User login
Safety and Usefulness of Free Fat Grafts After Microdiscectomy Using an Access Cannula: A Prospective Pilot Study and Literature Review
Hardware for the Heart: The Increasing Impact of Pacemakers, ICDs, and LVADs
Alicia S. Devine, JD, MD
Dr Devine is an assistant professor, department of emergency medicine, Eastern Virginia Medical School, Norfolk.
Disclosure: The author reports no conflict of interest.
Heart disease affects a growing number of patients each year. The causes of heart disease are diverse, but whether the etiology is ischemic or structural, the disease often progresses to the point where patients are at risk for fatal dysrhythmias and heart failure. Treatment modalities for heart disease range from lifestyle modification and medical management to interventional reperfusion, and often involve the surgical implantation of devices designed to improve cardiac function and/or to detect and terminate lethal dysrhythmias.
Over the past two decades, the use of automated implantable cardiac devices (AICDs) such as pacemakers, implantable cardioverter defibrillators (ICDs), and left ventricular assist devices (LVADs) has increased significantly. From 1993 to 2009, nearly 3 million patients received permanent pacemakers in the United States; in 2009 alone, over 188,000 were placed. From 2006 to 2011 (the period for which the most recent data are available), approximately 850,000 patients had an AICD implanted. For the 20-month period running from April 2010 to December 2011, nearly 260,000 patients received the device. Finally, from 2006 through 2013, over 9,000 LVADs were placed. Like the other cardiac devices discussed, the frequency of use continues to increase, with 3,834 LVADs placed in just the first 9 months of 2013.
Emergency physicians are expected to be able to stabilize and manage patients with these devices who present to the ED. Care for these patients requires an understanding of the components and function of the different devices as well as their complications. All of the devices are subject to complications from infection, bleeding, migration, or fracture of the component parts, and, more ominously, complete failure of the device. While the current generation of cardiac devices are much smaller in size than their initial prototypes, they are more technically complex, and consultation with cardiology after initial stabilization is recommended.
Cardiac Hardware
Management of the Patient With an Implanted Pacemaker
Martin Huecker, MD
Thomas Cunningham, MD
Dr Huecker is an assistant professor, department of emergency medicine, University of Louisville, Kentucky.
Dr Cunningham is chief resident, department of emergency medicine, University of Louisville, Kentucky.
Disclosure: The authors report no conflict of interest.
Introduction
Cardiac pacing was conceived in 1899, and the first successful pacemaker was implanted in 1960.1,2 New concepts and evolution of design have made pacemakers increasingly complex. Over the last decade, the rate of implantation has grown by over 50%.3 At the forefront of cardiac care, today’s EP must be proficient in the care of patients with cardiac pacemakers.
The pacemaker consists of a generator and its leads. The generator produces an electrical impulse that travels down the leads to depolarize myocardial tissue.4 A pacemaker corrects abnormal heart rhythms, using these electrical pulses to induce a novel sinus rhythm.5,6Table 1 summarizes the 2008 American College of Cardiology/American Heart Association Level I/II indications for pacemaker placement.
Permanent pacing involves fluoroscopic placement of leads into a chamber(s) of the heart. The generator is implanted most commonly in the left subcutaneous chest.7-9 A single-chamber pacemaker’s leads are located in either the right atrium or ventricle. Dual-chamber pacemakers function with one electrode in the atrium and one in the ventricle. A biventricular pacemaker, also known as cardiac resynchronization therapy (CRT) paces both ventricles via the septal walls.4,7,10
All pacemaker patients need prompt identification of the device manufacturer.8 Patients should carry identification cards. Chest X-ray may identify the device and will give information as to the location and structural integrity of wires. Interrogation should generally be performed in all patients and will provide valuable information such as battery status, current mode, rate, past rhythms, parameters to detect malignant rhythms, and therapeutic settings.4
Evaluation of the patient with a pacemaker begins with a detailed history and physical examination, including any complications involving the device. Clinicians should ask about pacemaker-related symptoms—ie, palpitations, light-headedness, syncope, or changes in exercise tolerance.3 As with all chest pain complaints in the ED, addressing abnormal vital signs and identification of myocardial infarction (MI) must precede other considerations.
Myocardial Infarction in the Pacemaker Patient
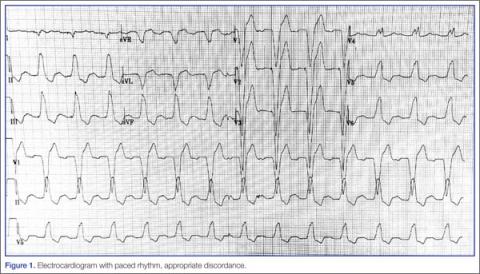
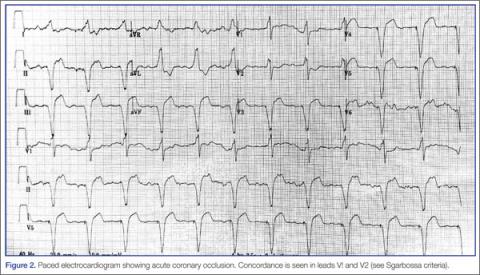
Because of the underlying rhythm induced by the cardiac pacemaker stimulation, acute coronary occlusion can be subtle.12 Since the pacemaker depolarizes the right ventricle, the delay in left ventricular depolarization is seen as left bundle branch block (LBBB) on electrocardiogram (ECG).13,14Figure 1 shows an ECG demonstrating paced rhythm and appropriate discordance, while the ECG in Figure 2 demonstrates acute coronary occlusion. Therefore, identification of coronary occlusion in the paced patient is done using the following Sgarbossa criteria:
- ST elevation ≥1 mm in a lead with upward (concordant) QRS complex; 5 points.
- ST depression ≥1 mm in lead V1, V2, or V3; 3 points.
- ST elevation ≥5 mm in a lead with downward (discordant) QRS complex; 2 points.13,15
An ECG demonstrating three points of Sgarbossa criteria yields a diagnosis of ST segment elevation MI with 98% specificity and 20% sensitivity.16 A modified Sgarbossa criteria replaces the absolute ST-elevation measurement (Sgarbossa criteria 3) with an ST/S ratio greater than -0.25. This yields a sensitivity of 90% and specificity of 90%.17
Pacemaker-Related Complications
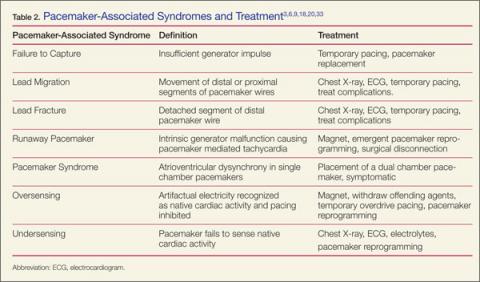
When ischemia is no longer a concern, address the device itself. Workup involves history and physical examination, with complete blood count, chest X-ray, cardiac biomarkers, basic metabolic panel, ultrasound, and device interrogation, as indicated. Table 2 provides a summary of associated pacemaker syndromes and treatment.
Infectious Complications
Patients with device-related infection can present with local or systemic signs, depending on time from implantation. Tenderness to palpation over the generator is sensitive for pocket infection. Although rare, pocket infections require urgent evaluation with mortality rates as high as 20%.18
Early (< 30 days) pocket complications are usually attributable to hematomas with or without infection. When infection is present, Staphylococcus aureus and Staphylococcus epidermidis are the most likely culprits. Up to 50% of isolates can be methicillin resistant S aureus.19 Although needle aspiration has been used in the past for evacuation and microbial identification, current recommendations do not advocate this approach.20 Incision and debridement are the mainstays of therapy. Over 70% of patients with pocket infections will have positive blood cultures and should receive antibiotic therapy with vancomycin.21
Patients with wound separation or pocket infection are at risk for lead infection, lead separation, and lead fracture with related thoracic involvement (ie, pneumonia, empyema, hemothorax, pneumothorax, or diaphragmatic rupture).20
Infectious complications greater than 30 days from implantation are more likely lead-related. Because of the risk for embolic disease to pulmonary or cardiac tissues, emergent line removal and empiric antibiotics are recommended.18 After admission, a transesophageal echocardiogram should be performed to evaluate for valvular involvement and baseline cardiac function.22-24
Physiologic Complications
Patients without ischemia or infection should be evaluated for device-related chest pain. Pain resulting from malfunction of the device usually occurs in the first 48 hours after implantation.9
Patients may present with chest pain related to lead migration or malposition. Perforation of the pleural cavity during the initial procedure can cause hemothorax or pneumothorax. Perforation of the myocardium can lead to hemopericardium and cardiac tamponade. Patients present with respiratory distress and cardiac dysfunction with or without pacing failure.4,9 Bedside cardiac ultrasound assists in assessing these complications and degree of severity.25
Lead migration occurs when a lead detaches from the generator and migrates. Complete separation from the generator may present as failure to capture and should be addressed before lead localization, as temporary pacing may be warranted. Leads coil and regress from patient tampering (ie, Twiddler’s Syndrome) or through spontaneous detachment.3
The ECG may detect functional leads that have migrated to the left heart (coronary sinus, entricular septal defect, perforation). Right bundle branch morphology, rather than the expected left bundle branch morphology, indicates a lead depolarizing the left ventricle.26,27
Lead fracture may occur at any time after implantation. In addition to the complications seen with lead separation and migration, lead fracture is associated with pulmonary vein thrombosis. Because of the volatile nature of fractured leads, patients present more frequently with pacemaker failure, dysrhythmias, and hemodynamic compromise. Temporary pacing may be necessary pending surgical intervention.4,20
Days to weeks postprocedure, patients are at risk for central venous thrombus due to creation of a thrombogenic environment. These thrombi can embolize to the pulmonary circulation and computed tomography pulmonary angiogram should be considered if suspicious.3
Electrical Complications
Failure to pace can be attributed to lead complication (ie, lead malposition, lead fracture), poor lead-tissue interface, or generator complication.28 Electrical complications arise from intrinsic generator malfunction, lack of pacemaker capture, oversensing/undersensing, and poor pacemaker output.29 Poor output results from battery failure, generator failure, or lead misplacement.9
Generator malfunction can produce unwanted tachycardia and exacerbate intrinsic poor cardiac function. Pacemaker-mediated tachycardia (PMT), pacemaker syndrome, and runaway pacemaker should be eliminated from the differential though interrogation and ECG.
Patients presenting with signs of hypotension and cardiac failure may have pacemaker syndrome. With single-chamber conduction, atrioventricular dysynchrony occurs, producing a lack of ventricular preload and poor cardiac output. Treatment includes symptomatic management and pacemaker replacement with a dual-chamber device. In the hemodynamically unstable patient, applications to increase the preload and reduce the afterload should be attempted.20,25
Trauma, battery failure, and intrinsic pacer malfunction can cause PMT such as runaway pacemaker. Application of a magnet has been shown effective only in some cases.3,30 Definitive therapy with emergent pacer reprogramming or surgical disconnection of pacer leads from the generator may be warranted.
Failure to capture occurs when the device electrical impulse is insufficient to depolarize the heart. Battery failure, generator failure, electrode impedance (from fibrosing of the electrodes), lead fracture or malposition, and long QT syndrome are all causes of failure to capture.29 Chest X-ray, ECG, device interrogation, and electrolyte measurement are imperative. The patient with intrinsic generator failure usually requires admission and surgical correction or replacement.3
Oversensing occurs when the device incorrectly interprets artifactual electricity as intrinsic cardiac depolarization. This results in a lack of cardiac stimulation by the pacemaker and can lead to heart block. Shivering, fasciculations from depolarizing neuromuscular blockade, and external interference can cause oversensing. Nonmedical causes include cell phones, security gates, Taser guns, magnets, and iPods.28 Iatrogenic causes include electrosurgery, LVADs, radiation therapy, magnetic resonance imaging (MRI), cardioversion, and lithotripsy.31,32 Treatment involves withdrawing the offending agent, then either placing a magnet over the generator to activate its asynchronous mode or temporary overdrive pacing.26,28,31
Undersensing occurs when the pacer fails to sense intrinsic cardiac activity. The result is competitive asynchronous activity between the native cardiac depolarization and the pacemaker impulses. Introduction of new intrinsic rhythms from lead complications (lead fracture, lead migration), ischemia (premature ventricular contraction, premature atrial contraction), or underlying cardiac disease (atrial fibrillation, right BBB [RBBB], LBBB) can precipitate undersensing.4,5,30 These patients are prone to arrhythmias and decompensation of cardiac function. Management requires identifying the cause of the underlying arrhythmia.29 Chest X-ray, ECG, device interrogation, and electrolyte measurement are useful diagnostics for patients with new arrhythmias or ischemia.3,14,27
Conclusion
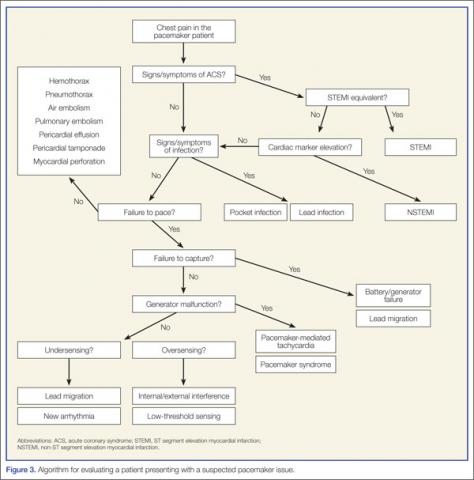
To assist the EP in evaluating a patient with a suspected pacemaker problem, we propose the algorithm presented in Figure 3.
Recent advancements and the increased prevalence of pacemakers require the EPs to be facile with their operating systems and morbidity. A detailed history and physical examination, along with utilization of simple diagnostics and device interrogation, can prove sufficient to diagnose most pacemaker-related complaints. Acute coronary syndrome and serious infections may be subtle, so a high level of suspicion should be maintained. With a knowledgeable EP and a supportive team, pacemaker complications can be successfully managed.
Managing Implantable Cardioverter Defibrillator Shock Complications
Dustin G. Leigh, MD; Cameron R. Wangsgard, MD; Daniel Cabrera, MD
Dr Leigh is a chief resident, department of emergency medicine, Mayo Clinic, Rochester, Minnesota. Dr Wangsgard is a chief resident, department of emergency medicine, Mayo Clinic, Rochester, Minnesota. Dr Cabrera is an assistant professor of emergency medicine, Mayo Clinic, Rochester, Minnesota.
Disclosure: The authors report no conflict of interest.
Introduction
Despite significant advances in emergency medical care and resuscitation techniques, sudden cardiac death remains a major public health problem, accounting for approximately 450,000 deaths annually in the United States.1 Moreover, the vast majority of people who suffer an out-of-hospital cardiac arrest will not survive. This is often the end result of fatal ventricular arrhythmias, including ventricular fibrillation (VF) and ventricular tachycardia (VT). The most effective therapy is rapid electrical defibrillation.2
During the 1970s, Mirowski and Mower developed the concept of an implantable defibrillator device that could monitor and analyze cardiac rhythms with automatic delivery of defibrillating shocks after detecting VF.3,4 In 1980, the first clinical implantation of a cardiac defibrillation device was performed. Development continued steadily until the 1996 the Multicenter Automatic Defibrillator Implantation Trial was prematurely aborted when a statistically significant reduction in mortality (54%) was recognized in patients who received ICD therapy instead of antiarrhythmic therapy.5,6 This was followed by large prospective, randomized, multicenter studies establishing that ICD therapy is effective for primary prevention of sudden death.7 Based on these developments, the ICD has rapidly evolved from a therapy of last resort for patients with recurrent malignant arrhythmias to the standard of care in the primary and secondary prevention of sudden cardiac death, and more recently as cardiac resynchronization devices in patients with congestive heart failure (CHF).3
These developments have led to a dramatic increase in the use of the ICD for monitoring and treatment of VT and VF. The dismal survival rate after cardiac arrest provides a strong impetus to identify high risk patients of sudden cardiac death resulting from VF/VT by primary prevention with an ICD.2,5 More than 100,000 ICDs are implanted annually in the United States.1 As a result of increased prevalence, the EP will often encounter patients who have received an ICD shock or complication of the device. Thus, experienced a general knowledge of implantation, components, complications, and acute management is crucial for clinicians who may care for these patients.
Indications
Implantable cardioverter defibrillators are generally indicated for the primary and secondary prevention of sudden cardiac death.8 The commonly accepted indications for ICD use are summarized here:
Primary Prevention
- Patients with previous MI and LV ejection fraction (LVEF) < 30%
- Patients with cardiomyopathy, New York Heart Association functional class III or IV and LVEF < 35%.
Secondary Prevention
- I Patients with an episode of sustained or unstable VT/VF with no reversible cause.
- I Patients with nonprovoked VT/VF with concomitant structural heart disease (valvular, ischemic, hypertrophic, infiltrative, dilated, channelopathies).
ICD Design
Current ICDs are third-generation device, only slightly larger than pacemakers. ICDs are small (25-45 mm), reliable, and contain sophisticated electrophysiologic analysis algorithms. They can store and report a large number of variables, such as ECGs, defibrillation logs, various energies, lead impedance, as well as battery charge.3,9 Stevenson et al1 describe four major functions of the ICD: sensing of electrical activity from the heart, detection of appropriate therapy, provision of therapy to terminate VT/VF, and pacing for bradycardia and/or CRT.
Components
The components of an ICD can be organized in the following manner:
I Pacing/sensing electrodes. Contemporary units complete these functions through use of two electrodes; one at the distal tip of the lead and one several millimeters back (bipolar leads).1
I Defibrillation electrodes/coils. The defibrillation electrode is a small coil of wire that has a relatively large surface area and extends along the distal aspect of the ventricular lead, positioned at the apex. This lead delivers current directly to the myocardium.11,12 Both the sensing and defibrillation electrodes are often housed in the same, single wire.
I Pulse generator. The pulse generator contains a microprocessor with sensing circuitry as well as high voltage capacitors, a battery, and memory storage component. Modern battery life is typically 5 to 7 years (frequency of shocks will lead to early termination of the battery life).2,11 Some ICDs have automatic self-checks of battery life and will emit a tone when the battery is low or near failure; these patients should be promptly evaluated and referred to the electrophysiologist as indicated.
Functions
The original concept of the ICD was to sense a potentially lethal dysrhythmia and to provide an appropriate therapy. As ICD technology has evolved, the number and variety of available programming and therapies have dramatically increased. Detection of the cardiac rhythm was designed initially to only detect ventricular fibrillation. With current generation models, the ventricular sensing lead filters the incoming signal to eliminate unwanted low frequency components (eg, T-waveand baseline drift) and high frequency components (eg, skeletal muscle electrical activity).3,13 Newer ICDs have the capability for remote monitoring and communication via telephone line or the Internet.
During implantation, the device is programmed with analysis criteria. Criteria for therapy are largely based on the rate, duration, polarity, and waveform of the signal sensed. When the device detects a signal fulfilling the preprogrammed criteria for VT/VF, it selects the appropriate tier of treatment as follows:
I Antitachycardia pacing (ATP). Ventricular tachycardia, particularly reentrant VT associated with scar formation from a prior MI, can sometimes be terminated by pacing the ventricle at a rate slightly faster than the tachycardia. This form of therapy involves the delivery of short bursts (eg, eight beats) of rapid ventricular pacing to terminate VT.14,15 This therapy is low voltage and usually not felt by patients. Antitachycardia pacing successfully terminates VT in over 80% of those with sustained dysrhythmia.16 In the Pain-FREE Rx II trial, data indicate ATP could successfully treat not only standard but rapid VT as well; outcomes revealed a 70% reduction in shocks without adverse effects.5,16
I Synchronized cardioversion. Typically, VT is an organized rhythm. Synchronization of the shock (delivered on R wave peak) and conversion can often be accomplished with low voltage. This helps to minimize discomfort and avoids defibrillation, which potentially could lead to degeneration of VT to VF.
I Defibrillation. This is the delivery of an unsynchronized shock during the cardiac cycle. This can be accomplished through a range of energies. Initial shocks are often programmed for lower energies to reduce capacitor charge time and expedite therapy. Typically, shocks are set to 5 to 10 joules above the defibrillatory threshold (determined at time of implantation).9,16
I Cardiac pacing. All models now have pacing modes similar to single- or dual-chamber pacers.
Implantation
Original ICDs were placed into the intraabdominal cavity through a large thoracotomy. With current-generation ICDs, leads are typically placed transvenously (subclavian, axillary, or cephalic vein), which has led to fewer perioperative complications, including shorter procedure time, shorter hospital stay, and lower costs as compared to abdominal implantation.5,17
The pulse generator remains subcutaneous or submuscular in the pectoral region. An electrode is advanced into the endocardium of the right ventricle apex; dual-chamber ICDs have an additional electrode placed in the right atrial appendage and biventricular ICDs have a third electrode placed transcutaneously in a branch off the coronary sinus.
At the time of the procedure, the electrophysiology team implanting the ICD will configure the diagnostic and therapeutic options; in particular, the defibrillatory threshold will be determined for each specific patient and the device set up with this value.
Complications
Acute complications in the peri-implantation period range from 4% to 5%.18 These are similar to other transvenous procedures and include bleeding, air embolism, infection, lead dislodgment, hemopneumothorax, and rarely death (perioperative mortality 0.2%-0.4%).2,19 Long-term complications may present consistent with other indwelling artificial hardware. Subclavian vein thrombosis with pulmonary thromboembolization, superior vena cava syndrome, as well as lead colonization with infection, are potential complications. superior vena cava thrombosis has been demonstrated in up to 40% of patients. These complications often present insidiously and the clinician should retain a high degree of suspicion.
Infection of the pocket or leads has been observed in up to 7%. Technical causes leading to inappropriate shock include faulty components, oversensing of electrical noise, lead fracture, electromagnetic interference, oversensing of diaphragm myopotentials, oversensing of T-waves, and double counting of QRS complexes.22
Lead complications can include infection, dislodgement (most will occur in the first 3 months after placement), fracture, and insulation defects. Lead failure rates have been reported at up to 1% to 9% at 2 years and as high as 40% at 8 years. Failure occurs secondary to insulation defects (26%), artifact oversensing (24%), fracture (24%), and 26% of the time secondary to infection.3,23
Cardiac perforation is uncommon but potentially devastating. These cases almost always occur with lead manipulation or repair of a screw in the lead; this rarely would lead to clinical significance but possibly the most emergent manifestation would be cardiac tamponade. Chest pain with signs and symptoms of tamponade require prompt diagnosis. Suspect this in the patient with a newly paced RBBB pattern on ECG, diaphragmatic contractions (hiccups), and pericardial effusion. Eighty percent of such perforations with tamponades will occur in first 4 days after implantation, and a chest X-ray or the echocardiogram can help confirm the diagnosis.
Pulse-generator complications include migration, skin erosion, and premature battery depletion.24 Twiddler’s syndrome after pacemaker insertion is a well-described syndrome in which twisting or rotating of the device in the pocket (from constant patient manipulation) results in device malfunction, and Boyle et al describe a similar scenario occurring after ICDs are implanted.25 The authors suggest that an increase in bradycardic pacing threshold or lead impedance may be the initial presentation; however, the possibility that the device failed to sense or treat arrhythmias also should be considered.
Lastly, several studies have documented a statistically significant adverse effect on quality of life in patients living with ICDs. Patients often describe a shock as “being struck by a truck”.22 This may result in depression and anxiety; both are especially prevalent in those who receive frequent shocks. It may be important to consider anxiolytics, support groups, or outpatient referral.2,22,26,27
Management of the Patient With an ICD in the Emergency Department
Patients with ICDs will present to the ED with a variety of complaints, ranging from general/non-specific (eg, dizziness) to life threatening (eg, cardiac arrest). The following section systematizes the approach to these patients.
Frequently, patients with ICDs will present with the complaint of having been shocked. In those patients, the most important initial step is to determine if the shock was appropriate. Initial management should include placement of a cardiac monitor and a rapid 12-lead ECG. A general assessment for the etiology of the shock may reveal a patient’s clinical deterioration, a change in medical therapy, or electrolyte imbalance.2 An accurate history of the surrounding events is key in determining the reason for patients presenting after receiving a shock. A history of chest pain or strenuous physical activity that preceded the shock may indicate, respectively, an appropriate shock from cardiac ischemia or an inappropriate shock caused by skeletal muscle activity. Also, presentations such as a fall following an episode of syncope may represent an ICD-related event and this possibility needs to be considered during the management of these patients.
Clinically Stable Patients After Isolated Shocks
For the patient who received an isolated shock and afterwards is asymptomatic, perform a general assessment as above. Often these patients have experienced an episode of sustained VT that was appropriately recognized and treated.1 For those who feel ill following a shock, emergent assessment is required for the possibility of a resultant arrhythmia following inappropriate shock (eg, device malfunction or battery depletion) or underlying active acute medical illness such as acute coronary syndrome. Always consider interrogation of the device, which will confirm appropriate shock delivery and successful termination of VT/VF. Interrogation also may reveal signs of altered impedance, which may be treated by ICD reprogramming or lead revision in the case of lead malfunction.2 Look for alternative explanations for inappropriate shocks. For example, obtain a chest X-ray to assess proper position of pulse generator or look for presence of lead fracture or migration. Lead fractures tend to occur at three sites: (1) the origin of the lead at the pulse generator, (2) the venous entry site, and (3) within the heart. A basic metabolic panel may reveal hypokalemia or hypomagnesemia leading to lower threshold for dysrhythmia. It is also important to inquire about new medication regimens. Patients with ICDs also are often on multiple cardiac medications, which could lead to alteration in the QT interval or to electrolyte imbalance.
We recommend contacting and discussing the care of patients who present after ICD shocks with the treating electrophysiologist or cardiologist whether or not the shock is considered appropriate.
Patients who have an ongoing arrhythmia when evaluated emergently should be managed according to advanced cardiac life support (ACLS) guidelines, regardless of the presence of an ICD,1 particularly in cases of cardiac arrest from a non-shockable rhythm.
Initially, the shocks should be presumed to be appropriate. Presence of VT/VF in setting of shock would be consistent with appropriate shock delivery. Next, the clinician needs to consider if shock delivery was effective and if it achieved termination of malignant ventricular arrhythmia. Patients with persistent VT/VF despite delivery of a shock may have ICDs with inadequate voltage in the batteries to terminate; external shocks and intravenous (IV) antiarrhythmic medications may be required and should be administered per ACLS guidelines.
When patients present with multiple shocks, the shocks are typically appropriate and often triggered by episodes of VT/VF. Treatment of the underlying causes is the priority; the patient may have sustained or recurrent VT/VF as a result of an acute event, such as cardiac ischemia, hypokalemia, or severe acute heart failure exacerbation. Aggressive reperfusion, management of potassium imbalance, and circulatory support are paramount.
Inappropriate shocks most commonly are delivered for supraventricular tachycardias such as atrial fibrillation that is incorrectly interpreted by the ICD as VT/VF. In these cases, the treatment is the same as for a patient without an ICD (eg, IV diltiazem to slow atrial fibrillation with rapid ventricular response).
In patients experiencing multiple inappropriate ICD shocks, the device can be immediately disarmed by placing a magnet over the ICD pocket until the electrophysiologist can reprogram it. This will not inhibit baseline/backup pacing. However, while a magnet is in place, neither supraventricular tachycardias nor VT/VF will be detected.1 If appropriate shock delivery has been performed for ventricular dysrhythmia, these patients must remain on a cardiac monitor under close medical observation. It is good practice to assume device failure after application of a magnet, and appropriate management strategies include placing external defibrillators pads on the patient’s chest. Fortunately, most ICDs will resume normal function following magnet removal.
In the Canadian Journal of Cardiology (1996), Kowey defined electrical storm as a state of cardiac electrical instability characterized by multiple episodes of ventricular tachycardia (VT storm) or ventricular fibrillation (VF storm) within a relatively short period of time.28,30,31,32
In the patient with an ICD, the generally accepted definition is occurrence of two or more appropriate therapies (antitachycardia pacing or shocks) in a 24-hour period. Triggers may include drug toxicity, electrolyte disturbances (hypokalemia and hypomagnesemia being the most common culprits), new or worsened heart failure, or myocardial ischemia, which account for more than a quarter of all episodes. Electrical storm usually heralds a life-threatening acute pathology placing these patients at immediate high risk of death.28 Immediate communication and consultation with the electrophysiology team is recommended.
Left Ventricular Assist Devices: From Mystery to Mastery
Alicia S. Devine, JD, MD
Dr Devine is an assistant professor, department of emergency medicine, Eastern Virginia Medical School, Norfolk.
Disclosure: The author reports no conflict of interest.
Approximately 5.7 million people in the United States have heart failure, and complications from heart failure represent 668,000 ED visits annually. Heart failure is the primary cause of death in 55,000 people each year. Half of patients die within 5 years of being diagnosed with heart failure.1
Heart failure is initially managed medically; however, some patients become refractory to medical treatment and require heart transplant. Unfortunately, the demand for donor hearts far exceeds the supply, and patients can spend a long time waiting for a donor heart. In addition, not all patients are candidates for transplantation. Left ventricular assist devices are mechanical devices implanted in patients with advanced heart failure in order to provide circulatory support when medications alone are not efficacious. LVADs have been associated with improved survival for heart failure patients.
There are generally two indications for LVAD support: as a bridge to transplant for patients waiting for a donor heart, or as destination therapy for patients who are not candidates for heart transplant. Some patients have had improvement in their cardiac function after LVAD implantation and are able to have the LVAD explanted, leading to a third use for LVADs: bridge to recovery.
LVADs have been in use for over 30 years, and they have evolved during that time to become smaller in size with much fewer complications. Initial models operated with a pulsatile-flow pump that, while adequate in terms of blood flow, contained several parts susceptible to breaking down. Early models were large and cumbersome, especially for smaller patients. New-generation LVADs use a continuous-flow design with either a centrifugal or an axial flow pump with a single moving part, the impeller. The continuous-flow LVADs are quieter, smaller, and significantly more durable than the earlier, pulsatile-flow LVADs. These improvements have expanded the pool of eligible patients to include children and smaller adults.2 Moreover, continuous-flow LVADs provide greater rates of survival and quality of life than the earlier pulsatile-flow models.3
There are fewer adverse events overall with the continuous-flow LVADs compared with the pulsatile-flow LVADs. The number of LVADs implanted each year continues to increase, and more than 95% of these are continuous-flow. As more and more advanced heart failure patients are receiving these devices, emergency physicians should have a basic familiarity with their function and their common complications.4
There are several manufacturers and types of continuous-flow LVADs, but they generally consist of a pump that is surgically implanted into the abdominal or chest cavity of the patient with an inflow cannula positioned in the left ventricle and an outflow cannula inserted into the ascending aorta. The device draws blood from the ventricle and directs it to the aorta. There is a driveline connected to the internal pump that exits the body through the abdominal or chest wall and connects to a system controller. The controller is usually housed in a garment worn by patients that also includes the external battery that powers the LVAD. The controller can also be powered by a base unit that can be plugged into an electrical outlet.5 Patients with continuous-flow LVADs are anticoagulated with warfarin with a target international normalized ratio (INR) of 1.5 to 2.5 and will usually be on an antiplatelet agent as well.2
LVAD patients are typically managed by a team of providers that includes a VAD coordinator; a cardiologist and/or a cardiothoracic surgeon; and a perfusionist, who should be notified as soon as the patient arrives in the ED. Patients understand that it is vital that their LVAD be powered at all times and will usually arrive in the ED with their charged backup batteries. If a power base is available in the hospital, the LVAD can be connected to it to save battery life. If power is interrupted to the LVAD, the pump will stop working. This can be fatal to patients with severe aortic insufficiency who have had their outflow tract surgically occluded and are therefore completely dependent on the LVAD.2
With continuous-flow LVADs, blood is pumped continuously, and a constant, machine-like murmur will be heard on auscultation rather than the typical heart sounds. LVAD patients may not have palpable arterial pulses, and in that case a doppler of the brachial artery and a manual blood pressure cuff are used to listen for the start of Korotkoff sounds as the cuff is released. The pressure at which the first sound is heard is used as an estimate of the mean arterial pressure (MAP). Left ventricular assist device patients should have a MAP between 70 and 90 mm Hg. An accurate pulse oximetry reading may not be attainable, and some centers use cerebral oximetry to obtain oxygenation status.2
The EP should examine all of the connections from the percutaneous lead to the controller and from the controller to the batteries to ensure that they are intact. The exit site for the percutaneous lead should also be examined for evidence of trauma and signs of infection. The exit site is a potential nidus for infection, and even minor trauma from a pull or tug on the lead can damage the tissue and seed an infection. Emergency physicians should ask LVAD patients about any recent trauma to the driveline.6,7
The ED evaluation for an LVAD patient should be focused toward the patient’s chief complaint, recognizing that often patients with LVADs presenting to the ED will have vague complaints of malaise or weakness that may represent a serious pathologic process. Infection, bleeding, thrombosis, and problems with volume status are common reasons for ED visits by LVAD patients.3,5
Infection
In addition to infections in the lung, skin, and urinary tract, patients with LVADs are at risk for infectious complications relating to their device. Implantation of an LVAD involves a sternal incision, the creation of an internal pocket for the LVAD, and a driveline connecting the internal LVAD with an external power source. An infection in any one of these locations can lead to endocarditis, bacteremia, and sepsis.6
Driveline and/or pocket infections are very common, affecting up to 36% of patients with continuous-flow LVADs.8 The exit site for the driveline is an access point for the entry of pathogens, and can be the source of infections in the driveline or in the pump pocket. Pump pocket infections can also occur from exposure to pathogens during surgery or in the immediate postoperative period. In addition, the pump itself can become infected from similar sources, as well as from bacteremia or fungemia from infections in the urine, lung, or central catheters.6
Infections in the driveline will often present with obvious signs such as purulent drainage, erythema, and tenderness at the exit site, but providers should have a high index of suspicion if there is dehiscence at the exit site or even persistent serous drainage from the site, as these can suggest a driveline infection. Pump pocket infections and device-related endocarditis can present with vague symptoms such as weight loss, malaise, and a low-grade fever.
A thorough evaluation should be undertaken in all LVAD patients with a suspected infection to detect a source, and cultures of blood, urine, and the driveline exit site should be obtained. Imaging techniques frequently used when considering device-related infections include ultrasound of the pump pocket and echocardiography to evaluate for endocarditis. Computed tomography is also used to evaluate for device-related infections.6,7,9,10 LVADs are not compatible with MRI.11
The majority of device-related infections are caused by bacteria, although fungal and viral species can be the source as well. Common pathogens implicated include S aureus, S epidermidis, enterococci, Pseudomonas aeruginosa, Klebsiella species and Enterobacter species. Empiric antibiotics with both gram-positive and gram-negative coverage should be initiated for suspected infection related to the device. If the infection has spread to the pump pocket or the device, patients may need surgery for drainage and possible removal of the device.6,7,9,10
Bleeding and Thrombosis
Bleeding complications occur with pulsatile-flow and continuous-flow LVADs at the same rate, and represent one of the most common adverse events seen in LVAD patients. Sites of bleeding include intracranial, nasal cavity, genitourinary tract, and gastrointestinal (GI).11
Interestingly, GI bleeding occurs at a much higher rate in patients with continuous-flow LVADs than in patients with pulsatile-flow devices.2,5,11,12 Patients with continuous-flow LVADs are anticoagulated with warfarin (to a target INR of between 1.5 and 2.5) and an antiplatelet agent to prevent pump thrombosis as well as other thromboembolic events.11 In addition to the effects of warfarin and aspirin, several other factors contribute to the increased incidence of GI bleeding, including an acquired von Willebrand disease and the development of small bowel angiodysplasias from the alteration in vascular hemodynamics from the continuous flow.13,14,15
Emergency physicians should have a high index of suspicion for a bleeding event in patients with an LVAD presenting to the ED. The evaluation of GI bleeding in LVAD patients is the same as in patients without LVADs, and management includes resuscitation with fluids, blood transfusion, and careful correction of coagulopathy. Gastrointestinal bleeding in an LVAD patient necessitates a consultation with a gastroenterologist and admission to the hospital.11
Pump thrombosis, though rare, can result in death and must be considered in cases of MAP < 60 mm Hg and/or an increased power requirement accompanied by a decrease in pulsatility index and flow. Markers of hemolysis such as elevated lactate dehydrogenase or hemoglobinuria also suggest pump thrombosis. Interrogation of the LVAD by the perfusionist is imperative when LVAD patients present to the ED. Echocardiography is the modality of choice in evaluating suspected pump thrombosis. Treatment may require replacement of the pump, or in some cases, anticoagulation or thrombolysis.2,11
Volume Status
Patients with LVADs can present with complaints of weakness and/or dizziness that can be due to dehydration and/or electrolyte deficiencies. Often, these patients will continue to restrict their salt and fluid intake after device implantation. They are frequently on diuretics, which can contribute to these problems. Checking and repleting electrolytes as well as administering a gentle bolus of IV fluids in patients with a MAP < 60 mm Hg will often correct the hypovolemia and electrolyte abnormalities. Evaluation for sepsis, pump thrombosis, and cannula malposition as causes of hypotension should be undertaken in the appropriate circumstances.2,11 Severe hypovolemia can interfere with effective LVAD function if it leads to the collapse of the left ventricle over the inflow cannula. Bedside ultrasound can be a useful adjunct in the evaluation of cannula position and volume status.2 An emergent consult with a cardiovascular surgeon is indicated in the event of pump thrombosis or cannula malposition.
Conclusion
The number of LVADs implanted each year continues to grow, and EPs need to have a basic familiarity with these devices and how to manage typical complaints seen in the ED. Patients and their caregivers have been given extensive education and training on the care and management of their LVAD components and can be a valuable source of information. They should bring the devices with them to the ED, along with the names and phone numbers of all of the members of their VAD treatment team, who should be called shortly after the patient’s arrival, as well as backup charged batteries to power their LVAD.
A priority is ensuring that all of the LVAD connections are intact and that there is adequate power to the device. A perfusionist will need to interrogate the controller if there is any concern about its function, including alarms sounding or lights flashing. The manufacturer’s website can be accessed if necessary for further information.
Cardiac Hardware Management of the Patient With an Implanted Pacemaker
- Chardack WM, Gage AA, Greatbatch W. A transistorized, self-contained, implantable pacemaker for the long-term correction of complete heart block. Surgery. 1960;48:643-654.
- Beck H, Boden WE, Patibandla S, Kireyev D, Gupta V, Campagna F, et al. 50th anniversary of the first successful permanent pacemaker implantation in the United States: historical review and future directions. Am J Cardiol. 2010;106(6):810-818.
- McMullan J, Valento M, Attari M, Venkat A. Care of the pacemaker/implantable cardioverter defibrillator patient in the ED. Am J Emerg Med. 2007;25(7):812-822.
- Kaszala K, Huizar JF, Ellenbogen KA. Contemporary pacemakers: what the primary care physician needs to know. Mayo Clin Proc. 2008;83(10):1170-1186.
- Park DS, Fishman GI. The cardiac conduction system. Circulation. 2011;123(8):904-915.
- Gregoratos G. Indications and Recommendations for Pacemaker Therapy. Am Fam Phys. 2005;71(8):1563-1570.
- Vardas PE, Simantirakis EN, Kanoupakis EM. New developments in cardiac pacemakers. Circulation. 2013;127(23):2343-2350.
- Cheng A, Tereshchenko LG. Evolutionary innovations in cardiac pacing. J Electrocardiol. 2011;44(6):611-615.
- Stone KR, McPherson CA. Assessment and management of patients with pacemakers and implantable cardioverter defibrillators. Crit Care Med. 2004;32(4 Suppl):S155-S165.
- Bernstein AD, Daubert JC, Fletcher RD, Hayes DL, Luderitz B, Reynolds DW, et al. The revised NASPE/BPEG generic code for antibradycardia, adaptive-rate, and multisite pacing. North American Society of Pacing and Electrophysiology/British Pacing and Electrophysiology Group. Pacing and clinical electrophysiology : Pacing Clin Electrophysiol. 2002;25(2):260-264.
- Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA, 3rd, Freedman RA, Gettes LS, et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Am J Cardiol. 2008;51(21):e1-e62.
- Chang AM, Shofer FS, Tabas JA, Magid DJ, McCusker CM, Hollander JE. Lack of association between left bundle-branch block and acute myocardial infarction in symptomatic ED patients. Am J Emerg Med. 2009;27(8):916-921.
- Sgarbossa EB, Pinski SL, Barbagelata A, Underwood DA, Gates KB, Topol EJ, et al. Electrocardiographic diagnosis of evolving acute myocardial infarction in the presence of left bundle-branch block. GUSTO-1 (Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries) Investigators. N Engl J Med. 1996;334(8):481-487.
- Venkatachalam KL. Common pitfalls in interpreting pacemaker electrocardiograms in the emergency department. J Electrocardiol. 2011;44(6):616-621.
- Sgarbossa EB, Pinski SL, Topol EJ, Califf RM, Barbagelata A, Goodman SG, et al. Acute myocardial infarction and complete bundle branch block at hospital admission: clinical characteristics and outcome in the thrombolytic era. GUSTO-I Investigators. Global Utilization of Streptokinase and t-PA [tissue-type plasminogen activator] for Occluded Coronary Arteries. J Am Coll Cardiol. 1998;31(1):105-110.
- Tabas JA, Rodriguez RM, Seligman HK, Goldschlager NF. Electrocardiographic criteria for detecting acute myocardial infarction in patients with left bundle branch block: a meta-analysis. Ann Emerg Med. 2008;52(4):329-336 e1.
- Smith SW, Dodd KW, Henry TD, Dvorak DM, Pearce LA. Diagnosis of ST-elevation myocardial infarction in the presence of left bundle branch block with the ST-elevation to S-wave ratio in a modified Sgarbossa rule. Ann Emerg Med. 2012;60(6):766-776.
- Nof E, Epstein LM. Complications of cardiac implants: handling device infections. Eur Heart J. 2013;34(3):229-236.
- Tarakji KG, Wilkoff BL. Management of cardiac implantable electronic device infections: the challenges of understanding the scope of the problem and its associated mortality. Expert Rev ardiovasc Ther. 2013;11(5):607-616.
- Balachander J, Rajagopal S. Pacemaker trouble shooting and follow up. Indian Heart J. 2011;63(4):356-370.
- Klug D, Wallet F, Lacroix D, Marquie C, Kouakam C, Kacet S, et al. Local symptoms at the site of pacemaker implantation indicate latent systemic infection. Heart. 2004;90(8):882-886.
- Kwak YL, Shim JK. Assessment of endocarditis and intracardiac masses by TEE. Int Anesthesiol Clin. 2008;46(2):105-120.
- Ryan EW, Bolger AF. Transesophageal echocardiography (TEE) in the evaluation of infective endocarditis. Cardiol Clin. 2000;18(4):773-787.
- Baddour LM. Cardiac device infection--or not. Circulation. 2010;121(15):1686-1687.
