User login
Competing Cardiovascular Outcomes
When to Worry About Incidental Renal and Adrenal Masses
› Use computed tomography studies and the Bosniak classification system to
guide management of renal cystic masses. A
› Perform laboratory tests for hypercortisolism, hyperaldosteronism, and hypersecretion of catecholamines (pheochromocytoma) on any patient with an incidental adrenal mass, regardless of signs or symptoms. C
› Refer patients with adrenal masses >4 cm for surgical evaluation. Refer any individual who has a history of malignancy and an adrenal mass for oncologic evaluation. B
Strength of recommendation (SOR)
A. Good-quality patient-oriented evidence
B. Inconsistent or limited-quality patient-oriented evidence
C. Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Jane C, a 76-year-old patient, reports lower abdominal discomfort and increased bowel movements. Her left lower quadrant is tender to palpation, without signs of a surgical abdomen, and vital signs are normal. Laboratory studies are also normal, except for mild anemia and a positive fecal occult blood test. Abdominal and pelvic computed tomography (CT), with and without contrast, are negative for acute pathology, but a 1.7-cm lesion is found in the upper pole of the left kidney. What is your next step?
Renal or adrenal masses may be discovered during imaging studies for complaints unrelated to the kidneys or adrenals. Detection of incidentalomas has increased dramatically, keeping pace with the growing use of ultrasonography, CT, and magnetic resonance imaging (MRI) for abdominal, chest, and back complaints.1
Family physicians can evaluate most of these masses and determine the need for referral by using clinical judgment, appropriate imaging studies, and screening laboratory tests. In the pages that follow, we present a systematic approach for evaluating these incidentalomas and determining when consultation or referral is needed.
Incidental renal masses are common
Lesions are commonly found in normal kidneys, and the incidence increases with age. Approximately one-third of individuals age 50 and older will have at least one renal cyst on CT.2
Most incidental renal masses are benign cysts requiring no further evaluation. Other possibilities include indeterminate or malignant cysts or solid masses, which may be malignant or benign. Inflammatory renal lesions from infection, infarction, or trauma also occur, but these tend to be symptomatic and are rarely found incidentally.
Classification of renal cysts—not based on size
Cysts are the most common adult renal masses. Typically they are unilocular and located in the renal cortex, frequently extending to the renal surface.3 Renal function is usually preserved, regardless of the cyst’s location or size. Careful examination of adjacent tissue is essential, as secondary cysts may form when solid tumors obstruct tubules of normal parenchyma. Cystic lesions containing enhancing soft tissue unattached to the wall or septa likely are malignant.4
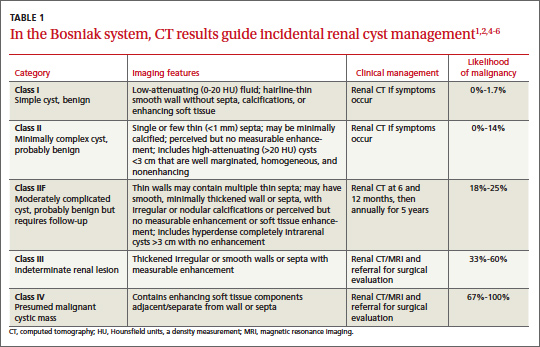
The Bosniak classification system, with 5 classes based on CT characteristics
(TABLE 1), is a useful guide for managing renal cystic lesions.4 Size is not an important feature in the Bosniak system; small cysts may be malignant and larger ones benign. Small cysts may grow into larger benign lesions, occasionally causing flank or abdominal pain, palpable masses, or hematuria.
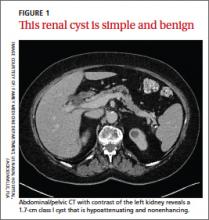
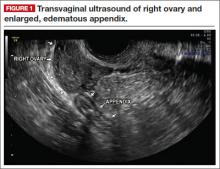
Simple cysts. Renal cysts that meet Bosniak class I criteria can be confidently labeled benign and need no further evaluation (FIGURE 1). Simple renal cysts on CT have homogenous low-attenuating fluid and thin nonenhancing walls without septa.4
On ultrasound, simple renal cysts show spherical or ovoid shape without internal echoes, a thin smooth wall separate from the surrounding parenchyma, and posterior wall enhancement caused by increased transmission through the water-filled cyst. The likelihood of malignancy is extremely low in a renal cyst that meets these criteria, which have a reported accuracy of 98% to 100%.3 Thus, no further evaluation is required if an obviously benign simple cyst is first noted on an adequate ultrasound. Inadequate ultrasound visualization or evidence of calcifications, septa, or multiple chambers calls for prompt renal CT.
CASE The mass on Ms. C’s left kidney is hypoattenuating and nonenhancing on CT. It meets Bosniak criteria for a benign simple cyst (class I) and requires no further evaluation or follow-up. Colonoscopy detects multiple colonic polyps that are removed, and the patient does well.
Mildly complicated cysts. Less diagnostic certainty characterizes cysts with mild abnormalities that keep them from being labeled as simple. Bosniak classes II and IIF describe mildly abnormal renal cysts. Class II cysts can be dismissed, whereas class IIF cysts require follow-up.
Class II cysts may contain a few hairline septa, fine calcium deposits in walls or septa, or an unmeasurable enhancement of the walls. A hyperattenuating but nonenhancing fluid also is described as category II. Small homogeneous cysts <3 cm, without enhancement but hyperattenuated, are reliably considered benign and need not be evaluated.2,7
Class IIF cysts may have multiple hairline-thin septa with unmeasurable enhancement or minimal smooth thickening or irregular/nodular calcifications of wall or septa without enhancing soft tissue components. Hyperattenuating cystic lesions >3 cm and intrarenal “noncortical” cysts are included in this category. Class IIF cysts require follow-up at 6 months with CT or MRI, then annually for at least 5 years.8
Obviously complicated cysts. Bosniak class III is indeterminate—neither benign nor clearly malignant. Class III cysts may have thickened borders or septa with measurable enhancement, or they may be multilocular, hemorrhagic, or infected. In 5 case series, 29 of 57 class III lesions proved to be malignant.5 MRI may characterize these lesions more definitively than CT prior to urologic referral.
Malignant cysts. Bosniak class IV renal lesions are clearly malignant, with large heterogeneous cysts or necrotic components, shaggy thickened walls, or enhancing soft tissue components separate from the wall or septa. Their unequivocal appearance results from solid tumor necrosis and liquefaction. Diagnosis is straightforward, and excision is indicated.2
A closer look at solid renal masses
Solid renal masses usually consist of enhancing tissue with little or no fluid. The goal of evaluation is to exclude malignancies, such as renal cell cancer, lymphomas, sarcomas, or metastasis. Benign solid masses include renal adenomas, angiomyolipomas, and oncocytomas, among others.
Several lesions can be diagnosed by appearance or symptoms:
Angiomyolipomas are recognized by their fat content within a noncalcified mass. Unenhanced CT usually is sufficient for diagnosis, unless the mass is very small or has atypical features.9
Vascular lesions can be identified because they enhance to the same degree as the vasculature. With the exception of inflammatory or vascular abnormalities, all enhancing lesions that do not contain fat should be presumed to be malignant.
In patients with a known extrarenal primary malignancy, 50% to 85% of incidental solid renal masses will represent metastatic disease.10 Percutaneous biopsy may be warranted to differentiate metastatic lesions from a secondary, primary (ie, renal cell carcinoma), or benign process.11
A study of 2770 solid renal mass excisions revealed that 12.8% were benign, with a direct relationship between malignancy and size. Masses <1 cm were benign 44% of the time.12 Early identification of small renal carcinomas may improve survival rates. Although renal cell carcinomas <3 cm in diameter have low metastatic potential, a solid, nonfat-containing mass should be evaluated for aggressive nephron-sparing surgery.6,13
Incidental adrenal masses occur infrequently
Adrenal incidentalomas are defined as radiographically identified masses >1 cm in diameter.14 They are much less common than their renal counterparts, with a reported prevalence of 0.35% to 5% on CT.15 Because the adrenal glands are hormonally active and receive substantial blood flow, metastatic, hormonally active, and nonfunctional causes for adrenal masses need to be considered.16
Adrenal pathology
Adrenal masses may be characterized by increased or normal adrenal function. Hyperfunctioning syndromes include hypercortisolism, hyperaldosteronism, adrenogenital hypersecretion of adrenocortical origin, and pheochromocytomas of the medulla. Symptom evaluation of these syndromes is important, but not sufficient to rule out a hyperfunctioning syndrome.
In a retrospective review of inapparent adrenal masses, ≤13% of pheochromocytomas were clinically silent.17 Therefore, laboratory testing is necessary for an incidental adrenal mass.
Nonfunctional lesions include adenomas, metastases, cysts, myelolipomas, hemorrhage, and adrenal carcinomas. These masses require evaluation for the possibility of cancer, the most common of which is metastasis. In patients with an extra-adrenal malignancy, the likelihood of malignancy in an incidental adrenal mass is at least 50%.18 An adrenal mass representing metastasis of a previously unrecognized cancer is exceedingly rare.19
Primary adrenal carcinoma is also rare, with an estimated incidence of 2 cases per one million in the general population. For patients with adrenal masses, the prevalence of carcinoma increases with lesion size (2% for tumors <4 cm, 6% for tumors 4-6 cm, and 25% for tumors >6 cm in diameter). 17 For this reason, tumors >4 cm in diameter are usually surgically resected in patients with no previous cancer history, unless radiologic criteria demonstrate clearly benign characteristics.
Although adrenal carcinomas are considered nonfunctioning, some evidence suggests they produce low levels of cortisol that may be associated with clinical features of metabolic syndrome.20
CT is first choice for adrenal mass evaluation
Dedicated adrenal CT with both unenhanced and delayed contrast-enhanced images is the most reliable study to evaluate an adrenal mass, according to the American College of Radiology. Consider another study only in patients with contrast allergy, renal compromise, or cancer history.21
Unenhanced CT can diagnose the approximately 70% of adenomas that are small, well-defined round masses with homogenous low-density lipid deposition.22 Delayed contrast enhancement can characterize most of the remaining 30%.23 Unenhanced CT with attenuation values of <10 Hounsfield units (HU) can diagnose adenomas with 71% specificity and 98% sensitivity,24 and can often diagnose simple cysts and myelolipomas, as well.
Other imaging options. MRI is an alternative to CT for patients with contraindications for contrast or radiation exposure. MRI provides less spatial resolution than CT, but chemical shift imaging can measure cytoplasmic lipid content similar to unenhanced CT. A small study found chemical shift MRI more reliable than unenhanced CT, but less reliable than CT with delayed contrast enhancement.25
Positron emission tomography (PET) is useful to noninvasively evaluate biochemical and physiologic processes. PET-CT incorporates unenhanced CT density measurements to improve PET accuracy. In a patient with a history of cancer, PET-CT has a sensitivity of 93% to 100% and a specificity of 95% in differentiating benign from malignant adrenal tumors.26
When to order a biopsy
The need for biopsy has decreased as imaging has improved, but biopsy is required whenever diagnostic imaging fails to differentiatea lesion as benign or malignant. CT guided biopsy provides diagnostic accuracy of 85% to 95%.27 Complications such as pneumothorax, hemorrhage, and bacteremia occur in 3% to 9% of biopsies. Before any adrenal biopsy, measure plasma-free metanephrines to exclude undiagnosed pheochromocytoma, which could precipitate a hypertensive crisis if untreated.22
These 3 laboratory screening tests are critical
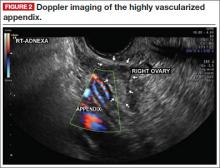
Family physicians can perform the initial biochemical evaluation of an adrenal incidentaloma. Guidance is available from the National Institutes of Health (NIH)28 and the American Academy of Clinical Endocrinologists (AACE) (FIGURE 2).29
Regardless of signs or symptoms, perform screening laboratory tests for 3 types of adrenal hyperfunction: hypercortisolism, hyperaldosteronism, and hypersecretion of catecholamines (pheochromocytoma). Screening tests are not recommended for androgen hypersecretion, which is extremely rare and causes recognizable symptoms such as hirsutism (Table 2).29
Hypercortisolism occurs in approximately 5% of adrenal incidentalomas.30 An overnight dexamethasone suppression test (DST) is most reliable for screening, with sensitivity >95% for Cushing syndrome.31 The patient takes a 1-mg dose of oral dexamethasone at 11 pm, and a fasting plasma cortisol sample is drawn the next day at 8 am.
Dexamethasone binds to glucocorticoid receptors in the pituitary gland, suppressing adrenocorticotropic hormone secretion. Cortisol will be depressed the next morning unless the adrenal mass produces cortisol autonomously. Patients with a DST >5 mcg/dL—highly suggestive of Cushing syndrome—require further evaluation, and we suggest referral to an endocrinologist.
Hyperaldosteronism is seen in 1% to 2% of adrenal incidentalomas.32 The aldosterone- to-renin ratio (ARR) is recommended as a screening test for hyperaldosteronism, with an ARR >20 requiring further testing.33 Medications that may affect the ARR include beta-blockers, spironolactone, clonidine, diuretics, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers.29
Refer a patient with evidence of hyperaldosteronism to an endocrinologist and a surgeon with experience in managing these lesions. If the ARR test result suggests an aldosterone excess, a salt-loading test is used to verify failure of aldosterone suppression. Adrenal venous sampling is often performed prior to surgical removal to confirm that an incidentaloma is the source of hyperaldosteronism.
Pheochromocytoma. Approximately 5% of incidental adrenal lesions are pheochromocytomas.30 Many patients with these epinephrine/norepinephrine secreting tumors do not show the classic symptom triad of headache, palpitations, and diaphoresis, and approximately half have normal blood pressure.34
Identifying a pheochromocytoma is important in any patient requiring surgery or biopsy, as surgical manipulation can cause a potentially fatal intraoperative catecholamine surge. Presurgical medical management can mitigate this reaction.
A plasma-free metanephrines test, which has 95% sensitivity, is the most reliable test for pheochromocytoma.35 Medications, including tricyclic antidepressants, decongestants, amphetamines, reserpine, and phenoxybenzamine, can cause falsepositive results.29 Confirm a positive plasma-free metanephrines test with a 24-hour fractionated urine metanephrines test, and refer the patient to an endocrinologist.
Managing adrenal incidentalomas
Refer all patients with adrenal masses >4 cm for surgical evaluation because of the risk of malignancy; all patients who have a history of malignancy and an adrenal mass of any size require a referral to an oncologist. Perform the AACE-recommended 3-element biochemical workup for all masses, with the exception of definitively diagnosed cysts or myelolipomas.
Refer to an endocrinologist all patients with abnormal screening laboratory results, regardless of adrenal mass size, as well as patients with concerning clinical findings. Initiate cardiovascular, diabetes, and bone density evaluation and management for metabolic syndrome.20
Monitoring after a negative workup
Little evidence exists to guide monitoring of small adrenal incidentalomas (<4 cm) with a negative workup. The 2002 NIH report recommended annual radiologic follow-up for 5 years,28 whereas the 2009 AACE guidelines recommend radiographic follow-up at 3 to 6 months, then at one and 2 years.29
Evidence indicates that 14% of lesions will enlarge in 2 years, although the clinical significance of enlargement is unknown. Some authors argue against CT monitoring because the risk of adrenal mass progression is similar to the malignancy risk posed by 3 years of radiation exposure with CT.20
Some guidelines recommend repeat biochemical screening every 3 to 4 years.28,29 AACE guidelines quote a 47% rate of progression over 3 years, but most adrenal masses progress to subclinical Cushing syndrome— a condition of uncertain significance. Subclinical Cushing’s has not been reported to progress to the overt syndrome, and new catecholamine or aldosterone secretion is rare.
Many endocrinologists reduce the frequency of follow-up, depending on the type of adrenal mass (cyst or solid) and its size. AACE suggests CT for adenomas one to 4 cm at 12 months. AACE and NIH recommend hormonal evaluation annually for 4 years. Adrenal cysts or myelolipoma in patients without cancer need no follow-up.29
CORRESPONDENCE
James C. Higgins, DO, CAPT, MC, USN, Ret., Naval Hospital Jacksonville, Family Medicine Department, 2080 Child Street, Box 1000, Jacksonville, FL 32214;
[email protected]
1. Berland LL, Silverman SG, Gore RM, et al. Managing incidental findings on abdominal CT: white paper of the ACR incidental findings committee. J Am Coll Radiol. 2010;7:754-773.
2. Silverman S, Israel G, Herts B, et al. Management of the incidental renal mass. Radiology. 2008;249:16-31.
3. Curry NS, Bissada NK. Radiologic evaluation of small and indeterminate renal masses. Urol Clin North Am. 1997;24:493-505.
4. Bosniak MA. The current radiological approach to renal cysts. Radiology. 1986;158:1-10.
5. Harisinghani M, Maher M, Gervais D, et al. Incidence of malignancy in complex cystic renal masses (Bosniak category III): should imaging guided biopsy precede surgery? AJR Am J Roentgenol. 2003;180:755-758.
6. Remzi M, Ozsoy M, Klingler HC. Are small renal tumors harmless? Analysis of histopathological features according to tumors less than 4 cm in diameter. J Urol. 2006;176:896-899.
7. Jonisch AI, Rubinowitz A, Mutalik P, et al. Can high attenuation renal cysts be differentiated from renal cell carcinoma at unenhanced computed tomography? Radiology. 2007;243:445-450.
8. Israel GM, Bosniak MA. Follow-up CT of moderately complex cystic lesions of the kidney. AJR Am J Roentgenol. 2003;181: 627-633.
9. Bosniak MA, Megibow AJ, Hulnick DH, et al. CT diagnosis of renal angiomyolipoma: the importance of detecting small amounts of fat. AJR Am J Roentengol. 1988;151:497-501.
10. Mitnick JS, Bosniak MA, Rothberg M, et al. Metastatic neoplasm to the kidney studied by computed tomography and sonogram. J Comput Assist Tomogr. 1985;9:43-49.
11. Rybicki FJ, Shu KM, Cibas ES, et al. Percutaneous biopsy of renal masses: sensitivity and negative predictive value stratified by clinical setting and size of masses. AJR Am J Roentgenol. 2003;180:1281-1287.
12. Frank I, Blure MI, Cheville JC, et al. Solid renal tumors: an analysis of pathological features related to tumor size. J Urol. 2003;170:2217-2220.
13. Hollingsworth JM, Miller DC, Daignault S, et al. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst. 2006;98:1331-1334.
14. Geelhoed GW, Spiegel CT. “Incidental” adrenal cyst: a correctable lesion possibly associated with hypertension. South Med J. 1981;74:626-630.
15. Davenport C, Liew A, Doherty B, et al. The prevalence of adrenal incidentaloma in routine clinical practice. Endocrine. 2011;40: 80-83.
16. Cook DM, Loriaux LD. The incidental adrenal mass. Am J Med. 1996;101:88 94.
17. Mansmann G, Lau J, Balk E, et al. The clinically inapparent adrenal mass: update in diagnosis and management. Endocr Rev. 2004;25:309-340.
18. Androulakis II, Kaltsas G, Piatitis G, et al. The clinical significance of adrenal incidentalomas. Eur J Clin Invest. 2011;41: 552-560.
19. Lee JE, Evans DB, Hickey RC, et al. Unknown primary cancer presenting as an adrenal mass: frequency and implications for diagnostic evaluation of adrenal incidentalomas. Surgery. 1998;124:1115-1122.
20. Aron D, Terzolo M, Cawood TJ. Adrenal incidentalomas. Best Pract Res Clin Endocrinol Metab. 2012;26:69-82.
21. ACR appropriateness criteria: incidentally discovered adrenal mass. American College of Radiology. Available at: http://www.acr.org/~/media/ACR/Documents/AppCriteria/Diagnostic/IncidentallyDiscoveredAdrenalMass.pdf. Accessed November 20, 2012.
22. Song JH, Mayo-Smith WW. Incidentally discovered adrenal mass. Radiol Clin North Am. 2011;49:361-368.
23. Korobkin M, Brodeur FJ, Francis IR, et al. CT time-attenuation washout curves of adrenal adenomas and nonadenomas. AJR Am J Roentgenol. 1998;170:747-752.
24. Boland GW, Lee MJ, Gazelle GS, et al. Characterization of adrenal masses using unenhanced CT: an analysis of the CT literature. AJR Am J Roentgenol. 1998;171:201-204.
25. Park BK, Kim CK, Kim B, et al. Chemical shift MR imaging of hyperattenuating (>10 HU) adrenal masses: does it still have a role? Radiology. 2004;231:711-716.
26. Boland GW, Blake MA, Holakere NS, et al. PET/CT for the characterization of adrenal masses in patients with cancer: qualitative vs quantitative accuracy in 150 consecutive patients. AJR Am J Roentgenol. 2009;192:956-962.
27. Paulsen SD, Nghiem HV, Korobkin M, et al. Changing role of imaging- guided percutaneous biopsy of adrenal masses: evaluation of 50 adrenal biopsies. AJR Am J Roentgenol. 2004;182:1033-1037
28. Grumbach MM, Biller BMK, Braunstein GD, et al. Management of the clinically inapparent adrenal mass (“incidentalomas”). Ann Intern Med. 2003;138:424-429.
29. Zeiger MA, Thompson GB, Quan-Yang D, et al. American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons medical guidelines for the management of adrenal incidentalomas. Endocr Pract. 2009;15(suppl 1):1-20.
30. Young WF. The incidentally discovered adrenal mass. N Engl J Med. 2007; 356:601-610.
31. Deutschbein T, Unger N, Hinrichs J, et al. Late-night and lowdose dexamethasone-suppressed cortisol in saliva and serum for the diagnosis of cortisol-secreting adrenal adenomas. Eur J Endocrinol. 2009;161:747-753.
32. Bernini G, Moretti A, Gianfranco A, et al. Primary aldosteronism in normokalemic patients with adrenal incidentalomas. Eur J Endocrinol. 2002;146:523-529.
33. Montori VM, Young WF Jr. Use of plasma aldosterone concentration-to-plasma renin activity ratio as a screening test for primary aldosteronism: a systematic review of the literature. Endocrinol Metab Clin North Am. 2002;31:619-632.
34. Motta-Ramirez GA, Remer EM, Herts BR, et al. Comparison of CT findings in symptomatic and incidentally discovered pheochromocytomas. AJR Am J Roentgenol. 2005;185:684-688.
35. Pacak K, Eisenhofer G, Grossman A. The incidentally discovered adrenal mass. N Engl J Med. 2007;356:2005.
› Use computed tomography studies and the Bosniak classification system to
guide management of renal cystic masses. A
› Perform laboratory tests for hypercortisolism, hyperaldosteronism, and hypersecretion of catecholamines (pheochromocytoma) on any patient with an incidental adrenal mass, regardless of signs or symptoms. C
› Refer patients with adrenal masses >4 cm for surgical evaluation. Refer any individual who has a history of malignancy and an adrenal mass for oncologic evaluation. B
Strength of recommendation (SOR)
A. Good-quality patient-oriented evidence
B. Inconsistent or limited-quality patient-oriented evidence
C. Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Jane C, a 76-year-old patient, reports lower abdominal discomfort and increased bowel movements. Her left lower quadrant is tender to palpation, without signs of a surgical abdomen, and vital signs are normal. Laboratory studies are also normal, except for mild anemia and a positive fecal occult blood test. Abdominal and pelvic computed tomography (CT), with and without contrast, are negative for acute pathology, but a 1.7-cm lesion is found in the upper pole of the left kidney. What is your next step?
Renal or adrenal masses may be discovered during imaging studies for complaints unrelated to the kidneys or adrenals. Detection of incidentalomas has increased dramatically, keeping pace with the growing use of ultrasonography, CT, and magnetic resonance imaging (MRI) for abdominal, chest, and back complaints.1
Family physicians can evaluate most of these masses and determine the need for referral by using clinical judgment, appropriate imaging studies, and screening laboratory tests. In the pages that follow, we present a systematic approach for evaluating these incidentalomas and determining when consultation or referral is needed.
Incidental renal masses are common
Lesions are commonly found in normal kidneys, and the incidence increases with age. Approximately one-third of individuals age 50 and older will have at least one renal cyst on CT.2
Most incidental renal masses are benign cysts requiring no further evaluation. Other possibilities include indeterminate or malignant cysts or solid masses, which may be malignant or benign. Inflammatory renal lesions from infection, infarction, or trauma also occur, but these tend to be symptomatic and are rarely found incidentally.

Classification of renal cysts—not based on size
Cysts are the most common adult renal masses. Typically they are unilocular and located in the renal cortex, frequently extending to the renal surface.3 Renal function is usually preserved, regardless of the cyst’s location or size. Careful examination of adjacent tissue is essential, as secondary cysts may form when solid tumors obstruct tubules of normal parenchyma. Cystic lesions containing enhancing soft tissue unattached to the wall or septa likely are malignant.4
The Bosniak classification system, with 5 classes based on CT characteristics
(TABLE 1), is a useful guide for managing renal cystic lesions.4 Size is not an important feature in the Bosniak system; small cysts may be malignant and larger ones benign. Small cysts may grow into larger benign lesions, occasionally causing flank or abdominal pain, palpable masses, or hematuria.
Simple cysts. Renal cysts that meet Bosniak class I criteria can be confidently labeled benign and need no further evaluation (FIGURE 1). Simple renal cysts on CT have homogenous low-attenuating fluid and thin nonenhancing walls without septa.4
On ultrasound, simple renal cysts show spherical or ovoid shape without internal echoes, a thin smooth wall separate from the surrounding parenchyma, and posterior wall enhancement caused by increased transmission through the water-filled cyst. The likelihood of malignancy is extremely low in a renal cyst that meets these criteria, which have a reported accuracy of 98% to 100%.3 Thus, no further evaluation is required if an obviously benign simple cyst is first noted on an adequate ultrasound. Inadequate ultrasound visualization or evidence of calcifications, septa, or multiple chambers calls for prompt renal CT.
CASE The mass on Ms. C’s left kidney is hypoattenuating and nonenhancing on CT. It meets Bosniak criteria for a benign simple cyst (class I) and requires no further evaluation or follow-up. Colonoscopy detects multiple colonic polyps that are removed, and the patient does well.
Mildly complicated cysts. Less diagnostic certainty characterizes cysts with mild abnormalities that keep them from being labeled as simple. Bosniak classes II and IIF describe mildly abnormal renal cysts. Class II cysts can be dismissed, whereas class IIF cysts require follow-up.
Class II cysts may contain a few hairline septa, fine calcium deposits in walls or septa, or an unmeasurable enhancement of the walls. A hyperattenuating but nonenhancing fluid also is described as category II. Small homogeneous cysts <3 cm, without enhancement but hyperattenuated, are reliably considered benign and need not be evaluated.2,7
Class IIF cysts may have multiple hairline-thin septa with unmeasurable enhancement or minimal smooth thickening or irregular/nodular calcifications of wall or septa without enhancing soft tissue components. Hyperattenuating cystic lesions >3 cm and intrarenal “noncortical” cysts are included in this category. Class IIF cysts require follow-up at 6 months with CT or MRI, then annually for at least 5 years.8
Obviously complicated cysts. Bosniak class III is indeterminate—neither benign nor clearly malignant. Class III cysts may have thickened borders or septa with measurable enhancement, or they may be multilocular, hemorrhagic, or infected. In 5 case series, 29 of 57 class III lesions proved to be malignant.5 MRI may characterize these lesions more definitively than CT prior to urologic referral.
