User login
Oophorectomy or salpingectomy—which makes more sense?
CASE: PATIENT OPTS FOR HYSTERECTOMY, ASKS ABOUT OOPHORECTOMY
Your 46-year-old patient reports increasingly severe dysmenorrhea at her annual visit, and a pelvic examination reveals an enlarged uterus. You order pelvic magnetic resonance imaging, which shows extensive adenomyosis.
After you counsel the patient about her options, she elects to undergo laparoscopic supracervical hysterectomy and asks whether she should have her ovaries removed at the time of surgery. She has no family history of ovarian or breast cancer.
What would you recommend for this woman, based on her situation and current medical research?
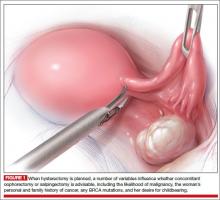
A prophylactic procedure should be considered only if 1) there is a reasonable expectation that it will benefit the patient and 2) there is evidence that, without it, the individual will be at high risk for disease.1 Bilateral oophorectomy at the time of hysterectomy for benign disease often has been recommended for women older than age 45 to prevent the subsequent development of ovarian cancer (FIGURES 1 and 2).
The 2002 Women’s Health Initiative report suggested that exogenous hormone use was associated with a slight increase in the risk of breast cancer.2 After its publication, the rate of oophorectomy at the time of hysterectomy declined slightly, likely reflecting women’s desire to preserve their own source of estrogen.3 For women younger than age 50, further slight declines in the rate of oophorectomy were seen from 2002 to 2010. However, in the United States, almost 300,000 women still undergo “prophylactic” bilateral salpingo-oophorectomy every year.4
The lifetime risk of ovarian cancer among women with a BRCA 1 mutation is 36% to 46%, and it is 10% to 27% among women with a BRCA 2 mutation. Annual screening for ovarian cancer using transvaginal ultrasound and CA 125 has not been effective even among this group of women and is not recommended.5 There is universal agreement that women with these mutations should strongly consider oophorectomy once they have completed childbearing.6 Genetic counseling and testing for these genetic mutations now are readily available.
In the general population of US women, the lifetime risk of ovarian cancer is 1.4%. The risk varies between populations, however. For white women with 3 or more term pregnancies and 4 or more years of oral contraceptive use, the lifetime risk is only 3 women in every 1,000 (0.3%).7
KNOW THE FULL RANGE OF RISKS ASSOCIATED WITH OOPHORECTOMY
After menopause and throughout a woman’s life, the ovaries continue to produce androgens, which are converted to estrone. Many studies suggest that endogenous estrogen is beneficial to the heart, bones, and brain.
A 2009 study from the Nurses’ Health Study (NHS) database found that, among women who underwent hysterectomy with oophorectomy, there were more cases of coronary heart disease (CHD), stroke, and lung cancer, compared with women who had hysterectomy with ovarian conservation.8
A subsequent NHS report focused on long-term mortality and found that, after 28 years of follow-up, women who had a hysterectomy and bilateral oophorectomy had a higher risk of dying from CHD (hazard ratio [HR], 1.23), colorectal cancer (HR, 1.49), lung cancer (HR, 1.29), and all causes (HR, 1.13) than did women who had hysterectomy and ovarian conservation.9 During the 28 years, 44 of 13,302 women (0.9%) died of ovarian cancer. At no age was there a survival advantage in the oophorectomy group. A Mayo Clinic study found similar results.10
Additional studies of the Mayo population found higher risks of anxiety, depression, dementia or cognitive impairment, and Parkinsonism in women who had their ovaries removed.11 Also, about 90% of premenopausal women experience vasomotor symptoms following oophorectomy; many women also experience mood changes, a decline in feelings of well-being, lower sexual desire, sleep disturbances, and headaches.
Overall, the evidence suggests that the removal of healthy ovaries does not meet the requirements for a prophylactic intervention.
EXOGENOUS ESTROGEN IS NOT A PRACTICAL STRATEGY AFTER OOPHORECTOMY
In the NHS studies, women who underwent hysterectomy and bilateral oophorectomy before age 50 but did not use subsequent estrogen therapy had a higher risk of all-cause mortality than women who did use estrogen (HR, 1.41).9 An early response to this finding was to advocate oophorectomy followed by the initiation of menopausal hormone therapy and statins to ward off any negative cardiovascular effects. However, data indicate that only 17% of women continue to take estrogen 5 years after the initial prescription, and only 18% of women still take statins 1 year after their first prescription.12 Even these figures are overstated because they do not include women who never see a doctor, those who see a doctor but don’t get a prescription, and those who never fill their first prescription.
Clearly, oophorectomy followed by initiation of estrogen and statins for women younger than 50 is unlikely to be effective.
THE LIKELIHOOD OF FUTURE ADNEXAL SURGERY IS LOW
Only about 6.2% of women who undergo hysterectomy with ovarian conservation require reoperation over the succeeding 20 years. The risk for age-matched women without hysterectomy is 4.8%, so the absolute difference is only 1.4% over 20 years.13
Although asymptomatic ovarian cysts are rather prevalent (6.6%) in postmenopausal women, they do not undergo transformation to cancer and usually resolve spontaneously.14 Therefore, the majority of these cysts do not need to be removed.
The suggestion that oophorectomy can avert the need for future adnexal surgery appears to be unfounded.
OVARIAN CANCER DOES NOT COME FROM THE OVARY
Seventy percent of epithelial ovarian cancers are of the serous high-grade and clinically aggressive type. The ovary contains no epithelial cells.15 Almost all high-grade cancers are associated with p53 mutations. Cancer precursor lesions called serous tubal intraepithelial cancer (STIC) have been found in the fallopian tubes of both BRCA-positive and BRCA-negative women, but no corresponding precursor lesions have ever been found in the ovary. Moreover, STIC precursor lesions have p53 mutations matching those found in high-grade serous “ovarian” cancers, but no similar p53 mutations have been found in low-grade, more indolent and treatable cancers found inside the ovary (ie, Stage 1). Therefore, the deadly form of ovarian cancer is, in fact, tubal cancer.
THE CASE FOR SALPINGECTOMY
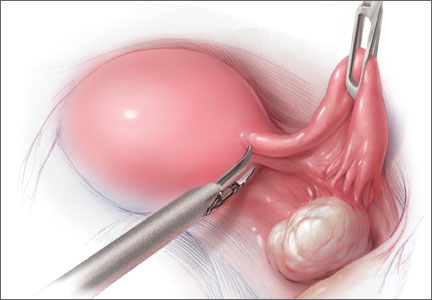
Because convincing evidence points to the tubal origin of ovarian cancer, some experts have proposed salpingectomy for prophylaxis. Salpingectomy should remove the source of aggressive cancers and preserve functioning ovaries. However, some wondered whether salpingectomy would compromise collateral circulation to the ovaries and predispose women to early ovarian failure.
A recent study of 79 women found similar antral follicle counts and mean ovarian diameters (as measured sonographically) and similar serum levels of anti-Müllerian hormone and follicle-stimulating hormone at baseline (prior to salpingectomy) and 3 months following surgery.16 Therefore, bilateral salpingectomy may be a reasonable choice for women who have completed childbearing and who are considering pelvic surgery. As the Society of Gynecologic Oncologists stated in recent guidelines: “For women at average risk of ovarian cancer, salpingectomy should be discussed and considered prior to abdominal or pelvic surgery, hysterectomy, or in lieu of tubal ligation.”17
CASE: RESOLVED
After you review the risks and benefits of prophylactic oophorectomy versus prophylactic salpingectomy, the patient chooses the latter option and undergoes a successful surgery.
BOTTOM LINE: IN WOMEN WITH AN AVERAGE RISK OF OVARIAN CANCER, SALPINGECTOMY IS PREFERRED
Reasonable evidence now suggests that oophorectomy is associated with higher risks of CHD, colorectal and lung cancers, and overall mortality. Almost all high-grade serous cancers arise from the fallopian tubes, not the ovaries. Therefore, for women at average risk for ovarian cancer who have completed childbearing, salpingectomy should be considered at the time of pelvic surgery.
After decades of failure to achieve early diagnosis or curative treatment of “ovarian” cancer, we finally may have a way to reduce the incidence of this deadly disease.
TELL US WHAT YOU THINK!
Share your thoughts on this article or on any topic relevant to ObGyns and women’s health practitioners. Tell us which topics you’d like to see covered in future issues, and what challenges you face in daily practice. We will consider publishing your letter and in a future issue.
Send your letter to: [email protected] Please include the city and state in which you practice.
Stay in touch! Your feedback is important to us!
- Hodges F, Svoboda J, Van Howe RS. Prophylactic interventions on children: balancing human rights with public health. J Med Ethics. 2002;28(1):10–16.
- Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333.
- Perera HK, Ananth CV, Richards CA, et al. Variation in ovarian conservation in women undergoing hysterectomy for benign indications. Obstet Gynecol. 2013;121(4):717–726.
- Whiteman MK, Hillis SD, Jamieson DJ, et al. Inpatient hysterectomy surveillance in the United States, 2000-2004. Am J Obstet Gynecol. 2008;198:34.e1–e7.
- Evans GR, Gaarenstroom KN, Stirling D, et al. Screening for familial ovarian cancer: poor survival of BRCA1/2 related cancers. J Med Genet. 2009;46(9):593–597.
- Kauff ND, Domchek SM, Friebel TM, et al. Risk-reducing salpingo-oophorectomy for the prevention of BRCA1- and BRCA2-associated breast and gynecologic cancer: A multicenter, prospective study. J Clin Oncol. 2008;26(8):1331–1337.
- Hartge P, Whittemore AS, Itnyre J, McGowan L, Cramer D. Rates and risks of ovarian cancer in subgroups of white women in the United States. The Collaborative Ovarian Cancer Group. Obstet Gynecol. 1994;84(5):760–764.
- Parker W, Broder M, Chang E, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the Nurses’ Health Study. Obstet Gynecol. 2009;113(5):1027–1037.
- Parker WH, Feskanich D, Broder MS, et al. Long-term mortality associated with oophorectomy compared with ovarian conservation in the Nurses’ Health Study. Obstet Gynecol. 2013;121(4):709–716.
- Rocca WA, Grossardt BR, de Andrade M, Malkasian GD, Melton LJ III. Survival patterns after oophorectomy in premenopausal women: A population-based cohort study. Lancet Oncol. 2006;7(10):821–828.
- Rocca W, Bower J, Maraganore D, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69(11):1074–1083.
- Sprague BL, Trentham-Dietz A, Cronin KA. A sustained decline in postmenopausal hormone use: Results from the national health and nutrition examination survey, 1999–2010. Obstet Gynecol. 2012;120(3):595–603.
- Casiano ER, Trabuco EC, Bharucha AE, et al. Risk of oophorectomy after hysterectomy. Obstet Gynecol. 2013;121(5):1069–1074.
- Pavlik EJ, Ueland FR, Miller RW, et al. Frequency and disposition of ovarian abnormalities followed with serial transvaginal ultrasonography. Obstet Gynecol. 2013;122(2 Pt 1):210–217.
- Kurman RJ, Shih IM. The origin and pathogenesis of epithelial ovarian cancer: A proposed unifying theory. Am J Surg Pathol. 2010;34(3):433–443.
- Morelli M, Venturella R, Mocciaro R, et al. Prophylactic salpingectomy in premenopausal low-risk women for ovarian cancer: primum non nocere. Gynecol Oncol. 2013;129(6):448–451.
- SGO Clinical Practice Statement: Salpingectomy for Ovarian Cancer Prevention. Society of Gynecologic Oncology. November 2013. https://www.sgo.org/clinical-practice/guidelines/sgo-clinical-practice-statement-salpingectomy-for-ovarian-cancer-prevention. Accessed February 10, 2014.
CASE: PATIENT OPTS FOR HYSTERECTOMY, ASKS ABOUT OOPHORECTOMY
Your 46-year-old patient reports increasingly severe dysmenorrhea at her annual visit, and a pelvic examination reveals an enlarged uterus. You order pelvic magnetic resonance imaging, which shows extensive adenomyosis.
After you counsel the patient about her options, she elects to undergo laparoscopic supracervical hysterectomy and asks whether she should have her ovaries removed at the time of surgery. She has no family history of ovarian or breast cancer.
What would you recommend for this woman, based on her situation and current medical research?
A prophylactic procedure should be considered only if 1) there is a reasonable expectation that it will benefit the patient and 2) there is evidence that, without it, the individual will be at high risk for disease.1 Bilateral oophorectomy at the time of hysterectomy for benign disease often has been recommended for women older than age 45 to prevent the subsequent development of ovarian cancer (FIGURES 1 and 2).
The 2002 Women’s Health Initiative report suggested that exogenous hormone use was associated with a slight increase in the risk of breast cancer.2 After its publication, the rate of oophorectomy at the time of hysterectomy declined slightly, likely reflecting women’s desire to preserve their own source of estrogen.3 For women younger than age 50, further slight declines in the rate of oophorectomy were seen from 2002 to 2010. However, in the United States, almost 300,000 women still undergo “prophylactic” bilateral salpingo-oophorectomy every year.4
The lifetime risk of ovarian cancer among women with a BRCA 1 mutation is 36% to 46%, and it is 10% to 27% among women with a BRCA 2 mutation. Annual screening for ovarian cancer using transvaginal ultrasound and CA 125 has not been effective even among this group of women and is not recommended.5 There is universal agreement that women with these mutations should strongly consider oophorectomy once they have completed childbearing.6 Genetic counseling and testing for these genetic mutations now are readily available.
In the general population of US women, the lifetime risk of ovarian cancer is 1.4%. The risk varies between populations, however. For white women with 3 or more term pregnancies and 4 or more years of oral contraceptive use, the lifetime risk is only 3 women in every 1,000 (0.3%).7
KNOW THE FULL RANGE OF RISKS ASSOCIATED WITH OOPHORECTOMY
After menopause and throughout a woman’s life, the ovaries continue to produce androgens, which are converted to estrone. Many studies suggest that endogenous estrogen is beneficial to the heart, bones, and brain.
A 2009 study from the Nurses’ Health Study (NHS) database found that, among women who underwent hysterectomy with oophorectomy, there were more cases of coronary heart disease (CHD), stroke, and lung cancer, compared with women who had hysterectomy with ovarian conservation.8
A subsequent NHS report focused on long-term mortality and found that, after 28 years of follow-up, women who had a hysterectomy and bilateral oophorectomy had a higher risk of dying from CHD (hazard ratio [HR], 1.23), colorectal cancer (HR, 1.49), lung cancer (HR, 1.29), and all causes (HR, 1.13) than did women who had hysterectomy and ovarian conservation.9 During the 28 years, 44 of 13,302 women (0.9%) died of ovarian cancer. At no age was there a survival advantage in the oophorectomy group. A Mayo Clinic study found similar results.10
Additional studies of the Mayo population found higher risks of anxiety, depression, dementia or cognitive impairment, and Parkinsonism in women who had their ovaries removed.11 Also, about 90% of premenopausal women experience vasomotor symptoms following oophorectomy; many women also experience mood changes, a decline in feelings of well-being, lower sexual desire, sleep disturbances, and headaches.
Overall, the evidence suggests that the removal of healthy ovaries does not meet the requirements for a prophylactic intervention.
EXOGENOUS ESTROGEN IS NOT A PRACTICAL STRATEGY AFTER OOPHORECTOMY
In the NHS studies, women who underwent hysterectomy and bilateral oophorectomy before age 50 but did not use subsequent estrogen therapy had a higher risk of all-cause mortality than women who did use estrogen (HR, 1.41).9 An early response to this finding was to advocate oophorectomy followed by the initiation of menopausal hormone therapy and statins to ward off any negative cardiovascular effects. However, data indicate that only 17% of women continue to take estrogen 5 years after the initial prescription, and only 18% of women still take statins 1 year after their first prescription.12 Even these figures are overstated because they do not include women who never see a doctor, those who see a doctor but don’t get a prescription, and those who never fill their first prescription.
Clearly, oophorectomy followed by initiation of estrogen and statins for women younger than 50 is unlikely to be effective.
THE LIKELIHOOD OF FUTURE ADNEXAL SURGERY IS LOW
Only about 6.2% of women who undergo hysterectomy with ovarian conservation require reoperation over the succeeding 20 years. The risk for age-matched women without hysterectomy is 4.8%, so the absolute difference is only 1.4% over 20 years.13
Although asymptomatic ovarian cysts are rather prevalent (6.6%) in postmenopausal women, they do not undergo transformation to cancer and usually resolve spontaneously.14 Therefore, the majority of these cysts do not need to be removed.
The suggestion that oophorectomy can avert the need for future adnexal surgery appears to be unfounded.
OVARIAN CANCER DOES NOT COME FROM THE OVARY
Seventy percent of epithelial ovarian cancers are of the serous high-grade and clinically aggressive type. The ovary contains no epithelial cells.15 Almost all high-grade cancers are associated with p53 mutations. Cancer precursor lesions called serous tubal intraepithelial cancer (STIC) have been found in the fallopian tubes of both BRCA-positive and BRCA-negative women, but no corresponding precursor lesions have ever been found in the ovary. Moreover, STIC precursor lesions have p53 mutations matching those found in high-grade serous “ovarian” cancers, but no similar p53 mutations have been found in low-grade, more indolent and treatable cancers found inside the ovary (ie, Stage 1). Therefore, the deadly form of ovarian cancer is, in fact, tubal cancer.
THE CASE FOR SALPINGECTOMY
Because convincing evidence points to the tubal origin of ovarian cancer, some experts have proposed salpingectomy for prophylaxis. Salpingectomy should remove the source of aggressive cancers and preserve functioning ovaries. However, some wondered whether salpingectomy would compromise collateral circulation to the ovaries and predispose women to early ovarian failure.
A recent study of 79 women found similar antral follicle counts and mean ovarian diameters (as measured sonographically) and similar serum levels of anti-Müllerian hormone and follicle-stimulating hormone at baseline (prior to salpingectomy) and 3 months following surgery.16 Therefore, bilateral salpingectomy may be a reasonable choice for women who have completed childbearing and who are considering pelvic surgery. As the Society of Gynecologic Oncologists stated in recent guidelines: “For women at average risk of ovarian cancer, salpingectomy should be discussed and considered prior to abdominal or pelvic surgery, hysterectomy, or in lieu of tubal ligation.”17
CASE: RESOLVED
After you review the risks and benefits of prophylactic oophorectomy versus prophylactic salpingectomy, the patient chooses the latter option and undergoes a successful surgery.
BOTTOM LINE: IN WOMEN WITH AN AVERAGE RISK OF OVARIAN CANCER, SALPINGECTOMY IS PREFERRED
Reasonable evidence now suggests that oophorectomy is associated with higher risks of CHD, colorectal and lung cancers, and overall mortality. Almost all high-grade serous cancers arise from the fallopian tubes, not the ovaries. Therefore, for women at average risk for ovarian cancer who have completed childbearing, salpingectomy should be considered at the time of pelvic surgery.
After decades of failure to achieve early diagnosis or curative treatment of “ovarian” cancer, we finally may have a way to reduce the incidence of this deadly disease.
TELL US WHAT YOU THINK!
Share your thoughts on this article or on any topic relevant to ObGyns and women’s health practitioners. Tell us which topics you’d like to see covered in future issues, and what challenges you face in daily practice. We will consider publishing your letter and in a future issue.
Send your letter to: [email protected] Please include the city and state in which you practice.
Stay in touch! Your feedback is important to us!
CASE: PATIENT OPTS FOR HYSTERECTOMY, ASKS ABOUT OOPHORECTOMY
Your 46-year-old patient reports increasingly severe dysmenorrhea at her annual visit, and a pelvic examination reveals an enlarged uterus. You order pelvic magnetic resonance imaging, which shows extensive adenomyosis.
After you counsel the patient about her options, she elects to undergo laparoscopic supracervical hysterectomy and asks whether she should have her ovaries removed at the time of surgery. She has no family history of ovarian or breast cancer.
What would you recommend for this woman, based on her situation and current medical research?
A prophylactic procedure should be considered only if 1) there is a reasonable expectation that it will benefit the patient and 2) there is evidence that, without it, the individual will be at high risk for disease.1 Bilateral oophorectomy at the time of hysterectomy for benign disease often has been recommended for women older than age 45 to prevent the subsequent development of ovarian cancer (FIGURES 1 and 2).
The 2002 Women’s Health Initiative report suggested that exogenous hormone use was associated with a slight increase in the risk of breast cancer.2 After its publication, the rate of oophorectomy at the time of hysterectomy declined slightly, likely reflecting women’s desire to preserve their own source of estrogen.3 For women younger than age 50, further slight declines in the rate of oophorectomy were seen from 2002 to 2010. However, in the United States, almost 300,000 women still undergo “prophylactic” bilateral salpingo-oophorectomy every year.4
The lifetime risk of ovarian cancer among women with a BRCA 1 mutation is 36% to 46%, and it is 10% to 27% among women with a BRCA 2 mutation. Annual screening for ovarian cancer using transvaginal ultrasound and CA 125 has not been effective even among this group of women and is not recommended.5 There is universal agreement that women with these mutations should strongly consider oophorectomy once they have completed childbearing.6 Genetic counseling and testing for these genetic mutations now are readily available.
In the general population of US women, the lifetime risk of ovarian cancer is 1.4%. The risk varies between populations, however. For white women with 3 or more term pregnancies and 4 or more years of oral contraceptive use, the lifetime risk is only 3 women in every 1,000 (0.3%).7
KNOW THE FULL RANGE OF RISKS ASSOCIATED WITH OOPHORECTOMY
After menopause and throughout a woman’s life, the ovaries continue to produce androgens, which are converted to estrone. Many studies suggest that endogenous estrogen is beneficial to the heart, bones, and brain.
A 2009 study from the Nurses’ Health Study (NHS) database found that, among women who underwent hysterectomy with oophorectomy, there were more cases of coronary heart disease (CHD), stroke, and lung cancer, compared with women who had hysterectomy with ovarian conservation.8
A subsequent NHS report focused on long-term mortality and found that, after 28 years of follow-up, women who had a hysterectomy and bilateral oophorectomy had a higher risk of dying from CHD (hazard ratio [HR], 1.23), colorectal cancer (HR, 1.49), lung cancer (HR, 1.29), and all causes (HR, 1.13) than did women who had hysterectomy and ovarian conservation.9 During the 28 years, 44 of 13,302 women (0.9%) died of ovarian cancer. At no age was there a survival advantage in the oophorectomy group. A Mayo Clinic study found similar results.10
Additional studies of the Mayo population found higher risks of anxiety, depression, dementia or cognitive impairment, and Parkinsonism in women who had their ovaries removed.11 Also, about 90% of premenopausal women experience vasomotor symptoms following oophorectomy; many women also experience mood changes, a decline in feelings of well-being, lower sexual desire, sleep disturbances, and headaches.
Overall, the evidence suggests that the removal of healthy ovaries does not meet the requirements for a prophylactic intervention.
EXOGENOUS ESTROGEN IS NOT A PRACTICAL STRATEGY AFTER OOPHORECTOMY
In the NHS studies, women who underwent hysterectomy and bilateral oophorectomy before age 50 but did not use subsequent estrogen therapy had a higher risk of all-cause mortality than women who did use estrogen (HR, 1.41).9 An early response to this finding was to advocate oophorectomy followed by the initiation of menopausal hormone therapy and statins to ward off any negative cardiovascular effects. However, data indicate that only 17% of women continue to take estrogen 5 years after the initial prescription, and only 18% of women still take statins 1 year after their first prescription.12 Even these figures are overstated because they do not include women who never see a doctor, those who see a doctor but don’t get a prescription, and those who never fill their first prescription.
Clearly, oophorectomy followed by initiation of estrogen and statins for women younger than 50 is unlikely to be effective.
THE LIKELIHOOD OF FUTURE ADNEXAL SURGERY IS LOW
Only about 6.2% of women who undergo hysterectomy with ovarian conservation require reoperation over the succeeding 20 years. The risk for age-matched women without hysterectomy is 4.8%, so the absolute difference is only 1.4% over 20 years.13
Although asymptomatic ovarian cysts are rather prevalent (6.6%) in postmenopausal women, they do not undergo transformation to cancer and usually resolve spontaneously.14 Therefore, the majority of these cysts do not need to be removed.
The suggestion that oophorectomy can avert the need for future adnexal surgery appears to be unfounded.
OVARIAN CANCER DOES NOT COME FROM THE OVARY
Seventy percent of epithelial ovarian cancers are of the serous high-grade and clinically aggressive type. The ovary contains no epithelial cells.15 Almost all high-grade cancers are associated with p53 mutations. Cancer precursor lesions called serous tubal intraepithelial cancer (STIC) have been found in the fallopian tubes of both BRCA-positive and BRCA-negative women, but no corresponding precursor lesions have ever been found in the ovary. Moreover, STIC precursor lesions have p53 mutations matching those found in high-grade serous “ovarian” cancers, but no similar p53 mutations have been found in low-grade, more indolent and treatable cancers found inside the ovary (ie, Stage 1). Therefore, the deadly form of ovarian cancer is, in fact, tubal cancer.
THE CASE FOR SALPINGECTOMY
Because convincing evidence points to the tubal origin of ovarian cancer, some experts have proposed salpingectomy for prophylaxis. Salpingectomy should remove the source of aggressive cancers and preserve functioning ovaries. However, some wondered whether salpingectomy would compromise collateral circulation to the ovaries and predispose women to early ovarian failure.
A recent study of 79 women found similar antral follicle counts and mean ovarian diameters (as measured sonographically) and similar serum levels of anti-Müllerian hormone and follicle-stimulating hormone at baseline (prior to salpingectomy) and 3 months following surgery.16 Therefore, bilateral salpingectomy may be a reasonable choice for women who have completed childbearing and who are considering pelvic surgery. As the Society of Gynecologic Oncologists stated in recent guidelines: “For women at average risk of ovarian cancer, salpingectomy should be discussed and considered prior to abdominal or pelvic surgery, hysterectomy, or in lieu of tubal ligation.”17
CASE: RESOLVED
After you review the risks and benefits of prophylactic oophorectomy versus prophylactic salpingectomy, the patient chooses the latter option and undergoes a successful surgery.
BOTTOM LINE: IN WOMEN WITH AN AVERAGE RISK OF OVARIAN CANCER, SALPINGECTOMY IS PREFERRED
Reasonable evidence now suggests that oophorectomy is associated with higher risks of CHD, colorectal and lung cancers, and overall mortality. Almost all high-grade serous cancers arise from the fallopian tubes, not the ovaries. Therefore, for women at average risk for ovarian cancer who have completed childbearing, salpingectomy should be considered at the time of pelvic surgery.
After decades of failure to achieve early diagnosis or curative treatment of “ovarian” cancer, we finally may have a way to reduce the incidence of this deadly disease.
TELL US WHAT YOU THINK!
Share your thoughts on this article or on any topic relevant to ObGyns and women’s health practitioners. Tell us which topics you’d like to see covered in future issues, and what challenges you face in daily practice. We will consider publishing your letter and in a future issue.
Send your letter to: [email protected] Please include the city and state in which you practice.
Stay in touch! Your feedback is important to us!
- Hodges F, Svoboda J, Van Howe RS. Prophylactic interventions on children: balancing human rights with public health. J Med Ethics. 2002;28(1):10–16.
- Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333.
- Perera HK, Ananth CV, Richards CA, et al. Variation in ovarian conservation in women undergoing hysterectomy for benign indications. Obstet Gynecol. 2013;121(4):717–726.
- Whiteman MK, Hillis SD, Jamieson DJ, et al. Inpatient hysterectomy surveillance in the United States, 2000-2004. Am J Obstet Gynecol. 2008;198:34.e1–e7.
- Evans GR, Gaarenstroom KN, Stirling D, et al. Screening for familial ovarian cancer: poor survival of BRCA1/2 related cancers. J Med Genet. 2009;46(9):593–597.
- Kauff ND, Domchek SM, Friebel TM, et al. Risk-reducing salpingo-oophorectomy for the prevention of BRCA1- and BRCA2-associated breast and gynecologic cancer: A multicenter, prospective study. J Clin Oncol. 2008;26(8):1331–1337.
- Hartge P, Whittemore AS, Itnyre J, McGowan L, Cramer D. Rates and risks of ovarian cancer in subgroups of white women in the United States. The Collaborative Ovarian Cancer Group. Obstet Gynecol. 1994;84(5):760–764.
- Parker W, Broder M, Chang E, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the Nurses’ Health Study. Obstet Gynecol. 2009;113(5):1027–1037.
- Parker WH, Feskanich D, Broder MS, et al. Long-term mortality associated with oophorectomy compared with ovarian conservation in the Nurses’ Health Study. Obstet Gynecol. 2013;121(4):709–716.
- Rocca WA, Grossardt BR, de Andrade M, Malkasian GD, Melton LJ III. Survival patterns after oophorectomy in premenopausal women: A population-based cohort study. Lancet Oncol. 2006;7(10):821–828.
- Rocca W, Bower J, Maraganore D, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69(11):1074–1083.
- Sprague BL, Trentham-Dietz A, Cronin KA. A sustained decline in postmenopausal hormone use: Results from the national health and nutrition examination survey, 1999–2010. Obstet Gynecol. 2012;120(3):595–603.
- Casiano ER, Trabuco EC, Bharucha AE, et al. Risk of oophorectomy after hysterectomy. Obstet Gynecol. 2013;121(5):1069–1074.
- Pavlik EJ, Ueland FR, Miller RW, et al. Frequency and disposition of ovarian abnormalities followed with serial transvaginal ultrasonography. Obstet Gynecol. 2013;122(2 Pt 1):210–217.
- Kurman RJ, Shih IM. The origin and pathogenesis of epithelial ovarian cancer: A proposed unifying theory. Am J Surg Pathol. 2010;34(3):433–443.
- Morelli M, Venturella R, Mocciaro R, et al. Prophylactic salpingectomy in premenopausal low-risk women for ovarian cancer: primum non nocere. Gynecol Oncol. 2013;129(6):448–451.
- SGO Clinical Practice Statement: Salpingectomy for Ovarian Cancer Prevention. Society of Gynecologic Oncology. November 2013. https://www.sgo.org/clinical-practice/guidelines/sgo-clinical-practice-statement-salpingectomy-for-ovarian-cancer-prevention. Accessed February 10, 2014.
- Hodges F, Svoboda J, Van Howe RS. Prophylactic interventions on children: balancing human rights with public health. J Med Ethics. 2002;28(1):10–16.
- Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333.
- Perera HK, Ananth CV, Richards CA, et al. Variation in ovarian conservation in women undergoing hysterectomy for benign indications. Obstet Gynecol. 2013;121(4):717–726.
- Whiteman MK, Hillis SD, Jamieson DJ, et al. Inpatient hysterectomy surveillance in the United States, 2000-2004. Am J Obstet Gynecol. 2008;198:34.e1–e7.
- Evans GR, Gaarenstroom KN, Stirling D, et al. Screening for familial ovarian cancer: poor survival of BRCA1/2 related cancers. J Med Genet. 2009;46(9):593–597.
- Kauff ND, Domchek SM, Friebel TM, et al. Risk-reducing salpingo-oophorectomy for the prevention of BRCA1- and BRCA2-associated breast and gynecologic cancer: A multicenter, prospective study. J Clin Oncol. 2008;26(8):1331–1337.
- Hartge P, Whittemore AS, Itnyre J, McGowan L, Cramer D. Rates and risks of ovarian cancer in subgroups of white women in the United States. The Collaborative Ovarian Cancer Group. Obstet Gynecol. 1994;84(5):760–764.
- Parker W, Broder M, Chang E, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the Nurses’ Health Study. Obstet Gynecol. 2009;113(5):1027–1037.
- Parker WH, Feskanich D, Broder MS, et al. Long-term mortality associated with oophorectomy compared with ovarian conservation in the Nurses’ Health Study. Obstet Gynecol. 2013;121(4):709–716.
- Rocca WA, Grossardt BR, de Andrade M, Malkasian GD, Melton LJ III. Survival patterns after oophorectomy in premenopausal women: A population-based cohort study. Lancet Oncol. 2006;7(10):821–828.
- Rocca W, Bower J, Maraganore D, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69(11):1074–1083.
- Sprague BL, Trentham-Dietz A, Cronin KA. A sustained decline in postmenopausal hormone use: Results from the national health and nutrition examination survey, 1999–2010. Obstet Gynecol. 2012;120(3):595–603.
- Casiano ER, Trabuco EC, Bharucha AE, et al. Risk of oophorectomy after hysterectomy. Obstet Gynecol. 2013;121(5):1069–1074.
- Pavlik EJ, Ueland FR, Miller RW, et al. Frequency and disposition of ovarian abnormalities followed with serial transvaginal ultrasonography. Obstet Gynecol. 2013;122(2 Pt 1):210–217.
- Kurman RJ, Shih IM. The origin and pathogenesis of epithelial ovarian cancer: A proposed unifying theory. Am J Surg Pathol. 2010;34(3):433–443.
- Morelli M, Venturella R, Mocciaro R, et al. Prophylactic salpingectomy in premenopausal low-risk women for ovarian cancer: primum non nocere. Gynecol Oncol. 2013;129(6):448–451.
- SGO Clinical Practice Statement: Salpingectomy for Ovarian Cancer Prevention. Society of Gynecologic Oncology. November 2013. https://www.sgo.org/clinical-practice/guidelines/sgo-clinical-practice-statement-salpingectomy-for-ovarian-cancer-prevention. Accessed February 10, 2014.
5 Common Eye Complaints
Knowing how to respond when patients present with problems involving the eye is crucial for family practice clinicians. Yet it is often difficult to know whether to treat or refer and which signs and symptoms are indicative of an ophthalmologic emergency with the potential to cause loss of sight.
Categorizing ophthalmologic conditions based on patients’ chief complaints can narrow the differential diagnosis. In this article, common complaints such as “I can’t see,” “I’m seeing things,” and “My eye hurts” are used to highlight disorders—both benign and emergent—associated with each.
Continue for the first problem... "I can't see"
1.”I CAN’T SEE”
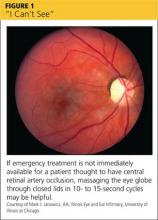
Patients may use words and phrases such as “cloudy vision,“ “a veil over my eyes,” or “fuzziness” to describe diminished vision. Some will report black areas within their visual field; others will have a loss of peripheral vision or total vision loss in one eye, or possibly even both. Some causes of vision problems, such as cataracts, are not emergencies. Causes of more severe (but painless) vision loss include central retinal artery occlusion (CRAO; see Figure 1) or vein occlusion (CRVO), giant cell arteritis (GCA), stroke or transient ischemic attack (TIA), nonarteritic anterior ischemic optic neuropathy (NAION), and nonorganic (functional) vision loss (see Table).1-11
When the cause is ischemic
Patients with CRAO experience acute loss of vision in one eye, usually occurring within seconds to minutes. Most patients with CRVO will have a similar presentation, depending on the presence or absence of ischemia and involvement of the macula. Those with branch retinal vein occlusion may have no vision loss at all.1-3
Risk factors for CRAO include cardiovascular disease, hypertension, diabetes, and other disorders associated with systemic inflammation. In patients older than 60, it is also important to consider GCA (to be discussed shortly) as a cause of CRAO.
In patients with CRAO, an eye exam will show profoundly decreased visual acuity, and the swinging light test (see “Use this mnemonic to ensure a comprehensive eye exam”) will reveal a relative afferent pupillary defect (RAPD). Fundoscopy is diagnostic, revealing a pale retina due to decreased blood flow.4 Emergent referral to ophthalmology is indicated to establish a definitive diagnosis and initiate treatment based on the cause of the occlusion. If emergency care is not immediately available, massaging the eye globe through closed lids, then releasing, in 10- to 15-second cycles, may be helpful.5
Risk factors for CRVO include age older than 65 and a number of chronic conditions. One analysis attributed 48% of cases to hypertension, 20% to hyperlipidemia, and 5% to diabetes.3 Fundoscopy will reveal dilated veins, retinal hemorrhages, and cotton wool spots, which look like puffy white patches on the retina.6
As with CRAO, an urgent ophthalmology referral is critical to establish the diagnosis and develop a treatment plan. Outcomes are poor in patients with visual acuity of 20/200 or worse at the time of diagnosis.7,8
GCA. Patients with GCA may develop arteritic ischemic optic neuropathy, resulting in vision loss in one or both eyes. Risk factors for GCA include age (> 50), polymyalgia rheumatica, Caucasian race, and female sex. Systemic symptoms include fever, muscle aches, headache, jaw claudication, and scalp pain.6
The swinging light test will reveal an RAPD;1,2 fundoscopy findings typically include disk edema and disk hemorrhages, or a pale retina if GCA is associated with CRAO.6 Testing, including an erythrocyte sedimentation rate and a C-reactive protein, will provide supportive evidence, and biopsy of the temporal artery will confirm the diagnosis.4
Blindness from GCA is often profound. Bilateral disease is treated immediately with high-dose corticosteroids; when just one eye is affected, high-dose steroids should also be started right away to prevent vision loss in the other eye. Whenever GCA is suspected, initiate treatment and provide an urgent referral to an ophthalmologist for biopsy and further treatment.6
Strokes and TIAs that affect vision may be a result of ischemia of the visual cortex or the eye itself. Visual cortex ischemia will present as a homonymous visual field cut between the eyes; TIAs that affect only one eye (known as amaurosis fugax) are associated with ischemia to the optic nerve or retina.
Patients with amaurosis fugax will experience unilateral loss of vision that extends like a dark shade from the top or bottom periphery to the center of vision. When a TIA is the cause, vision will return to normal within minutes. The underlying pathology is usually carotid artery atherosclerosis. If left untreated, evidence suggests that 30% to 50% of patients will have a stroke within a month.9
Visual acuity may or may not be decreased, depending on whether the ischemia involves the macula. Symptoms suggestive of amaurosis fugax should prompt an urgent ophthalmology referral, while patients with persistent vision loss or visual field deficit require urgent referral to a stroke treatment center.9
NAION is also associated with acute monocular vision loss, particularly in older patients.10 Visual acuity will be markedly decreased, and fundoscopic exam will show a swollen and hemorrhagic optic disc. The vision loss can be profound and is usually permanent; neither medical nor surgical treatment has been shown to improve outcomes.10
When the cause is functional
Functional (nonorganic) visual disturbances should also be considered when sudden blindness is reported. Nonorganic vision loss has a number of causes, and patients present with a range of chief complaints, making diagnosis complex. Because some patients will have organic disease with a component of functional vision loss, it is best to refer individuals whom you suspect of having functional vision loss to an ophthalmologist for testing and a definitive diagnosis. Treatment includes psychological support and reassurance that vision will return.11
Continue for the second problem... "I'm seeing things"
2. “I’M SEEING THINGS”
Patients with this problem often use words such as “flashes,” “floaters” “worms,” or “lights,” and various colors and unusual shapes to describe what they see. When this phenomenon is accompanied by decreased visual acuity, emergent or urgent referral is required. Normal vision in a patient who reports “seeing things” calls for careful consideration of the etiology and referral if the diagnosis is uncertain or the suspected disorder is sight-threatening (see Table).4,12-14 Migraine and psychiatric disorders should be considered if suggested by history. (Patients with ocular migraine—which may or may not be associated with a headache—may also report seeing light patterns off to one side, typically lasting 20 to 45 minutes.)
Vitreous or retinal detachment
Patients with vitreous detachment, which is far more common and less serious than retinal detachment, report seeing new floaters or peripheral flashing lights in one eye. Risk factors for vitreous detachment include myopia, older age, eye trauma, and previous eye surgery.4 Physical examination and visual acuity will be normal unless there is an accompanying retinal detachment.12
A full ophthalmologic evaluation is indicated to detect or rule out a retinal detachment or tear—which has been found to co-occur with acute vitreous detachment in 14% of cases.13 Those who present with decreased visual acuity or a visual field defect or who describe a “curtain of darkness” are at risk for retinal detachment and require a same-day referral.13
Like patients with vitreous detachment, those with a retinal detachment will report new floaters or peripheral flashing lights (see Figure 2).12 The presence of vitreous hemorrhage or pigment, which can be seen in a slit lamp exam, is associated with increased risk for retinal detachment, as is a subjective report of vision loss.13
When retinal detachment is suspected, immediate referral to an ophthalmologist is needed.13 Reattachment surgery has good outcomes, especially if it is performed prior to macular involvement or within the first three days of macular detachment.14
Continue for the second problem... "My eye hurts and is red"
3. “MY EYE HURTS AND IS RED”
Patients with painful, red eyes are at risk for a variety of sight-threatening conditions, including iritis (anterior uveitis), keratitis, and acute angle closure glaucoma, as well as eye trauma (see Table).1,2,4,12,15-27 Decreased visual acuity in a patient with painful, red eyes warrants an urgent or emergent ophthalmologic referral.
When to suspect iritis
Patients with iritis will complain of vision loss, pain, photophobia, and redness. An eye exam will reveal injection of the conjunctiva around the cornea. Visual acuity is often decreased. Pupillary reaction may be sluggish, and the pupil may be smaller or larger than the other eye,4 but a normal pupil size does not exclude iritis in a patient with unilateral eye pain and ciliary injection.15
Iritis is often idiopathic, but risk factors include chronic inflammatory conditions such as ankylosing spondylitis, ulcerative colitis, and Crohn’s disease.16
Treatment with topical steroids is recommended.16 Urgent referral for long-term management of iritis is needed.17
Keratitis has varied causes
Patients with keratitis present with eye pain or foreign body sensation, redness, blurred vision, and photophobia. Examination of the eye will show injection of the conjunctiva surrounding the cornea, and possible corneal defects or opacities; visual acuity may be normal or decreased. The cause varies, based on whether keratitis is bacterial, viral, or noninfectious.
Risk factors for bacterial keratitis include extended wear of contact lenses, eye trauma, eye surgery, and systemic disease such as diabetes, while viral keratitis often follows a case of viral conjunctivitis and herpes simplex keratitis often involves reactivation of the virus. Causes of noninfectious keratitis include flash burns, dry eye or blepharitis, snow blindness, and sunburn.18
Treatment with topical antibiotics is effective for bacterial keratitis, but follow-up referral is needed because the infection could lead to loss of sight.19 Herpes simplex keratitis, which may appear as a mild corneal ulcer (a slit lamp examination will show the classic branching dendritic lesion), can be managed with topical antiviral medications,20 but here, too, an ophthalmologic referral is recommended to look for deeper corneal infiltrates that could lead to vision loss.20,21 Topical numbing medications should not be prescribed for patients with eye problems, as their extended use can lead to infection, corneal thinning, or even perforation of the cornea.22
Blurred vision, pain suggest acute angle
closure glaucoma
Patients with acute angle closure glaucoma present with blurred vision, deep eye pain or brow ache, and frequently, nausea and vomiting.23 Some patients report seeing halos around lights, as well.
Risk factors for acute angle closure glaucoma include older age, Asian descent, farsightedness, family history, and female sex. Attacks are commonly idiopathic, but some are associated with routine pupillary dilation during eye exams.24
On examination, the cornea will be cloudy due to edema and the pupil will be mid-dilated and fixed.12 Typically, intraocular pressure in the affected eye will be elevated, an indication that the nausea and vomiting are associated with this disorder rather than a gastrointestinal condition.23 Emergent referral is needed to preserve vision.25
Eye trauma: What you’ll see, when to act
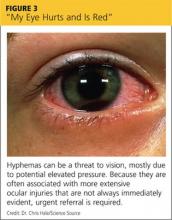
Hyphema. In patients with a hyphema—typically the result of eye trauma—you’ll usually see a meniscus of blood in front of the iris in the anterior chamber (see Figure 3). If the patient was supine before the evaluation, however, you’ll see red discoloration of the iris. Hyphemas can be a threat to vision, mostly due to potential elevated pressure. Because they are often associated with more extensive ocular injuries that are not always immediately evident, urgent referral is required.26
More significant blunt trauma can cause globe rupture, resulting in both eye pain and loss of vision. Flooding the eye with fluorescein before examining it may make it possible to see a dark or green stream from the ruptured globe.
If you suspect a globe rupture, immediately stop your exam. Do not touch the eye. Instead, protect the eye—with a metal or plastic shield and an antiemetic to prevent pressure and Valsalva strain—and obtain an emergency ophthalmology consult.2,4
Chemical burns. Patients who incur chemical burns of the eye should irrigate the injured eye right away. The physical exam should be delayed until irrigation reaches an endpoint of neutral pH, as measured with Nitrazine paper.4,27 Alkali burns are particularly destructive to the eye and require longer irrigation.27
An emergent ophthalmology referral is needed for all alkali burns of the eye, as well as for any patient whose visual acuity does not return to baseline after irrigation. Slit lamp examination showing a deep corneal injury is also reason for an ophthalmology referral.1,2
4. “MY EYE IS RED” (BUT PAIN FREE)
When a patient seeks care for a red eye that’s not painful, the history and physical will help you determine whether the condition is benign or emergent. Orbital cellulitis, which we’ll discuss shortly, is the most dangerous condition related to this presentation,4,9,28-32 requiring inpatient management and ophthalmology referral (see Table).
Conjunctivitis. The entire conjunctiva will be red and discharge will be present, but visual acuity will be normal. Conjunctivitis can be viral or bacterial; office-based testing is now available for viral conjunctivitis caused by adenovirus. Treating bacterial conjunctivitis with antibiotic drops or ointment speeds recovery (see Figure 4).29 When the cause is viral, standard treatment is supportive, with emphasis on preventing viral spread. Some antiviral preparations are being investigated as potential treatments for adenovirus conjunctivitis.28
Periorbital and orbital cellulitis. Redness surrounding the eye can be caused by preseptal (commonly called periorbital) or orbital cellulitis. The clinical presentation of these two conditions is similar, including redness, lid edema, and tenderness. However, periorbital cellulitis is more commonly seen after minor trauma to the eyelid skin or related to a stye or chalazion. Orbital cellulitis, which is considerably more serious, is typically associated with sinus disease or abscess.30
Patients with orbital cellulitis will present with restricted eye movements, decreased visual acuity, proptosis, and possibly an RAPD. These patients will often have pain as well. A fine-cut CT of the orbits aids in diagnosis.31
Care for each is different. Oral antibiotics are usually sufficient for patients with periorbital cellulitis. But for orbital cellulitis, a same-day ophthalmology referral and hospitalization for treatment with parenteral antibiotics is required.9,32
Subconjunctival hemorrhage—dramatic but harmless
While dramatic in appearance, subconjunctival hemorrhage generally does not affect vision. It may be the result of trauma to the globe but can also occur spontaneously.
On physical exam, you’ll see bleeding into the conjunctiva that stops at the edge of the cornea. Visual acuity will be normal, as will the remainder of the eye examination. Abnormal vision, pain, or significant or recurrent bleeding should prompt a search for an alternative diagnosis. No treatment is needed for a simple subconjunctival hemorrhage.4
5. “MY EYE HURTS”
Patients complaining of eye pain with or without vision changes—and without redness—usually have a medical history that leads to the diagnosis (see Table).1,2,4,33-38 Physical exam findings are compatible with the history.
