User login
Reunification: The Silent War of Families and Returning Troops
Is Your Patient at High Risk for Breast Cancer?
Some 200,000 women in the United States are diagnosed with breast cancer each year. Among them, 15% to 20% have a family history of breast cancer and/or other cancers, and an additional 5% to 10% have a hereditary form of the disease.1 Although great strides are being taken in the early detection and treatment of breast cancer, this disease remains the second leading cause of cancer deaths among women.
Equally important to early detection and improved treatment options is an understanding of factors that increase women’s risk for breast cancer, and the identification and management of women at increased risk—as well as identifying women who should be referred for high-risk evaluation and management. Interventions that limit the risk, whether offered by the primary care provider or a breast cancer specialist, can ultimately prevent some breast cancers from developing.
Risk assessment for breast cancer and an overview of high-risk evaluation and management provide the basis for this article.
Risk Factors
A variety of factors—some modifiable, some not—increase a woman’s risk for breast cancer (see Table 12). Factors associated with particularly increased risk include:
• Family history of breast cancer in a first-degree relative or of breast or ovarian cancer in two or more close relatives1-3
• Prior breast biopsy findings that revealed atypical ductal hyperplasia (ADH), atypical lobular hyperplasia (ALH), or lobular carcinoma in situ (LCIS)
• Personal or family history of a BRCA1 or BRCA2 mutation
• Having undergone radiation to the chest wall between ages 10 and 30
• Personal or family history of other rare hereditary breast cancer syndromes,4 such as Cowden syndrome, Li-Fraumeni syndrome, or Peutz-Jeghers syndrome.5-7
To understand high-risk factors in the overall context of breast cancer, it is important to understand the differences between sporadic, familial, and hereditary cancers. The majority of breast cancers, approximately 70%, occur sporadically.8 Sporadic cancers may be caused by random events occurring among or within cells, radiation exposure, or environmental or other unknown factors.
Among breast cancers, 15% to 20% are familial8,9—ie, a recognized pattern of cancers occurs in the patient’s family, but affected members have tested negative for known genetic mutations. Familial cancers may be influenced by not-yet-identified genetic mutations or by environmental factors. As families usually share the same environment, dietary habits, and lifestyles, there may be an association between those factors and families with a cancer history.
Anyone who has a personal or family history of a mutation in either of the breast or ovarian cancer susceptibility genes, BRCA1 and BRCA2, has a significant risk for breast cancer. These are hereditary autosomal dominant mutations, inherited from an affected parent. Hereditary cancer syndromes, such as those associated with BRCA1 or BRCA2, are relatively rare. Only 5% to 10% of breast cancers are considered hereditary; of those, approximately 80% are related to mutations in BRCA1 or BRCA2.10
Several “red flags” suggest the possibility of a hereditary type of breast cancer syndrome. Genetic risk is primarily identified through thorough history taking, which must address both the maternal and paternal sides of the family—over three generations, if possible.10 Table 210-14 identifies these red flags.
Atypical cells identified on breast biopsy also increase breast cancer risk. The presence of ADH or ALH increases a woman’s risk four to five times higher than the average.2 LCIS is associated with a 10-fold increase in breast cancer risk.9 The overall incidence of breast cancer in women with LCIS is estimated at 22.3%.3
Radiation exposure to the chest wall, particularly when administered between ages 10 and 30, also increases the risk for breast cancer. This usually occurs in female patients who have undergone mantle-field radiation treatment for another cancer, such as Hodgkin’s disease or non-Hodgkin’s lymphoma.15,16 Risk associated with this type of treatment can be as great as 12 times the normal risk. Patients treated before or during adolescence appear to be at highest risk.2 Persons exposed to other types of radiation (eg, survivors of atomic weapons) also have increased breast cancer risk.1
Rare genetic syndromes not associated with BRCA1 or BRCA2 have been associated with breast cancer as well. Cowden, Li-Fraumeni, and Peutz-Jeghers syndromes are all known hereditary breast cancer syndromes.4-7 Although these syndromes account for less than 1% of all breast cancers,10 it is important to be able to identify a patient who is at risk for harboring a genetic mutation that causes one of these syndromes—and to understand how to manage a woman who is already affected.
Cowden syndrome is an autosomal dominant disorder caused by a mutation in the PTEN gene.4,17 This syndrome carries a 25% to 50% risk for breast cancer.18 Other findings associated with Cowden syndrome include facial or buccal lesions, fibrocystic breast disease, benign thyroid conditions (eg, goiter), nonmedullary thyroid cancer, endometrial cancer, macrocephaly, uterine fibroids, and gastrointestinal hamartomas.3,10
Li-Fraumeni syndrome is a highly penetrant, autosomal dominant disorder caused by a mutation in the TP53 gene.4,6,19,20 Affected women’s overall cancer risk is 50% by age 35 and 90% lifelong. Breast cancer risk is estimated at 50% by age 50.3 Multiple primary cancers, including early-onset breast cancer (ie, before age 40), sarcoma, leukemia, childhood brain tumors, and adrenocortical carcinoma, are all associated with Li-Fraumeni.6,10,19,21
Peutz-Jeghers syndrome is also an autosomal dominant condition, caused by a mutation in the STK11 gene.3,22,23 The mean age of breast cancer diagnosis in affected women is 44. Hallmark features of Peutz-Jeghers include gastrointestinal hamartomas (often discovered during childhood24) and cancers of the colon, small bowel, pancreas, uterus, thyroid, lung, and breast. The most commonly reported malignancies in patients with Peutz-Jeghers syndrome are breast cancer and colon cancer. Associated phenotypic features include pigmented spots on the lips, buccal mucosa, and skin.10,24
Risk Assessment Tools
Women with any of the identified high-risk factors should be referred to a provider experienced in high-risk breast cancer assessment and management. Many comprehensive breast centers are adding high-risk programs to their array of services. Researching those programs in your area will facilitate the process of referral for women in your practice who are identified as high risk.
In efforts to identify patients at high risk for breast cancer, the importance of thorough history taking cannot be overstated. It is imperative that both the maternal and paternal sides of the family be assessed, as genetic mutations can be acquired through the patient’s mother or father.10
A variety of tools to assess breast cancer risk are available. The one most commonly used is the Gail risk assessment model,25 which was developed at the National Cancer Institute. A modified version, validated during the Breast Cancer Prevention Trial,26 calculates five-year and lifetime risk based on the following criteria:
• Current age
• Age at menarche
• Age at first live birth
• Race
• Number of first-degree relatives with a history of breast cancer
• Number of previous breast biopsies
• History of atypical cells on previous breast biopsy.
The Gail model has certain limitations. It cannot be used to assess women younger than 3527 or older than 85. Also, age at breast cancer diagnosis, non–first-degree relatives with breast cancer, paternal cancer history, ovarian cancer history, and ethnicity are not included among its considerations.3
The Claus model28 assesses risk in women with a family history of breast cancer more accurately than the Gail model, but it does not incorporate male breast cancer, ovarian cancer, or ethnicity.9
Several models are available to predict the risk for carrying a BRCA1 or BRCA2 mutation. The Myriad Genetic Laboratories database,29 the BRCAPRO model,30,31 the Manchester scoring system,32,33 the Tyrer-Duffy-Cuzick (IBIS) model,34 and the BOADICEA model35 all provide probability data, but each has its limitations. Breast specialists who provide high-risk assessment services calculate risk using a variety of models and choosing the most appropriate model(s) for each patient.36,37 Women with a high probability of carrying a mutation, based on history and findings from the risk assessment model, are referred for genetics counseling and testing.
Reducing the Risk
Risk reduction is an integral component of high-risk breast cancer management, and several strategies are currently recommended, including these:
• Exercise for 45 to 60 minutes at least five times per week.2,38 In women who engage in at least five hours of vigorous exercise each week, a 0.62 relative risk for breast cancer has been reported.38,39
• Limit alcohol intake to fewer than one to two drinks per day. A number of studies have documented a 30% to 50% increase in the incidence of breast cancer among women who consume alcohol in greater quantities.38,40
• Avoid obesity. Compared with women who have maintained their weight since age 18, those who gain 55 lb or more by menopause onset have a 1.45 relative risk for breast cancer.38,41
• Breastfeed infants. Lactation for two years or longer decreases the lifetime risk for breast cancer by 50%.3
• Follow a low-fat, high-fiber diet, rich in fresh fruits and vegetables. Increasing fruit and vegetable intake by even one serving per day has been associated with a 9% decrease in breast cancer incidence.38,42
Chemoprevention for At-Risk Women
Another risk reduction strategy for women at particularly high risk for breast cancer is chemoprevention. Currently, two medications, tamoxifen and raloxifene, are FDA approved for breast cancer risk reduction in women at high risk. These agents are offered to women with specific high-risk factors, including:
• Findings of LCIS, ADH, or ALH on previous breast biopsy
• A five-year probability of breast cancer exceeding 1.7%, based on a validated risk assessment model (eg, the Gail model, BRCAPRO)
• Presence of the BRCA1 or BRCA2 mutation.
Tamoxifen is a selective estrogen receptor modulator (SERM) that blocks the effects of estrogen on breast tissue.43 Often referred to as an “antiestrogen,” it is approved for use in both premenopausal and postmenopausal women. In the Breast Cancer Prevention Trial,44 women at high risk for breast cancer who took tamoxifen for five years had about a 50% risk reduction for invasive breast cancer and a 30% risk reduction in noninvasive breast cancer. The usual dose of tamoxifen is 20 mg/d.
Adverse effects associated with tamoxifen use, however, include blood clots, stroke, and uterine cancer. Women should not take tamoxifen if they have a history of cataracts, are current hormone therapy users, are planning a pregnancy or have the potential for becoming pregnant, or have a history of stroke, deep venous thrombosis, or pulmonary embolus. Less serious adverse effects include menopausal symptoms, menstrual irregularities, headache, fatigue, nausea, and skin irritation.43
Raloxifene, though more commonly prescribed to prevent and treat osteoporosis, has been shown to reduce the risk for invasive breast cancer by 56% to 72%, compared with placebo45,46; it exerts estrogenic effects on bone and antiestrogenic effects on breast and endometrial tissue.47 This SERM is approved for breast cancer risk reduction only in postmenopausal women.48 Recommended use is 60 mg/d for five years.
Although raloxifene provides a risk reduction benefit comparable to that of tamoxifen against invasive breast cancer (ie, incidence rates of 4.4 and 4.3, respectively, per 1,000 women per year49), it does not appear to reduce the risk for noninvasive breast cancer (ie, ductal carcinoma in situ).50 Potential major complications attributed to raloxifene use include blood clots, stroke, and uterine cancer, although the risk for uterine cancer is lower than with tamoxifen use.49 Minor adverse effects include leg cramps, menopausal symptoms, edema of the extremities, and flulike symptoms. Contraindications are comparable to those associated with tamoxifen.48
Screening Recommendations
Combined with mammography and breast ultrasound, the use of MRI to screen high-risk women is now being recommended, according to guidelines published in March 2007 by the American Cancer Society.20 Patient factors suggesting greatest benefit from annual MRI screening (combined with mammography) are listed in Table 3.4,20,51-55
In addition to patient-driven reduction strategies and the provider-initiated interventions for surveillance and management, monthly breast self-examination is encouraged, as are clinical breast examinations every six months.
Even with the most aggressive risk reduction program, not all breast cancers can be prevented. A key objective of high-risk screening and management is to identify patients with breast cancer at the earliest possible stage so that a cure is more likely to be achieved.
Conclusion
Identifying and screening women at high risk for breast cancer are essential skills for all primary care providers. Maintaining a comprehensive list of referral sources for high-risk management and genetic counseling services in your area will allow you to partner with other professionals to provide patients with the best possible care. Women feel empowered by education, particularly the newly acquired knowledge about breast cancer risk reduction—and reassured, knowing that their providers are interested and well informed in this complex area of women’s health.
1. National Cancer Institute. Genetics of breast and ovarian cancer (2008). www.cancer.gov/cancertopics/pdq/genetics/breast-and-ovarian. Accessed September 23, 2008.
2. American Cancer Society. Detailed Guide: Breast Cancer. What are the risk factors for breast cancer? 2007. www.cancer.org/docroot/CRI/content/CRI_2_4_2X_What_are_the_risk_factors_for_breast_cancer_5.asp. Accessed September 23, 2008.
3. Vogel V. Handbook of Breast Cancer Risk Assessment. Sudbury, MA: Jones and Bartlett; 2004.
4. Garber JE, Offit K. Hereditary cancer predisposition syndromes. J Clin Oncol. 2005;23(2):276-292.
5. Kelly P. Hereditary breast cancer considering Cowden syndrome: a case study. Cancer Nurs. 2003;26(5):370-375.
6. Li FP, Fraumeni JF Jr. Soft-tissue sarcomas, breast cancer, and other neoplasms: a familial syndrome? Ann Intern Med. 1969;71(4):747-752.
7. Giardiello FM, Welsh SB, Hamilton SR, et al. Increased risk of cancer in the Peutz-Jeghers syndrome. N Engl J Med. 1987;316(24):1511-1514.
8. Myriad Genetic Laboratories. Making Informed Decisions: Testing and Management for Hereditary Breast and Ovarian Cancer [video]. www.myriadtests.com/breast-cancer-patient-video.htm. Accessed September 23, 2008.
9. Korde LA, Calzone KA, Zujewski J. Assessing breast cancer risk: genetic factors are not the whole story. Postgrad Med. 2004;116(4):6-8, 11-14, 19-20.
10. Thull DL, Vogel VG. Recognition and management of hereditary breast cancer syndromes. Oncologist. 2004;9(1):13-24.
11. Breast Cancer Linkage Consortium. Cancer risks in BRCA2 mutation carriers. J Natl Cancer Inst. 1999;91(15):1310-1316.
12. Krainer M, Silva-Arrieta S, FitzGerald MG, et al. Differential contributions of BRCA1 and BRCA2 to early-onset breast cancer. N Engl J Med. 1997;336(20):1416-1421.
13. FitzGerald MG, MacDonald DH, Krainer M, et al. Germ-line BRCA1 mutations in Jewish and non-Jewish women with early-onset breast cancer. N Engl J Med. 1996;334(3):143-149.
14. Malone KE, Daling JR, Neal C, et al. Frequency of BRCA1/BRCA2 mutations in a population-based sample of young breast carcinoma cases. Cancer. 2000;88(6):1393-1402.
15. Travis LB, Hill D, Dores GM, et al. Cumulative absolute breast cancer risk for young women treated for Hodgkin lymphoma. J Natl Cancer Inst. 2005;97(19):1428-1437.
16. Longo DL. Radiation therapy in Hodgkin disease: why risk a Pyrrhic victory? [editorial]. J Natl Cancer Inst. 2005;97(19): 1394-1395.
17. Nelen MR, Padberg GW, Peeters EA, et al. Localization of the gene for Cowden disease to chromosome 10q22-23. Nat Genet. 1996;13(1):114-116.
18. Eng C. Genetics of Cowden syndrome: through the looking glass of oncology. Int J Oncol. 1998;12(3):701-710.
19. Li FP, Fraumeni JF Jr, Mulvihill JJ, et al. A cancer family syndrome in twenty-four kindreds. Cancer Res. 1988;48(18):5358-5362.
20. Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75-89.
21. Malkin D, Li FP, Strong LC, et al. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science. 1990;250(4985):1233-1238.
22. Schumacher V, Vogel T, Leube B, et al. STK11 genotyping and cancer risk in Peutz-Jeghers syndrome. J Med Genet. 2005;42(5):428-435.
23. Boardman LA, Thibodeau SN, Schaid DJ, et al. Increased risk for cancer in patients with the Peutz-Jeghers syndrome. Ann Intern Med. 1998;128(11):896-899.
24. Vasen HF. Clinical diagnosis and management of hereditary colorectal cancer syndromes. J Clin Oncol. 2000;18(21 Suppl):81S-92S.
25. Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81(24):1879-1886.
26. Costantino JP, Gail MH, Pee D, et al. Validation studies for models projecting the risk of invasive and total breast cancer incidence. J Natl Cancer Inst. 1999;91(18):1541-1548.
27. Rockhill B, Spiegelman D, Byrne C, et al. Validation of the Gail et al. model of breast cancer risk prediction and implications for chemoprevention. J Natl Cancer Inst. 2001;93(5): 358-366.
28. Claus EB, Risch N, Thompson WD. Autosomal dominant inheritance of early-onset breast cancer: implications for risk prediction. Cancer. 1994;73(3):643-651.
29. Easton DF, Deffenbaugh AM, Pruss D, et al. A systematic genetic assessment of 1,433 sequence variants of unknown clinical significance in the BRCA1 and BRCA2 breast cancer–predisposition genes. Am J Hum Genet. 2007;81(5):873-883.
30. Parmigiani G, Berry D, Aguilar O. Determining carrier probabilities for breast cancer–susceptibility genes BRCA1 and BRCA2. Am J Hum Genet. 1998;62(1):145-158.
31. Berry DA, Iversen ES Jr, Gudbjartsson DF, et al. BRCAPRO validation, sensitivity of genetic testing of BRCA1/BRCA2, and prevalence of other breast cancer susceptibility genes. J Clin Oncol. 2002;20(11):2701-2712.
32. Evans DG, Eccles DM, Rahman N, et al. A new scoring system for the chances of identifying a BRCA1/2 mutation outperforms existing models including BRCAPRO. J Med Genet. 2004;41(6):474-480.
33. Evans DG, Lalloo F, Wallace A, Rahman N. Update on the Manchester Scoring System for BRCA1 and BRCA2 testing.
J Med Genet. 2005;42(7):e39.
34. Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23(7):1111-1130.
35. Antoniou AC, Pharaoh PP, Smith P, Easton DF. The BOADICEA model of genetic susceptibility to breast and ovarian cancer. Br J Cancer. 2004;91(8):1580-1590.
36. Antoniou AC, Hardy R, Walker L, et al. Predicting the likelihood of carrying a BRCA1 or BRCA2 mutation: validation of BOADICEA, BRCAPRO, IBIS, Myriad and the Manchester scoring system using data from UK genetics clinics. J Med Genet. 2008;45(7):425-431.
37. Thirthagiri E, Lee SY, Kang P, et al. Evaluation of BRCA1 and BRCA2 mutations and risk-prediction models in a typical Asian country (Malaysia) with a relatively low incidence of breast cancer. Breast Cancer Res. 2008;10(4):R59.
38. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology.™ Breast Cancer Risk Reduction. V.I.2008. www.nccn.org/professionals/physician_gls/PDF/breast_risk.pdf. Accessed September 23, 2008.
39. Tehard B, Friedenreich CM, Oppert JM, Clavel-Chapelon F. Effect of physical activity on women at increased risk of breast cancer: results from the E3N cohort study. Cancer Epidemiol Biomarkers Prev. 2006;15(1):57-64.
40. Terry MB, Zhang FF, Kabat G, et al. Lifetime alcohol intake and breast cancer risk. Ann Epidemiol. 2006;16(3):230-240.
41. Eliassen AH, Colditz GA, Rosner B, et al. Adult weight change and risk of postmenopausal breast cancer. JAMA. 2006;296(2):193-201.
42. Prentice RL, Caan B, Chlebowski RT, et al. Low-fat dietary pattern and risk of invasive breast cancer: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295(6):629-642.
43. US Food and Drug Administration, Center for Drug Evaluation and Research. Tamoxifen information. www.fda.gov/cder/news/tamoxifen/default.htm. Accessed September 23, 2008.
44. Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90(18):1371-1388.
45. Cauley JA, Norton L, Lippman ME, et al. Continued breast cancer risk reduction in postmenopausal women treated with raloxifene: 4-year results from the MORE trial: Multiple Outcomes of Raloxifene Evaluation. Breast Cancer Res Treat. 2001;65(2):125-134.
46. Martino S, Cauley JA, Barrett-Connor E, et al; CORE In—vestigators. Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst. 2004;96(23):1751-1761.
47. Cummings SR, Eckert S, Krueger KA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial: Multiple Outcomes of Raloxifene Evaluation [erratum in: JAMA. 1999;282(22):2124]. JAMA. 1999;281(23):2189-2197.
48. US Food and Drug Administration, Center for Drug Evaluation and Research. FDA approves new uses for Evista (raloxifene hydrochloride). www.fda.gov/cder/Offices/OODP/whatsnew/raloxifene.htm. Accessed September 23, 2008.
49. Vogel VG, Costantino JP, Wickerham DL, et al; National Surgical Adjuvant Breast and Bowel Project (NSABP). Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295(23):2727-2741.
50. Grady D, Cauley JA, Geiger MJ, et al; Raloxifene Use for the Heart Trial Investigators. Reduced incidence of invasive breast cancer with raloxifene among women at increased coronary risk. J Natl Cancer Inst. 2008;100(12):854-861.
51. Kriege M, Brekelmans CT, Boetes C, et al; Magnetic Resonance Imaging Screening Study Group. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351(5):427-437.
52. Leach MO, Boggis CR, Dixon AK, et al; MARIBS Study Group. Screening with magnetic resonance imaging and mammography of a UK population at high familial risk of breast cancer: a prospective multicentre cohort study (MARIBS). Lancet. 2005;365(9473):1769-1778.
53. Lehman CD, Blume JD, Weatherall P, et al; International Breast MRI Consortium Working Group. Screening women at high risk for breast cancer with mammography and magnetic resonance imaging. Cancer. 2005;103(9):1898-1905.
54. Sardanelli F, Podo F. Breast MR imaging in women at high-risk of breast cancer: is something changing in early breast cancer detection? Eur Radiol. 2007;17(4):873-887.
55. Warner E, Plewes DB, Hill KA, et al. Surveillance of BRCA1 and BRCA2 mutation carriers with magnetic resonance imaging, ultrasound, mammography, and clinical breast examination. JAMA. 2004;292(11):1317-1325.
Some 200,000 women in the United States are diagnosed with breast cancer each year. Among them, 15% to 20% have a family history of breast cancer and/or other cancers, and an additional 5% to 10% have a hereditary form of the disease.1 Although great strides are being taken in the early detection and treatment of breast cancer, this disease remains the second leading cause of cancer deaths among women.
Equally important to early detection and improved treatment options is an understanding of factors that increase women’s risk for breast cancer, and the identification and management of women at increased risk—as well as identifying women who should be referred for high-risk evaluation and management. Interventions that limit the risk, whether offered by the primary care provider or a breast cancer specialist, can ultimately prevent some breast cancers from developing.
Risk assessment for breast cancer and an overview of high-risk evaluation and management provide the basis for this article.
Risk Factors
A variety of factors—some modifiable, some not—increase a woman’s risk for breast cancer (see Table 12). Factors associated with particularly increased risk include:
• Family history of breast cancer in a first-degree relative or of breast or ovarian cancer in two or more close relatives1-3
• Prior breast biopsy findings that revealed atypical ductal hyperplasia (ADH), atypical lobular hyperplasia (ALH), or lobular carcinoma in situ (LCIS)
• Personal or family history of a BRCA1 or BRCA2 mutation
• Having undergone radiation to the chest wall between ages 10 and 30
• Personal or family history of other rare hereditary breast cancer syndromes,4 such as Cowden syndrome, Li-Fraumeni syndrome, or Peutz-Jeghers syndrome.5-7
To understand high-risk factors in the overall context of breast cancer, it is important to understand the differences between sporadic, familial, and hereditary cancers. The majority of breast cancers, approximately 70%, occur sporadically.8 Sporadic cancers may be caused by random events occurring among or within cells, radiation exposure, or environmental or other unknown factors.
Among breast cancers, 15% to 20% are familial8,9—ie, a recognized pattern of cancers occurs in the patient’s family, but affected members have tested negative for known genetic mutations. Familial cancers may be influenced by not-yet-identified genetic mutations or by environmental factors. As families usually share the same environment, dietary habits, and lifestyles, there may be an association between those factors and families with a cancer history.
Anyone who has a personal or family history of a mutation in either of the breast or ovarian cancer susceptibility genes, BRCA1 and BRCA2, has a significant risk for breast cancer. These are hereditary autosomal dominant mutations, inherited from an affected parent. Hereditary cancer syndromes, such as those associated with BRCA1 or BRCA2, are relatively rare. Only 5% to 10% of breast cancers are considered hereditary; of those, approximately 80% are related to mutations in BRCA1 or BRCA2.10
Several “red flags” suggest the possibility of a hereditary type of breast cancer syndrome. Genetic risk is primarily identified through thorough history taking, which must address both the maternal and paternal sides of the family—over three generations, if possible.10 Table 210-14 identifies these red flags.
Atypical cells identified on breast biopsy also increase breast cancer risk. The presence of ADH or ALH increases a woman’s risk four to five times higher than the average.2 LCIS is associated with a 10-fold increase in breast cancer risk.9 The overall incidence of breast cancer in women with LCIS is estimated at 22.3%.3
Radiation exposure to the chest wall, particularly when administered between ages 10 and 30, also increases the risk for breast cancer. This usually occurs in female patients who have undergone mantle-field radiation treatment for another cancer, such as Hodgkin’s disease or non-Hodgkin’s lymphoma.15,16 Risk associated with this type of treatment can be as great as 12 times the normal risk. Patients treated before or during adolescence appear to be at highest risk.2 Persons exposed to other types of radiation (eg, survivors of atomic weapons) also have increased breast cancer risk.1
Rare genetic syndromes not associated with BRCA1 or BRCA2 have been associated with breast cancer as well. Cowden, Li-Fraumeni, and Peutz-Jeghers syndromes are all known hereditary breast cancer syndromes.4-7 Although these syndromes account for less than 1% of all breast cancers,10 it is important to be able to identify a patient who is at risk for harboring a genetic mutation that causes one of these syndromes—and to understand how to manage a woman who is already affected.
Cowden syndrome is an autosomal dominant disorder caused by a mutation in the PTEN gene.4,17 This syndrome carries a 25% to 50% risk for breast cancer.18 Other findings associated with Cowden syndrome include facial or buccal lesions, fibrocystic breast disease, benign thyroid conditions (eg, goiter), nonmedullary thyroid cancer, endometrial cancer, macrocephaly, uterine fibroids, and gastrointestinal hamartomas.3,10
Li-Fraumeni syndrome is a highly penetrant, autosomal dominant disorder caused by a mutation in the TP53 gene.4,6,19,20 Affected women’s overall cancer risk is 50% by age 35 and 90% lifelong. Breast cancer risk is estimated at 50% by age 50.3 Multiple primary cancers, including early-onset breast cancer (ie, before age 40), sarcoma, leukemia, childhood brain tumors, and adrenocortical carcinoma, are all associated with Li-Fraumeni.6,10,19,21
Peutz-Jeghers syndrome is also an autosomal dominant condition, caused by a mutation in the STK11 gene.3,22,23 The mean age of breast cancer diagnosis in affected women is 44. Hallmark features of Peutz-Jeghers include gastrointestinal hamartomas (often discovered during childhood24) and cancers of the colon, small bowel, pancreas, uterus, thyroid, lung, and breast. The most commonly reported malignancies in patients with Peutz-Jeghers syndrome are breast cancer and colon cancer. Associated phenotypic features include pigmented spots on the lips, buccal mucosa, and skin.10,24
Risk Assessment Tools
Women with any of the identified high-risk factors should be referred to a provider experienced in high-risk breast cancer assessment and management. Many comprehensive breast centers are adding high-risk programs to their array of services. Researching those programs in your area will facilitate the process of referral for women in your practice who are identified as high risk.
In efforts to identify patients at high risk for breast cancer, the importance of thorough history taking cannot be overstated. It is imperative that both the maternal and paternal sides of the family be assessed, as genetic mutations can be acquired through the patient’s mother or father.10
A variety of tools to assess breast cancer risk are available. The one most commonly used is the Gail risk assessment model,25 which was developed at the National Cancer Institute. A modified version, validated during the Breast Cancer Prevention Trial,26 calculates five-year and lifetime risk based on the following criteria:
• Current age
• Age at menarche
• Age at first live birth
• Race
• Number of first-degree relatives with a history of breast cancer
• Number of previous breast biopsies
• History of atypical cells on previous breast biopsy.
The Gail model has certain limitations. It cannot be used to assess women younger than 3527 or older than 85. Also, age at breast cancer diagnosis, non–first-degree relatives with breast cancer, paternal cancer history, ovarian cancer history, and ethnicity are not included among its considerations.3
The Claus model28 assesses risk in women with a family history of breast cancer more accurately than the Gail model, but it does not incorporate male breast cancer, ovarian cancer, or ethnicity.9
Several models are available to predict the risk for carrying a BRCA1 or BRCA2 mutation. The Myriad Genetic Laboratories database,29 the BRCAPRO model,30,31 the Manchester scoring system,32,33 the Tyrer-Duffy-Cuzick (IBIS) model,34 and the BOADICEA model35 all provide probability data, but each has its limitations. Breast specialists who provide high-risk assessment services calculate risk using a variety of models and choosing the most appropriate model(s) for each patient.36,37 Women with a high probability of carrying a mutation, based on history and findings from the risk assessment model, are referred for genetics counseling and testing.
Reducing the Risk
Risk reduction is an integral component of high-risk breast cancer management, and several strategies are currently recommended, including these:
• Exercise for 45 to 60 minutes at least five times per week.2,38 In women who engage in at least five hours of vigorous exercise each week, a 0.62 relative risk for breast cancer has been reported.38,39
• Limit alcohol intake to fewer than one to two drinks per day. A number of studies have documented a 30% to 50% increase in the incidence of breast cancer among women who consume alcohol in greater quantities.38,40
• Avoid obesity. Compared with women who have maintained their weight since age 18, those who gain 55 lb or more by menopause onset have a 1.45 relative risk for breast cancer.38,41
• Breastfeed infants. Lactation for two years or longer decreases the lifetime risk for breast cancer by 50%.3
• Follow a low-fat, high-fiber diet, rich in fresh fruits and vegetables. Increasing fruit and vegetable intake by even one serving per day has been associated with a 9% decrease in breast cancer incidence.38,42
Chemoprevention for At-Risk Women
Another risk reduction strategy for women at particularly high risk for breast cancer is chemoprevention. Currently, two medications, tamoxifen and raloxifene, are FDA approved for breast cancer risk reduction in women at high risk. These agents are offered to women with specific high-risk factors, including:
• Findings of LCIS, ADH, or ALH on previous breast biopsy
• A five-year probability of breast cancer exceeding 1.7%, based on a validated risk assessment model (eg, the Gail model, BRCAPRO)
• Presence of the BRCA1 or BRCA2 mutation.
Tamoxifen is a selective estrogen receptor modulator (SERM) that blocks the effects of estrogen on breast tissue.43 Often referred to as an “antiestrogen,” it is approved for use in both premenopausal and postmenopausal women. In the Breast Cancer Prevention Trial,44 women at high risk for breast cancer who took tamoxifen for five years had about a 50% risk reduction for invasive breast cancer and a 30% risk reduction in noninvasive breast cancer. The usual dose of tamoxifen is 20 mg/d.
Adverse effects associated with tamoxifen use, however, include blood clots, stroke, and uterine cancer. Women should not take tamoxifen if they have a history of cataracts, are current hormone therapy users, are planning a pregnancy or have the potential for becoming pregnant, or have a history of stroke, deep venous thrombosis, or pulmonary embolus. Less serious adverse effects include menopausal symptoms, menstrual irregularities, headache, fatigue, nausea, and skin irritation.43
Raloxifene, though more commonly prescribed to prevent and treat osteoporosis, has been shown to reduce the risk for invasive breast cancer by 56% to 72%, compared with placebo45,46; it exerts estrogenic effects on bone and antiestrogenic effects on breast and endometrial tissue.47 This SERM is approved for breast cancer risk reduction only in postmenopausal women.48 Recommended use is 60 mg/d for five years.
Although raloxifene provides a risk reduction benefit comparable to that of tamoxifen against invasive breast cancer (ie, incidence rates of 4.4 and 4.3, respectively, per 1,000 women per year49), it does not appear to reduce the risk for noninvasive breast cancer (ie, ductal carcinoma in situ).50 Potential major complications attributed to raloxifene use include blood clots, stroke, and uterine cancer, although the risk for uterine cancer is lower than with tamoxifen use.49 Minor adverse effects include leg cramps, menopausal symptoms, edema of the extremities, and flulike symptoms. Contraindications are comparable to those associated with tamoxifen.48
Screening Recommendations
Combined with mammography and breast ultrasound, the use of MRI to screen high-risk women is now being recommended, according to guidelines published in March 2007 by the American Cancer Society.20 Patient factors suggesting greatest benefit from annual MRI screening (combined with mammography) are listed in Table 3.4,20,51-55
In addition to patient-driven reduction strategies and the provider-initiated interventions for surveillance and management, monthly breast self-examination is encouraged, as are clinical breast examinations every six months.
Even with the most aggressive risk reduction program, not all breast cancers can be prevented. A key objective of high-risk screening and management is to identify patients with breast cancer at the earliest possible stage so that a cure is more likely to be achieved.
Conclusion
Identifying and screening women at high risk for breast cancer are essential skills for all primary care providers. Maintaining a comprehensive list of referral sources for high-risk management and genetic counseling services in your area will allow you to partner with other professionals to provide patients with the best possible care. Women feel empowered by education, particularly the newly acquired knowledge about breast cancer risk reduction—and reassured, knowing that their providers are interested and well informed in this complex area of women’s health.
Some 200,000 women in the United States are diagnosed with breast cancer each year. Among them, 15% to 20% have a family history of breast cancer and/or other cancers, and an additional 5% to 10% have a hereditary form of the disease.1 Although great strides are being taken in the early detection and treatment of breast cancer, this disease remains the second leading cause of cancer deaths among women.
Equally important to early detection and improved treatment options is an understanding of factors that increase women’s risk for breast cancer, and the identification and management of women at increased risk—as well as identifying women who should be referred for high-risk evaluation and management. Interventions that limit the risk, whether offered by the primary care provider or a breast cancer specialist, can ultimately prevent some breast cancers from developing.
Risk assessment for breast cancer and an overview of high-risk evaluation and management provide the basis for this article.
Risk Factors
A variety of factors—some modifiable, some not—increase a woman’s risk for breast cancer (see Table 12). Factors associated with particularly increased risk include:
• Family history of breast cancer in a first-degree relative or of breast or ovarian cancer in two or more close relatives1-3
• Prior breast biopsy findings that revealed atypical ductal hyperplasia (ADH), atypical lobular hyperplasia (ALH), or lobular carcinoma in situ (LCIS)
• Personal or family history of a BRCA1 or BRCA2 mutation
• Having undergone radiation to the chest wall between ages 10 and 30
• Personal or family history of other rare hereditary breast cancer syndromes,4 such as Cowden syndrome, Li-Fraumeni syndrome, or Peutz-Jeghers syndrome.5-7
To understand high-risk factors in the overall context of breast cancer, it is important to understand the differences between sporadic, familial, and hereditary cancers. The majority of breast cancers, approximately 70%, occur sporadically.8 Sporadic cancers may be caused by random events occurring among or within cells, radiation exposure, or environmental or other unknown factors.
Among breast cancers, 15% to 20% are familial8,9—ie, a recognized pattern of cancers occurs in the patient’s family, but affected members have tested negative for known genetic mutations. Familial cancers may be influenced by not-yet-identified genetic mutations or by environmental factors. As families usually share the same environment, dietary habits, and lifestyles, there may be an association between those factors and families with a cancer history.
Anyone who has a personal or family history of a mutation in either of the breast or ovarian cancer susceptibility genes, BRCA1 and BRCA2, has a significant risk for breast cancer. These are hereditary autosomal dominant mutations, inherited from an affected parent. Hereditary cancer syndromes, such as those associated with BRCA1 or BRCA2, are relatively rare. Only 5% to 10% of breast cancers are considered hereditary; of those, approximately 80% are related to mutations in BRCA1 or BRCA2.10
Several “red flags” suggest the possibility of a hereditary type of breast cancer syndrome. Genetic risk is primarily identified through thorough history taking, which must address both the maternal and paternal sides of the family—over three generations, if possible.10 Table 210-14 identifies these red flags.
Atypical cells identified on breast biopsy also increase breast cancer risk. The presence of ADH or ALH increases a woman’s risk four to five times higher than the average.2 LCIS is associated with a 10-fold increase in breast cancer risk.9 The overall incidence of breast cancer in women with LCIS is estimated at 22.3%.3
Radiation exposure to the chest wall, particularly when administered between ages 10 and 30, also increases the risk for breast cancer. This usually occurs in female patients who have undergone mantle-field radiation treatment for another cancer, such as Hodgkin’s disease or non-Hodgkin’s lymphoma.15,16 Risk associated with this type of treatment can be as great as 12 times the normal risk. Patients treated before or during adolescence appear to be at highest risk.2 Persons exposed to other types of radiation (eg, survivors of atomic weapons) also have increased breast cancer risk.1
Rare genetic syndromes not associated with BRCA1 or BRCA2 have been associated with breast cancer as well. Cowden, Li-Fraumeni, and Peutz-Jeghers syndromes are all known hereditary breast cancer syndromes.4-7 Although these syndromes account for less than 1% of all breast cancers,10 it is important to be able to identify a patient who is at risk for harboring a genetic mutation that causes one of these syndromes—and to understand how to manage a woman who is already affected.
Cowden syndrome is an autosomal dominant disorder caused by a mutation in the PTEN gene.4,17 This syndrome carries a 25% to 50% risk for breast cancer.18 Other findings associated with Cowden syndrome include facial or buccal lesions, fibrocystic breast disease, benign thyroid conditions (eg, goiter), nonmedullary thyroid cancer, endometrial cancer, macrocephaly, uterine fibroids, and gastrointestinal hamartomas.3,10
Li-Fraumeni syndrome is a highly penetrant, autosomal dominant disorder caused by a mutation in the TP53 gene.4,6,19,20 Affected women’s overall cancer risk is 50% by age 35 and 90% lifelong. Breast cancer risk is estimated at 50% by age 50.3 Multiple primary cancers, including early-onset breast cancer (ie, before age 40), sarcoma, leukemia, childhood brain tumors, and adrenocortical carcinoma, are all associated with Li-Fraumeni.6,10,19,21
Peutz-Jeghers syndrome is also an autosomal dominant condition, caused by a mutation in the STK11 gene.3,22,23 The mean age of breast cancer diagnosis in affected women is 44. Hallmark features of Peutz-Jeghers include gastrointestinal hamartomas (often discovered during childhood24) and cancers of the colon, small bowel, pancreas, uterus, thyroid, lung, and breast. The most commonly reported malignancies in patients with Peutz-Jeghers syndrome are breast cancer and colon cancer. Associated phenotypic features include pigmented spots on the lips, buccal mucosa, and skin.10,24
Risk Assessment Tools
Women with any of the identified high-risk factors should be referred to a provider experienced in high-risk breast cancer assessment and management. Many comprehensive breast centers are adding high-risk programs to their array of services. Researching those programs in your area will facilitate the process of referral for women in your practice who are identified as high risk.
In efforts to identify patients at high risk for breast cancer, the importance of thorough history taking cannot be overstated. It is imperative that both the maternal and paternal sides of the family be assessed, as genetic mutations can be acquired through the patient’s mother or father.10
A variety of tools to assess breast cancer risk are available. The one most commonly used is the Gail risk assessment model,25 which was developed at the National Cancer Institute. A modified version, validated during the Breast Cancer Prevention Trial,26 calculates five-year and lifetime risk based on the following criteria:
• Current age
• Age at menarche
• Age at first live birth
• Race
• Number of first-degree relatives with a history of breast cancer
• Number of previous breast biopsies
• History of atypical cells on previous breast biopsy.
