexidine solution, results found decreased acquisition of VRE by 50% (4.35 vs. 2.19 cases/1000 patient days, P < 0.008) following the introduction of daily chlorhexidine bathing. Furthermore, compared with 16 of 270 patients colonized with VRE who subsequently developed VRE bacteremia at baseline, only 4 of 226 VRE-colonized patients bathed with chlorhexidine in the intervention period developed a BSI, translating into a relative risk reduction of 3.35 (95% CI, 1.13–9.87; P < 0.035). Patients colonized with VRE were 3 times less likely to develop VRE bacteremia when bathed with chlorhexidine compared with regular bathing. Despite the success of this protocol for VRE, when analyzed by individual organism no significant reductions in MRSA acquisition or BSI were reported. This finding is similarly corroborated by a trial conducted in the pediatric ICU setting which found an overall reduction in bacteremia with daily chlorhexidine washes but no significant decrease in cases due to S. aureus [155].
The results of these studies suggest that daily bathing with chlorhexidine should be part of routine practice in health care, especially in ICUs where endemic MRSA or VRE rates are high. Whether there is benefit in other settings needs to be studied.
In addition to chlorhexidine washes, other decolonization techniques have been proposed to reduce colonization and the spread of HAIs in the ICU setting. A randomized controlled trial of daily 5% tea tree oil body washes for the prevention of MRSA colonization failed to significantly reduce rates compared to standard soap body washes [156]. Another proposed decolonization intervention that has not been widely adopted in the United States due to concerns related to development of resistant organisms is selective digestive decontamination (SDD) or selective oropharyngeal decontamination (SOD) with antimicrobial agents [157,158]. In terms of clinical benefit, SDD/SOD have been found to decrease MDRO infection rate [159] and mortality [160].
Cohorting
There is insufficient evidence to conclude that cohorting isolated patients is of benefit for routine use in the endemic ICU setting. A few studies, mainly in the outbreak setting, have examined this approach and the results are conflicting [161,162]. Pending further studies in this area, it is reasonable to cohort patients colonized with the same microorganisms, especially if patients cannot be placed in single rooms.
CONCLUSION
The emergence of MRSA and VRE has led to a resurgence of interest and emphasis on infection control practices and prevention. CDC guidelines to help health care practitioners manage these MDROs in the hospital and ICU-setting exist; however, many questions remain regarding best practice.
Prevention of MRSA and VRE needs to be a 2-pronged approach—antimicrobial stewardship [163] and infection control. A robust antimicrobial stewardship program to optimize and minimize inappropriate antibiotic use is necessary in every institution. From the infection prevention standpoint, it is unclear if systematic identification of MRSA and VRE colonization followed by contact precautions is useful in reducing transmission. It is clear that a strong institutional climate of promoting patient safety and a culture of infection prevention will help in reducing MRSA and VRE facility-wide. It also appears that universal gowning and gloving may be useful for reducing MRSA, but not VRE, transmission. While universal decolonization with mupirocin is efficacious in reducing MRSA, this strategy is not recommended because of promoting mupirocin resistance. However, the use of daily bathing with chlorhexidine represents a relatively low-cost, high-yield intervention that should be adopted. Pending further data, patients known to be colonized or infected with MRSA should be placed in contact precuations as is current practice in most institutions. Finally, in this era of MDROs, hand hygiene remains our best defense against the spread of pathogens in the health care environment.
Note: This article does not represent the views of the Department of Veterans Affairs.
Corresponding author: Nasia Safdar, MD, Willam S. Middleton Memorial Veterans Affairs Hospital, 2500 Overlook Terrace, Madison, WI 53705, [email protected].
Funding/support: This work is funded by a MERIT award from the Department of Veterans Affairs to Nasia Safdar.
Financial disclosures: None.
REFERENCES
1. Burton DC, Edwards JR, Horan TC, et al. Methicillin-resistant Staphylococcus aureus central line-associated bloodstream infections in US intensive care units, 1997–2007. JAMA 2009;301:727–36.
2. LeDell K, Muto CA, Jarvis WR, Farr BM. SHEA guideline for preventing nosocomial transmission of multidrug-resistant strains of Staphylococcus aureus and Enterococcus. Infect Control Hosp Epidemiol 2003;24:639–41.
3. Giske CG, Monnet DL, Cars O, Carmeli Y. Clinical and economic impact of common multidrug-resistant gram-negative bacilli. Antimicrob Agents Chemother 2008;52:813–21.
4. Klevens RM, Edwards JR, Richards CL Jr, et al. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Pub Health Rep 2007;122:160–6.
5. Schwaber MJ, Carmeli Y. The effect of antimicrobial resistance on patient outcomes: importance of proper evaluation of appropriate therapy. Crit Care 2009;13:106.
6. Grundmann H, Hori S, Winter B, et al. Risk factors for the transmission of methicillin-resistant Staphylococcus aureus in an adult intensive care unit: fitting a model to the data. J Inf Dis 2002;185:481–8.
7. Huang SS, Rifas-Shiman SL, Warren DK, et al. Improving methicillin-resistant Staphylococcus aureus surveillance and reporting in intensive care units. J Infect Dis 2007;195:330–8.
8. Muder RR, Cunningham C, McCray E, et al. Implementation of an industrial systems-engineering approach to reduce the incidence of methicillin-resistant Staphylococcus aureus infection. Infect Control Hosp Epidemiol 2008;29:702–8.
9. Hidron AI, Edwards JR, Patel J, et al. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol 2008;29:996–1011.
10. Pelz RK, Lipsett PA, Swoboda SM, et al. Vancomycin-sensitive and vancomycin-resistant enterococcal infections in the ICU: attributable costs and outcomes. Intensive Care Med 2002;28:692–7.
11. Warren DK, Kollef MH, Seiler SM, et al. The epidemiology of vancomycin-resistant Enterococcus colonization in a medical intensive care unit. Infect Control Hosp Epidemiol 2003;24:257–63.
12. Kohlenberg A, Schwab F, Meyer E, et al. Regional trends in multidrug-resistant infections in German intensive care units: a real-time model for epidemiological monitoring and analysis. J Hosp Infect 2009;73:239–45.
13. Deurenberg RH, Stobberingh EE. The evolution of Staphylococcus aureus. Infect Genet Evol 2008;8:747–63.
14. Gordon RJ, Lowy FD. Pathogenesis of methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis 2008;46 Suppl 5:S350–9.
15. Zhang K, McClure JA, Elsayed S, Conly JM. Novel staphylococcal cassette chromosome mec type, tentatively designated type VIII, harboring class A mec and type 4 ccr gene complexes in a Canadian epidemic strain of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 2009;53:531–40.
16. Chatterjee SS, Otto M. Improved understanding of factors driving methicillin-resistant Staphylococcus aureus epidemic waves. Clin Epidemiol 2013;5:205–17.
17. Otto M. MRSA virulence and spread. Cell Microbiol 2012;14:1513–21.
18. Vandenesch F, Naimi T, Enright MC, et al. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg Infect Dis 2003;9:978–84.
19. Li M, Diep BA, Villaruz AE, et al. Evolution of virulence in epidemic community-associated methicillin-resistant Staphylococcus aureus. Proc Natl Acad Sci U S A 2009;106:5883–8.
20. Courvalin P. Vancomycin resistance in gram-positive cocci. Clin Infect Dis 2006;42 Suppl 1:S25–34.
21. Gold HS. Vancomycin-resistant enterococci: mechanisms and clinical observations. Clin Infect Dis 2001;33:210–9.
22. Arias CA, Murray BE. The rise of the Enterococcus: beyond vancomycin resistance. Nat Rev Microbiol 2012;10:266–78.
23. Adcock PM, Pastor P, Medley F, Patterson JE, Murphy TV. Methicillin-resistant Staphylococcus aureus in two child care centers. J Infect Dis 1998;178:577–80.
24. Dietrich DW, Auld DB, Mermel LA. Community-acquired methicillin-resistant Staphylococcus aureus in southern New England children. Pediatrics 2004;113:e347–52.
25. Groom AV, Wolsey DH, Naimi TS, et al. Community-acquired methicillin-resistant Staphylococcus aureus in a rural American Indian community. JAMA 2001;286:1201–5.
26. Hewlett AL, Falk PS, Hughes KS, Mayhall CG. Epidemiology of methicillin-resistant Staphylococcus aureus in a university medical center day care facility. Infect Control Hosp Epidemiol 2009;30:985–92.
27. Kazakova SV, Hageman JC, Matava M, et al. A clone of methicillin-resistant Staphylococcus aureus among professional football players. N Engl J Med 2005;352:468–75.
28. Landrum ML, Neumann C, Cook C, et al. Epidemiology of Staphylococcus aureus blood and skin and soft tissue infections in the US military health system, 2005-2010. JAMA 2012;308:50–9.
29. Lindenmayer JM, Schoenfeld S, O’Grady R, Carney JK. Methicillin-resistant Staphylococcus aureus in a high school wrestling team and the surrounding community. Arch Intern Med 1998;158:895–9.
30. Malcolm B. The rise of methicillin-resistant staphylococcus aureus in U.S. correctional populations. J Correct Health Care 2011;17:254–65.
31. Nerby JM, Gorwitz R, Lesher L, et al. Risk factors for household transmission of community-associated methicillin-resistant Staphylococcus aureus. Pediatr Infect Dis J 2011;30:927–32.
32. Stemper ME, Shukla SK, Reed KD. Emergence and spread of community-associated methicillin-resistant Staphylococcus aureus in rural Wisconsin, 1989 to 1999. J Clin Microbiol 2004;42:5673–80.
33. Turabelidze G, Lin M, Wolkoff B, et al. Personal hygiene and methicillin-resistant Staphylococcus aureus infection. Emerg Infect Dis 2006;12:422–7.
34. Ellis MW, Hospenthal DR, Dooley DP, et al. Natural history of community-acquired methicillin-resistant Staphylococcus aureus colonization and infection in soldiers. Clin Infect Dis 2004;39:971–9.
35. Forster AJ, Oake N, Roth V, et al. Patient-level factors associated with methicillin-resistant Staphylococcus aureus carriage at hospital admission: a systematic review. Am J Infect Control 2013;41:214–20.
36. McKinnell JA, Miller LG, Eells SJ, et al. A systematic literature review and meta-analysis of factors associated with methicillin-resistant staphylococcus aureus colonization at time of hospital or intensive care unit admission. Infect Contol Hosp Epidemiol 2013;34:1077–86.
37. Furuno JP, McGregor JC, Harris AD, et al. Identifying groups at high risk for carriage of antibiotic-resistant bacteria. Arch Intern Med 2006;166:580–5.
38. Jernigan JA, Pullen AL, Flowers L, et al. Prevalence of and risk factors for colonization with methicillin-resistant Staphylococcus aureus at the time of hospital admission. Infect Control Hosp Epidemiol 2003;24:409–14.
39. Horner C, Parnell P, Hall D, Kearns A, Heritage J, Wilcox M. Meticillin-resistant Staphylococcus aureus in elderly residents of care homes: colonization rates and molecular epidemiology. J Hosp Infect 2013;83:212–8.
40. Allegranzi B, Pittet D. Role of hand hygiene in healthcare-associated infection prevention. J Hosp Infect 2009;73:305–15.
41. Boyce JM. Methicillin-resistant Staphylococcus aureus. Detection, epidemiology, and control measures. Infect Dis Clin North Am 1989;3:901–13.
42. Jernigan JA. Methicillin-resistant Staphylococcus aureus colonization among health care personnel in the emergency department: what does it tell us? Ann Emerg Med 2008;52:534–6.
43. Carnicer-Pont D, Bailey KA, Mason BW, Walker AM, Evans MR, Salmon RL. Risk factors for hospital-acquired methicillin-resistant Staphylococcus aureus bacteraemia: a case-control study. Epidemiol Infect 2006;134:1167–73.
44. Graffunder EM, Venezia RA. Risk factors associated with nosocomial methicillin-resistant Staphylococcus aureus (MRSA) infection including previous use of antimicrobials. J Antimicrob Chemother 2002;49:999–1005.
45. Thompson RL, Cabezudo I, Wenzel RP. Epidemiology of nosocomial infections caused by methicillin-resistant Staphylococcus aureus. Ann Intern Med 1982;97:309–17.
46. Davis KA, Stewart JJ, Crouch HK,et al. Methicillin-resistant Staphylococcus aureus (MRSA) nares colonization at hospital admission and its effect on subsequent MRSA infection. Clin Infect Dis 2004;39:776–82.
47. Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev 1997;10:505–20.
48. Safdar N, Bradley EA. The risk of infection after nasal colonization with Staphylococcus aureus. Am J Med 2008;121:310–5.
49. Wertheim HFL, Vos MC, Ott A, et al. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet 2004;364:703–5.
50. Garrouste-Orgeas M, Timsit JF, Kallel H, et al. Colonization with methicillin-resistant Staphylococcus aureus in ICU patients: morbidity, mortality, and glycopeptide use. Infect Control Hosp Epidemiol 2001;22:687–92.
51. Honda H, Krauss MJ, Coopersmith CM, et al. Staphylococcus aureus nasal colonization and subsequent infection in intensive care unit patients: does methicillin resistance matter? Infect Control Hosp Epidemiol;31:584–91.
52. von Eiff C, Becker K, Machka K, et al. Nasal carriage as a source of Staphylococcus aureus bacteremia. N Engl J Med 2001;344:11–6.
53. Pujol M, Pea C, Pallares R, et al. Nosocomial Staphylococcus aureus bacteremia among nasal carriers of methicillin-resistant and methicillin-susceptible strains. Am J Med 1996;100:509–16.
54. Rocha LA, Marques Ribas R, da Costa Darini AL, Gontijo Filho PP. Relationship between nasal colonization and ventilator-associated pneumonia and the role of the environment in transmission of Staphylococcus aureus in intensive care units. Am J Infect Control 2013;41:236–40.
55. Corne P, Marchandin Hln, Jonquet O, Campos J, Bauls A-L. Molecular evidence that nasal carriage of Staphylococcus aureus plays a role in respiratory tract infections of critically ill patients. J Clin Microbiol 2005;43:3491–3.
56. Quezada Joaquin NM, Diekema DJ, Perencevich EN, et al. Long-term risk for readmission, methicillin-resistant Staphylococcus aureus (MRSA) infection, and death among MRSA-colonized veterans. Antimicrob Agents Chemother 2013;57:1169–72.
57. Lin MY, Hayden MK. Methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococcus: recognition and prevention in intensive care units. Crit Care Med 2010;38:S335–44.
58. Carmeli Y, Eliopoulos GM, Samore MH. Antecedent treatment with different antibiotic agents as a risk factor for vancomycin-resistant Enterococcus. Emerg Infect Dis 2002;8:802–7.
59. Ostrowsky BE, Venkataraman L, D’Agata EM, et al. Vancomycin-resistant enterococci in intensive care units: high frequency of stool carriage during a non-outbreak period. Arch Intern Med 1999;159:1467–72.
60. Bonten MJ, Hayden MK, Nathan C, et al. Epidemiology of colonisation of patients and environment with vancomycin-resistant enterococci. Lancet 1996;348:1615–9.
61. Ostrowsky BE, Trick WE, Sohn AH, et al. Control of vancomycin-resistant enterococcus in health care facilities in a region. N Engl J Med 2001;344:1427–33.
62. Padiglione AA, Wolfe R, Grabsch EA, et al. Risk factors for new detection of vancomycin-resistant enterococci in acute-care hospitals that employ strict infection control procedures. Antimicrob Agents Chemother 2003;47:2492–8.
63. Batistao DW, Gontijo-Filho PP, Conceicao N, et al. Risk factors for vancomycin-resistant enterococci colonisation in critically ill patients. Mem Inst Oswaldo Cruz 2012;107:57–63.
64. Furtado GH, Martins ST, Coutinho AP, et al. Prevalence and factors associated with rectal vancomycin-resistant enterococci colonization in two intensive care units in Sao Paulo, Brazil. Braz J Infect Dis 2005;9:64–9.
65. Huang SS, Datta R, Rifas-Shiman S, et al. Colonization with antibiotic-susceptible strains protects against methicillin-resistant Staphylococcus aureus but not vancomycin-resistant enterococci acquisition: a nested case-control study. Crit Care 2011;15:R210.
66. Pan SC, Wang JT, Chen YC, et al. Incidence of and risk factors for infection or colonization of vancomycin-resistant enterococci in patients in the intensive care unit. PLoS One 2012;7:e47297.
67. Se YB, Chun HJ, Yi HJ, et al. Incidence and risk factors of infection caused by vancomycin-resistant enterococcus colonization in neurosurgical intensive care unit patients. J Korean Neurosurg Soc 2009;46:123–9.
68. Healthcare Infection Control Practices Advisory Committee (HICPAC). Management of multidrug-resistant organisms in healthcare settings, 2006. Accessed 11 Oct 2013 at www.cdc.gov/hicpac/mdro/mdro_toc.html.
69. Malani PN. Preventing infections in the ICU: one size does not fit all. JAMA 2013;310:1567–8.
70. Recommendations for preventing the spread of vancomycin resistance. Recommendations of the Hospital Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep 1995;44:1–13.
71. de Bruin MA, Riley LW. Does vancomycin prescribing intervention affect vancomycin-resistant enterococcus infection and colonization in hospitals? A systematic review. BMC Infect Dis 2007;7:24.
72. Adachi W, Bolding F, Armstrong R. Experience with vancomycin education and order sheet to limit vancomycin use. Hosp Pharm 1997:1370–3.
73. Fridkin SK, Lawton R, Edwards JR, et al. Monitoring antimicrobial use and resistance: comparison with a national benchmark on reducing vancomycin use and vancomycin-resistant enterococci. Emerg Infect Dis 2002;8:702–7.
74. Guglielmo BJ, Dudas V, Maewal I, et al. Impact of a series of interventions in vancomycin prescribing on use and prevalence of vancomycin-resistant enterococci. Jt Comm J Qual Patient Saf 2005;31:469–75.
75. Lautenbach E, LaRosa LA, Marr AM, et al. Changes in the prevalence of vancomycin-resistant enterococci in response to antimicrobial formulary interventions: impact of progressive restrictions on use of vancomycin and third-generation cephalosporins. Clin Infect Dis 2003;36:440–6.
76. Morgan AS, Brennan PJ, Fishman NO. Impact of a vancomycin restriction policy on use and cost of vancomycin and incidence of vancomycin-resistant Enterococcus. Ann Pharmacother 1997;31:970–3.
77. Anglim AM, Klym B, Byers KE, et al. Effect of a vancomycin restriction policy on ordering practices during an outbreak of vancomycin-resistant Enterococcus faecium. Arch Intern Med 1997;157:1132–6.
78. Montecalvo MA, Jarvis WR, Uman J, et al. Infection-control measures reduce transmission of vancomycin-resistant enterococci in an endemic setting. Ann Intern Med 1999;131:269–72.
79. Morris JG Jr, Shay DK, Hebden JN, et al. Enterococci resistant to multiple antimicrobial agents, including vancomycin. Establishment of endemicity in a university medical center. Ann Intern Med 1995;123:250–9.
80. Quale J, Landman D, Saurina G, et al. Manipulation of a hospital antimicrobial formulary to control an outbreak of vancomycin-resistant enterococci. Clin Infect Dis 1996;23:1020-5.
81. Rubin LG, Tucci V, Cercenado E, et al. Vancomycin-resistant Enterococcus faecium in hospitalized children. Infect Control Hosp Epidemiol 1992;13:700–5.
82. Lai KK, Kelley AL, Melvin ZS, et al. Failure to eradicate vancomycin-resistant enterococci in a university hospital and the cost of barrier precautions. Infect Control Hosp Epidemiol 1998;19:647–52.
83. Shaikh ZH, Osting CA, Hanna HA, et al. Effectiveness of a multifaceted infection control policy in reducing vancomycin usage and vancomycin-resistant enterococci at a tertiary care cancer centre. J Hosp Infect 2002;51:52–8.
84. Lafaurie M, Porcher R, Donay JL, et al. Reduction of fluoroquinolone use is associated with a decrease in methicillin-resistant Staphylococcus aureus and fluoroquinolone-resistant Pseudomonas aeruginosa isolation rates: a 10 year study. J Antimicrob Chemother 2012;67:1010–5.
85. Parienti JJ, Cattoir V, Thibon P, et al. Hospital-wide modification of fluoroquinolone policy and meticillin-resistant Staphylococcus aureus rates: a 10-year interrupted time-series analysis. J Hosp Infect 2011;78:118–22.
86. Safdar N, Abad C. Educational interventions for prevention of healthcare-associated infection: a systematic review. Crit Care Med 2008;36:933–40.
87. Boyce JM. It is time for action: improving hand hygiene in hospitals. Ann Intern Med 1999;130:153–5.
88. Jackson M, Chiarello LA, Gaynes RP, Gerberding JL. Nurse staffing and healthcare-associated infections: proceedings from a working group meeting. J Nurs Adm 2002;32:314–22.
89. Kuzu N, Ozer F, Aydemir S, et al. Compliance with hand hygiene and glove use in a university-affiliated hospital. Infect Control Hosp Epidemiol 2005;26:312–5.
90. Larson E, Killien M. Factors influencing handwashing behavior of patient care personnel. Am J Infect Control 1982;10:93–9.
91. Larson E, Kretzer EK. Compliance with handwashing and barrier precautions. J Hosp Infect 1995;30 Suppl:88–106.
92. Naikoba S, Hayward A. The effectiveness of interventions aimed at increasing handwashing in healthcare workers - a systematic review. J Hosp Infect 2001;47:173–80.
93. Pittet D, Simon A, Hugonnet S, et al. Hand hygiene among physicians: performance, beliefs, and perceptions. Ann Intern Med 2004;141:1–8.
94. Trick WE, Vernon MO, Welbel SF, et al. Multicenter intervention program to increase adherence to hand hygiene recommendations and glove use and to reduce the incidence of antimicrobial resistance. Infect Control Hosp Epidemiol 2007;28:42–9.
95. Wisniewski MF, Kim S, Trick WE, et al. Effect of education on hand hygiene beliefs and practices: a 5-year program. Infect Control Hosp Epidemiol 2007;28:88–91.
96. Rupp ME, Fitzgerald T, Puumala S, et al. Prospective, controlled, cross-over trial of alcohol-based hand gel in critical care units. Infect Control Hosp Epidemiol 2008;29:8–15.
97. Venkatesh AK, Lankford MG, Rooney DM, et al. Use of electronic alerts to enhance hand hygiene compliance and decrease transmission of vancomycin-resistant Enterococcus in a hematology unit. Am J Infect Control 2008;36:199–205.
98. Silvestri L, Petros AJ, Sarginson RE, et al. Handwashing in the intensive care unit: a big measure with modest effects. J Hosp Infect 2005;59:172–9.
99. Akyol A, Ulusoy H, Ozen I. Handwashing: a simple, economical and effective method for preventing nosocomial infections in intensive care units. J Hosp Infect 2006;62:395–405.
100. Simmons B, Bryant J, Neiman K, et al. The role of handwashing in prevention of endemic intensive care unit infections. Infect Control Hosp Epidemiol 1990;11:589–94.
101. Tenorio AR, Badri SM, Sahgal NB, et al. Effectiveness of gloves in the prevention of hand carriage of vancomycin-resistant enterococcus species by health care workers after patient care. Clin Infect Dis 2001;32:826–9.
102. Slaughter S, Hayden MK, Nathan C, et al. A comparison of the effect of universal use of gloves and gowns with that of glove use alone on acquisition of vancomycin-resistant enterococci in a medical intensive care unit. Ann Intern Med 1996;125:448–56.
103. Harris AD, Pineles L, Belton B, et al. Universal glove and gown use and acquisition of antibiotic-resistant bacteria in the ICU: a randomized trial. JAMA 2013;310:1571–80.
104. Dietze B, Rath A, Wendt C, Martiny H. Survival of MRSA on sterile goods packaging. J Hosp Infect 2001;49:255–61.
105. Hardy KJ, Oppenheim BA, Gossain S, et al. A study of the relationship between environmental contamination with methicillin-resistant Staphylococcus aureus (MRSA) and patients’ acquisition of MRSA. Infect Control Hosp Epidemiol 2006;27:127–32.
106. Jawad A, Heritage J, Snelling AM, et al. Influence of relative humidity and suspending menstrua on survival of Acinetobacter spp. on dry surfaces. J Clin Microbiol 1996;34:2881–7.
107. Boyce JM, Havill NL, Otter JA, Adams NM. Widespread environmental contamination associated with patients with diarrhea and methicillin-resistant Staphylococcus aureus colonization of the gastrointestinal tract. Infect Control Hosp Epidemiol 2007;28:1142–7.
108. Boyce JM, Potter-Bynoe G, Chenevert C, King T. Environmental contamination due to methicillin-resistant Staphylococcus aureus: possible infection control implications. Infect Control Hosp Epidemiol 1997;18:622–7.
109. Sexton T, Clarke P, O’Neill E, et al. Environmental reservoirs of methicillin-resistant Staphylococcus aureus in isolation rooms: correlation with patient isolates and implications for hospital hygiene. J Hosp Infect 2006;62:187–94.
110. Dancer SJ. Importance of the environment in meticillin-resistant Staphylococcus aureus acquisition: the case for hospital cleaning. Lancet infect dis 2008;8:101–13.
111. Dancer SJ, White LF, Lamb J, et al. Measuring the effect of enhanced cleaning in a UK hospital: a prospective cross-over study. BMC med 2009;7.
112. Rampling A, Wiseman S, Davis L, et al. Evidence that hospital hygiene is important in the control of methicillin-resistant Staphylococcus aureus. J Hosp Infect 2001;49:109–16.
113. Wilson APR, Smyth D, Moore G, et al. The impact of enhanced cleaning within the intensive care unit on contamination of the near-patient environment with hospital pathogens: a randomized crossover study in critical care units in two hospitals. Crit Care Med 2011;39:651–8.
114. Hess AS, Shardell M, Johnson JK, et al. A randomized controlled trial of enhanced cleaning to reduce contamination of healthcare worker gowns and gloves with multidrug-resistant bacteria. Infection Control Hosp Epidemiol 2013;34:487–93.
115. Falk PS, Winnike J, Woodmansee C, et al. Outbreak of vancomycin-resistant enterococci in a burn unit. Infect Control Hosp Epidemiol 2000;21:575–82.
116. Livornese LL Jr, Dias S, Samel C, et al. Hospital-acquired infection with vancomycin-resistant Enterococcus faecium transmitted by electronic thermometers. Ann Intern Med 1992;117:112–6.
117. Porwancher R, Sheth A, Remphrey S, et al. Epidemiological study of hospital-acquired infection with vancomycin-resistant Enterococcus faecium: possible transmission by an electronic ear-probe thermometer. Infect Control Hosp Epidemiol 1997;18:771–3.
118. Donskey CJ, Chowdhry TK, Hecker MT, et al. Effect of antibiotic therapy on the density of vancomycin-resistant enterococci in the stool of colonized patients. N Engl J Med 2000;343:1925–32.
119. Neely AN, Maley MP. Survival of enterococci and staphylococci on hospital fabrics and plastic. J Clin Microbiol 2000;38:724–6.
120. Noskin GA, Bednarz P, Suriano T, et al. Persistent contamination of fabric-covered furniture by vancomycin-resistant enterococci: implications for upholstery selection in hospitals. Am J Infect Control 2000;28:311–3.
121. Noskin GA, Stosor V, Cooper I, Peterson LR. Recovery of vancomycin-resistant enterococci on fingertips and environmental surfaces. Infect Control Hosp Epidemiol 1995;16:577–81.
122. Smith TL, Iwen PC, Olson SB, Rupp ME. Environmental contamination with vancomycin-resistant enterococci in an outpatient setting. Infect Control Hosp Epidemiol 1998;19:515–8.
123. Wendt C, Wiesenthal B, Dietz E, Ruden H. Survival of vancomycin-resistant and vancomycin-susceptible enterococci on dry surfaces. J Clin Microbiol 1998;36:3734–6.
124. Bhalla A, Pultz NJ, Gries DM, et al. Acquisition of nosocomial pathogens on hands after contact with environmental surfaces near hospitalized patients. Infect Control Hosp Epidemiol 2004;25:164–7.
125. Ray AJ, Hoyen CK, Taub TF, et al. Nosocomial transmission of vancomycin-resistant enterococci from surfaces. JAMA 2002;287:1400–1.
126. Hayden MK, Bonten MJ, Blom DW, et al. Reduction in acquisition of vancomycin-resistant enterococcus after enforcement of routine environmental cleaning measures. Clin Infect Dis 2006;42:1552–60.
127. Byers KE, Durbin LJ, Simonton BM, et al. Disinfection of hospital rooms contaminated with vancomycin-resistant Enterococcus faecium. Infect Control Hosp Epidemiol 1998;19:261–4.
128. Goodman ER, Platt R, Bass R, et al. Impact of an environmental cleaning intervention on the presence of methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci on surfaces in intensive care unit rooms. Infect Control Hosp Epidemiol 2008;29:593–9.
129. Dancer SJ. The role of environmental cleaning in the control of hospital-acquired infection. J Hosp Infect 2009;73:378–85.
130. Centers for Disease Control and Prevention. Methicillin-resistant Staphylococcus aureus (MRSA) infections. Accessed 11 Oct 2013 at www.cdc.gov/mrsa/index.html.
131. Edmond MB, Wenzel RP. Targeted decolonization to prevent ICU infections. N Engl J Med 2013;369:1471.
132. Lai KK, Fontecchio S, Melvin Z, Baker SP. Impact of alcohol-based, waterless hand antiseptic on the incidence of infection and colonization with methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci. Infect Control Hosp Epidemiol 2006;27:1018–24.
133. Ostrowsky B, Steinberg JT, Farr B, et al. Reality check: should we try to detect and isolate vancomycin-resistant enterococci patients? Infect Control Hosp Epidemiol 2001;22:116–9.
134. Harbarth S, Sax H, Uckay I, et al. A predictive model for identifying surgical patients at risk of methicillin-resistant Staphylococcus aureus carriage on admission. J Am Coll Surg 2008;207:683–9.
135. Jarvis WR. Targeted decolonization to prevent ICU infections. N Engl J Med 2013;369:1469.
136. Krause R, Honigl M, Zollner-Schwetz I. Targeted decolonization to prevent ICU infections. N Engl J Med;369:1469–70.
137. Huskins WC, Huckabee CM, O’Grady NP, et al. Intervention to reduce transmission of resistant bacteria in intensive care. N Engl J Med;364:1407–18.
138. Robicsek A, Beaumont JL, Paule SM, et al. Universal surveillance for methicillin-resistant Staphylococcus aureus in 3 affiliated hospitals. Ann Intern Med 2008;148:409–18.
139. Jain R, Kralovic SM, Evans ME, et al. Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. N Engl J Med 2011;364:1419–30.
140. Gurieva T, Bootsma MCJ, Bonten MJM. Successful Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections revisited. Clin Infect Dis 2012:54:1618–20.
141. Gavalda L, Masuet C, Beltran J, et al. Comparative cost of selective screening to prevent transmission of methicillin-resistant Staphylococcus aureus (MRSA), compared with the attributable costs of MRSA infection. Infection control and hospital epidemiology 2006;27:1264–6.
142. Otter JA, Herdman MT, Williams B, et al. Low prevalence of methicillin-resistant Staphylococcus aureus carriage at hospital admission: implications for risk-factor-based vs universal screening. J Hosp Infect 2013;83:114–21.
143. Harbarth S, Hawkey PM, Tenover F, et al. Update on screening and clinical diagnosis of methicillin-resistant Staphylococcus aureus (MRSA). Int J Antimicrob Agents 2011;37:110–7.
144. Calfee DP, Giannetta ET, Durbin LJ, et al. Control of endemic vancomycin-resistant Enterococcus among inpatients at a university hospital. Clin Infect Dis 2003;37:326–32.
145. Hendrix CW, Hammond JM, Swoboda SM, et al. Surveillance strategies and impact of vancomycin-resistant enterococcal colonization and infection in critically ill patients. Ann Surg 2001;233:259–65.
146. Muto CA, Giannetta ET, Durbin LJ, et al. Cost-effectiveness of perirectal surveillance cultures for controlling vancomycin-resistant Enterococcus. Infect Control Hosp Epidemiol 2002;23:429–35.
147. Price CS, Paule S, Noskin GA, Peterson LR. Active surveillance reduces the incidence of vancomycin-resistant enterococcal bacteremia. Clin Infect Dis 2003;37:921–8.
148. Shadel BN, Puzniak LA, Gillespie KN, et al. Surveillance for vancomycin-resistant enterococci: type, rates, costs, and implications. Infect Control Hosp Epidemiol 2006;27:1068–75.
149. Siddiqui AH, Harris AD, Hebden J, et al. The effect of active surveillance for vancomycin-resistant enterococci in high-risk units on vancomycin-resistant enterococci incidence hospital-wide. Am J Infect Control 2002;30:40–3.
150. Sandri AM, Dalarosa MG, Ruschel de Alcantara L, et al. Reduction in incidence of nosocomial methicillin-resistant Staphylococcus aureus (MRSA) infection in an intensive care unit: role of treatment with mupirocin ointment and chlorhexidine baths for nasal carriers of MRSA. Infect Control Hosp Epidemiol 2006;27:185–7.
151. Huang SS, Septimus E, Kleinman K, et al. Targeted versus universal decolonization to prevent ICU infection. N Engl J Med 2013;368:2255–65.
152. Bleasdale SC, Trick WE, Gonzalez IM, et al. Effectiveness of chlorhexidine bathing to reduce catheter-associated bloodstream infections in medical intensive care unit patients. Arch Intern Med 2007;167:2073–9.
153. Climo MW, Sepkowitz KA, Zuccotti G, et al. The effect of daily bathing with chlorhexidine on the acquisition of methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus, and healthcare-associated bloodstream infections: results of a quasi-experimental multicenter trial. Crit Care Med 2009;37:1858–65.
154. Vernon MO, Hayden MK, Trick WE, et al. Chlorhexidine gluconate to cleanse patients in a medical intensive care unit: the effectiveness of source control to reduce the bioburden of vancomycin-resistant enterococci. Arch Intern Med 2006;166:306–12.
155. Milstone AM, Elward A, Song X, et al. Daily chlorhexidine bathing to reduce bacteraemia in critically ill children: a multicentre, cluster-randomised, crossover trial. Lancet 2013;381:1099–106.
156. Blackwood B, Thompson G, McMullan R, et al. Tea tree oil (5%) body wash versus standard care (Johnson’s Baby Softwash) to prevent colonization with methicillin-resistant Staphylococcus aureus in critically ill adults: a randomized controlled trial. J Antimicrob Chemother 2013;68:1193–9.
157. Daneman N, Sarwar S, Fowler RA, et al. Effect of selective decontamination on antimicrobial resistance in intensive care units: a systematic review and meta-analysis. Lancet Infect Dis 2013;13:328–41.
158. Verwaest C, Verhaegen J, Ferdinande P, et al. Randomized, controlled trial of selective digestive decontamination in 600 mechanically ventilated patients in a multidisciplinary intensive care unit. Crit Care Med 1997;25:63–71.
159. de Smet AMGA, Kluytmans JAJW, Blok HEM, et al. Selective digestive tract decontamination and selective oropharyngeal decontamination and antibiotic resistance in patients in intensive-care units: an open-label, clustered group-randomised, crossover study. Lancet Infect Dis 2011;11:372–80.
160. de Jonge E, Schultz MJ, Spanjaard L, et al. Effects of selective decontamination of digestive tract on mortality and acquisition of resistant bacteria in intensive care: a randomised controlled trial. Lancet 2003;362:1011–6.
161. Cepeda JA, Whitehouse T, Cooper B, et al. Isolation of patients in single rooms or cohorts to reduce spread of MRSA in intensive-care units: prospective two-centre study. Lancet 2005;365:295–304.
162. Dhaliwal J, McGeer A. Does isolation prevent the spread of methicillin-resistant Staphylococcus aureus? CMAJ 2005;172:875.
163. Kollef MH, Micek ST. Antimicrobial stewardship programs: mandatory for all ICUs. Crit Care 2012;16:179.
164. McKinnell JA, Huang SS, Eells SJ, et al. Quantifying the impact of extranasal testing of body sites for methicillin-resistant Staphylococcus aureus colonization at the time of hospital or intensive care unit admission. Infect Control Hosp Epidemiol 2013;34:161–70.
165. Denkinger CM, Grant AD, Denkinger M, et al. Increased multi-drug resistance among the elderly on admission to the hospital—a 12-year surveillance study. Arch Gerontol Geriatr 2013;56:227–30.
166. Boisseau D, Alfandari S, Gauzit R, et al. Staphylococcus aureus nasal carriage during the infectious diseases national congress in France. Med Mal Infect 2012;42:435–9.
167. Fritz SA, Hogan PG, Hayek G, et al. Staphylococcus aureus colonization in children with community-associated Staphylococcus aureus skin infections and their household contacts. Arch Pediatr Adolesc Med 2012;166:551–7.
168. Rafee Y, Abdel-Haq N, Asmar B, et al. Increased prevalence of methicillin-resistant Staphylococcus aureus nasal colonization in household contacts of children with community acquired disease. BMC Infect Dis 2012;12:45.
169. Schechter-Perkins EM, Mitchell PM, Murray KA, et al. Prevalence and predictors of nasal and extranasal staphylococcal colonization in patients presenting to the emergency department. Ann Emerg Med 2011;57:492–9.
170. Bisaga A, Paquette K, Sabatini L, Lovell E. A prevalence study of methicillin-resistant staphylococcus aureus colonization in emergency department health care workers. Ann Emerg Med 2008;52:525–8.
171. Suffoletto B, Cannon E, Ilkhanipour K, Yealy D. Prevalence of Staphylococcus aureus nasal colonization in emergency department personnel. Ann Emerg Med 2008;52:529–33.
172. Young DM, Harris HW, Charlebois ED, et al. An epidemic of methicillin-resistant Staphylococcus aureus soft tissue infections among medically underserved patients. Arch Surg 2004;139:947-51; discussion 51–3.
173. Salgado CD, Farr BM, Calfee DP. Community-acquired methicillin-resistant Staphylococcus aureus: a meta-analysis of prevalence and risk factors. Clin Infect Dis 2003;36:131–9.
From the Department of Medicine, Infectious Disease Practice and Innovations, The Medical City, Pasig City, Philippines (Dr. Abad), the Division of Emergency Medicine, University of Wisconsin Medical School, Madison, WI (Dr. Pulia), University of Wisconsin Hospital and Clinics, Madison, WI (Ms. Krupp), and the Willam S. Middleton Memorial Veterans Affairs Hospital, Madison, WI (Dr. Safdar).
Patients in intensive care units (ICUs) are at greatly increased risk of developing health care-associated infections (HAIs) [1]. More than 70% of the bacteria that cause HAIs are resistant to at least one of the antimicrobials commonly used to treat these infections [2]. Two such pathogens, methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE) are responsible for a considerable proportion of ICU infections that are associated with increased morbidity, mortality, and costs [3–5]. In this review, we discuss the epidemiology of colonization and infection by MRSA and VRE and provide an update on practices for prevention of transmission and infection by MRSA and VRE in the ICU.
EPIDEMIOLOGY AND MECHANISMS OF RESISTANCE
MRSA is the major cause of HAIs worldwide [6]. Among ICUs in the United States, the proportion of methicillin resistance among S. aureus isolates increased from 35.9% in 1992 to 64.4% in 2003 [4]. Approximately 8% of patients are colonized with MRSA upon admission, and an average of 5% will acquire MRSA colonization in the ICU [7,8]. A comparison study of academic tertiary care facilities found medical ICUs had higher MRSA admission prevalence rates than surgical ICUs, whereas surgical ICUs had a higher incidence rate [7]. Enteroccoccus is the third most common pathogen associated with HAIs, with 33% resistant to vancomycin [9]. VRE infection is associated with increased ICU cost and increased length of stay [10]. Incidence of ICU-acquired VRE varies among regions and countries. For example, in the United States, Warren et al [11] reported a VRE incidence of 27 cases per 1000 patient ICU days, whereas Kohlenberg et al [12] reported a mean incidence of 0.29 cases per 1000 patient ICU days in Germany.