Malignant cysts. Bosniak class IV renal lesions are clearly malignant, with large heterogeneous cysts or necrotic components, shaggy thickened walls, or enhancing soft tissue components separate from the wall or septa. Their unequivocal appearance results from solid tumor necrosis and liquefaction. Diagnosis is straightforward, and excision is indicated.2
A closer look at solid renal masses
Solid renal masses usually consist of enhancing tissue with little or no fluid. The goal of evaluation is to exclude malignancies, such as renal cell cancer, lymphomas, sarcomas, or metastasis. Benign solid masses include renal adenomas, angiomyolipomas, and oncocytomas, among others.
Several lesions can be diagnosed by appearance or symptoms:
Angiomyolipomas are recognized by their fat content within a noncalcified mass. Unenhanced CT usually is sufficient for diagnosis, unless the mass is very small or has atypical features.9
Vascular lesions can be identified because they enhance to the same degree as the vasculature. With the exception of inflammatory or vascular abnormalities, all enhancing lesions that do not contain fat should be presumed to be malignant.
In patients with a known extrarenal primary malignancy, 50% to 85% of incidental solid renal masses will represent metastatic disease.10 Percutaneous biopsy may be warranted to differentiate metastatic lesions from a secondary, primary (ie, renal cell carcinoma), or benign process.11
A study of 2770 solid renal mass excisions revealed that 12.8% were benign, with a direct relationship between malignancy and size. Masses <1 cm were benign 44% of the time.12 Early identification of small renal carcinomas may improve survival rates. Although renal cell carcinomas <3 cm in diameter have low metastatic potential, a solid, nonfat-containing mass should be evaluated for aggressive nephron-sparing surgery.6,13
Incidental adrenal masses occur infrequently
Adrenal incidentalomas are defined as radiographically identified masses >1 cm in diameter.14 They are much less common than their renal counterparts, with a reported prevalence of 0.35% to 5% on CT.15 Because the adrenal glands are hormonally active and receive substantial blood flow, metastatic, hormonally active, and nonfunctional causes for adrenal masses need to be considered.16
Adrenal pathology
Adrenal masses may be characterized by increased or normal adrenal function. Hyperfunctioning syndromes include hypercortisolism, hyperaldosteronism, adrenogenital hypersecretion of adrenocortical origin, and pheochromocytomas of the medulla. Symptom evaluation of these syndromes is important, but not sufficient to rule out a hyperfunctioning syndrome.
In a retrospective review of inapparent adrenal masses, ≤13% of pheochromocytomas were clinically silent.17 Therefore, laboratory testing is necessary for an incidental adrenal mass.
Nonfunctional lesions include adenomas, metastases, cysts, myelolipomas, hemorrhage, and adrenal carcinomas. These masses require evaluation for the possibility of cancer, the most common of which is metastasis. In patients with an extra-adrenal malignancy, the likelihood of malignancy in an incidental adrenal mass is at least 50%.18 An adrenal mass representing metastasis of a previously unrecognized cancer is exceedingly rare.19
Primary adrenal carcinoma is also rare, with an estimated incidence of 2 cases per one million in the general population. For patients with adrenal masses, the prevalence of carcinoma increases with lesion size (2% for tumors <4 cm, 6% for tumors 4-6 cm, and 25% for tumors >6 cm in diameter). 17 For this reason, tumors >4 cm in diameter are usually surgically resected in patients with no previous cancer history, unless radiologic criteria demonstrate clearly benign characteristics.
Although adrenal carcinomas are considered nonfunctioning, some evidence suggests they produce low levels of cortisol that may be associated with clinical features of metabolic syndrome.20
CT is first choice for adrenal mass evaluation
Dedicated adrenal CT with both unenhanced and delayed contrast-enhanced images is the most reliable study to evaluate an adrenal mass, according to the American College of Radiology. Consider another study only in patients with contrast allergy, renal compromise, or cancer history.21
Unenhanced CT can diagnose the approximately 70% of adenomas that are small, well-defined round masses with homogenous low-density lipid deposition.22 Delayed contrast enhancement can characterize most of the remaining 30%.23 Unenhanced CT with attenuation values of <10 Hounsfield units (HU) can diagnose adenomas with 71% specificity and 98% sensitivity,24 and can often diagnose simple cysts and myelolipomas, as well.
Other imaging options. MRI is an alternative to CT for patients with contraindications for contrast or radiation exposure. MRI provides less spatial resolution than CT, but chemical shift imaging can measure cytoplasmic lipid content similar to unenhanced CT. A small study found chemical shift MRI more reliable than unenhanced CT, but less reliable than CT with delayed contrast enhancement.25
Positron emission tomography (PET) is useful to noninvasively evaluate biochemical and physiologic processes. PET-CT incorporates unenhanced CT density measurements to improve PET accuracy. In a patient with a history of cancer, PET-CT has a sensitivity of 93% to 100% and a specificity of 95% in differentiating benign from malignant adrenal tumors.26
When to order a biopsy
The need for biopsy has decreased as imaging has improved, but biopsy is required whenever diagnostic imaging fails to differentiatea lesion as benign or malignant. CT guided biopsy provides diagnostic accuracy of 85% to 95%.27 Complications such as pneumothorax, hemorrhage, and bacteremia occur in 3% to 9% of biopsies. Before any adrenal biopsy, measure plasma-free metanephrines to exclude undiagnosed pheochromocytoma, which could precipitate a hypertensive crisis if untreated.22
These 3 laboratory screening tests are critical
Family physicians can perform the initial biochemical evaluation of an adrenal incidentaloma. Guidance is available from the National Institutes of Health (NIH)28 and the American Academy of Clinical Endocrinologists (AACE) (FIGURE 2).29
Regardless of signs or symptoms, perform screening laboratory tests for 3 types of adrenal hyperfunction: hypercortisolism, hyperaldosteronism, and hypersecretion of catecholamines (pheochromocytoma). Screening tests are not recommended for androgen hypersecretion, which is extremely rare and causes recognizable symptoms such as hirsutism (Table 2).29
Hypercortisolism occurs in approximately 5% of adrenal incidentalomas.30 An overnight dexamethasone suppression test (DST) is most reliable for screening, with sensitivity >95% for Cushing syndrome.31 The patient takes a 1-mg dose of oral dexamethasone at 11 pm, and a fasting plasma cortisol sample is drawn the next day at 8 am.
Dexamethasone binds to glucocorticoid receptors in the pituitary gland, suppressing adrenocorticotropic hormone secretion. Cortisol will be depressed the next morning unless the adrenal mass produces cortisol autonomously. Patients with a DST >5 mcg/dL—highly suggestive of Cushing syndrome—require further evaluation, and we suggest referral to an endocrinologist.
Hyperaldosteronism is seen in 1% to 2% of adrenal incidentalomas.32 The aldosterone- to-renin ratio (ARR) is recommended as a screening test for hyperaldosteronism, with an ARR >20 requiring further testing.33 Medications that may affect the ARR include beta-blockers, spironolactone, clonidine, diuretics, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers.29
Refer a patient with evidence of hyperaldosteronism to an endocrinologist and a surgeon with experience in managing these lesions. If the ARR test result suggests an aldosterone excess, a salt-loading test is used to verify failure of aldosterone suppression. Adrenal venous sampling is often performed prior to surgical removal to confirm that an incidentaloma is the source of hyperaldosteronism.
Pheochromocytoma. Approximately 5% of incidental adrenal lesions are pheochromocytomas.30 Many patients with these epinephrine/norepinephrine secreting tumors do not show the classic symptom triad of headache, palpitations, and diaphoresis, and approximately half have normal blood pressure.34
Identifying a pheochromocytoma is important in any patient requiring surgery or biopsy, as surgical manipulation can cause a potentially fatal intraoperative catecholamine surge. Presurgical medical management can mitigate this reaction.
A plasma-free metanephrines test, which has 95% sensitivity, is the most reliable test for pheochromocytoma.35 Medications, including tricyclic antidepressants, decongestants, amphetamines, reserpine, and phenoxybenzamine, can cause falsepositive results.29 Confirm a positive plasma-free metanephrines test with a 24-hour fractionated urine metanephrines test, and refer the patient to an endocrinologist.
Managing adrenal incidentalomas
Refer all patients with adrenal masses >4 cm for surgical evaluation because of the risk of malignancy; all patients who have a history of malignancy and an adrenal mass of any size require a referral to an oncologist. Perform the AACE-recommended 3-element biochemical workup for all masses, with the exception of definitively diagnosed cysts or myelolipomas.
Refer to an endocrinologist all patients with abnormal screening laboratory results, regardless of adrenal mass size, as well as patients with concerning clinical findings. Initiate cardiovascular, diabetes, and bone density evaluation and management for metabolic syndrome.20
Monitoring after a negative workup
Little evidence exists to guide monitoring of small adrenal incidentalomas (<4 cm) with a negative workup. The 2002 NIH report recommended annual radiologic follow-up for 5 years,28 whereas the 2009 AACE guidelines recommend radiographic follow-up at 3 to 6 months, then at one and 2 years.29
Evidence indicates that 14% of lesions will enlarge in 2 years, although the clinical significance of enlargement is unknown. Some authors argue against CT monitoring because the risk of adrenal mass progression is similar to the malignancy risk posed by 3 years of radiation exposure with CT.20
Some guidelines recommend repeat biochemical screening every 3 to 4 years.28,29 AACE guidelines quote a 47% rate of progression over 3 years, but most adrenal masses progress to subclinical Cushing syndrome— a condition of uncertain significance. Subclinical Cushing’s has not been reported to progress to the overt syndrome, and new catecholamine or aldosterone secretion is rare.
Many endocrinologists reduce the frequency of follow-up, depending on the type of adrenal mass (cyst or solid) and its size. AACE suggests CT for adenomas one to 4 cm at 12 months. AACE and NIH recommend hormonal evaluation annually for 4 years. Adrenal cysts or myelolipoma in patients without cancer need no follow-up.29
CORRESPONDENCE
James C. Higgins, DO, CAPT, MC, USN, Ret., Naval Hospital Jacksonville, Family Medicine Department, 2080 Child Street, Box 1000, Jacksonville, FL 32214;
[email protected]
› Use computed tomography studies and the Bosniak classification system to
guide management of renal cystic masses. A
› Perform laboratory tests for hypercortisolism, hyperaldosteronism, and hypersecretion of catecholamines (pheochromocytoma) on any patient with an incidental adrenal mass, regardless of signs or symptoms. C
› Refer patients with adrenal masses >4 cm for surgical evaluation. Refer any individual who has a history of malignancy and an adrenal mass for oncologic evaluation. B
Strength of recommendation (SOR)
A. Good-quality patient-oriented evidence
B. Inconsistent or limited-quality patient-oriented evidence
C. Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Jane C, a 76-year-old patient, reports lower abdominal discomfort and increased bowel movements. Her left lower quadrant is tender to palpation, without signs of a surgical abdomen, and vital signs are normal. Laboratory studies are also normal, except for mild anemia and a positive fecal occult blood test. Abdominal and pelvic computed tomography (CT), with and without contrast, are negative for acute pathology, but a 1.7-cm lesion is found in the upper pole of the left kidney. What is your next step?
Renal or adrenal masses may be discovered during imaging studies for complaints unrelated to the kidneys or adrenals. Detection of incidentalomas has increased dramatically, keeping pace with the growing use of ultrasonography, CT, and magnetic resonance imaging (MRI) for abdominal, chest, and back complaints.1
Family physicians can evaluate most of these masses and determine the need for referral by using clinical judgment, appropriate imaging studies, and screening laboratory tests. In the pages that follow, we present a systematic approach for evaluating these incidentalomas and determining when consultation or referral is needed.
Incidental renal masses are common
Lesions are commonly found in normal kidneys, and the incidence increases with age. Approximately one-third of individuals age 50 and older will have at least one renal cyst on CT.2
Most incidental renal masses are benign cysts requiring no further evaluation. Other possibilities include indeterminate or malignant cysts or solid masses, which may be malignant or benign. Inflammatory renal lesions from infection, infarction, or trauma also occur, but these tend to be symptomatic and are rarely found incidentally.

Classification of renal cysts—not based on size
Cysts are the most common adult renal masses. Typically they are unilocular and located in the renal cortex, frequently extending to the renal surface.3 Renal function is usually preserved, regardless of the cyst’s location or size. Careful examination of adjacent tissue is essential, as secondary cysts may form when solid tumors obstruct tubules of normal parenchyma. Cystic lesions containing enhancing soft tissue unattached to the wall or septa likely are malignant.4
The Bosniak classification system, with 5 classes based on CT characteristics
(TABLE 1), is a useful guide for managing renal cystic lesions.4 Size is not an important feature in the Bosniak system; small cysts may be malignant and larger ones benign. Small cysts may grow into larger benign lesions, occasionally causing flank or abdominal pain, palpable masses, or hematuria.
Simple cysts. Renal cysts that meet Bosniak class I criteria can be confidently labeled benign and need no further evaluation (FIGURE 1). Simple renal cysts on CT have homogenous low-attenuating fluid and thin nonenhancing walls without septa.4
On ultrasound, simple renal cysts show spherical or ovoid shape without internal echoes, a thin smooth wall separate from the surrounding parenchyma, and posterior wall enhancement caused by increased transmission through the water-filled cyst. The likelihood of malignancy is extremely low in a renal cyst that meets these criteria, which have a reported accuracy of 98% to 100%.3 Thus, no further evaluation is required if an obviously benign simple cyst is first noted on an adequate ultrasound. Inadequate ultrasound visualization or evidence of calcifications, septa, or multiple chambers calls for prompt renal CT.
CASE The mass on Ms. C’s left kidney is hypoattenuating and nonenhancing on CT. It meets Bosniak criteria for a benign simple cyst (class I) and requires no further evaluation or follow-up. Colonoscopy detects multiple colonic polyps that are removed, and the patient does well.
Mildly complicated cysts. Less diagnostic certainty characterizes cysts with mild abnormalities that keep them from being labeled as simple. Bosniak classes II and IIF describe mildly abnormal renal cysts. Class II cysts can be dismissed, whereas class IIF cysts require follow-up.
Class II cysts may contain a few hairline septa, fine calcium deposits in walls or septa, or an unmeasurable enhancement of the walls. A hyperattenuating but nonenhancing fluid also is described as category II. Small homogeneous cysts <3 cm, without enhancement but hyperattenuated, are reliably considered benign and need not be evaluated.2,7
Class IIF cysts may have multiple hairline-thin septa with unmeasurable enhancement or minimal smooth thickening or irregular/nodular calcifications of wall or septa without enhancing soft tissue components. Hyperattenuating cystic lesions >3 cm and intrarenal “noncortical” cysts are included in this category. Class IIF cysts require follow-up at 6 months with CT or MRI, then annually for at least 5 years.8
Obviously complicated cysts. Bosniak class III is indeterminate—neither benign nor clearly malignant. Class III cysts may have thickened borders or septa with measurable enhancement, or they may be multilocular, hemorrhagic, or infected. In 5 case series, 29 of 57 class III lesions proved to be malignant.5 MRI may characterize these lesions more definitively than CT prior to urologic referral.
Malignant cysts. Bosniak class IV renal lesions are clearly malignant, with large heterogeneous cysts or necrotic components, shaggy thickened walls, or enhancing soft tissue components separate from the wall or septa. Their unequivocal appearance results from solid tumor necrosis and liquefaction. Diagnosis is straightforward, and excision is indicated.2
A closer look at solid renal masses
Solid renal masses usually consist of enhancing tissue with little or no fluid. The goal of evaluation is to exclude malignancies, such as renal cell cancer, lymphomas, sarcomas, or metastasis. Benign solid masses include renal adenomas, angiomyolipomas, and oncocytomas, among others.
Several lesions can be diagnosed by appearance or symptoms:
Angiomyolipomas are recognized by their fat content within a noncalcified mass. Unenhanced CT usually is sufficient for diagnosis, unless the mass is very small or has atypical features.9
Vascular lesions can be identified because they enhance to the same degree as the vasculature. With the exception of inflammatory or vascular abnormalities, all enhancing lesions that do not contain fat should be presumed to be malignant.
In patients with a known extrarenal primary malignancy, 50% to 85% of incidental solid renal masses will represent metastatic disease.10 Percutaneous biopsy may be warranted to differentiate metastatic lesions from a secondary, primary (ie, renal cell carcinoma), or benign process.11
A study of 2770 solid renal mass excisions revealed that 12.8% were benign, with a direct relationship between malignancy and size. Masses <1 cm were benign 44% of the time.12 Early identification of small renal carcinomas may improve survival rates. Although renal cell carcinomas <3 cm in diameter have low metastatic potential, a solid, nonfat-containing mass should be evaluated for aggressive nephron-sparing surgery.6,13
Incidental adrenal masses occur infrequently
Adrenal incidentalomas are defined as radiographically identified masses >1 cm in diameter.14 They are much less common than their renal counterparts, with a reported prevalence of 0.35% to 5% on CT.15 Because the adrenal glands are hormonally active and receive substantial blood flow, metastatic, hormonally active, and nonfunctional causes for adrenal masses need to be considered.16
Adrenal pathology
Adrenal masses may be characterized by increased or normal adrenal function. Hyperfunctioning syndromes include hypercortisolism, hyperaldosteronism, adrenogenital hypersecretion of adrenocortical origin, and pheochromocytomas of the medulla. Symptom evaluation of these syndromes is important, but not sufficient to rule out a hyperfunctioning syndrome.
In a retrospective review of inapparent adrenal masses, ≤13% of pheochromocytomas were clinically silent.17 Therefore, laboratory testing is necessary for an incidental adrenal mass.
Nonfunctional lesions include adenomas, metastases, cysts, myelolipomas, hemorrhage, and adrenal carcinomas. These masses require evaluation for the possibility of cancer, the most common of which is metastasis. In patients with an extra-adrenal malignancy, the likelihood of malignancy in an incidental adrenal mass is at least 50%.18 An adrenal mass representing metastasis of a previously unrecognized cancer is exceedingly rare.19
Primary adrenal carcinoma is also rare, with an estimated incidence of 2 cases per one million in the general population. For patients with adrenal masses, the prevalence of carcinoma increases with lesion size (2% for tumors <4 cm, 6% for tumors 4-6 cm, and 25% for tumors >6 cm in diameter). 17 For this reason, tumors >4 cm in diameter are usually surgically resected in patients with no previous cancer history, unless radiologic criteria demonstrate clearly benign characteristics.
Although adrenal carcinomas are considered nonfunctioning, some evidence suggests they produce low levels of cortisol that may be associated with clinical features of metabolic syndrome.20
CT is first choice for adrenal mass evaluation
Dedicated adrenal CT with both unenhanced and delayed contrast-enhanced images is the most reliable study to evaluate an adrenal mass, according to the American College of Radiology. Consider another study only in patients with contrast allergy, renal compromise, or cancer history.21
Unenhanced CT can diagnose the approximately 70% of adenomas that are small, well-defined round masses with homogenous low-density lipid deposition.22 Delayed contrast enhancement can characterize most of the remaining 30%.23 Unenhanced CT with attenuation values of <10 Hounsfield units (HU) can diagnose adenomas with 71% specificity and 98% sensitivity,24 and can often diagnose simple cysts and myelolipomas, as well.
Other imaging options. MRI is an alternative to CT for patients with contraindications for contrast or radiation exposure. MRI provides less spatial resolution than CT, but chemical shift imaging can measure cytoplasmic lipid content similar to unenhanced CT. A small study found chemical shift MRI more reliable than unenhanced CT, but less reliable than CT with delayed contrast enhancement.25
Positron emission tomography (PET) is useful to noninvasively evaluate biochemical and physiologic processes. PET-CT incorporates unenhanced CT density measurements to improve PET accuracy. In a patient with a history of cancer, PET-CT has a sensitivity of 93% to 100% and a specificity of 95% in differentiating benign from malignant adrenal tumors.26
When to order a biopsy
The need for biopsy has decreased as imaging has improved, but biopsy is required whenever diagnostic imaging fails to differentiatea lesion as benign or malignant. CT guided biopsy provides diagnostic accuracy of 85% to 95%.27 Complications such as pneumothorax, hemorrhage, and bacteremia occur in 3% to 9% of biopsies. Before any adrenal biopsy, measure plasma-free metanephrines to exclude undiagnosed pheochromocytoma, which could precipitate a hypertensive crisis if untreated.22
These 3 laboratory screening tests are critical
Family physicians can perform the initial biochemical evaluation of an adrenal incidentaloma. Guidance is available from the National Institutes of Health (NIH)28 and the American Academy of Clinical Endocrinologists (AACE) (FIGURE 2).29
Regardless of signs or symptoms, perform screening laboratory tests for 3 types of adrenal hyperfunction: hypercortisolism, hyperaldosteronism, and hypersecretion of catecholamines (pheochromocytoma). Screening tests are not recommended for androgen hypersecretion, which is extremely rare and causes recognizable symptoms such as hirsutism (Table 2).29
Hypercortisolism occurs in approximately 5% of adrenal incidentalomas.30 An overnight dexamethasone suppression test (DST) is most reliable for screening, with sensitivity >95% for Cushing syndrome.31 The patient takes a 1-mg dose of oral dexamethasone at 11 pm, and a fasting plasma cortisol sample is drawn the next day at 8 am.
Dexamethasone binds to glucocorticoid receptors in the pituitary gland, suppressing adrenocorticotropic hormone secretion. Cortisol will be depressed the next morning unless the adrenal mass produces cortisol autonomously. Patients with a DST >5 mcg/dL—highly suggestive of Cushing syndrome—require further evaluation, and we suggest referral to an endocrinologist.
Hyperaldosteronism is seen in 1% to 2% of adrenal incidentalomas.32 The aldosterone- to-renin ratio (ARR) is recommended as a screening test for hyperaldosteronism, with an ARR >20 requiring further testing.33 Medications that may affect the ARR include beta-blockers, spironolactone, clonidine, diuretics, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers.29
Refer a patient with evidence of hyperaldosteronism to an endocrinologist and a surgeon with experience in managing these lesions. If the ARR test result suggests an aldosterone excess, a salt-loading test is used to verify failure of aldosterone suppression. Adrenal venous sampling is often performed prior to surgical removal to confirm that an incidentaloma is the source of hyperaldosteronism.
Pheochromocytoma. Approximately 5% of incidental adrenal lesions are pheochromocytomas.30 Many patients with these epinephrine/norepinephrine secreting tumors do not show the classic symptom triad of headache, palpitations, and diaphoresis, and approximately half have normal blood pressure.34
Identifying a pheochromocytoma is important in any patient requiring surgery or biopsy, as surgical manipulation can cause a potentially fatal intraoperative catecholamine surge. Presurgical medical management can mitigate this reaction.
A plasma-free metanephrines test, which has 95% sensitivity, is the most reliable test for pheochromocytoma.35 Medications, including tricyclic antidepressants, decongestants, amphetamines, reserpine, and phenoxybenzamine, can cause falsepositive results.29 Confirm a positive plasma-free metanephrines test with a 24-hour fractionated urine metanephrines test, and refer the patient to an endocrinologist.
Managing adrenal incidentalomas
Refer all patients with adrenal masses >4 cm for surgical evaluation because of the risk of malignancy; all patients who have a history of malignancy and an adrenal mass of any size require a referral to an oncologist. Perform the AACE-recommended 3-element biochemical workup for all masses, with the exception of definitively diagnosed cysts or myelolipomas.
Refer to an endocrinologist all patients with abnormal screening laboratory results, regardless of adrenal mass size, as well as patients with concerning clinical findings. Initiate cardiovascular, diabetes, and bone density evaluation and management for metabolic syndrome.20
Monitoring after a negative workup
Little evidence exists to guide monitoring of small adrenal incidentalomas (<4 cm) with a negative workup. The 2002 NIH report recommended annual radiologic follow-up for 5 years,28 whereas the 2009 AACE guidelines recommend radiographic follow-up at 3 to 6 months, then at one and 2 years.29
Evidence indicates that 14% of lesions will enlarge in 2 years, although the clinical significance of enlargement is unknown. Some authors argue against CT monitoring because the risk of adrenal mass progression is similar to the malignancy risk posed by 3 years of radiation exposure with CT.20
Some guidelines recommend repeat biochemical screening every 3 to 4 years.28,29 AACE guidelines quote a 47% rate of progression over 3 years, but most adrenal masses progress to subclinical Cushing syndrome— a condition of uncertain significance. Subclinical Cushing’s has not been reported to progress to the overt syndrome, and new catecholamine or aldosterone secretion is rare.
Many endocrinologists reduce the frequency of follow-up, depending on the type of adrenal mass (cyst or solid) and its size. AACE suggests CT for adenomas one to 4 cm at 12 months. AACE and NIH recommend hormonal evaluation annually for 4 years. Adrenal cysts or myelolipoma in patients without cancer need no follow-up.29
CORRESPONDENCE
James C. Higgins, DO, CAPT, MC, USN, Ret., Naval Hospital Jacksonville, Family Medicine Department, 2080 Child Street, Box 1000, Jacksonville, FL 32214;
[email protected]
1. Berland LL, Silverman SG, Gore RM, et al. Managing incidental findings on abdominal CT: white paper of the ACR incidental findings committee. J Am Coll Radiol. 2010;7:754-773.
2. Silverman S, Israel G, Herts B, et al. Management of the incidental renal mass. Radiology. 2008;249:16-31.
3. Curry NS, Bissada NK. Radiologic evaluation of small and indeterminate renal masses. Urol Clin North Am. 1997;24:493-505.
4. Bosniak MA. The current radiological approach to renal cysts. Radiology. 1986;158:1-10.
5. Harisinghani M, Maher M, Gervais D, et al. Incidence of malignancy in complex cystic renal masses (Bosniak category III): should imaging guided biopsy precede surgery? AJR Am J Roentgenol. 2003;180:755-758.
6. Remzi M, Ozsoy M, Klingler HC. Are small renal tumors harmless? Analysis of histopathological features according to tumors less than 4 cm in diameter. J Urol. 2006;176:896-899.
7. Jonisch AI, Rubinowitz A, Mutalik P, et al. Can high attenuation renal cysts be differentiated from renal cell carcinoma at unenhanced computed tomography? Radiology. 2007;243:445-450.
8. Israel GM, Bosniak MA. Follow-up CT of moderately complex cystic lesions of the kidney. AJR Am J Roentgenol. 2003;181: 627-633.
9. Bosniak MA, Megibow AJ, Hulnick DH, et al. CT diagnosis of renal angiomyolipoma: the importance of detecting small amounts of fat. AJR Am J Roentengol. 1988;151:497-501.
10. Mitnick JS, Bosniak MA, Rothberg M, et al. Metastatic neoplasm to the kidney studied by computed tomography and sonogram. J Comput Assist Tomogr. 1985;9:43-49.
11. Rybicki FJ, Shu KM, Cibas ES, et al. Percutaneous biopsy of renal masses: sensitivity and negative predictive value stratified by clinical setting and size of masses. AJR Am J Roentgenol. 2003;180:1281-1287.
12. Frank I, Blure MI, Cheville JC, et al. Solid renal tumors: an analysis of pathological features related to tumor size. J Urol. 2003;170:2217-2220.