The Gail model has certain limitations. It cannot be used to assess women younger than 3527 or older than 85. Also, age at breast cancer diagnosis, non–first-degree relatives with breast cancer, paternal cancer history, ovarian cancer history, and ethnicity are not included among its considerations.3
The Claus model28 assesses risk in women with a family history of breast cancer more accurately than the Gail model, but it does not incorporate male breast cancer, ovarian cancer, or ethnicity.9
Several models are available to predict the risk for carrying a BRCA1 or BRCA2 mutation. The Myriad Genetic Laboratories database,29 the BRCAPRO model,30,31 the Manchester scoring system,32,33 the Tyrer-Duffy-Cuzick (IBIS) model,34 and the BOADICEA model35 all provide probability data, but each has its limitations. Breast specialists who provide high-risk assessment services calculate risk using a variety of models and choosing the most appropriate model(s) for each patient.36,37 Women with a high probability of carrying a mutation, based on history and findings from the risk assessment model, are referred for genetics counseling and testing.
Reducing the Risk
Risk reduction is an integral component of high-risk breast cancer management, and several strategies are currently recommended, including these:
• Exercise for 45 to 60 minutes at least five times per week.2,38 In women who engage in at least five hours of vigorous exercise each week, a 0.62 relative risk for breast cancer has been reported.38,39
• Limit alcohol intake to fewer than one to two drinks per day. A number of studies have documented a 30% to 50% increase in the incidence of breast cancer among women who consume alcohol in greater quantities.38,40
• Avoid obesity. Compared with women who have maintained their weight since age 18, those who gain 55 lb or more by menopause onset have a 1.45 relative risk for breast cancer.38,41
• Breastfeed infants. Lactation for two years or longer decreases the lifetime risk for breast cancer by 50%.3
• Follow a low-fat, high-fiber diet, rich in fresh fruits and vegetables. Increasing fruit and vegetable intake by even one serving per day has been associated with a 9% decrease in breast cancer incidence.38,42
Chemoprevention for At-Risk Women
Another risk reduction strategy for women at particularly high risk for breast cancer is chemoprevention. Currently, two medications, tamoxifen and raloxifene, are FDA approved for breast cancer risk reduction in women at high risk. These agents are offered to women with specific high-risk factors, including:
• Findings of LCIS, ADH, or ALH on previous breast biopsy
• A five-year probability of breast cancer exceeding 1.7%, based on a validated risk assessment model (eg, the Gail model, BRCAPRO)
• Presence of the BRCA1 or BRCA2 mutation.
Tamoxifen is a selective estrogen receptor modulator (SERM) that blocks the effects of estrogen on breast tissue.43 Often referred to as an “antiestrogen,” it is approved for use in both premenopausal and postmenopausal women. In the Breast Cancer Prevention Trial,44 women at high risk for breast cancer who took tamoxifen for five years had about a 50% risk reduction for invasive breast cancer and a 30% risk reduction in noninvasive breast cancer. The usual dose of tamoxifen is 20 mg/d.
Adverse effects associated with tamoxifen use, however, include blood clots, stroke, and uterine cancer. Women should not take tamoxifen if they have a history of cataracts, are current hormone therapy users, are planning a pregnancy or have the potential for becoming pregnant, or have a history of stroke, deep venous thrombosis, or pulmonary embolus. Less serious adverse effects include menopausal symptoms, menstrual irregularities, headache, fatigue, nausea, and skin irritation.43
Raloxifene, though more commonly prescribed to prevent and treat osteoporosis, has been shown to reduce the risk for invasive breast cancer by 56% to 72%, compared with placebo45,46; it exerts estrogenic effects on bone and antiestrogenic effects on breast and endometrial tissue.47 This SERM is approved for breast cancer risk reduction only in postmenopausal women.48 Recommended use is 60 mg/d for five years.
Although raloxifene provides a risk reduction benefit comparable to that of tamoxifen against invasive breast cancer (ie, incidence rates of 4.4 and 4.3, respectively, per 1,000 women per year49), it does not appear to reduce the risk for noninvasive breast cancer (ie, ductal carcinoma in situ).50 Potential major complications attributed to raloxifene use include blood clots, stroke, and uterine cancer, although the risk for uterine cancer is lower than with tamoxifen use.49 Minor adverse effects include leg cramps, menopausal symptoms, edema of the extremities, and flulike symptoms. Contraindications are comparable to those associated with tamoxifen.48
Screening Recommendations
Combined with mammography and breast ultrasound, the use of MRI to screen high-risk women is now being recommended, according to guidelines published in March 2007 by the American Cancer Society.20 Patient factors suggesting greatest benefit from annual MRI screening (combined with mammography) are listed in Table 3.4,20,51-55
In addition to patient-driven reduction strategies and the provider-initiated interventions for surveillance and management, monthly breast self-examination is encouraged, as are clinical breast examinations every six months.
Even with the most aggressive risk reduction program, not all breast cancers can be prevented. A key objective of high-risk screening and management is to identify patients with breast cancer at the earliest possible stage so that a cure is more likely to be achieved.
Conclusion
Identifying and screening women at high risk for breast cancer are essential skills for all primary care providers. Maintaining a comprehensive list of referral sources for high-risk management and genetic counseling services in your area will allow you to partner with other professionals to provide patients with the best possible care. Women feel empowered by education, particularly the newly acquired knowledge about breast cancer risk reduction—and reassured, knowing that their providers are interested and well informed in this complex area of women’s health.
1. National Cancer Institute. Genetics of breast and ovarian cancer (2008). www.cancer.gov/cancertopics/pdq/genetics/breast-and-ovarian. Accessed September 23, 2008.
2. American Cancer Society. Detailed Guide: Breast Cancer. What are the risk factors for breast cancer? 2007. www.cancer.org/docroot/CRI/content/CRI_2_4_2X_What_are_the_risk_factors_for_breast_cancer_5.asp. Accessed September 23, 2008.
3. Vogel V. Handbook of Breast Cancer Risk Assessment. Sudbury, MA: Jones and Bartlett; 2004.
4. Garber JE, Offit K. Hereditary cancer predisposition syndromes. J Clin Oncol. 2005;23(2):276-292.
5. Kelly P. Hereditary breast cancer considering Cowden syndrome: a case study. Cancer Nurs. 2003;26(5):370-375.
6. Li FP, Fraumeni JF Jr. Soft-tissue sarcomas, breast cancer, and other neoplasms: a familial syndrome? Ann Intern Med. 1969;71(4):747-752.
7. Giardiello FM, Welsh SB, Hamilton SR, et al. Increased risk of cancer in the Peutz-Jeghers syndrome. N Engl J Med. 1987;316(24):1511-1514.
8. Myriad Genetic Laboratories. Making Informed Decisions: Testing and Management for Hereditary Breast and Ovarian Cancer [video]. www.myriadtests.com/breast-cancer-patient-video.htm. Accessed September 23, 2008.
9. Korde LA, Calzone KA, Zujewski J. Assessing breast cancer risk: genetic factors are not the whole story. Postgrad Med. 2004;116(4):6-8, 11-14, 19-20.
10. Thull DL, Vogel VG. Recognition and management of hereditary breast cancer syndromes. Oncologist. 2004;9(1):13-24.
11. Breast Cancer Linkage Consortium. Cancer risks in BRCA2 mutation carriers. J Natl Cancer Inst. 1999;91(15):1310-1316.
12. Krainer M, Silva-Arrieta S, FitzGerald MG, et al. Differential contributions of BRCA1 and BRCA2 to early-onset breast cancer. N Engl J Med. 1997;336(20):1416-1421.
13. FitzGerald MG, MacDonald DH, Krainer M, et al. Germ-line BRCA1 mutations in Jewish and non-Jewish women with early-onset breast cancer. N Engl J Med. 1996;334(3):143-149.
14. Malone KE, Daling JR, Neal C, et al. Frequency of BRCA1/BRCA2 mutations in a population-based sample of young breast carcinoma cases. Cancer. 2000;88(6):1393-1402.
15. Travis LB, Hill D, Dores GM, et al. Cumulative absolute breast cancer risk for young women treated for Hodgkin lymphoma. J Natl Cancer Inst. 2005;97(19):1428-1437.
16. Longo DL. Radiation therapy in Hodgkin disease: why risk a Pyrrhic victory? [editorial]. J Natl Cancer Inst. 2005;97(19): 1394-1395.
17. Nelen MR, Padberg GW, Peeters EA, et al. Localization of the gene for Cowden disease to chromosome 10q22-23. Nat Genet. 1996;13(1):114-116.
18. Eng C. Genetics of Cowden syndrome: through the looking glass of oncology. Int J Oncol. 1998;12(3):701-710.
19. Li FP, Fraumeni JF Jr, Mulvihill JJ, et al. A cancer family syndrome in twenty-four kindreds. Cancer Res. 1988;48(18):5358-5362.
20. Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75-89.
21. Malkin D, Li FP, Strong LC, et al. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science. 1990;250(4985):1233-1238.
22. Schumacher V, Vogel T, Leube B, et al. STK11 genotyping and cancer risk in Peutz-Jeghers syndrome. J Med Genet. 2005;42(5):428-435.
23. Boardman LA, Thibodeau SN, Schaid DJ, et al. Increased risk for cancer in patients with the Peutz-Jeghers syndrome. Ann Intern Med. 1998;128(11):896-899.
24. Vasen HF. Clinical diagnosis and management of hereditary colorectal cancer syndromes. J Clin Oncol. 2000;18(21 Suppl):81S-92S.
25. Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81(24):1879-1886.
26. Costantino JP, Gail MH, Pee D, et al. Validation studies for models projecting the risk of invasive and total breast cancer incidence. J Natl Cancer Inst. 1999;91(18):1541-1548.
27. Rockhill B, Spiegelman D, Byrne C, et al. Validation of the Gail et al. model of breast cancer risk prediction and implications for chemoprevention. J Natl Cancer Inst. 2001;93(5): 358-366.
28. Claus EB, Risch N, Thompson WD. Autosomal dominant inheritance of early-onset breast cancer: implications for risk prediction. Cancer. 1994;73(3):643-651.
29. Easton DF, Deffenbaugh AM, Pruss D, et al. A systematic genetic assessment of 1,433 sequence variants of unknown clinical significance in the BRCA1 and BRCA2 breast cancer–predisposition genes. Am J Hum Genet. 2007;81(5):873-883.
30. Parmigiani G, Berry D, Aguilar O. Determining carrier probabilities for breast cancer–susceptibility genes BRCA1 and BRCA2. Am J Hum Genet. 1998;62(1):145-158.
31. Berry DA, Iversen ES Jr, Gudbjartsson DF, et al. BRCAPRO validation, sensitivity of genetic testing of BRCA1/BRCA2, and prevalence of other breast cancer susceptibility genes. J Clin Oncol. 2002;20(11):2701-2712.
32. Evans DG, Eccles DM, Rahman N, et al. A new scoring system for the chances of identifying a BRCA1/2 mutation outperforms existing models including BRCAPRO. J Med Genet. 2004;41(6):474-480.
33. Evans DG, Lalloo F, Wallace A, Rahman N. Update on the Manchester Scoring System for BRCA1 and BRCA2 testing.
J Med Genet. 2005;42(7):e39.
34. Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23(7):1111-1130.
35. Antoniou AC, Pharaoh PP, Smith P, Easton DF. The BOADICEA model of genetic susceptibility to breast and ovarian cancer. Br J Cancer. 2004;91(8):1580-1590.
36. Antoniou AC, Hardy R, Walker L, et al. Predicting the likelihood of carrying a BRCA1 or BRCA2 mutation: validation of BOADICEA, BRCAPRO, IBIS, Myriad and the Manchester scoring system using data from UK genetics clinics. J Med Genet. 2008;45(7):425-431.
37. Thirthagiri E, Lee SY, Kang P, et al. Evaluation of BRCA1 and BRCA2 mutations and risk-prediction models in a typical Asian country (Malaysia) with a relatively low incidence of breast cancer. Breast Cancer Res. 2008;10(4):R59.
38. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology.™ Breast Cancer Risk Reduction. V.I.2008. www.nccn.org/professionals/physician_gls/PDF/breast_risk.pdf. Accessed September 23, 2008.
39. Tehard B, Friedenreich CM, Oppert JM, Clavel-Chapelon F. Effect of physical activity on women at increased risk of breast cancer: results from the E3N cohort study. Cancer Epidemiol Biomarkers Prev. 2006;15(1):57-64.
40. Terry MB, Zhang FF, Kabat G, et al. Lifetime alcohol intake and breast cancer risk. Ann Epidemiol. 2006;16(3):230-240.
41. Eliassen AH, Colditz GA, Rosner B, et al. Adult weight change and risk of postmenopausal breast cancer. JAMA. 2006;296(2):193-201.
42. Prentice RL, Caan B, Chlebowski RT, et al. Low-fat dietary pattern and risk of invasive breast cancer: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295(6):629-642.
43. US Food and Drug Administration, Center for Drug Evaluation and Research. Tamoxifen information. www.fda.gov/cder/news/tamoxifen/default.htm. Accessed September 23, 2008.
44. Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90(18):1371-1388.
45. Cauley JA, Norton L, Lippman ME, et al. Continued breast cancer risk reduction in postmenopausal women treated with raloxifene: 4-year results from the MORE trial: Multiple Outcomes of Raloxifene Evaluation. Breast Cancer Res Treat. 2001;65(2):125-134.
46. Martino S, Cauley JA, Barrett-Connor E, et al; CORE In—vestigators. Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst. 2004;96(23):1751-1761.
47. Cummings SR, Eckert S, Krueger KA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial: Multiple Outcomes of Raloxifene Evaluation [erratum in: JAMA. 1999;282(22):2124]. JAMA. 1999;281(23):2189-2197.
48. US Food and Drug Administration, Center for Drug Evaluation and Research. FDA approves new uses for Evista (raloxifene hydrochloride). www.fda.gov/cder/Offices/OODP/whatsnew/raloxifene.htm. Accessed September 23, 2008.
49. Vogel VG, Costantino JP, Wickerham DL, et al; National Surgical Adjuvant Breast and Bowel Project (NSABP). Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295(23):2727-2741.
50. Grady D, Cauley JA, Geiger MJ, et al; Raloxifene Use for the Heart Trial Investigators. Reduced incidence of invasive breast cancer with raloxifene among women at increased coronary risk. J Natl Cancer Inst. 2008;100(12):854-861.
51. Kriege M, Brekelmans CT, Boetes C, et al; Magnetic Resonance Imaging Screening Study Group. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351(5):427-437.
52. Leach MO, Boggis CR, Dixon AK, et al; MARIBS Study Group. Screening with magnetic resonance imaging and mammography of a UK population at high familial risk of breast cancer: a prospective multicentre cohort study (MARIBS). Lancet. 2005;365(9473):1769-1778.
53. Lehman CD, Blume JD, Weatherall P, et al; International Breast MRI Consortium Working Group. Screening women at high risk for breast cancer with mammography and magnetic resonance imaging. Cancer. 2005;103(9):1898-1905.
54. Sardanelli F, Podo F. Breast MR imaging in women at high-risk of breast cancer: is something changing in early breast cancer detection? Eur Radiol. 2007;17(4):873-887.
55. Warner E, Plewes DB, Hill KA, et al. Surveillance of BRCA1 and BRCA2 mutation carriers with magnetic resonance imaging, ultrasound, mammography, and clinical breast examination. JAMA. 2004;292(11):1317-1325.
1. National Cancer Institute. Genetics of breast and ovarian cancer (2008). www.cancer.gov/cancertopics/pdq/genetics/breast-and-ovarian. Accessed September 23, 2008.
2. American Cancer Society. Detailed Guide: Breast Cancer. What are the risk factors for breast cancer? 2007. www.cancer.org/docroot/CRI/content/CRI_2_4_2X_What_are_the_risk_factors_for_breast_cancer_5.asp. Accessed September 23, 2008.
3. Vogel V. Handbook of Breast Cancer Risk Assessment. Sudbury, MA: Jones and Bartlett; 2004.
4. Garber JE, Offit K. Hereditary cancer predisposition syndromes. J Clin Oncol. 2005;23(2):276-292.
5. Kelly P. Hereditary breast cancer considering Cowden syndrome: a case study. Cancer Nurs. 2003;26(5):370-375.
6. Li FP, Fraumeni JF Jr. Soft-tissue sarcomas, breast cancer, and other neoplasms: a familial syndrome? Ann Intern Med. 1969;71(4):747-752.
7. Giardiello FM, Welsh SB, Hamilton SR, et al. Increased risk of cancer in the Peutz-Jeghers syndrome. N Engl J Med. 1987;316(24):1511-1514.
8. Myriad Genetic Laboratories. Making Informed Decisions: Testing and Management for Hereditary Breast and Ovarian Cancer [video]. www.myriadtests.com/breast-cancer-patient-video.htm. Accessed September 23, 2008.
9. Korde LA, Calzone KA, Zujewski J. Assessing breast cancer risk: genetic factors are not the whole story. Postgrad Med. 2004;116(4):6-8, 11-14, 19-20.
10. Thull DL, Vogel VG. Recognition and management of hereditary breast cancer syndromes. Oncologist. 2004;9(1):13-24.
11. Breast Cancer Linkage Consortium. Cancer risks in BRCA2 mutation carriers. J Natl Cancer Inst. 1999;91(15):1310-1316.
12. Krainer M, Silva-Arrieta S, FitzGerald MG, et al. Differential contributions of BRCA1 and BRCA2 to early-onset breast cancer. N Engl J Med. 1997;336(20):1416-1421.
13. FitzGerald MG, MacDonald DH, Krainer M, et al. Germ-line BRCA1 mutations in Jewish and non-Jewish women with early-onset breast cancer. N Engl J Med. 1996;334(3):143-149.
14. Malone KE, Daling JR, Neal C, et al. Frequency of BRCA1/BRCA2 mutations in a population-based sample of young breast carcinoma cases. Cancer. 2000;88(6):1393-1402.
15. Travis LB, Hill D, Dores GM, et al. Cumulative absolute breast cancer risk for young women treated for Hodgkin lymphoma. J Natl Cancer Inst. 2005;97(19):1428-1437.
16. Longo DL. Radiation therapy in Hodgkin disease: why risk a Pyrrhic victory? [editorial]. J Natl Cancer Inst. 2005;97(19): 1394-1395.
17. Nelen MR, Padberg GW, Peeters EA, et al. Localization of the gene for Cowden disease to chromosome 10q22-23. Nat Genet. 1996;13(1):114-116.
18. Eng C. Genetics of Cowden syndrome: through the looking glass of oncology. Int J Oncol. 1998;12(3):701-710.
19. Li FP, Fraumeni JF Jr, Mulvihill JJ, et al. A cancer family syndrome in twenty-four kindreds. Cancer Res. 1988;48(18):5358-5362.
20. Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75-89.
21. Malkin D, Li FP, Strong LC, et al. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science. 1990;250(4985):1233-1238.
22. Schumacher V, Vogel T, Leube B, et al. STK11 genotyping and cancer risk in Peutz-Jeghers syndrome. J Med Genet. 2005;42(5):428-435.
23. Boardman LA, Thibodeau SN, Schaid DJ, et al. Increased risk for cancer in patients with the Peutz-Jeghers syndrome. Ann Intern Med. 1998;128(11):896-899.
24. Vasen HF. Clinical diagnosis and management of hereditary colorectal cancer syndromes. J Clin Oncol. 2000;18(21 Suppl):81S-92S.
25. Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81(24):1879-1886.
26. Costantino JP, Gail MH, Pee D, et al. Validation studies for models projecting the risk of invasive and total breast cancer incidence. J Natl Cancer Inst. 1999;91(18):1541-1548.
27. Rockhill B, Spiegelman D, Byrne C, et al. Validation of the Gail et al. model of breast cancer risk prediction and implications for chemoprevention. J Natl Cancer Inst. 2001;93(5): 358-366.
28. Claus EB, Risch N, Thompson WD. Autosomal dominant inheritance of early-onset breast cancer: implications for risk prediction. Cancer. 1994;73(3):643-651.
29. Easton DF, Deffenbaugh AM, Pruss D, et al. A systematic genetic assessment of 1,433 sequence variants of unknown clinical significance in the BRCA1 and BRCA2 breast cancer–predisposition genes. Am J Hum Genet. 2007;81(5):873-883.
30. Parmigiani G, Berry D, Aguilar O. Determining carrier probabilities for breast cancer–susceptibility genes BRCA1 and BRCA2. Am J Hum Genet. 1998;62(1):145-158.
31. Berry DA, Iversen ES Jr, Gudbjartsson DF, et al. BRCAPRO validation, sensitivity of genetic testing of BRCA1/BRCA2, and prevalence of other breast cancer susceptibility genes. J Clin Oncol. 2002;20(11):2701-2712.
32. Evans DG, Eccles DM, Rahman N, et al. A new scoring system for the chances of identifying a BRCA1/2 mutation outperforms existing models including BRCAPRO. J Med Genet. 2004;41(6):474-480.
33. Evans DG, Lalloo F, Wallace A, Rahman N. Update on the Manchester Scoring System for BRCA1 and BRCA2 testing.
J Med Genet. 2005;42(7):e39.
34. Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23(7):1111-1130.
35. Antoniou AC, Pharaoh PP, Smith P, Easton DF. The BOADICEA model of genetic susceptibility to breast and ovarian cancer. Br J Cancer. 2004;91(8):1580-1590.
36. Antoniou AC, Hardy R, Walker L, et al. Predicting the likelihood of carrying a BRCA1 or BRCA2 mutation: validation of BOADICEA, BRCAPRO, IBIS, Myriad and the Manchester scoring system using data from UK genetics clinics. J Med Genet. 2008;45(7):425-431.
37. Thirthagiri E, Lee SY, Kang P, et al. Evaluation of BRCA1 and BRCA2 mutations and risk-prediction models in a typical Asian country (Malaysia) with a relatively low incidence of breast cancer. Breast Cancer Res. 2008;10(4):R59.
38. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology.™ Breast Cancer Risk Reduction. V.I.2008. www.nccn.org/professionals/physician_gls/PDF/breast_risk.pdf. Accessed September 23, 2008.
39. Tehard B, Friedenreich CM, Oppert JM, Clavel-Chapelon F. Effect of physical activity on women at increased risk of breast cancer: results from the E3N cohort study. Cancer Epidemiol Biomarkers Prev. 2006;15(1):57-64.
40. Terry MB, Zhang FF, Kabat G, et al. Lifetime alcohol intake and breast cancer risk. Ann Epidemiol. 2006;16(3):230-240.
41. Eliassen AH, Colditz GA, Rosner B, et al. Adult weight change and risk of postmenopausal breast cancer. JAMA. 2006;296(2):193-201.
42. Prentice RL, Caan B, Chlebowski RT, et al. Low-fat dietary pattern and risk of invasive breast cancer: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295(6):629-642.
43. US Food and Drug Administration, Center for Drug Evaluation and Research. Tamoxifen information. www.fda.gov/cder/news/tamoxifen/default.htm. Accessed September 23, 2008.
44. Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90(18):1371-1388.
45. Cauley JA, Norton L, Lippman ME, et al. Continued breast cancer risk reduction in postmenopausal women treated with raloxifene: 4-year results from the MORE trial: Multiple Outcomes of Raloxifene Evaluation. Breast Cancer Res Treat. 2001;65(2):125-134.
46. Martino S, Cauley JA, Barrett-Connor E, et al; CORE In—vestigators. Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst. 2004;96(23):1751-1761.
47. Cummings SR, Eckert S, Krueger KA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial: Multiple Outcomes of Raloxifene Evaluation [erratum in: JAMA. 1999;282(22):2124]. JAMA. 1999;281(23):2189-2197.
48. US Food and Drug Administration, Center for Drug Evaluation and Research. FDA approves new uses for Evista (raloxifene hydrochloride). www.fda.gov/cder/Offices/OODP/whatsnew/raloxifene.htm. Accessed September 23, 2008.
49. Vogel VG, Costantino JP, Wickerham DL, et al; National Surgical Adjuvant Breast and Bowel Project (NSABP). Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295(23):2727-2741.
50. Grady D, Cauley JA, Geiger MJ, et al; Raloxifene Use for the Heart Trial Investigators. Reduced incidence of invasive breast cancer with raloxifene among women at increased coronary risk. J Natl Cancer Inst. 2008;100(12):854-861.
51. Kriege M, Brekelmans CT, Boetes C, et al; Magnetic Resonance Imaging Screening Study Group. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351(5):427-437.
52. Leach MO, Boggis CR, Dixon AK, et al; MARIBS Study Group. Screening with magnetic resonance imaging and mammography of a UK population at high familial risk of breast cancer: a prospective multicentre cohort study (MARIBS). Lancet. 2005;365(9473):1769-1778.
53. Lehman CD, Blume JD, Weatherall P, et al; International Breast MRI Consortium Working Group. Screening women at high risk for breast cancer with mammography and magnetic resonance imaging. Cancer. 2005;103(9):1898-1905.
54. Sardanelli F, Podo F. Breast MR imaging in women at high-risk of breast cancer: is something changing in early breast cancer detection? Eur Radiol. 2007;17(4):873-887.
55. Warner E, Plewes DB, Hill KA, et al. Surveillance of BRCA1 and BRCA2 mutation carriers with magnetic resonance imaging, ultrasound, mammography, and clinical breast examination. JAMA. 2004;292(11):1317-1325.
Reconstruction of the Failed Acetabular Component Using Cemented Shells and Impaction Grafting in Revision Hip Arthroplasty
Update on pelvic surgery
The authors report no financial relationships relevant to this article.
Over the past 10 years, the midurethral sling has replaced the Burch urethropexy as the most common surgical procedure for correcting stress urinary incontinence (SUI). In this “Update” on midurethral slings, we highlight three recently published studies that compare popular surgical approaches to SUI:
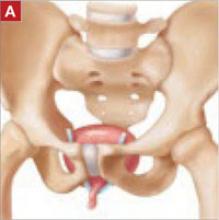
- the original tension-free vaginal tape (TVT) technique (FIGURE [“A”])
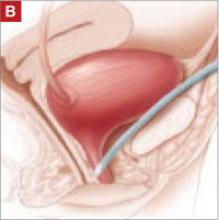
- the suprapubic urethral support sling (SPARC) (FIGURE [“B”])
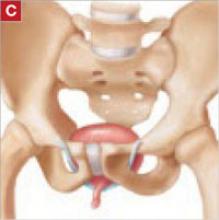
- the transobturator tape (TOT) technique (FIGURE [“C”])
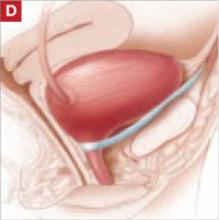
- the traditional pubovaginal sling (PVS), placed at the bladder neck (FIGURE [“D”]).
FIGURE [“A”] Four options for a midurethral sling to correct stress urinary incontinence: Tension-free vaginal tape (TVT) technique
FIGURE [“B”] Four options for a midurethral sling to correct stress urinary incontinence: Suprapubic urethral support sling (SPARC)
FIGURE [“C”] Four options for a midurethral sling to correct stress urinary incontinence: Transobturator tape (TOT) technique
FIGURE [“D”] Four options for a midurethral sling to correct stress urinary incontinence: Pubovaginal sling (PVS)
We’ve had a decade-plus of experience with the sling
The midurethral sling, first introduced as the tension-free vaginal tape, or TVT (Gynecare), was quick to be adopted because:
- it offers a minimally invasive approach
- it is highly efficacious
- serious adverse events are rare.
TVT utilizes a 5-mm trocar that is passed from the vagina through the retropubic space, exiting via small suprapubic incisions. A strip of permanent polypropylene mesh attached to these trocars is placed under the midportion of the urethra (FIGURE [“A”]).
We now have 11 years of follow-up data to support the use of the TVT midurethral sling for SUI.1
As TVT gained popularity, surgical equipment manufacturers developed various “kits,” so to speak, for placing a midurethral sling. Many have included innovations that have theoretical advantages over traditional TVT. Some place smaller, 3-mm trocars in a similar “bottom-up” fashion, as the TVT sling does; others utilize smaller trocars that are placed “top down” through the retropubic space into the vagina.
A later generation of slings uses the transobturator approach, to avoid blind passage of trocars through the retropubic space. These slings can be placed “in to out” or “out to in,” and rest in a slightly different orientation under the midurethra.
In an effort to make the procedure even more minimally invasive, some manufacturers now offer slings that are placed through one vaginal incision, thereby avoiding additional suprapubic or groin incisions. Other kits have made alterations to the polypropylene mesh by heat-sealing the material or applying a coating.
Such modifications haven’t always been improvements—some sling kits carried a higher incidence of mesh-related complications, and certain ones were removed from the market. And, although the number of commercially available midurethral sling kits has exploded, we’ve seen scant data published that compare the traditional TVT method with alternative approaches. Those alternatives may be considered midurethral slings, but we haven’t known whether minor variations in technique, or in the instrumentation, translate to improvements in long-term efficacy.
More readjustments for retention are needed after SPARC (vs. TVT)
Lord HE, Taylor JD, Finn JC, et al. A randomized controlled equivalence trial of short-term complications and efficacy of tension-free vaginal tape and suprapubic urethral support sling for treating stress incontinence. BJU Int. 2006;98:367–376.
This randomized, controlled trial compared TVT with SPARC to treat SUI. The study was designed as an equivalence trial: the investigators sought to determine if the “newer” intervention of the two (SPARC) is therapeutically equivalent to the existing intervention (TVT)—not whether one is superior. They therefore looked to see if patients who underwent TVT and those who underwent SPARC had the same rate (within a 5% margin) of bladder injury and other secondary outcomes.
Subjects were eligible to participate if they had SUI on the basis of urodynamic or clinical parameters. They were unaware of their assigned treatment, underwent TVT or SPARC, and were reevaluated 6 weeks postoperatively. Intraoperative, postoperative, and 6-week follow-up data were recorded by the study surgeon.
Three hundred and one patients were enrolled; 147 underwent TVT and 154 underwent SPARC. The groups were similar in regard to all baseline characteristics.
No significant difference was noted between the groups in the primary outcome, which was the rate of bladder perforation (TVT, 0.7%; SPARC, 1.9% [p=.62]). This effect remained after controlling for age, parity, prior urinary incontinence surgery, other concomitant surgery, and the surgeon’s level of experience. There were no intergroup differences in perioperative blood loss, urgency, or objective cure of SUI (defined as negative cough stress test) 6 weeks after surgery.
Subjects who underwent SPARC were more likely to experience urinary retention that required surgical readjustment of the sling (SPARC, 10 of 154; TVT, none [p=.002]). Although the objective cure rate was similar across groups, the subjective cure rate was significantly different (TVT, 87.1%; SPARC, 76.5% [p=.03]).
Regression analysis revealed that subjects who had prior surgery for urinary incontinence and those whose surgery was performed by a comparatively less experienced physician were more likely to report persistence of SUI symptoms.
This study reflects general clinical practice, in that it was conducted across a heterogeneous sample of subjects who had both primary and recurrent stress incontinence. Although the rate of bladder perforation was equivalent across groups, more patients who underwent SPARC required loosening of the sling postoperatively to relieve urinary retention.
These data suggest that the SPARC sling may be more difficult to adjust correctly even though it is designed with a tensioning suture. The difficulty may be a consequence of 1) smaller-caliber trocar tunnels or 2) the “top-down” approach less accurately locating the sling at the midportion of the urethra.
This study would have been more rigorous and the results, stronger, if postoperative assessment was made by a blinded examiner. An exceptional positive aspect of study design was that the investigators considered the surgeon’s level of experience—a variable that can certainly affect outcome.
Barber MD, Kleeman S, Karram MM, et al. Transobturator tape compared with tension-free vaginal tape for the treatment of stress urinary incontinence. Obstet Gynecol. 2008;111:611–621.
This randomized, controlled trial compared the efficacy of TVT with the transobturator tape (TOT) technique. Like Lord and colleagues’ study just discussed, it was conducted as an equivalence trial—to determine whether TOT is equivalent to TVT.
The primary outcome was abnormal bladder function 12 months after surgery, defined as the presence of any of the following:
- incontinence symptoms
- positive cough stress test
- retreatment for SUI
- treatment for postoperative urinary retention.
Women who had urodynamic stress incontinence were recruited from three academic centers; excluded were women who had detrusor overactivity, postvoid residual volume >100 mL, prior sling surgery, or contraindications to a midurethral sling.
For the retropubic approach, TVT was used. For the transobturator approach, the Monarc Subfascial Hammock (American Medical Systems) was used. Here, the tape is placed in an “outside-in” fashion.
Subjects completed a baseline bladder diary and a series of validated questionnaires. Postoperatively, subjects were followed for 2 years. Follow-up data included validated questionnaires, bladder diary, pelvic organ prolapse quantification, cough stress test, and postvoid residual volume determination. It was not possible to blind subjects or surgeons, but all postoperative assessments and exams were performed by a blinded nurse.
The investigators sought to determine if TVT and TOT yielded an equivalent (within a 15% margin) rate of abnormal bladder function.
Eventually, 170 patients underwent randomization and surgery (88, TVT; 82, TOT). Baseline demographic, clinical, and incontinence severity data were similar across groups.
Bladder perforation was more common with TVT than with TOT (7% and 0, respectively [p=.02]). Abnormal bladder function was noted in 46.6% of TVT subjects and in 42.7% of TOT subjects, with a noninferiority test demonstrating equivalence (p=.006). One year after surgery, 79% of patients in the TVT group and 82% of patients in the TOT group reported that bladder symptoms were “much better” or “very much better” (p=.88). No significant difference was noted between groups in any of the questionnaire responses after surgery.
This study has many strengths, including rigorous assessments, use of a blinded nurse-examiner to collect postoperative data, and a battery of validated questionnaires used throughout the study. In addition, the primary outcome measure, abnormal bladder function, is defined by stringent criteria that combine subjective and objective components, efficacy, and adverse events.
It will be interesting to see if the efficacy of TOT is maintained over time. The authors of the article point out that several transobturator sling kits are available, utilizing various trocar shapes, different approaches (i.e., “in to out”), and different types of mesh; this may mean variable rates of complications and different degrees of efficacy from one kit to the next.
Also notable in this study is that subjects had relatively high Valsalva leak-point pressures (approaching 100 cm H2O) in both groups.
Which technique is best for SUI with intrinsic sphincter deficiency?
Jeon MJ, Jung HJ, Chung SM, et al. Comparison of the treatment outcome of pubovaginal sling, tension-free vaginal tape, and transobturator tape for stress urinary incontinence with intrinsic sphincter deficiency. Am J Obstet Gynecol. 2008;199:76.e1–76.e4.
This retrospective cohort study was designed to evaluate techniques for treating severe SUI. Researchers were mainly interested in patients who had intrinsic sphincter deficiency (ISD), defined as a Valsalva leak-point pressure <60 cm H2O or maximal urethral closure pressure <20 cm H2O.
The pubovaginal (bladder neck) sling (PVS) has been considered the gold standard therapeutic option for patients who have ISD. Recently, however, data have shown satisfactory outcomes using TVT in this setting.2,3 The aim of this study, therefore, was to compare PVS, TVT, and TOT for treating SUI in patients who had ISD. (Note: The researchers used Uratape [Mentor-Purgès] for the transobturator sling.)
The study included 253 subjects who had ISD and who underwent surgical intervention (87, PVS; 94, TVT; 92, TOT); women who had detrusor overactivity and voiding dysfunction were excluded. Follow-up assessments were performed at 1, 3, 6, and 12 months and annually thereafter. Outcomes studied included complications and rates of cure; the latter was defined as 1) the absence of subjective complaints of leakage and 2) a negative cough stress test.
Median follow-up was 36, 24, and 12 months in the PVS, TVT, and TOT groups, respectively. All groups were similar in regard to baseline clinical and demographic characteristics. Bladder perforation was rare (PVS, 1; TVT and TOT, 0). No significant difference was noted across techniques in the rate of de novo urgency, voiding dysfunction, reoperation for urinary retention, and recurrent urinary tract infection.
Two years after surgery, the cure rate for the three procedures differed significantly: PVS and TVT, 87% each; TOT, 35% (p<.0001). A Cox proportional hazards regression model revealed that the risk of treatment failure with PVS was no different than it was for TVT. However, this model demonstrated that the risk of failure was 4.6 times higher for TOT compared with PVS (p<.0001).
This study is subject to the limitations of any retrospective study. It is unique, however, in that investigators focused on a more severe sample of subjects with ISD. In addition, the authors of the study used the appropriate statistical techniques to attempt to control for potential confounders.
Although the rate of cure was higher with TVT than with TOT, the rate of voiding dysfunction (i.e., the need for catheterization longer than 1 month after surgery) and de novo urgency was higher with TVT as well. This finding suggests that TVT provides more compressive force around the urethra than TOT does; on the other hand, it is possible instead that the difference arises in the method of tensioning of various types of sling.
Last, the study surgeon conducted the postoperative evaluations and was not blinded. This may have introduced bias into the assessments.
As more long-term data become available about different approaches to placing a midurethral sling, it’s likely that we will learn that not all techniques are equal. A customized approach—one that takes into account the individual patient’s clinical parameters—may be necessary to yield long-term efficacy with a sling.
Although, as the authors of this Update discuss, there are several surgical approaches to stress urinary incontinence (tension-free vaginal tape, suprapubic urethral support sling, transobturator tape, pubovaginal sling placed at the bladder neck), coding for the procedure is limited to a single Current Procedural Terminology (CPT) code when surgery is performed via a vaginal approach. CPT code 57288 ( Sling operation for stress incontinence [e.g., fascia or synthetic] ) has been assigned 21.59 relative value units in 2008 and should be reported no matter what type of sling is placed or what method is used to place it.
Failed placement
On occasion, sling material erodes or creates other problems for the patient, such that it must be removed or revised. To report correction of this adverse outcome, bill with 57287 (Removal or revision of sling for stress incontinence [e.g., fascia or synthetic]). If revision must be performed within the global period for the original procedure by the surgeon who placed the sling, append modifier -78 (Unplanned return to the operating/procedure room by the same physician following initial procedure for a related procedure during the postoperative period) to the revision code.
Minimally invasive placement
If you perform a sling procedure laparoscopically, report 51992 (Laparoscopy, surgical; sling operation for stress incontinence [e.g., fascia or synthetic]) instead. No corresponding code exists for laparoscopic revision of a sling procedure; under CPT rules, your only course is to report 51999 (Unlisted laparoscopy procedure, bladder).—MELANIE WITT, RN, CPC-OBGYN, MA
1. Nilsson CG, Palva K, Rezapour M, Falconer C. Eleven years prospective follow-up of the tension-free vaginal tape procedure for treatment of stress urinary incontinence. Int Urygynecol J Pelvic Floor Dysfunct. 2008;19:1043-1047.
2. Rezapour M, Falconer C, Ulmsten U. Tension-free vaginal tape (TVT) in stress incontinent women with intrinsic sphincter deficiency (ISD)—a long-term follow-up. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12(Suppl 2):S12-S14.
3. Meschia M, Pifarotti P, Buonaguidi A, Gattei U, Spennacchio M. Tension-free vaginal tape (TVT) for treatment of stress urinary incontinence in women with low-pressure urethra. Eur J Obstet Gynecol Reprod Biol. 2005;122:118-121.
The authors report no financial relationships relevant to this article.
Over the past 10 years, the midurethral sling has replaced the Burch urethropexy as the most common surgical procedure for correcting stress urinary incontinence (SUI). In this “Update” on midurethral slings, we highlight three recently published studies that compare popular surgical approaches to SUI:
- the original tension-free vaginal tape (TVT) technique (FIGURE [“A”])
- the suprapubic urethral support sling (SPARC) (FIGURE [“B”])
- the transobturator tape (TOT) technique (FIGURE [“C”])
- the traditional pubovaginal sling (PVS), placed at the bladder neck (FIGURE [“D”]).
