User login
Vaccinations for the ObGyn’s toolbox
CASE 1st prenatal appointment for young, pregnant migrant
A 21-year-old primigravid woman at 12 weeks’ gestation recently immigrated to the United States from an impoverished rural area of Southeast Asia. On the first prenatal appointment, she is noted to have no evidence of immunity to rubella, measles, or varicella. Her hepatitis B surface antigen and hepatitis C antibody tests are negative. She also has negative test results for gonorrhea, chlamydia, syphilis, and HIV infection. Her pap test is negative.
- What vaccinations should this patient receive during her pregnancy?
- What additional vaccinations are indicated postpartum?
Preventive vaccinations: What to know
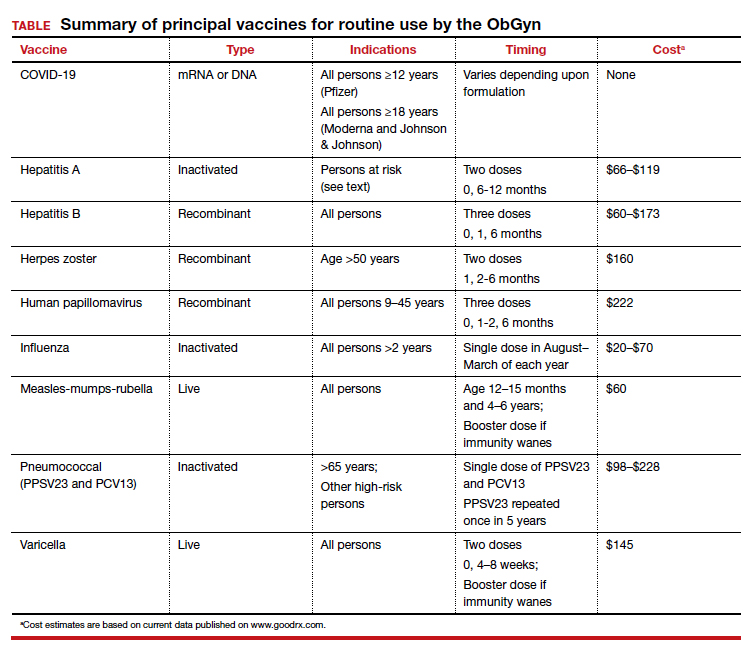
As ObGyns, we serve as the primary care physician for many women throughout their early and middle decades of life. Accordingly, we have an obligation to be well informed about preventive health services such as vaccinations. The purpose of this article is to review the principal vaccines with which ObGyns should be familiar. I will discuss the vaccines in alphabetical order and then focus on the indications and timing for each vaccine and the relative cost of each immunization. Key points are summarized in the TABLE.
COVID-19 vaccine
In the latter part of 2020 and early part of 2021, three COVID-19 vaccines received emergency use authorization (EUA) from the US Food and Drug Administration (FDA) for individuals 16 years of age and older (Pfizer-BioNTech) and 18 years of age and older (Moderna and Johnson & Johnson).1 The cost of their administration is borne by the federal government. Two of the vaccines are mRNA agents—Moderna and Pfizer-BioNTech. Both are administered in a 2-dose series, separated by 4 and 3 weeks, respectively. The efficacy of these vaccines in preventing serious or critical illness approaches 95%. The Pfizer-BioNTech vaccine has now been fully FDA approved for administration to individuals older than age 16, with EUA for those down to age 12. Full approval of the Moderna vaccine will not be far behind. Because of some evidence suggesting waning immunity over time and because of growing concerns about the increased transmissibility of the delta variant of the virus, the FDA has been strongly considering a recommendation for a third (booster) dose of each of these vaccines, administered 8 months after the second dose for all eligible Americans. On September 17, 2021, the FDA advisory committee recommended a booster for the Pfizer-BioNTech vaccine for people older than age 65 and for those over the age of 16 at high risk for severe COVID-19. Several days later, full FDA approval was granted for this recommendation. Subsequently, the Director of the Centers for Disease Control and Prevention (CDC) included health care workers and pregnant women in the group for whom the booster is recommended.
The third vaccine formulation is the Johnson & Johnson DNA vaccine, which is prepared with a human adenovirus vector. This vaccine is administered in a single intramuscular dose and has a reported efficacy of 66% to 85%, though it may approach 95% in preventing critical illness. The FDA is expected to announce decisions about booster doses for the Johnson & Johnson and Moderna vaccines in the coming weeks.
Although initial trials of the COVID-19vaccines excluded pregnant and lactating women, the vaccines are safe in pregnancy or postpartum. In fact the vaccines do not contain either a killed or attenuated viral particle that is capable of transmitting infection. Therefore, both the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine now support routine immunization during pregnancy.
A recent report by Shimabukuro and colleagues2 demonstrated that the risk of vaccine-related complications in pregnant women receiving the Pfizer-BioNTech or Moderna vaccines was no different than in nonpregnant patients and that there was no evidence of teratogenic effects. The trial included more than 35,000 pregnant women; 2.3% were vaccinated in the periconception period, 28.6% in the first trimester, 43.3% in the second trimester, and 25.7% in the third trimester. Given this, and in light of isolated reports of unusual thromboembolic complications associated with the Johnson & Johnson vaccine, I strongly recommend use of either the Moderna or Pfizer-BioNTech vaccine in our prenatal and postpartum patients.
Continue to: Hepatitis A vaccine...
Hepatitis A vaccine
The hepatitis A vaccine is an inactivated vaccine and is safe for use in pregnancy. It is available in two monovalent preparations—Havrix (GlaxoSmithKline) and Vaqta (Merck & Co.) and is administered in a 2-dose intramuscular injection at time zero and 6 to 12 months later.3 The vaccine is also available in a bivalent form with recombinant hepatitis B vaccine—Twinrix (GlaxoSmithKline). When administered in this form, the vaccine should be given at time zero, 1 month, and 6 months. The wholesale cost of the monovalent vaccine is $66 to $119, depending upon whether the provider uses a multi-dose or a single-dose vial. The cost of Twinrix is $149.
The hepatitis A vaccine is indicated for select pregnant and nonpregnant patients:
- international travelers
- intravenous drug users
- those with occupational exposure (eg, individuals who work in a primate laboratory)
- residents and staff in chronic care facilities
- individuals with chronic liver disease
- individuals with clotting factor disorders
- residents in endemic areas.
Hepatitis B vaccine
The hepatitis B vaccine is a recombinant vaccine that contains an inactivated portion of the hepatitis B surface antigen. It was originally produced in two monovalent formulations: Engerix B (GlaxoSmithKline) and Recombivax-HB (Merck & Co.). These original formulations are given in a 3-dose series at time zero, 1 month, and 6 months. Recently, a new and more potent formulation was introduced into clinical practice. Heplisav-B (Dynavax Technologies Co.) is also a recombinant vaccine that contains a boosting adjuvant. It is programed to be administered in a 2-dose series at time zero and 1 month.4-6
The wholesale cost of the monovalent vaccines varies from $60 to $173, depending upon use of a multi-dose vial versus a single-use vial. The cost of Heplisav-B varies from $146 to $173, depending upon use of a prefilled syringe versus a single-dose vial.
Although the hepatitis B vaccine should be part of the childhood immunization series, it also should be administered to any pregnant woman who has not been vaccinated previously or who does not already have evidence of immunity as a result of natural infection.
Continue to: Herpes zoster vaccine...
Herpes zoster vaccine
Herpes zoster infection (shingles) can be a particularly disabling condition in older patients and results from reactivation of a latent varicella-zoster infection. Shingles can cause extremely painful skin lesions, threaten the patient’s vision, and result in long-lasting postherpetic neuralgia. Both cellular and hormonal immunity are essential to protect against recurrent infection.
The original herpes zoster vaccine (Zoster Vaccine Live; ZVL, Zostavax) is no longer produced in the United States because it is not as effective as the newer vaccine—Recombinant Zoster Vaccine (Shingrix, GlaxoSmithKline).7,8 The antigen in the new vaccine is a component of the surface glycoprotein E, and it is combined with an adjuvant to enhance immunoreactivity. The vaccine is given intramuscularly in two doses at time zero and again at 2 to 6 months and is indicated for all individuals >50 years, including those who may have had an episode of shingles. This newer vaccine is 97% effective in patients >50 years and 90% effective in patients >70. The wholesale cost of each injection is about $160.
Human papillomavirus vaccine
The HPV vaccine (Gardasil-9, Merck & Co.) is a recombinant 9-valent vaccine directed against the human papillomavirus. It induces immunity to serotypes 6 and 11 (which cause 90% of genital warts), 16 and 18 (which cause 80% of genital cancers), and 31, 33, 45, 52, and 58 (viral strains that are responsible for both genital and oropharyngeal cancers). The vaccine is administered intramuscularly in a 3-dose series at time zero, 1-2 months, and 6 months. The principal target groups for the vaccine are males and females, ages 9 to 45 years. Ideally, children of both sexes should receive this vaccine prior to the onset of sexual activity. The wholesale cost of each vaccine injection is approximately $222.9
Influenza vaccine
The inactivated, intramuscular flu vaccine is recommended for anyone over age 2, including pregnant women. Although pregnant women are not more likely to acquire flu compared with those who are not pregnant, if they do become infected, they are likely to become more seriously ill, with higher mortality. Accordingly, all pregnant women should receive, in any trimester, the inactivated flu vaccine beginning in the late summer and early fall of each year and extending through March of the next year.10,11
Multiple formulations of the inactivated vaccine are marketed, all targeting two strains of influenza A and two strains of influenza B. The components of the vaccine vary each year as scientists try to match the new vaccine with the most highly prevalent strains in the previous flu season. The vaccine should be administered in a single intramuscular dose. The cost varies from approximately $20 to $70.
The intranasal influenza vaccine is a live virus vaccine that is intended primarily for children and should not be administered in pregnancy. In addition, there is a higher dose of the inactivated quadrivalent vaccine that is available for administration to patients over age 65. This higher dose is more likely to cause adverse effects and is not indicated in pregnancy.
Continue to: Measles, mumps, rubella vaccine (MMR)...
Measles, mumps, rubella vaccine (MMR)
The MMR is a standard component of the childhood vaccination series. The trivalent preparation is a live, attenuated vaccine that is typically given subcutaneously in a 2-dose series. The first dose is administered at age 12-15 months, and the second dose at age 4-6 years. The vaccine is highly immunogenic, with vaccine-induced immunity usually life-long. In some patients, however, immunity wanes over time. Accordingly, all pregnant women should be screened for immunity to rubella since, of the 3, this infection poses the greatest risk to the fetus. Women who do not have evidence of immunity should be advised to avoid contact with children who may have a viral exanthem. They should then receive a booster dose of the vaccine immediately postpartum and should practice secure contraception for 1 month. The vaccine cost is approximately $60.
Pneumococcal vaccine
The inactivated pneumococcal vaccine is produced in two forms, both of which are safe for administration in pregnancy.12 The original vaccine, introduced in 1983, was PPSV23 (Pneumovax 23, Merck & Co), a 23-serovalent vaccine that was intended primarily for adults. This vaccine is administered in a single subcutaneous or intramuscular dose. The newest vaccine, introduced in 2010, is PCV13 (Prevnar 13, Pfizer Inc), a 13-serovalent vaccine. It was intended primarily for children, in whom it is administered in a 4-dose series beginning at 6 to 8 weeks of age. The cost of the former is approximately $98 to $120; the cost of the latter is $228.
Vaccination against pneumococcal infection is routinely indicated for those older than the age of 65 and for the following at-risk patients, including those who are pregnant11:
- individuals who have had a splenectomy or who have a medical illness that produces functional asplenia (eg, sickle cell anemia)
- individuals with chronic cardiac, pulmonic, hepatic, or renal disease
- individuals with immunosuppressive conditions such as HIV infection or a disseminated malignancy
- individuals who have a cochlear implant
- individuals who have a chronic leak of cerebrospinal fluid.
The recommendations for timing of these 2 vaccines in adults can initially appear confusing. Put most simply, if a high-risk patient first receives the PCV13 vaccine, she should receive the PPSV23 vaccine in about 8 weeks. The PPSV23 vaccine should be repeated in 5 years. If an at-risk patient initially receives the PPSV23 vaccine, the PCV13 vaccine should be given 1 year later.12
Tdap vaccine
The Tdap vaccine contains tetanus toxoid, reduced diptheria toxoid, and an acellular component of the pertussis bacterium. Although it has long been part of the childhood vaccinations series, immunity to each component, particularly pertussis, tends to wane over time.
Pertussis poses a serious risk to the health of the pregnant woman and the newborn infant. Accordingly, the Advisory Committee on Immunization Practices (ACIP), CDC, and the ACOG now advise administration of a booster dose of this vaccine in the early third trimester of each pregnancy.13-15 The vaccine should be administered as a single intramuscular injection. The approximate cost of the vaccine is $64 to $71, depending upon whether the provider uses a single-dose vial or a single-dose prefilled syringe. In nonpregnant patients, the ACIP currently recommends administration of a booster dose of the vaccine every 10 years, primarily to provide durable protection against tetanus.
Continue to: Varicella vaccine...
Varicella vaccine
The varicella vaccine is also one of the main components of the childhood immunization series. This live virus vaccine can be administered subcutaneously as a monovalent agent or as a quadrivalent agent in association with the MMR vaccine.
Pregnant women who do not have a well-documented history of natural infection should be tested for IgG antibody to the varicella-zoster virus at the time of their first prenatal appointment. Interestingly, approximately 70% of patients with an uncertain history actually have immunity when tested. If the patient lacks immunity, she should be vaccinated immediately postpartum.16,17 The vaccine should be administered in a 2-dose series at time zero and then 4 to 8 weeks later. Patients should adhere to secure contraception from the time of the first dose until 1 month after the second dose. The cost of each dose of the vaccine is approximately $145.
Adverse effects of vaccination
All vaccines have many of the same side effects. The most common is simply a reaction at the site of injection, characterized by pain, increased warmth, erythema, swelling, and tenderness. Other common side effects include systemic manifestations, such as low-grade fever, nausea and vomiting, malaise, fatigue, headache, lymphadenopathy, myalgias, and arthralgias. Some vaccines, notably varicella, herpes zoster, measles, and rubella may cause a disseminated rash. Most of these minor side effects are easily managed by rest, hydration, and administration of an analgesic such as acetaminophen or ibuprofen. More serious side effects include rare complications such as anaphylaxis, Bell palsy, Guillain-Barre syndrome, and venous thromboembolism (Johnson & Johnson COVID-19 vaccine). Any of the vaccines discussed above should not be given, or given only with extreme caution, to an individual who has experienced any of these reactions with a previous vaccine.
Barriers to vaccination
Although the vaccines reviewed above are highly effective in preventing serious illness in recipients, the medical profession’s “report card” in ensuring adherence with vaccine protocols is not optimal. In fact, it probably merits a grade no higher than C+, with vaccination rates in the range of 50% to 70%.
One of the major barriers to vaccination is lack of detailed information about vaccine efficacy and safety on the part of both provider and patient. Another is the problem of misinformation (eg, the persistent belief on the part of some individuals that vaccines may cause a serious problem, such as autism).18,19 Another important barrier to widespread vaccination is the logistical problem associated with proper scheduling of multidose regimens (such as those for hepatitis A and B, varicella, and COVID-19). A final barrier, and in my own university-based practice, the most important obstacle is the expense of vaccination. Most, but not all, private insurance companies provide coverage for vaccines approved by the Centers for Disease Control and Prevention and the US Preventive Services Task Force. However, public insurance agencies often provide disappointingly inconsistent coverage for essential vaccines.
By keeping well informed about the most recent public health recommendations for vaccinations for adults and by leading important initiatives within our own practices, we should be able to overcome the first 3 barriers listed above. For example, Morgan and colleagues20 recently achieved a 97% success rate with Tdap administration in pregnancy by placing a best-practice alert in the patients’ electronic medical records. Surmounting the final barrier will require intense effort on the part of individual practitioners and professional organizations to advocate for coverage for essential vaccinations for our patients.
CASE Resolved
This patient was raised in an area of the world where her family did not have easy access to medical care. Accordingly, she did not receive the usual childhood vaccines, such as measles, mumps, rubella, varicella, hepatitis B, and almost certainly, tetanus, diphtheria, and pertussis (Tdap), and the HPV vaccine. The MMR vaccine and the varicella vaccine are live virus vaccines and should not be given during pregnancy. However, these vaccines should be administered postpartum, and the patient should be instructed to practice secure contraception for a minimum of 1 month following vaccination. She also should be offered the HPV vaccine postpartum. During pregnancy, she definitely should receive the COVID-19 vaccine, the 3-dose hepatitis B vaccine series, the influenza vaccine, and Tdap. If her present living conditions place her at risk for hepatitis A, she also should be vaccinated against this illness. ●
- Rasmussen SA, Kelley CF, Horton JP, et al. Coronavirus disease 2019 (COVID-19) vaccines and pregnancy. What obstetricians need to know. Obstet Gynecol. 2021;137:408-414. doi: 10.1097/AOG.0000000000004290.
- Shimabukuro TT, Kim SY, Myers RT, et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N Engl J Med. 2021;384:2273-2282. doi: 10.1056/NEJMoa2104983.
- Duff B, Duff P. Hepatitis A vaccine: ready for prime time. Obstet Gynecol. 1998;91:468-471. doi: 10.1016/s0029-7844(97)00669-8.
- Omer SB. Maternal immunization. N Engl J Med. 2017;376:1256-1267. doi: 10.1056/NEJMra1509044.
- Dionne-Odom J, Tita AT, Silverman NS. Society for Maternal-Fetal Medicine Consult Series: #38: hepatitis B in pregnancy screening, treatment, and prevention of vertical transmission. Am J Obstet Gynecol. 2016;214:6-14. doi: http://dx.doi.org/10.1016/j.ajog.2015.09.100.
- Yawetz S. Immunizations during pregnancy. UpToDate, January 15, 2021.
- Cunningham Al, Lal H, Kovac M, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016:375:1019-1032. doi: 10.1056/NEJMoa1603800.
- Albrecht MA, Levin MJ. Vaccination for the prevention of shingles (herpes zoster). UpToDate, July 6, 2020.
- ACOG Committee Opinion. Human papillomavirus vaccination. Obstet Gynecol. 2006;108:699-705. doi: 10.1097/00006250-200609000-00047.
- Callaghan WM, Creanga AA, Jamieson DJ. Pregnancy-related mortality resulting from influenza in the United States during the 2009-2010 pandemic. Obstet Gynecol. 2015;126:486-490. doi: 10.1097/AOG.0000000000000996.
- ACOG Committee Opinion. Influenza vaccination during pregnancy. Obstet Gynecol. 2014;124:648-651. doi: 10.1097/01.AOG.0000453599.11566.11.
- Scheller NM, Pasternak B, Molgaard-Nielsen D, et al. Quadrivalent HPV vaccination and the risk of adverse pregnancy outcomes. N Engl J Med. 2017;376:1223-1233. doi: 10.1056/NEJMoa1612296.
- Moumne O, Duff P. Treatment and prevention of pneumococcal infection. Clin Obstet Gynecol. 2019;62:781-789. doi: 10.1097/GRF.0000000000000451.
- ACOG Committee Opinion. Update on immunization and pregnancy: tetanus, diphtheria, and pertussis vaccination. Obstet Gynecol. 2017;130:668-669. doi: 10.1097/AOG.0000000000002293.
- Sukumaran L, McCarthy NL, Kharbanda EO, et al. Safety of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis and influenza vaccinations in pregnancy. Obstet Gynecol. 2015;126:1069-1074. doi: 10.1097/AOG.0000000000001066.
- Duff P. Varicella in pregnancy: five priorities for clinicians. Infect Dis Obstet Gynecol. 1994;1:163-165. doi: 10.1155/S1064744994000013.
- Duff P. Varicella vaccine. Infect Dis Obstet Gynecol. 1996;4:63-65. doi: 10.1155/S1064744996000142.
- Desmond A, Offit PA. On the shoulders of giants--from Jenner's cowpox to mRNA COVID vaccines. N Engl. J Med. 2021;384:1081-1083. doi: 10.1056/NEJMp2034334.
- Poland GA, Jacobson RM. The age-old struggle against the antivaccinationists. N Engl J Med. 2011;364:97-99. doi: 10.1056/NEJMp1010594.
- Morgan JL, Baggari SR, Chung W, et al. Association of a best-practice alert and prenatal administration with tetanus toxoid, reduced diptheria toxoid, and acellular pertussis vaccination rates. Obstet Gynecol. 2015;126:333-337. doi: 10.1097/AOG.0000000000000975.

CASE 1st prenatal appointment for young, pregnant migrant
A 21-year-old primigravid woman at 12 weeks’ gestation recently immigrated to the United States from an impoverished rural area of Southeast Asia. On the first prenatal appointment, she is noted to have no evidence of immunity to rubella, measles, or varicella. Her hepatitis B surface antigen and hepatitis C antibody tests are negative. She also has negative test results for gonorrhea, chlamydia, syphilis, and HIV infection. Her pap test is negative.
- What vaccinations should this patient receive during her pregnancy?
- What additional vaccinations are indicated postpartum?
Preventive vaccinations: What to know
As ObGyns, we serve as the primary care physician for many women throughout their early and middle decades of life. Accordingly, we have an obligation to be well informed about preventive health services such as vaccinations. The purpose of this article is to review the principal vaccines with which ObGyns should be familiar. I will discuss the vaccines in alphabetical order and then focus on the indications and timing for each vaccine and the relative cost of each immunization. Key points are summarized in the TABLE.

COVID-19 vaccine
In the latter part of 2020 and early part of 2021, three COVID-19 vaccines received emergency use authorization (EUA) from the US Food and Drug Administration (FDA) for individuals 16 years of age and older (Pfizer-BioNTech) and 18 years of age and older (Moderna and Johnson & Johnson).1 The cost of their administration is borne by the federal government. Two of the vaccines are mRNA agents—Moderna and Pfizer-BioNTech. Both are administered in a 2-dose series, separated by 4 and 3 weeks, respectively. The efficacy of these vaccines in preventing serious or critical illness approaches 95%. The Pfizer-BioNTech vaccine has now been fully FDA approved for administration to individuals older than age 16, with EUA for those down to age 12. Full approval of the Moderna vaccine will not be far behind. Because of some evidence suggesting waning immunity over time and because of growing concerns about the increased transmissibility of the delta variant of the virus, the FDA has been strongly considering a recommendation for a third (booster) dose of each of these vaccines, administered 8 months after the second dose for all eligible Americans. On September 17, 2021, the FDA advisory committee recommended a booster for the Pfizer-BioNTech vaccine for people older than age 65 and for those over the age of 16 at high risk for severe COVID-19. Several days later, full FDA approval was granted for this recommendation. Subsequently, the Director of the Centers for Disease Control and Prevention (CDC) included health care workers and pregnant women in the group for whom the booster is recommended.
