User login
How is a woman determined to have dense breast tissue?
Breasts that are heterogeneously dense or extremely dense on mammography are considered “dense breasts.” Breast density matters for 2 reasons: Dense tissue can mask cancer on a mammogram, and having dense breasts increases the risk of developing breast cancer.
Breast density measurement
A woman’s breast density is usually determined during her breast cancer screening with mammography by her radiologist through visual evaluation of the images taken. Breast density also can be measured from individual mammograms by computer software, and it can be estimated on computed tomography (CT) scan and magnetic resonance imaging (MRI). In the United States, information about breast density is usually included in a report sent from the radiologist to the referring clinician after a mammogram is taken, and may also be included in the patient letter following up screening mammography. In Europe, national reporting guidelines for physicians vary.
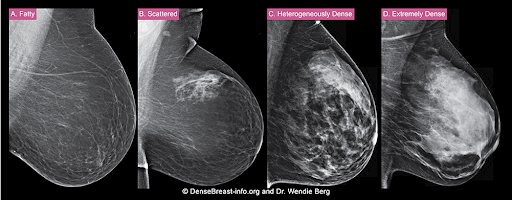
The density of a woman’s breast tissue is described using one of four BI-RADS® breast composition categories1 as shown in the FIGURE.
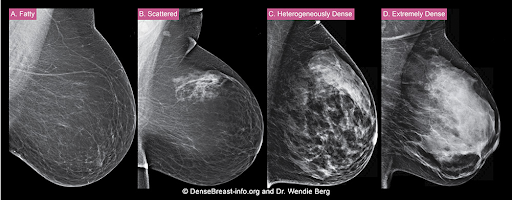
A. ALMOST ENTIRELY FATTY – On a mammogram, most of the tissue appears dark gray or black, while small amounts of dense (or fibroglandular) tissue display as light gray or white. About 13% of women aged 40 to 74 have breasts considered to be “fatty.”2
B. SCATTERED FIBROGLANDULAR DENSITY – There are scattered areas of dense (fibroglandular) tissue mixed with fat. Even in breasts with scattered areas of breast tissue, cancers can sometimes be missed when they look like areas of normal tissue or are within an area of denser tissue. About 43% of women aged 40 to 74 have breasts with scattered fibroglandular tissue.2
C. HETEROGENEOUSLY DENSE – There are large portions of the breast where dense (fibroglandular) tissue could hide small masses. About 36% of all women aged 40 to 74 have heterogeneously dense breasts.2
D. EXTREMELY DENSE – Most of the breast appears to consist of dense (fibroglandular) tissue, creating a “white out” situation and making it extremely difficult to see through and lowering the sensitivity of mammography. About 7% of all women aged 40 to 74 have extremely dense breasts.2
Factors that may impact breast density
Age. Breasts tend to become less dense as women get older, especially after menopause (as the glandular tissue atrophies and the breasts may appear more fatty-replaced).
Postmenopausal hormone therapy. An increase in mammographic density is more common among women taking continuous combined hormonal therapy than for those using oral low-dose estrogen or transdermal estrogen therapy.
Lactation. Breast density increases with lactation.
Weight changes. Weight gain can increase the amount of fat relative to dense tissue, resulting in slightly lower density as a proportion of breast tissue overall. Similarly, weight loss can decrease the amount of fat in the breasts, making breast density appear greater overall. Importantly, there is no change in the amount of glandular tissue; only the relative proportions change.
Tamoxifen or aromatase inhibitors. These medications can slightly reduce breast density.
Because breast density may change with age and other factors, it should be assessed every year.
For more information, visit medically sourced DenseBreast-info.org.
Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
1. Sickles EA, D’Orsi CJ, Bassett LW, et al. ACR BI-RADS Mammography. ACR BI-RADS Atlas, Breast Imaging Reporting and Data System. Reston, VA: American College of Radiology; 2013.
2. Sprague BL, Gangnon RE, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014;106:dju255. doi: 10.1093/jnci/dju255.
Breasts that are heterogeneously dense or extremely dense on mammography are considered “dense breasts.” Breast density matters for 2 reasons: Dense tissue can mask cancer on a mammogram, and having dense breasts increases the risk of developing breast cancer.
Breast density measurement
A woman’s breast density is usually determined during her breast cancer screening with mammography by her radiologist through visual evaluation of the images taken. Breast density also can be measured from individual mammograms by computer software, and it can be estimated on computed tomography (CT) scan and magnetic resonance imaging (MRI). In the United States, information about breast density is usually included in a report sent from the radiologist to the referring clinician after a mammogram is taken, and may also be included in the patient letter following up screening mammography. In Europe, national reporting guidelines for physicians vary.
The density of a woman’s breast tissue is described using one of four BI-RADS® breast composition categories1 as shown in the FIGURE.

A. ALMOST ENTIRELY FATTY – On a mammogram, most of the tissue appears dark gray or black, while small amounts of dense (or fibroglandular) tissue display as light gray or white. About 13% of women aged 40 to 74 have breasts considered to be “fatty.”2
B. SCATTERED FIBROGLANDULAR DENSITY – There are scattered areas of dense (fibroglandular) tissue mixed with fat. Even in breasts with scattered areas of breast tissue, cancers can sometimes be missed when they look like areas of normal tissue or are within an area of denser tissue. About 43% of women aged 40 to 74 have breasts with scattered fibroglandular tissue.2
C. HETEROGENEOUSLY DENSE – There are large portions of the breast where dense (fibroglandular) tissue could hide small masses. About 36% of all women aged 40 to 74 have heterogeneously dense breasts.2
D. EXTREMELY DENSE – Most of the breast appears to consist of dense (fibroglandular) tissue, creating a “white out” situation and making it extremely difficult to see through and lowering the sensitivity of mammography. About 7% of all women aged 40 to 74 have extremely dense breasts.2
Factors that may impact breast density
Age. Breasts tend to become less dense as women get older, especially after menopause (as the glandular tissue atrophies and the breasts may appear more fatty-replaced).
Postmenopausal hormone therapy. An increase in mammographic density is more common among women taking continuous combined hormonal therapy than for those using oral low-dose estrogen or transdermal estrogen therapy.
Lactation. Breast density increases with lactation.
Weight changes. Weight gain can increase the amount of fat relative to dense tissue, resulting in slightly lower density as a proportion of breast tissue overall. Similarly, weight loss can decrease the amount of fat in the breasts, making breast density appear greater overall. Importantly, there is no change in the amount of glandular tissue; only the relative proportions change.
Tamoxifen or aromatase inhibitors. These medications can slightly reduce breast density.
Because breast density may change with age and other factors, it should be assessed every year.
For more information, visit medically sourced DenseBreast-info.org.
Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
Breasts that are heterogeneously dense or extremely dense on mammography are considered “dense breasts.” Breast density matters for 2 reasons: Dense tissue can mask cancer on a mammogram, and having dense breasts increases the risk of developing breast cancer.
Breast density measurement
A woman’s breast density is usually determined during her breast cancer screening with mammography by her radiologist through visual evaluation of the images taken. Breast density also can be measured from individual mammograms by computer software, and it can be estimated on computed tomography (CT) scan and magnetic resonance imaging (MRI). In the United States, information about breast density is usually included in a report sent from the radiologist to the referring clinician after a mammogram is taken, and may also be included in the patient letter following up screening mammography. In Europe, national reporting guidelines for physicians vary.
The density of a woman’s breast tissue is described using one of four BI-RADS® breast composition categories1 as shown in the FIGURE.

A. ALMOST ENTIRELY FATTY – On a mammogram, most of the tissue appears dark gray or black, while small amounts of dense (or fibroglandular) tissue display as light gray or white. About 13% of women aged 40 to 74 have breasts considered to be “fatty.”2
B. SCATTERED FIBROGLANDULAR DENSITY – There are scattered areas of dense (fibroglandular) tissue mixed with fat. Even in breasts with scattered areas of breast tissue, cancers can sometimes be missed when they look like areas of normal tissue or are within an area of denser tissue. About 43% of women aged 40 to 74 have breasts with scattered fibroglandular tissue.2
C. HETEROGENEOUSLY DENSE – There are large portions of the breast where dense (fibroglandular) tissue could hide small masses. About 36% of all women aged 40 to 74 have heterogeneously dense breasts.2
D. EXTREMELY DENSE – Most of the breast appears to consist of dense (fibroglandular) tissue, creating a “white out” situation and making it extremely difficult to see through and lowering the sensitivity of mammography. About 7% of all women aged 40 to 74 have extremely dense breasts.2
Factors that may impact breast density
Age. Breasts tend to become less dense as women get older, especially after menopause (as the glandular tissue atrophies and the breasts may appear more fatty-replaced).
Postmenopausal hormone therapy. An increase in mammographic density is more common among women taking continuous combined hormonal therapy than for those using oral low-dose estrogen or transdermal estrogen therapy.
Lactation. Breast density increases with lactation.
Weight changes. Weight gain can increase the amount of fat relative to dense tissue, resulting in slightly lower density as a proportion of breast tissue overall. Similarly, weight loss can decrease the amount of fat in the breasts, making breast density appear greater overall. Importantly, there is no change in the amount of glandular tissue; only the relative proportions change.
Tamoxifen or aromatase inhibitors. These medications can slightly reduce breast density.
Because breast density may change with age and other factors, it should be assessed every year.
For more information, visit medically sourced DenseBreast-info.org.
Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
1. Sickles EA, D’Orsi CJ, Bassett LW, et al. ACR BI-RADS Mammography. ACR BI-RADS Atlas, Breast Imaging Reporting and Data System. Reston, VA: American College of Radiology; 2013.
2. Sprague BL, Gangnon RE, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014;106:dju255. doi: 10.1093/jnci/dju255.
1. Sickles EA, D’Orsi CJ, Bassett LW, et al. ACR BI-RADS Mammography. ACR BI-RADS Atlas, Breast Imaging Reporting and Data System. Reston, VA: American College of Radiology; 2013.
2. Sprague BL, Gangnon RE, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014;106:dju255. doi: 10.1093/jnci/dju255.
Translating the 2019 AAD-NPF Guidelines of Care for Psoriasis With Attention to Comorbidities
Psoriasis is a chronic and relapsing systemic inflammatory disease that predisposes patients to a host of other conditions. It is believed that these widespread effects are due to chronic inflammation and cytokine activation, which affect multiple body processes and lead to the development of various comorbidities that need to be proactively managed.
In April 2019, the American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) released recommendation guidelines for managing psoriasis in adults with an emphasis on common disease comorbidities, including psoriatic arthritis (PsA), cardiovascular disease (CVD), inflammatory bowel disease (IBD), metabolic syndrome, and mood disorders. Psychosocial wellness, mental health, and quality of life (QOL) measures in relation to psoriatic disease also were discussed.1
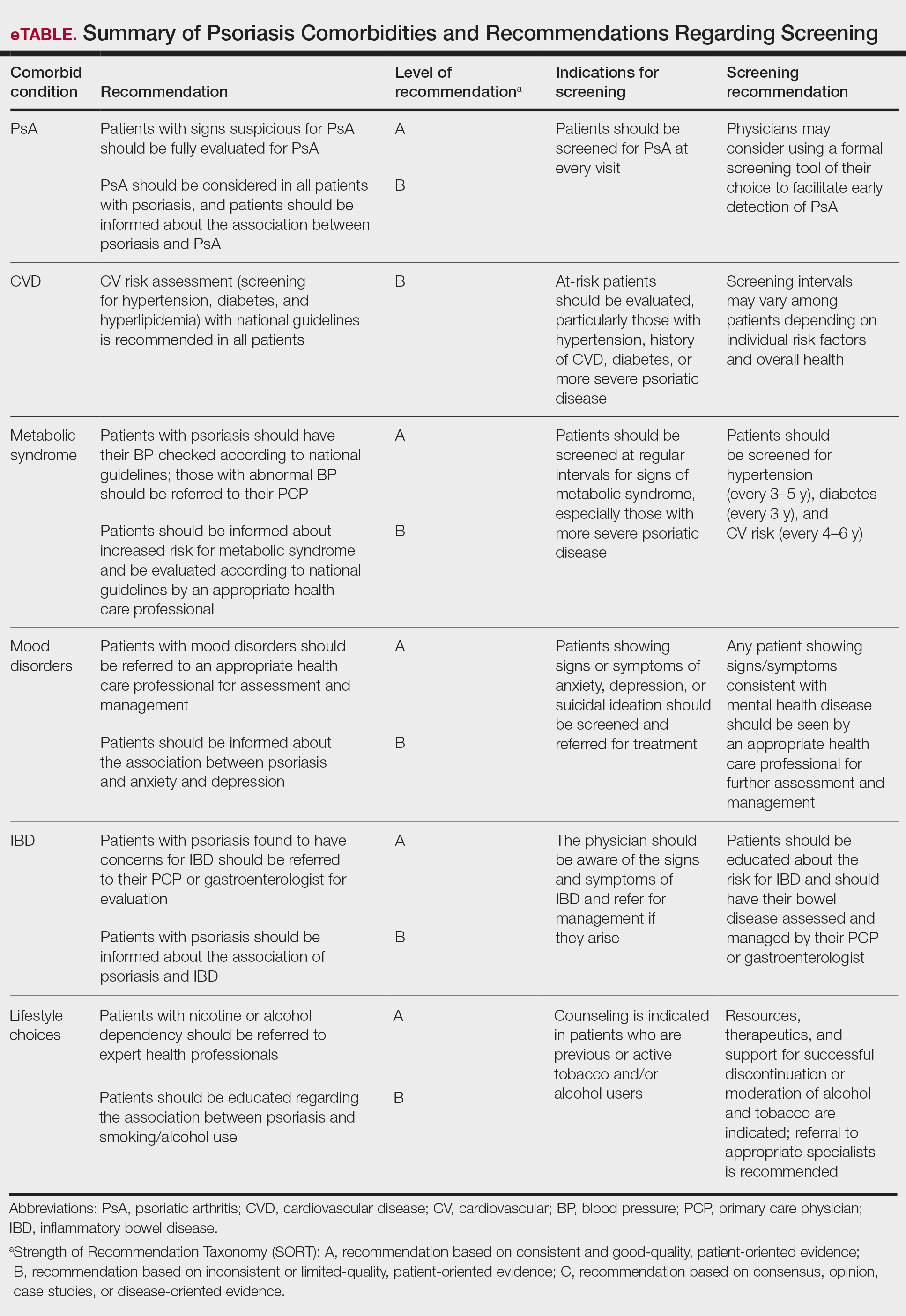
The AAD-NPF guidelines address current screening, monitoring, education, and treatment recommendations for the management of psoriatic comorbidities. The Table and eTable summarize the screening recommendations. These guidelines aim to assist dermatologists with comprehensive disease management by addressing potential extracutaneous manifestations of psoriasis in adults.

Screening and Risk Assessment
Patients with psoriasis should receive a thorough history and physical examination to assess disease severity and risk for potential comorbidities. Patients with greater disease severity—as measured by body surface area (BSA) involvement and type of therapy required—have a greater risk for other disease-related comorbidities, specifically metabolic syndrome, renal disease, chronic obstructive pulmonary disease (COPD), obstructive sleep apnea, uveitis, IBD, malignancy, and increased mortality.2 Because the likelihood of comorbidities is greatest with severe disease, more frequent monitoring is recommended for these patients.
Psoriatic Arthritis
Patients with psoriasis need to be evaluated for PsA at every visit. Patients presenting with signs and symptoms suspicious for PsA—joint swelling, peripheral joint involvement, and joint inflammation—warrant further evaluation and consultation. Early detection and treatment of PsA is essential for preventing unnecessary suffering and progressive joint destruction.3
There are several PsA screening questionnaires currently available, including the Psoriatic Arthritis Screening Evaluation, Psoriasis Epidemiology Screening Tool, and Toronto Psoriatic Arthritis Screen. No significant differences in sensitivity and specificity were found among these questionnaires when using the Classification Criteria for Psoriatic Arthritis as the gold standard. All 3 questionnaires—the Psoriatic Arthritis Screening Evaluation and the Psoriasis Epidemiology Screening Tool were developed for use in dermatology and rheumatology clinics, and the Toronto Psoriatic Arthritis Screen was developed for use in the primary care setting—were found to be effective in dermatology/rheumatology clinics and primary care clinics, respectively.3 False-positive results predominantly were seen in patients with degenerative joint disease or osteoarthritis. Dermatologists should conduct a thorough physical examination to distinguish PsA from degenerative joint disease. Imaging and laboratory tests to evaluate for signs of systemic inflammation (erythrocyte sedimentation rate, C-reactive protein) also can be helpful in distinguishing the 2 conditions; however, these metrics have not been shown to contribute to PsA diagnosis.1 Full rheumatologic consultation is warranted in challenging cases.
Cardiovascular Disease
Primary care physicians (PCPs) are recommended to screen patients for CVD risk factors using height, weight, blood pressure, blood glucose, hemoglobin A1C, lipid levels, abdominal circumference, and body mass index (BMI). Lifestyle modifications such as smoking cessation, exercise, and dietary changes are encouraged to achieve and maintain a normal BMI.
Dermatologists also need to give special consideration to comorbidities when selecting medications and/or therapies for disease management. Patients on TNF inhibitors have a lower risk for MI compared with patients using topical medications, phototherapy, and other oral agents.10 Additionally, patients on TNF inhibitors have a lower risk for occurrence of major adverse cardiovascular events compared with patients treated with methotrexate or phototherapy.11,12
Metabolic Syndrome
Numerous studies have demonstrated an association between psoriasis and metabolic syndrome. Patients with increased BSA involvement and
The association between psoriasis and weight loss has been analyzed in several studies. Weight loss, particularly in obese patients, has been shown to improve psoriasis severity, as measured by psoriasis area and severity index score and QOL measures.15 Another study found that gastric bypass was associated with a significant risk reduction in the development of psoriasis (P=.004) and the disease prognosis (P=.02 for severe psoriasis; P=.01 for PsA).16 Therefore, patients with moderate to severe psoriasis are recommended to have their obesity status determined according to national guidelines. For patients with a BMI above 40 kg/m2 and standard weight-loss measures fail, bariatric surgery is recommended. Additionally, the impact of psoriasis medications on weight has been studied. Apremilast has been associated with weight loss, whereas etanercept and infliximab have been linked to weight gain.17,18
An association between psoriasis and hypertension also has been demonstrated by studies, especially among patients with severe disease. Therefore, patients with moderate to severe psoriasis are recommended to have their blood pressure evaluated according to national guidelines, and those with a blood pressure of 140/90 mm Hg or higher should be referred to their PCP for assessment and treatment. Current evidence does not support restrictions on antihypertensive medications in patients with psoriasis. Physicians should be aware of the potential for cyclosporine to induce hypertension, which should be treated, specifically with amlodipine.19
Many studies have demonstrated an association between psoriasis and dyslipidemia, though the results are somewhat conflicting. In 2018, the American Heart Association and the American College of Cardiology deemed psoriasis as an atherosclerotic CVD risk-enhancing condition, favoring early initiation of statin therapy. Because dyslipidemia plays a prominent role in atherosclerosis and CVD, patients with moderate to severe psoriasis are recommended to undergo periodic screening with lipid tests (eg, fasting total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides).20 Patients with elevated fasting triglycerides or low-density lipoprotein cholesterol should be referred to their PCP for further management. Certain psoriasis medications also have been linked to dyslipidemia. Acitretin and cyclosporine are known to adversely affect lipid levels, so patients treated with either agent should undergo routine monitoring of serum lipid levels.
Psoriasis is strongly associated with diabetes mellitus. Because of the increased risk for diabetes in patients with severe disease, regular monitoring of fasting blood glucose and/or hemoglobin A1C levels in patients with moderate to severe psoriasis is recommended. Patients who meet criteria for prediabetes or diabetes should be referred to their PCP for further assessment and management.21,22
Mood Disorders
Psoriasis affects QOL and can have a major impact on patients’ interpersonal relationships. Studies have shown an association between psoriasis and mood disorders, specifically depression and anxiety. Unfortunately, patients with mood disorders are less likely to seek intervention for their skin disease, which poses a tremendous treatment barrier. Dermatologists should regularly monitor patients for psychiatric symptoms so that resources and treatments can be offered.
Certain psoriasis therapies have been shown to help alleviate associated depression and anxiety. Improvements in Beck Depression Inventory and Hamilton Depression Rating Scale scores were seen with etanercept.23 Adalimumab and ustekinumab showed improvement in Dermatology Life Quality Index compared with placebo.24,25 Patients receiving Goeckerman treatment also had improvement in anxiety and depression scores compared with conventional therapy.26 Biologic medications had the largest impact on improving depression symptoms compared with conventional systemic therapy and phototherapy.27 The recommendations support use of biologics and the Goeckerman regimen for the concomitant treatment of mood disorders and psoriasis.
Renal Disease
Studies have supported an association between psoriasis and chronic kidney disease (CKD), independent of risk factors including vascular disease, hypertension, and diabetes. The prevalence of moderate to advanced CKD also has been found to be directly related to increasing BSA affected by psoriasis.28 Patients should receive testing of blood urea nitrogen, creatinine, and urine microalbumin levels to assess for occult renal disease. In addition, physicians should be cautious when prescribing nephrotoxic drugs (nonsteroidal anti-inflammatory drugs and cyclosporine) and renally excreted agents (methotrexate and apremilast) because of the risk for underlying renal disease in patients with psoriasis. If newly acquired renal disease is suspected, physicians should withhold the offending agents. Patients with psoriasis with CKD are recommended to follow up with their PCP or nephrologist for evaluation and management.
Pulmonary Disease
Psoriasis also has an independent association with COPD. Patients with psoriasis have a higher likelihood of developing COPD (hazard ratio, 2.35; 95% CI, 1.42-3.89; P<.01) than controls.29 The prevalence of COPD also was found to correlate with psoriasis severity. Dermatologists should educate patients about the association between smoking and psoriasis as well as advise patients to discontinue smoking to reduce their risk for developing COPD and cancer.
Patients with psoriasis also are at an increased risk for obstructive sleep apnea. Obstructive sleep apnea should be considered in patients with risk factors including snoring, obesity, hypertension, or diabetes.
Inflammatory Bowel Disease
Patients with psoriasis have an increased risk for developing IBD. The prevalence ratios of both Crohn disease (2.49) and ulcerative colitis (1.64) are increased in patients with psoriasis relative to patients without psoriasis.30 Physicians need to be aware of the association between psoriasis and IBD and the effect that their coexistence may have on treatment choice for patients.
Adalimumab and infliximab are approved for the treatment of IBD, and certolizumab and ustekinumab are approved for Crohn disease. Use of TNF inhibitors in patients with IBD may cause psoriasiform lesions to develop.31 Nonetheless, treatment should be individualized and psoriasiform lesions treated with standard psoriasis measures. Psoriasis patients with IBD are recommended to avoid IL-17–inhibitor therapy, given its potential to worsen IBD flares.
Malignancy
Psoriasis patients aged 0 to 79 years have a greater overall risk for malignancy compared with patients without psoriasis.32 Patients with psoriasis have an increased risk for respiratory tract cancer, upper aerodigestive tract cancer, urinary tract cancer, and non-Hodgkin lymphoma.33 A mild association exists between PsA and lymphoma, nonmelanoma skin cancer (NMSC), and lung cancer.34 More severe psoriasis is associated with greater risk for lymphoma and NMSC. Dermatologists are recommended to educate patients on their risk for certain malignancies and to refer patients to specialists upon suspicion of malignancy.
Risk for malignancy has been shown to be affected by psoriasis treatments. Patients treated with UVB have reduced overall cancer rates for all age groups (hazard ratio, 0.52; P=.3), while those treated with psoralen plus UVA have an increased incidence of
Lifestyle Choices and QOL
A crucial aspect of successful psoriasis management is patient education. The strongest recommendations support lifestyle changes, such as smoking cessation and limitation of alcohol use. A tactful discussion regarding substance use, work productivity, interpersonal relationships, and sexual function can address substantial effects of psoriasis on QOL so that support and resources can be provided.
Final Thoughts
Management of psoriasis is multifaceted and involves screening, education, monitoring, and collaboration with PCPs and specialists. Regular follow-up with a dermatologist and PCP is strongly recommended for patients with psoriasis given the systemic nature of the disease. The 2019 AAD-NPF recommendations provide important information for dermatologists to coordinate care for complicated psoriasis cases, but clinical judgment is paramount when making medical decisions. The consideration of comorbidities is critical for developing a comprehensive treatment approach, and this approach will lead to better health outcomes and improved QOL for patients with psoriasis.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
- Gelfand JM, Troxel AB, Lewis JD, et al. The risk of mortality in patients with psoriasis: results from a population-based study. Arch Dermatol. 2007;143:1493-1499.
- Coates LC, Aslam T, Al Balushi F, et al. Comparison of three screening tools to detect psoriatic arthritis in patients with psoriasis (CONTEST study). Br J Dermatol. 2013;168:802-807.
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3168-3209.
- Lerman JB, Joshi AA, Chaturvedi A, et al. Coronary plaque characterization in psoriasis reveals high-risk features that improve after treatment in a prospective observational study. Circulation. 2017;136:263-276.
- Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735-1741.
- Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411-2418.
- Dunlay SM, Weston SA, Redfield MM, et al. Tumor necrosis factor-alpha and mortality in heart failure: a community study. Circulation. 2008;118:625-631.
- Russell SD, Saval MA, Robbins JL, et al. New York Heart Association functional class predicts exercise parameters in the current era. Am Heart J. 2009;158(4 suppl):S24-S30.
- Wu JJ, Poon K-YT, Channual JC, et al. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148:1244-1250.
- Wu JJ, Guerin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90.
- Wu JJ, Sundaram M, Cloutier M, et al. The risk of cardiovascular events in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus phototherapy: an observational cohort study. J Am Acad Dermatol. 2018;79:60-68.
- Gami AS, Witt BJ, Howard DE, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403-414.
- Langan SM, Seminara NM, Shin DB, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol. 2012;132:556-562.
- Jensen P, Zachariae C, Christensen R, et al. Effect of weight loss on the severity of psoriasis: a randomized clinical study. JAMA Dermatol. 2013;149:795-801.
- Egeberg A, Sørensen JA, Gislason GH, et al. Incidence and prognosis of psoriasis and psoriatic arthritis in patients undergoing bariatric surgery. JAMA Surg. 2017;152:344-349.
- Crowley J, Thaçi D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for ≥156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77:310-317.e1. doi:10.1016/j.jaad.2017.01.052
- Gisondi P, Del Giglio M, Di Francesco V, et al. Weight loss improves the response of obese patients with moderate-to-severe chronic plaque psoriasis to low-dose cyclosporine therapy: a randomized, controlled, investigator-blinded clinical trial. Am J Clin Nutr. 2008;88:1242-1247.
- Leenen FHH, Coletta E, Davies RA. Prevention of renal dysfunction and hypertension by amlodipine after heart transplant. Am J Cardiol. 2007;100:531-535.
- Goff DC Jr, Lloyd-Jones DM, Bennet G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk. Circulation. 2014;129(suppl 2):S49-S73.
- American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care. 2014;37(suppl 1):S14-S80.
- Ratner RE, Diabetes Prevention Program Research Group. An update on the diabetes prevention program. Endocr Pract. 2006;12(suppl 1):20-24.
- Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29-35.
- Kimball AB, Edson-Heredia E, Zhu B, et al. Understanding the relationship between pruritus severity and work productivity in patients with moderate-to-severe psoriasis: sleep problems are a mediating factor. J Drugs Dermatol. 2016;15:183-188.
- Langley RG, Tsai T-F, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br J Dermatol. 2018;178:114-123.
- Chern E, Yau D, Ho J-C, et al. Positive effect of modified Goeckerman regimen on quality of life and psychosocial distress in moderate and severe psoriasis. Acta Derm Venereol. 2011;91:447-451.
- Strober B, Gooderham M, de Jong EMGJ, et al. Depressive symptoms, depression, and the effect of biologic therapy among patients in Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Am Acad Dermatol. 2018;78:70-80.
- Wan J, Wang S, Haynes K, et al. Risk of moderate to advanced kidney disease in patients with psoriasis: population based cohort study. BMJ. 2013;347:f5961. doi:10.1136/bmj.f5961
- Chiang Y-Y, Lin H-W. Association between psoriasis and chronic obstructive pulmonary disease: a population-based study in Taiwan. J Eur Acad Dermatol Venereol. 2012;26:59-65.
- Cohen AD, Dreiher J, Birkenfeld S. Psoriasis associated with ulcerative colitis and Crohn’s disease. J Eur Acad Dermatol Venereol. 2009;23:561-565.
- Denadai R, Teixeira FV, Saad-Hossne R. The onset of psoriasis during the treatment of inflammatory bowel diseases with infliximab: should biological therapy be suspended? Arq Gastroenterol. 2012;49:172-176.
- Chen Y-J, Wu C-Y, Chen T-J, et al. The risk of cancer in patients with psoriasis: a population-based cohort study in Taiwan. J Am Acad Dermatol. 2011;65:84-91.
- Pouplard C, Brenaut E, Horreau C, et al. Risk of cancer in psoriasis: a systematic review and meta-analysis of epidemiological studies. J Eur Acad Dermatol Venereol. 2013;27(suppl 3):36-46.
- Chiesa Fuxench ZC, Shin DB, Ogdie Beatty A, et al. The risk of cancer in patients with psoriasis: a population-based cohort study in the health improvement network. JAMA Dermatol. 2016;152:282-290.
- Burmester GR, Panaccione R, Gordon KB, et al. Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn’s disease. Ann Rheum Dis. 2013;72:517-524.
- Dommasch ED, Abuabara K, Shin DB, et al. The risk of infection and malignancy with tumor necrosis factor antagonists in adults with psoriatic disease: a systematic review and meta-analysis of randomized controlled trials. J Am Acad Dermatol. 2011;64:1035-1050.
- Gordon KB, Papp KA, Langley RG, et al. Long-term safety experience of ustekinumab in patients with moderate to severe psoriasis (part II of II): results from analyses of infections and malignancy from pooled phase II and III clinical trials. J Am Acad Dermatol. 2012;66:742-751.
Psoriasis is a chronic and relapsing systemic inflammatory disease that predisposes patients to a host of other conditions. It is believed that these widespread effects are due to chronic inflammation and cytokine activation, which affect multiple body processes and lead to the development of various comorbidities that need to be proactively managed.
In April 2019, the American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) released recommendation guidelines for managing psoriasis in adults with an emphasis on common disease comorbidities, including psoriatic arthritis (PsA), cardiovascular disease (CVD), inflammatory bowel disease (IBD), metabolic syndrome, and mood disorders. Psychosocial wellness, mental health, and quality of life (QOL) measures in relation to psoriatic disease also were discussed.1
The AAD-NPF guidelines address current screening, monitoring, education, and treatment recommendations for the management of psoriatic comorbidities. The Table and eTable summarize the screening recommendations. These guidelines aim to assist dermatologists with comprehensive disease management by addressing potential extracutaneous manifestations of psoriasis in adults.


Screening and Risk Assessment
Patients with psoriasis should receive a thorough history and physical examination to assess disease severity and risk for potential comorbidities. Patients with greater disease severity—as measured by body surface area (BSA) involvement and type of therapy required—have a greater risk for other disease-related comorbidities, specifically metabolic syndrome, renal disease, chronic obstructive pulmonary disease (COPD), obstructive sleep apnea, uveitis, IBD, malignancy, and increased mortality.2 Because the likelihood of comorbidities is greatest with severe disease, more frequent monitoring is recommended for these patients.
Psoriatic Arthritis
Patients with psoriasis need to be evaluated for PsA at every visit. Patients presenting with signs and symptoms suspicious for PsA—joint swelling, peripheral joint involvement, and joint inflammation—warrant further evaluation and consultation. Early detection and treatment of PsA is essential for preventing unnecessary suffering and progressive joint destruction.3
There are several PsA screening questionnaires currently available, including the Psoriatic Arthritis Screening Evaluation, Psoriasis Epidemiology Screening Tool, and Toronto Psoriatic Arthritis Screen. No significant differences in sensitivity and specificity were found among these questionnaires when using the Classification Criteria for Psoriatic Arthritis as the gold standard. All 3 questionnaires—the Psoriatic Arthritis Screening Evaluation and the Psoriasis Epidemiology Screening Tool were developed for use in dermatology and rheumatology clinics, and the Toronto Psoriatic Arthritis Screen was developed for use in the primary care setting—were found to be effective in dermatology/rheumatology clinics and primary care clinics, respectively.3 False-positive results predominantly were seen in patients with degenerative joint disease or osteoarthritis. Dermatologists should conduct a thorough physical examination to distinguish PsA from degenerative joint disease. Imaging and laboratory tests to evaluate for signs of systemic inflammation (erythrocyte sedimentation rate, C-reactive protein) also can be helpful in distinguishing the 2 conditions; however, these metrics have not been shown to contribute to PsA diagnosis.1 Full rheumatologic consultation is warranted in challenging cases.
Cardiovascular Disease
Primary care physicians (PCPs) are recommended to screen patients for CVD risk factors using height, weight, blood pressure, blood glucose, hemoglobin A1C, lipid levels, abdominal circumference, and body mass index (BMI). Lifestyle modifications such as smoking cessation, exercise, and dietary changes are encouraged to achieve and maintain a normal BMI.
Dermatologists also need to give special consideration to comorbidities when selecting medications and/or therapies for disease management. Patients on TNF inhibitors have a lower risk for MI compared with patients using topical medications, phototherapy, and other oral agents.10 Additionally, patients on TNF inhibitors have a lower risk for occurrence of major adverse cardiovascular events compared with patients treated with methotrexate or phototherapy.11,12
Metabolic Syndrome
Numerous studies have demonstrated an association between psoriasis and metabolic syndrome. Patients with increased BSA involvement and
The association between psoriasis and weight loss has been analyzed in several studies. Weight loss, particularly in obese patients, has been shown to improve psoriasis severity, as measured by psoriasis area and severity index score and QOL measures.15 Another study found that gastric bypass was associated with a significant risk reduction in the development of psoriasis (P=.004) and the disease prognosis (P=.02 for severe psoriasis; P=.01 for PsA).16 Therefore, patients with moderate to severe psoriasis are recommended to have their obesity status determined according to national guidelines. For patients with a BMI above 40 kg/m2 and standard weight-loss measures fail, bariatric surgery is recommended. Additionally, the impact of psoriasis medications on weight has been studied. Apremilast has been associated with weight loss, whereas etanercept and infliximab have been linked to weight gain.17,18
An association between psoriasis and hypertension also has been demonstrated by studies, especially among patients with severe disease. Therefore, patients with moderate to severe psoriasis are recommended to have their blood pressure evaluated according to national guidelines, and those with a blood pressure of 140/90 mm Hg or higher should be referred to their PCP for assessment and treatment. Current evidence does not support restrictions on antihypertensive medications in patients with psoriasis. Physicians should be aware of the potential for cyclosporine to induce hypertension, which should be treated, specifically with amlodipine.19
Many studies have demonstrated an association between psoriasis and dyslipidemia, though the results are somewhat conflicting. In 2018, the American Heart Association and the American College of Cardiology deemed psoriasis as an atherosclerotic CVD risk-enhancing condition, favoring early initiation of statin therapy. Because dyslipidemia plays a prominent role in atherosclerosis and CVD, patients with moderate to severe psoriasis are recommended to undergo periodic screening with lipid tests (eg, fasting total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides).20 Patients with elevated fasting triglycerides or low-density lipoprotein cholesterol should be referred to their PCP for further management. Certain psoriasis medications also have been linked to dyslipidemia. Acitretin and cyclosporine are known to adversely affect lipid levels, so patients treated with either agent should undergo routine monitoring of serum lipid levels.
Psoriasis is strongly associated with diabetes mellitus. Because of the increased risk for diabetes in patients with severe disease, regular monitoring of fasting blood glucose and/or hemoglobin A1C levels in patients with moderate to severe psoriasis is recommended. Patients who meet criteria for prediabetes or diabetes should be referred to their PCP for further assessment and management.21,22
Mood Disorders
Psoriasis affects QOL and can have a major impact on patients’ interpersonal relationships. Studies have shown an association between psoriasis and mood disorders, specifically depression and anxiety. Unfortunately, patients with mood disorders are less likely to seek intervention for their skin disease, which poses a tremendous treatment barrier. Dermatologists should regularly monitor patients for psychiatric symptoms so that resources and treatments can be offered.
Certain psoriasis therapies have been shown to help alleviate associated depression and anxiety. Improvements in Beck Depression Inventory and Hamilton Depression Rating Scale scores were seen with etanercept.23 Adalimumab and ustekinumab showed improvement in Dermatology Life Quality Index compared with placebo.24,25 Patients receiving Goeckerman treatment also had improvement in anxiety and depression scores compared with conventional therapy.26 Biologic medications had the largest impact on improving depression symptoms compared with conventional systemic therapy and phototherapy.27 The recommendations support use of biologics and the Goeckerman regimen for the concomitant treatment of mood disorders and psoriasis.
Renal Disease
Studies have supported an association between psoriasis and chronic kidney disease (CKD), independent of risk factors including vascular disease, hypertension, and diabetes. The prevalence of moderate to advanced CKD also has been found to be directly related to increasing BSA affected by psoriasis.28 Patients should receive testing of blood urea nitrogen, creatinine, and urine microalbumin levels to assess for occult renal disease. In addition, physicians should be cautious when prescribing nephrotoxic drugs (nonsteroidal anti-inflammatory drugs and cyclosporine) and renally excreted agents (methotrexate and apremilast) because of the risk for underlying renal disease in patients with psoriasis. If newly acquired renal disease is suspected, physicians should withhold the offending agents. Patients with psoriasis with CKD are recommended to follow up with their PCP or nephrologist for evaluation and management.
Pulmonary Disease
Psoriasis also has an independent association with COPD. Patients with psoriasis have a higher likelihood of developing COPD (hazard ratio, 2.35; 95% CI, 1.42-3.89; P<.01) than controls.29 The prevalence of COPD also was found to correlate with psoriasis severity. Dermatologists should educate patients about the association between smoking and psoriasis as well as advise patients to discontinue smoking to reduce their risk for developing COPD and cancer.
Patients with psoriasis also are at an increased risk for obstructive sleep apnea. Obstructive sleep apnea should be considered in patients with risk factors including snoring, obesity, hypertension, or diabetes.
Inflammatory Bowel Disease
Patients with psoriasis have an increased risk for developing IBD. The prevalence ratios of both Crohn disease (2.49) and ulcerative colitis (1.64) are increased in patients with psoriasis relative to patients without psoriasis.30 Physicians need to be aware of the association between psoriasis and IBD and the effect that their coexistence may have on treatment choice for patients.
Adalimumab and infliximab are approved for the treatment of IBD, and certolizumab and ustekinumab are approved for Crohn disease. Use of TNF inhibitors in patients with IBD may cause psoriasiform lesions to develop.31 Nonetheless, treatment should be individualized and psoriasiform lesions treated with standard psoriasis measures. Psoriasis patients with IBD are recommended to avoid IL-17–inhibitor therapy, given its potential to worsen IBD flares.
Malignancy
Psoriasis patients aged 0 to 79 years have a greater overall risk for malignancy compared with patients without psoriasis.32 Patients with psoriasis have an increased risk for respiratory tract cancer, upper aerodigestive tract cancer, urinary tract cancer, and non-Hodgkin lymphoma.33 A mild association exists between PsA and lymphoma, nonmelanoma skin cancer (NMSC), and lung cancer.34 More severe psoriasis is associated with greater risk for lymphoma and NMSC. Dermatologists are recommended to educate patients on their risk for certain malignancies and to refer patients to specialists upon suspicion of malignancy.
Risk for malignancy has been shown to be affected by psoriasis treatments. Patients treated with UVB have reduced overall cancer rates for all age groups (hazard ratio, 0.52; P=.3), while those treated with psoralen plus UVA have an increased incidence of
Lifestyle Choices and QOL
A crucial aspect of successful psoriasis management is patient education. The strongest recommendations support lifestyle changes, such as smoking cessation and limitation of alcohol use. A tactful discussion regarding substance use, work productivity, interpersonal relationships, and sexual function can address substantial effects of psoriasis on QOL so that support and resources can be provided.
Final Thoughts
Management of psoriasis is multifaceted and involves screening, education, monitoring, and collaboration with PCPs and specialists. Regular follow-up with a dermatologist and PCP is strongly recommended for patients with psoriasis given the systemic nature of the disease. The 2019 AAD-NPF recommendations provide important information for dermatologists to coordinate care for complicated psoriasis cases, but clinical judgment is paramount when making medical decisions. The consideration of comorbidities is critical for developing a comprehensive treatment approach, and this approach will lead to better health outcomes and improved QOL for patients with psoriasis.
Psoriasis is a chronic and relapsing systemic inflammatory disease that predisposes patients to a host of other conditions. It is believed that these widespread effects are due to chronic inflammation and cytokine activation, which affect multiple body processes and lead to the development of various comorbidities that need to be proactively managed.
In April 2019, the American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) released recommendation guidelines for managing psoriasis in adults with an emphasis on common disease comorbidities, including psoriatic arthritis (PsA), cardiovascular disease (CVD), inflammatory bowel disease (IBD), metabolic syndrome, and mood disorders. Psychosocial wellness, mental health, and quality of life (QOL) measures in relation to psoriatic disease also were discussed.1
The AAD-NPF guidelines address current screening, monitoring, education, and treatment recommendations for the management of psoriatic comorbidities. The Table and eTable summarize the screening recommendations. These guidelines aim to assist dermatologists with comprehensive disease management by addressing potential extracutaneous manifestations of psoriasis in adults.