FIGURE [“A”] Four options for a midurethral sling to correct stress urinary incontinence: Tension-free vaginal tape (TVT) technique
FIGURE [“B”] Four options for a midurethral sling to correct stress urinary incontinence: Suprapubic urethral support sling (SPARC)
FIGURE [“C”] Four options for a midurethral sling to correct stress urinary incontinence: Transobturator tape (TOT) technique
FIGURE [“D”] Four options for a midurethral sling to correct stress urinary incontinence: Pubovaginal sling (PVS)
We’ve had a decade-plus of experience with the sling
The midurethral sling, first introduced as the tension-free vaginal tape, or TVT (Gynecare), was quick to be adopted because:
- it offers a minimally invasive approach
- it is highly efficacious
- serious adverse events are rare.
TVT utilizes a 5-mm trocar that is passed from the vagina through the retropubic space, exiting via small suprapubic incisions. A strip of permanent polypropylene mesh attached to these trocars is placed under the midportion of the urethra (FIGURE [“A”]).
We now have 11 years of follow-up data to support the use of the TVT midurethral sling for SUI.1
As TVT gained popularity, surgical equipment manufacturers developed various “kits,” so to speak, for placing a midurethral sling. Many have included innovations that have theoretical advantages over traditional TVT. Some place smaller, 3-mm trocars in a similar “bottom-up” fashion, as the TVT sling does; others utilize smaller trocars that are placed “top down” through the retropubic space into the vagina.
A later generation of slings uses the transobturator approach, to avoid blind passage of trocars through the retropubic space. These slings can be placed “in to out” or “out to in,” and rest in a slightly different orientation under the midurethra.
In an effort to make the procedure even more minimally invasive, some manufacturers now offer slings that are placed through one vaginal incision, thereby avoiding additional suprapubic or groin incisions. Other kits have made alterations to the polypropylene mesh by heat-sealing the material or applying a coating.
Such modifications haven’t always been improvements—some sling kits carried a higher incidence of mesh-related complications, and certain ones were removed from the market. And, although the number of commercially available midurethral sling kits has exploded, we’ve seen scant data published that compare the traditional TVT method with alternative approaches. Those alternatives may be considered midurethral slings, but we haven’t known whether minor variations in technique, or in the instrumentation, translate to improvements in long-term efficacy.
More readjustments for retention are needed after SPARC (vs. TVT)
Lord HE, Taylor JD, Finn JC, et al. A randomized controlled equivalence trial of short-term complications and efficacy of tension-free vaginal tape and suprapubic urethral support sling for treating stress incontinence. BJU Int. 2006;98:367–376.
This randomized, controlled trial compared TVT with SPARC to treat SUI. The study was designed as an equivalence trial: the investigators sought to determine if the “newer” intervention of the two (SPARC) is therapeutically equivalent to the existing intervention (TVT)—not whether one is superior. They therefore looked to see if patients who underwent TVT and those who underwent SPARC had the same rate (within a 5% margin) of bladder injury and other secondary outcomes.
Subjects were eligible to participate if they had SUI on the basis of urodynamic or clinical parameters. They were unaware of their assigned treatment, underwent TVT or SPARC, and were reevaluated 6 weeks postoperatively. Intraoperative, postoperative, and 6-week follow-up data were recorded by the study surgeon.
Three hundred and one patients were enrolled; 147 underwent TVT and 154 underwent SPARC. The groups were similar in regard to all baseline characteristics.
No significant difference was noted between the groups in the primary outcome, which was the rate of bladder perforation (TVT, 0.7%; SPARC, 1.9% [p=.62]). This effect remained after controlling for age, parity, prior urinary incontinence surgery, other concomitant surgery, and the surgeon’s level of experience. There were no intergroup differences in perioperative blood loss, urgency, or objective cure of SUI (defined as negative cough stress test) 6 weeks after surgery.
Subjects who underwent SPARC were more likely to experience urinary retention that required surgical readjustment of the sling (SPARC, 10 of 154; TVT, none [p=.002]). Although the objective cure rate was similar across groups, the subjective cure rate was significantly different (TVT, 87.1%; SPARC, 76.5% [p=.03]).
Regression analysis revealed that subjects who had prior surgery for urinary incontinence and those whose surgery was performed by a comparatively less experienced physician were more likely to report persistence of SUI symptoms.
This study reflects general clinical practice, in that it was conducted across a heterogeneous sample of subjects who had both primary and recurrent stress incontinence. Although the rate of bladder perforation was equivalent across groups, more patients who underwent SPARC required loosening of the sling postoperatively to relieve urinary retention.
These data suggest that the SPARC sling may be more difficult to adjust correctly even though it is designed with a tensioning suture. The difficulty may be a consequence of 1) smaller-caliber trocar tunnels or 2) the “top-down” approach less accurately locating the sling at the midportion of the urethra.
This study would have been more rigorous and the results, stronger, if postoperative assessment was made by a blinded examiner. An exceptional positive aspect of study design was that the investigators considered the surgeon’s level of experience—a variable that can certainly affect outcome.
Barber MD, Kleeman S, Karram MM, et al. Transobturator tape compared with tension-free vaginal tape for the treatment of stress urinary incontinence. Obstet Gynecol. 2008;111:611–621.
This randomized, controlled trial compared the efficacy of TVT with the transobturator tape (TOT) technique. Like Lord and colleagues’ study just discussed, it was conducted as an equivalence trial—to determine whether TOT is equivalent to TVT.
The primary outcome was abnormal bladder function 12 months after surgery, defined as the presence of any of the following:
- incontinence symptoms
- positive cough stress test
- retreatment for SUI
- treatment for postoperative urinary retention.
Women who had urodynamic stress incontinence were recruited from three academic centers; excluded were women who had detrusor overactivity, postvoid residual volume >100 mL, prior sling surgery, or contraindications to a midurethral sling.
For the retropubic approach, TVT was used. For the transobturator approach, the Monarc Subfascial Hammock (American Medical Systems) was used. Here, the tape is placed in an “outside-in” fashion.
Subjects completed a baseline bladder diary and a series of validated questionnaires. Postoperatively, subjects were followed for 2 years. Follow-up data included validated questionnaires, bladder diary, pelvic organ prolapse quantification, cough stress test, and postvoid residual volume determination. It was not possible to blind subjects or surgeons, but all postoperative assessments and exams were performed by a blinded nurse.
The investigators sought to determine if TVT and TOT yielded an equivalent (within a 15% margin) rate of abnormal bladder function.
Eventually, 170 patients underwent randomization and surgery (88, TVT; 82, TOT). Baseline demographic, clinical, and incontinence severity data were similar across groups.
Bladder perforation was more common with TVT than with TOT (7% and 0, respectively [p=.02]). Abnormal bladder function was noted in 46.6% of TVT subjects and in 42.7% of TOT subjects, with a noninferiority test demonstrating equivalence (p=.006). One year after surgery, 79% of patients in the TVT group and 82% of patients in the TOT group reported that bladder symptoms were “much better” or “very much better” (p=.88). No significant difference was noted between groups in any of the questionnaire responses after surgery.
This study has many strengths, including rigorous assessments, use of a blinded nurse-examiner to collect postoperative data, and a battery of validated questionnaires used throughout the study. In addition, the primary outcome measure, abnormal bladder function, is defined by stringent criteria that combine subjective and objective components, efficacy, and adverse events.
It will be interesting to see if the efficacy of TOT is maintained over time. The authors of the article point out that several transobturator sling kits are available, utilizing various trocar shapes, different approaches (i.e., “in to out”), and different types of mesh; this may mean variable rates of complications and different degrees of efficacy from one kit to the next.
Also notable in this study is that subjects had relatively high Valsalva leak-point pressures (approaching 100 cm H2O) in both groups.
Which technique is best for SUI with intrinsic sphincter deficiency?
Jeon MJ, Jung HJ, Chung SM, et al. Comparison of the treatment outcome of pubovaginal sling, tension-free vaginal tape, and transobturator tape for stress urinary incontinence with intrinsic sphincter deficiency. Am J Obstet Gynecol. 2008;199:76.e1–76.e4.
This retrospective cohort study was designed to evaluate techniques for treating severe SUI. Researchers were mainly interested in patients who had intrinsic sphincter deficiency (ISD), defined as a Valsalva leak-point pressure <60 cm H2O or maximal urethral closure pressure <20 cm H2O.
The pubovaginal (bladder neck) sling (PVS) has been considered the gold standard therapeutic option for patients who have ISD. Recently, however, data have shown satisfactory outcomes using TVT in this setting.2,3 The aim of this study, therefore, was to compare PVS, TVT, and TOT for treating SUI in patients who had ISD. (Note: The researchers used Uratape [Mentor-Purgès] for the transobturator sling.)
The study included 253 subjects who had ISD and who underwent surgical intervention (87, PVS; 94, TVT; 92, TOT); women who had detrusor overactivity and voiding dysfunction were excluded. Follow-up assessments were performed at 1, 3, 6, and 12 months and annually thereafter. Outcomes studied included complications and rates of cure; the latter was defined as 1) the absence of subjective complaints of leakage and 2) a negative cough stress test.
Median follow-up was 36, 24, and 12 months in the PVS, TVT, and TOT groups, respectively. All groups were similar in regard to baseline clinical and demographic characteristics. Bladder perforation was rare (PVS, 1; TVT and TOT, 0). No significant difference was noted across techniques in the rate of de novo urgency, voiding dysfunction, reoperation for urinary retention, and recurrent urinary tract infection.
Two years after surgery, the cure rate for the three procedures differed significantly: PVS and TVT, 87% each; TOT, 35% (p<.0001). A Cox proportional hazards regression model revealed that the risk of treatment failure with PVS was no different than it was for TVT. However, this model demonstrated that the risk of failure was 4.6 times higher for TOT compared with PVS (p<.0001).
This study is subject to the limitations of any retrospective study. It is unique, however, in that investigators focused on a more severe sample of subjects with ISD. In addition, the authors of the study used the appropriate statistical techniques to attempt to control for potential confounders.
Although the rate of cure was higher with TVT than with TOT, the rate of voiding dysfunction (i.e., the need for catheterization longer than 1 month after surgery) and de novo urgency was higher with TVT as well. This finding suggests that TVT provides more compressive force around the urethra than TOT does; on the other hand, it is possible instead that the difference arises in the method of tensioning of various types of sling.
Last, the study surgeon conducted the postoperative evaluations and was not blinded. This may have introduced bias into the assessments.
As more long-term data become available about different approaches to placing a midurethral sling, it’s likely that we will learn that not all techniques are equal. A customized approach—one that takes into account the individual patient’s clinical parameters—may be necessary to yield long-term efficacy with a sling.
Although, as the authors of this Update discuss, there are several surgical approaches to stress urinary incontinence (tension-free vaginal tape, suprapubic urethral support sling, transobturator tape, pubovaginal sling placed at the bladder neck), coding for the procedure is limited to a single Current Procedural Terminology (CPT) code when surgery is performed via a vaginal approach. CPT code 57288 ( Sling operation for stress incontinence [e.g., fascia or synthetic] ) has been assigned 21.59 relative value units in 2008 and should be reported no matter what type of sling is placed or what method is used to place it.
Failed placement
On occasion, sling material erodes or creates other problems for the patient, such that it must be removed or revised. To report correction of this adverse outcome, bill with 57287 (Removal or revision of sling for stress incontinence [e.g., fascia or synthetic]). If revision must be performed within the global period for the original procedure by the surgeon who placed the sling, append modifier -78 (Unplanned return to the operating/procedure room by the same physician following initial procedure for a related procedure during the postoperative period) to the revision code.
Minimally invasive placement
If you perform a sling procedure laparoscopically, report 51992 (Laparoscopy, surgical; sling operation for stress incontinence [e.g., fascia or synthetic]) instead. No corresponding code exists for laparoscopic revision of a sling procedure; under CPT rules, your only course is to report 51999 (Unlisted laparoscopy procedure, bladder).—MELANIE WITT, RN, CPC-OBGYN, MA
The authors report no financial relationships relevant to this article.
Over the past 10 years, the midurethral sling has replaced the Burch urethropexy as the most common surgical procedure for correcting stress urinary incontinence (SUI). In this “Update” on midurethral slings, we highlight three recently published studies that compare popular surgical approaches to SUI:
- the original tension-free vaginal tape (TVT) technique (FIGURE [“A”])
- the suprapubic urethral support sling (SPARC) (FIGURE [“B”])
- the transobturator tape (TOT) technique (FIGURE [“C”])
- the traditional pubovaginal sling (PVS), placed at the bladder neck (FIGURE [“D”]).
FIGURE [“A”] Four options for a midurethral sling to correct stress urinary incontinence: Tension-free vaginal tape (TVT) technique
FIGURE [“B”] Four options for a midurethral sling to correct stress urinary incontinence: Suprapubic urethral support sling (SPARC)
FIGURE [“C”] Four options for a midurethral sling to correct stress urinary incontinence: Transobturator tape (TOT) technique
FIGURE [“D”] Four options for a midurethral sling to correct stress urinary incontinence: Pubovaginal sling (PVS)
We’ve had a decade-plus of experience with the sling
The midurethral sling, first introduced as the tension-free vaginal tape, or TVT (Gynecare), was quick to be adopted because:
- it offers a minimally invasive approach
- it is highly efficacious
- serious adverse events are rare.
TVT utilizes a 5-mm trocar that is passed from the vagina through the retropubic space, exiting via small suprapubic incisions. A strip of permanent polypropylene mesh attached to these trocars is placed under the midportion of the urethra (FIGURE [“A”]).
We now have 11 years of follow-up data to support the use of the TVT midurethral sling for SUI.1
As TVT gained popularity, surgical equipment manufacturers developed various “kits,” so to speak, for placing a midurethral sling. Many have included innovations that have theoretical advantages over traditional TVT. Some place smaller, 3-mm trocars in a similar “bottom-up” fashion, as the TVT sling does; others utilize smaller trocars that are placed “top down” through the retropubic space into the vagina.
A later generation of slings uses the transobturator approach, to avoid blind passage of trocars through the retropubic space. These slings can be placed “in to out” or “out to in,” and rest in a slightly different orientation under the midurethra.
In an effort to make the procedure even more minimally invasive, some manufacturers now offer slings that are placed through one vaginal incision, thereby avoiding additional suprapubic or groin incisions. Other kits have made alterations to the polypropylene mesh by heat-sealing the material or applying a coating.
Such modifications haven’t always been improvements—some sling kits carried a higher incidence of mesh-related complications, and certain ones were removed from the market. And, although the number of commercially available midurethral sling kits has exploded, we’ve seen scant data published that compare the traditional TVT method with alternative approaches. Those alternatives may be considered midurethral slings, but we haven’t known whether minor variations in technique, or in the instrumentation, translate to improvements in long-term efficacy.
More readjustments for retention are needed after SPARC (vs. TVT)
Lord HE, Taylor JD, Finn JC, et al. A randomized controlled equivalence trial of short-term complications and efficacy of tension-free vaginal tape and suprapubic urethral support sling for treating stress incontinence. BJU Int. 2006;98:367–376.
This randomized, controlled trial compared TVT with SPARC to treat SUI. The study was designed as an equivalence trial: the investigators sought to determine if the “newer” intervention of the two (SPARC) is therapeutically equivalent to the existing intervention (TVT)—not whether one is superior. They therefore looked to see if patients who underwent TVT and those who underwent SPARC had the same rate (within a 5% margin) of bladder injury and other secondary outcomes.
Subjects were eligible to participate if they had SUI on the basis of urodynamic or clinical parameters. They were unaware of their assigned treatment, underwent TVT or SPARC, and were reevaluated 6 weeks postoperatively. Intraoperative, postoperative, and 6-week follow-up data were recorded by the study surgeon.
Three hundred and one patients were enrolled; 147 underwent TVT and 154 underwent SPARC. The groups were similar in regard to all baseline characteristics.
No significant difference was noted between the groups in the primary outcome, which was the rate of bladder perforation (TVT, 0.7%; SPARC, 1.9% [p=.62]). This effect remained after controlling for age, parity, prior urinary incontinence surgery, other concomitant surgery, and the surgeon’s level of experience. There were no intergroup differences in perioperative blood loss, urgency, or objective cure of SUI (defined as negative cough stress test) 6 weeks after surgery.
Subjects who underwent SPARC were more likely to experience urinary retention that required surgical readjustment of the sling (SPARC, 10 of 154; TVT, none [p=.002]). Although the objective cure rate was similar across groups, the subjective cure rate was significantly different (TVT, 87.1%; SPARC, 76.5% [p=.03]).
Regression analysis revealed that subjects who had prior surgery for urinary incontinence and those whose surgery was performed by a comparatively less experienced physician were more likely to report persistence of SUI symptoms.
This study reflects general clinical practice, in that it was conducted across a heterogeneous sample of subjects who had both primary and recurrent stress incontinence. Although the rate of bladder perforation was equivalent across groups, more patients who underwent SPARC required loosening of the sling postoperatively to relieve urinary retention.
These data suggest that the SPARC sling may be more difficult to adjust correctly even though it is designed with a tensioning suture. The difficulty may be a consequence of 1) smaller-caliber trocar tunnels or 2) the “top-down” approach less accurately locating the sling at the midportion of the urethra.
This study would have been more rigorous and the results, stronger, if postoperative assessment was made by a blinded examiner. An exceptional positive aspect of study design was that the investigators considered the surgeon’s level of experience—a variable that can certainly affect outcome.
Barber MD, Kleeman S, Karram MM, et al. Transobturator tape compared with tension-free vaginal tape for the treatment of stress urinary incontinence. Obstet Gynecol. 2008;111:611–621.
This randomized, controlled trial compared the efficacy of TVT with the transobturator tape (TOT) technique. Like Lord and colleagues’ study just discussed, it was conducted as an equivalence trial—to determine whether TOT is equivalent to TVT.
The primary outcome was abnormal bladder function 12 months after surgery, defined as the presence of any of the following:
- incontinence symptoms
- positive cough stress test
- retreatment for SUI
- treatment for postoperative urinary retention.
Women who had urodynamic stress incontinence were recruited from three academic centers; excluded were women who had detrusor overactivity, postvoid residual volume >100 mL, prior sling surgery, or contraindications to a midurethral sling.
For the retropubic approach, TVT was used. For the transobturator approach, the Monarc Subfascial Hammock (American Medical Systems) was used. Here, the tape is placed in an “outside-in” fashion.
Subjects completed a baseline bladder diary and a series of validated questionnaires. Postoperatively, subjects were followed for 2 years. Follow-up data included validated questionnaires, bladder diary, pelvic organ prolapse quantification, cough stress test, and postvoid residual volume determination. It was not possible to blind subjects or surgeons, but all postoperative assessments and exams were performed by a blinded nurse.
The investigators sought to determine if TVT and TOT yielded an equivalent (within a 15% margin) rate of abnormal bladder function.
Eventually, 170 patients underwent randomization and surgery (88, TVT; 82, TOT). Baseline demographic, clinical, and incontinence severity data were similar across groups.
Bladder perforation was more common with TVT than with TOT (7% and 0, respectively [p=.02]). Abnormal bladder function was noted in 46.6% of TVT subjects and in 42.7% of TOT subjects, with a noninferiority test demonstrating equivalence (p=.006). One year after surgery, 79% of patients in the TVT group and 82% of patients in the TOT group reported that bladder symptoms were “much better” or “very much better” (p=.88). No significant difference was noted between groups in any of the questionnaire responses after surgery.
This study has many strengths, including rigorous assessments, use of a blinded nurse-examiner to collect postoperative data, and a battery of validated questionnaires used throughout the study. In addition, the primary outcome measure, abnormal bladder function, is defined by stringent criteria that combine subjective and objective components, efficacy, and adverse events.
It will be interesting to see if the efficacy of TOT is maintained over time. The authors of the article point out that several transobturator sling kits are available, utilizing various trocar shapes, different approaches (i.e., “in to out”), and different types of mesh; this may mean variable rates of complications and different degrees of efficacy from one kit to the next.
Also notable in this study is that subjects had relatively high Valsalva leak-point pressures (approaching 100 cm H2O) in both groups.
Which technique is best for SUI with intrinsic sphincter deficiency?
Jeon MJ, Jung HJ, Chung SM, et al. Comparison of the treatment outcome of pubovaginal sling, tension-free vaginal tape, and transobturator tape for stress urinary incontinence with intrinsic sphincter deficiency. Am J Obstet Gynecol. 2008;199:76.e1–76.e4.
This retrospective cohort study was designed to evaluate techniques for treating severe SUI. Researchers were mainly interested in patients who had intrinsic sphincter deficiency (ISD), defined as a Valsalva leak-point pressure <60 cm H2O or maximal urethral closure pressure <20 cm H2O.
The pubovaginal (bladder neck) sling (PVS) has been considered the gold standard therapeutic option for patients who have ISD. Recently, however, data have shown satisfactory outcomes using TVT in this setting.2,3 The aim of this study, therefore, was to compare PVS, TVT, and TOT for treating SUI in patients who had ISD. (Note: The researchers used Uratape [Mentor-Purgès] for the transobturator sling.)
The study included 253 subjects who had ISD and who underwent surgical intervention (87, PVS; 94, TVT; 92, TOT); women who had detrusor overactivity and voiding dysfunction were excluded. Follow-up assessments were performed at 1, 3, 6, and 12 months and annually thereafter. Outcomes studied included complications and rates of cure; the latter was defined as 1) the absence of subjective complaints of leakage and 2) a negative cough stress test.
Median follow-up was 36, 24, and 12 months in the PVS, TVT, and TOT groups, respectively. All groups were similar in regard to baseline clinical and demographic characteristics. Bladder perforation was rare (PVS, 1; TVT and TOT, 0). No significant difference was noted across techniques in the rate of de novo urgency, voiding dysfunction, reoperation for urinary retention, and recurrent urinary tract infection.
Two years after surgery, the cure rate for the three procedures differed significantly: PVS and TVT, 87% each; TOT, 35% (p<.0001). A Cox proportional hazards regression model revealed that the risk of treatment failure with PVS was no different than it was for TVT. However, this model demonstrated that the risk of failure was 4.6 times higher for TOT compared with PVS (p<.0001).
This study is subject to the limitations of any retrospective study. It is unique, however, in that investigators focused on a more severe sample of subjects with ISD. In addition, the authors of the study used the appropriate statistical techniques to attempt to control for potential confounders.
Although the rate of cure was higher with TVT than with TOT, the rate of voiding dysfunction (i.e., the need for catheterization longer than 1 month after surgery) and de novo urgency was higher with TVT as well. This finding suggests that TVT provides more compressive force around the urethra than TOT does; on the other hand, it is possible instead that the difference arises in the method of tensioning of various types of sling.
Last, the study surgeon conducted the postoperative evaluations and was not blinded. This may have introduced bias into the assessments.
As more long-term data become available about different approaches to placing a midurethral sling, it’s likely that we will learn that not all techniques are equal. A customized approach—one that takes into account the individual patient’s clinical parameters—may be necessary to yield long-term efficacy with a sling.
Although, as the authors of this Update discuss, there are several surgical approaches to stress urinary incontinence (tension-free vaginal tape, suprapubic urethral support sling, transobturator tape, pubovaginal sling placed at the bladder neck), coding for the procedure is limited to a single Current Procedural Terminology (CPT) code when surgery is performed via a vaginal approach. CPT code 57288 ( Sling operation for stress incontinence [e.g., fascia or synthetic] ) has been assigned 21.59 relative value units in 2008 and should be reported no matter what type of sling is placed or what method is used to place it.
Failed placement
On occasion, sling material erodes or creates other problems for the patient, such that it must be removed or revised. To report correction of this adverse outcome, bill with 57287 (Removal or revision of sling for stress incontinence [e.g., fascia or synthetic]). If revision must be performed within the global period for the original procedure by the surgeon who placed the sling, append modifier -78 (Unplanned return to the operating/procedure room by the same physician following initial procedure for a related procedure during the postoperative period) to the revision code.
Minimally invasive placement
If you perform a sling procedure laparoscopically, report 51992 (Laparoscopy, surgical; sling operation for stress incontinence [e.g., fascia or synthetic]) instead. No corresponding code exists for laparoscopic revision of a sling procedure; under CPT rules, your only course is to report 51999 (Unlisted laparoscopy procedure, bladder).—MELANIE WITT, RN, CPC-OBGYN, MA
1. Nilsson CG, Palva K, Rezapour M, Falconer C. Eleven years prospective follow-up of the tension-free vaginal tape procedure for treatment of stress urinary incontinence. Int Urygynecol J Pelvic Floor Dysfunct. 2008;19:1043-1047.
2. Rezapour M, Falconer C, Ulmsten U. Tension-free vaginal tape (TVT) in stress incontinent women with intrinsic sphincter deficiency (ISD)—a long-term follow-up. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12(Suppl 2):S12-S14.
3. Meschia M, Pifarotti P, Buonaguidi A, Gattei U, Spennacchio M. Tension-free vaginal tape (TVT) for treatment of stress urinary incontinence in women with low-pressure urethra. Eur J Obstet Gynecol Reprod Biol. 2005;122:118-121.
1. Nilsson CG, Palva K, Rezapour M, Falconer C. Eleven years prospective follow-up of the tension-free vaginal tape procedure for treatment of stress urinary incontinence. Int Urygynecol J Pelvic Floor Dysfunct. 2008;19:1043-1047.
2. Rezapour M, Falconer C, Ulmsten U. Tension-free vaginal tape (TVT) in stress incontinent women with intrinsic sphincter deficiency (ISD)—a long-term follow-up. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12(Suppl 2):S12-S14.
3. Meschia M, Pifarotti P, Buonaguidi A, Gattei U, Spennacchio M. Tension-free vaginal tape (TVT) for treatment of stress urinary incontinence in women with low-pressure urethra. Eur J Obstet Gynecol Reprod Biol. 2005;122:118-121.
Fetal thrombophilia, perinatal stroke, and novel ideas about CP
The authors report no financial relationships relevant to this article.
Thrombosis is hypothesized to be the more common mechanism underlying cerebral palsy in many cases of maternal or fetal thrombophilia; for that reason, understanding the impact of maternal and fetal thrombophilia on pregnancy outcome is of paramount importance when counseling patients.
Is a maternal and fetal thrombophilia work-up needed in women who give birth to a term infant with cerebral palsy? Prospective studies are needed to evaluate whether that is the case. In this article, we review the literature on fetal thrombophilia and its role in explaining some cases of perinatal stroke that lead, ultimately, to cerebral palsy.
The several causes of cerebral palsy
Cerebral palsy is the most common chronic motor disability of childhood. Approximately 2 to 2.5 of every 1,000 children are given a diagnosis of this disorder every year.1,2 The condition appears early in life; it is not the result of recognized progressive disease.1 Risk factors for cerebral palsy are multiple and heterogenous1,3,4-6:
- Prematurity. The risk of developing cerebral palsy correlates inversely with gestational age.7,8 A premature infant who weighs less than 1,500 g at birth has a risk of cerebral palsy that is 20 to 30 times greater than that of a full-term, normal-weight newborn.3,4
- Hypoxia and ischemia. These are the conditions most often implicated as the cause of cerebral palsy. Fetal heart-rate monitoring was introduced in the 1960s in the hope that interventions to prevent hypoxia and ischemia would reduce the incidence of cerebral palsy. But monitoring has not had that effect—most likely, because some cases of cerebral palsy are caused by perinatal stroke.9 In fact, a large, population-based study has demonstrated that potentially asphyxiating obstetrical conditions account for only about 6% of cases of cerebral palsy.6
- Thrombophilia. Several recent studies report an association between fetal thrombophilia and both neonatal stroke and cerebral palsy.10-14 That association provides a possible explanation for adverse pregnancy outcomes that have otherwise been ascribed to events during delivery.15-23 Although thrombophilia is a recognized risk factor for cerebral palsy, the strength of the association has still not been fully investigated. TABLE 1 and TABLE 2 summarize studies that have examined this association. Given the rarity of both inherited thrombophilias and cerebral palsy, however, an enormous number of cases would be required to fully establish a causal relationship.
TABLE 1
Case reports reveal an association
between fetal thrombophilias and cerebral palsy
| Thrombophilias present | |||
|---|---|---|---|
| Study (type) | Cases of CP | Number | Type |
| Harum et al36 (case report) | 1 | 1 | Factor V Leiden |
| Thorarensen et al37 (case report) | 3 | 3 | Factor V Leiden |
| Lynch et al2 (case series) | 8 | 8 | Factor V Leiden |
| Halliday et al38 (case series) | 55 | 5 | Factor V Leiden; prothrombin mutation |
| Smith et al39 (case series) | 38 | 7 | Factor VIIIc |
| Nelson et al40 (case series) | 31 | 20 | Factor V Leiden; protein C deficiency |
TABLE 2
How often is a fetal thrombophilia
the likely underlying cause of cerebral palsy?
| Thrombophilia* | Prevalence of CP† | Odds ratio |
|---|---|---|
| Factor V Leiden | 6.3% | 0.62 (0.37–1.05) |
| Prothrombin gene | 5.2% | 1.11 (0.59–2.06) |
| MTHFR 677 | 54.1% | 1.27 (0.97–1.66) |
| MTHFR 1298 | 39.4% | 1.08 (0.69–1.19) |
| MTHFR 677/1298 | 15.1% | 1.18 (0.82–1.69) |
| * Heterozygous or homozygous | ||
| † Among 354 subjects with thrombophilia studied41 | ||
| Key: MTHFR, methyltetrahydrofolate reductase | ||
“Thrombophilia” describes a spectrum of congenital or acquired coagulation disorders associated with venous and arterial thrombosis.24 These disorders can occur in the mother or in the fetus, or in both concomitantly.
Fetal thrombophilia has a reported incidence of 2.4 to 5.1 cases for every 100,000 births.25 Whereas maternal thrombophilia has a substantially higher incidence, both maternal and fetal thrombophilia can lead to adverse maternal and fetal events.
The incidence of specific inherited fetal thrombophilias is summarized in TABLE 3. Maternal thrombophilia is generally associated with various adverse pregnancy outcomes, particularly cerebral palsy and perinatal stroke.9,26
TABLE 3
Inherited thrombophilias among the general population
| Study | Number | Factor V Leiden | Protein gene mutation | MTHFR |
|---|---|---|---|---|
| Gibson et al41 (2003) | 708 | 9.8% | 4.7% | 15.1%* |
| Dizon-Townson et al42 (2005) | 4,033 | 3.0% | Not reported | Not reported |
| Infante-Rivard et al43 (2002) | 472 | 3.3% | 1.3% | 43% to 49% |
| Stanley-Christian et al44 (2005) | 14 | 0 | 0 | 0 |
| Currie et al45 (2002) | 46 | 13.0% | Not reported | Not reported |
| Livingston et al46 (2001) | 92 | 0 | 2% | 4% |
| Schlembach et al47 (2003) | 28 | 4.0% | 2% | Not reported |
| Dizon-Townson et al48 (1997) | 130 | 8.6% | Not reported | Not reported |
| * Heterozygous and homozygous carriers of MTHFR C677T and A1298C | ||||
| Key: MTHFR, methyltetrahydrofolate reductase | ||||
Thrombophilia leads to thrombosis at the maternal or fetal interface (FIGURE):
- When thrombosis occurs on the maternal side, the consequence may be severe preeclampsia, intrauterine growth restriction, abruptio placenta, or fetal loss.27-29
- Thrombosis on the fetal side can be a source of emboli that bypass hepatic and pulmonary circulation and travel to the fetal brain.30 As a result, the newborn can sustain a catastrophic event such as perinatal arterial stroke via arterial thrombosis, cerebral sinus venous thrombosis, or renal vein thrombosis.25
Thrombophilia can lead to thrombosis at the maternal or the fetal interface
Thrombosis on the maternal side may lead to severe preeclampsia, intrauterine growth restriction, abruptio placenta, or fetal loss. Thrombosis on the fetal side can be a source of emboli that bypass hepatic and pulmonary circulation and travel to the fetal brain and cause a catastrophic event, such as perinatal arterial stroke via arterial thrombosis, cerebral sinus venous thrombosis, or renal vein thrombosis.
Perinatal and neonatal stroke
Perinatal stroke is defined as a cerebrovascular event that occurs between 28 weeks of gestation and 28 days of postnatal age.30 Incidence is approximately 17 to 93 cases for every 100,000 live births.9
Neonatal stroke occurs in approximately 1 of every 4,000 live births.30 In addition, 1 in every 2,300 to 4,000 newborns is given a diagnosis of ischemic stroke in the nursery.9
Stroke and cerebral palsy
Arterial ischemic stroke in the newborn accounts for 50% to 70% of cases of congenital hemiplegic cerebral palsy.11 Factor V Leiden mutation, prothrombin gene mutation, and a deficiency of protein C, protein S, and antithrombin III have, taken together in two studies, been identified in more than 50% of cerebral ischemic strokes.31,32 In addition to these thrombophilias, important risk factors for perinatal and neonatal stroke include:
- thrombosis in placental villi or vessels
- infection
- use of an intravascular catheter.33
The mechanism that underlies perinatal stroke is a thromboembolic event that originates from either an intracranial or extracranial vessel, the heart, or the placenta.10 A recent meta-analysis by Haywood and colleagues found a statistically significant correlation between protein C deficiency, MTHFR C677T (methyltetrahydrofolate reductase), and the first occurrence of arterial ischemic stroke in a pediatric population.34 Associations between specific thrombophilias and perinatal stroke, as well as pediatric stroke, have been demonstrated (TABLE 4), but we want to emphasize that the absolute risks in these populations are very small.34,35 In addition, the infrequency of these thrombophilias in the general population (TABLE 3) means that their positive predictive value is extremely low.
TABLE 4
Fetal thrombophilia is detected in as many as two thirds of study cases of perinatal and neonatal stroke
| Type of thrombophilia | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Infants | Thrombophilia | FVL | APCR | ACA | AT | PC | PS |
| Golomb et al31 | 22 | 14 (63%) * | 1 * | 3 * | 12 * | 0 | 0 | 0 |
| Bonduel et al32 | 30 | 9 (30%) † | n/a | n/a | n/a | 2 | 1 | 2 |
| deVeber et al49 | 92 | 35 (38%) ‡ | 0 | 6 | 23‡ | 10‡ | 6‡ | 3‡ |
| Mercuri et al50 | 24 | 10 (42%) | 5 | n/a | n/a | 0 | 0 | 0 |
| Günther et al35 | 91 | 62 (68%) | 17 | n/a | 3 | 0 | 6 | 0 |
| Govaert et al51 | 40 | 3 (8%) | 3 | n/a | n/a | n/a | n/a | n/a |
| * FVL, APCR, and ACA diagnoses overlapped. | ||||||||
| † Three patients had anticardiolipin antibody and plasminogen deficiency. | ||||||||
| ‡ Of 35 children, 21 had multiple abnormalities (combined coagulation deficiencies). | ||||||||
| Key: ACA, anticardiolipin antibody; APCR, activated protein C resistance; AT, antithrombin deficiency; FVL, factor V Leiden; PC, protein C deficiency; PS, protein S deficiency; n/a, not available or not studied. | ||||||||
Brain injury
The brain is the largest and most vulnerable fetal organ susceptible to thrombi that are formed either in the placenta or elsewhere.16 A review of cases of cerebral palsy has revealed a pathologic finding, fetal thrombotic vasculopathy (FTV), that has been associated with brain injury.16 Arias and colleagues17 and Kraus18 have observed a correlation among cerebral palsy, a thrombophilic state, and FTV.
Furthermore, Redline found that the presence of severe fetal vascular lesions correlated highly with neurologic impairment and cerebral palsy.19
What is the take-home message?
Regrettably for patients and their offspring, evidence about the relationship between thrombophilia and an adverse neurologic outcome is insufficiently strong to offer much in the way of definitive recommendations for the obstetrician.
We can, however, make some tentative recommendations on management:
Consider screening. When cerebral palsy occurs in association with perinatal stroke, fetal and maternal screening for thrombophilia can be performed.34 The recommended thrombophilia panel comprises tests for:
- factor V Leiden
- prothrombin G20210
- anticardiolipin antibody
- MTHFR mutation.10
Family screening has also been suggested in cases of 1) multiple prothrombotic risk factors in an affected newborn and 2) a positive family history.9
The cost-effectiveness of screening for thrombophilia has not been evaluated in prospective studies, because the positive predictive value of such screening is extremely low.
Consider offering prophylaxis, with cautions. A mother whose baby has been given a diagnosis of thrombophilia and fetal or neonatal stroke can be offered thromboprophylaxis (heparin and aspirin) during any subsequent pregnancy. The usefulness of this intervention has not been well studied and is based solely on expert opinion, however, so it is imperative to counsel patients on the risks and benefits of prophylactic therapy beforehand.
1. American College of Obstetricians and Gynecologists and American Academy of Pediatrics. Neonatal Encephalopathy and Cerebral Palsy: Defining the Pathogenesis and Pathophysiology. Washington DC: The American College of Obstetricians and Gynecologists; September 2003.
2. Lynch JK, Nelson KB, Curry CJ, Grether JK. Cerebrovascular disorders in children with the factor V Leiden mutation. J Child Neurol. 2001;16:735-744.
3. Gibson CS, MacLennan AH, Goldwater PN, Dekker GA. Antenatal causes of cerebral palsy: associations between inherited thrombophilias, viral and bacterial infection, and inherited susceptibility to infection. Obstet Gynecol Surv. 2003;58:209-220.
4. Ramin SM, Gilstrap LC. Other factors/conditions associated with cerebral palsy. Semin Perinatol. 2000;24:196-199.
5. Nelson KB, Dambrosia JM, Ting TY, Grether JK. Uncertain value of electronic fetal monitoring in predicting cerebral palsy. N Engl J Med. 1996;334:613-618.
6. Nelson KB, Grether JK. Potentially asphyxiating conditions and spastic cerebral palsy in infants of normal birth weight. Am J Obstet Gynecol. 1998;179:507-513.
7. Himmelman K, Hagberg G, Beckung E, Hagberg B, Uvebrant P. The changing panorama of cerebral palsy in Sweden. IX. Prevalence and origin in the birth-year period 1995–1998. Acta Paediatr. 2005;94:287-294.
8. Winter S, Autry A, Boyle C, Yeargin-Allsopp M. Trends in the prevalence of cerebral palsy in a population-based study. Pediatrics. 2002;110:1220-1225.
9. Nelson KB. Thrombophilias, Thrombosis and Outcome in Pregnancy, Mother, and Child Symposium. Society of Maternal– Fetal Medicine 26th Annual Meeting. Miami Beach, Fla; 2006.
10. Nelson KB, Lynch JK. Stroke in newborn infants. Lancet Neurol. 2004;3:150-158.
11. Lee J, Croen LA, Backstrand KH, et al. Maternal and infant characteristics associated with perinatal arterial stroke in the infant. JAMA. 2005;293:723-729.
12. Sarig G, Brenner B. Coagulation, inflammation and pregnancy complications. Lancet. 2004;363:96-97.
13. Fattal-Valevski A, Kenet G, Kupferminc MJ, et al. Role of thrombophilic risk factors in children with non-stroke cerebral palsy. Thromb Res. 2005;116:133-137.
14. Steiner M, Hodes MZ, Shreve M, Sundberg S, Edson JR. Postoperative stroke in a child with cerebral palsy heterozygous for factor V Leiden. J Pediatr Hematol Oncol. 2000;22:262-264.
15. Kraus FT. Perinatal pathology, the placenta and litigation. Human Pathol. 2003;34:517-521.
16. Kraus FT, Acheen VI. Fetal thrombotic vasculopathy in the placenta: cerebral thrombi and infarcts, coagulopathies and cerebral palsy. Hum Pathol. 1999;30:759-769.