The third vaccine formulation is the Johnson & Johnson DNA vaccine, which is prepared with a human adenovirus vector. This vaccine is administered in a single intramuscular dose and has a reported efficacy of 66% to 85%, though it may approach 95% in preventing critical illness. The FDA is expected to announce decisions about booster doses for the Johnson & Johnson and Moderna vaccines in the coming weeks.
Although initial trials of the COVID-19vaccines excluded pregnant and lactating women, the vaccines are safe in pregnancy or postpartum. In fact the vaccines do not contain either a killed or attenuated viral particle that is capable of transmitting infection. Therefore, both the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine now support routine immunization during pregnancy.
A recent report by Shimabukuro and colleagues2 demonstrated that the risk of vaccine-related complications in pregnant women receiving the Pfizer-BioNTech or Moderna vaccines was no different than in nonpregnant patients and that there was no evidence of teratogenic effects. The trial included more than 35,000 pregnant women; 2.3% were vaccinated in the periconception period, 28.6% in the first trimester, 43.3% in the second trimester, and 25.7% in the third trimester. Given this, and in light of isolated reports of unusual thromboembolic complications associated with the Johnson & Johnson vaccine, I strongly recommend use of either the Moderna or Pfizer-BioNTech vaccine in our prenatal and postpartum patients.
Continue to: Hepatitis A vaccine...
Hepatitis A vaccine
The hepatitis A vaccine is an inactivated vaccine and is safe for use in pregnancy. It is available in two monovalent preparations—Havrix (GlaxoSmithKline) and Vaqta (Merck & Co.) and is administered in a 2-dose intramuscular injection at time zero and 6 to 12 months later.3 The vaccine is also available in a bivalent form with recombinant hepatitis B vaccine—Twinrix (GlaxoSmithKline). When administered in this form, the vaccine should be given at time zero, 1 month, and 6 months. The wholesale cost of the monovalent vaccine is $66 to $119, depending upon whether the provider uses a multi-dose or a single-dose vial. The cost of Twinrix is $149.
The hepatitis A vaccine is indicated for select pregnant and nonpregnant patients:
- international travelers
- intravenous drug users
- those with occupational exposure (eg, individuals who work in a primate laboratory)
- residents and staff in chronic care facilities
- individuals with chronic liver disease
- individuals with clotting factor disorders
- residents in endemic areas.
Hepatitis B vaccine
The hepatitis B vaccine is a recombinant vaccine that contains an inactivated portion of the hepatitis B surface antigen. It was originally produced in two monovalent formulations: Engerix B (GlaxoSmithKline) and Recombivax-HB (Merck & Co.). These original formulations are given in a 3-dose series at time zero, 1 month, and 6 months. Recently, a new and more potent formulation was introduced into clinical practice. Heplisav-B (Dynavax Technologies Co.) is also a recombinant vaccine that contains a boosting adjuvant. It is programed to be administered in a 2-dose series at time zero and 1 month.4-6
The wholesale cost of the monovalent vaccines varies from $60 to $173, depending upon use of a multi-dose vial versus a single-use vial. The cost of Heplisav-B varies from $146 to $173, depending upon use of a prefilled syringe versus a single-dose vial.
Although the hepatitis B vaccine should be part of the childhood immunization series, it also should be administered to any pregnant woman who has not been vaccinated previously or who does not already have evidence of immunity as a result of natural infection.
Continue to: Herpes zoster vaccine...
Herpes zoster vaccine
Herpes zoster infection (shingles) can be a particularly disabling condition in older patients and results from reactivation of a latent varicella-zoster infection. Shingles can cause extremely painful skin lesions, threaten the patient’s vision, and result in long-lasting postherpetic neuralgia. Both cellular and hormonal immunity are essential to protect against recurrent infection.
The original herpes zoster vaccine (Zoster Vaccine Live; ZVL, Zostavax) is no longer produced in the United States because it is not as effective as the newer vaccine—Recombinant Zoster Vaccine (Shingrix, GlaxoSmithKline).7,8 The antigen in the new vaccine is a component of the surface glycoprotein E, and it is combined with an adjuvant to enhance immunoreactivity. The vaccine is given intramuscularly in two doses at time zero and again at 2 to 6 months and is indicated for all individuals >50 years, including those who may have had an episode of shingles. This newer vaccine is 97% effective in patients >50 years and 90% effective in patients >70. The wholesale cost of each injection is about $160.
Human papillomavirus vaccine
The HPV vaccine (Gardasil-9, Merck & Co.) is a recombinant 9-valent vaccine directed against the human papillomavirus. It induces immunity to serotypes 6 and 11 (which cause 90% of genital warts), 16 and 18 (which cause 80% of genital cancers), and 31, 33, 45, 52, and 58 (viral strains that are responsible for both genital and oropharyngeal cancers). The vaccine is administered intramuscularly in a 3-dose series at time zero, 1-2 months, and 6 months. The principal target groups for the vaccine are males and females, ages 9 to 45 years. Ideally, children of both sexes should receive this vaccine prior to the onset of sexual activity. The wholesale cost of each vaccine injection is approximately $222.9
Influenza vaccine
The inactivated, intramuscular flu vaccine is recommended for anyone over age 2, including pregnant women. Although pregnant women are not more likely to acquire flu compared with those who are not pregnant, if they do become infected, they are likely to become more seriously ill, with higher mortality. Accordingly, all pregnant women should receive, in any trimester, the inactivated flu vaccine beginning in the late summer and early fall of each year and extending through March of the next year.10,11
Multiple formulations of the inactivated vaccine are marketed, all targeting two strains of influenza A and two strains of influenza B. The components of the vaccine vary each year as scientists try to match the new vaccine with the most highly prevalent strains in the previous flu season. The vaccine should be administered in a single intramuscular dose. The cost varies from approximately $20 to $70.
The intranasal influenza vaccine is a live virus vaccine that is intended primarily for children and should not be administered in pregnancy. In addition, there is a higher dose of the inactivated quadrivalent vaccine that is available for administration to patients over age 65. This higher dose is more likely to cause adverse effects and is not indicated in pregnancy.
Continue to: Measles, mumps, rubella vaccine (MMR)...
Measles, mumps, rubella vaccine (MMR)
The MMR is a standard component of the childhood vaccination series. The trivalent preparation is a live, attenuated vaccine that is typically given subcutaneously in a 2-dose series. The first dose is administered at age 12-15 months, and the second dose at age 4-6 years. The vaccine is highly immunogenic, with vaccine-induced immunity usually life-long. In some patients, however, immunity wanes over time. Accordingly, all pregnant women should be screened for immunity to rubella since, of the 3, this infection poses the greatest risk to the fetus. Women who do not have evidence of immunity should be advised to avoid contact with children who may have a viral exanthem. They should then receive a booster dose of the vaccine immediately postpartum and should practice secure contraception for 1 month. The vaccine cost is approximately $60.
Pneumococcal vaccine
The inactivated pneumococcal vaccine is produced in two forms, both of which are safe for administration in pregnancy.12 The original vaccine, introduced in 1983, was PPSV23 (Pneumovax 23, Merck & Co), a 23-serovalent vaccine that was intended primarily for adults. This vaccine is administered in a single subcutaneous or intramuscular dose. The newest vaccine, introduced in 2010, is PCV13 (Prevnar 13, Pfizer Inc), a 13-serovalent vaccine. It was intended primarily for children, in whom it is administered in a 4-dose series beginning at 6 to 8 weeks of age. The cost of the former is approximately $98 to $120; the cost of the latter is $228.
Vaccination against pneumococcal infection is routinely indicated for those older than the age of 65 and for the following at-risk patients, including those who are pregnant11:
- individuals who have had a splenectomy or who have a medical illness that produces functional asplenia (eg, sickle cell anemia)
- individuals with chronic cardiac, pulmonic, hepatic, or renal disease
- individuals with immunosuppressive conditions such as HIV infection or a disseminated malignancy
- individuals who have a cochlear implant
- individuals who have a chronic leak of cerebrospinal fluid.
The recommendations for timing of these 2 vaccines in adults can initially appear confusing. Put most simply, if a high-risk patient first receives the PCV13 vaccine, she should receive the PPSV23 vaccine in about 8 weeks. The PPSV23 vaccine should be repeated in 5 years. If an at-risk patient initially receives the PPSV23 vaccine, the PCV13 vaccine should be given 1 year later.12
Tdap vaccine
The Tdap vaccine contains tetanus toxoid, reduced diptheria toxoid, and an acellular component of the pertussis bacterium. Although it has long been part of the childhood vaccinations series, immunity to each component, particularly pertussis, tends to wane over time.
Pertussis poses a serious risk to the health of the pregnant woman and the newborn infant. Accordingly, the Advisory Committee on Immunization Practices (ACIP), CDC, and the ACOG now advise administration of a booster dose of this vaccine in the early third trimester of each pregnancy.13-15 The vaccine should be administered as a single intramuscular injection. The approximate cost of the vaccine is $64 to $71, depending upon whether the provider uses a single-dose vial or a single-dose prefilled syringe. In nonpregnant patients, the ACIP currently recommends administration of a booster dose of the vaccine every 10 years, primarily to provide durable protection against tetanus.
Continue to: Varicella vaccine...
Varicella vaccine
The varicella vaccine is also one of the main components of the childhood immunization series. This live virus vaccine can be administered subcutaneously as a monovalent agent or as a quadrivalent agent in association with the MMR vaccine.
Pregnant women who do not have a well-documented history of natural infection should be tested for IgG antibody to the varicella-zoster virus at the time of their first prenatal appointment. Interestingly, approximately 70% of patients with an uncertain history actually have immunity when tested. If the patient lacks immunity, she should be vaccinated immediately postpartum.16,17 The vaccine should be administered in a 2-dose series at time zero and then 4 to 8 weeks later. Patients should adhere to secure contraception from the time of the first dose until 1 month after the second dose. The cost of each dose of the vaccine is approximately $145.
Adverse effects of vaccination
All vaccines have many of the same side effects. The most common is simply a reaction at the site of injection, characterized by pain, increased warmth, erythema, swelling, and tenderness. Other common side effects include systemic manifestations, such as low-grade fever, nausea and vomiting, malaise, fatigue, headache, lymphadenopathy, myalgias, and arthralgias. Some vaccines, notably varicella, herpes zoster, measles, and rubella may cause a disseminated rash. Most of these minor side effects are easily managed by rest, hydration, and administration of an analgesic such as acetaminophen or ibuprofen. More serious side effects include rare complications such as anaphylaxis, Bell palsy, Guillain-Barre syndrome, and venous thromboembolism (Johnson & Johnson COVID-19 vaccine). Any of the vaccines discussed above should not be given, or given only with extreme caution, to an individual who has experienced any of these reactions with a previous vaccine.
Barriers to vaccination
Although the vaccines reviewed above are highly effective in preventing serious illness in recipients, the medical profession’s “report card” in ensuring adherence with vaccine protocols is not optimal. In fact, it probably merits a grade no higher than C+, with vaccination rates in the range of 50% to 70%.
One of the major barriers to vaccination is lack of detailed information about vaccine efficacy and safety on the part of both provider and patient. Another is the problem of misinformation (eg, the persistent belief on the part of some individuals that vaccines may cause a serious problem, such as autism).18,19 Another important barrier to widespread vaccination is the logistical problem associated with proper scheduling of multidose regimens (such as those for hepatitis A and B, varicella, and COVID-19). A final barrier, and in my own university-based practice, the most important obstacle is the expense of vaccination. Most, but not all, private insurance companies provide coverage for vaccines approved by the Centers for Disease Control and Prevention and the US Preventive Services Task Force. However, public insurance agencies often provide disappointingly inconsistent coverage for essential vaccines.
By keeping well informed about the most recent public health recommendations for vaccinations for adults and by leading important initiatives within our own practices, we should be able to overcome the first 3 barriers listed above. For example, Morgan and colleagues20 recently achieved a 97% success rate with Tdap administration in pregnancy by placing a best-practice alert in the patients’ electronic medical records. Surmounting the final barrier will require intense effort on the part of individual practitioners and professional organizations to advocate for coverage for essential vaccinations for our patients.
CASE Resolved
This patient was raised in an area of the world where her family did not have easy access to medical care. Accordingly, she did not receive the usual childhood vaccines, such as measles, mumps, rubella, varicella, hepatitis B, and almost certainly, tetanus, diphtheria, and pertussis (Tdap), and the HPV vaccine. The MMR vaccine and the varicella vaccine are live virus vaccines and should not be given during pregnancy. However, these vaccines should be administered postpartum, and the patient should be instructed to practice secure contraception for a minimum of 1 month following vaccination. She also should be offered the HPV vaccine postpartum. During pregnancy, she definitely should receive the COVID-19 vaccine, the 3-dose hepatitis B vaccine series, the influenza vaccine, and Tdap. If her present living conditions place her at risk for hepatitis A, she also should be vaccinated against this illness. ●

CASE 1st prenatal appointment for young, pregnant migrant
A 21-year-old primigravid woman at 12 weeks’ gestation recently immigrated to the United States from an impoverished rural area of Southeast Asia. On the first prenatal appointment, she is noted to have no evidence of immunity to rubella, measles, or varicella. Her hepatitis B surface antigen and hepatitis C antibody tests are negative. She also has negative test results for gonorrhea, chlamydia, syphilis, and HIV infection. Her pap test is negative.
- What vaccinations should this patient receive during her pregnancy?
- What additional vaccinations are indicated postpartum?
Preventive vaccinations: What to know
As ObGyns, we serve as the primary care physician for many women throughout their early and middle decades of life. Accordingly, we have an obligation to be well informed about preventive health services such as vaccinations. The purpose of this article is to review the principal vaccines with which ObGyns should be familiar. I will discuss the vaccines in alphabetical order and then focus on the indications and timing for each vaccine and the relative cost of each immunization. Key points are summarized in the TABLE.

COVID-19 vaccine
In the latter part of 2020 and early part of 2021, three COVID-19 vaccines received emergency use authorization (EUA) from the US Food and Drug Administration (FDA) for individuals 16 years of age and older (Pfizer-BioNTech) and 18 years of age and older (Moderna and Johnson & Johnson).1 The cost of their administration is borne by the federal government. Two of the vaccines are mRNA agents—Moderna and Pfizer-BioNTech. Both are administered in a 2-dose series, separated by 4 and 3 weeks, respectively. The efficacy of these vaccines in preventing serious or critical illness approaches 95%. The Pfizer-BioNTech vaccine has now been fully FDA approved for administration to individuals older than age 16, with EUA for those down to age 12. Full approval of the Moderna vaccine will not be far behind. Because of some evidence suggesting waning immunity over time and because of growing concerns about the increased transmissibility of the delta variant of the virus, the FDA has been strongly considering a recommendation for a third (booster) dose of each of these vaccines, administered 8 months after the second dose for all eligible Americans. On September 17, 2021, the FDA advisory committee recommended a booster for the Pfizer-BioNTech vaccine for people older than age 65 and for those over the age of 16 at high risk for severe COVID-19. Several days later, full FDA approval was granted for this recommendation. Subsequently, the Director of the Centers for Disease Control and Prevention (CDC) included health care workers and pregnant women in the group for whom the booster is recommended.
The third vaccine formulation is the Johnson & Johnson DNA vaccine, which is prepared with a human adenovirus vector. This vaccine is administered in a single intramuscular dose and has a reported efficacy of 66% to 85%, though it may approach 95% in preventing critical illness. The FDA is expected to announce decisions about booster doses for the Johnson & Johnson and Moderna vaccines in the coming weeks.
Although initial trials of the COVID-19vaccines excluded pregnant and lactating women, the vaccines are safe in pregnancy or postpartum. In fact the vaccines do not contain either a killed or attenuated viral particle that is capable of transmitting infection. Therefore, both the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine now support routine immunization during pregnancy.
A recent report by Shimabukuro and colleagues2 demonstrated that the risk of vaccine-related complications in pregnant women receiving the Pfizer-BioNTech or Moderna vaccines was no different than in nonpregnant patients and that there was no evidence of teratogenic effects. The trial included more than 35,000 pregnant women; 2.3% were vaccinated in the periconception period, 28.6% in the first trimester, 43.3% in the second trimester, and 25.7% in the third trimester. Given this, and in light of isolated reports of unusual thromboembolic complications associated with the Johnson & Johnson vaccine, I strongly recommend use of either the Moderna or Pfizer-BioNTech vaccine in our prenatal and postpartum patients.
Continue to: Hepatitis A vaccine...
Hepatitis A vaccine
The hepatitis A vaccine is an inactivated vaccine and is safe for use in pregnancy. It is available in two monovalent preparations—Havrix (GlaxoSmithKline) and Vaqta (Merck & Co.) and is administered in a 2-dose intramuscular injection at time zero and 6 to 12 months later.3 The vaccine is also available in a bivalent form with recombinant hepatitis B vaccine—Twinrix (GlaxoSmithKline). When administered in this form, the vaccine should be given at time zero, 1 month, and 6 months. The wholesale cost of the monovalent vaccine is $66 to $119, depending upon whether the provider uses a multi-dose or a single-dose vial. The cost of Twinrix is $149.
The hepatitis A vaccine is indicated for select pregnant and nonpregnant patients:
- international travelers
- intravenous drug users
- those with occupational exposure (eg, individuals who work in a primate laboratory)
- residents and staff in chronic care facilities
- individuals with chronic liver disease
- individuals with clotting factor disorders
- residents in endemic areas.
Hepatitis B vaccine
The hepatitis B vaccine is a recombinant vaccine that contains an inactivated portion of the hepatitis B surface antigen. It was originally produced in two monovalent formulations: Engerix B (GlaxoSmithKline) and Recombivax-HB (Merck & Co.). These original formulations are given in a 3-dose series at time zero, 1 month, and 6 months. Recently, a new and more potent formulation was introduced into clinical practice. Heplisav-B (Dynavax Technologies Co.) is also a recombinant vaccine that contains a boosting adjuvant. It is programed to be administered in a 2-dose series at time zero and 1 month.4-6
The wholesale cost of the monovalent vaccines varies from $60 to $173, depending upon use of a multi-dose vial versus a single-use vial. The cost of Heplisav-B varies from $146 to $173, depending upon use of a prefilled syringe versus a single-dose vial.
Although the hepatitis B vaccine should be part of the childhood immunization series, it also should be administered to any pregnant woman who has not been vaccinated previously or who does not already have evidence of immunity as a result of natural infection.
Continue to: Herpes zoster vaccine...
Herpes zoster vaccine
Herpes zoster infection (shingles) can be a particularly disabling condition in older patients and results from reactivation of a latent varicella-zoster infection. Shingles can cause extremely painful skin lesions, threaten the patient’s vision, and result in long-lasting postherpetic neuralgia. Both cellular and hormonal immunity are essential to protect against recurrent infection.
The original herpes zoster vaccine (Zoster Vaccine Live; ZVL, Zostavax) is no longer produced in the United States because it is not as effective as the newer vaccine—Recombinant Zoster Vaccine (Shingrix, GlaxoSmithKline).7,8 The antigen in the new vaccine is a component of the surface glycoprotein E, and it is combined with an adjuvant to enhance immunoreactivity. The vaccine is given intramuscularly in two doses at time zero and again at 2 to 6 months and is indicated for all individuals >50 years, including those who may have had an episode of shingles. This newer vaccine is 97% effective in patients >50 years and 90% effective in patients >70. The wholesale cost of each injection is about $160.
Human papillomavirus vaccine
The HPV vaccine (Gardasil-9, Merck & Co.) is a recombinant 9-valent vaccine directed against the human papillomavirus. It induces immunity to serotypes 6 and 11 (which cause 90% of genital warts), 16 and 18 (which cause 80% of genital cancers), and 31, 33, 45, 52, and 58 (viral strains that are responsible for both genital and oropharyngeal cancers). The vaccine is administered intramuscularly in a 3-dose series at time zero, 1-2 months, and 6 months. The principal target groups for the vaccine are males and females, ages 9 to 45 years. Ideally, children of both sexes should receive this vaccine prior to the onset of sexual activity. The wholesale cost of each vaccine injection is approximately $222.9
Influenza vaccine
The inactivated, intramuscular flu vaccine is recommended for anyone over age 2, including pregnant women. Although pregnant women are not more likely to acquire flu compared with those who are not pregnant, if they do become infected, they are likely to become more seriously ill, with higher mortality. Accordingly, all pregnant women should receive, in any trimester, the inactivated flu vaccine beginning in the late summer and early fall of each year and extending through March of the next year.10,11
Multiple formulations of the inactivated vaccine are marketed, all targeting two strains of influenza A and two strains of influenza B. The components of the vaccine vary each year as scientists try to match the new vaccine with the most highly prevalent strains in the previous flu season. The vaccine should be administered in a single intramuscular dose. The cost varies from approximately $20 to $70.
The intranasal influenza vaccine is a live virus vaccine that is intended primarily for children and should not be administered in pregnancy. In addition, there is a higher dose of the inactivated quadrivalent vaccine that is available for administration to patients over age 65. This higher dose is more likely to cause adverse effects and is not indicated in pregnancy.
Continue to: Measles, mumps, rubella vaccine (MMR)...
Measles, mumps, rubella vaccine (MMR)
The MMR is a standard component of the childhood vaccination series. The trivalent preparation is a live, attenuated vaccine that is typically given subcutaneously in a 2-dose series. The first dose is administered at age 12-15 months, and the second dose at age 4-6 years. The vaccine is highly immunogenic, with vaccine-induced immunity usually life-long. In some patients, however, immunity wanes over time. Accordingly, all pregnant women should be screened for immunity to rubella since, of the 3, this infection poses the greatest risk to the fetus. Women who do not have evidence of immunity should be advised to avoid contact with children who may have a viral exanthem. They should then receive a booster dose of the vaccine immediately postpartum and should practice secure contraception for 1 month. The vaccine cost is approximately $60.
Pneumococcal vaccine
The inactivated pneumococcal vaccine is produced in two forms, both of which are safe for administration in pregnancy.12 The original vaccine, introduced in 1983, was PPSV23 (Pneumovax 23, Merck & Co), a 23-serovalent vaccine that was intended primarily for adults. This vaccine is administered in a single subcutaneous or intramuscular dose. The newest vaccine, introduced in 2010, is PCV13 (Prevnar 13, Pfizer Inc), a 13-serovalent vaccine. It was intended primarily for children, in whom it is administered in a 4-dose series beginning at 6 to 8 weeks of age. The cost of the former is approximately $98 to $120; the cost of the latter is $228.