13. Hollingsworth JM, Miller DC, Daignault S, et al. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst. 2006;98:1331-1334.
14. Geelhoed GW, Spiegel CT. “Incidental” adrenal cyst: a correctable lesion possibly associated with hypertension. South Med J. 1981;74:626-630.
15. Davenport C, Liew A, Doherty B, et al. The prevalence of adrenal incidentaloma in routine clinical practice. Endocrine. 2011;40: 80-83.
16. Cook DM, Loriaux LD. The incidental adrenal mass. Am J Med. 1996;101:88 94.
17. Mansmann G, Lau J, Balk E, et al. The clinically inapparent adrenal mass: update in diagnosis and management. Endocr Rev. 2004;25:309-340.
18. Androulakis II, Kaltsas G, Piatitis G, et al. The clinical significance of adrenal incidentalomas. Eur J Clin Invest. 2011;41: 552-560.
19. Lee JE, Evans DB, Hickey RC, et al. Unknown primary cancer presenting as an adrenal mass: frequency and implications for diagnostic evaluation of adrenal incidentalomas. Surgery. 1998;124:1115-1122.
20. Aron D, Terzolo M, Cawood TJ. Adrenal incidentalomas. Best Pract Res Clin Endocrinol Metab. 2012;26:69-82.
21. ACR appropriateness criteria: incidentally discovered adrenal mass. American College of Radiology. Available at: http://www.acr.org/~/media/ACR/Documents/AppCriteria/Diagnostic/IncidentallyDiscoveredAdrenalMass.pdf. Accessed November 20, 2012.
22. Song JH, Mayo-Smith WW. Incidentally discovered adrenal mass. Radiol Clin North Am. 2011;49:361-368.
23. Korobkin M, Brodeur FJ, Francis IR, et al. CT time-attenuation washout curves of adrenal adenomas and nonadenomas. AJR Am J Roentgenol. 1998;170:747-752.
24. Boland GW, Lee MJ, Gazelle GS, et al. Characterization of adrenal masses using unenhanced CT: an analysis of the CT literature. AJR Am J Roentgenol. 1998;171:201-204.
25. Park BK, Kim CK, Kim B, et al. Chemical shift MR imaging of hyperattenuating (>10 HU) adrenal masses: does it still have a role? Radiology. 2004;231:711-716.
26. Boland GW, Blake MA, Holakere NS, et al. PET/CT for the characterization of adrenal masses in patients with cancer: qualitative vs quantitative accuracy in 150 consecutive patients. AJR Am J Roentgenol. 2009;192:956-962.
27. Paulsen SD, Nghiem HV, Korobkin M, et al. Changing role of imaging- guided percutaneous biopsy of adrenal masses: evaluation of 50 adrenal biopsies. AJR Am J Roentgenol. 2004;182:1033-1037
28. Grumbach MM, Biller BMK, Braunstein GD, et al. Management of the clinically inapparent adrenal mass (“incidentalomas”). Ann Intern Med. 2003;138:424-429.
29. Zeiger MA, Thompson GB, Quan-Yang D, et al. American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons medical guidelines for the management of adrenal incidentalomas. Endocr Pract. 2009;15(suppl 1):1-20.
30. Young WF. The incidentally discovered adrenal mass. N Engl J Med. 2007; 356:601-610.
31. Deutschbein T, Unger N, Hinrichs J, et al. Late-night and lowdose dexamethasone-suppressed cortisol in saliva and serum for the diagnosis of cortisol-secreting adrenal adenomas. Eur J Endocrinol. 2009;161:747-753.
32. Bernini G, Moretti A, Gianfranco A, et al. Primary aldosteronism in normokalemic patients with adrenal incidentalomas. Eur J Endocrinol. 2002;146:523-529.
33. Montori VM, Young WF Jr. Use of plasma aldosterone concentration-to-plasma renin activity ratio as a screening test for primary aldosteronism: a systematic review of the literature. Endocrinol Metab Clin North Am. 2002;31:619-632.
34. Motta-Ramirez GA, Remer EM, Herts BR, et al. Comparison of CT findings in symptomatic and incidentally discovered pheochromocytomas. AJR Am J Roentgenol. 2005;185:684-688.
35. Pacak K, Eisenhofer G, Grossman A. The incidentally discovered adrenal mass. N Engl J Med. 2007;356:2005.
1. Berland LL, Silverman SG, Gore RM, et al. Managing incidental findings on abdominal CT: white paper of the ACR incidental findings committee. J Am Coll Radiol. 2010;7:754-773.
2. Silverman S, Israel G, Herts B, et al. Management of the incidental renal mass. Radiology. 2008;249:16-31.
3. Curry NS, Bissada NK. Radiologic evaluation of small and indeterminate renal masses. Urol Clin North Am. 1997;24:493-505.
4. Bosniak MA. The current radiological approach to renal cysts. Radiology. 1986;158:1-10.
5. Harisinghani M, Maher M, Gervais D, et al. Incidence of malignancy in complex cystic renal masses (Bosniak category III): should imaging guided biopsy precede surgery? AJR Am J Roentgenol. 2003;180:755-758.
6. Remzi M, Ozsoy M, Klingler HC. Are small renal tumors harmless? Analysis of histopathological features according to tumors less than 4 cm in diameter. J Urol. 2006;176:896-899.
7. Jonisch AI, Rubinowitz A, Mutalik P, et al. Can high attenuation renal cysts be differentiated from renal cell carcinoma at unenhanced computed tomography? Radiology. 2007;243:445-450.
8. Israel GM, Bosniak MA. Follow-up CT of moderately complex cystic lesions of the kidney. AJR Am J Roentgenol. 2003;181: 627-633.
9. Bosniak MA, Megibow AJ, Hulnick DH, et al. CT diagnosis of renal angiomyolipoma: the importance of detecting small amounts of fat. AJR Am J Roentengol. 1988;151:497-501.
10. Mitnick JS, Bosniak MA, Rothberg M, et al. Metastatic neoplasm to the kidney studied by computed tomography and sonogram. J Comput Assist Tomogr. 1985;9:43-49.
11. Rybicki FJ, Shu KM, Cibas ES, et al. Percutaneous biopsy of renal masses: sensitivity and negative predictive value stratified by clinical setting and size of masses. AJR Am J Roentgenol. 2003;180:1281-1287.
12. Frank I, Blure MI, Cheville JC, et al. Solid renal tumors: an analysis of pathological features related to tumor size. J Urol. 2003;170:2217-2220.
13. Hollingsworth JM, Miller DC, Daignault S, et al. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst. 2006;98:1331-1334.
14. Geelhoed GW, Spiegel CT. “Incidental” adrenal cyst: a correctable lesion possibly associated with hypertension. South Med J. 1981;74:626-630.
15. Davenport C, Liew A, Doherty B, et al. The prevalence of adrenal incidentaloma in routine clinical practice. Endocrine. 2011;40: 80-83.
16. Cook DM, Loriaux LD. The incidental adrenal mass. Am J Med. 1996;101:88 94.
17. Mansmann G, Lau J, Balk E, et al. The clinically inapparent adrenal mass: update in diagnosis and management. Endocr Rev. 2004;25:309-340.
18. Androulakis II, Kaltsas G, Piatitis G, et al. The clinical significance of adrenal incidentalomas. Eur J Clin Invest. 2011;41: 552-560.
19. Lee JE, Evans DB, Hickey RC, et al. Unknown primary cancer presenting as an adrenal mass: frequency and implications for diagnostic evaluation of adrenal incidentalomas. Surgery. 1998;124:1115-1122.
20. Aron D, Terzolo M, Cawood TJ. Adrenal incidentalomas. Best Pract Res Clin Endocrinol Metab. 2012;26:69-82.
21. ACR appropriateness criteria: incidentally discovered adrenal mass. American College of Radiology. Available at: http://www.acr.org/~/media/ACR/Documents/AppCriteria/Diagnostic/IncidentallyDiscoveredAdrenalMass.pdf. Accessed November 20, 2012.
22. Song JH, Mayo-Smith WW. Incidentally discovered adrenal mass. Radiol Clin North Am. 2011;49:361-368.
23. Korobkin M, Brodeur FJ, Francis IR, et al. CT time-attenuation washout curves of adrenal adenomas and nonadenomas. AJR Am J Roentgenol. 1998;170:747-752.
24. Boland GW, Lee MJ, Gazelle GS, et al. Characterization of adrenal masses using unenhanced CT: an analysis of the CT literature. AJR Am J Roentgenol. 1998;171:201-204.
25. Park BK, Kim CK, Kim B, et al. Chemical shift MR imaging of hyperattenuating (>10 HU) adrenal masses: does it still have a role? Radiology. 2004;231:711-716.
26. Boland GW, Blake MA, Holakere NS, et al. PET/CT for the characterization of adrenal masses in patients with cancer: qualitative vs quantitative accuracy in 150 consecutive patients. AJR Am J Roentgenol. 2009;192:956-962.
27. Paulsen SD, Nghiem HV, Korobkin M, et al. Changing role of imaging- guided percutaneous biopsy of adrenal masses: evaluation of 50 adrenal biopsies. AJR Am J Roentgenol. 2004;182:1033-1037
28. Grumbach MM, Biller BMK, Braunstein GD, et al. Management of the clinically inapparent adrenal mass (“incidentalomas”). Ann Intern Med. 2003;138:424-429.
29. Zeiger MA, Thompson GB, Quan-Yang D, et al. American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons medical guidelines for the management of adrenal incidentalomas. Endocr Pract. 2009;15(suppl 1):1-20.
30. Young WF. The incidentally discovered adrenal mass. N Engl J Med. 2007; 356:601-610.
31. Deutschbein T, Unger N, Hinrichs J, et al. Late-night and lowdose dexamethasone-suppressed cortisol in saliva and serum for the diagnosis of cortisol-secreting adrenal adenomas. Eur J Endocrinol. 2009;161:747-753.
32. Bernini G, Moretti A, Gianfranco A, et al. Primary aldosteronism in normokalemic patients with adrenal incidentalomas. Eur J Endocrinol. 2002;146:523-529.
33. Montori VM, Young WF Jr. Use of plasma aldosterone concentration-to-plasma renin activity ratio as a screening test for primary aldosteronism: a systematic review of the literature. Endocrinol Metab Clin North Am. 2002;31:619-632.
34. Motta-Ramirez GA, Remer EM, Herts BR, et al. Comparison of CT findings in symptomatic and incidentally discovered pheochromocytomas. AJR Am J Roentgenol. 2005;185:684-688.
35. Pacak K, Eisenhofer G, Grossman A. The incidentally discovered adrenal mass. N Engl J Med. 2007;356:2005.
Plantar Fasciitis: How Best to Treat?
› Use plantar fascia specific stretching to decrease pain in patients with plantar fasciitis. A
› Consider recommending prefabricated orthoses, including night splints, to decrease pain. A
› Consider using extracorporeal shock wave therapy for plantar fascial pain. A
Strength of recommendation (SOR)
A. Good-quality patient-oriented evidence
B. Inconsistent or limited-quality patient-oriented evidence
C. Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE A 43-year-old obese woman seeks advice for left heel pain she has had for 2 months. Before the onset of pain, her activity level had increased as part of a weight loss program. Her pain is at its worst in the morning, with her first few steps; it decreases with continued walking and intensifies again after being on her feet all day. There is no history of trauma, and she reports no paresthesias or radiation of the pain. Her medical history is otherwise unremarkable. She has used ibuprofen sparingly, with limited relief.
If you were this patient’s physician, how would you proceed with her care?
Plantar fasciitis (PF) is a common cause of heel pain that affects up to 10% of the US population and accounts for approximately 600,000 outpatient visits annually.1 The plantar fascia is a dense, fibrous membrane spanning the length of the foot. It originates at the medial calcaneal tubercle, attaches to the phalanges, and provides stability and arch support to the foot. The etiology of PF is unknown, but predisposing factors include overtraining, obesity, pes planus, decreased ankle dorsiflexion, and inappropriate footwear.2 Limited dorsiflexion due to tightness of the Achilles tendon strains the plantar fascia and can lead to PF. Histology shows minimal inflammatory changes, and some experts advocate the term plantar fasciosis to counter the misperception that it is primarily an inflammatory condition.3
A patient’s history and physical exam findings are the basis for confirming or dismissing a diagnosis of PF. Radiologic studies, used judiciously, can rule out important alternative diagnoses that should not be overlooked. Multiple treatment options range from conservative to surgical interventions, although studies of the effectiveness of each modality have had conflicting results. Clinical practice guidelines generally advocate a stepwise approach to treatment.
Diagnosis
The differential diagnosis of PF (TABLE) includes significant disorders such as calcaneal stress fracture, entrapment neuropathies (eg, tarsal tunnel syndrome), calcaneal tumor, Paget’s disease, and systemic arthritidies.4,5
What to look for in the history and physical exam
Severe heel pain upon initial weight bearing in the morning or after prolonged periods of inactivity is pathognomonic for PF.2 Initially the pain presents diffusely, but over time it localizes to the area of the medial calcaneal tubercle. Pain typically subsides with activity but may return with prolonged weight bearing, as it did with the patient in the opening case.
Test range of motion of the foot and ankle. Although this is not needed for diagnosing PF, some patients will exhibit limited ankle dorsiflexion, a predisposing factor for PF.4,6 Look for heel pad swelling, inflammation, or atrophy, and palpate the heel, plantar fascia, and calcaneal tubercle. Lastly, evaluate for gait abnormalities and the presence of sensory deficits or hypesthesias.4
The most common exam finding in PF is pain at the medial calcaneal tubercle, which may be exacerbated with passive ankle dorsiflexion or first digit extension.2,4 If paresthesias occur with percussion inferior to the medial malleolus, suspect possible nerve entrapment or tarsal tunnel syndrome. Tenderness with heel compression (squeeze test) may indicate a calcaneal fracture or apophysitis.
Imaging is useful to rule out alternative disorders
Radiologic studies generally do not contribute to the diagnosis or management of PF, but they can assist in ruling out alternative causes of heel pain or in reevaluation if symptoms of PF persist after 3 to 6 months of treatment.
Plain films lack the sensitivity to detect plantar fasciitis. While a plantar calcaneal spur is often seen on radiography, it does not confirm the diagnosis, correlate with severity of symptoms, or predict prognosis.4 Despite this deficiency, plain radiography remains the initial choice of imaging modalities, particularly to rule out other conditions.
Ultrasound accurately diagnoses plantar fasciitis. Plantar fascia thickness of more than 4.0 mm is diagnostic of PF.7 Additionally, a decrease in plantar fascia thickness correlates with a decrease in pain levels, and thus ultrasound can aid in monitoring treatment progress.8 If results of plain films and ultrasound are inconclusive and clinical concern for an alternative diagnosis warrants additional expense, consider arranging for magnetic resonance imaging.9
Noninvasive treatments
Conservative therapies remain the preferred approach to treating PF, successfully managing 85% to 90% of cases.10,11 A 2010 clinical practice guideline from the American College of Foot and Ankle Surgeons recommends conservative treatments such as nonsteroidal inflammatory drugs (NSAIDs), stretching, and prefabricated orthotics for the initial management of plantar heel pain.4 Emphasize to patients that it may take 6 to 12 months for symptoms to resolve.4
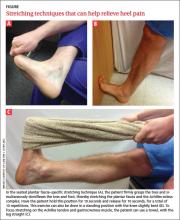
Stretching and trigger-point manual therapy are effective
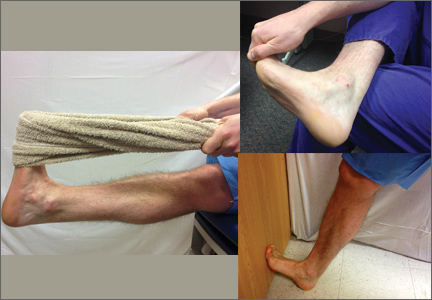
The traditional primary treatment modality for PF has been early initiation of an Achilles-soleus (heel-cord) muscle–stretching program. However, studies have shown that plantar fascia–specific stretching (PFSS) (FIGURE) significantly diminishes or eliminates heel pain when compared with traditional stretching movements, and is useful in treating chronic recalcitrant heel pain.12,13 PFSS has also yielded results superior to low-dose shock wave therapy.14
In a 2011 study, adding myofascial trigger-point manual therapy to a PFSS routine improved self-reported physical function and pain vs stretching alone.15 This manual therapy technique is specialized and should be administered only by trained physical therapists. Data are limited and mixed regarding the effectiveness of deep tissue massage, iontophoresis, or eccentric stretching of the plantar fascia to alleviate plantar fascial pain. Support for therapies such as rest, ice, heat, and massage has largely been anecdotal.
NSAIDs for PF lack good evidence
Nonsteroidal anti-inflammatory drugs (NSAIDs) are often prescribed to treat PF, despite a lack of evidence supporting their use. A small randomized, placebo-controlled double-blind study established a trend toward improvement in pain and disability scores with the use of NSAIDs. However, no statistically significant difference was noted in the measures between the NSAID and placebo groups at 1, 2, and 6 months.16 We found no studies that demonstrate a significant reduction in pain or improvement in function with the use of NSAIDs alone.
Although NSAIDs carry their own risks, they may work for some patients. And studies showing a lack of significant pain reduction may have been underpowered. If patients are willing to accept the risks of NSAID use, it would be reasonable to prescribe a therapeutic trial.
Orthotics and night splints can help, depending on comfort and compliance
Foot orthotics help prevent overpronation and attenuate tensile forces on the plantar fascia. A 2009 meta-analysis confirmed that both prefabricated and custom-made foot orthotics can decrease pain.17 One prospective study showed that 95% of patients had improvement in PF symptoms after 8 weeks of treatment with prefabricated orthotics.18 A Cochrane review found no difference in pain reduction between custom and prefabricated foot orthotics.19 A recent study demonstrated that rocker sole shoes—a type of therapeutic footwear with a more rounded outsole contour—combined with custom orthotics significantly reduced pain during walking compared with either modality alone.20 More research needs to be conducted into the use of rocker sole shoes before recommending them to PF patients.
Night splints help keep the foot and ankle in a neutral position, or slightly dorsiflexed, while patients sleep. Several studies have shown a reduction in pain with the use of night splints alone.17,21,22 Patient comfort and compliance tend to be the limiting factors in their use. Anterior splints are better tolerated than posterior splints.23
Shock wave therapy has better long-term results than steroid injections
Shock waves used to treat PF are thought to invoke extracellular responses that cause neovascularization and induce tissue repair and regeneration. A 2012 review article concluded that most research confirms that extracorporeal shock wave therapy (ESWT) reduces PF pain and improves function in 34% to 88% of cases.24 ESWT is comparable to surgical plantar fasciotomy without the operative risks, and yields better long-term effects in recalcitrant PF compared with corticosteroid injections (CSI).24 Many studies are underway to validate the effectiveness of ESWT. Currently, expense or lack of availability limits its use in some communities.
Invasive treatments
Corticosteroid injections may be used for more than just refractory pain
CSI have historically been reserved for recalcitrant heel pain. However, one systematic review cites evidence in support of CSI for the short-term management of plantar fascia pain.25 Compared with placebo, CSI reduces pain at both 6 and 12 weeks and decreases plantar fascia thickness.26 Additionally, the American College of Foot and Ankle Surgeons lists CSI as an acceptable first-line treatment for PF.4
The most common complication of CSI is postinjection pain. Other complications, such as fat pad atrophy, rarely occur.27 While the evidence is limited, CSI may be part of an initial approach to treating PF in addition to heel-cord or plantar fascia-specific stretching, particularly for patients who desire an expedited return to normal activity.
Platelet-rich plasma therapy holds promise
Platelet-rich plasma (PRP) has been gaining popularity as a treatment for PF pain. PRP is a component of whole blood that is centrifuged to a concentrated state, treated with an activating agent, and injected into the affected area. Theoretically, injected PRP increases the release of reparative growth factors, enhancing the healing process.28 PRP has been shown to be as effective in reducing pain scores as CSI at 3 weeks and 6 months.29 PRP also decreases plantar fascia thickness and improves pain scores and functional ability.30
To date, no trials have compared PRP with placebo injections. Postprocedural pain is the most common risk with PRP. While limited evidence exists, PRP seems to be a relatively safe and effective therapeutic alternative for treating chronic PF.
Surgery only when conservative measures fail
Reserve surgery for those who have not responded adequately after 6 to 12 months of conservative therapy.5 Endoscopic plantar fascia release is superior to traditional open surgery.31 Heel spur resection is no longer routinely practiced. Patients undergoing surgery should expect a return to normal activity in approximately 2 to 3 months, and up to 35% of patients may continue to have symptoms after surgical intervention.2,31
Treatment options in perspective
Treat conservatively at first. Stretching the plantar fascia and heel cord, using prefabricated orthotics, and wearing night splints are backed by firm clinical evidence of benefit. Acute treatment of PF may also include CSI, especially for patients who are athletic or otherwise active and wish to return to full function as soon as possible, and are willing to accept the risks associated with CSI.
ESWT improves pain and function scores and may also relieve pain in patients with recalcitrant PF pain. PRP has limited but promising evidence for patients with chronic PF pain. Surgical intervention remains the last line of therapy and is not always effective at reducing pain.
CASE You prescribe a conservative treatment program of plantar fascia–specific stretches and prefabricated orthoses for the patient in the opening scenario. At one month, her pain drops by 30%. At 6 months, her pain disappears, and she resumes a daily aerobic exercise program to assist in weight loss.
CORRESPONDENCE
Carlton J. Covey, MD, Nellis Family Medicine Residency, 99MDOS/SGOF, 4700 Las Vegas Boulevard N, Las Vegas, NV 89191;
[email protected]
1. Riddle DL, Schappert SM. Volume of ambulatory care visits and patterns of care for patients diagnosed with plantar fasciitis: a national study of medical doctors. Foot Ankle Int. 2004;25: 303-310.
2. Glazer JL. An approach to the diagnosis and treatment of plantar fasciitis. Phys Sportsmed. 2009;37:74-79.
3. Lemont H, Ammirati KM, Usen N. Plantar fasciitis: a degenerative process (fasciosis) without inflammation. J Am Podiatr Med Assoc. 2003;93:234-237.
4. Thomas JL, Christensen JC, Kravitz SR, et al. The diagnosis and treatment of heal pain: a clinical practice guideline – revision 2010. J Foot Ankle Surg. 2010;49(suppl):S1-S19.
5. Neufeld SK, Cerrato R. Plantar fasciitis: evaluation and treatment. J Am Acad Orthop Surg. 2008;16:338-346.
6. Singh D, Angel J, Bentley G, et al. Fortnightly review: plantar fasciitis. BMJ. 1997;315:172-175.
7. McMillan AM, Landorf KB, Barrett JT, et al. Diagnostic imaging for chronic plantar heel pain: a systematic review and metaanalysis. J Foot Ankle Res. 2009;2:32.
8. Mahowald S, Legge BS, Grady JF. The correlation between plantar fascia thickness and symptoms of plantar fasciitis. J Am Podiatr Med Assoc. 2011;101:385-389.
9. American College of Radiology. ACR appropriateness criteria. Chronic foot pain. Available at: http://www.acr.org/~/media/ACR/Documents/AppCriteria/Diagnostic/ChronicFootPain.pdf. Accessed November 11, 2012.
10. Gill LH. Plantar fasciitis: diagnosis and conservative treatment. J Am Acad Orthop Surg. 1997;5:109-117.
11. Martin RL, Irrgang JJ, Conti SF. Outcome study of subjects with insertional plantar fasciitis. Foot Ankle Int. 1998;19:803-811.
12. DiGiovanni BF, Nawoczenski DA, Malay DP, et al. Plantar fasciaspecific stretching exercise improves outcomes in patients with chronic plantar fasciitis: a prospective clinic trial with two-year follow up. J Bone Joint Surg Am. 2006;88:1775-1781.
13. Sweeting D, Parish B, Hooper L, et al. The effectiveness of manual stretching in the treatment of plantar heel pain: a systemic review. J Foot Ankle Res. 2011;4:1-13.
14. Rompe JD, Cacchio A, Lowell W, et al. Plantar fascia-specific stretching versus radial shock-wave therapy as initial treatment of plantar fasciopathy. J Bone Joint Surg Am. 2010;92:2514-2522.
15. Renan-Ordine R, Alburquerque-Sendin F, Rodriques De Souza DP, et al. Effectiveness of myofascial trigger point manual therapy combined with a self stretching protocol for the management of plantar heel pain: a randomized controlled trial. J Orthop Sports Phys Ther. 2011;41:43-50.
16. Donley BG, Moore T, Sferra J, et al. The efficacy of oral nonsteroidal anti-inflammatory medication (NSAID) in the treatment of plantar fasciitis: a randomized, prospective, placebo-controlled study. Foot Ankle Int. 2007;28:20-23.
17. Lee SY, McKeon P, Hertel J. Does the use of orthoses improve selfreported pain and function measures in patients with plantar fasciitis? A meta-analysis. Phys Ther Sport. 2009;10:12-18.
18. Pfeffer G, Bacchetti P, Deland J, et al. Comparison of custom and prefabricated orthoses in the initial treatment of proximal plantar fasciitis. Foot Ankle Int. 1999;20:214-221.
19. Hawke F, Burns J, Radford JA, et al. Custom-made foot orthoses for the treatment of foot pain. Cochrane Database Syst Rev. 2008;(3):CD006801.
20. Fong DT, Pang KY, Chung MM, et al. Evaluation of combined prescription of rocker sole shoes and custom-made foot orthoses for the treatment of plantar fasciitis. Clin Biomech. 2012;27: 1072-1077.
21. Berlet GC, Anderson RB, Davis H. A prospective trial of night splinting in the treatment of recalcitrant plantar fasciitis: the Ankle Dorsiflexion Dynasplint. Orthopedics. 2002;25: 1273-1275.
22. Roos E, Engstrom M, Soderberg B. Foot orthoses for the treatment of plantar fasciitis. Foot Ankle Int. 2006;27:606-611.
23. Goff JD, Crawford R. Diagnosis and treatment of plantar fasciitis. Am Fam Physician. 2011;84:676-682.
24. Wang CJ. Extracorporeal shockwave therapy in musculoskeletal disorders. J Orthop Surg Res. 2012;7:11.
25. Landorf KB, Menz HB. Plantar heel pain and fasciitis. Clin Evid (Online). 2008;2008:1111.
26. Ball EM, McKeeman HM, Patterson C, et al. Steroid injection for inferior heel pain: a randomized controlled trial. Ann Rheum Dis. 2013;72:996-1002.
27. Uden H, Boesch E, Kumar S. Plantar fasciitis – to jab or support? A systematic review of the current best evidence. J Multidiscip Healthc. 2011;4:155-164.
28. Shetty VD. Platelet-rich plasma: a ‘feeling’ and ‘hope’ ailing athletes. Br J Sports Med. 2010;44(suppl 1):i1-i82.
29. Aksahin E, Dogruyol D, Yüksel HY, et al. The comparison of the effect of corticosteroids and platelet-rich plasma (PRP) for the treatment of plantar fasciitis. Arch Orthop Trauma Surg. 2012;132:781-785.
30. Ragab EM, Othman AM. Platelets rich plasma for treatment of chronic plantar fasciitis. Arch Orthop Trauma Surg. 2012;132:1065-1070.