17. Arias F, Romero R, Joist H, Kraus FT. Thrombophilia: a mechanism of disease in women with adverse pregnancy outcome and thrombotic lesions in the placenta. J Matern Fetal Med. 1998;7:277-286.
18. Kraus FT. Cerebral palsy and thrombi in placental vessels in the fetus: insights from litigation. Hum Pathol. 1997;28:246-248.
19. Redline RW. Severe fetal placental vascular lesions in term infants with neurologic impairment. Am J Obstet Gynecol. 2005;192:452-457.
20. Kraus FT. Placental thrombi and related problems. Semin Diagn Pathol. 1993;10:275-283.
21. Rayne SC, Kraus FT. Placental thrombi and other vascular lesions: classification, morphology and clinical correlations. Pathol Res Pract. 1993;189:2-17.
22. Grafe MR. The correlation of prenatal brain damage and placental pathology. J Neuropathol Exp Neurol. 1994;53:407-415.
23. Redline RW, O’Riordan MA. Placental lesions associated with cerebral palsy and neurologic impairment following term birth. Arch Pathol Lab Med. 2000;124:1785-1791.
24. Paidas MJ, Ku DH, Arkel YS. Screening and management of inherited thrombophilias in the setting of adverse pregnancy outcome. Clin Perinatol. 2004;31:783-805.
25. Kenet G, Nowak-Göttl U. Fetal and neonatal thrombophilia. Obstet Gynecol Clin North Am. 2006;33:457-466.
26. Kujovich JL. Thrombophilia and pregnancy complications. Am J Obstet Gynecol. 2004;191:412-414.
27. Stella CL, How HY, Sibai BM. Thrombophilia and adverse maternal–perinatal outcome: controversies in screening and management. Am J Perinatol. 2006;23:499-506.
28. Stella CL, Sibai BM. Thrombophilia and adverse maternal–perinatal outcome. Clin Obstet Gynecol. 2006;49:850-860.
29. Sibai BM. Thrombophilia and severe preeclampsia: time to screen and treat in future pregnancies? Hypertension. 2005;46:1252-1253.
30. Lynch JK, Hirtz DG, DeVeber G, Nelson KB. Report of the National Institute of Neurologic Disorders and Stroke workshop on perinatal and childhood stroke. Pediatrics. 2002;109:116-123.
31. Golomb MR, MacGregor DL, Domi T, et al. Presumed pre- or perinatal arterial ischemic stroke: risk factors and outcomes. Ann Neurol. 2001;50:163-168.
32. Bonduel M, Sciuccati G, Hepner M, Torres AF, Pieroni G, Frontroth JP. Prethrombotic disorders in children with arterial ischemic stroke and sinovenous thrombosis. Arch Neurol. 1999;56:967-971.
33. Andrew ME, Monagle P, deVeber G, Chan AK. Thromboembolic disease and antithrombotic therapy in newborns. Hematology Am Soc Hematol Educ Program. 2001;358:374.-
34. Haywood S, Leisner R, Pindora S, Ganesan V. Thrombophilia and first arterial ischaemic stroke: a systematic review. Arch Dis Child. 2005;90:402-405.
35. Günther G, Junker R, Sträter R, et al. Childhood Stroke Study Group. Symptomatic ischemic stroke in full-term neonates: role of acquired and genetic prothrombotic risk factors. Stroke. 2000;31:2437-2441.
36. Harum KH, Hoon AH, Jr, Kato GJ, Casella JF, Breiter SN, Johnston MV. Homozygous factor-V mutation as a genetic cause of perinatal thrombosis and cerebral palsy. Dev Med Child Neurol. 1999;41:777-780.
37. Thorarensen O, Ryan S, Hunter J, Younkin DP. Factor V Leiden mutation: an unrecognized cause of hemiplegic cerebral palsy, neonatal stroke, and placental thrombosis. Ann Neurol. 1997;42:372-375.
38. Halliday JL, Reddihough D, Byron K, Ekert H, Ditchfield M. Hemiplegic cerebral palsy and factor V Leiden mutation. J Med Genet. 2000;37:787-789.
39. Smith RA, Skelton M, Howard M, Levene M. Is thrombophilia a factor in the development of hemiplegic cerebral palsy? Dev Med Child Neurol. 2001;43:724-730.
40. Nelson KB, Dambrosia JM, Grether JK, Phillips TM. Neonatal cytokines and coagulation factors in children with cerebral palsy. Ann Neurol. 1998;44:665-675.
41. Gibson CS, MacLennan A, Hague B, et al. Fetal thrombophilic polymorphisms are not a risk factor for cerebral palsy. Am J Obstet Gynecol. 2003;189 Suppl 1:S75.-
42. Dizon-Townson D, Miller C, Sibai BM, et al. National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. The relationship of the factor V Leiden mutation and pregnancy outcomes for mother and fetus. Obstet Gynecol. 2005;106:517-524.
43. Infante-Rivard C, Rivard GE, Yotov WV, et al. Absence of association of thrombophilia polymorphisms with intrauterine growth restriction. N Engl J Med. 2002;347:19-25.
44. Stanley-Christian H, Ghidini A, Sacher R, Shemirani M. Fetal genotype for specific inherited thrombophilia is not associated with severe preeclampsia. J Soc Gynecol Investig. 2005;12:198-201.
45. Currie L, Peek M, McNiven M, Prosser I, Mansour J, Ridgway J. Is there an increased maternal–infant prevalence of Factor V Leiden in association with severe pre-eclampsia? BJOG. 2002;109:191-196.
46. Livingston JC, Barton JR, Park V, Haddad B, Phillips O, Sibai BM. Maternal and fetal inherited thrombophilias are not related to the development of severe preeclampsia. Am J Obstet Gynecol. 2001;185:153-157.
47. Schlembach D, Beinder E, Zingsem J, Wunsiedler U, Beckmann MW, Fischer T. Association of maternal and/or fetal factor V Leiden and G20210A prothrombin mutation with HELLP syndrome and intrauterine growth restriction. Clin Sci (Lond). 2003;105:279-285.
48. Dizon-Townson DS, Meline L, Nelson LM, Varner M, Ward K. Fetal carriers of the factor V Leiden mutation are prone to miscarriage and placental infarction. Am J Obstet Gynecol. 1997;177:402-405.
49. deVeber G, Monagle P, Chan A, et al. Prothrombotic disorders in infants and children with cerebral thromboembolism. Arch Neurol. 1998;55:1539-1543.
50. Mercuri E, Cowan F, Gupte G, et al. Prothrombotic disorders and abnormal neurodevelopmental outcome in infants with neonatal cerebral infarction. Pediatrics. 2001;107:1400-1404.
51. Govaert P, Matthys E, Zecic A, Roelens F, Oostra A, Vanzieleghem B. Perinatal cortical infarction within middle cerebral artery trunks. Arch Dis Child Fetal Neonatal Ed. 2000;82:F59-F63.
The authors report no financial relationships relevant to this article.
Thrombosis is hypothesized to be the more common mechanism underlying cerebral palsy in many cases of maternal or fetal thrombophilia; for that reason, understanding the impact of maternal and fetal thrombophilia on pregnancy outcome is of paramount importance when counseling patients.
Is a maternal and fetal thrombophilia work-up needed in women who give birth to a term infant with cerebral palsy? Prospective studies are needed to evaluate whether that is the case. In this article, we review the literature on fetal thrombophilia and its role in explaining some cases of perinatal stroke that lead, ultimately, to cerebral palsy.
The several causes of cerebral palsy
Cerebral palsy is the most common chronic motor disability of childhood. Approximately 2 to 2.5 of every 1,000 children are given a diagnosis of this disorder every year.1,2 The condition appears early in life; it is not the result of recognized progressive disease.1 Risk factors for cerebral palsy are multiple and heterogenous1,3,4-6:
- Prematurity. The risk of developing cerebral palsy correlates inversely with gestational age.7,8 A premature infant who weighs less than 1,500 g at birth has a risk of cerebral palsy that is 20 to 30 times greater than that of a full-term, normal-weight newborn.3,4
- Hypoxia and ischemia. These are the conditions most often implicated as the cause of cerebral palsy. Fetal heart-rate monitoring was introduced in the 1960s in the hope that interventions to prevent hypoxia and ischemia would reduce the incidence of cerebral palsy. But monitoring has not had that effect—most likely, because some cases of cerebral palsy are caused by perinatal stroke.9 In fact, a large, population-based study has demonstrated that potentially asphyxiating obstetrical conditions account for only about 6% of cases of cerebral palsy.6
- Thrombophilia. Several recent studies report an association between fetal thrombophilia and both neonatal stroke and cerebral palsy.10-14 That association provides a possible explanation for adverse pregnancy outcomes that have otherwise been ascribed to events during delivery.15-23 Although thrombophilia is a recognized risk factor for cerebral palsy, the strength of the association has still not been fully investigated. TABLE 1 and TABLE 2 summarize studies that have examined this association. Given the rarity of both inherited thrombophilias and cerebral palsy, however, an enormous number of cases would be required to fully establish a causal relationship.
TABLE 1
Case reports reveal an association
between fetal thrombophilias and cerebral palsy
| Thrombophilias present | |||
|---|---|---|---|
| Study (type) | Cases of CP | Number | Type |
| Harum et al36 (case report) | 1 | 1 | Factor V Leiden |
| Thorarensen et al37 (case report) | 3 | 3 | Factor V Leiden |
| Lynch et al2 (case series) | 8 | 8 | Factor V Leiden |
| Halliday et al38 (case series) | 55 | 5 | Factor V Leiden; prothrombin mutation |
| Smith et al39 (case series) | 38 | 7 | Factor VIIIc |
| Nelson et al40 (case series) | 31 | 20 | Factor V Leiden; protein C deficiency |
TABLE 2
How often is a fetal thrombophilia
the likely underlying cause of cerebral palsy?
| Thrombophilia* | Prevalence of CP† | Odds ratio |
|---|---|---|
| Factor V Leiden | 6.3% | 0.62 (0.37–1.05) |
| Prothrombin gene | 5.2% | 1.11 (0.59–2.06) |
| MTHFR 677 | 54.1% | 1.27 (0.97–1.66) |
| MTHFR 1298 | 39.4% | 1.08 (0.69–1.19) |
| MTHFR 677/1298 | 15.1% | 1.18 (0.82–1.69) |
| * Heterozygous or homozygous | ||
| † Among 354 subjects with thrombophilia studied41 | ||
| Key: MTHFR, methyltetrahydrofolate reductase | ||
“Thrombophilia” describes a spectrum of congenital or acquired coagulation disorders associated with venous and arterial thrombosis.24 These disorders can occur in the mother or in the fetus, or in both concomitantly.
Fetal thrombophilia has a reported incidence of 2.4 to 5.1 cases for every 100,000 births.25 Whereas maternal thrombophilia has a substantially higher incidence, both maternal and fetal thrombophilia can lead to adverse maternal and fetal events.
The incidence of specific inherited fetal thrombophilias is summarized in TABLE 3. Maternal thrombophilia is generally associated with various adverse pregnancy outcomes, particularly cerebral palsy and perinatal stroke.9,26
TABLE 3
Inherited thrombophilias among the general population
| Study | Number | Factor V Leiden | Protein gene mutation | MTHFR |
|---|---|---|---|---|
| Gibson et al41 (2003) | 708 | 9.8% | 4.7% | 15.1%* |
| Dizon-Townson et al42 (2005) | 4,033 | 3.0% | Not reported | Not reported |
| Infante-Rivard et al43 (2002) | 472 | 3.3% | 1.3% | 43% to 49% |
| Stanley-Christian et al44 (2005) | 14 | 0 | 0 | 0 |
| Currie et al45 (2002) | 46 | 13.0% | Not reported | Not reported |
| Livingston et al46 (2001) | 92 | 0 | 2% | 4% |
| Schlembach et al47 (2003) | 28 | 4.0% | 2% | Not reported |
| Dizon-Townson et al48 (1997) | 130 | 8.6% | Not reported | Not reported |
| * Heterozygous and homozygous carriers of MTHFR C677T and A1298C | ||||
| Key: MTHFR, methyltetrahydrofolate reductase | ||||
Thrombophilia leads to thrombosis at the maternal or fetal interface (FIGURE):
- When thrombosis occurs on the maternal side, the consequence may be severe preeclampsia, intrauterine growth restriction, abruptio placenta, or fetal loss.27-29
- Thrombosis on the fetal side can be a source of emboli that bypass hepatic and pulmonary circulation and travel to the fetal brain.30 As a result, the newborn can sustain a catastrophic event such as perinatal arterial stroke via arterial thrombosis, cerebral sinus venous thrombosis, or renal vein thrombosis.25
Thrombophilia can lead to thrombosis at the maternal or the fetal interface
Thrombosis on the maternal side may lead to severe preeclampsia, intrauterine growth restriction, abruptio placenta, or fetal loss. Thrombosis on the fetal side can be a source of emboli that bypass hepatic and pulmonary circulation and travel to the fetal brain and cause a catastrophic event, such as perinatal arterial stroke via arterial thrombosis, cerebral sinus venous thrombosis, or renal vein thrombosis.
Perinatal and neonatal stroke
Perinatal stroke is defined as a cerebrovascular event that occurs between 28 weeks of gestation and 28 days of postnatal age.30 Incidence is approximately 17 to 93 cases for every 100,000 live births.9
Neonatal stroke occurs in approximately 1 of every 4,000 live births.30 In addition, 1 in every 2,300 to 4,000 newborns is given a diagnosis of ischemic stroke in the nursery.9
Stroke and cerebral palsy
Arterial ischemic stroke in the newborn accounts for 50% to 70% of cases of congenital hemiplegic cerebral palsy.11 Factor V Leiden mutation, prothrombin gene mutation, and a deficiency of protein C, protein S, and antithrombin III have, taken together in two studies, been identified in more than 50% of cerebral ischemic strokes.31,32 In addition to these thrombophilias, important risk factors for perinatal and neonatal stroke include:
- thrombosis in placental villi or vessels
- infection
- use of an intravascular catheter.33
The mechanism that underlies perinatal stroke is a thromboembolic event that originates from either an intracranial or extracranial vessel, the heart, or the placenta.10 A recent meta-analysis by Haywood and colleagues found a statistically significant correlation between protein C deficiency, MTHFR C677T (methyltetrahydrofolate reductase), and the first occurrence of arterial ischemic stroke in a pediatric population.34 Associations between specific thrombophilias and perinatal stroke, as well as pediatric stroke, have been demonstrated (TABLE 4), but we want to emphasize that the absolute risks in these populations are very small.34,35 In addition, the infrequency of these thrombophilias in the general population (TABLE 3) means that their positive predictive value is extremely low.
TABLE 4
Fetal thrombophilia is detected in as many as two thirds of study cases of perinatal and neonatal stroke
| Type of thrombophilia | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Infants | Thrombophilia | FVL | APCR | ACA | AT | PC | PS |
| Golomb et al31 | 22 | 14 (63%) * | 1 * | 3 * | 12 * | 0 | 0 | 0 |
| Bonduel et al32 | 30 | 9 (30%) † | n/a | n/a | n/a | 2 | 1 | 2 |
| deVeber et al49 | 92 | 35 (38%) ‡ | 0 | 6 | 23‡ | 10‡ | 6‡ | 3‡ |
| Mercuri et al50 | 24 | 10 (42%) | 5 | n/a | n/a | 0 | 0 | 0 |
| Günther et al35 | 91 | 62 (68%) | 17 | n/a | 3 | 0 | 6 | 0 |
| Govaert et al51 | 40 | 3 (8%) | 3 | n/a | n/a | n/a | n/a | n/a |
| * FVL, APCR, and ACA diagnoses overlapped. | ||||||||
| † Three patients had anticardiolipin antibody and plasminogen deficiency. | ||||||||
| ‡ Of 35 children, 21 had multiple abnormalities (combined coagulation deficiencies). | ||||||||
| Key: ACA, anticardiolipin antibody; APCR, activated protein C resistance; AT, antithrombin deficiency; FVL, factor V Leiden; PC, protein C deficiency; PS, protein S deficiency; n/a, not available or not studied. | ||||||||
Brain injury
The brain is the largest and most vulnerable fetal organ susceptible to thrombi that are formed either in the placenta or elsewhere.16 A review of cases of cerebral palsy has revealed a pathologic finding, fetal thrombotic vasculopathy (FTV), that has been associated with brain injury.16 Arias and colleagues17 and Kraus18 have observed a correlation among cerebral palsy, a thrombophilic state, and FTV.
Furthermore, Redline found that the presence of severe fetal vascular lesions correlated highly with neurologic impairment and cerebral palsy.19
What is the take-home message?
Regrettably for patients and their offspring, evidence about the relationship between thrombophilia and an adverse neurologic outcome is insufficiently strong to offer much in the way of definitive recommendations for the obstetrician.
We can, however, make some tentative recommendations on management:
Consider screening. When cerebral palsy occurs in association with perinatal stroke, fetal and maternal screening for thrombophilia can be performed.34 The recommended thrombophilia panel comprises tests for:
- factor V Leiden
- prothrombin G20210
- anticardiolipin antibody
- MTHFR mutation.10
Family screening has also been suggested in cases of 1) multiple prothrombotic risk factors in an affected newborn and 2) a positive family history.9
The cost-effectiveness of screening for thrombophilia has not been evaluated in prospective studies, because the positive predictive value of such screening is extremely low.
Consider offering prophylaxis, with cautions. A mother whose baby has been given a diagnosis of thrombophilia and fetal or neonatal stroke can be offered thromboprophylaxis (heparin and aspirin) during any subsequent pregnancy. The usefulness of this intervention has not been well studied and is based solely on expert opinion, however, so it is imperative to counsel patients on the risks and benefits of prophylactic therapy beforehand.
The authors report no financial relationships relevant to this article.
Thrombosis is hypothesized to be the more common mechanism underlying cerebral palsy in many cases of maternal or fetal thrombophilia; for that reason, understanding the impact of maternal and fetal thrombophilia on pregnancy outcome is of paramount importance when counseling patients.
Is a maternal and fetal thrombophilia work-up needed in women who give birth to a term infant with cerebral palsy? Prospective studies are needed to evaluate whether that is the case. In this article, we review the literature on fetal thrombophilia and its role in explaining some cases of perinatal stroke that lead, ultimately, to cerebral palsy.
The several causes of cerebral palsy
Cerebral palsy is the most common chronic motor disability of childhood. Approximately 2 to 2.5 of every 1,000 children are given a diagnosis of this disorder every year.1,2 The condition appears early in life; it is not the result of recognized progressive disease.1 Risk factors for cerebral palsy are multiple and heterogenous1,3,4-6:
- Prematurity. The risk of developing cerebral palsy correlates inversely with gestational age.7,8 A premature infant who weighs less than 1,500 g at birth has a risk of cerebral palsy that is 20 to 30 times greater than that of a full-term, normal-weight newborn.3,4
- Hypoxia and ischemia. These are the conditions most often implicated as the cause of cerebral palsy. Fetal heart-rate monitoring was introduced in the 1960s in the hope that interventions to prevent hypoxia and ischemia would reduce the incidence of cerebral palsy. But monitoring has not had that effect—most likely, because some cases of cerebral palsy are caused by perinatal stroke.9 In fact, a large, population-based study has demonstrated that potentially asphyxiating obstetrical conditions account for only about 6% of cases of cerebral palsy.6
- Thrombophilia. Several recent studies report an association between fetal thrombophilia and both neonatal stroke and cerebral palsy.10-14 That association provides a possible explanation for adverse pregnancy outcomes that have otherwise been ascribed to events during delivery.15-23 Although thrombophilia is a recognized risk factor for cerebral palsy, the strength of the association has still not been fully investigated. TABLE 1 and TABLE 2 summarize studies that have examined this association. Given the rarity of both inherited thrombophilias and cerebral palsy, however, an enormous number of cases would be required to fully establish a causal relationship.
TABLE 1
Case reports reveal an association
between fetal thrombophilias and cerebral palsy
| Thrombophilias present | |||
|---|---|---|---|
| Study (type) | Cases of CP | Number | Type |
| Harum et al36 (case report) | 1 | 1 | Factor V Leiden |
| Thorarensen et al37 (case report) | 3 | 3 | Factor V Leiden |
| Lynch et al2 (case series) | 8 | 8 | Factor V Leiden |
| Halliday et al38 (case series) | 55 | 5 | Factor V Leiden; prothrombin mutation |
| Smith et al39 (case series) | 38 | 7 | Factor VIIIc |
| Nelson et al40 (case series) | 31 | 20 | Factor V Leiden; protein C deficiency |
TABLE 2
How often is a fetal thrombophilia
the likely underlying cause of cerebral palsy?
| Thrombophilia* | Prevalence of CP† | Odds ratio |
|---|---|---|
| Factor V Leiden | 6.3% | 0.62 (0.37–1.05) |
| Prothrombin gene | 5.2% | 1.11 (0.59–2.06) |
| MTHFR 677 | 54.1% | 1.27 (0.97–1.66) |
| MTHFR 1298 | 39.4% | 1.08 (0.69–1.19) |
| MTHFR 677/1298 | 15.1% | 1.18 (0.82–1.69) |
| * Heterozygous or homozygous | ||
| † Among 354 subjects with thrombophilia studied41 | ||
| Key: MTHFR, methyltetrahydrofolate reductase | ||
“Thrombophilia” describes a spectrum of congenital or acquired coagulation disorders associated with venous and arterial thrombosis.24 These disorders can occur in the mother or in the fetus, or in both concomitantly.
Fetal thrombophilia has a reported incidence of 2.4 to 5.1 cases for every 100,000 births.25 Whereas maternal thrombophilia has a substantially higher incidence, both maternal and fetal thrombophilia can lead to adverse maternal and fetal events.
The incidence of specific inherited fetal thrombophilias is summarized in TABLE 3. Maternal thrombophilia is generally associated with various adverse pregnancy outcomes, particularly cerebral palsy and perinatal stroke.9,26
TABLE 3
Inherited thrombophilias among the general population
| Study | Number | Factor V Leiden | Protein gene mutation | MTHFR |
|---|---|---|---|---|
| Gibson et al41 (2003) | 708 | 9.8% | 4.7% | 15.1%* |
| Dizon-Townson et al42 (2005) | 4,033 | 3.0% | Not reported | Not reported |
| Infante-Rivard et al43 (2002) | 472 | 3.3% | 1.3% | 43% to 49% |
| Stanley-Christian et al44 (2005) | 14 | 0 | 0 | 0 |
| Currie et al45 (2002) | 46 | 13.0% | Not reported | Not reported |
| Livingston et al46 (2001) | 92 | 0 | 2% | 4% |
| Schlembach et al47 (2003) | 28 | 4.0% | 2% | Not reported |
| Dizon-Townson et al48 (1997) | 130 | 8.6% | Not reported | Not reported |
| * Heterozygous and homozygous carriers of MTHFR C677T and A1298C | ||||
| Key: MTHFR, methyltetrahydrofolate reductase | ||||
Thrombophilia leads to thrombosis at the maternal or fetal interface (FIGURE):
- When thrombosis occurs on the maternal side, the consequence may be severe preeclampsia, intrauterine growth restriction, abruptio placenta, or fetal loss.27-29
- Thrombosis on the fetal side can be a source of emboli that bypass hepatic and pulmonary circulation and travel to the fetal brain.30 As a result, the newborn can sustain a catastrophic event such as perinatal arterial stroke via arterial thrombosis, cerebral sinus venous thrombosis, or renal vein thrombosis.25
Thrombophilia can lead to thrombosis at the maternal or the fetal interface
Thrombosis on the maternal side may lead to severe preeclampsia, intrauterine growth restriction, abruptio placenta, or fetal loss. Thrombosis on the fetal side can be a source of emboli that bypass hepatic and pulmonary circulation and travel to the fetal brain and cause a catastrophic event, such as perinatal arterial stroke via arterial thrombosis, cerebral sinus venous thrombosis, or renal vein thrombosis.
Perinatal and neonatal stroke
Perinatal stroke is defined as a cerebrovascular event that occurs between 28 weeks of gestation and 28 days of postnatal age.30 Incidence is approximately 17 to 93 cases for every 100,000 live births.9
Neonatal stroke occurs in approximately 1 of every 4,000 live births.30 In addition, 1 in every 2,300 to 4,000 newborns is given a diagnosis of ischemic stroke in the nursery.9
Stroke and cerebral palsy
Arterial ischemic stroke in the newborn accounts for 50% to 70% of cases of congenital hemiplegic cerebral palsy.11 Factor V Leiden mutation, prothrombin gene mutation, and a deficiency of protein C, protein S, and antithrombin III have, taken together in two studies, been identified in more than 50% of cerebral ischemic strokes.31,32 In addition to these thrombophilias, important risk factors for perinatal and neonatal stroke include:
- thrombosis in placental villi or vessels
- infection
- use of an intravascular catheter.33
The mechanism that underlies perinatal stroke is a thromboembolic event that originates from either an intracranial or extracranial vessel, the heart, or the placenta.10 A recent meta-analysis by Haywood and colleagues found a statistically significant correlation between protein C deficiency, MTHFR C677T (methyltetrahydrofolate reductase), and the first occurrence of arterial ischemic stroke in a pediatric population.34 Associations between specific thrombophilias and perinatal stroke, as well as pediatric stroke, have been demonstrated (TABLE 4), but we want to emphasize that the absolute risks in these populations are very small.34,35 In addition, the infrequency of these thrombophilias in the general population (TABLE 3) means that their positive predictive value is extremely low.
TABLE 4
Fetal thrombophilia is detected in as many as two thirds of study cases of perinatal and neonatal stroke
| Type of thrombophilia | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Infants | Thrombophilia | FVL | APCR | ACA | AT | PC | PS |
| Golomb et al31 | 22 | 14 (63%) * | 1 * | 3 * | 12 * | 0 | 0 | 0 |
| Bonduel et al32 | 30 | 9 (30%) † | n/a | n/a | n/a | 2 | 1 | 2 |
| deVeber et al49 | 92 | 35 (38%) ‡ | 0 | 6 | 23‡ | 10‡ | 6‡ | 3‡ |
| Mercuri et al50 | 24 | 10 (42%) | 5 | n/a | n/a | 0 | 0 | 0 |
| Günther et al35 | 91 | 62 (68%) | 17 | n/a | 3 | 0 | 6 | 0 |
| Govaert et al51 | 40 | 3 (8%) | 3 | n/a | n/a | n/a | n/a | n/a |
| * FVL, APCR, and ACA diagnoses overlapped. | ||||||||
| † Three patients had anticardiolipin antibody and plasminogen deficiency. | ||||||||
| ‡ Of 35 children, 21 had multiple abnormalities (combined coagulation deficiencies). | ||||||||
| Key: ACA, anticardiolipin antibody; APCR, activated protein C resistance; AT, antithrombin deficiency; FVL, factor V Leiden; PC, protein C deficiency; PS, protein S deficiency; n/a, not available or not studied. | ||||||||
Brain injury
The brain is the largest and most vulnerable fetal organ susceptible to thrombi that are formed either in the placenta or elsewhere.16 A review of cases of cerebral palsy has revealed a pathologic finding, fetal thrombotic vasculopathy (FTV), that has been associated with brain injury.16 Arias and colleagues17 and Kraus18 have observed a correlation among cerebral palsy, a thrombophilic state, and FTV.
Furthermore, Redline found that the presence of severe fetal vascular lesions correlated highly with neurologic impairment and cerebral palsy.19
What is the take-home message?
Regrettably for patients and their offspring, evidence about the relationship between thrombophilia and an adverse neurologic outcome is insufficiently strong to offer much in the way of definitive recommendations for the obstetrician.
We can, however, make some tentative recommendations on management:
Consider screening. When cerebral palsy occurs in association with perinatal stroke, fetal and maternal screening for thrombophilia can be performed.34 The recommended thrombophilia panel comprises tests for:
- factor V Leiden
- prothrombin G20210
- anticardiolipin antibody
- MTHFR mutation.10
Family screening has also been suggested in cases of 1) multiple prothrombotic risk factors in an affected newborn and 2) a positive family history.9
The cost-effectiveness of screening for thrombophilia has not been evaluated in prospective studies, because the positive predictive value of such screening is extremely low.
Consider offering prophylaxis, with cautions. A mother whose baby has been given a diagnosis of thrombophilia and fetal or neonatal stroke can be offered thromboprophylaxis (heparin and aspirin) during any subsequent pregnancy. The usefulness of this intervention has not been well studied and is based solely on expert opinion, however, so it is imperative to counsel patients on the risks and benefits of prophylactic therapy beforehand.
1. American College of Obstetricians and Gynecologists and American Academy of Pediatrics. Neonatal Encephalopathy and Cerebral Palsy: Defining the Pathogenesis and Pathophysiology. Washington DC: The American College of Obstetricians and Gynecologists; September 2003.
2. Lynch JK, Nelson KB, Curry CJ, Grether JK. Cerebrovascular disorders in children with the factor V Leiden mutation. J Child Neurol. 2001;16:735-744.
3. Gibson CS, MacLennan AH, Goldwater PN, Dekker GA. Antenatal causes of cerebral palsy: associations between inherited thrombophilias, viral and bacterial infection, and inherited susceptibility to infection. Obstet Gynecol Surv. 2003;58:209-220.
4. Ramin SM, Gilstrap LC. Other factors/conditions associated with cerebral palsy. Semin Perinatol. 2000;24:196-199.
5. Nelson KB, Dambrosia JM, Ting TY, Grether JK. Uncertain value of electronic fetal monitoring in predicting cerebral palsy. N Engl J Med. 1996;334:613-618.
6. Nelson KB, Grether JK. Potentially asphyxiating conditions and spastic cerebral palsy in infants of normal birth weight. Am J Obstet Gynecol. 1998;179:507-513.
7. Himmelman K, Hagberg G, Beckung E, Hagberg B, Uvebrant P. The changing panorama of cerebral palsy in Sweden. IX. Prevalence and origin in the birth-year period 1995–1998. Acta Paediatr. 2005;94:287-294.
8. Winter S, Autry A, Boyle C, Yeargin-Allsopp M. Trends in the prevalence of cerebral palsy in a population-based study. Pediatrics. 2002;110:1220-1225.
9. Nelson KB. Thrombophilias, Thrombosis and Outcome in Pregnancy, Mother, and Child Symposium. Society of Maternal– Fetal Medicine 26th Annual Meeting. Miami Beach, Fla; 2006.
10. Nelson KB, Lynch JK. Stroke in newborn infants. Lancet Neurol. 2004;3:150-158.
11. Lee J, Croen LA, Backstrand KH, et al. Maternal and infant characteristics associated with perinatal arterial stroke in the infant. JAMA. 2005;293:723-729.
12. Sarig G, Brenner B. Coagulation, inflammation and pregnancy complications. Lancet. 2004;363:96-97.
13. Fattal-Valevski A, Kenet G, Kupferminc MJ, et al. Role of thrombophilic risk factors in children with non-stroke cerebral palsy. Thromb Res. 2005;116:133-137.
14. Steiner M, Hodes MZ, Shreve M, Sundberg S, Edson JR. Postoperative stroke in a child with cerebral palsy heterozygous for factor V Leiden. J Pediatr Hematol Oncol. 2000;22:262-264.
15. Kraus FT. Perinatal pathology, the placenta and litigation. Human Pathol. 2003;34:517-521.
16. Kraus FT, Acheen VI. Fetal thrombotic vasculopathy in the placenta: cerebral thrombi and infarcts, coagulopathies and cerebral palsy. Hum Pathol. 1999;30:759-769.
17. Arias F, Romero R, Joist H, Kraus FT. Thrombophilia: a mechanism of disease in women with adverse pregnancy outcome and thrombotic lesions in the placenta. J Matern Fetal Med. 1998;7:277-286.
18. Kraus FT. Cerebral palsy and thrombi in placental vessels in the fetus: insights from litigation. Hum Pathol. 1997;28:246-248.
19. Redline RW. Severe fetal placental vascular lesions in term infants with neurologic impairment. Am J Obstet Gynecol. 2005;192:452-457.
20. Kraus FT. Placental thrombi and related problems. Semin Diagn Pathol. 1993;10:275-283.
21. Rayne SC, Kraus FT. Placental thrombi and other vascular lesions: classification, morphology and clinical correlations. Pathol Res Pract. 1993;189:2-17.
22. Grafe MR. The correlation of prenatal brain damage and placental pathology. J Neuropathol Exp Neurol. 1994;53:407-415.
23. Redline RW, O’Riordan MA. Placental lesions associated with cerebral palsy and neurologic impairment following term birth. Arch Pathol Lab Med. 2000;124:1785-1791.
24. Paidas MJ, Ku DH, Arkel YS. Screening and management of inherited thrombophilias in the setting of adverse pregnancy outcome. Clin Perinatol. 2004;31:783-805.
25. Kenet G, Nowak-Göttl U. Fetal and neonatal thrombophilia. Obstet Gynecol Clin North Am. 2006;33:457-466.
26. Kujovich JL. Thrombophilia and pregnancy complications. Am J Obstet Gynecol. 2004;191:412-414.
27. Stella CL, How HY, Sibai BM. Thrombophilia and adverse maternal–perinatal outcome: controversies in screening and management. Am J Perinatol. 2006;23:499-506.
28. Stella CL, Sibai BM. Thrombophilia and adverse maternal–perinatal outcome. Clin Obstet Gynecol. 2006;49:850-860.
29. Sibai BM. Thrombophilia and severe preeclampsia: time to screen and treat in future pregnancies? Hypertension. 2005;46:1252-1253.
30. Lynch JK, Hirtz DG, DeVeber G, Nelson KB. Report of the National Institute of Neurologic Disorders and Stroke workshop on perinatal and childhood stroke. Pediatrics. 2002;109:116-123.
31. Golomb MR, MacGregor DL, Domi T, et al. Presumed pre- or perinatal arterial ischemic stroke: risk factors and outcomes. Ann Neurol. 2001;50:163-168.
32. Bonduel M, Sciuccati G, Hepner M, Torres AF, Pieroni G, Frontroth JP. Prethrombotic disorders in children with arterial ischemic stroke and sinovenous thrombosis. Arch Neurol. 1999;56:967-971.
33. Andrew ME, Monagle P, deVeber G, Chan AK. Thromboembolic disease and antithrombotic therapy in newborns. Hematology Am Soc Hematol Educ Program. 2001;358:374.-
34. Haywood S, Leisner R, Pindora S, Ganesan V. Thrombophilia and first arterial ischaemic stroke: a systematic review. Arch Dis Child. 2005;90:402-405.
35. Günther G, Junker R, Sträter R, et al. Childhood Stroke Study Group. Symptomatic ischemic stroke in full-term neonates: role of acquired and genetic prothrombotic risk factors. Stroke. 2000;31:2437-2441.
36. Harum KH, Hoon AH, Jr, Kato GJ, Casella JF, Breiter SN, Johnston MV. Homozygous factor-V mutation as a genetic cause of perinatal thrombosis and cerebral palsy. Dev Med Child Neurol. 1999;41:777-780.
37. Thorarensen O, Ryan S, Hunter J, Younkin DP. Factor V Leiden mutation: an unrecognized cause of hemiplegic cerebral palsy, neonatal stroke, and placental thrombosis. Ann Neurol. 1997;42:372-375.
38. Halliday JL, Reddihough D, Byron K, Ekert H, Ditchfield M. Hemiplegic cerebral palsy and factor V Leiden mutation. J Med Genet. 2000;37:787-789.
39. Smith RA, Skelton M, Howard M, Levene M. Is thrombophilia a factor in the development of hemiplegic cerebral palsy? Dev Med Child Neurol. 2001;43:724-730.
40. Nelson KB, Dambrosia JM, Grether JK, Phillips TM. Neonatal cytokines and coagulation factors in children with cerebral palsy. Ann Neurol. 1998;44:665-675.
41. Gibson CS, MacLennan A, Hague B, et al. Fetal thrombophilic polymorphisms are not a risk factor for cerebral palsy. Am J Obstet Gynecol. 2003;189 Suppl 1:S75.-
42. Dizon-Townson D, Miller C, Sibai BM, et al. National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. The relationship of the factor V Leiden mutation and pregnancy outcomes for mother and fetus. Obstet Gynecol. 2005;106:517-524.
43. Infante-Rivard C, Rivard GE, Yotov WV, et al. Absence of association of thrombophilia polymorphisms with intrauterine growth restriction. N Engl J Med. 2002;347:19-25.
44. Stanley-Christian H, Ghidini A, Sacher R, Shemirani M. Fetal genotype for specific inherited thrombophilia is not associated with severe preeclampsia. J Soc Gynecol Investig. 2005;12:198-201.
45. Currie L, Peek M, McNiven M, Prosser I, Mansour J, Ridgway J. Is there an increased maternal–infant prevalence of Factor V Leiden in association with severe pre-eclampsia? BJOG. 2002;109:191-196.
46. Livingston JC, Barton JR, Park V, Haddad B, Phillips O, Sibai BM. Maternal and fetal inherited thrombophilias are not related to the development of severe preeclampsia. Am J Obstet Gynecol. 2001;185:153-157.
47. Schlembach D, Beinder E, Zingsem J, Wunsiedler U, Beckmann MW, Fischer T. Association of maternal and/or fetal factor V Leiden and G20210A prothrombin mutation with HELLP syndrome and intrauterine growth restriction. Clin Sci (Lond). 2003;105:279-285.
48. Dizon-Townson DS, Meline L, Nelson LM, Varner M, Ward K. Fetal carriers of the factor V Leiden mutation are prone to miscarriage and placental infarction. Am J Obstet Gynecol. 1997;177:402-405.
49. deVeber G, Monagle P, Chan A, et al. Prothrombotic disorders in infants and children with cerebral thromboembolism. Arch Neurol. 1998;55:1539-1543.
50. Mercuri E, Cowan F, Gupte G, et al. Prothrombotic disorders and abnormal neurodevelopmental outcome in infants with neonatal cerebral infarction. Pediatrics. 2001;107:1400-1404.
51. Govaert P, Matthys E, Zecic A, Roelens F, Oostra A, Vanzieleghem B. Perinatal cortical infarction within middle cerebral artery trunks. Arch Dis Child Fetal Neonatal Ed. 2000;82:F59-F63.
1. American College of Obstetricians and Gynecologists and American Academy of Pediatrics. Neonatal Encephalopathy and Cerebral Palsy: Defining the Pathogenesis and Pathophysiology. Washington DC: The American College of Obstetricians and Gynecologists; September 2003.
2. Lynch JK, Nelson KB, Curry CJ, Grether JK. Cerebrovascular disorders in children with the factor V Leiden mutation. J Child Neurol. 2001;16:735-744.
3. Gibson CS, MacLennan AH, Goldwater PN, Dekker GA. Antenatal causes of cerebral palsy: associations between inherited thrombophilias, viral and bacterial infection, and inherited susceptibility to infection. Obstet Gynecol Surv. 2003;58:209-220.
4. Ramin SM, Gilstrap LC. Other factors/conditions associated with cerebral palsy. Semin Perinatol. 2000;24:196-199.
5. Nelson KB, Dambrosia JM, Ting TY, Grether JK. Uncertain value of electronic fetal monitoring in predicting cerebral palsy. N Engl J Med. 1996;334:613-618.
6. Nelson KB, Grether JK. Potentially asphyxiating conditions and spastic cerebral palsy in infants of normal birth weight. Am J Obstet Gynecol. 1998;179:507-513.
7. Himmelman K, Hagberg G, Beckung E, Hagberg B, Uvebrant P. The changing panorama of cerebral palsy in Sweden. IX. Prevalence and origin in the birth-year period 1995–1998. Acta Paediatr. 2005;94:287-294.