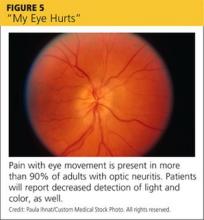
Optic neuritis. Patients with optic neuritis have acute to subacute vision loss, usually in one eye but sometimes bilaterally, lasting hours to days (see Figure 5). Optic neuritis is more common in women and in those ages 15 to 45, with an incidence of five in 100,000 among Caucasians.33 Pain with eye movement is present in more than 90% of adults with optic neuritis34 and is also common in children.35
In addition to vision loss, patients will report decreased detection of light and color,6 and examination will reveal an RAPD.1,2 Vision returns without treatment to the same extent as with treatment, but treatment will speed recovery.36 Patients with optic neuritis require an urgent referral to an ophthalmologist or neurologist to evaluate for multiple sclerosis, which develops in about 30% of those with optic neuritis.4,33
Corneal abrasion. Pain, localized to the surface of the eye, will be the primary complaint of patients with a corneal abrasion, who may or may not have loss of vision. Larger and deeper abrasions are extremely painful, while smaller corneal abrasions may be experienced as a foreign body sensation. The typical patient with a corneal abrasion is likely to have had trauma to the eye.37
Fluorescein is used to examine the patient with a suspected abrasion to highlight the epithelial defect.1 Visual acuity needs to be tested and checked using a pinhole if it is below baseline.37 Treatment protocols range from artificial tears to antibiotic drops or ointments. Topical steroids should be given to patients only by an ophthalmologist.4
Is patching necessary? In a systematic review comparing outcomes based on the use of patching versus not patching on the first day of injury, patients who were not given patches fared the same or better than those whose eyes were patched, both in terms of healing time and pain relief. Primary care providers can treat most corneal abrasions, and symptoms typically resolve in two days.38
REFERENCES
1. Wright JL, Wightman JM. Red and painful eye. In: Marx JA, Hockberger RS, Walls RM, et al, eds. Rosen’s Emergency Medicine: Concepts and Clinical Practice. 7th ed. Philadelphia, PA: Mosby Elsevier; 2009:chap 32.
2. Knoop KJ, Dennis WR, Hedges JR. Ophthalmologic procedures. In: Roberts JR, Hedges JR, eds. Clinical Procedures in Emergency Medicine. 5th ed. Philadelphia, PA: Saunders Elsevier;2009:chap 63.
3. Ehlers JP, Fekrat S. Retinal vein occlusion: beyond the acute event. Surv Ophthalmol. 2011;56:281-299.
4. Sharma R, Brunette DD. Ophthalmology. In: Marx JA, Hockberger RS, Walls RM, et al, eds. Rosen’s Emergency Medicine: Concepts and Clinical Practice. 7th ed. Philadelphia, PA: Mosby Elsevier; 2009:chap 69.
5. Cugati S, Varma DD, Chen CS, et al. Treatment options for central retinal artery occlusion. Curr Treat Options Neurol. 2013;15:63-77.
6. Matson M, Fujimoto L. Bilateral arteritic anterior ischemic optic neuropathy. Optometry. 2011;82:622-631.
7. McIntosh RL, Rogers SL, Lim L, et al. Natural history of central retinal vein occlusion: an evidence-based systematic review. Ophthalmology. 2010; 117:1113-1123.
8. Wong TY, Scott IU. Retinal-vein occlusion. N Engl J Med. 2010;363:2135-2144.
9. Crouch ER, Crouch ER, Grant T. Ophthalmology. In: Rakel RE, ed. Textbook of Family Medicine. 8th ed. Philadelphia, PA: Saunders Elsevier; 2011:chap 41.
10. Dickersin K, Manheimer E, Li T. Surgery for nonarteritic anterior ischemic optic neuropathy. Cochrane Database Syst Rev. 2012;(1):CD001538.
11. Thurtell MJ, Tomsak RL. Neuro-ophthalmology: afferent visual system. In: Daroff RB, Fenichel GM, Jankovic J, et al, eds. Bradley’s Neurology in Clinical Practice. 6th ed. Los Angeles, CA: Saunders Elsevier; 2012:chap 36.
12. Yanoff M, Cameron D. Diseases of the visual system. In: Goldman L, Schafer AI, eds. Cecil Medicine. 24th ed. Philadelphia, PA: Saunders Elsevier; 2011: chap 431.
13. Hollands H, Johnson D, Brox A, et al. Acute-onset floaters and flashes: is this patient at risk for retinal detachment? JAMA. 2009;302:2243-2249.
14. D’Amico DJ. Primary retinal detachment. N Engl J Med. 2008;359:2346-2354.
15. Hunsley T, Lee C. Does a normal-shaped pupil exclude the diagnosis of iritis? Best evidence topic reports. Towards evidence-based emergency medicine: best BETs from the Manchester Royal Infirmary. Emerg Med J. 2006;23:
872-877.
16. Islam N, Pavesio C. Uveitis (acute anterior). Clin Evid. 2010;4:705.
17. Grunwald L, Newcomb CW, Daniel E, et al. Risk of relapse in primary acute anterior uveitis. Ophthalmology. 2011;118:1911-1915.
18. Thomas PA, Geraldine P. Infectious keratitis. Curr Opin Infect Dis. 2007;20: 129-141.
19. Suwan-Apichon O, Reyes JM, Herretes S, et al. Topical corticosteroids as adjunctive therapy for bacterial keratitis. Cochrane Database Syst Rev. 2007;(4):CD005430.
20. Morris D, Latham E. Ulcers in the eye. J Emerg Med. 2012;42:62-64.
21. Wilhelmus KR. Antiviral treatment and other therapeutic interventions for herpes simplex virus epithelial keratitis. Cochrane Database Syst Rev. 2010;(12):CD002898.
22. Yagci A, Bozkurt B, Egrilmez S, et al. Topical anesthetic abuse keratopathy: a commonly overlooked health care problem. Cornea. 2011;30:571-575.
23. Cholongitas E, Pipili C, Dasenaki M. Acute angle closure glaucoma presented with nausea and epigastric pain. Dig Dis Sci. 2008;53:1430-1431.
24. White J. Diagnosis and management of acute angle-closure glaucoma. Emerg Nurse. 2011;19:27.
25. Lama DSC, Thama CCY, Laia JSM, et al. Current approaches to the management of acute primary angle closure. Curr Opin Ophthalmol. 2007;18:
146-151.
26. Gharaibeh A, Savage HI, Scherer RW, et al. Medical interventions for traumatic hyphema. Cochrane Database Syst Rev. 2011;(1):CD005431.
27. Connor AJ, Severn P. Use of a control test to aid pH assessment of chemical eye injuries. Emerg Med J. 2009;26:811-812.
28. Sambursky R, Trattler W, Tauber S, et al. Sensitivity and specificity of the AdenoPlus test for diagnosing adenoviral conjunctivitis. JAMA Ophthalmol. 2013;131:17-22.
29. Sheikh A, Hurwitz B. Antibiotics versus placebo for acute bacterial conjunctivitis. Cochrane Database Syst Rev. 2006;(2):CD001211.
30. Papier A, Tuttle DJ, Mahara TJ. Differential diagnosis of the swollen red eyelid. Am Fam Physician. 2007;76:1815-1824.
31. Howe L, Jones NS. Guidelines for the management of periorbital cellulitis/abscess. Clin Otolaryngol. 2004;29:725-728.
32. Mahalingam-Dhingra A, Lander L, Preciado DA, et al. Orbital and periorbital infections: a national perspective. Arch Otolaryngol Head Neck Surg. 2011;137:769-773.
33. Germann CA, Baumann MR, Hamzavi S. Ophthalmic diagnoses in the ED: optic neuritis. Am J Emerg Med. 2007;25:834-837.
34. Balcer LJ. Optic neuritis. N Engl J Med. 2006;354:1273-1280.
35. Olitsky SE, Hug D, Plummer L, et al. Abnormalities of the optic nerve. In: Kliegman RM, Behrman RE, Jenson HB, et al, eds. Nelson Textbook of Pediatrics. 19th ed. Philadelphia, PA: Saunders Elsevier; 2011:chap 623.
36. Gal RL, Vedula SS, Beck R. Corticosteroids for treating optic neuritis. Cochrane Database Syst Rev. 2012;(4):CD001430.
37. Aslam SA, Sheth HG, Vaughan AJ. Emergency management of corneal injuries. Injury. 2007;38:594-597.
38. Turner A, Rabiu M. Patching for corneal abrasion. Cochrane Database Syst Rev. 2006;(2):CD004764.
Knowing how to respond when patients present with problems involving the eye is crucial for family practice clinicians. Yet it is often difficult to know whether to treat or refer and which signs and symptoms are indicative of an ophthalmologic emergency with the potential to cause loss of sight.
Categorizing ophthalmologic conditions based on patients’ chief complaints can narrow the differential diagnosis. In this article, common complaints such as “I can’t see,” “I’m seeing things,” and “My eye hurts” are used to highlight disorders—both benign and emergent—associated with each.
Continue for the first problem... "I can't see"
1.”I CAN’T SEE”
Patients may use words and phrases such as “cloudy vision,“ “a veil over my eyes,” or “fuzziness” to describe diminished vision. Some will report black areas within their visual field; others will have a loss of peripheral vision or total vision loss in one eye, or possibly even both. Some causes of vision problems, such as cataracts, are not emergencies. Causes of more severe (but painless) vision loss include central retinal artery occlusion (CRAO; see Figure 1) or vein occlusion (CRVO), giant cell arteritis (GCA), stroke or transient ischemic attack (TIA), nonarteritic anterior ischemic optic neuropathy (NAION), and nonorganic (functional) vision loss (see Table).1-11
When the cause is ischemic
Patients with CRAO experience acute loss of vision in one eye, usually occurring within seconds to minutes. Most patients with CRVO will have a similar presentation, depending on the presence or absence of ischemia and involvement of the macula. Those with branch retinal vein occlusion may have no vision loss at all.1-3
Risk factors for CRAO include cardiovascular disease, hypertension, diabetes, and other disorders associated with systemic inflammation. In patients older than 60, it is also important to consider GCA (to be discussed shortly) as a cause of CRAO.
In patients with CRAO, an eye exam will show profoundly decreased visual acuity, and the swinging light test (see “Use this mnemonic to ensure a comprehensive eye exam”) will reveal a relative afferent pupillary defect (RAPD). Fundoscopy is diagnostic, revealing a pale retina due to decreased blood flow.4 Emergent referral to ophthalmology is indicated to establish a definitive diagnosis and initiate treatment based on the cause of the occlusion. If emergency care is not immediately available, massaging the eye globe through closed lids, then releasing, in 10- to 15-second cycles, may be helpful.5
Risk factors for CRVO include age older than 65 and a number of chronic conditions. One analysis attributed 48% of cases to hypertension, 20% to hyperlipidemia, and 5% to diabetes.3 Fundoscopy will reveal dilated veins, retinal hemorrhages, and cotton wool spots, which look like puffy white patches on the retina.6
As with CRAO, an urgent ophthalmology referral is critical to establish the diagnosis and develop a treatment plan. Outcomes are poor in patients with visual acuity of 20/200 or worse at the time of diagnosis.7,8
GCA. Patients with GCA may develop arteritic ischemic optic neuropathy, resulting in vision loss in one or both eyes. Risk factors for GCA include age (> 50), polymyalgia rheumatica, Caucasian race, and female sex. Systemic symptoms include fever, muscle aches, headache, jaw claudication, and scalp pain.6
The swinging light test will reveal an RAPD;1,2 fundoscopy findings typically include disk edema and disk hemorrhages, or a pale retina if GCA is associated with CRAO.6 Testing, including an erythrocyte sedimentation rate and a C-reactive protein, will provide supportive evidence, and biopsy of the temporal artery will confirm the diagnosis.4
Blindness from GCA is often profound. Bilateral disease is treated immediately with high-dose corticosteroids; when just one eye is affected, high-dose steroids should also be started right away to prevent vision loss in the other eye. Whenever GCA is suspected, initiate treatment and provide an urgent referral to an ophthalmologist for biopsy and further treatment.6
Strokes and TIAs that affect vision may be a result of ischemia of the visual cortex or the eye itself. Visual cortex ischemia will present as a homonymous visual field cut between the eyes; TIAs that affect only one eye (known as amaurosis fugax) are associated with ischemia to the optic nerve or retina.
Patients with amaurosis fugax will experience unilateral loss of vision that extends like a dark shade from the top or bottom periphery to the center of vision. When a TIA is the cause, vision will return to normal within minutes. The underlying pathology is usually carotid artery atherosclerosis. If left untreated, evidence suggests that 30% to 50% of patients will have a stroke within a month.9
Visual acuity may or may not be decreased, depending on whether the ischemia involves the macula. Symptoms suggestive of amaurosis fugax should prompt an urgent ophthalmology referral, while patients with persistent vision loss or visual field deficit require urgent referral to a stroke treatment center.9
NAION is also associated with acute monocular vision loss, particularly in older patients.10 Visual acuity will be markedly decreased, and fundoscopic exam will show a swollen and hemorrhagic optic disc. The vision loss can be profound and is usually permanent; neither medical nor surgical treatment has been shown to improve outcomes.10
When the cause is functional
Functional (nonorganic) visual disturbances should also be considered when sudden blindness is reported. Nonorganic vision loss has a number of causes, and patients present with a range of chief complaints, making diagnosis complex. Because some patients will have organic disease with a component of functional vision loss, it is best to refer individuals whom you suspect of having functional vision loss to an ophthalmologist for testing and a definitive diagnosis. Treatment includes psychological support and reassurance that vision will return.11
Continue for the second problem... "I'm seeing things"
2. “I’M SEEING THINGS”
Patients with this problem often use words such as “flashes,” “floaters” “worms,” or “lights,” and various colors and unusual shapes to describe what they see. When this phenomenon is accompanied by decreased visual acuity, emergent or urgent referral is required. Normal vision in a patient who reports “seeing things” calls for careful consideration of the etiology and referral if the diagnosis is uncertain or the suspected disorder is sight-threatening (see Table).4,12-14 Migraine and psychiatric disorders should be considered if suggested by history. (Patients with ocular migraine—which may or may not be associated with a headache—may also report seeing light patterns off to one side, typically lasting 20 to 45 minutes.)
Vitreous or retinal detachment
Patients with vitreous detachment, which is far more common and less serious than retinal detachment, report seeing new floaters or peripheral flashing lights in one eye. Risk factors for vitreous detachment include myopia, older age, eye trauma, and previous eye surgery.4 Physical examination and visual acuity will be normal unless there is an accompanying retinal detachment.12
A full ophthalmologic evaluation is indicated to detect or rule out a retinal detachment or tear—which has been found to co-occur with acute vitreous detachment in 14% of cases.13 Those who present with decreased visual acuity or a visual field defect or who describe a “curtain of darkness” are at risk for retinal detachment and require a same-day referral.13
Like patients with vitreous detachment, those with a retinal detachment will report new floaters or peripheral flashing lights (see Figure 2).12 The presence of vitreous hemorrhage or pigment, which can be seen in a slit lamp exam, is associated with increased risk for retinal detachment, as is a subjective report of vision loss.13
When retinal detachment is suspected, immediate referral to an ophthalmologist is needed.13 Reattachment surgery has good outcomes, especially if it is performed prior to macular involvement or within the first three days of macular detachment.14
Continue for the second problem... "My eye hurts and is red"
3. “MY EYE HURTS AND IS RED”
Patients with painful, red eyes are at risk for a variety of sight-threatening conditions, including iritis (anterior uveitis), keratitis, and acute angle closure glaucoma, as well as eye trauma (see Table).1,2,4,12,15-27 Decreased visual acuity in a patient with painful, red eyes warrants an urgent or emergent ophthalmologic referral.
When to suspect iritis
Patients with iritis will complain of vision loss, pain, photophobia, and redness. An eye exam will reveal injection of the conjunctiva around the cornea. Visual acuity is often decreased. Pupillary reaction may be sluggish, and the pupil may be smaller or larger than the other eye,4 but a normal pupil size does not exclude iritis in a patient with unilateral eye pain and ciliary injection.15
Iritis is often idiopathic, but risk factors include chronic inflammatory conditions such as ankylosing spondylitis, ulcerative colitis, and Crohn’s disease.16
Treatment with topical steroids is recommended.16 Urgent referral for long-term management of iritis is needed.17
Keratitis has varied causes
Patients with keratitis present with eye pain or foreign body sensation, redness, blurred vision, and photophobia. Examination of the eye will show injection of the conjunctiva surrounding the cornea, and possible corneal defects or opacities; visual acuity may be normal or decreased. The cause varies, based on whether keratitis is bacterial, viral, or noninfectious.
Risk factors for bacterial keratitis include extended wear of contact lenses, eye trauma, eye surgery, and systemic disease such as diabetes, while viral keratitis often follows a case of viral conjunctivitis and herpes simplex keratitis often involves reactivation of the virus. Causes of noninfectious keratitis include flash burns, dry eye or blepharitis, snow blindness, and sunburn.18
Treatment with topical antibiotics is effective for bacterial keratitis, but follow-up referral is needed because the infection could lead to loss of sight.19 Herpes simplex keratitis, which may appear as a mild corneal ulcer (a slit lamp examination will show the classic branching dendritic lesion), can be managed with topical antiviral medications,20 but here, too, an ophthalmologic referral is recommended to look for deeper corneal infiltrates that could lead to vision loss.20,21 Topical numbing medications should not be prescribed for patients with eye problems, as their extended use can lead to infection, corneal thinning, or even perforation of the cornea.22
Blurred vision, pain suggest acute angle
closure glaucoma
Patients with acute angle closure glaucoma present with blurred vision, deep eye pain or brow ache, and frequently, nausea and vomiting.23 Some patients report seeing halos around lights, as well.
Risk factors for acute angle closure glaucoma include older age, Asian descent, farsightedness, family history, and female sex. Attacks are commonly idiopathic, but some are associated with routine pupillary dilation during eye exams.24
On examination, the cornea will be cloudy due to edema and the pupil will be mid-dilated and fixed.12 Typically, intraocular pressure in the affected eye will be elevated, an indication that the nausea and vomiting are associated with this disorder rather than a gastrointestinal condition.23 Emergent referral is needed to preserve vision.25
Eye trauma: What you’ll see, when to act
Hyphema. In patients with a hyphema—typically the result of eye trauma—you’ll usually see a meniscus of blood in front of the iris in the anterior chamber (see Figure 3). If the patient was supine before the evaluation, however, you’ll see red discoloration of the iris. Hyphemas can be a threat to vision, mostly due to potential elevated pressure. Because they are often associated with more extensive ocular injuries that are not always immediately evident, urgent referral is required.26
More significant blunt trauma can cause globe rupture, resulting in both eye pain and loss of vision. Flooding the eye with fluorescein before examining it may make it possible to see a dark or green stream from the ruptured globe.
If you suspect a globe rupture, immediately stop your exam. Do not touch the eye. Instead, protect the eye—with a metal or plastic shield and an antiemetic to prevent pressure and Valsalva strain—and obtain an emergency ophthalmology consult.2,4
Chemical burns. Patients who incur chemical burns of the eye should irrigate the injured eye right away. The physical exam should be delayed until irrigation reaches an endpoint of neutral pH, as measured with Nitrazine paper.4,27 Alkali burns are particularly destructive to the eye and require longer irrigation.27
An emergent ophthalmology referral is needed for all alkali burns of the eye, as well as for any patient whose visual acuity does not return to baseline after irrigation. Slit lamp examination showing a deep corneal injury is also reason for an ophthalmology referral.1,2
4. “MY EYE IS RED” (BUT PAIN FREE)
When a patient seeks care for a red eye that’s not painful, the history and physical will help you determine whether the condition is benign or emergent. Orbital cellulitis, which we’ll discuss shortly, is the most dangerous condition related to this presentation,4,9,28-32 requiring inpatient management and ophthalmology referral (see Table).
Conjunctivitis. The entire conjunctiva will be red and discharge will be present, but visual acuity will be normal. Conjunctivitis can be viral or bacterial; office-based testing is now available for viral conjunctivitis caused by adenovirus. Treating bacterial conjunctivitis with antibiotic drops or ointment speeds recovery (see Figure 4).29 When the cause is viral, standard treatment is supportive, with emphasis on preventing viral spread. Some antiviral preparations are being investigated as potential treatments for adenovirus conjunctivitis.28
Periorbital and orbital cellulitis. Redness surrounding the eye can be caused by preseptal (commonly called periorbital) or orbital cellulitis. The clinical presentation of these two conditions is similar, including redness, lid edema, and tenderness. However, periorbital cellulitis is more commonly seen after minor trauma to the eyelid skin or related to a stye or chalazion. Orbital cellulitis, which is considerably more serious, is typically associated with sinus disease or abscess.30
Patients with orbital cellulitis will present with restricted eye movements, decreased visual acuity, proptosis, and possibly an RAPD. These patients will often have pain as well. A fine-cut CT of the orbits aids in diagnosis.31
Care for each is different. Oral antibiotics are usually sufficient for patients with periorbital cellulitis. But for orbital cellulitis, a same-day ophthalmology referral and hospitalization for treatment with parenteral antibiotics is required.9,32
Subconjunctival hemorrhage—dramatic but harmless
While dramatic in appearance, subconjunctival hemorrhage generally does not affect vision. It may be the result of trauma to the globe but can also occur spontaneously.
On physical exam, you’ll see bleeding into the conjunctiva that stops at the edge of the cornea. Visual acuity will be normal, as will the remainder of the eye examination. Abnormal vision, pain, or significant or recurrent bleeding should prompt a search for an alternative diagnosis. No treatment is needed for a simple subconjunctival hemorrhage.4
5. “MY EYE HURTS”
Patients complaining of eye pain with or without vision changes—and without redness—usually have a medical history that leads to the diagnosis (see Table).1,2,4,33-38 Physical exam findings are compatible with the history.
Optic neuritis. Patients with optic neuritis have acute to subacute vision loss, usually in one eye but sometimes bilaterally, lasting hours to days (see Figure 5). Optic neuritis is more common in women and in those ages 15 to 45, with an incidence of five in 100,000 among Caucasians.33 Pain with eye movement is present in more than 90% of adults with optic neuritis34 and is also common in children.35
In addition to vision loss, patients will report decreased detection of light and color,6 and examination will reveal an RAPD.1,2 Vision returns without treatment to the same extent as with treatment, but treatment will speed recovery.36 Patients with optic neuritis require an urgent referral to an ophthalmologist or neurologist to evaluate for multiple sclerosis, which develops in about 30% of those with optic neuritis.4,33
Corneal abrasion. Pain, localized to the surface of the eye, will be the primary complaint of patients with a corneal abrasion, who may or may not have loss of vision. Larger and deeper abrasions are extremely painful, while smaller corneal abrasions may be experienced as a foreign body sensation. The typical patient with a corneal abrasion is likely to have had trauma to the eye.37
Fluorescein is used to examine the patient with a suspected abrasion to highlight the epithelial defect.1 Visual acuity needs to be tested and checked using a pinhole if it is below baseline.37 Treatment protocols range from artificial tears to antibiotic drops or ointments. Topical steroids should be given to patients only by an ophthalmologist.4
Is patching necessary? In a systematic review comparing outcomes based on the use of patching versus not patching on the first day of injury, patients who were not given patches fared the same or better than those whose eyes were patched, both in terms of healing time and pain relief. Primary care providers can treat most corneal abrasions, and symptoms typically resolve in two days.38
REFERENCES
1. Wright JL, Wightman JM. Red and painful eye. In: Marx JA, Hockberger RS, Walls RM, et al, eds. Rosen’s Emergency Medicine: Concepts and Clinical Practice. 7th ed. Philadelphia, PA: Mosby Elsevier; 2009:chap 32.
2. Knoop KJ, Dennis WR, Hedges JR. Ophthalmologic procedures. In: Roberts JR, Hedges JR, eds. Clinical Procedures in Emergency Medicine. 5th ed. Philadelphia, PA: Saunders Elsevier;2009:chap 63.
3. Ehlers JP, Fekrat S. Retinal vein occlusion: beyond the acute event. Surv Ophthalmol. 2011;56:281-299.
4. Sharma R, Brunette DD. Ophthalmology. In: Marx JA, Hockberger RS, Walls RM, et al, eds. Rosen’s Emergency Medicine: Concepts and Clinical Practice. 7th ed. Philadelphia, PA: Mosby Elsevier; 2009:chap 69.
5. Cugati S, Varma DD, Chen CS, et al. Treatment options for central retinal artery occlusion. Curr Treat Options Neurol. 2013;15:63-77.
6. Matson M, Fujimoto L. Bilateral arteritic anterior ischemic optic neuropathy. Optometry. 2011;82:622-631.
7. McIntosh RL, Rogers SL, Lim L, et al. Natural history of central retinal vein occlusion: an evidence-based systematic review. Ophthalmology. 2010; 117:1113-1123.
8. Wong TY, Scott IU. Retinal-vein occlusion. N Engl J Med. 2010;363:2135-2144.
9. Crouch ER, Crouch ER, Grant T. Ophthalmology. In: Rakel RE, ed. Textbook of Family Medicine. 8th ed. Philadelphia, PA: Saunders Elsevier; 2011:chap 41.
10. Dickersin K, Manheimer E, Li T. Surgery for nonarteritic anterior ischemic optic neuropathy. Cochrane Database Syst Rev. 2012;(1):CD001538.
11. Thurtell MJ, Tomsak RL. Neuro-ophthalmology: afferent visual system. In: Daroff RB, Fenichel GM, Jankovic J, et al, eds. Bradley’s Neurology in Clinical Practice. 6th ed. Los Angeles, CA: Saunders Elsevier; 2012:chap 36.
12. Yanoff M, Cameron D. Diseases of the visual system. In: Goldman L, Schafer AI, eds. Cecil Medicine. 24th ed. Philadelphia, PA: Saunders Elsevier; 2011: chap 431.
13. Hollands H, Johnson D, Brox A, et al. Acute-onset floaters and flashes: is this patient at risk for retinal detachment? JAMA. 2009;302:2243-2249.
14. D’Amico DJ. Primary retinal detachment. N Engl J Med. 2008;359:2346-2354.
15. Hunsley T, Lee C. Does a normal-shaped pupil exclude the diagnosis of iritis? Best evidence topic reports. Towards evidence-based emergency medicine: best BETs from the Manchester Royal Infirmary. Emerg Med J. 2006;23:
872-877.
16. Islam N, Pavesio C. Uveitis (acute anterior). Clin Evid. 2010;4:705.
17. Grunwald L, Newcomb CW, Daniel E, et al. Risk of relapse in primary acute anterior uveitis. Ophthalmology. 2011;118:1911-1915.
18. Thomas PA, Geraldine P. Infectious keratitis. Curr Opin Infect Dis. 2007;20: 129-141.
19. Suwan-Apichon O, Reyes JM, Herretes S, et al. Topical corticosteroids as adjunctive therapy for bacterial keratitis. Cochrane Database Syst Rev. 2007;(4):CD005430.
20. Morris D, Latham E. Ulcers in the eye. J Emerg Med. 2012;42:62-64.
21. Wilhelmus KR. Antiviral treatment and other therapeutic interventions for herpes simplex virus epithelial keratitis. Cochrane Database Syst Rev. 2010;(12):CD002898.
22. Yagci A, Bozkurt B, Egrilmez S, et al. Topical anesthetic abuse keratopathy: a commonly overlooked health care problem. Cornea. 2011;30:571-575.
23. Cholongitas E, Pipili C, Dasenaki M. Acute angle closure glaucoma presented with nausea and epigastric pain. Dig Dis Sci. 2008;53:1430-1431.
24. White J. Diagnosis and management of acute angle-closure glaucoma. Emerg Nurse. 2011;19:27.
25. Lama DSC, Thama CCY, Laia JSM, et al. Current approaches to the management of acute primary angle closure. Curr Opin Ophthalmol. 2007;18:
146-151.
26. Gharaibeh A, Savage HI, Scherer RW, et al. Medical interventions for traumatic hyphema. Cochrane Database Syst Rev. 2011;(1):CD005431.
27. Connor AJ, Severn P. Use of a control test to aid pH assessment of chemical eye injuries. Emerg Med J. 2009;26:811-812.
28. Sambursky R, Trattler W, Tauber S, et al. Sensitivity and specificity of the AdenoPlus test for diagnosing adenoviral conjunctivitis. JAMA Ophthalmol. 2013;131:17-22.
29. Sheikh A, Hurwitz B. Antibiotics versus placebo for acute bacterial conjunctivitis. Cochrane Database Syst Rev. 2006;(2):CD001211.
30. Papier A, Tuttle DJ, Mahara TJ. Differential diagnosis of the swollen red eyelid. Am Fam Physician. 2007;76:1815-1824.
31. Howe L, Jones NS. Guidelines for the management of periorbital cellulitis/abscess. Clin Otolaryngol. 2004;29:725-728.
32. Mahalingam-Dhingra A, Lander L, Preciado DA, et al. Orbital and periorbital infections: a national perspective. Arch Otolaryngol Head Neck Surg. 2011;137:769-773.
33. Germann CA, Baumann MR, Hamzavi S. Ophthalmic diagnoses in the ED: optic neuritis. Am J Emerg Med. 2007;25:834-837.
34. Balcer LJ. Optic neuritis. N Engl J Med. 2006;354:1273-1280.
35. Olitsky SE, Hug D, Plummer L, et al. Abnormalities of the optic nerve. In: Kliegman RM, Behrman RE, Jenson HB, et al, eds. Nelson Textbook of Pediatrics. 19th ed. Philadelphia, PA: Saunders Elsevier; 2011:chap 623.
36. Gal RL, Vedula SS, Beck R. Corticosteroids for treating optic neuritis. Cochrane Database Syst Rev. 2012;(4):CD001430.
37. Aslam SA, Sheth HG, Vaughan AJ. Emergency management of corneal injuries. Injury. 2007;38:594-597.
38. Turner A, Rabiu M. Patching for corneal abrasion. Cochrane Database Syst Rev. 2006;(2):CD004764.
Knowing how to respond when patients present with problems involving the eye is crucial for family practice clinicians. Yet it is often difficult to know whether to treat or refer and which signs and symptoms are indicative of an ophthalmologic emergency with the potential to cause loss of sight.
Categorizing ophthalmologic conditions based on patients’ chief complaints can narrow the differential diagnosis. In this article, common complaints such as “I can’t see,” “I’m seeing things,” and “My eye hurts” are used to highlight disorders—both benign and emergent—associated with each.
Continue for the first problem... "I can't see"
1.”I CAN’T SEE”
Patients may use words and phrases such as “cloudy vision,“ “a veil over my eyes,” or “fuzziness” to describe diminished vision. Some will report black areas within their visual field; others will have a loss of peripheral vision or total vision loss in one eye, or possibly even both. Some causes of vision problems, such as cataracts, are not emergencies. Causes of more severe (but painless) vision loss include central retinal artery occlusion (CRAO; see Figure 1) or vein occlusion (CRVO), giant cell arteritis (GCA), stroke or transient ischemic attack (TIA), nonarteritic anterior ischemic optic neuropathy (NAION), and nonorganic (functional) vision loss (see Table).1-11
When the cause is ischemic
Patients with CRAO experience acute loss of vision in one eye, usually occurring within seconds to minutes. Most patients with CRVO will have a similar presentation, depending on the presence or absence of ischemia and involvement of the macula. Those with branch retinal vein occlusion may have no vision loss at all.1-3
Risk factors for CRAO include cardiovascular disease, hypertension, diabetes, and other disorders associated with systemic inflammation. In patients older than 60, it is also important to consider GCA (to be discussed shortly) as a cause of CRAO.
In patients with CRAO, an eye exam will show profoundly decreased visual acuity, and the swinging light test (see “Use this mnemonic to ensure a comprehensive eye exam”) will reveal a relative afferent pupillary defect (RAPD). Fundoscopy is diagnostic, revealing a pale retina due to decreased blood flow.4 Emergent referral to ophthalmology is indicated to establish a definitive diagnosis and initiate treatment based on the cause of the occlusion. If emergency care is not immediately available, massaging the eye globe through closed lids, then releasing, in 10- to 15-second cycles, may be helpful.5
Risk factors for CRVO include age older than 65 and a number of chronic conditions. One analysis attributed 48% of cases to hypertension, 20% to hyperlipidemia, and 5% to diabetes.3 Fundoscopy will reveal dilated veins, retinal hemorrhages, and cotton wool spots, which look like puffy white patches on the retina.6
As with CRAO, an urgent ophthalmology referral is critical to establish the diagnosis and develop a treatment plan. Outcomes are poor in patients with visual acuity of 20/200 or worse at the time of diagnosis.7,8
GCA. Patients with GCA may develop arteritic ischemic optic neuropathy, resulting in vision loss in one or both eyes. Risk factors for GCA include age (> 50), polymyalgia rheumatica, Caucasian race, and female sex. Systemic symptoms include fever, muscle aches, headache, jaw claudication, and scalp pain.6
The swinging light test will reveal an RAPD;1,2 fundoscopy findings typically include disk edema and disk hemorrhages, or a pale retina if GCA is associated with CRAO.6 Testing, including an erythrocyte sedimentation rate and a C-reactive protein, will provide supportive evidence, and biopsy of the temporal artery will confirm the diagnosis.4
Blindness from GCA is often profound. Bilateral disease is treated immediately with high-dose corticosteroids; when just one eye is affected, high-dose steroids should also be started right away to prevent vision loss in the other eye. Whenever GCA is suspected, initiate treatment and provide an urgent referral to an ophthalmologist for biopsy and further treatment.6
Strokes and TIAs that affect vision may be a result of ischemia of the visual cortex or the eye itself. Visual cortex ischemia will present as a homonymous visual field cut between the eyes; TIAs that affect only one eye (known as amaurosis fugax) are associated with ischemia to the optic nerve or retina.
Patients with amaurosis fugax will experience unilateral loss of vision that extends like a dark shade from the top or bottom periphery to the center of vision. When a TIA is the cause, vision will return to normal within minutes. The underlying pathology is usually carotid artery atherosclerosis. If left untreated, evidence suggests that 30% to 50% of patients will have a stroke within a month.9
Visual acuity may or may not be decreased, depending on whether the ischemia involves the macula. Symptoms suggestive of amaurosis fugax should prompt an urgent ophthalmology referral, while patients with persistent vision loss or visual field deficit require urgent referral to a stroke treatment center.9
NAION is also associated with acute monocular vision loss, particularly in older patients.10 Visual acuity will be markedly decreased, and fundoscopic exam will show a swollen and hemorrhagic optic disc. The vision loss can be profound and is usually permanent; neither medical nor surgical treatment has been shown to improve outcomes.10
When the cause is functional
Functional (nonorganic) visual disturbances should also be considered when sudden blindness is reported. Nonorganic vision loss has a number of causes, and patients present with a range of chief complaints, making diagnosis complex. Because some patients will have organic disease with a component of functional vision loss, it is best to refer individuals whom you suspect of having functional vision loss to an ophthalmologist for testing and a definitive diagnosis. Treatment includes psychological support and reassurance that vision will return.11
Continue for the second problem... "I'm seeing things"
2. “I’M SEEING THINGS”
Patients with this problem often use words such as “flashes,” “floaters” “worms,” or “lights,” and various colors and unusual shapes to describe what they see. When this phenomenon is accompanied by decreased visual acuity, emergent or urgent referral is required. Normal vision in a patient who reports “seeing things” calls for careful consideration of the etiology and referral if the diagnosis is uncertain or the suspected disorder is sight-threatening (see Table).4,12-14 Migraine and psychiatric disorders should be considered if suggested by history. (Patients with ocular migraine—which may or may not be associated with a headache—may also report seeing light patterns off to one side, typically lasting 20 to 45 minutes.)
Vitreous or retinal detachment
Patients with vitreous detachment, which is far more common and less serious than retinal detachment, report seeing new floaters or peripheral flashing lights in one eye. Risk factors for vitreous detachment include myopia, older age, eye trauma, and previous eye surgery.4 Physical examination and visual acuity will be normal unless there is an accompanying retinal detachment.12
A full ophthalmologic evaluation is indicated to detect or rule out a retinal detachment or tear—which has been found to co-occur with acute vitreous detachment in 14% of cases.13 Those who present with decreased visual acuity or a visual field defect or who describe a “curtain of darkness” are at risk for retinal detachment and require a same-day referral.13
Like patients with vitreous detachment, those with a retinal detachment will report new floaters or peripheral flashing lights (see Figure 2).12 The presence of vitreous hemorrhage or pigment, which can be seen in a slit lamp exam, is associated with increased risk for retinal detachment, as is a subjective report of vision loss.13
When retinal detachment is suspected, immediate referral to an ophthalmologist is needed.13 Reattachment surgery has good outcomes, especially if it is performed prior to macular involvement or within the first three days of macular detachment.14
Continue for the second problem... "My eye hurts and is red"
3. “MY EYE HURTS AND IS RED”
Patients with painful, red eyes are at risk for a variety of sight-threatening conditions, including iritis (anterior uveitis), keratitis, and acute angle closure glaucoma, as well as eye trauma (see Table).1,2,4,12,15-27 Decreased visual acuity in a patient with painful, red eyes warrants an urgent or emergent ophthalmologic referral.
When to suspect iritis
Patients with iritis will complain of vision loss, pain, photophobia, and redness. An eye exam will reveal injection of the conjunctiva around the cornea. Visual acuity is often decreased. Pupillary reaction may be sluggish, and the pupil may be smaller or larger than the other eye,4 but a normal pupil size does not exclude iritis in a patient with unilateral eye pain and ciliary injection.15
Iritis is often idiopathic, but risk factors include chronic inflammatory conditions such as ankylosing spondylitis, ulcerative colitis, and Crohn’s disease.16
Treatment with topical steroids is recommended.16 Urgent referral for long-term management of iritis is needed.17
Keratitis has varied causes
Patients with keratitis present with eye pain or foreign body sensation, redness, blurred vision, and photophobia. Examination of the eye will show injection of the conjunctiva surrounding the cornea, and possible corneal defects or opacities; visual acuity may be normal or decreased. The cause varies, based on whether keratitis is bacterial, viral, or noninfectious.
Risk factors for bacterial keratitis include extended wear of contact lenses, eye trauma, eye surgery, and systemic disease such as diabetes, while viral keratitis often follows a case of viral conjunctivitis and herpes simplex keratitis often involves reactivation of the virus. Causes of noninfectious keratitis include flash burns, dry eye or blepharitis, snow blindness, and sunburn.18
Treatment with topical antibiotics is effective for bacterial keratitis, but follow-up referral is needed because the infection could lead to loss of sight.19 Herpes simplex keratitis, which may appear as a mild corneal ulcer (a slit lamp examination will show the classic branching dendritic lesion), can be managed with topical antiviral medications,20 but here, too, an ophthalmologic referral is recommended to look for deeper corneal infiltrates that could lead to vision loss.20,21 Topical numbing medications should not be prescribed for patients with eye problems, as their extended use can lead to infection, corneal thinning, or even perforation of the cornea.22
Blurred vision, pain suggest acute angle
closure glaucoma
Patients with acute angle closure glaucoma present with blurred vision, deep eye pain or brow ache, and frequently, nausea and vomiting.23 Some patients report seeing halos around lights, as well.
Risk factors for acute angle closure glaucoma include older age, Asian descent, farsightedness, family history, and female sex. Attacks are commonly idiopathic, but some are associated with routine pupillary dilation during eye exams.24
On examination, the cornea will be cloudy due to edema and the pupil will be mid-dilated and fixed.12 Typically, intraocular pressure in the affected eye will be elevated, an indication that the nausea and vomiting are associated with this disorder rather than a gastrointestinal condition.23 Emergent referral is needed to preserve vision.25
Eye trauma: What you’ll see, when to act
Hyphema. In patients with a hyphema—typically the result of eye trauma—you’ll usually see a meniscus of blood in front of the iris in the anterior chamber (see Figure 3). If the patient was supine before the evaluation, however, you’ll see red discoloration of the iris. Hyphemas can be a threat to vision, mostly due to potential elevated pressure. Because they are often associated with more extensive ocular injuries that are not always immediately evident, urgent referral is required.26
More significant blunt trauma can cause globe rupture, resulting in both eye pain and loss of vision. Flooding the eye with fluorescein before examining it may make it possible to see a dark or green stream from the ruptured globe.
If you suspect a globe rupture, immediately stop your exam. Do not touch the eye. Instead, protect the eye—with a metal or plastic shield and an antiemetic to prevent pressure and Valsalva strain—and obtain an emergency ophthalmology consult.2,4
Chemical burns. Patients who incur chemical burns of the eye should irrigate the injured eye right away. The physical exam should be delayed until irrigation reaches an endpoint of neutral pH, as measured with Nitrazine paper.4,27 Alkali burns are particularly destructive to the eye and require longer irrigation.27
An emergent ophthalmology referral is needed for all alkali burns of the eye, as well as for any patient whose visual acuity does not return to baseline after irrigation. Slit lamp examination showing a deep corneal injury is also reason for an ophthalmology referral.1,2
4. “MY EYE IS RED” (BUT PAIN FREE)
When a patient seeks care for a red eye that’s not painful, the history and physical will help you determine whether the condition is benign or emergent. Orbital cellulitis, which we’ll discuss shortly, is the most dangerous condition related to this presentation,4,9,28-32 requiring inpatient management and ophthalmology referral (see Table).
Conjunctivitis. The entire conjunctiva will be red and discharge will be present, but visual acuity will be normal. Conjunctivitis can be viral or bacterial; office-based testing is now available for viral conjunctivitis caused by adenovirus. Treating bacterial conjunctivitis with antibiotic drops or ointment speeds recovery (see Figure 4).29 When the cause is viral, standard treatment is supportive, with emphasis on preventing viral spread. Some antiviral preparations are being investigated as potential treatments for adenovirus conjunctivitis.28
Periorbital and orbital cellulitis. Redness surrounding the eye can be caused by preseptal (commonly called periorbital) or orbital cellulitis. The clinical presentation of these two conditions is similar, including redness, lid edema, and tenderness. However, periorbital cellulitis is more commonly seen after minor trauma to the eyelid skin or related to a stye or chalazion. Orbital cellulitis, which is considerably more serious, is typically associated with sinus disease or abscess.30
Patients with orbital cellulitis will present with restricted eye movements, decreased visual acuity, proptosis, and possibly an RAPD. These patients will often have pain as well. A fine-cut CT of the orbits aids in diagnosis.31
Care for each is different. Oral antibiotics are usually sufficient for patients with periorbital cellulitis. But for orbital cellulitis, a same-day ophthalmology referral and hospitalization for treatment with parenteral antibiotics is required.9,32
Subconjunctival hemorrhage—dramatic but harmless
While dramatic in appearance, subconjunctival hemorrhage generally does not affect vision. It may be the result of trauma to the globe but can also occur spontaneously.
On physical exam, you’ll see bleeding into the conjunctiva that stops at the edge of the cornea. Visual acuity will be normal, as will the remainder of the eye examination. Abnormal vision, pain, or significant or recurrent bleeding should prompt a search for an alternative diagnosis. No treatment is needed for a simple subconjunctival hemorrhage.4
5. “MY EYE HURTS”
Patients complaining of eye pain with or without vision changes—and without redness—usually have a medical history that leads to the diagnosis (see Table).1,2,4,33-38 Physical exam findings are compatible with the history.
Optic neuritis. Patients with optic neuritis have acute to subacute vision loss, usually in one eye but sometimes bilaterally, lasting hours to days (see Figure 5). Optic neuritis is more common in women and in those ages 15 to 45, with an incidence of five in 100,000 among Caucasians.33 Pain with eye movement is present in more than 90% of adults with optic neuritis34 and is also common in children.35
In addition to vision loss, patients will report decreased detection of light and color,6 and examination will reveal an RAPD.1,2 Vision returns without treatment to the same extent as with treatment, but treatment will speed recovery.36 Patients with optic neuritis require an urgent referral to an ophthalmologist or neurologist to evaluate for multiple sclerosis, which develops in about 30% of those with optic neuritis.4,33
Corneal abrasion. Pain, localized to the surface of the eye, will be the primary complaint of patients with a corneal abrasion, who may or may not have loss of vision. Larger and deeper abrasions are extremely painful, while smaller corneal abrasions may be experienced as a foreign body sensation. The typical patient with a corneal abrasion is likely to have had trauma to the eye.37
Fluorescein is used to examine the patient with a suspected abrasion to highlight the epithelial defect.1 Visual acuity needs to be tested and checked using a pinhole if it is below baseline.37 Treatment protocols range from artificial tears to antibiotic drops or ointments. Topical steroids should be given to patients only by an ophthalmologist.4
Is patching necessary? In a systematic review comparing outcomes based on the use of patching versus not patching on the first day of injury, patients who were not given patches fared the same or better than those whose eyes were patched, both in terms of healing time and pain relief. Primary care providers can treat most corneal abrasions, and symptoms typically resolve in two days.38
REFERENCES
1. Wright JL, Wightman JM. Red and painful eye. In: Marx JA, Hockberger RS, Walls RM, et al, eds. Rosen’s Emergency Medicine: Concepts and Clinical Practice. 7th ed. Philadelphia, PA: Mosby Elsevier; 2009:chap 32.
2. Knoop KJ, Dennis WR, Hedges JR. Ophthalmologic procedures. In: Roberts JR, Hedges JR, eds. Clinical Procedures in Emergency Medicine. 5th ed. Philadelphia, PA: Saunders Elsevier;2009:chap 63.
3. Ehlers JP, Fekrat S. Retinal vein occlusion: beyond the acute event. Surv Ophthalmol. 2011;56:281-299.
4. Sharma R, Brunette DD. Ophthalmology. In: Marx JA, Hockberger RS, Walls RM, et al, eds. Rosen’s Emergency Medicine: Concepts and Clinical Practice. 7th ed. Philadelphia, PA: Mosby Elsevier; 2009:chap 69.
5. Cugati S, Varma DD, Chen CS, et al. Treatment options for central retinal artery occlusion. Curr Treat Options Neurol. 2013;15:63-77.
6. Matson M, Fujimoto L. Bilateral arteritic anterior ischemic optic neuropathy. Optometry. 2011;82:622-631.
7. McIntosh RL, Rogers SL, Lim L, et al. Natural history of central retinal vein occlusion: an evidence-based systematic review. Ophthalmology. 2010; 117:1113-1123.
8. Wong TY, Scott IU. Retinal-vein occlusion. N Engl J Med. 2010;363:2135-2144.
9. Crouch ER, Crouch ER, Grant T. Ophthalmology. In: Rakel RE, ed. Textbook of Family Medicine. 8th ed. Philadelphia, PA: Saunders Elsevier; 2011:chap 41.
10. Dickersin K, Manheimer E, Li T. Surgery for nonarteritic anterior ischemic optic neuropathy. Cochrane Database Syst Rev. 2012;(1):CD001538.
11. Thurtell MJ, Tomsak RL. Neuro-ophthalmology: afferent visual system. In: Daroff RB, Fenichel GM, Jankovic J, et al, eds. Bradley’s Neurology in Clinical Practice. 6th ed. Los Angeles, CA: Saunders Elsevier; 2012:chap 36.
12. Yanoff M, Cameron D. Diseases of the visual system. In: Goldman L, Schafer AI, eds. Cecil Medicine. 24th ed. Philadelphia, PA: Saunders Elsevier; 2011: chap 431.
13. Hollands H, Johnson D, Brox A, et al. Acute-onset floaters and flashes: is this patient at risk for retinal detachment? JAMA. 2009;302:2243-2249.
14. D’Amico DJ. Primary retinal detachment. N Engl J Med. 2008;359:2346-2354.
15. Hunsley T, Lee C. Does a normal-shaped pupil exclude the diagnosis of iritis? Best evidence topic reports. Towards evidence-based emergency medicine: best BETs from the Manchester Royal Infirmary. Emerg Med J. 2006;23:
872-877.