31. Saxena A. Uniportal endoscopic plantar fasciotomy: a prospective study on athletic patients. Foot Ankle Int. 2004;25:882-889.
› Use plantar fascia specific stretching to decrease pain in patients with plantar fasciitis. A
› Consider recommending prefabricated orthoses, including night splints, to decrease pain. A
› Consider using extracorporeal shock wave therapy for plantar fascial pain. A
Strength of recommendation (SOR)
A. Good-quality patient-oriented evidence
B. Inconsistent or limited-quality patient-oriented evidence
C. Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE A 43-year-old obese woman seeks advice for left heel pain she has had for 2 months. Before the onset of pain, her activity level had increased as part of a weight loss program. Her pain is at its worst in the morning, with her first few steps; it decreases with continued walking and intensifies again after being on her feet all day. There is no history of trauma, and she reports no paresthesias or radiation of the pain. Her medical history is otherwise unremarkable. She has used ibuprofen sparingly, with limited relief.
If you were this patient’s physician, how would you proceed with her care?
Plantar fasciitis (PF) is a common cause of heel pain that affects up to 10% of the US population and accounts for approximately 600,000 outpatient visits annually.1 The plantar fascia is a dense, fibrous membrane spanning the length of the foot. It originates at the medial calcaneal tubercle, attaches to the phalanges, and provides stability and arch support to the foot. The etiology of PF is unknown, but predisposing factors include overtraining, obesity, pes planus, decreased ankle dorsiflexion, and inappropriate footwear.2 Limited dorsiflexion due to tightness of the Achilles tendon strains the plantar fascia and can lead to PF. Histology shows minimal inflammatory changes, and some experts advocate the term plantar fasciosis to counter the misperception that it is primarily an inflammatory condition.3
A patient’s history and physical exam findings are the basis for confirming or dismissing a diagnosis of PF. Radiologic studies, used judiciously, can rule out important alternative diagnoses that should not be overlooked. Multiple treatment options range from conservative to surgical interventions, although studies of the effectiveness of each modality have had conflicting results. Clinical practice guidelines generally advocate a stepwise approach to treatment.
Diagnosis
The differential diagnosis of PF (TABLE) includes significant disorders such as calcaneal stress fracture, entrapment neuropathies (eg, tarsal tunnel syndrome), calcaneal tumor, Paget’s disease, and systemic arthritidies.4,5
What to look for in the history and physical exam
Severe heel pain upon initial weight bearing in the morning or after prolonged periods of inactivity is pathognomonic for PF.2 Initially the pain presents diffusely, but over time it localizes to the area of the medial calcaneal tubercle. Pain typically subsides with activity but may return with prolonged weight bearing, as it did with the patient in the opening case.
Test range of motion of the foot and ankle. Although this is not needed for diagnosing PF, some patients will exhibit limited ankle dorsiflexion, a predisposing factor for PF.4,6 Look for heel pad swelling, inflammation, or atrophy, and palpate the heel, plantar fascia, and calcaneal tubercle. Lastly, evaluate for gait abnormalities and the presence of sensory deficits or hypesthesias.4
The most common exam finding in PF is pain at the medial calcaneal tubercle, which may be exacerbated with passive ankle dorsiflexion or first digit extension.2,4 If paresthesias occur with percussion inferior to the medial malleolus, suspect possible nerve entrapment or tarsal tunnel syndrome. Tenderness with heel compression (squeeze test) may indicate a calcaneal fracture or apophysitis.
Imaging is useful to rule out alternative disorders
Radiologic studies generally do not contribute to the diagnosis or management of PF, but they can assist in ruling out alternative causes of heel pain or in reevaluation if symptoms of PF persist after 3 to 6 months of treatment.
Plain films lack the sensitivity to detect plantar fasciitis. While a plantar calcaneal spur is often seen on radiography, it does not confirm the diagnosis, correlate with severity of symptoms, or predict prognosis.4 Despite this deficiency, plain radiography remains the initial choice of imaging modalities, particularly to rule out other conditions.
Ultrasound accurately diagnoses plantar fasciitis. Plantar fascia thickness of more than 4.0 mm is diagnostic of PF.7 Additionally, a decrease in plantar fascia thickness correlates with a decrease in pain levels, and thus ultrasound can aid in monitoring treatment progress.8 If results of plain films and ultrasound are inconclusive and clinical concern for an alternative diagnosis warrants additional expense, consider arranging for magnetic resonance imaging.9
Noninvasive treatments
Conservative therapies remain the preferred approach to treating PF, successfully managing 85% to 90% of cases.10,11 A 2010 clinical practice guideline from the American College of Foot and Ankle Surgeons recommends conservative treatments such as nonsteroidal inflammatory drugs (NSAIDs), stretching, and prefabricated orthotics for the initial management of plantar heel pain.4 Emphasize to patients that it may take 6 to 12 months for symptoms to resolve.4
Stretching and trigger-point manual therapy are effective
The traditional primary treatment modality for PF has been early initiation of an Achilles-soleus (heel-cord) muscle–stretching program. However, studies have shown that plantar fascia–specific stretching (PFSS) (FIGURE) significantly diminishes or eliminates heel pain when compared with traditional stretching movements, and is useful in treating chronic recalcitrant heel pain.12,13 PFSS has also yielded results superior to low-dose shock wave therapy.14
In a 2011 study, adding myofascial trigger-point manual therapy to a PFSS routine improved self-reported physical function and pain vs stretching alone.15 This manual therapy technique is specialized and should be administered only by trained physical therapists. Data are limited and mixed regarding the effectiveness of deep tissue massage, iontophoresis, or eccentric stretching of the plantar fascia to alleviate plantar fascial pain. Support for therapies such as rest, ice, heat, and massage has largely been anecdotal.
NSAIDs for PF lack good evidence
Nonsteroidal anti-inflammatory drugs (NSAIDs) are often prescribed to treat PF, despite a lack of evidence supporting their use. A small randomized, placebo-controlled double-blind study established a trend toward improvement in pain and disability scores with the use of NSAIDs. However, no statistically significant difference was noted in the measures between the NSAID and placebo groups at 1, 2, and 6 months.16 We found no studies that demonstrate a significant reduction in pain or improvement in function with the use of NSAIDs alone.
Although NSAIDs carry their own risks, they may work for some patients. And studies showing a lack of significant pain reduction may have been underpowered. If patients are willing to accept the risks of NSAID use, it would be reasonable to prescribe a therapeutic trial.
Orthotics and night splints can help, depending on comfort and compliance
Foot orthotics help prevent overpronation and attenuate tensile forces on the plantar fascia. A 2009 meta-analysis confirmed that both prefabricated and custom-made foot orthotics can decrease pain.17 One prospective study showed that 95% of patients had improvement in PF symptoms after 8 weeks of treatment with prefabricated orthotics.18 A Cochrane review found no difference in pain reduction between custom and prefabricated foot orthotics.19 A recent study demonstrated that rocker sole shoes—a type of therapeutic footwear with a more rounded outsole contour—combined with custom orthotics significantly reduced pain during walking compared with either modality alone.20 More research needs to be conducted into the use of rocker sole shoes before recommending them to PF patients.
Night splints help keep the foot and ankle in a neutral position, or slightly dorsiflexed, while patients sleep. Several studies have shown a reduction in pain with the use of night splints alone.17,21,22 Patient comfort and compliance tend to be the limiting factors in their use. Anterior splints are better tolerated than posterior splints.23
Shock wave therapy has better long-term results than steroid injections
Shock waves used to treat PF are thought to invoke extracellular responses that cause neovascularization and induce tissue repair and regeneration. A 2012 review article concluded that most research confirms that extracorporeal shock wave therapy (ESWT) reduces PF pain and improves function in 34% to 88% of cases.24 ESWT is comparable to surgical plantar fasciotomy without the operative risks, and yields better long-term effects in recalcitrant PF compared with corticosteroid injections (CSI).24 Many studies are underway to validate the effectiveness of ESWT. Currently, expense or lack of availability limits its use in some communities.
Invasive treatments
Corticosteroid injections may be used for more than just refractory pain
CSI have historically been reserved for recalcitrant heel pain. However, one systematic review cites evidence in support of CSI for the short-term management of plantar fascia pain.25 Compared with placebo, CSI reduces pain at both 6 and 12 weeks and decreases plantar fascia thickness.26 Additionally, the American College of Foot and Ankle Surgeons lists CSI as an acceptable first-line treatment for PF.4
The most common complication of CSI is postinjection pain. Other complications, such as fat pad atrophy, rarely occur.27 While the evidence is limited, CSI may be part of an initial approach to treating PF in addition to heel-cord or plantar fascia-specific stretching, particularly for patients who desire an expedited return to normal activity.
Platelet-rich plasma therapy holds promise
Platelet-rich plasma (PRP) has been gaining popularity as a treatment for PF pain. PRP is a component of whole blood that is centrifuged to a concentrated state, treated with an activating agent, and injected into the affected area. Theoretically, injected PRP increases the release of reparative growth factors, enhancing the healing process.28 PRP has been shown to be as effective in reducing pain scores as CSI at 3 weeks and 6 months.29 PRP also decreases plantar fascia thickness and improves pain scores and functional ability.30
To date, no trials have compared PRP with placebo injections. Postprocedural pain is the most common risk with PRP. While limited evidence exists, PRP seems to be a relatively safe and effective therapeutic alternative for treating chronic PF.
Surgery only when conservative measures fail
Reserve surgery for those who have not responded adequately after 6 to 12 months of conservative therapy.5 Endoscopic plantar fascia release is superior to traditional open surgery.31 Heel spur resection is no longer routinely practiced. Patients undergoing surgery should expect a return to normal activity in approximately 2 to 3 months, and up to 35% of patients may continue to have symptoms after surgical intervention.2,31
Treatment options in perspective
Treat conservatively at first. Stretching the plantar fascia and heel cord, using prefabricated orthotics, and wearing night splints are backed by firm clinical evidence of benefit. Acute treatment of PF may also include CSI, especially for patients who are athletic or otherwise active and wish to return to full function as soon as possible, and are willing to accept the risks associated with CSI.
ESWT improves pain and function scores and may also relieve pain in patients with recalcitrant PF pain. PRP has limited but promising evidence for patients with chronic PF pain. Surgical intervention remains the last line of therapy and is not always effective at reducing pain.
CASE You prescribe a conservative treatment program of plantar fascia–specific stretches and prefabricated orthoses for the patient in the opening scenario. At one month, her pain drops by 30%. At 6 months, her pain disappears, and she resumes a daily aerobic exercise program to assist in weight loss.
CORRESPONDENCE
Carlton J. Covey, MD, Nellis Family Medicine Residency, 99MDOS/SGOF, 4700 Las Vegas Boulevard N, Las Vegas, NV 89191;
[email protected]
› Use plantar fascia specific stretching to decrease pain in patients with plantar fasciitis. A
› Consider recommending prefabricated orthoses, including night splints, to decrease pain. A
› Consider using extracorporeal shock wave therapy for plantar fascial pain. A
Strength of recommendation (SOR)
A. Good-quality patient-oriented evidence
B. Inconsistent or limited-quality patient-oriented evidence
C. Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE A 43-year-old obese woman seeks advice for left heel pain she has had for 2 months. Before the onset of pain, her activity level had increased as part of a weight loss program. Her pain is at its worst in the morning, with her first few steps; it decreases with continued walking and intensifies again after being on her feet all day. There is no history of trauma, and she reports no paresthesias or radiation of the pain. Her medical history is otherwise unremarkable. She has used ibuprofen sparingly, with limited relief.
If you were this patient’s physician, how would you proceed with her care?
Plantar fasciitis (PF) is a common cause of heel pain that affects up to 10% of the US population and accounts for approximately 600,000 outpatient visits annually.1 The plantar fascia is a dense, fibrous membrane spanning the length of the foot. It originates at the medial calcaneal tubercle, attaches to the phalanges, and provides stability and arch support to the foot. The etiology of PF is unknown, but predisposing factors include overtraining, obesity, pes planus, decreased ankle dorsiflexion, and inappropriate footwear.2 Limited dorsiflexion due to tightness of the Achilles tendon strains the plantar fascia and can lead to PF. Histology shows minimal inflammatory changes, and some experts advocate the term plantar fasciosis to counter the misperception that it is primarily an inflammatory condition.3
A patient’s history and physical exam findings are the basis for confirming or dismissing a diagnosis of PF. Radiologic studies, used judiciously, can rule out important alternative diagnoses that should not be overlooked. Multiple treatment options range from conservative to surgical interventions, although studies of the effectiveness of each modality have had conflicting results. Clinical practice guidelines generally advocate a stepwise approach to treatment.
Diagnosis
The differential diagnosis of PF (TABLE) includes significant disorders such as calcaneal stress fracture, entrapment neuropathies (eg, tarsal tunnel syndrome), calcaneal tumor, Paget’s disease, and systemic arthritidies.4,5
What to look for in the history and physical exam
Severe heel pain upon initial weight bearing in the morning or after prolonged periods of inactivity is pathognomonic for PF.2 Initially the pain presents diffusely, but over time it localizes to the area of the medial calcaneal tubercle. Pain typically subsides with activity but may return with prolonged weight bearing, as it did with the patient in the opening case.
Test range of motion of the foot and ankle. Although this is not needed for diagnosing PF, some patients will exhibit limited ankle dorsiflexion, a predisposing factor for PF.4,6 Look for heel pad swelling, inflammation, or atrophy, and palpate the heel, plantar fascia, and calcaneal tubercle. Lastly, evaluate for gait abnormalities and the presence of sensory deficits or hypesthesias.4
The most common exam finding in PF is pain at the medial calcaneal tubercle, which may be exacerbated with passive ankle dorsiflexion or first digit extension.2,4 If paresthesias occur with percussion inferior to the medial malleolus, suspect possible nerve entrapment or tarsal tunnel syndrome. Tenderness with heel compression (squeeze test) may indicate a calcaneal fracture or apophysitis.
Imaging is useful to rule out alternative disorders
Radiologic studies generally do not contribute to the diagnosis or management of PF, but they can assist in ruling out alternative causes of heel pain or in reevaluation if symptoms of PF persist after 3 to 6 months of treatment.
Plain films lack the sensitivity to detect plantar fasciitis. While a plantar calcaneal spur is often seen on radiography, it does not confirm the diagnosis, correlate with severity of symptoms, or predict prognosis.4 Despite this deficiency, plain radiography remains the initial choice of imaging modalities, particularly to rule out other conditions.
Ultrasound accurately diagnoses plantar fasciitis. Plantar fascia thickness of more than 4.0 mm is diagnostic of PF.7 Additionally, a decrease in plantar fascia thickness correlates with a decrease in pain levels, and thus ultrasound can aid in monitoring treatment progress.8 If results of plain films and ultrasound are inconclusive and clinical concern for an alternative diagnosis warrants additional expense, consider arranging for magnetic resonance imaging.9
Noninvasive treatments
Conservative therapies remain the preferred approach to treating PF, successfully managing 85% to 90% of cases.10,11 A 2010 clinical practice guideline from the American College of Foot and Ankle Surgeons recommends conservative treatments such as nonsteroidal inflammatory drugs (NSAIDs), stretching, and prefabricated orthotics for the initial management of plantar heel pain.4 Emphasize to patients that it may take 6 to 12 months for symptoms to resolve.4
Stretching and trigger-point manual therapy are effective
The traditional primary treatment modality for PF has been early initiation of an Achilles-soleus (heel-cord) muscle–stretching program. However, studies have shown that plantar fascia–specific stretching (PFSS) (FIGURE) significantly diminishes or eliminates heel pain when compared with traditional stretching movements, and is useful in treating chronic recalcitrant heel pain.12,13 PFSS has also yielded results superior to low-dose shock wave therapy.14
In a 2011 study, adding myofascial trigger-point manual therapy to a PFSS routine improved self-reported physical function and pain vs stretching alone.15 This manual therapy technique is specialized and should be administered only by trained physical therapists. Data are limited and mixed regarding the effectiveness of deep tissue massage, iontophoresis, or eccentric stretching of the plantar fascia to alleviate plantar fascial pain. Support for therapies such as rest, ice, heat, and massage has largely been anecdotal.
NSAIDs for PF lack good evidence
Nonsteroidal anti-inflammatory drugs (NSAIDs) are often prescribed to treat PF, despite a lack of evidence supporting their use. A small randomized, placebo-controlled double-blind study established a trend toward improvement in pain and disability scores with the use of NSAIDs. However, no statistically significant difference was noted in the measures between the NSAID and placebo groups at 1, 2, and 6 months.16 We found no studies that demonstrate a significant reduction in pain or improvement in function with the use of NSAIDs alone.
Although NSAIDs carry their own risks, they may work for some patients. And studies showing a lack of significant pain reduction may have been underpowered. If patients are willing to accept the risks of NSAID use, it would be reasonable to prescribe a therapeutic trial.
Orthotics and night splints can help, depending on comfort and compliance
Foot orthotics help prevent overpronation and attenuate tensile forces on the plantar fascia. A 2009 meta-analysis confirmed that both prefabricated and custom-made foot orthotics can decrease pain.17 One prospective study showed that 95% of patients had improvement in PF symptoms after 8 weeks of treatment with prefabricated orthotics.18 A Cochrane review found no difference in pain reduction between custom and prefabricated foot orthotics.19 A recent study demonstrated that rocker sole shoes—a type of therapeutic footwear with a more rounded outsole contour—combined with custom orthotics significantly reduced pain during walking compared with either modality alone.20 More research needs to be conducted into the use of rocker sole shoes before recommending them to PF patients.
Night splints help keep the foot and ankle in a neutral position, or slightly dorsiflexed, while patients sleep. Several studies have shown a reduction in pain with the use of night splints alone.17,21,22 Patient comfort and compliance tend to be the limiting factors in their use. Anterior splints are better tolerated than posterior splints.23
Shock wave therapy has better long-term results than steroid injections
Shock waves used to treat PF are thought to invoke extracellular responses that cause neovascularization and induce tissue repair and regeneration. A 2012 review article concluded that most research confirms that extracorporeal shock wave therapy (ESWT) reduces PF pain and improves function in 34% to 88% of cases.24 ESWT is comparable to surgical plantar fasciotomy without the operative risks, and yields better long-term effects in recalcitrant PF compared with corticosteroid injections (CSI).24 Many studies are underway to validate the effectiveness of ESWT. Currently, expense or lack of availability limits its use in some communities.
Invasive treatments
Corticosteroid injections may be used for more than just refractory pain
CSI have historically been reserved for recalcitrant heel pain. However, one systematic review cites evidence in support of CSI for the short-term management of plantar fascia pain.25 Compared with placebo, CSI reduces pain at both 6 and 12 weeks and decreases plantar fascia thickness.26 Additionally, the American College of Foot and Ankle Surgeons lists CSI as an acceptable first-line treatment for PF.4
The most common complication of CSI is postinjection pain. Other complications, such as fat pad atrophy, rarely occur.27 While the evidence is limited, CSI may be part of an initial approach to treating PF in addition to heel-cord or plantar fascia-specific stretching, particularly for patients who desire an expedited return to normal activity.
Platelet-rich plasma therapy holds promise
Platelet-rich plasma (PRP) has been gaining popularity as a treatment for PF pain. PRP is a component of whole blood that is centrifuged to a concentrated state, treated with an activating agent, and injected into the affected area. Theoretically, injected PRP increases the release of reparative growth factors, enhancing the healing process.28 PRP has been shown to be as effective in reducing pain scores as CSI at 3 weeks and 6 months.29 PRP also decreases plantar fascia thickness and improves pain scores and functional ability.30
To date, no trials have compared PRP with placebo injections. Postprocedural pain is the most common risk with PRP. While limited evidence exists, PRP seems to be a relatively safe and effective therapeutic alternative for treating chronic PF.
Surgery only when conservative measures fail
Reserve surgery for those who have not responded adequately after 6 to 12 months of conservative therapy.5 Endoscopic plantar fascia release is superior to traditional open surgery.31 Heel spur resection is no longer routinely practiced. Patients undergoing surgery should expect a return to normal activity in approximately 2 to 3 months, and up to 35% of patients may continue to have symptoms after surgical intervention.2,31
Treatment options in perspective
Treat conservatively at first. Stretching the plantar fascia and heel cord, using prefabricated orthotics, and wearing night splints are backed by firm clinical evidence of benefit. Acute treatment of PF may also include CSI, especially for patients who are athletic or otherwise active and wish to return to full function as soon as possible, and are willing to accept the risks associated with CSI.
ESWT improves pain and function scores and may also relieve pain in patients with recalcitrant PF pain. PRP has limited but promising evidence for patients with chronic PF pain. Surgical intervention remains the last line of therapy and is not always effective at reducing pain.
CASE You prescribe a conservative treatment program of plantar fascia–specific stretches and prefabricated orthoses for the patient in the opening scenario. At one month, her pain drops by 30%. At 6 months, her pain disappears, and she resumes a daily aerobic exercise program to assist in weight loss.
CORRESPONDENCE
Carlton J. Covey, MD, Nellis Family Medicine Residency, 99MDOS/SGOF, 4700 Las Vegas Boulevard N, Las Vegas, NV 89191;
[email protected]
1. Riddle DL, Schappert SM. Volume of ambulatory care visits and patterns of care for patients diagnosed with plantar fasciitis: a national study of medical doctors. Foot Ankle Int. 2004;25: 303-310.
2. Glazer JL. An approach to the diagnosis and treatment of plantar fasciitis. Phys Sportsmed. 2009;37:74-79.
3. Lemont H, Ammirati KM, Usen N. Plantar fasciitis: a degenerative process (fasciosis) without inflammation. J Am Podiatr Med Assoc. 2003;93:234-237.
4. Thomas JL, Christensen JC, Kravitz SR, et al. The diagnosis and treatment of heal pain: a clinical practice guideline – revision 2010. J Foot Ankle Surg. 2010;49(suppl):S1-S19.
5. Neufeld SK, Cerrato R. Plantar fasciitis: evaluation and treatment. J Am Acad Orthop Surg. 2008;16:338-346.
6. Singh D, Angel J, Bentley G, et al. Fortnightly review: plantar fasciitis. BMJ. 1997;315:172-175.
7. McMillan AM, Landorf KB, Barrett JT, et al. Diagnostic imaging for chronic plantar heel pain: a systematic review and metaanalysis. J Foot Ankle Res. 2009;2:32.
8. Mahowald S, Legge BS, Grady JF. The correlation between plantar fascia thickness and symptoms of plantar fasciitis. J Am Podiatr Med Assoc. 2011;101:385-389.
9. American College of Radiology. ACR appropriateness criteria. Chronic foot pain. Available at: http://www.acr.org/~/media/ACR/Documents/AppCriteria/Diagnostic/ChronicFootPain.pdf. Accessed November 11, 2012.
10. Gill LH. Plantar fasciitis: diagnosis and conservative treatment. J Am Acad Orthop Surg. 1997;5:109-117.
11. Martin RL, Irrgang JJ, Conti SF. Outcome study of subjects with insertional plantar fasciitis. Foot Ankle Int. 1998;19:803-811.
12. DiGiovanni BF, Nawoczenski DA, Malay DP, et al. Plantar fasciaspecific stretching exercise improves outcomes in patients with chronic plantar fasciitis: a prospective clinic trial with two-year follow up. J Bone Joint Surg Am. 2006;88:1775-1781.
13. Sweeting D, Parish B, Hooper L, et al. The effectiveness of manual stretching in the treatment of plantar heel pain: a systemic review. J Foot Ankle Res. 2011;4:1-13.
14. Rompe JD, Cacchio A, Lowell W, et al. Plantar fascia-specific stretching versus radial shock-wave therapy as initial treatment of plantar fasciopathy. J Bone Joint Surg Am. 2010;92:2514-2522.
15. Renan-Ordine R, Alburquerque-Sendin F, Rodriques De Souza DP, et al. Effectiveness of myofascial trigger point manual therapy combined with a self stretching protocol for the management of plantar heel pain: a randomized controlled trial. J Orthop Sports Phys Ther. 2011;41:43-50.
16. Donley BG, Moore T, Sferra J, et al. The efficacy of oral nonsteroidal anti-inflammatory medication (NSAID) in the treatment of plantar fasciitis: a randomized, prospective, placebo-controlled study. Foot Ankle Int. 2007;28:20-23.
17. Lee SY, McKeon P, Hertel J. Does the use of orthoses improve selfreported pain and function measures in patients with plantar fasciitis? A meta-analysis. Phys Ther Sport. 2009;10:12-18.
18. Pfeffer G, Bacchetti P, Deland J, et al. Comparison of custom and prefabricated orthoses in the initial treatment of proximal plantar fasciitis. Foot Ankle Int. 1999;20:214-221.
19. Hawke F, Burns J, Radford JA, et al. Custom-made foot orthoses for the treatment of foot pain. Cochrane Database Syst Rev. 2008;(3):CD006801.
20. Fong DT, Pang KY, Chung MM, et al. Evaluation of combined prescription of rocker sole shoes and custom-made foot orthoses for the treatment of plantar fasciitis. Clin Biomech. 2012;27: 1072-1077.
21. Berlet GC, Anderson RB, Davis H. A prospective trial of night splinting in the treatment of recalcitrant plantar fasciitis: the Ankle Dorsiflexion Dynasplint. Orthopedics. 2002;25: 1273-1275.
22. Roos E, Engstrom M, Soderberg B. Foot orthoses for the treatment of plantar fasciitis. Foot Ankle Int. 2006;27:606-611.
23. Goff JD, Crawford R. Diagnosis and treatment of plantar fasciitis. Am Fam Physician. 2011;84:676-682.
24. Wang CJ. Extracorporeal shockwave therapy in musculoskeletal disorders. J Orthop Surg Res. 2012;7:11.
25. Landorf KB, Menz HB. Plantar heel pain and fasciitis. Clin Evid (Online). 2008;2008:1111.
26. Ball EM, McKeeman HM, Patterson C, et al. Steroid injection for inferior heel pain: a randomized controlled trial. Ann Rheum Dis. 2013;72:996-1002.
27. Uden H, Boesch E, Kumar S. Plantar fasciitis – to jab or support? A systematic review of the current best evidence. J Multidiscip Healthc. 2011;4:155-164.
28. Shetty VD. Platelet-rich plasma: a ‘feeling’ and ‘hope’ ailing athletes. Br J Sports Med. 2010;44(suppl 1):i1-i82.
29. Aksahin E, Dogruyol D, Yüksel HY, et al. The comparison of the effect of corticosteroids and platelet-rich plasma (PRP) for the treatment of plantar fasciitis. Arch Orthop Trauma Surg. 2012;132:781-785.
30. Ragab EM, Othman AM. Platelets rich plasma for treatment of chronic plantar fasciitis. Arch Orthop Trauma Surg. 2012;132:1065-1070.
31. Saxena A. Uniportal endoscopic plantar fasciotomy: a prospective study on athletic patients. Foot Ankle Int. 2004;25:882-889.
1. Riddle DL, Schappert SM. Volume of ambulatory care visits and patterns of care for patients diagnosed with plantar fasciitis: a national study of medical doctors. Foot Ankle Int. 2004;25: 303-310.
2. Glazer JL. An approach to the diagnosis and treatment of plantar fasciitis. Phys Sportsmed. 2009;37:74-79.
3. Lemont H, Ammirati KM, Usen N. Plantar fasciitis: a degenerative process (fasciosis) without inflammation. J Am Podiatr Med Assoc. 2003;93:234-237.
