User login
The Role of Process Improvements in Reducing Heart Failure Readmissions
From the Department of Medicine, Division of Cardiology, Northwestern University Feinberg School of Medicine, Chicago, IL.
Objective: To review selected process-of-care interventions that can be applied both during the hospitalization and during the transitional care period to help address the persistent challenge of heart failure readmissions.
Methods: Review of the literature.
Results: Process-of-care interventions that can be implemented to reduce readmissions of heart failure patients include: accurately identifying heart failure patients; providing disease education; titrating guideline-directed medical therapy; ensuring discharge readiness; arranging close discharge follow-up; identifying and addressing social barriers; following up by telephone; using home health; and addressing comorbidities. Importantly, the heart failure hospitalization is an opportunity to set up outpatient success, and setting up feedback loops can aid in post-discharge monitoring.
Conclusion: We encourage teams to consider local capabilities when selecting processes to improve; begin by improving something small to build capacity and team morale, and continually iterate and reexamine processes, as health care systems are continually evolving.
Keywords: heart failure; process improvement; quality improvement; readmission; rehospitalization; transitional care.
The growing population of patients affected by heart failure continues to challenge health systems. The increasing prevalence is paralleled by the rising costs of managing heart failure, which are projected to grow from $30.7 billion in 2012 to $69.8 billion in 2030.1 A significant portion of these costs relate to readmission after an index heart failure hospitalization. The statistics are staggering: for patients hospitalized with heart failure, approximately 15% to 20% are readmitted within 30 days.2,3 Though recent temporal trends suggest a modest reduction in readmission rates, there is a concerning correlation with increasing mortality,3 and a recognition that readmission rate decreases may relate to subtle changes in coding-based risk adjustment.4 Despite these concerns, efforts to reduce readmissions after heart failure hospitalization command significant attention.
Process improvement methodologies may be helpful in reducing hospital readmissions. Various approaches have been employed, and results have been mixed. An analysis of 70 participating hospitals in the American Heart Association’s Get With the Guidelines initiative found that, while overall readmission rates declined by 1.0% over 3 years, only 1 hospital achieved a 20% reduction in readmission rates.5
It is notably difficult to reduce readmissions after heart failure hospitalization. One challenge is that patients with heart failure often have multiple comorbidities, and approximately 50% to 60% of 30-day readmissions after heart failure hospitalization arise from noncardiac causes.1 Another challenge is that a significant fraction of readmissions in general—perhaps 75%—may not be avoidable.6
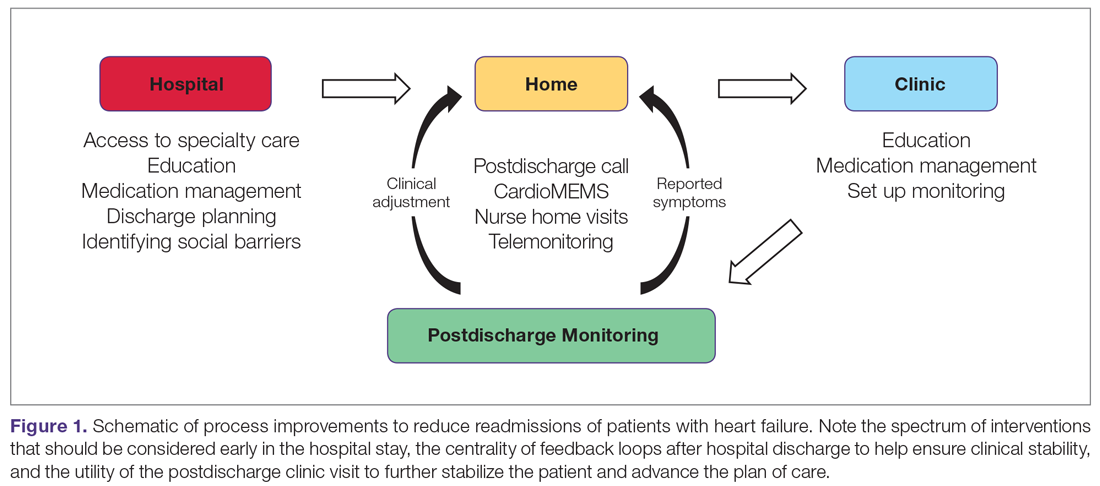
Recent excellent systematic reviews and meta-analyses provide comprehensive overviews of process improvement strategies that can be used to reduce readmissions after heart failure hospitalizations.7-9 Yet despite this extensive knowledge, few reports discuss the process of actually implementing these changes: the process of process improvement. Here, we seek to not only highlight some of the most promising potential interventions to reduce heart failure readmissions, but also to discuss a process improvement framework to help engender success, using our experience as a case study. We schematize process improvement efforts as having several distinct phases (Figure 1): processes delivered during the hospitalization and prior to discharge; feedback loops set up to maintain clinical stability at home; and the postdischarge clinic visit as an opportunity to further stabilize the patient and advance the plan of care. The discussion of these interventions follows this organization.
During Hospitalization
The heart failure hospitalization can be used as an opportunity to set up outpatient success, with several goals to target during the index admission. One goal is identifying the root causes of the heart failure syndrome and correcting those root causes, if possible. For example, patients in whom the heart failure syndrome is secondary to valvular heart disease may benefit from transcatheter aortic valve replacement.10 Another clinical goal is decongesting the patient, which is associated with lower readmission rates.11,12 These goals focus on the medical aspects of heart failure care. However, beyond these medical aspects, a patient must be equipped to successfully manage the disease at home.
To support medical and nonmedical interventions for hospitalized heart failure patients, a critical first step is identifying patients with heart failure. This accomplishes at least 2 objectives. First, early identification allows early initiation of interventions, such as heart failure education and social work evaluation. Early initiation of these interventions allows sufficient time during the hospitalization to make meaningful progress on these fronts. Second, early identification allows an opportunity for the delivery of cardiology specialty care, which may help with identifying and correcting root causes of the heart failure syndrome. Such access to cardiology has been shown to improve inpatient mortality and readmission rates.13
In smaller hospitals, identification of patients with heart failure can be as simple as reviewing overnight admissions. More advanced strategies, such as screeners based on brain natriuretic peptide (BNP) levels and administration of intravenous diuretics, can be employed.14,15 In the near future, deep learning-based natural language processing will be applied to mine full-text data in the electronic health record to identify heart failure hospitalizations.16
In the hospital, patients can also receive education about heart failure disease management. This education is a cornerstone of reducing heart failure readmissions. A recent systematic review of nurse education interventions demonstrated reductions in readmissions, hospitalizations, and costs.17 However, the efficacy of heart failure education hinges on many other variables. For patients to adhere to water restriction and daily weights, for example, there must also be patient understanding, compliance, and accessibility to providers to recommend how to strike the fluid balance. Education is therefore necessary, but not sufficient, for setting up outpatient success.
The hospitalization also represents an important time to start or uptitrate guideline-directed medical therapy (GDMT) for heart failure. Doing so takes advantage of an important opportunity to reduce the risk of readmission and even reverse the disease process.18 Uptitration of GDMT in patients with heart failure with reduced ejection fraction is associated with a decreased risk of mortality, while discontinuation is associated with an increased risk of mortality.19 However, recent registry data indicate that intensity of GDMT is just as likely to be decreased as increased during the hospitalization.20 Nevertheless, predischarge initiation of medications may be associated with higher attained doses in follow-up.21
Preparing for Discharge
Preparing a patient for discharge after a heart failure hospitalization involves stabilizing the medical condition as well as ensuring that the patient and caregivers have the medication, equipment, and self-care resources at home necessary to manage the condition. Several frameworks have been put forth to help care teams analyze a patient’s readiness for discharge. One is the B-PREPARED score,22 a validated instrument to discriminate among patients with regard to their readiness to discharge from the hospital. This instrument highlights the importance of several key factors that should be addressed during the discharge process, including counseling and written instructions about medications and their side effects; information about equipment needs and community resources; and information on activity levels and restrictions. Nurse education and discharge coordination can improve patients’ perception of discharge readiness,23 although whether this discharge readiness translates into improved readmission rates appears to depend on the specific follow-up intervention design.9
Prior to discharge, it is important to arrange postdischarge follow-up appointments, as emphasized by the American College of Cardiology/American Heart Association (ACC/AHA) guidelines.24 The use of nurse navigators can help with planning follow-up appointments. For example, the ACC Patient Navigator Program was applied in a single-center study of 120 patients randomized to the program versus usual care.25 This study found a significant increase in patient education and follow-up appointments compared to usual care, and a numerical decrease in hospital readmissions, although the finding was not statistically significant.25
A third critical component of preparing for discharge is identifying and addressing social barriers to care. In a study of patients stratified by household income, patients in the lowest income quartile had a higher readmission rate than patients in the highest income quartile.26 Poverty also correlates with heart failure mortality.27 Social factors play an important role in many aspects of patients’ ability to manage their health, including self-care, medication adherence, and ability to follow-up. Identifying these social factors prior to discharge is the first step to addressing them. While few studies specifically address the role of social workers in the management of heart failure care, the general medical literature suggests that social workers embedded in transitional care teams can augment readmission reduction efforts.28
After Discharge
Patients recently discharged from the hospital who have not yet attended their postdischarge appointment are in an incredibly vulnerable phase of care. Patients who are discharged from the hospital may not yet be connected with outpatient care. During this initial transitional care period, feedback loops involving patient communication back to the clinic, and clinic communication back to the patient, are critical to helping patients remain stable. For example, consider monitoring weights daily after hospital discharge. A patient at home can report increasing weights to a provider, who can then recommend an increased dose of diuretic. The patient can complete the feedback loop by taking the extra medication and monitoring the return of weight back to normal.
While daily weight monitoring is a simple process improvement that relies on the principle of establishing feedback loops, many other strategies exist. One commonly employed tool is the postdischarge telephone follow-up call, which is often coupled with other interventions in a comprehensive care bundle.8 During the telephone call, several process-of-care defects can be corrected, including missing medications or missing information on appointment times.
Beyond the telephone, newer technologies show promise for helping develop feedback loops for patients at home. One such technology is telemonitoring, whereby physiologic information such as weight, heart rate, and blood pressure is collected and sent back to a monitoring center. While the principle holds promise, several studies have not demonstrated significantly different outcomes as compared to usual care.13,29 Another promising technology is the CardioMEMS device (Abbott, Inc., Atlanta, GA), which can remotely transmit the pulmonary artery pressure, a physiologic signal which correlates with volume overload. There is now strong evidence supporting the efficacy of pulmonary artery pressure–guided heart failure management.30,31
Finally, home visits can be an efficient way to communicate symptoms, enable clinical assessment, and provide recommendations. One program that implemented home visits, 24-hour nurses available by call, and telephone follow-up showed a statistically significant reduction in readmissions.32 Furthermore, a meta-analysis of randomized controlled trials comparing home health to usual care showed decreased readmissions and mortality.33 The efficacy may be in strengthening the feedback loop—home care improves compliance with weight monitoring, fluid restriction, and medications.34 These studies provide a strong rationale for the benefits of home health in stabilizing heart failure patients postdischarge. Indeed, nurse home visits were 1 of the 2 process interventions in a Cochrane review of randomized controlled trials that were shown to statistically significantly decrease readmissions and mortality.9 These data underscore the importance of feedback loops for helping ensure patients are clinically stable.
Postdischarge Follow-Up Clinic Visit
The first clinic appointment postdischarge is an important check-in to help advance patient care. Several key tasks can be achieved during the postdischarge visit. First, the patient can be clinically stabilized by adjusting diuretic therapy. If the patient is clinically stable, GDMT can be uptitrated. Second, education around symptoms, medications, diet, and exercise can be reinforced. Finally, clinicians can help connect patients to other members of the multidisciplinary care team, including specialist care, home health, or cardiac rehabilitation.
Achieving 7-day follow-up visits after discharge has been a point of emphasis in national guidelines.24 The ACC promotes a “See You in 7” challenge, advising that all patients discharged with a diagnosis of heart failure have a follow-up appointment within 7 days. Yet based on the latest available data, arrival rates to the postdischarge clinic are dismal, hovering around 30%.35 In a multicenter observational study of hospitals participating in the “See You in 7” collaborative, hospitals were able to increase their 7-day follow-up appointment rates by 2% to 3%, and also noted an absolute decrease in readmission rates by 1% to 2%.36 We have demonstrated, using a mathematical approach called queuing theory, that discharge appointment wait times and clinic access can be significantly improved by providing a modest capacity buffer to clinic availability.37 Those interested in applying this model to their own clinical practice may do so with a free online calculator at http://hfresearch.org.
Another important aspect of postdischarge follow-up is appropriate management of the comorbidity burden, which, as noted, is often significant in patients hospitalized with heart failure.38 For instance, in recent cohorts of hospitalized heart failure patients, the incidence of hypertension was 78%, coronary artery disease was more than 50%, atrial fibrillation was more than 40%, and diabetes was nearly 40%.39 Given this burden of comorbidity, it is not surprising that only 35% of readmissions after an index heart failure hospitalization are for recurrent heart failure.40 Coordinating care among primary care physicians and relevant subspecialists is thus essential. Phone calls and secure electronic messages are very helpful in achieving this. There is increasing interest in more nimble care models, such as the patient-centered specialty practice41 or the dyspnea clinic, to help bring coordinated resources to the patient.42
Process of Process Improvement: Our Experiences
The previous sections outline a series of potential process improvements clinical teams and health systems can implement to impact heart failure readmissions. A plan on paper, however, does not equal a plan in actuality. How does one go about implementing these changes? We offer our local experience starting a heart failure transitional care program as a case study, then draw lessons learned as a set of practical tips for local teams to employ. What we hope to highlight is that there is a large difference between a completed process for transitional care of heart failure patients, and the process of developing that process itself. The former is the hardware, the latter is the software. The latter does not typically get highlighted, but it is absolutely critical to unlocking the capabilities of a team and the institution.
In 2015, Northwestern Memorial Hospital adopted a novel payment arrangement from the Center for Medicare and Medicaid Services for Medicare patients being discharged from the hospital with heart failure. Known as Bundled Payments for Care Improvement,43 this bundled payment model incentivized Northwestern Memorial Hospital charge, principally by reducing hospital readmissions and by collaborating with skilled nursing facilities to control length of stay.
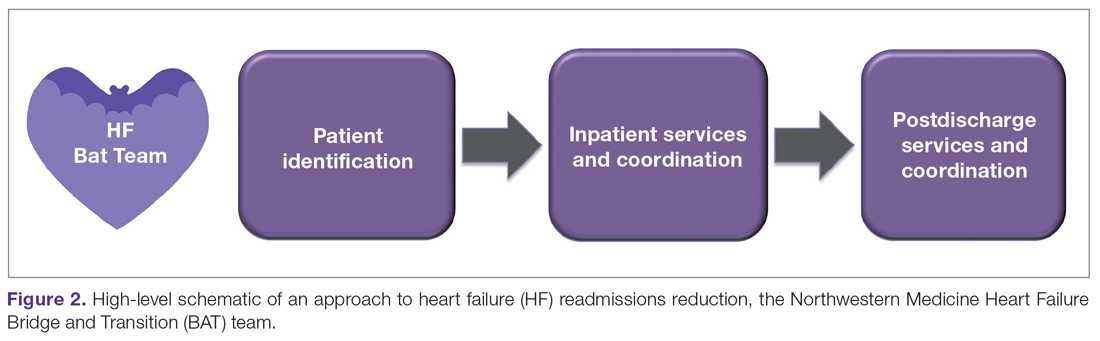
We approached this problem by drawing on the available literature,44,45 and by first creating a schematic of our high-level approach, which comprised 3 major elements (Figure 2): identification of hospitalized heart failure patients, delivery of a care bundle to hospitalized heart failure patients in hospital, and coordinating postdischarge care, centered on a telephone call and a postdischarge visit.
We then proceeded by building out, in stepwise fashion, each component of our value chain, using Agile techniques as a guiding principle.46 Agile, a productivity and process improvement mindset with roots in software development, emphasizes tackling 1 problem at a time, building out new features sequentially and completely, recognizing that the end user does not derive value from a program until new functionality is available for use. Rather than wholesale monolithic change, Agile emphasizes rapid iteration, prototyping, and discarding innovations not found to be helpful. The notion is to stand up new, incremental features rapidly, with each incremental improvement delivering value and helping to accelerate overall change.
Our experience building a robust way to identify heart failure cases is a good example of Agile process improvement in practice. At our hospital, identification of patients with heart failure was a challenge because more than half of heart failure patients are admitted to noncardiology floors. We developed a simple electronic health record query to detect heart failure patients, relying on parameters such as administration of intravenous diuretic or levels of BNP exceeding 100 ng/dL. We deployed this query, finding very high sensitivity for detection of heart failure patients.14 Patients found to have heart failure were then populated into a list in the electronic health record, which made patients’ heart failure status visible to all members of the health care team. Using this list, we were able to automate several processes necessary for heart failure care. For example, the list made it possible for cardiologists to know if there was a patient who perhaps needed cardiology consultation. Nurse navigators could know which patients needed heart failure education without having to be actively consulted by the admitting team. The same nurse navigators could then know upon discharge which patients needed a follow-up telephone call at 48 hours.
This list of heart failure patients was the end product, which was built through prototyping and iteration. For example, with our initial BNP cutoff of 300 ng/dL, we recognized we were missing several cases, and lowered the cutoff for the screener to 100 ng/dL. When we were satisfied this process was working well, we moved on to the next problem to tackle, avoiding trying to work on too many things at once. By doing so, we were able to focus our process improvement resources on 1 problem at a time, building up a suite of interventions. For our hospital, we settled on a bundle of interventions, captured by the mnemonic HEART:
Heart doctor sees patient in the hospital
Education about heart failure in the hospital
After-visit summary with 7-day appointment printed
Reach out to the patient by telephone within 72 hours
Treat the patient in clinic by the 7-day visit
Conclusion
We would like to emphasize that the elements of our heart failure readmissions interventions were not all put in place at once. This was an iterative process that proceeded in a stepwise fashion, with each step improving the care of our patients. We learned a number of lessons from our experience. First, we would advise that teams not try to do everything. One program simply cannot implement all possible readmission reduction interventions, and certainly not all at once. Trade-offs should be made, and interventions more likely to succeed in the local environment should be prioritized. In addition, interventions that do not fit and do not create synergy with the local practice environment should not be pursued.
Second, we would advise teams to start small, tackling a known problem in heart failure transitions of care first. This initial intuition is often right. An example might be improving 7-day appointments upon discharge. Starting with a problem that can be tackled builds process improvement muscle and improves team morale. Third, we would advise teams to consistently iterate on designs, tweaking and improving performance. Complex organizations always evolve; processes that work 1 year may fail the next because another element of the organization may have changed.
Finally, the framework presented in Figure 1 may be helpful in guiding how to structure interventions. Considering interventions to be delivered in the hospital, interventions to be delivered in the clinic, and how to set up feedback loops to support patients as outpatients help develop a comprehensive heart failure readmissions reduction program.
Corresponding author: R. Kannan Mutharasan, MD, Northwestern University Feinberg School of Medicine, 676 North Saint Clair St., Arkes Pavilion, Suite 7-038, Chicago, IL 60611;[email protected].
Financial disclosures: None.
1. Ziaeian B, Fonarow GC. The prevention of hospital readmissions in heart failure. Prog Cardiovasc Dis. 2016;58:379-385.
2. Kwok CS, Seferovic PM, Van Spall HG, et al. Early unplanned readmissions after admission to hospital with heart failure. Am J Cardiol. 2019;124:736-745.
3. Fonarow GC, Konstam MA, Yancy CW. The hospital readmission reduction program is associated with fewer readmissions, more deaths: time to reconsider. J Am Coll Cardiol. 2017;70:1931-1934.
4. Ody C, Msall L, Dafny LS, et al. Decreases in readmissions credited to medicare’s program to reduce hospital readmissions have been overstated. Health Aff (Millwood). 2019;38:36-43.
5. Bergethon KE, Ju C, DeVore AD, et al. Trends in 30-day readmission rates for patients hospitalized with heart failure: findings from the Get With The Guidelines-Heart Failure Registry. Circ Heart Fail. 2016;9.
6. van Walraven C, Jennings A, Forster AJ. A meta-analysis of hospital 30-day avoidable readmission rates. J Eval Clin Pract. 2012;18(6):1211-1218.
7. Albert NM. A systematic review of transitional-care strategies to reduce rehospitalization in patients with heart failure. Heart Lung. 2016;45:100-113.
8. Takeda A, Martin N, Taylor RS, Taylor SJ. Disease management interventions for heart failure. Cochrane Database Syst Rev. 2019;1:CD002752.
9. Van Spall HGC, Rahman T, Mytton O, et al. Comparative effectiveness of transitional care services in patients discharged from the hospital with heart failure: a systematic review and network meta-analysis. Eur J Heart Fail. 2017;19:1427-1443.
10. Reardon MJ, Van Mieghem NM, Popma JJ, et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2017;376:1321-1331.
11. Lala A, McNulty SE, Mentz RJ, et al. Relief and recurrence of congestion during and after hospitalization for acute heart failure: insights from Diuretic Optimization Strategy Evaluation in Acute Decompensated Heart Failure (DOSE-AHF) and Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARESS-HF). Circ Heart Fail. 2015;8:741-748.
12. Ambrosy AP, Pang PS, Khan S, et al. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: findings from the EVEREST trial. Eur Heart J. 2013;34:835-843.
13. Driscoll A, Meagher S, Kennedy R, et al. What is the impact of systems of care for heart failure on patients diagnosed with heart failure: a systematic review. BMC Cardiovasc Disord. 2016;16(1):195.
14. Ahmad FS, Wehbe RM, Kansal P, et al. Targeting the correct population when designing transitional care programs for medicare patients hospitalized with heart failure. JAMA Cardiol. 2017;2:1274-1275.
15. Blecker S, Sontag D, Horwitz LI, et al. Early identification of patients with acute decompensated heart failure. J Card Fail. 2018;24:357-362.
16. Lee J, Yoon W, Kim S, et al. BioBERT: a pre-trained biomedical language representation model for biomedical text mining. Bioinformatics. 2020;36:1234-1240.
17. Rice H, Say R, Betihavas V. The effect of nurse-led education on hospitalisation, readmission, quality of life and cost in adults with heart failure. A systematic review. Patient Educ Couns. 2018;101:363-374.
18. Hollenberg SM, Warner Stevenson L, Ahmad T, et al. 2019 ACC expert consensus decision pathway on risk assessment, management, and clinical trajectory of patients hospitalized with heart failure: A report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2019;74:1966-2011.
19. Tran RH, Aldemerdash A, Chang P, et al. Guideline-directed medical therapy and survival following hospitalization in patients with heart failure. Pharmacotherapy. 2018;38:406-416.
20. Greene SJ, Fonarow GC, DeVore AD, et al. Titration of medical therapy for heart failure with reduced ejection fraction. J Am Coll Cardiol. 2019;73:2365-2383.
21. Gattis WA, O’Connor CM, Gallup DS, et al;, IMPACT-HF Investigators and Coordinators. Predischarge initiation of carvedilol in patients hospitalized for decompensated heart failure: results of the Initiation Management Predischarge: Process for Assessment of Carvedilol Therapy in Heart Failure (IMPACT-HF) trial. J Am Coll Cardiol. 2004;43:1534-1541.
22. Graumlich JF, Novotny NL, Aldag JC. Brief scale measuring patient preparedness for hospital discharge to home: Psychometric properties. J Hosp Med. 2008;3:446-454.
23. Van Spall HGC, Lee SF, Xie F, et al. Effect of patient-centered transitional care services on clinical outcomes in patients hospitalized for heart failure: The PACT-HF Randomized Clinical Trial. JAMA. 2019;321:753-761.
24. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240-327.
25. Di Palo KE, Patel K, Assafin M, Piña IL. Implementation of a patient navigator program to reduce 30-day heart failure readmission rate. Prog Cardiovasc Dis. 2017;60:259-266.
26. Patil S, Shah M, Patel B, et al. Readmissions among patients admitted with acute decompensated heart failure based on income quartiles. Mayo Clin Proc. 2019;94:1939-1950.
27. Ahmad K, Chen EW, Nazir U, et al. Regional variation in the association of poverty and heart failure mortality in the 3135 counties of the united states. J Am Heart Assoc. 2019;8:e012422.
28. Bellon JE, Bilderback A, Ahuja-Yende NS, et al. University of Pittsburgh medical center home transitions multidisciplinary care coordination reduces readmissions for older adults. J Am Geriatr Soc. 2019;67:156-163.
29. Rosen D, McCall JD, Primack BA. Telehealth protocol to prevent readmission among high-risk patients with congestive heart failure. Am J Med. 2017;130:1326-1330.
30. Heywood JT, Jermyn R, Shavelle D, et al. Impact of practice-based management of pulmonary artery pressures in 2000 patients implanted with the CardioMEMS sensor. Circulation. 2017;135:1509-1517.
31. Abraham WT, Adamson PB, Bourge RC, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377:658-666.
32. Drozda JP, Smith DA, Freiman PC, et al. Heart failure readmission reduction. Am J Med Qual. 2017;32:134-140.
33. Malik AH, Malik SS, Aronow WS; MAGIC (Meta-analysis And oriGinal Investigation in Cardiology) investigators. Effect of home-based follow-up intervention on readmissions and mortality in heart failure patients: a meta-analysis. Future Cardiol. 2019;15:377-386.
34. Strano A, Briggs A, Powell N, et al. Home healthcare visits following hospital discharge: does the timing of visits affect 30-day hospital readmission rates for heart failure patients? Home Healthc Now. 2019;37:152-157.
35. DeVore AD, Cox M, Eapen ZJ, et al. Temporal trends and variation in early scheduled follow-up after a hospitalization for heart failure: findings from get with the guidelines-heart failure. Circ Heart Fail. 2016;9.
36. Baker H, Oliver-McNeil S, Deng L, Hummel SL. Regional hospital collaboration and outcomes in medicare heart failure patients: see you in 7. JACC Heart Fail. 2015;3:765-773.
37. Mutharasan RK, Ahmad FS, Gurvich I, et al. Buffer or suffer: redesigning heart failure postdischarge clinic using queuing theory. Circ Cardiovasc Qual Outcomes. 2018;11:e004351.
38. Ziaeian B, Hernandez AF, DeVore AD, et al. Long-term outcomes for heart failure patients with and without diabetes: From the Get With The Guidelines-Heart Failure Registry. Am Heart J. 2019;211:1-10.
39. Greene SJ, Butler J, Albert NM, et al. Medical therapy for heart failure with reduced ejection fraction: The CHAMP-HF Registry. J Am Coll Cardiol. 2018;72:351-366.
40. Dharmarajan K, Hsieh AF, Lin Z, et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309:355-363.
41. Ward L, Powell RE, Scharf ML, et al. Patient-centered specialty practice: defining the role of specialists in value-based health care. Chest. 2017;151:930-935.
42. Ryan JJ, Waxman AB. The dyspnea clinic. Circulation. 2018;137:1994-1996.
43. Oseran AS, Howard SE, Blumenthal DM. Factors associated with participation in cardiac episode payments included in medicare’s bundled payments for care improvement initiative. JAMA Cardiol. 2018;3:761-766.
44. Takeda A, Taylor SJC, Taylor RS, et al. Clinical service organisation for heart failure. Cochrane Database Syst Rev. 2012;(9):CD002752.
45. Albert NM, Barnason S, Deswal A, et al. Transitions of care in heart failure: a scientific statement from the American Heart Association. Circ Heart Fail. 2015;8:384-409.
46. Manifesto for Agile Software Development. http://agilemanifesto.org/ Accessed March 6, 2020.
From the Department of Medicine, Division of Cardiology, Northwestern University Feinberg School of Medicine, Chicago, IL.
Objective: To review selected process-of-care interventions that can be applied both during the hospitalization and during the transitional care period to help address the persistent challenge of heart failure readmissions.
Methods: Review of the literature.
Results: Process-of-care interventions that can be implemented to reduce readmissions of heart failure patients include: accurately identifying heart failure patients; providing disease education; titrating guideline-directed medical therapy; ensuring discharge readiness; arranging close discharge follow-up; identifying and addressing social barriers; following up by telephone; using home health; and addressing comorbidities. Importantly, the heart failure hospitalization is an opportunity to set up outpatient success, and setting up feedback loops can aid in post-discharge monitoring.
Conclusion: We encourage teams to consider local capabilities when selecting processes to improve; begin by improving something small to build capacity and team morale, and continually iterate and reexamine processes, as health care systems are continually evolving.
Keywords: heart failure; process improvement; quality improvement; readmission; rehospitalization; transitional care.
The growing population of patients affected by heart failure continues to challenge health systems. The increasing prevalence is paralleled by the rising costs of managing heart failure, which are projected to grow from $30.7 billion in 2012 to $69.8 billion in 2030.1 A significant portion of these costs relate to readmission after an index heart failure hospitalization. The statistics are staggering: for patients hospitalized with heart failure, approximately 15% to 20% are readmitted within 30 days.2,3 Though recent temporal trends suggest a modest reduction in readmission rates, there is a concerning correlation with increasing mortality,3 and a recognition that readmission rate decreases may relate to subtle changes in coding-based risk adjustment.4 Despite these concerns, efforts to reduce readmissions after heart failure hospitalization command significant attention.
Process improvement methodologies may be helpful in reducing hospital readmissions. Various approaches have been employed, and results have been mixed. An analysis of 70 participating hospitals in the American Heart Association’s Get With the Guidelines initiative found that, while overall readmission rates declined by 1.0% over 3 years, only 1 hospital achieved a 20% reduction in readmission rates.5
It is notably difficult to reduce readmissions after heart failure hospitalization. One challenge is that patients with heart failure often have multiple comorbidities, and approximately 50% to 60% of 30-day readmissions after heart failure hospitalization arise from noncardiac causes.1 Another challenge is that a significant fraction of readmissions in general—perhaps 75%—may not be avoidable.6
Recent excellent systematic reviews and meta-analyses provide comprehensive overviews of process improvement strategies that can be used to reduce readmissions after heart failure hospitalizations.7-9 Yet despite this extensive knowledge, few reports discuss the process of actually implementing these changes: the process of process improvement. Here, we seek to not only highlight some of the most promising potential interventions to reduce heart failure readmissions, but also to discuss a process improvement framework to help engender success, using our experience as a case study. We schematize process improvement efforts as having several distinct phases (Figure 1): processes delivered during the hospitalization and prior to discharge; feedback loops set up to maintain clinical stability at home; and the postdischarge clinic visit as an opportunity to further stabilize the patient and advance the plan of care. The discussion of these interventions follows this organization.

During Hospitalization
The heart failure hospitalization can be used as an opportunity to set up outpatient success, with several goals to target during the index admission. One goal is identifying the root causes of the heart failure syndrome and correcting those root causes, if possible. For example, patients in whom the heart failure syndrome is secondary to valvular heart disease may benefit from transcatheter aortic valve replacement.10 Another clinical goal is decongesting the patient, which is associated with lower readmission rates.11,12 These goals focus on the medical aspects of heart failure care. However, beyond these medical aspects, a patient must be equipped to successfully manage the disease at home.
To support medical and nonmedical interventions for hospitalized heart failure patients, a critical first step is identifying patients with heart failure. This accomplishes at least 2 objectives. First, early identification allows early initiation of interventions, such as heart failure education and social work evaluation. Early initiation of these interventions allows sufficient time during the hospitalization to make meaningful progress on these fronts. Second, early identification allows an opportunity for the delivery of cardiology specialty care, which may help with identifying and correcting root causes of the heart failure syndrome. Such access to cardiology has been shown to improve inpatient mortality and readmission rates.13
In smaller hospitals, identification of patients with heart failure can be as simple as reviewing overnight admissions. More advanced strategies, such as screeners based on brain natriuretic peptide (BNP) levels and administration of intravenous diuretics, can be employed.14,15 In the near future, deep learning-based natural language processing will be applied to mine full-text data in the electronic health record to identify heart failure hospitalizations.16
In the hospital, patients can also receive education about heart failure disease management. This education is a cornerstone of reducing heart failure readmissions. A recent systematic review of nurse education interventions demonstrated reductions in readmissions, hospitalizations, and costs.17 However, the efficacy of heart failure education hinges on many other variables. For patients to adhere to water restriction and daily weights, for example, there must also be patient understanding, compliance, and accessibility to providers to recommend how to strike the fluid balance. Education is therefore necessary, but not sufficient, for setting up outpatient success.
The hospitalization also represents an important time to start or uptitrate guideline-directed medical therapy (GDMT) for heart failure. Doing so takes advantage of an important opportunity to reduce the risk of readmission and even reverse the disease process.18 Uptitration of GDMT in patients with heart failure with reduced ejection fraction is associated with a decreased risk of mortality, while discontinuation is associated with an increased risk of mortality.19 However, recent registry data indicate that intensity of GDMT is just as likely to be decreased as increased during the hospitalization.20 Nevertheless, predischarge initiation of medications may be associated with higher attained doses in follow-up.21
Preparing for Discharge
Preparing a patient for discharge after a heart failure hospitalization involves stabilizing the medical condition as well as ensuring that the patient and caregivers have the medication, equipment, and self-care resources at home necessary to manage the condition. Several frameworks have been put forth to help care teams analyze a patient’s readiness for discharge. One is the B-PREPARED score,22 a validated instrument to discriminate among patients with regard to their readiness to discharge from the hospital. This instrument highlights the importance of several key factors that should be addressed during the discharge process, including counseling and written instructions about medications and their side effects; information about equipment needs and community resources; and information on activity levels and restrictions. Nurse education and discharge coordination can improve patients’ perception of discharge readiness,23 although whether this discharge readiness translates into improved readmission rates appears to depend on the specific follow-up intervention design.9
Prior to discharge, it is important to arrange postdischarge follow-up appointments, as emphasized by the American College of Cardiology/American Heart Association (ACC/AHA) guidelines.24 The use of nurse navigators can help with planning follow-up appointments. For example, the ACC Patient Navigator Program was applied in a single-center study of 120 patients randomized to the program versus usual care.25 This study found a significant increase in patient education and follow-up appointments compared to usual care, and a numerical decrease in hospital readmissions, although the finding was not statistically significant.25
A third critical component of preparing for discharge is identifying and addressing social barriers to care. In a study of patients stratified by household income, patients in the lowest income quartile had a higher readmission rate than patients in the highest income quartile.26 Poverty also correlates with heart failure mortality.27 Social factors play an important role in many aspects of patients’ ability to manage their health, including self-care, medication adherence, and ability to follow-up. Identifying these social factors prior to discharge is the first step to addressing them. While few studies specifically address the role of social workers in the management of heart failure care, the general medical literature suggests that social workers embedded in transitional care teams can augment readmission reduction efforts.28
After Discharge
Patients recently discharged from the hospital who have not yet attended their postdischarge appointment are in an incredibly vulnerable phase of care. Patients who are discharged from the hospital may not yet be connected with outpatient care. During this initial transitional care period, feedback loops involving patient communication back to the clinic, and clinic communication back to the patient, are critical to helping patients remain stable. For example, consider monitoring weights daily after hospital discharge. A patient at home can report increasing weights to a provider, who can then recommend an increased dose of diuretic. The patient can complete the feedback loop by taking the extra medication and monitoring the return of weight back to normal.
While daily weight monitoring is a simple process improvement that relies on the principle of establishing feedback loops, many other strategies exist. One commonly employed tool is the postdischarge telephone follow-up call, which is often coupled with other interventions in a comprehensive care bundle.8 During the telephone call, several process-of-care defects can be corrected, including missing medications or missing information on appointment times.
Beyond the telephone, newer technologies show promise for helping develop feedback loops for patients at home. One such technology is telemonitoring, whereby physiologic information such as weight, heart rate, and blood pressure is collected and sent back to a monitoring center. While the principle holds promise, several studies have not demonstrated significantly different outcomes as compared to usual care.13,29 Another promising technology is the CardioMEMS device (Abbott, Inc., Atlanta, GA), which can remotely transmit the pulmonary artery pressure, a physiologic signal which correlates with volume overload. There is now strong evidence supporting the efficacy of pulmonary artery pressure–guided heart failure management.30,31
Finally, home visits can be an efficient way to communicate symptoms, enable clinical assessment, and provide recommendations. One program that implemented home visits, 24-hour nurses available by call, and telephone follow-up showed a statistically significant reduction in readmissions.32 Furthermore, a meta-analysis of randomized controlled trials comparing home health to usual care showed decreased readmissions and mortality.33 The efficacy may be in strengthening the feedback loop—home care improves compliance with weight monitoring, fluid restriction, and medications.34 These studies provide a strong rationale for the benefits of home health in stabilizing heart failure patients postdischarge. Indeed, nurse home visits were 1 of the 2 process interventions in a Cochrane review of randomized controlled trials that were shown to statistically significantly decrease readmissions and mortality.9 These data underscore the importance of feedback loops for helping ensure patients are clinically stable.
Postdischarge Follow-Up Clinic Visit
The first clinic appointment postdischarge is an important check-in to help advance patient care. Several key tasks can be achieved during the postdischarge visit. First, the patient can be clinically stabilized by adjusting diuretic therapy. If the patient is clinically stable, GDMT can be uptitrated. Second, education around symptoms, medications, diet, and exercise can be reinforced. Finally, clinicians can help connect patients to other members of the multidisciplinary care team, including specialist care, home health, or cardiac rehabilitation.
Achieving 7-day follow-up visits after discharge has been a point of emphasis in national guidelines.24 The ACC promotes a “See You in 7” challenge, advising that all patients discharged with a diagnosis of heart failure have a follow-up appointment within 7 days. Yet based on the latest available data, arrival rates to the postdischarge clinic are dismal, hovering around 30%.35 In a multicenter observational study of hospitals participating in the “See You in 7” collaborative, hospitals were able to increase their 7-day follow-up appointment rates by 2% to 3%, and also noted an absolute decrease in readmission rates by 1% to 2%.36 We have demonstrated, using a mathematical approach called queuing theory, that discharge appointment wait times and clinic access can be significantly improved by providing a modest capacity buffer to clinic availability.37 Those interested in applying this model to their own clinical practice may do so with a free online calculator at http://hfresearch.org.
Another important aspect of postdischarge follow-up is appropriate management of the comorbidity burden, which, as noted, is often significant in patients hospitalized with heart failure.38 For instance, in recent cohorts of hospitalized heart failure patients, the incidence of hypertension was 78%, coronary artery disease was more than 50%, atrial fibrillation was more than 40%, and diabetes was nearly 40%.39 Given this burden of comorbidity, it is not surprising that only 35% of readmissions after an index heart failure hospitalization are for recurrent heart failure.40 Coordinating care among primary care physicians and relevant subspecialists is thus essential. Phone calls and secure electronic messages are very helpful in achieving this. There is increasing interest in more nimble care models, such as the patient-centered specialty practice41 or the dyspnea clinic, to help bring coordinated resources to the patient.42
Process of Process Improvement: Our Experiences
The previous sections outline a series of potential process improvements clinical teams and health systems can implement to impact heart failure readmissions. A plan on paper, however, does not equal a plan in actuality. How does one go about implementing these changes? We offer our local experience starting a heart failure transitional care program as a case study, then draw lessons learned as a set of practical tips for local teams to employ. What we hope to highlight is that there is a large difference between a completed process for transitional care of heart failure patients, and the process of developing that process itself. The former is the hardware, the latter is the software. The latter does not typically get highlighted, but it is absolutely critical to unlocking the capabilities of a team and the institution.
In 2015, Northwestern Memorial Hospital adopted a novel payment arrangement from the Center for Medicare and Medicaid Services for Medicare patients being discharged from the hospital with heart failure. Known as Bundled Payments for Care Improvement,43 this bundled payment model incentivized Northwestern Memorial Hospital charge, principally by reducing hospital readmissions and by collaborating with skilled nursing facilities to control length of stay.
We approached this problem by drawing on the available literature,44,45 and by first creating a schematic of our high-level approach, which comprised 3 major elements (Figure 2): identification of hospitalized heart failure patients, delivery of a care bundle to hospitalized heart failure patients in hospital, and coordinating postdischarge care, centered on a telephone call and a postdischarge visit.

