User login
2020 Update on Menopause
The term genitourinary syndrome of menopause (GSM) refers to the bothersome symptoms and physical findings associated with estrogen deficiency that involve the labia, vestibular tissue, clitoris, vagina, urethra, and bladder.1 GSM is associated with genital irritation, dryness, and burning; urinary symptoms including urgency, dysuria, and recurrent urinary tract infections; and sexual symptoms including vaginal dryness and pain. Vulvovaginal atrophy (VVA) represents a component of GSM.
GSM is highly prevalent, affecting more than three-quarters of menopausal women. In contrast to menopausal vasomotor symptoms, which often are most severe and frequent in recently menopausal women, GSM commonly presents years following menopause. Unfortunately, VVA symptoms may have a substantial negative impact on women’s quality of life.
In this 2020 Menopause Update, I review a large observational study that provides reassurance to clinicians and patients regarding the safety of the best-studied prescription treatment for GSM—vaginal estrogen. Because some women should not use vaginal estrogen and others choose not to use it, nonhormonal management of GSM is important. Dr. JoAnn Pinkerton provides details on a randomized clinical trial that compared the use of fractionated CO2 laser therapy with vaginal estrogen for the treatment of GSM. In addition, Dr. JoAnn Manson discusses recent studies that found lower health risks with vaginal estrogen use compared with systemic estrogen therapy.
Diagnosing GSM
GSM can be diagnosed presumptively based on a characteristic history in a menopausal patient. Performing a pelvic examination, however, allows clinicians to exclude other conditions that may present with similar symptoms, such as lichen sclerosus, Candida infection, and malignancy.
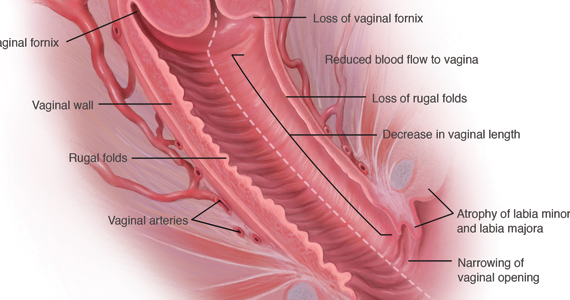
During inspection of the external genitalia, the clinician may note loss of the fat pad in the labia majora and mons as well as a reduction in labia minora pigmentation and tissue. The urethral meatus often becomes erythematous and prominent. If vaginal or introital narrowing is present, use of a pediatric (ultrathin) speculum reduces patient discomfort. The vaginal mucosa may appear smooth due to loss of rugation; it also may appear shiny and dry. Bleeding (friability) on contact with a spatula or cotton-tipped swab may occur. In addition, the vaginal fornices may become attenuated, leaving the cervix flush with the vaginal apex.
GSM can be diagnosed without laboratory assessment. However, vaginal pH, if measured, is characteristically higher than 5.0; microscopic wet prep often reveals many white blood cells, immature epithelial cells (large nuclei), and reduced or absent lactobacilli.2
Nonhormonal management of GSM
Water, silicone-based, and oil-based lubricants reduce the friction and discomfort associated with sexual activity. By contrast, vaginal moisturizers act longer than lubricants and can be applied several times weekly or daily. Natural oils, including olive and coconut oil, may be useful both as lubricants and as moisturizers. Aqueous lidocaine 4%, applied to vestibular tissue with cotton balls prior to penetration, reduces dyspareunia in women with GSM.3
Vaginal estrogen therapy
When nonhormonal management does not sufficiently reduce GSM symptoms, use of low-dose vaginal estrogen enhances thickness and elasticity of genital tissue and improves vaginal blood flow. Vaginal estrogen creams, tablets, an insert, and a ring are marketed in the United States. Although clinical improvement may be apparent within several weeks of initiating vaginal estrogen, the full benefit of treatment becomes apparent after 2 to 3 months.3
Despite the availability and effectiveness of low-dose vaginal estrogen, fears regarding the safety of menopausal hormone therapy have resulted in the underutilization of vaginal estrogen.4,5 Unfortunately, the package labeling for low-dose vaginal estrogen can exacerbate these fears.
Continue to: Nurses’ Health Study report...
Nurses’ Health Study report provides reassurance on long-term safety of vaginal estrogen
Bhupathiraju SN, Grodstein F, Stampfer MJ, et al. Vaginal estrogen use and chronic disease risk in the Nurses’ Health Study. Menopause. 2018;26:603-610
Bhupathiraju and colleagues published a report from the long-running Nurses’ Health prospective cohort study on the health outcomes associated with the use of vaginal estrogen.
Recap of the study
Starting in 1982, participants in the Nurses’Health Study were asked to report their use of vaginal estrogen via a validated questionnaire. For the years 1982 to 2012, investigators analyzed data from 896 and 52,901 women who had and had not used vaginal estrogen, respectively. The mean duration of vaginal estrogen use was 36 months.
In an analysis adjusted for numerous factors, the investigators observed no statistically significant differences in risk for cardiovascular outcomes (myocardial infarction, stroke, deep vein thrombosis, and pulmonary embolism) or invasive cancers (colorectal, endometrial, ovarian, or breast).
Findings uphold safety of vaginal estrogen
This landmark study provides reassurance that 3 years of use of vaginal estrogen does not increase the risk of cardiovascular events or invasive breast cancer, findings that hopefully will allow clinicians and women to feel comfortable regarding the safety of vaginal estrogen. A study of vaginal estrogen from the Women’s Health Initiative provided similar reassurance. Recent research supports guidance from The North American Menopause Society and the American College of Obstetricians and Gynecologists that vaginal estrogen can be used indefinitely, if indicated, and that use of concomitant progestin is not recommended in women who use vaginal estrogen and have an intact uterus.6,7
I agree with the authors, who point out that since treatment of GSM may need to be continued long term (even indefinitely), it would be helpful to have data that assessed the safety of longer-duration use of vaginal estrogen.
Results from Bhupathiraju and colleagues’ analysis of data from the Nurses’ Health Study on the 3-year safety of vaginal estrogen use encourage clinicians to recommend and women to use this safe and effective treatment for GSM.
How CO2 fractionated vaginal laser therapy compares with vaginal estrogen for relief of GSM symptoms
Paraiso MF, Ferrando CA, Sokol ER, et al. A randomized clinical trial comparing vaginal laser therapy to vaginal estrogen therapy in women with genitourinary syndrome of menopause: the VeLVET trial. Menopause. 2020;27:50-56.
Up to 50% to 60% of postmenopausal women experience GSM symptoms. However, many fewer receive treatment, either because they do not understand that the symptoms are related to menopause or they are not aware that safe and effective treatment is available. Sadly, many women are not asked about their symptoms or are embarrassed to tell providers.
GSM affects relationships and quality of life. Vaginal lubricants or moisturizers may provide relief. US Food and Drug Administration (FDA)–approved therapies include low-dose vaginal estrogen, available as a vaginal tablet, cream, suppository, and ring; intravaginal dehydroepiandrosterone (DHEA); and oral ospemifene, a selective estrogen replacement modulator. If women have an estrogen-sensitive breast or uterine cancer, an oncologist should be involved in decisions about vaginal hormonal therapy.
Energy-based devices such as vaginal lasers appear to induce wound healing; stimulate collagen and elastin fiber formation through increased storage of glycogen; and activate fibroblasts, which leads to increased extracellular matrix and restoration of vaginal pH.
These lasers are FDA approved for use in gynecology but not specifically for the treatment of GSM. In July 2016, the FDA issued a safety alert that energy-based devices, while approved for use in gynecology, have not been approved or adequately tested for menopausal vaginal conditions, and safety concerns include reports of vaginal burns.8 Lacking are publications of adequately powered randomized, sham-con-trolled trials to determine if laser therapy works better for women with GSM than placebos, moisturizers, or vaginal hormone therapies.
Recently, investigators conducted a multicenter, randomized, single-blinded trial of vaginal laser therapy and estrogen cream for treatment of GSM.
Continue to: Details of the study...
Details of the study
Paraiso and colleagues aimed to compare the 6-month efficacy and safety of fractionated CO2 vaginal laser therapy with that of estrogen vaginal cream for the treatment of vaginal dryness/GSM.
Participants randomly assigned to the estrogen therapy arm applied conjugated estrogen cream 0.5 g vaginally daily for 14 days, followed by twice weekly application for 24 weeks (a low-dose vaginal estrogen therapy). Participants randomly assigned to laser therapy underwent 3 vaginal treatments at a minimum of 6 weeks apart.
Sixty-nine women were enrolled in the trial before enrollment was closed because the FDA required that the sponsor obtain and maintain an investigational device exemption. Of 62 women who completed 6 months’ treatment, 30 received 3 laser treatments and 32 received estrogen cream.
The primary outcome compared subjective improvement in vaginal dryness using the visual analog scale (VAS) between the 2 groups at 6 months. Secondary outcomes included comparisons of the vaginal health index (VHI) and vaginal maturation index (VMI), the effect of GSM on quality of life, the effect of treatment on sexual function and urinary symptoms, and patient satisfaction.
Study findings
Efficacy. Laser therapy and estrogen therapy were found to be similarly effective except on the VMI, which favored estrogen. On patient global impression, 85.8% of laser-treated women rated their improvement as ‘‘better or much better’’ and 78.5% reported being either ‘‘satisfied or very satisfied,’’ compared with 70% and 73.3%, respectively, in the estrogen group, a statistically nonsignificant difference.
On linear regression, the investigators found a nonsignificant mean difference in female sexual function index scores. While VMI scores remained higher in the estrogen-treated group (adjusted P = .02), baseline and 6-month follow-up VMI data were available for only 34 participants (16 laser treated, 18 estrogen treated).
Regarding long-term effectiveness, 20% to 25% of the women in the laser-treated group needed further treatment after 1 year while the estrogen cream continued to work as long as it was used as prescribed.
Adverse effects. The incidence of vaginal bleeding was similar in the 2 groups: 6.7% in the laser group and 6.3% in the estrogen group. In the laser therapy group, 3% expe-rienced vaginal pain, discharge, and bladder infections, while in the estrogen cream group, 3% reported breast tenderness, migraine headaches, and abdominal cramping.
Takeaways. This small randomized, open-label (not blinded) trial provides pilot data on the effectiveness of vaginal CO2 laser compared with vaginal estrogen in treating vaginal atrophy, quality-of-life symptoms, sexual function, and urinary symptoms. Adverse events were minimal. Patient global impression of improvement and satisfaction improved for both vaginal laser and vaginal estrogen therapy.
Continue to: Study strengths and limitations...
Study strengths and limitations
To show noninferiority of vaginal laser therapy to vaginal estrogen, 196 study participants were needed. However, after 38% had been enrolled, the FDA sent a warning letter to the Foundation for Female Health Awareness, which required obtainment of an investigational device exemption for the laser and addition of a sham treatment arm.9 Instead of redesigning the trial and reconsenting the participants, the investigators closed the study, and analysis was performed only on the 62 participants who completed the study; vaginal maturation was assessed only in 34 participants.
The study lacked a placebo or sham control, which increases the risk of bias, while small numbers limit the strength of the findings. Longer-term evaluation of the effects of laser therapy beyond 6 months is needed to allow assessment of the effects of scarring on vaginal health, sexual function, and urinary issues.
Discussing therapy with patients
Despite this study’s preliminary findings, and until more robust data are available, providers should discuss the benefits and risks of all available treatment options for vaginal symptoms, including over-the-counter lubricants, vaginal moisturizers, FDA-approved vaginal hormone therapies (such as vaginal estrogen and intravaginal dehydroepiandrosterone), and systemic therapies, such as hormone therapy and ospemifene, to determine the best treatment for the individual woman with GSM.
In a healthy postmenopausal woman with bothersome GSM symptoms not responsive to lubricants and moisturizers, I recommend FDA-approved vaginal therapies as first-line treatment if there are no contraindications. For women with breast cancer, I involve their oncologist. If a patient asks about vaginal laser treatment, I share that vaginal energy-based therapies, such as the vaginal laser, have not been approved for menopausal vaginal concerns. In addition to the possibility of adverse events or unsuccessful treatment, there are significant out-of-pocket costs and the potential need for ongoing therapy after the initial 3 laser treatments.
For GSM that does not respond to lubricants and moisturizers, many FDA-approved vaginal and systemic therapies are available to treat vaginal symptoms. Vaginal laser treatment is a promising therapy for vaginal symptoms of GSM that needs further testing to determine its efficacy, safety, and long-term effects. If discussing vaginal energy-based therapies with patients, include the current lack of FDA approval for specific vaginal indications, potential adverse effects, the need for ongoing retreatment, and out-of-pocket costs.
JoAnn E. Manson, MD, DrPH, NCMP
Having more appropriate, evidence-based labeling of low-dose vaginal estrogen continues to be a high priority for The North American Menopause Society (NAMS), the International Society for the Study of Women’s Sexual Health (ISSWSH), and other professional societies.
NAMS and the Working Group on Women’s Health and Well-Being in Menopause had submitted a citizen’s petition to the US Food and Drug Administration (FDA) in 2016 requesting modification of the label—including removal of the “black box warning”—for low-dose vaginal estrogen products. The petition was, disappointingly, denied in 2018.1
Currently, the class labeling, which was based on the results of randomized trials with systemic hormone therapy, is not applicable to low-dose vaginal estrogen, and the inclusion of the black box warning has led to serious underutilization of an effective and safe treatment for a very common and life-altering condition, the genitourinary syndrome of menopause (GSM). This condition affects nearly half of postmenopausal women. It tends to be chronic and progressive and, unlike hot flashes and vasomotor symptoms, it does not remit or decline over time, and it affects women’s health and quality of life.
While removal of the black box warning would be appropriate, labeling should include emphatic reminders for women that if they have any bleeding or spotting they should seek medical attention immediately, and if they have a history of breast cancer or other estrogen-sensitive cancers they should talk with their oncologist prior to starting treatment with low-dose vaginal estrogen. Although the text would still inform women of research results on systemic hormone therapy, it would explain the differences between low-dose vaginal estrogen and systemic therapy.
Studies show vaginal estrogen has good safety profile
In the last several years, large, observational studies of low-dose vaginal estrogen have suggested that this treatment is not associated with an increase in cardiovascular disease, pulmonary embolism, venous thrombosis, cancer, or dementia—conditions listed in the black box warning that were linked to systemic estrogen therapy plus synthetic progestin. Recent data from the Nurses’ Health Study, for example, demonstrated that 3 years of vaginal estrogen use did not increase the risk of cardiovascular events or invasive breast cancer.
Women’s Health Initiative. In a prospective observational cohort study, Crandall and colleagues used data from participants in the Women’s Health Initiative Observational Study to determine the association between use of vaginal estrogen and risk of a global index event (GIE), defined as time to first occurrence of coronary heart disease, invasive breast cancer, stroke, pulmonary embolism, hip fracture, colorectal cancer, endometrial cancer, or death from any cause.2
Women were recruited from multiple clinical centers, were aged 50 to 79 years at baseline, and did not use systemic estrogen therapy during follow-up. The study included 45,663 women and median follow-up was 7.2 years. The investigators collected data on women’s self-reported use of vaginal estrogen as well as the development of the conditions defined above.
In women with a uterus, there was no significant difference between vaginal estrogen users and nonusers in the risk of stroke, invasive breast cancer, colorectal cancer, endometrial cancer, pulmonary embolism, or deep vein thrombosis. The risks of coronary heart disease, fracture, all-cause mortality, and GIE were lower in vaginal estrogen users than in nonusers (GIE adjusted hazard ratio [HR], 0.68; 95% confidence interval [CI], 0.55–0.86).
In women who had undergone hysterectomy, the risks of the individual GIE components and the overall GIE were not significantly different in users of vaginal estrogen compared with nonusers (GIE adjusted HR, 0.94; 95% CI, 0.70–1.26).
The investigators concluded that the risks of cardiovascular disease and cancer were not increased in postmenopausal women who used vaginal estrogen. Thus, this study offers reassurance on the treatment’s safety.2
Meta-analysis on menopausal hormone therapy and breast cancer risk. Further evidence now indicates that low-dose vaginal estrogen is not linked to chronic health conditions. In a large meta-analysis published in 2019, investigators looked at different types of hormone therapies—oral estrogen plus progestin, transdermal estrogen and progestin, estrogen alone, low-dose vaginal estrogen—and their relationship to breast cancer risk.3
Information on individual participants was obtained from 58 studies, 24 prospective and 34 retrospective. Breast cancer relative risks (RR) during years 5 to 14 of current hormone use were assessed according to the main hormonal contituents, doses, and modes of delivery of the last-used menopausal hormone therapy. For all systemic estrogen-only preparations, the RR was 1.33 (95% CI, 1.28–1.38), while for all estrogen-progestogen preparations, the RR was 2.08 (95% CI, 2.02–2.15). For transdermal estrogen, the RR was 1.35 (95% CI, 1.25–1.46). In contrast, for vaginal estrogen, the RR was 1.09 (95% CI, 0.97–1.23).3
Thus, the analysis found that in all the studies that had been done to date, there was no evidence of increased risk of breast cancer with vaginal estrogen therapy.
The evidence is growing that low-dose vaginal estrogen is different from systemic estrogen in terms of its safety profile and benefit-risk pattern. It is important for the FDA to consider these data and revise the vaginal estrogen label.
On the horizon: New estradiol reference ranges
It would be useful if we could accurately compare estradiol levels in women treated with vaginal estrogen against those of women treated with systemic estrogen therapy. In September 2019, NAMS held a workshop with the goal of establishing reference ranges for estradiol in postmenopausal women.4 It is very important to have good, reliable laboratory assays for estradiol and estrone, and to have a clear understanding of what is a reference range, that is, the range of estradiol levels in postmenopausal women who are not treated with estrogen. That way, you can observe what the estradiol blood levels are in women treated with low-dose vaginal estrogen or those treated with systemic estrogen versus the levels observed among postmenopausal women not receiving any estrogen product.
With the reference range information, we could look at data on the blood levels of estradiol with low-dose vaginal estrogen from the various studies available, as well as the increasing evidence from observational studies of the safety of low-dose vaginal estrogen to better understand its relationship with health. If these studies demonstrate that, with certain doses and formulations of low-dose vaginal estrogen, blood estradiol levels stay within the reference range of postmenopausal estradiol levels, it would inform the labeling modifications of these products. We need this information for future discussions with the FDA.
The laboratory assay technology used for such an investigation is primarily liquid chromatography with tandem mass spectrometry, the so-called LC-MS/MS assay. With use of this technology, the reference range for estradiol may be less than 10 picograms per milliliter. Previously, a very wide and inconsistent range—about 5 to 30 picograms per milliliter—was considered a “normal” range.
NAMS is championing the efforts to define a true evidence-based reference range that would represent the range of levels seen in postmenopausal women.5 This effort has been spearheaded by Dr. Richard Santen and colleagues. Using the more sensitive and specific LC-MS/MS assay will enable researchers and clinicians to better understand how levels on low-dose vaginal estrogen relate to the reference range for postmenopausal women. We are hoping to work together with researchers to establish these reference ranges, and to use that information to look at how low-dose vaginal estrogen compares to levels in untreated postmenopausal women, as well as to levels in women on systemic estrogen.
Hopefully, establishing the reference range can be done in an expeditious and timely way, with discussions with the FDA resuming shortly thereafter.
References
1.NAMS Citizen’s Petition and FDA Response, June 7, 2018. http://www.menopause.org/docs/
2. Crandall CJ, Hovey KM, Andrews CA, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the Women’s Health Initiative Observational Study. Menopause. 2018;25:11-20.
3. Collaborative Group on Hormonal Factors in Breast Cancer. Type and timing of menopausal hormone therapy and breast cancer risk: individual participant meta-analysis of the worldwide epidemiological evidence. Lancet. 2019;394:1159-1168.
4. Santen RJ, Pinkerton JV, Liu JH, et al. Workshop on normal reference ranges for estradiol in postmenopausal women, September 2019, Chicago, Illinois. Menopause. May 4, 2020. doi:10.1097/GME.0000000000001556.
5. Pinkerton JV, Liu JH, Santoro NF, et al. Workshop on normal reference ranges for estradiol in postmenopausal women: commentary from The North American Menopause Society on low-dose vaginal estrogen therapy. Menopause. 2020;27:611-613.
- Portman DJ, Gass ML. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and The North American Menopause Society. Maturitas. 2014;79:349-354.
- Kaunitz AM, Manson JE. Management of menopausal symptoms. Obstet Gynecol. 2015;126:859-876.
- Shifren JL. Genitourinary syndrome of menopause. Clin Obstet Gynecol. 2018;61:508-516.
- Manson JE, Kaunitz AM. Menopause management—getting clinical care back on track. N Engl J Med. 2016;374:803-806.
- Kingsberg SA, Krychman M, Graham S, et al. The women’s EMPOWER survey: identifying women’s perceptions on vulvar and vaginal atrophy and its treatment. J Sex Med. 2017;14:413-424.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 Hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin No. 141: Management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216.
- US Food and Drug Administration website. FDA warns against the use of energy-based devices to perform vaginal ‘rejuvenation’ or vaginal cosmetic procedures: FDA safety communication. https://www.fda.gov/medical-devices/safety-communications/fda-warns-against-use-energy-based-devices-perform-vaginal-rejuvenation-or-vaginal-cosmetic. Updated November 20, 2018. Accessed May 21, 2020.
- US Food and Drug Administration website. Letters to industry. https://www.fda.gov/medical-devices/industry-medical-devices/letters-industry. July 24, 2018. Accessed May 21, 2020
The term genitourinary syndrome of menopause (GSM) refers to the bothersome symptoms and physical findings associated with estrogen deficiency that involve the labia, vestibular tissue, clitoris, vagina, urethra, and bladder.1 GSM is associated with genital irritation, dryness, and burning; urinary symptoms including urgency, dysuria, and recurrent urinary tract infections; and sexual symptoms including vaginal dryness and pain. Vulvovaginal atrophy (VVA) represents a component of GSM.
GSM is highly prevalent, affecting more than three-quarters of menopausal women. In contrast to menopausal vasomotor symptoms, which often are most severe and frequent in recently menopausal women, GSM commonly presents years following menopause. Unfortunately, VVA symptoms may have a substantial negative impact on women’s quality of life.
In this 2020 Menopause Update, I review a large observational study that provides reassurance to clinicians and patients regarding the safety of the best-studied prescription treatment for GSM—vaginal estrogen. Because some women should not use vaginal estrogen and others choose not to use it, nonhormonal management of GSM is important. Dr. JoAnn Pinkerton provides details on a randomized clinical trial that compared the use of fractionated CO2 laser therapy with vaginal estrogen for the treatment of GSM. In addition, Dr. JoAnn Manson discusses recent studies that found lower health risks with vaginal estrogen use compared with systemic estrogen therapy.
Diagnosing GSM
GSM can be diagnosed presumptively based on a characteristic history in a menopausal patient. Performing a pelvic examination, however, allows clinicians to exclude other conditions that may present with similar symptoms, such as lichen sclerosus, Candida infection, and malignancy.
During inspection of the external genitalia, the clinician may note loss of the fat pad in the labia majora and mons as well as a reduction in labia minora pigmentation and tissue. The urethral meatus often becomes erythematous and prominent. If vaginal or introital narrowing is present, use of a pediatric (ultrathin) speculum reduces patient discomfort. The vaginal mucosa may appear smooth due to loss of rugation; it also may appear shiny and dry. Bleeding (friability) on contact with a spatula or cotton-tipped swab may occur. In addition, the vaginal fornices may become attenuated, leaving the cervix flush with the vaginal apex.
GSM can be diagnosed without laboratory assessment. However, vaginal pH, if measured, is characteristically higher than 5.0; microscopic wet prep often reveals many white blood cells, immature epithelial cells (large nuclei), and reduced or absent lactobacilli.2

Nonhormonal management of GSM
Water, silicone-based, and oil-based lubricants reduce the friction and discomfort associated with sexual activity. By contrast, vaginal moisturizers act longer than lubricants and can be applied several times weekly or daily. Natural oils, including olive and coconut oil, may be useful both as lubricants and as moisturizers. Aqueous lidocaine 4%, applied to vestibular tissue with cotton balls prior to penetration, reduces dyspareunia in women with GSM.3
Vaginal estrogen therapy
When nonhormonal management does not sufficiently reduce GSM symptoms, use of low-dose vaginal estrogen enhances thickness and elasticity of genital tissue and improves vaginal blood flow. Vaginal estrogen creams, tablets, an insert, and a ring are marketed in the United States. Although clinical improvement may be apparent within several weeks of initiating vaginal estrogen, the full benefit of treatment becomes apparent after 2 to 3 months.3
Despite the availability and effectiveness of low-dose vaginal estrogen, fears regarding the safety of menopausal hormone therapy have resulted in the underutilization of vaginal estrogen.4,5 Unfortunately, the package labeling for low-dose vaginal estrogen can exacerbate these fears.
Continue to: Nurses’ Health Study report...
Nurses’ Health Study report provides reassurance on long-term safety of vaginal estrogen
Bhupathiraju SN, Grodstein F, Stampfer MJ, et al. Vaginal estrogen use and chronic disease risk in the Nurses’ Health Study. Menopause. 2018;26:603-610
Bhupathiraju and colleagues published a report from the long-running Nurses’ Health prospective cohort study on the health outcomes associated with the use of vaginal estrogen.
Recap of the study
Starting in 1982, participants in the Nurses’Health Study were asked to report their use of vaginal estrogen via a validated questionnaire. For the years 1982 to 2012, investigators analyzed data from 896 and 52,901 women who had and had not used vaginal estrogen, respectively. The mean duration of vaginal estrogen use was 36 months.
In an analysis adjusted for numerous factors, the investigators observed no statistically significant differences in risk for cardiovascular outcomes (myocardial infarction, stroke, deep vein thrombosis, and pulmonary embolism) or invasive cancers (colorectal, endometrial, ovarian, or breast).
Findings uphold safety of vaginal estrogen
This landmark study provides reassurance that 3 years of use of vaginal estrogen does not increase the risk of cardiovascular events or invasive breast cancer, findings that hopefully will allow clinicians and women to feel comfortable regarding the safety of vaginal estrogen. A study of vaginal estrogen from the Women’s Health Initiative provided similar reassurance. Recent research supports guidance from The North American Menopause Society and the American College of Obstetricians and Gynecologists that vaginal estrogen can be used indefinitely, if indicated, and that use of concomitant progestin is not recommended in women who use vaginal estrogen and have an intact uterus.6,7
I agree with the authors, who point out that since treatment of GSM may need to be continued long term (even indefinitely), it would be helpful to have data that assessed the safety of longer-duration use of vaginal estrogen.
Results from Bhupathiraju and colleagues’ analysis of data from the Nurses’ Health Study on the 3-year safety of vaginal estrogen use encourage clinicians to recommend and women to use this safe and effective treatment for GSM.
How CO2 fractionated vaginal laser therapy compares with vaginal estrogen for relief of GSM symptoms
Paraiso MF, Ferrando CA, Sokol ER, et al. A randomized clinical trial comparing vaginal laser therapy to vaginal estrogen therapy in women with genitourinary syndrome of menopause: the VeLVET trial. Menopause. 2020;27:50-56.
Up to 50% to 60% of postmenopausal women experience GSM symptoms. However, many fewer receive treatment, either because they do not understand that the symptoms are related to menopause or they are not aware that safe and effective treatment is available. Sadly, many women are not asked about their symptoms or are embarrassed to tell providers.
GSM affects relationships and quality of life. Vaginal lubricants or moisturizers may provide relief. US Food and Drug Administration (FDA)–approved therapies include low-dose vaginal estrogen, available as a vaginal tablet, cream, suppository, and ring; intravaginal dehydroepiandrosterone (DHEA); and oral ospemifene, a selective estrogen replacement modulator. If women have an estrogen-sensitive breast or uterine cancer, an oncologist should be involved in decisions about vaginal hormonal therapy.
Energy-based devices such as vaginal lasers appear to induce wound healing; stimulate collagen and elastin fiber formation through increased storage of glycogen; and activate fibroblasts, which leads to increased extracellular matrix and restoration of vaginal pH.
These lasers are FDA approved for use in gynecology but not specifically for the treatment of GSM. In July 2016, the FDA issued a safety alert that energy-based devices, while approved for use in gynecology, have not been approved or adequately tested for menopausal vaginal conditions, and safety concerns include reports of vaginal burns.8 Lacking are publications of adequately powered randomized, sham-con-trolled trials to determine if laser therapy works better for women with GSM than placebos, moisturizers, or vaginal hormone therapies.
Recently, investigators conducted a multicenter, randomized, single-blinded trial of vaginal laser therapy and estrogen cream for treatment of GSM.
Continue to: Details of the study...
Details of the study
Paraiso and colleagues aimed to compare the 6-month efficacy and safety of fractionated CO2 vaginal laser therapy with that of estrogen vaginal cream for the treatment of vaginal dryness/GSM.
Participants randomly assigned to the estrogen therapy arm applied conjugated estrogen cream 0.5 g vaginally daily for 14 days, followed by twice weekly application for 24 weeks (a low-dose vaginal estrogen therapy). Participants randomly assigned to laser therapy underwent 3 vaginal treatments at a minimum of 6 weeks apart.
Sixty-nine women were enrolled in the trial before enrollment was closed because the FDA required that the sponsor obtain and maintain an investigational device exemption. Of 62 women who completed 6 months’ treatment, 30 received 3 laser treatments and 32 received estrogen cream.
The primary outcome compared subjective improvement in vaginal dryness using the visual analog scale (VAS) between the 2 groups at 6 months. Secondary outcomes included comparisons of the vaginal health index (VHI) and vaginal maturation index (VMI), the effect of GSM on quality of life, the effect of treatment on sexual function and urinary symptoms, and patient satisfaction.
Study findings
Efficacy. Laser therapy and estrogen therapy were found to be similarly effective except on the VMI, which favored estrogen. On patient global impression, 85.8% of laser-treated women rated their improvement as ‘‘better or much better’’ and 78.5% reported being either ‘‘satisfied or very satisfied,’’ compared with 70% and 73.3%, respectively, in the estrogen group, a statistically nonsignificant difference.
On linear regression, the investigators found a nonsignificant mean difference in female sexual function index scores. While VMI scores remained higher in the estrogen-treated group (adjusted P = .02), baseline and 6-month follow-up VMI data were available for only 34 participants (16 laser treated, 18 estrogen treated).
Regarding long-term effectiveness, 20% to 25% of the women in the laser-treated group needed further treatment after 1 year while the estrogen cream continued to work as long as it was used as prescribed.
Adverse effects. The incidence of vaginal bleeding was similar in the 2 groups: 6.7% in the laser group and 6.3% in the estrogen group. In the laser therapy group, 3% expe-rienced vaginal pain, discharge, and bladder infections, while in the estrogen cream group, 3% reported breast tenderness, migraine headaches, and abdominal cramping.
Takeaways. This small randomized, open-label (not blinded) trial provides pilot data on the effectiveness of vaginal CO2 laser compared with vaginal estrogen in treating vaginal atrophy, quality-of-life symptoms, sexual function, and urinary symptoms. Adverse events were minimal. Patient global impression of improvement and satisfaction improved for both vaginal laser and vaginal estrogen therapy.
Continue to: Study strengths and limitations...
Study strengths and limitations
To show noninferiority of vaginal laser therapy to vaginal estrogen, 196 study participants were needed. However, after 38% had been enrolled, the FDA sent a warning letter to the Foundation for Female Health Awareness, which required obtainment of an investigational device exemption for the laser and addition of a sham treatment arm.9 Instead of redesigning the trial and reconsenting the participants, the investigators closed the study, and analysis was performed only on the 62 participants who completed the study; vaginal maturation was assessed only in 34 participants.
The study lacked a placebo or sham control, which increases the risk of bias, while small numbers limit the strength of the findings. Longer-term evaluation of the effects of laser therapy beyond 6 months is needed to allow assessment of the effects of scarring on vaginal health, sexual function, and urinary issues.
Discussing therapy with patients
Despite this study’s preliminary findings, and until more robust data are available, providers should discuss the benefits and risks of all available treatment options for vaginal symptoms, including over-the-counter lubricants, vaginal moisturizers, FDA-approved vaginal hormone therapies (such as vaginal estrogen and intravaginal dehydroepiandrosterone), and systemic therapies, such as hormone therapy and ospemifene, to determine the best treatment for the individual woman with GSM.
In a healthy postmenopausal woman with bothersome GSM symptoms not responsive to lubricants and moisturizers, I recommend FDA-approved vaginal therapies as first-line treatment if there are no contraindications. For women with breast cancer, I involve their oncologist. If a patient asks about vaginal laser treatment, I share that vaginal energy-based therapies, such as the vaginal laser, have not been approved for menopausal vaginal concerns. In addition to the possibility of adverse events or unsuccessful treatment, there are significant out-of-pocket costs and the potential need for ongoing therapy after the initial 3 laser treatments.
For GSM that does not respond to lubricants and moisturizers, many FDA-approved vaginal and systemic therapies are available to treat vaginal symptoms. Vaginal laser treatment is a promising therapy for vaginal symptoms of GSM that needs further testing to determine its efficacy, safety, and long-term effects. If discussing vaginal energy-based therapies with patients, include the current lack of FDA approval for specific vaginal indications, potential adverse effects, the need for ongoing retreatment, and out-of-pocket costs.
JoAnn E. Manson, MD, DrPH, NCMP
Having more appropriate, evidence-based labeling of low-dose vaginal estrogen continues to be a high priority for The North American Menopause Society (NAMS), the International Society for the Study of Women’s Sexual Health (ISSWSH), and other professional societies.
NAMS and the Working Group on Women’s Health and Well-Being in Menopause had submitted a citizen’s petition to the US Food and Drug Administration (FDA) in 2016 requesting modification of the label—including removal of the “black box warning”—for low-dose vaginal estrogen products. The petition was, disappointingly, denied in 2018.1
Currently, the class labeling, which was based on the results of randomized trials with systemic hormone therapy, is not applicable to low-dose vaginal estrogen, and the inclusion of the black box warning has led to serious underutilization of an effective and safe treatment for a very common and life-altering condition, the genitourinary syndrome of menopause (GSM). This condition affects nearly half of postmenopausal women. It tends to be chronic and progressive and, unlike hot flashes and vasomotor symptoms, it does not remit or decline over time, and it affects women’s health and quality of life.
While removal of the black box warning would be appropriate, labeling should include emphatic reminders for women that if they have any bleeding or spotting they should seek medical attention immediately, and if they have a history of breast cancer or other estrogen-sensitive cancers they should talk with their oncologist prior to starting treatment with low-dose vaginal estrogen. Although the text would still inform women of research results on systemic hormone therapy, it would explain the differences between low-dose vaginal estrogen and systemic therapy.
Studies show vaginal estrogen has good safety profile
In the last several years, large, observational studies of low-dose vaginal estrogen have suggested that this treatment is not associated with an increase in cardiovascular disease, pulmonary embolism, venous thrombosis, cancer, or dementia—conditions listed in the black box warning that were linked to systemic estrogen therapy plus synthetic progestin. Recent data from the Nurses’ Health Study, for example, demonstrated that 3 years of vaginal estrogen use did not increase the risk of cardiovascular events or invasive breast cancer.
Women’s Health Initiative. In a prospective observational cohort study, Crandall and colleagues used data from participants in the Women’s Health Initiative Observational Study to determine the association between use of vaginal estrogen and risk of a global index event (GIE), defined as time to first occurrence of coronary heart disease, invasive breast cancer, stroke, pulmonary embolism, hip fracture, colorectal cancer, endometrial cancer, or death from any cause.2
Women were recruited from multiple clinical centers, were aged 50 to 79 years at baseline, and did not use systemic estrogen therapy during follow-up. The study included 45,663 women and median follow-up was 7.2 years. The investigators collected data on women’s self-reported use of vaginal estrogen as well as the development of the conditions defined above.
In women with a uterus, there was no significant difference between vaginal estrogen users and nonusers in the risk of stroke, invasive breast cancer, colorectal cancer, endometrial cancer, pulmonary embolism, or deep vein thrombosis. The risks of coronary heart disease, fracture, all-cause mortality, and GIE were lower in vaginal estrogen users than in nonusers (GIE adjusted hazard ratio [HR], 0.68; 95% confidence interval [CI], 0.55–0.86).
In women who had undergone hysterectomy, the risks of the individual GIE components and the overall GIE were not significantly different in users of vaginal estrogen compared with nonusers (GIE adjusted HR, 0.94; 95% CI, 0.70–1.26).
The investigators concluded that the risks of cardiovascular disease and cancer were not increased in postmenopausal women who used vaginal estrogen. Thus, this study offers reassurance on the treatment’s safety.2
Meta-analysis on menopausal hormone therapy and breast cancer risk. Further evidence now indicates that low-dose vaginal estrogen is not linked to chronic health conditions. In a large meta-analysis published in 2019, investigators looked at different types of hormone therapies—oral estrogen plus progestin, transdermal estrogen and progestin, estrogen alone, low-dose vaginal estrogen—and their relationship to breast cancer risk.3
Information on individual participants was obtained from 58 studies, 24 prospective and 34 retrospective. Breast cancer relative risks (RR) during years 5 to 14 of current hormone use were assessed according to the main hormonal contituents, doses, and modes of delivery of the last-used menopausal hormone therapy. For all systemic estrogen-only preparations, the RR was 1.33 (95% CI, 1.28–1.38), while for all estrogen-progestogen preparations, the RR was 2.08 (95% CI, 2.02–2.15). For transdermal estrogen, the RR was 1.35 (95% CI, 1.25–1.46). In contrast, for vaginal estrogen, the RR was 1.09 (95% CI, 0.97–1.23).3
Thus, the analysis found that in all the studies that had been done to date, there was no evidence of increased risk of breast cancer with vaginal estrogen therapy.
The evidence is growing that low-dose vaginal estrogen is different from systemic estrogen in terms of its safety profile and benefit-risk pattern. It is important for the FDA to consider these data and revise the vaginal estrogen label.
On the horizon: New estradiol reference ranges
It would be useful if we could accurately compare estradiol levels in women treated with vaginal estrogen against those of women treated with systemic estrogen therapy. In September 2019, NAMS held a workshop with the goal of establishing reference ranges for estradiol in postmenopausal women.4 It is very important to have good, reliable laboratory assays for estradiol and estrone, and to have a clear understanding of what is a reference range, that is, the range of estradiol levels in postmenopausal women who are not treated with estrogen. That way, you can observe what the estradiol blood levels are in women treated with low-dose vaginal estrogen or those treated with systemic estrogen versus the levels observed among postmenopausal women not receiving any estrogen product.
With the reference range information, we could look at data on the blood levels of estradiol with low-dose vaginal estrogen from the various studies available, as well as the increasing evidence from observational studies of the safety of low-dose vaginal estrogen to better understand its relationship with health. If these studies demonstrate that, with certain doses and formulations of low-dose vaginal estrogen, blood estradiol levels stay within the reference range of postmenopausal estradiol levels, it would inform the labeling modifications of these products. We need this information for future discussions with the FDA.
The laboratory assay technology used for such an investigation is primarily liquid chromatography with tandem mass spectrometry, the so-called LC-MS/MS assay. With use of this technology, the reference range for estradiol may be less than 10 picograms per milliliter. Previously, a very wide and inconsistent range—about 5 to 30 picograms per milliliter—was considered a “normal” range.
NAMS is championing the efforts to define a true evidence-based reference range that would represent the range of levels seen in postmenopausal women.5 This effort has been spearheaded by Dr. Richard Santen and colleagues. Using the more sensitive and specific LC-MS/MS assay will enable researchers and clinicians to better understand how levels on low-dose vaginal estrogen relate to the reference range for postmenopausal women. We are hoping to work together with researchers to establish these reference ranges, and to use that information to look at how low-dose vaginal estrogen compares to levels in untreated postmenopausal women, as well as to levels in women on systemic estrogen.
Hopefully, establishing the reference range can be done in an expeditious and timely way, with discussions with the FDA resuming shortly thereafter.
References
1.NAMS Citizen’s Petition and FDA Response, June 7, 2018. http://www.menopause.org/docs/
2. Crandall CJ, Hovey KM, Andrews CA, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the Women’s Health Initiative Observational Study. Menopause. 2018;25:11-20.
3. Collaborative Group on Hormonal Factors in Breast Cancer. Type and timing of menopausal hormone therapy and breast cancer risk: individual participant meta-analysis of the worldwide epidemiological evidence. Lancet. 2019;394:1159-1168.
4. Santen RJ, Pinkerton JV, Liu JH, et al. Workshop on normal reference ranges for estradiol in postmenopausal women, September 2019, Chicago, Illinois. Menopause. May 4, 2020. doi:10.1097/GME.0000000000001556.
