User login
The STD epidemic: Why we need to care about this escalating problem
The sexually transmitted disease (STD) epidemic in the United States is intensifying, and it disproportionately impacts high-risk communities. In 2018, rates of reportable STDs, including syphilis and Neisseria gonorrhoeae and Chlamydia trachomatis infections, reached an all-time high.1 That year, there were 1.8 million cases of chlamydia (increased 19% since 2014), 583,405 cases of gonorrhea (increased 63% since 2014), and 35,063 cases of primary and secondary syphilis (71% increase from 2014).1
Cases of newborn syphilis have more than doubled in 4 years, with rates reaching a 20-year high.1
This surge has not received the attention it deserves given the broad-reaching impact of these infections on women’s health and maternal-child health.2 As ObGyns, we are on the front line, and we need to be engaged in evidence-based strategies and population-based health initiatives to expedite diagnoses and treatment and to reduce the ongoing spread of these infections.
Disparities exist and continue to fuel this epidemic
The STD burden is disproportionately high among reproductive-aged women, and half of all reported STDs occur in women aged 15 to 24 years. African American women have rates up to 12 times higher than white women.3,4 Substantial geographic variability also exists, with the South, Southeast, and West having some of the highest STD rates.
These disparities are fueled by inequalities in socioeconomic status (SES), including employment, insurance, education, incarceration, stress/trauma exposure, and discrimination.5-7 Those with lower SES often have trouble accessing and affording quality health care, including sexual health services. Access to quality health care, including STD prevention and treatment, that meets the needs of lower SES populations is key to reducing STD disparities in the United States; however, access likely will be insufficient unless the structural inequities that drive these disparities are addressed.
Clinical consequences for women, infants, and mothers
STDs are most prevalent among reproductive-aged women and can lead to pelvic inflammatory disease, infertility, ectopic pregnancy,4,8 and increased risk of acquiring human immunodeficiency virus (HIV). STDs during pregnancy present additional consequences. Congenital syphilis is perhaps the most salient, with neonates experiencing substantial disability or death.
In addition, STDs contribute to overall peripartum and long-term adverse health outcomes.4,9,10 Untreated chlamydia infection, for example, is associated with neonatal pneumonia, neonatal conjunctivitis, low birth weight, premature rupture of membranes, preterm labor, and postpartum endometritis.2,11 Untreated gonorrhea is linked to disseminated gonococcal infection in the newborn, neonatal conjunctivitis, low birth weight, miscarriage, premature rupture of membranes, preterm labor, and chorioamnionitis.2,12
As preterm birth is the leading cause of infant morbidity and mortality and disproportionately affects African American women and women in the southeastern United States,13 there is a critical public heath need to improve STD screening, treatment, and prevention of reinfection among high-risk pregnant women.
Quality clinical services for STDs: Areas for focus
More and more, STDs are being diagnosed in primary care settings. In January 2020, the Centers for Disease Control and Prevention (CDC) released a document, referred to as STD QCS (quality clinical services), that outlines recommendations for basic and specialty-level STD clinical services.14 ObGyns and other clinicians who provide primary care should meet the basic recommendations as a minimum.
The STD QCS outlines 8 recommendation areas: sexual history and physical examination, prevention, screening,
Continue to: Sexual history and physical examination...
Sexual history and physical examination
A complete sexual history and risk assessment should be performed at a complete initial or annual visit and as indicated. Routinely updating the sexual history and risk assessment is important to normalize these questions within the frame of the person’s overall health, and it may be valuable in reducing stigma. This routine approach may be important particularly for younger patients and others whose risk for STDs may change frequently and dramatically.
Creating a safe space that permits privacy and assurance of confidentiality may help build trust and set the stage for disclosure. The American College of Obstetricians and Gynecologists recommends that all young people have time alone without parents for confidential counseling and discussion.15 All states allow minors to consent for STD services themselves, although 11 states limit this to those beyond a certain age.16
The CDC recommends using the 5 P’s—partners, practices, protection, past history of STDs, and prevention of pregnancy—as a guide for discussion.14 ObGyns are more likely than other providers to perform this screening routinely. While a pelvic examination should be available for STD evaluation as needed, it is not required for routine screening.
Prevention
ObGyns should employ several recommendations for STD prevention. These include providing or referring patients for vaccination against hepatitis B and human papillomavirus and providing brief STD/HIV prevention counseling along with contraceptive counseling. ObGyns should be familiar with HIV pre-exposure prophylaxis (PrEP) and nonoccupational postexposure prophylaxis (nPEP) and provide risk assessment, education, and referral or link to HIV care. Providing these services would improve access to care and further remove barriers to care. ObGyns also could consider providing condoms in their offices.14
Screening
STD screening of women at risk is critical since more than 80% of infected women are asymptomatic.8 Because young people are disproportionately experiencing STDs, annual screening for chlamydia and gonorrhea is recommended for women younger than 25 years. For women older than 25, those at increased risk can be screened.
Risk factors for chlamydia infection include having new or multiple sex partners, sex partners with concurrent partners, or sex partners who have an STD. For gonorrhea, risk factors include living in a high-morbidity area, having a previous or coexisting STD, new or multiple sex partners, inconsistent condom use in people who are not in a mutually monogamous relationship, and exchanging sex for money or drugs. Screening for syphilis in nonpregnant women is recommended for those who have had any sexual activity with a person recently diagnosed with syphilis or those who personally display signs or symptoms of infection.17
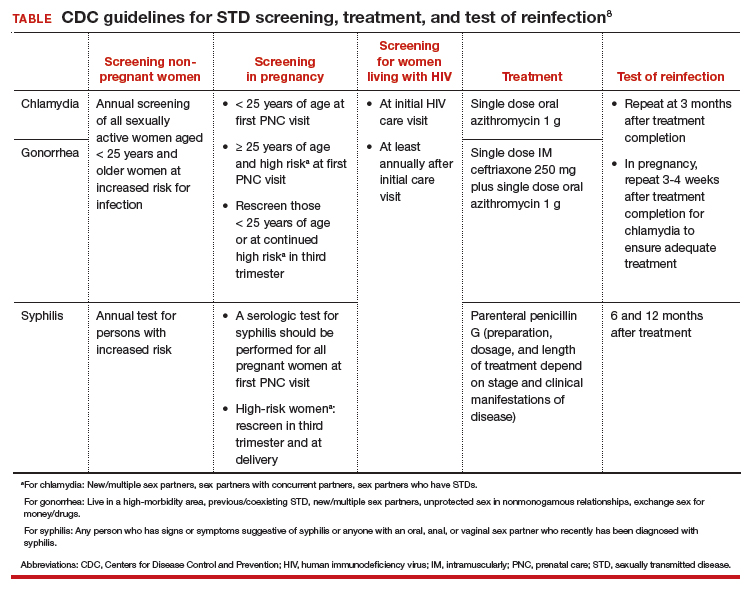
STD screening is especially important for pregnant women, and treatment of infections may improve pregnancy outcomes. The CDC recommends screening at the first prenatal care visit for chlamydia and gonorrhea in pregnant women younger than 25 years of age and in older pregnant women at increased risk; women younger than 25 years or at continued high risk should be rescreened in their third trimester. The CDC recommends screening all women for syphilis at their first prenatal care visit and rescreening those at high risk in the third trimester and at delivery (TABLE).18
Continue to: Partner services...
Partner services
Clearly outlined partner management services is paramount for preventing STD reinfection.14 Reinfection rates for chlamydia and gonorrhea among young women are high and vary by study population.19 At a minimum, ObGyns should counsel patients with an STD that their partner(s) should be notified and encouraged to seek services.
For states in which it is legal, expedited partner therapy (EPT)—the clinician provides medication for the partner without seeing the partner—should be provided for chlamydia or gonorrhea if the partner is unlikely to access timely care. EPT is legal in most states. (To check the legal status of EPT in your state, visit https://www.cdc.gov/std/ept/legal/default.htm.) Research is needed to evaluate optimal strategies for effective implementation of EPT services in different clinical settings.
Laboratory tests
ObGyns should be able to provide a wide range of laboratory evaluations (for example, a nucleic acid amplification test [NAAT] for genital chlamydia and gonorrhea, quantitative nontreponemal serologic test for syphilis, treponemal serologic test for syphilis) that can be ordered for screening or diagnostic purposes. To improve rates of recommended screening, consider having clinic-level policies that support screening, such as standing orders, express or walk-in screening appointments, lab panels, and reflex testing.
Further, having rapid results or point-of-care testing available would help decrease lags in time to treatment. Delays in treatment are particularly important in lower-resource communities; thus, point-of-care testing may be especially valuable with immediate access to treatment on site.
Treatment
Adequate and timely treatment of STDs is critical to decrease sequelae and the likelihood of transmission to others. Treatment is evolving, particularly for gonorrhea. Over the past several years, gonorrhea has become resistant to 6 previously recommended treatment options.20 Since 2015, the CDC recommends dual therapy for gonorrhea with an injection of ceftriaxone and oral azithromycin.
The first-line recommended treatments for bacterial STDs are listed in the TABLE. When possible, it is preferred to offer directly observed therapy at the time of the visit. This decreases the time to treatment and ensures that therapy is completed.
A call to action for ObGyns
Clinicians have multiple opportunities to address and reduce the surge of STDs in the United States. We play a critical role in screening, diagnosing, and treating patients, and it is thus imperative to be up-to-date on the recommended guidelines. Further, clinicians can advocate for more rapid testing modalities, with the goal of obtaining point-of-care testing results when possible and implementing strategies to improve partner treatment.
While a positive STD result may be associated with significant patient distress, it also may be an opportunity for enhancing the patient-provider relationship, coupling education with motivational approaches to help patients increase protective health behaviors.
It is critical to approach clinical care in a nonjudgmental manner to improve patients’ comfort in their relationship with the health care system. ●
- Be aware of up-to-date screening, treatment, and follow-up recommendations for STDs
- Develop strategies to maximize partner treatment, including expedited partner therapy
- Identify high-risk individuals for whom counseling on HIV and unintended pregnancy prevention strategies can be reinforced, including PrEP and contraception
- Create a clinical environment that normalizes STD testing and destigmatizes infection
- Integrate client-centered counseling to improve protective health behaviors
Abbreviations: HIV, human immunodeficiency virus; PrEP, pre-exposure prophylaxis; STD, sexually transmitted disease.
- Centers for Disease Control and Prevention. 2018 STD surveillance report. https://www.cdc.gov/nchhstp /newsroom/2019/2018-STD-surveillance-report.html. Accessed March 19, 2020.
- Centers for Disease Control and Prevention. Sexually transmitted diseases (STDs): STDs during pregnancy—CDC fact sheet (detailed). www.cdc.gov/std/pregnancy/stdfact -sheet-pregnancy-detailed.htm. Accessed March 19, 2020.
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2017: STDs in racial and ethnic minorities 2017. https://www.cdc.gov/std/stats17 /minorities.htm. Accessed March 19, 2020.
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2017: STDs in women and infants. https://www.cdc.gov/std/stats17/womenandinf .htm. Accessed March 19, 2020.
- Semega JL, Fontenot KR, Kollar MA; US Census Bureau. Income and poverty in the United States: 2016. Washington, DC: US Government Printing Office; 2017. https://www.census.gov/content/dam/Census/library /publications/2017/demo/P60-259.pdf. Accessed March 19, 2020.
- Harling G, Subramanian S, Barnighausen T, et al. Socioeconomic disparities in sexually transmitted infections among young adults in the United States: examining the interaction between income and race/ethnicity. Sex Transm Dis. 2013;40:575-581.