Understanding the mechanisms that allow development of resistant strains of S. aureus and Enterococcus species is important to devise preventive strategies. Methicillin resistance in MRSA is determined by the staphylococcal cassette chromosome mec (SCCmec), a mobile genetic element that carries the mecA gene. The mecA gene codes for an additional penicillin-binding protein (PBP) that has a reduced affinity towards methicillin (PBP2a/PBP2'). This results in a reduced ability to bind to the bacterial cell wall and inhibit synthesis [13,14]. Study of molecular epidemiology has identified MRSA as originating from 8 major variants of the mecA gene [15]. The majority of MRSA infections are caused by strains belonging to a few internationally disseminated clones [14]. The first identified strains were associated with infections in hospitalized patients (hospital-associated MRSA), but community-associated MRSA strains have since emerged and have become established globally, including in health care institutions [16].
Community-acquired MRSA can cause severe infections in health hosts [17]; possible explanations include increased CA-MRSA virulence due to the acquisition of mobile genetic elements, namely those containing Panton-Valentine leukocidin (PVL) [18] or increased expression of core genome-encoded virulence genes, such as phenol-soluble modulin (PSM) cytolysins, α-toxin, and other virulence determinants [19].
Enterococcus is intrinsically resistant to several antimicrobial drugs, with resistance to vancomycin encoded by several clusters of genes known as vancomycin resistance gene clusters (eg, vanA, vanB). The gene clusters generate resistance through multiple pathways which encode enzymes to determine the structure of peptidoglycan precursors [20,21]. Genetically diverse, hospital-associated VRE outbreaks have been associated with single clones, multiple clones, and changing molecular epidemiology over time [21]. While up to 25% of the VRE genome includes acquired elements, the majority of hospital-associated infections are traced to a few clonal complexes, which differ from community-associated VRE strains [22].
The evolution of these efficient mechanisms that promote drug resistance has made it extremely challenging to eradicate organisms such as MRSA and VRE. However, advances in recent years have furthered our understanding of the epidemiology, pathogenesis, and methods of prevention and containment.
RISK FACTORS FOR COLONIZATION AND INFECTION
MRSA
The risk factors underlying MRSA colonization and infection in the ICU setting can be categorized as either patient/host or environmental factors. A wide range of patient-level factors is associated with MRSA colonization upon admission. General principles regarding the transmission of MRSA in the community include close contact with colonized or infected individuals, breaks in the skin, crowded living conditions and poor hygiene. These factors, alone or in combination, are thought to underlie observed outbreaks among sports teams, military personnel, correction facilities, American Indian communities, and daycare centers [23–34].
Two recently published systematic reviews have summarized important patient-level factors associated with MRSA colonization at the time of hospital admission. Forster et al [35] examined 27 studies and identified previous admission to hospital, transfer from nursing home or long-term care facility, and previous antibiotic use as the top 3 factors associated with MRSA colonization. A similar review conducted by McKinnell and colleagues [36] found that prior hospitalization, nursing home contact, recent antibiotic use, and exposure to health care-associated pathogens (MRSA carriage, VRE carriage, or Clostridium difficile infection) were found to portend the highest risk. Specific comorbid conditions also conveyed an increased risk, including congestive heart failure, chronic wounds/bedsores, diabetes mellitus, pulmonary disease, immunosuppression, urinary catheter, and renal failure/dialysis. It is clear that health care contacts, especially recent hospitalization, residence in a long-term care facility, and antibiotic use, are significant risk factors for MRSA colonization [37–39].
 In contrast to those already colonized with MRSA, some patients acquire MRSA during hospitalization. In these cases, transmission via hands of health care workers is likely the most common mechanism for spread of MRSA [6,40–42]. An understaffed ICU has also been cited as a potential risk factor for ICU MRSA transmission, perhaps due to sacrifices in hand hygiene practices by overextended staff [6]. Additional factors associated with increased risk of nosocomial MRSA acquisition include duration of antibiotic therapy, exposure to quinolone or macrolide antibiotics, length of hospital stay, enteral feeding, post-surgical status, insertion of central line or urinary catheter during admission, ICU admission, and proximity to another patient with MRSA infection or colonization [43–45]. A summary of risk factors for MRSA acquisition is shown in Table 1.
In contrast to those already colonized with MRSA, some patients acquire MRSA during hospitalization. In these cases, transmission via hands of health care workers is likely the most common mechanism for spread of MRSA [6,40–42]. An understaffed ICU has also been cited as a potential risk factor for ICU MRSA transmission, perhaps due to sacrifices in hand hygiene practices by overextended staff [6]. Additional factors associated with increased risk of nosocomial MRSA acquisition include duration of antibiotic therapy, exposure to quinolone or macrolide antibiotics, length of hospital stay, enteral feeding, post-surgical status, insertion of central line or urinary catheter during admission, ICU admission, and proximity to another patient with MRSA infection or colonization [43–45]. A summary of risk factors for MRSA acquisition is shown in Table 1.
Regardless of whether MRSA colonization precedes admission or occurs due to nosocomial spread, it is associated with increased risk of developing a HAI [46–49]. In 2 large prospective observational cohort studies, the hazard ratios of MRSA colonization developing into S. aureus infections during the ICU stay were 3.84 and 4.70, respectively [50,51]. High levels of concordance between MRSA colonization strains and HAI strains have also been reported [52]. Nasal colonization with S. aureus has also been identified as an independent risk factor for developing ventilator-associated pneumonia (VAP) and bacteremia [53,54]. A case series of ICU patients with S. aureus nasal colonization who developed lower respiratory tract infections demonstrated genetically identical nasal and bronchial strains in 15/16 cases [55]. This finding strongly suggests that nasopharyngeal colonization with S. aureus contaminates oral secretions that are aspirated by critically ill patients, resulting in subsequent pneumonia. In a long-term outcomes study among a matched cohort of veterans, MRSA colonization was associated with an increased risk of infection-related readmission and mortality [56]. These findings reflect the critically important nature of measures designed to curb nosocomial transmission and acquisition of MRSA, especially among the vulnerable ICU population.
VRE
As with MRSA, risk factors associated with VRE colonization include both patient-level and ICU-level (or environmental) factors [57]. Examples of patient-level factors include previous antimicrobial exposure [58–62], underlying medical illnesses such as chronic renal failure requiring hemodialysis [11,63], length of hospital or ICU stay [11,59,64,65], and recent exposure to health care facilities. ICU-level factors of relevance are the prevalence of VRE in the unit, with high levels of endemicity leading to higher risk of colonization and transmission.
Antibiotic use is a major risk factor for VRE acquisition, although the type and class of antibiotic varies considerably across studies; the most frequently identified antibiotics are broad-spectrum cephalosporins, vancomycin, and anti-anaerobic agents [58,62,64]. Patients with chronic liver disease and post-transplantation are at exceedingly high risk for VRE acquisition [59]. In a recent study by Pan [66], for example, the authors found that the incidence of newly acquired VRE was 21.9 per 1000 patient-days in an ICU setting. On multivariate analysis, the authors found that, similar to other reports [11,59,67], length of stay in the ICU was associated with increased risk of VRE acquisition, with each additional day of stay increasing risk of VRE by 1.03 times. Warren et al undertook a prospective cohort study involving 519 patients admitted to the ICU for more than 48 hours [11]. Seventy-four (21%) of 352 patients were subsequently colonized with VRE. The median time to development of a positive VRE culture after ICU admission was 6 days. Increased mean APACHE II score on ICU admission (P = 0.002), sucralfate use (P = 0.003), vasopressor use (P = 0.01), tracheostomy in the ICU (P = 0.02), and C. difficile diarrhea (P = 0.002) appeared to be associated with VRE acquisition.
 It appears that VRE acquisition is often associated with the sick subgroup of patients, and risk factors generally associated with VRE colonization and infection co-relate with disease chronicity and severity of illness. Length of hospitalization, ICU stay, hemodialysis, or transplantation may all be markers of disease severity. A summary of risk factors for VRE acquisition is shown in Table 2.
It appears that VRE acquisition is often associated with the sick subgroup of patients, and risk factors generally associated with VRE colonization and infection co-relate with disease chronicity and severity of illness. Length of hospitalization, ICU stay, hemodialysis, or transplantation may all be markers of disease severity. A summary of risk factors for VRE acquisition is shown in Table 2.
REDUCING TRANSMISSION—MRSA AND VRE PREVENTION STRATEGIES
 Evidence-based guidelines developed by the Centers for Disease Control (CDC) Hospital Infection Control Practices Advisory Committee (HICPAC) for prevention of MRSA and VRE are available [68]. Several recently conducted well-designed clinical trials also provide additional insight that may be particularly helpful in the ICU setting [69]. A summary of the MRSA prevention guidelines issued by the CDC and included in its “MRSA toolkit” is provided in Table 3. A similar guideline on prevention of VRE [70], published more than a decade ago, has similar elements. Table 3 shows a side-by-side comparison of these elements. Unfortunately, despite these guidelines and extensive research regarding prevention and control, considerable controversy exists as to the most effective approaches. As such, these recommendations should be tailored to meet the needs of the specific ICU setting.
Evidence-based guidelines developed by the Centers for Disease Control (CDC) Hospital Infection Control Practices Advisory Committee (HICPAC) for prevention of MRSA and VRE are available [68]. Several recently conducted well-designed clinical trials also provide additional insight that may be particularly helpful in the ICU setting [69]. A summary of the MRSA prevention guidelines issued by the CDC and included in its “MRSA toolkit” is provided in Table 3. A similar guideline on prevention of VRE [70], published more than a decade ago, has similar elements. Table 3 shows a side-by-side comparison of these elements. Unfortunately, despite these guidelines and extensive research regarding prevention and control, considerable controversy exists as to the most effective approaches. As such, these recommendations should be tailored to meet the needs of the specific ICU setting.
Antimicrobial Stewardship
Antibiotic use is a major driver of antibiotic resistance. A meta-analysis by de Bruin and Riley [71] studied the effect of vancomycin usage on VRE colonization and infection. A total of 12 articles describing 13 studies were included; none were randomized controlled trials. All studies were quasi-experimental and lacked control groups. Among all studies, less than half (46%) implemented vancomycin reduction measures as the sole type of intervention [72–76]. The remaining studies implemented other infection control modalities and or restricted the use of other antimicrobials [77–83]. Although all studies that implemented vancomycin restriction alone as a single strategy showed a decline in vancomycin usage, only 2 of these [74,75] showed a relative risk reduction in VRE acquisition post-intervention. Also, studies that restricted vancomycin alone revealed a trend towards lower efficacy in reducing VRE colonization and infection (33%) when compared with those that used additional measures (71%). While judicious antibiotic use should always be practiced, the evidence for vancomycin restriction as a sole intervention to control VRE is scant. It may be that other antibiotics are as big or bigger drivers of resistance in enterococci than vancomycin. For example, a growing body of literature supports antibiotic restriction, especially fluoroquinolones, for reducing MRSA. In several time-series quasi-experimental studies, restriction of fluorquinolones was associated with reduced trends in MRSA infections in the acute care setting, and consideration should be given to monitor and optimize fluoroquinolone use in the ICU setting [84,85].
Antimicrobial stewardship programs are fundamental to optimizing antibiotic use in the ICU and the authors strongly recommend that all ICUs should have such a program in place.
Educational Interventions
Infection control and multidrug-resistant organism (MDRO)–specific education programs for health care workers is a core principle of the CDC’s prevention guidelines. The HICPAC VRE guideline also explicitly states “continuing education programs for hospital staff (including attending and consulting physicians, medical residents, and students; pharmacy, nursing, and laboratory personnel; and other direct patient-care providers) should include information concerning the epidemiology of VRE and the potential impact of this pathogen on the cost and outcome of patient care [70].” A systematic review published in 2008 [86] that included 26 studies showed that such interventions to prevent HCAIs are usually successful; in this review, 20 of 26 studies showed a statistically significant decrease in infection rates, with risk ratios ranging from 0 to 1.6. Education was usually part of a broader array of infection control interventions. While clearly essential, education alone is unlikely to have a sustained impact on reducing MRSA and VRE infections.
Infection Control Measures
Major infection control interventions include hand hygiene, the use of personal protective equipment (PPE), and cohorting. These measures can be grouped into “horizontal” (or global) vs. “vertical” (or targeted) strategies. Although not mutually exclusive, horizontal approaches are designed to have an impact on multiple pathogens (pathogen nonspecific), whereas vertical approaches are designed to reduce the impact of specific pathogens (such as VRE). For the purposes of this review, we will discuss both strategies for containment of MRSA and VRE. Horizontal strategies include hand hygiene, universal gloving and/or gowning, environmental cleaning, and daily bathing with chlorhexidine. Vertical strategies include screening for either MRSA or VRE followed by placement in contact precautions and decolonization with mupirocin.
Hand Hygiene
Hand washing is fundamental to reducing transmission of MDROs in health care institutions; however, optimal compliance is hard to achieve and sustain. Barriers to adherence may include unavailability of sinks or hand hygiene materials (eg, alcohol-based gels, gloves) time constraints, forgetfulness, or lack of knowledge [87–95]. Several monitoring strategies have been evaluated to increase compliance with hand hygiene. Most involve direct observation followed by performance assessment and feedback.
Trials examining the impact of improvements in hand hygiene compliance on HAIs in the ICU setting have largely found benefit, although not all studies showed a decline in HAI. In a prospective crossover trial, Rupp et al [96] found dramatic improvements in compliance with hand gel availability, but this did not translate to decreased nosocomial MRSA infections. Venkatesh et al [97] carried out a before-and-after interventional prospective study in a hematology unit in a tertiary level hospital to evaluate the use of an electronic method of surveillance to determine compliance with hand hygiene. The authors also used rates of horizontal transmission of VRE as a secondary end-point. Results of the study showed that hand hygiene compliance improved from 36.3% at baseline to 70.1%. This represented an OR of 4.1 (95% confidence interval, 3.7–4.5), which the authors attributed to the use of automated alerts. VRE transmission rates before and during intervention were not statistically different, but the rates of infection were lower at 1.0 per month in comparison with 4.7 infections per month in the preceding 6 months (P = 0.096).
While improved hand hygiene may result in significant reductions in HAIs [40], research indicates hand hygiene alone influences about 40% of infections in the ICU setting [98]. As such, hand hygiene should be viewed as a necessary component of a comprehensive infection control program [99]. Despite the success of hand hygiene in reducing HAIs in the ICU, effective strategies to improve compliance remain elusive even under study conditions and further research is needed in this area [100].
Personal Protective Equipment
Tenorio et al [101] conducted a study to assess the effectiveness of gloving in the prevention of hand carriage of VRE by health care workers. The study showed that among 50 health care workers who had contact with patients colonized with VRE, 6 carried a similar patient strain even prior to known contact, and 17 of 44 (69%) had a patient-related VRE strain on their gloves after contact. This suggests a relatively high rate of colonization after usual patient-care contact. Factors associated with acquisition of VRE on gloves included duration of contact, contact with a patient’s body fluids, presence of diarrhea in a patient, mean VRE colony counts on a patient’s skin, and number of body sites colonized with VRE. Although gloves reduced the risk of VRE acquisition of VRE by 71% (ie, 12/17 did not have VRE on their hands after de-gloving) the protection afforded by gloves was incomplete. As such, hand hygiene after glove removal is recommended.
Slaughter et al [102] compared the use of personal protective equipment in the acquisition of VRE. During this study, 93 patients in glove-and-gown rooms and 88 patients in glove-only rooms had similar rates of VRE at baseline entry into the ICU and after the intervention. Mean times to colonization among the patients who became colonized were 8.0 days in the glove-and-gown group and 7.1 days in the glove-only group. None of these comparisons were statistically significant and the authors concluded that the universal use of gown and gloves was no better than the use of gloves alone in preventing VRE colonization.
A recent cluster randomized trial compared the effect of universal PPE (ie, gowning and gloving) with usual care for reducing acquisition of MRSA or VRE as a composite outcome [103]. The study did not find that universal gowning and gloving reduced VRE or MRSA acquisition but found a 40% decline in MRSA acquisition in the intervention ICUs compared with baseline rates of MRSA. No major adverse effects of universal gowning and gloving were noted in this study. A thoughtful editorial commenting on this article proposes that several aspects of the study deserve consideration, including the possibility of false-negative screening tests for VRE, which may have partially accounted for the negative primary outcome [69].
Based on these studies, it appears that the use of barrier precautions may be of value more for MRSA than VRE but further studies are needed to examine its impact on other types of pathogens, including new and emerging MDROs. Until further evidence becomes available, routine gowning and gloving may be of value in units with a high prevalence of MRSA.
Environmental Cleaning
Accumulating data suggests that the environment may play a major role in transmission of pathogens. MRSA has the ability to survive for days to weeks on inanimate objects [104–107]. Environmental contamination results in contamination of staff clothing and gloves [107,108] and is highly correlated with colonization strains among inpatients [109]. Although some studies of enhanced cleaning techniques and increased environmental services staff time have demonstrated reductions in MRSA outbreaks [110–112], the results are not universally favorable [113,114] and further studies are needed to examine the impact of environmental cleaning on rates of MRSA colonization or infection.
Several studies have implicated contaminated equipment as vectors for transmission of VRE during outbreaks [115–117], but the direction of fomite transfer from patient to environment has been difficult to ascertain. VRE have been found frequently on a variety of inanimate objects and surfaces in different health care environments [118–123], including gloved or ungloved hands of health care workers [101,124,125]. Hayden et al [126] determined the effect of improved environmental cleaning on VRE acquisition rates. This study was a pre-and-post intervention study carried out in a 21-bed medical intensive care unit (MICU) in a tertiary hospital over several phases. The intervention included the creation of a unique and improved cleaning program, as well as in-services to housekeeper services, education of the MICU staff, and a hand hygiene campaign. The results of the study showed decreased acquisition of VRE from 33.47 cases per 1000 patient days at risk in period 1 to 10.40 cases per 1000 patient-days at risk by period 4 of the study. Increased environmental cleaning was also associated with reduced growth of VRE from environmental cultures. At baseline, weekly contamination rates were 0.15 and 0.1 for samples obtained before and after cleaning, respectively. Culture positivity decreased to 0.07 and 0.04 for before and after cleaning in period 2 and then remained at low levels during the remainder of the study. It is important to note that the method for disinfecting used in this study was the “bucket method” as promoted by Byers [127]. This study provides further support for the importance of an environmental reservoir and of environmental decontamination to prevent endemic cross-transmission of VRE [126].
Goodman et al [128] used similar interventions but added a feedback tool using a black-light monitoring system (ie, use of an invisible, nontoxic marker to delineate areas that are adequately or inadequately cleaned) to reduce the likelihood of isolating either MRSA or VRE from an ICU environment. This study also showed favorable results, and notably, the use of the black-light monitoring system identified specific areas that were typically inadequately disinfected. Results showed that flat, horizontal surfaces (eg, countertops, bedside tray tables, and hamper tops) were adequately cleaned more often than small, vertical surfaces (eg, doorknobs, toilet handles, light switches, and electronics).
Part of the controversy surrounding the impact of environmental cleaning is the difficulty in determining its individual value as part of an overall infection control bundle [129]. A proposed area of demonstrable impact for environmental cleaning are frequently touched sites which are more likely to be contaminated with pathogens. Focusing on these “hot-bed” areas of the care environment may offer a useful adjunct to other infection control measures [129].
Active Surveillance
Active surveillance refers to periodic screening for asymp-tomatic carriers followed by placement of colonized patients in contact isolation. This practice is highly variable across institutions, as the evidence supporting this practice is conflicting and there are concerns about the cost of implementing this approach without solid evidence [70,130,131]. Despite lack of randomized controlled trials to guide this practice for MRSA prevention, many hospitals utilize MRSA surveillance and it is mandated by law in 9 states [132,133].
A prospective, interventional cohort study of universal MRSA screening on admission to surgical wards failed to reduce nosocomial MRSA infections [134]. Most recently, a pragmatic, cluster-randomized ICU trial reported that universal decolonization with chlorhexidine wipes and mupirocin use was more effective than screening and isolation in reducing rates of MRSA clinical isolates [65]. However, concerns regarding the risk of mupirocin resistance have been expressed [135,136]. The only randomized trial that compared active surveillance for MRSA and VRE followed by contact precautions to usual care did not find a benefit to active surveillance.
Huskins et al [137], in a large, cluster-randomized trial of 19 ICUs from different hospitals, determined the utility of using a culture-based active surveillance and contact isolation, compared with usual care (contact isolation for patients colonized with MRSA or VRE) as identified by existing hospital protocols, to reduce the incidence of colonization or infection with MRSA or VRE. In this trial, which spanned 6 months and involved 3488 participants, the authors found no significant difference between the intervention and control ICUs in terms of MRSA and VRE colonization or infection rates.
Conflicting with these findings is an observational study comparing MRSA infection rates before and after institution of a universal screening protocol, which demonstrated a 69.6% (CI, –89.2% to –19.6%]; P = 0.03) reduction in hospital wide MRSA prevalence density with screening [138]. The “MRSA bundle” implemented in 2007 at VA hospitals nationwide, which included universal screening, produced a 62% (P < 0.001) reduction in MRSA ICU infections; the relative contribution of the various bundle components is uncertain [139,140].
A proposed cost-saving alternative to universal screening is selective screening based on risk factor assessment [141]. The effectiveness of this type of program depends on creating a clinical decision-making tool capable of accurately identifying high-risk individuals while also accounting for the different risk factor profiles between HA-MRSA and CA-MRSA [142]. It has been proposed that targeted screening protocols may be more cost-effective in settings with < 5% prevalence of MRSA colonization on admission [143].
Many studies [61,144–149] have shown that active surveillance against VRE is cost-effective. For example, Calfee et al [144] showed that an established active surveillance program results in control of endemic VRE in high-risk patients. The infection control program was established in response to a hospital-wide VRE outbreak, and was sustained after the outbreak was controlled. The study by Calfee et al spanned 5 years and was performed at a tertiary-level university hospital, where cultures from perirectal areas were used to identify high-risk patients who were asymptomatically colonized with VRE. During the latter 2 years, 768 new cases of VRE colonization were detected among 69,672 admissions (1.1% of admissions), of which 730 (95.1%) were identified by active surveillance methods. This implies that routine clinical cultures would probably have missed the majority of colonized patients. During this period, the incidence of VRE infection was likewise extremely low at 0.12/1000 patient days (ie, 90 nosocomial VRE infections were identified in 83 patients during 743,956 days of patient care). Sixty-nine of the 83 patients (83%) who developed nosocomial VRE infections were found to be colonized with VRE by surveillance culture before the onset of infection.
Patient Decolonization
Chlorhexidine gluconate has been used in several settings to control outbreaks and infections related to MRSA and VRE due to its broad-spectrum activity against these pathogens. Chlorhexidine-based solutions reduce the density of skin colonization with pathogens such as MRSA and VRE (skin asepsis), thus lowering the risk for horizontal transmission between health care workers and patients.
Decolonization with chlorhexidine as an MRSA infection reduction technique has demonstrated benefit in the ICU setting [150]. The previously mentioned large, cluster-randomized ICU trial by Huang and colleagues found universal decolonization with twice-daily intranasal mupirocin for 5 days and daily bathing with chlorhexidine-impregnated cloths for the entire ICU stay was superior to targeted decolonization of known MRSA carriers in preventing overall MRSA isolates. However, universal decolonization failed to show a reduction in MRSA bacteremia [151], and concerns about mupirocin resistance may limit the applicability of this approach.
There are now several studies [152–154] that show decreased acquisition of VRE with use of daily chlorhexidine bathing. In a study including 1787 ICU patients, Vernon et al found [154] that the reducing microbial density of VRE on patient’s skin by using chlorhexidine led to decreased transmission. In another study by Climo et al [153] that involved 6 ICUs at 4 academic centers and measured the incidence of MRSA and VRE colonization and blood stream infections (BSI) during a period of bathing with routine soap for 6 months compared with a 6-month period where all admitted patients received daily bathing with a chlorhexidine solution, results found decreased acquisition of VRE by 50% (4.35 vs. 2.19 cases/1000 patient days, P < 0.008) following the introduction of daily chlorhexidine bathing. Furthermore, compared with 16 of 270 patients colonized with VRE who subsequently developed VRE bacteremia at baseline, only 4 of 226 VRE-colonized patients bathed with chlorhexidine in the intervention period developed a BSI, translating into a relative risk reduction of 3.35 (95% CI, 1.13–9.87; P < 0.035). Patients colonized with VRE were 3 times less likely to develop VRE bacteremia when bathed with chlorhexidine compared with regular bathing. Despite the success of this protocol for VRE, when analyzed by individual organism no significant reductions in MRSA acquisition or BSI were reported. This finding is similarly corroborated by a trial conducted in the pediatric ICU setting which found an overall reduction in bacteremia with daily chlorhexidine washes but no significant decrease in cases due to S. aureus [155].
The results of these studies suggest that daily bathing with chlorhexidine should be part of routine practice in health care, especially in ICUs where endemic MRSA or VRE rates are high. Whether there is benefit in other settings needs to be studied.
In addition to chlorhexidine washes, other decolonization techniques have been proposed to reduce colonization and the spread of HAIs in the ICU setting. A randomized controlled trial of daily 5% tea tree oil body washes for the prevention of MRSA colonization failed to significantly reduce rates compared to standard soap body washes [156]. Another proposed decolonization intervention that has not been widely adopted in the United States due to concerns related to development of resistant organisms is selective digestive decontamination (SDD) or selective oropharyngeal decontamination (SOD) with antimicrobial agents [157,158]. In terms of clinical benefit, SDD/SOD have been found to decrease MDRO infection rate [159] and mortality [160].
Cohorting
There is insufficient evidence to conclude that cohorting isolated patients is of benefit for routine use in the endemic ICU setting. A few studies, mainly in the outbreak setting, have examined this approach and the results are conflicting [161,162]. Pending further studies in this area, it is reasonable to cohort patients colonized with the same microorganisms, especially if patients cannot be placed in single rooms.
CONCLUSION
The emergence of MRSA and VRE has led to a resurgence of interest and emphasis on infection control practices and prevention. CDC guidelines to help health care practitioners manage these MDROs in the hospital and ICU-setting exist; however, many questions remain regarding best practice.
Prevention of MRSA and VRE needs to be a 2-pronged approach—antimicrobial stewardship [163] and infection control. A robust antimicrobial stewardship program to optimize and minimize inappropriate antibiotic use is necessary in every institution. From the infection prevention standpoint, it is unclear if systematic identification of MRSA and VRE colonization followed by contact precautions is useful in reducing transmission. It is clear that a strong institutional climate of promoting patient safety and a culture of infection prevention will help in reducing MRSA and VRE facility-wide. It also appears that universal gowning and gloving may be useful for reducing MRSA, but not VRE, transmission. While universal decolonization with mupirocin is efficacious in reducing MRSA, this strategy is not recommended because of promoting mupirocin resistance. However, the use of daily bathing with chlorhexidine represents a relatively low-cost, high-yield intervention that should be adopted. Pending further data, patients known to be colonized or infected with MRSA should be placed in contact precuations as is current practice in most institutions. Finally, in this era of MDROs, hand hygiene remains our best defense against the spread of pathogens in the health care environment.
Note: This article does not represent the views of the Department of Veterans Affairs.
Corresponding author: Nasia Safdar, MD, Willam S. Middleton Memorial Veterans Affairs Hospital, 2500 Overlook Terrace, Madison, WI 53705, [email protected].
Funding/support: This work is funded by a MERIT award from the Department of Veterans Affairs to Nasia Safdar.
Financial disclosures: None.
REFERENCES
1. Burton DC, Edwards JR, Horan TC, et al. Methicillin-resistant Staphylococcus aureus central line-associated bloodstream infections in US intensive care units, 1997–2007. JAMA 2009;301:727–36.
2. LeDell K, Muto CA, Jarvis WR, Farr BM. SHEA guideline for preventing nosocomial transmission of multidrug-resistant strains of Staphylococcus aureus and Enterococcus. Infect Control Hosp Epidemiol 2003;24:639–41.
3. Giske CG, Monnet DL, Cars O, Carmeli Y. Clinical and economic impact of common multidrug-resistant gram-negative bacilli. Antimicrob Agents Chemother 2008;52:813–21.
4. Klevens RM, Edwards JR, Richards CL Jr, et al. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Pub Health Rep 2007;122:160–6.
5. Schwaber MJ, Carmeli Y. The effect of antimicrobial resistance on patient outcomes: importance of proper evaluation of appropriate therapy. Crit Care 2009;13:106.
6. Grundmann H, Hori S, Winter B, et al. Risk factors for the transmission of methicillin-resistant Staphylococcus aureus in an adult intensive care unit: fitting a model to the data. J Inf Dis 2002;185:481–8.
7. Huang SS, Rifas-Shiman SL, Warren DK, et al. Improving methicillin-resistant Staphylococcus aureus surveillance and reporting in intensive care units. J Infect Dis 2007;195:330–8.
8. Muder RR, Cunningham C, McCray E, et al. Implementation of an industrial systems-engineering approach to reduce the incidence of methicillin-resistant Staphylococcus aureus infection. Infect Control Hosp Epidemiol 2008;29:702–8.
9. Hidron AI, Edwards JR, Patel J, et al. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol 2008;29:996–1011.
10. Pelz RK, Lipsett PA, Swoboda SM, et al. Vancomycin-sensitive and vancomycin-resistant enterococcal infections in the ICU: attributable costs and outcomes. Intensive Care Med 2002;28:692–7.
11. Warren DK, Kollef MH, Seiler SM, et al. The epidemiology of vancomycin-resistant Enterococcus colonization in a medical intensive care unit. Infect Control Hosp Epidemiol 2003;24:257–63.
12. Kohlenberg A, Schwab F, Meyer E, et al. Regional trends in multidrug-resistant infections in German intensive care units: a real-time model for epidemiological monitoring and analysis. J Hosp Infect 2009;73:239–45.
13. Deurenberg RH, Stobberingh EE. The evolution of Staphylococcus aureus. Infect Genet Evol 2008;8:747–63.
14. Gordon RJ, Lowy FD. Pathogenesis of methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis 2008;46 Suppl 5:S350–9.
15. Zhang K, McClure JA, Elsayed S, Conly JM. Novel staphylococcal cassette chromosome mec type, tentatively designated type VIII, harboring class A mec and type 4 ccr gene complexes in a Canadian epidemic strain of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 2009;53:531–40.
16. Chatterjee SS, Otto M. Improved understanding of factors driving methicillin-resistant Staphylococcus aureus epidemic waves. Clin Epidemiol 2013;5:205–17.
17. Otto M. MRSA virulence and spread. Cell Microbiol 2012;14:1513–21.
18. Vandenesch F, Naimi T, Enright MC, et al. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg Infect Dis 2003;9:978–84.
19. Li M, Diep BA, Villaruz AE, et al. Evolution of virulence in epidemic community-associated methicillin-resistant Staphylococcus aureus. Proc Natl Acad Sci U S A 2009;106:5883–8.
20. Courvalin P. Vancomycin resistance in gram-positive cocci. Clin Infect Dis 2006;42 Suppl 1:S25–34.
21. Gold HS. Vancomycin-resistant enterococci: mechanisms and clinical observations. Clin Infect Dis 2001;33:210–9.
22. Arias CA, Murray BE. The rise of the Enterococcus: beyond vancomycin resistance. Nat Rev Microbiol 2012;10:266–78.
23. Adcock PM, Pastor P, Medley F, Patterson JE, Murphy TV. Methicillin-resistant Staphylococcus aureus in two child care centers. J Infect Dis 1998;178:577–80.
24. Dietrich DW, Auld DB, Mermel LA. Community-acquired methicillin-resistant Staphylococcus aureus in southern New England children. Pediatrics 2004;113:e347–52.
25. Groom AV, Wolsey DH, Naimi TS, et al. Community-acquired methicillin-resistant Staphylococcus aureus in a rural American Indian community. JAMA 2001;286:1201–5.
26. Hewlett AL, Falk PS, Hughes KS, Mayhall CG. Epidemiology of methicillin-resistant Staphylococcus aureus in a university medical center day care facility. Infect Control Hosp Epidemiol 2009;30:985–92.
27. Kazakova SV, Hageman JC, Matava M, et al. A clone of methicillin-resistant Staphylococcus aureus among professional football players. N Engl J Med 2005;352:468–75.
28. Landrum ML, Neumann C, Cook C, et al. Epidemiology of Staphylococcus aureus blood and skin and soft tissue infections in the US military health system, 2005-2010. JAMA 2012;308:50–9.
29. Lindenmayer JM, Schoenfeld S, O’Grady R, Carney JK. Methicillin-resistant Staphylococcus aureus in a high school wrestling team and the surrounding community. Arch Intern Med 1998;158:895–9.
30. Malcolm B. The rise of methicillin-resistant staphylococcus aureus in U.S. correctional populations. J Correct Health Care 2011;17:254–65.
31. Nerby JM, Gorwitz R, Lesher L, et al. Risk factors for household transmission of community-associated methicillin-resistant Staphylococcus aureus. Pediatr Infect Dis J 2011;30:927–32.
32. Stemper ME, Shukla SK, Reed KD. Emergence and spread of community-associated methicillin-resistant Staphylococcus aureus in rural Wisconsin, 1989 to 1999. J Clin Microbiol 2004;42:5673–80.
33. Turabelidze G, Lin M, Wolkoff B, et al. Personal hygiene and methicillin-resistant Staphylococcus aureus infection. Emerg Infect Dis 2006;12:422–7.
34. Ellis MW, Hospenthal DR, Dooley DP, et al. Natural history of community-acquired methicillin-resistant Staphylococcus aureus colonization and infection in soldiers. Clin Infect Dis 2004;39:971–9.
35. Forster AJ, Oake N, Roth V, et al. Patient-level factors associated with methicillin-resistant Staphylococcus aureus carriage at hospital admission: a systematic review. Am J Infect Control 2013;41:214–20.
36. McKinnell JA, Miller LG, Eells SJ, et al. A systematic literature review and meta-analysis of factors associated with methicillin-resistant staphylococcus aureus colonization at time of hospital or intensive care unit admission. Infect Contol Hosp Epidemiol 2013;34:1077–86.
37. Furuno JP, McGregor JC, Harris AD, et al. Identifying groups at high risk for carriage of antibiotic-resistant bacteria. Arch Intern Med 2006;166:580–5.
38. Jernigan JA, Pullen AL, Flowers L, et al. Prevalence of and risk factors for colonization with methicillin-resistant Staphylococcus aureus at the time of hospital admission. Infect Control Hosp Epidemiol 2003;24:409–14.
39. Horner C, Parnell P, Hall D, Kearns A, Heritage J, Wilcox M. Meticillin-resistant Staphylococcus aureus in elderly residents of care homes: colonization rates and molecular epidemiology. J Hosp Infect 2013;83:212–8.
40. Allegranzi B, Pittet D. Role of hand hygiene in healthcare-associated infection prevention. J Hosp Infect 2009;73:305–15.
41. Boyce JM. Methicillin-resistant Staphylococcus aureus. Detection, epidemiology, and control measures. Infect Dis Clin North Am 1989;3:901–13.
42. Jernigan JA. Methicillin-resistant Staphylococcus aureus colonization among health care personnel in the emergency department: what does it tell us? Ann Emerg Med 2008;52:534–6.
43. Carnicer-Pont D, Bailey KA, Mason BW, Walker AM, Evans MR, Salmon RL. Risk factors for hospital-acquired methicillin-resistant Staphylococcus aureus bacteraemia: a case-control study. Epidemiol Infect 2006;134:1167–73.
44. Graffunder EM, Venezia RA. Risk factors associated with nosocomial methicillin-resistant Staphylococcus aureus (MRSA) infection including previous use of antimicrobials. J Antimicrob Chemother 2002;49:999–1005.
45. Thompson RL, Cabezudo I, Wenzel RP. Epidemiology of nosocomial infections caused by methicillin-resistant Staphylococcus aureus. Ann Intern Med 1982;97:309–17.
46. Davis KA, Stewart JJ, Crouch HK,et al. Methicillin-resistant Staphylococcus aureus (MRSA) nares colonization at hospital admission and its effect on subsequent MRSA infection. Clin Infect Dis 2004;39:776–82.
47. Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev 1997;10:505–20.
48. Safdar N, Bradley EA. The risk of infection after nasal colonization with Staphylococcus aureus. Am J Med 2008;121:310–5.
49. Wertheim HFL, Vos MC, Ott A, et al. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet 2004;364:703–5.
50. Garrouste-Orgeas M, Timsit JF, Kallel H, et al. Colonization with methicillin-resistant Staphylococcus aureus in ICU patients: morbidity, mortality, and glycopeptide use. Infect Control Hosp Epidemiol 2001;22:687–92.
51. Honda H, Krauss MJ, Coopersmith CM, et al. Staphylococcus aureus nasal colonization and subsequent infection in intensive care unit patients: does methicillin resistance matter? Infect Control Hosp Epidemiol;31:584–91.
52. von Eiff C, Becker K, Machka K, et al. Nasal carriage as a source of Staphylococcus aureus bacteremia. N Engl J Med 2001;344:11–6.
53. Pujol M, Pea C, Pallares R, et al. Nosocomial Staphylococcus aureus bacteremia among nasal carriers of methicillin-resistant and methicillin-susceptible strains. Am J Med 1996;100:509–16.
54. Rocha LA, Marques Ribas R, da Costa Darini AL, Gontijo Filho PP. Relationship between nasal colonization and ventilator-associated pneumonia and the role of the environment in transmission of Staphylococcus aureus in intensive care units. Am J Infect Control 2013;41:236–40.
55. Corne P, Marchandin Hln, Jonquet O, Campos J, Bauls A-L. Molecular evidence that nasal carriage of Staphylococcus aureus plays a role in respiratory tract infections of critically ill patients. J Clin Microbiol 2005;43:3491–3.
56. Quezada Joaquin NM, Diekema DJ, Perencevich EN, et al. Long-term risk for readmission, methicillin-resistant Staphylococcus aureus (MRSA) infection, and death among MRSA-colonized veterans. Antimicrob Agents Chemother 2013;57:1169–72.
57. Lin MY, Hayden MK. Methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococcus: recognition and prevention in intensive care units. Crit Care Med 2010;38:S335–44.
58. Carmeli Y, Eliopoulos GM, Samore MH. Antecedent treatment with different antibiotic agents as a risk factor for vancomycin-resistant Enterococcus. Emerg Infect Dis 2002;8:802–7.
59. Ostrowsky BE, Venkataraman L, D’Agata EM, et al. Vancomycin-resistant enterococci in intensive care units: high frequency of stool carriage during a non-outbreak period. Arch Intern Med 1999;159:1467–72.
60. Bonten MJ, Hayden MK, Nathan C, et al. Epidemiology of colonisation of patients and environment with vancomycin-resistant enterococci. Lancet 1996;348:1615–9.
61. Ostrowsky BE, Trick WE, Sohn AH, et al. Control of vancomycin-resistant enterococcus in health care facilities in a region. N Engl J Med 2001;344:1427–33.
62. Padiglione AA, Wolfe R, Grabsch EA, et al. Risk factors for new detection of vancomycin-resistant enterococci in acute-care hospitals that employ strict infection control procedures. Antimicrob Agents Chemother 2003;47:2492–8.
63. Batistao DW, Gontijo-Filho PP, Conceicao N, et al. Risk factors for vancomycin-resistant enterococci colonisation in critically ill patients. Mem Inst Oswaldo Cruz 2012;107:57–63.
64. Furtado GH, Martins ST, Coutinho AP, et al. Prevalence and factors associated with rectal vancomycin-resistant enterococci colonization in two intensive care units in Sao Paulo, Brazil. Braz J Infect Dis 2005;9:64–9.
65. Huang SS, Datta R, Rifas-Shiman S, et al. Colonization with antibiotic-susceptible strains protects against methicillin-resistant Staphylococcus aureus but not vancomycin-resistant enterococci acquisition: a nested case-control study. Crit Care 2011;15:R210.
66. Pan SC, Wang JT, Chen YC, et al. Incidence of and risk factors for infection or colonization of vancomycin-resistant enterococci in patients in the intensive care unit. PLoS One 2012;7:e47297.
67. Se YB, Chun HJ, Yi HJ, et al. Incidence and risk factors of infection caused by vancomycin-resistant enterococcus colonization in neurosurgical intensive care unit patients. J Korean Neurosurg Soc 2009;46:123–9.
68. Healthcare Infection Control Practices Advisory Committee (HICPAC). Management of multidrug-resistant organisms in healthcare settings, 2006. Accessed 11 Oct 2013 at www.cdc.gov/hicpac/mdro/mdro_toc.html.
69. Malani PN. Preventing infections in the ICU: one size does not fit all. JAMA 2013;310:1567–8.
70. Recommendations for preventing the spread of vancomycin resistance. Recommendations of the Hospital Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep 1995;44:1–13.
71. de Bruin MA, Riley LW. Does vancomycin prescribing intervention affect vancomycin-resistant enterococcus infection and colonization in hospitals? A systematic review. BMC Infect Dis 2007;7:24.