Screening and Risk Assessment
Patients with psoriasis should receive a thorough history and physical examination to assess disease severity and risk for potential comorbidities. Patients with greater disease severity—as measured by body surface area (BSA) involvement and type of therapy required—have a greater risk for other disease-related comorbidities, specifically metabolic syndrome, renal disease, chronic obstructive pulmonary disease (COPD), obstructive sleep apnea, uveitis, IBD, malignancy, and increased mortality.2 Because the likelihood of comorbidities is greatest with severe disease, more frequent monitoring is recommended for these patients.
Psoriatic Arthritis
Patients with psoriasis need to be evaluated for PsA at every visit. Patients presenting with signs and symptoms suspicious for PsA—joint swelling, peripheral joint involvement, and joint inflammation—warrant further evaluation and consultation. Early detection and treatment of PsA is essential for preventing unnecessary suffering and progressive joint destruction.3
There are several PsA screening questionnaires currently available, including the Psoriatic Arthritis Screening Evaluation, Psoriasis Epidemiology Screening Tool, and Toronto Psoriatic Arthritis Screen. No significant differences in sensitivity and specificity were found among these questionnaires when using the Classification Criteria for Psoriatic Arthritis as the gold standard. All 3 questionnaires—the Psoriatic Arthritis Screening Evaluation and the Psoriasis Epidemiology Screening Tool were developed for use in dermatology and rheumatology clinics, and the Toronto Psoriatic Arthritis Screen was developed for use in the primary care setting—were found to be effective in dermatology/rheumatology clinics and primary care clinics, respectively.3 False-positive results predominantly were seen in patients with degenerative joint disease or osteoarthritis. Dermatologists should conduct a thorough physical examination to distinguish PsA from degenerative joint disease. Imaging and laboratory tests to evaluate for signs of systemic inflammation (erythrocyte sedimentation rate, C-reactive protein) also can be helpful in distinguishing the 2 conditions; however, these metrics have not been shown to contribute to PsA diagnosis.1 Full rheumatologic consultation is warranted in challenging cases.
Cardiovascular Disease
Primary care physicians (PCPs) are recommended to screen patients for CVD risk factors using height, weight, blood pressure, blood glucose, hemoglobin A1C, lipid levels, abdominal circumference, and body mass index (BMI). Lifestyle modifications such as smoking cessation, exercise, and dietary changes are encouraged to achieve and maintain a normal BMI.
Dermatologists also need to give special consideration to comorbidities when selecting medications and/or therapies for disease management. Patients on TNF inhibitors have a lower risk for MI compared with patients using topical medications, phototherapy, and other oral agents.10 Additionally, patients on TNF inhibitors have a lower risk for occurrence of major adverse cardiovascular events compared with patients treated with methotrexate or phototherapy.11,12
Metabolic Syndrome
Numerous studies have demonstrated an association between psoriasis and metabolic syndrome. Patients with increased BSA involvement and
The association between psoriasis and weight loss has been analyzed in several studies. Weight loss, particularly in obese patients, has been shown to improve psoriasis severity, as measured by psoriasis area and severity index score and QOL measures.15 Another study found that gastric bypass was associated with a significant risk reduction in the development of psoriasis (P=.004) and the disease prognosis (P=.02 for severe psoriasis; P=.01 for PsA).16 Therefore, patients with moderate to severe psoriasis are recommended to have their obesity status determined according to national guidelines. For patients with a BMI above 40 kg/m2 and standard weight-loss measures fail, bariatric surgery is recommended. Additionally, the impact of psoriasis medications on weight has been studied. Apremilast has been associated with weight loss, whereas etanercept and infliximab have been linked to weight gain.17,18
An association between psoriasis and hypertension also has been demonstrated by studies, especially among patients with severe disease. Therefore, patients with moderate to severe psoriasis are recommended to have their blood pressure evaluated according to national guidelines, and those with a blood pressure of 140/90 mm Hg or higher should be referred to their PCP for assessment and treatment. Current evidence does not support restrictions on antihypertensive medications in patients with psoriasis. Physicians should be aware of the potential for cyclosporine to induce hypertension, which should be treated, specifically with amlodipine.19
Many studies have demonstrated an association between psoriasis and dyslipidemia, though the results are somewhat conflicting. In 2018, the American Heart Association and the American College of Cardiology deemed psoriasis as an atherosclerotic CVD risk-enhancing condition, favoring early initiation of statin therapy. Because dyslipidemia plays a prominent role in atherosclerosis and CVD, patients with moderate to severe psoriasis are recommended to undergo periodic screening with lipid tests (eg, fasting total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides).20 Patients with elevated fasting triglycerides or low-density lipoprotein cholesterol should be referred to their PCP for further management. Certain psoriasis medications also have been linked to dyslipidemia. Acitretin and cyclosporine are known to adversely affect lipid levels, so patients treated with either agent should undergo routine monitoring of serum lipid levels.
Psoriasis is strongly associated with diabetes mellitus. Because of the increased risk for diabetes in patients with severe disease, regular monitoring of fasting blood glucose and/or hemoglobin A1C levels in patients with moderate to severe psoriasis is recommended. Patients who meet criteria for prediabetes or diabetes should be referred to their PCP for further assessment and management.21,22
Mood Disorders
Psoriasis affects QOL and can have a major impact on patients’ interpersonal relationships. Studies have shown an association between psoriasis and mood disorders, specifically depression and anxiety. Unfortunately, patients with mood disorders are less likely to seek intervention for their skin disease, which poses a tremendous treatment barrier. Dermatologists should regularly monitor patients for psychiatric symptoms so that resources and treatments can be offered.
Certain psoriasis therapies have been shown to help alleviate associated depression and anxiety. Improvements in Beck Depression Inventory and Hamilton Depression Rating Scale scores were seen with etanercept.23 Adalimumab and ustekinumab showed improvement in Dermatology Life Quality Index compared with placebo.24,25 Patients receiving Goeckerman treatment also had improvement in anxiety and depression scores compared with conventional therapy.26 Biologic medications had the largest impact on improving depression symptoms compared with conventional systemic therapy and phototherapy.27 The recommendations support use of biologics and the Goeckerman regimen for the concomitant treatment of mood disorders and psoriasis.
Renal Disease
Studies have supported an association between psoriasis and chronic kidney disease (CKD), independent of risk factors including vascular disease, hypertension, and diabetes. The prevalence of moderate to advanced CKD also has been found to be directly related to increasing BSA affected by psoriasis.28 Patients should receive testing of blood urea nitrogen, creatinine, and urine microalbumin levels to assess for occult renal disease. In addition, physicians should be cautious when prescribing nephrotoxic drugs (nonsteroidal anti-inflammatory drugs and cyclosporine) and renally excreted agents (methotrexate and apremilast) because of the risk for underlying renal disease in patients with psoriasis. If newly acquired renal disease is suspected, physicians should withhold the offending agents. Patients with psoriasis with CKD are recommended to follow up with their PCP or nephrologist for evaluation and management.
Pulmonary Disease
Psoriasis also has an independent association with COPD. Patients with psoriasis have a higher likelihood of developing COPD (hazard ratio, 2.35; 95% CI, 1.42-3.89; P<.01) than controls.29 The prevalence of COPD also was found to correlate with psoriasis severity. Dermatologists should educate patients about the association between smoking and psoriasis as well as advise patients to discontinue smoking to reduce their risk for developing COPD and cancer.
Patients with psoriasis also are at an increased risk for obstructive sleep apnea. Obstructive sleep apnea should be considered in patients with risk factors including snoring, obesity, hypertension, or diabetes.
Inflammatory Bowel Disease
Patients with psoriasis have an increased risk for developing IBD. The prevalence ratios of both Crohn disease (2.49) and ulcerative colitis (1.64) are increased in patients with psoriasis relative to patients without psoriasis.30 Physicians need to be aware of the association between psoriasis and IBD and the effect that their coexistence may have on treatment choice for patients.
Adalimumab and infliximab are approved for the treatment of IBD, and certolizumab and ustekinumab are approved for Crohn disease. Use of TNF inhibitors in patients with IBD may cause psoriasiform lesions to develop.31 Nonetheless, treatment should be individualized and psoriasiform lesions treated with standard psoriasis measures. Psoriasis patients with IBD are recommended to avoid IL-17–inhibitor therapy, given its potential to worsen IBD flares.
Malignancy
Psoriasis patients aged 0 to 79 years have a greater overall risk for malignancy compared with patients without psoriasis.32 Patients with psoriasis have an increased risk for respiratory tract cancer, upper aerodigestive tract cancer, urinary tract cancer, and non-Hodgkin lymphoma.33 A mild association exists between PsA and lymphoma, nonmelanoma skin cancer (NMSC), and lung cancer.34 More severe psoriasis is associated with greater risk for lymphoma and NMSC. Dermatologists are recommended to educate patients on their risk for certain malignancies and to refer patients to specialists upon suspicion of malignancy.
Risk for malignancy has been shown to be affected by psoriasis treatments. Patients treated with UVB have reduced overall cancer rates for all age groups (hazard ratio, 0.52; P=.3), while those treated with psoralen plus UVA have an increased incidence of
Lifestyle Choices and QOL
A crucial aspect of successful psoriasis management is patient education. The strongest recommendations support lifestyle changes, such as smoking cessation and limitation of alcohol use. A tactful discussion regarding substance use, work productivity, interpersonal relationships, and sexual function can address substantial effects of psoriasis on QOL so that support and resources can be provided.
Final Thoughts
Management of psoriasis is multifaceted and involves screening, education, monitoring, and collaboration with PCPs and specialists. Regular follow-up with a dermatologist and PCP is strongly recommended for patients with psoriasis given the systemic nature of the disease. The 2019 AAD-NPF recommendations provide important information for dermatologists to coordinate care for complicated psoriasis cases, but clinical judgment is paramount when making medical decisions. The consideration of comorbidities is critical for developing a comprehensive treatment approach, and this approach will lead to better health outcomes and improved QOL for patients with psoriasis.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
- Gelfand JM, Troxel AB, Lewis JD, et al. The risk of mortality in patients with psoriasis: results from a population-based study. Arch Dermatol. 2007;143:1493-1499.
- Coates LC, Aslam T, Al Balushi F, et al. Comparison of three screening tools to detect psoriatic arthritis in patients with psoriasis (CONTEST study). Br J Dermatol. 2013;168:802-807.
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3168-3209.
- Lerman JB, Joshi AA, Chaturvedi A, et al. Coronary plaque characterization in psoriasis reveals high-risk features that improve after treatment in a prospective observational study. Circulation. 2017;136:263-276.
- Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735-1741.
- Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411-2418.
- Dunlay SM, Weston SA, Redfield MM, et al. Tumor necrosis factor-alpha and mortality in heart failure: a community study. Circulation. 2008;118:625-631.
- Russell SD, Saval MA, Robbins JL, et al. New York Heart Association functional class predicts exercise parameters in the current era. Am Heart J. 2009;158(4 suppl):S24-S30.
- Wu JJ, Poon K-YT, Channual JC, et al. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148:1244-1250.
- Wu JJ, Guerin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90.
- Wu JJ, Sundaram M, Cloutier M, et al. The risk of cardiovascular events in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus phototherapy: an observational cohort study. J Am Acad Dermatol. 2018;79:60-68.
- Gami AS, Witt BJ, Howard DE, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403-414.
- Langan SM, Seminara NM, Shin DB, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol. 2012;132:556-562.
- Jensen P, Zachariae C, Christensen R, et al. Effect of weight loss on the severity of psoriasis: a randomized clinical study. JAMA Dermatol. 2013;149:795-801.
- Egeberg A, Sørensen JA, Gislason GH, et al. Incidence and prognosis of psoriasis and psoriatic arthritis in patients undergoing bariatric surgery. JAMA Surg. 2017;152:344-349.
- Crowley J, Thaçi D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for ≥156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77:310-317.e1. doi:10.1016/j.jaad.2017.01.052
- Gisondi P, Del Giglio M, Di Francesco V, et al. Weight loss improves the response of obese patients with moderate-to-severe chronic plaque psoriasis to low-dose cyclosporine therapy: a randomized, controlled, investigator-blinded clinical trial. Am J Clin Nutr. 2008;88:1242-1247.
- Leenen FHH, Coletta E, Davies RA. Prevention of renal dysfunction and hypertension by amlodipine after heart transplant. Am J Cardiol. 2007;100:531-535.
- Goff DC Jr, Lloyd-Jones DM, Bennet G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk. Circulation. 2014;129(suppl 2):S49-S73.
- American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care. 2014;37(suppl 1):S14-S80.
- Ratner RE, Diabetes Prevention Program Research Group. An update on the diabetes prevention program. Endocr Pract. 2006;12(suppl 1):20-24.
- Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29-35.
- Kimball AB, Edson-Heredia E, Zhu B, et al. Understanding the relationship between pruritus severity and work productivity in patients with moderate-to-severe psoriasis: sleep problems are a mediating factor. J Drugs Dermatol. 2016;15:183-188.
- Langley RG, Tsai T-F, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br J Dermatol. 2018;178:114-123.
- Chern E, Yau D, Ho J-C, et al. Positive effect of modified Goeckerman regimen on quality of life and psychosocial distress in moderate and severe psoriasis. Acta Derm Venereol. 2011;91:447-451.
- Strober B, Gooderham M, de Jong EMGJ, et al. Depressive symptoms, depression, and the effect of biologic therapy among patients in Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Am Acad Dermatol. 2018;78:70-80.
- Wan J, Wang S, Haynes K, et al. Risk of moderate to advanced kidney disease in patients with psoriasis: population based cohort study. BMJ. 2013;347:f5961. doi:10.1136/bmj.f5961
- Chiang Y-Y, Lin H-W. Association between psoriasis and chronic obstructive pulmonary disease: a population-based study in Taiwan. J Eur Acad Dermatol Venereol. 2012;26:59-65.
- Cohen AD, Dreiher J, Birkenfeld S. Psoriasis associated with ulcerative colitis and Crohn’s disease. J Eur Acad Dermatol Venereol. 2009;23:561-565.
- Denadai R, Teixeira FV, Saad-Hossne R. The onset of psoriasis during the treatment of inflammatory bowel diseases with infliximab: should biological therapy be suspended? Arq Gastroenterol. 2012;49:172-176.
- Chen Y-J, Wu C-Y, Chen T-J, et al. The risk of cancer in patients with psoriasis: a population-based cohort study in Taiwan. J Am Acad Dermatol. 2011;65:84-91.
- Pouplard C, Brenaut E, Horreau C, et al. Risk of cancer in psoriasis: a systematic review and meta-analysis of epidemiological studies. J Eur Acad Dermatol Venereol. 2013;27(suppl 3):36-46.
- Chiesa Fuxench ZC, Shin DB, Ogdie Beatty A, et al. The risk of cancer in patients with psoriasis: a population-based cohort study in the health improvement network. JAMA Dermatol. 2016;152:282-290.
- Burmester GR, Panaccione R, Gordon KB, et al. Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn’s disease. Ann Rheum Dis. 2013;72:517-524.
- Dommasch ED, Abuabara K, Shin DB, et al. The risk of infection and malignancy with tumor necrosis factor antagonists in adults with psoriatic disease: a systematic review and meta-analysis of randomized controlled trials. J Am Acad Dermatol. 2011;64:1035-1050.
- Gordon KB, Papp KA, Langley RG, et al. Long-term safety experience of ustekinumab in patients with moderate to severe psoriasis (part II of II): results from analyses of infections and malignancy from pooled phase II and III clinical trials. J Am Acad Dermatol. 2012;66:742-751.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
- Gelfand JM, Troxel AB, Lewis JD, et al. The risk of mortality in patients with psoriasis: results from a population-based study. Arch Dermatol. 2007;143:1493-1499.
- Coates LC, Aslam T, Al Balushi F, et al. Comparison of three screening tools to detect psoriatic arthritis in patients with psoriasis (CONTEST study). Br J Dermatol. 2013;168:802-807.
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3168-3209.
- Lerman JB, Joshi AA, Chaturvedi A, et al. Coronary plaque characterization in psoriasis reveals high-risk features that improve after treatment in a prospective observational study. Circulation. 2017;136:263-276.
- Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735-1741.
- Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411-2418.
- Dunlay SM, Weston SA, Redfield MM, et al. Tumor necrosis factor-alpha and mortality in heart failure: a community study. Circulation. 2008;118:625-631.
- Russell SD, Saval MA, Robbins JL, et al. New York Heart Association functional class predicts exercise parameters in the current era. Am Heart J. 2009;158(4 suppl):S24-S30.
- Wu JJ, Poon K-YT, Channual JC, et al. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148:1244-1250.
- Wu JJ, Guerin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90.
- Wu JJ, Sundaram M, Cloutier M, et al. The risk of cardiovascular events in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus phototherapy: an observational cohort study. J Am Acad Dermatol. 2018;79:60-68.
- Gami AS, Witt BJ, Howard DE, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403-414.
- Langan SM, Seminara NM, Shin DB, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol. 2012;132:556-562.
- Jensen P, Zachariae C, Christensen R, et al. Effect of weight loss on the severity of psoriasis: a randomized clinical study. JAMA Dermatol. 2013;149:795-801.
- Egeberg A, Sørensen JA, Gislason GH, et al. Incidence and prognosis of psoriasis and psoriatic arthritis in patients undergoing bariatric surgery. JAMA Surg. 2017;152:344-349.
- Crowley J, Thaçi D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for ≥156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77:310-317.e1. doi:10.1016/j.jaad.2017.01.052
- Gisondi P, Del Giglio M, Di Francesco V, et al. Weight loss improves the response of obese patients with moderate-to-severe chronic plaque psoriasis to low-dose cyclosporine therapy: a randomized, controlled, investigator-blinded clinical trial. Am J Clin Nutr. 2008;88:1242-1247.
- Leenen FHH, Coletta E, Davies RA. Prevention of renal dysfunction and hypertension by amlodipine after heart transplant. Am J Cardiol. 2007;100:531-535.
- Goff DC Jr, Lloyd-Jones DM, Bennet G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk. Circulation. 2014;129(suppl 2):S49-S73.
- American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care. 2014;37(suppl 1):S14-S80.
- Ratner RE, Diabetes Prevention Program Research Group. An update on the diabetes prevention program. Endocr Pract. 2006;12(suppl 1):20-24.
- Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29-35.
- Kimball AB, Edson-Heredia E, Zhu B, et al. Understanding the relationship between pruritus severity and work productivity in patients with moderate-to-severe psoriasis: sleep problems are a mediating factor. J Drugs Dermatol. 2016;15:183-188.
- Langley RG, Tsai T-F, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br J Dermatol. 2018;178:114-123.
- Chern E, Yau D, Ho J-C, et al. Positive effect of modified Goeckerman regimen on quality of life and psychosocial distress in moderate and severe psoriasis. Acta Derm Venereol. 2011;91:447-451.
- Strober B, Gooderham M, de Jong EMGJ, et al. Depressive symptoms, depression, and the effect of biologic therapy among patients in Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Am Acad Dermatol. 2018;78:70-80.
- Wan J, Wang S, Haynes K, et al. Risk of moderate to advanced kidney disease in patients with psoriasis: population based cohort study. BMJ. 2013;347:f5961. doi:10.1136/bmj.f5961
- Chiang Y-Y, Lin H-W. Association between psoriasis and chronic obstructive pulmonary disease: a population-based study in Taiwan. J Eur Acad Dermatol Venereol. 2012;26:59-65.
- Cohen AD, Dreiher J, Birkenfeld S. Psoriasis associated with ulcerative colitis and Crohn’s disease. J Eur Acad Dermatol Venereol. 2009;23:561-565.
- Denadai R, Teixeira FV, Saad-Hossne R. The onset of psoriasis during the treatment of inflammatory bowel diseases with infliximab: should biological therapy be suspended? Arq Gastroenterol. 2012;49:172-176.
- Chen Y-J, Wu C-Y, Chen T-J, et al. The risk of cancer in patients with psoriasis: a population-based cohort study in Taiwan. J Am Acad Dermatol. 2011;65:84-91.
- Pouplard C, Brenaut E, Horreau C, et al. Risk of cancer in psoriasis: a systematic review and meta-analysis of epidemiological studies. J Eur Acad Dermatol Venereol. 2013;27(suppl 3):36-46.
- Chiesa Fuxench ZC, Shin DB, Ogdie Beatty A, et al. The risk of cancer in patients with psoriasis: a population-based cohort study in the health improvement network. JAMA Dermatol. 2016;152:282-290.
- Burmester GR, Panaccione R, Gordon KB, et al. Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn’s disease. Ann Rheum Dis. 2013;72:517-524.
- Dommasch ED, Abuabara K, Shin DB, et al. The risk of infection and malignancy with tumor necrosis factor antagonists in adults with psoriatic disease: a systematic review and meta-analysis of randomized controlled trials. J Am Acad Dermatol. 2011;64:1035-1050.
- Gordon KB, Papp KA, Langley RG, et al. Long-term safety experience of ustekinumab in patients with moderate to severe psoriasis (part II of II): results from analyses of infections and malignancy from pooled phase II and III clinical trials. J Am Acad Dermatol. 2012;66:742-751.
Practice Points
- Educating patients about psoriasis and its extracutaneous manifestations, available treatment options, and the impact of lifestyle choices is advised to maximize their patient’s disease awareness and to promote a collaborative physician-patient partnership.
- Physicians are strongly recommended to screen patients with psoriasis for the presence of disease comorbidities to ensure comprehensive management of their disease.
- Managing psoriasis as a multisystem inflammatory disorder requires the combined effort of dermatologists and other specialists to prevent and treat disease comorbidities and enhance patients’ quality of life.
Update on Biologics for Psoriasis in Clinical Practice
Biologics have transformed the management of moderate to severe psoriasis. There currently are 11 biologics approved by the US Food and Drug Administration (Table) for psoriasis treatment that have been affirmed by various clinical studies. This article provides dosing initiation, maintenance information, and updated clinical data using phase 3 studies (N=8) published between May 2020 and February 2021. Generic names of the 11 biologics were searched separately in the PubMed database within the specified date range. Subsequent results were reviewed by title and selected for phase 3 and 4 trials. Clinical data in this review focus on reducing patient disease burden by allocating a biologic best fit for each patient’s individual health profile.

IL-17A Inhibitors Update
Secukinumab is safe and efficacious for skin clearance in the presence of comorbidities and can be used for improving plaque psoriasis and palmoplantar pustular psoriasis. An extension of a phase 3 randomized controlled trial (RCT)—2PRECISE—evaluated the efficacy and safety of secukinumab dosing at 300 mg (n=79) and 150 mg (n=80) in adults with moderate to severe palmoplantar pustular psoriasis (palmoplantar psoriasis area and severity index [PPPASI] score ≥12 and dermatology life quality index [DLQI] ≥10) over 148 weeks.1 Extension patients were included from the 52-week 2PRECISE study per the investigator’s judgement of a meaningful clinical response (exact criteria not described). All treatment groups demonstrated a mean (SD) PPPASI of 22.7 (9.5) by the extension trial’s start. Results affirmed that clinical response waned after week 148 in all groups excluding placebo/secukinumab 150 mg, which maintained a mean (SD) PPPASI of 22.7 (9.5). The most frequent adverse events were nasopharyngitis, pustular psoriasis, headache, and pruritus.1
Comorbidities do not have a major impact on secukinumab’s efficacy. A post hoc analysis of 4 phase 3 RCTs—ERASURE, FIXTURE, FEATURE, and JUNCTURE—gathered data from adult patients (N=2401) to assess baseline comorbidities with efficacy and safety of secukinumab vs etanercept after 12 weeks of treatment.2 Sixty-one percent (n=1469) had at least 1 comorbidity, most frequently obesity, hypertension, psoriatic arthritis, hyperlipidemia, or diabetes mellitus. All patient groups had a greater likelihood of a psoriasis area and severity index (PASI) response with any dose of secukinumab vs patients with comorbidities who were taking etanercept or placebo (P<.05) at week 12. All groups had a greater likelihood of achieving investigator global assessment scores of 0/1 (clear/almost clear) vs patients with comorbidities taking etanercept or placebo (P<.05). Baseline comorbidities did not significantly affect treatment response, except obesity, which was associated with decreased probability of achieving all PASI and investigator global assessment (P<.01) responses. Secukinumab-treated patients with and without comorbidities had equivalent likelihood of treatment-emergent adverse events (TEAEs).2
Brodalumab is an effective biologic that has shown long-term safety with continuous administration. Continuous brodalumab and brodalumab after placebo demonstrated impactful skin clearance after 120 weeks in AMAGINE-1, a phase 3 RCT involving adults (N=442) with moderate to severe plaque psoriasis.3 Patients randomized to brodalumab 210 mg (n=222) or placebo (n=220) were rerandomized according to initial treatment response. In patients switching from brodalumab to placebo at week 12, 55% and 94% achieved PASI 75 at week 20 and week 120, respectively, and 75% reached PASI 100 at week 120. Of patients with static physician global assessment (sPGA) scores of 0/1 (clear/almost clear) at week 12 who were rerandomized to brodalumab, 96% and 80% (using observed data) achieved PASI 75 and PASI 100, respectively. Mean (SD) time to return of skin disease following withdrawal of brodalumab was 74.7 (50.5) days. Treatment-emergent adverse events included headaches, arthralgia, diarrhea, and nausea. Suicidal ideation was rare (this study had 1 completed suicide), and authors cited that no causal association has been made between brodalumab and suicidality. Brodalumab also demonstrated favorable treatment response in patients who underwent a lapse in treatment, offering real-world value, as intermittent treatment administration can occur because of personal or financial reasons.3
Ixekizumab is associated with more rapid skin clearance, better resolution of nail psoriasis, and superior improvement in quality-of-life measures when compared with guselkumab. The phase 3 study IXORA-R compared skin and nail clearance as well as patient-reported outcomes over 24 weeks with ixekizumab 80 mg (n=520) vs guselkumab 100 mg (n=507) in adults with moderate to severe plaque psoriasis.4 Ixekizumab (50%) was shown to be no worse than guselkumab (52%; difference, –2.3%) using a noninferiority test (noninferiority margin of –11.4%). The treatments exhibited similar efficacy, with no significant difference in proportion of patients reaching PASI 100 (P=.41). Ixekizumab patients tended to have skin clearance sooner than guselkumab patients, reaching PASI 50/75/90 and PASI 100 in a median time that was 2 weeks and 7.5 weeks earlier, respectively. More ixekizumab patients (52%) achieved clear nails vs guselkumab patients (31%; P=.007). Ixekizumab patients reported greater satisfaction with their skin disease affecting quality of life (DLQI), with more DLQI 0/1 (no effect at all on patient’s life) scores and being itch free (P<.05). Ixekizumab was associated with significantly more days of complete skin clearance (PASI 100) vs guselkumab (55.6 days vs 42.2 days; P<.001). Although an upper respiratory tract infection was the most common TEAE, the proportion of TEAEs was similar between treatments.4
IL-23 Inhibitors Update
Tildrakizumab has similar long-term skin clearance efficacy and safety in patients with psoriasis with and without comorbid metabolic syndrome (MetS). A post hoc analysis of 2 phase 2 RCTs (reSURFACE 1/2) involving adults (N=338 and N=307) with moderate to severe plaque psoriasis assessed long-term efficacy (3 years), drug survival, and safety for 5 years of continuous tildrakizumab 100 mg and 200 mg in adults with comorbid MetS.5 Although no difference in efficacy was concluded, greater body mass index of the MetS population was shown to be associated with lower biologic efficacy compared to the general population. The proportion of patients who achieved PASI 75 at week 52 was comparable in patients with MetS and patients without MetS (tildrakizumab 100 mg, 85% and 86% vs 86% and 94% for reSURFACE 1/2, respectively; tildrakizumab 200 mg, 76% and 87% vs 76% and 87% for reSURFACE 1/2, respectively).5
Tildrakizumab also demonstrated efficacy and safety for up to 5 years in 2 other phase 3 RCTs with no dose-related differences in frequency of injections and malignancies. Tildrakizumab 100 mg is the recommended dose. The 200-mg dose can be utilized in patients with a high burden of disease and disability. reSURFACE 1 and reSURFACE 2 involved adults with chronic moderate to severe plaque psoriasis randomized to tildrakizumab 100 mg, 200 mg, or placebo with the option of long-term extension to week 244 if patients reached 50% or greater improvement from baseline PASI score.6 Patients in reSURFACE 2 also were randomized to etanercept 50 mg with partial responders and nonresponders at week 28 switching to tildrakizumab 200 mg until week 244. Extension results showed PASI 75 achievement in 88.7% (95% CI, 84.6%-92.1%) of patients taking tildrakizumab 100 mg (n=235), 92.5% (95% CI, 88.1%-95.7%) of patients taking tildrakizumab 200 mg (n=176), and 81.3% (95% CI, 72.6%-88.2%) of patients taking etanercept/partial nonresponders (n=85). The most common TEAE was nasopharyngitis (10.5/100 patient-years for tildrakizumab 100 mg and 10.7/100 patient-years for tildrakizumab 200 mg). The frequency of severe infections (eg, diverticulitis, pneumonia, cellulitis, appendicitis) was 1.2 per 100 patient-years for tildrakizumab 100 mg and 1.3 per 100 patient-years for tildrakizumab 200 mg.6
Risankizumab and tildrakizumab require the lowest number of injections, thereby providing sustainable skin clearance with a convenient injection dosing schedule for patients. Risankizumab efficacy (8.2% with inferiority margin of 12%) was noninferior to secukinumab when assessing the proportion of PASI 90 responders at week 16 (after 2 doses of risankizumab vs 7 doses of secukinumab).7 IMMerge, an international phase 3 RCT, involved adults (N=327) with moderate to severe plaque psoriasis to compare the safety and efficacy of risankizumab 150 mg (n=164) vs secukinumab 300 mg (n=163) up to 52 weeks. A greater proportion of the risankizumab arm (86.6%) achieved PASI 90 in 52 weeks compared to the secukinumab arm (57.1%). Superior skin clearance (PASI 90) at week 52 was achieved after 5 doses with risankizumab vs 16 doses of secukinumab. Risankizumab TEAEs were nasopharyngitis, upper respiratory tract infection, headache, arthralgia, diarrhea, and bronchitis.7
Continuous risankizumab treatment shows substantially stronger skin clearing performance compared with intermittent treatment following drug withdrawal, demonstrating that treatment gaps minimize therapeutic response. IMMhance, an international phase 3 RCT involving adults (N=507) with moderate to severe plaque psoriasis, evaluated the safety and efficacy with risankizumab 150 mg after 52 weeks and 104 weeks.8 Part A randomized patients to risankizumab 150 mg (n=407) or placebo (n=100). Part B rerandomized patients at week 28 to continue risankizumab 150 mg or placebo (designated as withdrawal of treatment; later re-treated with risankizumab 150 mg if patients had sPGA ≥3). At week 52, significantly more patients reached sPGA score of 0/1 with risankizumab/risankizumab (n=97 [87.4%]) vs risankizumab/placebo (n=138 [61.3%]; P<.001). At week 104, significantly more patients reached an sPGA score of 0/1 with risankizumab/risankizumab (n=90 [81.1%]) vs risankizumab/placebo (n=16 [7.1%]; P<.001). Risankizumab exhibited longevity following withdrawal, as median time to loss of response and relapse was 42 weeks (sPGA ≥3). The extent of TEAEs was similar between risankizumab and placebo and included nasopharyngitis, upper respiratory tract infection, headache, and back pain.8
Final Thoughts
Biologics for psoriasis help produce intended results for skin disease clearance and are tools for precision medicine. Recent data demonstrate safe, durable, and continuous efficacy with biologics, which offer patients a better chance of treatment success. This guide may serve as a quick reference for biologic selection with special consideration of individual disease characteristics and comorbidities.
- Mrowietz U, Bachelez H, Burden AD, et al. Efficacy and safety of secukinumab in moderate to severe palmoplantar pustular psoriasis over 148 weeks: extension of the 2PRECISE study. J Am Acad Dermatol. 2021;84:552-554. doi:10.1016/j.jaad.2020.06.038
- Gottlieb AB, Wu JJ, Griffiths CEM, et al. Clinical efficacy and safety of secukinumab in patients with psoriasis and comorbidities: pooled analysis of 4 phase 3 clinical trials [published online October 21, 2020]. J Dermatolog Treat. doi:10.1080/09546634.2020.1832187
- Papp K, Menter A, Leonardi C, et al. Long-term efficacy and safety of brodalumab in psoriasis through 120 weeks and after withdrawal and retreatment: subgroup analysis of a randomized phase III trial (AMAGINE-1). Br J Dermatol. 2020;183:1037-1048. doi:10.1111/bjd.19132
- Blauvelt A, Leonardi C, Elewski B, et al. A head-to-head comparison of ixekizumab vs. guselkumab in patients with moderate-to-severe plaque psoriasis: 24-week efficacy and safety results from a randomized, double-blinded trial. Br J Dermatol. 2021;184:1047-1058. doi:10.1111/bjd.19509
- Lebwohl MG, Leonardi CL, Mehta NN, et al. Tildrakizumab efficacy, drug survival, and safety are comparable in patients with psoriasis with and without metabolic syndrome: long-term results from 2 phase 3 randomized controlled studies (reSURFACE 1 and reSURFACE 2). J Am Acad Dermatol. 2021;84:398-407. doi:10.1016/j.jaad.2020.09.047
- Thaci D, Piaserico S, Warren RB, et al. Five-year efficacy and safety of tildrakizumab in patients with moderate-to-severe psoriasis who respond at week 28: pooled analyses of two randomized phase III clinical trials (reSURFACE 1 and reSURFACE 2)[published online February 5, 2021]. Br J Dermatol. doi:10.1111/bjd.19866
- Warren RB, Blauvelt A, Poulin Y, et al. Efficacy and safety of risankizumab vs. secukinumab in patients with moderate-to-severe plaque psoriasis (IMMerge): results from a phase III, randomized, open-label, efficacy-assessor-blinded clinical trial. Br J Dermatol. 2021;184:50-59. doi:10.1111/bjd.19341
- Blauvelt A, Leonardi CL, Gooderham M, et al. Efficacy and safety of continuous risankizumab therapy vs treatment withdrawal in patients with moderate to severe plaque psoriasis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:649-658. doi:10.1001/jamadermatol.2020.0723
Biologics have transformed the management of moderate to severe psoriasis. There currently are 11 biologics approved by the US Food and Drug Administration (Table) for psoriasis treatment that have been affirmed by various clinical studies. This article provides dosing initiation, maintenance information, and updated clinical data using phase 3 studies (N=8) published between May 2020 and February 2021. Generic names of the 11 biologics were searched separately in the PubMed database within the specified date range. Subsequent results were reviewed by title and selected for phase 3 and 4 trials. Clinical data in this review focus on reducing patient disease burden by allocating a biologic best fit for each patient’s individual health profile.

IL-17A Inhibitors Update
Secukinumab is safe and efficacious for skin clearance in the presence of comorbidities and can be used for improving plaque psoriasis and palmoplantar pustular psoriasis. An extension of a phase 3 randomized controlled trial (RCT)—2PRECISE—evaluated the efficacy and safety of secukinumab dosing at 300 mg (n=79) and 150 mg (n=80) in adults with moderate to severe palmoplantar pustular psoriasis (palmoplantar psoriasis area and severity index [PPPASI] score ≥12 and dermatology life quality index [DLQI] ≥10) over 148 weeks.1 Extension patients were included from the 52-week 2PRECISE study per the investigator’s judgement of a meaningful clinical response (exact criteria not described). All treatment groups demonstrated a mean (SD) PPPASI of 22.7 (9.5) by the extension trial’s start. Results affirmed that clinical response waned after week 148 in all groups excluding placebo/secukinumab 150 mg, which maintained a mean (SD) PPPASI of 22.7 (9.5). The most frequent adverse events were nasopharyngitis, pustular psoriasis, headache, and pruritus.1
Comorbidities do not have a major impact on secukinumab’s efficacy. A post hoc analysis of 4 phase 3 RCTs—ERASURE, FIXTURE, FEATURE, and JUNCTURE—gathered data from adult patients (N=2401) to assess baseline comorbidities with efficacy and safety of secukinumab vs etanercept after 12 weeks of treatment.2 Sixty-one percent (n=1469) had at least 1 comorbidity, most frequently obesity, hypertension, psoriatic arthritis, hyperlipidemia, or diabetes mellitus. All patient groups had a greater likelihood of a psoriasis area and severity index (PASI) response with any dose of secukinumab vs patients with comorbidities who were taking etanercept or placebo (P<.05) at week 12. All groups had a greater likelihood of achieving investigator global assessment scores of 0/1 (clear/almost clear) vs patients with comorbidities taking etanercept or placebo (P<.05). Baseline comorbidities did not significantly affect treatment response, except obesity, which was associated with decreased probability of achieving all PASI and investigator global assessment (P<.01) responses. Secukinumab-treated patients with and without comorbidities had equivalent likelihood of treatment-emergent adverse events (TEAEs).2
Brodalumab is an effective biologic that has shown long-term safety with continuous administration. Continuous brodalumab and brodalumab after placebo demonstrated impactful skin clearance after 120 weeks in AMAGINE-1, a phase 3 RCT involving adults (N=442) with moderate to severe plaque psoriasis.3 Patients randomized to brodalumab 210 mg (n=222) or placebo (n=220) were rerandomized according to initial treatment response. In patients switching from brodalumab to placebo at week 12, 55% and 94% achieved PASI 75 at week 20 and week 120, respectively, and 75% reached PASI 100 at week 120. Of patients with static physician global assessment (sPGA) scores of 0/1 (clear/almost clear) at week 12 who were rerandomized to brodalumab, 96% and 80% (using observed data) achieved PASI 75 and PASI 100, respectively. Mean (SD) time to return of skin disease following withdrawal of brodalumab was 74.7 (50.5) days. Treatment-emergent adverse events included headaches, arthralgia, diarrhea, and nausea. Suicidal ideation was rare (this study had 1 completed suicide), and authors cited that no causal association has been made between brodalumab and suicidality. Brodalumab also demonstrated favorable treatment response in patients who underwent a lapse in treatment, offering real-world value, as intermittent treatment administration can occur because of personal or financial reasons.3
Ixekizumab is associated with more rapid skin clearance, better resolution of nail psoriasis, and superior improvement in quality-of-life measures when compared with guselkumab. The phase 3 study IXORA-R compared skin and nail clearance as well as patient-reported outcomes over 24 weeks with ixekizumab 80 mg (n=520) vs guselkumab 100 mg (n=507) in adults with moderate to severe plaque psoriasis.4 Ixekizumab (50%) was shown to be no worse than guselkumab (52%; difference, –2.3%) using a noninferiority test (noninferiority margin of –11.4%). The treatments exhibited similar efficacy, with no significant difference in proportion of patients reaching PASI 100 (P=.41). Ixekizumab patients tended to have skin clearance sooner than guselkumab patients, reaching PASI 50/75/90 and PASI 100 in a median time that was 2 weeks and 7.5 weeks earlier, respectively. More ixekizumab patients (52%) achieved clear nails vs guselkumab patients (31%; P=.007). Ixekizumab patients reported greater satisfaction with their skin disease affecting quality of life (DLQI), with more DLQI 0/1 (no effect at all on patient’s life) scores and being itch free (P<.05). Ixekizumab was associated with significantly more days of complete skin clearance (PASI 100) vs guselkumab (55.6 days vs 42.2 days; P<.001). Although an upper respiratory tract infection was the most common TEAE, the proportion of TEAEs was similar between treatments.4
IL-23 Inhibitors Update
Tildrakizumab has similar long-term skin clearance efficacy and safety in patients with psoriasis with and without comorbid metabolic syndrome (MetS). A post hoc analysis of 2 phase 2 RCTs (reSURFACE 1/2) involving adults (N=338 and N=307) with moderate to severe plaque psoriasis assessed long-term efficacy (3 years), drug survival, and safety for 5 years of continuous tildrakizumab 100 mg and 200 mg in adults with comorbid MetS.5 Although no difference in efficacy was concluded, greater body mass index of the MetS population was shown to be associated with lower biologic efficacy compared to the general population. The proportion of patients who achieved PASI 75 at week 52 was comparable in patients with MetS and patients without MetS (tildrakizumab 100 mg, 85% and 86% vs 86% and 94% for reSURFACE 1/2, respectively; tildrakizumab 200 mg, 76% and 87% vs 76% and 87% for reSURFACE 1/2, respectively).5
Tildrakizumab also demonstrated efficacy and safety for up to 5 years in 2 other phase 3 RCTs with no dose-related differences in frequency of injections and malignancies. Tildrakizumab 100 mg is the recommended dose. The 200-mg dose can be utilized in patients with a high burden of disease and disability. reSURFACE 1 and reSURFACE 2 involved adults with chronic moderate to severe plaque psoriasis randomized to tildrakizumab 100 mg, 200 mg, or placebo with the option of long-term extension to week 244 if patients reached 50% or greater improvement from baseline PASI score.6 Patients in reSURFACE 2 also were randomized to etanercept 50 mg with partial responders and nonresponders at week 28 switching to tildrakizumab 200 mg until week 244. Extension results showed PASI 75 achievement in 88.7% (95% CI, 84.6%-92.1%) of patients taking tildrakizumab 100 mg (n=235), 92.5% (95% CI, 88.1%-95.7%) of patients taking tildrakizumab 200 mg (n=176), and 81.3% (95% CI, 72.6%-88.2%) of patients taking etanercept/partial nonresponders (n=85). The most common TEAE was nasopharyngitis (10.5/100 patient-years for tildrakizumab 100 mg and 10.7/100 patient-years for tildrakizumab 200 mg). The frequency of severe infections (eg, diverticulitis, pneumonia, cellulitis, appendicitis) was 1.2 per 100 patient-years for tildrakizumab 100 mg and 1.3 per 100 patient-years for tildrakizumab 200 mg.6
Risankizumab and tildrakizumab require the lowest number of injections, thereby providing sustainable skin clearance with a convenient injection dosing schedule for patients. Risankizumab efficacy (8.2% with inferiority margin of 12%) was noninferior to secukinumab when assessing the proportion of PASI 90 responders at week 16 (after 2 doses of risankizumab vs 7 doses of secukinumab).7 IMMerge, an international phase 3 RCT, involved adults (N=327) with moderate to severe plaque psoriasis to compare the safety and efficacy of risankizumab 150 mg (n=164) vs secukinumab 300 mg (n=163) up to 52 weeks. A greater proportion of the risankizumab arm (86.6%) achieved PASI 90 in 52 weeks compared to the secukinumab arm (57.1%). Superior skin clearance (PASI 90) at week 52 was achieved after 5 doses with risankizumab vs 16 doses of secukinumab. Risankizumab TEAEs were nasopharyngitis, upper respiratory tract infection, headache, arthralgia, diarrhea, and bronchitis.7
Continuous risankizumab treatment shows substantially stronger skin clearing performance compared with intermittent treatment following drug withdrawal, demonstrating that treatment gaps minimize therapeutic response. IMMhance, an international phase 3 RCT involving adults (N=507) with moderate to severe plaque psoriasis, evaluated the safety and efficacy with risankizumab 150 mg after 52 weeks and 104 weeks.8 Part A randomized patients to risankizumab 150 mg (n=407) or placebo (n=100). Part B rerandomized patients at week 28 to continue risankizumab 150 mg or placebo (designated as withdrawal of treatment; later re-treated with risankizumab 150 mg if patients had sPGA ≥3). At week 52, significantly more patients reached sPGA score of 0/1 with risankizumab/risankizumab (n=97 [87.4%]) vs risankizumab/placebo (n=138 [61.3%]; P<.001). At week 104, significantly more patients reached an sPGA score of 0/1 with risankizumab/risankizumab (n=90 [81.1%]) vs risankizumab/placebo (n=16 [7.1%]; P<.001). Risankizumab exhibited longevity following withdrawal, as median time to loss of response and relapse was 42 weeks (sPGA ≥3). The extent of TEAEs was similar between risankizumab and placebo and included nasopharyngitis, upper respiratory tract infection, headache, and back pain.8
Final Thoughts
Biologics for psoriasis help produce intended results for skin disease clearance and are tools for precision medicine. Recent data demonstrate safe, durable, and continuous efficacy with biologics, which offer patients a better chance of treatment success. This guide may serve as a quick reference for biologic selection with special consideration of individual disease characteristics and comorbidities.
Biologics have transformed the management of moderate to severe psoriasis. There currently are 11 biologics approved by the US Food and Drug Administration (Table) for psoriasis treatment that have been affirmed by various clinical studies. This article provides dosing initiation, maintenance information, and updated clinical data using phase 3 studies (N=8) published between May 2020 and February 2021. Generic names of the 11 biologics were searched separately in the PubMed database within the specified date range. Subsequent results were reviewed by title and selected for phase 3 and 4 trials. Clinical data in this review focus on reducing patient disease burden by allocating a biologic best fit for each patient’s individual health profile.