Vaccination against pneumococcal infection is routinely indicated for those older than the age of 65 and for the following at-risk patients, including those who are pregnant11:
- individuals who have had a splenectomy or who have a medical illness that produces functional asplenia (eg, sickle cell anemia)
- individuals with chronic cardiac, pulmonic, hepatic, or renal disease
- individuals with immunosuppressive conditions such as HIV infection or a disseminated malignancy
- individuals who have a cochlear implant
- individuals who have a chronic leak of cerebrospinal fluid.
The recommendations for timing of these 2 vaccines in adults can initially appear confusing. Put most simply, if a high-risk patient first receives the PCV13 vaccine, she should receive the PPSV23 vaccine in about 8 weeks. The PPSV23 vaccine should be repeated in 5 years. If an at-risk patient initially receives the PPSV23 vaccine, the PCV13 vaccine should be given 1 year later.12
Tdap vaccine
The Tdap vaccine contains tetanus toxoid, reduced diptheria toxoid, and an acellular component of the pertussis bacterium. Although it has long been part of the childhood vaccinations series, immunity to each component, particularly pertussis, tends to wane over time.
Pertussis poses a serious risk to the health of the pregnant woman and the newborn infant. Accordingly, the Advisory Committee on Immunization Practices (ACIP), CDC, and the ACOG now advise administration of a booster dose of this vaccine in the early third trimester of each pregnancy.13-15 The vaccine should be administered as a single intramuscular injection. The approximate cost of the vaccine is $64 to $71, depending upon whether the provider uses a single-dose vial or a single-dose prefilled syringe. In nonpregnant patients, the ACIP currently recommends administration of a booster dose of the vaccine every 10 years, primarily to provide durable protection against tetanus.
Continue to: Varicella vaccine...
Varicella vaccine
The varicella vaccine is also one of the main components of the childhood immunization series. This live virus vaccine can be administered subcutaneously as a monovalent agent or as a quadrivalent agent in association with the MMR vaccine.
Pregnant women who do not have a well-documented history of natural infection should be tested for IgG antibody to the varicella-zoster virus at the time of their first prenatal appointment. Interestingly, approximately 70% of patients with an uncertain history actually have immunity when tested. If the patient lacks immunity, she should be vaccinated immediately postpartum.16,17 The vaccine should be administered in a 2-dose series at time zero and then 4 to 8 weeks later. Patients should adhere to secure contraception from the time of the first dose until 1 month after the second dose. The cost of each dose of the vaccine is approximately $145.
Adverse effects of vaccination
All vaccines have many of the same side effects. The most common is simply a reaction at the site of injection, characterized by pain, increased warmth, erythema, swelling, and tenderness. Other common side effects include systemic manifestations, such as low-grade fever, nausea and vomiting, malaise, fatigue, headache, lymphadenopathy, myalgias, and arthralgias. Some vaccines, notably varicella, herpes zoster, measles, and rubella may cause a disseminated rash. Most of these minor side effects are easily managed by rest, hydration, and administration of an analgesic such as acetaminophen or ibuprofen. More serious side effects include rare complications such as anaphylaxis, Bell palsy, Guillain-Barre syndrome, and venous thromboembolism (Johnson & Johnson COVID-19 vaccine). Any of the vaccines discussed above should not be given, or given only with extreme caution, to an individual who has experienced any of these reactions with a previous vaccine.
Barriers to vaccination
Although the vaccines reviewed above are highly effective in preventing serious illness in recipients, the medical profession’s “report card” in ensuring adherence with vaccine protocols is not optimal. In fact, it probably merits a grade no higher than C+, with vaccination rates in the range of 50% to 70%.
One of the major barriers to vaccination is lack of detailed information about vaccine efficacy and safety on the part of both provider and patient. Another is the problem of misinformation (eg, the persistent belief on the part of some individuals that vaccines may cause a serious problem, such as autism).18,19 Another important barrier to widespread vaccination is the logistical problem associated with proper scheduling of multidose regimens (such as those for hepatitis A and B, varicella, and COVID-19). A final barrier, and in my own university-based practice, the most important obstacle is the expense of vaccination. Most, but not all, private insurance companies provide coverage for vaccines approved by the Centers for Disease Control and Prevention and the US Preventive Services Task Force. However, public insurance agencies often provide disappointingly inconsistent coverage for essential vaccines.
By keeping well informed about the most recent public health recommendations for vaccinations for adults and by leading important initiatives within our own practices, we should be able to overcome the first 3 barriers listed above. For example, Morgan and colleagues20 recently achieved a 97% success rate with Tdap administration in pregnancy by placing a best-practice alert in the patients’ electronic medical records. Surmounting the final barrier will require intense effort on the part of individual practitioners and professional organizations to advocate for coverage for essential vaccinations for our patients.
CASE Resolved
This patient was raised in an area of the world where her family did not have easy access to medical care. Accordingly, she did not receive the usual childhood vaccines, such as measles, mumps, rubella, varicella, hepatitis B, and almost certainly, tetanus, diphtheria, and pertussis (Tdap), and the HPV vaccine. The MMR vaccine and the varicella vaccine are live virus vaccines and should not be given during pregnancy. However, these vaccines should be administered postpartum, and the patient should be instructed to practice secure contraception for a minimum of 1 month following vaccination. She also should be offered the HPV vaccine postpartum. During pregnancy, she definitely should receive the COVID-19 vaccine, the 3-dose hepatitis B vaccine series, the influenza vaccine, and Tdap. If her present living conditions place her at risk for hepatitis A, she also should be vaccinated against this illness. ●
- Rasmussen SA, Kelley CF, Horton JP, et al. Coronavirus disease 2019 (COVID-19) vaccines and pregnancy. What obstetricians need to know. Obstet Gynecol. 2021;137:408-414. doi: 10.1097/AOG.0000000000004290.
- Shimabukuro TT, Kim SY, Myers RT, et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N Engl J Med. 2021;384:2273-2282. doi: 10.1056/NEJMoa2104983.
- Duff B, Duff P. Hepatitis A vaccine: ready for prime time. Obstet Gynecol. 1998;91:468-471. doi: 10.1016/s0029-7844(97)00669-8.
- Omer SB. Maternal immunization. N Engl J Med. 2017;376:1256-1267. doi: 10.1056/NEJMra1509044.
- Dionne-Odom J, Tita AT, Silverman NS. Society for Maternal-Fetal Medicine Consult Series: #38: hepatitis B in pregnancy screening, treatment, and prevention of vertical transmission. Am J Obstet Gynecol. 2016;214:6-14. doi: http://dx.doi.org/10.1016/j.ajog.2015.09.100.
- Yawetz S. Immunizations during pregnancy. UpToDate, January 15, 2021.
- Cunningham Al, Lal H, Kovac M, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016:375:1019-1032. doi: 10.1056/NEJMoa1603800.
- Albrecht MA, Levin MJ. Vaccination for the prevention of shingles (herpes zoster). UpToDate, July 6, 2020.
- ACOG Committee Opinion. Human papillomavirus vaccination. Obstet Gynecol. 2006;108:699-705. doi: 10.1097/00006250-200609000-00047.
- Callaghan WM, Creanga AA, Jamieson DJ. Pregnancy-related mortality resulting from influenza in the United States during the 2009-2010 pandemic. Obstet Gynecol. 2015;126:486-490. doi: 10.1097/AOG.0000000000000996.
- ACOG Committee Opinion. Influenza vaccination during pregnancy. Obstet Gynecol. 2014;124:648-651. doi: 10.1097/01.AOG.0000453599.11566.11.
- Scheller NM, Pasternak B, Molgaard-Nielsen D, et al. Quadrivalent HPV vaccination and the risk of adverse pregnancy outcomes. N Engl J Med. 2017;376:1223-1233. doi: 10.1056/NEJMoa1612296.
- Moumne O, Duff P. Treatment and prevention of pneumococcal infection. Clin Obstet Gynecol. 2019;62:781-789. doi: 10.1097/GRF.0000000000000451.
- ACOG Committee Opinion. Update on immunization and pregnancy: tetanus, diphtheria, and pertussis vaccination. Obstet Gynecol. 2017;130:668-669. doi: 10.1097/AOG.0000000000002293.
- Sukumaran L, McCarthy NL, Kharbanda EO, et al. Safety of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis and influenza vaccinations in pregnancy. Obstet Gynecol. 2015;126:1069-1074. doi: 10.1097/AOG.0000000000001066.
- Duff P. Varicella in pregnancy: five priorities for clinicians. Infect Dis Obstet Gynecol. 1994;1:163-165. doi: 10.1155/S1064744994000013.
- Duff P. Varicella vaccine. Infect Dis Obstet Gynecol. 1996;4:63-65. doi: 10.1155/S1064744996000142.
- Desmond A, Offit PA. On the shoulders of giants--from Jenner's cowpox to mRNA COVID vaccines. N Engl. J Med. 2021;384:1081-1083. doi: 10.1056/NEJMp2034334.
- Poland GA, Jacobson RM. The age-old struggle against the antivaccinationists. N Engl J Med. 2011;364:97-99. doi: 10.1056/NEJMp1010594.
- Morgan JL, Baggari SR, Chung W, et al. Association of a best-practice alert and prenatal administration with tetanus toxoid, reduced diptheria toxoid, and acellular pertussis vaccination rates. Obstet Gynecol. 2015;126:333-337. doi: 10.1097/AOG.0000000000000975.
- Rasmussen SA, Kelley CF, Horton JP, et al. Coronavirus disease 2019 (COVID-19) vaccines and pregnancy. What obstetricians need to know. Obstet Gynecol. 2021;137:408-414. doi: 10.1097/AOG.0000000000004290.
- Shimabukuro TT, Kim SY, Myers RT, et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N Engl J Med. 2021;384:2273-2282. doi: 10.1056/NEJMoa2104983.
- Duff B, Duff P. Hepatitis A vaccine: ready for prime time. Obstet Gynecol. 1998;91:468-471. doi: 10.1016/s0029-7844(97)00669-8.
- Omer SB. Maternal immunization. N Engl J Med. 2017;376:1256-1267. doi: 10.1056/NEJMra1509044.
- Dionne-Odom J, Tita AT, Silverman NS. Society for Maternal-Fetal Medicine Consult Series: #38: hepatitis B in pregnancy screening, treatment, and prevention of vertical transmission. Am J Obstet Gynecol. 2016;214:6-14. doi: http://dx.doi.org/10.1016/j.ajog.2015.09.100.
- Yawetz S. Immunizations during pregnancy. UpToDate, January 15, 2021.
- Cunningham Al, Lal H, Kovac M, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016:375:1019-1032. doi: 10.1056/NEJMoa1603800.
- Albrecht MA, Levin MJ. Vaccination for the prevention of shingles (herpes zoster). UpToDate, July 6, 2020.
- ACOG Committee Opinion. Human papillomavirus vaccination. Obstet Gynecol. 2006;108:699-705. doi: 10.1097/00006250-200609000-00047.
- Callaghan WM, Creanga AA, Jamieson DJ. Pregnancy-related mortality resulting from influenza in the United States during the 2009-2010 pandemic. Obstet Gynecol. 2015;126:486-490. doi: 10.1097/AOG.0000000000000996.
- ACOG Committee Opinion. Influenza vaccination during pregnancy. Obstet Gynecol. 2014;124:648-651. doi: 10.1097/01.AOG.0000453599.11566.11.
- Scheller NM, Pasternak B, Molgaard-Nielsen D, et al. Quadrivalent HPV vaccination and the risk of adverse pregnancy outcomes. N Engl J Med. 2017;376:1223-1233. doi: 10.1056/NEJMoa1612296.
- Moumne O, Duff P. Treatment and prevention of pneumococcal infection. Clin Obstet Gynecol. 2019;62:781-789. doi: 10.1097/GRF.0000000000000451.
- ACOG Committee Opinion. Update on immunization and pregnancy: tetanus, diphtheria, and pertussis vaccination. Obstet Gynecol. 2017;130:668-669. doi: 10.1097/AOG.0000000000002293.
- Sukumaran L, McCarthy NL, Kharbanda EO, et al. Safety of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis and influenza vaccinations in pregnancy. Obstet Gynecol. 2015;126:1069-1074. doi: 10.1097/AOG.0000000000001066.
- Duff P. Varicella in pregnancy: five priorities for clinicians. Infect Dis Obstet Gynecol. 1994;1:163-165. doi: 10.1155/S1064744994000013.
- Duff P. Varicella vaccine. Infect Dis Obstet Gynecol. 1996;4:63-65. doi: 10.1155/S1064744996000142.
- Desmond A, Offit PA. On the shoulders of giants--from Jenner's cowpox to mRNA COVID vaccines. N Engl. J Med. 2021;384:1081-1083. doi: 10.1056/NEJMp2034334.
- Poland GA, Jacobson RM. The age-old struggle against the antivaccinationists. N Engl J Med. 2011;364:97-99. doi: 10.1056/NEJMp1010594.
- Morgan JL, Baggari SR, Chung W, et al. Association of a best-practice alert and prenatal administration with tetanus toxoid, reduced diptheria toxoid, and acellular pertussis vaccination rates. Obstet Gynecol. 2015;126:333-337. doi: 10.1097/AOG.0000000000000975.
Novel and Alternative Strategies for Management of Panitumumab-Induced Hypomagnesemia
Background
Panitumumab is an epidermal growth factor receptor (EGFR) inhibiting monoclonal antibody approved for the treatment of RAS wild-type metastatic colorectal cancer (mCRC), which has an incidence of hypomagnesemia of approximately 35%. Grade 3 or 4 hypomagnesemia occurs in roughly 7% of patients, which can lead to serious complications such as seizures and arrhythmias. In one study, hypomagnesemia led to discontinuation of targeted therapy in 3% of patients. Currently, there is no standardized prophylactic strategy or treatment protocol for panitumumab-induced hypomagnesemia. In cases of refractory hypomagnesemia, it is recommended to discontinue panitumumab, even if the patient is deriving clinical benefit.
Case Report
This 59-year-old male was diagnosed with RAS wild-type mCRC and had already progressed through multiple lines of treatment. Panitumumab was initiated with good response; however, the drug was discontinued due to grade 4 hypomagnesemia, despite intravenous and oral supplementation. As the patient progressed through further lines of treatment, the decision was made to retry panitumumab. Grade 2-3 hypomagnesemia persisted throughout treatment, requiring frequent magnesium infusions. Innovative and alternative treatment options were investigated in an effort to improve his quality of life. In addition to oral and intravenous magnesium replacement, an ambulatory elastomeric pump, traditionally used for fluorouracil administration, was repurposed to deliver between 6 and 24 grams of magnesium sulfate over 24 to 72 hours. The pump was generally well tolerated with the exception of mild skin irritation around the port site, which prevented a transition to longer infusion times. The ambulatory elastomeric pump decreased the frequency of healthcare visits and improved the hypomagnesemia sufficiently to continue treatment with panitumumab, although levels did not fully normalize. A two-week trial of amiloride was also attempted to decrease renal magnesium wasting. Amiloride normalized magnesium levels but had to be discontinued due to asymptomatic hyperkalemia. This case report suggests that amiloride and magnesium replacement via ambulatory elastomeric pumps may be safe and effective treatment options for panitumumab-induced refractory hypomagnesemia in mCRC, potentially improving quality of life and allowing beneficial anti-cancer treatments to continue. Future studies should further evaluate optimization of amiloride and intravenous magnesium replacement via ambulatory elastomeric pump.
Background
Panitumumab is an epidermal growth factor receptor (EGFR) inhibiting monoclonal antibody approved for the treatment of RAS wild-type metastatic colorectal cancer (mCRC), which has an incidence of hypomagnesemia of approximately 35%. Grade 3 or 4 hypomagnesemia occurs in roughly 7% of patients, which can lead to serious complications such as seizures and arrhythmias. In one study, hypomagnesemia led to discontinuation of targeted therapy in 3% of patients. Currently, there is no standardized prophylactic strategy or treatment protocol for panitumumab-induced hypomagnesemia. In cases of refractory hypomagnesemia, it is recommended to discontinue panitumumab, even if the patient is deriving clinical benefit.
Case Report
This 59-year-old male was diagnosed with RAS wild-type mCRC and had already progressed through multiple lines of treatment. Panitumumab was initiated with good response; however, the drug was discontinued due to grade 4 hypomagnesemia, despite intravenous and oral supplementation. As the patient progressed through further lines of treatment, the decision was made to retry panitumumab. Grade 2-3 hypomagnesemia persisted throughout treatment, requiring frequent magnesium infusions. Innovative and alternative treatment options were investigated in an effort to improve his quality of life. In addition to oral and intravenous magnesium replacement, an ambulatory elastomeric pump, traditionally used for fluorouracil administration, was repurposed to deliver between 6 and 24 grams of magnesium sulfate over 24 to 72 hours. The pump was generally well tolerated with the exception of mild skin irritation around the port site, which prevented a transition to longer infusion times. The ambulatory elastomeric pump decreased the frequency of healthcare visits and improved the hypomagnesemia sufficiently to continue treatment with panitumumab, although levels did not fully normalize. A two-week trial of amiloride was also attempted to decrease renal magnesium wasting. Amiloride normalized magnesium levels but had to be discontinued due to asymptomatic hyperkalemia. This case report suggests that amiloride and magnesium replacement via ambulatory elastomeric pumps may be safe and effective treatment options for panitumumab-induced refractory hypomagnesemia in mCRC, potentially improving quality of life and allowing beneficial anti-cancer treatments to continue. Future studies should further evaluate optimization of amiloride and intravenous magnesium replacement via ambulatory elastomeric pump.
Background
Panitumumab is an epidermal growth factor receptor (EGFR) inhibiting monoclonal antibody approved for the treatment of RAS wild-type metastatic colorectal cancer (mCRC), which has an incidence of hypomagnesemia of approximately 35%. Grade 3 or 4 hypomagnesemia occurs in roughly 7% of patients, which can lead to serious complications such as seizures and arrhythmias. In one study, hypomagnesemia led to discontinuation of targeted therapy in 3% of patients. Currently, there is no standardized prophylactic strategy or treatment protocol for panitumumab-induced hypomagnesemia. In cases of refractory hypomagnesemia, it is recommended to discontinue panitumumab, even if the patient is deriving clinical benefit.
Case Report
This 59-year-old male was diagnosed with RAS wild-type mCRC and had already progressed through multiple lines of treatment. Panitumumab was initiated with good response; however, the drug was discontinued due to grade 4 hypomagnesemia, despite intravenous and oral supplementation. As the patient progressed through further lines of treatment, the decision was made to retry panitumumab. Grade 2-3 hypomagnesemia persisted throughout treatment, requiring frequent magnesium infusions. Innovative and alternative treatment options were investigated in an effort to improve his quality of life. In addition to oral and intravenous magnesium replacement, an ambulatory elastomeric pump, traditionally used for fluorouracil administration, was repurposed to deliver between 6 and 24 grams of magnesium sulfate over 24 to 72 hours. The pump was generally well tolerated with the exception of mild skin irritation around the port site, which prevented a transition to longer infusion times. The ambulatory elastomeric pump decreased the frequency of healthcare visits and improved the hypomagnesemia sufficiently to continue treatment with panitumumab, although levels did not fully normalize. A two-week trial of amiloride was also attempted to decrease renal magnesium wasting. Amiloride normalized magnesium levels but had to be discontinued due to asymptomatic hyperkalemia. This case report suggests that amiloride and magnesium replacement via ambulatory elastomeric pumps may be safe and effective treatment options for panitumumab-induced refractory hypomagnesemia in mCRC, potentially improving quality of life and allowing beneficial anti-cancer treatments to continue. Future studies should further evaluate optimization of amiloride and intravenous magnesium replacement via ambulatory elastomeric pump.
The new transdermal contraceptive patch expands available contraceptive options: Does it offer protection with less VTE risk?
The first transdermal contraceptive patch was approved by the US Food and Drug Administration (FDA) in 2001.1 A 2018 survey revealed that 5% of women in the United States between the ages of 15 and 49 years reported the use of a short-acting hormonal contraceptive method (ie, vaginal ring, transdermal patch, injectable) within the past month, with just 0.3% reporting the use of a transdermal patch.2 Transdermal contraceptive patches are an effective form of birth control that may be a convenient option for patients who do not want to take a daily oral contraceptive pill but want similar efficacy and tolerability. Typical failure rates of patches are similar to that of combined oral contraceptives (COCs).1,3
While transdermal hormone delivery results in less peaks and troughs of estrogen compared with COCs, the total estrogen exposure is higher than with COCs; therefore, the risk for venous thromboembolism (VTE) with previously available patches is about twice as high.1 Twirla (Agile), an ethinyl estradiol (EE)/levonorgestrel (LNG) patch, delivers a low and consistent daily dose of hormones over 3 patches replaced once weekly, with no patch on the fourth week.3 Twirla contains 120 μg/day LNG and 30 μg/day EE. OrthoEvra, FDA approved in 2001 as mentioned, contains 150 μg/day norelgestromin and 35 μg/day EE.1 A reduction of the EE dose in COCs has been associated with lower risk for VTE.4
The addition of Twirla to the market offers another contraceptive option for patients who opt for a weekly, self-administered method.
How much lower is the VTE risk?
OBG
Barbara Levy, MD: The reality is we can’t designate a reduction of risk, except, in general, when the dose of ethinyl estradiol is lower, we think that the VTE risk is lower. There has not been a head-to-head comparison in a large enough population to be able to say that the risk is reduced by a certain factor. We just look at the overall exposure to estrogen and say, “In general, for VTE risk, a lower dose is a better thing for women.”
That being said, look at birth control pills, like COCs. We don’t have actual numbers to say that a 30-μg pill is this much less risky than a 35-μg pill. We just put it into a hierarchy, and that’s what we can do with the patch. We can say that, in general, lower is better for VTE risk, but no one can provide absolute numbers.
Continue to: Efficacy...
Efficacy
OBG
Dr. Levy: You have to look at the pivotal trials and look at what the efficacy was in a trial setting. In the real-world setting, the effectiveness is never quite as good as it is in a clinical trial. I think the bottom line for all of us is that combined oral contraception, meaning estrogen with progestin, is equivalently effective across the different options that are available for women. Efficacy really isn’t the factor to use to distinguish which one I’m going to pick. It is about the patient’s convenience and many other factors. But in terms of its clinical effectiveness in preventing pregnancy, from a very practical standpoint, I think we consider them all the same.