We then proceeded by building out, in stepwise fashion, each component of our value chain, using Agile techniques as a guiding principle.46 Agile, a productivity and process improvement mindset with roots in software development, emphasizes tackling 1 problem at a time, building out new features sequentially and completely, recognizing that the end user does not derive value from a program until new functionality is available for use. Rather than wholesale monolithic change, Agile emphasizes rapid iteration, prototyping, and discarding innovations not found to be helpful. The notion is to stand up new, incremental features rapidly, with each incremental improvement delivering value and helping to accelerate overall change.
Our experience building a robust way to identify heart failure cases is a good example of Agile process improvement in practice. At our hospital, identification of patients with heart failure was a challenge because more than half of heart failure patients are admitted to noncardiology floors. We developed a simple electronic health record query to detect heart failure patients, relying on parameters such as administration of intravenous diuretic or levels of BNP exceeding 100 ng/dL. We deployed this query, finding very high sensitivity for detection of heart failure patients.14 Patients found to have heart failure were then populated into a list in the electronic health record, which made patients’ heart failure status visible to all members of the health care team. Using this list, we were able to automate several processes necessary for heart failure care. For example, the list made it possible for cardiologists to know if there was a patient who perhaps needed cardiology consultation. Nurse navigators could know which patients needed heart failure education without having to be actively consulted by the admitting team. The same nurse navigators could then know upon discharge which patients needed a follow-up telephone call at 48 hours.
This list of heart failure patients was the end product, which was built through prototyping and iteration. For example, with our initial BNP cutoff of 300 ng/dL, we recognized we were missing several cases, and lowered the cutoff for the screener to 100 ng/dL. When we were satisfied this process was working well, we moved on to the next problem to tackle, avoiding trying to work on too many things at once. By doing so, we were able to focus our process improvement resources on 1 problem at a time, building up a suite of interventions. For our hospital, we settled on a bundle of interventions, captured by the mnemonic HEART:
Heart doctor sees patient in the hospital
Education about heart failure in the hospital
After-visit summary with 7-day appointment printed
Reach out to the patient by telephone within 72 hours
Treat the patient in clinic by the 7-day visit
Conclusion
We would like to emphasize that the elements of our heart failure readmissions interventions were not all put in place at once. This was an iterative process that proceeded in a stepwise fashion, with each step improving the care of our patients. We learned a number of lessons from our experience. First, we would advise that teams not try to do everything. One program simply cannot implement all possible readmission reduction interventions, and certainly not all at once. Trade-offs should be made, and interventions more likely to succeed in the local environment should be prioritized. In addition, interventions that do not fit and do not create synergy with the local practice environment should not be pursued.
Second, we would advise teams to start small, tackling a known problem in heart failure transitions of care first. This initial intuition is often right. An example might be improving 7-day appointments upon discharge. Starting with a problem that can be tackled builds process improvement muscle and improves team morale. Third, we would advise teams to consistently iterate on designs, tweaking and improving performance. Complex organizations always evolve; processes that work 1 year may fail the next because another element of the organization may have changed.
Finally, the framework presented in Figure 1 may be helpful in guiding how to structure interventions. Considering interventions to be delivered in the hospital, interventions to be delivered in the clinic, and how to set up feedback loops to support patients as outpatients help develop a comprehensive heart failure readmissions reduction program.
Corresponding author: R. Kannan Mutharasan, MD, Northwestern University Feinberg School of Medicine, 676 North Saint Clair St., Arkes Pavilion, Suite 7-038, Chicago, IL 60611;[email protected].
Financial disclosures: None.
From the Department of Medicine, Division of Cardiology, Northwestern University Feinberg School of Medicine, Chicago, IL.
Objective: To review selected process-of-care interventions that can be applied both during the hospitalization and during the transitional care period to help address the persistent challenge of heart failure readmissions.
Methods: Review of the literature.
Results: Process-of-care interventions that can be implemented to reduce readmissions of heart failure patients include: accurately identifying heart failure patients; providing disease education; titrating guideline-directed medical therapy; ensuring discharge readiness; arranging close discharge follow-up; identifying and addressing social barriers; following up by telephone; using home health; and addressing comorbidities. Importantly, the heart failure hospitalization is an opportunity to set up outpatient success, and setting up feedback loops can aid in post-discharge monitoring.
Conclusion: We encourage teams to consider local capabilities when selecting processes to improve; begin by improving something small to build capacity and team morale, and continually iterate and reexamine processes, as health care systems are continually evolving.
Keywords: heart failure; process improvement; quality improvement; readmission; rehospitalization; transitional care.
The growing population of patients affected by heart failure continues to challenge health systems. The increasing prevalence is paralleled by the rising costs of managing heart failure, which are projected to grow from $30.7 billion in 2012 to $69.8 billion in 2030.1 A significant portion of these costs relate to readmission after an index heart failure hospitalization. The statistics are staggering: for patients hospitalized with heart failure, approximately 15% to 20% are readmitted within 30 days.2,3 Though recent temporal trends suggest a modest reduction in readmission rates, there is a concerning correlation with increasing mortality,3 and a recognition that readmission rate decreases may relate to subtle changes in coding-based risk adjustment.4 Despite these concerns, efforts to reduce readmissions after heart failure hospitalization command significant attention.
Process improvement methodologies may be helpful in reducing hospital readmissions. Various approaches have been employed, and results have been mixed. An analysis of 70 participating hospitals in the American Heart Association’s Get With the Guidelines initiative found that, while overall readmission rates declined by 1.0% over 3 years, only 1 hospital achieved a 20% reduction in readmission rates.5
It is notably difficult to reduce readmissions after heart failure hospitalization. One challenge is that patients with heart failure often have multiple comorbidities, and approximately 50% to 60% of 30-day readmissions after heart failure hospitalization arise from noncardiac causes.1 Another challenge is that a significant fraction of readmissions in general—perhaps 75%—may not be avoidable.6
Recent excellent systematic reviews and meta-analyses provide comprehensive overviews of process improvement strategies that can be used to reduce readmissions after heart failure hospitalizations.7-9 Yet despite this extensive knowledge, few reports discuss the process of actually implementing these changes: the process of process improvement. Here, we seek to not only highlight some of the most promising potential interventions to reduce heart failure readmissions, but also to discuss a process improvement framework to help engender success, using our experience as a case study. We schematize process improvement efforts as having several distinct phases (Figure 1): processes delivered during the hospitalization and prior to discharge; feedback loops set up to maintain clinical stability at home; and the postdischarge clinic visit as an opportunity to further stabilize the patient and advance the plan of care. The discussion of these interventions follows this organization.

During Hospitalization
The heart failure hospitalization can be used as an opportunity to set up outpatient success, with several goals to target during the index admission. One goal is identifying the root causes of the heart failure syndrome and correcting those root causes, if possible. For example, patients in whom the heart failure syndrome is secondary to valvular heart disease may benefit from transcatheter aortic valve replacement.10 Another clinical goal is decongesting the patient, which is associated with lower readmission rates.11,12 These goals focus on the medical aspects of heart failure care. However, beyond these medical aspects, a patient must be equipped to successfully manage the disease at home.
To support medical and nonmedical interventions for hospitalized heart failure patients, a critical first step is identifying patients with heart failure. This accomplishes at least 2 objectives. First, early identification allows early initiation of interventions, such as heart failure education and social work evaluation. Early initiation of these interventions allows sufficient time during the hospitalization to make meaningful progress on these fronts. Second, early identification allows an opportunity for the delivery of cardiology specialty care, which may help with identifying and correcting root causes of the heart failure syndrome. Such access to cardiology has been shown to improve inpatient mortality and readmission rates.13
In smaller hospitals, identification of patients with heart failure can be as simple as reviewing overnight admissions. More advanced strategies, such as screeners based on brain natriuretic peptide (BNP) levels and administration of intravenous diuretics, can be employed.14,15 In the near future, deep learning-based natural language processing will be applied to mine full-text data in the electronic health record to identify heart failure hospitalizations.16
In the hospital, patients can also receive education about heart failure disease management. This education is a cornerstone of reducing heart failure readmissions. A recent systematic review of nurse education interventions demonstrated reductions in readmissions, hospitalizations, and costs.17 However, the efficacy of heart failure education hinges on many other variables. For patients to adhere to water restriction and daily weights, for example, there must also be patient understanding, compliance, and accessibility to providers to recommend how to strike the fluid balance. Education is therefore necessary, but not sufficient, for setting up outpatient success.
The hospitalization also represents an important time to start or uptitrate guideline-directed medical therapy (GDMT) for heart failure. Doing so takes advantage of an important opportunity to reduce the risk of readmission and even reverse the disease process.18 Uptitration of GDMT in patients with heart failure with reduced ejection fraction is associated with a decreased risk of mortality, while discontinuation is associated with an increased risk of mortality.19 However, recent registry data indicate that intensity of GDMT is just as likely to be decreased as increased during the hospitalization.20 Nevertheless, predischarge initiation of medications may be associated with higher attained doses in follow-up.21
Preparing for Discharge
Preparing a patient for discharge after a heart failure hospitalization involves stabilizing the medical condition as well as ensuring that the patient and caregivers have the medication, equipment, and self-care resources at home necessary to manage the condition. Several frameworks have been put forth to help care teams analyze a patient’s readiness for discharge. One is the B-PREPARED score,22 a validated instrument to discriminate among patients with regard to their readiness to discharge from the hospital. This instrument highlights the importance of several key factors that should be addressed during the discharge process, including counseling and written instructions about medications and their side effects; information about equipment needs and community resources; and information on activity levels and restrictions. Nurse education and discharge coordination can improve patients’ perception of discharge readiness,23 although whether this discharge readiness translates into improved readmission rates appears to depend on the specific follow-up intervention design.9
Prior to discharge, it is important to arrange postdischarge follow-up appointments, as emphasized by the American College of Cardiology/American Heart Association (ACC/AHA) guidelines.24 The use of nurse navigators can help with planning follow-up appointments. For example, the ACC Patient Navigator Program was applied in a single-center study of 120 patients randomized to the program versus usual care.25 This study found a significant increase in patient education and follow-up appointments compared to usual care, and a numerical decrease in hospital readmissions, although the finding was not statistically significant.25
A third critical component of preparing for discharge is identifying and addressing social barriers to care. In a study of patients stratified by household income, patients in the lowest income quartile had a higher readmission rate than patients in the highest income quartile.26 Poverty also correlates with heart failure mortality.27 Social factors play an important role in many aspects of patients’ ability to manage their health, including self-care, medication adherence, and ability to follow-up. Identifying these social factors prior to discharge is the first step to addressing them. While few studies specifically address the role of social workers in the management of heart failure care, the general medical literature suggests that social workers embedded in transitional care teams can augment readmission reduction efforts.28
After Discharge
Patients recently discharged from the hospital who have not yet attended their postdischarge appointment are in an incredibly vulnerable phase of care. Patients who are discharged from the hospital may not yet be connected with outpatient care. During this initial transitional care period, feedback loops involving patient communication back to the clinic, and clinic communication back to the patient, are critical to helping patients remain stable. For example, consider monitoring weights daily after hospital discharge. A patient at home can report increasing weights to a provider, who can then recommend an increased dose of diuretic. The patient can complete the feedback loop by taking the extra medication and monitoring the return of weight back to normal.
While daily weight monitoring is a simple process improvement that relies on the principle of establishing feedback loops, many other strategies exist. One commonly employed tool is the postdischarge telephone follow-up call, which is often coupled with other interventions in a comprehensive care bundle.8 During the telephone call, several process-of-care defects can be corrected, including missing medications or missing information on appointment times.
Beyond the telephone, newer technologies show promise for helping develop feedback loops for patients at home. One such technology is telemonitoring, whereby physiologic information such as weight, heart rate, and blood pressure is collected and sent back to a monitoring center. While the principle holds promise, several studies have not demonstrated significantly different outcomes as compared to usual care.13,29 Another promising technology is the CardioMEMS device (Abbott, Inc., Atlanta, GA), which can remotely transmit the pulmonary artery pressure, a physiologic signal which correlates with volume overload. There is now strong evidence supporting the efficacy of pulmonary artery pressure–guided heart failure management.30,31
Finally, home visits can be an efficient way to communicate symptoms, enable clinical assessment, and provide recommendations. One program that implemented home visits, 24-hour nurses available by call, and telephone follow-up showed a statistically significant reduction in readmissions.32 Furthermore, a meta-analysis of randomized controlled trials comparing home health to usual care showed decreased readmissions and mortality.33 The efficacy may be in strengthening the feedback loop—home care improves compliance with weight monitoring, fluid restriction, and medications.34 These studies provide a strong rationale for the benefits of home health in stabilizing heart failure patients postdischarge. Indeed, nurse home visits were 1 of the 2 process interventions in a Cochrane review of randomized controlled trials that were shown to statistically significantly decrease readmissions and mortality.9 These data underscore the importance of feedback loops for helping ensure patients are clinically stable.
Postdischarge Follow-Up Clinic Visit
The first clinic appointment postdischarge is an important check-in to help advance patient care. Several key tasks can be achieved during the postdischarge visit. First, the patient can be clinically stabilized by adjusting diuretic therapy. If the patient is clinically stable, GDMT can be uptitrated. Second, education around symptoms, medications, diet, and exercise can be reinforced. Finally, clinicians can help connect patients to other members of the multidisciplinary care team, including specialist care, home health, or cardiac rehabilitation.
Achieving 7-day follow-up visits after discharge has been a point of emphasis in national guidelines.24 The ACC promotes a “See You in 7” challenge, advising that all patients discharged with a diagnosis of heart failure have a follow-up appointment within 7 days. Yet based on the latest available data, arrival rates to the postdischarge clinic are dismal, hovering around 30%.35 In a multicenter observational study of hospitals participating in the “See You in 7” collaborative, hospitals were able to increase their 7-day follow-up appointment rates by 2% to 3%, and also noted an absolute decrease in readmission rates by 1% to 2%.36 We have demonstrated, using a mathematical approach called queuing theory, that discharge appointment wait times and clinic access can be significantly improved by providing a modest capacity buffer to clinic availability.37 Those interested in applying this model to their own clinical practice may do so with a free online calculator at http://hfresearch.org.
Another important aspect of postdischarge follow-up is appropriate management of the comorbidity burden, which, as noted, is often significant in patients hospitalized with heart failure.38 For instance, in recent cohorts of hospitalized heart failure patients, the incidence of hypertension was 78%, coronary artery disease was more than 50%, atrial fibrillation was more than 40%, and diabetes was nearly 40%.39 Given this burden of comorbidity, it is not surprising that only 35% of readmissions after an index heart failure hospitalization are for recurrent heart failure.40 Coordinating care among primary care physicians and relevant subspecialists is thus essential. Phone calls and secure electronic messages are very helpful in achieving this. There is increasing interest in more nimble care models, such as the patient-centered specialty practice41 or the dyspnea clinic, to help bring coordinated resources to the patient.42
Process of Process Improvement: Our Experiences
The previous sections outline a series of potential process improvements clinical teams and health systems can implement to impact heart failure readmissions. A plan on paper, however, does not equal a plan in actuality. How does one go about implementing these changes? We offer our local experience starting a heart failure transitional care program as a case study, then draw lessons learned as a set of practical tips for local teams to employ. What we hope to highlight is that there is a large difference between a completed process for transitional care of heart failure patients, and the process of developing that process itself. The former is the hardware, the latter is the software. The latter does not typically get highlighted, but it is absolutely critical to unlocking the capabilities of a team and the institution.
In 2015, Northwestern Memorial Hospital adopted a novel payment arrangement from the Center for Medicare and Medicaid Services for Medicare patients being discharged from the hospital with heart failure. Known as Bundled Payments for Care Improvement,43 this bundled payment model incentivized Northwestern Memorial Hospital charge, principally by reducing hospital readmissions and by collaborating with skilled nursing facilities to control length of stay.
We approached this problem by drawing on the available literature,44,45 and by first creating a schematic of our high-level approach, which comprised 3 major elements (Figure 2): identification of hospitalized heart failure patients, delivery of a care bundle to hospitalized heart failure patients in hospital, and coordinating postdischarge care, centered on a telephone call and a postdischarge visit.