16. Islam N, Pavesio C. Uveitis (acute anterior). Clin Evid. 2010;4:705.
17. Grunwald L, Newcomb CW, Daniel E, et al. Risk of relapse in primary acute anterior uveitis. Ophthalmology. 2011;118:1911-1915.
18. Thomas PA, Geraldine P. Infectious keratitis. Curr Opin Infect Dis. 2007;20: 129-141.
19. Suwan-Apichon O, Reyes JM, Herretes S, et al. Topical corticosteroids as adjunctive therapy for bacterial keratitis. Cochrane Database Syst Rev. 2007;(4):CD005430.
20. Morris D, Latham E. Ulcers in the eye. J Emerg Med. 2012;42:62-64.
21. Wilhelmus KR. Antiviral treatment and other therapeutic interventions for herpes simplex virus epithelial keratitis. Cochrane Database Syst Rev. 2010;(12):CD002898.
22. Yagci A, Bozkurt B, Egrilmez S, et al. Topical anesthetic abuse keratopathy: a commonly overlooked health care problem. Cornea. 2011;30:571-575.
23. Cholongitas E, Pipili C, Dasenaki M. Acute angle closure glaucoma presented with nausea and epigastric pain. Dig Dis Sci. 2008;53:1430-1431.
24. White J. Diagnosis and management of acute angle-closure glaucoma. Emerg Nurse. 2011;19:27.
25. Lama DSC, Thama CCY, Laia JSM, et al. Current approaches to the management of acute primary angle closure. Curr Opin Ophthalmol. 2007;18:
146-151.
26. Gharaibeh A, Savage HI, Scherer RW, et al. Medical interventions for traumatic hyphema. Cochrane Database Syst Rev. 2011;(1):CD005431.
27. Connor AJ, Severn P. Use of a control test to aid pH assessment of chemical eye injuries. Emerg Med J. 2009;26:811-812.
28. Sambursky R, Trattler W, Tauber S, et al. Sensitivity and specificity of the AdenoPlus test for diagnosing adenoviral conjunctivitis. JAMA Ophthalmol. 2013;131:17-22.
29. Sheikh A, Hurwitz B. Antibiotics versus placebo for acute bacterial conjunctivitis. Cochrane Database Syst Rev. 2006;(2):CD001211.
30. Papier A, Tuttle DJ, Mahara TJ. Differential diagnosis of the swollen red eyelid. Am Fam Physician. 2007;76:1815-1824.
31. Howe L, Jones NS. Guidelines for the management of periorbital cellulitis/abscess. Clin Otolaryngol. 2004;29:725-728.
32. Mahalingam-Dhingra A, Lander L, Preciado DA, et al. Orbital and periorbital infections: a national perspective. Arch Otolaryngol Head Neck Surg. 2011;137:769-773.
33. Germann CA, Baumann MR, Hamzavi S. Ophthalmic diagnoses in the ED: optic neuritis. Am J Emerg Med. 2007;25:834-837.
34. Balcer LJ. Optic neuritis. N Engl J Med. 2006;354:1273-1280.
35. Olitsky SE, Hug D, Plummer L, et al. Abnormalities of the optic nerve. In: Kliegman RM, Behrman RE, Jenson HB, et al, eds. Nelson Textbook of Pediatrics. 19th ed. Philadelphia, PA: Saunders Elsevier; 2011:chap 623.
36. Gal RL, Vedula SS, Beck R. Corticosteroids for treating optic neuritis. Cochrane Database Syst Rev. 2012;(4):CD001430.
37. Aslam SA, Sheth HG, Vaughan AJ. Emergency management of corneal injuries. Injury. 2007;38:594-597.
38. Turner A, Rabiu M. Patching for corneal abrasion. Cochrane Database Syst Rev. 2006;(2):CD004764.
2014 Update on abnormal uterine bleeding
As recently defined by the International Federation of Gynecology and Obstetrics (FIGO)—and endorsed by the American College of Obstetricians and Gynecologists—the term “abnormal uterine bleeding” (AUB) now describes any departure from normal menstrual bleeding.1 To determine the most appropriate intervention for this widespread problem, FIGO proposed that clinicians consider potential contributors to the clinical problem by investigating and categorizing patients according to the following system:
- Polyp
- Adenomyosis
- Leiomyoma
- Malignancy and hyperplasia
- Coagulopathy
- Ovulatory disorders
- Endometrial dysfunction
- Iatrogenic
- Not otherwise classified.
A given individual may be found to have one or more of these features, but not all of the features may contribute to the AUB. To facilitate their use, these nine causes are more commonly identified using the acronym PALM-COEIN.
In this article, I focus on three of these categories, presenting recent data on AUB associated with leiomyomata (AUB-L) or adenomyosis (AUB-A), and AUB of an iatrogenic nature (AUB-I).
AUB-L: SATISFACTION RATES ARE SIMILAR 5 YEARS AFTER FIBROID TREATMENT BY SURGERY OR UTERINE ARTERY EMBOLIZATION
Gupta JK, Sinha A, Lumsden MA, Hickey M. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev. 2012;5:CD005073. doi:10.1002/14651858.CD005073.pub3.
Women who undergo uterine artery embolization (UAE) for the treatment of symptomatic uterine fibroids are just as satisfied with the outcome as women treated with hysterectomy or myomectomy, according to this 2012 review from the Cochrane Database.
Gupta and colleagues found similar patient-satisfaction rates at 5 years (odds ratio [OR] 0.9; 95% confidence interval [CI], 0.45–1.8), although women undergoing UAE were more likely to require additional interventions within 2 years (56 additional interventions per 1,000 women for surgery vs 250 per 1,000 women for UAE; OR, 5.64).
Details and general findings
Gupta and colleagues selected randomized, controlled trials comparing UAE with surgery:
- three trials of UAE versus abdominal hysterectomy (n = 291)
- one trial of UAE versus hysterectomy or myomectomy (the specific surgery was determined by patient preference) (n = 157)
- one trial of UAE versus myomectomy in women desiring future childbearing (n = 121).
In these trials, UAE was bilateral and involved the use of permanent embolic material.
Among the findings:
- Costs were lower with UAE, as assessed by measuring the duration of the procedure, length of hospitalization, and time to resumption of normal activities.
- Ovarian-failure rates were comparable between women in the UAE and surgery groups. Ovarian function was assessed by measuring follicle-stimulating hormone (FSH), although FSH thresholds varied in some of the studies.
- Pregnancy was less likely after UAE than after myomectomy. In the trial comparing UAE with myomectomy, 26 women later tried to conceive after UAE versus 40 after myomectomy. Significantly fewer women became pregnant after UAE (OR, 0.29; 95% CI, 0.10–0.85).
Related Article: Update on Fertility G. David Adamson, MD; Mary E. Abusief, MD (February 2014)
Bleeding outcomes were not measured
Strengths of this systematic review are its inclusion of high-quality, randomized, controlled trials and its assessment of ovarian-failure rates. However, a major weakness is the fact that its design does not allow for discrete evaluation of bleeding outcomes. Nor can its findings be broken down by the type of leiomyoma being treated.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
This review demonstrates that women are satisfied with outcomes five years after UAE and that ovarian failure is not more common after UAE than after surgery. Although the available evidence demonstrates that pregnancy following UAE is possible, women requiring a surgical procedure for AUB-L who are uncertain about their childbearing plans or who are hoping to conceive should be encouraged to select myomectomy as their intervention of choice.
AUB-A: FOR ADENOMYOSIS-ASSOCIATED AUB, CONSIDER THE LNG-IUS AS AN ALTERNATIVE TO HYSTERECTOMY
Ozdegirmenci O, Kayikcioglu F, Akgul MA, et al. Comparison of levonorgestrel intrauterine system versus hysterectomy on efficacy and quality of life in patients with adenomyosis. Fertil Steril. 2011;95(2):497–502.
In a small randomized, controlled trial of the levonorgestrel-releasing intrauterine system (LNG-IUS; Mirena) versus hysterectomy for adenomyosis-associated AUB, women allocated to the LNG-IUS experienced a reduction in bleeding and comparable gains in hemoglobin values during the first year of use. Both the LNG-IUS and hysterectomy improved health-related quality of life, but the LNG-IUS was associated with superior improvements in measures of psychological and social functioning.
Related Article: Update: Minimally invasive gynecology Amy Garcia, MD (April 2013)
Details and general findings of the trial
Eighty-six women were enrolled in the trial after exclusion of endometrial pathology as a cause of their heavy menstrual bleeding and after transvaginal ultrasound and magnetic resonance imaging findings were consistent with the diagnosis of adenomyosis. Participants then were randomly assigned to undergo hysterectomy or insertion of an LNG-IUS (43 women in each group). At baseline, the mean (SD) age was 44.28 (4.36) years among women in the LNG-IUS group versus 46.38 (3.76) years among women undergoing hysterectomy (P = .032), a statistical difference that I suspect is not clinically significant.
Menstrual bleeding, hemoglobin levels, and quality of life were assessed prior to insertion or surgery, and again at 6- and 12-month follow-up. Eleven women in the hysterectomy group were lost to follow-up.
General findings of the trial include:
- Women in the LNG-IUS group had a mean reduction in the volume of menstrual bleeding—as measured by the number of pads used—from two pads to one pad at 6 months, remaining at that level until 12 months. Serum hemoglobin levels increased from a median of just over
11 g/dL at the time of insertion to 13 g/dL at 6 months and slightly higher at 12 months. In the five self-reported quality-of-life domains assessed (physical, psychological, social, environmental, and a national environmental domain), women using the LNG-IUS demonstrated improvement in all five. - Women in the hysterectomy group were treated using an abdominal surgical approach, with one patient experiencing postoperative wound infection that required secondary suture. Postoperative pathologic analysis found that 21 of these women (65.6%) had adenomyosis, six women (18.8%) had myomas, three women (9.4%) had both adenomyosis and a myoma, and two women (6.2%) had a normal uterus. Serum hemoglobin levels increased from a median of roughly 10.5 g/dL at the time of treatment to 13 g/dL at 6 months and slightly higher at 12 months. (There were no statistically significant differences in hemoglobin values between the LNG-IUS and hysterectomy groups at any point in the study.) Quality of life improved in three of the five domains assessed (physical and both environmental domains).
Although 11 women were lost to follow-up, this trial appeared to have an adequate sample size to examine the selected outcomes, and the population was well defined.
Two weaknesses were the limited follow-up (only 12 months) and the use of quality-of-life measures designed for a Turkish population (the trial was conducted in Turkey), which may or may not be fully applicable to a US population.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
The relationship of adenomyosis to gynecologic symptoms, including heavy menstrual bleeding and dysmenorrhea, needs further study. However, this trial confirmed that transvaginal ultrasound is helpful in the nonsurgical diagnosis of adenomyosis and suggests that the LNG-IUS may be as effective at 1 year as hysterectomy for the treatment of adenomyosis-associated heavy menstrual bleeding (AUB-A).
Clinicians who perform office-based ultrasound to assess AUB should familiarize themselves with the criteria for ultrasonic diagnosis of adenomyosis. These criteria include the presence of heterogeneous myometrial echogenicity, a loss of clarity of the endo-myometrial interface, typically radially oriented linear striations, the appearance of myometrial cysts, and an overall globular enlarged uterus characterized by asymmetric thickening of the myometrium.2
In patients with heavy menstrual bleeding who have these findings, particularly if there is coexistent dysmenorrhea and uterine tenderness, it behooves the clinician to consider the LNG-IUS as first-line therapy, especially for women who wish to preserve fertility, but also for women for whom fertility is not an issue.
There is some evidence that the therapeutic effect of the LNG-IUS containing 20 µg of levonorgestrel may start to fade at 2 or 3 years, a possibility that should be shared with patients.3 Other features, such as cavity size, thickness of the myometrium, and the coexistence of clinically relevant leiomyomas, have not been evaluated but may have an impact on the clinical response.
AUB-I: LOW-DOSE DOXYCYCLINE REDUCES THE TIME TO AMENORRHEA IN USERS OF CONTINUOUS ORAL CONTRACEPTIVES
Kaneshiro B, Edelman A, Carlson NE, Nichols M, Forbes MM, Jensen J. A randomized controlled trial of subantimicrobial-dose doxycycline to prevent unscheduled bleeding with continuous oral contraceptive pill use. Contraception. 2012;85(4):351–358.
Unscheduled bleeding is the most common complaint among women who use continuous combination oral contraceptives (OCs). Because unscheduled bleeding has been correlated with the upregulation of matrix metalloprotineases (MMPs), Kaneshiro and colleagues conducted a randomized, controlled trial of doxycycline (an MMP inhibitor) versus placebo among users of continuous OCs. The addition of doxycycline to the OC regimen did not significantly reduce unscheduled bleeding during the first 84 days of use, but it did shorten the time required to achieve amenorrhea (mean of 61.7 days for doxycycline vs 85.2 days for placebo; standard error [SE], 7.7 vs 6.7, respectively; P = .03).
Related Article: Big step forward and downward: An OC with 10 μg of estrogen Robert L. Barbieri, MD (Editorial, May 2011)
Details and general findings of the trial
Participants (n = 65) were healthy women aged 18 to 45 years who had no contraindications to continuous use of combination OCs. Prior to enrollment, they all had used cyclic combination contraception (pill, patch, or ring) without unscheduled bleeding, thereby avoiding the “transition bleeding” that often occurs when continuous OCs are initiated.
All women in the trial were started on continuous OCs (20 µg ethinyl estradiol with 100 µg levonorgestrel; Aviane) and then randomly assigned to receive one of the following for 84 days in addition to the OC:
- doxycycline 40 mg daily (controlled-release Oracea), a subantimicrobial dose
- placebo.
After 84 days, doxycycline was discontinued, and participants were observed for an additional 28 days on the OC regimen alone for the documentation of bleeding patterns.
General findings:
- The number of bleeding and spotting days decreased in both groups over the course of the study.
- During the first 84 days of the trial, bleeding and spotting occurred among a median of 11 and 17 women in the doxycycline and placebo groups, respectively, and bleeding alone (without spotting) occurred in a median 3 and 4 women in the doxycycline and placebo groups, respectively.
- During the 28-day observation period, bleeding and spotting occurred among a median of 0 and 6 women in the doxycycline and placebo groups, respectively. Bleeding alone (without spotting) was absent in both groups.
- Women in the doxycycline group were significantly less likely to report side effects such as headache, depressed mood, and abdominal cramping. However, they were more likely to prefer continuous OCs without doxycycline, compared with women receiving placebo (16.1% vs 10.7%).
WHAT THIS EVIDENCE MEANS FOR PRACTICE
This trial increases our insight into AUB associated with the use of progestins and suggests that concomitant doxycycline may reduce unscheduled bleeding and spotting in women using continuous combination OCs. The trial was of adequate sample size for the primary outcomes, lending credence to its findings, although longer-term data would be helpful.
I have included this trial for two reasons:
It offers useful information regarding the mechanisms and potential prevention or reduction of AUB-I in users of continuous combined estrogen-progestin contraception.
Doxycycline is one of the agents covered in a Cochrane review of high-quality research into AUB-I in women using progestin-only products, including injectables, implantables, intrauterine systems, and oral agents.4 Estrogens have been shown to have some value in reducing breakthrough bleeding associated with depot medroxyprogesterone acetate, and individual use of tranexamic acid or doxycycline has shown value in terminating an episode of breakthrough bleeding in women using progestin-only contraceptives.
TELL US WHAT YOU THINK!
Share your thoughts on this article or on any topic relevant to ObGyns and women’s health practitioners. Tell us which topics you’d like to see covered in future issues, and what challenges you face in daily practice. We will consider publishing your letter and in a future issue.
Send your letter to: [email protected] Please include the city and state in which you practice.
Stay in touch! Your feedback is important to us!
- Munro MG, Critchley HO, Broder MS, Fraser IS; FIGO Working Group on Menstrual Disorders. The FIGO classification for causes of abnormal bleeding in the reproductive years. Fertil Steril. 2011;95(7):2204–2208.
- Champaneria R, Abedin P, Daniels J, Balogun M, Khan KS. Ultrasound scan and magnetic resonance imaging for the diagnosis of adenomyosis: Systematic review comparing test accuracy. Acta Obstet Gynecol Scand. 2010;89(11):1374–1384.
- Cho S, Nam A, Kim H, et al. Clinical effects of the levonorgestrel-releasing intrauterine device in patients with adenomyosis. Am J Obstet Gynecol. 2008;198(4):373.e1–e7.
- Abdel-Aleem H, d’Arcangues C, Vogelsong KM, Gaffield ML, Gulmezoglu AM. Treatment of vaginal bleeding irregularities induced by progestin-only contraceptives. Cochrane Database Syst Rev. 2013;10:CD003449.
As recently defined by the International Federation of Gynecology and Obstetrics (FIGO)—and endorsed by the American College of Obstetricians and Gynecologists—the term “abnormal uterine bleeding” (AUB) now describes any departure from normal menstrual bleeding.1 To determine the most appropriate intervention for this widespread problem, FIGO proposed that clinicians consider potential contributors to the clinical problem by investigating and categorizing patients according to the following system:
- Polyp
- Adenomyosis
- Leiomyoma
- Malignancy and hyperplasia
- Coagulopathy
- Ovulatory disorders
- Endometrial dysfunction
- Iatrogenic
- Not otherwise classified.
A given individual may be found to have one or more of these features, but not all of the features may contribute to the AUB. To facilitate their use, these nine causes are more commonly identified using the acronym PALM-COEIN.
In this article, I focus on three of these categories, presenting recent data on AUB associated with leiomyomata (AUB-L) or adenomyosis (AUB-A), and AUB of an iatrogenic nature (AUB-I).
AUB-L: SATISFACTION RATES ARE SIMILAR 5 YEARS AFTER FIBROID TREATMENT BY SURGERY OR UTERINE ARTERY EMBOLIZATION
Gupta JK, Sinha A, Lumsden MA, Hickey M. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev. 2012;5:CD005073. doi:10.1002/14651858.CD005073.pub3.
Women who undergo uterine artery embolization (UAE) for the treatment of symptomatic uterine fibroids are just as satisfied with the outcome as women treated with hysterectomy or myomectomy, according to this 2012 review from the Cochrane Database.
Gupta and colleagues found similar patient-satisfaction rates at 5 years (odds ratio [OR] 0.9; 95% confidence interval [CI], 0.45–1.8), although women undergoing UAE were more likely to require additional interventions within 2 years (56 additional interventions per 1,000 women for surgery vs 250 per 1,000 women for UAE; OR, 5.64).
Details and general findings
Gupta and colleagues selected randomized, controlled trials comparing UAE with surgery:
- three trials of UAE versus abdominal hysterectomy (n = 291)
- one trial of UAE versus hysterectomy or myomectomy (the specific surgery was determined by patient preference) (n = 157)
- one trial of UAE versus myomectomy in women desiring future childbearing (n = 121).
In these trials, UAE was bilateral and involved the use of permanent embolic material.
Among the findings:
- Costs were lower with UAE, as assessed by measuring the duration of the procedure, length of hospitalization, and time to resumption of normal activities.
- Ovarian-failure rates were comparable between women in the UAE and surgery groups. Ovarian function was assessed by measuring follicle-stimulating hormone (FSH), although FSH thresholds varied in some of the studies.
- Pregnancy was less likely after UAE than after myomectomy. In the trial comparing UAE with myomectomy, 26 women later tried to conceive after UAE versus 40 after myomectomy. Significantly fewer women became pregnant after UAE (OR, 0.29; 95% CI, 0.10–0.85).
Related Article: Update on Fertility G. David Adamson, MD; Mary E. Abusief, MD (February 2014)
Bleeding outcomes were not measured
Strengths of this systematic review are its inclusion of high-quality, randomized, controlled trials and its assessment of ovarian-failure rates. However, a major weakness is the fact that its design does not allow for discrete evaluation of bleeding outcomes. Nor can its findings be broken down by the type of leiomyoma being treated.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
This review demonstrates that women are satisfied with outcomes five years after UAE and that ovarian failure is not more common after UAE than after surgery. Although the available evidence demonstrates that pregnancy following UAE is possible, women requiring a surgical procedure for AUB-L who are uncertain about their childbearing plans or who are hoping to conceive should be encouraged to select myomectomy as their intervention of choice.
AUB-A: FOR ADENOMYOSIS-ASSOCIATED AUB, CONSIDER THE LNG-IUS AS AN ALTERNATIVE TO HYSTERECTOMY
Ozdegirmenci O, Kayikcioglu F, Akgul MA, et al. Comparison of levonorgestrel intrauterine system versus hysterectomy on efficacy and quality of life in patients with adenomyosis. Fertil Steril. 2011;95(2):497–502.
In a small randomized, controlled trial of the levonorgestrel-releasing intrauterine system (LNG-IUS; Mirena) versus hysterectomy for adenomyosis-associated AUB, women allocated to the LNG-IUS experienced a reduction in bleeding and comparable gains in hemoglobin values during the first year of use. Both the LNG-IUS and hysterectomy improved health-related quality of life, but the LNG-IUS was associated with superior improvements in measures of psychological and social functioning.
Related Article: Update: Minimally invasive gynecology Amy Garcia, MD (April 2013)
Details and general findings of the trial
Eighty-six women were enrolled in the trial after exclusion of endometrial pathology as a cause of their heavy menstrual bleeding and after transvaginal ultrasound and magnetic resonance imaging findings were consistent with the diagnosis of adenomyosis. Participants then were randomly assigned to undergo hysterectomy or insertion of an LNG-IUS (43 women in each group). At baseline, the mean (SD) age was 44.28 (4.36) years among women in the LNG-IUS group versus 46.38 (3.76) years among women undergoing hysterectomy (P = .032), a statistical difference that I suspect is not clinically significant.
Menstrual bleeding, hemoglobin levels, and quality of life were assessed prior to insertion or surgery, and again at 6- and 12-month follow-up. Eleven women in the hysterectomy group were lost to follow-up.
General findings of the trial include:
- Women in the LNG-IUS group had a mean reduction in the volume of menstrual bleeding—as measured by the number of pads used—from two pads to one pad at 6 months, remaining at that level until 12 months. Serum hemoglobin levels increased from a median of just over
11 g/dL at the time of insertion to 13 g/dL at 6 months and slightly higher at 12 months. In the five self-reported quality-of-life domains assessed (physical, psychological, social, environmental, and a national environmental domain), women using the LNG-IUS demonstrated improvement in all five. - Women in the hysterectomy group were treated using an abdominal surgical approach, with one patient experiencing postoperative wound infection that required secondary suture. Postoperative pathologic analysis found that 21 of these women (65.6%) had adenomyosis, six women (18.8%) had myomas, three women (9.4%) had both adenomyosis and a myoma, and two women (6.2%) had a normal uterus. Serum hemoglobin levels increased from a median of roughly 10.5 g/dL at the time of treatment to 13 g/dL at 6 months and slightly higher at 12 months. (There were no statistically significant differences in hemoglobin values between the LNG-IUS and hysterectomy groups at any point in the study.) Quality of life improved in three of the five domains assessed (physical and both environmental domains).
Although 11 women were lost to follow-up, this trial appeared to have an adequate sample size to examine the selected outcomes, and the population was well defined.
Two weaknesses were the limited follow-up (only 12 months) and the use of quality-of-life measures designed for a Turkish population (the trial was conducted in Turkey), which may or may not be fully applicable to a US population.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
The relationship of adenomyosis to gynecologic symptoms, including heavy menstrual bleeding and dysmenorrhea, needs further study. However, this trial confirmed that transvaginal ultrasound is helpful in the nonsurgical diagnosis of adenomyosis and suggests that the LNG-IUS may be as effective at 1 year as hysterectomy for the treatment of adenomyosis-associated heavy menstrual bleeding (AUB-A).
Clinicians who perform office-based ultrasound to assess AUB should familiarize themselves with the criteria for ultrasonic diagnosis of adenomyosis. These criteria include the presence of heterogeneous myometrial echogenicity, a loss of clarity of the endo-myometrial interface, typically radially oriented linear striations, the appearance of myometrial cysts, and an overall globular enlarged uterus characterized by asymmetric thickening of the myometrium.2
In patients with heavy menstrual bleeding who have these findings, particularly if there is coexistent dysmenorrhea and uterine tenderness, it behooves the clinician to consider the LNG-IUS as first-line therapy, especially for women who wish to preserve fertility, but also for women for whom fertility is not an issue.
There is some evidence that the therapeutic effect of the LNG-IUS containing 20 µg of levonorgestrel may start to fade at 2 or 3 years, a possibility that should be shared with patients.3 Other features, such as cavity size, thickness of the myometrium, and the coexistence of clinically relevant leiomyomas, have not been evaluated but may have an impact on the clinical response.
AUB-I: LOW-DOSE DOXYCYCLINE REDUCES THE TIME TO AMENORRHEA IN USERS OF CONTINUOUS ORAL CONTRACEPTIVES
Kaneshiro B, Edelman A, Carlson NE, Nichols M, Forbes MM, Jensen J. A randomized controlled trial of subantimicrobial-dose doxycycline to prevent unscheduled bleeding with continuous oral contraceptive pill use. Contraception. 2012;85(4):351–358.
Unscheduled bleeding is the most common complaint among women who use continuous combination oral contraceptives (OCs). Because unscheduled bleeding has been correlated with the upregulation of matrix metalloprotineases (MMPs), Kaneshiro and colleagues conducted a randomized, controlled trial of doxycycline (an MMP inhibitor) versus placebo among users of continuous OCs. The addition of doxycycline to the OC regimen did not significantly reduce unscheduled bleeding during the first 84 days of use, but it did shorten the time required to achieve amenorrhea (mean of 61.7 days for doxycycline vs 85.2 days for placebo; standard error [SE], 7.7 vs 6.7, respectively; P = .03).
Related Article: Big step forward and downward: An OC with 10 μg of estrogen Robert L. Barbieri, MD (Editorial, May 2011)
Details and general findings of the trial
Participants (n = 65) were healthy women aged 18 to 45 years who had no contraindications to continuous use of combination OCs. Prior to enrollment, they all had used cyclic combination contraception (pill, patch, or ring) without unscheduled bleeding, thereby avoiding the “transition bleeding” that often occurs when continuous OCs are initiated.
All women in the trial were started on continuous OCs (20 µg ethinyl estradiol with 100 µg levonorgestrel; Aviane) and then randomly assigned to receive one of the following for 84 days in addition to the OC:
- doxycycline 40 mg daily (controlled-release Oracea), a subantimicrobial dose
- placebo.
After 84 days, doxycycline was discontinued, and participants were observed for an additional 28 days on the OC regimen alone for the documentation of bleeding patterns.
General findings:
- The number of bleeding and spotting days decreased in both groups over the course of the study.
- During the first 84 days of the trial, bleeding and spotting occurred among a median of 11 and 17 women in the doxycycline and placebo groups, respectively, and bleeding alone (without spotting) occurred in a median 3 and 4 women in the doxycycline and placebo groups, respectively.
- During the 28-day observation period, bleeding and spotting occurred among a median of 0 and 6 women in the doxycycline and placebo groups, respectively. Bleeding alone (without spotting) was absent in both groups.
- Women in the doxycycline group were significantly less likely to report side effects such as headache, depressed mood, and abdominal cramping. However, they were more likely to prefer continuous OCs without doxycycline, compared with women receiving placebo (16.1% vs 10.7%).
WHAT THIS EVIDENCE MEANS FOR PRACTICE
This trial increases our insight into AUB associated with the use of progestins and suggests that concomitant doxycycline may reduce unscheduled bleeding and spotting in women using continuous combination OCs. The trial was of adequate sample size for the primary outcomes, lending credence to its findings, although longer-term data would be helpful.
I have included this trial for two reasons:
It offers useful information regarding the mechanisms and potential prevention or reduction of AUB-I in users of continuous combined estrogen-progestin contraception.
Doxycycline is one of the agents covered in a Cochrane review of high-quality research into AUB-I in women using progestin-only products, including injectables, implantables, intrauterine systems, and oral agents.4 Estrogens have been shown to have some value in reducing breakthrough bleeding associated with depot medroxyprogesterone acetate, and individual use of tranexamic acid or doxycycline has shown value in terminating an episode of breakthrough bleeding in women using progestin-only contraceptives.
TELL US WHAT YOU THINK!
Share your thoughts on this article or on any topic relevant to ObGyns and women’s health practitioners. Tell us which topics you’d like to see covered in future issues, and what challenges you face in daily practice. We will consider publishing your letter and in a future issue.
Send your letter to: [email protected] Please include the city and state in which you practice.
Stay in touch! Your feedback is important to us!
As recently defined by the International Federation of Gynecology and Obstetrics (FIGO)—and endorsed by the American College of Obstetricians and Gynecologists—the term “abnormal uterine bleeding” (AUB) now describes any departure from normal menstrual bleeding.1 To determine the most appropriate intervention for this widespread problem, FIGO proposed that clinicians consider potential contributors to the clinical problem by investigating and categorizing patients according to the following system:
- Polyp
- Adenomyosis
- Leiomyoma
- Malignancy and hyperplasia
- Coagulopathy
- Ovulatory disorders
- Endometrial dysfunction
- Iatrogenic
- Not otherwise classified.
A given individual may be found to have one or more of these features, but not all of the features may contribute to the AUB. To facilitate their use, these nine causes are more commonly identified using the acronym PALM-COEIN.
In this article, I focus on three of these categories, presenting recent data on AUB associated with leiomyomata (AUB-L) or adenomyosis (AUB-A), and AUB of an iatrogenic nature (AUB-I).
AUB-L: SATISFACTION RATES ARE SIMILAR 5 YEARS AFTER FIBROID TREATMENT BY SURGERY OR UTERINE ARTERY EMBOLIZATION
Gupta JK, Sinha A, Lumsden MA, Hickey M. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev. 2012;5:CD005073. doi:10.1002/14651858.CD005073.pub3.
Women who undergo uterine artery embolization (UAE) for the treatment of symptomatic uterine fibroids are just as satisfied with the outcome as women treated with hysterectomy or myomectomy, according to this 2012 review from the Cochrane Database.
Gupta and colleagues found similar patient-satisfaction rates at 5 years (odds ratio [OR] 0.9; 95% confidence interval [CI], 0.45–1.8), although women undergoing UAE were more likely to require additional interventions within 2 years (56 additional interventions per 1,000 women for surgery vs 250 per 1,000 women for UAE; OR, 5.64).
Details and general findings
Gupta and colleagues selected randomized, controlled trials comparing UAE with surgery:
- three trials of UAE versus abdominal hysterectomy (n = 291)
- one trial of UAE versus hysterectomy or myomectomy (the specific surgery was determined by patient preference) (n = 157)
- one trial of UAE versus myomectomy in women desiring future childbearing (n = 121).
In these trials, UAE was bilateral and involved the use of permanent embolic material.
Among the findings:
- Costs were lower with UAE, as assessed by measuring the duration of the procedure, length of hospitalization, and time to resumption of normal activities.
- Ovarian-failure rates were comparable between women in the UAE and surgery groups. Ovarian function was assessed by measuring follicle-stimulating hormone (FSH), although FSH thresholds varied in some of the studies.
- Pregnancy was less likely after UAE than after myomectomy. In the trial comparing UAE with myomectomy, 26 women later tried to conceive after UAE versus 40 after myomectomy. Significantly fewer women became pregnant after UAE (OR, 0.29; 95% CI, 0.10–0.85).
Related Article: Update on Fertility G. David Adamson, MD; Mary E. Abusief, MD (February 2014)
Bleeding outcomes were not measured
Strengths of this systematic review are its inclusion of high-quality, randomized, controlled trials and its assessment of ovarian-failure rates. However, a major weakness is the fact that its design does not allow for discrete evaluation of bleeding outcomes. Nor can its findings be broken down by the type of leiomyoma being treated.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
This review demonstrates that women are satisfied with outcomes five years after UAE and that ovarian failure is not more common after UAE than after surgery. Although the available evidence demonstrates that pregnancy following UAE is possible, women requiring a surgical procedure for AUB-L who are uncertain about their childbearing plans or who are hoping to conceive should be encouraged to select myomectomy as their intervention of choice.
AUB-A: FOR ADENOMYOSIS-ASSOCIATED AUB, CONSIDER THE LNG-IUS AS AN ALTERNATIVE TO HYSTERECTOMY
Ozdegirmenci O, Kayikcioglu F, Akgul MA, et al. Comparison of levonorgestrel intrauterine system versus hysterectomy on efficacy and quality of life in patients with adenomyosis. Fertil Steril. 2011;95(2):497–502.
In a small randomized, controlled trial of the levonorgestrel-releasing intrauterine system (LNG-IUS; Mirena) versus hysterectomy for adenomyosis-associated AUB, women allocated to the LNG-IUS experienced a reduction in bleeding and comparable gains in hemoglobin values during the first year of use. Both the LNG-IUS and hysterectomy improved health-related quality of life, but the LNG-IUS was associated with superior improvements in measures of psychological and social functioning.
Related Article: Update: Minimally invasive gynecology Amy Garcia, MD (April 2013)
Details and general findings of the trial
Eighty-six women were enrolled in the trial after exclusion of endometrial pathology as a cause of their heavy menstrual bleeding and after transvaginal ultrasound and magnetic resonance imaging findings were consistent with the diagnosis of adenomyosis. Participants then were randomly assigned to undergo hysterectomy or insertion of an LNG-IUS (43 women in each group). At baseline, the mean (SD) age was 44.28 (4.36) years among women in the LNG-IUS group versus 46.38 (3.76) years among women undergoing hysterectomy (P = .032), a statistical difference that I suspect is not clinically significant.
Menstrual bleeding, hemoglobin levels, and quality of life were assessed prior to insertion or surgery, and again at 6- and 12-month follow-up. Eleven women in the hysterectomy group were lost to follow-up.
General findings of the trial include:
- Women in the LNG-IUS group had a mean reduction in the volume of menstrual bleeding—as measured by the number of pads used—from two pads to one pad at 6 months, remaining at that level until 12 months. Serum hemoglobin levels increased from a median of just over
11 g/dL at the time of insertion to 13 g/dL at 6 months and slightly higher at 12 months. In the five self-reported quality-of-life domains assessed (physical, psychological, social, environmental, and a national environmental domain), women using the LNG-IUS demonstrated improvement in all five. - Women in the hysterectomy group were treated using an abdominal surgical approach, with one patient experiencing postoperative wound infection that required secondary suture. Postoperative pathologic analysis found that 21 of these women (65.6%) had adenomyosis, six women (18.8%) had myomas, three women (9.4%) had both adenomyosis and a myoma, and two women (6.2%) had a normal uterus. Serum hemoglobin levels increased from a median of roughly 10.5 g/dL at the time of treatment to 13 g/dL at 6 months and slightly higher at 12 months. (There were no statistically significant differences in hemoglobin values between the LNG-IUS and hysterectomy groups at any point in the study.) Quality of life improved in three of the five domains assessed (physical and both environmental domains).
Although 11 women were lost to follow-up, this trial appeared to have an adequate sample size to examine the selected outcomes, and the population was well defined.
Two weaknesses were the limited follow-up (only 12 months) and the use of quality-of-life measures designed for a Turkish population (the trial was conducted in Turkey), which may or may not be fully applicable to a US population.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
The relationship of adenomyosis to gynecologic symptoms, including heavy menstrual bleeding and dysmenorrhea, needs further study. However, this trial confirmed that transvaginal ultrasound is helpful in the nonsurgical diagnosis of adenomyosis and suggests that the LNG-IUS may be as effective at 1 year as hysterectomy for the treatment of adenomyosis-associated heavy menstrual bleeding (AUB-A).
Clinicians who perform office-based ultrasound to assess AUB should familiarize themselves with the criteria for ultrasonic diagnosis of adenomyosis. These criteria include the presence of heterogeneous myometrial echogenicity, a loss of clarity of the endo-myometrial interface, typically radially oriented linear striations, the appearance of myometrial cysts, and an overall globular enlarged uterus characterized by asymmetric thickening of the myometrium.2
In patients with heavy menstrual bleeding who have these findings, particularly if there is coexistent dysmenorrhea and uterine tenderness, it behooves the clinician to consider the LNG-IUS as first-line therapy, especially for women who wish to preserve fertility, but also for women for whom fertility is not an issue.
There is some evidence that the therapeutic effect of the LNG-IUS containing 20 µg of levonorgestrel may start to fade at 2 or 3 years, a possibility that should be shared with patients.3 Other features, such as cavity size, thickness of the myometrium, and the coexistence of clinically relevant leiomyomas, have not been evaluated but may have an impact on the clinical response.
AUB-I: LOW-DOSE DOXYCYCLINE REDUCES THE TIME TO AMENORRHEA IN USERS OF CONTINUOUS ORAL CONTRACEPTIVES
Kaneshiro B, Edelman A, Carlson NE, Nichols M, Forbes MM, Jensen J. A randomized controlled trial of subantimicrobial-dose doxycycline to prevent unscheduled bleeding with continuous oral contraceptive pill use. Contraception. 2012;85(4):351–358.
Unscheduled bleeding is the most common complaint among women who use continuous combination oral contraceptives (OCs). Because unscheduled bleeding has been correlated with the upregulation of matrix metalloprotineases (MMPs), Kaneshiro and colleagues conducted a randomized, controlled trial of doxycycline (an MMP inhibitor) versus placebo among users of continuous OCs. The addition of doxycycline to the OC regimen did not significantly reduce unscheduled bleeding during the first 84 days of use, but it did shorten the time required to achieve amenorrhea (mean of 61.7 days for doxycycline vs 85.2 days for placebo; standard error [SE], 7.7 vs 6.7, respectively; P = .03).
Related Article: Big step forward and downward: An OC with 10 μg of estrogen Robert L. Barbieri, MD (Editorial, May 2011)
Details and general findings of the trial
Participants (n = 65) were healthy women aged 18 to 45 years who had no contraindications to continuous use of combination OCs. Prior to enrollment, they all had used cyclic combination contraception (pill, patch, or ring) without unscheduled bleeding, thereby avoiding the “transition bleeding” that often occurs when continuous OCs are initiated.
All women in the trial were started on continuous OCs (20 µg ethinyl estradiol with 100 µg levonorgestrel; Aviane) and then randomly assigned to receive one of the following for 84 days in addition to the OC:
- doxycycline 40 mg daily (controlled-release Oracea), a subantimicrobial dose
- placebo.
After 84 days, doxycycline was discontinued, and participants were observed for an additional 28 days on the OC regimen alone for the documentation of bleeding patterns.
General findings:
- The number of bleeding and spotting days decreased in both groups over the course of the study.
- During the first 84 days of the trial, bleeding and spotting occurred among a median of 11 and 17 women in the doxycycline and placebo groups, respectively, and bleeding alone (without spotting) occurred in a median 3 and 4 women in the doxycycline and placebo groups, respectively.
- During the 28-day observation period, bleeding and spotting occurred among a median of 0 and 6 women in the doxycycline and placebo groups, respectively. Bleeding alone (without spotting) was absent in both groups.
- Women in the doxycycline group were significantly less likely to report side effects such as headache, depressed mood, and abdominal cramping. However, they were more likely to prefer continuous OCs without doxycycline, compared with women receiving placebo (16.1% vs 10.7%).
WHAT THIS EVIDENCE MEANS FOR PRACTICE
This trial increases our insight into AUB associated with the use of progestins and suggests that concomitant doxycycline may reduce unscheduled bleeding and spotting in women using continuous combination OCs. The trial was of adequate sample size for the primary outcomes, lending credence to its findings, although longer-term data would be helpful.
I have included this trial for two reasons:
It offers useful information regarding the mechanisms and potential prevention or reduction of AUB-I in users of continuous combined estrogen-progestin contraception.
Doxycycline is one of the agents covered in a Cochrane review of high-quality research into AUB-I in women using progestin-only products, including injectables, implantables, intrauterine systems, and oral agents.4 Estrogens have been shown to have some value in reducing breakthrough bleeding associated with depot medroxyprogesterone acetate, and individual use of tranexamic acid or doxycycline has shown value in terminating an episode of breakthrough bleeding in women using progestin-only contraceptives.
TELL US WHAT YOU THINK!
Share your thoughts on this article or on any topic relevant to ObGyns and women’s health practitioners. Tell us which topics you’d like to see covered in future issues, and what challenges you face in daily practice. We will consider publishing your letter and in a future issue.
Send your letter to: [email protected] Please include the city and state in which you practice.
Stay in touch! Your feedback is important to us!
- Munro MG, Critchley HO, Broder MS, Fraser IS; FIGO Working Group on Menstrual Disorders. The FIGO classification for causes of abnormal bleeding in the reproductive years. Fertil Steril. 2011;95(7):2204–2208.
- Champaneria R, Abedin P, Daniels J, Balogun M, Khan KS. Ultrasound scan and magnetic resonance imaging for the diagnosis of adenomyosis: Systematic review comparing test accuracy. Acta Obstet Gynecol Scand. 2010;89(11):1374–1384.
- Cho S, Nam A, Kim H, et al. Clinical effects of the levonorgestrel-releasing intrauterine device in patients with adenomyosis. Am J Obstet Gynecol. 2008;198(4):373.e1–e7.
- Abdel-Aleem H, d’Arcangues C, Vogelsong KM, Gaffield ML, Gulmezoglu AM. Treatment of vaginal bleeding irregularities induced by progestin-only contraceptives. Cochrane Database Syst Rev. 2013;10:CD003449.
- Munro MG, Critchley HO, Broder MS, Fraser IS; FIGO Working Group on Menstrual Disorders. The FIGO classification for causes of abnormal bleeding in the reproductive years. Fertil Steril. 2011;95(7):2204–2208.
- Champaneria R, Abedin P, Daniels J, Balogun M, Khan KS. Ultrasound scan and magnetic resonance imaging for the diagnosis of adenomyosis: Systematic review comparing test accuracy. Acta Obstet Gynecol Scand. 2010;89(11):1374–1384.
- Cho S, Nam A, Kim H, et al. Clinical effects of the levonorgestrel-releasing intrauterine device in patients with adenomyosis. Am J Obstet Gynecol. 2008;198(4):373.e1–e7.
- Abdel-Aleem H, d’Arcangues C, Vogelsong KM, Gaffield ML, Gulmezoglu AM. Treatment of vaginal bleeding irregularities induced by progestin-only contraceptives. Cochrane Database Syst Rev. 2013;10:CD003449.
The Nonmotor Symptoms of Parkinson’s Disease: Update on Diagnosis and Treatment
From the Department of Neurology, Movement Disorders Division, University of Pittsburgh Medical Center, Pittsburgh, PA.
Abstract
- Objective: To review the prevalence, diagnosis, and treatment of the nonmotor symptoms (NMS) associated with Parkinson’s disease (PD).
- Methods: Narrative review of the literature.
- Results: The NMS of PD are becoming increasingly recognized as having a critical role in the impact of this neurodegenerative movement disorder. This has led to significant investigative efforts to identify new or better NMS therapies. The preponderance of PD patients will be diagnosed with 1 or multiple NMS during the course of their disease, with many of these symptoms occurring months or even years prior to receiving the PD diagnosis. Despite the high prevalence and impact on disease burden, NMS often go undetected due to a lack of reporting by patients or insufficient interrogation by physicians. Further complicating NMS management is that only a few therapies have the level of evidence needed to support their use in the treatment of NMS.
- Conclusion: The practitioner needs to be aware of NMS and conduct thorough patient questioning in order to recognize, diagnose, and address NMS in PD patients.
Parkinson’s disease (PD) is a neurodegenerative movement disorder with an estimated prevalence of 1% to 2% among  the population over the age of 65 years [1]. Recognition and clinical diagnosis of PD is primarily made based on the cardinal motor features, including rigidity, tremor, bradykinesia, and postural instability. The motor symptoms are neuropathologically associated with accumulation of alpha-synuclein with Lewy body formation and neurodegeneration of the nigrostriatal dopamine system. Postmortem evaluation of the brains of PD patients has revealed more widespread degeneration in nondopaminergic systems, including several brainstem nuclei (raphe nucleus, locus ceruleus, dorsal vagal nucleus), limbic and neocortical structures, as well as the peripheral autonomic system [2,3].
the population over the age of 65 years [1]. Recognition and clinical diagnosis of PD is primarily made based on the cardinal motor features, including rigidity, tremor, bradykinesia, and postural instability. The motor symptoms are neuropathologically associated with accumulation of alpha-synuclein with Lewy body formation and neurodegeneration of the nigrostriatal dopamine system. Postmortem evaluation of the brains of PD patients has revealed more widespread degeneration in nondopaminergic systems, including several brainstem nuclei (raphe nucleus, locus ceruleus, dorsal vagal nucleus), limbic and neocortical structures, as well as the peripheral autonomic system [2,3].
 The nonmotor symptoms (NMS) of PD are the clinical manifestations of this extensive degeneration, which suggests that NMS are intrinsic and fundamental features of PD. NMS are exceedingly common, and up to 90% of PD patients will experience nonmotor features, including depression, anxiety, sleep disturbances, cognitive impairment, and dysautonomia [4,5] (Table).
The nonmotor symptoms (NMS) of PD are the clinical manifestations of this extensive degeneration, which suggests that NMS are intrinsic and fundamental features of PD. NMS are exceedingly common, and up to 90% of PD patients will experience nonmotor features, including depression, anxiety, sleep disturbances, cognitive impairment, and dysautonomia [4,5] (Table).
NMS have a greater impact on quality of life as compared to the motor symptoms [6,7], but are frequently underrecognized [8]. Evidence suggests that unless there is systematic and specific interrogation by practioners, NMS will elude recognition [9–11]. Recognizing NMS as part of PD is complicated by the fact that these symptoms are common in the general population and not specific for PD [12,13]. NMS can occur at any stage of the disease and may predate diagnosis [12], although as PD progresses the NMS become more prevalent, with a greater impact on health care costs and institutionalization rates than motor features [14,15].
Neuropsychiatric Symptoms
Depression
Epidemiology and Diagnosis
Depression is one of the most common neuropsychiatric manifestations observed in PD patients, with prevalence reports between 4% and 72%, though likely to be closer to 30% to 45% [16–20]. The severity of depression in the PD population has been shown to be greater than in patients with matched chronic disabilities [21,22] and also greater than in the general population over the age of 65 years [23]. The onset of depression can occur at any stage of the disease, even predating the diagnosis. Additionally, depression has more than twice the impact on health status than motor symptoms [24].
Though the mechanisms are not fully understood, it is suspected that psychosocial as well as neuropathological changes contribute to the pathogenesis of depression in PD. In a study comparing 104 PD patients and 61 patients with equivalent disability scores, functional disability was found to be responsible for only 9% of the variation of depression scores [22]. The increased prevalence of depression in PD patients can in part be explained by the neuropathological changes seen in post-mortem studies. Two neurotransmitters that are fundamental in the pathogenesis of depression are serotonin, from the raphe nuclei, and norepinepherine, from the locus ceruleus [20]. Both of these brainstem structures demonstrate alpha-synucleinopathy-associated degeneration and these changes can precede the development of motor dysfunction [3].