Considering route of administration
OBG
Dr. Levy: I think there’s always a benefit in having lots of choices. And for some women, being able to put a patch on once a week is much more convenient, easier to remember, and delivers a very consistent dose of hormone absorbed through the skin, which is different than taking a pill in the morning when your levels go up quickly then diminish over the day. The hormones are higher at a certain time, and then they drop off, so there might be some advantages for people who are very sensitive to swings in hormonal levels. There’s also a convenience factor, where for some people they will choose that. Other people might really dislike having a relatively large patch on their skin somewhere, or they may have skin sensitivity to the adhesive. Overall, I always think that having more options is better and individual girls/women will choose what works best for them.
Counseling tips
OBG
Dr. Levy: Like other patches that are available on the market, these are a once-a-week patch. The patch should be placed on clean, dry skin. No lotions, perfumes, or anything on the skin because you really want them to stick for the whole week, and it’s not going to stick if there’s anything oily on the skin. The first patch is placed on day 1 of a menstrual cycle, the first day of bleeding, and then changed weekly for 3 weeks. Then there’s a 7-day patch-free time in which one would expect to have a period.
In general, breakthrough bleeding was not a significant problem with the patch, but some women will have some irregular spotting and bleeding with any sort of hormonal treatment; some women may have no periods at all. In other words, the estrogen dose and progestin may be of a balance that allows the patient not to have periods. But, in general, most of the women in the trial had regular light menstrual flow during the week when their patch was not on.5
Continue to: Pricing...
Pricing
OBG
Dr. Levy: That’s a tricky question. Insurance plans through Obamacare, the Affordable Care Act, are required to cover every form of contraception. That means they must cover a patch. It doesn’t mean that they have to cover this patch. And because there are generics available of the other patch formulation, it is likely that this would be a higher tier, meaning that there may be a higher copay for someone who wanted to use Twirla versus one of the generic patches.
I can’t say that that’s universally the case, but my experience with most of the health insurance plans is that they tend to put barriers in the way for any of us to prescribe, and for women to use, brand-name products. So Twirla is new on the market; it’s a brand-name product. It may work much better for some people; and in those cases, the health care provider might have to send a letter to the insurance company saying why this one is medically necessary for a patient. There probably will be some hoops to go through for coverage without a copay. I think coverage will be there, but there may be a substantial copay because of the tier level.
OBG
Dr. Levy: I don’t think that the payer is going to buy that argument unless the requirement is to use a patch. If the patient, for example, has some sort of gastrointestinal disease where they don’t absorb things well, so pills don’t work well, we might get to the place where they have to have a patch. If the patient has a lot of breast tenderness or has symptoms on the generic patch that delivers a higher level of estrogen, then we would have to document those symptoms to say, “She’s not tolerating this one and, therefore, we need to go to that one.” So, I think as prescribers we would have to justify not only the lower dose but also the form.
As a clinician, I would always like to put somebody on the lower dose. We do think lower is better, but we have to be sensitive to the costs of all of these things too. I’m very sensitive to my patients’ out-of-pocket costs because, in the end, if the costs are a lot of money or she can only afford one month at a time, then she may miss a window where she may not have the money to buy next month’s supply when it’s due, and get pregnant. We have to balance all of those things as we’re thinking through the best option for an individual.
We have more to learn
OBG
Dr. Levy: I think it’s always exciting when we have new products available, and there’s a lot more we’ll learn as Twirla comes into commercial use and millions instead of thousands of people are using it. Overall, I think it’s fantastic that there’s ongoing research and that there are new products out there. And kudos to the company for doing the research and for getting approval, and I’m looking forward to learning more about it.
- Galzote RA, Rafie S, Teal R, et al. Transdermal delivery of combined hormonal contraception: a review of the current literature. Int J Womens Health. 2017;9:315-321.
- Contraceptive use in the United States. Guttmacher website. Published May 2021. Accessed August 29, 2021. https://www.guttmacher.org/fact-sheet/contraceptive-method-use-united-states#.
- US National Library of Medicine. Estrogen and progestin (transdermal patch contraceptives). MedlinePlus website. Updated February 15, 2021. Accessed August 29, 2021. https://medlineplus.gov/druginfo/meds/a602006.html.
- American College of Obstetricians and Gynecologists Practice Bulletin No. 206: use of hormonal contraception in women with coexisting medical conditions [published correction appears in: ACOG Committee on Practice Bulletins—gynecology. Obstet Gynecol. 2019;133:1288.] Obstet Gynecol. 2019;133:E128-E150.
- Nelson AL, Kaunitz AM, Kroll R, et al. Efficacy, safety, and tolerability of a levonorgestrel/ethinyl estradiol transdermal delivery system: phase 3 clinical trial results. Contraception. 2021;103:137-143. doi: 10.1016/j.contraception.2020.
11.011.
The first transdermal contraceptive patch was approved by the US Food and Drug Administration (FDA) in 2001.1 A 2018 survey revealed that 5% of women in the United States between the ages of 15 and 49 years reported the use of a short-acting hormonal contraceptive method (ie, vaginal ring, transdermal patch, injectable) within the past month, with just 0.3% reporting the use of a transdermal patch.2 Transdermal contraceptive patches are an effective form of birth control that may be a convenient option for patients who do not want to take a daily oral contraceptive pill but want similar efficacy and tolerability. Typical failure rates of patches are similar to that of combined oral contraceptives (COCs).1,3
While transdermal hormone delivery results in less peaks and troughs of estrogen compared with COCs, the total estrogen exposure is higher than with COCs; therefore, the risk for venous thromboembolism (VTE) with previously available patches is about twice as high.1 Twirla (Agile), an ethinyl estradiol (EE)/levonorgestrel (LNG) patch, delivers a low and consistent daily dose of hormones over 3 patches replaced once weekly, with no patch on the fourth week.3 Twirla contains 120 μg/day LNG and 30 μg/day EE. OrthoEvra, FDA approved in 2001 as mentioned, contains 150 μg/day norelgestromin and 35 μg/day EE.1 A reduction of the EE dose in COCs has been associated with lower risk for VTE.4
The addition of Twirla to the market offers another contraceptive option for patients who opt for a weekly, self-administered method.
How much lower is the VTE risk?
OBG
Barbara Levy, MD: The reality is we can’t designate a reduction of risk, except, in general, when the dose of ethinyl estradiol is lower, we think that the VTE risk is lower. There has not been a head-to-head comparison in a large enough population to be able to say that the risk is reduced by a certain factor. We just look at the overall exposure to estrogen and say, “In general, for VTE risk, a lower dose is a better thing for women.”
That being said, look at birth control pills, like COCs. We don’t have actual numbers to say that a 30-μg pill is this much less risky than a 35-μg pill. We just put it into a hierarchy, and that’s what we can do with the patch. We can say that, in general, lower is better for VTE risk, but no one can provide absolute numbers.
Continue to: Efficacy...
Efficacy
OBG
Dr. Levy: You have to look at the pivotal trials and look at what the efficacy was in a trial setting. In the real-world setting, the effectiveness is never quite as good as it is in a clinical trial. I think the bottom line for all of us is that combined oral contraception, meaning estrogen with progestin, is equivalently effective across the different options that are available for women. Efficacy really isn’t the factor to use to distinguish which one I’m going to pick. It is about the patient’s convenience and many other factors. But in terms of its clinical effectiveness in preventing pregnancy, from a very practical standpoint, I think we consider them all the same.
Considering route of administration
OBG
Dr. Levy: I think there’s always a benefit in having lots of choices. And for some women, being able to put a patch on once a week is much more convenient, easier to remember, and delivers a very consistent dose of hormone absorbed through the skin, which is different than taking a pill in the morning when your levels go up quickly then diminish over the day. The hormones are higher at a certain time, and then they drop off, so there might be some advantages for people who are very sensitive to swings in hormonal levels. There’s also a convenience factor, where for some people they will choose that. Other people might really dislike having a relatively large patch on their skin somewhere, or they may have skin sensitivity to the adhesive. Overall, I always think that having more options is better and individual girls/women will choose what works best for them.
Counseling tips
OBG
Dr. Levy: Like other patches that are available on the market, these are a once-a-week patch. The patch should be placed on clean, dry skin. No lotions, perfumes, or anything on the skin because you really want them to stick for the whole week, and it’s not going to stick if there’s anything oily on the skin. The first patch is placed on day 1 of a menstrual cycle, the first day of bleeding, and then changed weekly for 3 weeks. Then there’s a 7-day patch-free time in which one would expect to have a period.
In general, breakthrough bleeding was not a significant problem with the patch, but some women will have some irregular spotting and bleeding with any sort of hormonal treatment; some women may have no periods at all. In other words, the estrogen dose and progestin may be of a balance that allows the patient not to have periods. But, in general, most of the women in the trial had regular light menstrual flow during the week when their patch was not on.5
Continue to: Pricing...
Pricing
OBG
Dr. Levy: That’s a tricky question. Insurance plans through Obamacare, the Affordable Care Act, are required to cover every form of contraception. That means they must cover a patch. It doesn’t mean that they have to cover this patch. And because there are generics available of the other patch formulation, it is likely that this would be a higher tier, meaning that there may be a higher copay for someone who wanted to use Twirla versus one of the generic patches.
I can’t say that that’s universally the case, but my experience with most of the health insurance plans is that they tend to put barriers in the way for any of us to prescribe, and for women to use, brand-name products. So Twirla is new on the market; it’s a brand-name product. It may work much better for some people; and in those cases, the health care provider might have to send a letter to the insurance company saying why this one is medically necessary for a patient. There probably will be some hoops to go through for coverage without a copay. I think coverage will be there, but there may be a substantial copay because of the tier level.
OBG
Dr. Levy: I don’t think that the payer is going to buy that argument unless the requirement is to use a patch. If the patient, for example, has some sort of gastrointestinal disease where they don’t absorb things well, so pills don’t work well, we might get to the place where they have to have a patch. If the patient has a lot of breast tenderness or has symptoms on the generic patch that delivers a higher level of estrogen, then we would have to document those symptoms to say, “She’s not tolerating this one and, therefore, we need to go to that one.” So, I think as prescribers we would have to justify not only the lower dose but also the form.
As a clinician, I would always like to put somebody on the lower dose. We do think lower is better, but we have to be sensitive to the costs of all of these things too. I’m very sensitive to my patients’ out-of-pocket costs because, in the end, if the costs are a lot of money or she can only afford one month at a time, then she may miss a window where she may not have the money to buy next month’s supply when it’s due, and get pregnant. We have to balance all of those things as we’re thinking through the best option for an individual.
We have more to learn
OBG
Dr. Levy: I think it’s always exciting when we have new products available, and there’s a lot more we’ll learn as Twirla comes into commercial use and millions instead of thousands of people are using it. Overall, I think it’s fantastic that there’s ongoing research and that there are new products out there. And kudos to the company for doing the research and for getting approval, and I’m looking forward to learning more about it.
The first transdermal contraceptive patch was approved by the US Food and Drug Administration (FDA) in 2001.1 A 2018 survey revealed that 5% of women in the United States between the ages of 15 and 49 years reported the use of a short-acting hormonal contraceptive method (ie, vaginal ring, transdermal patch, injectable) within the past month, with just 0.3% reporting the use of a transdermal patch.2 Transdermal contraceptive patches are an effective form of birth control that may be a convenient option for patients who do not want to take a daily oral contraceptive pill but want similar efficacy and tolerability. Typical failure rates of patches are similar to that of combined oral contraceptives (COCs).1,3
While transdermal hormone delivery results in less peaks and troughs of estrogen compared with COCs, the total estrogen exposure is higher than with COCs; therefore, the risk for venous thromboembolism (VTE) with previously available patches is about twice as high.1 Twirla (Agile), an ethinyl estradiol (EE)/levonorgestrel (LNG) patch, delivers a low and consistent daily dose of hormones over 3 patches replaced once weekly, with no patch on the fourth week.3 Twirla contains 120 μg/day LNG and 30 μg/day EE. OrthoEvra, FDA approved in 2001 as mentioned, contains 150 μg/day norelgestromin and 35 μg/day EE.1 A reduction of the EE dose in COCs has been associated with lower risk for VTE.4
The addition of Twirla to the market offers another contraceptive option for patients who opt for a weekly, self-administered method.
How much lower is the VTE risk?
OBG
Barbara Levy, MD: The reality is we can’t designate a reduction of risk, except, in general, when the dose of ethinyl estradiol is lower, we think that the VTE risk is lower. There has not been a head-to-head comparison in a large enough population to be able to say that the risk is reduced by a certain factor. We just look at the overall exposure to estrogen and say, “In general, for VTE risk, a lower dose is a better thing for women.”
That being said, look at birth control pills, like COCs. We don’t have actual numbers to say that a 30-μg pill is this much less risky than a 35-μg pill. We just put it into a hierarchy, and that’s what we can do with the patch. We can say that, in general, lower is better for VTE risk, but no one can provide absolute numbers.
Continue to: Efficacy...
Efficacy
OBG
Dr. Levy: You have to look at the pivotal trials and look at what the efficacy was in a trial setting. In the real-world setting, the effectiveness is never quite as good as it is in a clinical trial. I think the bottom line for all of us is that combined oral contraception, meaning estrogen with progestin, is equivalently effective across the different options that are available for women. Efficacy really isn’t the factor to use to distinguish which one I’m going to pick. It is about the patient’s convenience and many other factors. But in terms of its clinical effectiveness in preventing pregnancy, from a very practical standpoint, I think we consider them all the same.
Considering route of administration
OBG
Dr. Levy: I think there’s always a benefit in having lots of choices. And for some women, being able to put a patch on once a week is much more convenient, easier to remember, and delivers a very consistent dose of hormone absorbed through the skin, which is different than taking a pill in the morning when your levels go up quickly then diminish over the day. The hormones are higher at a certain time, and then they drop off, so there might be some advantages for people who are very sensitive to swings in hormonal levels. There’s also a convenience factor, where for some people they will choose that. Other people might really dislike having a relatively large patch on their skin somewhere, or they may have skin sensitivity to the adhesive. Overall, I always think that having more options is better and individual girls/women will choose what works best for them.
Counseling tips
OBG
Dr. Levy: Like other patches that are available on the market, these are a once-a-week patch. The patch should be placed on clean, dry skin. No lotions, perfumes, or anything on the skin because you really want them to stick for the whole week, and it’s not going to stick if there’s anything oily on the skin. The first patch is placed on day 1 of a menstrual cycle, the first day of bleeding, and then changed weekly for 3 weeks. Then there’s a 7-day patch-free time in which one would expect to have a period.
In general, breakthrough bleeding was not a significant problem with the patch, but some women will have some irregular spotting and bleeding with any sort of hormonal treatment; some women may have no periods at all. In other words, the estrogen dose and progestin may be of a balance that allows the patient not to have periods. But, in general, most of the women in the trial had regular light menstrual flow during the week when their patch was not on.5
Continue to: Pricing...
Pricing
OBG
Dr. Levy: That’s a tricky question. Insurance plans through Obamacare, the Affordable Care Act, are required to cover every form of contraception. That means they must cover a patch. It doesn’t mean that they have to cover this patch. And because there are generics available of the other patch formulation, it is likely that this would be a higher tier, meaning that there may be a higher copay for someone who wanted to use Twirla versus one of the generic patches.
I can’t say that that’s universally the case, but my experience with most of the health insurance plans is that they tend to put barriers in the way for any of us to prescribe, and for women to use, brand-name products. So Twirla is new on the market; it’s a brand-name product. It may work much better for some people; and in those cases, the health care provider might have to send a letter to the insurance company saying why this one is medically necessary for a patient. There probably will be some hoops to go through for coverage without a copay. I think coverage will be there, but there may be a substantial copay because of the tier level.
OBG
Dr. Levy: I don’t think that the payer is going to buy that argument unless the requirement is to use a patch. If the patient, for example, has some sort of gastrointestinal disease where they don’t absorb things well, so pills don’t work well, we might get to the place where they have to have a patch. If the patient has a lot of breast tenderness or has symptoms on the generic patch that delivers a higher level of estrogen, then we would have to document those symptoms to say, “She’s not tolerating this one and, therefore, we need to go to that one.” So, I think as prescribers we would have to justify not only the lower dose but also the form.
As a clinician, I would always like to put somebody on the lower dose. We do think lower is better, but we have to be sensitive to the costs of all of these things too. I’m very sensitive to my patients’ out-of-pocket costs because, in the end, if the costs are a lot of money or she can only afford one month at a time, then she may miss a window where she may not have the money to buy next month’s supply when it’s due, and get pregnant. We have to balance all of those things as we’re thinking through the best option for an individual.
We have more to learn
OBG
Dr. Levy: I think it’s always exciting when we have new products available, and there’s a lot more we’ll learn as Twirla comes into commercial use and millions instead of thousands of people are using it. Overall, I think it’s fantastic that there’s ongoing research and that there are new products out there. And kudos to the company for doing the research and for getting approval, and I’m looking forward to learning more about it.
- Galzote RA, Rafie S, Teal R, et al. Transdermal delivery of combined hormonal contraception: a review of the current literature. Int J Womens Health. 2017;9:315-321.
- Contraceptive use in the United States. Guttmacher website. Published May 2021. Accessed August 29, 2021. https://www.guttmacher.org/fact-sheet/contraceptive-method-use-united-states#.
- US National Library of Medicine. Estrogen and progestin (transdermal patch contraceptives). MedlinePlus website. Updated February 15, 2021. Accessed August 29, 2021. https://medlineplus.gov/druginfo/meds/a602006.html.
- American College of Obstetricians and Gynecologists Practice Bulletin No. 206: use of hormonal contraception in women with coexisting medical conditions [published correction appears in: ACOG Committee on Practice Bulletins—gynecology. Obstet Gynecol. 2019;133:1288.] Obstet Gynecol. 2019;133:E128-E150.
- Nelson AL, Kaunitz AM, Kroll R, et al. Efficacy, safety, and tolerability of a levonorgestrel/ethinyl estradiol transdermal delivery system: phase 3 clinical trial results. Contraception. 2021;103:137-143. doi: 10.1016/j.contraception.2020.
11.011.
- Galzote RA, Rafie S, Teal R, et al. Transdermal delivery of combined hormonal contraception: a review of the current literature. Int J Womens Health. 2017;9:315-321.
- Contraceptive use in the United States. Guttmacher website. Published May 2021. Accessed August 29, 2021. https://www.guttmacher.org/fact-sheet/contraceptive-method-use-united-states#.
- US National Library of Medicine. Estrogen and progestin (transdermal patch contraceptives). MedlinePlus website. Updated February 15, 2021. Accessed August 29, 2021. https://medlineplus.gov/druginfo/meds/a602006.html.
- American College of Obstetricians and Gynecologists Practice Bulletin No. 206: use of hormonal contraception in women with coexisting medical conditions [published correction appears in: ACOG Committee on Practice Bulletins—gynecology. Obstet Gynecol. 2019;133:1288.] Obstet Gynecol. 2019;133:E128-E150.
- Nelson AL, Kaunitz AM, Kroll R, et al. Efficacy, safety, and tolerability of a levonorgestrel/ethinyl estradiol transdermal delivery system: phase 3 clinical trial results. Contraception. 2021;103:137-143. doi: 10.1016/j.contraception.2020.
11.011.
ITP after COVID-19 Vaccination at the Salisbury VA Healthcare System: Case Studies
Background
An association between vaccines and the rare development of immune thrombocytopenic purpura (ITP) has been reported in the literature. More recently, there have been a few case reports published describing patients developing ITP shortly after COVID- 19 vaccination, but this has not been reported specifically in the Veteran population. The SVAHCS has three cases of Veterans diagnosed with new or relapsed ITP within two months of receiving the second COVID-19 vaccine (all Pfizer brand). The treatment(s) and current outcome for each patient is summarized below.
Case Reports
Case 1 is a 78-year-old male Veteran who received his second COVID-19 vaccine on 2/10/21. Patient was diagnosed with ITP 4/27/21, hospitalized multiple times and treated with pulse dexamethasone, prednisone taper, rituximab IV weekly and romiplostim injections. Currently, patient has a thrombocytosis and romiplostim injections are on hold. Case 2 is a 90-yearold male Veteran who received his second COVID-19 vaccine on 3/16/21. Patient was diagnosed on 5/3/21 and treated with pulse dexamethasone, prednisone taper and rituximab IV weekly. Platelet count is currently normal. Case 3 is a 75-year-old male Veteran who received his second COVID-19 vaccine on 2/1/21. He has a history of ITP diagnosed 12/12/14 that has been well controlled with weekly romiplostim injections until 4/9/21. Patient was hospitalized and treated with pulse dexamethasone and prednisone taper. Upon discharge, therapy was changed from romiplostim to fostamatinib. Currently, platelet count recovered and is stable.
Conclusions
The two Veterans with de novo ITP exhibited resistant disease and had prolonged treatment courses, taking approximately a month to recover their platelet counts. In contrast, the Veteran with relapsed ITP exhibited a faster recovery period of approximately two weeks. In the safety trials conducted for the Pfizer COVID-19 vaccine, participants received vaccination or placebo and had a follow-up for an average of two months which may explain why ITP was not reported as a possible association until after marketing. After treating the above cases, the SVAHCS plans to use thrombopoietin receptor agonists (TPO-RAs) earlier in the treatment of ITP that may be associated with the COVID-19 vaccine as this has recently been recommended in case reports from the general population.
Background
An association between vaccines and the rare development of immune thrombocytopenic purpura (ITP) has been reported in the literature. More recently, there have been a few case reports published describing patients developing ITP shortly after COVID- 19 vaccination, but this has not been reported specifically in the Veteran population. The SVAHCS has three cases of Veterans diagnosed with new or relapsed ITP within two months of receiving the second COVID-19 vaccine (all Pfizer brand). The treatment(s) and current outcome for each patient is summarized below.
Case Reports
Case 1 is a 78-year-old male Veteran who received his second COVID-19 vaccine on 2/10/21. Patient was diagnosed with ITP 4/27/21, hospitalized multiple times and treated with pulse dexamethasone, prednisone taper, rituximab IV weekly and romiplostim injections. Currently, patient has a thrombocytosis and romiplostim injections are on hold. Case 2 is a 90-yearold male Veteran who received his second COVID-19 vaccine on 3/16/21. Patient was diagnosed on 5/3/21 and treated with pulse dexamethasone, prednisone taper and rituximab IV weekly. Platelet count is currently normal. Case 3 is a 75-year-old male Veteran who received his second COVID-19 vaccine on 2/1/21. He has a history of ITP diagnosed 12/12/14 that has been well controlled with weekly romiplostim injections until 4/9/21. Patient was hospitalized and treated with pulse dexamethasone and prednisone taper. Upon discharge, therapy was changed from romiplostim to fostamatinib. Currently, platelet count recovered and is stable.