8. Winter S, Autry A, Boyle C, Yeargin-Allsopp M. Trends in the prevalence of cerebral palsy in a population-based study. Pediatrics. 2002;110:1220-1225.
9. Nelson KB. Thrombophilias, Thrombosis and Outcome in Pregnancy, Mother, and Child Symposium. Society of Maternal– Fetal Medicine 26th Annual Meeting. Miami Beach, Fla; 2006.
10. Nelson KB, Lynch JK. Stroke in newborn infants. Lancet Neurol. 2004;3:150-158.
11. Lee J, Croen LA, Backstrand KH, et al. Maternal and infant characteristics associated with perinatal arterial stroke in the infant. JAMA. 2005;293:723-729.
12. Sarig G, Brenner B. Coagulation, inflammation and pregnancy complications. Lancet. 2004;363:96-97.
13. Fattal-Valevski A, Kenet G, Kupferminc MJ, et al. Role of thrombophilic risk factors in children with non-stroke cerebral palsy. Thromb Res. 2005;116:133-137.
14. Steiner M, Hodes MZ, Shreve M, Sundberg S, Edson JR. Postoperative stroke in a child with cerebral palsy heterozygous for factor V Leiden. J Pediatr Hematol Oncol. 2000;22:262-264.
15. Kraus FT. Perinatal pathology, the placenta and litigation. Human Pathol. 2003;34:517-521.
16. Kraus FT, Acheen VI. Fetal thrombotic vasculopathy in the placenta: cerebral thrombi and infarcts, coagulopathies and cerebral palsy. Hum Pathol. 1999;30:759-769.
17. Arias F, Romero R, Joist H, Kraus FT. Thrombophilia: a mechanism of disease in women with adverse pregnancy outcome and thrombotic lesions in the placenta. J Matern Fetal Med. 1998;7:277-286.
18. Kraus FT. Cerebral palsy and thrombi in placental vessels in the fetus: insights from litigation. Hum Pathol. 1997;28:246-248.
19. Redline RW. Severe fetal placental vascular lesions in term infants with neurologic impairment. Am J Obstet Gynecol. 2005;192:452-457.
20. Kraus FT. Placental thrombi and related problems. Semin Diagn Pathol. 1993;10:275-283.
21. Rayne SC, Kraus FT. Placental thrombi and other vascular lesions: classification, morphology and clinical correlations. Pathol Res Pract. 1993;189:2-17.
22. Grafe MR. The correlation of prenatal brain damage and placental pathology. J Neuropathol Exp Neurol. 1994;53:407-415.
23. Redline RW, O’Riordan MA. Placental lesions associated with cerebral palsy and neurologic impairment following term birth. Arch Pathol Lab Med. 2000;124:1785-1791.
24. Paidas MJ, Ku DH, Arkel YS. Screening and management of inherited thrombophilias in the setting of adverse pregnancy outcome. Clin Perinatol. 2004;31:783-805.
25. Kenet G, Nowak-Göttl U. Fetal and neonatal thrombophilia. Obstet Gynecol Clin North Am. 2006;33:457-466.
26. Kujovich JL. Thrombophilia and pregnancy complications. Am J Obstet Gynecol. 2004;191:412-414.
27. Stella CL, How HY, Sibai BM. Thrombophilia and adverse maternal–perinatal outcome: controversies in screening and management. Am J Perinatol. 2006;23:499-506.
28. Stella CL, Sibai BM. Thrombophilia and adverse maternal–perinatal outcome. Clin Obstet Gynecol. 2006;49:850-860.
29. Sibai BM. Thrombophilia and severe preeclampsia: time to screen and treat in future pregnancies? Hypertension. 2005;46:1252-1253.
30. Lynch JK, Hirtz DG, DeVeber G, Nelson KB. Report of the National Institute of Neurologic Disorders and Stroke workshop on perinatal and childhood stroke. Pediatrics. 2002;109:116-123.
31. Golomb MR, MacGregor DL, Domi T, et al. Presumed pre- or perinatal arterial ischemic stroke: risk factors and outcomes. Ann Neurol. 2001;50:163-168.
32. Bonduel M, Sciuccati G, Hepner M, Torres AF, Pieroni G, Frontroth JP. Prethrombotic disorders in children with arterial ischemic stroke and sinovenous thrombosis. Arch Neurol. 1999;56:967-971.
33. Andrew ME, Monagle P, deVeber G, Chan AK. Thromboembolic disease and antithrombotic therapy in newborns. Hematology Am Soc Hematol Educ Program. 2001;358:374.-
34. Haywood S, Leisner R, Pindora S, Ganesan V. Thrombophilia and first arterial ischaemic stroke: a systematic review. Arch Dis Child. 2005;90:402-405.
35. Günther G, Junker R, Sträter R, et al. Childhood Stroke Study Group. Symptomatic ischemic stroke in full-term neonates: role of acquired and genetic prothrombotic risk factors. Stroke. 2000;31:2437-2441.
36. Harum KH, Hoon AH, Jr, Kato GJ, Casella JF, Breiter SN, Johnston MV. Homozygous factor-V mutation as a genetic cause of perinatal thrombosis and cerebral palsy. Dev Med Child Neurol. 1999;41:777-780.
37. Thorarensen O, Ryan S, Hunter J, Younkin DP. Factor V Leiden mutation: an unrecognized cause of hemiplegic cerebral palsy, neonatal stroke, and placental thrombosis. Ann Neurol. 1997;42:372-375.
38. Halliday JL, Reddihough D, Byron K, Ekert H, Ditchfield M. Hemiplegic cerebral palsy and factor V Leiden mutation. J Med Genet. 2000;37:787-789.
39. Smith RA, Skelton M, Howard M, Levene M. Is thrombophilia a factor in the development of hemiplegic cerebral palsy? Dev Med Child Neurol. 2001;43:724-730.
40. Nelson KB, Dambrosia JM, Grether JK, Phillips TM. Neonatal cytokines and coagulation factors in children with cerebral palsy. Ann Neurol. 1998;44:665-675.
41. Gibson CS, MacLennan A, Hague B, et al. Fetal thrombophilic polymorphisms are not a risk factor for cerebral palsy. Am J Obstet Gynecol. 2003;189 Suppl 1:S75.-
42. Dizon-Townson D, Miller C, Sibai BM, et al. National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. The relationship of the factor V Leiden mutation and pregnancy outcomes for mother and fetus. Obstet Gynecol. 2005;106:517-524.
43. Infante-Rivard C, Rivard GE, Yotov WV, et al. Absence of association of thrombophilia polymorphisms with intrauterine growth restriction. N Engl J Med. 2002;347:19-25.
44. Stanley-Christian H, Ghidini A, Sacher R, Shemirani M. Fetal genotype for specific inherited thrombophilia is not associated with severe preeclampsia. J Soc Gynecol Investig. 2005;12:198-201.
45. Currie L, Peek M, McNiven M, Prosser I, Mansour J, Ridgway J. Is there an increased maternal–infant prevalence of Factor V Leiden in association with severe pre-eclampsia? BJOG. 2002;109:191-196.
46. Livingston JC, Barton JR, Park V, Haddad B, Phillips O, Sibai BM. Maternal and fetal inherited thrombophilias are not related to the development of severe preeclampsia. Am J Obstet Gynecol. 2001;185:153-157.
47. Schlembach D, Beinder E, Zingsem J, Wunsiedler U, Beckmann MW, Fischer T. Association of maternal and/or fetal factor V Leiden and G20210A prothrombin mutation with HELLP syndrome and intrauterine growth restriction. Clin Sci (Lond). 2003;105:279-285.
48. Dizon-Townson DS, Meline L, Nelson LM, Varner M, Ward K. Fetal carriers of the factor V Leiden mutation are prone to miscarriage and placental infarction. Am J Obstet Gynecol. 1997;177:402-405.
49. deVeber G, Monagle P, Chan A, et al. Prothrombotic disorders in infants and children with cerebral thromboembolism. Arch Neurol. 1998;55:1539-1543.
50. Mercuri E, Cowan F, Gupte G, et al. Prothrombotic disorders and abnormal neurodevelopmental outcome in infants with neonatal cerebral infarction. Pediatrics. 2001;107:1400-1404.
51. Govaert P, Matthys E, Zecic A, Roelens F, Oostra A, Vanzieleghem B. Perinatal cortical infarction within middle cerebral artery trunks. Arch Dis Child Fetal Neonatal Ed. 2000;82:F59-F63.
Sport-Related Mild Traumatic Brain Injury
For the highly motivated athlete, and often from a parent’s point of view, the return to play after a mild traumatic brain injury (mTBI, or concussion) may affect future scholarship and professional prospects, but it also carries the risk of further injury and permanent disability. Recognition of sport-related mTBI has been described as the most challenging aspect of managing this particular injury.1 Research has shown that patients, athletes,2-7 and health care providers8-10 all lack knowledge regarding some aspect of mTBI, and appropriate education is crucial.
Management of the athlete with mTBI requires both acute and follow-up care, using assessment tools found to be sensitive to detect deficits in cognition, balance, and coordination.
CASE STUDY
An 18-year-old high school football player was tackled during a Saturday afternoon game; on the previous play, he had run 80 yards following an interception. The tackle caused both his ear pads and his chinstrap to break, but he did not lose consciousness. Within two minutes, he was evaluated on the sidelines by the team’s physician assistant and its certified athletic trainer, during which he became nauseated and vomited several times. The player also complained of a new-onset headache and some dizziness. Three weeks earlier, he had been diagnosed with an mTBI; he recovered fully and was medically cleared to play one week later.
On the sidelines immediately after the current injury, the athlete underwent a neurologic examination that yielded no focal neurologic findings. He was transported to the local emergency department (ED) because of the headache and vomiting. The ED provider made a diagnosis of “forehead contusion” and told the patient that he “did not have a brain injury since there was no loss of consciousness.” CT was not ordered, and the athlete was prescribed ibuprofen for his headache.
The following Monday, the athlete was reevaluated by the team PA and the PA’s supervising physician. The athlete reported some residual headache but said the dizziness, nausea, and vomiting had resolved shortly after the injury. His neurologic exam was unremarkable, and although no baseline data were available, results from the Automated Neuropsychological Assessment Metrics (ANAM)11 computerized test demonstrated deficits in reaction time, problem solving, and short-term memory, in comparison with age-matched individuals. CT with contrast performed at that time was negative for hematoma or intracranial swelling.
The athlete was diagnosed with a resolving mTBI and postconcussion syndrome. The consensus was that the vomiting was most likely not a result of the head injury but rather was triggered by the physical exertion of having sprinted 80 yards on the previous play. He was restricted from any exercise and all contact sports until he was asymptomatic, both at rest and during physical activity. The athlete and his mother were informed about the risks of second-impact syndrome.12,13 Follow-up ANAM testing was suggested, but the patient did not return to the office for the test.
mTBI IN THE YOUNGER PATIENT
This case is not an isolated occurrence. In the United States, annual estimates of sport-related traumatic brain injuries, predominantly concussions, range from 1.6 million to 3.8 million.14 According to recent data from injury surveillance systems, concussions sustained by high school athletes represent a greater proportion of sport-related injuries (8.9%) than do those among college athletes (5.8%). Female athletes sustain sport-related mTBI and associated injuries at a higher rate and proportion than do males participating in the same sports.15
Sustaining a sport-related mTBI at an early age is of particular concern: The brain is still developing, and younger patients have an enhanced potential for cumulative effects and prolonged cognitive deficits. Athletes with even one previous mTBI are at increased risk for future mTBI (adjusted risk ratio, 1.4, compared with athletes who have never sustained such an injury).16 Of even greater concern, high school athletes tend to experience delays in cognitive and symptomatic recovery following a concussive injury.17
A growing body of literature has demonstrated difficulties in recognizing and managing mTBIs at all levels of play and within various patient populations.1,18,19 One survey of mTBI evaluation in primary care settings revealed that only 33% of practitioners responsible for sideline coverage used a standard, objective protocol, and an additional 31% used no mTBI guidelines. Among the latter, 71% cited a lack of knowledge, and 16% said they found existing guidelines confusing.10
Another study revealed that hospital discharge instructions for children sustaining an athletic mTBI were inadequate in 69.7% of cases.9 Among these patients, 13% were instructed to return to activity too soon, and 87% were given no instructions at all. The need to better educate athletes, parents, coaches, and health care professionals about the potential seriousness of sport-related mTBI and safe return to the playing field is clear.1,20
This discussion will address new advances in mTBI management and return-to-play decisions for adolescent athletes in the primary care setting.
TERMINOLOGY AND PATHOPHYSIOLOGY
The terminology associated with mTBI has been evolving along with an enhanced understanding of its pathology and etiology. Since more than 90% of sport-related traumatic brain injuries (TBIs) are considered “mild,” the term mTBI is often being used in place of concussion.14 Considering the long-lasting effects of more severe TBI, the contemporary term mTBI more accurately portrays the seriousness of even a seemingly minor injury, often minimized as a “ding” or “bell ringer.” These lay terms do not convey the magnitude and extent of injury sustained and are often thought by nonmedical persons to refer to a different, unrelated injury.6
Since the Concussion in Sport Group18 first met in 2001, several features of sport-related mTBI have been described in an effort to clarify its definition. These include an injury that
(1) is caused by a direct blow to the head or an indirect blow elsewhere in the body that transmits an impulsive force to the head
(2) may cause an immediate and short-lived alteration in neurologic function
(3) may cause neuropathologic changes but typically reflects a functional disturbance rather than a structural injury
(4) is represented by a gradient of clinical symptoms that often resolve sequentially, often without loss of consciousness
(5) is predominantly associated with negative findings on conventional neuroimaging studies (eg, CT, MRI).18
When an athlete sustains an impact to the head, external forces create accelerations and decelerations of the brain within the skull. These forces create the classic coup-contrecoup injury,1 in which the brain impacts the skull at the initial point of contact, with a second point of injury on the directly opposite side of the brain. In some cases, rotational forces occur when the skull is impacted in such a way that the brain rotates about its axis, causing shear and stretch forces on the brain tissue. Either mechanism of injury can trigger a chain of metabolic events in the brain that result in decreased blood flow, increased glucose utilization, and neurotransmitter dysfunction. All of these are thought to contribute to the transient neurologic deficits associated with mTBI.21,22
If a patient who is recovering from mTBI sustains a second head injury before metabolic changes caused by the first injury have fully resolved, a second-impact syndrome (SIS)12,13,16 can develop (see Figure 112,13,16 ). SIS results in massive, rapid brain edema, causing increased intracranial pressure and eventual brain herniation. The majority of cases of SIS are reported in patients younger than 18 and are thought to be the result of altered autoregulation of cerebral blood flow.12 In pediatric athletes, therefore, the proper recognition of mTBI, its effective management, and the return-to-play determination are crucial to decrease the risk of SIS.
INITIAL EVALUATION
The clinical course and management of mTBI can be separated into two distinct components.23 The first is the initial or acute evaluation, which should occur as close to the time of injury as possible. Evaluation of an acute mTBI centers on the history and physical examination at the time of injury; its focus is to determine whether a neurosurgical emergency exists and what course of treatment is needed. Tools such as the Standard Assessment of Concussion (SAC)24 and Folstein’s Mini–Mental State Examination (MMSE)25 should be used to assess the extent of initial cognitive impairment, while other tools, such as the Balance Error Scoring System26 test for motor impairment, should be combined with the neurologic physical exam to formulate the differential diagnosis.
No two brain injuries are alike. The clinician must key into the mechanism of injury, initial and current symptoms (including headache, confusion, and amnesia),16 and positive and negative neurologic findings. Differentials that must be ruled out immediately include skull fracture, cerebral contusion, and epidural hematomas.
Along with the physical findings associated with mTBI, those suggesting more severe injury may include acute localized swelling, deformity, prolonged loss of consciousness, intractable vomiting, and often multiple positive neurologic exam findings, such as cranial nerve abnormalities and motor weakness. Any one of these findings in the initial evaluation warrants activation of emergency medical services, including immediate transport to an ED equipped to manage a neurosurgical emergency.
Since 1974, at least 25 different scales have been used to help practitioners evaluating mTBI assign a grade of severity.1 Although grading scales can be helpful to objectify subjective symptoms, they vary considerably, and none has been shown valid, reliable, or sensitive through published research. Furthermore, an assigned grade cannot reliably express the severity of injury or the prognosis for recovery in each case.1,17
DIAGNOSTIC IMAGING
In mTBI management, the sole purpose of diagnostic imaging is to rule out a more severe structural injury, such as intracranial hemorrhage or hematoma. Symptoms commonly associated with mTBI, such as nausea, vomiting, headache, and visual disturbances, are also cardinal signs of a mass effect resulting from both subdural and epidural hematomas. When these symptoms occur in the acute phase of mTBI management, immediate CT without contrast is imperative to rule out skull fracture and intracranial hemorrhage. Note: Negative imaging test results in the presence of mTBI symptoms do not rule out mTBI, a functional injury; they only confirm that no structural pathology exists.1
Clinicians must also be aware that because a subdural hematoma accumulates slowly, positive findings may not be evident on CT or MRI for seven to 14 days after the initial injury. Thus, the sudden return or worsening of mTBI symptoms that were previously resolved or stable warrants evaluation for a slower (and more commonly fatal) chronic subdural hematoma.1 In this emergent case, CT without IV contrast should be performed first to rule out acute hemorrhage or any mass effect. However, because of the lysing effect of clotted blood, CT with IV contrast or MRI is needed to definitively determine dural and gray-matter reactions to an occult bleed.27
At this time, there is no gold standard among imaging techniques to capture the functional disturbances often noted with sport-related mTBI. However, in one recent study of functional MRI (fMRI) use following sport-related mTBI, athletes with evidence of hyperactivation on their initial post-mTBI scan took longer to recover, based on symptom presentation and neurocognitive testing.28 Though currently used for research purposes, fMRI appears to demonstrate measurable metabolic changes in the brain even after symptoms have been resolved. This promising modality may soon provide helpful neurophysiologic data for the clinical assessment and management of sport-related mTBI.
SYMPTOM SURVEILLANCE
The second component of clinical management of mTBI is the follow-up and surveillance of symptoms and neurologic limitations over time. This is essential for making clinical decisions, including the appropriate time to return the athlete safely to sports or work, so as to avoid further injury.
While the acute component of mTBI management relies on the objective nature of physical examination and neuroimaging, follow-up and surveillance are heavily dependent on the subjective symptoms (see Table 11,18), particularly when physical examination and neuroimaging findings are unremarkable.27,29 Therefore, sensitive and specific clinical tools are needed to accurately assess the various elements of cognition and psychological functioning that are most commonly impaired by mTBI.30
COGNITIVE ASSESSMENT
The metabolic changes that occur after cerebral injury have been shown to cause temporary deficits in normal cognition.23,31 Within the first 24 hours of injury, mild to moderate cognitive impairment is noted across all domains, with the greatest deficits occurring in global functioning, memory acquisition, and delayed memory. Deficits in these areas have been shown to resolve within seven to 10 days following the initial injury.31 It is helpful for these cognitive domains to have been clinically evaluated before injury (baseline), as postinjury evaluation can more effectively detect the extent of debilitation caused by mTBI; subsequent reevaluations can be used to monitor the rate of recovery.
Neuropsychological testing has been studied extensively to determine its value and use in the assessment of mTBI.18,32 Currently, two types of testing are used. Traditional neuropsychological testing comprises a battery of pencil-and-paper exercises administered and interpreted by a psychologist to evaluate cognition and identify areas of deficit following mTBI.33 Although these tests produce a wealth of data, they are expensive, they may require a referral, and administering them can take longer than four hours.
The newest form of neuropsychological testing involves computer-based protocols. Though not yet fully validated, these tools require less time to administer than traditional testing and are commonly used in the sports medicine community (see Table 21,11,33-35 ). Computer-based testing, which may be conducted in the school’s computer lab, has the potential to make preseason baseline testing feasible for large numbers of athletes. Other advantages are ease of administration, a time requirement of about 30 minutes, and the availability of multiple versions to control for the effects of practice.1,23
Two approaches have been suggested for effective use of neuropsychological testing in both components of mTBI management1,30:
First, perform baseline testing at the start of the athletic season, before exposure to injury (possibly within the preparticipation physical), then retest the injured athlete once he or she reports being asymptomatic. Return to play may be considered once the injured athlete scores at or above baseline testing.
Second, perform baseline testing at the start of the season, then retest the injured athlete at set time intervals, charting improvement and rate of recovery. This serial assessment can provide a patient recovery curve for the clinician.30
Results from any neuropsychological testing protocol may be more valuable to the trained practitioner (usually a neurophysiologist, although developers of computer-based testing offer training and credentialing) than are subjective symptoms alone. However, clinicians must use these results as only one variable in the return-to-play decision.23 Cognitive testing is most reliable when baseline testing is included, but this may not be feasible in all settings. If baseline data are not available, normative data for the population of interest (eg, high school level) may be used; these are often available from the manufacturer of the computerized cognitive testing platform.
MOTOR CONTROL ASSESSMENT
Immediately following mTBI, a subtle yet significant degree of motor impairment may develop, often lasting beyond the acute phase of injury. This impairment may affect proprioception, fine and gross motor control, reaction time, and postural stability (balance), all of which are necessary and vital components of athletics. Any impairment in motor control will not only have a negative effect on athletic performance; it will undoubtedly increase the likelihood of a second and possibly more severe injury.
In the neurologic physical examination, results from the tests that are traditionally used to assess motor pathways and coordination have been subject to the individual clinician’s skill and interpretation.26 Several new tests have been developed to assess postural stability more objectively, producing data that give the clinician insight into the brain’s ability to organize incoming sensory information and respond appropriately to environmental changes.36 The gold standard for evaluating postural stability has been the force platform system, which measures vertical ground reaction forces as the body’s center of mass moves around a fixed base of support.26,37
This tool detects even minute alterations in the athlete’s postural stability as stress is exerted on the visual, vestibular, and somatosensory modalities; it can quantify the accuracy and timing of the subject’s motor responses. In recent studies of athletes with mTBI, vestibular and visual alterations caused a deficit in mean stability that peaked at 24 hours postinjury and lasted as long as 10 days before returning to baseline scores.36,38
Although performing force platform testing requires very little time, it is ordinarily administered by an otolaryngologist or an audiologist and usually requires a referral. Cost and limited accessibility make it impractical for obtaining baseline testing in entire teams or other large groups.
Compared with force platform testing, the Balance Error Scoring System (BESS)26 test has also been shown to produce reliable and valid assessments of postural stability.26,39 This low-cost alternative can be used in the clinic or on the sidelines to identify subtle impairments in postural stability, making baseline assessment for large groups feasible and helping clinicians safely return an injured athlete to play.
The BESS tests the athlete’s balance in three different positions, with eyes closed, on both firm and soft foam surfaces. Postural stability is determined by totaling the number of errors made during six 20-second trials (see Figure 2 and Table 3,26,39 page 21). Comparison studies have shown that athletes who experience mTBI have an increased number of errors on days 1 through 5 postinjury, compared with noninjured controls.36
DETERMINING RETURN TO PLAY
Despite the current emphasis on evidence-based practice and the possible consequences of premature participation in sports, the return-to-play decision often depends more on speculation than quantifiable data. The clinician is challenged to synthesize as much subjective and objective data as are available—patient-reported symptomatology, adjunctive assessment scores, and physical examination findings, and preferably with input from a management team that may include a certified athletic trainer, the team physician, a neurosurgeon, and a neuropsychiatrist.1,17
The student-athlete’s teachers should not be overlooked as observers of baseline classroom performance and possible dysfunction and impairment related to the injury. Parents and coaches may offer additional information, but the possibility of bias must be considered.
Only when the athlete reports that symptoms have fully resolved, it is generally agreed, should return to play even be considered.1,18,19 Then, provided that the athlete has regained at least baseline cognitive function and postural stability, a return-to-play progression can begin (see Table 41,19).
Athletes should be moved slowly through these stages, with ongoing supervision and reevaluation after each stage for possible recurrence of symptoms. On average, this progression takes three to five days.
CONCLUSION
No two mTBIs are alike. The mechanism of injury, the degree of neurologic dysfunction, and the time needed for recovery ultimately have no correlation. No athlete should return to play while mTBI symptoms persist, whether at rest or following exercise. If an athlete sustains an injury resulting in loss of consciousness (whatever the duration) or experiences amnesia, return to play that day should not be considered until further evaluation and/or neuroimaging can be performed.
It is imperative that everyone involved with student athletes, both medically and scholastically, be educated about the signs, symptoms, and management of mTBI to ensure a smooth return to school, sports, and other routine activities.
The CDC has begun to address this issue with several Heads Up tool kits for high school coaches, youth sports supervisors, and medical providers. These are available at www.cdc.gov/ncipc/tbi/TBI.htm.
REFERENCES
1. Guskiewicz KM, Bruce SL, Cantu RC, et al. National Athletic Trainers’ Association Position Statement: Management of Sport-Related Concussion. J Athl Train. 2004; 39(3):280-297.
2. Delaney JS, Abuzeyad F, Correa JA, Foxford R. Recognition and characteristics of concussions in the emergency department population. J Emerg Med. 2005;29(2): 189-197.
3. Kaut KP, DePompei R, Kerr J, Congeni J. Reports of head injury and symptom knowledge among college athletes: implications for assessment and educational intervention. Clin J Sport Med. 2003;13(4):213-221.
4. LaBotz M, Martin MR, Kimura IF, et al. A comparison of a preparticipation evaluation history form and a symptom-based concussion survey in the identification of previous head injury in collegiate athletes. Clin J Sport Med. 2005;15(2):73-78.
5. McCrea M, Hammeke T, Olsen G, et al. Unreported concussion in high school football players: implications for prevention. Clin J Sport Med. 2004;14(1):13-17.
6. Valovich McLeod TC, Bay RC, Heil J, McVeigh SD. Identification of sport and recreational activity concussion history through the preparticipation screening and a symptom survey in young athletes. Clin J Sport Med. 2008;18(3):235-240.
7. Williamson IJ, Goodman D. Converging evidence for the under-reporting of concussions in youth ice hockey. Br J Sports Med. 2006;40(2):128-132.
8. Bazarian JJ, Veenema T, Brayer AF, Lee E. Knowledge of concussion guidelines among practitioners caring for children. Clin Pediatr (Phila). 2001;40(4):207-212.
9. Genuardi FJ, King WD. Inappropriate discharge instructions for youth athletes hospitalized for concussion. Pediatrics. 1995;95(2):216-218.
10. Pleacher MD, Dexter WW. Concussion management by primary care providers. Br J Sports Med. 2006;40(1):e2.
11. Levinson DM, Reeves DL. Monitoring recovery from traumatic brain injury using automated neuropsychological assessment metrics (ANAM V1.0). Arch Clin Neuropsychol. 1997;12(2):155-166.
12. Cantu RC. Second-impact syndrome. Clin Sports Med. 1998;17(1):37-44.
13. McCrory PR, Berkovic SF. Second impact syndrome. Neurology. 1998;50(3):677-683.
14. Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21(5):375-378.
15. Gessel LM, Fields SK, Collins CL, et al. Concussions among United States high school and collegiate athletes. J Athl Train. 2007;42(4):495-503.
16. Guskiewicz KM, McCrea M, Marshall SW, et al. Cumulative effects associated with recurrent concussion in collegiate football players: the NCAA Concussion Study. JAMA. 2003;290(19):2549-2555.
17. Field M, Collins MW, Lovell MR, Maroon J. Does age play a role in recovery from sports-related concussion? A comparison of high school and collegiate athletes. J Pediatr. 2003;142(5):546-553.
18. Aubry M, Cantu RC, Dvorak J, et al. Summary and agreement statement of the First International Conference on Concussion in Sport, Vienna 2001: recommendations for the improvement of safety and health of athletes who may suffer concussive injuries. Br J Sports Med. 2002;36(1):6-10.
19. McCrory P, Johnston K, Meeuwisse W, et al. Summary and agreement statement of the 2nd International Conference on Concussion in Sport, Prague 2004. Clin J Sport Med. 2005;15(2):48-55.
20. Centers for Disease Control and Prevention. Nonfatal traumatic brain injuries from sports and recreation activities—United States, 2001-2005. MMWR Morb Mortal Wkly Rep. 2007;56(29):733-737.
21. Giza CC, Hovda DA. Ionic and metabolic consequences of concussion. In: Cantu RC, ed. Neurologic Athletic Head and Spine Injuries. Philadelphia, PA: WB Saunders Co; 2000:80-100.
22. Giza CC, Hovda DA. The neurometabolic cascade of concussion. J Athl Train. 2001;36(3):228-235.
23. Guskiewicz KM, Cantu RC. The concussion puzzle: evaluation of the sport-related concussion. Am J Med Sports. 2004;6:13-21.
24. McCrea M, Randolph C, Kelly JP. The Standardized Assessment of Concussion (SAC): Manual for Administration, Scoring and Interpretation. 2nd ed. Waukesha, WI: CNS Inc; 2000.
25. Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198.
26. Riemann BL, Guskiewicz KM. Effects of mild head injury on postural stability as measured through clinical balance testing. J Athl Train. 2000;35(1):19-25.
27. Borg J, Holm L, Cassidy JD, et al. Diagnostic procedures in mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med. 2004;43(43 Suppl):61-75.
28. Lovell MR, Pardini JE, Welling J, et al. Functional brain abnormalities are related to clinical recovery and time to return-to-play in athletes. Neurosurgery. 2007;61(2): 352-359.
29. Chan RC. Base rate of post-concussion symptoms among normal people and its neuropsychological correlates. Clin Rehabil. 2001;15(3):266-273.
30. Randolph C, McCrea M, Barr WB. Is neuropsychological testing useful in the management of sport-related concussion? J Athl Train. 2005;40(3):139-152.
31. Belanger HG, Vanderploeg RD. The neuropsychological impact of sports-related concussion: a meta-analysis. J Int Neuropsychol Soc. 2005;11(4):345-357.
32. Guskiewicz KM, Bruce SL, Cantu RC, et al; National Athletic Trainers’ Association. Research based recommendations on management of sport related concussion: summary of the National Athletic Trainers’ Association position statement. Br J Sports Med. 2006;40(1):6-10.
33. Collie A, Darby D, Maruff P. Computerised cognitive assessment of athletes with sports related head injury. Br J Sports Med. 2001;35(5):297-302.
34. Erlanger D, Saliba E, Barth J, et al. Monitoring resolution of postconcussion symptoms in athletes: preliminary results of a Web-based neuropsychological test protocol. J Athl Train. 2001;36(3):280-287.
35. Schatz P, Pardini JE, Lovell MR, et al. Sensitivity and specificity of the ImPACT Test Battery for concussion in athletes. Arch Clin Neuropsychol. 2006;21(1):91-99.
36. Guskiewicz KM, Ross SE, Marshall SW. Postural stability and neuropsychological deficits after concussion in collegiate athletes. J Athl Train. 2001;36(3):263-273.
37. Guskiewicz KM. Postural stability assessment following concussion: one piece of the puzzle. Clin J Sport Med. 2001;11(3):182-189.
38. Peterson CL, Ferrara MS, Mrazik M, et al. Evaluation of neuropsychological domain scores and postural stability following cerebral concussion in sports. Clin J Sport Med. 2003;13(4):230-237.
39. Riemann BL, Guskiewicz KM, Shields EW. Relationship between clinical and forceplate measure of postural stability. J Sport Rehabil. 1999;8:71-82.
For the highly motivated athlete, and often from a parent’s point of view, the return to play after a mild traumatic brain injury (mTBI, or concussion) may affect future scholarship and professional prospects, but it also carries the risk of further injury and permanent disability. Recognition of sport-related mTBI has been described as the most challenging aspect of managing this particular injury.1 Research has shown that patients, athletes,2-7 and health care providers8-10 all lack knowledge regarding some aspect of mTBI, and appropriate education is crucial.
Management of the athlete with mTBI requires both acute and follow-up care, using assessment tools found to be sensitive to detect deficits in cognition, balance, and coordination.
CASE STUDY
An 18-year-old high school football player was tackled during a Saturday afternoon game; on the previous play, he had run 80 yards following an interception. The tackle caused both his ear pads and his chinstrap to break, but he did not lose consciousness. Within two minutes, he was evaluated on the sidelines by the team’s physician assistant and its certified athletic trainer, during which he became nauseated and vomited several times. The player also complained of a new-onset headache and some dizziness. Three weeks earlier, he had been diagnosed with an mTBI; he recovered fully and was medically cleared to play one week later.
On the sidelines immediately after the current injury, the athlete underwent a neurologic examination that yielded no focal neurologic findings. He was transported to the local emergency department (ED) because of the headache and vomiting. The ED provider made a diagnosis of “forehead contusion” and told the patient that he “did not have a brain injury since there was no loss of consciousness.” CT was not ordered, and the athlete was prescribed ibuprofen for his headache.
The following Monday, the athlete was reevaluated by the team PA and the PA’s supervising physician. The athlete reported some residual headache but said the dizziness, nausea, and vomiting had resolved shortly after the injury. His neurologic exam was unremarkable, and although no baseline data were available, results from the Automated Neuropsychological Assessment Metrics (ANAM)11 computerized test demonstrated deficits in reaction time, problem solving, and short-term memory, in comparison with age-matched individuals. CT with contrast performed at that time was negative for hematoma or intracranial swelling.
The athlete was diagnosed with a resolving mTBI and postconcussion syndrome. The consensus was that the vomiting was most likely not a result of the head injury but rather was triggered by the physical exertion of having sprinted 80 yards on the previous play. He was restricted from any exercise and all contact sports until he was asymptomatic, both at rest and during physical activity. The athlete and his mother were informed about the risks of second-impact syndrome.12,13 Follow-up ANAM testing was suggested, but the patient did not return to the office for the test.
mTBI IN THE YOUNGER PATIENT
This case is not an isolated occurrence. In the United States, annual estimates of sport-related traumatic brain injuries, predominantly concussions, range from 1.6 million to 3.8 million.14 According to recent data from injury surveillance systems, concussions sustained by high school athletes represent a greater proportion of sport-related injuries (8.9%) than do those among college athletes (5.8%). Female athletes sustain sport-related mTBI and associated injuries at a higher rate and proportion than do males participating in the same sports.15
Sustaining a sport-related mTBI at an early age is of particular concern: The brain is still developing, and younger patients have an enhanced potential for cumulative effects and prolonged cognitive deficits. Athletes with even one previous mTBI are at increased risk for future mTBI (adjusted risk ratio, 1.4, compared with athletes who have never sustained such an injury).16 Of even greater concern, high school athletes tend to experience delays in cognitive and symptomatic recovery following a concussive injury.17
A growing body of literature has demonstrated difficulties in recognizing and managing mTBIs at all levels of play and within various patient populations.1,18,19 One survey of mTBI evaluation in primary care settings revealed that only 33% of practitioners responsible for sideline coverage used a standard, objective protocol, and an additional 31% used no mTBI guidelines. Among the latter, 71% cited a lack of knowledge, and 16% said they found existing guidelines confusing.10
Another study revealed that hospital discharge instructions for children sustaining an athletic mTBI were inadequate in 69.7% of cases.9 Among these patients, 13% were instructed to return to activity too soon, and 87% were given no instructions at all. The need to better educate athletes, parents, coaches, and health care professionals about the potential seriousness of sport-related mTBI and safe return to the playing field is clear.1,20
This discussion will address new advances in mTBI management and return-to-play decisions for adolescent athletes in the primary care setting.
TERMINOLOGY AND PATHOPHYSIOLOGY
The terminology associated with mTBI has been evolving along with an enhanced understanding of its pathology and etiology. Since more than 90% of sport-related traumatic brain injuries (TBIs) are considered “mild,” the term mTBI is often being used in place of concussion.14 Considering the long-lasting effects of more severe TBI, the contemporary term mTBI more accurately portrays the seriousness of even a seemingly minor injury, often minimized as a “ding” or “bell ringer.” These lay terms do not convey the magnitude and extent of injury sustained and are often thought by nonmedical persons to refer to a different, unrelated injury.6
Since the Concussion in Sport Group18 first met in 2001, several features of sport-related mTBI have been described in an effort to clarify its definition. These include an injury that
(1) is caused by a direct blow to the head or an indirect blow elsewhere in the body that transmits an impulsive force to the head
(2) may cause an immediate and short-lived alteration in neurologic function
(3) may cause neuropathologic changes but typically reflects a functional disturbance rather than a structural injury
(4) is represented by a gradient of clinical symptoms that often resolve sequentially, often without loss of consciousness
(5) is predominantly associated with negative findings on conventional neuroimaging studies (eg, CT, MRI).18
When an athlete sustains an impact to the head, external forces create accelerations and decelerations of the brain within the skull. These forces create the classic coup-contrecoup injury,1 in which the brain impacts the skull at the initial point of contact, with a second point of injury on the directly opposite side of the brain. In some cases, rotational forces occur when the skull is impacted in such a way that the brain rotates about its axis, causing shear and stretch forces on the brain tissue. Either mechanism of injury can trigger a chain of metabolic events in the brain that result in decreased blood flow, increased glucose utilization, and neurotransmitter dysfunction. All of these are thought to contribute to the transient neurologic deficits associated with mTBI.21,22
If a patient who is recovering from mTBI sustains a second head injury before metabolic changes caused by the first injury have fully resolved, a second-impact syndrome (SIS)12,13,16 can develop (see Figure 112,13,16 ). SIS results in massive, rapid brain edema, causing increased intracranial pressure and eventual brain herniation. The majority of cases of SIS are reported in patients younger than 18 and are thought to be the result of altered autoregulation of cerebral blood flow.12 In pediatric athletes, therefore, the proper recognition of mTBI, its effective management, and the return-to-play determination are crucial to decrease the risk of SIS.
INITIAL EVALUATION
The clinical course and management of mTBI can be separated into two distinct components.23 The first is the initial or acute evaluation, which should occur as close to the time of injury as possible. Evaluation of an acute mTBI centers on the history and physical examination at the time of injury; its focus is to determine whether a neurosurgical emergency exists and what course of treatment is needed. Tools such as the Standard Assessment of Concussion (SAC)24 and Folstein’s Mini–Mental State Examination (MMSE)25 should be used to assess the extent of initial cognitive impairment, while other tools, such as the Balance Error Scoring System26 test for motor impairment, should be combined with the neurologic physical exam to formulate the differential diagnosis.
No two brain injuries are alike. The clinician must key into the mechanism of injury, initial and current symptoms (including headache, confusion, and amnesia),16 and positive and negative neurologic findings. Differentials that must be ruled out immediately include skull fracture, cerebral contusion, and epidural hematomas.
Along with the physical findings associated with mTBI, those suggesting more severe injury may include acute localized swelling, deformity, prolonged loss of consciousness, intractable vomiting, and often multiple positive neurologic exam findings, such as cranial nerve abnormalities and motor weakness. Any one of these findings in the initial evaluation warrants activation of emergency medical services, including immediate transport to an ED equipped to manage a neurosurgical emergency.