We then proceeded by building out, in stepwise fashion, each component of our value chain, using Agile techniques as a guiding principle.46 Agile, a productivity and process improvement mindset with roots in software development, emphasizes tackling 1 problem at a time, building out new features sequentially and completely, recognizing that the end user does not derive value from a program until new functionality is available for use. Rather than wholesale monolithic change, Agile emphasizes rapid iteration, prototyping, and discarding innovations not found to be helpful. The notion is to stand up new, incremental features rapidly, with each incremental improvement delivering value and helping to accelerate overall change.
Our experience building a robust way to identify heart failure cases is a good example of Agile process improvement in practice. At our hospital, identification of patients with heart failure was a challenge because more than half of heart failure patients are admitted to noncardiology floors. We developed a simple electronic health record query to detect heart failure patients, relying on parameters such as administration of intravenous diuretic or levels of BNP exceeding 100 ng/dL. We deployed this query, finding very high sensitivity for detection of heart failure patients.14 Patients found to have heart failure were then populated into a list in the electronic health record, which made patients’ heart failure status visible to all members of the health care team. Using this list, we were able to automate several processes necessary for heart failure care. For example, the list made it possible for cardiologists to know if there was a patient who perhaps needed cardiology consultation. Nurse navigators could know which patients needed heart failure education without having to be actively consulted by the admitting team. The same nurse navigators could then know upon discharge which patients needed a follow-up telephone call at 48 hours.
This list of heart failure patients was the end product, which was built through prototyping and iteration. For example, with our initial BNP cutoff of 300 ng/dL, we recognized we were missing several cases, and lowered the cutoff for the screener to 100 ng/dL. When we were satisfied this process was working well, we moved on to the next problem to tackle, avoiding trying to work on too many things at once. By doing so, we were able to focus our process improvement resources on 1 problem at a time, building up a suite of interventions. For our hospital, we settled on a bundle of interventions, captured by the mnemonic HEART:
Heart doctor sees patient in the hospital
Education about heart failure in the hospital
After-visit summary with 7-day appointment printed
Reach out to the patient by telephone within 72 hours
Treat the patient in clinic by the 7-day visit
Conclusion
We would like to emphasize that the elements of our heart failure readmissions interventions were not all put in place at once. This was an iterative process that proceeded in a stepwise fashion, with each step improving the care of our patients. We learned a number of lessons from our experience. First, we would advise that teams not try to do everything. One program simply cannot implement all possible readmission reduction interventions, and certainly not all at once. Trade-offs should be made, and interventions more likely to succeed in the local environment should be prioritized. In addition, interventions that do not fit and do not create synergy with the local practice environment should not be pursued.
Second, we would advise teams to start small, tackling a known problem in heart failure transitions of care first. This initial intuition is often right. An example might be improving 7-day appointments upon discharge. Starting with a problem that can be tackled builds process improvement muscle and improves team morale. Third, we would advise teams to consistently iterate on designs, tweaking and improving performance. Complex organizations always evolve; processes that work 1 year may fail the next because another element of the organization may have changed.
Finally, the framework presented in Figure 1 may be helpful in guiding how to structure interventions. Considering interventions to be delivered in the hospital, interventions to be delivered in the clinic, and how to set up feedback loops to support patients as outpatients help develop a comprehensive heart failure readmissions reduction program.
Corresponding author: R. Kannan Mutharasan, MD, Northwestern University Feinberg School of Medicine, 676 North Saint Clair St., Arkes Pavilion, Suite 7-038, Chicago, IL 60611;[email protected].
Financial disclosures: None.
1. Ziaeian B, Fonarow GC. The prevention of hospital readmissions in heart failure. Prog Cardiovasc Dis. 2016;58:379-385.
2. Kwok CS, Seferovic PM, Van Spall HG, et al. Early unplanned readmissions after admission to hospital with heart failure. Am J Cardiol. 2019;124:736-745.
3. Fonarow GC, Konstam MA, Yancy CW. The hospital readmission reduction program is associated with fewer readmissions, more deaths: time to reconsider. J Am Coll Cardiol. 2017;70:1931-1934.
4. Ody C, Msall L, Dafny LS, et al. Decreases in readmissions credited to medicare’s program to reduce hospital readmissions have been overstated. Health Aff (Millwood). 2019;38:36-43.
5. Bergethon KE, Ju C, DeVore AD, et al. Trends in 30-day readmission rates for patients hospitalized with heart failure: findings from the Get With The Guidelines-Heart Failure Registry. Circ Heart Fail. 2016;9.
6. van Walraven C, Jennings A, Forster AJ. A meta-analysis of hospital 30-day avoidable readmission rates. J Eval Clin Pract. 2012;18(6):1211-1218.
7. Albert NM. A systematic review of transitional-care strategies to reduce rehospitalization in patients with heart failure. Heart Lung. 2016;45:100-113.
8. Takeda A, Martin N, Taylor RS, Taylor SJ. Disease management interventions for heart failure. Cochrane Database Syst Rev. 2019;1:CD002752.
9. Van Spall HGC, Rahman T, Mytton O, et al. Comparative effectiveness of transitional care services in patients discharged from the hospital with heart failure: a systematic review and network meta-analysis. Eur J Heart Fail. 2017;19:1427-1443.
10. Reardon MJ, Van Mieghem NM, Popma JJ, et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2017;376:1321-1331.
11. Lala A, McNulty SE, Mentz RJ, et al. Relief and recurrence of congestion during and after hospitalization for acute heart failure: insights from Diuretic Optimization Strategy Evaluation in Acute Decompensated Heart Failure (DOSE-AHF) and Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARESS-HF). Circ Heart Fail. 2015;8:741-748.
12. Ambrosy AP, Pang PS, Khan S, et al. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: findings from the EVEREST trial. Eur Heart J. 2013;34:835-843.
13. Driscoll A, Meagher S, Kennedy R, et al. What is the impact of systems of care for heart failure on patients diagnosed with heart failure: a systematic review. BMC Cardiovasc Disord. 2016;16(1):195.
14. Ahmad FS, Wehbe RM, Kansal P, et al. Targeting the correct population when designing transitional care programs for medicare patients hospitalized with heart failure. JAMA Cardiol. 2017;2:1274-1275.
15. Blecker S, Sontag D, Horwitz LI, et al. Early identification of patients with acute decompensated heart failure. J Card Fail. 2018;24:357-362.
16. Lee J, Yoon W, Kim S, et al. BioBERT: a pre-trained biomedical language representation model for biomedical text mining. Bioinformatics. 2020;36:1234-1240.
17. Rice H, Say R, Betihavas V. The effect of nurse-led education on hospitalisation, readmission, quality of life and cost in adults with heart failure. A systematic review. Patient Educ Couns. 2018;101:363-374.
18. Hollenberg SM, Warner Stevenson L, Ahmad T, et al. 2019 ACC expert consensus decision pathway on risk assessment, management, and clinical trajectory of patients hospitalized with heart failure: A report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2019;74:1966-2011.
19. Tran RH, Aldemerdash A, Chang P, et al. Guideline-directed medical therapy and survival following hospitalization in patients with heart failure. Pharmacotherapy. 2018;38:406-416.
20. Greene SJ, Fonarow GC, DeVore AD, et al. Titration of medical therapy for heart failure with reduced ejection fraction. J Am Coll Cardiol. 2019;73:2365-2383.
21. Gattis WA, O’Connor CM, Gallup DS, et al;, IMPACT-HF Investigators and Coordinators. Predischarge initiation of carvedilol in patients hospitalized for decompensated heart failure: results of the Initiation Management Predischarge: Process for Assessment of Carvedilol Therapy in Heart Failure (IMPACT-HF) trial. J Am Coll Cardiol. 2004;43:1534-1541.
22. Graumlich JF, Novotny NL, Aldag JC. Brief scale measuring patient preparedness for hospital discharge to home: Psychometric properties. J Hosp Med. 2008;3:446-454.
23. Van Spall HGC, Lee SF, Xie F, et al. Effect of patient-centered transitional care services on clinical outcomes in patients hospitalized for heart failure: The PACT-HF Randomized Clinical Trial. JAMA. 2019;321:753-761.
24. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240-327.
25. Di Palo KE, Patel K, Assafin M, Piña IL. Implementation of a patient navigator program to reduce 30-day heart failure readmission rate. Prog Cardiovasc Dis. 2017;60:259-266.
26. Patil S, Shah M, Patel B, et al. Readmissions among patients admitted with acute decompensated heart failure based on income quartiles. Mayo Clin Proc. 2019;94:1939-1950.
27. Ahmad K, Chen EW, Nazir U, et al. Regional variation in the association of poverty and heart failure mortality in the 3135 counties of the united states. J Am Heart Assoc. 2019;8:e012422.
28. Bellon JE, Bilderback A, Ahuja-Yende NS, et al. University of Pittsburgh medical center home transitions multidisciplinary care coordination reduces readmissions for older adults. J Am Geriatr Soc. 2019;67:156-163.
29. Rosen D, McCall JD, Primack BA. Telehealth protocol to prevent readmission among high-risk patients with congestive heart failure. Am J Med. 2017;130:1326-1330.
30. Heywood JT, Jermyn R, Shavelle D, et al. Impact of practice-based management of pulmonary artery pressures in 2000 patients implanted with the CardioMEMS sensor. Circulation. 2017;135:1509-1517.
31. Abraham WT, Adamson PB, Bourge RC, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377:658-666.
32. Drozda JP, Smith DA, Freiman PC, et al. Heart failure readmission reduction. Am J Med Qual. 2017;32:134-140.
33. Malik AH, Malik SS, Aronow WS; MAGIC (Meta-analysis And oriGinal Investigation in Cardiology) investigators. Effect of home-based follow-up intervention on readmissions and mortality in heart failure patients: a meta-analysis. Future Cardiol. 2019;15:377-386.
34. Strano A, Briggs A, Powell N, et al. Home healthcare visits following hospital discharge: does the timing of visits affect 30-day hospital readmission rates for heart failure patients? Home Healthc Now. 2019;37:152-157.
35. DeVore AD, Cox M, Eapen ZJ, et al. Temporal trends and variation in early scheduled follow-up after a hospitalization for heart failure: findings from get with the guidelines-heart failure. Circ Heart Fail. 2016;9.
36. Baker H, Oliver-McNeil S, Deng L, Hummel SL. Regional hospital collaboration and outcomes in medicare heart failure patients: see you in 7. JACC Heart Fail. 2015;3:765-773.
37. Mutharasan RK, Ahmad FS, Gurvich I, et al. Buffer or suffer: redesigning heart failure postdischarge clinic using queuing theory. Circ Cardiovasc Qual Outcomes. 2018;11:e004351.
38. Ziaeian B, Hernandez AF, DeVore AD, et al. Long-term outcomes for heart failure patients with and without diabetes: From the Get With The Guidelines-Heart Failure Registry. Am Heart J. 2019;211:1-10.
39. Greene SJ, Butler J, Albert NM, et al. Medical therapy for heart failure with reduced ejection fraction: The CHAMP-HF Registry. J Am Coll Cardiol. 2018;72:351-366.
40. Dharmarajan K, Hsieh AF, Lin Z, et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309:355-363.
41. Ward L, Powell RE, Scharf ML, et al. Patient-centered specialty practice: defining the role of specialists in value-based health care. Chest. 2017;151:930-935.
42. Ryan JJ, Waxman AB. The dyspnea clinic. Circulation. 2018;137:1994-1996.
43. Oseran AS, Howard SE, Blumenthal DM. Factors associated with participation in cardiac episode payments included in medicare’s bundled payments for care improvement initiative. JAMA Cardiol. 2018;3:761-766.
44. Takeda A, Taylor SJC, Taylor RS, et al. Clinical service organisation for heart failure. Cochrane Database Syst Rev. 2012;(9):CD002752.
45. Albert NM, Barnason S, Deswal A, et al. Transitions of care in heart failure: a scientific statement from the American Heart Association. Circ Heart Fail. 2015;8:384-409.
46. Manifesto for Agile Software Development. http://agilemanifesto.org/ Accessed March 6, 2020.
1. Ziaeian B, Fonarow GC. The prevention of hospital readmissions in heart failure. Prog Cardiovasc Dis. 2016;58:379-385.
2. Kwok CS, Seferovic PM, Van Spall HG, et al. Early unplanned readmissions after admission to hospital with heart failure. Am J Cardiol. 2019;124:736-745.
3. Fonarow GC, Konstam MA, Yancy CW. The hospital readmission reduction program is associated with fewer readmissions, more deaths: time to reconsider. J Am Coll Cardiol. 2017;70:1931-1934.
4. Ody C, Msall L, Dafny LS, et al. Decreases in readmissions credited to medicare’s program to reduce hospital readmissions have been overstated. Health Aff (Millwood). 2019;38:36-43.
5. Bergethon KE, Ju C, DeVore AD, et al. Trends in 30-day readmission rates for patients hospitalized with heart failure: findings from the Get With The Guidelines-Heart Failure Registry. Circ Heart Fail. 2016;9.
6. van Walraven C, Jennings A, Forster AJ. A meta-analysis of hospital 30-day avoidable readmission rates. J Eval Clin Pract. 2012;18(6):1211-1218.
7. Albert NM. A systematic review of transitional-care strategies to reduce rehospitalization in patients with heart failure. Heart Lung. 2016;45:100-113.
8. Takeda A, Martin N, Taylor RS, Taylor SJ. Disease management interventions for heart failure. Cochrane Database Syst Rev. 2019;1:CD002752.
9. Van Spall HGC, Rahman T, Mytton O, et al. Comparative effectiveness of transitional care services in patients discharged from the hospital with heart failure: a systematic review and network meta-analysis. Eur J Heart Fail. 2017;19:1427-1443.
10. Reardon MJ, Van Mieghem NM, Popma JJ, et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2017;376:1321-1331.
11. Lala A, McNulty SE, Mentz RJ, et al. Relief and recurrence of congestion during and after hospitalization for acute heart failure: insights from Diuretic Optimization Strategy Evaluation in Acute Decompensated Heart Failure (DOSE-AHF) and Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARESS-HF). Circ Heart Fail. 2015;8:741-748.
12. Ambrosy AP, Pang PS, Khan S, et al. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: findings from the EVEREST trial. Eur Heart J. 2013;34:835-843.
13. Driscoll A, Meagher S, Kennedy R, et al. What is the impact of systems of care for heart failure on patients diagnosed with heart failure: a systematic review. BMC Cardiovasc Disord. 2016;16(1):195.
14. Ahmad FS, Wehbe RM, Kansal P, et al. Targeting the correct population when designing transitional care programs for medicare patients hospitalized with heart failure. JAMA Cardiol. 2017;2:1274-1275.
15. Blecker S, Sontag D, Horwitz LI, et al. Early identification of patients with acute decompensated heart failure. J Card Fail. 2018;24:357-362.
16. Lee J, Yoon W, Kim S, et al. BioBERT: a pre-trained biomedical language representation model for biomedical text mining. Bioinformatics. 2020;36:1234-1240.
17. Rice H, Say R, Betihavas V. The effect of nurse-led education on hospitalisation, readmission, quality of life and cost in adults with heart failure. A systematic review. Patient Educ Couns. 2018;101:363-374.
18. Hollenberg SM, Warner Stevenson L, Ahmad T, et al. 2019 ACC expert consensus decision pathway on risk assessment, management, and clinical trajectory of patients hospitalized with heart failure: A report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2019;74:1966-2011.
19. Tran RH, Aldemerdash A, Chang P, et al. Guideline-directed medical therapy and survival following hospitalization in patients with heart failure. Pharmacotherapy. 2018;38:406-416.
20. Greene SJ, Fonarow GC, DeVore AD, et al. Titration of medical therapy for heart failure with reduced ejection fraction. J Am Coll Cardiol. 2019;73:2365-2383.
21. Gattis WA, O’Connor CM, Gallup DS, et al;, IMPACT-HF Investigators and Coordinators. Predischarge initiation of carvedilol in patients hospitalized for decompensated heart failure: results of the Initiation Management Predischarge: Process for Assessment of Carvedilol Therapy in Heart Failure (IMPACT-HF) trial. J Am Coll Cardiol. 2004;43:1534-1541.
22. Graumlich JF, Novotny NL, Aldag JC. Brief scale measuring patient preparedness for hospital discharge to home: Psychometric properties. J Hosp Med. 2008;3:446-454.
23. Van Spall HGC, Lee SF, Xie F, et al. Effect of patient-centered transitional care services on clinical outcomes in patients hospitalized for heart failure: The PACT-HF Randomized Clinical Trial. JAMA. 2019;321:753-761.
24. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240-327.
25. Di Palo KE, Patel K, Assafin M, Piña IL. Implementation of a patient navigator program to reduce 30-day heart failure readmission rate. Prog Cardiovasc Dis. 2017;60:259-266.
26. Patil S, Shah M, Patel B, et al. Readmissions among patients admitted with acute decompensated heart failure based on income quartiles. Mayo Clin Proc. 2019;94:1939-1950.
27. Ahmad K, Chen EW, Nazir U, et al. Regional variation in the association of poverty and heart failure mortality in the 3135 counties of the united states. J Am Heart Assoc. 2019;8:e012422.
28. Bellon JE, Bilderback A, Ahuja-Yende NS, et al. University of Pittsburgh medical center home transitions multidisciplinary care coordination reduces readmissions for older adults. J Am Geriatr Soc. 2019;67:156-163.
29. Rosen D, McCall JD, Primack BA. Telehealth protocol to prevent readmission among high-risk patients with congestive heart failure. Am J Med. 2017;130:1326-1330.
30. Heywood JT, Jermyn R, Shavelle D, et al. Impact of practice-based management of pulmonary artery pressures in 2000 patients implanted with the CardioMEMS sensor. Circulation. 2017;135:1509-1517.
31. Abraham WT, Adamson PB, Bourge RC, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377:658-666.
32. Drozda JP, Smith DA, Freiman PC, et al. Heart failure readmission reduction. Am J Med Qual. 2017;32:134-140.
33. Malik AH, Malik SS, Aronow WS; MAGIC (Meta-analysis And oriGinal Investigation in Cardiology) investigators. Effect of home-based follow-up intervention on readmissions and mortality in heart failure patients: a meta-analysis. Future Cardiol. 2019;15:377-386.
34. Strano A, Briggs A, Powell N, et al. Home healthcare visits following hospital discharge: does the timing of visits affect 30-day hospital readmission rates for heart failure patients? Home Healthc Now. 2019;37:152-157.
35. DeVore AD, Cox M, Eapen ZJ, et al. Temporal trends and variation in early scheduled follow-up after a hospitalization for heart failure: findings from get with the guidelines-heart failure. Circ Heart Fail. 2016;9.
36. Baker H, Oliver-McNeil S, Deng L, Hummel SL. Regional hospital collaboration and outcomes in medicare heart failure patients: see you in 7. JACC Heart Fail. 2015;3:765-773.
37. Mutharasan RK, Ahmad FS, Gurvich I, et al. Buffer or suffer: redesigning heart failure postdischarge clinic using queuing theory. Circ Cardiovasc Qual Outcomes. 2018;11:e004351.
38. Ziaeian B, Hernandez AF, DeVore AD, et al. Long-term outcomes for heart failure patients with and without diabetes: From the Get With The Guidelines-Heart Failure Registry. Am Heart J. 2019;211:1-10.
39. Greene SJ, Butler J, Albert NM, et al. Medical therapy for heart failure with reduced ejection fraction: The CHAMP-HF Registry. J Am Coll Cardiol. 2018;72:351-366.
40. Dharmarajan K, Hsieh AF, Lin Z, et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309:355-363.
41. Ward L, Powell RE, Scharf ML, et al. Patient-centered specialty practice: defining the role of specialists in value-based health care. Chest. 2017;151:930-935.
42. Ryan JJ, Waxman AB. The dyspnea clinic. Circulation. 2018;137:1994-1996.
43. Oseran AS, Howard SE, Blumenthal DM. Factors associated with participation in cardiac episode payments included in medicare’s bundled payments for care improvement initiative. JAMA Cardiol. 2018;3:761-766.
44. Takeda A, Taylor SJC, Taylor RS, et al. Clinical service organisation for heart failure. Cochrane Database Syst Rev. 2012;(9):CD002752.
45. Albert NM, Barnason S, Deswal A, et al. Transitions of care in heart failure: a scientific statement from the American Heart Association. Circ Heart Fail. 2015;8:384-409.
46. Manifesto for Agile Software Development. http://agilemanifesto.org/ Accessed March 6, 2020.
Candidiasis: The essentials of diagnosis and treatment
CASE Woman with vulvar itch and white vaginal discharge
A 26-year-old sexually active nulligravid woman requests evaluation for moderately intense “itching in the vagina and on the vulva.” She uses combination oral contraceptives and has 2 current sexual partners. On physical examination, you note a thick, white, curd-like discharge that is adherent to the vaginal epithelium. The vulva is erythematous, and small “satellite lesions” are evident in the intertriginous folds.
- What is the most likely diagnosis?
- How should you treat this patient?
Approximately 75% of all women will have at least 1 episode of vulvovaginal candidiasis (VVC) in their lifetime.1 Candida albicans, the most commonly identified organism in these infections, colonizes the vagina of many individuals commensally; higher rates of colonization occur in women with diabetes, obesity, recent use of broad-spectrum antibiotics, steroid use and immunosuppression, and in women who are pregnant. Of special interest, pregnant women have an increased risk of symptomatic infection, and they respond less favorably to conventional treatment regimens.1
Deconstructing C albicans and other species
Historically, in more than 90% of cases, C albicans is the principal cause of VVC. While it remains the most prevalent Candida species in the United States, over the last 15 years studies have demonstrated that in some countries, such as India and Nigeria, C albicans constitutes less than half of the cultured species in women with VVC. This observation may be due to the widespread availability and use of common antifungal medications, which leads to resistance and selection for resistant species.1,2
In asymptomatic women, vaginal colonies of C albicans grow in the yeast form. This condition is usually well tolerated by the host and does not cause a major immune response. In periods of stress for the host micro- and mycobiomes, however (dysbiosis, immune suppression, trauma), C albicans is induced into morphogenesis, proliferating and forming hyphae that are thought to activate the host immune response. The vaginal epithelium becomes sensitized to the presence of C albicans and recruits large numbers of neutrophils that, in turn, drive the pathophysiology of VVC.3
There is a theory that the separation of the urethra and anus by the vagina has exerted evolutionary pressure to maintain the presence of commensal C albicans yeast colonies in the vagina. C albicans exerts an antagonistic effect on many bacteria and, therefore, may act as a “microbiologic barrier” between the anus and the urethra to prevent urinary tract infections that, before the modern antibiotic era, may have caused serious morbidity and even mortality.3
Other organisms that cause VVC include C glabrata, C parapsilosis, and C tropicalis. Ex vivo experiments have shown that co-infection of C albicans with C glabrata enhances the ability of C glabrata to invade tissue.2 C glabrata is more frequently resistant to commonly used antifungal compounds than C albicans,2,4 which suggests that identifying the specific fungal pathogen is becoming increasingly important in planning targeted therapy.
Continue to: A common infection...
A common infection
While three-quarters of women will experience VVC at least once in their lifetime, between 40% and 45% will experience it more than once, and 5% to 8% will develop recurrent VVC. Among pregnant women, 15% will develop symptomatic VVC.1,2
However, because VVC is not a reportable disease and antifungal medication is available over the counter without physician consultation, these numbers likely underestimate the true incidence of the infection.4
Complications in pregnancy
Vaginal infections, including VVC, bacterial vaginosis (BV), and trichomoniasis, may be associated with 40% of preterm deliveries.5 The high concentrations of estrogen and progesterone during pregnancy create a uniquely glycogen-rich vaginal environment in which Candida species can flourish.2,4 Even asymptomatic colonization of the vagina with Candida species has been associated with preterm labor, preterm birth, and low birth weight.1,6 This association appears to have more severe consequences if VVC occurs in the second trimester compared with the first trimester.6
Additionally, congenital candidiasis of the newborn may result from intrauterine Candida infection or heavy maternal vaginal colonization at delivery, and the infection is evident within 24 hours of birth. It presents typically as oropharyngeal candidiasis (thrush) of the newborn.1
Clinical manifestations of infection
The classic manifestations of Candida infection are similar in both the pregnant and nonpregnant patient: acute vaginal and vulvar pruritus and thick, white, malodorous “cottage cheese” vaginal discharge.1,4 Exercise caution, however, in treating presumptively based on these symptoms alone, especially in pregnancy, because they are not specific to candidiasis.4 Vaginal discharge is not always present, and it may vary in appearance and odor. Pruritus is the most specific symptom of Candida infection, but studies show that it is an accurate predictor in only 38% of cases.7
Other common signs and symptoms include the sensation of burning, dysuria, dyspareunia, fissures, excoriations, and pruritus ani. Physical examination demonstrates erythema and swelling of labial, vulvar, and vaginal structures, with a normal cervix and an adherent white or off-white discharge. When the discharge is removed from the vaginal wall, small bleeding points may appear.1,4
Making the diagnosis
As mentioned, history alone is not sufficient to make a definitive diagnosis of candidiasis. The diagnosis should be made by examining vaginal secretions under a microscope or by culture.4 A wet mount and KOH (potassium hydroxide) prep help differentiate VVC, BV, and trichomoniasis. Culture is particularly valuable in identifying less common fungal organisms, such as C glabrata and C tropicalis.
Vaginal pH testing is not conclusive for Candida because vaginal pH is normal in VVC. However, pH assessment can rule in other causative organisms if the value is abnormal (that is, elevated pH of 4.5 or greater with BV and trichomoniasis).1
Treatment options
Acute infection. A pregnant woman who tests positive for VVC may safely be treated in any trimester with a 7-day course of a topical azole.8 If the patient prefers the convenience of oral therapy, after the first trimester, oral fluconazole, 150 mg on day 1 and day 3, may be used for treatment. Note that fluconazole has been associated with an increased risk of spontaneous abortion and cardiac septal defects when used in the first trimester.1
The Centers for Disease Control and Prevention recommends a number of topical treatments for VVC (TABLE).8 Several of these drugs are available over the counter without a prescription. Topical azoles are more effective than nystatin in treating VVC, and posttreatment cultures are negative in up to 90% of treated patients.8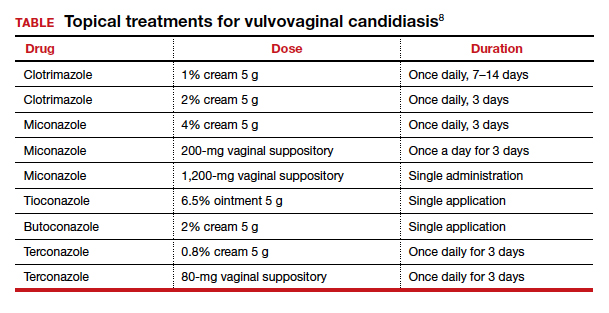
Recurrent infections. Recurrent VVC is defined as 4 or more episodes of symptomatic VVC within 12 months.8 Typical first-line treatment of recurrent infections in nonpregnant patients is a 6-month course of fluconazole, 150 mg weekly.9,10 As noted, however, fluconazole should not be used in the first trimester of pregnancy. It is acceptable therapy thereafter for patients who have troublesome recurrent or persistent infections.
Continue to: Strategies for preventing recurrence...
Strategies for preventing recurrence
While it is logical to consider antimycotic prophylaxis in women with a history of recurring VVC and/or a significant number of known risk factors, data suggest that extended prophylaxis with an azole does not consistently achieve long-term elimination of vaginal Candida organisms after cessation of the azole.9
At-risk women should be counseled to make lifestyle adjustments, such as wearing breathable cotton clothing, particularly undergarments; promptly changing out of damp clothing; and forgoing the use of commercial intravaginal feminine hygiene products.
Recent research has shown that the use of Saccharomyces cerevisiae–based probiotics has promise for controlling the burden of C albicans in women receiving antifungal drugs for VVC and also for preventing recurrence; however, this approach has undergone limited testing in humans, and its efficacy and safety in pregnancy is unknown.11 ●
- Duff P. Maternal and fetal infection. In: Resnik R, Lockwood CJ, Moore TR, et al, eds. Creasy and Resnik's Maternal-Fetal Medicine: Principles and Practice. 8th ed. Philadelphia, PA: Elsevier; 2019:862.
- Goncalves B, Ferreira C, Alves CT, et al. Vulvovaginal candidiasis: epidemiology, microbiology and risk factors. Crit Rev Microbiol. 2016;42:905-927.
- Hall RA, Noverr MC. Fungal interactions with the human host: exploring the spectrum of symbiosis. Curr Opin Microbiol. 2017;40:58-64.
- Sobel JD. Vulvovaginal candidosis. Lancet. 2007;369:1961-1971.
- Holzer I, Farr A, Kiss H; et al. The colonization with Candida species is more harmful in the second trimester of pregnancy. Arch Gynecol Obstet. 2017;295:891-895.
- Farr A, Kiss H, Holzer I, et al. Effect of asymptomatic vaginal colonization with Candida albicans on pregnancy outcome. Acta Obstet Gynecol Scand. 2015;94:989-996.
- Anderson MR, Klink K, Cohrssen A. Evaluation of vaginal complaints. JAMA. 2004;291:1368-1379.
- Workowski KA, Bolan GA, Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1-137.
- Sobel JD, Wiesenfeld HC, Martens M, et al. Maintenance fluconazole therapy for recurrent vulvovaginal candidiasis. N Engl J Med. 2004;351:876-883.
- US Food and Drug Administration. FDA Drug Safety Communication: Use of long-term, high-dose Diflucan (fluconazole) during pregnancy may be associated with birth defects in infants. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communicationuse-long-term-high-dose-diflucan-fluconazole-during-pregnancy-may-be#. Updated August 4, 2017. Accessed July 6, 2020.
- Gaziano R, Sabbatini S, Roselletti E, et al. Saccharomyces cerevisiae-based probiotics as novel antimicrobial agents to prevent and treat vaginal infections. Front Microbiol. 2020;11:718.
CASE Woman with vulvar itch and white vaginal discharge
A 26-year-old sexually active nulligravid woman requests evaluation for moderately intense “itching in the vagina and on the vulva.” She uses combination oral contraceptives and has 2 current sexual partners. On physical examination, you note a thick, white, curd-like discharge that is adherent to the vaginal epithelium. The vulva is erythematous, and small “satellite lesions” are evident in the intertriginous folds.
- What is the most likely diagnosis?
- How should you treat this patient?
Approximately 75% of all women will have at least 1 episode of vulvovaginal candidiasis (VVC) in their lifetime.1 Candida albicans, the most commonly identified organism in these infections, colonizes the vagina of many individuals commensally; higher rates of colonization occur in women with diabetes, obesity, recent use of broad-spectrum antibiotics, steroid use and immunosuppression, and in women who are pregnant. Of special interest, pregnant women have an increased risk of symptomatic infection, and they respond less favorably to conventional treatment regimens.1
Deconstructing C albicans and other species
Historically, in more than 90% of cases, C albicans is the principal cause of VVC. While it remains the most prevalent Candida species in the United States, over the last 15 years studies have demonstrated that in some countries, such as India and Nigeria, C albicans constitutes less than half of the cultured species in women with VVC. This observation may be due to the widespread availability and use of common antifungal medications, which leads to resistance and selection for resistant species.1,2
In asymptomatic women, vaginal colonies of C albicans grow in the yeast form. This condition is usually well tolerated by the host and does not cause a major immune response. In periods of stress for the host micro- and mycobiomes, however (dysbiosis, immune suppression, trauma), C albicans is induced into morphogenesis, proliferating and forming hyphae that are thought to activate the host immune response. The vaginal epithelium becomes sensitized to the presence of C albicans and recruits large numbers of neutrophils that, in turn, drive the pathophysiology of VVC.3
There is a theory that the separation of the urethra and anus by the vagina has exerted evolutionary pressure to maintain the presence of commensal C albicans yeast colonies in the vagina. C albicans exerts an antagonistic effect on many bacteria and, therefore, may act as a “microbiologic barrier” between the anus and the urethra to prevent urinary tract infections that, before the modern antibiotic era, may have caused serious morbidity and even mortality.3
Other organisms that cause VVC include C glabrata, C parapsilosis, and C tropicalis. Ex vivo experiments have shown that co-infection of C albicans with C glabrata enhances the ability of C glabrata to invade tissue.2 C glabrata is more frequently resistant to commonly used antifungal compounds than C albicans,2,4 which suggests that identifying the specific fungal pathogen is becoming increasingly important in planning targeted therapy.
Continue to: A common infection...
A common infection
While three-quarters of women will experience VVC at least once in their lifetime, between 40% and 45% will experience it more than once, and 5% to 8% will develop recurrent VVC. Among pregnant women, 15% will develop symptomatic VVC.1,2
However, because VVC is not a reportable disease and antifungal medication is available over the counter without physician consultation, these numbers likely underestimate the true incidence of the infection.4
Complications in pregnancy
Vaginal infections, including VVC, bacterial vaginosis (BV), and trichomoniasis, may be associated with 40% of preterm deliveries.5 The high concentrations of estrogen and progesterone during pregnancy create a uniquely glycogen-rich vaginal environment in which Candida species can flourish.2,4 Even asymptomatic colonization of the vagina with Candida species has been associated with preterm labor, preterm birth, and low birth weight.1,6 This association appears to have more severe consequences if VVC occurs in the second trimester compared with the first trimester.6
Additionally, congenital candidiasis of the newborn may result from intrauterine Candida infection or heavy maternal vaginal colonization at delivery, and the infection is evident within 24 hours of birth. It presents typically as oropharyngeal candidiasis (thrush) of the newborn.1
Clinical manifestations of infection
The classic manifestations of Candida infection are similar in both the pregnant and nonpregnant patient: acute vaginal and vulvar pruritus and thick, white, malodorous “cottage cheese” vaginal discharge.1,4 Exercise caution, however, in treating presumptively based on these symptoms alone, especially in pregnancy, because they are not specific to candidiasis.4 Vaginal discharge is not always present, and it may vary in appearance and odor. Pruritus is the most specific symptom of Candida infection, but studies show that it is an accurate predictor in only 38% of cases.7
Other common signs and symptoms include the sensation of burning, dysuria, dyspareunia, fissures, excoriations, and pruritus ani. Physical examination demonstrates erythema and swelling of labial, vulvar, and vaginal structures, with a normal cervix and an adherent white or off-white discharge. When the discharge is removed from the vaginal wall, small bleeding points may appear.1,4
Making the diagnosis
As mentioned, history alone is not sufficient to make a definitive diagnosis of candidiasis. The diagnosis should be made by examining vaginal secretions under a microscope or by culture.4 A wet mount and KOH (potassium hydroxide) prep help differentiate VVC, BV, and trichomoniasis. Culture is particularly valuable in identifying less common fungal organisms, such as C glabrata and C tropicalis.
Vaginal pH testing is not conclusive for Candida because vaginal pH is normal in VVC. However, pH assessment can rule in other causative organisms if the value is abnormal (that is, elevated pH of 4.5 or greater with BV and trichomoniasis).1
Treatment options
Acute infection. A pregnant woman who tests positive for VVC may safely be treated in any trimester with a 7-day course of a topical azole.8 If the patient prefers the convenience of oral therapy, after the first trimester, oral fluconazole, 150 mg on day 1 and day 3, may be used for treatment. Note that fluconazole has been associated with an increased risk of spontaneous abortion and cardiac septal defects when used in the first trimester.1
The Centers for Disease Control and Prevention recommends a number of topical treatments for VVC (TABLE).8 Several of these drugs are available over the counter without a prescription. Topical azoles are more effective than nystatin in treating VVC, and posttreatment cultures are negative in up to 90% of treated patients.8
Recurrent infections. Recurrent VVC is defined as 4 or more episodes of symptomatic VVC within 12 months.8 Typical first-line treatment of recurrent infections in nonpregnant patients is a 6-month course of fluconazole, 150 mg weekly.9,10 As noted, however, fluconazole should not be used in the first trimester of pregnancy. It is acceptable therapy thereafter for patients who have troublesome recurrent or persistent infections.
Continue to: Strategies for preventing recurrence...
Strategies for preventing recurrence
While it is logical to consider antimycotic prophylaxis in women with a history of recurring VVC and/or a significant number of known risk factors, data suggest that extended prophylaxis with an azole does not consistently achieve long-term elimination of vaginal Candida organisms after cessation of the azole.9
At-risk women should be counseled to make lifestyle adjustments, such as wearing breathable cotton clothing, particularly undergarments; promptly changing out of damp clothing; and forgoing the use of commercial intravaginal feminine hygiene products.
Recent research has shown that the use of Saccharomyces cerevisiae–based probiotics has promise for controlling the burden of C albicans in women receiving antifungal drugs for VVC and also for preventing recurrence; however, this approach has undergone limited testing in humans, and its efficacy and safety in pregnancy is unknown.11 ●
CASE Woman with vulvar itch and white vaginal discharge
A 26-year-old sexually active nulligravid woman requests evaluation for moderately intense “itching in the vagina and on the vulva.” She uses combination oral contraceptives and has 2 current sexual partners. On physical examination, you note a thick, white, curd-like discharge that is adherent to the vaginal epithelium. The vulva is erythematous, and small “satellite lesions” are evident in the intertriginous folds.
- What is the most likely diagnosis?
- How should you treat this patient?
Approximately 75% of all women will have at least 1 episode of vulvovaginal candidiasis (VVC) in their lifetime.1 Candida albicans, the most commonly identified organism in these infections, colonizes the vagina of many individuals commensally; higher rates of colonization occur in women with diabetes, obesity, recent use of broad-spectrum antibiotics, steroid use and immunosuppression, and in women who are pregnant. Of special interest, pregnant women have an increased risk of symptomatic infection, and they respond less favorably to conventional treatment regimens.1
Deconstructing C albicans and other species
Historically, in more than 90% of cases, C albicans is the principal cause of VVC. While it remains the most prevalent Candida species in the United States, over the last 15 years studies have demonstrated that in some countries, such as India and Nigeria, C albicans constitutes less than half of the cultured species in women with VVC. This observation may be due to the widespread availability and use of common antifungal medications, which leads to resistance and selection for resistant species.1,2
In asymptomatic women, vaginal colonies of C albicans grow in the yeast form. This condition is usually well tolerated by the host and does not cause a major immune response. In periods of stress for the host micro- and mycobiomes, however (dysbiosis, immune suppression, trauma), C albicans is induced into morphogenesis, proliferating and forming hyphae that are thought to activate the host immune response. The vaginal epithelium becomes sensitized to the presence of C albicans and recruits large numbers of neutrophils that, in turn, drive the pathophysiology of VVC.3
There is a theory that the separation of the urethra and anus by the vagina has exerted evolutionary pressure to maintain the presence of commensal C albicans yeast colonies in the vagina. C albicans exerts an antagonistic effect on many bacteria and, therefore, may act as a “microbiologic barrier” between the anus and the urethra to prevent urinary tract infections that, before the modern antibiotic era, may have caused serious morbidity and even mortality.3
Other organisms that cause VVC include C glabrata, C parapsilosis, and C tropicalis. Ex vivo experiments have shown that co-infection of C albicans with C glabrata enhances the ability of C glabrata to invade tissue.2 C glabrata is more frequently resistant to commonly used antifungal compounds than C albicans,2,4 which suggests that identifying the specific fungal pathogen is becoming increasingly important in planning targeted therapy.
Continue to: A common infection...
A common infection
While three-quarters of women will experience VVC at least once in their lifetime, between 40% and 45% will experience it more than once, and 5% to 8% will develop recurrent VVC. Among pregnant women, 15% will develop symptomatic VVC.1,2
However, because VVC is not a reportable disease and antifungal medication is available over the counter without physician consultation, these numbers likely underestimate the true incidence of the infection.4
Complications in pregnancy
Vaginal infections, including VVC, bacterial vaginosis (BV), and trichomoniasis, may be associated with 40% of preterm deliveries.5 The high concentrations of estrogen and progesterone during pregnancy create a uniquely glycogen-rich vaginal environment in which Candida species can flourish.2,4 Even asymptomatic colonization of the vagina with Candida species has been associated with preterm labor, preterm birth, and low birth weight.1,6 This association appears to have more severe consequences if VVC occurs in the second trimester compared with the first trimester.6
Additionally, congenital candidiasis of the newborn may result from intrauterine Candida infection or heavy maternal vaginal colonization at delivery, and the infection is evident within 24 hours of birth. It presents typically as oropharyngeal candidiasis (thrush) of the newborn.1
Clinical manifestations of infection
The classic manifestations of Candida infection are similar in both the pregnant and nonpregnant patient: acute vaginal and vulvar pruritus and thick, white, malodorous “cottage cheese” vaginal discharge.1,4 Exercise caution, however, in treating presumptively based on these symptoms alone, especially in pregnancy, because they are not specific to candidiasis.4 Vaginal discharge is not always present, and it may vary in appearance and odor. Pruritus is the most specific symptom of Candida infection, but studies show that it is an accurate predictor in only 38% of cases.7
Other common signs and symptoms include the sensation of burning, dysuria, dyspareunia, fissures, excoriations, and pruritus ani. Physical examination demonstrates erythema and swelling of labial, vulvar, and vaginal structures, with a normal cervix and an adherent white or off-white discharge. When the discharge is removed from the vaginal wall, small bleeding points may appear.1,4
Making the diagnosis
As mentioned, history alone is not sufficient to make a definitive diagnosis of candidiasis. The diagnosis should be made by examining vaginal secretions under a microscope or by culture.4 A wet mount and KOH (potassium hydroxide) prep help differentiate VVC, BV, and trichomoniasis. Culture is particularly valuable in identifying less common fungal organisms, such as C glabrata and C tropicalis.
Vaginal pH testing is not conclusive for Candida because vaginal pH is normal in VVC. However, pH assessment can rule in other causative organisms if the value is abnormal (that is, elevated pH of 4.5 or greater with BV and trichomoniasis).1
Treatment options
Acute infection. A pregnant woman who tests positive for VVC may safely be treated in any trimester with a 7-day course of a topical azole.8 If the patient prefers the convenience of oral therapy, after the first trimester, oral fluconazole, 150 mg on day 1 and day 3, may be used for treatment. Note that fluconazole has been associated with an increased risk of spontaneous abortion and cardiac septal defects when used in the first trimester.1
The Centers for Disease Control and Prevention recommends a number of topical treatments for VVC (TABLE).8 Several of these drugs are available over the counter without a prescription. Topical azoles are more effective than nystatin in treating VVC, and posttreatment cultures are negative in up to 90% of treated patients.8
Recurrent infections. Recurrent VVC is defined as 4 or more episodes of symptomatic VVC within 12 months.8 Typical first-line treatment of recurrent infections in nonpregnant patients is a 6-month course of fluconazole, 150 mg weekly.9,10 As noted, however, fluconazole should not be used in the first trimester of pregnancy. It is acceptable therapy thereafter for patients who have troublesome recurrent or persistent infections.
Continue to: Strategies for preventing recurrence...
Strategies for preventing recurrence
While it is logical to consider antimycotic prophylaxis in women with a history of recurring VVC and/or a significant number of known risk factors, data suggest that extended prophylaxis with an azole does not consistently achieve long-term elimination of vaginal Candida organisms after cessation of the azole.9
At-risk women should be counseled to make lifestyle adjustments, such as wearing breathable cotton clothing, particularly undergarments; promptly changing out of damp clothing; and forgoing the use of commercial intravaginal feminine hygiene products.
Recent research has shown that the use of Saccharomyces cerevisiae–based probiotics has promise for controlling the burden of C albicans in women receiving antifungal drugs for VVC and also for preventing recurrence; however, this approach has undergone limited testing in humans, and its efficacy and safety in pregnancy is unknown.11 ●
- Duff P. Maternal and fetal infection. In: Resnik R, Lockwood CJ, Moore TR, et al, eds. Creasy and Resnik's Maternal-Fetal Medicine: Principles and Practice. 8th ed. Philadelphia, PA: Elsevier; 2019:862.
- Goncalves B, Ferreira C, Alves CT, et al. Vulvovaginal candidiasis: epidemiology, microbiology and risk factors. Crit Rev Microbiol. 2016;42:905-927.
- Hall RA, Noverr MC. Fungal interactions with the human host: exploring the spectrum of symbiosis. Curr Opin Microbiol. 2017;40:58-64.
- Sobel JD. Vulvovaginal candidosis. Lancet. 2007;369:1961-1971.
- Holzer I, Farr A, Kiss H; et al. The colonization with Candida species is more harmful in the second trimester of pregnancy. Arch Gynecol Obstet. 2017;295:891-895.
- Farr A, Kiss H, Holzer I, et al. Effect of asymptomatic vaginal colonization with Candida albicans on pregnancy outcome. Acta Obstet Gynecol Scand. 2015;94:989-996.
- Anderson MR, Klink K, Cohrssen A. Evaluation of vaginal complaints. JAMA. 2004;291:1368-1379.
- Workowski KA, Bolan GA, Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1-137.
- Sobel JD, Wiesenfeld HC, Martens M, et al. Maintenance fluconazole therapy for recurrent vulvovaginal candidiasis. N Engl J Med. 2004;351:876-883.
- US Food and Drug Administration. FDA Drug Safety Communication: Use of long-term, high-dose Diflucan (fluconazole) during pregnancy may be associated with birth defects in infants. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communicationuse-long-term-high-dose-diflucan-fluconazole-during-pregnancy-may-be#. Updated August 4, 2017. Accessed July 6, 2020.
- Gaziano R, Sabbatini S, Roselletti E, et al. Saccharomyces cerevisiae-based probiotics as novel antimicrobial agents to prevent and treat vaginal infections. Front Microbiol. 2020;11:718.
- Duff P. Maternal and fetal infection. In: Resnik R, Lockwood CJ, Moore TR, et al, eds. Creasy and Resnik's Maternal-Fetal Medicine: Principles and Practice. 8th ed. Philadelphia, PA: Elsevier; 2019:862.
- Goncalves B, Ferreira C, Alves CT, et al. Vulvovaginal candidiasis: epidemiology, microbiology and risk factors. Crit Rev Microbiol. 2016;42:905-927.
- Hall RA, Noverr MC. Fungal interactions with the human host: exploring the spectrum of symbiosis. Curr Opin Microbiol. 2017;40:58-64.
- Sobel JD. Vulvovaginal candidosis. Lancet. 2007;369:1961-1971.
- Holzer I, Farr A, Kiss H; et al. The colonization with Candida species is more harmful in the second trimester of pregnancy. Arch Gynecol Obstet. 2017;295:891-895.
- Farr A, Kiss H, Holzer I, et al. Effect of asymptomatic vaginal colonization with Candida albicans on pregnancy outcome. Acta Obstet Gynecol Scand. 2015;94:989-996.
- Anderson MR, Klink K, Cohrssen A. Evaluation of vaginal complaints. JAMA. 2004;291:1368-1379.
- Workowski KA, Bolan GA, Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1-137.
- Sobel JD, Wiesenfeld HC, Martens M, et al. Maintenance fluconazole therapy for recurrent vulvovaginal candidiasis. N Engl J Med. 2004;351:876-883.
- US Food and Drug Administration. FDA Drug Safety Communication: Use of long-term, high-dose Diflucan (fluconazole) during pregnancy may be associated with birth defects in infants. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communicationuse-long-term-high-dose-diflucan-fluconazole-during-pregnancy-may-be#. Updated August 4, 2017. Accessed July 6, 2020.
- Gaziano R, Sabbatini S, Roselletti E, et al. Saccharomyces cerevisiae-based probiotics as novel antimicrobial agents to prevent and treat vaginal infections. Front Microbiol. 2020;11:718.
2020 Update on abnormal uterine bleeding
Abnormal uterine bleeding (AUB) continues to be a top reason that women present for gynecologic care. In general, our approach to the management of AUB is to diagnose causes before we prescribe therapy and to offer conservative therapies initially and progress to more invasive measures if indicated.
In this Update, we highlight several new studies that provide evidence for preferential use of certain medical and surgical therapies. In considering conservative therapy for the treatment of AUB, we take a closer look at the efficacy of cyclic progestogens. Another important issue, as more types of endometrial ablation (EA) are being developed and are coming into the market, is the need for additional guidance regarding decisions about EA versus progestin-releasing intrauterine devices (IUDs). Lastly, an unintended consequence of an increased cesarean delivery rate is the development of isthmocele, also known as cesarean scar defect or uterine niche. These defects, which can be bothersome and cause abnormal bleeding, are treated with various techniques. Within the last year, 2 systematic reviews that compare the efficacy of several different approaches and provide guidance have been published.
Is it time to retire cyclic progestogens for the treatment of heavy menstrual bleeding?
Bofill Rodriguez M, Lethaby A, Low C, et al. Cyclical progestogens for heavy menstrual bleeding. Cochrane Database Syst Rev. 2019;(8):CD001016.
In a recent Cochrane Database Systematic Review, Bofill Rodriguez and colleagues looked at the efficacy, safety, and tolerability of oral progestogen therapy for heavy menstrual bleeding.1 They considered progestogen (medroxyprogesterone acetate or norethisterone) in short-cycle use (7 to 10 days in the luteal phase) and long-cycle use (21 days per cycle) in a review of 15 randomized clinical trials (RCTs) that included a total of 1,071 women. As this topic had not been updated in 12 years, this review was essential in demonstrating changes that occurred over the past decade.
The primary outcomes of the analysis were menstrual blood loss and treatment satisfaction. Secondary outcomes included the number of days of bleeding, quality of life, adherence and acceptability of treatment, adverse events, and costs.
Classic progestogens fall short compared with newer approaches
Analysis of the data revealed that short-cycle progestogen was inferior to treatment with tranexamic acid, danazol, and the 65-µg progesterone-releasing IUD (Pg-IUD). Of note, the 65-µg Pg-IUD has been off the market since 2001, and danazol is rarely used in current practice. Furthermore, based on 2 trials, cyclic progestogens demonstrated no clear benefit over nonsteroidal anti-inflammatory drugs. Additionally, long-cycle progestogen therapy was found to be inferior to the 52-mg levonorgestrel-releasing IUD (LNG-IUD), tranexamic acid, and ormeloxifene.
It should be noted that the quality of evidence is still lacking for progestogen therapy, and this study's main limitation is bias, as the women and the researchers were aware of the treatments that were given. This review is helpful, however, for emphasizing the advantage of tranexamic acid and LNG-IUD use in clinical care.
The takeaway. Although it may not necessarily be time to retire the use of cyclic oral progestogens, the 52-mg LNG-IUD or tranexamic acid may be more successful for treating AUB in women who are appropriate candidates.
Cyclic progestogen therapy appears to be less effective for the treatment of AUB when compared with tranexamic acid and the LNG-IUD. It does not appear to be more helpful than nonsteroidal anti-inflammatory drugs. We frequently offer and prescribe tranexamic acid, 1,300 mg 3 times daily, as a medical alternative to hormonal therapy for up to 5 days monthly for women without thromboembolism risk. Lukes and colleagues published an RCT in 2010 that demonstrated a 40% reduction of bleeding in tranexamic acid–treated women compared with an 8.2% reduction in the placebo group.2
Continue to: Endometrial ablation...
Endometrial ablation: New evidence informs when it could (and could not) be the best option
Bergeron C, Laberge PY, Boutin A, et al. Endometrial ablation or resection versus levonorgestrel intra-uterine system for the treatment of women with heavy menstrual bleeding and a normal uterine cavity: a systematic review with meta-analysis. Hum Reprod Update. 2020;26:302-311.
Vitale SG, Ferrero S, Ciebiera M, et al. Hysteroscopic endometrial resection vs hysterectomy for abnormal uterine bleeding: impact on quality of life and sexuality. Evidence from a systematic review of randomized controlled trials. Curr Opin Obstet Gynecol. 2020;32:159-165.
Two systematic reviews evaluated the efficacy of EA in women with abnormal uterine bleeding. One compared EA with the LNG-IUD and reported on safety and efficacy, while the other compared EA with hysterectomy and reported on quality of life.
Bergeron and colleagues reviewed 13 studies that included 884 women to compare the efficacy and safety of EA or resection with the LNG-IUD for the treatment of premenopausal women with AUB.3 They found no significant differences between EA and the LNG-IUD in terms of subsequent hysterectomy (risk ratio [RR] = 1.3; 95% confidence interval [CI], 0.60-2.11). It was not surprising that, when looking at age, EA was associated with a higher risk for hysterectomy in women younger than age 42 (RR = 5.26; 95% CI, 1.21-22.91). Conversely, subsequent hysterectomy was less likely with EA compared to LNG-IUD use in women older than 42 years. However, statistical significance was not reached in the older group (RR = 0.51; 95% CI, 0.21-1.24).
In the systematic review by Vitale and colleagues, 9 studies met inclusion criteria for a comparison of EA and hysterectomy, with the objective of ascertaining improvement in quality of life and several other measures.4
Although there was significant heterogeneity between assessment tools, both treatment groups experienced similar improvements in quality of life during the first year. However, hysterectomy was more advantageous in terms of improving uterine bleeding and satisfaction in the long term when compared with EA.4
As EA is considered, it is important to continue to counsel about the efficacy of the LNG-IUD, as well as its decreased associated morbidity. Additionally, EA is particularly less effective in younger women.
Continue to: Laparoscopy is best approach for isthomocele management, with caveats...
Laparoscopy is best approach for isthomocele management, with caveats
He Y, Zhong J, Zhou W, et al. Four surgical strategies for the treatment of cesarean scar defect: a systematic review and network meta-analysis. J Minim Invasive Gynecol. 2020;27:593-602.
Vitale SG, Ludwin A, Vilos GA, et al. From hysteroscopy to laparoendoscopic surgery: what is the best surgical approach for symptomatic isthmocele? A systematic review and meta-analysis. Arch Gynecol Obstet. 2020;301:33-52.
The isthmocele (cesarean scar defect, uterine niche), a known complication of cesarean delivery, represents a myometrial defect in the anterior uterine wall that often presents as abnormal uterine bleeding. It also can be a site for pregnancy-related complications, such as invasive placentation, placenta previa, and uterine rupture.
Two systematic reviews compared surgical strategies for treating isthmocele, including laparoscopy, hysteroscopy, combined laparoscopy and hysteroscopy, laparotomy, and vaginal repair.
Laparoscopy reduced isthmocele-associated AUB better than other techniques
A review by He and colleagues analyzed data from 10 pertinent studies (4 RCTs and 6 observational studies) that included 858 patients in total.5 Treatments compared were laparoscopy, hysteroscopy, combined laparoscopy with hysteroscopy, and vaginal repair for reduction of AUB and isthmocele and diverticulum depth.
The authors found no difference in intraoperative bleeding between the 4 surgical methods (laparotomy was not included in this review). Hysteroscopic surgery was associated with the shortest operative time, while laparoscopy was the longest surgery. In terms of reducing intermittent abnormal bleeding and scar depth, laparoscopic surgery performed better than the other 3 methods.
Approach considerations in isthmocele repair
Vitale and colleagues conducted a systematic review that included 33 publications (28 focused on a single surgical technique, 5 compared different techniques) to examine the effectiveness and risks of various surgical approaches for isthmocele in women with AUB, infertility, or for prevention of obstetric complications.6
Results of their analysis in general favored a laparoscopic approach for patients who desired future fertility, with an improvement rate of 92.7%. Hysteroscopic correction had an 85% improvement rate, and vaginal correction had an 82.5% improvement rate.
Although there were no high-level data to suggest a threshold for myometrial thickness in recommending a surgical approach, the authors provided a helpful algorithm for choosing a route based on a patient's fertility desires. For the asymptomatic patient, they suggest no treatment. In symptomatic patients, the laparoscopic approach is the gold standard but requires significant laparoscopic surgical skill, and a hysteroscopic approach may be considered as an alternative route if the residual myometrial defect is greater than 2.5 to 3.5 mm. For patients who are not considering future reproduction, hysteroscopy is the gold standard as long as the residual myometrial thickness is greater than 2.5 to 3.5 mm.
The takeaway. Of the several methods used for surgical isthmocele management, the laparoscopic approach reduced intermittent abnormal bleeding and scar depth better than other methods. It also was associated with the longest surgical duration. Hysteroscopic surgery was the quickest procedure to perform and is effective in removing the upper valve to promote the elimination of the hematocele and symptoms of abnormal bleeding; however, it does not change the anatomic aspects of the isthmocele in terms of myometrial thickness. Some authors suggested that deciding on the surgical route should be based on fertility desires and the residual thickness of the myometrium. ●
In terms of isthmocele repair, the laparoscopic approach is preferred in patients who desire fertility, as long as the surgeon possesses the skill set to perform this difficult surgery, and as long as the residual myometrium is thicker than 2.5 to 3.5 mm.
- Bofill Rodriguez M, Lethaby A, Low C, et al. Cyclical progestogens for heavy menstrual bleeding. Cochrane Database Syst Rev. 2019;(8):CD001016.
- Lukes AS, Moore KA, Muse KN, et al. Tranexamic acid treatment for heavy menstrual bleeding: a randomized controlled study. Obstet Gynecol. 2010;116:865-875.
- Bergeron C, Laberge PY, Boutin A, et al. Endometrial ablation or resection versus levonorgestrel intra-uterine system for the treatment of women with heavy menstrual bleeding and a normal uterine cavity: a systematic review with meta-analysis. Hum Reprod Update. 2020;26:302-311.
- Vitale SG, Ferrero S, Ciebiera M, et al. Hysteroscopic endometrial resection vs hysterectomy for abnormal uterine bleeding: impact on quality of life and sexuality. Evidence from a systematic review of randomized controlled trials. Curr Opin Obstet Gynecol. 2020;32:159-165.
- He Y, Zhong J, Zhou W, et al. Four surgical strategies for the treatment of cesarean scar defect: a systematic review and network meta-analysis. J Minim Invasive Gynecol. 2020;27:593-602.
- Vitale SG, Ludwin A, Vilos GA, et al. From hysteroscopy to laparoendoscopic surgery: what is the best surgical approach for symptomatic isthmocele? A systematic review and meta-analysis. Arch Gynecol Obstet. 2020;301:33-52.