IL-17A Inhibitors Update
Secukinumab is safe and efficacious for skin clearance in the presence of comorbidities and can be used for improving plaque psoriasis and palmoplantar pustular psoriasis. An extension of a phase 3 randomized controlled trial (RCT)—2PRECISE—evaluated the efficacy and safety of secukinumab dosing at 300 mg (n=79) and 150 mg (n=80) in adults with moderate to severe palmoplantar pustular psoriasis (palmoplantar psoriasis area and severity index [PPPASI] score ≥12 and dermatology life quality index [DLQI] ≥10) over 148 weeks.1 Extension patients were included from the 52-week 2PRECISE study per the investigator’s judgement of a meaningful clinical response (exact criteria not described). All treatment groups demonstrated a mean (SD) PPPASI of 22.7 (9.5) by the extension trial’s start. Results affirmed that clinical response waned after week 148 in all groups excluding placebo/secukinumab 150 mg, which maintained a mean (SD) PPPASI of 22.7 (9.5). The most frequent adverse events were nasopharyngitis, pustular psoriasis, headache, and pruritus.1
Comorbidities do not have a major impact on secukinumab’s efficacy. A post hoc analysis of 4 phase 3 RCTs—ERASURE, FIXTURE, FEATURE, and JUNCTURE—gathered data from adult patients (N=2401) to assess baseline comorbidities with efficacy and safety of secukinumab vs etanercept after 12 weeks of treatment.2 Sixty-one percent (n=1469) had at least 1 comorbidity, most frequently obesity, hypertension, psoriatic arthritis, hyperlipidemia, or diabetes mellitus. All patient groups had a greater likelihood of a psoriasis area and severity index (PASI) response with any dose of secukinumab vs patients with comorbidities who were taking etanercept or placebo (P<.05) at week 12. All groups had a greater likelihood of achieving investigator global assessment scores of 0/1 (clear/almost clear) vs patients with comorbidities taking etanercept or placebo (P<.05). Baseline comorbidities did not significantly affect treatment response, except obesity, which was associated with decreased probability of achieving all PASI and investigator global assessment (P<.01) responses. Secukinumab-treated patients with and without comorbidities had equivalent likelihood of treatment-emergent adverse events (TEAEs).2
Brodalumab is an effective biologic that has shown long-term safety with continuous administration. Continuous brodalumab and brodalumab after placebo demonstrated impactful skin clearance after 120 weeks in AMAGINE-1, a phase 3 RCT involving adults (N=442) with moderate to severe plaque psoriasis.3 Patients randomized to brodalumab 210 mg (n=222) or placebo (n=220) were rerandomized according to initial treatment response. In patients switching from brodalumab to placebo at week 12, 55% and 94% achieved PASI 75 at week 20 and week 120, respectively, and 75% reached PASI 100 at week 120. Of patients with static physician global assessment (sPGA) scores of 0/1 (clear/almost clear) at week 12 who were rerandomized to brodalumab, 96% and 80% (using observed data) achieved PASI 75 and PASI 100, respectively. Mean (SD) time to return of skin disease following withdrawal of brodalumab was 74.7 (50.5) days. Treatment-emergent adverse events included headaches, arthralgia, diarrhea, and nausea. Suicidal ideation was rare (this study had 1 completed suicide), and authors cited that no causal association has been made between brodalumab and suicidality. Brodalumab also demonstrated favorable treatment response in patients who underwent a lapse in treatment, offering real-world value, as intermittent treatment administration can occur because of personal or financial reasons.3
Ixekizumab is associated with more rapid skin clearance, better resolution of nail psoriasis, and superior improvement in quality-of-life measures when compared with guselkumab. The phase 3 study IXORA-R compared skin and nail clearance as well as patient-reported outcomes over 24 weeks with ixekizumab 80 mg (n=520) vs guselkumab 100 mg (n=507) in adults with moderate to severe plaque psoriasis.4 Ixekizumab (50%) was shown to be no worse than guselkumab (52%; difference, –2.3%) using a noninferiority test (noninferiority margin of –11.4%). The treatments exhibited similar efficacy, with no significant difference in proportion of patients reaching PASI 100 (P=.41). Ixekizumab patients tended to have skin clearance sooner than guselkumab patients, reaching PASI 50/75/90 and PASI 100 in a median time that was 2 weeks and 7.5 weeks earlier, respectively. More ixekizumab patients (52%) achieved clear nails vs guselkumab patients (31%; P=.007). Ixekizumab patients reported greater satisfaction with their skin disease affecting quality of life (DLQI), with more DLQI 0/1 (no effect at all on patient’s life) scores and being itch free (P<.05). Ixekizumab was associated with significantly more days of complete skin clearance (PASI 100) vs guselkumab (55.6 days vs 42.2 days; P<.001). Although an upper respiratory tract infection was the most common TEAE, the proportion of TEAEs was similar between treatments.4
IL-23 Inhibitors Update
Tildrakizumab has similar long-term skin clearance efficacy and safety in patients with psoriasis with and without comorbid metabolic syndrome (MetS). A post hoc analysis of 2 phase 2 RCTs (reSURFACE 1/2) involving adults (N=338 and N=307) with moderate to severe plaque psoriasis assessed long-term efficacy (3 years), drug survival, and safety for 5 years of continuous tildrakizumab 100 mg and 200 mg in adults with comorbid MetS.5 Although no difference in efficacy was concluded, greater body mass index of the MetS population was shown to be associated with lower biologic efficacy compared to the general population. The proportion of patients who achieved PASI 75 at week 52 was comparable in patients with MetS and patients without MetS (tildrakizumab 100 mg, 85% and 86% vs 86% and 94% for reSURFACE 1/2, respectively; tildrakizumab 200 mg, 76% and 87% vs 76% and 87% for reSURFACE 1/2, respectively).5
Tildrakizumab also demonstrated efficacy and safety for up to 5 years in 2 other phase 3 RCTs with no dose-related differences in frequency of injections and malignancies. Tildrakizumab 100 mg is the recommended dose. The 200-mg dose can be utilized in patients with a high burden of disease and disability. reSURFACE 1 and reSURFACE 2 involved adults with chronic moderate to severe plaque psoriasis randomized to tildrakizumab 100 mg, 200 mg, or placebo with the option of long-term extension to week 244 if patients reached 50% or greater improvement from baseline PASI score.6 Patients in reSURFACE 2 also were randomized to etanercept 50 mg with partial responders and nonresponders at week 28 switching to tildrakizumab 200 mg until week 244. Extension results showed PASI 75 achievement in 88.7% (95% CI, 84.6%-92.1%) of patients taking tildrakizumab 100 mg (n=235), 92.5% (95% CI, 88.1%-95.7%) of patients taking tildrakizumab 200 mg (n=176), and 81.3% (95% CI, 72.6%-88.2%) of patients taking etanercept/partial nonresponders (n=85). The most common TEAE was nasopharyngitis (10.5/100 patient-years for tildrakizumab 100 mg and 10.7/100 patient-years for tildrakizumab 200 mg). The frequency of severe infections (eg, diverticulitis, pneumonia, cellulitis, appendicitis) was 1.2 per 100 patient-years for tildrakizumab 100 mg and 1.3 per 100 patient-years for tildrakizumab 200 mg.6
Risankizumab and tildrakizumab require the lowest number of injections, thereby providing sustainable skin clearance with a convenient injection dosing schedule for patients. Risankizumab efficacy (8.2% with inferiority margin of 12%) was noninferior to secukinumab when assessing the proportion of PASI 90 responders at week 16 (after 2 doses of risankizumab vs 7 doses of secukinumab).7 IMMerge, an international phase 3 RCT, involved adults (N=327) with moderate to severe plaque psoriasis to compare the safety and efficacy of risankizumab 150 mg (n=164) vs secukinumab 300 mg (n=163) up to 52 weeks. A greater proportion of the risankizumab arm (86.6%) achieved PASI 90 in 52 weeks compared to the secukinumab arm (57.1%). Superior skin clearance (PASI 90) at week 52 was achieved after 5 doses with risankizumab vs 16 doses of secukinumab. Risankizumab TEAEs were nasopharyngitis, upper respiratory tract infection, headache, arthralgia, diarrhea, and bronchitis.7
Continuous risankizumab treatment shows substantially stronger skin clearing performance compared with intermittent treatment following drug withdrawal, demonstrating that treatment gaps minimize therapeutic response. IMMhance, an international phase 3 RCT involving adults (N=507) with moderate to severe plaque psoriasis, evaluated the safety and efficacy with risankizumab 150 mg after 52 weeks and 104 weeks.8 Part A randomized patients to risankizumab 150 mg (n=407) or placebo (n=100). Part B rerandomized patients at week 28 to continue risankizumab 150 mg or placebo (designated as withdrawal of treatment; later re-treated with risankizumab 150 mg if patients had sPGA ≥3). At week 52, significantly more patients reached sPGA score of 0/1 with risankizumab/risankizumab (n=97 [87.4%]) vs risankizumab/placebo (n=138 [61.3%]; P<.001). At week 104, significantly more patients reached an sPGA score of 0/1 with risankizumab/risankizumab (n=90 [81.1%]) vs risankizumab/placebo (n=16 [7.1%]; P<.001). Risankizumab exhibited longevity following withdrawal, as median time to loss of response and relapse was 42 weeks (sPGA ≥3). The extent of TEAEs was similar between risankizumab and placebo and included nasopharyngitis, upper respiratory tract infection, headache, and back pain.8
Final Thoughts
Biologics for psoriasis help produce intended results for skin disease clearance and are tools for precision medicine. Recent data demonstrate safe, durable, and continuous efficacy with biologics, which offer patients a better chance of treatment success. This guide may serve as a quick reference for biologic selection with special consideration of individual disease characteristics and comorbidities.
- Mrowietz U, Bachelez H, Burden AD, et al. Efficacy and safety of secukinumab in moderate to severe palmoplantar pustular psoriasis over 148 weeks: extension of the 2PRECISE study. J Am Acad Dermatol. 2021;84:552-554. doi:10.1016/j.jaad.2020.06.038
- Gottlieb AB, Wu JJ, Griffiths CEM, et al. Clinical efficacy and safety of secukinumab in patients with psoriasis and comorbidities: pooled analysis of 4 phase 3 clinical trials [published online October 21, 2020]. J Dermatolog Treat. doi:10.1080/09546634.2020.1832187
- Papp K, Menter A, Leonardi C, et al. Long-term efficacy and safety of brodalumab in psoriasis through 120 weeks and after withdrawal and retreatment: subgroup analysis of a randomized phase III trial (AMAGINE-1). Br J Dermatol. 2020;183:1037-1048. doi:10.1111/bjd.19132
- Blauvelt A, Leonardi C, Elewski B, et al. A head-to-head comparison of ixekizumab vs. guselkumab in patients with moderate-to-severe plaque psoriasis: 24-week efficacy and safety results from a randomized, double-blinded trial. Br J Dermatol. 2021;184:1047-1058. doi:10.1111/bjd.19509
- Lebwohl MG, Leonardi CL, Mehta NN, et al. Tildrakizumab efficacy, drug survival, and safety are comparable in patients with psoriasis with and without metabolic syndrome: long-term results from 2 phase 3 randomized controlled studies (reSURFACE 1 and reSURFACE 2). J Am Acad Dermatol. 2021;84:398-407. doi:10.1016/j.jaad.2020.09.047
- Thaci D, Piaserico S, Warren RB, et al. Five-year efficacy and safety of tildrakizumab in patients with moderate-to-severe psoriasis who respond at week 28: pooled analyses of two randomized phase III clinical trials (reSURFACE 1 and reSURFACE 2)[published online February 5, 2021]. Br J Dermatol. doi:10.1111/bjd.19866
- Warren RB, Blauvelt A, Poulin Y, et al. Efficacy and safety of risankizumab vs. secukinumab in patients with moderate-to-severe plaque psoriasis (IMMerge): results from a phase III, randomized, open-label, efficacy-assessor-blinded clinical trial. Br J Dermatol. 2021;184:50-59. doi:10.1111/bjd.19341
- Blauvelt A, Leonardi CL, Gooderham M, et al. Efficacy and safety of continuous risankizumab therapy vs treatment withdrawal in patients with moderate to severe plaque psoriasis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:649-658. doi:10.1001/jamadermatol.2020.0723
- Mrowietz U, Bachelez H, Burden AD, et al. Efficacy and safety of secukinumab in moderate to severe palmoplantar pustular psoriasis over 148 weeks: extension of the 2PRECISE study. J Am Acad Dermatol. 2021;84:552-554. doi:10.1016/j.jaad.2020.06.038
- Gottlieb AB, Wu JJ, Griffiths CEM, et al. Clinical efficacy and safety of secukinumab in patients with psoriasis and comorbidities: pooled analysis of 4 phase 3 clinical trials [published online October 21, 2020]. J Dermatolog Treat. doi:10.1080/09546634.2020.1832187
- Papp K, Menter A, Leonardi C, et al. Long-term efficacy and safety of brodalumab in psoriasis through 120 weeks and after withdrawal and retreatment: subgroup analysis of a randomized phase III trial (AMAGINE-1). Br J Dermatol. 2020;183:1037-1048. doi:10.1111/bjd.19132
- Blauvelt A, Leonardi C, Elewski B, et al. A head-to-head comparison of ixekizumab vs. guselkumab in patients with moderate-to-severe plaque psoriasis: 24-week efficacy and safety results from a randomized, double-blinded trial. Br J Dermatol. 2021;184:1047-1058. doi:10.1111/bjd.19509
- Lebwohl MG, Leonardi CL, Mehta NN, et al. Tildrakizumab efficacy, drug survival, and safety are comparable in patients with psoriasis with and without metabolic syndrome: long-term results from 2 phase 3 randomized controlled studies (reSURFACE 1 and reSURFACE 2). J Am Acad Dermatol. 2021;84:398-407. doi:10.1016/j.jaad.2020.09.047
- Thaci D, Piaserico S, Warren RB, et al. Five-year efficacy and safety of tildrakizumab in patients with moderate-to-severe psoriasis who respond at week 28: pooled analyses of two randomized phase III clinical trials (reSURFACE 1 and reSURFACE 2)[published online February 5, 2021]. Br J Dermatol. doi:10.1111/bjd.19866
- Warren RB, Blauvelt A, Poulin Y, et al. Efficacy and safety of risankizumab vs. secukinumab in patients with moderate-to-severe plaque psoriasis (IMMerge): results from a phase III, randomized, open-label, efficacy-assessor-blinded clinical trial. Br J Dermatol. 2021;184:50-59. doi:10.1111/bjd.19341
- Blauvelt A, Leonardi CL, Gooderham M, et al. Efficacy and safety of continuous risankizumab therapy vs treatment withdrawal in patients with moderate to severe plaque psoriasis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:649-658. doi:10.1001/jamadermatol.2020.0723
Practice Points
- Choosing a biologic best fit for each patient’s individual health profile can reduce psoriasis disease burden.
- Clinicians should educate psoriasis patients that biologics are safe for most comorbidities, and conditions such as obesity have been associated with poorer therapeutic response.
- It is important to discuss possible side effects of biologics with patients and reassure them that mild side effects are the most common during therapy.
Bone Health in Patients With Prostate Cancer: An Evidence-Based Algorithm
Prostate cancer (PC) is the most commonly and newly diagnosed nonskin cancer and the second leading cause of cancer death in men in the United States. About 191,930 cases and about 33,330 deaths from PC were expected for the year 2020.1 About 1 in 41 men will die of PC. Most men diagnosed with PC are aged > 65 years and do not die of their disease. The 5-year survival rate of localized and regional disease is nearly 100%, and disease with distant metastases is 31%. As a result, more than 3.1 million men in the United States who have been diagnosed with PC are still alive today.1 Among veterans, there is a substantial population living with PC. Skolarus and Hawley reported in 2014 that an estimated 200,000 veterans with PC were survivors and 12,000 were newly diagnosed.2
In PC, skeletal strength can be affected by several factors, such as aging, malnutrition, androgen-deprivation therapy (ADT), and bone metastasis.3,4 In fact, most men can live the rest of their life with PC by using strategies to monitor and treat it, once it shows either radiographic or chemical signs of progression.5 ADT is the standard of care to treat hormone-sensitive PC, which is associated with significant skeletal-related adverse effects (AEs).6,7
Men undergoing ADT are 4 times more likely to develop substantial bone deficiency, Shahinian and colleagues found that in men surviving 5 years after PC diagnosis, 19.4% of those who received ADT had a fracture compared with 12% in men who did not (P < .001). The authors established a significant relation between the number of doses of gonadotropin-releasing hormone given in the first 12 months and the risk of fracture.8 Of those who progressed to metastatic disease, the first metastatic nonnodal site is most commonly to the bone.9 Advanced PC is characterized by increased bone turnover, which further raises concerns for bone health and patient performance.10
Skeletal-related events (SREs) include pathologic fracture, spinal cord compression, palliative radiation, or surgery to bone, and change in antineoplastic therapy secondary to bone pain. The concept of bone health refers to the prevention, diagnosis, and treatment of idiopathic, pathogenic, and treatment-related bone loss and delay or prevention of SREs.6,11 Guidelines and expert groups have recommended screening for osteoporosis at the start of ADT with bone mineral density testing, ensuring adequate calcium and vitamin D intake, modifying lifestyle behaviors (smoking cessation, alcohol moderation, and regular exercise), and prescribing bisphosphonates or receptor-activated nuclear factor κ-B ligand inhibitor, denosumab, for men with osteoporosis or who are at general high-fracture risk.12,13 The overuse of these medications results in undue cost to patients as well as AEs, such as osteonecrosis of the jaw (ONJ), hypocalcemia, and bone/joint pains.14-17 There are evidence-based guidelines for appropriate use of bisphosphonates and denosumab for delay and prevention of SREs in the setting of advanced PC.18 These doses also typically differ in frequency to those of osteoporosis.19 We summarize the evidence and guidance for health care providers who care for patients with PC at various stages and complications from both disease-related and treatment-related comorbidities.
Bone-Strengthening Agents
Overall, there is evidence to support the use of bone-strengthening agents in patients with osteopenia/osteoporosis in the prevention of SREs with significant risk factors for progressive bone demineralization, such as lifestyle factors and, in particular, treatments such as ADT. Bone-remodeling agents for treatment of bony metastasis have been shown to provide therapeutic advantage only in limited instances in the castration-resistant PC (CRPC) setting. Hence, in patients with hormone-sensitive PC due to medication-related AEs, treatment with bone-strengthening agents is indicated only if the patient has a significant preexisting risk for fracture from osteopenia/osteoporosis (Table). The Figure depicts an algorithm for the management of bone health in men with PC who are being treated with ADT.
Denosumab and bisphosphonates have an established role in preventing SREs in metastatic CRPC.20 The choice of denosumab or a bisphosphonate typically varies based on the indication, possible AEs, and cost of therapy. There are multiple studies involving initiation of these agents at various stages of disease to improve both time to progression as well as management of SREs. There is a lack of evidence that bisphosphonates prevent metastatic-bone lesions in castration-sensitive PC; therefore, prophylactic use of this agent is not recommended in patients unless they have significant bone demineralization.21,22
Medication-induced ONJ is a severe AE of both denosumab and bisphosphonate therapies. Data from recent trials showed that higher dosing and prolonged duration of denosumab and bisphosphonate therapies further increased risk of ONJ by 1.8% and 1.3%, respectively.15 Careful history taking and discussions with the patient and if possible their dentist on how to reduce risk are recommended. It is good practice for the patient to complete a dental evaluation prior to starting IV bisphosphonates or denosumab. Dental evaluations should be performed routinely at 3- to 12-month intervals throughout therapy based on individualized risk assessment.23 The benefits of using bisphosphonates to prevent fractures associated with osteoporosis outweigh the risk of ONJ in high-risk populations, but not in all patients with PC. A case-by-case basis and evaluation of risk factors should be performed prior to administering bone-modifying therapy. The long-term safety of IV bisphosphonates has not been adequately studied in controlled trials, and concerns regarding long-term complications, including renal toxicity, ONJ, and atypical femoral fractures, remain with prolonged therapy.24,25
The CALGB 70604 (Alliance) trial compared 3-month dosing to monthly treatment with zoledronic acid (ZA), showing no inferiority to lower frequency dosing.26 A Cochrane review of clinical trials found that in patients with advanced PC, bisphosphonates were found to provide roughly 58 fewer SREs per 1000 on average.27 A phase 3 study showed a modest benefit to denosumab vs ZA in the CRPC group regarding incidence of SREs. The rates of SREs were 289 of 951 patients in the bisphosphonate group, and 241 of 950 patients in the denosumab group (30.4% vs 25.3%; hazard ratio [HR], 0.78; 95% CI, 0.66-0.93; P = .005).28 In 2020, the American Society of Clinical Oncology endorsed the Cancer Care Ontario guidelines for prostate bone health care.18 Adequate supplementation is necessary in all patients treated with a bisphosphonate or denosumab to prevent treatment-related hypocalcemia. Typically, daily supplementation with a minimum of calcium 500 mg and vitamin D 400 IU is recommended.16
Bone Health in Patients
Nonmetastatic Hormone-Sensitive PC
ADT forms the backbone of treatment for patients with local and advanced metastatic castration-sensitive PC along with surgical and focal radiotherapy options. Cancer treatment-induced bone loss is known to occur with prolonged use of ADT. The ZEUS trial found no prevention of bone metastasis in patients with high-risk localized PC with the use of ZA in the absence of bone metastasis. A Kaplan-Meier estimated proportion of bone metastases after a median follow-up of 4.8 years was found to be not statistically significant: 14.7% in the ZA group vs 13.2% in the control/placebo group.29 The STAMPEDE trial showed no significant overall survival (OS) benefit with the addition of ZA to ADT vs ADT alone (HR, 0.94; 95% CI, 0.79-1.11; P = .45), 5-year survival with ADT alone was 55% compared to ADT plus ZA with 57% 5-year survival.30 The RADAR trial showed that at 5 years in high Gleason score patients, use of ZA in the absence of bone metastasis was beneficial, but not in low- or intermediate-risk patients. However, at 10-year analysis there was no significant difference in any of the high-stratified groups with or without ZA.31
The PR04 trial showed no effect on OS with clodronate compared with placebo in nonmetastatic castration-sensitive PC, with a HR of 1.12 (95% CI, 0.89-1.42; P = .94). The estimated 5-year survival was 80% with placebo and 78% with clodronate; 10-year survival rates were 51% with placebo and 48% with clodronate.32 Data from the HALT trial showed an increased bone mineral density and reduced risk of new vertebral fractures vs placebo (1.5% vs 3.9%, respectively) in the absence of metastatic bone lesions and a reduction in new vertebral fractures in patients with nonmetastatic PC.33 Most of these studies showed no benefit with the addition of ZA to nonmetastatic PC; although, the HALT trial provides evidence to support use of denosumab in patients with nonmetastatic PC for preventing vertebral fragility fractures in men receiving ADT.
Metastatic Hormone-Sensitive PC
ZA is often used to treat men with metastatic castration-sensitive PC despite limited efficacy and safety data. The CALGB 90202 (Alliance) trial authors found that the early use of ZA was not associated with increased time to first SRE. The median time to first SRE was 31.9 months in the ZA group (95% CI, 24.2-40.3) and 29.8 months in the placebo group (stratified HR, 0.97; 95% CI, 0-1.17; 1-sided stratified log-rank P = .39).34 OS was similar between the groups (HR, 0.88; 95% CI, 0.70-1.12; P = .29) as were reported AEs.34 Results from these studies suggest limited benefit in treating patients with metastatic hormone-sensitive PC with bisphosphonates without other medical indications for use. Additional studies suggest similar results for treatment with denosumab to that of bisphosphonate therapies.35
Nonmetastatic CRPC
Reasonable interest among treating clinicians exists to be able to delay or prevent the development of metastatic bone disease in patients who are showing biochemical signs of castration resistance but have not yet developed distant metastatic disease. Time to progression on ADT to castration resistance usually occurs 2 to 3 years following initiation of treatment. This typically occurs in patients with rising prostate-specific antigen (PSA). As per the Prostate Cancer Working Group 3, in the absence of radiologic progression, CRPC is defined by a 25% increase from the nadir (considering a starting value of ≥ 1 ng/mL), with a minimum rise of 2 ng/mL in the setting of castrate serum testosterone < 50 ng/dL despite good adherence to an ADT regimen, with proven serologic castration either by undetectable or a near undetectable nadir of serum testosterone concentration. Therapeutic implications include prevention of SREs as well as time to metastatic bone lesions. The Zometa 704 trial examined the use of ZA to reduce time to first metastatic bone lesion in the setting of patients with nonmetastatic CRPC.36 The trial was discontinued prematurely due to low patient accrual, but initial analysis provided information on the natural history of a rising PSA in this patient population. At 2 years, one-third of patients had developed bone metastases. Median bone metastasis-free survival was 30 months. Median time to first bone metastasis and OS were not reached. Baseline PSA and PSA velocity independently predicted a shorter time to first bone metastasis, metastasis-free survival, and OS.36
Denosumab was also studied in the setting of nonmetastatic CRPC in the Denosumab 147 trial. The study enrolled 1432 patients and found a significantly increased bone metastasis-free survival by a median of 4.2 months over placebo (HR, 0.85; 95% CI, 0.73-0.98; P = .03). Denosumab significantly delayed time to first bone metastasis (HR, 0.84; 95% CI, 0.71-0.98; P = .03). OS was similar between groups (HR, 1.01; 95% CI, 0.85-1.20; P = .91). Rates of AEs and serious AEs were similar between groups, except for ONJ and hypocalcemia. The rates of ONJ for denosumab were 1%, 3%, 4% in years 1,2, 3, respectively; overall, < 5% (n = 33). Hypocalcemia occurred in < 2% (n = 12) in denosumab-treated patients. The authors concluded that in men with CRPC, denosumab significantly prolonged bone metastasis–free survival and delayed time-to-bone metastasis.37 These 2 studies suggest a role of receptor-activated nuclear factor κ-B ligand inhibitor denosumab in patients with nonmetastatic CRPC in the appropriate setting. There were delays in bony metastatic disease, but no difference in OS. Rare denosumab treatment–related specific AEs were noted. Hence, denosumab is not recommended for use in this setting.
Metastatic CRPC
Castration resistance typically occurs 2 to 3 years following initiation of ADT and the most common extranodal site of disease is within the bone in metastatic PC. Disease progression within bones after ADT can be challenging given both the nature of progressive cancer with osteoblastic metastatic lesions and the prolonged effects of ADT on unaffected bone. The Zometa 039 study compared ZA with placebo and found a significant difference in SREs (38% and 49%, respectively; P .03). No survival benefit was observed with the addition of ZA. Use of other bisphosphonates pamidronate and clodronate did not have a similar degree of benefit.38,39
A phase 3 study of 1904 patients found that denosumab was superior to ZA in delaying the time to first on-study SRE (HR, 0.82; 95% CI, 0.71-0.95) and reducing rates of multiple SREs (HR, 0.82; 95% CI, 0.71-0.94).40 This was later confirmed with an additional study that demonstrated treatment with denosumab significantly reduced the risk of developing a first symptomatic SRE, defined as a pathologic fracture, spinal cord compression, necessity for radiation, or surgery (HR, 0.78; 95% CI, 0.66-0.93; P = .005) and first and subsequent symptomatic SREs (rate ratio, 0.78; 95% CI, 0.65-0.92; P = .004) compared with ZA.28 These findings suggest a continued role of denosumab in the treatment of advanced metastatic CRPC from both control of bone disease as well as quality of life and palliation of cancer-related symptoms.
Radium-223 dichloride (radium-223) is an α-emitting radionuclide for treatment of metastatic CRPC with bone metastasis, but otherwise no additional metastatic sites. Radium-223 is a calcium-mimetic that preferentially accumulates into areas of high-bone turnover, such as where bone metastases tend to occur. Radium-223 induces apoptosis of tumor cells through double-stranded DNA breaks. Studies have shown radium-223 to prolong OS and time-to-first symptomatic SRE.41 The ERA-223 trial showed that when radium-223 was combined with abiraterone acetate, there was an increase in fragility fracture risk compared with placebo combined with abiraterone. Data from the study revealed that the median symptomatic SRE-free survival was 22.3 months (95% CI, 20.4-24.8) in the radium-223 group and 26.0 months (21.8-28.3) in the placebo group. Concurrent treatment with abiraterone acetate plus prednisone or prednisolone and radium-223 was associated with increased fracture risk. Osteoporotic fractures were the most common type of fracture in the radium-223 group and of all fracture types, differed the most between the study groups.42
Conclusions
Convincing evidence supports the ongoing use of bisphosphonates and denosumab in patients with osteoporosis, significant osteopenia with risk factors, and in patients with CRPC with bone metastasis. Bone metastases can cause considerable morbidity and mortality among men with advanced PC. Pain, fracture, and neurologic injury can occur with metastatic bone lesions as well as with ADT-related bone loss. Prevention of SREs in patients with PC is a reasonable goal in PC survivors while being mindful of managing the risks of these therapies.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7-30. doi:10.3322/caac.21590
2. Skolarus TA, Hawley ST. Prostate cancer survivorship care in the Veterans Health Administration. Fed Pract. 2014;31(8):10-17.
3. Gartrell BA, Coleman R, Efstathiou E, et al. Metastatic prostate cancer and the bone: significance and therapeutic options. Eur Urol. 2015;68(5):850-858. doi:10.1016/j.eururo.2015.06.039
4. Bolla M, de Reijke TM, Van Tienhoven G, et al. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med. 2009;360(24):2516-2527. doi:10.1056/NEJMoa0810095
5. Welch HG, Albertsen PC. Reconsidering Prostate cancer mortality—The future of PSA screening. N Engl J Med. 2020;382(16):1557-1563. doi:10.1056/NEJMms1914228
6. Coleman R, Body JJ, Aapro M, Hadji P, Herrstedt J; ESMO Guidelines Working Group. Bone health in cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol. 2014;25 (suppl 3):iii124-137. doi:10.1093/annonc/mdu103
7. Saylor PJ, Smith MR. Adverse effects of androgen deprivation therapy: defining the problem and promoting health among men with prostate cancer. J Natl Compr Canc Netw. 2010;8(2):211-223. doi:10.6004/jnccn.2010.0014
8. Shahinian VB, Kuo Y-F, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352(2):154-164. doi:10.1056/NEJMoa041943
9. Sartor O, de Bono JS. Metastatic prostate cancer. N Engl J Med. 2018;378(7):645-657. doi:10.1056/NEJMra1701695
10. Saad F, Eastham JA, Smith MR. Biochemical markers of bone turnover and clinical outcomes in men with prostate cancer. Urol Oncol. 2012;30(4):369-378. doi:10.1016/j.urolonc.2010.08.007
11. Cosman F, de Beur SJ, LeBoff MS, et al; National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359-2381. doi:10.1007/s00198-014-2794-2
12. Alibhai SMH, Zukotynski K, Walker-Dilks C, et al; Cancer Care Ontario Genitourinary Cancer Disease Site Group. Bone health and bone-targeted therapies for prostate cancer: a programme in evidence-based care - Cancer Care Ontario Clinical Practice Guideline. Clin Oncol (R Coll Radiol). 2017;29(6):348-355. doi:10.1016/j.clon.2017.01.007
13. LEE CE. A comprehensive bone-health management approach with men with prostate cancer recieving androgen deprivation therapy. Curr Oncol. 2011;18(4):e163-172. doi:10.3747/co.v18i4.746
14. Kennel KA, Drake MT. Adverse effects of bisphosphonates: Implications for osteoporosis management. Mayo Clin Proc. 2009;84(7):632-638. doi:10.1016/S0025-6196(11)60752-0
15. Saad F, Brown JE, Van Poznak C, et al. Incidence, risk factors, and outcomes of osteonecrosis of the jaw: integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastases. Ann Oncol. 2012;23(5):1341-1347. doi:10.1093/annonc/mdr435
16. Body J-J, Bone HG, de Boer RH, et al. Hypocalcaemia in patients with metastatic bone disease treated with denosumab. Eur J Cancer. 2015;51(13):1812-1821. doi:10.1016/j.ejca.2015.05.016
17. Wysowski DK, Chang JT. Alendronate and risedronate: reports of severe bone, joint, and muscle pain. Arch Intern Med. 2005;165(3):346-347. doi:10.1001/archinte.165.3.346-b
18. Saylor PJ, Rumble RB, Tagawa S, et al. Bone health and bone-targeted therapies for prostate cancer: ASCO endorsement of a cancer care Ontario guideline. J Clin Oncol. 2020;38(15):1736-1743. doi:10.1200/JCO.19.03148
19. Saad F, Gleason DM, Murray R, et al; Zoledronic Acid Prostate Cancer Study Group. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst. 2004;96(11):879-882. doi:10.1093/jnci/djh141
20. Saad F, Gleason DM, Murray R, et al; Zoledronic Acid Prostate Cancer Study Group. A randomized, placebo-controlled trial of zoledronic zcid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94(19):1458-1468. doi:10.1093/jnci/94.19.1458
21. Aapro M, Saad F. Bone-modifying agents in the treatment of bone metastases in patients with advanced genitourinary malignancies: a focus on zoledronic acid. Ther Adv Urol. 2012;4(2):85-101. doi:10.1177/1756287212441234
22. Cianferotti L, Bertoldo F, Carini M, et al. The prevention of fragility fractures in patients with non-metastatic prostate cancer: a position statement by the international osteoporosis foundation. Oncotarget. 2017;8(43):75646-75663. doi:10.18632/oncotarget.17980
23. Ruggiero S, Gralow J, Marx RE, et al. Practical guidelines for the prevention, diagnosis, and treatment of osteonecrosis of the jaw in patients with cancer. J Oncol Pract. 2006;2(1):7-14. doi:10.1200/JOP.2006.2.1.7
24. Corraini P, Heide-Jørgensen U, Schøodt M, et al. Osteonecrosis of the jaw and survival of patients with cancer: a nationwide cohort study in Denmark. Cancer Med. 2017;6(10):2271-2277. doi:10.1002/cam4.1173
25. Watts NB, Diab DL. Long-term use of bisphosphonates in osteoporosis. J Clin Endocrinol Metab. 2010;95(4):1555-1565. doi:10.1210/jc.2009-1947
26. Himelstein AL, Foster JC, Khatcheressian JL, et al. Effect of longer interval vs standard dosing of zoledronic acid on skeletal events in patients with bone metastases: a randomized clinical trial. JAMA. 2017;317(1):48-58. doi:10.1001/jama.2016.19425
27. Macherey S, Monsef I, Jahn F, et al. Bisphosphonates for advanced prostate cancer. Cochrane Database Syst Rev. 2017;12(12):CD006250. doi:10.1002/14651858.CD006250.pub2
28. Smith MR, Coleman RE, Klotz L, et al. Denosumab for the prevention of skeletal complications in metastatic castration-resistant prostate cancer: comparison of skeletal-related events and symptomatic skeletal events. Ann Oncol. 2015;26(2):368-374. doi:10.1093/annonc/mdu519
29. Wirth M, Tammela T, Cicalese V, et al. Prevention of bone metastases in patients with high-risk nonmetastatic prostate cancer treated with zoledronic acid: efficacy and safety results of the Zometa European Study (ZEUS). Eur Urol. 2015;67(3):482-491. doi:10.1016/j.eururo.2014.02.014
30. James ND, Sydes MR, Clarke NW, et al; STAMPEDE Investigators. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387(10024):1163-1177. doi:10.1016/S0140-6736(15)01037-5
31. Denham JW, Joseph D, Lamb DS, et al. Short-term androgen suppression and radiotherapy versus intermediate-term androgen suppression and radiotherapy, with or without zoledronic acid, in men with locally advanced prostate cancer (TROG 03.04 RADAR): 10-year results from a randomised, phase 3, factorial trial. Lancet Oncol. 2019;20(2):267-281. doi:10.1016/S1470-2045(18)30757-5
32. Dearnaley DP, Mason MD, Parmar MK, Sanders K, Sydes MR. Adjuvant therapy with oral sodium clodronate in locally advanced and metastatic prostate cancer: long-term overall survival results from the MRC PR04 and PR05 randomised controlled trials. Lancet Oncol. 2009;10(9):872-876. doi:10.1016/S1470-2045(09)70201-3
33. Smith MR, Egerdie B, Toriz NH, et al; Denosumab HALT Prostate Cancer Study Group. Denosumab in men receiving androgen-deprivation therapy for prostate Cancer. N Engl J Med. 2009;361(8):745-755. doi:10.1056/NEJMoa0809003
34. Smith MR, Halabi S, Ryan CJ, et al. Randomized controlled trial of early zoledronic acid in men with castration-sensitive prostate cancer and bone metastases: results of CALGB 90202 (alliance). J Clin Oncol. 2014;32(11):1143-1150. doi:10.1200/JCO.2013.51.6500
35. Kozyrakis D, Paridis D, Perikleous S, Malizos K, Zarkadas A, Tsagkalis A. The current role of osteoclast inhibitors in patients with prostate cancer. Adv Urol. 2018;2018:1525832. doi:10.1155/2018/1525832
36. Smith MR, Kabbinavar F, Saad F, et al. Natural history of rising serum prostate-specific antigen in men with castrate nonmetastatic prostate cancer. J Clin Oncol. 2005;23(13):2918-2925. doi:10.1200/JCO.2005.01.529
37. Smith MR, Saad F, Coleman R, et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet. 2012;379(9810):39-46. doi:10.1016/S0140-6736(11)61226-9
38. Small EJ, Smith MR, Seaman JJ, Petrone S, Kowalski MO. Combined analysis of two multicenter, randomized, placebo-controlled studies of pamidronate disodium for the palliation of bone pain in men with metastatic prostate cancer. J Clin Oncol. 2003;21(23):4277-4284. doi:10.1200/JCO.2003.05.147
39. Ernst DS, Tannock IF, Winquist EW, et al. Randomized, double-blind, controlled trial of mitoxantrone/prednisone and clodronate versus mitoxantrone/prednisone and placebo in patients with hormone-refractory prostate cancer and pain. J Clin Oncol. 2003;21(17):3335-3342. doi:10.1200/JCO.2003.03.042
40. Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377(9768):813-822. doi:10.1016/S0140-6736(10)62344-6
41. Parker C, Nilsson S, Heinrich D, et al; ALSYMPCA Investigators Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213-223. doi:10.1056/NEJMoa1213755
42. Smith M, Parker C, Saad F, et al. Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(3):408-419. doi:10.1016/S1470-2045(18)30860-X
43. Smith MR, Saad F, Shore ND, et al. Effect of denosumab on prolonging bone-metastasis-free survival (BMFS) in men with nonmetastatic castrate-resistant prostate cancer (CRPC) presenting with aggressive PSA kinetics. J Clin Oncol. 2012;30(5_suppl):6-6.
44. Saad F, Gleason DM, Murray R, et al; Zoledronic Acid Prostate Cancer Study Group. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94(19):1458-1468. doi:10.1093/jnci/94.19.1458
Prostate cancer (PC) is the most commonly and newly diagnosed nonskin cancer and the second leading cause of cancer death in men in the United States. About 191,930 cases and about 33,330 deaths from PC were expected for the year 2020.1 About 1 in 41 men will die of PC. Most men diagnosed with PC are aged > 65 years and do not die of their disease. The 5-year survival rate of localized and regional disease is nearly 100%, and disease with distant metastases is 31%. As a result, more than 3.1 million men in the United States who have been diagnosed with PC are still alive today.1 Among veterans, there is a substantial population living with PC. Skolarus and Hawley reported in 2014 that an estimated 200,000 veterans with PC were survivors and 12,000 were newly diagnosed.2
In PC, skeletal strength can be affected by several factors, such as aging, malnutrition, androgen-deprivation therapy (ADT), and bone metastasis.3,4 In fact, most men can live the rest of their life with PC by using strategies to monitor and treat it, once it shows either radiographic or chemical signs of progression.5 ADT is the standard of care to treat hormone-sensitive PC, which is associated with significant skeletal-related adverse effects (AEs).6,7
Men undergoing ADT are 4 times more likely to develop substantial bone deficiency, Shahinian and colleagues found that in men surviving 5 years after PC diagnosis, 19.4% of those who received ADT had a fracture compared with 12% in men who did not (P < .001). The authors established a significant relation between the number of doses of gonadotropin-releasing hormone given in the first 12 months and the risk of fracture.8 Of those who progressed to metastatic disease, the first metastatic nonnodal site is most commonly to the bone.9 Advanced PC is characterized by increased bone turnover, which further raises concerns for bone health and patient performance.10
Skeletal-related events (SREs) include pathologic fracture, spinal cord compression, palliative radiation, or surgery to bone, and change in antineoplastic therapy secondary to bone pain. The concept of bone health refers to the prevention, diagnosis, and treatment of idiopathic, pathogenic, and treatment-related bone loss and delay or prevention of SREs.6,11 Guidelines and expert groups have recommended screening for osteoporosis at the start of ADT with bone mineral density testing, ensuring adequate calcium and vitamin D intake, modifying lifestyle behaviors (smoking cessation, alcohol moderation, and regular exercise), and prescribing bisphosphonates or receptor-activated nuclear factor κ-B ligand inhibitor, denosumab, for men with osteoporosis or who are at general high-fracture risk.12,13 The overuse of these medications results in undue cost to patients as well as AEs, such as osteonecrosis of the jaw (ONJ), hypocalcemia, and bone/joint pains.14-17 There are evidence-based guidelines for appropriate use of bisphosphonates and denosumab for delay and prevention of SREs in the setting of advanced PC.18 These doses also typically differ in frequency to those of osteoporosis.19 We summarize the evidence and guidance for health care providers who care for patients with PC at various stages and complications from both disease-related and treatment-related comorbidities.
Bone-Strengthening Agents
Overall, there is evidence to support the use of bone-strengthening agents in patients with osteopenia/osteoporosis in the prevention of SREs with significant risk factors for progressive bone demineralization, such as lifestyle factors and, in particular, treatments such as ADT. Bone-remodeling agents for treatment of bony metastasis have been shown to provide therapeutic advantage only in limited instances in the castration-resistant PC (CRPC) setting. Hence, in patients with hormone-sensitive PC due to medication-related AEs, treatment with bone-strengthening agents is indicated only if the patient has a significant preexisting risk for fracture from osteopenia/osteoporosis (Table). The Figure depicts an algorithm for the management of bone health in men with PC who are being treated with ADT.
Denosumab and bisphosphonates have an established role in preventing SREs in metastatic CRPC.20 The choice of denosumab or a bisphosphonate typically varies based on the indication, possible AEs, and cost of therapy. There are multiple studies involving initiation of these agents at various stages of disease to improve both time to progression as well as management of SREs. There is a lack of evidence that bisphosphonates prevent metastatic-bone lesions in castration-sensitive PC; therefore, prophylactic use of this agent is not recommended in patients unless they have significant bone demineralization.21,22
Medication-induced ONJ is a severe AE of both denosumab and bisphosphonate therapies. Data from recent trials showed that higher dosing and prolonged duration of denosumab and bisphosphonate therapies further increased risk of ONJ by 1.8% and 1.3%, respectively.15 Careful history taking and discussions with the patient and if possible their dentist on how to reduce risk are recommended. It is good practice for the patient to complete a dental evaluation prior to starting IV bisphosphonates or denosumab. Dental evaluations should be performed routinely at 3- to 12-month intervals throughout therapy based on individualized risk assessment.23 The benefits of using bisphosphonates to prevent fractures associated with osteoporosis outweigh the risk of ONJ in high-risk populations, but not in all patients with PC. A case-by-case basis and evaluation of risk factors should be performed prior to administering bone-modifying therapy. The long-term safety of IV bisphosphonates has not been adequately studied in controlled trials, and concerns regarding long-term complications, including renal toxicity, ONJ, and atypical femoral fractures, remain with prolonged therapy.24,25
The CALGB 70604 (Alliance) trial compared 3-month dosing to monthly treatment with zoledronic acid (ZA), showing no inferiority to lower frequency dosing.26 A Cochrane review of clinical trials found that in patients with advanced PC, bisphosphonates were found to provide roughly 58 fewer SREs per 1000 on average.27 A phase 3 study showed a modest benefit to denosumab vs ZA in the CRPC group regarding incidence of SREs. The rates of SREs were 289 of 951 patients in the bisphosphonate group, and 241 of 950 patients in the denosumab group (30.4% vs 25.3%; hazard ratio [HR], 0.78; 95% CI, 0.66-0.93; P = .005).28 In 2020, the American Society of Clinical Oncology endorsed the Cancer Care Ontario guidelines for prostate bone health care.18 Adequate supplementation is necessary in all patients treated with a bisphosphonate or denosumab to prevent treatment-related hypocalcemia. Typically, daily supplementation with a minimum of calcium 500 mg and vitamin D 400 IU is recommended.16
Bone Health in Patients
Nonmetastatic Hormone-Sensitive PC
ADT forms the backbone of treatment for patients with local and advanced metastatic castration-sensitive PC along with surgical and focal radiotherapy options. Cancer treatment-induced bone loss is known to occur with prolonged use of ADT. The ZEUS trial found no prevention of bone metastasis in patients with high-risk localized PC with the use of ZA in the absence of bone metastasis. A Kaplan-Meier estimated proportion of bone metastases after a median follow-up of 4.8 years was found to be not statistically significant: 14.7% in the ZA group vs 13.2% in the control/placebo group.29 The STAMPEDE trial showed no significant overall survival (OS) benefit with the addition of ZA to ADT vs ADT alone (HR, 0.94; 95% CI, 0.79-1.11; P = .45), 5-year survival with ADT alone was 55% compared to ADT plus ZA with 57% 5-year survival.30 The RADAR trial showed that at 5 years in high Gleason score patients, use of ZA in the absence of bone metastasis was beneficial, but not in low- or intermediate-risk patients. However, at 10-year analysis there was no significant difference in any of the high-stratified groups with or without ZA.31
The PR04 trial showed no effect on OS with clodronate compared with placebo in nonmetastatic castration-sensitive PC, with a HR of 1.12 (95% CI, 0.89-1.42; P = .94). The estimated 5-year survival was 80% with placebo and 78% with clodronate; 10-year survival rates were 51% with placebo and 48% with clodronate.32 Data from the HALT trial showed an increased bone mineral density and reduced risk of new vertebral fractures vs placebo (1.5% vs 3.9%, respectively) in the absence of metastatic bone lesions and a reduction in new vertebral fractures in patients with nonmetastatic PC.33 Most of these studies showed no benefit with the addition of ZA to nonmetastatic PC; although, the HALT trial provides evidence to support use of denosumab in patients with nonmetastatic PC for preventing vertebral fragility fractures in men receiving ADT.
Metastatic Hormone-Sensitive PC
ZA is often used to treat men with metastatic castration-sensitive PC despite limited efficacy and safety data. The CALGB 90202 (Alliance) trial authors found that the early use of ZA was not associated with increased time to first SRE. The median time to first SRE was 31.9 months in the ZA group (95% CI, 24.2-40.3) and 29.8 months in the placebo group (stratified HR, 0.97; 95% CI, 0-1.17; 1-sided stratified log-rank P = .39).34 OS was similar between the groups (HR, 0.88; 95% CI, 0.70-1.12; P = .29) as were reported AEs.34 Results from these studies suggest limited benefit in treating patients with metastatic hormone-sensitive PC with bisphosphonates without other medical indications for use. Additional studies suggest similar results for treatment with denosumab to that of bisphosphonate therapies.35
Nonmetastatic CRPC
Reasonable interest among treating clinicians exists to be able to delay or prevent the development of metastatic bone disease in patients who are showing biochemical signs of castration resistance but have not yet developed distant metastatic disease. Time to progression on ADT to castration resistance usually occurs 2 to 3 years following initiation of treatment. This typically occurs in patients with rising prostate-specific antigen (PSA). As per the Prostate Cancer Working Group 3, in the absence of radiologic progression, CRPC is defined by a 25% increase from the nadir (considering a starting value of ≥ 1 ng/mL), with a minimum rise of 2 ng/mL in the setting of castrate serum testosterone < 50 ng/dL despite good adherence to an ADT regimen, with proven serologic castration either by undetectable or a near undetectable nadir of serum testosterone concentration. Therapeutic implications include prevention of SREs as well as time to metastatic bone lesions. The Zometa 704 trial examined the use of ZA to reduce time to first metastatic bone lesion in the setting of patients with nonmetastatic CRPC.36 The trial was discontinued prematurely due to low patient accrual, but initial analysis provided information on the natural history of a rising PSA in this patient population. At 2 years, one-third of patients had developed bone metastases. Median bone metastasis-free survival was 30 months. Median time to first bone metastasis and OS were not reached. Baseline PSA and PSA velocity independently predicted a shorter time to first bone metastasis, metastasis-free survival, and OS.36
Denosumab was also studied in the setting of nonmetastatic CRPC in the Denosumab 147 trial. The study enrolled 1432 patients and found a significantly increased bone metastasis-free survival by a median of 4.2 months over placebo (HR, 0.85; 95% CI, 0.73-0.98; P = .03). Denosumab significantly delayed time to first bone metastasis (HR, 0.84; 95% CI, 0.71-0.98; P = .03). OS was similar between groups (HR, 1.01; 95% CI, 0.85-1.20; P = .91). Rates of AEs and serious AEs were similar between groups, except for ONJ and hypocalcemia. The rates of ONJ for denosumab were 1%, 3%, 4% in years 1,2, 3, respectively; overall, < 5% (n = 33). Hypocalcemia occurred in < 2% (n = 12) in denosumab-treated patients. The authors concluded that in men with CRPC, denosumab significantly prolonged bone metastasis–free survival and delayed time-to-bone metastasis.37 These 2 studies suggest a role of receptor-activated nuclear factor κ-B ligand inhibitor denosumab in patients with nonmetastatic CRPC in the appropriate setting. There were delays in bony metastatic disease, but no difference in OS. Rare denosumab treatment–related specific AEs were noted. Hence, denosumab is not recommended for use in this setting.
Metastatic CRPC
Castration resistance typically occurs 2 to 3 years following initiation of ADT and the most common extranodal site of disease is within the bone in metastatic PC. Disease progression within bones after ADT can be challenging given both the nature of progressive cancer with osteoblastic metastatic lesions and the prolonged effects of ADT on unaffected bone. The Zometa 039 study compared ZA with placebo and found a significant difference in SREs (38% and 49%, respectively; P .03). No survival benefit was observed with the addition of ZA. Use of other bisphosphonates pamidronate and clodronate did not have a similar degree of benefit.38,39
A phase 3 study of 1904 patients found that denosumab was superior to ZA in delaying the time to first on-study SRE (HR, 0.82; 95% CI, 0.71-0.95) and reducing rates of multiple SREs (HR, 0.82; 95% CI, 0.71-0.94).40 This was later confirmed with an additional study that demonstrated treatment with denosumab significantly reduced the risk of developing a first symptomatic SRE, defined as a pathologic fracture, spinal cord compression, necessity for radiation, or surgery (HR, 0.78; 95% CI, 0.66-0.93; P = .005) and first and subsequent symptomatic SREs (rate ratio, 0.78; 95% CI, 0.65-0.92; P = .004) compared with ZA.28 These findings suggest a continued role of denosumab in the treatment of advanced metastatic CRPC from both control of bone disease as well as quality of life and palliation of cancer-related symptoms.
Radium-223 dichloride (radium-223) is an α-emitting radionuclide for treatment of metastatic CRPC with bone metastasis, but otherwise no additional metastatic sites. Radium-223 is a calcium-mimetic that preferentially accumulates into areas of high-bone turnover, such as where bone metastases tend to occur. Radium-223 induces apoptosis of tumor cells through double-stranded DNA breaks. Studies have shown radium-223 to prolong OS and time-to-first symptomatic SRE.41 The ERA-223 trial showed that when radium-223 was combined with abiraterone acetate, there was an increase in fragility fracture risk compared with placebo combined with abiraterone. Data from the study revealed that the median symptomatic SRE-free survival was 22.3 months (95% CI, 20.4-24.8) in the radium-223 group and 26.0 months (21.8-28.3) in the placebo group. Concurrent treatment with abiraterone acetate plus prednisone or prednisolone and radium-223 was associated with increased fracture risk. Osteoporotic fractures were the most common type of fracture in the radium-223 group and of all fracture types, differed the most between the study groups.42
Conclusions
Convincing evidence supports the ongoing use of bisphosphonates and denosumab in patients with osteoporosis, significant osteopenia with risk factors, and in patients with CRPC with bone metastasis. Bone metastases can cause considerable morbidity and mortality among men with advanced PC. Pain, fracture, and neurologic injury can occur with metastatic bone lesions as well as with ADT-related bone loss. Prevention of SREs in patients with PC is a reasonable goal in PC survivors while being mindful of managing the risks of these therapies.
Prostate cancer (PC) is the most commonly and newly diagnosed nonskin cancer and the second leading cause of cancer death in men in the United States. About 191,930 cases and about 33,330 deaths from PC were expected for the year 2020.1 About 1 in 41 men will die of PC. Most men diagnosed with PC are aged > 65 years and do not die of their disease. The 5-year survival rate of localized and regional disease is nearly 100%, and disease with distant metastases is 31%. As a result, more than 3.1 million men in the United States who have been diagnosed with PC are still alive today.1 Among veterans, there is a substantial population living with PC. Skolarus and Hawley reported in 2014 that an estimated 200,000 veterans with PC were survivors and 12,000 were newly diagnosed.2
In PC, skeletal strength can be affected by several factors, such as aging, malnutrition, androgen-deprivation therapy (ADT), and bone metastasis.3,4 In fact, most men can live the rest of their life with PC by using strategies to monitor and treat it, once it shows either radiographic or chemical signs of progression.5 ADT is the standard of care to treat hormone-sensitive PC, which is associated with significant skeletal-related adverse effects (AEs).6,7
Men undergoing ADT are 4 times more likely to develop substantial bone deficiency, Shahinian and colleagues found that in men surviving 5 years after PC diagnosis, 19.4% of those who received ADT had a fracture compared with 12% in men who did not (P < .001). The authors established a significant relation between the number of doses of gonadotropin-releasing hormone given in the first 12 months and the risk of fracture.8 Of those who progressed to metastatic disease, the first metastatic nonnodal site is most commonly to the bone.9 Advanced PC is characterized by increased bone turnover, which further raises concerns for bone health and patient performance.10
Skeletal-related events (SREs) include pathologic fracture, spinal cord compression, palliative radiation, or surgery to bone, and change in antineoplastic therapy secondary to bone pain. The concept of bone health refers to the prevention, diagnosis, and treatment of idiopathic, pathogenic, and treatment-related bone loss and delay or prevention of SREs.6,11 Guidelines and expert groups have recommended screening for osteoporosis at the start of ADT with bone mineral density testing, ensuring adequate calcium and vitamin D intake, modifying lifestyle behaviors (smoking cessation, alcohol moderation, and regular exercise), and prescribing bisphosphonates or receptor-activated nuclear factor κ-B ligand inhibitor, denosumab, for men with osteoporosis or who are at general high-fracture risk.12,13 The overuse of these medications results in undue cost to patients as well as AEs, such as osteonecrosis of the jaw (ONJ), hypocalcemia, and bone/joint pains.14-17 There are evidence-based guidelines for appropriate use of bisphosphonates and denosumab for delay and prevention of SREs in the setting of advanced PC.18 These doses also typically differ in frequency to those of osteoporosis.19 We summarize the evidence and guidance for health care providers who care for patients with PC at various stages and complications from both disease-related and treatment-related comorbidities.
Bone-Strengthening Agents
Overall, there is evidence to support the use of bone-strengthening agents in patients with osteopenia/osteoporosis in the prevention of SREs with significant risk factors for progressive bone demineralization, such as lifestyle factors and, in particular, treatments such as ADT. Bone-remodeling agents for treatment of bony metastasis have been shown to provide therapeutic advantage only in limited instances in the castration-resistant PC (CRPC) setting. Hence, in patients with hormone-sensitive PC due to medication-related AEs, treatment with bone-strengthening agents is indicated only if the patient has a significant preexisting risk for fracture from osteopenia/osteoporosis (Table). The Figure depicts an algorithm for the management of bone health in men with PC who are being treated with ADT.
Denosumab and bisphosphonates have an established role in preventing SREs in metastatic CRPC.20 The choice of denosumab or a bisphosphonate typically varies based on the indication, possible AEs, and cost of therapy. There are multiple studies involving initiation of these agents at various stages of disease to improve both time to progression as well as management of SREs. There is a lack of evidence that bisphosphonates prevent metastatic-bone lesions in castration-sensitive PC; therefore, prophylactic use of this agent is not recommended in patients unless they have significant bone demineralization.21,22
Medication-induced ONJ is a severe AE of both denosumab and bisphosphonate therapies. Data from recent trials showed that higher dosing and prolonged duration of denosumab and bisphosphonate therapies further increased risk of ONJ by 1.8% and 1.3%, respectively.15 Careful history taking and discussions with the patient and if possible their dentist on how to reduce risk are recommended. It is good practice for the patient to complete a dental evaluation prior to starting IV bisphosphonates or denosumab. Dental evaluations should be performed routinely at 3- to 12-month intervals throughout therapy based on individualized risk assessment.23 The benefits of using bisphosphonates to prevent fractures associated with osteoporosis outweigh the risk of ONJ in high-risk populations, but not in all patients with PC. A case-by-case basis and evaluation of risk factors should be performed prior to administering bone-modifying therapy. The long-term safety of IV bisphosphonates has not been adequately studied in controlled trials, and concerns regarding long-term complications, including renal toxicity, ONJ, and atypical femoral fractures, remain with prolonged therapy.24,25
The CALGB 70604 (Alliance) trial compared 3-month dosing to monthly treatment with zoledronic acid (ZA), showing no inferiority to lower frequency dosing.26 A Cochrane review of clinical trials found that in patients with advanced PC, bisphosphonates were found to provide roughly 58 fewer SREs per 1000 on average.27 A phase 3 study showed a modest benefit to denosumab vs ZA in the CRPC group regarding incidence of SREs. The rates of SREs were 289 of 951 patients in the bisphosphonate group, and 241 of 950 patients in the denosumab group (30.4% vs 25.3%; hazard ratio [HR], 0.78; 95% CI, 0.66-0.93; P = .005).28 In 2020, the American Society of Clinical Oncology endorsed the Cancer Care Ontario guidelines for prostate bone health care.18 Adequate supplementation is necessary in all patients treated with a bisphosphonate or denosumab to prevent treatment-related hypocalcemia. Typically, daily supplementation with a minimum of calcium 500 mg and vitamin D 400 IU is recommended.16
Bone Health in Patients
Nonmetastatic Hormone-Sensitive PC
ADT forms the backbone of treatment for patients with local and advanced metastatic castration-sensitive PC along with surgical and focal radiotherapy options. Cancer treatment-induced bone loss is known to occur with prolonged use of ADT. The ZEUS trial found no prevention of bone metastasis in patients with high-risk localized PC with the use of ZA in the absence of bone metastasis. A Kaplan-Meier estimated proportion of bone metastases after a median follow-up of 4.8 years was found to be not statistically significant: 14.7% in the ZA group vs 13.2% in the control/placebo group.29 The STAMPEDE trial showed no significant overall survival (OS) benefit with the addition of ZA to ADT vs ADT alone (HR, 0.94; 95% CI, 0.79-1.11; P = .45), 5-year survival with ADT alone was 55% compared to ADT plus ZA with 57% 5-year survival.30 The RADAR trial showed that at 5 years in high Gleason score patients, use of ZA in the absence of bone metastasis was beneficial, but not in low- or intermediate-risk patients. However, at 10-year analysis there was no significant difference in any of the high-stratified groups with or without ZA.31
The PR04 trial showed no effect on OS with clodronate compared with placebo in nonmetastatic castration-sensitive PC, with a HR of 1.12 (95% CI, 0.89-1.42; P = .94). The estimated 5-year survival was 80% with placebo and 78% with clodronate; 10-year survival rates were 51% with placebo and 48% with clodronate.32 Data from the HALT trial showed an increased bone mineral density and reduced risk of new vertebral fractures vs placebo (1.5% vs 3.9%, respectively) in the absence of metastatic bone lesions and a reduction in new vertebral fractures in patients with nonmetastatic PC.33 Most of these studies showed no benefit with the addition of ZA to nonmetastatic PC; although, the HALT trial provides evidence to support use of denosumab in patients with nonmetastatic PC for preventing vertebral fragility fractures in men receiving ADT.
Metastatic Hormone-Sensitive PC
ZA is often used to treat men with metastatic castration-sensitive PC despite limited efficacy and safety data. The CALGB 90202 (Alliance) trial authors found that the early use of ZA was not associated with increased time to first SRE. The median time to first SRE was 31.9 months in the ZA group (95% CI, 24.2-40.3) and 29.8 months in the placebo group (stratified HR, 0.97; 95% CI, 0-1.17; 1-sided stratified log-rank P = .39).34 OS was similar between the groups (HR, 0.88; 95% CI, 0.70-1.12; P = .29) as were reported AEs.34 Results from these studies suggest limited benefit in treating patients with metastatic hormone-sensitive PC with bisphosphonates without other medical indications for use. Additional studies suggest similar results for treatment with denosumab to that of bisphosphonate therapies.35
Nonmetastatic CRPC
Reasonable interest among treating clinicians exists to be able to delay or prevent the development of metastatic bone disease in patients who are showing biochemical signs of castration resistance but have not yet developed distant metastatic disease. Time to progression on ADT to castration resistance usually occurs 2 to 3 years following initiation of treatment. This typically occurs in patients with rising prostate-specific antigen (PSA). As per the Prostate Cancer Working Group 3, in the absence of radiologic progression, CRPC is defined by a 25% increase from the nadir (considering a starting value of ≥ 1 ng/mL), with a minimum rise of 2 ng/mL in the setting of castrate serum testosterone < 50 ng/dL despite good adherence to an ADT regimen, with proven serologic castration either by undetectable or a near undetectable nadir of serum testosterone concentration. Therapeutic implications include prevention of SREs as well as time to metastatic bone lesions. The Zometa 704 trial examined the use of ZA to reduce time to first metastatic bone lesion in the setting of patients with nonmetastatic CRPC.36 The trial was discontinued prematurely due to low patient accrual, but initial analysis provided information on the natural history of a rising PSA in this patient population. At 2 years, one-third of patients had developed bone metastases. Median bone metastasis-free survival was 30 months. Median time to first bone metastasis and OS were not reached. Baseline PSA and PSA velocity independently predicted a shorter time to first bone metastasis, metastasis-free survival, and OS.36
Denosumab was also studied in the setting of nonmetastatic CRPC in the Denosumab 147 trial. The study enrolled 1432 patients and found a significantly increased bone metastasis-free survival by a median of 4.2 months over placebo (HR, 0.85; 95% CI, 0.73-0.98; P = .03). Denosumab significantly delayed time to first bone metastasis (HR, 0.84; 95% CI, 0.71-0.98; P = .03). OS was similar between groups (HR, 1.01; 95% CI, 0.85-1.20; P = .91). Rates of AEs and serious AEs were similar between groups, except for ONJ and hypocalcemia. The rates of ONJ for denosumab were 1%, 3%, 4% in years 1,2, 3, respectively; overall, < 5% (n = 33). Hypocalcemia occurred in < 2% (n = 12) in denosumab-treated patients. The authors concluded that in men with CRPC, denosumab significantly prolonged bone metastasis–free survival and delayed time-to-bone metastasis.37 These 2 studies suggest a role of receptor-activated nuclear factor κ-B ligand inhibitor denosumab in patients with nonmetastatic CRPC in the appropriate setting. There were delays in bony metastatic disease, but no difference in OS. Rare denosumab treatment–related specific AEs were noted. Hence, denosumab is not recommended for use in this setting.
Metastatic CRPC
Castration resistance typically occurs 2 to 3 years following initiation of ADT and the most common extranodal site of disease is within the bone in metastatic PC. Disease progression within bones after ADT can be challenging given both the nature of progressive cancer with osteoblastic metastatic lesions and the prolonged effects of ADT on unaffected bone. The Zometa 039 study compared ZA with placebo and found a significant difference in SREs (38% and 49%, respectively; P .03). No survival benefit was observed with the addition of ZA. Use of other bisphosphonates pamidronate and clodronate did not have a similar degree of benefit.38,39
A phase 3 study of 1904 patients found that denosumab was superior to ZA in delaying the time to first on-study SRE (HR, 0.82; 95% CI, 0.71-0.95) and reducing rates of multiple SREs (HR, 0.82; 95% CI, 0.71-0.94).40 This was later confirmed with an additional study that demonstrated treatment with denosumab significantly reduced the risk of developing a first symptomatic SRE, defined as a pathologic fracture, spinal cord compression, necessity for radiation, or surgery (HR, 0.78; 95% CI, 0.66-0.93; P = .005) and first and subsequent symptomatic SREs (rate ratio, 0.78; 95% CI, 0.65-0.92; P = .004) compared with ZA.28 These findings suggest a continued role of denosumab in the treatment of advanced metastatic CRPC from both control of bone disease as well as quality of life and palliation of cancer-related symptoms.
Radium-223 dichloride (radium-223) is an α-emitting radionuclide for treatment of metastatic CRPC with bone metastasis, but otherwise no additional metastatic sites. Radium-223 is a calcium-mimetic that preferentially accumulates into areas of high-bone turnover, such as where bone metastases tend to occur. Radium-223 induces apoptosis of tumor cells through double-stranded DNA breaks. Studies have shown radium-223 to prolong OS and time-to-first symptomatic SRE.41 The ERA-223 trial showed that when radium-223 was combined with abiraterone acetate, there was an increase in fragility fracture risk compared with placebo combined with abiraterone. Data from the study revealed that the median symptomatic SRE-free survival was 22.3 months (95% CI, 20.4-24.8) in the radium-223 group and 26.0 months (21.8-28.3) in the placebo group. Concurrent treatment with abiraterone acetate plus prednisone or prednisolone and radium-223 was associated with increased fracture risk. Osteoporotic fractures were the most common type of fracture in the radium-223 group and of all fracture types, differed the most between the study groups.42
Conclusions
Convincing evidence supports the ongoing use of bisphosphonates and denosumab in patients with osteoporosis, significant osteopenia with risk factors, and in patients with CRPC with bone metastasis. Bone metastases can cause considerable morbidity and mortality among men with advanced PC. Pain, fracture, and neurologic injury can occur with metastatic bone lesions as well as with ADT-related bone loss. Prevention of SREs in patients with PC is a reasonable goal in PC survivors while being mindful of managing the risks of these therapies.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7-30. doi:10.3322/caac.21590
2. Skolarus TA, Hawley ST. Prostate cancer survivorship care in the Veterans Health Administration. Fed Pract. 2014;31(8):10-17.
3. Gartrell BA, Coleman R, Efstathiou E, et al. Metastatic prostate cancer and the bone: significance and therapeutic options. Eur Urol. 2015;68(5):850-858. doi:10.1016/j.eururo.2015.06.039
4. Bolla M, de Reijke TM, Van Tienhoven G, et al. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med. 2009;360(24):2516-2527. doi:10.1056/NEJMoa0810095
5. Welch HG, Albertsen PC. Reconsidering Prostate cancer mortality—The future of PSA screening. N Engl J Med. 2020;382(16):1557-1563. doi:10.1056/NEJMms1914228
6. Coleman R, Body JJ, Aapro M, Hadji P, Herrstedt J; ESMO Guidelines Working Group. Bone health in cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol. 2014;25 (suppl 3):iii124-137. doi:10.1093/annonc/mdu103
7. Saylor PJ, Smith MR. Adverse effects of androgen deprivation therapy: defining the problem and promoting health among men with prostate cancer. J Natl Compr Canc Netw. 2010;8(2):211-223. doi:10.6004/jnccn.2010.0014
8. Shahinian VB, Kuo Y-F, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352(2):154-164. doi:10.1056/NEJMoa041943
9. Sartor O, de Bono JS. Metastatic prostate cancer. N Engl J Med. 2018;378(7):645-657. doi:10.1056/NEJMra1701695
10. Saad F, Eastham JA, Smith MR. Biochemical markers of bone turnover and clinical outcomes in men with prostate cancer. Urol Oncol. 2012;30(4):369-378. doi:10.1016/j.urolonc.2010.08.007
11. Cosman F, de Beur SJ, LeBoff MS, et al; National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359-2381. doi:10.1007/s00198-014-2794-2
12. Alibhai SMH, Zukotynski K, Walker-Dilks C, et al; Cancer Care Ontario Genitourinary Cancer Disease Site Group. Bone health and bone-targeted therapies for prostate cancer: a programme in evidence-based care - Cancer Care Ontario Clinical Practice Guideline. Clin Oncol (R Coll Radiol). 2017;29(6):348-355. doi:10.1016/j.clon.2017.01.007
13. LEE CE. A comprehensive bone-health management approach with men with prostate cancer recieving androgen deprivation therapy. Curr Oncol. 2011;18(4):e163-172. doi:10.3747/co.v18i4.746
14. Kennel KA, Drake MT. Adverse effects of bisphosphonates: Implications for osteoporosis management. Mayo Clin Proc. 2009;84(7):632-638. doi:10.1016/S0025-6196(11)60752-0
15. Saad F, Brown JE, Van Poznak C, et al. Incidence, risk factors, and outcomes of osteonecrosis of the jaw: integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastases. Ann Oncol. 2012;23(5):1341-1347. doi:10.1093/annonc/mdr435
16. Body J-J, Bone HG, de Boer RH, et al. Hypocalcaemia in patients with metastatic bone disease treated with denosumab. Eur J Cancer. 2015;51(13):1812-1821. doi:10.1016/j.ejca.2015.05.016
17. Wysowski DK, Chang JT. Alendronate and risedronate: reports of severe bone, joint, and muscle pain. Arch Intern Med. 2005;165(3):346-347. doi:10.1001/archinte.165.3.346-b
18. Saylor PJ, Rumble RB, Tagawa S, et al. Bone health and bone-targeted therapies for prostate cancer: ASCO endorsement of a cancer care Ontario guideline. J Clin Oncol. 2020;38(15):1736-1743. doi:10.1200/JCO.19.03148
19. Saad F, Gleason DM, Murray R, et al; Zoledronic Acid Prostate Cancer Study Group. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst. 2004;96(11):879-882. doi:10.1093/jnci/djh141
20. Saad F, Gleason DM, Murray R, et al; Zoledronic Acid Prostate Cancer Study Group. A randomized, placebo-controlled trial of zoledronic zcid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94(19):1458-1468. doi:10.1093/jnci/94.19.1458
21. Aapro M, Saad F. Bone-modifying agents in the treatment of bone metastases in patients with advanced genitourinary malignancies: a focus on zoledronic acid. Ther Adv Urol. 2012;4(2):85-101. doi:10.1177/1756287212441234
22. Cianferotti L, Bertoldo F, Carini M, et al. The prevention of fragility fractures in patients with non-metastatic prostate cancer: a position statement by the international osteoporosis foundation. Oncotarget. 2017;8(43):75646-75663. doi:10.18632/oncotarget.17980
23. Ruggiero S, Gralow J, Marx RE, et al. Practical guidelines for the prevention, diagnosis, and treatment of osteonecrosis of the jaw in patients with cancer. J Oncol Pract. 2006;2(1):7-14. doi:10.1200/JOP.2006.2.1.7
24. Corraini P, Heide-Jørgensen U, Schøodt M, et al. Osteonecrosis of the jaw and survival of patients with cancer: a nationwide cohort study in Denmark. Cancer Med. 2017;6(10):2271-2277. doi:10.1002/cam4.1173
25. Watts NB, Diab DL. Long-term use of bisphosphonates in osteoporosis. J Clin Endocrinol Metab. 2010;95(4):1555-1565. doi:10.1210/jc.2009-1947
26. Himelstein AL, Foster JC, Khatcheressian JL, et al. Effect of longer interval vs standard dosing of zoledronic acid on skeletal events in patients with bone metastases: a randomized clinical trial. JAMA. 2017;317(1):48-58. doi:10.1001/jama.2016.19425
27. Macherey S, Monsef I, Jahn F, et al. Bisphosphonates for advanced prostate cancer. Cochrane Database Syst Rev. 2017;12(12):CD006250. doi:10.1002/14651858.CD006250.pub2
28. Smith MR, Coleman RE, Klotz L, et al. Denosumab for the prevention of skeletal complications in metastatic castration-resistant prostate cancer: comparison of skeletal-related events and symptomatic skeletal events. Ann Oncol. 2015;26(2):368-374. doi:10.1093/annonc/mdu519
29. Wirth M, Tammela T, Cicalese V, et al. Prevention of bone metastases in patients with high-risk nonmetastatic prostate cancer treated with zoledronic acid: efficacy and safety results of the Zometa European Study (ZEUS). Eur Urol. 2015;67(3):482-491. doi:10.1016/j.eururo.2014.02.014
30. James ND, Sydes MR, Clarke NW, et al; STAMPEDE Investigators. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387(10024):1163-1177. doi:10.1016/S0140-6736(15)01037-5
31. Denham JW, Joseph D, Lamb DS, et al. Short-term androgen suppression and radiotherapy versus intermediate-term androgen suppression and radiotherapy, with or without zoledronic acid, in men with locally advanced prostate cancer (TROG 03.04 RADAR): 10-year results from a randomised, phase 3, factorial trial. Lancet Oncol. 2019;20(2):267-281. doi:10.1016/S1470-2045(18)30757-5
32. Dearnaley DP, Mason MD, Parmar MK, Sanders K, Sydes MR. Adjuvant therapy with oral sodium clodronate in locally advanced and metastatic prostate cancer: long-term overall survival results from the MRC PR04 and PR05 randomised controlled trials. Lancet Oncol. 2009;10(9):872-876. doi:10.1016/S1470-2045(09)70201-3
33. Smith MR, Egerdie B, Toriz NH, et al; Denosumab HALT Prostate Cancer Study Group. Denosumab in men receiving androgen-deprivation therapy for prostate Cancer. N Engl J Med. 2009;361(8):745-755. doi:10.1056/NEJMoa0809003
34. Smith MR, Halabi S, Ryan CJ, et al. Randomized controlled trial of early zoledronic acid in men with castration-sensitive prostate cancer and bone metastases: results of CALGB 90202 (alliance). J Clin Oncol. 2014;32(11):1143-1150. doi:10.1200/JCO.2013.51.6500
35. Kozyrakis D, Paridis D, Perikleous S, Malizos K, Zarkadas A, Tsagkalis A. The current role of osteoclast inhibitors in patients with prostate cancer. Adv Urol. 2018;2018:1525832. doi:10.1155/2018/1525832
36. Smith MR, Kabbinavar F, Saad F, et al. Natural history of rising serum prostate-specific antigen in men with castrate nonmetastatic prostate cancer. J Clin Oncol. 2005;23(13):2918-2925. doi:10.1200/JCO.2005.01.529
37. Smith MR, Saad F, Coleman R, et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet. 2012;379(9810):39-46. doi:10.1016/S0140-6736(11)61226-9
38. Small EJ, Smith MR, Seaman JJ, Petrone S, Kowalski MO. Combined analysis of two multicenter, randomized, placebo-controlled studies of pamidronate disodium for the palliation of bone pain in men with metastatic prostate cancer. J Clin Oncol. 2003;21(23):4277-4284. doi:10.1200/JCO.2003.05.147
39. Ernst DS, Tannock IF, Winquist EW, et al. Randomized, double-blind, controlled trial of mitoxantrone/prednisone and clodronate versus mitoxantrone/prednisone and placebo in patients with hormone-refractory prostate cancer and pain. J Clin Oncol. 2003;21(17):3335-3342. doi:10.1200/JCO.2003.03.042
40. Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377(9768):813-822. doi:10.1016/S0140-6736(10)62344-6
41. Parker C, Nilsson S, Heinrich D, et al; ALSYMPCA Investigators Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213-223. doi:10.1056/NEJMoa1213755
42. Smith M, Parker C, Saad F, et al. Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(3):408-419. doi:10.1016/S1470-2045(18)30860-X
43. Smith MR, Saad F, Shore ND, et al. Effect of denosumab on prolonging bone-metastasis-free survival (BMFS) in men with nonmetastatic castrate-resistant prostate cancer (CRPC) presenting with aggressive PSA kinetics. J Clin Oncol. 2012;30(5_suppl):6-6.
44. Saad F, Gleason DM, Murray R, et al; Zoledronic Acid Prostate Cancer Study Group. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94(19):1458-1468. doi:10.1093/jnci/94.19.1458
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7-30. doi:10.3322/caac.21590
2. Skolarus TA, Hawley ST. Prostate cancer survivorship care in the Veterans Health Administration. Fed Pract. 2014;31(8):10-17.
3. Gartrell BA, Coleman R, Efstathiou E, et al. Metastatic prostate cancer and the bone: significance and therapeutic options. Eur Urol. 2015;68(5):850-858. doi:10.1016/j.eururo.2015.06.039
4. Bolla M, de Reijke TM, Van Tienhoven G, et al. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med. 2009;360(24):2516-2527. doi:10.1056/NEJMoa0810095
5. Welch HG, Albertsen PC. Reconsidering Prostate cancer mortality—The future of PSA screening. N Engl J Med. 2020;382(16):1557-1563. doi:10.1056/NEJMms1914228
6. Coleman R, Body JJ, Aapro M, Hadji P, Herrstedt J; ESMO Guidelines Working Group. Bone health in cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol. 2014;25 (suppl 3):iii124-137. doi:10.1093/annonc/mdu103
7. Saylor PJ, Smith MR. Adverse effects of androgen deprivation therapy: defining the problem and promoting health among men with prostate cancer. J Natl Compr Canc Netw. 2010;8(2):211-223. doi:10.6004/jnccn.2010.0014
8. Shahinian VB, Kuo Y-F, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352(2):154-164. doi:10.1056/NEJMoa041943
9. Sartor O, de Bono JS. Metastatic prostate cancer. N Engl J Med. 2018;378(7):645-657. doi:10.1056/NEJMra1701695
10. Saad F, Eastham JA, Smith MR. Biochemical markers of bone turnover and clinical outcomes in men with prostate cancer. Urol Oncol. 2012;30(4):369-378. doi:10.1016/j.urolonc.2010.08.007
11. Cosman F, de Beur SJ, LeBoff MS, et al; National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359-2381. doi:10.1007/s00198-014-2794-2
12. Alibhai SMH, Zukotynski K, Walker-Dilks C, et al; Cancer Care Ontario Genitourinary Cancer Disease Site Group. Bone health and bone-targeted therapies for prostate cancer: a programme in evidence-based care - Cancer Care Ontario Clinical Practice Guideline. Clin Oncol (R Coll Radiol). 2017;29(6):348-355. doi:10.1016/j.clon.2017.01.007
13. LEE CE. A comprehensive bone-health management approach with men with prostate cancer recieving androgen deprivation therapy. Curr Oncol. 2011;18(4):e163-172. doi:10.3747/co.v18i4.746
14. Kennel KA, Drake MT. Adverse effects of bisphosphonates: Implications for osteoporosis management. Mayo Clin Proc. 2009;84(7):632-638. doi:10.1016/S0025-6196(11)60752-0
15. Saad F, Brown JE, Van Poznak C, et al. Incidence, risk factors, and outcomes of osteonecrosis of the jaw: integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastases. Ann Oncol. 2012;23(5):1341-1347. doi:10.1093/annonc/mdr435
16. Body J-J, Bone HG, de Boer RH, et al. Hypocalcaemia in patients with metastatic bone disease treated with denosumab. Eur J Cancer. 2015;51(13):1812-1821. doi:10.1016/j.ejca.2015.05.016
17. Wysowski DK, Chang JT. Alendronate and risedronate: reports of severe bone, joint, and muscle pain. Arch Intern Med. 2005;165(3):346-347. doi:10.1001/archinte.165.3.346-b
18. Saylor PJ, Rumble RB, Tagawa S, et al. Bone health and bone-targeted therapies for prostate cancer: ASCO endorsement of a cancer care Ontario guideline. J Clin Oncol. 2020;38(15):1736-1743. doi:10.1200/JCO.19.03148
19. Saad F, Gleason DM, Murray R, et al; Zoledronic Acid Prostate Cancer Study Group. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst. 2004;96(11):879-882. doi:10.1093/jnci/djh141
20. Saad F, Gleason DM, Murray R, et al; Zoledronic Acid Prostate Cancer Study Group. A randomized, placebo-controlled trial of zoledronic zcid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94(19):1458-1468. doi:10.1093/jnci/94.19.1458
21. Aapro M, Saad F. Bone-modifying agents in the treatment of bone metastases in patients with advanced genitourinary malignancies: a focus on zoledronic acid. Ther Adv Urol. 2012;4(2):85-101. doi:10.1177/1756287212441234
22. Cianferotti L, Bertoldo F, Carini M, et al. The prevention of fragility fractures in patients with non-metastatic prostate cancer: a position statement by the international osteoporosis foundation. Oncotarget. 2017;8(43):75646-75663. doi:10.18632/oncotarget.17980
23. Ruggiero S, Gralow J, Marx RE, et al. Practical guidelines for the prevention, diagnosis, and treatment of osteonecrosis of the jaw in patients with cancer. J Oncol Pract. 2006;2(1):7-14. doi:10.1200/JOP.2006.2.1.7
24. Corraini P, Heide-Jørgensen U, Schøodt M, et al. Osteonecrosis of the jaw and survival of patients with cancer: a nationwide cohort study in Denmark. Cancer Med. 2017;6(10):2271-2277. doi:10.1002/cam4.1173
25. Watts NB, Diab DL. Long-term use of bisphosphonates in osteoporosis. J Clin Endocrinol Metab. 2010;95(4):1555-1565. doi:10.1210/jc.2009-1947
26. Himelstein AL, Foster JC, Khatcheressian JL, et al. Effect of longer interval vs standard dosing of zoledronic acid on skeletal events in patients with bone metastases: a randomized clinical trial. JAMA. 2017;317(1):48-58. doi:10.1001/jama.2016.19425
27. Macherey S, Monsef I, Jahn F, et al. Bisphosphonates for advanced prostate cancer. Cochrane Database Syst Rev. 2017;12(12):CD006250. doi:10.1002/14651858.CD006250.pub2
28. Smith MR, Coleman RE, Klotz L, et al. Denosumab for the prevention of skeletal complications in metastatic castration-resistant prostate cancer: comparison of skeletal-related events and symptomatic skeletal events. Ann Oncol. 2015;26(2):368-374. doi:10.1093/annonc/mdu519
29. Wirth M, Tammela T, Cicalese V, et al. Prevention of bone metastases in patients with high-risk nonmetastatic prostate cancer treated with zoledronic acid: efficacy and safety results of the Zometa European Study (ZEUS). Eur Urol. 2015;67(3):482-491. doi:10.1016/j.eururo.2014.02.014
30. James ND, Sydes MR, Clarke NW, et al; STAMPEDE Investigators. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387(10024):1163-1177. doi:10.1016/S0140-6736(15)01037-5
31. Denham JW, Joseph D, Lamb DS, et al. Short-term androgen suppression and radiotherapy versus intermediate-term androgen suppression and radiotherapy, with or without zoledronic acid, in men with locally advanced prostate cancer (TROG 03.04 RADAR): 10-year results from a randomised, phase 3, factorial trial. Lancet Oncol. 2019;20(2):267-281. doi:10.1016/S1470-2045(18)30757-5
32. Dearnaley DP, Mason MD, Parmar MK, Sanders K, Sydes MR. Adjuvant therapy with oral sodium clodronate in locally advanced and metastatic prostate cancer: long-term overall survival results from the MRC PR04 and PR05 randomised controlled trials. Lancet Oncol. 2009;10(9):872-876. doi:10.1016/S1470-2045(09)70201-3
33. Smith MR, Egerdie B, Toriz NH, et al; Denosumab HALT Prostate Cancer Study Group. Denosumab in men receiving androgen-deprivation therapy for prostate Cancer. N Engl J Med. 2009;361(8):745-755. doi:10.1056/NEJMoa0809003
34. Smith MR, Halabi S, Ryan CJ, et al. Randomized controlled trial of early zoledronic acid in men with castration-sensitive prostate cancer and bone metastases: results of CALGB 90202 (alliance). J Clin Oncol. 2014;32(11):1143-1150. doi:10.1200/JCO.2013.51.6500
35. Kozyrakis D, Paridis D, Perikleous S, Malizos K, Zarkadas A, Tsagkalis A. The current role of osteoclast inhibitors in patients with prostate cancer. Adv Urol. 2018;2018:1525832. doi:10.1155/2018/1525832
36. Smith MR, Kabbinavar F, Saad F, et al. Natural history of rising serum prostate-specific antigen in men with castrate nonmetastatic prostate cancer. J Clin Oncol. 2005;23(13):2918-2925. doi:10.1200/JCO.2005.01.529
37. Smith MR, Saad F, Coleman R, et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet. 2012;379(9810):39-46. doi:10.1016/S0140-6736(11)61226-9
38. Small EJ, Smith MR, Seaman JJ, Petrone S, Kowalski MO. Combined analysis of two multicenter, randomized, placebo-controlled studies of pamidronate disodium for the palliation of bone pain in men with metastatic prostate cancer. J Clin Oncol. 2003;21(23):4277-4284. doi:10.1200/JCO.2003.05.147
39. Ernst DS, Tannock IF, Winquist EW, et al. Randomized, double-blind, controlled trial of mitoxantrone/prednisone and clodronate versus mitoxantrone/prednisone and placebo in patients with hormone-refractory prostate cancer and pain. J Clin Oncol. 2003;21(17):3335-3342. doi:10.1200/JCO.2003.03.042
40. Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377(9768):813-822. doi:10.1016/S0140-6736(10)62344-6
41. Parker C, Nilsson S, Heinrich D, et al; ALSYMPCA Investigators Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213-223. doi:10.1056/NEJMoa1213755
42. Smith M, Parker C, Saad F, et al. Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(3):408-419. doi:10.1016/S1470-2045(18)30860-X
43. Smith MR, Saad F, Shore ND, et al. Effect of denosumab on prolonging bone-metastasis-free survival (BMFS) in men with nonmetastatic castrate-resistant prostate cancer (CRPC) presenting with aggressive PSA kinetics. J Clin Oncol. 2012;30(5_suppl):6-6.
44. Saad F, Gleason DM, Murray R, et al; Zoledronic Acid Prostate Cancer Study Group. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94(19):1458-1468. doi:10.1093/jnci/94.19.1458
2021 Update on female sexual health
The approach to diagnosis and treatment of female sexual function continues to be a challenge for women’s health professionals. The search for a female “little blue pill” remains elusive as researchers struggle to understand the mechanisms that underlie the complex aspects of female sexual health. This Update will review the recent literature on the use of fractional CO2 laser for treatment of female sexual dysfunction and vulvovaginal symptoms. Bottom line: While the quality of the studies is poor overall, fractional CO2 laser treatment seems to temporarily improve symptoms of genitourinary syndrome of menopause (GSM). The duration of response, cost, and the overall long-term impact on sexual health remain in question.
A retrospective look at CO2 laser and postmenopausal GSM
Filippini M, Luvero D, Salvatore S, et al. Efficacy of fractional CO2 laser treatment in postmenopausal women with genitourinary syndrome: a multicenter study. Menopause. 2019;27:43-49. doi: 10.1097/GME. 0000000000001428.
Researchers conducted a retrospective, multicenter study of postmenopausal women with at least one symptom of GSM, including itching, burning, dyspareunia with penetration, and dryness.
Study details
A total of 171 of the 645 women (26.5%) were oncology patients. Women were excluded from analysis if they used any form of topical therapy within 15 days; had prolapse stage 2 or greater; or had any infection, abscess, or anatomical deformity precluding treatment with the laser.
Patients underwent gynecologic examination and were given a questionnaire to assess vulvovaginal symptoms. Exams occurred monthly during treatment (average, 6.5 months), at 6- and 12-months posttreatment, and then annually. No topical therapy was advised during or after treatment.
Patients received either 3 or 4 fractional CO2 laser treatments to the vulva and/or vagina depending on symptom location and type. Higher power settings of the same laser were used to treat vaginal symptoms (40W; 1,000 microseconds) versus vulvar symptoms (25W; 500 microseconds). Treatment sessions were 5 to 6 minutes. The study authors used a visual analog rating scale (VAS) for “atrophy and related symptoms,” tested vaginal pH, and completed the Vaginal Health Index Score. VAS scores were obtained from the patients prior to the initial laser intervention and 1 month after the final treatment.
Results
There were statistically significant improvements in dryness, vaginal orifice pain, dyspareunia, itching, and burning for both the 3-treatment and 4-treatment cohorts. The delta of improvement was then compared for the 2 subgroups; curiously, there was greater improvement of symptoms such as dryness (65% vs 61%), itching (78% vs 72%), burning (72% vs 67%), and vaginal orifice pain (67% vs 60%) in the group that received 3 cycles than in the group that received 4 cycles.
With regard to vaginal pH improvement, the 4-cycle group performed better than the 3-cycle group (1% improvement in the 4-cycle group vs 6% in the 3-cycle group). Although vaginal pH reduction was somewhat better in the group that received 4 treatments, and the pre versus posttreatment percentages were statistically significantly different, the clinical significance of a pH difference between 5.72 and 5.53 is questionable, especially since there was a greater difference in baseline pH between the two cohorts (6.08 in the 4-cycle group vs 5.59 in the 3-cycle group).
There were no reported adverse events related to the fractional laser treatments, and 6% of the patients underwent additional laser treatments during the followup timeframe of 8 to 20 months.
This was a retrospective study with no control or comparison group and short-term follow-up. The VAS scores were obtained 1 month after the final treatment. Failure to request additional treatment at 8 to 20 months cannot be used to infer that the therapeutic improvements recorded at 1 month were enduring. In addition, although the large number of patients in this study may lead to statistical significance, clinical significance is still questionable. Given the lack of a comparison group and the very short follow-up, it is hard to draw any scientifically valid conclusions from this study.
Continue to: Randomized data on CO2 laser vs Kegels for sexual dysfunction...
Randomized data on CO2 laser vs Kegels for sexual dysfunction
Lou W, Chen F, Xu T, et al. A randomized controlled study of vaginal fractional CO2 laser therapy for female sexual dysfunction. Lasers Med Sci. March 15, 2021. doi: 10.1007/s10103-021-03260-x.
In a small randomized controlled trial (RCT) conducted in China, Lou and colleagues identified premenopausal women at “high risk” for sexual dysfunction as determined by the Chinese version of the Female Sexual Function Index (CFSFI).
Details of the study
A total of 84 women (mean age, 36.5 years) were included in the study. All the participants were heterosexual and married or with a long-term partner. The domain of sexual dysfunction was not considered. Women were excluded if they had no current heterosexual partner; had genital malformation, urinary incontinence, or prolapse stage 2 or higher; a history of pelvic floor mesh treatment; current gynecologic malignancy; abnormal cervical cytology; or were currently pregnant or postpartum. In addition, women were excluded if they had been treated previously for sexual dysfunction or mental “disease.” The cohort was randomized to receive fractional CO2 laser treatments (three 15-minute treatments 1 month apart at 60W, 1,000 microseconds) or coached Kegel exercises (10 exercises repeated twice daily at least 3 times/week and monitored by physical therapists at biweekly clinic visits). Sexual distress was evaluated by using the Female Sexual Distress Scale-Revised (FSDSR). Outcomes measured were pelvic floor muscle strength and scores on the CFSFI and FSDSR. Data were obtained at 3, 6, 9, and 12 months after initiation of therapy.
Both groups showed improvement
The laser cohort showed slightly more improvement in scale scores at 6 and 12 months. Specifically, the laser group had better scores on lubrication and overall satisfaction, with moderate effect size; neither group had improvements in arousal, desire, or orgasm. The Kegel group showed a significant improvement in pelvic floor strength and orgasm at 12 months, an improvement not seen in the laser cohort. Both groups showed gradual improvement in the FSDSR, with the laser group reporting a lower score (10.0) at 12 months posttreatment relative to the Kegel group (11.1). Again, these were modest effects as baseline scores for both cohorts were around 12.5. There were minimal safety signals in the laser group, with 22.5% of women reporting scant bloody discharge posttreatment and 72.5% describing mild discomfort (1 on a 1–10 VAS scale) during the procedure.
This study is problematic in several areas. Although it was a prospective, randomized trial, it was not blinded, and the therapeutic interventions were markedly different in nature and requirement for individual patient motivation. The experiences of sexual dysfunction among the participants were not stratified by type—arousal, desire, lubrication, orgasm, or pain. All patients had regular cyclic menses; however, the authors do not report on contraceptive methods, hormonal therapy, or other comorbid conditions that could impact sexual health. The cohorts may or may not have been similar in baseline types of sexual dissatisfaction.
CO2 laser for lichen sclerosus: Is it effective?
Pagano T, Conforti A, Buonfantino C, et al. Effect of rescue fractional microablative CO2 laser on symptoms and sexual dysfunction in women affected by vulvar lichen sclerosus resistant to long-term use of topic corticosteroid: a prospective longitudinal study. Menopause. 2020;27:418-422. doi: 10.1097 /GME.0000000000001482.
Burkett LS, Siddique M, Zeymo A, et al. Clobetasol compared with fractionated carbon dioxide laser for lichen sclerosus: a randomized controlled trial. Obstet Gynecol. 2021;137:968-978. doi: 10.1097 /AOG.0000000000004332.
Mitchell L, Goldstein AT, Heller D, et al. Fractionated carbon dioxide laser for the treatment of vulvar lichen sclerosus: a randomized controlled trial. Obstet Gynecol. 2021;137:979-987. doi: 10.1097 /AOG.0000000000004409.
High potency corticosteroid ointment is the current standard treatment for lichen sclerosus. Alternative options for disease that is refractory to steroids are limited. Three studies published in the past year explored the CO2 laser’s ability to treat lichen sclerosus symptoms and resultant sexual dysfunction—Pagano and colleagues conducted a small prospective study and Burkett and colleagues and Mitchell et al conducted small RCTs.
Details of the Pagano study
Three premenopausal and 37 postmenopausal women with refractory lichen sclerosus (defined as no improvement after 4 cycles of ultra-high potency steroids) were included in the study. Lichen sclerosus was uniformly biopsy confirmed. Women using topical or systemic hormones were excluded. VAS was administered prior to initial treatment and after each of 2 fractional CO2 treatments (25–30 W; 1,000 microseconds) 30 to 40 days apart to determine severity of vulvar itching, dyspareunia with penetration, vulvar dryness, sexual dysfunction, and procedure discomfort. Follow-up was conducted at 1 month after the final treatment. VAS score for the primary outcome of vulvar itching declined from 8 pretreatment to 6 after the first treatment and to 3 after the second. There was no significant treatment-related pain reported.
The authors acknowledged the limitations of their study; it was a relatively small sample size, nonrandomized and had short-term follow-up of a mixed patient population and no sham or control group. The short-term improvements reported in the study patients may not be sustained without ongoing treatment for a lifelong chronic disease, and the long-term potential for development of squamous cell carcinoma may or may not be ameliorated.
Continue to: Burkett et al: RCT study 1...
Burkett et al: RCT study 1
A total of 52 postmenopausal patients with biopsy-proven lichen sclerosus were randomly assigned to clobetasol or CO2 laser; 51 women completed 6-month follow-up. The outcomes were stratified by prior high-potency steroid use. The steroid cohort used clobetasol 0.05% nightly for 1 month, 3 times per week for 2 months, then as needed. The laser cohort received 3 treatments (26 W; 800 microseconds) 4 to 6 weeks apart. Overall adherence was only 75% in the clobetasol group, compared with 96% in the laser group. The authors found treatment efficacy of CO2 laser therapy only in the group of patients who had prior treatment with high potency topical corticosteroids. They conclude that, …“Despite previously optimistic results in well designed clinical trials of fractionated CO2 for genitourinary syndrome of menopause, and in noncontrolled case series for vulvar lichen sclerosus, our study failed to show any significant benefit of monotherapy of fractionated CO2 for vulvar lichen sclerosus. There may be a role for fractionated CO2 as an adjuvant therapy along with topical ultrapotent corticosteroids in vulvar lichen sclerosus.”
Mitchell et al: RCT study 2
This was a double blind, placebo-controlled, and histologically validated study of fractional CO2 for treatment of lichen sclerosus in 35 women; 17 in the treatment arm and 18 in the sham laser encounters. At least a 4-week no treatment period of topical steroids was required before monotherapy with CO2 laser was initiated.
The authors found no difference in their primary outcome—histopathology scale scores—after 5 treatments over 24 weeks. Secondary endpoints were changes in the CSS (Clinical Scoring System for Vulvar Lichen Sclerosus), a validated instrument that includes both a clinician’s examination of the severity of disease and a patient’s report of the severity of her symptoms. The patient score is the total of 4 domains: itching, soreness, burning, and dyspareunia. The clinician objective examination documents fissures, erosions, hyperkeratosis, agglutination, stenosis, and atrophy. At the conclusion of treatment there were no significant differences in the patient reported symptoms or the clinical findings between the active treatment and sham groups.
As a monotherapy, CO2 laser therapy is not effective in treating lichen sclerosus, although it may help improve symptoms as an adjunct to high potency steroid therapy when topical treatment alone has failed to provide adequate response.
Conclusion
The quality of evidence to support the use of the CO2 laser for improvement in sexual dysfunction is poor. Although patient satisfaction scores improved overall, and most specifically for symptoms related to GSM, the lack of blinding; inappropriate or no control groups; the very short-term outcomes; and for one of the studies, the lack of a clear definition of sexual dysfunction, make it difficult to draw meaningful conclusions for clinical care.
For GSM, we know that topical estrogen therapy works—and with little to no systemic absorption. The CO2 laser should be studied in comparison to this gold standard, with consideration of costs and potential long-term harms in addition to patient satisfaction and short-term measures of improvement. In addition, and very importantly, it is our professional responsibility to present the evidence for safety of topical estrogens to our professional colleagues as well as to our patients with estrogen-dependent cancers so that they understand the value of estrogen as a safe and appropriate alternative to expensive and potentially short-term interventions such as CO2 laser treatment. ●