Since 1974, at least 25 different scales have been used to help practitioners evaluating mTBI assign a grade of severity.1 Although grading scales can be helpful to objectify subjective symptoms, they vary considerably, and none has been shown valid, reliable, or sensitive through published research. Furthermore, an assigned grade cannot reliably express the severity of injury or the prognosis for recovery in each case.1,17
DIAGNOSTIC IMAGING
In mTBI management, the sole purpose of diagnostic imaging is to rule out a more severe structural injury, such as intracranial hemorrhage or hematoma. Symptoms commonly associated with mTBI, such as nausea, vomiting, headache, and visual disturbances, are also cardinal signs of a mass effect resulting from both subdural and epidural hematomas. When these symptoms occur in the acute phase of mTBI management, immediate CT without contrast is imperative to rule out skull fracture and intracranial hemorrhage. Note: Negative imaging test results in the presence of mTBI symptoms do not rule out mTBI, a functional injury; they only confirm that no structural pathology exists.1
Clinicians must also be aware that because a subdural hematoma accumulates slowly, positive findings may not be evident on CT or MRI for seven to 14 days after the initial injury. Thus, the sudden return or worsening of mTBI symptoms that were previously resolved or stable warrants evaluation for a slower (and more commonly fatal) chronic subdural hematoma.1 In this emergent case, CT without IV contrast should be performed first to rule out acute hemorrhage or any mass effect. However, because of the lysing effect of clotted blood, CT with IV contrast or MRI is needed to definitively determine dural and gray-matter reactions to an occult bleed.27
At this time, there is no gold standard among imaging techniques to capture the functional disturbances often noted with sport-related mTBI. However, in one recent study of functional MRI (fMRI) use following sport-related mTBI, athletes with evidence of hyperactivation on their initial post-mTBI scan took longer to recover, based on symptom presentation and neurocognitive testing.28 Though currently used for research purposes, fMRI appears to demonstrate measurable metabolic changes in the brain even after symptoms have been resolved. This promising modality may soon provide helpful neurophysiologic data for the clinical assessment and management of sport-related mTBI.
SYMPTOM SURVEILLANCE
The second component of clinical management of mTBI is the follow-up and surveillance of symptoms and neurologic limitations over time. This is essential for making clinical decisions, including the appropriate time to return the athlete safely to sports or work, so as to avoid further injury.
While the acute component of mTBI management relies on the objective nature of physical examination and neuroimaging, follow-up and surveillance are heavily dependent on the subjective symptoms (see Table 11,18), particularly when physical examination and neuroimaging findings are unremarkable.27,29 Therefore, sensitive and specific clinical tools are needed to accurately assess the various elements of cognition and psychological functioning that are most commonly impaired by mTBI.30
COGNITIVE ASSESSMENT
The metabolic changes that occur after cerebral injury have been shown to cause temporary deficits in normal cognition.23,31 Within the first 24 hours of injury, mild to moderate cognitive impairment is noted across all domains, with the greatest deficits occurring in global functioning, memory acquisition, and delayed memory. Deficits in these areas have been shown to resolve within seven to 10 days following the initial injury.31 It is helpful for these cognitive domains to have been clinically evaluated before injury (baseline), as postinjury evaluation can more effectively detect the extent of debilitation caused by mTBI; subsequent reevaluations can be used to monitor the rate of recovery.
Neuropsychological testing has been studied extensively to determine its value and use in the assessment of mTBI.18,32 Currently, two types of testing are used. Traditional neuropsychological testing comprises a battery of pencil-and-paper exercises administered and interpreted by a psychologist to evaluate cognition and identify areas of deficit following mTBI.33 Although these tests produce a wealth of data, they are expensive, they may require a referral, and administering them can take longer than four hours.
The newest form of neuropsychological testing involves computer-based protocols. Though not yet fully validated, these tools require less time to administer than traditional testing and are commonly used in the sports medicine community (see Table 21,11,33-35 ). Computer-based testing, which may be conducted in the school’s computer lab, has the potential to make preseason baseline testing feasible for large numbers of athletes. Other advantages are ease of administration, a time requirement of about 30 minutes, and the availability of multiple versions to control for the effects of practice.1,23
Two approaches have been suggested for effective use of neuropsychological testing in both components of mTBI management1,30:
First, perform baseline testing at the start of the athletic season, before exposure to injury (possibly within the preparticipation physical), then retest the injured athlete once he or she reports being asymptomatic. Return to play may be considered once the injured athlete scores at or above baseline testing.
Second, perform baseline testing at the start of the season, then retest the injured athlete at set time intervals, charting improvement and rate of recovery. This serial assessment can provide a patient recovery curve for the clinician.30
Results from any neuropsychological testing protocol may be more valuable to the trained practitioner (usually a neurophysiologist, although developers of computer-based testing offer training and credentialing) than are subjective symptoms alone. However, clinicians must use these results as only one variable in the return-to-play decision.23 Cognitive testing is most reliable when baseline testing is included, but this may not be feasible in all settings. If baseline data are not available, normative data for the population of interest (eg, high school level) may be used; these are often available from the manufacturer of the computerized cognitive testing platform.
MOTOR CONTROL ASSESSMENT
Immediately following mTBI, a subtle yet significant degree of motor impairment may develop, often lasting beyond the acute phase of injury. This impairment may affect proprioception, fine and gross motor control, reaction time, and postural stability (balance), all of which are necessary and vital components of athletics. Any impairment in motor control will not only have a negative effect on athletic performance; it will undoubtedly increase the likelihood of a second and possibly more severe injury.
In the neurologic physical examination, results from the tests that are traditionally used to assess motor pathways and coordination have been subject to the individual clinician’s skill and interpretation.26 Several new tests have been developed to assess postural stability more objectively, producing data that give the clinician insight into the brain’s ability to organize incoming sensory information and respond appropriately to environmental changes.36 The gold standard for evaluating postural stability has been the force platform system, which measures vertical ground reaction forces as the body’s center of mass moves around a fixed base of support.26,37
This tool detects even minute alterations in the athlete’s postural stability as stress is exerted on the visual, vestibular, and somatosensory modalities; it can quantify the accuracy and timing of the subject’s motor responses. In recent studies of athletes with mTBI, vestibular and visual alterations caused a deficit in mean stability that peaked at 24 hours postinjury and lasted as long as 10 days before returning to baseline scores.36,38
Although performing force platform testing requires very little time, it is ordinarily administered by an otolaryngologist or an audiologist and usually requires a referral. Cost and limited accessibility make it impractical for obtaining baseline testing in entire teams or other large groups.
Compared with force platform testing, the Balance Error Scoring System (BESS)26 test has also been shown to produce reliable and valid assessments of postural stability.26,39 This low-cost alternative can be used in the clinic or on the sidelines to identify subtle impairments in postural stability, making baseline assessment for large groups feasible and helping clinicians safely return an injured athlete to play.
The BESS tests the athlete’s balance in three different positions, with eyes closed, on both firm and soft foam surfaces. Postural stability is determined by totaling the number of errors made during six 20-second trials (see Figure 2 and Table 3,26,39 page 21). Comparison studies have shown that athletes who experience mTBI have an increased number of errors on days 1 through 5 postinjury, compared with noninjured controls.36
DETERMINING RETURN TO PLAY
Despite the current emphasis on evidence-based practice and the possible consequences of premature participation in sports, the return-to-play decision often depends more on speculation than quantifiable data. The clinician is challenged to synthesize as much subjective and objective data as are available—patient-reported symptomatology, adjunctive assessment scores, and physical examination findings, and preferably with input from a management team that may include a certified athletic trainer, the team physician, a neurosurgeon, and a neuropsychiatrist.1,17
The student-athlete’s teachers should not be overlooked as observers of baseline classroom performance and possible dysfunction and impairment related to the injury. Parents and coaches may offer additional information, but the possibility of bias must be considered.
Only when the athlete reports that symptoms have fully resolved, it is generally agreed, should return to play even be considered.1,18,19 Then, provided that the athlete has regained at least baseline cognitive function and postural stability, a return-to-play progression can begin (see Table 41,19).
Athletes should be moved slowly through these stages, with ongoing supervision and reevaluation after each stage for possible recurrence of symptoms. On average, this progression takes three to five days.
CONCLUSION
No two mTBIs are alike. The mechanism of injury, the degree of neurologic dysfunction, and the time needed for recovery ultimately have no correlation. No athlete should return to play while mTBI symptoms persist, whether at rest or following exercise. If an athlete sustains an injury resulting in loss of consciousness (whatever the duration) or experiences amnesia, return to play that day should not be considered until further evaluation and/or neuroimaging can be performed.
It is imperative that everyone involved with student athletes, both medically and scholastically, be educated about the signs, symptoms, and management of mTBI to ensure a smooth return to school, sports, and other routine activities.
The CDC has begun to address this issue with several Heads Up tool kits for high school coaches, youth sports supervisors, and medical providers. These are available at www.cdc.gov/ncipc/tbi/TBI.htm.
REFERENCES
1. Guskiewicz KM, Bruce SL, Cantu RC, et al. National Athletic Trainers’ Association Position Statement: Management of Sport-Related Concussion. J Athl Train. 2004; 39(3):280-297.
2. Delaney JS, Abuzeyad F, Correa JA, Foxford R. Recognition and characteristics of concussions in the emergency department population. J Emerg Med. 2005;29(2): 189-197.
3. Kaut KP, DePompei R, Kerr J, Congeni J. Reports of head injury and symptom knowledge among college athletes: implications for assessment and educational intervention. Clin J Sport Med. 2003;13(4):213-221.
4. LaBotz M, Martin MR, Kimura IF, et al. A comparison of a preparticipation evaluation history form and a symptom-based concussion survey in the identification of previous head injury in collegiate athletes. Clin J Sport Med. 2005;15(2):73-78.
5. McCrea M, Hammeke T, Olsen G, et al. Unreported concussion in high school football players: implications for prevention. Clin J Sport Med. 2004;14(1):13-17.
6. Valovich McLeod TC, Bay RC, Heil J, McVeigh SD. Identification of sport and recreational activity concussion history through the preparticipation screening and a symptom survey in young athletes. Clin J Sport Med. 2008;18(3):235-240.
7. Williamson IJ, Goodman D. Converging evidence for the under-reporting of concussions in youth ice hockey. Br J Sports Med. 2006;40(2):128-132.
8. Bazarian JJ, Veenema T, Brayer AF, Lee E. Knowledge of concussion guidelines among practitioners caring for children. Clin Pediatr (Phila). 2001;40(4):207-212.
9. Genuardi FJ, King WD. Inappropriate discharge instructions for youth athletes hospitalized for concussion. Pediatrics. 1995;95(2):216-218.
10. Pleacher MD, Dexter WW. Concussion management by primary care providers. Br J Sports Med. 2006;40(1):e2.
11. Levinson DM, Reeves DL. Monitoring recovery from traumatic brain injury using automated neuropsychological assessment metrics (ANAM V1.0). Arch Clin Neuropsychol. 1997;12(2):155-166.
12. Cantu RC. Second-impact syndrome. Clin Sports Med. 1998;17(1):37-44.
13. McCrory PR, Berkovic SF. Second impact syndrome. Neurology. 1998;50(3):677-683.
14. Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21(5):375-378.
15. Gessel LM, Fields SK, Collins CL, et al. Concussions among United States high school and collegiate athletes. J Athl Train. 2007;42(4):495-503.
16. Guskiewicz KM, McCrea M, Marshall SW, et al. Cumulative effects associated with recurrent concussion in collegiate football players: the NCAA Concussion Study. JAMA. 2003;290(19):2549-2555.
17. Field M, Collins MW, Lovell MR, Maroon J. Does age play a role in recovery from sports-related concussion? A comparison of high school and collegiate athletes. J Pediatr. 2003;142(5):546-553.
18. Aubry M, Cantu RC, Dvorak J, et al. Summary and agreement statement of the First International Conference on Concussion in Sport, Vienna 2001: recommendations for the improvement of safety and health of athletes who may suffer concussive injuries. Br J Sports Med. 2002;36(1):6-10.
19. McCrory P, Johnston K, Meeuwisse W, et al. Summary and agreement statement of the 2nd International Conference on Concussion in Sport, Prague 2004. Clin J Sport Med. 2005;15(2):48-55.
20. Centers for Disease Control and Prevention. Nonfatal traumatic brain injuries from sports and recreation activities—United States, 2001-2005. MMWR Morb Mortal Wkly Rep. 2007;56(29):733-737.
21. Giza CC, Hovda DA. Ionic and metabolic consequences of concussion. In: Cantu RC, ed. Neurologic Athletic Head and Spine Injuries. Philadelphia, PA: WB Saunders Co; 2000:80-100.
22. Giza CC, Hovda DA. The neurometabolic cascade of concussion. J Athl Train. 2001;36(3):228-235.
23. Guskiewicz KM, Cantu RC. The concussion puzzle: evaluation of the sport-related concussion. Am J Med Sports. 2004;6:13-21.
24. McCrea M, Randolph C, Kelly JP. The Standardized Assessment of Concussion (SAC): Manual for Administration, Scoring and Interpretation. 2nd ed. Waukesha, WI: CNS Inc; 2000.
25. Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198.
26. Riemann BL, Guskiewicz KM. Effects of mild head injury on postural stability as measured through clinical balance testing. J Athl Train. 2000;35(1):19-25.
27. Borg J, Holm L, Cassidy JD, et al. Diagnostic procedures in mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med. 2004;43(43 Suppl):61-75.
28. Lovell MR, Pardini JE, Welling J, et al. Functional brain abnormalities are related to clinical recovery and time to return-to-play in athletes. Neurosurgery. 2007;61(2): 352-359.
29. Chan RC. Base rate of post-concussion symptoms among normal people and its neuropsychological correlates. Clin Rehabil. 2001;15(3):266-273.
30. Randolph C, McCrea M, Barr WB. Is neuropsychological testing useful in the management of sport-related concussion? J Athl Train. 2005;40(3):139-152.
31. Belanger HG, Vanderploeg RD. The neuropsychological impact of sports-related concussion: a meta-analysis. J Int Neuropsychol Soc. 2005;11(4):345-357.
32. Guskiewicz KM, Bruce SL, Cantu RC, et al; National Athletic Trainers’ Association. Research based recommendations on management of sport related concussion: summary of the National Athletic Trainers’ Association position statement. Br J Sports Med. 2006;40(1):6-10.
33. Collie A, Darby D, Maruff P. Computerised cognitive assessment of athletes with sports related head injury. Br J Sports Med. 2001;35(5):297-302.
34. Erlanger D, Saliba E, Barth J, et al. Monitoring resolution of postconcussion symptoms in athletes: preliminary results of a Web-based neuropsychological test protocol. J Athl Train. 2001;36(3):280-287.
35. Schatz P, Pardini JE, Lovell MR, et al. Sensitivity and specificity of the ImPACT Test Battery for concussion in athletes. Arch Clin Neuropsychol. 2006;21(1):91-99.
36. Guskiewicz KM, Ross SE, Marshall SW. Postural stability and neuropsychological deficits after concussion in collegiate athletes. J Athl Train. 2001;36(3):263-273.
37. Guskiewicz KM. Postural stability assessment following concussion: one piece of the puzzle. Clin J Sport Med. 2001;11(3):182-189.
38. Peterson CL, Ferrara MS, Mrazik M, et al. Evaluation of neuropsychological domain scores and postural stability following cerebral concussion in sports. Clin J Sport Med. 2003;13(4):230-237.
39. Riemann BL, Guskiewicz KM, Shields EW. Relationship between clinical and forceplate measure of postural stability. J Sport Rehabil. 1999;8:71-82.
For the highly motivated athlete, and often from a parent’s point of view, the return to play after a mild traumatic brain injury (mTBI, or concussion) may affect future scholarship and professional prospects, but it also carries the risk of further injury and permanent disability. Recognition of sport-related mTBI has been described as the most challenging aspect of managing this particular injury.1 Research has shown that patients, athletes,2-7 and health care providers8-10 all lack knowledge regarding some aspect of mTBI, and appropriate education is crucial.
Management of the athlete with mTBI requires both acute and follow-up care, using assessment tools found to be sensitive to detect deficits in cognition, balance, and coordination.
CASE STUDY
An 18-year-old high school football player was tackled during a Saturday afternoon game; on the previous play, he had run 80 yards following an interception. The tackle caused both his ear pads and his chinstrap to break, but he did not lose consciousness. Within two minutes, he was evaluated on the sidelines by the team’s physician assistant and its certified athletic trainer, during which he became nauseated and vomited several times. The player also complained of a new-onset headache and some dizziness. Three weeks earlier, he had been diagnosed with an mTBI; he recovered fully and was medically cleared to play one week later.
On the sidelines immediately after the current injury, the athlete underwent a neurologic examination that yielded no focal neurologic findings. He was transported to the local emergency department (ED) because of the headache and vomiting. The ED provider made a diagnosis of “forehead contusion” and told the patient that he “did not have a brain injury since there was no loss of consciousness.” CT was not ordered, and the athlete was prescribed ibuprofen for his headache.
The following Monday, the athlete was reevaluated by the team PA and the PA’s supervising physician. The athlete reported some residual headache but said the dizziness, nausea, and vomiting had resolved shortly after the injury. His neurologic exam was unremarkable, and although no baseline data were available, results from the Automated Neuropsychological Assessment Metrics (ANAM)11 computerized test demonstrated deficits in reaction time, problem solving, and short-term memory, in comparison with age-matched individuals. CT with contrast performed at that time was negative for hematoma or intracranial swelling.
The athlete was diagnosed with a resolving mTBI and postconcussion syndrome. The consensus was that the vomiting was most likely not a result of the head injury but rather was triggered by the physical exertion of having sprinted 80 yards on the previous play. He was restricted from any exercise and all contact sports until he was asymptomatic, both at rest and during physical activity. The athlete and his mother were informed about the risks of second-impact syndrome.12,13 Follow-up ANAM testing was suggested, but the patient did not return to the office for the test.
mTBI IN THE YOUNGER PATIENT
This case is not an isolated occurrence. In the United States, annual estimates of sport-related traumatic brain injuries, predominantly concussions, range from 1.6 million to 3.8 million.14 According to recent data from injury surveillance systems, concussions sustained by high school athletes represent a greater proportion of sport-related injuries (8.9%) than do those among college athletes (5.8%). Female athletes sustain sport-related mTBI and associated injuries at a higher rate and proportion than do males participating in the same sports.15
Sustaining a sport-related mTBI at an early age is of particular concern: The brain is still developing, and younger patients have an enhanced potential for cumulative effects and prolonged cognitive deficits. Athletes with even one previous mTBI are at increased risk for future mTBI (adjusted risk ratio, 1.4, compared with athletes who have never sustained such an injury).16 Of even greater concern, high school athletes tend to experience delays in cognitive and symptomatic recovery following a concussive injury.17
A growing body of literature has demonstrated difficulties in recognizing and managing mTBIs at all levels of play and within various patient populations.1,18,19 One survey of mTBI evaluation in primary care settings revealed that only 33% of practitioners responsible for sideline coverage used a standard, objective protocol, and an additional 31% used no mTBI guidelines. Among the latter, 71% cited a lack of knowledge, and 16% said they found existing guidelines confusing.10
Another study revealed that hospital discharge instructions for children sustaining an athletic mTBI were inadequate in 69.7% of cases.9 Among these patients, 13% were instructed to return to activity too soon, and 87% were given no instructions at all. The need to better educate athletes, parents, coaches, and health care professionals about the potential seriousness of sport-related mTBI and safe return to the playing field is clear.1,20
This discussion will address new advances in mTBI management and return-to-play decisions for adolescent athletes in the primary care setting.
TERMINOLOGY AND PATHOPHYSIOLOGY
The terminology associated with mTBI has been evolving along with an enhanced understanding of its pathology and etiology. Since more than 90% of sport-related traumatic brain injuries (TBIs) are considered “mild,” the term mTBI is often being used in place of concussion.14 Considering the long-lasting effects of more severe TBI, the contemporary term mTBI more accurately portrays the seriousness of even a seemingly minor injury, often minimized as a “ding” or “bell ringer.” These lay terms do not convey the magnitude and extent of injury sustained and are often thought by nonmedical persons to refer to a different, unrelated injury.6
Since the Concussion in Sport Group18 first met in 2001, several features of sport-related mTBI have been described in an effort to clarify its definition. These include an injury that
(1) is caused by a direct blow to the head or an indirect blow elsewhere in the body that transmits an impulsive force to the head
(2) may cause an immediate and short-lived alteration in neurologic function
(3) may cause neuropathologic changes but typically reflects a functional disturbance rather than a structural injury
(4) is represented by a gradient of clinical symptoms that often resolve sequentially, often without loss of consciousness
(5) is predominantly associated with negative findings on conventional neuroimaging studies (eg, CT, MRI).18
When an athlete sustains an impact to the head, external forces create accelerations and decelerations of the brain within the skull. These forces create the classic coup-contrecoup injury,1 in which the brain impacts the skull at the initial point of contact, with a second point of injury on the directly opposite side of the brain. In some cases, rotational forces occur when the skull is impacted in such a way that the brain rotates about its axis, causing shear and stretch forces on the brain tissue. Either mechanism of injury can trigger a chain of metabolic events in the brain that result in decreased blood flow, increased glucose utilization, and neurotransmitter dysfunction. All of these are thought to contribute to the transient neurologic deficits associated with mTBI.21,22
If a patient who is recovering from mTBI sustains a second head injury before metabolic changes caused by the first injury have fully resolved, a second-impact syndrome (SIS)12,13,16 can develop (see Figure 112,13,16 ). SIS results in massive, rapid brain edema, causing increased intracranial pressure and eventual brain herniation. The majority of cases of SIS are reported in patients younger than 18 and are thought to be the result of altered autoregulation of cerebral blood flow.12 In pediatric athletes, therefore, the proper recognition of mTBI, its effective management, and the return-to-play determination are crucial to decrease the risk of SIS.
INITIAL EVALUATION
The clinical course and management of mTBI can be separated into two distinct components.23 The first is the initial or acute evaluation, which should occur as close to the time of injury as possible. Evaluation of an acute mTBI centers on the history and physical examination at the time of injury; its focus is to determine whether a neurosurgical emergency exists and what course of treatment is needed. Tools such as the Standard Assessment of Concussion (SAC)24 and Folstein’s Mini–Mental State Examination (MMSE)25 should be used to assess the extent of initial cognitive impairment, while other tools, such as the Balance Error Scoring System26 test for motor impairment, should be combined with the neurologic physical exam to formulate the differential diagnosis.
No two brain injuries are alike. The clinician must key into the mechanism of injury, initial and current symptoms (including headache, confusion, and amnesia),16 and positive and negative neurologic findings. Differentials that must be ruled out immediately include skull fracture, cerebral contusion, and epidural hematomas.
Along with the physical findings associated with mTBI, those suggesting more severe injury may include acute localized swelling, deformity, prolonged loss of consciousness, intractable vomiting, and often multiple positive neurologic exam findings, such as cranial nerve abnormalities and motor weakness. Any one of these findings in the initial evaluation warrants activation of emergency medical services, including immediate transport to an ED equipped to manage a neurosurgical emergency.
Since 1974, at least 25 different scales have been used to help practitioners evaluating mTBI assign a grade of severity.1 Although grading scales can be helpful to objectify subjective symptoms, they vary considerably, and none has been shown valid, reliable, or sensitive through published research. Furthermore, an assigned grade cannot reliably express the severity of injury or the prognosis for recovery in each case.1,17
DIAGNOSTIC IMAGING
In mTBI management, the sole purpose of diagnostic imaging is to rule out a more severe structural injury, such as intracranial hemorrhage or hematoma. Symptoms commonly associated with mTBI, such as nausea, vomiting, headache, and visual disturbances, are also cardinal signs of a mass effect resulting from both subdural and epidural hematomas. When these symptoms occur in the acute phase of mTBI management, immediate CT without contrast is imperative to rule out skull fracture and intracranial hemorrhage. Note: Negative imaging test results in the presence of mTBI symptoms do not rule out mTBI, a functional injury; they only confirm that no structural pathology exists.1
Clinicians must also be aware that because a subdural hematoma accumulates slowly, positive findings may not be evident on CT or MRI for seven to 14 days after the initial injury. Thus, the sudden return or worsening of mTBI symptoms that were previously resolved or stable warrants evaluation for a slower (and more commonly fatal) chronic subdural hematoma.1 In this emergent case, CT without IV contrast should be performed first to rule out acute hemorrhage or any mass effect. However, because of the lysing effect of clotted blood, CT with IV contrast or MRI is needed to definitively determine dural and gray-matter reactions to an occult bleed.27
At this time, there is no gold standard among imaging techniques to capture the functional disturbances often noted with sport-related mTBI. However, in one recent study of functional MRI (fMRI) use following sport-related mTBI, athletes with evidence of hyperactivation on their initial post-mTBI scan took longer to recover, based on symptom presentation and neurocognitive testing.28 Though currently used for research purposes, fMRI appears to demonstrate measurable metabolic changes in the brain even after symptoms have been resolved. This promising modality may soon provide helpful neurophysiologic data for the clinical assessment and management of sport-related mTBI.
SYMPTOM SURVEILLANCE
The second component of clinical management of mTBI is the follow-up and surveillance of symptoms and neurologic limitations over time. This is essential for making clinical decisions, including the appropriate time to return the athlete safely to sports or work, so as to avoid further injury.
While the acute component of mTBI management relies on the objective nature of physical examination and neuroimaging, follow-up and surveillance are heavily dependent on the subjective symptoms (see Table 11,18), particularly when physical examination and neuroimaging findings are unremarkable.27,29 Therefore, sensitive and specific clinical tools are needed to accurately assess the various elements of cognition and psychological functioning that are most commonly impaired by mTBI.30
COGNITIVE ASSESSMENT
The metabolic changes that occur after cerebral injury have been shown to cause temporary deficits in normal cognition.23,31 Within the first 24 hours of injury, mild to moderate cognitive impairment is noted across all domains, with the greatest deficits occurring in global functioning, memory acquisition, and delayed memory. Deficits in these areas have been shown to resolve within seven to 10 days following the initial injury.31 It is helpful for these cognitive domains to have been clinically evaluated before injury (baseline), as postinjury evaluation can more effectively detect the extent of debilitation caused by mTBI; subsequent reevaluations can be used to monitor the rate of recovery.
Neuropsychological testing has been studied extensively to determine its value and use in the assessment of mTBI.18,32 Currently, two types of testing are used. Traditional neuropsychological testing comprises a battery of pencil-and-paper exercises administered and interpreted by a psychologist to evaluate cognition and identify areas of deficit following mTBI.33 Although these tests produce a wealth of data, they are expensive, they may require a referral, and administering them can take longer than four hours.
The newest form of neuropsychological testing involves computer-based protocols. Though not yet fully validated, these tools require less time to administer than traditional testing and are commonly used in the sports medicine community (see Table 21,11,33-35 ). Computer-based testing, which may be conducted in the school’s computer lab, has the potential to make preseason baseline testing feasible for large numbers of athletes. Other advantages are ease of administration, a time requirement of about 30 minutes, and the availability of multiple versions to control for the effects of practice.1,23
Two approaches have been suggested for effective use of neuropsychological testing in both components of mTBI management1,30:
First, perform baseline testing at the start of the athletic season, before exposure to injury (possibly within the preparticipation physical), then retest the injured athlete once he or she reports being asymptomatic. Return to play may be considered once the injured athlete scores at or above baseline testing.
Second, perform baseline testing at the start of the season, then retest the injured athlete at set time intervals, charting improvement and rate of recovery. This serial assessment can provide a patient recovery curve for the clinician.30
Results from any neuropsychological testing protocol may be more valuable to the trained practitioner (usually a neurophysiologist, although developers of computer-based testing offer training and credentialing) than are subjective symptoms alone. However, clinicians must use these results as only one variable in the return-to-play decision.23 Cognitive testing is most reliable when baseline testing is included, but this may not be feasible in all settings. If baseline data are not available, normative data for the population of interest (eg, high school level) may be used; these are often available from the manufacturer of the computerized cognitive testing platform.
MOTOR CONTROL ASSESSMENT
Immediately following mTBI, a subtle yet significant degree of motor impairment may develop, often lasting beyond the acute phase of injury. This impairment may affect proprioception, fine and gross motor control, reaction time, and postural stability (balance), all of which are necessary and vital components of athletics. Any impairment in motor control will not only have a negative effect on athletic performance; it will undoubtedly increase the likelihood of a second and possibly more severe injury.
In the neurologic physical examination, results from the tests that are traditionally used to assess motor pathways and coordination have been subject to the individual clinician’s skill and interpretation.26 Several new tests have been developed to assess postural stability more objectively, producing data that give the clinician insight into the brain’s ability to organize incoming sensory information and respond appropriately to environmental changes.36 The gold standard for evaluating postural stability has been the force platform system, which measures vertical ground reaction forces as the body’s center of mass moves around a fixed base of support.26,37
This tool detects even minute alterations in the athlete’s postural stability as stress is exerted on the visual, vestibular, and somatosensory modalities; it can quantify the accuracy and timing of the subject’s motor responses. In recent studies of athletes with mTBI, vestibular and visual alterations caused a deficit in mean stability that peaked at 24 hours postinjury and lasted as long as 10 days before returning to baseline scores.36,38
Although performing force platform testing requires very little time, it is ordinarily administered by an otolaryngologist or an audiologist and usually requires a referral. Cost and limited accessibility make it impractical for obtaining baseline testing in entire teams or other large groups.
Compared with force platform testing, the Balance Error Scoring System (BESS)26 test has also been shown to produce reliable and valid assessments of postural stability.26,39 This low-cost alternative can be used in the clinic or on the sidelines to identify subtle impairments in postural stability, making baseline assessment for large groups feasible and helping clinicians safely return an injured athlete to play.
The BESS tests the athlete’s balance in three different positions, with eyes closed, on both firm and soft foam surfaces. Postural stability is determined by totaling the number of errors made during six 20-second trials (see Figure 2 and Table 3,26,39 page 21). Comparison studies have shown that athletes who experience mTBI have an increased number of errors on days 1 through 5 postinjury, compared with noninjured controls.36
DETERMINING RETURN TO PLAY
Despite the current emphasis on evidence-based practice and the possible consequences of premature participation in sports, the return-to-play decision often depends more on speculation than quantifiable data. The clinician is challenged to synthesize as much subjective and objective data as are available—patient-reported symptomatology, adjunctive assessment scores, and physical examination findings, and preferably with input from a management team that may include a certified athletic trainer, the team physician, a neurosurgeon, and a neuropsychiatrist.1,17
The student-athlete’s teachers should not be overlooked as observers of baseline classroom performance and possible dysfunction and impairment related to the injury. Parents and coaches may offer additional information, but the possibility of bias must be considered.
Only when the athlete reports that symptoms have fully resolved, it is generally agreed, should return to play even be considered.1,18,19 Then, provided that the athlete has regained at least baseline cognitive function and postural stability, a return-to-play progression can begin (see Table 41,19).
Athletes should be moved slowly through these stages, with ongoing supervision and reevaluation after each stage for possible recurrence of symptoms. On average, this progression takes three to five days.
CONCLUSION
No two mTBIs are alike. The mechanism of injury, the degree of neurologic dysfunction, and the time needed for recovery ultimately have no correlation. No athlete should return to play while mTBI symptoms persist, whether at rest or following exercise. If an athlete sustains an injury resulting in loss of consciousness (whatever the duration) or experiences amnesia, return to play that day should not be considered until further evaluation and/or neuroimaging can be performed.
It is imperative that everyone involved with student athletes, both medically and scholastically, be educated about the signs, symptoms, and management of mTBI to ensure a smooth return to school, sports, and other routine activities.
The CDC has begun to address this issue with several Heads Up tool kits for high school coaches, youth sports supervisors, and medical providers. These are available at www.cdc.gov/ncipc/tbi/TBI.htm.
REFERENCES
1. Guskiewicz KM, Bruce SL, Cantu RC, et al. National Athletic Trainers’ Association Position Statement: Management of Sport-Related Concussion. J Athl Train. 2004; 39(3):280-297.
2. Delaney JS, Abuzeyad F, Correa JA, Foxford R. Recognition and characteristics of concussions in the emergency department population. J Emerg Med. 2005;29(2): 189-197.
3. Kaut KP, DePompei R, Kerr J, Congeni J. Reports of head injury and symptom knowledge among college athletes: implications for assessment and educational intervention. Clin J Sport Med. 2003;13(4):213-221.
4. LaBotz M, Martin MR, Kimura IF, et al. A comparison of a preparticipation evaluation history form and a symptom-based concussion survey in the identification of previous head injury in collegiate athletes. Clin J Sport Med. 2005;15(2):73-78.
5. McCrea M, Hammeke T, Olsen G, et al. Unreported concussion in high school football players: implications for prevention. Clin J Sport Med. 2004;14(1):13-17.
6. Valovich McLeod TC, Bay RC, Heil J, McVeigh SD. Identification of sport and recreational activity concussion history through the preparticipation screening and a symptom survey in young athletes. Clin J Sport Med. 2008;18(3):235-240.
7. Williamson IJ, Goodman D. Converging evidence for the under-reporting of concussions in youth ice hockey. Br J Sports Med. 2006;40(2):128-132.
8. Bazarian JJ, Veenema T, Brayer AF, Lee E. Knowledge of concussion guidelines among practitioners caring for children. Clin Pediatr (Phila). 2001;40(4):207-212.
9. Genuardi FJ, King WD. Inappropriate discharge instructions for youth athletes hospitalized for concussion. Pediatrics. 1995;95(2):216-218.
10. Pleacher MD, Dexter WW. Concussion management by primary care providers. Br J Sports Med. 2006;40(1):e2.
11. Levinson DM, Reeves DL. Monitoring recovery from traumatic brain injury using automated neuropsychological assessment metrics (ANAM V1.0). Arch Clin Neuropsychol. 1997;12(2):155-166.
12. Cantu RC. Second-impact syndrome. Clin Sports Med. 1998;17(1):37-44.
13. McCrory PR, Berkovic SF. Second impact syndrome. Neurology. 1998;50(3):677-683.
14. Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21(5):375-378.
15. Gessel LM, Fields SK, Collins CL, et al. Concussions among United States high school and collegiate athletes. J Athl Train. 2007;42(4):495-503.
16. Guskiewicz KM, McCrea M, Marshall SW, et al. Cumulative effects associated with recurrent concussion in collegiate football players: the NCAA Concussion Study. JAMA. 2003;290(19):2549-2555.
17. Field M, Collins MW, Lovell MR, Maroon J. Does age play a role in recovery from sports-related concussion? A comparison of high school and collegiate athletes. J Pediatr. 2003;142(5):546-553.
18. Aubry M, Cantu RC, Dvorak J, et al. Summary and agreement statement of the First International Conference on Concussion in Sport, Vienna 2001: recommendations for the improvement of safety and health of athletes who may suffer concussive injuries. Br J Sports Med. 2002;36(1):6-10.
19. McCrory P, Johnston K, Meeuwisse W, et al. Summary and agreement statement of the 2nd International Conference on Concussion in Sport, Prague 2004. Clin J Sport Med. 2005;15(2):48-55.
20. Centers for Disease Control and Prevention. Nonfatal traumatic brain injuries from sports and recreation activities—United States, 2001-2005. MMWR Morb Mortal Wkly Rep. 2007;56(29):733-737.
21. Giza CC, Hovda DA. Ionic and metabolic consequences of concussion. In: Cantu RC, ed. Neurologic Athletic Head and Spine Injuries. Philadelphia, PA: WB Saunders Co; 2000:80-100.
22. Giza CC, Hovda DA. The neurometabolic cascade of concussion. J Athl Train. 2001;36(3):228-235.
23. Guskiewicz KM, Cantu RC. The concussion puzzle: evaluation of the sport-related concussion. Am J Med Sports. 2004;6:13-21.
24. McCrea M, Randolph C, Kelly JP. The Standardized Assessment of Concussion (SAC): Manual for Administration, Scoring and Interpretation. 2nd ed. Waukesha, WI: CNS Inc; 2000.
25. Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198.
26. Riemann BL, Guskiewicz KM. Effects of mild head injury on postural stability as measured through clinical balance testing. J Athl Train. 2000;35(1):19-25.
27. Borg J, Holm L, Cassidy JD, et al. Diagnostic procedures in mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med. 2004;43(43 Suppl):61-75.
28. Lovell MR, Pardini JE, Welling J, et al. Functional brain abnormalities are related to clinical recovery and time to return-to-play in athletes. Neurosurgery. 2007;61(2): 352-359.
29. Chan RC. Base rate of post-concussion symptoms among normal people and its neuropsychological correlates. Clin Rehabil. 2001;15(3):266-273.
30. Randolph C, McCrea M, Barr WB. Is neuropsychological testing useful in the management of sport-related concussion? J Athl Train. 2005;40(3):139-152.
31. Belanger HG, Vanderploeg RD. The neuropsychological impact of sports-related concussion: a meta-analysis. J Int Neuropsychol Soc. 2005;11(4):345-357.
32. Guskiewicz KM, Bruce SL, Cantu RC, et al; National Athletic Trainers’ Association. Research based recommendations on management of sport related concussion: summary of the National Athletic Trainers’ Association position statement. Br J Sports Med. 2006;40(1):6-10.
33. Collie A, Darby D, Maruff P. Computerised cognitive assessment of athletes with sports related head injury. Br J Sports Med. 2001;35(5):297-302.
34. Erlanger D, Saliba E, Barth J, et al. Monitoring resolution of postconcussion symptoms in athletes: preliminary results of a Web-based neuropsychological test protocol. J Athl Train. 2001;36(3):280-287.
35. Schatz P, Pardini JE, Lovell MR, et al. Sensitivity and specificity of the ImPACT Test Battery for concussion in athletes. Arch Clin Neuropsychol. 2006;21(1):91-99.
36. Guskiewicz KM, Ross SE, Marshall SW. Postural stability and neuropsychological deficits after concussion in collegiate athletes. J Athl Train. 2001;36(3):263-273.
37. Guskiewicz KM. Postural stability assessment following concussion: one piece of the puzzle. Clin J Sport Med. 2001;11(3):182-189.
38. Peterson CL, Ferrara MS, Mrazik M, et al. Evaluation of neuropsychological domain scores and postural stability following cerebral concussion in sports. Clin J Sport Med. 2003;13(4):230-237.
39. Riemann BL, Guskiewicz KM, Shields EW. Relationship between clinical and forceplate measure of postural stability. J Sport Rehabil. 1999;8:71-82.
Superior Mesenteric Artery Syndrome Following Laparoscopic Gastric Banding Procedure
Goal Attainment in Patients with Type 2 Diabetes, Part 2
ADHD in Young Adults
Attention-deficit/hyperactivity disorder (ADHD) was once thought to be limited to overactive or inattentive children. Yet recent studies have shown that ADHD has a 50% to 60% persistence rate into adulthood and may affect as many as seven million adults in the United States today, impairing the ability of many to function productively.1,2 A significant number of adolescents previously diagnosed with ADHD but not currently receiving treatment are emerging into young adulthood. Some patients are prompted to seek help when their ADHD symptoms interfere with daily functioning; in others, ADHD is identified when they seek treatment for other conditions.
Most older ADHD patients initially present in the primary care setting,3 where practitioners may be reluctant to treat them because:
(1) The diagnosis of ADHD is subjective and purely clinical.
(2) It is unclear how the presently published diagnostic criteria should be applied to adults.
(3) The ADHD treatments proven most effective are schedule II psychostimulants, which have a certain potential for abuse.4
This article summarizes a review of currently accepted practice in primary care for recognizing and treating ADHD in the young adult patient.