Diagnosing depression in PD is complicated by the fact that there is overlap between other PD symptoms and clinical features of depression (ie, amotivation, bradykinesia, fatigue, and sleep disturbances). However, many depressed PD patients are less likely to report feelings of guilt or failure and tend to have higher rates of anxiety [9,20,25]. Typically, PD patients are more likely to be diagnosed with minor depression or dysthymia rather than a major depressive disorder [19,20]. Formal testing through systematic questionnaires are diagnostically useful in the clinic, and serial testing can reveal changes over time to guide more effective treatment. Validated tools to evaluate depression in PD include the Beck Depression Inventory, Hamilton Depression Rating Scale, Montgomery-Asberg Depression Rating Scale, Geriatric DRS, and Hospital Anxiety and Depression scale [20].
Treatment Options
Treatment of depression in PD demonstrates generally poorer responses to typical antidepressants and side effects that may worsen other PD symptoms. Selective serotonin reuptake inhibitors (SSRIs) have been widely used as there are generally few drug-drug interactions and minimal effect on motor symptoms; however, several studies have demonstrated little benefit on depression in PD [26]. In a randomized, double-blind, placebo-controlled trial of the antidepressants paroxetine and venlafaxine, both were found to be effective and well tolerated [27]. Tricyclic anti-depressants (TCAs) have also demonstrated efficacy. In randomized controlled trials comparing TCAs to SSRIs, a greater benefit on depression symptoms has been found with TCAs [28–30]. The use of TCAs, however, is limited by anticholinergic side effects that occasionally worsen orthostatic hypotension or cognitive impairment [15,31]. Dopamine agonists have also been studied in depressed PD patients. In a randomized, double-blind, placebo-controlled trial [32] and a prospective observational study [33], pramipexole demonstrated significant improvements in depression symptoms. Ropinirole also demonstrated significant symptomatic improvement [34]. These studies suggest that while SSRIs are commonly used, evidence is accumulating to support the role of TCAs, SNRIs, and dopamine agonists in the treatment of depression in PD.
Other therapies have also been tried in pharmacologic-resistant patients. Electroconvulsive therapy has been reported to improve both depression and motor symptoms [35,36]; however, this is a treatment reserved for patients with severe and drug-refractory depression. A randomized controlled trial investigating cognitive behavioral therapy has also demonstrated improvement of depression scores [37]. The role of physical activity as treatment for depression in PD patients is unclear. As described in a recent review by Loprinzi et al [38], the literature is contradictory, with one group experiencing reduced depression but with no signficant effect in several other studies.
Anxiety
Epidemiology and Diagnosis
The prevalence of anxiety in PD patients is about 40% [39], which is 2 times greater than in the general population [9]. Anxiety may worsen PD symptoms, especially tremor and cognition. Risk factors for anxiety include the female gender, greater motor fluctuations, prior history of anxiety, and younger age of PD onset [40]. As with depression, some patients also report worsening of anxious symptoms during “off” states [41]. Screening tools that have been validated to help practitioners identify anxiety in PD include the Hospital Anxiety and Depression Scale, Beck Anxiety Inventory, Zung Self-rating Anxiety Scale, Spielberger State Trait Anxiety Inventory, and Hamilton Anxiety Rating Scale [15].
Treatment Options
The treatment of diagnosed anxiety in PD is primarily with benzodiazepines, which are particularly beneficial in patients whose tremors are exacerbated by anxiety or stress. The use of benzodiazepines has not been evaluated by a randomized controlled trial and use should be limited given the potential risks of sedation, cognitive effects, and psychomotor agitation. Other case studies have found benefit with serotonergic medications like fluoxetine or citalopram (especially with concomitant depression) or with optimization of levodopa therapy [42,43].
Hallucinations, Delusions, and Psychosis
Epidemiology
The prevalence of visual hallucinations in PD patients is about 20% to 40% [44,45]. Risk factors for psychotic symptoms include cognitive impairment, advanced age, prolonged duration of disease, depression, severe dysautonomia, and sleep disorders [46–48]. Early recognition of hallucinations is critical because of a strong correlation between the manifestation of psychosis and the need for nursing home placement or hospitalization. With early and effective treatment there is a decreased need for placement and a reduction on caregiver burden [44,49].
Treatment Options
Hallucinations can occur in delirium and it is important to first rule out an underlying infection or an offending medication, especially if there is a sudden onset or worsening of symptoms. Psychotic symptoms have been reported in drug-naive patients, though they are often iatrogenically induced with dopaminergic agents. All antiparkinsonian medications are capable of inducing or exacerbating hallucinations [9,50]. Additionally, psychotic symptoms tend to improve when dopaminergic agonists are reduced or eliminated. However, there is no clear relationship between the dose of dopaminergic agents and manifestation of hallucinations [48,51,52]. If hallucinations persist or there are motor complications that arise from reduction of dopaminergic agents, initiation of clozapine has been demonstrated to be efficacious in a rater-blinded prospective study and in a retrospective analysis [53–55]; however, regular monitoring for neutropenia is required. Quetiapine has demonstrated similar benefit without significant effects on motor symptoms in a randomized, rater-blinded study and in an evidence-based review [56,57]. It is also important to review or eliminate other medications that may contribute to hallucinations.
Cognitive Impairment
Epidemiology
The prevalence of dementia in the PD population is 20% to 40% [58], though almost 80% of PD patients ultimately develop cognitive decline [59]. Overall, a PD patient is 6 times more likely to develop dementia than someone in the general population [60]. There may be parallel progression of cognitive impairment and motor symptoms, but there is no correlation with overall duration of disease [60,61]. Risk factors linked with the presence of dementia include older age at onset of PD, presence of hallucinations, and male gender [62,63].
Cognitive dysfunction can be detected early in PD through neuropsychological testing; however, impairment of cognition is often insidious and may not be appreciated until symptoms become severe. Several screening tools have been used to evaluate for cognitive impairment in PD including the Mini-Mental State Exam (MMSE), Montreal Cognitive Assessment (MoCA), Mini-Mental Parkinson, Scales for Outcomes of Parkinson’s disease–Cognition, and others. Accumulating evidence, however, is suggestive of the superiority of the MoCA in the detection of cognitive deficits associated
with PD [64].
Dementia is a substantial burden for the caregiver and is a significant contributor to mortality in PD patients [65]. Cognitive impairment often presents with other behavioral symptoms, which further hastens placement outside the home and increases cost of caring for PD patients [49,66].
Cognitive impairment in Parkinson’s disease is typically associated with degeneration of primarily subcortical structures. PD patients with mild cognitive impairment were found to have deficits most significantly in memory, executive function, memory, and language abilities [67]. A recent study by Mak et al evaluated grey matter volumes by structural MRI in PD patients with evidence of mild cognitive impairment by MMSE and MoCA as compared with findings in cognitively intact patients. This demonstrated decreased brain volumes in areas that correlate with affected cognitive domains including the left insula, left superior frontal and left middle temporal areas [68].
Treatment Options
Prior to initiation of therapy, it is important to evaluate the patient for depression and to rule out pseudodementia. Bradyphrenia, or slowness of thought, should also be considered, as this symptom may also lead to an incorrect dementia diagnosis. Lastly, a thorough review of medications should be performed and offending agents including anticholinergics, TCAs, dopamine agonists, and amantadine should be discontinued as these can worsen cognition.
Rivastigmine has demonstrated modest improvement in cognitive performance in PD patients with dementia in a large multicenter, placebo-controlled study [69]. Other cholinesterase inhibitors (ie, donepezil or galantamine) are not recommended at this time due to limited studies or contradictory results in the literature [31,54]. Caution is advised with use of cholinesterase inhibitors as they may worsen tremor or autonomic dysfunction; also, use is limited by nausea or other gastrointestinal symptoms. Memantine, an NMDA receptor antagonist, has also been investigated in randomized, double-blind, placebo-controlled trials and demonstrated modest improvement of cognition and is generally well tolerated [70,71].
Nonpharmacologic therapy includes physical exercise, which has demonstrated improvement in memory tasks and processing speed [72]. Cognitive training has been less rigorously studied; however, a recent single-blinded controlled study demonstrated significant improvement of learning and memory in PD patients who completed computer-based cognitive training [73].
Compulsive Disorders
Impulse Control Disorders
Impulse control disorders (ICDs) are inappropriate behaviors resulting from a failure to resist an impulse, which leads to pleasure-seeking activities at the expense of relationships and ability to function socially. In PD, ICDs are expressed as pathologic gambling, hypersexuality, binge eating, compulsive shopping, and excessive spending [9,66]. The prevalence of all ICDs in PD is 15% to 20% and a patient may be diagnosed with multiple ICDs [74]. Dopamine agonist use has been implicated in the development of ICDs and this risk is further increased with the addition of levodopa [75,76]. Clinical features associated with ICDs include young age of onset, male gender, family history of addiction, depression or anxiety, and disinhibition or impulsive traits [77,78].
Traditionally, treatment consists of reduction or elimination of dopamine agonists, though adjustment of levodopa therapy may also be necessary. Amantadine as an adjunct therapy has been shown in a randomized, double-blind crossover study to reduce impulsivity in a few patients with pathologic gambling [79].
Dopamine Dysregulation Syndrome
Dopamine dysregulation syndrome (DDS) is characterized by compulsive use of dopaminergic medications beyond what is needed to treat parkinsonian symptoms, and is associated with social impairment. Patients describe addictive symptoms like craving or intense desire to obtain more dopaminergic medication [9,74]. Like ICDs, treatment of DDS consists of modification to dopaminergic medications, though patients with DDS may also require psychiatric evaluation and treatment.
Punding
Punding is another compulsive disorder that is defined as an intense fascination with objects and is associated with repetitive handling, manipulation, sorting, or arrangement of the items [80]. Occurrence of punding has been associated with higher total daily levels of levodopa, although one study has also implicated dopamine agonists [15,81]. As with the other compulsive disorders, punding also tends to respond well to reduction or discontinuation of levodopa. Studies have demonstrated modest benefit with SSRIs or atypical antipsychotics in long-term follow-up [82,83], though one study reported worsening of punding with quetiapine [84].
Apathy
Epidemiology and Treatment
Apathy is often characterized by a loss of motivation or inability to initiate goal-directed behavior, which results in dependence on others for activities of daily living and increases caregiver burden [85]. Patients demonstrate indifference, lack of interest, or inability to express or describe emotion. The apathetic patient may lack spontaneous and voluntary activity, and their affect display is often flattened [86].
With a prevalence of 30% to 50% [87], apathy is as common as depression in PD patients [66,88]. Risk factors associated with apathy include advanced age, severity of depression, severity of motor dysfunction, and dementia [89]. Apathy is frequently mistaken for depression given the significant overlap in symptoms; however, the patient with pure apathy will deny sadness or depressed feelings. It is also important to distinguish apathy from motor impairment or cognitive dysfunction that could explain the behavioral changes. No medications have reliably been shown to improve apathy, though it may be improved with initiation of dopaminergic therapy, especially early in the course [86,90].
Sleep Disorders
The original report of PD by James Parkinson describes sleep disturbances and daytime somnolence [91], which suggests that sleep disorders may be an intrinsic feature of the neurodegenerative process of PD itself.
REM Behavioral Disorder
Epidemiology and Diagnosis
Rapid eye movement behavioral disorder (RBD) is a parasomnia characterized by vocalizations and motor activity during dreaming due to loss of normal atonia associated with rapid eye movement (REM) sleep. Patients enact their dreams, which may lead to violent behaviors that can injure the patient or their bed partner. RBD is seen in 25% to 50% of PD patients [92,93], with variability depending on diagnostic technique and patient selection. Polysomnography is the most important diagnostic tool and demonstrates increased chin tone and limb movements during REM sleep in RBD [94,95]. Diagnosis can also be made clinically with patient and bed partner reports, though sensitivity is only approximately 30% [15].
Interestingly, many studies are now investigating the relationship between presence of RBD and later onset of neurodegenerative disorders. Multiple studies have shown that 40% to 65% of patients diagnosed with idiopathic RBD later develop an alpha-synucleinopathy, which includes PD, dementia with Lewy bodies, or multiple system atrophy within 10 years [92,95]. Prior studies report that as many as 90% of patients with idiopathic RBD develop neurodegenerative synucleinopathy when followed over 14 years [96]. Idiopathic RBD is currently being investigated as a potential clinical marker of pre-symptomatic PD in a multicenter observational study. If RBD is an early marker for neurodegenerative disease, it may be used to identify patients for neuroprotective trials as treatments are developed.
Treatment Options
Low-dose clonazepam (0.25–1 mg) is the mainstay of therapy, especially for patients that injure themselves or bed partners [97]; however, the use of benzodiazepines is historical and there remain no randomized controlled double-blind studies to evaluate the efficacy of clonazepam. Use of clonazepam may be limited by daytime sedation, confusion, or psychomotor agitation [31,97,98]. Melatonin (doses between 3–12 mg at bedtime) has also demonstrated benefit in RBD in a double-blind, placebo-controlled trial and in a small case series, with fewer side effects and no addiction potential as compared to clonazepam [99,100]. Case reports also support the use of several other effective medications, including cholinesterase inhibitors (rivastigmine and donepezil) and dopaminergic agents (pramipexole and levodopa) [15,20].
Restless Leg Syndrome and Periodic Limb Movements in Sleep
Epidemiology
Restless leg syndrome (RLS) and periodic limb movements in sleep (PLMS) cause disruptions of sleep and have an important impact on quality of sleep in PD patients. RLS is described as a strong urge to move the legs, accompanied by an uncomfortable sensation that is exacerbated at rest and relieved by movement. RLS is more frequently diagnosed in patients with PD, though prevalence reports vary widely [15]. Secondary causes for RLS should be investigated including iron deficiency, uremia and polyneuropathy. Several case reports demonstrate onset or worsening of RLS with use of antidepressants [101, 102] or antipsychotics like risperidone, aripiprazole, and quetiapine [103,104].
PLMS occurs in approximately 80% to 90% of patients with RLS, though may be present independently, and when seen on polysomnography is supportive of RLS [105]. PLMS is characterized by repetitive dorsiflexion of the foot, extension of the great toe, and may be accompanied by flexion of the knee and hip. The prevalence of PLMS in PD is approximately 60% and correlates with severity of PD motor features [106].
Treatment Options
Treatment of RLS should be initiated with nonpharmacologic therapies including good sleep hygiene, exercise, leg massage, and heat or ice packs [105,107]. Dopamine (DA) agonists are the primary treatment for RLS; however, even modest adjustments in levodopa can be helpful. One drawback to levodopa therapy is augmentation (a worsening or reappearance of symptoms) when serum levels fall due to the short half-life of levodopa [107,108]. DA agonists are less likely to cause augmentation. Both pramipexole and ropinirole have been extensively investigated in controlled, randomized, double-blind studies with benefits in 70% to 90% of patients with RLS and PLMS; however, there is a risk of developing compulsive behaviors [109–112]. Another option for PD patients is rotigotine, which has demonstrated improvement of RLS symptoms in a randomized, double-blind, placebo-controlled trial and has the added benefit that it may also help with motor symptoms [113,114].
More recently, gabapentin enacarbil has demonstrated improvement of moderate to severe RLS and was well tolerated in multiple randomized, double-blind, placebo-controlled trials [107,115,116]. Lastly, opioids (tramadol, oxycodone, codeine) have been shown to be effective, especially in the treatment of RLS that is refractory to other treatments [105,107].
Insomnia
Epidemiology
The most common sleep disorder in PD is insomnia, with a prevalence between 37% to 88% [14,117]. Insomnia is associated with difficulty in initiation or maintenance of sleep. Disruption of sleep typically leads to daytime somnolence and patient reports of a strong impact on motor disability and overall quality of life. There are several contributors to insomnia in PD patients including nocturia, depression, RLS, dystonia, and akinesia/rigidity/difficulty turning in bed [118].
Treatment Options
The use of carbidopa/levodopa controlled-release formulations at bedtime is associated with improved sleep duration and nocturnal akinesia, although it does not demonstrate a significant improvement in overall sleep ratings [54]. Hypnotics like eszopiclone and zolpidem have also demonstrated improved quality of sleep in limited controlled trials and a meta-analysis, but use is limited by sedation, dizziness, and falls [54,119]. Benzodiazepines improve sleep latency, but there is a risk of cognitive impairment, tolerance, and falls [117,120]. Melatonin at 3 to 5 mg and 50 mg doses have been investigated in 2 randomized, double-blind, placebo-controlled trials; however, there was a modest benefit and it was concluded that there is insufficient evidence to support the use of melatonin [54]. Nevertheless, melatonin is well tolerated and may be tried with minimal risk [54]. More recently, a randomized controlled trial using doxepin has demonstrated improvement of insomnia scores and was generally well tolerated [121].
Excessive Daytime Sleepiness and Abrupt Sleep Onset
EDS and Fatigue: Epidemiology and Treatment
A common complaint by PD patients is excessive daytime sleepiness (EDS), which can be verified with multiple sleep latency testing. EDS frequency varies in the literature, but is seen in approximately 15% to 50% of PD patients [4,122]. The etiology is usually multifactorial, with insomnia, dysautonomia, and depression as contributing factors [117]. A longer duration of symptoms, greater total load of levodopa, cognitive decline, and male gender are all risk factors for EDS [122,123]. It has been proposed that EDS is an intrinsic feature of PD; however, there is also an association with the use of antiparkinsonian medications. A randomized controlled trial demonstrated that use of the dopamine agonist pramipexole was associated with greater somnolence as compared to levodopa therapy (35% vs. 13%); however, this difference was only seen during the initial escalation phase [124]. Additionally, the combined use of dopamine agonists and levodopa has shown an even greater risk of EDS [125]. The evidence for the use of stimulants for EDS is lacking. The few studies conducted with modafinil have not demonstrated a robust improvement of EDS [126–128]. Other stimulants like methylphenidate have been studied with improvement of Epworth Sleepiness Score, though no randomized control trials have been undertaken [129].
It is important to distinguish EDS, a propensity for daytime sleep, from fatigue or excessive tiredness associated with mental or physical exertion [117]. Fatigue is often multifactorial and may be related to insomnia, sleep apnea, sedating effects of medications, frequent awakenings from nocturia, and degeneration of brain areas regulating sleep/wake cycles related to the underlying disease process [20, 117]. It is also important to consider depression and dementia in the differential, as these disorders may be erroneously be diagnosed as fatigue. Treatment of fatigue should include regular mild exercise, maintenance of a stimulating environment, removal of sedating medications, and management of intrinsic sleep disorders if present [117]. The use of stimulants for fatigue is controversial. A small randomized controlled trial (n = 48) using modafinil demonstrated improvement on the global clinical impression scale for fatigue but no significant change on the Fatigue Severity Scale; this study was limited by the power and points to the need for a larger study [130].
Sleep Attacks: Epidemiology and Treatment
Abrupt sleep onset, or “sleep attacks,” occurs when transition from wake to sleep is unavoidable and may occur without warning. Sleep attacks are threefold more likely to occur in patients using DA agonists, with an associated dose-related increase in risk [131]. Adjustment or elimination of DA agonists often improves sleep attacks, though it is important to address concurrent EDS if present. Nonpharmacologic treatments to consider include mild exercise, early morning bright light exposure, and a stimulating environment [117].
Sleep-Disordered Breathing/Obstructive Sleep Apnea
Epidemiology and Treatment
Sleep-disordered breathing (SDB) consists of either a deficit in the drive to breathe as in central sleep apnea, or may be due to an blockage of the airway as seen in obstructive sleep apnea (OSA). Apnea leads to oxygen desaturations that consequently trigger awakenings throughout the night, which in turn is experienced by the patient as daytime somnolence [117]. The prevalence of SDB and OSA is variable in the literature, ranging from no increased risk in PD patients [132,133] to 50% prevalence in PD patients [134,135]. Discussions with bed partners, history of snoring, and clinical reports of EDS or daytime fatigue are important indicators of SDB. Polysomnography confirms the diagnosis and can direct treatment, which frequently includes application of CPAP devices during sleep.
Autinomic Dysfunction
Orthostatic Hypotension
Epidemiology and Diagnosis
Orthostatic hypotension (OH) is defined as a 20-mm Hg fall in systolic blood pressure or 10-mm Hg drop in diastolic blood pressure within 3 minutes of a change in position. The prevalence of OH in PD patients is 30% to 60% [136,137]. Symptoms of OH can occur early in the disease and may precede diagnosis of PD [137]. Patients experience OH as dizziness, drowsiness, palpitations, nausea, or loss of consciousness. Additionally, falls and supine hypertension that accompany OH are associated with increased risk of morbidity and mortality in PD patients [138]. Several medications used in the treatment of PD can exacerbate OH, including levodopa, DA agonists, MAO-B inhibitors, and TCAs [139].
Treatment Options
First-line therapies for OH include nonpharmacologic methods such as compression stockings, sleeping with head elevated to 30 degrees, increased water and salt intake, more frequent small meals, and slowly changing position [140]. Additionally, it is important to discuss the removal or reduction of all antihypertensives with the patient’s PCP. Fludrocortisone (a mineralacorticoid) and domperidone (a peripheral dopamine antagonist not currently approved for use in the United States) modestly improved OH in a 2-phase, randomized, controlled, double-blind, crossover trial [141]. Pyridostigmine has also demonstrated improvement of standing blood pressure and OH symptoms in a double-blind, randomized cross-over study and has the additional benefit of not worsening supine hypertension [142]. Other effective treatments include midodrine, per a randomized, double-blind multicenter study [143], as well as droxidopa in a double-blind, crossover, placebo-controlled study [144]. Currently there is insufficient evidence to support the preferential use of any specific agent in the treatment of OH in PD.
Gastrointestinal Dysmotility
Constipation: Epidemiology and Treatment
Constipation is reported by nearly 60% of PD patients [145]. Constipation can precede the development of motor symptoms of PD, and the prevalence of GI disturbances increases with age and longer duration of disease. Nearly one third of patients will have been diagnosed with a GI disturbance within the year prior to PD diagnosis [146], which is associated with an increased risk for the development PD [147]. People with constipation (defined as < 1 bowel movement per day) but without a PD diagnosis had more nigral Lewy body degeneration postmortem [148] compared with people without constipation.
Treatments for constipation include dietary modification, increased fluid intake, and mild exercise. Macrogol significantly improved constipation in PD patients and was very well tolerated in a randomized placebo-controlled study [149]. Lubiprostone, a GI active prostaglandin, is also effective in the short-term treatment of constipation in a placebo-controlled trial [150].
Gastroparesis: Epidemiology and Treatment
Gastroparesis, like constipation, is related to enteric dopaminergic cell loss and degeneration of the dorsal motor nucleus of the vagus [151]. Patients experience gastroparesis as early satiety, full sensation, and nausea. Decreased gastric motility leads to retention of food as well as medications, which can slow absorption and delay onset of action for many medications including levodopa. Domperidone has both prokinetic and antiemetic properties, which have been beneficial in the treatment of gastroparesis [152], but its use is not currently approved in the United States.
Dysphagia: Epidemiology and Treatment
Dysphagia is associated with more advanced stages of PD as well as a significant increase in morbidity. Swallow exercises have demonstrated improvement of dysphagia [153]. The impact of levodopa therapy on dysphagia in the literature is controversial. Videofluoroscopic examination is the most common method for evaluation of swallowing disorders and provides important information for speech-language pathologists regarding recommendations for dietary modifications [154]. Adjustment of medication regimens to avoid an oral route is also helpful. This includes Parcopa, orally disintegrating carbidopa/levodopa tablets, and transdermal approaches like the rotigotine patch. For some patients, enteral nutrition is needed and placement of nasogastric tubes or percutaneous endoscopic gastrostomy tubes are an option.
Sialorrhea (Drooling)
Epidemiology
Difficulty handling oral secretions due to impaired or infrequent swallowing results in sialorrhea in up to 75% of PD patients [155], which is a significant embarrassment for most patients [156]. PD patients with drooling have difficulty speaking, eating, and engaging in social interactions, which significantly impacts perceived quality of life [157].
Treatment Options
Botulinum toxin (A and B) injections into the submandibular or parotid glands have demonstrated efficacy in multiple double-blind, randomized, placebo-controlled studies for the treatment of sialorrhea in PD patients; however, injections are associated with greater invasiveness and cost [158–160]. Glycopyrrolate, an anticholinergic drug, was also efficacious in the treatment of sialorrhea in the short term in a double-blind, randomized, placebo-controlled study [161]. Alternatively, gum chewing increases swallow frequency, improves drooling, and also shows a benefit with dysphagia [162].
Genitourinary Disturbances
Bladdery dysfunction: Epidemiology and Treatment
Bladder dysfunction in PD is often secondary to hyperactivity of the detrusor muscle leading to urinary urgency, increased urinary frequency, and nocturia. Less commonly, hypoactive detrusor muscle causes difficulty with initiation of urination, delayed bladder emptying, and recurrent infections. Urinary disturbances may occur before the onset of motor symptoms or early on in the disease course [12]. Disease severity is associated with greater urinary disturbances, and more than 50% of advanced PD patients report severe bladder symptoms [163].
Anticholinergic medications such as oxybutynin, solifenacin, and tolterodine are commonly used in the treatment of detrusor hyperactivity and demonstrate significant improvement in detrusor pressure in a recent systemic review and meta-analysis [164]. PD patients on these agents should be closely monitored for side effects including cognitive impairment, somnolence, hallucinations, confusion, and blurred vision. Other treatments include botulinum toxin injections into the detrusor muscle, which has demonstrated safety and efficacy in a recent systematic review [165].
Erectile dysfunction: Epidemiology and Treatment
Erectile dysfunction (ED) is reported by more than 60% of male PD patients [145] and is thought to be related to hypothalamic dysfunction and modification of the dopamine-oxytocin pathway [166]. Effects of PD medications, cognitive impairment, fatigue, apathy, and low testosterone contribute to loss of libido and ED [20,167]. Phosphodiesterase inhibitors such as sildenafil, vardenafil, and tadalafil are possibly useful in the treatment of ED in PD patients, though randomized trials have been limited [166,168]. Apomorphine sublingually is another medication that has demonstrated improvement of ED in a double-blind, crossover study and can be considered for patients with contraindications to phosphodiesterase inhibitors [169].
Sensory Symptoms
Pain
Epidemiology
Sensory disturbances in PD include diminished ability to identify odors, visual abnormalities (blurred vision, abnormal color perception, double vision), and pain. Pain is the most disabling sensory disturbance, though frequently underreported. Nearly two thirds of PD patients report pain, [170], though only half of patients receive any treatment [171]. Pain may also be a presenting symptom that precedes the clinical diagnosis of PD [172,173].
Treatment Options
There are several types of pain described by PD patients, the most common of which is musculoskeletal, typically involving the shoulder. Other types include dystonic, radicular, and central pain [174]. First-line treatment of musculoskeletal complaints includes nonsteroidal anti-inflammatory drugs (NSAIDs) and physiotherapy. Modification of levodopa regimen (including altering timing and frequency or adding controlled release formulations) can often provide relief for dystonic pain, and also for central pain for some patients [173, 174]. Deep brain stimulation, with subthalamic nucleus or globus pallidus targets, has demonstrated improvement with dystonic, central, and musculoskeletal pain in a small clinical study [175].
Conclusion
NMS are an intrinsic part of PD, may predate diagnosis, and substantially affect the majority of patients with PD. For many of these patients, NMS have a greater impact on quality of life and health care costs than the cardinal motor symptoms that define the disease. Many of these symptoms are not recognized by practioners and often are not volunteered by PD patients, making it important for practitioners to routinely and directly inquire about NMS. Treatment of NMS in PD is challenging, and only a few therapies have the level of evidence needed to support their use in the treatment of these problems. Nevertheless, proper recognition and addressing of these symptoms afford the clinician an opportunity to make a positive and potentially significant impact on the PD patient’s quality of life.
Corresponding author: Samay Jain, MD, MS, Dept of Neurology, 811 Kaufmann Bldg, Pittsburgh, PA 15213, [email protected].
Financial disclosures: None.
1. Alves G, Forsaa EB, Pedersen KF, et al. Epidemiology of Parkinson's disease. J Neurol 2008;255 Suppl 5:18–32.
2. Stern MB, Lang A, Poewe W. Toward a redefinition of Parkinson's disease. Mov Disord 2012;27:54–60.
3. Braak H, Del Tredici K, Rub U, et al. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging 2003;24:197–211.
4. Tandberg E, Larsen JP, Karlsen K. A community-based study of sleep disorders in patients with Parkinson's disease. Mov Disord 1998;13:895–9.
5. Shulman LM, Taback RL, Bean J, Weiner WJ. Comorbidity of the nonmotor symptoms of Parkinson's disease. Mov Disord 2001;16:507–10.
6. Park A, Stacy M. Non-motor symptoms in Parkinson's disease. J Neurol 2009;256 Suppl 3:293–8.
7. Chaudhuri KR, Schapira AH. Non-motor symptoms of Parkinson's disease: dopaminergic pathophysiology and treatment. Lancet Neurol 2009;8:464–74.
8. Shulman LM, Taback RL, Rabinstein AA, Weiner WJ. Non-recognition of depression and other non-motor symptoms in Parkinson's disease. Parkinsonism Relat Disord 2002;8:193–7.
9. Bonnet AM, Jutras MF, Czernecki V, et al. Nonmotor symptoms in Parkinson's disease in 2012: relevant clinical aspects. Parkinsons Dis 2012:2012:198316.
10. Chaudhuri KR, Healy DG, Schapira AH. Non-motor symptoms of Parkinson's disease: diagnosis and management. Lancet Neurol 2006;5:235–45.
11. Hussl AK, Seppi K, Poewe W. Nonmotor symptoms in Parkinson's disease. Expert Rev Neurother 2013;13:581–3.
12. O'Sullivan SS, Williams DR, Gallagher DA, et al. Nonmotor symptoms as presenting complaints in Parkinson's disease: a clinicopathological study. Mov Disord 2008;23:101–6.
13. Lang AE. A critical appraisal of the premotor symptoms of Parkinson's disease: potential usefulness in early diagnosis and design of neuroprotective trials. Mov Disord 2011;26:775–83.
14. Barone P, Antonini A, Colosimo C, et al. The PRIAMO study: A multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson's disease. Mov Disord 2009;24:1641–9.
15. Bernal-Pacheco O, Limotai N, Go CL, Fernandez HH. Nonmotor manifestations in Parkinson disease. Neurologist 2012;18:1–16.
16. Lemke MR, Fuchs G, Gemende I, et al. Depression and Parkinson's disease. J Neurol 2004;251 Suppl 6: VI/24–7.
17. Ravina, B, Camicioli R, Como PG, et al, The impact of depressive symptoms in early Parkinson disease. Neurology 2007;69:342–7.
18. Jasinska-Myga B, Putzke JD Wider C, et al. Depression in Parkinson's disease. Can J Neurol Sci 2010;37:61–6.
19. Slaughter JR, Slaughter KA, Nichols D, et al. Prevalence, clinical manifestations, etiology, and treatment of depression in Parkinson's disease. J Neuropsychiatry Clin Neurosci 2001;13:187–96.
20. Simuni T, Sethi K. Nonmotor manifestations of Parkinson's disease. Ann Neurol 2008;64 Suppl 2:S65–80.
21. Ehmann TS, Beninger RJ, Gawel MJ, Riopelle RJ. Depressive symptoms in Parkinson's disease: a comparison with disabled control subjects. J Geriatr Psychiatry Neurol 1990;3:3–9.
22. Menza MA, Mark MH. Parkinson's disease and depression: the relationship to disability and personality. J Neuropsychiatry Clin Neurosci 1994;6:165–9.
23. CDC. Current depression among adults–United States, 2006 and 2008. Morb Mort Weekly Rep 2010;59:1229–35.
24. Hinnell C, Hurt CS, Landau S, et al. Nonmotor versus motor symptoms: how much do they matter to health status in Parkinson's disease? Mov Disord 2012;27:236–41.
25. Cummings JL. Depression and Parkinson's disease: a review. Am J Psychiatry 1992;149:443–54.
26. Weintraub D, Morales KH, Moberg PJ, et al. Antidepressant studies in Parkinson's disease: a review and meta-analysis. Mov Disord 2005;20:1161–9.
27. Richard IH, McDermott MP, Kurlan R, et al. A randomized, double-blind, placebo-controlled trial of antidepressants in Parkinson disease. Neurology 2012;78:1229–36.
28. Menza M, Dobkin RD, Marin H, et al. A controlled trial of antidepressants in patients with Parkinson disease and depression. Neurology 2009;72:886–92.
29. Okun MS, Fernandez HH. Will tricyclic antidepressants make a comeback for depressed Parkinson disease patients? Neurology 2009;72:868–9.
30. Antonini A, Tesei S, Zecchinelli A, et al. Randomized study of sertraline and low-dose amitriptyline in patients with Parkinson's disease and depression: effect on quality of life. Mov Disord 2006;21:1119–22.
31. Pedrosa DJ, Timmermann L. Review: management of Parkinson's disease. Neuropsychiatr Dis Treat 2013;9: 321–40.
32. Barone P, Poewe W, Albrecht S, et al. Pramipexole for the treatment of depressive symptoms in patients with Parkinson's disease: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 2010;9:573–80.
33. Lemke MR, Brecht HM, Koester J, et al. Anhedonia, depression, and motor functioning in Parkinson's disease during treatment with pramipexole. J Neuropsychiatry Clin Neurosci 2005;17:214–20.
34. Rektorova I, Balaz M, Svatova J, et al. Effects of ropinirole on nonmotor symptoms of Parkinson disease: a prospective multicenter study. Clin Neuropharmacol 2008;31:261–6.
35. Van der Wurff FB, Stek ML, Hoogendijk WL, Beekman AT. Electroconvulsive therapy for the depressed elderly. Cochrane Database Syst Rev 2003(2):CD003593.
36. Okun MS, Watts RL. Depression associated with Parkinson's disease: clinical features and treatment. Neurology 2002;58(4 Suppl 1):S63–70.
37. Dobkin RD, Menza M, Allen LA, et al. Cognitive-behavioral therapy for depression in Parkinson's disease: a randomized, controlled trial. Am J Psychiatry 2013;168:1066–74.
38. Loprinzi PD, Herod SM, Cardinal BJ, Noakes TD. Physical activity and the brain: A review of this dynamic, bi-directional relationship. Brain Res 2013;1539:95–104.
39. Richard IH. Anxiety disorders in Parkinson's disease. Adv Neurol 2005;96:42–55.
40. Leentjens AF, Dujardin K, Marsh L, et al. Symptomatology and markers of anxiety disorders in Parkinson's disease: a cross-sectional study. Mov Disord 2011;26:484–92.
41. Witjas T, Kaphan E, Azulay JP, et al. Nonmotor fluctuations in Parkinson's disease: frequent and disabling. Neurology 2002;59:408–13.
42. Olanow CW, Stern MB, Sethi K. The scientific and clinical basis for the treatment of Parkinson disease Neurology 2009;72(21 Suppl 4):S1–136.
43. Richard IH, Schiffer RB, Kurlan R. Anxiety and Parkinson's disease. J Neuropsychiatry Clin Neurosci 1996;8:383–92.
44. Fenelon G, Alves G. Epidemiology of psychosis in Parkinson's disease. J Neurol Sci 2010;289:12–7.
45. Papapetropoulos S, Katzen H, Schrag A, et al. A questionnaire-based (UM-PDHQ) study of hallucinations in Parkinson's disease. BMC Neurol 2008;8:21.
46. Fenelon G, Mahieux F, Huon R, Ziegler M. Hallucinations in Parkinson's disease: prevalence, phenomenology and risk factors. Brain 2000;123 (Pt 4):733–45.
47. Papapetropoulos S, Mash DC. Psychotic symptoms in Parkinson's disease. From description to etiology. J Neurol 2005;252:753–64.
48. Aarsland D, Larsen JP, Cummins JL, Laake K. Prevalence and clinical correlates of psychotic symptoms in Parkinson disease: a community-based study. Arch Neurol 1999;56:595–601.
49. Aarsland D, Larsen JP, Tandberg E, Laake K. Predictors of nursing home placement in Parkinson's disease: a population-based, prospective study. J Am Geriatr Soc 2000;48:938–42.
50. Lohle M, Storch A, Reichmann H, Beyond tremor and rigidity: non-motor features of Parkinson's disease. J Neural Transm 2009;116:1483–92.
51. Fenelon G. Psychosis in Parkinson's disease: phenomenology, frequency, risk factors, and current understanding of pathophysiologic mechanisms. CNS Spectr 2008;13(3 Suppl 4):18–25.
52. Merims D, Shabtai H, Korczyn AD, et al. Antiparkinsonian medication is not a risk factor for the development of hallucinations in Parkinson's disease. J Neural Transm 2004;111:1447–53.
53. Merims D, Balas M, Peretz C, et al. Rater-blinded, prospective comparison: quetiapine versus clozapine for Parkinson's disease psychosis. Clin Neuropharmacol 2006;29:331–7.
54. Seppi K, Weintraub D, Coelho M, et al. The Movement Disorder Society Evidence-Based Medicine Review Update: Treatments for the non-motor symptoms of Parkinson's disease. Mov Disord 2011;26 Suppl 3:S42–80.
55. Fernandez HH, Donnelly EM, Friedman JH. Long-term outcome of clozapine use for psychosis in parkinsonian patients. Mov Disord 2004;19:831–3.
56. Morgante L, Epifanio A, Spina E, et al. Quetiapine and clozapine in parkinsonian patients with dopaminergic psychosis. Clin Neuropharmacol 2004;27:153–6.
57. Miyasaki JM, Shannon K, Voon V, et al. Practice parameter: evaluation and treatment of depression, psychosis, and dementia in Parkinson disease (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2006;66:996–1002.
58. Riedel O, Klotsche J, Spottke A, et al. Cognitive impairment in 873 patients with idiopathic Parkinson's disease. Results from the German Study on Epidemiology of Parkinson's Disease with Dementia (GEPAD). J Neurol 2008;255:255–64.
59. Aarsland D, Andersen K, Larsen JP, et al. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch Neurol 2003;60:387–92.
60. Aarsland D, Andersen K, Larsen JP, et al. Risk of dementia in Parkinson's disease: a community-based, prospective study. Neurology 2001;56:730–6.
61. Riggeal BD, Crucian GP, Seignourel P, et al. Cognitive decline tracks motor progression and not disease duration in Parkinson patients. Neuropsychiatr Dis Treat 2007;3:955–8.
62. Hughes TA, Ross HF, Musa S, et al. A 10-year study of the incidence of and factors predicting dementia in Parkinson's disease. Neurology 2000;54:1596–602.
63. Hobson P, Meara J. Risk and incidence of dementia in a cohort of older subjects with Parkinson's disease in the United Kingdom. Mov Disord 2004;19:1043–9.
64. Hoops S, Nazem S, Siderowf AD, et al. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology 2009;73:1738–45.
65. Louis ED, Marder K, Cote L, et al. Mortality from Parkinson disease. Arch Neurol 1997;54:260–4.
66. Fernandez HH. Nonmotor complications of Parkinson disease. Cleve Clin J Med 2012;79 Suppl 2:S14–8.
67. Sollinger AB, Goldstein FC, Lah JJ, et al. Mild cognitive impairment in Parkinson's disease: subtypes and motor characteristics. Parkinsonism Relat Disord 2010;16:177–80.
68. Mak E, Zhou J, Tan LC, et al. Cognitive deficits in mild Parkinson's disease are associated with distinct areas of grey matter atrophy. J Neurol Neurosurg Psychiatry 2013 Oct 16.
69. Emre M, Aarsland D, Albanese A, et al. Rivastigmine for dementia associated with Parkinson's disease. N Engl J Med 2004;351:2509–18.
70. Aarsland D, Ballard C, Walker Z, et al. Memantine in patients with Parkinson's disease dementia or dementia with Lewy bodies: a double-blind, placebo-controlled, multicentre trial. Lancet Neurol 2009;8:613–8.
71. Leroi I, Overshott R, Byrne EJ, et al. Randomized controlled trial of memantine in dementia associated with Parkinson's disease. Mov Disord 2009;24:1217–21.
72. Speelman AD, van de Warrenburg BP, van Nimwegen M, et al. How might physical activity benefit patients with Parkinson disease? Nat Rev Neurol 2013;7:528–34.
73. Naismith SL, Mowszowski L, Diamond K, Lewis SJ. Improving memory in Parkinson's disease: a healthy brain ageing cognitive training program. Mov Disord 2013;28:1097–103.
74. Weintraub D, Koester J, Potenza MN, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol 2010;67:589–95.
75. Voon V, Reynolds B, Brezing C, et al. Impulsive choice and response in dopamine agonist-related impulse control behaviors. Psychopharmacology (Berl) 2010;207:645–59.
76. Weintraub D, Siderowf AD, Potenza MN, et al. Association of dopamine agonist use with impulse control disorders in Parkinson disease. Arch Neurol 2006; 63:969–73.
77. Pontone G, Williams JR, Bassett SS, Marsh L. Clinical features associated with impulse control disorders in Parkinson disease. Neurology 2006;67:1258–61.
78. Voon V, Mehta AR, Hallett M. Impulse control disorders in Parkinson's disease: recent advances. Curr Opin Neurol 2011;24:324–30.
79. Thomas A, Bonanni L, Gambi F, et al. Pathological gambling in Parkinson disease is reduced by amantadine. Ann Neurol 2010;68:400–4.
80. Miyasaki JM, Al Hassan K, Lang AE, Voon V. Punding prevalence in Parkinson's disease. Mov Disord 2007;22:1179–81.
81. Nguyen FN, Chang YL, Okun MS, et al. Prevalence and characteristics of punding and repetitive behaviors among Parkinson patients in North-Central Florida. Int J Geriatr Psychiatry 2010;25:540–1.
82. Sohtaoglu M, Demiray DY, Kenangil G, et al. Long term follow-up of Parkinson's disease patients with impulse control disorders. Parkinsonism Relat Disord 2010;16:334–7.
83. Antonini A, Cilia R. Behavioural adverse effects of dopaminergic treatments in Parkinson's disease: incidence, neurobiological basis, management and prevention. Drug Saf 2009;
32:475–88.
84. Miwa H, Morita S, Nakanishi I, Kondo T, Stereotyped behaviors or punding after quetiapine administration in Parkinson's disease. Parkinsonism Relat Disord 2004;10:177–80.
85. Skorvanek M, Rosenberger J, Gdovinova Z, et al. Apathy in elderly nondemented patients with parkinson's disease: clinical determinants and relationship to quality of life. J Geriatr Psychiatry Neurol 2013;26:237–43.
86. Marin RS, Fogel BS, Hawkins J, et al. Apathy: a treatable syndrome. J Neuropsychiatry Clin Neurosci 1995;7:23–30.
87. Pluck GC, Brown RG. Apathy in Parkinson's disease. J Neurol Neurosurg Psychiatry 2002;73:636–42.
88. Kirsch-Darrow L, Fernandez HH, Marsiske M, et al. Dissociating apathy and depression in Parkinson disease. Neurology 2006;67:33–8.
89. Pedersen KF, Alves G, Aarsland D, Larsen JP. Occurrence and risk factors for apathy in Parkinson disease: a 4-year prospective longitudinal study. J Neurol Neurosurg Psychiatry 2009;80:1279–82.
90. Czernecki V, Pillon B, Houeto JL, et al. Motivation, reward, and Parkinson's disease: influence of dopatherapy. Neuropsychologia 2002;40:2257–67.
91. Parkinson J. An essay on the shaking palsy. Sherwood, Neely, and Jones; 1817.
92. Schenck CH, Mahowald WM. REM sleep behavior disorder: clinical, developmental, and neuroscience perspectives 16 years after its formal identification in SLEEP. Sleep 2002;25:120–38.
93. Sixel-Doring F, Trautmann E, Mollenhauer B, Trenkwalder C. Associated factors for REM sleep behavior disorder in Parkinson disease. Neurology 2011;77:1048–54.
94. Eisensehr I, v Lindeiner H, Jager M, Noachtar S. REM sleep behavior disorder in sleep-disordered patients with versus without Parkinson's disease: is there a need for polysomnography? J Neurol Sci 2001;186:7–11.
95. Postuma RB, Gagnon JF, Montplaisir JY. REM sleep behavior disorder and prodromal neurodegeneration - where are we headed? Tremor Other Hyperkinet Mov (N Y) 2013;3.
96. Iranzo A, Tolosa E, Gelpi E, et al. Neurodegenerative disease status and post-mortem pathology in idiopathic rapid-eye-movement sleep behaviour disorder: an observational cohort study. Lancet Neurol 2013;12: 443–53.
97. Schenck CH, Mahowald MW. Rapid eye movement sleep parasomnias. Neurol Clin 2005;23:1107–26.
98. Anderson KN, Shneerson JM. Drug treatment of REM sleep behavior disorder: the use of drug therapies other than clonazepam. J Clin Sleep Med 2009;5:235–9.
99. Boeve BF, Silber MH, Ferman TJ. Melatonin for treatment of REM sleep behavior disorder in neurologic disorders: results in 14 patients. Sleep Med 2003;4:281–4.
100. Kunz D, Mahlberg R. A two-part, double-blind, placebo-controlled trial of exogenous melatonin in REM sleep behaviour disorder. J Sleep Res 2010;19:591–6.
101. Perroud N, Lazignac C, Baleydier B, et al. Restless legs syndrome induced by citalopram: a psychiatric emergency? Gen Hosp Psychiatry 2007;29:72–4.
102. Buskova J, Vorlova T, Pisko J, Sonka K, Severe sleep-related movement disorder induced by sertraline. Sleep Med 2012;13:769–70.
103. Rittmannsberger H, Werl R. Restless legs syndrome induced by quetiapine: report of seven cases and review of the literature. Int J Neuropsychopharmacol 2013;16:1427–31.
104. Perez-Lloret S, Rey MV, Bondon-Guitton E, et al. Drugs associated with restless legs syndrome: a case/noncase study in the French pharmacovigilance database. J Clin Psychopharmacol 2012;32:824–7.
105. Aurora RN, Kristo DA, Bista SR, et al. The treatment of restless legs syndrome and periodic limb movement disorder in adults-an update for 2012: practice parameters with an evidence-based systematic review and meta-analyses: an American Academy of Sleep Medicine Clinical Practice Guideline. Sleep 2012;35:1039–62.
106. Covassin N, Neikrug AB, Liu L, et al. Clinical correlates of periodic limb movements in sleep in Parkinson's disease. J Neurol Sci 2012;316:131–6.
107. Rios Romenets S, Postuma RB. Treatment of restless legs syndrome. Curr Treat Options Neurol 2013;15:396-409.
108. Hogl B, Paulus W, Clarenbach P, Trenkwalder C, Restless legs syndrome: diagnostic assessment and the advantages and risks of dopaminergic treatment. J Neurol 2006;253 Suppl 4:IV22-8.
109. Garcia-Borreguero D, Kohnen R, Silber MH, et al. The long-term treatment of restless legs syndrome/Willis-Ekbom disease: evidence-based guidelines and clinical consensus best practice guidance: a report from the International Restless Legs Syndrome Study Group. Sleep Med 2013;14:675–84.
110. Montagna P, Hornyak M, Ulfberg J, et al. Randomized trial of pramipexole for patients with restless legs syndrome (RLS) and RLS-related impairment of mood. Sleep Med 2011;12:34–40.
111. Partinen M, Hirvonen K, Jama L, et al. Efficacy and safety of pramipexole in idiopathic restless legs syndrome: a polysomnographic dose-finding study--the PRELUDE study. Sleep Med 2006;7:407–17.