Cheryl Iglesia, MD
Dr. Iglesia is Director, Section of Female Pelvic Medicine and Reconstructive Surgery, MedStar Washington Hospital Center, and Professor, Departments of ObGyn and Urology, Georgetown University School of Medicine, Washington, DC. She is a member of the OBG Management Board of Editors.
Barbara Levy, MD: Cheryl, you have more experience with use of the energy-based cosmetic laser than most ObGyns, and I thought that speaking with you about this technology would be of benefit, not only to me in learning more about the hands-on experience of a lead researcher and practitioner but also readers who are hearing more and more about the growth of cosmetic gynecology in general. Thank you for taking the time today.
Cheryl Iglesia, MD: I’m happy to speak about this with you, Barbara.
Dr. Levy: Specifically, I would like to talk about use of these technologies for sexual dysfunction. In the last few years some of the available data have been on the CO2 laser versus physical therapy, which is not an appropriate comparison.1
Dr. Iglesia: There have been limited data, and less randomized, controlled data, on laser and radiofrequency energies for cosmetic gynecology, and in fact these devices remain unapproved for any gynecologic indication. In 2018 the US Food and Drug Administration (FDA) issued a Safety Communication about the use of energy-based devices to perform vaginal rejuvenation or cosmetic procedures. The International Urogynecological Association (IUGA) issued a consensus statement echoing concerns about the devices, and an International Continence Society/International Society for the Study of Vulvovaginal Disease Best Practice Consensus Statement did not recommend the laser for “routine treatment of vaginal atrophy or urinary incontinence unless treatment is part of a well-designed trial or with special arrangements for clinical governance, consent, and audit.”2
In May 2020, as evidence remains limited (although 522 studies are ongoing in coordination with the FDA), the American Urogynecologic Society (AUGS) published a clinical consensus statement from a panel of experts in female pelvic medicine and reconstructive surgery. The panel had about 90% consensus that there is short-term efficacy for the laser with GSM and dyspareunia. But we only have outcomes data that lasts a maximum of 1 year.2
A problem with our VeLVET trial,3 which was published in Menopause, and the Cruz and colleagues’ trial from South America,4 both of which compared the CO2 laser to estrogen and had randomized groups, was that they were limited by the outcome measures used, none of which were consistently validated. But these studies also had small numbers of participants and short-term follow-up. So I don’t think there are much existing data that are promising for supporting energy-based treatment for GSM.
We also have just-published data on the laser for lichen sclerosus.5 For the AUGS panel, there was about 80% consensus for energy-based-device use and lichen sclerosus.2 According to Mitchell et al, who conducted a small, randomized, sham-controlled trial, CO2 laser resulted in no significant difference in histopathology scale score between active and sham arms.5
Future trials may want to assess laser as a mechanism for improved local drug delivery (eg, use of combined laser plus local estrogen for GSM or combined laser plus topical steroid for lichen sclerosus). I am also aware that properly designed laser versus sham studies are underway.
Dr. Levy: What about for stress urinary incontinence (SUI)? I don’t think these technologies are going to work.
Dr. Iglesia: For the AUGS panel, there was only about 70% consensus for energy-based-device use and SUI,2 and I’m one of the naysayers. The pathophysiology of SUI is so multifactorial that it’s hard to believe that laser or radiofrequency wand therapy could have sustained improvements, especially since prior radiofrequency therapy from the last decade (for instance, Renessa, Novasys Medical) did not show long-term efficacy.
Understanding lasers and coordinating care
Dr. Levy: We don’t know what the long-term outcomes are for the CO2 laser and GSM.
Dr. Iglesia: I agree with you, and I think there needs to be an understanding of the mechanism of how lasers work, whether it be erbium (Er:YAG), which is the most common, or CO2. Erbium and CO2 lasers, which are on the far-infrared spectrum, target the chromophore, water. My feeling is that, when you look at results from the Cruz trial,4 or even our trial that compared vaginal estrogen with laser,3 when there is severe GSM and high pH with virtually no water present in the tissues, that laser is not going to properly function. But I don’t think we know exactly what optimal pretreatment is necessary, and that is one of the problems. Furthermore, when intravaginal lasers are done and no adequate speculum exam is conducted prior to introducing the laser, there could be discharge or old creams present that block the mirrors necessary to adequately fire the fractionated laser beams.
Unfortunately, oftentimes these devices are marketed to women with breast cancer, who may be taking aromatase inhibitors, which cause the no-water problem; they dry out everything. They are effective for preventing breast cancer recurrence, but they cause severe atrophy (perhaps worse than many of the other selective estrogen-receptor modulators), with a resultant high vaginal pH. If we can bring that pH level down, closer to the normal 4.5 range so that we could have some level of moisture, and add estrogen first, the overall treatment approach will probably be more effective. We still do not know what happens after 1 year, though, and how often touch-ups need to be performed.
In fact, when working with a patient with breast cancer, I will speak with her oncologist; I will collaborate to put in place a treatment plan that may include initial pretreatment with low-dose vaginal estrogen followed by laser treatment for vaginal atrophy. But I will make sure I use the lowest dose. Sometimes when the patient comes back, the estrogen’s worked so well she’ll say, “Oh, I’m happy, so I don’t need the laser anymore.” A balanced conversation is necessary, especially with cancer survivors.
Informing patients and colleagues
Dr. Levy: I completely agree, and I think one of the key points here is that our purpose is to serve our patients. The data demonstrate that low doses of vaginal estrogen are not harmful for women who are being treated for or who have recovered from breast cancer. It is our ethical obligation to convince these women and their oncologists that ongoing treatment with vaginal estrogen not only will help their GSM but also their overactive bladder and their risk of urinary tract infections and other things. We could be exploiting patients who are really fearful of using any estrogen because of a perceived cancer risk. We could actually be validating their fear by telling them we have an alternative treatment for which they have to pay cash.
Treatment access
Dr. Iglesia: Yes, these are not cosmetic conditions that we are treating. So my goal when evaluating treatment for refractory GSM or lichen sclerosus is to find optimal energy-based therapies with the hope that one day these will be approved gynecologic conditions by the US FDA for laser and wand therapies and that they will ultimately not be out-of-pocket expenses but rather therapies covered by insurance.
Dr. Levy: Great. I understand that AUGS/IUGA have been working on a terminology algorithm to help distinguish between procedures being performed to resolve a medical problem such as prolapse or incontinence versus those designed to be cosmetic.
Dr. Iglesia: Yes, there is a big document from experts in both societies out for public comment right now. It will hopefully be published soon.
Outstanding questions remain
Dr. Levy: Really, we as ObGyns shouldn’t be quick to incorporate these things into our practices without high-quality studies demonstrating value. I have a major concern about these devices in the long term. When you look at fractional CO2 use on the face, for instance, which is a much different type of skin than the vagina, the laser builds collagen—but we don’t have long-term outcome results. The vagina is supposed to be an elastic tissue, so what is the risk of long-term scarring there? Yes, the laser builds collagen in the vaginal epithelium, but what does it do to scarring in the rest of the tissue? We don’t have answers to that.
Dr. Iglesia: And that is the question—how does histology equate with function? Well, I would go with what the patients are reporting.
Dr. Levy: Absolutely. But the thing about vaginal low-dose estrogen is that it is something that the oncologists or the ObGyns could be implementing with patients while they are undergoing cancer therapy, while in their menopausal transition, to preserve vulvovaginal function as opposed to trying to regain it.
Dr. Iglesia: Certainly, although it still needs to be determined when that type of approach would actually be contraindicated.
Dr. Levy: Thank you, Cheryl, for your valuable insights.
Dr. Iglesia: Of course. Thank you. ●
References
1. Lou W, Chen F, Xu T, et al. A randomized controlled study of vaginal fractional CO2 laser therapy for female sexual dysfunction. Lasers Med Sci. March 15, 2021. doi: 10.1007/s10103-021-03260-x.
2. Alshiek J, Garcia B, Minassian V, et al. Vaginal energy-based devices. Female Pelvic Med Reconstr Surg. 2020;26:287-298. doi: 10.1097 /SPV.0000000000000872.
3. Paraiso MF, Ferrando CA, et al. A randomized clinical trial comparing vaginal laser therapy to vaginal estrogen therapy in women with genitourinary syndrome of menopause: the VeLVET Trial. Menopause. 2020;27:50-56. doi: 10.1097/GME.0000000000001416.
4. Cruz VL, Steiner ML, et al. Randomized, double-blind, placebo-controlled clinical trial for evaluating the efficacy of fractional CO2 laser compared with topical estriol in the treatment of vaginal atrophy in postmenopausal women. Menopause. 2018;25:21-28. doi: 10.1097 /GME.0000000000000955.
5. Mitchell L, Goldstein A, Heller D, et al. Fractionated carbon dioxide laser for the treatment of vulvar lichen sclerosus: a randomized controlled trial. Obstet Gynecol. 2021;137:979-987. doi: 10.1097/AOG.0000000000004409.