4. Thomas JL, Christensen JC, Kravitz SR, et al. The diagnosis and treatment of heal pain: a clinical practice guideline – revision 2010. J Foot Ankle Surg. 2010;49(suppl):S1-S19.
5. Neufeld SK, Cerrato R. Plantar fasciitis: evaluation and treatment. J Am Acad Orthop Surg. 2008;16:338-346.
6. Singh D, Angel J, Bentley G, et al. Fortnightly review: plantar fasciitis. BMJ. 1997;315:172-175.
7. McMillan AM, Landorf KB, Barrett JT, et al. Diagnostic imaging for chronic plantar heel pain: a systematic review and metaanalysis. J Foot Ankle Res. 2009;2:32.
8. Mahowald S, Legge BS, Grady JF. The correlation between plantar fascia thickness and symptoms of plantar fasciitis. J Am Podiatr Med Assoc. 2011;101:385-389.
9. American College of Radiology. ACR appropriateness criteria. Chronic foot pain. Available at: http://www.acr.org/~/media/ACR/Documents/AppCriteria/Diagnostic/ChronicFootPain.pdf. Accessed November 11, 2012.
10. Gill LH. Plantar fasciitis: diagnosis and conservative treatment. J Am Acad Orthop Surg. 1997;5:109-117.
11. Martin RL, Irrgang JJ, Conti SF. Outcome study of subjects with insertional plantar fasciitis. Foot Ankle Int. 1998;19:803-811.
12. DiGiovanni BF, Nawoczenski DA, Malay DP, et al. Plantar fasciaspecific stretching exercise improves outcomes in patients with chronic plantar fasciitis: a prospective clinic trial with two-year follow up. J Bone Joint Surg Am. 2006;88:1775-1781.
13. Sweeting D, Parish B, Hooper L, et al. The effectiveness of manual stretching in the treatment of plantar heel pain: a systemic review. J Foot Ankle Res. 2011;4:1-13.
14. Rompe JD, Cacchio A, Lowell W, et al. Plantar fascia-specific stretching versus radial shock-wave therapy as initial treatment of plantar fasciopathy. J Bone Joint Surg Am. 2010;92:2514-2522.
15. Renan-Ordine R, Alburquerque-Sendin F, Rodriques De Souza DP, et al. Effectiveness of myofascial trigger point manual therapy combined with a self stretching protocol for the management of plantar heel pain: a randomized controlled trial. J Orthop Sports Phys Ther. 2011;41:43-50.
16. Donley BG, Moore T, Sferra J, et al. The efficacy of oral nonsteroidal anti-inflammatory medication (NSAID) in the treatment of plantar fasciitis: a randomized, prospective, placebo-controlled study. Foot Ankle Int. 2007;28:20-23.
17. Lee SY, McKeon P, Hertel J. Does the use of orthoses improve selfreported pain and function measures in patients with plantar fasciitis? A meta-analysis. Phys Ther Sport. 2009;10:12-18.
18. Pfeffer G, Bacchetti P, Deland J, et al. Comparison of custom and prefabricated orthoses in the initial treatment of proximal plantar fasciitis. Foot Ankle Int. 1999;20:214-221.
19. Hawke F, Burns J, Radford JA, et al. Custom-made foot orthoses for the treatment of foot pain. Cochrane Database Syst Rev. 2008;(3):CD006801.
20. Fong DT, Pang KY, Chung MM, et al. Evaluation of combined prescription of rocker sole shoes and custom-made foot orthoses for the treatment of plantar fasciitis. Clin Biomech. 2012;27: 1072-1077.
21. Berlet GC, Anderson RB, Davis H. A prospective trial of night splinting in the treatment of recalcitrant plantar fasciitis: the Ankle Dorsiflexion Dynasplint. Orthopedics. 2002;25: 1273-1275.
22. Roos E, Engstrom M, Soderberg B. Foot orthoses for the treatment of plantar fasciitis. Foot Ankle Int. 2006;27:606-611.
23. Goff JD, Crawford R. Diagnosis and treatment of plantar fasciitis. Am Fam Physician. 2011;84:676-682.
24. Wang CJ. Extracorporeal shockwave therapy in musculoskeletal disorders. J Orthop Surg Res. 2012;7:11.
25. Landorf KB, Menz HB. Plantar heel pain and fasciitis. Clin Evid (Online). 2008;2008:1111.
26. Ball EM, McKeeman HM, Patterson C, et al. Steroid injection for inferior heel pain: a randomized controlled trial. Ann Rheum Dis. 2013;72:996-1002.
27. Uden H, Boesch E, Kumar S. Plantar fasciitis – to jab or support? A systematic review of the current best evidence. J Multidiscip Healthc. 2011;4:155-164.
28. Shetty VD. Platelet-rich plasma: a ‘feeling’ and ‘hope’ ailing athletes. Br J Sports Med. 2010;44(suppl 1):i1-i82.
29. Aksahin E, Dogruyol D, Yüksel HY, et al. The comparison of the effect of corticosteroids and platelet-rich plasma (PRP) for the treatment of plantar fasciitis. Arch Orthop Trauma Surg. 2012;132:781-785.
30. Ragab EM, Othman AM. Platelets rich plasma for treatment of chronic plantar fasciitis. Arch Orthop Trauma Surg. 2012;132:1065-1070.
31. Saxena A. Uniportal endoscopic plantar fasciotomy: a prospective study on athletic patients. Foot Ankle Int. 2004;25:882-889.
The Pros and Cons of Using Larger Femoral Heads in Total Hip Arthroplasty
Colorectal cancer risk increased with bariatric surgery
The risk of colorectal cancer was significantly increased among people who had undergone obesity surgery in a retrospective cohort study of more than 77,000 obese patients enrolled in a Swedish registry.
The increased risk for colorectal cancer was associated with all three bariatric procedures – vertical banded gastroplasty, adjustable gastric banding, and Roux-en-Y gastric bypass – and increased further over time, reported Dr. Maryam Derogar, of the Karolinska Institutet, Stockholm, and her associates. No such pattern over time was seen among the obese patients who did not have surgery.
"Our data suggest that increased colorectal cancer risk may be a long-term consequence of such surgery," they concluded. If the association is confirmed, they added, "it should stimulate research addressing colonoscopic evaluation of the incidence of colorectal adenomatous polyps after obesity surgery with a view to defining an optimum colonoscopy surveillance strategy for the increasing number of patients who undergo obesity surgery. The study was published online in the Annals of Surgery (2013 [doi:10.1097/SLA.0b013e318288463a]).
To address their "unexpected" finding in an earlier study of an apparent increase in the risk of colorectal cancer after obesity surgery, but no increase in the risk of other cancers related to obesity, they conducted a retrospective cohort study using national registry data between 1980 and 2009, of 15,095 obese patients who had undergone obesity surgery and 62,016 patients who had been diagnosed with obesity but did not undergo surgery. They calculated the colorectal cancer risk using the standardized incidence ratio (SIR), the observed number of cases divided by the number of expected cases in that group.
Over a median of 10 years, there were 70 colorectal cancers in the obesity surgery group; and over a median of 7 years, 373 among those who had no surgery. The SIR for colorectal cancer among those who had surgery was 1.60, which was statistically significant. Among those who had no surgery, there was a small, insignificant increase in risk group (a SIR of 1.26). In the surgery group, the risk increased over time in men and women, up to a twofold increased risk among those patients followed for at least 10 years, a pattern than was not observed in the obese patients who had no surgery.
The "substantial increase in colorectal cancer risk, above that associated with excess body weight alone, more than 10 years after surgery is compatible with the long natural history of colorectal carcinogenesis from normal mucosa to a malignant colorectal cancer," the authors wrote. Why the risk was increased is not clear, but one possible explanation could be that the malabsorption effects of the gastric bypass procedure results in local mucosal changes, the authors speculated. Previously, they had identified rectal mucosal hyperproliferation in patients who had undergone obesity surgery, present at least 3 years after the procedure, a finding that was "associated with increased mucosal expression of the protumorigenic cytokine macrophage migration inhibitory factor," they wrote.
The study’s strengths included the size of the sample, long follow-up, and the validity of Swedish national registry data, while the limitations included the retrospective design and the lack of data on body weight over time.
As in the United States and other countries, obesity has been increasing in Sweden, with a corresponding increase in bariatric surgery. Over the last 20 years, the prevalence of obesity in Sweden has doubled, and the annual number of obesity operations performed has increased from 1,500 in 2006 to almost 4,000 in 2009, according to the authors.
The study was supported by the Swedish Research Council. The authors had no conflicts of interest to declare.
The risk of colorectal cancer was significantly increased among people who had undergone obesity surgery in a retrospective cohort study of more than 77,000 obese patients enrolled in a Swedish registry.
The increased risk for colorectal cancer was associated with all three bariatric procedures – vertical banded gastroplasty, adjustable gastric banding, and Roux-en-Y gastric bypass – and increased further over time, reported Dr. Maryam Derogar, of the Karolinska Institutet, Stockholm, and her associates. No such pattern over time was seen among the obese patients who did not have surgery.
"Our data suggest that increased colorectal cancer risk may be a long-term consequence of such surgery," they concluded. If the association is confirmed, they added, "it should stimulate research addressing colonoscopic evaluation of the incidence of colorectal adenomatous polyps after obesity surgery with a view to defining an optimum colonoscopy surveillance strategy for the increasing number of patients who undergo obesity surgery. The study was published online in the Annals of Surgery (2013 [doi:10.1097/SLA.0b013e318288463a]).
To address their "unexpected" finding in an earlier study of an apparent increase in the risk of colorectal cancer after obesity surgery, but no increase in the risk of other cancers related to obesity, they conducted a retrospective cohort study using national registry data between 1980 and 2009, of 15,095 obese patients who had undergone obesity surgery and 62,016 patients who had been diagnosed with obesity but did not undergo surgery. They calculated the colorectal cancer risk using the standardized incidence ratio (SIR), the observed number of cases divided by the number of expected cases in that group.
Over a median of 10 years, there were 70 colorectal cancers in the obesity surgery group; and over a median of 7 years, 373 among those who had no surgery. The SIR for colorectal cancer among those who had surgery was 1.60, which was statistically significant. Among those who had no surgery, there was a small, insignificant increase in risk group (a SIR of 1.26). In the surgery group, the risk increased over time in men and women, up to a twofold increased risk among those patients followed for at least 10 years, a pattern than was not observed in the obese patients who had no surgery.
The "substantial increase in colorectal cancer risk, above that associated with excess body weight alone, more than 10 years after surgery is compatible with the long natural history of colorectal carcinogenesis from normal mucosa to a malignant colorectal cancer," the authors wrote. Why the risk was increased is not clear, but one possible explanation could be that the malabsorption effects of the gastric bypass procedure results in local mucosal changes, the authors speculated. Previously, they had identified rectal mucosal hyperproliferation in patients who had undergone obesity surgery, present at least 3 years after the procedure, a finding that was "associated with increased mucosal expression of the protumorigenic cytokine macrophage migration inhibitory factor," they wrote.
The study’s strengths included the size of the sample, long follow-up, and the validity of Swedish national registry data, while the limitations included the retrospective design and the lack of data on body weight over time.
As in the United States and other countries, obesity has been increasing in Sweden, with a corresponding increase in bariatric surgery. Over the last 20 years, the prevalence of obesity in Sweden has doubled, and the annual number of obesity operations performed has increased from 1,500 in 2006 to almost 4,000 in 2009, according to the authors.
The study was supported by the Swedish Research Council. The authors had no conflicts of interest to declare.
The risk of colorectal cancer was significantly increased among people who had undergone obesity surgery in a retrospective cohort study of more than 77,000 obese patients enrolled in a Swedish registry.
The increased risk for colorectal cancer was associated with all three bariatric procedures – vertical banded gastroplasty, adjustable gastric banding, and Roux-en-Y gastric bypass – and increased further over time, reported Dr. Maryam Derogar, of the Karolinska Institutet, Stockholm, and her associates. No such pattern over time was seen among the obese patients who did not have surgery.
"Our data suggest that increased colorectal cancer risk may be a long-term consequence of such surgery," they concluded. If the association is confirmed, they added, "it should stimulate research addressing colonoscopic evaluation of the incidence of colorectal adenomatous polyps after obesity surgery with a view to defining an optimum colonoscopy surveillance strategy for the increasing number of patients who undergo obesity surgery. The study was published online in the Annals of Surgery (2013 [doi:10.1097/SLA.0b013e318288463a]).
To address their "unexpected" finding in an earlier study of an apparent increase in the risk of colorectal cancer after obesity surgery, but no increase in the risk of other cancers related to obesity, they conducted a retrospective cohort study using national registry data between 1980 and 2009, of 15,095 obese patients who had undergone obesity surgery and 62,016 patients who had been diagnosed with obesity but did not undergo surgery. They calculated the colorectal cancer risk using the standardized incidence ratio (SIR), the observed number of cases divided by the number of expected cases in that group.
Over a median of 10 years, there were 70 colorectal cancers in the obesity surgery group; and over a median of 7 years, 373 among those who had no surgery. The SIR for colorectal cancer among those who had surgery was 1.60, which was statistically significant. Among those who had no surgery, there was a small, insignificant increase in risk group (a SIR of 1.26). In the surgery group, the risk increased over time in men and women, up to a twofold increased risk among those patients followed for at least 10 years, a pattern than was not observed in the obese patients who had no surgery.
The "substantial increase in colorectal cancer risk, above that associated with excess body weight alone, more than 10 years after surgery is compatible with the long natural history of colorectal carcinogenesis from normal mucosa to a malignant colorectal cancer," the authors wrote. Why the risk was increased is not clear, but one possible explanation could be that the malabsorption effects of the gastric bypass procedure results in local mucosal changes, the authors speculated. Previously, they had identified rectal mucosal hyperproliferation in patients who had undergone obesity surgery, present at least 3 years after the procedure, a finding that was "associated with increased mucosal expression of the protumorigenic cytokine macrophage migration inhibitory factor," they wrote.
The study’s strengths included the size of the sample, long follow-up, and the validity of Swedish national registry data, while the limitations included the retrospective design and the lack of data on body weight over time.
As in the United States and other countries, obesity has been increasing in Sweden, with a corresponding increase in bariatric surgery. Over the last 20 years, the prevalence of obesity in Sweden has doubled, and the annual number of obesity operations performed has increased from 1,500 in 2006 to almost 4,000 in 2009, according to the authors.
The study was supported by the Swedish Research Council. The authors had no conflicts of interest to declare.
FROM THE ANNALS OF SURGERY
What is causing her abdominal pain?
CASE: A healthy, nulliparous, 20-year-old woman visits her ObGyn to report a 2-day history of right lower quadrant pain. Her last menstrual period was appropriately timed and normal. She is not sexually active. Upon examination, she exhibits minimal left and right lower quadrant tenderness. Examination with the vaginal speculum reveals no vaginal or cervical abnormalities, and bimanual examination reveals a uterus of normal size, with cervical motion tenderness but no adnexal fullness or mass. For this reason, transvaginal ultrasound (TVUS) is performed. It reveals an enlarged, edematous appendix adjacent to a normal-appearing right ovary (FIGURE 1).
No uterine or ovarian pathology is noted. Because the appendix is enlarged on TVUS, Doppler interrogation is added, which shows abundant vascularity of the appendix (FIGURE 2).
What do these findings suggest?
Acute appendicitis is the most likely diagnosis, as both the physical findings and ultrasound imaging point to it. The patient is referred to a general surgeon, who examines her, noting that she is afebrile, with tenderness in the right lower quadrant. She exhibits localized guarding at McBurneys point, with mild rebound. There is no sign of organomegaly. Bowel sounds are normal, with no distention. The patient undergoes laparoscopy, which confirms the diagnosis. The appendix is resected successfully, and rupture is averted (FIGURE 3).
The patient is discharged home on the first postoperative day. At a follow-up visit 2 weeks later, she is fully recovered and has returned to full and normal activity.
Clinicians who are familiar with the appearance of an inflamed appendix on TVUS may be able to expedite the management of women with appendicitis, avoiding the potential delay, expense, and radiation exposure associated with computed tomography imaging of the abdomen and pelvis.
Acknowledgment
The authors are grateful to Grace J. Horton, RDMS, and Christine L. Bubier, AS, RT(R), RDMS, who generated the images in this case.
Do you have a DIAGNOSTIC IN-SIGHT?
Submit a query for your image-based case! [email protected]
CASE: A healthy, nulliparous, 20-year-old woman visits her ObGyn to report a 2-day history of right lower quadrant pain. Her last menstrual period was appropriately timed and normal. She is not sexually active. Upon examination, she exhibits minimal left and right lower quadrant tenderness. Examination with the vaginal speculum reveals no vaginal or cervical abnormalities, and bimanual examination reveals a uterus of normal size, with cervical motion tenderness but no adnexal fullness or mass. For this reason, transvaginal ultrasound (TVUS) is performed. It reveals an enlarged, edematous appendix adjacent to a normal-appearing right ovary (FIGURE 1).
No uterine or ovarian pathology is noted. Because the appendix is enlarged on TVUS, Doppler interrogation is added, which shows abundant vascularity of the appendix (FIGURE 2).
What do these findings suggest?
Acute appendicitis is the most likely diagnosis, as both the physical findings and ultrasound imaging point to it. The patient is referred to a general surgeon, who examines her, noting that she is afebrile, with tenderness in the right lower quadrant. She exhibits localized guarding at McBurneys point, with mild rebound. There is no sign of organomegaly. Bowel sounds are normal, with no distention. The patient undergoes laparoscopy, which confirms the diagnosis. The appendix is resected successfully, and rupture is averted (FIGURE 3).
The patient is discharged home on the first postoperative day. At a follow-up visit 2 weeks later, she is fully recovered and has returned to full and normal activity.
Clinicians who are familiar with the appearance of an inflamed appendix on TVUS may be able to expedite the management of women with appendicitis, avoiding the potential delay, expense, and radiation exposure associated with computed tomography imaging of the abdomen and pelvis.
Acknowledgment
The authors are grateful to Grace J. Horton, RDMS, and Christine L. Bubier, AS, RT(R), RDMS, who generated the images in this case.
Do you have a DIAGNOSTIC IN-SIGHT?
Submit a query for your image-based case! [email protected]
CASE: A healthy, nulliparous, 20-year-old woman visits her ObGyn to report a 2-day history of right lower quadrant pain. Her last menstrual period was appropriately timed and normal. She is not sexually active. Upon examination, she exhibits minimal left and right lower quadrant tenderness. Examination with the vaginal speculum reveals no vaginal or cervical abnormalities, and bimanual examination reveals a uterus of normal size, with cervical motion tenderness but no adnexal fullness or mass. For this reason, transvaginal ultrasound (TVUS) is performed. It reveals an enlarged, edematous appendix adjacent to a normal-appearing right ovary (FIGURE 1).
No uterine or ovarian pathology is noted. Because the appendix is enlarged on TVUS, Doppler interrogation is added, which shows abundant vascularity of the appendix (FIGURE 2).
What do these findings suggest?
Acute appendicitis is the most likely diagnosis, as both the physical findings and ultrasound imaging point to it. The patient is referred to a general surgeon, who examines her, noting that she is afebrile, with tenderness in the right lower quadrant. She exhibits localized guarding at McBurneys point, with mild rebound. There is no sign of organomegaly. Bowel sounds are normal, with no distention. The patient undergoes laparoscopy, which confirms the diagnosis. The appendix is resected successfully, and rupture is averted (FIGURE 3).
The patient is discharged home on the first postoperative day. At a follow-up visit 2 weeks later, she is fully recovered and has returned to full and normal activity.
Clinicians who are familiar with the appearance of an inflamed appendix on TVUS may be able to expedite the management of women with appendicitis, avoiding the potential delay, expense, and radiation exposure associated with computed tomography imaging of the abdomen and pelvis.
Acknowledgment
The authors are grateful to Grace J. Horton, RDMS, and Christine L. Bubier, AS, RT(R), RDMS, who generated the images in this case.
Do you have a DIAGNOSTIC IN-SIGHT?
Submit a query for your image-based case! [email protected]
Acute Compartment Syndrome: To Save a Limb
Acute compartment syndrome (ACS) is a condition in which elevated pressures in the confined space of a closed fascial compartment lead to vascular compromise. Typically, ACS develops in the distal extremities after a traumatic event, such as a fracture, crush, or burn injury. A dangerous cycle ensues, involving increased compartmental pressure, decreased tissue perfusion, and continuing ischemia; fluids leak from the vasculature, perpetuating the process.1
Diagnosis of ACS requires a high level of clinical suspicion combined with a keen understanding of the risk factors for ACS and its pathophysiology, as well as astute awareness of the subtle clinical exam findings that usually accompany objective pressure measurements. Though most common in the extremities, ACS may also develop in the buttock, pelvis, or abdominal or spinal musculature.2-5
Prompt recognition and management of ACS are critical: This condition is considered a surgical emergency requiring immediate attention. Urgent decompression by fasciotomy is the definitive treatment6—an essential intervention to prevent critical tissue ischemia and necrosis. Failure to release the fascia in a timely manner can result in poor outcomes for patients, including but not limited to chronic pain, paralysis, sensory or motor deficits, loss of limb, deformity, and renal failure secondary to rhabdomyolysis.7,8
Clinical assessment and severity of injury are the two main factors that lead to prompt diagnosis and treatment of ACS. However, distracting injuries or an unconsciousness patient may interfere with the assessment, decreasing its accuracy.7 Heightened awareness of ACS is paramount so that clinicians can better recognize the condition before complications arise.
EPIDEMIOLOGY
ACS is typically associated with long bone injuries after significant trauma or crush injuries (eg, in a motor vehicle collision). Less often, severe burns, gun shot wounds, snake bites, poor anticoagulation, prolonged surgery (including procedures involving prolonged elevation of a limb),9 nephrotic syndrome, IV infiltrations, and other volume-expanding pathologies can present a similar risk.10-15 Essentially, ACS is a self-perpetuating process resulting from either increased compartmental content (eg, bleeding, edema) or reduced compartment size (eg, tight casting, burns).7,16
Ninety percent of cases of ACS in the extremities occur in men,15 and males younger than 35 have the greatest incidence of posttraumatic ACS.15,17,18 Although fractures account for nearly 70% of confirmed cases of ACS, soft-tissue injuries (particularly vascular injuries15) are also associated with ACS.18 Clinicians should be aware of a key misconception: that open fractures relieve intracompartmental pressure, reducing the risk for ACS. Rather, the damage and inflammation that occur in open fractures pose the same risk for ACS as do closed fractures.15
PATIENT PRESENTATION
A key consideration, in addition to the history and mechanism of injury or illness, is that patient’s description of the pain: The conscious patient will complain of pain that worsens progressively over time and that usually seems out of proportion to the physical exam findings or apparent injury.19 For this reason, serial assessments are recommended; worsening pain is indicative of rapid evolution of ACS, with the possibility of irreversible necrosis.20
Pain with passive stretch is an excellent indicator that the pathology is progressing.2 The “classic P” signs and symptoms of ACS are progressively later findings that represent markedly significant ischemia and injury already in progress: paresthesia, paresis/paralysis, poikilothermia (the inability of the patient to maintain a constant core temperature), pulselessness, and pallor.17
Diminishment of pain suggests a poor prognosis, as this indicates that the tissue is likely nonviable and necrotic by that point.1
Clinicians must be mindful that, in patients with central or peripheral nerve deficits or in those who have undergone nerve blocks or regional anesthesia, pain may be absent; in these instances, the risk for delayed diagnosis is increased.14,21 Furthermore, pain tolerance varies among patients, and distracting injuries may also cloud the clinical picture.
The diagnosis of ACS is not easily made. Thus, clinical suspicion should be elevated in any high-energy scenario: an extensive burn, overly restrictive casting, or volume-expanding disorder—especially in patients who present with pain that is out of proportion to the injury or is worsening progressively.1,7
PHYSICAL EXAMINATION
Physical examination and assessment should be repeated every two to four hours. The examining clinician should focus on pain characteristics, the skin, and the “classic P” signs mentioned above. Marked tenderness out of proportion to the injury, unmanageable pain, and pain on passive movement of the limb are the strongest indicators of developing ACS. These findings are nearly universal in the conscious patient, but they can be confused with primary pain of the injury itself. Additional findings of sensory or motor impairments may help raise the clinician’s suspicion for ACS but are nonspecific.1,7,23 Palpable tenseness is a common observation in ACS, but a clinician’s subjective measurement of skin firmness has little value as an objective strategy.22 Absence of distal pulses, decreased skin temperature, and pallor are very late signs that pressures are sufficiently high to cause complete arterial occlusion.20 Thus, clinicians should not wait for absent pulses or pallor to begin treatment. Suspicion for ACS should arise as soon as pain associated with trauma or surgery intensifies or becomes unmanageable.
DIAGNOSTIC TOOLS
ACS is diagnosed based on the previously mentioned clinical signs, along with objective evidence of decreased tissue perfusion in the affected limbs.24 Direct intracompartmental pressure measurements, obtained using a needle- or catheter-based technique, are the most common means of identifying ACS.7 However, near infrared spectroscopy and infrared imaging have also been found helpful.7, 25
Direct Intracompartmental Pressure Monitoring
Supported by clinical findings, direct measurement of the intracompartmental pressure (ICP) using a needle transducer is the most accurate method for detecting ACS and guiding treatment choices. Several measurement options are available, including a handheld needle manometer, which records single-pressure readings, or a wick or slit catheter, which can record continuous pressures.13 The choice between devices is based on facility/provider preference, as any commercial pressure device can be used with similar accuracies. When no such device is available, an 18-gauge needle with a set-up resembling an arterial line is another option.1
When using these instruments, it is essential for the provider to implement proper sterile technique and to record pressures from compartments within a 5-cm radius of the injury site.17 Furthermore, clinicians should be aware that the ICP can vary greatly and that tolerance to increased pressure varies with the patient’s diastolic blood pressure.