- Ghani SN, Kirkpatrick JN, Spencer KT, Smith GL, Burke MC, Kim SS, et al. Rapid assessment of left ventricular systolic function in a pacemaker clinic using a hand-carried ultrasound device. J Interv Card Electrophysiol. 2006;16(1):39-43.
- Scheibly K. Pacemaker timing and electrocardiogram interpretation. AACN Adv Crit Care. 2010;21(4):386-396.
- Zimetbaum PJ, Josephson ME. Use of the electrocardiogram in acute myocardial infarction. N Engl J Med. 2003;348(10):933-940.
- Misiri J, Kusumoto F, Goldschlager N. Electromagnetic interference and implanted cardiac devices: the nonmedical environment (part I). Clin Cardiol. 2012;35(5):276-280.
- Trohman RG, Kim MH, Pinski SL. Cardiac pacing: the state of the art. Lancet. 2004;364(9446):1701-1719.
- Kramer DB, Mitchell SL, Brock DW. Deactivation of pacemakers and implantable cardioverter-defibrillators. Prog Cardiovasc Dis. 2012;55(3):290-299.
- Misiri J, Kusumoto F, Goldschlager N. Electromagnetic interference and implanted cardiac devices: the medical environment (part II). Clin Cardiol. 2012;35(6):321-328.
- Zikria JF, Machnicki S, Rhim E, Bhatti T, Graham RE. MRI of patients with cardiac pacemakers: a review of the medical literature. Am J Roentgenol. 2011;196(2):390-401.
- Cai Q, Mehta N, Sgarbossa EB, Pinski SL, Wagner GS, Califf RM, et al. The left bundle-branch block puzzle in the 2013 ST-elevation myocardial infarction guideline: from falsely declaring emergency to denying reperfusion in a high-risk population. Are the Sgarbossa Criteria ready for prime time? Am Heart J. 2013;166(3):409-413.
Managing Implantable Cardioverter Defibrillator Shock Complications
- Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2-e220.
- Slaughter MS, Pagani FD, Rogers JG, et al. Clinical management of continuous-flow left ventricular assist devices in advanced heart failure. J Heart Lung Transplant. 2010;29(4 Suppl):S1-S39.
- Slaughter MS, Rogers JG, Milano GC, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009; 361(23):2241-2251.
- Kirklin JK, Naftel DC, Kormos RL, et al. Fifth INTERMACS annual report: risk factor analysis from more than 6,000 mechanical circulatory support patients. J Heart Lung Transplant. 2013;32(2):141-156.
- Miller LW, Pagani FD, Russell SD, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357(9):885-896.
- Califano S, Pagani FD, Malani PN. Left ventricular assist device-associated infections. Infect Dis Clin N Am. 2012;26(1):77-87.
- Peredo D, Conte JV. Left ventricular assist device driveline infections. Cardiol Clin. 2011;29(4):515-527.
- Schaffer JM, Allen JG, Weiss ES, et al. Infectious complications after pulsatile-flow and continuous-flow left ventricular assist device implantation.
J Heart Lung Transplant. 2011;30(2):164-174. - Gordon RJ, Quagliarello B, Lowy FD. Ventricular assist device-related infections. Lancet Infect Dis. 2006;6(7):426-437.
- Maniar S, Kondareddy S, Topkara VK. Left ventricular assist-device-related infections: past, present and future. Expert Rev Med Devices. 2011;8(5):627-634.
- Klein T, Jacob M. Management of implantable assisted circulation devices. Cardiol Clin. 2012;30:673-682
- John RJ, Kamdar F, Liao K, et al. Improved survival and decreasing incidence of adverse events with the HeartMate II left ventricular assist device as bridge-to-transplant therapy. Ann Thorac Surg. 2008;86:1227-1235.
- Klovaite J, Gustafsson F, Mortensen SA, Sander K, Nielson LB. Severely impaired von Willebrand factor-dependent platelet aggregation in patients with a continuous-flow left ventricular assist device (HeartMate II). J Am Coll Cardiol. 2009;53(23):2162-2167.
- Stern DR, Kazam J, Edwards P, et al. Increased incidence of gastrointestinal bleeding following implantation of the HeartMate II LVAD. J Card Surg. 2010:25(3):352-356.
- Kushnir VM, Sharma S, Ewald GA, et al. Evaluation of GI bleeding after implantation of left ventricular assist device. Gastrointest Endoscopy. 2012;75(5):973-979.
Left Ventricular Assist Devices: From Mystery to Mastery
- Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2-e220.
- Slaughter MS, Pagani FD, Rogers JG, et al. Clinical management of continuous-flow left ventricular assist devices in advanced heart failure. J Heart Lung Transplant. 2010;29(4 Suppl):S1-S39.
- Slaughter MS, Rogers JG, Milano GC, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009; 361(23):2241-2251.
- Kirklin JK, Naftel DC, Kormos RL, et al. Fifth INTERMACS annual report: risk factor analysis from more than 6,000 mechanical circulatory support patients. J Heart Lung Transplant. 2013;32(2):141-156.
- Miller LW, Pagani FD, Russell SD, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357(9):885-896.
- Califano S, Pagani FD, Malani PN. Left ventricular assist device-associated infections. Infect Dis Clin N Am. 2012;26(1):77-87.
- Peredo D, Conte JV. Left ventricular assist device driveline infections. Cardiol Clin. 2011;29(4):515-527.
- Schaffer JM, Allen JG, Weiss ES, et al. Infectious complications after pulsatile-flow and continuous-flow left ventricular assist device implantation. J Heart Lung Transplant. 2011;30(2):164-174.
- Gordon RJ, Quagliarello B, Lowy FD. Ventricular assist device-related infections. Lancet Infect Dis. 2006;6(7):426-437.
- Maniar S, Kondareddy S, Topkara VK. Left ventricular assist-device-related infections: past, present and future. Expert Rev Med Devices. 2011;8(5):627-634.
- Klein T, Jacob M. Management of implantable assisted circulation devices. Cardiol Clin. 2012;30:673-682
- John RJ, Kamdar F, Liao K, et al. Improved survival and decreasing incidence of adverse events with the HeartMate II left ventricular assist device as bridge-to-transplant therapy. Ann Thorac Surg. 2008;86:1227-1235.
- Klovaite J, Gustafsson F, Mortensen SA, Sander K, Nielson LB. Severely impaired von Willebrand factor-dependent platelet aggregation in patients with a continuous-flow left ventricular assist device (HeartMate II). J Am Coll Cardiol. 2009;53(23):2162-2167.
- Stern DR, Kazam J, Edwards P, et al. Increased incidence of gastrointestinal bleeding following implantation of the HeartMate II LVAD. J Card Surg. 2010:25(3):352-356.
- Kushnir VM, Sharma S, Ewald GA, et al. Evaluation of GI bleeding after implantation of left ventricular assist device. Gastrointest Endoscopy. 2012;75(5):973-979.
Alicia S. Devine, JD, MD
Dr Devine is an assistant professor, department of emergency medicine, Eastern Virginia Medical School, Norfolk.
Disclosure: The author reports no conflict of interest.
Heart disease affects a growing number of patients each year. The causes of heart disease are diverse, but whether the etiology is ischemic or structural, the disease often progresses to the point where patients are at risk for fatal dysrhythmias and heart failure. Treatment modalities for heart disease range from lifestyle modification and medical management to interventional reperfusion, and often involve the surgical implantation of devices designed to improve cardiac function and/or to detect and terminate lethal dysrhythmias.
Over the past two decades, the use of automated implantable cardiac devices (AICDs) such as pacemakers, implantable cardioverter defibrillators (ICDs), and left ventricular assist devices (LVADs) has increased significantly. From 1993 to 2009, nearly 3 million patients received permanent pacemakers in the United States; in 2009 alone, over 188,000 were placed. From 2006 to 2011 (the period for which the most recent data are available), approximately 850,000 patients had an AICD implanted. For the 20-month period running from April 2010 to December 2011, nearly 260,000 patients received the device. Finally, from 2006 through 2013, over 9,000 LVADs were placed. Like the other cardiac devices discussed, the frequency of use continues to increase, with 3,834 LVADs placed in just the first 9 months of 2013.
Emergency physicians are expected to be able to stabilize and manage patients with these devices who present to the ED. Care for these patients requires an understanding of the components and function of the different devices as well as their complications. All of the devices are subject to complications from infection, bleeding, migration, or fracture of the component parts, and, more ominously, complete failure of the device. While the current generation of cardiac devices are much smaller in size than their initial prototypes, they are more technically complex, and consultation with cardiology after initial stabilization is recommended.
Cardiac Hardware
Management of the Patient With an Implanted Pacemaker
Martin Huecker, MD
Thomas Cunningham, MD
Dr Huecker is an assistant professor, department of emergency medicine, University of Louisville, Kentucky.
Dr Cunningham is chief resident, department of emergency medicine, University of Louisville, Kentucky.
Disclosure: The authors report no conflict of interest.
Introduction
Cardiac pacing was conceived in 1899, and the first successful pacemaker was implanted in 1960.1,2 New concepts and evolution of design have made pacemakers increasingly complex. Over the last decade, the rate of implantation has grown by over 50%.3 At the forefront of cardiac care, today’s EP must be proficient in the care of patients with cardiac pacemakers.
The pacemaker consists of a generator and its leads. The generator produces an electrical impulse that travels down the leads to depolarize myocardial tissue.4 A pacemaker corrects abnormal heart rhythms, using these electrical pulses to induce a novel sinus rhythm.5,6Table 1 summarizes the 2008 American College of Cardiology/American Heart Association Level I/II indications for pacemaker placement.
Permanent pacing involves fluoroscopic placement of leads into a chamber(s) of the heart. The generator is implanted most commonly in the left subcutaneous chest.7-9 A single-chamber pacemaker’s leads are located in either the right atrium or ventricle. Dual-chamber pacemakers function with one electrode in the atrium and one in the ventricle. A biventricular pacemaker, also known as cardiac resynchronization therapy (CRT) paces both ventricles via the septal walls.4,7,10
All pacemaker patients need prompt identification of the device manufacturer.8 Patients should carry identification cards. Chest X-ray may identify the device and will give information as to the location and structural integrity of wires. Interrogation should generally be performed in all patients and will provide valuable information such as battery status, current mode, rate, past rhythms, parameters to detect malignant rhythms, and therapeutic settings.4
Evaluation of the patient with a pacemaker begins with a detailed history and physical examination, including any complications involving the device. Clinicians should ask about pacemaker-related symptoms—ie, palpitations, light-headedness, syncope, or changes in exercise tolerance.3 As with all chest pain complaints in the ED, addressing abnormal vital signs and identification of myocardial infarction (MI) must precede other considerations.
Myocardial Infarction in the Pacemaker Patient
Because of the underlying rhythm induced by the cardiac pacemaker stimulation, acute coronary occlusion can be subtle.12 Since the pacemaker depolarizes the right ventricle, the delay in left ventricular depolarization is seen as left bundle branch block (LBBB) on electrocardiogram (ECG).13,14Figure 1 shows an ECG demonstrating paced rhythm and appropriate discordance, while the ECG in Figure 2 demonstrates acute coronary occlusion. Therefore, identification of coronary occlusion in the paced patient is done using the following Sgarbossa criteria:
- ST elevation ≥1 mm in a lead with upward (concordant) QRS complex; 5 points.
- ST depression ≥1 mm in lead V1, V2, or V3; 3 points.
- ST elevation ≥5 mm in a lead with downward (discordant) QRS complex; 2 points.13,15
An ECG demonstrating three points of Sgarbossa criteria yields a diagnosis of ST segment elevation MI with 98% specificity and 20% sensitivity.16 A modified Sgarbossa criteria replaces the absolute ST-elevation measurement (Sgarbossa criteria 3) with an ST/S ratio greater than -0.25. This yields a sensitivity of 90% and specificity of 90%.17
Pacemaker-Related Complications
When ischemia is no longer a concern, address the device itself. Workup involves history and physical examination, with complete blood count, chest X-ray, cardiac biomarkers, basic metabolic panel, ultrasound, and device interrogation, as indicated. Table 2 provides a summary of associated pacemaker syndromes and treatment.
Infectious Complications
Patients with device-related infection can present with local or systemic signs, depending on time from implantation. Tenderness to palpation over the generator is sensitive for pocket infection. Although rare, pocket infections require urgent evaluation with mortality rates as high as 20%.18
Early (< 30 days) pocket complications are usually attributable to hematomas with or without infection. When infection is present, Staphylococcus aureus and Staphylococcus epidermidis are the most likely culprits. Up to 50% of isolates can be methicillin resistant S aureus.19 Although needle aspiration has been used in the past for evacuation and microbial identification, current recommendations do not advocate this approach.20 Incision and debridement are the mainstays of therapy. Over 70% of patients with pocket infections will have positive blood cultures and should receive antibiotic therapy with vancomycin.21
Patients with wound separation or pocket infection are at risk for lead infection, lead separation, and lead fracture with related thoracic involvement (ie, pneumonia, empyema, hemothorax, pneumothorax, or diaphragmatic rupture).20
Infectious complications greater than 30 days from implantation are more likely lead-related. Because of the risk for embolic disease to pulmonary or cardiac tissues, emergent line removal and empiric antibiotics are recommended.18 After admission, a transesophageal echocardiogram should be performed to evaluate for valvular involvement and baseline cardiac function.22-24
Physiologic Complications
Patients without ischemia or infection should be evaluated for device-related chest pain. Pain resulting from malfunction of the device usually occurs in the first 48 hours after implantation.9
Patients may present with chest pain related to lead migration or malposition. Perforation of the pleural cavity during the initial procedure can cause hemothorax or pneumothorax. Perforation of the myocardium can lead to hemopericardium and cardiac tamponade. Patients present with respiratory distress and cardiac dysfunction with or without pacing failure.4,9 Bedside cardiac ultrasound assists in assessing these complications and degree of severity.25
Lead migration occurs when a lead detaches from the generator and migrates. Complete separation from the generator may present as failure to capture and should be addressed before lead localization, as temporary pacing may be warranted. Leads coil and regress from patient tampering (ie, Twiddler’s Syndrome) or through spontaneous detachment.3
The ECG may detect functional leads that have migrated to the left heart (coronary sinus, entricular septal defect, perforation). Right bundle branch morphology, rather than the expected left bundle branch morphology, indicates a lead depolarizing the left ventricle.26,27
Lead fracture may occur at any time after implantation. In addition to the complications seen with lead separation and migration, lead fracture is associated with pulmonary vein thrombosis. Because of the volatile nature of fractured leads, patients present more frequently with pacemaker failure, dysrhythmias, and hemodynamic compromise. Temporary pacing may be necessary pending surgical intervention.4,20
Days to weeks postprocedure, patients are at risk for central venous thrombus due to creation of a thrombogenic environment. These thrombi can embolize to the pulmonary circulation and computed tomography pulmonary angiogram should be considered if suspicious.3
Electrical Complications
Failure to pace can be attributed to lead complication (ie, lead malposition, lead fracture), poor lead-tissue interface, or generator complication.28 Electrical complications arise from intrinsic generator malfunction, lack of pacemaker capture, oversensing/undersensing, and poor pacemaker output.29 Poor output results from battery failure, generator failure, or lead misplacement.9
Generator malfunction can produce unwanted tachycardia and exacerbate intrinsic poor cardiac function. Pacemaker-mediated tachycardia (PMT), pacemaker syndrome, and runaway pacemaker should be eliminated from the differential though interrogation and ECG.
Patients presenting with signs of hypotension and cardiac failure may have pacemaker syndrome. With single-chamber conduction, atrioventricular dysynchrony occurs, producing a lack of ventricular preload and poor cardiac output. Treatment includes symptomatic management and pacemaker replacement with a dual-chamber device. In the hemodynamically unstable patient, applications to increase the preload and reduce the afterload should be attempted.20,25
Trauma, battery failure, and intrinsic pacer malfunction can cause PMT such as runaway pacemaker. Application of a magnet has been shown effective only in some cases.3,30 Definitive therapy with emergent pacer reprogramming or surgical disconnection of pacer leads from the generator may be warranted.
Failure to capture occurs when the device electrical impulse is insufficient to depolarize the heart. Battery failure, generator failure, electrode impedance (from fibrosing of the electrodes), lead fracture or malposition, and long QT syndrome are all causes of failure to capture.29 Chest X-ray, ECG, device interrogation, and electrolyte measurement are imperative. The patient with intrinsic generator failure usually requires admission and surgical correction or replacement.3
Oversensing occurs when the device incorrectly interprets artifactual electricity as intrinsic cardiac depolarization. This results in a lack of cardiac stimulation by the pacemaker and can lead to heart block. Shivering, fasciculations from depolarizing neuromuscular blockade, and external interference can cause oversensing. Nonmedical causes include cell phones, security gates, Taser guns, magnets, and iPods.28 Iatrogenic causes include electrosurgery, LVADs, radiation therapy, magnetic resonance imaging (MRI), cardioversion, and lithotripsy.31,32 Treatment involves withdrawing the offending agent, then either placing a magnet over the generator to activate its asynchronous mode or temporary overdrive pacing.26,28,31
Undersensing occurs when the pacer fails to sense intrinsic cardiac activity. The result is competitive asynchronous activity between the native cardiac depolarization and the pacemaker impulses. Introduction of new intrinsic rhythms from lead complications (lead fracture, lead migration), ischemia (premature ventricular contraction, premature atrial contraction), or underlying cardiac disease (atrial fibrillation, right BBB [RBBB], LBBB) can precipitate undersensing.4,5,30 These patients are prone to arrhythmias and decompensation of cardiac function. Management requires identifying the cause of the underlying arrhythmia.29 Chest X-ray, ECG, device interrogation, and electrolyte measurement are useful diagnostics for patients with new arrhythmias or ischemia.3,14,27
Conclusion
To assist the EP in evaluating a patient with a suspected pacemaker problem, we propose the algorithm presented in Figure 3.
Recent advancements and the increased prevalence of pacemakers require the EPs to be facile with their operating systems and morbidity. A detailed history and physical examination, along with utilization of simple diagnostics and device interrogation, can prove sufficient to diagnose most pacemaker-related complaints. Acute coronary syndrome and serious infections may be subtle, so a high level of suspicion should be maintained. With a knowledgeable EP and a supportive team, pacemaker complications can be successfully managed.
Managing Implantable Cardioverter Defibrillator Shock Complications
Dustin G. Leigh, MD; Cameron R. Wangsgard, MD; Daniel Cabrera, MD
Dr Leigh is a chief resident, department of emergency medicine, Mayo Clinic, Rochester, Minnesota. Dr Wangsgard is a chief resident, department of emergency medicine, Mayo Clinic, Rochester, Minnesota. Dr Cabrera is an assistant professor of emergency medicine, Mayo Clinic, Rochester, Minnesota.
Disclosure: The authors report no conflict of interest.
Introduction
Despite significant advances in emergency medical care and resuscitation techniques, sudden cardiac death remains a major public health problem, accounting for approximately 450,000 deaths annually in the United States.1 Moreover, the vast majority of people who suffer an out-of-hospital cardiac arrest will not survive. This is often the end result of fatal ventricular arrhythmias, including ventricular fibrillation (VF) and ventricular tachycardia (VT). The most effective therapy is rapid electrical defibrillation.2
During the 1970s, Mirowski and Mower developed the concept of an implantable defibrillator device that could monitor and analyze cardiac rhythms with automatic delivery of defibrillating shocks after detecting VF.3,4 In 1980, the first clinical implantation of a cardiac defibrillation device was performed. Development continued steadily until the 1996 the Multicenter Automatic Defibrillator Implantation Trial was prematurely aborted when a statistically significant reduction in mortality (54%) was recognized in patients who received ICD therapy instead of antiarrhythmic therapy.5,6 This was followed by large prospective, randomized, multicenter studies establishing that ICD therapy is effective for primary prevention of sudden death.7 Based on these developments, the ICD has rapidly evolved from a therapy of last resort for patients with recurrent malignant arrhythmias to the standard of care in the primary and secondary prevention of sudden cardiac death, and more recently as cardiac resynchronization devices in patients with congestive heart failure (CHF).3
These developments have led to a dramatic increase in the use of the ICD for monitoring and treatment of VT and VF. The dismal survival rate after cardiac arrest provides a strong impetus to identify high risk patients of sudden cardiac death resulting from VF/VT by primary prevention with an ICD.2,5 More than 100,000 ICDs are implanted annually in the United States.1 As a result of increased prevalence, the EP will often encounter patients who have received an ICD shock or complication of the device. Thus, experienced a general knowledge of implantation, components, complications, and acute management is crucial for clinicians who may care for these patients.
Indications
Implantable cardioverter defibrillators are generally indicated for the primary and secondary prevention of sudden cardiac death.8 The commonly accepted indications for ICD use are summarized here:
Primary Prevention
- Patients with previous MI and LV ejection fraction (LVEF) < 30%
- Patients with cardiomyopathy, New York Heart Association functional class III or IV and LVEF < 35%.
Secondary Prevention
- I Patients with an episode of sustained or unstable VT/VF with no reversible cause.
- I Patients with nonprovoked VT/VF with concomitant structural heart disease (valvular, ischemic, hypertrophic, infiltrative, dilated, channelopathies).
ICD Design
Current ICDs are third-generation device, only slightly larger than pacemakers. ICDs are small (25-45 mm), reliable, and contain sophisticated electrophysiologic analysis algorithms. They can store and report a large number of variables, such as ECGs, defibrillation logs, various energies, lead impedance, as well as battery charge.3,9 Stevenson et al1 describe four major functions of the ICD: sensing of electrical activity from the heart, detection of appropriate therapy, provision of therapy to terminate VT/VF, and pacing for bradycardia and/or CRT.
Components
The components of an ICD can be organized in the following manner:
I Pacing/sensing electrodes. Contemporary units complete these functions through use of two electrodes; one at the distal tip of the lead and one several millimeters back (bipolar leads).1
I Defibrillation electrodes/coils. The defibrillation electrode is a small coil of wire that has a relatively large surface area and extends along the distal aspect of the ventricular lead, positioned at the apex. This lead delivers current directly to the myocardium.11,12 Both the sensing and defibrillation electrodes are often housed in the same, single wire.
I Pulse generator. The pulse generator contains a microprocessor with sensing circuitry as well as high voltage capacitors, a battery, and memory storage component. Modern battery life is typically 5 to 7 years (frequency of shocks will lead to early termination of the battery life).2,11 Some ICDs have automatic self-checks of battery life and will emit a tone when the battery is low or near failure; these patients should be promptly evaluated and referred to the electrophysiologist as indicated.
Functions
The original concept of the ICD was to sense a potentially lethal dysrhythmia and to provide an appropriate therapy. As ICD technology has evolved, the number and variety of available programming and therapies have dramatically increased. Detection of the cardiac rhythm was designed initially to only detect ventricular fibrillation. With current generation models, the ventricular sensing lead filters the incoming signal to eliminate unwanted low frequency components (eg, T-waveand baseline drift) and high frequency components (eg, skeletal muscle electrical activity).3,13 Newer ICDs have the capability for remote monitoring and communication via telephone line or the Internet.
During implantation, the device is programmed with analysis criteria. Criteria for therapy are largely based on the rate, duration, polarity, and waveform of the signal sensed. When the device detects a signal fulfilling the preprogrammed criteria for VT/VF, it selects the appropriate tier of treatment as follows:
I Antitachycardia pacing (ATP). Ventricular tachycardia, particularly reentrant VT associated with scar formation from a prior MI, can sometimes be terminated by pacing the ventricle at a rate slightly faster than the tachycardia. This form of therapy involves the delivery of short bursts (eg, eight beats) of rapid ventricular pacing to terminate VT.14,15 This therapy is low voltage and usually not felt by patients. Antitachycardia pacing successfully terminates VT in over 80% of those with sustained dysrhythmia.16 In the Pain-FREE Rx II trial, data indicate ATP could successfully treat not only standard but rapid VT as well; outcomes revealed a 70% reduction in shocks without adverse effects.5,16
I Synchronized cardioversion. Typically, VT is an organized rhythm. Synchronization of the shock (delivered on R wave peak) and conversion can often be accomplished with low voltage. This helps to minimize discomfort and avoids defibrillation, which potentially could lead to degeneration of VT to VF.
I Defibrillation. This is the delivery of an unsynchronized shock during the cardiac cycle. This can be accomplished through a range of energies. Initial shocks are often programmed for lower energies to reduce capacitor charge time and expedite therapy. Typically, shocks are set to 5 to 10 joules above the defibrillatory threshold (determined at time of implantation).9,16
I Cardiac pacing. All models now have pacing modes similar to single- or dual-chamber pacers.
Implantation
Original ICDs were placed into the intraabdominal cavity through a large thoracotomy. With current-generation ICDs, leads are typically placed transvenously (subclavian, axillary, or cephalic vein), which has led to fewer perioperative complications, including shorter procedure time, shorter hospital stay, and lower costs as compared to abdominal implantation.5,17
The pulse generator remains subcutaneous or submuscular in the pectoral region. An electrode is advanced into the endocardium of the right ventricle apex; dual-chamber ICDs have an additional electrode placed in the right atrial appendage and biventricular ICDs have a third electrode placed transcutaneously in a branch off the coronary sinus.
At the time of the procedure, the electrophysiology team implanting the ICD will configure the diagnostic and therapeutic options; in particular, the defibrillatory threshold will be determined for each specific patient and the device set up with this value.
Complications
Acute complications in the peri-implantation period range from 4% to 5%.18 These are similar to other transvenous procedures and include bleeding, air embolism, infection, lead dislodgment, hemopneumothorax, and rarely death (perioperative mortality 0.2%-0.4%).2,19 Long-term complications may present consistent with other indwelling artificial hardware. Subclavian vein thrombosis with pulmonary thromboembolization, superior vena cava syndrome, as well as lead colonization with infection, are potential complications. superior vena cava thrombosis has been demonstrated in up to 40% of patients. These complications often present insidiously and the clinician should retain a high degree of suspicion.
Infection of the pocket or leads has been observed in up to 7%. Technical causes leading to inappropriate shock include faulty components, oversensing of electrical noise, lead fracture, electromagnetic interference, oversensing of diaphragm myopotentials, oversensing of T-waves, and double counting of QRS complexes.22
Lead complications can include infection, dislodgement (most will occur in the first 3 months after placement), fracture, and insulation defects. Lead failure rates have been reported at up to 1% to 9% at 2 years and as high as 40% at 8 years. Failure occurs secondary to insulation defects (26%), artifact oversensing (24%), fracture (24%), and 26% of the time secondary to infection.3,23
Cardiac perforation is uncommon but potentially devastating. These cases almost always occur with lead manipulation or repair of a screw in the lead; this rarely would lead to clinical significance but possibly the most emergent manifestation would be cardiac tamponade. Chest pain with signs and symptoms of tamponade require prompt diagnosis. Suspect this in the patient with a newly paced RBBB pattern on ECG, diaphragmatic contractions (hiccups), and pericardial effusion. Eighty percent of such perforations with tamponades will occur in first 4 days after implantation, and a chest X-ray or the echocardiogram can help confirm the diagnosis.
Pulse-generator complications include migration, skin erosion, and premature battery depletion.24 Twiddler’s syndrome after pacemaker insertion is a well-described syndrome in which twisting or rotating of the device in the pocket (from constant patient manipulation) results in device malfunction, and Boyle et al describe a similar scenario occurring after ICDs are implanted.25 The authors suggest that an increase in bradycardic pacing threshold or lead impedance may be the initial presentation; however, the possibility that the device failed to sense or treat arrhythmias also should be considered.
Lastly, several studies have documented a statistically significant adverse effect on quality of life in patients living with ICDs. Patients often describe a shock as “being struck by a truck”.22 This may result in depression and anxiety; both are especially prevalent in those who receive frequent shocks. It may be important to consider anxiolytics, support groups, or outpatient referral.2,22,26,27
Management of the Patient With an ICD in the Emergency Department
Patients with ICDs will present to the ED with a variety of complaints, ranging from general/non-specific (eg, dizziness) to life threatening (eg, cardiac arrest). The following section systematizes the approach to these patients.
Frequently, patients with ICDs will present with the complaint of having been shocked. In those patients, the most important initial step is to determine if the shock was appropriate. Initial management should include placement of a cardiac monitor and a rapid 12-lead ECG. A general assessment for the etiology of the shock may reveal a patient’s clinical deterioration, a change in medical therapy, or electrolyte imbalance.2 An accurate history of the surrounding events is key in determining the reason for patients presenting after receiving a shock. A history of chest pain or strenuous physical activity that preceded the shock may indicate, respectively, an appropriate shock from cardiac ischemia or an inappropriate shock caused by skeletal muscle activity. Also, presentations such as a fall following an episode of syncope may represent an ICD-related event and this possibility needs to be considered during the management of these patients.
Clinically Stable Patients After Isolated Shocks
For the patient who received an isolated shock and afterwards is asymptomatic, perform a general assessment as above. Often these patients have experienced an episode of sustained VT that was appropriately recognized and treated.1 For those who feel ill following a shock, emergent assessment is required for the possibility of a resultant arrhythmia following inappropriate shock (eg, device malfunction or battery depletion) or underlying active acute medical illness such as acute coronary syndrome. Always consider interrogation of the device, which will confirm appropriate shock delivery and successful termination of VT/VF. Interrogation also may reveal signs of altered impedance, which may be treated by ICD reprogramming or lead revision in the case of lead malfunction.2 Look for alternative explanations for inappropriate shocks. For example, obtain a chest X-ray to assess proper position of pulse generator or look for presence of lead fracture or migration. Lead fractures tend to occur at three sites: (1) the origin of the lead at the pulse generator, (2) the venous entry site, and (3) within the heart. A basic metabolic panel may reveal hypokalemia or hypomagnesemia leading to lower threshold for dysrhythmia. It is also important to inquire about new medication regimens. Patients with ICDs also are often on multiple cardiac medications, which could lead to alteration in the QT interval or to electrolyte imbalance.
We recommend contacting and discussing the care of patients who present after ICD shocks with the treating electrophysiologist or cardiologist whether or not the shock is considered appropriate.
Patients who have an ongoing arrhythmia when evaluated emergently should be managed according to advanced cardiac life support (ACLS) guidelines, regardless of the presence of an ICD,1 particularly in cases of cardiac arrest from a non-shockable rhythm.
Initially, the shocks should be presumed to be appropriate. Presence of VT/VF in setting of shock would be consistent with appropriate shock delivery. Next, the clinician needs to consider if shock delivery was effective and if it achieved termination of malignant ventricular arrhythmia. Patients with persistent VT/VF despite delivery of a shock may have ICDs with inadequate voltage in the batteries to terminate; external shocks and intravenous (IV) antiarrhythmic medications may be required and should be administered per ACLS guidelines.
When patients present with multiple shocks, the shocks are typically appropriate and often triggered by episodes of VT/VF. Treatment of the underlying causes is the priority; the patient may have sustained or recurrent VT/VF as a result of an acute event, such as cardiac ischemia, hypokalemia, or severe acute heart failure exacerbation. Aggressive reperfusion, management of potassium imbalance, and circulatory support are paramount.
Inappropriate shocks most commonly are delivered for supraventricular tachycardias such as atrial fibrillation that is incorrectly interpreted by the ICD as VT/VF. In these cases, the treatment is the same as for a patient without an ICD (eg, IV diltiazem to slow atrial fibrillation with rapid ventricular response).
In patients experiencing multiple inappropriate ICD shocks, the device can be immediately disarmed by placing a magnet over the ICD pocket until the electrophysiologist can reprogram it. This will not inhibit baseline/backup pacing. However, while a magnet is in place, neither supraventricular tachycardias nor VT/VF will be detected.1 If appropriate shock delivery has been performed for ventricular dysrhythmia, these patients must remain on a cardiac monitor under close medical observation. It is good practice to assume device failure after application of a magnet, and appropriate management strategies include placing external defibrillators pads on the patient’s chest. Fortunately, most ICDs will resume normal function following magnet removal.
In the Canadian Journal of Cardiology (1996), Kowey defined electrical storm as a state of cardiac electrical instability characterized by multiple episodes of ventricular tachycardia (VT storm) or ventricular fibrillation (VF storm) within a relatively short period of time.28,30,31,32
In the patient with an ICD, the generally accepted definition is occurrence of two or more appropriate therapies (antitachycardia pacing or shocks) in a 24-hour period. Triggers may include drug toxicity, electrolyte disturbances (hypokalemia and hypomagnesemia being the most common culprits), new or worsened heart failure, or myocardial ischemia, which account for more than a quarter of all episodes. Electrical storm usually heralds a life-threatening acute pathology placing these patients at immediate high risk of death.28 Immediate communication and consultation with the electrophysiology team is recommended.
Left Ventricular Assist Devices: From Mystery to Mastery
Alicia S. Devine, JD, MD
Dr Devine is an assistant professor, department of emergency medicine, Eastern Virginia Medical School, Norfolk.
Disclosure: The author reports no conflict of interest.
Approximately 5.7 million people in the United States have heart failure, and complications from heart failure represent 668,000 ED visits annually. Heart failure is the primary cause of death in 55,000 people each year. Half of patients die within 5 years of being diagnosed with heart failure.1
Heart failure is initially managed medically; however, some patients become refractory to medical treatment and require heart transplant. Unfortunately, the demand for donor hearts far exceeds the supply, and patients can spend a long time waiting for a donor heart. In addition, not all patients are candidates for transplantation. Left ventricular assist devices are mechanical devices implanted in patients with advanced heart failure in order to provide circulatory support when medications alone are not efficacious. LVADs have been associated with improved survival for heart failure patients.
There are generally two indications for LVAD support: as a bridge to transplant for patients waiting for a donor heart, or as destination therapy for patients who are not candidates for heart transplant. Some patients have had improvement in their cardiac function after LVAD implantation and are able to have the LVAD explanted, leading to a third use for LVADs: bridge to recovery.
LVADs have been in use for over 30 years, and they have evolved during that time to become smaller in size with much fewer complications. Initial models operated with a pulsatile-flow pump that, while adequate in terms of blood flow, contained several parts susceptible to breaking down. Early models were large and cumbersome, especially for smaller patients. New-generation LVADs use a continuous-flow design with either a centrifugal or an axial flow pump with a single moving part, the impeller. The continuous-flow LVADs are quieter, smaller, and significantly more durable than the earlier, pulsatile-flow LVADs. These improvements have expanded the pool of eligible patients to include children and smaller adults.2 Moreover, continuous-flow LVADs provide greater rates of survival and quality of life than the earlier pulsatile-flow models.3
There are fewer adverse events overall with the continuous-flow LVADs compared with the pulsatile-flow LVADs. The number of LVADs implanted each year continues to increase, and more than 95% of these are continuous-flow. As more and more advanced heart failure patients are receiving these devices, emergency physicians should have a basic familiarity with their function and their common complications.4
There are several manufacturers and types of continuous-flow LVADs, but they generally consist of a pump that is surgically implanted into the abdominal or chest cavity of the patient with an inflow cannula positioned in the left ventricle and an outflow cannula inserted into the ascending aorta. The device draws blood from the ventricle and directs it to the aorta. There is a driveline connected to the internal pump that exits the body through the abdominal or chest wall and connects to a system controller. The controller is usually housed in a garment worn by patients that also includes the external battery that powers the LVAD. The controller can also be powered by a base unit that can be plugged into an electrical outlet.5 Patients with continuous-flow LVADs are anticoagulated with warfarin with a target international normalized ratio (INR) of 1.5 to 2.5 and will usually be on an antiplatelet agent as well.2
LVAD patients are typically managed by a team of providers that includes a VAD coordinator; a cardiologist and/or a cardiothoracic surgeon; and a perfusionist, who should be notified as soon as the patient arrives in the ED. Patients understand that it is vital that their LVAD be powered at all times and will usually arrive in the ED with their charged backup batteries. If a power base is available in the hospital, the LVAD can be connected to it to save battery life. If power is interrupted to the LVAD, the pump will stop working. This can be fatal to patients with severe aortic insufficiency who have had their outflow tract surgically occluded and are therefore completely dependent on the LVAD.2
With continuous-flow LVADs, blood is pumped continuously, and a constant, machine-like murmur will be heard on auscultation rather than the typical heart sounds. LVAD patients may not have palpable arterial pulses, and in that case a doppler of the brachial artery and a manual blood pressure cuff are used to listen for the start of Korotkoff sounds as the cuff is released. The pressure at which the first sound is heard is used as an estimate of the mean arterial pressure (MAP). Left ventricular assist device patients should have a MAP between 70 and 90 mm Hg. An accurate pulse oximetry reading may not be attainable, and some centers use cerebral oximetry to obtain oxygenation status.2
The EP should examine all of the connections from the percutaneous lead to the controller and from the controller to the batteries to ensure that they are intact. The exit site for the percutaneous lead should also be examined for evidence of trauma and signs of infection. The exit site is a potential nidus for infection, and even minor trauma from a pull or tug on the lead can damage the tissue and seed an infection. Emergency physicians should ask LVAD patients about any recent trauma to the driveline.6,7
The ED evaluation for an LVAD patient should be focused toward the patient’s chief complaint, recognizing that often patients with LVADs presenting to the ED will have vague complaints of malaise or weakness that may represent a serious pathologic process. Infection, bleeding, thrombosis, and problems with volume status are common reasons for ED visits by LVAD patients.3,5
Infection
In addition to infections in the lung, skin, and urinary tract, patients with LVADs are at risk for infectious complications relating to their device. Implantation of an LVAD involves a sternal incision, the creation of an internal pocket for the LVAD, and a driveline connecting the internal LVAD with an external power source. An infection in any one of these locations can lead to endocarditis, bacteremia, and sepsis.6
Driveline and/or pocket infections are very common, affecting up to 36% of patients with continuous-flow LVADs.8 The exit site for the driveline is an access point for the entry of pathogens, and can be the source of infections in the driveline or in the pump pocket. Pump pocket infections can also occur from exposure to pathogens during surgery or in the immediate postoperative period. In addition, the pump itself can become infected from similar sources, as well as from bacteremia or fungemia from infections in the urine, lung, or central catheters.6
Infections in the driveline will often present with obvious signs such as purulent drainage, erythema, and tenderness at the exit site, but providers should have a high index of suspicion if there is dehiscence at the exit site or even persistent serous drainage from the site, as these can suggest a driveline infection. Pump pocket infections and device-related endocarditis can present with vague symptoms such as weight loss, malaise, and a low-grade fever.
A thorough evaluation should be undertaken in all LVAD patients with a suspected infection to detect a source, and cultures of blood, urine, and the driveline exit site should be obtained. Imaging techniques frequently used when considering device-related infections include ultrasound of the pump pocket and echocardiography to evaluate for endocarditis. Computed tomography is also used to evaluate for device-related infections.6,7,9,10 LVADs are not compatible with MRI.11
The majority of device-related infections are caused by bacteria, although fungal and viral species can be the source as well. Common pathogens implicated include S aureus, S epidermidis, enterococci, Pseudomonas aeruginosa, Klebsiella species and Enterobacter species. Empiric antibiotics with both gram-positive and gram-negative coverage should be initiated for suspected infection related to the device. If the infection has spread to the pump pocket or the device, patients may need surgery for drainage and possible removal of the device.6,7,9,10
Bleeding and Thrombosis
Bleeding complications occur with pulsatile-flow and continuous-flow LVADs at the same rate, and represent one of the most common adverse events seen in LVAD patients. Sites of bleeding include intracranial, nasal cavity, genitourinary tract, and gastrointestinal (GI).11
Interestingly, GI bleeding occurs at a much higher rate in patients with continuous-flow LVADs than in patients with pulsatile-flow devices.2,5,11,12 Patients with continuous-flow LVADs are anticoagulated with warfarin (to a target INR of between 1.5 and 2.5) and an antiplatelet agent to prevent pump thrombosis as well as other thromboembolic events.11 In addition to the effects of warfarin and aspirin, several other factors contribute to the increased incidence of GI bleeding, including an acquired von Willebrand disease and the development of small bowel angiodysplasias from the alteration in vascular hemodynamics from the continuous flow.13,14,15
Emergency physicians should have a high index of suspicion for a bleeding event in patients with an LVAD presenting to the ED. The evaluation of GI bleeding in LVAD patients is the same as in patients without LVADs, and management includes resuscitation with fluids, blood transfusion, and careful correction of coagulopathy. Gastrointestinal bleeding in an LVAD patient necessitates a consultation with a gastroenterologist and admission to the hospital.11
Pump thrombosis, though rare, can result in death and must be considered in cases of MAP < 60 mm Hg and/or an increased power requirement accompanied by a decrease in pulsatility index and flow. Markers of hemolysis such as elevated lactate dehydrogenase or hemoglobinuria also suggest pump thrombosis. Interrogation of the LVAD by the perfusionist is imperative when LVAD patients present to the ED. Echocardiography is the modality of choice in evaluating suspected pump thrombosis. Treatment may require replacement of the pump, or in some cases, anticoagulation or thrombolysis.2,11
Volume Status
Patients with LVADs can present with complaints of weakness and/or dizziness that can be due to dehydration and/or electrolyte deficiencies. Often, these patients will continue to restrict their salt and fluid intake after device implantation. They are frequently on diuretics, which can contribute to these problems. Checking and repleting electrolytes as well as administering a gentle bolus of IV fluids in patients with a MAP < 60 mm Hg will often correct the hypovolemia and electrolyte abnormalities. Evaluation for sepsis, pump thrombosis, and cannula malposition as causes of hypotension should be undertaken in the appropriate circumstances.2,11 Severe hypovolemia can interfere with effective LVAD function if it leads to the collapse of the left ventricle over the inflow cannula. Bedside ultrasound can be a useful adjunct in the evaluation of cannula position and volume status.2 An emergent consult with a cardiovascular surgeon is indicated in the event of pump thrombosis or cannula malposition.
Conclusion
The number of LVADs implanted each year continues to grow, and EPs need to have a basic familiarity with these devices and how to manage typical complaints seen in the ED. Patients and their caregivers have been given extensive education and training on the care and management of their LVAD components and can be a valuable source of information. They should bring the devices with them to the ED, along with the names and phone numbers of all of the members of their VAD treatment team, who should be called shortly after the patient’s arrival, as well as backup charged batteries to power their LVAD.
A priority is ensuring that all of the LVAD connections are intact and that there is adequate power to the device. A perfusionist will need to interrogate the controller if there is any concern about its function, including alarms sounding or lights flashing. The manufacturer’s website can be accessed if necessary for further information.
Alicia S. Devine, JD, MD
Dr Devine is an assistant professor, department of emergency medicine, Eastern Virginia Medical School, Norfolk.
Disclosure: The author reports no conflict of interest.
Heart disease affects a growing number of patients each year. The causes of heart disease are diverse, but whether the etiology is ischemic or structural, the disease often progresses to the point where patients are at risk for fatal dysrhythmias and heart failure. Treatment modalities for heart disease range from lifestyle modification and medical management to interventional reperfusion, and often involve the surgical implantation of devices designed to improve cardiac function and/or to detect and terminate lethal dysrhythmias.