Abnormal uterine bleeding (AUB) continues to be a top reason that women present for gynecologic care. In general, our approach to the management of AUB is to diagnose causes before we prescribe therapy and to offer conservative therapies initially and progress to more invasive measures if indicated.
In this Update, we highlight several new studies that provide evidence for preferential use of certain medical and surgical therapies. In considering conservative therapy for the treatment of AUB, we take a closer look at the efficacy of cyclic progestogens. Another important issue, as more types of endometrial ablation (EA) are being developed and are coming into the market, is the need for additional guidance regarding decisions about EA versus progestin-releasing intrauterine devices (IUDs). Lastly, an unintended consequence of an increased cesarean delivery rate is the development of isthmocele, also known as cesarean scar defect or uterine niche. These defects, which can be bothersome and cause abnormal bleeding, are treated with various techniques. Within the last year, 2 systematic reviews that compare the efficacy of several different approaches and provide guidance have been published.
Is it time to retire cyclic progestogens for the treatment of heavy menstrual bleeding?
Bofill Rodriguez M, Lethaby A, Low C, et al. Cyclical progestogens for heavy menstrual bleeding. Cochrane Database Syst Rev. 2019;(8):CD001016.
In a recent Cochrane Database Systematic Review, Bofill Rodriguez and colleagues looked at the efficacy, safety, and tolerability of oral progestogen therapy for heavy menstrual bleeding.1 They considered progestogen (medroxyprogesterone acetate or norethisterone) in short-cycle use (7 to 10 days in the luteal phase) and long-cycle use (21 days per cycle) in a review of 15 randomized clinical trials (RCTs) that included a total of 1,071 women. As this topic had not been updated in 12 years, this review was essential in demonstrating changes that occurred over the past decade.
The primary outcomes of the analysis were menstrual blood loss and treatment satisfaction. Secondary outcomes included the number of days of bleeding, quality of life, adherence and acceptability of treatment, adverse events, and costs.
Classic progestogens fall short compared with newer approaches
Analysis of the data revealed that short-cycle progestogen was inferior to treatment with tranexamic acid, danazol, and the 65-µg progesterone-releasing IUD (Pg-IUD). Of note, the 65-µg Pg-IUD has been off the market since 2001, and danazol is rarely used in current practice. Furthermore, based on 2 trials, cyclic progestogens demonstrated no clear benefit over nonsteroidal anti-inflammatory drugs. Additionally, long-cycle progestogen therapy was found to be inferior to the 52-mg levonorgestrel-releasing IUD (LNG-IUD), tranexamic acid, and ormeloxifene.
It should be noted that the quality of evidence is still lacking for progestogen therapy, and this study's main limitation is bias, as the women and the researchers were aware of the treatments that were given. This review is helpful, however, for emphasizing the advantage of tranexamic acid and LNG-IUD use in clinical care.
The takeaway. Although it may not necessarily be time to retire the use of cyclic oral progestogens, the 52-mg LNG-IUD or tranexamic acid may be more successful for treating AUB in women who are appropriate candidates.
Cyclic progestogen therapy appears to be less effective for the treatment of AUB when compared with tranexamic acid and the LNG-IUD. It does not appear to be more helpful than nonsteroidal anti-inflammatory drugs. We frequently offer and prescribe tranexamic acid, 1,300 mg 3 times daily, as a medical alternative to hormonal therapy for up to 5 days monthly for women without thromboembolism risk. Lukes and colleagues published an RCT in 2010 that demonstrated a 40% reduction of bleeding in tranexamic acid–treated women compared with an 8.2% reduction in the placebo group.2
Continue to: Endometrial ablation...
Endometrial ablation: New evidence informs when it could (and could not) be the best option
Bergeron C, Laberge PY, Boutin A, et al. Endometrial ablation or resection versus levonorgestrel intra-uterine system for the treatment of women with heavy menstrual bleeding and a normal uterine cavity: a systematic review with meta-analysis. Hum Reprod Update. 2020;26:302-311.
Vitale SG, Ferrero S, Ciebiera M, et al. Hysteroscopic endometrial resection vs hysterectomy for abnormal uterine bleeding: impact on quality of life and sexuality. Evidence from a systematic review of randomized controlled trials. Curr Opin Obstet Gynecol. 2020;32:159-165.
Two systematic reviews evaluated the efficacy of EA in women with abnormal uterine bleeding. One compared EA with the LNG-IUD and reported on safety and efficacy, while the other compared EA with hysterectomy and reported on quality of life.
Bergeron and colleagues reviewed 13 studies that included 884 women to compare the efficacy and safety of EA or resection with the LNG-IUD for the treatment of premenopausal women with AUB.3 They found no significant differences between EA and the LNG-IUD in terms of subsequent hysterectomy (risk ratio [RR] = 1.3; 95% confidence interval [CI], 0.60-2.11). It was not surprising that, when looking at age, EA was associated with a higher risk for hysterectomy in women younger than age 42 (RR = 5.26; 95% CI, 1.21-22.91). Conversely, subsequent hysterectomy was less likely with EA compared to LNG-IUD use in women older than 42 years. However, statistical significance was not reached in the older group (RR = 0.51; 95% CI, 0.21-1.24).
In the systematic review by Vitale and colleagues, 9 studies met inclusion criteria for a comparison of EA and hysterectomy, with the objective of ascertaining improvement in quality of life and several other measures.4
Although there was significant heterogeneity between assessment tools, both treatment groups experienced similar improvements in quality of life during the first year. However, hysterectomy was more advantageous in terms of improving uterine bleeding and satisfaction in the long term when compared with EA.4
As EA is considered, it is important to continue to counsel about the efficacy of the LNG-IUD, as well as its decreased associated morbidity. Additionally, EA is particularly less effective in younger women.
Continue to: Laparoscopy is best approach for isthomocele management, with caveats...
Laparoscopy is best approach for isthomocele management, with caveats
He Y, Zhong J, Zhou W, et al. Four surgical strategies for the treatment of cesarean scar defect: a systematic review and network meta-analysis. J Minim Invasive Gynecol. 2020;27:593-602.
Vitale SG, Ludwin A, Vilos GA, et al. From hysteroscopy to laparoendoscopic surgery: what is the best surgical approach for symptomatic isthmocele? A systematic review and meta-analysis. Arch Gynecol Obstet. 2020;301:33-52.
The isthmocele (cesarean scar defect, uterine niche), a known complication of cesarean delivery, represents a myometrial defect in the anterior uterine wall that often presents as abnormal uterine bleeding. It also can be a site for pregnancy-related complications, such as invasive placentation, placenta previa, and uterine rupture.
Two systematic reviews compared surgical strategies for treating isthmocele, including laparoscopy, hysteroscopy, combined laparoscopy and hysteroscopy, laparotomy, and vaginal repair.
Laparoscopy reduced isthmocele-associated AUB better than other techniques
A review by He and colleagues analyzed data from 10 pertinent studies (4 RCTs and 6 observational studies) that included 858 patients in total.5 Treatments compared were laparoscopy, hysteroscopy, combined laparoscopy with hysteroscopy, and vaginal repair for reduction of AUB and isthmocele and diverticulum depth.
The authors found no difference in intraoperative bleeding between the 4 surgical methods (laparotomy was not included in this review). Hysteroscopic surgery was associated with the shortest operative time, while laparoscopy was the longest surgery. In terms of reducing intermittent abnormal bleeding and scar depth, laparoscopic surgery performed better than the other 3 methods.
Approach considerations in isthmocele repair
Vitale and colleagues conducted a systematic review that included 33 publications (28 focused on a single surgical technique, 5 compared different techniques) to examine the effectiveness and risks of various surgical approaches for isthmocele in women with AUB, infertility, or for prevention of obstetric complications.6
Results of their analysis in general favored a laparoscopic approach for patients who desired future fertility, with an improvement rate of 92.7%. Hysteroscopic correction had an 85% improvement rate, and vaginal correction had an 82.5% improvement rate.
Although there were no high-level data to suggest a threshold for myometrial thickness in recommending a surgical approach, the authors provided a helpful algorithm for choosing a route based on a patient's fertility desires. For the asymptomatic patient, they suggest no treatment. In symptomatic patients, the laparoscopic approach is the gold standard but requires significant laparoscopic surgical skill, and a hysteroscopic approach may be considered as an alternative route if the residual myometrial defect is greater than 2.5 to 3.5 mm. For patients who are not considering future reproduction, hysteroscopy is the gold standard as long as the residual myometrial thickness is greater than 2.5 to 3.5 mm.
The takeaway. Of the several methods used for surgical isthmocele management, the laparoscopic approach reduced intermittent abnormal bleeding and scar depth better than other methods. It also was associated with the longest surgical duration. Hysteroscopic surgery was the quickest procedure to perform and is effective in removing the upper valve to promote the elimination of the hematocele and symptoms of abnormal bleeding; however, it does not change the anatomic aspects of the isthmocele in terms of myometrial thickness. Some authors suggested that deciding on the surgical route should be based on fertility desires and the residual thickness of the myometrium. ●
In terms of isthmocele repair, the laparoscopic approach is preferred in patients who desire fertility, as long as the surgeon possesses the skill set to perform this difficult surgery, and as long as the residual myometrium is thicker than 2.5 to 3.5 mm.

Abnormal uterine bleeding (AUB) continues to be a top reason that women present for gynecologic care. In general, our approach to the management of AUB is to diagnose causes before we prescribe therapy and to offer conservative therapies initially and progress to more invasive measures if indicated.
In this Update, we highlight several new studies that provide evidence for preferential use of certain medical and surgical therapies. In considering conservative therapy for the treatment of AUB, we take a closer look at the efficacy of cyclic progestogens. Another important issue, as more types of endometrial ablation (EA) are being developed and are coming into the market, is the need for additional guidance regarding decisions about EA versus progestin-releasing intrauterine devices (IUDs). Lastly, an unintended consequence of an increased cesarean delivery rate is the development of isthmocele, also known as cesarean scar defect or uterine niche. These defects, which can be bothersome and cause abnormal bleeding, are treated with various techniques. Within the last year, 2 systematic reviews that compare the efficacy of several different approaches and provide guidance have been published.
Is it time to retire cyclic progestogens for the treatment of heavy menstrual bleeding?
Bofill Rodriguez M, Lethaby A, Low C, et al. Cyclical progestogens for heavy menstrual bleeding. Cochrane Database Syst Rev. 2019;(8):CD001016.
In a recent Cochrane Database Systematic Review, Bofill Rodriguez and colleagues looked at the efficacy, safety, and tolerability of oral progestogen therapy for heavy menstrual bleeding.1 They considered progestogen (medroxyprogesterone acetate or norethisterone) in short-cycle use (7 to 10 days in the luteal phase) and long-cycle use (21 days per cycle) in a review of 15 randomized clinical trials (RCTs) that included a total of 1,071 women. As this topic had not been updated in 12 years, this review was essential in demonstrating changes that occurred over the past decade.
The primary outcomes of the analysis were menstrual blood loss and treatment satisfaction. Secondary outcomes included the number of days of bleeding, quality of life, adherence and acceptability of treatment, adverse events, and costs.
Classic progestogens fall short compared with newer approaches
Analysis of the data revealed that short-cycle progestogen was inferior to treatment with tranexamic acid, danazol, and the 65-µg progesterone-releasing IUD (Pg-IUD). Of note, the 65-µg Pg-IUD has been off the market since 2001, and danazol is rarely used in current practice. Furthermore, based on 2 trials, cyclic progestogens demonstrated no clear benefit over nonsteroidal anti-inflammatory drugs. Additionally, long-cycle progestogen therapy was found to be inferior to the 52-mg levonorgestrel-releasing IUD (LNG-IUD), tranexamic acid, and ormeloxifene.
It should be noted that the quality of evidence is still lacking for progestogen therapy, and this study's main limitation is bias, as the women and the researchers were aware of the treatments that were given. This review is helpful, however, for emphasizing the advantage of tranexamic acid and LNG-IUD use in clinical care.
The takeaway. Although it may not necessarily be time to retire the use of cyclic oral progestogens, the 52-mg LNG-IUD or tranexamic acid may be more successful for treating AUB in women who are appropriate candidates.
Cyclic progestogen therapy appears to be less effective for the treatment of AUB when compared with tranexamic acid and the LNG-IUD. It does not appear to be more helpful than nonsteroidal anti-inflammatory drugs. We frequently offer and prescribe tranexamic acid, 1,300 mg 3 times daily, as a medical alternative to hormonal therapy for up to 5 days monthly for women without thromboembolism risk. Lukes and colleagues published an RCT in 2010 that demonstrated a 40% reduction of bleeding in tranexamic acid–treated women compared with an 8.2% reduction in the placebo group.2
Continue to: Endometrial ablation...
Endometrial ablation: New evidence informs when it could (and could not) be the best option
Bergeron C, Laberge PY, Boutin A, et al. Endometrial ablation or resection versus levonorgestrel intra-uterine system for the treatment of women with heavy menstrual bleeding and a normal uterine cavity: a systematic review with meta-analysis. Hum Reprod Update. 2020;26:302-311.
Vitale SG, Ferrero S, Ciebiera M, et al. Hysteroscopic endometrial resection vs hysterectomy for abnormal uterine bleeding: impact on quality of life and sexuality. Evidence from a systematic review of randomized controlled trials. Curr Opin Obstet Gynecol. 2020;32:159-165.
Two systematic reviews evaluated the efficacy of EA in women with abnormal uterine bleeding. One compared EA with the LNG-IUD and reported on safety and efficacy, while the other compared EA with hysterectomy and reported on quality of life.
Bergeron and colleagues reviewed 13 studies that included 884 women to compare the efficacy and safety of EA or resection with the LNG-IUD for the treatment of premenopausal women with AUB.3 They found no significant differences between EA and the LNG-IUD in terms of subsequent hysterectomy (risk ratio [RR] = 1.3; 95% confidence interval [CI], 0.60-2.11). It was not surprising that, when looking at age, EA was associated with a higher risk for hysterectomy in women younger than age 42 (RR = 5.26; 95% CI, 1.21-22.91). Conversely, subsequent hysterectomy was less likely with EA compared to LNG-IUD use in women older than 42 years. However, statistical significance was not reached in the older group (RR = 0.51; 95% CI, 0.21-1.24).
In the systematic review by Vitale and colleagues, 9 studies met inclusion criteria for a comparison of EA and hysterectomy, with the objective of ascertaining improvement in quality of life and several other measures.4
Although there was significant heterogeneity between assessment tools, both treatment groups experienced similar improvements in quality of life during the first year. However, hysterectomy was more advantageous in terms of improving uterine bleeding and satisfaction in the long term when compared with EA.4
As EA is considered, it is important to continue to counsel about the efficacy of the LNG-IUD, as well as its decreased associated morbidity. Additionally, EA is particularly less effective in younger women.
Continue to: Laparoscopy is best approach for isthomocele management, with caveats...
Laparoscopy is best approach for isthomocele management, with caveats
He Y, Zhong J, Zhou W, et al. Four surgical strategies for the treatment of cesarean scar defect: a systematic review and network meta-analysis. J Minim Invasive Gynecol. 2020;27:593-602.
Vitale SG, Ludwin A, Vilos GA, et al. From hysteroscopy to laparoendoscopic surgery: what is the best surgical approach for symptomatic isthmocele? A systematic review and meta-analysis. Arch Gynecol Obstet. 2020;301:33-52.
The isthmocele (cesarean scar defect, uterine niche), a known complication of cesarean delivery, represents a myometrial defect in the anterior uterine wall that often presents as abnormal uterine bleeding. It also can be a site for pregnancy-related complications, such as invasive placentation, placenta previa, and uterine rupture.
Two systematic reviews compared surgical strategies for treating isthmocele, including laparoscopy, hysteroscopy, combined laparoscopy and hysteroscopy, laparotomy, and vaginal repair.
Laparoscopy reduced isthmocele-associated AUB better than other techniques
A review by He and colleagues analyzed data from 10 pertinent studies (4 RCTs and 6 observational studies) that included 858 patients in total.5 Treatments compared were laparoscopy, hysteroscopy, combined laparoscopy with hysteroscopy, and vaginal repair for reduction of AUB and isthmocele and diverticulum depth.
The authors found no difference in intraoperative bleeding between the 4 surgical methods (laparotomy was not included in this review). Hysteroscopic surgery was associated with the shortest operative time, while laparoscopy was the longest surgery. In terms of reducing intermittent abnormal bleeding and scar depth, laparoscopic surgery performed better than the other 3 methods.
Approach considerations in isthmocele repair
Vitale and colleagues conducted a systematic review that included 33 publications (28 focused on a single surgical technique, 5 compared different techniques) to examine the effectiveness and risks of various surgical approaches for isthmocele in women with AUB, infertility, or for prevention of obstetric complications.6
Results of their analysis in general favored a laparoscopic approach for patients who desired future fertility, with an improvement rate of 92.7%. Hysteroscopic correction had an 85% improvement rate, and vaginal correction had an 82.5% improvement rate.
Although there were no high-level data to suggest a threshold for myometrial thickness in recommending a surgical approach, the authors provided a helpful algorithm for choosing a route based on a patient's fertility desires. For the asymptomatic patient, they suggest no treatment. In symptomatic patients, the laparoscopic approach is the gold standard but requires significant laparoscopic surgical skill, and a hysteroscopic approach may be considered as an alternative route if the residual myometrial defect is greater than 2.5 to 3.5 mm. For patients who are not considering future reproduction, hysteroscopy is the gold standard as long as the residual myometrial thickness is greater than 2.5 to 3.5 mm.
The takeaway. Of the several methods used for surgical isthmocele management, the laparoscopic approach reduced intermittent abnormal bleeding and scar depth better than other methods. It also was associated with the longest surgical duration. Hysteroscopic surgery was the quickest procedure to perform and is effective in removing the upper valve to promote the elimination of the hematocele and symptoms of abnormal bleeding; however, it does not change the anatomic aspects of the isthmocele in terms of myometrial thickness. Some authors suggested that deciding on the surgical route should be based on fertility desires and the residual thickness of the myometrium. ●
In terms of isthmocele repair, the laparoscopic approach is preferred in patients who desire fertility, as long as the surgeon possesses the skill set to perform this difficult surgery, and as long as the residual myometrium is thicker than 2.5 to 3.5 mm.
- Bofill Rodriguez M, Lethaby A, Low C, et al. Cyclical progestogens for heavy menstrual bleeding. Cochrane Database Syst Rev. 2019;(8):CD001016.
- Lukes AS, Moore KA, Muse KN, et al. Tranexamic acid treatment for heavy menstrual bleeding: a randomized controlled study. Obstet Gynecol. 2010;116:865-875.
- Bergeron C, Laberge PY, Boutin A, et al. Endometrial ablation or resection versus levonorgestrel intra-uterine system for the treatment of women with heavy menstrual bleeding and a normal uterine cavity: a systematic review with meta-analysis. Hum Reprod Update. 2020;26:302-311.
- Vitale SG, Ferrero S, Ciebiera M, et al. Hysteroscopic endometrial resection vs hysterectomy for abnormal uterine bleeding: impact on quality of life and sexuality. Evidence from a systematic review of randomized controlled trials. Curr Opin Obstet Gynecol. 2020;32:159-165.
- He Y, Zhong J, Zhou W, et al. Four surgical strategies for the treatment of cesarean scar defect: a systematic review and network meta-analysis. J Minim Invasive Gynecol. 2020;27:593-602.
- Vitale SG, Ludwin A, Vilos GA, et al. From hysteroscopy to laparoendoscopic surgery: what is the best surgical approach for symptomatic isthmocele? A systematic review and meta-analysis. Arch Gynecol Obstet. 2020;301:33-52.
- Bofill Rodriguez M, Lethaby A, Low C, et al. Cyclical progestogens for heavy menstrual bleeding. Cochrane Database Syst Rev. 2019;(8):CD001016.
- Lukes AS, Moore KA, Muse KN, et al. Tranexamic acid treatment for heavy menstrual bleeding: a randomized controlled study. Obstet Gynecol. 2010;116:865-875.
- Bergeron C, Laberge PY, Boutin A, et al. Endometrial ablation or resection versus levonorgestrel intra-uterine system for the treatment of women with heavy menstrual bleeding and a normal uterine cavity: a systematic review with meta-analysis. Hum Reprod Update. 2020;26:302-311.
- Vitale SG, Ferrero S, Ciebiera M, et al. Hysteroscopic endometrial resection vs hysterectomy for abnormal uterine bleeding: impact on quality of life and sexuality. Evidence from a systematic review of randomized controlled trials. Curr Opin Obstet Gynecol. 2020;32:159-165.
- He Y, Zhong J, Zhou W, et al. Four surgical strategies for the treatment of cesarean scar defect: a systematic review and network meta-analysis. J Minim Invasive Gynecol. 2020;27:593-602.
- Vitale SG, Ludwin A, Vilos GA, et al. From hysteroscopy to laparoendoscopic surgery: what is the best surgical approach for symptomatic isthmocele? A systematic review and meta-analysis. Arch Gynecol Obstet. 2020;301:33-52.
Evidence-based management of early pregnancy loss
The American College of Obstetricians and Gynecologists (ACOG) defines early pregnancy loss (EPL) as a nonviable, intrauterine pregnancy up to 12 6/7 weeks’ gestation.1 The term EPL has been used interchangeably with miscarriage, spontaneous abortion, and early pregnancy failure; the preferred terms among US women who experience pregnancy loss are EPL and miscarriage.2 EPL is the most common complication of early pregnancy and accounts for up to 15% to 20% of clinically recognized pregnancies.3
The most common cause of EPL is a chromosomal abnormality (TABLE 1). Other common etiologies include structural abnormalities, such as uterine fibroids or polyps. Risk factors for EPL include maternal age, prior pregnancy loss, and various maternal conditions and medication and substance use (TABLE 2).


Definitive diagnosis of EPL often requires more than 1 ultrasonography scan or other examination to determine whether a pregnancy is nonviable versus too early to confirm viability. The consensus guidelines from the Society of Radiologists in Ultrasound provide transvaginal ultrasonographic criteria to diagnose EPL (TABLE 3).4 Two of the diagnostic criteria require only 1 ultrasonography scan while the others require repeat ultrasonography.
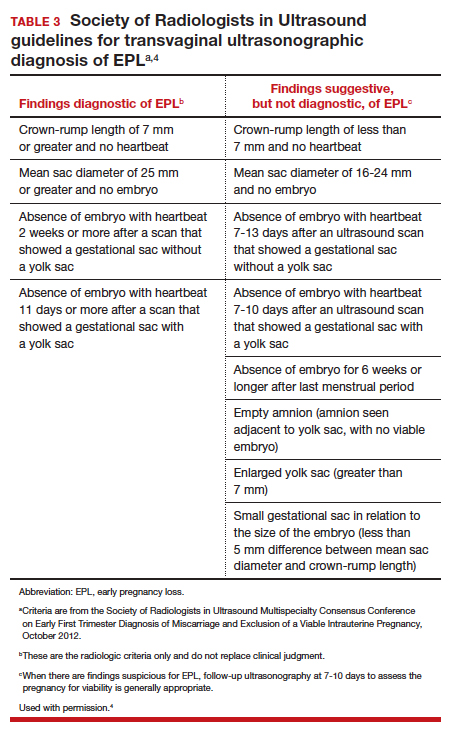
Note that a definitive diagnosis may be more important to some patients than others due to differing pregnancy intent and/or desirableness. Patients may choose to take action in terms of medication or uterine aspiration based on suspicion of EPL, or they may wish to end the pregnancy regardless of EPL diagnosis.
Management options for EPL
EPL can be managed expectantly, with medication, or with uterine aspiration. These methods have different risks and benefits, and in most cases all should be made available to women who experience EPL.5-7
Expectant management
Expectant management involves waiting for the body to spontaneously expel the nonviable pregnancy. In the absence of any signs of infection, hemodynamic instability, or other medical instability, it is safe and reasonable to wait a month or more before intervening, according to patient choice. Expectant management is up to 80% effective.8
Medication management
Medication management entails using mifepristone and misoprostol, or misoprostol alone, to cause uterine contractions to expel the pregnancy. A landmark study demonstrated that medication management of EPL with the combination of mifepristone and misoprostol is significantly more effective than misoprostol alone.9 While the mean cost of mifepristone is approximately $90 per dose, its addition is cost-effective given the increased efficacy.10
The evidence-based combination regimen is to provide mifepristone 200 mg orally, followed 24 hours later by misoprostol 800 µg vaginally, for a success rate of 87.8% by 8 days, and 91.2% by 30 days posttreatment. Success rates can be increased further by adding a second dose of misoprostol to take as needed.5
We strongly recommend using the combination regimen if you have access to mifepristone. If you do not have access to mifepristone in your clinical setting, perhaps this indication for use can help facilitate getting it onto your formulary. (See “Ordering mifepristone” below.)
Without access to mifepristone, medication abortion still should be offered after discussing the decreased efficacy with patients. The first-trimester misoprostol-only regimen for EPL is to give misoprostol 800 µg buccally, vaginally, or sublingually, with a second dose if there is no effect (TABLE 4).1,5 For losses after 9 weeks, some data suggest adding additional doses of misoprostol 400 µg every 3 hours until expulsion.11
- There are 2 distributors of mifepristone in the United States. Danco (www.earlyoptionpill.com) distributes the branded Mifeprex and GenBioPro (www.genbiopro.com) distributes generic mifepristone.
- To order mifepristone, 1 health care provider from your clinic or facility must read and sign the distributor’s prescriber agreement and account setup form. These forms and instructions can be found on each distributor’s website. Future orders can be made by calling the distributor directly (Danco: 1-877-432-7596; GenBioPro: 1-855-643-3463).
- The shelf life of mifepristone is 18 months.
- Each patient who receives mifepristone needs to read and sign a patient agreement (available on distributor websites), as required by the US Food and Drug Administration–approved Risk Evaluation and Mitigation Strategy (REMS) program.
Continue to: Uterine aspiration...
Uterine aspiration
Uterine aspiration is the third management option for EPL and is virtually 100% successful. Although aspiration is used when expectant or medication management fails, it is also a first-line option based on patient choice or contraindications to the other 2 management options.
We recommend either manual vacuum aspiration (MVA) or electric vacuum aspiration (EVA); sharp curettage almost never should be used. Uterine aspiration can be performed safely in a clinic, emergency department, or operating room (OR) setting, depending on patient characteristics and desires.12-14 For various reasons, many patients prefer outpatient management. These reasons may include avoiding the costs and delays associated with OR management, wanting more control over who performs the procedure, or avoiding more significant/general anesthesia. MVA in the outpatient setting is the most cost-effective approach to uterine aspiration.15
Choosing a management approach
There are virtually no contraindications for uterine aspiration. Expectant and medication management are contraindicated (and uterine aspiration is recommended) in the setting of bleeding disorders, anticoagulation, suspected intrauterine infection, suspected molar pregnancy, significant cardiopulmonary disease, or any condition for which heavy, unsupervised bleeding might be dangerous.1 Uterine aspiration offers immediate resolution, with a procedure usually lasting 3 to 10 minutes. By contrast, expectant and medication management offer a less predictable time to resolution and, often, a more prolonged period of active pregnancy expulsion.
In the absence of a contraindication, patient choice should determine which management option is used. All 3 options are similarly safe and effective, and the differences that do exist are acceptable to patients as long as they are allowed to access their preferred EPL management method.5,6,16 Patient satisfaction is associated directly with the ability to choose the method of preference.
Managing pain
Pain management should be offered to all women diagnosed with EPL. Those who choose expectant or medication management likely will require only oral nonsteroidal anti-inflammatory drugs (NSAIDs). A minority may require the addition of a small number of narcotic pain pills.17
Women who choose uterine aspiration also should be offered pain management. All patients should be given a paracervical block; other medications can include NSAIDs, an oral benzodiazepine, intravenous (IV) sedation, or even general anesthesia/monitored airway care.17
Patients’ expectations about pain management should be addressed directly during initial counseling. This may help patients decide what type of management and treatment location they might prefer.
Checking blood type: Is it necessary?
The ACOG practice bulletin for EPL states, “administration of Rh D immune globulin should be considered in cases of early pregnancy loss, especially those that are later in the first trimester.”1 A growing body of evidence indicates that Rho(D) immune globulin likely is unnecessary in early pregnancy.
A recent prospective cohort study of 42 women who were at 5 to 12 weeks’ gestation found that the fetal red blood cell concentration was below the calculated threshold for Rh sensitization.18 In light of recent evidence, the National Abortion Federation now recommends foregoing Rh testing and provision of Rh immune globulin at less than 8 weeks’ gestation for uterine aspiration and at less than 10 weeks’ gestation for medication abortion.19
We feel there is sufficient evidence to forego Rh testing in EPL at similar gestational ages, although this is not yet reflected in US societal guidelines. (It is already standard practice in some countries.) Although the risk of Rh alloimmunization is low, the risk of significant consequences in the event of Rh alloimmunization is high. Currently, it also is reasonable to continue giving Rho(D) immune globulin to Rh-negative patients who experience EPL at any gestational age. A lower dose (50 µg) is sufficient for EPL; the standard 300-µg dose also is acceptable.20
We anticipate that society and ACOG guidelines will change in the next few years as the body of evidence increases, and practice should change to reflect new guidance.
Continue to: Prophylactic antibiotics...
Prophylactic antibiotics
The risk of infection with EPL is low overall regardless of the management approach.1 Prophylactic antibiotics are recommended for patients undergoing uterine aspiration but are not necessary in the setting of expectant or medication management. We recommend prophylaxis with 1 dose of oral doxycycline 200 mg or oral azithromycin 500 mg approximately 30 minutes to 1 hour prior to uterine aspiration.21 Alternatives include 1 dose of oral metronidazole 500 mg or, if the patient is unable to take oral medications, IV cefazolin 2 g.
A multisite international randomized controlled trial concluded that antibiotic prophylaxis before uterine aspiration for EPL did not significantly reduce the risk of infection.22 However, there was a significant reduction in pelvic infection with antibiotic administration for the subgroup of women who underwent MVA, which is our recommended approach (along with EVA, and opposed to sharp curettage) for outpatient EPL management.
Follow-up after EPL
In-person follow-up after treatment of EPL is not medically necessary. A repeat ultrasonography 1 to 2 weeks after expectant or medication management can be helpful to confirm completion of the process, and clinicians should focus on presence or absence of a gestational sac to determine if further management is needed.1
Follow-up by telemedicine or phone also is an option and may be preferred in the following situations:
- the patient lives far from the clinic
- travel to the clinic is difficult or expensive
- the patient has child-care issues
- there is a global pandemic necessitating physical distancing.
If the patient’s reported history and symptoms are consistent with a completed process, no further intervention is indicated.
If ongoing EPL is a concern, ask the patient to come in for an evaluation and ultrasonography. If visiting the clinic is still a challenge, following with urine or serum human chorionic gonadotropin (HCG) levels also is acceptable. Experts recommend waiting 4 weeks before expecting a negative urine HCG measurement, although up to 25% of women with a completed EPL will still have a positive test at 4 weeks.23,24
A postprocedure serum HCG is more helpful if a preprocedure HCG level already is known. Numerous studies have evaluated phone follow-up after medication abortion and it is reasonable to translate these practices to follow-up after EPL, recognizing that direct data looking at alternative EPL follow-up are much more limited.23,25-30
The benefit of HCG follow-up at a scheduled time (such as 1 week) is less clear for EPL than for medication abortion, as HCG trends are less predictable in the setting of EPL. However, if the pregnancy has passed, a significant drop in the HCG level would be expected. It is important to take into account the patient’s history and clinical symptoms and consider in-person evaluation with possible ultrasonography if there is concern that the pregnancy tissue has not passed.
Pay attention to mental health
It is critical to assess the patient’s mental and emotional health. This should be done both at the time of EPL diagnosis and management and again at follow-up. Both patients and their partners can struggle after experiencing EPL, and they may suffer from prolonged posttraumatic stress.31
Often, EPL occurs before people have shared the news about their pregnancy. This can amplify the sense of isolation and sadness many women report. Equally critical is recognizing that not all women who experience EPL grieve, and clinicians should normalize patient experiences and feelings. Provider language is important. We recommend use of these questions and phrases:
- I’m so sorry for your loss.
- How are you feeling?
- How have you been doing since I saw you last?
- Your friends/family/partner may be grieving differently or at a different pace than you—this is normal.
- Just because the EPL process is complete doesn’t necessarily mean your processing and/or grieving is over.
- Whatever you’re feeling is okay.
Continue to: Address desire for future pregnancy or contraception...
Address desire for future pregnancy or contraception
No additional workup is necessary after EPL unless a patient is experiencing recurrent pregnancy loss. We do recommend discussing plans for future conception. If a patient wants to conceive again as soon as possible, she can start trying when she feels emotionally ready (even before her next menstrual period). One study found that the ability to conceive and those pregnancy outcomes were the same when patients were randomly assigned to start trying immediately versus waiting 3 months after EPL.32
Alternatively, a patient may want to prevent pregnancy after EPL, and this information should be explicitly elicited and addressed with comprehensive contraception counseling as needed. All forms of contraception can be initiated immediately on successful management of EPL. All contraceptive methods, including an intrauterine device, can be initiated immediately following uterine aspiration.1,33,34
Patients should be reminded that if they delay contraception initiation by more than 7 days, they are potentially at risk for pregnancy.35 Most importantly, clinicians should not make assumptions about future pregnancy desires and should ask open-ended questions to provide appropriate patient counseling.
Finally, patients may feel additional anxiety in a subsequent pregnancy. It is helpful to acknowledge this and perhaps even offer earlier and more frequent visits in early pregnancy to help reduce anxiety. EPL is commonly experienced, and unfortunately it is sometimes poorly addressed by clinicians.
We hope this guidance will help you provide excellent, evidence-based, and sensitive care that will not only manage your patient’s EPL but also make the experience as positive as possible. ●
- Early pregnancy loss (EPL) is common, occurring in up to 15% to 20% of clinically recognized pregnancies.
- EPL can be managed expectantly, with medication, or by uterine aspiration.
- There are virtually no contraindications to uterine aspiration.
- Contraindications to expectant or medication management include any situation in which heavy, unsupervised bleeding might be dangerous.
- In the absence of contraindications, patient preference should dictate the management approach.
- Mifepristone-misoprostol is more effective than misoprostol alone.
- Manual uterine aspiration in the outpatient setting is the most cost-effective approach to uterine evacuation.
- Rh testing is not necessary at less than 8 weeks’ gestation if choosing uterine aspiration, or at less than 10 weeks’ gestation if choosing expectant or medication management.
- Antibiotic prophylaxis is indicated for uterine aspiration, but not for expectant or medication management.
- Ultrasonography follow-up should focus on presence or absence of gestational sac.
- There are viable telemedicine and phone follow-up options that do not require repeat ultrasonography or in-person evaluation.
- There is no need to delay future conception once EPL management is confirmed to be complete.
- It is okay to initiate any contraceptive method immediately on completed management of EPL.
- Feelings toward EPL can be complex and varied; it is helpful to normalize your patients’ experiences, ask open-ended questions, and provide support as needed.
- American College of Obstetricians and Gynecologists. Practice bulletin No. 200: early pregnancy loss. Obstet Gynecol. 2018;132:e197-e207.
- Clement EG, Horvath S, McAllister A, et al. The language of first-trimester nonviable pregnancy: patient-reported preferences and clarity. Obstet Gynecol. 2019;133:149-154.
- Ventura SJ, Curtin SC, Abma JC, et al. Estimated pregnancy rates and rates of pregnancy outcomes for the United States, 1990-2008. Natl Vital Stat Rep. 2012;60:1-21.
- Doubilet PM, Benson CB, Bourne T, et al; Society of Radiologists in Ultrasound Multispecialty Panel on Early First Trimester Diagnosis of Miscarriage and Exclusion of a Viable Uterine Pregnancy. Diagnostic criteria for nonviable pregnancy early in the first trimester. N Engl J Med. 2013;369:1443-1451.
- Zhang J, Gilles JM, Barnhart K, et al. A comparison of medical management with misoprostol and surgical management for early pregnancy failure. N Engl J Med. 2005;353:761-769.
- Nanda K, Peloggia A, Grimes D, et al. Expectant care versus surgical treatment for miscarriage. Cochrane Database Syst Rev. 2006(2):CD003518.
- Neilson JP, Hickey M, Vazquez J. Medical treatment for early fetal death (less than 24 weeks). Cochrane Database Syst Rev. 2006(3):CD002253.
- Luise C, Jermy K, May C, et al. Outcome of expectant management of spontaneous first trimester miscarriage: observational study. BMJ. 2002;324:873-875.
- Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Nagendra D, Koelper N, Loza-Avalos SE, et al. Cost-effectiveness of mifepristone pretreatment for the medical management of nonviable early pregnancy: secondary analysis of a randomized clinical trial. JAMA Netw Open. 2020;3:e201594.
- World Health Organization. Safe Abortion: Technical and Policy Guidance for Health Systems. 2nd ed. Geneva, Switzerland: World Health Organization; 2012.
- Wiebe E, Janssen P. Management of spontaneous abortion in family practices and hospitals. Fam Med. 1998;30:293-296.
- Harris LH, Dalton VK, Johnson TR. Surgical management of early pregnancy failure: history, politics, and safe, cost-effective care. Am J Obstet Gynecol. 2007;196:445.e1-e5.
- Dalton VK, Harris L, Weisman CS, et al. Patient preferences, satisfaction, and resource use in office evacuation of early pregnancy failure. Obstet Gynecol. 2006;108:103-110.
- Rausch M, Lorch S, Chung K, et al. A cost-effectiveness analysis of surgical versus medical management of early pregnancy loss. Fertil Steril. 2012;97:355-360.
- Trinder J, Brocklehurst P, Porter R, et al. Management of miscarriage: expectant, medical, or surgical? Results of randomised controlled trial (Miscarriage Treatment [MIST] trial). BMJ. 2006;332:1235-1240.
- Calvache JA, Delgado-Noguera MF, Lesaffre E, et al. Anaesthesia for evacuation of incomplete miscarriage. Cochrane Database System Rev. 2012(4):CD008681.
- Horvath S, Tsao P, Huang ZY, et al. The concentration of fetal red blood cells in first-trimester pregnant women undergoing uterine aspiration is below the calculated threshold for Rh sensitization. Contraception. 2020;102:1-6.
- National Abortion Federation. 2020 clinical policy guidelines for abortion care. https://www.prochoice.org/education-and-advocacy/cpg. Accessed June 9, 2020.
- American College of Obstetricians and Gynecologists. Practice bulletin No. 181: prevention of Rh D alloimmunization. Obstet Gynecol. 2017;130:e59-e70.
- American College of Obstetricians and Gynecologists. Practice bulletin No. 104: antibiotic prophylaxis for gynecologic procedures. Obstet Gynecol. 2009;113:1180-1189.
- Lissauer D, Wilson A, Hewitt CA, et al. A randomized trial of prophylactic antibiotics for miscarriage surgery. N Engl J Med. 2019;380:1012-1021.
- Perriera L, Reeves MF, Chen BA, et al. Feasibility of telephone follow-up after medical abortion. Contraception. 2010:81:143-149.
- Barnhart K, Sammel MD, Chung K, et al. Decline of serum human chorionic gonadotropin and spontaneous complete abortion: defining the normal curve. Obstet Gynecol. 2004;104(5 pt 1):975-981.
- Chen MJ, Rounds KM, Creinin MD, et al. Comparing office and telephone follow-up after medical abortion. Contraception. 2016;94:122-126.
- Clark W, Bracken H, Tanenhaus J, et al. Alternatives to a routine follow-up visit for early medical abortion. Obstet Gynecol. 2010;115(2 pt 1):264-272.
- Jackson AV, Dayananda I, Fortin JM, et al. Can women accurately assess the outcome of medical abortion based on symptoms alone? Contraception. 2012;85:192-197.
- Raymond EG, Tan YL, Grant M, et al. Self-assessment of medical abortion outcome using symptoms and home pregnancy testing. Contraception. 2018;97:324-328.
- Raymond EG, Shochet T, Bracken H. Low-sensitivity urine pregnancy testing to assess medical abortion outcome: a systematic review. Contraception. 2018;98:30-35.
- Raymond EG, Grossman D, Mark A, et al. Commentary: no-test medication abortion: a sample protocol for increasing access during a pandemic and beyond. Contraception. 2020;101:361-366.
- Farren J, Jalmbrant M, Ameye L, et al. Post-traumatic stress, anxiety and depression following miscarriage or ectopic pregnancy: a prospective cohort study. BMJ Open. 2016;6:e011864.
- Schliep KC, Mitchell EM, Mumford SL, et al. Trying to conceive after an early pregnancy loss: an assessment on how long couples should wait. Obstet Gynecol. 2016;127:204-212. DOI: 0.1097/AOG.0000000000001159.
- American College of Obstetricians and Gynecologists. Committee opinion No. 642: increasing access to contraceptive implants and intrauterine devices to reduce unintended pregnancy. Obstet Gynecol. 2015;126:e44-e48.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. US medical eligibility criteria (US MEC) for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-103.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. US selected practice recommendations for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-66.
The American College of Obstetricians and Gynecologists (ACOG) defines early pregnancy loss (EPL) as a nonviable, intrauterine pregnancy up to 12 6/7 weeks’ gestation.1 The term EPL has been used interchangeably with miscarriage, spontaneous abortion, and early pregnancy failure; the preferred terms among US women who experience pregnancy loss are EPL and miscarriage.2 EPL is the most common complication of early pregnancy and accounts for up to 15% to 20% of clinically recognized pregnancies.3
The most common cause of EPL is a chromosomal abnormality (TABLE 1). Other common etiologies include structural abnormalities, such as uterine fibroids or polyps. Risk factors for EPL include maternal age, prior pregnancy loss, and various maternal conditions and medication and substance use (TABLE 2).