112. Trenkwalder C Garcia-Borreguero D, Montagna P, et al. Ropinirole in the treatment of restless legs syndrome: results from the TREAT RLS 1 study, a 12 week, randomised, placebo controlled study in 10 European countries. J Neurol Neurosurg Psychiatry 2004;75:92–7.
113. Hening WA, Allen RP, Ondo WG, et al. Rotigotine improves restless legs syndrome: a 6-month randomized, double-blind, placebo-controlled trial in the United States. Mov Disord 2010;25:1675–83.
114. Oertel WH, Benes H, Garcia-Borreguero D, et al. Rotigotine transdermal patch in moderate to severe idiopathic restless legs syndrome: a randomized, placebo-controlled polysomnographic study. Sleep Med 2010;11:848–56.
115. Lee DO, Ziman RB, Perkins AT, et al. A randomized, double-blind, placebo-controlled study to assess the efficacy and tolerability of gabapentin enacarbil in subjects with restless legs syndrome. J Clin Sleep Med 2011;7:282–92.
116. Kushida CA, Walters AS, Becker P, et al. A randomized, double-blind, placebo-controlled, crossover study of XP13512/GSK1838262 in the treatment of patients with primary restless legs syndrome. Sleep 2009;32:159–68.
117. Menza M, Dobkin RD, Marin H, Bienfait K. Sleep disturbances in Parkinson's disease. Mov Disord 2010;25 Suppl 1:S117–22.
118. Louter M, van Sloun RJ, Pevernagie DA, et al. Subjectively impaired bed mobility in Parkinson disease affects sleep efficiency. Sleep Med 2013;14:668–74.
119. Menza M, Dobkin RD, Marin H, et al. Treatment of insomnia in Parkinson's disease: a controlled trial of eszopiclone and placebo. Mov Disord 2010;25:1708–14.
120. Nowell PD, Mazumdar S, Buysse DJ, et al. Benzodiazepines and zolpidem for chronic insomnia: a meta-analysis of treatment efficacy. JAMA 1997;278:2170–7.
121. Rios Romenets S, Creti L, Fichten C, et al. Doxepin and cognitive behavioural therapy for insomnia in patients with Parkinson's disease – a randomized study. Parkinsonism Relat Disord 2013;19:670–5.
122. Ondo WG, Dat Vuong K, Khan H, et al. Daytime sleepiness and other sleep disorders in Parkinson's disease. Neurology 2001;57:1392–6.
123. Razmy A, Lang AE, Shapiro CM, Predictors of impaired daytime sleep and wakefulness in patients with Parkinson disease treated with older (ergot) vs newer (nonergot) dopamine agonists. Arch Neurol 2004;61:97–102.
124. Holloway RG, Shoulson I, Fahn S, et al. Pramipexole vs levodopa as initial treatment for Parkinson disease: a 4-year randomized controlled trial. Arch Neurol 2004;61:1044–53.
125. Paus S, Brecht HM, Koster J, et al. Sleep attacks, daytime sleepiness, and dopamine agonists in Parkinson's disease. Mov Disord 2003;18:659–67.
126. Hogl B, Saletu M, Brandauer E, et al. Modafinil for the treatment of daytime sleepiness in Parkinson's disease: a double-blind, randomized, crossover, placebo-controlled polygraphic trial. Sleep 2002;25:905–9.
127. Ondo WG, Fayle R, Atassi F, Jankovic J. Modafinil for daytime somnolence in Parkinson's disease: double blind, placebo controlled parallel trial. J Neurol Neurosurg Psychiatry 2005;76:1636-–9.
128. Adler CH, Caviness JN, Hentz JG, et al. Randomized trial of modafinil for treating subjective daytime sleepiness in patients with Parkinson's disease. Mov Disord 2003;18:287–93.
129. Devos D, Krystkowiak P, Clement F, et al. Improvement of gait by chronic, high doses of methylphenidate in patients with advanced Parkinson's disease. J Neurol Neurosurg Psychiatry 2007;78:470–5.
130. Tyne HL, Taylor J, Baker GA, Steiger MJ. Modafinil for Parkinson's disease fatigue. J Neurol 2010;257:452–6.
131. Avorn J, Schneeweiss S, Sudarsky LR, et al. Sudden uncontrollable somnolence and medication use in Parkinson disease. Arch Neurol 2005;62:1242–8.
132. Trotti LM, Bliwise DL. No increased risk of obstructive sleep apnea in Parkinson's disease. Mov Disord 2010;25:2246–9.
133. da Silva-Junior FP, do Prado GF, Barbosa ER, et al. Sleep disordered breathing in Parkinson's disease: A critical appraisal. Sleep Med Rev 2013 Jul 22.
134. Noradina AT, Karim NA, Hamidon BB, et al. Sleep-disordered breathing in patients with Parkinson's disease. Singapore Med J 2010;51:60–4.
135. Oerlemans WG, de Weerd AW. The prevalence of sleep disorders in patients with Parkinson's disease. A self-reported, community-based survey. Sleep Med 2002;3:147–9.
136. Low PA. Prevalence of orthostatic hypotension. Clin Auton Res 2008;18 Suppl 1:8–13.
137. Goldstein DS. Orthostatic hypotension as an early finding in Parkinson's disease. Clin Auton Res 2006;16:46–54.
138. Sharabi Y, Goldstein DS. Mechanisms of orthostatic hypotension and supine hypertension in Parkinson disease. J Neurol Sci 2011;310:123–8.
139. Sanchez-Ferro A, Benito-Leon J, Gomez-Esteban JC. The management of orthostatic hypotension in Parkinson's disease. Front Neurol 2013;4:64.
140. Lahrmann H, Cortelli P, Hilz M, et al. EFNS guidelines on the diagnosis and management of orthostatic hypotension. Eur J Neurol 2006;13:930–6.
141. Schoffer KL, Henderson RD, O'Maley K, O'Sullivan JD. Nonpharmacological treatment, fludrocortisone, and domperidone for orthostatic hypotension in Parkinson's disease. Mov Disord 2007;22:1543–9.
142. Singer W, Sandroni P, Opfer-Gehrking TL, et al. Pyridostigmine treatment trial in neurogenic orthostatic hypotension. Arch Neurol 2006;63:513–8.
143. Low PA, Gilden JL, Freeman R, et al. Efficacy of midodrine vs placebo in neurogenic orthostatic hypotension. A randomized, double-blind multicenter study. Midodrine Study Group. JAMA 1997;277:1046–51.
144. Kaufmann H. L-dihydroxyphenylserine (Droxidopa): a new therapy for neurogenic orthostatic hypotension: the US experience. Clin Auton Res 2008;18 Suppl 1:19–24.
145. Magerkurth C, Schnitzer R, Braune S. Symptoms of autonomic failure in Parkinson's disease: prevalence and impact on daily life. Clin Auton Res 2005;15:76–82.
146. Makaroff L, Gunn A, Gervasoni C, Richy F. Gastrointestinal Disorders in Parkinson's Disease: Prevalence and Health Outcomes in a US Claims Database. J Parkinsons Dis 2011;1:65–74.
147. Abbott RD, Petrovitch H, White LR, et al. Frequency of bowel movements and the future risk of Parkinson's disease. Neurology 2001;57:456–62.
148. Abbott RD, Ross GW, Petrovitch H, et al. Bowel movement frequency in late-life and incidental Lewy bodies. Mov Disord 2007;22:1581–6.
149. Zangaglia R, Martignoni E, Glorioso M, et al. Macrogol for the treatment of constipation in Parkinson's disease. A randomized placebo-controlled study. Mov Disord 2007;22:1239–44.
150. Ondo WG, Kenney C, Sullivan K, et al. Placebo-controlled trial of lubiprostone for constipation associated with Parkinson disease. Neurology 2012;78:1650–4.
151. Cersosimo MG, Benarroch EE. Neural control of the gastrointestinal tract: implications for Parkinson disease. Mov Disord 2008;23:1065–75.
152. Reddymasu SC, Soykan I, McCallum RW. Domperidone: review of pharmacology and clinical applications in gastroenterology. Am J Gastroenterol 2007;102:2036–45.
153. Argolo N, Sampaio M, Pinho P, et al. Do swallowing exercises improve swallowing dynamic and quality of life in Parkinson's disease? NeuroRehabilitation 2013;32:949–55.
154. Troche MS, Sapienza CM, Rosenbek JC. Effects of bolus consistency on timing and safety of swallow in patients with Parkinson's disease. Dysphagia 2008;23:26–32.
155. Edwards LL, Quigley EM, Pfeiffer RF. Gastrointestinal dysfunction in Parkinson's disease: frequency and pathophysiology. Neurology 1992;42:726–32.
156. Kalf JG, Smit AM, Bloem BR, et al. Impact of drooling in Parkinson's disease. J Neurol 2007;254:1227–32.
157. Leibner J, Ramjit A, Sedig L, et al. The impact of and the factors associated with drooling in Parkinson's disease. Parkinsonism Relat Disord 2010;16:475–7.
158. Mancini F, Zangaglia R, Cristina S, et al. Double-blind, placebo-controlled study to evaluate the efficacy and safety of botulinum toxin type A in the treatment of drooling in parkinsonism. Mov Disord 2003;18:685–8.
159. Lagalla G, Millevolte M, Capecci M, et al. Long-lasting benefits of botulinum toxin type B in Parkinson's disease-related drooling. J Neurol 2009;256:563–7.
160. Lagalla G, Millevolte N, Capecci M, et al. Botulinum toxin type A for drooling in Parkinson's disease: a double-blind, randomized, placebo-controlled study. Mov Disord 2006;21:704–7.
161. Arbouw ME, Movig KL, Koopmann M, et al. Glycopyrrolate for sialorrhea in Parkinson disease: a randomized, double-blind, crossover trial. Neurology 2010; 74:1203–7.
162. South AR, Somers, Jog MS. Gum chewing improves swallow frequency and latency in Parkinson patients: a preliminary study. Neurology 2010;74:1198–202.
163. Winge K, Nielsen KK. Bladder dysfunction in advanced Parkinson's disease. Neurourol Urodyn 2012;31:1279–83.
164. Madhuvrata P, Singh PM, Hasafa Z, Abdel-Fattah M. Anticholinergic drugs for adult neurogenic detrusor overactivity: a systematic review and meta-analysis. Eur Urol 2012;62:816–30.
165. Soljanik I. Efficacy and safety of botulinum toxin a intradetrusor injections in adults with neurogenic detrusor overactivity/neurogenic overactive bladder: a systematic review. Drugs 2013;73:1055–66.
166. Sakakibara R, Uchiyama T, Yamanishi T, Kishi M. Genitourinary dysfunction in Parkinson's disease. Mov Disord 2010;25:2–12.
167. Kummer A, Cardoso F, Teixeira AL. Loss of libido in Parkinson's disease. J Sex Med 2009;6:1024–31.
168. Lombardi G, Nelli F, Celso M, et al. Treating erectile dysfunction and central neurological diseases with oral phosphodiesterase type 5 inhibitors. Review of the literature. J Sex Med 2012 9(4): 970–85.
169. Dula E, Bukofzer S, Perdok R, George M. Double-blind, crossover comparison of 3 mg apomorphine SL with placebo and with 4 mg apomorphine SL in male erectile dysfunction. Eur Urol 2001;39(5): 558–3
170. Negre-Pages L, Regragui W, Bouhassira D, et al. Chronic pain in Parkinson's disease: the cross-sectional French DoPaMiP survey. Mov Disord 2008;23:1361–9.
171. Beiske AG, Loge JH, Ronningen A, Svensson E. Pain in Parkinson's disease: Prevalence and characteristics. Pain 2009. 141:173–7.
172. Lin CH, Wu RM, H.Y. Chang HY, et al. Preceding pain symptoms and Parkinson's disease: a nationwide population-based cohort study. Eur J Neurol 2013;20:1398–404.
173. Ha AD, Jankovic J. Pain in Parkinson's disease. Mov Disord 2012; 27:485–91.
174. Ford B. Pain in Parkinson's disease. Mov Disord 2010;25 Suppl 1:S98–103.
175. Loher TJ, Burgunder JM, Weber S, et al. Effect of chronic pallidal deep brain stimulation on off period dystonia and sensory symptoms in advanced Parkinson's disease. J Neurol Neurosurg Psychiatry 2002;73:395–9.
176. Ramirez-Ruiz B, Marti MJ, Tolosa E, et al. Longitudinal evaluation of cerebral morphological changes in Parkinson's disease with and without dementia. J Neurol 2005;252:1345–52.
177. Jellinger KA. Formation and development of Lewy pathology: a critical update. J Neurol 2009;256 Suppl 3:270–9.
178. Levy R, Dubois B. Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cereb Cortex 2006;16:916–28.
179. Connor JR, Boyer PJ, Menzies SL, et al. Neuropathological examination suggests impaired brain iron acquisition in restless legs syndrome. Neurology 2003;61: 304–9.
180. Earley CJ, Barker P, Horská A, Allen RP. MRI-determined regional brain iron concentrations in early- and late-onset restless legs syndrome. Sleep Med 2006;7: 458–61.
181. Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci 2001;24:726–31.
182. Boeve BF, Silber MH, Saper CB, et al. Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease. Brain 2007;130(Pt 11): 2770–88.
183. Benarroch EE, Schmeichel AM, Paris J. Involvement of the ventrolateral medulla in parkinsonism with autonomic failure. Neurology 2000;54:963–8.
184. Chudler EH, Dong WK. The role of the basal ganglia in nociception and pain. Pain 1995;60:3–38.
185. Wolters E. Non-motor extranigral signs and symptoms in Parkinson's disease. Parkinsonism Relat Disord 2009;15 Suppl 3:S6–12.
186. Davidsdottir S, Cronin-Golomb A, Lee A. Visual and spatial symptoms in Parkinson's disease. Vision Res 2005;45:1285–96.
From the Department of Neurology, Movement Disorders Division, University of Pittsburgh Medical Center, Pittsburgh, PA.
Abstract
- Objective: To review the prevalence, diagnosis, and treatment of the nonmotor symptoms (NMS) associated with Parkinson’s disease (PD).
- Methods: Narrative review of the literature.
- Results: The NMS of PD are becoming increasingly recognized as having a critical role in the impact of this neurodegenerative movement disorder. This has led to significant investigative efforts to identify new or better NMS therapies. The preponderance of PD patients will be diagnosed with 1 or multiple NMS during the course of their disease, with many of these symptoms occurring months or even years prior to receiving the PD diagnosis. Despite the high prevalence and impact on disease burden, NMS often go undetected due to a lack of reporting by patients or insufficient interrogation by physicians. Further complicating NMS management is that only a few therapies have the level of evidence needed to support their use in the treatment of NMS.
- Conclusion: The practitioner needs to be aware of NMS and conduct thorough patient questioning in order to recognize, diagnose, and address NMS in PD patients.
Parkinson’s disease (PD) is a neurodegenerative movement disorder with an estimated prevalence of 1% to 2% among  the population over the age of 65 years [1]. Recognition and clinical diagnosis of PD is primarily made based on the cardinal motor features, including rigidity, tremor, bradykinesia, and postural instability. The motor symptoms are neuropathologically associated with accumulation of alpha-synuclein with Lewy body formation and neurodegeneration of the nigrostriatal dopamine system. Postmortem evaluation of the brains of PD patients has revealed more widespread degeneration in nondopaminergic systems, including several brainstem nuclei (raphe nucleus, locus ceruleus, dorsal vagal nucleus), limbic and neocortical structures, as well as the peripheral autonomic system [2,3].
the population over the age of 65 years [1]. Recognition and clinical diagnosis of PD is primarily made based on the cardinal motor features, including rigidity, tremor, bradykinesia, and postural instability. The motor symptoms are neuropathologically associated with accumulation of alpha-synuclein with Lewy body formation and neurodegeneration of the nigrostriatal dopamine system. Postmortem evaluation of the brains of PD patients has revealed more widespread degeneration in nondopaminergic systems, including several brainstem nuclei (raphe nucleus, locus ceruleus, dorsal vagal nucleus), limbic and neocortical structures, as well as the peripheral autonomic system [2,3].
 The nonmotor symptoms (NMS) of PD are the clinical manifestations of this extensive degeneration, which suggests that NMS are intrinsic and fundamental features of PD. NMS are exceedingly common, and up to 90% of PD patients will experience nonmotor features, including depression, anxiety, sleep disturbances, cognitive impairment, and dysautonomia [4,5] (Table).
The nonmotor symptoms (NMS) of PD are the clinical manifestations of this extensive degeneration, which suggests that NMS are intrinsic and fundamental features of PD. NMS are exceedingly common, and up to 90% of PD patients will experience nonmotor features, including depression, anxiety, sleep disturbances, cognitive impairment, and dysautonomia [4,5] (Table).
NMS have a greater impact on quality of life as compared to the motor symptoms [6,7], but are frequently underrecognized [8]. Evidence suggests that unless there is systematic and specific interrogation by practioners, NMS will elude recognition [9–11]. Recognizing NMS as part of PD is complicated by the fact that these symptoms are common in the general population and not specific for PD [12,13]. NMS can occur at any stage of the disease and may predate diagnosis [12], although as PD progresses the NMS become more prevalent, with a greater impact on health care costs and institutionalization rates than motor features [14,15].
Neuropsychiatric Symptoms
Depression
Epidemiology and Diagnosis
Depression is one of the most common neuropsychiatric manifestations observed in PD patients, with prevalence reports between 4% and 72%, though likely to be closer to 30% to 45% [16–20]. The severity of depression in the PD population has been shown to be greater than in patients with matched chronic disabilities [21,22] and also greater than in the general population over the age of 65 years [23]. The onset of depression can occur at any stage of the disease, even predating the diagnosis. Additionally, depression has more than twice the impact on health status than motor symptoms [24].
Though the mechanisms are not fully understood, it is suspected that psychosocial as well as neuropathological changes contribute to the pathogenesis of depression in PD. In a study comparing 104 PD patients and 61 patients with equivalent disability scores, functional disability was found to be responsible for only 9% of the variation of depression scores [22]. The increased prevalence of depression in PD patients can in part be explained by the neuropathological changes seen in post-mortem studies. Two neurotransmitters that are fundamental in the pathogenesis of depression are serotonin, from the raphe nuclei, and norepinepherine, from the locus ceruleus [20]. Both of these brainstem structures demonstrate alpha-synucleinopathy-associated degeneration and these changes can precede the development of motor dysfunction [3].
Diagnosing depression in PD is complicated by the fact that there is overlap between other PD symptoms and clinical features of depression (ie, amotivation, bradykinesia, fatigue, and sleep disturbances). However, many depressed PD patients are less likely to report feelings of guilt or failure and tend to have higher rates of anxiety [9,20,25]. Typically, PD patients are more likely to be diagnosed with minor depression or dysthymia rather than a major depressive disorder [19,20]. Formal testing through systematic questionnaires are diagnostically useful in the clinic, and serial testing can reveal changes over time to guide more effective treatment. Validated tools to evaluate depression in PD include the Beck Depression Inventory, Hamilton Depression Rating Scale, Montgomery-Asberg Depression Rating Scale, Geriatric DRS, and Hospital Anxiety and Depression scale [20].
Treatment Options
Treatment of depression in PD demonstrates generally poorer responses to typical antidepressants and side effects that may worsen other PD symptoms. Selective serotonin reuptake inhibitors (SSRIs) have been widely used as there are generally few drug-drug interactions and minimal effect on motor symptoms; however, several studies have demonstrated little benefit on depression in PD [26]. In a randomized, double-blind, placebo-controlled trial of the antidepressants paroxetine and venlafaxine, both were found to be effective and well tolerated [27]. Tricyclic anti-depressants (TCAs) have also demonstrated efficacy. In randomized controlled trials comparing TCAs to SSRIs, a greater benefit on depression symptoms has been found with TCAs [28–30]. The use of TCAs, however, is limited by anticholinergic side effects that occasionally worsen orthostatic hypotension or cognitive impairment [15,31]. Dopamine agonists have also been studied in depressed PD patients. In a randomized, double-blind, placebo-controlled trial [32] and a prospective observational study [33], pramipexole demonstrated significant improvements in depression symptoms. Ropinirole also demonstrated significant symptomatic improvement [34]. These studies suggest that while SSRIs are commonly used, evidence is accumulating to support the role of TCAs, SNRIs, and dopamine agonists in the treatment of depression in PD.
Other therapies have also been tried in pharmacologic-resistant patients. Electroconvulsive therapy has been reported to improve both depression and motor symptoms [35,36]; however, this is a treatment reserved for patients with severe and drug-refractory depression. A randomized controlled trial investigating cognitive behavioral therapy has also demonstrated improvement of depression scores [37]. The role of physical activity as treatment for depression in PD patients is unclear. As described in a recent review by Loprinzi et al [38], the literature is contradictory, with one group experiencing reduced depression but with no signficant effect in several other studies.
Anxiety
Epidemiology and Diagnosis
The prevalence of anxiety in PD patients is about 40% [39], which is 2 times greater than in the general population [9]. Anxiety may worsen PD symptoms, especially tremor and cognition. Risk factors for anxiety include the female gender, greater motor fluctuations, prior history of anxiety, and younger age of PD onset [40]. As with depression, some patients also report worsening of anxious symptoms during “off” states [41]. Screening tools that have been validated to help practitioners identify anxiety in PD include the Hospital Anxiety and Depression Scale, Beck Anxiety Inventory, Zung Self-rating Anxiety Scale, Spielberger State Trait Anxiety Inventory, and Hamilton Anxiety Rating Scale [15].
Treatment Options
The treatment of diagnosed anxiety in PD is primarily with benzodiazepines, which are particularly beneficial in patients whose tremors are exacerbated by anxiety or stress. The use of benzodiazepines has not been evaluated by a randomized controlled trial and use should be limited given the potential risks of sedation, cognitive effects, and psychomotor agitation. Other case studies have found benefit with serotonergic medications like fluoxetine or citalopram (especially with concomitant depression) or with optimization of levodopa therapy [42,43].
Hallucinations, Delusions, and Psychosis
Epidemiology
The prevalence of visual hallucinations in PD patients is about 20% to 40% [44,45]. Risk factors for psychotic symptoms include cognitive impairment, advanced age, prolonged duration of disease, depression, severe dysautonomia, and sleep disorders [46–48]. Early recognition of hallucinations is critical because of a strong correlation between the manifestation of psychosis and the need for nursing home placement or hospitalization. With early and effective treatment there is a decreased need for placement and a reduction on caregiver burden [44,49].
Treatment Options
Hallucinations can occur in delirium and it is important to first rule out an underlying infection or an offending medication, especially if there is a sudden onset or worsening of symptoms. Psychotic symptoms have been reported in drug-naive patients, though they are often iatrogenically induced with dopaminergic agents. All antiparkinsonian medications are capable of inducing or exacerbating hallucinations [9,50]. Additionally, psychotic symptoms tend to improve when dopaminergic agonists are reduced or eliminated. However, there is no clear relationship between the dose of dopaminergic agents and manifestation of hallucinations [48,51,52]. If hallucinations persist or there are motor complications that arise from reduction of dopaminergic agents, initiation of clozapine has been demonstrated to be efficacious in a rater-blinded prospective study and in a retrospective analysis [53–55]; however, regular monitoring for neutropenia is required. Quetiapine has demonstrated similar benefit without significant effects on motor symptoms in a randomized, rater-blinded study and in an evidence-based review [56,57]. It is also important to review or eliminate other medications that may contribute to hallucinations.
Cognitive Impairment
Epidemiology
The prevalence of dementia in the PD population is 20% to 40% [58], though almost 80% of PD patients ultimately develop cognitive decline [59]. Overall, a PD patient is 6 times more likely to develop dementia than someone in the general population [60]. There may be parallel progression of cognitive impairment and motor symptoms, but there is no correlation with overall duration of disease [60,61]. Risk factors linked with the presence of dementia include older age at onset of PD, presence of hallucinations, and male gender [62,63].
Cognitive dysfunction can be detected early in PD through neuropsychological testing; however, impairment of cognition is often insidious and may not be appreciated until symptoms become severe. Several screening tools have been used to evaluate for cognitive impairment in PD including the Mini-Mental State Exam (MMSE), Montreal Cognitive Assessment (MoCA), Mini-Mental Parkinson, Scales for Outcomes of Parkinson’s disease–Cognition, and others. Accumulating evidence, however, is suggestive of the superiority of the MoCA in the detection of cognitive deficits associated
with PD [64].
Dementia is a substantial burden for the caregiver and is a significant contributor to mortality in PD patients [65]. Cognitive impairment often presents with other behavioral symptoms, which further hastens placement outside the home and increases cost of caring for PD patients [49,66].
Cognitive impairment in Parkinson’s disease is typically associated with degeneration of primarily subcortical structures. PD patients with mild cognitive impairment were found to have deficits most significantly in memory, executive function, memory, and language abilities [67]. A recent study by Mak et al evaluated grey matter volumes by structural MRI in PD patients with evidence of mild cognitive impairment by MMSE and MoCA as compared with findings in cognitively intact patients. This demonstrated decreased brain volumes in areas that correlate with affected cognitive domains including the left insula, left superior frontal and left middle temporal areas [68].
Treatment Options
Prior to initiation of therapy, it is important to evaluate the patient for depression and to rule out pseudodementia. Bradyphrenia, or slowness of thought, should also be considered, as this symptom may also lead to an incorrect dementia diagnosis. Lastly, a thorough review of medications should be performed and offending agents including anticholinergics, TCAs, dopamine agonists, and amantadine should be discontinued as these can worsen cognition.
Rivastigmine has demonstrated modest improvement in cognitive performance in PD patients with dementia in a large multicenter, placebo-controlled study [69]. Other cholinesterase inhibitors (ie, donepezil or galantamine) are not recommended at this time due to limited studies or contradictory results in the literature [31,54]. Caution is advised with use of cholinesterase inhibitors as they may worsen tremor or autonomic dysfunction; also, use is limited by nausea or other gastrointestinal symptoms. Memantine, an NMDA receptor antagonist, has also been investigated in randomized, double-blind, placebo-controlled trials and demonstrated modest improvement of cognition and is generally well tolerated [70,71].
Nonpharmacologic therapy includes physical exercise, which has demonstrated improvement in memory tasks and processing speed [72]. Cognitive training has been less rigorously studied; however, a recent single-blinded controlled study demonstrated significant improvement of learning and memory in PD patients who completed computer-based cognitive training [73].
Compulsive Disorders
Impulse Control Disorders
Impulse control disorders (ICDs) are inappropriate behaviors resulting from a failure to resist an impulse, which leads to pleasure-seeking activities at the expense of relationships and ability to function socially. In PD, ICDs are expressed as pathologic gambling, hypersexuality, binge eating, compulsive shopping, and excessive spending [9,66]. The prevalence of all ICDs in PD is 15% to 20% and a patient may be diagnosed with multiple ICDs [74]. Dopamine agonist use has been implicated in the development of ICDs and this risk is further increased with the addition of levodopa [75,76]. Clinical features associated with ICDs include young age of onset, male gender, family history of addiction, depression or anxiety, and disinhibition or impulsive traits [77,78].
Traditionally, treatment consists of reduction or elimination of dopamine agonists, though adjustment of levodopa therapy may also be necessary. Amantadine as an adjunct therapy has been shown in a randomized, double-blind crossover study to reduce impulsivity in a few patients with pathologic gambling [79].
Dopamine Dysregulation Syndrome
Dopamine dysregulation syndrome (DDS) is characterized by compulsive use of dopaminergic medications beyond what is needed to treat parkinsonian symptoms, and is associated with social impairment. Patients describe addictive symptoms like craving or intense desire to obtain more dopaminergic medication [9,74]. Like ICDs, treatment of DDS consists of modification to dopaminergic medications, though patients with DDS may also require psychiatric evaluation and treatment.
Punding
Punding is another compulsive disorder that is defined as an intense fascination with objects and is associated with repetitive handling, manipulation, sorting, or arrangement of the items [80]. Occurrence of punding has been associated with higher total daily levels of levodopa, although one study has also implicated dopamine agonists [15,81]. As with the other compulsive disorders, punding also tends to respond well to reduction or discontinuation of levodopa. Studies have demonstrated modest benefit with SSRIs or atypical antipsychotics in long-term follow-up [82,83], though one study reported worsening of punding with quetiapine [84].
Apathy
Epidemiology and Treatment
Apathy is often characterized by a loss of motivation or inability to initiate goal-directed behavior, which results in dependence on others for activities of daily living and increases caregiver burden [85]. Patients demonstrate indifference, lack of interest, or inability to express or describe emotion. The apathetic patient may lack spontaneous and voluntary activity, and their affect display is often flattened [86].
With a prevalence of 30% to 50% [87], apathy is as common as depression in PD patients [66,88]. Risk factors associated with apathy include advanced age, severity of depression, severity of motor dysfunction, and dementia [89]. Apathy is frequently mistaken for depression given the significant overlap in symptoms; however, the patient with pure apathy will deny sadness or depressed feelings. It is also important to distinguish apathy from motor impairment or cognitive dysfunction that could explain the behavioral changes. No medications have reliably been shown to improve apathy, though it may be improved with initiation of dopaminergic therapy, especially early in the course [86,90].
Sleep Disorders
The original report of PD by James Parkinson describes sleep disturbances and daytime somnolence [91], which suggests that sleep disorders may be an intrinsic feature of the neurodegenerative process of PD itself.
REM Behavioral Disorder
Epidemiology and Diagnosis
Rapid eye movement behavioral disorder (RBD) is a parasomnia characterized by vocalizations and motor activity during dreaming due to loss of normal atonia associated with rapid eye movement (REM) sleep. Patients enact their dreams, which may lead to violent behaviors that can injure the patient or their bed partner. RBD is seen in 25% to 50% of PD patients [92,93], with variability depending on diagnostic technique and patient selection. Polysomnography is the most important diagnostic tool and demonstrates increased chin tone and limb movements during REM sleep in RBD [94,95]. Diagnosis can also be made clinically with patient and bed partner reports, though sensitivity is only approximately 30% [15].
Interestingly, many studies are now investigating the relationship between presence of RBD and later onset of neurodegenerative disorders. Multiple studies have shown that 40% to 65% of patients diagnosed with idiopathic RBD later develop an alpha-synucleinopathy, which includes PD, dementia with Lewy bodies, or multiple system atrophy within 10 years [92,95]. Prior studies report that as many as 90% of patients with idiopathic RBD develop neurodegenerative synucleinopathy when followed over 14 years [96]. Idiopathic RBD is currently being investigated as a potential clinical marker of pre-symptomatic PD in a multicenter observational study. If RBD is an early marker for neurodegenerative disease, it may be used to identify patients for neuroprotective trials as treatments are developed.
Treatment Options
Low-dose clonazepam (0.25–1 mg) is the mainstay of therapy, especially for patients that injure themselves or bed partners [97]; however, the use of benzodiazepines is historical and there remain no randomized controlled double-blind studies to evaluate the efficacy of clonazepam. Use of clonazepam may be limited by daytime sedation, confusion, or psychomotor agitation [31,97,98]. Melatonin (doses between 3–12 mg at bedtime) has also demonstrated benefit in RBD in a double-blind, placebo-controlled trial and in a small case series, with fewer side effects and no addiction potential as compared to clonazepam [99,100]. Case reports also support the use of several other effective medications, including cholinesterase inhibitors (rivastigmine and donepezil) and dopaminergic agents (pramipexole and levodopa) [15,20].
Restless Leg Syndrome and Periodic Limb Movements in Sleep
Epidemiology
Restless leg syndrome (RLS) and periodic limb movements in sleep (PLMS) cause disruptions of sleep and have an important impact on quality of sleep in PD patients. RLS is described as a strong urge to move the legs, accompanied by an uncomfortable sensation that is exacerbated at rest and relieved by movement. RLS is more frequently diagnosed in patients with PD, though prevalence reports vary widely [15]. Secondary causes for RLS should be investigated including iron deficiency, uremia and polyneuropathy. Several case reports demonstrate onset or worsening of RLS with use of antidepressants [101, 102] or antipsychotics like risperidone, aripiprazole, and quetiapine [103,104].
PLMS occurs in approximately 80% to 90% of patients with RLS, though may be present independently, and when seen on polysomnography is supportive of RLS [105]. PLMS is characterized by repetitive dorsiflexion of the foot, extension of the great toe, and may be accompanied by flexion of the knee and hip. The prevalence of PLMS in PD is approximately 60% and correlates with severity of PD motor features [106].
Treatment Options
Treatment of RLS should be initiated with nonpharmacologic therapies including good sleep hygiene, exercise, leg massage, and heat or ice packs [105,107]. Dopamine (DA) agonists are the primary treatment for RLS; however, even modest adjustments in levodopa can be helpful. One drawback to levodopa therapy is augmentation (a worsening or reappearance of symptoms) when serum levels fall due to the short half-life of levodopa [107,108]. DA agonists are less likely to cause augmentation. Both pramipexole and ropinirole have been extensively investigated in controlled, randomized, double-blind studies with benefits in 70% to 90% of patients with RLS and PLMS; however, there is a risk of developing compulsive behaviors [109–112]. Another option for PD patients is rotigotine, which has demonstrated improvement of RLS symptoms in a randomized, double-blind, placebo-controlled trial and has the added benefit that it may also help with motor symptoms [113,114].
More recently, gabapentin enacarbil has demonstrated improvement of moderate to severe RLS and was well tolerated in multiple randomized, double-blind, placebo-controlled trials [107,115,116]. Lastly, opioids (tramadol, oxycodone, codeine) have been shown to be effective, especially in the treatment of RLS that is refractory to other treatments [105,107].
Insomnia
Epidemiology
The most common sleep disorder in PD is insomnia, with a prevalence between 37% to 88% [14,117]. Insomnia is associated with difficulty in initiation or maintenance of sleep. Disruption of sleep typically leads to daytime somnolence and patient reports of a strong impact on motor disability and overall quality of life. There are several contributors to insomnia in PD patients including nocturia, depression, RLS, dystonia, and akinesia/rigidity/difficulty turning in bed [118].
Treatment Options
The use of carbidopa/levodopa controlled-release formulations at bedtime is associated with improved sleep duration and nocturnal akinesia, although it does not demonstrate a significant improvement in overall sleep ratings [54]. Hypnotics like eszopiclone and zolpidem have also demonstrated improved quality of sleep in limited controlled trials and a meta-analysis, but use is limited by sedation, dizziness, and falls [54,119]. Benzodiazepines improve sleep latency, but there is a risk of cognitive impairment, tolerance, and falls [117,120]. Melatonin at 3 to 5 mg and 50 mg doses have been investigated in 2 randomized, double-blind, placebo-controlled trials; however, there was a modest benefit and it was concluded that there is insufficient evidence to support the use of melatonin [54]. Nevertheless, melatonin is well tolerated and may be tried with minimal risk [54]. More recently, a randomized controlled trial using doxepin has demonstrated improvement of insomnia scores and was generally well tolerated [121].
Excessive Daytime Sleepiness and Abrupt Sleep Onset
EDS and Fatigue: Epidemiology and Treatment
A common complaint by PD patients is excessive daytime sleepiness (EDS), which can be verified with multiple sleep latency testing. EDS frequency varies in the literature, but is seen in approximately 15% to 50% of PD patients [4,122]. The etiology is usually multifactorial, with insomnia, dysautonomia, and depression as contributing factors [117]. A longer duration of symptoms, greater total load of levodopa, cognitive decline, and male gender are all risk factors for EDS [122,123]. It has been proposed that EDS is an intrinsic feature of PD; however, there is also an association with the use of antiparkinsonian medications. A randomized controlled trial demonstrated that use of the dopamine agonist pramipexole was associated with greater somnolence as compared to levodopa therapy (35% vs. 13%); however, this difference was only seen during the initial escalation phase [124]. Additionally, the combined use of dopamine agonists and levodopa has shown an even greater risk of EDS [125]. The evidence for the use of stimulants for EDS is lacking. The few studies conducted with modafinil have not demonstrated a robust improvement of EDS [126–128]. Other stimulants like methylphenidate have been studied with improvement of Epworth Sleepiness Score, though no randomized control trials have been undertaken [129].
It is important to distinguish EDS, a propensity for daytime sleep, from fatigue or excessive tiredness associated with mental or physical exertion [117]. Fatigue is often multifactorial and may be related to insomnia, sleep apnea, sedating effects of medications, frequent awakenings from nocturia, and degeneration of brain areas regulating sleep/wake cycles related to the underlying disease process [20, 117]. It is also important to consider depression and dementia in the differential, as these disorders may be erroneously be diagnosed as fatigue. Treatment of fatigue should include regular mild exercise, maintenance of a stimulating environment, removal of sedating medications, and management of intrinsic sleep disorders if present [117]. The use of stimulants for fatigue is controversial. A small randomized controlled trial (n = 48) using modafinil demonstrated improvement on the global clinical impression scale for fatigue but no significant change on the Fatigue Severity Scale; this study was limited by the power and points to the need for a larger study [130].
Sleep Attacks: Epidemiology and Treatment
Abrupt sleep onset, or “sleep attacks,” occurs when transition from wake to sleep is unavoidable and may occur without warning. Sleep attacks are threefold more likely to occur in patients using DA agonists, with an associated dose-related increase in risk [131]. Adjustment or elimination of DA agonists often improves sleep attacks, though it is important to address concurrent EDS if present. Nonpharmacologic treatments to consider include mild exercise, early morning bright light exposure, and a stimulating environment [117].
Sleep-Disordered Breathing/Obstructive Sleep Apnea
Epidemiology and Treatment
Sleep-disordered breathing (SDB) consists of either a deficit in the drive to breathe as in central sleep apnea, or may be due to an blockage of the airway as seen in obstructive sleep apnea (OSA). Apnea leads to oxygen desaturations that consequently trigger awakenings throughout the night, which in turn is experienced by the patient as daytime somnolence [117]. The prevalence of SDB and OSA is variable in the literature, ranging from no increased risk in PD patients [132,133] to 50% prevalence in PD patients [134,135]. Discussions with bed partners, history of snoring, and clinical reports of EDS or daytime fatigue are important indicators of SDB. Polysomnography confirms the diagnosis and can direct treatment, which frequently includes application of CPAP devices during sleep.
Autinomic Dysfunction
Orthostatic Hypotension
Epidemiology and Diagnosis
Orthostatic hypotension (OH) is defined as a 20-mm Hg fall in systolic blood pressure or 10-mm Hg drop in diastolic blood pressure within 3 minutes of a change in position. The prevalence of OH in PD patients is 30% to 60% [136,137]. Symptoms of OH can occur early in the disease and may precede diagnosis of PD [137]. Patients experience OH as dizziness, drowsiness, palpitations, nausea, or loss of consciousness. Additionally, falls and supine hypertension that accompany OH are associated with increased risk of morbidity and mortality in PD patients [138]. Several medications used in the treatment of PD can exacerbate OH, including levodopa, DA agonists, MAO-B inhibitors, and TCAs [139].
Treatment Options
First-line therapies for OH include nonpharmacologic methods such as compression stockings, sleeping with head elevated to 30 degrees, increased water and salt intake, more frequent small meals, and slowly changing position [140]. Additionally, it is important to discuss the removal or reduction of all antihypertensives with the patient’s PCP. Fludrocortisone (a mineralacorticoid) and domperidone (a peripheral dopamine antagonist not currently approved for use in the United States) modestly improved OH in a 2-phase, randomized, controlled, double-blind, crossover trial [141]. Pyridostigmine has also demonstrated improvement of standing blood pressure and OH symptoms in a double-blind, randomized cross-over study and has the additional benefit of not worsening supine hypertension [142]. Other effective treatments include midodrine, per a randomized, double-blind multicenter study [143], as well as droxidopa in a double-blind, crossover, placebo-controlled study [144]. Currently there is insufficient evidence to support the preferential use of any specific agent in the treatment of OH in PD.
Gastrointestinal Dysmotility
Constipation: Epidemiology and Treatment
Constipation is reported by nearly 60% of PD patients [145]. Constipation can precede the development of motor symptoms of PD, and the prevalence of GI disturbances increases with age and longer duration of disease. Nearly one third of patients will have been diagnosed with a GI disturbance within the year prior to PD diagnosis [146], which is associated with an increased risk for the development PD [147]. People with constipation (defined as < 1 bowel movement per day) but without a PD diagnosis had more nigral Lewy body degeneration postmortem [148] compared with people without constipation.
Treatments for constipation include dietary modification, increased fluid intake, and mild exercise. Macrogol significantly improved constipation in PD patients and was very well tolerated in a randomized placebo-controlled study [149]. Lubiprostone, a GI active prostaglandin, is also effective in the short-term treatment of constipation in a placebo-controlled trial [150].
Gastroparesis: Epidemiology and Treatment
Gastroparesis, like constipation, is related to enteric dopaminergic cell loss and degeneration of the dorsal motor nucleus of the vagus [151]. Patients experience gastroparesis as early satiety, full sensation, and nausea. Decreased gastric motility leads to retention of food as well as medications, which can slow absorption and delay onset of action for many medications including levodopa. Domperidone has both prokinetic and antiemetic properties, which have been beneficial in the treatment of gastroparesis [152], but its use is not currently approved in the United States.
Dysphagia: Epidemiology and Treatment
Dysphagia is associated with more advanced stages of PD as well as a significant increase in morbidity. Swallow exercises have demonstrated improvement of dysphagia [153]. The impact of levodopa therapy on dysphagia in the literature is controversial. Videofluoroscopic examination is the most common method for evaluation of swallowing disorders and provides important information for speech-language pathologists regarding recommendations for dietary modifications [154]. Adjustment of medication regimens to avoid an oral route is also helpful. This includes Parcopa, orally disintegrating carbidopa/levodopa tablets, and transdermal approaches like the rotigotine patch. For some patients, enteral nutrition is needed and placement of nasogastric tubes or percutaneous endoscopic gastrostomy tubes are an option.
Sialorrhea (Drooling)
Epidemiology
Difficulty handling oral secretions due to impaired or infrequent swallowing results in sialorrhea in up to 75% of PD patients [155], which is a significant embarrassment for most patients [156]. PD patients with drooling have difficulty speaking, eating, and engaging in social interactions, which significantly impacts perceived quality of life [157].
Treatment Options
Botulinum toxin (A and B) injections into the submandibular or parotid glands have demonstrated efficacy in multiple double-blind, randomized, placebo-controlled studies for the treatment of sialorrhea in PD patients; however, injections are associated with greater invasiveness and cost [158–160]. Glycopyrrolate, an anticholinergic drug, was also efficacious in the treatment of sialorrhea in the short term in a double-blind, randomized, placebo-controlled study [161]. Alternatively, gum chewing increases swallow frequency, improves drooling, and also shows a benefit with dysphagia [162].
Genitourinary Disturbances
Bladdery dysfunction: Epidemiology and Treatment
Bladder dysfunction in PD is often secondary to hyperactivity of the detrusor muscle leading to urinary urgency, increased urinary frequency, and nocturia. Less commonly, hypoactive detrusor muscle causes difficulty with initiation of urination, delayed bladder emptying, and recurrent infections. Urinary disturbances may occur before the onset of motor symptoms or early on in the disease course [12]. Disease severity is associated with greater urinary disturbances, and more than 50% of advanced PD patients report severe bladder symptoms [163].
Anticholinergic medications such as oxybutynin, solifenacin, and tolterodine are commonly used in the treatment of detrusor hyperactivity and demonstrate significant improvement in detrusor pressure in a recent systemic review and meta-analysis [164]. PD patients on these agents should be closely monitored for side effects including cognitive impairment, somnolence, hallucinations, confusion, and blurred vision. Other treatments include botulinum toxin injections into the detrusor muscle, which has demonstrated safety and efficacy in a recent systematic review [165].
Erectile dysfunction: Epidemiology and Treatment
Erectile dysfunction (ED) is reported by more than 60% of male PD patients [145] and is thought to be related to hypothalamic dysfunction and modification of the dopamine-oxytocin pathway [166]. Effects of PD medications, cognitive impairment, fatigue, apathy, and low testosterone contribute to loss of libido and ED [20,167]. Phosphodiesterase inhibitors such as sildenafil, vardenafil, and tadalafil are possibly useful in the treatment of ED in PD patients, though randomized trials have been limited [166,168]. Apomorphine sublingually is another medication that has demonstrated improvement of ED in a double-blind, crossover study and can be considered for patients with contraindications to phosphodiesterase inhibitors [169].
Sensory Symptoms
Pain
Epidemiology
Sensory disturbances in PD include diminished ability to identify odors, visual abnormalities (blurred vision, abnormal color perception, double vision), and pain. Pain is the most disabling sensory disturbance, though frequently underreported. Nearly two thirds of PD patients report pain, [170], though only half of patients receive any treatment [171]. Pain may also be a presenting symptom that precedes the clinical diagnosis of PD [172,173].
Treatment Options
There are several types of pain described by PD patients, the most common of which is musculoskeletal, typically involving the shoulder. Other types include dystonic, radicular, and central pain [174]. First-line treatment of musculoskeletal complaints includes nonsteroidal anti-inflammatory drugs (NSAIDs) and physiotherapy. Modification of levodopa regimen (including altering timing and frequency or adding controlled release formulations) can often provide relief for dystonic pain, and also for central pain for some patients [173, 174]. Deep brain stimulation, with subthalamic nucleus or globus pallidus targets, has demonstrated improvement with dystonic, central, and musculoskeletal pain in a small clinical study [175].
Conclusion
NMS are an intrinsic part of PD, may predate diagnosis, and substantially affect the majority of patients with PD. For many of these patients, NMS have a greater impact on quality of life and health care costs than the cardinal motor symptoms that define the disease. Many of these symptoms are not recognized by practioners and often are not volunteered by PD patients, making it important for practitioners to routinely and directly inquire about NMS. Treatment of NMS in PD is challenging, and only a few therapies have the level of evidence needed to support their use in the treatment of these problems. Nevertheless, proper recognition and addressing of these symptoms afford the clinician an opportunity to make a positive and potentially significant impact on the PD patient’s quality of life.
Corresponding author: Samay Jain, MD, MS, Dept of Neurology, 811 Kaufmann Bldg, Pittsburgh, PA 15213, [email protected].
Financial disclosures: None.
From the Department of Neurology, Movement Disorders Division, University of Pittsburgh Medical Center, Pittsburgh, PA.
Abstract
- Objective: To review the prevalence, diagnosis, and treatment of the nonmotor symptoms (NMS) associated with Parkinson’s disease (PD).
- Methods: Narrative review of the literature.
- Results: The NMS of PD are becoming increasingly recognized as having a critical role in the impact of this neurodegenerative movement disorder. This has led to significant investigative efforts to identify new or better NMS therapies. The preponderance of PD patients will be diagnosed with 1 or multiple NMS during the course of their disease, with many of these symptoms occurring months or even years prior to receiving the PD diagnosis. Despite the high prevalence and impact on disease burden, NMS often go undetected due to a lack of reporting by patients or insufficient interrogation by physicians. Further complicating NMS management is that only a few therapies have the level of evidence needed to support their use in the treatment of NMS.
- Conclusion: The practitioner needs to be aware of NMS and conduct thorough patient questioning in order to recognize, diagnose, and address NMS in PD patients.