Over the past two decades, the use of automated implantable cardiac devices (AICDs) such as pacemakers, implantable cardioverter defibrillators (ICDs), and left ventricular assist devices (LVADs) has increased significantly. From 1993 to 2009, nearly 3 million patients received permanent pacemakers in the United States; in 2009 alone, over 188,000 were placed. From 2006 to 2011 (the period for which the most recent data are available), approximately 850,000 patients had an AICD implanted. For the 20-month period running from April 2010 to December 2011, nearly 260,000 patients received the device. Finally, from 2006 through 2013, over 9,000 LVADs were placed. Like the other cardiac devices discussed, the frequency of use continues to increase, with 3,834 LVADs placed in just the first 9 months of 2013.
Emergency physicians are expected to be able to stabilize and manage patients with these devices who present to the ED. Care for these patients requires an understanding of the components and function of the different devices as well as their complications. All of the devices are subject to complications from infection, bleeding, migration, or fracture of the component parts, and, more ominously, complete failure of the device. While the current generation of cardiac devices are much smaller in size than their initial prototypes, they are more technically complex, and consultation with cardiology after initial stabilization is recommended.
Cardiac Hardware
Management of the Patient With an Implanted Pacemaker
Martin Huecker, MD
Thomas Cunningham, MD
Dr Huecker is an assistant professor, department of emergency medicine, University of Louisville, Kentucky.
Dr Cunningham is chief resident, department of emergency medicine, University of Louisville, Kentucky.
Disclosure: The authors report no conflict of interest.
Introduction
Cardiac pacing was conceived in 1899, and the first successful pacemaker was implanted in 1960.1,2 New concepts and evolution of design have made pacemakers increasingly complex. Over the last decade, the rate of implantation has grown by over 50%.3 At the forefront of cardiac care, today’s EP must be proficient in the care of patients with cardiac pacemakers.
The pacemaker consists of a generator and its leads. The generator produces an electrical impulse that travels down the leads to depolarize myocardial tissue.4 A pacemaker corrects abnormal heart rhythms, using these electrical pulses to induce a novel sinus rhythm.5,6Table 1 summarizes the 2008 American College of Cardiology/American Heart Association Level I/II indications for pacemaker placement.
Permanent pacing involves fluoroscopic placement of leads into a chamber(s) of the heart. The generator is implanted most commonly in the left subcutaneous chest.7-9 A single-chamber pacemaker’s leads are located in either the right atrium or ventricle. Dual-chamber pacemakers function with one electrode in the atrium and one in the ventricle. A biventricular pacemaker, also known as cardiac resynchronization therapy (CRT) paces both ventricles via the septal walls.4,7,10
All pacemaker patients need prompt identification of the device manufacturer.8 Patients should carry identification cards. Chest X-ray may identify the device and will give information as to the location and structural integrity of wires. Interrogation should generally be performed in all patients and will provide valuable information such as battery status, current mode, rate, past rhythms, parameters to detect malignant rhythms, and therapeutic settings.4
Evaluation of the patient with a pacemaker begins with a detailed history and physical examination, including any complications involving the device. Clinicians should ask about pacemaker-related symptoms—ie, palpitations, light-headedness, syncope, or changes in exercise tolerance.3 As with all chest pain complaints in the ED, addressing abnormal vital signs and identification of myocardial infarction (MI) must precede other considerations.
Myocardial Infarction in the Pacemaker Patient
Because of the underlying rhythm induced by the cardiac pacemaker stimulation, acute coronary occlusion can be subtle.12 Since the pacemaker depolarizes the right ventricle, the delay in left ventricular depolarization is seen as left bundle branch block (LBBB) on electrocardiogram (ECG).13,14Figure 1 shows an ECG demonstrating paced rhythm and appropriate discordance, while the ECG in Figure 2 demonstrates acute coronary occlusion. Therefore, identification of coronary occlusion in the paced patient is done using the following Sgarbossa criteria:
- ST elevation ≥1 mm in a lead with upward (concordant) QRS complex; 5 points.
- ST depression ≥1 mm in lead V1, V2, or V3; 3 points.
- ST elevation ≥5 mm in a lead with downward (discordant) QRS complex; 2 points.13,15
An ECG demonstrating three points of Sgarbossa criteria yields a diagnosis of ST segment elevation MI with 98% specificity and 20% sensitivity.16 A modified Sgarbossa criteria replaces the absolute ST-elevation measurement (Sgarbossa criteria 3) with an ST/S ratio greater than -0.25. This yields a sensitivity of 90% and specificity of 90%.17
Pacemaker-Related Complications
When ischemia is no longer a concern, address the device itself. Workup involves history and physical examination, with complete blood count, chest X-ray, cardiac biomarkers, basic metabolic panel, ultrasound, and device interrogation, as indicated. Table 2 provides a summary of associated pacemaker syndromes and treatment.
Infectious Complications
Patients with device-related infection can present with local or systemic signs, depending on time from implantation. Tenderness to palpation over the generator is sensitive for pocket infection. Although rare, pocket infections require urgent evaluation with mortality rates as high as 20%.18
Early (< 30 days) pocket complications are usually attributable to hematomas with or without infection. When infection is present, Staphylococcus aureus and Staphylococcus epidermidis are the most likely culprits. Up to 50% of isolates can be methicillin resistant S aureus.19 Although needle aspiration has been used in the past for evacuation and microbial identification, current recommendations do not advocate this approach.20 Incision and debridement are the mainstays of therapy. Over 70% of patients with pocket infections will have positive blood cultures and should receive antibiotic therapy with vancomycin.21
Patients with wound separation or pocket infection are at risk for lead infection, lead separation, and lead fracture with related thoracic involvement (ie, pneumonia, empyema, hemothorax, pneumothorax, or diaphragmatic rupture).20
Infectious complications greater than 30 days from implantation are more likely lead-related. Because of the risk for embolic disease to pulmonary or cardiac tissues, emergent line removal and empiric antibiotics are recommended.18 After admission, a transesophageal echocardiogram should be performed to evaluate for valvular involvement and baseline cardiac function.22-24
Physiologic Complications
Patients without ischemia or infection should be evaluated for device-related chest pain. Pain resulting from malfunction of the device usually occurs in the first 48 hours after implantation.9
Patients may present with chest pain related to lead migration or malposition. Perforation of the pleural cavity during the initial procedure can cause hemothorax or pneumothorax. Perforation of the myocardium can lead to hemopericardium and cardiac tamponade. Patients present with respiratory distress and cardiac dysfunction with or without pacing failure.4,9 Bedside cardiac ultrasound assists in assessing these complications and degree of severity.25
Lead migration occurs when a lead detaches from the generator and migrates. Complete separation from the generator may present as failure to capture and should be addressed before lead localization, as temporary pacing may be warranted. Leads coil and regress from patient tampering (ie, Twiddler’s Syndrome) or through spontaneous detachment.3
The ECG may detect functional leads that have migrated to the left heart (coronary sinus, entricular septal defect, perforation). Right bundle branch morphology, rather than the expected left bundle branch morphology, indicates a lead depolarizing the left ventricle.26,27
Lead fracture may occur at any time after implantation. In addition to the complications seen with lead separation and migration, lead fracture is associated with pulmonary vein thrombosis. Because of the volatile nature of fractured leads, patients present more frequently with pacemaker failure, dysrhythmias, and hemodynamic compromise. Temporary pacing may be necessary pending surgical intervention.4,20
Days to weeks postprocedure, patients are at risk for central venous thrombus due to creation of a thrombogenic environment. These thrombi can embolize to the pulmonary circulation and computed tomography pulmonary angiogram should be considered if suspicious.3
Electrical Complications
Failure to pace can be attributed to lead complication (ie, lead malposition, lead fracture), poor lead-tissue interface, or generator complication.28 Electrical complications arise from intrinsic generator malfunction, lack of pacemaker capture, oversensing/undersensing, and poor pacemaker output.29 Poor output results from battery failure, generator failure, or lead misplacement.9
Generator malfunction can produce unwanted tachycardia and exacerbate intrinsic poor cardiac function. Pacemaker-mediated tachycardia (PMT), pacemaker syndrome, and runaway pacemaker should be eliminated from the differential though interrogation and ECG.
Patients presenting with signs of hypotension and cardiac failure may have pacemaker syndrome. With single-chamber conduction, atrioventricular dysynchrony occurs, producing a lack of ventricular preload and poor cardiac output. Treatment includes symptomatic management and pacemaker replacement with a dual-chamber device. In the hemodynamically unstable patient, applications to increase the preload and reduce the afterload should be attempted.20,25
Trauma, battery failure, and intrinsic pacer malfunction can cause PMT such as runaway pacemaker. Application of a magnet has been shown effective only in some cases.3,30 Definitive therapy with emergent pacer reprogramming or surgical disconnection of pacer leads from the generator may be warranted.
Failure to capture occurs when the device electrical impulse is insufficient to depolarize the heart. Battery failure, generator failure, electrode impedance (from fibrosing of the electrodes), lead fracture or malposition, and long QT syndrome are all causes of failure to capture.29 Chest X-ray, ECG, device interrogation, and electrolyte measurement are imperative. The patient with intrinsic generator failure usually requires admission and surgical correction or replacement.3
Oversensing occurs when the device incorrectly interprets artifactual electricity as intrinsic cardiac depolarization. This results in a lack of cardiac stimulation by the pacemaker and can lead to heart block. Shivering, fasciculations from depolarizing neuromuscular blockade, and external interference can cause oversensing. Nonmedical causes include cell phones, security gates, Taser guns, magnets, and iPods.28 Iatrogenic causes include electrosurgery, LVADs, radiation therapy, magnetic resonance imaging (MRI), cardioversion, and lithotripsy.31,32 Treatment involves withdrawing the offending agent, then either placing a magnet over the generator to activate its asynchronous mode or temporary overdrive pacing.26,28,31
Undersensing occurs when the pacer fails to sense intrinsic cardiac activity. The result is competitive asynchronous activity between the native cardiac depolarization and the pacemaker impulses. Introduction of new intrinsic rhythms from lead complications (lead fracture, lead migration), ischemia (premature ventricular contraction, premature atrial contraction), or underlying cardiac disease (atrial fibrillation, right BBB [RBBB], LBBB) can precipitate undersensing.4,5,30 These patients are prone to arrhythmias and decompensation of cardiac function. Management requires identifying the cause of the underlying arrhythmia.29 Chest X-ray, ECG, device interrogation, and electrolyte measurement are useful diagnostics for patients with new arrhythmias or ischemia.3,14,27
Conclusion
To assist the EP in evaluating a patient with a suspected pacemaker problem, we propose the algorithm presented in Figure 3.
Recent advancements and the increased prevalence of pacemakers require the EPs to be facile with their operating systems and morbidity. A detailed history and physical examination, along with utilization of simple diagnostics and device interrogation, can prove sufficient to diagnose most pacemaker-related complaints. Acute coronary syndrome and serious infections may be subtle, so a high level of suspicion should be maintained. With a knowledgeable EP and a supportive team, pacemaker complications can be successfully managed.
Managing Implantable Cardioverter Defibrillator Shock Complications
Dustin G. Leigh, MD; Cameron R. Wangsgard, MD; Daniel Cabrera, MD
Dr Leigh is a chief resident, department of emergency medicine, Mayo Clinic, Rochester, Minnesota. Dr Wangsgard is a chief resident, department of emergency medicine, Mayo Clinic, Rochester, Minnesota. Dr Cabrera is an assistant professor of emergency medicine, Mayo Clinic, Rochester, Minnesota.
Disclosure: The authors report no conflict of interest.
Introduction
Despite significant advances in emergency medical care and resuscitation techniques, sudden cardiac death remains a major public health problem, accounting for approximately 450,000 deaths annually in the United States.1 Moreover, the vast majority of people who suffer an out-of-hospital cardiac arrest will not survive. This is often the end result of fatal ventricular arrhythmias, including ventricular fibrillation (VF) and ventricular tachycardia (VT). The most effective therapy is rapid electrical defibrillation.2
During the 1970s, Mirowski and Mower developed the concept of an implantable defibrillator device that could monitor and analyze cardiac rhythms with automatic delivery of defibrillating shocks after detecting VF.3,4 In 1980, the first clinical implantation of a cardiac defibrillation device was performed. Development continued steadily until the 1996 the Multicenter Automatic Defibrillator Implantation Trial was prematurely aborted when a statistically significant reduction in mortality (54%) was recognized in patients who received ICD therapy instead of antiarrhythmic therapy.5,6 This was followed by large prospective, randomized, multicenter studies establishing that ICD therapy is effective for primary prevention of sudden death.7 Based on these developments, the ICD has rapidly evolved from a therapy of last resort for patients with recurrent malignant arrhythmias to the standard of care in the primary and secondary prevention of sudden cardiac death, and more recently as cardiac resynchronization devices in patients with congestive heart failure (CHF).3
These developments have led to a dramatic increase in the use of the ICD for monitoring and treatment of VT and VF. The dismal survival rate after cardiac arrest provides a strong impetus to identify high risk patients of sudden cardiac death resulting from VF/VT by primary prevention with an ICD.2,5 More than 100,000 ICDs are implanted annually in the United States.1 As a result of increased prevalence, the EP will often encounter patients who have received an ICD shock or complication of the device. Thus, experienced a general knowledge of implantation, components, complications, and acute management is crucial for clinicians who may care for these patients.
Indications
Implantable cardioverter defibrillators are generally indicated for the primary and secondary prevention of sudden cardiac death.8 The commonly accepted indications for ICD use are summarized here:
Primary Prevention
- Patients with previous MI and LV ejection fraction (LVEF) < 30%
- Patients with cardiomyopathy, New York Heart Association functional class III or IV and LVEF < 35%.
Secondary Prevention
- I Patients with an episode of sustained or unstable VT/VF with no reversible cause.
- I Patients with nonprovoked VT/VF with concomitant structural heart disease (valvular, ischemic, hypertrophic, infiltrative, dilated, channelopathies).
ICD Design
Current ICDs are third-generation device, only slightly larger than pacemakers. ICDs are small (25-45 mm), reliable, and contain sophisticated electrophysiologic analysis algorithms. They can store and report a large number of variables, such as ECGs, defibrillation logs, various energies, lead impedance, as well as battery charge.3,9 Stevenson et al1 describe four major functions of the ICD: sensing of electrical activity from the heart, detection of appropriate therapy, provision of therapy to terminate VT/VF, and pacing for bradycardia and/or CRT.
Components
The components of an ICD can be organized in the following manner:
I Pacing/sensing electrodes. Contemporary units complete these functions through use of two electrodes; one at the distal tip of the lead and one several millimeters back (bipolar leads).1
I Defibrillation electrodes/coils. The defibrillation electrode is a small coil of wire that has a relatively large surface area and extends along the distal aspect of the ventricular lead, positioned at the apex. This lead delivers current directly to the myocardium.11,12 Both the sensing and defibrillation electrodes are often housed in the same, single wire.
I Pulse generator. The pulse generator contains a microprocessor with sensing circuitry as well as high voltage capacitors, a battery, and memory storage component. Modern battery life is typically 5 to 7 years (frequency of shocks will lead to early termination of the battery life).2,11 Some ICDs have automatic self-checks of battery life and will emit a tone when the battery is low or near failure; these patients should be promptly evaluated and referred to the electrophysiologist as indicated.
Functions
The original concept of the ICD was to sense a potentially lethal dysrhythmia and to provide an appropriate therapy. As ICD technology has evolved, the number and variety of available programming and therapies have dramatically increased. Detection of the cardiac rhythm was designed initially to only detect ventricular fibrillation. With current generation models, the ventricular sensing lead filters the incoming signal to eliminate unwanted low frequency components (eg, T-waveand baseline drift) and high frequency components (eg, skeletal muscle electrical activity).3,13 Newer ICDs have the capability for remote monitoring and communication via telephone line or the Internet.
During implantation, the device is programmed with analysis criteria. Criteria for therapy are largely based on the rate, duration, polarity, and waveform of the signal sensed. When the device detects a signal fulfilling the preprogrammed criteria for VT/VF, it selects the appropriate tier of treatment as follows:
I Antitachycardia pacing (ATP). Ventricular tachycardia, particularly reentrant VT associated with scar formation from a prior MI, can sometimes be terminated by pacing the ventricle at a rate slightly faster than the tachycardia. This form of therapy involves the delivery of short bursts (eg, eight beats) of rapid ventricular pacing to terminate VT.14,15 This therapy is low voltage and usually not felt by patients. Antitachycardia pacing successfully terminates VT in over 80% of those with sustained dysrhythmia.16 In the Pain-FREE Rx II trial, data indicate ATP could successfully treat not only standard but rapid VT as well; outcomes revealed a 70% reduction in shocks without adverse effects.5,16
I Synchronized cardioversion. Typically, VT is an organized rhythm. Synchronization of the shock (delivered on R wave peak) and conversion can often be accomplished with low voltage. This helps to minimize discomfort and avoids defibrillation, which potentially could lead to degeneration of VT to VF.
I Defibrillation. This is the delivery of an unsynchronized shock during the cardiac cycle. This can be accomplished through a range of energies. Initial shocks are often programmed for lower energies to reduce capacitor charge time and expedite therapy. Typically, shocks are set to 5 to 10 joules above the defibrillatory threshold (determined at time of implantation).9,16
I Cardiac pacing. All models now have pacing modes similar to single- or dual-chamber pacers.
Implantation
Original ICDs were placed into the intraabdominal cavity through a large thoracotomy. With current-generation ICDs, leads are typically placed transvenously (subclavian, axillary, or cephalic vein), which has led to fewer perioperative complications, including shorter procedure time, shorter hospital stay, and lower costs as compared to abdominal implantation.5,17
The pulse generator remains subcutaneous or submuscular in the pectoral region. An electrode is advanced into the endocardium of the right ventricle apex; dual-chamber ICDs have an additional electrode placed in the right atrial appendage and biventricular ICDs have a third electrode placed transcutaneously in a branch off the coronary sinus.
At the time of the procedure, the electrophysiology team implanting the ICD will configure the diagnostic and therapeutic options; in particular, the defibrillatory threshold will be determined for each specific patient and the device set up with this value.
Complications
Acute complications in the peri-implantation period range from 4% to 5%.18 These are similar to other transvenous procedures and include bleeding, air embolism, infection, lead dislodgment, hemopneumothorax, and rarely death (perioperative mortality 0.2%-0.4%).2,19 Long-term complications may present consistent with other indwelling artificial hardware. Subclavian vein thrombosis with pulmonary thromboembolization, superior vena cava syndrome, as well as lead colonization with infection, are potential complications. superior vena cava thrombosis has been demonstrated in up to 40% of patients. These complications often present insidiously and the clinician should retain a high degree of suspicion.
Infection of the pocket or leads has been observed in up to 7%. Technical causes leading to inappropriate shock include faulty components, oversensing of electrical noise, lead fracture, electromagnetic interference, oversensing of diaphragm myopotentials, oversensing of T-waves, and double counting of QRS complexes.22
Lead complications can include infection, dislodgement (most will occur in the first 3 months after placement), fracture, and insulation defects. Lead failure rates have been reported at up to 1% to 9% at 2 years and as high as 40% at 8 years. Failure occurs secondary to insulation defects (26%), artifact oversensing (24%), fracture (24%), and 26% of the time secondary to infection.3,23
Cardiac perforation is uncommon but potentially devastating. These cases almost always occur with lead manipulation or repair of a screw in the lead; this rarely would lead to clinical significance but possibly the most emergent manifestation would be cardiac tamponade. Chest pain with signs and symptoms of tamponade require prompt diagnosis. Suspect this in the patient with a newly paced RBBB pattern on ECG, diaphragmatic contractions (hiccups), and pericardial effusion. Eighty percent of such perforations with tamponades will occur in first 4 days after implantation, and a chest X-ray or the echocardiogram can help confirm the diagnosis.
Pulse-generator complications include migration, skin erosion, and premature battery depletion.24 Twiddler’s syndrome after pacemaker insertion is a well-described syndrome in which twisting or rotating of the device in the pocket (from constant patient manipulation) results in device malfunction, and Boyle et al describe a similar scenario occurring after ICDs are implanted.25 The authors suggest that an increase in bradycardic pacing threshold or lead impedance may be the initial presentation; however, the possibility that the device failed to sense or treat arrhythmias also should be considered.
Lastly, several studies have documented a statistically significant adverse effect on quality of life in patients living with ICDs. Patients often describe a shock as “being struck by a truck”.22 This may result in depression and anxiety; both are especially prevalent in those who receive frequent shocks. It may be important to consider anxiolytics, support groups, or outpatient referral.2,22,26,27
Management of the Patient With an ICD in the Emergency Department
Patients with ICDs will present to the ED with a variety of complaints, ranging from general/non-specific (eg, dizziness) to life threatening (eg, cardiac arrest). The following section systematizes the approach to these patients.
Frequently, patients with ICDs will present with the complaint of having been shocked. In those patients, the most important initial step is to determine if the shock was appropriate. Initial management should include placement of a cardiac monitor and a rapid 12-lead ECG. A general assessment for the etiology of the shock may reveal a patient’s clinical deterioration, a change in medical therapy, or electrolyte imbalance.2 An accurate history of the surrounding events is key in determining the reason for patients presenting after receiving a shock. A history of chest pain or strenuous physical activity that preceded the shock may indicate, respectively, an appropriate shock from cardiac ischemia or an inappropriate shock caused by skeletal muscle activity. Also, presentations such as a fall following an episode of syncope may represent an ICD-related event and this possibility needs to be considered during the management of these patients.
Clinically Stable Patients After Isolated Shocks
For the patient who received an isolated shock and afterwards is asymptomatic, perform a general assessment as above. Often these patients have experienced an episode of sustained VT that was appropriately recognized and treated.1 For those who feel ill following a shock, emergent assessment is required for the possibility of a resultant arrhythmia following inappropriate shock (eg, device malfunction or battery depletion) or underlying active acute medical illness such as acute coronary syndrome. Always consider interrogation of the device, which will confirm appropriate shock delivery and successful termination of VT/VF. Interrogation also may reveal signs of altered impedance, which may be treated by ICD reprogramming or lead revision in the case of lead malfunction.2 Look for alternative explanations for inappropriate shocks. For example, obtain a chest X-ray to assess proper position of pulse generator or look for presence of lead fracture or migration. Lead fractures tend to occur at three sites: (1) the origin of the lead at the pulse generator, (2) the venous entry site, and (3) within the heart. A basic metabolic panel may reveal hypokalemia or hypomagnesemia leading to lower threshold for dysrhythmia. It is also important to inquire about new medication regimens. Patients with ICDs also are often on multiple cardiac medications, which could lead to alteration in the QT interval or to electrolyte imbalance.
We recommend contacting and discussing the care of patients who present after ICD shocks with the treating electrophysiologist or cardiologist whether or not the shock is considered appropriate.
Patients who have an ongoing arrhythmia when evaluated emergently should be managed according to advanced cardiac life support (ACLS) guidelines, regardless of the presence of an ICD,1 particularly in cases of cardiac arrest from a non-shockable rhythm.
Initially, the shocks should be presumed to be appropriate. Presence of VT/VF in setting of shock would be consistent with appropriate shock delivery. Next, the clinician needs to consider if shock delivery was effective and if it achieved termination of malignant ventricular arrhythmia. Patients with persistent VT/VF despite delivery of a shock may have ICDs with inadequate voltage in the batteries to terminate; external shocks and intravenous (IV) antiarrhythmic medications may be required and should be administered per ACLS guidelines.
When patients present with multiple shocks, the shocks are typically appropriate and often triggered by episodes of VT/VF. Treatment of the underlying causes is the priority; the patient may have sustained or recurrent VT/VF as a result of an acute event, such as cardiac ischemia, hypokalemia, or severe acute heart failure exacerbation. Aggressive reperfusion, management of potassium imbalance, and circulatory support are paramount.
Inappropriate shocks most commonly are delivered for supraventricular tachycardias such as atrial fibrillation that is incorrectly interpreted by the ICD as VT/VF. In these cases, the treatment is the same as for a patient without an ICD (eg, IV diltiazem to slow atrial fibrillation with rapid ventricular response).
In patients experiencing multiple inappropriate ICD shocks, the device can be immediately disarmed by placing a magnet over the ICD pocket until the electrophysiologist can reprogram it. This will not inhibit baseline/backup pacing. However, while a magnet is in place, neither supraventricular tachycardias nor VT/VF will be detected.1 If appropriate shock delivery has been performed for ventricular dysrhythmia, these patients must remain on a cardiac monitor under close medical observation. It is good practice to assume device failure after application of a magnet, and appropriate management strategies include placing external defibrillators pads on the patient’s chest. Fortunately, most ICDs will resume normal function following magnet removal.
In the Canadian Journal of Cardiology (1996), Kowey defined electrical storm as a state of cardiac electrical instability characterized by multiple episodes of ventricular tachycardia (VT storm) or ventricular fibrillation (VF storm) within a relatively short period of time.28,30,31,32
In the patient with an ICD, the generally accepted definition is occurrence of two or more appropriate therapies (antitachycardia pacing or shocks) in a 24-hour period. Triggers may include drug toxicity, electrolyte disturbances (hypokalemia and hypomagnesemia being the most common culprits), new or worsened heart failure, or myocardial ischemia, which account for more than a quarter of all episodes. Electrical storm usually heralds a life-threatening acute pathology placing these patients at immediate high risk of death.28 Immediate communication and consultation with the electrophysiology team is recommended.
Left Ventricular Assist Devices: From Mystery to Mastery
Alicia S. Devine, JD, MD
Dr Devine is an assistant professor, department of emergency medicine, Eastern Virginia Medical School, Norfolk.
Disclosure: The author reports no conflict of interest.
Approximately 5.7 million people in the United States have heart failure, and complications from heart failure represent 668,000 ED visits annually. Heart failure is the primary cause of death in 55,000 people each year. Half of patients die within 5 years of being diagnosed with heart failure.1
Heart failure is initially managed medically; however, some patients become refractory to medical treatment and require heart transplant. Unfortunately, the demand for donor hearts far exceeds the supply, and patients can spend a long time waiting for a donor heart. In addition, not all patients are candidates for transplantation. Left ventricular assist devices are mechanical devices implanted in patients with advanced heart failure in order to provide circulatory support when medications alone are not efficacious. LVADs have been associated with improved survival for heart failure patients.
There are generally two indications for LVAD support: as a bridge to transplant for patients waiting for a donor heart, or as destination therapy for patients who are not candidates for heart transplant. Some patients have had improvement in their cardiac function after LVAD implantation and are able to have the LVAD explanted, leading to a third use for LVADs: bridge to recovery.
LVADs have been in use for over 30 years, and they have evolved during that time to become smaller in size with much fewer complications. Initial models operated with a pulsatile-flow pump that, while adequate in terms of blood flow, contained several parts susceptible to breaking down. Early models were large and cumbersome, especially for smaller patients. New-generation LVADs use a continuous-flow design with either a centrifugal or an axial flow pump with a single moving part, the impeller. The continuous-flow LVADs are quieter, smaller, and significantly more durable than the earlier, pulsatile-flow LVADs. These improvements have expanded the pool of eligible patients to include children and smaller adults.2 Moreover, continuous-flow LVADs provide greater rates of survival and quality of life than the earlier pulsatile-flow models.3
There are fewer adverse events overall with the continuous-flow LVADs compared with the pulsatile-flow LVADs. The number of LVADs implanted each year continues to increase, and more than 95% of these are continuous-flow. As more and more advanced heart failure patients are receiving these devices, emergency physicians should have a basic familiarity with their function and their common complications.4
There are several manufacturers and types of continuous-flow LVADs, but they generally consist of a pump that is surgically implanted into the abdominal or chest cavity of the patient with an inflow cannula positioned in the left ventricle and an outflow cannula inserted into the ascending aorta. The device draws blood from the ventricle and directs it to the aorta. There is a driveline connected to the internal pump that exits the body through the abdominal or chest wall and connects to a system controller. The controller is usually housed in a garment worn by patients that also includes the external battery that powers the LVAD. The controller can also be powered by a base unit that can be plugged into an electrical outlet.5 Patients with continuous-flow LVADs are anticoagulated with warfarin with a target international normalized ratio (INR) of 1.5 to 2.5 and will usually be on an antiplatelet agent as well.2
LVAD patients are typically managed by a team of providers that includes a VAD coordinator; a cardiologist and/or a cardiothoracic surgeon; and a perfusionist, who should be notified as soon as the patient arrives in the ED. Patients understand that it is vital that their LVAD be powered at all times and will usually arrive in the ED with their charged backup batteries. If a power base is available in the hospital, the LVAD can be connected to it to save battery life. If power is interrupted to the LVAD, the pump will stop working. This can be fatal to patients with severe aortic insufficiency who have had their outflow tract surgically occluded and are therefore completely dependent on the LVAD.2
With continuous-flow LVADs, blood is pumped continuously, and a constant, machine-like murmur will be heard on auscultation rather than the typical heart sounds. LVAD patients may not have palpable arterial pulses, and in that case a doppler of the brachial artery and a manual blood pressure cuff are used to listen for the start of Korotkoff sounds as the cuff is released. The pressure at which the first sound is heard is used as an estimate of the mean arterial pressure (MAP). Left ventricular assist device patients should have a MAP between 70 and 90 mm Hg. An accurate pulse oximetry reading may not be attainable, and some centers use cerebral oximetry to obtain oxygenation status.2
The EP should examine all of the connections from the percutaneous lead to the controller and from the controller to the batteries to ensure that they are intact. The exit site for the percutaneous lead should also be examined for evidence of trauma and signs of infection. The exit site is a potential nidus for infection, and even minor trauma from a pull or tug on the lead can damage the tissue and seed an infection. Emergency physicians should ask LVAD patients about any recent trauma to the driveline.6,7
The ED evaluation for an LVAD patient should be focused toward the patient’s chief complaint, recognizing that often patients with LVADs presenting to the ED will have vague complaints of malaise or weakness that may represent a serious pathologic process. Infection, bleeding, thrombosis, and problems with volume status are common reasons for ED visits by LVAD patients.3,5
Infection
In addition to infections in the lung, skin, and urinary tract, patients with LVADs are at risk for infectious complications relating to their device. Implantation of an LVAD involves a sternal incision, the creation of an internal pocket for the LVAD, and a driveline connecting the internal LVAD with an external power source. An infection in any one of these locations can lead to endocarditis, bacteremia, and sepsis.6
Driveline and/or pocket infections are very common, affecting up to 36% of patients with continuous-flow LVADs.8 The exit site for the driveline is an access point for the entry of pathogens, and can be the source of infections in the driveline or in the pump pocket. Pump pocket infections can also occur from exposure to pathogens during surgery or in the immediate postoperative period. In addition, the pump itself can become infected from similar sources, as well as from bacteremia or fungemia from infections in the urine, lung, or central catheters.6
Infections in the driveline will often present with obvious signs such as purulent drainage, erythema, and tenderness at the exit site, but providers should have a high index of suspicion if there is dehiscence at the exit site or even persistent serous drainage from the site, as these can suggest a driveline infection. Pump pocket infections and device-related endocarditis can present with vague symptoms such as weight loss, malaise, and a low-grade fever.
A thorough evaluation should be undertaken in all LVAD patients with a suspected infection to detect a source, and cultures of blood, urine, and the driveline exit site should be obtained. Imaging techniques frequently used when considering device-related infections include ultrasound of the pump pocket and echocardiography to evaluate for endocarditis. Computed tomography is also used to evaluate for device-related infections.6,7,9,10 LVADs are not compatible with MRI.11
The majority of device-related infections are caused by bacteria, although fungal and viral species can be the source as well. Common pathogens implicated include S aureus, S epidermidis, enterococci, Pseudomonas aeruginosa, Klebsiella species and Enterobacter species. Empiric antibiotics with both gram-positive and gram-negative coverage should be initiated for suspected infection related to the device. If the infection has spread to the pump pocket or the device, patients may need surgery for drainage and possible removal of the device.6,7,9,10
Bleeding and Thrombosis
Bleeding complications occur with pulsatile-flow and continuous-flow LVADs at the same rate, and represent one of the most common adverse events seen in LVAD patients. Sites of bleeding include intracranial, nasal cavity, genitourinary tract, and gastrointestinal (GI).11
Interestingly, GI bleeding occurs at a much higher rate in patients with continuous-flow LVADs than in patients with pulsatile-flow devices.2,5,11,12 Patients with continuous-flow LVADs are anticoagulated with warfarin (to a target INR of between 1.5 and 2.5) and an antiplatelet agent to prevent pump thrombosis as well as other thromboembolic events.11 In addition to the effects of warfarin and aspirin, several other factors contribute to the increased incidence of GI bleeding, including an acquired von Willebrand disease and the development of small bowel angiodysplasias from the alteration in vascular hemodynamics from the continuous flow.13,14,15
Emergency physicians should have a high index of suspicion for a bleeding event in patients with an LVAD presenting to the ED. The evaluation of GI bleeding in LVAD patients is the same as in patients without LVADs, and management includes resuscitation with fluids, blood transfusion, and careful correction of coagulopathy. Gastrointestinal bleeding in an LVAD patient necessitates a consultation with a gastroenterologist and admission to the hospital.11
Pump thrombosis, though rare, can result in death and must be considered in cases of MAP < 60 mm Hg and/or an increased power requirement accompanied by a decrease in pulsatility index and flow. Markers of hemolysis such as elevated lactate dehydrogenase or hemoglobinuria also suggest pump thrombosis. Interrogation of the LVAD by the perfusionist is imperative when LVAD patients present to the ED. Echocardiography is the modality of choice in evaluating suspected pump thrombosis. Treatment may require replacement of the pump, or in some cases, anticoagulation or thrombolysis.2,11
Volume Status
Patients with LVADs can present with complaints of weakness and/or dizziness that can be due to dehydration and/or electrolyte deficiencies. Often, these patients will continue to restrict their salt and fluid intake after device implantation. They are frequently on diuretics, which can contribute to these problems. Checking and repleting electrolytes as well as administering a gentle bolus of IV fluids in patients with a MAP < 60 mm Hg will often correct the hypovolemia and electrolyte abnormalities. Evaluation for sepsis, pump thrombosis, and cannula malposition as causes of hypotension should be undertaken in the appropriate circumstances.2,11 Severe hypovolemia can interfere with effective LVAD function if it leads to the collapse of the left ventricle over the inflow cannula. Bedside ultrasound can be a useful adjunct in the evaluation of cannula position and volume status.2 An emergent consult with a cardiovascular surgeon is indicated in the event of pump thrombosis or cannula malposition.
Conclusion
The number of LVADs implanted each year continues to grow, and EPs need to have a basic familiarity with these devices and how to manage typical complaints seen in the ED. Patients and their caregivers have been given extensive education and training on the care and management of their LVAD components and can be a valuable source of information. They should bring the devices with them to the ED, along with the names and phone numbers of all of the members of their VAD treatment team, who should be called shortly after the patient’s arrival, as well as backup charged batteries to power their LVAD.
A priority is ensuring that all of the LVAD connections are intact and that there is adequate power to the device. A perfusionist will need to interrogate the controller if there is any concern about its function, including alarms sounding or lights flashing. The manufacturer’s website can be accessed if necessary for further information.
Cardiac Hardware Management of the Patient With an Implanted Pacemaker
- Chardack WM, Gage AA, Greatbatch W. A transistorized, self-contained, implantable pacemaker for the long-term correction of complete heart block. Surgery. 1960;48:643-654.
- Beck H, Boden WE, Patibandla S, Kireyev D, Gupta V, Campagna F, et al. 50th anniversary of the first successful permanent pacemaker implantation in the United States: historical review and future directions. Am J Cardiol. 2010;106(6):810-818.
- McMullan J, Valento M, Attari M, Venkat A. Care of the pacemaker/implantable cardioverter defibrillator patient in the ED. Am J Emerg Med. 2007;25(7):812-822.
- Kaszala K, Huizar JF, Ellenbogen KA. Contemporary pacemakers: what the primary care physician needs to know. Mayo Clin Proc. 2008;83(10):1170-1186.
- Park DS, Fishman GI. The cardiac conduction system. Circulation. 2011;123(8):904-915.
- Gregoratos G. Indications and Recommendations for Pacemaker Therapy. Am Fam Phys. 2005;71(8):1563-1570.
- Vardas PE, Simantirakis EN, Kanoupakis EM. New developments in cardiac pacemakers. Circulation. 2013;127(23):2343-2350.
- Cheng A, Tereshchenko LG. Evolutionary innovations in cardiac pacing. J Electrocardiol. 2011;44(6):611-615.
- Stone KR, McPherson CA. Assessment and management of patients with pacemakers and implantable cardioverter defibrillators. Crit Care Med. 2004;32(4 Suppl):S155-S165.
- Bernstein AD, Daubert JC, Fletcher RD, Hayes DL, Luderitz B, Reynolds DW, et al. The revised NASPE/BPEG generic code for antibradycardia, adaptive-rate, and multisite pacing. North American Society of Pacing and Electrophysiology/British Pacing and Electrophysiology Group. Pacing and clinical electrophysiology : Pacing Clin Electrophysiol. 2002;25(2):260-264.
- Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA, 3rd, Freedman RA, Gettes LS, et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Am J Cardiol. 2008;51(21):e1-e62.
- Chang AM, Shofer FS, Tabas JA, Magid DJ, McCusker CM, Hollander JE. Lack of association between left bundle-branch block and acute myocardial infarction in symptomatic ED patients. Am J Emerg Med. 2009;27(8):916-921.
- Sgarbossa EB, Pinski SL, Barbagelata A, Underwood DA, Gates KB, Topol EJ, et al. Electrocardiographic diagnosis of evolving acute myocardial infarction in the presence of left bundle-branch block. GUSTO-1 (Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries) Investigators. N Engl J Med. 1996;334(8):481-487.
- Venkatachalam KL. Common pitfalls in interpreting pacemaker electrocardiograms in the emergency department. J Electrocardiol. 2011;44(6):616-621.
- Sgarbossa EB, Pinski SL, Topol EJ, Califf RM, Barbagelata A, Goodman SG, et al. Acute myocardial infarction and complete bundle branch block at hospital admission: clinical characteristics and outcome in the thrombolytic era. GUSTO-I Investigators. Global Utilization of Streptokinase and t-PA [tissue-type plasminogen activator] for Occluded Coronary Arteries. J Am Coll Cardiol. 1998;31(1):105-110.
- Tabas JA, Rodriguez RM, Seligman HK, Goldschlager NF. Electrocardiographic criteria for detecting acute myocardial infarction in patients with left bundle branch block: a meta-analysis. Ann Emerg Med. 2008;52(4):329-336 e1.
- Smith SW, Dodd KW, Henry TD, Dvorak DM, Pearce LA. Diagnosis of ST-elevation myocardial infarction in the presence of left bundle branch block with the ST-elevation to S-wave ratio in a modified Sgarbossa rule. Ann Emerg Med. 2012;60(6):766-776.
- Nof E, Epstein LM. Complications of cardiac implants: handling device infections. Eur Heart J. 2013;34(3):229-236.
- Tarakji KG, Wilkoff BL. Management of cardiac implantable electronic device infections: the challenges of understanding the scope of the problem and its associated mortality. Expert Rev ardiovasc Ther. 2013;11(5):607-616.
- Balachander J, Rajagopal S. Pacemaker trouble shooting and follow up. Indian Heart J. 2011;63(4):356-370.
- Klug D, Wallet F, Lacroix D, Marquie C, Kouakam C, Kacet S, et al. Local symptoms at the site of pacemaker implantation indicate latent systemic infection. Heart. 2004;90(8):882-886.
- Kwak YL, Shim JK. Assessment of endocarditis and intracardiac masses by TEE. Int Anesthesiol Clin. 2008;46(2):105-120.
- Ryan EW, Bolger AF. Transesophageal echocardiography (TEE) in the evaluation of infective endocarditis. Cardiol Clin. 2000;18(4):773-787.
- Baddour LM. Cardiac device infection--or not. Circulation. 2010;121(15):1686-1687.
- Ghani SN, Kirkpatrick JN, Spencer KT, Smith GL, Burke MC, Kim SS, et al. Rapid assessment of left ventricular systolic function in a pacemaker clinic using a hand-carried ultrasound device. J Interv Card Electrophysiol. 2006;16(1):39-43.
- Scheibly K. Pacemaker timing and electrocardiogram interpretation. AACN Adv Crit Care. 2010;21(4):386-396.
- Zimetbaum PJ, Josephson ME. Use of the electrocardiogram in acute myocardial infarction. N Engl J Med. 2003;348(10):933-940.
- Misiri J, Kusumoto F, Goldschlager N. Electromagnetic interference and implanted cardiac devices: the nonmedical environment (part I). Clin Cardiol. 2012;35(5):276-280.
- Trohman RG, Kim MH, Pinski SL. Cardiac pacing: the state of the art. Lancet. 2004;364(9446):1701-1719.
- Kramer DB, Mitchell SL, Brock DW. Deactivation of pacemakers and implantable cardioverter-defibrillators. Prog Cardiovasc Dis. 2012;55(3):290-299.
- Misiri J, Kusumoto F, Goldschlager N. Electromagnetic interference and implanted cardiac devices: the medical environment (part II). Clin Cardiol. 2012;35(6):321-328.
- Zikria JF, Machnicki S, Rhim E, Bhatti T, Graham RE. MRI of patients with cardiac pacemakers: a review of the medical literature. Am J Roentgenol. 2011;196(2):390-401.
- Cai Q, Mehta N, Sgarbossa EB, Pinski SL, Wagner GS, Califf RM, et al. The left bundle-branch block puzzle in the 2013 ST-elevation myocardial infarction guideline: from falsely declaring emergency to denying reperfusion in a high-risk population. Are the Sgarbossa Criteria ready for prime time? Am Heart J. 2013;166(3):409-413.
Managing Implantable Cardioverter Defibrillator Shock Complications
- Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2-e220.
- Slaughter MS, Pagani FD, Rogers JG, et al. Clinical management of continuous-flow left ventricular assist devices in advanced heart failure. J Heart Lung Transplant. 2010;29(4 Suppl):S1-S39.
- Slaughter MS, Rogers JG, Milano GC, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009; 361(23):2241-2251.
- Kirklin JK, Naftel DC, Kormos RL, et al. Fifth INTERMACS annual report: risk factor analysis from more than 6,000 mechanical circulatory support patients. J Heart Lung Transplant. 2013;32(2):141-156.
- Miller LW, Pagani FD, Russell SD, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357(9):885-896.
- Califano S, Pagani FD, Malani PN. Left ventricular assist device-associated infections. Infect Dis Clin N Am. 2012;26(1):77-87.
- Peredo D, Conte JV. Left ventricular assist device driveline infections. Cardiol Clin. 2011;29(4):515-527.
- Schaffer JM, Allen JG, Weiss ES, et al. Infectious complications after pulsatile-flow and continuous-flow left ventricular assist device implantation.
J Heart Lung Transplant. 2011;30(2):164-174. - Gordon RJ, Quagliarello B, Lowy FD. Ventricular assist device-related infections. Lancet Infect Dis. 2006;6(7):426-437.