Conclusions
The two Veterans with de novo ITP exhibited resistant disease and had prolonged treatment courses, taking approximately a month to recover their platelet counts. In contrast, the Veteran with relapsed ITP exhibited a faster recovery period of approximately two weeks. In the safety trials conducted for the Pfizer COVID-19 vaccine, participants received vaccination or placebo and had a follow-up for an average of two months which may explain why ITP was not reported as a possible association until after marketing. After treating the above cases, the SVAHCS plans to use thrombopoietin receptor agonists (TPO-RAs) earlier in the treatment of ITP that may be associated with the COVID-19 vaccine as this has recently been recommended in case reports from the general population.
Background
An association between vaccines and the rare development of immune thrombocytopenic purpura (ITP) has been reported in the literature. More recently, there have been a few case reports published describing patients developing ITP shortly after COVID- 19 vaccination, but this has not been reported specifically in the Veteran population. The SVAHCS has three cases of Veterans diagnosed with new or relapsed ITP within two months of receiving the second COVID-19 vaccine (all Pfizer brand). The treatment(s) and current outcome for each patient is summarized below.
Case Reports
Case 1 is a 78-year-old male Veteran who received his second COVID-19 vaccine on 2/10/21. Patient was diagnosed with ITP 4/27/21, hospitalized multiple times and treated with pulse dexamethasone, prednisone taper, rituximab IV weekly and romiplostim injections. Currently, patient has a thrombocytosis and romiplostim injections are on hold. Case 2 is a 90-yearold male Veteran who received his second COVID-19 vaccine on 3/16/21. Patient was diagnosed on 5/3/21 and treated with pulse dexamethasone, prednisone taper and rituximab IV weekly. Platelet count is currently normal. Case 3 is a 75-year-old male Veteran who received his second COVID-19 vaccine on 2/1/21. He has a history of ITP diagnosed 12/12/14 that has been well controlled with weekly romiplostim injections until 4/9/21. Patient was hospitalized and treated with pulse dexamethasone and prednisone taper. Upon discharge, therapy was changed from romiplostim to fostamatinib. Currently, platelet count recovered and is stable.
Conclusions
The two Veterans with de novo ITP exhibited resistant disease and had prolonged treatment courses, taking approximately a month to recover their platelet counts. In contrast, the Veteran with relapsed ITP exhibited a faster recovery period of approximately two weeks. In the safety trials conducted for the Pfizer COVID-19 vaccine, participants received vaccination or placebo and had a follow-up for an average of two months which may explain why ITP was not reported as a possible association until after marketing. After treating the above cases, the SVAHCS plans to use thrombopoietin receptor agonists (TPO-RAs) earlier in the treatment of ITP that may be associated with the COVID-19 vaccine as this has recently been recommended in case reports from the general population.
Evans’ Syndrome in Undiagnosed Small Lymphocytic Lymphoma: Case Report and Literature Review
Background
Evans’ syndrome is a rare entity characterized by concomitant or sequential multilineage cytopenia particularly autoimmune hemolytic anemia, ITP and very rarely autoimmune neutropenia. Although more common in young adults, it can occur in elderly usually associated with malignancies like CLL.
Case Report
A 74 years old Veteran presented with complaints of fatigue and worsening dyspnea on exertion. His physical exam was unremarkable except jaundice. His labs were significant for macrocytic anemia with Hemoglobin of 7.4g/dl compared to 11.7g/dl 6 months prior, MCV 106.9 fL, LDH 809U/L, indirect bilirubin 4.1mg/dl, absolute reticulocyte 0.16M/uL, Haptoglobin <15mg/dl and Positive DAT. Platelets were mildly decreased at 111K/ul. No lymphocytosis was noted. Initially, the hemolysis was thought to be cephalosporin- related given that the patient had taken cephalexin recently for cellulitis. As part of the workup for anemia, the patient underwent EGD and colonoscopy which was initially unrevealing. However, random biopsies from the descending colon and terminal ileum returned with a small lymphocytic infiltrate consistent with SLL/CLL. Cytogenetics showed trisomy-12 which is associated with intermediate prognosis for CLL. PET scan done subsequently revealed only a reactive marrow and an enlarged 15.8cm non-hypermetabolic spleen. This veteran having anemia, positive DAT, thrombocytopenia, and splenomegaly got diagnosed with Evans’s syndrome. This syndrome was the initial manifestation of his underlying CLL. We started the patient on a prednisone taper for 4 weeks to which anemia and thrombocytopenia barely responded, ultimately Rituximab 375mg/m2 x4 weekly doses was started which led to complete resolution of anemia and thrombocytopenia. We closely followed the patient and monitored CBC and hemolytic markers. The patient relapsed in two years which was subsequently managed with another course of Rituximab 375mg/m2 x4 weekly doses.
Conclusions
This case report aims to call attention to this relatively rare entity which is difficult to treat and often associated with frequent relapses. Though rare, physicians should maintain high suspicion for this syndrome in patients with multi-lineage cytopenia which are usually not even responding well to the common treatment for cytopenia. Furthermore, there is room for improvement in Evans’ syndrome management since mortality remains higher in these patients than in those with isolated autoimmuce cytopenias.
Background
Evans’ syndrome is a rare entity characterized by concomitant or sequential multilineage cytopenia particularly autoimmune hemolytic anemia, ITP and very rarely autoimmune neutropenia. Although more common in young adults, it can occur in elderly usually associated with malignancies like CLL.
Case Report
A 74 years old Veteran presented with complaints of fatigue and worsening dyspnea on exertion. His physical exam was unremarkable except jaundice. His labs were significant for macrocytic anemia with Hemoglobin of 7.4g/dl compared to 11.7g/dl 6 months prior, MCV 106.9 fL, LDH 809U/L, indirect bilirubin 4.1mg/dl, absolute reticulocyte 0.16M/uL, Haptoglobin <15mg/dl and Positive DAT. Platelets were mildly decreased at 111K/ul. No lymphocytosis was noted. Initially, the hemolysis was thought to be cephalosporin- related given that the patient had taken cephalexin recently for cellulitis. As part of the workup for anemia, the patient underwent EGD and colonoscopy which was initially unrevealing. However, random biopsies from the descending colon and terminal ileum returned with a small lymphocytic infiltrate consistent with SLL/CLL. Cytogenetics showed trisomy-12 which is associated with intermediate prognosis for CLL. PET scan done subsequently revealed only a reactive marrow and an enlarged 15.8cm non-hypermetabolic spleen. This veteran having anemia, positive DAT, thrombocytopenia, and splenomegaly got diagnosed with Evans’s syndrome. This syndrome was the initial manifestation of his underlying CLL. We started the patient on a prednisone taper for 4 weeks to which anemia and thrombocytopenia barely responded, ultimately Rituximab 375mg/m2 x4 weekly doses was started which led to complete resolution of anemia and thrombocytopenia. We closely followed the patient and monitored CBC and hemolytic markers. The patient relapsed in two years which was subsequently managed with another course of Rituximab 375mg/m2 x4 weekly doses.
Conclusions
This case report aims to call attention to this relatively rare entity which is difficult to treat and often associated with frequent relapses. Though rare, physicians should maintain high suspicion for this syndrome in patients with multi-lineage cytopenia which are usually not even responding well to the common treatment for cytopenia. Furthermore, there is room for improvement in Evans’ syndrome management since mortality remains higher in these patients than in those with isolated autoimmuce cytopenias.
Background
Evans’ syndrome is a rare entity characterized by concomitant or sequential multilineage cytopenia particularly autoimmune hemolytic anemia, ITP and very rarely autoimmune neutropenia. Although more common in young adults, it can occur in elderly usually associated with malignancies like CLL.
Case Report
A 74 years old Veteran presented with complaints of fatigue and worsening dyspnea on exertion. His physical exam was unremarkable except jaundice. His labs were significant for macrocytic anemia with Hemoglobin of 7.4g/dl compared to 11.7g/dl 6 months prior, MCV 106.9 fL, LDH 809U/L, indirect bilirubin 4.1mg/dl, absolute reticulocyte 0.16M/uL, Haptoglobin <15mg/dl and Positive DAT. Platelets were mildly decreased at 111K/ul. No lymphocytosis was noted. Initially, the hemolysis was thought to be cephalosporin- related given that the patient had taken cephalexin recently for cellulitis. As part of the workup for anemia, the patient underwent EGD and colonoscopy which was initially unrevealing. However, random biopsies from the descending colon and terminal ileum returned with a small lymphocytic infiltrate consistent with SLL/CLL. Cytogenetics showed trisomy-12 which is associated with intermediate prognosis for CLL. PET scan done subsequently revealed only a reactive marrow and an enlarged 15.8cm non-hypermetabolic spleen. This veteran having anemia, positive DAT, thrombocytopenia, and splenomegaly got diagnosed with Evans’s syndrome. This syndrome was the initial manifestation of his underlying CLL. We started the patient on a prednisone taper for 4 weeks to which anemia and thrombocytopenia barely responded, ultimately Rituximab 375mg/m2 x4 weekly doses was started which led to complete resolution of anemia and thrombocytopenia. We closely followed the patient and monitored CBC and hemolytic markers. The patient relapsed in two years which was subsequently managed with another course of Rituximab 375mg/m2 x4 weekly doses.
Conclusions
This case report aims to call attention to this relatively rare entity which is difficult to treat and often associated with frequent relapses. Though rare, physicians should maintain high suspicion for this syndrome in patients with multi-lineage cytopenia which are usually not even responding well to the common treatment for cytopenia. Furthermore, there is room for improvement in Evans’ syndrome management since mortality remains higher in these patients than in those with isolated autoimmuce cytopenias.
Development of Debilitating Neuropathy After Two Cycles of Pembrolizumab
Case Report
73-year-old white male presented with large right shoulder soft tissue mass (17x5 cm) near the scapula, and was subsequently sent for surgical resection by his primary care. Pathology showed nodular melanoma with positive margin, lymphovascular invasion and neurotropism present with high mitosis. PET-CT scan showed positive uptake in axillary and supraclavicular lymph nodes as well as uptake in the left proximal tibia. Biopsy of the bone was also positive for melanoma. Molecular study showed BRAF mutation at L597, high tumor mutation burden (24 mutations/Mb), and PD-L1 positive in 60% of tumor cells and PD-1 was positive in immune cells, but not in tumor cells. One other distinct feature of this clinical presentation was the abundance of macrophages (CD68+) in the tumor microenvironment. Patient was initiated therapy with pembrolizumab. However, three weeks after his second cycle, he was admitted to hospital due to severe weakness in both upper extremities and pain at night. He also experienced a new onset of polyarthralgia in both hands, unable to play musical instruments. He was started on steroid treatment and showed significant improvement. Once steroid was tapered off, the sensation of pain substantially decreased but persisted. EMG showed right median motor neuropathy and left median sensory neuropathy. Blood test detected ANA positive, and as TSH was high, levothyroxine was initiated.
Outcome
His PET-CT scan showed improvement after only two cycles of treatment and has remained stable for over ten months without any treatment (patient elected to stop pembrolizumab treatment due to frequent traveling). We have performed a more detailed study of the macrophages in his tumor sample and interestingly, the majority of macrophages were type-1 (CD 80+), with some, type-2 macrophages (CD163+). It is known that type-1 macrophages are pro-inflammatory and have antitumor effect, while type-2 macrophages have opposite effect and often promote tumor growth and metastasis. This could explain the side effect and long duration of response despite only two cycles of pembrolizumab treatment. Characteristics of macrophages in melanoma tumor samples may be an important parameter to predict side effect and tumor response beyond PD1 or PD-L1 expression.
Case Report
73-year-old white male presented with large right shoulder soft tissue mass (17x5 cm) near the scapula, and was subsequently sent for surgical resection by his primary care. Pathology showed nodular melanoma with positive margin, lymphovascular invasion and neurotropism present with high mitosis. PET-CT scan showed positive uptake in axillary and supraclavicular lymph nodes as well as uptake in the left proximal tibia. Biopsy of the bone was also positive for melanoma. Molecular study showed BRAF mutation at L597, high tumor mutation burden (24 mutations/Mb), and PD-L1 positive in 60% of tumor cells and PD-1 was positive in immune cells, but not in tumor cells. One other distinct feature of this clinical presentation was the abundance of macrophages (CD68+) in the tumor microenvironment. Patient was initiated therapy with pembrolizumab. However, three weeks after his second cycle, he was admitted to hospital due to severe weakness in both upper extremities and pain at night. He also experienced a new onset of polyarthralgia in both hands, unable to play musical instruments. He was started on steroid treatment and showed significant improvement. Once steroid was tapered off, the sensation of pain substantially decreased but persisted. EMG showed right median motor neuropathy and left median sensory neuropathy. Blood test detected ANA positive, and as TSH was high, levothyroxine was initiated.
Outcome
His PET-CT scan showed improvement after only two cycles of treatment and has remained stable for over ten months without any treatment (patient elected to stop pembrolizumab treatment due to frequent traveling). We have performed a more detailed study of the macrophages in his tumor sample and interestingly, the majority of macrophages were type-1 (CD 80+), with some, type-2 macrophages (CD163+). It is known that type-1 macrophages are pro-inflammatory and have antitumor effect, while type-2 macrophages have opposite effect and often promote tumor growth and metastasis. This could explain the side effect and long duration of response despite only two cycles of pembrolizumab treatment. Characteristics of macrophages in melanoma tumor samples may be an important parameter to predict side effect and tumor response beyond PD1 or PD-L1 expression.
Case Report
73-year-old white male presented with large right shoulder soft tissue mass (17x5 cm) near the scapula, and was subsequently sent for surgical resection by his primary care. Pathology showed nodular melanoma with positive margin, lymphovascular invasion and neurotropism present with high mitosis. PET-CT scan showed positive uptake in axillary and supraclavicular lymph nodes as well as uptake in the left proximal tibia. Biopsy of the bone was also positive for melanoma. Molecular study showed BRAF mutation at L597, high tumor mutation burden (24 mutations/Mb), and PD-L1 positive in 60% of tumor cells and PD-1 was positive in immune cells, but not in tumor cells. One other distinct feature of this clinical presentation was the abundance of macrophages (CD68+) in the tumor microenvironment. Patient was initiated therapy with pembrolizumab. However, three weeks after his second cycle, he was admitted to hospital due to severe weakness in both upper extremities and pain at night. He also experienced a new onset of polyarthralgia in both hands, unable to play musical instruments. He was started on steroid treatment and showed significant improvement. Once steroid was tapered off, the sensation of pain substantially decreased but persisted. EMG showed right median motor neuropathy and left median sensory neuropathy. Blood test detected ANA positive, and as TSH was high, levothyroxine was initiated.
Outcome
His PET-CT scan showed improvement after only two cycles of treatment and has remained stable for over ten months without any treatment (patient elected to stop pembrolizumab treatment due to frequent traveling). We have performed a more detailed study of the macrophages in his tumor sample and interestingly, the majority of macrophages were type-1 (CD 80+), with some, type-2 macrophages (CD163+). It is known that type-1 macrophages are pro-inflammatory and have antitumor effect, while type-2 macrophages have opposite effect and often promote tumor growth and metastasis. This could explain the side effect and long duration of response despite only two cycles of pembrolizumab treatment. Characteristics of macrophages in melanoma tumor samples may be an important parameter to predict side effect and tumor response beyond PD1 or PD-L1 expression.
2021 Update on pelvic floor disorders
With the increasing prevalence of pelvic floor disorders among our aging population, women’s health clinicians should be prepared to counsel patients on treatment options and posttreatment expectations. In this Update, we will review recent literature on surgical treatments for pelvic organ prolapse (POP) and stress urinary incontinence (SUI). We also include our review of an award-winning and practice-changing study on office-based pessary care. Lastly, we will finish with a summary of a recent Society of Gynecologic Surgeons collaborative systematic review on sexual function after surgery.
5-year RCT data on hysteropexy vs hysterectomy for POP
Nager CW, Visco AG, Richter HE, et al; National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Effect of sacrospinous hysteropexy with graft vs vaginal hysterectomy with uterosacral ligament suspension on treatment failure in women with uterovaginal prolapse: 5-year results of a randomized clinical trial. Am J Obstet Gynecol. 2021;225:153. e1-153.e31. doi: 10.1016/j.ajog.2021.03.012.
The Pelvic Floor Disorders Network conducted a multisite randomized superiority trial comparing sacrospinous hysteropexy with mesh graft to vaginal hysterectomy with uterosacral ligament suspension for POP.
Study details
Postmenopausal women who desired surgery for symptomatic uterovaginal prolapse were randomly assigned to sacrospinous hysteropexy with polypropylene mesh graft using the Uphold-LITE device (Boston Scientific) versus vaginal hysterectomy with uterosacral ligament suspension. Participants were masked to treatment allocation and completed study visits at 6-month intervals through 60 months. Quantitative prolapse POP-Q exams were performed and patients completed multiple validated questionnaires regarding the presence; severity; and impact of prolapse, urinary, bowel, and pelvic pain symptoms.
Results
A total of 183 postmenopausal women were randomized, and 156 (81 hysteropexy and 75 hysterectomy) patients completed 5-year follow up with no demographic differences between the 2 intervention groups. Operative time was statistically less in the hysteropexy group (111.5 min vs 156.7 min). There were fewer treatment failures (a composite including retreatment for prolapse, prolapse beyond the hymen, and/or bothersome bulge symptoms) in the hysteropexy than in the hysterectomy group (37% vs 54%, respectively) at 5 years of follow up. However, most patients with treatment failure were classified as an intermittent failure, with only 16% of hysteropexy patients and 22% of hysterectomy patients classified as persistent failures. There were no meaningful differences between patient-reported outcomes. Hysteropexy had an 8% mesh exposure risk, with none requiring surgical management.
This study represents the highest quality randomized trial design and boasts high patient retention rates and 5-year follow up. Findings support further investigation on the use of polypropylene mesh for POP. In April of 2019, the US Food and Drug Administration halted the selling and distribution of vaginal mesh products for prolapse repair given the lack of safety outcomes, concerns about mesh exposure rates, and possible increased rates of pelvic pain and adverse events. This study invites pelvic reconstructive surgeons to revisit the debate of hysteropexy versus hysterectomy and synthetic mesh versus native tissue repairs. The 8% mesh exposure rate represents a challenge for the future design and development of vaginal implant materials, weighing the balancing of improved long-term efficacy with the safety and complication concerns.
Continue to: Preliminary 12-month data for a single-incision sling for surgical management of SUI...
Preliminary 12-month data for a single-incision sling for surgical management of SUI
Erickson T, Roovers JP, Gheiler E, et al. A multicenter prospective study evaluating efficacy and safety of a single-incision sling procedure for stress urinary incontinence. J Minim Invasive Gynecol. 2021;28:93-99. doi: 10.1016/j.jmig.2020.04.014.
In this industry-sponsored study, researchers compared a novel single-incision sling to currently available midurethral slings for SUI with 12-month outcomes and adverse event details. However, results are primarily descriptive with no statistical testing.
Study details
Patients were eligible for inclusion in this prospective, nonrandomized cohort study if SUI was their primary incontinence symptom, with confirmatory office testing. Exclusion criteria included POP greater than stage 2, prior SUI surgery, plans for future pregnancy, elevated postvoid residuals, or concomitant surgical procedures. The single-incision Altis (Coloplast) sling was compared to all commercially available transobturator and retropubic midurethral slings. The primary outcome of this study was reduction in 24-hour pad weights, and secondary outcomes included negative cough-stress test and subjective patient-reported outcomes via validated questionnaires.
Results
A total of 184 women were enrolled in the Altis group and 171 in the comparator other sling group. Symptom severity was similar between groups, but more patients in the comparator group had mixed urinary incontinence, and more patients in the Altis group had intrinsic sphincter deficiency. The Altis group had a higher proportion of “dry patients,” but otherwise the outcomes were similar between the 2 groups, including negative cough-stress test and patientreported outcomes. Two patients in the Altis group and 7 patients in the comparator group underwent device revisions. Again, statistical analysis was not performed.
Single-incision slings may reduce the risk of groin pain associated with transobturator slings and may be a good option for patients who desire less mesh burden than the traditional retropubic slings or who are not good candidates. This trial suggests that the Altis single-incision sling may be similar in outcomes and adverse events to currently available midurethral slings, but further, more rigorous trials are underway to fully evaluate this—including a US-based multicenter randomized trial of Altis single-incision slings versus retropubic slings (ClinicalTrials.gov Identifier: NCT03520114).
Office-based pessary care can be safely spaced out to 24 weeks without an increase in erosions
Propst K, Mellen C, O’Sullivan DM, et al. Timing of office-based pessary care: a randomized controlled trial. Obstet Gynecol. 2020;135:100-105. doi: 10.1097 /AOG.0000000000003580.
For women already using a pessary without issues, extending office visits to every 6 months does not increase rates of vaginal epithelial abnormalities, according to results of this randomized controlled trial.
Study details
Women already using a Gelhorn, ring, or incontinence dish pessary for POP, SUI, or both were randomized to continue routine care with office evaluation every 12 weeks versus the extended-care cohort (with office evaluation every 24 weeks). Women were excluded if they removed and replaced the pessary themselves or if there was a presence of vaginal epithelial abnormalities, such as erosion or granulation tissue.
Results
The rate of vaginal epithelium erosion was 7.4% in the routine arm and 1.7% in the extended-care arm, meeting criteria for noninferiority of extended care. The majority of patients with office visits every 24 weeks preferred the less frequent examinations, and there was no difference in degree of bother due to vaginal discharge. There was also no difference in the percentage of patients with unscheduled visits. The only factors associated with vaginal epithelium abnormalities were prior abnormalities and lifetime duration of pessary use.
As there are currently no evidenced-based guidelines for pessary care, this study contributes data to support extended office-based care up to 24 weeks, a common practice in the United Kingdom. During the COVID-19 pandemic, with reduced health care access, these findings should be reassuring to clinicians and patients.
Continue to: How can we counsel patients regarding changes in sexual activity and function after surgery for POP?...
How can we counsel patients regarding changes in sexual activity and function after surgery for POP?