Parkinson’s disease (PD) is a neurodegenerative movement disorder with an estimated prevalence of 1% to 2% among  the population over the age of 65 years [1]. Recognition and clinical diagnosis of PD is primarily made based on the cardinal motor features, including rigidity, tremor, bradykinesia, and postural instability. The motor symptoms are neuropathologically associated with accumulation of alpha-synuclein with Lewy body formation and neurodegeneration of the nigrostriatal dopamine system. Postmortem evaluation of the brains of PD patients has revealed more widespread degeneration in nondopaminergic systems, including several brainstem nuclei (raphe nucleus, locus ceruleus, dorsal vagal nucleus), limbic and neocortical structures, as well as the peripheral autonomic system [2,3].
the population over the age of 65 years [1]. Recognition and clinical diagnosis of PD is primarily made based on the cardinal motor features, including rigidity, tremor, bradykinesia, and postural instability. The motor symptoms are neuropathologically associated with accumulation of alpha-synuclein with Lewy body formation and neurodegeneration of the nigrostriatal dopamine system. Postmortem evaluation of the brains of PD patients has revealed more widespread degeneration in nondopaminergic systems, including several brainstem nuclei (raphe nucleus, locus ceruleus, dorsal vagal nucleus), limbic and neocortical structures, as well as the peripheral autonomic system [2,3].
 The nonmotor symptoms (NMS) of PD are the clinical manifestations of this extensive degeneration, which suggests that NMS are intrinsic and fundamental features of PD. NMS are exceedingly common, and up to 90% of PD patients will experience nonmotor features, including depression, anxiety, sleep disturbances, cognitive impairment, and dysautonomia [4,5] (Table).
The nonmotor symptoms (NMS) of PD are the clinical manifestations of this extensive degeneration, which suggests that NMS are intrinsic and fundamental features of PD. NMS are exceedingly common, and up to 90% of PD patients will experience nonmotor features, including depression, anxiety, sleep disturbances, cognitive impairment, and dysautonomia [4,5] (Table).
NMS have a greater impact on quality of life as compared to the motor symptoms [6,7], but are frequently underrecognized [8]. Evidence suggests that unless there is systematic and specific interrogation by practioners, NMS will elude recognition [9–11]. Recognizing NMS as part of PD is complicated by the fact that these symptoms are common in the general population and not specific for PD [12,13]. NMS can occur at any stage of the disease and may predate diagnosis [12], although as PD progresses the NMS become more prevalent, with a greater impact on health care costs and institutionalization rates than motor features [14,15].
Neuropsychiatric Symptoms
Depression
Epidemiology and Diagnosis
Depression is one of the most common neuropsychiatric manifestations observed in PD patients, with prevalence reports between 4% and 72%, though likely to be closer to 30% to 45% [16–20]. The severity of depression in the PD population has been shown to be greater than in patients with matched chronic disabilities [21,22] and also greater than in the general population over the age of 65 years [23]. The onset of depression can occur at any stage of the disease, even predating the diagnosis. Additionally, depression has more than twice the impact on health status than motor symptoms [24].
Though the mechanisms are not fully understood, it is suspected that psychosocial as well as neuropathological changes contribute to the pathogenesis of depression in PD. In a study comparing 104 PD patients and 61 patients with equivalent disability scores, functional disability was found to be responsible for only 9% of the variation of depression scores [22]. The increased prevalence of depression in PD patients can in part be explained by the neuropathological changes seen in post-mortem studies. Two neurotransmitters that are fundamental in the pathogenesis of depression are serotonin, from the raphe nuclei, and norepinepherine, from the locus ceruleus [20]. Both of these brainstem structures demonstrate alpha-synucleinopathy-associated degeneration and these changes can precede the development of motor dysfunction [3].
Diagnosing depression in PD is complicated by the fact that there is overlap between other PD symptoms and clinical features of depression (ie, amotivation, bradykinesia, fatigue, and sleep disturbances). However, many depressed PD patients are less likely to report feelings of guilt or failure and tend to have higher rates of anxiety [9,20,25]. Typically, PD patients are more likely to be diagnosed with minor depression or dysthymia rather than a major depressive disorder [19,20]. Formal testing through systematic questionnaires are diagnostically useful in the clinic, and serial testing can reveal changes over time to guide more effective treatment. Validated tools to evaluate depression in PD include the Beck Depression Inventory, Hamilton Depression Rating Scale, Montgomery-Asberg Depression Rating Scale, Geriatric DRS, and Hospital Anxiety and Depression scale [20].
Treatment Options
Treatment of depression in PD demonstrates generally poorer responses to typical antidepressants and side effects that may worsen other PD symptoms. Selective serotonin reuptake inhibitors (SSRIs) have been widely used as there are generally few drug-drug interactions and minimal effect on motor symptoms; however, several studies have demonstrated little benefit on depression in PD [26]. In a randomized, double-blind, placebo-controlled trial of the antidepressants paroxetine and venlafaxine, both were found to be effective and well tolerated [27]. Tricyclic anti-depressants (TCAs) have also demonstrated efficacy. In randomized controlled trials comparing TCAs to SSRIs, a greater benefit on depression symptoms has been found with TCAs [28–30]. The use of TCAs, however, is limited by anticholinergic side effects that occasionally worsen orthostatic hypotension or cognitive impairment [15,31]. Dopamine agonists have also been studied in depressed PD patients. In a randomized, double-blind, placebo-controlled trial [32] and a prospective observational study [33], pramipexole demonstrated significant improvements in depression symptoms. Ropinirole also demonstrated significant symptomatic improvement [34]. These studies suggest that while SSRIs are commonly used, evidence is accumulating to support the role of TCAs, SNRIs, and dopamine agonists in the treatment of depression in PD.
Other therapies have also been tried in pharmacologic-resistant patients. Electroconvulsive therapy has been reported to improve both depression and motor symptoms [35,36]; however, this is a treatment reserved for patients with severe and drug-refractory depression. A randomized controlled trial investigating cognitive behavioral therapy has also demonstrated improvement of depression scores [37]. The role of physical activity as treatment for depression in PD patients is unclear. As described in a recent review by Loprinzi et al [38], the literature is contradictory, with one group experiencing reduced depression but with no signficant effect in several other studies.
Anxiety
Epidemiology and Diagnosis
The prevalence of anxiety in PD patients is about 40% [39], which is 2 times greater than in the general population [9]. Anxiety may worsen PD symptoms, especially tremor and cognition. Risk factors for anxiety include the female gender, greater motor fluctuations, prior history of anxiety, and younger age of PD onset [40]. As with depression, some patients also report worsening of anxious symptoms during “off” states [41]. Screening tools that have been validated to help practitioners identify anxiety in PD include the Hospital Anxiety and Depression Scale, Beck Anxiety Inventory, Zung Self-rating Anxiety Scale, Spielberger State Trait Anxiety Inventory, and Hamilton Anxiety Rating Scale [15].
Treatment Options
The treatment of diagnosed anxiety in PD is primarily with benzodiazepines, which are particularly beneficial in patients whose tremors are exacerbated by anxiety or stress. The use of benzodiazepines has not been evaluated by a randomized controlled trial and use should be limited given the potential risks of sedation, cognitive effects, and psychomotor agitation. Other case studies have found benefit with serotonergic medications like fluoxetine or citalopram (especially with concomitant depression) or with optimization of levodopa therapy [42,43].
Hallucinations, Delusions, and Psychosis
Epidemiology
The prevalence of visual hallucinations in PD patients is about 20% to 40% [44,45]. Risk factors for psychotic symptoms include cognitive impairment, advanced age, prolonged duration of disease, depression, severe dysautonomia, and sleep disorders [46–48]. Early recognition of hallucinations is critical because of a strong correlation between the manifestation of psychosis and the need for nursing home placement or hospitalization. With early and effective treatment there is a decreased need for placement and a reduction on caregiver burden [44,49].
Treatment Options
Hallucinations can occur in delirium and it is important to first rule out an underlying infection or an offending medication, especially if there is a sudden onset or worsening of symptoms. Psychotic symptoms have been reported in drug-naive patients, though they are often iatrogenically induced with dopaminergic agents. All antiparkinsonian medications are capable of inducing or exacerbating hallucinations [9,50]. Additionally, psychotic symptoms tend to improve when dopaminergic agonists are reduced or eliminated. However, there is no clear relationship between the dose of dopaminergic agents and manifestation of hallucinations [48,51,52]. If hallucinations persist or there are motor complications that arise from reduction of dopaminergic agents, initiation of clozapine has been demonstrated to be efficacious in a rater-blinded prospective study and in a retrospective analysis [53–55]; however, regular monitoring for neutropenia is required. Quetiapine has demonstrated similar benefit without significant effects on motor symptoms in a randomized, rater-blinded study and in an evidence-based review [56,57]. It is also important to review or eliminate other medications that may contribute to hallucinations.
Cognitive Impairment
Epidemiology
The prevalence of dementia in the PD population is 20% to 40% [58], though almost 80% of PD patients ultimately develop cognitive decline [59]. Overall, a PD patient is 6 times more likely to develop dementia than someone in the general population [60]. There may be parallel progression of cognitive impairment and motor symptoms, but there is no correlation with overall duration of disease [60,61]. Risk factors linked with the presence of dementia include older age at onset of PD, presence of hallucinations, and male gender [62,63].
Cognitive dysfunction can be detected early in PD through neuropsychological testing; however, impairment of cognition is often insidious and may not be appreciated until symptoms become severe. Several screening tools have been used to evaluate for cognitive impairment in PD including the Mini-Mental State Exam (MMSE), Montreal Cognitive Assessment (MoCA), Mini-Mental Parkinson, Scales for Outcomes of Parkinson’s disease–Cognition, and others. Accumulating evidence, however, is suggestive of the superiority of the MoCA in the detection of cognitive deficits associated
with PD [64].
Dementia is a substantial burden for the caregiver and is a significant contributor to mortality in PD patients [65]. Cognitive impairment often presents with other behavioral symptoms, which further hastens placement outside the home and increases cost of caring for PD patients [49,66].
Cognitive impairment in Parkinson’s disease is typically associated with degeneration of primarily subcortical structures. PD patients with mild cognitive impairment were found to have deficits most significantly in memory, executive function, memory, and language abilities [67]. A recent study by Mak et al evaluated grey matter volumes by structural MRI in PD patients with evidence of mild cognitive impairment by MMSE and MoCA as compared with findings in cognitively intact patients. This demonstrated decreased brain volumes in areas that correlate with affected cognitive domains including the left insula, left superior frontal and left middle temporal areas [68].
Treatment Options
Prior to initiation of therapy, it is important to evaluate the patient for depression and to rule out pseudodementia. Bradyphrenia, or slowness of thought, should also be considered, as this symptom may also lead to an incorrect dementia diagnosis. Lastly, a thorough review of medications should be performed and offending agents including anticholinergics, TCAs, dopamine agonists, and amantadine should be discontinued as these can worsen cognition.
Rivastigmine has demonstrated modest improvement in cognitive performance in PD patients with dementia in a large multicenter, placebo-controlled study [69]. Other cholinesterase inhibitors (ie, donepezil or galantamine) are not recommended at this time due to limited studies or contradictory results in the literature [31,54]. Caution is advised with use of cholinesterase inhibitors as they may worsen tremor or autonomic dysfunction; also, use is limited by nausea or other gastrointestinal symptoms. Memantine, an NMDA receptor antagonist, has also been investigated in randomized, double-blind, placebo-controlled trials and demonstrated modest improvement of cognition and is generally well tolerated [70,71].
Nonpharmacologic therapy includes physical exercise, which has demonstrated improvement in memory tasks and processing speed [72]. Cognitive training has been less rigorously studied; however, a recent single-blinded controlled study demonstrated significant improvement of learning and memory in PD patients who completed computer-based cognitive training [73].
Compulsive Disorders
Impulse Control Disorders
Impulse control disorders (ICDs) are inappropriate behaviors resulting from a failure to resist an impulse, which leads to pleasure-seeking activities at the expense of relationships and ability to function socially. In PD, ICDs are expressed as pathologic gambling, hypersexuality, binge eating, compulsive shopping, and excessive spending [9,66]. The prevalence of all ICDs in PD is 15% to 20% and a patient may be diagnosed with multiple ICDs [74]. Dopamine agonist use has been implicated in the development of ICDs and this risk is further increased with the addition of levodopa [75,76]. Clinical features associated with ICDs include young age of onset, male gender, family history of addiction, depression or anxiety, and disinhibition or impulsive traits [77,78].
Traditionally, treatment consists of reduction or elimination of dopamine agonists, though adjustment of levodopa therapy may also be necessary. Amantadine as an adjunct therapy has been shown in a randomized, double-blind crossover study to reduce impulsivity in a few patients with pathologic gambling [79].
Dopamine Dysregulation Syndrome
Dopamine dysregulation syndrome (DDS) is characterized by compulsive use of dopaminergic medications beyond what is needed to treat parkinsonian symptoms, and is associated with social impairment. Patients describe addictive symptoms like craving or intense desire to obtain more dopaminergic medication [9,74]. Like ICDs, treatment of DDS consists of modification to dopaminergic medications, though patients with DDS may also require psychiatric evaluation and treatment.
Punding
Punding is another compulsive disorder that is defined as an intense fascination with objects and is associated with repetitive handling, manipulation, sorting, or arrangement of the items [80]. Occurrence of punding has been associated with higher total daily levels of levodopa, although one study has also implicated dopamine agonists [15,81]. As with the other compulsive disorders, punding also tends to respond well to reduction or discontinuation of levodopa. Studies have demonstrated modest benefit with SSRIs or atypical antipsychotics in long-term follow-up [82,83], though one study reported worsening of punding with quetiapine [84].
Apathy
Epidemiology and Treatment
Apathy is often characterized by a loss of motivation or inability to initiate goal-directed behavior, which results in dependence on others for activities of daily living and increases caregiver burden [85]. Patients demonstrate indifference, lack of interest, or inability to express or describe emotion. The apathetic patient may lack spontaneous and voluntary activity, and their affect display is often flattened [86].
With a prevalence of 30% to 50% [87], apathy is as common as depression in PD patients [66,88]. Risk factors associated with apathy include advanced age, severity of depression, severity of motor dysfunction, and dementia [89]. Apathy is frequently mistaken for depression given the significant overlap in symptoms; however, the patient with pure apathy will deny sadness or depressed feelings. It is also important to distinguish apathy from motor impairment or cognitive dysfunction that could explain the behavioral changes. No medications have reliably been shown to improve apathy, though it may be improved with initiation of dopaminergic therapy, especially early in the course [86,90].
Sleep Disorders
The original report of PD by James Parkinson describes sleep disturbances and daytime somnolence [91], which suggests that sleep disorders may be an intrinsic feature of the neurodegenerative process of PD itself.
REM Behavioral Disorder
Epidemiology and Diagnosis
Rapid eye movement behavioral disorder (RBD) is a parasomnia characterized by vocalizations and motor activity during dreaming due to loss of normal atonia associated with rapid eye movement (REM) sleep. Patients enact their dreams, which may lead to violent behaviors that can injure the patient or their bed partner. RBD is seen in 25% to 50% of PD patients [92,93], with variability depending on diagnostic technique and patient selection. Polysomnography is the most important diagnostic tool and demonstrates increased chin tone and limb movements during REM sleep in RBD [94,95]. Diagnosis can also be made clinically with patient and bed partner reports, though sensitivity is only approximately 30% [15].
Interestingly, many studies are now investigating the relationship between presence of RBD and later onset of neurodegenerative disorders. Multiple studies have shown that 40% to 65% of patients diagnosed with idiopathic RBD later develop an alpha-synucleinopathy, which includes PD, dementia with Lewy bodies, or multiple system atrophy within 10 years [92,95]. Prior studies report that as many as 90% of patients with idiopathic RBD develop neurodegenerative synucleinopathy when followed over 14 years [96]. Idiopathic RBD is currently being investigated as a potential clinical marker of pre-symptomatic PD in a multicenter observational study. If RBD is an early marker for neurodegenerative disease, it may be used to identify patients for neuroprotective trials as treatments are developed.
Treatment Options
Low-dose clonazepam (0.25–1 mg) is the mainstay of therapy, especially for patients that injure themselves or bed partners [97]; however, the use of benzodiazepines is historical and there remain no randomized controlled double-blind studies to evaluate the efficacy of clonazepam. Use of clonazepam may be limited by daytime sedation, confusion, or psychomotor agitation [31,97,98]. Melatonin (doses between 3–12 mg at bedtime) has also demonstrated benefit in RBD in a double-blind, placebo-controlled trial and in a small case series, with fewer side effects and no addiction potential as compared to clonazepam [99,100]. Case reports also support the use of several other effective medications, including cholinesterase inhibitors (rivastigmine and donepezil) and dopaminergic agents (pramipexole and levodopa) [15,20].
Restless Leg Syndrome and Periodic Limb Movements in Sleep
Epidemiology
Restless leg syndrome (RLS) and periodic limb movements in sleep (PLMS) cause disruptions of sleep and have an important impact on quality of sleep in PD patients. RLS is described as a strong urge to move the legs, accompanied by an uncomfortable sensation that is exacerbated at rest and relieved by movement. RLS is more frequently diagnosed in patients with PD, though prevalence reports vary widely [15]. Secondary causes for RLS should be investigated including iron deficiency, uremia and polyneuropathy. Several case reports demonstrate onset or worsening of RLS with use of antidepressants [101, 102] or antipsychotics like risperidone, aripiprazole, and quetiapine [103,104].
PLMS occurs in approximately 80% to 90% of patients with RLS, though may be present independently, and when seen on polysomnography is supportive of RLS [105]. PLMS is characterized by repetitive dorsiflexion of the foot, extension of the great toe, and may be accompanied by flexion of the knee and hip. The prevalence of PLMS in PD is approximately 60% and correlates with severity of PD motor features [106].
Treatment Options
Treatment of RLS should be initiated with nonpharmacologic therapies including good sleep hygiene, exercise, leg massage, and heat or ice packs [105,107]. Dopamine (DA) agonists are the primary treatment for RLS; however, even modest adjustments in levodopa can be helpful. One drawback to levodopa therapy is augmentation (a worsening or reappearance of symptoms) when serum levels fall due to the short half-life of levodopa [107,108]. DA agonists are less likely to cause augmentation. Both pramipexole and ropinirole have been extensively investigated in controlled, randomized, double-blind studies with benefits in 70% to 90% of patients with RLS and PLMS; however, there is a risk of developing compulsive behaviors [109–112]. Another option for PD patients is rotigotine, which has demonstrated improvement of RLS symptoms in a randomized, double-blind, placebo-controlled trial and has the added benefit that it may also help with motor symptoms [113,114].
More recently, gabapentin enacarbil has demonstrated improvement of moderate to severe RLS and was well tolerated in multiple randomized, double-blind, placebo-controlled trials [107,115,116]. Lastly, opioids (tramadol, oxycodone, codeine) have been shown to be effective, especially in the treatment of RLS that is refractory to other treatments [105,107].
Insomnia
Epidemiology
The most common sleep disorder in PD is insomnia, with a prevalence between 37% to 88% [14,117]. Insomnia is associated with difficulty in initiation or maintenance of sleep. Disruption of sleep typically leads to daytime somnolence and patient reports of a strong impact on motor disability and overall quality of life. There are several contributors to insomnia in PD patients including nocturia, depression, RLS, dystonia, and akinesia/rigidity/difficulty turning in bed [118].
Treatment Options
The use of carbidopa/levodopa controlled-release formulations at bedtime is associated with improved sleep duration and nocturnal akinesia, although it does not demonstrate a significant improvement in overall sleep ratings [54]. Hypnotics like eszopiclone and zolpidem have also demonstrated improved quality of sleep in limited controlled trials and a meta-analysis, but use is limited by sedation, dizziness, and falls [54,119]. Benzodiazepines improve sleep latency, but there is a risk of cognitive impairment, tolerance, and falls [117,120]. Melatonin at 3 to 5 mg and 50 mg doses have been investigated in 2 randomized, double-blind, placebo-controlled trials; however, there was a modest benefit and it was concluded that there is insufficient evidence to support the use of melatonin [54]. Nevertheless, melatonin is well tolerated and may be tried with minimal risk [54]. More recently, a randomized controlled trial using doxepin has demonstrated improvement of insomnia scores and was generally well tolerated [121].
Excessive Daytime Sleepiness and Abrupt Sleep Onset
EDS and Fatigue: Epidemiology and Treatment
A common complaint by PD patients is excessive daytime sleepiness (EDS), which can be verified with multiple sleep latency testing. EDS frequency varies in the literature, but is seen in approximately 15% to 50% of PD patients [4,122]. The etiology is usually multifactorial, with insomnia, dysautonomia, and depression as contributing factors [117]. A longer duration of symptoms, greater total load of levodopa, cognitive decline, and male gender are all risk factors for EDS [122,123]. It has been proposed that EDS is an intrinsic feature of PD; however, there is also an association with the use of antiparkinsonian medications. A randomized controlled trial demonstrated that use of the dopamine agonist pramipexole was associated with greater somnolence as compared to levodopa therapy (35% vs. 13%); however, this difference was only seen during the initial escalation phase [124]. Additionally, the combined use of dopamine agonists and levodopa has shown an even greater risk of EDS [125]. The evidence for the use of stimulants for EDS is lacking. The few studies conducted with modafinil have not demonstrated a robust improvement of EDS [126–128]. Other stimulants like methylphenidate have been studied with improvement of Epworth Sleepiness Score, though no randomized control trials have been undertaken [129].
It is important to distinguish EDS, a propensity for daytime sleep, from fatigue or excessive tiredness associated with mental or physical exertion [117]. Fatigue is often multifactorial and may be related to insomnia, sleep apnea, sedating effects of medications, frequent awakenings from nocturia, and degeneration of brain areas regulating sleep/wake cycles related to the underlying disease process [20, 117]. It is also important to consider depression and dementia in the differential, as these disorders may be erroneously be diagnosed as fatigue. Treatment of fatigue should include regular mild exercise, maintenance of a stimulating environment, removal of sedating medications, and management of intrinsic sleep disorders if present [117]. The use of stimulants for fatigue is controversial. A small randomized controlled trial (n = 48) using modafinil demonstrated improvement on the global clinical impression scale for fatigue but no significant change on the Fatigue Severity Scale; this study was limited by the power and points to the need for a larger study [130].
Sleep Attacks: Epidemiology and Treatment
Abrupt sleep onset, or “sleep attacks,” occurs when transition from wake to sleep is unavoidable and may occur without warning. Sleep attacks are threefold more likely to occur in patients using DA agonists, with an associated dose-related increase in risk [131]. Adjustment or elimination of DA agonists often improves sleep attacks, though it is important to address concurrent EDS if present. Nonpharmacologic treatments to consider include mild exercise, early morning bright light exposure, and a stimulating environment [117].
Sleep-Disordered Breathing/Obstructive Sleep Apnea
Epidemiology and Treatment
Sleep-disordered breathing (SDB) consists of either a deficit in the drive to breathe as in central sleep apnea, or may be due to an blockage of the airway as seen in obstructive sleep apnea (OSA). Apnea leads to oxygen desaturations that consequently trigger awakenings throughout the night, which in turn is experienced by the patient as daytime somnolence [117]. The prevalence of SDB and OSA is variable in the literature, ranging from no increased risk in PD patients [132,133] to 50% prevalence in PD patients [134,135]. Discussions with bed partners, history of snoring, and clinical reports of EDS or daytime fatigue are important indicators of SDB. Polysomnography confirms the diagnosis and can direct treatment, which frequently includes application of CPAP devices during sleep.
Autinomic Dysfunction
Orthostatic Hypotension
Epidemiology and Diagnosis
Orthostatic hypotension (OH) is defined as a 20-mm Hg fall in systolic blood pressure or 10-mm Hg drop in diastolic blood pressure within 3 minutes of a change in position. The prevalence of OH in PD patients is 30% to 60% [136,137]. Symptoms of OH can occur early in the disease and may precede diagnosis of PD [137]. Patients experience OH as dizziness, drowsiness, palpitations, nausea, or loss of consciousness. Additionally, falls and supine hypertension that accompany OH are associated with increased risk of morbidity and mortality in PD patients [138]. Several medications used in the treatment of PD can exacerbate OH, including levodopa, DA agonists, MAO-B inhibitors, and TCAs [139].
Treatment Options
First-line therapies for OH include nonpharmacologic methods such as compression stockings, sleeping with head elevated to 30 degrees, increased water and salt intake, more frequent small meals, and slowly changing position [140]. Additionally, it is important to discuss the removal or reduction of all antihypertensives with the patient’s PCP. Fludrocortisone (a mineralacorticoid) and domperidone (a peripheral dopamine antagonist not currently approved for use in the United States) modestly improved OH in a 2-phase, randomized, controlled, double-blind, crossover trial [141]. Pyridostigmine has also demonstrated improvement of standing blood pressure and OH symptoms in a double-blind, randomized cross-over study and has the additional benefit of not worsening supine hypertension [142]. Other effective treatments include midodrine, per a randomized, double-blind multicenter study [143], as well as droxidopa in a double-blind, crossover, placebo-controlled study [144]. Currently there is insufficient evidence to support the preferential use of any specific agent in the treatment of OH in PD.
Gastrointestinal Dysmotility
Constipation: Epidemiology and Treatment
Constipation is reported by nearly 60% of PD patients [145]. Constipation can precede the development of motor symptoms of PD, and the prevalence of GI disturbances increases with age and longer duration of disease. Nearly one third of patients will have been diagnosed with a GI disturbance within the year prior to PD diagnosis [146], which is associated with an increased risk for the development PD [147]. People with constipation (defined as < 1 bowel movement per day) but without a PD diagnosis had more nigral Lewy body degeneration postmortem [148] compared with people without constipation.
Treatments for constipation include dietary modification, increased fluid intake, and mild exercise. Macrogol significantly improved constipation in PD patients and was very well tolerated in a randomized placebo-controlled study [149]. Lubiprostone, a GI active prostaglandin, is also effective in the short-term treatment of constipation in a placebo-controlled trial [150].
Gastroparesis: Epidemiology and Treatment
Gastroparesis, like constipation, is related to enteric dopaminergic cell loss and degeneration of the dorsal motor nucleus of the vagus [151]. Patients experience gastroparesis as early satiety, full sensation, and nausea. Decreased gastric motility leads to retention of food as well as medications, which can slow absorption and delay onset of action for many medications including levodopa. Domperidone has both prokinetic and antiemetic properties, which have been beneficial in the treatment of gastroparesis [152], but its use is not currently approved in the United States.
Dysphagia: Epidemiology and Treatment
Dysphagia is associated with more advanced stages of PD as well as a significant increase in morbidity. Swallow exercises have demonstrated improvement of dysphagia [153]. The impact of levodopa therapy on dysphagia in the literature is controversial. Videofluoroscopic examination is the most common method for evaluation of swallowing disorders and provides important information for speech-language pathologists regarding recommendations for dietary modifications [154]. Adjustment of medication regimens to avoid an oral route is also helpful. This includes Parcopa, orally disintegrating carbidopa/levodopa tablets, and transdermal approaches like the rotigotine patch. For some patients, enteral nutrition is needed and placement of nasogastric tubes or percutaneous endoscopic gastrostomy tubes are an option.
Sialorrhea (Drooling)
Epidemiology
Difficulty handling oral secretions due to impaired or infrequent swallowing results in sialorrhea in up to 75% of PD patients [155], which is a significant embarrassment for most patients [156]. PD patients with drooling have difficulty speaking, eating, and engaging in social interactions, which significantly impacts perceived quality of life [157].
Treatment Options
Botulinum toxin (A and B) injections into the submandibular or parotid glands have demonstrated efficacy in multiple double-blind, randomized, placebo-controlled studies for the treatment of sialorrhea in PD patients; however, injections are associated with greater invasiveness and cost [158–160]. Glycopyrrolate, an anticholinergic drug, was also efficacious in the treatment of sialorrhea in the short term in a double-blind, randomized, placebo-controlled study [161]. Alternatively, gum chewing increases swallow frequency, improves drooling, and also shows a benefit with dysphagia [162].
Genitourinary Disturbances
Bladdery dysfunction: Epidemiology and Treatment
Bladder dysfunction in PD is often secondary to hyperactivity of the detrusor muscle leading to urinary urgency, increased urinary frequency, and nocturia. Less commonly, hypoactive detrusor muscle causes difficulty with initiation of urination, delayed bladder emptying, and recurrent infections. Urinary disturbances may occur before the onset of motor symptoms or early on in the disease course [12]. Disease severity is associated with greater urinary disturbances, and more than 50% of advanced PD patients report severe bladder symptoms [163].
Anticholinergic medications such as oxybutynin, solifenacin, and tolterodine are commonly used in the treatment of detrusor hyperactivity and demonstrate significant improvement in detrusor pressure in a recent systemic review and meta-analysis [164]. PD patients on these agents should be closely monitored for side effects including cognitive impairment, somnolence, hallucinations, confusion, and blurred vision. Other treatments include botulinum toxin injections into the detrusor muscle, which has demonstrated safety and efficacy in a recent systematic review [165].
Erectile dysfunction: Epidemiology and Treatment
Erectile dysfunction (ED) is reported by more than 60% of male PD patients [145] and is thought to be related to hypothalamic dysfunction and modification of the dopamine-oxytocin pathway [166]. Effects of PD medications, cognitive impairment, fatigue, apathy, and low testosterone contribute to loss of libido and ED [20,167]. Phosphodiesterase inhibitors such as sildenafil, vardenafil, and tadalafil are possibly useful in the treatment of ED in PD patients, though randomized trials have been limited [166,168]. Apomorphine sublingually is another medication that has demonstrated improvement of ED in a double-blind, crossover study and can be considered for patients with contraindications to phosphodiesterase inhibitors [169].
Sensory Symptoms
Pain
Epidemiology
Sensory disturbances in PD include diminished ability to identify odors, visual abnormalities (blurred vision, abnormal color perception, double vision), and pain. Pain is the most disabling sensory disturbance, though frequently underreported. Nearly two thirds of PD patients report pain, [170], though only half of patients receive any treatment [171]. Pain may also be a presenting symptom that precedes the clinical diagnosis of PD [172,173].
Treatment Options
There are several types of pain described by PD patients, the most common of which is musculoskeletal, typically involving the shoulder. Other types include dystonic, radicular, and central pain [174]. First-line treatment of musculoskeletal complaints includes nonsteroidal anti-inflammatory drugs (NSAIDs) and physiotherapy. Modification of levodopa regimen (including altering timing and frequency or adding controlled release formulations) can often provide relief for dystonic pain, and also for central pain for some patients [173, 174]. Deep brain stimulation, with subthalamic nucleus or globus pallidus targets, has demonstrated improvement with dystonic, central, and musculoskeletal pain in a small clinical study [175].
Conclusion
NMS are an intrinsic part of PD, may predate diagnosis, and substantially affect the majority of patients with PD. For many of these patients, NMS have a greater impact on quality of life and health care costs than the cardinal motor symptoms that define the disease. Many of these symptoms are not recognized by practioners and often are not volunteered by PD patients, making it important for practitioners to routinely and directly inquire about NMS. Treatment of NMS in PD is challenging, and only a few therapies have the level of evidence needed to support their use in the treatment of these problems. Nevertheless, proper recognition and addressing of these symptoms afford the clinician an opportunity to make a positive and potentially significant impact on the PD patient’s quality of life.
Corresponding author: Samay Jain, MD, MS, Dept of Neurology, 811 Kaufmann Bldg, Pittsburgh, PA 15213, [email protected].
Financial disclosures: None.
1. Alves G, Forsaa EB, Pedersen KF, et al. Epidemiology of Parkinson's disease. J Neurol 2008;255 Suppl 5:18–32.
2. Stern MB, Lang A, Poewe W. Toward a redefinition of Parkinson's disease. Mov Disord 2012;27:54–60.
3. Braak H, Del Tredici K, Rub U, et al. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging 2003;24:197–211.
4. Tandberg E, Larsen JP, Karlsen K. A community-based study of sleep disorders in patients with Parkinson's disease. Mov Disord 1998;13:895–9.
5. Shulman LM, Taback RL, Bean J, Weiner WJ. Comorbidity of the nonmotor symptoms of Parkinson's disease. Mov Disord 2001;16:507–10.
6. Park A, Stacy M. Non-motor symptoms in Parkinson's disease. J Neurol 2009;256 Suppl 3:293–8.
7. Chaudhuri KR, Schapira AH. Non-motor symptoms of Parkinson's disease: dopaminergic pathophysiology and treatment. Lancet Neurol 2009;8:464–74.
8. Shulman LM, Taback RL, Rabinstein AA, Weiner WJ. Non-recognition of depression and other non-motor symptoms in Parkinson's disease. Parkinsonism Relat Disord 2002;8:193–7.
9. Bonnet AM, Jutras MF, Czernecki V, et al. Nonmotor symptoms in Parkinson's disease in 2012: relevant clinical aspects. Parkinsons Dis 2012:2012:198316.
10. Chaudhuri KR, Healy DG, Schapira AH. Non-motor symptoms of Parkinson's disease: diagnosis and management. Lancet Neurol 2006;5:235–45.
11. Hussl AK, Seppi K, Poewe W. Nonmotor symptoms in Parkinson's disease. Expert Rev Neurother 2013;13:581–3.
12. O'Sullivan SS, Williams DR, Gallagher DA, et al. Nonmotor symptoms as presenting complaints in Parkinson's disease: a clinicopathological study. Mov Disord 2008;23:101–6.
13. Lang AE. A critical appraisal of the premotor symptoms of Parkinson's disease: potential usefulness in early diagnosis and design of neuroprotective trials. Mov Disord 2011;26:775–83.
14. Barone P, Antonini A, Colosimo C, et al. The PRIAMO study: A multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson's disease. Mov Disord 2009;24:1641–9.
15. Bernal-Pacheco O, Limotai N, Go CL, Fernandez HH. Nonmotor manifestations in Parkinson disease. Neurologist 2012;18:1–16.
16. Lemke MR, Fuchs G, Gemende I, et al. Depression and Parkinson's disease. J Neurol 2004;251 Suppl 6: VI/24–7.
17. Ravina, B, Camicioli R, Como PG, et al, The impact of depressive symptoms in early Parkinson disease. Neurology 2007;69:342–7.
18. Jasinska-Myga B, Putzke JD Wider C, et al. Depression in Parkinson's disease. Can J Neurol Sci 2010;37:61–6.
19. Slaughter JR, Slaughter KA, Nichols D, et al. Prevalence, clinical manifestations, etiology, and treatment of depression in Parkinson's disease. J Neuropsychiatry Clin Neurosci 2001;13:187–96.
20. Simuni T, Sethi K. Nonmotor manifestations of Parkinson's disease. Ann Neurol 2008;64 Suppl 2:S65–80.
21. Ehmann TS, Beninger RJ, Gawel MJ, Riopelle RJ. Depressive symptoms in Parkinson's disease: a comparison with disabled control subjects. J Geriatr Psychiatry Neurol 1990;3:3–9.
22. Menza MA, Mark MH. Parkinson's disease and depression: the relationship to disability and personality. J Neuropsychiatry Clin Neurosci 1994;6:165–9.
23. CDC. Current depression among adults–United States, 2006 and 2008. Morb Mort Weekly Rep 2010;59:1229–35.
24. Hinnell C, Hurt CS, Landau S, et al. Nonmotor versus motor symptoms: how much do they matter to health status in Parkinson's disease? Mov Disord 2012;27:236–41.
25. Cummings JL. Depression and Parkinson's disease: a review. Am J Psychiatry 1992;149:443–54.
26. Weintraub D, Morales KH, Moberg PJ, et al. Antidepressant studies in Parkinson's disease: a review and meta-analysis. Mov Disord 2005;20:1161–9.
27. Richard IH, McDermott MP, Kurlan R, et al. A randomized, double-blind, placebo-controlled trial of antidepressants in Parkinson disease. Neurology 2012;78:1229–36.
28. Menza M, Dobkin RD, Marin H, et al. A controlled trial of antidepressants in patients with Parkinson disease and depression. Neurology 2009;72:886–92.
29. Okun MS, Fernandez HH. Will tricyclic antidepressants make a comeback for depressed Parkinson disease patients? Neurology 2009;72:868–9.
30. Antonini A, Tesei S, Zecchinelli A, et al. Randomized study of sertraline and low-dose amitriptyline in patients with Parkinson's disease and depression: effect on quality of life. Mov Disord 2006;21:1119–22.
31. Pedrosa DJ, Timmermann L. Review: management of Parkinson's disease. Neuropsychiatr Dis Treat 2013;9: 321–40.
32. Barone P, Poewe W, Albrecht S, et al. Pramipexole for the treatment of depressive symptoms in patients with Parkinson's disease: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 2010;9:573–80.
33. Lemke MR, Brecht HM, Koester J, et al. Anhedonia, depression, and motor functioning in Parkinson's disease during treatment with pramipexole. J Neuropsychiatry Clin Neurosci 2005;17:214–20.
34. Rektorova I, Balaz M, Svatova J, et al. Effects of ropinirole on nonmotor symptoms of Parkinson disease: a prospective multicenter study. Clin Neuropharmacol 2008;31:261–6.
35. Van der Wurff FB, Stek ML, Hoogendijk WL, Beekman AT. Electroconvulsive therapy for the depressed elderly. Cochrane Database Syst Rev 2003(2):CD003593.
36. Okun MS, Watts RL. Depression associated with Parkinson's disease: clinical features and treatment. Neurology 2002;58(4 Suppl 1):S63–70.
37. Dobkin RD, Menza M, Allen LA, et al. Cognitive-behavioral therapy for depression in Parkinson's disease: a randomized, controlled trial. Am J Psychiatry 2013;168:1066–74.
38. Loprinzi PD, Herod SM, Cardinal BJ, Noakes TD. Physical activity and the brain: A review of this dynamic, bi-directional relationship. Brain Res 2013;1539:95–104.
39. Richard IH. Anxiety disorders in Parkinson's disease. Adv Neurol 2005;96:42–55.
40. Leentjens AF, Dujardin K, Marsh L, et al. Symptomatology and markers of anxiety disorders in Parkinson's disease: a cross-sectional study. Mov Disord 2011;26:484–92.
41. Witjas T, Kaphan E, Azulay JP, et al. Nonmotor fluctuations in Parkinson's disease: frequent and disabling. Neurology 2002;59:408–13.
42. Olanow CW, Stern MB, Sethi K. The scientific and clinical basis for the treatment of Parkinson disease Neurology 2009;72(21 Suppl 4):S1–136.
43. Richard IH, Schiffer RB, Kurlan R. Anxiety and Parkinson's disease. J Neuropsychiatry Clin Neurosci 1996;8:383–92.
44. Fenelon G, Alves G. Epidemiology of psychosis in Parkinson's disease. J Neurol Sci 2010;289:12–7.
45. Papapetropoulos S, Katzen H, Schrag A, et al. A questionnaire-based (UM-PDHQ) study of hallucinations in Parkinson's disease. BMC Neurol 2008;8:21.
46. Fenelon G, Mahieux F, Huon R, Ziegler M. Hallucinations in Parkinson's disease: prevalence, phenomenology and risk factors. Brain 2000;123 (Pt 4):733–45.
47. Papapetropoulos S, Mash DC. Psychotic symptoms in Parkinson's disease. From description to etiology. J Neurol 2005;252:753–64.
48. Aarsland D, Larsen JP, Cummins JL, Laake K. Prevalence and clinical correlates of psychotic symptoms in Parkinson disease: a community-based study. Arch Neurol 1999;56:595–601.
49. Aarsland D, Larsen JP, Tandberg E, Laake K. Predictors of nursing home placement in Parkinson's disease: a population-based, prospective study. J Am Geriatr Soc 2000;48:938–42.
50. Lohle M, Storch A, Reichmann H, Beyond tremor and rigidity: non-motor features of Parkinson's disease. J Neural Transm 2009;116:1483–92.
51. Fenelon G. Psychosis in Parkinson's disease: phenomenology, frequency, risk factors, and current understanding of pathophysiologic mechanisms. CNS Spectr 2008;13(3 Suppl 4):18–25.
52. Merims D, Shabtai H, Korczyn AD, et al. Antiparkinsonian medication is not a risk factor for the development of hallucinations in Parkinson's disease. J Neural Transm 2004;111:1447–53.
53. Merims D, Balas M, Peretz C, et al. Rater-blinded, prospective comparison: quetiapine versus clozapine for Parkinson's disease psychosis. Clin Neuropharmacol 2006;29:331–7.
54. Seppi K, Weintraub D, Coelho M, et al. The Movement Disorder Society Evidence-Based Medicine Review Update: Treatments for the non-motor symptoms of Parkinson's disease. Mov Disord 2011;26 Suppl 3:S42–80.
55. Fernandez HH, Donnelly EM, Friedman JH. Long-term outcome of clozapine use for psychosis in parkinsonian patients. Mov Disord 2004;19:831–3.
56. Morgante L, Epifanio A, Spina E, et al. Quetiapine and clozapine in parkinsonian patients with dopaminergic psychosis. Clin Neuropharmacol 2004;27:153–6.
57. Miyasaki JM, Shannon K, Voon V, et al. Practice parameter: evaluation and treatment of depression, psychosis, and dementia in Parkinson disease (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2006;66:996–1002.
58. Riedel O, Klotsche J, Spottke A, et al. Cognitive impairment in 873 patients with idiopathic Parkinson's disease. Results from the German Study on Epidemiology of Parkinson's Disease with Dementia (GEPAD). J Neurol 2008;255:255–64.
59. Aarsland D, Andersen K, Larsen JP, et al. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch Neurol 2003;60:387–92.
60. Aarsland D, Andersen K, Larsen JP, et al. Risk of dementia in Parkinson's disease: a community-based, prospective study. Neurology 2001;56:730–6.
61. Riggeal BD, Crucian GP, Seignourel P, et al. Cognitive decline tracks motor progression and not disease duration in Parkinson patients. Neuropsychiatr Dis Treat 2007;3:955–8.
62. Hughes TA, Ross HF, Musa S, et al. A 10-year study of the incidence of and factors predicting dementia in Parkinson's disease. Neurology 2000;54:1596–602.
63. Hobson P, Meara J. Risk and incidence of dementia in a cohort of older subjects with Parkinson's disease in the United Kingdom. Mov Disord 2004;19:1043–9.
64. Hoops S, Nazem S, Siderowf AD, et al. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology 2009;73:1738–45.
65. Louis ED, Marder K, Cote L, et al. Mortality from Parkinson disease. Arch Neurol 1997;54:260–4.
66. Fernandez HH. Nonmotor complications of Parkinson disease. Cleve Clin J Med 2012;79 Suppl 2:S14–8.
67. Sollinger AB, Goldstein FC, Lah JJ, et al. Mild cognitive impairment in Parkinson's disease: subtypes and motor characteristics. Parkinsonism Relat Disord 2010;16:177–80.
68. Mak E, Zhou J, Tan LC, et al. Cognitive deficits in mild Parkinson's disease are associated with distinct areas of grey matter atrophy. J Neurol Neurosurg Psychiatry 2013 Oct 16.
69. Emre M, Aarsland D, Albanese A, et al. Rivastigmine for dementia associated with Parkinson's disease. N Engl J Med 2004;351:2509–18.
70. Aarsland D, Ballard C, Walker Z, et al. Memantine in patients with Parkinson's disease dementia or dementia with Lewy bodies: a double-blind, placebo-controlled, multicentre trial. Lancet Neurol 2009;8:613–8.
71. Leroi I, Overshott R, Byrne EJ, et al. Randomized controlled trial of memantine in dementia associated with Parkinson's disease. Mov Disord 2009;24:1217–21.
72. Speelman AD, van de Warrenburg BP, van Nimwegen M, et al. How might physical activity benefit patients with Parkinson disease? Nat Rev Neurol 2013;7:528–34.
73. Naismith SL, Mowszowski L, Diamond K, Lewis SJ. Improving memory in Parkinson's disease: a healthy brain ageing cognitive training program. Mov Disord 2013;28:1097–103.
74. Weintraub D, Koester J, Potenza MN, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol 2010;67:589–95.
75. Voon V, Reynolds B, Brezing C, et al. Impulsive choice and response in dopamine agonist-related impulse control behaviors. Psychopharmacology (Berl) 2010;207:645–59.
76. Weintraub D, Siderowf AD, Potenza MN, et al. Association of dopamine agonist use with impulse control disorders in Parkinson disease. Arch Neurol 2006; 63:969–73.
77. Pontone G, Williams JR, Bassett SS, Marsh L. Clinical features associated with impulse control disorders in Parkinson disease. Neurology 2006;67:1258–61.
78. Voon V, Mehta AR, Hallett M. Impulse control disorders in Parkinson's disease: recent advances. Curr Opin Neurol 2011;24:324–30.
79. Thomas A, Bonanni L, Gambi F, et al. Pathological gambling in Parkinson disease is reduced by amantadine. Ann Neurol 2010;68:400–4.
80. Miyasaki JM, Al Hassan K, Lang AE, Voon V. Punding prevalence in Parkinson's disease. Mov Disord 2007;22:1179–81.
81. Nguyen FN, Chang YL, Okun MS, et al. Prevalence and characteristics of punding and repetitive behaviors among Parkinson patients in North-Central Florida. Int J Geriatr Psychiatry 2010;25:540–1.
82. Sohtaoglu M, Demiray DY, Kenangil G, et al. Long term follow-up of Parkinson's disease patients with impulse control disorders. Parkinsonism Relat Disord 2010;16:334–7.
83. Antonini A, Cilia R. Behavioural adverse effects of dopaminergic treatments in Parkinson's disease: incidence, neurobiological basis, management and prevention. Drug Saf 2009;
32:475–88.
84. Miwa H, Morita S, Nakanishi I, Kondo T, Stereotyped behaviors or punding after quetiapine administration in Parkinson's disease. Parkinsonism Relat Disord 2004;10:177–80.
85. Skorvanek M, Rosenberger J, Gdovinova Z, et al. Apathy in elderly nondemented patients with parkinson's disease: clinical determinants and relationship to quality of life. J Geriatr Psychiatry Neurol 2013;26:237–43.
86. Marin RS, Fogel BS, Hawkins J, et al. Apathy: a treatable syndrome. J Neuropsychiatry Clin Neurosci 1995;7:23–30.
87. Pluck GC, Brown RG. Apathy in Parkinson's disease. J Neurol Neurosurg Psychiatry 2002;73:636–42.
88. Kirsch-Darrow L, Fernandez HH, Marsiske M, et al. Dissociating apathy and depression in Parkinson disease. Neurology 2006;67:33–8.
89. Pedersen KF, Alves G, Aarsland D, Larsen JP. Occurrence and risk factors for apathy in Parkinson disease: a 4-year prospective longitudinal study. J Neurol Neurosurg Psychiatry 2009;80:1279–82.
90. Czernecki V, Pillon B, Houeto JL, et al. Motivation, reward, and Parkinson's disease: influence of dopatherapy. Neuropsychologia 2002;40:2257–67.
91. Parkinson J. An essay on the shaking palsy. Sherwood, Neely, and Jones; 1817.
92. Schenck CH, Mahowald WM. REM sleep behavior disorder: clinical, developmental, and neuroscience perspectives 16 years after its formal identification in SLEEP. Sleep 2002;25:120–38.
93. Sixel-Doring F, Trautmann E, Mollenhauer B, Trenkwalder C. Associated factors for REM sleep behavior disorder in Parkinson disease. Neurology 2011;77:1048–54.
94. Eisensehr I, v Lindeiner H, Jager M, Noachtar S. REM sleep behavior disorder in sleep-disordered patients with versus without Parkinson's disease: is there a need for polysomnography? J Neurol Sci 2001;186:7–11.