- Maniar S, Kondareddy S, Topkara VK. Left ventricular assist-device-related infections: past, present and future. Expert Rev Med Devices. 2011;8(5):627-634.
- Klein T, Jacob M. Management of implantable assisted circulation devices. Cardiol Clin. 2012;30:673-682
- John RJ, Kamdar F, Liao K, et al. Improved survival and decreasing incidence of adverse events with the HeartMate II left ventricular assist device as bridge-to-transplant therapy. Ann Thorac Surg. 2008;86:1227-1235.
- Klovaite J, Gustafsson F, Mortensen SA, Sander K, Nielson LB. Severely impaired von Willebrand factor-dependent platelet aggregation in patients with a continuous-flow left ventricular assist device (HeartMate II). J Am Coll Cardiol. 2009;53(23):2162-2167.
- Stern DR, Kazam J, Edwards P, et al. Increased incidence of gastrointestinal bleeding following implantation of the HeartMate II LVAD. J Card Surg. 2010:25(3):352-356.
- Kushnir VM, Sharma S, Ewald GA, et al. Evaluation of GI bleeding after implantation of left ventricular assist device. Gastrointest Endoscopy. 2012;75(5):973-979.
Left Ventricular Assist Devices: From Mystery to Mastery
- Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2-e220.
- Slaughter MS, Pagani FD, Rogers JG, et al. Clinical management of continuous-flow left ventricular assist devices in advanced heart failure. J Heart Lung Transplant. 2010;29(4 Suppl):S1-S39.
- Slaughter MS, Rogers JG, Milano GC, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009; 361(23):2241-2251.
- Kirklin JK, Naftel DC, Kormos RL, et al. Fifth INTERMACS annual report: risk factor analysis from more than 6,000 mechanical circulatory support patients. J Heart Lung Transplant. 2013;32(2):141-156.
- Miller LW, Pagani FD, Russell SD, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357(9):885-896.
- Califano S, Pagani FD, Malani PN. Left ventricular assist device-associated infections. Infect Dis Clin N Am. 2012;26(1):77-87.
- Peredo D, Conte JV. Left ventricular assist device driveline infections. Cardiol Clin. 2011;29(4):515-527.
- Schaffer JM, Allen JG, Weiss ES, et al. Infectious complications after pulsatile-flow and continuous-flow left ventricular assist device implantation. J Heart Lung Transplant. 2011;30(2):164-174.
- Gordon RJ, Quagliarello B, Lowy FD. Ventricular assist device-related infections. Lancet Infect Dis. 2006;6(7):426-437.
- Maniar S, Kondareddy S, Topkara VK. Left ventricular assist-device-related infections: past, present and future. Expert Rev Med Devices. 2011;8(5):627-634.
- Klein T, Jacob M. Management of implantable assisted circulation devices. Cardiol Clin. 2012;30:673-682
- John RJ, Kamdar F, Liao K, et al. Improved survival and decreasing incidence of adverse events with the HeartMate II left ventricular assist device as bridge-to-transplant therapy. Ann Thorac Surg. 2008;86:1227-1235.
- Klovaite J, Gustafsson F, Mortensen SA, Sander K, Nielson LB. Severely impaired von Willebrand factor-dependent platelet aggregation in patients with a continuous-flow left ventricular assist device (HeartMate II). J Am Coll Cardiol. 2009;53(23):2162-2167.
- Stern DR, Kazam J, Edwards P, et al. Increased incidence of gastrointestinal bleeding following implantation of the HeartMate II LVAD. J Card Surg. 2010:25(3):352-356.
- Kushnir VM, Sharma S, Ewald GA, et al. Evaluation of GI bleeding after implantation of left ventricular assist device. Gastrointest Endoscopy. 2012;75(5):973-979.
Cardiac Hardware Management of the Patient With an Implanted Pacemaker
- Chardack WM, Gage AA, Greatbatch W. A transistorized, self-contained, implantable pacemaker for the long-term correction of complete heart block. Surgery. 1960;48:643-654.
- Beck H, Boden WE, Patibandla S, Kireyev D, Gupta V, Campagna F, et al. 50th anniversary of the first successful permanent pacemaker implantation in the United States: historical review and future directions. Am J Cardiol. 2010;106(6):810-818.
- McMullan J, Valento M, Attari M, Venkat A. Care of the pacemaker/implantable cardioverter defibrillator patient in the ED. Am J Emerg Med. 2007;25(7):812-822.
- Kaszala K, Huizar JF, Ellenbogen KA. Contemporary pacemakers: what the primary care physician needs to know. Mayo Clin Proc. 2008;83(10):1170-1186.
- Park DS, Fishman GI. The cardiac conduction system. Circulation. 2011;123(8):904-915.
- Gregoratos G. Indications and Recommendations for Pacemaker Therapy. Am Fam Phys. 2005;71(8):1563-1570.
- Vardas PE, Simantirakis EN, Kanoupakis EM. New developments in cardiac pacemakers. Circulation. 2013;127(23):2343-2350.
- Cheng A, Tereshchenko LG. Evolutionary innovations in cardiac pacing. J Electrocardiol. 2011;44(6):611-615.
- Stone KR, McPherson CA. Assessment and management of patients with pacemakers and implantable cardioverter defibrillators. Crit Care Med. 2004;32(4 Suppl):S155-S165.
- Bernstein AD, Daubert JC, Fletcher RD, Hayes DL, Luderitz B, Reynolds DW, et al. The revised NASPE/BPEG generic code for antibradycardia, adaptive-rate, and multisite pacing. North American Society of Pacing and Electrophysiology/British Pacing and Electrophysiology Group. Pacing and clinical electrophysiology : Pacing Clin Electrophysiol. 2002;25(2):260-264.
- Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA, 3rd, Freedman RA, Gettes LS, et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Am J Cardiol. 2008;51(21):e1-e62.
- Chang AM, Shofer FS, Tabas JA, Magid DJ, McCusker CM, Hollander JE. Lack of association between left bundle-branch block and acute myocardial infarction in symptomatic ED patients. Am J Emerg Med. 2009;27(8):916-921.
- Sgarbossa EB, Pinski SL, Barbagelata A, Underwood DA, Gates KB, Topol EJ, et al. Electrocardiographic diagnosis of evolving acute myocardial infarction in the presence of left bundle-branch block. GUSTO-1 (Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries) Investigators. N Engl J Med. 1996;334(8):481-487.
- Venkatachalam KL. Common pitfalls in interpreting pacemaker electrocardiograms in the emergency department. J Electrocardiol. 2011;44(6):616-621.
- Sgarbossa EB, Pinski SL, Topol EJ, Califf RM, Barbagelata A, Goodman SG, et al. Acute myocardial infarction and complete bundle branch block at hospital admission: clinical characteristics and outcome in the thrombolytic era. GUSTO-I Investigators. Global Utilization of Streptokinase and t-PA [tissue-type plasminogen activator] for Occluded Coronary Arteries. J Am Coll Cardiol. 1998;31(1):105-110.
- Tabas JA, Rodriguez RM, Seligman HK, Goldschlager NF. Electrocardiographic criteria for detecting acute myocardial infarction in patients with left bundle branch block: a meta-analysis. Ann Emerg Med. 2008;52(4):329-336 e1.
- Smith SW, Dodd KW, Henry TD, Dvorak DM, Pearce LA. Diagnosis of ST-elevation myocardial infarction in the presence of left bundle branch block with the ST-elevation to S-wave ratio in a modified Sgarbossa rule. Ann Emerg Med. 2012;60(6):766-776.
- Nof E, Epstein LM. Complications of cardiac implants: handling device infections. Eur Heart J. 2013;34(3):229-236.
- Tarakji KG, Wilkoff BL. Management of cardiac implantable electronic device infections: the challenges of understanding the scope of the problem and its associated mortality. Expert Rev ardiovasc Ther. 2013;11(5):607-616.
- Balachander J, Rajagopal S. Pacemaker trouble shooting and follow up. Indian Heart J. 2011;63(4):356-370.
- Klug D, Wallet F, Lacroix D, Marquie C, Kouakam C, Kacet S, et al. Local symptoms at the site of pacemaker implantation indicate latent systemic infection. Heart. 2004;90(8):882-886.
- Kwak YL, Shim JK. Assessment of endocarditis and intracardiac masses by TEE. Int Anesthesiol Clin. 2008;46(2):105-120.
- Ryan EW, Bolger AF. Transesophageal echocardiography (TEE) in the evaluation of infective endocarditis. Cardiol Clin. 2000;18(4):773-787.
- Baddour LM. Cardiac device infection--or not. Circulation. 2010;121(15):1686-1687.
- Ghani SN, Kirkpatrick JN, Spencer KT, Smith GL, Burke MC, Kim SS, et al. Rapid assessment of left ventricular systolic function in a pacemaker clinic using a hand-carried ultrasound device. J Interv Card Electrophysiol. 2006;16(1):39-43.
- Scheibly K. Pacemaker timing and electrocardiogram interpretation. AACN Adv Crit Care. 2010;21(4):386-396.
- Zimetbaum PJ, Josephson ME. Use of the electrocardiogram in acute myocardial infarction. N Engl J Med. 2003;348(10):933-940.
- Misiri J, Kusumoto F, Goldschlager N. Electromagnetic interference and implanted cardiac devices: the nonmedical environment (part I). Clin Cardiol. 2012;35(5):276-280.
- Trohman RG, Kim MH, Pinski SL. Cardiac pacing: the state of the art. Lancet. 2004;364(9446):1701-1719.
- Kramer DB, Mitchell SL, Brock DW. Deactivation of pacemakers and implantable cardioverter-defibrillators. Prog Cardiovasc Dis. 2012;55(3):290-299.
- Misiri J, Kusumoto F, Goldschlager N. Electromagnetic interference and implanted cardiac devices: the medical environment (part II). Clin Cardiol. 2012;35(6):321-328.
- Zikria JF, Machnicki S, Rhim E, Bhatti T, Graham RE. MRI of patients with cardiac pacemakers: a review of the medical literature. Am J Roentgenol. 2011;196(2):390-401.
- Cai Q, Mehta N, Sgarbossa EB, Pinski SL, Wagner GS, Califf RM, et al. The left bundle-branch block puzzle in the 2013 ST-elevation myocardial infarction guideline: from falsely declaring emergency to denying reperfusion in a high-risk population. Are the Sgarbossa Criteria ready for prime time? Am Heart J. 2013;166(3):409-413.
Managing Implantable Cardioverter Defibrillator Shock Complications
- Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2-e220.
- Slaughter MS, Pagani FD, Rogers JG, et al. Clinical management of continuous-flow left ventricular assist devices in advanced heart failure. J Heart Lung Transplant. 2010;29(4 Suppl):S1-S39.
- Slaughter MS, Rogers JG, Milano GC, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009; 361(23):2241-2251.
- Kirklin JK, Naftel DC, Kormos RL, et al. Fifth INTERMACS annual report: risk factor analysis from more than 6,000 mechanical circulatory support patients. J Heart Lung Transplant. 2013;32(2):141-156.
- Miller LW, Pagani FD, Russell SD, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357(9):885-896.
- Califano S, Pagani FD, Malani PN. Left ventricular assist device-associated infections. Infect Dis Clin N Am. 2012;26(1):77-87.
- Peredo D, Conte JV. Left ventricular assist device driveline infections. Cardiol Clin. 2011;29(4):515-527.
- Schaffer JM, Allen JG, Weiss ES, et al. Infectious complications after pulsatile-flow and continuous-flow left ventricular assist device implantation.
J Heart Lung Transplant. 2011;30(2):164-174. - Gordon RJ, Quagliarello B, Lowy FD. Ventricular assist device-related infections. Lancet Infect Dis. 2006;6(7):426-437.
- Maniar S, Kondareddy S, Topkara VK. Left ventricular assist-device-related infections: past, present and future. Expert Rev Med Devices. 2011;8(5):627-634.
- Klein T, Jacob M. Management of implantable assisted circulation devices. Cardiol Clin. 2012;30:673-682
- John RJ, Kamdar F, Liao K, et al. Improved survival and decreasing incidence of adverse events with the HeartMate II left ventricular assist device as bridge-to-transplant therapy. Ann Thorac Surg. 2008;86:1227-1235.
- Klovaite J, Gustafsson F, Mortensen SA, Sander K, Nielson LB. Severely impaired von Willebrand factor-dependent platelet aggregation in patients with a continuous-flow left ventricular assist device (HeartMate II). J Am Coll Cardiol. 2009;53(23):2162-2167.
- Stern DR, Kazam J, Edwards P, et al. Increased incidence of gastrointestinal bleeding following implantation of the HeartMate II LVAD. J Card Surg. 2010:25(3):352-356.
- Kushnir VM, Sharma S, Ewald GA, et al. Evaluation of GI bleeding after implantation of left ventricular assist device. Gastrointest Endoscopy. 2012;75(5):973-979.
Left Ventricular Assist Devices: From Mystery to Mastery
- Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2-e220.
- Slaughter MS, Pagani FD, Rogers JG, et al. Clinical management of continuous-flow left ventricular assist devices in advanced heart failure. J Heart Lung Transplant. 2010;29(4 Suppl):S1-S39.
- Slaughter MS, Rogers JG, Milano GC, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009; 361(23):2241-2251.
- Kirklin JK, Naftel DC, Kormos RL, et al. Fifth INTERMACS annual report: risk factor analysis from more than 6,000 mechanical circulatory support patients. J Heart Lung Transplant. 2013;32(2):141-156.
- Miller LW, Pagani FD, Russell SD, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357(9):885-896.
- Califano S, Pagani FD, Malani PN. Left ventricular assist device-associated infections. Infect Dis Clin N Am. 2012;26(1):77-87.
- Peredo D, Conte JV. Left ventricular assist device driveline infections. Cardiol Clin. 2011;29(4):515-527.
- Schaffer JM, Allen JG, Weiss ES, et al. Infectious complications after pulsatile-flow and continuous-flow left ventricular assist device implantation. J Heart Lung Transplant. 2011;30(2):164-174.
- Gordon RJ, Quagliarello B, Lowy FD. Ventricular assist device-related infections. Lancet Infect Dis. 2006;6(7):426-437.
- Maniar S, Kondareddy S, Topkara VK. Left ventricular assist-device-related infections: past, present and future. Expert Rev Med Devices. 2011;8(5):627-634.
- Klein T, Jacob M. Management of implantable assisted circulation devices. Cardiol Clin. 2012;30:673-682
- John RJ, Kamdar F, Liao K, et al. Improved survival and decreasing incidence of adverse events with the HeartMate II left ventricular assist device as bridge-to-transplant therapy. Ann Thorac Surg. 2008;86:1227-1235.
- Klovaite J, Gustafsson F, Mortensen SA, Sander K, Nielson LB. Severely impaired von Willebrand factor-dependent platelet aggregation in patients with a continuous-flow left ventricular assist device (HeartMate II). J Am Coll Cardiol. 2009;53(23):2162-2167.
- Stern DR, Kazam J, Edwards P, et al. Increased incidence of gastrointestinal bleeding following implantation of the HeartMate II LVAD. J Card Surg. 2010:25(3):352-356.
- Kushnir VM, Sharma S, Ewald GA, et al. Evaluation of GI bleeding after implantation of left ventricular assist device. Gastrointest Endoscopy. 2012;75(5):973-979.
von Willebrand Disease: Approach to Diagnosis and Management
von Willebrand disease (VWD) is an inherited bleeding disorder caused by deficient or defective plasma von Willebrand factor (VWF). VWF is an adhesive multimeric plasma glycoprotein that performs 2 major functions in hemostasis: it mediates platelet adhesion to injured subendothelium via glycoprotein 1bα (GPIbα), and it binds and stabilizes factor VIII (FVIII) in circulation, protecting it from proteolytic degradation by enzymes. The current VWD classification recognizes 3 types. In order to understand the role of the numerous laboratory investigations as well as the classification of VWD, it is important to review the structure and function of the VWF subunit. Bleeding symptoms reflect the defect in primary hemostasis: mucocutaneous bleeding and excessive bleeding after surgery or trauma. Treatment focuses on increasing VWF levels with desmopressin (1-deamino-8-D-arginine vasopressin, DDAVP) or clotting factor concentrates containing both VWF and FVIII (VWF/FVIII concentrate). Nonspecific treatment options include antifibrinolytic agents (tranexamic acid) and hormone therapy (oral contraceptive pill).
To read the full article in PDF:
von Willebrand disease (VWD) is an inherited bleeding disorder caused by deficient or defective plasma von Willebrand factor (VWF). VWF is an adhesive multimeric plasma glycoprotein that performs 2 major functions in hemostasis: it mediates platelet adhesion to injured subendothelium via glycoprotein 1bα (GPIbα), and it binds and stabilizes factor VIII (FVIII) in circulation, protecting it from proteolytic degradation by enzymes. The current VWD classification recognizes 3 types. In order to understand the role of the numerous laboratory investigations as well as the classification of VWD, it is important to review the structure and function of the VWF subunit. Bleeding symptoms reflect the defect in primary hemostasis: mucocutaneous bleeding and excessive bleeding after surgery or trauma. Treatment focuses on increasing VWF levels with desmopressin (1-deamino-8-D-arginine vasopressin, DDAVP) or clotting factor concentrates containing both VWF and FVIII (VWF/FVIII concentrate). Nonspecific treatment options include antifibrinolytic agents (tranexamic acid) and hormone therapy (oral contraceptive pill).
To read the full article in PDF:
von Willebrand disease (VWD) is an inherited bleeding disorder caused by deficient or defective plasma von Willebrand factor (VWF). VWF is an adhesive multimeric plasma glycoprotein that performs 2 major functions in hemostasis: it mediates platelet adhesion to injured subendothelium via glycoprotein 1bα (GPIbα), and it binds and stabilizes factor VIII (FVIII) in circulation, protecting it from proteolytic degradation by enzymes. The current VWD classification recognizes 3 types. In order to understand the role of the numerous laboratory investigations as well as the classification of VWD, it is important to review the structure and function of the VWF subunit. Bleeding symptoms reflect the defect in primary hemostasis: mucocutaneous bleeding and excessive bleeding after surgery or trauma. Treatment focuses on increasing VWF levels with desmopressin (1-deamino-8-D-arginine vasopressin, DDAVP) or clotting factor concentrates containing both VWF and FVIII (VWF/FVIII concentrate). Nonspecific treatment options include antifibrinolytic agents (tranexamic acid) and hormone therapy (oral contraceptive pill).
To read the full article in PDF:
Tiny Bubbles: Or, the Dangers of Cleaning Fruit
A previously healthy 32-year-old man presented to the emergency department (ED) after unintentionally ingesting a mouthful of concentrated (35%) hydrogen peroxide (H2O2) from an unmarked bottle he kept in his refrigerator. Upon realizing his error, he immediately drank a liter of water, which promptly induced vomiting. In the ED, the patient complained of mild throat and chest discomfort as well as “abdominal fullness.”
His initial vital signs included a blood pressure of 140/92 mm Hg; heart rate, 93 beats/min; respiratory rate, 18 breaths/min; and temperature, 96.4°F. His O2 saturation was 98% on room air. Physical examination revealed tenderness in the epigastric region with no peritoneal findings. Oropharynx and chest examination were normal, and standard laboratory investigations were all within normal limits.
WHAT ARE THE POTENTIAL EXPOSURES TO HYDROGEN PEROXIDE?
Hydrogen peroxide is a colorless and odorless liquid. Solutions with concentrations ranging from 3% to 5% have many household applications, including use as a wound disinfectant and dentifrice; dilute solutions are also utilized for similar purposes in the hospital setting. Industrial-strength H2O2 (concentrations of 10% to 35%) is employed to bleach textiles and paper, and higher concentrations (70% to 90%) are used as an oxygen source for rocket engines.
Consumer application of concentrated H2O2 solutions has become increasingly common. Some, like this patient, clean the surfaces of fruits and vegetables with H2O2 to decrease transmission of bacteria during cutting.1 More concerning, however, is the purported medicinal benefits of ingesting “food-grade” (35%) H2O2 mixed with water—touted on many Internet sites as a treatment for illnesses such as emphysema, cancer, anemia, and HIV.2 Sometimes referred to as “hyperoxygenation therapy,” this so-called treatment has not been approved by the FDA for any such purpose.3 When diluted sufficiently, this concoction is not harmful but is unlikely to provide any health benefits.
Continue reading for the toxic effects of concentrated hydrogen peroxide...
WHAT ARE THE TOXIC EFFECTS OF CONCENTRATED HYDROGEN PEROXIDE?
Injury from concentrated H2O2 consumption is primarily from either direct caustic injury or the embolic obstruction of blood flow. Following ingestion, the enzyme catalase metabolizes the breakdown of H2O2 in accordance with the following equation: 2H2O2(aq) → 2H2O(l) + O2(g) + heat. A single milliliter of 35% H2O2 results in the liberation of 100 mL of O2. (The more common 3% household solution generates 10 mL of oxygen per 1 mL of H2O2.) The creation of a large intragastric pressure gradient from the liberation of gas, coupled with the caustic and exothermic injury of the bowel mucosa, may contribute to the movement of oxygen through epithelial interstices into the circulation.
In addition, and perhaps more importantly, absorption of intact H2O2 with subsequent metabolism by catalase in the blood liberates oxygen directly within the vasculature. Oxygen bubbles may coalesce in blood circulation and occlude vascular flow. In canine studies, elevated oxygen tension in the portal venous system led to cessation of mesenteric flow in arteries and veins, though the mechanism of action is unclear.4 Furthermore, coalescence of bubbles can lead to disruption of bowel-cell architecture, fibrin plugging of capillaries, venous thrombosis, and infarction of tissues.4
Cases of cardiac and cerebral gas embolism have been reported and present similarly to patients with diving-related decompression injuries (eg, stroke-like syndromes).5,6 The proposed mechanism for these latter effects involves the metabolism of H2O2 in the systemic circulation with production of oxygen bubbles. In the presence of an atrial septal defect, bubbles may move from the right atrium to the arterial circulation.7
Toxicity and death from H2O2 exposure associated with the historical treatment of inspissated meconium,4 as well as the irrigation of wounds,8 has been reported in the medical literature. Ingestion of a 3% solution is generally benign, resulting at worst in gastrointestinal symptoms or throat irritation.9 Rarely does significant toxicity occur at this low concentration,5 with the vast majority of such cases involving concentrated solutions of 35%.
Continue reading for the case continuation...
CASE CONTINUATION
Based on this patient’s continued symptoms, an abdominal radiograph was obtained to assess the presence of portal venous air. Although radiographic findings were normal, continued abdominal examination findings warranted a subsequent abdominal CT scan, which revealed the presence of extensive air throughout the portal venous system (see the figure).
DO ALL PATIENTS PRESENTING WITH H2O2 INGESTION REQUIRE IMAGING TO ASSESS FOR THE PRESENCE OF PORTAL VENOUS AIR?
Reportedly, ingestion of as little as a “sip” or “mouthful” of 35% H2O2 has resulted in venous and arterial gas embolism,6 occasionally with severe consequences, but no current consensus guidelines exist regarding imaging requirements. Some toxicologists and hyperbaric physicians believe that the presence of portal venous air does not adversely impact a patient’s prognosis or necessitate treatment, and therefore a work-up is unnecessary.
Others, however, suggest that the presence of portal venous air indicates oversaturation of oxygen in the blood, placing the patient at increased risk for cardiac and cerebral air embolism. Neither one of these theories is well supported in the literature. Although practice patterns vary by institution, it is reasonable that all patients presenting with abdominal complaints after ingestion of H2O2 undergo CT imaging to assess for portal venous air.
Continue reading to find out what to do if portal venous air is detected...
IF PORTAL VENOUS AIR IS DETECTED, DO PATIENTS REQUIRE HYPERBARIC OXYGEN THERAPY?
The management of patients with portal venous gas following H2O2 ingestion is controversial and has not been established. Hyperbaric oxygen therapy involves increasing the ambient pressure by several atmospheres inside a specially designed chamber—the same therapy used for diving-related bubble injury.
Hyperbaric therapy increases the amount of oxygen that can be dissolved in the blood, thereby decreasing bubble formation and allowing transport of dissolved oxygen to the lungs, where it can be exhaled. Some patients with portal venous air experience significant pain and portal venous hypertension, which may respond rapidly to this therapy.10
Based on available literature, hyperbaric therapy is reasonable for patients with significant abdominal pain and portal venous air following H2O2 ingestion; less controversial is the role of hyperbaric therapy in those with cerebral air embolism. Multiple case reports of patients with significant neurologic findings demonstrate resolution of symptoms following hyperbaric therapy.6
Continue reading for the case conclusion...
CASE CONCLUSION
Hyperbaric oxygen therapy was recommended for the patient in this case, but transfer to a hyperbaric facility was not possible. He was instead admitted to the hospital for continuous monitoring. Over the next 12 hours, his symptoms gradually resolved, and a repeat CT the following day showed complete resolution of the portal venous gas. The patient was subsequently discharged without any sequelae.
REFERENCES
1. Ukuku DO, Bari ML, Kawamoto S, Isshiki K. Use of hydrogen peroxide in combination with nisin, sodium lactate and citric acid for reducing transfer of bacterial pathogens from whole melon surfaces to fresh-cut pieces. Int J Food Microbiol. 2005;104(2):225-233.
2. 35% H2O2 hydrogen peroxide food grade certified benefits. The One Minute Miracle Web site. www.theoneminutemiracleinc.com/pages/h2o2-benefits/. Accessed January 20, 2013.
3. FDA. FDA warns consumers against drinking high-strength hydrogen peroxide for medicinal use: ingestion can lead to serious health risk and death [news release]. July 27, 2006. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2006/ucm108701.htm. Accessed January 20, 2013.
4. Shaw A, Cooperman A, Fusco J. Gas embolism produced by hydrogen peroxide. N Engl J Med. 1967;277(5):238-241.
5. Cina SJ, Downs JC, Conradi SE. Hydrogen peroxide: a source of lethal oxygen embolism. Case report and review of the literature. Am J Forensic Med Pathol. 1994;15(1):44-50.
6. Rider SP, Jackson SB, Rusyniak DE. Cerebral air gas embolism from concentrated hydrogen peroxide ingestion. Clin Toxicol (Phila). 2008;46(9):815-818.
7. French LK, Horowitz BZ, McKeown NJ. Hydrogen peroxide ingestion associated with portal venous gas and treatment with hyperbaric oxygen: a case series and review of the literature. Clin Toxicol (Phila). 2010;48(6):533-538.
8. Bassan MM, Dudai M, Shalev O. Near-fatal systemic oxygen embolism due to wound irrigation with hydrogen peroxide. Postgrad Med J. 1982;58(681):448-450.
9. Henry MC, Wheeler J, Mofenson HC, et al. Hydrogen peroxide 3% exposures. J Toxicol Clin Toxicol. 1996;34(3):323-327.
10. Papafragkou S, Gasparyan A, Batista R, Scott P. Treatment of portal venous gas embolism with hyperbaric oxygen after accidental ingestion of hydrogen peroxide: a case report and review of the literature. J Emerg Med. 2012;43(1):e21-e23
A previously healthy 32-year-old man presented to the emergency department (ED) after unintentionally ingesting a mouthful of concentrated (35%) hydrogen peroxide (H2O2) from an unmarked bottle he kept in his refrigerator. Upon realizing his error, he immediately drank a liter of water, which promptly induced vomiting. In the ED, the patient complained of mild throat and chest discomfort as well as “abdominal fullness.”
His initial vital signs included a blood pressure of 140/92 mm Hg; heart rate, 93 beats/min; respiratory rate, 18 breaths/min; and temperature, 96.4°F. His O2 saturation was 98% on room air. Physical examination revealed tenderness in the epigastric region with no peritoneal findings. Oropharynx and chest examination were normal, and standard laboratory investigations were all within normal limits.
WHAT ARE THE POTENTIAL EXPOSURES TO HYDROGEN PEROXIDE?
Hydrogen peroxide is a colorless and odorless liquid. Solutions with concentrations ranging from 3% to 5% have many household applications, including use as a wound disinfectant and dentifrice; dilute solutions are also utilized for similar purposes in the hospital setting. Industrial-strength H2O2 (concentrations of 10% to 35%) is employed to bleach textiles and paper, and higher concentrations (70% to 90%) are used as an oxygen source for rocket engines.
Consumer application of concentrated H2O2 solutions has become increasingly common. Some, like this patient, clean the surfaces of fruits and vegetables with H2O2 to decrease transmission of bacteria during cutting.1 More concerning, however, is the purported medicinal benefits of ingesting “food-grade” (35%) H2O2 mixed with water—touted on many Internet sites as a treatment for illnesses such as emphysema, cancer, anemia, and HIV.2 Sometimes referred to as “hyperoxygenation therapy,” this so-called treatment has not been approved by the FDA for any such purpose.3 When diluted sufficiently, this concoction is not harmful but is unlikely to provide any health benefits.
Continue reading for the toxic effects of concentrated hydrogen peroxide...
WHAT ARE THE TOXIC EFFECTS OF CONCENTRATED HYDROGEN PEROXIDE?
Injury from concentrated H2O2 consumption is primarily from either direct caustic injury or the embolic obstruction of blood flow. Following ingestion, the enzyme catalase metabolizes the breakdown of H2O2 in accordance with the following equation: 2H2O2(aq) → 2H2O(l) + O2(g) + heat. A single milliliter of 35% H2O2 results in the liberation of 100 mL of O2. (The more common 3% household solution generates 10 mL of oxygen per 1 mL of H2O2.) The creation of a large intragastric pressure gradient from the liberation of gas, coupled with the caustic and exothermic injury of the bowel mucosa, may contribute to the movement of oxygen through epithelial interstices into the circulation.
In addition, and perhaps more importantly, absorption of intact H2O2 with subsequent metabolism by catalase in the blood liberates oxygen directly within the vasculature. Oxygen bubbles may coalesce in blood circulation and occlude vascular flow. In canine studies, elevated oxygen tension in the portal venous system led to cessation of mesenteric flow in arteries and veins, though the mechanism of action is unclear.4 Furthermore, coalescence of bubbles can lead to disruption of bowel-cell architecture, fibrin plugging of capillaries, venous thrombosis, and infarction of tissues.4
Cases of cardiac and cerebral gas embolism have been reported and present similarly to patients with diving-related decompression injuries (eg, stroke-like syndromes).5,6 The proposed mechanism for these latter effects involves the metabolism of H2O2 in the systemic circulation with production of oxygen bubbles. In the presence of an atrial septal defect, bubbles may move from the right atrium to the arterial circulation.7
Toxicity and death from H2O2 exposure associated with the historical treatment of inspissated meconium,4 as well as the irrigation of wounds,8 has been reported in the medical literature. Ingestion of a 3% solution is generally benign, resulting at worst in gastrointestinal symptoms or throat irritation.9 Rarely does significant toxicity occur at this low concentration,5 with the vast majority of such cases involving concentrated solutions of 35%.
Continue reading for the case continuation...
CASE CONTINUATION
Based on this patient’s continued symptoms, an abdominal radiograph was obtained to assess the presence of portal venous air. Although radiographic findings were normal, continued abdominal examination findings warranted a subsequent abdominal CT scan, which revealed the presence of extensive air throughout the portal venous system (see the figure).
DO ALL PATIENTS PRESENTING WITH H2O2 INGESTION REQUIRE IMAGING TO ASSESS FOR THE PRESENCE OF PORTAL VENOUS AIR?
Reportedly, ingestion of as little as a “sip” or “mouthful” of 35% H2O2 has resulted in venous and arterial gas embolism,6 occasionally with severe consequences, but no current consensus guidelines exist regarding imaging requirements. Some toxicologists and hyperbaric physicians believe that the presence of portal venous air does not adversely impact a patient’s prognosis or necessitate treatment, and therefore a work-up is unnecessary.
Others, however, suggest that the presence of portal venous air indicates oversaturation of oxygen in the blood, placing the patient at increased risk for cardiac and cerebral air embolism. Neither one of these theories is well supported in the literature. Although practice patterns vary by institution, it is reasonable that all patients presenting with abdominal complaints after ingestion of H2O2 undergo CT imaging to assess for portal venous air.
Continue reading to find out what to do if portal venous air is detected...
IF PORTAL VENOUS AIR IS DETECTED, DO PATIENTS REQUIRE HYPERBARIC OXYGEN THERAPY?
The management of patients with portal venous gas following H2O2 ingestion is controversial and has not been established. Hyperbaric oxygen therapy involves increasing the ambient pressure by several atmospheres inside a specially designed chamber—the same therapy used for diving-related bubble injury.
Hyperbaric therapy increases the amount of oxygen that can be dissolved in the blood, thereby decreasing bubble formation and allowing transport of dissolved oxygen to the lungs, where it can be exhaled. Some patients with portal venous air experience significant pain and portal venous hypertension, which may respond rapidly to this therapy.10
Based on available literature, hyperbaric therapy is reasonable for patients with significant abdominal pain and portal venous air following H2O2 ingestion; less controversial is the role of hyperbaric therapy in those with cerebral air embolism. Multiple case reports of patients with significant neurologic findings demonstrate resolution of symptoms following hyperbaric therapy.6
Continue reading for the case conclusion...
CASE CONCLUSION
Hyperbaric oxygen therapy was recommended for the patient in this case, but transfer to a hyperbaric facility was not possible. He was instead admitted to the hospital for continuous monitoring. Over the next 12 hours, his symptoms gradually resolved, and a repeat CT the following day showed complete resolution of the portal venous gas. The patient was subsequently discharged without any sequelae.
REFERENCES
1. Ukuku DO, Bari ML, Kawamoto S, Isshiki K. Use of hydrogen peroxide in combination with nisin, sodium lactate and citric acid for reducing transfer of bacterial pathogens from whole melon surfaces to fresh-cut pieces. Int J Food Microbiol. 2005;104(2):225-233.
2. 35% H2O2 hydrogen peroxide food grade certified benefits. The One Minute Miracle Web site. www.theoneminutemiracleinc.com/pages/h2o2-benefits/. Accessed January 20, 2013.
3. FDA. FDA warns consumers against drinking high-strength hydrogen peroxide for medicinal use: ingestion can lead to serious health risk and death [news release]. July 27, 2006. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2006/ucm108701.htm. Accessed January 20, 2013.
4. Shaw A, Cooperman A, Fusco J. Gas embolism produced by hydrogen peroxide. N Engl J Med. 1967;277(5):238-241.
5. Cina SJ, Downs JC, Conradi SE. Hydrogen peroxide: a source of lethal oxygen embolism. Case report and review of the literature. Am J Forensic Med Pathol. 1994;15(1):44-50.
6. Rider SP, Jackson SB, Rusyniak DE. Cerebral air gas embolism from concentrated hydrogen peroxide ingestion. Clin Toxicol (Phila). 2008;46(9):815-818.
7. French LK, Horowitz BZ, McKeown NJ. Hydrogen peroxide ingestion associated with portal venous gas and treatment with hyperbaric oxygen: a case series and review of the literature. Clin Toxicol (Phila). 2010;48(6):533-538.
8. Bassan MM, Dudai M, Shalev O. Near-fatal systemic oxygen embolism due to wound irrigation with hydrogen peroxide. Postgrad Med J. 1982;58(681):448-450.
9. Henry MC, Wheeler J, Mofenson HC, et al. Hydrogen peroxide 3% exposures. J Toxicol Clin Toxicol. 1996;34(3):323-327.
10. Papafragkou S, Gasparyan A, Batista R, Scott P. Treatment of portal venous gas embolism with hyperbaric oxygen after accidental ingestion of hydrogen peroxide: a case report and review of the literature. J Emerg Med. 2012;43(1):e21-e23
A previously healthy 32-year-old man presented to the emergency department (ED) after unintentionally ingesting a mouthful of concentrated (35%) hydrogen peroxide (H2O2) from an unmarked bottle he kept in his refrigerator. Upon realizing his error, he immediately drank a liter of water, which promptly induced vomiting. In the ED, the patient complained of mild throat and chest discomfort as well as “abdominal fullness.”
His initial vital signs included a blood pressure of 140/92 mm Hg; heart rate, 93 beats/min; respiratory rate, 18 breaths/min; and temperature, 96.4°F. His O2 saturation was 98% on room air. Physical examination revealed tenderness in the epigastric region with no peritoneal findings. Oropharynx and chest examination were normal, and standard laboratory investigations were all within normal limits.
WHAT ARE THE POTENTIAL EXPOSURES TO HYDROGEN PEROXIDE?
Hydrogen peroxide is a colorless and odorless liquid. Solutions with concentrations ranging from 3% to 5% have many household applications, including use as a wound disinfectant and dentifrice; dilute solutions are also utilized for similar purposes in the hospital setting. Industrial-strength H2O2 (concentrations of 10% to 35%) is employed to bleach textiles and paper, and higher concentrations (70% to 90%) are used as an oxygen source for rocket engines.
Consumer application of concentrated H2O2 solutions has become increasingly common. Some, like this patient, clean the surfaces of fruits and vegetables with H2O2 to decrease transmission of bacteria during cutting.1 More concerning, however, is the purported medicinal benefits of ingesting “food-grade” (35%) H2O2 mixed with water—touted on many Internet sites as a treatment for illnesses such as emphysema, cancer, anemia, and HIV.2 Sometimes referred to as “hyperoxygenation therapy,” this so-called treatment has not been approved by the FDA for any such purpose.3 When diluted sufficiently, this concoction is not harmful but is unlikely to provide any health benefits.
Continue reading for the toxic effects of concentrated hydrogen peroxide...
WHAT ARE THE TOXIC EFFECTS OF CONCENTRATED HYDROGEN PEROXIDE?
Injury from concentrated H2O2 consumption is primarily from either direct caustic injury or the embolic obstruction of blood flow. Following ingestion, the enzyme catalase metabolizes the breakdown of H2O2 in accordance with the following equation: 2H2O2(aq) → 2H2O(l) + O2(g) + heat. A single milliliter of 35% H2O2 results in the liberation of 100 mL of O2. (The more common 3% household solution generates 10 mL of oxygen per 1 mL of H2O2.) The creation of a large intragastric pressure gradient from the liberation of gas, coupled with the caustic and exothermic injury of the bowel mucosa, may contribute to the movement of oxygen through epithelial interstices into the circulation.
In addition, and perhaps more importantly, absorption of intact H2O2 with subsequent metabolism by catalase in the blood liberates oxygen directly within the vasculature. Oxygen bubbles may coalesce in blood circulation and occlude vascular flow. In canine studies, elevated oxygen tension in the portal venous system led to cessation of mesenteric flow in arteries and veins, though the mechanism of action is unclear.4 Furthermore, coalescence of bubbles can lead to disruption of bowel-cell architecture, fibrin plugging of capillaries, venous thrombosis, and infarction of tissues.4
Cases of cardiac and cerebral gas embolism have been reported and present similarly to patients with diving-related decompression injuries (eg, stroke-like syndromes).5,6 The proposed mechanism for these latter effects involves the metabolism of H2O2 in the systemic circulation with production of oxygen bubbles. In the presence of an atrial septal defect, bubbles may move from the right atrium to the arterial circulation.7
Toxicity and death from H2O2 exposure associated with the historical treatment of inspissated meconium,4 as well as the irrigation of wounds,8 has been reported in the medical literature. Ingestion of a 3% solution is generally benign, resulting at worst in gastrointestinal symptoms or throat irritation.9 Rarely does significant toxicity occur at this low concentration,5 with the vast majority of such cases involving concentrated solutions of 35%.
Continue reading for the case continuation...
CASE CONTINUATION
Based on this patient’s continued symptoms, an abdominal radiograph was obtained to assess the presence of portal venous air. Although radiographic findings were normal, continued abdominal examination findings warranted a subsequent abdominal CT scan, which revealed the presence of extensive air throughout the portal venous system (see the figure).
DO ALL PATIENTS PRESENTING WITH H2O2 INGESTION REQUIRE IMAGING TO ASSESS FOR THE PRESENCE OF PORTAL VENOUS AIR?
Reportedly, ingestion of as little as a “sip” or “mouthful” of 35% H2O2 has resulted in venous and arterial gas embolism,6 occasionally with severe consequences, but no current consensus guidelines exist regarding imaging requirements. Some toxicologists and hyperbaric physicians believe that the presence of portal venous air does not adversely impact a patient’s prognosis or necessitate treatment, and therefore a work-up is unnecessary.
Others, however, suggest that the presence of portal venous air indicates oversaturation of oxygen in the blood, placing the patient at increased risk for cardiac and cerebral air embolism. Neither one of these theories is well supported in the literature. Although practice patterns vary by institution, it is reasonable that all patients presenting with abdominal complaints after ingestion of H2O2 undergo CT imaging to assess for portal venous air.
Continue reading to find out what to do if portal venous air is detected...
IF PORTAL VENOUS AIR IS DETECTED, DO PATIENTS REQUIRE HYPERBARIC OXYGEN THERAPY?
The management of patients with portal venous gas following H2O2 ingestion is controversial and has not been established. Hyperbaric oxygen therapy involves increasing the ambient pressure by several atmospheres inside a specially designed chamber—the same therapy used for diving-related bubble injury.
Hyperbaric therapy increases the amount of oxygen that can be dissolved in the blood, thereby decreasing bubble formation and allowing transport of dissolved oxygen to the lungs, where it can be exhaled. Some patients with portal venous air experience significant pain and portal venous hypertension, which may respond rapidly to this therapy.10
Based on available literature, hyperbaric therapy is reasonable for patients with significant abdominal pain and portal venous air following H2O2 ingestion; less controversial is the role of hyperbaric therapy in those with cerebral air embolism. Multiple case reports of patients with significant neurologic findings demonstrate resolution of symptoms following hyperbaric therapy.6
Continue reading for the case conclusion...
CASE CONCLUSION
Hyperbaric oxygen therapy was recommended for the patient in this case, but transfer to a hyperbaric facility was not possible. He was instead admitted to the hospital for continuous monitoring. Over the next 12 hours, his symptoms gradually resolved, and a repeat CT the following day showed complete resolution of the portal venous gas. The patient was subsequently discharged without any sequelae.
REFERENCES
1. Ukuku DO, Bari ML, Kawamoto S, Isshiki K. Use of hydrogen peroxide in combination with nisin, sodium lactate and citric acid for reducing transfer of bacterial pathogens from whole melon surfaces to fresh-cut pieces. Int J Food Microbiol. 2005;104(2):225-233.
2. 35% H2O2 hydrogen peroxide food grade certified benefits. The One Minute Miracle Web site. www.theoneminutemiracleinc.com/pages/h2o2-benefits/. Accessed January 20, 2013.
3. FDA. FDA warns consumers against drinking high-strength hydrogen peroxide for medicinal use: ingestion can lead to serious health risk and death [news release]. July 27, 2006. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2006/ucm108701.htm. Accessed January 20, 2013.
4. Shaw A, Cooperman A, Fusco J. Gas embolism produced by hydrogen peroxide. N Engl J Med. 1967;277(5):238-241.
5. Cina SJ, Downs JC, Conradi SE. Hydrogen peroxide: a source of lethal oxygen embolism. Case report and review of the literature. Am J Forensic Med Pathol. 1994;15(1):44-50.