- Meyer PA, Penman-Aguilar A, Campbell VA, et al; Centers for Disease Control and Prevention. Conclusion and future directions: CDC Health Disparities and Inequalities Report— United States, 2013. MMWR Suppl. 2013;62(3):184-186.
- Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03): 1-137.
- Elliott B, Brunham RC, Laga M, et al. Maternal gonococcal infection as a preventable risk factor for low birth weight. J Infect Dis. 1990;161:531-536.
- Warr AJ, Pintye J, Kinuthia J, et al. Sexually transmitted infections during pregnancy and subsequent risk of stillbirth and infant mortality in Kenya: a prospective study. Sex Transm Infect. 2019;95:60-66.
- Andrews WW, Goldenberg RL, Mercer B, et al. The Preterm Prediction Study: association of second-trimester genitourinary chlamydia infection with subsequent spontaneous preterm birth. Am J Obstet Gynecol. 2000;183:662-668.
- Alger LS, Lovchik JC, Hebel JR, et al. The association of Chlamydia trachomatis, Neisseria gonorrhoeae, and group B streptococci with preterm rupture of the membranes and pregnancy outcome. Am J Obstet Gynecol. 1988;159:397-404.
- March of Dimes. Maternal, infant, and child health in the United States, 2016. https://www.marchofdimes.org /materials/March-of-Dimes-2016-Databook.pdf. Accessed March 19, 2020.
- Barrow RY, Ahmed F, Bolan GA, et al. Recommendations for providing quality sexually transmitted diseases clinical services, 2020. MMWR Recomm Rep. 2020;68(5):1-20.
- American College of Obstetricians and Gynecologists. ACOG committee opinion No. 598: The initial reproductive health visit. May 2014. https:// www.acog.org/-/media /project/acog/acogorg/clinical/files/committee-opinion /articles/2014/05/the-initial-reproductive-health-visit.pdf. Accessed March 31, 2020.
- Guttmacher Institute. An overview of consent to reproductive health services by young people. March 1, 2020. https://www .guttmacher.org/state-policy/explore/overview-minors -consent-law. Accessed March 19, 2020.
- Centers for Disease Control and Prevention. Pocket guide for providers: Syphilis: a provider’s guide to treatment and prevention. 2017. https://www.cdc.gov/std/syphilis /Syphilis-Pocket-Guide-FINAL-508.pdf. Accessed March 19, 2020.
- Centers for Disease Control and Prevention. 2015 Sexually transmitted diseases treatment guidelines: syphilis during pregnancy. https://www.cdc.gov/std/tg2015/syphilis -pregnancy.htm. Accessed March 19, 2020.
- Hosenfeld CB, Workowski KA, Berman S, et al. Repeat infection with chlamydia and gonorrhea among females: a systematic review of the literature. Sex Transm Dis. 2009;36:478-489.
- Bodie M, Gale-Rowe M, Alexandre S, et al. Addressing the rising rates of gonorrhea and drug-resistant gonorrhea: there is no time like the present. Can Commun Dis Rep. 2019;45:54-62.
The sexually transmitted disease (STD) epidemic in the United States is intensifying, and it disproportionately impacts high-risk communities. In 2018, rates of reportable STDs, including syphilis and Neisseria gonorrhoeae and Chlamydia trachomatis infections, reached an all-time high.1 That year, there were 1.8 million cases of chlamydia (increased 19% since 2014), 583,405 cases of gonorrhea (increased 63% since 2014), and 35,063 cases of primary and secondary syphilis (71% increase from 2014).1
Cases of newborn syphilis have more than doubled in 4 years, with rates reaching a 20-year high.1
This surge has not received the attention it deserves given the broad-reaching impact of these infections on women’s health and maternal-child health.2 As ObGyns, we are on the front line, and we need to be engaged in evidence-based strategies and population-based health initiatives to expedite diagnoses and treatment and to reduce the ongoing spread of these infections.

Disparities exist and continue to fuel this epidemic
The STD burden is disproportionately high among reproductive-aged women, and half of all reported STDs occur in women aged 15 to 24 years. African American women have rates up to 12 times higher than white women.3,4 Substantial geographic variability also exists, with the South, Southeast, and West having some of the highest STD rates.
These disparities are fueled by inequalities in socioeconomic status (SES), including employment, insurance, education, incarceration, stress/trauma exposure, and discrimination.5-7 Those with lower SES often have trouble accessing and affording quality health care, including sexual health services. Access to quality health care, including STD prevention and treatment, that meets the needs of lower SES populations is key to reducing STD disparities in the United States; however, access likely will be insufficient unless the structural inequities that drive these disparities are addressed.
Clinical consequences for women, infants, and mothers
STDs are most prevalent among reproductive-aged women and can lead to pelvic inflammatory disease, infertility, ectopic pregnancy,4,8 and increased risk of acquiring human immunodeficiency virus (HIV). STDs during pregnancy present additional consequences. Congenital syphilis is perhaps the most salient, with neonates experiencing substantial disability or death.
In addition, STDs contribute to overall peripartum and long-term adverse health outcomes.4,9,10 Untreated chlamydia infection, for example, is associated with neonatal pneumonia, neonatal conjunctivitis, low birth weight, premature rupture of membranes, preterm labor, and postpartum endometritis.2,11 Untreated gonorrhea is linked to disseminated gonococcal infection in the newborn, neonatal conjunctivitis, low birth weight, miscarriage, premature rupture of membranes, preterm labor, and chorioamnionitis.2,12
As preterm birth is the leading cause of infant morbidity and mortality and disproportionately affects African American women and women in the southeastern United States,13 there is a critical public heath need to improve STD screening, treatment, and prevention of reinfection among high-risk pregnant women.
Quality clinical services for STDs: Areas for focus
More and more, STDs are being diagnosed in primary care settings. In January 2020, the Centers for Disease Control and Prevention (CDC) released a document, referred to as STD QCS (quality clinical services), that outlines recommendations for basic and specialty-level STD clinical services.14 ObGyns and other clinicians who provide primary care should meet the basic recommendations as a minimum.
The STD QCS outlines 8 recommendation areas: sexual history and physical examination, prevention, screening,
Continue to: Sexual history and physical examination...
Sexual history and physical examination
A complete sexual history and risk assessment should be performed at a complete initial or annual visit and as indicated. Routinely updating the sexual history and risk assessment is important to normalize these questions within the frame of the person’s overall health, and it may be valuable in reducing stigma. This routine approach may be important particularly for younger patients and others whose risk for STDs may change frequently and dramatically.
Creating a safe space that permits privacy and assurance of confidentiality may help build trust and set the stage for disclosure. The American College of Obstetricians and Gynecologists recommends that all young people have time alone without parents for confidential counseling and discussion.15 All states allow minors to consent for STD services themselves, although 11 states limit this to those beyond a certain age.16
The CDC recommends using the 5 P’s—partners, practices, protection, past history of STDs, and prevention of pregnancy—as a guide for discussion.14 ObGyns are more likely than other providers to perform this screening routinely. While a pelvic examination should be available for STD evaluation as needed, it is not required for routine screening.
Prevention
ObGyns should employ several recommendations for STD prevention. These include providing or referring patients for vaccination against hepatitis B and human papillomavirus and providing brief STD/HIV prevention counseling along with contraceptive counseling. ObGyns should be familiar with HIV pre-exposure prophylaxis (PrEP) and nonoccupational postexposure prophylaxis (nPEP) and provide risk assessment, education, and referral or link to HIV care. Providing these services would improve access to care and further remove barriers to care. ObGyns also could consider providing condoms in their offices.14
Screening
STD screening of women at risk is critical since more than 80% of infected women are asymptomatic.8 Because young people are disproportionately experiencing STDs, annual screening for chlamydia and gonorrhea is recommended for women younger than 25 years. For women older than 25, those at increased risk can be screened.
Risk factors for chlamydia infection include having new or multiple sex partners, sex partners with concurrent partners, or sex partners who have an STD. For gonorrhea, risk factors include living in a high-morbidity area, having a previous or coexisting STD, new or multiple sex partners, inconsistent condom use in people who are not in a mutually monogamous relationship, and exchanging sex for money or drugs. Screening for syphilis in nonpregnant women is recommended for those who have had any sexual activity with a person recently diagnosed with syphilis or those who personally display signs or symptoms of infection.17
STD screening is especially important for pregnant women, and treatment of infections may improve pregnancy outcomes. The CDC recommends screening at the first prenatal care visit for chlamydia and gonorrhea in pregnant women younger than 25 years of age and in older pregnant women at increased risk; women younger than 25 years or at continued high risk should be rescreened in their third trimester. The CDC recommends screening all women for syphilis at their first prenatal care visit and rescreening those at high risk in the third trimester and at delivery (TABLE).18

Continue to: Partner services...
Partner services
Clearly outlined partner management services is paramount for preventing STD reinfection.14 Reinfection rates for chlamydia and gonorrhea among young women are high and vary by study population.19 At a minimum, ObGyns should counsel patients with an STD that their partner(s) should be notified and encouraged to seek services.
For states in which it is legal, expedited partner therapy (EPT)—the clinician provides medication for the partner without seeing the partner—should be provided for chlamydia or gonorrhea if the partner is unlikely to access timely care. EPT is legal in most states. (To check the legal status of EPT in your state, visit https://www.cdc.gov/std/ept/legal/default.htm.) Research is needed to evaluate optimal strategies for effective implementation of EPT services in different clinical settings.
Laboratory tests
ObGyns should be able to provide a wide range of laboratory evaluations (for example, a nucleic acid amplification test [NAAT] for genital chlamydia and gonorrhea, quantitative nontreponemal serologic test for syphilis, treponemal serologic test for syphilis) that can be ordered for screening or diagnostic purposes. To improve rates of recommended screening, consider having clinic-level policies that support screening, such as standing orders, express or walk-in screening appointments, lab panels, and reflex testing.
Further, having rapid results or point-of-care testing available would help decrease lags in time to treatment. Delays in treatment are particularly important in lower-resource communities; thus, point-of-care testing may be especially valuable with immediate access to treatment on site.
Treatment
Adequate and timely treatment of STDs is critical to decrease sequelae and the likelihood of transmission to others. Treatment is evolving, particularly for gonorrhea. Over the past several years, gonorrhea has become resistant to 6 previously recommended treatment options.20 Since 2015, the CDC recommends dual therapy for gonorrhea with an injection of ceftriaxone and oral azithromycin.
The first-line recommended treatments for bacterial STDs are listed in the TABLE. When possible, it is preferred to offer directly observed therapy at the time of the visit. This decreases the time to treatment and ensures that therapy is completed.
A call to action for ObGyns
Clinicians have multiple opportunities to address and reduce the surge of STDs in the United States. We play a critical role in screening, diagnosing, and treating patients, and it is thus imperative to be up-to-date on the recommended guidelines. Further, clinicians can advocate for more rapid testing modalities, with the goal of obtaining point-of-care testing results when possible and implementing strategies to improve partner treatment.
While a positive STD result may be associated with significant patient distress, it also may be an opportunity for enhancing the patient-provider relationship, coupling education with motivational approaches to help patients increase protective health behaviors.
It is critical to approach clinical care in a nonjudgmental manner to improve patients’ comfort in their relationship with the health care system. ●
- Be aware of up-to-date screening, treatment, and follow-up recommendations for STDs
- Develop strategies to maximize partner treatment, including expedited partner therapy
- Identify high-risk individuals for whom counseling on HIV and unintended pregnancy prevention strategies can be reinforced, including PrEP and contraception
- Create a clinical environment that normalizes STD testing and destigmatizes infection
- Integrate client-centered counseling to improve protective health behaviors
Abbreviations: HIV, human immunodeficiency virus; PrEP, pre-exposure prophylaxis; STD, sexually transmitted disease.