Definitive diagnosis of EPL often requires more than 1 ultrasonography scan or other examination to determine whether a pregnancy is nonviable versus too early to confirm viability. The consensus guidelines from the Society of Radiologists in Ultrasound provide transvaginal ultrasonographic criteria to diagnose EPL (TABLE 3).4 Two of the diagnostic criteria require only 1 ultrasonography scan while the others require repeat ultrasonography.

Note that a definitive diagnosis may be more important to some patients than others due to differing pregnancy intent and/or desirableness. Patients may choose to take action in terms of medication or uterine aspiration based on suspicion of EPL, or they may wish to end the pregnancy regardless of EPL diagnosis.
Management options for EPL
EPL can be managed expectantly, with medication, or with uterine aspiration. These methods have different risks and benefits, and in most cases all should be made available to women who experience EPL.5-7
Expectant management
Expectant management involves waiting for the body to spontaneously expel the nonviable pregnancy. In the absence of any signs of infection, hemodynamic instability, or other medical instability, it is safe and reasonable to wait a month or more before intervening, according to patient choice. Expectant management is up to 80% effective.8
Medication management
Medication management entails using mifepristone and misoprostol, or misoprostol alone, to cause uterine contractions to expel the pregnancy. A landmark study demonstrated that medication management of EPL with the combination of mifepristone and misoprostol is significantly more effective than misoprostol alone.9 While the mean cost of mifepristone is approximately $90 per dose, its addition is cost-effective given the increased efficacy.10
The evidence-based combination regimen is to provide mifepristone 200 mg orally, followed 24 hours later by misoprostol 800 µg vaginally, for a success rate of 87.8% by 8 days, and 91.2% by 30 days posttreatment. Success rates can be increased further by adding a second dose of misoprostol to take as needed.5
We strongly recommend using the combination regimen if you have access to mifepristone. If you do not have access to mifepristone in your clinical setting, perhaps this indication for use can help facilitate getting it onto your formulary. (See “Ordering mifepristone” below.)
Without access to mifepristone, medication abortion still should be offered after discussing the decreased efficacy with patients. The first-trimester misoprostol-only regimen for EPL is to give misoprostol 800 µg buccally, vaginally, or sublingually, with a second dose if there is no effect (TABLE 4).1,5 For losses after 9 weeks, some data suggest adding additional doses of misoprostol 400 µg every 3 hours until expulsion.11
- There are 2 distributors of mifepristone in the United States. Danco (www.earlyoptionpill.com) distributes the branded Mifeprex and GenBioPro (www.genbiopro.com) distributes generic mifepristone.
- To order mifepristone, 1 health care provider from your clinic or facility must read and sign the distributor’s prescriber agreement and account setup form. These forms and instructions can be found on each distributor’s website. Future orders can be made by calling the distributor directly (Danco: 1-877-432-7596; GenBioPro: 1-855-643-3463).
- The shelf life of mifepristone is 18 months.
- Each patient who receives mifepristone needs to read and sign a patient agreement (available on distributor websites), as required by the US Food and Drug Administration–approved Risk Evaluation and Mitigation Strategy (REMS) program.

Continue to: Uterine aspiration...
Uterine aspiration
Uterine aspiration is the third management option for EPL and is virtually 100% successful. Although aspiration is used when expectant or medication management fails, it is also a first-line option based on patient choice or contraindications to the other 2 management options.
We recommend either manual vacuum aspiration (MVA) or electric vacuum aspiration (EVA); sharp curettage almost never should be used. Uterine aspiration can be performed safely in a clinic, emergency department, or operating room (OR) setting, depending on patient characteristics and desires.12-14 For various reasons, many patients prefer outpatient management. These reasons may include avoiding the costs and delays associated with OR management, wanting more control over who performs the procedure, or avoiding more significant/general anesthesia. MVA in the outpatient setting is the most cost-effective approach to uterine aspiration.15
Choosing a management approach
There are virtually no contraindications for uterine aspiration. Expectant and medication management are contraindicated (and uterine aspiration is recommended) in the setting of bleeding disorders, anticoagulation, suspected intrauterine infection, suspected molar pregnancy, significant cardiopulmonary disease, or any condition for which heavy, unsupervised bleeding might be dangerous.1 Uterine aspiration offers immediate resolution, with a procedure usually lasting 3 to 10 minutes. By contrast, expectant and medication management offer a less predictable time to resolution and, often, a more prolonged period of active pregnancy expulsion.
In the absence of a contraindication, patient choice should determine which management option is used. All 3 options are similarly safe and effective, and the differences that do exist are acceptable to patients as long as they are allowed to access their preferred EPL management method.5,6,16 Patient satisfaction is associated directly with the ability to choose the method of preference.
Managing pain
Pain management should be offered to all women diagnosed with EPL. Those who choose expectant or medication management likely will require only oral nonsteroidal anti-inflammatory drugs (NSAIDs). A minority may require the addition of a small number of narcotic pain pills.17
Women who choose uterine aspiration also should be offered pain management. All patients should be given a paracervical block; other medications can include NSAIDs, an oral benzodiazepine, intravenous (IV) sedation, or even general anesthesia/monitored airway care.17
Patients’ expectations about pain management should be addressed directly during initial counseling. This may help patients decide what type of management and treatment location they might prefer.
Checking blood type: Is it necessary?
The ACOG practice bulletin for EPL states, “administration of Rh D immune globulin should be considered in cases of early pregnancy loss, especially those that are later in the first trimester.”1 A growing body of evidence indicates that Rho(D) immune globulin likely is unnecessary in early pregnancy.
A recent prospective cohort study of 42 women who were at 5 to 12 weeks’ gestation found that the fetal red blood cell concentration was below the calculated threshold for Rh sensitization.18 In light of recent evidence, the National Abortion Federation now recommends foregoing Rh testing and provision of Rh immune globulin at less than 8 weeks’ gestation for uterine aspiration and at less than 10 weeks’ gestation for medication abortion.19
We feel there is sufficient evidence to forego Rh testing in EPL at similar gestational ages, although this is not yet reflected in US societal guidelines. (It is already standard practice in some countries.) Although the risk of Rh alloimmunization is low, the risk of significant consequences in the event of Rh alloimmunization is high. Currently, it also is reasonable to continue giving Rho(D) immune globulin to Rh-negative patients who experience EPL at any gestational age. A lower dose (50 µg) is sufficient for EPL; the standard 300-µg dose also is acceptable.20
We anticipate that society and ACOG guidelines will change in the next few years as the body of evidence increases, and practice should change to reflect new guidance.
Continue to: Prophylactic antibiotics...
Prophylactic antibiotics
The risk of infection with EPL is low overall regardless of the management approach.1 Prophylactic antibiotics are recommended for patients undergoing uterine aspiration but are not necessary in the setting of expectant or medication management. We recommend prophylaxis with 1 dose of oral doxycycline 200 mg or oral azithromycin 500 mg approximately 30 minutes to 1 hour prior to uterine aspiration.21 Alternatives include 1 dose of oral metronidazole 500 mg or, if the patient is unable to take oral medications, IV cefazolin 2 g.
A multisite international randomized controlled trial concluded that antibiotic prophylaxis before uterine aspiration for EPL did not significantly reduce the risk of infection.22 However, there was a significant reduction in pelvic infection with antibiotic administration for the subgroup of women who underwent MVA, which is our recommended approach (along with EVA, and opposed to sharp curettage) for outpatient EPL management.
Follow-up after EPL
In-person follow-up after treatment of EPL is not medically necessary. A repeat ultrasonography 1 to 2 weeks after expectant or medication management can be helpful to confirm completion of the process, and clinicians should focus on presence or absence of a gestational sac to determine if further management is needed.1
Follow-up by telemedicine or phone also is an option and may be preferred in the following situations:
- the patient lives far from the clinic
- travel to the clinic is difficult or expensive
- the patient has child-care issues
- there is a global pandemic necessitating physical distancing.
If the patient’s reported history and symptoms are consistent with a completed process, no further intervention is indicated.
If ongoing EPL is a concern, ask the patient to come in for an evaluation and ultrasonography. If visiting the clinic is still a challenge, following with urine or serum human chorionic gonadotropin (HCG) levels also is acceptable. Experts recommend waiting 4 weeks before expecting a negative urine HCG measurement, although up to 25% of women with a completed EPL will still have a positive test at 4 weeks.23,24
A postprocedure serum HCG is more helpful if a preprocedure HCG level already is known. Numerous studies have evaluated phone follow-up after medication abortion and it is reasonable to translate these practices to follow-up after EPL, recognizing that direct data looking at alternative EPL follow-up are much more limited.23,25-30
The benefit of HCG follow-up at a scheduled time (such as 1 week) is less clear for EPL than for medication abortion, as HCG trends are less predictable in the setting of EPL. However, if the pregnancy has passed, a significant drop in the HCG level would be expected. It is important to take into account the patient’s history and clinical symptoms and consider in-person evaluation with possible ultrasonography if there is concern that the pregnancy tissue has not passed.
Pay attention to mental health
It is critical to assess the patient’s mental and emotional health. This should be done both at the time of EPL diagnosis and management and again at follow-up. Both patients and their partners can struggle after experiencing EPL, and they may suffer from prolonged posttraumatic stress.31
Often, EPL occurs before people have shared the news about their pregnancy. This can amplify the sense of isolation and sadness many women report. Equally critical is recognizing that not all women who experience EPL grieve, and clinicians should normalize patient experiences and feelings. Provider language is important. We recommend use of these questions and phrases:
- I’m so sorry for your loss.
- How are you feeling?
- How have you been doing since I saw you last?
- Your friends/family/partner may be grieving differently or at a different pace than you—this is normal.
- Just because the EPL process is complete doesn’t necessarily mean your processing and/or grieving is over.
- Whatever you’re feeling is okay.
Continue to: Address desire for future pregnancy or contraception...
Address desire for future pregnancy or contraception
No additional workup is necessary after EPL unless a patient is experiencing recurrent pregnancy loss. We do recommend discussing plans for future conception. If a patient wants to conceive again as soon as possible, she can start trying when she feels emotionally ready (even before her next menstrual period). One study found that the ability to conceive and those pregnancy outcomes were the same when patients were randomly assigned to start trying immediately versus waiting 3 months after EPL.32
Alternatively, a patient may want to prevent pregnancy after EPL, and this information should be explicitly elicited and addressed with comprehensive contraception counseling as needed. All forms of contraception can be initiated immediately on successful management of EPL. All contraceptive methods, including an intrauterine device, can be initiated immediately following uterine aspiration.1,33,34
Patients should be reminded that if they delay contraception initiation by more than 7 days, they are potentially at risk for pregnancy.35 Most importantly, clinicians should not make assumptions about future pregnancy desires and should ask open-ended questions to provide appropriate patient counseling.
Finally, patients may feel additional anxiety in a subsequent pregnancy. It is helpful to acknowledge this and perhaps even offer earlier and more frequent visits in early pregnancy to help reduce anxiety. EPL is commonly experienced, and unfortunately it is sometimes poorly addressed by clinicians.
We hope this guidance will help you provide excellent, evidence-based, and sensitive care that will not only manage your patient’s EPL but also make the experience as positive as possible. ●
- Early pregnancy loss (EPL) is common, occurring in up to 15% to 20% of clinically recognized pregnancies.
- EPL can be managed expectantly, with medication, or by uterine aspiration.
- There are virtually no contraindications to uterine aspiration.
- Contraindications to expectant or medication management include any situation in which heavy, unsupervised bleeding might be dangerous.
- In the absence of contraindications, patient preference should dictate the management approach.
- Mifepristone-misoprostol is more effective than misoprostol alone.
- Manual uterine aspiration in the outpatient setting is the most cost-effective approach to uterine evacuation.
- Rh testing is not necessary at less than 8 weeks’ gestation if choosing uterine aspiration, or at less than 10 weeks’ gestation if choosing expectant or medication management.
- Antibiotic prophylaxis is indicated for uterine aspiration, but not for expectant or medication management.
- Ultrasonography follow-up should focus on presence or absence of gestational sac.
- There are viable telemedicine and phone follow-up options that do not require repeat ultrasonography or in-person evaluation.
- There is no need to delay future conception once EPL management is confirmed to be complete.
- It is okay to initiate any contraceptive method immediately on completed management of EPL.
- Feelings toward EPL can be complex and varied; it is helpful to normalize your patients’ experiences, ask open-ended questions, and provide support as needed.
The American College of Obstetricians and Gynecologists (ACOG) defines early pregnancy loss (EPL) as a nonviable, intrauterine pregnancy up to 12 6/7 weeks’ gestation.1 The term EPL has been used interchangeably with miscarriage, spontaneous abortion, and early pregnancy failure; the preferred terms among US women who experience pregnancy loss are EPL and miscarriage.2 EPL is the most common complication of early pregnancy and accounts for up to 15% to 20% of clinically recognized pregnancies.3
The most common cause of EPL is a chromosomal abnormality (TABLE 1). Other common etiologies include structural abnormalities, such as uterine fibroids or polyps. Risk factors for EPL include maternal age, prior pregnancy loss, and various maternal conditions and medication and substance use (TABLE 2).


Definitive diagnosis of EPL often requires more than 1 ultrasonography scan or other examination to determine whether a pregnancy is nonviable versus too early to confirm viability. The consensus guidelines from the Society of Radiologists in Ultrasound provide transvaginal ultrasonographic criteria to diagnose EPL (TABLE 3).4 Two of the diagnostic criteria require only 1 ultrasonography scan while the others require repeat ultrasonography.

Note that a definitive diagnosis may be more important to some patients than others due to differing pregnancy intent and/or desirableness. Patients may choose to take action in terms of medication or uterine aspiration based on suspicion of EPL, or they may wish to end the pregnancy regardless of EPL diagnosis.
Management options for EPL
EPL can be managed expectantly, with medication, or with uterine aspiration. These methods have different risks and benefits, and in most cases all should be made available to women who experience EPL.5-7
Expectant management
Expectant management involves waiting for the body to spontaneously expel the nonviable pregnancy. In the absence of any signs of infection, hemodynamic instability, or other medical instability, it is safe and reasonable to wait a month or more before intervening, according to patient choice. Expectant management is up to 80% effective.8
Medication management
Medication management entails using mifepristone and misoprostol, or misoprostol alone, to cause uterine contractions to expel the pregnancy. A landmark study demonstrated that medication management of EPL with the combination of mifepristone and misoprostol is significantly more effective than misoprostol alone.9 While the mean cost of mifepristone is approximately $90 per dose, its addition is cost-effective given the increased efficacy.10
The evidence-based combination regimen is to provide mifepristone 200 mg orally, followed 24 hours later by misoprostol 800 µg vaginally, for a success rate of 87.8% by 8 days, and 91.2% by 30 days posttreatment. Success rates can be increased further by adding a second dose of misoprostol to take as needed.5
We strongly recommend using the combination regimen if you have access to mifepristone. If you do not have access to mifepristone in your clinical setting, perhaps this indication for use can help facilitate getting it onto your formulary. (See “Ordering mifepristone” below.)
Without access to mifepristone, medication abortion still should be offered after discussing the decreased efficacy with patients. The first-trimester misoprostol-only regimen for EPL is to give misoprostol 800 µg buccally, vaginally, or sublingually, with a second dose if there is no effect (TABLE 4).1,5 For losses after 9 weeks, some data suggest adding additional doses of misoprostol 400 µg every 3 hours until expulsion.11
- There are 2 distributors of mifepristone in the United States. Danco (www.earlyoptionpill.com) distributes the branded Mifeprex and GenBioPro (www.genbiopro.com) distributes generic mifepristone.
- To order mifepristone, 1 health care provider from your clinic or facility must read and sign the distributor’s prescriber agreement and account setup form. These forms and instructions can be found on each distributor’s website. Future orders can be made by calling the distributor directly (Danco: 1-877-432-7596; GenBioPro: 1-855-643-3463).
- The shelf life of mifepristone is 18 months.
- Each patient who receives mifepristone needs to read and sign a patient agreement (available on distributor websites), as required by the US Food and Drug Administration–approved Risk Evaluation and Mitigation Strategy (REMS) program.