5. Pinkerton JV, Liu JH, Santoro NF, et al. Workshop on normal reference ranges for estradiol in postmenopausal women: commentary from The North American Menopause Society on low-dose vaginal estrogen therapy. Menopause. 2020;27:611-613.
The term genitourinary syndrome of menopause (GSM) refers to the bothersome symptoms and physical findings associated with estrogen deficiency that involve the labia, vestibular tissue, clitoris, vagina, urethra, and bladder.1 GSM is associated with genital irritation, dryness, and burning; urinary symptoms including urgency, dysuria, and recurrent urinary tract infections; and sexual symptoms including vaginal dryness and pain. Vulvovaginal atrophy (VVA) represents a component of GSM.
GSM is highly prevalent, affecting more than three-quarters of menopausal women. In contrast to menopausal vasomotor symptoms, which often are most severe and frequent in recently menopausal women, GSM commonly presents years following menopause. Unfortunately, VVA symptoms may have a substantial negative impact on women’s quality of life.
In this 2020 Menopause Update, I review a large observational study that provides reassurance to clinicians and patients regarding the safety of the best-studied prescription treatment for GSM—vaginal estrogen. Because some women should not use vaginal estrogen and others choose not to use it, nonhormonal management of GSM is important. Dr. JoAnn Pinkerton provides details on a randomized clinical trial that compared the use of fractionated CO2 laser therapy with vaginal estrogen for the treatment of GSM. In addition, Dr. JoAnn Manson discusses recent studies that found lower health risks with vaginal estrogen use compared with systemic estrogen therapy.
Diagnosing GSM
GSM can be diagnosed presumptively based on a characteristic history in a menopausal patient. Performing a pelvic examination, however, allows clinicians to exclude other conditions that may present with similar symptoms, such as lichen sclerosus, Candida infection, and malignancy.
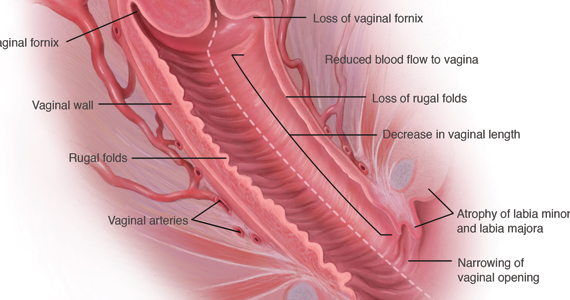
During inspection of the external genitalia, the clinician may note loss of the fat pad in the labia majora and mons as well as a reduction in labia minora pigmentation and tissue. The urethral meatus often becomes erythematous and prominent. If vaginal or introital narrowing is present, use of a pediatric (ultrathin) speculum reduces patient discomfort. The vaginal mucosa may appear smooth due to loss of rugation; it also may appear shiny and dry. Bleeding (friability) on contact with a spatula or cotton-tipped swab may occur. In addition, the vaginal fornices may become attenuated, leaving the cervix flush with the vaginal apex.
GSM can be diagnosed without laboratory assessment. However, vaginal pH, if measured, is characteristically higher than 5.0; microscopic wet prep often reveals many white blood cells, immature epithelial cells (large nuclei), and reduced or absent lactobacilli.2

Nonhormonal management of GSM
Water, silicone-based, and oil-based lubricants reduce the friction and discomfort associated with sexual activity. By contrast, vaginal moisturizers act longer than lubricants and can be applied several times weekly or daily. Natural oils, including olive and coconut oil, may be useful both as lubricants and as moisturizers. Aqueous lidocaine 4%, applied to vestibular tissue with cotton balls prior to penetration, reduces dyspareunia in women with GSM.3
Vaginal estrogen therapy
When nonhormonal management does not sufficiently reduce GSM symptoms, use of low-dose vaginal estrogen enhances thickness and elasticity of genital tissue and improves vaginal blood flow. Vaginal estrogen creams, tablets, an insert, and a ring are marketed in the United States. Although clinical improvement may be apparent within several weeks of initiating vaginal estrogen, the full benefit of treatment becomes apparent after 2 to 3 months.3
Despite the availability and effectiveness of low-dose vaginal estrogen, fears regarding the safety of menopausal hormone therapy have resulted in the underutilization of vaginal estrogen.4,5 Unfortunately, the package labeling for low-dose vaginal estrogen can exacerbate these fears.
Continue to: Nurses’ Health Study report...
Nurses’ Health Study report provides reassurance on long-term safety of vaginal estrogen
Bhupathiraju SN, Grodstein F, Stampfer MJ, et al. Vaginal estrogen use and chronic disease risk in the Nurses’ Health Study. Menopause. 2018;26:603-610
Bhupathiraju and colleagues published a report from the long-running Nurses’ Health prospective cohort study on the health outcomes associated with the use of vaginal estrogen.
Recap of the study
Starting in 1982, participants in the Nurses’Health Study were asked to report their use of vaginal estrogen via a validated questionnaire. For the years 1982 to 2012, investigators analyzed data from 896 and 52,901 women who had and had not used vaginal estrogen, respectively. The mean duration of vaginal estrogen use was 36 months.
In an analysis adjusted for numerous factors, the investigators observed no statistically significant differences in risk for cardiovascular outcomes (myocardial infarction, stroke, deep vein thrombosis, and pulmonary embolism) or invasive cancers (colorectal, endometrial, ovarian, or breast).
Findings uphold safety of vaginal estrogen
This landmark study provides reassurance that 3 years of use of vaginal estrogen does not increase the risk of cardiovascular events or invasive breast cancer, findings that hopefully will allow clinicians and women to feel comfortable regarding the safety of vaginal estrogen. A study of vaginal estrogen from the Women’s Health Initiative provided similar reassurance. Recent research supports guidance from The North American Menopause Society and the American College of Obstetricians and Gynecologists that vaginal estrogen can be used indefinitely, if indicated, and that use of concomitant progestin is not recommended in women who use vaginal estrogen and have an intact uterus.6,7
I agree with the authors, who point out that since treatment of GSM may need to be continued long term (even indefinitely), it would be helpful to have data that assessed the safety of longer-duration use of vaginal estrogen.
Results from Bhupathiraju and colleagues’ analysis of data from the Nurses’ Health Study on the 3-year safety of vaginal estrogen use encourage clinicians to recommend and women to use this safe and effective treatment for GSM.
How CO2 fractionated vaginal laser therapy compares with vaginal estrogen for relief of GSM symptoms
Paraiso MF, Ferrando CA, Sokol ER, et al. A randomized clinical trial comparing vaginal laser therapy to vaginal estrogen therapy in women with genitourinary syndrome of menopause: the VeLVET trial. Menopause. 2020;27:50-56.
Up to 50% to 60% of postmenopausal women experience GSM symptoms. However, many fewer receive treatment, either because they do not understand that the symptoms are related to menopause or they are not aware that safe and effective treatment is available. Sadly, many women are not asked about their symptoms or are embarrassed to tell providers.
GSM affects relationships and quality of life. Vaginal lubricants or moisturizers may provide relief. US Food and Drug Administration (FDA)–approved therapies include low-dose vaginal estrogen, available as a vaginal tablet, cream, suppository, and ring; intravaginal dehydroepiandrosterone (DHEA); and oral ospemifene, a selective estrogen replacement modulator. If women have an estrogen-sensitive breast or uterine cancer, an oncologist should be involved in decisions about vaginal hormonal therapy.
Energy-based devices such as vaginal lasers appear to induce wound healing; stimulate collagen and elastin fiber formation through increased storage of glycogen; and activate fibroblasts, which leads to increased extracellular matrix and restoration of vaginal pH.
These lasers are FDA approved for use in gynecology but not specifically for the treatment of GSM. In July 2016, the FDA issued a safety alert that energy-based devices, while approved for use in gynecology, have not been approved or adequately tested for menopausal vaginal conditions, and safety concerns include reports of vaginal burns.8 Lacking are publications of adequately powered randomized, sham-con-trolled trials to determine if laser therapy works better for women with GSM than placebos, moisturizers, or vaginal hormone therapies.
Recently, investigators conducted a multicenter, randomized, single-blinded trial of vaginal laser therapy and estrogen cream for treatment of GSM.
Continue to: Details of the study...
Details of the study
Paraiso and colleagues aimed to compare the 6-month efficacy and safety of fractionated CO2 vaginal laser therapy with that of estrogen vaginal cream for the treatment of vaginal dryness/GSM.
Participants randomly assigned to the estrogen therapy arm applied conjugated estrogen cream 0.5 g vaginally daily for 14 days, followed by twice weekly application for 24 weeks (a low-dose vaginal estrogen therapy). Participants randomly assigned to laser therapy underwent 3 vaginal treatments at a minimum of 6 weeks apart.
Sixty-nine women were enrolled in the trial before enrollment was closed because the FDA required that the sponsor obtain and maintain an investigational device exemption. Of 62 women who completed 6 months’ treatment, 30 received 3 laser treatments and 32 received estrogen cream.
The primary outcome compared subjective improvement in vaginal dryness using the visual analog scale (VAS) between the 2 groups at 6 months. Secondary outcomes included comparisons of the vaginal health index (VHI) and vaginal maturation index (VMI), the effect of GSM on quality of life, the effect of treatment on sexual function and urinary symptoms, and patient satisfaction.
Study findings
Efficacy. Laser therapy and estrogen therapy were found to be similarly effective except on the VMI, which favored estrogen. On patient global impression, 85.8% of laser-treated women rated their improvement as ‘‘better or much better’’ and 78.5% reported being either ‘‘satisfied or very satisfied,’’ compared with 70% and 73.3%, respectively, in the estrogen group, a statistically nonsignificant difference.
On linear regression, the investigators found a nonsignificant mean difference in female sexual function index scores. While VMI scores remained higher in the estrogen-treated group (adjusted P = .02), baseline and 6-month follow-up VMI data were available for only 34 participants (16 laser treated, 18 estrogen treated).
Regarding long-term effectiveness, 20% to 25% of the women in the laser-treated group needed further treatment after 1 year while the estrogen cream continued to work as long as it was used as prescribed.
Adverse effects. The incidence of vaginal bleeding was similar in the 2 groups: 6.7% in the laser group and 6.3% in the estrogen group. In the laser therapy group, 3% expe-rienced vaginal pain, discharge, and bladder infections, while in the estrogen cream group, 3% reported breast tenderness, migraine headaches, and abdominal cramping.
Takeaways. This small randomized, open-label (not blinded) trial provides pilot data on the effectiveness of vaginal CO2 laser compared with vaginal estrogen in treating vaginal atrophy, quality-of-life symptoms, sexual function, and urinary symptoms. Adverse events were minimal. Patient global impression of improvement and satisfaction improved for both vaginal laser and vaginal estrogen therapy.
Continue to: Study strengths and limitations...
Study strengths and limitations
To show noninferiority of vaginal laser therapy to vaginal estrogen, 196 study participants were needed. However, after 38% had been enrolled, the FDA sent a warning letter to the Foundation for Female Health Awareness, which required obtainment of an investigational device exemption for the laser and addition of a sham treatment arm.9 Instead of redesigning the trial and reconsenting the participants, the investigators closed the study, and analysis was performed only on the 62 participants who completed the study; vaginal maturation was assessed only in 34 participants.
The study lacked a placebo or sham control, which increases the risk of bias, while small numbers limit the strength of the findings. Longer-term evaluation of the effects of laser therapy beyond 6 months is needed to allow assessment of the effects of scarring on vaginal health, sexual function, and urinary issues.
Discussing therapy with patients
Despite this study’s preliminary findings, and until more robust data are available, providers should discuss the benefits and risks of all available treatment options for vaginal symptoms, including over-the-counter lubricants, vaginal moisturizers, FDA-approved vaginal hormone therapies (such as vaginal estrogen and intravaginal dehydroepiandrosterone), and systemic therapies, such as hormone therapy and ospemifene, to determine the best treatment for the individual woman with GSM.
In a healthy postmenopausal woman with bothersome GSM symptoms not responsive to lubricants and moisturizers, I recommend FDA-approved vaginal therapies as first-line treatment if there are no contraindications. For women with breast cancer, I involve their oncologist. If a patient asks about vaginal laser treatment, I share that vaginal energy-based therapies, such as the vaginal laser, have not been approved for menopausal vaginal concerns. In addition to the possibility of adverse events or unsuccessful treatment, there are significant out-of-pocket costs and the potential need for ongoing therapy after the initial 3 laser treatments.
For GSM that does not respond to lubricants and moisturizers, many FDA-approved vaginal and systemic therapies are available to treat vaginal symptoms. Vaginal laser treatment is a promising therapy for vaginal symptoms of GSM that needs further testing to determine its efficacy, safety, and long-term effects. If discussing vaginal energy-based therapies with patients, include the current lack of FDA approval for specific vaginal indications, potential adverse effects, the need for ongoing retreatment, and out-of-pocket costs.
JoAnn E. Manson, MD, DrPH, NCMP
Having more appropriate, evidence-based labeling of low-dose vaginal estrogen continues to be a high priority for The North American Menopause Society (NAMS), the International Society for the Study of Women’s Sexual Health (ISSWSH), and other professional societies.
NAMS and the Working Group on Women’s Health and Well-Being in Menopause had submitted a citizen’s petition to the US Food and Drug Administration (FDA) in 2016 requesting modification of the label—including removal of the “black box warning”—for low-dose vaginal estrogen products. The petition was, disappointingly, denied in 2018.1
Currently, the class labeling, which was based on the results of randomized trials with systemic hormone therapy, is not applicable to low-dose vaginal estrogen, and the inclusion of the black box warning has led to serious underutilization of an effective and safe treatment for a very common and life-altering condition, the genitourinary syndrome of menopause (GSM). This condition affects nearly half of postmenopausal women. It tends to be chronic and progressive and, unlike hot flashes and vasomotor symptoms, it does not remit or decline over time, and it affects women’s health and quality of life.
While removal of the black box warning would be appropriate, labeling should include emphatic reminders for women that if they have any bleeding or spotting they should seek medical attention immediately, and if they have a history of breast cancer or other estrogen-sensitive cancers they should talk with their oncologist prior to starting treatment with low-dose vaginal estrogen. Although the text would still inform women of research results on systemic hormone therapy, it would explain the differences between low-dose vaginal estrogen and systemic therapy.
Studies show vaginal estrogen has good safety profile
In the last several years, large, observational studies of low-dose vaginal estrogen have suggested that this treatment is not associated with an increase in cardiovascular disease, pulmonary embolism, venous thrombosis, cancer, or dementia—conditions listed in the black box warning that were linked to systemic estrogen therapy plus synthetic progestin. Recent data from the Nurses’ Health Study, for example, demonstrated that 3 years of vaginal estrogen use did not increase the risk of cardiovascular events or invasive breast cancer.
Women’s Health Initiative. In a prospective observational cohort study, Crandall and colleagues used data from participants in the Women’s Health Initiative Observational Study to determine the association between use of vaginal estrogen and risk of a global index event (GIE), defined as time to first occurrence of coronary heart disease, invasive breast cancer, stroke, pulmonary embolism, hip fracture, colorectal cancer, endometrial cancer, or death from any cause.2
Women were recruited from multiple clinical centers, were aged 50 to 79 years at baseline, and did not use systemic estrogen therapy during follow-up. The study included 45,663 women and median follow-up was 7.2 years. The investigators collected data on women’s self-reported use of vaginal estrogen as well as the development of the conditions defined above.
In women with a uterus, there was no significant difference between vaginal estrogen users and nonusers in the risk of stroke, invasive breast cancer, colorectal cancer, endometrial cancer, pulmonary embolism, or deep vein thrombosis. The risks of coronary heart disease, fracture, all-cause mortality, and GIE were lower in vaginal estrogen users than in nonusers (GIE adjusted hazard ratio [HR], 0.68; 95% confidence interval [CI], 0.55–0.86).
In women who had undergone hysterectomy, the risks of the individual GIE components and the overall GIE were not significantly different in users of vaginal estrogen compared with nonusers (GIE adjusted HR, 0.94; 95% CI, 0.70–1.26).
The investigators concluded that the risks of cardiovascular disease and cancer were not increased in postmenopausal women who used vaginal estrogen. Thus, this study offers reassurance on the treatment’s safety.2
Meta-analysis on menopausal hormone therapy and breast cancer risk. Further evidence now indicates that low-dose vaginal estrogen is not linked to chronic health conditions. In a large meta-analysis published in 2019, investigators looked at different types of hormone therapies—oral estrogen plus progestin, transdermal estrogen and progestin, estrogen alone, low-dose vaginal estrogen—and their relationship to breast cancer risk.3
Information on individual participants was obtained from 58 studies, 24 prospective and 34 retrospective. Breast cancer relative risks (RR) during years 5 to 14 of current hormone use were assessed according to the main hormonal contituents, doses, and modes of delivery of the last-used menopausal hormone therapy. For all systemic estrogen-only preparations, the RR was 1.33 (95% CI, 1.28–1.38), while for all estrogen-progestogen preparations, the RR was 2.08 (95% CI, 2.02–2.15). For transdermal estrogen, the RR was 1.35 (95% CI, 1.25–1.46). In contrast, for vaginal estrogen, the RR was 1.09 (95% CI, 0.97–1.23).3
Thus, the analysis found that in all the studies that had been done to date, there was no evidence of increased risk of breast cancer with vaginal estrogen therapy.
The evidence is growing that low-dose vaginal estrogen is different from systemic estrogen in terms of its safety profile and benefit-risk pattern. It is important for the FDA to consider these data and revise the vaginal estrogen label.
On the horizon: New estradiol reference ranges
It would be useful if we could accurately compare estradiol levels in women treated with vaginal estrogen against those of women treated with systemic estrogen therapy. In September 2019, NAMS held a workshop with the goal of establishing reference ranges for estradiol in postmenopausal women.4 It is very important to have good, reliable laboratory assays for estradiol and estrone, and to have a clear understanding of what is a reference range, that is, the range of estradiol levels in postmenopausal women who are not treated with estrogen. That way, you can observe what the estradiol blood levels are in women treated with low-dose vaginal estrogen or those treated with systemic estrogen versus the levels observed among postmenopausal women not receiving any estrogen product.
With the reference range information, we could look at data on the blood levels of estradiol with low-dose vaginal estrogen from the various studies available, as well as the increasing evidence from observational studies of the safety of low-dose vaginal estrogen to better understand its relationship with health. If these studies demonstrate that, with certain doses and formulations of low-dose vaginal estrogen, blood estradiol levels stay within the reference range of postmenopausal estradiol levels, it would inform the labeling modifications of these products. We need this information for future discussions with the FDA.
The laboratory assay technology used for such an investigation is primarily liquid chromatography with tandem mass spectrometry, the so-called LC-MS/MS assay. With use of this technology, the reference range for estradiol may be less than 10 picograms per milliliter. Previously, a very wide and inconsistent range—about 5 to 30 picograms per milliliter—was considered a “normal” range.
NAMS is championing the efforts to define a true evidence-based reference range that would represent the range of levels seen in postmenopausal women.5 This effort has been spearheaded by Dr. Richard Santen and colleagues. Using the more sensitive and specific LC-MS/MS assay will enable researchers and clinicians to better understand how levels on low-dose vaginal estrogen relate to the reference range for postmenopausal women. We are hoping to work together with researchers to establish these reference ranges, and to use that information to look at how low-dose vaginal estrogen compares to levels in untreated postmenopausal women, as well as to levels in women on systemic estrogen.
Hopefully, establishing the reference range can be done in an expeditious and timely way, with discussions with the FDA resuming shortly thereafter.
References
1.NAMS Citizen’s Petition and FDA Response, June 7, 2018. http://www.menopause.org/docs/
2. Crandall CJ, Hovey KM, Andrews CA, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the Women’s Health Initiative Observational Study. Menopause. 2018;25:11-20.
3. Collaborative Group on Hormonal Factors in Breast Cancer. Type and timing of menopausal hormone therapy and breast cancer risk: individual participant meta-analysis of the worldwide epidemiological evidence. Lancet. 2019;394:1159-1168.
4. Santen RJ, Pinkerton JV, Liu JH, et al. Workshop on normal reference ranges for estradiol in postmenopausal women, September 2019, Chicago, Illinois. Menopause. May 4, 2020. doi:10.1097/GME.0000000000001556.
5. Pinkerton JV, Liu JH, Santoro NF, et al. Workshop on normal reference ranges for estradiol in postmenopausal women: commentary from The North American Menopause Society on low-dose vaginal estrogen therapy. Menopause. 2020;27:611-613.
- Portman DJ, Gass ML. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and The North American Menopause Society. Maturitas. 2014;79:349-354.
- Kaunitz AM, Manson JE. Management of menopausal symptoms. Obstet Gynecol. 2015;126:859-876.
- Shifren JL. Genitourinary syndrome of menopause. Clin Obstet Gynecol. 2018;61:508-516.
- Manson JE, Kaunitz AM. Menopause management—getting clinical care back on track. N Engl J Med. 2016;374:803-806.
- Kingsberg SA, Krychman M, Graham S, et al. The women’s EMPOWER survey: identifying women’s perceptions on vulvar and vaginal atrophy and its treatment. J Sex Med. 2017;14:413-424.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 Hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin No. 141: Management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216.
- US Food and Drug Administration website. FDA warns against the use of energy-based devices to perform vaginal ‘rejuvenation’ or vaginal cosmetic procedures: FDA safety communication. https://www.fda.gov/medical-devices/safety-communications/fda-warns-against-use-energy-based-devices-perform-vaginal-rejuvenation-or-vaginal-cosmetic. Updated November 20, 2018. Accessed May 21, 2020.
- US Food and Drug Administration website. Letters to industry. https://www.fda.gov/medical-devices/industry-medical-devices/letters-industry. July 24, 2018. Accessed May 21, 2020
- Portman DJ, Gass ML. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and The North American Menopause Society. Maturitas. 2014;79:349-354.
- Kaunitz AM, Manson JE. Management of menopausal symptoms. Obstet Gynecol. 2015;126:859-876.
- Shifren JL. Genitourinary syndrome of menopause. Clin Obstet Gynecol. 2018;61:508-516.
- Manson JE, Kaunitz AM. Menopause management—getting clinical care back on track. N Engl J Med. 2016;374:803-806.
- Kingsberg SA, Krychman M, Graham S, et al. The women’s EMPOWER survey: identifying women’s perceptions on vulvar and vaginal atrophy and its treatment. J Sex Med. 2017;14:413-424.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 Hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin No. 141: Management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216.
- US Food and Drug Administration website. FDA warns against the use of energy-based devices to perform vaginal ‘rejuvenation’ or vaginal cosmetic procedures: FDA safety communication. https://www.fda.gov/medical-devices/safety-communications/fda-warns-against-use-energy-based-devices-perform-vaginal-rejuvenation-or-vaginal-cosmetic. Updated November 20, 2018. Accessed May 21, 2020.
- US Food and Drug Administration website. Letters to industry. https://www.fda.gov/medical-devices/industry-medical-devices/letters-industry. July 24, 2018. Accessed May 21, 2020
ASCCP guidelines for managing abnormal cervical cancer tests: What’s new?
The 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors Consensus Guidelines, which represent a consensus of nearly 20 professional organizations and patient advocates, are a culmination of almost 10 years of research.1 With the last version issued in 2012,2 these latest guidelines offer the most recent recommendations regarding safely triaging women with abnormal cervical cancer screening results.
According to the consensus, research has shown that risk-based management allows clinicians to better discriminate women who will likely develop precancer from those who can safely continue with routine screening. As you will hear from guidelines coauthor Dr. Warner Huh, one of the most important differences between these guidelines and the 2012 version is a new emphasis on the principle of “equal management for equal risk.” Essentially, this insures that all women who have the same amount of risk for progression to precancer or cancer are managed the same.
The guidelines were once again published in the Journal of Lower Genital Tract Disease, and the tables they reference are publicly available. Additionally, ASCCP is developing a new management guidelines app to facilitate the use of the guidelines on smartphones and computers. With the publicly available risk tables, and the ASCCP navigation app, the guidelines will more easily accommodate updates as new information and technology become available.
OBG Management : The latest ASCCP guidelines, published in April, represent a “paradigm shift” from results to risk-based guidelines. Can you explain what this means and why the shift was undertaken?
Warner K. Huh, MD: Yes, the shift occurred because we needed to focus less on algorithms and more on risk. We started promulgating a concept of “equal management for equal risk” back in 2012. What this means is that if we have a method to look up a risk score based on relevant test results and other pieces of information, then all patients with that score should be managed in the same manner. We wanted that to be the underlying principle.
Focusing on risk tables also makes it easier to incorporate any future technologies used for risk estimation without having to rebuild algorithms from scratch. ASCCP is developing a new management guidelines app to streamline navigation of the guidelines. This app makes them easier for clinicians to use; they simply plug in certain variables from the patient’s history and receive 1 of 5 outputs: treatment, colposcopy, or surveillance at 1, 3, or 5 years.
The only drawbacks, if you view them as such, are that the clinician must plug in all the variables, and then must sit back and trust in what we have done. Clinicians have to trust that the system works and will simplify the clinical decision making.
We spent a lot of time determining what the risk thresholds should be. Some may argue they are arbitrary, but the decisions were data-driven, and carefully, thoughtfully vetted; we deliberated about whether the cut points actually made sense clinically to a practicing clinician base. The clinical action thresholds refer to a specific percentage below which a woman falls into one bucket and above which she falls into another bucket.
The other element that is unique about the guidelines is that instead of looking at the patient’s current screening result in isolation, the user sees it along with the prior one because prior history dictates subsequent risk.
It’s important that clinicians understand why this system is so markedly different from what we have done previously, and why risk-based guidelines make infinitely more sense than algorithmic ones. It’s because: 1) they can be easier to use; 2) they incorporate new data more efficiently and effectively than algorithm-based guidelines; and 3) they can incorporate future technologies seamlessly rather than having to create yet another algorithm.
Continue to: OBG Management : What do clinicians need in order to execute the guidelines?...
OBG Management : What do clinicians need in order to execute the guidelines?
Dr. Huh: Nothing. All of the information needed—the guidelines article and risk tables—are publicly available. However, to make navigation of the guidelines easier, the plan is for the app that I mentioned. I have the app on my phone and am actively beta testing it now. We’re planning on creating a web-based application as well, that will allow users to access the Internet and their electronic health record system so that they can plug in information directly from patient charts. The web-based app will be similar to the web-based Breast Cancer Surveillance Consortium’s Risk Calculator (https://tools.bcsc-scc.org/BC5yearRisk/calculator.htm). You will pull it up, plug in the requested information, including the patient’s age; their Pap smear and genotyping results; and their previous screening history.
OBG Management : When will the app be available for users?
Dr. Huh: It will be available for release on June 8.
OBG Management : Were HPV vaccination levels incorporated into the new guidelines?
OBG Management : Have recommendations regarding colposcopy changed?
Dr. Huh: Not really. About 3 years ago, we created basic colposcopy guidelines—the ASCCP Colposcopy Standards—so everything about colposcopy references back to those guidelines. Those colposcopy standards covered terminology and risk-based colposcopy, which actually aligns beautifully with these guidelines.
OBG Management : To narrow in on some changes from the prior guidelines, can colposcopy be deferred in certain patients?
Dr. Huh: Yes. Not everyone who has an abnormal screening test needs to come back for colposcopy.
OBG Management : How has guidance for expedited treatment or treatment without colposcopic biopsy changed?
Dr. Huh: This was heavily debated within not only the treatment group that I co-chaired with Richard Guido, MD, but also within the entire steering committee. The recommendation is that if the patient has an immediate risk of CIN 3 that is >60%, the patient should go straight to treatment without a colposcopic biopsy. The main reason for this is that you do not want to biopsy a patient and then lose them to follow-up.
When a woman has >60% immediate risk of CIN 3, we are fairly certain that colposcopy is not going to change management ultimately, so we recommend that patients receive treatment right away. We have already been doing this for 15 to 20 years, so this is not a new concept. It is just more formally codified here by assigning a percentage to the risk. Those who have between 25% and 60% immediate risk of CIN 3 should receive immediate colposcopy. We realize that not all clinicians have the ability to do this, so if clinicians can’t treat immediately, we recommend they do whatever they can to prevent losing the patient to follow-up.
OBG Management : How should a positive primary HPV screening test be managed?
Dr. Huh: If a woman has a positive primary HPV screening test, genotyping should be performed. If genotyping reveals HPV 16 or 18, then the patient should proceed to colposcopy. If genotyping reveals other forms of HPV, reflex cytology or a Pap smear should follow. ●
- Perkins RB, Guido RS, Castle PE, et al, for the 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors. J Low Genit Tract Dis. 2020;24(2):102-131.
- Massad LS, Einstein MH, Huh WK, et al, for the 2012 ASCCP Consensus Guidelines Conference. 2012 Updated Consensus Guidelines for the Management of Abnormal Cervical Cancer Screening Tests and Cancer Precursors. J Low Genit Tract Dis. 2013;17(5):S1-S27.
The 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors Consensus Guidelines, which represent a consensus of nearly 20 professional organizations and patient advocates, are a culmination of almost 10 years of research.1 With the last version issued in 2012,2 these latest guidelines offer the most recent recommendations regarding safely triaging women with abnormal cervical cancer screening results.
According to the consensus, research has shown that risk-based management allows clinicians to better discriminate women who will likely develop precancer from those who can safely continue with routine screening. As you will hear from guidelines coauthor Dr. Warner Huh, one of the most important differences between these guidelines and the 2012 version is a new emphasis on the principle of “equal management for equal risk.” Essentially, this insures that all women who have the same amount of risk for progression to precancer or cancer are managed the same.
The guidelines were once again published in the Journal of Lower Genital Tract Disease, and the tables they reference are publicly available. Additionally, ASCCP is developing a new management guidelines app to facilitate the use of the guidelines on smartphones and computers. With the publicly available risk tables, and the ASCCP navigation app, the guidelines will more easily accommodate updates as new information and technology become available.
OBG Management : The latest ASCCP guidelines, published in April, represent a “paradigm shift” from results to risk-based guidelines. Can you explain what this means and why the shift was undertaken?
Warner K. Huh, MD: Yes, the shift occurred because we needed to focus less on algorithms and more on risk. We started promulgating a concept of “equal management for equal risk” back in 2012. What this means is that if we have a method to look up a risk score based on relevant test results and other pieces of information, then all patients with that score should be managed in the same manner. We wanted that to be the underlying principle.
Focusing on risk tables also makes it easier to incorporate any future technologies used for risk estimation without having to rebuild algorithms from scratch. ASCCP is developing a new management guidelines app to streamline navigation of the guidelines. This app makes them easier for clinicians to use; they simply plug in certain variables from the patient’s history and receive 1 of 5 outputs: treatment, colposcopy, or surveillance at 1, 3, or 5 years.
The only drawbacks, if you view them as such, are that the clinician must plug in all the variables, and then must sit back and trust in what we have done. Clinicians have to trust that the system works and will simplify the clinical decision making.
We spent a lot of time determining what the risk thresholds should be. Some may argue they are arbitrary, but the decisions were data-driven, and carefully, thoughtfully vetted; we deliberated about whether the cut points actually made sense clinically to a practicing clinician base. The clinical action thresholds refer to a specific percentage below which a woman falls into one bucket and above which she falls into another bucket.
The other element that is unique about the guidelines is that instead of looking at the patient’s current screening result in isolation, the user sees it along with the prior one because prior history dictates subsequent risk.
It’s important that clinicians understand why this system is so markedly different from what we have done previously, and why risk-based guidelines make infinitely more sense than algorithmic ones. It’s because: 1) they can be easier to use; 2) they incorporate new data more efficiently and effectively than algorithm-based guidelines; and 3) they can incorporate future technologies seamlessly rather than having to create yet another algorithm.
Continue to: OBG Management : What do clinicians need in order to execute the guidelines?...
OBG Management : What do clinicians need in order to execute the guidelines?
Dr. Huh: Nothing. All of the information needed—the guidelines article and risk tables—are publicly available. However, to make navigation of the guidelines easier, the plan is for the app that I mentioned. I have the app on my phone and am actively beta testing it now. We’re planning on creating a web-based application as well, that will allow users to access the Internet and their electronic health record system so that they can plug in information directly from patient charts. The web-based app will be similar to the web-based Breast Cancer Surveillance Consortium’s Risk Calculator (https://tools.bcsc-scc.org/BC5yearRisk/calculator.htm). You will pull it up, plug in the requested information, including the patient’s age; their Pap smear and genotyping results; and their previous screening history.
OBG Management : When will the app be available for users?
Dr. Huh: It will be available for release on June 8.
OBG Management : Were HPV vaccination levels incorporated into the new guidelines?
OBG Management : Have recommendations regarding colposcopy changed?
Dr. Huh: Not really. About 3 years ago, we created basic colposcopy guidelines—the ASCCP Colposcopy Standards—so everything about colposcopy references back to those guidelines. Those colposcopy standards covered terminology and risk-based colposcopy, which actually aligns beautifully with these guidelines.
OBG Management : To narrow in on some changes from the prior guidelines, can colposcopy be deferred in certain patients?
Dr. Huh: Yes. Not everyone who has an abnormal screening test needs to come back for colposcopy.
OBG Management : How has guidance for expedited treatment or treatment without colposcopic biopsy changed?
Dr. Huh: This was heavily debated within not only the treatment group that I co-chaired with Richard Guido, MD, but also within the entire steering committee. The recommendation is that if the patient has an immediate risk of CIN 3 that is >60%, the patient should go straight to treatment without a colposcopic biopsy. The main reason for this is that you do not want to biopsy a patient and then lose them to follow-up.
When a woman has >60% immediate risk of CIN 3, we are fairly certain that colposcopy is not going to change management ultimately, so we recommend that patients receive treatment right away. We have already been doing this for 15 to 20 years, so this is not a new concept. It is just more formally codified here by assigning a percentage to the risk. Those who have between 25% and 60% immediate risk of CIN 3 should receive immediate colposcopy. We realize that not all clinicians have the ability to do this, so if clinicians can’t treat immediately, we recommend they do whatever they can to prevent losing the patient to follow-up.
OBG Management : How should a positive primary HPV screening test be managed?
Dr. Huh: If a woman has a positive primary HPV screening test, genotyping should be performed. If genotyping reveals HPV 16 or 18, then the patient should proceed to colposcopy. If genotyping reveals other forms of HPV, reflex cytology or a Pap smear should follow. ●
The 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors Consensus Guidelines, which represent a consensus of nearly 20 professional organizations and patient advocates, are a culmination of almost 10 years of research.1 With the last version issued in 2012,2 these latest guidelines offer the most recent recommendations regarding safely triaging women with abnormal cervical cancer screening results.
According to the consensus, research has shown that risk-based management allows clinicians to better discriminate women who will likely develop precancer from those who can safely continue with routine screening. As you will hear from guidelines coauthor Dr. Warner Huh, one of the most important differences between these guidelines and the 2012 version is a new emphasis on the principle of “equal management for equal risk.” Essentially, this insures that all women who have the same amount of risk for progression to precancer or cancer are managed the same.
The guidelines were once again published in the Journal of Lower Genital Tract Disease, and the tables they reference are publicly available. Additionally, ASCCP is developing a new management guidelines app to facilitate the use of the guidelines on smartphones and computers. With the publicly available risk tables, and the ASCCP navigation app, the guidelines will more easily accommodate updates as new information and technology become available.
OBG Management : The latest ASCCP guidelines, published in April, represent a “paradigm shift” from results to risk-based guidelines. Can you explain what this means and why the shift was undertaken?
Warner K. Huh, MD: Yes, the shift occurred because we needed to focus less on algorithms and more on risk. We started promulgating a concept of “equal management for equal risk” back in 2012. What this means is that if we have a method to look up a risk score based on relevant test results and other pieces of information, then all patients with that score should be managed in the same manner. We wanted that to be the underlying principle.
Focusing on risk tables also makes it easier to incorporate any future technologies used for risk estimation without having to rebuild algorithms from scratch. ASCCP is developing a new management guidelines app to streamline navigation of the guidelines. This app makes them easier for clinicians to use; they simply plug in certain variables from the patient’s history and receive 1 of 5 outputs: treatment, colposcopy, or surveillance at 1, 3, or 5 years.
The only drawbacks, if you view them as such, are that the clinician must plug in all the variables, and then must sit back and trust in what we have done. Clinicians have to trust that the system works and will simplify the clinical decision making.
We spent a lot of time determining what the risk thresholds should be. Some may argue they are arbitrary, but the decisions were data-driven, and carefully, thoughtfully vetted; we deliberated about whether the cut points actually made sense clinically to a practicing clinician base. The clinical action thresholds refer to a specific percentage below which a woman falls into one bucket and above which she falls into another bucket.
The other element that is unique about the guidelines is that instead of looking at the patient’s current screening result in isolation, the user sees it along with the prior one because prior history dictates subsequent risk.
It’s important that clinicians understand why this system is so markedly different from what we have done previously, and why risk-based guidelines make infinitely more sense than algorithmic ones. It’s because: 1) they can be easier to use; 2) they incorporate new data more efficiently and effectively than algorithm-based guidelines; and 3) they can incorporate future technologies seamlessly rather than having to create yet another algorithm.
Continue to: OBG Management : What do clinicians need in order to execute the guidelines?...
OBG Management : What do clinicians need in order to execute the guidelines?
Dr. Huh: Nothing. All of the information needed—the guidelines article and risk tables—are publicly available. However, to make navigation of the guidelines easier, the plan is for the app that I mentioned. I have the app on my phone and am actively beta testing it now. We’re planning on creating a web-based application as well, that will allow users to access the Internet and their electronic health record system so that they can plug in information directly from patient charts. The web-based app will be similar to the web-based Breast Cancer Surveillance Consortium’s Risk Calculator (https://tools.bcsc-scc.org/BC5yearRisk/calculator.htm). You will pull it up, plug in the requested information, including the patient’s age; their Pap smear and genotyping results; and their previous screening history.
OBG Management : When will the app be available for users?
Dr. Huh: It will be available for release on June 8.
OBG Management : Were HPV vaccination levels incorporated into the new guidelines?
OBG Management : Have recommendations regarding colposcopy changed?
Dr. Huh: Not really. About 3 years ago, we created basic colposcopy guidelines—the ASCCP Colposcopy Standards—so everything about colposcopy references back to those guidelines. Those colposcopy standards covered terminology and risk-based colposcopy, which actually aligns beautifully with these guidelines.
OBG Management : To narrow in on some changes from the prior guidelines, can colposcopy be deferred in certain patients?
Dr. Huh: Yes. Not everyone who has an abnormal screening test needs to come back for colposcopy.
OBG Management : How has guidance for expedited treatment or treatment without colposcopic biopsy changed?
Dr. Huh: This was heavily debated within not only the treatment group that I co-chaired with Richard Guido, MD, but also within the entire steering committee. The recommendation is that if the patient has an immediate risk of CIN 3 that is >60%, the patient should go straight to treatment without a colposcopic biopsy. The main reason for this is that you do not want to biopsy a patient and then lose them to follow-up.
When a woman has >60% immediate risk of CIN 3, we are fairly certain that colposcopy is not going to change management ultimately, so we recommend that patients receive treatment right away. We have already been doing this for 15 to 20 years, so this is not a new concept. It is just more formally codified here by assigning a percentage to the risk. Those who have between 25% and 60% immediate risk of CIN 3 should receive immediate colposcopy. We realize that not all clinicians have the ability to do this, so if clinicians can’t treat immediately, we recommend they do whatever they can to prevent losing the patient to follow-up.
OBG Management : How should a positive primary HPV screening test be managed?
Dr. Huh: If a woman has a positive primary HPV screening test, genotyping should be performed. If genotyping reveals HPV 16 or 18, then the patient should proceed to colposcopy. If genotyping reveals other forms of HPV, reflex cytology or a Pap smear should follow. ●
- Perkins RB, Guido RS, Castle PE, et al, for the 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors. J Low Genit Tract Dis. 2020;24(2):102-131.
- Massad LS, Einstein MH, Huh WK, et al, for the 2012 ASCCP Consensus Guidelines Conference. 2012 Updated Consensus Guidelines for the Management of Abnormal Cervical Cancer Screening Tests and Cancer Precursors. J Low Genit Tract Dis. 2013;17(5):S1-S27.
- Perkins RB, Guido RS, Castle PE, et al, for the 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors. J Low Genit Tract Dis. 2020;24(2):102-131.
- Massad LS, Einstein MH, Huh WK, et al, for the 2012 ASCCP Consensus Guidelines Conference. 2012 Updated Consensus Guidelines for the Management of Abnormal Cervical Cancer Screening Tests and Cancer Precursors. J Low Genit Tract Dis. 2013;17(5):S1-S27.
Team-Based Hypertension Management in Outpatient Settings
From Western University of Health Sciences College of Pharmacy, Department of Pharmacy Practice and Administration, Pomona, CA.
Abstract
- Objective: To review the current literature regarding the clinical effectiveness and cost-effectiveness of implementing hypertension team-based care (TBC) interventions in the outpatient setting, and discuss challenges to implementation.