Normal capillary perfusion pressure (diastolic blood pressure minus compartment pressure) is approximately 30 mm Hg. Therefore, absolute ICP readings above 30 mm Hg can be diagnostic of ACS and indicative of fasciotomy. Likewise, a perfusion pressure below 30 mm Hg is also diagnostic. Based on consensus, either calculation can be used.16,17
Infrared Spectroscopy and Imaging
Barker and colleagues7 describe use of near infrared spectroscopy (NIRS) as a method to diagnose lower-extremity ACS. Noninvasive skin probes can detect the absorption spectra of mixed venous hemoglobin levels beneath the skin to determine oxygen saturation (StO2). StO2 measured by NIRS is location dependent, enabling the clinician to monitor oxygenation levels in any illuminated tissue. NIRS has great potential for use in confirming a diagnosis of ACS since decreased perfusion pressure from elevated ICP correlates well with decreased StO2. However, this technique has limitations that currently prevent its widespread use as a diagnostic tool.7
Use of noninvasive infrared imaging has also been investigated. Katz and colleagues25 describe the use of a long-wave infrared camera and thermographic imaging analysis software to detect differences in surface temperatures in patients’ limbs after blunt trauma. The researchers were able to correlate declines in temperature with decreased blood flow. They proposed that this modality be used to make the diagnosis of ACS before the development of muscle ischemia and necrosis. This software is still being investigated.25
Imaging Studies
CT or MRI can be helpful in identifying swelling, hematomas, or areas of necrosis, but their specificity is not sufficient to confirm elevated compartmental pressures24; additionally, MRI cannot distinguish between swelling resulting from soft-tissue injury and swelling in muscles affected by ACS.26, 27
Ultrasound also has potential as a diagnostic tool, as it helps clinicians visualize soft-tissue structures and assess the patency of large arteries and veins; the absence of venous outflow may suggest ACS. However, the efficacy of ultrasound in diagnosing ACS has proven inconsistent, and it is not recommended over direct ICP measurements. Instead, ultrasound can be relied on as an adjunctive modality.13
Blood Tests
Laboratory tests cannot contribute toward the diagnosis of ACS, but assessment of renal function and skeletal muscle breakdown is important to identify potential complications of ACS. Obtaining baseline levels in blood urea nitrogen and creatinine will help identify changes in kidney function, while potassium, urates, creatine phosphokinase (CPK), and myoglobin can be measured to assess for muscle breakdown. Findings of myoglobinuria with elevated CPK are strongly indicative of rhabdomyolysis, which can easily precipitate acute renal failure.8
Results from a complete blood count or prothrombin time/partial thromboplastin time may facilitate monitoring for blood loss or identification of contributing bleeding disorders. Because surgery remains the definitive treatment for ACS, a type and screen is essential in the workup, as blood products and transfusion are likely to be required during treatment.
MANAGEMENT
Fasciotomy remains the standard of care for patients presenting with the clinical signs and symptoms of increased compartment pressure consistent with ACS.16 Researchers engaged in animal studies and human case reports have shown that fasciotomy must be performed within six hours of injury to prevent adverse outcomes.8,16,28
As definitive treatment for ACS, a complete fasciotomy of all compartments in the vicinity of the injury should be performed. The most effective approach for fasciotomy consists of two long skin incisions (ie, double-incision radical dissection, to prevent concomitant increased pressure within the boundary of the skin), on opposite aspects of the affected limb, to ensure that all compartmental fascia can be decompressed.17,26,28
Delayed primary intention on postop day 5 is the preferred method for fasciotomy wound closure. Wounds should remain open to allow limb swelling to subside. In severe cases, when delayed primary intention cannot be performed in the window of time described, split-thickness skin grafts can be used. Postoperative wounds should be packed open with bulky dressings and changed daily. Negative-pressure wound dressings can be used for improved and accelerated wound closure.29
In the setting of ACS or limb ischemia, it is important to consider the possibility that tissue destruction will lead to significant myoglobinuria and potentially rhabdomyolysis.8 In patients presenting with crush injuries, or trauma patients who experience a significant rise in creatine kinase, the kidneys should be protected via extracellular resuscitation with isotonic fluids. The goal of resuscitation is to maintain urinary output of at least 200 mL/h to prevent renal failure.29
PATIENT EDUCATION
Patients who undergo fasciotomy within 12 hours of onset of signs and symptoms of ACS retain normal limb function in 68% of cases. However, this outcome falls to 8% if fasciotomy is delayed longer than 12 hours.30 Patients should understand that return to normal function usually takes two to three months and requires active participation by the patient. Furthermore, 20% of patients have some motor and sensory deficits at one year postfasciotomy.17
FOLLOW-UP
After fasciotomy, patients will require adequate pain control and an extensive rehabilitation program. Early physical therapy should progress slowly, with focus on range-of-motion and stretching exercises. Once patients have regained the ability to ambulate, resistance exercises and moderate exercise activities should be implemented to return them to their regular activities.31
CONCLUSION
ACS figures significantly in the long-term morbidity and mortality associated with trauma. Clinical research and laboratory science have indicated that ACS must be treated within six hours to prevent life-long deformity and disability. New diagnostic and therapeutic approaches must be investigated to improve outcomes. The most widely accepted surgical approach is the double-incision radical dissection of all fascia within the affected limb.
Appropriate management must include protection of the patient’s kidneys, given the risk for rhabdomyolysis, as well as extensive postoperative physical therapy. Given the invasive treatment required for ACS, progression toward full recovery is a long and difficult process. However, with prompt recognition and early intervention, full return to normal function is possible, with little to no deformity or dysfunction.
REFERECNES
1. Murdock M, Murdoch MM. Compartment syndrome: a review of the literature. Clin Podiatr Med Surg. 2012;29:301-310, viii.
2. Osteen KD, Haque SH. Bilateral gluteal compartment syndrome following right total knee revision: a case report. Ochsner J. 2012;12:141-144.
3. Paryavi E, Jobin CM, Ludwig SC, et al. Acute exertional lumbar paraspinal compartment syndrome. Spine. 2010;35:E1529-E1533.
4. Bosch U, Tscherne H. The pelvic compartment syndrome. Arch Ortho Trauma Surg. 1992;111:314-317.
5. Maeda A, Wakabayashi K, Suzuki H. Acute limb ischemia due to abdominal compartment syndrome. Catheter Cardiovasc Interv. 2013 Jun 19. [Epub ahead of print]
6. Masquelet AC. Acute compartment syndrome of the leg: pressure measurement and fasciotomy. Orthop Trumatol Surg Res. 2010;96:913-917.
7. Barker T, Midwinter M, Porter K. The diagnosis of acute lower limb compartment syndrome: applications of near infrared spectroscopy. Trauma. 2011;13:125-136.
8. Bosch X, Poch E, Grau JM. Rhabdomyolysis and acute kidney injury. N Engl J Med. 2009;361:62-72.
9. Karmaniolou I, Staikou C. Compartment syndrome as a complication of the lithotomy position. West Indian Med J. 2010;59:698-701.
10. Pietrangiolillo Z, Frassoldati R, Leonelli V, et al. Compartment syndrome after viper-bite in toddler: case report and review of literature. Acta Biomed. 2012;83:44-50.
11. Kakkar R, Ellis M, Fearon PV. Compartment syndrome of the thigh as a complication of anticoagulant therapy in a patient with a left ventricular assist device (Berlin Heart). Gen Thorac Cardiovasc Surg. 2010;58: 477-479.
12. Balogh ZJ, Leppäniemi A. Patient populations at risk for intra-abdominal hypertension and abdominal compartment syndrome. Am Surg. 2011;77 suppl 1:S12-S16.
13. Zimmerman DC, Kapoor T, Elfond M, Scott P. Spontaneous compartment syndrome of the upper arm in a patient receiving anticoagulation therapy. J Emerg Med. 2013;44:e53-e56.
14. Olson SA. Acute compartment syndrome in lower extremity musculoskeletal trauma. J Am Acad Orthop Surg. 2005;13(7):436-444.
15. Branco BC, Inaba K, Barmparas G, et al. Incidence and predictors for the need for fasciotomy after extremity trauma: a 10-year review in a mature level I trauma centre. Injury. 2011;42:1157-1163.
16. Wall CJ, Richardson, Lowe AJ, et al. Survey of management of acute, traumatic compartment syndrome of the leg in Australia. ANZ J Surg. 2007;77:733-737.
17. Wall CJ, Lynch J, Harris IA, et al; Liverpool (Sydney) and Royal Melbourne Hospitals. Clinical practice guidelines for the management of acute limb compartment syndrome following trauma. ANZ J Surg. 2010;80:151-156.
18. McQueen MM, Gaston P, Court-Brown CM. Acute compartment syndrome: who is at risk? J Bone Joint Surg. 2000;82:200-203.
19. Khan M, Hodkinson SL. Acute compartment syndrome—presenting as severe pain in an extremity out of proportion with the injury. J R Army Med Corps. 1997;143:165-166.
20. Percival TJ, White JM, Ricci MA. Compartment syndrome in the setting of vascular injury. Perspect Vasc Surg Endovasc Ther. 2011;23(2):119-124.
21. Badhe S, Baiju D, Elliot R, et al. The ‘silent’ compartment syndrome. Injury. 2009;40:220-222.
22. Shuler FD, Dietz MJ. Physicians’ ability to manually detect isolated elevations in leg intracompartmental pressure. J Bone Joint Surg Am. 2010;92:361-367.
23. Taylor RM, Sullivan MP, Mehta S. Acute compartment syndrome: obtaining diagnosis, providing treatment, and minimizing medicolegal risk. Curr Rev Musculoskelet Med. 2012;5(3):206-213.
24. McDonald S, Bearcroft P. Compartment syndromes. Semin Musculoskelet Radiol. 2010;14:236-244.
25. Katz LM, Nauriyal V, Nagaraj S, et al. Infrared imaging of trauma patients for detection of acute compartment syndrome of the leg. Crit Care Med. 2008;36:1756-1761.
26. Shadgan B, Menon M, Sanders D, et al. Current thinking about acute compartment syndrome of the lower extremity. Can J Surg. 2010;53:329-334.
27. Rominger M, Lukosch C, Bachmann G, et al. Compartment syndrome: value of MR imaging. Radiology. 1995;197:296.
28. Kashuk JL, Moore EE, Pinski S, et al. Lower extremity compartment syndrome in the acute care surgery paradigm: safety lessons learned. Patient Saf Surg. 2009;3(1):11.
29. Rasul AT Jr. Acute compartment syndrome (2011). emedicine.medscape.com/article/307668-overview#showall. Accessed June 25, 2013.
30. Sheridan GW, Matsen FA 3rd. Fasciotomy in the treatment of the acute compartment syndrome. J Bone Joint Surg Am. 1976;58(1):112-115.
31. Schubert AG. Exertional compartment syndrome: review of the literature and proposed rehabilitation guidelines following surgical release. Intern J Sports Phys Ther. 2011;6:126-141.
Acute compartment syndrome (ACS) is a condition in which elevated pressures in the confined space of a closed fascial compartment lead to vascular compromise. Typically, ACS develops in the distal extremities after a traumatic event, such as a fracture, crush, or burn injury. A dangerous cycle ensues, involving increased compartmental pressure, decreased tissue perfusion, and continuing ischemia; fluids leak from the vasculature, perpetuating the process.1
Diagnosis of ACS requires a high level of clinical suspicion combined with a keen understanding of the risk factors for ACS and its pathophysiology, as well as astute awareness of the subtle clinical exam findings that usually accompany objective pressure measurements. Though most common in the extremities, ACS may also develop in the buttock, pelvis, or abdominal or spinal musculature.2-5
Prompt recognition and management of ACS are critical: This condition is considered a surgical emergency requiring immediate attention. Urgent decompression by fasciotomy is the definitive treatment6—an essential intervention to prevent critical tissue ischemia and necrosis. Failure to release the fascia in a timely manner can result in poor outcomes for patients, including but not limited to chronic pain, paralysis, sensory or motor deficits, loss of limb, deformity, and renal failure secondary to rhabdomyolysis.7,8
Clinical assessment and severity of injury are the two main factors that lead to prompt diagnosis and treatment of ACS. However, distracting injuries or an unconsciousness patient may interfere with the assessment, decreasing its accuracy.7 Heightened awareness of ACS is paramount so that clinicians can better recognize the condition before complications arise.
EPIDEMIOLOGY
ACS is typically associated with long bone injuries after significant trauma or crush injuries (eg, in a motor vehicle collision). Less often, severe burns, gun shot wounds, snake bites, poor anticoagulation, prolonged surgery (including procedures involving prolonged elevation of a limb),9 nephrotic syndrome, IV infiltrations, and other volume-expanding pathologies can present a similar risk.10-15 Essentially, ACS is a self-perpetuating process resulting from either increased compartmental content (eg, bleeding, edema) or reduced compartment size (eg, tight casting, burns).7,16
Ninety percent of cases of ACS in the extremities occur in men,15 and males younger than 35 have the greatest incidence of posttraumatic ACS.15,17,18 Although fractures account for nearly 70% of confirmed cases of ACS, soft-tissue injuries (particularly vascular injuries15) are also associated with ACS.18 Clinicians should be aware of a key misconception: that open fractures relieve intracompartmental pressure, reducing the risk for ACS. Rather, the damage and inflammation that occur in open fractures pose the same risk for ACS as do closed fractures.15
PATIENT PRESENTATION
A key consideration, in addition to the history and mechanism of injury or illness, is that patient’s description of the pain: The conscious patient will complain of pain that worsens progressively over time and that usually seems out of proportion to the physical exam findings or apparent injury.19 For this reason, serial assessments are recommended; worsening pain is indicative of rapid evolution of ACS, with the possibility of irreversible necrosis.20
Pain with passive stretch is an excellent indicator that the pathology is progressing.2 The “classic P” signs and symptoms of ACS are progressively later findings that represent markedly significant ischemia and injury already in progress: paresthesia, paresis/paralysis, poikilothermia (the inability of the patient to maintain a constant core temperature), pulselessness, and pallor.17
Diminishment of pain suggests a poor prognosis, as this indicates that the tissue is likely nonviable and necrotic by that point.1
Clinicians must be mindful that, in patients with central or peripheral nerve deficits or in those who have undergone nerve blocks or regional anesthesia, pain may be absent; in these instances, the risk for delayed diagnosis is increased.14,21 Furthermore, pain tolerance varies among patients, and distracting injuries may also cloud the clinical picture.
The diagnosis of ACS is not easily made. Thus, clinical suspicion should be elevated in any high-energy scenario: an extensive burn, overly restrictive casting, or volume-expanding disorder—especially in patients who present with pain that is out of proportion to the injury or is worsening progressively.1,7
PHYSICAL EXAMINATION
Physical examination and assessment should be repeated every two to four hours. The examining clinician should focus on pain characteristics, the skin, and the “classic P” signs mentioned above. Marked tenderness out of proportion to the injury, unmanageable pain, and pain on passive movement of the limb are the strongest indicators of developing ACS. These findings are nearly universal in the conscious patient, but they can be confused with primary pain of the injury itself. Additional findings of sensory or motor impairments may help raise the clinician’s suspicion for ACS but are nonspecific.1,7,23 Palpable tenseness is a common observation in ACS, but a clinician’s subjective measurement of skin firmness has little value as an objective strategy.22 Absence of distal pulses, decreased skin temperature, and pallor are very late signs that pressures are sufficiently high to cause complete arterial occlusion.20 Thus, clinicians should not wait for absent pulses or pallor to begin treatment. Suspicion for ACS should arise as soon as pain associated with trauma or surgery intensifies or becomes unmanageable.
DIAGNOSTIC TOOLS
ACS is diagnosed based on the previously mentioned clinical signs, along with objective evidence of decreased tissue perfusion in the affected limbs.24 Direct intracompartmental pressure measurements, obtained using a needle- or catheter-based technique, are the most common means of identifying ACS.7 However, near infrared spectroscopy and infrared imaging have also been found helpful.7, 25
Direct Intracompartmental Pressure Monitoring
Supported by clinical findings, direct measurement of the intracompartmental pressure (ICP) using a needle transducer is the most accurate method for detecting ACS and guiding treatment choices. Several measurement options are available, including a handheld needle manometer, which records single-pressure readings, or a wick or slit catheter, which can record continuous pressures.13 The choice between devices is based on facility/provider preference, as any commercial pressure device can be used with similar accuracies. When no such device is available, an 18-gauge needle with a set-up resembling an arterial line is another option.1
When using these instruments, it is essential for the provider to implement proper sterile technique and to record pressures from compartments within a 5-cm radius of the injury site.17 Furthermore, clinicians should be aware that the ICP can vary greatly and that tolerance to increased pressure varies with the patient’s diastolic blood pressure.
Normal capillary perfusion pressure (diastolic blood pressure minus compartment pressure) is approximately 30 mm Hg. Therefore, absolute ICP readings above 30 mm Hg can be diagnostic of ACS and indicative of fasciotomy. Likewise, a perfusion pressure below 30 mm Hg is also diagnostic. Based on consensus, either calculation can be used.16,17
Infrared Spectroscopy and Imaging
Barker and colleagues7 describe use of near infrared spectroscopy (NIRS) as a method to diagnose lower-extremity ACS. Noninvasive skin probes can detect the absorption spectra of mixed venous hemoglobin levels beneath the skin to determine oxygen saturation (StO2). StO2 measured by NIRS is location dependent, enabling the clinician to monitor oxygenation levels in any illuminated tissue. NIRS has great potential for use in confirming a diagnosis of ACS since decreased perfusion pressure from elevated ICP correlates well with decreased StO2. However, this technique has limitations that currently prevent its widespread use as a diagnostic tool.7
Use of noninvasive infrared imaging has also been investigated. Katz and colleagues25 describe the use of a long-wave infrared camera and thermographic imaging analysis software to detect differences in surface temperatures in patients’ limbs after blunt trauma. The researchers were able to correlate declines in temperature with decreased blood flow. They proposed that this modality be used to make the diagnosis of ACS before the development of muscle ischemia and necrosis. This software is still being investigated.25
Imaging Studies
CT or MRI can be helpful in identifying swelling, hematomas, or areas of necrosis, but their specificity is not sufficient to confirm elevated compartmental pressures24; additionally, MRI cannot distinguish between swelling resulting from soft-tissue injury and swelling in muscles affected by ACS.26, 27
Ultrasound also has potential as a diagnostic tool, as it helps clinicians visualize soft-tissue structures and assess the patency of large arteries and veins; the absence of venous outflow may suggest ACS. However, the efficacy of ultrasound in diagnosing ACS has proven inconsistent, and it is not recommended over direct ICP measurements. Instead, ultrasound can be relied on as an adjunctive modality.13
Blood Tests
Laboratory tests cannot contribute toward the diagnosis of ACS, but assessment of renal function and skeletal muscle breakdown is important to identify potential complications of ACS. Obtaining baseline levels in blood urea nitrogen and creatinine will help identify changes in kidney function, while potassium, urates, creatine phosphokinase (CPK), and myoglobin can be measured to assess for muscle breakdown. Findings of myoglobinuria with elevated CPK are strongly indicative of rhabdomyolysis, which can easily precipitate acute renal failure.8
Results from a complete blood count or prothrombin time/partial thromboplastin time may facilitate monitoring for blood loss or identification of contributing bleeding disorders. Because surgery remains the definitive treatment for ACS, a type and screen is essential in the workup, as blood products and transfusion are likely to be required during treatment.
MANAGEMENT
Fasciotomy remains the standard of care for patients presenting with the clinical signs and symptoms of increased compartment pressure consistent with ACS.16 Researchers engaged in animal studies and human case reports have shown that fasciotomy must be performed within six hours of injury to prevent adverse outcomes.8,16,28
As definitive treatment for ACS, a complete fasciotomy of all compartments in the vicinity of the injury should be performed. The most effective approach for fasciotomy consists of two long skin incisions (ie, double-incision radical dissection, to prevent concomitant increased pressure within the boundary of the skin), on opposite aspects of the affected limb, to ensure that all compartmental fascia can be decompressed.17,26,28
Delayed primary intention on postop day 5 is the preferred method for fasciotomy wound closure. Wounds should remain open to allow limb swelling to subside. In severe cases, when delayed primary intention cannot be performed in the window of time described, split-thickness skin grafts can be used. Postoperative wounds should be packed open with bulky dressings and changed daily. Negative-pressure wound dressings can be used for improved and accelerated wound closure.29
In the setting of ACS or limb ischemia, it is important to consider the possibility that tissue destruction will lead to significant myoglobinuria and potentially rhabdomyolysis.8 In patients presenting with crush injuries, or trauma patients who experience a significant rise in creatine kinase, the kidneys should be protected via extracellular resuscitation with isotonic fluids. The goal of resuscitation is to maintain urinary output of at least 200 mL/h to prevent renal failure.29
PATIENT EDUCATION
Patients who undergo fasciotomy within 12 hours of onset of signs and symptoms of ACS retain normal limb function in 68% of cases. However, this outcome falls to 8% if fasciotomy is delayed longer than 12 hours.30 Patients should understand that return to normal function usually takes two to three months and requires active participation by the patient. Furthermore, 20% of patients have some motor and sensory deficits at one year postfasciotomy.17
FOLLOW-UP
After fasciotomy, patients will require adequate pain control and an extensive rehabilitation program. Early physical therapy should progress slowly, with focus on range-of-motion and stretching exercises. Once patients have regained the ability to ambulate, resistance exercises and moderate exercise activities should be implemented to return them to their regular activities.31
CONCLUSION
ACS figures significantly in the long-term morbidity and mortality associated with trauma. Clinical research and laboratory science have indicated that ACS must be treated within six hours to prevent life-long deformity and disability. New diagnostic and therapeutic approaches must be investigated to improve outcomes. The most widely accepted surgical approach is the double-incision radical dissection of all fascia within the affected limb.
Appropriate management must include protection of the patient’s kidneys, given the risk for rhabdomyolysis, as well as extensive postoperative physical therapy. Given the invasive treatment required for ACS, progression toward full recovery is a long and difficult process. However, with prompt recognition and early intervention, full return to normal function is possible, with little to no deformity or dysfunction.
REFERECNES
1. Murdock M, Murdoch MM. Compartment syndrome: a review of the literature. Clin Podiatr Med Surg. 2012;29:301-310, viii.
2. Osteen KD, Haque SH. Bilateral gluteal compartment syndrome following right total knee revision: a case report. Ochsner J. 2012;12:141-144.
3. Paryavi E, Jobin CM, Ludwig SC, et al. Acute exertional lumbar paraspinal compartment syndrome. Spine. 2010;35:E1529-E1533.
4. Bosch U, Tscherne H. The pelvic compartment syndrome. Arch Ortho Trauma Surg. 1992;111:314-317.
5. Maeda A, Wakabayashi K, Suzuki H. Acute limb ischemia due to abdominal compartment syndrome. Catheter Cardiovasc Interv. 2013 Jun 19. [Epub ahead of print]
6. Masquelet AC. Acute compartment syndrome of the leg: pressure measurement and fasciotomy. Orthop Trumatol Surg Res. 2010;96:913-917.
7. Barker T, Midwinter M, Porter K. The diagnosis of acute lower limb compartment syndrome: applications of near infrared spectroscopy. Trauma. 2011;13:125-136.
8. Bosch X, Poch E, Grau JM. Rhabdomyolysis and acute kidney injury. N Engl J Med. 2009;361:62-72.
9. Karmaniolou I, Staikou C. Compartment syndrome as a complication of the lithotomy position. West Indian Med J. 2010;59:698-701.
10. Pietrangiolillo Z, Frassoldati R, Leonelli V, et al. Compartment syndrome after viper-bite in toddler: case report and review of literature. Acta Biomed. 2012;83:44-50.
11. Kakkar R, Ellis M, Fearon PV. Compartment syndrome of the thigh as a complication of anticoagulant therapy in a patient with a left ventricular assist device (Berlin Heart). Gen Thorac Cardiovasc Surg. 2010;58: 477-479.
12. Balogh ZJ, Leppäniemi A. Patient populations at risk for intra-abdominal hypertension and abdominal compartment syndrome. Am Surg. 2011;77 suppl 1:S12-S16.
13. Zimmerman DC, Kapoor T, Elfond M, Scott P. Spontaneous compartment syndrome of the upper arm in a patient receiving anticoagulation therapy. J Emerg Med. 2013;44:e53-e56.
14. Olson SA. Acute compartment syndrome in lower extremity musculoskeletal trauma. J Am Acad Orthop Surg. 2005;13(7):436-444.
15. Branco BC, Inaba K, Barmparas G, et al. Incidence and predictors for the need for fasciotomy after extremity trauma: a 10-year review in a mature level I trauma centre. Injury. 2011;42:1157-1163.
16. Wall CJ, Richardson, Lowe AJ, et al. Survey of management of acute, traumatic compartment syndrome of the leg in Australia. ANZ J Surg. 2007;77:733-737.
17. Wall CJ, Lynch J, Harris IA, et al; Liverpool (Sydney) and Royal Melbourne Hospitals. Clinical practice guidelines for the management of acute limb compartment syndrome following trauma. ANZ J Surg. 2010;80:151-156.
18. McQueen MM, Gaston P, Court-Brown CM. Acute compartment syndrome: who is at risk? J Bone Joint Surg. 2000;82:200-203.
19. Khan M, Hodkinson SL. Acute compartment syndrome—presenting as severe pain in an extremity out of proportion with the injury. J R Army Med Corps. 1997;143:165-166.
20. Percival TJ, White JM, Ricci MA. Compartment syndrome in the setting of vascular injury. Perspect Vasc Surg Endovasc Ther. 2011;23(2):119-124.
21. Badhe S, Baiju D, Elliot R, et al. The ‘silent’ compartment syndrome. Injury. 2009;40:220-222.
22. Shuler FD, Dietz MJ. Physicians’ ability to manually detect isolated elevations in leg intracompartmental pressure. J Bone Joint Surg Am. 2010;92:361-367.
23. Taylor RM, Sullivan MP, Mehta S. Acute compartment syndrome: obtaining diagnosis, providing treatment, and minimizing medicolegal risk. Curr Rev Musculoskelet Med. 2012;5(3):206-213.
24. McDonald S, Bearcroft P. Compartment syndromes. Semin Musculoskelet Radiol. 2010;14:236-244.
25. Katz LM, Nauriyal V, Nagaraj S, et al. Infrared imaging of trauma patients for detection of acute compartment syndrome of the leg. Crit Care Med. 2008;36:1756-1761.
26. Shadgan B, Menon M, Sanders D, et al. Current thinking about acute compartment syndrome of the lower extremity. Can J Surg. 2010;53:329-334.
27. Rominger M, Lukosch C, Bachmann G, et al. Compartment syndrome: value of MR imaging. Radiology. 1995;197:296.
28. Kashuk JL, Moore EE, Pinski S, et al. Lower extremity compartment syndrome in the acute care surgery paradigm: safety lessons learned. Patient Saf Surg. 2009;3(1):11.
29. Rasul AT Jr. Acute compartment syndrome (2011). emedicine.medscape.com/article/307668-overview#showall. Accessed June 25, 2013.
30. Sheridan GW, Matsen FA 3rd. Fasciotomy in the treatment of the acute compartment syndrome. J Bone Joint Surg Am. 1976;58(1):112-115.
31. Schubert AG. Exertional compartment syndrome: review of the literature and proposed rehabilitation guidelines following surgical release. Intern J Sports Phys Ther. 2011;6:126-141.
Acute compartment syndrome (ACS) is a condition in which elevated pressures in the confined space of a closed fascial compartment lead to vascular compromise. Typically, ACS develops in the distal extremities after a traumatic event, such as a fracture, crush, or burn injury. A dangerous cycle ensues, involving increased compartmental pressure, decreased tissue perfusion, and continuing ischemia; fluids leak from the vasculature, perpetuating the process.1
Diagnosis of ACS requires a high level of clinical suspicion combined with a keen understanding of the risk factors for ACS and its pathophysiology, as well as astute awareness of the subtle clinical exam findings that usually accompany objective pressure measurements. Though most common in the extremities, ACS may also develop in the buttock, pelvis, or abdominal or spinal musculature.2-5
Prompt recognition and management of ACS are critical: This condition is considered a surgical emergency requiring immediate attention. Urgent decompression by fasciotomy is the definitive treatment6—an essential intervention to prevent critical tissue ischemia and necrosis. Failure to release the fascia in a timely manner can result in poor outcomes for patients, including but not limited to chronic pain, paralysis, sensory or motor deficits, loss of limb, deformity, and renal failure secondary to rhabdomyolysis.7,8
Clinical assessment and severity of injury are the two main factors that lead to prompt diagnosis and treatment of ACS. However, distracting injuries or an unconsciousness patient may interfere with the assessment, decreasing its accuracy.7 Heightened awareness of ACS is paramount so that clinicians can better recognize the condition before complications arise.