72. Adachi W, Bolding F, Armstrong R. Experience with vancomycin education and order sheet to limit vancomycin use. Hosp Pharm 1997:1370–3.
73. Fridkin SK, Lawton R, Edwards JR, et al. Monitoring antimicrobial use and resistance: comparison with a national benchmark on reducing vancomycin use and vancomycin-resistant enterococci. Emerg Infect Dis 2002;8:702–7.
74. Guglielmo BJ, Dudas V, Maewal I, et al. Impact of a series of interventions in vancomycin prescribing on use and prevalence of vancomycin-resistant enterococci. Jt Comm J Qual Patient Saf 2005;31:469–75.
75. Lautenbach E, LaRosa LA, Marr AM, et al. Changes in the prevalence of vancomycin-resistant enterococci in response to antimicrobial formulary interventions: impact of progressive restrictions on use of vancomycin and third-generation cephalosporins. Clin Infect Dis 2003;36:440–6.
76. Morgan AS, Brennan PJ, Fishman NO. Impact of a vancomycin restriction policy on use and cost of vancomycin and incidence of vancomycin-resistant Enterococcus. Ann Pharmacother 1997;31:970–3.
77. Anglim AM, Klym B, Byers KE, et al. Effect of a vancomycin restriction policy on ordering practices during an outbreak of vancomycin-resistant Enterococcus faecium. Arch Intern Med 1997;157:1132–6.
78. Montecalvo MA, Jarvis WR, Uman J, et al. Infection-control measures reduce transmission of vancomycin-resistant enterococci in an endemic setting. Ann Intern Med 1999;131:269–72.
79. Morris JG Jr, Shay DK, Hebden JN, et al. Enterococci resistant to multiple antimicrobial agents, including vancomycin. Establishment of endemicity in a university medical center. Ann Intern Med 1995;123:250–9.
80. Quale J, Landman D, Saurina G, et al. Manipulation of a hospital antimicrobial formulary to control an outbreak of vancomycin-resistant enterococci. Clin Infect Dis 1996;23:1020-5.
81. Rubin LG, Tucci V, Cercenado E, et al. Vancomycin-resistant Enterococcus faecium in hospitalized children. Infect Control Hosp Epidemiol 1992;13:700–5.
82. Lai KK, Kelley AL, Melvin ZS, et al. Failure to eradicate vancomycin-resistant enterococci in a university hospital and the cost of barrier precautions. Infect Control Hosp Epidemiol 1998;19:647–52.
83. Shaikh ZH, Osting CA, Hanna HA, et al. Effectiveness of a multifaceted infection control policy in reducing vancomycin usage and vancomycin-resistant enterococci at a tertiary care cancer centre. J Hosp Infect 2002;51:52–8.
84. Lafaurie M, Porcher R, Donay JL, et al. Reduction of fluoroquinolone use is associated with a decrease in methicillin-resistant Staphylococcus aureus and fluoroquinolone-resistant Pseudomonas aeruginosa isolation rates: a 10 year study. J Antimicrob Chemother 2012;67:1010–5.
85. Parienti JJ, Cattoir V, Thibon P, et al. Hospital-wide modification of fluoroquinolone policy and meticillin-resistant Staphylococcus aureus rates: a 10-year interrupted time-series analysis. J Hosp Infect 2011;78:118–22.
86. Safdar N, Abad C. Educational interventions for prevention of healthcare-associated infection: a systematic review. Crit Care Med 2008;36:933–40.
87. Boyce JM. It is time for action: improving hand hygiene in hospitals. Ann Intern Med 1999;130:153–5.
88. Jackson M, Chiarello LA, Gaynes RP, Gerberding JL. Nurse staffing and healthcare-associated infections: proceedings from a working group meeting. J Nurs Adm 2002;32:314–22.
89. Kuzu N, Ozer F, Aydemir S, et al. Compliance with hand hygiene and glove use in a university-affiliated hospital. Infect Control Hosp Epidemiol 2005;26:312–5.
90. Larson E, Killien M. Factors influencing handwashing behavior of patient care personnel. Am J Infect Control 1982;10:93–9.
91. Larson E, Kretzer EK. Compliance with handwashing and barrier precautions. J Hosp Infect 1995;30 Suppl:88–106.
92. Naikoba S, Hayward A. The effectiveness of interventions aimed at increasing handwashing in healthcare workers - a systematic review. J Hosp Infect 2001;47:173–80.
93. Pittet D, Simon A, Hugonnet S, et al. Hand hygiene among physicians: performance, beliefs, and perceptions. Ann Intern Med 2004;141:1–8.
94. Trick WE, Vernon MO, Welbel SF, et al. Multicenter intervention program to increase adherence to hand hygiene recommendations and glove use and to reduce the incidence of antimicrobial resistance. Infect Control Hosp Epidemiol 2007;28:42–9.
95. Wisniewski MF, Kim S, Trick WE, et al. Effect of education on hand hygiene beliefs and practices: a 5-year program. Infect Control Hosp Epidemiol 2007;28:88–91.
96. Rupp ME, Fitzgerald T, Puumala S, et al. Prospective, controlled, cross-over trial of alcohol-based hand gel in critical care units. Infect Control Hosp Epidemiol 2008;29:8–15.
97. Venkatesh AK, Lankford MG, Rooney DM, et al. Use of electronic alerts to enhance hand hygiene compliance and decrease transmission of vancomycin-resistant Enterococcus in a hematology unit. Am J Infect Control 2008;36:199–205.
98. Silvestri L, Petros AJ, Sarginson RE, et al. Handwashing in the intensive care unit: a big measure with modest effects. J Hosp Infect 2005;59:172–9.
99. Akyol A, Ulusoy H, Ozen I. Handwashing: a simple, economical and effective method for preventing nosocomial infections in intensive care units. J Hosp Infect 2006;62:395–405.
100. Simmons B, Bryant J, Neiman K, et al. The role of handwashing in prevention of endemic intensive care unit infections. Infect Control Hosp Epidemiol 1990;11:589–94.
101. Tenorio AR, Badri SM, Sahgal NB, et al. Effectiveness of gloves in the prevention of hand carriage of vancomycin-resistant enterococcus species by health care workers after patient care. Clin Infect Dis 2001;32:826–9.
102. Slaughter S, Hayden MK, Nathan C, et al. A comparison of the effect of universal use of gloves and gowns with that of glove use alone on acquisition of vancomycin-resistant enterococci in a medical intensive care unit. Ann Intern Med 1996;125:448–56.
103. Harris AD, Pineles L, Belton B, et al. Universal glove and gown use and acquisition of antibiotic-resistant bacteria in the ICU: a randomized trial. JAMA 2013;310:1571–80.
104. Dietze B, Rath A, Wendt C, Martiny H. Survival of MRSA on sterile goods packaging. J Hosp Infect 2001;49:255–61.
105. Hardy KJ, Oppenheim BA, Gossain S, et al. A study of the relationship between environmental contamination with methicillin-resistant Staphylococcus aureus (MRSA) and patients’ acquisition of MRSA. Infect Control Hosp Epidemiol 2006;27:127–32.
106. Jawad A, Heritage J, Snelling AM, et al. Influence of relative humidity and suspending menstrua on survival of Acinetobacter spp. on dry surfaces. J Clin Microbiol 1996;34:2881–7.
107. Boyce JM, Havill NL, Otter JA, Adams NM. Widespread environmental contamination associated with patients with diarrhea and methicillin-resistant Staphylococcus aureus colonization of the gastrointestinal tract. Infect Control Hosp Epidemiol 2007;28:1142–7.
108. Boyce JM, Potter-Bynoe G, Chenevert C, King T. Environmental contamination due to methicillin-resistant Staphylococcus aureus: possible infection control implications. Infect Control Hosp Epidemiol 1997;18:622–7.
109. Sexton T, Clarke P, O’Neill E, et al. Environmental reservoirs of methicillin-resistant Staphylococcus aureus in isolation rooms: correlation with patient isolates and implications for hospital hygiene. J Hosp Infect 2006;62:187–94.
110. Dancer SJ. Importance of the environment in meticillin-resistant Staphylococcus aureus acquisition: the case for hospital cleaning. Lancet infect dis 2008;8:101–13.
111. Dancer SJ, White LF, Lamb J, et al. Measuring the effect of enhanced cleaning in a UK hospital: a prospective cross-over study. BMC med 2009;7.
112. Rampling A, Wiseman S, Davis L, et al. Evidence that hospital hygiene is important in the control of methicillin-resistant Staphylococcus aureus. J Hosp Infect 2001;49:109–16.
113. Wilson APR, Smyth D, Moore G, et al. The impact of enhanced cleaning within the intensive care unit on contamination of the near-patient environment with hospital pathogens: a randomized crossover study in critical care units in two hospitals. Crit Care Med 2011;39:651–8.
114. Hess AS, Shardell M, Johnson JK, et al. A randomized controlled trial of enhanced cleaning to reduce contamination of healthcare worker gowns and gloves with multidrug-resistant bacteria. Infection Control Hosp Epidemiol 2013;34:487–93.
115. Falk PS, Winnike J, Woodmansee C, et al. Outbreak of vancomycin-resistant enterococci in a burn unit. Infect Control Hosp Epidemiol 2000;21:575–82.
116. Livornese LL Jr, Dias S, Samel C, et al. Hospital-acquired infection with vancomycin-resistant Enterococcus faecium transmitted by electronic thermometers. Ann Intern Med 1992;117:112–6.
117. Porwancher R, Sheth A, Remphrey S, et al. Epidemiological study of hospital-acquired infection with vancomycin-resistant Enterococcus faecium: possible transmission by an electronic ear-probe thermometer. Infect Control Hosp Epidemiol 1997;18:771–3.
118. Donskey CJ, Chowdhry TK, Hecker MT, et al. Effect of antibiotic therapy on the density of vancomycin-resistant enterococci in the stool of colonized patients. N Engl J Med 2000;343:1925–32.
119. Neely AN, Maley MP. Survival of enterococci and staphylococci on hospital fabrics and plastic. J Clin Microbiol 2000;38:724–6.
120. Noskin GA, Bednarz P, Suriano T, et al. Persistent contamination of fabric-covered furniture by vancomycin-resistant enterococci: implications for upholstery selection in hospitals. Am J Infect Control 2000;28:311–3.
121. Noskin GA, Stosor V, Cooper I, Peterson LR. Recovery of vancomycin-resistant enterococci on fingertips and environmental surfaces. Infect Control Hosp Epidemiol 1995;16:577–81.
122. Smith TL, Iwen PC, Olson SB, Rupp ME. Environmental contamination with vancomycin-resistant enterococci in an outpatient setting. Infect Control Hosp Epidemiol 1998;19:515–8.
123. Wendt C, Wiesenthal B, Dietz E, Ruden H. Survival of vancomycin-resistant and vancomycin-susceptible enterococci on dry surfaces. J Clin Microbiol 1998;36:3734–6.
124. Bhalla A, Pultz NJ, Gries DM, et al. Acquisition of nosocomial pathogens on hands after contact with environmental surfaces near hospitalized patients. Infect Control Hosp Epidemiol 2004;25:164–7.
125. Ray AJ, Hoyen CK, Taub TF, et al. Nosocomial transmission of vancomycin-resistant enterococci from surfaces. JAMA 2002;287:1400–1.
126. Hayden MK, Bonten MJ, Blom DW, et al. Reduction in acquisition of vancomycin-resistant enterococcus after enforcement of routine environmental cleaning measures. Clin Infect Dis 2006;42:1552–60.
127. Byers KE, Durbin LJ, Simonton BM, et al. Disinfection of hospital rooms contaminated with vancomycin-resistant Enterococcus faecium. Infect Control Hosp Epidemiol 1998;19:261–4.
128. Goodman ER, Platt R, Bass R, et al. Impact of an environmental cleaning intervention on the presence of methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci on surfaces in intensive care unit rooms. Infect Control Hosp Epidemiol 2008;29:593–9.
129. Dancer SJ. The role of environmental cleaning in the control of hospital-acquired infection. J Hosp Infect 2009;73:378–85.
130. Centers for Disease Control and Prevention. Methicillin-resistant Staphylococcus aureus (MRSA) infections. Accessed 11 Oct 2013 at www.cdc.gov/mrsa/index.html.
131. Edmond MB, Wenzel RP. Targeted decolonization to prevent ICU infections. N Engl J Med 2013;369:1471.
132. Lai KK, Fontecchio S, Melvin Z, Baker SP. Impact of alcohol-based, waterless hand antiseptic on the incidence of infection and colonization with methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci. Infect Control Hosp Epidemiol 2006;27:1018–24.
133. Ostrowsky B, Steinberg JT, Farr B, et al. Reality check: should we try to detect and isolate vancomycin-resistant enterococci patients? Infect Control Hosp Epidemiol 2001;22:116–9.
134. Harbarth S, Sax H, Uckay I, et al. A predictive model for identifying surgical patients at risk of methicillin-resistant Staphylococcus aureus carriage on admission. J Am Coll Surg 2008;207:683–9.
135. Jarvis WR. Targeted decolonization to prevent ICU infections. N Engl J Med 2013;369:1469.
136. Krause R, Honigl M, Zollner-Schwetz I. Targeted decolonization to prevent ICU infections. N Engl J Med;369:1469–70.
137. Huskins WC, Huckabee CM, O’Grady NP, et al. Intervention to reduce transmission of resistant bacteria in intensive care. N Engl J Med;364:1407–18.
138. Robicsek A, Beaumont JL, Paule SM, et al. Universal surveillance for methicillin-resistant Staphylococcus aureus in 3 affiliated hospitals. Ann Intern Med 2008;148:409–18.
139. Jain R, Kralovic SM, Evans ME, et al. Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. N Engl J Med 2011;364:1419–30.
140. Gurieva T, Bootsma MCJ, Bonten MJM. Successful Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections revisited. Clin Infect Dis 2012:54:1618–20.
141. Gavalda L, Masuet C, Beltran J, et al. Comparative cost of selective screening to prevent transmission of methicillin-resistant Staphylococcus aureus (MRSA), compared with the attributable costs of MRSA infection. Infection control and hospital epidemiology 2006;27:1264–6.
142. Otter JA, Herdman MT, Williams B, et al. Low prevalence of methicillin-resistant Staphylococcus aureus carriage at hospital admission: implications for risk-factor-based vs universal screening. J Hosp Infect 2013;83:114–21.
143. Harbarth S, Hawkey PM, Tenover F, et al. Update on screening and clinical diagnosis of methicillin-resistant Staphylococcus aureus (MRSA). Int J Antimicrob Agents 2011;37:110–7.
144. Calfee DP, Giannetta ET, Durbin LJ, et al. Control of endemic vancomycin-resistant Enterococcus among inpatients at a university hospital. Clin Infect Dis 2003;37:326–32.
145. Hendrix CW, Hammond JM, Swoboda SM, et al. Surveillance strategies and impact of vancomycin-resistant enterococcal colonization and infection in critically ill patients. Ann Surg 2001;233:259–65.
146. Muto CA, Giannetta ET, Durbin LJ, et al. Cost-effectiveness of perirectal surveillance cultures for controlling vancomycin-resistant Enterococcus. Infect Control Hosp Epidemiol 2002;23:429–35.
147. Price CS, Paule S, Noskin GA, Peterson LR. Active surveillance reduces the incidence of vancomycin-resistant enterococcal bacteremia. Clin Infect Dis 2003;37:921–8.
148. Shadel BN, Puzniak LA, Gillespie KN, et al. Surveillance for vancomycin-resistant enterococci: type, rates, costs, and implications. Infect Control Hosp Epidemiol 2006;27:1068–75.
149. Siddiqui AH, Harris AD, Hebden J, et al. The effect of active surveillance for vancomycin-resistant enterococci in high-risk units on vancomycin-resistant enterococci incidence hospital-wide. Am J Infect Control 2002;30:40–3.
150. Sandri AM, Dalarosa MG, Ruschel de Alcantara L, et al. Reduction in incidence of nosocomial methicillin-resistant Staphylococcus aureus (MRSA) infection in an intensive care unit: role of treatment with mupirocin ointment and chlorhexidine baths for nasal carriers of MRSA. Infect Control Hosp Epidemiol 2006;27:185–7.
151. Huang SS, Septimus E, Kleinman K, et al. Targeted versus universal decolonization to prevent ICU infection. N Engl J Med 2013;368:2255–65.
152. Bleasdale SC, Trick WE, Gonzalez IM, et al. Effectiveness of chlorhexidine bathing to reduce catheter-associated bloodstream infections in medical intensive care unit patients. Arch Intern Med 2007;167:2073–9.
153. Climo MW, Sepkowitz KA, Zuccotti G, et al. The effect of daily bathing with chlorhexidine on the acquisition of methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus, and healthcare-associated bloodstream infections: results of a quasi-experimental multicenter trial. Crit Care Med 2009;37:1858–65.
154. Vernon MO, Hayden MK, Trick WE, et al. Chlorhexidine gluconate to cleanse patients in a medical intensive care unit: the effectiveness of source control to reduce the bioburden of vancomycin-resistant enterococci. Arch Intern Med 2006;166:306–12.
155. Milstone AM, Elward A, Song X, et al. Daily chlorhexidine bathing to reduce bacteraemia in critically ill children: a multicentre, cluster-randomised, crossover trial. Lancet 2013;381:1099–106.
156. Blackwood B, Thompson G, McMullan R, et al. Tea tree oil (5%) body wash versus standard care (Johnson’s Baby Softwash) to prevent colonization with methicillin-resistant Staphylococcus aureus in critically ill adults: a randomized controlled trial. J Antimicrob Chemother 2013;68:1193–9.
157. Daneman N, Sarwar S, Fowler RA, et al. Effect of selective decontamination on antimicrobial resistance in intensive care units: a systematic review and meta-analysis. Lancet Infect Dis 2013;13:328–41.
158. Verwaest C, Verhaegen J, Ferdinande P, et al. Randomized, controlled trial of selective digestive decontamination in 600 mechanically ventilated patients in a multidisciplinary intensive care unit. Crit Care Med 1997;25:63–71.
159. de Smet AMGA, Kluytmans JAJW, Blok HEM, et al. Selective digestive tract decontamination and selective oropharyngeal decontamination and antibiotic resistance in patients in intensive-care units: an open-label, clustered group-randomised, crossover study. Lancet Infect Dis 2011;11:372–80.
160. de Jonge E, Schultz MJ, Spanjaard L, et al. Effects of selective decontamination of digestive tract on mortality and acquisition of resistant bacteria in intensive care: a randomised controlled trial. Lancet 2003;362:1011–6.
161. Cepeda JA, Whitehouse T, Cooper B, et al. Isolation of patients in single rooms or cohorts to reduce spread of MRSA in intensive-care units: prospective two-centre study. Lancet 2005;365:295–304.
162. Dhaliwal J, McGeer A. Does isolation prevent the spread of methicillin-resistant Staphylococcus aureus? CMAJ 2005;172:875.
163. Kollef MH, Micek ST. Antimicrobial stewardship programs: mandatory for all ICUs. Crit Care 2012;16:179.
164. McKinnell JA, Huang SS, Eells SJ, et al. Quantifying the impact of extranasal testing of body sites for methicillin-resistant Staphylococcus aureus colonization at the time of hospital or intensive care unit admission. Infect Control Hosp Epidemiol 2013;34:161–70.
165. Denkinger CM, Grant AD, Denkinger M, et al. Increased multi-drug resistance among the elderly on admission to the hospital—a 12-year surveillance study. Arch Gerontol Geriatr 2013;56:227–30.
166. Boisseau D, Alfandari S, Gauzit R, et al. Staphylococcus aureus nasal carriage during the infectious diseases national congress in France. Med Mal Infect 2012;42:435–9.
167. Fritz SA, Hogan PG, Hayek G, et al. Staphylococcus aureus colonization in children with community-associated Staphylococcus aureus skin infections and their household contacts. Arch Pediatr Adolesc Med 2012;166:551–7.
168. Rafee Y, Abdel-Haq N, Asmar B, et al. Increased prevalence of methicillin-resistant Staphylococcus aureus nasal colonization in household contacts of children with community acquired disease. BMC Infect Dis 2012;12:45.
169. Schechter-Perkins EM, Mitchell PM, Murray KA, et al. Prevalence and predictors of nasal and extranasal staphylococcal colonization in patients presenting to the emergency department. Ann Emerg Med 2011;57:492–9.
170. Bisaga A, Paquette K, Sabatini L, Lovell E. A prevalence study of methicillin-resistant staphylococcus aureus colonization in emergency department health care workers. Ann Emerg Med 2008;52:525–8.
171. Suffoletto B, Cannon E, Ilkhanipour K, Yealy D. Prevalence of Staphylococcus aureus nasal colonization in emergency department personnel. Ann Emerg Med 2008;52:529–33.
172. Young DM, Harris HW, Charlebois ED, et al. An epidemic of methicillin-resistant Staphylococcus aureus soft tissue infections among medically underserved patients. Arch Surg 2004;139:947-51; discussion 51–3.
173. Salgado CD, Farr BM, Calfee DP. Community-acquired methicillin-resistant Staphylococcus aureus: a meta-analysis of prevalence and risk factors. Clin Infect Dis 2003;36:131–9.
From the Department of Medicine, Infectious Disease Practice and Innovations, The Medical City, Pasig City, Philippines (Dr. Abad), the Division of Emergency Medicine, University of Wisconsin Medical School, Madison, WI (Dr. Pulia), University of Wisconsin Hospital and Clinics, Madison, WI (Ms. Krupp), and the Willam S. Middleton Memorial Veterans Affairs Hospital, Madison, WI (Dr. Safdar).
Patients in intensive care units (ICUs) are at greatly increased risk of developing health care-associated infections (HAIs) [1]. More than 70% of the bacteria that cause HAIs are resistant to at least one of the antimicrobials commonly used to treat these infections [2]. Two such pathogens, methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE) are responsible for a considerable proportion of ICU infections that are associated with increased morbidity, mortality, and costs [3–5]. In this review, we discuss the epidemiology of colonization and infection by MRSA and VRE and provide an update on practices for prevention of transmission and infection by MRSA and VRE in the ICU.
EPIDEMIOLOGY AND MECHANISMS OF RESISTANCE
MRSA is the major cause of HAIs worldwide [6]. Among ICUs in the United States, the proportion of methicillin resistance among S. aureus isolates increased from 35.9% in 1992 to 64.4% in 2003 [4]. Approximately 8% of patients are colonized with MRSA upon admission, and an average of 5% will acquire MRSA colonization in the ICU [7,8]. A comparison study of academic tertiary care facilities found medical ICUs had higher MRSA admission prevalence rates than surgical ICUs, whereas surgical ICUs had a higher incidence rate [7]. Enteroccoccus is the third most common pathogen associated with HAIs, with 33% resistant to vancomycin [9]. VRE infection is associated with increased ICU cost and increased length of stay [10]. Incidence of ICU-acquired VRE varies among regions and countries. For example, in the United States, Warren et al [11] reported a VRE incidence of 27 cases per 1000 patient ICU days, whereas Kohlenberg et al [12] reported a mean incidence of 0.29 cases per 1000 patient ICU days in Germany.
Understanding the mechanisms that allow development of resistant strains of S. aureus and Enterococcus species is important to devise preventive strategies. Methicillin resistance in MRSA is determined by the staphylococcal cassette chromosome mec (SCCmec), a mobile genetic element that carries the mecA gene. The mecA gene codes for an additional penicillin-binding protein (PBP) that has a reduced affinity towards methicillin (PBP2a/PBP2'). This results in a reduced ability to bind to the bacterial cell wall and inhibit synthesis [13,14]. Study of molecular epidemiology has identified MRSA as originating from 8 major variants of the mecA gene [15]. The majority of MRSA infections are caused by strains belonging to a few internationally disseminated clones [14]. The first identified strains were associated with infections in hospitalized patients (hospital-associated MRSA), but community-associated MRSA strains have since emerged and have become established globally, including in health care institutions [16].
Community-acquired MRSA can cause severe infections in health hosts [17]; possible explanations include increased CA-MRSA virulence due to the acquisition of mobile genetic elements, namely those containing Panton-Valentine leukocidin (PVL) [18] or increased expression of core genome-encoded virulence genes, such as phenol-soluble modulin (PSM) cytolysins, α-toxin, and other virulence determinants [19].
Enterococcus is intrinsically resistant to several antimicrobial drugs, with resistance to vancomycin encoded by several clusters of genes known as vancomycin resistance gene clusters (eg, vanA, vanB). The gene clusters generate resistance through multiple pathways which encode enzymes to determine the structure of peptidoglycan precursors [20,21]. Genetically diverse, hospital-associated VRE outbreaks have been associated with single clones, multiple clones, and changing molecular epidemiology over time [21]. While up to 25% of the VRE genome includes acquired elements, the majority of hospital-associated infections are traced to a few clonal complexes, which differ from community-associated VRE strains [22].
The evolution of these efficient mechanisms that promote drug resistance has made it extremely challenging to eradicate organisms such as MRSA and VRE. However, advances in recent years have furthered our understanding of the epidemiology, pathogenesis, and methods of prevention and containment.
RISK FACTORS FOR COLONIZATION AND INFECTION
MRSA
The risk factors underlying MRSA colonization and infection in the ICU setting can be categorized as either patient/host or environmental factors. A wide range of patient-level factors is associated with MRSA colonization upon admission. General principles regarding the transmission of MRSA in the community include close contact with colonized or infected individuals, breaks in the skin, crowded living conditions and poor hygiene. These factors, alone or in combination, are thought to underlie observed outbreaks among sports teams, military personnel, correction facilities, American Indian communities, and daycare centers [23–34].
Two recently published systematic reviews have summarized important patient-level factors associated with MRSA colonization at the time of hospital admission. Forster et al [35] examined 27 studies and identified previous admission to hospital, transfer from nursing home or long-term care facility, and previous antibiotic use as the top 3 factors associated with MRSA colonization. A similar review conducted by McKinnell and colleagues [36] found that prior hospitalization, nursing home contact, recent antibiotic use, and exposure to health care-associated pathogens (MRSA carriage, VRE carriage, or Clostridium difficile infection) were found to portend the highest risk. Specific comorbid conditions also conveyed an increased risk, including congestive heart failure, chronic wounds/bedsores, diabetes mellitus, pulmonary disease, immunosuppression, urinary catheter, and renal failure/dialysis. It is clear that health care contacts, especially recent hospitalization, residence in a long-term care facility, and antibiotic use, are significant risk factors for MRSA colonization [37–39].
 In contrast to those already colonized with MRSA, some patients acquire MRSA during hospitalization. In these cases, transmission via hands of health care workers is likely the most common mechanism for spread of MRSA [6,40–42]. An understaffed ICU has also been cited as a potential risk factor for ICU MRSA transmission, perhaps due to sacrifices in hand hygiene practices by overextended staff [6]. Additional factors associated with increased risk of nosocomial MRSA acquisition include duration of antibiotic therapy, exposure to quinolone or macrolide antibiotics, length of hospital stay, enteral feeding, post-surgical status, insertion of central line or urinary catheter during admission, ICU admission, and proximity to another patient with MRSA infection or colonization [43–45]. A summary of risk factors for MRSA acquisition is shown in Table 1.
In contrast to those already colonized with MRSA, some patients acquire MRSA during hospitalization. In these cases, transmission via hands of health care workers is likely the most common mechanism for spread of MRSA [6,40–42]. An understaffed ICU has also been cited as a potential risk factor for ICU MRSA transmission, perhaps due to sacrifices in hand hygiene practices by overextended staff [6]. Additional factors associated with increased risk of nosocomial MRSA acquisition include duration of antibiotic therapy, exposure to quinolone or macrolide antibiotics, length of hospital stay, enteral feeding, post-surgical status, insertion of central line or urinary catheter during admission, ICU admission, and proximity to another patient with MRSA infection or colonization [43–45]. A summary of risk factors for MRSA acquisition is shown in Table 1.
Regardless of whether MRSA colonization precedes admission or occurs due to nosocomial spread, it is associated with increased risk of developing a HAI [46–49]. In 2 large prospective observational cohort studies, the hazard ratios of MRSA colonization developing into S. aureus infections during the ICU stay were 3.84 and 4.70, respectively [50,51]. High levels of concordance between MRSA colonization strains and HAI strains have also been reported [52]. Nasal colonization with S. aureus has also been identified as an independent risk factor for developing ventilator-associated pneumonia (VAP) and bacteremia [53,54]. A case series of ICU patients with S. aureus nasal colonization who developed lower respiratory tract infections demonstrated genetically identical nasal and bronchial strains in 15/16 cases [55]. This finding strongly suggests that nasopharyngeal colonization with S. aureus contaminates oral secretions that are aspirated by critically ill patients, resulting in subsequent pneumonia. In a long-term outcomes study among a matched cohort of veterans, MRSA colonization was associated with an increased risk of infection-related readmission and mortality [56]. These findings reflect the critically important nature of measures designed to curb nosocomial transmission and acquisition of MRSA, especially among the vulnerable ICU population.
VRE
As with MRSA, risk factors associated with VRE colonization include both patient-level and ICU-level (or environmental) factors [57]. Examples of patient-level factors include previous antimicrobial exposure [58–62], underlying medical illnesses such as chronic renal failure requiring hemodialysis [11,63], length of hospital or ICU stay [11,59,64,65], and recent exposure to health care facilities. ICU-level factors of relevance are the prevalence of VRE in the unit, with high levels of endemicity leading to higher risk of colonization and transmission.
Antibiotic use is a major risk factor for VRE acquisition, although the type and class of antibiotic varies considerably across studies; the most frequently identified antibiotics are broad-spectrum cephalosporins, vancomycin, and anti-anaerobic agents [58,62,64]. Patients with chronic liver disease and post-transplantation are at exceedingly high risk for VRE acquisition [59]. In a recent study by Pan [66], for example, the authors found that the incidence of newly acquired VRE was 21.9 per 1000 patient-days in an ICU setting. On multivariate analysis, the authors found that, similar to other reports [11,59,67], length of stay in the ICU was associated with increased risk of VRE acquisition, with each additional day of stay increasing risk of VRE by 1.03 times. Warren et al undertook a prospective cohort study involving 519 patients admitted to the ICU for more than 48 hours [11]. Seventy-four (21%) of 352 patients were subsequently colonized with VRE. The median time to development of a positive VRE culture after ICU admission was 6 days. Increased mean APACHE II score on ICU admission (P = 0.002), sucralfate use (P = 0.003), vasopressor use (P = 0.01), tracheostomy in the ICU (P = 0.02), and C. difficile diarrhea (P = 0.002) appeared to be associated with VRE acquisition.
 It appears that VRE acquisition is often associated with the sick subgroup of patients, and risk factors generally associated with VRE colonization and infection co-relate with disease chronicity and severity of illness. Length of hospitalization, ICU stay, hemodialysis, or transplantation may all be markers of disease severity. A summary of risk factors for VRE acquisition is shown in Table 2.
It appears that VRE acquisition is often associated with the sick subgroup of patients, and risk factors generally associated with VRE colonization and infection co-relate with disease chronicity and severity of illness. Length of hospitalization, ICU stay, hemodialysis, or transplantation may all be markers of disease severity. A summary of risk factors for VRE acquisition is shown in Table 2.
REDUCING TRANSMISSION—MRSA AND VRE PREVENTION STRATEGIES
 Evidence-based guidelines developed by the Centers for Disease Control (CDC) Hospital Infection Control Practices Advisory Committee (HICPAC) for prevention of MRSA and VRE are available [68]. Several recently conducted well-designed clinical trials also provide additional insight that may be particularly helpful in the ICU setting [69]. A summary of the MRSA prevention guidelines issued by the CDC and included in its “MRSA toolkit” is provided in Table 3. A similar guideline on prevention of VRE [70], published more than a decade ago, has similar elements. Table 3 shows a side-by-side comparison of these elements. Unfortunately, despite these guidelines and extensive research regarding prevention and control, considerable controversy exists as to the most effective approaches. As such, these recommendations should be tailored to meet the needs of the specific ICU setting.
Evidence-based guidelines developed by the Centers for Disease Control (CDC) Hospital Infection Control Practices Advisory Committee (HICPAC) for prevention of MRSA and VRE are available [68]. Several recently conducted well-designed clinical trials also provide additional insight that may be particularly helpful in the ICU setting [69]. A summary of the MRSA prevention guidelines issued by the CDC and included in its “MRSA toolkit” is provided in Table 3. A similar guideline on prevention of VRE [70], published more than a decade ago, has similar elements. Table 3 shows a side-by-side comparison of these elements. Unfortunately, despite these guidelines and extensive research regarding prevention and control, considerable controversy exists as to the most effective approaches. As such, these recommendations should be tailored to meet the needs of the specific ICU setting.
Antimicrobial Stewardship
Antibiotic use is a major driver of antibiotic resistance. A meta-analysis by de Bruin and Riley [71] studied the effect of vancomycin usage on VRE colonization and infection. A total of 12 articles describing 13 studies were included; none were randomized controlled trials. All studies were quasi-experimental and lacked control groups. Among all studies, less than half (46%) implemented vancomycin reduction measures as the sole type of intervention [72–76]. The remaining studies implemented other infection control modalities and or restricted the use of other antimicrobials [77–83]. Although all studies that implemented vancomycin restriction alone as a single strategy showed a decline in vancomycin usage, only 2 of these [74,75] showed a relative risk reduction in VRE acquisition post-intervention. Also, studies that restricted vancomycin alone revealed a trend towards lower efficacy in reducing VRE colonization and infection (33%) when compared with those that used additional measures (71%). While judicious antibiotic use should always be practiced, the evidence for vancomycin restriction as a sole intervention to control VRE is scant. It may be that other antibiotics are as big or bigger drivers of resistance in enterococci than vancomycin. For example, a growing body of literature supports antibiotic restriction, especially fluoroquinolones, for reducing MRSA. In several time-series quasi-experimental studies, restriction of fluorquinolones was associated with reduced trends in MRSA infections in the acute care setting, and consideration should be given to monitor and optimize fluoroquinolone use in the ICU setting [84,85].
Antimicrobial stewardship programs are fundamental to optimizing antibiotic use in the ICU and the authors strongly recommend that all ICUs should have such a program in place.
Educational Interventions
Infection control and multidrug-resistant organism (MDRO)–specific education programs for health care workers is a core principle of the CDC’s prevention guidelines. The HICPAC VRE guideline also explicitly states “continuing education programs for hospital staff (including attending and consulting physicians, medical residents, and students; pharmacy, nursing, and laboratory personnel; and other direct patient-care providers) should include information concerning the epidemiology of VRE and the potential impact of this pathogen on the cost and outcome of patient care [70].” A systematic review published in 2008 [86] that included 26 studies showed that such interventions to prevent HCAIs are usually successful; in this review, 20 of 26 studies showed a statistically significant decrease in infection rates, with risk ratios ranging from 0 to 1.6. Education was usually part of a broader array of infection control interventions. While clearly essential, education alone is unlikely to have a sustained impact on reducing MRSA and VRE infections.
Infection Control Measures
Major infection control interventions include hand hygiene, the use of personal protective equipment (PPE), and cohorting. These measures can be grouped into “horizontal” (or global) vs. “vertical” (or targeted) strategies. Although not mutually exclusive, horizontal approaches are designed to have an impact on multiple pathogens (pathogen nonspecific), whereas vertical approaches are designed to reduce the impact of specific pathogens (such as VRE). For the purposes of this review, we will discuss both strategies for containment of MRSA and VRE. Horizontal strategies include hand hygiene, universal gloving and/or gowning, environmental cleaning, and daily bathing with chlorhexidine. Vertical strategies include screening for either MRSA or VRE followed by placement in contact precautions and decolonization with mupirocin.
Hand Hygiene
Hand washing is fundamental to reducing transmission of MDROs in health care institutions; however, optimal compliance is hard to achieve and sustain. Barriers to adherence may include unavailability of sinks or hand hygiene materials (eg, alcohol-based gels, gloves) time constraints, forgetfulness, or lack of knowledge [87–95]. Several monitoring strategies have been evaluated to increase compliance with hand hygiene. Most involve direct observation followed by performance assessment and feedback.
Trials examining the impact of improvements in hand hygiene compliance on HAIs in the ICU setting have largely found benefit, although not all studies showed a decline in HAI. In a prospective crossover trial, Rupp et al [96] found dramatic improvements in compliance with hand gel availability, but this did not translate to decreased nosocomial MRSA infections. Venkatesh et al [97] carried out a before-and-after interventional prospective study in a hematology unit in a tertiary level hospital to evaluate the use of an electronic method of surveillance to determine compliance with hand hygiene. The authors also used rates of horizontal transmission of VRE as a secondary end-point. Results of the study showed that hand hygiene compliance improved from 36.3% at baseline to 70.1%. This represented an OR of 4.1 (95% confidence interval, 3.7–4.5), which the authors attributed to the use of automated alerts. VRE transmission rates before and during intervention were not statistically different, but the rates of infection were lower at 1.0 per month in comparison with 4.7 infections per month in the preceding 6 months (P = 0.096).
While improved hand hygiene may result in significant reductions in HAIs [40], research indicates hand hygiene alone influences about 40% of infections in the ICU setting [98]. As such, hand hygiene should be viewed as a necessary component of a comprehensive infection control program [99]. Despite the success of hand hygiene in reducing HAIs in the ICU, effective strategies to improve compliance remain elusive even under study conditions and further research is needed in this area [100].
Personal Protective Equipment
Tenorio et al [101] conducted a study to assess the effectiveness of gloving in the prevention of hand carriage of VRE by health care workers. The study showed that among 50 health care workers who had contact with patients colonized with VRE, 6 carried a similar patient strain even prior to known contact, and 17 of 44 (69%) had a patient-related VRE strain on their gloves after contact. This suggests a relatively high rate of colonization after usual patient-care contact. Factors associated with acquisition of VRE on gloves included duration of contact, contact with a patient’s body fluids, presence of diarrhea in a patient, mean VRE colony counts on a patient’s skin, and number of body sites colonized with VRE. Although gloves reduced the risk of VRE acquisition of VRE by 71% (ie, 12/17 did not have VRE on their hands after de-gloving) the protection afforded by gloves was incomplete. As such, hand hygiene after glove removal is recommended.
Slaughter et al [102] compared the use of personal protective equipment in the acquisition of VRE. During this study, 93 patients in glove-and-gown rooms and 88 patients in glove-only rooms had similar rates of VRE at baseline entry into the ICU and after the intervention. Mean times to colonization among the patients who became colonized were 8.0 days in the glove-and-gown group and 7.1 days in the glove-only group. None of these comparisons were statistically significant and the authors concluded that the universal use of gown and gloves was no better than the use of gloves alone in preventing VRE colonization.
A recent cluster randomized trial compared the effect of universal PPE (ie, gowning and gloving) with usual care for reducing acquisition of MRSA or VRE as a composite outcome [103]. The study did not find that universal gowning and gloving reduced VRE or MRSA acquisition but found a 40% decline in MRSA acquisition in the intervention ICUs compared with baseline rates of MRSA. No major adverse effects of universal gowning and gloving were noted in this study. A thoughtful editorial commenting on this article proposes that several aspects of the study deserve consideration, including the possibility of false-negative screening tests for VRE, which may have partially accounted for the negative primary outcome [69].
Based on these studies, it appears that the use of barrier precautions may be of value more for MRSA than VRE but further studies are needed to examine its impact on other types of pathogens, including new and emerging MDROs. Until further evidence becomes available, routine gowning and gloving may be of value in units with a high prevalence of MRSA.
Environmental Cleaning
Accumulating data suggests that the environment may play a major role in transmission of pathogens. MRSA has the ability to survive for days to weeks on inanimate objects [104–107]. Environmental contamination results in contamination of staff clothing and gloves [107,108] and is highly correlated with colonization strains among inpatients [109]. Although some studies of enhanced cleaning techniques and increased environmental services staff time have demonstrated reductions in MRSA outbreaks [110–112], the results are not universally favorable [113,114] and further studies are needed to examine the impact of environmental cleaning on rates of MRSA colonization or infection.