- Methods: A literature review was conducted of meta-analyses, systematic reviews, and randomized controlled trials comparing TBC models to usual care for hypertension management.
- Results: Compared to usual care, TBC models have demonstrated greater blood pressure reductions and improved blood pressure control rates. Evidence was strongest for models involving nurses and pharmacists whose roles included medication management, patient education and counseling, coordination of care and follow-up, population health management, and performance measurement with quality improvement. Although TBC results in an increase in health care costs, the overall long-term benefits support the cost-effectiveness of these models over usual care. The most common barriers to TBC implementation include underutilization of technology, stakeholder engagement, and reimbursement issues.
- Conclusion: Hypertension TBC models have been shown to be clinically effective and cost-effective, but continued research comparing different models is warranted to determine which combination of health professionals and interventions is most impactful and cost-effective in practice. An implementation science approach, in which TBC models unique to each organization’s situation are created, will be useful to identify and overcome challenges and provide a solid foundation for sustainment.
Keywords: blood pressure; pharmacist; nurse; nurse practitioner; cost-effectiveness; team-based care.
Approximately 1 in 3 US adults—or about 100 million people—have high blood pressure, and only about half (48%) have their blood pressure under control.1 Effective blood pressure management has been shown to decrease the incidence of stroke, heart attack, and heart failure.2-4 The American College of Cardiology/American Heart Association (ACC/AHA) 2017 blood pressure guidelines recommended lower thresholds for diagnosing hypertension and initiating antihypertensive medication, and intensified the blood pressure goal to less than 130/80 mm Hg.5 Changing practice standards to more intensive blood pressure goals requires significant adjustments by clinicians and health care systems. In fact, new guideline uptake is often delayed, ignored, or sparsely applied.6 Due to this dramatic change in hypertension practice standards, the ACC/AHA guidelines support interdisciplinary team-based care (TBC) for hypertension management.5,7 Additionally, the Centers for Disease Control and Prevention (CDC) and the Community Preventive Services Task Force (CPSTF) promote TBC to improve blood pressure control in their initiatives to prevent heart disease and stroke.8,9
The National Academy of Medicine defines TBC as “the provision of health services to individuals, families, and/or their communities by at least 2 healthcare providers who work collaboratively with patients and their caregivers—to the extent preferred by each patient—to accomplish shared goals within and across settings to achieve coordinated, high-quality care.”10 Specific goals for TBC in hypertension treatment are listed in Table 1, and a checklist of key elements of TBC to consider before implementation are presented in Table 2.
TBC has been shown to have many advantages, including increased access to care due to expanded hours of operation and shorter wait times.11 Team-based models also provide effective and efficient delivery of patient education, behavioral health care, and care coordination.12-14 Patients are more likely to receive high-quality care when multiple providers, each with varied expertise, are on the health care team.11,15 Furthermore, clinicians report improved professional job satisfaction related to their ability to practice in environments where they are encouraged to work at the top of their licenses.16 Consequently, TBC has been accepted as a vital part of the patient-centered medical home (PCMH) model.17-19 Standards set by the National Committee for Quality Assurance (NCQA) include TBC as a requirement health systems must meet in order to achieve the highest level of PCMH recognition. While a team-based approach offers substantial benefits and is recognized as a marker of quality, implementation has presented various challenges, and the sustainability of these models in care settings has been questioned.20

In this article, we review the current literature regarding the clinical effectiveness and cost-effectiveness of implementing hypertension TBC interventions in the outpatient setting. We also discuss the challenges and opportunities of implementing this strategy in health systems and community settings in the United States.
Evidence of Impact and Effectiveness
Various models of hypertension TBC have been shown to increase the proportion of individuals with controlled blood pressure and to lead to a reduction in both systolic (SBP) and diastolic blood pressure (DBP), resulting in a strong recommendation for TBC approaches by the 2017 ACC/AHA blood pressure guidelines.5,21-25 There is great diversity in the types of hypertension treatment models studied, with few utilizing physician specialists and most utilizing nonphysician providers, such as community health workers, physician assistants, nurses, nurse practitioners, dietitians, social workers, and pharmacists.22,26-29 These professionals share duties of hypertension management with primary care physicians to reduce the burden of responsibility for care on any single provider type. TBC is patient-centered, and typically includes interprofessional collaboration, treatment algorithms, adherence counseling, frequent follow-up, home blood pressure monitoring, and patient self-management education.
Numerous studies have supported implementation of TBC in recent years. A systematic review and meta-analysis of 100 trials of hypertension TBC involving 55,920 patients concluded that the most effective blood pressure–lowering strategies use multilevel, multicomponent approaches to address barriers to hypertension control. Nonphysician providers are often involved in measuring blood pressure, ordering and assessing laboratory tests, and titrating medications.30 Compared with usual care, TBC with physician medication titration resulted in reductions in mean SBP and DBP (6.2 mm Hg and 2.7 mm Hg, respectively), while TBC with nonphysician medication titration also resulted in reductions in mean SBP and DBP (7.1 mm Hg and 3.1 mm Hg, respectively). Nurses and pharmacists are specifically mentioned by the 2017 ACC/AHA blood pressure guidelines as essential members of the hypertension treatment team.5 Randomized controlled trials (RCTs) and meta-analyses of TBC involving nurse or pharmacist interventions demonstrated greater reductions in SBP and/or greater attainment of blood pressure goals compared to usual care.21,26,31,32 The literature supports the roles of nurses and pharmacists in hypertension management in all aspects of care, including medication management, patient education and counseling, coordination of care and follow-up, population health management, and performance measurement with quality improvement.33
Nurses
Nurses are commonly part of TBC hypertension management programs. One meta-analysis and systematic review of international RCTs compared nurse, nurse prescriber (United Kingdom), and nurse practitioner interventions for hypertension with usual care. Interventions that included a stepped treatment algorithm and nurse prescribing showed greater reductions in SBP (8.2 mm Hg and 8.9 mm Hg, respectively) compared to usual care.31 Similarly, models that utilized telephone monitoring demonstrated greater achievement of blood pressure targets, while those that involved home monitoring showed significant reductions in blood pressure. Another international meta-analysis and systematic review of 11 nurse-led interventions in hypertensive patients with diabetes demonstrated a 5.8 mm Hg mean decrease in SBP compared to physician-led care. However, nurse-led care was not superior in achievement of study targets.34
A recent meta-analysis and systematic review, performed by Shaw and colleagues, sought to determine whether nurse-led protocols are effective for outpatient management of adults with diabetes, hypertension, and hyperlipidemia. All of the included studies involved a registered nurse who titrated medications by following a protocol, and most were RCTs comparing the nurse protocols to usual care. Overall, mean SBP and DBP decreased by 3.86 mm Hg and 1.56 mm Hg, respectively, while blood glucose and lipid levels were also reduced compared to usual care.24
Limited RCT data have been published since the Shaw et al meta-analysis. A single-blind RCT was performed in an urban community health care center in China among patients with uncontrolled blood pressure (SBP ≥ 140 mm Hg and/or DBP ≥ 90 mm Hg).35 The study group received care via a nurse-led model, which included a delivery design system, decision support, clinical information system, and self-management support, and the control group received usual care. At 12 weeks, patients in the study group had significantly lower blood pressure than control patients, with mean SBP/DBP reduction of 14.37/7.43 mm Hg and 5.10/2.69 mm Hg, respectively (P < 0.01). Improved medication adherence and increased patient satisfaction were other benefits of the nurse-led model.
Nurse case managers (NCM) also play a critical role in hypertension management, coordinating health care services to meet patient health needs. Ogedegbe sought to evaluate the comparative effectiveness of home blood pressure telemonitoring (HBPTM)+NCM versus HBPTM alone on SBP reduction in black and Hispanic stroke survivors.36,37 NCMs evaluated patient profiles, counseled patients on target lifestyle behaviors, and reviewed home blood pressure data. At 6 months, SBP declined by 13.63 mm Hg from baseline in the HBPTM+NCM group and 6.31 mm Hg in the HBPTM alone group (P < 0.0001). At 12 months, SBP in the HBPTM+NCM group declined by 14.76 mm Hg, while blood pressure in the HBPTM alone group declined by 5.53 mm Hg (P < 0.0001).
Pharmacists
Clinical pharmacists are also widely utilized in TBC models for hypertension management. Typical models involve pharmacists entering into collaborative practice agreements with physicians, leading to optimization of medications, avoidance of adverse drug events, and transitional care activities focusing on medication reconciliation and patient education in outpatient settings.30,38 The largest and most recent meta-analysis of pharmacist interventions, conducted in 2014 by Santschi et al,23 combined 2 previous systematic reviews to include a total of 39 RCTs with 14,224 patients.32,39 Pharmacist interventions included patient education, recommendations to physicians, and medication management. Compared with usual care, pharmacist interventions showed greater reductions in SBP (7.6 mm Hg) and DBP (3.9 mm Hg).23
Numerous studies substantiating the impact of pharmacist interventions on clinical outcomes have heavily influenced clinical practice and guideline development. Carter et al conducted a prospective, multi-state, cluster-randomized trial in 32 primary care clinics to evaluate whether clinics randomized to receive the pharmacist-physician collaborative care model (PPCCM) achieved better blood pressure outcomes versus clinics randomized to usual care.25 Investigators enrolled 625 patients with uncontrolled hypertension, 50% of whom had a prior diagnosis of diabetes mellitus or chronic kidney disease. The primary outcome of blood pressure control at 9 months in the intervention clinics compared to the control clinics was 43% and 34%, respectively (P = 0.059). The difference in mean SBP/DBP between the intervention and control clinics for all patients at 9 months was −6.1/−2.9 mm Hg. In a post-hoc analysis of patients with chronic kidney disease and diabetes, the pharmacist-intervention group had a significantly greater mean SBP reduction and higher blood pressure control rates compared to usual care at 9 months.40
A pre-specified secondary analysis from the Carter et al study determined that, in patients from racial minority groups, the mean SBP was 7.3 mm Hg lower in those who received the intervention compared to those in the control group (P = 0.0042).41 In patients with less than 12 years of education, those in the intervention group had a mean SBP 8.1 mm Hg lower than the SBP of those in the control group (P = 0.0001). Similar reductions in blood pressure occurred in patients with low income, Medicaid beneficiaries, or those without insurance. This study demonstrated that pharmacist interventions reduced racial and socioeconomic disparities in blood pressure treatment.
Other studies of pharmacist interventions in underserved populations have yielded positive results. In a retrospective review of uninsured patients, blood pressure control rates in a pharmacist-driven primary care clinic ranked in the 90th percentile of NCQA benchmarks, and was superior to the 2013 reported mean for commercial insurers.42 Similarly, another retrospective cohort study of a PPCCM on time to goal blood pressure in uninsured patients with hypertension showed the median time to blood pressure goal was 36 days in the PPCCM cohort versus 259 days in usual care cohorts (P < 0.001).43 A post-hoc analysis revealed the mean time-in-therapeutic blood pressure range was 46.2% ± 24.3% in the PPCCM group and 24.8% ± 27.4% in the usual care group (P < 0.0001). The blood pressure control rates at 12 months were 89% in the PPCCM group compared with 50% in the usual care group (P < 0.0001).44
Tsuyuki et al conducted the RxACTION study, a multicenter RCT evaluating the effectiveness of enhanced pharmacist care versus usual care in 23 Canadian community pharmacies and outpatient clinics following a 6-month intervention.45 Enhanced pharmacy services included pharmacist assessment of and counseling about cardiovascular disease risk and blood pressure control, review of current antihypertensive medications, and prescribing/titrating drug therapy, as needed, through independent prescriptive authority. Compared to the usual care group (n = 67), the intervention group had a reduction in SBP of 6.6 mm Hg (P = 0.006) and in DBP of 3.2 mm Hg (P = 0.01). This study expanded the pharmacists’ scope of practice, showing evidence for enhancing pharmacist roles on the hypertension care team. Tsuyuki et al also conducted the RxEACH randomized trial, which evaluated community pharmacist cardiovascular risk reduction interventions and showed an improvement in SBP and DBP, with reported results comparable to RxACTION.46
Victor et al conducted the landmark Black Barbershop Study, a cluster RCT involving 319 non-Hispanic black male patients with hypertension from 52 black-owned barbershops.47,48 Barbershops were assigned to 1 of 2 groups. The control group consisted of barbers who encouraged lifestyle modifications and made referrals to primary care providers. The intervention group had pharmacists who met regularly with participants at the barbershops and measured blood pressure, encouraged lifestyle changes, and prescribed drug therapy under collaborative practice agreements with physicians. Both groups demonstrated improvements in blood pressure outcomes, but the intervention group showed greater improvement in SBP and achievement of blood pressure goals compared to the control group. The results in the intervention group proved sustainable over the course of a year, even after the frequency of pharmacists’ visits was reduced. At 6 months, the mean SBP fell by 27.0 mm Hg (to 125.8 mm Hg) in the intervention group, as compared to a 9.3 mm Hg (to 145.4 mm Hg) reduction in the control group (P < 0.001), and blood pressure less than 130/80 mm Hg was achieved among 63.6% of the participants in the intervention group versus 11.7% in the control group (P < 0.001).
This community-level trial brought pharmacists to the barbershop and made them an essential part of the health care team through the endorsement of the barber, who the participants trusted and with whom they had a relationship. Long-standing issues related to distrust of the medical profession by this population were addressed, and trusted community barbershops were utilized as safe spaces for health care delivery. Health care professionals should consider utilizing community locations that other minority populations perceive as social centers and safe places, to reduce health disparities and barriers to care. However, models that bring care to patients need further economic and feasibility evaluations.
Other Health Care Professionals and Future Studies
In addition to models led by nurses and pharmacists, studies have also assessed models of TBC incorporating other health care professionals, including registered dietitians, medical assistants, community health workers, and health coaches (NCT02674464).49,50 Ongoing studies are also looking at the impact of TBC on underserved communities (NCT02674464, NCT03504124). Involving a variety of health care professionals with different communities and populations in TBC studies is warranted to determine the optimal settings in which to utilize different skill sets.
The Impress Study involves nurses who are assessing lifestyle risk and developing an action plan according to a standardized procedure, which may be advantageous given the degree of heterogeneity found in other TBC models.51 There are also studies underway or recently published that compare different components of TBC in order to determine which combination of TBC elements is preferred. Some of these have shown the benefits of using clinical decision-support systems (through a guideline-based treatment protocol) or training programs with ongoing support.52,53 Continued research comparing different TBC models is needed to determine which combination of health professionals and interventions is most impactful in practice.
Cost-Effectiveness
According to the CDC, TBC in hypertension management has proven to be cost-effective.54 Systematic reviews and meta-analyses assessing the cost-effectiveness of TBC in hypertension management have been conducted.26,27,29,55-58 While the general consensus supports this approach as being cost-effective, these determinations are based on studies that are widely heterogeneous. In each of these studies, different types of costs are taken into account when determining cost-effectiveness. The range of costs can be quite wide, depending on how they are calculated, making it difficult to determine the true cost-effectiveness of different TBC models.
Intervention cost is represented by the amount of money spent to implement and maintain the intervention beyond the cost of usual care or the cost without the intervention. For TBC, intervention cost consists of personnel resources such as provider time, patient time, and non-personnel resources, including rent and utilities. Studies show that intervention costs for TBC can range from $35 to $1350 per person per year (mean, $618; median, $428).27,56 One analysis, based on 20 studies comparing TBC to usual care, calculated an intervention cost of $284 per person per year,55 while another study showed an intervention cost of $525 per enrollee per year.56 Intervention cost can vary by the type of provider that is used, the amount of time spent per patient, and the setting where services are provided. Overall, the intervention cost of implementing TBC for hypertension management is consistently higher than the cost of usual care.
Health care cost is another factor to consider. It is the difference in the cost of health care products and services that are utilized in the process of TBC, as compared to care that is provided in the absence of TBC. Health care costs include the costs associated with hospitalizations, outpatient visits, emergency room visits, and medications. One study estimated a median health care cost of hypertension TBC of $65 per person per year.55 Overall, studies evaluating the impact of TBC for hypertension management on health care costs were mixed, with some showing that TBC resulted in an increase in health care cost, and others showing a savings compared to usual care.58 The variability in health care costs was due to the different number of health care components and comorbidities of the patients included in the studies. Also, study duration affected the estimated health care costs of TBC. Most studies did not assess long-term health care cost savings that could be achieved from prolonged blood pressure control.58 When considering both intervention and health care cost, Jacob et al estimated that TBC increased overall net cost by a median value of $329 per person per year.55 While some studies did attribute an overall reduction in health care costs to TBC for hypertension management, on average, team-based models increased health care costs compared to usual care.27,29,55,58,59
However, health care costs do not take into account the long-term reductions in morbidity and mortality or increased quality-adjusted life years (QALY) that result from improved blood pressure control attributed to TBC. In most cost-effectiveness studies, an intervention is considered to be cost-effective if the cost per QALY gained is less than the accepted threshold of $50,000.55 One study estimated that the cost per QALY of TBC in hypertension management is $4763,55,60 while another study estimated a median cost per QALY of $9716 to $13,992.55 A systematic review of 34 international studies estimated the median cost per QALY to be $13,986, ranging from $6683 to $58,610.57 The wide range in cost can be attributed to the variability in interventions, health outcomes used to measure effectiveness, and the settings and countries where the studies were conducted. In another study, a TBC intervention involving pharmacists resulted in a cost per QALY of $26,800.61 The intervention was found to be cost-effective for higher-risk patients, defined as those having diabetes, a smoking history, dyslipidemia, or obesity. For patients who did not have these risk factors, the cost per QALY increased to $43,330.61 Thus, the patient population should be considered before implementing a TBC model. Furthermore, the increased use of technology, allowing for more efficient provision of services and communication between providers, could reduce intervention costs and lead to increased cost efficacy in these models.
The variation in the models used for TBC makes it difficult to draw conclusions on the cost-effectiveness of these interventions. Although it is apparent that TBC in general is cost-effective, more studies are needed comparing different team-based models to determine which specific ones are most cost-effective.
Challenges to Implementation of Team-Based Care
Recognizing and addressing the challenges inherent to a TBC approach is important to the sustainability of such a model within various settings and institutions. Numerous studies conducted on team-based models have identified common challenges that appear to be consistent across multiple settings. These challenges can be categorized as financial, provider-specific, and technology.
Financial Barriers
Although studies have demonstrated the cost-effectiveness of controlling hypertension and preventing serious complications, health systems are still confronted with the challenge of covering the cost for TBC implementation and maintenance.29 The 2 main financial barriers for TBC services are stakeholder engagement and reimbursement for services. According to Kennelty et al, stakeholder engagement is key to the sustainability of the service.27 However, decisions by stakeholders on cost are influenced by many factors, which include available funds, perceived value, and estimates for return on investment. Additionally, interventions must align with the organization’s mission and vision and be feasible to implement, and organizations must have the capacity for administrative support.29 These various financial decisions may greatly influence the sustainability of a TBC model.
The reimbursement challenges for individual providers are an additional barrier to the sustainability of the service. In the United States, most providers are reimbursed via fee-for-service payment plans, but these plans do not reimburse all clinical providers because they are not all recognized as licensed providers.62,63 For example, pharmacists are not recognized by the Centers for Medicare & Medicaid Services as licensed health care providers, which limits their ability to be reimbursed for clinical services provided outside of a traditional dispensing role. Furthermore, state laws determine the services nonphysician providers can offer and how they are recognized for reimbursement by tertiary payers. For instance, pharmacist roles, such as ordering labs and modifying or prescribing medication regimens, vary greatly between states.7,63,64
Financial barriers are a major challenge facing the sustainability of a TBC hypertension service, so including all stakeholders in the decision-making process may improve the organization’s ability to sustain the service.
Provider-Specific Barriers
Notable barriers that are attributed to providers include lack of knowledge, lack of time, lack of initiative to change blood pressure medications, and inability to reach intensive blood pressure goals set in guidelines.29 Studies such as the SPRINT trial have significantly impacted clinical guideline cut-offs for blood pressure, but reaching the intensive blood pressure goals from clinical trials is difficult to emulate in clinical practice.65 In a typical clinical setting, providers may lack the confidence to make adjustments in therapy based on a single blood pressure measurement, and clinical inertia, defined as failure of health care providers to modify therapy when indicated,66 may contribute to the inability to achieve blood pressure goals. Many factors contribute to clinical inertia, including lack of knowledge, time, or clinical protocols on how to modify therapy, causing providers to delay clinical decisions. Implementing site-specific protocols and utilizing hypertension specialist health care professionals in TBC can address the barriers contributing to clinical inertia.
Technology Barriers
A common barrier in a variety of services, but especially prevalent in a TBC service, is access to an electronic health record (EHR) for all providers treating the patient. Some providers who are not directly tied to the same clinical site as the patient’s primary care provider may not have adequate access to the full EHR. For example, pharmacists who are managing hypertension in a TBC model in a community pharmacy may have access only to health information from prescription records. Patient interviews may not provide the pharmacist with adequate information about laboratory results, vitals, and other medical information and history for the patient, making it difficult for the pharmacist to make a proper recommendation for treatment.27 Depending on the setting, communication between providers may be a barrier in achieving optimal outcomes, especially when providers do not have access to a shared medical record.
In addition, patients often lack access to technology used to manage hypertension. Many new technologies exist that aid patients in managing their blood pressure, such as smart phone applications to track blood pressure readings and alarms to remind patients to take their medications. Studies have shown that telemonitoring of blood pressure measurements and management of hypertension, especially in combination with TBC, is effective and reduces costs compared to usual care.67 However, the lack of equal access to the various technologies available may inhibit the success of a TBC hypertension program. Patients may lack access, knowledge, or financial means to utilize the various methods available for managing their hypertension electronically.29
Conclusion
Incorporating nonphysician providers into the health care team for the treatment of hypertension has proven to be more effective than usual care and has been recognized by recent guidelines as a best practice approach to achieving blood pressure goals. Multiple studies have demonstrated that TBC utilizing nurses and pharmacists can improve blood pressure management. While adding members to the team increases health care costs, the long-term benefits of achieving optimal blood pressure goals contribute to the overall cost-effectiveness of TBC strategies over usual care. However, comparisons between different TBC models are warranted to determine which combination of health care professionals and/or interventions is most effective. Cost-analysis estimates are difficult to compare due to widely varied methodology and variance in the models that have been employed. Studies must consider pathways to overcoming reimbursement issues, provider-specific challenges, and technology barriers. Follow-up and monitoring after initiation of drug therapy for hypertension control should include systematic strategies to help improve blood pressure, including use of home blood pressure monitoring, TBC, and telehealth strategies. Future implementation science approaches to hypertension TBC models within specific clinic settings will be useful to identify and overcome challenges and will help to determine the populations who will benefit most, allowing for greater success in sustaining TBC models.
Corresponding author: Shawn R. Smith, PharmD, 309 E. 2nd Street, Pomona, CA 91766; [email protected].
Financial disclosures: None.
1. Fryar CD, Ostchega Y, Hales CM, et al. Hypertension prevalence and control among adults: United States, 2015–2016. NCHS Data Brief. 2017(289):1-8.
2. Ambrosius WT, Sink KM, Foy CG, et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: The Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials. 2014;11:532-546.
3. Lawes CM, Bennett DA, Feigin VL, Rodgers A. Blood pressure and stroke: an overview of published reviews. Stroke. 2017;35:776-785.
4. Zanchetti A, Thomopoulos C, Parati G. Randomized controlled trials of blood pressure lowering in hypertension: A critical reappraisal. Circ Res. 2015;116:1058-1073.
5. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/ AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127-e248.
6. Grol R. Successes and failures in the implementation of evidence-based guidelines for clinical practice. Med Care. 2001;39:II46-II54.
7. Brush JE, Handberg EM, Biga C, et al. 2015 ACC health policy statement on cardiovascular team-based care and the role of advanced practice providers. J Am Coll Cardiol. 2015;65:2118-2136.
8. Centers for Disease Control and Prevention. Best practices for cardiovascular disease prevention programs: a guide to effective health care system interventions and community programs linked to clinical services, promoting team-based care to improve high blood pressure control. www.cdc.gov/dhdsp/pubs/guides/best-practices/team-based-care.htm. Accessed April 30, 2020.
9. Centers for Disease Control and Prevention. Task Force recommends team-based care for improving blood pressure [press release]. May 15, 2012. www.cdc.gov/media/releases/2012/p0515_bp_control.html
10. Mitchell P, Wynia M, Golden R, et al. Core principles & values of effective team-based health care. 2012. Institute of Medicine, Washington, DC.
11. Campbell SM, Hann M, Hacker J, et al. Identifying predictors of high-quality care in English general practice: observational study. BMJ. 2001;323:784-787.
12. Shojania KG, Ranji SR, McDonald KM, et al. Effects of quality improvement strategies for type 2 diabetes on glycemic control: a meta-regression analysis. JAMA. 2006;296:427-440.
13. Walsh JM, McDonald KM, Shojania KG, et al. Quality improvement strategies for hypertension management: a systematic review. Med Care. 2006;44:646-657.
14. Wagner E. The role of patient care teams in chronic disease management. BMJ. 2000;320:560-572.
15. Coleman K, Reid R. Continuous and team-based healing relationships: improving patient care through teams. In: Phillips KE, Weir V, eds. Safety Net Medical Home Initiative Implementation Guide Series. 2nd ed. Seattle, WA: Qualis Health and The MacColl Center for Health Care Innovation at the Group Health Research Institute; 2013.
16. Sinsky CA, Willard-Grace R, Schutzbank AM, et al. In search of joy in practice: a report of 23 high-functioning primary care practices. Ann Fam Med. 2013;11:272-278
17. Howard J, Etz RS, Crocker JB, et al. Maximizing the patient-centered medical home (PCMH) by choosing words wisely. J Am Board Fam Med. 2016;29:248-253.
18. Solberg LI, Crain AL, Tillema JO, et al. Challenges of medical home transformation reported by 118 patient-centered medical home (PCMH) leaders. J Am Board Fam Med. 2014;27:449-457.
19. Crabtree BF, Chase SM, Wise CG, et al. Evaluation of patient centered medical home practice transformation initiatives. Med Care. 2011;49:10-16.
20. Carter BL. Blood pressure control—implementing a team approach. US Cardiol. 2011;8:108-113.
21. Carter BL, Rogers M, Daly J, et al. The potency of team-based care interventions for hypertension: a meta-analysis. Arch Intern Med. 2009;169:1748-1755.
22. Proia KK, Thota AB, Njie GJ, et al. Team-based care and improved blood pressure control: a community guide systematic review. Am J Prev Med. 2014;47:86-99.
23. Santschi V, Chiolero A, Colosimo AL, et al. Improving blood pressure control through pharmacist interventions: a meta-analysis of randomized controlled trials. J Am Heart Assoc. 2014;3:e000718.
24. Shaw RJ, McDuffie JR, Hendrix CC, et al. Effects of nurse-managed protocols in the outpatient management of adults with chronic conditions: a systematic review and meta-analysis. Ann Intern Med. 2014;161:113-121.
25. Carter BL, Coffey CS, Ardery G, et al. Cluster-randomized trial of a physician/pharmacist collaborative model to improve blood pressure control. Circ Cardiovasc Qual Outcomes. 2015;8:235-243
26. Carter BL, Bosworth HB, Green BB. The hypertension team: the role of the pharmacist, nurse and teamwork in hypertension therapy. J Clin Hypertens. 2012;14:51-65.
27. Kennelty KA, Polgreen LA, Carter BL. Team-based care with pharmacists to improve blood pressure: a review of recent literature. Curr Hypertens Rep. 2018;20:1.
28. Brownstein JN, Chowdhury FM, Norris SL, et al. Effectiveness of community health workers in the care of people with hypertension. Am J Prev Med. 2007;32:435-447.
29. Derington CG, King JB, Bryant KB, et al. Cost-effectiveness and challenges of implementing intensive blood pressure goals and team-based care. Curr Hypertens Rep. 2019;21:91.
30. Mills KT, Obst KM, Shen W, et al. Comparative effectiveness of implementation strategies for blood pressure control in hypertensive patients: a systematic review and meta-analysis. Ann Intern Med. 2018;168:110-120.
31. Clark CE, Smith LFP, Taylor RS, et al. Nurse led interventions to improve control of blood pressure in people with hypertension: systematic review and meta-analysis. BMJ. 2010;341:c3995.
32. Santschi V, Chiolero A, Burnand B, et al. Impact of pharmacist care in the management of cardiovascular disease risk factors: a systematic review and meta-analysis of randomized trials. Arch Intern Med. 2011;171:1441-1453.
33. Dennison Himmelfarb CR, Commodore-Mensah Y, Hill MN. Expanding the role of nurses to improve hypertension care and control globally. Ann Glob Health. 2016;82:243-253.
34. Clark CE, Smith LFP, Taylor RS, Campbell JL. Nurse-led interventions used to improve control of high blood pressure in people with diabetes: a systematic review and meta-analysis. DiabetMed. 2011;28:250-261.
35. Zhu X, Wong FKY, Wu CLH. Development and evaluation of a nurse-led hypertension management model: A randomized controlled trial. Int J Nurs Stud. 2018;77:171-178.
36. Spruill TM, Williams O, Teresi JA, et al. Comparative effectiveness of home blood pressure telemonitoring (HBPTM) plus nurse case management versus HBPTM alone among Black and Hispanic stroke survivors: study protocol for a randomized controlled trial. Trials. 2015;16:97.
37. Ogedegbe G. Comparative effectiveness of home BP telemonitoring plus nurse case management (HBPTM+NCM) versus HBPTM alone on systolic BP (SBP) reduction among minority stroke survivors. International Stroke Conference 2020; February 19-21, 2020; Los Angeles, CA. Abstract LB19.
38. Dunn SP, Birtcher KK, Beavers CJ, et al. The role of the clinical pharmacist in the care of patients with cardiovascular disease. J Am Coll Cardiol. 2015;66:2129-2139.
39. Santschi V, Chiolero A, Paradis G et al. Pharmacist interventions to improve cardiovascular disease risk factors in diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Care. 2012;35:2706-2717.
40. Anderegg MD, Gums TH, Uribe L, et al. Pharmacist intervention for blood pressure control in patients with diabetes and/or chronic kidney disease. Pharmacotherapy. 2018;38:309-318.
41. Anderegg MD, Gums TH, Uribe L et al. Physician-pharmacist collaborative management: narrowing the socioeconomic blood pressure gap. Hypertension. 2016;68:1314-1320.
42. Sisson EM, Dixon DL, Kildow DC, et al. Effectiveness of a pharmacist-physician team-based collaboration to improve long-term blood pressure control at an inner-city safety-net clinic. Pharmacotherapy. 2016;36:342-347.
43. Dixon DL, Sisson EM, Parod ED, et al. Pharmacist-physician collaborative care model and time to goal blood pressure in the uninsured population. J Clin Hypertens (Greenwich). 2018;20:88-95.
44. Dixon DL, Parod ED, Sisson EM et al. Impact of a pharmacist-physician collaborative care model on time-in-therapeutic blood pressure range in patients with hypertension. J Am Coll Clin Pharm. 2020;3:404-409.
45. Tsuyuki RT, Houle SK, Charrois TL, et al. Randomized trial of the effect of pharmacist prescribing on improving blood pressure in the community: the Alberta Clinical Trial in Optimizing Hypertension (RxACTION). Circulation. 2015;132:93-100.
46. Tsuyuki RT, Al Hamarneh YN, Jones CA, et al. The effectiveness of pharmacist interventions on cardiovascular risk: The Multicenter Randomized Controlled RxEACH trial. J Am Coll Cardiol. 2016;67:2846-2854.
47. Victor RG, Lynch K, Li N, et al. A cluster-randomized trial of blood-pressure reduction in black barbershops. N Engl J Med. 2018;378:1291-1301.
48. Victor RG, Blyler CA, Li N et al. Sustainability of blood pressure reduction in black barbershops. Circulation. 2019;139:10-19.
49. Panattoni L, Hurlimann L, Wilson C, et al. Workflow standardization of a novel team care model to improve chronic care: a quasi-experimental study. BMC Health Serv Res. 2017;17:286.
50. Chang AR, Bonaparte H, Yule C. Randomized controlled trial comparing a self-guided vs. dietitian-led approach using web-based tools to lower blood pressure: study design and rationale. International Stroke Conference 2020; February 19-21, 2020; Los Angeles, CA. Abstract P169.
51. Stephen C, Halcomb E, Mcinnes S, et al. Improving blood pressure control in primary care: The ImPress study. Int J Nurs Stud. 2019;95:28-33.
52. He J, Shi X, Lin M. Comparative effectiveness of implementation strategies on cardiovascular risk factor control in patients with diabetes: The D4C cluster randomized trial. International Stroke Conference 2020; February 19-21, 2020; Los Angeles, CA. Abstract 17.
53. Jafar TH, Gandhi M, de Silva HA, et al. A community-based intervention for managing hypertension in rural South Asia. N Engl J Med. 2020;382:717-726.
54. Centers for Disease Control and Prevention. Promoting team-based care to improve high blood pressure control. www.cdc.gov/dhdsp/pubs/guides/best-practices/team-based-care.htm. Accessed April 30, 2020.
55. Jacob V, Chattopadhyay SK, Thota AB, et al. Economics of team-based care in controlling blood pressure: a community guide systematic review. Am J Prev Med. 2015;49:772-783.
56. Dehmer SP, Baker-Goering MM, Maciosek MV, et al. Modeled health and economic impact of team-based care for hypertension. Am J Prev Med. 2016;50(5 suppl 1):S34-S44.
57. Zhang D, Wang G, Joo H. A systematic review of economic evidence on community hypertension interventions. Am J Prev Med. 2017;53:S121-S130.
58. Community Preventive Services Task Force. Cardiovascular disease: team-based care to improve blood pressure control. 2011. www.thecommunityguide.org/findings/cardiovascular-disease-team-based-care-improve-blood-pressure-control. Accessed April 30, 2020.
59. Kulchaitanaroaj P, Brooks JM, Ardery G et al. Incremental costs associated with physician and pharmacist collaboration to improve blood pressure control. Pharmacotherapy. 2012;32:772-780.
60. Mason JM, Freemantle N, Gibson JM, New JP. Specialist nurse-led clinics to improve control of hypertension and hyperlipidemia in diabetes. Diabetes Care. 2005;28:40-46.
61. Kulchaitanaroaj P, Brooks JM, Chaiyakunapruk N et al. Cost-utility analysis of physician-pharmacist collaborative intervention for treating hypertension compared with usual care. J Hypertens. 2017;35:178-187.
62. Lall D, Engel N, Devadasan N, et al. Models of care for chronic conditions in low/middle-income countries: a ‘best fit’ framework synthesis. BMJ Glob Health. 2018;3:e001077.
63. Bodenheimer T, Chen E, Bennett HD. Confronting the growing burden of chronic disease: can the U.S. health care workforce do the job? Health Aff (Millwood). 2009;28:64-74.
64. Smith M, Bates DW, Bodenheimer T, Cleary PD. Why pharmacists belong in the medical home. Health Aff (Millwood). 2010;29:906-913.
65. Wright JT, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
66. Phillips LS, Branch WT, Cook CB, et al. Clinical inertia. Ann Intern Med. 2001;135:825-834.
67. McManus RJ, Mant J, Franssen M, et al. Efficacy of self-monitored blood pressure, with or without telemonitoring, for titration of antihypertensive medication (TASMINH4): an unmasked randomised controlled trial. Lancet. 2018;391:949-959.
68. Tucker KL, Sheppard JP, Stevens R, et al. Self-monitoring of blood pressure in hypertension: a systematic review and individual patient data meta-analysis. PLoS Med. 2017;14:e1002389.
69. Casey DE, Thomas RJ, Bhalla V, et al. 2019 AHA/ACC clinical performance and quality measures for adults with high blood pressure: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures. J Am Coll Cardiol. 2019;74:2661-2706.
From Western University of Health Sciences College of Pharmacy, Department of Pharmacy Practice and Administration, Pomona, CA.
Abstract
- Objective: To review the current literature regarding the clinical effectiveness and cost-effectiveness of implementing hypertension team-based care (TBC) interventions in the outpatient setting, and discuss challenges to implementation.
- Methods: A literature review was conducted of meta-analyses, systematic reviews, and randomized controlled trials comparing TBC models to usual care for hypertension management.
- Results: Compared to usual care, TBC models have demonstrated greater blood pressure reductions and improved blood pressure control rates. Evidence was strongest for models involving nurses and pharmacists whose roles included medication management, patient education and counseling, coordination of care and follow-up, population health management, and performance measurement with quality improvement. Although TBC results in an increase in health care costs, the overall long-term benefits support the cost-effectiveness of these models over usual care. The most common barriers to TBC implementation include underutilization of technology, stakeholder engagement, and reimbursement issues.
- Conclusion: Hypertension TBC models have been shown to be clinically effective and cost-effective, but continued research comparing different models is warranted to determine which combination of health professionals and interventions is most impactful and cost-effective in practice. An implementation science approach, in which TBC models unique to each organization’s situation are created, will be useful to identify and overcome challenges and provide a solid foundation for sustainment.
Keywords: blood pressure; pharmacist; nurse; nurse practitioner; cost-effectiveness; team-based care.
Approximately 1 in 3 US adults—or about 100 million people—have high blood pressure, and only about half (48%) have their blood pressure under control.1 Effective blood pressure management has been shown to decrease the incidence of stroke, heart attack, and heart failure.2-4 The American College of Cardiology/American Heart Association (ACC/AHA) 2017 blood pressure guidelines recommended lower thresholds for diagnosing hypertension and initiating antihypertensive medication, and intensified the blood pressure goal to less than 130/80 mm Hg.5 Changing practice standards to more intensive blood pressure goals requires significant adjustments by clinicians and health care systems. In fact, new guideline uptake is often delayed, ignored, or sparsely applied.6 Due to this dramatic change in hypertension practice standards, the ACC/AHA guidelines support interdisciplinary team-based care (TBC) for hypertension management.5,7 Additionally, the Centers for Disease Control and Prevention (CDC) and the Community Preventive Services Task Force (CPSTF) promote TBC to improve blood pressure control in their initiatives to prevent heart disease and stroke.8,9
The National Academy of Medicine defines TBC as “the provision of health services to individuals, families, and/or their communities by at least 2 healthcare providers who work collaboratively with patients and their caregivers—to the extent preferred by each patient—to accomplish shared goals within and across settings to achieve coordinated, high-quality care.”10 Specific goals for TBC in hypertension treatment are listed in Table 1, and a checklist of key elements of TBC to consider before implementation are presented in Table 2.

TBC has been shown to have many advantages, including increased access to care due to expanded hours of operation and shorter wait times.11 Team-based models also provide effective and efficient delivery of patient education, behavioral health care, and care coordination.12-14 Patients are more likely to receive high-quality care when multiple providers, each with varied expertise, are on the health care team.11,15 Furthermore, clinicians report improved professional job satisfaction related to their ability to practice in environments where they are encouraged to work at the top of their licenses.16 Consequently, TBC has been accepted as a vital part of the patient-centered medical home (PCMH) model.17-19 Standards set by the National Committee for Quality Assurance (NCQA) include TBC as a requirement health systems must meet in order to achieve the highest level of PCMH recognition. While a team-based approach offers substantial benefits and is recognized as a marker of quality, implementation has presented various challenges, and the sustainability of these models in care settings has been questioned.20

In this article, we review the current literature regarding the clinical effectiveness and cost-effectiveness of implementing hypertension TBC interventions in the outpatient setting. We also discuss the challenges and opportunities of implementing this strategy in health systems and community settings in the United States.
Evidence of Impact and Effectiveness
Various models of hypertension TBC have been shown to increase the proportion of individuals with controlled blood pressure and to lead to a reduction in both systolic (SBP) and diastolic blood pressure (DBP), resulting in a strong recommendation for TBC approaches by the 2017 ACC/AHA blood pressure guidelines.5,21-25 There is great diversity in the types of hypertension treatment models studied, with few utilizing physician specialists and most utilizing nonphysician providers, such as community health workers, physician assistants, nurses, nurse practitioners, dietitians, social workers, and pharmacists.22,26-29 These professionals share duties of hypertension management with primary care physicians to reduce the burden of responsibility for care on any single provider type. TBC is patient-centered, and typically includes interprofessional collaboration, treatment algorithms, adherence counseling, frequent follow-up, home blood pressure monitoring, and patient self-management education.