The approach to diagnosis and treatment of female sexual function continues to be a challenge for women’s health professionals. The search for a female “little blue pill” remains elusive as researchers struggle to understand the mechanisms that underlie the complex aspects of female sexual health. This Update will review the recent literature on the use of fractional CO2 laser for treatment of female sexual dysfunction and vulvovaginal symptoms. Bottom line: While the quality of the studies is poor overall, fractional CO2 laser treatment seems to temporarily improve symptoms of genitourinary syndrome of menopause (GSM). The duration of response, cost, and the overall long-term impact on sexual health remain in question.
A retrospective look at CO2 laser and postmenopausal GSM
Filippini M, Luvero D, Salvatore S, et al. Efficacy of fractional CO2 laser treatment in postmenopausal women with genitourinary syndrome: a multicenter study. Menopause. 2019;27:43-49. doi: 10.1097/GME. 0000000000001428.
Researchers conducted a retrospective, multicenter study of postmenopausal women with at least one symptom of GSM, including itching, burning, dyspareunia with penetration, and dryness.
Study details
A total of 171 of the 645 women (26.5%) were oncology patients. Women were excluded from analysis if they used any form of topical therapy within 15 days; had prolapse stage 2 or greater; or had any infection, abscess, or anatomical deformity precluding treatment with the laser.
Patients underwent gynecologic examination and were given a questionnaire to assess vulvovaginal symptoms. Exams occurred monthly during treatment (average, 6.5 months), at 6- and 12-months posttreatment, and then annually. No topical therapy was advised during or after treatment.
Patients received either 3 or 4 fractional CO2 laser treatments to the vulva and/or vagina depending on symptom location and type. Higher power settings of the same laser were used to treat vaginal symptoms (40W; 1,000 microseconds) versus vulvar symptoms (25W; 500 microseconds). Treatment sessions were 5 to 6 minutes. The study authors used a visual analog rating scale (VAS) for “atrophy and related symptoms,” tested vaginal pH, and completed the Vaginal Health Index Score. VAS scores were obtained from the patients prior to the initial laser intervention and 1 month after the final treatment.
Results
There were statistically significant improvements in dryness, vaginal orifice pain, dyspareunia, itching, and burning for both the 3-treatment and 4-treatment cohorts. The delta of improvement was then compared for the 2 subgroups; curiously, there was greater improvement of symptoms such as dryness (65% vs 61%), itching (78% vs 72%), burning (72% vs 67%), and vaginal orifice pain (67% vs 60%) in the group that received 3 cycles than in the group that received 4 cycles.
With regard to vaginal pH improvement, the 4-cycle group performed better than the 3-cycle group (1% improvement in the 4-cycle group vs 6% in the 3-cycle group). Although vaginal pH reduction was somewhat better in the group that received 4 treatments, and the pre versus posttreatment percentages were statistically significantly different, the clinical significance of a pH difference between 5.72 and 5.53 is questionable, especially since there was a greater difference in baseline pH between the two cohorts (6.08 in the 4-cycle group vs 5.59 in the 3-cycle group).
There were no reported adverse events related to the fractional laser treatments, and 6% of the patients underwent additional laser treatments during the followup timeframe of 8 to 20 months.
This was a retrospective study with no control or comparison group and short-term follow-up. The VAS scores were obtained 1 month after the final treatment. Failure to request additional treatment at 8 to 20 months cannot be used to infer that the therapeutic improvements recorded at 1 month were enduring. In addition, although the large number of patients in this study may lead to statistical significance, clinical significance is still questionable. Given the lack of a comparison group and the very short follow-up, it is hard to draw any scientifically valid conclusions from this study.
Continue to: Randomized data on CO2 laser vs Kegels for sexual dysfunction...
Randomized data on CO2 laser vs Kegels for sexual dysfunction
Lou W, Chen F, Xu T, et al. A randomized controlled study of vaginal fractional CO2 laser therapy for female sexual dysfunction. Lasers Med Sci. March 15, 2021. doi: 10.1007/s10103-021-03260-x.
In a small randomized controlled trial (RCT) conducted in China, Lou and colleagues identified premenopausal women at “high risk” for sexual dysfunction as determined by the Chinese version of the Female Sexual Function Index (CFSFI).
Details of the study
A total of 84 women (mean age, 36.5 years) were included in the study. All the participants were heterosexual and married or with a long-term partner. The domain of sexual dysfunction was not considered. Women were excluded if they had no current heterosexual partner; had genital malformation, urinary incontinence, or prolapse stage 2 or higher; a history of pelvic floor mesh treatment; current gynecologic malignancy; abnormal cervical cytology; or were currently pregnant or postpartum. In addition, women were excluded if they had been treated previously for sexual dysfunction or mental “disease.” The cohort was randomized to receive fractional CO2 laser treatments (three 15-minute treatments 1 month apart at 60W, 1,000 microseconds) or coached Kegel exercises (10 exercises repeated twice daily at least 3 times/week and monitored by physical therapists at biweekly clinic visits). Sexual distress was evaluated by using the Female Sexual Distress Scale-Revised (FSDSR). Outcomes measured were pelvic floor muscle strength and scores on the CFSFI and FSDSR. Data were obtained at 3, 6, 9, and 12 months after initiation of therapy.
Both groups showed improvement
The laser cohort showed slightly more improvement in scale scores at 6 and 12 months. Specifically, the laser group had better scores on lubrication and overall satisfaction, with moderate effect size; neither group had improvements in arousal, desire, or orgasm. The Kegel group showed a significant improvement in pelvic floor strength and orgasm at 12 months, an improvement not seen in the laser cohort. Both groups showed gradual improvement in the FSDSR, with the laser group reporting a lower score (10.0) at 12 months posttreatment relative to the Kegel group (11.1). Again, these were modest effects as baseline scores for both cohorts were around 12.5. There were minimal safety signals in the laser group, with 22.5% of women reporting scant bloody discharge posttreatment and 72.5% describing mild discomfort (1 on a 1–10 VAS scale) during the procedure.
This study is problematic in several areas. Although it was a prospective, randomized trial, it was not blinded, and the therapeutic interventions were markedly different in nature and requirement for individual patient motivation. The experiences of sexual dysfunction among the participants were not stratified by type—arousal, desire, lubrication, orgasm, or pain. All patients had regular cyclic menses; however, the authors do not report on contraceptive methods, hormonal therapy, or other comorbid conditions that could impact sexual health. The cohorts may or may not have been similar in baseline types of sexual dissatisfaction.
CO2 laser for lichen sclerosus: Is it effective?
Pagano T, Conforti A, Buonfantino C, et al. Effect of rescue fractional microablative CO2 laser on symptoms and sexual dysfunction in women affected by vulvar lichen sclerosus resistant to long-term use of topic corticosteroid: a prospective longitudinal study. Menopause. 2020;27:418-422. doi: 10.1097 /GME.0000000000001482.
Burkett LS, Siddique M, Zeymo A, et al. Clobetasol compared with fractionated carbon dioxide laser for lichen sclerosus: a randomized controlled trial. Obstet Gynecol. 2021;137:968-978. doi: 10.1097 /AOG.0000000000004332.
Mitchell L, Goldstein AT, Heller D, et al. Fractionated carbon dioxide laser for the treatment of vulvar lichen sclerosus: a randomized controlled trial. Obstet Gynecol. 2021;137:979-987. doi: 10.1097 /AOG.0000000000004409.
High potency corticosteroid ointment is the current standard treatment for lichen sclerosus. Alternative options for disease that is refractory to steroids are limited. Three studies published in the past year explored the CO2 laser’s ability to treat lichen sclerosus symptoms and resultant sexual dysfunction—Pagano and colleagues conducted a small prospective study and Burkett and colleagues and Mitchell et al conducted small RCTs.
Details of the Pagano study
Three premenopausal and 37 postmenopausal women with refractory lichen sclerosus (defined as no improvement after 4 cycles of ultra-high potency steroids) were included in the study. Lichen sclerosus was uniformly biopsy confirmed. Women using topical or systemic hormones were excluded. VAS was administered prior to initial treatment and after each of 2 fractional CO2 treatments (25–30 W; 1,000 microseconds) 30 to 40 days apart to determine severity of vulvar itching, dyspareunia with penetration, vulvar dryness, sexual dysfunction, and procedure discomfort. Follow-up was conducted at 1 month after the final treatment. VAS score for the primary outcome of vulvar itching declined from 8 pretreatment to 6 after the first treatment and to 3 after the second. There was no significant treatment-related pain reported.
The authors acknowledged the limitations of their study; it was a relatively small sample size, nonrandomized and had short-term follow-up of a mixed patient population and no sham or control group. The short-term improvements reported in the study patients may not be sustained without ongoing treatment for a lifelong chronic disease, and the long-term potential for development of squamous cell carcinoma may or may not be ameliorated.
Continue to: Burkett et al: RCT study 1...
Burkett et al: RCT study 1
A total of 52 postmenopausal patients with biopsy-proven lichen sclerosus were randomly assigned to clobetasol or CO2 laser; 51 women completed 6-month follow-up. The outcomes were stratified by prior high-potency steroid use. The steroid cohort used clobetasol 0.05% nightly for 1 month, 3 times per week for 2 months, then as needed. The laser cohort received 3 treatments (26 W; 800 microseconds) 4 to 6 weeks apart. Overall adherence was only 75% in the clobetasol group, compared with 96% in the laser group. The authors found treatment efficacy of CO2 laser therapy only in the group of patients who had prior treatment with high potency topical corticosteroids. They conclude that, …“Despite previously optimistic results in well designed clinical trials of fractionated CO2 for genitourinary syndrome of menopause, and in noncontrolled case series for vulvar lichen sclerosus, our study failed to show any significant benefit of monotherapy of fractionated CO2 for vulvar lichen sclerosus. There may be a role for fractionated CO2 as an adjuvant therapy along with topical ultrapotent corticosteroids in vulvar lichen sclerosus.”
Mitchell et al: RCT study 2
This was a double blind, placebo-controlled, and histologically validated study of fractional CO2 for treatment of lichen sclerosus in 35 women; 17 in the treatment arm and 18 in the sham laser encounters. At least a 4-week no treatment period of topical steroids was required before monotherapy with CO2 laser was initiated.
The authors found no difference in their primary outcome—histopathology scale scores—after 5 treatments over 24 weeks. Secondary endpoints were changes in the CSS (Clinical Scoring System for Vulvar Lichen Sclerosus), a validated instrument that includes both a clinician’s examination of the severity of disease and a patient’s report of the severity of her symptoms. The patient score is the total of 4 domains: itching, soreness, burning, and dyspareunia. The clinician objective examination documents fissures, erosions, hyperkeratosis, agglutination, stenosis, and atrophy. At the conclusion of treatment there were no significant differences in the patient reported symptoms or the clinical findings between the active treatment and sham groups.
As a monotherapy, CO2 laser therapy is not effective in treating lichen sclerosus, although it may help improve symptoms as an adjunct to high potency steroid therapy when topical treatment alone has failed to provide adequate response.
Conclusion
The quality of evidence to support the use of the CO2 laser for improvement in sexual dysfunction is poor. Although patient satisfaction scores improved overall, and most specifically for symptoms related to GSM, the lack of blinding; inappropriate or no control groups; the very short-term outcomes; and for one of the studies, the lack of a clear definition of sexual dysfunction, make it difficult to draw meaningful conclusions for clinical care.
For GSM, we know that topical estrogen therapy works—and with little to no systemic absorption. The CO2 laser should be studied in comparison to this gold standard, with consideration of costs and potential long-term harms in addition to patient satisfaction and short-term measures of improvement. In addition, and very importantly, it is our professional responsibility to present the evidence for safety of topical estrogens to our professional colleagues as well as to our patients with estrogen-dependent cancers so that they understand the value of estrogen as a safe and appropriate alternative to expensive and potentially short-term interventions such as CO2 laser treatment. ●

Cheryl Iglesia, MD
Dr. Iglesia is Director, Section of Female Pelvic Medicine and Reconstructive Surgery, MedStar Washington Hospital Center, and Professor, Departments of ObGyn and Urology, Georgetown University School of Medicine, Washington, DC. She is a member of the OBG Management Board of Editors.
Barbara Levy, MD: Cheryl, you have more experience with use of the energy-based cosmetic laser than most ObGyns, and I thought that speaking with you about this technology would be of benefit, not only to me in learning more about the hands-on experience of a lead researcher and practitioner but also readers who are hearing more and more about the growth of cosmetic gynecology in general. Thank you for taking the time today.
Cheryl Iglesia, MD: I’m happy to speak about this with you, Barbara.
Dr. Levy: Specifically, I would like to talk about use of these technologies for sexual dysfunction. In the last few years some of the available data have been on the CO2 laser versus physical therapy, which is not an appropriate comparison.1
Dr. Iglesia: There have been limited data, and less randomized, controlled data, on laser and radiofrequency energies for cosmetic gynecology, and in fact these devices remain unapproved for any gynecologic indication. In 2018 the US Food and Drug Administration (FDA) issued a Safety Communication about the use of energy-based devices to perform vaginal rejuvenation or cosmetic procedures. The International Urogynecological Association (IUGA) issued a consensus statement echoing concerns about the devices, and an International Continence Society/International Society for the Study of Vulvovaginal Disease Best Practice Consensus Statement did not recommend the laser for “routine treatment of vaginal atrophy or urinary incontinence unless treatment is part of a well-designed trial or with special arrangements for clinical governance, consent, and audit.”2
In May 2020, as evidence remains limited (although 522 studies are ongoing in coordination with the FDA), the American Urogynecologic Society (AUGS) published a clinical consensus statement from a panel of experts in female pelvic medicine and reconstructive surgery. The panel had about 90% consensus that there is short-term efficacy for the laser with GSM and dyspareunia. But we only have outcomes data that lasts a maximum of 1 year.2
A problem with our VeLVET trial,3 which was published in Menopause, and the Cruz and colleagues’ trial from South America,4 both of which compared the CO2 laser to estrogen and had randomized groups, was that they were limited by the outcome measures used, none of which were consistently validated. But these studies also had small numbers of participants and short-term follow-up. So I don’t think there are much existing data that are promising for supporting energy-based treatment for GSM.
We also have just-published data on the laser for lichen sclerosus.5 For the AUGS panel, there was about 80% consensus for energy-based-device use and lichen sclerosus.2 According to Mitchell et al, who conducted a small, randomized, sham-controlled trial, CO2 laser resulted in no significant difference in histopathology scale score between active and sham arms.5
Future trials may want to assess laser as a mechanism for improved local drug delivery (eg, use of combined laser plus local estrogen for GSM or combined laser plus topical steroid for lichen sclerosus). I am also aware that properly designed laser versus sham studies are underway.
Dr. Levy: What about for stress urinary incontinence (SUI)? I don’t think these technologies are going to work.
Dr. Iglesia: For the AUGS panel, there was only about 70% consensus for energy-based-device use and SUI,2 and I’m one of the naysayers. The pathophysiology of SUI is so multifactorial that it’s hard to believe that laser or radiofrequency wand therapy could have sustained improvements, especially since prior radiofrequency therapy from the last decade (for instance, Renessa, Novasys Medical) did not show long-term efficacy.
Understanding lasers and coordinating care
Dr. Levy: We don’t know what the long-term outcomes are for the CO2 laser and GSM.
Dr. Iglesia: I agree with you, and I think there needs to be an understanding of the mechanism of how lasers work, whether it be erbium (Er:YAG), which is the most common, or CO2. Erbium and CO2 lasers, which are on the far-infrared spectrum, target the chromophore, water. My feeling is that, when you look at results from the Cruz trial,4 or even our trial that compared vaginal estrogen with laser,3 when there is severe GSM and high pH with virtually no water present in the tissues, that laser is not going to properly function. But I don’t think we know exactly what optimal pretreatment is necessary, and that is one of the problems. Furthermore, when intravaginal lasers are done and no adequate speculum exam is conducted prior to introducing the laser, there could be discharge or old creams present that block the mirrors necessary to adequately fire the fractionated laser beams.
Unfortunately, oftentimes these devices are marketed to women with breast cancer, who may be taking aromatase inhibitors, which cause the no-water problem; they dry out everything. They are effective for preventing breast cancer recurrence, but they cause severe atrophy (perhaps worse than many of the other selective estrogen-receptor modulators), with a resultant high vaginal pH. If we can bring that pH level down, closer to the normal 4.5 range so that we could have some level of moisture, and add estrogen first, the overall treatment approach will probably be more effective. We still do not know what happens after 1 year, though, and how often touch-ups need to be performed.
In fact, when working with a patient with breast cancer, I will speak with her oncologist; I will collaborate to put in place a treatment plan that may include initial pretreatment with low-dose vaginal estrogen followed by laser treatment for vaginal atrophy. But I will make sure I use the lowest dose. Sometimes when the patient comes back, the estrogen’s worked so well she’ll say, “Oh, I’m happy, so I don’t need the laser anymore.” A balanced conversation is necessary, especially with cancer survivors.
Informing patients and colleagues
Dr. Levy: I completely agree, and I think one of the key points here is that our purpose is to serve our patients. The data demonstrate that low doses of vaginal estrogen are not harmful for women who are being treated for or who have recovered from breast cancer. It is our ethical obligation to convince these women and their oncologists that ongoing treatment with vaginal estrogen not only will help their GSM but also their overactive bladder and their risk of urinary tract infections and other things. We could be exploiting patients who are really fearful of using any estrogen because of a perceived cancer risk. We could actually be validating their fear by telling them we have an alternative treatment for which they have to pay cash.
Treatment access
Dr. Iglesia: Yes, these are not cosmetic conditions that we are treating. So my goal when evaluating treatment for refractory GSM or lichen sclerosus is to find optimal energy-based therapies with the hope that one day these will be approved gynecologic conditions by the US FDA for laser and wand therapies and that they will ultimately not be out-of-pocket expenses but rather therapies covered by insurance.
Dr. Levy: Great. I understand that AUGS/IUGA have been working on a terminology algorithm to help distinguish between procedures being performed to resolve a medical problem such as prolapse or incontinence versus those designed to be cosmetic.
Dr. Iglesia: Yes, there is a big document from experts in both societies out for public comment right now. It will hopefully be published soon.
Outstanding questions remain
Dr. Levy: Really, we as ObGyns shouldn’t be quick to incorporate these things into our practices without high-quality studies demonstrating value. I have a major concern about these devices in the long term. When you look at fractional CO2 use on the face, for instance, which is a much different type of skin than the vagina, the laser builds collagen—but we don’t have long-term outcome results. The vagina is supposed to be an elastic tissue, so what is the risk of long-term scarring there? Yes, the laser builds collagen in the vaginal epithelium, but what does it do to scarring in the rest of the tissue? We don’t have answers to that.
Dr. Iglesia: And that is the question—how does histology equate with function? Well, I would go with what the patients are reporting.
Dr. Levy: Absolutely. But the thing about vaginal low-dose estrogen is that it is something that the oncologists or the ObGyns could be implementing with patients while they are undergoing cancer therapy, while in their menopausal transition, to preserve vulvovaginal function as opposed to trying to regain it.
Dr. Iglesia: Certainly, although it still needs to be determined when that type of approach would actually be contraindicated.
Dr. Levy: Thank you, Cheryl, for your valuable insights.
Dr. Iglesia: Of course. Thank you. ●
References
1. Lou W, Chen F, Xu T, et al. A randomized controlled study of vaginal fractional CO2 laser therapy for female sexual dysfunction. Lasers Med Sci. March 15, 2021. doi: 10.1007/s10103-021-03260-x.
2. Alshiek J, Garcia B, Minassian V, et al. Vaginal energy-based devices. Female Pelvic Med Reconstr Surg. 2020;26:287-298. doi: 10.1097 /SPV.0000000000000872.
3. Paraiso MF, Ferrando CA, et al. A randomized clinical trial comparing vaginal laser therapy to vaginal estrogen therapy in women with genitourinary syndrome of menopause: the VeLVET Trial. Menopause. 2020;27:50-56. doi: 10.1097/GME.0000000000001416.
4. Cruz VL, Steiner ML, et al. Randomized, double-blind, placebo-controlled clinical trial for evaluating the efficacy of fractional CO2 laser compared with topical estriol in the treatment of vaginal atrophy in postmenopausal women. Menopause. 2018;25:21-28. doi: 10.1097 /GME.0000000000000955.
5. Mitchell L, Goldstein A, Heller D, et al. Fractionated carbon dioxide laser for the treatment of vulvar lichen sclerosus: a randomized controlled trial. Obstet Gynecol. 2021;137:979-987. doi: 10.1097/AOG.0000000000004409.

The approach to diagnosis and treatment of female sexual function continues to be a challenge for women’s health professionals. The search for a female “little blue pill” remains elusive as researchers struggle to understand the mechanisms that underlie the complex aspects of female sexual health. This Update will review the recent literature on the use of fractional CO2 laser for treatment of female sexual dysfunction and vulvovaginal symptoms. Bottom line: While the quality of the studies is poor overall, fractional CO2 laser treatment seems to temporarily improve symptoms of genitourinary syndrome of menopause (GSM). The duration of response, cost, and the overall long-term impact on sexual health remain in question.
A retrospective look at CO2 laser and postmenopausal GSM
Filippini M, Luvero D, Salvatore S, et al. Efficacy of fractional CO2 laser treatment in postmenopausal women with genitourinary syndrome: a multicenter study. Menopause. 2019;27:43-49. doi: 10.1097/GME. 0000000000001428.
Researchers conducted a retrospective, multicenter study of postmenopausal women with at least one symptom of GSM, including itching, burning, dyspareunia with penetration, and dryness.
Study details
A total of 171 of the 645 women (26.5%) were oncology patients. Women were excluded from analysis if they used any form of topical therapy within 15 days; had prolapse stage 2 or greater; or had any infection, abscess, or anatomical deformity precluding treatment with the laser.
Patients underwent gynecologic examination and were given a questionnaire to assess vulvovaginal symptoms. Exams occurred monthly during treatment (average, 6.5 months), at 6- and 12-months posttreatment, and then annually. No topical therapy was advised during or after treatment.
Patients received either 3 or 4 fractional CO2 laser treatments to the vulva and/or vagina depending on symptom location and type. Higher power settings of the same laser were used to treat vaginal symptoms (40W; 1,000 microseconds) versus vulvar symptoms (25W; 500 microseconds). Treatment sessions were 5 to 6 minutes. The study authors used a visual analog rating scale (VAS) for “atrophy and related symptoms,” tested vaginal pH, and completed the Vaginal Health Index Score. VAS scores were obtained from the patients prior to the initial laser intervention and 1 month after the final treatment.
Results
There were statistically significant improvements in dryness, vaginal orifice pain, dyspareunia, itching, and burning for both the 3-treatment and 4-treatment cohorts. The delta of improvement was then compared for the 2 subgroups; curiously, there was greater improvement of symptoms such as dryness (65% vs 61%), itching (78% vs 72%), burning (72% vs 67%), and vaginal orifice pain (67% vs 60%) in the group that received 3 cycles than in the group that received 4 cycles.
With regard to vaginal pH improvement, the 4-cycle group performed better than the 3-cycle group (1% improvement in the 4-cycle group vs 6% in the 3-cycle group). Although vaginal pH reduction was somewhat better in the group that received 4 treatments, and the pre versus posttreatment percentages were statistically significantly different, the clinical significance of a pH difference between 5.72 and 5.53 is questionable, especially since there was a greater difference in baseline pH between the two cohorts (6.08 in the 4-cycle group vs 5.59 in the 3-cycle group).
There were no reported adverse events related to the fractional laser treatments, and 6% of the patients underwent additional laser treatments during the followup timeframe of 8 to 20 months.
This was a retrospective study with no control or comparison group and short-term follow-up. The VAS scores were obtained 1 month after the final treatment. Failure to request additional treatment at 8 to 20 months cannot be used to infer that the therapeutic improvements recorded at 1 month were enduring. In addition, although the large number of patients in this study may lead to statistical significance, clinical significance is still questionable. Given the lack of a comparison group and the very short follow-up, it is hard to draw any scientifically valid conclusions from this study.
Continue to: Randomized data on CO2 laser vs Kegels for sexual dysfunction...
Randomized data on CO2 laser vs Kegels for sexual dysfunction
Lou W, Chen F, Xu T, et al. A randomized controlled study of vaginal fractional CO2 laser therapy for female sexual dysfunction. Lasers Med Sci. March 15, 2021. doi: 10.1007/s10103-021-03260-x.
In a small randomized controlled trial (RCT) conducted in China, Lou and colleagues identified premenopausal women at “high risk” for sexual dysfunction as determined by the Chinese version of the Female Sexual Function Index (CFSFI).
Details of the study
A total of 84 women (mean age, 36.5 years) were included in the study. All the participants were heterosexual and married or with a long-term partner. The domain of sexual dysfunction was not considered. Women were excluded if they had no current heterosexual partner; had genital malformation, urinary incontinence, or prolapse stage 2 or higher; a history of pelvic floor mesh treatment; current gynecologic malignancy; abnormal cervical cytology; or were currently pregnant or postpartum. In addition, women were excluded if they had been treated previously for sexual dysfunction or mental “disease.” The cohort was randomized to receive fractional CO2 laser treatments (three 15-minute treatments 1 month apart at 60W, 1,000 microseconds) or coached Kegel exercises (10 exercises repeated twice daily at least 3 times/week and monitored by physical therapists at biweekly clinic visits). Sexual distress was evaluated by using the Female Sexual Distress Scale-Revised (FSDSR). Outcomes measured were pelvic floor muscle strength and scores on the CFSFI and FSDSR. Data were obtained at 3, 6, 9, and 12 months after initiation of therapy.
Both groups showed improvement
The laser cohort showed slightly more improvement in scale scores at 6 and 12 months. Specifically, the laser group had better scores on lubrication and overall satisfaction, with moderate effect size; neither group had improvements in arousal, desire, or orgasm. The Kegel group showed a significant improvement in pelvic floor strength and orgasm at 12 months, an improvement not seen in the laser cohort. Both groups showed gradual improvement in the FSDSR, with the laser group reporting a lower score (10.0) at 12 months posttreatment relative to the Kegel group (11.1). Again, these were modest effects as baseline scores for both cohorts were around 12.5. There were minimal safety signals in the laser group, with 22.5% of women reporting scant bloody discharge posttreatment and 72.5% describing mild discomfort (1 on a 1–10 VAS scale) during the procedure.
This study is problematic in several areas. Although it was a prospective, randomized trial, it was not blinded, and the therapeutic interventions were markedly different in nature and requirement for individual patient motivation. The experiences of sexual dysfunction among the participants were not stratified by type—arousal, desire, lubrication, orgasm, or pain. All patients had regular cyclic menses; however, the authors do not report on contraceptive methods, hormonal therapy, or other comorbid conditions that could impact sexual health. The cohorts may or may not have been similar in baseline types of sexual dissatisfaction.
CO2 laser for lichen sclerosus: Is it effective?
Pagano T, Conforti A, Buonfantino C, et al. Effect of rescue fractional microablative CO2 laser on symptoms and sexual dysfunction in women affected by vulvar lichen sclerosus resistant to long-term use of topic corticosteroid: a prospective longitudinal study. Menopause. 2020;27:418-422. doi: 10.1097 /GME.0000000000001482.
Burkett LS, Siddique M, Zeymo A, et al. Clobetasol compared with fractionated carbon dioxide laser for lichen sclerosus: a randomized controlled trial. Obstet Gynecol. 2021;137:968-978. doi: 10.1097 /AOG.0000000000004332.
Mitchell L, Goldstein AT, Heller D, et al. Fractionated carbon dioxide laser for the treatment of vulvar lichen sclerosus: a randomized controlled trial. Obstet Gynecol. 2021;137:979-987. doi: 10.1097 /AOG.0000000000004409.
High potency corticosteroid ointment is the current standard treatment for lichen sclerosus. Alternative options for disease that is refractory to steroids are limited. Three studies published in the past year explored the CO2 laser’s ability to treat lichen sclerosus symptoms and resultant sexual dysfunction—Pagano and colleagues conducted a small prospective study and Burkett and colleagues and Mitchell et al conducted small RCTs.
Details of the Pagano study
Three premenopausal and 37 postmenopausal women with refractory lichen sclerosus (defined as no improvement after 4 cycles of ultra-high potency steroids) were included in the study. Lichen sclerosus was uniformly biopsy confirmed. Women using topical or systemic hormones were excluded. VAS was administered prior to initial treatment and after each of 2 fractional CO2 treatments (25–30 W; 1,000 microseconds) 30 to 40 days apart to determine severity of vulvar itching, dyspareunia with penetration, vulvar dryness, sexual dysfunction, and procedure discomfort. Follow-up was conducted at 1 month after the final treatment. VAS score for the primary outcome of vulvar itching declined from 8 pretreatment to 6 after the first treatment and to 3 after the second. There was no significant treatment-related pain reported.
The authors acknowledged the limitations of their study; it was a relatively small sample size, nonrandomized and had short-term follow-up of a mixed patient population and no sham or control group. The short-term improvements reported in the study patients may not be sustained without ongoing treatment for a lifelong chronic disease, and the long-term potential for development of squamous cell carcinoma may or may not be ameliorated.
Continue to: Burkett et al: RCT study 1...
Burkett et al: RCT study 1
A total of 52 postmenopausal patients with biopsy-proven lichen sclerosus were randomly assigned to clobetasol or CO2 laser; 51 women completed 6-month follow-up. The outcomes were stratified by prior high-potency steroid use. The steroid cohort used clobetasol 0.05% nightly for 1 month, 3 times per week for 2 months, then as needed. The laser cohort received 3 treatments (26 W; 800 microseconds) 4 to 6 weeks apart. Overall adherence was only 75% in the clobetasol group, compared with 96% in the laser group. The authors found treatment efficacy of CO2 laser therapy only in the group of patients who had prior treatment with high potency topical corticosteroids. They conclude that, …“Despite previously optimistic results in well designed clinical trials of fractionated CO2 for genitourinary syndrome of menopause, and in noncontrolled case series for vulvar lichen sclerosus, our study failed to show any significant benefit of monotherapy of fractionated CO2 for vulvar lichen sclerosus. There may be a role for fractionated CO2 as an adjuvant therapy along with topical ultrapotent corticosteroids in vulvar lichen sclerosus.”
Mitchell et al: RCT study 2
This was a double blind, placebo-controlled, and histologically validated study of fractional CO2 for treatment of lichen sclerosus in 35 women; 17 in the treatment arm and 18 in the sham laser encounters. At least a 4-week no treatment period of topical steroids was required before monotherapy with CO2 laser was initiated.
The authors found no difference in their primary outcome—histopathology scale scores—after 5 treatments over 24 weeks. Secondary endpoints were changes in the CSS (Clinical Scoring System for Vulvar Lichen Sclerosus), a validated instrument that includes both a clinician’s examination of the severity of disease and a patient’s report of the severity of her symptoms. The patient score is the total of 4 domains: itching, soreness, burning, and dyspareunia. The clinician objective examination documents fissures, erosions, hyperkeratosis, agglutination, stenosis, and atrophy. At the conclusion of treatment there were no significant differences in the patient reported symptoms or the clinical findings between the active treatment and sham groups.
As a monotherapy, CO2 laser therapy is not effective in treating lichen sclerosus, although it may help improve symptoms as an adjunct to high potency steroid therapy when topical treatment alone has failed to provide adequate response.
Conclusion
The quality of evidence to support the use of the CO2 laser for improvement in sexual dysfunction is poor. Although patient satisfaction scores improved overall, and most specifically for symptoms related to GSM, the lack of blinding; inappropriate or no control groups; the very short-term outcomes; and for one of the studies, the lack of a clear definition of sexual dysfunction, make it difficult to draw meaningful conclusions for clinical care.
For GSM, we know that topical estrogen therapy works—and with little to no systemic absorption. The CO2 laser should be studied in comparison to this gold standard, with consideration of costs and potential long-term harms in addition to patient satisfaction and short-term measures of improvement. In addition, and very importantly, it is our professional responsibility to present the evidence for safety of topical estrogens to our professional colleagues as well as to our patients with estrogen-dependent cancers so that they understand the value of estrogen as a safe and appropriate alternative to expensive and potentially short-term interventions such as CO2 laser treatment. ●