EPIDEMIOLOGY
ACS is typically associated with long bone injuries after significant trauma or crush injuries (eg, in a motor vehicle collision). Less often, severe burns, gun shot wounds, snake bites, poor anticoagulation, prolonged surgery (including procedures involving prolonged elevation of a limb),9 nephrotic syndrome, IV infiltrations, and other volume-expanding pathologies can present a similar risk.10-15 Essentially, ACS is a self-perpetuating process resulting from either increased compartmental content (eg, bleeding, edema) or reduced compartment size (eg, tight casting, burns).7,16
Ninety percent of cases of ACS in the extremities occur in men,15 and males younger than 35 have the greatest incidence of posttraumatic ACS.15,17,18 Although fractures account for nearly 70% of confirmed cases of ACS, soft-tissue injuries (particularly vascular injuries15) are also associated with ACS.18 Clinicians should be aware of a key misconception: that open fractures relieve intracompartmental pressure, reducing the risk for ACS. Rather, the damage and inflammation that occur in open fractures pose the same risk for ACS as do closed fractures.15
PATIENT PRESENTATION
A key consideration, in addition to the history and mechanism of injury or illness, is that patient’s description of the pain: The conscious patient will complain of pain that worsens progressively over time and that usually seems out of proportion to the physical exam findings or apparent injury.19 For this reason, serial assessments are recommended; worsening pain is indicative of rapid evolution of ACS, with the possibility of irreversible necrosis.20
Pain with passive stretch is an excellent indicator that the pathology is progressing.2 The “classic P” signs and symptoms of ACS are progressively later findings that represent markedly significant ischemia and injury already in progress: paresthesia, paresis/paralysis, poikilothermia (the inability of the patient to maintain a constant core temperature), pulselessness, and pallor.17
Diminishment of pain suggests a poor prognosis, as this indicates that the tissue is likely nonviable and necrotic by that point.1
Clinicians must be mindful that, in patients with central or peripheral nerve deficits or in those who have undergone nerve blocks or regional anesthesia, pain may be absent; in these instances, the risk for delayed diagnosis is increased.14,21 Furthermore, pain tolerance varies among patients, and distracting injuries may also cloud the clinical picture.
The diagnosis of ACS is not easily made. Thus, clinical suspicion should be elevated in any high-energy scenario: an extensive burn, overly restrictive casting, or volume-expanding disorder—especially in patients who present with pain that is out of proportion to the injury or is worsening progressively.1,7
PHYSICAL EXAMINATION
Physical examination and assessment should be repeated every two to four hours. The examining clinician should focus on pain characteristics, the skin, and the “classic P” signs mentioned above. Marked tenderness out of proportion to the injury, unmanageable pain, and pain on passive movement of the limb are the strongest indicators of developing ACS. These findings are nearly universal in the conscious patient, but they can be confused with primary pain of the injury itself. Additional findings of sensory or motor impairments may help raise the clinician’s suspicion for ACS but are nonspecific.1,7,23 Palpable tenseness is a common observation in ACS, but a clinician’s subjective measurement of skin firmness has little value as an objective strategy.22 Absence of distal pulses, decreased skin temperature, and pallor are very late signs that pressures are sufficiently high to cause complete arterial occlusion.20 Thus, clinicians should not wait for absent pulses or pallor to begin treatment. Suspicion for ACS should arise as soon as pain associated with trauma or surgery intensifies or becomes unmanageable.
DIAGNOSTIC TOOLS
ACS is diagnosed based on the previously mentioned clinical signs, along with objective evidence of decreased tissue perfusion in the affected limbs.24 Direct intracompartmental pressure measurements, obtained using a needle- or catheter-based technique, are the most common means of identifying ACS.7 However, near infrared spectroscopy and infrared imaging have also been found helpful.7, 25
Direct Intracompartmental Pressure Monitoring
Supported by clinical findings, direct measurement of the intracompartmental pressure (ICP) using a needle transducer is the most accurate method for detecting ACS and guiding treatment choices. Several measurement options are available, including a handheld needle manometer, which records single-pressure readings, or a wick or slit catheter, which can record continuous pressures.13 The choice between devices is based on facility/provider preference, as any commercial pressure device can be used with similar accuracies. When no such device is available, an 18-gauge needle with a set-up resembling an arterial line is another option.1
When using these instruments, it is essential for the provider to implement proper sterile technique and to record pressures from compartments within a 5-cm radius of the injury site.17 Furthermore, clinicians should be aware that the ICP can vary greatly and that tolerance to increased pressure varies with the patient’s diastolic blood pressure.
Normal capillary perfusion pressure (diastolic blood pressure minus compartment pressure) is approximately 30 mm Hg. Therefore, absolute ICP readings above 30 mm Hg can be diagnostic of ACS and indicative of fasciotomy. Likewise, a perfusion pressure below 30 mm Hg is also diagnostic. Based on consensus, either calculation can be used.16,17
Infrared Spectroscopy and Imaging
Barker and colleagues7 describe use of near infrared spectroscopy (NIRS) as a method to diagnose lower-extremity ACS. Noninvasive skin probes can detect the absorption spectra of mixed venous hemoglobin levels beneath the skin to determine oxygen saturation (StO2). StO2 measured by NIRS is location dependent, enabling the clinician to monitor oxygenation levels in any illuminated tissue. NIRS has great potential for use in confirming a diagnosis of ACS since decreased perfusion pressure from elevated ICP correlates well with decreased StO2. However, this technique has limitations that currently prevent its widespread use as a diagnostic tool.7
Use of noninvasive infrared imaging has also been investigated. Katz and colleagues25 describe the use of a long-wave infrared camera and thermographic imaging analysis software to detect differences in surface temperatures in patients’ limbs after blunt trauma. The researchers were able to correlate declines in temperature with decreased blood flow. They proposed that this modality be used to make the diagnosis of ACS before the development of muscle ischemia and necrosis. This software is still being investigated.25
Imaging Studies
CT or MRI can be helpful in identifying swelling, hematomas, or areas of necrosis, but their specificity is not sufficient to confirm elevated compartmental pressures24; additionally, MRI cannot distinguish between swelling resulting from soft-tissue injury and swelling in muscles affected by ACS.26, 27
Ultrasound also has potential as a diagnostic tool, as it helps clinicians visualize soft-tissue structures and assess the patency of large arteries and veins; the absence of venous outflow may suggest ACS. However, the efficacy of ultrasound in diagnosing ACS has proven inconsistent, and it is not recommended over direct ICP measurements. Instead, ultrasound can be relied on as an adjunctive modality.13
Blood Tests
Laboratory tests cannot contribute toward the diagnosis of ACS, but assessment of renal function and skeletal muscle breakdown is important to identify potential complications of ACS. Obtaining baseline levels in blood urea nitrogen and creatinine will help identify changes in kidney function, while potassium, urates, creatine phosphokinase (CPK), and myoglobin can be measured to assess for muscle breakdown. Findings of myoglobinuria with elevated CPK are strongly indicative of rhabdomyolysis, which can easily precipitate acute renal failure.8
Results from a complete blood count or prothrombin time/partial thromboplastin time may facilitate monitoring for blood loss or identification of contributing bleeding disorders. Because surgery remains the definitive treatment for ACS, a type and screen is essential in the workup, as blood products and transfusion are likely to be required during treatment.
MANAGEMENT
Fasciotomy remains the standard of care for patients presenting with the clinical signs and symptoms of increased compartment pressure consistent with ACS.16 Researchers engaged in animal studies and human case reports have shown that fasciotomy must be performed within six hours of injury to prevent adverse outcomes.8,16,28
As definitive treatment for ACS, a complete fasciotomy of all compartments in the vicinity of the injury should be performed. The most effective approach for fasciotomy consists of two long skin incisions (ie, double-incision radical dissection, to prevent concomitant increased pressure within the boundary of the skin), on opposite aspects of the affected limb, to ensure that all compartmental fascia can be decompressed.17,26,28
Delayed primary intention on postop day 5 is the preferred method for fasciotomy wound closure. Wounds should remain open to allow limb swelling to subside. In severe cases, when delayed primary intention cannot be performed in the window of time described, split-thickness skin grafts can be used. Postoperative wounds should be packed open with bulky dressings and changed daily. Negative-pressure wound dressings can be used for improved and accelerated wound closure.29
In the setting of ACS or limb ischemia, it is important to consider the possibility that tissue destruction will lead to significant myoglobinuria and potentially rhabdomyolysis.8 In patients presenting with crush injuries, or trauma patients who experience a significant rise in creatine kinase, the kidneys should be protected via extracellular resuscitation with isotonic fluids. The goal of resuscitation is to maintain urinary output of at least 200 mL/h to prevent renal failure.29
PATIENT EDUCATION
Patients who undergo fasciotomy within 12 hours of onset of signs and symptoms of ACS retain normal limb function in 68% of cases. However, this outcome falls to 8% if fasciotomy is delayed longer than 12 hours.30 Patients should understand that return to normal function usually takes two to three months and requires active participation by the patient. Furthermore, 20% of patients have some motor and sensory deficits at one year postfasciotomy.17
FOLLOW-UP
After fasciotomy, patients will require adequate pain control and an extensive rehabilitation program. Early physical therapy should progress slowly, with focus on range-of-motion and stretching exercises. Once patients have regained the ability to ambulate, resistance exercises and moderate exercise activities should be implemented to return them to their regular activities.31
CONCLUSION
ACS figures significantly in the long-term morbidity and mortality associated with trauma. Clinical research and laboratory science have indicated that ACS must be treated within six hours to prevent life-long deformity and disability. New diagnostic and therapeutic approaches must be investigated to improve outcomes. The most widely accepted surgical approach is the double-incision radical dissection of all fascia within the affected limb.
Appropriate management must include protection of the patient’s kidneys, given the risk for rhabdomyolysis, as well as extensive postoperative physical therapy. Given the invasive treatment required for ACS, progression toward full recovery is a long and difficult process. However, with prompt recognition and early intervention, full return to normal function is possible, with little to no deformity or dysfunction.
REFERECNES
1. Murdock M, Murdoch MM. Compartment syndrome: a review of the literature. Clin Podiatr Med Surg. 2012;29:301-310, viii.
2. Osteen KD, Haque SH. Bilateral gluteal compartment syndrome following right total knee revision: a case report. Ochsner J. 2012;12:141-144.
3. Paryavi E, Jobin CM, Ludwig SC, et al. Acute exertional lumbar paraspinal compartment syndrome. Spine. 2010;35:E1529-E1533.
4. Bosch U, Tscherne H. The pelvic compartment syndrome. Arch Ortho Trauma Surg. 1992;111:314-317.
5. Maeda A, Wakabayashi K, Suzuki H. Acute limb ischemia due to abdominal compartment syndrome. Catheter Cardiovasc Interv. 2013 Jun 19. [Epub ahead of print]
6. Masquelet AC. Acute compartment syndrome of the leg: pressure measurement and fasciotomy. Orthop Trumatol Surg Res. 2010;96:913-917.
7. Barker T, Midwinter M, Porter K. The diagnosis of acute lower limb compartment syndrome: applications of near infrared spectroscopy. Trauma. 2011;13:125-136.
8. Bosch X, Poch E, Grau JM. Rhabdomyolysis and acute kidney injury. N Engl J Med. 2009;361:62-72.
9. Karmaniolou I, Staikou C. Compartment syndrome as a complication of the lithotomy position. West Indian Med J. 2010;59:698-701.
10. Pietrangiolillo Z, Frassoldati R, Leonelli V, et al. Compartment syndrome after viper-bite in toddler: case report and review of literature. Acta Biomed. 2012;83:44-50.
11. Kakkar R, Ellis M, Fearon PV. Compartment syndrome of the thigh as a complication of anticoagulant therapy in a patient with a left ventricular assist device (Berlin Heart). Gen Thorac Cardiovasc Surg. 2010;58: 477-479.
12. Balogh ZJ, Leppäniemi A. Patient populations at risk for intra-abdominal hypertension and abdominal compartment syndrome. Am Surg. 2011;77 suppl 1:S12-S16.
13. Zimmerman DC, Kapoor T, Elfond M, Scott P. Spontaneous compartment syndrome of the upper arm in a patient receiving anticoagulation therapy. J Emerg Med. 2013;44:e53-e56.
14. Olson SA. Acute compartment syndrome in lower extremity musculoskeletal trauma. J Am Acad Orthop Surg. 2005;13(7):436-444.
15. Branco BC, Inaba K, Barmparas G, et al. Incidence and predictors for the need for fasciotomy after extremity trauma: a 10-year review in a mature level I trauma centre. Injury. 2011;42:1157-1163.
16. Wall CJ, Richardson, Lowe AJ, et al. Survey of management of acute, traumatic compartment syndrome of the leg in Australia. ANZ J Surg. 2007;77:733-737.
17. Wall CJ, Lynch J, Harris IA, et al; Liverpool (Sydney) and Royal Melbourne Hospitals. Clinical practice guidelines for the management of acute limb compartment syndrome following trauma. ANZ J Surg. 2010;80:151-156.
18. McQueen MM, Gaston P, Court-Brown CM. Acute compartment syndrome: who is at risk? J Bone Joint Surg. 2000;82:200-203.
19. Khan M, Hodkinson SL. Acute compartment syndrome—presenting as severe pain in an extremity out of proportion with the injury. J R Army Med Corps. 1997;143:165-166.
20. Percival TJ, White JM, Ricci MA. Compartment syndrome in the setting of vascular injury. Perspect Vasc Surg Endovasc Ther. 2011;23(2):119-124.
21. Badhe S, Baiju D, Elliot R, et al. The ‘silent’ compartment syndrome. Injury. 2009;40:220-222.
22. Shuler FD, Dietz MJ. Physicians’ ability to manually detect isolated elevations in leg intracompartmental pressure. J Bone Joint Surg Am. 2010;92:361-367.
23. Taylor RM, Sullivan MP, Mehta S. Acute compartment syndrome: obtaining diagnosis, providing treatment, and minimizing medicolegal risk. Curr Rev Musculoskelet Med. 2012;5(3):206-213.
24. McDonald S, Bearcroft P. Compartment syndromes. Semin Musculoskelet Radiol. 2010;14:236-244.
25. Katz LM, Nauriyal V, Nagaraj S, et al. Infrared imaging of trauma patients for detection of acute compartment syndrome of the leg. Crit Care Med. 2008;36:1756-1761.
26. Shadgan B, Menon M, Sanders D, et al. Current thinking about acute compartment syndrome of the lower extremity. Can J Surg. 2010;53:329-334.
27. Rominger M, Lukosch C, Bachmann G, et al. Compartment syndrome: value of MR imaging. Radiology. 1995;197:296.
28. Kashuk JL, Moore EE, Pinski S, et al. Lower extremity compartment syndrome in the acute care surgery paradigm: safety lessons learned. Patient Saf Surg. 2009;3(1):11.
29. Rasul AT Jr. Acute compartment syndrome (2011). emedicine.medscape.com/article/307668-overview#showall. Accessed June 25, 2013.
30. Sheridan GW, Matsen FA 3rd. Fasciotomy in the treatment of the acute compartment syndrome. J Bone Joint Surg Am. 1976;58(1):112-115.
31. Schubert AG. Exertional compartment syndrome: review of the literature and proposed rehabilitation guidelines following surgical release. Intern J Sports Phys Ther. 2011;6:126-141.
How to Choose a Contraceptive for Your Postpartum Patient
Q: What’s a vital aspect of the care we provide to postpartum patients?
A: Optimal timing of evaluation for contraception.
Good timing minimizes the likelihood that postpartum contraception will be initiated too early or too late to be effective.
The choice of a contraceptive method for a postpartum woman also requires a careful balancing act. On one side: the risks of contraception to the mother and her newborn. On the other: the risk for unintended pregnancy. Among the concerns that need to be addressed in contraceptive decision-making are:
• Whether the woman has resumed sexual intercourse
• Infant feeding practices
• Risk for venous thromboembolism (VTE)
• Logistics of various long-acting reversible contraceptives and tubal sterilization.
In this article, we outline the components of effective contraceptive counseling and decision-making. We also summarize recent recommendations from the CDC on the use of various contraceptive methods during the postpartum period.
FIRST: START AT THREE WEEKS
The traditional six-week postpartum visit was timed to take place after complete involution of the uterus following vaginal delivery. However, involution occurs too late to prevent unintended pregnancy because ovulation can—and often does—occur as early as the fourth postpartum week among nonbreastfeeding women.
In the past, when it was more common to fit a contraceptive diaphragm after pregnancy, six weeks may have been the best timing for the visit. Today, given the high safety and efficacy of modern contraceptive methods (even when initiated before complete involution), as well as the importance of safe birth spacing, the routine postpartum visit is more appropriately scheduled at three weeks for women who have had an uneventful delivery.
In some cases, of course, it may be appropriate to schedule a visit even earlier, depending on the medical needs of the mother (which may include staple removal after cesarean delivery, follow-up blood pressure assessment for patients who have gestational hypertension, etc). That said, the first postpartum visit should be routinely scheduled for no later than three weeks for healthy women who have had an uncomplicated delivery.1
The data support this approach. In one study, 57% of women reported the resumption of intercourse by the sixth postpartum week.2 A routine three-week postpartum visit instead of a visit at six weeks would reduce unmet contraceptive needs among this group of women.
HOW INFANT FEEDING PRACTICES COME INTO PLAY
Both the American Congress of Obstetricians and Gynecologists (ACOG) and the American Academy of Pediatrics recommend six months of exclusive breastfeeding because of recognized health benefits for both the mother and her infant. Exclusive breastfeeding is also a requirement if a woman desires to use breastfeeding as a contraceptive method.
Healthy People 2010 is a set of US health objectives that includes goals for breastfeeding rates. Although the percentage of infants who were ever breastfed has reached the 75% target of Healthy People 2010, according to data from the National Health and Nutrition Examination Survey (NHANES), the percentage of infants who were breastfed at six months of age has changed only minimally.3 For Mexican-American infants, that rate is 40%, compared with 35% for non-Hispanic whites and 20% for non-Hispanic black infants.3 Rates of exclusive breastfeeding are even lower, highlighting the importance of early breastfeeding support and contraceptive guidance during the postpartum period—support and guidance that can be offered at a three-week postpartum visit.
EXTENT OF BREASTFEEDING TO BE ASSESSED
Full or nearly full breastfeeding should be encouraged, along with frequent feeding of the infant. In addition, the contraceptive effect of lactation during the first six months of breastfeeding should be emphasized (see sidebar on the lactational amenorrhea method [LAM] of contraception).
Keep in mind, however, that a substantial number of nursing mothers who are not breastfeeding exclusively will ovulate before the six-week postpartum visit. Data suggest that approximately 50% of all nonbreastfeeding women will ovulate before the six-week visit, with some ovulating as early as postpartum day 25.4
For this reason, you need to determine the extent of breastfeeding at the three-week visit to determine whether LAM is a contraceptive option for your patient. Full or nearly full breastfeeding means that the vast majority of feeding is breastfeeding and that breastfeeding is not replaced by any other kind of feeding. Frequent feeding means that the infant is breastfed when hungry, be it day or night, which implies at least one nighttime feeding. If evaluation at the three-week visit indicates that breastfeeding is no longer full or nearly full and frequent, another form of contraception should be initiated.5
For most women, the benefits of initiating a progestin-only or nonhormonal method of contraception at this time outweigh the risks, regardless of breastfeeding status, according to the CDC’s medical eligibility criteria for contraceptive use.6
HOW THE RISK FOR VTE AFFECTS THE CHOICE OF CONTRACEPTIVE
The hematologic changes of normal pregnancy shift coagulability and fibrinolytic systems toward a state of hypercoagulability. This physiologic process reduces the risk for puerperal hemorrhage; however, it also predisposes women to VTE during pregnancy and into the postpartum period. Studies assessing the risk for VTE in postpartum women indicate that it increases by a factor of 22 to 84 during the first six weeks, compared with the risk in nonpregnant, nonpostpartum women of reproductive age.7 This heightened risk is most pronounced immediately after delivery, declining rapidly over the first 21 days after delivery and returning to a near-baseline level by 42 days postpartum.
By the time of the recommended three-week postpartum visit, the period of highest VTE risk has passed. For women who are no longer breastfeeding, the benefits of all hormonal contraceptive methods, including those that contain estrogen, outweigh their risks, according to a newly released update to recommendations from the CDC (see Table 1).6 Although combined oral contraceptives are known to increase the risk for VTE by a factor of 3 to 7, data suggest that healthy women who do not have additional risk factors for VTE (eg, thrombophilia, obesity, smoking, or age 35 or older) can use them safely.6
The updated recommendations discourage use of estrogen-containing contraceptives before 21 days postpartum because they present an unacceptable level of risk (regardless of breastfeeding status). But they allow the use of combined hormonal contraceptives in otherwise healthy, breastfeeding women after 30 days postpartum. For women who have additional risk factors for VTE, the risks of combined hormonal contraceptives outweigh the benefits until six weeks postpartum, regardless of breastfeeding status.6
In contrast, progestin-only and nonhormonal contraceptive methods can be safely initiated by both breastfeeding and nonbreastfeeding women before 21 days postpartum, which means that women can begin using them before discharge from the hospital.
WHEN TO CONSIDER LARC OR STERILIZATION
Long-acting reversible contraceptives (LARC) are an important postpartum contraceptive option because they offer highly effective protection against pregnancy that can begin as soon as the placenta is delivered. LARC methods include contraceptive implants and intrauterine devices (IUDs).
According to the CDC’s medical eligibility criteria for contraceptive use, implants can be placed immediately after delivery of the placenta without restriction.8
The copper IUD can be placed within 10 minutes after delivery of the placenta without restriction. If this window is missed, the benefits of inserting the IUD still outweigh the risks. Because four weeks postpartum is another time when the copper IUD can be inserted without restriction, the three-week visit is a reasonable time to screen and schedule a patient for insertion.
The benefits of insertion of the levonorgestrel-releasing intrauterine system (LNG-IUS) are also believed to outweigh the risks before four weeks postpartum. Like the copper IUD, the LNG-IUS can be inserted without restriction at four weeks postpartum or later.
There is no need for a pelvic exam at the three-week postpartum visit among women who undergo immediate postplacental insertion of the copper IUD or LNG-IUS. In fact, women can delay the exam until involution is complete.
Sterilization is best after complete involution
Interval tubal sterilization by laparoscopic, bilateral tubal fulguration or hysteroscopic microinsert placement is one of the most effective ways to prevent pregnancy. Both methods are best performed after the completion of involution and the return of normal coagulation; scheduling can take place at the three-week postpartum visit.
Given the benefit of depot medroxyprogesterone acetate (DMPA) in endometrial suppression before hysteroscopic sterilization, it is reasonable to consider administering DMPA at the three-week postpartum visit in anticipation of surgery after involution is complete.
THE BOTTOM LINE
Since most contraceptive methods can be safely initiated at or shortly after a three-weeks’ postpartum visit, there is no longer any reason to time the routine postpartum visit to coincide with the completion of involution. For healthy women who have had an uneventful delivery, the routine postpartum visit should occur at three weeks.
REFERENCES
1. Speroff L, Mishell DR. The postpartum visit: it’s time for a change in order to optimally initiate contraception. Contraception. 2008;78(2): 90–98.
2. Connolly A, Thorp J, Pahel L. Effects of pregnancy and childbirth on postpartum sexual function: a longitudinal prospective study. Int Urogynecol J Pelvic Floor Dysfunct. 2005; 16(4):263–267.
3. McDowell MA, Wang C-Y, Kennedy-Stephenson J. Breastfeeding in the United States: Findings from the National Health and Nutrition Examination Surveys 1999–2006. NCHS Data Briefs. 2008;5:1–8.
4. Jackson E, Glasier A. Return of ovulation and menses in postpartum, nonlactating women: a systematic review. Obstet Gynecol. 2011;117(3):657–662.
5. Kennedy K, Rivera R, McNeilly A. Consensus statement on the use of breastfeeding as a family planning method. Contraception. 1988; 39(5):477–496.
6. Centers for Disease Control and Prevention. Update to CDC’s US Medical Eligibility Criteria for Contraceptive Use, 2010: Revised recommendations for the use of contraceptive methods during the postpartum period. MMWR. 2011;60(26):878–883.
7. Jackson E, Curtis K, Gaffield M. Risk of venous thromboembolism during the postpartum period: a systematic review. Obstet Gynecol. 2011;117(3):691–703.
8. Centers for Disease Control and Prevention. US Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR. 2010;59(No. RR-4):1–86.
9. Kletzky OA, Marrs RP, Howard WF, McCormick W, Mishell DR Jr. Prolactin synthesis and release during pregnancy and puerperium. Am J Obstet Gynecol. 1980;136(4):545–550.
10. Labbok MH, Hight-Laukaran V, Peterson AE, Fletcher V, von Hertzen H, Van Look PF. Multicenter study of the Lactional Amenorrhea Method (LAM): I. Efficacy, duration, and implications for clinical application. Contraception. 1997;55(6):327–336.
11. Valdes V, Labbok MH, Pugin E, Perez A. The efficacy of the Lactational Amenorrhea Method (LAM) among working women. Contraception. 2000;62(5):217–219.
Q: What’s a vital aspect of the care we provide to postpartum patients?
A: Optimal timing of evaluation for contraception.
Good timing minimizes the likelihood that postpartum contraception will be initiated too early or too late to be effective.
The choice of a contraceptive method for a postpartum woman also requires a careful balancing act. On one side: the risks of contraception to the mother and her newborn. On the other: the risk for unintended pregnancy. Among the concerns that need to be addressed in contraceptive decision-making are:
• Whether the woman has resumed sexual intercourse
• Infant feeding practices
• Risk for venous thromboembolism (VTE)
• Logistics of various long-acting reversible contraceptives and tubal sterilization.
In this article, we outline the components of effective contraceptive counseling and decision-making. We also summarize recent recommendations from the CDC on the use of various contraceptive methods during the postpartum period.
FIRST: START AT THREE WEEKS
The traditional six-week postpartum visit was timed to take place after complete involution of the uterus following vaginal delivery. However, involution occurs too late to prevent unintended pregnancy because ovulation can—and often does—occur as early as the fourth postpartum week among nonbreastfeeding women.
In the past, when it was more common to fit a contraceptive diaphragm after pregnancy, six weeks may have been the best timing for the visit. Today, given the high safety and efficacy of modern contraceptive methods (even when initiated before complete involution), as well as the importance of safe birth spacing, the routine postpartum visit is more appropriately scheduled at three weeks for women who have had an uneventful delivery.
In some cases, of course, it may be appropriate to schedule a visit even earlier, depending on the medical needs of the mother (which may include staple removal after cesarean delivery, follow-up blood pressure assessment for patients who have gestational hypertension, etc). That said, the first postpartum visit should be routinely scheduled for no later than three weeks for healthy women who have had an uncomplicated delivery.1
The data support this approach. In one study, 57% of women reported the resumption of intercourse by the sixth postpartum week.2 A routine three-week postpartum visit instead of a visit at six weeks would reduce unmet contraceptive needs among this group of women.