Numerous studies have supported implementation of TBC in recent years. A systematic review and meta-analysis of 100 trials of hypertension TBC involving 55,920 patients concluded that the most effective blood pressure–lowering strategies use multilevel, multicomponent approaches to address barriers to hypertension control. Nonphysician providers are often involved in measuring blood pressure, ordering and assessing laboratory tests, and titrating medications.30 Compared with usual care, TBC with physician medication titration resulted in reductions in mean SBP and DBP (6.2 mm Hg and 2.7 mm Hg, respectively), while TBC with nonphysician medication titration also resulted in reductions in mean SBP and DBP (7.1 mm Hg and 3.1 mm Hg, respectively). Nurses and pharmacists are specifically mentioned by the 2017 ACC/AHA blood pressure guidelines as essential members of the hypertension treatment team.5 Randomized controlled trials (RCTs) and meta-analyses of TBC involving nurse or pharmacist interventions demonstrated greater reductions in SBP and/or greater attainment of blood pressure goals compared to usual care.21,26,31,32 The literature supports the roles of nurses and pharmacists in hypertension management in all aspects of care, including medication management, patient education and counseling, coordination of care and follow-up, population health management, and performance measurement with quality improvement.33
Nurses
Nurses are commonly part of TBC hypertension management programs. One meta-analysis and systematic review of international RCTs compared nurse, nurse prescriber (United Kingdom), and nurse practitioner interventions for hypertension with usual care. Interventions that included a stepped treatment algorithm and nurse prescribing showed greater reductions in SBP (8.2 mm Hg and 8.9 mm Hg, respectively) compared to usual care.31 Similarly, models that utilized telephone monitoring demonstrated greater achievement of blood pressure targets, while those that involved home monitoring showed significant reductions in blood pressure. Another international meta-analysis and systematic review of 11 nurse-led interventions in hypertensive patients with diabetes demonstrated a 5.8 mm Hg mean decrease in SBP compared to physician-led care. However, nurse-led care was not superior in achievement of study targets.34
A recent meta-analysis and systematic review, performed by Shaw and colleagues, sought to determine whether nurse-led protocols are effective for outpatient management of adults with diabetes, hypertension, and hyperlipidemia. All of the included studies involved a registered nurse who titrated medications by following a protocol, and most were RCTs comparing the nurse protocols to usual care. Overall, mean SBP and DBP decreased by 3.86 mm Hg and 1.56 mm Hg, respectively, while blood glucose and lipid levels were also reduced compared to usual care.24
Limited RCT data have been published since the Shaw et al meta-analysis. A single-blind RCT was performed in an urban community health care center in China among patients with uncontrolled blood pressure (SBP ≥ 140 mm Hg and/or DBP ≥ 90 mm Hg).35 The study group received care via a nurse-led model, which included a delivery design system, decision support, clinical information system, and self-management support, and the control group received usual care. At 12 weeks, patients in the study group had significantly lower blood pressure than control patients, with mean SBP/DBP reduction of 14.37/7.43 mm Hg and 5.10/2.69 mm Hg, respectively (P < 0.01). Improved medication adherence and increased patient satisfaction were other benefits of the nurse-led model.
Nurse case managers (NCM) also play a critical role in hypertension management, coordinating health care services to meet patient health needs. Ogedegbe sought to evaluate the comparative effectiveness of home blood pressure telemonitoring (HBPTM)+NCM versus HBPTM alone on SBP reduction in black and Hispanic stroke survivors.36,37 NCMs evaluated patient profiles, counseled patients on target lifestyle behaviors, and reviewed home blood pressure data. At 6 months, SBP declined by 13.63 mm Hg from baseline in the HBPTM+NCM group and 6.31 mm Hg in the HBPTM alone group (P < 0.0001). At 12 months, SBP in the HBPTM+NCM group declined by 14.76 mm Hg, while blood pressure in the HBPTM alone group declined by 5.53 mm Hg (P < 0.0001).
Pharmacists
Clinical pharmacists are also widely utilized in TBC models for hypertension management. Typical models involve pharmacists entering into collaborative practice agreements with physicians, leading to optimization of medications, avoidance of adverse drug events, and transitional care activities focusing on medication reconciliation and patient education in outpatient settings.30,38 The largest and most recent meta-analysis of pharmacist interventions, conducted in 2014 by Santschi et al,23 combined 2 previous systematic reviews to include a total of 39 RCTs with 14,224 patients.32,39 Pharmacist interventions included patient education, recommendations to physicians, and medication management. Compared with usual care, pharmacist interventions showed greater reductions in SBP (7.6 mm Hg) and DBP (3.9 mm Hg).23
Numerous studies substantiating the impact of pharmacist interventions on clinical outcomes have heavily influenced clinical practice and guideline development. Carter et al conducted a prospective, multi-state, cluster-randomized trial in 32 primary care clinics to evaluate whether clinics randomized to receive the pharmacist-physician collaborative care model (PPCCM) achieved better blood pressure outcomes versus clinics randomized to usual care.25 Investigators enrolled 625 patients with uncontrolled hypertension, 50% of whom had a prior diagnosis of diabetes mellitus or chronic kidney disease. The primary outcome of blood pressure control at 9 months in the intervention clinics compared to the control clinics was 43% and 34%, respectively (P = 0.059). The difference in mean SBP/DBP between the intervention and control clinics for all patients at 9 months was −6.1/−2.9 mm Hg. In a post-hoc analysis of patients with chronic kidney disease and diabetes, the pharmacist-intervention group had a significantly greater mean SBP reduction and higher blood pressure control rates compared to usual care at 9 months.40
A pre-specified secondary analysis from the Carter et al study determined that, in patients from racial minority groups, the mean SBP was 7.3 mm Hg lower in those who received the intervention compared to those in the control group (P = 0.0042).41 In patients with less than 12 years of education, those in the intervention group had a mean SBP 8.1 mm Hg lower than the SBP of those in the control group (P = 0.0001). Similar reductions in blood pressure occurred in patients with low income, Medicaid beneficiaries, or those without insurance. This study demonstrated that pharmacist interventions reduced racial and socioeconomic disparities in blood pressure treatment.
Other studies of pharmacist interventions in underserved populations have yielded positive results. In a retrospective review of uninsured patients, blood pressure control rates in a pharmacist-driven primary care clinic ranked in the 90th percentile of NCQA benchmarks, and was superior to the 2013 reported mean for commercial insurers.42 Similarly, another retrospective cohort study of a PPCCM on time to goal blood pressure in uninsured patients with hypertension showed the median time to blood pressure goal was 36 days in the PPCCM cohort versus 259 days in usual care cohorts (P < 0.001).43 A post-hoc analysis revealed the mean time-in-therapeutic blood pressure range was 46.2% ± 24.3% in the PPCCM group and 24.8% ± 27.4% in the usual care group (P < 0.0001). The blood pressure control rates at 12 months were 89% in the PPCCM group compared with 50% in the usual care group (P < 0.0001).44
Tsuyuki et al conducted the RxACTION study, a multicenter RCT evaluating the effectiveness of enhanced pharmacist care versus usual care in 23 Canadian community pharmacies and outpatient clinics following a 6-month intervention.45 Enhanced pharmacy services included pharmacist assessment of and counseling about cardiovascular disease risk and blood pressure control, review of current antihypertensive medications, and prescribing/titrating drug therapy, as needed, through independent prescriptive authority. Compared to the usual care group (n = 67), the intervention group had a reduction in SBP of 6.6 mm Hg (P = 0.006) and in DBP of 3.2 mm Hg (P = 0.01). This study expanded the pharmacists’ scope of practice, showing evidence for enhancing pharmacist roles on the hypertension care team. Tsuyuki et al also conducted the RxEACH randomized trial, which evaluated community pharmacist cardiovascular risk reduction interventions and showed an improvement in SBP and DBP, with reported results comparable to RxACTION.46
Victor et al conducted the landmark Black Barbershop Study, a cluster RCT involving 319 non-Hispanic black male patients with hypertension from 52 black-owned barbershops.47,48 Barbershops were assigned to 1 of 2 groups. The control group consisted of barbers who encouraged lifestyle modifications and made referrals to primary care providers. The intervention group had pharmacists who met regularly with participants at the barbershops and measured blood pressure, encouraged lifestyle changes, and prescribed drug therapy under collaborative practice agreements with physicians. Both groups demonstrated improvements in blood pressure outcomes, but the intervention group showed greater improvement in SBP and achievement of blood pressure goals compared to the control group. The results in the intervention group proved sustainable over the course of a year, even after the frequency of pharmacists’ visits was reduced. At 6 months, the mean SBP fell by 27.0 mm Hg (to 125.8 mm Hg) in the intervention group, as compared to a 9.3 mm Hg (to 145.4 mm Hg) reduction in the control group (P < 0.001), and blood pressure less than 130/80 mm Hg was achieved among 63.6% of the participants in the intervention group versus 11.7% in the control group (P < 0.001).
This community-level trial brought pharmacists to the barbershop and made them an essential part of the health care team through the endorsement of the barber, who the participants trusted and with whom they had a relationship. Long-standing issues related to distrust of the medical profession by this population were addressed, and trusted community barbershops were utilized as safe spaces for health care delivery. Health care professionals should consider utilizing community locations that other minority populations perceive as social centers and safe places, to reduce health disparities and barriers to care. However, models that bring care to patients need further economic and feasibility evaluations.
Other Health Care Professionals and Future Studies
In addition to models led by nurses and pharmacists, studies have also assessed models of TBC incorporating other health care professionals, including registered dietitians, medical assistants, community health workers, and health coaches (NCT02674464).49,50 Ongoing studies are also looking at the impact of TBC on underserved communities (NCT02674464, NCT03504124). Involving a variety of health care professionals with different communities and populations in TBC studies is warranted to determine the optimal settings in which to utilize different skill sets.
The Impress Study involves nurses who are assessing lifestyle risk and developing an action plan according to a standardized procedure, which may be advantageous given the degree of heterogeneity found in other TBC models.51 There are also studies underway or recently published that compare different components of TBC in order to determine which combination of TBC elements is preferred. Some of these have shown the benefits of using clinical decision-support systems (through a guideline-based treatment protocol) or training programs with ongoing support.52,53 Continued research comparing different TBC models is needed to determine which combination of health professionals and interventions is most impactful in practice.
Cost-Effectiveness
According to the CDC, TBC in hypertension management has proven to be cost-effective.54 Systematic reviews and meta-analyses assessing the cost-effectiveness of TBC in hypertension management have been conducted.26,27,29,55-58 While the general consensus supports this approach as being cost-effective, these determinations are based on studies that are widely heterogeneous. In each of these studies, different types of costs are taken into account when determining cost-effectiveness. The range of costs can be quite wide, depending on how they are calculated, making it difficult to determine the true cost-effectiveness of different TBC models.
Intervention cost is represented by the amount of money spent to implement and maintain the intervention beyond the cost of usual care or the cost without the intervention. For TBC, intervention cost consists of personnel resources such as provider time, patient time, and non-personnel resources, including rent and utilities. Studies show that intervention costs for TBC can range from $35 to $1350 per person per year (mean, $618; median, $428).27,56 One analysis, based on 20 studies comparing TBC to usual care, calculated an intervention cost of $284 per person per year,55 while another study showed an intervention cost of $525 per enrollee per year.56 Intervention cost can vary by the type of provider that is used, the amount of time spent per patient, and the setting where services are provided. Overall, the intervention cost of implementing TBC for hypertension management is consistently higher than the cost of usual care.
Health care cost is another factor to consider. It is the difference in the cost of health care products and services that are utilized in the process of TBC, as compared to care that is provided in the absence of TBC. Health care costs include the costs associated with hospitalizations, outpatient visits, emergency room visits, and medications. One study estimated a median health care cost of hypertension TBC of $65 per person per year.55 Overall, studies evaluating the impact of TBC for hypertension management on health care costs were mixed, with some showing that TBC resulted in an increase in health care cost, and others showing a savings compared to usual care.58 The variability in health care costs was due to the different number of health care components and comorbidities of the patients included in the studies. Also, study duration affected the estimated health care costs of TBC. Most studies did not assess long-term health care cost savings that could be achieved from prolonged blood pressure control.58 When considering both intervention and health care cost, Jacob et al estimated that TBC increased overall net cost by a median value of $329 per person per year.55 While some studies did attribute an overall reduction in health care costs to TBC for hypertension management, on average, team-based models increased health care costs compared to usual care.27,29,55,58,59
However, health care costs do not take into account the long-term reductions in morbidity and mortality or increased quality-adjusted life years (QALY) that result from improved blood pressure control attributed to TBC. In most cost-effectiveness studies, an intervention is considered to be cost-effective if the cost per QALY gained is less than the accepted threshold of $50,000.55 One study estimated that the cost per QALY of TBC in hypertension management is $4763,55,60 while another study estimated a median cost per QALY of $9716 to $13,992.55 A systematic review of 34 international studies estimated the median cost per QALY to be $13,986, ranging from $6683 to $58,610.57 The wide range in cost can be attributed to the variability in interventions, health outcomes used to measure effectiveness, and the settings and countries where the studies were conducted. In another study, a TBC intervention involving pharmacists resulted in a cost per QALY of $26,800.61 The intervention was found to be cost-effective for higher-risk patients, defined as those having diabetes, a smoking history, dyslipidemia, or obesity. For patients who did not have these risk factors, the cost per QALY increased to $43,330.61 Thus, the patient population should be considered before implementing a TBC model. Furthermore, the increased use of technology, allowing for more efficient provision of services and communication between providers, could reduce intervention costs and lead to increased cost efficacy in these models.
The variation in the models used for TBC makes it difficult to draw conclusions on the cost-effectiveness of these interventions. Although it is apparent that TBC in general is cost-effective, more studies are needed comparing different team-based models to determine which specific ones are most cost-effective.
Challenges to Implementation of Team-Based Care
Recognizing and addressing the challenges inherent to a TBC approach is important to the sustainability of such a model within various settings and institutions. Numerous studies conducted on team-based models have identified common challenges that appear to be consistent across multiple settings. These challenges can be categorized as financial, provider-specific, and technology.
Financial Barriers
Although studies have demonstrated the cost-effectiveness of controlling hypertension and preventing serious complications, health systems are still confronted with the challenge of covering the cost for TBC implementation and maintenance.29 The 2 main financial barriers for TBC services are stakeholder engagement and reimbursement for services. According to Kennelty et al, stakeholder engagement is key to the sustainability of the service.27 However, decisions by stakeholders on cost are influenced by many factors, which include available funds, perceived value, and estimates for return on investment. Additionally, interventions must align with the organization’s mission and vision and be feasible to implement, and organizations must have the capacity for administrative support.29 These various financial decisions may greatly influence the sustainability of a TBC model.
The reimbursement challenges for individual providers are an additional barrier to the sustainability of the service. In the United States, most providers are reimbursed via fee-for-service payment plans, but these plans do not reimburse all clinical providers because they are not all recognized as licensed providers.62,63 For example, pharmacists are not recognized by the Centers for Medicare & Medicaid Services as licensed health care providers, which limits their ability to be reimbursed for clinical services provided outside of a traditional dispensing role. Furthermore, state laws determine the services nonphysician providers can offer and how they are recognized for reimbursement by tertiary payers. For instance, pharmacist roles, such as ordering labs and modifying or prescribing medication regimens, vary greatly between states.7,63,64
Financial barriers are a major challenge facing the sustainability of a TBC hypertension service, so including all stakeholders in the decision-making process may improve the organization’s ability to sustain the service.
Provider-Specific Barriers
Notable barriers that are attributed to providers include lack of knowledge, lack of time, lack of initiative to change blood pressure medications, and inability to reach intensive blood pressure goals set in guidelines.29 Studies such as the SPRINT trial have significantly impacted clinical guideline cut-offs for blood pressure, but reaching the intensive blood pressure goals from clinical trials is difficult to emulate in clinical practice.65 In a typical clinical setting, providers may lack the confidence to make adjustments in therapy based on a single blood pressure measurement, and clinical inertia, defined as failure of health care providers to modify therapy when indicated,66 may contribute to the inability to achieve blood pressure goals. Many factors contribute to clinical inertia, including lack of knowledge, time, or clinical protocols on how to modify therapy, causing providers to delay clinical decisions. Implementing site-specific protocols and utilizing hypertension specialist health care professionals in TBC can address the barriers contributing to clinical inertia.
Technology Barriers
A common barrier in a variety of services, but especially prevalent in a TBC service, is access to an electronic health record (EHR) for all providers treating the patient. Some providers who are not directly tied to the same clinical site as the patient’s primary care provider may not have adequate access to the full EHR. For example, pharmacists who are managing hypertension in a TBC model in a community pharmacy may have access only to health information from prescription records. Patient interviews may not provide the pharmacist with adequate information about laboratory results, vitals, and other medical information and history for the patient, making it difficult for the pharmacist to make a proper recommendation for treatment.27 Depending on the setting, communication between providers may be a barrier in achieving optimal outcomes, especially when providers do not have access to a shared medical record.
In addition, patients often lack access to technology used to manage hypertension. Many new technologies exist that aid patients in managing their blood pressure, such as smart phone applications to track blood pressure readings and alarms to remind patients to take their medications. Studies have shown that telemonitoring of blood pressure measurements and management of hypertension, especially in combination with TBC, is effective and reduces costs compared to usual care.67 However, the lack of equal access to the various technologies available may inhibit the success of a TBC hypertension program. Patients may lack access, knowledge, or financial means to utilize the various methods available for managing their hypertension electronically.29
Conclusion
Incorporating nonphysician providers into the health care team for the treatment of hypertension has proven to be more effective than usual care and has been recognized by recent guidelines as a best practice approach to achieving blood pressure goals. Multiple studies have demonstrated that TBC utilizing nurses and pharmacists can improve blood pressure management. While adding members to the team increases health care costs, the long-term benefits of achieving optimal blood pressure goals contribute to the overall cost-effectiveness of TBC strategies over usual care. However, comparisons between different TBC models are warranted to determine which combination of health care professionals and/or interventions is most effective. Cost-analysis estimates are difficult to compare due to widely varied methodology and variance in the models that have been employed. Studies must consider pathways to overcoming reimbursement issues, provider-specific challenges, and technology barriers. Follow-up and monitoring after initiation of drug therapy for hypertension control should include systematic strategies to help improve blood pressure, including use of home blood pressure monitoring, TBC, and telehealth strategies. Future implementation science approaches to hypertension TBC models within specific clinic settings will be useful to identify and overcome challenges and will help to determine the populations who will benefit most, allowing for greater success in sustaining TBC models.
Corresponding author: Shawn R. Smith, PharmD, 309 E. 2nd Street, Pomona, CA 91766; [email protected].
Financial disclosures: None.
From Western University of Health Sciences College of Pharmacy, Department of Pharmacy Practice and Administration, Pomona, CA.
Abstract
- Objective: To review the current literature regarding the clinical effectiveness and cost-effectiveness of implementing hypertension team-based care (TBC) interventions in the outpatient setting, and discuss challenges to implementation.
- Methods: A literature review was conducted of meta-analyses, systematic reviews, and randomized controlled trials comparing TBC models to usual care for hypertension management.
- Results: Compared to usual care, TBC models have demonstrated greater blood pressure reductions and improved blood pressure control rates. Evidence was strongest for models involving nurses and pharmacists whose roles included medication management, patient education and counseling, coordination of care and follow-up, population health management, and performance measurement with quality improvement. Although TBC results in an increase in health care costs, the overall long-term benefits support the cost-effectiveness of these models over usual care. The most common barriers to TBC implementation include underutilization of technology, stakeholder engagement, and reimbursement issues.
- Conclusion: Hypertension TBC models have been shown to be clinically effective and cost-effective, but continued research comparing different models is warranted to determine which combination of health professionals and interventions is most impactful and cost-effective in practice. An implementation science approach, in which TBC models unique to each organization’s situation are created, will be useful to identify and overcome challenges and provide a solid foundation for sustainment.
Keywords: blood pressure; pharmacist; nurse; nurse practitioner; cost-effectiveness; team-based care.
Approximately 1 in 3 US adults—or about 100 million people—have high blood pressure, and only about half (48%) have their blood pressure under control.1 Effective blood pressure management has been shown to decrease the incidence of stroke, heart attack, and heart failure.2-4 The American College of Cardiology/American Heart Association (ACC/AHA) 2017 blood pressure guidelines recommended lower thresholds for diagnosing hypertension and initiating antihypertensive medication, and intensified the blood pressure goal to less than 130/80 mm Hg.5 Changing practice standards to more intensive blood pressure goals requires significant adjustments by clinicians and health care systems. In fact, new guideline uptake is often delayed, ignored, or sparsely applied.6 Due to this dramatic change in hypertension practice standards, the ACC/AHA guidelines support interdisciplinary team-based care (TBC) for hypertension management.5,7 Additionally, the Centers for Disease Control and Prevention (CDC) and the Community Preventive Services Task Force (CPSTF) promote TBC to improve blood pressure control in their initiatives to prevent heart disease and stroke.8,9
The National Academy of Medicine defines TBC as “the provision of health services to individuals, families, and/or their communities by at least 2 healthcare providers who work collaboratively with patients and their caregivers—to the extent preferred by each patient—to accomplish shared goals within and across settings to achieve coordinated, high-quality care.”10 Specific goals for TBC in hypertension treatment are listed in Table 1, and a checklist of key elements of TBC to consider before implementation are presented in Table 2.

TBC has been shown to have many advantages, including increased access to care due to expanded hours of operation and shorter wait times.11 Team-based models also provide effective and efficient delivery of patient education, behavioral health care, and care coordination.12-14 Patients are more likely to receive high-quality care when multiple providers, each with varied expertise, are on the health care team.11,15 Furthermore, clinicians report improved professional job satisfaction related to their ability to practice in environments where they are encouraged to work at the top of their licenses.16 Consequently, TBC has been accepted as a vital part of the patient-centered medical home (PCMH) model.17-19 Standards set by the National Committee for Quality Assurance (NCQA) include TBC as a requirement health systems must meet in order to achieve the highest level of PCMH recognition. While a team-based approach offers substantial benefits and is recognized as a marker of quality, implementation has presented various challenges, and the sustainability of these models in care settings has been questioned.20

In this article, we review the current literature regarding the clinical effectiveness and cost-effectiveness of implementing hypertension TBC interventions in the outpatient setting. We also discuss the challenges and opportunities of implementing this strategy in health systems and community settings in the United States.
Evidence of Impact and Effectiveness
Various models of hypertension TBC have been shown to increase the proportion of individuals with controlled blood pressure and to lead to a reduction in both systolic (SBP) and diastolic blood pressure (DBP), resulting in a strong recommendation for TBC approaches by the 2017 ACC/AHA blood pressure guidelines.5,21-25 There is great diversity in the types of hypertension treatment models studied, with few utilizing physician specialists and most utilizing nonphysician providers, such as community health workers, physician assistants, nurses, nurse practitioners, dietitians, social workers, and pharmacists.22,26-29 These professionals share duties of hypertension management with primary care physicians to reduce the burden of responsibility for care on any single provider type. TBC is patient-centered, and typically includes interprofessional collaboration, treatment algorithms, adherence counseling, frequent follow-up, home blood pressure monitoring, and patient self-management education.
Numerous studies have supported implementation of TBC in recent years. A systematic review and meta-analysis of 100 trials of hypertension TBC involving 55,920 patients concluded that the most effective blood pressure–lowering strategies use multilevel, multicomponent approaches to address barriers to hypertension control. Nonphysician providers are often involved in measuring blood pressure, ordering and assessing laboratory tests, and titrating medications.30 Compared with usual care, TBC with physician medication titration resulted in reductions in mean SBP and DBP (6.2 mm Hg and 2.7 mm Hg, respectively), while TBC with nonphysician medication titration also resulted in reductions in mean SBP and DBP (7.1 mm Hg and 3.1 mm Hg, respectively). Nurses and pharmacists are specifically mentioned by the 2017 ACC/AHA blood pressure guidelines as essential members of the hypertension treatment team.5 Randomized controlled trials (RCTs) and meta-analyses of TBC involving nurse or pharmacist interventions demonstrated greater reductions in SBP and/or greater attainment of blood pressure goals compared to usual care.21,26,31,32 The literature supports the roles of nurses and pharmacists in hypertension management in all aspects of care, including medication management, patient education and counseling, coordination of care and follow-up, population health management, and performance measurement with quality improvement.33
Nurses
Nurses are commonly part of TBC hypertension management programs. One meta-analysis and systematic review of international RCTs compared nurse, nurse prescriber (United Kingdom), and nurse practitioner interventions for hypertension with usual care. Interventions that included a stepped treatment algorithm and nurse prescribing showed greater reductions in SBP (8.2 mm Hg and 8.9 mm Hg, respectively) compared to usual care.31 Similarly, models that utilized telephone monitoring demonstrated greater achievement of blood pressure targets, while those that involved home monitoring showed significant reductions in blood pressure. Another international meta-analysis and systematic review of 11 nurse-led interventions in hypertensive patients with diabetes demonstrated a 5.8 mm Hg mean decrease in SBP compared to physician-led care. However, nurse-led care was not superior in achievement of study targets.34
A recent meta-analysis and systematic review, performed by Shaw and colleagues, sought to determine whether nurse-led protocols are effective for outpatient management of adults with diabetes, hypertension, and hyperlipidemia. All of the included studies involved a registered nurse who titrated medications by following a protocol, and most were RCTs comparing the nurse protocols to usual care. Overall, mean SBP and DBP decreased by 3.86 mm Hg and 1.56 mm Hg, respectively, while blood glucose and lipid levels were also reduced compared to usual care.24
Limited RCT data have been published since the Shaw et al meta-analysis. A single-blind RCT was performed in an urban community health care center in China among patients with uncontrolled blood pressure (SBP ≥ 140 mm Hg and/or DBP ≥ 90 mm Hg).35 The study group received care via a nurse-led model, which included a delivery design system, decision support, clinical information system, and self-management support, and the control group received usual care. At 12 weeks, patients in the study group had significantly lower blood pressure than control patients, with mean SBP/DBP reduction of 14.37/7.43 mm Hg and 5.10/2.69 mm Hg, respectively (P < 0.01). Improved medication adherence and increased patient satisfaction were other benefits of the nurse-led model.
Nurse case managers (NCM) also play a critical role in hypertension management, coordinating health care services to meet patient health needs. Ogedegbe sought to evaluate the comparative effectiveness of home blood pressure telemonitoring (HBPTM)+NCM versus HBPTM alone on SBP reduction in black and Hispanic stroke survivors.36,37 NCMs evaluated patient profiles, counseled patients on target lifestyle behaviors, and reviewed home blood pressure data. At 6 months, SBP declined by 13.63 mm Hg from baseline in the HBPTM+NCM group and 6.31 mm Hg in the HBPTM alone group (P < 0.0001). At 12 months, SBP in the HBPTM+NCM group declined by 14.76 mm Hg, while blood pressure in the HBPTM alone group declined by 5.53 mm Hg (P < 0.0001).
Pharmacists
Clinical pharmacists are also widely utilized in TBC models for hypertension management. Typical models involve pharmacists entering into collaborative practice agreements with physicians, leading to optimization of medications, avoidance of adverse drug events, and transitional care activities focusing on medication reconciliation and patient education in outpatient settings.30,38 The largest and most recent meta-analysis of pharmacist interventions, conducted in 2014 by Santschi et al,23 combined 2 previous systematic reviews to include a total of 39 RCTs with 14,224 patients.32,39 Pharmacist interventions included patient education, recommendations to physicians, and medication management. Compared with usual care, pharmacist interventions showed greater reductions in SBP (7.6 mm Hg) and DBP (3.9 mm Hg).23
Numerous studies substantiating the impact of pharmacist interventions on clinical outcomes have heavily influenced clinical practice and guideline development. Carter et al conducted a prospective, multi-state, cluster-randomized trial in 32 primary care clinics to evaluate whether clinics randomized to receive the pharmacist-physician collaborative care model (PPCCM) achieved better blood pressure outcomes versus clinics randomized to usual care.25 Investigators enrolled 625 patients with uncontrolled hypertension, 50% of whom had a prior diagnosis of diabetes mellitus or chronic kidney disease. The primary outcome of blood pressure control at 9 months in the intervention clinics compared to the control clinics was 43% and 34%, respectively (P = 0.059). The difference in mean SBP/DBP between the intervention and control clinics for all patients at 9 months was −6.1/−2.9 mm Hg. In a post-hoc analysis of patients with chronic kidney disease and diabetes, the pharmacist-intervention group had a significantly greater mean SBP reduction and higher blood pressure control rates compared to usual care at 9 months.40
A pre-specified secondary analysis from the Carter et al study determined that, in patients from racial minority groups, the mean SBP was 7.3 mm Hg lower in those who received the intervention compared to those in the control group (P = 0.0042).41 In patients with less than 12 years of education, those in the intervention group had a mean SBP 8.1 mm Hg lower than the SBP of those in the control group (P = 0.0001). Similar reductions in blood pressure occurred in patients with low income, Medicaid beneficiaries, or those without insurance. This study demonstrated that pharmacist interventions reduced racial and socioeconomic disparities in blood pressure treatment.
Other studies of pharmacist interventions in underserved populations have yielded positive results. In a retrospective review of uninsured patients, blood pressure control rates in a pharmacist-driven primary care clinic ranked in the 90th percentile of NCQA benchmarks, and was superior to the 2013 reported mean for commercial insurers.42 Similarly, another retrospective cohort study of a PPCCM on time to goal blood pressure in uninsured patients with hypertension showed the median time to blood pressure goal was 36 days in the PPCCM cohort versus 259 days in usual care cohorts (P < 0.001).43 A post-hoc analysis revealed the mean time-in-therapeutic blood pressure range was 46.2% ± 24.3% in the PPCCM group and 24.8% ± 27.4% in the usual care group (P < 0.0001). The blood pressure control rates at 12 months were 89% in the PPCCM group compared with 50% in the usual care group (P < 0.0001).44
Tsuyuki et al conducted the RxACTION study, a multicenter RCT evaluating the effectiveness of enhanced pharmacist care versus usual care in 23 Canadian community pharmacies and outpatient clinics following a 6-month intervention.45 Enhanced pharmacy services included pharmacist assessment of and counseling about cardiovascular disease risk and blood pressure control, review of current antihypertensive medications, and prescribing/titrating drug therapy, as needed, through independent prescriptive authority. Compared to the usual care group (n = 67), the intervention group had a reduction in SBP of 6.6 mm Hg (P = 0.006) and in DBP of 3.2 mm Hg (P = 0.01). This study expanded the pharmacists’ scope of practice, showing evidence for enhancing pharmacist roles on the hypertension care team. Tsuyuki et al also conducted the RxEACH randomized trial, which evaluated community pharmacist cardiovascular risk reduction interventions and showed an improvement in SBP and DBP, with reported results comparable to RxACTION.46
Victor et al conducted the landmark Black Barbershop Study, a cluster RCT involving 319 non-Hispanic black male patients with hypertension from 52 black-owned barbershops.47,48 Barbershops were assigned to 1 of 2 groups. The control group consisted of barbers who encouraged lifestyle modifications and made referrals to primary care providers. The intervention group had pharmacists who met regularly with participants at the barbershops and measured blood pressure, encouraged lifestyle changes, and prescribed drug therapy under collaborative practice agreements with physicians. Both groups demonstrated improvements in blood pressure outcomes, but the intervention group showed greater improvement in SBP and achievement of blood pressure goals compared to the control group. The results in the intervention group proved sustainable over the course of a year, even after the frequency of pharmacists’ visits was reduced. At 6 months, the mean SBP fell by 27.0 mm Hg (to 125.8 mm Hg) in the intervention group, as compared to a 9.3 mm Hg (to 145.4 mm Hg) reduction in the control group (P < 0.001), and blood pressure less than 130/80 mm Hg was achieved among 63.6% of the participants in the intervention group versus 11.7% in the control group (P < 0.001).
This community-level trial brought pharmacists to the barbershop and made them an essential part of the health care team through the endorsement of the barber, who the participants trusted and with whom they had a relationship. Long-standing issues related to distrust of the medical profession by this population were addressed, and trusted community barbershops were utilized as safe spaces for health care delivery. Health care professionals should consider utilizing community locations that other minority populations perceive as social centers and safe places, to reduce health disparities and barriers to care. However, models that bring care to patients need further economic and feasibility evaluations.
Other Health Care Professionals and Future Studies
In addition to models led by nurses and pharmacists, studies have also assessed models of TBC incorporating other health care professionals, including registered dietitians, medical assistants, community health workers, and health coaches (NCT02674464).49,50 Ongoing studies are also looking at the impact of TBC on underserved communities (NCT02674464, NCT03504124). Involving a variety of health care professionals with different communities and populations in TBC studies is warranted to determine the optimal settings in which to utilize different skill sets.
The Impress Study involves nurses who are assessing lifestyle risk and developing an action plan according to a standardized procedure, which may be advantageous given the degree of heterogeneity found in other TBC models.51 There are also studies underway or recently published that compare different components of TBC in order to determine which combination of TBC elements is preferred. Some of these have shown the benefits of using clinical decision-support systems (through a guideline-based treatment protocol) or training programs with ongoing support.52,53 Continued research comparing different TBC models is needed to determine which combination of health professionals and interventions is most impactful in practice.
Cost-Effectiveness
According to the CDC, TBC in hypertension management has proven to be cost-effective.54 Systematic reviews and meta-analyses assessing the cost-effectiveness of TBC in hypertension management have been conducted.26,27,29,55-58 While the general consensus supports this approach as being cost-effective, these determinations are based on studies that are widely heterogeneous. In each of these studies, different types of costs are taken into account when determining cost-effectiveness. The range of costs can be quite wide, depending on how they are calculated, making it difficult to determine the true cost-effectiveness of different TBC models.
Intervention cost is represented by the amount of money spent to implement and maintain the intervention beyond the cost of usual care or the cost without the intervention. For TBC, intervention cost consists of personnel resources such as provider time, patient time, and non-personnel resources, including rent and utilities. Studies show that intervention costs for TBC can range from $35 to $1350 per person per year (mean, $618; median, $428).27,56 One analysis, based on 20 studies comparing TBC to usual care, calculated an intervention cost of $284 per person per year,55 while another study showed an intervention cost of $525 per enrollee per year.56 Intervention cost can vary by the type of provider that is used, the amount of time spent per patient, and the setting where services are provided. Overall, the intervention cost of implementing TBC for hypertension management is consistently higher than the cost of usual care.
Health care cost is another factor to consider. It is the difference in the cost of health care products and services that are utilized in the process of TBC, as compared to care that is provided in the absence of TBC. Health care costs include the costs associated with hospitalizations, outpatient visits, emergency room visits, and medications. One study estimated a median health care cost of hypertension TBC of $65 per person per year.55 Overall, studies evaluating the impact of TBC for hypertension management on health care costs were mixed, with some showing that TBC resulted in an increase in health care cost, and others showing a savings compared to usual care.58 The variability in health care costs was due to the different number of health care components and comorbidities of the patients included in the studies. Also, study duration affected the estimated health care costs of TBC. Most studies did not assess long-term health care cost savings that could be achieved from prolonged blood pressure control.58 When considering both intervention and health care cost, Jacob et al estimated that TBC increased overall net cost by a median value of $329 per person per year.55 While some studies did attribute an overall reduction in health care costs to TBC for hypertension management, on average, team-based models increased health care costs compared to usual care.27,29,55,58,59
However, health care costs do not take into account the long-term reductions in morbidity and mortality or increased quality-adjusted life years (QALY) that result from improved blood pressure control attributed to TBC. In most cost-effectiveness studies, an intervention is considered to be cost-effective if the cost per QALY gained is less than the accepted threshold of $50,000.55 One study estimated that the cost per QALY of TBC in hypertension management is $4763,55,60 while another study estimated a median cost per QALY of $9716 to $13,992.55 A systematic review of 34 international studies estimated the median cost per QALY to be $13,986, ranging from $6683 to $58,610.57 The wide range in cost can be attributed to the variability in interventions, health outcomes used to measure effectiveness, and the settings and countries where the studies were conducted. In another study, a TBC intervention involving pharmacists resulted in a cost per QALY of $26,800.61 The intervention was found to be cost-effective for higher-risk patients, defined as those having diabetes, a smoking history, dyslipidemia, or obesity. For patients who did not have these risk factors, the cost per QALY increased to $43,330.61 Thus, the patient population should be considered before implementing a TBC model. Furthermore, the increased use of technology, allowing for more efficient provision of services and communication between providers, could reduce intervention costs and lead to increased cost efficacy in these models.
The variation in the models used for TBC makes it difficult to draw conclusions on the cost-effectiveness of these interventions. Although it is apparent that TBC in general is cost-effective, more studies are needed comparing different team-based models to determine which specific ones are most cost-effective.
Challenges to Implementation of Team-Based Care
Recognizing and addressing the challenges inherent to a TBC approach is important to the sustainability of such a model within various settings and institutions. Numerous studies conducted on team-based models have identified common challenges that appear to be consistent across multiple settings. These challenges can be categorized as financial, provider-specific, and technology.
Financial Barriers
Although studies have demonstrated the cost-effectiveness of controlling hypertension and preventing serious complications, health systems are still confronted with the challenge of covering the cost for TBC implementation and maintenance.29 The 2 main financial barriers for TBC services are stakeholder engagement and reimbursement for services. According to Kennelty et al, stakeholder engagement is key to the sustainability of the service.27 However, decisions by stakeholders on cost are influenced by many factors, which include available funds, perceived value, and estimates for return on investment. Additionally, interventions must align with the organization’s mission and vision and be feasible to implement, and organizations must have the capacity for administrative support.29 These various financial decisions may greatly influence the sustainability of a TBC model.
The reimbursement challenges for individual providers are an additional barrier to the sustainability of the service. In the United States, most providers are reimbursed via fee-for-service payment plans, but these plans do not reimburse all clinical providers because they are not all recognized as licensed providers.62,63 For example, pharmacists are not recognized by the Centers for Medicare & Medicaid Services as licensed health care providers, which limits their ability to be reimbursed for clinical services provided outside of a traditional dispensing role. Furthermore, state laws determine the services nonphysician providers can offer and how they are recognized for reimbursement by tertiary payers. For instance, pharmacist roles, such as ordering labs and modifying or prescribing medication regimens, vary greatly between states.7,63,64
Financial barriers are a major challenge facing the sustainability of a TBC hypertension service, so including all stakeholders in the decision-making process may improve the organization’s ability to sustain the service.
Provider-Specific Barriers
Notable barriers that are attributed to providers include lack of knowledge, lack of time, lack of initiative to change blood pressure medications, and inability to reach intensive blood pressure goals set in guidelines.29 Studies such as the SPRINT trial have significantly impacted clinical guideline cut-offs for blood pressure, but reaching the intensive blood pressure goals from clinical trials is difficult to emulate in clinical practice.65 In a typical clinical setting, providers may lack the confidence to make adjustments in therapy based on a single blood pressure measurement, and clinical inertia, defined as failure of health care providers to modify therapy when indicated,66 may contribute to the inability to achieve blood pressure goals. Many factors contribute to clinical inertia, including lack of knowledge, time, or clinical protocols on how to modify therapy, causing providers to delay clinical decisions. Implementing site-specific protocols and utilizing hypertension specialist health care professionals in TBC can address the barriers contributing to clinical inertia.
Technology Barriers
A common barrier in a variety of services, but especially prevalent in a TBC service, is access to an electronic health record (EHR) for all providers treating the patient. Some providers who are not directly tied to the same clinical site as the patient’s primary care provider may not have adequate access to the full EHR. For example, pharmacists who are managing hypertension in a TBC model in a community pharmacy may have access only to health information from prescription records. Patient interviews may not provide the pharmacist with adequate information about laboratory results, vitals, and other medical information and history for the patient, making it difficult for the pharmacist to make a proper recommendation for treatment.27 Depending on the setting, communication between providers may be a barrier in achieving optimal outcomes, especially when providers do not have access to a shared medical record.
In addition, patients often lack access to technology used to manage hypertension. Many new technologies exist that aid patients in managing their blood pressure, such as smart phone applications to track blood pressure readings and alarms to remind patients to take their medications. Studies have shown that telemonitoring of blood pressure measurements and management of hypertension, especially in combination with TBC, is effective and reduces costs compared to usual care.67 However, the lack of equal access to the various technologies available may inhibit the success of a TBC hypertension program. Patients may lack access, knowledge, or financial means to utilize the various methods available for managing their hypertension electronically.29
Conclusion
Incorporating nonphysician providers into the health care team for the treatment of hypertension has proven to be more effective than usual care and has been recognized by recent guidelines as a best practice approach to achieving blood pressure goals. Multiple studies have demonstrated that TBC utilizing nurses and pharmacists can improve blood pressure management. While adding members to the team increases health care costs, the long-term benefits of achieving optimal blood pressure goals contribute to the overall cost-effectiveness of TBC strategies over usual care. However, comparisons between different TBC models are warranted to determine which combination of health care professionals and/or interventions is most effective. Cost-analysis estimates are difficult to compare due to widely varied methodology and variance in the models that have been employed. Studies must consider pathways to overcoming reimbursement issues, provider-specific challenges, and technology barriers. Follow-up and monitoring after initiation of drug therapy for hypertension control should include systematic strategies to help improve blood pressure, including use of home blood pressure monitoring, TBC, and telehealth strategies. Future implementation science approaches to hypertension TBC models within specific clinic settings will be useful to identify and overcome challenges and will help to determine the populations who will benefit most, allowing for greater success in sustaining TBC models.