Cheryl Iglesia, MD
Dr. Iglesia is Director, Section of Female Pelvic Medicine and Reconstructive Surgery, MedStar Washington Hospital Center, and Professor, Departments of ObGyn and Urology, Georgetown University School of Medicine, Washington, DC. She is a member of the OBG Management Board of Editors.
Barbara Levy, MD: Cheryl, you have more experience with use of the energy-based cosmetic laser than most ObGyns, and I thought that speaking with you about this technology would be of benefit, not only to me in learning more about the hands-on experience of a lead researcher and practitioner but also readers who are hearing more and more about the growth of cosmetic gynecology in general. Thank you for taking the time today.
Cheryl Iglesia, MD: I’m happy to speak about this with you, Barbara.
Dr. Levy: Specifically, I would like to talk about use of these technologies for sexual dysfunction. In the last few years some of the available data have been on the CO2 laser versus physical therapy, which is not an appropriate comparison.1
Dr. Iglesia: There have been limited data, and less randomized, controlled data, on laser and radiofrequency energies for cosmetic gynecology, and in fact these devices remain unapproved for any gynecologic indication. In 2018 the US Food and Drug Administration (FDA) issued a Safety Communication about the use of energy-based devices to perform vaginal rejuvenation or cosmetic procedures. The International Urogynecological Association (IUGA) issued a consensus statement echoing concerns about the devices, and an International Continence Society/International Society for the Study of Vulvovaginal Disease Best Practice Consensus Statement did not recommend the laser for “routine treatment of vaginal atrophy or urinary incontinence unless treatment is part of a well-designed trial or with special arrangements for clinical governance, consent, and audit.”2
In May 2020, as evidence remains limited (although 522 studies are ongoing in coordination with the FDA), the American Urogynecologic Society (AUGS) published a clinical consensus statement from a panel of experts in female pelvic medicine and reconstructive surgery. The panel had about 90% consensus that there is short-term efficacy for the laser with GSM and dyspareunia. But we only have outcomes data that lasts a maximum of 1 year.2
A problem with our VeLVET trial,3 which was published in Menopause, and the Cruz and colleagues’ trial from South America,4 both of which compared the CO2 laser to estrogen and had randomized groups, was that they were limited by the outcome measures used, none of which were consistently validated. But these studies also had small numbers of participants and short-term follow-up. So I don’t think there are much existing data that are promising for supporting energy-based treatment for GSM.
We also have just-published data on the laser for lichen sclerosus.5 For the AUGS panel, there was about 80% consensus for energy-based-device use and lichen sclerosus.2 According to Mitchell et al, who conducted a small, randomized, sham-controlled trial, CO2 laser resulted in no significant difference in histopathology scale score between active and sham arms.5
Future trials may want to assess laser as a mechanism for improved local drug delivery (eg, use of combined laser plus local estrogen for GSM or combined laser plus topical steroid for lichen sclerosus). I am also aware that properly designed laser versus sham studies are underway.
Dr. Levy: What about for stress urinary incontinence (SUI)? I don’t think these technologies are going to work.
Dr. Iglesia: For the AUGS panel, there was only about 70% consensus for energy-based-device use and SUI,2 and I’m one of the naysayers. The pathophysiology of SUI is so multifactorial that it’s hard to believe that laser or radiofrequency wand therapy could have sustained improvements, especially since prior radiofrequency therapy from the last decade (for instance, Renessa, Novasys Medical) did not show long-term efficacy.
Understanding lasers and coordinating care
Dr. Levy: We don’t know what the long-term outcomes are for the CO2 laser and GSM.
Dr. Iglesia: I agree with you, and I think there needs to be an understanding of the mechanism of how lasers work, whether it be erbium (Er:YAG), which is the most common, or CO2. Erbium and CO2 lasers, which are on the far-infrared spectrum, target the chromophore, water. My feeling is that, when you look at results from the Cruz trial,4 or even our trial that compared vaginal estrogen with laser,3 when there is severe GSM and high pH with virtually no water present in the tissues, that laser is not going to properly function. But I don’t think we know exactly what optimal pretreatment is necessary, and that is one of the problems. Furthermore, when intravaginal lasers are done and no adequate speculum exam is conducted prior to introducing the laser, there could be discharge or old creams present that block the mirrors necessary to adequately fire the fractionated laser beams.
Unfortunately, oftentimes these devices are marketed to women with breast cancer, who may be taking aromatase inhibitors, which cause the no-water problem; they dry out everything. They are effective for preventing breast cancer recurrence, but they cause severe atrophy (perhaps worse than many of the other selective estrogen-receptor modulators), with a resultant high vaginal pH. If we can bring that pH level down, closer to the normal 4.5 range so that we could have some level of moisture, and add estrogen first, the overall treatment approach will probably be more effective. We still do not know what happens after 1 year, though, and how often touch-ups need to be performed.
In fact, when working with a patient with breast cancer, I will speak with her oncologist; I will collaborate to put in place a treatment plan that may include initial pretreatment with low-dose vaginal estrogen followed by laser treatment for vaginal atrophy. But I will make sure I use the lowest dose. Sometimes when the patient comes back, the estrogen’s worked so well she’ll say, “Oh, I’m happy, so I don’t need the laser anymore.” A balanced conversation is necessary, especially with cancer survivors.
Informing patients and colleagues
Dr. Levy: I completely agree, and I think one of the key points here is that our purpose is to serve our patients. The data demonstrate that low doses of vaginal estrogen are not harmful for women who are being treated for or who have recovered from breast cancer. It is our ethical obligation to convince these women and their oncologists that ongoing treatment with vaginal estrogen not only will help their GSM but also their overactive bladder and their risk of urinary tract infections and other things. We could be exploiting patients who are really fearful of using any estrogen because of a perceived cancer risk. We could actually be validating their fear by telling them we have an alternative treatment for which they have to pay cash.
Treatment access
Dr. Iglesia: Yes, these are not cosmetic conditions that we are treating. So my goal when evaluating treatment for refractory GSM or lichen sclerosus is to find optimal energy-based therapies with the hope that one day these will be approved gynecologic conditions by the US FDA for laser and wand therapies and that they will ultimately not be out-of-pocket expenses but rather therapies covered by insurance.
Dr. Levy: Great. I understand that AUGS/IUGA have been working on a terminology algorithm to help distinguish between procedures being performed to resolve a medical problem such as prolapse or incontinence versus those designed to be cosmetic.
Dr. Iglesia: Yes, there is a big document from experts in both societies out for public comment right now. It will hopefully be published soon.
Outstanding questions remain
Dr. Levy: Really, we as ObGyns shouldn’t be quick to incorporate these things into our practices without high-quality studies demonstrating value. I have a major concern about these devices in the long term. When you look at fractional CO2 use on the face, for instance, which is a much different type of skin than the vagina, the laser builds collagen—but we don’t have long-term outcome results. The vagina is supposed to be an elastic tissue, so what is the risk of long-term scarring there? Yes, the laser builds collagen in the vaginal epithelium, but what does it do to scarring in the rest of the tissue? We don’t have answers to that.
Dr. Iglesia: And that is the question—how does histology equate with function? Well, I would go with what the patients are reporting.
Dr. Levy: Absolutely. But the thing about vaginal low-dose estrogen is that it is something that the oncologists or the ObGyns could be implementing with patients while they are undergoing cancer therapy, while in their menopausal transition, to preserve vulvovaginal function as opposed to trying to regain it.
Dr. Iglesia: Certainly, although it still needs to be determined when that type of approach would actually be contraindicated.
Dr. Levy: Thank you, Cheryl, for your valuable insights.
Dr. Iglesia: Of course. Thank you. ●
References
1. Lou W, Chen F, Xu T, et al. A randomized controlled study of vaginal fractional CO2 laser therapy for female sexual dysfunction. Lasers Med Sci. March 15, 2021. doi: 10.1007/s10103-021-03260-x.
2. Alshiek J, Garcia B, Minassian V, et al. Vaginal energy-based devices. Female Pelvic Med Reconstr Surg. 2020;26:287-298. doi: 10.1097 /SPV.0000000000000872.
3. Paraiso MF, Ferrando CA, et al. A randomized clinical trial comparing vaginal laser therapy to vaginal estrogen therapy in women with genitourinary syndrome of menopause: the VeLVET Trial. Menopause. 2020;27:50-56. doi: 10.1097/GME.0000000000001416.
4. Cruz VL, Steiner ML, et al. Randomized, double-blind, placebo-controlled clinical trial for evaluating the efficacy of fractional CO2 laser compared with topical estriol in the treatment of vaginal atrophy in postmenopausal women. Menopause. 2018;25:21-28. doi: 10.1097 /GME.0000000000000955.
5. Mitchell L, Goldstein A, Heller D, et al. Fractionated carbon dioxide laser for the treatment of vulvar lichen sclerosus: a randomized controlled trial. Obstet Gynecol. 2021;137:979-987. doi: 10.1097/AOG.0000000000004409.
Dismantling racism in your personal and professional spheres
On May 25, 2020, George Floyd was murdered by a White police officer who held his knee on Floyd’s neck for nine and a half minutes. Nine and a half minutes. George Floyd was not the first Black person killed by law enforcement. He has not been the last. Much has been written about why Floyd’s murder sparked unprecedented worldwide outrage despite being far from unprecedented itself. We cannot be so naive as to think what happened was new, and we should not ignore the tireless work that so many have been doing to fight racism up to this point. But for many who have been stirred to do something for the first time, especially White people, the question has been,
“What do I do?” The answer is, do the work.
This article is centered on anti-Black racism with a focus on medicine. We recognize that there is racism against other minoritized groups. Each group deserves attention and to have their stories told. We recognize intersectionality and that an individual has multiple identities and that these may compound the marginalization they experience. This too deserves attention.
However, we cannot satisfactorily explore any of these concepts within the confines of a single article. Our intention is to use this forum to promote further conversation, specifically about anti-Black racism in medicine. We hope it compels you to begin learning to recognize and dismantle racism in yourself and your surroundings, both at home and at work.
Being a health care provider requires lifelong learning. If we practiced only what we learned in training, our patients could suffer. So we continually seek out updated research and guidelines to best treat our patients. Understanding how racism impacts your patients, colleagues, family, and friends is your responsibility as much as understanding guidelines for standards of care. We must resist the urge to feel this is someone else’s duty. It is the job of each and every one of us. We must do the work.
Race is real but it’s not biologic
It is imperative to understand that race is not a biologic category. Phenotypic differences between humans do not reliably map to racial categories. Racial categories themselves have morphed over the centuries, and interpretation of race has been litigated in this country since its founding.1 People who identify as a given race do not have inherent biology that is different from those who identify as another race. It may then be tempting to try to erase race from our thinking, and, indeed, the idea of being “color blind” was long worn as a badge of honor signifying a commitment to equality. So this is the tension: if race exists, it must be a biologic trait and with it must go other inherent traits. But if race is not a biologic entity, perhaps it is not real and, therefore, should be ignored. In fact, neither is true. Race is not based on genetic or biologic inheritance, but it is a social and political categorization that is real and has very real ramifications. As we will discuss further, race does have a biologic impact on individuals. The mechanism by which that happens is racism.
Continue to: What is racism, and who is racist?...
What is racism, and who is racist?
Various definitions of racism have been offered:
- prejudice, discrimination, or antagonism directed against a person or people on the basis of their membership in a particular racial or ethnic group, typically one that is minoritized or marginalized2
- a belief that race is a fundamental determinant of human traits and capacities and that racial differences produce an inherent superiority of a particular race3
- the systemic oppression of a racial group to the social, economic, and political advantage of another; a political or social system founded on racism and designed to execute its principles.3
The common themes in these definitions are power, hierarchy, and oppression. Racism is a fabricated system to justify and reinforce power for some and disenfranchisement for others based on race. The system is pervasive and beneficial to the group that it serves.
Ibram X. Kendi posits that all racism is structural racism: “‘Institutional racism’ and ‘structural racism’ and ‘systemic racism’ are redundant. Racism itself is institutional, structural, and systemic.”4 This is not saying that individuals don’t enact racism, but it emphasizes that racism is not the action of a “few bad apples.” Furthermore, it underscores that race was created to bolster power structures ensuring White dominance. The racism that has followed, in all of its forms, is both because these ideas were created in the first place and to perpetuate that ongoing power structure.4
Dorothy Roberts, JD, writes in her book Fatal Invention that, while grouping people and creating hierarchy has always happened amongst humans, there is a specific history in our country of using race to create and perpetuate the dominance of White people and the subjugation of Black people.
Kendi also asserts that there is no neutrality with regard to racism—there is racist and antiracist: “A racist: one who is supporting a racist policy through their actions or inaction or expressing a racist idea. An antiracist: one who is supporting an antiracist policy through their actions or expressing an antiracist idea.”4 He describes all people as moving in and out of being racist and antiracist, and states “being an antiracist requires persistent self-awareness, constant self-criticism, and regular self-examination.”4 In thinking about race and racism in this way, we all must grapple with our own racism, but in so doing are taking a step toward antiracism.
History is important
Among the most important things one can do in a journey to dismantle racism is learn the history of racism.
The infrastructure and institutions of our nation were created on a foundation of slavery, including the origins of American medicine and gynecology. Physicians in the antebellum South performed inspections of enslaved people’s bodies to certify them for sale.5 The ability to assign market value to a Black person’s body was published as an essential physician competency.5
Gynecology has a particularly painful history with regard to slavery. By 1808, transatlantic slave trade was banned in the United States and, as Dr. Cooper Owens describes in her book Medical Bondage: Race, Gender, and the Origins of American Gynecology, this made reproduction of enslaved people within the United States a priority for slave owners and those invested in an economy that depended on slavery.6 Gynecologists were permitted unrestricted access to enslaved women for experiments to optimize reproduction. Many of these physicians became prominent voices adding to the canon of racialized medicine. Medical journals themselves gained reverence because of heightened interest in keeping enslaved people alive and just well enough to work and reproduce.6 Today, we hold sacred the relationship between a patient and their physician. We must understand that there was no such relationship between a doctor and an enslaved person. The relationship was between the doctor and slave owner.6,7 Slavery does not allow for the autonomy of the enslaved. This is the context in which we must understand the discoveries of gynecologists during that time.
Despite the abolition of slavery with the passage of the 13th amendment, racist policies remained ubiquitous in the United States. Segregation of Black people was codified not only in the Jim Crow South but also in the North. Interracial marriage was outlawed by all but 9 states.
While there are numerous federal policies that led to cumulative and egregious disadvantage for Black Americans, one powerful example is redlining. In 1934 the Federal Housing Administration was created, and by insuring private mortgages, the FHA made it easier for eligible home buyers to obtain financing. The FHA used a system of maps that graded neighborhoods. Racial composition of neighborhoods was overtly used as a component of grading, and the presence of Black people led a neighborhood to be downgraded or redlined.8,9 This meant Black people were largely ineligible for FHA-backed loans. In The Color of Law, Richard Rothstein writes, “Today’s residential segregation in the North, South, Midwest, and West is not the unintended consequence of individual choices and of otherwise well-meaning law or regulation but of unhidden public policy that explicitly segregated every metropolitan area in the United States.The policy was so systematic and forceful that its effects endure to the present time.”9
Though these specific policies are no longer in place, many correlations have been found between historically redlined neighborhoods and higher rates of diseases today, including diabetes, hypertension, asthma, and preterm deliveries.10 These policies also have played a role in creating the wealth gap—directly by limiting the opportunity for home ownership, which translates to intergenerational wealth, and indirectly by the disinvestment in neighborhoods where Black people live, leading to reduced access to quality education, decreased employment opportunities, and increased environmental hazards.8,11
Continue to: Health disparities...
Health disparities
The numerous health disparities, more accurately termed health inequities, suffered by racial minority groups is well documented.12
COVID-19 death and vaccination-rate inequities. Early in the COVID-19 pandemic, data emerged that racial minorities were being disparately affected.13 In December 2020, the Centers for Disease Control and Prevention (CDC) reported that Hispanic or Latino, non-Hispanic Black, and non-Hispanic American Indian or Alaska Native people had all died at higher rates than White Americans.14 These racial groups had higher hospitalization rates across age groups and, after adjusting for age, rates of hospitalization were 2.8 to 3.4 times higher.15 We are continuing to learn what factors contribute to these inequities, but it has highlighted how racist policies have led to disparate access to health care, or even clean air, clean water, and nutritious food, and left communities of color more vulnerable to severe illness and death from COVID-19. With the advent of vaccines for COVID-19, we continue to see racial disparities as Black Americans have the lowest rates of vaccination.16 All of these inequities have to be understood in the context of the racist structures that exist in our society. As medical providers, we must understand and help to dismantle these structures.
Pregnancy-related mortality (PRM) inequities. A powerful example of a persistent health inequity in our field is the well-known disparity in pregnancy-related mortality when examining this outcome by race. Per CDC analysis of data on PRM from 2007–2016, Black women died at a rate 3.2 times higher than White women. This disparity was even greater in patients older than 30 years of age. When they compared rates while controlling for the highest level of education, the disparity is even more pronounced: PRM rate for those with a college degree or higher was 5.2 times greater for Black people compared with White people.16The CDC also reported that, in 2018, the infant mortality for non-Hispanic Black infants was 10.8 per 1,000 live births, compared with 4.6 per 1,000 live births for White infants. This is a rate 2.4-times higher for Black infants.17 Dr. Cooper Owens and Dr. Fett note in their article, “Black maternal and infant health: Historical legacies of slavery,” that in 1850 this rate was 1.6-times higher for Black infants, which means the inequity was worse in 2018 America than in the antebellum South.5
The role of patient experience
As discussed, governmental policies have created persistent inequities in wealth, access to health care, and exposure to environmental toxins, among many other disparities. However, the data finding that highly educated Black pregnant patients suffer markedly increased risk of maternal death, indicate that inequities cannot be attributed only to education or lack of access to health care. This is where some will once again lean on the idea that there is something inherently different about Black people. But if we know that race was created and is not an empiric category, we must consider the social variables impacting Black patients’ experience.
As Linda Blount, President and CEO of the Black Women’s Health Imperative, put it, “Race is not a risk factor. It is the lived experience of being a Black woman in this society that is the risk factor.”18 So how much of these inequities can be accounted for by differential treatment of Black patients? There is, for example, data on the disproportionately lower rates of Black renal transplant recipients and inordinately higher rates of amputations among Black patients.19,20 None of us wants to think we are treating our Black patients differently, but the data demand that we ask ourselves if we are. Some of this is built into the system. For example, in their article “Hidden in plain sight—Reconsidering the use of race correction in clinical algorithms,” Vyas and colleagues outline a list of calculators and algorithms that include race.21 This means we may be using these calculators and changing outcomes for our patients based on their race. This is only one example of racism hidden within guidelines and standards of care.
The existence of racism on an interpersonal level also cannot be denied. This could lead to differential care specifically, but also can manifest by way of the toll it takes on a patient generally. This is the concept of allostatic load or weathering: the chronic stress of experiencing racism creates detrimental physiologic change. There is ongoing research into epigenetic modifications from stress that could be impacting health outcomes in Black populations.
Continue to: What is the work we need to do?...
What is the work we need to do?
Become educated. We have discussed taking the initiative to learn about the history of racism, including the legacies of slavery and the ongoing impact of racism on health. This knowledge is foundational and sometimes transformative. It allows us to see opportunities for antiracism and gives us the knowledge to begin meaningful conversations.
Take action. We must take inventory within our lives. What are our spheres of influence? What are our resources? Where can we make an impact? Right now, you can take out a pen and paper and write down all the roles you play. Look for opportunities in personal interactions and daily routines. Unfortunately, there will be many opportunities to speak up against racism—although this is rarely easy. Find articles, podcasts, and workshops on upstander training. One framework to respond to microaggressions has been proposed by faculty at Boston University Medical Center using the acronym LIFT (Lights on, Impact vs Intent, Full stop, Teach).22 It advises highlighting, clarifying, and directly addressing problematic comments with such statements as “I heard you say…” or “What did you mean by that comment?”, or a more direct “Statements like that are not OK with me,” or a teaching statement of “I read an article that made me think differently about comments like the one you made...”22 How and when to employ these strategies takes deliberate practice and will be uncomfortable. But we must do the work.
Practice empathetic listening. In a podcast discussion with Brené Brown on creating transformative cultures, Aiko Bethea, a leader in diversity and equity innovation, implores listeners to believe people of color.23,24 Draw on the history you’ve learned and understand the context in which Black people live in our society. Don’t brush off your Black friend who is upset about being stopped by security. That wasn’t the first time she was in that situation. Take seriously your patient’s concern that they are not being treated appropriately because of being Black. At the same time, do not think of Black people as a monolith or a stereotype. Respect people’s individuality.
Teach our kids all of this. We must also find ways to make change on a larger scale—within our practices, hospitals, medical schools, places of worship, town councils, school boards, state legislatures, and so on. If you are in a faculty position, you can reach out to leadership to scrutinize the curriculum while also ensuring that what and how you are teaching aligns with your antiracist principles. Question the theories, calculators, and algorithms being used and taught. Inquire about policies around recruitment of trainees and faculty as well as promotion, and implement strategies to make this inclusive and equitable. If you run a practice, you can ensure hiring and compensation policies are equitable. Examine patient access and barriers that your minoritized patients are facing, and address those barriers. Share resources and tools that you find helpful and develop a community of colleagues to develop with and hold one another accountable.
In her June 2020 article, An Open Letter to Corporate America, Philanthropy, Academia, etc: What now?, Bethea lays out an extensive framework for approaching antiracism at a high level.25 Among the principles she emphasizes is that the work of diversity, equity, and inclusion should not be siloed and cannot continue to be undervalued. It must be viewed as leadership and engaged in by leadership. The work of diversity, equity, and inclusion for any given institution must be explicit, intentional, measured, and transparent. Within that work, antiracism deserves individual attention. This work must center the people of color for whom you are pursuing equity. White people must resist the urge to make this about them.25
Drs. Esther Choo and J. Nwando Olayiwola present their proposals for combating racism in two 2020 Lancet articles.26,27 They discuss anticipating failure and backlash and learning from them but not being derailed by them. They emphasize the need for ongoing, serious financial investment and transformation in leadership. They also point out the need for data, discouraging more research on well-established inequities while recommending investigating interventions.26,27 If you are in leadership positions, read these articles and many more. Enact these principles. Make the investment. If you are not in such a position, find ways to hold your organization’s leadership accountable. Find ways to get a seat at the table and steer the conversation. In medicine, we have to make change at every level of our organizations. That will include the very difficult work of changing climate and culture. In addition, we have to look not only within our organizations but also to the communities we serve. Those voices must be valued in this conversation.
Will this take time? Yes. Will this be hard? Yes. Can you do everything? No. Can you do your part? Yes! Do the work.
- Roberts D. Fatal Invention: How Science, Politics and Big Business Re-create Race in the Twenty-First Century. The New Press: New York, New York; 2012.
- Definition of racism in English. Lexico web site. https://www.lexico. com/en/definition/racism. Accessed July 30, 2021.
- Definition of racism. Merriam-Webster web site. https://www .merriam-webster.com/dictionary/racism. Accessed July 30, 2021.
- Kendi IX. How To Be an Antiracist. One World: New York, NY; 2019.
- Cooper Owens D, Fett SM. Black maternal and infant health: historical legacies of slavery. Am J Public Health. 2019;109:1342-1345. doi: 10.2105/AJPH.2019.305243.
- Cooper Owens D. Medical Bondage: Race, Gender, and the Origins of American Gynecology. University of Georgia Press: Athens, GA; 2017.
- Washington H. Medical Apartheid: The Dark History of Medical Experimentation on Black Americans from Colonial Times to the Present. Anchor Books: New York, NY; 2006.
- Coates T. The case for reparations. The Atlantic. 2014;313.5:54-71.
- Rothstein R. The Color of the Law: A Forgotten History of How our Government Segregated America. Liveright Publishing Corporation: New York, NY; 2017.
- Nelson RK, Ayers EL; The Digital Scholarship Lab and the National Community Reinvestment Coalition. American Panorama, ed. Not Even Past: Social Vulnerability and the Legacy of Redlining. https://dsl.richmond.edu/socialvulnerability. Accessed July 30, 2021.
- Williams DR, Lawrence JA, Davis BA. Racism and health: evidence and needed research. Annu Rev Public Health. 2019;40:105-125. doi: 10.1146 /annurev-publhealth-040218-043750.
- Institute of Medicine (US) Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care. Smedley BD, Stith AY, Nelson AR, eds. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. National Academies Press: Washington, DC; 2003.
- Artiga S, Corallo B, Pham O. Racial disparities in COVID-19: key findings from available data and analysis. KFF web site. August 17, 2020. https://www.kff.org/racial-equity-and-health-policy/issue-brief /racial-disparities-covid-19-key-findings-available-data-analysis/. Accessed July 30, 2021.
- Disparities in deaths from COVID-19. Centers for Disease Control and Prevention web site. https://www.cdc.gov/coronavirus/2019-ncov /community/health-equity/racial-ethnic-disparities/disparities -deaths.html. Updated December 10, 2020. Accessed July 30, 2021.
- Disparities in COVID-19 hospitalizations. Centers for Disease Control and Prevention web site. https://www.cdc.gov/coronavirus/2019 -ncov/community/health-equity/racial-ethnic-disparities/disparities -hospitalization.html. Updated July 28, 2021. Accessed July 30, 2021.
- COVID data tracker. Centers for Disease Control and Prevention web site. https://covid.cdc.gov/covid-data-tracker/#vaccination -demographics-trends. Accessed July 30, 2021.
- Infant mortality. Centers for Disease Control and Prevention web site. https://www.cdc.gov/reproductivehealth/maternalinfanthealth /infantmortality.htm. Last reviewed September 2020. Accessed July 30, 2021.
- Roeder A. America is failing its Black mothers. Harvard Public Health. Winter 2019. https://www.hsph.harvard.edu/magazine/magazine _article/america-is-failing-its-black-mothers/. Accessed July 30, 2021.
- Ku E, Lee BK, McCulloch CE, et al. Racial and ethnic disparities in kidney transplant access within a theoretical context of medical eligibility. Transplantation. 2020;104:1437-1444. doi: 10.1097/TP .0000000000002962.
- Arya S, Binney Z, Khakharia A, et al. Race and socioeconomic status independently affect risk of major amputation in peripheral artery disease. J Am Heart Assoc. 2018;7:e007425. doi: 10.1161 /JAHA.117.007425.
- Vyas DA, Eisenstein LG, Jones DS, et al. Hidden in plain sight— reconsidering the use of race correction in clinical algorithms. N Engl J Med. 2020;383:874-882. doi: 10.1056/NEJMms2004740.
- A Curriculum to Increase Faculty Engagement in the CLER Program. Boston University Medical Center web site. https://www.bumc .bu.edu/facdev-medicine/files/2020/05/Bystander-Training-for -Microaggressions-Executive-Summary.pdf. Accessed July 30, 2021.
- Brenè with Aiko Bethea on inclusivity at work: the heart of hard conversations. Spotify web site. https://open.spotify.com/episod e/3IODQ37EurkFf0zMNhazqI?si=wJIZgzpWTDCF1QVhwAdhiw. Accessed July 30, 2021.
- Brenè with Aiko Bethea on creating transformative cultures. Spotify web site. https://open.spotify.com/episode/7K47gQF5Ruc7MAXxEN q6jI?si=X0pzd2NnRAGwMD-bkyg-VQ. Accessed July 30, 2021.
- Bethea A. An open letter to corporate America, philanthropy, academia, etc.: What now? June 1, 2020. https://aikobethea.medium. com/an-open-letter-to-corporate-america-philanthropy-academiaetc-what-now-8b2d3a310f22. Accessed July 30, 2021.
- Choo E. Seven things organisations should be doing to combat racism. Lancet. 2020;396:157. doi:10.1016/S0140-6736(20)31565-8.
- Olayiwola JN, Choo E. Seven more things organisations should be doing to combat racism. Lancet. 2020;396:593. doi: 10.1016/S0140 -6736(20)31718-9.
On May 25, 2020, George Floyd was murdered by a White police officer who held his knee on Floyd’s neck for nine and a half minutes. Nine and a half minutes. George Floyd was not the first Black person killed by law enforcement. He has not been the last. Much has been written about why Floyd’s murder sparked unprecedented worldwide outrage despite being far from unprecedented itself. We cannot be so naive as to think what happened was new, and we should not ignore the tireless work that so many have been doing to fight racism up to this point. But for many who have been stirred to do something for the first time, especially White people, the question has been,
“What do I do?” The answer is, do the work.
This article is centered on anti-Black racism with a focus on medicine. We recognize that there is racism against other minoritized groups. Each group deserves attention and to have their stories told. We recognize intersectionality and that an individual has multiple identities and that these may compound the marginalization they experience. This too deserves attention.
However, we cannot satisfactorily explore any of these concepts within the confines of a single article. Our intention is to use this forum to promote further conversation, specifically about anti-Black racism in medicine. We hope it compels you to begin learning to recognize and dismantle racism in yourself and your surroundings, both at home and at work.
Being a health care provider requires lifelong learning. If we practiced only what we learned in training, our patients could suffer. So we continually seek out updated research and guidelines to best treat our patients. Understanding how racism impacts your patients, colleagues, family, and friends is your responsibility as much as understanding guidelines for standards of care. We must resist the urge to feel this is someone else’s duty. It is the job of each and every one of us. We must do the work.
Race is real but it’s not biologic
It is imperative to understand that race is not a biologic category. Phenotypic differences between humans do not reliably map to racial categories. Racial categories themselves have morphed over the centuries, and interpretation of race has been litigated in this country since its founding.1 People who identify as a given race do not have inherent biology that is different from those who identify as another race. It may then be tempting to try to erase race from our thinking, and, indeed, the idea of being “color blind” was long worn as a badge of honor signifying a commitment to equality. So this is the tension: if race exists, it must be a biologic trait and with it must go other inherent traits. But if race is not a biologic entity, perhaps it is not real and, therefore, should be ignored. In fact, neither is true. Race is not based on genetic or biologic inheritance, but it is a social and political categorization that is real and has very real ramifications. As we will discuss further, race does have a biologic impact on individuals. The mechanism by which that happens is racism.
Continue to: What is racism, and who is racist?...
What is racism, and who is racist?
Various definitions of racism have been offered:
- prejudice, discrimination, or antagonism directed against a person or people on the basis of their membership in a particular racial or ethnic group, typically one that is minoritized or marginalized2
- a belief that race is a fundamental determinant of human traits and capacities and that racial differences produce an inherent superiority of a particular race3
- the systemic oppression of a racial group to the social, economic, and political advantage of another; a political or social system founded on racism and designed to execute its principles.3
The common themes in these definitions are power, hierarchy, and oppression. Racism is a fabricated system to justify and reinforce power for some and disenfranchisement for others based on race. The system is pervasive and beneficial to the group that it serves.
Ibram X. Kendi posits that all racism is structural racism: “‘Institutional racism’ and ‘structural racism’ and ‘systemic racism’ are redundant. Racism itself is institutional, structural, and systemic.”4 This is not saying that individuals don’t enact racism, but it emphasizes that racism is not the action of a “few bad apples.” Furthermore, it underscores that race was created to bolster power structures ensuring White dominance. The racism that has followed, in all of its forms, is both because these ideas were created in the first place and to perpetuate that ongoing power structure.4
Dorothy Roberts, JD, writes in her book Fatal Invention that, while grouping people and creating hierarchy has always happened amongst humans, there is a specific history in our country of using race to create and perpetuate the dominance of White people and the subjugation of Black people.
Kendi also asserts that there is no neutrality with regard to racism—there is racist and antiracist: “A racist: one who is supporting a racist policy through their actions or inaction or expressing a racist idea. An antiracist: one who is supporting an antiracist policy through their actions or expressing an antiracist idea.”4 He describes all people as moving in and out of being racist and antiracist, and states “being an antiracist requires persistent self-awareness, constant self-criticism, and regular self-examination.”4 In thinking about race and racism in this way, we all must grapple with our own racism, but in so doing are taking a step toward antiracism.
History is important
Among the most important things one can do in a journey to dismantle racism is learn the history of racism.
The infrastructure and institutions of our nation were created on a foundation of slavery, including the origins of American medicine and gynecology. Physicians in the antebellum South performed inspections of enslaved people’s bodies to certify them for sale.5 The ability to assign market value to a Black person’s body was published as an essential physician competency.5
Gynecology has a particularly painful history with regard to slavery. By 1808, transatlantic slave trade was banned in the United States and, as Dr. Cooper Owens describes in her book Medical Bondage: Race, Gender, and the Origins of American Gynecology, this made reproduction of enslaved people within the United States a priority for slave owners and those invested in an economy that depended on slavery.6 Gynecologists were permitted unrestricted access to enslaved women for experiments to optimize reproduction. Many of these physicians became prominent voices adding to the canon of racialized medicine. Medical journals themselves gained reverence because of heightened interest in keeping enslaved people alive and just well enough to work and reproduce.6 Today, we hold sacred the relationship between a patient and their physician. We must understand that there was no such relationship between a doctor and an enslaved person. The relationship was between the doctor and slave owner.6,7 Slavery does not allow for the autonomy of the enslaved. This is the context in which we must understand the discoveries of gynecologists during that time.
Despite the abolition of slavery with the passage of the 13th amendment, racist policies remained ubiquitous in the United States. Segregation of Black people was codified not only in the Jim Crow South but also in the North. Interracial marriage was outlawed by all but 9 states.
While there are numerous federal policies that led to cumulative and egregious disadvantage for Black Americans, one powerful example is redlining. In 1934 the Federal Housing Administration was created, and by insuring private mortgages, the FHA made it easier for eligible home buyers to obtain financing. The FHA used a system of maps that graded neighborhoods. Racial composition of neighborhoods was overtly used as a component of grading, and the presence of Black people led a neighborhood to be downgraded or redlined.8,9 This meant Black people were largely ineligible for FHA-backed loans. In The Color of Law, Richard Rothstein writes, “Today’s residential segregation in the North, South, Midwest, and West is not the unintended consequence of individual choices and of otherwise well-meaning law or regulation but of unhidden public policy that explicitly segregated every metropolitan area in the United States.The policy was so systematic and forceful that its effects endure to the present time.”9
Though these specific policies are no longer in place, many correlations have been found between historically redlined neighborhoods and higher rates of diseases today, including diabetes, hypertension, asthma, and preterm deliveries.10 These policies also have played a role in creating the wealth gap—directly by limiting the opportunity for home ownership, which translates to intergenerational wealth, and indirectly by the disinvestment in neighborhoods where Black people live, leading to reduced access to quality education, decreased employment opportunities, and increased environmental hazards.8,11
Continue to: Health disparities...
Health disparities
The numerous health disparities, more accurately termed health inequities, suffered by racial minority groups is well documented.12
COVID-19 death and vaccination-rate inequities. Early in the COVID-19 pandemic, data emerged that racial minorities were being disparately affected.13 In December 2020, the Centers for Disease Control and Prevention (CDC) reported that Hispanic or Latino, non-Hispanic Black, and non-Hispanic American Indian or Alaska Native people had all died at higher rates than White Americans.14 These racial groups had higher hospitalization rates across age groups and, after adjusting for age, rates of hospitalization were 2.8 to 3.4 times higher.15 We are continuing to learn what factors contribute to these inequities, but it has highlighted how racist policies have led to disparate access to health care, or even clean air, clean water, and nutritious food, and left communities of color more vulnerable to severe illness and death from COVID-19. With the advent of vaccines for COVID-19, we continue to see racial disparities as Black Americans have the lowest rates of vaccination.16 All of these inequities have to be understood in the context of the racist structures that exist in our society. As medical providers, we must understand and help to dismantle these structures.
Pregnancy-related mortality (PRM) inequities. A powerful example of a persistent health inequity in our field is the well-known disparity in pregnancy-related mortality when examining this outcome by race. Per CDC analysis of data on PRM from 2007–2016, Black women died at a rate 3.2 times higher than White women. This disparity was even greater in patients older than 30 years of age. When they compared rates while controlling for the highest level of education, the disparity is even more pronounced: PRM rate for those with a college degree or higher was 5.2 times greater for Black people compared with White people.16The CDC also reported that, in 2018, the infant mortality for non-Hispanic Black infants was 10.8 per 1,000 live births, compared with 4.6 per 1,000 live births for White infants. This is a rate 2.4-times higher for Black infants.17 Dr. Cooper Owens and Dr. Fett note in their article, “Black maternal and infant health: Historical legacies of slavery,” that in 1850 this rate was 1.6-times higher for Black infants, which means the inequity was worse in 2018 America than in the antebellum South.5
The role of patient experience
As discussed, governmental policies have created persistent inequities in wealth, access to health care, and exposure to environmental toxins, among many other disparities. However, the data finding that highly educated Black pregnant patients suffer markedly increased risk of maternal death, indicate that inequities cannot be attributed only to education or lack of access to health care. This is where some will once again lean on the idea that there is something inherently different about Black people. But if we know that race was created and is not an empiric category, we must consider the social variables impacting Black patients’ experience.
As Linda Blount, President and CEO of the Black Women’s Health Imperative, put it, “Race is not a risk factor. It is the lived experience of being a Black woman in this society that is the risk factor.”18 So how much of these inequities can be accounted for by differential treatment of Black patients? There is, for example, data on the disproportionately lower rates of Black renal transplant recipients and inordinately higher rates of amputations among Black patients.19,20 None of us wants to think we are treating our Black patients differently, but the data demand that we ask ourselves if we are. Some of this is built into the system. For example, in their article “Hidden in plain sight—Reconsidering the use of race correction in clinical algorithms,” Vyas and colleagues outline a list of calculators and algorithms that include race.21 This means we may be using these calculators and changing outcomes for our patients based on their race. This is only one example of racism hidden within guidelines and standards of care.
The existence of racism on an interpersonal level also cannot be denied. This could lead to differential care specifically, but also can manifest by way of the toll it takes on a patient generally. This is the concept of allostatic load or weathering: the chronic stress of experiencing racism creates detrimental physiologic change. There is ongoing research into epigenetic modifications from stress that could be impacting health outcomes in Black populations.
Continue to: What is the work we need to do?...
What is the work we need to do?
Become educated. We have discussed taking the initiative to learn about the history of racism, including the legacies of slavery and the ongoing impact of racism on health. This knowledge is foundational and sometimes transformative. It allows us to see opportunities for antiracism and gives us the knowledge to begin meaningful conversations.
Take action. We must take inventory within our lives. What are our spheres of influence? What are our resources? Where can we make an impact? Right now, you can take out a pen and paper and write down all the roles you play. Look for opportunities in personal interactions and daily routines. Unfortunately, there will be many opportunities to speak up against racism—although this is rarely easy. Find articles, podcasts, and workshops on upstander training. One framework to respond to microaggressions has been proposed by faculty at Boston University Medical Center using the acronym LIFT (Lights on, Impact vs Intent, Full stop, Teach).22 It advises highlighting, clarifying, and directly addressing problematic comments with such statements as “I heard you say…” or “What did you mean by that comment?”, or a more direct “Statements like that are not OK with me,” or a teaching statement of “I read an article that made me think differently about comments like the one you made...”22 How and when to employ these strategies takes deliberate practice and will be uncomfortable. But we must do the work.
Practice empathetic listening. In a podcast discussion with Brené Brown on creating transformative cultures, Aiko Bethea, a leader in diversity and equity innovation, implores listeners to believe people of color.23,24 Draw on the history you’ve learned and understand the context in which Black people live in our society. Don’t brush off your Black friend who is upset about being stopped by security. That wasn’t the first time she was in that situation. Take seriously your patient’s concern that they are not being treated appropriately because of being Black. At the same time, do not think of Black people as a monolith or a stereotype. Respect people’s individuality.
Teach our kids all of this. We must also find ways to make change on a larger scale—within our practices, hospitals, medical schools, places of worship, town councils, school boards, state legislatures, and so on. If you are in a faculty position, you can reach out to leadership to scrutinize the curriculum while also ensuring that what and how you are teaching aligns with your antiracist principles. Question the theories, calculators, and algorithms being used and taught. Inquire about policies around recruitment of trainees and faculty as well as promotion, and implement strategies to make this inclusive and equitable. If you run a practice, you can ensure hiring and compensation policies are equitable. Examine patient access and barriers that your minoritized patients are facing, and address those barriers. Share resources and tools that you find helpful and develop a community of colleagues to develop with and hold one another accountable.
In her June 2020 article, An Open Letter to Corporate America, Philanthropy, Academia, etc: What now?, Bethea lays out an extensive framework for approaching antiracism at a high level.25 Among the principles she emphasizes is that the work of diversity, equity, and inclusion should not be siloed and cannot continue to be undervalued. It must be viewed as leadership and engaged in by leadership. The work of diversity, equity, and inclusion for any given institution must be explicit, intentional, measured, and transparent. Within that work, antiracism deserves individual attention. This work must center the people of color for whom you are pursuing equity. White people must resist the urge to make this about them.25
Drs. Esther Choo and J. Nwando Olayiwola present their proposals for combating racism in two 2020 Lancet articles.26,27 They discuss anticipating failure and backlash and learning from them but not being derailed by them. They emphasize the need for ongoing, serious financial investment and transformation in leadership. They also point out the need for data, discouraging more research on well-established inequities while recommending investigating interventions.26,27 If you are in leadership positions, read these articles and many more. Enact these principles. Make the investment. If you are not in such a position, find ways to hold your organization’s leadership accountable. Find ways to get a seat at the table and steer the conversation. In medicine, we have to make change at every level of our organizations. That will include the very difficult work of changing climate and culture. In addition, we have to look not only within our organizations but also to the communities we serve. Those voices must be valued in this conversation.
Will this take time? Yes. Will this be hard? Yes. Can you do everything? No. Can you do your part? Yes! Do the work.
On May 25, 2020, George Floyd was murdered by a White police officer who held his knee on Floyd’s neck for nine and a half minutes. Nine and a half minutes. George Floyd was not the first Black person killed by law enforcement. He has not been the last. Much has been written about why Floyd’s murder sparked unprecedented worldwide outrage despite being far from unprecedented itself. We cannot be so naive as to think what happened was new, and we should not ignore the tireless work that so many have been doing to fight racism up to this point. But for many who have been stirred to do something for the first time, especially White people, the question has been,
“What do I do?” The answer is, do the work.
This article is centered on anti-Black racism with a focus on medicine. We recognize that there is racism against other minoritized groups. Each group deserves attention and to have their stories told. We recognize intersectionality and that an individual has multiple identities and that these may compound the marginalization they experience. This too deserves attention.
However, we cannot satisfactorily explore any of these concepts within the confines of a single article. Our intention is to use this forum to promote further conversation, specifically about anti-Black racism in medicine. We hope it compels you to begin learning to recognize and dismantle racism in yourself and your surroundings, both at home and at work.
Being a health care provider requires lifelong learning. If we practiced only what we learned in training, our patients could suffer. So we continually seek out updated research and guidelines to best treat our patients. Understanding how racism impacts your patients, colleagues, family, and friends is your responsibility as much as understanding guidelines for standards of care. We must resist the urge to feel this is someone else’s duty. It is the job of each and every one of us. We must do the work.
Race is real but it’s not biologic
It is imperative to understand that race is not a biologic category. Phenotypic differences between humans do not reliably map to racial categories. Racial categories themselves have morphed over the centuries, and interpretation of race has been litigated in this country since its founding.1 People who identify as a given race do not have inherent biology that is different from those who identify as another race. It may then be tempting to try to erase race from our thinking, and, indeed, the idea of being “color blind” was long worn as a badge of honor signifying a commitment to equality. So this is the tension: if race exists, it must be a biologic trait and with it must go other inherent traits. But if race is not a biologic entity, perhaps it is not real and, therefore, should be ignored. In fact, neither is true. Race is not based on genetic or biologic inheritance, but it is a social and political categorization that is real and has very real ramifications. As we will discuss further, race does have a biologic impact on individuals. The mechanism by which that happens is racism.
Continue to: What is racism, and who is racist?...
What is racism, and who is racist?
Various definitions of racism have been offered:
- prejudice, discrimination, or antagonism directed against a person or people on the basis of their membership in a particular racial or ethnic group, typically one that is minoritized or marginalized2
- a belief that race is a fundamental determinant of human traits and capacities and that racial differences produce an inherent superiority of a particular race3
- the systemic oppression of a racial group to the social, economic, and political advantage of another; a political or social system founded on racism and designed to execute its principles.3
The common themes in these definitions are power, hierarchy, and oppression. Racism is a fabricated system to justify and reinforce power for some and disenfranchisement for others based on race. The system is pervasive and beneficial to the group that it serves.
Ibram X. Kendi posits that all racism is structural racism: “‘Institutional racism’ and ‘structural racism’ and ‘systemic racism’ are redundant. Racism itself is institutional, structural, and systemic.”4 This is not saying that individuals don’t enact racism, but it emphasizes that racism is not the action of a “few bad apples.” Furthermore, it underscores that race was created to bolster power structures ensuring White dominance. The racism that has followed, in all of its forms, is both because these ideas were created in the first place and to perpetuate that ongoing power structure.4
Dorothy Roberts, JD, writes in her book Fatal Invention that, while grouping people and creating hierarchy has always happened amongst humans, there is a specific history in our country of using race to create and perpetuate the dominance of White people and the subjugation of Black people.
Kendi also asserts that there is no neutrality with regard to racism—there is racist and antiracist: “A racist: one who is supporting a racist policy through their actions or inaction or expressing a racist idea. An antiracist: one who is supporting an antiracist policy through their actions or expressing an antiracist idea.”4 He describes all people as moving in and out of being racist and antiracist, and states “being an antiracist requires persistent self-awareness, constant self-criticism, and regular self-examination.”4 In thinking about race and racism in this way, we all must grapple with our own racism, but in so doing are taking a step toward antiracism.
History is important
Among the most important things one can do in a journey to dismantle racism is learn the history of racism.
The infrastructure and institutions of our nation were created on a foundation of slavery, including the origins of American medicine and gynecology. Physicians in the antebellum South performed inspections of enslaved people’s bodies to certify them for sale.5 The ability to assign market value to a Black person’s body was published as an essential physician competency.5
Gynecology has a particularly painful history with regard to slavery. By 1808, transatlantic slave trade was banned in the United States and, as Dr. Cooper Owens describes in her book Medical Bondage: Race, Gender, and the Origins of American Gynecology, this made reproduction of enslaved people within the United States a priority for slave owners and those invested in an economy that depended on slavery.6 Gynecologists were permitted unrestricted access to enslaved women for experiments to optimize reproduction. Many of these physicians became prominent voices adding to the canon of racialized medicine. Medical journals themselves gained reverence because of heightened interest in keeping enslaved people alive and just well enough to work and reproduce.6 Today, we hold sacred the relationship between a patient and their physician. We must understand that there was no such relationship between a doctor and an enslaved person. The relationship was between the doctor and slave owner.6,7 Slavery does not allow for the autonomy of the enslaved. This is the context in which we must understand the discoveries of gynecologists during that time.
Despite the abolition of slavery with the passage of the 13th amendment, racist policies remained ubiquitous in the United States. Segregation of Black people was codified not only in the Jim Crow South but also in the North. Interracial marriage was outlawed by all but 9 states.
While there are numerous federal policies that led to cumulative and egregious disadvantage for Black Americans, one powerful example is redlining. In 1934 the Federal Housing Administration was created, and by insuring private mortgages, the FHA made it easier for eligible home buyers to obtain financing. The FHA used a system of maps that graded neighborhoods. Racial composition of neighborhoods was overtly used as a component of grading, and the presence of Black people led a neighborhood to be downgraded or redlined.8,9 This meant Black people were largely ineligible for FHA-backed loans. In The Color of Law, Richard Rothstein writes, “Today’s residential segregation in the North, South, Midwest, and West is not the unintended consequence of individual choices and of otherwise well-meaning law or regulation but of unhidden public policy that explicitly segregated every metropolitan area in the United States.The policy was so systematic and forceful that its effects endure to the present time.”9
Though these specific policies are no longer in place, many correlations have been found between historically redlined neighborhoods and higher rates of diseases today, including diabetes, hypertension, asthma, and preterm deliveries.10 These policies also have played a role in creating the wealth gap—directly by limiting the opportunity for home ownership, which translates to intergenerational wealth, and indirectly by the disinvestment in neighborhoods where Black people live, leading to reduced access to quality education, decreased employment opportunities, and increased environmental hazards.8,11
Continue to: Health disparities...
Health disparities
The numerous health disparities, more accurately termed health inequities, suffered by racial minority groups is well documented.12
COVID-19 death and vaccination-rate inequities. Early in the COVID-19 pandemic, data emerged that racial minorities were being disparately affected.13 In December 2020, the Centers for Disease Control and Prevention (CDC) reported that Hispanic or Latino, non-Hispanic Black, and non-Hispanic American Indian or Alaska Native people had all died at higher rates than White Americans.14 These racial groups had higher hospitalization rates across age groups and, after adjusting for age, rates of hospitalization were 2.8 to 3.4 times higher.15 We are continuing to learn what factors contribute to these inequities, but it has highlighted how racist policies have led to disparate access to health care, or even clean air, clean water, and nutritious food, and left communities of color more vulnerable to severe illness and death from COVID-19. With the advent of vaccines for COVID-19, we continue to see racial disparities as Black Americans have the lowest rates of vaccination.16 All of these inequities have to be understood in the context of the racist structures that exist in our society. As medical providers, we must understand and help to dismantle these structures.
Pregnancy-related mortality (PRM) inequities. A powerful example of a persistent health inequity in our field is the well-known disparity in pregnancy-related mortality when examining this outcome by race. Per CDC analysis of data on PRM from 2007–2016, Black women died at a rate 3.2 times higher than White women. This disparity was even greater in patients older than 30 years of age. When they compared rates while controlling for the highest level of education, the disparity is even more pronounced: PRM rate for those with a college degree or higher was 5.2 times greater for Black people compared with White people.16The CDC also reported that, in 2018, the infant mortality for non-Hispanic Black infants was 10.8 per 1,000 live births, compared with 4.6 per 1,000 live births for White infants. This is a rate 2.4-times higher for Black infants.17 Dr. Cooper Owens and Dr. Fett note in their article, “Black maternal and infant health: Historical legacies of slavery,” that in 1850 this rate was 1.6-times higher for Black infants, which means the inequity was worse in 2018 America than in the antebellum South.5
The role of patient experience
As discussed, governmental policies have created persistent inequities in wealth, access to health care, and exposure to environmental toxins, among many other disparities. However, the data finding that highly educated Black pregnant patients suffer markedly increased risk of maternal death, indicate that inequities cannot be attributed only to education or lack of access to health care. This is where some will once again lean on the idea that there is something inherently different about Black people. But if we know that race was created and is not an empiric category, we must consider the social variables impacting Black patients’ experience.
As Linda Blount, President and CEO of the Black Women’s Health Imperative, put it, “Race is not a risk factor. It is the lived experience of being a Black woman in this society that is the risk factor.”18 So how much of these inequities can be accounted for by differential treatment of Black patients? There is, for example, data on the disproportionately lower rates of Black renal transplant recipients and inordinately higher rates of amputations among Black patients.19,20 None of us wants to think we are treating our Black patients differently, but the data demand that we ask ourselves if we are. Some of this is built into the system. For example, in their article “Hidden in plain sight—Reconsidering the use of race correction in clinical algorithms,” Vyas and colleagues outline a list of calculators and algorithms that include race.21 This means we may be using these calculators and changing outcomes for our patients based on their race. This is only one example of racism hidden within guidelines and standards of care.
The existence of racism on an interpersonal level also cannot be denied. This could lead to differential care specifically, but also can manifest by way of the toll it takes on a patient generally. This is the concept of allostatic load or weathering: the chronic stress of experiencing racism creates detrimental physiologic change. There is ongoing research into epigenetic modifications from stress that could be impacting health outcomes in Black populations.
Continue to: What is the work we need to do?...
What is the work we need to do?
Become educated. We have discussed taking the initiative to learn about the history of racism, including the legacies of slavery and the ongoing impact of racism on health. This knowledge is foundational and sometimes transformative. It allows us to see opportunities for antiracism and gives us the knowledge to begin meaningful conversations.
Take action. We must take inventory within our lives. What are our spheres of influence? What are our resources? Where can we make an impact? Right now, you can take out a pen and paper and write down all the roles you play. Look for opportunities in personal interactions and daily routines. Unfortunately, there will be many opportunities to speak up against racism—although this is rarely easy. Find articles, podcasts, and workshops on upstander training. One framework to respond to microaggressions has been proposed by faculty at Boston University Medical Center using the acronym LIFT (Lights on, Impact vs Intent, Full stop, Teach).22 It advises highlighting, clarifying, and directly addressing problematic comments with such statements as “I heard you say…” or “What did you mean by that comment?”, or a more direct “Statements like that are not OK with me,” or a teaching statement of “I read an article that made me think differently about comments like the one you made...”22 How and when to employ these strategies takes deliberate practice and will be uncomfortable. But we must do the work.
Practice empathetic listening. In a podcast discussion with Brené Brown on creating transformative cultures, Aiko Bethea, a leader in diversity and equity innovation, implores listeners to believe people of color.23,24 Draw on the history you’ve learned and understand the context in which Black people live in our society. Don’t brush off your Black friend who is upset about being stopped by security. That wasn’t the first time she was in that situation. Take seriously your patient’s concern that they are not being treated appropriately because of being Black. At the same time, do not think of Black people as a monolith or a stereotype. Respect people’s individuality.
Teach our kids all of this. We must also find ways to make change on a larger scale—within our practices, hospitals, medical schools, places of worship, town councils, school boards, state legislatures, and so on. If you are in a faculty position, you can reach out to leadership to scrutinize the curriculum while also ensuring that what and how you are teaching aligns with your antiracist principles. Question the theories, calculators, and algorithms being used and taught. Inquire about policies around recruitment of trainees and faculty as well as promotion, and implement strategies to make this inclusive and equitable. If you run a practice, you can ensure hiring and compensation policies are equitable. Examine patient access and barriers that your minoritized patients are facing, and address those barriers. Share resources and tools that you find helpful and develop a community of colleagues to develop with and hold one another accountable.
In her June 2020 article, An Open Letter to Corporate America, Philanthropy, Academia, etc: What now?, Bethea lays out an extensive framework for approaching antiracism at a high level.25 Among the principles she emphasizes is that the work of diversity, equity, and inclusion should not be siloed and cannot continue to be undervalued. It must be viewed as leadership and engaged in by leadership. The work of diversity, equity, and inclusion for any given institution must be explicit, intentional, measured, and transparent. Within that work, antiracism deserves individual attention. This work must center the people of color for whom you are pursuing equity. White people must resist the urge to make this about them.25
Drs. Esther Choo and J. Nwando Olayiwola present their proposals for combating racism in two 2020 Lancet articles.26,27 They discuss anticipating failure and backlash and learning from them but not being derailed by them. They emphasize the need for ongoing, serious financial investment and transformation in leadership. They also point out the need for data, discouraging more research on well-established inequities while recommending investigating interventions.26,27 If you are in leadership positions, read these articles and many more. Enact these principles. Make the investment. If you are not in such a position, find ways to hold your organization’s leadership accountable. Find ways to get a seat at the table and steer the conversation. In medicine, we have to make change at every level of our organizations. That will include the very difficult work of changing climate and culture. In addition, we have to look not only within our organizations but also to the communities we serve. Those voices must be valued in this conversation.
Will this take time? Yes. Will this be hard? Yes. Can you do everything? No. Can you do your part? Yes! Do the work.
- Roberts D. Fatal Invention: How Science, Politics and Big Business Re-create Race in the Twenty-First Century. The New Press: New York, New York; 2012.
- Definition of racism in English. Lexico web site. https://www.lexico. com/en/definition/racism. Accessed July 30, 2021.
- Definition of racism. Merriam-Webster web site. https://www .merriam-webster.com/dictionary/racism. Accessed July 30, 2021.
- Kendi IX. How To Be an Antiracist. One World: New York, NY; 2019.
- Cooper Owens D, Fett SM. Black maternal and infant health: historical legacies of slavery. Am J Public Health. 2019;109:1342-1345. doi: 10.2105/AJPH.2019.305243.
- Cooper Owens D. Medical Bondage: Race, Gender, and the Origins of American Gynecology. University of Georgia Press: Athens, GA; 2017.
- Washington H. Medical Apartheid: The Dark History of Medical Experimentation on Black Americans from Colonial Times to the Present. Anchor Books: New York, NY; 2006.
- Coates T. The case for reparations. The Atlantic. 2014;313.5:54-71.
- Rothstein R. The Color of the Law: A Forgotten History of How our Government Segregated America. Liveright Publishing Corporation: New York, NY; 2017.
- Nelson RK, Ayers EL; The Digital Scholarship Lab and the National Community Reinvestment Coalition. American Panorama, ed. Not Even Past: Social Vulnerability and the Legacy of Redlining. https://dsl.richmond.edu/socialvulnerability. Accessed July 30, 2021.
- Williams DR, Lawrence JA, Davis BA. Racism and health: evidence and needed research. Annu Rev Public Health. 2019;40:105-125. doi: 10.1146 /annurev-publhealth-040218-043750.
- Institute of Medicine (US) Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care. Smedley BD, Stith AY, Nelson AR, eds. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. National Academies Press: Washington, DC; 2003.
- Artiga S, Corallo B, Pham O. Racial disparities in COVID-19: key findings from available data and analysis. KFF web site. August 17, 2020. https://www.kff.org/racial-equity-and-health-policy/issue-brief /racial-disparities-covid-19-key-findings-available-data-analysis/. Accessed July 30, 2021.
- Disparities in deaths from COVID-19. Centers for Disease Control and Prevention web site. https://www.cdc.gov/coronavirus/2019-ncov /community/health-equity/racial-ethnic-disparities/disparities -deaths.html. Updated December 10, 2020. Accessed July 30, 2021.
- Disparities in COVID-19 hospitalizations. Centers for Disease Control and Prevention web site. https://www.cdc.gov/coronavirus/2019 -ncov/community/health-equity/racial-ethnic-disparities/disparities -hospitalization.html. Updated July 28, 2021. Accessed July 30, 2021.
- COVID data tracker. Centers for Disease Control and Prevention web site. https://covid.cdc.gov/covid-data-tracker/#vaccination -demographics-trends. Accessed July 30, 2021.
- Infant mortality. Centers for Disease Control and Prevention web site. https://www.cdc.gov/reproductivehealth/maternalinfanthealth /infantmortality.htm. Last reviewed September 2020. Accessed July 30, 2021.
- Roeder A. America is failing its Black mothers. Harvard Public Health. Winter 2019. https://www.hsph.harvard.edu/magazine/magazine _article/america-is-failing-its-black-mothers/. Accessed July 30, 2021.
- Ku E, Lee BK, McCulloch CE, et al. Racial and ethnic disparities in kidney transplant access within a theoretical context of medical eligibility. Transplantation. 2020;104:1437-1444. doi: 10.1097/TP .0000000000002962.
- Arya S, Binney Z, Khakharia A, et al. Race and socioeconomic status independently affect risk of major amputation in peripheral artery disease. J Am Heart Assoc. 2018;7:e007425. doi: 10.1161 /JAHA.117.007425.
- Vyas DA, Eisenstein LG, Jones DS, et al. Hidden in plain sight— reconsidering the use of race correction in clinical algorithms. N Engl J Med. 2020;383:874-882. doi: 10.1056/NEJMms2004740.
- A Curriculum to Increase Faculty Engagement in the CLER Program. Boston University Medical Center web site. https://www.bumc .bu.edu/facdev-medicine/files/2020/05/Bystander-Training-for -Microaggressions-Executive-Summary.pdf. Accessed July 30, 2021.
- Brenè with Aiko Bethea on inclusivity at work: the heart of hard conversations. Spotify web site. https://open.spotify.com/episod e/3IODQ37EurkFf0zMNhazqI?si=wJIZgzpWTDCF1QVhwAdhiw. Accessed July 30, 2021.
- Brenè with Aiko Bethea on creating transformative cultures. Spotify web site. https://open.spotify.com/episode/7K47gQF5Ruc7MAXxEN q6jI?si=X0pzd2NnRAGwMD-bkyg-VQ. Accessed July 30, 2021.
- Bethea A. An open letter to corporate America, philanthropy, academia, etc.: What now? June 1, 2020. https://aikobethea.medium. com/an-open-letter-to-corporate-america-philanthropy-academiaetc-what-now-8b2d3a310f22. Accessed July 30, 2021.
- Choo E. Seven things organisations should be doing to combat racism. Lancet. 2020;396:157. doi:10.1016/S0140-6736(20)31565-8.
- Olayiwola JN, Choo E. Seven more things organisations should be doing to combat racism. Lancet. 2020;396:593. doi: 10.1016/S0140 -6736(20)31718-9.
- Roberts D. Fatal Invention: How Science, Politics and Big Business Re-create Race in the Twenty-First Century. The New Press: New York, New York; 2012.
- Definition of racism in English. Lexico web site. https://www.lexico. com/en/definition/racism. Accessed July 30, 2021.
- Definition of racism. Merriam-Webster web site. https://www .merriam-webster.com/dictionary/racism. Accessed July 30, 2021.
- Kendi IX. How To Be an Antiracist. One World: New York, NY; 2019.
- Cooper Owens D, Fett SM. Black maternal and infant health: historical legacies of slavery. Am J Public Health. 2019;109:1342-1345. doi: 10.2105/AJPH.2019.305243.
- Cooper Owens D. Medical Bondage: Race, Gender, and the Origins of American Gynecology. University of Georgia Press: Athens, GA; 2017.
- Washington H. Medical Apartheid: The Dark History of Medical Experimentation on Black Americans from Colonial Times to the Present. Anchor Books: New York, NY; 2006.
- Coates T. The case for reparations. The Atlantic. 2014;313.5:54-71.
- Rothstein R. The Color of the Law: A Forgotten History of How our Government Segregated America. Liveright Publishing Corporation: New York, NY; 2017.
- Nelson RK, Ayers EL; The Digital Scholarship Lab and the National Community Reinvestment Coalition. American Panorama, ed. Not Even Past: Social Vulnerability and the Legacy of Redlining. https://dsl.richmond.edu/socialvulnerability. Accessed July 30, 2021.
- Williams DR, Lawrence JA, Davis BA. Racism and health: evidence and needed research. Annu Rev Public Health. 2019;40:105-125. doi: 10.1146 /annurev-publhealth-040218-043750.
- Institute of Medicine (US) Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care. Smedley BD, Stith AY, Nelson AR, eds. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. National Academies Press: Washington, DC; 2003.
- Artiga S, Corallo B, Pham O. Racial disparities in COVID-19: key findings from available data and analysis. KFF web site. August 17, 2020. https://www.kff.org/racial-equity-and-health-policy/issue-brief /racial-disparities-covid-19-key-findings-available-data-analysis/. Accessed July 30, 2021.
- Disparities in deaths from COVID-19. Centers for Disease Control and Prevention web site. https://www.cdc.gov/coronavirus/2019-ncov /community/health-equity/racial-ethnic-disparities/disparities -deaths.html. Updated December 10, 2020. Accessed July 30, 2021.
- Disparities in COVID-19 hospitalizations. Centers for Disease Control and Prevention web site. https://www.cdc.gov/coronavirus/2019 -ncov/community/health-equity/racial-ethnic-disparities/disparities -hospitalization.html. Updated July 28, 2021. Accessed July 30, 2021.
- COVID data tracker. Centers for Disease Control and Prevention web site. https://covid.cdc.gov/covid-data-tracker/#vaccination -demographics-trends. Accessed July 30, 2021.
- Infant mortality. Centers for Disease Control and Prevention web site. https://www.cdc.gov/reproductivehealth/maternalinfanthealth /infantmortality.htm. Last reviewed September 2020. Accessed July 30, 2021.
- Roeder A. America is failing its Black mothers. Harvard Public Health. Winter 2019. https://www.hsph.harvard.edu/magazine/magazine _article/america-is-failing-its-black-mothers/. Accessed July 30, 2021.
- Ku E, Lee BK, McCulloch CE, et al. Racial and ethnic disparities in kidney transplant access within a theoretical context of medical eligibility. Transplantation. 2020;104:1437-1444. doi: 10.1097/TP .0000000000002962.
- Arya S, Binney Z, Khakharia A, et al. Race and socioeconomic status independently affect risk of major amputation in peripheral artery disease. J Am Heart Assoc. 2018;7:e007425. doi: 10.1161 /JAHA.117.007425.
- Vyas DA, Eisenstein LG, Jones DS, et al. Hidden in plain sight— reconsidering the use of race correction in clinical algorithms. N Engl J Med. 2020;383:874-882. doi: 10.1056/NEJMms2004740.
- A Curriculum to Increase Faculty Engagement in the CLER Program. Boston University Medical Center web site. https://www.bumc .bu.edu/facdev-medicine/files/2020/05/Bystander-Training-for -Microaggressions-Executive-Summary.pdf. Accessed July 30, 2021.
- Brenè with Aiko Bethea on inclusivity at work: the heart of hard conversations. Spotify web site. https://open.spotify.com/episod e/3IODQ37EurkFf0zMNhazqI?si=wJIZgzpWTDCF1QVhwAdhiw. Accessed July 30, 2021.
- Brenè with Aiko Bethea on creating transformative cultures. Spotify web site. https://open.spotify.com/episode/7K47gQF5Ruc7MAXxEN q6jI?si=X0pzd2NnRAGwMD-bkyg-VQ. Accessed July 30, 2021.
- Bethea A. An open letter to corporate America, philanthropy, academia, etc.: What now? June 1, 2020. https://aikobethea.medium. com/an-open-letter-to-corporate-america-philanthropy-academiaetc-what-now-8b2d3a310f22. Accessed July 30, 2021.
- Choo E. Seven things organisations should be doing to combat racism. Lancet. 2020;396:157. doi:10.1016/S0140-6736(20)31565-8.
- Olayiwola JN, Choo E. Seven more things organisations should be doing to combat racism. Lancet. 2020;396:593. doi: 10.1016/S0140 -6736(20)31718-9.
2021 Update on abnormal uterine bleeding
Abnormal uterine bleeding (AUB) continues to be a top-10 reason why women present for gynecologic care, which makes keeping up with clinical therapies important. Over the past year, we have learned a tremendous amount about elagolix with hormonal add-back therapy for the treatment of bleeding associated with uterine fibroids. In this Update, we provide an overview from 3 randomized clinical trials on the recent US Food and Drug Administration (FDA)-approved drug, elagolix with hormonal add-back therapy (approved May 29, 2020). In addition, we review the data on the Cerene cryotherapy device (Channel Medsystems), as one might rightly ask, do we need another endometrial ablation device? We will address that question, as this device has some unique features that gynecologists should be aware of. Last, we review a study on the importance of considering quality of life in patients with uterine fibroids, which provides sobering information on the psychosocial aspects of uterine fibroids that all clinicians who care for such patients should be aware of.
Endometrial ablation with a new cryotherapy device: Is less more?
Curlin HL, Cintron LC, Anderson TL. A prospective, multicenter, clinical trial evaluating the safety and effectiveness of the Cerene device to treat heavy menstrual bleeding. J Minim Invasive Gynecol. 2021;28:899-908.
The phrase “less is more,” in the world of architecture and design, is often associated with Ludwig Mies van der Rohe (1886–1969). One could argue that this principle is one key advantage with the addition of yet another non-resectoscopic endometrial ablation device. The Cerene cryotherapy device, FDA approved in 2019, is presented as a simple, disposable device for in-office use that takes advantage of natural cryoanesthesia and results in less tissue destruction than many other ablation methods.
Device reduces bleeding and permits greater ability for future evaluation
Recently, Curlin and colleagues conducted a prospective, multicenter clinical trial to evaluate the safety and efficacy of the Cerene device in reducing menstrual blood loss.1 They followed 230 patients over 12 months and found that 81% (77% with intention-to-treat analysis) met the primary end point of a pictorial blood loss assessment chart (PBLAC) score of 75 or lower. Clinically, this translated to 44% of patients experiencing light bleeding; 27%, eumenorrhea; and 10%, amenorrhea. This is clearly “less” in terms of the rate of amenorrhea in most endometrial ablation studies. However, this also may translate into “more” ability to evaluate the endometrial cavity in the future, as 97% of the patients were able to undergo hysteroscopy at the 12-month mark and, of those, 93% were able to have the entire endometrial cavity assessed.
Further, of 97 patients who had a tubal sterilization, none had symptoms or evidence of postablation tubal sterilization syndrome. Three patients were unable to undergo hysteroscopy due to pain intolerance (2) or cervical stenosis (1). This is important because some gynecologists have expressed concern over intrauterine synechiae, which may result in scarring and associated future difficulty in assessing the endometrium for possible cancer.
Details about the device
The Cerene device is a single use, disposable device that uses cryothermal energy from nitrous oxide that results in a liquid-to-gas phase change within a polyurethane balloon (resulting in a temperature of -86°C) and delivered through a 6-mm sheath. It may be used in uterine cavities that measure between 2.5 and 6.5 cm in length, corresponding to approximately 10 cm in a uterine sound measurement. Treatment time is 2.5 minutes of nitrous oxide flow.
As mentioned, another benefit claimed is that the Cerene device’s cryoanalgesia properties enable the procedure to be more tolerated in the office setting. Of the 230 patients studied in the Curlin trial, no procedures were performed under general anesthesia.1 Medications used included paracervical block (PCB) only (8%), PCB plus nonsteroidal anti-inflammatory drugs (19.8%), PCB plus oral narcotics/anxiolytics (69%), and PCB plus intravenous sedation (2.9%), showing that this device is ideally suited for in-office use.
The rate of serious adverse events was 2.5% (7 total events in 6 patients within 12 months). All serious adverse events were reviewed by a Clinical Events Committee and none were deemed to be device-related events.
Long-term outcomes remain to be seen
For physicians and patients who worry about the ability to access the endometrial cavity in the future, less may be more. It will be interesting to see what the long-term outcomes show with use of the Cerene cryotherapy device, and whether a lower amenorrhea rate will translate into a higher repeat intervention rate or not. Of course, not all are minimalists. As the architect Robert Venturi (1925–2018) was quoted as saying, “Less is a bore.”
The new Cerene cryotherapy endometrial ablation method meets the FDA’s target for reduction of menstrual blood loss, but it has a slightly lower amenorrhea rate than other devices. Its most significant features are the potential for improved analgesia for in-office use and the possibility that there may be less scarring of the endometrial cavity for future assessment if needed.
Continue to: QoL assessment in women with fibroids is useful in evaluating treatment success...
QoL assessment in women with fibroids is useful in evaluating treatment success
Go VAA, Thomas MC, Singh B, et al. A systematic review of the psychosocial impact of fibroids before and after treatment. Am J Obstet Gynecol. 2020;223:674- 708.e8.
In many studies that assess AUB, the primary emphasis generally is placed on quantitation of menstrual bleeding by using PBLAC and alkaline hematin scores. In a systematic review, Go and colleagues argue the case for the importance of measuring the psychosocial impact of abnormal bleeding, emphasizing the concerning finding that many women with fibroids report lower vitality and lower social function scores than women with breast cancer.2
Fibroids associated with inconvenience—and anxiety
The authors analyzed and reviewed 18 randomized trials and 39 observational studies after screening 3,625 records from electronic database searches, with the goal to include only studies with validated quality of life (QoL) questionnaires that were administered both before and after treatment. A highlighted aspect of the reviewed studies was that “control” and “concern” subscales were most affected by fibroids, noting the inconvenience and anxiety that are related to the unpredictable onset and intensity of menses and the feeling of loss of control over one’s health and future.
This systematic review is important because although previous research has shown that fibroids significantly affect QoL, the psychosocial burden of fibroid symptoms had not been compared across different QoL instruments for both disease-specific and general validated health subscales.
Disability levels with fibroids are similar to those with other chronic diseases
Go and colleagues further reported that uterine fibroids have considerable psychosocial impact and lead to poor overall QoL physically and emotionally, with diminished sexual function and increased urinary or defecatory issues. Women with fibroids experienced a level of disability that was similar to that seen in other chronic diseases, and their vitality scores were lower than those associated with heart disease, diabetes, and as mentioned, breast cancer.
The authors concluded that “although objective clinical measures are important to establish a comprehensive understanding of health status, patient reported QoL outcomes play a critical role in evaluating success of a therapy.” They suggested that a larger emphasis on patient-centered care may help to mitigate the psychosocial effects of fibroids.
The study by Go and colleagues highlights the significant psychosocial aspects of the heavy menstrual bleeding associated with fibroids, and the authors found that many women with fibroids score in the range of those with other significant diseases, such as breast cancer and diabetes.
We have noted the trend of including QoL in research, and Go and colleagues make an excellent and compelling argument for this trend using quantitative analysis. It is important to consider this not only in our design of future research but also, and perhaps more importantly, in our clinical care of women as we try to better understand what they are experiencing.
Continue to: What have we learned over the past year about elagolix for uterine fibroids?...
What have we learned over the past year about elagolix for uterine fibroids?
Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
Simon JA, Al-Hendy A, Archer DF, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326.
Al-Hendy A, Bradley L, Owens CD, et al. Predictors of response for elagolix with add-back therapy in women with heavy menstrual bleeding associated with uterine fibroids. Am J Obstet Gynecol. 2021:224-72.e1-72.e50.
Data from the Elaris UF-1 and UF-2 6-month, phase 3 trials3 and the results of the Elaris UF-EXTEND trial with a 6-month extension (totaling 12 months of use)4 were published in 2020, and the 12-month results were discussed in OBG Management (2020;32[7]:35, 39-40). An additional data analysis from the same researchers assessed the effect of elagolix with hormonal add-back therapy in a number of patient subgroups.5 These 3 publications have added to our knowledge of this therapy, and it is worth reviewing them in this context
Design of the elagolix plus hormonal add-back therapy trials
The initial UF-1 and UF-2 trials were 2 identical, double-blind, randomized, placebo-controlled, 6-month, phase 3 trials designed to evaluate the safety and efficacy of elagolix and hormonal add-back therapy.3 UF-1 was conducted at 76 sites in the United States from December 2015 through December 2018, whereas UF-2 was conducted at 77 sites in the United States and Canada from February 2016 through January 2019; the trials were registered separately. Both trials had a 2:1:1 randomization of elagolix (300 mg twice daily) with hormonal add-back therapy (estradiol 1 mg and norethindrone acetate 0.5 mg daily), elagolix alone (300 mg twice daily), or placebo.
In the 6-month studies, the primary end point was both menstrual blood loss of less than 80 mL and at least a 50% reduction of menstrual blood loss as measured by the alkaline hematin method.3 Among several secondary end points was the assessment of QoL using the Uterine Fibroid Symptom QoL questionnaire (UFS-QoL).
Trial results. In UF-1, 68.5% of 206 women, and in UF-2, 76.5% of 189 women, respectively, taking elagolix with add-back therapy met the primary objective. Among women taking elagolix alone, in UF-1, 84.1% of 104 women, and in UF-2, 77% of 95 women, respectively, met criteria. There was improvement in UFS-QoL scores in women receiving elagolix plus add-back therapy with a reduction of symptom severity of -33.2 in UF-1 and -41.4 in UF-2, as compared with the placebo-treated groups (-10.3 and -7.7, respectively).
Adverse effects. Elagolix was associated with a low incidence of serious adverse effects, and the addition of hormonal add-back therapy attenuated the decreases in bone mineral density observed with elagolix alone. In both UF-1 and UF-2 trials, bone mineral density did not differ significantly in the groups of women who received elagolix with hormonal addback therapy versus placebo.
The extension trial results
Of note, in the 12-month study (6-month extension), the authors reported that 87.9% of the women taking elagolix with hormonal add-back therapy met the primary objective.4 Among the women taking elagolix alone, 89.4% met the primary objective.
In a review of the AbbVie-funded extension study, the editorial comments in the Obstetrical and Gynecological Survey expressed concern over the high proportion of data loss, comparing the number of patients joining the extended trial, patients who completed an additional 6 months of treatment, and patients who completed the posttreatment follow-up period of “up to 12 months.”6 Approximately one-third of patients were lost between initial enrollment to the subset who completed follow-up. There was concern that “losses of that magnitude pose a serious threat to validity.”6
Effectiveness in subgroups
Al-Hendy and colleagues analyzed data from the Elaris UF-1 and UF-2 trials to see if the outcomes for elagolix with hormonal addback therapy demonstrated safety and efficacy in subgroups of patients of varying ages, races and ethnicities, baseline menstrual blood loss, body mass indices, fibroid location, and uterine and fibroid volume.5
Results. In all subgroups, they found a statistically significant reduction in blood loss in mean menstrual blood loss volume for those treated with elagolix plus hormonal addback therapy compared with those treated with placebo. As well, in terms of QoL, among all subgroups, the mean change in symptom severity score as well as health-related QoL total score from baseline to month 6 was statistically significantly greater than the mean change in the placebo group.
The bottom line
Elagolix with hormonal add-back therapy appears to be a safe and effective method to reduce menstrual blood loss associated with uterine fibroids. It also has a favorable effect on QoL and appears to have benefits in subgroups of women of varying ages, races and ethnicities, baseline menstrual blood loss, body mass indices, fibroid location, and uterine and fibroid volume. ●
Elagolix plus hormonal add-back therapy provides several advantages to fibroid care, including a pill form that, as a gonadotropin-releasing hormone (GnRH) antagonist, provides much quicker action than GnRH agonists. The hormonal add-back feature seems to improve QoL measures and has a favorable reported bleeding reduction rate. It also appears to be reasonably safe. Although the studies reviewed here may have some weaknesses, it helps to have another therapy to offer to women who have blood loss associated with fibroids. Deciding on the drug’s optimal clinical use has not been fully explored, as it may be a short-term solution to a long-term problem and may not be ideal for all patients with fibroids. Elagolix and hormonal add-back therapy may be advantageous for patients who need to stop bleeding quickly and are trying to decide about their reproductive plans, for patients close to menopause who need a therapy to bridge this gap, and for patients trying to obtain relief between pregnancies.
- Curlin HL, Cintron LC, Anderson TL. A prospective, multicenter, clinical trial evaluating the safety and effectiveness of the Cerene device to treat heavy menstrual bleeding. J Minim Invasive Gynecol. 2021;28:899-908.
- Go VAA, Thomas MC, Singh B, et al. A systematic review of the psychosocial impact of fibroids before and after treatment. Am J Obstet Gynecol. 2020;223:674-708.e8.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
- Simon JA, Al-Hendy A, Archer DF, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326.
- Al-Hendy A, Bradley L, Owens CD, et al. Predictors of response for elagolix with add-back therapy in women with heavy menstrual bleeding associated with uterine fibroids. Am J Obstet Gynecol. 2021:224-72.e1-72.e50.
- Obstetrical & Gynecological Survey. 2020;75:545-547.
Abnormal uterine bleeding (AUB) continues to be a top-10 reason why women present for gynecologic care, which makes keeping up with clinical therapies important. Over the past year, we have learned a tremendous amount about elagolix with hormonal add-back therapy for the treatment of bleeding associated with uterine fibroids. In this Update, we provide an overview from 3 randomized clinical trials on the recent US Food and Drug Administration (FDA)-approved drug, elagolix with hormonal add-back therapy (approved May 29, 2020). In addition, we review the data on the Cerene cryotherapy device (Channel Medsystems), as one might rightly ask, do we need another endometrial ablation device? We will address that question, as this device has some unique features that gynecologists should be aware of. Last, we review a study on the importance of considering quality of life in patients with uterine fibroids, which provides sobering information on the psychosocial aspects of uterine fibroids that all clinicians who care for such patients should be aware of.
Endometrial ablation with a new cryotherapy device: Is less more?
Curlin HL, Cintron LC, Anderson TL. A prospective, multicenter, clinical trial evaluating the safety and effectiveness of the Cerene device to treat heavy menstrual bleeding. J Minim Invasive Gynecol. 2021;28:899-908.
The phrase “less is more,” in the world of architecture and design, is often associated with Ludwig Mies van der Rohe (1886–1969). One could argue that this principle is one key advantage with the addition of yet another non-resectoscopic endometrial ablation device. The Cerene cryotherapy device, FDA approved in 2019, is presented as a simple, disposable device for in-office use that takes advantage of natural cryoanesthesia and results in less tissue destruction than many other ablation methods.
Device reduces bleeding and permits greater ability for future evaluation
Recently, Curlin and colleagues conducted a prospective, multicenter clinical trial to evaluate the safety and efficacy of the Cerene device in reducing menstrual blood loss.1 They followed 230 patients over 12 months and found that 81% (77% with intention-to-treat analysis) met the primary end point of a pictorial blood loss assessment chart (PBLAC) score of 75 or lower. Clinically, this translated to 44% of patients experiencing light bleeding; 27%, eumenorrhea; and 10%, amenorrhea. This is clearly “less” in terms of the rate of amenorrhea in most endometrial ablation studies. However, this also may translate into “more” ability to evaluate the endometrial cavity in the future, as 97% of the patients were able to undergo hysteroscopy at the 12-month mark and, of those, 93% were able to have the entire endometrial cavity assessed.
Further, of 97 patients who had a tubal sterilization, none had symptoms or evidence of postablation tubal sterilization syndrome. Three patients were unable to undergo hysteroscopy due to pain intolerance (2) or cervical stenosis (1). This is important because some gynecologists have expressed concern over intrauterine synechiae, which may result in scarring and associated future difficulty in assessing the endometrium for possible cancer.
Details about the device
The Cerene device is a single use, disposable device that uses cryothermal energy from nitrous oxide that results in a liquid-to-gas phase change within a polyurethane balloon (resulting in a temperature of -86°C) and delivered through a 6-mm sheath. It may be used in uterine cavities that measure between 2.5 and 6.5 cm in length, corresponding to approximately 10 cm in a uterine sound measurement. Treatment time is 2.5 minutes of nitrous oxide flow.
As mentioned, another benefit claimed is that the Cerene device’s cryoanalgesia properties enable the procedure to be more tolerated in the office setting. Of the 230 patients studied in the Curlin trial, no procedures were performed under general anesthesia.1 Medications used included paracervical block (PCB) only (8%), PCB plus nonsteroidal anti-inflammatory drugs (19.8%), PCB plus oral narcotics/anxiolytics (69%), and PCB plus intravenous sedation (2.9%), showing that this device is ideally suited for in-office use.
The rate of serious adverse events was 2.5% (7 total events in 6 patients within 12 months). All serious adverse events were reviewed by a Clinical Events Committee and none were deemed to be device-related events.
Long-term outcomes remain to be seen
For physicians and patients who worry about the ability to access the endometrial cavity in the future, less may be more. It will be interesting to see what the long-term outcomes show with use of the Cerene cryotherapy device, and whether a lower amenorrhea rate will translate into a higher repeat intervention rate or not. Of course, not all are minimalists. As the architect Robert Venturi (1925–2018) was quoted as saying, “Less is a bore.”
The new Cerene cryotherapy endometrial ablation method meets the FDA’s target for reduction of menstrual blood loss, but it has a slightly lower amenorrhea rate than other devices. Its most significant features are the potential for improved analgesia for in-office use and the possibility that there may be less scarring of the endometrial cavity for future assessment if needed.
Continue to: QoL assessment in women with fibroids is useful in evaluating treatment success...
QoL assessment in women with fibroids is useful in evaluating treatment success
Go VAA, Thomas MC, Singh B, et al. A systematic review of the psychosocial impact of fibroids before and after treatment. Am J Obstet Gynecol. 2020;223:674- 708.e8.
In many studies that assess AUB, the primary emphasis generally is placed on quantitation of menstrual bleeding by using PBLAC and alkaline hematin scores. In a systematic review, Go and colleagues argue the case for the importance of measuring the psychosocial impact of abnormal bleeding, emphasizing the concerning finding that many women with fibroids report lower vitality and lower social function scores than women with breast cancer.2
Fibroids associated with inconvenience—and anxiety
The authors analyzed and reviewed 18 randomized trials and 39 observational studies after screening 3,625 records from electronic database searches, with the goal to include only studies with validated quality of life (QoL) questionnaires that were administered both before and after treatment. A highlighted aspect of the reviewed studies was that “control” and “concern” subscales were most affected by fibroids, noting the inconvenience and anxiety that are related to the unpredictable onset and intensity of menses and the feeling of loss of control over one’s health and future.
This systematic review is important because although previous research has shown that fibroids significantly affect QoL, the psychosocial burden of fibroid symptoms had not been compared across different QoL instruments for both disease-specific and general validated health subscales.
Disability levels with fibroids are similar to those with other chronic diseases
Go and colleagues further reported that uterine fibroids have considerable psychosocial impact and lead to poor overall QoL physically and emotionally, with diminished sexual function and increased urinary or defecatory issues. Women with fibroids experienced a level of disability that was similar to that seen in other chronic diseases, and their vitality scores were lower than those associated with heart disease, diabetes, and as mentioned, breast cancer.
The authors concluded that “although objective clinical measures are important to establish a comprehensive understanding of health status, patient reported QoL outcomes play a critical role in evaluating success of a therapy.” They suggested that a larger emphasis on patient-centered care may help to mitigate the psychosocial effects of fibroids.
The study by Go and colleagues highlights the significant psychosocial aspects of the heavy menstrual bleeding associated with fibroids, and the authors found that many women with fibroids score in the range of those with other significant diseases, such as breast cancer and diabetes.
We have noted the trend of including QoL in research, and Go and colleagues make an excellent and compelling argument for this trend using quantitative analysis. It is important to consider this not only in our design of future research but also, and perhaps more importantly, in our clinical care of women as we try to better understand what they are experiencing.
Continue to: What have we learned over the past year about elagolix for uterine fibroids?...
What have we learned over the past year about elagolix for uterine fibroids?
Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
Simon JA, Al-Hendy A, Archer DF, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326.
Al-Hendy A, Bradley L, Owens CD, et al. Predictors of response for elagolix with add-back therapy in women with heavy menstrual bleeding associated with uterine fibroids. Am J Obstet Gynecol. 2021:224-72.e1-72.e50.
Data from the Elaris UF-1 and UF-2 6-month, phase 3 trials3 and the results of the Elaris UF-EXTEND trial with a 6-month extension (totaling 12 months of use)4 were published in 2020, and the 12-month results were discussed in OBG Management (2020;32[7]:35, 39-40). An additional data analysis from the same researchers assessed the effect of elagolix with hormonal add-back therapy in a number of patient subgroups.5 These 3 publications have added to our knowledge of this therapy, and it is worth reviewing them in this context
Design of the elagolix plus hormonal add-back therapy trials
The initial UF-1 and UF-2 trials were 2 identical, double-blind, randomized, placebo-controlled, 6-month, phase 3 trials designed to evaluate the safety and efficacy of elagolix and hormonal add-back therapy.3 UF-1 was conducted at 76 sites in the United States from December 2015 through December 2018, whereas UF-2 was conducted at 77 sites in the United States and Canada from February 2016 through January 2019; the trials were registered separately. Both trials had a 2:1:1 randomization of elagolix (300 mg twice daily) with hormonal add-back therapy (estradiol 1 mg and norethindrone acetate 0.5 mg daily), elagolix alone (300 mg twice daily), or placebo.
In the 6-month studies, the primary end point was both menstrual blood loss of less than 80 mL and at least a 50% reduction of menstrual blood loss as measured by the alkaline hematin method.3 Among several secondary end points was the assessment of QoL using the Uterine Fibroid Symptom QoL questionnaire (UFS-QoL).
Trial results. In UF-1, 68.5% of 206 women, and in UF-2, 76.5% of 189 women, respectively, taking elagolix with add-back therapy met the primary objective. Among women taking elagolix alone, in UF-1, 84.1% of 104 women, and in UF-2, 77% of 95 women, respectively, met criteria. There was improvement in UFS-QoL scores in women receiving elagolix plus add-back therapy with a reduction of symptom severity of -33.2 in UF-1 and -41.4 in UF-2, as compared with the placebo-treated groups (-10.3 and -7.7, respectively).
Adverse effects. Elagolix was associated with a low incidence of serious adverse effects, and the addition of hormonal add-back therapy attenuated the decreases in bone mineral density observed with elagolix alone. In both UF-1 and UF-2 trials, bone mineral density did not differ significantly in the groups of women who received elagolix with hormonal addback therapy versus placebo.
The extension trial results
Of note, in the 12-month study (6-month extension), the authors reported that 87.9% of the women taking elagolix with hormonal add-back therapy met the primary objective.4 Among the women taking elagolix alone, 89.4% met the primary objective.
In a review of the AbbVie-funded extension study, the editorial comments in the Obstetrical and Gynecological Survey expressed concern over the high proportion of data loss, comparing the number of patients joining the extended trial, patients who completed an additional 6 months of treatment, and patients who completed the posttreatment follow-up period of “up to 12 months.”6 Approximately one-third of patients were lost between initial enrollment to the subset who completed follow-up. There was concern that “losses of that magnitude pose a serious threat to validity.”6
Effectiveness in subgroups
Al-Hendy and colleagues analyzed data from the Elaris UF-1 and UF-2 trials to see if the outcomes for elagolix with hormonal addback therapy demonstrated safety and efficacy in subgroups of patients of varying ages, races and ethnicities, baseline menstrual blood loss, body mass indices, fibroid location, and uterine and fibroid volume.5
Results. In all subgroups, they found a statistically significant reduction in blood loss in mean menstrual blood loss volume for those treated with elagolix plus hormonal addback therapy compared with those treated with placebo. As well, in terms of QoL, among all subgroups, the mean change in symptom severity score as well as health-related QoL total score from baseline to month 6 was statistically significantly greater than the mean change in the placebo group.
The bottom line
Elagolix with hormonal add-back therapy appears to be a safe and effective method to reduce menstrual blood loss associated with uterine fibroids. It also has a favorable effect on QoL and appears to have benefits in subgroups of women of varying ages, races and ethnicities, baseline menstrual blood loss, body mass indices, fibroid location, and uterine and fibroid volume. ●
Elagolix plus hormonal add-back therapy provides several advantages to fibroid care, including a pill form that, as a gonadotropin-releasing hormone (GnRH) antagonist, provides much quicker action than GnRH agonists. The hormonal add-back feature seems to improve QoL measures and has a favorable reported bleeding reduction rate. It also appears to be reasonably safe. Although the studies reviewed here may have some weaknesses, it helps to have another therapy to offer to women who have blood loss associated with fibroids. Deciding on the drug’s optimal clinical use has not been fully explored, as it may be a short-term solution to a long-term problem and may not be ideal for all patients with fibroids. Elagolix and hormonal add-back therapy may be advantageous for patients who need to stop bleeding quickly and are trying to decide about their reproductive plans, for patients close to menopause who need a therapy to bridge this gap, and for patients trying to obtain relief between pregnancies.
Abnormal uterine bleeding (AUB) continues to be a top-10 reason why women present for gynecologic care, which makes keeping up with clinical therapies important. Over the past year, we have learned a tremendous amount about elagolix with hormonal add-back therapy for the treatment of bleeding associated with uterine fibroids. In this Update, we provide an overview from 3 randomized clinical trials on the recent US Food and Drug Administration (FDA)-approved drug, elagolix with hormonal add-back therapy (approved May 29, 2020). In addition, we review the data on the Cerene cryotherapy device (Channel Medsystems), as one might rightly ask, do we need another endometrial ablation device? We will address that question, as this device has some unique features that gynecologists should be aware of. Last, we review a study on the importance of considering quality of life in patients with uterine fibroids, which provides sobering information on the psychosocial aspects of uterine fibroids that all clinicians who care for such patients should be aware of.
Endometrial ablation with a new cryotherapy device: Is less more?
Curlin HL, Cintron LC, Anderson TL. A prospective, multicenter, clinical trial evaluating the safety and effectiveness of the Cerene device to treat heavy menstrual bleeding. J Minim Invasive Gynecol. 2021;28:899-908.
The phrase “less is more,” in the world of architecture and design, is often associated with Ludwig Mies van der Rohe (1886–1969). One could argue that this principle is one key advantage with the addition of yet another non-resectoscopic endometrial ablation device. The Cerene cryotherapy device, FDA approved in 2019, is presented as a simple, disposable device for in-office use that takes advantage of natural cryoanesthesia and results in less tissue destruction than many other ablation methods.
Device reduces bleeding and permits greater ability for future evaluation
Recently, Curlin and colleagues conducted a prospective, multicenter clinical trial to evaluate the safety and efficacy of the Cerene device in reducing menstrual blood loss.1 They followed 230 patients over 12 months and found that 81% (77% with intention-to-treat analysis) met the primary end point of a pictorial blood loss assessment chart (PBLAC) score of 75 or lower. Clinically, this translated to 44% of patients experiencing light bleeding; 27%, eumenorrhea; and 10%, amenorrhea. This is clearly “less” in terms of the rate of amenorrhea in most endometrial ablation studies. However, this also may translate into “more” ability to evaluate the endometrial cavity in the future, as 97% of the patients were able to undergo hysteroscopy at the 12-month mark and, of those, 93% were able to have the entire endometrial cavity assessed.
Further, of 97 patients who had a tubal sterilization, none had symptoms or evidence of postablation tubal sterilization syndrome. Three patients were unable to undergo hysteroscopy due to pain intolerance (2) or cervical stenosis (1). This is important because some gynecologists have expressed concern over intrauterine synechiae, which may result in scarring and associated future difficulty in assessing the endometrium for possible cancer.
Details about the device
The Cerene device is a single use, disposable device that uses cryothermal energy from nitrous oxide that results in a liquid-to-gas phase change within a polyurethane balloon (resulting in a temperature of -86°C) and delivered through a 6-mm sheath. It may be used in uterine cavities that measure between 2.5 and 6.5 cm in length, corresponding to approximately 10 cm in a uterine sound measurement. Treatment time is 2.5 minutes of nitrous oxide flow.
As mentioned, another benefit claimed is that the Cerene device’s cryoanalgesia properties enable the procedure to be more tolerated in the office setting. Of the 230 patients studied in the Curlin trial, no procedures were performed under general anesthesia.1 Medications used included paracervical block (PCB) only (8%), PCB plus nonsteroidal anti-inflammatory drugs (19.8%), PCB plus oral narcotics/anxiolytics (69%), and PCB plus intravenous sedation (2.9%), showing that this device is ideally suited for in-office use.
The rate of serious adverse events was 2.5% (7 total events in 6 patients within 12 months). All serious adverse events were reviewed by a Clinical Events Committee and none were deemed to be device-related events.
Long-term outcomes remain to be seen
For physicians and patients who worry about the ability to access the endometrial cavity in the future, less may be more. It will be interesting to see what the long-term outcomes show with use of the Cerene cryotherapy device, and whether a lower amenorrhea rate will translate into a higher repeat intervention rate or not. Of course, not all are minimalists. As the architect Robert Venturi (1925–2018) was quoted as saying, “Less is a bore.”
The new Cerene cryotherapy endometrial ablation method meets the FDA’s target for reduction of menstrual blood loss, but it has a slightly lower amenorrhea rate than other devices. Its most significant features are the potential for improved analgesia for in-office use and the possibility that there may be less scarring of the endometrial cavity for future assessment if needed.
Continue to: QoL assessment in women with fibroids is useful in evaluating treatment success...
QoL assessment in women with fibroids is useful in evaluating treatment success
Go VAA, Thomas MC, Singh B, et al. A systematic review of the psychosocial impact of fibroids before and after treatment. Am J Obstet Gynecol. 2020;223:674- 708.e8.
In many studies that assess AUB, the primary emphasis generally is placed on quantitation of menstrual bleeding by using PBLAC and alkaline hematin scores. In a systematic review, Go and colleagues argue the case for the importance of measuring the psychosocial impact of abnormal bleeding, emphasizing the concerning finding that many women with fibroids report lower vitality and lower social function scores than women with breast cancer.2
Fibroids associated with inconvenience—and anxiety
The authors analyzed and reviewed 18 randomized trials and 39 observational studies after screening 3,625 records from electronic database searches, with the goal to include only studies with validated quality of life (QoL) questionnaires that were administered both before and after treatment. A highlighted aspect of the reviewed studies was that “control” and “concern” subscales were most affected by fibroids, noting the inconvenience and anxiety that are related to the unpredictable onset and intensity of menses and the feeling of loss of control over one’s health and future.
This systematic review is important because although previous research has shown that fibroids significantly affect QoL, the psychosocial burden of fibroid symptoms had not been compared across different QoL instruments for both disease-specific and general validated health subscales.
Disability levels with fibroids are similar to those with other chronic diseases
Go and colleagues further reported that uterine fibroids have considerable psychosocial impact and lead to poor overall QoL physically and emotionally, with diminished sexual function and increased urinary or defecatory issues. Women with fibroids experienced a level of disability that was similar to that seen in other chronic diseases, and their vitality scores were lower than those associated with heart disease, diabetes, and as mentioned, breast cancer.
The authors concluded that “although objective clinical measures are important to establish a comprehensive understanding of health status, patient reported QoL outcomes play a critical role in evaluating success of a therapy.” They suggested that a larger emphasis on patient-centered care may help to mitigate the psychosocial effects of fibroids.
The study by Go and colleagues highlights the significant psychosocial aspects of the heavy menstrual bleeding associated with fibroids, and the authors found that many women with fibroids score in the range of those with other significant diseases, such as breast cancer and diabetes.
We have noted the trend of including QoL in research, and Go and colleagues make an excellent and compelling argument for this trend using quantitative analysis. It is important to consider this not only in our design of future research but also, and perhaps more importantly, in our clinical care of women as we try to better understand what they are experiencing.
Continue to: What have we learned over the past year about elagolix for uterine fibroids?...
What have we learned over the past year about elagolix for uterine fibroids?
Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
Simon JA, Al-Hendy A, Archer DF, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326.
Al-Hendy A, Bradley L, Owens CD, et al. Predictors of response for elagolix with add-back therapy in women with heavy menstrual bleeding associated with uterine fibroids. Am J Obstet Gynecol. 2021:224-72.e1-72.e50.
Data from the Elaris UF-1 and UF-2 6-month, phase 3 trials3 and the results of the Elaris UF-EXTEND trial with a 6-month extension (totaling 12 months of use)4 were published in 2020, and the 12-month results were discussed in OBG Management (2020;32[7]:35, 39-40). An additional data analysis from the same researchers assessed the effect of elagolix with hormonal add-back therapy in a number of patient subgroups.5 These 3 publications have added to our knowledge of this therapy, and it is worth reviewing them in this context
Design of the elagolix plus hormonal add-back therapy trials
The initial UF-1 and UF-2 trials were 2 identical, double-blind, randomized, placebo-controlled, 6-month, phase 3 trials designed to evaluate the safety and efficacy of elagolix and hormonal add-back therapy.3 UF-1 was conducted at 76 sites in the United States from December 2015 through December 2018, whereas UF-2 was conducted at 77 sites in the United States and Canada from February 2016 through January 2019; the trials were registered separately. Both trials had a 2:1:1 randomization of elagolix (300 mg twice daily) with hormonal add-back therapy (estradiol 1 mg and norethindrone acetate 0.5 mg daily), elagolix alone (300 mg twice daily), or placebo.
In the 6-month studies, the primary end point was both menstrual blood loss of less than 80 mL and at least a 50% reduction of menstrual blood loss as measured by the alkaline hematin method.3 Among several secondary end points was the assessment of QoL using the Uterine Fibroid Symptom QoL questionnaire (UFS-QoL).
Trial results. In UF-1, 68.5% of 206 women, and in UF-2, 76.5% of 189 women, respectively, taking elagolix with add-back therapy met the primary objective. Among women taking elagolix alone, in UF-1, 84.1% of 104 women, and in UF-2, 77% of 95 women, respectively, met criteria. There was improvement in UFS-QoL scores in women receiving elagolix plus add-back therapy with a reduction of symptom severity of -33.2 in UF-1 and -41.4 in UF-2, as compared with the placebo-treated groups (-10.3 and -7.7, respectively).
Adverse effects. Elagolix was associated with a low incidence of serious adverse effects, and the addition of hormonal add-back therapy attenuated the decreases in bone mineral density observed with elagolix alone. In both UF-1 and UF-2 trials, bone mineral density did not differ significantly in the groups of women who received elagolix with hormonal addback therapy versus placebo.
The extension trial results
Of note, in the 12-month study (6-month extension), the authors reported that 87.9% of the women taking elagolix with hormonal add-back therapy met the primary objective.4 Among the women taking elagolix alone, 89.4% met the primary objective.
In a review of the AbbVie-funded extension study, the editorial comments in the Obstetrical and Gynecological Survey expressed concern over the high proportion of data loss, comparing the number of patients joining the extended trial, patients who completed an additional 6 months of treatment, and patients who completed the posttreatment follow-up period of “up to 12 months.”6 Approximately one-third of patients were lost between initial enrollment to the subset who completed follow-up. There was concern that “losses of that magnitude pose a serious threat to validity.”6
Effectiveness in subgroups
Al-Hendy and colleagues analyzed data from the Elaris UF-1 and UF-2 trials to see if the outcomes for elagolix with hormonal addback therapy demonstrated safety and efficacy in subgroups of patients of varying ages, races and ethnicities, baseline menstrual blood loss, body mass indices, fibroid location, and uterine and fibroid volume.5
Results. In all subgroups, they found a statistically significant reduction in blood loss in mean menstrual blood loss volume for those treated with elagolix plus hormonal addback therapy compared with those treated with placebo. As well, in terms of QoL, among all subgroups, the mean change in symptom severity score as well as health-related QoL total score from baseline to month 6 was statistically significantly greater than the mean change in the placebo group.
The bottom line
Elagolix with hormonal add-back therapy appears to be a safe and effective method to reduce menstrual blood loss associated with uterine fibroids. It also has a favorable effect on QoL and appears to have benefits in subgroups of women of varying ages, races and ethnicities, baseline menstrual blood loss, body mass indices, fibroid location, and uterine and fibroid volume. ●
Elagolix plus hormonal add-back therapy provides several advantages to fibroid care, including a pill form that, as a gonadotropin-releasing hormone (GnRH) antagonist, provides much quicker action than GnRH agonists. The hormonal add-back feature seems to improve QoL measures and has a favorable reported bleeding reduction rate. It also appears to be reasonably safe. Although the studies reviewed here may have some weaknesses, it helps to have another therapy to offer to women who have blood loss associated with fibroids. Deciding on the drug’s optimal clinical use has not been fully explored, as it may be a short-term solution to a long-term problem and may not be ideal for all patients with fibroids. Elagolix and hormonal add-back therapy may be advantageous for patients who need to stop bleeding quickly and are trying to decide about their reproductive plans, for patients close to menopause who need a therapy to bridge this gap, and for patients trying to obtain relief between pregnancies.
- Curlin HL, Cintron LC, Anderson TL. A prospective, multicenter, clinical trial evaluating the safety and effectiveness of the Cerene device to treat heavy menstrual bleeding. J Minim Invasive Gynecol. 2021;28:899-908.
- Go VAA, Thomas MC, Singh B, et al. A systematic review of the psychosocial impact of fibroids before and after treatment. Am J Obstet Gynecol. 2020;223:674-708.e8.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
- Simon JA, Al-Hendy A, Archer DF, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326.
- Al-Hendy A, Bradley L, Owens CD, et al. Predictors of response for elagolix with add-back therapy in women with heavy menstrual bleeding associated with uterine fibroids. Am J Obstet Gynecol. 2021:224-72.e1-72.e50.
- Obstetrical & Gynecological Survey. 2020;75:545-547.
- Curlin HL, Cintron LC, Anderson TL. A prospective, multicenter, clinical trial evaluating the safety and effectiveness of the Cerene device to treat heavy menstrual bleeding. J Minim Invasive Gynecol. 2021;28:899-908.
- Go VAA, Thomas MC, Singh B, et al. A systematic review of the psychosocial impact of fibroids before and after treatment. Am J Obstet Gynecol. 2020;223:674-708.e8.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
- Simon JA, Al-Hendy A, Archer DF, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326.
- Al-Hendy A, Bradley L, Owens CD, et al. Predictors of response for elagolix with add-back therapy in women with heavy menstrual bleeding associated with uterine fibroids. Am J Obstet Gynecol. 2021:224-72.e1-72.e50.
- Obstetrical & Gynecological Survey. 2020;75:545-547.
VTE prevention: Patient selection and treatment planning throughout pregnancy
Pregnancy and the postpartum period are times of increased risk for venous thromboembolism (VTE). While VTE is a rare event overall, it is responsible for more than 9% of maternal deaths in the United States.1 The increased risk of VTE exists throughout pregnancy, rising in the third trimester.2 The highest-risk period is the first 6 weeks postpartum, likely peaking in the first 2 to 3 weeks and returning to baseline at about 12 weeks postpartum.2,3
To reduce this source of maternal harm, the National Partnership for Maternal Safety and the Council on Patient Safety in Women’s Health Care recommend the use of VTE prevention bundles. Bundles include standard assessment of risk during prenatal care, any admission to the hospital, and postpartum coupled with standard recommendations for treatment.4-6 Multiple published guidelines are available for prevention of VTE in pregnancy, and they provide varying recommendations on patient selection and treatment. Many of these recommendations are based on low quality of evidence, making the choice of standard practice difficult.
In this article, I attempt to simplify patient selection and treatment based on currently published guidelines from the American College of Obstetricians and Gynecologists (ACOG), Royal College of Obstetricians and Gynaecologists (RCOG), American College of Chest Physicians (CHEST), American Society of Hematology (ASH), and expert opinion.
Determining VTE risk and need for prophylaxis
CASE 1 Woman with factor V Leiden
A 25-year-old woman (G1P0) presents for her initial prenatal visit. She says she is a carrier for factor V Leiden but has never had a clot. She was tested after her sister had a VTE. She asks, does she need VTE prophylaxis before her delivery?
What are the considerations and options for this patient?
Options for VTE prophylaxis
Before considering patients at risk for VTE, it is helpful to review the options for prophylaxis. Patients can undergo clinical surveillance or routine care with attention to VTE symptoms and a low threshold for workup.
There are 3 categories of chemoprophylaxis for prevention of VTE. (TABLE 1 offers examples of dosing regimens.) No strategy has been proven optimal over another:
- prophylactic-dose: the lowest, fixed dose.
- intermediate-dose: lacks a standard definition and is any dose higher than prophylactic-dose but lower than therapeutic-dose. This includes fixed twice-daily doses, weight-based doses, and incrementally increasing doses.
- therapeutic-dose: typically used for treatment but mentioned here since patients with high-risk conditions may use it for prevention of VTE.
The preferred agent for VTE chemoprophylaxis is low molecular weight heparin (LMWH; dalteparin, enoxaparin). LMWH has a lower risk of complications than unfractionated heparin (UFH) and can be injected once daily. LMWH and UFH do not cross the placenta. LMWH and UFH are safe in breastfeeding. Oral direct thrombin inhibitors and anti-Xa inhibitors are not recommended in pregnancy or lactation at this time. Warfarin is avoided in pregnancy except in situations with mechanical heart valves, which will not be addressed here. Patients taking warfarin for long-term anticoagulation can transition back while breastfeeding with appropriate bridging.
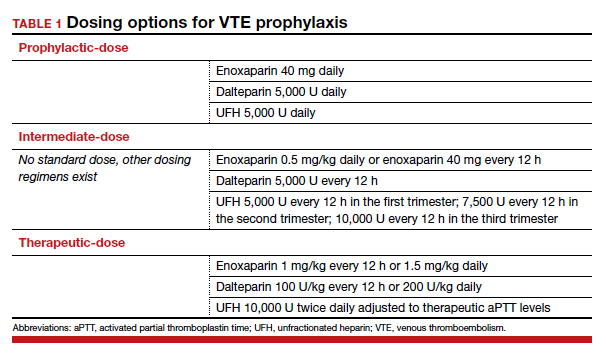
Expert opinion recommends antepartum chemoprophylaxis when there is a 2% to 3% risk of VTE in pregnancy.7-9 This is balanced against an approximately 2% overall risk of bleeding, with less than 1% risk of bleeding antepartum.9
Continue to: Risk factors for VTE...
Risk factors for VTE
History of VTE. The most important risk factor for VTE is a personal history of prior VTE.6 Recurrence risks have been widely reported and depend on the factors surrounding the initial event. For patients with a prior provoked deep vein thrombosis (DVT; associated with trauma or surgery), the antepartum VTE risk likely is less than 1%, and VTE chemoprophylaxis is not recommended antepartum.7
For patients with a prior VTE that was not associated with surgery or trauma (unprovoked), the risk is approximately 3%; for prior VTE related to pregnancy or hormonal contraception, the risk is approximately 6%.7 For both of these groups, prophylactic-dose antepartum is recommended. Patients with recurrent VTE are often taking long-term anticoagulation. Anyone on long-term anticoagulation should be placed on therapeutic-dose antepartum. For patients not receiving long-term anticoagulation, consider a hematology consultation when available, and begin an intermediate-dose or therapeutic-dose regimen after assessing other risk factors and the risk of bleeding and discussing treatment with the patient.
Thrombophilias. The next most important risk factor is the presence of inherited thrombophilias.6 Factor V homozygote, prothrombin G20210A mutation homozygote, antithrombin deficiency, and combined factor V heterozygote and prothrombin G20210A heterozygote (also called compound heterozygote) have the strongest association with VTE in pregnancy.8 It is recommended that patients with these high-risk thrombophilias receive prophylactic-dose antepartum.8
Factor V heterozygote, prothrombin G20210A mutation heterozygote, and protein C or protein S deficiency are considered low-risk thrombophilias. Patients with low-risk thrombophilias and no personal history of VTE or first-degree relative with VTE can be monitored with clinical surveillance antepartum. However, if a family history of VTE or other risk factors for VTE are present, antepartum prophylactic-dose is recommended. Clinical factors to consider antepartum include obesity, age older than 35 years, parity of 3 or higher, varicose veins, immobility, smoking, assisted reproductive technology use, multiple gestation, and preeclampsia.10
Antiphospholipid syndrome (APS) is another high-risk condition. For patients not taking long-term anticoagulation antepartum, prophylactic-dose is recommended. For patients on long-term anticoagulation, therapeutic-dose is recommended.
Other medical conditions. Patients with medical conditions that place them at high risk for VTE may warrant prophylactic-dose antepartum. These include active cancer, active systemic lupus erythematosus, sickle cell disease, nephropathy, and inflammatory bowel disease.10 This decision can be made in conjunction with other specialists caring for the patient.
Antepartum prophylactic-dose is not recommended for low-risk patients as there is less than 1% risk of VTE.7 (TABLE 2 summarizes antepartum chemoprophylaxis recommendations.)
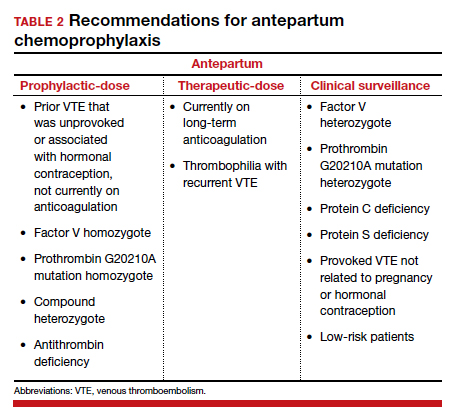
CASE 1 continued Patient develops another VTE risk factor
The patient is being followed with clinical surveillance. At 19 weeks’ gestation, she presents to the emergency department with shortness of breath and fever. She is diagnosed with COVID-19 and is admitted by a medicine service. They call the OB team to ask for recommendations regarding anticoagulation.
What should the next steps include?
Hospitalization and nonobstetric surgery are risk factors for VTE. Many hospitals use a standardized assessment for all inpatients, such as the Padua or Caprini VTE risk assessment scores. These can be modified for use in pregnant patients, although neither scoring system is currently validated for use in pregnancy.5 For any pregnant patient admitted to the hospital, mechanical prophylaxis is recommended.
COVID-19. Infection with the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and its associated clinical syndrome, COVID-19, is associated with increased rates of VTE. Recommendations for pregnant patients with COVID-19 are the same as for the general population. During hospitalization for COVID-19, pregnant patients should be placed on prophylactic-dose chemoprophylaxis. Patients should not be discharged home on chemoprophylaxis, and patients managed as outpatients for their disease do not need chemoprophylaxis.11
Management approach. Prophylactic-dose administration is recommended during hospital stay for all patients admitted with anticipated length of stay of 3 days or longer and who are not at high risk for bleeding or delivery.10 Both LMWH and UFH are options for inpatients. For any nonobstetric surgery or admission, LMWH may be most appropriate. However, as most obstetrics admissions are at increased risk for delivery, UFH 5,000 U twice daily to 3 times daily is the best option to increase the chances for neuraxial anesthesia. (I review anesthesia considerations for delivery later in this article.) For patients at high risk for bleeding or delivery, mechanical prophylaxis alone, with elastic stockings or pneumatic compression devices, can be used.
Continue to: CASE 1 continued Patient is discharged home...
CASE 1 continued Patient is discharged home
The patient received enoxaparin while she was in the hospital. She is now discharged and doing well. She asks, will she need anticoagulation prophylaxis after delivery?
How would you counsel her?
Chemoprophylaxis in the postpartum period
With no risk of fetal harm and a higher risk of VTE per day, the threshold for chemoprophylaxis is lower in the postpartum period. The risk of postpartum bleeding is less than 1%, with the most common complication being wound hematomas (0.61%).9 For this case patient, the COVID-19 diagnosis does not alter the recommendations for postpartum chemoprophylaxis. Additionally, as the need for neuraxial anesthesia has passed, the use of intermediate-dose chemoprophylaxis over prophylactic-dose is advocated in the postpartum period, especially in obese patients.12
As mentioned previously, there is no standard definition of intermediate-dose. Data suggest that a weight-based intermediate-dose is most likely to achieve therapeutic levels of anti-Xa in this high-risk population compared with a fixed dose.13,14 For example, enoxaparin 0.5 mg/kg twice daily is recommended for patients with class 3 obesity or higher by the Society for Maternal-Fetal Medicine.12
As a rule, anyone who was on chemoprophylaxis antepartum should be continued on at least an equivalent dose for 6 weeks postpartum. Postpartum, patients with any prior DVT should take prophylactic-dose or intermediate-dose chemoprophylaxis for 6 weeks. Patients with a known high-risk thrombophilia should receive prophylactic-dose or intermediate-dose chemoprophylaxis postpartum for 6 weeks. For patients with a low-risk thrombophilia, prophylactic-dose or intermediate-dose chemoprophylaxis is recommended for 6 weeks.
For low-risk patients without prior VTE or thrombophilia, standardized risk assessment is recommended.
Cesarean delivery
Cesarean delivery (CD) is a risk factor for postpartum VTE.9 A universal chemoprophylaxis strategy has not been proven in this patient population. Mechanical prophylaxis with sequential compression devices is recommended for all patients undergoing CD pre-procedure and until patients are fully ambulatory.8,9 Early ambulation also should be encouraged.
Many risk assessment models are available for postoperative VTE prevention, and they have widely different chemoprophylaxis rates. Studies have shown chemoprophylaxis rates of 85% by RCOG, 1% by ACOG, 35% by CHEST, 94% by Caprini, and less than 1% by Padua.15,16 In addition to the antepartum patient-specific risk factors mentioned, postpartum risk factors include infection, postpartum hemorrhage, and transfusion. Based on data extrapolated from the nonobstetric literature, chemoprophylaxis is recommended until discharge from the hospital unless risk factors are expected to continue.9
Neuraxial anesthesia
For patients who require postpartum chemoprophylaxis, the Society for Obstetric Anesthesia and Perinatology (SOAP) offers evidence-based guidelines for use after neuraxial anesthesia. UFH can be initiated 1 hour or longer after a neuraxial procedure and 1 hour or longer after catheter removal. Prophylactic-dose LMWH can be restarted at 12 hours or longer after a neuraxial procedure and at 4 to 6 hours or longer after catheter removal. For patients restarting intermediate-dose or therapeutic-dose, the recommendations are to wait 24 hours or longer after a neuraxial procedure and 4 hours or longer after catheter removal.17 Timing can be individualized based on the patient’s risk of hemorrhage and surgical bleeding. Although it may be tempting to delay chemoprophylaxis in the setting of bleeding, postpartum hemorrhage and transfusion increase the risks of VTE. In this setting, it is best to consider the use of UFH, which safely can be started earlier than LMWH.
For patients without neuraxial anesthesia, ACOG recommends chemoprophylaxis 4 to 6 hours after vaginal delivery and 6 to 12 hours after CD.8 (TABLE 3 summarizes recommendations for postpartum chemoprophylaxis.)
Continue to: Adjusting the anticoagulation regimen...
Adjusting the anticoagulation regimen
CASE 2 Pregnant woman with prior VTE
A 36-year-old woman (G1P0) with prior VTE is taking enoxaparin 40 mg daily. She asks, does she need any blood work for her anticoagulation?
What would you test for?
Increased renal clearance of LMWH and increased volume of distribution during pregnancy has led to the consideration of monitoring anti-Xa levels. There are no published standards or recommendations for dose adjustment. At this time, anti-Xa level monitoring antepartum is not recommended, but it may be considered when a patient is at the extremes of weight. With a weight-based strategy in the postpartum period, monitoring is not recommended as studies show a higher likelihood of therapeutic anti-Xa levels with this approach.13,14 This is an active area of research, and these recommendations may change.
For prophylactic-dose or intermediate-dose anticoagulation, a peak anti-Xa level of 0.2 to 0.6 U/mL is generally accepted as the target. For therapeutic-dose, a peak anti-Xa level of 0.6 to 1.2 U/mL is generally accepted as the therapeutic range. This blood draw must be collected 4 hours after the third dose.
CASE 2 continued Anticoagulation considerations nearing delivery
The patient is now at 36 weeks’ gestation, and she asks, what should be done regarding her anticoagulation prior to delivery?
What would be an appropriate approach?
Traditionally, patients were transitioned to UFH at 36 weeks and allowed to present in spontaneous labor to increase the likelihood of neuraxial anesthesia. The alternative is to continue prophylactic-dose LMWH until a scheduled delivery. While the SOAP guidelines establish the timeframe that is safe to proceed with neuraxial anesthesia, there is variation in practice, so consider discussing this with your anesthesia providers.
SOAP considers prophylactic-dose UFH to be 5,000 U 2 to 3 times per day. In this setting, neuraxial anesthesia can be placed more than 4 to 6 hours from the last dose.17 But due to the pharmacokinetics of pregnancy, ACOG recommends 10,000 U in the third trimester.8 This dose is considered intermediate-dose by SOAP, and 12 hours or longer plus a normal activated partial thromboplastin time (aPTT) or undetectable anti-Xa level are required prior to neuraxial anesthesia. This is the same time allowed for prophylactic-dose LMWH without lab work. Prophylactic-dose LMWH is considered to be enoxaparin 40 mg or less daily or 30 mg twice daily, and dalteparin 5,000 U daily. For therapeutic-dose LMWH or UFH, 24 hours or more from last dose is recommended prior to neuraxial anesthesia. For intermediate-dose LMWH, data are limited to recommend anything between 12 and 24 hours.17
In my practice, we favor a shared decision-making approach with patients. We discuss the likelihood of labor prior to 39 weeks based on a patient’s history, the importance of neuraxial anesthesia to the patient, and the importance of the number of daily injections. Most patients continue enoxaparin until a scheduled induction, and they are instructed to skip their dose if labor symptoms begin. Patients at high risk for preterm delivery can be transitioned to heparin earlier than 36 weeks. ●
- Creanga AA, Syverson C, Seed K, et al. Pregnancy-related mortality in the United States, 2011-2013. Obstet Gynecol. 2017;130:366-373. doi: 10.1097/AOG.0000000000002114.
- Kourlaba G, Relakis J, Kontodimas S, et al. A systematic review and meta-analysis of the epidemiology and burden of venous thromboembolism among pregnant women. Int J Gynaecol Obstet. 2016;132:4-10. doi: 10.1016/j.ijgo.2015.06.054.
- Sultan AA, West J, Tata LJ, et al. Risk of first venous thromboembolism in and around pregnancy: a population-based cohort study. Br J Haematol. 2012;156:366-373. doi: 10.1111/j.1365-2141.2011.08956.x.
- American College of Obstetricians and Gynecologists. Council on Patient Safety in Women’s Health Care: maternal venous thromboembolism (+AIM). 2015. https://safehealthcareforeverywoman.org/council/patient-safety-bundles/maternal-safety-bundles/maternal-venous-thromboembolism-aim/. Accessed February 26, 2021.
- Urato AC, Abi-Jaoude E, Abramson J, et al. National Partnership for Maternal Safety: consensus bundle on venous thromboembolism. Obstet Gynecol. 2019;134:1115-1117. doi: 10.1097/AOG.0000000000003540.
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics. ACOG practice bulletin no. 196: thromboembolism in pregnancy. Obstet Gynecol. 2018;132:e1-e17. doi: 10.1097/AOG.0000000000002706.
- Bates SM, Rajasekhar A, Middeldorp S, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: venous thromboembolism in the context of pregnancy. Blood Adv. 2018;2:3317-3359. doi: 10.1182/bloodadvances.2018024802.
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 197: inherited thrombophilias in pregnancy. Obstet Gynecol. 2018;132:e18-e34. doi: 10.1097/AOG.0000000000002703.
- Bates SM, Greer IA, Middeldorp S, et al. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2, suppl):e691S-e736S. doi: 10.1378/chest.11-2300.
- Lamont MC, McDermott C, Thomson AJ, et al. United Kingdom recommendations for obstetric venous thromboembolism prophylaxis: evidence and rationale. Semin Perinatol. 2019;43:222-228. doi: 10.1053/j.semperi.2019.03.008.
- National Institutes of Health. COVID-19 Treatment Guidelines Panel. Coronavirus disease 2019 (COVID-19) treatment guidelines. https://www.covid19treatmentguidelines.nih.gov/. Accessed February 26, 2021.
- Society for Maternal-Fetal Medicine (SMFM); Pacheco LD, Saade G, Metz TD. Society for Maternal-Fetal Medicine Consult Series #51: thromboembolism prophylaxis for cesarean delivery. Am J Obstet Gynecol. 2020;223:B11-B17. doi: 10.1016/j.ajog.2020.04.032.
- Overcash RT, Somers AT, LaCoursiere DY. Enoxaparin dosing after cesarean delivery in morbidly obese women. Obstet Gynecol. 2015;125:1371-1376. doi: 10.1097/AOG.0000000000000873.
- Hiscock RJ, Casey E, Simmons SW, et al. Peak plasma anti-Xa levels after first and third doses of enoxaparin in women receiving weight-based thromboprophylaxis following caesarean section: a prospective cohort study. Int J Obstet Anesth. 2013;22:280-288. doi: 10.1016/j.ijoa.2013.05.008.
- Palmerola KL, D’Alton ME, Brock CO, et al. A comparison of recommendations for pharmacologic thromboembolism prophylaxis after caesarean delivery from three major guidelines. BJOG. 2016;123:2157-2162. doi: 10.1111/1471-0528.13706.
- Tran JP, Stribling SS, Ibezim UC, et al. Performance of risk assessment models for peripartum thromboprophylaxis. Reprod Sci. 2019;26:1243-1248. doi: 10.1177/1933719118813197.
- Leffert L, Butwick A, Carvalho B, et al; members of the SOAP VTE Taskforce. The Society for Obstetric Anesthesia and Perinatology consensus statement on the anesthetic management of pregnant and postpartum women receiving thromboprophylaxis or higher dose anticoagulants. Anesth Analg. 2018;126:928-944. doi: 10.1213/ANE.0000000000002530.