The sexually transmitted disease (STD) epidemic in the United States is intensifying, and it disproportionately impacts high-risk communities. In 2018, rates of reportable STDs, including syphilis and Neisseria gonorrhoeae and Chlamydia trachomatis infections, reached an all-time high.1 That year, there were 1.8 million cases of chlamydia (increased 19% since 2014), 583,405 cases of gonorrhea (increased 63% since 2014), and 35,063 cases of primary and secondary syphilis (71% increase from 2014).1
Cases of newborn syphilis have more than doubled in 4 years, with rates reaching a 20-year high.1
This surge has not received the attention it deserves given the broad-reaching impact of these infections on women’s health and maternal-child health.2 As ObGyns, we are on the front line, and we need to be engaged in evidence-based strategies and population-based health initiatives to expedite diagnoses and treatment and to reduce the ongoing spread of these infections.

Disparities exist and continue to fuel this epidemic
The STD burden is disproportionately high among reproductive-aged women, and half of all reported STDs occur in women aged 15 to 24 years. African American women have rates up to 12 times higher than white women.3,4 Substantial geographic variability also exists, with the South, Southeast, and West having some of the highest STD rates.
These disparities are fueled by inequalities in socioeconomic status (SES), including employment, insurance, education, incarceration, stress/trauma exposure, and discrimination.5-7 Those with lower SES often have trouble accessing and affording quality health care, including sexual health services. Access to quality health care, including STD prevention and treatment, that meets the needs of lower SES populations is key to reducing STD disparities in the United States; however, access likely will be insufficient unless the structural inequities that drive these disparities are addressed.
Clinical consequences for women, infants, and mothers
STDs are most prevalent among reproductive-aged women and can lead to pelvic inflammatory disease, infertility, ectopic pregnancy,4,8 and increased risk of acquiring human immunodeficiency virus (HIV). STDs during pregnancy present additional consequences. Congenital syphilis is perhaps the most salient, with neonates experiencing substantial disability or death.
In addition, STDs contribute to overall peripartum and long-term adverse health outcomes.4,9,10 Untreated chlamydia infection, for example, is associated with neonatal pneumonia, neonatal conjunctivitis, low birth weight, premature rupture of membranes, preterm labor, and postpartum endometritis.2,11 Untreated gonorrhea is linked to disseminated gonococcal infection in the newborn, neonatal conjunctivitis, low birth weight, miscarriage, premature rupture of membranes, preterm labor, and chorioamnionitis.2,12
As preterm birth is the leading cause of infant morbidity and mortality and disproportionately affects African American women and women in the southeastern United States,13 there is a critical public heath need to improve STD screening, treatment, and prevention of reinfection among high-risk pregnant women.
Quality clinical services for STDs: Areas for focus
More and more, STDs are being diagnosed in primary care settings. In January 2020, the Centers for Disease Control and Prevention (CDC) released a document, referred to as STD QCS (quality clinical services), that outlines recommendations for basic and specialty-level STD clinical services.14 ObGyns and other clinicians who provide primary care should meet the basic recommendations as a minimum.
The STD QCS outlines 8 recommendation areas: sexual history and physical examination, prevention, screening,
Continue to: Sexual history and physical examination...
Sexual history and physical examination
A complete sexual history and risk assessment should be performed at a complete initial or annual visit and as indicated. Routinely updating the sexual history and risk assessment is important to normalize these questions within the frame of the person’s overall health, and it may be valuable in reducing stigma. This routine approach may be important particularly for younger patients and others whose risk for STDs may change frequently and dramatically.
Creating a safe space that permits privacy and assurance of confidentiality may help build trust and set the stage for disclosure. The American College of Obstetricians and Gynecologists recommends that all young people have time alone without parents for confidential counseling and discussion.15 All states allow minors to consent for STD services themselves, although 11 states limit this to those beyond a certain age.16
The CDC recommends using the 5 P’s—partners, practices, protection, past history of STDs, and prevention of pregnancy—as a guide for discussion.14 ObGyns are more likely than other providers to perform this screening routinely. While a pelvic examination should be available for STD evaluation as needed, it is not required for routine screening.
Prevention
ObGyns should employ several recommendations for STD prevention. These include providing or referring patients for vaccination against hepatitis B and human papillomavirus and providing brief STD/HIV prevention counseling along with contraceptive counseling. ObGyns should be familiar with HIV pre-exposure prophylaxis (PrEP) and nonoccupational postexposure prophylaxis (nPEP) and provide risk assessment, education, and referral or link to HIV care. Providing these services would improve access to care and further remove barriers to care. ObGyns also could consider providing condoms in their offices.14
Screening
STD screening of women at risk is critical since more than 80% of infected women are asymptomatic.8 Because young people are disproportionately experiencing STDs, annual screening for chlamydia and gonorrhea is recommended for women younger than 25 years. For women older than 25, those at increased risk can be screened.
Risk factors for chlamydia infection include having new or multiple sex partners, sex partners with concurrent partners, or sex partners who have an STD. For gonorrhea, risk factors include living in a high-morbidity area, having a previous or coexisting STD, new or multiple sex partners, inconsistent condom use in people who are not in a mutually monogamous relationship, and exchanging sex for money or drugs. Screening for syphilis in nonpregnant women is recommended for those who have had any sexual activity with a person recently diagnosed with syphilis or those who personally display signs or symptoms of infection.17
STD screening is especially important for pregnant women, and treatment of infections may improve pregnancy outcomes. The CDC recommends screening at the first prenatal care visit for chlamydia and gonorrhea in pregnant women younger than 25 years of age and in older pregnant women at increased risk; women younger than 25 years or at continued high risk should be rescreened in their third trimester. The CDC recommends screening all women for syphilis at their first prenatal care visit and rescreening those at high risk in the third trimester and at delivery (TABLE).18

Continue to: Partner services...
Partner services
Clearly outlined partner management services is paramount for preventing STD reinfection.14 Reinfection rates for chlamydia and gonorrhea among young women are high and vary by study population.19 At a minimum, ObGyns should counsel patients with an STD that their partner(s) should be notified and encouraged to seek services.
For states in which it is legal, expedited partner therapy (EPT)—the clinician provides medication for the partner without seeing the partner—should be provided for chlamydia or gonorrhea if the partner is unlikely to access timely care. EPT is legal in most states. (To check the legal status of EPT in your state, visit https://www.cdc.gov/std/ept/legal/default.htm.) Research is needed to evaluate optimal strategies for effective implementation of EPT services in different clinical settings.
Laboratory tests
ObGyns should be able to provide a wide range of laboratory evaluations (for example, a nucleic acid amplification test [NAAT] for genital chlamydia and gonorrhea, quantitative nontreponemal serologic test for syphilis, treponemal serologic test for syphilis) that can be ordered for screening or diagnostic purposes. To improve rates of recommended screening, consider having clinic-level policies that support screening, such as standing orders, express or walk-in screening appointments, lab panels, and reflex testing.
Further, having rapid results or point-of-care testing available would help decrease lags in time to treatment. Delays in treatment are particularly important in lower-resource communities; thus, point-of-care testing may be especially valuable with immediate access to treatment on site.
Treatment
Adequate and timely treatment of STDs is critical to decrease sequelae and the likelihood of transmission to others. Treatment is evolving, particularly for gonorrhea. Over the past several years, gonorrhea has become resistant to 6 previously recommended treatment options.20 Since 2015, the CDC recommends dual therapy for gonorrhea with an injection of ceftriaxone and oral azithromycin.
The first-line recommended treatments for bacterial STDs are listed in the TABLE. When possible, it is preferred to offer directly observed therapy at the time of the visit. This decreases the time to treatment and ensures that therapy is completed.
A call to action for ObGyns
Clinicians have multiple opportunities to address and reduce the surge of STDs in the United States. We play a critical role in screening, diagnosing, and treating patients, and it is thus imperative to be up-to-date on the recommended guidelines. Further, clinicians can advocate for more rapid testing modalities, with the goal of obtaining point-of-care testing results when possible and implementing strategies to improve partner treatment.
While a positive STD result may be associated with significant patient distress, it also may be an opportunity for enhancing the patient-provider relationship, coupling education with motivational approaches to help patients increase protective health behaviors.
It is critical to approach clinical care in a nonjudgmental manner to improve patients’ comfort in their relationship with the health care system. ●
- Be aware of up-to-date screening, treatment, and follow-up recommendations for STDs
- Develop strategies to maximize partner treatment, including expedited partner therapy
- Identify high-risk individuals for whom counseling on HIV and unintended pregnancy prevention strategies can be reinforced, including PrEP and contraception
- Create a clinical environment that normalizes STD testing and destigmatizes infection
- Integrate client-centered counseling to improve protective health behaviors
Abbreviations: HIV, human immunodeficiency virus; PrEP, pre-exposure prophylaxis; STD, sexually transmitted disease.
- Centers for Disease Control and Prevention. 2018 STD surveillance report. https://www.cdc.gov/nchhstp /newsroom/2019/2018-STD-surveillance-report.html. Accessed March 19, 2020.
- Centers for Disease Control and Prevention. Sexually transmitted diseases (STDs): STDs during pregnancy—CDC fact sheet (detailed). www.cdc.gov/std/pregnancy/stdfact -sheet-pregnancy-detailed.htm. Accessed March 19, 2020.
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2017: STDs in racial and ethnic minorities 2017. https://www.cdc.gov/std/stats17 /minorities.htm. Accessed March 19, 2020.
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2017: STDs in women and infants. https://www.cdc.gov/std/stats17/womenandinf .htm. Accessed March 19, 2020.
- Semega JL, Fontenot KR, Kollar MA; US Census Bureau. Income and poverty in the United States: 2016. Washington, DC: US Government Printing Office; 2017. https://www.census.gov/content/dam/Census/library /publications/2017/demo/P60-259.pdf. Accessed March 19, 2020.
- Harling G, Subramanian S, Barnighausen T, et al. Socioeconomic disparities in sexually transmitted infections among young adults in the United States: examining the interaction between income and race/ethnicity. Sex Transm Dis. 2013;40:575-581.
- Meyer PA, Penman-Aguilar A, Campbell VA, et al; Centers for Disease Control and Prevention. Conclusion and future directions: CDC Health Disparities and Inequalities Report— United States, 2013. MMWR Suppl. 2013;62(3):184-186.
- Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03): 1-137.
- Elliott B, Brunham RC, Laga M, et al. Maternal gonococcal infection as a preventable risk factor for low birth weight. J Infect Dis. 1990;161:531-536.
- Warr AJ, Pintye J, Kinuthia J, et al. Sexually transmitted infections during pregnancy and subsequent risk of stillbirth and infant mortality in Kenya: a prospective study. Sex Transm Infect. 2019;95:60-66.
- Andrews WW, Goldenberg RL, Mercer B, et al. The Preterm Prediction Study: association of second-trimester genitourinary chlamydia infection with subsequent spontaneous preterm birth. Am J Obstet Gynecol. 2000;183:662-668.
- Alger LS, Lovchik JC, Hebel JR, et al. The association of Chlamydia trachomatis, Neisseria gonorrhoeae, and group B streptococci with preterm rupture of the membranes and pregnancy outcome. Am J Obstet Gynecol. 1988;159:397-404.
- March of Dimes. Maternal, infant, and child health in the United States, 2016. https://www.marchofdimes.org /materials/March-of-Dimes-2016-Databook.pdf. Accessed March 19, 2020.
- Barrow RY, Ahmed F, Bolan GA, et al. Recommendations for providing quality sexually transmitted diseases clinical services, 2020. MMWR Recomm Rep. 2020;68(5):1-20.