Corresponding author: Shawn R. Smith, PharmD, 309 E. 2nd Street, Pomona, CA 91766; [email protected].
Financial disclosures: None.
1. Fryar CD, Ostchega Y, Hales CM, et al. Hypertension prevalence and control among adults: United States, 2015–2016. NCHS Data Brief. 2017(289):1-8.
2. Ambrosius WT, Sink KM, Foy CG, et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: The Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials. 2014;11:532-546.
3. Lawes CM, Bennett DA, Feigin VL, Rodgers A. Blood pressure and stroke: an overview of published reviews. Stroke. 2017;35:776-785.
4. Zanchetti A, Thomopoulos C, Parati G. Randomized controlled trials of blood pressure lowering in hypertension: A critical reappraisal. Circ Res. 2015;116:1058-1073.
5. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/ AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127-e248.
6. Grol R. Successes and failures in the implementation of evidence-based guidelines for clinical practice. Med Care. 2001;39:II46-II54.
7. Brush JE, Handberg EM, Biga C, et al. 2015 ACC health policy statement on cardiovascular team-based care and the role of advanced practice providers. J Am Coll Cardiol. 2015;65:2118-2136.
8. Centers for Disease Control and Prevention. Best practices for cardiovascular disease prevention programs: a guide to effective health care system interventions and community programs linked to clinical services, promoting team-based care to improve high blood pressure control. www.cdc.gov/dhdsp/pubs/guides/best-practices/team-based-care.htm. Accessed April 30, 2020.
9. Centers for Disease Control and Prevention. Task Force recommends team-based care for improving blood pressure [press release]. May 15, 2012. www.cdc.gov/media/releases/2012/p0515_bp_control.html
10. Mitchell P, Wynia M, Golden R, et al. Core principles & values of effective team-based health care. 2012. Institute of Medicine, Washington, DC.
11. Campbell SM, Hann M, Hacker J, et al. Identifying predictors of high-quality care in English general practice: observational study. BMJ. 2001;323:784-787.
12. Shojania KG, Ranji SR, McDonald KM, et al. Effects of quality improvement strategies for type 2 diabetes on glycemic control: a meta-regression analysis. JAMA. 2006;296:427-440.
13. Walsh JM, McDonald KM, Shojania KG, et al. Quality improvement strategies for hypertension management: a systematic review. Med Care. 2006;44:646-657.
14. Wagner E. The role of patient care teams in chronic disease management. BMJ. 2000;320:560-572.
15. Coleman K, Reid R. Continuous and team-based healing relationships: improving patient care through teams. In: Phillips KE, Weir V, eds. Safety Net Medical Home Initiative Implementation Guide Series. 2nd ed. Seattle, WA: Qualis Health and The MacColl Center for Health Care Innovation at the Group Health Research Institute; 2013.
16. Sinsky CA, Willard-Grace R, Schutzbank AM, et al. In search of joy in practice: a report of 23 high-functioning primary care practices. Ann Fam Med. 2013;11:272-278
17. Howard J, Etz RS, Crocker JB, et al. Maximizing the patient-centered medical home (PCMH) by choosing words wisely. J Am Board Fam Med. 2016;29:248-253.
18. Solberg LI, Crain AL, Tillema JO, et al. Challenges of medical home transformation reported by 118 patient-centered medical home (PCMH) leaders. J Am Board Fam Med. 2014;27:449-457.
19. Crabtree BF, Chase SM, Wise CG, et al. Evaluation of patient centered medical home practice transformation initiatives. Med Care. 2011;49:10-16.
20. Carter BL. Blood pressure control—implementing a team approach. US Cardiol. 2011;8:108-113.
21. Carter BL, Rogers M, Daly J, et al. The potency of team-based care interventions for hypertension: a meta-analysis. Arch Intern Med. 2009;169:1748-1755.
22. Proia KK, Thota AB, Njie GJ, et al. Team-based care and improved blood pressure control: a community guide systematic review. Am J Prev Med. 2014;47:86-99.
23. Santschi V, Chiolero A, Colosimo AL, et al. Improving blood pressure control through pharmacist interventions: a meta-analysis of randomized controlled trials. J Am Heart Assoc. 2014;3:e000718.
24. Shaw RJ, McDuffie JR, Hendrix CC, et al. Effects of nurse-managed protocols in the outpatient management of adults with chronic conditions: a systematic review and meta-analysis. Ann Intern Med. 2014;161:113-121.
25. Carter BL, Coffey CS, Ardery G, et al. Cluster-randomized trial of a physician/pharmacist collaborative model to improve blood pressure control. Circ Cardiovasc Qual Outcomes. 2015;8:235-243
26. Carter BL, Bosworth HB, Green BB. The hypertension team: the role of the pharmacist, nurse and teamwork in hypertension therapy. J Clin Hypertens. 2012;14:51-65.
27. Kennelty KA, Polgreen LA, Carter BL. Team-based care with pharmacists to improve blood pressure: a review of recent literature. Curr Hypertens Rep. 2018;20:1.
28. Brownstein JN, Chowdhury FM, Norris SL, et al. Effectiveness of community health workers in the care of people with hypertension. Am J Prev Med. 2007;32:435-447.
29. Derington CG, King JB, Bryant KB, et al. Cost-effectiveness and challenges of implementing intensive blood pressure goals and team-based care. Curr Hypertens Rep. 2019;21:91.
30. Mills KT, Obst KM, Shen W, et al. Comparative effectiveness of implementation strategies for blood pressure control in hypertensive patients: a systematic review and meta-analysis. Ann Intern Med. 2018;168:110-120.
31. Clark CE, Smith LFP, Taylor RS, et al. Nurse led interventions to improve control of blood pressure in people with hypertension: systematic review and meta-analysis. BMJ. 2010;341:c3995.
32. Santschi V, Chiolero A, Burnand B, et al. Impact of pharmacist care in the management of cardiovascular disease risk factors: a systematic review and meta-analysis of randomized trials. Arch Intern Med. 2011;171:1441-1453.
33. Dennison Himmelfarb CR, Commodore-Mensah Y, Hill MN. Expanding the role of nurses to improve hypertension care and control globally. Ann Glob Health. 2016;82:243-253.
34. Clark CE, Smith LFP, Taylor RS, Campbell JL. Nurse-led interventions used to improve control of high blood pressure in people with diabetes: a systematic review and meta-analysis. DiabetMed. 2011;28:250-261.
35. Zhu X, Wong FKY, Wu CLH. Development and evaluation of a nurse-led hypertension management model: A randomized controlled trial. Int J Nurs Stud. 2018;77:171-178.
36. Spruill TM, Williams O, Teresi JA, et al. Comparative effectiveness of home blood pressure telemonitoring (HBPTM) plus nurse case management versus HBPTM alone among Black and Hispanic stroke survivors: study protocol for a randomized controlled trial. Trials. 2015;16:97.
37. Ogedegbe G. Comparative effectiveness of home BP telemonitoring plus nurse case management (HBPTM+NCM) versus HBPTM alone on systolic BP (SBP) reduction among minority stroke survivors. International Stroke Conference 2020; February 19-21, 2020; Los Angeles, CA. Abstract LB19.
38. Dunn SP, Birtcher KK, Beavers CJ, et al. The role of the clinical pharmacist in the care of patients with cardiovascular disease. J Am Coll Cardiol. 2015;66:2129-2139.
39. Santschi V, Chiolero A, Paradis G et al. Pharmacist interventions to improve cardiovascular disease risk factors in diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Care. 2012;35:2706-2717.
40. Anderegg MD, Gums TH, Uribe L, et al. Pharmacist intervention for blood pressure control in patients with diabetes and/or chronic kidney disease. Pharmacotherapy. 2018;38:309-318.
41. Anderegg MD, Gums TH, Uribe L et al. Physician-pharmacist collaborative management: narrowing the socioeconomic blood pressure gap. Hypertension. 2016;68:1314-1320.
42. Sisson EM, Dixon DL, Kildow DC, et al. Effectiveness of a pharmacist-physician team-based collaboration to improve long-term blood pressure control at an inner-city safety-net clinic. Pharmacotherapy. 2016;36:342-347.
43. Dixon DL, Sisson EM, Parod ED, et al. Pharmacist-physician collaborative care model and time to goal blood pressure in the uninsured population. J Clin Hypertens (Greenwich). 2018;20:88-95.
44. Dixon DL, Parod ED, Sisson EM et al. Impact of a pharmacist-physician collaborative care model on time-in-therapeutic blood pressure range in patients with hypertension. J Am Coll Clin Pharm. 2020;3:404-409.
45. Tsuyuki RT, Houle SK, Charrois TL, et al. Randomized trial of the effect of pharmacist prescribing on improving blood pressure in the community: the Alberta Clinical Trial in Optimizing Hypertension (RxACTION). Circulation. 2015;132:93-100.
46. Tsuyuki RT, Al Hamarneh YN, Jones CA, et al. The effectiveness of pharmacist interventions on cardiovascular risk: The Multicenter Randomized Controlled RxEACH trial. J Am Coll Cardiol. 2016;67:2846-2854.
47. Victor RG, Lynch K, Li N, et al. A cluster-randomized trial of blood-pressure reduction in black barbershops. N Engl J Med. 2018;378:1291-1301.
48. Victor RG, Blyler CA, Li N et al. Sustainability of blood pressure reduction in black barbershops. Circulation. 2019;139:10-19.
49. Panattoni L, Hurlimann L, Wilson C, et al. Workflow standardization of a novel team care model to improve chronic care: a quasi-experimental study. BMC Health Serv Res. 2017;17:286.
50. Chang AR, Bonaparte H, Yule C. Randomized controlled trial comparing a self-guided vs. dietitian-led approach using web-based tools to lower blood pressure: study design and rationale. International Stroke Conference 2020; February 19-21, 2020; Los Angeles, CA. Abstract P169.
51. Stephen C, Halcomb E, Mcinnes S, et al. Improving blood pressure control in primary care: The ImPress study. Int J Nurs Stud. 2019;95:28-33.
52. He J, Shi X, Lin M. Comparative effectiveness of implementation strategies on cardiovascular risk factor control in patients with diabetes: The D4C cluster randomized trial. International Stroke Conference 2020; February 19-21, 2020; Los Angeles, CA. Abstract 17.
53. Jafar TH, Gandhi M, de Silva HA, et al. A community-based intervention for managing hypertension in rural South Asia. N Engl J Med. 2020;382:717-726.
54. Centers for Disease Control and Prevention. Promoting team-based care to improve high blood pressure control. www.cdc.gov/dhdsp/pubs/guides/best-practices/team-based-care.htm. Accessed April 30, 2020.
55. Jacob V, Chattopadhyay SK, Thota AB, et al. Economics of team-based care in controlling blood pressure: a community guide systematic review. Am J Prev Med. 2015;49:772-783.
56. Dehmer SP, Baker-Goering MM, Maciosek MV, et al. Modeled health and economic impact of team-based care for hypertension. Am J Prev Med. 2016;50(5 suppl 1):S34-S44.
57. Zhang D, Wang G, Joo H. A systematic review of economic evidence on community hypertension interventions. Am J Prev Med. 2017;53:S121-S130.
58. Community Preventive Services Task Force. Cardiovascular disease: team-based care to improve blood pressure control. 2011. www.thecommunityguide.org/findings/cardiovascular-disease-team-based-care-improve-blood-pressure-control. Accessed April 30, 2020.
59. Kulchaitanaroaj P, Brooks JM, Ardery G et al. Incremental costs associated with physician and pharmacist collaboration to improve blood pressure control. Pharmacotherapy. 2012;32:772-780.
60. Mason JM, Freemantle N, Gibson JM, New JP. Specialist nurse-led clinics to improve control of hypertension and hyperlipidemia in diabetes. Diabetes Care. 2005;28:40-46.
61. Kulchaitanaroaj P, Brooks JM, Chaiyakunapruk N et al. Cost-utility analysis of physician-pharmacist collaborative intervention for treating hypertension compared with usual care. J Hypertens. 2017;35:178-187.
62. Lall D, Engel N, Devadasan N, et al. Models of care for chronic conditions in low/middle-income countries: a ‘best fit’ framework synthesis. BMJ Glob Health. 2018;3:e001077.
63. Bodenheimer T, Chen E, Bennett HD. Confronting the growing burden of chronic disease: can the U.S. health care workforce do the job? Health Aff (Millwood). 2009;28:64-74.
64. Smith M, Bates DW, Bodenheimer T, Cleary PD. Why pharmacists belong in the medical home. Health Aff (Millwood). 2010;29:906-913.
65. Wright JT, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
66. Phillips LS, Branch WT, Cook CB, et al. Clinical inertia. Ann Intern Med. 2001;135:825-834.
67. McManus RJ, Mant J, Franssen M, et al. Efficacy of self-monitored blood pressure, with or without telemonitoring, for titration of antihypertensive medication (TASMINH4): an unmasked randomised controlled trial. Lancet. 2018;391:949-959.
68. Tucker KL, Sheppard JP, Stevens R, et al. Self-monitoring of blood pressure in hypertension: a systematic review and individual patient data meta-analysis. PLoS Med. 2017;14:e1002389.
69. Casey DE, Thomas RJ, Bhalla V, et al. 2019 AHA/ACC clinical performance and quality measures for adults with high blood pressure: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures. J Am Coll Cardiol. 2019;74:2661-2706.
1. Fryar CD, Ostchega Y, Hales CM, et al. Hypertension prevalence and control among adults: United States, 2015–2016. NCHS Data Brief. 2017(289):1-8.
2. Ambrosius WT, Sink KM, Foy CG, et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: The Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials. 2014;11:532-546.
3. Lawes CM, Bennett DA, Feigin VL, Rodgers A. Blood pressure and stroke: an overview of published reviews. Stroke. 2017;35:776-785.
4. Zanchetti A, Thomopoulos C, Parati G. Randomized controlled trials of blood pressure lowering in hypertension: A critical reappraisal. Circ Res. 2015;116:1058-1073.
5. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/ AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127-e248.
6. Grol R. Successes and failures in the implementation of evidence-based guidelines for clinical practice. Med Care. 2001;39:II46-II54.
7. Brush JE, Handberg EM, Biga C, et al. 2015 ACC health policy statement on cardiovascular team-based care and the role of advanced practice providers. J Am Coll Cardiol. 2015;65:2118-2136.
8. Centers for Disease Control and Prevention. Best practices for cardiovascular disease prevention programs: a guide to effective health care system interventions and community programs linked to clinical services, promoting team-based care to improve high blood pressure control. www.cdc.gov/dhdsp/pubs/guides/best-practices/team-based-care.htm. Accessed April 30, 2020.
9. Centers for Disease Control and Prevention. Task Force recommends team-based care for improving blood pressure [press release]. May 15, 2012. www.cdc.gov/media/releases/2012/p0515_bp_control.html
10. Mitchell P, Wynia M, Golden R, et al. Core principles & values of effective team-based health care. 2012. Institute of Medicine, Washington, DC.
11. Campbell SM, Hann M, Hacker J, et al. Identifying predictors of high-quality care in English general practice: observational study. BMJ. 2001;323:784-787.
12. Shojania KG, Ranji SR, McDonald KM, et al. Effects of quality improvement strategies for type 2 diabetes on glycemic control: a meta-regression analysis. JAMA. 2006;296:427-440.
13. Walsh JM, McDonald KM, Shojania KG, et al. Quality improvement strategies for hypertension management: a systematic review. Med Care. 2006;44:646-657.
14. Wagner E. The role of patient care teams in chronic disease management. BMJ. 2000;320:560-572.
15. Coleman K, Reid R. Continuous and team-based healing relationships: improving patient care through teams. In: Phillips KE, Weir V, eds. Safety Net Medical Home Initiative Implementation Guide Series. 2nd ed. Seattle, WA: Qualis Health and The MacColl Center for Health Care Innovation at the Group Health Research Institute; 2013.
16. Sinsky CA, Willard-Grace R, Schutzbank AM, et al. In search of joy in practice: a report of 23 high-functioning primary care practices. Ann Fam Med. 2013;11:272-278
17. Howard J, Etz RS, Crocker JB, et al. Maximizing the patient-centered medical home (PCMH) by choosing words wisely. J Am Board Fam Med. 2016;29:248-253.
18. Solberg LI, Crain AL, Tillema JO, et al. Challenges of medical home transformation reported by 118 patient-centered medical home (PCMH) leaders. J Am Board Fam Med. 2014;27:449-457.
19. Crabtree BF, Chase SM, Wise CG, et al. Evaluation of patient centered medical home practice transformation initiatives. Med Care. 2011;49:10-16.
20. Carter BL. Blood pressure control—implementing a team approach. US Cardiol. 2011;8:108-113.
21. Carter BL, Rogers M, Daly J, et al. The potency of team-based care interventions for hypertension: a meta-analysis. Arch Intern Med. 2009;169:1748-1755.
22. Proia KK, Thota AB, Njie GJ, et al. Team-based care and improved blood pressure control: a community guide systematic review. Am J Prev Med. 2014;47:86-99.
23. Santschi V, Chiolero A, Colosimo AL, et al. Improving blood pressure control through pharmacist interventions: a meta-analysis of randomized controlled trials. J Am Heart Assoc. 2014;3:e000718.
24. Shaw RJ, McDuffie JR, Hendrix CC, et al. Effects of nurse-managed protocols in the outpatient management of adults with chronic conditions: a systematic review and meta-analysis. Ann Intern Med. 2014;161:113-121.
25. Carter BL, Coffey CS, Ardery G, et al. Cluster-randomized trial of a physician/pharmacist collaborative model to improve blood pressure control. Circ Cardiovasc Qual Outcomes. 2015;8:235-243
26. Carter BL, Bosworth HB, Green BB. The hypertension team: the role of the pharmacist, nurse and teamwork in hypertension therapy. J Clin Hypertens. 2012;14:51-65.
27. Kennelty KA, Polgreen LA, Carter BL. Team-based care with pharmacists to improve blood pressure: a review of recent literature. Curr Hypertens Rep. 2018;20:1.
28. Brownstein JN, Chowdhury FM, Norris SL, et al. Effectiveness of community health workers in the care of people with hypertension. Am J Prev Med. 2007;32:435-447.
29. Derington CG, King JB, Bryant KB, et al. Cost-effectiveness and challenges of implementing intensive blood pressure goals and team-based care. Curr Hypertens Rep. 2019;21:91.
30. Mills KT, Obst KM, Shen W, et al. Comparative effectiveness of implementation strategies for blood pressure control in hypertensive patients: a systematic review and meta-analysis. Ann Intern Med. 2018;168:110-120.
31. Clark CE, Smith LFP, Taylor RS, et al. Nurse led interventions to improve control of blood pressure in people with hypertension: systematic review and meta-analysis. BMJ. 2010;341:c3995.
32. Santschi V, Chiolero A, Burnand B, et al. Impact of pharmacist care in the management of cardiovascular disease risk factors: a systematic review and meta-analysis of randomized trials. Arch Intern Med. 2011;171:1441-1453.
33. Dennison Himmelfarb CR, Commodore-Mensah Y, Hill MN. Expanding the role of nurses to improve hypertension care and control globally. Ann Glob Health. 2016;82:243-253.
34. Clark CE, Smith LFP, Taylor RS, Campbell JL. Nurse-led interventions used to improve control of high blood pressure in people with diabetes: a systematic review and meta-analysis. DiabetMed. 2011;28:250-261.
35. Zhu X, Wong FKY, Wu CLH. Development and evaluation of a nurse-led hypertension management model: A randomized controlled trial. Int J Nurs Stud. 2018;77:171-178.
36. Spruill TM, Williams O, Teresi JA, et al. Comparative effectiveness of home blood pressure telemonitoring (HBPTM) plus nurse case management versus HBPTM alone among Black and Hispanic stroke survivors: study protocol for a randomized controlled trial. Trials. 2015;16:97.
37. Ogedegbe G. Comparative effectiveness of home BP telemonitoring plus nurse case management (HBPTM+NCM) versus HBPTM alone on systolic BP (SBP) reduction among minority stroke survivors. International Stroke Conference 2020; February 19-21, 2020; Los Angeles, CA. Abstract LB19.
38. Dunn SP, Birtcher KK, Beavers CJ, et al. The role of the clinical pharmacist in the care of patients with cardiovascular disease. J Am Coll Cardiol. 2015;66:2129-2139.
39. Santschi V, Chiolero A, Paradis G et al. Pharmacist interventions to improve cardiovascular disease risk factors in diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Care. 2012;35:2706-2717.
40. Anderegg MD, Gums TH, Uribe L, et al. Pharmacist intervention for blood pressure control in patients with diabetes and/or chronic kidney disease. Pharmacotherapy. 2018;38:309-318.
41. Anderegg MD, Gums TH, Uribe L et al. Physician-pharmacist collaborative management: narrowing the socioeconomic blood pressure gap. Hypertension. 2016;68:1314-1320.
42. Sisson EM, Dixon DL, Kildow DC, et al. Effectiveness of a pharmacist-physician team-based collaboration to improve long-term blood pressure control at an inner-city safety-net clinic. Pharmacotherapy. 2016;36:342-347.
43. Dixon DL, Sisson EM, Parod ED, et al. Pharmacist-physician collaborative care model and time to goal blood pressure in the uninsured population. J Clin Hypertens (Greenwich). 2018;20:88-95.
44. Dixon DL, Parod ED, Sisson EM et al. Impact of a pharmacist-physician collaborative care model on time-in-therapeutic blood pressure range in patients with hypertension. J Am Coll Clin Pharm. 2020;3:404-409.
45. Tsuyuki RT, Houle SK, Charrois TL, et al. Randomized trial of the effect of pharmacist prescribing on improving blood pressure in the community: the Alberta Clinical Trial in Optimizing Hypertension (RxACTION). Circulation. 2015;132:93-100.
46. Tsuyuki RT, Al Hamarneh YN, Jones CA, et al. The effectiveness of pharmacist interventions on cardiovascular risk: The Multicenter Randomized Controlled RxEACH trial. J Am Coll Cardiol. 2016;67:2846-2854.
47. Victor RG, Lynch K, Li N, et al. A cluster-randomized trial of blood-pressure reduction in black barbershops. N Engl J Med. 2018;378:1291-1301.
48. Victor RG, Blyler CA, Li N et al. Sustainability of blood pressure reduction in black barbershops. Circulation. 2019;139:10-19.
49. Panattoni L, Hurlimann L, Wilson C, et al. Workflow standardization of a novel team care model to improve chronic care: a quasi-experimental study. BMC Health Serv Res. 2017;17:286.
50. Chang AR, Bonaparte H, Yule C. Randomized controlled trial comparing a self-guided vs. dietitian-led approach using web-based tools to lower blood pressure: study design and rationale. International Stroke Conference 2020; February 19-21, 2020; Los Angeles, CA. Abstract P169.
51. Stephen C, Halcomb E, Mcinnes S, et al. Improving blood pressure control in primary care: The ImPress study. Int J Nurs Stud. 2019;95:28-33.
52. He J, Shi X, Lin M. Comparative effectiveness of implementation strategies on cardiovascular risk factor control in patients with diabetes: The D4C cluster randomized trial. International Stroke Conference 2020; February 19-21, 2020; Los Angeles, CA. Abstract 17.
53. Jafar TH, Gandhi M, de Silva HA, et al. A community-based intervention for managing hypertension in rural South Asia. N Engl J Med. 2020;382:717-726.
54. Centers for Disease Control and Prevention. Promoting team-based care to improve high blood pressure control. www.cdc.gov/dhdsp/pubs/guides/best-practices/team-based-care.htm. Accessed April 30, 2020.
55. Jacob V, Chattopadhyay SK, Thota AB, et al. Economics of team-based care in controlling blood pressure: a community guide systematic review. Am J Prev Med. 2015;49:772-783.
56. Dehmer SP, Baker-Goering MM, Maciosek MV, et al. Modeled health and economic impact of team-based care for hypertension. Am J Prev Med. 2016;50(5 suppl 1):S34-S44.
57. Zhang D, Wang G, Joo H. A systematic review of economic evidence on community hypertension interventions. Am J Prev Med. 2017;53:S121-S130.
58. Community Preventive Services Task Force. Cardiovascular disease: team-based care to improve blood pressure control. 2011. www.thecommunityguide.org/findings/cardiovascular-disease-team-based-care-improve-blood-pressure-control. Accessed April 30, 2020.
59. Kulchaitanaroaj P, Brooks JM, Ardery G et al. Incremental costs associated with physician and pharmacist collaboration to improve blood pressure control. Pharmacotherapy. 2012;32:772-780.
60. Mason JM, Freemantle N, Gibson JM, New JP. Specialist nurse-led clinics to improve control of hypertension and hyperlipidemia in diabetes. Diabetes Care. 2005;28:40-46.
61. Kulchaitanaroaj P, Brooks JM, Chaiyakunapruk N et al. Cost-utility analysis of physician-pharmacist collaborative intervention for treating hypertension compared with usual care. J Hypertens. 2017;35:178-187.
62. Lall D, Engel N, Devadasan N, et al. Models of care for chronic conditions in low/middle-income countries: a ‘best fit’ framework synthesis. BMJ Glob Health. 2018;3:e001077.
63. Bodenheimer T, Chen E, Bennett HD. Confronting the growing burden of chronic disease: can the U.S. health care workforce do the job? Health Aff (Millwood). 2009;28:64-74.
64. Smith M, Bates DW, Bodenheimer T, Cleary PD. Why pharmacists belong in the medical home. Health Aff (Millwood). 2010;29:906-913.
65. Wright JT, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
66. Phillips LS, Branch WT, Cook CB, et al. Clinical inertia. Ann Intern Med. 2001;135:825-834.
67. McManus RJ, Mant J, Franssen M, et al. Efficacy of self-monitored blood pressure, with or without telemonitoring, for titration of antihypertensive medication (TASMINH4): an unmasked randomised controlled trial. Lancet. 2018;391:949-959.
68. Tucker KL, Sheppard JP, Stevens R, et al. Self-monitoring of blood pressure in hypertension: a systematic review and individual patient data meta-analysis. PLoS Med. 2017;14:e1002389.
69. Casey DE, Thomas RJ, Bhalla V, et al. 2019 AHA/ACC clinical performance and quality measures for adults with high blood pressure: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures. J Am Coll Cardiol. 2019;74:2661-2706.
Atypical Features of COVID-19: A Literature Review
From the University of Florida College of Medicine, Division of Infectious Diseases and Global Medicine, Gainesville, FL.
Abstract
- Objective: To review current reports on atypical manifestations of coronavirus disease 2019 (COVID-19).
- Methods: Review of the literature.
- Results: Evidence regarding atypical features of COVID-19 is accumulating. SARS-CoV-2 can infect human cells that express the angiotensin-converting enzyme 2 receptor, which would allow for a broad spectrum of illnesses affecting the renal, cardiac, and gastrointestinal organ systems. Neurologic, cutaneous, and musculoskeletal manifestations have also been reported. The potential for SARS-CoV-2 to induce a hypercoagulable state provides another avenue for the virus to indirectly damage various organ systems, as evidenced by reports of cerebrovascular disease, myocardial injury, and a chilblain-like rash in patients with COVID-19.
- Conclusion: Because the signs and symptoms of COVID-19 may occur with varying frequency across populations, it is important to keep differentials broad when assessing patients with a clinical illness that may indeed be COVID-19.
Keywords: coronavirus; severe acute respiratory syndrome coronavirus-2; SARS-CoV-2; pandemic.
Coronavirus disease 2019 (COVID-19), the syndrome caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), was first reported in Wuhan, China, in early December 2019.1 Since then, the virus has spread quickly around the world, with the World Health Organization (WHO) declaring the coronavirus outbreak a global pandemic on March 11, 2020. As of May 21, 2020, more than 5,000,000 cases of COVID-19 have been confirmed, and more than 328,000 deaths related to COVID-19 have been reported globally.2 These numbers are expected to increase, due to the reproduction number (R0) of SARS-CoV-2. R0 represents the number of new infections generated by an infectious person in a totally naïve population.3 The WHO estimates that the R0 of SARS-CoV-2 is 1.95, with other estimates ranging from 1.4 to 6.49.3 To control the pathogen, the R0 needs to be brought under a value of 1.
A fundamental tool in lowering the R0 is prompt testing and isolation of those who display signs and symptoms of infection. SARS-CoV-2 is still a novel pathogen about which we know relatively little. The common symptoms of COVID-19 are now well known—including fever, fatigue, anorexia, cough, and shortness of breath—but atypical manifestations of this viral continue to be reported and described. To help clinicians across specialties and settings identify patients with possible infection, we have summarized findings from current reports on COVID-19 manifestations involving the renal, cardiac, gastrointestinal (GI), and other organ systems.
Renal
During the 2003 SARS-CoV-1 outbreak, acute kidney injury (AKI) was an uncommon complication of the infection, but early reports suggest that AKI may occur more commonly with COVID-19.4 In a study of 193 patients with laboratory-confirmed COVID-19 treated in 3 Chinese hospitals, 59% presented with proteinuria, 44% with hematuria, 14% with increased blood urea nitrogen, and 10% with increased levels of serum creatinine.4 These markers, indicative of AKI, may be associated with increased mortality. Among this cohort, those with AKI had a mortality risk 5.3 times higher than those who did not have AKI.4 The pathophysiology of renal disease in COVID-19 may be related to dehydration or inflammatory mediators, causing decreased renal perfusion and cytokine storm, but evidence also suggests that SARS-CoV-2 is able to directly infect kidney cells.5 The virus infects cells by using angiotensin-converting enzyme 2 (ACE2) on the cell membrane as a cell entry receptor; ACE2 is expressed on the kidney, heart, and GI cells, and this may allow SARS-CoV-2 to directly infect and damage these organs. Other potential mechanisms of renal injury include overproduction of proinflammatory cytokines and administration of nephrotoxic drugs. No matter the mechanism, however, increased serum creatinine and blood urea nitrogen correlate with an increased likelihood of requiring intensive care unit (ICU) admission.6 Therefore, clinicians should carefully monitor renal function in patients with COVID-19.
Cardiac
In a report of 138 Chinese patients hospitalized for COVID-19, 36 required ICU admission: 44.4% of these had arrhythmias and 22.2% had developed acute cardiac injury.6 In addition, the cardiac cell injury biomarker troponin I was more likely to be elevated in ICU patients.6 A study of 21 patients admitted to the ICU in Washington State found elevated levels of brain natriuretic peptide.7 These biomarkers reflect the presence of myocardial stress, but do not necessarily indicate direct myocardial infection. Case reports of fulminant myocarditis in those with COVID-19 have begun to surface, however.8,9 An examination of 68 deaths in persons with COVID-19 concluded that 7% were caused by myocarditis with circulatory failure.10
The pathophysiology of myocardial injury in COVID-19 is likely multifactorial. This includes increased inflammatory mediators, hypoxemia, and metabolic changes that can directly damage myocardial tissue. These factors can also exacerbate comorbid conditions, such as coronary artery disease, leading to ischemia and dysfunction of preexisting electrical conduction abnormalities. However, pathologic evidence of myocarditis and the presence of the ACE2 receptor, which may be a mediator of cardiac function, on cardiac muscle cells suggest that SARS-CoV-2 is capable of directly infecting and damaging myocardial cells. Other proposed mechanisms include infection-mediated downregulation of ACE2, causing cardiac dysfunction, or thrombus formation.11 Although respiratory failure is the most common source of advanced illness in COVID-19 patients, myocarditis and arrhythmias can be life-threatening manifestations of the disease.
Gastrointestinal
As noted, ACE2 is expressed in the GI tract. In 73 patients hospitalized for COVID-19, 53.4% tested positive for SARS-CoV-2 RNA in stool, and 23.4% continued to have RNA-positive stool samples even after their respiratory samples tested negative.12 These findings suggest the potential for SARS-CoV-2 to spread through fecal-oral transmission in those who are asymptomatic, pre-symptomatic, or symptomatic. This mode of transmission has yet to be determined conclusively, and more research is needed. However, GI symptoms have been reported in persons with COVID-19. Among 138 hospitalized patients, 10.1% had complaints of diarrhea and nausea and 3.6% reported vomiting.6 Those who reported nausea and diarrhea noted that they developed these symptoms 1 to 2 days before they developed fever.6 Also, among a cohort of 1099 Chinese patients with COVID-19, 3.8% complained of diarrhea.13 Although diarrhea does not occur in a majority of patients, GI complaints, such as nausea, vomiting, or diarrhea, should raise clinical suspicion for COVID-19, and in known areas of active transmission, testing of patients with GI symptoms is likely warranted.
Ocular
Ocular manifestations of COVID-19 are now being described, and should be taken into consideration when examining a patient. In a study of 38 patients with COVID-19 from Hubei province, China, 31.6% had ocular findings consistent with conjunctivitis, including conjunctival hyperemia, chemosis, epiphora, and increased ocular secretions.14 SARS-CoV-2 was detected in conjunctival and nasopharyngeal samples in 2 patients from this cohort. Conjunctival congestion was reported in a cohort of 1099 patients with COVID-19 treated at multiple centers throughout China, but at a much lower incidence, approximately 0.8%.13 Because SARS-CoV-2 can cause conjunctival disease and has been detected in samples from the external surface of the eye, it appears the virus is transmissible from tears or contact with the eye itself.
Neurologic
Common reported neurologic symptoms include dizziness, headache, impaired consciousness, ataxia, and cerebrovascular events. In a cohort of 214 patients from Wuhan, China, 36.4% had some form of neurological insult.15 These symptoms were more common in those with severe illness (P = 0.02).15 Two interesting neurologic symptoms that have been described are anosmia (loss of smell) and ageusia (loss of taste), which are being found primarily in tandem. It is still unclear how many people with COVID-19 are experiencing these symptoms, but a report from Italy estimates 19.4% of 320 patients examined had chemosensory dysfunction.16 The aforementioned report from Wuhan, China, found that 5.1% had anosmia and 5.6% had ageusia.15 The presence of anosmia/ageusia in some patients suggests that SARS-CoV-2 may enter the central nervous system (CNS) through a retrograde neuronal route.15 In addition, a case report from Japan described a 24-year-old man who presented with meningitis/encephalitis and had SARS-CoV-2 RNA present in his cerebrospinal fluid, showing that SARS-CoV-2 can penetrate into the CNS.17
SARS-CoV-2 may also have an association with Guillain–Barré syndrome, as this condition was reported in 5 patients from 3 hospitals in Northern Italy.18 The symptoms of Guillain–Barré syndrome presented 5 to 10 days after the typical COVID-19 symptoms, and evolved over 36 hours to 4 days afterwards. Four of the 5 patients experienced flaccid tetraparesis or tetraplegia, and 3 required mechanical ventilation.18
Another possible cause of neurologic injury in COVID-19 is damage to endothelial cells in cerebral blood vessels, causing thrombus formation and possibly increasing the risk of acute ischemic stroke.15,19 Supporting this mechanism of injury, significantly lower platelet counts were noted in patients with CNS symptoms (P = 0.005).15 Other hematological impacts of COVID-19 have been reported, particularly hypercoagulability, as evidenced by elevated D-dimer levels.13,20 This hypercoagulable state is linked to overproduction of proinflammatory cytokines (cytokine storm), leading to dysregulation of coagulation pathways and reduced concentrations of anticoagulants, such as protein C, antithrombin III, and tissue factor pathway inhibitor.21
Cutaneous
Cutaneous findings emerging in persons with COVID-19 demonstrate features of small-vessel and capillary occlusion, including erythematous skin eruptions and petechial rash. One report from Italy noted that 20.4% of patients with COVID-19 (n = 88) had a cutaneous finding, with a cutaneous manifestation developing in 8 at the onset of illness and in 10 following hospital admission.22 Fourteen patients had an erythematous rash, primarily on the trunk, with 3 patients having a diffuse urticarial appearing rash, and 1 patient developing vesicles.22 The severity of illness did not appear to correlate with the cutaneous manifestation, and the lesions healed within a few days.
One case report described a patient from Bangkok who was thought to be suffering from dengue fever, but was found to have SARS-CoV-2 infection. He initially presented with skin rash and petechiae, and later developed respiratory disease.23
Other dermatologic findings of COVID-19 resemble chilblains disease, colloquially referred to as “COVID toes.” Two women, 27 and 35 years old, presented to a dermatology clinic in Qatar with a chief complaint of skin rash, described as red-purple papules on the dorsal aspects of the fingers bilaterally.22 Both patients had an unremarkable medical and drug history, but recent travel to the United Kingdom dictated SARS-CoV-2 screening, which was positive.24 An Italian case report describes a 23-year-old man who tested positive for SARS-CoV-2 and had violaceous plaques on an erythematous background on his feet, without any lesions on his hands.25 Since chilblains is less common in the warmer months and these events correspond with the COVID-19 pandemic, SARS-CoV-2 infection is the suspected etiology. The pathophysiology of these lesions is unclear, and more research is needed. As more data become available, we may see cutaneous manifestations in patients with COVID-19 similar to those commonly reported with other viral infectious processes.
Musculoskeletal
Of 138 patients hospitalized in Wuhan, China, for COVID-19, 34.8% presented with myalgia; the presence of myalgia does not appear to be correlated with an increased likelihood of ICU admission.6 Myalgia or arthralgia was also reported in 14.9% among the cohort of 1099 COVID-19 patients in China.13 These musculoskeletal symptoms are described among large muscle groups found in the extremities, trunk, and back, and should raise suspicion in patients who present with other signs and symptoms concerning for COVID-19.
Conclusion
Evidence regarding atypical features of COVID-19 is accumulating. SARS-CoV-2 can infect a human cells that express the ACE2 receptor, which would allow for a broad spectrum of illnesses. The potential for SARS-CoV-2 to induce a hypercoagulable state allows it to indirectly damage various organ systems,20 leading to cerebrovascular disease, myocardial injury, and a chilblain-like rash. Clinicians must be aware of these unique features, as early recognition of persons who present with COVID-19 will allow for prompt testing, institution of infection control and isolation practices, and treatment, as needed, among those infected. Also, this is a pandemic involving a novel virus affecting different populations throughout the world, and these signs and symptoms may occur with varying frequency across populations. Therefore, it is important to keep differentials broad when assessing patients with a clinical illness that may indeed be COVID-19.
Corresponding author: Norman L. Beatty, MD, [email protected].
Financial disclosures: None.
1. WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020 [press release]. World Health Organization; March 11, 2020.
2. Coronavirus COVID-19 Global Cases by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. Johns Hopkins CSSE. https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6 Accessed May 15, 2020.
3. Liu Y, Gayle AA, Wilder-Smith A, Rocklöv J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J Travel Med. 2020;27(2):taaa021. doi:10.1093/jtm/taaa021
4. Li Z, Wu M, Guo J, et al. Caution on kidney dysfunctions of 2019-nCoV patients. medRxiv preprint. doi: 10.1101/2020.02.08.20021212
5. Li W, Moore MJ, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450-454. doi: 10.1038/nature02145.
6. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. doi:10.1001/jama.2020.1585
7. Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323:1612‐1614. doi:10.1001/jama.2020.4326
8. Chen C, Zhou Y, Wang DW. SARS-CoV-2: a potential novel etiology of fulminant myocarditis. Herz. 2020;45:230-232. doi: 10.1007/s00059-020-04909-z
9. Hu H, Ma F, Wei X, Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. 2020 Mar 16;ehaa190. doi: 10.1093/eurheartj/ehaa190
10. Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846-848. doi:10.1007/s00134-020-05991-x
11. Akhmerov A, Marban E. COVID-19 and the heart. Circ Res. 2020;126:1443-1455. doi:10.1161/CIRCRESAHA.120.317055
12. Xiao F, Tang M, Zheng X, et al. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158:1831-1833. doi: 10.1053/j.gastro.2020.02.055
13. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1078-1720. doi: 10.1056/NEJMoa2002032
14. Wu P, Duan F, Luo C, et al. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020 Mar 31;e201291. doi: 10.1001/jamaophthalmol.2020.1291
15. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020 Apr 10. doi: 10.1001/jamaneurol.2020.1127
16. Vaira LA, Salzano G, Deiana G, De Riu G. Anosmia and ageusia: common findings in COVID-19 patients. Laryngoscope. 2020 Apr 1. doi: 10.1002/lary.28692
17. Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS-coronavirus-2. Int J Infect Dis. 2020;94:55-58. doi: 10.1016/j.ijid.2020.03.062
18. Toscano G, Palmerini F, Ravaglia S, et al. Guillain–Barré syndrome associated with SARS-CoV-2. N Engl J Med. 2020 Apr 17;NEJMc2009191. doi:10.1056/nejmc2009191
19. Dafer RM, Osteraas ND, Biller J. Acute stroke care in the coronavirus disease 2019 pandemic. J Stroke Cerebrovascular Dis. 2020 Apr 17:104881. doi: 10.1016/j.jstrokecerebrovasdis.2020.104881
20. Terpos E, Ntanasis-Stathopoulos I, Elalamy I, et al. Hematological findings and complications of COVID-19. Am J Hematol. 2020;10.1002/ajh.25829. doi:10.1002/ajh.25829
21. Jose RJ, Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med. 2020;S2213-2600(20)30216-2. doi:10.1016/S2213-2600(20)30216-2
22. Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020 Mar 26. doi: 10.1111/jdv.16387
23. Joob B, Wiwanitkit V. COVID-19 can present with a rash and be mistaken for dengue. J Am Acad Dermatol. 2020;82(5):e177. doi: 10.1016/j.jaad.2020.03.036
24. Alramthan A, Aldaraji W. A Case of COVID‐19 presenting in clinical picture resembling chilblains disease. First report from the Middle East. Clin Exp Dermatol. 2020 Apr 17. doi: 10.1111/ced.14243
25. Kolivras A, Dehavay F, Delplace D, et al. Coronavirus (COVID-19) infection–induced chilblains: a case report with histopathologic findings. JAAD Case Rep. 2020 Apr 18. doi: 10.1016/j.jdcr.2020.04.011
From the University of Florida College of Medicine, Division of Infectious Diseases and Global Medicine, Gainesville, FL.