Pregnancy and the postpartum period are times of increased risk for venous thromboembolism (VTE). While VTE is a rare event overall, it is responsible for more than 9% of maternal deaths in the United States.1 The increased risk of VTE exists throughout pregnancy, rising in the third trimester.2 The highest-risk period is the first 6 weeks postpartum, likely peaking in the first 2 to 3 weeks and returning to baseline at about 12 weeks postpartum.2,3
To reduce this source of maternal harm, the National Partnership for Maternal Safety and the Council on Patient Safety in Women’s Health Care recommend the use of VTE prevention bundles. Bundles include standard assessment of risk during prenatal care, any admission to the hospital, and postpartum coupled with standard recommendations for treatment.4-6 Multiple published guidelines are available for prevention of VTE in pregnancy, and they provide varying recommendations on patient selection and treatment. Many of these recommendations are based on low quality of evidence, making the choice of standard practice difficult.
In this article, I attempt to simplify patient selection and treatment based on currently published guidelines from the American College of Obstetricians and Gynecologists (ACOG), Royal College of Obstetricians and Gynaecologists (RCOG), American College of Chest Physicians (CHEST), American Society of Hematology (ASH), and expert opinion.
Determining VTE risk and need for prophylaxis
CASE 1 Woman with factor V Leiden
A 25-year-old woman (G1P0) presents for her initial prenatal visit. She says she is a carrier for factor V Leiden but has never had a clot. She was tested after her sister had a VTE. She asks, does she need VTE prophylaxis before her delivery?
What are the considerations and options for this patient?
Options for VTE prophylaxis
Before considering patients at risk for VTE, it is helpful to review the options for prophylaxis. Patients can undergo clinical surveillance or routine care with attention to VTE symptoms and a low threshold for workup.
There are 3 categories of chemoprophylaxis for prevention of VTE. (TABLE 1 offers examples of dosing regimens.) No strategy has been proven optimal over another:
- prophylactic-dose: the lowest, fixed dose.
- intermediate-dose: lacks a standard definition and is any dose higher than prophylactic-dose but lower than therapeutic-dose. This includes fixed twice-daily doses, weight-based doses, and incrementally increasing doses.
- therapeutic-dose: typically used for treatment but mentioned here since patients with high-risk conditions may use it for prevention of VTE.
The preferred agent for VTE chemoprophylaxis is low molecular weight heparin (LMWH; dalteparin, enoxaparin). LMWH has a lower risk of complications than unfractionated heparin (UFH) and can be injected once daily. LMWH and UFH do not cross the placenta. LMWH and UFH are safe in breastfeeding. Oral direct thrombin inhibitors and anti-Xa inhibitors are not recommended in pregnancy or lactation at this time. Warfarin is avoided in pregnancy except in situations with mechanical heart valves, which will not be addressed here. Patients taking warfarin for long-term anticoagulation can transition back while breastfeeding with appropriate bridging.