6. Rider SP, Jackson SB, Rusyniak DE. Cerebral air gas embolism from concentrated hydrogen peroxide ingestion. Clin Toxicol (Phila). 2008;46(9):815-818.
7. French LK, Horowitz BZ, McKeown NJ. Hydrogen peroxide ingestion associated with portal venous gas and treatment with hyperbaric oxygen: a case series and review of the literature. Clin Toxicol (Phila). 2010;48(6):533-538.
8. Bassan MM, Dudai M, Shalev O. Near-fatal systemic oxygen embolism due to wound irrigation with hydrogen peroxide. Postgrad Med J. 1982;58(681):448-450.
9. Henry MC, Wheeler J, Mofenson HC, et al. Hydrogen peroxide 3% exposures. J Toxicol Clin Toxicol. 1996;34(3):323-327.
10. Papafragkou S, Gasparyan A, Batista R, Scott P. Treatment of portal venous gas embolism with hyperbaric oxygen after accidental ingestion of hydrogen peroxide: a case report and review of the literature. J Emerg Med. 2012;43(1):e21-e23
Woman, 78, With Dyspnea, Dry Cough, and Fatigue
A 78-year-old woman presented to the emergency department (ED) complaining of shortness of breath, a dry nonproductive cough, fatigue, hypoxia, and general malaise lasting for several months and worsening over a two-week period. She denied having fever, chills, hemoptysis, weight loss, headache, rashes, or joint pain. She reported sweats, decrease in appetite, wheezing, cough without sputum production, and slight swelling of the legs. The patient complained of chest pain upon admission, but it resolved quickly.
The patient, a retired widow with five grown children, denied recent surgery or exposure to sick people, had not travelled, and reported no changes in her home environment. She claimed to have no pets but admitted to currently smoking about four cigarettes a day; she had previously smoked, on average, three packs of cigarettes per day for 60 years. She denied using alcohol or drugs, including intravenous agents.
The patient’s medical history was significant for paroxysmal atrial fibrillation. She had also been diagnosed with chronic obstructive pulmonary disease (COPD), transient ischemic attack, patent foramen ovale, hyperlipidemia, seizure disorder, and hypothyroidism. She had no known HIV risk factors and had had no exposure to asbestos or tuberculosis.
The patient’s current medications included amiodarone (200 mg/d) for four years; valproic acid (500 mg/d); aspirin (325 mg/d); levothyroxine (50 g/d); rosuvastatin (10 mg/d); daily warfarin, dosed according to the international normalized ratio (INR); and budesonide/formoterol (160/4.5 mg, one puff bid). She denied having any drug allergies.
Physical examination in the ED revealed a pulse of 63 beats/min; blood pressure, 108/50 mm Hg; and respiratory rate, 16 to 20 breaths/min. The patient’s O2 saturation was 84% on room air; 82% to 84% on 4 L to 6 L of supplemental oxygen; 87% to 92% with a venturi mask; and 95% on biphasic positive airway pressure (BiPAP) device. She was afebrile with hypoxia and able to speak in full sentences. Crackles were detected in the upper lung fields, best heard anteriorly, as well as a few scattered wheezes and rhonchi. Her heart sounds were normal with a regular rhythm; her extremities exhibited trace edema bilaterally. The remainder of the physical exam was normal.
The patient’s laboratory values included a normal white blood cell (WBC) count, elevated lactic acid dehydrogenase (LDH) at 448 IU/L (reference range, 84 to 246 IU/L), and no eosinophils. The erythrocyte sedimentation rate (ESR) was not measured on admission. Blood analysis of her N-terminal pro-brain natriuretic peptide (NT-proBNP) was 4,877 pg/mL; for women older than 75, a level higher than 1,800 pg/mL is abnormal.
A chest x-ray was performed on admission, showing hyperinflation of the lungs with mild coarsening of the lung markings. A bandlike area of opacity in the right lower lobe with bilateral apical pleural thickening was noted (see Figure 1). Noncontrast CT of the chest revealed diffuse upper lobe ground glass opacities in both lungs, extending into the right middle lobe and lingula as well the superior segments of the lower lobes, with areas of emphysema and septal thickening. Numerous nodules, some of which appeared cavitary, were apparent in the lower lobes.
A two-dimensional echocardiogram demonstrated normal left ventricular size and systolic function, mild tricuspid regurgitation without evidence of pulmonary hypertension, and mild left atrial enlargement.
The patient was admitted to the cardiac unit for evaluation. While there, she received one dose of methylprednisolone (125 mg IV), three doses of ipratropium bromide/albuterol, one dose of ceftriaxone (1 g IV), and one dose of azithromycin (500 mg po). In the absence of significant leg edema and an elevation of jugular venous distention with a normal two-dimensional echocardiogram, heart failure was ruled out. The chest pains reported on initial presentation were ultimately felt to be noncardiac in nature.
After the patient was transferred to the medical floor with an initial diagnosis of exacerbation of her COPD, she was treated with antibiotics, nebulizers, and corticosteroids. She continued to experience episodes of O2 desaturation while on 4 L to 6 L of oxygen via nasal cannula and on a venturi mask. She was then placed on a BiPAP device, set to 12/5, and 50% Fio2 (fraction of inspired oxygen), which improved her oxygenation.
Her hypoxia prompted further radiographic studies. The resulting chest CT scan showed ground glass opacities located primarily in the upper lung areas, greater on the right than on the left side (see Figure 2). The radiologist suggested that the hypoxia was caused by an infection, but because the patient’s presenting symptoms were chronic in nature, drug-induced causes were considered as well. Amiodarone was discontinued.
Cardiology was consulted and agreed that stopping amiodarone was acceptable since the patient was in sinus rhythm at the time. The patient continued to take antibiotics and prednisone. Her symptoms slowly improved during hospitalization, and she required less oxygen. Based on the patient’s presentation, physical exam findings, imaging studies, and laboratory findings, amiodarone-induced pulmonary toxicity (APT) was diagnosed.
She was discharged home on supplemental oxygen at 4 L via cannula, a tapering dosage of prednisone, and metered-dose inhalers for fluticasone/salmeterol and tiotropium bromide. She also had outpatient appointments scheduled, one with the pulmonologist to follow up on her imaging studies and to manage the prednisone taper and the other with the cardiologist to manage her atrial fibrillation.
At pulmonology two months later, she had a chest x-ray (see Figure 3) and pulmonary function tests (PFTs). The patient reported feeling progressively better in the past month. Her dyspnea on exertion had improved, and she did not require supplemental oxygen anymore. She stopped smoking cigarettes.
The patient continued to use fluticasone/salmeterol but stopped tiotropium bromide. On physical exam, her O2 saturation was 95% on room air, heart rhythm and rate were regular, and her lungs revealed very minimal crackles at the right base but were otherwise clear.
The plan specified continuing the prednisone taper. The patient was asked to call the office if she had any worsening shortness of breath, cough, and sputum production. She was also encouraged to continue refraining from smoking cigarettes. This patient had done very well, with near complete resolution of symptoms and a clear chest x-ray.
Continue reading for discussion...
DISCUSSION
Amiodarone, a highly effective antiarrhythmic drug, is FDA approved for suppressing ventricular fibrillation and ventricular tachycardia. It is also used off-label as a second- or third-line choice for atrial fibrillation.1
Standard of care requires that, prior to starting amiodarone therapy, patients have a baseline chest x-ray and PFTs with diffusing capacity performed. Thereafter, the patient should be monitored with annual chest x-rays, with one performed promptly if new symptoms develop. Serial PFTs have not offered any benefit for monitoring, but a decrease of more than 15% in total lung capacity or more than 20% in diffusing capacity from baseline is consistent with APT.2
Adverse effects, both cardiac and noncardiac, are common with amiodarone therapy. They include proarrhythmias, bradycardia, and heart block, as well as thyroid and liver dysfunctions; dermatologic conditions such as blue-gray discoloration of the skin and photosensitivity; neurologic effects such as ataxia, paresthesias, and tremor; ocular problems, including corneal microdeposits; gastrointestinal problems such as nausea, anorexia, and constipation; and lung problems such as pulmonary toxicity, pleural effusion, and pleural thickening.3-6 Of these, pulmonary toxicity is the most severe and life threatening.7
APT, also known as amiodarone pneumonitis and amiodarone lung, typically manifests from a few months to a year and a half after treatment is commenced.6 APT can occur even after the drug is discontinued, because amiodarone has a very long elimination half-life of approximately 15 to 45 days and a tendency to concentrate in organs with high blood perfusion and in adipose tissues.8 Patients taking 400 mg/d for two months or longer or 200 mg/d for more than two years are considered at higher risk for APT.9 The severity of disease appears to correlate with the cumulative dose and length of treatment.10
Numerous risk factors for pulmonary toxicity have been reported, including high drug dosage, pre-existing lung disease, patient age, and prior surgery (see Table 1).11 According to an analysis of a database of 237 patients, only age and duration of amiodarone therapy were significant risk factors for APT.9 Its incidence is not precisely known; reported rates range from 1% to 17%.6,12,13
Presentation with such nonspecific symptoms as shortness of breath, nonproductive cough, fatigue, hypoxia, and general malaise is typical for many pulmonary and cardiac illnesses (see Table 2), making APT difficult to diagnose.14 Occasionally, rapid onset with progression to pneumonitis and respiratory failure masquerades as acute respiratory distress syndrome (ARDS).15
Notable, however, is that APT can manifest with nonproductive cough and dyspnea in 50% to 75% of cases. In addition, presenting symptoms will include fever (33% to 50% of cases) with associated malaise, fatigue, chest pain, and weight loss. In patients with APT, the physical exam usually reveals bilateral crackles on inspiration, but diffuse rales may be heard as well.11
Laboratory studies are not very helpful in diagnosing APT. Patients may present with nonspecific elevated WBCs without eosinophilia and an elevated LDH level.11 An elevated ESR may be detected before symptoms of APT manifest and can be present at the time of diagnosis.6
Imaging studies are far more helpful and specific in diagnosing APT. The typical chest x-ray shows bilateral patchy diffuse infiltrates.12 CT of the chest is usually more revealing, demonstrating ground glass opacities in the periphery and subpleural thickening, especially where infiltrates are denser. This thickening may result in pleuritic chest pain.6
The right upper lobe is more often affected in these cases than the left lung.6 Numerous pulmonary nodules in the upper lobes are found rarely and can be confused with lung cancer. These nodules are likely the result of an accumulation of the drug in areas of previous inflammation; a lung mass should prompt the addition of APT in the differential.2,16
APT is a diagnosis of exclusion, requiring clinical suspicion, drug history, imaging, and consideration of the differential. The presence of three or more clinical factors supports a diagnosis of APT (see Table 3).11
Once APT is recognized, the first action is to have the patient stop taking amiodarone, followed by the administration of corticosteroids (eg, prednisone 40 to 60 mg/d11) for four to 12 months.17 Patients, especially those with underlying lung disease, will typically require temporary oxygen supplementation until hypoxia resolves. Even after the drug has been discontinued, some patients experience worsening symptoms before they see improvement simply because the drug can persist in lung tissue for up to a year following cessation of therapy.6
If APT is diagnosed early, the prognosis is favorable. In one study, a significant number of APT patients stabilized or improved after withdrawal of the drug, regardless of concurrent treatment with corticosteroids.18 Follow-up studies, both imaging and PFT, indicate complete clearing of lung opacities in the majority of patients treated for APT.19 Radiologic improvement may be seen six months after cessation of amiodarone.20 Patients who develop ARDS tend to do poorly and have a mortality rate of approximately 50%.11
Continue reading for the conclusion...
CONCLUSION
Among patients who are taking long-term or high-dose amiodarone, particularly those older than 60, new-onset nonproductive cough and dyspnea signal the need for pulmonary and cardiac work-up. Once the diagnosis of APT is made, treatment is straightforward: Withdraw the amiodarone, and initiate corticosteroid therapy.
REFERENCES
1. Fuster V, Rydén LE, Asinger RW, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to Develop Guidelines for the Management of Patients With Atrial Fibrillation); North American Society of Pacing and Electrophysiology. ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation: executive summary. Circulation. 2001; 104(17):2118-2150.
2. Jarand J, Lee A, Leigh R. Amiodaronoma: an unusual form of amiodarone-induced pulmonary toxicity. CMAJ. 2007;176(10):1411-1413.
3. Connolly S. Evidence-based analysis of amiodarone efficacy and safety. Circulation. 1999;100:2025-2034.
4. Amiodarone Trials Meta-Analysis Investigators. Effect of prophylactic amiodarone on mortality after acute myocardial infarction and in congestive heart failure: meta-analysis of individual data from 6500 patients in randomised trials. Lancet. 1997;350(9089):1417-1424.
5. Pollak PT. Clinical organ toxicity of antiarrhythmic compounds: ocular and pulmonary manifestations. Am J Cardiol. 1999;84(9A):37R-45R.
6. Camus P, Martin W, Rosenow E. Amiodarone pulmonary toxicity. Clin Chest Med. 2004;25(1):65-75.
7. Rady MY, Ryan T, Starr NJ. Preoperative therapy with amiodarone and the incidence of acute organ dysfunction after cardiac surgery. Anesth Analg. 1997;85(3):489-497.
8. Canada A, Lesko L, Haffajee C, et al. Amiodarone for tachyarrhythmias: kinetics, and efficacy. Drug Intell Clin Pharm. 1983;17(2):100-104.
9. Ernawati DK, Stafford L, Hughes JD. Amiodarone-induced pulmonary toxicity. Br J Clin Pharmacol. 2008;66(1):82-87.
10. Liu FL, Cohen RD, Downar E, et al. Amiodarone pulmonary toxicity: functional and ultrastructural evaluation. Thorax. 1986;41(2):100-105.
11. Chan E, King TE. Amiodarone pulmonary toxicity. UpToDate. 2013. www.uptodate.com/contents/amiodarone-pulmonary-toxicity. Accessed January 17, 2014.
12. Wolkove N, Baltzan M. Amiodarone pulmonary toxicity. Can Respir J. 2009;16(2):43-48.
13. Jackevicius CA, Tom A, Essebag V, et al. Population-level incidence and risk factors for pulmonary toxicity associated with amiodarone. Am J Cardiol. 2011;108:705-710.
14. Jessurun G, Crijns H. Amiodarone pulmonary toxicity [editorial]. BMJ. 1997;314(7081):619-620.
15. Nacca N, Castigliano B, Yuhico L, et al. Severe amiodarone induced pulmonary toxicity. J Thorac Dis. 2012;4(6):667-670.
16. Arnon R, Raz I, Chajek-Shaul T, et al. Amiodarone pulmonary toxicity presenting as a solitary lung mass. Chest. 1988;93(2):425-427.
17. Yamada Y, Shiga T, Matsuda N, et al. Incidence and predictors of pulmonary toxicity in Japanese patients receiving low-dose amiodarone. Circ J. 2007;71(10):1610-1616.
18. Coudert B, Bailly F, Lombard JN, et al. Amiodarone pneumonitis: bronchoalveolar lavage findings in 15 patients and review of the literature. Chest. 1992;102(4):1005-1012.
19. Vernhet H, Bousquet C, Durand G, et al. Reversible amiodarone-induced lung disease: HRCT findings. Eur Radiol. 2001;11(9):1697-1703.
20. Olson LK, Forrest JV, Friedman PJ, et al. Pneumonitis after amiodarone therapy. Radiology. 1984;150(2):327-330.
A 78-year-old woman presented to the emergency department (ED) complaining of shortness of breath, a dry nonproductive cough, fatigue, hypoxia, and general malaise lasting for several months and worsening over a two-week period. She denied having fever, chills, hemoptysis, weight loss, headache, rashes, or joint pain. She reported sweats, decrease in appetite, wheezing, cough without sputum production, and slight swelling of the legs. The patient complained of chest pain upon admission, but it resolved quickly.
The patient, a retired widow with five grown children, denied recent surgery or exposure to sick people, had not travelled, and reported no changes in her home environment. She claimed to have no pets but admitted to currently smoking about four cigarettes a day; she had previously smoked, on average, three packs of cigarettes per day for 60 years. She denied using alcohol or drugs, including intravenous agents.
The patient’s medical history was significant for paroxysmal atrial fibrillation. She had also been diagnosed with chronic obstructive pulmonary disease (COPD), transient ischemic attack, patent foramen ovale, hyperlipidemia, seizure disorder, and hypothyroidism. She had no known HIV risk factors and had had no exposure to asbestos or tuberculosis.
The patient’s current medications included amiodarone (200 mg/d) for four years; valproic acid (500 mg/d); aspirin (325 mg/d); levothyroxine (50 g/d); rosuvastatin (10 mg/d); daily warfarin, dosed according to the international normalized ratio (INR); and budesonide/formoterol (160/4.5 mg, one puff bid). She denied having any drug allergies.
Physical examination in the ED revealed a pulse of 63 beats/min; blood pressure, 108/50 mm Hg; and respiratory rate, 16 to 20 breaths/min. The patient’s O2 saturation was 84% on room air; 82% to 84% on 4 L to 6 L of supplemental oxygen; 87% to 92% with a venturi mask; and 95% on biphasic positive airway pressure (BiPAP) device. She was afebrile with hypoxia and able to speak in full sentences. Crackles were detected in the upper lung fields, best heard anteriorly, as well as a few scattered wheezes and rhonchi. Her heart sounds were normal with a regular rhythm; her extremities exhibited trace edema bilaterally. The remainder of the physical exam was normal.
The patient’s laboratory values included a normal white blood cell (WBC) count, elevated lactic acid dehydrogenase (LDH) at 448 IU/L (reference range, 84 to 246 IU/L), and no eosinophils. The erythrocyte sedimentation rate (ESR) was not measured on admission. Blood analysis of her N-terminal pro-brain natriuretic peptide (NT-proBNP) was 4,877 pg/mL; for women older than 75, a level higher than 1,800 pg/mL is abnormal.
A chest x-ray was performed on admission, showing hyperinflation of the lungs with mild coarsening of the lung markings. A bandlike area of opacity in the right lower lobe with bilateral apical pleural thickening was noted (see Figure 1). Noncontrast CT of the chest revealed diffuse upper lobe ground glass opacities in both lungs, extending into the right middle lobe and lingula as well the superior segments of the lower lobes, with areas of emphysema and septal thickening. Numerous nodules, some of which appeared cavitary, were apparent in the lower lobes.
A two-dimensional echocardiogram demonstrated normal left ventricular size and systolic function, mild tricuspid regurgitation without evidence of pulmonary hypertension, and mild left atrial enlargement.
The patient was admitted to the cardiac unit for evaluation. While there, she received one dose of methylprednisolone (125 mg IV), three doses of ipratropium bromide/albuterol, one dose of ceftriaxone (1 g IV), and one dose of azithromycin (500 mg po). In the absence of significant leg edema and an elevation of jugular venous distention with a normal two-dimensional echocardiogram, heart failure was ruled out. The chest pains reported on initial presentation were ultimately felt to be noncardiac in nature.
After the patient was transferred to the medical floor with an initial diagnosis of exacerbation of her COPD, she was treated with antibiotics, nebulizers, and corticosteroids. She continued to experience episodes of O2 desaturation while on 4 L to 6 L of oxygen via nasal cannula and on a venturi mask. She was then placed on a BiPAP device, set to 12/5, and 50% Fio2 (fraction of inspired oxygen), which improved her oxygenation.
Her hypoxia prompted further radiographic studies. The resulting chest CT scan showed ground glass opacities located primarily in the upper lung areas, greater on the right than on the left side (see Figure 2). The radiologist suggested that the hypoxia was caused by an infection, but because the patient’s presenting symptoms were chronic in nature, drug-induced causes were considered as well. Amiodarone was discontinued.
Cardiology was consulted and agreed that stopping amiodarone was acceptable since the patient was in sinus rhythm at the time. The patient continued to take antibiotics and prednisone. Her symptoms slowly improved during hospitalization, and she required less oxygen. Based on the patient’s presentation, physical exam findings, imaging studies, and laboratory findings, amiodarone-induced pulmonary toxicity (APT) was diagnosed.
She was discharged home on supplemental oxygen at 4 L via cannula, a tapering dosage of prednisone, and metered-dose inhalers for fluticasone/salmeterol and tiotropium bromide. She also had outpatient appointments scheduled, one with the pulmonologist to follow up on her imaging studies and to manage the prednisone taper and the other with the cardiologist to manage her atrial fibrillation.
At pulmonology two months later, she had a chest x-ray (see Figure 3) and pulmonary function tests (PFTs). The patient reported feeling progressively better in the past month. Her dyspnea on exertion had improved, and she did not require supplemental oxygen anymore. She stopped smoking cigarettes.
The patient continued to use fluticasone/salmeterol but stopped tiotropium bromide. On physical exam, her O2 saturation was 95% on room air, heart rhythm and rate were regular, and her lungs revealed very minimal crackles at the right base but were otherwise clear.
The plan specified continuing the prednisone taper. The patient was asked to call the office if she had any worsening shortness of breath, cough, and sputum production. She was also encouraged to continue refraining from smoking cigarettes. This patient had done very well, with near complete resolution of symptoms and a clear chest x-ray.
Continue reading for discussion...
DISCUSSION
Amiodarone, a highly effective antiarrhythmic drug, is FDA approved for suppressing ventricular fibrillation and ventricular tachycardia. It is also used off-label as a second- or third-line choice for atrial fibrillation.1
Standard of care requires that, prior to starting amiodarone therapy, patients have a baseline chest x-ray and PFTs with diffusing capacity performed. Thereafter, the patient should be monitored with annual chest x-rays, with one performed promptly if new symptoms develop. Serial PFTs have not offered any benefit for monitoring, but a decrease of more than 15% in total lung capacity or more than 20% in diffusing capacity from baseline is consistent with APT.2
Adverse effects, both cardiac and noncardiac, are common with amiodarone therapy. They include proarrhythmias, bradycardia, and heart block, as well as thyroid and liver dysfunctions; dermatologic conditions such as blue-gray discoloration of the skin and photosensitivity; neurologic effects such as ataxia, paresthesias, and tremor; ocular problems, including corneal microdeposits; gastrointestinal problems such as nausea, anorexia, and constipation; and lung problems such as pulmonary toxicity, pleural effusion, and pleural thickening.3-6 Of these, pulmonary toxicity is the most severe and life threatening.7
APT, also known as amiodarone pneumonitis and amiodarone lung, typically manifests from a few months to a year and a half after treatment is commenced.6 APT can occur even after the drug is discontinued, because amiodarone has a very long elimination half-life of approximately 15 to 45 days and a tendency to concentrate in organs with high blood perfusion and in adipose tissues.8 Patients taking 400 mg/d for two months or longer or 200 mg/d for more than two years are considered at higher risk for APT.9 The severity of disease appears to correlate with the cumulative dose and length of treatment.10
Numerous risk factors for pulmonary toxicity have been reported, including high drug dosage, pre-existing lung disease, patient age, and prior surgery (see Table 1).11 According to an analysis of a database of 237 patients, only age and duration of amiodarone therapy were significant risk factors for APT.9 Its incidence is not precisely known; reported rates range from 1% to 17%.6,12,13
Presentation with such nonspecific symptoms as shortness of breath, nonproductive cough, fatigue, hypoxia, and general malaise is typical for many pulmonary and cardiac illnesses (see Table 2), making APT difficult to diagnose.14 Occasionally, rapid onset with progression to pneumonitis and respiratory failure masquerades as acute respiratory distress syndrome (ARDS).15
Notable, however, is that APT can manifest with nonproductive cough and dyspnea in 50% to 75% of cases. In addition, presenting symptoms will include fever (33% to 50% of cases) with associated malaise, fatigue, chest pain, and weight loss. In patients with APT, the physical exam usually reveals bilateral crackles on inspiration, but diffuse rales may be heard as well.11
Laboratory studies are not very helpful in diagnosing APT. Patients may present with nonspecific elevated WBCs without eosinophilia and an elevated LDH level.11 An elevated ESR may be detected before symptoms of APT manifest and can be present at the time of diagnosis.6
Imaging studies are far more helpful and specific in diagnosing APT. The typical chest x-ray shows bilateral patchy diffuse infiltrates.12 CT of the chest is usually more revealing, demonstrating ground glass opacities in the periphery and subpleural thickening, especially where infiltrates are denser. This thickening may result in pleuritic chest pain.6
The right upper lobe is more often affected in these cases than the left lung.6 Numerous pulmonary nodules in the upper lobes are found rarely and can be confused with lung cancer. These nodules are likely the result of an accumulation of the drug in areas of previous inflammation; a lung mass should prompt the addition of APT in the differential.2,16
APT is a diagnosis of exclusion, requiring clinical suspicion, drug history, imaging, and consideration of the differential. The presence of three or more clinical factors supports a diagnosis of APT (see Table 3).11
Once APT is recognized, the first action is to have the patient stop taking amiodarone, followed by the administration of corticosteroids (eg, prednisone 40 to 60 mg/d11) for four to 12 months.17 Patients, especially those with underlying lung disease, will typically require temporary oxygen supplementation until hypoxia resolves. Even after the drug has been discontinued, some patients experience worsening symptoms before they see improvement simply because the drug can persist in lung tissue for up to a year following cessation of therapy.6
If APT is diagnosed early, the prognosis is favorable. In one study, a significant number of APT patients stabilized or improved after withdrawal of the drug, regardless of concurrent treatment with corticosteroids.18 Follow-up studies, both imaging and PFT, indicate complete clearing of lung opacities in the majority of patients treated for APT.19 Radiologic improvement may be seen six months after cessation of amiodarone.20 Patients who develop ARDS tend to do poorly and have a mortality rate of approximately 50%.11
Continue reading for the conclusion...
CONCLUSION
Among patients who are taking long-term or high-dose amiodarone, particularly those older than 60, new-onset nonproductive cough and dyspnea signal the need for pulmonary and cardiac work-up. Once the diagnosis of APT is made, treatment is straightforward: Withdraw the amiodarone, and initiate corticosteroid therapy.
REFERENCES
1. Fuster V, Rydén LE, Asinger RW, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to Develop Guidelines for the Management of Patients With Atrial Fibrillation); North American Society of Pacing and Electrophysiology. ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation: executive summary. Circulation. 2001; 104(17):2118-2150.
2. Jarand J, Lee A, Leigh R. Amiodaronoma: an unusual form of amiodarone-induced pulmonary toxicity. CMAJ. 2007;176(10):1411-1413.
3. Connolly S. Evidence-based analysis of amiodarone efficacy and safety. Circulation. 1999;100:2025-2034.
4. Amiodarone Trials Meta-Analysis Investigators. Effect of prophylactic amiodarone on mortality after acute myocardial infarction and in congestive heart failure: meta-analysis of individual data from 6500 patients in randomised trials. Lancet. 1997;350(9089):1417-1424.
5. Pollak PT. Clinical organ toxicity of antiarrhythmic compounds: ocular and pulmonary manifestations. Am J Cardiol. 1999;84(9A):37R-45R.
6. Camus P, Martin W, Rosenow E. Amiodarone pulmonary toxicity. Clin Chest Med. 2004;25(1):65-75.
7. Rady MY, Ryan T, Starr NJ. Preoperative therapy with amiodarone and the incidence of acute organ dysfunction after cardiac surgery. Anesth Analg. 1997;85(3):489-497.
8. Canada A, Lesko L, Haffajee C, et al. Amiodarone for tachyarrhythmias: kinetics, and efficacy. Drug Intell Clin Pharm. 1983;17(2):100-104.
9. Ernawati DK, Stafford L, Hughes JD. Amiodarone-induced pulmonary toxicity. Br J Clin Pharmacol. 2008;66(1):82-87.
10. Liu FL, Cohen RD, Downar E, et al. Amiodarone pulmonary toxicity: functional and ultrastructural evaluation. Thorax. 1986;41(2):100-105.
11. Chan E, King TE. Amiodarone pulmonary toxicity. UpToDate. 2013. www.uptodate.com/contents/amiodarone-pulmonary-toxicity. Accessed January 17, 2014.
12. Wolkove N, Baltzan M. Amiodarone pulmonary toxicity. Can Respir J. 2009;16(2):43-48.
13. Jackevicius CA, Tom A, Essebag V, et al. Population-level incidence and risk factors for pulmonary toxicity associated with amiodarone. Am J Cardiol. 2011;108:705-710.
14. Jessurun G, Crijns H. Amiodarone pulmonary toxicity [editorial]. BMJ. 1997;314(7081):619-620.
15. Nacca N, Castigliano B, Yuhico L, et al. Severe amiodarone induced pulmonary toxicity. J Thorac Dis. 2012;4(6):667-670.
16. Arnon R, Raz I, Chajek-Shaul T, et al. Amiodarone pulmonary toxicity presenting as a solitary lung mass. Chest. 1988;93(2):425-427.
17. Yamada Y, Shiga T, Matsuda N, et al. Incidence and predictors of pulmonary toxicity in Japanese patients receiving low-dose amiodarone. Circ J. 2007;71(10):1610-1616.
18. Coudert B, Bailly F, Lombard JN, et al. Amiodarone pneumonitis: bronchoalveolar lavage findings in 15 patients and review of the literature. Chest. 1992;102(4):1005-1012.
19. Vernhet H, Bousquet C, Durand G, et al. Reversible amiodarone-induced lung disease: HRCT findings. Eur Radiol. 2001;11(9):1697-1703.
20. Olson LK, Forrest JV, Friedman PJ, et al. Pneumonitis after amiodarone therapy. Radiology. 1984;150(2):327-330.
A 78-year-old woman presented to the emergency department (ED) complaining of shortness of breath, a dry nonproductive cough, fatigue, hypoxia, and general malaise lasting for several months and worsening over a two-week period. She denied having fever, chills, hemoptysis, weight loss, headache, rashes, or joint pain. She reported sweats, decrease in appetite, wheezing, cough without sputum production, and slight swelling of the legs. The patient complained of chest pain upon admission, but it resolved quickly.
The patient, a retired widow with five grown children, denied recent surgery or exposure to sick people, had not travelled, and reported no changes in her home environment. She claimed to have no pets but admitted to currently smoking about four cigarettes a day; she had previously smoked, on average, three packs of cigarettes per day for 60 years. She denied using alcohol or drugs, including intravenous agents.
The patient’s medical history was significant for paroxysmal atrial fibrillation. She had also been diagnosed with chronic obstructive pulmonary disease (COPD), transient ischemic attack, patent foramen ovale, hyperlipidemia, seizure disorder, and hypothyroidism. She had no known HIV risk factors and had had no exposure to asbestos or tuberculosis.
The patient’s current medications included amiodarone (200 mg/d) for four years; valproic acid (500 mg/d); aspirin (325 mg/d); levothyroxine (50 g/d); rosuvastatin (10 mg/d); daily warfarin, dosed according to the international normalized ratio (INR); and budesonide/formoterol (160/4.5 mg, one puff bid). She denied having any drug allergies.
Physical examination in the ED revealed a pulse of 63 beats/min; blood pressure, 108/50 mm Hg; and respiratory rate, 16 to 20 breaths/min. The patient’s O2 saturation was 84% on room air; 82% to 84% on 4 L to 6 L of supplemental oxygen; 87% to 92% with a venturi mask; and 95% on biphasic positive airway pressure (BiPAP) device. She was afebrile with hypoxia and able to speak in full sentences. Crackles were detected in the upper lung fields, best heard anteriorly, as well as a few scattered wheezes and rhonchi. Her heart sounds were normal with a regular rhythm; her extremities exhibited trace edema bilaterally. The remainder of the physical exam was normal.
The patient’s laboratory values included a normal white blood cell (WBC) count, elevated lactic acid dehydrogenase (LDH) at 448 IU/L (reference range, 84 to 246 IU/L), and no eosinophils. The erythrocyte sedimentation rate (ESR) was not measured on admission. Blood analysis of her N-terminal pro-brain natriuretic peptide (NT-proBNP) was 4,877 pg/mL; for women older than 75, a level higher than 1,800 pg/mL is abnormal.
A chest x-ray was performed on admission, showing hyperinflation of the lungs with mild coarsening of the lung markings. A bandlike area of opacity in the right lower lobe with bilateral apical pleural thickening was noted (see Figure 1). Noncontrast CT of the chest revealed diffuse upper lobe ground glass opacities in both lungs, extending into the right middle lobe and lingula as well the superior segments of the lower lobes, with areas of emphysema and septal thickening. Numerous nodules, some of which appeared cavitary, were apparent in the lower lobes.
A two-dimensional echocardiogram demonstrated normal left ventricular size and systolic function, mild tricuspid regurgitation without evidence of pulmonary hypertension, and mild left atrial enlargement.
The patient was admitted to the cardiac unit for evaluation. While there, she received one dose of methylprednisolone (125 mg IV), three doses of ipratropium bromide/albuterol, one dose of ceftriaxone (1 g IV), and one dose of azithromycin (500 mg po). In the absence of significant leg edema and an elevation of jugular venous distention with a normal two-dimensional echocardiogram, heart failure was ruled out. The chest pains reported on initial presentation were ultimately felt to be noncardiac in nature.
After the patient was transferred to the medical floor with an initial diagnosis of exacerbation of her COPD, she was treated with antibiotics, nebulizers, and corticosteroids. She continued to experience episodes of O2 desaturation while on 4 L to 6 L of oxygen via nasal cannula and on a venturi mask. She was then placed on a BiPAP device, set to 12/5, and 50% Fio2 (fraction of inspired oxygen), which improved her oxygenation.
Her hypoxia prompted further radiographic studies. The resulting chest CT scan showed ground glass opacities located primarily in the upper lung areas, greater on the right than on the left side (see Figure 2). The radiologist suggested that the hypoxia was caused by an infection, but because the patient’s presenting symptoms were chronic in nature, drug-induced causes were considered as well. Amiodarone was discontinued.
Cardiology was consulted and agreed that stopping amiodarone was acceptable since the patient was in sinus rhythm at the time. The patient continued to take antibiotics and prednisone. Her symptoms slowly improved during hospitalization, and she required less oxygen. Based on the patient’s presentation, physical exam findings, imaging studies, and laboratory findings, amiodarone-induced pulmonary toxicity (APT) was diagnosed.
She was discharged home on supplemental oxygen at 4 L via cannula, a tapering dosage of prednisone, and metered-dose inhalers for fluticasone/salmeterol and tiotropium bromide. She also had outpatient appointments scheduled, one with the pulmonologist to follow up on her imaging studies and to manage the prednisone taper and the other with the cardiologist to manage her atrial fibrillation.
At pulmonology two months later, she had a chest x-ray (see Figure 3) and pulmonary function tests (PFTs). The patient reported feeling progressively better in the past month. Her dyspnea on exertion had improved, and she did not require supplemental oxygen anymore. She stopped smoking cigarettes.
The patient continued to use fluticasone/salmeterol but stopped tiotropium bromide. On physical exam, her O2 saturation was 95% on room air, heart rhythm and rate were regular, and her lungs revealed very minimal crackles at the right base but were otherwise clear.
The plan specified continuing the prednisone taper. The patient was asked to call the office if she had any worsening shortness of breath, cough, and sputum production. She was also encouraged to continue refraining from smoking cigarettes. This patient had done very well, with near complete resolution of symptoms and a clear chest x-ray.
Continue reading for discussion...
DISCUSSION
Amiodarone, a highly effective antiarrhythmic drug, is FDA approved for suppressing ventricular fibrillation and ventricular tachycardia. It is also used off-label as a second- or third-line choice for atrial fibrillation.1
Standard of care requires that, prior to starting amiodarone therapy, patients have a baseline chest x-ray and PFTs with diffusing capacity performed. Thereafter, the patient should be monitored with annual chest x-rays, with one performed promptly if new symptoms develop. Serial PFTs have not offered any benefit for monitoring, but a decrease of more than 15% in total lung capacity or more than 20% in diffusing capacity from baseline is consistent with APT.2
Adverse effects, both cardiac and noncardiac, are common with amiodarone therapy. They include proarrhythmias, bradycardia, and heart block, as well as thyroid and liver dysfunctions; dermatologic conditions such as blue-gray discoloration of the skin and photosensitivity; neurologic effects such as ataxia, paresthesias, and tremor; ocular problems, including corneal microdeposits; gastrointestinal problems such as nausea, anorexia, and constipation; and lung problems such as pulmonary toxicity, pleural effusion, and pleural thickening.3-6 Of these, pulmonary toxicity is the most severe and life threatening.7
APT, also known as amiodarone pneumonitis and amiodarone lung, typically manifests from a few months to a year and a half after treatment is commenced.6 APT can occur even after the drug is discontinued, because amiodarone has a very long elimination half-life of approximately 15 to 45 days and a tendency to concentrate in organs with high blood perfusion and in adipose tissues.8 Patients taking 400 mg/d for two months or longer or 200 mg/d for more than two years are considered at higher risk for APT.9 The severity of disease appears to correlate with the cumulative dose and length of treatment.10
Numerous risk factors for pulmonary toxicity have been reported, including high drug dosage, pre-existing lung disease, patient age, and prior surgery (see Table 1).11 According to an analysis of a database of 237 patients, only age and duration of amiodarone therapy were significant risk factors for APT.9 Its incidence is not precisely known; reported rates range from 1% to 17%.6,12,13
Presentation with such nonspecific symptoms as shortness of breath, nonproductive cough, fatigue, hypoxia, and general malaise is typical for many pulmonary and cardiac illnesses (see Table 2), making APT difficult to diagnose.14 Occasionally, rapid onset with progression to pneumonitis and respiratory failure masquerades as acute respiratory distress syndrome (ARDS).15
Notable, however, is that APT can manifest with nonproductive cough and dyspnea in 50% to 75% of cases. In addition, presenting symptoms will include fever (33% to 50% of cases) with associated malaise, fatigue, chest pain, and weight loss. In patients with APT, the physical exam usually reveals bilateral crackles on inspiration, but diffuse rales may be heard as well.11
Laboratory studies are not very helpful in diagnosing APT. Patients may present with nonspecific elevated WBCs without eosinophilia and an elevated LDH level.11 An elevated ESR may be detected before symptoms of APT manifest and can be present at the time of diagnosis.6
Imaging studies are far more helpful and specific in diagnosing APT. The typical chest x-ray shows bilateral patchy diffuse infiltrates.12 CT of the chest is usually more revealing, demonstrating ground glass opacities in the periphery and subpleural thickening, especially where infiltrates are denser. This thickening may result in pleuritic chest pain.6
The right upper lobe is more often affected in these cases than the left lung.6 Numerous pulmonary nodules in the upper lobes are found rarely and can be confused with lung cancer. These nodules are likely the result of an accumulation of the drug in areas of previous inflammation; a lung mass should prompt the addition of APT in the differential.2,16
APT is a diagnosis of exclusion, requiring clinical suspicion, drug history, imaging, and consideration of the differential. The presence of three or more clinical factors supports a diagnosis of APT (see Table 3).11
Once APT is recognized, the first action is to have the patient stop taking amiodarone, followed by the administration of corticosteroids (eg, prednisone 40 to 60 mg/d11) for four to 12 months.17 Patients, especially those with underlying lung disease, will typically require temporary oxygen supplementation until hypoxia resolves. Even after the drug has been discontinued, some patients experience worsening symptoms before they see improvement simply because the drug can persist in lung tissue for up to a year following cessation of therapy.6
If APT is diagnosed early, the prognosis is favorable. In one study, a significant number of APT patients stabilized or improved after withdrawal of the drug, regardless of concurrent treatment with corticosteroids.18 Follow-up studies, both imaging and PFT, indicate complete clearing of lung opacities in the majority of patients treated for APT.19 Radiologic improvement may be seen six months after cessation of amiodarone.20 Patients who develop ARDS tend to do poorly and have a mortality rate of approximately 50%.11
Continue reading for the conclusion...
CONCLUSION
Among patients who are taking long-term or high-dose amiodarone, particularly those older than 60, new-onset nonproductive cough and dyspnea signal the need for pulmonary and cardiac work-up. Once the diagnosis of APT is made, treatment is straightforward: Withdraw the amiodarone, and initiate corticosteroid therapy.
REFERENCES
1. Fuster V, Rydén LE, Asinger RW, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to Develop Guidelines for the Management of Patients With Atrial Fibrillation); North American Society of Pacing and Electrophysiology. ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation: executive summary. Circulation. 2001; 104(17):2118-2150.
2. Jarand J, Lee A, Leigh R. Amiodaronoma: an unusual form of amiodarone-induced pulmonary toxicity. CMAJ. 2007;176(10):1411-1413.
3. Connolly S. Evidence-based analysis of amiodarone efficacy and safety. Circulation. 1999;100:2025-2034.
4. Amiodarone Trials Meta-Analysis Investigators. Effect of prophylactic amiodarone on mortality after acute myocardial infarction and in congestive heart failure: meta-analysis of individual data from 6500 patients in randomised trials. Lancet. 1997;350(9089):1417-1424.
5. Pollak PT. Clinical organ toxicity of antiarrhythmic compounds: ocular and pulmonary manifestations. Am J Cardiol. 1999;84(9A):37R-45R.
6. Camus P, Martin W, Rosenow E. Amiodarone pulmonary toxicity. Clin Chest Med. 2004;25(1):65-75.
7. Rady MY, Ryan T, Starr NJ. Preoperative therapy with amiodarone and the incidence of acute organ dysfunction after cardiac surgery. Anesth Analg. 1997;85(3):489-497.
8. Canada A, Lesko L, Haffajee C, et al. Amiodarone for tachyarrhythmias: kinetics, and efficacy. Drug Intell Clin Pharm. 1983;17(2):100-104.
9. Ernawati DK, Stafford L, Hughes JD. Amiodarone-induced pulmonary toxicity. Br J Clin Pharmacol. 2008;66(1):82-87.
10. Liu FL, Cohen RD, Downar E, et al. Amiodarone pulmonary toxicity: functional and ultrastructural evaluation. Thorax. 1986;41(2):100-105.
11. Chan E, King TE. Amiodarone pulmonary toxicity. UpToDate. 2013. www.uptodate.com/contents/amiodarone-pulmonary-toxicity. Accessed January 17, 2014.
12. Wolkove N, Baltzan M. Amiodarone pulmonary toxicity. Can Respir J. 2009;16(2):43-48.
13. Jackevicius CA, Tom A, Essebag V, et al. Population-level incidence and risk factors for pulmonary toxicity associated with amiodarone. Am J Cardiol. 2011;108:705-710.
14. Jessurun G, Crijns H. Amiodarone pulmonary toxicity [editorial]. BMJ. 1997;314(7081):619-620.
15. Nacca N, Castigliano B, Yuhico L, et al. Severe amiodarone induced pulmonary toxicity. J Thorac Dis. 2012;4(6):667-670.
16. Arnon R, Raz I, Chajek-Shaul T, et al. Amiodarone pulmonary toxicity presenting as a solitary lung mass. Chest. 1988;93(2):425-427.