Abstract
- Objective: To review current reports on atypical manifestations of coronavirus disease 2019 (COVID-19).
- Methods: Review of the literature.
- Results: Evidence regarding atypical features of COVID-19 is accumulating. SARS-CoV-2 can infect human cells that express the angiotensin-converting enzyme 2 receptor, which would allow for a broad spectrum of illnesses affecting the renal, cardiac, and gastrointestinal organ systems. Neurologic, cutaneous, and musculoskeletal manifestations have also been reported. The potential for SARS-CoV-2 to induce a hypercoagulable state provides another avenue for the virus to indirectly damage various organ systems, as evidenced by reports of cerebrovascular disease, myocardial injury, and a chilblain-like rash in patients with COVID-19.
- Conclusion: Because the signs and symptoms of COVID-19 may occur with varying frequency across populations, it is important to keep differentials broad when assessing patients with a clinical illness that may indeed be COVID-19.
Keywords: coronavirus; severe acute respiratory syndrome coronavirus-2; SARS-CoV-2; pandemic.
Coronavirus disease 2019 (COVID-19), the syndrome caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), was first reported in Wuhan, China, in early December 2019.1 Since then, the virus has spread quickly around the world, with the World Health Organization (WHO) declaring the coronavirus outbreak a global pandemic on March 11, 2020. As of May 21, 2020, more than 5,000,000 cases of COVID-19 have been confirmed, and more than 328,000 deaths related to COVID-19 have been reported globally.2 These numbers are expected to increase, due to the reproduction number (R0) of SARS-CoV-2. R0 represents the number of new infections generated by an infectious person in a totally naïve population.3 The WHO estimates that the R0 of SARS-CoV-2 is 1.95, with other estimates ranging from 1.4 to 6.49.3 To control the pathogen, the R0 needs to be brought under a value of 1.
A fundamental tool in lowering the R0 is prompt testing and isolation of those who display signs and symptoms of infection. SARS-CoV-2 is still a novel pathogen about which we know relatively little. The common symptoms of COVID-19 are now well known—including fever, fatigue, anorexia, cough, and shortness of breath—but atypical manifestations of this viral continue to be reported and described. To help clinicians across specialties and settings identify patients with possible infection, we have summarized findings from current reports on COVID-19 manifestations involving the renal, cardiac, gastrointestinal (GI), and other organ systems.
Renal
During the 2003 SARS-CoV-1 outbreak, acute kidney injury (AKI) was an uncommon complication of the infection, but early reports suggest that AKI may occur more commonly with COVID-19.4 In a study of 193 patients with laboratory-confirmed COVID-19 treated in 3 Chinese hospitals, 59% presented with proteinuria, 44% with hematuria, 14% with increased blood urea nitrogen, and 10% with increased levels of serum creatinine.4 These markers, indicative of AKI, may be associated with increased mortality. Among this cohort, those with AKI had a mortality risk 5.3 times higher than those who did not have AKI.4 The pathophysiology of renal disease in COVID-19 may be related to dehydration or inflammatory mediators, causing decreased renal perfusion and cytokine storm, but evidence also suggests that SARS-CoV-2 is able to directly infect kidney cells.5 The virus infects cells by using angiotensin-converting enzyme 2 (ACE2) on the cell membrane as a cell entry receptor; ACE2 is expressed on the kidney, heart, and GI cells, and this may allow SARS-CoV-2 to directly infect and damage these organs. Other potential mechanisms of renal injury include overproduction of proinflammatory cytokines and administration of nephrotoxic drugs. No matter the mechanism, however, increased serum creatinine and blood urea nitrogen correlate with an increased likelihood of requiring intensive care unit (ICU) admission.6 Therefore, clinicians should carefully monitor renal function in patients with COVID-19.
Cardiac
In a report of 138 Chinese patients hospitalized for COVID-19, 36 required ICU admission: 44.4% of these had arrhythmias and 22.2% had developed acute cardiac injury.6 In addition, the cardiac cell injury biomarker troponin I was more likely to be elevated in ICU patients.6 A study of 21 patients admitted to the ICU in Washington State found elevated levels of brain natriuretic peptide.7 These biomarkers reflect the presence of myocardial stress, but do not necessarily indicate direct myocardial infection. Case reports of fulminant myocarditis in those with COVID-19 have begun to surface, however.8,9 An examination of 68 deaths in persons with COVID-19 concluded that 7% were caused by myocarditis with circulatory failure.10
The pathophysiology of myocardial injury in COVID-19 is likely multifactorial. This includes increased inflammatory mediators, hypoxemia, and metabolic changes that can directly damage myocardial tissue. These factors can also exacerbate comorbid conditions, such as coronary artery disease, leading to ischemia and dysfunction of preexisting electrical conduction abnormalities. However, pathologic evidence of myocarditis and the presence of the ACE2 receptor, which may be a mediator of cardiac function, on cardiac muscle cells suggest that SARS-CoV-2 is capable of directly infecting and damaging myocardial cells. Other proposed mechanisms include infection-mediated downregulation of ACE2, causing cardiac dysfunction, or thrombus formation.11 Although respiratory failure is the most common source of advanced illness in COVID-19 patients, myocarditis and arrhythmias can be life-threatening manifestations of the disease.
Gastrointestinal
As noted, ACE2 is expressed in the GI tract. In 73 patients hospitalized for COVID-19, 53.4% tested positive for SARS-CoV-2 RNA in stool, and 23.4% continued to have RNA-positive stool samples even after their respiratory samples tested negative.12 These findings suggest the potential for SARS-CoV-2 to spread through fecal-oral transmission in those who are asymptomatic, pre-symptomatic, or symptomatic. This mode of transmission has yet to be determined conclusively, and more research is needed. However, GI symptoms have been reported in persons with COVID-19. Among 138 hospitalized patients, 10.1% had complaints of diarrhea and nausea and 3.6% reported vomiting.6 Those who reported nausea and diarrhea noted that they developed these symptoms 1 to 2 days before they developed fever.6 Also, among a cohort of 1099 Chinese patients with COVID-19, 3.8% complained of diarrhea.13 Although diarrhea does not occur in a majority of patients, GI complaints, such as nausea, vomiting, or diarrhea, should raise clinical suspicion for COVID-19, and in known areas of active transmission, testing of patients with GI symptoms is likely warranted.
Ocular
Ocular manifestations of COVID-19 are now being described, and should be taken into consideration when examining a patient. In a study of 38 patients with COVID-19 from Hubei province, China, 31.6% had ocular findings consistent with conjunctivitis, including conjunctival hyperemia, chemosis, epiphora, and increased ocular secretions.14 SARS-CoV-2 was detected in conjunctival and nasopharyngeal samples in 2 patients from this cohort. Conjunctival congestion was reported in a cohort of 1099 patients with COVID-19 treated at multiple centers throughout China, but at a much lower incidence, approximately 0.8%.13 Because SARS-CoV-2 can cause conjunctival disease and has been detected in samples from the external surface of the eye, it appears the virus is transmissible from tears or contact with the eye itself.
Neurologic
Common reported neurologic symptoms include dizziness, headache, impaired consciousness, ataxia, and cerebrovascular events. In a cohort of 214 patients from Wuhan, China, 36.4% had some form of neurological insult.15 These symptoms were more common in those with severe illness (P = 0.02).15 Two interesting neurologic symptoms that have been described are anosmia (loss of smell) and ageusia (loss of taste), which are being found primarily in tandem. It is still unclear how many people with COVID-19 are experiencing these symptoms, but a report from Italy estimates 19.4% of 320 patients examined had chemosensory dysfunction.16 The aforementioned report from Wuhan, China, found that 5.1% had anosmia and 5.6% had ageusia.15 The presence of anosmia/ageusia in some patients suggests that SARS-CoV-2 may enter the central nervous system (CNS) through a retrograde neuronal route.15 In addition, a case report from Japan described a 24-year-old man who presented with meningitis/encephalitis and had SARS-CoV-2 RNA present in his cerebrospinal fluid, showing that SARS-CoV-2 can penetrate into the CNS.17
SARS-CoV-2 may also have an association with Guillain–Barré syndrome, as this condition was reported in 5 patients from 3 hospitals in Northern Italy.18 The symptoms of Guillain–Barré syndrome presented 5 to 10 days after the typical COVID-19 symptoms, and evolved over 36 hours to 4 days afterwards. Four of the 5 patients experienced flaccid tetraparesis or tetraplegia, and 3 required mechanical ventilation.18
Another possible cause of neurologic injury in COVID-19 is damage to endothelial cells in cerebral blood vessels, causing thrombus formation and possibly increasing the risk of acute ischemic stroke.15,19 Supporting this mechanism of injury, significantly lower platelet counts were noted in patients with CNS symptoms (P = 0.005).15 Other hematological impacts of COVID-19 have been reported, particularly hypercoagulability, as evidenced by elevated D-dimer levels.13,20 This hypercoagulable state is linked to overproduction of proinflammatory cytokines (cytokine storm), leading to dysregulation of coagulation pathways and reduced concentrations of anticoagulants, such as protein C, antithrombin III, and tissue factor pathway inhibitor.21
Cutaneous
Cutaneous findings emerging in persons with COVID-19 demonstrate features of small-vessel and capillary occlusion, including erythematous skin eruptions and petechial rash. One report from Italy noted that 20.4% of patients with COVID-19 (n = 88) had a cutaneous finding, with a cutaneous manifestation developing in 8 at the onset of illness and in 10 following hospital admission.22 Fourteen patients had an erythematous rash, primarily on the trunk, with 3 patients having a diffuse urticarial appearing rash, and 1 patient developing vesicles.22 The severity of illness did not appear to correlate with the cutaneous manifestation, and the lesions healed within a few days.
One case report described a patient from Bangkok who was thought to be suffering from dengue fever, but was found to have SARS-CoV-2 infection. He initially presented with skin rash and petechiae, and later developed respiratory disease.23
Other dermatologic findings of COVID-19 resemble chilblains disease, colloquially referred to as “COVID toes.” Two women, 27 and 35 years old, presented to a dermatology clinic in Qatar with a chief complaint of skin rash, described as red-purple papules on the dorsal aspects of the fingers bilaterally.22 Both patients had an unremarkable medical and drug history, but recent travel to the United Kingdom dictated SARS-CoV-2 screening, which was positive.24 An Italian case report describes a 23-year-old man who tested positive for SARS-CoV-2 and had violaceous plaques on an erythematous background on his feet, without any lesions on his hands.25 Since chilblains is less common in the warmer months and these events correspond with the COVID-19 pandemic, SARS-CoV-2 infection is the suspected etiology. The pathophysiology of these lesions is unclear, and more research is needed. As more data become available, we may see cutaneous manifestations in patients with COVID-19 similar to those commonly reported with other viral infectious processes.
Musculoskeletal
Of 138 patients hospitalized in Wuhan, China, for COVID-19, 34.8% presented with myalgia; the presence of myalgia does not appear to be correlated with an increased likelihood of ICU admission.6 Myalgia or arthralgia was also reported in 14.9% among the cohort of 1099 COVID-19 patients in China.13 These musculoskeletal symptoms are described among large muscle groups found in the extremities, trunk, and back, and should raise suspicion in patients who present with other signs and symptoms concerning for COVID-19.
Conclusion
Evidence regarding atypical features of COVID-19 is accumulating. SARS-CoV-2 can infect a human cells that express the ACE2 receptor, which would allow for a broad spectrum of illnesses. The potential for SARS-CoV-2 to induce a hypercoagulable state allows it to indirectly damage various organ systems,20 leading to cerebrovascular disease, myocardial injury, and a chilblain-like rash. Clinicians must be aware of these unique features, as early recognition of persons who present with COVID-19 will allow for prompt testing, institution of infection control and isolation practices, and treatment, as needed, among those infected. Also, this is a pandemic involving a novel virus affecting different populations throughout the world, and these signs and symptoms may occur with varying frequency across populations. Therefore, it is important to keep differentials broad when assessing patients with a clinical illness that may indeed be COVID-19.
Corresponding author: Norman L. Beatty, MD, [email protected].
Financial disclosures: None.
From the University of Florida College of Medicine, Division of Infectious Diseases and Global Medicine, Gainesville, FL.
Abstract
- Objective: To review current reports on atypical manifestations of coronavirus disease 2019 (COVID-19).
- Methods: Review of the literature.
- Results: Evidence regarding atypical features of COVID-19 is accumulating. SARS-CoV-2 can infect human cells that express the angiotensin-converting enzyme 2 receptor, which would allow for a broad spectrum of illnesses affecting the renal, cardiac, and gastrointestinal organ systems. Neurologic, cutaneous, and musculoskeletal manifestations have also been reported. The potential for SARS-CoV-2 to induce a hypercoagulable state provides another avenue for the virus to indirectly damage various organ systems, as evidenced by reports of cerebrovascular disease, myocardial injury, and a chilblain-like rash in patients with COVID-19.
- Conclusion: Because the signs and symptoms of COVID-19 may occur with varying frequency across populations, it is important to keep differentials broad when assessing patients with a clinical illness that may indeed be COVID-19.
Keywords: coronavirus; severe acute respiratory syndrome coronavirus-2; SARS-CoV-2; pandemic.
Coronavirus disease 2019 (COVID-19), the syndrome caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), was first reported in Wuhan, China, in early December 2019.1 Since then, the virus has spread quickly around the world, with the World Health Organization (WHO) declaring the coronavirus outbreak a global pandemic on March 11, 2020. As of May 21, 2020, more than 5,000,000 cases of COVID-19 have been confirmed, and more than 328,000 deaths related to COVID-19 have been reported globally.2 These numbers are expected to increase, due to the reproduction number (R0) of SARS-CoV-2. R0 represents the number of new infections generated by an infectious person in a totally naïve population.3 The WHO estimates that the R0 of SARS-CoV-2 is 1.95, with other estimates ranging from 1.4 to 6.49.3 To control the pathogen, the R0 needs to be brought under a value of 1.
A fundamental tool in lowering the R0 is prompt testing and isolation of those who display signs and symptoms of infection. SARS-CoV-2 is still a novel pathogen about which we know relatively little. The common symptoms of COVID-19 are now well known—including fever, fatigue, anorexia, cough, and shortness of breath—but atypical manifestations of this viral continue to be reported and described. To help clinicians across specialties and settings identify patients with possible infection, we have summarized findings from current reports on COVID-19 manifestations involving the renal, cardiac, gastrointestinal (GI), and other organ systems.
Renal
During the 2003 SARS-CoV-1 outbreak, acute kidney injury (AKI) was an uncommon complication of the infection, but early reports suggest that AKI may occur more commonly with COVID-19.4 In a study of 193 patients with laboratory-confirmed COVID-19 treated in 3 Chinese hospitals, 59% presented with proteinuria, 44% with hematuria, 14% with increased blood urea nitrogen, and 10% with increased levels of serum creatinine.4 These markers, indicative of AKI, may be associated with increased mortality. Among this cohort, those with AKI had a mortality risk 5.3 times higher than those who did not have AKI.4 The pathophysiology of renal disease in COVID-19 may be related to dehydration or inflammatory mediators, causing decreased renal perfusion and cytokine storm, but evidence also suggests that SARS-CoV-2 is able to directly infect kidney cells.5 The virus infects cells by using angiotensin-converting enzyme 2 (ACE2) on the cell membrane as a cell entry receptor; ACE2 is expressed on the kidney, heart, and GI cells, and this may allow SARS-CoV-2 to directly infect and damage these organs. Other potential mechanisms of renal injury include overproduction of proinflammatory cytokines and administration of nephrotoxic drugs. No matter the mechanism, however, increased serum creatinine and blood urea nitrogen correlate with an increased likelihood of requiring intensive care unit (ICU) admission.6 Therefore, clinicians should carefully monitor renal function in patients with COVID-19.
Cardiac
In a report of 138 Chinese patients hospitalized for COVID-19, 36 required ICU admission: 44.4% of these had arrhythmias and 22.2% had developed acute cardiac injury.6 In addition, the cardiac cell injury biomarker troponin I was more likely to be elevated in ICU patients.6 A study of 21 patients admitted to the ICU in Washington State found elevated levels of brain natriuretic peptide.7 These biomarkers reflect the presence of myocardial stress, but do not necessarily indicate direct myocardial infection. Case reports of fulminant myocarditis in those with COVID-19 have begun to surface, however.8,9 An examination of 68 deaths in persons with COVID-19 concluded that 7% were caused by myocarditis with circulatory failure.10
The pathophysiology of myocardial injury in COVID-19 is likely multifactorial. This includes increased inflammatory mediators, hypoxemia, and metabolic changes that can directly damage myocardial tissue. These factors can also exacerbate comorbid conditions, such as coronary artery disease, leading to ischemia and dysfunction of preexisting electrical conduction abnormalities. However, pathologic evidence of myocarditis and the presence of the ACE2 receptor, which may be a mediator of cardiac function, on cardiac muscle cells suggest that SARS-CoV-2 is capable of directly infecting and damaging myocardial cells. Other proposed mechanisms include infection-mediated downregulation of ACE2, causing cardiac dysfunction, or thrombus formation.11 Although respiratory failure is the most common source of advanced illness in COVID-19 patients, myocarditis and arrhythmias can be life-threatening manifestations of the disease.
Gastrointestinal
As noted, ACE2 is expressed in the GI tract. In 73 patients hospitalized for COVID-19, 53.4% tested positive for SARS-CoV-2 RNA in stool, and 23.4% continued to have RNA-positive stool samples even after their respiratory samples tested negative.12 These findings suggest the potential for SARS-CoV-2 to spread through fecal-oral transmission in those who are asymptomatic, pre-symptomatic, or symptomatic. This mode of transmission has yet to be determined conclusively, and more research is needed. However, GI symptoms have been reported in persons with COVID-19. Among 138 hospitalized patients, 10.1% had complaints of diarrhea and nausea and 3.6% reported vomiting.6 Those who reported nausea and diarrhea noted that they developed these symptoms 1 to 2 days before they developed fever.6 Also, among a cohort of 1099 Chinese patients with COVID-19, 3.8% complained of diarrhea.13 Although diarrhea does not occur in a majority of patients, GI complaints, such as nausea, vomiting, or diarrhea, should raise clinical suspicion for COVID-19, and in known areas of active transmission, testing of patients with GI symptoms is likely warranted.
Ocular
Ocular manifestations of COVID-19 are now being described, and should be taken into consideration when examining a patient. In a study of 38 patients with COVID-19 from Hubei province, China, 31.6% had ocular findings consistent with conjunctivitis, including conjunctival hyperemia, chemosis, epiphora, and increased ocular secretions.14 SARS-CoV-2 was detected in conjunctival and nasopharyngeal samples in 2 patients from this cohort. Conjunctival congestion was reported in a cohort of 1099 patients with COVID-19 treated at multiple centers throughout China, but at a much lower incidence, approximately 0.8%.13 Because SARS-CoV-2 can cause conjunctival disease and has been detected in samples from the external surface of the eye, it appears the virus is transmissible from tears or contact with the eye itself.
Neurologic
Common reported neurologic symptoms include dizziness, headache, impaired consciousness, ataxia, and cerebrovascular events. In a cohort of 214 patients from Wuhan, China, 36.4% had some form of neurological insult.15 These symptoms were more common in those with severe illness (P = 0.02).15 Two interesting neurologic symptoms that have been described are anosmia (loss of smell) and ageusia (loss of taste), which are being found primarily in tandem. It is still unclear how many people with COVID-19 are experiencing these symptoms, but a report from Italy estimates 19.4% of 320 patients examined had chemosensory dysfunction.16 The aforementioned report from Wuhan, China, found that 5.1% had anosmia and 5.6% had ageusia.15 The presence of anosmia/ageusia in some patients suggests that SARS-CoV-2 may enter the central nervous system (CNS) through a retrograde neuronal route.15 In addition, a case report from Japan described a 24-year-old man who presented with meningitis/encephalitis and had SARS-CoV-2 RNA present in his cerebrospinal fluid, showing that SARS-CoV-2 can penetrate into the CNS.17
SARS-CoV-2 may also have an association with Guillain–Barré syndrome, as this condition was reported in 5 patients from 3 hospitals in Northern Italy.18 The symptoms of Guillain–Barré syndrome presented 5 to 10 days after the typical COVID-19 symptoms, and evolved over 36 hours to 4 days afterwards. Four of the 5 patients experienced flaccid tetraparesis or tetraplegia, and 3 required mechanical ventilation.18
Another possible cause of neurologic injury in COVID-19 is damage to endothelial cells in cerebral blood vessels, causing thrombus formation and possibly increasing the risk of acute ischemic stroke.15,19 Supporting this mechanism of injury, significantly lower platelet counts were noted in patients with CNS symptoms (P = 0.005).15 Other hematological impacts of COVID-19 have been reported, particularly hypercoagulability, as evidenced by elevated D-dimer levels.13,20 This hypercoagulable state is linked to overproduction of proinflammatory cytokines (cytokine storm), leading to dysregulation of coagulation pathways and reduced concentrations of anticoagulants, such as protein C, antithrombin III, and tissue factor pathway inhibitor.21
Cutaneous
Cutaneous findings emerging in persons with COVID-19 demonstrate features of small-vessel and capillary occlusion, including erythematous skin eruptions and petechial rash. One report from Italy noted that 20.4% of patients with COVID-19 (n = 88) had a cutaneous finding, with a cutaneous manifestation developing in 8 at the onset of illness and in 10 following hospital admission.22 Fourteen patients had an erythematous rash, primarily on the trunk, with 3 patients having a diffuse urticarial appearing rash, and 1 patient developing vesicles.22 The severity of illness did not appear to correlate with the cutaneous manifestation, and the lesions healed within a few days.
One case report described a patient from Bangkok who was thought to be suffering from dengue fever, but was found to have SARS-CoV-2 infection. He initially presented with skin rash and petechiae, and later developed respiratory disease.23
Other dermatologic findings of COVID-19 resemble chilblains disease, colloquially referred to as “COVID toes.” Two women, 27 and 35 years old, presented to a dermatology clinic in Qatar with a chief complaint of skin rash, described as red-purple papules on the dorsal aspects of the fingers bilaterally.22 Both patients had an unremarkable medical and drug history, but recent travel to the United Kingdom dictated SARS-CoV-2 screening, which was positive.24 An Italian case report describes a 23-year-old man who tested positive for SARS-CoV-2 and had violaceous plaques on an erythematous background on his feet, without any lesions on his hands.25 Since chilblains is less common in the warmer months and these events correspond with the COVID-19 pandemic, SARS-CoV-2 infection is the suspected etiology. The pathophysiology of these lesions is unclear, and more research is needed. As more data become available, we may see cutaneous manifestations in patients with COVID-19 similar to those commonly reported with other viral infectious processes.
Musculoskeletal
Of 138 patients hospitalized in Wuhan, China, for COVID-19, 34.8% presented with myalgia; the presence of myalgia does not appear to be correlated with an increased likelihood of ICU admission.6 Myalgia or arthralgia was also reported in 14.9% among the cohort of 1099 COVID-19 patients in China.13 These musculoskeletal symptoms are described among large muscle groups found in the extremities, trunk, and back, and should raise suspicion in patients who present with other signs and symptoms concerning for COVID-19.
Conclusion
Evidence regarding atypical features of COVID-19 is accumulating. SARS-CoV-2 can infect a human cells that express the ACE2 receptor, which would allow for a broad spectrum of illnesses. The potential for SARS-CoV-2 to induce a hypercoagulable state allows it to indirectly damage various organ systems,20 leading to cerebrovascular disease, myocardial injury, and a chilblain-like rash. Clinicians must be aware of these unique features, as early recognition of persons who present with COVID-19 will allow for prompt testing, institution of infection control and isolation practices, and treatment, as needed, among those infected. Also, this is a pandemic involving a novel virus affecting different populations throughout the world, and these signs and symptoms may occur with varying frequency across populations. Therefore, it is important to keep differentials broad when assessing patients with a clinical illness that may indeed be COVID-19.
Corresponding author: Norman L. Beatty, MD, [email protected].
Financial disclosures: None.
1. WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020 [press release]. World Health Organization; March 11, 2020.
2. Coronavirus COVID-19 Global Cases by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. Johns Hopkins CSSE. https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6 Accessed May 15, 2020.
3. Liu Y, Gayle AA, Wilder-Smith A, Rocklöv J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J Travel Med. 2020;27(2):taaa021. doi:10.1093/jtm/taaa021
4. Li Z, Wu M, Guo J, et al. Caution on kidney dysfunctions of 2019-nCoV patients. medRxiv preprint. doi: 10.1101/2020.02.08.20021212
5. Li W, Moore MJ, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450-454. doi: 10.1038/nature02145.
6. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. doi:10.1001/jama.2020.1585
7. Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323:1612‐1614. doi:10.1001/jama.2020.4326
8. Chen C, Zhou Y, Wang DW. SARS-CoV-2: a potential novel etiology of fulminant myocarditis. Herz. 2020;45:230-232. doi: 10.1007/s00059-020-04909-z
9. Hu H, Ma F, Wei X, Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. 2020 Mar 16;ehaa190. doi: 10.1093/eurheartj/ehaa190
10. Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846-848. doi:10.1007/s00134-020-05991-x
11. Akhmerov A, Marban E. COVID-19 and the heart. Circ Res. 2020;126:1443-1455. doi:10.1161/CIRCRESAHA.120.317055
12. Xiao F, Tang M, Zheng X, et al. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158:1831-1833. doi: 10.1053/j.gastro.2020.02.055
13. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1078-1720. doi: 10.1056/NEJMoa2002032
14. Wu P, Duan F, Luo C, et al. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020 Mar 31;e201291. doi: 10.1001/jamaophthalmol.2020.1291
15. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020 Apr 10. doi: 10.1001/jamaneurol.2020.1127
16. Vaira LA, Salzano G, Deiana G, De Riu G. Anosmia and ageusia: common findings in COVID-19 patients. Laryngoscope. 2020 Apr 1. doi: 10.1002/lary.28692
17. Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS-coronavirus-2. Int J Infect Dis. 2020;94:55-58. doi: 10.1016/j.ijid.2020.03.062
18. Toscano G, Palmerini F, Ravaglia S, et al. Guillain–Barré syndrome associated with SARS-CoV-2. N Engl J Med. 2020 Apr 17;NEJMc2009191. doi:10.1056/nejmc2009191
19. Dafer RM, Osteraas ND, Biller J. Acute stroke care in the coronavirus disease 2019 pandemic. J Stroke Cerebrovascular Dis. 2020 Apr 17:104881. doi: 10.1016/j.jstrokecerebrovasdis.2020.104881
20. Terpos E, Ntanasis-Stathopoulos I, Elalamy I, et al. Hematological findings and complications of COVID-19. Am J Hematol. 2020;10.1002/ajh.25829. doi:10.1002/ajh.25829
21. Jose RJ, Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med. 2020;S2213-2600(20)30216-2. doi:10.1016/S2213-2600(20)30216-2
22. Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020 Mar 26. doi: 10.1111/jdv.16387
23. Joob B, Wiwanitkit V. COVID-19 can present with a rash and be mistaken for dengue. J Am Acad Dermatol. 2020;82(5):e177. doi: 10.1016/j.jaad.2020.03.036
24. Alramthan A, Aldaraji W. A Case of COVID‐19 presenting in clinical picture resembling chilblains disease. First report from the Middle East. Clin Exp Dermatol. 2020 Apr 17. doi: 10.1111/ced.14243
25. Kolivras A, Dehavay F, Delplace D, et al. Coronavirus (COVID-19) infection–induced chilblains: a case report with histopathologic findings. JAAD Case Rep. 2020 Apr 18. doi: 10.1016/j.jdcr.2020.04.011
1. WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020 [press release]. World Health Organization; March 11, 2020.
2. Coronavirus COVID-19 Global Cases by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. Johns Hopkins CSSE. https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6 Accessed May 15, 2020.
3. Liu Y, Gayle AA, Wilder-Smith A, Rocklöv J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J Travel Med. 2020;27(2):taaa021. doi:10.1093/jtm/taaa021
4. Li Z, Wu M, Guo J, et al. Caution on kidney dysfunctions of 2019-nCoV patients. medRxiv preprint. doi: 10.1101/2020.02.08.20021212
5. Li W, Moore MJ, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450-454. doi: 10.1038/nature02145.
6. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. doi:10.1001/jama.2020.1585
7. Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323:1612‐1614. doi:10.1001/jama.2020.4326
8. Chen C, Zhou Y, Wang DW. SARS-CoV-2: a potential novel etiology of fulminant myocarditis. Herz. 2020;45:230-232. doi: 10.1007/s00059-020-04909-z
9. Hu H, Ma F, Wei X, Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. 2020 Mar 16;ehaa190. doi: 10.1093/eurheartj/ehaa190
10. Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846-848. doi:10.1007/s00134-020-05991-x
11. Akhmerov A, Marban E. COVID-19 and the heart. Circ Res. 2020;126:1443-1455. doi:10.1161/CIRCRESAHA.120.317055
12. Xiao F, Tang M, Zheng X, et al. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158:1831-1833. doi: 10.1053/j.gastro.2020.02.055
13. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1078-1720. doi: 10.1056/NEJMoa2002032
14. Wu P, Duan F, Luo C, et al. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020 Mar 31;e201291. doi: 10.1001/jamaophthalmol.2020.1291
15. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020 Apr 10. doi: 10.1001/jamaneurol.2020.1127
16. Vaira LA, Salzano G, Deiana G, De Riu G. Anosmia and ageusia: common findings in COVID-19 patients. Laryngoscope. 2020 Apr 1. doi: 10.1002/lary.28692
17. Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS-coronavirus-2. Int J Infect Dis. 2020;94:55-58. doi: 10.1016/j.ijid.2020.03.062
18. Toscano G, Palmerini F, Ravaglia S, et al. Guillain–Barré syndrome associated with SARS-CoV-2. N Engl J Med. 2020 Apr 17;NEJMc2009191. doi:10.1056/nejmc2009191
19. Dafer RM, Osteraas ND, Biller J. Acute stroke care in the coronavirus disease 2019 pandemic. J Stroke Cerebrovascular Dis. 2020 Apr 17:104881. doi: 10.1016/j.jstrokecerebrovasdis.2020.104881
20. Terpos E, Ntanasis-Stathopoulos I, Elalamy I, et al. Hematological findings and complications of COVID-19. Am J Hematol. 2020;10.1002/ajh.25829. doi:10.1002/ajh.25829
21. Jose RJ, Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med. 2020;S2213-2600(20)30216-2. doi:10.1016/S2213-2600(20)30216-2
22. Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020 Mar 26. doi: 10.1111/jdv.16387
23. Joob B, Wiwanitkit V. COVID-19 can present with a rash and be mistaken for dengue. J Am Acad Dermatol. 2020;82(5):e177. doi: 10.1016/j.jaad.2020.03.036
24. Alramthan A, Aldaraji W. A Case of COVID‐19 presenting in clinical picture resembling chilblains disease. First report from the Middle East. Clin Exp Dermatol. 2020 Apr 17. doi: 10.1111/ced.14243
25. Kolivras A, Dehavay F, Delplace D, et al. Coronavirus (COVID-19) infection–induced chilblains: a case report with histopathologic findings. JAAD Case Rep. 2020 Apr 18. doi: 10.1016/j.jdcr.2020.04.011
Respiratory particles generated by speech can remain airborne for up to 14 minutes
Stadnytskyi and colleagues explored the size of droplets created by speech using a highly sensitive laser system. They reported in PNAS that speaking resulted in the generation of a high number of medium-sized droplets (10- to 100-µm in diameter). Under the conditions of their experiment (27% humidity and 23° C) they reported that speech probably generates droplets that originate at a size of 12 to 21 µm in diameter and quickly dehydrate to an estimated diameter of 4 µm. The 4 µm-sized particles had a falling rate of only 0.06 cm·s−1 and remained airborne for 8 to 14 minutes.1
As reported by Hamner and colleagues, on March 10, 2020, 61 persons attended a 2.5-hour choir practice. One choir member had symptoms of an upper respiratory infection that began on March 7. Eventually that choir member tested positive for SARS-CoV-2. Of the 60 remaining persons, 52 (86.7%) eventually developed an upper respiratory illness. In total, 33 cases of SARS-CoV-2 were confirmed by nucleic acid testing and 20 probable cases were diagnosed (these individuals declined testing). The choir attendees developed symptoms at a median of 3 days following the practice, with a range of 1 to 12 days. Three of the 53 ill people were hospitalized, and two died.2
The Stadnytskyi study suggests that speech generates large respiratory droplets that dehydrate into very small droplets that may remain in the air for an extended period of time. If the SARS-CoV-2 virus were in the original large droplet, the rapid dehydration of the droplet would result in prolonged airborne presence of the virus and enhance its infectivity.
The Hamner study highlights the importance of vocalization and respiratory particles in transmitting the SARS-CoV-2 virus. For clinicians and patients, both studies support many recommendations to reduce viral transmission, including:
- all clinicians and patients need to wear face masks
- all clinicians and patients should avoid face-to-face contact if alternative approaches to communication are possible
- all clinicians and patients should avoid gathering in large groups or crowded public spaces and need to maintain physical distancing.
The COVID pandemic has dramatically changed how we practice medicine and socialize.
- Stadnytskyi V, Bax CE, Bax A, et al. The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission. PNAS. May 13, 2020. https://doi.org/10.1073/pnas.2006874117.
- Hamner L, Dubbel P, Capron I, et al. High SARS-CoV-2 attack rate following exposure at choir practice—Skagit County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69:606-610. Early release, May 12, 2020.
Stadnytskyi and colleagues explored the size of droplets created by speech using a highly sensitive laser system. They reported in PNAS that speaking resulted in the generation of a high number of medium-sized droplets (10- to 100-µm in diameter). Under the conditions of their experiment (27% humidity and 23° C) they reported that speech probably generates droplets that originate at a size of 12 to 21 µm in diameter and quickly dehydrate to an estimated diameter of 4 µm. The 4 µm-sized particles had a falling rate of only 0.06 cm·s−1 and remained airborne for 8 to 14 minutes.1
As reported by Hamner and colleagues, on March 10, 2020, 61 persons attended a 2.5-hour choir practice. One choir member had symptoms of an upper respiratory infection that began on March 7. Eventually that choir member tested positive for SARS-CoV-2. Of the 60 remaining persons, 52 (86.7%) eventually developed an upper respiratory illness. In total, 33 cases of SARS-CoV-2 were confirmed by nucleic acid testing and 20 probable cases were diagnosed (these individuals declined testing). The choir attendees developed symptoms at a median of 3 days following the practice, with a range of 1 to 12 days. Three of the 53 ill people were hospitalized, and two died.2
The Stadnytskyi study suggests that speech generates large respiratory droplets that dehydrate into very small droplets that may remain in the air for an extended period of time. If the SARS-CoV-2 virus were in the original large droplet, the rapid dehydration of the droplet would result in prolonged airborne presence of the virus and enhance its infectivity.
The Hamner study highlights the importance of vocalization and respiratory particles in transmitting the SARS-CoV-2 virus. For clinicians and patients, both studies support many recommendations to reduce viral transmission, including:
- all clinicians and patients need to wear face masks
- all clinicians and patients should avoid face-to-face contact if alternative approaches to communication are possible
- all clinicians and patients should avoid gathering in large groups or crowded public spaces and need to maintain physical distancing.
The COVID pandemic has dramatically changed how we practice medicine and socialize.
Stadnytskyi and colleagues explored the size of droplets created by speech using a highly sensitive laser system. They reported in PNAS that speaking resulted in the generation of a high number of medium-sized droplets (10- to 100-µm in diameter). Under the conditions of their experiment (27% humidity and 23° C) they reported that speech probably generates droplets that originate at a size of 12 to 21 µm in diameter and quickly dehydrate to an estimated diameter of 4 µm. The 4 µm-sized particles had a falling rate of only 0.06 cm·s−1 and remained airborne for 8 to 14 minutes.1
As reported by Hamner and colleagues, on March 10, 2020, 61 persons attended a 2.5-hour choir practice. One choir member had symptoms of an upper respiratory infection that began on March 7. Eventually that choir member tested positive for SARS-CoV-2. Of the 60 remaining persons, 52 (86.7%) eventually developed an upper respiratory illness. In total, 33 cases of SARS-CoV-2 were confirmed by nucleic acid testing and 20 probable cases were diagnosed (these individuals declined testing). The choir attendees developed symptoms at a median of 3 days following the practice, with a range of 1 to 12 days. Three of the 53 ill people were hospitalized, and two died.2
The Stadnytskyi study suggests that speech generates large respiratory droplets that dehydrate into very small droplets that may remain in the air for an extended period of time. If the SARS-CoV-2 virus were in the original large droplet, the rapid dehydration of the droplet would result in prolonged airborne presence of the virus and enhance its infectivity.
The Hamner study highlights the importance of vocalization and respiratory particles in transmitting the SARS-CoV-2 virus. For clinicians and patients, both studies support many recommendations to reduce viral transmission, including:
- all clinicians and patients need to wear face masks
- all clinicians and patients should avoid face-to-face contact if alternative approaches to communication are possible
- all clinicians and patients should avoid gathering in large groups or crowded public spaces and need to maintain physical distancing.
The COVID pandemic has dramatically changed how we practice medicine and socialize.
- Stadnytskyi V, Bax CE, Bax A, et al. The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission. PNAS. May 13, 2020. https://doi.org/10.1073/pnas.2006874117.
- Hamner L, Dubbel P, Capron I, et al. High SARS-CoV-2 attack rate following exposure at choir practice—Skagit County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69:606-610. Early release, May 12, 2020.
- Stadnytskyi V, Bax CE, Bax A, et al. The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission. PNAS. May 13, 2020. https://doi.org/10.1073/pnas.2006874117.
- Hamner L, Dubbel P, Capron I, et al. High SARS-CoV-2 attack rate following exposure at choir practice—Skagit County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69:606-610. Early release, May 12, 2020.
Managing Trichomonas vaginalis infections
CASE Woman with malodorous vaginal discharge
A 26-year-old nulligravid woman with 2 current sexual partners requests evaluation because she has a yellow-green frothy vaginal discharge that is slightly malodorous. One of her sexual partners has noted a similar discharge from his urethra. On physical examination, the clinician notes that the patient’s discharge emanates from the vaginal mucosa, and the exocervix has multiple punctate hemorrhages. Considerations in this case include:
- What is the most likely diagnosis?
- How should this patient be evaluated and treated?
- Should the patient’s sexual partners be treated?
This clinical scenario is most consistent with a trichomonas infection, although other conditions, including bacterial vaginosis, gonorrhea, and chlamydia infection, must be considered in the differential diagnosis.
In this article, we examine the microbiology, epidemiology, clinical manifestations, and diagnosis and treatment of this common sexually transmitted infection (STI).
The causative microbe
Trichomonas vaginalis is a free-living flagellated protozoan that accounts for almost half of all nonviral STIs globally. It has a predilection for the mucosal epithelium of the genitourinary tract, including the vagina and urethra. Humans are the only known host for T vaginalis. The infection is transmitted through sexual intercourse, and the organism reproduces through binary fission in the lower genital tract of women and in the urethra and prostate of men.
This anaerobic trophozoite has 4 flagella anteriorly and 1 flagellum that projects posteriorly, with an undulating membrane that gives its characteristic motile appearance on saline microscopy.1
T vaginalis infection causes major mechanical stress on epithelial cells, which results in disruption of the plasma cell membrane and, ultimately, cell death. The necrotic cell fragments are then phagocytosed by trichomonads, thus accelerating the infection.2
Groups at risk
Trichomonal infections are not reportable to public health authorities, which makes assessing the true prevalence of infection difficult.