Continue to: Uterine aspiration...
Uterine aspiration
Uterine aspiration is the third management option for EPL and is virtually 100% successful. Although aspiration is used when expectant or medication management fails, it is also a first-line option based on patient choice or contraindications to the other 2 management options.
We recommend either manual vacuum aspiration (MVA) or electric vacuum aspiration (EVA); sharp curettage almost never should be used. Uterine aspiration can be performed safely in a clinic, emergency department, or operating room (OR) setting, depending on patient characteristics and desires.12-14 For various reasons, many patients prefer outpatient management. These reasons may include avoiding the costs and delays associated with OR management, wanting more control over who performs the procedure, or avoiding more significant/general anesthesia. MVA in the outpatient setting is the most cost-effective approach to uterine aspiration.15
Choosing a management approach
There are virtually no contraindications for uterine aspiration. Expectant and medication management are contraindicated (and uterine aspiration is recommended) in the setting of bleeding disorders, anticoagulation, suspected intrauterine infection, suspected molar pregnancy, significant cardiopulmonary disease, or any condition for which heavy, unsupervised bleeding might be dangerous.1 Uterine aspiration offers immediate resolution, with a procedure usually lasting 3 to 10 minutes. By contrast, expectant and medication management offer a less predictable time to resolution and, often, a more prolonged period of active pregnancy expulsion.
In the absence of a contraindication, patient choice should determine which management option is used. All 3 options are similarly safe and effective, and the differences that do exist are acceptable to patients as long as they are allowed to access their preferred EPL management method.5,6,16 Patient satisfaction is associated directly with the ability to choose the method of preference.
Managing pain
Pain management should be offered to all women diagnosed with EPL. Those who choose expectant or medication management likely will require only oral nonsteroidal anti-inflammatory drugs (NSAIDs). A minority may require the addition of a small number of narcotic pain pills.17
Women who choose uterine aspiration also should be offered pain management. All patients should be given a paracervical block; other medications can include NSAIDs, an oral benzodiazepine, intravenous (IV) sedation, or even general anesthesia/monitored airway care.17
Patients’ expectations about pain management should be addressed directly during initial counseling. This may help patients decide what type of management and treatment location they might prefer.
Checking blood type: Is it necessary?
The ACOG practice bulletin for EPL states, “administration of Rh D immune globulin should be considered in cases of early pregnancy loss, especially those that are later in the first trimester.”1 A growing body of evidence indicates that Rho(D) immune globulin likely is unnecessary in early pregnancy.
A recent prospective cohort study of 42 women who were at 5 to 12 weeks’ gestation found that the fetal red blood cell concentration was below the calculated threshold for Rh sensitization.18 In light of recent evidence, the National Abortion Federation now recommends foregoing Rh testing and provision of Rh immune globulin at less than 8 weeks’ gestation for uterine aspiration and at less than 10 weeks’ gestation for medication abortion.19
We feel there is sufficient evidence to forego Rh testing in EPL at similar gestational ages, although this is not yet reflected in US societal guidelines. (It is already standard practice in some countries.) Although the risk of Rh alloimmunization is low, the risk of significant consequences in the event of Rh alloimmunization is high. Currently, it also is reasonable to continue giving Rho(D) immune globulin to Rh-negative patients who experience EPL at any gestational age. A lower dose (50 µg) is sufficient for EPL; the standard 300-µg dose also is acceptable.20
We anticipate that society and ACOG guidelines will change in the next few years as the body of evidence increases, and practice should change to reflect new guidance.
Continue to: Prophylactic antibiotics...
Prophylactic antibiotics
The risk of infection with EPL is low overall regardless of the management approach.1 Prophylactic antibiotics are recommended for patients undergoing uterine aspiration but are not necessary in the setting of expectant or medication management. We recommend prophylaxis with 1 dose of oral doxycycline 200 mg or oral azithromycin 500 mg approximately 30 minutes to 1 hour prior to uterine aspiration.21 Alternatives include 1 dose of oral metronidazole 500 mg or, if the patient is unable to take oral medications, IV cefazolin 2 g.
A multisite international randomized controlled trial concluded that antibiotic prophylaxis before uterine aspiration for EPL did not significantly reduce the risk of infection.22 However, there was a significant reduction in pelvic infection with antibiotic administration for the subgroup of women who underwent MVA, which is our recommended approach (along with EVA, and opposed to sharp curettage) for outpatient EPL management.
Follow-up after EPL
In-person follow-up after treatment of EPL is not medically necessary. A repeat ultrasonography 1 to 2 weeks after expectant or medication management can be helpful to confirm completion of the process, and clinicians should focus on presence or absence of a gestational sac to determine if further management is needed.1
Follow-up by telemedicine or phone also is an option and may be preferred in the following situations:
- the patient lives far from the clinic
- travel to the clinic is difficult or expensive
- the patient has child-care issues
- there is a global pandemic necessitating physical distancing.
If the patient’s reported history and symptoms are consistent with a completed process, no further intervention is indicated.
If ongoing EPL is a concern, ask the patient to come in for an evaluation and ultrasonography. If visiting the clinic is still a challenge, following with urine or serum human chorionic gonadotropin (HCG) levels also is acceptable. Experts recommend waiting 4 weeks before expecting a negative urine HCG measurement, although up to 25% of women with a completed EPL will still have a positive test at 4 weeks.23,24
A postprocedure serum HCG is more helpful if a preprocedure HCG level already is known. Numerous studies have evaluated phone follow-up after medication abortion and it is reasonable to translate these practices to follow-up after EPL, recognizing that direct data looking at alternative EPL follow-up are much more limited.23,25-30
The benefit of HCG follow-up at a scheduled time (such as 1 week) is less clear for EPL than for medication abortion, as HCG trends are less predictable in the setting of EPL. However, if the pregnancy has passed, a significant drop in the HCG level would be expected. It is important to take into account the patient’s history and clinical symptoms and consider in-person evaluation with possible ultrasonography if there is concern that the pregnancy tissue has not passed.
Pay attention to mental health
It is critical to assess the patient’s mental and emotional health. This should be done both at the time of EPL diagnosis and management and again at follow-up. Both patients and their partners can struggle after experiencing EPL, and they may suffer from prolonged posttraumatic stress.31
Often, EPL occurs before people have shared the news about their pregnancy. This can amplify the sense of isolation and sadness many women report. Equally critical is recognizing that not all women who experience EPL grieve, and clinicians should normalize patient experiences and feelings. Provider language is important. We recommend use of these questions and phrases:
- I’m so sorry for your loss.
- How are you feeling?
- How have you been doing since I saw you last?
- Your friends/family/partner may be grieving differently or at a different pace than you—this is normal.
- Just because the EPL process is complete doesn’t necessarily mean your processing and/or grieving is over.
- Whatever you’re feeling is okay.
Continue to: Address desire for future pregnancy or contraception...
Address desire for future pregnancy or contraception
No additional workup is necessary after EPL unless a patient is experiencing recurrent pregnancy loss. We do recommend discussing plans for future conception. If a patient wants to conceive again as soon as possible, she can start trying when she feels emotionally ready (even before her next menstrual period). One study found that the ability to conceive and those pregnancy outcomes were the same when patients were randomly assigned to start trying immediately versus waiting 3 months after EPL.32
Alternatively, a patient may want to prevent pregnancy after EPL, and this information should be explicitly elicited and addressed with comprehensive contraception counseling as needed. All forms of contraception can be initiated immediately on successful management of EPL. All contraceptive methods, including an intrauterine device, can be initiated immediately following uterine aspiration.1,33,34
Patients should be reminded that if they delay contraception initiation by more than 7 days, they are potentially at risk for pregnancy.35 Most importantly, clinicians should not make assumptions about future pregnancy desires and should ask open-ended questions to provide appropriate patient counseling.
Finally, patients may feel additional anxiety in a subsequent pregnancy. It is helpful to acknowledge this and perhaps even offer earlier and more frequent visits in early pregnancy to help reduce anxiety. EPL is commonly experienced, and unfortunately it is sometimes poorly addressed by clinicians.
We hope this guidance will help you provide excellent, evidence-based, and sensitive care that will not only manage your patient’s EPL but also make the experience as positive as possible. ●
- Early pregnancy loss (EPL) is common, occurring in up to 15% to 20% of clinically recognized pregnancies.
- EPL can be managed expectantly, with medication, or by uterine aspiration.
- There are virtually no contraindications to uterine aspiration.
- Contraindications to expectant or medication management include any situation in which heavy, unsupervised bleeding might be dangerous.
- In the absence of contraindications, patient preference should dictate the management approach.
- Mifepristone-misoprostol is more effective than misoprostol alone.
- Manual uterine aspiration in the outpatient setting is the most cost-effective approach to uterine evacuation.
- Rh testing is not necessary at less than 8 weeks’ gestation if choosing uterine aspiration, or at less than 10 weeks’ gestation if choosing expectant or medication management.
- Antibiotic prophylaxis is indicated for uterine aspiration, but not for expectant or medication management.
- Ultrasonography follow-up should focus on presence or absence of gestational sac.
- There are viable telemedicine and phone follow-up options that do not require repeat ultrasonography or in-person evaluation.
- There is no need to delay future conception once EPL management is confirmed to be complete.
- It is okay to initiate any contraceptive method immediately on completed management of EPL.
- Feelings toward EPL can be complex and varied; it is helpful to normalize your patients’ experiences, ask open-ended questions, and provide support as needed.
- American College of Obstetricians and Gynecologists. Practice bulletin No. 200: early pregnancy loss. Obstet Gynecol. 2018;132:e197-e207.
- Clement EG, Horvath S, McAllister A, et al. The language of first-trimester nonviable pregnancy: patient-reported preferences and clarity. Obstet Gynecol. 2019;133:149-154.
- Ventura SJ, Curtin SC, Abma JC, et al. Estimated pregnancy rates and rates of pregnancy outcomes for the United States, 1990-2008. Natl Vital Stat Rep. 2012;60:1-21.
- Doubilet PM, Benson CB, Bourne T, et al; Society of Radiologists in Ultrasound Multispecialty Panel on Early First Trimester Diagnosis of Miscarriage and Exclusion of a Viable Uterine Pregnancy. Diagnostic criteria for nonviable pregnancy early in the first trimester. N Engl J Med. 2013;369:1443-1451.
- Zhang J, Gilles JM, Barnhart K, et al. A comparison of medical management with misoprostol and surgical management for early pregnancy failure. N Engl J Med. 2005;353:761-769.
- Nanda K, Peloggia A, Grimes D, et al. Expectant care versus surgical treatment for miscarriage. Cochrane Database Syst Rev. 2006(2):CD003518.
- Neilson JP, Hickey M, Vazquez J. Medical treatment for early fetal death (less than 24 weeks). Cochrane Database Syst Rev. 2006(3):CD002253.
- Luise C, Jermy K, May C, et al. Outcome of expectant management of spontaneous first trimester miscarriage: observational study. BMJ. 2002;324:873-875.
- Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Nagendra D, Koelper N, Loza-Avalos SE, et al. Cost-effectiveness of mifepristone pretreatment for the medical management of nonviable early pregnancy: secondary analysis of a randomized clinical trial. JAMA Netw Open. 2020;3:e201594.
- World Health Organization. Safe Abortion: Technical and Policy Guidance for Health Systems. 2nd ed. Geneva, Switzerland: World Health Organization; 2012.
- Wiebe E, Janssen P. Management of spontaneous abortion in family practices and hospitals. Fam Med. 1998;30:293-296.
- Harris LH, Dalton VK, Johnson TR. Surgical management of early pregnancy failure: history, politics, and safe, cost-effective care. Am J Obstet Gynecol. 2007;196:445.e1-e5.
- Dalton VK, Harris L, Weisman CS, et al. Patient preferences, satisfaction, and resource use in office evacuation of early pregnancy failure. Obstet Gynecol. 2006;108:103-110.
- Rausch M, Lorch S, Chung K, et al. A cost-effectiveness analysis of surgical versus medical management of early pregnancy loss. Fertil Steril. 2012;97:355-360.
- Trinder J, Brocklehurst P, Porter R, et al. Management of miscarriage: expectant, medical, or surgical? Results of randomised controlled trial (Miscarriage Treatment [MIST] trial). BMJ. 2006;332:1235-1240.
- Calvache JA, Delgado-Noguera MF, Lesaffre E, et al. Anaesthesia for evacuation of incomplete miscarriage. Cochrane Database System Rev. 2012(4):CD008681.
- Horvath S, Tsao P, Huang ZY, et al. The concentration of fetal red blood cells in first-trimester pregnant women undergoing uterine aspiration is below the calculated threshold for Rh sensitization. Contraception. 2020;102:1-6.
- National Abortion Federation. 2020 clinical policy guidelines for abortion care. https://www.prochoice.org/education-and-advocacy/cpg. Accessed June 9, 2020.
- American College of Obstetricians and Gynecologists. Practice bulletin No. 181: prevention of Rh D alloimmunization. Obstet Gynecol. 2017;130:e59-e70.
- American College of Obstetricians and Gynecologists. Practice bulletin No. 104: antibiotic prophylaxis for gynecologic procedures. Obstet Gynecol. 2009;113:1180-1189.
- Lissauer D, Wilson A, Hewitt CA, et al. A randomized trial of prophylactic antibiotics for miscarriage surgery. N Engl J Med. 2019;380:1012-1021.
- Perriera L, Reeves MF, Chen BA, et al. Feasibility of telephone follow-up after medical abortion. Contraception. 2010:81:143-149.
- Barnhart K, Sammel MD, Chung K, et al. Decline of serum human chorionic gonadotropin and spontaneous complete abortion: defining the normal curve. Obstet Gynecol. 2004;104(5 pt 1):975-981.
- Chen MJ, Rounds KM, Creinin MD, et al. Comparing office and telephone follow-up after medical abortion. Contraception. 2016;94:122-126.
- Clark W, Bracken H, Tanenhaus J, et al. Alternatives to a routine follow-up visit for early medical abortion. Obstet Gynecol. 2010;115(2 pt 1):264-272.
- Jackson AV, Dayananda I, Fortin JM, et al. Can women accurately assess the outcome of medical abortion based on symptoms alone? Contraception. 2012;85:192-197.
- Raymond EG, Tan YL, Grant M, et al. Self-assessment of medical abortion outcome using symptoms and home pregnancy testing. Contraception. 2018;97:324-328.
- Raymond EG, Shochet T, Bracken H. Low-sensitivity urine pregnancy testing to assess medical abortion outcome: a systematic review. Contraception. 2018;98:30-35.
- Raymond EG, Grossman D, Mark A, et al. Commentary: no-test medication abortion: a sample protocol for increasing access during a pandemic and beyond. Contraception. 2020;101:361-366.
- Farren J, Jalmbrant M, Ameye L, et al. Post-traumatic stress, anxiety and depression following miscarriage or ectopic pregnancy: a prospective cohort study. BMJ Open. 2016;6:e011864.
- Schliep KC, Mitchell EM, Mumford SL, et al. Trying to conceive after an early pregnancy loss: an assessment on how long couples should wait. Obstet Gynecol. 2016;127:204-212. DOI: 0.1097/AOG.0000000000001159.
- American College of Obstetricians and Gynecologists. Committee opinion No. 642: increasing access to contraceptive implants and intrauterine devices to reduce unintended pregnancy. Obstet Gynecol. 2015;126:e44-e48.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. US medical eligibility criteria (US MEC) for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-103.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. US selected practice recommendations for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-66.
- American College of Obstetricians and Gynecologists. Practice bulletin No. 200: early pregnancy loss. Obstet Gynecol. 2018;132:e197-e207.
- Clement EG, Horvath S, McAllister A, et al. The language of first-trimester nonviable pregnancy: patient-reported preferences and clarity. Obstet Gynecol. 2019;133:149-154.
- Ventura SJ, Curtin SC, Abma JC, et al. Estimated pregnancy rates and rates of pregnancy outcomes for the United States, 1990-2008. Natl Vital Stat Rep. 2012;60:1-21.
- Doubilet PM, Benson CB, Bourne T, et al; Society of Radiologists in Ultrasound Multispecialty Panel on Early First Trimester Diagnosis of Miscarriage and Exclusion of a Viable Uterine Pregnancy. Diagnostic criteria for nonviable pregnancy early in the first trimester. N Engl J Med. 2013;369:1443-1451.
- Zhang J, Gilles JM, Barnhart K, et al. A comparison of medical management with misoprostol and surgical management for early pregnancy failure. N Engl J Med. 2005;353:761-769.
- Nanda K, Peloggia A, Grimes D, et al. Expectant care versus surgical treatment for miscarriage. Cochrane Database Syst Rev. 2006(2):CD003518.
- Neilson JP, Hickey M, Vazquez J. Medical treatment for early fetal death (less than 24 weeks). Cochrane Database Syst Rev. 2006(3):CD002253.
- Luise C, Jermy K, May C, et al. Outcome of expectant management of spontaneous first trimester miscarriage: observational study. BMJ. 2002;324:873-875.
- Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Nagendra D, Koelper N, Loza-Avalos SE, et al. Cost-effectiveness of mifepristone pretreatment for the medical management of nonviable early pregnancy: secondary analysis of a randomized clinical trial. JAMA Netw Open. 2020;3:e201594.
- World Health Organization. Safe Abortion: Technical and Policy Guidance for Health Systems. 2nd ed. Geneva, Switzerland: World Health Organization; 2012.
- Wiebe E, Janssen P. Management of spontaneous abortion in family practices and hospitals. Fam Med. 1998;30:293-296.
- Harris LH, Dalton VK, Johnson TR. Surgical management of early pregnancy failure: history, politics, and safe, cost-effective care. Am J Obstet Gynecol. 2007;196:445.e1-e5.
- Dalton VK, Harris L, Weisman CS, et al. Patient preferences, satisfaction, and resource use in office evacuation of early pregnancy failure. Obstet Gynecol. 2006;108:103-110.
- Rausch M, Lorch S, Chung K, et al. A cost-effectiveness analysis of surgical versus medical management of early pregnancy loss. Fertil Steril. 2012;97:355-360.
- Trinder J, Brocklehurst P, Porter R, et al. Management of miscarriage: expectant, medical, or surgical? Results of randomised controlled trial (Miscarriage Treatment [MIST] trial). BMJ. 2006;332:1235-1240.
- Calvache JA, Delgado-Noguera MF, Lesaffre E, et al. Anaesthesia for evacuation of incomplete miscarriage. Cochrane Database System Rev. 2012(4):CD008681.
- Horvath S, Tsao P, Huang ZY, et al. The concentration of fetal red blood cells in first-trimester pregnant women undergoing uterine aspiration is below the calculated threshold for Rh sensitization. Contraception. 2020;102:1-6.
- National Abortion Federation. 2020 clinical policy guidelines for abortion care. https://www.prochoice.org/education-and-advocacy/cpg. Accessed June 9, 2020.
- American College of Obstetricians and Gynecologists. Practice bulletin No. 181: prevention of Rh D alloimmunization. Obstet Gynecol. 2017;130:e59-e70.
- American College of Obstetricians and Gynecologists. Practice bulletin No. 104: antibiotic prophylaxis for gynecologic procedures. Obstet Gynecol. 2009;113:1180-1189.
- Lissauer D, Wilson A, Hewitt CA, et al. A randomized trial of prophylactic antibiotics for miscarriage surgery. N Engl J Med. 2019;380:1012-1021.
- Perriera L, Reeves MF, Chen BA, et al. Feasibility of telephone follow-up after medical abortion. Contraception. 2010:81:143-149.
- Barnhart K, Sammel MD, Chung K, et al. Decline of serum human chorionic gonadotropin and spontaneous complete abortion: defining the normal curve. Obstet Gynecol. 2004;104(5 pt 1):975-981.
- Chen MJ, Rounds KM, Creinin MD, et al. Comparing office and telephone follow-up after medical abortion. Contraception. 2016;94:122-126.
- Clark W, Bracken H, Tanenhaus J, et al. Alternatives to a routine follow-up visit for early medical abortion. Obstet Gynecol. 2010;115(2 pt 1):264-272.
- Jackson AV, Dayananda I, Fortin JM, et al. Can women accurately assess the outcome of medical abortion based on symptoms alone? Contraception. 2012;85:192-197.
- Raymond EG, Tan YL, Grant M, et al. Self-assessment of medical abortion outcome using symptoms and home pregnancy testing. Contraception. 2018;97:324-328.
- Raymond EG, Shochet T, Bracken H. Low-sensitivity urine pregnancy testing to assess medical abortion outcome: a systematic review. Contraception. 2018;98:30-35.
- Raymond EG, Grossman D, Mark A, et al. Commentary: no-test medication abortion: a sample protocol for increasing access during a pandemic and beyond. Contraception. 2020;101:361-366.
- Farren J, Jalmbrant M, Ameye L, et al. Post-traumatic stress, anxiety and depression following miscarriage or ectopic pregnancy: a prospective cohort study. BMJ Open. 2016;6:e011864.
- Schliep KC, Mitchell EM, Mumford SL, et al. Trying to conceive after an early pregnancy loss: an assessment on how long couples should wait. Obstet Gynecol. 2016;127:204-212. DOI: 0.1097/AOG.0000000000001159.
- American College of Obstetricians and Gynecologists. Committee opinion No. 642: increasing access to contraceptive implants and intrauterine devices to reduce unintended pregnancy. Obstet Gynecol. 2015;126:e44-e48.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. US medical eligibility criteria (US MEC) for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-103.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. US selected practice recommendations for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-66.
Telemedicine: Navigating legal issues
In the first 2 articles of this series, “Telemedicine: A primer for today’s ObGyn” and “Telemedicine: Common hurdles and proper coding for ObGyns,” which appeared in the May and June issues of
Legal issues surrounding telemedicine
There are numerous legal, regulatory, and compliance issues that existed before the pandemic that likely will continue to be of concern postpandemic. Although the recent 1135 waiver (allowing Medicare to pay for office, hospital, and other visits furnished via telehealth)1 and other regulations are now in place for almost every aspect of telemedicine, virtual medicine is not a free-for-all (even though it may seem like it). Practicing ethical telemedicine entails abiding by numerous federal and state-specific laws and requirements. It is important to be aware of the laws in each state in which your patients are located and to practice according to the requirements of these laws. This often requires consultation with an experienced health care attorney who is knowledgeable about the use of telemedicine and who can help you with issues surrounding:
- Malpractice insurance. It is an important first step to contact your practice’s malpractice insurance carrier and confirm coverage for telemedicine visits. Telemedicine visits are considered the same as in-person visits when determining scope of practice and malpractice liability. Nevertheless, a best practice is to have written verification from your malpractice carrier about the types of telemedicine services and claims for which your ObGyn practice is covered. Additionally, if you care for patients virtually who live in a state in which you are not licensed, check with your carrier to determine if potential claims will be covered.
- Corporate practice laws. These laws require that your practice be governed by a health care professional and not someone with a nonmedical background. This becomes important if you are looking to create a virtual practice in another state. States that prohibit the corporate practice of medicine have state-specific mandates that require strict adherence. Consult with a health care attorney before entering into a business arrangement with a nonphysician or corporate entity.
- Delegation agreement requirements. These laws require physician collaboration and/or supervision of allied health care workers such as nurse practitioners (NPs) and physician assistants (PAs) and may limit the number of allied health care providers that a physician may supervise. Many states are allowing allied health care workers to practice at the top of their license, but this is still state specific. Thus, it is an important issue to consider, especially for practices that rely heavily on the services of advanced practice registered nurses (APRNs), for example, who have a broad scope of practice and who may be qualified to care for many common ObGyn problems.
- Informed consent requirements. Some states have no requirements regarding consent for a virtual visit. Others require either written or verbal consent. In states that do not require informed consent, it is best practice to nevertheless obtain either written or oral consent and to document in the patient’s record that consent was obtained before initiating a virtual visit. The consent should follow state-mandated disclosures, as well as the practice’s policies regarding billing, scheduling, and cancellations of telemedicine visits.
- Interstate licensing laws. Because of the COVID-19 pandemic, federal and state licensure waivers are in place to allow physicians to care for patients outside the physician’s home state, but these waivers likely will be lifted postpandemic. Once waivers are lifted, physicians will need to be licensed not only in the state in which they practice but also in the state where the patient is located at the time of treatment. Even physicians who practice in states that belong to the Interstate Medical Licensure Compact2 must apply for and obtain a license to practice within Compact member states. Membership in the Interstate Medical Licensure Compact expedites the licensure process, but does not alleviate the need to obtain a license to practice in each member state. To ensure compliance with interstate licensure laws, seek advice from a health care attorney specializing in telemedicine.
- Drug monitoring laws. The Ryan Haight Online Pharmacy Consumer Protection Act of 20083 implemented a requirement that physicians have at least one in-person, face-to-face visit with patients before prescribing a controlled substance for the first time. Because state laws may vary, we suggest consulting with a health care attorney to understand your state’s requirements for prescribing controlled substances to new patients and when using telemedicine (see “Prescription drugs” at https://www.cdc.gov/phlp/publications/topic/prescription.html for more information).
- Data privacy and security. From a content perspective, health care data and personally identifiable information are extremely rich, which makes electronic health records (EHRs), or the digital form of patients’ medical histories and other data, particularly tempting targets for hackers and cyber criminals. We caution that services such as Facetime and Skype are not encrypted; they have been granted waivers for telemedicine use, but these waivers are probably not going to be permanent once the COVID-19 crisis passes.
- HIPAA compliance. Generally—and certainly under normal circumstances—telemedicine is subject to the same rules governing protected health information (PHI) as any other technology and process used in physician practices. The Health Insurance Portability and Accountability Act (HIPAA) Security Rule includes guidelines on telemedicine and stipulates that only authorized users should have access to ePHI, that a system of secure communication must be established to protect the security of ePHI, and that a system to monitor communications must be maintained, among other requirements.4 Third parties that provide telemedicine, data storage, and other services, with a few exceptions, must have a business associate agreement (BAA) with a covered entity. Covered entities include health care providers, health plans, and health and health care clearinghouses. Such an agreement should include specific language that ensures that HIPAA requirements will be met and that governs permitted and required uses of PHI, strictly limits other uses of PHI, and establishes appropriate safeguards and steps that must be taken in the event of a breach or disallowed disclosure of PHI. Best practice requires that providers establish robust protocols, policies, and processes for handling sensitive information.
During the COVID-19 pandemic, however, certain HIPAA restrictions relating to telemedicine have been temporarily waived by the US Department of Health and Human Services (HHS). More specifically, HHS Secretary Alex Azar has exercised his authority to waive sanctions against covered hospitals for noncompliance with requirements: to obtain a patient’s consent to speak with family members or friends involved in the patient’s care, to distribute a notice of privacy practices, to request privacy restrictions, to request confidential communications, and the use of nonpublic facing audio and video communications products, among others.5 These are temporary measures only; once the national public health emergency has passed or at the HHS Secretary’s discretion based on new developments, this position on discretionary nonenforcement may end.
Continue to: Crisis creates opportunity: The future of telemedicine...
Crisis creates opportunity: The future of telemedicine
It was just a few years ago when the use of telemedicine was relegated to treating patients in only rural areas or those located a great distance from brick and mortar practices. But the pandemic, along with the coincident relaxation of the Centers for Medicare and Medicaid Services’ (CMS) requirements for conducting telemedicine visits has made the technology highly attractive to ObGyns who can now treat many patients 24/7 from their homes using laptops and even mobile devices. In addition, the pandemic has prompted an expansion of current procedural terminology (CPT) codes that makes it possible to bill patients for telemedicine services and be appropriately compensated.
Thus, as awful as COVID-19 is, we can conclude that it has provided us with opportunities. We predict that when the crisis has abated, although the current relaxation of HIPAA guidelines will probably be rescinded, restrictions will not likely return to precoronavirus status; changes will certainly be made, and telemedicine will likely become part and parcel of caring for ObGyn patients.
Telemedicine has been used successfully for years to improve patient access to medical care while reducing health care costs. In 2016, an estimated 61% of US health care institutions and 40% to 50% of US hospitals used telemedicine.6 And according to the results of a survey of America’s physicians conducted in April 2020, almost half (48%) are treating patients through telemedicine, which is up from just 18% 2 years ago.7
Letting loose the genie in the bottle
Widespread use of telemedicine traditionally has been limited by low reimbursement rates and interstate licensing and practice issues, but we predict that the use of telemedicine is going to significantly increase in the future. Here’s why:8 Disruptive innovation was defined by Professor Clayton Christensen of the Harvard Business School in 1997.9 Disruptive innovation explains the process by which a disruptive force spurs the development of simple, convenient, and affordable solutions that then replace processes that are expensive and complicated. According to Christensen, a critical element of the process is a technology that makes a product or service more accessible to a larger number of people while reducing cost and increasing ease of use. For example, innovations making equipment for dialysis cheaper and simpler helped make it possible to administer the treatment in neighborhood clinics, rather than in centralized hospitals, thus disrupting the hospital’s share of the dialysis business.
The concept of telemedicine and the technology for its implementation have been available for more than 15 years. However, it was the coronavirus that released the genie from the bottle, serving as the disruptive force to release the innovation. Telemedicine has demonstrated that the technology offers solutions that address patients’ urgent, unmet needs for access to care at an affordable price and that enhances the productivity of the ObGyn. The result is simple, convenient, and affordable; patients can readily access the medical care they need to effectively maintain their health or manage conditions that arise.
Telemedicine has reached a level of critical mass. Data suggest that patients, especially younger ones, have accepted and appreciate the use of this technology.10 It gives patients more opportunities to receive health care in their homes or at work where they feel more comfortable and less anxious than they do in physicians’ offices.
Several other health care issues may be altered by telemedicine.
The physician shortage. If the data are to be believed, there will be a significant shortage of physicians—and perhaps ObGyns—in the near future.11 Telemedicine can help the problem by making it possible to provide medical care not only in rural areas where there are no ObGyns but also in urban areas where a shortage may be looming.
Continuing medical education (CME). CME is moving from large, expensive, in-person conferences to virtual conferences and online learning.
The American health care budget is bloated with expenses exceeding $3 trillion.12 Telemedicine can help reduce health care costs by facilitating patient appointments that do not require office staff or many of the overhead expenses associated with brick and mortar operations. Telemedicine reduces the financial impact of patient no-shows. Because patients are keen on participating, the use of telemedicine likely will improve patient engagement and clinical outcomes. Telemedicine already has a reputation of reducing unnecessary office and emergency room visits and hospital admissions.13
Clinical trials. One of the obstacles to overcome in the early stages of a clinical trial is finding participants. Telemedicine will make patient recruitment more straightforward. And because telemedicine makes distance from the office a nonissue, recruiters will be less restricted by geographic boundaries.
In addition, telemedicine allows for the participants of the trial to stay in their homes most of the time while wearing remote monitoring devices. Such devices would enable trial researchers to spot deviations from patients’ baseline readings.
The bottom line
COVID-19 has provided the opportunity for us to see how telemedicine can contribute to reducing the spread of infectious diseases by protecting physicians, their staff, and patients themselves. Once the COVID-19 crisis has passed, it is likely that telemedicine will continue to move health care delivery from the hospital or clinic into the home. The growth and integration of information and communication technologies into health care delivery holds great potential for patients, providers, and payers in health systems of the future. ●
CVS is using telemedicine to complement the company’s retail “Minute Clinic,” which offers routine preventive and clinical services, such as vaccine administration, disease screenings, treatment for minor illnesses and injuries, and monitoring of chronic conditions—services that traditionally were provided in physician’s offices only. These clinics are open 7 days per week, providing services on a walk-in basis at an affordable price—about $60 per visit compared with an average of $150 for an uninsured patient to see a primary care physician in his/her office.1 While this seems to be fulfilling an unmet need for patients, the service may prove disruptive to traditional health care delivery by removing a lucrative source of income from physicians.
Reference
1. CVS Health. CVS Health’s MinuteClinic introduces new virtual care offering. August 8, 2018. https://cvshealth.com/newsroom/press-releases/cvs-healths-minuteclinic-introduces-new-virtual-care-offering. Accessed June 16, 2020.
- CMS.gov. 1135 Waiver – At A Glance.https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/SurveyCertEmergPrep/Downloads/1135-Waivers-At-A-Glance.pdf. Accessed June 16, 2020.
- Interstate Medical Licensure Compact. https://www.imlcc.org/. Accessed June 16, 2020.
- American Psychiatric Association. The Ryan Haight OnlinePharmacy Consumer Protection Act of 2008. https://www.psychiatry.org/psychiatrists/practice/telepsychiatry/toolkit/ryan-haight-act. Accessed June 16, 2020.
- American Medical Association. HIPAA security rule and riskanalysis. https://www.ama-assn.org/practice-management/hipaa/hipaa-security-rule-risk-analysis#:~:text=The%20HIPAA%20Security%20Rule%20requires,and%20security%20of%20this%20information. Accessed June 16, 2020.
- HHS.gov. Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. Content last reviewed on March 30, 2020.https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html. Accessed June 16, 2020.
- Mahar J, Rosencrance J, Rasmussen P. The Future of Telemedicine (And What’s in the Way). Consult QD. March 1,2019. https://consultqd.clevelandclinic.org/the-future-of-telemedicine-and-whats-in-the-way. Accessed June 23, 2020.
- Merritt Hawkins. Survey: Physician Practice Patterns Changing As A Result Of COVID-19. April 22, 2020.https://www.merritthawkins.com/news-and-insights/media-room/press/-Physician-Practice-Patterns-Changing-as-a-Result-of-COVID-19/. Accessed June 17, 2020.
- The Medical Futurist. COVID-19 and the rise of telemedicine.March 31, 2020. https://medicalfuturist.com/covid-19-was-needed-for-telemedicine-to-finally-go-mainstream/. Accessed June 16, 2020.
- Christensen C, Euchner J. Managing disruption: an interview with Clayton Christensen. Research-Technology Management. 2011;54:1, 11-17.
- Wordstream. 4 major trends for post-COVID-19 world. Last updated May 1, 2020. https://www.wordstream.com/blog/ws/2020/03/23/covid-19-business-trends. Accessed June16, 2020.
- Rosenberg J. Physician shortage likely to impact ob/gyn workforce in coming years. AJMC. September 21, 2019. https://www.ajmc.com/newsroom/physician-shortage-likely-to-impact-obgyn-workforce-in-coming-years. Accessed June 16, 2020.
- CMS.gov. National Health Expenditure Data: Historical. Page last modified December 17, 2019. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NationalHealthAccountsHistorical. Accessed June 17, 2020.
- Cohen JK. Study: Telehealth program reduces unnecessary ED visits by 6.7%. Hospital Review. February 27, 2017.https://www.beckershospitalreview.com/telehealth/study-telehealth-program-reduces-unnecessary-ed-visits-by-6-7.html. Accessed June 23, 2020.
In the first 2 articles of this series, “Telemedicine: A primer for today’s ObGyn” and “Telemedicine: Common hurdles and proper coding for ObGyns,” which appeared in the May and June issues of
Legal issues surrounding telemedicine
There are numerous legal, regulatory, and compliance issues that existed before the pandemic that likely will continue to be of concern postpandemic. Although the recent 1135 waiver (allowing Medicare to pay for office, hospital, and other visits furnished via telehealth)1 and other regulations are now in place for almost every aspect of telemedicine, virtual medicine is not a free-for-all (even though it may seem like it). Practicing ethical telemedicine entails abiding by numerous federal and state-specific laws and requirements. It is important to be aware of the laws in each state in which your patients are located and to practice according to the requirements of these laws. This often requires consultation with an experienced health care attorney who is knowledgeable about the use of telemedicine and who can help you with issues surrounding:
- Malpractice insurance. It is an important first step to contact your practice’s malpractice insurance carrier and confirm coverage for telemedicine visits. Telemedicine visits are considered the same as in-person visits when determining scope of practice and malpractice liability. Nevertheless, a best practice is to have written verification from your malpractice carrier about the types of telemedicine services and claims for which your ObGyn practice is covered. Additionally, if you care for patients virtually who live in a state in which you are not licensed, check with your carrier to determine if potential claims will be covered.
- Corporate practice laws. These laws require that your practice be governed by a health care professional and not someone with a nonmedical background. This becomes important if you are looking to create a virtual practice in another state. States that prohibit the corporate practice of medicine have state-specific mandates that require strict adherence. Consult with a health care attorney before entering into a business arrangement with a nonphysician or corporate entity.
- Delegation agreement requirements. These laws require physician collaboration and/or supervision of allied health care workers such as nurse practitioners (NPs) and physician assistants (PAs) and may limit the number of allied health care providers that a physician may supervise. Many states are allowing allied health care workers to practice at the top of their license, but this is still state specific. Thus, it is an important issue to consider, especially for practices that rely heavily on the services of advanced practice registered nurses (APRNs), for example, who have a broad scope of practice and who may be qualified to care for many common ObGyn problems.
- Informed consent requirements. Some states have no requirements regarding consent for a virtual visit. Others require either written or verbal consent. In states that do not require informed consent, it is best practice to nevertheless obtain either written or oral consent and to document in the patient’s record that consent was obtained before initiating a virtual visit. The consent should follow state-mandated disclosures, as well as the practice’s policies regarding billing, scheduling, and cancellations of telemedicine visits.
- Interstate licensing laws. Because of the COVID-19 pandemic, federal and state licensure waivers are in place to allow physicians to care for patients outside the physician’s home state, but these waivers likely will be lifted postpandemic. Once waivers are lifted, physicians will need to be licensed not only in the state in which they practice but also in the state where the patient is located at the time of treatment. Even physicians who practice in states that belong to the Interstate Medical Licensure Compact2 must apply for and obtain a license to practice within Compact member states. Membership in the Interstate Medical Licensure Compact expedites the licensure process, but does not alleviate the need to obtain a license to practice in each member state. To ensure compliance with interstate licensure laws, seek advice from a health care attorney specializing in telemedicine.
- Drug monitoring laws. The Ryan Haight Online Pharmacy Consumer Protection Act of 20083 implemented a requirement that physicians have at least one in-person, face-to-face visit with patients before prescribing a controlled substance for the first time. Because state laws may vary, we suggest consulting with a health care attorney to understand your state’s requirements for prescribing controlled substances to new patients and when using telemedicine (see “Prescription drugs” at https://www.cdc.gov/phlp/publications/topic/prescription.html for more information).
- Data privacy and security. From a content perspective, health care data and personally identifiable information are extremely rich, which makes electronic health records (EHRs), or the digital form of patients’ medical histories and other data, particularly tempting targets for hackers and cyber criminals. We caution that services such as Facetime and Skype are not encrypted; they have been granted waivers for telemedicine use, but these waivers are probably not going to be permanent once the COVID-19 crisis passes.
- HIPAA compliance. Generally—and certainly under normal circumstances—telemedicine is subject to the same rules governing protected health information (PHI) as any other technology and process used in physician practices. The Health Insurance Portability and Accountability Act (HIPAA) Security Rule includes guidelines on telemedicine and stipulates that only authorized users should have access to ePHI, that a system of secure communication must be established to protect the security of ePHI, and that a system to monitor communications must be maintained, among other requirements.4 Third parties that provide telemedicine, data storage, and other services, with a few exceptions, must have a business associate agreement (BAA) with a covered entity. Covered entities include health care providers, health plans, and health and health care clearinghouses. Such an agreement should include specific language that ensures that HIPAA requirements will be met and that governs permitted and required uses of PHI, strictly limits other uses of PHI, and establishes appropriate safeguards and steps that must be taken in the event of a breach or disallowed disclosure of PHI. Best practice requires that providers establish robust protocols, policies, and processes for handling sensitive information.
During the COVID-19 pandemic, however, certain HIPAA restrictions relating to telemedicine have been temporarily waived by the US Department of Health and Human Services (HHS). More specifically, HHS Secretary Alex Azar has exercised his authority to waive sanctions against covered hospitals for noncompliance with requirements: to obtain a patient’s consent to speak with family members or friends involved in the patient’s care, to distribute a notice of privacy practices, to request privacy restrictions, to request confidential communications, and the use of nonpublic facing audio and video communications products, among others.5 These are temporary measures only; once the national public health emergency has passed or at the HHS Secretary’s discretion based on new developments, this position on discretionary nonenforcement may end.
Continue to: Crisis creates opportunity: The future of telemedicine...
Crisis creates opportunity: The future of telemedicine
It was just a few years ago when the use of telemedicine was relegated to treating patients in only rural areas or those located a great distance from brick and mortar practices. But the pandemic, along with the coincident relaxation of the Centers for Medicare and Medicaid Services’ (CMS) requirements for conducting telemedicine visits has made the technology highly attractive to ObGyns who can now treat many patients 24/7 from their homes using laptops and even mobile devices. In addition, the pandemic has prompted an expansion of current procedural terminology (CPT) codes that makes it possible to bill patients for telemedicine services and be appropriately compensated.
Thus, as awful as COVID-19 is, we can conclude that it has provided us with opportunities. We predict that when the crisis has abated, although the current relaxation of HIPAA guidelines will probably be rescinded, restrictions will not likely return to precoronavirus status; changes will certainly be made, and telemedicine will likely become part and parcel of caring for ObGyn patients.
Telemedicine has been used successfully for years to improve patient access to medical care while reducing health care costs. In 2016, an estimated 61% of US health care institutions and 40% to 50% of US hospitals used telemedicine.6 And according to the results of a survey of America’s physicians conducted in April 2020, almost half (48%) are treating patients through telemedicine, which is up from just 18% 2 years ago.7
Letting loose the genie in the bottle
Widespread use of telemedicine traditionally has been limited by low reimbursement rates and interstate licensing and practice issues, but we predict that the use of telemedicine is going to significantly increase in the future. Here’s why:8 Disruptive innovation was defined by Professor Clayton Christensen of the Harvard Business School in 1997.9 Disruptive innovation explains the process by which a disruptive force spurs the development of simple, convenient, and affordable solutions that then replace processes that are expensive and complicated. According to Christensen, a critical element of the process is a technology that makes a product or service more accessible to a larger number of people while reducing cost and increasing ease of use. For example, innovations making equipment for dialysis cheaper and simpler helped make it possible to administer the treatment in neighborhood clinics, rather than in centralized hospitals, thus disrupting the hospital’s share of the dialysis business.
The concept of telemedicine and the technology for its implementation have been available for more than 15 years. However, it was the coronavirus that released the genie from the bottle, serving as the disruptive force to release the innovation. Telemedicine has demonstrated that the technology offers solutions that address patients’ urgent, unmet needs for access to care at an affordable price and that enhances the productivity of the ObGyn. The result is simple, convenient, and affordable; patients can readily access the medical care they need to effectively maintain their health or manage conditions that arise.
Telemedicine has reached a level of critical mass. Data suggest that patients, especially younger ones, have accepted and appreciate the use of this technology.10 It gives patients more opportunities to receive health care in their homes or at work where they feel more comfortable and less anxious than they do in physicians’ offices.
Several other health care issues may be altered by telemedicine.
The physician shortage. If the data are to be believed, there will be a significant shortage of physicians—and perhaps ObGyns—in the near future.11 Telemedicine can help the problem by making it possible to provide medical care not only in rural areas where there are no ObGyns but also in urban areas where a shortage may be looming.
Continuing medical education (CME). CME is moving from large, expensive, in-person conferences to virtual conferences and online learning.
The American health care budget is bloated with expenses exceeding $3 trillion.12 Telemedicine can help reduce health care costs by facilitating patient appointments that do not require office staff or many of the overhead expenses associated with brick and mortar operations. Telemedicine reduces the financial impact of patient no-shows. Because patients are keen on participating, the use of telemedicine likely will improve patient engagement and clinical outcomes. Telemedicine already has a reputation of reducing unnecessary office and emergency room visits and hospital admissions.13
Clinical trials. One of the obstacles to overcome in the early stages of a clinical trial is finding participants. Telemedicine will make patient recruitment more straightforward. And because telemedicine makes distance from the office a nonissue, recruiters will be less restricted by geographic boundaries.
In addition, telemedicine allows for the participants of the trial to stay in their homes most of the time while wearing remote monitoring devices. Such devices would enable trial researchers to spot deviations from patients’ baseline readings.
The bottom line
COVID-19 has provided the opportunity for us to see how telemedicine can contribute to reducing the spread of infectious diseases by protecting physicians, their staff, and patients themselves. Once the COVID-19 crisis has passed, it is likely that telemedicine will continue to move health care delivery from the hospital or clinic into the home. The growth and integration of information and communication technologies into health care delivery holds great potential for patients, providers, and payers in health systems of the future. ●
CVS is using telemedicine to complement the company’s retail “Minute Clinic,” which offers routine preventive and clinical services, such as vaccine administration, disease screenings, treatment for minor illnesses and injuries, and monitoring of chronic conditions—services that traditionally were provided in physician’s offices only. These clinics are open 7 days per week, providing services on a walk-in basis at an affordable price—about $60 per visit compared with an average of $150 for an uninsured patient to see a primary care physician in his/her office.1 While this seems to be fulfilling an unmet need for patients, the service may prove disruptive to traditional health care delivery by removing a lucrative source of income from physicians.
Reference
1. CVS Health. CVS Health’s MinuteClinic introduces new virtual care offering. August 8, 2018. https://cvshealth.com/newsroom/press-releases/cvs-healths-minuteclinic-introduces-new-virtual-care-offering. Accessed June 16, 2020.
In the first 2 articles of this series, “Telemedicine: A primer for today’s ObGyn” and “Telemedicine: Common hurdles and proper coding for ObGyns,” which appeared in the May and June issues of
Legal issues surrounding telemedicine
There are numerous legal, regulatory, and compliance issues that existed before the pandemic that likely will continue to be of concern postpandemic. Although the recent 1135 waiver (allowing Medicare to pay for office, hospital, and other visits furnished via telehealth)1 and other regulations are now in place for almost every aspect of telemedicine, virtual medicine is not a free-for-all (even though it may seem like it). Practicing ethical telemedicine entails abiding by numerous federal and state-specific laws and requirements. It is important to be aware of the laws in each state in which your patients are located and to practice according to the requirements of these laws. This often requires consultation with an experienced health care attorney who is knowledgeable about the use of telemedicine and who can help you with issues surrounding:
- Malpractice insurance. It is an important first step to contact your practice’s malpractice insurance carrier and confirm coverage for telemedicine visits. Telemedicine visits are considered the same as in-person visits when determining scope of practice and malpractice liability. Nevertheless, a best practice is to have written verification from your malpractice carrier about the types of telemedicine services and claims for which your ObGyn practice is covered. Additionally, if you care for patients virtually who live in a state in which you are not licensed, check with your carrier to determine if potential claims will be covered.
- Corporate practice laws. These laws require that your practice be governed by a health care professional and not someone with a nonmedical background. This becomes important if you are looking to create a virtual practice in another state. States that prohibit the corporate practice of medicine have state-specific mandates that require strict adherence. Consult with a health care attorney before entering into a business arrangement with a nonphysician or corporate entity.
- Delegation agreement requirements. These laws require physician collaboration and/or supervision of allied health care workers such as nurse practitioners (NPs) and physician assistants (PAs) and may limit the number of allied health care providers that a physician may supervise. Many states are allowing allied health care workers to practice at the top of their license, but this is still state specific. Thus, it is an important issue to consider, especially for practices that rely heavily on the services of advanced practice registered nurses (APRNs), for example, who have a broad scope of practice and who may be qualified to care for many common ObGyn problems.
- Informed consent requirements. Some states have no requirements regarding consent for a virtual visit. Others require either written or verbal consent. In states that do not require informed consent, it is best practice to nevertheless obtain either written or oral consent and to document in the patient’s record that consent was obtained before initiating a virtual visit. The consent should follow state-mandated disclosures, as well as the practice’s policies regarding billing, scheduling, and cancellations of telemedicine visits.
- Interstate licensing laws. Because of the COVID-19 pandemic, federal and state licensure waivers are in place to allow physicians to care for patients outside the physician’s home state, but these waivers likely will be lifted postpandemic. Once waivers are lifted, physicians will need to be licensed not only in the state in which they practice but also in the state where the patient is located at the time of treatment. Even physicians who practice in states that belong to the Interstate Medical Licensure Compact2 must apply for and obtain a license to practice within Compact member states. Membership in the Interstate Medical Licensure Compact expedites the licensure process, but does not alleviate the need to obtain a license to practice in each member state. To ensure compliance with interstate licensure laws, seek advice from a health care attorney specializing in telemedicine.
- Drug monitoring laws. The Ryan Haight Online Pharmacy Consumer Protection Act of 20083 implemented a requirement that physicians have at least one in-person, face-to-face visit with patients before prescribing a controlled substance for the first time. Because state laws may vary, we suggest consulting with a health care attorney to understand your state’s requirements for prescribing controlled substances to new patients and when using telemedicine (see “Prescription drugs” at https://www.cdc.gov/phlp/publications/topic/prescription.html for more information).
- Data privacy and security. From a content perspective, health care data and personally identifiable information are extremely rich, which makes electronic health records (EHRs), or the digital form of patients’ medical histories and other data, particularly tempting targets for hackers and cyber criminals. We caution that services such as Facetime and Skype are not encrypted; they have been granted waivers for telemedicine use, but these waivers are probably not going to be permanent once the COVID-19 crisis passes.
- HIPAA compliance. Generally—and certainly under normal circumstances—telemedicine is subject to the same rules governing protected health information (PHI) as any other technology and process used in physician practices. The Health Insurance Portability and Accountability Act (HIPAA) Security Rule includes guidelines on telemedicine and stipulates that only authorized users should have access to ePHI, that a system of secure communication must be established to protect the security of ePHI, and that a system to monitor communications must be maintained, among other requirements.4 Third parties that provide telemedicine, data storage, and other services, with a few exceptions, must have a business associate agreement (BAA) with a covered entity. Covered entities include health care providers, health plans, and health and health care clearinghouses. Such an agreement should include specific language that ensures that HIPAA requirements will be met and that governs permitted and required uses of PHI, strictly limits other uses of PHI, and establishes appropriate safeguards and steps that must be taken in the event of a breach or disallowed disclosure of PHI. Best practice requires that providers establish robust protocols, policies, and processes for handling sensitive information.
During the COVID-19 pandemic, however, certain HIPAA restrictions relating to telemedicine have been temporarily waived by the US Department of Health and Human Services (HHS). More specifically, HHS Secretary Alex Azar has exercised his authority to waive sanctions against covered hospitals for noncompliance with requirements: to obtain a patient’s consent to speak with family members or friends involved in the patient’s care, to distribute a notice of privacy practices, to request privacy restrictions, to request confidential communications, and the use of nonpublic facing audio and video communications products, among others.5 These are temporary measures only; once the national public health emergency has passed or at the HHS Secretary’s discretion based on new developments, this position on discretionary nonenforcement may end.
Continue to: Crisis creates opportunity: The future of telemedicine...
Crisis creates opportunity: The future of telemedicine
It was just a few years ago when the use of telemedicine was relegated to treating patients in only rural areas or those located a great distance from brick and mortar practices. But the pandemic, along with the coincident relaxation of the Centers for Medicare and Medicaid Services’ (CMS) requirements for conducting telemedicine visits has made the technology highly attractive to ObGyns who can now treat many patients 24/7 from their homes using laptops and even mobile devices. In addition, the pandemic has prompted an expansion of current procedural terminology (CPT) codes that makes it possible to bill patients for telemedicine services and be appropriately compensated.
Thus, as awful as COVID-19 is, we can conclude that it has provided us with opportunities. We predict that when the crisis has abated, although the current relaxation of HIPAA guidelines will probably be rescinded, restrictions will not likely return to precoronavirus status; changes will certainly be made, and telemedicine will likely become part and parcel of caring for ObGyn patients.
Telemedicine has been used successfully for years to improve patient access to medical care while reducing health care costs. In 2016, an estimated 61% of US health care institutions and 40% to 50% of US hospitals used telemedicine.6 And according to the results of a survey of America’s physicians conducted in April 2020, almost half (48%) are treating patients through telemedicine, which is up from just 18% 2 years ago.7
Letting loose the genie in the bottle
Widespread use of telemedicine traditionally has been limited by low reimbursement rates and interstate licensing and practice issues, but we predict that the use of telemedicine is going to significantly increase in the future. Here’s why:8 Disruptive innovation was defined by Professor Clayton Christensen of the Harvard Business School in 1997.9 Disruptive innovation explains the process by which a disruptive force spurs the development of simple, convenient, and affordable solutions that then replace processes that are expensive and complicated. According to Christensen, a critical element of the process is a technology that makes a product or service more accessible to a larger number of people while reducing cost and increasing ease of use. For example, innovations making equipment for dialysis cheaper and simpler helped make it possible to administer the treatment in neighborhood clinics, rather than in centralized hospitals, thus disrupting the hospital’s share of the dialysis business.
The concept of telemedicine and the technology for its implementation have been available for more than 15 years. However, it was the coronavirus that released the genie from the bottle, serving as the disruptive force to release the innovation. Telemedicine has demonstrated that the technology offers solutions that address patients’ urgent, unmet needs for access to care at an affordable price and that enhances the productivity of the ObGyn. The result is simple, convenient, and affordable; patients can readily access the medical care they need to effectively maintain their health or manage conditions that arise.
Telemedicine has reached a level of critical mass. Data suggest that patients, especially younger ones, have accepted and appreciate the use of this technology.10 It gives patients more opportunities to receive health care in their homes or at work where they feel more comfortable and less anxious than they do in physicians’ offices.
Several other health care issues may be altered by telemedicine.
The physician shortage. If the data are to be believed, there will be a significant shortage of physicians—and perhaps ObGyns—in the near future.11 Telemedicine can help the problem by making it possible to provide medical care not only in rural areas where there are no ObGyns but also in urban areas where a shortage may be looming.
Continuing medical education (CME). CME is moving from large, expensive, in-person conferences to virtual conferences and online learning.
The American health care budget is bloated with expenses exceeding $3 trillion.12 Telemedicine can help reduce health care costs by facilitating patient appointments that do not require office staff or many of the overhead expenses associated with brick and mortar operations. Telemedicine reduces the financial impact of patient no-shows. Because patients are keen on participating, the use of telemedicine likely will improve patient engagement and clinical outcomes. Telemedicine already has a reputation of reducing unnecessary office and emergency room visits and hospital admissions.13
Clinical trials. One of the obstacles to overcome in the early stages of a clinical trial is finding participants. Telemedicine will make patient recruitment more straightforward. And because telemedicine makes distance from the office a nonissue, recruiters will be less restricted by geographic boundaries.
In addition, telemedicine allows for the participants of the trial to stay in their homes most of the time while wearing remote monitoring devices. Such devices would enable trial researchers to spot deviations from patients’ baseline readings.
The bottom line
COVID-19 has provided the opportunity for us to see how telemedicine can contribute to reducing the spread of infectious diseases by protecting physicians, their staff, and patients themselves. Once the COVID-19 crisis has passed, it is likely that telemedicine will continue to move health care delivery from the hospital or clinic into the home. The growth and integration of information and communication technologies into health care delivery holds great potential for patients, providers, and payers in health systems of the future. ●
CVS is using telemedicine to complement the company’s retail “Minute Clinic,” which offers routine preventive and clinical services, such as vaccine administration, disease screenings, treatment for minor illnesses and injuries, and monitoring of chronic conditions—services that traditionally were provided in physician’s offices only. These clinics are open 7 days per week, providing services on a walk-in basis at an affordable price—about $60 per visit compared with an average of $150 for an uninsured patient to see a primary care physician in his/her office.1 While this seems to be fulfilling an unmet need for patients, the service may prove disruptive to traditional health care delivery by removing a lucrative source of income from physicians.
Reference
1. CVS Health. CVS Health’s MinuteClinic introduces new virtual care offering. August 8, 2018. https://cvshealth.com/newsroom/press-releases/cvs-healths-minuteclinic-introduces-new-virtual-care-offering. Accessed June 16, 2020.
- CMS.gov. 1135 Waiver – At A Glance.https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/SurveyCertEmergPrep/Downloads/1135-Waivers-At-A-Glance.pdf. Accessed June 16, 2020.
- Interstate Medical Licensure Compact. https://www.imlcc.org/. Accessed June 16, 2020.
- American Psychiatric Association. The Ryan Haight OnlinePharmacy Consumer Protection Act of 2008. https://www.psychiatry.org/psychiatrists/practice/telepsychiatry/toolkit/ryan-haight-act. Accessed June 16, 2020.
- American Medical Association. HIPAA security rule and riskanalysis. https://www.ama-assn.org/practice-management/hipaa/hipaa-security-rule-risk-analysis#:~:text=The%20HIPAA%20Security%20Rule%20requires,and%20security%20of%20this%20information. Accessed June 16, 2020.
- HHS.gov. Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. Content last reviewed on March 30, 2020.https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html. Accessed June 16, 2020.
- Mahar J, Rosencrance J, Rasmussen P. The Future of Telemedicine (And What’s in the Way). Consult QD. March 1,2019. https://consultqd.clevelandclinic.org/the-future-of-telemedicine-and-whats-in-the-way. Accessed June 23, 2020.
- Merritt Hawkins. Survey: Physician Practice Patterns Changing As A Result Of COVID-19. April 22, 2020.https://www.merritthawkins.com/news-and-insights/media-room/press/-Physician-Practice-Patterns-Changing-as-a-Result-of-COVID-19/. Accessed June 17, 2020.
- The Medical Futurist. COVID-19 and the rise of telemedicine.March 31, 2020. https://medicalfuturist.com/covid-19-was-needed-for-telemedicine-to-finally-go-mainstream/. Accessed June 16, 2020.
- Christensen C, Euchner J. Managing disruption: an interview with Clayton Christensen. Research-Technology Management. 2011;54:1, 11-17.
- Wordstream. 4 major trends for post-COVID-19 world. Last updated May 1, 2020. https://www.wordstream.com/blog/ws/2020/03/23/covid-19-business-trends. Accessed June16, 2020.
- Rosenberg J. Physician shortage likely to impact ob/gyn workforce in coming years. AJMC. September 21, 2019. https://www.ajmc.com/newsroom/physician-shortage-likely-to-impact-obgyn-workforce-in-coming-years. Accessed June 16, 2020.
- CMS.gov. National Health Expenditure Data: Historical. Page last modified December 17, 2019. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NationalHealthAccountsHistorical. Accessed June 17, 2020.
- Cohen JK. Study: Telehealth program reduces unnecessary ED visits by 6.7%. Hospital Review. February 27, 2017.https://www.beckershospitalreview.com/telehealth/study-telehealth-program-reduces-unnecessary-ed-visits-by-6-7.html. Accessed June 23, 2020.
- CMS.gov. 1135 Waiver – At A Glance.https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/SurveyCertEmergPrep/Downloads/1135-Waivers-At-A-Glance.pdf. Accessed June 16, 2020.
- Interstate Medical Licensure Compact. https://www.imlcc.org/. Accessed June 16, 2020.
- American Psychiatric Association. The Ryan Haight OnlinePharmacy Consumer Protection Act of 2008. https://www.psychiatry.org/psychiatrists/practice/telepsychiatry/toolkit/ryan-haight-act. Accessed June 16, 2020.
- American Medical Association. HIPAA security rule and riskanalysis. https://www.ama-assn.org/practice-management/hipaa/hipaa-security-rule-risk-analysis#:~:text=The%20HIPAA%20Security%20Rule%20requires,and%20security%20of%20this%20information. Accessed June 16, 2020.
- HHS.gov. Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. Content last reviewed on March 30, 2020.https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html. Accessed June 16, 2020.
- Mahar J, Rosencrance J, Rasmussen P. The Future of Telemedicine (And What’s in the Way). Consult QD. March 1,2019. https://consultqd.clevelandclinic.org/the-future-of-telemedicine-and-whats-in-the-way. Accessed June 23, 2020.
- Merritt Hawkins. Survey: Physician Practice Patterns Changing As A Result Of COVID-19. April 22, 2020.https://www.merritthawkins.com/news-and-insights/media-room/press/-Physician-Practice-Patterns-Changing-as-a-Result-of-COVID-19/. Accessed June 17, 2020.
- The Medical Futurist. COVID-19 and the rise of telemedicine.March 31, 2020. https://medicalfuturist.com/covid-19-was-needed-for-telemedicine-to-finally-go-mainstream/. Accessed June 16, 2020.
- Christensen C, Euchner J. Managing disruption: an interview with Clayton Christensen. Research-Technology Management. 2011;54:1, 11-17.
- Wordstream. 4 major trends for post-COVID-19 world. Last updated May 1, 2020. https://www.wordstream.com/blog/ws/2020/03/23/covid-19-business-trends. Accessed June16, 2020.
- Rosenberg J. Physician shortage likely to impact ob/gyn workforce in coming years. AJMC. September 21, 2019. https://www.ajmc.com/newsroom/physician-shortage-likely-to-impact-obgyn-workforce-in-coming-years. Accessed June 16, 2020.
- CMS.gov. National Health Expenditure Data: Historical. Page last modified December 17, 2019. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NationalHealthAccountsHistorical. Accessed June 17, 2020.
- Cohen JK. Study: Telehealth program reduces unnecessary ED visits by 6.7%. Hospital Review. February 27, 2017.https://www.beckershospitalreview.com/telehealth/study-telehealth-program-reduces-unnecessary-ed-visits-by-6-7.html. Accessed June 23, 2020.
Open Clinical Trials for Patients With COVID-19
Finding effective treatment or a vaccine for COVID-19, the disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has placed significant strains on the global health care system. The National Library of Medicine database lists > 1,800 trials that are aimed at addressing COVID-19-related health care. Already, trials developed by the US Department of Veterans Affairs (VA), US Department of Defense (DoD), and the National Institute of Allergy and Infectious Diseases have provided important data on effective treatment options. The clinical trials listed below are all open as of May 31, 2020 and have trial sites at VA and DoD facilities. For additional information and full inclusion/exclusion criteria, please consult clinicaltrials.gov.
Adaptive COVID-19 Treatment Trial (ACTT)
This study is an adaptive, randomized, double-blind, placebo-controlled trial to evaluate the safety and efficacy of novel therapeutic agents in hospitalized adults diagnosed with COVID-19. The study will compare different investigational therapeutic agents to a control arm. ID: NCT04280705
Sponsor: National Institute of Allergy and Infectious Diseases
Contact: Central Contact ([email protected])
Locations: VA Palo Alto Health Care System, California; Naval Medical Center San Diego, California; Southeast Louisiana Veterans Health Care System, New Orleans; Walter Reed National Military Medical Center, Bethesda, Maryland; National Institutes of Health - Clinical Center, National Institute of Allergy and Infectious Diseases Laboratory Of Immunoregulation, Bethesda, Maryland; Brooke Army Medical Center, Fort Sam Houston, Texas; Madigan Army Medical Center, Tacoma, Washington
Study to Evaluate the Safety and Antiviral Activity of Remdesivir (GS-5734) in Participants With Severe Coronavirus Disease (COVID-19)
The primary objective of this study is to evaluate the efficacy of 2 remdesivir (RDV) regimens with respect to clinical status assessed by a 7-point ordinal scale on Day 11 (NCT04292730) or Day 14 (NCT04292899).
ID: NCT04292730/NCT04292899
Sponsor: Gilead Sciences
Contact: Gilead Clinical Study Information Center (833-445-3230)
Location: James J. Peters VA Medical Center, Bronx, New York
Expanded Access Remdesivir (RDV; GS-5734)
The treatment of communicable Novel Coronavirus of 2019 with Remdesivir (RDV; GS-5734) also known as severe acute respiratory syndrome coronavirus 2.
ID: NCT04302766
Sponsor: US Army Medical Research and Development Command
Contact: Sandi Parriott ([email protected])
A Study to Evaluate the Safety and Efficacy of Tocilizumab in Patients With Severe COVID-19 Pneumonia (COVACTA)
This study will evaluate the efficacy, safety, pharmacodynamics, and pharmacokinetics of tocilizumab (TCZ) compared with a matching placebo in combination with standard of care (SOC) in hospitalized patients with severe COVID-19 pneumonia.
ID: NCT04320615
Sponsor: Hoffmann-La Roche
Location: James J Peters VA Medical Center, Bronx, New York
Administration of Intravenous Vitamin C in Novel Coronavirus Infection (COVID-19) and Decreased Oxygenation (AVoCaDO)
Previous research has shown that high dose intravenous vitamin C (HDIVC) may benefit patients with sepsis, acute lung injury (ALI), and the acute respiratory distress syndrome (ARDS). However, it is not known if early administration of HDIVC could prevent progression to ARDS. We hypothesize that HDIVC is safe and tolerable in COVID-19 subjects given early or late in the disease course and may reduce the risk of respiratory failure requiring mechanical ventilation and development of ARDS along with reductions in supplemental oxygen demand and inflammatory markers.
ID: NCT04357782
Sponsor: Hunter Holmes Mcguire VA Medical CenterContact: Brian Davis ([email protected])
Location: Hunter Holmes Mcguire VA Medical Center, Richmond, Virginia
Treatment Of CORONAVIRUS DISEASE 2019 (COVID-19) With Anti-Sars-CoV-2 Convalescent Plasma (ASCoV2CP)
This is an expanded access open-label, single-arm, multi-site protocol to provide convalescent plasma as a treatment for patients diagnosed with severe, or life-threatening COVID-19.
ID: NCT04360486
Sponsor: US Army Medical Research and Development Command
Contact: Andrew Cap ([email protected])
VA Remote and Equitable Access to COVID-19 Healthcare Delivery (VA-REACH TRIAL) (VA-REACH)
We propose a 3-arm randomized control trial to determine the efficacy of hydroxychloroquine or azithromycin in treating mild to moderate COVID-19 among veterans in the outpatient setting.
ID: NCT04363203
Sponsor: Salomeh Keyhani
Location: San Francisco VA Health Care System, California
A Study to Evaluate the Safety and Efficacy of MSTT1041A (Astegolimab) or UTTR1147A in Patients With Severe COVID-19 Pneumonia (COVASTIL)
This is a Phase II, randomized, double-blind, placebo-controlled, multicenter study to assess the efficacy and safety of MSTT1041A (astegolimab) or UTTR1147A in combination with standard of care (SOC) compared with matching placebo in combination with SOC in patients hospitalized with severe coronavirus disease 2019 (COVID-19) pneumonia.
ID: NCT04386616
Sponsor: Genentech
Contact: Study ID Number: GA42469 ([email protected])
Location: Southeast Louisiana Veterans Health Care System, New Orleans
Hormonal Intervention for the Treatment in Veterans With COVID-19 Requiring Hospitalization (HITCH)
The purpose of this study is to determine if temporary androgen suppression improves the clinical outcomes of veterans who are hospitalized to an acute care ward due to COVID-19.ID: NCT04397718
Sponsor: VA Office of Research and Development
Contact: Matthew B Rettig ([email protected]), Nicholas Nickols ([email protected])
Locations: VA Greater Los Angeles Healthcare System, California; VA NY Harbor Healthcare System, New York; VA Puget Sound Health Care System, Seattle, Washington
Adaptive COVID-19 Treatment Trial 2 (ACTT-II)
ACTT-II will evaluate the combination of baricitinib and remdesivir compared to remdesivir alone. Subjects will be assessed daily while hospitalized. If the subjects are discharged from the hospital, they will have a study visit at Days 15, 22, and 29.
ID: NCT04401579
Sponsor: National Institute of Allergy and Infectious Diseases (NIAID)
Contact: Central Contact ([email protected])
Locations: VA Palo Alto Health Care System, California; Naval Medical Center San Diego, California; Rocky Mountain Regional Veteran Affairs Medical Center, Aurora, Colorado; Southeast Louisiana Veterans Health Care System, New Orleans; Walter Reed National Military Medical Center, Bethesda, Maryland; National Institutes of Health - Clinical Center, National Institute of Allergy and Infectious Diseases Laboratory Of Immunoregulation, Bethesda, Maryland; Brooke Army Medical Center, Fort Sam Houston, Texas; Madigan Army Medical Center, Tacoma, Washington
Finding effective treatment or a vaccine for COVID-19, the disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has placed significant strains on the global health care system. The National Library of Medicine database lists > 1,800 trials that are aimed at addressing COVID-19-related health care. Already, trials developed by the US Department of Veterans Affairs (VA), US Department of Defense (DoD), and the National Institute of Allergy and Infectious Diseases have provided important data on effective treatment options. The clinical trials listed below are all open as of May 31, 2020 and have trial sites at VA and DoD facilities. For additional information and full inclusion/exclusion criteria, please consult clinicaltrials.gov.
Adaptive COVID-19 Treatment Trial (ACTT)
This study is an adaptive, randomized, double-blind, placebo-controlled trial to evaluate the safety and efficacy of novel therapeutic agents in hospitalized adults diagnosed with COVID-19. The study will compare different investigational therapeutic agents to a control arm. ID: NCT04280705
Sponsor: National Institute of Allergy and Infectious Diseases
Contact: Central Contact ([email protected])
Locations: VA Palo Alto Health Care System, California; Naval Medical Center San Diego, California; Southeast Louisiana Veterans Health Care System, New Orleans; Walter Reed National Military Medical Center, Bethesda, Maryland; National Institutes of Health - Clinical Center, National Institute of Allergy and Infectious Diseases Laboratory Of Immunoregulation, Bethesda, Maryland; Brooke Army Medical Center, Fort Sam Houston, Texas; Madigan Army Medical Center, Tacoma, Washington
Study to Evaluate the Safety and Antiviral Activity of Remdesivir (GS-5734) in Participants With Severe Coronavirus Disease (COVID-19)
The primary objective of this study is to evaluate the efficacy of 2 remdesivir (RDV) regimens with respect to clinical status assessed by a 7-point ordinal scale on Day 11 (NCT04292730) or Day 14 (NCT04292899).
ID: NCT04292730/NCT04292899
Sponsor: Gilead Sciences
Contact: Gilead Clinical Study Information Center (833-445-3230)
Location: James J. Peters VA Medical Center, Bronx, New York
Expanded Access Remdesivir (RDV; GS-5734)
The treatment of communicable Novel Coronavirus of 2019 with Remdesivir (RDV; GS-5734) also known as severe acute respiratory syndrome coronavirus 2.
ID: NCT04302766
Sponsor: US Army Medical Research and Development Command
Contact: Sandi Parriott ([email protected])
A Study to Evaluate the Safety and Efficacy of Tocilizumab in Patients With Severe COVID-19 Pneumonia (COVACTA)
This study will evaluate the efficacy, safety, pharmacodynamics, and pharmacokinetics of tocilizumab (TCZ) compared with a matching placebo in combination with standard of care (SOC) in hospitalized patients with severe COVID-19 pneumonia.
ID: NCT04320615
Sponsor: Hoffmann-La Roche
Location: James J Peters VA Medical Center, Bronx, New York
Administration of Intravenous Vitamin C in Novel Coronavirus Infection (COVID-19) and Decreased Oxygenation (AVoCaDO)
Previous research has shown that high dose intravenous vitamin C (HDIVC) may benefit patients with sepsis, acute lung injury (ALI), and the acute respiratory distress syndrome (ARDS). However, it is not known if early administration of HDIVC could prevent progression to ARDS. We hypothesize that HDIVC is safe and tolerable in COVID-19 subjects given early or late in the disease course and may reduce the risk of respiratory failure requiring mechanical ventilation and development of ARDS along with reductions in supplemental oxygen demand and inflammatory markers.
ID: NCT04357782
Sponsor: Hunter Holmes Mcguire VA Medical CenterContact: Brian Davis ([email protected])
Location: Hunter Holmes Mcguire VA Medical Center, Richmond, Virginia
Treatment Of CORONAVIRUS DISEASE 2019 (COVID-19) With Anti-Sars-CoV-2 Convalescent Plasma (ASCoV2CP)
This is an expanded access open-label, single-arm, multi-site protocol to provide convalescent plasma as a treatment for patients diagnosed with severe, or life-threatening COVID-19.
ID: NCT04360486
Sponsor: US Army Medical Research and Development Command
Contact: Andrew Cap ([email protected])
VA Remote and Equitable Access to COVID-19 Healthcare Delivery (VA-REACH TRIAL) (VA-REACH)
We propose a 3-arm randomized control trial to determine the efficacy of hydroxychloroquine or azithromycin in treating mild to moderate COVID-19 among veterans in the outpatient setting.
ID: NCT04363203
Sponsor: Salomeh Keyhani
Location: San Francisco VA Health Care System, California
A Study to Evaluate the Safety and Efficacy of MSTT1041A (Astegolimab) or UTTR1147A in Patients With Severe COVID-19 Pneumonia (COVASTIL)
This is a Phase II, randomized, double-blind, placebo-controlled, multicenter study to assess the efficacy and safety of MSTT1041A (astegolimab) or UTTR1147A in combination with standard of care (SOC) compared with matching placebo in combination with SOC in patients hospitalized with severe coronavirus disease 2019 (COVID-19) pneumonia.
ID: NCT04386616
Sponsor: Genentech
Contact: Study ID Number: GA42469 ([email protected])
Location: Southeast Louisiana Veterans Health Care System, New Orleans
Hormonal Intervention for the Treatment in Veterans With COVID-19 Requiring Hospitalization (HITCH)
The purpose of this study is to determine if temporary androgen suppression improves the clinical outcomes of veterans who are hospitalized to an acute care ward due to COVID-19.ID: NCT04397718
Sponsor: VA Office of Research and Development
Contact: Matthew B Rettig ([email protected]), Nicholas Nickols ([email protected])
Locations: VA Greater Los Angeles Healthcare System, California; VA NY Harbor Healthcare System, New York; VA Puget Sound Health Care System, Seattle, Washington
Adaptive COVID-19 Treatment Trial 2 (ACTT-II)
ACTT-II will evaluate the combination of baricitinib and remdesivir compared to remdesivir alone. Subjects will be assessed daily while hospitalized. If the subjects are discharged from the hospital, they will have a study visit at Days 15, 22, and 29.
ID: NCT04401579
Sponsor: National Institute of Allergy and Infectious Diseases (NIAID)
Contact: Central Contact ([email protected])
Locations: VA Palo Alto Health Care System, California; Naval Medical Center San Diego, California; Rocky Mountain Regional Veteran Affairs Medical Center, Aurora, Colorado; Southeast Louisiana Veterans Health Care System, New Orleans; Walter Reed National Military Medical Center, Bethesda, Maryland; National Institutes of Health - Clinical Center, National Institute of Allergy and Infectious Diseases Laboratory Of Immunoregulation, Bethesda, Maryland; Brooke Army Medical Center, Fort Sam Houston, Texas; Madigan Army Medical Center, Tacoma, Washington
Finding effective treatment or a vaccine for COVID-19, the disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has placed significant strains on the global health care system. The National Library of Medicine database lists > 1,800 trials that are aimed at addressing COVID-19-related health care. Already, trials developed by the US Department of Veterans Affairs (VA), US Department of Defense (DoD), and the National Institute of Allergy and Infectious Diseases have provided important data on effective treatment options. The clinical trials listed below are all open as of May 31, 2020 and have trial sites at VA and DoD facilities. For additional information and full inclusion/exclusion criteria, please consult clinicaltrials.gov.
Adaptive COVID-19 Treatment Trial (ACTT)
This study is an adaptive, randomized, double-blind, placebo-controlled trial to evaluate the safety and efficacy of novel therapeutic agents in hospitalized adults diagnosed with COVID-19. The study will compare different investigational therapeutic agents to a control arm. ID: NCT04280705
Sponsor: National Institute of Allergy and Infectious Diseases
Contact: Central Contact ([email protected])
Locations: VA Palo Alto Health Care System, California; Naval Medical Center San Diego, California; Southeast Louisiana Veterans Health Care System, New Orleans; Walter Reed National Military Medical Center, Bethesda, Maryland; National Institutes of Health - Clinical Center, National Institute of Allergy and Infectious Diseases Laboratory Of Immunoregulation, Bethesda, Maryland; Brooke Army Medical Center, Fort Sam Houston, Texas; Madigan Army Medical Center, Tacoma, Washington
Study to Evaluate the Safety and Antiviral Activity of Remdesivir (GS-5734) in Participants With Severe Coronavirus Disease (COVID-19)
The primary objective of this study is to evaluate the efficacy of 2 remdesivir (RDV) regimens with respect to clinical status assessed by a 7-point ordinal scale on Day 11 (NCT04292730) or Day 14 (NCT04292899).
ID: NCT04292730/NCT04292899
Sponsor: Gilead Sciences
Contact: Gilead Clinical Study Information Center (833-445-3230)
Location: James J. Peters VA Medical Center, Bronx, New York
Expanded Access Remdesivir (RDV; GS-5734)
The treatment of communicable Novel Coronavirus of 2019 with Remdesivir (RDV; GS-5734) also known as severe acute respiratory syndrome coronavirus 2.
ID: NCT04302766
Sponsor: US Army Medical Research and Development Command
Contact: Sandi Parriott ([email protected])
A Study to Evaluate the Safety and Efficacy of Tocilizumab in Patients With Severe COVID-19 Pneumonia (COVACTA)
This study will evaluate the efficacy, safety, pharmacodynamics, and pharmacokinetics of tocilizumab (TCZ) compared with a matching placebo in combination with standard of care (SOC) in hospitalized patients with severe COVID-19 pneumonia.
ID: NCT04320615
Sponsor: Hoffmann-La Roche
Location: James J Peters VA Medical Center, Bronx, New York
Administration of Intravenous Vitamin C in Novel Coronavirus Infection (COVID-19) and Decreased Oxygenation (AVoCaDO)
Previous research has shown that high dose intravenous vitamin C (HDIVC) may benefit patients with sepsis, acute lung injury (ALI), and the acute respiratory distress syndrome (ARDS). However, it is not known if early administration of HDIVC could prevent progression to ARDS. We hypothesize that HDIVC is safe and tolerable in COVID-19 subjects given early or late in the disease course and may reduce the risk of respiratory failure requiring mechanical ventilation and development of ARDS along with reductions in supplemental oxygen demand and inflammatory markers.
ID: NCT04357782
Sponsor: Hunter Holmes Mcguire VA Medical CenterContact: Brian Davis ([email protected])
Location: Hunter Holmes Mcguire VA Medical Center, Richmond, Virginia
Treatment Of CORONAVIRUS DISEASE 2019 (COVID-19) With Anti-Sars-CoV-2 Convalescent Plasma (ASCoV2CP)
This is an expanded access open-label, single-arm, multi-site protocol to provide convalescent plasma as a treatment for patients diagnosed with severe, or life-threatening COVID-19.
ID: NCT04360486
Sponsor: US Army Medical Research and Development Command
Contact: Andrew Cap ([email protected])
VA Remote and Equitable Access to COVID-19 Healthcare Delivery (VA-REACH TRIAL) (VA-REACH)
We propose a 3-arm randomized control trial to determine the efficacy of hydroxychloroquine or azithromycin in treating mild to moderate COVID-19 among veterans in the outpatient setting.
ID: NCT04363203
Sponsor: Salomeh Keyhani
Location: San Francisco VA Health Care System, California
A Study to Evaluate the Safety and Efficacy of MSTT1041A (Astegolimab) or UTTR1147A in Patients With Severe COVID-19 Pneumonia (COVASTIL)
This is a Phase II, randomized, double-blind, placebo-controlled, multicenter study to assess the efficacy and safety of MSTT1041A (astegolimab) or UTTR1147A in combination with standard of care (SOC) compared with matching placebo in combination with SOC in patients hospitalized with severe coronavirus disease 2019 (COVID-19) pneumonia.
ID: NCT04386616
Sponsor: Genentech
Contact: Study ID Number: GA42469 ([email protected])
Location: Southeast Louisiana Veterans Health Care System, New Orleans
Hormonal Intervention for the Treatment in Veterans With COVID-19 Requiring Hospitalization (HITCH)
The purpose of this study is to determine if temporary androgen suppression improves the clinical outcomes of veterans who are hospitalized to an acute care ward due to COVID-19.ID: NCT04397718
Sponsor: VA Office of Research and Development
Contact: Matthew B Rettig ([email protected]), Nicholas Nickols ([email protected])
Locations: VA Greater Los Angeles Healthcare System, California; VA NY Harbor Healthcare System, New York; VA Puget Sound Health Care System, Seattle, Washington
Adaptive COVID-19 Treatment Trial 2 (ACTT-II)
ACTT-II will evaluate the combination of baricitinib and remdesivir compared to remdesivir alone. Subjects will be assessed daily while hospitalized. If the subjects are discharged from the hospital, they will have a study visit at Days 15, 22, and 29.
ID: NCT04401579
Sponsor: National Institute of Allergy and Infectious Diseases (NIAID)
Contact: Central Contact ([email protected])
Locations: VA Palo Alto Health Care System, California; Naval Medical Center San Diego, California; Rocky Mountain Regional Veteran Affairs Medical Center, Aurora, Colorado; Southeast Louisiana Veterans Health Care System, New Orleans; Walter Reed National Military Medical Center, Bethesda, Maryland; National Institutes of Health - Clinical Center, National Institute of Allergy and Infectious Diseases Laboratory Of Immunoregulation, Bethesda, Maryland; Brooke Army Medical Center, Fort Sam Houston, Texas; Madigan Army Medical Center, Tacoma, Washington
Telemedicine: Common hurdles and proper coding for ObGyns
Since the COVID-19 pandemic began, many significant changes have occurred that have made the implementation of telemedicine easier and more attractive for gynecologic practices. In the first article in this series, we discussed the benefits of telemedicine to physicians and patients, how to get started using telemedicine, and implementing a workflow. This article will discuss the common hurdles in the process and the proper coding to use to insure reimbursement for services rendered.
Barriers to implementing telemedicine
Incorrect assumptions
Latecomers to telemedicine often assume that patients prefer face-to-face visits when, in fact, many may prefer the convenience of virtual visits. More than 50% of patients who are surveyed about their experience with telemedicine say that online tools have helped improve their relationship with their providers.1 Telemedicine has grown astronomically during the COVID-19 pandemic to the point where many patients now expect their health care providers to be able to conduct virtual visits. Practices that do not offer telemedicine may find their patients seeking services elsewhere. Nearly two-thirds of health care professionals expect their commitment to telemedicine to increase significantly in the next 3 years.2 Of those providers who have not yet adopted the practice, nearly 85% expect to implement telemedicine in the near future.3 COVID-19 has motivated the increased use of telemedicine to enhance the communication with patients, making it possible for patients to have enhanced access to health care during this pandemic while minimizing infectious transmission of COVID-19 to physicians and their staff.4
Admittedly, telemedicine is not appropriate for all patients. In general, situations that do not lend themselves to telemedicine are those for which an in-person visit is required to evaluate the patient via a physical examination, to perform a protocol-driven procedure, or provide an aggressive intervention. Additional patients for whom telemedicine may be inappropriate include those with cognitive disorders, those with language barriers, those with emergency situations that warrant an office visit or a visit to the emergency department, and patients who do not have access to the technology to conduct a virtual visit.
Cost and complexity
The process of implementing electronic health records (EHRs) left a bitter taste in the mouths of many health care professionals. But EHRs are complicated and expensive. Implementation often resulted in lost productivity. Because the learning curve was so steep, many physicians had to decrease the number of patients they saw before becoming comfortable with the conversion from paper charts to an EHR.
Telemedicine implementation is much less onerous and expensive. Telemedicine is available as a cloud-based platform, which requires less information technology (IT) support and less hardware and software. The technology required for patients to participate in telemedicine is nearly ubiquitous. According to the Pew Research Center, 96% of Americans own a cell phone (81% have a smart phone), and more than half (52%) own a tablet, so the basic equipment to connect patients to providers is already in place.5
On the provider side, the basic equipment required for a telemedicine program is a computer with video and audio capabilities and a broadband connection that is fast enough to show video in real time and to provide high-quality viewing of any images to be reviewed.
The growth in telemedicine means that telemedicine options are now more diverse, with many more affordable solutions. However, most telemedicine programs do require the purchase and set-up of new technology and equipment and the training of staff—some of which may be outside the budgets of health care providers in smaller independent practices. Many gynecologists have technology budgets that are already stretched thin. And for patients who do not have access to a smartphone or computer with Internet access, real-time telemedicine may be out of reach.
But with new guidelines put forth by the Centers for Medicare and Medicaid Services (CMS) in March 2020, connectivity can take place inexpensively using free platforms such as Google Hangouts, Skype, Facetime, and Facebook Messenger. If a non‒HIPAA-compliant platform is used initially, conversion to a HIPAA-compliant platform is recommended.6 These platforms do not require the purchase of, or subscription to, any expensive hardware or software. The disadvantages of these programs are the lack of documentation, the failure to be Health Insurance Portability and Accountability Act (HIPAA)-compliant, and the lack of encryption; however, these disadvantages are no longer an issue after the new CMS guidelines.
Depending on the magnitude of the program, IT assistance may be needed to get started. It is imperative that the telemedicine program is interoperable with the EHR and the billing program. Otherwise, double and triple entry will erase the efficiency provided by conducting a virtual visit.
Continue to: Licensing...
Licensing
Another concern or barrier is a license to participate in telemedicine. The March 15, 2020, approval of telemedicine states that physicians who are licensed in the state where the patient is located do not require any additional license or permission to conduct virtual visits.7 CMS has temporarily waived the requirement that out-of-state providers be licensed in the state where they are providing services when they are licensed in another state. For questions regarding licensure, contact your State Board of Medicine or Department of Health for information on requirements for licenses across state lines (see “Resources,” at the end of the article).
Informed consent
Just like with any other aspect of providing care for patients, obtaining informed consent is paramount. Not only is getting informed patient consent a recommended best practice of the American Telemedicine Association (ATA), but it is actually a legal requirement in many states and could be a condition of getting paid, depending on the payer. To check the requirements regarding patient consent in your state, look at The National Telehealth Policy Resource Center’s state map (see “Resources.")
Some states do not have any requirements regarding consent for a virtual visit. Others require verbal consent. Even if it is not a legal requirement in your state, consider making it a part of your practice’s policy to obtain written or verbal consent and to document in the patient’s record that consent was obtained prior to the virtual visit so that you are protected when using this new technology.
Because telemedicine is a new way of receiving care for many patients, it is important to let them know how it works including how patient confidentiality and privacy are handled, what technical equipment is required, and what they should expect in terms of scheduling, cancellations, and billing policies. A sample consent form for telemedicine use is shown in FIGURE 1.