Antosh DD, Dieter AA, Balk EM, et al. Sexual function after pelvic organ prolapse surgery: a systematic review comparing different approaches to pelvic floor repair. Am J Obstet Gynecol. 2021;2:S0002-9378(21)00610-4. doi: 10.1016/j.ajog.2021.05.042.
A secondary analysis of a recent systematic review found overall moderate- to high-quality evidence that were no differences in total dyspareunia, de novo dyspareunia, and scores on a validated sexual function questionnaire (PISQ12) when comparing postoperative sexual function outcomes of native tissue repair to sacrocolpopexy, transvaginal mesh, or biologic graft. Rates of postoperative dyspareunia were higher for transvaginal mesh than for sacrocolpopexy.
Study details
The Society of Gynecologic Surgeons Systematic Review Group identified 43 original prospective, comparative studies of reconstructive prolapse surgery that reported sexual function outcomes when comparing 2 different types of POP procedures. Thirty-seven of those studies were randomized controlled trials. Specifically, they looked at data comparing outcomes for native tissue versus sacrocolpopexy, native tissue versus transvaginal mesh, native tissue versus biologic graft, and transvaginal mesh versus sacrocolpopexy.
Results
Overall, the prevalence of postoperative dyspareunia was lower than preoperatively after all surgery types. The only statistical difference in this review demonstrated higher postoperative prevalence of dyspareunia after transvaginal mesh than sacrocolpopexy, based on 2 studies. When comparing native tissue prolapse repair to transvaginal mesh, sacrocolpopexy, or biologic grafts, there were no significant differences in sexual activity, baseline, or postoperative total dyspareunia, de-novo dyspareunia, or sexual function changes as measured by the PISQ12 validated questionnaire. ●
This systematic review further contributes to the growing evidence that, regardless of surgical approach to POP, sexual function generally improves and dyspareunia rates generally decrease postoperatively, with overall low rates of de novo dyspareunia. This will help patients and providers select the best-fit surgical approach without concern for worsened sexual function. It also underscores the need for inclusion of standardized sexual function terminology use and sexual health outcomes in future prolapse surgery research.

With the increasing prevalence of pelvic floor disorders among our aging population, women’s health clinicians should be prepared to counsel patients on treatment options and posttreatment expectations. In this Update, we will review recent literature on surgical treatments for pelvic organ prolapse (POP) and stress urinary incontinence (SUI). We also include our review of an award-winning and practice-changing study on office-based pessary care. Lastly, we will finish with a summary of a recent Society of Gynecologic Surgeons collaborative systematic review on sexual function after surgery.
5-year RCT data on hysteropexy vs hysterectomy for POP
Nager CW, Visco AG, Richter HE, et al; National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Effect of sacrospinous hysteropexy with graft vs vaginal hysterectomy with uterosacral ligament suspension on treatment failure in women with uterovaginal prolapse: 5-year results of a randomized clinical trial. Am J Obstet Gynecol. 2021;225:153. e1-153.e31. doi: 10.1016/j.ajog.2021.03.012.
The Pelvic Floor Disorders Network conducted a multisite randomized superiority trial comparing sacrospinous hysteropexy with mesh graft to vaginal hysterectomy with uterosacral ligament suspension for POP.
Study details
Postmenopausal women who desired surgery for symptomatic uterovaginal prolapse were randomly assigned to sacrospinous hysteropexy with polypropylene mesh graft using the Uphold-LITE device (Boston Scientific) versus vaginal hysterectomy with uterosacral ligament suspension. Participants were masked to treatment allocation and completed study visits at 6-month intervals through 60 months. Quantitative prolapse POP-Q exams were performed and patients completed multiple validated questionnaires regarding the presence; severity; and impact of prolapse, urinary, bowel, and pelvic pain symptoms.
Results
A total of 183 postmenopausal women were randomized, and 156 (81 hysteropexy and 75 hysterectomy) patients completed 5-year follow up with no demographic differences between the 2 intervention groups. Operative time was statistically less in the hysteropexy group (111.5 min vs 156.7 min). There were fewer treatment failures (a composite including retreatment for prolapse, prolapse beyond the hymen, and/or bothersome bulge symptoms) in the hysteropexy than in the hysterectomy group (37% vs 54%, respectively) at 5 years of follow up. However, most patients with treatment failure were classified as an intermittent failure, with only 16% of hysteropexy patients and 22% of hysterectomy patients classified as persistent failures. There were no meaningful differences between patient-reported outcomes. Hysteropexy had an 8% mesh exposure risk, with none requiring surgical management.
This study represents the highest quality randomized trial design and boasts high patient retention rates and 5-year follow up. Findings support further investigation on the use of polypropylene mesh for POP. In April of 2019, the US Food and Drug Administration halted the selling and distribution of vaginal mesh products for prolapse repair given the lack of safety outcomes, concerns about mesh exposure rates, and possible increased rates of pelvic pain and adverse events. This study invites pelvic reconstructive surgeons to revisit the debate of hysteropexy versus hysterectomy and synthetic mesh versus native tissue repairs. The 8% mesh exposure rate represents a challenge for the future design and development of vaginal implant materials, weighing the balancing of improved long-term efficacy with the safety and complication concerns.
Continue to: Preliminary 12-month data for a single-incision sling for surgical management of SUI...
Preliminary 12-month data for a single-incision sling for surgical management of SUI
Erickson T, Roovers JP, Gheiler E, et al. A multicenter prospective study evaluating efficacy and safety of a single-incision sling procedure for stress urinary incontinence. J Minim Invasive Gynecol. 2021;28:93-99. doi: 10.1016/j.jmig.2020.04.014.
In this industry-sponsored study, researchers compared a novel single-incision sling to currently available midurethral slings for SUI with 12-month outcomes and adverse event details. However, results are primarily descriptive with no statistical testing.
Study details
Patients were eligible for inclusion in this prospective, nonrandomized cohort study if SUI was their primary incontinence symptom, with confirmatory office testing. Exclusion criteria included POP greater than stage 2, prior SUI surgery, plans for future pregnancy, elevated postvoid residuals, or concomitant surgical procedures. The single-incision Altis (Coloplast) sling was compared to all commercially available transobturator and retropubic midurethral slings. The primary outcome of this study was reduction in 24-hour pad weights, and secondary outcomes included negative cough-stress test and subjective patient-reported outcomes via validated questionnaires.
Results
A total of 184 women were enrolled in the Altis group and 171 in the comparator other sling group. Symptom severity was similar between groups, but more patients in the comparator group had mixed urinary incontinence, and more patients in the Altis group had intrinsic sphincter deficiency. The Altis group had a higher proportion of “dry patients,” but otherwise the outcomes were similar between the 2 groups, including negative cough-stress test and patientreported outcomes. Two patients in the Altis group and 7 patients in the comparator group underwent device revisions. Again, statistical analysis was not performed.
Single-incision slings may reduce the risk of groin pain associated with transobturator slings and may be a good option for patients who desire less mesh burden than the traditional retropubic slings or who are not good candidates. This trial suggests that the Altis single-incision sling may be similar in outcomes and adverse events to currently available midurethral slings, but further, more rigorous trials are underway to fully evaluate this—including a US-based multicenter randomized trial of Altis single-incision slings versus retropubic slings (ClinicalTrials.gov Identifier: NCT03520114).
Office-based pessary care can be safely spaced out to 24 weeks without an increase in erosions
Propst K, Mellen C, O’Sullivan DM, et al. Timing of office-based pessary care: a randomized controlled trial. Obstet Gynecol. 2020;135:100-105. doi: 10.1097 /AOG.0000000000003580.
For women already using a pessary without issues, extending office visits to every 6 months does not increase rates of vaginal epithelial abnormalities, according to results of this randomized controlled trial.
Study details
Women already using a Gelhorn, ring, or incontinence dish pessary for POP, SUI, or both were randomized to continue routine care with office evaluation every 12 weeks versus the extended-care cohort (with office evaluation every 24 weeks). Women were excluded if they removed and replaced the pessary themselves or if there was a presence of vaginal epithelial abnormalities, such as erosion or granulation tissue.
Results
The rate of vaginal epithelium erosion was 7.4% in the routine arm and 1.7% in the extended-care arm, meeting criteria for noninferiority of extended care. The majority of patients with office visits every 24 weeks preferred the less frequent examinations, and there was no difference in degree of bother due to vaginal discharge. There was also no difference in the percentage of patients with unscheduled visits. The only factors associated with vaginal epithelium abnormalities were prior abnormalities and lifetime duration of pessary use.
As there are currently no evidenced-based guidelines for pessary care, this study contributes data to support extended office-based care up to 24 weeks, a common practice in the United Kingdom. During the COVID-19 pandemic, with reduced health care access, these findings should be reassuring to clinicians and patients.
Continue to: How can we counsel patients regarding changes in sexual activity and function after surgery for POP?...
How can we counsel patients regarding changes in sexual activity and function after surgery for POP?
Antosh DD, Dieter AA, Balk EM, et al. Sexual function after pelvic organ prolapse surgery: a systematic review comparing different approaches to pelvic floor repair. Am J Obstet Gynecol. 2021;2:S0002-9378(21)00610-4. doi: 10.1016/j.ajog.2021.05.042.
A secondary analysis of a recent systematic review found overall moderate- to high-quality evidence that were no differences in total dyspareunia, de novo dyspareunia, and scores on a validated sexual function questionnaire (PISQ12) when comparing postoperative sexual function outcomes of native tissue repair to sacrocolpopexy, transvaginal mesh, or biologic graft. Rates of postoperative dyspareunia were higher for transvaginal mesh than for sacrocolpopexy.
Study details
The Society of Gynecologic Surgeons Systematic Review Group identified 43 original prospective, comparative studies of reconstructive prolapse surgery that reported sexual function outcomes when comparing 2 different types of POP procedures. Thirty-seven of those studies were randomized controlled trials. Specifically, they looked at data comparing outcomes for native tissue versus sacrocolpopexy, native tissue versus transvaginal mesh, native tissue versus biologic graft, and transvaginal mesh versus sacrocolpopexy.
Results
Overall, the prevalence of postoperative dyspareunia was lower than preoperatively after all surgery types. The only statistical difference in this review demonstrated higher postoperative prevalence of dyspareunia after transvaginal mesh than sacrocolpopexy, based on 2 studies. When comparing native tissue prolapse repair to transvaginal mesh, sacrocolpopexy, or biologic grafts, there were no significant differences in sexual activity, baseline, or postoperative total dyspareunia, de-novo dyspareunia, or sexual function changes as measured by the PISQ12 validated questionnaire. ●
This systematic review further contributes to the growing evidence that, regardless of surgical approach to POP, sexual function generally improves and dyspareunia rates generally decrease postoperatively, with overall low rates of de novo dyspareunia. This will help patients and providers select the best-fit surgical approach without concern for worsened sexual function. It also underscores the need for inclusion of standardized sexual function terminology use and sexual health outcomes in future prolapse surgery research.

With the increasing prevalence of pelvic floor disorders among our aging population, women’s health clinicians should be prepared to counsel patients on treatment options and posttreatment expectations. In this Update, we will review recent literature on surgical treatments for pelvic organ prolapse (POP) and stress urinary incontinence (SUI). We also include our review of an award-winning and practice-changing study on office-based pessary care. Lastly, we will finish with a summary of a recent Society of Gynecologic Surgeons collaborative systematic review on sexual function after surgery.
5-year RCT data on hysteropexy vs hysterectomy for POP
Nager CW, Visco AG, Richter HE, et al; National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Effect of sacrospinous hysteropexy with graft vs vaginal hysterectomy with uterosacral ligament suspension on treatment failure in women with uterovaginal prolapse: 5-year results of a randomized clinical trial. Am J Obstet Gynecol. 2021;225:153. e1-153.e31. doi: 10.1016/j.ajog.2021.03.012.
The Pelvic Floor Disorders Network conducted a multisite randomized superiority trial comparing sacrospinous hysteropexy with mesh graft to vaginal hysterectomy with uterosacral ligament suspension for POP.
Study details
Postmenopausal women who desired surgery for symptomatic uterovaginal prolapse were randomly assigned to sacrospinous hysteropexy with polypropylene mesh graft using the Uphold-LITE device (Boston Scientific) versus vaginal hysterectomy with uterosacral ligament suspension. Participants were masked to treatment allocation and completed study visits at 6-month intervals through 60 months. Quantitative prolapse POP-Q exams were performed and patients completed multiple validated questionnaires regarding the presence; severity; and impact of prolapse, urinary, bowel, and pelvic pain symptoms.
Results
A total of 183 postmenopausal women were randomized, and 156 (81 hysteropexy and 75 hysterectomy) patients completed 5-year follow up with no demographic differences between the 2 intervention groups. Operative time was statistically less in the hysteropexy group (111.5 min vs 156.7 min). There were fewer treatment failures (a composite including retreatment for prolapse, prolapse beyond the hymen, and/or bothersome bulge symptoms) in the hysteropexy than in the hysterectomy group (37% vs 54%, respectively) at 5 years of follow up. However, most patients with treatment failure were classified as an intermittent failure, with only 16% of hysteropexy patients and 22% of hysterectomy patients classified as persistent failures. There were no meaningful differences between patient-reported outcomes. Hysteropexy had an 8% mesh exposure risk, with none requiring surgical management.
This study represents the highest quality randomized trial design and boasts high patient retention rates and 5-year follow up. Findings support further investigation on the use of polypropylene mesh for POP. In April of 2019, the US Food and Drug Administration halted the selling and distribution of vaginal mesh products for prolapse repair given the lack of safety outcomes, concerns about mesh exposure rates, and possible increased rates of pelvic pain and adverse events. This study invites pelvic reconstructive surgeons to revisit the debate of hysteropexy versus hysterectomy and synthetic mesh versus native tissue repairs. The 8% mesh exposure rate represents a challenge for the future design and development of vaginal implant materials, weighing the balancing of improved long-term efficacy with the safety and complication concerns.
Continue to: Preliminary 12-month data for a single-incision sling for surgical management of SUI...
Preliminary 12-month data for a single-incision sling for surgical management of SUI
Erickson T, Roovers JP, Gheiler E, et al. A multicenter prospective study evaluating efficacy and safety of a single-incision sling procedure for stress urinary incontinence. J Minim Invasive Gynecol. 2021;28:93-99. doi: 10.1016/j.jmig.2020.04.014.
In this industry-sponsored study, researchers compared a novel single-incision sling to currently available midurethral slings for SUI with 12-month outcomes and adverse event details. However, results are primarily descriptive with no statistical testing.
Study details
Patients were eligible for inclusion in this prospective, nonrandomized cohort study if SUI was their primary incontinence symptom, with confirmatory office testing. Exclusion criteria included POP greater than stage 2, prior SUI surgery, plans for future pregnancy, elevated postvoid residuals, or concomitant surgical procedures. The single-incision Altis (Coloplast) sling was compared to all commercially available transobturator and retropubic midurethral slings. The primary outcome of this study was reduction in 24-hour pad weights, and secondary outcomes included negative cough-stress test and subjective patient-reported outcomes via validated questionnaires.
Results
A total of 184 women were enrolled in the Altis group and 171 in the comparator other sling group. Symptom severity was similar between groups, but more patients in the comparator group had mixed urinary incontinence, and more patients in the Altis group had intrinsic sphincter deficiency. The Altis group had a higher proportion of “dry patients,” but otherwise the outcomes were similar between the 2 groups, including negative cough-stress test and patientreported outcomes. Two patients in the Altis group and 7 patients in the comparator group underwent device revisions. Again, statistical analysis was not performed.
Single-incision slings may reduce the risk of groin pain associated with transobturator slings and may be a good option for patients who desire less mesh burden than the traditional retropubic slings or who are not good candidates. This trial suggests that the Altis single-incision sling may be similar in outcomes and adverse events to currently available midurethral slings, but further, more rigorous trials are underway to fully evaluate this—including a US-based multicenter randomized trial of Altis single-incision slings versus retropubic slings (ClinicalTrials.gov Identifier: NCT03520114).
Office-based pessary care can be safely spaced out to 24 weeks without an increase in erosions
Propst K, Mellen C, O’Sullivan DM, et al. Timing of office-based pessary care: a randomized controlled trial. Obstet Gynecol. 2020;135:100-105. doi: 10.1097 /AOG.0000000000003580.
For women already using a pessary without issues, extending office visits to every 6 months does not increase rates of vaginal epithelial abnormalities, according to results of this randomized controlled trial.
Study details
Women already using a Gelhorn, ring, or incontinence dish pessary for POP, SUI, or both were randomized to continue routine care with office evaluation every 12 weeks versus the extended-care cohort (with office evaluation every 24 weeks). Women were excluded if they removed and replaced the pessary themselves or if there was a presence of vaginal epithelial abnormalities, such as erosion or granulation tissue.
Results
The rate of vaginal epithelium erosion was 7.4% in the routine arm and 1.7% in the extended-care arm, meeting criteria for noninferiority of extended care. The majority of patients with office visits every 24 weeks preferred the less frequent examinations, and there was no difference in degree of bother due to vaginal discharge. There was also no difference in the percentage of patients with unscheduled visits. The only factors associated with vaginal epithelium abnormalities were prior abnormalities and lifetime duration of pessary use.
As there are currently no evidenced-based guidelines for pessary care, this study contributes data to support extended office-based care up to 24 weeks, a common practice in the United Kingdom. During the COVID-19 pandemic, with reduced health care access, these findings should be reassuring to clinicians and patients.
Continue to: How can we counsel patients regarding changes in sexual activity and function after surgery for POP?...
How can we counsel patients regarding changes in sexual activity and function after surgery for POP?
Antosh DD, Dieter AA, Balk EM, et al. Sexual function after pelvic organ prolapse surgery: a systematic review comparing different approaches to pelvic floor repair. Am J Obstet Gynecol. 2021;2:S0002-9378(21)00610-4. doi: 10.1016/j.ajog.2021.05.042.
A secondary analysis of a recent systematic review found overall moderate- to high-quality evidence that were no differences in total dyspareunia, de novo dyspareunia, and scores on a validated sexual function questionnaire (PISQ12) when comparing postoperative sexual function outcomes of native tissue repair to sacrocolpopexy, transvaginal mesh, or biologic graft. Rates of postoperative dyspareunia were higher for transvaginal mesh than for sacrocolpopexy.
Study details
The Society of Gynecologic Surgeons Systematic Review Group identified 43 original prospective, comparative studies of reconstructive prolapse surgery that reported sexual function outcomes when comparing 2 different types of POP procedures. Thirty-seven of those studies were randomized controlled trials. Specifically, they looked at data comparing outcomes for native tissue versus sacrocolpopexy, native tissue versus transvaginal mesh, native tissue versus biologic graft, and transvaginal mesh versus sacrocolpopexy.
Results
Overall, the prevalence of postoperative dyspareunia was lower than preoperatively after all surgery types. The only statistical difference in this review demonstrated higher postoperative prevalence of dyspareunia after transvaginal mesh than sacrocolpopexy, based on 2 studies. When comparing native tissue prolapse repair to transvaginal mesh, sacrocolpopexy, or biologic grafts, there were no significant differences in sexual activity, baseline, or postoperative total dyspareunia, de-novo dyspareunia, or sexual function changes as measured by the PISQ12 validated questionnaire. ●
This systematic review further contributes to the growing evidence that, regardless of surgical approach to POP, sexual function generally improves and dyspareunia rates generally decrease postoperatively, with overall low rates of de novo dyspareunia. This will help patients and providers select the best-fit surgical approach without concern for worsened sexual function. It also underscores the need for inclusion of standardized sexual function terminology use and sexual health outcomes in future prolapse surgery research.
Double Hit: Epstein-Barr Virus Causing Infectious Mononucleosis Followed by Hemolytic Uremic Syndrome
Introduction
Epstein-Barr virus (EBV) is a herpes virus that commonly causes infectious mononucleosis (IM) and linked to different hematological conditions. Here we present a case of EBV-triggered Hemolytic Uremic Syndrome (HUS) with pulmonary involvement.
Case Presentation
A 20-year-old male presented with fever, thrombocytopenia, and splenomegaly. Acute EBV serology was positive. Creatinine and hemoglobin were normal. He was diagnosed with IM. platelet count improved within 3 weeks. 4 weeks later, he returned with severe hemoptysis. Hgb 6.8g/dL, platelet 133,000/uL, lactate dehydrogenase 969u/L, creatinine 21mg/dL, and schistocytes on peripheral smear. Chest computed tomography showed bilateral opacities consistent with diffuse alveolar hemorrhage (DAH). Emergent hemodialysis and plasmapheresis were started. Infectious work up was negative. Autoimmune work up was also negative (anti-neutrophil cytoplasmic, anti-basement membrane antibodies, ANA). Aadamts13 activity was 62% (normal ~66%) ruling out thrombotic thrombocytopenic purpura (TTP). Kidney biopsy revealed thrombotic microangiopathic process. The patient was eventually diagnosed with HUS and treated with Eculizumab. 4 months later his renal function has partially recovered and no longer needs hemodialysis.
Discussion
HUS is a rare entity that is known to be triggered by different underlying pathologies. However, its link to EBV remains unclear. Literature review has revealed only two cases of EBV-triggered HUS, even though almost 90-95% of adults are EBV-seropositive. What unique about our case is the patient initially presented with documented IM, and HUS happened a month later. This raises the theory that HUS could be a sequela of the infection, rather than an effect of acute viral phase and this is the first case to report such correlation. The other unique thing is pulmonary involvement in HUS. With consultation with pulmonary service, we believe our patient had DAH based on clinical and radiographic findings. To our knowledge this is the first case to show this association.
Conclusion
EBV is a common virus with high seropositivity among world’s population. Its link to HUS remains unclear and needs more investigation. Providers should recognize HUS as a complication of EBV infection, either in the acute phase or as a sequela. Adolescents are at higher risk for such complication since IM is common in this population.
Introduction
Epstein-Barr virus (EBV) is a herpes virus that commonly causes infectious mononucleosis (IM) and linked to different hematological conditions. Here we present a case of EBV-triggered Hemolytic Uremic Syndrome (HUS) with pulmonary involvement.
Case Presentation
A 20-year-old male presented with fever, thrombocytopenia, and splenomegaly. Acute EBV serology was positive. Creatinine and hemoglobin were normal. He was diagnosed with IM. platelet count improved within 3 weeks. 4 weeks later, he returned with severe hemoptysis. Hgb 6.8g/dL, platelet 133,000/uL, lactate dehydrogenase 969u/L, creatinine 21mg/dL, and schistocytes on peripheral smear. Chest computed tomography showed bilateral opacities consistent with diffuse alveolar hemorrhage (DAH). Emergent hemodialysis and plasmapheresis were started. Infectious work up was negative. Autoimmune work up was also negative (anti-neutrophil cytoplasmic, anti-basement membrane antibodies, ANA). Aadamts13 activity was 62% (normal ~66%) ruling out thrombotic thrombocytopenic purpura (TTP). Kidney biopsy revealed thrombotic microangiopathic process. The patient was eventually diagnosed with HUS and treated with Eculizumab. 4 months later his renal function has partially recovered and no longer needs hemodialysis.