The World Health Organization estimated the incidence of infection to be more than 156 million cases globally in 2016, with a prevalence of 110.4 million people at any one time.3
The 2013-2014 National Health and Nutrition Examination Survey tested 4,057 men and women aged 18 to 59 years for T vaginalis and found a prevalence of 0.5% among men and 1.8% among women.4 The prevalence increased with age. There was a disproportionate burden of trichomonas infections in the non-Hispanic black population, with 4.2% of black men and 8.9% of black women affected.4
Targeted screening of urogenital samples for T vaginalis in a population of US women undergoing Chlamydia trachomatis/Neisseria gonorrhoeae screening demonstrated prevalence rates of 8.7%, 6.7%, and 1.7% for T vaginalis, C trachomatis, and N gonorrhoeae, respectively.5
Differences in prevalence estimates may be due to differences in the varying sensitivity of each testing modality and patient populations. In one study, nucleic acid amplification testing (NAAT) for T vaginalis detected rates as high as 11.3% in women and 6.1% in men undergoing evaluations at STI clinics.6
Continue to: Clinical manifestations of infection...
Clinical manifestations of infection
Most cases of T vaginalis remain in an asymptomatic carrier state, with up to 85% of women and 77% of men reporting no clinical symptoms.1 However, approximately one-third of asymptomatic carriers will experience symptoms within 6 months of infection acquisition. This latency in appearance of clinical symptoms certainly contributes to the high transmission rate of T vaginalis.
Infected men may experience purulent urethritis, dysuria, and postcoital pruritus. Common clinical symptoms in women include abnormal vaginal discharge that may be malodorous, purulent, thin, frothy, and yellow-green, as well as symptoms of dyspareunia and vulvar irritation. Punctate hemorrhages in the cervix (colpitis macularis) and vaginal walls (macular vaginitis) give the characteristic “strawberry appearance,” but these findings are seen in only 2% of affected women.7
Complications in ObGyn patients
Although T vaginalis once was regarded as more of an annoyance than a public health issue, awareness of the infection’s ramifications has increased in recent years. Because of these complications, treatment of both symptomatic and asymptomatic patients is clearly indicated.
Complications of trichomonal infection in men include balanoposthitis, epididymitis, prostatitis, urethritis, and infertility.7 In women, complications include infections of the adnexa, endometrium, and vestibular glands, as well as cervical neoplasia and increased co-infection rates with other STIs, such as bacterial vaginosis, chlamydia infection, gonorrhea, syphilis, and herpes simplex virus type 2.1
Infection in pregnancy. Adverse outcomes in pregnant women with T vaginalis infections at mid-gestation include low birth weight, preterm premature rupture of membranes, preterm delivery, and postpartum endometritis.8 A disproportionately larger share of the low birth weight rate associated with T vaginalis infections occurs in black women compared with white and Hispanic women.8 Perinatal transmission to newborns can cause fever; respiratory difficulties; urinary tract infections; nasal discharge; and, in female infants, vaginal discharge.9,10
Co-infection concerns. The increased rate of co-infection with human immunodeficiency virus type 1 (HIV-1) and T vaginalis is a major concern.11 One study found a higher concentration of HIV-1 in semen samples from men with T vaginalis and symptomatic urethritis.12 Further, T vaginalis was found in 17.4% of women with HIV screened at a public clinic in California, with almost 38% of black women affected.13 Trichomoniasis can increase the risk of HIV-1 acquisition by 1.52-fold (95% confidence interval, 1.04- to 2.24-fold), pointing toward a potential amplifying effect of T vaginalis on HIV transmission rates.14 This association may be based at least in part on the organism’s ability to cause microulcerations in the genital and urinary tract epithelium, thus creating pathways for other microorganisms to enter the vascular system.
Making the diagnosis
The nonspecific symptoms of T vaginalis create a wide differential to consider. Vaginal discharge may be due to bacterial vaginosis, vulvovaginal candidiasis, physiologic discharge, atrophy, and nonspecific inflammation. The presence of malodorous and discolored discharge increases the likelihood of bacterial vaginosis or T vaginalis infection. Pruritus often is associated with candidiasis co-infection.
The diagnosis of trichomoniasis can be confirmed in the outpatient office with the use of saline microscopy, an inexpensive test that is based on observation of motile trichomonads in a wet mount of vaginal fluid. The sensitivity of the wet mount ranges from 44% to 68% compared with culture. Culture, traditionally using Diamond’s medium, has a sensitivity of 81% to 94% and was long the gold standard; however, culture has been replaced largely by molecular and antigen testing.
Three US Food and Drug Administration (FDA)-approved NAATs for T vaginalis currently are on the market; all can detect co-infection with gonorrhea and chlamydia from the same specimen. These tests include the Aptima T vaginalis rRNA target assay (Hologic, Bedford, Massachusetts) and the BD ProbTec T vaginalis Qx (TVQ) amplified DNA assay (BD Diagnostics, Baltimore, Maryland), both of which require up to 8 hours to yield results. The Xpert T vaginalis (TV) assay (Cepheid, Sunnyvale, California) is the first NAAT that is FDA approved for use with male urine (in addition to female urine), and it yields results in 60 to 90 minutes. Sensitivity for these NAAT assays ranges from 88% to 100%.15
Point-of-care testing is preferred for rapid diagnosis and for helping the clinician provide same-visit treatment for STIs. The Solana trichomonas assay (Quidel, San Diego, California) detects T vaginalis DNA and can yield results within 40 minutes, but it requires specialized equipment for running the samples. The AmpliVue trichomonas assay (Quidel, San Diego, California) is similar to the Solana assay but it is contained within a small handheld cartridge that does not require additional equipment. Sensitivities are 92% to 98% for Solona and 90.7% to 100% for AmpliVue. The OSOM trichomonas rapid test (Sekisui, Framingham, Massachusetts) uses antigen-detection immunochromatography to provide results in 10 to 15 minutes, with 83% to 92% sensitivity and 99% specificity for vaginal specimens.15,16
Continue to: The TABLE provides a summary...
The TABLE provides a summary of the clinical performance of the various tests for T vaginalis. 15-18
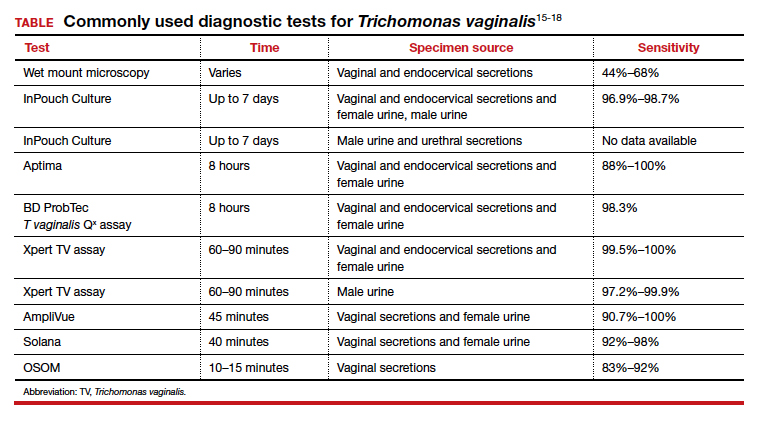
Treatment options
The 5-nitroimidazole agents, which include metronidazole and tinidazole, are the preferred agents for the treatment of trichomoniasis.
Dosing regimen. While a single oral dose of metronidazole 2 g has long been the mainstay of treatment for T vaginalis, this regimen recently has been questioned, at least in women, due to the high posttreatment positive rate of T vaginalis, which ranges from 5% to 37%.19,20 These cases may be due to reinfection by untreated sexual partners. They also may result from treatment failure, however, specifically inadequate treatment time.21 Overall, patients treated with single-dose metronidazole are 1.87 times more likely to experience treatment failure compared with those treated with a multidose regimen.19 Since many cases of T vaginalis infection are associated with bacterial vaginosis co-infection, recommending metronidazole 500 mg twice daily for 7 days is beneficial because this course provides optimal treatment for both infections.
Treatment during pregnancy. In the minds of some investigators, treatment of T vaginalis in asymptomatic pregnant women is problematic. One study demonstrated a similar to slightly increased risk of preterm delivery for metronidazole-treated patients compared with a placebo-treated group.22 Limitations of the study included atypical treatment dosing (2 doses of metronidazole 2 g given 48 hours apart at 16 to 23 weeks’ gestation and repeated at 24 to 29 weeks’ gestation) and a latency between the last dose of metronidazole and preterm delivery.22
We believe that all pregnant women, symptomatic or asymptomatic, should be treated because of the sexually transmitted nature of the infection and the probability that most asymptomatic carriers ultimately become symptomatic.
Cost of treatment. Generic oral metronidazole is very inexpensive. The approximate retail price for 14 metronidazole 500-mg tablets is $15.69 (www.goodrx.com). By contrast, a single-dose course of tinidazole (four 500-mg tablets) costs approximately $45. Accordingly, we reserve tinidazole for patients who have experienced a treatment failure with metronidazole or who cannot tolerate metronidazole.
Drug‒alcohol interaction. With both metronidazole and tinidazole, patients must abstain from alcohol during treatment and for 72 hours after completing therapy because these drugs have a disulfiram-like reaction with ethanol.
- Kissinger P. Epidemiology and treatment of trichomoniasis. Curr Infect Dis Rep. 2015;17:484.
- Midlej V, Benchimol M. Trichomonas vaginalis kills and eats—evidence for phagocytic activity as a cytopathic effect. Parasitology. 2010;137:65-76.
- Rowley J, Vander Hoorn S, Korenromp E, et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull World Health Organ. 2019;97:548–562P.
- Patel EU, Gaydos CA, Packman ZR, et al. Prevalence and correlates of Trichomonas vaginalis infection among men and women in the United States. Clin Infect Dis. 2018;67:211-217.
- Ginocchio CC, Chapin K, Smith JS, et al. Prevalence of Trichomonas vaginalis and coinfection with Chlamydia trachomatis and Neisseria gonorrhoeae in the United States as determined by the Aptima Trichomonas vaginalis nucleic acid amplification assay. J Clin Microbiol. 2012;50:2601-2608.
- Schwebke J, Merriweather A, Massingale S, et al. Screening for Trichomonas vaginalis in a large high-risk population: prevalence among men and women determined by nucleic acid amplification testing. Sex Transm Dis. 2018;45:e23-e24.
- Petrin D, Delgaty K, Bhatt R, et al. Clinical and microbiological aspects of Trichomonas vaginalis. Clin Microbiol Rev. 1998;11:300-317.
- Cotch MF, Pastorek JG II, Nugent RP, et al. Trichomonas vaginalis associated with low birth weight and preterm delivery. The Vaginal Infections and Prematurity Study Group. Sex Transm Dis. 1997;24:353-360.
- Smith LM, Wang M, Zangwill K, et al. Trichomonas vaginalis infection in a premature newborn. J Perinatol. 2002;22:502-503.
- Temesvári P, Kerekes A, Tege A, et al. Demonstration of Trichomonas vaginalis in tracheal aspirates in infants with early respiratory failure. J Matern Fetal Neonatal Med. 2002;11:347-349.
- Kissinger P, Adamski A. Trichomoniasis and HIV interactions: a review. Sex Transm Infect. 2013;89:426-433.
- Cohen MS, Hoffman IF, Royce RA, et al; AIDSCAP Malawi Research Group. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. Lancet. 1997;349:1868-1873.
- Sorvillo F, Kovacs A, Kerndt P, et al. Risk factors for trichomoniasis among women with human immunodeficiency virus (HIV) infection at a public clinic in Los Angeles County, California: implications for HIV prevention. Am J Trop Med Hyg. 1998;58:495-500.
- McClelland RS, Sangare L, Hassan WM, et al. Infection with Trichomonas vaginalis increases the risk of HIV-1 acquisition. J Infect Dis. 2007;195:698-702.
- Van Gerwen OT, Muzny CA. Recent advances in the epidemiology, diagnosis, and management of Trichomonas vaginalis infection. F1000Res. 2019;8:F1000 Faculty Rev-1666.
- Gaydos CA, Klausner JD, Pai NP, et al. Rapid and point-of-care tests for the diagnosis of Trichomonas vaginalis in women and men. Sex Transm Infect. 2017;93(S4):S31-S35.
- Rivers CA, Muzny CA, Schwebke JR. Diagnostic rates differ on the basis of the number of read days with the use of the InPouch culture system for Trichomonas vaginalis screening. J Clin Microbiol. 2013;51:3875-3876.
- Van Der Pol B, Williams JA, Taylor SN, et al. Detection of Trichomonas vaginalis DNA by use of self-obtained vaginal swabs with the BD ProbeTec Qx assay on the BD Viper System. J Clin Microbiol. 2014;52:885-889.
- Howe K, Kissinger P. Single-dose compared with multidose metronidazole for the treatment of trichomoniasis in women: a meta-analysis. Sex Transm Dis. 2017;44:29-34.
- Duff P. Should the length of treatment for trichomoniasis in women be reconsidered? OBG Manag. 2017;29(3):48-49.
- Krashin JW, Koumans EH, Bradshaw-Sydnor AC, et al. Trichomonas vaginalis prevalence, incidence, risk factors and antibiotic-resistance in an adolescent population. Sex Transm Dis. 2010;37:440-444.
- Klebanoff MA, Carey JC, Hauth JC, et al; National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Failure of metronidazole to prevent preterm delivery among pregnant women with asymptomatic Trichomonas vaginalis infection. N Engl J Med. 2001;345:487-493.
CASE Woman with malodorous vaginal discharge
A 26-year-old nulligravid woman with 2 current sexual partners requests evaluation because she has a yellow-green frothy vaginal discharge that is slightly malodorous. One of her sexual partners has noted a similar discharge from his urethra. On physical examination, the clinician notes that the patient’s discharge emanates from the vaginal mucosa, and the exocervix has multiple punctate hemorrhages. Considerations in this case include:
- What is the most likely diagnosis?
- How should this patient be evaluated and treated?
- Should the patient’s sexual partners be treated?
This clinical scenario is most consistent with a trichomonas infection, although other conditions, including bacterial vaginosis, gonorrhea, and chlamydia infection, must be considered in the differential diagnosis.
In this article, we examine the microbiology, epidemiology, clinical manifestations, and diagnosis and treatment of this common sexually transmitted infection (STI).
The causative microbe
Trichomonas vaginalis is a free-living flagellated protozoan that accounts for almost half of all nonviral STIs globally. It has a predilection for the mucosal epithelium of the genitourinary tract, including the vagina and urethra. Humans are the only known host for T vaginalis. The infection is transmitted through sexual intercourse, and the organism reproduces through binary fission in the lower genital tract of women and in the urethra and prostate of men.
This anaerobic trophozoite has 4 flagella anteriorly and 1 flagellum that projects posteriorly, with an undulating membrane that gives its characteristic motile appearance on saline microscopy.1
T vaginalis infection causes major mechanical stress on epithelial cells, which results in disruption of the plasma cell membrane and, ultimately, cell death. The necrotic cell fragments are then phagocytosed by trichomonads, thus accelerating the infection.2
Groups at risk
Trichomonal infections are not reportable to public health authorities, which makes assessing the true prevalence of infection difficult.
The World Health Organization estimated the incidence of infection to be more than 156 million cases globally in 2016, with a prevalence of 110.4 million people at any one time.3
The 2013-2014 National Health and Nutrition Examination Survey tested 4,057 men and women aged 18 to 59 years for T vaginalis and found a prevalence of 0.5% among men and 1.8% among women.4 The prevalence increased with age. There was a disproportionate burden of trichomonas infections in the non-Hispanic black population, with 4.2% of black men and 8.9% of black women affected.4
Targeted screening of urogenital samples for T vaginalis in a population of US women undergoing Chlamydia trachomatis/Neisseria gonorrhoeae screening demonstrated prevalence rates of 8.7%, 6.7%, and 1.7% for T vaginalis, C trachomatis, and N gonorrhoeae, respectively.5
Differences in prevalence estimates may be due to differences in the varying sensitivity of each testing modality and patient populations. In one study, nucleic acid amplification testing (NAAT) for T vaginalis detected rates as high as 11.3% in women and 6.1% in men undergoing evaluations at STI clinics.6
Continue to: Clinical manifestations of infection...
Clinical manifestations of infection
Most cases of T vaginalis remain in an asymptomatic carrier state, with up to 85% of women and 77% of men reporting no clinical symptoms.1 However, approximately one-third of asymptomatic carriers will experience symptoms within 6 months of infection acquisition. This latency in appearance of clinical symptoms certainly contributes to the high transmission rate of T vaginalis.
Infected men may experience purulent urethritis, dysuria, and postcoital pruritus. Common clinical symptoms in women include abnormal vaginal discharge that may be malodorous, purulent, thin, frothy, and yellow-green, as well as symptoms of dyspareunia and vulvar irritation. Punctate hemorrhages in the cervix (colpitis macularis) and vaginal walls (macular vaginitis) give the characteristic “strawberry appearance,” but these findings are seen in only 2% of affected women.7
Complications in ObGyn patients
Although T vaginalis once was regarded as more of an annoyance than a public health issue, awareness of the infection’s ramifications has increased in recent years. Because of these complications, treatment of both symptomatic and asymptomatic patients is clearly indicated.
Complications of trichomonal infection in men include balanoposthitis, epididymitis, prostatitis, urethritis, and infertility.7 In women, complications include infections of the adnexa, endometrium, and vestibular glands, as well as cervical neoplasia and increased co-infection rates with other STIs, such as bacterial vaginosis, chlamydia infection, gonorrhea, syphilis, and herpes simplex virus type 2.1
Infection in pregnancy. Adverse outcomes in pregnant women with T vaginalis infections at mid-gestation include low birth weight, preterm premature rupture of membranes, preterm delivery, and postpartum endometritis.8 A disproportionately larger share of the low birth weight rate associated with T vaginalis infections occurs in black women compared with white and Hispanic women.8 Perinatal transmission to newborns can cause fever; respiratory difficulties; urinary tract infections; nasal discharge; and, in female infants, vaginal discharge.9,10
Co-infection concerns. The increased rate of co-infection with human immunodeficiency virus type 1 (HIV-1) and T vaginalis is a major concern.11 One study found a higher concentration of HIV-1 in semen samples from men with T vaginalis and symptomatic urethritis.12 Further, T vaginalis was found in 17.4% of women with HIV screened at a public clinic in California, with almost 38% of black women affected.13 Trichomoniasis can increase the risk of HIV-1 acquisition by 1.52-fold (95% confidence interval, 1.04- to 2.24-fold), pointing toward a potential amplifying effect of T vaginalis on HIV transmission rates.14 This association may be based at least in part on the organism’s ability to cause microulcerations in the genital and urinary tract epithelium, thus creating pathways for other microorganisms to enter the vascular system.
Making the diagnosis
The nonspecific symptoms of T vaginalis create a wide differential to consider. Vaginal discharge may be due to bacterial vaginosis, vulvovaginal candidiasis, physiologic discharge, atrophy, and nonspecific inflammation. The presence of malodorous and discolored discharge increases the likelihood of bacterial vaginosis or T vaginalis infection. Pruritus often is associated with candidiasis co-infection.
The diagnosis of trichomoniasis can be confirmed in the outpatient office with the use of saline microscopy, an inexpensive test that is based on observation of motile trichomonads in a wet mount of vaginal fluid. The sensitivity of the wet mount ranges from 44% to 68% compared with culture. Culture, traditionally using Diamond’s medium, has a sensitivity of 81% to 94% and was long the gold standard; however, culture has been replaced largely by molecular and antigen testing.
Three US Food and Drug Administration (FDA)-approved NAATs for T vaginalis currently are on the market; all can detect co-infection with gonorrhea and chlamydia from the same specimen. These tests include the Aptima T vaginalis rRNA target assay (Hologic, Bedford, Massachusetts) and the BD ProbTec T vaginalis Qx (TVQ) amplified DNA assay (BD Diagnostics, Baltimore, Maryland), both of which require up to 8 hours to yield results. The Xpert T vaginalis (TV) assay (Cepheid, Sunnyvale, California) is the first NAAT that is FDA approved for use with male urine (in addition to female urine), and it yields results in 60 to 90 minutes. Sensitivity for these NAAT assays ranges from 88% to 100%.15
Point-of-care testing is preferred for rapid diagnosis and for helping the clinician provide same-visit treatment for STIs. The Solana trichomonas assay (Quidel, San Diego, California) detects T vaginalis DNA and can yield results within 40 minutes, but it requires specialized equipment for running the samples. The AmpliVue trichomonas assay (Quidel, San Diego, California) is similar to the Solana assay but it is contained within a small handheld cartridge that does not require additional equipment. Sensitivities are 92% to 98% for Solona and 90.7% to 100% for AmpliVue. The OSOM trichomonas rapid test (Sekisui, Framingham, Massachusetts) uses antigen-detection immunochromatography to provide results in 10 to 15 minutes, with 83% to 92% sensitivity and 99% specificity for vaginal specimens.15,16
Continue to: The TABLE provides a summary...
The TABLE provides a summary of the clinical performance of the various tests for T vaginalis. 15-18

Treatment options
The 5-nitroimidazole agents, which include metronidazole and tinidazole, are the preferred agents for the treatment of trichomoniasis.
Dosing regimen. While a single oral dose of metronidazole 2 g has long been the mainstay of treatment for T vaginalis, this regimen recently has been questioned, at least in women, due to the high posttreatment positive rate of T vaginalis, which ranges from 5% to 37%.19,20 These cases may be due to reinfection by untreated sexual partners. They also may result from treatment failure, however, specifically inadequate treatment time.21 Overall, patients treated with single-dose metronidazole are 1.87 times more likely to experience treatment failure compared with those treated with a multidose regimen.19 Since many cases of T vaginalis infection are associated with bacterial vaginosis co-infection, recommending metronidazole 500 mg twice daily for 7 days is beneficial because this course provides optimal treatment for both infections.
Treatment during pregnancy. In the minds of some investigators, treatment of T vaginalis in asymptomatic pregnant women is problematic. One study demonstrated a similar to slightly increased risk of preterm delivery for metronidazole-treated patients compared with a placebo-treated group.22 Limitations of the study included atypical treatment dosing (2 doses of metronidazole 2 g given 48 hours apart at 16 to 23 weeks’ gestation and repeated at 24 to 29 weeks’ gestation) and a latency between the last dose of metronidazole and preterm delivery.22
We believe that all pregnant women, symptomatic or asymptomatic, should be treated because of the sexually transmitted nature of the infection and the probability that most asymptomatic carriers ultimately become symptomatic.
Cost of treatment. Generic oral metronidazole is very inexpensive. The approximate retail price for 14 metronidazole 500-mg tablets is $15.69 (www.goodrx.com). By contrast, a single-dose course of tinidazole (four 500-mg tablets) costs approximately $45. Accordingly, we reserve tinidazole for patients who have experienced a treatment failure with metronidazole or who cannot tolerate metronidazole.
Drug‒alcohol interaction. With both metronidazole and tinidazole, patients must abstain from alcohol during treatment and for 72 hours after completing therapy because these drugs have a disulfiram-like reaction with ethanol.
CASE Woman with malodorous vaginal discharge
A 26-year-old nulligravid woman with 2 current sexual partners requests evaluation because she has a yellow-green frothy vaginal discharge that is slightly malodorous. One of her sexual partners has noted a similar discharge from his urethra. On physical examination, the clinician notes that the patient’s discharge emanates from the vaginal mucosa, and the exocervix has multiple punctate hemorrhages. Considerations in this case include:
- What is the most likely diagnosis?
- How should this patient be evaluated and treated?
- Should the patient’s sexual partners be treated?
This clinical scenario is most consistent with a trichomonas infection, although other conditions, including bacterial vaginosis, gonorrhea, and chlamydia infection, must be considered in the differential diagnosis.
In this article, we examine the microbiology, epidemiology, clinical manifestations, and diagnosis and treatment of this common sexually transmitted infection (STI).
The causative microbe
Trichomonas vaginalis is a free-living flagellated protozoan that accounts for almost half of all nonviral STIs globally. It has a predilection for the mucosal epithelium of the genitourinary tract, including the vagina and urethra. Humans are the only known host for T vaginalis. The infection is transmitted through sexual intercourse, and the organism reproduces through binary fission in the lower genital tract of women and in the urethra and prostate of men.
This anaerobic trophozoite has 4 flagella anteriorly and 1 flagellum that projects posteriorly, with an undulating membrane that gives its characteristic motile appearance on saline microscopy.1
T vaginalis infection causes major mechanical stress on epithelial cells, which results in disruption of the plasma cell membrane and, ultimately, cell death. The necrotic cell fragments are then phagocytosed by trichomonads, thus accelerating the infection.2
Groups at risk
Trichomonal infections are not reportable to public health authorities, which makes assessing the true prevalence of infection difficult.
The World Health Organization estimated the incidence of infection to be more than 156 million cases globally in 2016, with a prevalence of 110.4 million people at any one time.3
The 2013-2014 National Health and Nutrition Examination Survey tested 4,057 men and women aged 18 to 59 years for T vaginalis and found a prevalence of 0.5% among men and 1.8% among women.4 The prevalence increased with age. There was a disproportionate burden of trichomonas infections in the non-Hispanic black population, with 4.2% of black men and 8.9% of black women affected.4
Targeted screening of urogenital samples for T vaginalis in a population of US women undergoing Chlamydia trachomatis/Neisseria gonorrhoeae screening demonstrated prevalence rates of 8.7%, 6.7%, and 1.7% for T vaginalis, C trachomatis, and N gonorrhoeae, respectively.5
Differences in prevalence estimates may be due to differences in the varying sensitivity of each testing modality and patient populations. In one study, nucleic acid amplification testing (NAAT) for T vaginalis detected rates as high as 11.3% in women and 6.1% in men undergoing evaluations at STI clinics.6
Continue to: Clinical manifestations of infection...
Clinical manifestations of infection
Most cases of T vaginalis remain in an asymptomatic carrier state, with up to 85% of women and 77% of men reporting no clinical symptoms.1 However, approximately one-third of asymptomatic carriers will experience symptoms within 6 months of infection acquisition. This latency in appearance of clinical symptoms certainly contributes to the high transmission rate of T vaginalis.
Infected men may experience purulent urethritis, dysuria, and postcoital pruritus. Common clinical symptoms in women include abnormal vaginal discharge that may be malodorous, purulent, thin, frothy, and yellow-green, as well as symptoms of dyspareunia and vulvar irritation. Punctate hemorrhages in the cervix (colpitis macularis) and vaginal walls (macular vaginitis) give the characteristic “strawberry appearance,” but these findings are seen in only 2% of affected women.7
Complications in ObGyn patients
Although T vaginalis once was regarded as more of an annoyance than a public health issue, awareness of the infection’s ramifications has increased in recent years. Because of these complications, treatment of both symptomatic and asymptomatic patients is clearly indicated.
Complications of trichomonal infection in men include balanoposthitis, epididymitis, prostatitis, urethritis, and infertility.7 In women, complications include infections of the adnexa, endometrium, and vestibular glands, as well as cervical neoplasia and increased co-infection rates with other STIs, such as bacterial vaginosis, chlamydia infection, gonorrhea, syphilis, and herpes simplex virus type 2.1
Infection in pregnancy. Adverse outcomes in pregnant women with T vaginalis infections at mid-gestation include low birth weight, preterm premature rupture of membranes, preterm delivery, and postpartum endometritis.8 A disproportionately larger share of the low birth weight rate associated with T vaginalis infections occurs in black women compared with white and Hispanic women.8 Perinatal transmission to newborns can cause fever; respiratory difficulties; urinary tract infections; nasal discharge; and, in female infants, vaginal discharge.9,10
Co-infection concerns. The increased rate of co-infection with human immunodeficiency virus type 1 (HIV-1) and T vaginalis is a major concern.11 One study found a higher concentration of HIV-1 in semen samples from men with T vaginalis and symptomatic urethritis.12 Further, T vaginalis was found in 17.4% of women with HIV screened at a public clinic in California, with almost 38% of black women affected.13 Trichomoniasis can increase the risk of HIV-1 acquisition by 1.52-fold (95% confidence interval, 1.04- to 2.24-fold), pointing toward a potential amplifying effect of T vaginalis on HIV transmission rates.14 This association may be based at least in part on the organism’s ability to cause microulcerations in the genital and urinary tract epithelium, thus creating pathways for other microorganisms to enter the vascular system.
Making the diagnosis
The nonspecific symptoms of T vaginalis create a wide differential to consider. Vaginal discharge may be due to bacterial vaginosis, vulvovaginal candidiasis, physiologic discharge, atrophy, and nonspecific inflammation. The presence of malodorous and discolored discharge increases the likelihood of bacterial vaginosis or T vaginalis infection. Pruritus often is associated with candidiasis co-infection.
The diagnosis of trichomoniasis can be confirmed in the outpatient office with the use of saline microscopy, an inexpensive test that is based on observation of motile trichomonads in a wet mount of vaginal fluid. The sensitivity of the wet mount ranges from 44% to 68% compared with culture. Culture, traditionally using Diamond’s medium, has a sensitivity of 81% to 94% and was long the gold standard; however, culture has been replaced largely by molecular and antigen testing.
Three US Food and Drug Administration (FDA)-approved NAATs for T vaginalis currently are on the market; all can detect co-infection with gonorrhea and chlamydia from the same specimen. These tests include the Aptima T vaginalis rRNA target assay (Hologic, Bedford, Massachusetts) and the BD ProbTec T vaginalis Qx (TVQ) amplified DNA assay (BD Diagnostics, Baltimore, Maryland), both of which require up to 8 hours to yield results. The Xpert T vaginalis (TV) assay (Cepheid, Sunnyvale, California) is the first NAAT that is FDA approved for use with male urine (in addition to female urine), and it yields results in 60 to 90 minutes. Sensitivity for these NAAT assays ranges from 88% to 100%.15
Point-of-care testing is preferred for rapid diagnosis and for helping the clinician provide same-visit treatment for STIs. The Solana trichomonas assay (Quidel, San Diego, California) detects T vaginalis DNA and can yield results within 40 minutes, but it requires specialized equipment for running the samples. The AmpliVue trichomonas assay (Quidel, San Diego, California) is similar to the Solana assay but it is contained within a small handheld cartridge that does not require additional equipment. Sensitivities are 92% to 98% for Solona and 90.7% to 100% for AmpliVue. The OSOM trichomonas rapid test (Sekisui, Framingham, Massachusetts) uses antigen-detection immunochromatography to provide results in 10 to 15 minutes, with 83% to 92% sensitivity and 99% specificity for vaginal specimens.15,16
Continue to: The TABLE provides a summary...
The TABLE provides a summary of the clinical performance of the various tests for T vaginalis. 15-18

Treatment options
The 5-nitroimidazole agents, which include metronidazole and tinidazole, are the preferred agents for the treatment of trichomoniasis.
Dosing regimen. While a single oral dose of metronidazole 2 g has long been the mainstay of treatment for T vaginalis, this regimen recently has been questioned, at least in women, due to the high posttreatment positive rate of T vaginalis, which ranges from 5% to 37%.19,20 These cases may be due to reinfection by untreated sexual partners. They also may result from treatment failure, however, specifically inadequate treatment time.21 Overall, patients treated with single-dose metronidazole are 1.87 times more likely to experience treatment failure compared with those treated with a multidose regimen.19 Since many cases of T vaginalis infection are associated with bacterial vaginosis co-infection, recommending metronidazole 500 mg twice daily for 7 days is beneficial because this course provides optimal treatment for both infections.
Treatment during pregnancy. In the minds of some investigators, treatment of T vaginalis in asymptomatic pregnant women is problematic. One study demonstrated a similar to slightly increased risk of preterm delivery for metronidazole-treated patients compared with a placebo-treated group.22 Limitations of the study included atypical treatment dosing (2 doses of metronidazole 2 g given 48 hours apart at 16 to 23 weeks’ gestation and repeated at 24 to 29 weeks’ gestation) and a latency between the last dose of metronidazole and preterm delivery.22
We believe that all pregnant women, symptomatic or asymptomatic, should be treated because of the sexually transmitted nature of the infection and the probability that most asymptomatic carriers ultimately become symptomatic.
Cost of treatment. Generic oral metronidazole is very inexpensive. The approximate retail price for 14 metronidazole 500-mg tablets is $15.69 (www.goodrx.com). By contrast, a single-dose course of tinidazole (four 500-mg tablets) costs approximately $45. Accordingly, we reserve tinidazole for patients who have experienced a treatment failure with metronidazole or who cannot tolerate metronidazole.
Drug‒alcohol interaction. With both metronidazole and tinidazole, patients must abstain from alcohol during treatment and for 72 hours after completing therapy because these drugs have a disulfiram-like reaction with ethanol.
- Kissinger P. Epidemiology and treatment of trichomoniasis. Curr Infect Dis Rep. 2015;17:484.
- Midlej V, Benchimol M. Trichomonas vaginalis kills and eats—evidence for phagocytic activity as a cytopathic effect. Parasitology. 2010;137:65-76.
- Rowley J, Vander Hoorn S, Korenromp E, et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull World Health Organ. 2019;97:548–562P.
- Patel EU, Gaydos CA, Packman ZR, et al. Prevalence and correlates of Trichomonas vaginalis infection among men and women in the United States. Clin Infect Dis. 2018;67:211-217.
- Ginocchio CC, Chapin K, Smith JS, et al. Prevalence of Trichomonas vaginalis and coinfection with Chlamydia trachomatis and Neisseria gonorrhoeae in the United States as determined by the Aptima Trichomonas vaginalis nucleic acid amplification assay. J Clin Microbiol. 2012;50:2601-2608.
- Schwebke J, Merriweather A, Massingale S, et al. Screening for Trichomonas vaginalis in a large high-risk population: prevalence among men and women determined by nucleic acid amplification testing. Sex Transm Dis. 2018;45:e23-e24.
- Petrin D, Delgaty K, Bhatt R, et al. Clinical and microbiological aspects of Trichomonas vaginalis. Clin Microbiol Rev. 1998;11:300-317.
- Cotch MF, Pastorek JG II, Nugent RP, et al. Trichomonas vaginalis associated with low birth weight and preterm delivery. The Vaginal Infections and Prematurity Study Group. Sex Transm Dis. 1997;24:353-360.
- Smith LM, Wang M, Zangwill K, et al. Trichomonas vaginalis infection in a premature newborn. J Perinatol. 2002;22:502-503.
- Temesvári P, Kerekes A, Tege A, et al. Demonstration of Trichomonas vaginalis in tracheal aspirates in infants with early respiratory failure. J Matern Fetal Neonatal Med. 2002;11:347-349.
- Kissinger P, Adamski A. Trichomoniasis and HIV interactions: a review. Sex Transm Infect. 2013;89:426-433.
- Cohen MS, Hoffman IF, Royce RA, et al; AIDSCAP Malawi Research Group. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. Lancet. 1997;349:1868-1873.
- Sorvillo F, Kovacs A, Kerndt P, et al. Risk factors for trichomoniasis among women with human immunodeficiency virus (HIV) infection at a public clinic in Los Angeles County, California: implications for HIV prevention. Am J Trop Med Hyg. 1998;58:495-500.
- McClelland RS, Sangare L, Hassan WM, et al. Infection with Trichomonas vaginalis increases the risk of HIV-1 acquisition. J Infect Dis. 2007;195:698-702.
- Van Gerwen OT, Muzny CA. Recent advances in the epidemiology, diagnosis, and management of Trichomonas vaginalis infection. F1000Res. 2019;8:F1000 Faculty Rev-1666.
- Gaydos CA, Klausner JD, Pai NP, et al. Rapid and point-of-care tests for the diagnosis of Trichomonas vaginalis in women and men. Sex Transm Infect. 2017;93(S4):S31-S35.
- Rivers CA, Muzny CA, Schwebke JR. Diagnostic rates differ on the basis of the number of read days with the use of the InPouch culture system for Trichomonas vaginalis screening. J Clin Microbiol. 2013;51:3875-3876.
- Van Der Pol B, Williams JA, Taylor SN, et al. Detection of Trichomonas vaginalis DNA by use of self-obtained vaginal swabs with the BD ProbeTec Qx assay on the BD Viper System. J Clin Microbiol. 2014;52:885-889.
- Howe K, Kissinger P. Single-dose compared with multidose metronidazole for the treatment of trichomoniasis in women: a meta-analysis. Sex Transm Dis. 2017;44:29-34.
- Duff P. Should the length of treatment for trichomoniasis in women be reconsidered? OBG Manag. 2017;29(3):48-49.
- Krashin JW, Koumans EH, Bradshaw-Sydnor AC, et al. Trichomonas vaginalis prevalence, incidence, risk factors and antibiotic-resistance in an adolescent population. Sex Transm Dis. 2010;37:440-444.
- Klebanoff MA, Carey JC, Hauth JC, et al; National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Failure of metronidazole to prevent preterm delivery among pregnant women with asymptomatic Trichomonas vaginalis infection. N Engl J Med. 2001;345:487-493.
- Kissinger P. Epidemiology and treatment of trichomoniasis. Curr Infect Dis Rep. 2015;17:484.
- Midlej V, Benchimol M. Trichomonas vaginalis kills and eats—evidence for phagocytic activity as a cytopathic effect. Parasitology. 2010;137:65-76.
- Rowley J, Vander Hoorn S, Korenromp E, et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull World Health Organ. 2019;97:548–562P.
- Patel EU, Gaydos CA, Packman ZR, et al. Prevalence and correlates of Trichomonas vaginalis infection among men and women in the United States. Clin Infect Dis. 2018;67:211-217.
- Ginocchio CC, Chapin K, Smith JS, et al. Prevalence of Trichomonas vaginalis and coinfection with Chlamydia trachomatis and Neisseria gonorrhoeae in the United States as determined by the Aptima Trichomonas vaginalis nucleic acid amplification assay. J Clin Microbiol. 2012;50:2601-2608.
- Schwebke J, Merriweather A, Massingale S, et al. Screening for Trichomonas vaginalis in a large high-risk population: prevalence among men and women determined by nucleic acid amplification testing. Sex Transm Dis. 2018;45:e23-e24.
- Petrin D, Delgaty K, Bhatt R, et al. Clinical and microbiological aspects of Trichomonas vaginalis. Clin Microbiol Rev. 1998;11:300-317.
- Cotch MF, Pastorek JG II, Nugent RP, et al. Trichomonas vaginalis associated with low birth weight and preterm delivery. The Vaginal Infections and Prematurity Study Group. Sex Transm Dis. 1997;24:353-360.
- Smith LM, Wang M, Zangwill K, et al. Trichomonas vaginalis infection in a premature newborn. J Perinatol. 2002;22:502-503.
- Temesvári P, Kerekes A, Tege A, et al. Demonstration of Trichomonas vaginalis in tracheal aspirates in infants with early respiratory failure. J Matern Fetal Neonatal Med. 2002;11:347-349.
- Kissinger P, Adamski A. Trichomoniasis and HIV interactions: a review. Sex Transm Infect. 2013;89:426-433.
- Cohen MS, Hoffman IF, Royce RA, et al; AIDSCAP Malawi Research Group. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. Lancet. 1997;349:1868-1873.
- Sorvillo F, Kovacs A, Kerndt P, et al. Risk factors for trichomoniasis among women with human immunodeficiency virus (HIV) infection at a public clinic in Los Angeles County, California: implications for HIV prevention. Am J Trop Med Hyg. 1998;58:495-500.
- McClelland RS, Sangare L, Hassan WM, et al. Infection with Trichomonas vaginalis increases the risk of HIV-1 acquisition. J Infect Dis. 2007;195:698-702.
- Van Gerwen OT, Muzny CA. Recent advances in the epidemiology, diagnosis, and management of Trichomonas vaginalis infection. F1000Res. 2019;8:F1000 Faculty Rev-1666.
- Gaydos CA, Klausner JD, Pai NP, et al. Rapid and point-of-care tests for the diagnosis of Trichomonas vaginalis in women and men. Sex Transm Infect. 2017;93(S4):S31-S35.
- Rivers CA, Muzny CA, Schwebke JR. Diagnostic rates differ on the basis of the number of read days with the use of the InPouch culture system for Trichomonas vaginalis screening. J Clin Microbiol. 2013;51:3875-3876.
- Van Der Pol B, Williams JA, Taylor SN, et al. Detection of Trichomonas vaginalis DNA by use of self-obtained vaginal swabs with the BD ProbeTec Qx assay on the BD Viper System. J Clin Microbiol. 2014;52:885-889.
- Howe K, Kissinger P. Single-dose compared with multidose metronidazole for the treatment of trichomoniasis in women: a meta-analysis. Sex Transm Dis. 2017;44:29-34.
- Duff P. Should the length of treatment for trichomoniasis in women be reconsidered? OBG Manag. 2017;29(3):48-49.