Several studies have implicated contaminated equipment as vectors for transmission of VRE during outbreaks [115–117], but the direction of fomite transfer from patient to environment has been difficult to ascertain. VRE have been found frequently on a variety of inanimate objects and surfaces in different health care environments [118–123], including gloved or ungloved hands of health care workers [101,124,125]. Hayden et al [126] determined the effect of improved environmental cleaning on VRE acquisition rates. This study was a pre-and-post intervention study carried out in a 21-bed medical intensive care unit (MICU) in a tertiary hospital over several phases. The intervention included the creation of a unique and improved cleaning program, as well as in-services to housekeeper services, education of the MICU staff, and a hand hygiene campaign. The results of the study showed decreased acquisition of VRE from 33.47 cases per 1000 patient days at risk in period 1 to 10.40 cases per 1000 patient-days at risk by period 4 of the study. Increased environmental cleaning was also associated with reduced growth of VRE from environmental cultures. At baseline, weekly contamination rates were 0.15 and 0.1 for samples obtained before and after cleaning, respectively. Culture positivity decreased to 0.07 and 0.04 for before and after cleaning in period 2 and then remained at low levels during the remainder of the study. It is important to note that the method for disinfecting used in this study was the “bucket method” as promoted by Byers [127]. This study provides further support for the importance of an environmental reservoir and of environmental decontamination to prevent endemic cross-transmission of VRE [126].
Goodman et al [128] used similar interventions but added a feedback tool using a black-light monitoring system (ie, use of an invisible, nontoxic marker to delineate areas that are adequately or inadequately cleaned) to reduce the likelihood of isolating either MRSA or VRE from an ICU environment. This study also showed favorable results, and notably, the use of the black-light monitoring system identified specific areas that were typically inadequately disinfected. Results showed that flat, horizontal surfaces (eg, countertops, bedside tray tables, and hamper tops) were adequately cleaned more often than small, vertical surfaces (eg, doorknobs, toilet handles, light switches, and electronics).
Part of the controversy surrounding the impact of environmental cleaning is the difficulty in determining its individual value as part of an overall infection control bundle [129]. A proposed area of demonstrable impact for environmental cleaning are frequently touched sites which are more likely to be contaminated with pathogens. Focusing on these “hot-bed” areas of the care environment may offer a useful adjunct to other infection control measures [129].
Active Surveillance
Active surveillance refers to periodic screening for asymp-tomatic carriers followed by placement of colonized patients in contact isolation. This practice is highly variable across institutions, as the evidence supporting this practice is conflicting and there are concerns about the cost of implementing this approach without solid evidence [70,130,131]. Despite lack of randomized controlled trials to guide this practice for MRSA prevention, many hospitals utilize MRSA surveillance and it is mandated by law in 9 states [132,133].
A prospective, interventional cohort study of universal MRSA screening on admission to surgical wards failed to reduce nosocomial MRSA infections [134]. Most recently, a pragmatic, cluster-randomized ICU trial reported that universal decolonization with chlorhexidine wipes and mupirocin use was more effective than screening and isolation in reducing rates of MRSA clinical isolates [65]. However, concerns regarding the risk of mupirocin resistance have been expressed [135,136]. The only randomized trial that compared active surveillance for MRSA and VRE followed by contact precautions to usual care did not find a benefit to active surveillance.
Huskins et al [137], in a large, cluster-randomized trial of 19 ICUs from different hospitals, determined the utility of using a culture-based active surveillance and contact isolation, compared with usual care (contact isolation for patients colonized with MRSA or VRE) as identified by existing hospital protocols, to reduce the incidence of colonization or infection with MRSA or VRE. In this trial, which spanned 6 months and involved 3488 participants, the authors found no significant difference between the intervention and control ICUs in terms of MRSA and VRE colonization or infection rates.
Conflicting with these findings is an observational study comparing MRSA infection rates before and after institution of a universal screening protocol, which demonstrated a 69.6% (CI, –89.2% to –19.6%]; P = 0.03) reduction in hospital wide MRSA prevalence density with screening [138]. The “MRSA bundle” implemented in 2007 at VA hospitals nationwide, which included universal screening, produced a 62% (P < 0.001) reduction in MRSA ICU infections; the relative contribution of the various bundle components is uncertain [139,140].
A proposed cost-saving alternative to universal screening is selective screening based on risk factor assessment [141]. The effectiveness of this type of program depends on creating a clinical decision-making tool capable of accurately identifying high-risk individuals while also accounting for the different risk factor profiles between HA-MRSA and CA-MRSA [142]. It has been proposed that targeted screening protocols may be more cost-effective in settings with < 5% prevalence of MRSA colonization on admission [143].
Many studies [61,144–149] have shown that active surveillance against VRE is cost-effective. For example, Calfee et al [144] showed that an established active surveillance program results in control of endemic VRE in high-risk patients. The infection control program was established in response to a hospital-wide VRE outbreak, and was sustained after the outbreak was controlled. The study by Calfee et al spanned 5 years and was performed at a tertiary-level university hospital, where cultures from perirectal areas were used to identify high-risk patients who were asymptomatically colonized with VRE. During the latter 2 years, 768 new cases of VRE colonization were detected among 69,672 admissions (1.1% of admissions), of which 730 (95.1%) were identified by active surveillance methods. This implies that routine clinical cultures would probably have missed the majority of colonized patients. During this period, the incidence of VRE infection was likewise extremely low at 0.12/1000 patient days (ie, 90 nosocomial VRE infections were identified in 83 patients during 743,956 days of patient care). Sixty-nine of the 83 patients (83%) who developed nosocomial VRE infections were found to be colonized with VRE by surveillance culture before the onset of infection.
Patient Decolonization
Chlorhexidine gluconate has been used in several settings to control outbreaks and infections related to MRSA and VRE due to its broad-spectrum activity against these pathogens. Chlorhexidine-based solutions reduce the density of skin colonization with pathogens such as MRSA and VRE (skin asepsis), thus lowering the risk for horizontal transmission between health care workers and patients.
Decolonization with chlorhexidine as an MRSA infection reduction technique has demonstrated benefit in the ICU setting [150]. The previously mentioned large, cluster-randomized ICU trial by Huang and colleagues found universal decolonization with twice-daily intranasal mupirocin for 5 days and daily bathing with chlorhexidine-impregnated cloths for the entire ICU stay was superior to targeted decolonization of known MRSA carriers in preventing overall MRSA isolates. However, universal decolonization failed to show a reduction in MRSA bacteremia [151], and concerns about mupirocin resistance may limit the applicability of this approach.
There are now several studies [152–154] that show decreased acquisition of VRE with use of daily chlorhexidine bathing. In a study including 1787 ICU patients, Vernon et al found [154] that the reducing microbial density of VRE on patient’s skin by using chlorhexidine led to decreased transmission. In another study by Climo et al [153] that involved 6 ICUs at 4 academic centers and measured the incidence of MRSA and VRE colonization and blood stream infections (BSI) during a period of bathing with routine soap for 6 months compared with a 6-month period where all admitted patients received daily bathing with a chlorhexidine solution, results found decreased acquisition of VRE by 50% (4.35 vs. 2.19 cases/1000 patient days, P < 0.008) following the introduction of daily chlorhexidine bathing. Furthermore, compared with 16 of 270 patients colonized with VRE who subsequently developed VRE bacteremia at baseline, only 4 of 226 VRE-colonized patients bathed with chlorhexidine in the intervention period developed a BSI, translating into a relative risk reduction of 3.35 (95% CI, 1.13–9.87; P < 0.035). Patients colonized with VRE were 3 times less likely to develop VRE bacteremia when bathed with chlorhexidine compared with regular bathing. Despite the success of this protocol for VRE, when analyzed by individual organism no significant reductions in MRSA acquisition or BSI were reported. This finding is similarly corroborated by a trial conducted in the pediatric ICU setting which found an overall reduction in bacteremia with daily chlorhexidine washes but no significant decrease in cases due to S. aureus [155].
The results of these studies suggest that daily bathing with chlorhexidine should be part of routine practice in health care, especially in ICUs where endemic MRSA or VRE rates are high. Whether there is benefit in other settings needs to be studied.
In addition to chlorhexidine washes, other decolonization techniques have been proposed to reduce colonization and the spread of HAIs in the ICU setting. A randomized controlled trial of daily 5% tea tree oil body washes for the prevention of MRSA colonization failed to significantly reduce rates compared to standard soap body washes [156]. Another proposed decolonization intervention that has not been widely adopted in the United States due to concerns related to development of resistant organisms is selective digestive decontamination (SDD) or selective oropharyngeal decontamination (SOD) with antimicrobial agents [157,158]. In terms of clinical benefit, SDD/SOD have been found to decrease MDRO infection rate [159] and mortality [160].
Cohorting
There is insufficient evidence to conclude that cohorting isolated patients is of benefit for routine use in the endemic ICU setting. A few studies, mainly in the outbreak setting, have examined this approach and the results are conflicting [161,162]. Pending further studies in this area, it is reasonable to cohort patients colonized with the same microorganisms, especially if patients cannot be placed in single rooms.
CONCLUSION
The emergence of MRSA and VRE has led to a resurgence of interest and emphasis on infection control practices and prevention. CDC guidelines to help health care practitioners manage these MDROs in the hospital and ICU-setting exist; however, many questions remain regarding best practice.
Prevention of MRSA and VRE needs to be a 2-pronged approach—antimicrobial stewardship [163] and infection control. A robust antimicrobial stewardship program to optimize and minimize inappropriate antibiotic use is necessary in every institution. From the infection prevention standpoint, it is unclear if systematic identification of MRSA and VRE colonization followed by contact precautions is useful in reducing transmission. It is clear that a strong institutional climate of promoting patient safety and a culture of infection prevention will help in reducing MRSA and VRE facility-wide. It also appears that universal gowning and gloving may be useful for reducing MRSA, but not VRE, transmission. While universal decolonization with mupirocin is efficacious in reducing MRSA, this strategy is not recommended because of promoting mupirocin resistance. However, the use of daily bathing with chlorhexidine represents a relatively low-cost, high-yield intervention that should be adopted. Pending further data, patients known to be colonized or infected with MRSA should be placed in contact precuations as is current practice in most institutions. Finally, in this era of MDROs, hand hygiene remains our best defense against the spread of pathogens in the health care environment.
Note: This article does not represent the views of the Department of Veterans Affairs.
Corresponding author: Nasia Safdar, MD, Willam S. Middleton Memorial Veterans Affairs Hospital, 2500 Overlook Terrace, Madison, WI 53705, [email protected].
Funding/support: This work is funded by a MERIT award from the Department of Veterans Affairs to Nasia Safdar.
Financial disclosures: None.
REFERENCES
1. Burton DC, Edwards JR, Horan TC, et al. Methicillin-resistant Staphylococcus aureus central line-associated bloodstream infections in US intensive care units, 1997–2007. JAMA 2009;301:727–36.
2. LeDell K, Muto CA, Jarvis WR, Farr BM. SHEA guideline for preventing nosocomial transmission of multidrug-resistant strains of Staphylococcus aureus and Enterococcus. Infect Control Hosp Epidemiol 2003;24:639–41.
3. Giske CG, Monnet DL, Cars O, Carmeli Y. Clinical and economic impact of common multidrug-resistant gram-negative bacilli. Antimicrob Agents Chemother 2008;52:813–21.
4. Klevens RM, Edwards JR, Richards CL Jr, et al. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Pub Health Rep 2007;122:160–6.
5. Schwaber MJ, Carmeli Y. The effect of antimicrobial resistance on patient outcomes: importance of proper evaluation of appropriate therapy. Crit Care 2009;13:106.
6. Grundmann H, Hori S, Winter B, et al. Risk factors for the transmission of methicillin-resistant Staphylococcus aureus in an adult intensive care unit: fitting a model to the data. J Inf Dis 2002;185:481–8.
7. Huang SS, Rifas-Shiman SL, Warren DK, et al. Improving methicillin-resistant Staphylococcus aureus surveillance and reporting in intensive care units. J Infect Dis 2007;195:330–8.
8. Muder RR, Cunningham C, McCray E, et al. Implementation of an industrial systems-engineering approach to reduce the incidence of methicillin-resistant Staphylococcus aureus infection. Infect Control Hosp Epidemiol 2008;29:702–8.
9. Hidron AI, Edwards JR, Patel J, et al. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol 2008;29:996–1011.
10. Pelz RK, Lipsett PA, Swoboda SM, et al. Vancomycin-sensitive and vancomycin-resistant enterococcal infections in the ICU: attributable costs and outcomes. Intensive Care Med 2002;28:692–7.
11. Warren DK, Kollef MH, Seiler SM, et al. The epidemiology of vancomycin-resistant Enterococcus colonization in a medical intensive care unit. Infect Control Hosp Epidemiol 2003;24:257–63.
12. Kohlenberg A, Schwab F, Meyer E, et al. Regional trends in multidrug-resistant infections in German intensive care units: a real-time model for epidemiological monitoring and analysis. J Hosp Infect 2009;73:239–45.
13. Deurenberg RH, Stobberingh EE. The evolution of Staphylococcus aureus. Infect Genet Evol 2008;8:747–63.
14. Gordon RJ, Lowy FD. Pathogenesis of methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis 2008;46 Suppl 5:S350–9.
15. Zhang K, McClure JA, Elsayed S, Conly JM. Novel staphylococcal cassette chromosome mec type, tentatively designated type VIII, harboring class A mec and type 4 ccr gene complexes in a Canadian epidemic strain of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 2009;53:531–40.
16. Chatterjee SS, Otto M. Improved understanding of factors driving methicillin-resistant Staphylococcus aureus epidemic waves. Clin Epidemiol 2013;5:205–17.
17. Otto M. MRSA virulence and spread. Cell Microbiol 2012;14:1513–21.
18. Vandenesch F, Naimi T, Enright MC, et al. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg Infect Dis 2003;9:978–84.
19. Li M, Diep BA, Villaruz AE, et al. Evolution of virulence in epidemic community-associated methicillin-resistant Staphylococcus aureus. Proc Natl Acad Sci U S A 2009;106:5883–8.
20. Courvalin P. Vancomycin resistance in gram-positive cocci. Clin Infect Dis 2006;42 Suppl 1:S25–34.
21. Gold HS. Vancomycin-resistant enterococci: mechanisms and clinical observations. Clin Infect Dis 2001;33:210–9.
22. Arias CA, Murray BE. The rise of the Enterococcus: beyond vancomycin resistance. Nat Rev Microbiol 2012;10:266–78.
23. Adcock PM, Pastor P, Medley F, Patterson JE, Murphy TV. Methicillin-resistant Staphylococcus aureus in two child care centers. J Infect Dis 1998;178:577–80.
24. Dietrich DW, Auld DB, Mermel LA. Community-acquired methicillin-resistant Staphylococcus aureus in southern New England children. Pediatrics 2004;113:e347–52.
25. Groom AV, Wolsey DH, Naimi TS, et al. Community-acquired methicillin-resistant Staphylococcus aureus in a rural American Indian community. JAMA 2001;286:1201–5.
26. Hewlett AL, Falk PS, Hughes KS, Mayhall CG. Epidemiology of methicillin-resistant Staphylococcus aureus in a university medical center day care facility. Infect Control Hosp Epidemiol 2009;30:985–92.
27. Kazakova SV, Hageman JC, Matava M, et al. A clone of methicillin-resistant Staphylococcus aureus among professional football players. N Engl J Med 2005;352:468–75.
28. Landrum ML, Neumann C, Cook C, et al. Epidemiology of Staphylococcus aureus blood and skin and soft tissue infections in the US military health system, 2005-2010. JAMA 2012;308:50–9.
29. Lindenmayer JM, Schoenfeld S, O’Grady R, Carney JK. Methicillin-resistant Staphylococcus aureus in a high school wrestling team and the surrounding community. Arch Intern Med 1998;158:895–9.
30. Malcolm B. The rise of methicillin-resistant staphylococcus aureus in U.S. correctional populations. J Correct Health Care 2011;17:254–65.
31. Nerby JM, Gorwitz R, Lesher L, et al. Risk factors for household transmission of community-associated methicillin-resistant Staphylococcus aureus. Pediatr Infect Dis J 2011;30:927–32.
32. Stemper ME, Shukla SK, Reed KD. Emergence and spread of community-associated methicillin-resistant Staphylococcus aureus in rural Wisconsin, 1989 to 1999. J Clin Microbiol 2004;42:5673–80.
33. Turabelidze G, Lin M, Wolkoff B, et al. Personal hygiene and methicillin-resistant Staphylococcus aureus infection. Emerg Infect Dis 2006;12:422–7.
34. Ellis MW, Hospenthal DR, Dooley DP, et al. Natural history of community-acquired methicillin-resistant Staphylococcus aureus colonization and infection in soldiers. Clin Infect Dis 2004;39:971–9.
35. Forster AJ, Oake N, Roth V, et al. Patient-level factors associated with methicillin-resistant Staphylococcus aureus carriage at hospital admission: a systematic review. Am J Infect Control 2013;41:214–20.
36. McKinnell JA, Miller LG, Eells SJ, et al. A systematic literature review and meta-analysis of factors associated with methicillin-resistant staphylococcus aureus colonization at time of hospital or intensive care unit admission. Infect Contol Hosp Epidemiol 2013;34:1077–86.
37. Furuno JP, McGregor JC, Harris AD, et al. Identifying groups at high risk for carriage of antibiotic-resistant bacteria. Arch Intern Med 2006;166:580–5.
38. Jernigan JA, Pullen AL, Flowers L, et al. Prevalence of and risk factors for colonization with methicillin-resistant Staphylococcus aureus at the time of hospital admission. Infect Control Hosp Epidemiol 2003;24:409–14.
39. Horner C, Parnell P, Hall D, Kearns A, Heritage J, Wilcox M. Meticillin-resistant Staphylococcus aureus in elderly residents of care homes: colonization rates and molecular epidemiology. J Hosp Infect 2013;83:212–8.
40. Allegranzi B, Pittet D. Role of hand hygiene in healthcare-associated infection prevention. J Hosp Infect 2009;73:305–15.
41. Boyce JM. Methicillin-resistant Staphylococcus aureus. Detection, epidemiology, and control measures. Infect Dis Clin North Am 1989;3:901–13.
42. Jernigan JA. Methicillin-resistant Staphylococcus aureus colonization among health care personnel in the emergency department: what does it tell us? Ann Emerg Med 2008;52:534–6.
43. Carnicer-Pont D, Bailey KA, Mason BW, Walker AM, Evans MR, Salmon RL. Risk factors for hospital-acquired methicillin-resistant Staphylococcus aureus bacteraemia: a case-control study. Epidemiol Infect 2006;134:1167–73.
44. Graffunder EM, Venezia RA. Risk factors associated with nosocomial methicillin-resistant Staphylococcus aureus (MRSA) infection including previous use of antimicrobials. J Antimicrob Chemother 2002;49:999–1005.
45. Thompson RL, Cabezudo I, Wenzel RP. Epidemiology of nosocomial infections caused by methicillin-resistant Staphylococcus aureus. Ann Intern Med 1982;97:309–17.
46. Davis KA, Stewart JJ, Crouch HK,et al. Methicillin-resistant Staphylococcus aureus (MRSA) nares colonization at hospital admission and its effect on subsequent MRSA infection. Clin Infect Dis 2004;39:776–82.
47. Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev 1997;10:505–20.
48. Safdar N, Bradley EA. The risk of infection after nasal colonization with Staphylococcus aureus. Am J Med 2008;121:310–5.
49. Wertheim HFL, Vos MC, Ott A, et al. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet 2004;364:703–5.
50. Garrouste-Orgeas M, Timsit JF, Kallel H, et al. Colonization with methicillin-resistant Staphylococcus aureus in ICU patients: morbidity, mortality, and glycopeptide use. Infect Control Hosp Epidemiol 2001;22:687–92.
51. Honda H, Krauss MJ, Coopersmith CM, et al. Staphylococcus aureus nasal colonization and subsequent infection in intensive care unit patients: does methicillin resistance matter? Infect Control Hosp Epidemiol;31:584–91.
52. von Eiff C, Becker K, Machka K, et al. Nasal carriage as a source of Staphylococcus aureus bacteremia. N Engl J Med 2001;344:11–6.
53. Pujol M, Pea C, Pallares R, et al. Nosocomial Staphylococcus aureus bacteremia among nasal carriers of methicillin-resistant and methicillin-susceptible strains. Am J Med 1996;100:509–16.
54. Rocha LA, Marques Ribas R, da Costa Darini AL, Gontijo Filho PP. Relationship between nasal colonization and ventilator-associated pneumonia and the role of the environment in transmission of Staphylococcus aureus in intensive care units. Am J Infect Control 2013;41:236–40.
55. Corne P, Marchandin Hln, Jonquet O, Campos J, Bauls A-L. Molecular evidence that nasal carriage of Staphylococcus aureus plays a role in respiratory tract infections of critically ill patients. J Clin Microbiol 2005;43:3491–3.
56. Quezada Joaquin NM, Diekema DJ, Perencevich EN, et al. Long-term risk for readmission, methicillin-resistant Staphylococcus aureus (MRSA) infection, and death among MRSA-colonized veterans. Antimicrob Agents Chemother 2013;57:1169–72.
57. Lin MY, Hayden MK. Methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococcus: recognition and prevention in intensive care units. Crit Care Med 2010;38:S335–44.
58. Carmeli Y, Eliopoulos GM, Samore MH. Antecedent treatment with different antibiotic agents as a risk factor for vancomycin-resistant Enterococcus. Emerg Infect Dis 2002;8:802–7.
59. Ostrowsky BE, Venkataraman L, D’Agata EM, et al. Vancomycin-resistant enterococci in intensive care units: high frequency of stool carriage during a non-outbreak period. Arch Intern Med 1999;159:1467–72.
60. Bonten MJ, Hayden MK, Nathan C, et al. Epidemiology of colonisation of patients and environment with vancomycin-resistant enterococci. Lancet 1996;348:1615–9.
61. Ostrowsky BE, Trick WE, Sohn AH, et al. Control of vancomycin-resistant enterococcus in health care facilities in a region. N Engl J Med 2001;344:1427–33.
62. Padiglione AA, Wolfe R, Grabsch EA, et al. Risk factors for new detection of vancomycin-resistant enterococci in acute-care hospitals that employ strict infection control procedures. Antimicrob Agents Chemother 2003;47:2492–8.
63. Batistao DW, Gontijo-Filho PP, Conceicao N, et al. Risk factors for vancomycin-resistant enterococci colonisation in critically ill patients. Mem Inst Oswaldo Cruz 2012;107:57–63.
64. Furtado GH, Martins ST, Coutinho AP, et al. Prevalence and factors associated with rectal vancomycin-resistant enterococci colonization in two intensive care units in Sao Paulo, Brazil. Braz J Infect Dis 2005;9:64–9.
65. Huang SS, Datta R, Rifas-Shiman S, et al. Colonization with antibiotic-susceptible strains protects against methicillin-resistant Staphylococcus aureus but not vancomycin-resistant enterococci acquisition: a nested case-control study. Crit Care 2011;15:R210.
66. Pan SC, Wang JT, Chen YC, et al. Incidence of and risk factors for infection or colonization of vancomycin-resistant enterococci in patients in the intensive care unit. PLoS One 2012;7:e47297.
67. Se YB, Chun HJ, Yi HJ, et al. Incidence and risk factors of infection caused by vancomycin-resistant enterococcus colonization in neurosurgical intensive care unit patients. J Korean Neurosurg Soc 2009;46:123–9.
68. Healthcare Infection Control Practices Advisory Committee (HICPAC). Management of multidrug-resistant organisms in healthcare settings, 2006. Accessed 11 Oct 2013 at www.cdc.gov/hicpac/mdro/mdro_toc.html.
69. Malani PN. Preventing infections in the ICU: one size does not fit all. JAMA 2013;310:1567–8.
70. Recommendations for preventing the spread of vancomycin resistance. Recommendations of the Hospital Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep 1995;44:1–13.
71. de Bruin MA, Riley LW. Does vancomycin prescribing intervention affect vancomycin-resistant enterococcus infection and colonization in hospitals? A systematic review. BMC Infect Dis 2007;7:24.
72. Adachi W, Bolding F, Armstrong R. Experience with vancomycin education and order sheet to limit vancomycin use. Hosp Pharm 1997:1370–3.
73. Fridkin SK, Lawton R, Edwards JR, et al. Monitoring antimicrobial use and resistance: comparison with a national benchmark on reducing vancomycin use and vancomycin-resistant enterococci. Emerg Infect Dis 2002;8:702–7.
74. Guglielmo BJ, Dudas V, Maewal I, et al. Impact of a series of interventions in vancomycin prescribing on use and prevalence of vancomycin-resistant enterococci. Jt Comm J Qual Patient Saf 2005;31:469–75.
75. Lautenbach E, LaRosa LA, Marr AM, et al. Changes in the prevalence of vancomycin-resistant enterococci in response to antimicrobial formulary interventions: impact of progressive restrictions on use of vancomycin and third-generation cephalosporins. Clin Infect Dis 2003;36:440–6.
76. Morgan AS, Brennan PJ, Fishman NO. Impact of a vancomycin restriction policy on use and cost of vancomycin and incidence of vancomycin-resistant Enterococcus. Ann Pharmacother 1997;31:970–3.
77. Anglim AM, Klym B, Byers KE, et al. Effect of a vancomycin restriction policy on ordering practices during an outbreak of vancomycin-resistant Enterococcus faecium. Arch Intern Med 1997;157:1132–6.
78. Montecalvo MA, Jarvis WR, Uman J, et al. Infection-control measures reduce transmission of vancomycin-resistant enterococci in an endemic setting. Ann Intern Med 1999;131:269–72.
79. Morris JG Jr, Shay DK, Hebden JN, et al. Enterococci resistant to multiple antimicrobial agents, including vancomycin. Establishment of endemicity in a university medical center. Ann Intern Med 1995;123:250–9.
80. Quale J, Landman D, Saurina G, et al. Manipulation of a hospital antimicrobial formulary to control an outbreak of vancomycin-resistant enterococci. Clin Infect Dis 1996;23:1020-5.
81. Rubin LG, Tucci V, Cercenado E, et al. Vancomycin-resistant Enterococcus faecium in hospitalized children. Infect Control Hosp Epidemiol 1992;13:700–5.
82. Lai KK, Kelley AL, Melvin ZS, et al. Failure to eradicate vancomycin-resistant enterococci in a university hospital and the cost of barrier precautions. Infect Control Hosp Epidemiol 1998;19:647–52.
83. Shaikh ZH, Osting CA, Hanna HA, et al. Effectiveness of a multifaceted infection control policy in reducing vancomycin usage and vancomycin-resistant enterococci at a tertiary care cancer centre. J Hosp Infect 2002;51:52–8.
84. Lafaurie M, Porcher R, Donay JL, et al. Reduction of fluoroquinolone use is associated with a decrease in methicillin-resistant Staphylococcus aureus and fluoroquinolone-resistant Pseudomonas aeruginosa isolation rates: a 10 year study. J Antimicrob Chemother 2012;67:1010–5.
85. Parienti JJ, Cattoir V, Thibon P, et al. Hospital-wide modification of fluoroquinolone policy and meticillin-resistant Staphylococcus aureus rates: a 10-year interrupted time-series analysis. J Hosp Infect 2011;78:118–22.
86. Safdar N, Abad C. Educational interventions for prevention of healthcare-associated infection: a systematic review. Crit Care Med 2008;36:933–40.
87. Boyce JM. It is time for action: improving hand hygiene in hospitals. Ann Intern Med 1999;130:153–5.
88. Jackson M, Chiarello LA, Gaynes RP, Gerberding JL. Nurse staffing and healthcare-associated infections: proceedings from a working group meeting. J Nurs Adm 2002;32:314–22.
89. Kuzu N, Ozer F, Aydemir S, et al. Compliance with hand hygiene and glove use in a university-affiliated hospital. Infect Control Hosp Epidemiol 2005;26:312–5.
90. Larson E, Killien M. Factors influencing handwashing behavior of patient care personnel. Am J Infect Control 1982;10:93–9.
91. Larson E, Kretzer EK. Compliance with handwashing and barrier precautions. J Hosp Infect 1995;30 Suppl:88–106.
92. Naikoba S, Hayward A. The effectiveness of interventions aimed at increasing handwashing in healthcare workers - a systematic review. J Hosp Infect 2001;47:173–80.
93. Pittet D, Simon A, Hugonnet S, et al. Hand hygiene among physicians: performance, beliefs, and perceptions. Ann Intern Med 2004;141:1–8.
94. Trick WE, Vernon MO, Welbel SF, et al. Multicenter intervention program to increase adherence to hand hygiene recommendations and glove use and to reduce the incidence of antimicrobial resistance. Infect Control Hosp Epidemiol 2007;28:42–9.
95. Wisniewski MF, Kim S, Trick WE, et al. Effect of education on hand hygiene beliefs and practices: a 5-year program. Infect Control Hosp Epidemiol 2007;28:88–91.
96. Rupp ME, Fitzgerald T, Puumala S, et al. Prospective, controlled, cross-over trial of alcohol-based hand gel in critical care units. Infect Control Hosp Epidemiol 2008;29:8–15.
97. Venkatesh AK, Lankford MG, Rooney DM, et al. Use of electronic alerts to enhance hand hygiene compliance and decrease transmission of vancomycin-resistant Enterococcus in a hematology unit. Am J Infect Control 2008;36:199–205.
98. Silvestri L, Petros AJ, Sarginson RE, et al. Handwashing in the intensive care unit: a big measure with modest effects. J Hosp Infect 2005;59:172–9.
99. Akyol A, Ulusoy H, Ozen I. Handwashing: a simple, economical and effective method for preventing nosocomial infections in intensive care units. J Hosp Infect 2006;62:395–405.
100. Simmons B, Bryant J, Neiman K, et al. The role of handwashing in prevention of endemic intensive care unit infections. Infect Control Hosp Epidemiol 1990;11:589–94.
101. Tenorio AR, Badri SM, Sahgal NB, et al. Effectiveness of gloves in the prevention of hand carriage of vancomycin-resistant enterococcus species by health care workers after patient care. Clin Infect Dis 2001;32:826–9.
102. Slaughter S, Hayden MK, Nathan C, et al. A comparison of the effect of universal use of gloves and gowns with that of glove use alone on acquisition of vancomycin-resistant enterococci in a medical intensive care unit. Ann Intern Med 1996;125:448–56.
103. Harris AD, Pineles L, Belton B, et al. Universal glove and gown use and acquisition of antibiotic-resistant bacteria in the ICU: a randomized trial. JAMA 2013;310:1571–80.
104. Dietze B, Rath A, Wendt C, Martiny H. Survival of MRSA on sterile goods packaging. J Hosp Infect 2001;49:255–61.
105. Hardy KJ, Oppenheim BA, Gossain S, et al. A study of the relationship between environmental contamination with methicillin-resistant Staphylococcus aureus (MRSA) and patients’ acquisition of MRSA. Infect Control Hosp Epidemiol 2006;27:127–32.
106. Jawad A, Heritage J, Snelling AM, et al. Influence of relative humidity and suspending menstrua on survival of Acinetobacter spp. on dry surfaces. J Clin Microbiol 1996;34:2881–7.
107. Boyce JM, Havill NL, Otter JA, Adams NM. Widespread environmental contamination associated with patients with diarrhea and methicillin-resistant Staphylococcus aureus colonization of the gastrointestinal tract. Infect Control Hosp Epidemiol 2007;28:1142–7.
108. Boyce JM, Potter-Bynoe G, Chenevert C, King T. Environmental contamination due to methicillin-resistant Staphylococcus aureus: possible infection control implications. Infect Control Hosp Epidemiol 1997;18:622–7.
109. Sexton T, Clarke P, O’Neill E, et al. Environmental reservoirs of methicillin-resistant Staphylococcus aureus in isolation rooms: correlation with patient isolates and implications for hospital hygiene. J Hosp Infect 2006;62:187–94.
110. Dancer SJ. Importance of the environment in meticillin-resistant Staphylococcus aureus acquisition: the case for hospital cleaning. Lancet infect dis 2008;8:101–13.
111. Dancer SJ, White LF, Lamb J, et al. Measuring the effect of enhanced cleaning in a UK hospital: a prospective cross-over study. BMC med 2009;7.
112. Rampling A, Wiseman S, Davis L, et al. Evidence that hospital hygiene is important in the control of methicillin-resistant Staphylococcus aureus. J Hosp Infect 2001;49:109–16.
113. Wilson APR, Smyth D, Moore G, et al. The impact of enhanced cleaning within the intensive care unit on contamination of the near-patient environment with hospital pathogens: a randomized crossover study in critical care units in two hospitals. Crit Care Med 2011;39:651–8.
114. Hess AS, Shardell M, Johnson JK, et al. A randomized controlled trial of enhanced cleaning to reduce contamination of healthcare worker gowns and gloves with multidrug-resistant bacteria. Infection Control Hosp Epidemiol 2013;34:487–93.
115. Falk PS, Winnike J, Woodmansee C, et al. Outbreak of vancomycin-resistant enterococci in a burn unit. Infect Control Hosp Epidemiol 2000;21:575–82.
116. Livornese LL Jr, Dias S, Samel C, et al. Hospital-acquired infection with vancomycin-resistant Enterococcus faecium transmitted by electronic thermometers. Ann Intern Med 1992;117:112–6.
117. Porwancher R, Sheth A, Remphrey S, et al. Epidemiological study of hospital-acquired infection with vancomycin-resistant Enterococcus faecium: possible transmission by an electronic ear-probe thermometer. Infect Control Hosp Epidemiol 1997;18:771–3.
118. Donskey CJ, Chowdhry TK, Hecker MT, et al. Effect of antibiotic therapy on the density of vancomycin-resistant enterococci in the stool of colonized patients. N Engl J Med 2000;343:1925–32.
119. Neely AN, Maley MP. Survival of enterococci and staphylococci on hospital fabrics and plastic. J Clin Microbiol 2000;38:724–6.
120. Noskin GA, Bednarz P, Suriano T, et al. Persistent contamination of fabric-covered furniture by vancomycin-resistant enterococci: implications for upholstery selection in hospitals. Am J Infect Control 2000;28:311–3.
121. Noskin GA, Stosor V, Cooper I, Peterson LR. Recovery of vancomycin-resistant enterococci on fingertips and environmental surfaces. Infect Control Hosp Epidemiol 1995;16:577–81.
122. Smith TL, Iwen PC, Olson SB, Rupp ME. Environmental contamination with vancomycin-resistant enterococci in an outpatient setting. Infect Control Hosp Epidemiol 1998;19:515–8.
123. Wendt C, Wiesenthal B, Dietz E, Ruden H. Survival of vancomycin-resistant and vancomycin-susceptible enterococci on dry surfaces. J Clin Microbiol 1998;36:3734–6.
124. Bhalla A, Pultz NJ, Gries DM, et al. Acquisition of nosocomial pathogens on hands after contact with environmental surfaces near hospitalized patients. Infect Control Hosp Epidemiol 2004;25:164–7.
125. Ray AJ, Hoyen CK, Taub TF, et al. Nosocomial transmission of vancomycin-resistant enterococci from surfaces. JAMA 2002;287:1400–1.
126. Hayden MK, Bonten MJ, Blom DW, et al. Reduction in acquisition of vancomycin-resistant enterococcus after enforcement of routine environmental cleaning measures. Clin Infect Dis 2006;42:1552–60.
127. Byers KE, Durbin LJ, Simonton BM, et al. Disinfection of hospital rooms contaminated with vancomycin-resistant Enterococcus faecium. Infect Control Hosp Epidemiol 1998;19:261–4.
128. Goodman ER, Platt R, Bass R, et al. Impact of an environmental cleaning intervention on the presence of methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci on surfaces in intensive care unit rooms. Infect Control Hosp Epidemiol 2008;29:593–9.
129. Dancer SJ. The role of environmental cleaning in the control of hospital-acquired infection. J Hosp Infect 2009;73:378–85.
130. Centers for Disease Control and Prevention. Methicillin-resistant Staphylococcus aureus (MRSA) infections. Accessed 11 Oct 2013 at www.cdc.gov/mrsa/index.html.
131. Edmond MB, Wenzel RP. Targeted decolonization to prevent ICU infections. N Engl J Med 2013;369:1471.
132. Lai KK, Fontecchio S, Melvin Z, Baker SP. Impact of alcohol-based, waterless hand antiseptic on the incidence of infection and colonization with methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci. Infect Control Hosp Epidemiol 2006;27:1018–24.
133. Ostrowsky B, Steinberg JT, Farr B, et al. Reality check: should we try to detect and isolate vancomycin-resistant enterococci patients? Infect Control Hosp Epidemiol 2001;22:116–9.
134. Harbarth S, Sax H, Uckay I, et al. A predictive model for identifying surgical patients at risk of methicillin-resistant Staphylococcus aureus carriage on admission. J Am Coll Surg 2008;207:683–9.
135. Jarvis WR. Targeted decolonization to prevent ICU infections. N Engl J Med 2013;369:1469.
136. Krause R, Honigl M, Zollner-Schwetz I. Targeted decolonization to prevent ICU infections. N Engl J Med;369:1469–70.
137. Huskins WC, Huckabee CM, O’Grady NP, et al. Intervention to reduce transmission of resistant bacteria in intensive care. N Engl J Med;364:1407–18.
138. Robicsek A, Beaumont JL, Paule SM, et al. Universal surveillance for methicillin-resistant Staphylococcus aureus in 3 affiliated hospitals. Ann Intern Med 2008;148:409–18.
139. Jain R, Kralovic SM, Evans ME, et al. Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. N Engl J Med 2011;364:1419–30.
140. Gurieva T, Bootsma MCJ, Bonten MJM. Successful Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections revisited. Clin Infect Dis 2012:54:1618–20.
141. Gavalda L, Masuet C, Beltran J, et al. Comparative cost of selective screening to prevent transmission of methicillin-resistant Staphylococcus aureus (MRSA), compared with the attributable costs of MRSA infection. Infection control and hospital epidemiology 2006;27:1264–6.
142. Otter JA, Herdman MT, Williams B, et al. Low prevalence of methicillin-resistant Staphylococcus aureus carriage at hospital admission: implications for risk-factor-based vs universal screening. J Hosp Infect 2013;83:114–21.
143. Harbarth S, Hawkey PM, Tenover F, et al. Update on screening and clinical diagnosis of methicillin-resistant Staphylococcus aureus (MRSA). Int J Antimicrob Agents 2011;37:110–7.
144. Calfee DP, Giannetta ET, Durbin LJ, et al. Control of endemic vancomycin-resistant Enterococcus among inpatients at a university hospital. Clin Infect Dis 2003;37:326–32.
145. Hendrix CW, Hammond JM, Swoboda SM, et al. Surveillance strategies and impact of vancomycin-resistant enterococcal colonization and infection in critically ill patients. Ann Surg 2001;233:259–65.
146. Muto CA, Giannetta ET, Durbin LJ, et al. Cost-effectiveness of perirectal surveillance cultures for controlling vancomycin-resistant Enterococcus. Infect Control Hosp Epidemiol 2002;23:429–35.
147. Price CS, Paule S, Noskin GA, Peterson LR. Active surveillance reduces the incidence of vancomycin-resistant enterococcal bacteremia. Clin Infect Dis 2003;37:921–8.
148. Shadel BN, Puzniak LA, Gillespie KN, et al. Surveillance for vancomycin-resistant enterococci: type, rates, costs, and implications. Infect Control Hosp Epidemiol 2006;27:1068–75.
149. Siddiqui AH, Harris AD, Hebden J, et al. The effect of active surveillance for vancomycin-resistant enterococci in high-risk units on vancomycin-resistant enterococci incidence hospital-wide. Am J Infect Control 2002;30:40–3.
150. Sandri AM, Dalarosa MG, Ruschel de Alcantara L, et al. Reduction in incidence of nosocomial methicillin-resistant Staphylococcus aureus (MRSA) infection in an intensive care unit: role of treatment with mupirocin ointment and chlorhexidine baths for nasal carriers of MRSA. Infect Control Hosp Epidemiol 2006;27:185–7.
151. Huang SS, Septimus E, Kleinman K, et al. Targeted versus universal decolonization to prevent ICU infection. N Engl J Med 2013;368:2255–65.
152. Bleasdale SC, Trick WE, Gonzalez IM, et al. Effectiveness of chlorhexidine bathing to reduce catheter-associated bloodstream infections in medical intensive care unit patients. Arch Intern Med 2007;167:2073–9.
153. Climo MW, Sepkowitz KA, Zuccotti G, et al. The effect of daily bathing with chlorhexidine on the acquisition of methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus, and healthcare-associated bloodstream infections: results of a quasi-experimental multicenter trial. Crit Care Med 2009;37:1858–65.
154. Vernon MO, Hayden MK, Trick WE, et al. Chlorhexidine gluconate to cleanse patients in a medical intensive care unit: the effectiveness of source control to reduce the bioburden of vancomycin-resistant enterococci. Arch Intern Med 2006;166:306–12.
155. Milstone AM, Elward A, Song X, et al. Daily chlorhexidine bathing to reduce bacteraemia in critically ill children: a multicentre, cluster-randomised, crossover trial. Lancet 2013;381:1099–106.