- American College of Obstetricians and Gynecologists. ACOG committee opinion No. 598: The initial reproductive health visit. May 2014. https:// www.acog.org/-/media /project/acog/acogorg/clinical/files/committee-opinion /articles/2014/05/the-initial-reproductive-health-visit.pdf. Accessed March 31, 2020.
- Guttmacher Institute. An overview of consent to reproductive health services by young people. March 1, 2020. https://www .guttmacher.org/state-policy/explore/overview-minors -consent-law. Accessed March 19, 2020.
- Centers for Disease Control and Prevention. Pocket guide for providers: Syphilis: a provider’s guide to treatment and prevention. 2017. https://www.cdc.gov/std/syphilis /Syphilis-Pocket-Guide-FINAL-508.pdf. Accessed March 19, 2020.
- Centers for Disease Control and Prevention. 2015 Sexually transmitted diseases treatment guidelines: syphilis during pregnancy. https://www.cdc.gov/std/tg2015/syphilis -pregnancy.htm. Accessed March 19, 2020.
- Hosenfeld CB, Workowski KA, Berman S, et al. Repeat infection with chlamydia and gonorrhea among females: a systematic review of the literature. Sex Transm Dis. 2009;36:478-489.
- Bodie M, Gale-Rowe M, Alexandre S, et al. Addressing the rising rates of gonorrhea and drug-resistant gonorrhea: there is no time like the present. Can Commun Dis Rep. 2019;45:54-62.
- Centers for Disease Control and Prevention. 2018 STD surveillance report. https://www.cdc.gov/nchhstp /newsroom/2019/2018-STD-surveillance-report.html. Accessed March 19, 2020.
- Centers for Disease Control and Prevention. Sexually transmitted diseases (STDs): STDs during pregnancy—CDC fact sheet (detailed). www.cdc.gov/std/pregnancy/stdfact -sheet-pregnancy-detailed.htm. Accessed March 19, 2020.
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2017: STDs in racial and ethnic minorities 2017. https://www.cdc.gov/std/stats17 /minorities.htm. Accessed March 19, 2020.
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2017: STDs in women and infants. https://www.cdc.gov/std/stats17/womenandinf .htm. Accessed March 19, 2020.
- Semega JL, Fontenot KR, Kollar MA; US Census Bureau. Income and poverty in the United States: 2016. Washington, DC: US Government Printing Office; 2017. https://www.census.gov/content/dam/Census/library /publications/2017/demo/P60-259.pdf. Accessed March 19, 2020.
- Harling G, Subramanian S, Barnighausen T, et al. Socioeconomic disparities in sexually transmitted infections among young adults in the United States: examining the interaction between income and race/ethnicity. Sex Transm Dis. 2013;40:575-581.
- Meyer PA, Penman-Aguilar A, Campbell VA, et al; Centers for Disease Control and Prevention. Conclusion and future directions: CDC Health Disparities and Inequalities Report— United States, 2013. MMWR Suppl. 2013;62(3):184-186.
- Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03): 1-137.
- Elliott B, Brunham RC, Laga M, et al. Maternal gonococcal infection as a preventable risk factor for low birth weight. J Infect Dis. 1990;161:531-536.
- Warr AJ, Pintye J, Kinuthia J, et al. Sexually transmitted infections during pregnancy and subsequent risk of stillbirth and infant mortality in Kenya: a prospective study. Sex Transm Infect. 2019;95:60-66.
- Andrews WW, Goldenberg RL, Mercer B, et al. The Preterm Prediction Study: association of second-trimester genitourinary chlamydia infection with subsequent spontaneous preterm birth. Am J Obstet Gynecol. 2000;183:662-668.
- Alger LS, Lovchik JC, Hebel JR, et al. The association of Chlamydia trachomatis, Neisseria gonorrhoeae, and group B streptococci with preterm rupture of the membranes and pregnancy outcome. Am J Obstet Gynecol. 1988;159:397-404.
- March of Dimes. Maternal, infant, and child health in the United States, 2016. https://www.marchofdimes.org /materials/March-of-Dimes-2016-Databook.pdf. Accessed March 19, 2020.
- Barrow RY, Ahmed F, Bolan GA, et al. Recommendations for providing quality sexually transmitted diseases clinical services, 2020. MMWR Recomm Rep. 2020;68(5):1-20.
- American College of Obstetricians and Gynecologists. ACOG committee opinion No. 598: The initial reproductive health visit. May 2014. https:// www.acog.org/-/media /project/acog/acogorg/clinical/files/committee-opinion /articles/2014/05/the-initial-reproductive-health-visit.pdf. Accessed March 31, 2020.
- Guttmacher Institute. An overview of consent to reproductive health services by young people. March 1, 2020. https://www .guttmacher.org/state-policy/explore/overview-minors -consent-law. Accessed March 19, 2020.
- Centers for Disease Control and Prevention. Pocket guide for providers: Syphilis: a provider’s guide to treatment and prevention. 2017. https://www.cdc.gov/std/syphilis /Syphilis-Pocket-Guide-FINAL-508.pdf. Accessed March 19, 2020.
- Centers for Disease Control and Prevention. 2015 Sexually transmitted diseases treatment guidelines: syphilis during pregnancy. https://www.cdc.gov/std/tg2015/syphilis -pregnancy.htm. Accessed March 19, 2020.
- Hosenfeld CB, Workowski KA, Berman S, et al. Repeat infection with chlamydia and gonorrhea among females: a systematic review of the literature. Sex Transm Dis. 2009;36:478-489.
- Bodie M, Gale-Rowe M, Alexandre S, et al. Addressing the rising rates of gonorrhea and drug-resistant gonorrhea: there is no time like the present. Can Commun Dis Rep. 2019;45:54-62.
Evaluating and managing the patient with nipple discharge
CASE Young woman with discharge from one nipple
A 26-year-old African American woman presents with a 10-month history of left nipple discharge. The patient describes the discharge as spontaneous, colored dark brown to yellow, and occurring from a single opening in the nipple. The discharge is associated with left breast pain and fullness, without a palpable lump. The patient has no family or personal history of breast cancer.
Nipple discharge is the third most common breast-related symptom (after palpable masses and breast pain), with an estimated prevalence of 5% to 8% among premenopausal women.1 While most causes of nipple discharge reflect benign issues, approximately 5% to 12% of breast cancers have nipple discharge as the only symptom.2 Not surprisingly, nipple discharge creates anxiety for both patients and clinicians.
In this article, we—a breast imaging radiologist, gynecologist, and breast surgeon—outline key steps for evaluating and managing patients with nipple discharge.
Two types of nipple discharge
Nipple discharge can be characterized as physiologic or pathologic. The distinction is based on the patient’s history in conjunction with the clinical breast exam.
Physiologic nipple discharge often is bilateral, nonspontaneous, and white, yellow, green, or brown (TABLE).3 It often is due to nipple stimulation, and the patient can elicit discharge by manually manipulating the breast. Usually, multiple ducts are involved. Galactorrhea refers specifically to milky discharge and occurs most commonly during pregnancy or lactation.2 Galactorrhea that is not associated with pregnancy or lactation often is related to elevated prolactin or thyroid-stimulating hormone levels or to medications. One study reported that no cancers were found when discharge was nonspontaneous and colored or milky.4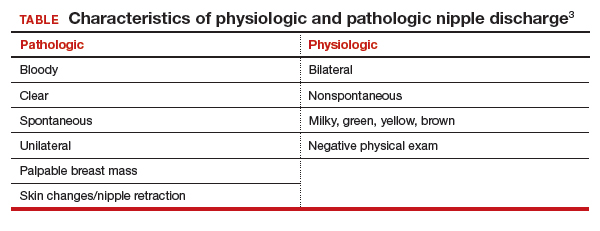
Pathologic nipple discharge is defined as a spontaneous, bloody, clear, or single-duct discharge. A palpable mass in the same breast automatically increases the suspicion of the discharge, regardless of its color or spontaneity.2 The most common cause of pathologic nipple discharge is an intraductal papilloma, a benign epithelial tumor, which accounts for approximately 57% of cases.5
Although the risk of malignancy is low for all patients with nipple discharge, increasing age is associated with increased risk of breast cancer. One study demonstrated that among women aged 40 to 60 years presenting with nipple discharge, the prevalence of invasive cancer is 10%, and the percentage jumps to 32% among women older than 60.6
Breast exam. For any patient with nonlactational nipple discharge, we recommend a thorough breast examination. Deep palpation of all quadrants of the symptomatic breast, especially near the nipple areolar complex, should elicit nipple discharge without any direct squeezing of the nipple. If the patient’s history and physical exam are consistent with physiologic discharge, no further workup is needed. Reassure the patient and recommend appropriate breast cancer screening. Encourage the patient to decrease stimulation or manual manipulation of the nipples if the discharge bothers her.
Continue to: CASE Continued: Workup...
CASE Continued: Workup
On physical exam, the patient’s breasts are noted to be cup size DDD and asymmetric, with the left breast larger than the right; there is no contour deformity. There is no skin or nipple retraction, skin rash, swelling, or nipple changes bilaterally. No dominant masses are appreciated bilaterally. Manual compression elicits no nipple discharge.
Although the discharge is nonbloody, its spontaneity, unilaterality, and single-duct/orifice origin suggest a pathologic cause. The patient is referred for breast imaging.
Imaging workup for pathologic discharge
The American College of Radiology (ACR) Appropriateness Criteria is a useful tool that provides an evidence-based, easy-to-use algorithm for breast imaging in the patient with pathologic nipple discharge (FIGURE 1).6 The algorithm is categorized by patient age, with diagnostic mammography recommended for women aged 30 and older.6 Diagnostic mammography is recommended if the patient has not had a mammogram study in the last 6 months.6 For patients with no prior mammograms, we recommend bilateral diagnostic mammography to compare symmetry of the breasts.
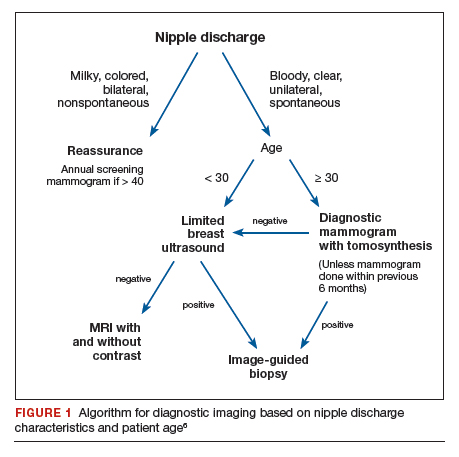
Currently, no studies show that digital breast tomosynthesis (3-D mammography) has a benefit compared with standard 2-D mammography in women with pathologic nipple discharge.6 Given the increased sensitivity of digital breast tomosynthesis for cancer detection, however, in our practice it is standard to use tomosynthesis in the diagnostic evaluation of most patients.
Mammography
On mammography, ductal carcinoma in situ (DCIS) usually presents as calcifications. Both the morphology and distribution of calcifications are used to characterize them as suspicious or, typically, benign. DCIS usually presents as fine pleomorphic or fine linear branching calcifications in a segmental or linear distribution. In patients with pathologic nipple discharge and no other symptoms, the radiologist must closely examine the retroareolar region of the breast to assess for faint calcifications. Magnification views also can be performed to better characterize calcifications.
The sensitivity of mammography for nipple discharge varies in the literature, ranging from approximately 15% to 68%, with a specificity range of 38% to 98%.6 This results in a relatively low positive predictive value but a high negative predictive value of 90%.7 Mammographic sensitivity largely is limited by increased breast density. As more data emerge on the utility of digital breast tomosynthesis in dense breasts, mammographic sensitivity for nipple discharge will likely increase.