95. Postuma RB, Gagnon JF, Montplaisir JY. REM sleep behavior disorder and prodromal neurodegeneration - where are we headed? Tremor Other Hyperkinet Mov (N Y) 2013;3.
96. Iranzo A, Tolosa E, Gelpi E, et al. Neurodegenerative disease status and post-mortem pathology in idiopathic rapid-eye-movement sleep behaviour disorder: an observational cohort study. Lancet Neurol 2013;12: 443–53.
97. Schenck CH, Mahowald MW. Rapid eye movement sleep parasomnias. Neurol Clin 2005;23:1107–26.
98. Anderson KN, Shneerson JM. Drug treatment of REM sleep behavior disorder: the use of drug therapies other than clonazepam. J Clin Sleep Med 2009;5:235–9.
99. Boeve BF, Silber MH, Ferman TJ. Melatonin for treatment of REM sleep behavior disorder in neurologic disorders: results in 14 patients. Sleep Med 2003;4:281–4.
100. Kunz D, Mahlberg R. A two-part, double-blind, placebo-controlled trial of exogenous melatonin in REM sleep behaviour disorder. J Sleep Res 2010;19:591–6.
101. Perroud N, Lazignac C, Baleydier B, et al. Restless legs syndrome induced by citalopram: a psychiatric emergency? Gen Hosp Psychiatry 2007;29:72–4.
102. Buskova J, Vorlova T, Pisko J, Sonka K, Severe sleep-related movement disorder induced by sertraline. Sleep Med 2012;13:769–70.
103. Rittmannsberger H, Werl R. Restless legs syndrome induced by quetiapine: report of seven cases and review of the literature. Int J Neuropsychopharmacol 2013;16:1427–31.
104. Perez-Lloret S, Rey MV, Bondon-Guitton E, et al. Drugs associated with restless legs syndrome: a case/noncase study in the French pharmacovigilance database. J Clin Psychopharmacol 2012;32:824–7.
105. Aurora RN, Kristo DA, Bista SR, et al. The treatment of restless legs syndrome and periodic limb movement disorder in adults-an update for 2012: practice parameters with an evidence-based systematic review and meta-analyses: an American Academy of Sleep Medicine Clinical Practice Guideline. Sleep 2012;35:1039–62.
106. Covassin N, Neikrug AB, Liu L, et al. Clinical correlates of periodic limb movements in sleep in Parkinson's disease. J Neurol Sci 2012;316:131–6.
107. Rios Romenets S, Postuma RB. Treatment of restless legs syndrome. Curr Treat Options Neurol 2013;15:396-409.
108. Hogl B, Paulus W, Clarenbach P, Trenkwalder C, Restless legs syndrome: diagnostic assessment and the advantages and risks of dopaminergic treatment. J Neurol 2006;253 Suppl 4:IV22-8.
109. Garcia-Borreguero D, Kohnen R, Silber MH, et al. The long-term treatment of restless legs syndrome/Willis-Ekbom disease: evidence-based guidelines and clinical consensus best practice guidance: a report from the International Restless Legs Syndrome Study Group. Sleep Med 2013;14:675–84.
110. Montagna P, Hornyak M, Ulfberg J, et al. Randomized trial of pramipexole for patients with restless legs syndrome (RLS) and RLS-related impairment of mood. Sleep Med 2011;12:34–40.
111. Partinen M, Hirvonen K, Jama L, et al. Efficacy and safety of pramipexole in idiopathic restless legs syndrome: a polysomnographic dose-finding study--the PRELUDE study. Sleep Med 2006;7:407–17.
112. Trenkwalder C Garcia-Borreguero D, Montagna P, et al. Ropinirole in the treatment of restless legs syndrome: results from the TREAT RLS 1 study, a 12 week, randomised, placebo controlled study in 10 European countries. J Neurol Neurosurg Psychiatry 2004;75:92–7.
113. Hening WA, Allen RP, Ondo WG, et al. Rotigotine improves restless legs syndrome: a 6-month randomized, double-blind, placebo-controlled trial in the United States. Mov Disord 2010;25:1675–83.
114. Oertel WH, Benes H, Garcia-Borreguero D, et al. Rotigotine transdermal patch in moderate to severe idiopathic restless legs syndrome: a randomized, placebo-controlled polysomnographic study. Sleep Med 2010;11:848–56.
115. Lee DO, Ziman RB, Perkins AT, et al. A randomized, double-blind, placebo-controlled study to assess the efficacy and tolerability of gabapentin enacarbil in subjects with restless legs syndrome. J Clin Sleep Med 2011;7:282–92.
116. Kushida CA, Walters AS, Becker P, et al. A randomized, double-blind, placebo-controlled, crossover study of XP13512/GSK1838262 in the treatment of patients with primary restless legs syndrome. Sleep 2009;32:159–68.
117. Menza M, Dobkin RD, Marin H, Bienfait K. Sleep disturbances in Parkinson's disease. Mov Disord 2010;25 Suppl 1:S117–22.
118. Louter M, van Sloun RJ, Pevernagie DA, et al. Subjectively impaired bed mobility in Parkinson disease affects sleep efficiency. Sleep Med 2013;14:668–74.
119. Menza M, Dobkin RD, Marin H, et al. Treatment of insomnia in Parkinson's disease: a controlled trial of eszopiclone and placebo. Mov Disord 2010;25:1708–14.
120. Nowell PD, Mazumdar S, Buysse DJ, et al. Benzodiazepines and zolpidem for chronic insomnia: a meta-analysis of treatment efficacy. JAMA 1997;278:2170–7.
121. Rios Romenets S, Creti L, Fichten C, et al. Doxepin and cognitive behavioural therapy for insomnia in patients with Parkinson's disease – a randomized study. Parkinsonism Relat Disord 2013;19:670–5.
122. Ondo WG, Dat Vuong K, Khan H, et al. Daytime sleepiness and other sleep disorders in Parkinson's disease. Neurology 2001;57:1392–6.
123. Razmy A, Lang AE, Shapiro CM, Predictors of impaired daytime sleep and wakefulness in patients with Parkinson disease treated with older (ergot) vs newer (nonergot) dopamine agonists. Arch Neurol 2004;61:97–102.
124. Holloway RG, Shoulson I, Fahn S, et al. Pramipexole vs levodopa as initial treatment for Parkinson disease: a 4-year randomized controlled trial. Arch Neurol 2004;61:1044–53.
125. Paus S, Brecht HM, Koster J, et al. Sleep attacks, daytime sleepiness, and dopamine agonists in Parkinson's disease. Mov Disord 2003;18:659–67.
126. Hogl B, Saletu M, Brandauer E, et al. Modafinil for the treatment of daytime sleepiness in Parkinson's disease: a double-blind, randomized, crossover, placebo-controlled polygraphic trial. Sleep 2002;25:905–9.
127. Ondo WG, Fayle R, Atassi F, Jankovic J. Modafinil for daytime somnolence in Parkinson's disease: double blind, placebo controlled parallel trial. J Neurol Neurosurg Psychiatry 2005;76:1636-–9.
128. Adler CH, Caviness JN, Hentz JG, et al. Randomized trial of modafinil for treating subjective daytime sleepiness in patients with Parkinson's disease. Mov Disord 2003;18:287–93.
129. Devos D, Krystkowiak P, Clement F, et al. Improvement of gait by chronic, high doses of methylphenidate in patients with advanced Parkinson's disease. J Neurol Neurosurg Psychiatry 2007;78:470–5.
130. Tyne HL, Taylor J, Baker GA, Steiger MJ. Modafinil for Parkinson's disease fatigue. J Neurol 2010;257:452–6.
131. Avorn J, Schneeweiss S, Sudarsky LR, et al. Sudden uncontrollable somnolence and medication use in Parkinson disease. Arch Neurol 2005;62:1242–8.
132. Trotti LM, Bliwise DL. No increased risk of obstructive sleep apnea in Parkinson's disease. Mov Disord 2010;25:2246–9.
133. da Silva-Junior FP, do Prado GF, Barbosa ER, et al. Sleep disordered breathing in Parkinson's disease: A critical appraisal. Sleep Med Rev 2013 Jul 22.
134. Noradina AT, Karim NA, Hamidon BB, et al. Sleep-disordered breathing in patients with Parkinson's disease. Singapore Med J 2010;51:60–4.
135. Oerlemans WG, de Weerd AW. The prevalence of sleep disorders in patients with Parkinson's disease. A self-reported, community-based survey. Sleep Med 2002;3:147–9.
136. Low PA. Prevalence of orthostatic hypotension. Clin Auton Res 2008;18 Suppl 1:8–13.
137. Goldstein DS. Orthostatic hypotension as an early finding in Parkinson's disease. Clin Auton Res 2006;16:46–54.
138. Sharabi Y, Goldstein DS. Mechanisms of orthostatic hypotension and supine hypertension in Parkinson disease. J Neurol Sci 2011;310:123–8.
139. Sanchez-Ferro A, Benito-Leon J, Gomez-Esteban JC. The management of orthostatic hypotension in Parkinson's disease. Front Neurol 2013;4:64.
140. Lahrmann H, Cortelli P, Hilz M, et al. EFNS guidelines on the diagnosis and management of orthostatic hypotension. Eur J Neurol 2006;13:930–6.
141. Schoffer KL, Henderson RD, O'Maley K, O'Sullivan JD. Nonpharmacological treatment, fludrocortisone, and domperidone for orthostatic hypotension in Parkinson's disease. Mov Disord 2007;22:1543–9.
142. Singer W, Sandroni P, Opfer-Gehrking TL, et al. Pyridostigmine treatment trial in neurogenic orthostatic hypotension. Arch Neurol 2006;63:513–8.
143. Low PA, Gilden JL, Freeman R, et al. Efficacy of midodrine vs placebo in neurogenic orthostatic hypotension. A randomized, double-blind multicenter study. Midodrine Study Group. JAMA 1997;277:1046–51.
144. Kaufmann H. L-dihydroxyphenylserine (Droxidopa): a new therapy for neurogenic orthostatic hypotension: the US experience. Clin Auton Res 2008;18 Suppl 1:19–24.
145. Magerkurth C, Schnitzer R, Braune S. Symptoms of autonomic failure in Parkinson's disease: prevalence and impact on daily life. Clin Auton Res 2005;15:76–82.
146. Makaroff L, Gunn A, Gervasoni C, Richy F. Gastrointestinal Disorders in Parkinson's Disease: Prevalence and Health Outcomes in a US Claims Database. J Parkinsons Dis 2011;1:65–74.
147. Abbott RD, Petrovitch H, White LR, et al. Frequency of bowel movements and the future risk of Parkinson's disease. Neurology 2001;57:456–62.
148. Abbott RD, Ross GW, Petrovitch H, et al. Bowel movement frequency in late-life and incidental Lewy bodies. Mov Disord 2007;22:1581–6.
149. Zangaglia R, Martignoni E, Glorioso M, et al. Macrogol for the treatment of constipation in Parkinson's disease. A randomized placebo-controlled study. Mov Disord 2007;22:1239–44.
150. Ondo WG, Kenney C, Sullivan K, et al. Placebo-controlled trial of lubiprostone for constipation associated with Parkinson disease. Neurology 2012;78:1650–4.
151. Cersosimo MG, Benarroch EE. Neural control of the gastrointestinal tract: implications for Parkinson disease. Mov Disord 2008;23:1065–75.
152. Reddymasu SC, Soykan I, McCallum RW. Domperidone: review of pharmacology and clinical applications in gastroenterology. Am J Gastroenterol 2007;102:2036–45.
153. Argolo N, Sampaio M, Pinho P, et al. Do swallowing exercises improve swallowing dynamic and quality of life in Parkinson's disease? NeuroRehabilitation 2013;32:949–55.
154. Troche MS, Sapienza CM, Rosenbek JC. Effects of bolus consistency on timing and safety of swallow in patients with Parkinson's disease. Dysphagia 2008;23:26–32.
155. Edwards LL, Quigley EM, Pfeiffer RF. Gastrointestinal dysfunction in Parkinson's disease: frequency and pathophysiology. Neurology 1992;42:726–32.
156. Kalf JG, Smit AM, Bloem BR, et al. Impact of drooling in Parkinson's disease. J Neurol 2007;254:1227–32.
157. Leibner J, Ramjit A, Sedig L, et al. The impact of and the factors associated with drooling in Parkinson's disease. Parkinsonism Relat Disord 2010;16:475–7.
158. Mancini F, Zangaglia R, Cristina S, et al. Double-blind, placebo-controlled study to evaluate the efficacy and safety of botulinum toxin type A in the treatment of drooling in parkinsonism. Mov Disord 2003;18:685–8.
159. Lagalla G, Millevolte M, Capecci M, et al. Long-lasting benefits of botulinum toxin type B in Parkinson's disease-related drooling. J Neurol 2009;256:563–7.
160. Lagalla G, Millevolte N, Capecci M, et al. Botulinum toxin type A for drooling in Parkinson's disease: a double-blind, randomized, placebo-controlled study. Mov Disord 2006;21:704–7.
161. Arbouw ME, Movig KL, Koopmann M, et al. Glycopyrrolate for sialorrhea in Parkinson disease: a randomized, double-blind, crossover trial. Neurology 2010; 74:1203–7.
162. South AR, Somers, Jog MS. Gum chewing improves swallow frequency and latency in Parkinson patients: a preliminary study. Neurology 2010;74:1198–202.
163. Winge K, Nielsen KK. Bladder dysfunction in advanced Parkinson's disease. Neurourol Urodyn 2012;31:1279–83.
164. Madhuvrata P, Singh PM, Hasafa Z, Abdel-Fattah M. Anticholinergic drugs for adult neurogenic detrusor overactivity: a systematic review and meta-analysis. Eur Urol 2012;62:816–30.
165. Soljanik I. Efficacy and safety of botulinum toxin a intradetrusor injections in adults with neurogenic detrusor overactivity/neurogenic overactive bladder: a systematic review. Drugs 2013;73:1055–66.
166. Sakakibara R, Uchiyama T, Yamanishi T, Kishi M. Genitourinary dysfunction in Parkinson's disease. Mov Disord 2010;25:2–12.
167. Kummer A, Cardoso F, Teixeira AL. Loss of libido in Parkinson's disease. J Sex Med 2009;6:1024–31.
168. Lombardi G, Nelli F, Celso M, et al. Treating erectile dysfunction and central neurological diseases with oral phosphodiesterase type 5 inhibitors. Review of the literature. J Sex Med 2012 9(4): 970–85.
169. Dula E, Bukofzer S, Perdok R, George M. Double-blind, crossover comparison of 3 mg apomorphine SL with placebo and with 4 mg apomorphine SL in male erectile dysfunction. Eur Urol 2001;39(5): 558–3
170. Negre-Pages L, Regragui W, Bouhassira D, et al. Chronic pain in Parkinson's disease: the cross-sectional French DoPaMiP survey. Mov Disord 2008;23:1361–9.
171. Beiske AG, Loge JH, Ronningen A, Svensson E. Pain in Parkinson's disease: Prevalence and characteristics. Pain 2009. 141:173–7.
172. Lin CH, Wu RM, H.Y. Chang HY, et al. Preceding pain symptoms and Parkinson's disease: a nationwide population-based cohort study. Eur J Neurol 2013;20:1398–404.
173. Ha AD, Jankovic J. Pain in Parkinson's disease. Mov Disord 2012; 27:485–91.
174. Ford B. Pain in Parkinson's disease. Mov Disord 2010;25 Suppl 1:S98–103.
175. Loher TJ, Burgunder JM, Weber S, et al. Effect of chronic pallidal deep brain stimulation on off period dystonia and sensory symptoms in advanced Parkinson's disease. J Neurol Neurosurg Psychiatry 2002;73:395–9.
176. Ramirez-Ruiz B, Marti MJ, Tolosa E, et al. Longitudinal evaluation of cerebral morphological changes in Parkinson's disease with and without dementia. J Neurol 2005;252:1345–52.
177. Jellinger KA. Formation and development of Lewy pathology: a critical update. J Neurol 2009;256 Suppl 3:270–9.
178. Levy R, Dubois B. Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cereb Cortex 2006;16:916–28.
179. Connor JR, Boyer PJ, Menzies SL, et al. Neuropathological examination suggests impaired brain iron acquisition in restless legs syndrome. Neurology 2003;61: 304–9.
180. Earley CJ, Barker P, Horská A, Allen RP. MRI-determined regional brain iron concentrations in early- and late-onset restless legs syndrome. Sleep Med 2006;7: 458–61.
181. Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci 2001;24:726–31.
182. Boeve BF, Silber MH, Saper CB, et al. Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease. Brain 2007;130(Pt 11): 2770–88.
183. Benarroch EE, Schmeichel AM, Paris J. Involvement of the ventrolateral medulla in parkinsonism with autonomic failure. Neurology 2000;54:963–8.
184. Chudler EH, Dong WK. The role of the basal ganglia in nociception and pain. Pain 1995;60:3–38.
185. Wolters E. Non-motor extranigral signs and symptoms in Parkinson's disease. Parkinsonism Relat Disord 2009;15 Suppl 3:S6–12.
186. Davidsdottir S, Cronin-Golomb A, Lee A. Visual and spatial symptoms in Parkinson's disease. Vision Res 2005;45:1285–96.
1. Alves G, Forsaa EB, Pedersen KF, et al. Epidemiology of Parkinson's disease. J Neurol 2008;255 Suppl 5:18–32.
2. Stern MB, Lang A, Poewe W. Toward a redefinition of Parkinson's disease. Mov Disord 2012;27:54–60.
3. Braak H, Del Tredici K, Rub U, et al. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging 2003;24:197–211.
4. Tandberg E, Larsen JP, Karlsen K. A community-based study of sleep disorders in patients with Parkinson's disease. Mov Disord 1998;13:895–9.
5. Shulman LM, Taback RL, Bean J, Weiner WJ. Comorbidity of the nonmotor symptoms of Parkinson's disease. Mov Disord 2001;16:507–10.
6. Park A, Stacy M. Non-motor symptoms in Parkinson's disease. J Neurol 2009;256 Suppl 3:293–8.
7. Chaudhuri KR, Schapira AH. Non-motor symptoms of Parkinson's disease: dopaminergic pathophysiology and treatment. Lancet Neurol 2009;8:464–74.
8. Shulman LM, Taback RL, Rabinstein AA, Weiner WJ. Non-recognition of depression and other non-motor symptoms in Parkinson's disease. Parkinsonism Relat Disord 2002;8:193–7.
9. Bonnet AM, Jutras MF, Czernecki V, et al. Nonmotor symptoms in Parkinson's disease in 2012: relevant clinical aspects. Parkinsons Dis 2012:2012:198316.
10. Chaudhuri KR, Healy DG, Schapira AH. Non-motor symptoms of Parkinson's disease: diagnosis and management. Lancet Neurol 2006;5:235–45.
11. Hussl AK, Seppi K, Poewe W. Nonmotor symptoms in Parkinson's disease. Expert Rev Neurother 2013;13:581–3.
12. O'Sullivan SS, Williams DR, Gallagher DA, et al. Nonmotor symptoms as presenting complaints in Parkinson's disease: a clinicopathological study. Mov Disord 2008;23:101–6.
13. Lang AE. A critical appraisal of the premotor symptoms of Parkinson's disease: potential usefulness in early diagnosis and design of neuroprotective trials. Mov Disord 2011;26:775–83.
14. Barone P, Antonini A, Colosimo C, et al. The PRIAMO study: A multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson's disease. Mov Disord 2009;24:1641–9.
15. Bernal-Pacheco O, Limotai N, Go CL, Fernandez HH. Nonmotor manifestations in Parkinson disease. Neurologist 2012;18:1–16.
16. Lemke MR, Fuchs G, Gemende I, et al. Depression and Parkinson's disease. J Neurol 2004;251 Suppl 6: VI/24–7.
17. Ravina, B, Camicioli R, Como PG, et al, The impact of depressive symptoms in early Parkinson disease. Neurology 2007;69:342–7.
18. Jasinska-Myga B, Putzke JD Wider C, et al. Depression in Parkinson's disease. Can J Neurol Sci 2010;37:61–6.
19. Slaughter JR, Slaughter KA, Nichols D, et al. Prevalence, clinical manifestations, etiology, and treatment of depression in Parkinson's disease. J Neuropsychiatry Clin Neurosci 2001;13:187–96.
20. Simuni T, Sethi K. Nonmotor manifestations of Parkinson's disease. Ann Neurol 2008;64 Suppl 2:S65–80.
21. Ehmann TS, Beninger RJ, Gawel MJ, Riopelle RJ. Depressive symptoms in Parkinson's disease: a comparison with disabled control subjects. J Geriatr Psychiatry Neurol 1990;3:3–9.
22. Menza MA, Mark MH. Parkinson's disease and depression: the relationship to disability and personality. J Neuropsychiatry Clin Neurosci 1994;6:165–9.
23. CDC. Current depression among adults–United States, 2006 and 2008. Morb Mort Weekly Rep 2010;59:1229–35.
24. Hinnell C, Hurt CS, Landau S, et al. Nonmotor versus motor symptoms: how much do they matter to health status in Parkinson's disease? Mov Disord 2012;27:236–41.
25. Cummings JL. Depression and Parkinson's disease: a review. Am J Psychiatry 1992;149:443–54.
26. Weintraub D, Morales KH, Moberg PJ, et al. Antidepressant studies in Parkinson's disease: a review and meta-analysis. Mov Disord 2005;20:1161–9.
27. Richard IH, McDermott MP, Kurlan R, et al. A randomized, double-blind, placebo-controlled trial of antidepressants in Parkinson disease. Neurology 2012;78:1229–36.
28. Menza M, Dobkin RD, Marin H, et al. A controlled trial of antidepressants in patients with Parkinson disease and depression. Neurology 2009;72:886–92.
29. Okun MS, Fernandez HH. Will tricyclic antidepressants make a comeback for depressed Parkinson disease patients? Neurology 2009;72:868–9.
30. Antonini A, Tesei S, Zecchinelli A, et al. Randomized study of sertraline and low-dose amitriptyline in patients with Parkinson's disease and depression: effect on quality of life. Mov Disord 2006;21:1119–22.
31. Pedrosa DJ, Timmermann L. Review: management of Parkinson's disease. Neuropsychiatr Dis Treat 2013;9: 321–40.
32. Barone P, Poewe W, Albrecht S, et al. Pramipexole for the treatment of depressive symptoms in patients with Parkinson's disease: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 2010;9:573–80.
33. Lemke MR, Brecht HM, Koester J, et al. Anhedonia, depression, and motor functioning in Parkinson's disease during treatment with pramipexole. J Neuropsychiatry Clin Neurosci 2005;17:214–20.
34. Rektorova I, Balaz M, Svatova J, et al. Effects of ropinirole on nonmotor symptoms of Parkinson disease: a prospective multicenter study. Clin Neuropharmacol 2008;31:261–6.
35. Van der Wurff FB, Stek ML, Hoogendijk WL, Beekman AT. Electroconvulsive therapy for the depressed elderly. Cochrane Database Syst Rev 2003(2):CD003593.
36. Okun MS, Watts RL. Depression associated with Parkinson's disease: clinical features and treatment. Neurology 2002;58(4 Suppl 1):S63–70.
37. Dobkin RD, Menza M, Allen LA, et al. Cognitive-behavioral therapy for depression in Parkinson's disease: a randomized, controlled trial. Am J Psychiatry 2013;168:1066–74.
38. Loprinzi PD, Herod SM, Cardinal BJ, Noakes TD. Physical activity and the brain: A review of this dynamic, bi-directional relationship. Brain Res 2013;1539:95–104.
39. Richard IH. Anxiety disorders in Parkinson's disease. Adv Neurol 2005;96:42–55.
40. Leentjens AF, Dujardin K, Marsh L, et al. Symptomatology and markers of anxiety disorders in Parkinson's disease: a cross-sectional study. Mov Disord 2011;26:484–92.
41. Witjas T, Kaphan E, Azulay JP, et al. Nonmotor fluctuations in Parkinson's disease: frequent and disabling. Neurology 2002;59:408–13.
42. Olanow CW, Stern MB, Sethi K. The scientific and clinical basis for the treatment of Parkinson disease Neurology 2009;72(21 Suppl 4):S1–136.
43. Richard IH, Schiffer RB, Kurlan R. Anxiety and Parkinson's disease. J Neuropsychiatry Clin Neurosci 1996;8:383–92.
44. Fenelon G, Alves G. Epidemiology of psychosis in Parkinson's disease. J Neurol Sci 2010;289:12–7.
45. Papapetropoulos S, Katzen H, Schrag A, et al. A questionnaire-based (UM-PDHQ) study of hallucinations in Parkinson's disease. BMC Neurol 2008;8:21.
46. Fenelon G, Mahieux F, Huon R, Ziegler M. Hallucinations in Parkinson's disease: prevalence, phenomenology and risk factors. Brain 2000;123 (Pt 4):733–45.
47. Papapetropoulos S, Mash DC. Psychotic symptoms in Parkinson's disease. From description to etiology. J Neurol 2005;252:753–64.
48. Aarsland D, Larsen JP, Cummins JL, Laake K. Prevalence and clinical correlates of psychotic symptoms in Parkinson disease: a community-based study. Arch Neurol 1999;56:595–601.
49. Aarsland D, Larsen JP, Tandberg E, Laake K. Predictors of nursing home placement in Parkinson's disease: a population-based, prospective study. J Am Geriatr Soc 2000;48:938–42.
50. Lohle M, Storch A, Reichmann H, Beyond tremor and rigidity: non-motor features of Parkinson's disease. J Neural Transm 2009;116:1483–92.
51. Fenelon G. Psychosis in Parkinson's disease: phenomenology, frequency, risk factors, and current understanding of pathophysiologic mechanisms. CNS Spectr 2008;13(3 Suppl 4):18–25.
52. Merims D, Shabtai H, Korczyn AD, et al. Antiparkinsonian medication is not a risk factor for the development of hallucinations in Parkinson's disease. J Neural Transm 2004;111:1447–53.
53. Merims D, Balas M, Peretz C, et al. Rater-blinded, prospective comparison: quetiapine versus clozapine for Parkinson's disease psychosis. Clin Neuropharmacol 2006;29:331–7.
54. Seppi K, Weintraub D, Coelho M, et al. The Movement Disorder Society Evidence-Based Medicine Review Update: Treatments for the non-motor symptoms of Parkinson's disease. Mov Disord 2011;26 Suppl 3:S42–80.
55. Fernandez HH, Donnelly EM, Friedman JH. Long-term outcome of clozapine use for psychosis in parkinsonian patients. Mov Disord 2004;19:831–3.
56. Morgante L, Epifanio A, Spina E, et al. Quetiapine and clozapine in parkinsonian patients with dopaminergic psychosis. Clin Neuropharmacol 2004;27:153–6.
57. Miyasaki JM, Shannon K, Voon V, et al. Practice parameter: evaluation and treatment of depression, psychosis, and dementia in Parkinson disease (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2006;66:996–1002.
58. Riedel O, Klotsche J, Spottke A, et al. Cognitive impairment in 873 patients with idiopathic Parkinson's disease. Results from the German Study on Epidemiology of Parkinson's Disease with Dementia (GEPAD). J Neurol 2008;255:255–64.
59. Aarsland D, Andersen K, Larsen JP, et al. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch Neurol 2003;60:387–92.
60. Aarsland D, Andersen K, Larsen JP, et al. Risk of dementia in Parkinson's disease: a community-based, prospective study. Neurology 2001;56:730–6.
61. Riggeal BD, Crucian GP, Seignourel P, et al. Cognitive decline tracks motor progression and not disease duration in Parkinson patients. Neuropsychiatr Dis Treat 2007;3:955–8.
62. Hughes TA, Ross HF, Musa S, et al. A 10-year study of the incidence of and factors predicting dementia in Parkinson's disease. Neurology 2000;54:1596–602.
63. Hobson P, Meara J. Risk and incidence of dementia in a cohort of older subjects with Parkinson's disease in the United Kingdom. Mov Disord 2004;19:1043–9.
64. Hoops S, Nazem S, Siderowf AD, et al. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology 2009;73:1738–45.
65. Louis ED, Marder K, Cote L, et al. Mortality from Parkinson disease. Arch Neurol 1997;54:260–4.
66. Fernandez HH. Nonmotor complications of Parkinson disease. Cleve Clin J Med 2012;79 Suppl 2:S14–8.
67. Sollinger AB, Goldstein FC, Lah JJ, et al. Mild cognitive impairment in Parkinson's disease: subtypes and motor characteristics. Parkinsonism Relat Disord 2010;16:177–80.
68. Mak E, Zhou J, Tan LC, et al. Cognitive deficits in mild Parkinson's disease are associated with distinct areas of grey matter atrophy. J Neurol Neurosurg Psychiatry 2013 Oct 16.
69. Emre M, Aarsland D, Albanese A, et al. Rivastigmine for dementia associated with Parkinson's disease. N Engl J Med 2004;351:2509–18.
70. Aarsland D, Ballard C, Walker Z, et al. Memantine in patients with Parkinson's disease dementia or dementia with Lewy bodies: a double-blind, placebo-controlled, multicentre trial. Lancet Neurol 2009;8:613–8.
71. Leroi I, Overshott R, Byrne EJ, et al. Randomized controlled trial of memantine in dementia associated with Parkinson's disease. Mov Disord 2009;24:1217–21.
72. Speelman AD, van de Warrenburg BP, van Nimwegen M, et al. How might physical activity benefit patients with Parkinson disease? Nat Rev Neurol 2013;7:528–34.
73. Naismith SL, Mowszowski L, Diamond K, Lewis SJ. Improving memory in Parkinson's disease: a healthy brain ageing cognitive training program. Mov Disord 2013;28:1097–103.
74. Weintraub D, Koester J, Potenza MN, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol 2010;67:589–95.
75. Voon V, Reynolds B, Brezing C, et al. Impulsive choice and response in dopamine agonist-related impulse control behaviors. Psychopharmacology (Berl) 2010;207:645–59.
76. Weintraub D, Siderowf AD, Potenza MN, et al. Association of dopamine agonist use with impulse control disorders in Parkinson disease. Arch Neurol 2006; 63:969–73.
77. Pontone G, Williams JR, Bassett SS, Marsh L. Clinical features associated with impulse control disorders in Parkinson disease. Neurology 2006;67:1258–61.
78. Voon V, Mehta AR, Hallett M. Impulse control disorders in Parkinson's disease: recent advances. Curr Opin Neurol 2011;24:324–30.
79. Thomas A, Bonanni L, Gambi F, et al. Pathological gambling in Parkinson disease is reduced by amantadine. Ann Neurol 2010;68:400–4.
80. Miyasaki JM, Al Hassan K, Lang AE, Voon V. Punding prevalence in Parkinson's disease. Mov Disord 2007;22:1179–81.
81. Nguyen FN, Chang YL, Okun MS, et al. Prevalence and characteristics of punding and repetitive behaviors among Parkinson patients in North-Central Florida. Int J Geriatr Psychiatry 2010;25:540–1.
82. Sohtaoglu M, Demiray DY, Kenangil G, et al. Long term follow-up of Parkinson's disease patients with impulse control disorders. Parkinsonism Relat Disord 2010;16:334–7.
83. Antonini A, Cilia R. Behavioural adverse effects of dopaminergic treatments in Parkinson's disease: incidence, neurobiological basis, management and prevention. Drug Saf 2009;
32:475–88.
84. Miwa H, Morita S, Nakanishi I, Kondo T, Stereotyped behaviors or punding after quetiapine administration in Parkinson's disease. Parkinsonism Relat Disord 2004;10:177–80.
85. Skorvanek M, Rosenberger J, Gdovinova Z, et al. Apathy in elderly nondemented patients with parkinson's disease: clinical determinants and relationship to quality of life. J Geriatr Psychiatry Neurol 2013;26:237–43.
86. Marin RS, Fogel BS, Hawkins J, et al. Apathy: a treatable syndrome. J Neuropsychiatry Clin Neurosci 1995;7:23–30.
87. Pluck GC, Brown RG. Apathy in Parkinson's disease. J Neurol Neurosurg Psychiatry 2002;73:636–42.
88. Kirsch-Darrow L, Fernandez HH, Marsiske M, et al. Dissociating apathy and depression in Parkinson disease. Neurology 2006;67:33–8.
89. Pedersen KF, Alves G, Aarsland D, Larsen JP. Occurrence and risk factors for apathy in Parkinson disease: a 4-year prospective longitudinal study. J Neurol Neurosurg Psychiatry 2009;80:1279–82.
90. Czernecki V, Pillon B, Houeto JL, et al. Motivation, reward, and Parkinson's disease: influence of dopatherapy. Neuropsychologia 2002;40:2257–67.
91. Parkinson J. An essay on the shaking palsy. Sherwood, Neely, and Jones; 1817.
92. Schenck CH, Mahowald WM. REM sleep behavior disorder: clinical, developmental, and neuroscience perspectives 16 years after its formal identification in SLEEP. Sleep 2002;25:120–38.
93. Sixel-Doring F, Trautmann E, Mollenhauer B, Trenkwalder C. Associated factors for REM sleep behavior disorder in Parkinson disease. Neurology 2011;77:1048–54.
94. Eisensehr I, v Lindeiner H, Jager M, Noachtar S. REM sleep behavior disorder in sleep-disordered patients with versus without Parkinson's disease: is there a need for polysomnography? J Neurol Sci 2001;186:7–11.
95. Postuma RB, Gagnon JF, Montplaisir JY. REM sleep behavior disorder and prodromal neurodegeneration - where are we headed? Tremor Other Hyperkinet Mov (N Y) 2013;3.
96. Iranzo A, Tolosa E, Gelpi E, et al. Neurodegenerative disease status and post-mortem pathology in idiopathic rapid-eye-movement sleep behaviour disorder: an observational cohort study. Lancet Neurol 2013;12: 443–53.
97. Schenck CH, Mahowald MW. Rapid eye movement sleep parasomnias. Neurol Clin 2005;23:1107–26.
98. Anderson KN, Shneerson JM. Drug treatment of REM sleep behavior disorder: the use of drug therapies other than clonazepam. J Clin Sleep Med 2009;5:235–9.
99. Boeve BF, Silber MH, Ferman TJ. Melatonin for treatment of REM sleep behavior disorder in neurologic disorders: results in 14 patients. Sleep Med 2003;4:281–4.
100. Kunz D, Mahlberg R. A two-part, double-blind, placebo-controlled trial of exogenous melatonin in REM sleep behaviour disorder. J Sleep Res 2010;19:591–6.
101. Perroud N, Lazignac C, Baleydier B, et al. Restless legs syndrome induced by citalopram: a psychiatric emergency? Gen Hosp Psychiatry 2007;29:72–4.
102. Buskova J, Vorlova T, Pisko J, Sonka K, Severe sleep-related movement disorder induced by sertraline. Sleep Med 2012;13:769–70.
103. Rittmannsberger H, Werl R. Restless legs syndrome induced by quetiapine: report of seven cases and review of the literature. Int J Neuropsychopharmacol 2013;16:1427–31.
104. Perez-Lloret S, Rey MV, Bondon-Guitton E, et al. Drugs associated with restless legs syndrome: a case/noncase study in the French pharmacovigilance database. J Clin Psychopharmacol 2012;32:824–7.
105. Aurora RN, Kristo DA, Bista SR, et al. The treatment of restless legs syndrome and periodic limb movement disorder in adults-an update for 2012: practice parameters with an evidence-based systematic review and meta-analyses: an American Academy of Sleep Medicine Clinical Practice Guideline. Sleep 2012;35:1039–62.
106. Covassin N, Neikrug AB, Liu L, et al. Clinical correlates of periodic limb movements in sleep in Parkinson's disease. J Neurol Sci 2012;316:131–6.
107. Rios Romenets S, Postuma RB. Treatment of restless legs syndrome. Curr Treat Options Neurol 2013;15:396-409.
108. Hogl B, Paulus W, Clarenbach P, Trenkwalder C, Restless legs syndrome: diagnostic assessment and the advantages and risks of dopaminergic treatment. J Neurol 2006;253 Suppl 4:IV22-8.
109. Garcia-Borreguero D, Kohnen R, Silber MH, et al. The long-term treatment of restless legs syndrome/Willis-Ekbom disease: evidence-based guidelines and clinical consensus best practice guidance: a report from the International Restless Legs Syndrome Study Group. Sleep Med 2013;14:675–84.
110. Montagna P, Hornyak M, Ulfberg J, et al. Randomized trial of pramipexole for patients with restless legs syndrome (RLS) and RLS-related impairment of mood. Sleep Med 2011;12:34–40.
111. Partinen M, Hirvonen K, Jama L, et al. Efficacy and safety of pramipexole in idiopathic restless legs syndrome: a polysomnographic dose-finding study--the PRELUDE study. Sleep Med 2006;7:407–17.
112. Trenkwalder C Garcia-Borreguero D, Montagna P, et al. Ropinirole in the treatment of restless legs syndrome: results from the TREAT RLS 1 study, a 12 week, randomised, placebo controlled study in 10 European countries. J Neurol Neurosurg Psychiatry 2004;75:92–7.
113. Hening WA, Allen RP, Ondo WG, et al. Rotigotine improves restless legs syndrome: a 6-month randomized, double-blind, placebo-controlled trial in the United States. Mov Disord 2010;25:1675–83.
114. Oertel WH, Benes H, Garcia-Borreguero D, et al. Rotigotine transdermal patch in moderate to severe idiopathic restless legs syndrome: a randomized, placebo-controlled polysomnographic study. Sleep Med 2010;11:848–56.
115. Lee DO, Ziman RB, Perkins AT, et al. A randomized, double-blind, placebo-controlled study to assess the efficacy and tolerability of gabapentin enacarbil in subjects with restless legs syndrome. J Clin Sleep Med 2011;7:282–92.
116. Kushida CA, Walters AS, Becker P, et al. A randomized, double-blind, placebo-controlled, crossover study of XP13512/GSK1838262 in the treatment of patients with primary restless legs syndrome. Sleep 2009;32:159–68.
117. Menza M, Dobkin RD, Marin H, Bienfait K. Sleep disturbances in Parkinson's disease. Mov Disord 2010;25 Suppl 1:S117–22.
118. Louter M, van Sloun RJ, Pevernagie DA, et al. Subjectively impaired bed mobility in Parkinson disease affects sleep efficiency. Sleep Med 2013;14:668–74.
119. Menza M, Dobkin RD, Marin H, et al. Treatment of insomnia in Parkinson's disease: a controlled trial of eszopiclone and placebo. Mov Disord 2010;25:1708–14.
120. Nowell PD, Mazumdar S, Buysse DJ, et al. Benzodiazepines and zolpidem for chronic insomnia: a meta-analysis of treatment efficacy. JAMA 1997;278:2170–7.
121. Rios Romenets S, Creti L, Fichten C, et al. Doxepin and cognitive behavioural therapy for insomnia in patients with Parkinson's disease – a randomized study. Parkinsonism Relat Disord 2013;19:670–5.
122. Ondo WG, Dat Vuong K, Khan H, et al. Daytime sleepiness and other sleep disorders in Parkinson's disease. Neurology 2001;57:1392–6.
123. Razmy A, Lang AE, Shapiro CM, Predictors of impaired daytime sleep and wakefulness in patients with Parkinson disease treated with older (ergot) vs newer (nonergot) dopamine agonists. Arch Neurol 2004;61:97–102.
124. Holloway RG, Shoulson I, Fahn S, et al. Pramipexole vs levodopa as initial treatment for Parkinson disease: a 4-year randomized controlled trial. Arch Neurol 2004;61:1044–53.
125. Paus S, Brecht HM, Koster J, et al. Sleep attacks, daytime sleepiness, and dopamine agonists in Parkinson's disease. Mov Disord 2003;18:659–67.
126. Hogl B, Saletu M, Brandauer E, et al. Modafinil for the treatment of daytime sleepiness in Parkinson's disease: a double-blind, randomized, crossover, placebo-controlled polygraphic trial. Sleep 2002;25:905–9.
127. Ondo WG, Fayle R, Atassi F, Jankovic J. Modafinil for daytime somnolence in Parkinson's disease: double blind, placebo controlled parallel trial. J Neurol Neurosurg Psychiatry 2005;76:1636-–9.
128. Adler CH, Caviness JN, Hentz JG, et al. Randomized trial of modafinil for treating subjective daytime sleepiness in patients with Parkinson's disease. Mov Disord 2003;18:287–93.
129. Devos D, Krystkowiak P, Clement F, et al. Improvement of gait by chronic, high doses of methylphenidate in patients with advanced Parkinson's disease. J Neurol Neurosurg Psychiatry 2007;78:470–5.
130. Tyne HL, Taylor J, Baker GA, Steiger MJ. Modafinil for Parkinson's disease fatigue. J Neurol 2010;257:452–6.
131. Avorn J, Schneeweiss S, Sudarsky LR, et al. Sudden uncontrollable somnolence and medication use in Parkinson disease. Arch Neurol 2005;62:1242–8.
132. Trotti LM, Bliwise DL. No increased risk of obstructive sleep apnea in Parkinson's disease. Mov Disord 2010;25:2246–9.
133. da Silva-Junior FP, do Prado GF, Barbosa ER, et al. Sleep disordered breathing in Parkinson's disease: A critical appraisal. Sleep Med Rev 2013 Jul 22.
134. Noradina AT, Karim NA, Hamidon BB, et al. Sleep-disordered breathing in patients with Parkinson's disease. Singapore Med J 2010;51:60–4.
135. Oerlemans WG, de Weerd AW. The prevalence of sleep disorders in patients with Parkinson's disease. A self-reported, community-based survey. Sleep Med 2002;3:147–9.
136. Low PA. Prevalence of orthostatic hypotension. Clin Auton Res 2008;18 Suppl 1:8–13.
137. Goldstein DS. Orthostatic hypotension as an early finding in Parkinson's disease. Clin Auton Res 2006;16:46–54.
138. Sharabi Y, Goldstein DS. Mechanisms of orthostatic hypotension and supine hypertension in Parkinson disease. J Neurol Sci 2011;310:123–8.
139. Sanchez-Ferro A, Benito-Leon J, Gomez-Esteban JC. The management of orthostatic hypotension in Parkinson's disease. Front Neurol 2013;4:64.
140. Lahrmann H, Cortelli P, Hilz M, et al. EFNS guidelines on the diagnosis and management of orthostatic hypotension. Eur J Neurol 2006;13:930–6.
141. Schoffer KL, Henderson RD, O'Maley K, O'Sullivan JD. Nonpharmacological treatment, fludrocortisone, and domperidone for orthostatic hypotension in Parkinson's disease. Mov Disord 2007;22:1543–9.
142. Singer W, Sandroni P, Opfer-Gehrking TL, et al. Pyridostigmine treatment trial in neurogenic orthostatic hypotension. Arch Neurol 2006;63:513–8.
143. Low PA, Gilden JL, Freeman R, et al. Efficacy of midodrine vs placebo in neurogenic orthostatic hypotension. A randomized, double-blind multicenter study. Midodrine Study Group. JAMA 1997;277:1046–51.
144. Kaufmann H. L-dihydroxyphenylserine (Droxidopa): a new therapy for neurogenic orthostatic hypotension: the US experience. Clin Auton Res 2008;18 Suppl 1:19–24.
145. Magerkurth C, Schnitzer R, Braune S. Symptoms of autonomic failure in Parkinson's disease: prevalence and impact on daily life. Clin Auton Res 2005;15:76–82.
146. Makaroff L, Gunn A, Gervasoni C, Richy F. Gastrointestinal Disorders in Parkinson's Disease: Prevalence and Health Outcomes in a US Claims Database. J Parkinsons Dis 2011;1:65–74.
147. Abbott RD, Petrovitch H, White LR, et al. Frequency of bowel movements and the future risk of Parkinson's disease. Neurology 2001;57:456–62.
148. Abbott RD, Ross GW, Petrovitch H, et al. Bowel movement frequency in late-life and incidental Lewy bodies. Mov Disord 2007;22:1581–6.
149. Zangaglia R, Martignoni E, Glorioso M, et al. Macrogol for the treatment of constipation in Parkinson's disease. A randomized placebo-controlled study. Mov Disord 2007;22:1239–44.
150. Ondo WG, Kenney C, Sullivan K, et al. Placebo-controlled trial of lubiprostone for constipation associated with Parkinson disease. Neurology 2012;78:1650–4.
151. Cersosimo MG, Benarroch EE. Neural control of the gastrointestinal tract: implications for Parkinson disease. Mov Disord 2008;23:1065–75.
152. Reddymasu SC, Soykan I, McCallum RW. Domperidone: review of pharmacology and clinical applications in gastroenterology. Am J Gastroenterol 2007;102:2036–45.
153. Argolo N, Sampaio M, Pinho P, et al. Do swallowing exercises improve swallowing dynamic and quality of life in Parkinson's disease? NeuroRehabilitation 2013;32:949–55.
154. Troche MS, Sapienza CM, Rosenbek JC. Effects of bolus consistency on timing and safety of swallow in patients with Parkinson's disease. Dysphagia 2008;23:26–32.
155. Edwards LL, Quigley EM, Pfeiffer RF. Gastrointestinal dysfunction in Parkinson's disease: frequency and pathophysiology. Neurology 1992;42:726–32.
156. Kalf JG, Smit AM, Bloem BR, et al. Impact of drooling in Parkinson's disease. J Neurol 2007;254:1227–32.
157. Leibner J, Ramjit A, Sedig L, et al. The impact of and the factors associated with drooling in Parkinson's disease. Parkinsonism Relat Disord 2010;16:475–7.
158. Mancini F, Zangaglia R, Cristina S, et al. Double-blind, placebo-controlled study to evaluate the efficacy and safety of botulinum toxin type A in the treatment of drooling in parkinsonism. Mov Disord 2003;18:685–8.
159. Lagalla G, Millevolte M, Capecci M, et al. Long-lasting benefits of botulinum toxin type B in Parkinson's disease-related drooling. J Neurol 2009;256:563–7.
160. Lagalla G, Millevolte N, Capecci M, et al. Botulinum toxin type A for drooling in Parkinson's disease: a double-blind, randomized, placebo-controlled study. Mov Disord 2006;21:704–7.
161. Arbouw ME, Movig KL, Koopmann M, et al. Glycopyrrolate for sialorrhea in Parkinson disease: a randomized, double-blind, crossover trial. Neurology 2010; 74:1203–7.
162. South AR, Somers, Jog MS. Gum chewing improves swallow frequency and latency in Parkinson patients: a preliminary study. Neurology 2010;74:1198–202.
163. Winge K, Nielsen KK. Bladder dysfunction in advanced Parkinson's disease. Neurourol Urodyn 2012;31:1279–83.
164. Madhuvrata P, Singh PM, Hasafa Z, Abdel-Fattah M. Anticholinergic drugs for adult neurogenic detrusor overactivity: a systematic review and meta-analysis. Eur Urol 2012;62:816–30.
165. Soljanik I. Efficacy and safety of botulinum toxin a intradetrusor injections in adults with neurogenic detrusor overactivity/neurogenic overactive bladder: a systematic review. Drugs 2013;73:1055–66.
166. Sakakibara R, Uchiyama T, Yamanishi T, Kishi M. Genitourinary dysfunction in Parkinson's disease. Mov Disord 2010;25:2–12.