156. Blackwood B, Thompson G, McMullan R, et al. Tea tree oil (5%) body wash versus standard care (Johnson’s Baby Softwash) to prevent colonization with methicillin-resistant Staphylococcus aureus in critically ill adults: a randomized controlled trial. J Antimicrob Chemother 2013;68:1193–9.
157. Daneman N, Sarwar S, Fowler RA, et al. Effect of selective decontamination on antimicrobial resistance in intensive care units: a systematic review and meta-analysis. Lancet Infect Dis 2013;13:328–41.
158. Verwaest C, Verhaegen J, Ferdinande P, et al. Randomized, controlled trial of selective digestive decontamination in 600 mechanically ventilated patients in a multidisciplinary intensive care unit. Crit Care Med 1997;25:63–71.
159. de Smet AMGA, Kluytmans JAJW, Blok HEM, et al. Selective digestive tract decontamination and selective oropharyngeal decontamination and antibiotic resistance in patients in intensive-care units: an open-label, clustered group-randomised, crossover study. Lancet Infect Dis 2011;11:372–80.
160. de Jonge E, Schultz MJ, Spanjaard L, et al. Effects of selective decontamination of digestive tract on mortality and acquisition of resistant bacteria in intensive care: a randomised controlled trial. Lancet 2003;362:1011–6.
161. Cepeda JA, Whitehouse T, Cooper B, et al. Isolation of patients in single rooms or cohorts to reduce spread of MRSA in intensive-care units: prospective two-centre study. Lancet 2005;365:295–304.
162. Dhaliwal J, McGeer A. Does isolation prevent the spread of methicillin-resistant Staphylococcus aureus? CMAJ 2005;172:875.
163. Kollef MH, Micek ST. Antimicrobial stewardship programs: mandatory for all ICUs. Crit Care 2012;16:179.
164. McKinnell JA, Huang SS, Eells SJ, et al. Quantifying the impact of extranasal testing of body sites for methicillin-resistant Staphylococcus aureus colonization at the time of hospital or intensive care unit admission. Infect Control Hosp Epidemiol 2013;34:161–70.
165. Denkinger CM, Grant AD, Denkinger M, et al. Increased multi-drug resistance among the elderly on admission to the hospital—a 12-year surveillance study. Arch Gerontol Geriatr 2013;56:227–30.
166. Boisseau D, Alfandari S, Gauzit R, et al. Staphylococcus aureus nasal carriage during the infectious diseases national congress in France. Med Mal Infect 2012;42:435–9.
167. Fritz SA, Hogan PG, Hayek G, et al. Staphylococcus aureus colonization in children with community-associated Staphylococcus aureus skin infections and their household contacts. Arch Pediatr Adolesc Med 2012;166:551–7.
168. Rafee Y, Abdel-Haq N, Asmar B, et al. Increased prevalence of methicillin-resistant Staphylococcus aureus nasal colonization in household contacts of children with community acquired disease. BMC Infect Dis 2012;12:45.
169. Schechter-Perkins EM, Mitchell PM, Murray KA, et al. Prevalence and predictors of nasal and extranasal staphylococcal colonization in patients presenting to the emergency department. Ann Emerg Med 2011;57:492–9.
170. Bisaga A, Paquette K, Sabatini L, Lovell E. A prevalence study of methicillin-resistant staphylococcus aureus colonization in emergency department health care workers. Ann Emerg Med 2008;52:525–8.
171. Suffoletto B, Cannon E, Ilkhanipour K, Yealy D. Prevalence of Staphylococcus aureus nasal colonization in emergency department personnel. Ann Emerg Med 2008;52:529–33.
172. Young DM, Harris HW, Charlebois ED, et al. An epidemic of methicillin-resistant Staphylococcus aureus soft tissue infections among medically underserved patients. Arch Surg 2004;139:947-51; discussion 51–3.
173. Salgado CD, Farr BM, Calfee DP. Community-acquired methicillin-resistant Staphylococcus aureus: a meta-analysis of prevalence and risk factors. Clin Infect Dis 2003;36:131–9.
Pain, Anxiety, and Dementia: A Catastrophic Outcome
Advanced psychiatric illness and dementia create a wide range of barriers to health care. These patients are unable to provide reliable details with respect to their illness or even discuss basic features of their medical history, forcing providers to rely on contributions from caregiver reports and medical records. Confounding the limits on medical information, physical examinations are often abbreviated or completely refused because of the patient’s distrust, discomfort, or delusion. Over time, the involvement of consulting services may amplify the impact of these barriers as the need for diagnostic and therapeutic interventions emerge. Meanwhile, this delay in definitive management opens a window of risk for deterioration, in which patients cannot be relied on to report important clinical changes.
This case report describes a patient with significant cognitive dysfunction who developed a rare and devastating complication of a hematologic disorder. As the case illustrates, transferring a patient from the psychiatric ward to Internal Medicine (IM) can create unique diagnostic and management challenges.
CASE REPORT
A 64-year-old man developed hematochezia after having been hospitalized in a locked psychiatric ward for the preceding 6 months following a suicide attempt. The episode of hematochezia occurred while on anticoagulation treatment with warfarin for chronic lower extremity deep venous thrombosis (DVT), which prompted the IM consultation. The patient’s past medical history was notable for dementia, hypothyroidism, Crohn disease, and primary sclerosing cholangitis.
The IM Consult Service recommended holding anticoagulation therapy and reversing the coagulopathy with vitamin K. The patient’s stool returned hemoccult and toxin positive for Clostridium difficile (C difficile). The hematochezia was attributed to the infection with C difficile in the setting of anticoagulation. Oral metronidazole was started. Hemoglobin remained stable without further episodes of bleeding. Seven days after the episode of hematochezia, the patient experienced worsening generalized pain and new skin findings. He was transferred to the general medical ward for further management.
The patient’s medical records revealed early cognitive decline with recommendations for supervised residential care as early as age 59 years. An extensive neurocognitive assessment indicated a diagnosis of semantic dementia. He also had a history of recurrent DVT with anticoagulation therapy for > 10 years with no prior workup for a hypercoagulable state. A recent baseline mental status report described a childlike demeanor, profound global speech deficits with marked difficulty understanding even basic medical concepts (eg, the need for a peripheral intravenous catheter), and generalized anxiety disorder complicated by hyperesthesia. The patient frequently refused physical examinations and blood draws as a result. He devoted himself to simple puzzles of kittens and puppies.
Vital signs were normal as were the head and neck, pulmonary, cardiac, and abdominal examinations. The patient’s neurocognitive examination was remarkable for his dependence on instrumental activities of daily living, global aphasia, impaired short- and long-term recall, and poor judgment. He scored 21 out of 30 on a recent mini-mental state examination: failure to achieve 3-word recall; disorientation to month, season, hospital, and county; and an inability to write a sentence or identify a pen. Otherwise, he had fluent speech, facial symmetry, intact strength and sensation throughout, and normal reflexes.
A skin examination revealed diffuse tender subcutaneous lesions. The largest lesion was about 5 cm, located in the left anterolateral thigh. Smaller lesions of about 1 cm were noted in the abdominal wall, right thigh, and bilateral upper extremities. An exquisitely tender, well-demarcated 20-cm elliptical lesion with central necrosis and an erythematous border developed in the left axilla the following day (Figure 1A). Pain limited adduction of the left arm.
The initial laboratory evaluation demonstrated a stable hemoglobin level of 11.1 g/dL, a platelet count of 128 k/mL, and no leukocytosis. Electrolytes and renal indexes were normal. D-dimer and fibrin split products were > 10,000 ng/mL and 20 mg/mL, respectively. Fibrinogen level was 351 mg/dL. The prothrombin time and international normalized ratio were 15.1 seconds and 1.4, respectively. The activated partial thromboplastin time (aPTT) was measured at 40 seconds. High sensitivity C-reactive protein was 4.59. Recent head imaging included a brain magnetic resonance imaging (MRI) notable for enlarged sulci and ventricles with temporal predominance. Positron emission tomography (PET) brain imaging was significant for diffuse hypometabolism in bilateral parietal and temporal lobes with preservation of sensorimotor and occipital cortexes. There was no clear radiographic evidence of cerebral embolic phenomenon or focal cerebrovascular events.
Enoxaparin treatment was initiated for a suspected hypercoagulable state. Ceftriaxone was administered for a urinary tract infection (UTI). Despite premedication, the bedside biopsy of his necrotic skin lesion was aborted due to severe anxiety and generalized somatic pain. A surgical excisional biopsy was thus obtained under general anesthesia. Enoxaparin was held the night before and the morning of surgery. There were no immediate complications related to the biopsy, and malignancy was not seen on intraoperative frozen sections.
Generalized somatic pain persisted the morning after the surgical biopsy, but the patient remained clinically unchanged. An hour later, he was found unresponsive with no pulse. Despite extensive resuscitative efforts, the patient died.
Postmortem
There was a high index of suspicion for a hemostatic perturbation given the skin findings and recent manipulation of anticoagulation with a prior thrombotic event. The axillary lesion closely resembled warfarin-related skin necrosis. Management included enoxaparin with supportive care, pending definitive pathologic findings.
Postmortem examination confirmed diffuse multiorgan involvement similar to the process seen in the thigh biopsy. Ischemic injury secondary to small vessel microthrombi were evident in the skin, subcutaneous fat, large bowel, urinary bladder, and associated pericystic fat (Figure 1B). Interpretation of the surgical thigh biopsy became available after the patient died. It demonstrated infarcted fat with fat necrosis and hemorrhage (Figure 2).
The results of the laboratory investigations for thrombophilia also came back after the patient died. A potent lupus anticoagulant (LA) was demonstrated. It manifested primarily in the intrinsic pathway as a strongly positive LA-sensitive-aPTT (delta time = 20.5 seconds) assay with a weakly positive dilute Russell’s viper venom time assay. The antigenic specificity of the LA antibodies was not uncovered, as the plasma levels of both IgM and IgG anticardiolipin and anti-Β2-glycoprotein-I antibodies were within the reference range. Factor (F) II and FV genotyping revealed wild-type FV, and the prothrombin gene G20210A was without mutation.
Assays for plasma levels of protein S and antithrombin activity were also normal, which excluded deficiencies in these proteins. The assay for protein C activity was slightly decreased. This may have exacerbated the hemostatic imbalance caused by the LA, as the FVII level had normalized. However, the etiology of the protein C deficiency is not clear. Considerations include (1) a warfarin disequilibrium state due to the discontinuation of oral anticoagulation and institution of vitamin K therapy; (2) an epiphenomenon resulting from active thromboses; or (3) a possible hereditary protein C deficiency.
The definitive diagnosis of the catastrophic antiphospholipid antibody (APA) syndrome relies on multiorgan failure in < 1 week, histopathologic evidence of small vessel thrombosis, and a positive LA.1 The study patient fulfilled these criteria (Table).
DISCUSSION
Catastrophic progression of APA syndrome is an infrequent and devastating complication of this autoimmune disorder with a mortality rate of nearly 50%.1 Antiphospholipid antibody syndrome typically presents with thromboses of the larger vessels, and it more commonly affects the venous system. In contrast, diffuse small vessel thromboses underlie the pathogenesis of catastrophic APA syndrome (CAPS).2 This catastrophic progression occurs in < 1 out of 100 patients with the APA syndrome, more frequently in women (69%), and over an age range of 7 decades (mean 38 years).2 A case series analysis identified older age (aged > 36 years), history of systemic lupus erythematous, and broader organ involvement as prognostic indicators of a poor outcome. Better outcomes are associated with thrombocytopenia and anticoagulation treatment. However, gender did not influence mortality.3
Prevention is key to APA management, given the lack of efficacious treatment.2 Preventive measures are focused on avoiding triggers and aggressively treating those triggers that may arise. Possible triggers in this case included cessation of anticoagulation due to hematochezia and in anticipation of surgery, infection (C difficile colitis, suspected necrotic skin wound super infection, and a UTI), and biopsy-related trauma.
Initial clinical stability in this patient with abrupt decompensation along with pending laboratory and pathology results limited the opportunity for more aggressive therapeutic intervention for CAPS. Moreover, the relative sparing of the cardiopulmonary and renal systems contrasted with the more classical systemic involvement usually seen in CAPS. Second-line therapies for CAPS include plasma exchange and high-dose steroids.2 Third-line therapeutics include immunosuppressive agents, such as cyclophosphamide.2
The rapid decompensation, described on postoperative day 1, after a low-risk surgical biopsy highlights the importance of perioperative care in patients with this autoimmune condition. Following a review of surgical cases, Erkan and colleagues concluded that standard antithrombotic regimens for general and orthopedic surgery are likely to undertreat patients with APA syndrome.4 They recommend the following guidelines in place of standard antithrombotic management: preoperative platelet count > 100 k/µL, higher threshold before proceeding with surgery/interventional procedures, limiting intravascular manipulations, and minimizing periods without anticoagulation therapy.4
A case report of a 31-year-old female undergoing mitral valve replacement complicated postoperatively by CAPS-associated biventricular failure, despite preoperative transition of warfarin to unfractionated heparin, illustrates this significant perioperative risk.5 Evidence-based guidelines recommend holding enoxaparin 24 hours before surgery and 24 hours after invasive procedures in patients requiring bridging anticoagulation therapy.6
Treatment of the patient in this case was complicated by his cognitive impairment. Dementia is a less common but well-documented consequence of APA syndrome. A case review of 28 patients with the APA syndrome and dementia suggests an early onset of cognitive decline with a mean age of 49 years. There may be no clear preceding history of stroke in > 50% of patients.7 Interestingly, dementia followed initial manifestations of disease by an average of 3.5 years, even in some patients receiving anticoagulation therapy.7
A nuclear medicine study of 22 patients with APA syndrome and mild neuropsychiatric symptoms demonstrated a 73% incidence of cerebral hypoperfusion (55% diffuse and 18% local) based on PET imaging despite unremarkable MRI findings.8 Extended periods of hypoperfusion secondary to arterial thromboses in the temporal and parietal lobes may have been the primary etiology for dementia in this case. As such, the coexistence of neurologic abnormalities and a hypercoagulability state warrants a thorough diagnostic workup for similar disorders, despite the higher prevalence of dementia in advanced age.
Unfortunately, this patient’s cognitive disorder prevented a timely and less invasive bedside biopsy and required a surgical biopsy for which anticoagulation therapy was interrupted. A less invasive biopsy and timelier laboratory findings may have avoided triggers, including trauma from the surgical biopsy and interruptions in anticoagulation therapy, which may have contributed to the onset of CAPS.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Asherson RA, Cervera R, Piette J, et al. Catastrophic antiphospholipid syndrome: Clues to the pathogenesis from a series of 80 patients. Medicine (Baltimore). 2001;80(6):355-377.
2. Cervera R, Asherson RA. Multiorgan failure due to rapid occlusive vascular disease in antiphospholipid syndrome: The ‘catastrophic’ antiphospholipid syndrome. APLAR J Rheumatol. 2004;7(3):254-262.
3. Bayraktar UD, Erkan D, Bucciarelli S, Epinosa G, Asherson R; Catastrophic Antiphospholipid Syndrome Project Group. The clinical spectrum of catastrophic antiphospholipid syndrome in the absence and presence of lupus. J Rheumatol. 2007;34(2):346-352.
4. Erkan D, Leibowitz E, Berman J, Lockshin MD. Perioperative medical management of antiphospholipid syndrome: Hospital for special surgery experience, review of literature, and recommendations. J Rheumatol. 2002;29(4):843-849.
5. Dornan RIP. Acute postoperative biventricular failure associated with antiphospholipid antibody syndrome. Br J Anaesth. 2004;92(5):748-754.
6. Douketis JD, Berger PD, Dunn AS, et al; American College of Chest Physicians. The perioperative management of antithrombotic therapy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(suppl 6):S299-S339.
7. Gómez-Puerta JA, Cervera R, Calvo LM, et al. Dementia associated with the antiphospolipid syndrome: Clinical and radiological characteristics of 30 patients. Rheumatology (Oxford). 2005;44(1):95-99.
8. Kao C-H, Lan J-L, Hsieh J-F, Ho Y-J, ChangLai S-P, Lee J-K, et al. Evaluation of regional cerebral blood flow with 99mTc-HMPAO in primary antiphospholipid antibody syndrome. J Nucl Med. 1999;40:1446-1450.
Advanced psychiatric illness and dementia create a wide range of barriers to health care. These patients are unable to provide reliable details with respect to their illness or even discuss basic features of their medical history, forcing providers to rely on contributions from caregiver reports and medical records. Confounding the limits on medical information, physical examinations are often abbreviated or completely refused because of the patient’s distrust, discomfort, or delusion. Over time, the involvement of consulting services may amplify the impact of these barriers as the need for diagnostic and therapeutic interventions emerge. Meanwhile, this delay in definitive management opens a window of risk for deterioration, in which patients cannot be relied on to report important clinical changes.
This case report describes a patient with significant cognitive dysfunction who developed a rare and devastating complication of a hematologic disorder. As the case illustrates, transferring a patient from the psychiatric ward to Internal Medicine (IM) can create unique diagnostic and management challenges.
CASE REPORT
A 64-year-old man developed hematochezia after having been hospitalized in a locked psychiatric ward for the preceding 6 months following a suicide attempt. The episode of hematochezia occurred while on anticoagulation treatment with warfarin for chronic lower extremity deep venous thrombosis (DVT), which prompted the IM consultation. The patient’s past medical history was notable for dementia, hypothyroidism, Crohn disease, and primary sclerosing cholangitis.
The IM Consult Service recommended holding anticoagulation therapy and reversing the coagulopathy with vitamin K. The patient’s stool returned hemoccult and toxin positive for Clostridium difficile (C difficile). The hematochezia was attributed to the infection with C difficile in the setting of anticoagulation. Oral metronidazole was started. Hemoglobin remained stable without further episodes of bleeding. Seven days after the episode of hematochezia, the patient experienced worsening generalized pain and new skin findings. He was transferred to the general medical ward for further management.
The patient’s medical records revealed early cognitive decline with recommendations for supervised residential care as early as age 59 years. An extensive neurocognitive assessment indicated a diagnosis of semantic dementia. He also had a history of recurrent DVT with anticoagulation therapy for > 10 years with no prior workup for a hypercoagulable state. A recent baseline mental status report described a childlike demeanor, profound global speech deficits with marked difficulty understanding even basic medical concepts (eg, the need for a peripheral intravenous catheter), and generalized anxiety disorder complicated by hyperesthesia. The patient frequently refused physical examinations and blood draws as a result. He devoted himself to simple puzzles of kittens and puppies.
Vital signs were normal as were the head and neck, pulmonary, cardiac, and abdominal examinations. The patient’s neurocognitive examination was remarkable for his dependence on instrumental activities of daily living, global aphasia, impaired short- and long-term recall, and poor judgment. He scored 21 out of 30 on a recent mini-mental state examination: failure to achieve 3-word recall; disorientation to month, season, hospital, and county; and an inability to write a sentence or identify a pen. Otherwise, he had fluent speech, facial symmetry, intact strength and sensation throughout, and normal reflexes.
A skin examination revealed diffuse tender subcutaneous lesions. The largest lesion was about 5 cm, located in the left anterolateral thigh. Smaller lesions of about 1 cm were noted in the abdominal wall, right thigh, and bilateral upper extremities. An exquisitely tender, well-demarcated 20-cm elliptical lesion with central necrosis and an erythematous border developed in the left axilla the following day (Figure 1A). Pain limited adduction of the left arm.
The initial laboratory evaluation demonstrated a stable hemoglobin level of 11.1 g/dL, a platelet count of 128 k/mL, and no leukocytosis. Electrolytes and renal indexes were normal. D-dimer and fibrin split products were > 10,000 ng/mL and 20 mg/mL, respectively. Fibrinogen level was 351 mg/dL. The prothrombin time and international normalized ratio were 15.1 seconds and 1.4, respectively. The activated partial thromboplastin time (aPTT) was measured at 40 seconds. High sensitivity C-reactive protein was 4.59. Recent head imaging included a brain magnetic resonance imaging (MRI) notable for enlarged sulci and ventricles with temporal predominance. Positron emission tomography (PET) brain imaging was significant for diffuse hypometabolism in bilateral parietal and temporal lobes with preservation of sensorimotor and occipital cortexes. There was no clear radiographic evidence of cerebral embolic phenomenon or focal cerebrovascular events.
Enoxaparin treatment was initiated for a suspected hypercoagulable state. Ceftriaxone was administered for a urinary tract infection (UTI). Despite premedication, the bedside biopsy of his necrotic skin lesion was aborted due to severe anxiety and generalized somatic pain. A surgical excisional biopsy was thus obtained under general anesthesia. Enoxaparin was held the night before and the morning of surgery. There were no immediate complications related to the biopsy, and malignancy was not seen on intraoperative frozen sections.
Generalized somatic pain persisted the morning after the surgical biopsy, but the patient remained clinically unchanged. An hour later, he was found unresponsive with no pulse. Despite extensive resuscitative efforts, the patient died.
Postmortem
There was a high index of suspicion for a hemostatic perturbation given the skin findings and recent manipulation of anticoagulation with a prior thrombotic event. The axillary lesion closely resembled warfarin-related skin necrosis. Management included enoxaparin with supportive care, pending definitive pathologic findings.
Postmortem examination confirmed diffuse multiorgan involvement similar to the process seen in the thigh biopsy. Ischemic injury secondary to small vessel microthrombi were evident in the skin, subcutaneous fat, large bowel, urinary bladder, and associated pericystic fat (Figure 1B). Interpretation of the surgical thigh biopsy became available after the patient died. It demonstrated infarcted fat with fat necrosis and hemorrhage (Figure 2).
The results of the laboratory investigations for thrombophilia also came back after the patient died. A potent lupus anticoagulant (LA) was demonstrated. It manifested primarily in the intrinsic pathway as a strongly positive LA-sensitive-aPTT (delta time = 20.5 seconds) assay with a weakly positive dilute Russell’s viper venom time assay. The antigenic specificity of the LA antibodies was not uncovered, as the plasma levels of both IgM and IgG anticardiolipin and anti-Β2-glycoprotein-I antibodies were within the reference range. Factor (F) II and FV genotyping revealed wild-type FV, and the prothrombin gene G20210A was without mutation.
Assays for plasma levels of protein S and antithrombin activity were also normal, which excluded deficiencies in these proteins. The assay for protein C activity was slightly decreased. This may have exacerbated the hemostatic imbalance caused by the LA, as the FVII level had normalized. However, the etiology of the protein C deficiency is not clear. Considerations include (1) a warfarin disequilibrium state due to the discontinuation of oral anticoagulation and institution of vitamin K therapy; (2) an epiphenomenon resulting from active thromboses; or (3) a possible hereditary protein C deficiency.
The definitive diagnosis of the catastrophic antiphospholipid antibody (APA) syndrome relies on multiorgan failure in < 1 week, histopathologic evidence of small vessel thrombosis, and a positive LA.1 The study patient fulfilled these criteria (Table).
DISCUSSION
Catastrophic progression of APA syndrome is an infrequent and devastating complication of this autoimmune disorder with a mortality rate of nearly 50%.1 Antiphospholipid antibody syndrome typically presents with thromboses of the larger vessels, and it more commonly affects the venous system. In contrast, diffuse small vessel thromboses underlie the pathogenesis of catastrophic APA syndrome (CAPS).2 This catastrophic progression occurs in < 1 out of 100 patients with the APA syndrome, more frequently in women (69%), and over an age range of 7 decades (mean 38 years).2 A case series analysis identified older age (aged > 36 years), history of systemic lupus erythematous, and broader organ involvement as prognostic indicators of a poor outcome. Better outcomes are associated with thrombocytopenia and anticoagulation treatment. However, gender did not influence mortality.3
Prevention is key to APA management, given the lack of efficacious treatment.2 Preventive measures are focused on avoiding triggers and aggressively treating those triggers that may arise. Possible triggers in this case included cessation of anticoagulation due to hematochezia and in anticipation of surgery, infection (C difficile colitis, suspected necrotic skin wound super infection, and a UTI), and biopsy-related trauma.
Initial clinical stability in this patient with abrupt decompensation along with pending laboratory and pathology results limited the opportunity for more aggressive therapeutic intervention for CAPS. Moreover, the relative sparing of the cardiopulmonary and renal systems contrasted with the more classical systemic involvement usually seen in CAPS. Second-line therapies for CAPS include plasma exchange and high-dose steroids.2 Third-line therapeutics include immunosuppressive agents, such as cyclophosphamide.2
The rapid decompensation, described on postoperative day 1, after a low-risk surgical biopsy highlights the importance of perioperative care in patients with this autoimmune condition. Following a review of surgical cases, Erkan and colleagues concluded that standard antithrombotic regimens for general and orthopedic surgery are likely to undertreat patients with APA syndrome.4 They recommend the following guidelines in place of standard antithrombotic management: preoperative platelet count > 100 k/µL, higher threshold before proceeding with surgery/interventional procedures, limiting intravascular manipulations, and minimizing periods without anticoagulation therapy.4
A case report of a 31-year-old female undergoing mitral valve replacement complicated postoperatively by CAPS-associated biventricular failure, despite preoperative transition of warfarin to unfractionated heparin, illustrates this significant perioperative risk.5 Evidence-based guidelines recommend holding enoxaparin 24 hours before surgery and 24 hours after invasive procedures in patients requiring bridging anticoagulation therapy.6
Treatment of the patient in this case was complicated by his cognitive impairment. Dementia is a less common but well-documented consequence of APA syndrome. A case review of 28 patients with the APA syndrome and dementia suggests an early onset of cognitive decline with a mean age of 49 years. There may be no clear preceding history of stroke in > 50% of patients.7 Interestingly, dementia followed initial manifestations of disease by an average of 3.5 years, even in some patients receiving anticoagulation therapy.7
A nuclear medicine study of 22 patients with APA syndrome and mild neuropsychiatric symptoms demonstrated a 73% incidence of cerebral hypoperfusion (55% diffuse and 18% local) based on PET imaging despite unremarkable MRI findings.8 Extended periods of hypoperfusion secondary to arterial thromboses in the temporal and parietal lobes may have been the primary etiology for dementia in this case. As such, the coexistence of neurologic abnormalities and a hypercoagulability state warrants a thorough diagnostic workup for similar disorders, despite the higher prevalence of dementia in advanced age.
Unfortunately, this patient’s cognitive disorder prevented a timely and less invasive bedside biopsy and required a surgical biopsy for which anticoagulation therapy was interrupted. A less invasive biopsy and timelier laboratory findings may have avoided triggers, including trauma from the surgical biopsy and interruptions in anticoagulation therapy, which may have contributed to the onset of CAPS.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Advanced psychiatric illness and dementia create a wide range of barriers to health care. These patients are unable to provide reliable details with respect to their illness or even discuss basic features of their medical history, forcing providers to rely on contributions from caregiver reports and medical records. Confounding the limits on medical information, physical examinations are often abbreviated or completely refused because of the patient’s distrust, discomfort, or delusion. Over time, the involvement of consulting services may amplify the impact of these barriers as the need for diagnostic and therapeutic interventions emerge. Meanwhile, this delay in definitive management opens a window of risk for deterioration, in which patients cannot be relied on to report important clinical changes.
This case report describes a patient with significant cognitive dysfunction who developed a rare and devastating complication of a hematologic disorder. As the case illustrates, transferring a patient from the psychiatric ward to Internal Medicine (IM) can create unique diagnostic and management challenges.
CASE REPORT
A 64-year-old man developed hematochezia after having been hospitalized in a locked psychiatric ward for the preceding 6 months following a suicide attempt. The episode of hematochezia occurred while on anticoagulation treatment with warfarin for chronic lower extremity deep venous thrombosis (DVT), which prompted the IM consultation. The patient’s past medical history was notable for dementia, hypothyroidism, Crohn disease, and primary sclerosing cholangitis.
The IM Consult Service recommended holding anticoagulation therapy and reversing the coagulopathy with vitamin K. The patient’s stool returned hemoccult and toxin positive for Clostridium difficile (C difficile). The hematochezia was attributed to the infection with C difficile in the setting of anticoagulation. Oral metronidazole was started. Hemoglobin remained stable without further episodes of bleeding. Seven days after the episode of hematochezia, the patient experienced worsening generalized pain and new skin findings. He was transferred to the general medical ward for further management.
The patient’s medical records revealed early cognitive decline with recommendations for supervised residential care as early as age 59 years. An extensive neurocognitive assessment indicated a diagnosis of semantic dementia. He also had a history of recurrent DVT with anticoagulation therapy for > 10 years with no prior workup for a hypercoagulable state. A recent baseline mental status report described a childlike demeanor, profound global speech deficits with marked difficulty understanding even basic medical concepts (eg, the need for a peripheral intravenous catheter), and generalized anxiety disorder complicated by hyperesthesia. The patient frequently refused physical examinations and blood draws as a result. He devoted himself to simple puzzles of kittens and puppies.
Vital signs were normal as were the head and neck, pulmonary, cardiac, and abdominal examinations. The patient’s neurocognitive examination was remarkable for his dependence on instrumental activities of daily living, global aphasia, impaired short- and long-term recall, and poor judgment. He scored 21 out of 30 on a recent mini-mental state examination: failure to achieve 3-word recall; disorientation to month, season, hospital, and county; and an inability to write a sentence or identify a pen. Otherwise, he had fluent speech, facial symmetry, intact strength and sensation throughout, and normal reflexes.
A skin examination revealed diffuse tender subcutaneous lesions. The largest lesion was about 5 cm, located in the left anterolateral thigh. Smaller lesions of about 1 cm were noted in the abdominal wall, right thigh, and bilateral upper extremities. An exquisitely tender, well-demarcated 20-cm elliptical lesion with central necrosis and an erythematous border developed in the left axilla the following day (Figure 1A). Pain limited adduction of the left arm.
The initial laboratory evaluation demonstrated a stable hemoglobin level of 11.1 g/dL, a platelet count of 128 k/mL, and no leukocytosis. Electrolytes and renal indexes were normal. D-dimer and fibrin split products were > 10,000 ng/mL and 20 mg/mL, respectively. Fibrinogen level was 351 mg/dL. The prothrombin time and international normalized ratio were 15.1 seconds and 1.4, respectively. The activated partial thromboplastin time (aPTT) was measured at 40 seconds. High sensitivity C-reactive protein was 4.59. Recent head imaging included a brain magnetic resonance imaging (MRI) notable for enlarged sulci and ventricles with temporal predominance. Positron emission tomography (PET) brain imaging was significant for diffuse hypometabolism in bilateral parietal and temporal lobes with preservation of sensorimotor and occipital cortexes. There was no clear radiographic evidence of cerebral embolic phenomenon or focal cerebrovascular events.
Enoxaparin treatment was initiated for a suspected hypercoagulable state. Ceftriaxone was administered for a urinary tract infection (UTI). Despite premedication, the bedside biopsy of his necrotic skin lesion was aborted due to severe anxiety and generalized somatic pain. A surgical excisional biopsy was thus obtained under general anesthesia. Enoxaparin was held the night before and the morning of surgery. There were no immediate complications related to the biopsy, and malignancy was not seen on intraoperative frozen sections.
Generalized somatic pain persisted the morning after the surgical biopsy, but the patient remained clinically unchanged. An hour later, he was found unresponsive with no pulse. Despite extensive resuscitative efforts, the patient died.
Postmortem
There was a high index of suspicion for a hemostatic perturbation given the skin findings and recent manipulation of anticoagulation with a prior thrombotic event. The axillary lesion closely resembled warfarin-related skin necrosis. Management included enoxaparin with supportive care, pending definitive pathologic findings.
Postmortem examination confirmed diffuse multiorgan involvement similar to the process seen in the thigh biopsy. Ischemic injury secondary to small vessel microthrombi were evident in the skin, subcutaneous fat, large bowel, urinary bladder, and associated pericystic fat (Figure 1B). Interpretation of the surgical thigh biopsy became available after the patient died. It demonstrated infarcted fat with fat necrosis and hemorrhage (Figure 2).
The results of the laboratory investigations for thrombophilia also came back after the patient died. A potent lupus anticoagulant (LA) was demonstrated. It manifested primarily in the intrinsic pathway as a strongly positive LA-sensitive-aPTT (delta time = 20.5 seconds) assay with a weakly positive dilute Russell’s viper venom time assay. The antigenic specificity of the LA antibodies was not uncovered, as the plasma levels of both IgM and IgG anticardiolipin and anti-Β2-glycoprotein-I antibodies were within the reference range. Factor (F) II and FV genotyping revealed wild-type FV, and the prothrombin gene G20210A was without mutation.
Assays for plasma levels of protein S and antithrombin activity were also normal, which excluded deficiencies in these proteins. The assay for protein C activity was slightly decreased. This may have exacerbated the hemostatic imbalance caused by the LA, as the FVII level had normalized. However, the etiology of the protein C deficiency is not clear. Considerations include (1) a warfarin disequilibrium state due to the discontinuation of oral anticoagulation and institution of vitamin K therapy; (2) an epiphenomenon resulting from active thromboses; or (3) a possible hereditary protein C deficiency.
The definitive diagnosis of the catastrophic antiphospholipid antibody (APA) syndrome relies on multiorgan failure in < 1 week, histopathologic evidence of small vessel thrombosis, and a positive LA.1 The study patient fulfilled these criteria (Table).
DISCUSSION
Catastrophic progression of APA syndrome is an infrequent and devastating complication of this autoimmune disorder with a mortality rate of nearly 50%.1 Antiphospholipid antibody syndrome typically presents with thromboses of the larger vessels, and it more commonly affects the venous system. In contrast, diffuse small vessel thromboses underlie the pathogenesis of catastrophic APA syndrome (CAPS).2 This catastrophic progression occurs in < 1 out of 100 patients with the APA syndrome, more frequently in women (69%), and over an age range of 7 decades (mean 38 years).2 A case series analysis identified older age (aged > 36 years), history of systemic lupus erythematous, and broader organ involvement as prognostic indicators of a poor outcome. Better outcomes are associated with thrombocytopenia and anticoagulation treatment. However, gender did not influence mortality.3
Prevention is key to APA management, given the lack of efficacious treatment.2 Preventive measures are focused on avoiding triggers and aggressively treating those triggers that may arise. Possible triggers in this case included cessation of anticoagulation due to hematochezia and in anticipation of surgery, infection (C difficile colitis, suspected necrotic skin wound super infection, and a UTI), and biopsy-related trauma.
Initial clinical stability in this patient with abrupt decompensation along with pending laboratory and pathology results limited the opportunity for more aggressive therapeutic intervention for CAPS. Moreover, the relative sparing of the cardiopulmonary and renal systems contrasted with the more classical systemic involvement usually seen in CAPS. Second-line therapies for CAPS include plasma exchange and high-dose steroids.2 Third-line therapeutics include immunosuppressive agents, such as cyclophosphamide.2
The rapid decompensation, described on postoperative day 1, after a low-risk surgical biopsy highlights the importance of perioperative care in patients with this autoimmune condition. Following a review of surgical cases, Erkan and colleagues concluded that standard antithrombotic regimens for general and orthopedic surgery are likely to undertreat patients with APA syndrome.4 They recommend the following guidelines in place of standard antithrombotic management: preoperative platelet count > 100 k/µL, higher threshold before proceeding with surgery/interventional procedures, limiting intravascular manipulations, and minimizing periods without anticoagulation therapy.4
A case report of a 31-year-old female undergoing mitral valve replacement complicated postoperatively by CAPS-associated biventricular failure, despite preoperative transition of warfarin to unfractionated heparin, illustrates this significant perioperative risk.5 Evidence-based guidelines recommend holding enoxaparin 24 hours before surgery and 24 hours after invasive procedures in patients requiring bridging anticoagulation therapy.6
Treatment of the patient in this case was complicated by his cognitive impairment. Dementia is a less common but well-documented consequence of APA syndrome. A case review of 28 patients with the APA syndrome and dementia suggests an early onset of cognitive decline with a mean age of 49 years. There may be no clear preceding history of stroke in > 50% of patients.7 Interestingly, dementia followed initial manifestations of disease by an average of 3.5 years, even in some patients receiving anticoagulation therapy.7
A nuclear medicine study of 22 patients with APA syndrome and mild neuropsychiatric symptoms demonstrated a 73% incidence of cerebral hypoperfusion (55% diffuse and 18% local) based on PET imaging despite unremarkable MRI findings.8 Extended periods of hypoperfusion secondary to arterial thromboses in the temporal and parietal lobes may have been the primary etiology for dementia in this case. As such, the coexistence of neurologic abnormalities and a hypercoagulability state warrants a thorough diagnostic workup for similar disorders, despite the higher prevalence of dementia in advanced age.
Unfortunately, this patient’s cognitive disorder prevented a timely and less invasive bedside biopsy and required a surgical biopsy for which anticoagulation therapy was interrupted. A less invasive biopsy and timelier laboratory findings may have avoided triggers, including trauma from the surgical biopsy and interruptions in anticoagulation therapy, which may have contributed to the onset of CAPS.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Asherson RA, Cervera R, Piette J, et al. Catastrophic antiphospholipid syndrome: Clues to the pathogenesis from a series of 80 patients. Medicine (Baltimore). 2001;80(6):355-377.
2. Cervera R, Asherson RA. Multiorgan failure due to rapid occlusive vascular disease in antiphospholipid syndrome: The ‘catastrophic’ antiphospholipid syndrome. APLAR J Rheumatol. 2004;7(3):254-262.
3. Bayraktar UD, Erkan D, Bucciarelli S, Epinosa G, Asherson R; Catastrophic Antiphospholipid Syndrome Project Group. The clinical spectrum of catastrophic antiphospholipid syndrome in the absence and presence of lupus. J Rheumatol. 2007;34(2):346-352.
4. Erkan D, Leibowitz E, Berman J, Lockshin MD. Perioperative medical management of antiphospholipid syndrome: Hospital for special surgery experience, review of literature, and recommendations. J Rheumatol. 2002;29(4):843-849.
5. Dornan RIP. Acute postoperative biventricular failure associated with antiphospholipid antibody syndrome. Br J Anaesth. 2004;92(5):748-754.
6. Douketis JD, Berger PD, Dunn AS, et al; American College of Chest Physicians. The perioperative management of antithrombotic therapy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(suppl 6):S299-S339.
7. Gómez-Puerta JA, Cervera R, Calvo LM, et al. Dementia associated with the antiphospolipid syndrome: Clinical and radiological characteristics of 30 patients. Rheumatology (Oxford). 2005;44(1):95-99.
8. Kao C-H, Lan J-L, Hsieh J-F, Ho Y-J, ChangLai S-P, Lee J-K, et al. Evaluation of regional cerebral blood flow with 99mTc-HMPAO in primary antiphospholipid antibody syndrome. J Nucl Med. 1999;40:1446-1450.
1. Asherson RA, Cervera R, Piette J, et al. Catastrophic antiphospholipid syndrome: Clues to the pathogenesis from a series of 80 patients. Medicine (Baltimore). 2001;80(6):355-377.
2. Cervera R, Asherson RA. Multiorgan failure due to rapid occlusive vascular disease in antiphospholipid syndrome: The ‘catastrophic’ antiphospholipid syndrome. APLAR J Rheumatol. 2004;7(3):254-262.
3. Bayraktar UD, Erkan D, Bucciarelli S, Epinosa G, Asherson R; Catastrophic Antiphospholipid Syndrome Project Group. The clinical spectrum of catastrophic antiphospholipid syndrome in the absence and presence of lupus. J Rheumatol. 2007;34(2):346-352.
4. Erkan D, Leibowitz E, Berman J, Lockshin MD. Perioperative medical management of antiphospholipid syndrome: Hospital for special surgery experience, review of literature, and recommendations. J Rheumatol. 2002;29(4):843-849.
5. Dornan RIP. Acute postoperative biventricular failure associated with antiphospholipid antibody syndrome. Br J Anaesth. 2004;92(5):748-754.
6. Douketis JD, Berger PD, Dunn AS, et al; American College of Chest Physicians. The perioperative management of antithrombotic therapy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(suppl 6):S299-S339.
7. Gómez-Puerta JA, Cervera R, Calvo LM, et al. Dementia associated with the antiphospolipid syndrome: Clinical and radiological characteristics of 30 patients. Rheumatology (Oxford). 2005;44(1):95-99.
8. Kao C-H, Lan J-L, Hsieh J-F, Ho Y-J, ChangLai S-P, Lee J-K, et al. Evaluation of regional cerebral blood flow with 99mTc-HMPAO in primary antiphospholipid antibody syndrome. J Nucl Med. 1999;40:1446-1450.