Ultrasonography
As an adjunct to mammography, the ACR Appropriateness Criteria recommends targeted (or “limited”) ultrasonography of the retroareolar region of the affected breast for patients aged 30 and older. Ultrasonography is useful to assess for intraductal masses and architectural distortion, and it has higher sensitivity but lower specificity than mammography. The sensitivity of ultrasonography for detecting breast cancer in patients presenting with nipple discharge is reported to be 56% to 80%.6 Ultrasonography can identify lesions not visible mammographically in 63% to 69% of cases.8 Although DCIS usually presents as calcifications, it also can present as an intraductal mass on ultrasonography.
The ACR recommends targeted ultrasonography for patients with nipple discharge and a negative mammogram, or to evaluate a suspicious mammographic abnormality such as architectural distortion, focal asymmetry, or a mass.6 For patient comfort, ultrasonography is the preferred modality for image-guided biopsy.
For women younger than 30 years, targeted ultrasonography is the initial imaging study of choice, according to the ACR criteria.6 Women younger than 30 years with pathologic nipple discharge have a very low risk of breast cancer and tend to have higher breast density, making mammography less useful. Although the radiation dose from mammography is negligible given technological improvements and dose-reduction techniques, ultrasonography remains the preferred initial imaging modality in young women, not only for nipple discharge but also for palpable lumps and focal breast pain.
Mammography is used as an adjunct to ultrasonography in women younger than 30 years when a suspicious abnormality is detected on ultrasonography, such as an intraductal mass or architectural distortion. In these cases, mammography can be used to assess for extent of disease or to visualize suspicious calcifications not well seen on ultrasonography.
For practical purposes regarding which imaging study to order for a patient, it is most efficient to order both a diagnostic mammogram (with tomosynthesis, if possible) and a targeted ultrasound scan of the affected breast. Even if both orders are not needed, having them available increases efficiency for both the radiologist and the ordering physician.
Continue to: CASE Continued: Imaging findings...
CASE Continued: Imaging findings
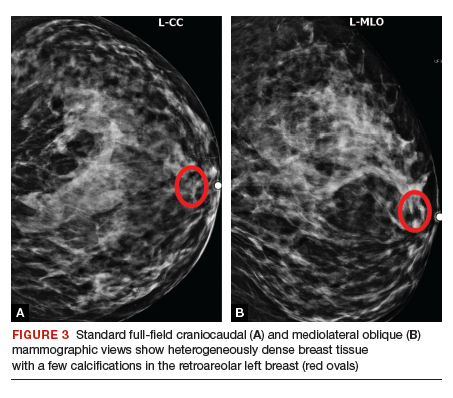
Given her age, the patient initially undergoes targeted ultrasonography. The grayscale image (FIGURE 2) demonstrates multiple mildly dilated ducts (white arrows) with surrounding hyperechogenicity of the fat (red arrows), indicating soft tissue edema. No intraductal mass is imaged. Given that the ultrasonography findings are not completely negative and are equivocal for malignancy, bilateral diagnostic mammography (FIGURE 3, left breast only) is performed. Standard full-field craniocaudal (FIGURE 3A) and mediolateral oblique (FIGURE 3B) mammographic views demonstrate a heterogeneously dense breast with a few calcifications in the retroareolar left breast (red ovals). No associated mass or architectural distortions are noted. The mammographic and sonographic findings do not reveal a definitive biopsy target.

Ductography
When a suspicious abnormality is visualized on either mammography or ultrasonography, the standard of care is to perform an image-guided biopsy of the abnormality. When the standard workup is negative or equivocal, the standard of care historically was to perform ductography.
Ductography is an invasive procedure that involves cannulating the suspicious duct with a small catheter and injecting radiopaque dye into the duct under mammographic guidance. While the sensitivity of ductography is higher than that of both mammography and ultrasonography, its specificity is lower than that of either modality.
Most cases of pathologic discharge are spontaneous and are not reproducible on the day of the procedure. If the procedural radiologist cannot visualize the duct that is producing the discharge, the procedure cannot be performed. Although most patients tolerate the procedure well, ductography produces patient discomfort from cannulation of the duct and injection of contrast.
Magnetic resonance imaging
Dynamic contrast-enhanced magnetic resonance imaging (MRI) is the most sensitive imaging study for evaluating pathologic nipple discharge, and it has largely replaced ductography as an adjunct to mammography and ultrasonography. MRI’s sensitivity for detecting breast cancer ranges from 93% to 100%.6 In addition, MRI allows visualization of the entire breast and areas peripheral to the field of view of a standard ductogram or ultrasound scan.9
Clinicians commonly ask, “Why not skip the mammogram and ultrasound scan and go straight to MRI, since it is so much more sensitive?” Breast MRI has several limitations, including relatively low specificity, cost, use of intravenous contrast, and patient discomfort (that is, claustrophobia, prone positioning). MRI should be utilized for pathologic discharge only when the mammogram and/or targeted ultrasound scans are negative or equivocal.
CASE Continued: Additional imaging
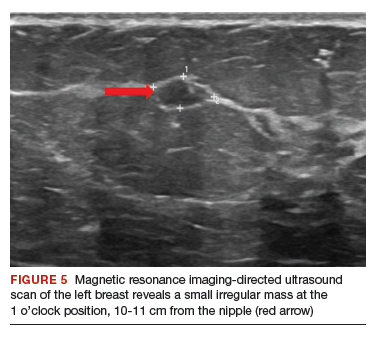
A contrast-enhanced MRI of the breasts (FIGURE 4) demonstrates a large area of non-mass enhancement (red oval) in the left breast, which involves most of the upper breast extending from the nipple to the posterior breast tissue; it measures approximately 7.3 x 14 x 9.1 cm in transverse, anteroposterior, and craniocaudal dimensions, respectively. There is no evidence of left pectoralis muscle involvement. An MRI-directed second look left breast ultrasonography (FIGURE 5) is performed, revealing a small irregular mass in the left breast 1 o’clock position, 10 to 11 cm from the nipple (red arrow). This area had not been imaged in the prior ultrasound scan due to its posterior location far from the nipple. Ultrasound-guided core needle biopsy is performed; moderately differentiated invasive ductal carcinoma (IDC) with high-grade DCIS is found.

Continue to: When to refer for surgery...
When to refer for surgery
No surgical evaluation or intervention is needed for physiologic nipple discharge. As mentioned previously, reassure the patient and recommend appropriate breast cancer screening. In the setting of pathologic discharge, however, referral to a breast surgeon may be indicated after appropriate imaging workup has been done.
Since abnormal imaging almost always results in a recommendation for image-guided biopsy, typically the biopsy is performed prior to the surgical consultation. Once the pathology report from the biopsy is available, the radiologist makes a radiologic-pathologic concordance statement and recommends surgical consultation. This process allows the surgeon to have all the necessary information at the initial visit.
However, in the setting of pathologic nipple discharge with normal breast imaging, the surgeon and patient may opt for close observation or surgery for definitive diagnosis. Surgical options include single-duct excision when nipple discharge is localized to one duct or central duct excision when nipple discharge cannot be localized to one duct.
CASE Continued: Follow-up
The patient was referred to a breast surgeon. Given the extent of disease in the left breast, breast conservation was not possible. The patient underwent left breast simple mastectomy with sentinel lymph node biopsy and prophylactic right simple mastectomy. Final pathology results revealed stage IA IDC with DCIS. Sentinel lymph nodes were negative for malignancy. The patient underwent adjuvant left chest wall radiation, endocrine therapy with tamoxifen, and implant reconstruction. After 2 years of follow-up, she is disease free.
In summary
Nipple discharge can be classified as physiologic or pathologic. For pathologic discharge, a thorough physical examination should be performed with subsequent imaging evaluation. First-line tools, based on patient age, include diagnostic mammography and targeted ultrasonography. Contrast-enhanced MRI is then recommended for negative or equivocal cases. All patients with pathologic nipple discharge should be referred to a breast surgeon following appropriate imaging evaluation. ●
- Alcock C, Layer GT. Predicting occult malignancy in nipple discharge. ANZ J Surg. 2010;80:646-649.
- Patel BK, Falcon S, Drukteinis J. Management of nipple discharge and the associated imaging findings. Am J Med. 2015;128:353-360.
- Mazzarello S, Arnaout A. Five things to know about nipple discharge. CMAJ. 2015;187:599.
- Goksel HA, Yagmurdur MC, Demirhan B, et al. Management strategies for patients with nipple discharge. Langenbecks Arch Surg. 2005;390:52-58.
- Vargas HI, Vargas MP, Eldrageely K, et al. Outcomes of clinical and surgical assessment of women with pathological nipple discharge. Am Surg. 2006;72:124-128.
- Expert Panel on Breast Imaging; Lee S, Tikha S, Moy L, et al. American College of Radiology Appropriateness Criteria: Evaluation of nipple discharge. https://acsearch.acr.org /docs/3099312/Narrative/. Accessed February 2, 2020.
- Cabioglu N, Hunt KK, Singletary SE, et al. Surgical decision making and factors determining a diagnosis of breast carcinoma in women presenting with nipple discharge. J Am Coll Surg. 2003;196:354-364.
- Morrogh M, Park A, Elkin EB, et al. Lessons learned from 416 cases of nipple discharge of the breast. Am J Surg. 2010;200:73-80.
- Morrogh M, Morris EA, Liberman L, et al. The predictive value of ductography and magnetic resonance imaging in the management of nipple discharge. Ann Surg Oncol. 2007;14:3369-3377.
CASE Young woman with discharge from one nipple
A 26-year-old African American woman presents with a 10-month history of left nipple discharge. The patient describes the discharge as spontaneous, colored dark brown to yellow, and occurring from a single opening in the nipple. The discharge is associated with left breast pain and fullness, without a palpable lump. The patient has no family or personal history of breast cancer.
Nipple discharge is the third most common breast-related symptom (after palpable masses and breast pain), with an estimated prevalence of 5% to 8% among premenopausal women.1 While most causes of nipple discharge reflect benign issues, approximately 5% to 12% of breast cancers have nipple discharge as the only symptom.2 Not surprisingly, nipple discharge creates anxiety for both patients and clinicians.
In this article, we—a breast imaging radiologist, gynecologist, and breast surgeon—outline key steps for evaluating and managing patients with nipple discharge.
Two types of nipple discharge
Nipple discharge can be characterized as physiologic or pathologic. The distinction is based on the patient’s history in conjunction with the clinical breast exam.
Physiologic nipple discharge often is bilateral, nonspontaneous, and white, yellow, green, or brown (TABLE).3 It often is due to nipple stimulation, and the patient can elicit discharge by manually manipulating the breast. Usually, multiple ducts are involved. Galactorrhea refers specifically to milky discharge and occurs most commonly during pregnancy or lactation.2 Galactorrhea that is not associated with pregnancy or lactation often is related to elevated prolactin or thyroid-stimulating hormone levels or to medications. One study reported that no cancers were found when discharge was nonspontaneous and colored or milky.4
Pathologic nipple discharge is defined as a spontaneous, bloody, clear, or single-duct discharge. A palpable mass in the same breast automatically increases the suspicion of the discharge, regardless of its color or spontaneity.2 The most common cause of pathologic nipple discharge is an intraductal papilloma, a benign epithelial tumor, which accounts for approximately 57% of cases.5
Although the risk of malignancy is low for all patients with nipple discharge, increasing age is associated with increased risk of breast cancer. One study demonstrated that among women aged 40 to 60 years presenting with nipple discharge, the prevalence of invasive cancer is 10%, and the percentage jumps to 32% among women older than 60.6
Breast exam. For any patient with nonlactational nipple discharge, we recommend a thorough breast examination. Deep palpation of all quadrants of the symptomatic breast, especially near the nipple areolar complex, should elicit nipple discharge without any direct squeezing of the nipple. If the patient’s history and physical exam are consistent with physiologic discharge, no further workup is needed. Reassure the patient and recommend appropriate breast cancer screening. Encourage the patient to decrease stimulation or manual manipulation of the nipples if the discharge bothers her.