17. Yamada Y, Shiga T, Matsuda N, et al. Incidence and predictors of pulmonary toxicity in Japanese patients receiving low-dose amiodarone. Circ J. 2007;71(10):1610-1616.
18. Coudert B, Bailly F, Lombard JN, et al. Amiodarone pneumonitis: bronchoalveolar lavage findings in 15 patients and review of the literature. Chest. 1992;102(4):1005-1012.
19. Vernhet H, Bousquet C, Durand G, et al. Reversible amiodarone-induced lung disease: HRCT findings. Eur Radiol. 2001;11(9):1697-1703.
20. Olson LK, Forrest JV, Friedman PJ, et al. Pneumonitis after amiodarone therapy. Radiology. 1984;150(2):327-330.
2014 Update on Fertility
These experts discuss three recent American Society for Reproductive Medicine Committee Opinions. The first is on the optimal use of the most widely prescribed medication for fertility, clomiphene citrate. The second highlights the currently recommended vaccinations for women who are of reproductive age. And the third is on the current evidence for prevention of postsurgical adhesions, which have the potential to cause infertility. Their discussions could affect how you approach your infertile patients.
SAFE, EFFECTIVE USE OF CLOMIPHENE
Practice Committee of the American Society for Reproductive Medicine. Use of clomiphene citrate in infertile women: A committee opinion. Fertil Steril. 2013;100(2):341–348.
Clomiphene citrate (CC) is the fertility medication most commonly used by gynecologists. However, important principles in its use often are not followed, resulting in suboptimal patient care. The American Society for Reproductive Medicine published a recent Committee Opinion on CC’s indications, use, and alternative treatments. We summarize the essential aspects of CC use.
Who should be treated?
CC can be used to treat both anovulation/oligo-ovulation and unexplained infertility, but it is not effective in hypothalamic amenorrhea or hypergonadotropic hypogonadism (usually premature ovarian insufficiency). Anovulation/oligo-ovulation may be due to polycystic ovary syndrome (PCOS), obesity, hypothalamic dysfunction related to eating disorders, weight, exercise, stress, hyperprolactinemia, pituitary tumors, or thyroid disease. The exact cause is often indeterminable, however.
Related Article: Polycystic ovary syndrome: Where we stand with diagnosis and treatment and where we're going Steven R. Lindheim, MD, MMM, and Leah Whigham, PhD (First of a 4-part series, September 2012)
There is no evidence CC is effective treatment for “luteal phase defect.” Unexplained infertility also can be treated with CC with intrauterine insemination (IUI).1
Pretreatment evaluation
Diagnosis of ovulatory dysfunction is usually made by menstrual history alone (normal menses, ≥24 and ≥35 days). Testing with luteal phase serum progesterone or serial transvaginal ultrasound generally is unnecessary.
Use the history, physical examination, and other testing, as necessary, to rule out other endocrinopathies, including diabetes mellitus (screening for impaired glucose tolerance), thyroid disorders (measurement of thyroid-stimulating hormone, or TSH), hyperprolactinemia (prolactin assessment), congenital adrenal hyperplasia (measurement of 17-alpha hydroxyprogesterone acetate), and virilization (assessment of testosterone and dehydroepiandrosterone sulfate, or DHEA-S).
If disease-specific treatment does not result in normal ovulation, then CC can be used. Although it may be difficult for them, obese women should be encouraged to lose weight. In infertile couples with a normal menstrual cycle and no other identifiable infertility factors, if hysterosalpingogram and semen analysis are normal, treatment of their unexplained infertility with CC and IUI may be effective. Ovulation induction or ovarian stimulation has little benefit when severe male, uterine, or tubal factors are present.
Treatment regimens
CC is usually given 50 mg/day orally for 5 days starting on the second to fifth spontaneous or progestin-induced menstrual cycle day, with equivalent treatment outcomes regardless of start day 2, 3, 4, or 5. If the patient’s response to this dose is inadequate, treatment can be increased 50 mg/day in each subsequent cycle, to a maximum of 250 mg/day. However, the maximum FDA-approved dose is 100 mg/day, and only 20% of patients respond when given doses higher than this. Obese patients may respond at the higher doses.
The luteinizing hormone (LH) surge occurs 5 to 12 days after the last CC dose is taken. There is no benefit to giving human chorionic gonadotropin (hCG) if the patient has a spontaneous LH surge. The pregnancy rate might actually be reduced by 25% when hCG is given unnecessarily.2
In anovulatory/oligo-ovulatory women, there is no benefit of IUI over timed intercourse for achieving pregnancy. For unexplained infertility, however, CC with timed intercourse does not appear effective, but CC combined with IUI is effective.3 Timed intercourse should occur approximately every 2 days (1–3 days) starting about 3 to 4 days before expected ovulation.
Treatment should continue 3 to 4 months. Younger patients (<35 years) with a short duration of infertility (<2 years) who respond to CC can receive up to 6 months of treatment. Treatment beyond 6 months is not recommended.
Ovulation and pregnancy rates
Half of anovulatory/oligo-ovulatory women will ovulate with a 50-mg dose of CC and half of the remaining will ovulate with a 100-mg dose. Among women who ovulate with CC, cumulative pregnancy rates for 50 mg/day, 100 mg/day, or 150 mg/day at 3 months are 50%, 45%, and 33%, respectively, and at 6 months are 62%, 66%, and 38%, respectively. In general, a 55% to 73% pregnancy rate can be expected.4 Increasing age, duration of infertility, and obesity are associated with lower pregnancy rates and treatment failure.
Alternative and adjunctive regimens
For patients who are not using progestin to induce menses and who have not responded with ovulation by day 14 to 21, longer courses of CC treatment (7 to 8 days) and a step-up protocol to the next highest CC dose are alternative regimens that may work in some cases.
Some anovulatory or oligo-ovulatory women with PCOS who do not respond to CC alone may respond to CC combined with metformin at 1,500 to 1,700 mg/day. Metformin combined with diet and exercise for weight loss is recommended. Metformin is associated with gastrointestinal side effects and rare hepatic toxicity or lactic acidosis; therefore, liver and renal functions should be assessed prior to treatment and monitored afterward.
Women with DHEA-S serum concentrations of 200 µg/dL or greater, and even some women with normal DHEA-S levels, may be more responsive to CC and achieve higher pregnancy rates when given dexamethasone 0.5 mg/daily on cycle days 3 to 12. Glucocorticoids have significant side effects and should be discontinued if treatment is unsuccessful or when pregnancy occurs.
Related Article: Clomiphene failure? Try adding dexamethasone to your clomiphene infertility regimen Robert L. Barbieri, MD (Editorial, May 2012)
Some CC-resistant anovulatory women and women with unexplained infertility may benefit from a trial of sequential CC/gonadotropin treatment consisting of standard CC treatment followed by human menopausal gonadotropins (hMG) or follicle-stimulating hormone (FSH) 75 to 150 IU/day for 3 days. Some, but not all, studies show pregnancy rates in these patients equivalent to those undergoing gonadotropin treatment alone (at a reduced cost). There are no studies directly comparing the treatment regimens, however, and risks of multiple pregnancy might be increased for patients taking both CC and gonadotropin, so this treatment should only be provided by clinicians with requisite training and experience.
Other alternatives to CC therapy in CC-resistant patients include aromatase inhibitors, tamoxifen, insulin-sensitizing agents, ovarian drilling, gonadotropins, and in vitro fertilization.
Monitoring of CC cycles
Objective evidence of ovulation is key to successful treatment. Ovulation predictor kits are more than 90% successful, if used properly, in identifying the LH surge 5 to 12 days after CC is finished (usually around cycle day 16 or 17). Ovulation occurs about one-half day to 2 days after the LH surge. Serum progesterone is the most certain test of prior ovulation (other than pregnancy) but cannot predict time of ovulation. Serial ultrasound shows the size and number of follicles and presumptive ovulation with follicle collapse, as well as echogenic corpus luteum and cul de sac fluid, but it is expensive and often not cost-effective.
It is prudent to postpone further treatment if the patient has large ovaries or a cyst, but routine baseline ultrasound monitoring is no longer considered necessary. However, regular contact with the patient should be maintained to review response to treatment and to ensure that any additional or alternative treatments are not delayed.
Side effects of CC treatment
Mood swings, visual disturbances, breast tenderness, pelvic discomfort, and nausea are reported in less than 10% of patients. Mild ovarian hyperstimulation syndrome (OHSS) is not uncommon, but severe OHSS is rare.
Related Article: Avoiding ovarian hyperstimulation syndrome G. David Adamson, MD (Audiocast, February 2011)
The major risk to CC treatment is twin (8% risk) and triplet (0.5% risk) pregnancies. There is no evidence of increased risk of congenital anomalies, miscarriage, or ovarian cancer.1,5,6
WHAT THIS EVIDENCE MEANS FOR PRACTICE
All gynecologists should be able to diagnose and treat infertility with clomiphene. It is effective for many patients with anovulatory/oligo-ovulatory infertility, and also for unexplained infertility when combined with IUI. Careful evaluation of fertility and endocrinologic status is necessary before treatment, as is monitoring during treatment. Although this treatment may appear to be simple, there are many important principles that need to be followed if treatment is to be effective and safe, and if the patient is to receive quality infertility care. Treatment is safe, (the major risk is multiple pregnancy) but should not be continued for more than 3 to 6 months.
STRIVE FOR PREPREGNANCY VACCINATION
Practice Committee of American Society for Reproductive Medicine. Vaccination guidelines for female infertility patients: A committee opinion. Fertil Steril. 2013;99(2):337–339.
Patients presenting for fertility treatment may have incomplete or unknown immunization status. Encounters with women who desire conception offer an opportunity for providers to optimize their patients’ health prior to pregnancy. Vaccination before or, when appropriate, during pregnancy protects women from preventable disease, decreases the risk for vertical fetal transmission, and enables the passage of maternal immunoglobulins to the fetus, conferring passive immunity to the newborn.
National standards for vaccination have been established by the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC). This yearly updated vaccination schedule is available at the CDC’s Web site (http://www.cdc.gov/vaccines/schedules/hcp/adult.html).7 Ideally, a woman’s immunization status should be evaluated and made complete prior to pregnancy. Some vaccines are safe and appropriate for administration during pregnancy, provided the benefits clearly outweigh the risks. The recommended vaccines during pregnancy include inactivated influenza (seasonal and H1N1) and the combined tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap).
Related Article: CDC urges flu vaccination for all, especially pregnant women (News for Your Practice, October 2013)
Many physicians avoid giving vaccinations during pregnancy because of the concern that a spontaneous abortion or congenital anomaly might be incorrectly attributed to vaccine administration, but few vaccines are contradicted during pregnancy. Those that are contraindicated are those containing live virus, including measles, mumps, and rubella (MMR); varicella; and herpes zoster. Concerns also have been raised regarding the safety of administering influenza vaccines containing the mercury-based preservative thimerosol. However, no scientific evidence has conclusively linked adverse effects on offspring with thimerosol-containing vaccines administered during pregnancy.
Immunizations recommended for women of reproductive age
Measles, mumps, rubella (MMR). This vaccine is recommended for all women lacking confirmed immunity to rubella. The vaccine contains live, attenuated virus and is given as a single dose. Women should avoid pregnancy for 1 month after vaccination.
Varicella. This vaccine is for all women lacking confirmed immunity to varicella. It also contains a live, attenuated virus. It is administered in two doses, 1 month apart, and women should avoid pregnancy for 1 month after vaccination.
Influenza. The flu vaccine is recommended annually for individuals 6 months of age and older. The injectable vaccine contains inactivated virus and may be administered during pregnancy—at any time but optimally in October or November because the flu season occurs January through March. (The intranasal influenza vaccine contains live, attenuated virus and should be avoided in pregnancy.) Either method is administered as a single dose.
Thimerosal is a mercury-based preservative used in vaccines, including the influenza vaccine, and is appropriate for use in pregnant women; studies have not shown an association between vaccines containing thimerosal and adverse effects in pregnant women or their offspring.
Tetanus-diptheria-pertussis (Tdap) and tetanus-diphtheria (Td). Tdap or Td is recommended for adults aged 19 to 64 years who have or anticipate having close contact with an infant less than 12 months of age. Due to the recent increase in pertussis infection, Tdap should be given to all women who have not previously received the vaccine and who are pregnant or might become pregnant. It can be given anytime during pregnancy, but optimal administration is during the third trimester or late second trimester (after 20 weeks’ gestation) to confer the greatest amount of fetal protection.
If the vaccine is not being administered during pregnancy, it should be given in the immediate postpartum period to ensure pertussis immunity and to reduce transmission to the newborn. Tdap is administered as a single dose of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis.
Non-routine vaccines include pneumococcus, hepatitis A, hepatitis B, and meningococcus (TABLE). These vaccines should be administered as indicated in high-risk patients.
Health-care providers caring for women with infertility are urged to assess patients’ immunization status prior to attempting pregnancy, to counsel patients about the importance of protecting them and their potential offspring from preventable disease, and to facilitate vaccination prior to conception attempts.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Vaccination is a very important aspect of pre-pregnancy care but is especially important for infertile women who desire pregnancy. Planning of infertility treatment should include assessment of the patient’s vaccination status and completion of appropriate vaccinations before infertility treatment is initiated.
DO CURRENT OPTIONS EFFECTIVELY PREVENT POSTSURGICAL ADHESIONS?
Practice Committee of American Society for Reproductive Medicine in collaboration with Society of Reproductive Surgeons. Pathogenesis, consequences, and control of peritoneal adhesions in gynecologic surgery: A committee opinion. Fertil Steril. 2013;99(6):1550–1555.
Postoperative adhesions are a natural consequence of surgery and a major problem in gynecology. They may cause postsurgical infertility, abdominal/pelvic pain, or bowel obstruction as well as complicate subsequent surgeries by increasing operative times and the risk of bowel injury. The American Society for Reproductive Medicine (ASRM) and the Society of Reproductive Surgeons (SRS) recently evaluated the epidemiology, pathogenesis, and clinical consequences of adhesion formation and the evidence behind strategies for reducing adhesion formation.
In their joint Committee Opinion, they noted that open and laparoscopic approaches to surgery carry comparable levels of risk for adhesion-related hospital readmission. Ovarian surgery has the highest risk for adhesion-related readmission, at 7.5 per 100 initial operations, and the incidence of small bowel obstruction after hysterectomy was found to be 1.6 per 100 procedures. Adhesion-related US health-care costs are estimated at approximately $1 billion annually.
The Societies noted that more severe adnexal adhesions are associated with lower pregnancy rates, and treatment of adnexal adhesions appears to improve pregnancy rates. Investigators found adhesions to cause about three-quarters of postoperative small bowel obstructions; however, the relationship between adhesions and pelvic pain remains unclear. It is thought that adhesions may cause visceral pain by impairing organ mobility, but there is no relationship between the extent of adhesions and the severity of pain. It appears that only dense adhesions involving the bowel are associated with chronic pelvic pain. Predicting the outcome of lysis of adnexal or bowel adhesions is difficult.
Reduction of adhesion formation
Theoretically, adhesions may be reduced by minimizing peritoneal injury during surgery, avoiding intraoperative reactive foreign bodies, reducing local inflammatory response, inhibiting the coagulation cascade and promoting fibrinolysis, or by placing barriers between damaged tissues.
Related Article: Update on Fertility G. David Adamson, MD (February 2008)
Careful surgical technique includes gentle tissue handling, meticulous hemostasis, excision of necrotic tissue, minimizing ischemia and desiccation, using fine and nonreactive suture, and preventing foreign-body reaction and infection, all “microsurgical principles.”
ASRM and SRS reported that the surgical approach (laparoscopy vs laparotomy) is much less important than the extent of tissue injury. However, laparoscopy may result in less tissue and organ handling and trauma, avoid contamination with foreign bodies, enable more precise tissue handling, and result in less postoperative infection. The pneumoperitoneum has a tamponade effect that facilitates hemostasis during laparoscopy, but the process also can be associated with peritoneal desiccation and reduced temperatures that can increase injury.
Laparoscopic myomectomy was found to have a 70% risk of postoperative adhesions, compared with a 90% risk after laparotomy. It is unclear whether peritoneal closure at laparotomy reduces or increases adhesions, but parietal peritoneal closure at primary cesarean delivery results in fewer dense and filmy adhesions.
Related Article: How to avoid intestinal and urinary tract injuries during gynecologic laparoscopy Michael Baggish, MD (Second of a 2-part series on laparoscopic complications, October 2012)
Adjuncts to surgical technique
SRM and SRS reported on three adjuncts to surgical technique that have been proposed to reduce the risk of postoperative adhesions: anti-inflammatory agents, peritoneal instillates, and adhesion barriers.
Dexamethasone, promethazine, and other local and systemic anti-inflammatory drugs and adhesion-reducing substances have not been found effective for reducing postoperative adhesions.
Peritoneal instillates—which create “hydroflotation” and include antibiotic solutions, 32% dextran 70, and crystalloid solutions such as normal saline and Ringer’s lactate with or without heparin or corticosteroids—have not been found effective.8 Icodextrin 4% (Adept Adhesion Reduction Solution, Baxter Healthcare) is FDA approved as an adjunct to good surgical technique for the reduction of postoperative adhesions in patients undergoing gynecologic laparoscopic adhesiolysis. However, a systematic review concluded that there is insufficient evidence for its use as an adhesion-preventing agent.8
Adhesion barriers may help reduce postoperative adhesions but cannot compensate for poor surgical technique. Although the bioresorbable membrane sodium hyaluronic acid and carboxymethyl cellulose (Seprafilm, Genzyme Corp) is FDA-approved, there is limited evidence that it prevents adhesions after myomectomy.9 Because it fragments easily, it is mostly used at laparotomy.
Oxidized regenerated cellulose (Interceed, Ethicon Women’s Health and Urology) is an FDA-approved absorbable adhesion barrier for use at laparotomy that requires no suturing and has been shown to reduce the incidence and extent of new and recurrent adhesions at both laparoscopy and laparotomy by 40% to 50%, although there is little evidence that this improves fertility.9 Complete hemostasis must be achieved to use Interceed, and the addition of heparin confers no benefit.
Another product is expanded polytetrafluoroethylene (ePTFE, Gore-Tex Surgical Membrane, WL Gore and Associates), a nonabsorbable adhesion barrier produced in thin sheets and approved by the FDA for peritoneal repair. ePTFE must be sutured to tissue and helps prevent adhesion formation and reformation regardless of the type of injury or whether complete hemostasis has been achieved. In a small trial, it decreased postmyomectomy adhesions.10 ePTFE also was more effective than oxidized regenerated cellulose in preventing adhesions after adnexal surgery.11 Its use has been limited by the need for suturing and later reoperation for removal, although it probably does not have to be removed if it will not interfere with normal organ function since it has been used as a pericardial graft for many years.12
Hyaluronic acid (HA) solution (Sepracoat, Genzyme) is a natural bioabsorbable component of the extracellular matrix. Women undergoing laparotomy have fewer new adhesions with HA solution, but it is not approved for use in the United States.13 Polyethylene glycol (PEG; SprayGel, Confluent Surgical) was effective in early clinical trials but is not FDA-approved.12 Fibrin sealant (Tisseel VH, Baxter Healthcare) has been reported to decrease the formation of adhesions after salpingostomy, salpingolysis, and ovariolysis. Because it is a biologic product derived from human blood donors, it poses a risk for transmission of infectious agents. It is FDA-approved for use in cardiothoracic surgery, splenic injuries, and colostomy closure for hemostasis.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Adhesions are the most common complication following gynecologic surgery, and they pose potential longstanding consequences to patients. There is no evidence that anti-inflammatory agents reduce postoperative adhesions and insufficient evidence to recommend peritoneal instillates. FDA-approved surgical barriers reduce postoperative adhesions but there is not substantial evidence that their use improves fertility, decreases pain, or reduces the incidence of postoperative bowel obstruction. All gynecologists need to understand the importance of using microsurgical principles rather than relying on adhesion barriers to reduce postoperative adhesions.
WE WANT TO HEAR FROM YOU!
Drop us a line and let us know what you think about current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: [email protected]
- Practice Committee of the American Society for Reproductive Medicine. Use of clomiphene citrate in infertile women: A committee opinion. Fertil Steril. 2013;100(2):341–348.
- George K, Nair R, Tharyan P. Ovulation triggers in anovulatory women undergoing ovulation induction. Cochrane Database Syst Rev. 2008;(3):CD006900.
- Deaton JL, Gibson M, Blackmer KM, Nakajima ST, Badger GJ, Brumsted JR. A randomized, controlled trial of clomiphene citrate and intrauterine insemination in couples with unexplained infertility or surgically corrected endometriosis. Fertil Steril. 1990;54(6):1083–1088.
- Thessaloniki ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Consensus on infertility treatment related to polycystic ovary syndrome. Fertil Steril. 2008;89(3):505–522.
- Reefhuis J, Honein MA, Schieve LA, Rasmussen SA; National Birth Defects Prevention Study. Use of clomiphene citrate and birth defects, National Birth Defects Prevention Study, 1997-2005. Hum Reprod. 2011;26(2):451–457.
- Silva Idos S, Wark PA, McCormack VA, et al. Ovulation-stimulation drugs and cancer risks: a long-term follow-up of a British cohort. Br J Cancer. 2009;100(11):1824–1831.
- Adult immunization schedules. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/vaccines/schedules/hcp/adult.html. Updated October 19, 2013. Accessed January 16, 2014.
- Metwally M, Watson A, Lilford R, Vandekerckhove P. Fluid and pharmacological agents for adhesion prevention after gynaecological surgery. Cochrane Database Syst Rev. 2006;(2):CD001298.
- Farquhar C, Vandekerckhove P, Watson A, Vail A, Wiseman D. Barrier agents for preventing adhesions after surgery for subfertility. Cochrane Database Syst Rev. 2000;(2):CD000475.
- The Myomectomy Adhesion Multicenter Study Group. An expanded polytetrafluoroethylene barrier (Gore-Tex Surgical Membrane) reduces post-myomectomy adhesion formation. Fertil Steril. 1995;63(3):491–493.
- Haney AF, Hesla J, Hurst BS, et al. Expanded polytetrafluoroethylene (Gore-Tex Surgical Membrane) is superior to oxidized regenerated cellulose (Interceed TC7+) in preventing adhesions. Fertil Steril. 1995;63(5):1021–1026.
- Alejandro G, Flores RM. Surgical management of tumors invading the superior vena cava. Ann Thorac Surg 2008;85(6):2144−2146.
- Diamond MP; The Sepracoat Adhesion Study Group. Reduction of de novo postsurgical adhesions by intraoperative precoating with Sepracoat (HAL-C) solution: A prospective, randomized blinded, placebo-controlled multicenter study. Fertil Steril. 1998;69(6):1067–1074.
These experts discuss three recent American Society for Reproductive Medicine Committee Opinions. The first is on the optimal use of the most widely prescribed medication for fertility, clomiphene citrate. The second highlights the currently recommended vaccinations for women who are of reproductive age. And the third is on the current evidence for prevention of postsurgical adhesions, which have the potential to cause infertility. Their discussions could affect how you approach your infertile patients.
SAFE, EFFECTIVE USE OF CLOMIPHENE
Practice Committee of the American Society for Reproductive Medicine. Use of clomiphene citrate in infertile women: A committee opinion. Fertil Steril. 2013;100(2):341–348.
Clomiphene citrate (CC) is the fertility medication most commonly used by gynecologists. However, important principles in its use often are not followed, resulting in suboptimal patient care. The American Society for Reproductive Medicine published a recent Committee Opinion on CC’s indications, use, and alternative treatments. We summarize the essential aspects of CC use.
Who should be treated?
CC can be used to treat both anovulation/oligo-ovulation and unexplained infertility, but it is not effective in hypothalamic amenorrhea or hypergonadotropic hypogonadism (usually premature ovarian insufficiency). Anovulation/oligo-ovulation may be due to polycystic ovary syndrome (PCOS), obesity, hypothalamic dysfunction related to eating disorders, weight, exercise, stress, hyperprolactinemia, pituitary tumors, or thyroid disease. The exact cause is often indeterminable, however.
Related Article: Polycystic ovary syndrome: Where we stand with diagnosis and treatment and where we're going Steven R. Lindheim, MD, MMM, and Leah Whigham, PhD (First of a 4-part series, September 2012)
There is no evidence CC is effective treatment for “luteal phase defect.” Unexplained infertility also can be treated with CC with intrauterine insemination (IUI).1
Pretreatment evaluation
Diagnosis of ovulatory dysfunction is usually made by menstrual history alone (normal menses, ≥24 and ≥35 days). Testing with luteal phase serum progesterone or serial transvaginal ultrasound generally is unnecessary.
Use the history, physical examination, and other testing, as necessary, to rule out other endocrinopathies, including diabetes mellitus (screening for impaired glucose tolerance), thyroid disorders (measurement of thyroid-stimulating hormone, or TSH), hyperprolactinemia (prolactin assessment), congenital adrenal hyperplasia (measurement of 17-alpha hydroxyprogesterone acetate), and virilization (assessment of testosterone and dehydroepiandrosterone sulfate, or DHEA-S).
If disease-specific treatment does not result in normal ovulation, then CC can be used. Although it may be difficult for them, obese women should be encouraged to lose weight. In infertile couples with a normal menstrual cycle and no other identifiable infertility factors, if hysterosalpingogram and semen analysis are normal, treatment of their unexplained infertility with CC and IUI may be effective. Ovulation induction or ovarian stimulation has little benefit when severe male, uterine, or tubal factors are present.
Treatment regimens
CC is usually given 50 mg/day orally for 5 days starting on the second to fifth spontaneous or progestin-induced menstrual cycle day, with equivalent treatment outcomes regardless of start day 2, 3, 4, or 5. If the patient’s response to this dose is inadequate, treatment can be increased 50 mg/day in each subsequent cycle, to a maximum of 250 mg/day. However, the maximum FDA-approved dose is 100 mg/day, and only 20% of patients respond when given doses higher than this. Obese patients may respond at the higher doses.
The luteinizing hormone (LH) surge occurs 5 to 12 days after the last CC dose is taken. There is no benefit to giving human chorionic gonadotropin (hCG) if the patient has a spontaneous LH surge. The pregnancy rate might actually be reduced by 25% when hCG is given unnecessarily.2
In anovulatory/oligo-ovulatory women, there is no benefit of IUI over timed intercourse for achieving pregnancy. For unexplained infertility, however, CC with timed intercourse does not appear effective, but CC combined with IUI is effective.3 Timed intercourse should occur approximately every 2 days (1–3 days) starting about 3 to 4 days before expected ovulation.
Treatment should continue 3 to 4 months. Younger patients (<35 years) with a short duration of infertility (<2 years) who respond to CC can receive up to 6 months of treatment. Treatment beyond 6 months is not recommended.
Ovulation and pregnancy rates
Half of anovulatory/oligo-ovulatory women will ovulate with a 50-mg dose of CC and half of the remaining will ovulate with a 100-mg dose. Among women who ovulate with CC, cumulative pregnancy rates for 50 mg/day, 100 mg/day, or 150 mg/day at 3 months are 50%, 45%, and 33%, respectively, and at 6 months are 62%, 66%, and 38%, respectively. In general, a 55% to 73% pregnancy rate can be expected.4 Increasing age, duration of infertility, and obesity are associated with lower pregnancy rates and treatment failure.
Alternative and adjunctive regimens
For patients who are not using progestin to induce menses and who have not responded with ovulation by day 14 to 21, longer courses of CC treatment (7 to 8 days) and a step-up protocol to the next highest CC dose are alternative regimens that may work in some cases.
Some anovulatory or oligo-ovulatory women with PCOS who do not respond to CC alone may respond to CC combined with metformin at 1,500 to 1,700 mg/day. Metformin combined with diet and exercise for weight loss is recommended. Metformin is associated with gastrointestinal side effects and rare hepatic toxicity or lactic acidosis; therefore, liver and renal functions should be assessed prior to treatment and monitored afterward.
Women with DHEA-S serum concentrations of 200 µg/dL or greater, and even some women with normal DHEA-S levels, may be more responsive to CC and achieve higher pregnancy rates when given dexamethasone 0.5 mg/daily on cycle days 3 to 12. Glucocorticoids have significant side effects and should be discontinued if treatment is unsuccessful or when pregnancy occurs.
Related Article: Clomiphene failure? Try adding dexamethasone to your clomiphene infertility regimen Robert L. Barbieri, MD (Editorial, May 2012)
Some CC-resistant anovulatory women and women with unexplained infertility may benefit from a trial of sequential CC/gonadotropin treatment consisting of standard CC treatment followed by human menopausal gonadotropins (hMG) or follicle-stimulating hormone (FSH) 75 to 150 IU/day for 3 days. Some, but not all, studies show pregnancy rates in these patients equivalent to those undergoing gonadotropin treatment alone (at a reduced cost). There are no studies directly comparing the treatment regimens, however, and risks of multiple pregnancy might be increased for patients taking both CC and gonadotropin, so this treatment should only be provided by clinicians with requisite training and experience.
Other alternatives to CC therapy in CC-resistant patients include aromatase inhibitors, tamoxifen, insulin-sensitizing agents, ovarian drilling, gonadotropins, and in vitro fertilization.
Monitoring of CC cycles
Objective evidence of ovulation is key to successful treatment. Ovulation predictor kits are more than 90% successful, if used properly, in identifying the LH surge 5 to 12 days after CC is finished (usually around cycle day 16 or 17). Ovulation occurs about one-half day to 2 days after the LH surge. Serum progesterone is the most certain test of prior ovulation (other than pregnancy) but cannot predict time of ovulation. Serial ultrasound shows the size and number of follicles and presumptive ovulation with follicle collapse, as well as echogenic corpus luteum and cul de sac fluid, but it is expensive and often not cost-effective.
It is prudent to postpone further treatment if the patient has large ovaries or a cyst, but routine baseline ultrasound monitoring is no longer considered necessary. However, regular contact with the patient should be maintained to review response to treatment and to ensure that any additional or alternative treatments are not delayed.
Side effects of CC treatment
Mood swings, visual disturbances, breast tenderness, pelvic discomfort, and nausea are reported in less than 10% of patients. Mild ovarian hyperstimulation syndrome (OHSS) is not uncommon, but severe OHSS is rare.
Related Article: Avoiding ovarian hyperstimulation syndrome G. David Adamson, MD (Audiocast, February 2011)
The major risk to CC treatment is twin (8% risk) and triplet (0.5% risk) pregnancies. There is no evidence of increased risk of congenital anomalies, miscarriage, or ovarian cancer.1,5,6
WHAT THIS EVIDENCE MEANS FOR PRACTICE
All gynecologists should be able to diagnose and treat infertility with clomiphene. It is effective for many patients with anovulatory/oligo-ovulatory infertility, and also for unexplained infertility when combined with IUI. Careful evaluation of fertility and endocrinologic status is necessary before treatment, as is monitoring during treatment. Although this treatment may appear to be simple, there are many important principles that need to be followed if treatment is to be effective and safe, and if the patient is to receive quality infertility care. Treatment is safe, (the major risk is multiple pregnancy) but should not be continued for more than 3 to 6 months.
STRIVE FOR PREPREGNANCY VACCINATION
Practice Committee of American Society for Reproductive Medicine. Vaccination guidelines for female infertility patients: A committee opinion. Fertil Steril. 2013;99(2):337–339.
Patients presenting for fertility treatment may have incomplete or unknown immunization status. Encounters with women who desire conception offer an opportunity for providers to optimize their patients’ health prior to pregnancy. Vaccination before or, when appropriate, during pregnancy protects women from preventable disease, decreases the risk for vertical fetal transmission, and enables the passage of maternal immunoglobulins to the fetus, conferring passive immunity to the newborn.
National standards for vaccination have been established by the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC). This yearly updated vaccination schedule is available at the CDC’s Web site (http://www.cdc.gov/vaccines/schedules/hcp/adult.html).7 Ideally, a woman’s immunization status should be evaluated and made complete prior to pregnancy. Some vaccines are safe and appropriate for administration during pregnancy, provided the benefits clearly outweigh the risks. The recommended vaccines during pregnancy include inactivated influenza (seasonal and H1N1) and the combined tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap).
Related Article: CDC urges flu vaccination for all, especially pregnant women (News for Your Practice, October 2013)
Many physicians avoid giving vaccinations during pregnancy because of the concern that a spontaneous abortion or congenital anomaly might be incorrectly attributed to vaccine administration, but few vaccines are contradicted during pregnancy. Those that are contraindicated are those containing live virus, including measles, mumps, and rubella (MMR); varicella; and herpes zoster. Concerns also have been raised regarding the safety of administering influenza vaccines containing the mercury-based preservative thimerosol. However, no scientific evidence has conclusively linked adverse effects on offspring with thimerosol-containing vaccines administered during pregnancy.
Immunizations recommended for women of reproductive age
Measles, mumps, rubella (MMR). This vaccine is recommended for all women lacking confirmed immunity to rubella. The vaccine contains live, attenuated virus and is given as a single dose. Women should avoid pregnancy for 1 month after vaccination.
Varicella. This vaccine is for all women lacking confirmed immunity to varicella. It also contains a live, attenuated virus. It is administered in two doses, 1 month apart, and women should avoid pregnancy for 1 month after vaccination.
Influenza. The flu vaccine is recommended annually for individuals 6 months of age and older. The injectable vaccine contains inactivated virus and may be administered during pregnancy—at any time but optimally in October or November because the flu season occurs January through March. (The intranasal influenza vaccine contains live, attenuated virus and should be avoided in pregnancy.) Either method is administered as a single dose.
Thimerosal is a mercury-based preservative used in vaccines, including the influenza vaccine, and is appropriate for use in pregnant women; studies have not shown an association between vaccines containing thimerosal and adverse effects in pregnant women or their offspring.
Tetanus-diptheria-pertussis (Tdap) and tetanus-diphtheria (Td). Tdap or Td is recommended for adults aged 19 to 64 years who have or anticipate having close contact with an infant less than 12 months of age. Due to the recent increase in pertussis infection, Tdap should be given to all women who have not previously received the vaccine and who are pregnant or might become pregnant. It can be given anytime during pregnancy, but optimal administration is during the third trimester or late second trimester (after 20 weeks’ gestation) to confer the greatest amount of fetal protection.
If the vaccine is not being administered during pregnancy, it should be given in the immediate postpartum period to ensure pertussis immunity and to reduce transmission to the newborn. Tdap is administered as a single dose of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis.
Non-routine vaccines include pneumococcus, hepatitis A, hepatitis B, and meningococcus (TABLE). These vaccines should be administered as indicated in high-risk patients.
Health-care providers caring for women with infertility are urged to assess patients’ immunization status prior to attempting pregnancy, to counsel patients about the importance of protecting them and their potential offspring from preventable disease, and to facilitate vaccination prior to conception attempts.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Vaccination is a very important aspect of pre-pregnancy care but is especially important for infertile women who desire pregnancy. Planning of infertility treatment should include assessment of the patient’s vaccination status and completion of appropriate vaccinations before infertility treatment is initiated.
DO CURRENT OPTIONS EFFECTIVELY PREVENT POSTSURGICAL ADHESIONS?
Practice Committee of American Society for Reproductive Medicine in collaboration with Society of Reproductive Surgeons. Pathogenesis, consequences, and control of peritoneal adhesions in gynecologic surgery: A committee opinion. Fertil Steril. 2013;99(6):1550–1555.
Postoperative adhesions are a natural consequence of surgery and a major problem in gynecology. They may cause postsurgical infertility, abdominal/pelvic pain, or bowel obstruction as well as complicate subsequent surgeries by increasing operative times and the risk of bowel injury. The American Society for Reproductive Medicine (ASRM) and the Society of Reproductive Surgeons (SRS) recently evaluated the epidemiology, pathogenesis, and clinical consequences of adhesion formation and the evidence behind strategies for reducing adhesion formation.
In their joint Committee Opinion, they noted that open and laparoscopic approaches to surgery carry comparable levels of risk for adhesion-related hospital readmission. Ovarian surgery has the highest risk for adhesion-related readmission, at 7.5 per 100 initial operations, and the incidence of small bowel obstruction after hysterectomy was found to be 1.6 per 100 procedures. Adhesion-related US health-care costs are estimated at approximately $1 billion annually.
The Societies noted that more severe adnexal adhesions are associated with lower pregnancy rates, and treatment of adnexal adhesions appears to improve pregnancy rates. Investigators found adhesions to cause about three-quarters of postoperative small bowel obstructions; however, the relationship between adhesions and pelvic pain remains unclear. It is thought that adhesions may cause visceral pain by impairing organ mobility, but there is no relationship between the extent of adhesions and the severity of pain. It appears that only dense adhesions involving the bowel are associated with chronic pelvic pain. Predicting the outcome of lysis of adnexal or bowel adhesions is difficult.
Reduction of adhesion formation
Theoretically, adhesions may be reduced by minimizing peritoneal injury during surgery, avoiding intraoperative reactive foreign bodies, reducing local inflammatory response, inhibiting the coagulation cascade and promoting fibrinolysis, or by placing barriers between damaged tissues.
Related Article: Update on Fertility G. David Adamson, MD (February 2008)
Careful surgical technique includes gentle tissue handling, meticulous hemostasis, excision of necrotic tissue, minimizing ischemia and desiccation, using fine and nonreactive suture, and preventing foreign-body reaction and infection, all “microsurgical principles.”
ASRM and SRS reported that the surgical approach (laparoscopy vs laparotomy) is much less important than the extent of tissue injury. However, laparoscopy may result in less tissue and organ handling and trauma, avoid contamination with foreign bodies, enable more precise tissue handling, and result in less postoperative infection. The pneumoperitoneum has a tamponade effect that facilitates hemostasis during laparoscopy, but the process also can be associated with peritoneal desiccation and reduced temperatures that can increase injury.
Laparoscopic myomectomy was found to have a 70% risk of postoperative adhesions, compared with a 90% risk after laparotomy. It is unclear whether peritoneal closure at laparotomy reduces or increases adhesions, but parietal peritoneal closure at primary cesarean delivery results in fewer dense and filmy adhesions.
Related Article: How to avoid intestinal and urinary tract injuries during gynecologic laparoscopy Michael Baggish, MD (Second of a 2-part series on laparoscopic complications, October 2012)
Adjuncts to surgical technique
SRM and SRS reported on three adjuncts to surgical technique that have been proposed to reduce the risk of postoperative adhesions: anti-inflammatory agents, peritoneal instillates, and adhesion barriers.
Dexamethasone, promethazine, and other local and systemic anti-inflammatory drugs and adhesion-reducing substances have not been found effective for reducing postoperative adhesions.
Peritoneal instillates—which create “hydroflotation” and include antibiotic solutions, 32% dextran 70, and crystalloid solutions such as normal saline and Ringer’s lactate with or without heparin or corticosteroids—have not been found effective.8 Icodextrin 4% (Adept Adhesion Reduction Solution, Baxter Healthcare) is FDA approved as an adjunct to good surgical technique for the reduction of postoperative adhesions in patients undergoing gynecologic laparoscopic adhesiolysis. However, a systematic review concluded that there is insufficient evidence for its use as an adhesion-preventing agent.8
Adhesion barriers may help reduce postoperative adhesions but cannot compensate for poor surgical technique. Although the bioresorbable membrane sodium hyaluronic acid and carboxymethyl cellulose (Seprafilm, Genzyme Corp) is FDA-approved, there is limited evidence that it prevents adhesions after myomectomy.9 Because it fragments easily, it is mostly used at laparotomy.
Oxidized regenerated cellulose (Interceed, Ethicon Women’s Health and Urology) is an FDA-approved absorbable adhesion barrier for use at laparotomy that requires no suturing and has been shown to reduce the incidence and extent of new and recurrent adhesions at both laparoscopy and laparotomy by 40% to 50%, although there is little evidence that this improves fertility.9 Complete hemostasis must be achieved to use Interceed, and the addition of heparin confers no benefit.
Another product is expanded polytetrafluoroethylene (ePTFE, Gore-Tex Surgical Membrane, WL Gore and Associates), a nonabsorbable adhesion barrier produced in thin sheets and approved by the FDA for peritoneal repair. ePTFE must be sutured to tissue and helps prevent adhesion formation and reformation regardless of the type of injury or whether complete hemostasis has been achieved. In a small trial, it decreased postmyomectomy adhesions.10 ePTFE also was more effective than oxidized regenerated cellulose in preventing adhesions after adnexal surgery.11 Its use has been limited by the need for suturing and later reoperation for removal, although it probably does not have to be removed if it will not interfere with normal organ function since it has been used as a pericardial graft for many years.12
Hyaluronic acid (HA) solution (Sepracoat, Genzyme) is a natural bioabsorbable component of the extracellular matrix. Women undergoing laparotomy have fewer new adhesions with HA solution, but it is not approved for use in the United States.13 Polyethylene glycol (PEG; SprayGel, Confluent Surgical) was effective in early clinical trials but is not FDA-approved.12 Fibrin sealant (Tisseel VH, Baxter Healthcare) has been reported to decrease the formation of adhesions after salpingostomy, salpingolysis, and ovariolysis. Because it is a biologic product derived from human blood donors, it poses a risk for transmission of infectious agents. It is FDA-approved for use in cardiothoracic surgery, splenic injuries, and colostomy closure for hemostasis.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Adhesions are the most common complication following gynecologic surgery, and they pose potential longstanding consequences to patients. There is no evidence that anti-inflammatory agents reduce postoperative adhesions and insufficient evidence to recommend peritoneal instillates. FDA-approved surgical barriers reduce postoperative adhesions but there is not substantial evidence that their use improves fertility, decreases pain, or reduces the incidence of postoperative bowel obstruction. All gynecologists need to understand the importance of using microsurgical principles rather than relying on adhesion barriers to reduce postoperative adhesions.
WE WANT TO HEAR FROM YOU!
Drop us a line and let us know what you think about current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: [email protected]
These experts discuss three recent American Society for Reproductive Medicine Committee Opinions. The first is on the optimal use of the most widely prescribed medication for fertility, clomiphene citrate. The second highlights the currently recommended vaccinations for women who are of reproductive age. And the third is on the current evidence for prevention of postsurgical adhesions, which have the potential to cause infertility. Their discussions could affect how you approach your infertile patients.
SAFE, EFFECTIVE USE OF CLOMIPHENE
Practice Committee of the American Society for Reproductive Medicine. Use of clomiphene citrate in infertile women: A committee opinion. Fertil Steril. 2013;100(2):341–348.
Clomiphene citrate (CC) is the fertility medication most commonly used by gynecologists. However, important principles in its use often are not followed, resulting in suboptimal patient care. The American Society for Reproductive Medicine published a recent Committee Opinion on CC’s indications, use, and alternative treatments. We summarize the essential aspects of CC use.
Who should be treated?
CC can be used to treat both anovulation/oligo-ovulation and unexplained infertility, but it is not effective in hypothalamic amenorrhea or hypergonadotropic hypogonadism (usually premature ovarian insufficiency). Anovulation/oligo-ovulation may be due to polycystic ovary syndrome (PCOS), obesity, hypothalamic dysfunction related to eating disorders, weight, exercise, stress, hyperprolactinemia, pituitary tumors, or thyroid disease. The exact cause is often indeterminable, however.