- Krashin JW, Koumans EH, Bradshaw-Sydnor AC, et al. Trichomonas vaginalis prevalence, incidence, risk factors and antibiotic-resistance in an adolescent population. Sex Transm Dis. 2010;37:440-444.
- Klebanoff MA, Carey JC, Hauth JC, et al; National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Failure of metronidazole to prevent preterm delivery among pregnant women with asymptomatic Trichomonas vaginalis infection. N Engl J Med. 2001;345:487-493.
Telemedicine: A primer for today’s ObGyn
If telemedicine had not yet begun to play a significant role in your ObGyn practice, it is almost certain to now as the COVID-19 pandemic demands new ways of caring for our patients while keeping others safe from disease. According to the American College of Obstetricians and Gynecologists (ACOG), the term “telemedicine” refers to delivering traditional clinical diagnosis and monitoring via technology (see “ACOG weighs in on telehealth”).1
Whether they realize it or not, most ObGyns have practiced a simple form of telemedicine when they take phone calls from patients who are seeking medication refills. In these cases, physicians either can call the pharmacy to refill the medication or suggest patients make an office appointment to receive a new prescription (much to the chagrin of many patients—especially millennials). Physicians who acquiesce to patients’ phone requests to have prescriptions filled or to others seeking free medical advice are not compensated for these services, yet are legally responsible for their actions and advice—a situation that does not make for good medicine.
This is where telemedicine can be an important addition to an ObGyn practice. Telemedicine saves the patient the time and effort of coming to the office, while providing compensation to the physician for his/her time and advice and providing a record of the interaction, all of which makes for far better medicine. This article—the first of 3 on the subject—discusses the process of integrating telemedicine into a practice with minimal time, energy, and expense.
Telemedicine and the ObGyn practice
Many ObGyn patients do not require an in-person visit in order to receive effective care. There is even the potential to provide prenatal care via telemedicine by replacing some of the many prenatal well-care office visits with at-home care for pregnant women with low-risk pregnancies. A typical virtual visit for a low-risk pregnancy includes utilizing home monitoring equipment to track fetal heart rate, maternal blood pressure, and fundal height.2
Practices typically use telemedicine platforms to manage one or both of the following types of encounters: 1) walk-in visits through the practice’s web site; for most of these, patients tend not to care which physicians they see; their priority is usually the first available provider; and 2) appointment-based consultations, where patients schedule video chats in advance, usually with a specific provider.
Although incorporating telemedicine into a practice may seem overwhelming, it requires minimal additional equipment, interfaces easily with a practice’s web site and electronic medical record (EMR) system, increases productivity, and improves workflow. And patients generally appreciate the option of not having to travel to the office for an appointment.
Most patients and physicians are already comfortable with their mobile phones, tablets, social media, and wearable technology, such as Fitbits. Telemedicine is a logical next step. And given the current situation with COVID-19, it is really not a matter of “if,” but rather “when” to incorporate telemedicine as a communication and practice tool, and the sooner the better.
Continue to: Getting started...
Getting started
Physicians and their colleagues and staff first need to become comfortable with telemedicine technology. Physicians can begin by using video communication for other purposes, such as for conducting staff meetings. They should practice starting and ending calls and adjusting audio volume and video quality to ensure good reception.
Selecting a video platform
TABLE 1 provides a list of the most popular video providers and the advantages and disadvantages of each, and TABLE 2 shows a list of free video chat apps. Apps are available that can:
- share and mark up lab tests, magnetic resonance images, and other medical documents without exposing the entire desktop
- securely send documents over a Health Insurance Portability and Accountability Act (HIPAA)-compliant video
- stream digital device images live while still seeing patients’ faces.
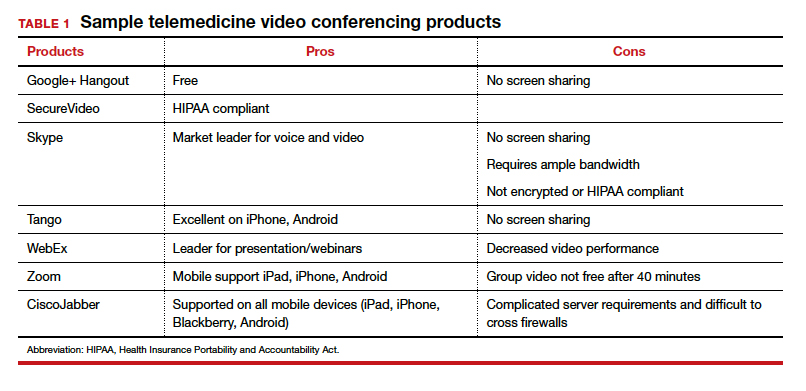
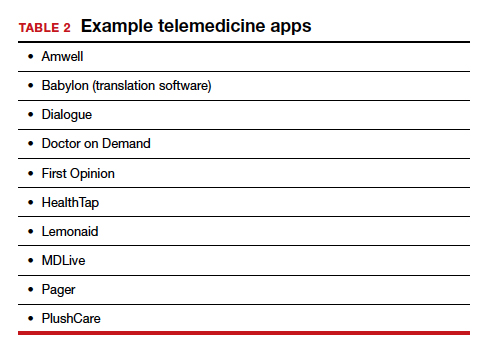
Physicians should make sure their implementation team has the necessary equipment, including webcams, microphones, and speakers, and they should take the time to do research and test out a few programs before selecting one for their practice. Consider appointing a telemedicine point person who is knowledgeable about the technology and can patiently explain it to others. And keep in mind that video chatting is dependent upon a fast, strong Internet connection that has sufficient bandwidth to transport a large amount of data. If your practice has connectivity problems, consider consulting with an information technology (IT) expert.
Testing it out and obtaining feedback
Once a team is comfortable using video within the practice, it is time to test it out with a few patients and perhaps a few payers. Most patients are eager to start using video for their medical encounters. Even senior patients are often willing to try consults via video. According to a recent survey, 64% of patients are willing to see a physician over video.3 And among those who were comfortable accepting an invitation to participate in a video encounter, increasing age was actually associated with a higher likelihood to accept an invite.
Physician colleagues, medical assistants, and nurse practitioners will need some basic telemedicine skills, and physicians and staff should be prepared to make video connections seamless for patients. Usually, patients need some guidance and encouragement, such as telling them to check their spam folder for their invites if the invites fail to arrive in their email inbox, adjusting audio settings, or setting up a webcam. In the beginning, ObGyns should make sure they build in plenty of buffer time for the unexpected, as there will certainly be some “bugs” that need to be worked out.
ObGyns should encourage and collect patient feedback to such questions as:
- What kinds of devices (laptop, mobile) do they prefer using?
- What kind of networks are they using (3G, corporate, home)?
- What features do they like? What features do they have a hard time finding?
- What do they like or not like about the video experience?
- Keep track of the types of questions patients ask, and be patient as patients become acclimated to the video consultation experience.
Continue to: Streamlining online workflow...
Streamlining online workflow
Armed with feedback from patients, it is time to start streamlining online workflow. Most ObGyns want to be able to manage video visits in a way that is similar to the way they manage face-to-face visits with patients. This may mean experimenting with a virtual waiting room. A virtual waiting room is a simple web page or link that can be sent to patients. On that page, patients sign in with minimal demographic information and select one of the time slots when the physician is available. Typically, these programs are designed to alert the physicians and/or staff when a patient enters the virtual waiting room. Patients have access to the online patient queue and can start a chat or video call when both parties are ready. Such a waiting room model serves as a stepping stone for new practices to familiarize themselves with video conferencing. This approach is also perfect for practices that already have a practice management system and just want to add a video component.
Influences on practice workflow
With good time management, telemedicine can improve the efficiency and productivity of your practice. Your daily schedule and management of patients will need some minor changes, but significant alterations to your existing schedule and workflow are generally unnecessary. One of the advantages of telemedicine is the convenience of prompt care and the easy access patients have to your practice. This decreases visits to the emergency department and to urgent care centers.
Consider scheduling telemedicine appointments at the end of the day when your staff has left the office, as no staff members are required for a telemedicine visit. Ideally, you should offer a set time to communicate with patients, as this avoids having to make multiple calls to reach a patient. Another advantage of telemedicine is that you can provide care in the evenings and on weekends if you want. Whereas before you might have been fielding calls from patients during these times and not being compensated, with telemedicine you can conduct a virtual visit from any location and any computer or mobile phone and receive remuneration for your care.
And while access to care has been a problem in many ObGyn practices, many additional patients can be accommodated into a busy ObGyn practice by using telemedicine.
Telemedicine and the coronavirus
The current health care crisis makes implementing telemedicine essential. Patients who think they may have COVID-19 or who have been diagnosed need to be quarantined. Such patients can be helped safely in the comfort of their own homes without endangering others. Patients can be triaged virtually. All those who are febrile or have respiratory symptoms can continue to avail themselves of virtual visits.
According to reports in the media, COVID-19 is stretching the health care workforce to its limits and creating a shortage, both because of the sheer number of cases and because health care workers are getting sick themselves. Physicians who test positive do not have to be completely removed from the workforce if they have the ability to care for patients remotely from their homes. And not incidentally the new environment has prompted the Centers for Medicaid and Medicare Services (CMS) and private payers to initiate national payment policies that create parity between office and telemedicine visits.4
Continue to: Bottom line...
Bottom line
Patient-driven care is the future, and telemedicine is part of that. Patients want to have ready access to their health care providers without having to devote hours to a medical encounter that could be completed in a matter of minutes via telemedicine.
In the next article in this series, we will review the proper coding for a telemedicine visit so that appropriate compensation is gleaned. We will also review the barriers to implementing telemedicine visits. The third article is written with the assistance of 2 health care attorneys, Anjali Dooley and Nadia de la Houssaye, who are experts in telemedicine and who have helped dozens of practices and hospitals implement the technology. They provide legal guidelines for ObGyns who are considering adding telemedicine to their practice. ●
The American College of Obstetricians and Gynecologists (ACOG) encourages all practices and facilities without telemedicine capabilities “to strategize about how telehealth could be integrated into their services as appropriate.”1 In doing so, they also encourage consideration of ways to care for those who may not have access to such technology or who do not know how to use it. They also explain that a number of federal telehealth policy changes have been made in response to the COVID-19 pandemic, and that most private health insurers are following suit.2 Such changes include:
- covering all telehealth visits for all traditional Medicare beneficiaries regardless of geographic location or originating site
- not requiring physicians to have a pre-existing relationship with a patient to provide a telehealth visit
- permitting the use of FaceTime, Skype, and other everyday communication technologies to provide telehealth visits.
A summary of the major telehealth policy changes, as well as information on how to code and bill for telehealth visits can be found at https://www.acog.org/clinical-information/physician-faqs/~/link .aspx?_id=3803296EAAD940C69525D4DD2679A00E&_z=z.
References
- American College of Obstetricians and Gynecologists. COVID-19 FAQs for obstetriciangynecologists, gynecology. https://www.acog.org/clinical-information/physician-faqs/covid19faqs-for-ob-gyns-gynecology. Accessed April 8, 2020.
- American College of Obstetricians and Gynecologists. Managing patients remotely: billing for digital and telehealth services. Updated April 2, 2020. https://www.acog.org/clinicalinformation/physician-faqs/~/link.aspx?_id=3803296EAAD940C69525D4DD2679A00E&_z=z. Accessed April 8, 2020.
- Implementing telehealth in practice. ACOG Committee Opinion. February 2020. https://www.acog.org/clinical /clinical-guidance/committee-opinion/articles/2020/02 /implementing-telehealth-in-practice. Accessed April 6, 2020.
- de Mooij MJM, Hodny RL, O’Neil DA, et al. OB nest: reimagining low-risk prenatal care. Mayo Clin Proc. 2018;93:458-466.
- Gardner MR, Jenkins SM, O’Neil DA, et al. Perceptions of video-based appointments from the patient’s home: a patient survey. Telemed J E Health. 2015;21:281-285.
- American College of Obstetricians and Gynecologists. Managing patients remotely: billing for digital and telehealth services. Updated April 2, 2020. https://www.acog.org /clinical-information/physician-faqs/~/link.aspx?_id=380 3296EAAD940C69525D4DD2679A00E&_z=z. Accessed April 8, 2020.
If telemedicine had not yet begun to play a significant role in your ObGyn practice, it is almost certain to now as the COVID-19 pandemic demands new ways of caring for our patients while keeping others safe from disease. According to the American College of Obstetricians and Gynecologists (ACOG), the term “telemedicine” refers to delivering traditional clinical diagnosis and monitoring via technology (see “ACOG weighs in on telehealth”).1
Whether they realize it or not, most ObGyns have practiced a simple form of telemedicine when they take phone calls from patients who are seeking medication refills. In these cases, physicians either can call the pharmacy to refill the medication or suggest patients make an office appointment to receive a new prescription (much to the chagrin of many patients—especially millennials). Physicians who acquiesce to patients’ phone requests to have prescriptions filled or to others seeking free medical advice are not compensated for these services, yet are legally responsible for their actions and advice—a situation that does not make for good medicine.
This is where telemedicine can be an important addition to an ObGyn practice. Telemedicine saves the patient the time and effort of coming to the office, while providing compensation to the physician for his/her time and advice and providing a record of the interaction, all of which makes for far better medicine. This article—the first of 3 on the subject—discusses the process of integrating telemedicine into a practice with minimal time, energy, and expense.
Telemedicine and the ObGyn practice
Many ObGyn patients do not require an in-person visit in order to receive effective care. There is even the potential to provide prenatal care via telemedicine by replacing some of the many prenatal well-care office visits with at-home care for pregnant women with low-risk pregnancies. A typical virtual visit for a low-risk pregnancy includes utilizing home monitoring equipment to track fetal heart rate, maternal blood pressure, and fundal height.2
Practices typically use telemedicine platforms to manage one or both of the following types of encounters: 1) walk-in visits through the practice’s web site; for most of these, patients tend not to care which physicians they see; their priority is usually the first available provider; and 2) appointment-based consultations, where patients schedule video chats in advance, usually with a specific provider.
Although incorporating telemedicine into a practice may seem overwhelming, it requires minimal additional equipment, interfaces easily with a practice’s web site and electronic medical record (EMR) system, increases productivity, and improves workflow. And patients generally appreciate the option of not having to travel to the office for an appointment.
Most patients and physicians are already comfortable with their mobile phones, tablets, social media, and wearable technology, such as Fitbits. Telemedicine is a logical next step. And given the current situation with COVID-19, it is really not a matter of “if,” but rather “when” to incorporate telemedicine as a communication and practice tool, and the sooner the better.
Continue to: Getting started...
Getting started
Physicians and their colleagues and staff first need to become comfortable with telemedicine technology. Physicians can begin by using video communication for other purposes, such as for conducting staff meetings. They should practice starting and ending calls and adjusting audio volume and video quality to ensure good reception.
Selecting a video platform
TABLE 1 provides a list of the most popular video providers and the advantages and disadvantages of each, and TABLE 2 shows a list of free video chat apps. Apps are available that can:
- share and mark up lab tests, magnetic resonance images, and other medical documents without exposing the entire desktop
- securely send documents over a Health Insurance Portability and Accountability Act (HIPAA)-compliant video
- stream digital device images live while still seeing patients’ faces.


Physicians should make sure their implementation team has the necessary equipment, including webcams, microphones, and speakers, and they should take the time to do research and test out a few programs before selecting one for their practice. Consider appointing a telemedicine point person who is knowledgeable about the technology and can patiently explain it to others. And keep in mind that video chatting is dependent upon a fast, strong Internet connection that has sufficient bandwidth to transport a large amount of data. If your practice has connectivity problems, consider consulting with an information technology (IT) expert.
Testing it out and obtaining feedback
Once a team is comfortable using video within the practice, it is time to test it out with a few patients and perhaps a few payers. Most patients are eager to start using video for their medical encounters. Even senior patients are often willing to try consults via video. According to a recent survey, 64% of patients are willing to see a physician over video.3 And among those who were comfortable accepting an invitation to participate in a video encounter, increasing age was actually associated with a higher likelihood to accept an invite.
Physician colleagues, medical assistants, and nurse practitioners will need some basic telemedicine skills, and physicians and staff should be prepared to make video connections seamless for patients. Usually, patients need some guidance and encouragement, such as telling them to check their spam folder for their invites if the invites fail to arrive in their email inbox, adjusting audio settings, or setting up a webcam. In the beginning, ObGyns should make sure they build in plenty of buffer time for the unexpected, as there will certainly be some “bugs” that need to be worked out.
ObGyns should encourage and collect patient feedback to such questions as:
- What kinds of devices (laptop, mobile) do they prefer using?
- What kind of networks are they using (3G, corporate, home)?
- What features do they like? What features do they have a hard time finding?
- What do they like or not like about the video experience?
- Keep track of the types of questions patients ask, and be patient as patients become acclimated to the video consultation experience.
Continue to: Streamlining online workflow...
Streamlining online workflow
Armed with feedback from patients, it is time to start streamlining online workflow. Most ObGyns want to be able to manage video visits in a way that is similar to the way they manage face-to-face visits with patients. This may mean experimenting with a virtual waiting room. A virtual waiting room is a simple web page or link that can be sent to patients. On that page, patients sign in with minimal demographic information and select one of the time slots when the physician is available. Typically, these programs are designed to alert the physicians and/or staff when a patient enters the virtual waiting room. Patients have access to the online patient queue and can start a chat or video call when both parties are ready. Such a waiting room model serves as a stepping stone for new practices to familiarize themselves with video conferencing. This approach is also perfect for practices that already have a practice management system and just want to add a video component.
Influences on practice workflow
With good time management, telemedicine can improve the efficiency and productivity of your practice. Your daily schedule and management of patients will need some minor changes, but significant alterations to your existing schedule and workflow are generally unnecessary. One of the advantages of telemedicine is the convenience of prompt care and the easy access patients have to your practice. This decreases visits to the emergency department and to urgent care centers.
Consider scheduling telemedicine appointments at the end of the day when your staff has left the office, as no staff members are required for a telemedicine visit. Ideally, you should offer a set time to communicate with patients, as this avoids having to make multiple calls to reach a patient. Another advantage of telemedicine is that you can provide care in the evenings and on weekends if you want. Whereas before you might have been fielding calls from patients during these times and not being compensated, with telemedicine you can conduct a virtual visit from any location and any computer or mobile phone and receive remuneration for your care.
And while access to care has been a problem in many ObGyn practices, many additional patients can be accommodated into a busy ObGyn practice by using telemedicine.
Telemedicine and the coronavirus
The current health care crisis makes implementing telemedicine essential. Patients who think they may have COVID-19 or who have been diagnosed need to be quarantined. Such patients can be helped safely in the comfort of their own homes without endangering others. Patients can be triaged virtually. All those who are febrile or have respiratory symptoms can continue to avail themselves of virtual visits.
According to reports in the media, COVID-19 is stretching the health care workforce to its limits and creating a shortage, both because of the sheer number of cases and because health care workers are getting sick themselves. Physicians who test positive do not have to be completely removed from the workforce if they have the ability to care for patients remotely from their homes. And not incidentally the new environment has prompted the Centers for Medicaid and Medicare Services (CMS) and private payers to initiate national payment policies that create parity between office and telemedicine visits.4
Continue to: Bottom line...
Bottom line
Patient-driven care is the future, and telemedicine is part of that. Patients want to have ready access to their health care providers without having to devote hours to a medical encounter that could be completed in a matter of minutes via telemedicine.
In the next article in this series, we will review the proper coding for a telemedicine visit so that appropriate compensation is gleaned. We will also review the barriers to implementing telemedicine visits. The third article is written with the assistance of 2 health care attorneys, Anjali Dooley and Nadia de la Houssaye, who are experts in telemedicine and who have helped dozens of practices and hospitals implement the technology. They provide legal guidelines for ObGyns who are considering adding telemedicine to their practice. ●
The American College of Obstetricians and Gynecologists (ACOG) encourages all practices and facilities without telemedicine capabilities “to strategize about how telehealth could be integrated into their services as appropriate.”1 In doing so, they also encourage consideration of ways to care for those who may not have access to such technology or who do not know how to use it. They also explain that a number of federal telehealth policy changes have been made in response to the COVID-19 pandemic, and that most private health insurers are following suit.2 Such changes include:
- covering all telehealth visits for all traditional Medicare beneficiaries regardless of geographic location or originating site
- not requiring physicians to have a pre-existing relationship with a patient to provide a telehealth visit
- permitting the use of FaceTime, Skype, and other everyday communication technologies to provide telehealth visits.
A summary of the major telehealth policy changes, as well as information on how to code and bill for telehealth visits can be found at https://www.acog.org/clinical-information/physician-faqs/~/link .aspx?_id=3803296EAAD940C69525D4DD2679A00E&_z=z.
References
- American College of Obstetricians and Gynecologists. COVID-19 FAQs for obstetriciangynecologists, gynecology. https://www.acog.org/clinical-information/physician-faqs/covid19faqs-for-ob-gyns-gynecology. Accessed April 8, 2020.
- American College of Obstetricians and Gynecologists. Managing patients remotely: billing for digital and telehealth services. Updated April 2, 2020. https://www.acog.org/clinicalinformation/physician-faqs/~/link.aspx?_id=3803296EAAD940C69525D4DD2679A00E&_z=z. Accessed April 8, 2020.
If telemedicine had not yet begun to play a significant role in your ObGyn practice, it is almost certain to now as the COVID-19 pandemic demands new ways of caring for our patients while keeping others safe from disease. According to the American College of Obstetricians and Gynecologists (ACOG), the term “telemedicine” refers to delivering traditional clinical diagnosis and monitoring via technology (see “ACOG weighs in on telehealth”).1
Whether they realize it or not, most ObGyns have practiced a simple form of telemedicine when they take phone calls from patients who are seeking medication refills. In these cases, physicians either can call the pharmacy to refill the medication or suggest patients make an office appointment to receive a new prescription (much to the chagrin of many patients—especially millennials). Physicians who acquiesce to patients’ phone requests to have prescriptions filled or to others seeking free medical advice are not compensated for these services, yet are legally responsible for their actions and advice—a situation that does not make for good medicine.
This is where telemedicine can be an important addition to an ObGyn practice. Telemedicine saves the patient the time and effort of coming to the office, while providing compensation to the physician for his/her time and advice and providing a record of the interaction, all of which makes for far better medicine. This article—the first of 3 on the subject—discusses the process of integrating telemedicine into a practice with minimal time, energy, and expense.
Telemedicine and the ObGyn practice
Many ObGyn patients do not require an in-person visit in order to receive effective care. There is even the potential to provide prenatal care via telemedicine by replacing some of the many prenatal well-care office visits with at-home care for pregnant women with low-risk pregnancies. A typical virtual visit for a low-risk pregnancy includes utilizing home monitoring equipment to track fetal heart rate, maternal blood pressure, and fundal height.2
Practices typically use telemedicine platforms to manage one or both of the following types of encounters: 1) walk-in visits through the practice’s web site; for most of these, patients tend not to care which physicians they see; their priority is usually the first available provider; and 2) appointment-based consultations, where patients schedule video chats in advance, usually with a specific provider.
Although incorporating telemedicine into a practice may seem overwhelming, it requires minimal additional equipment, interfaces easily with a practice’s web site and electronic medical record (EMR) system, increases productivity, and improves workflow. And patients generally appreciate the option of not having to travel to the office for an appointment.
Most patients and physicians are already comfortable with their mobile phones, tablets, social media, and wearable technology, such as Fitbits. Telemedicine is a logical next step. And given the current situation with COVID-19, it is really not a matter of “if,” but rather “when” to incorporate telemedicine as a communication and practice tool, and the sooner the better.
Continue to: Getting started...
Getting started
Physicians and their colleagues and staff first need to become comfortable with telemedicine technology. Physicians can begin by using video communication for other purposes, such as for conducting staff meetings. They should practice starting and ending calls and adjusting audio volume and video quality to ensure good reception.
Selecting a video platform
TABLE 1 provides a list of the most popular video providers and the advantages and disadvantages of each, and TABLE 2 shows a list of free video chat apps. Apps are available that can:
- share and mark up lab tests, magnetic resonance images, and other medical documents without exposing the entire desktop
- securely send documents over a Health Insurance Portability and Accountability Act (HIPAA)-compliant video
- stream digital device images live while still seeing patients’ faces.


Physicians should make sure their implementation team has the necessary equipment, including webcams, microphones, and speakers, and they should take the time to do research and test out a few programs before selecting one for their practice. Consider appointing a telemedicine point person who is knowledgeable about the technology and can patiently explain it to others. And keep in mind that video chatting is dependent upon a fast, strong Internet connection that has sufficient bandwidth to transport a large amount of data. If your practice has connectivity problems, consider consulting with an information technology (IT) expert.
Testing it out and obtaining feedback
Once a team is comfortable using video within the practice, it is time to test it out with a few patients and perhaps a few payers. Most patients are eager to start using video for their medical encounters. Even senior patients are often willing to try consults via video. According to a recent survey, 64% of patients are willing to see a physician over video.3 And among those who were comfortable accepting an invitation to participate in a video encounter, increasing age was actually associated with a higher likelihood to accept an invite.
Physician colleagues, medical assistants, and nurse practitioners will need some basic telemedicine skills, and physicians and staff should be prepared to make video connections seamless for patients. Usually, patients need some guidance and encouragement, such as telling them to check their spam folder for their invites if the invites fail to arrive in their email inbox, adjusting audio settings, or setting up a webcam. In the beginning, ObGyns should make sure they build in plenty of buffer time for the unexpected, as there will certainly be some “bugs” that need to be worked out.
ObGyns should encourage and collect patient feedback to such questions as:
- What kinds of devices (laptop, mobile) do they prefer using?
- What kind of networks are they using (3G, corporate, home)?
- What features do they like? What features do they have a hard time finding?
- What do they like or not like about the video experience?
- Keep track of the types of questions patients ask, and be patient as patients become acclimated to the video consultation experience.
Continue to: Streamlining online workflow...
Streamlining online workflow
Armed with feedback from patients, it is time to start streamlining online workflow. Most ObGyns want to be able to manage video visits in a way that is similar to the way they manage face-to-face visits with patients. This may mean experimenting with a virtual waiting room. A virtual waiting room is a simple web page or link that can be sent to patients. On that page, patients sign in with minimal demographic information and select one of the time slots when the physician is available. Typically, these programs are designed to alert the physicians and/or staff when a patient enters the virtual waiting room. Patients have access to the online patient queue and can start a chat or video call when both parties are ready. Such a waiting room model serves as a stepping stone for new practices to familiarize themselves with video conferencing. This approach is also perfect for practices that already have a practice management system and just want to add a video component.
Influences on practice workflow
With good time management, telemedicine can improve the efficiency and productivity of your practice. Your daily schedule and management of patients will need some minor changes, but significant alterations to your existing schedule and workflow are generally unnecessary. One of the advantages of telemedicine is the convenience of prompt care and the easy access patients have to your practice. This decreases visits to the emergency department and to urgent care centers.
Consider scheduling telemedicine appointments at the end of the day when your staff has left the office, as no staff members are required for a telemedicine visit. Ideally, you should offer a set time to communicate with patients, as this avoids having to make multiple calls to reach a patient. Another advantage of telemedicine is that you can provide care in the evenings and on weekends if you want. Whereas before you might have been fielding calls from patients during these times and not being compensated, with telemedicine you can conduct a virtual visit from any location and any computer or mobile phone and receive remuneration for your care.
And while access to care has been a problem in many ObGyn practices, many additional patients can be accommodated into a busy ObGyn practice by using telemedicine.
Telemedicine and the coronavirus
The current health care crisis makes implementing telemedicine essential. Patients who think they may have COVID-19 or who have been diagnosed need to be quarantined. Such patients can be helped safely in the comfort of their own homes without endangering others. Patients can be triaged virtually. All those who are febrile or have respiratory symptoms can continue to avail themselves of virtual visits.
According to reports in the media, COVID-19 is stretching the health care workforce to its limits and creating a shortage, both because of the sheer number of cases and because health care workers are getting sick themselves. Physicians who test positive do not have to be completely removed from the workforce if they have the ability to care for patients remotely from their homes. And not incidentally the new environment has prompted the Centers for Medicaid and Medicare Services (CMS) and private payers to initiate national payment policies that create parity between office and telemedicine visits.4
Continue to: Bottom line...
Bottom line
Patient-driven care is the future, and telemedicine is part of that. Patients want to have ready access to their health care providers without having to devote hours to a medical encounter that could be completed in a matter of minutes via telemedicine.
In the next article in this series, we will review the proper coding for a telemedicine visit so that appropriate compensation is gleaned. We will also review the barriers to implementing telemedicine visits. The third article is written with the assistance of 2 health care attorneys, Anjali Dooley and Nadia de la Houssaye, who are experts in telemedicine and who have helped dozens of practices and hospitals implement the technology. They provide legal guidelines for ObGyns who are considering adding telemedicine to their practice. ●
The American College of Obstetricians and Gynecologists (ACOG) encourages all practices and facilities without telemedicine capabilities “to strategize about how telehealth could be integrated into their services as appropriate.”1 In doing so, they also encourage consideration of ways to care for those who may not have access to such technology or who do not know how to use it. They also explain that a number of federal telehealth policy changes have been made in response to the COVID-19 pandemic, and that most private health insurers are following suit.2 Such changes include:
- covering all telehealth visits for all traditional Medicare beneficiaries regardless of geographic location or originating site
- not requiring physicians to have a pre-existing relationship with a patient to provide a telehealth visit
- permitting the use of FaceTime, Skype, and other everyday communication technologies to provide telehealth visits.
A summary of the major telehealth policy changes, as well as information on how to code and bill for telehealth visits can be found at https://www.acog.org/clinical-information/physician-faqs/~/link .aspx?_id=3803296EAAD940C69525D4DD2679A00E&_z=z.
References
- American College of Obstetricians and Gynecologists. COVID-19 FAQs for obstetriciangynecologists, gynecology. https://www.acog.org/clinical-information/physician-faqs/covid19faqs-for-ob-gyns-gynecology. Accessed April 8, 2020.
- American College of Obstetricians and Gynecologists. Managing patients remotely: billing for digital and telehealth services. Updated April 2, 2020. https://www.acog.org/clinicalinformation/physician-faqs/~/link.aspx?_id=3803296EAAD940C69525D4DD2679A00E&_z=z. Accessed April 8, 2020.
- Implementing telehealth in practice. ACOG Committee Opinion. February 2020. https://www.acog.org/clinical /clinical-guidance/committee-opinion/articles/2020/02 /implementing-telehealth-in-practice. Accessed April 6, 2020.
- de Mooij MJM, Hodny RL, O’Neil DA, et al. OB nest: reimagining low-risk prenatal care. Mayo Clin Proc. 2018;93:458-466.
- Gardner MR, Jenkins SM, O’Neil DA, et al. Perceptions of video-based appointments from the patient’s home: a patient survey. Telemed J E Health. 2015;21:281-285.
- American College of Obstetricians and Gynecologists. Managing patients remotely: billing for digital and telehealth services. Updated April 2, 2020. https://www.acog.org /clinical-information/physician-faqs/~/link.aspx?_id=380 3296EAAD940C69525D4DD2679A00E&_z=z. Accessed April 8, 2020.
- Implementing telehealth in practice. ACOG Committee Opinion. February 2020. https://www.acog.org/clinical /clinical-guidance/committee-opinion/articles/2020/02 /implementing-telehealth-in-practice. Accessed April 6, 2020.
- de Mooij MJM, Hodny RL, O’Neil DA, et al. OB nest: reimagining low-risk prenatal care. Mayo Clin Proc. 2018;93:458-466.
- Gardner MR, Jenkins SM, O’Neil DA, et al. Perceptions of video-based appointments from the patient’s home: a patient survey. Telemed J E Health. 2015;21:281-285.
- American College of Obstetricians and Gynecologists. Managing patients remotely: billing for digital and telehealth services. Updated April 2, 2020. https://www.acog.org /clinical-information/physician-faqs/~/link.aspx?_id=380 3296EAAD940C69525D4DD2679A00E&_z=z. Accessed April 8, 2020.
COVID-19 and pregnancy: Is miscarriage a risk?

- Are you treating pregnant patients with COVID-19? Take this brief survey: https://www.surveymonkey.com/r/CDZ7VFK
- Enroll your patients in PRIORITY: Pregnancy Coronavirus Outcomes Registry
- Second-Trimester Miscarriage in a Pregnant Woman With SARS-CoV-2 Infection JAMA. April 30, 2020

- Are you treating pregnant patients with COVID-19? Take this brief survey: https://www.surveymonkey.com/r/CDZ7VFK
- Enroll your patients in PRIORITY: Pregnancy Coronavirus Outcomes Registry
- Second-Trimester Miscarriage in a Pregnant Woman With SARS-CoV-2 Infection JAMA. April 30, 2020

- Are you treating pregnant patients with COVID-19? Take this brief survey: https://www.surveymonkey.com/r/CDZ7VFK
- Enroll your patients in PRIORITY: Pregnancy Coronavirus Outcomes Registry
- Second-Trimester Miscarriage in a Pregnant Woman With SARS-CoV-2 Infection JAMA. April 30, 2020
ACOG offers guidance on optimizing patient care in the midst of COVID-19
The American College of Obstetricians and Gynecologists (ACOG) posted a useful resource on its website on March 30 for clinicians practicing ambulatory gynecology. The guidance, “COVID-19 FAQs for Obstetrician–Gynecologists, Gynecology” (https://www.acog.org/), is based on expert opinion and is intended to supplement guidance from the Centers for Disease Control and Prevention as well as previously issued ACOG guidance.1
Which patients need to be seen, and when
The ACOG guidance provides examples of patients needing in-person appointments, video or telephone visits, or for whom deferral of a visit until after the COVID-19 outbreak would be appropriate. Highlights include:
In-person appointments
- suspected ectopic pregnancy
- profuse vaginal bleeding
Video or telephone visits
- contraceptive counseling and prescribing
- management of menopausal symptoms
Deferral of a visit until after the COVID-19 outbreak
- routine well-woman visits for average-risk patients.
Cervical screening
With respect to patients with abnormal cervical cancer screening results, ACOG recommends the ASCCP’s guidance that2:
- for patients with low-grade test results, colposcopy/cervical biopsies be deferred up to 6 to 12 months
- for patients with high-grade results, colposcopy/cervical biopsies be performed within 3 months.
Contraception
Regarding contraceptive services, the ACOG guidance suggests that placement of intrauterine devices (IUDs) and contraceptive implants should continue “where possible.” If initiation of long-acting reversible contraception (LARC) is not feasible, the guidance recommends that use of self-administered contraceptives (including subcutaneous injections, oral, transdermal patch, and vaginal ring contraception) be encouraged as a bridge to later initiation of LARC.
The guidance suggests that removal of IUDs and implants be postponed when possible.
Finally, the guidance suggests that patients with an existing IUD or implant who seek removal and replacement of their contraceptives be counseled regarding extended use of these devices.
Individualize your approach
ACOG emphasizes that no single solution applies to all situations and that each practice or clinic should evaluate the individual situation, including the availability of local and regional resources, staffing, and personal protective equipment; the prevalence of COVID-19 in the region; and the type of practice.
A roadmap for care
This guidance from ACOG should help clinicians caring for women during the COVID-19 outbreak to counsel and guide patients in a prudent manner.
- American College of Obstetricians and Gynecologists website. COVID-19 FAQs for obstetrician-gynecologists, gynecology. https://www.acog.org/clinical-information/physician-faqs/covid19-faqs-for-ob-gyns-gynecology. Accessed April 3, 2020.
- ASCCP website. ASCCP interim guidance for timing of diagnostic and treatment procedures for patients with abnormal cervical screening tests. https://www.asccp.org/covid-19. Accessed April 3, 2020.
The American College of Obstetricians and Gynecologists (ACOG) posted a useful resource on its website on March 30 for clinicians practicing ambulatory gynecology. The guidance, “COVID-19 FAQs for Obstetrician–Gynecologists, Gynecology” (https://www.acog.org/), is based on expert opinion and is intended to supplement guidance from the Centers for Disease Control and Prevention as well as previously issued ACOG guidance.1
Which patients need to be seen, and when
The ACOG guidance provides examples of patients needing in-person appointments, video or telephone visits, or for whom deferral of a visit until after the COVID-19 outbreak would be appropriate. Highlights include:
In-person appointments
- suspected ectopic pregnancy
- profuse vaginal bleeding
Video or telephone visits
- contraceptive counseling and prescribing
- management of menopausal symptoms
Deferral of a visit until after the COVID-19 outbreak
- routine well-woman visits for average-risk patients.
Cervical screening
With respect to patients with abnormal cervical cancer screening results, ACOG recommends the ASCCP’s guidance that2:
- for patients with low-grade test results, colposcopy/cervical biopsies be deferred up to 6 to 12 months
- for patients with high-grade results, colposcopy/cervical biopsies be performed within 3 months.
Contraception
Regarding contraceptive services, the ACOG guidance suggests that placement of intrauterine devices (IUDs) and contraceptive implants should continue “where possible.” If initiation of long-acting reversible contraception (LARC) is not feasible, the guidance recommends that use of self-administered contraceptives (including subcutaneous injections, oral, transdermal patch, and vaginal ring contraception) be encouraged as a bridge to later initiation of LARC.
The guidance suggests that removal of IUDs and implants be postponed when possible.
Finally, the guidance suggests that patients with an existing IUD or implant who seek removal and replacement of their contraceptives be counseled regarding extended use of these devices.
Individualize your approach
ACOG emphasizes that no single solution applies to all situations and that each practice or clinic should evaluate the individual situation, including the availability of local and regional resources, staffing, and personal protective equipment; the prevalence of COVID-19 in the region; and the type of practice.
A roadmap for care
This guidance from ACOG should help clinicians caring for women during the COVID-19 outbreak to counsel and guide patients in a prudent manner.
The American College of Obstetricians and Gynecologists (ACOG) posted a useful resource on its website on March 30 for clinicians practicing ambulatory gynecology. The guidance, “COVID-19 FAQs for Obstetrician–Gynecologists, Gynecology” (https://www.acog.org/), is based on expert opinion and is intended to supplement guidance from the Centers for Disease Control and Prevention as well as previously issued ACOG guidance.1
Which patients need to be seen, and when
The ACOG guidance provides examples of patients needing in-person appointments, video or telephone visits, or for whom deferral of a visit until after the COVID-19 outbreak would be appropriate. Highlights include:
In-person appointments
- suspected ectopic pregnancy
- profuse vaginal bleeding
Video or telephone visits
- contraceptive counseling and prescribing
- management of menopausal symptoms
Deferral of a visit until after the COVID-19 outbreak
- routine well-woman visits for average-risk patients.
Cervical screening
With respect to patients with abnormal cervical cancer screening results, ACOG recommends the ASCCP’s guidance that2:
- for patients with low-grade test results, colposcopy/cervical biopsies be deferred up to 6 to 12 months
- for patients with high-grade results, colposcopy/cervical biopsies be performed within 3 months.
Contraception
Regarding contraceptive services, the ACOG guidance suggests that placement of intrauterine devices (IUDs) and contraceptive implants should continue “where possible.” If initiation of long-acting reversible contraception (LARC) is not feasible, the guidance recommends that use of self-administered contraceptives (including subcutaneous injections, oral, transdermal patch, and vaginal ring contraception) be encouraged as a bridge to later initiation of LARC.
The guidance suggests that removal of IUDs and implants be postponed when possible.
Finally, the guidance suggests that patients with an existing IUD or implant who seek removal and replacement of their contraceptives be counseled regarding extended use of these devices.
Individualize your approach
ACOG emphasizes that no single solution applies to all situations and that each practice or clinic should evaluate the individual situation, including the availability of local and regional resources, staffing, and personal protective equipment; the prevalence of COVID-19 in the region; and the type of practice.
A roadmap for care
This guidance from ACOG should help clinicians caring for women during the COVID-19 outbreak to counsel and guide patients in a prudent manner.
- American College of Obstetricians and Gynecologists website. COVID-19 FAQs for obstetrician-gynecologists, gynecology. https://www.acog.org/clinical-information/physician-faqs/covid19-faqs-for-ob-gyns-gynecology. Accessed April 3, 2020.
- ASCCP website. ASCCP interim guidance for timing of diagnostic and treatment procedures for patients with abnormal cervical screening tests. https://www.asccp.org/covid-19. Accessed April 3, 2020.
- American College of Obstetricians and Gynecologists website. COVID-19 FAQs for obstetrician-gynecologists, gynecology. https://www.acog.org/clinical-information/physician-faqs/covid19-faqs-for-ob-gyns-gynecology. Accessed April 3, 2020.
- ASCCP website. ASCCP interim guidance for timing of diagnostic and treatment procedures for patients with abnormal cervical screening tests. https://www.asccp.org/covid-19. Accessed April 3, 2020.