Background
Between 1990 and 1998, the number of school-age children diagnosed with ADHD reportedly increased by 700%.5 The accompanying increase in use of schedule II stimulants6 (primarily methylphenidate and dexamphetamine) aroused some controversy, even though these medications were shown to be effective in reducing the inattentiveness, impulsivity, and hyperactivity associated with ADHD.7,8
Concerns regarding the indicated medications—possible abuse, associated adverse effects, inconvenience, stigma—prompted many parents of affected children to decline pharmacologic treatment.9 Among treated children, a large proportion discontinued their medications because they did not have a response or experienced intolerable adverse effects.10,11 Still others were never diagnosed. As a result, a significant number of adolescents with untreated or undertreated ADHD are now entering adulthood. Without treatment now, perhaps half of them will experience lifelong impairment resulting from ADHD and associated comorbidities (ie, conduct and oppositional-defiant disorder, antisocial personality disorder, substance abuse disorder, anxiety disorders, and depression).1,2,9,10,12
ADHD is believed to have a solid neurobiologic basis, but the condition has no known objective markers. Its diagnosis remains subjective and clinical, depending primarily on structured interviews conducted by trained practitioners.13 Unlike most behavioral disorders, which are first understood in adults, then extrapolated to children,14 ADHD has been recognized and treated in children since the late 1930s15 but has only recently been identified among significant numbers of adults.1 Thus, ADHD is currently best understood in children.14
The classic symptoms and signs of ADHD and its subtypes undergo subtle alterations as the patient matures.2,12,16 Hyperactivity wanes in adolescence and may be replaced by a restlessness that prompts the adult patient to change jobs and/or living quarters frequently, leading to an unstable lifestyle.12 Although impulsivity and inattention may persist through adolescence into adulthood, they are often obscured by both coping mechanisms (eg, choice of employment, conscious efforts by high achievers to overcome their disorganization)1 and behavioral comorbidities (depression, self-medication/substance abuse, personality disorders) that the patient may have developed.10,16 These developments may significantly complicate identification of the disorder in older patients.2,12,10,16
The adult with ADHD often exhibits low self-esteem, anxiety, depression, sleep disturbances,17 difficulties with personal relationships and jobs, and impulsivity, which can lead to trouble with the law.18 The costs to adult ADHD patients, their families, and the community are enormous, making it all the more important for health care professionals to understand this condition.
Diagnostic Criteria
The diagnosis of ADHD is based on criteria from the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition7,19(DSM-IV; see www.cdc gov/ncbddd/adhd/symptom.htm). ADHD may be widely acknowledged to affect adults, but only recently has an attempt been made to modify the DSM-IV criteria to accommodate the adult patient; changes so far have been limited to minor rewording. As the DSM-IV undergoes significant change at a conservative pace, individual practitioners must decide how best to apply the current criteria to adult patients.
Modifying the DSM-IV Criteria
Adult ADHD is a relatively new diagnostic category. Creating such categories to account for the symptoms of less impaired patients incurs the risk of ascribing pathology to conditions that lie at the margins of normality; hence the reluctance of DSM-IV editors to engage in rapid change. Weighed against their conservative approach, however, is the opportunity to treat individuals whose lives would benefit as a result. Thus, the editors of DSM-IV encourage its use as a guideline rather than a “cookbook.”20 The further practitioners move away from the comorbidities of mental health toward the merging of “soft” morbidity with normality, the more flexibility is required in applying the DSM-IV criteria.
For example, for a diagnosis of adult ADHD, one DSM-IV requirement is for the patient to have experienced onset of symptoms by age 7 (perhaps to distinguish it from confounding comorbidities that develop during or after adolescence). This seems unnecessarily restrictive and can be difficult to establish retrospectively. Even careful history-taking can be insufficient, as third-party observers are likely to be unavailable.9,13 Although recent studies have shown adult self-determined questionnaires to be an effective means of detecting ADHD impairment during childhood, many practitioners continue to question their validity.21 Proposals have been made to modify this age requirement, but agreement has not been reached on a new cutoff point (if one should be specified at all).
Because many “subthreshold” patients might benefit from pharmacotherapy, it has been suggested that the currently required number of DSM-IV criteria (six) should be relaxed. But what should that number be? Requiring only three criteria, researchers recently found, would result in 25% of all presenting patients qualifying for a diagnosis of ADHD.22 Most experts agree that a corresponding increase in psychostimulant use would be unjustified.
The willingness of practitioners to use the current DSM-IV criteria flexibly (eg, deciding what comprises a “clinically significant impairment”) will be determined by their level of comfort in diagnosing DSM-IV–defined disorders. Until continued research can provide more definitive diagnostic criteria for the adult with ADHD, a conservative approach may be advisable to avoid medicating patients unnecessarily.
Supplemental Rating Scales
Experts have developed several rating scales which, while not diagnostic, are nonetheless useful in identifying the adult who is impaired by ADHD but who may not strictly meet the DSM-IV criteria. These include:
• Wender Utah Rating Scale for Adult ADHD.23,24 One of the earliest and still most useful adjunctive rating scales for adult ADHD,23 this tool was originally developed to retrospectively identify childhood onset (before age 7) of ADHD symptoms. The original 61 items have been condensed and reorganized into a 25-item self-assessment questionnaire (see Table 123,24). The currently used Wender Utah scale seeks to elicit from the adult patient the core symptoms of ADHD.25
• Conners’ Adult ADHD Rating Scales (CAARS).26 This proprietary, 93-question, structured clinical interview allows the clinician to determine the presence of DSM-IV–defined symptoms of ADHD.27 Questions are grouped into nine symptom domains thought to encompass ADHD in adults, with responses categorized into four distinct problem areas21 (see Table 221,26,27). Although problematic responses appear to correlate highly with confirmed ADHD, a 15% misdiagnosis rate prevents use of the CAARS for definitive diagnosis.21
• Brown ADHD Rating Scale for Adults.28 This tool identifies five important symptom clusters (see Table 321,28). The Brown scale overlooks hyperactivity in adults and instead emphasizes executive functioning.
• Adult ADHD Self-Report Scale (ASRS-v1.1).29 This self-administered questionnaire, developed through the World Health Organization, assesses for 18 DSM-IV–designated ADHD symptoms, with the first six items found most predictive. If four of these six items are “scored” (ie, the patient responds “Often” or “Very often”), the assessment is considered highly consistent with adult ADHD, and further investigation is warranted. (For seven of the 18 items, an answer of “Sometimes” is also scored. See Table 4.29) Items 7 through 18 provide additional cues to distinguish specific symptoms or impairments.
No consensus exists regarding the reliable use of these rating scales to assist in the diagnosis of adult ADHD. As mentioned earlier, the role of patient self-reported symptoms of ADHD is subject to lingering controversy.27
ADHD Subtypes
Many practitioners find it aids diagnosis to divide ADHD into the subtypes shown in Table 519,27; subtypes 4 and 5 are often applicable in this patient population. According to Robison et al,30 however, women with ADHD are more likely to have the combined type (subtype 3) than men are (75% vs 62%, respectively), have a more complex presentation, and display greater impairment than men on all measures of ADHD symptoms.
Promise of Biomarkers
The neurobiologic basis of ADHD is poorly understood. Since medications known to have beneficial effects in ADHD alter dopamine levels,31 one prominent theory attributes the condition to dysfunction of dopamine neurotransmission and subsequent disruption of dopamine-modulated circuits among the frontal, striatal, and limbic regions of the brain.32 Early imaging studies using radioligands have shown a 70% increase in levels of the dopamine transporter molecule among subjects with ADHD, compared with non-ADHD controls.33 Subsequently, however, dopamine levels have been found to vary among subregions of the brain, suggesting that the explanation is probably more complex.32-34
ADHD medications also alter regulation of norepinephrine and possibly serotonin.31 The interplay in the brain between norepinephrine and dopamine is complex, and investigation of these processes is hampered by the current lack of a suitable radioligand that will bind selectively to the norepinephrine transporter molecule.35 Until an objective marker for ADHD is identified, the diagnosis of ADHD remains subjective and purely clinical.
Treatment
Current treatment recommendations for adult ADHD are almost exclusively pharmacologic. Effective, FDA-approved agents are methylphenidate, dextroamphetamine, and the nonstimulant atomoxetine. Treatment efficacy is determined by patient response, which is far from uniform or predictable. Selecting the optimal drug and dosage for each patient can be a lengthy process. Off-label use of other pharmacologic agents (eg, bupropion, clonidine, modafinil, and the tricyclic antidepressants31), combinations of agents, and medication for the ADHD patient with a history of substance abuse are treatments that are best left to a specialist.
Until recently, the role of behavioral therapy for children with ADHD had been somewhat discounted.36 In current research, behavioral therapy appears to help properly motivated adult patients understand their condition and develop appropriate coping skills.37 Self-referred adults with ADHD are usually motivated and compliant with prescribed treatment regimens.3
Currently, few therapeutic options are available for the ADHD-impaired adult who does not meet DSM-IV criteria for stimulant medications. If adults with confirmed ADHD benefit from behavioral therapies, however, their use to treat less impaired, subthreshold ADHD adults (for whom pharmacotherapy may not be warranted) is an intriguing possibility.
Conclusion
Previously considered a childhood-only disorder, ADHD is now known to persist into adulthood in many cases, often causing significant impairment in affected individuals. Ongoing research and brain imaging studies continue to improve our understanding of the neurobiologic basis for ADHD. Since no biomarker has yet been proved valid, diagnosis of ADHD remains subjective and clinical.
No consensus exists regarding the best diagnostic tools for ADHD in the young adult. Applying the DSM-IV criteria for ADHD to the adult patient is controversial. Until more objective data make it possible to modify these criteria, the primary care practitioner must rely on supplemental rating scales and other tools to gather diagnostic information.
Currently, schedule II stimulants and the FDA-approved nonstimulant atomoxetine are the most effective agents known for the adult patient with ADHD. Off-label medication use, combinations of medications, and the addition of behavioral therapy are best handled by a specialist.
1. Adler L, Cohen J. Diagnosis and evaluation of adults with attention-deficit/hyperactivity disorder. Psychiatr Clin North Am. 2004;27(2):187-201.
2. Wilens TE, Dodson W. A clinical perspective of attention-deficit/hyperactivity disorder into adulthood. J Clin Psychiatry. 2004;65(10):1301-1313.
3. Faraone SV, Spencer TJ, Montano CB, Biederman J. Attention-deficit/hyperactivity disorder in adults: a survey of current practice in psychiatry and primary care. Arch Intern Med. 2004;164(11):1221-1226.
4. Wilens TE, Gignac M, Swezey A, et al. Characteristics of adolescents and young adults with ADHD who divert or misuse their prescribed medications. J Am Acad Child Adolesc Psychiatry. 2006;45(4):408-414.
5. Diller LH. Running on Ritalin: A Physician Reflects on Children, Society, and Performance in a Pill. New York, NY: Bantam Books; 1999.
6. Zwi M, Pindoria S, Joughin C. Parent training interventions in attention-deficit/hyperactivity disorder (protocol). Cochrane Database Syst Rev. 2001(2):CD003018.
7. Kliegman RM, Marcdante KJ, Jenson HB, Behrman RE. Nelson Essentials of Pediatrics. 5th ed. Philadelphia, PA: Saunders; 2005.
8. National Institutes of Health Consensus Development Conference Statement: diagnosis and treatment of attention-deficit/hyperactivity (ADHD). J Am Acad Child Adolesc Psychiatry. 2000;39(2):182-193.
9. Adler LA. Clinical presentations of adult patients with ADHD. J Clin Psychiatry. 2004;65 Suppl 3:8-11.
10. Biederman J. Impact of comorbidity in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2004;65 Suppl 3:3-7.
11. Meaux JB, Hester C, Smith B, Shoptaw A. Stimulant medications: a trade-off? The lived experience of adolescents with ADHD. J Spec Pediatr Nurs. 2006;11(4):214-226.
12. Culpepper L. Primary care treatment of attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2006;67 Suppl 8:51-58.
13. McGough JJ, Barkley RA. Diagnostic controversies in adult attention deficit hyperactivity disorder. Am J Psychiatry. 2004;161(11):1948-1956.
14. Arnold LE. Alternative treatments for adults with attention-deficit hyperactivity disorder (ADHD). Ann N Y Acad Sci. 2001;931:310-341.
15. Bradley C. The behavior of children receiving benzedrine. Am J Psychiatry. 1937;94(3):577-585.
16. Wilens TE, Biederman J, Spencer TJ. Attention deficit/hyperactivity disorder across the lifespan. Annu Rev Med. 2002;53:113-131.
17. Gau SS, Kessler RC, Tseng WL, et al. Association between sleep problems and symptoms of attention-deficit/hyperactivity disorder in young adults. Sleep. 2007;30(2):195-201.
18. Barkley RA, Fischer M, Smallish L, Fletcher K. Young adult outcomes of hyperactive children: adaptive functioning in major life activities. J Am Acad Child Adolesc Psychiatry. 2006;45(2):192-202.
19. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. (Text Revision: DSM-IV-TR). Washington, DC: American Psychiatric Association; 2000:85-93.
20. Frances A, First MB, Pincus HA. DSM-IV Guidebook. Arlington, VA: American Psychiatric Publishing Group; 1995.
21. Bowes M. ADHD in adults: definition and diagnosis. Neuropsychiatry Rev. 2001;2(1):22, 24-25.
22. Faraone SV, Biederman J, Spencer T, et al. Diagnosing adult attention deficit hyperactivity disorder: are late onset and subthreshold diagnoses valid? Am J Psychiatry. 2006;163(10):1720-1729.
23. McCann BS, Scheele L, Ward N, Roy-Byrne P. Discriminant validity of the Wender Utah Rating Scale for attention-deficit/hyperactivity disorder in adults. J Neuropsychiatry Clin Neurosci. 2000;12(2):240-245.
24. Ward MF, Wender PH, Reimherr FW. The Wender Utah Rating Scale: an aid in the retrospective diagnosis of childhood attention deficit hyperactivity disorder. Am J Psychiatry. 1993;150(6):885-890.
25. McCann BS, Roy-Byrne P. Attention-deficit/hyperactivity disorder and learning disabilities in adults. Semin Clin Neuropsychiatry. 2000;5(3):191-197.
26. Conners CK, Erhart D, Sparrow E. Conners’ Adult ADHD Rating Scales, Technical Manual. New York, NY: Multi-Health Systems; 1999.
27. Murphy KR, Adler LA. Assessing attention-deficit/hyperactivity disorder in adults: focus on rating scales. J Clin Psychiatry. 2004;65 Suppl 3:12-17.
28. Brown TE. Brown Attention-Deficit Disorder Scales. San Antonio, TX: Psychological Corporation; 1996.
29. World Health Organization. Adult ADHD Self-Report Scale (ASRS-v1.1; 2003). www.med.nyu.edu/psych/assets/adhd screen18.pdf. Accessed August 21, 2008.
30. Robison RH, Reimherr FW, Marchant BK, et al. Gender differences in 2 clinical trials of adults with attention-deficit/hyperactivity disorder: a retrospective date analysis. J Clin Psychiatry. 2008;69(2):213-221.
31. Wilens TE. Mechanism of action of agents used in attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2006;67 Suppl 8:32-38.
32. Volkow ND, Wang GJ, Newcorn J, et al. Brain dopamine transporter levels in treatment and drug naïve adults with ADHD. Neuroimage. 2007;34(3):1182-1190.
33. Spencer TJ, Biederman J, Madras BK, et al. Further evidence of dopamine transporter dysregulation in ADHD: a controlled PET imaging study using altropane. Biol Psychiatry. 2007;62(9):1059-1061.
34. Spencer TJ, Biederman J, Madras BK, et al. In vivo neuroreceptor imaging in attention-deficit/hyperactivity disorder: a focus on the dopamine transporter. Biol Psychiatry. 2005;57(11):1293-1300.
35. Volkow ND, Wang GJ, Fowler JS, Ding YS. Imaging the effects of methylphenidate on brain dopamine: new model on its therapeutic actions for attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57(11):1410-1415.
36. MTA Cooperative Group. A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder: Multimodal Treatment Study of Children with ADHD. Arch Gen Psychiatry. 1999;56(12):1073-1086.
37. Safren SA. Cognitive-behavioral approaches to ADHD treatment in adulthood. J Clin Psychiatry. 2006;67 Suppl 8:46-50.
Attention-deficit/hyperactivity disorder (ADHD) was once thought to be limited to overactive or inattentive children. Yet recent studies have shown that ADHD has a 50% to 60% persistence rate into adulthood and may affect as many as seven million adults in the United States today, impairing the ability of many to function productively.1,2 A significant number of adolescents previously diagnosed with ADHD but not currently receiving treatment are emerging into young adulthood. Some patients are prompted to seek help when their ADHD symptoms interfere with daily functioning; in others, ADHD is identified when they seek treatment for other conditions.
Most older ADHD patients initially present in the primary care setting,3 where practitioners may be reluctant to treat them because:
(1) The diagnosis of ADHD is subjective and purely clinical.
(2) It is unclear how the presently published diagnostic criteria should be applied to adults.
(3) The ADHD treatments proven most effective are schedule II psychostimulants, which have a certain potential for abuse.4
This article summarizes a review of currently accepted practice in primary care for recognizing and treating ADHD in the young adult patient.
Background
Between 1990 and 1998, the number of school-age children diagnosed with ADHD reportedly increased by 700%.5 The accompanying increase in use of schedule II stimulants6 (primarily methylphenidate and dexamphetamine) aroused some controversy, even though these medications were shown to be effective in reducing the inattentiveness, impulsivity, and hyperactivity associated with ADHD.7,8
Concerns regarding the indicated medications—possible abuse, associated adverse effects, inconvenience, stigma—prompted many parents of affected children to decline pharmacologic treatment.9 Among treated children, a large proportion discontinued their medications because they did not have a response or experienced intolerable adverse effects.10,11 Still others were never diagnosed. As a result, a significant number of adolescents with untreated or undertreated ADHD are now entering adulthood. Without treatment now, perhaps half of them will experience lifelong impairment resulting from ADHD and associated comorbidities (ie, conduct and oppositional-defiant disorder, antisocial personality disorder, substance abuse disorder, anxiety disorders, and depression).1,2,9,10,12
ADHD is believed to have a solid neurobiologic basis, but the condition has no known objective markers. Its diagnosis remains subjective and clinical, depending primarily on structured interviews conducted by trained practitioners.13 Unlike most behavioral disorders, which are first understood in adults, then extrapolated to children,14 ADHD has been recognized and treated in children since the late 1930s15 but has only recently been identified among significant numbers of adults.1 Thus, ADHD is currently best understood in children.14
The classic symptoms and signs of ADHD and its subtypes undergo subtle alterations as the patient matures.2,12,16 Hyperactivity wanes in adolescence and may be replaced by a restlessness that prompts the adult patient to change jobs and/or living quarters frequently, leading to an unstable lifestyle.12 Although impulsivity and inattention may persist through adolescence into adulthood, they are often obscured by both coping mechanisms (eg, choice of employment, conscious efforts by high achievers to overcome their disorganization)1 and behavioral comorbidities (depression, self-medication/substance abuse, personality disorders) that the patient may have developed.10,16 These developments may significantly complicate identification of the disorder in older patients.2,12,10,16
The adult with ADHD often exhibits low self-esteem, anxiety, depression, sleep disturbances,17 difficulties with personal relationships and jobs, and impulsivity, which can lead to trouble with the law.18 The costs to adult ADHD patients, their families, and the community are enormous, making it all the more important for health care professionals to understand this condition.
Diagnostic Criteria
The diagnosis of ADHD is based on criteria from the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition7,19(DSM-IV; see www.cdc gov/ncbddd/adhd/symptom.htm). ADHD may be widely acknowledged to affect adults, but only recently has an attempt been made to modify the DSM-IV criteria to accommodate the adult patient; changes so far have been limited to minor rewording. As the DSM-IV undergoes significant change at a conservative pace, individual practitioners must decide how best to apply the current criteria to adult patients.
Modifying the DSM-IV Criteria
Adult ADHD is a relatively new diagnostic category. Creating such categories to account for the symptoms of less impaired patients incurs the risk of ascribing pathology to conditions that lie at the margins of normality; hence the reluctance of DSM-IV editors to engage in rapid change. Weighed against their conservative approach, however, is the opportunity to treat individuals whose lives would benefit as a result. Thus, the editors of DSM-IV encourage its use as a guideline rather than a “cookbook.”20 The further practitioners move away from the comorbidities of mental health toward the merging of “soft” morbidity with normality, the more flexibility is required in applying the DSM-IV criteria.
For example, for a diagnosis of adult ADHD, one DSM-IV requirement is for the patient to have experienced onset of symptoms by age 7 (perhaps to distinguish it from confounding comorbidities that develop during or after adolescence). This seems unnecessarily restrictive and can be difficult to establish retrospectively. Even careful history-taking can be insufficient, as third-party observers are likely to be unavailable.9,13 Although recent studies have shown adult self-determined questionnaires to be an effective means of detecting ADHD impairment during childhood, many practitioners continue to question their validity.21 Proposals have been made to modify this age requirement, but agreement has not been reached on a new cutoff point (if one should be specified at all).
Because many “subthreshold” patients might benefit from pharmacotherapy, it has been suggested that the currently required number of DSM-IV criteria (six) should be relaxed. But what should that number be? Requiring only three criteria, researchers recently found, would result in 25% of all presenting patients qualifying for a diagnosis of ADHD.22 Most experts agree that a corresponding increase in psychostimulant use would be unjustified.
The willingness of practitioners to use the current DSM-IV criteria flexibly (eg, deciding what comprises a “clinically significant impairment”) will be determined by their level of comfort in diagnosing DSM-IV–defined disorders. Until continued research can provide more definitive diagnostic criteria for the adult with ADHD, a conservative approach may be advisable to avoid medicating patients unnecessarily.
Supplemental Rating Scales
Experts have developed several rating scales which, while not diagnostic, are nonetheless useful in identifying the adult who is impaired by ADHD but who may not strictly meet the DSM-IV criteria. These include:
• Wender Utah Rating Scale for Adult ADHD.23,24 One of the earliest and still most useful adjunctive rating scales for adult ADHD,23 this tool was originally developed to retrospectively identify childhood onset (before age 7) of ADHD symptoms. The original 61 items have been condensed and reorganized into a 25-item self-assessment questionnaire (see Table 123,24). The currently used Wender Utah scale seeks to elicit from the adult patient the core symptoms of ADHD.25
• Conners’ Adult ADHD Rating Scales (CAARS).26 This proprietary, 93-question, structured clinical interview allows the clinician to determine the presence of DSM-IV–defined symptoms of ADHD.27 Questions are grouped into nine symptom domains thought to encompass ADHD in adults, with responses categorized into four distinct problem areas21 (see Table 221,26,27). Although problematic responses appear to correlate highly with confirmed ADHD, a 15% misdiagnosis rate prevents use of the CAARS for definitive diagnosis.21
• Brown ADHD Rating Scale for Adults.28 This tool identifies five important symptom clusters (see Table 321,28). The Brown scale overlooks hyperactivity in adults and instead emphasizes executive functioning.
• Adult ADHD Self-Report Scale (ASRS-v1.1).29 This self-administered questionnaire, developed through the World Health Organization, assesses for 18 DSM-IV–designated ADHD symptoms, with the first six items found most predictive. If four of these six items are “scored” (ie, the patient responds “Often” or “Very often”), the assessment is considered highly consistent with adult ADHD, and further investigation is warranted. (For seven of the 18 items, an answer of “Sometimes” is also scored. See Table 4.29) Items 7 through 18 provide additional cues to distinguish specific symptoms or impairments.
No consensus exists regarding the reliable use of these rating scales to assist in the diagnosis of adult ADHD. As mentioned earlier, the role of patient self-reported symptoms of ADHD is subject to lingering controversy.27
ADHD Subtypes
Many practitioners find it aids diagnosis to divide ADHD into the subtypes shown in Table 519,27; subtypes 4 and 5 are often applicable in this patient population. According to Robison et al,30 however, women with ADHD are more likely to have the combined type (subtype 3) than men are (75% vs 62%, respectively), have a more complex presentation, and display greater impairment than men on all measures of ADHD symptoms.
Promise of Biomarkers
The neurobiologic basis of ADHD is poorly understood. Since medications known to have beneficial effects in ADHD alter dopamine levels,31 one prominent theory attributes the condition to dysfunction of dopamine neurotransmission and subsequent disruption of dopamine-modulated circuits among the frontal, striatal, and limbic regions of the brain.32 Early imaging studies using radioligands have shown a 70% increase in levels of the dopamine transporter molecule among subjects with ADHD, compared with non-ADHD controls.33 Subsequently, however, dopamine levels have been found to vary among subregions of the brain, suggesting that the explanation is probably more complex.32-34
ADHD medications also alter regulation of norepinephrine and possibly serotonin.31 The interplay in the brain between norepinephrine and dopamine is complex, and investigation of these processes is hampered by the current lack of a suitable radioligand that will bind selectively to the norepinephrine transporter molecule.35 Until an objective marker for ADHD is identified, the diagnosis of ADHD remains subjective and purely clinical.
Treatment
Current treatment recommendations for adult ADHD are almost exclusively pharmacologic. Effective, FDA-approved agents are methylphenidate, dextroamphetamine, and the nonstimulant atomoxetine. Treatment efficacy is determined by patient response, which is far from uniform or predictable. Selecting the optimal drug and dosage for each patient can be a lengthy process. Off-label use of other pharmacologic agents (eg, bupropion, clonidine, modafinil, and the tricyclic antidepressants31), combinations of agents, and medication for the ADHD patient with a history of substance abuse are treatments that are best left to a specialist.
Until recently, the role of behavioral therapy for children with ADHD had been somewhat discounted.36 In current research, behavioral therapy appears to help properly motivated adult patients understand their condition and develop appropriate coping skills.37 Self-referred adults with ADHD are usually motivated and compliant with prescribed treatment regimens.3
Currently, few therapeutic options are available for the ADHD-impaired adult who does not meet DSM-IV criteria for stimulant medications. If adults with confirmed ADHD benefit from behavioral therapies, however, their use to treat less impaired, subthreshold ADHD adults (for whom pharmacotherapy may not be warranted) is an intriguing possibility.
Conclusion
Previously considered a childhood-only disorder, ADHD is now known to persist into adulthood in many cases, often causing significant impairment in affected individuals. Ongoing research and brain imaging studies continue to improve our understanding of the neurobiologic basis for ADHD. Since no biomarker has yet been proved valid, diagnosis of ADHD remains subjective and clinical.
No consensus exists regarding the best diagnostic tools for ADHD in the young adult. Applying the DSM-IV criteria for ADHD to the adult patient is controversial. Until more objective data make it possible to modify these criteria, the primary care practitioner must rely on supplemental rating scales and other tools to gather diagnostic information.
Currently, schedule II stimulants and the FDA-approved nonstimulant atomoxetine are the most effective agents known for the adult patient with ADHD. Off-label medication use, combinations of medications, and the addition of behavioral therapy are best handled by a specialist.
Attention-deficit/hyperactivity disorder (ADHD) was once thought to be limited to overactive or inattentive children. Yet recent studies have shown that ADHD has a 50% to 60% persistence rate into adulthood and may affect as many as seven million adults in the United States today, impairing the ability of many to function productively.1,2 A significant number of adolescents previously diagnosed with ADHD but not currently receiving treatment are emerging into young adulthood. Some patients are prompted to seek help when their ADHD symptoms interfere with daily functioning; in others, ADHD is identified when they seek treatment for other conditions.
Most older ADHD patients initially present in the primary care setting,3 where practitioners may be reluctant to treat them because:
(1) The diagnosis of ADHD is subjective and purely clinical.
(2) It is unclear how the presently published diagnostic criteria should be applied to adults.
(3) The ADHD treatments proven most effective are schedule II psychostimulants, which have a certain potential for abuse.4
This article summarizes a review of currently accepted practice in primary care for recognizing and treating ADHD in the young adult patient.
Background
Between 1990 and 1998, the number of school-age children diagnosed with ADHD reportedly increased by 700%.5 The accompanying increase in use of schedule II stimulants6 (primarily methylphenidate and dexamphetamine) aroused some controversy, even though these medications were shown to be effective in reducing the inattentiveness, impulsivity, and hyperactivity associated with ADHD.7,8
Concerns regarding the indicated medications—possible abuse, associated adverse effects, inconvenience, stigma—prompted many parents of affected children to decline pharmacologic treatment.9 Among treated children, a large proportion discontinued their medications because they did not have a response or experienced intolerable adverse effects.10,11 Still others were never diagnosed. As a result, a significant number of adolescents with untreated or undertreated ADHD are now entering adulthood. Without treatment now, perhaps half of them will experience lifelong impairment resulting from ADHD and associated comorbidities (ie, conduct and oppositional-defiant disorder, antisocial personality disorder, substance abuse disorder, anxiety disorders, and depression).1,2,9,10,12
ADHD is believed to have a solid neurobiologic basis, but the condition has no known objective markers. Its diagnosis remains subjective and clinical, depending primarily on structured interviews conducted by trained practitioners.13 Unlike most behavioral disorders, which are first understood in adults, then extrapolated to children,14 ADHD has been recognized and treated in children since the late 1930s15 but has only recently been identified among significant numbers of adults.1 Thus, ADHD is currently best understood in children.14
The classic symptoms and signs of ADHD and its subtypes undergo subtle alterations as the patient matures.2,12,16 Hyperactivity wanes in adolescence and may be replaced by a restlessness that prompts the adult patient to change jobs and/or living quarters frequently, leading to an unstable lifestyle.12 Although impulsivity and inattention may persist through adolescence into adulthood, they are often obscured by both coping mechanisms (eg, choice of employment, conscious efforts by high achievers to overcome their disorganization)1 and behavioral comorbidities (depression, self-medication/substance abuse, personality disorders) that the patient may have developed.10,16 These developments may significantly complicate identification of the disorder in older patients.2,12,10,16
The adult with ADHD often exhibits low self-esteem, anxiety, depression, sleep disturbances,17 difficulties with personal relationships and jobs, and impulsivity, which can lead to trouble with the law.18 The costs to adult ADHD patients, their families, and the community are enormous, making it all the more important for health care professionals to understand this condition.
Diagnostic Criteria
The diagnosis of ADHD is based on criteria from the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition7,19(DSM-IV; see www.cdc gov/ncbddd/adhd/symptom.htm). ADHD may be widely acknowledged to affect adults, but only recently has an attempt been made to modify the DSM-IV criteria to accommodate the adult patient; changes so far have been limited to minor rewording. As the DSM-IV undergoes significant change at a conservative pace, individual practitioners must decide how best to apply the current criteria to adult patients.
Modifying the DSM-IV Criteria
Adult ADHD is a relatively new diagnostic category. Creating such categories to account for the symptoms of less impaired patients incurs the risk of ascribing pathology to conditions that lie at the margins of normality; hence the reluctance of DSM-IV editors to engage in rapid change. Weighed against their conservative approach, however, is the opportunity to treat individuals whose lives would benefit as a result. Thus, the editors of DSM-IV encourage its use as a guideline rather than a “cookbook.”20 The further practitioners move away from the comorbidities of mental health toward the merging of “soft” morbidity with normality, the more flexibility is required in applying the DSM-IV criteria.
For example, for a diagnosis of adult ADHD, one DSM-IV requirement is for the patient to have experienced onset of symptoms by age 7 (perhaps to distinguish it from confounding comorbidities that develop during or after adolescence). This seems unnecessarily restrictive and can be difficult to establish retrospectively. Even careful history-taking can be insufficient, as third-party observers are likely to be unavailable.9,13 Although recent studies have shown adult self-determined questionnaires to be an effective means of detecting ADHD impairment during childhood, many practitioners continue to question their validity.21 Proposals have been made to modify this age requirement, but agreement has not been reached on a new cutoff point (if one should be specified at all).
Because many “subthreshold” patients might benefit from pharmacotherapy, it has been suggested that the currently required number of DSM-IV criteria (six) should be relaxed. But what should that number be? Requiring only three criteria, researchers recently found, would result in 25% of all presenting patients qualifying for a diagnosis of ADHD.22 Most experts agree that a corresponding increase in psychostimulant use would be unjustified.
The willingness of practitioners to use the current DSM-IV criteria flexibly (eg, deciding what comprises a “clinically significant impairment”) will be determined by their level of comfort in diagnosing DSM-IV–defined disorders. Until continued research can provide more definitive diagnostic criteria for the adult with ADHD, a conservative approach may be advisable to avoid medicating patients unnecessarily.
Supplemental Rating Scales
Experts have developed several rating scales which, while not diagnostic, are nonetheless useful in identifying the adult who is impaired by ADHD but who may not strictly meet the DSM-IV criteria. These include:
• Wender Utah Rating Scale for Adult ADHD.23,24 One of the earliest and still most useful adjunctive rating scales for adult ADHD,23 this tool was originally developed to retrospectively identify childhood onset (before age 7) of ADHD symptoms. The original 61 items have been condensed and reorganized into a 25-item self-assessment questionnaire (see Table 123,24). The currently used Wender Utah scale seeks to elicit from the adult patient the core symptoms of ADHD.25
• Conners’ Adult ADHD Rating Scales (CAARS).26 This proprietary, 93-question, structured clinical interview allows the clinician to determine the presence of DSM-IV–defined symptoms of ADHD.27 Questions are grouped into nine symptom domains thought to encompass ADHD in adults, with responses categorized into four distinct problem areas21 (see Table 221,26,27). Although problematic responses appear to correlate highly with confirmed ADHD, a 15% misdiagnosis rate prevents use of the CAARS for definitive diagnosis.21
• Brown ADHD Rating Scale for Adults.28 This tool identifies five important symptom clusters (see Table 321,28). The Brown scale overlooks hyperactivity in adults and instead emphasizes executive functioning.
• Adult ADHD Self-Report Scale (ASRS-v1.1).29 This self-administered questionnaire, developed through the World Health Organization, assesses for 18 DSM-IV–designated ADHD symptoms, with the first six items found most predictive. If four of these six items are “scored” (ie, the patient responds “Often” or “Very often”), the assessment is considered highly consistent with adult ADHD, and further investigation is warranted. (For seven of the 18 items, an answer of “Sometimes” is also scored. See Table 4.29) Items 7 through 18 provide additional cues to distinguish specific symptoms or impairments.
No consensus exists regarding the reliable use of these rating scales to assist in the diagnosis of adult ADHD. As mentioned earlier, the role of patient self-reported symptoms of ADHD is subject to lingering controversy.27
ADHD Subtypes
Many practitioners find it aids diagnosis to divide ADHD into the subtypes shown in Table 519,27; subtypes 4 and 5 are often applicable in this patient population. According to Robison et al,30 however, women with ADHD are more likely to have the combined type (subtype 3) than men are (75% vs 62%, respectively), have a more complex presentation, and display greater impairment than men on all measures of ADHD symptoms.
Promise of Biomarkers
The neurobiologic basis of ADHD is poorly understood. Since medications known to have beneficial effects in ADHD alter dopamine levels,31 one prominent theory attributes the condition to dysfunction of dopamine neurotransmission and subsequent disruption of dopamine-modulated circuits among the frontal, striatal, and limbic regions of the brain.32 Early imaging studies using radioligands have shown a 70% increase in levels of the dopamine transporter molecule among subjects with ADHD, compared with non-ADHD controls.33 Subsequently, however, dopamine levels have been found to vary among subregions of the brain, suggesting that the explanation is probably more complex.32-34
ADHD medications also alter regulation of norepinephrine and possibly serotonin.31 The interplay in the brain between norepinephrine and dopamine is complex, and investigation of these processes is hampered by the current lack of a suitable radioligand that will bind selectively to the norepinephrine transporter molecule.35 Until an objective marker for ADHD is identified, the diagnosis of ADHD remains subjective and purely clinical.
Treatment
Current treatment recommendations for adult ADHD are almost exclusively pharmacologic. Effective, FDA-approved agents are methylphenidate, dextroamphetamine, and the nonstimulant atomoxetine. Treatment efficacy is determined by patient response, which is far from uniform or predictable. Selecting the optimal drug and dosage for each patient can be a lengthy process. Off-label use of other pharmacologic agents (eg, bupropion, clonidine, modafinil, and the tricyclic antidepressants31), combinations of agents, and medication for the ADHD patient with a history of substance abuse are treatments that are best left to a specialist.
Until recently, the role of behavioral therapy for children with ADHD had been somewhat discounted.36 In current research, behavioral therapy appears to help properly motivated adult patients understand their condition and develop appropriate coping skills.37 Self-referred adults with ADHD are usually motivated and compliant with prescribed treatment regimens.3
Currently, few therapeutic options are available for the ADHD-impaired adult who does not meet DSM-IV criteria for stimulant medications. If adults with confirmed ADHD benefit from behavioral therapies, however, their use to treat less impaired, subthreshold ADHD adults (for whom pharmacotherapy may not be warranted) is an intriguing possibility.
Conclusion
Previously considered a childhood-only disorder, ADHD is now known to persist into adulthood in many cases, often causing significant impairment in affected individuals. Ongoing research and brain imaging studies continue to improve our understanding of the neurobiologic basis for ADHD. Since no biomarker has yet been proved valid, diagnosis of ADHD remains subjective and clinical.
No consensus exists regarding the best diagnostic tools for ADHD in the young adult. Applying the DSM-IV criteria for ADHD to the adult patient is controversial. Until more objective data make it possible to modify these criteria, the primary care practitioner must rely on supplemental rating scales and other tools to gather diagnostic information.
Currently, schedule II stimulants and the FDA-approved nonstimulant atomoxetine are the most effective agents known for the adult patient with ADHD. Off-label medication use, combinations of medications, and the addition of behavioral therapy are best handled by a specialist.
1. Adler L, Cohen J. Diagnosis and evaluation of adults with attention-deficit/hyperactivity disorder. Psychiatr Clin North Am. 2004;27(2):187-201.
2. Wilens TE, Dodson W. A clinical perspective of attention-deficit/hyperactivity disorder into adulthood. J Clin Psychiatry. 2004;65(10):1301-1313.
3. Faraone SV, Spencer TJ, Montano CB, Biederman J. Attention-deficit/hyperactivity disorder in adults: a survey of current practice in psychiatry and primary care. Arch Intern Med. 2004;164(11):1221-1226.
4. Wilens TE, Gignac M, Swezey A, et al. Characteristics of adolescents and young adults with ADHD who divert or misuse their prescribed medications. J Am Acad Child Adolesc Psychiatry. 2006;45(4):408-414.
5. Diller LH. Running on Ritalin: A Physician Reflects on Children, Society, and Performance in a Pill. New York, NY: Bantam Books; 1999.
6. Zwi M, Pindoria S, Joughin C. Parent training interventions in attention-deficit/hyperactivity disorder (protocol). Cochrane Database Syst Rev. 2001(2):CD003018.
7. Kliegman RM, Marcdante KJ, Jenson HB, Behrman RE. Nelson Essentials of Pediatrics. 5th ed. Philadelphia, PA: Saunders; 2005.
8. National Institutes of Health Consensus Development Conference Statement: diagnosis and treatment of attention-deficit/hyperactivity (ADHD). J Am Acad Child Adolesc Psychiatry. 2000;39(2):182-193.
9. Adler LA. Clinical presentations of adult patients with ADHD. J Clin Psychiatry. 2004;65 Suppl 3:8-11.
10. Biederman J. Impact of comorbidity in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2004;65 Suppl 3:3-7.
11. Meaux JB, Hester C, Smith B, Shoptaw A. Stimulant medications: a trade-off? The lived experience of adolescents with ADHD. J Spec Pediatr Nurs. 2006;11(4):214-226.
12. Culpepper L. Primary care treatment of attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2006;67 Suppl 8:51-58.
13. McGough JJ, Barkley RA. Diagnostic controversies in adult attention deficit hyperactivity disorder. Am J Psychiatry. 2004;161(11):1948-1956.
14. Arnold LE. Alternative treatments for adults with attention-deficit hyperactivity disorder (ADHD). Ann N Y Acad Sci. 2001;931:310-341.
15. Bradley C. The behavior of children receiving benzedrine. Am J Psychiatry. 1937;94(3):577-585.
16. Wilens TE, Biederman J, Spencer TJ. Attention deficit/hyperactivity disorder across the lifespan. Annu Rev Med. 2002;53:113-131.
17. Gau SS, Kessler RC, Tseng WL, et al. Association between sleep problems and symptoms of attention-deficit/hyperactivity disorder in young adults. Sleep. 2007;30(2):195-201.