Continue to: CASE Continued: Workup...
CASE Continued: Workup
On physical exam, the patient’s breasts are noted to be cup size DDD and asymmetric, with the left breast larger than the right; there is no contour deformity. There is no skin or nipple retraction, skin rash, swelling, or nipple changes bilaterally. No dominant masses are appreciated bilaterally. Manual compression elicits no nipple discharge.
Although the discharge is nonbloody, its spontaneity, unilaterality, and single-duct/orifice origin suggest a pathologic cause. The patient is referred for breast imaging.
Imaging workup for pathologic discharge
The American College of Radiology (ACR) Appropriateness Criteria is a useful tool that provides an evidence-based, easy-to-use algorithm for breast imaging in the patient with pathologic nipple discharge (FIGURE 1).6 The algorithm is categorized by patient age, with diagnostic mammography recommended for women aged 30 and older.6 Diagnostic mammography is recommended if the patient has not had a mammogram study in the last 6 months.6 For patients with no prior mammograms, we recommend bilateral diagnostic mammography to compare symmetry of the breasts.

Currently, no studies show that digital breast tomosynthesis (3-D mammography) has a benefit compared with standard 2-D mammography in women with pathologic nipple discharge.6 Given the increased sensitivity of digital breast tomosynthesis for cancer detection, however, in our practice it is standard to use tomosynthesis in the diagnostic evaluation of most patients.
Mammography
On mammography, ductal carcinoma in situ (DCIS) usually presents as calcifications. Both the morphology and distribution of calcifications are used to characterize them as suspicious or, typically, benign. DCIS usually presents as fine pleomorphic or fine linear branching calcifications in a segmental or linear distribution. In patients with pathologic nipple discharge and no other symptoms, the radiologist must closely examine the retroareolar region of the breast to assess for faint calcifications. Magnification views also can be performed to better characterize calcifications.
The sensitivity of mammography for nipple discharge varies in the literature, ranging from approximately 15% to 68%, with a specificity range of 38% to 98%.6 This results in a relatively low positive predictive value but a high negative predictive value of 90%.7 Mammographic sensitivity largely is limited by increased breast density. As more data emerge on the utility of digital breast tomosynthesis in dense breasts, mammographic sensitivity for nipple discharge will likely increase.
Ultrasonography
As an adjunct to mammography, the ACR Appropriateness Criteria recommends targeted (or “limited”) ultrasonography of the retroareolar region of the affected breast for patients aged 30 and older. Ultrasonography is useful to assess for intraductal masses and architectural distortion, and it has higher sensitivity but lower specificity than mammography. The sensitivity of ultrasonography for detecting breast cancer in patients presenting with nipple discharge is reported to be 56% to 80%.6 Ultrasonography can identify lesions not visible mammographically in 63% to 69% of cases.8 Although DCIS usually presents as calcifications, it also can present as an intraductal mass on ultrasonography.
The ACR recommends targeted ultrasonography for patients with nipple discharge and a negative mammogram, or to evaluate a suspicious mammographic abnormality such as architectural distortion, focal asymmetry, or a mass.6 For patient comfort, ultrasonography is the preferred modality for image-guided biopsy.
For women younger than 30 years, targeted ultrasonography is the initial imaging study of choice, according to the ACR criteria.6 Women younger than 30 years with pathologic nipple discharge have a very low risk of breast cancer and tend to have higher breast density, making mammography less useful. Although the radiation dose from mammography is negligible given technological improvements and dose-reduction techniques, ultrasonography remains the preferred initial imaging modality in young women, not only for nipple discharge but also for palpable lumps and focal breast pain.
Mammography is used as an adjunct to ultrasonography in women younger than 30 years when a suspicious abnormality is detected on ultrasonography, such as an intraductal mass or architectural distortion. In these cases, mammography can be used to assess for extent of disease or to visualize suspicious calcifications not well seen on ultrasonography.
For practical purposes regarding which imaging study to order for a patient, it is most efficient to order both a diagnostic mammogram (with tomosynthesis, if possible) and a targeted ultrasound scan of the affected breast. Even if both orders are not needed, having them available increases efficiency for both the radiologist and the ordering physician.
Continue to: CASE Continued: Imaging findings...
CASE Continued: Imaging findings
Given her age, the patient initially undergoes targeted ultrasonography. The grayscale image (FIGURE 2) demonstrates multiple mildly dilated ducts (white arrows) with surrounding hyperechogenicity of the fat (red arrows), indicating soft tissue edema. No intraductal mass is imaged. Given that the ultrasonography findings are not completely negative and are equivocal for malignancy, bilateral diagnostic mammography (FIGURE 3, left breast only) is performed. Standard full-field craniocaudal (FIGURE 3A) and mediolateral oblique (FIGURE 3B) mammographic views demonstrate a heterogeneously dense breast with a few calcifications in the retroareolar left breast (red ovals). No associated mass or architectural distortions are noted. The mammographic and sonographic findings do not reveal a definitive biopsy target.


Ductography
When a suspicious abnormality is visualized on either mammography or ultrasonography, the standard of care is to perform an image-guided biopsy of the abnormality. When the standard workup is negative or equivocal, the standard of care historically was to perform ductography.
Ductography is an invasive procedure that involves cannulating the suspicious duct with a small catheter and injecting radiopaque dye into the duct under mammographic guidance. While the sensitivity of ductography is higher than that of both mammography and ultrasonography, its specificity is lower than that of either modality.
Most cases of pathologic discharge are spontaneous and are not reproducible on the day of the procedure. If the procedural radiologist cannot visualize the duct that is producing the discharge, the procedure cannot be performed. Although most patients tolerate the procedure well, ductography produces patient discomfort from cannulation of the duct and injection of contrast.
Magnetic resonance imaging
Dynamic contrast-enhanced magnetic resonance imaging (MRI) is the most sensitive imaging study for evaluating pathologic nipple discharge, and it has largely replaced ductography as an adjunct to mammography and ultrasonography. MRI’s sensitivity for detecting breast cancer ranges from 93% to 100%.6 In addition, MRI allows visualization of the entire breast and areas peripheral to the field of view of a standard ductogram or ultrasound scan.9
Clinicians commonly ask, “Why not skip the mammogram and ultrasound scan and go straight to MRI, since it is so much more sensitive?” Breast MRI has several limitations, including relatively low specificity, cost, use of intravenous contrast, and patient discomfort (that is, claustrophobia, prone positioning). MRI should be utilized for pathologic discharge only when the mammogram and/or targeted ultrasound scans are negative or equivocal.
CASE Continued: Additional imaging
A contrast-enhanced MRI of the breasts (FIGURE 4) demonstrates a large area of non-mass enhancement (red oval) in the left breast, which involves most of the upper breast extending from the nipple to the posterior breast tissue; it measures approximately 7.3 x 14 x 9.1 cm in transverse, anteroposterior, and craniocaudal dimensions, respectively. There is no evidence of left pectoralis muscle involvement. An MRI-directed second look left breast ultrasonography (FIGURE 5) is performed, revealing a small irregular mass in the left breast 1 o’clock position, 10 to 11 cm from the nipple (red arrow). This area had not been imaged in the prior ultrasound scan due to its posterior location far from the nipple. Ultrasound-guided core needle biopsy is performed; moderately differentiated invasive ductal carcinoma (IDC) with high-grade DCIS is found.


Continue to: When to refer for surgery...
When to refer for surgery
No surgical evaluation or intervention is needed for physiologic nipple discharge. As mentioned previously, reassure the patient and recommend appropriate breast cancer screening. In the setting of pathologic discharge, however, referral to a breast surgeon may be indicated after appropriate imaging workup has been done.
Since abnormal imaging almost always results in a recommendation for image-guided biopsy, typically the biopsy is performed prior to the surgical consultation. Once the pathology report from the biopsy is available, the radiologist makes a radiologic-pathologic concordance statement and recommends surgical consultation. This process allows the surgeon to have all the necessary information at the initial visit.
However, in the setting of pathologic nipple discharge with normal breast imaging, the surgeon and patient may opt for close observation or surgery for definitive diagnosis. Surgical options include single-duct excision when nipple discharge is localized to one duct or central duct excision when nipple discharge cannot be localized to one duct.
CASE Continued: Follow-up
The patient was referred to a breast surgeon. Given the extent of disease in the left breast, breast conservation was not possible. The patient underwent left breast simple mastectomy with sentinel lymph node biopsy and prophylactic right simple mastectomy. Final pathology results revealed stage IA IDC with DCIS. Sentinel lymph nodes were negative for malignancy. The patient underwent adjuvant left chest wall radiation, endocrine therapy with tamoxifen, and implant reconstruction. After 2 years of follow-up, she is disease free.
In summary
Nipple discharge can be classified as physiologic or pathologic. For pathologic discharge, a thorough physical examination should be performed with subsequent imaging evaluation. First-line tools, based on patient age, include diagnostic mammography and targeted ultrasonography. Contrast-enhanced MRI is then recommended for negative or equivocal cases. All patients with pathologic nipple discharge should be referred to a breast surgeon following appropriate imaging evaluation. ●
CASE Young woman with discharge from one nipple
A 26-year-old African American woman presents with a 10-month history of left nipple discharge. The patient describes the discharge as spontaneous, colored dark brown to yellow, and occurring from a single opening in the nipple. The discharge is associated with left breast pain and fullness, without a palpable lump. The patient has no family or personal history of breast cancer.
Nipple discharge is the third most common breast-related symptom (after palpable masses and breast pain), with an estimated prevalence of 5% to 8% among premenopausal women.1 While most causes of nipple discharge reflect benign issues, approximately 5% to 12% of breast cancers have nipple discharge as the only symptom.2 Not surprisingly, nipple discharge creates anxiety for both patients and clinicians.
In this article, we—a breast imaging radiologist, gynecologist, and breast surgeon—outline key steps for evaluating and managing patients with nipple discharge.
Two types of nipple discharge
Nipple discharge can be characterized as physiologic or pathologic. The distinction is based on the patient’s history in conjunction with the clinical breast exam.
Physiologic nipple discharge often is bilateral, nonspontaneous, and white, yellow, green, or brown (TABLE).3 It often is due to nipple stimulation, and the patient can elicit discharge by manually manipulating the breast. Usually, multiple ducts are involved. Galactorrhea refers specifically to milky discharge and occurs most commonly during pregnancy or lactation.2 Galactorrhea that is not associated with pregnancy or lactation often is related to elevated prolactin or thyroid-stimulating hormone levels or to medications. One study reported that no cancers were found when discharge was nonspontaneous and colored or milky.4
Pathologic nipple discharge is defined as a spontaneous, bloody, clear, or single-duct discharge. A palpable mass in the same breast automatically increases the suspicion of the discharge, regardless of its color or spontaneity.2 The most common cause of pathologic nipple discharge is an intraductal papilloma, a benign epithelial tumor, which accounts for approximately 57% of cases.5
Although the risk of malignancy is low for all patients with nipple discharge, increasing age is associated with increased risk of breast cancer. One study demonstrated that among women aged 40 to 60 years presenting with nipple discharge, the prevalence of invasive cancer is 10%, and the percentage jumps to 32% among women older than 60.6
Breast exam. For any patient with nonlactational nipple discharge, we recommend a thorough breast examination. Deep palpation of all quadrants of the symptomatic breast, especially near the nipple areolar complex, should elicit nipple discharge without any direct squeezing of the nipple. If the patient’s history and physical exam are consistent with physiologic discharge, no further workup is needed. Reassure the patient and recommend appropriate breast cancer screening. Encourage the patient to decrease stimulation or manual manipulation of the nipples if the discharge bothers her.