Liability insurance
Another hurdle that must be considered is liability insurance for conducting virtual visits with patients. Gynecologists who are going to offer telemedicine care to patients should request proof in writing that their liability insurance policy covers telemedicine malpractice and that the coverage extends to other states should the patient be in another state from the state in which the gynecologist holds a license. Additionally, gynecologists who provide telemedicine care should check with liability insurers regarding any requirements or limitations to conducting a virtual visit with their patients and should document them. For example, the policy may require that the physician keep a written or recorded record of the visit in the EHR. If that is the case, then using Skype, Facebook, or Google for the virtual visit, which do not include documentation, would be less desirable.
Privacy
Certainly, there is concern about privacy, and HIPAA compliance is critical to telemedicine success. Because of the COVID-19 emergency, as of March 1, 2020, physicians may now communicate with patients, and provide telehealth services, through remote communications without penalties.8 With these changes in the HIPAA requirements, physicians may use applications that allow for video chats, including Apple FaceTime, Facebook Messenger video chat, Google Hangouts video, and Skype, to provide telehealth without risk that the Office for Civil Rights will impose a penalty for noncompliance with HIPAA rules. The consent for patients should mention that these “public” applications potentially introduce privacy risks. This is a motivation for gynecologists to consider one of the programs that promises encryption, privacy, and HIPAA compliance, such as Updox, Doxy.me, and Amazon Chime. It is also important to recognize that a virtual visit could result in colleagues (if the patient is in an office setting) or family members (if the patient is in the home environment) overhearing conversations between the health care professional and the patient. Therefore, we suggest that patients conduct virtual visits in locations in which they feel assured of some semblance of privacy.
Continue to: Compensation for telemedicine...
Compensation for telemedicine
Perhaps the biggest barrier to virtual health adoption has been compensation for telemedicine visits. Both commercial payers and CMS have been slow to enact formal policies for telemedicine reimbursement. Because of this, the common misconceptions (that providers cannot be reimbursed for telemedicine appointments or that compensation occurs at a reduced rate) have persisted, making telemedicine economically unappealing.
The good news is that this is changing; legislation in most states is quickly embracing virtual health visits as a result of the COVID-19 pandemic.9 In fact, as of January 1, 2020, telemedicine services are no longer considered “optional” coverage in Medicare Advantage plans.10 Nor are they required to have an additional fee. Instead, CMS now allows telemedicine as a standard, covered benefit in all plans, enabling beneficiaries to seek care from their homes rather than requiring them to go to a health care facility.11 In the past, telemedicine was restricted for use in rural areas or when patients resided a great distance from their health care providers. Starting March 6, 2020, and for the duration of the COVID-19 public health emergency, Medicare will make payment for professional services furnished to beneficiaries in all areas of the country in all settings regardless of location or distance between the patient and the health care provider.12
In addition, since March 15, 2020, CMS has expanded access to telemedicine services for all Medicare beneficiaries—not just those who have been diagnosed with COVID-19.13 The expanded access also applies to pre-COVID-19 coverage from physician offices, skilled nursing facilities, and hospitals. This means that Medicare will now make payments to physicians for telemedicine services provided in any health care facility or in a patient’s home, so that patients do not need to go to the physician’s office.
The facts are that there are parity laws and that commercial payers and CMS are required by state law to reimburse for telemedicine—often at the same rate as that for a comparable in-person visit. On the commercial side, there has been an increase in commercial parity legislation that requires health plans to cover virtual visits in the same way they cover face-to-face services. With the new guidelines for reimbursement, every state and Washington DC has parity laws in place. (To stay abreast of state-by-state changes in virtual health reimbursement, the Center for Connected Health Policy and the Advisory Board Primer are valuable resources. See “Resources.”) As long as the provider performs and documents the elements of history and decision-making, including the time spent counseling, and documents the visit as if a face-to-face visit occurred, then clinicians have a billable evaluation and management (E&M) visit.
Continue to: Virtual services for Medicare patients...
Virtual services for Medicare patients
There are 3 main types of virtual services gynecologists can provide to Medicare patients: Medicare telehealth visits, virtual check-ins, and e-visits.
Medicare telehealth visits. Largely because of the COVID-19 pandemic, Medicare patients may now use telecommunication technology for any services that previously occurred in an in-person communication. The gynecologist must use an interactive audio and video telecommunications system that permits real-time communication between the physician and the patient, and the patient should have a prior established relationship with the gynecologist with whom the telemedicine visit is taking place. The new guidelines indicate that the US Department of Health and Human Services (HHS) will not conduct audits to ensure that such a prior relationship exists for claims submitted during this public health emergency.14
The Current Procedural Terminology (CPT) codes for virtual visits using synchronous audio/visual communication are:
- 99201-99295, Office visit for a new patient
- 99211-99215, Office visit for an established patient.
Important modifiers for telemedicine visits include:
- modifier 02 for POS (place of service) for telehealth Medicare
- modifier 95 for commercial payers.
(A list of all available CPT codes for telehealth services from CMS can be found in “Resources.”)
Virtual check-ins. Established Medicare patients may have a brief communication with gynecologists the traditional way using a telephone or via live video. These brief virtual services, usually 5 to 10 minutes in duration, are initiated by the patient. The purpose of the virtual check-in is to determine if an office visit or a test or procedure is indicated.
Medicare pays for these “virtual check-ins” (or brief communication technology-based services) for patients to communicate with their physicians and avoid unnecessary trips to the office. These brief virtual check-ins are only for established patients. If an existing patient contacts the gynecologist’s office to ask a question or determine if an office visit is necessary, the gynecologist may bill for it using code G2012.
E-visits. Established Medicare patients may have non–face-to-face patient-initiated communications with their gynecologists without going to the physician’s office. These services can be billed only when the physician has an established relationship with the patient. The services may be billed using CPT codes 99421 to 99423. Coding for these visits is determined by the length of time the gynecologist spends online with the patient:
- 99421: Online digital evaluation and management service, for an established patient 5 to 10 minutes spent on the virtual visit
- 99422: 11 to 20 minutes
- 99423: ≥ 21 minutes.
Many clinicians want to immediately start the communication process with their patients. Many will avail themselves of the free video communication offered by Google Hangouts, Skype, Facetime, and Facebook Messenger. Since the March 15, 2020, relaxation of the HIPAA restrictions for telemedicine, it is now possible to have a virtual visit with a patient using one of the free, non–HIPAA-compliant connections. This type of visit is no different than a telephone call but with an added video component. Using these free technologies, a gynecologist can have an asynchronous visit with a patient (referred to as the store and forward method of sending information or medical images), which means that the service takes place in one direction with no opportunity for interaction with the patient. Asynchronous visits are akin to video text messages left for the patient. By contrast, a synchronous or real-time video visit with a patient is a 2-way communication that provides medical care without examining the patient.
Using triangulation
There are some downsides to telemedicine visits. First, virtual visits on Skype, FaceTime, and other non–HIPAA-compliant methods are not conducted on an encrypted website. Second, no documentation is created for the doctor-patient encounter. Finally, unless the physician keeps a record of these virtual visits and submits the interactions to the practice coders, there will be no billing and no reimbursement for the visits. In this scenario, physicians are legally responsible for their decision-making, prescription writing, and medical advice, but do not receive compensation for their efforts.
This can be remedied by using “triangulation,” which involves: 1. the physician, 2. the patient, and 3. a scribe or medical assistant who will record the visit. Before initiating the virtual visit using triangulation, it is imperative to ask the patient for permission if your medical assistant (or any other person in the office who functions as a scribe) will be listening to the conversation. It is important to explain that the person is there to take accurate notes and ascertain that the notes are entered into the EHR. Also, the scribe or assistant will record the time, date, and duration of the visit, which is a requirement for billing purposes. The scribe may also ascertain that the visit is properly coded and entered into the practice management system, and that a bill is submitted to the insurance company. By using triangulation, you have documentation that consent was obtained, that the visit took place, that notes were taken, and that the patient’s insurance company will be billed for the visit (see FIGURE 2 for a sample documentation form).

Continue to: Which CPT codes should I use?...
Which CPT codes should I use?
The answer depends on a number of factors, but a good rule of thumb is to use the same codes that you would use for an in-person appointment (CPT codes 99211-99215 for an established patient visit and 99201-99205 for a new patient visit). These are the most common CPT codes for outpatient gynecologic office visits whether they take place face-to-face or as a synchronous virtual visit (via a real-time interactive audio and video telecommunications system).
For example, the reimbursement for code 99213 has a range from $73 to $100. You may wonder how you can achieve the complexity requirements for a level-3 office visit without a physical examination. Whether as a face-to-face or virtual visit, documentation for these encounters requires 2 of 3 of the following components:
- expanded problem-focused history
- expanded problem-focused exam (not accomplished with telemedicine)
- low-complexity medical decision-making OR
- at least 15 minutes spent face to face with the patient if coding is based on time.
If a gynecologist reviews the results of a recent lab test for an estrogen-deficient patient and adjusts the estrogen dosage, writes a prescription, and spends 15 minutes communicating with the patient, he/she has met the complexity requirements for a code 99213. Because Level 3 and 4 visits (99214 and 99215) require a comprehensive physical examination, it is necessary to document the time spent with the patient (code 99214 requires 25 to 39 minutes of consultation and code 99215 requires ≥ 40 minutes).
Some final billing and coding advice
Always confirm telemedicine billing guidelines before beginning to conduct telemedicine visits. Consider starting a phone call to a payer armed with the fact that the payer is required by law to offer parity between telemedicine and face-to-face visits. Then ask which specific billing codes should be used.
Until you and your practice become comfortable with the process of, and the coding and billing for, telemedicine, consider using a telemedicine platform that has a built-in rules engine that offers recommendations for each telemedicine visit based on past claims data. These systems help gynecologists determine which CPT code to use and which modifiers are appropriate for the various insurance companies. In other words, the rules engine helps you submit a clean claim that is less likely to be denied and very likely to be paid. There are some vendors who are so confident that their rules engine will match the service with the proper CPT code and modifier that they guarantee full private payer reimbursement for telemedicine visits, or the vendor will reimburse the claim.
Watch for the third and final installment in this series, which was written with the assistance of 2 attorneys. It will review the legal guidelines for implementing telemedicine in a gynecologic practice and discuss the future of the technology. ●
- COVID-19 and Telehealth Coding Options as of March 20, 2020. https://www.ismanet.org/pdf/COVID-19andTelehealthcodes3-20-2020Updates.pdf.
- Federation of State Medical Boards. US States and Territories Modifying Licensure Requirements for Physicians in Response to COVID-19. Last updated May 26, 2020. https://www.fsmb.org/siteassets/advocacy/pdf/state-emergency-declarations-licensures-requirementscovid-19.pdf.
- Center for Connected Health Policy. Current State Laws and Reimbursement Policies https://www.cchpca.org/telehealth-policy/current-state-laws-and-reimbursement-policies.
- Centers for Medicare and Medicaid Services. List of Telehealth Services. Updated April 30, 2020. https://www.cms.gov/Medicare/Medicare-General-Information/Telehealth/Telehealth-Codes.
- American Medical Association. AMA quick guide to telemedicinein practice. Updated May 22, 2020. https://www.ama-assn.org/practice-management/digital/ama-quick-guide-telemedicine-practice.
- Eddy N. Patients increasingly trusting of remote care technology. Healthcare IT News. October 22, 2019. https://www.healthcareitnews.com/news/patients-increasingly-trusting-remote-care-technology-says-new-report. Accessed May 26, 2020.
- Welch BM, Harvey J, O’Connell NS, et al. Patient preferences for direct-to-consumer telemedicine services: a nationwide survey. BMC Health Serv Res. 2017;17:784.
- Tsai JM, Cheng MJ, Tsai HH, et al. Acceptance and resistance of telehealth: the perspective of dual-factor concepts in technology adoption. Int J Inform Manag. 2019;49:34-44.
- Hollander J, Carr BG. Virtually perfect? Telemedicine for COVID-19. N Engl J Med. 2020;382:1679-1681.
- Pew Research Center. Internet and Technology. Mobile Fact Sheet. June 12, 2019. https://www.pewresearch.org /internet/fact-sheet/mobile/. Accessed May 18, 2020.
- American Medical Association. AMA quick guide to telemedicine in practice. https://www.ama-assn.org/ practice-management/digital/ama-quick-guide-telemedicine- practice. Accessed March 20, 2020.
- Center for Connected Health Policy. Federal and state regulation updates. https://www.cchpca.org. Accessed March 20, 2020.
- The White House. Proclamation on declaring a national emergency concerning the novel coronavirus disease (Covid-19) outbreak. March 13, 2020. https://www.whitehouse.gov/presidential-actions/proclamation-declaring-national-emergency-concerning-novel-coronavirus-disease-covid-19-outbreak/. Accessed May 18, 2020.
- Center for Connected Health Policy. Quick glance state telehealth actions in response to COVID-19. https://www.cchpca.org/sites/default/files/2020-05/STATE%20TELEHEALTH%20ACTIONS%20IN%20RESPONSE%20TO%20COVID%20
OVERVIEW%205.5.2020_0.pdf. AccessedMay 13, 2020. - Medicare.gov. https://www.medicare.gov/sign-up-change -plans/types-of-medicare-health-plans/medicare-advantage-plans/how-do-medicare-advantage-plans-work. Accessed May 13, 2020.
- Centers for Medicare and Medicaid Services. CMS finalizes policies to bring innovative telehealth benefit to Medicare Advantage. April 5, 2019. https://www.cms.gov/newsroom /press-releases/cms-finalizes-policies-bring-innovative-telehealth-benefit-medicare-advantage. Accessed May 18,2020.
- Centers for Medicare & Medicaid Services. Medicare telemedicine health care provider fact sheet. https://www.cms.gov/newsroom/fact-sheets/medicare-telemedicine-health-care-provider-fact-sheet. Accessed May 30, 2020.
- Centers for Medicare & Medicaid Services. Medicare telehealth frequently asked questions. https://www.cms.gov/files/document/medicare-telehealth-frequently-asked-questions-faqs-31720.pdf.
- American Hospital Association. Coronavirus update: CMS broadens access to telehealth during Covid-19 public health emergency. https://www.aha.org/advisory/2020-03-17-coronavirus-update-cms-broadens-access-telehealth-during-covid-19-public-health. Accessed May 18, 2020.
Since the COVID-19 pandemic began, many significant changes have occurred that have made the implementation of telemedicine easier and more attractive for gynecologic practices. In the first article in this series, we discussed the benefits of telemedicine to physicians and patients, how to get started using telemedicine, and implementing a workflow. This article will discuss the common hurdles in the process and the proper coding to use to insure reimbursement for services rendered.
Barriers to implementing telemedicine
Incorrect assumptions
Latecomers to telemedicine often assume that patients prefer face-to-face visits when, in fact, many may prefer the convenience of virtual visits. More than 50% of patients who are surveyed about their experience with telemedicine say that online tools have helped improve their relationship with their providers.1 Telemedicine has grown astronomically during the COVID-19 pandemic to the point where many patients now expect their health care providers to be able to conduct virtual visits. Practices that do not offer telemedicine may find their patients seeking services elsewhere. Nearly two-thirds of health care professionals expect their commitment to telemedicine to increase significantly in the next 3 years.2 Of those providers who have not yet adopted the practice, nearly 85% expect to implement telemedicine in the near future.3 COVID-19 has motivated the increased use of telemedicine to enhance the communication with patients, making it possible for patients to have enhanced access to health care during this pandemic while minimizing infectious transmission of COVID-19 to physicians and their staff.4
Admittedly, telemedicine is not appropriate for all patients. In general, situations that do not lend themselves to telemedicine are those for which an in-person visit is required to evaluate the patient via a physical examination, to perform a protocol-driven procedure, or provide an aggressive intervention. Additional patients for whom telemedicine may be inappropriate include those with cognitive disorders, those with language barriers, those with emergency situations that warrant an office visit or a visit to the emergency department, and patients who do not have access to the technology to conduct a virtual visit.
Cost and complexity
The process of implementing electronic health records (EHRs) left a bitter taste in the mouths of many health care professionals. But EHRs are complicated and expensive. Implementation often resulted in lost productivity. Because the learning curve was so steep, many physicians had to decrease the number of patients they saw before becoming comfortable with the conversion from paper charts to an EHR.
Telemedicine implementation is much less onerous and expensive. Telemedicine is available as a cloud-based platform, which requires less information technology (IT) support and less hardware and software. The technology required for patients to participate in telemedicine is nearly ubiquitous. According to the Pew Research Center, 96% of Americans own a cell phone (81% have a smart phone), and more than half (52%) own a tablet, so the basic equipment to connect patients to providers is already in place.5
On the provider side, the basic equipment required for a telemedicine program is a computer with video and audio capabilities and a broadband connection that is fast enough to show video in real time and to provide high-quality viewing of any images to be reviewed.
The growth in telemedicine means that telemedicine options are now more diverse, with many more affordable solutions. However, most telemedicine programs do require the purchase and set-up of new technology and equipment and the training of staff—some of which may be outside the budgets of health care providers in smaller independent practices. Many gynecologists have technology budgets that are already stretched thin. And for patients who do not have access to a smartphone or computer with Internet access, real-time telemedicine may be out of reach.
But with new guidelines put forth by the Centers for Medicare and Medicaid Services (CMS) in March 2020, connectivity can take place inexpensively using free platforms such as Google Hangouts, Skype, Facetime, and Facebook Messenger. If a non‒HIPAA-compliant platform is used initially, conversion to a HIPAA-compliant platform is recommended.6 These platforms do not require the purchase of, or subscription to, any expensive hardware or software. The disadvantages of these programs are the lack of documentation, the failure to be Health Insurance Portability and Accountability Act (HIPAA)-compliant, and the lack of encryption; however, these disadvantages are no longer an issue after the new CMS guidelines.
Depending on the magnitude of the program, IT assistance may be needed to get started. It is imperative that the telemedicine program is interoperable with the EHR and the billing program. Otherwise, double and triple entry will erase the efficiency provided by conducting a virtual visit.
Continue to: Licensing...
Licensing
Another concern or barrier is a license to participate in telemedicine. The March 15, 2020, approval of telemedicine states that physicians who are licensed in the state where the patient is located do not require any additional license or permission to conduct virtual visits.7 CMS has temporarily waived the requirement that out-of-state providers be licensed in the state where they are providing services when they are licensed in another state. For questions regarding licensure, contact your State Board of Medicine or Department of Health for information on requirements for licenses across state lines (see “Resources,” at the end of the article).
Informed consent
Just like with any other aspect of providing care for patients, obtaining informed consent is paramount. Not only is getting informed patient consent a recommended best practice of the American Telemedicine Association (ATA), but it is actually a legal requirement in many states and could be a condition of getting paid, depending on the payer. To check the requirements regarding patient consent in your state, look at The National Telehealth Policy Resource Center’s state map (see “Resources.")
Some states do not have any requirements regarding consent for a virtual visit. Others require verbal consent. Even if it is not a legal requirement in your state, consider making it a part of your practice’s policy to obtain written or verbal consent and to document in the patient’s record that consent was obtained prior to the virtual visit so that you are protected when using this new technology.
Because telemedicine is a new way of receiving care for many patients, it is important to let them know how it works including how patient confidentiality and privacy are handled, what technical equipment is required, and what they should expect in terms of scheduling, cancellations, and billing policies. A sample consent form for telemedicine use is shown in FIGURE 1.