Discussion
HUS is a rare entity that is known to be triggered by different underlying pathologies. However, its link to EBV remains unclear. Literature review has revealed only two cases of EBV-triggered HUS, even though almost 90-95% of adults are EBV-seropositive. What unique about our case is the patient initially presented with documented IM, and HUS happened a month later. This raises the theory that HUS could be a sequela of the infection, rather than an effect of acute viral phase and this is the first case to report such correlation. The other unique thing is pulmonary involvement in HUS. With consultation with pulmonary service, we believe our patient had DAH based on clinical and radiographic findings. To our knowledge this is the first case to show this association.
Conclusion
EBV is a common virus with high seropositivity among world’s population. Its link to HUS remains unclear and needs more investigation. Providers should recognize HUS as a complication of EBV infection, either in the acute phase or as a sequela. Adolescents are at higher risk for such complication since IM is common in this population.
Introduction
Epstein-Barr virus (EBV) is a herpes virus that commonly causes infectious mononucleosis (IM) and linked to different hematological conditions. Here we present a case of EBV-triggered Hemolytic Uremic Syndrome (HUS) with pulmonary involvement.
Case Presentation
A 20-year-old male presented with fever, thrombocytopenia, and splenomegaly. Acute EBV serology was positive. Creatinine and hemoglobin were normal. He was diagnosed with IM. platelet count improved within 3 weeks. 4 weeks later, he returned with severe hemoptysis. Hgb 6.8g/dL, platelet 133,000/uL, lactate dehydrogenase 969u/L, creatinine 21mg/dL, and schistocytes on peripheral smear. Chest computed tomography showed bilateral opacities consistent with diffuse alveolar hemorrhage (DAH). Emergent hemodialysis and plasmapheresis were started. Infectious work up was negative. Autoimmune work up was also negative (anti-neutrophil cytoplasmic, anti-basement membrane antibodies, ANA). Aadamts13 activity was 62% (normal ~66%) ruling out thrombotic thrombocytopenic purpura (TTP). Kidney biopsy revealed thrombotic microangiopathic process. The patient was eventually diagnosed with HUS and treated with Eculizumab. 4 months later his renal function has partially recovered and no longer needs hemodialysis.
Discussion
HUS is a rare entity that is known to be triggered by different underlying pathologies. However, its link to EBV remains unclear. Literature review has revealed only two cases of EBV-triggered HUS, even though almost 90-95% of adults are EBV-seropositive. What unique about our case is the patient initially presented with documented IM, and HUS happened a month later. This raises the theory that HUS could be a sequela of the infection, rather than an effect of acute viral phase and this is the first case to report such correlation. The other unique thing is pulmonary involvement in HUS. With consultation with pulmonary service, we believe our patient had DAH based on clinical and radiographic findings. To our knowledge this is the first case to show this association.
Conclusion
EBV is a common virus with high seropositivity among world’s population. Its link to HUS remains unclear and needs more investigation. Providers should recognize HUS as a complication of EBV infection, either in the acute phase or as a sequela. Adolescents are at higher risk for such complication since IM is common in this population.
Autoeczematization: A Strange Id Reaction of the Skin
Autoeczematization (AE), or id reaction, is a disseminated eczematous reaction that occurs days or weeks after exposure to a primary stimulus, resulting from a release of antigen(s). Whitfield1 first described AE in 1921, when he postulated that the id reaction was due to sensitization of the skin after a primary stimulus. He called it “a form of auto-intoxication derived from changes in the patient’s own tissues.”1 The exact prevalence of id reactions is unknown; one study showed that 17% of patients with dermatophyte infections developed an id reaction, typically tinea pedis linked with vesicles on the palms.2 Tinea capitis is one of the most common causes of AE in children, which is frequently misdiagnosed as a drug reaction. Approximately 37% of patients diagnosed with stasis dermatitis develop an id reaction (Figure 1). A history of contact dermatitis is common in patients presenting with AE.2-6

Pathophysiology of Id Reactions
An abnormal immune response against autologous skin antigens may be responsible for the development of AE. Shelley5 postulated that hair follicles play an important role in id reactions, as Sharquie et al6 recently emphasized for many skin disorders. The pathogenesis of AE is uncertain, but circulating T lymphocytes play a role in this reaction. Normally, T cells are activated by a release of antigens after a primary exposure to a stimulus. However, overactivation of these T cells induces autoimmune reactions such as AE.7 Activated T lymphocytes express HLA-DR and IL-2 receptor, markers elevated in the peripheral blood of patients undergoing id reactions. After treatment, the levels of activated T lymphocytes decline. An increase in the number of CD25+ T cells and a decrease in the number of suppressor T cells in the blood may occur during an id reaction.7-9 Keratinocytes produce proinflammatory cytokines, such as thymic stromal erythropoietin, IL-25, and IL-33, that activate T cells.10-12 Therefore, the most likely pathogenesis of an id reaction is that T lymphocytes are activated at the primary reaction site due to proinflammatory cytokines released by keratinocytes. These activated T cells then travel systemically via hematogenous dissemination. The spread of activated T lymphocytes produces an eczematous reaction at secondary locations distant to the primary site.9
Clinical and Histopathological Features of Id Reactions
Clinically, AE is first evident as a vesicular dissemination that groups to form papules or nummular patches and usually is present on the legs, feet, arms, and/or trunk (Figure 2). The primary dermatitis is localized to the area that was the site of contact to the offending stimuli. This localized eczematous eruption begins with an acute or subacute onset. It has the appearance of small crusted vesicles with erythema (Figure 1). The first sign of AE is vesicles presenting near the primary site on flexural surfaces or on the hands and feet. A classic example is tinea pedis linked with vesicles on the palms and sides of the fingers, resembling dyshidrotic eczema. Sites of prior cutaneous trauma, such as dermatoses, scars, and burns, are common locations for early AE. In later stages, vesicles disseminate to the legs, arms, and trunk, where they group to form papules and nummular patches in a symmetrical pattern.5,13-15 These lesions may be extremely pruritic. The pruritus may be so intense that it interrupts daily activities and disrupts the ability to fall or stay asleep.16
Histologically, biopsy specimens show psoriasiform spongiotic dermatitis with mononuclear cells contained in the vesicles. Interstitial edema and perivascular lymphohistiocytic infiltrates are evident. Eosinophils also may be present. This pattern is not unique toid reactions.17-19 Although AE is a reaction pattern that may be due to a fungal or bacterial infection, the etiologic agent is not evident microscopically within the eczema itself.
Etiology of Id Reactions
Id reactions most commonly occur from either stasis dermatitis or tinea pedis, although a wide variety of other causes should be considered. Evaluation of the primary site rather than the id reaction may identify an infectious or parasitic agent. Sometimes the AE reaction is specifically named: dermatophytid with dermatophytosis, bacterid with a bacterial infectious process, and tuberculid with tuberculosis. Similarly, there may be reactions to underlying candidiasis, sporotrichosis, histoplasmosis, and other fungal infections that can cause a cutaneous id reaction.18,20-22Mycobacterium species, Pseudomonas, Staphylococcus, and Streptococcus are bacterial causes of AE.15,23-26 Viral infections that can cause an id reaction are herpes simplex virus and molluscum contagiosum.27-29 Scabies, leishmaniasis, and pediculosis capitis are parasitic infections that may be etiologic.14,30,31 In addition, noninfectious stimuli besides stasis dermatitis that can produce id reactions include medications, topical creams, tattoo ink, sutures, radiotherapy, and dyshidrotic eczema. The primary reaction to these agents is a localized dermatitis followed by the immunological response that induces a secondary reaction distant from the primary site.17,18,32-38
Differential Diagnoses
Differential diagnoses include other types of eczema and some vesicular eruptions. Irritant contact dermatitis is another dermatosis that presents as a widespread vesicular eruption due to repetitive exposure to toxic irritants. The rash is erythematous with pustules, blisters, and crusts. It is only found in areas directly exposed to irritants, as opposed to AE, which spreads to areas distant to the primary reaction site. Irritant contact dermatitis presents with more of a burning sensation, whereas AE is more pruritic.39,40 Allergic contact dermatitis presents with erythematous vesicles and papules and sometimes with bullae. There is edema and crust formation, which often can spread past the point of contact in later stages. Similar to AE, there is intense pruritus. However, allergic contact dermatitis most commonly is caused by exposure to metals, cosmetics, and fragrances, whereas infectious agents and stasis dermatitis are the most common causes of AE.40,41 It may be challenging to distinguish AE from other causes of widespread eczematous dissemination. Vesicular eruptions sometimes require distinction from AE, including herpetic infections, insect bite reactions, and drug eruptions.18,42
Treatment
The underlying condition should be treated to mitigate the inflammatory response causing the id reaction. If not skillfully orchestrated, the id reaction can reoccur. For infectious causes of AE, an antifungal, antibacterial, antiviral, or antiparasitic should be given. If stasis dermatitis is responsible for the id reaction, compression stockings and leg elevation are indicated. The id reaction itself is treated with systemic or topical corticosteroids and wet compresses if acute. The goal of these treatments is to reduce patient discomfort caused by the inflammation and pruritus.18,43
Conclusion
Id reactions are an unusual phenomenon that commonly occurs after fungal skin infections and stasis dermatitis. T lymphocytes and keratinocytes may play a key role in this reaction, with newer research further delineating the process and possibly providing enhanced treatment options. Therapy focuses on treating the underlying condition, supplemented with corticosteroids for the autoeczema.
- Whitfield A. Lumleian Lectures on Some Points in the Aetiology of Skin Diseases. Delivered before the Royal College of Physicians of London on March 10th, 15th, and 17th, 1921. Lecture II. Lancet. 1921;2:122-127.
- Cheng N, Rucker Wright D, Cohen BA. Dermatophytid in tinea capitis: rarely reported common phenomenon with clinical implications. Pediatrics. 2011;128:E453-E457.
- Schrom KP, Kobs A, Nedorost S. Clinical psoriasiform dermatitis following dupilumab use for autoeczematization secondary to chronic stasis dermatitis. Cureus. 2020;12:e7831. doi:10.7759/cureus.7831
- Templeton HJ, Lunsford CJ, Allington HV. Autosensitization dermatitis; report of five cases and protocol of an experiment. Arch Derm Syphilol. 1949;59:68-77.
- Shelley WB. Id reaction. In: Consultations in Dermatology. Saunders; 1972:262-267.
- Sharquie KE, Noaimi AA, Flayih RA. Clinical and histopathological findings in patients with follicular dermatoses: all skin diseases starts in the hair follicles as new hypothesis. Am J Clin Res Rev. 2020;4:17.
- Kasteler JS, Petersen MJ, Vance JE, et al. Circulating activated T lymphocytes in autoeczematization. Arch Dermatol. 1992;128:795-798.
- González-Amaro R, Baranda L, Abud-Mendoza C, et al. Autoeczematization is associated with abnormal immune recognition of autologous skin antigens. J Am Acad Dermatol. 1993;28:56-60.
- Cunningham MJ, Zone JJ, Petersen MJ, et al. Circulating activated (DR-positive) T lymphocytes in a patient with autoeczematization. J Am Acad Dermatol. 1986;14:1039-1041.
- Furue M, Ulzii D, Vu YH, et al. Pathogenesis of atopic dermatitis: current paradigm. Iran J Immunol. 2019;16:97-107.
- Uchi H, Terao H, Koga T, et al. Cytokines and chemokines in the epidermis. J Dermatol Sci. 2000;24(suppl 1):S29-S38.
- Bos JD, Kapsenberg ML. The skin immune system: progress in cutaneous biology. Immunol Today. 1993;14:75-78.
- Young AW Jr. Dynamics of autosensitization dermatitis; a clinical and microscopic concept of autoeczematization. AMA Arch Derm. 1958;77:495-502.
- Brenner S, Wolf R, Landau M. Scabid: an unusual id reaction to scabies. Int J Dermatol. 1993;32:128-129.
- Yamany T, Schwartz RA. Infectious eczematoid dermatitis: a comprehensive review. J Eur Acad Dermatol Venereol. 2015;29:203-208.
- Wang X, Li L, Shi X, et al. Itching and its related factors in subtypes of eczema: a cross-sectional multicenter study in tertiary hospitals of China. Sci Rep. 2018;8:10754.
- Price A, Tavazoie M, Meehan SA, et al. Id reaction associated with red tattoo ink. Cutis. 2018;102:E32-E34.
- Ilkit M, Durdu M, Karaks¸ M. Cutaneous id reactions: a comprehensive review of clinical manifestations, epidemiology, etiology, and management. Crit Rev Microbiol. 2012;38:191-202.
- Kaner SR. Dermatitis venenata of the feet with a generalized “id” reaction. J Am Podiatry Assoc. 1970;60:199-204.
- Jordan L, Jackson NA, Carter-Snell B, et al. Pustular tinea id reaction. Cutis. 2019;103:E3-E4.
- Crum N, Hardaway C, Graham B. Development of an idlike reaction during treatment for acute pulmonary histoplasmosis: a new cutaneous manifestation in histoplasmosis. J Am Acad Dermatol. 2003;48(2 suppl):S5-S6.
- Chirac A, Brzezinski P, Chiriac AE, et al. Autosensitisation (autoeczematisation) reactions in a case of diaper dermatitis candidiasis. Niger Med J. 2014;55:274-275.
- Singh PY, Sinha P, Baveja S, et al. Immune-mediated tuberculous uveitis—a rare association with papulonecrotic tuberculid. Indian J Ophthalmol. 2019;67:1207-1209.
- Urso B, Georgesen C, Harp J. Papulonecrotic tuberculid secondary to Mycobacterium avium complex. Cutis. 2019;104:E11-E13.
- Choudhri SH, Magro CM, Crowson AN, et al. An id reaction to Mycobacterium leprae: first documented case. Cutis. 1994;54:282-286.
- Park JW, Jeong GJ, Seo SJ, et al. Pseudomonas toe web infection and autosensitisation dermatitis: diagnostic and therapeutic challenge. Int Wound J. 2020;17:1543-1544. doi:10.1111/iwj.13386
- Netchiporouk E, Cohen BA. Recognizing and managing eczematous id reactions to molluscum contagiosum virus in children. Pediatrics. 2012;129:E1072-E1075.
- Aurelian L, Ono F, Burnett J. Herpes simplex virus (HSV)-associated erythema multiforme (HAEM): a viral disease with an autoimmune component. Dermatol Online J. 2003;9:1.
- Rocamora V, Romaní J, Puig L, et al. Id reaction to molluscum contagiosum. Pediatr Dermatol. 1996;13:349-350.
- Yes¸ilova Y, Özbilgin A, Turan E, et al. Clinical exacerbation developing during treatment of cutaneous leishmaniasis: an id reaction? Turkiye Parazitol Derg. 2014;38:281-282.
- Connor CJ, Selby JC, Wanat KA. Severe pediculosis capitus: a case of “crusted lice” with autoeczematization. Dermatol Online J. 2016;22:13030/qt7c91z913.
- Shelley WB. The autoimmune mechanism in clinical dermatology. Arch Dermatol. 1962;86:27-34.
- Bosworth A, Hull PR. Disseminated eczema following radiotherapy: a case report. J Cutan Med Surg. 2018;22:353-355.
- Lowther C, Miedler JD, Cockerell CJ. Id-like reaction to BCG therapy for bladder cancer. Cutis. 2013;91:145-151.
- Huerth KA, Glick PL, Glick ZR. Cutaneous id reaction after using cyanoacrylate for wound closure. Cutis. 2020;105:E11-E13.
- Amini S, Burdick AE, Janniger CK. Dyshidrotic eczema (pompholyx). Updated April 22, 2020. Accessed August 23, 2021. https://emedicine.medscape.com/article/1122527-overview
- Sundaresan S, Migden MR, Silapunt S. Stasis dermatitis: pathophysiology, evaluation, and management. Am J Clin Dermatol. 2017;18:383-390.
- Hughes JDM, Pratt MD. Allergic contact dermatitis and autoeczematization to proctosedyl® cream and proctomyxin® cream. Case Rep Dermatol. 2018;10:238-246.
- Bains SN, Nash P, Fonacier L. Irritant contact dermatitis. Clin Rev Allergy Immunol. 2019;56:99-109.
- Novak-Bilic´ G, Vucˇic´ M, Japundžic´ I, et al. Irritant and allergic contact dermatitis—skin lesion characteristics. Acta Clin Croat. 2018;57:713-720.
- Nassau S, Fonacier L. Allergic contact dermatitis. Med Clin North Am. 2020;104:61-76.
- Lewis DJ, Schlichte MJ, Dao H Jr. Atypical disseminated herpes zoster: management guidelines in immunocompromised patients. Cutis. 2017;100:321-330.
- Nedorost S, White S, Rowland DY, et al. Development and implementation of an order set to improve value of care for patients with severe stasis dermatitis. J Am Acad Dermatol. 2019;80:815-817.
Autoeczematization (AE), or id reaction, is a disseminated eczematous reaction that occurs days or weeks after exposure to a primary stimulus, resulting from a release of antigen(s). Whitfield1 first described AE in 1921, when he postulated that the id reaction was due to sensitization of the skin after a primary stimulus. He called it “a form of auto-intoxication derived from changes in the patient’s own tissues.”1 The exact prevalence of id reactions is unknown; one study showed that 17% of patients with dermatophyte infections developed an id reaction, typically tinea pedis linked with vesicles on the palms.2 Tinea capitis is one of the most common causes of AE in children, which is frequently misdiagnosed as a drug reaction. Approximately 37% of patients diagnosed with stasis dermatitis develop an id reaction (Figure 1). A history of contact dermatitis is common in patients presenting with AE.2-6

Pathophysiology of Id Reactions
An abnormal immune response against autologous skin antigens may be responsible for the development of AE. Shelley5 postulated that hair follicles play an important role in id reactions, as Sharquie et al6 recently emphasized for many skin disorders. The pathogenesis of AE is uncertain, but circulating T lymphocytes play a role in this reaction. Normally, T cells are activated by a release of antigens after a primary exposure to a stimulus. However, overactivation of these T cells induces autoimmune reactions such as AE.7 Activated T lymphocytes express HLA-DR and IL-2 receptor, markers elevated in the peripheral blood of patients undergoing id reactions. After treatment, the levels of activated T lymphocytes decline. An increase in the number of CD25+ T cells and a decrease in the number of suppressor T cells in the blood may occur during an id reaction.7-9 Keratinocytes produce proinflammatory cytokines, such as thymic stromal erythropoietin, IL-25, and IL-33, that activate T cells.10-12 Therefore, the most likely pathogenesis of an id reaction is that T lymphocytes are activated at the primary reaction site due to proinflammatory cytokines released by keratinocytes. These activated T cells then travel systemically via hematogenous dissemination. The spread of activated T lymphocytes produces an eczematous reaction at secondary locations distant to the primary site.9
Clinical and Histopathological Features of Id Reactions
Clinically, AE is first evident as a vesicular dissemination that groups to form papules or nummular patches and usually is present on the legs, feet, arms, and/or trunk (Figure 2). The primary dermatitis is localized to the area that was the site of contact to the offending stimuli. This localized eczematous eruption begins with an acute or subacute onset. It has the appearance of small crusted vesicles with erythema (Figure 1). The first sign of AE is vesicles presenting near the primary site on flexural surfaces or on the hands and feet. A classic example is tinea pedis linked with vesicles on the palms and sides of the fingers, resembling dyshidrotic eczema. Sites of prior cutaneous trauma, such as dermatoses, scars, and burns, are common locations for early AE. In later stages, vesicles disseminate to the legs, arms, and trunk, where they group to form papules and nummular patches in a symmetrical pattern.5,13-15 These lesions may be extremely pruritic. The pruritus may be so intense that it interrupts daily activities and disrupts the ability to fall or stay asleep.16

Histologically, biopsy specimens show psoriasiform spongiotic dermatitis with mononuclear cells contained in the vesicles. Interstitial edema and perivascular lymphohistiocytic infiltrates are evident. Eosinophils also may be present. This pattern is not unique toid reactions.17-19 Although AE is a reaction pattern that may be due to a fungal or bacterial infection, the etiologic agent is not evident microscopically within the eczema itself.
Etiology of Id Reactions
Id reactions most commonly occur from either stasis dermatitis or tinea pedis, although a wide variety of other causes should be considered. Evaluation of the primary site rather than the id reaction may identify an infectious or parasitic agent. Sometimes the AE reaction is specifically named: dermatophytid with dermatophytosis, bacterid with a bacterial infectious process, and tuberculid with tuberculosis. Similarly, there may be reactions to underlying candidiasis, sporotrichosis, histoplasmosis, and other fungal infections that can cause a cutaneous id reaction.18,20-22Mycobacterium species, Pseudomonas, Staphylococcus, and Streptococcus are bacterial causes of AE.15,23-26 Viral infections that can cause an id reaction are herpes simplex virus and molluscum contagiosum.27-29 Scabies, leishmaniasis, and pediculosis capitis are parasitic infections that may be etiologic.14,30,31 In addition, noninfectious stimuli besides stasis dermatitis that can produce id reactions include medications, topical creams, tattoo ink, sutures, radiotherapy, and dyshidrotic eczema. The primary reaction to these agents is a localized dermatitis followed by the immunological response that induces a secondary reaction distant from the primary site.17,18,32-38
Differential Diagnoses
Differential diagnoses include other types of eczema and some vesicular eruptions. Irritant contact dermatitis is another dermatosis that presents as a widespread vesicular eruption due to repetitive exposure to toxic irritants. The rash is erythematous with pustules, blisters, and crusts. It is only found in areas directly exposed to irritants, as opposed to AE, which spreads to areas distant to the primary reaction site. Irritant contact dermatitis presents with more of a burning sensation, whereas AE is more pruritic.39,40 Allergic contact dermatitis presents with erythematous vesicles and papules and sometimes with bullae. There is edema and crust formation, which often can spread past the point of contact in later stages. Similar to AE, there is intense pruritus. However, allergic contact dermatitis most commonly is caused by exposure to metals, cosmetics, and fragrances, whereas infectious agents and stasis dermatitis are the most common causes of AE.40,41 It may be challenging to distinguish AE from other causes of widespread eczematous dissemination. Vesicular eruptions sometimes require distinction from AE, including herpetic infections, insect bite reactions, and drug eruptions.18,42
Treatment
The underlying condition should be treated to mitigate the inflammatory response causing the id reaction. If not skillfully orchestrated, the id reaction can reoccur. For infectious causes of AE, an antifungal, antibacterial, antiviral, or antiparasitic should be given. If stasis dermatitis is responsible for the id reaction, compression stockings and leg elevation are indicated. The id reaction itself is treated with systemic or topical corticosteroids and wet compresses if acute. The goal of these treatments is to reduce patient discomfort caused by the inflammation and pruritus.18,43
Conclusion
Id reactions are an unusual phenomenon that commonly occurs after fungal skin infections and stasis dermatitis. T lymphocytes and keratinocytes may play a key role in this reaction, with newer research further delineating the process and possibly providing enhanced treatment options. Therapy focuses on treating the underlying condition, supplemented with corticosteroids for the autoeczema.