Related Article: Polycystic ovary syndrome: Where we stand with diagnosis and treatment and where we're going Steven R. Lindheim, MD, MMM, and Leah Whigham, PhD (First of a 4-part series, September 2012)
There is no evidence CC is effective treatment for “luteal phase defect.” Unexplained infertility also can be treated with CC with intrauterine insemination (IUI).1
Pretreatment evaluation
Diagnosis of ovulatory dysfunction is usually made by menstrual history alone (normal menses, ≥24 and ≥35 days). Testing with luteal phase serum progesterone or serial transvaginal ultrasound generally is unnecessary.
Use the history, physical examination, and other testing, as necessary, to rule out other endocrinopathies, including diabetes mellitus (screening for impaired glucose tolerance), thyroid disorders (measurement of thyroid-stimulating hormone, or TSH), hyperprolactinemia (prolactin assessment), congenital adrenal hyperplasia (measurement of 17-alpha hydroxyprogesterone acetate), and virilization (assessment of testosterone and dehydroepiandrosterone sulfate, or DHEA-S).
If disease-specific treatment does not result in normal ovulation, then CC can be used. Although it may be difficult for them, obese women should be encouraged to lose weight. In infertile couples with a normal menstrual cycle and no other identifiable infertility factors, if hysterosalpingogram and semen analysis are normal, treatment of their unexplained infertility with CC and IUI may be effective. Ovulation induction or ovarian stimulation has little benefit when severe male, uterine, or tubal factors are present.
Treatment regimens
CC is usually given 50 mg/day orally for 5 days starting on the second to fifth spontaneous or progestin-induced menstrual cycle day, with equivalent treatment outcomes regardless of start day 2, 3, 4, or 5. If the patient’s response to this dose is inadequate, treatment can be increased 50 mg/day in each subsequent cycle, to a maximum of 250 mg/day. However, the maximum FDA-approved dose is 100 mg/day, and only 20% of patients respond when given doses higher than this. Obese patients may respond at the higher doses.
The luteinizing hormone (LH) surge occurs 5 to 12 days after the last CC dose is taken. There is no benefit to giving human chorionic gonadotropin (hCG) if the patient has a spontaneous LH surge. The pregnancy rate might actually be reduced by 25% when hCG is given unnecessarily.2
In anovulatory/oligo-ovulatory women, there is no benefit of IUI over timed intercourse for achieving pregnancy. For unexplained infertility, however, CC with timed intercourse does not appear effective, but CC combined with IUI is effective.3 Timed intercourse should occur approximately every 2 days (1–3 days) starting about 3 to 4 days before expected ovulation.
Treatment should continue 3 to 4 months. Younger patients (<35 years) with a short duration of infertility (<2 years) who respond to CC can receive up to 6 months of treatment. Treatment beyond 6 months is not recommended.
Ovulation and pregnancy rates
Half of anovulatory/oligo-ovulatory women will ovulate with a 50-mg dose of CC and half of the remaining will ovulate with a 100-mg dose. Among women who ovulate with CC, cumulative pregnancy rates for 50 mg/day, 100 mg/day, or 150 mg/day at 3 months are 50%, 45%, and 33%, respectively, and at 6 months are 62%, 66%, and 38%, respectively. In general, a 55% to 73% pregnancy rate can be expected.4 Increasing age, duration of infertility, and obesity are associated with lower pregnancy rates and treatment failure.
Alternative and adjunctive regimens
For patients who are not using progestin to induce menses and who have not responded with ovulation by day 14 to 21, longer courses of CC treatment (7 to 8 days) and a step-up protocol to the next highest CC dose are alternative regimens that may work in some cases.
Some anovulatory or oligo-ovulatory women with PCOS who do not respond to CC alone may respond to CC combined with metformin at 1,500 to 1,700 mg/day. Metformin combined with diet and exercise for weight loss is recommended. Metformin is associated with gastrointestinal side effects and rare hepatic toxicity or lactic acidosis; therefore, liver and renal functions should be assessed prior to treatment and monitored afterward.
Women with DHEA-S serum concentrations of 200 µg/dL or greater, and even some women with normal DHEA-S levels, may be more responsive to CC and achieve higher pregnancy rates when given dexamethasone 0.5 mg/daily on cycle days 3 to 12. Glucocorticoids have significant side effects and should be discontinued if treatment is unsuccessful or when pregnancy occurs.
Related Article: Clomiphene failure? Try adding dexamethasone to your clomiphene infertility regimen Robert L. Barbieri, MD (Editorial, May 2012)
Some CC-resistant anovulatory women and women with unexplained infertility may benefit from a trial of sequential CC/gonadotropin treatment consisting of standard CC treatment followed by human menopausal gonadotropins (hMG) or follicle-stimulating hormone (FSH) 75 to 150 IU/day for 3 days. Some, but not all, studies show pregnancy rates in these patients equivalent to those undergoing gonadotropin treatment alone (at a reduced cost). There are no studies directly comparing the treatment regimens, however, and risks of multiple pregnancy might be increased for patients taking both CC and gonadotropin, so this treatment should only be provided by clinicians with requisite training and experience.
Other alternatives to CC therapy in CC-resistant patients include aromatase inhibitors, tamoxifen, insulin-sensitizing agents, ovarian drilling, gonadotropins, and in vitro fertilization.
Monitoring of CC cycles
Objective evidence of ovulation is key to successful treatment. Ovulation predictor kits are more than 90% successful, if used properly, in identifying the LH surge 5 to 12 days after CC is finished (usually around cycle day 16 or 17). Ovulation occurs about one-half day to 2 days after the LH surge. Serum progesterone is the most certain test of prior ovulation (other than pregnancy) but cannot predict time of ovulation. Serial ultrasound shows the size and number of follicles and presumptive ovulation with follicle collapse, as well as echogenic corpus luteum and cul de sac fluid, but it is expensive and often not cost-effective.
It is prudent to postpone further treatment if the patient has large ovaries or a cyst, but routine baseline ultrasound monitoring is no longer considered necessary. However, regular contact with the patient should be maintained to review response to treatment and to ensure that any additional or alternative treatments are not delayed.
Side effects of CC treatment
Mood swings, visual disturbances, breast tenderness, pelvic discomfort, and nausea are reported in less than 10% of patients. Mild ovarian hyperstimulation syndrome (OHSS) is not uncommon, but severe OHSS is rare.
Related Article: Avoiding ovarian hyperstimulation syndrome G. David Adamson, MD (Audiocast, February 2011)
The major risk to CC treatment is twin (8% risk) and triplet (0.5% risk) pregnancies. There is no evidence of increased risk of congenital anomalies, miscarriage, or ovarian cancer.1,5,6
WHAT THIS EVIDENCE MEANS FOR PRACTICE
All gynecologists should be able to diagnose and treat infertility with clomiphene. It is effective for many patients with anovulatory/oligo-ovulatory infertility, and also for unexplained infertility when combined with IUI. Careful evaluation of fertility and endocrinologic status is necessary before treatment, as is monitoring during treatment. Although this treatment may appear to be simple, there are many important principles that need to be followed if treatment is to be effective and safe, and if the patient is to receive quality infertility care. Treatment is safe, (the major risk is multiple pregnancy) but should not be continued for more than 3 to 6 months.
STRIVE FOR PREPREGNANCY VACCINATION
Practice Committee of American Society for Reproductive Medicine. Vaccination guidelines for female infertility patients: A committee opinion. Fertil Steril. 2013;99(2):337–339.
Patients presenting for fertility treatment may have incomplete or unknown immunization status. Encounters with women who desire conception offer an opportunity for providers to optimize their patients’ health prior to pregnancy. Vaccination before or, when appropriate, during pregnancy protects women from preventable disease, decreases the risk for vertical fetal transmission, and enables the passage of maternal immunoglobulins to the fetus, conferring passive immunity to the newborn.
National standards for vaccination have been established by the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC). This yearly updated vaccination schedule is available at the CDC’s Web site (http://www.cdc.gov/vaccines/schedules/hcp/adult.html).7 Ideally, a woman’s immunization status should be evaluated and made complete prior to pregnancy. Some vaccines are safe and appropriate for administration during pregnancy, provided the benefits clearly outweigh the risks. The recommended vaccines during pregnancy include inactivated influenza (seasonal and H1N1) and the combined tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap).
Related Article: CDC urges flu vaccination for all, especially pregnant women (News for Your Practice, October 2013)
Many physicians avoid giving vaccinations during pregnancy because of the concern that a spontaneous abortion or congenital anomaly might be incorrectly attributed to vaccine administration, but few vaccines are contradicted during pregnancy. Those that are contraindicated are those containing live virus, including measles, mumps, and rubella (MMR); varicella; and herpes zoster. Concerns also have been raised regarding the safety of administering influenza vaccines containing the mercury-based preservative thimerosol. However, no scientific evidence has conclusively linked adverse effects on offspring with thimerosol-containing vaccines administered during pregnancy.
Immunizations recommended for women of reproductive age
Measles, mumps, rubella (MMR). This vaccine is recommended for all women lacking confirmed immunity to rubella. The vaccine contains live, attenuated virus and is given as a single dose. Women should avoid pregnancy for 1 month after vaccination.
Varicella. This vaccine is for all women lacking confirmed immunity to varicella. It also contains a live, attenuated virus. It is administered in two doses, 1 month apart, and women should avoid pregnancy for 1 month after vaccination.
Influenza. The flu vaccine is recommended annually for individuals 6 months of age and older. The injectable vaccine contains inactivated virus and may be administered during pregnancy—at any time but optimally in October or November because the flu season occurs January through March. (The intranasal influenza vaccine contains live, attenuated virus and should be avoided in pregnancy.) Either method is administered as a single dose.
Thimerosal is a mercury-based preservative used in vaccines, including the influenza vaccine, and is appropriate for use in pregnant women; studies have not shown an association between vaccines containing thimerosal and adverse effects in pregnant women or their offspring.
Tetanus-diptheria-pertussis (Tdap) and tetanus-diphtheria (Td). Tdap or Td is recommended for adults aged 19 to 64 years who have or anticipate having close contact with an infant less than 12 months of age. Due to the recent increase in pertussis infection, Tdap should be given to all women who have not previously received the vaccine and who are pregnant or might become pregnant. It can be given anytime during pregnancy, but optimal administration is during the third trimester or late second trimester (after 20 weeks’ gestation) to confer the greatest amount of fetal protection.
If the vaccine is not being administered during pregnancy, it should be given in the immediate postpartum period to ensure pertussis immunity and to reduce transmission to the newborn. Tdap is administered as a single dose of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis.
Non-routine vaccines include pneumococcus, hepatitis A, hepatitis B, and meningococcus (TABLE). These vaccines should be administered as indicated in high-risk patients.
Health-care providers caring for women with infertility are urged to assess patients’ immunization status prior to attempting pregnancy, to counsel patients about the importance of protecting them and their potential offspring from preventable disease, and to facilitate vaccination prior to conception attempts.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Vaccination is a very important aspect of pre-pregnancy care but is especially important for infertile women who desire pregnancy. Planning of infertility treatment should include assessment of the patient’s vaccination status and completion of appropriate vaccinations before infertility treatment is initiated.
DO CURRENT OPTIONS EFFECTIVELY PREVENT POSTSURGICAL ADHESIONS?
Practice Committee of American Society for Reproductive Medicine in collaboration with Society of Reproductive Surgeons. Pathogenesis, consequences, and control of peritoneal adhesions in gynecologic surgery: A committee opinion. Fertil Steril. 2013;99(6):1550–1555.
Postoperative adhesions are a natural consequence of surgery and a major problem in gynecology. They may cause postsurgical infertility, abdominal/pelvic pain, or bowel obstruction as well as complicate subsequent surgeries by increasing operative times and the risk of bowel injury. The American Society for Reproductive Medicine (ASRM) and the Society of Reproductive Surgeons (SRS) recently evaluated the epidemiology, pathogenesis, and clinical consequences of adhesion formation and the evidence behind strategies for reducing adhesion formation.
In their joint Committee Opinion, they noted that open and laparoscopic approaches to surgery carry comparable levels of risk for adhesion-related hospital readmission. Ovarian surgery has the highest risk for adhesion-related readmission, at 7.5 per 100 initial operations, and the incidence of small bowel obstruction after hysterectomy was found to be 1.6 per 100 procedures. Adhesion-related US health-care costs are estimated at approximately $1 billion annually.
The Societies noted that more severe adnexal adhesions are associated with lower pregnancy rates, and treatment of adnexal adhesions appears to improve pregnancy rates. Investigators found adhesions to cause about three-quarters of postoperative small bowel obstructions; however, the relationship between adhesions and pelvic pain remains unclear. It is thought that adhesions may cause visceral pain by impairing organ mobility, but there is no relationship between the extent of adhesions and the severity of pain. It appears that only dense adhesions involving the bowel are associated with chronic pelvic pain. Predicting the outcome of lysis of adnexal or bowel adhesions is difficult.
Reduction of adhesion formation
Theoretically, adhesions may be reduced by minimizing peritoneal injury during surgery, avoiding intraoperative reactive foreign bodies, reducing local inflammatory response, inhibiting the coagulation cascade and promoting fibrinolysis, or by placing barriers between damaged tissues.
Related Article: Update on Fertility G. David Adamson, MD (February 2008)
Careful surgical technique includes gentle tissue handling, meticulous hemostasis, excision of necrotic tissue, minimizing ischemia and desiccation, using fine and nonreactive suture, and preventing foreign-body reaction and infection, all “microsurgical principles.”
ASRM and SRS reported that the surgical approach (laparoscopy vs laparotomy) is much less important than the extent of tissue injury. However, laparoscopy may result in less tissue and organ handling and trauma, avoid contamination with foreign bodies, enable more precise tissue handling, and result in less postoperative infection. The pneumoperitoneum has a tamponade effect that facilitates hemostasis during laparoscopy, but the process also can be associated with peritoneal desiccation and reduced temperatures that can increase injury.
Laparoscopic myomectomy was found to have a 70% risk of postoperative adhesions, compared with a 90% risk after laparotomy. It is unclear whether peritoneal closure at laparotomy reduces or increases adhesions, but parietal peritoneal closure at primary cesarean delivery results in fewer dense and filmy adhesions.
Related Article: How to avoid intestinal and urinary tract injuries during gynecologic laparoscopy Michael Baggish, MD (Second of a 2-part series on laparoscopic complications, October 2012)
Adjuncts to surgical technique
SRM and SRS reported on three adjuncts to surgical technique that have been proposed to reduce the risk of postoperative adhesions: anti-inflammatory agents, peritoneal instillates, and adhesion barriers.
Dexamethasone, promethazine, and other local and systemic anti-inflammatory drugs and adhesion-reducing substances have not been found effective for reducing postoperative adhesions.
Peritoneal instillates—which create “hydroflotation” and include antibiotic solutions, 32% dextran 70, and crystalloid solutions such as normal saline and Ringer’s lactate with or without heparin or corticosteroids—have not been found effective.8 Icodextrin 4% (Adept Adhesion Reduction Solution, Baxter Healthcare) is FDA approved as an adjunct to good surgical technique for the reduction of postoperative adhesions in patients undergoing gynecologic laparoscopic adhesiolysis. However, a systematic review concluded that there is insufficient evidence for its use as an adhesion-preventing agent.8
Adhesion barriers may help reduce postoperative adhesions but cannot compensate for poor surgical technique. Although the bioresorbable membrane sodium hyaluronic acid and carboxymethyl cellulose (Seprafilm, Genzyme Corp) is FDA-approved, there is limited evidence that it prevents adhesions after myomectomy.9 Because it fragments easily, it is mostly used at laparotomy.
Oxidized regenerated cellulose (Interceed, Ethicon Women’s Health and Urology) is an FDA-approved absorbable adhesion barrier for use at laparotomy that requires no suturing and has been shown to reduce the incidence and extent of new and recurrent adhesions at both laparoscopy and laparotomy by 40% to 50%, although there is little evidence that this improves fertility.9 Complete hemostasis must be achieved to use Interceed, and the addition of heparin confers no benefit.
Another product is expanded polytetrafluoroethylene (ePTFE, Gore-Tex Surgical Membrane, WL Gore and Associates), a nonabsorbable adhesion barrier produced in thin sheets and approved by the FDA for peritoneal repair. ePTFE must be sutured to tissue and helps prevent adhesion formation and reformation regardless of the type of injury or whether complete hemostasis has been achieved. In a small trial, it decreased postmyomectomy adhesions.10 ePTFE also was more effective than oxidized regenerated cellulose in preventing adhesions after adnexal surgery.11 Its use has been limited by the need for suturing and later reoperation for removal, although it probably does not have to be removed if it will not interfere with normal organ function since it has been used as a pericardial graft for many years.12
Hyaluronic acid (HA) solution (Sepracoat, Genzyme) is a natural bioabsorbable component of the extracellular matrix. Women undergoing laparotomy have fewer new adhesions with HA solution, but it is not approved for use in the United States.13 Polyethylene glycol (PEG; SprayGel, Confluent Surgical) was effective in early clinical trials but is not FDA-approved.12 Fibrin sealant (Tisseel VH, Baxter Healthcare) has been reported to decrease the formation of adhesions after salpingostomy, salpingolysis, and ovariolysis. Because it is a biologic product derived from human blood donors, it poses a risk for transmission of infectious agents. It is FDA-approved for use in cardiothoracic surgery, splenic injuries, and colostomy closure for hemostasis.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Adhesions are the most common complication following gynecologic surgery, and they pose potential longstanding consequences to patients. There is no evidence that anti-inflammatory agents reduce postoperative adhesions and insufficient evidence to recommend peritoneal instillates. FDA-approved surgical barriers reduce postoperative adhesions but there is not substantial evidence that their use improves fertility, decreases pain, or reduces the incidence of postoperative bowel obstruction. All gynecologists need to understand the importance of using microsurgical principles rather than relying on adhesion barriers to reduce postoperative adhesions.
WE WANT TO HEAR FROM YOU!
Drop us a line and let us know what you think about current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: [email protected]
- Practice Committee of the American Society for Reproductive Medicine. Use of clomiphene citrate in infertile women: A committee opinion. Fertil Steril. 2013;100(2):341–348.
- George K, Nair R, Tharyan P. Ovulation triggers in anovulatory women undergoing ovulation induction. Cochrane Database Syst Rev. 2008;(3):CD006900.
- Deaton JL, Gibson M, Blackmer KM, Nakajima ST, Badger GJ, Brumsted JR. A randomized, controlled trial of clomiphene citrate and intrauterine insemination in couples with unexplained infertility or surgically corrected endometriosis. Fertil Steril. 1990;54(6):1083–1088.
- Thessaloniki ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Consensus on infertility treatment related to polycystic ovary syndrome. Fertil Steril. 2008;89(3):505–522.
- Reefhuis J, Honein MA, Schieve LA, Rasmussen SA; National Birth Defects Prevention Study. Use of clomiphene citrate and birth defects, National Birth Defects Prevention Study, 1997-2005. Hum Reprod. 2011;26(2):451–457.
- Silva Idos S, Wark PA, McCormack VA, et al. Ovulation-stimulation drugs and cancer risks: a long-term follow-up of a British cohort. Br J Cancer. 2009;100(11):1824–1831.
- Adult immunization schedules. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/vaccines/schedules/hcp/adult.html. Updated October 19, 2013. Accessed January 16, 2014.
- Metwally M, Watson A, Lilford R, Vandekerckhove P. Fluid and pharmacological agents for adhesion prevention after gynaecological surgery. Cochrane Database Syst Rev. 2006;(2):CD001298.
- Farquhar C, Vandekerckhove P, Watson A, Vail A, Wiseman D. Barrier agents for preventing adhesions after surgery for subfertility. Cochrane Database Syst Rev. 2000;(2):CD000475.
- The Myomectomy Adhesion Multicenter Study Group. An expanded polytetrafluoroethylene barrier (Gore-Tex Surgical Membrane) reduces post-myomectomy adhesion formation. Fertil Steril. 1995;63(3):491–493.
- Haney AF, Hesla J, Hurst BS, et al. Expanded polytetrafluoroethylene (Gore-Tex Surgical Membrane) is superior to oxidized regenerated cellulose (Interceed TC7+) in preventing adhesions. Fertil Steril. 1995;63(5):1021–1026.
- Alejandro G, Flores RM. Surgical management of tumors invading the superior vena cava. Ann Thorac Surg 2008;85(6):2144−2146.
- Diamond MP; The Sepracoat Adhesion Study Group. Reduction of de novo postsurgical adhesions by intraoperative precoating with Sepracoat (HAL-C) solution: A prospective, randomized blinded, placebo-controlled multicenter study. Fertil Steril. 1998;69(6):1067–1074.
- Practice Committee of the American Society for Reproductive Medicine. Use of clomiphene citrate in infertile women: A committee opinion. Fertil Steril. 2013;100(2):341–348.
- George K, Nair R, Tharyan P. Ovulation triggers in anovulatory women undergoing ovulation induction. Cochrane Database Syst Rev. 2008;(3):CD006900.
- Deaton JL, Gibson M, Blackmer KM, Nakajima ST, Badger GJ, Brumsted JR. A randomized, controlled trial of clomiphene citrate and intrauterine insemination in couples with unexplained infertility or surgically corrected endometriosis. Fertil Steril. 1990;54(6):1083–1088.
- Thessaloniki ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Consensus on infertility treatment related to polycystic ovary syndrome. Fertil Steril. 2008;89(3):505–522.
- Reefhuis J, Honein MA, Schieve LA, Rasmussen SA; National Birth Defects Prevention Study. Use of clomiphene citrate and birth defects, National Birth Defects Prevention Study, 1997-2005. Hum Reprod. 2011;26(2):451–457.
- Silva Idos S, Wark PA, McCormack VA, et al. Ovulation-stimulation drugs and cancer risks: a long-term follow-up of a British cohort. Br J Cancer. 2009;100(11):1824–1831.
- Adult immunization schedules. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/vaccines/schedules/hcp/adult.html. Updated October 19, 2013. Accessed January 16, 2014.
- Metwally M, Watson A, Lilford R, Vandekerckhove P. Fluid and pharmacological agents for adhesion prevention after gynaecological surgery. Cochrane Database Syst Rev. 2006;(2):CD001298.
- Farquhar C, Vandekerckhove P, Watson A, Vail A, Wiseman D. Barrier agents for preventing adhesions after surgery for subfertility. Cochrane Database Syst Rev. 2000;(2):CD000475.
- The Myomectomy Adhesion Multicenter Study Group. An expanded polytetrafluoroethylene barrier (Gore-Tex Surgical Membrane) reduces post-myomectomy adhesion formation. Fertil Steril. 1995;63(3):491–493.
- Haney AF, Hesla J, Hurst BS, et al. Expanded polytetrafluoroethylene (Gore-Tex Surgical Membrane) is superior to oxidized regenerated cellulose (Interceed TC7+) in preventing adhesions. Fertil Steril. 1995;63(5):1021–1026.
- Alejandro G, Flores RM. Surgical management of tumors invading the superior vena cava. Ann Thorac Surg 2008;85(6):2144−2146.
- Diamond MP; The Sepracoat Adhesion Study Group. Reduction of de novo postsurgical adhesions by intraoperative precoating with Sepracoat (HAL-C) solution: A prospective, randomized blinded, placebo-controlled multicenter study. Fertil Steril. 1998;69(6):1067–1074.
Metastatic Spinal Cord Compression: A Review
Case
A 60-year-old man with stage IV hormoneindependent prostate cancer, with widely metastatic disease to the bone, presents to the ED with increased weakness and new onset of numbness in the lower extremities, which he states began earlier that day. After failing several lines of chemotherapy, he is currently being treated with hormonal therapy alone. Patient first noted weakness in the left lower extremity 5 days before presentation, which progressed to bilateral involvement, making ambulation difficult and requiring the use of a walker. He denies back pain or urinary or fecal incontinence. Regarding pain management, he had been recently treated at one of the pain clinics in the hospital and has continued on opioid medication at another institution. Until the past week, he states he had back pain without neurological deficits.
His vital signs are stable at presentation. Patient is obese but in no acute distress. His cardiopulmonary examination is unremarkable; abdominal examination is benign; and back examination is normal. On neurological examination, iliopsoas flexion is 4/5 bilaterally; the rest of the motor examination is normal, with toes downgoing bilaterally upon plantar stimulation. Diminished sensation to light touch is noted at the T4-T6 sensory level and below; patient also has diminished proprioception in his lower extremities.
Patient had undergone a whole body scan one month prior to presentation, which revealed increased tracer uptake of Technetium-99m in multiple areas in the thoracic and lumbar spine. The radiologist also reported bilateral involvement in the wrists, femurs, tibias, and humeri—all in concordance with multifocal bone disease noted in previous computed tomography scans.
How should you approach this case?
Overview of Metastatic Spinal Cord Compression
Malignant or metastatic spinal cord compression (MSCC) of the thecal sac is an ominous complication of advanced cancer and an oncologic emergency presenting clinically in approximately 3% to 10% of cancer-related deaths.1,2 Cancer patients have a median survival of 3 to 6 months from diagnosis of MSCC.1,3,4 This disease causes significant disability due to paralysis, sensory loss, protracted pain, and sphincter dysfunction.5 If left untreated, MSCC has the potential to cause paraplegia in almost all affected patients; therefore, prompt recognition and treatment are essential to maintain mobility and neurological function. Generally speaking, any cancer patient who presents with new or worsening back pain—even in the absence of neurological deficits—merits evaluation for spinal cord compression.6 Nevertheless, individual risk assessment is warranted.7
Epidemiology
In the United States, more than 20,000 cases of MSCC are reported each year.8 According to postmortem studies, this condition affects 5% to 36% of cancer patients.9,10 In a US nationwide study of 15,367 cases of MSCC,2 the mean age at hospitalization was 62 years, with 37% of cases occurring in women. In approximately 20% of cases, MSCC was the initial presentation of cancer4; this has been reflected in our experience at MD Anderson Cancer Center.
Cancers of the breast, lung, prostate, and multiple myeloma are the most frequent underlying conditions in MSCC.2,8 Its prevalence varies depending on tumor type, occurring in 0.2% of pancreatic cancers; however, MSCC may affect up to 7.9% to 15% of myelomas1,2 and 13% of lymphomas.2 Interestingly, 5.5% of patients with prostate cancer develop MSCC.2 According to a study by Lu et al,11 historical risk factors include known nonvertebral bony metastases and stage IV disease at the time of diagnosis.
The most common location of MSCC is the thoracic spine (69% of cases); 29% of cases occur at the lumbosacral level and 10% at the cervical area.12 Most likely this pattern follows the lymphatic drainage, as metastases from breast and lung cancers tend to be found in the thoracic spine. Pelvic and intra-abdominal malignancies most commonly migrate to the lumbar spine. Multiple spinal epidural metastases were noted in 31% of those who underwent complete imaging of the spine.12
Pathophysiology
Most cases of MSCC are epidural in origin, arising from the vertebral column in 85% of patients.8 Epidural spread is caused mainly by hematogenous mechanism through the Batson venous plexus,13 debilitating the bone and eventually causing vertebral collapse with compression of the spinal canal. Epidural spread is less likely caused by direct tumor extension (ie, erosion through the bone) or by direct deposition of tumor cells into the epidural space.14 Ultimate neuronal injury is thought to involve vasogenic edema,15 leading to ischemia13 through venous infarction, but there has been debate regarding this last phenomenon.16 In cases of paralysis, demyelination is striking.16
Clinical Presentation
Even though cancer accounts for less than 1% of episodes of low back pain, it is the most common systemic disease affecting the spine.17 An important clinical inquiry is to determine whether back pain in an established cancer patient can be ruled out without extensive imaging. Unfortunately, clinical examination alone cannot exclude MSCC. Because of the high specificity (0.98), any cancer patient with new back pain should be considered to have metastasis until proven otherwise.17
Symptoms in MSCC at presentation can be motor, sensory, and/or autonomic. Back pain varies depending on the site of metastasis, which can be referred, local, radicular, or a combination of all three.18 The primary complaint is pain in 83% to 96% of cases,19,20 though this is a nonspecific sign.
Previous studies have shown 40% to 64% of patients were not ambulatory at the time of diagnosis.19,25 Recent case series, however, report an increased number of ambulatory patients—possibly due to increased clinician awareness.26 In other cases, only 9% of patients were able to walk independently without aid.27 Loss of sensation, dense paraplegia, and incontinence are late findings and likely signal some degree of permanent disability.19
Misdiagnosis is a common issue in the ED setting. In an interesting retrospective study of 63 patients with spinal cord compression28 (not necessarily malignant), 18 (29%) were misdiagnosed.28 Consequently, there was a significant delay in diagnosis despite obvious neurological deficits at presentation.
Evaluation and Imaging
A detailed physical examination is essential to diagnosing MSCC. A thorough neurological examination, including sensation, strength, and reflexes should be carefully documented. If spinal instability is suspected, range-of-motion testing is contraindicated. The modified Frankel classification,29 adapted from the traumatic spine cord injury work by Frankel, et al,30 may be used to assess the degree of disability (Table).
Lu et al11 noted hyperreflexia and upward going Babinski reflex as common findings. Moreover, risk factors of decreased rectal sphincter tone and bladder were determinant for poor outcomes.
MRI studies should include the entire spine—not just the perceived area of interest— as up to 38% of patients have multiple-site metastases12 (Figure 1). Sensory deficits and mechanical pain may be present two to four vertebral levels away from the actual lesion.11 If MRI suggests cord compression, severity can be graded using the MSCC scale34 (Figure 2). Several scoring systems have been developed to aid in decision making concerning surgical treatment.
Management and Outcomes
The goal of therapy is symptom control and preservation of function. This requires a multidisciplinary approach and may involve radiation therapy and surgery, as well as medical efforts. Upon diagnosis and initiation of therapy, serial neurological evaluation should be undertaken. Neurovital signs should be scheduled to coincide with other nursing efforts to ease the burden of care and minimize patient discomfort.
The mainstay of medical therapy is treatment with corticosteroids.35 Initial trials have demonstrated that corticosteroids improve functional status in MSCC, but controversy exists regarding the effective dose. In a randomized, controlled trial by Sorensen et al,36 which sought to evaluate functional outcomes of highdose corticosteroids as an adjunct to radiotherapy, 57 patients received either high-dose dexamethasone or no corticosteroid therapy. Fifty-nine percent of patients in the dexamethasone group were ambulatory 6 months after treatment compared to 39% in the group who did not receive steroids.36
A patient without a biopsy-confirmed cancer diagnosis in need of corticosteroid treatment presents a dilemma. Plasmacytomas, thymomas, lymphomas, multiple myeloma, germ-cell tumors are very sensitive to corticosteroid therapy in patients with MSCC.38 However, corticosteroids given before tissue samples are obtained may hinder proper diagnosis and complicate future management.39,40 In the absence of neurological deficit, corticosteroids may be withheld and emergent consultation with neurosurgery and oncology should be obtained. If there is any question regarding the nature of the lesion, tissue diagnosis must be obtained without delay.
Strict bed rest (including logroll and bedpan use) should be instituted if there is suspicion of spinal cord instability. Patients with suspected involvement of the cervical spine should have a Philadelphia collar placed until spinal stability has been confirmed. In the United Kingdom, the National Institutes for Health Care Excellence guidelines recommend all patients with suspected cord compression be nursed in a flat position.22 Other institutions, however, do not believe that strict bed rest is necessary, as it is presumed that MSCC is inherently different from that caused by trauma. Authors supporting this position contend that the increased incidence of deep vein thrombosis, infection (particularly from the urinary tract), and decubitus ulcers outweighs the benefit of bed rest. Patient preference should be taken into consideration as those with good functional status may be quite resistant to bed rest. In cases where cord compression is strongly suspected, these patients should be educated on proper bed rest. The greatest predictors of outcome are ambulatory and functional status at the time of diagnosis (generally based on an Eastern Cooperative Oncology Group scale). Patients with a good functional status, limited disease, and a life expectancy of greater than 3 to 6 months may benefit from surgery.41 However, emergent surgical evaluation is required in patients not responding to radiotherapy or who received received only limited doses of radiotherapy, as well as those with spinal instability, direct cord compression due to a bony fragment, impending sphincter dysfunction, unknown primary tumor, or no paraplegia for >48 hours.15
Unfortunately, surgery is only indicated in 10% to 15% of MSCC cases.42 In the past two decades, significant improvements regarding new aggressive surgical techniques have been made, and include circumferential decompression of the spine and staged or single stage anterior posterior surgery with stabilization. 43 Additionally, the combination of surgery with radiotherapy has improved outcomes.44
Most patients benefit from short-course radiotherapy45 even when given palliatively. 46 Longer courses of radiotherapy are highly recommended for patients with a more favorable prognosis.47 Up to 10% of patients diagnosed with spinal cord compression will require treatment for disease recurrence.42 There is a limited role for chemotherapy, and in seminomas and lymphomas, results can be quite dramatic.38
Prevention
Lu et al11 found that only 54% of patients were aware that back pain should be reported to their physician. Delays in diagnosis and treatment are common and well described in the literature.21 Patients should be instructed to call their physician within 24 hours from the development of any new or worsening back pain, and should be advised to seek immediate care if they develop any neurological symptoms. To facilitate appropriate and prompt management of MSCC, hospitals should develop diagnostic algorithms to minimize delays in referral to a comprehensive center for further treatment.
Case Conclusion
Based on this patient’s symptoms and status at presentation, the emergency team determined he was at high risk for MSCC. An initial dosage of 10 mg dexamethasone was administered intravenously (IV), followed by 4 mg IV every 6 hours prior to imaging. An MRI without contrast of the cervical, thoracic, and lumbar spine showed cord compression with mild cord edema at T4 level, along with diffused osseous metastasis.
Upon diagnosis, patient was referred to radiation oncology for radiotherapy of the T2-T6 vertebral bodies. Three days after initiation of radiation therapy, his neurological function deteriorated with paraplegia and incontinence, and he was emergently evaluated for neurosurgery. Although T4 laminectomy and decompression of the spinal cord were performed without complication, patient did not recover neurological function. His hospital course was complicated by Ogilvie syndrome and episodes of delirium, and he was discharged to a rehabilitation facility 23 days after admission; paraplegia and urinary and bowel incontinence remained unchanged.
- Loblaw DA, Laperriere NJ, Mackillop WJ. A population-based study of malignant spinal cord compression in Ontario. Clin Oncol (R Coll Radiol). 2003;15(4):211-217.
- Mak KS, Lee LK, Mak RH, et al. Incidence and treatment patterns in hospitalizations for malignant spinal cord compression in the United States, 1998-2006. Int J Radiat Oncol Biol Phys. 2011;80(3):824-831.
- Constans JP, de Divitiis E, Donzelli R, Spaziante R, Meder JF, Haye C. Spinal metastases with
- neurological manifestations. Review of 600 cases. J Neurosurg. 1983;59(1):111-118.
- Schiff D, O’Neill BP, Suman VJ. Spinal epidural metastasis as the initial manifestation of malignancy: clinical features and diagnostic approach. Neurology. 1997;49(2):452-456.
- Loblaw DA, Perry J, Chambers A, Laperriere NJ. Systematic review of the diagnosis and management of malignant extradural spinal cord compression: the Cancer Care Ontario Practice Guidelines Initiative’s Neuro-Oncology Disease Site Group. J Clin Oncol. 2005;23(9):2028-2037.
- Levack P, Graham J, Collie D, et al. Don’t wait for a sensory level—listen to the symptoms: a prospective audit of the delays in diagnosis of malignant cord compression. Clin Oncol (R Coll Radiol). 2002;14(6):472-480.
- Talcott JA, Stomper PC, Drislane FW, et al. Assessing suspected spinal cord compression: a multidisciplinary outcomes analysis of 342 episodes. Support Care Cancer. 1999;7(1):31-38.
- Byrne TN. Spinal cord compression from epidural metastases. N Engl J Med. 1992;327(9):614-619.
- Abrams HL, Spiro R, Goldstein N. Metastases in carcinoma; analysis of 1000 autopsied cases. Cancer. 1950;3(1):74-85.
- Wong DA, Fornasier VL, MacNab I. Spinal metastases: the obvious, the occult, and the impostors. Spine (Phila Pa 1976). 1990;15(1):1-4.
- Lu C, Gonzalez RG, Jolesz FA, Wen PY, Talcott JA. Suspected spinal cord compression in cancer patients: a multidisciplinary risk assessment. J Support Oncol. 2005;3(4):305-312.
- Schiff D, O’Neill BP, Wang CH, O’Fallon JR. Neuroimaging and treatment implications of patients with multiple epidural spinal metastases. Cancer. 1998;83(8):1593-1601.
- Arguello F, Baggs RB, Duerst RE, Johnstone L, McQueen K, Frantz CN. Pathogenesis of vertebral metastasis and epidural spinal cord compression. Cancer. 1990;65(1):98-106.
- Schiff D. Spinal cord compression. Neurol Clin. 2003;21(1):67-86, viii.
- Prasad D, Schiff D. Malignant spinal-cord compression. Lancet Oncol. 2005;6(1):15-24.
- Helweg-Larsen S, Laursen H. Clinical and autopsy findings in spinal cord compression due to metastatic disease. Eur J Neurol. 1998;5(6):587-592.
- Deyo RA, Rainville J, Kent DL. What can the history and physical examination tell us about low back pain? JAMA. 199218. Abrahm JL, Banffy MB, Harris MB. Spinal cord compression in patients with advanced metastatic cancer: "all I care about is walking and living my life." JAMA. 2008;299(8):937-946.
- Bach F, Larsen BH, Rohde K, et al. Metastatic spinal cord compression. Occurrence, symptoms, clinical presentations and prognosis in 398 patients with spinal cord compression. Acta Neurochir (Wien). 1990;107(1-2):37-43.
- Gilbert RW, Kim JH, Posner JB. Epidural spinal cord compression from metastatic tumor: diagnosis and treatment. Ann Neurol. 1978;3(1):40-51.
- Husband DJ. Malignant spinal cord compression: prospective study of delays in referral and treatment. BMJ. 1998;317(7150):18-21.
- Metastatic Spinal Cord Compression: Diagnosis and Management of Patients at Risk of or with Metastatic Spinal Cord Compression. Cardiff UK: National Collaborating Centre for Cancer; 2008.
- Shiue K, Sahgal A, Chow E, et al. Management of metastatic spinal cord compression. Expert Rev Anticancer Ther. 2010;10(5):697-708.
- Hammack JE. Spinal cord disease in patients with cancer. Continuum (Minneap Minn). 2012;18(2):312-327.
- Helweg-Larsen S. Clinical outcome in metastatic spinal cord compression. A prospective study of 153 patients. Acta Neurol Scand. 1996;94(4):269-275.
- Rades D, Fehlauer F, Schulte R, et al. Prognostic factors for local control and survival after radiotherapy of metastatic spinal cord compression. J Clin Oncol. 2006;24(21):3388-3393.
- McLinton A, Hutchison C. Malignant spinal cord compression: a retrospective audit of clinical practice at a UK regional cancer centre. Br J Cancer. 2006;94(4):486-491.
- Dugas AF, Lucas JM, Edlow JA. Diagnosis of spinal cord compression in nontrauma patients in the emergency department. Acad Emerg Med. 2011;18(7):719-725.
- Ditunno JF, Jr, Young W, Donovan WH, Creasey metastatic spinal CORD compression 18 EMERGENCY MEDICINE I january 2014 www.emed-journal.com G. American Spinal Surgery Association. The international standards booklet for neurological and functional classification of spinal cord injury. American Spinal Injury Association. Paraplegia. 1994;32(2):70-80.
- Frankel HL, Hancock DO, Hyslop G, et al. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. I. Paraplegia. 1969;7(3):179-192.
- Portenoy RK, Galer BS, Salamon O, et al. Identification of epidural neoplasm. Radiography and bone scintigraphy in the symptomatic and asymptomatic spine. Cancer. 1989;64(11):2207-2213.
- Husband DJ, Grant KA, Romaniuk CS. MRI in the diagnosis and treatment of suspected malignant spinal cord compression. Br J Radiol. 2001;74(877):15-23.
- Carmody RF, Yang PJ, Seeley GW, Seeger JF, Unger EC, Johnson JE. Spinal cord compression due to metastatic disease: diagnosis with MR imaging versus myelography. Radiology. 1989;173(1):225-229.
- Bilsky MH, Laufer I, Fourney DR, et al. Reliability analysis of the epidural spinal cord compression scale. J Neurosurg Spine. 2010;13(3):324-328.
- Loblaw DA, Mitera G, Ford M, Laperriere NJ. A 2011 updated systematic review and clinical practice guideline for the management of malignant extradural spinal cord compression. Int J Radiat Oncol Biol Phys. 2012;84(2):312-317.
- Sorensen S, Helweg-Larsen S, Mouridsen H, Hansen HH. Effect of high-dose dexamethasone in carcinomatous metastatic spinal cord compression treated with radiotherapy: a randomised trial. Eur J Cancer. 1994;30A(1):22-27.
- Heimdal K, Hirschberg H, Slettebo H, Watne K, Nome O. High incidence of serious side effects of high-dose dexamethasone treatment in patients with epidural spinal cord compression. J Neurooncol. 1992;12(2):141-144.
- Posner JB, Howieson J, Cvitkovic E. "Disappearing" spinal cord compression: oncolytic effect of glucocorticoids (and other chemotherapeutic agents) on epidural metastases. Ann Neurol. 1977;2(5):409-413.
- Kan E, Levi I, Benharroch D. Alterations in the primary diagnosis of lymphomas pretreated with corticosteroid agents. Leuk Lymphoma. 2011;52(3):425-428.
- Borenstein SH, Gerstle T, Malkin D, Thorner P, Filler RM. The effects of prebiopsy cortico-steroid treatment on the diagnosis of mediastinal lymphoma. J Pediatr Surg. 2000;35(6):973-976.
- Akram H, Allibone J. Spinal surgery for palliation in malignant spinal cord compression. Clin Oncol (R Coll Radiol). 2010;22(9):792-800.
- Rades D, Abrahm JL. The role of radiotherapy for metastatic epidural spinal cord compression. Nat Rev Clin Oncol. 2010;7(10):590-598.
- Sundaresan N, Sachdev VP, Holland JF, et al. Surgical treatment of spinal cord compression from epidural metastasis. J Clin Oncol. 1995;13(9):2330-2335.
- Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366(9486):643-648.
- Rades D, Dahm-Daphi J, Rudat V, et al. Is shortcourse radiotherapy with high doses per fraction the appropriate regimen for metastatic spinal cord compression in colorectal cancer patients? Strahlenther Onkol. 2006;182(12):708-712.
- van den Hout WB, van der Linden YM, Steenland E, et al. Single- versus multiple-fraction radiotherapy in patients with painful bone metastases: cost-utility analysis based on a randomized trial. J Natl Cancer Inst. 2003;95(3):222-229.