Liability insurance
Another hurdle that must be considered is liability insurance for conducting virtual visits with patients. Gynecologists who are going to offer telemedicine care to patients should request proof in writing that their liability insurance policy covers telemedicine malpractice and that the coverage extends to other states should the patient be in another state from the state in which the gynecologist holds a license. Additionally, gynecologists who provide telemedicine care should check with liability insurers regarding any requirements or limitations to conducting a virtual visit with their patients and should document them. For example, the policy may require that the physician keep a written or recorded record of the visit in the EHR. If that is the case, then using Skype, Facebook, or Google for the virtual visit, which do not include documentation, would be less desirable.
Privacy
Certainly, there is concern about privacy, and HIPAA compliance is critical to telemedicine success. Because of the COVID-19 emergency, as of March 1, 2020, physicians may now communicate with patients, and provide telehealth services, through remote communications without penalties.8 With these changes in the HIPAA requirements, physicians may use applications that allow for video chats, including Apple FaceTime, Facebook Messenger video chat, Google Hangouts video, and Skype, to provide telehealth without risk that the Office for Civil Rights will impose a penalty for noncompliance with HIPAA rules. The consent for patients should mention that these “public” applications potentially introduce privacy risks. This is a motivation for gynecologists to consider one of the programs that promises encryption, privacy, and HIPAA compliance, such as Updox, Doxy.me, and Amazon Chime. It is also important to recognize that a virtual visit could result in colleagues (if the patient is in an office setting) or family members (if the patient is in the home environment) overhearing conversations between the health care professional and the patient. Therefore, we suggest that patients conduct virtual visits in locations in which they feel assured of some semblance of privacy.
Continue to: Compensation for telemedicine...
Compensation for telemedicine
Perhaps the biggest barrier to virtual health adoption has been compensation for telemedicine visits. Both commercial payers and CMS have been slow to enact formal policies for telemedicine reimbursement. Because of this, the common misconceptions (that providers cannot be reimbursed for telemedicine appointments or that compensation occurs at a reduced rate) have persisted, making telemedicine economically unappealing.
The good news is that this is changing; legislation in most states is quickly embracing virtual health visits as a result of the COVID-19 pandemic.9 In fact, as of January 1, 2020, telemedicine services are no longer considered “optional” coverage in Medicare Advantage plans.10 Nor are they required to have an additional fee. Instead, CMS now allows telemedicine as a standard, covered benefit in all plans, enabling beneficiaries to seek care from their homes rather than requiring them to go to a health care facility.11 In the past, telemedicine was restricted for use in rural areas or when patients resided a great distance from their health care providers. Starting March 6, 2020, and for the duration of the COVID-19 public health emergency, Medicare will make payment for professional services furnished to beneficiaries in all areas of the country in all settings regardless of location or distance between the patient and the health care provider.12
In addition, since March 15, 2020, CMS has expanded access to telemedicine services for all Medicare beneficiaries—not just those who have been diagnosed with COVID-19.13 The expanded access also applies to pre-COVID-19 coverage from physician offices, skilled nursing facilities, and hospitals. This means that Medicare will now make payments to physicians for telemedicine services provided in any health care facility or in a patient’s home, so that patients do not need to go to the physician’s office.
The facts are that there are parity laws and that commercial payers and CMS are required by state law to reimburse for telemedicine—often at the same rate as that for a comparable in-person visit. On the commercial side, there has been an increase in commercial parity legislation that requires health plans to cover virtual visits in the same way they cover face-to-face services. With the new guidelines for reimbursement, every state and Washington DC has parity laws in place. (To stay abreast of state-by-state changes in virtual health reimbursement, the Center for Connected Health Policy and the Advisory Board Primer are valuable resources. See “Resources.”) As long as the provider performs and documents the elements of history and decision-making, including the time spent counseling, and documents the visit as if a face-to-face visit occurred, then clinicians have a billable evaluation and management (E&M) visit.
Continue to: Virtual services for Medicare patients...
Virtual services for Medicare patients
There are 3 main types of virtual services gynecologists can provide to Medicare patients: Medicare telehealth visits, virtual check-ins, and e-visits.
Medicare telehealth visits. Largely because of the COVID-19 pandemic, Medicare patients may now use telecommunication technology for any services that previously occurred in an in-person communication. The gynecologist must use an interactive audio and video telecommunications system that permits real-time communication between the physician and the patient, and the patient should have a prior established relationship with the gynecologist with whom the telemedicine visit is taking place. The new guidelines indicate that the US Department of Health and Human Services (HHS) will not conduct audits to ensure that such a prior relationship exists for claims submitted during this public health emergency.14
The Current Procedural Terminology (CPT) codes for virtual visits using synchronous audio/visual communication are:
- 99201-99295, Office visit for a new patient
- 99211-99215, Office visit for an established patient.
Important modifiers for telemedicine visits include:
- modifier 02 for POS (place of service) for telehealth Medicare
- modifier 95 for commercial payers.
(A list of all available CPT codes for telehealth services from CMS can be found in “Resources.”)
Virtual check-ins. Established Medicare patients may have a brief communication with gynecologists the traditional way using a telephone or via live video. These brief virtual services, usually 5 to 10 minutes in duration, are initiated by the patient. The purpose of the virtual check-in is to determine if an office visit or a test or procedure is indicated.
Medicare pays for these “virtual check-ins” (or brief communication technology-based services) for patients to communicate with their physicians and avoid unnecessary trips to the office. These brief virtual check-ins are only for established patients. If an existing patient contacts the gynecologist’s office to ask a question or determine if an office visit is necessary, the gynecologist may bill for it using code G2012.
E-visits. Established Medicare patients may have non–face-to-face patient-initiated communications with their gynecologists without going to the physician’s office. These services can be billed only when the physician has an established relationship with the patient. The services may be billed using CPT codes 99421 to 99423. Coding for these visits is determined by the length of time the gynecologist spends online with the patient:
- 99421: Online digital evaluation and management service, for an established patient 5 to 10 minutes spent on the virtual visit
- 99422: 11 to 20 minutes
- 99423: ≥ 21 minutes.
Many clinicians want to immediately start the communication process with their patients. Many will avail themselves of the free video communication offered by Google Hangouts, Skype, Facetime, and Facebook Messenger. Since the March 15, 2020, relaxation of the HIPAA restrictions for telemedicine, it is now possible to have a virtual visit with a patient using one of the free, non–HIPAA-compliant connections. This type of visit is no different than a telephone call but with an added video component. Using these free technologies, a gynecologist can have an asynchronous visit with a patient (referred to as the store and forward method of sending information or medical images), which means that the service takes place in one direction with no opportunity for interaction with the patient. Asynchronous visits are akin to video text messages left for the patient. By contrast, a synchronous or real-time video visit with a patient is a 2-way communication that provides medical care without examining the patient.
Using triangulation
There are some downsides to telemedicine visits. First, virtual visits on Skype, FaceTime, and other non–HIPAA-compliant methods are not conducted on an encrypted website. Second, no documentation is created for the doctor-patient encounter. Finally, unless the physician keeps a record of these virtual visits and submits the interactions to the practice coders, there will be no billing and no reimbursement for the visits. In this scenario, physicians are legally responsible for their decision-making, prescription writing, and medical advice, but do not receive compensation for their efforts.
This can be remedied by using “triangulation,” which involves: 1. the physician, 2. the patient, and 3. a scribe or medical assistant who will record the visit. Before initiating the virtual visit using triangulation, it is imperative to ask the patient for permission if your medical assistant (or any other person in the office who functions as a scribe) will be listening to the conversation. It is important to explain that the person is there to take accurate notes and ascertain that the notes are entered into the EHR. Also, the scribe or assistant will record the time, date, and duration of the visit, which is a requirement for billing purposes. The scribe may also ascertain that the visit is properly coded and entered into the practice management system, and that a bill is submitted to the insurance company. By using triangulation, you have documentation that consent was obtained, that the visit took place, that notes were taken, and that the patient’s insurance company will be billed for the visit (see FIGURE 2 for a sample documentation form).

Continue to: Which CPT codes should I use?...
Which CPT codes should I use?
The answer depends on a number of factors, but a good rule of thumb is to use the same codes that you would use for an in-person appointment (CPT codes 99211-99215 for an established patient visit and 99201-99205 for a new patient visit). These are the most common CPT codes for outpatient gynecologic office visits whether they take place face-to-face or as a synchronous virtual visit (via a real-time interactive audio and video telecommunications system).
For example, the reimbursement for code 99213 has a range from $73 to $100. You may wonder how you can achieve the complexity requirements for a level-3 office visit without a physical examination. Whether as a face-to-face or virtual visit, documentation for these encounters requires 2 of 3 of the following components:
- expanded problem-focused history
- expanded problem-focused exam (not accomplished with telemedicine)
- low-complexity medical decision-making OR
- at least 15 minutes spent face to face with the patient if coding is based on time.
If a gynecologist reviews the results of a recent lab test for an estrogen-deficient patient and adjusts the estrogen dosage, writes a prescription, and spends 15 minutes communicating with the patient, he/she has met the complexity requirements for a code 99213. Because Level 3 and 4 visits (99214 and 99215) require a comprehensive physical examination, it is necessary to document the time spent with the patient (code 99214 requires 25 to 39 minutes of consultation and code 99215 requires ≥ 40 minutes).
Some final billing and coding advice
Always confirm telemedicine billing guidelines before beginning to conduct telemedicine visits. Consider starting a phone call to a payer armed with the fact that the payer is required by law to offer parity between telemedicine and face-to-face visits. Then ask which specific billing codes should be used.
Until you and your practice become comfortable with the process of, and the coding and billing for, telemedicine, consider using a telemedicine platform that has a built-in rules engine that offers recommendations for each telemedicine visit based on past claims data. These systems help gynecologists determine which CPT code to use and which modifiers are appropriate for the various insurance companies. In other words, the rules engine helps you submit a clean claim that is less likely to be denied and very likely to be paid. There are some vendors who are so confident that their rules engine will match the service with the proper CPT code and modifier that they guarantee full private payer reimbursement for telemedicine visits, or the vendor will reimburse the claim.
Watch for the third and final installment in this series, which was written with the assistance of 2 attorneys. It will review the legal guidelines for implementing telemedicine in a gynecologic practice and discuss the future of the technology. ●
- COVID-19 and Telehealth Coding Options as of March 20, 2020. https://www.ismanet.org/pdf/COVID-19andTelehealthcodes3-20-2020Updates.pdf.
- Federation of State Medical Boards. US States and Territories Modifying Licensure Requirements for Physicians in Response to COVID-19. Last updated May 26, 2020. https://www.fsmb.org/siteassets/advocacy/pdf/state-emergency-declarations-licensures-requirementscovid-19.pdf.
- Center for Connected Health Policy. Current State Laws and Reimbursement Policies https://www.cchpca.org/telehealth-policy/current-state-laws-and-reimbursement-policies.
- Centers for Medicare and Medicaid Services. List of Telehealth Services. Updated April 30, 2020. https://www.cms.gov/Medicare/Medicare-General-Information/Telehealth/Telehealth-Codes.
- American Medical Association. AMA quick guide to telemedicinein practice. Updated May 22, 2020. https://www.ama-assn.org/practice-management/digital/ama-quick-guide-telemedicine-practice.
Since the COVID-19 pandemic began, many significant changes have occurred that have made the implementation of telemedicine easier and more attractive for gynecologic practices. In the first article in this series, we discussed the benefits of telemedicine to physicians and patients, how to get started using telemedicine, and implementing a workflow. This article will discuss the common hurdles in the process and the proper coding to use to insure reimbursement for services rendered.
Barriers to implementing telemedicine
Incorrect assumptions
Latecomers to telemedicine often assume that patients prefer face-to-face visits when, in fact, many may prefer the convenience of virtual visits. More than 50% of patients who are surveyed about their experience with telemedicine say that online tools have helped improve their relationship with their providers.1 Telemedicine has grown astronomically during the COVID-19 pandemic to the point where many patients now expect their health care providers to be able to conduct virtual visits. Practices that do not offer telemedicine may find their patients seeking services elsewhere. Nearly two-thirds of health care professionals expect their commitment to telemedicine to increase significantly in the next 3 years.2 Of those providers who have not yet adopted the practice, nearly 85% expect to implement telemedicine in the near future.3 COVID-19 has motivated the increased use of telemedicine to enhance the communication with patients, making it possible for patients to have enhanced access to health care during this pandemic while minimizing infectious transmission of COVID-19 to physicians and their staff.4
Admittedly, telemedicine is not appropriate for all patients. In general, situations that do not lend themselves to telemedicine are those for which an in-person visit is required to evaluate the patient via a physical examination, to perform a protocol-driven procedure, or provide an aggressive intervention. Additional patients for whom telemedicine may be inappropriate include those with cognitive disorders, those with language barriers, those with emergency situations that warrant an office visit or a visit to the emergency department, and patients who do not have access to the technology to conduct a virtual visit.
Cost and complexity
The process of implementing electronic health records (EHRs) left a bitter taste in the mouths of many health care professionals. But EHRs are complicated and expensive. Implementation often resulted in lost productivity. Because the learning curve was so steep, many physicians had to decrease the number of patients they saw before becoming comfortable with the conversion from paper charts to an EHR.
Telemedicine implementation is much less onerous and expensive. Telemedicine is available as a cloud-based platform, which requires less information technology (IT) support and less hardware and software. The technology required for patients to participate in telemedicine is nearly ubiquitous. According to the Pew Research Center, 96% of Americans own a cell phone (81% have a smart phone), and more than half (52%) own a tablet, so the basic equipment to connect patients to providers is already in place.5
On the provider side, the basic equipment required for a telemedicine program is a computer with video and audio capabilities and a broadband connection that is fast enough to show video in real time and to provide high-quality viewing of any images to be reviewed.
The growth in telemedicine means that telemedicine options are now more diverse, with many more affordable solutions. However, most telemedicine programs do require the purchase and set-up of new technology and equipment and the training of staff—some of which may be outside the budgets of health care providers in smaller independent practices. Many gynecologists have technology budgets that are already stretched thin. And for patients who do not have access to a smartphone or computer with Internet access, real-time telemedicine may be out of reach.
But with new guidelines put forth by the Centers for Medicare and Medicaid Services (CMS) in March 2020, connectivity can take place inexpensively using free platforms such as Google Hangouts, Skype, Facetime, and Facebook Messenger. If a non‒HIPAA-compliant platform is used initially, conversion to a HIPAA-compliant platform is recommended.6 These platforms do not require the purchase of, or subscription to, any expensive hardware or software. The disadvantages of these programs are the lack of documentation, the failure to be Health Insurance Portability and Accountability Act (HIPAA)-compliant, and the lack of encryption; however, these disadvantages are no longer an issue after the new CMS guidelines.
Depending on the magnitude of the program, IT assistance may be needed to get started. It is imperative that the telemedicine program is interoperable with the EHR and the billing program. Otherwise, double and triple entry will erase the efficiency provided by conducting a virtual visit.
Continue to: Licensing...
Licensing
Another concern or barrier is a license to participate in telemedicine. The March 15, 2020, approval of telemedicine states that physicians who are licensed in the state where the patient is located do not require any additional license or permission to conduct virtual visits.7 CMS has temporarily waived the requirement that out-of-state providers be licensed in the state where they are providing services when they are licensed in another state. For questions regarding licensure, contact your State Board of Medicine or Department of Health for information on requirements for licenses across state lines (see “Resources,” at the end of the article).
Informed consent
Just like with any other aspect of providing care for patients, obtaining informed consent is paramount. Not only is getting informed patient consent a recommended best practice of the American Telemedicine Association (ATA), but it is actually a legal requirement in many states and could be a condition of getting paid, depending on the payer. To check the requirements regarding patient consent in your state, look at The National Telehealth Policy Resource Center’s state map (see “Resources.")
Some states do not have any requirements regarding consent for a virtual visit. Others require verbal consent. Even if it is not a legal requirement in your state, consider making it a part of your practice’s policy to obtain written or verbal consent and to document in the patient’s record that consent was obtained prior to the virtual visit so that you are protected when using this new technology.
Because telemedicine is a new way of receiving care for many patients, it is important to let them know how it works including how patient confidentiality and privacy are handled, what technical equipment is required, and what they should expect in terms of scheduling, cancellations, and billing policies. A sample consent form for telemedicine use is shown in FIGURE 1.


Liability insurance
Another hurdle that must be considered is liability insurance for conducting virtual visits with patients. Gynecologists who are going to offer telemedicine care to patients should request proof in writing that their liability insurance policy covers telemedicine malpractice and that the coverage extends to other states should the patient be in another state from the state in which the gynecologist holds a license. Additionally, gynecologists who provide telemedicine care should check with liability insurers regarding any requirements or limitations to conducting a virtual visit with their patients and should document them. For example, the policy may require that the physician keep a written or recorded record of the visit in the EHR. If that is the case, then using Skype, Facebook, or Google for the virtual visit, which do not include documentation, would be less desirable.
Privacy
Certainly, there is concern about privacy, and HIPAA compliance is critical to telemedicine success. Because of the COVID-19 emergency, as of March 1, 2020, physicians may now communicate with patients, and provide telehealth services, through remote communications without penalties.8 With these changes in the HIPAA requirements, physicians may use applications that allow for video chats, including Apple FaceTime, Facebook Messenger video chat, Google Hangouts video, and Skype, to provide telehealth without risk that the Office for Civil Rights will impose a penalty for noncompliance with HIPAA rules. The consent for patients should mention that these “public” applications potentially introduce privacy risks. This is a motivation for gynecologists to consider one of the programs that promises encryption, privacy, and HIPAA compliance, such as Updox, Doxy.me, and Amazon Chime. It is also important to recognize that a virtual visit could result in colleagues (if the patient is in an office setting) or family members (if the patient is in the home environment) overhearing conversations between the health care professional and the patient. Therefore, we suggest that patients conduct virtual visits in locations in which they feel assured of some semblance of privacy.
Continue to: Compensation for telemedicine...
Compensation for telemedicine
Perhaps the biggest barrier to virtual health adoption has been compensation for telemedicine visits. Both commercial payers and CMS have been slow to enact formal policies for telemedicine reimbursement. Because of this, the common misconceptions (that providers cannot be reimbursed for telemedicine appointments or that compensation occurs at a reduced rate) have persisted, making telemedicine economically unappealing.
The good news is that this is changing; legislation in most states is quickly embracing virtual health visits as a result of the COVID-19 pandemic.9 In fact, as of January 1, 2020, telemedicine services are no longer considered “optional” coverage in Medicare Advantage plans.10 Nor are they required to have an additional fee. Instead, CMS now allows telemedicine as a standard, covered benefit in all plans, enabling beneficiaries to seek care from their homes rather than requiring them to go to a health care facility.11 In the past, telemedicine was restricted for use in rural areas or when patients resided a great distance from their health care providers. Starting March 6, 2020, and for the duration of the COVID-19 public health emergency, Medicare will make payment for professional services furnished to beneficiaries in all areas of the country in all settings regardless of location or distance between the patient and the health care provider.12
In addition, since March 15, 2020, CMS has expanded access to telemedicine services for all Medicare beneficiaries—not just those who have been diagnosed with COVID-19.13 The expanded access also applies to pre-COVID-19 coverage from physician offices, skilled nursing facilities, and hospitals. This means that Medicare will now make payments to physicians for telemedicine services provided in any health care facility or in a patient’s home, so that patients do not need to go to the physician’s office.
The facts are that there are parity laws and that commercial payers and CMS are required by state law to reimburse for telemedicine—often at the same rate as that for a comparable in-person visit. On the commercial side, there has been an increase in commercial parity legislation that requires health plans to cover virtual visits in the same way they cover face-to-face services. With the new guidelines for reimbursement, every state and Washington DC has parity laws in place. (To stay abreast of state-by-state changes in virtual health reimbursement, the Center for Connected Health Policy and the Advisory Board Primer are valuable resources. See “Resources.”) As long as the provider performs and documents the elements of history and decision-making, including the time spent counseling, and documents the visit as if a face-to-face visit occurred, then clinicians have a billable evaluation and management (E&M) visit.
Continue to: Virtual services for Medicare patients...
Virtual services for Medicare patients
There are 3 main types of virtual services gynecologists can provide to Medicare patients: Medicare telehealth visits, virtual check-ins, and e-visits.
Medicare telehealth visits. Largely because of the COVID-19 pandemic, Medicare patients may now use telecommunication technology for any services that previously occurred in an in-person communication. The gynecologist must use an interactive audio and video telecommunications system that permits real-time communication between the physician and the patient, and the patient should have a prior established relationship with the gynecologist with whom the telemedicine visit is taking place. The new guidelines indicate that the US Department of Health and Human Services (HHS) will not conduct audits to ensure that such a prior relationship exists for claims submitted during this public health emergency.14
The Current Procedural Terminology (CPT) codes for virtual visits using synchronous audio/visual communication are:
- 99201-99295, Office visit for a new patient
- 99211-99215, Office visit for an established patient.
Important modifiers for telemedicine visits include:
- modifier 02 for POS (place of service) for telehealth Medicare
- modifier 95 for commercial payers.
(A list of all available CPT codes for telehealth services from CMS can be found in “Resources.”)
Virtual check-ins. Established Medicare patients may have a brief communication with gynecologists the traditional way using a telephone or via live video. These brief virtual services, usually 5 to 10 minutes in duration, are initiated by the patient. The purpose of the virtual check-in is to determine if an office visit or a test or procedure is indicated.
Medicare pays for these “virtual check-ins” (or brief communication technology-based services) for patients to communicate with their physicians and avoid unnecessary trips to the office. These brief virtual check-ins are only for established patients. If an existing patient contacts the gynecologist’s office to ask a question or determine if an office visit is necessary, the gynecologist may bill for it using code G2012.
E-visits. Established Medicare patients may have non–face-to-face patient-initiated communications with their gynecologists without going to the physician’s office. These services can be billed only when the physician has an established relationship with the patient. The services may be billed using CPT codes 99421 to 99423. Coding for these visits is determined by the length of time the gynecologist spends online with the patient:
- 99421: Online digital evaluation and management service, for an established patient 5 to 10 minutes spent on the virtual visit
- 99422: 11 to 20 minutes
- 99423: ≥ 21 minutes.
Many clinicians want to immediately start the communication process with their patients. Many will avail themselves of the free video communication offered by Google Hangouts, Skype, Facetime, and Facebook Messenger. Since the March 15, 2020, relaxation of the HIPAA restrictions for telemedicine, it is now possible to have a virtual visit with a patient using one of the free, non–HIPAA-compliant connections. This type of visit is no different than a telephone call but with an added video component. Using these free technologies, a gynecologist can have an asynchronous visit with a patient (referred to as the store and forward method of sending information or medical images), which means that the service takes place in one direction with no opportunity for interaction with the patient. Asynchronous visits are akin to video text messages left for the patient. By contrast, a synchronous or real-time video visit with a patient is a 2-way communication that provides medical care without examining the patient.
Using triangulation
There are some downsides to telemedicine visits. First, virtual visits on Skype, FaceTime, and other non–HIPAA-compliant methods are not conducted on an encrypted website. Second, no documentation is created for the doctor-patient encounter. Finally, unless the physician keeps a record of these virtual visits and submits the interactions to the practice coders, there will be no billing and no reimbursement for the visits. In this scenario, physicians are legally responsible for their decision-making, prescription writing, and medical advice, but do not receive compensation for their efforts.
This can be remedied by using “triangulation,” which involves: 1. the physician, 2. the patient, and 3. a scribe or medical assistant who will record the visit. Before initiating the virtual visit using triangulation, it is imperative to ask the patient for permission if your medical assistant (or any other person in the office who functions as a scribe) will be listening to the conversation. It is important to explain that the person is there to take accurate notes and ascertain that the notes are entered into the EHR. Also, the scribe or assistant will record the time, date, and duration of the visit, which is a requirement for billing purposes. The scribe may also ascertain that the visit is properly coded and entered into the practice management system, and that a bill is submitted to the insurance company. By using triangulation, you have documentation that consent was obtained, that the visit took place, that notes were taken, and that the patient’s insurance company will be billed for the visit (see FIGURE 2 for a sample documentation form).

Continue to: Which CPT codes should I use?...
Which CPT codes should I use?
The answer depends on a number of factors, but a good rule of thumb is to use the same codes that you would use for an in-person appointment (CPT codes 99211-99215 for an established patient visit and 99201-99205 for a new patient visit). These are the most common CPT codes for outpatient gynecologic office visits whether they take place face-to-face or as a synchronous virtual visit (via a real-time interactive audio and video telecommunications system).
For example, the reimbursement for code 99213 has a range from $73 to $100. You may wonder how you can achieve the complexity requirements for a level-3 office visit without a physical examination. Whether as a face-to-face or virtual visit, documentation for these encounters requires 2 of 3 of the following components:
- expanded problem-focused history
- expanded problem-focused exam (not accomplished with telemedicine)
- low-complexity medical decision-making OR
- at least 15 minutes spent face to face with the patient if coding is based on time.
If a gynecologist reviews the results of a recent lab test for an estrogen-deficient patient and adjusts the estrogen dosage, writes a prescription, and spends 15 minutes communicating with the patient, he/she has met the complexity requirements for a code 99213. Because Level 3 and 4 visits (99214 and 99215) require a comprehensive physical examination, it is necessary to document the time spent with the patient (code 99214 requires 25 to 39 minutes of consultation and code 99215 requires ≥ 40 minutes).
Some final billing and coding advice
Always confirm telemedicine billing guidelines before beginning to conduct telemedicine visits. Consider starting a phone call to a payer armed with the fact that the payer is required by law to offer parity between telemedicine and face-to-face visits. Then ask which specific billing codes should be used.
Until you and your practice become comfortable with the process of, and the coding and billing for, telemedicine, consider using a telemedicine platform that has a built-in rules engine that offers recommendations for each telemedicine visit based on past claims data. These systems help gynecologists determine which CPT code to use and which modifiers are appropriate for the various insurance companies. In other words, the rules engine helps you submit a clean claim that is less likely to be denied and very likely to be paid. There are some vendors who are so confident that their rules engine will match the service with the proper CPT code and modifier that they guarantee full private payer reimbursement for telemedicine visits, or the vendor will reimburse the claim.
Watch for the third and final installment in this series, which was written with the assistance of 2 attorneys. It will review the legal guidelines for implementing telemedicine in a gynecologic practice and discuss the future of the technology. ●
- COVID-19 and Telehealth Coding Options as of March 20, 2020. https://www.ismanet.org/pdf/COVID-19andTelehealthcodes3-20-2020Updates.pdf.
- Federation of State Medical Boards. US States and Territories Modifying Licensure Requirements for Physicians in Response to COVID-19. Last updated May 26, 2020. https://www.fsmb.org/siteassets/advocacy/pdf/state-emergency-declarations-licensures-requirementscovid-19.pdf.
- Center for Connected Health Policy. Current State Laws and Reimbursement Policies https://www.cchpca.org/telehealth-policy/current-state-laws-and-reimbursement-policies.
- Centers for Medicare and Medicaid Services. List of Telehealth Services. Updated April 30, 2020. https://www.cms.gov/Medicare/Medicare-General-Information/Telehealth/Telehealth-Codes.
- American Medical Association. AMA quick guide to telemedicinein practice. Updated May 22, 2020. https://www.ama-assn.org/practice-management/digital/ama-quick-guide-telemedicine-practice.
- Eddy N. Patients increasingly trusting of remote care technology. Healthcare IT News. October 22, 2019. https://www.healthcareitnews.com/news/patients-increasingly-trusting-remote-care-technology-says-new-report. Accessed May 26, 2020.
- Welch BM, Harvey J, O’Connell NS, et al. Patient preferences for direct-to-consumer telemedicine services: a nationwide survey. BMC Health Serv Res. 2017;17:784.
- Tsai JM, Cheng MJ, Tsai HH, et al. Acceptance and resistance of telehealth: the perspective of dual-factor concepts in technology adoption. Int J Inform Manag. 2019;49:34-44.
- Hollander J, Carr BG. Virtually perfect? Telemedicine for COVID-19. N Engl J Med. 2020;382:1679-1681.
- Pew Research Center. Internet and Technology. Mobile Fact Sheet. June 12, 2019. https://www.pewresearch.org /internet/fact-sheet/mobile/. Accessed May 18, 2020.
- American Medical Association. AMA quick guide to telemedicine in practice. https://www.ama-assn.org/ practice-management/digital/ama-quick-guide-telemedicine- practice. Accessed March 20, 2020.
- Center for Connected Health Policy. Federal and state regulation updates. https://www.cchpca.org. Accessed March 20, 2020.
- The White House. Proclamation on declaring a national emergency concerning the novel coronavirus disease (Covid-19) outbreak. March 13, 2020. https://www.whitehouse.gov/presidential-actions/proclamation-declaring-national-emergency-concerning-novel-coronavirus-disease-covid-19-outbreak/. Accessed May 18, 2020.
- Center for Connected Health Policy. Quick glance state telehealth actions in response to COVID-19. https://www.cchpca.org/sites/default/files/2020-05/STATE%20TELEHEALTH%20ACTIONS%20IN%20RESPONSE%20TO%20COVID%20
OVERVIEW%205.5.2020_0.pdf. AccessedMay 13, 2020. - Medicare.gov. https://www.medicare.gov/sign-up-change -plans/types-of-medicare-health-plans/medicare-advantage-plans/how-do-medicare-advantage-plans-work. Accessed May 13, 2020.
- Centers for Medicare and Medicaid Services. CMS finalizes policies to bring innovative telehealth benefit to Medicare Advantage. April 5, 2019. https://www.cms.gov/newsroom /press-releases/cms-finalizes-policies-bring-innovative-telehealth-benefit-medicare-advantage. Accessed May 18,2020.
- Centers for Medicare & Medicaid Services. Medicare telemedicine health care provider fact sheet. https://www.cms.gov/newsroom/fact-sheets/medicare-telemedicine-health-care-provider-fact-sheet. Accessed May 30, 2020.
- Centers for Medicare & Medicaid Services. Medicare telehealth frequently asked questions. https://www.cms.gov/files/document/medicare-telehealth-frequently-asked-questions-faqs-31720.pdf.
- American Hospital Association. Coronavirus update: CMS broadens access to telehealth during Covid-19 public health emergency. https://www.aha.org/advisory/2020-03-17-coronavirus-update-cms-broadens-access-telehealth-during-covid-19-public-health. Accessed May 18, 2020.
- Eddy N. Patients increasingly trusting of remote care technology. Healthcare IT News. October 22, 2019. https://www.healthcareitnews.com/news/patients-increasingly-trusting-remote-care-technology-says-new-report. Accessed May 26, 2020.
- Welch BM, Harvey J, O’Connell NS, et al. Patient preferences for direct-to-consumer telemedicine services: a nationwide survey. BMC Health Serv Res. 2017;17:784.
- Tsai JM, Cheng MJ, Tsai HH, et al. Acceptance and resistance of telehealth: the perspective of dual-factor concepts in technology adoption. Int J Inform Manag. 2019;49:34-44.
- Hollander J, Carr BG. Virtually perfect? Telemedicine for COVID-19. N Engl J Med. 2020;382:1679-1681.
- Pew Research Center. Internet and Technology. Mobile Fact Sheet. June 12, 2019. https://www.pewresearch.org /internet/fact-sheet/mobile/. Accessed May 18, 2020.
- American Medical Association. AMA quick guide to telemedicine in practice. https://www.ama-assn.org/ practice-management/digital/ama-quick-guide-telemedicine- practice. Accessed March 20, 2020.
- Center for Connected Health Policy. Federal and state regulation updates. https://www.cchpca.org. Accessed March 20, 2020.
- The White House. Proclamation on declaring a national emergency concerning the novel coronavirus disease (Covid-19) outbreak. March 13, 2020. https://www.whitehouse.gov/presidential-actions/proclamation-declaring-national-emergency-concerning-novel-coronavirus-disease-covid-19-outbreak/. Accessed May 18, 2020.
- Center for Connected Health Policy. Quick glance state telehealth actions in response to COVID-19. https://www.cchpca.org/sites/default/files/2020-05/STATE%20TELEHEALTH%20ACTIONS%20IN%20RESPONSE%20TO%20COVID%20
OVERVIEW%205.5.2020_0.pdf. AccessedMay 13, 2020. - Medicare.gov. https://www.medicare.gov/sign-up-change -plans/types-of-medicare-health-plans/medicare-advantage-plans/how-do-medicare-advantage-plans-work. Accessed May 13, 2020.
- Centers for Medicare and Medicaid Services. CMS finalizes policies to bring innovative telehealth benefit to Medicare Advantage. April 5, 2019. https://www.cms.gov/newsroom /press-releases/cms-finalizes-policies-bring-innovative-telehealth-benefit-medicare-advantage. Accessed May 18,2020.
- Centers for Medicare & Medicaid Services. Medicare telemedicine health care provider fact sheet. https://www.cms.gov/newsroom/fact-sheets/medicare-telemedicine-health-care-provider-fact-sheet. Accessed May 30, 2020.
- Centers for Medicare & Medicaid Services. Medicare telehealth frequently asked questions. https://www.cms.gov/files/document/medicare-telehealth-frequently-asked-questions-faqs-31720.pdf.
- American Hospital Association. Coronavirus update: CMS broadens access to telehealth during Covid-19 public health emergency. https://www.aha.org/advisory/2020-03-17-coronavirus-update-cms-broadens-access-telehealth-during-covid-19-public-health. Accessed May 18, 2020.