A Medical Specialty e-Consult Program in a VA Health Care System
The VA is charged with providing high-quality, efficient care for those who have served in the armed forces. Starting in 2009, leadership within the VA articulated a set of “transformational” initiatives, which included greater implementation of telemedicine to improve the coordination of veterans’ care and access to specialty care.
The VA Boston Healthcare System (VABHS) consists of 3 major facilities and multiple community-based outpatient clinics (CBOCs) across eastern Massachusetts. Most medical specialty care is concentrated near the single acute-care site, resulting in a diffuse network of primary and specialty care providers. Consequently, many patients travel beyond their primary medical facility to access specialists. To address this fragmentation, the Medical Service at VABHS implemented a system of electronic chart review consultations (e-Consults) to provide high-quality, efficient care while maintaining the integrity of the patient’s medical home and improving access to specialty providers.
Designed to address clinical questions that may not require a face-to-face (FTF) encounter, e-Consults are a form of store-and-forward telemedicine (SAFT), with information-sharing between requesting providers (typically primary care) and answering specialists, using the VA’s existing electronic health record (EHR), the Computerized Patient Record System (CPRS). Store-and-forward telemedicine increases patient access to specialists and timeliness in initiating a consultation while decreasing patient travel and associated costs.1-15
Although previous studies have usually found high concordance between SAFT and FTF consultations in both diagnosis and disease management, the feasibility of SAFT has been historically limited by the lack of an integrated shared EHR.16-18 The VA implemented CPRS in 1996 and was soon recognized as a fully integrated EHR, allowing any VA clinician to view any veteran’s medical record nationwide. This technology, combined with the geographically dispersed population of veterans and the concentration of specialty services within mostly urban tertiary care centers, makes the VA an optimal environment to benefit from implementation of SAFT.
This study examines the implementation of e-Consults in a large VA medical service with the primary goal of facilitating timely specialty care access. This article details the initial experience of implementation and clinicians’ early perceptions of the system.
E-Consult Implementation
The VABHS provides tertiary specialty care for much of New England. It also serves a primary care population of more than 30,000 veterans in eastern Massachusetts through a network of 3 larger primary care practices based at 3 main campuses and 5 smaller CBOCs. Most specialty care is provided by the 2 campuses in Boston.
Discussions with primary care leadership and e-mail announcements to primary care providers (PCPs) throughout VABHS announced the availability and purpose of the new e-Consult. The initiative was initially piloted within 2 specialties, allergy and cardiology, beginning in January 2011. After establishing the logistics for routing, processing, and encounter data capture, e-Consults were expanded to the remaining subspecialties by December 2011.
Within 9 months, 12 different specialties had e-Consult options: endocrinology, gastroenterology, hematology, infectious disease, nephrology, oncology, palliative care, pulmonary disease, rheumatology, and sleep medicine. The e-Consult use was tracked for this study on a monthly basis.
Process and Work Flow
Any PCP with consult ordering privileges can request an e-Consult. Expectations were set that e-Consults should be used to request help with nonemergent clinical issues and be completed within 3 nonholiday weekdays.
The e-Consult uses CPRS modular programming to create an event-based electronic consultation request. Once initiated, the consult is routed to the consulting service. Each specialty defines how to triage the e-Consult request; computer alerts can be immediately generated to a single individual or a defined team within each specialty. Options to alert or print consult requests to administrative personnel also exist. As with requests for FTF consultation requests, e-Consults allow specialists the options to deny a request if they feel it is not appropriate for their specialty. In addition, specialists are able to convert the e-Consult request to a FTF encounter, to forward the e-Consult to another specialty, or to add a “comment” before proceeding. These comments generate an immediate electronic alert to the requestor, thus providing a forum for asynchronous preconsultation dialogue between providers. Once the consultation is complete, the requestor receives an alert that directs them to view the completed consultation note in the EHR.
The entirety of the EHR is accessible to the consultant, including all primary care and specialty progress notes, inpatient and outpatient laboratory testing, and all diagnostic testing performed nationally within the VA.
User Feedback
Based on the literature, separate online questionnaires were developed to ascertain primary care clinicians’ and specialists’ perceptions of the e-Consult system. This 26-item survey for PCPs and 13-item survey for specialists assessed use of e-Consults, satisfaction with the system, and for primary care clinicians, perceived benefits of the e-Consult process
and specific feedback by specialty.
Nine months after full implementation, perceptions of e-Consult processes were solicited. All PCPs, staff physicians, nurse practitioners, and internal medicine residents assigned to a VABHS primary care clinic were surveyed. Eligible specialist consultants included attending physicians and fellows who were identified by the specialty chief as potentially having responded to an e-Consult in the past 6 months. The survey was administered in June 2012 and queried the previous 6 months’ experience.
The monthly number of e-Consults ranged from 96 to 137 over the 6-month study period. Cardiology and hematology were the most commonly requested e-Consults, with > 20 per specialty per month. The 146 cardiology e-Consults during those 6 months represented 20% of all new cardiology consults, whereas the 159 e-Consults to hematology represented 53% of their new consults. The least used specialties for e-Consults were allergy, oncology, palliative care, and rheumatology, each averaging < 2 e-Consults per month.
Provider Perceptions
Overall, 85 of the 163 providers completed the survey (52%). Permanent staff had a better response rate than did trainees: 58% vs 33% among PCPs (P < .02), and 71% vs 44% among specialists (P = .03).
Of the 47 primary care respondents, 4 residents and 2 staff physicians had not previously used e-Consults; all 4 residents and 1 staff physician stated that this was due to lack of awareness of the system. The remaining 41 PCPs had requested ≥ 1 e-Consult in the past 6 months. All 38 specialist provider respondents had answered ≥ 1 e-Consult in study period, with 9 providers completing > 20.
e-Consult Satisfaction
Overall, 93% of PCPs were satisfied with e-Consults, and none were dissatisfied, compared with 53% of specialty providers who were satisfied and 26% who were dissatisfied (P < .001) (Figure 1). All specialists who were dissatisfied were in the sections of cardiology or gastroenterology. Further inspection identified several differences between those specialty providers who were generally satisfied with e-Consults (n = 20) compared with those providers who were dissatisfied (n = 10). Eight PCPs reported being neither satisfied nor dissatisfied.
In analyses comparing satisfied specialists (n = 20) with dissatisfied specialists (n = 10), excluding the 8 respondents who were neither satisfied nor dissatisfied, those who were satisfied reported a lower rate of conversion of e-Consults to FTF visits than did their dissatisfied peers, 26% vs 52%, respectively (P = .01). Satisfied specialists were more likely to view e-Consults as replacing work they otherwise would have done, such as a replacement for a FTF consult and prior informal “curbside” consults, 79% vs 50%, respectively (P < .02). Satisfied specialists were somewhat more likely to complete their e-Consults, on average, in ≤ 15 minutes, compared with dissatisfied specialists, although this trend did not reach statistical significance (59% vs 49%, respectively; (P = .07) (Figure 2).
Improving Quality of Care
PCPs were asked to note which benefits were realized among patients for whom e-Consults replaced FTF visits. Nearly all (98%) indicated that the e-Consults enabled patients to avoid unnecessary travel, and 95% of PCPs indicated that they requested e-consults to receive faster clinical input about a patient. A total of 59% believed e-Consults helped their patients avoid additional copays, and 56% obtained specialty input for a patient who would otherwise refuse to travel. When an e-Consult did not avoid a FTF visit (ie, despite the e-Consult, the patient ultimately proceeded to a FTF consultation with the specialist), the majority of PCPs believed that the e-Consult provided them with reassurance and initiated diagnostic testing that would be useful during the patient’s subsequent FTF consult (Figure 3).
Overall, 61% of specialists agreed with the statement, “e-Consults improve the quality of care VA Boston Healthcare System provides.” Even when specialists perceived that e-Consults did not avoid FTF visits, most agreed that e-Consults helped initiate diagnostic testing prior to a FTF visit. In addition, satisfied specialists saw the benefit in reassuring the PCP and initiating additional management prior to a FTF visit.
Dynamics of e-Consults
Primary care providers reported that 78% of their requested e-Consults were completed within 2 days and 95% of all e-Consults were completed by day 3. In aggregate, primary care clinicians estimated that about one-third of their e-Consults replaced FTF visits; one-third replaced prior mechanisms for informal consultation (eg, “curbside” or e-mail); and one-third of e-Consults represented new requests that would not have involved specialty consultation in the absence of the e-Consult mechanism.
Specialists estimated that 27% of e-Consults were new work (ie, consultations that would not have occurred formally or informally in the absence of the e-Consult mechanism); 32% replaced FTF visits; and 42% were substitutes for prior informal communications. Specialists reported a wide range of time required to answer e-Consults. For specialists, on average, 54% of e-Consults took < 15 minutes to complete, but 20% took > 25 minutes, with 6% requiring ≥ 45 minutes to complete (Figure 2).
Discussion
This article reports the initial experience with the implementation of an e-Consult system for PCPs and medical subspecialists in a large VA health care system. Primary care providers were generally satisfied with the e-Consult system and reported that the system yielded tangible benefits to patients, such as quicker specialty input and avoidance of FTF visits and travel. Specialists were somewhat less satisfied than were their primary care colleagues; nevertheless, specialists perceived similar benefits for patients. Despite the general satisfaction among PCPs, > 1 in 4 specialists expressed dissatisfaction with the system.
Satisfaction with e-Consults may be influenced by the typology of the specialty itself. Some specialties (eg, hematology) rely more heavily on laboratory tests, compared with specialties such as cardiology, gastroenterology, or pulmonary whose subtleties of history and physical examination (H&P), along with review of imaging data, are more commonly required for clinical decision making.2 Hence, the EHR may allow some specialties to provide e-Consults with greater facility. For example, because the EHR enables clinicians to trend the results of years of complete blood counts with a few simple keystrokes, a hematologist may have more confidence in making a clinical assessment without seeing the patient based on historical information in the EHR.
Anecdotal evidence from specialists suggests discomfort when clinical input is based on H&P findings of others. While the sample size was small, this reasoning could explain the differences in satisfaction among specialties: Satisfied specialists may be taking slightly less time to complete their e-Consults, but they were less likely to view the e-Consult as new work and less likely to convert to a FTF consultation.
Store-and-forward telemedicine cannot completely replace FTF visits, but rather supplement them. If e-Consults obviate patient travel and copays while stimulating more timely completion of the consultation, the benefits of cost savings and improved specialty access by veterans merit further attention. When an e-Consult did not avoid a FTF consultation, the majority of clinicians perceived that the e-Consult allowed the PCP to initiate diagnostic testing or alterations in treatment prior to the eventual FTF consultation with the specialist. This finding could be considered as a proxy for increased coordination of care between primary care and specialty providers in anticipation of a FTF visit.
During the study period, workload capture for e-Consults was administratively fixed at the level of a brief consultation, regardless of the effort expended. Validation of the current finding regarding time spent completing consults has resulted in a change. On January 10, 2014, VA Central Office updated its policy to allow 3 levels of workload credit for e-Consults based on time: up to 15 minutes, 16 to 30 minutes, and > 30 minutes.
E-consults are just one example of innovations that broadly fall under the rubric of telehealth initiatives being deployed across VA health care facilities. Other examples of SAFT include pictures collected by technicians and sent to specialists for review to screen for diabetic retinopathy, a practice done within VA for many years.19 Clinical video telehealth provides real-time videoconferencing between patient and specialist and can obviate the need for long-distance patient travel. Initially used only for interviews, newer equipment amplifies sound and enhances optical imaging to allow thorough physical examinations when trained personnel are handling the equipment.
Each technology has its challenges for implementation, which vary in degree of coordination and cost. Overall, the application of new technologies and repurposing existing ones may be limited by creativity alone in the efforts to improve access and quality of the care provided to veterans. The Table highlights telehealth uses being deployed by VA.
Conclusion
Electronic consults have been well received by PCPs and most specialists in the VABHS, seeming to meet the goal of using telehealth to improve veterans’ access to specialty care and coordination of care between PCPs and specialists. While not examined in this initial report, e-Consults may lead to reduced costs, and this possibility should be further explored.
Despite concerns expressed by some specialists, most believed that e-Consults improved the quality of care their patients received. Future work needs to validate these findings, examine patient perspectives, delve more deeply into ways to improve and bring more value to the process, and address the specialty effort in relation to workload awarded.
Acknowledgements
The authors would like to thank Yisraela Elstein and Pauline Benedetti for their assistance in tracking workload data and Steven Simon for his detailed review of the manuscript. Funding for this work was supported by the Department of Veterans Affairs Specialty Care Transformation Initiative for the 21st Century—T21 innovation grant for telehealth initiatives.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Cusack CM, Pan E, Hook JM, et al. The Value of Provider-to-Provider Telehealth Technologies. Charlestown, MA: Center for Information Technology Leadership; 2007.
2. Horner K, Wagner E, Tufano J. Electronic consultations between primary and specialty care clinicians: Early insights. Issue Brief (Commonw Fund). 2011;23:1-14.
3. Angstman KB, Rohrer JE, Adamson SC, Chaudhry R. Impact of e-consults on return visits of primary care patients. Health Care Manag (Frederick). 2009;28(3):253-257.
4. Kim Y, Chen AH, Keith E, Yee HF Jr, Kushel MB. Not perfect, but better: Primary care providers’ experiences with electronic referrals in a safety net health system. J Gen Intern Med. 2009;24(5):614-619.
5. Angstman KB, Adamson SC, Furst JW, Houston MS, Rohrer JE. Provider satisfaction with virtual specialist consultations in a family medicine department. Health Care Manag (Frederick). 2009;28(1):14-18.
6. Mahnke CB, Jordan CP, Bergvall E, Person DA, Pinsker JE. The Pacific Asynchronous TeleHealth (PATH) system: Review of 1,000 pediatric teleconsultations. Telemed J E Health. 2011;17(1):35-39.
7. Sood S, Mbarika V, Jugoo S, et al. What is telemedicine? A collection of 104 peer-reviewed perspectives and theoretical underpinnings. Telemed J E Health. 2007;13(5):573-590.
8. Maeder A. Telehealth and remote access. Stud Health Technol Inform. 2010;151:239-254.
9. Schmidt T, Lappan CM, Hospenthal DR, Murray CK. Deployed provider satisfaction with infectious disease teleconsulation. Mil Med. 2011;176(12):1417-1420.
10. Verhoeven F, Tanja-Dijkstra K, Nijland N, Eysenbach G, van Gemert-Pijnen L. Asynchronous and synchronous teleconsultation for diabetes care: A systematic literature review. J Diabetes Sci Technol. 2010;4(3):666-684.
11. Krupinski E, Nypaver M, Poropatich R, Ellis D, Safwat R, Sapci H. Telemedicine/telehealth: An international perspective. Clinical applications in telemedicine/telehealth. Telemed J E Health. 2002;8(1):13-34.
12. Moreno-Ramirez D, Ferrandiz L, Ruiz-de-Casas A, et al. Economic evaluation of a store-and-forward teledermatology system for skin cancer patients. J Telemed Telecare. 2009;15(1):40-45.
13. Callahan CW, Malone F, Estroff D, Person DA. Effectiveness of an Internet-based store-and-forward telemedicine system for pediatric subspecialty consultation. Arch Pediatr Adolesc Med. 2005;159(4):389-393.
14. Gagnon MP, Duplantie J, Fortin JP, Landry R. Implementing telehealth to support medical practice in rural/remote regions: What are the conditions for success? Implement Sci. 2006;1:18.
15. Hailey D, Ohinmaa A, Roine R. Study quality and evidence of benefit in recent assessments of telemedicine. J Telemed Telecare. 2004;10(6):318-324.
16. High WA, Houston MS, Calobrisi SD, Drage LA, McEvoy MT. Assessment of the accuracy of low-cost store-and-forward teledermatology consultation. J Am Acad Dermatol. 2000;42(5 pt 1):776-783.
17. Houston MS, Myers JD, Levens SP, et al. Clinical consultations using store-and-forward telemedicine technology. Mayo Clin Proc. 1999;74(8):764-769.
18. Hersh WR, Hickam DH, Severance SM, Dana TL, Pyle Krages K, Helfand M. Diagnosis, access and outcomes: Update of a systematic review of telemedicine services. J Telemed Telecare. 2006;12(suppl 2):S3-S31.
19. Cavallerano AA, Conlin PR. Teleretinal imaging to screen for diabetic retinopathy in the Veterans Health Administration. J Diabetes Sci Technol. 2008;2(1):33-39.
20. Arora S, Thornton K, Murata G, et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med. 2011;364(23):2199-2207.
The VA is charged with providing high-quality, efficient care for those who have served in the armed forces. Starting in 2009, leadership within the VA articulated a set of “transformational” initiatives, which included greater implementation of telemedicine to improve the coordination of veterans’ care and access to specialty care.
The VA Boston Healthcare System (VABHS) consists of 3 major facilities and multiple community-based outpatient clinics (CBOCs) across eastern Massachusetts. Most medical specialty care is concentrated near the single acute-care site, resulting in a diffuse network of primary and specialty care providers. Consequently, many patients travel beyond their primary medical facility to access specialists. To address this fragmentation, the Medical Service at VABHS implemented a system of electronic chart review consultations (e-Consults) to provide high-quality, efficient care while maintaining the integrity of the patient’s medical home and improving access to specialty providers.
Designed to address clinical questions that may not require a face-to-face (FTF) encounter, e-Consults are a form of store-and-forward telemedicine (SAFT), with information-sharing between requesting providers (typically primary care) and answering specialists, using the VA’s existing electronic health record (EHR), the Computerized Patient Record System (CPRS). Store-and-forward telemedicine increases patient access to specialists and timeliness in initiating a consultation while decreasing patient travel and associated costs.1-15
Although previous studies have usually found high concordance between SAFT and FTF consultations in both diagnosis and disease management, the feasibility of SAFT has been historically limited by the lack of an integrated shared EHR.16-18 The VA implemented CPRS in 1996 and was soon recognized as a fully integrated EHR, allowing any VA clinician to view any veteran’s medical record nationwide. This technology, combined with the geographically dispersed population of veterans and the concentration of specialty services within mostly urban tertiary care centers, makes the VA an optimal environment to benefit from implementation of SAFT.
This study examines the implementation of e-Consults in a large VA medical service with the primary goal of facilitating timely specialty care access. This article details the initial experience of implementation and clinicians’ early perceptions of the system.
E-Consult Implementation
The VABHS provides tertiary specialty care for much of New England. It also serves a primary care population of more than 30,000 veterans in eastern Massachusetts through a network of 3 larger primary care practices based at 3 main campuses and 5 smaller CBOCs. Most specialty care is provided by the 2 campuses in Boston.
Discussions with primary care leadership and e-mail announcements to primary care providers (PCPs) throughout VABHS announced the availability and purpose of the new e-Consult. The initiative was initially piloted within 2 specialties, allergy and cardiology, beginning in January 2011. After establishing the logistics for routing, processing, and encounter data capture, e-Consults were expanded to the remaining subspecialties by December 2011.
Within 9 months, 12 different specialties had e-Consult options: endocrinology, gastroenterology, hematology, infectious disease, nephrology, oncology, palliative care, pulmonary disease, rheumatology, and sleep medicine. The e-Consult use was tracked for this study on a monthly basis.
Process and Work Flow
Any PCP with consult ordering privileges can request an e-Consult. Expectations were set that e-Consults should be used to request help with nonemergent clinical issues and be completed within 3 nonholiday weekdays.
The e-Consult uses CPRS modular programming to create an event-based electronic consultation request. Once initiated, the consult is routed to the consulting service. Each specialty defines how to triage the e-Consult request; computer alerts can be immediately generated to a single individual or a defined team within each specialty. Options to alert or print consult requests to administrative personnel also exist. As with requests for FTF consultation requests, e-Consults allow specialists the options to deny a request if they feel it is not appropriate for their specialty. In addition, specialists are able to convert the e-Consult request to a FTF encounter, to forward the e-Consult to another specialty, or to add a “comment” before proceeding. These comments generate an immediate electronic alert to the requestor, thus providing a forum for asynchronous preconsultation dialogue between providers. Once the consultation is complete, the requestor receives an alert that directs them to view the completed consultation note in the EHR.
The entirety of the EHR is accessible to the consultant, including all primary care and specialty progress notes, inpatient and outpatient laboratory testing, and all diagnostic testing performed nationally within the VA.
User Feedback
Based on the literature, separate online questionnaires were developed to ascertain primary care clinicians’ and specialists’ perceptions of the e-Consult system. This 26-item survey for PCPs and 13-item survey for specialists assessed use of e-Consults, satisfaction with the system, and for primary care clinicians, perceived benefits of the e-Consult process
and specific feedback by specialty.
Nine months after full implementation, perceptions of e-Consult processes were solicited. All PCPs, staff physicians, nurse practitioners, and internal medicine residents assigned to a VABHS primary care clinic were surveyed. Eligible specialist consultants included attending physicians and fellows who were identified by the specialty chief as potentially having responded to an e-Consult in the past 6 months. The survey was administered in June 2012 and queried the previous 6 months’ experience.
The monthly number of e-Consults ranged from 96 to 137 over the 6-month study period. Cardiology and hematology were the most commonly requested e-Consults, with > 20 per specialty per month. The 146 cardiology e-Consults during those 6 months represented 20% of all new cardiology consults, whereas the 159 e-Consults to hematology represented 53% of their new consults. The least used specialties for e-Consults were allergy, oncology, palliative care, and rheumatology, each averaging < 2 e-Consults per month.
Provider Perceptions
Overall, 85 of the 163 providers completed the survey (52%). Permanent staff had a better response rate than did trainees: 58% vs 33% among PCPs (P < .02), and 71% vs 44% among specialists (P = .03).
Of the 47 primary care respondents, 4 residents and 2 staff physicians had not previously used e-Consults; all 4 residents and 1 staff physician stated that this was due to lack of awareness of the system. The remaining 41 PCPs had requested ≥ 1 e-Consult in the past 6 months. All 38 specialist provider respondents had answered ≥ 1 e-Consult in study period, with 9 providers completing > 20.
e-Consult Satisfaction
Overall, 93% of PCPs were satisfied with e-Consults, and none were dissatisfied, compared with 53% of specialty providers who were satisfied and 26% who were dissatisfied (P < .001) (Figure 1). All specialists who were dissatisfied were in the sections of cardiology or gastroenterology. Further inspection identified several differences between those specialty providers who were generally satisfied with e-Consults (n = 20) compared with those providers who were dissatisfied (n = 10). Eight PCPs reported being neither satisfied nor dissatisfied.
In analyses comparing satisfied specialists (n = 20) with dissatisfied specialists (n = 10), excluding the 8 respondents who were neither satisfied nor dissatisfied, those who were satisfied reported a lower rate of conversion of e-Consults to FTF visits than did their dissatisfied peers, 26% vs 52%, respectively (P = .01). Satisfied specialists were more likely to view e-Consults as replacing work they otherwise would have done, such as a replacement for a FTF consult and prior informal “curbside” consults, 79% vs 50%, respectively (P < .02). Satisfied specialists were somewhat more likely to complete their e-Consults, on average, in ≤ 15 minutes, compared with dissatisfied specialists, although this trend did not reach statistical significance (59% vs 49%, respectively; (P = .07) (Figure 2).
Improving Quality of Care
PCPs were asked to note which benefits were realized among patients for whom e-Consults replaced FTF visits. Nearly all (98%) indicated that the e-Consults enabled patients to avoid unnecessary travel, and 95% of PCPs indicated that they requested e-consults to receive faster clinical input about a patient. A total of 59% believed e-Consults helped their patients avoid additional copays, and 56% obtained specialty input for a patient who would otherwise refuse to travel. When an e-Consult did not avoid a FTF visit (ie, despite the e-Consult, the patient ultimately proceeded to a FTF consultation with the specialist), the majority of PCPs believed that the e-Consult provided them with reassurance and initiated diagnostic testing that would be useful during the patient’s subsequent FTF consult (Figure 3).
Overall, 61% of specialists agreed with the statement, “e-Consults improve the quality of care VA Boston Healthcare System provides.” Even when specialists perceived that e-Consults did not avoid FTF visits, most agreed that e-Consults helped initiate diagnostic testing prior to a FTF visit. In addition, satisfied specialists saw the benefit in reassuring the PCP and initiating additional management prior to a FTF visit.
Dynamics of e-Consults
Primary care providers reported that 78% of their requested e-Consults were completed within 2 days and 95% of all e-Consults were completed by day 3. In aggregate, primary care clinicians estimated that about one-third of their e-Consults replaced FTF visits; one-third replaced prior mechanisms for informal consultation (eg, “curbside” or e-mail); and one-third of e-Consults represented new requests that would not have involved specialty consultation in the absence of the e-Consult mechanism.
Specialists estimated that 27% of e-Consults were new work (ie, consultations that would not have occurred formally or informally in the absence of the e-Consult mechanism); 32% replaced FTF visits; and 42% were substitutes for prior informal communications. Specialists reported a wide range of time required to answer e-Consults. For specialists, on average, 54% of e-Consults took < 15 minutes to complete, but 20% took > 25 minutes, with 6% requiring ≥ 45 minutes to complete (Figure 2).
Discussion
This article reports the initial experience with the implementation of an e-Consult system for PCPs and medical subspecialists in a large VA health care system. Primary care providers were generally satisfied with the e-Consult system and reported that the system yielded tangible benefits to patients, such as quicker specialty input and avoidance of FTF visits and travel. Specialists were somewhat less satisfied than were their primary care colleagues; nevertheless, specialists perceived similar benefits for patients. Despite the general satisfaction among PCPs, > 1 in 4 specialists expressed dissatisfaction with the system.
Satisfaction with e-Consults may be influenced by the typology of the specialty itself. Some specialties (eg, hematology) rely more heavily on laboratory tests, compared with specialties such as cardiology, gastroenterology, or pulmonary whose subtleties of history and physical examination (H&P), along with review of imaging data, are more commonly required for clinical decision making.2 Hence, the EHR may allow some specialties to provide e-Consults with greater facility. For example, because the EHR enables clinicians to trend the results of years of complete blood counts with a few simple keystrokes, a hematologist may have more confidence in making a clinical assessment without seeing the patient based on historical information in the EHR.
Anecdotal evidence from specialists suggests discomfort when clinical input is based on H&P findings of others. While the sample size was small, this reasoning could explain the differences in satisfaction among specialties: Satisfied specialists may be taking slightly less time to complete their e-Consults, but they were less likely to view the e-Consult as new work and less likely to convert to a FTF consultation.
Store-and-forward telemedicine cannot completely replace FTF visits, but rather supplement them. If e-Consults obviate patient travel and copays while stimulating more timely completion of the consultation, the benefits of cost savings and improved specialty access by veterans merit further attention. When an e-Consult did not avoid a FTF consultation, the majority of clinicians perceived that the e-Consult allowed the PCP to initiate diagnostic testing or alterations in treatment prior to the eventual FTF consultation with the specialist. This finding could be considered as a proxy for increased coordination of care between primary care and specialty providers in anticipation of a FTF visit.
During the study period, workload capture for e-Consults was administratively fixed at the level of a brief consultation, regardless of the effort expended. Validation of the current finding regarding time spent completing consults has resulted in a change. On January 10, 2014, VA Central Office updated its policy to allow 3 levels of workload credit for e-Consults based on time: up to 15 minutes, 16 to 30 minutes, and > 30 minutes.
E-consults are just one example of innovations that broadly fall under the rubric of telehealth initiatives being deployed across VA health care facilities. Other examples of SAFT include pictures collected by technicians and sent to specialists for review to screen for diabetic retinopathy, a practice done within VA for many years.19 Clinical video telehealth provides real-time videoconferencing between patient and specialist and can obviate the need for long-distance patient travel. Initially used only for interviews, newer equipment amplifies sound and enhances optical imaging to allow thorough physical examinations when trained personnel are handling the equipment.
Each technology has its challenges for implementation, which vary in degree of coordination and cost. Overall, the application of new technologies and repurposing existing ones may be limited by creativity alone in the efforts to improve access and quality of the care provided to veterans. The Table highlights telehealth uses being deployed by VA.
Conclusion
Electronic consults have been well received by PCPs and most specialists in the VABHS, seeming to meet the goal of using telehealth to improve veterans’ access to specialty care and coordination of care between PCPs and specialists. While not examined in this initial report, e-Consults may lead to reduced costs, and this possibility should be further explored.
Despite concerns expressed by some specialists, most believed that e-Consults improved the quality of care their patients received. Future work needs to validate these findings, examine patient perspectives, delve more deeply into ways to improve and bring more value to the process, and address the specialty effort in relation to workload awarded.
Acknowledgements
The authors would like to thank Yisraela Elstein and Pauline Benedetti for their assistance in tracking workload data and Steven Simon for his detailed review of the manuscript. Funding for this work was supported by the Department of Veterans Affairs Specialty Care Transformation Initiative for the 21st Century—T21 innovation grant for telehealth initiatives.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
The VA is charged with providing high-quality, efficient care for those who have served in the armed forces. Starting in 2009, leadership within the VA articulated a set of “transformational” initiatives, which included greater implementation of telemedicine to improve the coordination of veterans’ care and access to specialty care.
The VA Boston Healthcare System (VABHS) consists of 3 major facilities and multiple community-based outpatient clinics (CBOCs) across eastern Massachusetts. Most medical specialty care is concentrated near the single acute-care site, resulting in a diffuse network of primary and specialty care providers. Consequently, many patients travel beyond their primary medical facility to access specialists. To address this fragmentation, the Medical Service at VABHS implemented a system of electronic chart review consultations (e-Consults) to provide high-quality, efficient care while maintaining the integrity of the patient’s medical home and improving access to specialty providers.
Designed to address clinical questions that may not require a face-to-face (FTF) encounter, e-Consults are a form of store-and-forward telemedicine (SAFT), with information-sharing between requesting providers (typically primary care) and answering specialists, using the VA’s existing electronic health record (EHR), the Computerized Patient Record System (CPRS). Store-and-forward telemedicine increases patient access to specialists and timeliness in initiating a consultation while decreasing patient travel and associated costs.1-15
Although previous studies have usually found high concordance between SAFT and FTF consultations in both diagnosis and disease management, the feasibility of SAFT has been historically limited by the lack of an integrated shared EHR.16-18 The VA implemented CPRS in 1996 and was soon recognized as a fully integrated EHR, allowing any VA clinician to view any veteran’s medical record nationwide. This technology, combined with the geographically dispersed population of veterans and the concentration of specialty services within mostly urban tertiary care centers, makes the VA an optimal environment to benefit from implementation of SAFT.
This study examines the implementation of e-Consults in a large VA medical service with the primary goal of facilitating timely specialty care access. This article details the initial experience of implementation and clinicians’ early perceptions of the system.
E-Consult Implementation
The VABHS provides tertiary specialty care for much of New England. It also serves a primary care population of more than 30,000 veterans in eastern Massachusetts through a network of 3 larger primary care practices based at 3 main campuses and 5 smaller CBOCs. Most specialty care is provided by the 2 campuses in Boston.
Discussions with primary care leadership and e-mail announcements to primary care providers (PCPs) throughout VABHS announced the availability and purpose of the new e-Consult. The initiative was initially piloted within 2 specialties, allergy and cardiology, beginning in January 2011. After establishing the logistics for routing, processing, and encounter data capture, e-Consults were expanded to the remaining subspecialties by December 2011.
Within 9 months, 12 different specialties had e-Consult options: endocrinology, gastroenterology, hematology, infectious disease, nephrology, oncology, palliative care, pulmonary disease, rheumatology, and sleep medicine. The e-Consult use was tracked for this study on a monthly basis.
Process and Work Flow
Any PCP with consult ordering privileges can request an e-Consult. Expectations were set that e-Consults should be used to request help with nonemergent clinical issues and be completed within 3 nonholiday weekdays.
The e-Consult uses CPRS modular programming to create an event-based electronic consultation request. Once initiated, the consult is routed to the consulting service. Each specialty defines how to triage the e-Consult request; computer alerts can be immediately generated to a single individual or a defined team within each specialty. Options to alert or print consult requests to administrative personnel also exist. As with requests for FTF consultation requests, e-Consults allow specialists the options to deny a request if they feel it is not appropriate for their specialty. In addition, specialists are able to convert the e-Consult request to a FTF encounter, to forward the e-Consult to another specialty, or to add a “comment” before proceeding. These comments generate an immediate electronic alert to the requestor, thus providing a forum for asynchronous preconsultation dialogue between providers. Once the consultation is complete, the requestor receives an alert that directs them to view the completed consultation note in the EHR.
The entirety of the EHR is accessible to the consultant, including all primary care and specialty progress notes, inpatient and outpatient laboratory testing, and all diagnostic testing performed nationally within the VA.
User Feedback
Based on the literature, separate online questionnaires were developed to ascertain primary care clinicians’ and specialists’ perceptions of the e-Consult system. This 26-item survey for PCPs and 13-item survey for specialists assessed use of e-Consults, satisfaction with the system, and for primary care clinicians, perceived benefits of the e-Consult process
and specific feedback by specialty.
Nine months after full implementation, perceptions of e-Consult processes were solicited. All PCPs, staff physicians, nurse practitioners, and internal medicine residents assigned to a VABHS primary care clinic were surveyed. Eligible specialist consultants included attending physicians and fellows who were identified by the specialty chief as potentially having responded to an e-Consult in the past 6 months. The survey was administered in June 2012 and queried the previous 6 months’ experience.
The monthly number of e-Consults ranged from 96 to 137 over the 6-month study period. Cardiology and hematology were the most commonly requested e-Consults, with > 20 per specialty per month. The 146 cardiology e-Consults during those 6 months represented 20% of all new cardiology consults, whereas the 159 e-Consults to hematology represented 53% of their new consults. The least used specialties for e-Consults were allergy, oncology, palliative care, and rheumatology, each averaging < 2 e-Consults per month.
Provider Perceptions
Overall, 85 of the 163 providers completed the survey (52%). Permanent staff had a better response rate than did trainees: 58% vs 33% among PCPs (P < .02), and 71% vs 44% among specialists (P = .03).
Of the 47 primary care respondents, 4 residents and 2 staff physicians had not previously used e-Consults; all 4 residents and 1 staff physician stated that this was due to lack of awareness of the system. The remaining 41 PCPs had requested ≥ 1 e-Consult in the past 6 months. All 38 specialist provider respondents had answered ≥ 1 e-Consult in study period, with 9 providers completing > 20.
e-Consult Satisfaction
Overall, 93% of PCPs were satisfied with e-Consults, and none were dissatisfied, compared with 53% of specialty providers who were satisfied and 26% who were dissatisfied (P < .001) (Figure 1). All specialists who were dissatisfied were in the sections of cardiology or gastroenterology. Further inspection identified several differences between those specialty providers who were generally satisfied with e-Consults (n = 20) compared with those providers who were dissatisfied (n = 10). Eight PCPs reported being neither satisfied nor dissatisfied.
In analyses comparing satisfied specialists (n = 20) with dissatisfied specialists (n = 10), excluding the 8 respondents who were neither satisfied nor dissatisfied, those who were satisfied reported a lower rate of conversion of e-Consults to FTF visits than did their dissatisfied peers, 26% vs 52%, respectively (P = .01). Satisfied specialists were more likely to view e-Consults as replacing work they otherwise would have done, such as a replacement for a FTF consult and prior informal “curbside” consults, 79% vs 50%, respectively (P < .02). Satisfied specialists were somewhat more likely to complete their e-Consults, on average, in ≤ 15 minutes, compared with dissatisfied specialists, although this trend did not reach statistical significance (59% vs 49%, respectively; (P = .07) (Figure 2).
Improving Quality of Care
PCPs were asked to note which benefits were realized among patients for whom e-Consults replaced FTF visits. Nearly all (98%) indicated that the e-Consults enabled patients to avoid unnecessary travel, and 95% of PCPs indicated that they requested e-consults to receive faster clinical input about a patient. A total of 59% believed e-Consults helped their patients avoid additional copays, and 56% obtained specialty input for a patient who would otherwise refuse to travel. When an e-Consult did not avoid a FTF visit (ie, despite the e-Consult, the patient ultimately proceeded to a FTF consultation with the specialist), the majority of PCPs believed that the e-Consult provided them with reassurance and initiated diagnostic testing that would be useful during the patient’s subsequent FTF consult (Figure 3).
Overall, 61% of specialists agreed with the statement, “e-Consults improve the quality of care VA Boston Healthcare System provides.” Even when specialists perceived that e-Consults did not avoid FTF visits, most agreed that e-Consults helped initiate diagnostic testing prior to a FTF visit. In addition, satisfied specialists saw the benefit in reassuring the PCP and initiating additional management prior to a FTF visit.
Dynamics of e-Consults
Primary care providers reported that 78% of their requested e-Consults were completed within 2 days and 95% of all e-Consults were completed by day 3. In aggregate, primary care clinicians estimated that about one-third of their e-Consults replaced FTF visits; one-third replaced prior mechanisms for informal consultation (eg, “curbside” or e-mail); and one-third of e-Consults represented new requests that would not have involved specialty consultation in the absence of the e-Consult mechanism.
Specialists estimated that 27% of e-Consults were new work (ie, consultations that would not have occurred formally or informally in the absence of the e-Consult mechanism); 32% replaced FTF visits; and 42% were substitutes for prior informal communications. Specialists reported a wide range of time required to answer e-Consults. For specialists, on average, 54% of e-Consults took < 15 minutes to complete, but 20% took > 25 minutes, with 6% requiring ≥ 45 minutes to complete (Figure 2).
Discussion
This article reports the initial experience with the implementation of an e-Consult system for PCPs and medical subspecialists in a large VA health care system. Primary care providers were generally satisfied with the e-Consult system and reported that the system yielded tangible benefits to patients, such as quicker specialty input and avoidance of FTF visits and travel. Specialists were somewhat less satisfied than were their primary care colleagues; nevertheless, specialists perceived similar benefits for patients. Despite the general satisfaction among PCPs, > 1 in 4 specialists expressed dissatisfaction with the system.
Satisfaction with e-Consults may be influenced by the typology of the specialty itself. Some specialties (eg, hematology) rely more heavily on laboratory tests, compared with specialties such as cardiology, gastroenterology, or pulmonary whose subtleties of history and physical examination (H&P), along with review of imaging data, are more commonly required for clinical decision making.2 Hence, the EHR may allow some specialties to provide e-Consults with greater facility. For example, because the EHR enables clinicians to trend the results of years of complete blood counts with a few simple keystrokes, a hematologist may have more confidence in making a clinical assessment without seeing the patient based on historical information in the EHR.
Anecdotal evidence from specialists suggests discomfort when clinical input is based on H&P findings of others. While the sample size was small, this reasoning could explain the differences in satisfaction among specialties: Satisfied specialists may be taking slightly less time to complete their e-Consults, but they were less likely to view the e-Consult as new work and less likely to convert to a FTF consultation.
Store-and-forward telemedicine cannot completely replace FTF visits, but rather supplement them. If e-Consults obviate patient travel and copays while stimulating more timely completion of the consultation, the benefits of cost savings and improved specialty access by veterans merit further attention. When an e-Consult did not avoid a FTF consultation, the majority of clinicians perceived that the e-Consult allowed the PCP to initiate diagnostic testing or alterations in treatment prior to the eventual FTF consultation with the specialist. This finding could be considered as a proxy for increased coordination of care between primary care and specialty providers in anticipation of a FTF visit.
During the study period, workload capture for e-Consults was administratively fixed at the level of a brief consultation, regardless of the effort expended. Validation of the current finding regarding time spent completing consults has resulted in a change. On January 10, 2014, VA Central Office updated its policy to allow 3 levels of workload credit for e-Consults based on time: up to 15 minutes, 16 to 30 minutes, and > 30 minutes.
E-consults are just one example of innovations that broadly fall under the rubric of telehealth initiatives being deployed across VA health care facilities. Other examples of SAFT include pictures collected by technicians and sent to specialists for review to screen for diabetic retinopathy, a practice done within VA for many years.19 Clinical video telehealth provides real-time videoconferencing between patient and specialist and can obviate the need for long-distance patient travel. Initially used only for interviews, newer equipment amplifies sound and enhances optical imaging to allow thorough physical examinations when trained personnel are handling the equipment.
Each technology has its challenges for implementation, which vary in degree of coordination and cost. Overall, the application of new technologies and repurposing existing ones may be limited by creativity alone in the efforts to improve access and quality of the care provided to veterans. The Table highlights telehealth uses being deployed by VA.
Conclusion
Electronic consults have been well received by PCPs and most specialists in the VABHS, seeming to meet the goal of using telehealth to improve veterans’ access to specialty care and coordination of care between PCPs and specialists. While not examined in this initial report, e-Consults may lead to reduced costs, and this possibility should be further explored.