- Steenland E, Leer JW, van Houwelingen H, et al. The effect of a single fraction compared to multiple fractions on painful bone metastases: a global analysis of the Dutch Bone Metastasis Study. Radiother Oncol. 1999;52(2):101-109.
- Rades D, Hueppe M, Schild SE. A score to identify patients with metastatic spinal cord compression who may be candidates for best supportive care. Cancer. 2013;119(4):897-903.
- Guo Y, Palmer JL, Bianty J, Konzen B, Shin K, Bruera E. Advance directives and do-not-resuscitate orders in patients with cancer with metastatic spinal cord compression: advanced care planning implications. J Palliat Med. 2010;13(5):513-517.
Case
A 60-year-old man with stage IV hormoneindependent prostate cancer, with widely metastatic disease to the bone, presents to the ED with increased weakness and new onset of numbness in the lower extremities, which he states began earlier that day. After failing several lines of chemotherapy, he is currently being treated with hormonal therapy alone. Patient first noted weakness in the left lower extremity 5 days before presentation, which progressed to bilateral involvement, making ambulation difficult and requiring the use of a walker. He denies back pain or urinary or fecal incontinence. Regarding pain management, he had been recently treated at one of the pain clinics in the hospital and has continued on opioid medication at another institution. Until the past week, he states he had back pain without neurological deficits.
His vital signs are stable at presentation. Patient is obese but in no acute distress. His cardiopulmonary examination is unremarkable; abdominal examination is benign; and back examination is normal. On neurological examination, iliopsoas flexion is 4/5 bilaterally; the rest of the motor examination is normal, with toes downgoing bilaterally upon plantar stimulation. Diminished sensation to light touch is noted at the T4-T6 sensory level and below; patient also has diminished proprioception in his lower extremities.
Patient had undergone a whole body scan one month prior to presentation, which revealed increased tracer uptake of Technetium-99m in multiple areas in the thoracic and lumbar spine. The radiologist also reported bilateral involvement in the wrists, femurs, tibias, and humeri—all in concordance with multifocal bone disease noted in previous computed tomography scans.
How should you approach this case?
Overview of Metastatic Spinal Cord Compression
Malignant or metastatic spinal cord compression (MSCC) of the thecal sac is an ominous complication of advanced cancer and an oncologic emergency presenting clinically in approximately 3% to 10% of cancer-related deaths.1,2 Cancer patients have a median survival of 3 to 6 months from diagnosis of MSCC.1,3,4 This disease causes significant disability due to paralysis, sensory loss, protracted pain, and sphincter dysfunction.5 If left untreated, MSCC has the potential to cause paraplegia in almost all affected patients; therefore, prompt recognition and treatment are essential to maintain mobility and neurological function. Generally speaking, any cancer patient who presents with new or worsening back pain—even in the absence of neurological deficits—merits evaluation for spinal cord compression.6 Nevertheless, individual risk assessment is warranted.7
Epidemiology
In the United States, more than 20,000 cases of MSCC are reported each year.8 According to postmortem studies, this condition affects 5% to 36% of cancer patients.9,10 In a US nationwide study of 15,367 cases of MSCC,2 the mean age at hospitalization was 62 years, with 37% of cases occurring in women. In approximately 20% of cases, MSCC was the initial presentation of cancer4; this has been reflected in our experience at MD Anderson Cancer Center.
Cancers of the breast, lung, prostate, and multiple myeloma are the most frequent underlying conditions in MSCC.2,8 Its prevalence varies depending on tumor type, occurring in 0.2% of pancreatic cancers; however, MSCC may affect up to 7.9% to 15% of myelomas1,2 and 13% of lymphomas.2 Interestingly, 5.5% of patients with prostate cancer develop MSCC.2 According to a study by Lu et al,11 historical risk factors include known nonvertebral bony metastases and stage IV disease at the time of diagnosis.
The most common location of MSCC is the thoracic spine (69% of cases); 29% of cases occur at the lumbosacral level and 10% at the cervical area.12 Most likely this pattern follows the lymphatic drainage, as metastases from breast and lung cancers tend to be found in the thoracic spine. Pelvic and intra-abdominal malignancies most commonly migrate to the lumbar spine. Multiple spinal epidural metastases were noted in 31% of those who underwent complete imaging of the spine.12
Pathophysiology
Most cases of MSCC are epidural in origin, arising from the vertebral column in 85% of patients.8 Epidural spread is caused mainly by hematogenous mechanism through the Batson venous plexus,13 debilitating the bone and eventually causing vertebral collapse with compression of the spinal canal. Epidural spread is less likely caused by direct tumor extension (ie, erosion through the bone) or by direct deposition of tumor cells into the epidural space.14 Ultimate neuronal injury is thought to involve vasogenic edema,15 leading to ischemia13 through venous infarction, but there has been debate regarding this last phenomenon.16 In cases of paralysis, demyelination is striking.16
Clinical Presentation
Even though cancer accounts for less than 1% of episodes of low back pain, it is the most common systemic disease affecting the spine.17 An important clinical inquiry is to determine whether back pain in an established cancer patient can be ruled out without extensive imaging. Unfortunately, clinical examination alone cannot exclude MSCC. Because of the high specificity (0.98), any cancer patient with new back pain should be considered to have metastasis until proven otherwise.17
Symptoms in MSCC at presentation can be motor, sensory, and/or autonomic. Back pain varies depending on the site of metastasis, which can be referred, local, radicular, or a combination of all three.18 The primary complaint is pain in 83% to 96% of cases,19,20 though this is a nonspecific sign.
Previous studies have shown 40% to 64% of patients were not ambulatory at the time of diagnosis.19,25 Recent case series, however, report an increased number of ambulatory patients—possibly due to increased clinician awareness.26 In other cases, only 9% of patients were able to walk independently without aid.27 Loss of sensation, dense paraplegia, and incontinence are late findings and likely signal some degree of permanent disability.19
Misdiagnosis is a common issue in the ED setting. In an interesting retrospective study of 63 patients with spinal cord compression28 (not necessarily malignant), 18 (29%) were misdiagnosed.28 Consequently, there was a significant delay in diagnosis despite obvious neurological deficits at presentation.
Evaluation and Imaging
A detailed physical examination is essential to diagnosing MSCC. A thorough neurological examination, including sensation, strength, and reflexes should be carefully documented. If spinal instability is suspected, range-of-motion testing is contraindicated. The modified Frankel classification,29 adapted from the traumatic spine cord injury work by Frankel, et al,30 may be used to assess the degree of disability (Table).
Lu et al11 noted hyperreflexia and upward going Babinski reflex as common findings. Moreover, risk factors of decreased rectal sphincter tone and bladder were determinant for poor outcomes.
MRI studies should include the entire spine—not just the perceived area of interest— as up to 38% of patients have multiple-site metastases12 (Figure 1). Sensory deficits and mechanical pain may be present two to four vertebral levels away from the actual lesion.11 If MRI suggests cord compression, severity can be graded using the MSCC scale34 (Figure 2). Several scoring systems have been developed to aid in decision making concerning surgical treatment.
Management and Outcomes
The goal of therapy is symptom control and preservation of function. This requires a multidisciplinary approach and may involve radiation therapy and surgery, as well as medical efforts. Upon diagnosis and initiation of therapy, serial neurological evaluation should be undertaken. Neurovital signs should be scheduled to coincide with other nursing efforts to ease the burden of care and minimize patient discomfort.
The mainstay of medical therapy is treatment with corticosteroids.35 Initial trials have demonstrated that corticosteroids improve functional status in MSCC, but controversy exists regarding the effective dose. In a randomized, controlled trial by Sorensen et al,36 which sought to evaluate functional outcomes of highdose corticosteroids as an adjunct to radiotherapy, 57 patients received either high-dose dexamethasone or no corticosteroid therapy. Fifty-nine percent of patients in the dexamethasone group were ambulatory 6 months after treatment compared to 39% in the group who did not receive steroids.36
A patient without a biopsy-confirmed cancer diagnosis in need of corticosteroid treatment presents a dilemma. Plasmacytomas, thymomas, lymphomas, multiple myeloma, germ-cell tumors are very sensitive to corticosteroid therapy in patients with MSCC.38 However, corticosteroids given before tissue samples are obtained may hinder proper diagnosis and complicate future management.39,40 In the absence of neurological deficit, corticosteroids may be withheld and emergent consultation with neurosurgery and oncology should be obtained. If there is any question regarding the nature of the lesion, tissue diagnosis must be obtained without delay.
Strict bed rest (including logroll and bedpan use) should be instituted if there is suspicion of spinal cord instability. Patients with suspected involvement of the cervical spine should have a Philadelphia collar placed until spinal stability has been confirmed. In the United Kingdom, the National Institutes for Health Care Excellence guidelines recommend all patients with suspected cord compression be nursed in a flat position.22 Other institutions, however, do not believe that strict bed rest is necessary, as it is presumed that MSCC is inherently different from that caused by trauma. Authors supporting this position contend that the increased incidence of deep vein thrombosis, infection (particularly from the urinary tract), and decubitus ulcers outweighs the benefit of bed rest. Patient preference should be taken into consideration as those with good functional status may be quite resistant to bed rest. In cases where cord compression is strongly suspected, these patients should be educated on proper bed rest. The greatest predictors of outcome are ambulatory and functional status at the time of diagnosis (generally based on an Eastern Cooperative Oncology Group scale). Patients with a good functional status, limited disease, and a life expectancy of greater than 3 to 6 months may benefit from surgery.41 However, emergent surgical evaluation is required in patients not responding to radiotherapy or who received received only limited doses of radiotherapy, as well as those with spinal instability, direct cord compression due to a bony fragment, impending sphincter dysfunction, unknown primary tumor, or no paraplegia for >48 hours.15
Unfortunately, surgery is only indicated in 10% to 15% of MSCC cases.42 In the past two decades, significant improvements regarding new aggressive surgical techniques have been made, and include circumferential decompression of the spine and staged or single stage anterior posterior surgery with stabilization. 43 Additionally, the combination of surgery with radiotherapy has improved outcomes.44
Most patients benefit from short-course radiotherapy45 even when given palliatively. 46 Longer courses of radiotherapy are highly recommended for patients with a more favorable prognosis.47 Up to 10% of patients diagnosed with spinal cord compression will require treatment for disease recurrence.42 There is a limited role for chemotherapy, and in seminomas and lymphomas, results can be quite dramatic.38
Prevention
Lu et al11 found that only 54% of patients were aware that back pain should be reported to their physician. Delays in diagnosis and treatment are common and well described in the literature.21 Patients should be instructed to call their physician within 24 hours from the development of any new or worsening back pain, and should be advised to seek immediate care if they develop any neurological symptoms. To facilitate appropriate and prompt management of MSCC, hospitals should develop diagnostic algorithms to minimize delays in referral to a comprehensive center for further treatment.
Case Conclusion
Based on this patient’s symptoms and status at presentation, the emergency team determined he was at high risk for MSCC. An initial dosage of 10 mg dexamethasone was administered intravenously (IV), followed by 4 mg IV every 6 hours prior to imaging. An MRI without contrast of the cervical, thoracic, and lumbar spine showed cord compression with mild cord edema at T4 level, along with diffused osseous metastasis.
Upon diagnosis, patient was referred to radiation oncology for radiotherapy of the T2-T6 vertebral bodies. Three days after initiation of radiation therapy, his neurological function deteriorated with paraplegia and incontinence, and he was emergently evaluated for neurosurgery. Although T4 laminectomy and decompression of the spinal cord were performed without complication, patient did not recover neurological function. His hospital course was complicated by Ogilvie syndrome and episodes of delirium, and he was discharged to a rehabilitation facility 23 days after admission; paraplegia and urinary and bowel incontinence remained unchanged.
Case
A 60-year-old man with stage IV hormoneindependent prostate cancer, with widely metastatic disease to the bone, presents to the ED with increased weakness and new onset of numbness in the lower extremities, which he states began earlier that day. After failing several lines of chemotherapy, he is currently being treated with hormonal therapy alone. Patient first noted weakness in the left lower extremity 5 days before presentation, which progressed to bilateral involvement, making ambulation difficult and requiring the use of a walker. He denies back pain or urinary or fecal incontinence. Regarding pain management, he had been recently treated at one of the pain clinics in the hospital and has continued on opioid medication at another institution. Until the past week, he states he had back pain without neurological deficits.
His vital signs are stable at presentation. Patient is obese but in no acute distress. His cardiopulmonary examination is unremarkable; abdominal examination is benign; and back examination is normal. On neurological examination, iliopsoas flexion is 4/5 bilaterally; the rest of the motor examination is normal, with toes downgoing bilaterally upon plantar stimulation. Diminished sensation to light touch is noted at the T4-T6 sensory level and below; patient also has diminished proprioception in his lower extremities.
Patient had undergone a whole body scan one month prior to presentation, which revealed increased tracer uptake of Technetium-99m in multiple areas in the thoracic and lumbar spine. The radiologist also reported bilateral involvement in the wrists, femurs, tibias, and humeri—all in concordance with multifocal bone disease noted in previous computed tomography scans.
How should you approach this case?
Overview of Metastatic Spinal Cord Compression
Malignant or metastatic spinal cord compression (MSCC) of the thecal sac is an ominous complication of advanced cancer and an oncologic emergency presenting clinically in approximately 3% to 10% of cancer-related deaths.1,2 Cancer patients have a median survival of 3 to 6 months from diagnosis of MSCC.1,3,4 This disease causes significant disability due to paralysis, sensory loss, protracted pain, and sphincter dysfunction.5 If left untreated, MSCC has the potential to cause paraplegia in almost all affected patients; therefore, prompt recognition and treatment are essential to maintain mobility and neurological function. Generally speaking, any cancer patient who presents with new or worsening back pain—even in the absence of neurological deficits—merits evaluation for spinal cord compression.6 Nevertheless, individual risk assessment is warranted.7
Epidemiology
In the United States, more than 20,000 cases of MSCC are reported each year.8 According to postmortem studies, this condition affects 5% to 36% of cancer patients.9,10 In a US nationwide study of 15,367 cases of MSCC,2 the mean age at hospitalization was 62 years, with 37% of cases occurring in women. In approximately 20% of cases, MSCC was the initial presentation of cancer4; this has been reflected in our experience at MD Anderson Cancer Center.
Cancers of the breast, lung, prostate, and multiple myeloma are the most frequent underlying conditions in MSCC.2,8 Its prevalence varies depending on tumor type, occurring in 0.2% of pancreatic cancers; however, MSCC may affect up to 7.9% to 15% of myelomas1,2 and 13% of lymphomas.2 Interestingly, 5.5% of patients with prostate cancer develop MSCC.2 According to a study by Lu et al,11 historical risk factors include known nonvertebral bony metastases and stage IV disease at the time of diagnosis.
The most common location of MSCC is the thoracic spine (69% of cases); 29% of cases occur at the lumbosacral level and 10% at the cervical area.12 Most likely this pattern follows the lymphatic drainage, as metastases from breast and lung cancers tend to be found in the thoracic spine. Pelvic and intra-abdominal malignancies most commonly migrate to the lumbar spine. Multiple spinal epidural metastases were noted in 31% of those who underwent complete imaging of the spine.12
Pathophysiology
Most cases of MSCC are epidural in origin, arising from the vertebral column in 85% of patients.8 Epidural spread is caused mainly by hematogenous mechanism through the Batson venous plexus,13 debilitating the bone and eventually causing vertebral collapse with compression of the spinal canal. Epidural spread is less likely caused by direct tumor extension (ie, erosion through the bone) or by direct deposition of tumor cells into the epidural space.14 Ultimate neuronal injury is thought to involve vasogenic edema,15 leading to ischemia13 through venous infarction, but there has been debate regarding this last phenomenon.16 In cases of paralysis, demyelination is striking.16
Clinical Presentation
Even though cancer accounts for less than 1% of episodes of low back pain, it is the most common systemic disease affecting the spine.17 An important clinical inquiry is to determine whether back pain in an established cancer patient can be ruled out without extensive imaging. Unfortunately, clinical examination alone cannot exclude MSCC. Because of the high specificity (0.98), any cancer patient with new back pain should be considered to have metastasis until proven otherwise.17
Symptoms in MSCC at presentation can be motor, sensory, and/or autonomic. Back pain varies depending on the site of metastasis, which can be referred, local, radicular, or a combination of all three.18 The primary complaint is pain in 83% to 96% of cases,19,20 though this is a nonspecific sign.
Previous studies have shown 40% to 64% of patients were not ambulatory at the time of diagnosis.19,25 Recent case series, however, report an increased number of ambulatory patients—possibly due to increased clinician awareness.26 In other cases, only 9% of patients were able to walk independently without aid.27 Loss of sensation, dense paraplegia, and incontinence are late findings and likely signal some degree of permanent disability.19
Misdiagnosis is a common issue in the ED setting. In an interesting retrospective study of 63 patients with spinal cord compression28 (not necessarily malignant), 18 (29%) were misdiagnosed.28 Consequently, there was a significant delay in diagnosis despite obvious neurological deficits at presentation.
Evaluation and Imaging
A detailed physical examination is essential to diagnosing MSCC. A thorough neurological examination, including sensation, strength, and reflexes should be carefully documented. If spinal instability is suspected, range-of-motion testing is contraindicated. The modified Frankel classification,29 adapted from the traumatic spine cord injury work by Frankel, et al,30 may be used to assess the degree of disability (Table).
Lu et al11 noted hyperreflexia and upward going Babinski reflex as common findings. Moreover, risk factors of decreased rectal sphincter tone and bladder were determinant for poor outcomes.
MRI studies should include the entire spine—not just the perceived area of interest— as up to 38% of patients have multiple-site metastases12 (Figure 1). Sensory deficits and mechanical pain may be present two to four vertebral levels away from the actual lesion.11 If MRI suggests cord compression, severity can be graded using the MSCC scale34 (Figure 2). Several scoring systems have been developed to aid in decision making concerning surgical treatment.
Management and Outcomes
The goal of therapy is symptom control and preservation of function. This requires a multidisciplinary approach and may involve radiation therapy and surgery, as well as medical efforts. Upon diagnosis and initiation of therapy, serial neurological evaluation should be undertaken. Neurovital signs should be scheduled to coincide with other nursing efforts to ease the burden of care and minimize patient discomfort.
The mainstay of medical therapy is treatment with corticosteroids.35 Initial trials have demonstrated that corticosteroids improve functional status in MSCC, but controversy exists regarding the effective dose. In a randomized, controlled trial by Sorensen et al,36 which sought to evaluate functional outcomes of highdose corticosteroids as an adjunct to radiotherapy, 57 patients received either high-dose dexamethasone or no corticosteroid therapy. Fifty-nine percent of patients in the dexamethasone group were ambulatory 6 months after treatment compared to 39% in the group who did not receive steroids.36
A patient without a biopsy-confirmed cancer diagnosis in need of corticosteroid treatment presents a dilemma. Plasmacytomas, thymomas, lymphomas, multiple myeloma, germ-cell tumors are very sensitive to corticosteroid therapy in patients with MSCC.38 However, corticosteroids given before tissue samples are obtained may hinder proper diagnosis and complicate future management.39,40 In the absence of neurological deficit, corticosteroids may be withheld and emergent consultation with neurosurgery and oncology should be obtained. If there is any question regarding the nature of the lesion, tissue diagnosis must be obtained without delay.
Strict bed rest (including logroll and bedpan use) should be instituted if there is suspicion of spinal cord instability. Patients with suspected involvement of the cervical spine should have a Philadelphia collar placed until spinal stability has been confirmed. In the United Kingdom, the National Institutes for Health Care Excellence guidelines recommend all patients with suspected cord compression be nursed in a flat position.22 Other institutions, however, do not believe that strict bed rest is necessary, as it is presumed that MSCC is inherently different from that caused by trauma. Authors supporting this position contend that the increased incidence of deep vein thrombosis, infection (particularly from the urinary tract), and decubitus ulcers outweighs the benefit of bed rest. Patient preference should be taken into consideration as those with good functional status may be quite resistant to bed rest. In cases where cord compression is strongly suspected, these patients should be educated on proper bed rest. The greatest predictors of outcome are ambulatory and functional status at the time of diagnosis (generally based on an Eastern Cooperative Oncology Group scale). Patients with a good functional status, limited disease, and a life expectancy of greater than 3 to 6 months may benefit from surgery.41 However, emergent surgical evaluation is required in patients not responding to radiotherapy or who received received only limited doses of radiotherapy, as well as those with spinal instability, direct cord compression due to a bony fragment, impending sphincter dysfunction, unknown primary tumor, or no paraplegia for >48 hours.15
Unfortunately, surgery is only indicated in 10% to 15% of MSCC cases.42 In the past two decades, significant improvements regarding new aggressive surgical techniques have been made, and include circumferential decompression of the spine and staged or single stage anterior posterior surgery with stabilization. 43 Additionally, the combination of surgery with radiotherapy has improved outcomes.44
Most patients benefit from short-course radiotherapy45 even when given palliatively. 46 Longer courses of radiotherapy are highly recommended for patients with a more favorable prognosis.47 Up to 10% of patients diagnosed with spinal cord compression will require treatment for disease recurrence.42 There is a limited role for chemotherapy, and in seminomas and lymphomas, results can be quite dramatic.38
Prevention
Lu et al11 found that only 54% of patients were aware that back pain should be reported to their physician. Delays in diagnosis and treatment are common and well described in the literature.21 Patients should be instructed to call their physician within 24 hours from the development of any new or worsening back pain, and should be advised to seek immediate care if they develop any neurological symptoms. To facilitate appropriate and prompt management of MSCC, hospitals should develop diagnostic algorithms to minimize delays in referral to a comprehensive center for further treatment.
Case Conclusion
Based on this patient’s symptoms and status at presentation, the emergency team determined he was at high risk for MSCC. An initial dosage of 10 mg dexamethasone was administered intravenously (IV), followed by 4 mg IV every 6 hours prior to imaging. An MRI without contrast of the cervical, thoracic, and lumbar spine showed cord compression with mild cord edema at T4 level, along with diffused osseous metastasis.
Upon diagnosis, patient was referred to radiation oncology for radiotherapy of the T2-T6 vertebral bodies. Three days after initiation of radiation therapy, his neurological function deteriorated with paraplegia and incontinence, and he was emergently evaluated for neurosurgery. Although T4 laminectomy and decompression of the spinal cord were performed without complication, patient did not recover neurological function. His hospital course was complicated by Ogilvie syndrome and episodes of delirium, and he was discharged to a rehabilitation facility 23 days after admission; paraplegia and urinary and bowel incontinence remained unchanged.
- Loblaw DA, Laperriere NJ, Mackillop WJ. A population-based study of malignant spinal cord compression in Ontario. Clin Oncol (R Coll Radiol). 2003;15(4):211-217.
- Mak KS, Lee LK, Mak RH, et al. Incidence and treatment patterns in hospitalizations for malignant spinal cord compression in the United States, 1998-2006. Int J Radiat Oncol Biol Phys. 2011;80(3):824-831.
- Constans JP, de Divitiis E, Donzelli R, Spaziante R, Meder JF, Haye C. Spinal metastases with
- neurological manifestations. Review of 600 cases. J Neurosurg. 1983;59(1):111-118.
- Schiff D, O’Neill BP, Suman VJ. Spinal epidural metastasis as the initial manifestation of malignancy: clinical features and diagnostic approach. Neurology. 1997;49(2):452-456.
- Loblaw DA, Perry J, Chambers A, Laperriere NJ. Systematic review of the diagnosis and management of malignant extradural spinal cord compression: the Cancer Care Ontario Practice Guidelines Initiative’s Neuro-Oncology Disease Site Group. J Clin Oncol. 2005;23(9):2028-2037.
- Levack P, Graham J, Collie D, et al. Don’t wait for a sensory level—listen to the symptoms: a prospective audit of the delays in diagnosis of malignant cord compression. Clin Oncol (R Coll Radiol). 2002;14(6):472-480.
- Talcott JA, Stomper PC, Drislane FW, et al. Assessing suspected spinal cord compression: a multidisciplinary outcomes analysis of 342 episodes. Support Care Cancer. 1999;7(1):31-38.
- Byrne TN. Spinal cord compression from epidural metastases. N Engl J Med. 1992;327(9):614-619.
- Abrams HL, Spiro R, Goldstein N. Metastases in carcinoma; analysis of 1000 autopsied cases. Cancer. 1950;3(1):74-85.
- Wong DA, Fornasier VL, MacNab I. Spinal metastases: the obvious, the occult, and the impostors. Spine (Phila Pa 1976). 1990;15(1):1-4.
- Lu C, Gonzalez RG, Jolesz FA, Wen PY, Talcott JA. Suspected spinal cord compression in cancer patients: a multidisciplinary risk assessment. J Support Oncol. 2005;3(4):305-312.
- Schiff D, O’Neill BP, Wang CH, O’Fallon JR. Neuroimaging and treatment implications of patients with multiple epidural spinal metastases. Cancer. 1998;83(8):1593-1601.
- Arguello F, Baggs RB, Duerst RE, Johnstone L, McQueen K, Frantz CN. Pathogenesis of vertebral metastasis and epidural spinal cord compression. Cancer. 1990;65(1):98-106.
- Schiff D. Spinal cord compression. Neurol Clin. 2003;21(1):67-86, viii.
- Prasad D, Schiff D. Malignant spinal-cord compression. Lancet Oncol. 2005;6(1):15-24.
- Helweg-Larsen S, Laursen H. Clinical and autopsy findings in spinal cord compression due to metastatic disease. Eur J Neurol. 1998;5(6):587-592.
- Deyo RA, Rainville J, Kent DL. What can the history and physical examination tell us about low back pain? JAMA. 199218. Abrahm JL, Banffy MB, Harris MB. Spinal cord compression in patients with advanced metastatic cancer: "all I care about is walking and living my life." JAMA. 2008;299(8):937-946.
- Bach F, Larsen BH, Rohde K, et al. Metastatic spinal cord compression. Occurrence, symptoms, clinical presentations and prognosis in 398 patients with spinal cord compression. Acta Neurochir (Wien). 1990;107(1-2):37-43.
- Gilbert RW, Kim JH, Posner JB. Epidural spinal cord compression from metastatic tumor: diagnosis and treatment. Ann Neurol. 1978;3(1):40-51.
- Husband DJ. Malignant spinal cord compression: prospective study of delays in referral and treatment. BMJ. 1998;317(7150):18-21.
- Metastatic Spinal Cord Compression: Diagnosis and Management of Patients at Risk of or with Metastatic Spinal Cord Compression. Cardiff UK: National Collaborating Centre for Cancer; 2008.
- Shiue K, Sahgal A, Chow E, et al. Management of metastatic spinal cord compression. Expert Rev Anticancer Ther. 2010;10(5):697-708.
- Hammack JE. Spinal cord disease in patients with cancer. Continuum (Minneap Minn). 2012;18(2):312-327.
- Helweg-Larsen S. Clinical outcome in metastatic spinal cord compression. A prospective study of 153 patients. Acta Neurol Scand. 1996;94(4):269-275.
- Rades D, Fehlauer F, Schulte R, et al. Prognostic factors for local control and survival after radiotherapy of metastatic spinal cord compression. J Clin Oncol. 2006;24(21):3388-3393.
- McLinton A, Hutchison C. Malignant spinal cord compression: a retrospective audit of clinical practice at a UK regional cancer centre. Br J Cancer. 2006;94(4):486-491.
- Dugas AF, Lucas JM, Edlow JA. Diagnosis of spinal cord compression in nontrauma patients in the emergency department. Acad Emerg Med. 2011;18(7):719-725.
- Ditunno JF, Jr, Young W, Donovan WH, Creasey metastatic spinal CORD compression 18 EMERGENCY MEDICINE I january 2014 www.emed-journal.com G. American Spinal Surgery Association. The international standards booklet for neurological and functional classification of spinal cord injury. American Spinal Injury Association. Paraplegia. 1994;32(2):70-80.
- Frankel HL, Hancock DO, Hyslop G, et al. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. I. Paraplegia. 1969;7(3):179-192.
- Portenoy RK, Galer BS, Salamon O, et al. Identification of epidural neoplasm. Radiography and bone scintigraphy in the symptomatic and asymptomatic spine. Cancer. 1989;64(11):2207-2213.
- Husband DJ, Grant KA, Romaniuk CS. MRI in the diagnosis and treatment of suspected malignant spinal cord compression. Br J Radiol. 2001;74(877):15-23.
- Carmody RF, Yang PJ, Seeley GW, Seeger JF, Unger EC, Johnson JE. Spinal cord compression due to metastatic disease: diagnosis with MR imaging versus myelography. Radiology. 1989;173(1):225-229.
- Bilsky MH, Laufer I, Fourney DR, et al. Reliability analysis of the epidural spinal cord compression scale. J Neurosurg Spine. 2010;13(3):324-328.
- Loblaw DA, Mitera G, Ford M, Laperriere NJ. A 2011 updated systematic review and clinical practice guideline for the management of malignant extradural spinal cord compression. Int J Radiat Oncol Biol Phys. 2012;84(2):312-317.
- Sorensen S, Helweg-Larsen S, Mouridsen H, Hansen HH. Effect of high-dose dexamethasone in carcinomatous metastatic spinal cord compression treated with radiotherapy: a randomised trial. Eur J Cancer. 1994;30A(1):22-27.
- Heimdal K, Hirschberg H, Slettebo H, Watne K, Nome O. High incidence of serious side effects of high-dose dexamethasone treatment in patients with epidural spinal cord compression. J Neurooncol. 1992;12(2):141-144.
- Posner JB, Howieson J, Cvitkovic E. "Disappearing" spinal cord compression: oncolytic effect of glucocorticoids (and other chemotherapeutic agents) on epidural metastases. Ann Neurol. 1977;2(5):409-413.
- Kan E, Levi I, Benharroch D. Alterations in the primary diagnosis of lymphomas pretreated with corticosteroid agents. Leuk Lymphoma. 2011;52(3):425-428.
- Borenstein SH, Gerstle T, Malkin D, Thorner P, Filler RM. The effects of prebiopsy cortico-steroid treatment on the diagnosis of mediastinal lymphoma. J Pediatr Surg. 2000;35(6):973-976.
- Akram H, Allibone J. Spinal surgery for palliation in malignant spinal cord compression. Clin Oncol (R Coll Radiol). 2010;22(9):792-800.
- Rades D, Abrahm JL. The role of radiotherapy for metastatic epidural spinal cord compression. Nat Rev Clin Oncol. 2010;7(10):590-598.
- Sundaresan N, Sachdev VP, Holland JF, et al. Surgical treatment of spinal cord compression from epidural metastasis. J Clin Oncol. 1995;13(9):2330-2335.
- Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366(9486):643-648.
- Rades D, Dahm-Daphi J, Rudat V, et al. Is shortcourse radiotherapy with high doses per fraction the appropriate regimen for metastatic spinal cord compression in colorectal cancer patients? Strahlenther Onkol. 2006;182(12):708-712.
- van den Hout WB, van der Linden YM, Steenland E, et al. Single- versus multiple-fraction radiotherapy in patients with painful bone metastases: cost-utility analysis based on a randomized trial. J Natl Cancer Inst. 2003;95(3):222-229.
- Steenland E, Leer JW, van Houwelingen H, et al. The effect of a single fraction compared to multiple fractions on painful bone metastases: a global analysis of the Dutch Bone Metastasis Study. Radiother Oncol. 1999;52(2):101-109.
- Rades D, Hueppe M, Schild SE. A score to identify patients with metastatic spinal cord compression who may be candidates for best supportive care. Cancer. 2013;119(4):897-903.
- Guo Y, Palmer JL, Bianty J, Konzen B, Shin K, Bruera E. Advance directives and do-not-resuscitate orders in patients with cancer with metastatic spinal cord compression: advanced care planning implications. J Palliat Med. 2010;13(5):513-517.
- Loblaw DA, Laperriere NJ, Mackillop WJ. A population-based study of malignant spinal cord compression in Ontario. Clin Oncol (R Coll Radiol). 2003;15(4):211-217.
- Mak KS, Lee LK, Mak RH, et al. Incidence and treatment patterns in hospitalizations for malignant spinal cord compression in the United States, 1998-2006. Int J Radiat Oncol Biol Phys. 2011;80(3):824-831.
- Constans JP, de Divitiis E, Donzelli R, Spaziante R, Meder JF, Haye C. Spinal metastases with
- neurological manifestations. Review of 600 cases. J Neurosurg. 1983;59(1):111-118.
- Schiff D, O’Neill BP, Suman VJ. Spinal epidural metastasis as the initial manifestation of malignancy: clinical features and diagnostic approach. Neurology. 1997;49(2):452-456.
- Loblaw DA, Perry J, Chambers A, Laperriere NJ. Systematic review of the diagnosis and management of malignant extradural spinal cord compression: the Cancer Care Ontario Practice Guidelines Initiative’s Neuro-Oncology Disease Site Group. J Clin Oncol. 2005;23(9):2028-2037.
- Levack P, Graham J, Collie D, et al. Don’t wait for a sensory level—listen to the symptoms: a prospective audit of the delays in diagnosis of malignant cord compression. Clin Oncol (R Coll Radiol). 2002;14(6):472-480.
- Talcott JA, Stomper PC, Drislane FW, et al. Assessing suspected spinal cord compression: a multidisciplinary outcomes analysis of 342 episodes. Support Care Cancer. 1999;7(1):31-38.
- Byrne TN. Spinal cord compression from epidural metastases. N Engl J Med. 1992;327(9):614-619.
- Abrams HL, Spiro R, Goldstein N. Metastases in carcinoma; analysis of 1000 autopsied cases. Cancer. 1950;3(1):74-85.
- Wong DA, Fornasier VL, MacNab I. Spinal metastases: the obvious, the occult, and the impostors. Spine (Phila Pa 1976). 1990;15(1):1-4.
- Lu C, Gonzalez RG, Jolesz FA, Wen PY, Talcott JA. Suspected spinal cord compression in cancer patients: a multidisciplinary risk assessment. J Support Oncol. 2005;3(4):305-312.
- Schiff D, O’Neill BP, Wang CH, O’Fallon JR. Neuroimaging and treatment implications of patients with multiple epidural spinal metastases. Cancer. 1998;83(8):1593-1601.
- Arguello F, Baggs RB, Duerst RE, Johnstone L, McQueen K, Frantz CN. Pathogenesis of vertebral metastasis and epidural spinal cord compression. Cancer. 1990;65(1):98-106.
- Schiff D. Spinal cord compression. Neurol Clin. 2003;21(1):67-86, viii.
- Prasad D, Schiff D. Malignant spinal-cord compression. Lancet Oncol. 2005;6(1):15-24.
- Helweg-Larsen S, Laursen H. Clinical and autopsy findings in spinal cord compression due to metastatic disease. Eur J Neurol. 1998;5(6):587-592.
- Deyo RA, Rainville J, Kent DL. What can the history and physical examination tell us about low back pain? JAMA. 199218. Abrahm JL, Banffy MB, Harris MB. Spinal cord compression in patients with advanced metastatic cancer: "all I care about is walking and living my life." JAMA. 2008;299(8):937-946.
- Bach F, Larsen BH, Rohde K, et al. Metastatic spinal cord compression. Occurrence, symptoms, clinical presentations and prognosis in 398 patients with spinal cord compression. Acta Neurochir (Wien). 1990;107(1-2):37-43.
- Gilbert RW, Kim JH, Posner JB. Epidural spinal cord compression from metastatic tumor: diagnosis and treatment. Ann Neurol. 1978;3(1):40-51.
- Husband DJ. Malignant spinal cord compression: prospective study of delays in referral and treatment. BMJ. 1998;317(7150):18-21.
- Metastatic Spinal Cord Compression: Diagnosis and Management of Patients at Risk of or with Metastatic Spinal Cord Compression. Cardiff UK: National Collaborating Centre for Cancer; 2008.
- Shiue K, Sahgal A, Chow E, et al. Management of metastatic spinal cord compression. Expert Rev Anticancer Ther. 2010;10(5):697-708.
- Hammack JE. Spinal cord disease in patients with cancer. Continuum (Minneap Minn). 2012;18(2):312-327.
- Helweg-Larsen S. Clinical outcome in metastatic spinal cord compression. A prospective study of 153 patients. Acta Neurol Scand. 1996;94(4):269-275.
- Rades D, Fehlauer F, Schulte R, et al. Prognostic factors for local control and survival after radiotherapy of metastatic spinal cord compression. J Clin Oncol. 2006;24(21):3388-3393.
- McLinton A, Hutchison C. Malignant spinal cord compression: a retrospective audit of clinical practice at a UK regional cancer centre. Br J Cancer. 2006;94(4):486-491.
- Dugas AF, Lucas JM, Edlow JA. Diagnosis of spinal cord compression in nontrauma patients in the emergency department. Acad Emerg Med. 2011;18(7):719-725.
- Ditunno JF, Jr, Young W, Donovan WH, Creasey metastatic spinal CORD compression 18 EMERGENCY MEDICINE I january 2014 www.emed-journal.com G. American Spinal Surgery Association. The international standards booklet for neurological and functional classification of spinal cord injury. American Spinal Injury Association. Paraplegia. 1994;32(2):70-80.
- Frankel HL, Hancock DO, Hyslop G, et al. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. I. Paraplegia. 1969;7(3):179-192.
- Portenoy RK, Galer BS, Salamon O, et al. Identification of epidural neoplasm. Radiography and bone scintigraphy in the symptomatic and asymptomatic spine. Cancer. 1989;64(11):2207-2213.
- Husband DJ, Grant KA, Romaniuk CS. MRI in the diagnosis and treatment of suspected malignant spinal cord compression. Br J Radiol. 2001;74(877):15-23.
- Carmody RF, Yang PJ, Seeley GW, Seeger JF, Unger EC, Johnson JE. Spinal cord compression due to metastatic disease: diagnosis with MR imaging versus myelography. Radiology. 1989;173(1):225-229.
- Bilsky MH, Laufer I, Fourney DR, et al. Reliability analysis of the epidural spinal cord compression scale. J Neurosurg Spine. 2010;13(3):324-328.
- Loblaw DA, Mitera G, Ford M, Laperriere NJ. A 2011 updated systematic review and clinical practice guideline for the management of malignant extradural spinal cord compression. Int J Radiat Oncol Biol Phys. 2012;84(2):312-317.
- Sorensen S, Helweg-Larsen S, Mouridsen H, Hansen HH. Effect of high-dose dexamethasone in carcinomatous metastatic spinal cord compression treated with radiotherapy: a randomised trial. Eur J Cancer. 1994;30A(1):22-27.
- Heimdal K, Hirschberg H, Slettebo H, Watne K, Nome O. High incidence of serious side effects of high-dose dexamethasone treatment in patients with epidural spinal cord compression. J Neurooncol. 1992;12(2):141-144.
- Posner JB, Howieson J, Cvitkovic E. "Disappearing" spinal cord compression: oncolytic effect of glucocorticoids (and other chemotherapeutic agents) on epidural metastases. Ann Neurol. 1977;2(5):409-413.
- Kan E, Levi I, Benharroch D. Alterations in the primary diagnosis of lymphomas pretreated with corticosteroid agents. Leuk Lymphoma. 2011;52(3):425-428.
- Borenstein SH, Gerstle T, Malkin D, Thorner P, Filler RM. The effects of prebiopsy cortico-steroid treatment on the diagnosis of mediastinal lymphoma. J Pediatr Surg. 2000;35(6):973-976.
- Akram H, Allibone J. Spinal surgery for palliation in malignant spinal cord compression. Clin Oncol (R Coll Radiol). 2010;22(9):792-800.
- Rades D, Abrahm JL. The role of radiotherapy for metastatic epidural spinal cord compression. Nat Rev Clin Oncol. 2010;7(10):590-598.
- Sundaresan N, Sachdev VP, Holland JF, et al. Surgical treatment of spinal cord compression from epidural metastasis. J Clin Oncol. 1995;13(9):2330-2335.
- Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366(9486):643-648.
- Rades D, Dahm-Daphi J, Rudat V, et al. Is shortcourse radiotherapy with high doses per fraction the appropriate regimen for metastatic spinal cord compression in colorectal cancer patients? Strahlenther Onkol. 2006;182(12):708-712.
- van den Hout WB, van der Linden YM, Steenland E, et al. Single- versus multiple-fraction radiotherapy in patients with painful bone metastases: cost-utility analysis based on a randomized trial. J Natl Cancer Inst. 2003;95(3):222-229.
- Steenland E, Leer JW, van Houwelingen H, et al. The effect of a single fraction compared to multiple fractions on painful bone metastases: a global analysis of the Dutch Bone Metastasis Study. Radiother Oncol. 1999;52(2):101-109.
- Rades D, Hueppe M, Schild SE. A score to identify patients with metastatic spinal cord compression who may be candidates for best supportive care. Cancer. 2013;119(4):897-903.
- Guo Y, Palmer JL, Bianty J, Konzen B, Shin K, Bruera E. Advance directives and do-not-resuscitate orders in patients with cancer with metastatic spinal cord compression: advanced care planning implications. J Palliat Med. 2010;13(5):513-517.
Refining Transitions of Care: A Quality Improvement Project to Improve the Timeliness of Discharge Documentation
Swollen Tongue in an Immune-Compromised Host
Chlamydia trachomatis Infection: Screening and Management
Abstract
- Objective: To review current criteria and rationale for Chlamydia trachomatis screening, testing methods, and treatment of infection.
- Methods: Review of the literature.
- Results: C. trachomatis urogenital infections are an important public health problem. Screening for C. trachomatis in women age 25 and younger and men and women of any age at increased risk allows for the early treatment of disease, avoiding morbidity such as pelvic inflammatory disease, ectopic pregnancy, and chronic pelvic pain, and reducing health care costs.
- Conclusion: Current screening recommendations are not being implemented satisfactorily. Home-based methods of screening are acceptable and may improve universal screening rates.
Abstract
- Objective: To review current criteria and rationale for Chlamydia trachomatis screening, testing methods, and treatment of infection.
- Methods: Review of the literature.
- Results: C. trachomatis urogenital infections are an important public health problem. Screening for C. trachomatis in women age 25 and younger and men and women of any age at increased risk allows for the early treatment of disease, avoiding morbidity such as pelvic inflammatory disease, ectopic pregnancy, and chronic pelvic pain, and reducing health care costs.
- Conclusion: Current screening recommendations are not being implemented satisfactorily. Home-based methods of screening are acceptable and may improve universal screening rates.
Abstract
- Objective: To review current criteria and rationale for Chlamydia trachomatis screening, testing methods, and treatment of infection.
- Methods: Review of the literature.
- Results: C. trachomatis urogenital infections are an important public health problem. Screening for C. trachomatis in women age 25 and younger and men and women of any age at increased risk allows for the early treatment of disease, avoiding morbidity such as pelvic inflammatory disease, ectopic pregnancy, and chronic pelvic pain, and reducing health care costs.
- Conclusion: Current screening recommendations are not being implemented satisfactorily. Home-based methods of screening are acceptable and may improve universal screening rates.