18. Barkley RA, Fischer M, Smallish L, Fletcher K. Young adult outcomes of hyperactive children: adaptive functioning in major life activities. J Am Acad Child Adolesc Psychiatry. 2006;45(2):192-202.
19. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. (Text Revision: DSM-IV-TR). Washington, DC: American Psychiatric Association; 2000:85-93.
20. Frances A, First MB, Pincus HA. DSM-IV Guidebook. Arlington, VA: American Psychiatric Publishing Group; 1995.
21. Bowes M. ADHD in adults: definition and diagnosis. Neuropsychiatry Rev. 2001;2(1):22, 24-25.
22. Faraone SV, Biederman J, Spencer T, et al. Diagnosing adult attention deficit hyperactivity disorder: are late onset and subthreshold diagnoses valid? Am J Psychiatry. 2006;163(10):1720-1729.
23. McCann BS, Scheele L, Ward N, Roy-Byrne P. Discriminant validity of the Wender Utah Rating Scale for attention-deficit/hyperactivity disorder in adults. J Neuropsychiatry Clin Neurosci. 2000;12(2):240-245.
24. Ward MF, Wender PH, Reimherr FW. The Wender Utah Rating Scale: an aid in the retrospective diagnosis of childhood attention deficit hyperactivity disorder. Am J Psychiatry. 1993;150(6):885-890.
25. McCann BS, Roy-Byrne P. Attention-deficit/hyperactivity disorder and learning disabilities in adults. Semin Clin Neuropsychiatry. 2000;5(3):191-197.
26. Conners CK, Erhart D, Sparrow E. Conners’ Adult ADHD Rating Scales, Technical Manual. New York, NY: Multi-Health Systems; 1999.
27. Murphy KR, Adler LA. Assessing attention-deficit/hyperactivity disorder in adults: focus on rating scales. J Clin Psychiatry. 2004;65 Suppl 3:12-17.
28. Brown TE. Brown Attention-Deficit Disorder Scales. San Antonio, TX: Psychological Corporation; 1996.
29. World Health Organization. Adult ADHD Self-Report Scale (ASRS-v1.1; 2003). www.med.nyu.edu/psych/assets/adhd screen18.pdf. Accessed August 21, 2008.
30. Robison RH, Reimherr FW, Marchant BK, et al. Gender differences in 2 clinical trials of adults with attention-deficit/hyperactivity disorder: a retrospective date analysis. J Clin Psychiatry. 2008;69(2):213-221.
31. Wilens TE. Mechanism of action of agents used in attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2006;67 Suppl 8:32-38.
32. Volkow ND, Wang GJ, Newcorn J, et al. Brain dopamine transporter levels in treatment and drug naïve adults with ADHD. Neuroimage. 2007;34(3):1182-1190.
33. Spencer TJ, Biederman J, Madras BK, et al. Further evidence of dopamine transporter dysregulation in ADHD: a controlled PET imaging study using altropane. Biol Psychiatry. 2007;62(9):1059-1061.
34. Spencer TJ, Biederman J, Madras BK, et al. In vivo neuroreceptor imaging in attention-deficit/hyperactivity disorder: a focus on the dopamine transporter. Biol Psychiatry. 2005;57(11):1293-1300.
35. Volkow ND, Wang GJ, Fowler JS, Ding YS. Imaging the effects of methylphenidate on brain dopamine: new model on its therapeutic actions for attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57(11):1410-1415.
36. MTA Cooperative Group. A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder: Multimodal Treatment Study of Children with ADHD. Arch Gen Psychiatry. 1999;56(12):1073-1086.
37. Safren SA. Cognitive-behavioral approaches to ADHD treatment in adulthood. J Clin Psychiatry. 2006;67 Suppl 8:46-50.
1. Adler L, Cohen J. Diagnosis and evaluation of adults with attention-deficit/hyperactivity disorder. Psychiatr Clin North Am. 2004;27(2):187-201.
2. Wilens TE, Dodson W. A clinical perspective of attention-deficit/hyperactivity disorder into adulthood. J Clin Psychiatry. 2004;65(10):1301-1313.
3. Faraone SV, Spencer TJ, Montano CB, Biederman J. Attention-deficit/hyperactivity disorder in adults: a survey of current practice in psychiatry and primary care. Arch Intern Med. 2004;164(11):1221-1226.
4. Wilens TE, Gignac M, Swezey A, et al. Characteristics of adolescents and young adults with ADHD who divert or misuse their prescribed medications. J Am Acad Child Adolesc Psychiatry. 2006;45(4):408-414.
5. Diller LH. Running on Ritalin: A Physician Reflects on Children, Society, and Performance in a Pill. New York, NY: Bantam Books; 1999.
6. Zwi M, Pindoria S, Joughin C. Parent training interventions in attention-deficit/hyperactivity disorder (protocol). Cochrane Database Syst Rev. 2001(2):CD003018.
7. Kliegman RM, Marcdante KJ, Jenson HB, Behrman RE. Nelson Essentials of Pediatrics. 5th ed. Philadelphia, PA: Saunders; 2005.
8. National Institutes of Health Consensus Development Conference Statement: diagnosis and treatment of attention-deficit/hyperactivity (ADHD). J Am Acad Child Adolesc Psychiatry. 2000;39(2):182-193.
9. Adler LA. Clinical presentations of adult patients with ADHD. J Clin Psychiatry. 2004;65 Suppl 3:8-11.
10. Biederman J. Impact of comorbidity in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2004;65 Suppl 3:3-7.
11. Meaux JB, Hester C, Smith B, Shoptaw A. Stimulant medications: a trade-off? The lived experience of adolescents with ADHD. J Spec Pediatr Nurs. 2006;11(4):214-226.
12. Culpepper L. Primary care treatment of attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2006;67 Suppl 8:51-58.
13. McGough JJ, Barkley RA. Diagnostic controversies in adult attention deficit hyperactivity disorder. Am J Psychiatry. 2004;161(11):1948-1956.
14. Arnold LE. Alternative treatments for adults with attention-deficit hyperactivity disorder (ADHD). Ann N Y Acad Sci. 2001;931:310-341.
15. Bradley C. The behavior of children receiving benzedrine. Am J Psychiatry. 1937;94(3):577-585.
16. Wilens TE, Biederman J, Spencer TJ. Attention deficit/hyperactivity disorder across the lifespan. Annu Rev Med. 2002;53:113-131.
17. Gau SS, Kessler RC, Tseng WL, et al. Association between sleep problems and symptoms of attention-deficit/hyperactivity disorder in young adults. Sleep. 2007;30(2):195-201.
18. Barkley RA, Fischer M, Smallish L, Fletcher K. Young adult outcomes of hyperactive children: adaptive functioning in major life activities. J Am Acad Child Adolesc Psychiatry. 2006;45(2):192-202.
19. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. (Text Revision: DSM-IV-TR). Washington, DC: American Psychiatric Association; 2000:85-93.
20. Frances A, First MB, Pincus HA. DSM-IV Guidebook. Arlington, VA: American Psychiatric Publishing Group; 1995.
21. Bowes M. ADHD in adults: definition and diagnosis. Neuropsychiatry Rev. 2001;2(1):22, 24-25.
22. Faraone SV, Biederman J, Spencer T, et al. Diagnosing adult attention deficit hyperactivity disorder: are late onset and subthreshold diagnoses valid? Am J Psychiatry. 2006;163(10):1720-1729.
23. McCann BS, Scheele L, Ward N, Roy-Byrne P. Discriminant validity of the Wender Utah Rating Scale for attention-deficit/hyperactivity disorder in adults. J Neuropsychiatry Clin Neurosci. 2000;12(2):240-245.
24. Ward MF, Wender PH, Reimherr FW. The Wender Utah Rating Scale: an aid in the retrospective diagnosis of childhood attention deficit hyperactivity disorder. Am J Psychiatry. 1993;150(6):885-890.
25. McCann BS, Roy-Byrne P. Attention-deficit/hyperactivity disorder and learning disabilities in adults. Semin Clin Neuropsychiatry. 2000;5(3):191-197.
26. Conners CK, Erhart D, Sparrow E. Conners’ Adult ADHD Rating Scales, Technical Manual. New York, NY: Multi-Health Systems; 1999.
27. Murphy KR, Adler LA. Assessing attention-deficit/hyperactivity disorder in adults: focus on rating scales. J Clin Psychiatry. 2004;65 Suppl 3:12-17.
28. Brown TE. Brown Attention-Deficit Disorder Scales. San Antonio, TX: Psychological Corporation; 1996.
29. World Health Organization. Adult ADHD Self-Report Scale (ASRS-v1.1; 2003). www.med.nyu.edu/psych/assets/adhd screen18.pdf. Accessed August 21, 2008.
30. Robison RH, Reimherr FW, Marchant BK, et al. Gender differences in 2 clinical trials of adults with attention-deficit/hyperactivity disorder: a retrospective date analysis. J Clin Psychiatry. 2008;69(2):213-221.
31. Wilens TE. Mechanism of action of agents used in attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2006;67 Suppl 8:32-38.
32. Volkow ND, Wang GJ, Newcorn J, et al. Brain dopamine transporter levels in treatment and drug naïve adults with ADHD. Neuroimage. 2007;34(3):1182-1190.
33. Spencer TJ, Biederman J, Madras BK, et al. Further evidence of dopamine transporter dysregulation in ADHD: a controlled PET imaging study using altropane. Biol Psychiatry. 2007;62(9):1059-1061.
34. Spencer TJ, Biederman J, Madras BK, et al. In vivo neuroreceptor imaging in attention-deficit/hyperactivity disorder: a focus on the dopamine transporter. Biol Psychiatry. 2005;57(11):1293-1300.
35. Volkow ND, Wang GJ, Fowler JS, Ding YS. Imaging the effects of methylphenidate on brain dopamine: new model on its therapeutic actions for attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57(11):1410-1415.
36. MTA Cooperative Group. A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder: Multimodal Treatment Study of Children with ADHD. Arch Gen Psychiatry. 1999;56(12):1073-1086.
37. Safren SA. Cognitive-behavioral approaches to ADHD treatment in adulthood. J Clin Psychiatry. 2006;67 Suppl 8:46-50.
Topical hemostasis agents: Some tried and true, others too new
The author reports no financial relationships relevant to this article.
- What clinical problem is this device or drug designed to address?
- Is the problem important clinically for the well-being of my patients?
- What do I know about the basic science and physiology of the problem?
- Are there data supporting improvement in clinical outcomes, or do the studies look only at surrogate endpoints?
- Has the drug or device been studied in gynecologic patients? Is it approved for use in this population?
- Is there evidence that the new technology is more effective, safer, or less expensive than products I use now?
The process of evaluation is critical for us—as advocates for individual patients and as stewards of the diminishing resources available to treat patients in the United States.
With these questions in mind, let’s look at a surgical problem for which there are many new and potentially exciting products being promoted to gynecologic surgeons: the need to achieve hemostasis.
Bleeding is a serious clinical problem during surgery—one that can have a major impact on the well-being of the patient. We generally use sutures and electrosurgical instruments—both bipolar and monopolar—to control major vessels. Topical hemostasis agents may be useful, however, in areas where generalized oozing is present, or where the application of energy may endanger vital structures.
Many products are available for use in these situations. Some are tried and true; others, new or unapproved for use in gynecologic surgery. Your assessment and selection of the optimal product should take into account 1) the cause of bleeding and 2) the mechanism of action of the product.
Surgical bleeding has one of two causes
Although the science of coagulation is complex, bleeding occurs, from a surgical perspective, because of either of two problems:
- failure to control significant arterial and venous sources
- failure of normal clotting functions, such as vasoconstriction; platelet activation and plugging ( FIGURE ); and activation of the coagulation cascade.
No topical or systemic agent adequately controls Problem #1. To address Problem #2, we have several choices to augment hemostasis, including thermal, chemical, and mechanical means. In addition, newer agents deliver additional coagulation-enhancing products such as platelets and thrombin to the operative site to supplement the patient’s natural clotting process.
FIGURE How a clot forms
Tried and true technologies
Because of long-standing experience with porcine gelatin, oxidized regenerated cellulose (ORC), and microfibrillar collagen, the Food and Drug Administration recategorized them in 2002 as Class-II devices with a clear safety profile.
Porcine gelatin
This class of products (Gelfoam, Surgifoam, Spongistan) has been around since the 1940s. The products have no intrinsic hemostatic action. They absorb 45 times their weight in blood and provide a scaff old on which platelets come into close contact, initiating the release of intrinsic and extrinsic clotting mechanisms.
Oxidized regenerated cellulose
This class (Surgicel, Oxycel) has also been around since the 1940s. The agents have acidic properties, due to their low pH level, and achieve hemostasis via denaturation of blood proteins, mechanical activation of the clotting cascade, and local vasoconstriction.
Because of its low pH, ORC is bactericidal against many common pathogens of the reproductive tract. A few studies have explored laparoscopic application of ORC to achieve hemostasis at sites of uterine perforation and for tubal bleeding secondary to sterilization.1,2 Successful hemostasis of moderate bleeding was achieved without the need for suture or conversion to laparotomy in all cases without brisk arterial bleeding.
Another ORC product (Interceed) is often used in gynecologic surgery as a barrier to adhesion formation. Its degree of oxidation, weave, and pore size differ from those of Surgicel. It requires an absolutely dry operative field to prevent adhesions.
Microfibrillar collagen
Substances derived from bovine collagen were marketed in the 1970s and 1980s. Collagen provides binding sites for platelets, which degranulate, releasing coagulation factors and initiating the clotting cascade.
Microfibrillar collagen products are available in a variety of forms (powder, sheets, loaded syringes for endoscopic placement) and are applied with pressure directly to the bleeding site. Collagen (Instat, Helistat) is supplied as a sponge, whereas microfibrillar collagen (Avitene, Superstat, Actifoam, Helitene, Hemopad, Novacol) is a tenacious powder or sheet. Microfibrillar collagen, like porcine gelatin and ORC, is absorbed by the body over time.
No studies have evaluated microfibrillar collagen in gynecologic surgery, although case reports of successful application to sites of uterine perforation after dilation and curettage and to bleeding sites after vaginal or laparoscopic hysterectomy have been published.3,4
New products are largely unproven
Class-III devices require significantly more study before safety and efficacy can be demonstrated. The two types of products presented here—topical thrombin and tissue sealants—remain largely unproven.
Topical thrombin
This class of products has been available for more than 20 years. As a liquid (Thrombogen), topical thrombin can be supplied in a syringe and sprayed onto oozing sites. A liquid combination of collagen gelatin matrix and bovine thrombin (FloSeal) provides a structure on which clots can form; triggers topical conversion of fibrinogen to fibrin; and activates the clotting cascade. It was approved for use in 1999. CoStasis and Vitagel are similar products, approved in 2000 and 2006, respectively, that add plasma obtained from the patient at the beginning of the surgical procedure.
Thrombin and ORC don’t mix. The acidity of ORC inactivates thrombin; therefore, ORC products should not be used with any product containing bovine or human thrombin.
The theoretical advantage of products that use patients’ plasma is the addition of autologous clotting factors and platelets to the bovine collagen and thrombin mixture. Preliminary studies have shown:
- a reduction in postoperative pain in 20 orthopedic surgery patients randomized to platelet gel, compared with what was seen in 20 women in the control group5
- a reduction in the rate of sternal wound infection in cardiac surgery patients (0.3% with the gel; 1.8% without it)6
- a potentially shorter healing time when platelet gel is applied to surgical wounds.7
The facts. Labeling for topical thrombin specifically states that it is not for use in cases of infection or for postpartum hemorrhage or menorrhagia. Studies of topical thrombin products have used the time to cessation of bleeding as their primary effectiveness end-point. In practical terms, however, studies have demonstrated no reduction in the need for transfusion or chest tube drainage in re-operative cardiac surgery patients.8
Disadvantages of topical thrombin include the cost of the product (including the cost of a plasma-collection device) and the need for operating room staff to collect and combine the product for use. Topical thrombin also exposes the patient to the risk of antibody formation (see Bovine thrombin can trigger risky antibodies), catastrophic bleeding, and, even, death.
Products that contain bovine thrombin have some safety issues with regard to their antigenic reactivity. Patients may develop antibodies to the bovine product that cross-react with human thrombin and factor Va. Associated with all products that contain bovine thrombin is a black box warning that states that the product may be associated with severe bleeding, thrombosis, and, rarely, death, because of antibody formation.
In one case report, a very complicated patient who required systemic anticoagulation for a mechanical aortic valve underwent hysterectomy, with topical thrombin administered at the end of the procedure in an effort to avert postoperative hemorrhage.1 She developed antibodies to the bovine thrombin, which caused significant and severe coagulation defects.
No clinical studies have assessed these products in gynecologic surgery.
Reference
1. Sharma JB, Malhotra M, Pundir P. Laparoscopic oxidized cellulose (Surgicel) application for small uterine perforations. Int J Gynaecol Obstet. 2003;83:271-275.
This last set of products has been approved for use in cardiopulmonary bypass procedures, in patients who have splenic injury, and to close a colostomy. They are “tissue glues” that also have hemostatic properties.
Tisseel is a combination of human thrombin, human “sealer protein” (fibrinogen), and aprotinin, a synthetic inhibitor of fibrinolysis that prevents premature degradation of a clot once it has formed. In clinical studies, this product has reduced the need for splenectomy in patients who have bleeding that is difficult to control.
Disadvantages of tissue-sealing products. These products have not been studied in gynecologic patients. They have the significant disadvantage of containing products derived from pooled human plasma. Although precautions have been taken to reduce transmission of infectious disease, viral transmission may occur. Anaphylaxis is an additional risk.
Many products are available to help the ObGyn surgeon achieve hemostasis in tough situations. Most of the time, we face generalized oozing after treatment of extensive endometriosis or adhesiolysis; in these cases, older topical agents should serve us well. Patients who experience massive bleeding are not likely to benefit from the use of any of the products described in this article.
Extensive bleeding from uterine incisions—at cesarean section or after myomectomy—might respond to topical thrombin, platelet gel products, or tissue sealants, but these products have not been studied in our patients. They also are expensive and carry some risk for our patients.
Don’t overlook two strategies for extremely high-risk situations:
- Cell-saver technology can help avert transfusion in patients expected to lose a substantial amount of blood
- Intravenous recombinant activated factor VII (NovoSeven) can be life-saving for women who experience postpartum hemorrhage, placenta percreta, or retroperitoneal sarcoma and for whom our standard strategies have failed.—BARBARA S. LEVY, MD
1. Sharma JB, Malhotra M, Pundir P. Laparoscopic oxidized cellulose (Surgicel) application for small uterine perforations. Int J Gynaecol Obstet. 2003;83:271-275.
2. Sharma JB, Malhotra M. Topical oxidized cellulose for tubal hemorrhage hemostasis during laparoscopic sterilization. Int J Gynaecol Obstet. 2003;82:221-222.
3. Borten M, Friedman EA. Translaparoscopic hemostasis with microfibrillar collagen in lieu of laparotomy. A report of two cases. J Reprod Med. 1983;28:804-806.
4. Holub Z, Jabor A. Laparoscopic management of bleeding after laparoscopic or vaginal hysterectomy. JSLS. 2004;8:235-238.
5. Zavadil DP, Satterlee CC, Costigan JM, Holt DW, Shostrom VK. Autologous platelet gel and platelet-poor plasma reduce pain with total shoulder arthroplasty. J Extra Corpor Technol. 2007;39:177-182.
6. Trowbridge CC, Stammers AH, Woods E, Yen BR, Klayman M. Use of platelet gel and its effects on infection in cardiac surgery. J Extra Corpor Technol. 2005;37:381-386.
7. Hom DB, Linzie MB, Huang TC. The healing effects of autologous platelet gel on acute human skin wounds. Arch Facial Plast Surg. 2007;9:174-183.
8. Wajon P, Gibson J, Calcroft R, Hughes C, Thrift B. Intraoperative plateletpheresis and autologous platelet gel do not reduce chest tube drainage or allogeneic blood transfusion after reoperative coronary artery bypass graft. Anesth Analg. 2001;93:536-542.
The author reports no financial relationships relevant to this article.
- What clinical problem is this device or drug designed to address?
- Is the problem important clinically for the well-being of my patients?
- What do I know about the basic science and physiology of the problem?
- Are there data supporting improvement in clinical outcomes, or do the studies look only at surrogate endpoints?
- Has the drug or device been studied in gynecologic patients? Is it approved for use in this population?
- Is there evidence that the new technology is more effective, safer, or less expensive than products I use now?
The process of evaluation is critical for us—as advocates for individual patients and as stewards of the diminishing resources available to treat patients in the United States.
With these questions in mind, let’s look at a surgical problem for which there are many new and potentially exciting products being promoted to gynecologic surgeons: the need to achieve hemostasis.
Bleeding is a serious clinical problem during surgery—one that can have a major impact on the well-being of the patient. We generally use sutures and electrosurgical instruments—both bipolar and monopolar—to control major vessels. Topical hemostasis agents may be useful, however, in areas where generalized oozing is present, or where the application of energy may endanger vital structures.
Many products are available for use in these situations. Some are tried and true; others, new or unapproved for use in gynecologic surgery. Your assessment and selection of the optimal product should take into account 1) the cause of bleeding and 2) the mechanism of action of the product.
Surgical bleeding has one of two causes
Although the science of coagulation is complex, bleeding occurs, from a surgical perspective, because of either of two problems:
- failure to control significant arterial and venous sources
- failure of normal clotting functions, such as vasoconstriction; platelet activation and plugging ( FIGURE ); and activation of the coagulation cascade.
No topical or systemic agent adequately controls Problem #1. To address Problem #2, we have several choices to augment hemostasis, including thermal, chemical, and mechanical means. In addition, newer agents deliver additional coagulation-enhancing products such as platelets and thrombin to the operative site to supplement the patient’s natural clotting process.
FIGURE How a clot forms
Tried and true technologies
Because of long-standing experience with porcine gelatin, oxidized regenerated cellulose (ORC), and microfibrillar collagen, the Food and Drug Administration recategorized them in 2002 as Class-II devices with a clear safety profile.
Porcine gelatin
This class of products (Gelfoam, Surgifoam, Spongistan) has been around since the 1940s. The products have no intrinsic hemostatic action. They absorb 45 times their weight in blood and provide a scaff old on which platelets come into close contact, initiating the release of intrinsic and extrinsic clotting mechanisms.
Oxidized regenerated cellulose
This class (Surgicel, Oxycel) has also been around since the 1940s. The agents have acidic properties, due to their low pH level, and achieve hemostasis via denaturation of blood proteins, mechanical activation of the clotting cascade, and local vasoconstriction.
Because of its low pH, ORC is bactericidal against many common pathogens of the reproductive tract. A few studies have explored laparoscopic application of ORC to achieve hemostasis at sites of uterine perforation and for tubal bleeding secondary to sterilization.1,2 Successful hemostasis of moderate bleeding was achieved without the need for suture or conversion to laparotomy in all cases without brisk arterial bleeding.
Another ORC product (Interceed) is often used in gynecologic surgery as a barrier to adhesion formation. Its degree of oxidation, weave, and pore size differ from those of Surgicel. It requires an absolutely dry operative field to prevent adhesions.
Microfibrillar collagen
Substances derived from bovine collagen were marketed in the 1970s and 1980s. Collagen provides binding sites for platelets, which degranulate, releasing coagulation factors and initiating the clotting cascade.
Microfibrillar collagen products are available in a variety of forms (powder, sheets, loaded syringes for endoscopic placement) and are applied with pressure directly to the bleeding site. Collagen (Instat, Helistat) is supplied as a sponge, whereas microfibrillar collagen (Avitene, Superstat, Actifoam, Helitene, Hemopad, Novacol) is a tenacious powder or sheet. Microfibrillar collagen, like porcine gelatin and ORC, is absorbed by the body over time.
No studies have evaluated microfibrillar collagen in gynecologic surgery, although case reports of successful application to sites of uterine perforation after dilation and curettage and to bleeding sites after vaginal or laparoscopic hysterectomy have been published.3,4
New products are largely unproven
Class-III devices require significantly more study before safety and efficacy can be demonstrated. The two types of products presented here—topical thrombin and tissue sealants—remain largely unproven.
Topical thrombin
This class of products has been available for more than 20 years. As a liquid (Thrombogen), topical thrombin can be supplied in a syringe and sprayed onto oozing sites. A liquid combination of collagen gelatin matrix and bovine thrombin (FloSeal) provides a structure on which clots can form; triggers topical conversion of fibrinogen to fibrin; and activates the clotting cascade. It was approved for use in 1999. CoStasis and Vitagel are similar products, approved in 2000 and 2006, respectively, that add plasma obtained from the patient at the beginning of the surgical procedure.
Thrombin and ORC don’t mix. The acidity of ORC inactivates thrombin; therefore, ORC products should not be used with any product containing bovine or human thrombin.
The theoretical advantage of products that use patients’ plasma is the addition of autologous clotting factors and platelets to the bovine collagen and thrombin mixture. Preliminary studies have shown:
- a reduction in postoperative pain in 20 orthopedic surgery patients randomized to platelet gel, compared with what was seen in 20 women in the control group5
- a reduction in the rate of sternal wound infection in cardiac surgery patients (0.3% with the gel; 1.8% without it)6
- a potentially shorter healing time when platelet gel is applied to surgical wounds.7
The facts. Labeling for topical thrombin specifically states that it is not for use in cases of infection or for postpartum hemorrhage or menorrhagia. Studies of topical thrombin products have used the time to cessation of bleeding as their primary effectiveness end-point. In practical terms, however, studies have demonstrated no reduction in the need for transfusion or chest tube drainage in re-operative cardiac surgery patients.8
Disadvantages of topical thrombin include the cost of the product (including the cost of a plasma-collection device) and the need for operating room staff to collect and combine the product for use. Topical thrombin also exposes the patient to the risk of antibody formation (see Bovine thrombin can trigger risky antibodies), catastrophic bleeding, and, even, death.
Products that contain bovine thrombin have some safety issues with regard to their antigenic reactivity. Patients may develop antibodies to the bovine product that cross-react with human thrombin and factor Va. Associated with all products that contain bovine thrombin is a black box warning that states that the product may be associated with severe bleeding, thrombosis, and, rarely, death, because of antibody formation.
In one case report, a very complicated patient who required systemic anticoagulation for a mechanical aortic valve underwent hysterectomy, with topical thrombin administered at the end of the procedure in an effort to avert postoperative hemorrhage.1 She developed antibodies to the bovine thrombin, which caused significant and severe coagulation defects.
No clinical studies have assessed these products in gynecologic surgery.
Reference
1. Sharma JB, Malhotra M, Pundir P. Laparoscopic oxidized cellulose (Surgicel) application for small uterine perforations. Int J Gynaecol Obstet. 2003;83:271-275.
This last set of products has been approved for use in cardiopulmonary bypass procedures, in patients who have splenic injury, and to close a colostomy. They are “tissue glues” that also have hemostatic properties.
Tisseel is a combination of human thrombin, human “sealer protein” (fibrinogen), and aprotinin, a synthetic inhibitor of fibrinolysis that prevents premature degradation of a clot once it has formed. In clinical studies, this product has reduced the need for splenectomy in patients who have bleeding that is difficult to control.
Disadvantages of tissue-sealing products. These products have not been studied in gynecologic patients. They have the significant disadvantage of containing products derived from pooled human plasma. Although precautions have been taken to reduce transmission of infectious disease, viral transmission may occur. Anaphylaxis is an additional risk.
Many products are available to help the ObGyn surgeon achieve hemostasis in tough situations. Most of the time, we face generalized oozing after treatment of extensive endometriosis or adhesiolysis; in these cases, older topical agents should serve us well. Patients who experience massive bleeding are not likely to benefit from the use of any of the products described in this article.
Extensive bleeding from uterine incisions—at cesarean section or after myomectomy—might respond to topical thrombin, platelet gel products, or tissue sealants, but these products have not been studied in our patients. They also are expensive and carry some risk for our patients.
Don’t overlook two strategies for extremely high-risk situations:
- Cell-saver technology can help avert transfusion in patients expected to lose a substantial amount of blood
- Intravenous recombinant activated factor VII (NovoSeven) can be life-saving for women who experience postpartum hemorrhage, placenta percreta, or retroperitoneal sarcoma and for whom our standard strategies have failed.—BARBARA S. LEVY, MD
The author reports no financial relationships relevant to this article.
- What clinical problem is this device or drug designed to address?
- Is the problem important clinically for the well-being of my patients?
- What do I know about the basic science and physiology of the problem?
- Are there data supporting improvement in clinical outcomes, or do the studies look only at surrogate endpoints?
- Has the drug or device been studied in gynecologic patients? Is it approved for use in this population?
- Is there evidence that the new technology is more effective, safer, or less expensive than products I use now?
The process of evaluation is critical for us—as advocates for individual patients and as stewards of the diminishing resources available to treat patients in the United States.
With these questions in mind, let’s look at a surgical problem for which there are many new and potentially exciting products being promoted to gynecologic surgeons: the need to achieve hemostasis.
Bleeding is a serious clinical problem during surgery—one that can have a major impact on the well-being of the patient. We generally use sutures and electrosurgical instruments—both bipolar and monopolar—to control major vessels. Topical hemostasis agents may be useful, however, in areas where generalized oozing is present, or where the application of energy may endanger vital structures.
Many products are available for use in these situations. Some are tried and true; others, new or unapproved for use in gynecologic surgery. Your assessment and selection of the optimal product should take into account 1) the cause of bleeding and 2) the mechanism of action of the product.
Surgical bleeding has one of two causes
Although the science of coagulation is complex, bleeding occurs, from a surgical perspective, because of either of two problems:
- failure to control significant arterial and venous sources
- failure of normal clotting functions, such as vasoconstriction; platelet activation and plugging ( FIGURE ); and activation of the coagulation cascade.
No topical or systemic agent adequately controls Problem #1. To address Problem #2, we have several choices to augment hemostasis, including thermal, chemical, and mechanical means. In addition, newer agents deliver additional coagulation-enhancing products such as platelets and thrombin to the operative site to supplement the patient’s natural clotting process.
FIGURE How a clot forms
Tried and true technologies
Because of long-standing experience with porcine gelatin, oxidized regenerated cellulose (ORC), and microfibrillar collagen, the Food and Drug Administration recategorized them in 2002 as Class-II devices with a clear safety profile.
Porcine gelatin
This class of products (Gelfoam, Surgifoam, Spongistan) has been around since the 1940s. The products have no intrinsic hemostatic action. They absorb 45 times their weight in blood and provide a scaff old on which platelets come into close contact, initiating the release of intrinsic and extrinsic clotting mechanisms.
Oxidized regenerated cellulose
This class (Surgicel, Oxycel) has also been around since the 1940s. The agents have acidic properties, due to their low pH level, and achieve hemostasis via denaturation of blood proteins, mechanical activation of the clotting cascade, and local vasoconstriction.
Because of its low pH, ORC is bactericidal against many common pathogens of the reproductive tract. A few studies have explored laparoscopic application of ORC to achieve hemostasis at sites of uterine perforation and for tubal bleeding secondary to sterilization.1,2 Successful hemostasis of moderate bleeding was achieved without the need for suture or conversion to laparotomy in all cases without brisk arterial bleeding.
Another ORC product (Interceed) is often used in gynecologic surgery as a barrier to adhesion formation. Its degree of oxidation, weave, and pore size differ from those of Surgicel. It requires an absolutely dry operative field to prevent adhesions.
Microfibrillar collagen
Substances derived from bovine collagen were marketed in the 1970s and 1980s. Collagen provides binding sites for platelets, which degranulate, releasing coagulation factors and initiating the clotting cascade.
Microfibrillar collagen products are available in a variety of forms (powder, sheets, loaded syringes for endoscopic placement) and are applied with pressure directly to the bleeding site. Collagen (Instat, Helistat) is supplied as a sponge, whereas microfibrillar collagen (Avitene, Superstat, Actifoam, Helitene, Hemopad, Novacol) is a tenacious powder or sheet. Microfibrillar collagen, like porcine gelatin and ORC, is absorbed by the body over time.
No studies have evaluated microfibrillar collagen in gynecologic surgery, although case reports of successful application to sites of uterine perforation after dilation and curettage and to bleeding sites after vaginal or laparoscopic hysterectomy have been published.3,4
New products are largely unproven
Class-III devices require significantly more study before safety and efficacy can be demonstrated. The two types of products presented here—topical thrombin and tissue sealants—remain largely unproven.
Topical thrombin
This class of products has been available for more than 20 years. As a liquid (Thrombogen), topical thrombin can be supplied in a syringe and sprayed onto oozing sites. A liquid combination of collagen gelatin matrix and bovine thrombin (FloSeal) provides a structure on which clots can form; triggers topical conversion of fibrinogen to fibrin; and activates the clotting cascade. It was approved for use in 1999. CoStasis and Vitagel are similar products, approved in 2000 and 2006, respectively, that add plasma obtained from the patient at the beginning of the surgical procedure.
Thrombin and ORC don’t mix. The acidity of ORC inactivates thrombin; therefore, ORC products should not be used with any product containing bovine or human thrombin.
The theoretical advantage of products that use patients’ plasma is the addition of autologous clotting factors and platelets to the bovine collagen and thrombin mixture. Preliminary studies have shown:
- a reduction in postoperative pain in 20 orthopedic surgery patients randomized to platelet gel, compared with what was seen in 20 women in the control group5
- a reduction in the rate of sternal wound infection in cardiac surgery patients (0.3% with the gel; 1.8% without it)6
- a potentially shorter healing time when platelet gel is applied to surgical wounds.7
The facts. Labeling for topical thrombin specifically states that it is not for use in cases of infection or for postpartum hemorrhage or menorrhagia. Studies of topical thrombin products have used the time to cessation of bleeding as their primary effectiveness end-point. In practical terms, however, studies have demonstrated no reduction in the need for transfusion or chest tube drainage in re-operative cardiac surgery patients.8
Disadvantages of topical thrombin include the cost of the product (including the cost of a plasma-collection device) and the need for operating room staff to collect and combine the product for use. Topical thrombin also exposes the patient to the risk of antibody formation (see Bovine thrombin can trigger risky antibodies), catastrophic bleeding, and, even, death.
Products that contain bovine thrombin have some safety issues with regard to their antigenic reactivity. Patients may develop antibodies to the bovine product that cross-react with human thrombin and factor Va. Associated with all products that contain bovine thrombin is a black box warning that states that the product may be associated with severe bleeding, thrombosis, and, rarely, death, because of antibody formation.
In one case report, a very complicated patient who required systemic anticoagulation for a mechanical aortic valve underwent hysterectomy, with topical thrombin administered at the end of the procedure in an effort to avert postoperative hemorrhage.1 She developed antibodies to the bovine thrombin, which caused significant and severe coagulation defects.
No clinical studies have assessed these products in gynecologic surgery.
Reference
1. Sharma JB, Malhotra M, Pundir P. Laparoscopic oxidized cellulose (Surgicel) application for small uterine perforations. Int J Gynaecol Obstet. 2003;83:271-275.
This last set of products has been approved for use in cardiopulmonary bypass procedures, in patients who have splenic injury, and to close a colostomy. They are “tissue glues” that also have hemostatic properties.
Tisseel is a combination of human thrombin, human “sealer protein” (fibrinogen), and aprotinin, a synthetic inhibitor of fibrinolysis that prevents premature degradation of a clot once it has formed. In clinical studies, this product has reduced the need for splenectomy in patients who have bleeding that is difficult to control.
Disadvantages of tissue-sealing products. These products have not been studied in gynecologic patients. They have the significant disadvantage of containing products derived from pooled human plasma. Although precautions have been taken to reduce transmission of infectious disease, viral transmission may occur. Anaphylaxis is an additional risk.
Many products are available to help the ObGyn surgeon achieve hemostasis in tough situations. Most of the time, we face generalized oozing after treatment of extensive endometriosis or adhesiolysis; in these cases, older topical agents should serve us well. Patients who experience massive bleeding are not likely to benefit from the use of any of the products described in this article.
Extensive bleeding from uterine incisions—at cesarean section or after myomectomy—might respond to topical thrombin, platelet gel products, or tissue sealants, but these products have not been studied in our patients. They also are expensive and carry some risk for our patients.
Don’t overlook two strategies for extremely high-risk situations:
- Cell-saver technology can help avert transfusion in patients expected to lose a substantial amount of blood
- Intravenous recombinant activated factor VII (NovoSeven) can be life-saving for women who experience postpartum hemorrhage, placenta percreta, or retroperitoneal sarcoma and for whom our standard strategies have failed.—BARBARA S. LEVY, MD
1. Sharma JB, Malhotra M, Pundir P. Laparoscopic oxidized cellulose (Surgicel) application for small uterine perforations. Int J Gynaecol Obstet. 2003;83:271-275.
2. Sharma JB, Malhotra M. Topical oxidized cellulose for tubal hemorrhage hemostasis during laparoscopic sterilization. Int J Gynaecol Obstet. 2003;82:221-222.
3. Borten M, Friedman EA. Translaparoscopic hemostasis with microfibrillar collagen in lieu of laparotomy. A report of two cases. J Reprod Med. 1983;28:804-806.
4. Holub Z, Jabor A. Laparoscopic management of bleeding after laparoscopic or vaginal hysterectomy. JSLS. 2004;8:235-238.
5. Zavadil DP, Satterlee CC, Costigan JM, Holt DW, Shostrom VK. Autologous platelet gel and platelet-poor plasma reduce pain with total shoulder arthroplasty. J Extra Corpor Technol. 2007;39:177-182.
6. Trowbridge CC, Stammers AH, Woods E, Yen BR, Klayman M. Use of platelet gel and its effects on infection in cardiac surgery. J Extra Corpor Technol. 2005;37:381-386.
7. Hom DB, Linzie MB, Huang TC. The healing effects of autologous platelet gel on acute human skin wounds. Arch Facial Plast Surg. 2007;9:174-183.
8. Wajon P, Gibson J, Calcroft R, Hughes C, Thrift B. Intraoperative plateletpheresis and autologous platelet gel do not reduce chest tube drainage or allogeneic blood transfusion after reoperative coronary artery bypass graft. Anesth Analg. 2001;93:536-542.
1. Sharma JB, Malhotra M, Pundir P. Laparoscopic oxidized cellulose (Surgicel) application for small uterine perforations. Int J Gynaecol Obstet. 2003;83:271-275.
2. Sharma JB, Malhotra M. Topical oxidized cellulose for tubal hemorrhage hemostasis during laparoscopic sterilization. Int J Gynaecol Obstet. 2003;82:221-222.
3. Borten M, Friedman EA. Translaparoscopic hemostasis with microfibrillar collagen in lieu of laparotomy. A report of two cases. J Reprod Med. 1983;28:804-806.
4. Holub Z, Jabor A. Laparoscopic management of bleeding after laparoscopic or vaginal hysterectomy. JSLS. 2004;8:235-238.
5. Zavadil DP, Satterlee CC, Costigan JM, Holt DW, Shostrom VK. Autologous platelet gel and platelet-poor plasma reduce pain with total shoulder arthroplasty. J Extra Corpor Technol. 2007;39:177-182.
6. Trowbridge CC, Stammers AH, Woods E, Yen BR, Klayman M. Use of platelet gel and its effects on infection in cardiac surgery. J Extra Corpor Technol. 2005;37:381-386.
7. Hom DB, Linzie MB, Huang TC. The healing effects of autologous platelet gel on acute human skin wounds. Arch Facial Plast Surg. 2007;9:174-183.
8. Wajon P, Gibson J, Calcroft R, Hughes C, Thrift B. Intraoperative plateletpheresis and autologous platelet gel do not reduce chest tube drainage or allogeneic blood transfusion after reoperative coronary artery bypass graft. Anesth Analg. 2001;93:536-542.