Despite concerns expressed by some specialists, most believed that e-Consults improved the quality of care their patients received. Future work needs to validate these findings, examine patient perspectives, delve more deeply into ways to improve and bring more value to the process, and address the specialty effort in relation to workload awarded.
Acknowledgements
The authors would like to thank Yisraela Elstein and Pauline Benedetti for their assistance in tracking workload data and Steven Simon for his detailed review of the manuscript. Funding for this work was supported by the Department of Veterans Affairs Specialty Care Transformation Initiative for the 21st Century—T21 innovation grant for telehealth initiatives.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Cusack CM, Pan E, Hook JM, et al. The Value of Provider-to-Provider Telehealth Technologies. Charlestown, MA: Center for Information Technology Leadership; 2007.
2. Horner K, Wagner E, Tufano J. Electronic consultations between primary and specialty care clinicians: Early insights. Issue Brief (Commonw Fund). 2011;23:1-14.
3. Angstman KB, Rohrer JE, Adamson SC, Chaudhry R. Impact of e-consults on return visits of primary care patients. Health Care Manag (Frederick). 2009;28(3):253-257.
4. Kim Y, Chen AH, Keith E, Yee HF Jr, Kushel MB. Not perfect, but better: Primary care providers’ experiences with electronic referrals in a safety net health system. J Gen Intern Med. 2009;24(5):614-619.
5. Angstman KB, Adamson SC, Furst JW, Houston MS, Rohrer JE. Provider satisfaction with virtual specialist consultations in a family medicine department. Health Care Manag (Frederick). 2009;28(1):14-18.
6. Mahnke CB, Jordan CP, Bergvall E, Person DA, Pinsker JE. The Pacific Asynchronous TeleHealth (PATH) system: Review of 1,000 pediatric teleconsultations. Telemed J E Health. 2011;17(1):35-39.
7. Sood S, Mbarika V, Jugoo S, et al. What is telemedicine? A collection of 104 peer-reviewed perspectives and theoretical underpinnings. Telemed J E Health. 2007;13(5):573-590.
8. Maeder A. Telehealth and remote access. Stud Health Technol Inform. 2010;151:239-254.
9. Schmidt T, Lappan CM, Hospenthal DR, Murray CK. Deployed provider satisfaction with infectious disease teleconsulation. Mil Med. 2011;176(12):1417-1420.
10. Verhoeven F, Tanja-Dijkstra K, Nijland N, Eysenbach G, van Gemert-Pijnen L. Asynchronous and synchronous teleconsultation for diabetes care: A systematic literature review. J Diabetes Sci Technol. 2010;4(3):666-684.
11. Krupinski E, Nypaver M, Poropatich R, Ellis D, Safwat R, Sapci H. Telemedicine/telehealth: An international perspective. Clinical applications in telemedicine/telehealth. Telemed J E Health. 2002;8(1):13-34.
12. Moreno-Ramirez D, Ferrandiz L, Ruiz-de-Casas A, et al. Economic evaluation of a store-and-forward teledermatology system for skin cancer patients. J Telemed Telecare. 2009;15(1):40-45.
13. Callahan CW, Malone F, Estroff D, Person DA. Effectiveness of an Internet-based store-and-forward telemedicine system for pediatric subspecialty consultation. Arch Pediatr Adolesc Med. 2005;159(4):389-393.
14. Gagnon MP, Duplantie J, Fortin JP, Landry R. Implementing telehealth to support medical practice in rural/remote regions: What are the conditions for success? Implement Sci. 2006;1:18.
15. Hailey D, Ohinmaa A, Roine R. Study quality and evidence of benefit in recent assessments of telemedicine. J Telemed Telecare. 2004;10(6):318-324.
16. High WA, Houston MS, Calobrisi SD, Drage LA, McEvoy MT. Assessment of the accuracy of low-cost store-and-forward teledermatology consultation. J Am Acad Dermatol. 2000;42(5 pt 1):776-783.
17. Houston MS, Myers JD, Levens SP, et al. Clinical consultations using store-and-forward telemedicine technology. Mayo Clin Proc. 1999;74(8):764-769.
18. Hersh WR, Hickam DH, Severance SM, Dana TL, Pyle Krages K, Helfand M. Diagnosis, access and outcomes: Update of a systematic review of telemedicine services. J Telemed Telecare. 2006;12(suppl 2):S3-S31.
19. Cavallerano AA, Conlin PR. Teleretinal imaging to screen for diabetic retinopathy in the Veterans Health Administration. J Diabetes Sci Technol. 2008;2(1):33-39.
20. Arora S, Thornton K, Murata G, et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med. 2011;364(23):2199-2207.
1. Cusack CM, Pan E, Hook JM, et al. The Value of Provider-to-Provider Telehealth Technologies. Charlestown, MA: Center for Information Technology Leadership; 2007.
2. Horner K, Wagner E, Tufano J. Electronic consultations between primary and specialty care clinicians: Early insights. Issue Brief (Commonw Fund). 2011;23:1-14.
3. Angstman KB, Rohrer JE, Adamson SC, Chaudhry R. Impact of e-consults on return visits of primary care patients. Health Care Manag (Frederick). 2009;28(3):253-257.
4. Kim Y, Chen AH, Keith E, Yee HF Jr, Kushel MB. Not perfect, but better: Primary care providers’ experiences with electronic referrals in a safety net health system. J Gen Intern Med. 2009;24(5):614-619.
5. Angstman KB, Adamson SC, Furst JW, Houston MS, Rohrer JE. Provider satisfaction with virtual specialist consultations in a family medicine department. Health Care Manag (Frederick). 2009;28(1):14-18.
6. Mahnke CB, Jordan CP, Bergvall E, Person DA, Pinsker JE. The Pacific Asynchronous TeleHealth (PATH) system: Review of 1,000 pediatric teleconsultations. Telemed J E Health. 2011;17(1):35-39.
7. Sood S, Mbarika V, Jugoo S, et al. What is telemedicine? A collection of 104 peer-reviewed perspectives and theoretical underpinnings. Telemed J E Health. 2007;13(5):573-590.
8. Maeder A. Telehealth and remote access. Stud Health Technol Inform. 2010;151:239-254.
9. Schmidt T, Lappan CM, Hospenthal DR, Murray CK. Deployed provider satisfaction with infectious disease teleconsulation. Mil Med. 2011;176(12):1417-1420.
10. Verhoeven F, Tanja-Dijkstra K, Nijland N, Eysenbach G, van Gemert-Pijnen L. Asynchronous and synchronous teleconsultation for diabetes care: A systematic literature review. J Diabetes Sci Technol. 2010;4(3):666-684.
11. Krupinski E, Nypaver M, Poropatich R, Ellis D, Safwat R, Sapci H. Telemedicine/telehealth: An international perspective. Clinical applications in telemedicine/telehealth. Telemed J E Health. 2002;8(1):13-34.
12. Moreno-Ramirez D, Ferrandiz L, Ruiz-de-Casas A, et al. Economic evaluation of a store-and-forward teledermatology system for skin cancer patients. J Telemed Telecare. 2009;15(1):40-45.
13. Callahan CW, Malone F, Estroff D, Person DA. Effectiveness of an Internet-based store-and-forward telemedicine system for pediatric subspecialty consultation. Arch Pediatr Adolesc Med. 2005;159(4):389-393.
14. Gagnon MP, Duplantie J, Fortin JP, Landry R. Implementing telehealth to support medical practice in rural/remote regions: What are the conditions for success? Implement Sci. 2006;1:18.
15. Hailey D, Ohinmaa A, Roine R. Study quality and evidence of benefit in recent assessments of telemedicine. J Telemed Telecare. 2004;10(6):318-324.
16. High WA, Houston MS, Calobrisi SD, Drage LA, McEvoy MT. Assessment of the accuracy of low-cost store-and-forward teledermatology consultation. J Am Acad Dermatol. 2000;42(5 pt 1):776-783.
17. Houston MS, Myers JD, Levens SP, et al. Clinical consultations using store-and-forward telemedicine technology. Mayo Clin Proc. 1999;74(8):764-769.
18. Hersh WR, Hickam DH, Severance SM, Dana TL, Pyle Krages K, Helfand M. Diagnosis, access and outcomes: Update of a systematic review of telemedicine services. J Telemed Telecare. 2006;12(suppl 2):S3-S31.
19. Cavallerano AA, Conlin PR. Teleretinal imaging to screen for diabetic retinopathy in the Veterans Health Administration. J Diabetes Sci Technol. 2008;2(1):33-39.
20. Arora S, Thornton K, Murata G, et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med. 2011;364(23):2199-2207.
Chronic vulvar symptoms and dermatologic disruptions: How to make the correct diagnosis
Nearly one in every six women will experience chronic vulvar symptoms at some point, from ongoing itching to sensations of rawness, burning, or dyspareunia. Regrettably, clinicians generally are taught only a few possible causes for these symptoms, primarily infections such as yeast, bacterial vaginosis, herpes simplex virus, or anogenital warts. However, infections rarely produce chronic symptoms that do not respond, at least temporarily, to therapy.
In this two-part series, we focus on a total of 10 cases of vulvar symptoms, zeroing in on diagnosis and treatment. In this first part, we describe five patient scenarios illustrating the diagnosis and treatment of:
- lichen sclerosus
- vulvodynia
- lichen simplex chronicus
- lichen planus
- hidradenitis suppurativa.
In many chronic cases, more than one entity is the cause
Specific skin diseases, sensations of rawness from various external and internal irritants, neuropathy, and psychological issues are all much more common causes of chronic vulvar symptoms than infection. Moreover, most women with chronic vulvar symptoms have more than one entity producing their discomfort.
Very often, the cause of a patient’s symptoms is not clear at the first visit, with nonspecific redness or even normal skin seen on examination. Pathognomonic skin findings can be obscured by irritant contact dermatitis caused by unnecessary medications or overwashing, atrophic vaginitis, and/or rubbing and scratching. In such cases, obvious abnormalities must be eliminated and the patient reevaluated to definitively discover and treat the cause of the symptoms.
CASE 1. ANOGENITAL ITCHING AND DYSPAREUNIA
A 62-year-old woman schedules a visit to address her anogenital itching. She reports pain with scratching and has developed introital dyspareunia. On physical examination, you find a well-demarcated white plaque of thickened, crinkled skin (FIGURE 1). A wet mount shows parabasal cells and no lactobacilli.
Diagnosis: Lichen sclerosus and atrophic vagina.
Treatment: Halobetasol ointment, an ultra-potent topical corticosteroid, once or twice daily; along with estradiol cream (0.5 g intravaginally) 3 times a week.
Lichen sclerosus is a skin disease found most often on the vulva of postmenopausal women, although it also can affect prepubertal children and reproductive-age women. Lichen sclerosus is multifactorial in pathogenesis, including prominent autoimmune factors, local environmental factors, and genetic predisposition.1
Although there is no cure for lichen sclerosus, the symptoms and clinical abnormalities usually can be well managed with ultra-potent topical corticosteroids. However, scarring and architectural changes are not reversible. Moreover, poorly controlled lichen sclerosus exhibits malignant transformation on anogenital skin in about 3% of affected patients.
The standard of care is application of an ultra-potent topical corticosteroid ointment once or twice daily until the skin texture normalizes again. The most common of such corticosteroids are clobetasol, halobetasol, and betamethasone dipropionate in an augmented vehicle (betamethasone dipropionate in the usual vehicle is only a medium-high medication in terms of potency.) One of us (L.E.) finds that some women experience irritation with generic clobetasol.
The ointment form of the selected corticosteroid is preferred, as creams are irritating to the vulva in most women because they contain more alcohols and preservatives than ointments do. The amount to be used is very small—far smaller than the pea-sized amount often suggested. By using this smaller amount, we avoid spread to the surrounding hair-bearing skin, which is at greater risk for steroid dermatitis and atrophy than the modified mucous membranes.
Related video: Lichen sclerosis: My approach to treatment Michael Baggish, MD
Even asymptomatic lichen sclerosus can progress
Most vulvologists agree that when the skin normalizes (not when symptoms subside), it is best to either decrease the frequency of application of the ultra-potent corticosteroid to two or three times a week, or to continue daily use with a lower-potency corticosteroid such as triamcinolone ointment 0.1%. Discontinuation of therapy usually results in recurrence.2
Treatment should not be based solely on symptoms, as asymptomatic lichen sclerosus can progress and cause permanent scarring and an increased risk for squamous cell carcinoma.
Although no studies have shown a decreased risk for squamous cell carcinoma with ongoing use of a corticosteroid, vulvologists have observed that malignant transformation occurs uniformly in the setting of poorly controlled lichen sclerosus. Immune dysregulation and inflammation may play an important role, so careful management to minimize inflammation may help prevent a malignancy.3
Secondary treatment choices
Secondary choices for lichen sclerosus include the topical calcineurin inhibitors tacrolimus (Protopic) and pimecrolimus (Elidel) but not testosterone, which has been shown to be ineffective. Tacrolimus and pimecrolimus are useful but often burn upon application, and they are “black-boxed” for cutaneous squamous cell carcinoma and lymphoma. Therefore, although squamous cell carcinoma associated with their use is extraordinarily uncommon, patients should be advised of these risks, particularly because lichen sclerosus already exhibits this association.
Most postmenopausal women with lichen sclerosus also exhibit hypothyroidism, so they should be monitored for this. However, thyroid function testing in 18 children showed no evidence of hypothyroidism in that age group (L.E. unpublished data).
Estrogen replacement may be advised
Postmenopausal women who have prominent introital lichen sclerosus or dyspareunia should receive estrogen replacement of some type so that there is only one cause, rather than two, for their dyspareunia, thinning, fragility, and inelasticity.
Women with well-controlled lichen sclerosus should be followed twice a year to ensure that their disease remains suppressed with ongoing therapy, and to evaluate for active disease, adverse effects of therapy, and the appearance of dysplasia or squamous cell carcinoma.
Women with lichen sclerosus occasionally experience discomfort after their clinical skin disease has cleared. These women now have developed vulvodynia triggered by their lichen sclerosus.
Related series: Vulvar Pain Syndromes—A 3-part roundtable
Part 1. Making the correct diagnosis (September 2011)
Part 2. A bounty of treatments—but not all of them proven (October 2011)
Part 3. Causes and treatment of vestibulodynia (November 2011)
CASE 2. IS IT REALLY CHRONIC YEAST INFECTION?
A 36-year-old woman consults you about her history of chronic yeast infection that manifests as introital burning, discharge, and dyspareunia. She is otherwise healthy, except for irritable bowel syndrome and fibromyalgia.
Physical examination reveals a mild patchy redness of the vestibule and surrounding modified mucous membranes (FIGURE 2). Gentle probing with a cotton swab triggers exquisite pain in the vestibule, with slight extension to the labia minora. A wet mount shows no evidence of increased white blood cells, parabasal cells, clue cells, or yeast forms. Lactobacilli are abundant.
Diagnosis: Vulvodynia, with a nearly vestibulodynia pattern.
Treatment: Venlafaxine and pelvic floor physical therapy.
Vulvodynia is a genital pain syndrome defined as sensations of chronic burning, irritation, rawness, and soreness in the absence of objective disease and infection that could explain the discomfort. Vulvodynia occurs in approximately 7% to 8% of women.4
Vulvodynia generally is believed to be a multifactorial symptom, occurring as a result of pelvic floor dysfunction and neuropathic pain,5,6 with anxiety/depression issues exacerbating symptoms. Some recent studies have shown the presence of biochemical mediators of inflammation in the absence of clinical and histologic inflammation.7 Discomfort often is worsened by infections or the application of common irritants (creams, panty liners, soaps, some topical anesthetics). Estrogen deficiency is another common exacerbating factor.
Women tend to exhibit other pain syndromes such as chronic headaches, fibromyalgia, temperomandibular disorder, or premenstrual syndrome, as well as prominent anxiety, depression, sleep disorder, and so on.
Almost uniformly present are symptoms of pelvic floor dysfunction, such as constipation, irritable bowel syndrome, and interstitial cystitis or urinary symptoms in the absence of a urinary tract infection. These women also are frequently unusually intolerant of medications.
Classifying vulvodynia
There are two primary patterns of vulvodynia. The first and most common is vestibulodynia, formerly called vulvar vestibulitis. The term vestibulitis was eliminated to reflect the absence of clinical and histologic inflammation. Vestibulodynia refers to pain that is always limited to the vestibule. Generalized vulvodynia, however, extends beyond the vestibule, is migratory, or does not include the vestibule.
Several vulvologists have found that many patients exhibit features of both types of vulvodynia, and these patterns probably exist on a spectrum. The difference is probably unimportant in clinical practice, except that vestibulodynia can be treated with vestibulectomy.
How we manage vulvodynia
We focus on pelvic floor physical therapy and on the provision of medication for neuropathic pain, which is initiated at very small doses and gradually increased to active doses.8 The medications used and the ultimate doses often required include:
- amitriptyline or desipramine 150 mg
- gabapentin 600 to 1,200 mg three times daily
- venlafaxine XR 150 mg daily
- pregabalin 150 mg twice a day
- duloxetine 60 mg a day.
Compounded amitriptyline 2% with baclofen 2% cream applied three times daily is beneficial for many patients, and topical lidocaine jelly 2% or ointment 5% (which often burns) can help provide immediate temporary relief.
Most patients require sex therapy and counseling for maximal improvement. Women with vestibulodynia in whom these therapies fail are good candidates for vestibulectomy if their pain is strictly limited to the vestibule. Fortunately, most women do not require this aggressive therapy.
Related article: Successful treatment of chronic vaginitis Robert L. Barbieri, MD (Editorial; July 2013)
CASE 3. SEVERE ITCHING DISRUPTS SLEEP
A 34-year-old patient reports excruciating itching, with disruption of daily activities and sleep. She has been treated for candidiasis on multiple occasions, but in your office her wet mount and confirmatory culture are negative. Physical examination reveals a pink, lichenified plaque with excoriation (FIGURE 3).
Diagnosis: Lichen simplex chronicus.
Treatment: Ultra-potent corticosteroid ointment applied very sparingly twice daily and covered with petroleum jelly. You also order nighttime sedation with amitriptyline to break the itch-scratch cycle. When the patient’s itching resolves and her skin clears, you taper her off the corticosteroid, warning her that recurrence is likely, and instruct her to restart the medication immediately should itching recur.
Lichen simplex chronicus (formerly called squamous hyperplasia or hyperplastic dystrophy, and also known as eczema, neurodermatitis, or localized atopic dermatitis) occurs when irritation from any cause produces itching in a predisposed person. The subsequent scratching and rubbing both produce the rash and exacerbate the irritation that drives the itching, even after the original cause is gone. The rubbing and scratching perpetuate the irritation and itching, producing the “itch-scratch” cycle.
The appearance of lichen simplex chronicus is produced by rubbing (where the skin thickens and lichenifies) or scratching (where the skin becomes red with linear erosions, called excoriations, caused by fingernails).
The initial trigger for lichen simplex chronicus often is an infection—often yeast—but overwashing, stress, sweat, heat, urine, irritating lubricants, and use of panty liners also may precipitate the itching. At the office visit, the original infection or other cause of irritation often is no longer present, and only lichen simplex chronicus can be identified.
How to treat lichen simplex chronicus
Management of lichen simplex chronicus requires very sparing application of an ultra-potent topical corticosteroid (clobetasol, halobetasol, or betamethasone dipropionate in an augmented vehicle ointment) twice daily, with the ointment covered with petroleum jelly. Care also must be taken to avoid irritants.
In addition, nighttime sedation helps to interrupt the itch-scratch cycle by preventing rubbing during sleep.
When the skin appears normal and itching has resolved, taper the medication down or off, warning the patient that recurrence is common with any future irritation.
Restart therapy immediately upon recurrence to prevent lichenification and chronic problems.
Second-line medications include calcineurin inhibitors (tacrolimus or pimecrolimus). Although these agents do not contribute to atrophy, they are less effective than topical corticosteroids,9 cost more, and can cause burning upon application.
Unlike lichen sclerosus, lichen simplex chronicus does not always recur upon cessation of treatment, and there is no need for concern about an increased risk of malignancy or significant scarring.
Related article: New treatment option for vulvar and vaginal atrophy Andrew M. Kaunitz, MD (News for your Practice; May 2013)
CASE 4. ORAL AND VULVAR INVOLVEMENT
A 73-year-old patient seeks your help in alleviating longstanding introital itching and rawness, with dyspareunia. She has tried topical estradiol cream intravaginally three times weekly in combination with weekly fluconazole, to no avail.
Physical examination reveals deep red patches and erosions of the vestibule, with complete resorption of the labia minora (FIGURE 4). Patchy redness of the vagina is apparent as well, so you examine the patient’s mouth and find deep redness of the gingivae and erosions of the buccal mucosae, with surrounding white, lacy papules. A wet mount shows a marked increase in lymphocytes and parabasal cells, with a pH of more than 7.
Diagnosis: After correlating the vulvar and oral findings, you make a diagnosis of lichen planus.
Treatment: You initiate halobetasol ointment twice daily, to be applied to the vulva. You also continue vaginal estradiol cream but add hydrocortisone acetate 200 mg compounded vaginal suppositories nightly, as well as clobetasol gel to be applied to oral lesions three times a day. You follow the patient closely for secondary yeast of the mouth and vagina.
Erosive multimucosal lichen planus is a disease of cell-mediated immunity that overwhelmingly affects menopausal women. The most common surfaces involved are the mouth, vagina, rectal mucosa, and vulva; usually, at least two surfaces are affected. The esophagus, extra-auditory canals, nasal mucosa, and eyes also can be involved. Dry, extragenital skin usually is not affected in the setting of erosive vulvovaginal lichen planus.
Vulvar lichen planus most often is controlled with ultra-potent topical corticosteroids (again, clobetasol, halobetasol, or betamethasone dipropionate in an augmented vehicle), but other mucosal surfaces often are more difficult to manage. Although there is no definitive cure for this condition, careful local care, estrogen replacement, and suppression of oral and vulvovaginal candidiasis usually provide relief.
Calcineurin inhibitors (tacrolimus, pimecrolimus) sometimes are useful in patients who improve only partially after treatment with a topical corticosteroid, provided burning with application is tolerable.10 Systemic immunosuppressants such as hydroxychloroquine, methotrexate, mycophenolate mofetil, azathioprine, cyclosporine, cyclophosphamide, and tumor necrosis factor (TNF) alpha blockers (etanercept, adalimumab, infliximab), as well as oral retinoids, can be added for more recalcitrant disease.11
How to manage disease that affects the vagina
When the vagina is involved in lichen planus, treatment is important to prevent scarring, as well as rawness and pain from irritant contact dermatitis caused by purulent vaginal secretions. Occasionally, a 25-mg hydrocortisone acetate rectal suppository inserted into the vagina nightly improves vaginal lichen planus, but sometimes more potent suppositories, such as doses of 100 to 200 mg, may be compounded. Dilators should be inserted daily to prevent vaginal synechiae.
Oral involvement requires targeted treatment
The mouth is almost always involved in lichen planus. If a dermatologist is not involved in patient care, a prescription for dexamethasone/nystatin elixir (50:50) (5 mL swish, hold, and spit four times daily) can improve oral symptoms remarkably. Alternatively, clobetasol gel applied to affected areas of the mouth three or four times daily can be helpful. Secondary yeast of the vagina and mouth are common with the use of topical corticosteroids.
Careful clinical follow-up is advised
Like uncontrolled lichen sclerosus, erosive lichen planus of the vulva produces scarring and sometimes eventuates into squamous cell carcinoma. Therefore, careful clinical surveillance is warranted. And therapy must be continued to prevent recurrence of lichen planus (as it must be for lichen sclerosus), scarring, and to decrease the risk of squamous cell carcinoma. And like lichen sclerosus, lichen planus sometimes triggers vulvodynia.
CASE 5. MULTIPLE BOILS IN THE GROIN
A 31-year-old morbidly obese African American woman comes to your office with continually evolving boils in the groin. A culture shows Bacterioides spp, Escherichia coli, and Peptococcus spp. In the past, multiple courses of various antibiotics have provided only modest relief.
Physical examination reveals fluctuant nodules, scars, and draining sinus tracts of the hair-bearing vulva and crural crease (FIGURE 5). The axillae are clear.
Diagnosis: Hidradenitis suppurativa.
Treatment: The patient begins taking minocycline 100 mg twice daily. Because she is a smoker, you refer her to an aggressive primary care provider for smoking cessation and weight loss management.
Three months later, the patient is developing only about two nodules a month, managed by early intralesional injections of triamcinolone acetonide.
Hidradenitis suppurativa is sometimes called inverse acne because the underlying pathogenesis is similar to cystic acne. Follicular plugging with keratin debris occurs, with additional keratin, sebaceous material, and normal skin bacteria trapped below the occlusion and distending the follicle. As the follicle wall stretches, thins, and allows for leakage of keratin debris into surrounding dermis, a brisk foreign-body response produces a noninfectious abscess.
Hidradenitis suppurativa affects more than 2% of the population.12 It appears only in areas of the body that contain apocrine glands and in individuals who have double- or triple-outlet follicles that predispose them to follicular occlusion. Therefore, this disease has a genetic component.
Other risk factors include male sex, African genetic background, obesity, and smoking. The prevalence of metabolic syndrome is significantly higher in individuals with hidradenitis suppurativa than in the general population.13
Recommended management
Treatments include:
- chronic antibiotics with nonspecific anti-inflammatory activity (tetracyclines, erythromycin, clindamycin, and trimethoprim-sulfamethoxazole)
- intralesional injection of corticosteroids for early nodules (which often aborts their development)
- TNF alpha blockers (etanercept, adalimumab, infliximab)14–16
- surgical removal of affected skin—the definitive therapy.
Note, however, that anogenital hidradenitis often is too extensive for surgery to be practical. In patients who have localized hidradenitis, primary excision is an excellent early therapy, provided the patient is advised that recurrence may occur in apocrine-containing nearby skin. Aggressive curettage of the roof of the cysts has been performed by some clinicians with good response.
Don’t overlook adjuvant approaches
Smoking cessation and weight loss often are useful.
Other therapies backed by anecdotal evidence include oral contraceptives or spironolactone for their anti-androgen effect, as well as metformin, a more recently studied agent.
Local care with antibacterial soaps and topical antibiotics may be useful for some women.
MORE CASES TO COME
In Part 2 of this series, which will appear in the June 2014 issue of OBG Management, we will discuss the following cases:
- atrophic vagina and atrophic vaginitis
- contact dermatitis
- vulvar aphthae
- desquamative inflammatory vaginitis
- psoriasis.
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article. Send your letter to: [email protected] Please include the city and state in which you practice.
- Doulaveri G, Armira K, Kouris A, et al. Genital vulvar lichen sclerosus in monozygotic twin women: A case report and review of the literature. Case Rep Dermatol. 2013;5(3):321–325.
- Virgili A, Minghetti S, Borghi A, Corazza M. Proactive maintenance therapy with a topical corticosteroid for vulvar lichen sclerosus: Preliminary results of a randomized study. Br J Dermatol. 2013;168(6):1316–1324.
- Brodrick B, Belkin ZR, Goldstein AT. Influence of treatments on prognosis for vulvar lichen sclerosus: Facts and controversies. Clin Dermatol. 2013;31(6):780–786.
- Harlow BL, Kunitz CG, Nguyen RH, Rydell SA, Turner RM, MacLehose RF. Prevalence of symptoms consistent with a diagnosis of vulvodynia: Population-based estimates from two geographic regions. Am J Obstet Gynecol. 2014;210(1):40.e1–e8.
- Morin M, Bergeron S, Khalife S, Mayrand MH, Binik YM. Morphometry of the pelvic floor muscles in women with and without provoked vestibulodynia using 4D ultrasound. J Sex Med. 2014;11(3):776–785.
- Hampson JP, Reed BD, Clauw DJ, et al. Augmented central pain processing in vulvodynia. J Pain. 2013;14(6):579–589.
- Omoigui S. The biochemical origin of pain: the origin of all pain is inflammation and the inflammatory response. Part 2 of 3: Inflammatory profile of pain syndromes. Med Hypotheses. 2007;69(6):1169–1178.
- Haefner HK, Collins ME, Davis GD, et al. The vulvodynia guideline. J Low Genit Tract Dis. 2005;9(1):40–51.
- Frankel HC, Qureshi AA. Comparative effectiveness of topical calcineurin inhibitors in adult patients with atopic dermatitis. Am J Clin Dermatol. 2012;13(2):113–123.
- Samycia M, Lin AN. Efficacy of topical calcineurin inhibitors in lichen planus. J Cutan Med Surg. 2012;16(4):221–229.
- Mirowski GW, Goddard A. Treatment of vulvovaginal lichen planus. Dermatol Clin. 2010;28(4):717–725.
- Vinding GR, Miller IM, Zarchi K, et al. The prevalence of inverse recurrent suppuration: A population-based study of possible hidradenitis suppurativa [published online ahead of print December 16, 2013]. Br J Dermatol. doi:10.1111/bjd.12787.
- Gold DA, Reeder VJ, Mahan MG, Hamzavi IH. The prevalence of metabolic syndrome in patients with hidradenitis suppurativa. J Am Acad Dermatol. 2014;70(4):699–703.
- Scheinfeld N. Hidradenitis suppurativa: A practical review of possible medical treatments based on over 350 hidradenitis patients. Dermatol Online J. 2013;19(4):1.
- Kimball AB, Kerdel F, Adams D, et al. Adalimumab for the treatment of moderate to severe hidradenitis suppurativa:
A parallel randomized trial. Ann Intern Med. 2012;157(12):846–855. - Chinniah N, Cains GD. Moderate to severe hidradenitis suppurativa treated with biological therapies [published online ahead of print January 23, 2014]. Australas J Dermatol. doi:10.1111/ajd.12136.
Nearly one in every six women will experience chronic vulvar symptoms at some point, from ongoing itching to sensations of rawness, burning, or dyspareunia. Regrettably, clinicians generally are taught only a few possible causes for these symptoms, primarily infections such as yeast, bacterial vaginosis, herpes simplex virus, or anogenital warts. However, infections rarely produce chronic symptoms that do not respond, at least temporarily, to therapy.
In this two-part series, we focus on a total of 10 cases of vulvar symptoms, zeroing in on diagnosis and treatment. In this first part, we describe five patient scenarios illustrating the diagnosis and treatment of:
- lichen sclerosus
- vulvodynia
- lichen simplex chronicus
- lichen planus
- hidradenitis suppurativa.
In many chronic cases, more than one entity is the cause
Specific skin diseases, sensations of rawness from various external and internal irritants, neuropathy, and psychological issues are all much more common causes of chronic vulvar symptoms than infection. Moreover, most women with chronic vulvar symptoms have more than one entity producing their discomfort.
Very often, the cause of a patient’s symptoms is not clear at the first visit, with nonspecific redness or even normal skin seen on examination. Pathognomonic skin findings can be obscured by irritant contact dermatitis caused by unnecessary medications or overwashing, atrophic vaginitis, and/or rubbing and scratching. In such cases, obvious abnormalities must be eliminated and the patient reevaluated to definitively discover and treat the cause of the symptoms.
CASE 1. ANOGENITAL ITCHING AND DYSPAREUNIA
A 62-year-old woman schedules a visit to address her anogenital itching. She reports pain with scratching and has developed introital dyspareunia. On physical examination, you find a well-demarcated white plaque of thickened, crinkled skin (FIGURE 1). A wet mount shows parabasal cells and no lactobacilli.
Diagnosis: Lichen sclerosus and atrophic vagina.
Treatment: Halobetasol ointment, an ultra-potent topical corticosteroid, once or twice daily; along with estradiol cream (0.5 g intravaginally) 3 times a week.
Lichen sclerosus is a skin disease found most often on the vulva of postmenopausal women, although it also can affect prepubertal children and reproductive-age women. Lichen sclerosus is multifactorial in pathogenesis, including prominent autoimmune factors, local environmental factors, and genetic predisposition.1
Although there is no cure for lichen sclerosus, the symptoms and clinical abnormalities usually can be well managed with ultra-potent topical corticosteroids. However, scarring and architectural changes are not reversible. Moreover, poorly controlled lichen sclerosus exhibits malignant transformation on anogenital skin in about 3% of affected patients.
The standard of care is application of an ultra-potent topical corticosteroid ointment once or twice daily until the skin texture normalizes again. The most common of such corticosteroids are clobetasol, halobetasol, and betamethasone dipropionate in an augmented vehicle (betamethasone dipropionate in the usual vehicle is only a medium-high medication in terms of potency.) One of us (L.E.) finds that some women experience irritation with generic clobetasol.
The ointment form of the selected corticosteroid is preferred, as creams are irritating to the vulva in most women because they contain more alcohols and preservatives than ointments do. The amount to be used is very small—far smaller than the pea-sized amount often suggested. By using this smaller amount, we avoid spread to the surrounding hair-bearing skin, which is at greater risk for steroid dermatitis and atrophy than the modified mucous membranes.
Related video: Lichen sclerosis: My approach to treatment Michael Baggish, MD
Even asymptomatic lichen sclerosus can progress
Most vulvologists agree that when the skin normalizes (not when symptoms subside), it is best to either decrease the frequency of application of the ultra-potent corticosteroid to two or three times a week, or to continue daily use with a lower-potency corticosteroid such as triamcinolone ointment 0.1%. Discontinuation of therapy usually results in recurrence.2
Treatment should not be based solely on symptoms, as asymptomatic lichen sclerosus can progress and cause permanent scarring and an increased risk for squamous cell carcinoma.
Although no studies have shown a decreased risk for squamous cell carcinoma with ongoing use of a corticosteroid, vulvologists have observed that malignant transformation occurs uniformly in the setting of poorly controlled lichen sclerosus. Immune dysregulation and inflammation may play an important role, so careful management to minimize inflammation may help prevent a malignancy.3
Secondary treatment choices
Secondary choices for lichen sclerosus include the topical calcineurin inhibitors tacrolimus (Protopic) and pimecrolimus (Elidel) but not testosterone, which has been shown to be ineffective. Tacrolimus and pimecrolimus are useful but often burn upon application, and they are “black-boxed” for cutaneous squamous cell carcinoma and lymphoma. Therefore, although squamous cell carcinoma associated with their use is extraordinarily uncommon, patients should be advised of these risks, particularly because lichen sclerosus already exhibits this association.
Most postmenopausal women with lichen sclerosus also exhibit hypothyroidism, so they should be monitored for this. However, thyroid function testing in 18 children showed no evidence of hypothyroidism in that age group (L.E. unpublished data).
Estrogen replacement may be advised
Postmenopausal women who have prominent introital lichen sclerosus or dyspareunia should receive estrogen replacement of some type so that there is only one cause, rather than two, for their dyspareunia, thinning, fragility, and inelasticity.
Women with well-controlled lichen sclerosus should be followed twice a year to ensure that their disease remains suppressed with ongoing therapy, and to evaluate for active disease, adverse effects of therapy, and the appearance of dysplasia or squamous cell carcinoma.
Women with lichen sclerosus occasionally experience discomfort after their clinical skin disease has cleared. These women now have developed vulvodynia triggered by their lichen sclerosus.
Related series: Vulvar Pain Syndromes—A 3-part roundtable
Part 1. Making the correct diagnosis (September 2011)
Part 2. A bounty of treatments—but not all of them proven (October 2011)
Part 3. Causes and treatment of vestibulodynia (November 2011)
CASE 2. IS IT REALLY CHRONIC YEAST INFECTION?
A 36-year-old woman consults you about her history of chronic yeast infection that manifests as introital burning, discharge, and dyspareunia. She is otherwise healthy, except for irritable bowel syndrome and fibromyalgia.
Physical examination reveals a mild patchy redness of the vestibule and surrounding modified mucous membranes (FIGURE 2). Gentle probing with a cotton swab triggers exquisite pain in the vestibule, with slight extension to the labia minora. A wet mount shows no evidence of increased white blood cells, parabasal cells, clue cells, or yeast forms. Lactobacilli are abundant.
Diagnosis: Vulvodynia, with a nearly vestibulodynia pattern.
Treatment: Venlafaxine and pelvic floor physical therapy.
Vulvodynia is a genital pain syndrome defined as sensations of chronic burning, irritation, rawness, and soreness in the absence of objective disease and infection that could explain the discomfort. Vulvodynia occurs in approximately 7% to 8% of women.4
Vulvodynia generally is believed to be a multifactorial symptom, occurring as a result of pelvic floor dysfunction and neuropathic pain,5,6 with anxiety/depression issues exacerbating symptoms. Some recent studies have shown the presence of biochemical mediators of inflammation in the absence of clinical and histologic inflammation.7 Discomfort often is worsened by infections or the application of common irritants (creams, panty liners, soaps, some topical anesthetics). Estrogen deficiency is another common exacerbating factor.
Women tend to exhibit other pain syndromes such as chronic headaches, fibromyalgia, temperomandibular disorder, or premenstrual syndrome, as well as prominent anxiety, depression, sleep disorder, and so on.
Almost uniformly present are symptoms of pelvic floor dysfunction, such as constipation, irritable bowel syndrome, and interstitial cystitis or urinary symptoms in the absence of a urinary tract infection. These women also are frequently unusually intolerant of medications.
Classifying vulvodynia
There are two primary patterns of vulvodynia. The first and most common is vestibulodynia, formerly called vulvar vestibulitis. The term vestibulitis was eliminated to reflect the absence of clinical and histologic inflammation. Vestibulodynia refers to pain that is always limited to the vestibule. Generalized vulvodynia, however, extends beyond the vestibule, is migratory, or does not include the vestibule.
Several vulvologists have found that many patients exhibit features of both types of vulvodynia, and these patterns probably exist on a spectrum. The difference is probably unimportant in clinical practice, except that vestibulodynia can be treated with vestibulectomy.
How we manage vulvodynia
We focus on pelvic floor physical therapy and on the provision of medication for neuropathic pain, which is initiated at very small doses and gradually increased to active doses.8 The medications used and the ultimate doses often required include:
- amitriptyline or desipramine 150 mg
- gabapentin 600 to 1,200 mg three times daily
- venlafaxine XR 150 mg daily
- pregabalin 150 mg twice a day
- duloxetine 60 mg a day.
Compounded amitriptyline 2% with baclofen 2% cream applied three times daily is beneficial for many patients, and topical lidocaine jelly 2% or ointment 5% (which often burns) can help provide immediate temporary relief.
Most patients require sex therapy and counseling for maximal improvement. Women with vestibulodynia in whom these therapies fail are good candidates for vestibulectomy if their pain is strictly limited to the vestibule. Fortunately, most women do not require this aggressive therapy.
Related article: Successful treatment of chronic vaginitis Robert L. Barbieri, MD (Editorial; July 2013)
CASE 3. SEVERE ITCHING DISRUPTS SLEEP
A 34-year-old patient reports excruciating itching, with disruption of daily activities and sleep. She has been treated for candidiasis on multiple occasions, but in your office her wet mount and confirmatory culture are negative. Physical examination reveals a pink, lichenified plaque with excoriation (FIGURE 3).
Diagnosis: Lichen simplex chronicus.
Treatment: Ultra-potent corticosteroid ointment applied very sparingly twice daily and covered with petroleum jelly. You also order nighttime sedation with amitriptyline to break the itch-scratch cycle. When the patient’s itching resolves and her skin clears, you taper her off the corticosteroid, warning her that recurrence is likely, and instruct her to restart the medication immediately should itching recur.
Lichen simplex chronicus (formerly called squamous hyperplasia or hyperplastic dystrophy, and also known as eczema, neurodermatitis, or localized atopic dermatitis) occurs when irritation from any cause produces itching in a predisposed person. The subsequent scratching and rubbing both produce the rash and exacerbate the irritation that drives the itching, even after the original cause is gone. The rubbing and scratching perpetuate the irritation and itching, producing the “itch-scratch” cycle.
The appearance of lichen simplex chronicus is produced by rubbing (where the skin thickens and lichenifies) or scratching (where the skin becomes red with linear erosions, called excoriations, caused by fingernails).
The initial trigger for lichen simplex chronicus often is an infection—often yeast—but overwashing, stress, sweat, heat, urine, irritating lubricants, and use of panty liners also may precipitate the itching. At the office visit, the original infection or other cause of irritation often is no longer present, and only lichen simplex chronicus can be identified.
How to treat lichen simplex chronicus
Management of lichen simplex chronicus requires very sparing application of an ultra-potent topical corticosteroid (clobetasol, halobetasol, or betamethasone dipropionate in an augmented vehicle ointment) twice daily, with the ointment covered with petroleum jelly. Care also must be taken to avoid irritants.