Continue to: CASE Continued: Workup...
CASE Continued: Workup
On physical exam, the patient’s breasts are noted to be cup size DDD and asymmetric, with the left breast larger than the right; there is no contour deformity. There is no skin or nipple retraction, skin rash, swelling, or nipple changes bilaterally. No dominant masses are appreciated bilaterally. Manual compression elicits no nipple discharge.
Although the discharge is nonbloody, its spontaneity, unilaterality, and single-duct/orifice origin suggest a pathologic cause. The patient is referred for breast imaging.
Imaging workup for pathologic discharge
The American College of Radiology (ACR) Appropriateness Criteria is a useful tool that provides an evidence-based, easy-to-use algorithm for breast imaging in the patient with pathologic nipple discharge (FIGURE 1).6 The algorithm is categorized by patient age, with diagnostic mammography recommended for women aged 30 and older.6 Diagnostic mammography is recommended if the patient has not had a mammogram study in the last 6 months.6 For patients with no prior mammograms, we recommend bilateral diagnostic mammography to compare symmetry of the breasts.

Currently, no studies show that digital breast tomosynthesis (3-D mammography) has a benefit compared with standard 2-D mammography in women with pathologic nipple discharge.6 Given the increased sensitivity of digital breast tomosynthesis for cancer detection, however, in our practice it is standard to use tomosynthesis in the diagnostic evaluation of most patients.
Mammography
On mammography, ductal carcinoma in situ (DCIS) usually presents as calcifications. Both the morphology and distribution of calcifications are used to characterize them as suspicious or, typically, benign. DCIS usually presents as fine pleomorphic or fine linear branching calcifications in a segmental or linear distribution. In patients with pathologic nipple discharge and no other symptoms, the radiologist must closely examine the retroareolar region of the breast to assess for faint calcifications. Magnification views also can be performed to better characterize calcifications.
The sensitivity of mammography for nipple discharge varies in the literature, ranging from approximately 15% to 68%, with a specificity range of 38% to 98%.6 This results in a relatively low positive predictive value but a high negative predictive value of 90%.7 Mammographic sensitivity largely is limited by increased breast density. As more data emerge on the utility of digital breast tomosynthesis in dense breasts, mammographic sensitivity for nipple discharge will likely increase.
Ultrasonography
As an adjunct to mammography, the ACR Appropriateness Criteria recommends targeted (or “limited”) ultrasonography of the retroareolar region of the affected breast for patients aged 30 and older. Ultrasonography is useful to assess for intraductal masses and architectural distortion, and it has higher sensitivity but lower specificity than mammography. The sensitivity of ultrasonography for detecting breast cancer in patients presenting with nipple discharge is reported to be 56% to 80%.6 Ultrasonography can identify lesions not visible mammographically in 63% to 69% of cases.8 Although DCIS usually presents as calcifications, it also can present as an intraductal mass on ultrasonography.
The ACR recommends targeted ultrasonography for patients with nipple discharge and a negative mammogram, or to evaluate a suspicious mammographic abnormality such as architectural distortion, focal asymmetry, or a mass.6 For patient comfort, ultrasonography is the preferred modality for image-guided biopsy.
For women younger than 30 years, targeted ultrasonography is the initial imaging study of choice, according to the ACR criteria.6 Women younger than 30 years with pathologic nipple discharge have a very low risk of breast cancer and tend to have higher breast density, making mammography less useful. Although the radiation dose from mammography is negligible given technological improvements and dose-reduction techniques, ultrasonography remains the preferred initial imaging modality in young women, not only for nipple discharge but also for palpable lumps and focal breast pain.
Mammography is used as an adjunct to ultrasonography in women younger than 30 years when a suspicious abnormality is detected on ultrasonography, such as an intraductal mass or architectural distortion. In these cases, mammography can be used to assess for extent of disease or to visualize suspicious calcifications not well seen on ultrasonography.
For practical purposes regarding which imaging study to order for a patient, it is most efficient to order both a diagnostic mammogram (with tomosynthesis, if possible) and a targeted ultrasound scan of the affected breast. Even if both orders are not needed, having them available increases efficiency for both the radiologist and the ordering physician.
Continue to: CASE Continued: Imaging findings...
CASE Continued: Imaging findings
Given her age, the patient initially undergoes targeted ultrasonography. The grayscale image (FIGURE 2) demonstrates multiple mildly dilated ducts (white arrows) with surrounding hyperechogenicity of the fat (red arrows), indicating soft tissue edema. No intraductal mass is imaged. Given that the ultrasonography findings are not completely negative and are equivocal for malignancy, bilateral diagnostic mammography (FIGURE 3, left breast only) is performed. Standard full-field craniocaudal (FIGURE 3A) and mediolateral oblique (FIGURE 3B) mammographic views demonstrate a heterogeneously dense breast with a few calcifications in the retroareolar left breast (red ovals). No associated mass or architectural distortions are noted. The mammographic and sonographic findings do not reveal a definitive biopsy target.


Ductography
When a suspicious abnormality is visualized on either mammography or ultrasonography, the standard of care is to perform an image-guided biopsy of the abnormality. When the standard workup is negative or equivocal, the standard of care historically was to perform ductography.
Ductography is an invasive procedure that involves cannulating the suspicious duct with a small catheter and injecting radiopaque dye into the duct under mammographic guidance. While the sensitivity of ductography is higher than that of both mammography and ultrasonography, its specificity is lower than that of either modality.
Most cases of pathologic discharge are spontaneous and are not reproducible on the day of the procedure. If the procedural radiologist cannot visualize the duct that is producing the discharge, the procedure cannot be performed. Although most patients tolerate the procedure well, ductography produces patient discomfort from cannulation of the duct and injection of contrast.
Magnetic resonance imaging
Dynamic contrast-enhanced magnetic resonance imaging (MRI) is the most sensitive imaging study for evaluating pathologic nipple discharge, and it has largely replaced ductography as an adjunct to mammography and ultrasonography. MRI’s sensitivity for detecting breast cancer ranges from 93% to 100%.6 In addition, MRI allows visualization of the entire breast and areas peripheral to the field of view of a standard ductogram or ultrasound scan.9
Clinicians commonly ask, “Why not skip the mammogram and ultrasound scan and go straight to MRI, since it is so much more sensitive?” Breast MRI has several limitations, including relatively low specificity, cost, use of intravenous contrast, and patient discomfort (that is, claustrophobia, prone positioning). MRI should be utilized for pathologic discharge only when the mammogram and/or targeted ultrasound scans are negative or equivocal.
CASE Continued: Additional imaging
A contrast-enhanced MRI of the breasts (FIGURE 4) demonstrates a large area of non-mass enhancement (red oval) in the left breast, which involves most of the upper breast extending from the nipple to the posterior breast tissue; it measures approximately 7.3 x 14 x 9.1 cm in transverse, anteroposterior, and craniocaudal dimensions, respectively. There is no evidence of left pectoralis muscle involvement. An MRI-directed second look left breast ultrasonography (FIGURE 5) is performed, revealing a small irregular mass in the left breast 1 o’clock position, 10 to 11 cm from the nipple (red arrow). This area had not been imaged in the prior ultrasound scan due to its posterior location far from the nipple. Ultrasound-guided core needle biopsy is performed; moderately differentiated invasive ductal carcinoma (IDC) with high-grade DCIS is found.


Continue to: When to refer for surgery...
When to refer for surgery
No surgical evaluation or intervention is needed for physiologic nipple discharge. As mentioned previously, reassure the patient and recommend appropriate breast cancer screening. In the setting of pathologic discharge, however, referral to a breast surgeon may be indicated after appropriate imaging workup has been done.
Since abnormal imaging almost always results in a recommendation for image-guided biopsy, typically the biopsy is performed prior to the surgical consultation. Once the pathology report from the biopsy is available, the radiologist makes a radiologic-pathologic concordance statement and recommends surgical consultation. This process allows the surgeon to have all the necessary information at the initial visit.
However, in the setting of pathologic nipple discharge with normal breast imaging, the surgeon and patient may opt for close observation or surgery for definitive diagnosis. Surgical options include single-duct excision when nipple discharge is localized to one duct or central duct excision when nipple discharge cannot be localized to one duct.
CASE Continued: Follow-up
The patient was referred to a breast surgeon. Given the extent of disease in the left breast, breast conservation was not possible. The patient underwent left breast simple mastectomy with sentinel lymph node biopsy and prophylactic right simple mastectomy. Final pathology results revealed stage IA IDC with DCIS. Sentinel lymph nodes were negative for malignancy. The patient underwent adjuvant left chest wall radiation, endocrine therapy with tamoxifen, and implant reconstruction. After 2 years of follow-up, she is disease free.
In summary
Nipple discharge can be classified as physiologic or pathologic. For pathologic discharge, a thorough physical examination should be performed with subsequent imaging evaluation. First-line tools, based on patient age, include diagnostic mammography and targeted ultrasonography. Contrast-enhanced MRI is then recommended for negative or equivocal cases. All patients with pathologic nipple discharge should be referred to a breast surgeon following appropriate imaging evaluation. ●
- Alcock C, Layer GT. Predicting occult malignancy in nipple discharge. ANZ J Surg. 2010;80:646-649.
- Patel BK, Falcon S, Drukteinis J. Management of nipple discharge and the associated imaging findings. Am J Med. 2015;128:353-360.
- Mazzarello S, Arnaout A. Five things to know about nipple discharge. CMAJ. 2015;187:599.
- Goksel HA, Yagmurdur MC, Demirhan B, et al. Management strategies for patients with nipple discharge. Langenbecks Arch Surg. 2005;390:52-58.
- Vargas HI, Vargas MP, Eldrageely K, et al. Outcomes of clinical and surgical assessment of women with pathological nipple discharge. Am Surg. 2006;72:124-128.
- Expert Panel on Breast Imaging; Lee S, Tikha S, Moy L, et al. American College of Radiology Appropriateness Criteria: Evaluation of nipple discharge. https://acsearch.acr.org /docs/3099312/Narrative/. Accessed February 2, 2020.
- Cabioglu N, Hunt KK, Singletary SE, et al. Surgical decision making and factors determining a diagnosis of breast carcinoma in women presenting with nipple discharge. J Am Coll Surg. 2003;196:354-364.
- Morrogh M, Park A, Elkin EB, et al. Lessons learned from 416 cases of nipple discharge of the breast. Am J Surg. 2010;200:73-80.
- Morrogh M, Morris EA, Liberman L, et al. The predictive value of ductography and magnetic resonance imaging in the management of nipple discharge. Ann Surg Oncol. 2007;14:3369-3377.
- Alcock C, Layer GT. Predicting occult malignancy in nipple discharge. ANZ J Surg. 2010;80:646-649.
- Patel BK, Falcon S, Drukteinis J. Management of nipple discharge and the associated imaging findings. Am J Med. 2015;128:353-360.
- Mazzarello S, Arnaout A. Five things to know about nipple discharge. CMAJ. 2015;187:599.
- Goksel HA, Yagmurdur MC, Demirhan B, et al. Management strategies for patients with nipple discharge. Langenbecks Arch Surg. 2005;390:52-58.
- Vargas HI, Vargas MP, Eldrageely K, et al. Outcomes of clinical and surgical assessment of women with pathological nipple discharge. Am Surg. 2006;72:124-128.