Autoeczematization (AE), or id reaction, is a disseminated eczematous reaction that occurs days or weeks after exposure to a primary stimulus, resulting from a release of antigen(s). Whitfield1 first described AE in 1921, when he postulated that the id reaction was due to sensitization of the skin after a primary stimulus. He called it “a form of auto-intoxication derived from changes in the patient’s own tissues.”1 The exact prevalence of id reactions is unknown; one study showed that 17% of patients with dermatophyte infections developed an id reaction, typically tinea pedis linked with vesicles on the palms.2 Tinea capitis is one of the most common causes of AE in children, which is frequently misdiagnosed as a drug reaction. Approximately 37% of patients diagnosed with stasis dermatitis develop an id reaction (Figure 1). A history of contact dermatitis is common in patients presenting with AE.2-6

Pathophysiology of Id Reactions
An abnormal immune response against autologous skin antigens may be responsible for the development of AE. Shelley5 postulated that hair follicles play an important role in id reactions, as Sharquie et al6 recently emphasized for many skin disorders. The pathogenesis of AE is uncertain, but circulating T lymphocytes play a role in this reaction. Normally, T cells are activated by a release of antigens after a primary exposure to a stimulus. However, overactivation of these T cells induces autoimmune reactions such as AE.7 Activated T lymphocytes express HLA-DR and IL-2 receptor, markers elevated in the peripheral blood of patients undergoing id reactions. After treatment, the levels of activated T lymphocytes decline. An increase in the number of CD25+ T cells and a decrease in the number of suppressor T cells in the blood may occur during an id reaction.7-9 Keratinocytes produce proinflammatory cytokines, such as thymic stromal erythropoietin, IL-25, and IL-33, that activate T cells.10-12 Therefore, the most likely pathogenesis of an id reaction is that T lymphocytes are activated at the primary reaction site due to proinflammatory cytokines released by keratinocytes. These activated T cells then travel systemically via hematogenous dissemination. The spread of activated T lymphocytes produces an eczematous reaction at secondary locations distant to the primary site.9
Clinical and Histopathological Features of Id Reactions
Clinically, AE is first evident as a vesicular dissemination that groups to form papules or nummular patches and usually is present on the legs, feet, arms, and/or trunk (Figure 2). The primary dermatitis is localized to the area that was the site of contact to the offending stimuli. This localized eczematous eruption begins with an acute or subacute onset. It has the appearance of small crusted vesicles with erythema (Figure 1). The first sign of AE is vesicles presenting near the primary site on flexural surfaces or on the hands and feet. A classic example is tinea pedis linked with vesicles on the palms and sides of the fingers, resembling dyshidrotic eczema. Sites of prior cutaneous trauma, such as dermatoses, scars, and burns, are common locations for early AE. In later stages, vesicles disseminate to the legs, arms, and trunk, where they group to form papules and nummular patches in a symmetrical pattern.5,13-15 These lesions may be extremely pruritic. The pruritus may be so intense that it interrupts daily activities and disrupts the ability to fall or stay asleep.16

Histologically, biopsy specimens show psoriasiform spongiotic dermatitis with mononuclear cells contained in the vesicles. Interstitial edema and perivascular lymphohistiocytic infiltrates are evident. Eosinophils also may be present. This pattern is not unique toid reactions.17-19 Although AE is a reaction pattern that may be due to a fungal or bacterial infection, the etiologic agent is not evident microscopically within the eczema itself.
Etiology of Id Reactions
Id reactions most commonly occur from either stasis dermatitis or tinea pedis, although a wide variety of other causes should be considered. Evaluation of the primary site rather than the id reaction may identify an infectious or parasitic agent. Sometimes the AE reaction is specifically named: dermatophytid with dermatophytosis, bacterid with a bacterial infectious process, and tuberculid with tuberculosis. Similarly, there may be reactions to underlying candidiasis, sporotrichosis, histoplasmosis, and other fungal infections that can cause a cutaneous id reaction.18,20-22Mycobacterium species, Pseudomonas, Staphylococcus, and Streptococcus are bacterial causes of AE.15,23-26 Viral infections that can cause an id reaction are herpes simplex virus and molluscum contagiosum.27-29 Scabies, leishmaniasis, and pediculosis capitis are parasitic infections that may be etiologic.14,30,31 In addition, noninfectious stimuli besides stasis dermatitis that can produce id reactions include medications, topical creams, tattoo ink, sutures, radiotherapy, and dyshidrotic eczema. The primary reaction to these agents is a localized dermatitis followed by the immunological response that induces a secondary reaction distant from the primary site.17,18,32-38
Differential Diagnoses
Differential diagnoses include other types of eczema and some vesicular eruptions. Irritant contact dermatitis is another dermatosis that presents as a widespread vesicular eruption due to repetitive exposure to toxic irritants. The rash is erythematous with pustules, blisters, and crusts. It is only found in areas directly exposed to irritants, as opposed to AE, which spreads to areas distant to the primary reaction site. Irritant contact dermatitis presents with more of a burning sensation, whereas AE is more pruritic.39,40 Allergic contact dermatitis presents with erythematous vesicles and papules and sometimes with bullae. There is edema and crust formation, which often can spread past the point of contact in later stages. Similar to AE, there is intense pruritus. However, allergic contact dermatitis most commonly is caused by exposure to metals, cosmetics, and fragrances, whereas infectious agents and stasis dermatitis are the most common causes of AE.40,41 It may be challenging to distinguish AE from other causes of widespread eczematous dissemination. Vesicular eruptions sometimes require distinction from AE, including herpetic infections, insect bite reactions, and drug eruptions.18,42
Treatment
The underlying condition should be treated to mitigate the inflammatory response causing the id reaction. If not skillfully orchestrated, the id reaction can reoccur. For infectious causes of AE, an antifungal, antibacterial, antiviral, or antiparasitic should be given. If stasis dermatitis is responsible for the id reaction, compression stockings and leg elevation are indicated. The id reaction itself is treated with systemic or topical corticosteroids and wet compresses if acute. The goal of these treatments is to reduce patient discomfort caused by the inflammation and pruritus.18,43
Conclusion
Id reactions are an unusual phenomenon that commonly occurs after fungal skin infections and stasis dermatitis. T lymphocytes and keratinocytes may play a key role in this reaction, with newer research further delineating the process and possibly providing enhanced treatment options. Therapy focuses on treating the underlying condition, supplemented with corticosteroids for the autoeczema.
- Whitfield A. Lumleian Lectures on Some Points in the Aetiology of Skin Diseases. Delivered before the Royal College of Physicians of London on March 10th, 15th, and 17th, 1921. Lecture II. Lancet. 1921;2:122-127.
- Cheng N, Rucker Wright D, Cohen BA. Dermatophytid in tinea capitis: rarely reported common phenomenon with clinical implications. Pediatrics. 2011;128:E453-E457.
- Schrom KP, Kobs A, Nedorost S. Clinical psoriasiform dermatitis following dupilumab use for autoeczematization secondary to chronic stasis dermatitis. Cureus. 2020;12:e7831. doi:10.7759/cureus.7831
- Templeton HJ, Lunsford CJ, Allington HV. Autosensitization dermatitis; report of five cases and protocol of an experiment. Arch Derm Syphilol. 1949;59:68-77.
- Shelley WB. Id reaction. In: Consultations in Dermatology. Saunders; 1972:262-267.
- Sharquie KE, Noaimi AA, Flayih RA. Clinical and histopathological findings in patients with follicular dermatoses: all skin diseases starts in the hair follicles as new hypothesis. Am J Clin Res Rev. 2020;4:17.
- Kasteler JS, Petersen MJ, Vance JE, et al. Circulating activated T lymphocytes in autoeczematization. Arch Dermatol. 1992;128:795-798.
- González-Amaro R, Baranda L, Abud-Mendoza C, et al. Autoeczematization is associated with abnormal immune recognition of autologous skin antigens. J Am Acad Dermatol. 1993;28:56-60.
- Cunningham MJ, Zone JJ, Petersen MJ, et al. Circulating activated (DR-positive) T lymphocytes in a patient with autoeczematization. J Am Acad Dermatol. 1986;14:1039-1041.
- Furue M, Ulzii D, Vu YH, et al. Pathogenesis of atopic dermatitis: current paradigm. Iran J Immunol. 2019;16:97-107.
- Uchi H, Terao H, Koga T, et al. Cytokines and chemokines in the epidermis. J Dermatol Sci. 2000;24(suppl 1):S29-S38.
- Bos JD, Kapsenberg ML. The skin immune system: progress in cutaneous biology. Immunol Today. 1993;14:75-78.
- Young AW Jr. Dynamics of autosensitization dermatitis; a clinical and microscopic concept of autoeczematization. AMA Arch Derm. 1958;77:495-502.
- Brenner S, Wolf R, Landau M. Scabid: an unusual id reaction to scabies. Int J Dermatol. 1993;32:128-129.
- Yamany T, Schwartz RA. Infectious eczematoid dermatitis: a comprehensive review. J Eur Acad Dermatol Venereol. 2015;29:203-208.
- Wang X, Li L, Shi X, et al. Itching and its related factors in subtypes of eczema: a cross-sectional multicenter study in tertiary hospitals of China. Sci Rep. 2018;8:10754.
- Price A, Tavazoie M, Meehan SA, et al. Id reaction associated with red tattoo ink. Cutis. 2018;102:E32-E34.
- Ilkit M, Durdu M, Karaks¸ M. Cutaneous id reactions: a comprehensive review of clinical manifestations, epidemiology, etiology, and management. Crit Rev Microbiol. 2012;38:191-202.
- Kaner SR. Dermatitis venenata of the feet with a generalized “id” reaction. J Am Podiatry Assoc. 1970;60:199-204.
- Jordan L, Jackson NA, Carter-Snell B, et al. Pustular tinea id reaction. Cutis. 2019;103:E3-E4.
- Crum N, Hardaway C, Graham B. Development of an idlike reaction during treatment for acute pulmonary histoplasmosis: a new cutaneous manifestation in histoplasmosis. J Am Acad Dermatol. 2003;48(2 suppl):S5-S6.
- Chirac A, Brzezinski P, Chiriac AE, et al. Autosensitisation (autoeczematisation) reactions in a case of diaper dermatitis candidiasis. Niger Med J. 2014;55:274-275.
- Singh PY, Sinha P, Baveja S, et al. Immune-mediated tuberculous uveitis—a rare association with papulonecrotic tuberculid. Indian J Ophthalmol. 2019;67:1207-1209.
- Urso B, Georgesen C, Harp J. Papulonecrotic tuberculid secondary to Mycobacterium avium complex. Cutis. 2019;104:E11-E13.
- Choudhri SH, Magro CM, Crowson AN, et al. An id reaction to Mycobacterium leprae: first documented case. Cutis. 1994;54:282-286.
- Park JW, Jeong GJ, Seo SJ, et al. Pseudomonas toe web infection and autosensitisation dermatitis: diagnostic and therapeutic challenge. Int Wound J. 2020;17:1543-1544. doi:10.1111/iwj.13386
- Netchiporouk E, Cohen BA. Recognizing and managing eczematous id reactions to molluscum contagiosum virus in children. Pediatrics. 2012;129:E1072-E1075.
- Aurelian L, Ono F, Burnett J. Herpes simplex virus (HSV)-associated erythema multiforme (HAEM): a viral disease with an autoimmune component. Dermatol Online J. 2003;9:1.
- Rocamora V, Romaní J, Puig L, et al. Id reaction to molluscum contagiosum. Pediatr Dermatol. 1996;13:349-350.
- Yes¸ilova Y, Özbilgin A, Turan E, et al. Clinical exacerbation developing during treatment of cutaneous leishmaniasis: an id reaction? Turkiye Parazitol Derg. 2014;38:281-282.
- Connor CJ, Selby JC, Wanat KA. Severe pediculosis capitus: a case of “crusted lice” with autoeczematization. Dermatol Online J. 2016;22:13030/qt7c91z913.
- Shelley WB. The autoimmune mechanism in clinical dermatology. Arch Dermatol. 1962;86:27-34.
- Bosworth A, Hull PR. Disseminated eczema following radiotherapy: a case report. J Cutan Med Surg. 2018;22:353-355.
- Lowther C, Miedler JD, Cockerell CJ. Id-like reaction to BCG therapy for bladder cancer. Cutis. 2013;91:145-151.
- Huerth KA, Glick PL, Glick ZR. Cutaneous id reaction after using cyanoacrylate for wound closure. Cutis. 2020;105:E11-E13.
- Amini S, Burdick AE, Janniger CK. Dyshidrotic eczema (pompholyx). Updated April 22, 2020. Accessed August 23, 2021. https://emedicine.medscape.com/article/1122527-overview
- Sundaresan S, Migden MR, Silapunt S. Stasis dermatitis: pathophysiology, evaluation, and management. Am J Clin Dermatol. 2017;18:383-390.
- Hughes JDM, Pratt MD. Allergic contact dermatitis and autoeczematization to proctosedyl® cream and proctomyxin® cream. Case Rep Dermatol. 2018;10:238-246.
- Bains SN, Nash P, Fonacier L. Irritant contact dermatitis. Clin Rev Allergy Immunol. 2019;56:99-109.
- Novak-Bilic´ G, Vucˇic´ M, Japundžic´ I, et al. Irritant and allergic contact dermatitis—skin lesion characteristics. Acta Clin Croat. 2018;57:713-720.
- Nassau S, Fonacier L. Allergic contact dermatitis. Med Clin North Am. 2020;104:61-76.
- Lewis DJ, Schlichte MJ, Dao H Jr. Atypical disseminated herpes zoster: management guidelines in immunocompromised patients. Cutis. 2017;100:321-330.
- Nedorost S, White S, Rowland DY, et al. Development and implementation of an order set to improve value of care for patients with severe stasis dermatitis. J Am Acad Dermatol. 2019;80:815-817.
- Whitfield A. Lumleian Lectures on Some Points in the Aetiology of Skin Diseases. Delivered before the Royal College of Physicians of London on March 10th, 15th, and 17th, 1921. Lecture II. Lancet. 1921;2:122-127.
- Cheng N, Rucker Wright D, Cohen BA. Dermatophytid in tinea capitis: rarely reported common phenomenon with clinical implications. Pediatrics. 2011;128:E453-E457.
- Schrom KP, Kobs A, Nedorost S. Clinical psoriasiform dermatitis following dupilumab use for autoeczematization secondary to chronic stasis dermatitis. Cureus. 2020;12:e7831. doi:10.7759/cureus.7831
- Templeton HJ, Lunsford CJ, Allington HV. Autosensitization dermatitis; report of five cases and protocol of an experiment. Arch Derm Syphilol. 1949;59:68-77.
- Shelley WB. Id reaction. In: Consultations in Dermatology. Saunders; 1972:262-267.
- Sharquie KE, Noaimi AA, Flayih RA. Clinical and histopathological findings in patients with follicular dermatoses: all skin diseases starts in the hair follicles as new hypothesis. Am J Clin Res Rev. 2020;4:17.
- Kasteler JS, Petersen MJ, Vance JE, et al. Circulating activated T lymphocytes in autoeczematization. Arch Dermatol. 1992;128:795-798.
- González-Amaro R, Baranda L, Abud-Mendoza C, et al. Autoeczematization is associated with abnormal immune recognition of autologous skin antigens. J Am Acad Dermatol. 1993;28:56-60.
- Cunningham MJ, Zone JJ, Petersen MJ, et al. Circulating activated (DR-positive) T lymphocytes in a patient with autoeczematization. J Am Acad Dermatol. 1986;14:1039-1041.
- Furue M, Ulzii D, Vu YH, et al. Pathogenesis of atopic dermatitis: current paradigm. Iran J Immunol. 2019;16:97-107.
- Uchi H, Terao H, Koga T, et al. Cytokines and chemokines in the epidermis. J Dermatol Sci. 2000;24(suppl 1):S29-S38.
- Bos JD, Kapsenberg ML. The skin immune system: progress in cutaneous biology. Immunol Today. 1993;14:75-78.
- Young AW Jr. Dynamics of autosensitization dermatitis; a clinical and microscopic concept of autoeczematization. AMA Arch Derm. 1958;77:495-502.
- Brenner S, Wolf R, Landau M. Scabid: an unusual id reaction to scabies. Int J Dermatol. 1993;32:128-129.
- Yamany T, Schwartz RA. Infectious eczematoid dermatitis: a comprehensive review. J Eur Acad Dermatol Venereol. 2015;29:203-208.
- Wang X, Li L, Shi X, et al. Itching and its related factors in subtypes of eczema: a cross-sectional multicenter study in tertiary hospitals of China. Sci Rep. 2018;8:10754.
- Price A, Tavazoie M, Meehan SA, et al. Id reaction associated with red tattoo ink. Cutis. 2018;102:E32-E34.
- Ilkit M, Durdu M, Karaks¸ M. Cutaneous id reactions: a comprehensive review of clinical manifestations, epidemiology, etiology, and management. Crit Rev Microbiol. 2012;38:191-202.
- Kaner SR. Dermatitis venenata of the feet with a generalized “id” reaction. J Am Podiatry Assoc. 1970;60:199-204.
- Jordan L, Jackson NA, Carter-Snell B, et al. Pustular tinea id reaction. Cutis. 2019;103:E3-E4.
- Crum N, Hardaway C, Graham B. Development of an idlike reaction during treatment for acute pulmonary histoplasmosis: a new cutaneous manifestation in histoplasmosis. J Am Acad Dermatol. 2003;48(2 suppl):S5-S6.
- Chirac A, Brzezinski P, Chiriac AE, et al. Autosensitisation (autoeczematisation) reactions in a case of diaper dermatitis candidiasis. Niger Med J. 2014;55:274-275.
- Singh PY, Sinha P, Baveja S, et al. Immune-mediated tuberculous uveitis—a rare association with papulonecrotic tuberculid. Indian J Ophthalmol. 2019;67:1207-1209.
- Urso B, Georgesen C, Harp J. Papulonecrotic tuberculid secondary to Mycobacterium avium complex. Cutis. 2019;104:E11-E13.
- Choudhri SH, Magro CM, Crowson AN, et al. An id reaction to Mycobacterium leprae: first documented case. Cutis. 1994;54:282-286.
- Park JW, Jeong GJ, Seo SJ, et al. Pseudomonas toe web infection and autosensitisation dermatitis: diagnostic and therapeutic challenge. Int Wound J. 2020;17:1543-1544. doi:10.1111/iwj.13386
- Netchiporouk E, Cohen BA. Recognizing and managing eczematous id reactions to molluscum contagiosum virus in children. Pediatrics. 2012;129:E1072-E1075.
- Aurelian L, Ono F, Burnett J. Herpes simplex virus (HSV)-associated erythema multiforme (HAEM): a viral disease with an autoimmune component. Dermatol Online J. 2003;9:1.
- Rocamora V, Romaní J, Puig L, et al. Id reaction to molluscum contagiosum. Pediatr Dermatol. 1996;13:349-350.
- Yes¸ilova Y, Özbilgin A, Turan E, et al. Clinical exacerbation developing during treatment of cutaneous leishmaniasis: an id reaction? Turkiye Parazitol Derg. 2014;38:281-282.
- Connor CJ, Selby JC, Wanat KA. Severe pediculosis capitus: a case of “crusted lice” with autoeczematization. Dermatol Online J. 2016;22:13030/qt7c91z913.
- Shelley WB. The autoimmune mechanism in clinical dermatology. Arch Dermatol. 1962;86:27-34.
- Bosworth A, Hull PR. Disseminated eczema following radiotherapy: a case report. J Cutan Med Surg. 2018;22:353-355.
- Lowther C, Miedler JD, Cockerell CJ. Id-like reaction to BCG therapy for bladder cancer. Cutis. 2013;91:145-151.
- Huerth KA, Glick PL, Glick ZR. Cutaneous id reaction after using cyanoacrylate for wound closure. Cutis. 2020;105:E11-E13.
- Amini S, Burdick AE, Janniger CK. Dyshidrotic eczema (pompholyx). Updated April 22, 2020. Accessed August 23, 2021. https://emedicine.medscape.com/article/1122527-overview
- Sundaresan S, Migden MR, Silapunt S. Stasis dermatitis: pathophysiology, evaluation, and management. Am J Clin Dermatol. 2017;18:383-390.
- Hughes JDM, Pratt MD. Allergic contact dermatitis and autoeczematization to proctosedyl® cream and proctomyxin® cream. Case Rep Dermatol. 2018;10:238-246.
- Bains SN, Nash P, Fonacier L. Irritant contact dermatitis. Clin Rev Allergy Immunol. 2019;56:99-109.
- Novak-Bilic´ G, Vucˇic´ M, Japundžic´ I, et al. Irritant and allergic contact dermatitis—skin lesion characteristics. Acta Clin Croat. 2018;57:713-720.
- Nassau S, Fonacier L. Allergic contact dermatitis. Med Clin North Am. 2020;104:61-76.
- Lewis DJ, Schlichte MJ, Dao H Jr. Atypical disseminated herpes zoster: management guidelines in immunocompromised patients. Cutis. 2017;100:321-330.
- Nedorost S, White S, Rowland DY, et al. Development and implementation of an order set to improve value of care for patients with severe stasis dermatitis. J Am Acad Dermatol. 2019;80:815-817.
Practice Points
- Autoeczematization, or id reaction, is a disseminated reaction of the skin occurring at a site distant to a primary cutaneous infection or stimulus.
- T lymphocytes and keratinocytes are postulated to be involved in the pathogenesis of id reactions.
- Therapy includes treating the underlying pathology while providing topical corticosteroids for the autoeczematous lesions.