HOW INFANT FEEDING PRACTICES COME INTO PLAY
Both the American Congress of Obstetricians and Gynecologists (ACOG) and the American Academy of Pediatrics recommend six months of exclusive breastfeeding because of recognized health benefits for both the mother and her infant. Exclusive breastfeeding is also a requirement if a woman desires to use breastfeeding as a contraceptive method.
Healthy People 2010 is a set of US health objectives that includes goals for breastfeeding rates. Although the percentage of infants who were ever breastfed has reached the 75% target of Healthy People 2010, according to data from the National Health and Nutrition Examination Survey (NHANES), the percentage of infants who were breastfed at six months of age has changed only minimally.3 For Mexican-American infants, that rate is 40%, compared with 35% for non-Hispanic whites and 20% for non-Hispanic black infants.3 Rates of exclusive breastfeeding are even lower, highlighting the importance of early breastfeeding support and contraceptive guidance during the postpartum period—support and guidance that can be offered at a three-week postpartum visit.
EXTENT OF BREASTFEEDING TO BE ASSESSED
Full or nearly full breastfeeding should be encouraged, along with frequent feeding of the infant. In addition, the contraceptive effect of lactation during the first six months of breastfeeding should be emphasized (see sidebar on the lactational amenorrhea method [LAM] of contraception).
Keep in mind, however, that a substantial number of nursing mothers who are not breastfeeding exclusively will ovulate before the six-week postpartum visit. Data suggest that approximately 50% of all nonbreastfeeding women will ovulate before the six-week visit, with some ovulating as early as postpartum day 25.4
For this reason, you need to determine the extent of breastfeeding at the three-week visit to determine whether LAM is a contraceptive option for your patient. Full or nearly full breastfeeding means that the vast majority of feeding is breastfeeding and that breastfeeding is not replaced by any other kind of feeding. Frequent feeding means that the infant is breastfed when hungry, be it day or night, which implies at least one nighttime feeding. If evaluation at the three-week visit indicates that breastfeeding is no longer full or nearly full and frequent, another form of contraception should be initiated.5
For most women, the benefits of initiating a progestin-only or nonhormonal method of contraception at this time outweigh the risks, regardless of breastfeeding status, according to the CDC’s medical eligibility criteria for contraceptive use.6
HOW THE RISK FOR VTE AFFECTS THE CHOICE OF CONTRACEPTIVE
The hematologic changes of normal pregnancy shift coagulability and fibrinolytic systems toward a state of hypercoagulability. This physiologic process reduces the risk for puerperal hemorrhage; however, it also predisposes women to VTE during pregnancy and into the postpartum period. Studies assessing the risk for VTE in postpartum women indicate that it increases by a factor of 22 to 84 during the first six weeks, compared with the risk in nonpregnant, nonpostpartum women of reproductive age.7 This heightened risk is most pronounced immediately after delivery, declining rapidly over the first 21 days after delivery and returning to a near-baseline level by 42 days postpartum.
By the time of the recommended three-week postpartum visit, the period of highest VTE risk has passed. For women who are no longer breastfeeding, the benefits of all hormonal contraceptive methods, including those that contain estrogen, outweigh their risks, according to a newly released update to recommendations from the CDC (see Table 1).6 Although combined oral contraceptives are known to increase the risk for VTE by a factor of 3 to 7, data suggest that healthy women who do not have additional risk factors for VTE (eg, thrombophilia, obesity, smoking, or age 35 or older) can use them safely.6
The updated recommendations discourage use of estrogen-containing contraceptives before 21 days postpartum because they present an unacceptable level of risk (regardless of breastfeeding status). But they allow the use of combined hormonal contraceptives in otherwise healthy, breastfeeding women after 30 days postpartum. For women who have additional risk factors for VTE, the risks of combined hormonal contraceptives outweigh the benefits until six weeks postpartum, regardless of breastfeeding status.6
In contrast, progestin-only and nonhormonal contraceptive methods can be safely initiated by both breastfeeding and nonbreastfeeding women before 21 days postpartum, which means that women can begin using them before discharge from the hospital.
WHEN TO CONSIDER LARC OR STERILIZATION
Long-acting reversible contraceptives (LARC) are an important postpartum contraceptive option because they offer highly effective protection against pregnancy that can begin as soon as the placenta is delivered. LARC methods include contraceptive implants and intrauterine devices (IUDs).
According to the CDC’s medical eligibility criteria for contraceptive use, implants can be placed immediately after delivery of the placenta without restriction.8
The copper IUD can be placed within 10 minutes after delivery of the placenta without restriction. If this window is missed, the benefits of inserting the IUD still outweigh the risks. Because four weeks postpartum is another time when the copper IUD can be inserted without restriction, the three-week visit is a reasonable time to screen and schedule a patient for insertion.
The benefits of insertion of the levonorgestrel-releasing intrauterine system (LNG-IUS) are also believed to outweigh the risks before four weeks postpartum. Like the copper IUD, the LNG-IUS can be inserted without restriction at four weeks postpartum or later.
There is no need for a pelvic exam at the three-week postpartum visit among women who undergo immediate postplacental insertion of the copper IUD or LNG-IUS. In fact, women can delay the exam until involution is complete.
Sterilization is best after complete involution
Interval tubal sterilization by laparoscopic, bilateral tubal fulguration or hysteroscopic microinsert placement is one of the most effective ways to prevent pregnancy. Both methods are best performed after the completion of involution and the return of normal coagulation; scheduling can take place at the three-week postpartum visit.
Given the benefit of depot medroxyprogesterone acetate (DMPA) in endometrial suppression before hysteroscopic sterilization, it is reasonable to consider administering DMPA at the three-week postpartum visit in anticipation of surgery after involution is complete.
THE BOTTOM LINE
Since most contraceptive methods can be safely initiated at or shortly after a three-weeks’ postpartum visit, there is no longer any reason to time the routine postpartum visit to coincide with the completion of involution. For healthy women who have had an uneventful delivery, the routine postpartum visit should occur at three weeks.
REFERENCES
1. Speroff L, Mishell DR. The postpartum visit: it’s time for a change in order to optimally initiate contraception. Contraception. 2008;78(2): 90–98.
2. Connolly A, Thorp J, Pahel L. Effects of pregnancy and childbirth on postpartum sexual function: a longitudinal prospective study. Int Urogynecol J Pelvic Floor Dysfunct. 2005; 16(4):263–267.
3. McDowell MA, Wang C-Y, Kennedy-Stephenson J. Breastfeeding in the United States: Findings from the National Health and Nutrition Examination Surveys 1999–2006. NCHS Data Briefs. 2008;5:1–8.
4. Jackson E, Glasier A. Return of ovulation and menses in postpartum, nonlactating women: a systematic review. Obstet Gynecol. 2011;117(3):657–662.
5. Kennedy K, Rivera R, McNeilly A. Consensus statement on the use of breastfeeding as a family planning method. Contraception. 1988; 39(5):477–496.
6. Centers for Disease Control and Prevention. Update to CDC’s US Medical Eligibility Criteria for Contraceptive Use, 2010: Revised recommendations for the use of contraceptive methods during the postpartum period. MMWR. 2011;60(26):878–883.
7. Jackson E, Curtis K, Gaffield M. Risk of venous thromboembolism during the postpartum period: a systematic review. Obstet Gynecol. 2011;117(3):691–703.
8. Centers for Disease Control and Prevention. US Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR. 2010;59(No. RR-4):1–86.
9. Kletzky OA, Marrs RP, Howard WF, McCormick W, Mishell DR Jr. Prolactin synthesis and release during pregnancy and puerperium. Am J Obstet Gynecol. 1980;136(4):545–550.
10. Labbok MH, Hight-Laukaran V, Peterson AE, Fletcher V, von Hertzen H, Van Look PF. Multicenter study of the Lactional Amenorrhea Method (LAM): I. Efficacy, duration, and implications for clinical application. Contraception. 1997;55(6):327–336.
11. Valdes V, Labbok MH, Pugin E, Perez A. The efficacy of the Lactational Amenorrhea Method (LAM) among working women. Contraception. 2000;62(5):217–219.
Q: What’s a vital aspect of the care we provide to postpartum patients?
A: Optimal timing of evaluation for contraception.
Good timing minimizes the likelihood that postpartum contraception will be initiated too early or too late to be effective.
The choice of a contraceptive method for a postpartum woman also requires a careful balancing act. On one side: the risks of contraception to the mother and her newborn. On the other: the risk for unintended pregnancy. Among the concerns that need to be addressed in contraceptive decision-making are:
• Whether the woman has resumed sexual intercourse
• Infant feeding practices
• Risk for venous thromboembolism (VTE)
• Logistics of various long-acting reversible contraceptives and tubal sterilization.
In this article, we outline the components of effective contraceptive counseling and decision-making. We also summarize recent recommendations from the CDC on the use of various contraceptive methods during the postpartum period.
FIRST: START AT THREE WEEKS
The traditional six-week postpartum visit was timed to take place after complete involution of the uterus following vaginal delivery. However, involution occurs too late to prevent unintended pregnancy because ovulation can—and often does—occur as early as the fourth postpartum week among nonbreastfeeding women.
In the past, when it was more common to fit a contraceptive diaphragm after pregnancy, six weeks may have been the best timing for the visit. Today, given the high safety and efficacy of modern contraceptive methods (even when initiated before complete involution), as well as the importance of safe birth spacing, the routine postpartum visit is more appropriately scheduled at three weeks for women who have had an uneventful delivery.
In some cases, of course, it may be appropriate to schedule a visit even earlier, depending on the medical needs of the mother (which may include staple removal after cesarean delivery, follow-up blood pressure assessment for patients who have gestational hypertension, etc). That said, the first postpartum visit should be routinely scheduled for no later than three weeks for healthy women who have had an uncomplicated delivery.1
The data support this approach. In one study, 57% of women reported the resumption of intercourse by the sixth postpartum week.2 A routine three-week postpartum visit instead of a visit at six weeks would reduce unmet contraceptive needs among this group of women.
HOW INFANT FEEDING PRACTICES COME INTO PLAY
Both the American Congress of Obstetricians and Gynecologists (ACOG) and the American Academy of Pediatrics recommend six months of exclusive breastfeeding because of recognized health benefits for both the mother and her infant. Exclusive breastfeeding is also a requirement if a woman desires to use breastfeeding as a contraceptive method.
Healthy People 2010 is a set of US health objectives that includes goals for breastfeeding rates. Although the percentage of infants who were ever breastfed has reached the 75% target of Healthy People 2010, according to data from the National Health and Nutrition Examination Survey (NHANES), the percentage of infants who were breastfed at six months of age has changed only minimally.3 For Mexican-American infants, that rate is 40%, compared with 35% for non-Hispanic whites and 20% for non-Hispanic black infants.3 Rates of exclusive breastfeeding are even lower, highlighting the importance of early breastfeeding support and contraceptive guidance during the postpartum period—support and guidance that can be offered at a three-week postpartum visit.
EXTENT OF BREASTFEEDING TO BE ASSESSED
Full or nearly full breastfeeding should be encouraged, along with frequent feeding of the infant. In addition, the contraceptive effect of lactation during the first six months of breastfeeding should be emphasized (see sidebar on the lactational amenorrhea method [LAM] of contraception).
Keep in mind, however, that a substantial number of nursing mothers who are not breastfeeding exclusively will ovulate before the six-week postpartum visit. Data suggest that approximately 50% of all nonbreastfeeding women will ovulate before the six-week visit, with some ovulating as early as postpartum day 25.4
For this reason, you need to determine the extent of breastfeeding at the three-week visit to determine whether LAM is a contraceptive option for your patient. Full or nearly full breastfeeding means that the vast majority of feeding is breastfeeding and that breastfeeding is not replaced by any other kind of feeding. Frequent feeding means that the infant is breastfed when hungry, be it day or night, which implies at least one nighttime feeding. If evaluation at the three-week visit indicates that breastfeeding is no longer full or nearly full and frequent, another form of contraception should be initiated.5
For most women, the benefits of initiating a progestin-only or nonhormonal method of contraception at this time outweigh the risks, regardless of breastfeeding status, according to the CDC’s medical eligibility criteria for contraceptive use.6
HOW THE RISK FOR VTE AFFECTS THE CHOICE OF CONTRACEPTIVE
The hematologic changes of normal pregnancy shift coagulability and fibrinolytic systems toward a state of hypercoagulability. This physiologic process reduces the risk for puerperal hemorrhage; however, it also predisposes women to VTE during pregnancy and into the postpartum period. Studies assessing the risk for VTE in postpartum women indicate that it increases by a factor of 22 to 84 during the first six weeks, compared with the risk in nonpregnant, nonpostpartum women of reproductive age.7 This heightened risk is most pronounced immediately after delivery, declining rapidly over the first 21 days after delivery and returning to a near-baseline level by 42 days postpartum.
By the time of the recommended three-week postpartum visit, the period of highest VTE risk has passed. For women who are no longer breastfeeding, the benefits of all hormonal contraceptive methods, including those that contain estrogen, outweigh their risks, according to a newly released update to recommendations from the CDC (see Table 1).6 Although combined oral contraceptives are known to increase the risk for VTE by a factor of 3 to 7, data suggest that healthy women who do not have additional risk factors for VTE (eg, thrombophilia, obesity, smoking, or age 35 or older) can use them safely.6
The updated recommendations discourage use of estrogen-containing contraceptives before 21 days postpartum because they present an unacceptable level of risk (regardless of breastfeeding status). But they allow the use of combined hormonal contraceptives in otherwise healthy, breastfeeding women after 30 days postpartum. For women who have additional risk factors for VTE, the risks of combined hormonal contraceptives outweigh the benefits until six weeks postpartum, regardless of breastfeeding status.6
In contrast, progestin-only and nonhormonal contraceptive methods can be safely initiated by both breastfeeding and nonbreastfeeding women before 21 days postpartum, which means that women can begin using them before discharge from the hospital.
WHEN TO CONSIDER LARC OR STERILIZATION
Long-acting reversible contraceptives (LARC) are an important postpartum contraceptive option because they offer highly effective protection against pregnancy that can begin as soon as the placenta is delivered. LARC methods include contraceptive implants and intrauterine devices (IUDs).
According to the CDC’s medical eligibility criteria for contraceptive use, implants can be placed immediately after delivery of the placenta without restriction.8
The copper IUD can be placed within 10 minutes after delivery of the placenta without restriction. If this window is missed, the benefits of inserting the IUD still outweigh the risks. Because four weeks postpartum is another time when the copper IUD can be inserted without restriction, the three-week visit is a reasonable time to screen and schedule a patient for insertion.
The benefits of insertion of the levonorgestrel-releasing intrauterine system (LNG-IUS) are also believed to outweigh the risks before four weeks postpartum. Like the copper IUD, the LNG-IUS can be inserted without restriction at four weeks postpartum or later.
There is no need for a pelvic exam at the three-week postpartum visit among women who undergo immediate postplacental insertion of the copper IUD or LNG-IUS. In fact, women can delay the exam until involution is complete.
Sterilization is best after complete involution
Interval tubal sterilization by laparoscopic, bilateral tubal fulguration or hysteroscopic microinsert placement is one of the most effective ways to prevent pregnancy. Both methods are best performed after the completion of involution and the return of normal coagulation; scheduling can take place at the three-week postpartum visit.
Given the benefit of depot medroxyprogesterone acetate (DMPA) in endometrial suppression before hysteroscopic sterilization, it is reasonable to consider administering DMPA at the three-week postpartum visit in anticipation of surgery after involution is complete.
THE BOTTOM LINE
Since most contraceptive methods can be safely initiated at or shortly after a three-weeks’ postpartum visit, there is no longer any reason to time the routine postpartum visit to coincide with the completion of involution. For healthy women who have had an uneventful delivery, the routine postpartum visit should occur at three weeks.
REFERENCES
1. Speroff L, Mishell DR. The postpartum visit: it’s time for a change in order to optimally initiate contraception. Contraception. 2008;78(2): 90–98.
2. Connolly A, Thorp J, Pahel L. Effects of pregnancy and childbirth on postpartum sexual function: a longitudinal prospective study. Int Urogynecol J Pelvic Floor Dysfunct. 2005; 16(4):263–267.
3. McDowell MA, Wang C-Y, Kennedy-Stephenson J. Breastfeeding in the United States: Findings from the National Health and Nutrition Examination Surveys 1999–2006. NCHS Data Briefs. 2008;5:1–8.
4. Jackson E, Glasier A. Return of ovulation and menses in postpartum, nonlactating women: a systematic review. Obstet Gynecol. 2011;117(3):657–662.
5. Kennedy K, Rivera R, McNeilly A. Consensus statement on the use of breastfeeding as a family planning method. Contraception. 1988; 39(5):477–496.
6. Centers for Disease Control and Prevention. Update to CDC’s US Medical Eligibility Criteria for Contraceptive Use, 2010: Revised recommendations for the use of contraceptive methods during the postpartum period. MMWR. 2011;60(26):878–883.
7. Jackson E, Curtis K, Gaffield M. Risk of venous thromboembolism during the postpartum period: a systematic review. Obstet Gynecol. 2011;117(3):691–703.
8. Centers for Disease Control and Prevention. US Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR. 2010;59(No. RR-4):1–86.
9. Kletzky OA, Marrs RP, Howard WF, McCormick W, Mishell DR Jr. Prolactin synthesis and release during pregnancy and puerperium. Am J Obstet Gynecol. 1980;136(4):545–550.
10. Labbok MH, Hight-Laukaran V, Peterson AE, Fletcher V, von Hertzen H, Van Look PF. Multicenter study of the Lactional Amenorrhea Method (LAM): I. Efficacy, duration, and implications for clinical application. Contraception. 1997;55(6):327–336.
11. Valdes V, Labbok MH, Pugin E, Perez A. The efficacy of the Lactational Amenorrhea Method (LAM) among working women. Contraception. 2000;62(5):217–219.
Battle of the Contraceptives
Because half of US pregnancies continue to be unintended, rates of induced abortion in our patients remain high. In addition, unintended pregnancies lead to negative health and social consequences for women and infants. A report from the Contraceptive Choice Project, spearheaded by Dr. Jeffrey Peipert from the Department of Obstetrics and Gynecology, Washington University School of Medicine, St. Louis, Missouri, and published in New England Journal of Medicine, underscores the high efficacy of long-acting reversible contraceptives, a term referring to intrauterine devices (IUDs) and the contraceptive implant, in preventing unintended pregnancy in a US population.
Details of the study
Eligibility criteria for the Project included age 14 to 45, residence in the St. Louis, Missouri, area, and the need for contraception. The woman’s contraceptive of choice was made available at no charge, with most women choosing a long-acting method. The published study includes outcomes for
7,486 women who used oral contraceptives (OCs), the patch, the ring, an IUD, an implant, or depot medroxyprogesterone acetate (DMPA) injections.
Among women using OCs, the patch, or the ring, the pregnancy rate was 4.55 per 100 participant-years. This rate was nearly 22-fold higher than that observed in women using IUDs or the implant (hazard ratio, 21.8): that rate was 0.27 per 100 participant-years. A similar low rate of pregnancy was noted among women who chose DMPA and returned every three months for follow-up injections.
Among women younger than 21 who used OCs, the patch, or the ring, the rate of unintended pregnancy was twice as high as in older women using these same methods. By contrast, regardless of age, pregnancy rates were uniformly low among women using long-acting methods.
Study limitations
The authors point out that their study design was not randomized—participants were at high risk for unintended pregnancy and willing to begin using a new contraceptive method, which could have resulted in higher adherence rates and lower failure rates.
Access is ongoing
barrier to use
These important data from the Contraceptive Choice Project clarify that long-acting reversible contraceptives represent powerful tools to help women minimize unintended pregnancy and induced abortion, and that women will choose these methods if they are accessible.
The findings in this report also make it clear that rates of unintended pregnancy are particularly high among adolescents using shorter-acting hor monal contraceptives (OCs, patch, or ring) and that longer-acting contraceptives are particularly useful in our younger patients. Other recent reports have provided clear evidence that immediately providing long-acting contraceptives after childbirth or induced abortion reduces unintended pregnancy in these settings.1,2
In the US, inadequate access to long-acting reversible contraceptives continues to constrain use. Accordingly, insurance pol icies that fully cover longer-acting contraceptives could go a long way toward reducing the rate of unintended pregnancies and induced abortions in our patients.
If long-acting reversible contraceptives become more widely available in the US, I look forward to a time when the great majority of our patients’ pregnancies are planned and when far fewer women will face the troubling prospect of an induced abortion.
References
1. Tocce KM, Sheeder JL, Teal SB. Rapid repeat pregnancy in adolescents: do immediate postpartum contraceptive implants make a difference? Am J Obstet Gynecol. 2012;206(6):481.e1–e7.
2. Bednarek PH, Creinin MD, Reeves MF, Cwiak C, Espey E, Jensen JT; Post-Aspiration IUD Randomization (PAIR) Study Trial Group. Immediate versus delayed IUD insertion after uterine aspiration. N Engl J Med. 2011;364(23):2208–2217.
Because half of US pregnancies continue to be unintended, rates of induced abortion in our patients remain high. In addition, unintended pregnancies lead to negative health and social consequences for women and infants. A report from the Contraceptive Choice Project, spearheaded by Dr. Jeffrey Peipert from the Department of Obstetrics and Gynecology, Washington University School of Medicine, St. Louis, Missouri, and published in New England Journal of Medicine, underscores the high efficacy of long-acting reversible contraceptives, a term referring to intrauterine devices (IUDs) and the contraceptive implant, in preventing unintended pregnancy in a US population.
Details of the study
Eligibility criteria for the Project included age 14 to 45, residence in the St. Louis, Missouri, area, and the need for contraception. The woman’s contraceptive of choice was made available at no charge, with most women choosing a long-acting method. The published study includes outcomes for
7,486 women who used oral contraceptives (OCs), the patch, the ring, an IUD, an implant, or depot medroxyprogesterone acetate (DMPA) injections.
Among women using OCs, the patch, or the ring, the pregnancy rate was 4.55 per 100 participant-years. This rate was nearly 22-fold higher than that observed in women using IUDs or the implant (hazard ratio, 21.8): that rate was 0.27 per 100 participant-years. A similar low rate of pregnancy was noted among women who chose DMPA and returned every three months for follow-up injections.
Among women younger than 21 who used OCs, the patch, or the ring, the rate of unintended pregnancy was twice as high as in older women using these same methods. By contrast, regardless of age, pregnancy rates were uniformly low among women using long-acting methods.
Study limitations
The authors point out that their study design was not randomized—participants were at high risk for unintended pregnancy and willing to begin using a new contraceptive method, which could have resulted in higher adherence rates and lower failure rates.
Access is ongoing
barrier to use
These important data from the Contraceptive Choice Project clarify that long-acting reversible contraceptives represent powerful tools to help women minimize unintended pregnancy and induced abortion, and that women will choose these methods if they are accessible.
The findings in this report also make it clear that rates of unintended pregnancy are particularly high among adolescents using shorter-acting hor monal contraceptives (OCs, patch, or ring) and that longer-acting contraceptives are particularly useful in our younger patients. Other recent reports have provided clear evidence that immediately providing long-acting contraceptives after childbirth or induced abortion reduces unintended pregnancy in these settings.1,2
In the US, inadequate access to long-acting reversible contraceptives continues to constrain use. Accordingly, insurance pol icies that fully cover longer-acting contraceptives could go a long way toward reducing the rate of unintended pregnancies and induced abortions in our patients.
If long-acting reversible contraceptives become more widely available in the US, I look forward to a time when the great majority of our patients’ pregnancies are planned and when far fewer women will face the troubling prospect of an induced abortion.
References
1. Tocce KM, Sheeder JL, Teal SB. Rapid repeat pregnancy in adolescents: do immediate postpartum contraceptive implants make a difference? Am J Obstet Gynecol. 2012;206(6):481.e1–e7.
2. Bednarek PH, Creinin MD, Reeves MF, Cwiak C, Espey E, Jensen JT; Post-Aspiration IUD Randomization (PAIR) Study Trial Group. Immediate versus delayed IUD insertion after uterine aspiration. N Engl J Med. 2011;364(23):2208–2217.
Because half of US pregnancies continue to be unintended, rates of induced abortion in our patients remain high. In addition, unintended pregnancies lead to negative health and social consequences for women and infants. A report from the Contraceptive Choice Project, spearheaded by Dr. Jeffrey Peipert from the Department of Obstetrics and Gynecology, Washington University School of Medicine, St. Louis, Missouri, and published in New England Journal of Medicine, underscores the high efficacy of long-acting reversible contraceptives, a term referring to intrauterine devices (IUDs) and the contraceptive implant, in preventing unintended pregnancy in a US population.
Details of the study
Eligibility criteria for the Project included age 14 to 45, residence in the St. Louis, Missouri, area, and the need for contraception. The woman’s contraceptive of choice was made available at no charge, with most women choosing a long-acting method. The published study includes outcomes for
7,486 women who used oral contraceptives (OCs), the patch, the ring, an IUD, an implant, or depot medroxyprogesterone acetate (DMPA) injections.
Among women using OCs, the patch, or the ring, the pregnancy rate was 4.55 per 100 participant-years. This rate was nearly 22-fold higher than that observed in women using IUDs or the implant (hazard ratio, 21.8): that rate was 0.27 per 100 participant-years. A similar low rate of pregnancy was noted among women who chose DMPA and returned every three months for follow-up injections.
Among women younger than 21 who used OCs, the patch, or the ring, the rate of unintended pregnancy was twice as high as in older women using these same methods. By contrast, regardless of age, pregnancy rates were uniformly low among women using long-acting methods.
Study limitations
The authors point out that their study design was not randomized—participants were at high risk for unintended pregnancy and willing to begin using a new contraceptive method, which could have resulted in higher adherence rates and lower failure rates.
Access is ongoing
barrier to use
These important data from the Contraceptive Choice Project clarify that long-acting reversible contraceptives represent powerful tools to help women minimize unintended pregnancy and induced abortion, and that women will choose these methods if they are accessible.
The findings in this report also make it clear that rates of unintended pregnancy are particularly high among adolescents using shorter-acting hor monal contraceptives (OCs, patch, or ring) and that longer-acting contraceptives are particularly useful in our younger patients. Other recent reports have provided clear evidence that immediately providing long-acting contraceptives after childbirth or induced abortion reduces unintended pregnancy in these settings.1,2
In the US, inadequate access to long-acting reversible contraceptives continues to constrain use. Accordingly, insurance pol icies that fully cover longer-acting contraceptives could go a long way toward reducing the rate of unintended pregnancies and induced abortions in our patients.
If long-acting reversible contraceptives become more widely available in the US, I look forward to a time when the great majority of our patients’ pregnancies are planned and when far fewer women will face the troubling prospect of an induced abortion.
References
1. Tocce KM, Sheeder JL, Teal SB. Rapid repeat pregnancy in adolescents: do immediate postpartum contraceptive implants make a difference? Am J Obstet Gynecol. 2012;206(6):481.e1–e7.
2. Bednarek PH, Creinin MD, Reeves MF, Cwiak C, Espey E, Jensen JT; Post-Aspiration IUD Randomization (PAIR) Study Trial Group. Immediate versus delayed IUD insertion after uterine aspiration. N Engl J Med. 2011;364(23):2208–2217.