Expert opinion recommends antepartum chemoprophylaxis when there is a 2% to 3% risk of VTE in pregnancy.7-9 This is balanced against an approximately 2% overall risk of bleeding, with less than 1% risk of bleeding antepartum.9
Continue to: Risk factors for VTE...
Risk factors for VTE
History of VTE. The most important risk factor for VTE is a personal history of prior VTE.6 Recurrence risks have been widely reported and depend on the factors surrounding the initial event. For patients with a prior provoked deep vein thrombosis (DVT; associated with trauma or surgery), the antepartum VTE risk likely is less than 1%, and VTE chemoprophylaxis is not recommended antepartum.7
For patients with a prior VTE that was not associated with surgery or trauma (unprovoked), the risk is approximately 3%; for prior VTE related to pregnancy or hormonal contraception, the risk is approximately 6%.7 For both of these groups, prophylactic-dose antepartum is recommended. Patients with recurrent VTE are often taking long-term anticoagulation. Anyone on long-term anticoagulation should be placed on therapeutic-dose antepartum. For patients not receiving long-term anticoagulation, consider a hematology consultation when available, and begin an intermediate-dose or therapeutic-dose regimen after assessing other risk factors and the risk of bleeding and discussing treatment with the patient.
Thrombophilias. The next most important risk factor is the presence of inherited thrombophilias.6 Factor V homozygote, prothrombin G20210A mutation homozygote, antithrombin deficiency, and combined factor V heterozygote and prothrombin G20210A heterozygote (also called compound heterozygote) have the strongest association with VTE in pregnancy.8 It is recommended that patients with these high-risk thrombophilias receive prophylactic-dose antepartum.8
Factor V heterozygote, prothrombin G20210A mutation heterozygote, and protein C or protein S deficiency are considered low-risk thrombophilias. Patients with low-risk thrombophilias and no personal history of VTE or first-degree relative with VTE can be monitored with clinical surveillance antepartum. However, if a family history of VTE or other risk factors for VTE are present, antepartum prophylactic-dose is recommended. Clinical factors to consider antepartum include obesity, age older than 35 years, parity of 3 or higher, varicose veins, immobility, smoking, assisted reproductive technology use, multiple gestation, and preeclampsia.10
Antiphospholipid syndrome (APS) is another high-risk condition. For patients not taking long-term anticoagulation antepartum, prophylactic-dose is recommended. For patients on long-term anticoagulation, therapeutic-dose is recommended.
Other medical conditions. Patients with medical conditions that place them at high risk for VTE may warrant prophylactic-dose antepartum. These include active cancer, active systemic lupus erythematosus, sickle cell disease, nephropathy, and inflammatory bowel disease.10 This decision can be made in conjunction with other specialists caring for the patient.
Antepartum prophylactic-dose is not recommended for low-risk patients as there is less than 1% risk of VTE.7 (TABLE 2 summarizes antepartum chemoprophylaxis recommendations.)

CASE 1 continued Patient develops another VTE risk factor
The patient is being followed with clinical surveillance. At 19 weeks’ gestation, she presents to the emergency department with shortness of breath and fever. She is diagnosed with COVID-19 and is admitted by a medicine service. They call the OB team to ask for recommendations regarding anticoagulation.
What should the next steps include?
Hospitalization and nonobstetric surgery are risk factors for VTE. Many hospitals use a standardized assessment for all inpatients, such as the Padua or Caprini VTE risk assessment scores. These can be modified for use in pregnant patients, although neither scoring system is currently validated for use in pregnancy.5 For any pregnant patient admitted to the hospital, mechanical prophylaxis is recommended.
COVID-19. Infection with the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and its associated clinical syndrome, COVID-19, is associated with increased rates of VTE. Recommendations for pregnant patients with COVID-19 are the same as for the general population. During hospitalization for COVID-19, pregnant patients should be placed on prophylactic-dose chemoprophylaxis. Patients should not be discharged home on chemoprophylaxis, and patients managed as outpatients for their disease do not need chemoprophylaxis.11
Management approach. Prophylactic-dose administration is recommended during hospital stay for all patients admitted with anticipated length of stay of 3 days or longer and who are not at high risk for bleeding or delivery.10 Both LMWH and UFH are options for inpatients. For any nonobstetric surgery or admission, LMWH may be most appropriate. However, as most obstetrics admissions are at increased risk for delivery, UFH 5,000 U twice daily to 3 times daily is the best option to increase the chances for neuraxial anesthesia. (I review anesthesia considerations for delivery later in this article.) For patients at high risk for bleeding or delivery, mechanical prophylaxis alone, with elastic stockings or pneumatic compression devices, can be used.
Continue to: CASE 1 continued Patient is discharged home...
CASE 1 continued Patient is discharged home
The patient received enoxaparin while she was in the hospital. She is now discharged and doing well. She asks, will she need anticoagulation prophylaxis after delivery?
How would you counsel her?
Chemoprophylaxis in the postpartum period
With no risk of fetal harm and a higher risk of VTE per day, the threshold for chemoprophylaxis is lower in the postpartum period. The risk of postpartum bleeding is less than 1%, with the most common complication being wound hematomas (0.61%).9 For this case patient, the COVID-19 diagnosis does not alter the recommendations for postpartum chemoprophylaxis. Additionally, as the need for neuraxial anesthesia has passed, the use of intermediate-dose chemoprophylaxis over prophylactic-dose is advocated in the postpartum period, especially in obese patients.12
As mentioned previously, there is no standard definition of intermediate-dose. Data suggest that a weight-based intermediate-dose is most likely to achieve therapeutic levels of anti-Xa in this high-risk population compared with a fixed dose.13,14 For example, enoxaparin 0.5 mg/kg twice daily is recommended for patients with class 3 obesity or higher by the Society for Maternal-Fetal Medicine.12
As a rule, anyone who was on chemoprophylaxis antepartum should be continued on at least an equivalent dose for 6 weeks postpartum. Postpartum, patients with any prior DVT should take prophylactic-dose or intermediate-dose chemoprophylaxis for 6 weeks. Patients with a known high-risk thrombophilia should receive prophylactic-dose or intermediate-dose chemoprophylaxis postpartum for 6 weeks. For patients with a low-risk thrombophilia, prophylactic-dose or intermediate-dose chemoprophylaxis is recommended for 6 weeks.
For low-risk patients without prior VTE or thrombophilia, standardized risk assessment is recommended.
Cesarean delivery
Cesarean delivery (CD) is a risk factor for postpartum VTE.9 A universal chemoprophylaxis strategy has not been proven in this patient population. Mechanical prophylaxis with sequential compression devices is recommended for all patients undergoing CD pre-procedure and until patients are fully ambulatory.8,9 Early ambulation also should be encouraged.
Many risk assessment models are available for postoperative VTE prevention, and they have widely different chemoprophylaxis rates. Studies have shown chemoprophylaxis rates of 85% by RCOG, 1% by ACOG, 35% by CHEST, 94% by Caprini, and less than 1% by Padua.15,16 In addition to the antepartum patient-specific risk factors mentioned, postpartum risk factors include infection, postpartum hemorrhage, and transfusion. Based on data extrapolated from the nonobstetric literature, chemoprophylaxis is recommended until discharge from the hospital unless risk factors are expected to continue.9
Neuraxial anesthesia
For patients who require postpartum chemoprophylaxis, the Society for Obstetric Anesthesia and Perinatology (SOAP) offers evidence-based guidelines for use after neuraxial anesthesia. UFH can be initiated 1 hour or longer after a neuraxial procedure and 1 hour or longer after catheter removal. Prophylactic-dose LMWH can be restarted at 12 hours or longer after a neuraxial procedure and at 4 to 6 hours or longer after catheter removal. For patients restarting intermediate-dose or therapeutic-dose, the recommendations are to wait 24 hours or longer after a neuraxial procedure and 4 hours or longer after catheter removal.17 Timing can be individualized based on the patient’s risk of hemorrhage and surgical bleeding. Although it may be tempting to delay chemoprophylaxis in the setting of bleeding, postpartum hemorrhage and transfusion increase the risks of VTE. In this setting, it is best to consider the use of UFH, which safely can be started earlier than LMWH.
For patients without neuraxial anesthesia, ACOG recommends chemoprophylaxis 4 to 6 hours after vaginal delivery and 6 to 12 hours after CD.8 (TABLE 3 summarizes recommendations for postpartum chemoprophylaxis.)

Continue to: Adjusting the anticoagulation regimen...
Adjusting the anticoagulation regimen
CASE 2 Pregnant woman with prior VTE
A 36-year-old woman (G1P0) with prior VTE is taking enoxaparin 40 mg daily. She asks, does she need any blood work for her anticoagulation?
What would you test for?
Increased renal clearance of LMWH and increased volume of distribution during pregnancy has led to the consideration of monitoring anti-Xa levels. There are no published standards or recommendations for dose adjustment. At this time, anti-Xa level monitoring antepartum is not recommended, but it may be considered when a patient is at the extremes of weight. With a weight-based strategy in the postpartum period, monitoring is not recommended as studies show a higher likelihood of therapeutic anti-Xa levels with this approach.13,14 This is an active area of research, and these recommendations may change.
For prophylactic-dose or intermediate-dose anticoagulation, a peak anti-Xa level of 0.2 to 0.6 U/mL is generally accepted as the target. For therapeutic-dose, a peak anti-Xa level of 0.6 to 1.2 U/mL is generally accepted as the therapeutic range. This blood draw must be collected 4 hours after the third dose.
CASE 2 continued Anticoagulation considerations nearing delivery
The patient is now at 36 weeks’ gestation, and she asks, what should be done regarding her anticoagulation prior to delivery?
What would be an appropriate approach?
Traditionally, patients were transitioned to UFH at 36 weeks and allowed to present in spontaneous labor to increase the likelihood of neuraxial anesthesia. The alternative is to continue prophylactic-dose LMWH until a scheduled delivery. While the SOAP guidelines establish the timeframe that is safe to proceed with neuraxial anesthesia, there is variation in practice, so consider discussing this with your anesthesia providers.
SOAP considers prophylactic-dose UFH to be 5,000 U 2 to 3 times per day. In this setting, neuraxial anesthesia can be placed more than 4 to 6 hours from the last dose.17 But due to the pharmacokinetics of pregnancy, ACOG recommends 10,000 U in the third trimester.8 This dose is considered intermediate-dose by SOAP, and 12 hours or longer plus a normal activated partial thromboplastin time (aPTT) or undetectable anti-Xa level are required prior to neuraxial anesthesia. This is the same time allowed for prophylactic-dose LMWH without lab work. Prophylactic-dose LMWH is considered to be enoxaparin 40 mg or less daily or 30 mg twice daily, and dalteparin 5,000 U daily. For therapeutic-dose LMWH or UFH, 24 hours or more from last dose is recommended prior to neuraxial anesthesia. For intermediate-dose LMWH, data are limited to recommend anything between 12 and 24 hours.17
In my practice, we favor a shared decision-making approach with patients. We discuss the likelihood of labor prior to 39 weeks based on a patient’s history, the importance of neuraxial anesthesia to the patient, and the importance of the number of daily injections. Most patients continue enoxaparin until a scheduled induction, and they are instructed to skip their dose if labor symptoms begin. Patients at high risk for preterm delivery can be transitioned to heparin earlier than 36 weeks. ●

Pregnancy and the postpartum period are times of increased risk for venous thromboembolism (VTE). While VTE is a rare event overall, it is responsible for more than 9% of maternal deaths in the United States.1 The increased risk of VTE exists throughout pregnancy, rising in the third trimester.2 The highest-risk period is the first 6 weeks postpartum, likely peaking in the first 2 to 3 weeks and returning to baseline at about 12 weeks postpartum.2,3
To reduce this source of maternal harm, the National Partnership for Maternal Safety and the Council on Patient Safety in Women’s Health Care recommend the use of VTE prevention bundles. Bundles include standard assessment of risk during prenatal care, any admission to the hospital, and postpartum coupled with standard recommendations for treatment.4-6 Multiple published guidelines are available for prevention of VTE in pregnancy, and they provide varying recommendations on patient selection and treatment. Many of these recommendations are based on low quality of evidence, making the choice of standard practice difficult.
In this article, I attempt to simplify patient selection and treatment based on currently published guidelines from the American College of Obstetricians and Gynecologists (ACOG), Royal College of Obstetricians and Gynaecologists (RCOG), American College of Chest Physicians (CHEST), American Society of Hematology (ASH), and expert opinion.
Determining VTE risk and need for prophylaxis
CASE 1 Woman with factor V Leiden
A 25-year-old woman (G1P0) presents for her initial prenatal visit. She says she is a carrier for factor V Leiden but has never had a clot. She was tested after her sister had a VTE. She asks, does she need VTE prophylaxis before her delivery?
What are the considerations and options for this patient?
Options for VTE prophylaxis
Before considering patients at risk for VTE, it is helpful to review the options for prophylaxis. Patients can undergo clinical surveillance or routine care with attention to VTE symptoms and a low threshold for workup.
There are 3 categories of chemoprophylaxis for prevention of VTE. (TABLE 1 offers examples of dosing regimens.) No strategy has been proven optimal over another:
- prophylactic-dose: the lowest, fixed dose.
- intermediate-dose: lacks a standard definition and is any dose higher than prophylactic-dose but lower than therapeutic-dose. This includes fixed twice-daily doses, weight-based doses, and incrementally increasing doses.
- therapeutic-dose: typically used for treatment but mentioned here since patients with high-risk conditions may use it for prevention of VTE.
The preferred agent for VTE chemoprophylaxis is low molecular weight heparin (LMWH; dalteparin, enoxaparin). LMWH has a lower risk of complications than unfractionated heparin (UFH) and can be injected once daily. LMWH and UFH do not cross the placenta. LMWH and UFH are safe in breastfeeding. Oral direct thrombin inhibitors and anti-Xa inhibitors are not recommended in pregnancy or lactation at this time. Warfarin is avoided in pregnancy except in situations with mechanical heart valves, which will not be addressed here. Patients taking warfarin for long-term anticoagulation can transition back while breastfeeding with appropriate bridging.

Expert opinion recommends antepartum chemoprophylaxis when there is a 2% to 3% risk of VTE in pregnancy.7-9 This is balanced against an approximately 2% overall risk of bleeding, with less than 1% risk of bleeding antepartum.9
Continue to: Risk factors for VTE...
Risk factors for VTE
History of VTE. The most important risk factor for VTE is a personal history of prior VTE.6 Recurrence risks have been widely reported and depend on the factors surrounding the initial event. For patients with a prior provoked deep vein thrombosis (DVT; associated with trauma or surgery), the antepartum VTE risk likely is less than 1%, and VTE chemoprophylaxis is not recommended antepartum.7
For patients with a prior VTE that was not associated with surgery or trauma (unprovoked), the risk is approximately 3%; for prior VTE related to pregnancy or hormonal contraception, the risk is approximately 6%.7 For both of these groups, prophylactic-dose antepartum is recommended. Patients with recurrent VTE are often taking long-term anticoagulation. Anyone on long-term anticoagulation should be placed on therapeutic-dose antepartum. For patients not receiving long-term anticoagulation, consider a hematology consultation when available, and begin an intermediate-dose or therapeutic-dose regimen after assessing other risk factors and the risk of bleeding and discussing treatment with the patient.
Thrombophilias. The next most important risk factor is the presence of inherited thrombophilias.6 Factor V homozygote, prothrombin G20210A mutation homozygote, antithrombin deficiency, and combined factor V heterozygote and prothrombin G20210A heterozygote (also called compound heterozygote) have the strongest association with VTE in pregnancy.8 It is recommended that patients with these high-risk thrombophilias receive prophylactic-dose antepartum.8
Factor V heterozygote, prothrombin G20210A mutation heterozygote, and protein C or protein S deficiency are considered low-risk thrombophilias. Patients with low-risk thrombophilias and no personal history of VTE or first-degree relative with VTE can be monitored with clinical surveillance antepartum. However, if a family history of VTE or other risk factors for VTE are present, antepartum prophylactic-dose is recommended. Clinical factors to consider antepartum include obesity, age older than 35 years, parity of 3 or higher, varicose veins, immobility, smoking, assisted reproductive technology use, multiple gestation, and preeclampsia.10
Antiphospholipid syndrome (APS) is another high-risk condition. For patients not taking long-term anticoagulation antepartum, prophylactic-dose is recommended. For patients on long-term anticoagulation, therapeutic-dose is recommended.
Other medical conditions. Patients with medical conditions that place them at high risk for VTE may warrant prophylactic-dose antepartum. These include active cancer, active systemic lupus erythematosus, sickle cell disease, nephropathy, and inflammatory bowel disease.10 This decision can be made in conjunction with other specialists caring for the patient.
Antepartum prophylactic-dose is not recommended for low-risk patients as there is less than 1% risk of VTE.7 (TABLE 2 summarizes antepartum chemoprophylaxis recommendations.)

CASE 1 continued Patient develops another VTE risk factor
The patient is being followed with clinical surveillance. At 19 weeks’ gestation, she presents to the emergency department with shortness of breath and fever. She is diagnosed with COVID-19 and is admitted by a medicine service. They call the OB team to ask for recommendations regarding anticoagulation.
What should the next steps include?
Hospitalization and nonobstetric surgery are risk factors for VTE. Many hospitals use a standardized assessment for all inpatients, such as the Padua or Caprini VTE risk assessment scores. These can be modified for use in pregnant patients, although neither scoring system is currently validated for use in pregnancy.5 For any pregnant patient admitted to the hospital, mechanical prophylaxis is recommended.
COVID-19. Infection with the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and its associated clinical syndrome, COVID-19, is associated with increased rates of VTE. Recommendations for pregnant patients with COVID-19 are the same as for the general population. During hospitalization for COVID-19, pregnant patients should be placed on prophylactic-dose chemoprophylaxis. Patients should not be discharged home on chemoprophylaxis, and patients managed as outpatients for their disease do not need chemoprophylaxis.11
Management approach. Prophylactic-dose administration is recommended during hospital stay for all patients admitted with anticipated length of stay of 3 days or longer and who are not at high risk for bleeding or delivery.10 Both LMWH and UFH are options for inpatients. For any nonobstetric surgery or admission, LMWH may be most appropriate. However, as most obstetrics admissions are at increased risk for delivery, UFH 5,000 U twice daily to 3 times daily is the best option to increase the chances for neuraxial anesthesia. (I review anesthesia considerations for delivery later in this article.) For patients at high risk for bleeding or delivery, mechanical prophylaxis alone, with elastic stockings or pneumatic compression devices, can be used.
Continue to: CASE 1 continued Patient is discharged home...
CASE 1 continued Patient is discharged home
The patient received enoxaparin while she was in the hospital. She is now discharged and doing well. She asks, will she need anticoagulation prophylaxis after delivery?
How would you counsel her?
Chemoprophylaxis in the postpartum period
With no risk of fetal harm and a higher risk of VTE per day, the threshold for chemoprophylaxis is lower in the postpartum period. The risk of postpartum bleeding is less than 1%, with the most common complication being wound hematomas (0.61%).9 For this case patient, the COVID-19 diagnosis does not alter the recommendations for postpartum chemoprophylaxis. Additionally, as the need for neuraxial anesthesia has passed, the use of intermediate-dose chemoprophylaxis over prophylactic-dose is advocated in the postpartum period, especially in obese patients.12
As mentioned previously, there is no standard definition of intermediate-dose. Data suggest that a weight-based intermediate-dose is most likely to achieve therapeutic levels of anti-Xa in this high-risk population compared with a fixed dose.13,14 For example, enoxaparin 0.5 mg/kg twice daily is recommended for patients with class 3 obesity or higher by the Society for Maternal-Fetal Medicine.12
As a rule, anyone who was on chemoprophylaxis antepartum should be continued on at least an equivalent dose for 6 weeks postpartum. Postpartum, patients with any prior DVT should take prophylactic-dose or intermediate-dose chemoprophylaxis for 6 weeks. Patients with a known high-risk thrombophilia should receive prophylactic-dose or intermediate-dose chemoprophylaxis postpartum for 6 weeks. For patients with a low-risk thrombophilia, prophylactic-dose or intermediate-dose chemoprophylaxis is recommended for 6 weeks.
For low-risk patients without prior VTE or thrombophilia, standardized risk assessment is recommended.
Cesarean delivery
Cesarean delivery (CD) is a risk factor for postpartum VTE.9 A universal chemoprophylaxis strategy has not been proven in this patient population. Mechanical prophylaxis with sequential compression devices is recommended for all patients undergoing CD pre-procedure and until patients are fully ambulatory.8,9 Early ambulation also should be encouraged.
Many risk assessment models are available for postoperative VTE prevention, and they have widely different chemoprophylaxis rates. Studies have shown chemoprophylaxis rates of 85% by RCOG, 1% by ACOG, 35% by CHEST, 94% by Caprini, and less than 1% by Padua.15,16 In addition to the antepartum patient-specific risk factors mentioned, postpartum risk factors include infection, postpartum hemorrhage, and transfusion. Based on data extrapolated from the nonobstetric literature, chemoprophylaxis is recommended until discharge from the hospital unless risk factors are expected to continue.9
Neuraxial anesthesia
For patients who require postpartum chemoprophylaxis, the Society for Obstetric Anesthesia and Perinatology (SOAP) offers evidence-based guidelines for use after neuraxial anesthesia. UFH can be initiated 1 hour or longer after a neuraxial procedure and 1 hour or longer after catheter removal. Prophylactic-dose LMWH can be restarted at 12 hours or longer after a neuraxial procedure and at 4 to 6 hours or longer after catheter removal. For patients restarting intermediate-dose or therapeutic-dose, the recommendations are to wait 24 hours or longer after a neuraxial procedure and 4 hours or longer after catheter removal.17 Timing can be individualized based on the patient’s risk of hemorrhage and surgical bleeding. Although it may be tempting to delay chemoprophylaxis in the setting of bleeding, postpartum hemorrhage and transfusion increase the risks of VTE. In this setting, it is best to consider the use of UFH, which safely can be started earlier than LMWH.
For patients without neuraxial anesthesia, ACOG recommends chemoprophylaxis 4 to 6 hours after vaginal delivery and 6 to 12 hours after CD.8 (TABLE 3 summarizes recommendations for postpartum chemoprophylaxis.)

Continue to: Adjusting the anticoagulation regimen...
Adjusting the anticoagulation regimen
CASE 2 Pregnant woman with prior VTE
A 36-year-old woman (G1P0) with prior VTE is taking enoxaparin 40 mg daily. She asks, does she need any blood work for her anticoagulation?
What would you test for?
Increased renal clearance of LMWH and increased volume of distribution during pregnancy has led to the consideration of monitoring anti-Xa levels. There are no published standards or recommendations for dose adjustment. At this time, anti-Xa level monitoring antepartum is not recommended, but it may be considered when a patient is at the extremes of weight. With a weight-based strategy in the postpartum period, monitoring is not recommended as studies show a higher likelihood of therapeutic anti-Xa levels with this approach.13,14 This is an active area of research, and these recommendations may change.
For prophylactic-dose or intermediate-dose anticoagulation, a peak anti-Xa level of 0.2 to 0.6 U/mL is generally accepted as the target. For therapeutic-dose, a peak anti-Xa level of 0.6 to 1.2 U/mL is generally accepted as the therapeutic range. This blood draw must be collected 4 hours after the third dose.
CASE 2 continued Anticoagulation considerations nearing delivery
The patient is now at 36 weeks’ gestation, and she asks, what should be done regarding her anticoagulation prior to delivery?
What would be an appropriate approach?
Traditionally, patients were transitioned to UFH at 36 weeks and allowed to present in spontaneous labor to increase the likelihood of neuraxial anesthesia. The alternative is to continue prophylactic-dose LMWH until a scheduled delivery. While the SOAP guidelines establish the timeframe that is safe to proceed with neuraxial anesthesia, there is variation in practice, so consider discussing this with your anesthesia providers.
SOAP considers prophylactic-dose UFH to be 5,000 U 2 to 3 times per day. In this setting, neuraxial anesthesia can be placed more than 4 to 6 hours from the last dose.17 But due to the pharmacokinetics of pregnancy, ACOG recommends 10,000 U in the third trimester.8 This dose is considered intermediate-dose by SOAP, and 12 hours or longer plus a normal activated partial thromboplastin time (aPTT) or undetectable anti-Xa level are required prior to neuraxial anesthesia. This is the same time allowed for prophylactic-dose LMWH without lab work. Prophylactic-dose LMWH is considered to be enoxaparin 40 mg or less daily or 30 mg twice daily, and dalteparin 5,000 U daily. For therapeutic-dose LMWH or UFH, 24 hours or more from last dose is recommended prior to neuraxial anesthesia. For intermediate-dose LMWH, data are limited to recommend anything between 12 and 24 hours.17
In my practice, we favor a shared decision-making approach with patients. We discuss the likelihood of labor prior to 39 weeks based on a patient’s history, the importance of neuraxial anesthesia to the patient, and the importance of the number of daily injections. Most patients continue enoxaparin until a scheduled induction, and they are instructed to skip their dose if labor symptoms begin. Patients at high risk for preterm delivery can be transitioned to heparin earlier than 36 weeks. ●
- Creanga AA, Syverson C, Seed K, et al. Pregnancy-related mortality in the United States, 2011-2013. Obstet Gynecol. 2017;130:366-373. doi: 10.1097/AOG.0000000000002114.
- Kourlaba G, Relakis J, Kontodimas S, et al. A systematic review and meta-analysis of the epidemiology and burden of venous thromboembolism among pregnant women. Int J Gynaecol Obstet. 2016;132:4-10. doi: 10.1016/j.ijgo.2015.06.054.
- Sultan AA, West J, Tata LJ, et al. Risk of first venous thromboembolism in and around pregnancy: a population-based cohort study. Br J Haematol. 2012;156:366-373. doi: 10.1111/j.1365-2141.2011.08956.x.
- American College of Obstetricians and Gynecologists. Council on Patient Safety in Women’s Health Care: maternal venous thromboembolism (+AIM). 2015. https://safehealthcareforeverywoman.org/council/patient-safety-bundles/maternal-safety-bundles/maternal-venous-thromboembolism-aim/. Accessed February 26, 2021.
- Urato AC, Abi-Jaoude E, Abramson J, et al. National Partnership for Maternal Safety: consensus bundle on venous thromboembolism. Obstet Gynecol. 2019;134:1115-1117. doi: 10.1097/AOG.0000000000003540.
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics. ACOG practice bulletin no. 196: thromboembolism in pregnancy. Obstet Gynecol. 2018;132:e1-e17. doi: 10.1097/AOG.0000000000002706.
- Bates SM, Rajasekhar A, Middeldorp S, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: venous thromboembolism in the context of pregnancy. Blood Adv. 2018;2:3317-3359. doi: 10.1182/bloodadvances.2018024802.
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 197: inherited thrombophilias in pregnancy. Obstet Gynecol. 2018;132:e18-e34. doi: 10.1097/AOG.0000000000002703.
- Bates SM, Greer IA, Middeldorp S, et al. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2, suppl):e691S-e736S. doi: 10.1378/chest.11-2300.
- Lamont MC, McDermott C, Thomson AJ, et al. United Kingdom recommendations for obstetric venous thromboembolism prophylaxis: evidence and rationale. Semin Perinatol. 2019;43:222-228. doi: 10.1053/j.semperi.2019.03.008.
- National Institutes of Health. COVID-19 Treatment Guidelines Panel. Coronavirus disease 2019 (COVID-19) treatment guidelines. https://www.covid19treatmentguidelines.nih.gov/. Accessed February 26, 2021.
- Society for Maternal-Fetal Medicine (SMFM); Pacheco LD, Saade G, Metz TD. Society for Maternal-Fetal Medicine Consult Series #51: thromboembolism prophylaxis for cesarean delivery. Am J Obstet Gynecol. 2020;223:B11-B17. doi: 10.1016/j.ajog.2020.04.032.
- Overcash RT, Somers AT, LaCoursiere DY. Enoxaparin dosing after cesarean delivery in morbidly obese women. Obstet Gynecol. 2015;125:1371-1376. doi: 10.1097/AOG.0000000000000873.
- Hiscock RJ, Casey E, Simmons SW, et al. Peak plasma anti-Xa levels after first and third doses of enoxaparin in women receiving weight-based thromboprophylaxis following caesarean section: a prospective cohort study. Int J Obstet Anesth. 2013;22:280-288. doi: 10.1016/j.ijoa.2013.05.008.
- Palmerola KL, D’Alton ME, Brock CO, et al. A comparison of recommendations for pharmacologic thromboembolism prophylaxis after caesarean delivery from three major guidelines. BJOG. 2016;123:2157-2162. doi: 10.1111/1471-0528.13706.
- Tran JP, Stribling SS, Ibezim UC, et al. Performance of risk assessment models for peripartum thromboprophylaxis. Reprod Sci. 2019;26:1243-1248. doi: 10.1177/1933719118813197.
- Leffert L, Butwick A, Carvalho B, et al; members of the SOAP VTE Taskforce. The Society for Obstetric Anesthesia and Perinatology consensus statement on the anesthetic management of pregnant and postpartum women receiving thromboprophylaxis or higher dose anticoagulants. Anesth Analg. 2018;126:928-944. doi: 10.1213/ANE.0000000000002530.
- Creanga AA, Syverson C, Seed K, et al. Pregnancy-related mortality in the United States, 2011-2013. Obstet Gynecol. 2017;130:366-373. doi: 10.1097/AOG.0000000000002114.
- Kourlaba G, Relakis J, Kontodimas S, et al. A systematic review and meta-analysis of the epidemiology and burden of venous thromboembolism among pregnant women. Int J Gynaecol Obstet. 2016;132:4-10. doi: 10.1016/j.ijgo.2015.06.054.
- Sultan AA, West J, Tata LJ, et al. Risk of first venous thromboembolism in and around pregnancy: a population-based cohort study. Br J Haematol. 2012;156:366-373. doi: 10.1111/j.1365-2141.2011.08956.x.
- American College of Obstetricians and Gynecologists. Council on Patient Safety in Women’s Health Care: maternal venous thromboembolism (+AIM). 2015. https://safehealthcareforeverywoman.org/council/patient-safety-bundles/maternal-safety-bundles/maternal-venous-thromboembolism-aim/. Accessed February 26, 2021.
- Urato AC, Abi-Jaoude E, Abramson J, et al. National Partnership for Maternal Safety: consensus bundle on venous thromboembolism. Obstet Gynecol. 2019;134:1115-1117. doi: 10.1097/AOG.0000000000003540.
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics. ACOG practice bulletin no. 196: thromboembolism in pregnancy. Obstet Gynecol. 2018;132:e1-e17. doi: 10.1097/AOG.0000000000002706.
- Bates SM, Rajasekhar A, Middeldorp S, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: venous thromboembolism in the context of pregnancy. Blood Adv. 2018;2:3317-3359. doi: 10.1182/bloodadvances.2018024802.
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 197: inherited thrombophilias in pregnancy. Obstet Gynecol. 2018;132:e18-e34. doi: 10.1097/AOG.0000000000002703.
- Bates SM, Greer IA, Middeldorp S, et al. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2, suppl):e691S-e736S. doi: 10.1378/chest.11-2300.
- Lamont MC, McDermott C, Thomson AJ, et al. United Kingdom recommendations for obstetric venous thromboembolism prophylaxis: evidence and rationale. Semin Perinatol. 2019;43:222-228. doi: 10.1053/j.semperi.2019.03.008.
- National Institutes of Health. COVID-19 Treatment Guidelines Panel. Coronavirus disease 2019 (COVID-19) treatment guidelines. https://www.covid19treatmentguidelines.nih.gov/. Accessed February 26, 2021.
- Society for Maternal-Fetal Medicine (SMFM); Pacheco LD, Saade G, Metz TD. Society for Maternal-Fetal Medicine Consult Series #51: thromboembolism prophylaxis for cesarean delivery. Am J Obstet Gynecol. 2020;223:B11-B17. doi: 10.1016/j.ajog.2020.04.032.
- Overcash RT, Somers AT, LaCoursiere DY. Enoxaparin dosing after cesarean delivery in morbidly obese women. Obstet Gynecol. 2015;125:1371-1376. doi: 10.1097/AOG.0000000000000873.
- Hiscock RJ, Casey E, Simmons SW, et al. Peak plasma anti-Xa levels after first and third doses of enoxaparin in women receiving weight-based thromboprophylaxis following caesarean section: a prospective cohort study. Int J Obstet Anesth. 2013;22:280-288. doi: 10.1016/j.ijoa.2013.05.008.
- Palmerola KL, D’Alton ME, Brock CO, et al. A comparison of recommendations for pharmacologic thromboembolism prophylaxis after caesarean delivery from three major guidelines. BJOG. 2016;123:2157-2162. doi: 10.1111/1471-0528.13706.
- Tran JP, Stribling SS, Ibezim UC, et al. Performance of risk assessment models for peripartum thromboprophylaxis. Reprod Sci. 2019;26:1243-1248. doi: 10.1177/1933719118813197.
- Leffert L, Butwick A, Carvalho B, et al; members of the SOAP VTE Taskforce. The Society for Obstetric Anesthesia and Perinatology consensus statement on the anesthetic management of pregnant and postpartum women receiving thromboprophylaxis or higher dose anticoagulants. Anesth Analg. 2018;126:928-944. doi: 10.1213/ANE.0000000000002530.