In addition, nighttime sedation helps to interrupt the itch-scratch cycle by preventing rubbing during sleep.
When the skin appears normal and itching has resolved, taper the medication down or off, warning the patient that recurrence is common with any future irritation.
Restart therapy immediately upon recurrence to prevent lichenification and chronic problems.
Second-line medications include calcineurin inhibitors (tacrolimus or pimecrolimus). Although these agents do not contribute to atrophy, they are less effective than topical corticosteroids,9 cost more, and can cause burning upon application.
Unlike lichen sclerosus, lichen simplex chronicus does not always recur upon cessation of treatment, and there is no need for concern about an increased risk of malignancy or significant scarring.
Related article: New treatment option for vulvar and vaginal atrophy Andrew M. Kaunitz, MD (News for your Practice; May 2013)
CASE 4. ORAL AND VULVAR INVOLVEMENT
A 73-year-old patient seeks your help in alleviating longstanding introital itching and rawness, with dyspareunia. She has tried topical estradiol cream intravaginally three times weekly in combination with weekly fluconazole, to no avail.
Physical examination reveals deep red patches and erosions of the vestibule, with complete resorption of the labia minora (FIGURE 4). Patchy redness of the vagina is apparent as well, so you examine the patient’s mouth and find deep redness of the gingivae and erosions of the buccal mucosae, with surrounding white, lacy papules. A wet mount shows a marked increase in lymphocytes and parabasal cells, with a pH of more than 7.
Diagnosis: After correlating the vulvar and oral findings, you make a diagnosis of lichen planus.
Treatment: You initiate halobetasol ointment twice daily, to be applied to the vulva. You also continue vaginal estradiol cream but add hydrocortisone acetate 200 mg compounded vaginal suppositories nightly, as well as clobetasol gel to be applied to oral lesions three times a day. You follow the patient closely for secondary yeast of the mouth and vagina.
Erosive multimucosal lichen planus is a disease of cell-mediated immunity that overwhelmingly affects menopausal women. The most common surfaces involved are the mouth, vagina, rectal mucosa, and vulva; usually, at least two surfaces are affected. The esophagus, extra-auditory canals, nasal mucosa, and eyes also can be involved. Dry, extragenital skin usually is not affected in the setting of erosive vulvovaginal lichen planus.
Vulvar lichen planus most often is controlled with ultra-potent topical corticosteroids (again, clobetasol, halobetasol, or betamethasone dipropionate in an augmented vehicle), but other mucosal surfaces often are more difficult to manage. Although there is no definitive cure for this condition, careful local care, estrogen replacement, and suppression of oral and vulvovaginal candidiasis usually provide relief.
Calcineurin inhibitors (tacrolimus, pimecrolimus) sometimes are useful in patients who improve only partially after treatment with a topical corticosteroid, provided burning with application is tolerable.10 Systemic immunosuppressants such as hydroxychloroquine, methotrexate, mycophenolate mofetil, azathioprine, cyclosporine, cyclophosphamide, and tumor necrosis factor (TNF) alpha blockers (etanercept, adalimumab, infliximab), as well as oral retinoids, can be added for more recalcitrant disease.11
How to manage disease that affects the vagina
When the vagina is involved in lichen planus, treatment is important to prevent scarring, as well as rawness and pain from irritant contact dermatitis caused by purulent vaginal secretions. Occasionally, a 25-mg hydrocortisone acetate rectal suppository inserted into the vagina nightly improves vaginal lichen planus, but sometimes more potent suppositories, such as doses of 100 to 200 mg, may be compounded. Dilators should be inserted daily to prevent vaginal synechiae.
Oral involvement requires targeted treatment
The mouth is almost always involved in lichen planus. If a dermatologist is not involved in patient care, a prescription for dexamethasone/nystatin elixir (50:50) (5 mL swish, hold, and spit four times daily) can improve oral symptoms remarkably. Alternatively, clobetasol gel applied to affected areas of the mouth three or four times daily can be helpful. Secondary yeast of the vagina and mouth are common with the use of topical corticosteroids.
Careful clinical follow-up is advised
Like uncontrolled lichen sclerosus, erosive lichen planus of the vulva produces scarring and sometimes eventuates into squamous cell carcinoma. Therefore, careful clinical surveillance is warranted. And therapy must be continued to prevent recurrence of lichen planus (as it must be for lichen sclerosus), scarring, and to decrease the risk of squamous cell carcinoma. And like lichen sclerosus, lichen planus sometimes triggers vulvodynia.
CASE 5. MULTIPLE BOILS IN THE GROIN
A 31-year-old morbidly obese African American woman comes to your office with continually evolving boils in the groin. A culture shows Bacterioides spp, Escherichia coli, and Peptococcus spp. In the past, multiple courses of various antibiotics have provided only modest relief.
Physical examination reveals fluctuant nodules, scars, and draining sinus tracts of the hair-bearing vulva and crural crease (FIGURE 5). The axillae are clear.
Diagnosis: Hidradenitis suppurativa.
Treatment: The patient begins taking minocycline 100 mg twice daily. Because she is a smoker, you refer her to an aggressive primary care provider for smoking cessation and weight loss management.
Three months later, the patient is developing only about two nodules a month, managed by early intralesional injections of triamcinolone acetonide.
Hidradenitis suppurativa is sometimes called inverse acne because the underlying pathogenesis is similar to cystic acne. Follicular plugging with keratin debris occurs, with additional keratin, sebaceous material, and normal skin bacteria trapped below the occlusion and distending the follicle. As the follicle wall stretches, thins, and allows for leakage of keratin debris into surrounding dermis, a brisk foreign-body response produces a noninfectious abscess.
Hidradenitis suppurativa affects more than 2% of the population.12 It appears only in areas of the body that contain apocrine glands and in individuals who have double- or triple-outlet follicles that predispose them to follicular occlusion. Therefore, this disease has a genetic component.
Other risk factors include male sex, African genetic background, obesity, and smoking. The prevalence of metabolic syndrome is significantly higher in individuals with hidradenitis suppurativa than in the general population.13
Recommended management
Treatments include:
- chronic antibiotics with nonspecific anti-inflammatory activity (tetracyclines, erythromycin, clindamycin, and trimethoprim-sulfamethoxazole)
- intralesional injection of corticosteroids for early nodules (which often aborts their development)
- TNF alpha blockers (etanercept, adalimumab, infliximab)14–16
- surgical removal of affected skin—the definitive therapy.
Note, however, that anogenital hidradenitis often is too extensive for surgery to be practical. In patients who have localized hidradenitis, primary excision is an excellent early therapy, provided the patient is advised that recurrence may occur in apocrine-containing nearby skin. Aggressive curettage of the roof of the cysts has been performed by some clinicians with good response.
Don’t overlook adjuvant approaches
Smoking cessation and weight loss often are useful.
Other therapies backed by anecdotal evidence include oral contraceptives or spironolactone for their anti-androgen effect, as well as metformin, a more recently studied agent.
Local care with antibacterial soaps and topical antibiotics may be useful for some women.
MORE CASES TO COME
In Part 2 of this series, which will appear in the June 2014 issue of OBG Management, we will discuss the following cases:
- atrophic vagina and atrophic vaginitis
- contact dermatitis
- vulvar aphthae
- desquamative inflammatory vaginitis
- psoriasis.
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article. Send your letter to: [email protected] Please include the city and state in which you practice.
Nearly one in every six women will experience chronic vulvar symptoms at some point, from ongoing itching to sensations of rawness, burning, or dyspareunia. Regrettably, clinicians generally are taught only a few possible causes for these symptoms, primarily infections such as yeast, bacterial vaginosis, herpes simplex virus, or anogenital warts. However, infections rarely produce chronic symptoms that do not respond, at least temporarily, to therapy.
In this two-part series, we focus on a total of 10 cases of vulvar symptoms, zeroing in on diagnosis and treatment. In this first part, we describe five patient scenarios illustrating the diagnosis and treatment of:
- lichen sclerosus
- vulvodynia
- lichen simplex chronicus
- lichen planus
- hidradenitis suppurativa.
In many chronic cases, more than one entity is the cause
Specific skin diseases, sensations of rawness from various external and internal irritants, neuropathy, and psychological issues are all much more common causes of chronic vulvar symptoms than infection. Moreover, most women with chronic vulvar symptoms have more than one entity producing their discomfort.
Very often, the cause of a patient’s symptoms is not clear at the first visit, with nonspecific redness or even normal skin seen on examination. Pathognomonic skin findings can be obscured by irritant contact dermatitis caused by unnecessary medications or overwashing, atrophic vaginitis, and/or rubbing and scratching. In such cases, obvious abnormalities must be eliminated and the patient reevaluated to definitively discover and treat the cause of the symptoms.
CASE 1. ANOGENITAL ITCHING AND DYSPAREUNIA
A 62-year-old woman schedules a visit to address her anogenital itching. She reports pain with scratching and has developed introital dyspareunia. On physical examination, you find a well-demarcated white plaque of thickened, crinkled skin (FIGURE 1). A wet mount shows parabasal cells and no lactobacilli.
Diagnosis: Lichen sclerosus and atrophic vagina.
Treatment: Halobetasol ointment, an ultra-potent topical corticosteroid, once or twice daily; along with estradiol cream (0.5 g intravaginally) 3 times a week.
Lichen sclerosus is a skin disease found most often on the vulva of postmenopausal women, although it also can affect prepubertal children and reproductive-age women. Lichen sclerosus is multifactorial in pathogenesis, including prominent autoimmune factors, local environmental factors, and genetic predisposition.1
Although there is no cure for lichen sclerosus, the symptoms and clinical abnormalities usually can be well managed with ultra-potent topical corticosteroids. However, scarring and architectural changes are not reversible. Moreover, poorly controlled lichen sclerosus exhibits malignant transformation on anogenital skin in about 3% of affected patients.
The standard of care is application of an ultra-potent topical corticosteroid ointment once or twice daily until the skin texture normalizes again. The most common of such corticosteroids are clobetasol, halobetasol, and betamethasone dipropionate in an augmented vehicle (betamethasone dipropionate in the usual vehicle is only a medium-high medication in terms of potency.) One of us (L.E.) finds that some women experience irritation with generic clobetasol.
The ointment form of the selected corticosteroid is preferred, as creams are irritating to the vulva in most women because they contain more alcohols and preservatives than ointments do. The amount to be used is very small—far smaller than the pea-sized amount often suggested. By using this smaller amount, we avoid spread to the surrounding hair-bearing skin, which is at greater risk for steroid dermatitis and atrophy than the modified mucous membranes.
Related video: Lichen sclerosis: My approach to treatment Michael Baggish, MD
Even asymptomatic lichen sclerosus can progress
Most vulvologists agree that when the skin normalizes (not when symptoms subside), it is best to either decrease the frequency of application of the ultra-potent corticosteroid to two or three times a week, or to continue daily use with a lower-potency corticosteroid such as triamcinolone ointment 0.1%. Discontinuation of therapy usually results in recurrence.2
Treatment should not be based solely on symptoms, as asymptomatic lichen sclerosus can progress and cause permanent scarring and an increased risk for squamous cell carcinoma.
Although no studies have shown a decreased risk for squamous cell carcinoma with ongoing use of a corticosteroid, vulvologists have observed that malignant transformation occurs uniformly in the setting of poorly controlled lichen sclerosus. Immune dysregulation and inflammation may play an important role, so careful management to minimize inflammation may help prevent a malignancy.3
Secondary treatment choices
Secondary choices for lichen sclerosus include the topical calcineurin inhibitors tacrolimus (Protopic) and pimecrolimus (Elidel) but not testosterone, which has been shown to be ineffective. Tacrolimus and pimecrolimus are useful but often burn upon application, and they are “black-boxed” for cutaneous squamous cell carcinoma and lymphoma. Therefore, although squamous cell carcinoma associated with their use is extraordinarily uncommon, patients should be advised of these risks, particularly because lichen sclerosus already exhibits this association.
Most postmenopausal women with lichen sclerosus also exhibit hypothyroidism, so they should be monitored for this. However, thyroid function testing in 18 children showed no evidence of hypothyroidism in that age group (L.E. unpublished data).
Estrogen replacement may be advised
Postmenopausal women who have prominent introital lichen sclerosus or dyspareunia should receive estrogen replacement of some type so that there is only one cause, rather than two, for their dyspareunia, thinning, fragility, and inelasticity.
Women with well-controlled lichen sclerosus should be followed twice a year to ensure that their disease remains suppressed with ongoing therapy, and to evaluate for active disease, adverse effects of therapy, and the appearance of dysplasia or squamous cell carcinoma.
Women with lichen sclerosus occasionally experience discomfort after their clinical skin disease has cleared. These women now have developed vulvodynia triggered by their lichen sclerosus.
Related series: Vulvar Pain Syndromes—A 3-part roundtable
Part 1. Making the correct diagnosis (September 2011)
Part 2. A bounty of treatments—but not all of them proven (October 2011)
Part 3. Causes and treatment of vestibulodynia (November 2011)
CASE 2. IS IT REALLY CHRONIC YEAST INFECTION?
A 36-year-old woman consults you about her history of chronic yeast infection that manifests as introital burning, discharge, and dyspareunia. She is otherwise healthy, except for irritable bowel syndrome and fibromyalgia.
Physical examination reveals a mild patchy redness of the vestibule and surrounding modified mucous membranes (FIGURE 2). Gentle probing with a cotton swab triggers exquisite pain in the vestibule, with slight extension to the labia minora. A wet mount shows no evidence of increased white blood cells, parabasal cells, clue cells, or yeast forms. Lactobacilli are abundant.
Diagnosis: Vulvodynia, with a nearly vestibulodynia pattern.
Treatment: Venlafaxine and pelvic floor physical therapy.
Vulvodynia is a genital pain syndrome defined as sensations of chronic burning, irritation, rawness, and soreness in the absence of objective disease and infection that could explain the discomfort. Vulvodynia occurs in approximately 7% to 8% of women.4
Vulvodynia generally is believed to be a multifactorial symptom, occurring as a result of pelvic floor dysfunction and neuropathic pain,5,6 with anxiety/depression issues exacerbating symptoms. Some recent studies have shown the presence of biochemical mediators of inflammation in the absence of clinical and histologic inflammation.7 Discomfort often is worsened by infections or the application of common irritants (creams, panty liners, soaps, some topical anesthetics). Estrogen deficiency is another common exacerbating factor.
Women tend to exhibit other pain syndromes such as chronic headaches, fibromyalgia, temperomandibular disorder, or premenstrual syndrome, as well as prominent anxiety, depression, sleep disorder, and so on.
Almost uniformly present are symptoms of pelvic floor dysfunction, such as constipation, irritable bowel syndrome, and interstitial cystitis or urinary symptoms in the absence of a urinary tract infection. These women also are frequently unusually intolerant of medications.
Classifying vulvodynia
There are two primary patterns of vulvodynia. The first and most common is vestibulodynia, formerly called vulvar vestibulitis. The term vestibulitis was eliminated to reflect the absence of clinical and histologic inflammation. Vestibulodynia refers to pain that is always limited to the vestibule. Generalized vulvodynia, however, extends beyond the vestibule, is migratory, or does not include the vestibule.
Several vulvologists have found that many patients exhibit features of both types of vulvodynia, and these patterns probably exist on a spectrum. The difference is probably unimportant in clinical practice, except that vestibulodynia can be treated with vestibulectomy.
How we manage vulvodynia
We focus on pelvic floor physical therapy and on the provision of medication for neuropathic pain, which is initiated at very small doses and gradually increased to active doses.8 The medications used and the ultimate doses often required include:
- amitriptyline or desipramine 150 mg
- gabapentin 600 to 1,200 mg three times daily
- venlafaxine XR 150 mg daily
- pregabalin 150 mg twice a day
- duloxetine 60 mg a day.
Compounded amitriptyline 2% with baclofen 2% cream applied three times daily is beneficial for many patients, and topical lidocaine jelly 2% or ointment 5% (which often burns) can help provide immediate temporary relief.
Most patients require sex therapy and counseling for maximal improvement. Women with vestibulodynia in whom these therapies fail are good candidates for vestibulectomy if their pain is strictly limited to the vestibule. Fortunately, most women do not require this aggressive therapy.
Related article: Successful treatment of chronic vaginitis Robert L. Barbieri, MD (Editorial; July 2013)
CASE 3. SEVERE ITCHING DISRUPTS SLEEP
A 34-year-old patient reports excruciating itching, with disruption of daily activities and sleep. She has been treated for candidiasis on multiple occasions, but in your office her wet mount and confirmatory culture are negative. Physical examination reveals a pink, lichenified plaque with excoriation (FIGURE 3).
Diagnosis: Lichen simplex chronicus.
Treatment: Ultra-potent corticosteroid ointment applied very sparingly twice daily and covered with petroleum jelly. You also order nighttime sedation with amitriptyline to break the itch-scratch cycle. When the patient’s itching resolves and her skin clears, you taper her off the corticosteroid, warning her that recurrence is likely, and instruct her to restart the medication immediately should itching recur.
Lichen simplex chronicus (formerly called squamous hyperplasia or hyperplastic dystrophy, and also known as eczema, neurodermatitis, or localized atopic dermatitis) occurs when irritation from any cause produces itching in a predisposed person. The subsequent scratching and rubbing both produce the rash and exacerbate the irritation that drives the itching, even after the original cause is gone. The rubbing and scratching perpetuate the irritation and itching, producing the “itch-scratch” cycle.
The appearance of lichen simplex chronicus is produced by rubbing (where the skin thickens and lichenifies) or scratching (where the skin becomes red with linear erosions, called excoriations, caused by fingernails).
The initial trigger for lichen simplex chronicus often is an infection—often yeast—but overwashing, stress, sweat, heat, urine, irritating lubricants, and use of panty liners also may precipitate the itching. At the office visit, the original infection or other cause of irritation often is no longer present, and only lichen simplex chronicus can be identified.
How to treat lichen simplex chronicus
Management of lichen simplex chronicus requires very sparing application of an ultra-potent topical corticosteroid (clobetasol, halobetasol, or betamethasone dipropionate in an augmented vehicle ointment) twice daily, with the ointment covered with petroleum jelly. Care also must be taken to avoid irritants.
In addition, nighttime sedation helps to interrupt the itch-scratch cycle by preventing rubbing during sleep.
When the skin appears normal and itching has resolved, taper the medication down or off, warning the patient that recurrence is common with any future irritation.
Restart therapy immediately upon recurrence to prevent lichenification and chronic problems.
Second-line medications include calcineurin inhibitors (tacrolimus or pimecrolimus). Although these agents do not contribute to atrophy, they are less effective than topical corticosteroids,9 cost more, and can cause burning upon application.
Unlike lichen sclerosus, lichen simplex chronicus does not always recur upon cessation of treatment, and there is no need for concern about an increased risk of malignancy or significant scarring.
Related article: New treatment option for vulvar and vaginal atrophy Andrew M. Kaunitz, MD (News for your Practice; May 2013)
CASE 4. ORAL AND VULVAR INVOLVEMENT
A 73-year-old patient seeks your help in alleviating longstanding introital itching and rawness, with dyspareunia. She has tried topical estradiol cream intravaginally three times weekly in combination with weekly fluconazole, to no avail.
Physical examination reveals deep red patches and erosions of the vestibule, with complete resorption of the labia minora (FIGURE 4). Patchy redness of the vagina is apparent as well, so you examine the patient’s mouth and find deep redness of the gingivae and erosions of the buccal mucosae, with surrounding white, lacy papules. A wet mount shows a marked increase in lymphocytes and parabasal cells, with a pH of more than 7.
Diagnosis: After correlating the vulvar and oral findings, you make a diagnosis of lichen planus.
Treatment: You initiate halobetasol ointment twice daily, to be applied to the vulva. You also continue vaginal estradiol cream but add hydrocortisone acetate 200 mg compounded vaginal suppositories nightly, as well as clobetasol gel to be applied to oral lesions three times a day. You follow the patient closely for secondary yeast of the mouth and vagina.
Erosive multimucosal lichen planus is a disease of cell-mediated immunity that overwhelmingly affects menopausal women. The most common surfaces involved are the mouth, vagina, rectal mucosa, and vulva; usually, at least two surfaces are affected. The esophagus, extra-auditory canals, nasal mucosa, and eyes also can be involved. Dry, extragenital skin usually is not affected in the setting of erosive vulvovaginal lichen planus.
Vulvar lichen planus most often is controlled with ultra-potent topical corticosteroids (again, clobetasol, halobetasol, or betamethasone dipropionate in an augmented vehicle), but other mucosal surfaces often are more difficult to manage. Although there is no definitive cure for this condition, careful local care, estrogen replacement, and suppression of oral and vulvovaginal candidiasis usually provide relief.
Calcineurin inhibitors (tacrolimus, pimecrolimus) sometimes are useful in patients who improve only partially after treatment with a topical corticosteroid, provided burning with application is tolerable.10 Systemic immunosuppressants such as hydroxychloroquine, methotrexate, mycophenolate mofetil, azathioprine, cyclosporine, cyclophosphamide, and tumor necrosis factor (TNF) alpha blockers (etanercept, adalimumab, infliximab), as well as oral retinoids, can be added for more recalcitrant disease.11
How to manage disease that affects the vagina
When the vagina is involved in lichen planus, treatment is important to prevent scarring, as well as rawness and pain from irritant contact dermatitis caused by purulent vaginal secretions. Occasionally, a 25-mg hydrocortisone acetate rectal suppository inserted into the vagina nightly improves vaginal lichen planus, but sometimes more potent suppositories, such as doses of 100 to 200 mg, may be compounded. Dilators should be inserted daily to prevent vaginal synechiae.
Oral involvement requires targeted treatment
The mouth is almost always involved in lichen planus. If a dermatologist is not involved in patient care, a prescription for dexamethasone/nystatin elixir (50:50) (5 mL swish, hold, and spit four times daily) can improve oral symptoms remarkably. Alternatively, clobetasol gel applied to affected areas of the mouth three or four times daily can be helpful. Secondary yeast of the vagina and mouth are common with the use of topical corticosteroids.
Careful clinical follow-up is advised
Like uncontrolled lichen sclerosus, erosive lichen planus of the vulva produces scarring and sometimes eventuates into squamous cell carcinoma. Therefore, careful clinical surveillance is warranted. And therapy must be continued to prevent recurrence of lichen planus (as it must be for lichen sclerosus), scarring, and to decrease the risk of squamous cell carcinoma. And like lichen sclerosus, lichen planus sometimes triggers vulvodynia.
CASE 5. MULTIPLE BOILS IN THE GROIN
A 31-year-old morbidly obese African American woman comes to your office with continually evolving boils in the groin. A culture shows Bacterioides spp, Escherichia coli, and Peptococcus spp. In the past, multiple courses of various antibiotics have provided only modest relief.
Physical examination reveals fluctuant nodules, scars, and draining sinus tracts of the hair-bearing vulva and crural crease (FIGURE 5). The axillae are clear.
Diagnosis: Hidradenitis suppurativa.
Treatment: The patient begins taking minocycline 100 mg twice daily. Because she is a smoker, you refer her to an aggressive primary care provider for smoking cessation and weight loss management.
Three months later, the patient is developing only about two nodules a month, managed by early intralesional injections of triamcinolone acetonide.
Hidradenitis suppurativa is sometimes called inverse acne because the underlying pathogenesis is similar to cystic acne. Follicular plugging with keratin debris occurs, with additional keratin, sebaceous material, and normal skin bacteria trapped below the occlusion and distending the follicle. As the follicle wall stretches, thins, and allows for leakage of keratin debris into surrounding dermis, a brisk foreign-body response produces a noninfectious abscess.
Hidradenitis suppurativa affects more than 2% of the population.12 It appears only in areas of the body that contain apocrine glands and in individuals who have double- or triple-outlet follicles that predispose them to follicular occlusion. Therefore, this disease has a genetic component.
Other risk factors include male sex, African genetic background, obesity, and smoking. The prevalence of metabolic syndrome is significantly higher in individuals with hidradenitis suppurativa than in the general population.13
Recommended management
Treatments include:
- chronic antibiotics with nonspecific anti-inflammatory activity (tetracyclines, erythromycin, clindamycin, and trimethoprim-sulfamethoxazole)
- intralesional injection of corticosteroids for early nodules (which often aborts their development)
- TNF alpha blockers (etanercept, adalimumab, infliximab)14–16
- surgical removal of affected skin—the definitive therapy.
Note, however, that anogenital hidradenitis often is too extensive for surgery to be practical. In patients who have localized hidradenitis, primary excision is an excellent early therapy, provided the patient is advised that recurrence may occur in apocrine-containing nearby skin. Aggressive curettage of the roof of the cysts has been performed by some clinicians with good response.
Don’t overlook adjuvant approaches
Smoking cessation and weight loss often are useful.
Other therapies backed by anecdotal evidence include oral contraceptives or spironolactone for their anti-androgen effect, as well as metformin, a more recently studied agent.
Local care with antibacterial soaps and topical antibiotics may be useful for some women.
MORE CASES TO COME
In Part 2 of this series, which will appear in the June 2014 issue of OBG Management, we will discuss the following cases:
- atrophic vagina and atrophic vaginitis
- contact dermatitis
- vulvar aphthae
- desquamative inflammatory vaginitis
- psoriasis.
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article. Send your letter to: [email protected] Please include the city and state in which you practice.
- Doulaveri G, Armira K, Kouris A, et al. Genital vulvar lichen sclerosus in monozygotic twin women: A case report and review of the literature. Case Rep Dermatol. 2013;5(3):321–325.
- Virgili A, Minghetti S, Borghi A, Corazza M. Proactive maintenance therapy with a topical corticosteroid for vulvar lichen sclerosus: Preliminary results of a randomized study. Br J Dermatol. 2013;168(6):1316–1324.
- Brodrick B, Belkin ZR, Goldstein AT. Influence of treatments on prognosis for vulvar lichen sclerosus: Facts and controversies. Clin Dermatol. 2013;31(6):780–786.
- Harlow BL, Kunitz CG, Nguyen RH, Rydell SA, Turner RM, MacLehose RF. Prevalence of symptoms consistent with a diagnosis of vulvodynia: Population-based estimates from two geographic regions. Am J Obstet Gynecol. 2014;210(1):40.e1–e8.
- Morin M, Bergeron S, Khalife S, Mayrand MH, Binik YM. Morphometry of the pelvic floor muscles in women with and without provoked vestibulodynia using 4D ultrasound. J Sex Med. 2014;11(3):776–785.
- Hampson JP, Reed BD, Clauw DJ, et al. Augmented central pain processing in vulvodynia. J Pain. 2013;14(6):579–589.
- Omoigui S. The biochemical origin of pain: the origin of all pain is inflammation and the inflammatory response. Part 2 of 3: Inflammatory profile of pain syndromes. Med Hypotheses. 2007;69(6):1169–1178.
- Haefner HK, Collins ME, Davis GD, et al. The vulvodynia guideline. J Low Genit Tract Dis. 2005;9(1):40–51.
- Frankel HC, Qureshi AA. Comparative effectiveness of topical calcineurin inhibitors in adult patients with atopic dermatitis. Am J Clin Dermatol. 2012;13(2):113–123.
- Samycia M, Lin AN. Efficacy of topical calcineurin inhibitors in lichen planus. J Cutan Med Surg. 2012;16(4):221–229.
- Mirowski GW, Goddard A. Treatment of vulvovaginal lichen planus. Dermatol Clin. 2010;28(4):717–725.
- Vinding GR, Miller IM, Zarchi K, et al. The prevalence of inverse recurrent suppuration: A population-based study of possible hidradenitis suppurativa [published online ahead of print December 16, 2013]. Br J Dermatol. doi:10.1111/bjd.12787.
- Gold DA, Reeder VJ, Mahan MG, Hamzavi IH. The prevalence of metabolic syndrome in patients with hidradenitis suppurativa. J Am Acad Dermatol. 2014;70(4):699–703.
- Scheinfeld N. Hidradenitis suppurativa: A practical review of possible medical treatments based on over 350 hidradenitis patients. Dermatol Online J. 2013;19(4):1.
- Kimball AB, Kerdel F, Adams D, et al. Adalimumab for the treatment of moderate to severe hidradenitis suppurativa:
A parallel randomized trial. Ann Intern Med. 2012;157(12):846–855. - Chinniah N, Cains GD. Moderate to severe hidradenitis suppurativa treated with biological therapies [published online ahead of print January 23, 2014]. Australas J Dermatol. doi:10.1111/ajd.12136.
- Doulaveri G, Armira K, Kouris A, et al. Genital vulvar lichen sclerosus in monozygotic twin women: A case report and review of the literature. Case Rep Dermatol. 2013;5(3):321–325.
- Virgili A, Minghetti S, Borghi A, Corazza M. Proactive maintenance therapy with a topical corticosteroid for vulvar lichen sclerosus: Preliminary results of a randomized study. Br J Dermatol. 2013;168(6):1316–1324.
- Brodrick B, Belkin ZR, Goldstein AT. Influence of treatments on prognosis for vulvar lichen sclerosus: Facts and controversies. Clin Dermatol. 2013;31(6):780–786.
- Harlow BL, Kunitz CG, Nguyen RH, Rydell SA, Turner RM, MacLehose RF. Prevalence of symptoms consistent with a diagnosis of vulvodynia: Population-based estimates from two geographic regions. Am J Obstet Gynecol. 2014;210(1):40.e1–e8.
- Morin M, Bergeron S, Khalife S, Mayrand MH, Binik YM. Morphometry of the pelvic floor muscles in women with and without provoked vestibulodynia using 4D ultrasound. J Sex Med. 2014;11(3):776–785.
- Hampson JP, Reed BD, Clauw DJ, et al. Augmented central pain processing in vulvodynia. J Pain. 2013;14(6):579–589.
- Omoigui S. The biochemical origin of pain: the origin of all pain is inflammation and the inflammatory response. Part 2 of 3: Inflammatory profile of pain syndromes. Med Hypotheses. 2007;69(6):1169–1178.
- Haefner HK, Collins ME, Davis GD, et al. The vulvodynia guideline. J Low Genit Tract Dis. 2005;9(1):40–51.
- Frankel HC, Qureshi AA. Comparative effectiveness of topical calcineurin inhibitors in adult patients with atopic dermatitis. Am J Clin Dermatol. 2012;13(2):113–123.
- Samycia M, Lin AN. Efficacy of topical calcineurin inhibitors in lichen planus. J Cutan Med Surg. 2012;16(4):221–229.
- Mirowski GW, Goddard A. Treatment of vulvovaginal lichen planus. Dermatol Clin. 2010;28(4):717–725.
- Vinding GR, Miller IM, Zarchi K, et al. The prevalence of inverse recurrent suppuration: A population-based study of possible hidradenitis suppurativa [published online ahead of print December 16, 2013]. Br J Dermatol. doi:10.1111/bjd.12787.
- Gold DA, Reeder VJ, Mahan MG, Hamzavi IH. The prevalence of metabolic syndrome in patients with hidradenitis suppurativa. J Am Acad Dermatol. 2014;70(4):699–703.
- Scheinfeld N. Hidradenitis suppurativa: A practical review of possible medical treatments based on over 350 hidradenitis patients. Dermatol Online J. 2013;19(4):1.
- Kimball AB, Kerdel F, Adams D, et al. Adalimumab for the treatment of moderate to severe hidradenitis suppurativa:
A parallel randomized trial. Ann Intern Med. 2012;157(12):846–855. - Chinniah N, Cains GD. Moderate to severe hidradenitis suppurativa treated with biological therapies [published online ahead of print January 23, 2014]. Australas J Dermatol. doi:10.1111/ajd.12136.
Read Part 2: Chronic vulvar irritation, itching, and pain. What is the diagnosis? (June 2014)
Preventing Burnout With Cognitive Empathy
Empathy expressed by a practitioner is important to patients—but empathy is also important for the practitioner. According to researchers from University of Montreal in Canada, empathic concern and perspective taking when treating patients can help prevent physician burnout.
Empathy and sympathy are 2 different concepts, and they can lead to different outcomes, the researchers say. For instance, in hypothetical situations, sympathetic physicians who “feel” their patients’ pain have used more health care resources than empathic ones who “know” their patients’ pain. Some authors, the researchers point out, believe that sympathy can be detrimental to objectivity in decision making and lead to compassion fatigue and burnout.
In their study, the researchers aimed to identify the best balance between empathic concern for the patient and affective distance necessary to maintain clinical neutrality and the physician’s own emotional equilibrium.
They gave questionnaires to 294 general practitioners: the Maslach Burnout Inventory to measure emotional exhaustion, depersonalization, and personal accomplishment; a subscale of the Jefferson Scale of Physician Empathy (JSPE) to measure perspective taking (seeing things from the patient’s point of view); and the Toronto Empathy Questionnaire to rate emotional concern.
A high level of perspective taking was associated with lower burnout. But contrary to their expectations, the researchers found that a higher level of empathic concern was also significantly associated with a lower proportion of burnout. The odds of experiencing burnout were significantly lower when physicians scored high on perspective taking (P < .001), high on empathic concern (P < .05), and high on both (P < .001). Empathic concern had no effect when perspective taking was low. The researchers speculate that when physicians are good at adopting the point of view of their patients, their emotional reaction and prosocial helping behaviors reduce the effect of exposure to stress.
Burnout was significantly associated with living alone (P < .05) and, to a certain extent, being a woman. Women had higher levels of emotional exhaustion (P < .01), lower levels of depersonalization (P < .05), and higher levels of empathic concern (P < .001) than did men. Burnout was also associated with lower scores on the JSPE measure of “standing in the patient’s shoes.”
Being sympathetic rather than empathic can lead to empathic overarousal or personal distress, the researchers say. Physicians may have trouble maintaining a sense of ownership regarding whose emotions belong to whom. According to the researchers, professionals need a high level of emotional regulation skills, that is, cognitive empathy.
But cognitive empathy takes work, the researchers suggest. According to them, it isn’t always easy to maintain emotional concern while keeping a safe distance. However, the benefits of staying engaged—just not too engaged—can be good for practitioners in several ways. Remaining open to the patients’ experience may lead to better mental health in physicians, the researchers say, and physicians have reported in other research that showing interest in the patient protected them from monotony. Moreover, good relationships with patients were reflected in patient gratitude, the researchers note, and that was another source of strength for the physician. Their findings, they say, “go a step further,” suggesting that cognitive empathy, but not affective empathy, when used independently, will lead to lower burnout and higher well-being.
Teaching emotion regulation and mindfulness should be part of the curricula for health care practitioners, the researchers say. Training physicians to keep the right amount of distance can help them help their patients—and themselves.
Source
Lamothe M, Boujut E, Zenasni F, Sultan S. BMC Family Practice. 2014;15:15.
doi: 10.1186/1471-2296-15-15.
Empathy expressed by a practitioner is important to patients—but empathy is also important for the practitioner. According to researchers from University of Montreal in Canada, empathic concern and perspective taking when treating patients can help prevent physician burnout.
Empathy and sympathy are 2 different concepts, and they can lead to different outcomes, the researchers say. For instance, in hypothetical situations, sympathetic physicians who “feel” their patients’ pain have used more health care resources than empathic ones who “know” their patients’ pain. Some authors, the researchers point out, believe that sympathy can be detrimental to objectivity in decision making and lead to compassion fatigue and burnout.
In their study, the researchers aimed to identify the best balance between empathic concern for the patient and affective distance necessary to maintain clinical neutrality and the physician’s own emotional equilibrium.
They gave questionnaires to 294 general practitioners: the Maslach Burnout Inventory to measure emotional exhaustion, depersonalization, and personal accomplishment; a subscale of the Jefferson Scale of Physician Empathy (JSPE) to measure perspective taking (seeing things from the patient’s point of view); and the Toronto Empathy Questionnaire to rate emotional concern.
A high level of perspective taking was associated with lower burnout. But contrary to their expectations, the researchers found that a higher level of empathic concern was also significantly associated with a lower proportion of burnout. The odds of experiencing burnout were significantly lower when physicians scored high on perspective taking (P < .001), high on empathic concern (P < .05), and high on both (P < .001). Empathic concern had no effect when perspective taking was low. The researchers speculate that when physicians are good at adopting the point of view of their patients, their emotional reaction and prosocial helping behaviors reduce the effect of exposure to stress.
Burnout was significantly associated with living alone (P < .05) and, to a certain extent, being a woman. Women had higher levels of emotional exhaustion (P < .01), lower levels of depersonalization (P < .05), and higher levels of empathic concern (P < .001) than did men. Burnout was also associated with lower scores on the JSPE measure of “standing in the patient’s shoes.”
Being sympathetic rather than empathic can lead to empathic overarousal or personal distress, the researchers say. Physicians may have trouble maintaining a sense of ownership regarding whose emotions belong to whom. According to the researchers, professionals need a high level of emotional regulation skills, that is, cognitive empathy.
But cognitive empathy takes work, the researchers suggest. According to them, it isn’t always easy to maintain emotional concern while keeping a safe distance. However, the benefits of staying engaged—just not too engaged—can be good for practitioners in several ways. Remaining open to the patients’ experience may lead to better mental health in physicians, the researchers say, and physicians have reported in other research that showing interest in the patient protected them from monotony. Moreover, good relationships with patients were reflected in patient gratitude, the researchers note, and that was another source of strength for the physician. Their findings, they say, “go a step further,” suggesting that cognitive empathy, but not affective empathy, when used independently, will lead to lower burnout and higher well-being.
Teaching emotion regulation and mindfulness should be part of the curricula for health care practitioners, the researchers say. Training physicians to keep the right amount of distance can help them help their patients—and themselves.
Source
Lamothe M, Boujut E, Zenasni F, Sultan S. BMC Family Practice. 2014;15:15.
doi: 10.1186/1471-2296-15-15.
Empathy expressed by a practitioner is important to patients—but empathy is also important for the practitioner. According to researchers from University of Montreal in Canada, empathic concern and perspective taking when treating patients can help prevent physician burnout.
Empathy and sympathy are 2 different concepts, and they can lead to different outcomes, the researchers say. For instance, in hypothetical situations, sympathetic physicians who “feel” their patients’ pain have used more health care resources than empathic ones who “know” their patients’ pain. Some authors, the researchers point out, believe that sympathy can be detrimental to objectivity in decision making and lead to compassion fatigue and burnout.
In their study, the researchers aimed to identify the best balance between empathic concern for the patient and affective distance necessary to maintain clinical neutrality and the physician’s own emotional equilibrium.
They gave questionnaires to 294 general practitioners: the Maslach Burnout Inventory to measure emotional exhaustion, depersonalization, and personal accomplishment; a subscale of the Jefferson Scale of Physician Empathy (JSPE) to measure perspective taking (seeing things from the patient’s point of view); and the Toronto Empathy Questionnaire to rate emotional concern.
A high level of perspective taking was associated with lower burnout. But contrary to their expectations, the researchers found that a higher level of empathic concern was also significantly associated with a lower proportion of burnout. The odds of experiencing burnout were significantly lower when physicians scored high on perspective taking (P < .001), high on empathic concern (P < .05), and high on both (P < .001). Empathic concern had no effect when perspective taking was low. The researchers speculate that when physicians are good at adopting the point of view of their patients, their emotional reaction and prosocial helping behaviors reduce the effect of exposure to stress.
Burnout was significantly associated with living alone (P < .05) and, to a certain extent, being a woman. Women had higher levels of emotional exhaustion (P < .01), lower levels of depersonalization (P < .05), and higher levels of empathic concern (P < .001) than did men. Burnout was also associated with lower scores on the JSPE measure of “standing in the patient’s shoes.”
Being sympathetic rather than empathic can lead to empathic overarousal or personal distress, the researchers say. Physicians may have trouble maintaining a sense of ownership regarding whose emotions belong to whom. According to the researchers, professionals need a high level of emotional regulation skills, that is, cognitive empathy.
But cognitive empathy takes work, the researchers suggest. According to them, it isn’t always easy to maintain emotional concern while keeping a safe distance. However, the benefits of staying engaged—just not too engaged—can be good for practitioners in several ways. Remaining open to the patients’ experience may lead to better mental health in physicians, the researchers say, and physicians have reported in other research that showing interest in the patient protected them from monotony. Moreover, good relationships with patients were reflected in patient gratitude, the researchers note, and that was another source of strength for the physician. Their findings, they say, “go a step further,” suggesting that cognitive empathy, but not affective empathy, when used independently, will lead to lower burnout and higher well-being.
Teaching emotion regulation and mindfulness should be part of the curricula for health care practitioners, the researchers say. Training physicians to keep the right amount of distance can help them help their patients—and themselves.
Source
Lamothe M, Boujut E, Zenasni F, Sultan S. BMC Family Practice. 2014;15:15.
doi: 10.1186/1471-2296-15-15.