167. Kummer A, Cardoso F, Teixeira AL. Loss of libido in Parkinson's disease. J Sex Med 2009;6:1024–31.
168. Lombardi G, Nelli F, Celso M, et al. Treating erectile dysfunction and central neurological diseases with oral phosphodiesterase type 5 inhibitors. Review of the literature. J Sex Med 2012 9(4): 970–85.
169. Dula E, Bukofzer S, Perdok R, George M. Double-blind, crossover comparison of 3 mg apomorphine SL with placebo and with 4 mg apomorphine SL in male erectile dysfunction. Eur Urol 2001;39(5): 558–3
170. Negre-Pages L, Regragui W, Bouhassira D, et al. Chronic pain in Parkinson's disease: the cross-sectional French DoPaMiP survey. Mov Disord 2008;23:1361–9.
171. Beiske AG, Loge JH, Ronningen A, Svensson E. Pain in Parkinson's disease: Prevalence and characteristics. Pain 2009. 141:173–7.
172. Lin CH, Wu RM, H.Y. Chang HY, et al. Preceding pain symptoms and Parkinson's disease: a nationwide population-based cohort study. Eur J Neurol 2013;20:1398–404.
173. Ha AD, Jankovic J. Pain in Parkinson's disease. Mov Disord 2012; 27:485–91.
174. Ford B. Pain in Parkinson's disease. Mov Disord 2010;25 Suppl 1:S98–103.
175. Loher TJ, Burgunder JM, Weber S, et al. Effect of chronic pallidal deep brain stimulation on off period dystonia and sensory symptoms in advanced Parkinson's disease. J Neurol Neurosurg Psychiatry 2002;73:395–9.
176. Ramirez-Ruiz B, Marti MJ, Tolosa E, et al. Longitudinal evaluation of cerebral morphological changes in Parkinson's disease with and without dementia. J Neurol 2005;252:1345–52.
177. Jellinger KA. Formation and development of Lewy pathology: a critical update. J Neurol 2009;256 Suppl 3:270–9.
178. Levy R, Dubois B. Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cereb Cortex 2006;16:916–28.
179. Connor JR, Boyer PJ, Menzies SL, et al. Neuropathological examination suggests impaired brain iron acquisition in restless legs syndrome. Neurology 2003;61: 304–9.
180. Earley CJ, Barker P, Horská A, Allen RP. MRI-determined regional brain iron concentrations in early- and late-onset restless legs syndrome. Sleep Med 2006;7: 458–61.
181. Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci 2001;24:726–31.
182. Boeve BF, Silber MH, Saper CB, et al. Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease. Brain 2007;130(Pt 11): 2770–88.
183. Benarroch EE, Schmeichel AM, Paris J. Involvement of the ventrolateral medulla in parkinsonism with autonomic failure. Neurology 2000;54:963–8.
184. Chudler EH, Dong WK. The role of the basal ganglia in nociception and pain. Pain 1995;60:3–38.
185. Wolters E. Non-motor extranigral signs and symptoms in Parkinson's disease. Parkinsonism Relat Disord 2009;15 Suppl 3:S6–12.
186. Davidsdottir S, Cronin-Golomb A, Lee A. Visual and spatial symptoms in Parkinson's disease. Vision Res 2005;45:1285–96.
Assessment of Health Literacy as a Predictor of Asthma Exacerbation Among Puerto Rican Veteran Patients
Pseudomyocardial Infarction in Diabetic Ketoacidosis: A Clinical and Diagnostic Dilemma
Non–Daily-Dosed Rosuvastatin in Statin-Intolerant Veterans
Preventing Bilateral Injuries in SQ Injections
Irreducible Posterolateral Elbow Dislocation
Perinatal depression: what you can do to reduce its long-term effects
We’ve come a long way in our understanding of depression—and that’s a good thing. Consider the treatments popular in the late 18th and early 19th Centuries, for example, which included water immersion (short of drowning), spinning (to reorder the contents of the brain), and the induction of vomiting and administration of enemas, not to mention institutionalization.1 These modalities wouldn’t attract many patients (or clinicians) today.
And yet, even our distant forebears had some inkling of the potential for depression to continue from one generation to the next. As Trotula of Salerno noted around the 11th Century:
In other words, melancholy (aka depression) sometimes has its origins in the womb.
From our 21st Century vantage point, we understand this conclusion in more scientific terms. Data suggest than 14% to 23% of pregnant women will experience depressive symptoms during pregnancy,3 with the potential for long-term effects in the child. In the largest study to date on the effects of antenatal and postnatal parental depression on offspring, Pearson and colleagues found that children of mothers who are depressed during pregnancy are likely to experience depression themselves at age 18.4 Specifically, for each standard-deviation increase in the antenatal maternal depression score, offspring were 1.28 times more likely to have depression at age 18 (95% confidence interval [CI], 1.08–1.51; P = .003).4
Related Article: A talk about, then a plan for, antidepressants in pregnancy Danielle Carlin, MD, and Louann Brizendine, MD (May 2011)
Maternal depression in the postnatal period also was found to be a risk factor for depression in offspring, but only among mothers with “low education” (defined as either no education or compulsory education ending at or before age 16).4 For each standard-deviation increase in the postnatal maternal depression score in this population, offspring were 1.26 times more likely to have depression at age 18, compared with the children of nondepressed women (95% CI, 1.06–1.50; P = .01).4
Although antenatal depression in fathers was not associated with an increased incidence of depression in offspring, postnatal depression was—but only when the fathers had low education.4
As for the mechanism of transmission of depression from parent to child? Although Pearson and colleagues did not attempt to identify it, they did observe that the differential effects of maternal and paternal antenatal depression—with only maternal depression having an impact on offspring—suggest that, in pregnancy, maternal depression may be transmitted to her child “through the biological consequences of depression in utero.”4
Clearly, if it goes unchecked during pregnancy, maternal depression has the potential to ravage the life of both mother and child. In this article, I review guidance on the management of depression in pregnancy from the American College of Obstetricians and Gynecologists (ACOG) and the American Psychiatric Association (APA), and I offer insights from a perinatal psychiatrist on how ObGyns might adjust their practices to reduce the impact of depression on both mother and infant.
COMPLICATIONS OF PERINATAL DEPRESSION
In a joint report on depression and pregnancy from ACOG and the APA, Yonkers and colleagues noted that low birth weight, neonatal irritability, and diminished neonatal activity and attentiveness are among the adverse reproductive outcomes that have been associated with untreated maternal depression.3 Reproductive outcomes are more dire if maternal depression is severe or if the mother has bipolar disorder or postpartum psychosis, potentially including infanticide or death from suicide.5
Pregnancy complications such as vomiting, nausea, hyperemesis gravidarum, and preeclampsia appear to occur more frequently in depressed women than in nondepressed women, according to the ACOG/APA report,3 although this finding is based on limited data, notes Leena P. Mittal, MD, director of the Reproductive Psychiatry Consultation Service at Brigham and Women’s Hospital in Boston and instructor in psychiatry at Harvard Medical School.
“The trouble with those studies in general is the difficulty of controlling for both the severity of depression and the effects of treatment of depression—or the effects of treatment versus effects of the illness itself,” she says.
That difficulty is compounded by the likely use of multiple medications—
including nonpsychiatric agents—during pregnancy, “which makes it difficult to assess the impact of a single compound, such as an antidepressant, on maternal and fetal outcomes,” according to ACOG and the APA.3 (More than 80% of pregnant women take at least one dose of a medication.3)
HOW THE OBGYN CAN MAKE A DIFFERENCE
Because of the potential for adverse short- and long-term effects of perinatal depression, “there is a need to identify it and attempt to address it prior to the postpartum period,” Dr. Mittal says. “If a woman has depressive symptoms during pregnancy, it is important to try to direct her toward treatment—either by initiating treatment yourself or referring her to a psychiatrist or psychiatric care provider before she enters the postpartum period.” Once she’s postpartum, she will be exposed to additional variables that will influence the severity and duration of her depression, Dr. Mittal says.
Screen all pregnant women for depression
Dr. Mittal recommends routine screening of all perinatal women.
“The data are not entirely clear about the intervals at which these women should be screened,” she says, “but the recommendation would be screening at least once during pregnancy and then again postpartum. Some clinicians screen for depression during each trimester of pregnancy.”
At Dr. Mittal’s institution, such screening usually takes place at the patient’s first prenatal visit.
The screening tools with the most high-quality data backing them include the:
- Edinburgh Postnatal Depression Scale (EPDS). “Despite its name, this tool has been validated for use during pregnancy and for use in the nonperinatal woman as well,” Dr. Mittal notes. It also is in the public domain (http://www.fresno.ucsf.edu/pediatrics/downloads/edinburghscale.pdf). “It’s particularly useful during pregnancy because it assesses the woman for symptoms of depression at the same time that it separates those symptoms from the physical symptoms of pregnancy—there can be some overlap.” The EPDS is self-administered, brief (10 questions), and easily assessed by the clinician, with a score of 10 or above indicating a likelihood of depression.6 It has been validated in more than a dozen languages, as well.
- Patient Health Questionnaire (PHQ-9).7 This is another public-domain tool validated for use during pregnancy (http://www.cqaimh.org/pdf/tool_phq9.pdf). It is utilized widely in primary care and closely associated with depression criteria listed in the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders. Like the EPDS, it is self-administered, brief (9 questions), and easy to score. In general, PHQ-9 scores of 5, 10, 15, and 20 represent mild, moderate, moderately severe, and severe depression, respectively.8
Neither of these tools should override clinical judgment. Even with a positive score, clinical assessment is recommended. Nor are these tools designed to detect anxiety, personality disorders, and phobias.
Try to address the issue before conception
The best time to address perinatal depression, of course, with a conversation about prevention, is during the preconception period. Having time before pregnancy to determine the best perinatal management approach is especially valuable.
“What’s important for an ObGyn to consider when counseling someone who is contemplating pregnancy and who has a history of depression is the need to weigh the risks of treatment during pregnancy against the risks of nontreatment,” says Dr. Mittal. Two ways to do that are to assess the severity of her depressive symptoms—both currently and historically—and explore her response to treatment.
“Obviously, suicidality and psychosis suggest very severe illness, whether they are currently present or occurred in the past, and so does a history of psychiatric hospitalization,” says Dr. Mittal. “In such cases, the untreated illness itself carries significant risk, and when it is weighed against the perhaps smaller risk of antidepressant medication during pregnancy, the risk-benefit analysis likely is very different than it might be for someone with mild to moderate depression. I would definitely agree that addressing severity from the beginning is important.”
An understanding of the patient’s response to treatment also is beneficial. Has any treatment been helpful? If so, that information can guide the choice of treatment during pregnancy, says Dr. Mittal. Even knowing whether a woman has responded to nonpharmacologic therapy such as psychotherapy can help shape the treatment plan.
“It might mean that there’s a way to limit the risk of exposure to a variety of psychotropic medications,” Dr. Mittal says. “Or if the patient has had a good response to a particular medication, it might make sense to try that agent again—or, if she’s currently taking it, to stick with it.”
Even if preconception counseling is difficult to achieve, ObGyns see a large number of women of reproductive age during the course of routine gynecologic care.
“I do think it’s worth having a discussion about reproductive planning, especially in the context of their psychiatric illness or history, even if they aren’t currently planning a pregnancy,” says Dr. Mittal.
When to refer the patient to a psychiatrist
Again, the severity of symptoms comes into play.
“In severe mental illness—bipolar disorder, psychotic disorders, or a history of severe illness requiring psychiatric hospitalization—it is important to have a psychiatrist involved,” says Dr. Mittal.
“Even if the woman is stable during pregnancy, the postpartum risk—especially in bipolar disorder—is extremely high. The postpartum period is a vulnerable time, anyway, because obstetric care is coming to its end, and there’s a lot changing irrespective of mental illness. So a patient who’s at high risk for postpartum illness should have a psychiatrist on board as early as possible.”
Consultation with a psychiatrist is another option when managing women with severe depression, a significant psychiatric history, or refractory illness.
Should you prescribe antidepressant medication?
Dr. Mittal believes that ObGyns should feel fairly comfortable prescribing antidepressant medication to patients who have mild or moderate depression, provided that the initiation of such medication is the patient’s informed choice.
Once severe disease (including bipolar disorder and a history of suicidality or psychosis or psychiatric hospitalization) has been ruled out and a history indicates that the patient has mild to moderate symptoms and has responded to treatment, an ObGyn is well qualified to treat perinatal depression, says Dr. Mittal.
Typically, SSRIs are the first-line treatment for perinatal depression and generally have similar amounts of data about their risk in pregnancy. Paroxetine (Paxil) is the exception, as we have more data about the risk for cardiac defects in neonates exposed to it in utero, Dr. Mittal says.
SSRIs generally are found in low amounts in breast milk, although sertraline (Zoloft) generally is found in the smallest quantity, making it the most commonly used SSRI in pregnancy. Sertraline is followed by citalopram (Celexa), escitalopram (Lexapro), and fluoxetine (Prozac) in the respective amount of medication passed into breast milk.
The literature around the teratogenic risks of psychiatric medications is extremely diverse, she says. The “sum total” of the data suggests that SSRIs have relatively few teratogenic risks. “The overall story around SSRIs does not appear to suggest that they carry a risk of major malformations.”
Related Article: Antidepressants linked to pregnancy risks in infertility treatment (News for Your Practice, December 2012)
Dr. Mittal also recommends keeping in mind the possibility that psychotherapy alone is sometimes sufficient for a woman with mild to moderate depression.
“If she has a history of responding to psychotherapy alone and also has mild to moderate symptoms, I think a reasonable approach would be to try it again.”
“This is where preconception planning is especially useful,” she says. “If somebody with mild to moderate symptoms has never had a good trial of psychotherapy, the preconception period is a good time to determine whether it might be effective, to shape the optimal treatment plan.”
Two forms of psychotherapy have solid evidence of efficacy in perinatal depression:
- cognitive behavioral therapy (CBT) —an action-oriented approach that treats maladaptive thinking as the cause of pathologic behavior and “negative” emotions
- interpersonal psychotherapy (IPT)—a treatment in which the patient is educated about depression and its symptoms and her relation to the environment, especially social functioning. Unlike some other forms of therapy, IPT does not focus on underlying personality structures.
There are other forms of psychotherapy, but CBT and IPT have a large evidence base and are generally time-limited, rather than open-ended. They also are manualized and problem-focused, says Dr. Mittal.
How to prescribe an SSRI
SSRIs generally are initiated at a low dose and gradually titrated up (if necessary). A typical starting dose of sertraline, for example, would be 25 to 50 mg. The patient should be counseled about potential side effects, which include increased perspiration, somnolence or insomnia, nausea, diarrhea, headache, dizziness, and restlessness. These effects generally begin to subside the first week or two after initiation.
Sexual side effects such as reduced desire and difficulties with orgasm also may occur and generally do not diminish over time.
The patient also should be advised not to discontinue the SSRI abruptly, if at all possible, because of the risk that she might develop mild discontinuation syndrome. Although this syndrome is short-lived, self-limited, and non-life-threatening, it is uncomfortable. Symptoms include changes in mood or anxiety, shakiness, tremor, or gastrointestinal disturbance. If the patient elects to discontinue an SSRI, tapering over 4 to 7 days is preferable. However, in the event that the patient exhibits an adverse reaction or intolerance to antidepressant medication, immediate discontinuation may be appropriate, says Dr. Mittal.
After initiating SSRI therapy, follow-up in 2 weeks is appropriate, after which time oversight can be transferred to the patient’s primary care provider. In the United States, primary care physicians prescribe the bulk of SSRI medications.
It may take 6 to 8 weeks for the medication to begin to reduce depressive symptoms, although sleep and appetite sometimes improve within 1 or 2 weeks.
Avoid abrupt drug discontinuation in pregnancy
When asked to recommend one intervention that would have a big impact on reducing the burden of depression in pregnancy, Dr. Mittal zeroed in on the population of women who elect to discontinue antidepressant medication during pregnancy.
“I would suggest that ObGyns discourage these women against abrupt discontinuation,” she says. “There is a small body of literature that demonstrates that, in patients with significant illness—severe depression and bipolar disorder, certainly—abrupt discontinuation increases the likelihood of recurrence in the short period of time afterward. If medication is abruptly stopped when a woman discovers she’s pregnant, she’s likely to need to return to treatment during pregnancy because of recurrent symptoms. What happens in that case is that her pregnancy is exposed to both severe symptoms and the reinitiation of treatment, possibly including additional medications beyond the initial agent,” says Dr. Mittal.
Many women assume they should never get pregnant because of their mental health issues, their medications, or both, says Dr. Mittal. Or they believe they must stop their meds if they become pregnant. In fact, some patients report that they have been counseled to avoid medication in pregnancy by their psychiatrist or obstetrician!
“I have spoken to many psychiatrists who say they are not comfortable prescribing to pregnant women, so they either drop the patients or stop their meds!” she says.
When that happens, the patient should find another psychiatrist.
WE WANT TO HEAR FROM YOU!
Drop us a line and let us know what you think about current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: [email protected]
- Nemade R, Reiss NS, Dombeck M. Historical understandings of depression. Mentalhelp.net. http://www.mentalhelp.net/poc/view_doc.php?type=doc&id=12995&cn=5. Published September 19, 2007. Accessed January 13, 2014.
- Brockington I. A historical perspective on the psychiatry of motherhood. In: Perinatal Stress, Mood, and Anxiety Disorders. Basel, Switzerland: S Karger AG; 2005.
- Yonkers KA, Wisner KL, Stewart DE, et al. The management of depression during pregnancy: A report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Obstet Gynecol. 2009;114(3):703–713.
- Pearson RM, Evans J, Kounali D, et al. Maternal depression during pregnancy and the postnatal period. Risks and possible mechanisms for offspring depression at age 18 years [published online ahead of print October 9, 2013]. JAMA Psychiatry. doi:10.1001/jamapsychiatry.2013.2163.
- Hasser C, Brizendine L, Spielvogel A. SSRI use during pregnancy. Current Psychiatry. 2006;5(4):31–40.
- Edinburgh Postnatal Depression Scale. http://www.fresno.ucsf.edu/pediatrics/downloads/edinburghscale.pdf. Accessed January 14, 2014.
- Patient Health Questionnaire (PHQ-9). http://www.cqaimh.org/pdf/tool_phq9.pdf. Accessed January 14, 2014.
- Kroenke K, Spitzer R, Williams W. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613.
We’ve come a long way in our understanding of depression—and that’s a good thing. Consider the treatments popular in the late 18th and early 19th Centuries, for example, which included water immersion (short of drowning), spinning (to reorder the contents of the brain), and the induction of vomiting and administration of enemas, not to mention institutionalization.1 These modalities wouldn’t attract many patients (or clinicians) today.
And yet, even our distant forebears had some inkling of the potential for depression to continue from one generation to the next. As Trotula of Salerno noted around the 11th Century:
In other words, melancholy (aka depression) sometimes has its origins in the womb.
From our 21st Century vantage point, we understand this conclusion in more scientific terms. Data suggest than 14% to 23% of pregnant women will experience depressive symptoms during pregnancy,3 with the potential for long-term effects in the child. In the largest study to date on the effects of antenatal and postnatal parental depression on offspring, Pearson and colleagues found that children of mothers who are depressed during pregnancy are likely to experience depression themselves at age 18.4 Specifically, for each standard-deviation increase in the antenatal maternal depression score, offspring were 1.28 times more likely to have depression at age 18 (95% confidence interval [CI], 1.08–1.51; P = .003).4
Related Article: A talk about, then a plan for, antidepressants in pregnancy Danielle Carlin, MD, and Louann Brizendine, MD (May 2011)
Maternal depression in the postnatal period also was found to be a risk factor for depression in offspring, but only among mothers with “low education” (defined as either no education or compulsory education ending at or before age 16).4 For each standard-deviation increase in the postnatal maternal depression score in this population, offspring were 1.26 times more likely to have depression at age 18, compared with the children of nondepressed women (95% CI, 1.06–1.50; P = .01).4
Although antenatal depression in fathers was not associated with an increased incidence of depression in offspring, postnatal depression was—but only when the fathers had low education.4
As for the mechanism of transmission of depression from parent to child? Although Pearson and colleagues did not attempt to identify it, they did observe that the differential effects of maternal and paternal antenatal depression—with only maternal depression having an impact on offspring—suggest that, in pregnancy, maternal depression may be transmitted to her child “through the biological consequences of depression in utero.”4
Clearly, if it goes unchecked during pregnancy, maternal depression has the potential to ravage the life of both mother and child. In this article, I review guidance on the management of depression in pregnancy from the American College of Obstetricians and Gynecologists (ACOG) and the American Psychiatric Association (APA), and I offer insights from a perinatal psychiatrist on how ObGyns might adjust their practices to reduce the impact of depression on both mother and infant.
COMPLICATIONS OF PERINATAL DEPRESSION
In a joint report on depression and pregnancy from ACOG and the APA, Yonkers and colleagues noted that low birth weight, neonatal irritability, and diminished neonatal activity and attentiveness are among the adverse reproductive outcomes that have been associated with untreated maternal depression.3 Reproductive outcomes are more dire if maternal depression is severe or if the mother has bipolar disorder or postpartum psychosis, potentially including infanticide or death from suicide.5
Pregnancy complications such as vomiting, nausea, hyperemesis gravidarum, and preeclampsia appear to occur more frequently in depressed women than in nondepressed women, according to the ACOG/APA report,3 although this finding is based on limited data, notes Leena P. Mittal, MD, director of the Reproductive Psychiatry Consultation Service at Brigham and Women’s Hospital in Boston and instructor in psychiatry at Harvard Medical School.
“The trouble with those studies in general is the difficulty of controlling for both the severity of depression and the effects of treatment of depression—or the effects of treatment versus effects of the illness itself,” she says.
That difficulty is compounded by the likely use of multiple medications—
including nonpsychiatric agents—during pregnancy, “which makes it difficult to assess the impact of a single compound, such as an antidepressant, on maternal and fetal outcomes,” according to ACOG and the APA.3 (More than 80% of pregnant women take at least one dose of a medication.3)
HOW THE OBGYN CAN MAKE A DIFFERENCE
Because of the potential for adverse short- and long-term effects of perinatal depression, “there is a need to identify it and attempt to address it prior to the postpartum period,” Dr. Mittal says. “If a woman has depressive symptoms during pregnancy, it is important to try to direct her toward treatment—either by initiating treatment yourself or referring her to a psychiatrist or psychiatric care provider before she enters the postpartum period.” Once she’s postpartum, she will be exposed to additional variables that will influence the severity and duration of her depression, Dr. Mittal says.
Screen all pregnant women for depression
Dr. Mittal recommends routine screening of all perinatal women.
“The data are not entirely clear about the intervals at which these women should be screened,” she says, “but the recommendation would be screening at least once during pregnancy and then again postpartum. Some clinicians screen for depression during each trimester of pregnancy.”
At Dr. Mittal’s institution, such screening usually takes place at the patient’s first prenatal visit.
The screening tools with the most high-quality data backing them include the:
- Edinburgh Postnatal Depression Scale (EPDS). “Despite its name, this tool has been validated for use during pregnancy and for use in the nonperinatal woman as well,” Dr. Mittal notes. It also is in the public domain (http://www.fresno.ucsf.edu/pediatrics/downloads/edinburghscale.pdf). “It’s particularly useful during pregnancy because it assesses the woman for symptoms of depression at the same time that it separates those symptoms from the physical symptoms of pregnancy—there can be some overlap.” The EPDS is self-administered, brief (10 questions), and easily assessed by the clinician, with a score of 10 or above indicating a likelihood of depression.6 It has been validated in more than a dozen languages, as well.
- Patient Health Questionnaire (PHQ-9).7 This is another public-domain tool validated for use during pregnancy (http://www.cqaimh.org/pdf/tool_phq9.pdf). It is utilized widely in primary care and closely associated with depression criteria listed in the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders. Like the EPDS, it is self-administered, brief (9 questions), and easy to score. In general, PHQ-9 scores of 5, 10, 15, and 20 represent mild, moderate, moderately severe, and severe depression, respectively.8
Neither of these tools should override clinical judgment. Even with a positive score, clinical assessment is recommended. Nor are these tools designed to detect anxiety, personality disorders, and phobias.
Try to address the issue before conception
The best time to address perinatal depression, of course, with a conversation about prevention, is during the preconception period. Having time before pregnancy to determine the best perinatal management approach is especially valuable.
“What’s important for an ObGyn to consider when counseling someone who is contemplating pregnancy and who has a history of depression is the need to weigh the risks of treatment during pregnancy against the risks of nontreatment,” says Dr. Mittal. Two ways to do that are to assess the severity of her depressive symptoms—both currently and historically—and explore her response to treatment.
“Obviously, suicidality and psychosis suggest very severe illness, whether they are currently present or occurred in the past, and so does a history of psychiatric hospitalization,” says Dr. Mittal. “In such cases, the untreated illness itself carries significant risk, and when it is weighed against the perhaps smaller risk of antidepressant medication during pregnancy, the risk-benefit analysis likely is very different than it might be for someone with mild to moderate depression. I would definitely agree that addressing severity from the beginning is important.”
An understanding of the patient’s response to treatment also is beneficial. Has any treatment been helpful? If so, that information can guide the choice of treatment during pregnancy, says Dr. Mittal. Even knowing whether a woman has responded to nonpharmacologic therapy such as psychotherapy can help shape the treatment plan.
“It might mean that there’s a way to limit the risk of exposure to a variety of psychotropic medications,” Dr. Mittal says. “Or if the patient has had a good response to a particular medication, it might make sense to try that agent again—or, if she’s currently taking it, to stick with it.”
Even if preconception counseling is difficult to achieve, ObGyns see a large number of women of reproductive age during the course of routine gynecologic care.
“I do think it’s worth having a discussion about reproductive planning, especially in the context of their psychiatric illness or history, even if they aren’t currently planning a pregnancy,” says Dr. Mittal.
When to refer the patient to a psychiatrist
Again, the severity of symptoms comes into play.
“In severe mental illness—bipolar disorder, psychotic disorders, or a history of severe illness requiring psychiatric hospitalization—it is important to have a psychiatrist involved,” says Dr. Mittal.
“Even if the woman is stable during pregnancy, the postpartum risk—especially in bipolar disorder—is extremely high. The postpartum period is a vulnerable time, anyway, because obstetric care is coming to its end, and there’s a lot changing irrespective of mental illness. So a patient who’s at high risk for postpartum illness should have a psychiatrist on board as early as possible.”
Consultation with a psychiatrist is another option when managing women with severe depression, a significant psychiatric history, or refractory illness.
Should you prescribe antidepressant medication?
Dr. Mittal believes that ObGyns should feel fairly comfortable prescribing antidepressant medication to patients who have mild or moderate depression, provided that the initiation of such medication is the patient’s informed choice.
Once severe disease (including bipolar disorder and a history of suicidality or psychosis or psychiatric hospitalization) has been ruled out and a history indicates that the patient has mild to moderate symptoms and has responded to treatment, an ObGyn is well qualified to treat perinatal depression, says Dr. Mittal.
Typically, SSRIs are the first-line treatment for perinatal depression and generally have similar amounts of data about their risk in pregnancy. Paroxetine (Paxil) is the exception, as we have more data about the risk for cardiac defects in neonates exposed to it in utero, Dr. Mittal says.
SSRIs generally are found in low amounts in breast milk, although sertraline (Zoloft) generally is found in the smallest quantity, making it the most commonly used SSRI in pregnancy. Sertraline is followed by citalopram (Celexa), escitalopram (Lexapro), and fluoxetine (Prozac) in the respective amount of medication passed into breast milk.
The literature around the teratogenic risks of psychiatric medications is extremely diverse, she says. The “sum total” of the data suggests that SSRIs have relatively few teratogenic risks. “The overall story around SSRIs does not appear to suggest that they carry a risk of major malformations.”
Related Article: Antidepressants linked to pregnancy risks in infertility treatment (News for Your Practice, December 2012)
Dr. Mittal also recommends keeping in mind the possibility that psychotherapy alone is sometimes sufficient for a woman with mild to moderate depression.
“If she has a history of responding to psychotherapy alone and also has mild to moderate symptoms, I think a reasonable approach would be to try it again.”
“This is where preconception planning is especially useful,” she says. “If somebody with mild to moderate symptoms has never had a good trial of psychotherapy, the preconception period is a good time to determine whether it might be effective, to shape the optimal treatment plan.”
Two forms of psychotherapy have solid evidence of efficacy in perinatal depression:
- cognitive behavioral therapy (CBT) —an action-oriented approach that treats maladaptive thinking as the cause of pathologic behavior and “negative” emotions
- interpersonal psychotherapy (IPT)—a treatment in which the patient is educated about depression and its symptoms and her relation to the environment, especially social functioning. Unlike some other forms of therapy, IPT does not focus on underlying personality structures.
There are other forms of psychotherapy, but CBT and IPT have a large evidence base and are generally time-limited, rather than open-ended. They also are manualized and problem-focused, says Dr. Mittal.
How to prescribe an SSRI
SSRIs generally are initiated at a low dose and gradually titrated up (if necessary). A typical starting dose of sertraline, for example, would be 25 to 50 mg. The patient should be counseled about potential side effects, which include increased perspiration, somnolence or insomnia, nausea, diarrhea, headache, dizziness, and restlessness. These effects generally begin to subside the first week or two after initiation.
Sexual side effects such as reduced desire and difficulties with orgasm also may occur and generally do not diminish over time.
The patient also should be advised not to discontinue the SSRI abruptly, if at all possible, because of the risk that she might develop mild discontinuation syndrome. Although this syndrome is short-lived, self-limited, and non-life-threatening, it is uncomfortable. Symptoms include changes in mood or anxiety, shakiness, tremor, or gastrointestinal disturbance. If the patient elects to discontinue an SSRI, tapering over 4 to 7 days is preferable. However, in the event that the patient exhibits an adverse reaction or intolerance to antidepressant medication, immediate discontinuation may be appropriate, says Dr. Mittal.
After initiating SSRI therapy, follow-up in 2 weeks is appropriate, after which time oversight can be transferred to the patient’s primary care provider. In the United States, primary care physicians prescribe the bulk of SSRI medications.
It may take 6 to 8 weeks for the medication to begin to reduce depressive symptoms, although sleep and appetite sometimes improve within 1 or 2 weeks.
Avoid abrupt drug discontinuation in pregnancy
When asked to recommend one intervention that would have a big impact on reducing the burden of depression in pregnancy, Dr. Mittal zeroed in on the population of women who elect to discontinue antidepressant medication during pregnancy.
“I would suggest that ObGyns discourage these women against abrupt discontinuation,” she says. “There is a small body of literature that demonstrates that, in patients with significant illness—severe depression and bipolar disorder, certainly—abrupt discontinuation increases the likelihood of recurrence in the short period of time afterward. If medication is abruptly stopped when a woman discovers she’s pregnant, she’s likely to need to return to treatment during pregnancy because of recurrent symptoms. What happens in that case is that her pregnancy is exposed to both severe symptoms and the reinitiation of treatment, possibly including additional medications beyond the initial agent,” says Dr. Mittal.
Many women assume they should never get pregnant because of their mental health issues, their medications, or both, says Dr. Mittal. Or they believe they must stop their meds if they become pregnant. In fact, some patients report that they have been counseled to avoid medication in pregnancy by their psychiatrist or obstetrician!
“I have spoken to many psychiatrists who say they are not comfortable prescribing to pregnant women, so they either drop the patients or stop their meds!” she says.
When that happens, the patient should find another psychiatrist.
WE WANT TO HEAR FROM YOU!
Drop us a line and let us know what you think about current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: [email protected]
We’ve come a long way in our understanding of depression—and that’s a good thing. Consider the treatments popular in the late 18th and early 19th Centuries, for example, which included water immersion (short of drowning), spinning (to reorder the contents of the brain), and the induction of vomiting and administration of enemas, not to mention institutionalization.1 These modalities wouldn’t attract many patients (or clinicians) today.
And yet, even our distant forebears had some inkling of the potential for depression to continue from one generation to the next. As Trotula of Salerno noted around the 11th Century:
In other words, melancholy (aka depression) sometimes has its origins in the womb.
From our 21st Century vantage point, we understand this conclusion in more scientific terms. Data suggest than 14% to 23% of pregnant women will experience depressive symptoms during pregnancy,3 with the potential for long-term effects in the child. In the largest study to date on the effects of antenatal and postnatal parental depression on offspring, Pearson and colleagues found that children of mothers who are depressed during pregnancy are likely to experience depression themselves at age 18.4 Specifically, for each standard-deviation increase in the antenatal maternal depression score, offspring were 1.28 times more likely to have depression at age 18 (95% confidence interval [CI], 1.08–1.51; P = .003).4
Related Article: A talk about, then a plan for, antidepressants in pregnancy Danielle Carlin, MD, and Louann Brizendine, MD (May 2011)
Maternal depression in the postnatal period also was found to be a risk factor for depression in offspring, but only among mothers with “low education” (defined as either no education or compulsory education ending at or before age 16).4 For each standard-deviation increase in the postnatal maternal depression score in this population, offspring were 1.26 times more likely to have depression at age 18, compared with the children of nondepressed women (95% CI, 1.06–1.50; P = .01).4
Although antenatal depression in fathers was not associated with an increased incidence of depression in offspring, postnatal depression was—but only when the fathers had low education.4
As for the mechanism of transmission of depression from parent to child? Although Pearson and colleagues did not attempt to identify it, they did observe that the differential effects of maternal and paternal antenatal depression—with only maternal depression having an impact on offspring—suggest that, in pregnancy, maternal depression may be transmitted to her child “through the biological consequences of depression in utero.”4
Clearly, if it goes unchecked during pregnancy, maternal depression has the potential to ravage the life of both mother and child. In this article, I review guidance on the management of depression in pregnancy from the American College of Obstetricians and Gynecologists (ACOG) and the American Psychiatric Association (APA), and I offer insights from a perinatal psychiatrist on how ObGyns might adjust their practices to reduce the impact of depression on both mother and infant.
COMPLICATIONS OF PERINATAL DEPRESSION
In a joint report on depression and pregnancy from ACOG and the APA, Yonkers and colleagues noted that low birth weight, neonatal irritability, and diminished neonatal activity and attentiveness are among the adverse reproductive outcomes that have been associated with untreated maternal depression.3 Reproductive outcomes are more dire if maternal depression is severe or if the mother has bipolar disorder or postpartum psychosis, potentially including infanticide or death from suicide.5
Pregnancy complications such as vomiting, nausea, hyperemesis gravidarum, and preeclampsia appear to occur more frequently in depressed women than in nondepressed women, according to the ACOG/APA report,3 although this finding is based on limited data, notes Leena P. Mittal, MD, director of the Reproductive Psychiatry Consultation Service at Brigham and Women’s Hospital in Boston and instructor in psychiatry at Harvard Medical School.
“The trouble with those studies in general is the difficulty of controlling for both the severity of depression and the effects of treatment of depression—or the effects of treatment versus effects of the illness itself,” she says.
That difficulty is compounded by the likely use of multiple medications—
including nonpsychiatric agents—during pregnancy, “which makes it difficult to assess the impact of a single compound, such as an antidepressant, on maternal and fetal outcomes,” according to ACOG and the APA.3 (More than 80% of pregnant women take at least one dose of a medication.3)
HOW THE OBGYN CAN MAKE A DIFFERENCE
Because of the potential for adverse short- and long-term effects of perinatal depression, “there is a need to identify it and attempt to address it prior to the postpartum period,” Dr. Mittal says. “If a woman has depressive symptoms during pregnancy, it is important to try to direct her toward treatment—either by initiating treatment yourself or referring her to a psychiatrist or psychiatric care provider before she enters the postpartum period.” Once she’s postpartum, she will be exposed to additional variables that will influence the severity and duration of her depression, Dr. Mittal says.
Screen all pregnant women for depression
Dr. Mittal recommends routine screening of all perinatal women.
“The data are not entirely clear about the intervals at which these women should be screened,” she says, “but the recommendation would be screening at least once during pregnancy and then again postpartum. Some clinicians screen for depression during each trimester of pregnancy.”
At Dr. Mittal’s institution, such screening usually takes place at the patient’s first prenatal visit.
The screening tools with the most high-quality data backing them include the:
- Edinburgh Postnatal Depression Scale (EPDS). “Despite its name, this tool has been validated for use during pregnancy and for use in the nonperinatal woman as well,” Dr. Mittal notes. It also is in the public domain (http://www.fresno.ucsf.edu/pediatrics/downloads/edinburghscale.pdf). “It’s particularly useful during pregnancy because it assesses the woman for symptoms of depression at the same time that it separates those symptoms from the physical symptoms of pregnancy—there can be some overlap.” The EPDS is self-administered, brief (10 questions), and easily assessed by the clinician, with a score of 10 or above indicating a likelihood of depression.6 It has been validated in more than a dozen languages, as well.
- Patient Health Questionnaire (PHQ-9).7 This is another public-domain tool validated for use during pregnancy (http://www.cqaimh.org/pdf/tool_phq9.pdf). It is utilized widely in primary care and closely associated with depression criteria listed in the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders. Like the EPDS, it is self-administered, brief (9 questions), and easy to score. In general, PHQ-9 scores of 5, 10, 15, and 20 represent mild, moderate, moderately severe, and severe depression, respectively.8
Neither of these tools should override clinical judgment. Even with a positive score, clinical assessment is recommended. Nor are these tools designed to detect anxiety, personality disorders, and phobias.
Try to address the issue before conception
The best time to address perinatal depression, of course, with a conversation about prevention, is during the preconception period. Having time before pregnancy to determine the best perinatal management approach is especially valuable.
“What’s important for an ObGyn to consider when counseling someone who is contemplating pregnancy and who has a history of depression is the need to weigh the risks of treatment during pregnancy against the risks of nontreatment,” says Dr. Mittal. Two ways to do that are to assess the severity of her depressive symptoms—both currently and historically—and explore her response to treatment.
“Obviously, suicidality and psychosis suggest very severe illness, whether they are currently present or occurred in the past, and so does a history of psychiatric hospitalization,” says Dr. Mittal. “In such cases, the untreated illness itself carries significant risk, and when it is weighed against the perhaps smaller risk of antidepressant medication during pregnancy, the risk-benefit analysis likely is very different than it might be for someone with mild to moderate depression. I would definitely agree that addressing severity from the beginning is important.”
An understanding of the patient’s response to treatment also is beneficial. Has any treatment been helpful? If so, that information can guide the choice of treatment during pregnancy, says Dr. Mittal. Even knowing whether a woman has responded to nonpharmacologic therapy such as psychotherapy can help shape the treatment plan.
“It might mean that there’s a way to limit the risk of exposure to a variety of psychotropic medications,” Dr. Mittal says. “Or if the patient has had a good response to a particular medication, it might make sense to try that agent again—or, if she’s currently taking it, to stick with it.”
Even if preconception counseling is difficult to achieve, ObGyns see a large number of women of reproductive age during the course of routine gynecologic care.
“I do think it’s worth having a discussion about reproductive planning, especially in the context of their psychiatric illness or history, even if they aren’t currently planning a pregnancy,” says Dr. Mittal.
When to refer the patient to a psychiatrist
Again, the severity of symptoms comes into play.
“In severe mental illness—bipolar disorder, psychotic disorders, or a history of severe illness requiring psychiatric hospitalization—it is important to have a psychiatrist involved,” says Dr. Mittal.
“Even if the woman is stable during pregnancy, the postpartum risk—especially in bipolar disorder—is extremely high. The postpartum period is a vulnerable time, anyway, because obstetric care is coming to its end, and there’s a lot changing irrespective of mental illness. So a patient who’s at high risk for postpartum illness should have a psychiatrist on board as early as possible.”
Consultation with a psychiatrist is another option when managing women with severe depression, a significant psychiatric history, or refractory illness.
Should you prescribe antidepressant medication?
Dr. Mittal believes that ObGyns should feel fairly comfortable prescribing antidepressant medication to patients who have mild or moderate depression, provided that the initiation of such medication is the patient’s informed choice.
Once severe disease (including bipolar disorder and a history of suicidality or psychosis or psychiatric hospitalization) has been ruled out and a history indicates that the patient has mild to moderate symptoms and has responded to treatment, an ObGyn is well qualified to treat perinatal depression, says Dr. Mittal.
Typically, SSRIs are the first-line treatment for perinatal depression and generally have similar amounts of data about their risk in pregnancy. Paroxetine (Paxil) is the exception, as we have more data about the risk for cardiac defects in neonates exposed to it in utero, Dr. Mittal says.
SSRIs generally are found in low amounts in breast milk, although sertraline (Zoloft) generally is found in the smallest quantity, making it the most commonly used SSRI in pregnancy. Sertraline is followed by citalopram (Celexa), escitalopram (Lexapro), and fluoxetine (Prozac) in the respective amount of medication passed into breast milk.
The literature around the teratogenic risks of psychiatric medications is extremely diverse, she says. The “sum total” of the data suggests that SSRIs have relatively few teratogenic risks. “The overall story around SSRIs does not appear to suggest that they carry a risk of major malformations.”
Related Article: Antidepressants linked to pregnancy risks in infertility treatment (News for Your Practice, December 2012)
Dr. Mittal also recommends keeping in mind the possibility that psychotherapy alone is sometimes sufficient for a woman with mild to moderate depression.
“If she has a history of responding to psychotherapy alone and also has mild to moderate symptoms, I think a reasonable approach would be to try it again.”
“This is where preconception planning is especially useful,” she says. “If somebody with mild to moderate symptoms has never had a good trial of psychotherapy, the preconception period is a good time to determine whether it might be effective, to shape the optimal treatment plan.”
Two forms of psychotherapy have solid evidence of efficacy in perinatal depression:
- cognitive behavioral therapy (CBT) —an action-oriented approach that treats maladaptive thinking as the cause of pathologic behavior and “negative” emotions
- interpersonal psychotherapy (IPT)—a treatment in which the patient is educated about depression and its symptoms and her relation to the environment, especially social functioning. Unlike some other forms of therapy, IPT does not focus on underlying personality structures.
There are other forms of psychotherapy, but CBT and IPT have a large evidence base and are generally time-limited, rather than open-ended. They also are manualized and problem-focused, says Dr. Mittal.
How to prescribe an SSRI
SSRIs generally are initiated at a low dose and gradually titrated up (if necessary). A typical starting dose of sertraline, for example, would be 25 to 50 mg. The patient should be counseled about potential side effects, which include increased perspiration, somnolence or insomnia, nausea, diarrhea, headache, dizziness, and restlessness. These effects generally begin to subside the first week or two after initiation.
Sexual side effects such as reduced desire and difficulties with orgasm also may occur and generally do not diminish over time.
The patient also should be advised not to discontinue the SSRI abruptly, if at all possible, because of the risk that she might develop mild discontinuation syndrome. Although this syndrome is short-lived, self-limited, and non-life-threatening, it is uncomfortable. Symptoms include changes in mood or anxiety, shakiness, tremor, or gastrointestinal disturbance. If the patient elects to discontinue an SSRI, tapering over 4 to 7 days is preferable. However, in the event that the patient exhibits an adverse reaction or intolerance to antidepressant medication, immediate discontinuation may be appropriate, says Dr. Mittal.
After initiating SSRI therapy, follow-up in 2 weeks is appropriate, after which time oversight can be transferred to the patient’s primary care provider. In the United States, primary care physicians prescribe the bulk of SSRI medications.
It may take 6 to 8 weeks for the medication to begin to reduce depressive symptoms, although sleep and appetite sometimes improve within 1 or 2 weeks.
Avoid abrupt drug discontinuation in pregnancy
When asked to recommend one intervention that would have a big impact on reducing the burden of depression in pregnancy, Dr. Mittal zeroed in on the population of women who elect to discontinue antidepressant medication during pregnancy.
“I would suggest that ObGyns discourage these women against abrupt discontinuation,” she says. “There is a small body of literature that demonstrates that, in patients with significant illness—severe depression and bipolar disorder, certainly—abrupt discontinuation increases the likelihood of recurrence in the short period of time afterward. If medication is abruptly stopped when a woman discovers she’s pregnant, she’s likely to need to return to treatment during pregnancy because of recurrent symptoms. What happens in that case is that her pregnancy is exposed to both severe symptoms and the reinitiation of treatment, possibly including additional medications beyond the initial agent,” says Dr. Mittal.
Many women assume they should never get pregnant because of their mental health issues, their medications, or both, says Dr. Mittal. Or they believe they must stop their meds if they become pregnant. In fact, some patients report that they have been counseled to avoid medication in pregnancy by their psychiatrist or obstetrician!
“I have spoken to many psychiatrists who say they are not comfortable prescribing to pregnant women, so they either drop the patients or stop their meds!” she says.
When that happens, the patient should find another psychiatrist.
WE WANT TO HEAR FROM YOU!
Drop us a line and let us know what you think about current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: [email protected]
- Nemade R, Reiss NS, Dombeck M. Historical understandings of depression. Mentalhelp.net. http://www.mentalhelp.net/poc/view_doc.php?type=doc&id=12995&cn=5. Published September 19, 2007. Accessed January 13, 2014.
- Brockington I. A historical perspective on the psychiatry of motherhood. In: Perinatal Stress, Mood, and Anxiety Disorders. Basel, Switzerland: S Karger AG; 2005.
- Yonkers KA, Wisner KL, Stewart DE, et al. The management of depression during pregnancy: A report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Obstet Gynecol. 2009;114(3):703–713.
- Pearson RM, Evans J, Kounali D, et al. Maternal depression during pregnancy and the postnatal period. Risks and possible mechanisms for offspring depression at age 18 years [published online ahead of print October 9, 2013]. JAMA Psychiatry. doi:10.1001/jamapsychiatry.2013.2163.
- Hasser C, Brizendine L, Spielvogel A. SSRI use during pregnancy. Current Psychiatry. 2006;5(4):31–40.
- Edinburgh Postnatal Depression Scale. http://www.fresno.ucsf.edu/pediatrics/downloads/edinburghscale.pdf. Accessed January 14, 2014.
- Patient Health Questionnaire (PHQ-9). http://www.cqaimh.org/pdf/tool_phq9.pdf. Accessed January 14, 2014.
- Kroenke K, Spitzer R, Williams W. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613.
- Nemade R, Reiss NS, Dombeck M. Historical understandings of depression. Mentalhelp.net. http://www.mentalhelp.net/poc/view_doc.php?type=doc&id=12995&cn=5. Published September 19, 2007. Accessed January 13, 2014.
- Brockington I. A historical perspective on the psychiatry of motherhood. In: Perinatal Stress, Mood, and Anxiety Disorders. Basel, Switzerland: S Karger AG; 2005.
- Yonkers KA, Wisner KL, Stewart DE, et al. The management of depression during pregnancy: A report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Obstet Gynecol. 2009;114(3):703–713.
- Pearson RM, Evans J, Kounali D, et al. Maternal depression during pregnancy and the postnatal period. Risks and possible mechanisms for offspring depression at age 18 years [published online ahead of print October 9, 2013]. JAMA Psychiatry. doi:10.1001/jamapsychiatry.2013.2163.
- Hasser C, Brizendine L, Spielvogel A. SSRI use during pregnancy. Current Psychiatry. 2006;5(4):31–40.
- Edinburgh Postnatal Depression Scale. http://www.fresno.ucsf.edu/pediatrics/downloads/edinburghscale.pdf. Accessed January 14, 2014.
- Patient Health Questionnaire (PHQ-9). http://www.cqaimh.org/pdf/tool_phq9.pdf. Accessed January 14, 2014.
- Kroenke K, Spitzer R, Williams W. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613.