- Expert Panel on Breast Imaging; Lee S, Tikha S, Moy L, et al. American College of Radiology Appropriateness Criteria: Evaluation of nipple discharge. https://acsearch.acr.org /docs/3099312/Narrative/. Accessed February 2, 2020.
- Cabioglu N, Hunt KK, Singletary SE, et al. Surgical decision making and factors determining a diagnosis of breast carcinoma in women presenting with nipple discharge. J Am Coll Surg. 2003;196:354-364.
- Morrogh M, Park A, Elkin EB, et al. Lessons learned from 416 cases of nipple discharge of the breast. Am J Surg. 2010;200:73-80.
- Morrogh M, Morris EA, Liberman L, et al. The predictive value of ductography and magnetic resonance imaging in the management of nipple discharge. Ann Surg Oncol. 2007;14:3369-3377.
2020 Update on prenatal phenotyping
As prenatal genetic testing and imaging have advanced, the diagnosis of genetic disorders has moved from the postnatal to the prenatal time frame. This has largely been facilitated by the increasing use of exome sequencing (ES) in the prenatal setting. Two landmark trials published in January 2019 highlighted the overall diagnostic yields of prenatal ES as 8.5% and 10% in fetuses with normal karyotype and microarray.1,2
Although this is a huge step forward in prenatal diagnosis, ES is currently a manually curated, labor-intensive task. The process involves reviewing thousands of sequence variants for any given sample and prioritizing each variant based on bioinformatic data, prediction models, literature review, and specific patient characteristics. The patient characteristics, or phenotypic information, are critically important in prioritizing candidate variants.
To date, prenatal ES has been limited by the use of inconsistent terminology and the lack of well-understood prenatal phenotypes. In this Update, we highlight how recently published work draws attention to these critical gaps in prenatal diagnosis.
Standardizing phenotyping language in the prenatal setting
Tomar S, Sethi R, Lai PS. Specific phenotype semantics facilitate gene prioritization in clinical exome sequencing. Eur J Hum Genet. 2019;27:1389-1397.
Clinical ES in pediatric and adult populations is enhanced by the use of standardized vocabulary to describe disorders. Standardized language ensures that identified variants are filtered correctly and in a systematic fashion based on the patient characteristics that are provided. One commonly used platform is the Human Phenotype Ontology (HPO).
Tomar and colleagues assessed the impact of HPO-based clinical information on the performance of a gene prioritization tool.3 Gene prioritization (or simulation) tools are used for interpretation of ES data to help analysts efficiently sort through the thousands of variants in an individual’s genetic sequence. The performance, or accuracy, of a prioritization tool can be assessed by looking at the location of the disease-causing gene in the suggested gene list.
Continue to: Cohort of diagnosed patients and gene prioritization...
Cohort of diagnosed patients and gene prioritization
In this experimental model, Tomar and colleagues included 50 cases with neuromuscular disorders; all had available sequencing data, fully described phenotypes, and known causal genes. The authors varied the level of available clinical information in the HPO terms used for simulated variant analysis. Using 3 web-based gene prioritization tools on the 50 cases, they varied the HPO input to include a random selection of 10%, 30%, and 50% of HPO terms derived from deep phenotyping.
The 3 prioritization tools ranked input genes based on gene-phenotype associations that were derived from gene-phenotype databases. The authors then assessed the quality of the candidate gene lists by the location of the known causative gene on the generated rank lists. They repeated this analysis 4 times with different randomly selected HPO terms.
Inclusion of more HPO terms allowed for more accurate diagnoses in rare disorders
The authors found that the phenotype input for ES matters. When only 10% and 30% of the HPO terms were used to create a candidate gene list, the causative gene was less likely to be in the top portions of gene lists than when 50% or 100% of the available HPO terms were used.
For well-characterized disorders, use of the top 10% HPO terms performed as well as when all available HPO terms were used. For previously undescribed disease-gene associations, identification of the disease gene suffered with more limited HPO term availability.
What this study contributes
This study was a simulation of previously sequenced patients with neuromuscular disorders. It examined a small sample size for a narrow spectrum of disease. However, it clearly illustrated the principle that completeness of phenotypic information for ES pipelines is relevant for interpretation.
The quantity and quality of phenotype input into ES matters for assessing genetic variants. HPO terms have been developed to represent prenatal sonographic findings, and these have been extended to include gestational age of onset in some cases. Providing as much data as possible about the prenatal phenotype through accepted uniform vocabulary (such as HPO) will increase the likelihood that a prenatal diagnosis can be made.
Detailed description of prenatal findings is essential to diagnosis
Aarabi M, Sniezek O, Jiang H, et al. Importance of complete phenotyping in prenatal whole exome sequencing. Hum Genet. 2018;137:175-181.
In a retrospective cohort study, Aarabi and colleagues evaluated the diagnostic utility and limitations of ES in prenatal cases with structural birth defects.4
A case series study
The investigators included 20 pregnancies with structural birth defects that were referred to their center for prenatal diagnosis between 12 and 20 weeks’ gestation. All pregnancies had normal karyotype and microarray analyses prior to enrollment.
ES was performed on trio samples, which included fetal and parental DNA samples (extracted from peripheral blood). Reports provided by the commercial laboratories were normal for all cases and included no pathogenic or likely pathogenic variants. The laboratory provided the investigators with the FASTQ (genetic sequence) files for reanalysis, which was performed using both prenatal and postnatal detailed phenotypic information.
Continue to: Use of postnatal information facilitated diagnoses...
Use of postnatal information facilitated diagnoses
Reanalysis of ES data using detailed postnatal findings revealed a possible diagnosis in 20% of cases. Each case in which a diagnosis was made, detailed below, highlights an important limitation in our current ability to make prenatal diagnoses.
Case 1. A fetus was diagnosed prenatally with arthrogryposis, plagiocephaly, and club feet. After birth, the infant also was found to have generalized muscle weakness, elevated creatine phosphokinase, and congenital hip dislocation.
Reanalysis of the ES data revealed compound heterozygous missense variants in the nebulin gene (NEB). Although classified as variants of uncertain significance (VUS), these are consistent with the phenotype, the authors argued, and with the diagnosis of autosomal recessive nemaline myopathy 2.
Case 2. Prenatal diagnosis was made of a right limb anomaly, tetralogy of Fallot, intrauterine growth restriction, ambiguous genitalia, and dextrocardia. Postnatal evaluation revealed absent pulmonary valve syndrome, right arm dysplasia, pectus carinatum deformity, and failure to thrive.
In this case, ES with the postnatal information revealed a VUS in the NOTCH1 gene, which has been associated with Adams-Oliver syndrome. Although by strict criteria this variant is also of uncertain significance, Adams-Oliver syndrome is characterized, in part, by transverse limb defects and congenital heart disease, as was found in the proband.
Case 3. Prenatal ultrasonography revealed microcephaly and absence of the septum pellucidum. Postnatal magnetic resonance imaging revealed semi-lobar holoprosencephaly. A holoprosencephaly-specific gene panel revealed a deletion in the ZIC2 gene, which is known to cause holoprosencephaly.
Careful re-examination of the ES data revealed some abnormality in the ZIC2 signal, which might have been studied in greater detail and thereby detected if the prenatal diagnosis of holoprosencephaly had been made.
Case 4. An ultrasound evaluation at 12 weeks’ gestation revealed a cystic hygroma, short long bones, and possible absent hand and fibula. A postnatal fetal autopsy at 14 weeks showed split-hand and split-foot malformations, which were not appreciated on ultrasonography.
In filtering the ES data with this information, a pathogenic variant in the PRCN gene was identified as causal, and the diagnosis of Goltz syndrome was made.
Challenges facing prenatal diagnosis
A case series is inherently limited by its small sample size. Nevertheless, the authors suggest 2 major challenges in our ability to make the above diagnoses in the prenatal setting:
1) the prenatal assessment being limited to major structural abnormalities, and 2) commercial laboratories not having enough experience or volume to interpret the limited information provided by prenatal imaging.
Prenatal genetic diagnosis often is limited by incomplete information about the features seen on ultrasonography. Although not all features are visible prenatally, more diagnoses can be made if laboratories are provided with detailed information about the structural abnormalities that are seen. Furthermore, if ES does not provide a prenatal diagnosis, the data should be reviewed postnatally if more detailed phenotypic information becomes available.
Can AI technology be incorporated to make a genetic diagnosis?
Hsieh TC, Mensah MA, Pantel JT, et al. PEDIA: prioritization of exome data by image analysis. Genet Med. 2019;21:2807-2814.
Increasingly, ES is used in all types of undiagnosed, rare genetic diseases. Although there is a high diagnostic yield in many populations, ES’s clinical utility is limited by the labor-intensive process of interpreting each variant in the context of detailed phenotypic information. The widespread use of HPO would be one step toward standardizing the information that is entered into the analysis of ES data, but even HPO cannot capture certain visual clues.
Hsieh and colleagues attempted to use artificial intelligence (AI) for “next-generation phenotyping” to assess facial dysmorphology and integrate the information into variant classification.5 The authors described their approach of incorporating AI as “prioritization of exome data by image analysis” (PEDIA).
Continue to: Designing dysmorphology machine learning...
Designing dysmorphology machine learning
The cohort included 679 individuals with 105 different genetic disorders. All individuals had a previously confirmed molecular diagnosis that would be detected by ES. Each individual had a frontal facial photograph analyzed and detailed clinical features documented in HPO terms extracted by 2 clinicians.
A facial analysis software called DeepGestalt, trained on 17,000 patient images, was used to create a Gestalt score. Each individual had 4 different predicted gene scoring approaches:
- a molecular deleteriousness score
- facial analysis with the Gestalt score
- a combination of molecular deleteriousness score and HPO-based gene-prioritization tool (termed semantic similarity score)
- the PEDIA score, which included all 3 prior approaches.
A type of machine learning algorithm (support vector machine, or SVM) was applied, validated, and used to prioritize genes based on the combined scores.
AI seemed to improve diagnostic accuracy
Utilizing the combination of machine learning, HPO terms, and facial analysis software greatly improved the accuracy of variant classification predictions over any approach alone.
Using only the sequence variant and molecular deleteriousness score, the causative variant was ranked in the top 10 of all identified variants in less than 45% of cases. Adding the HPO-based gene prioritization tools increased the accuracy to 63% to 94%. Use of the PEDIA score, which incorporated all 3, increased the accuracy to 99% for the top 10 ranking.
Even more impressive improvements were made in the top 1 ranking accuracy rate, which went from 36% to 74% without facial image information to 86% to 89% with inclusion of DeepGestalt scores.
Study strengths and limitations
This study’s innovative application of facial analysis and machine learning, combined with HPO-driven variant classification, showed added benefit. To achieve this with available patient photographs and thorough phenotyping, previously diagnosed patients were used. Because complete ES information was not available for those patients, their known pathogenic variant was inserted into randomly selected exomes from the 1000 Genomes Project (healthy individuals). The authors additionally noted that the PEDIA score performance was diminished for rare disorders in which limited data were available.
The accuracy of gene prediction in pediatric and adult populations is enhanced by the use of computer-assisted image analysis and machine-learning algorithms. These computational methods may be employed to automate variant classification, making it more accurate, efficient, and less laborious. Detailed descriptions or characteristic images of prenatal findings also may allow this technology to be introduced in the prenatal setting.
- Lord J, McMullan DJ, Eberhardt RY, et al; for the Prenatal Assessment of Genomes and Exomes Consortium. Prenatal exome sequencing analysis in fetal structural anomalies detected by ultrasonography (PAGE): a cohort study. Lancet. 2019;393:747-757.
- Petrovski S, Aggarwal V, Giordano JL, et al. Whole-exome sequencing in the evaluation of fetal structural anomalies: a prospective cohort study. Lancet. 2019;393:758-767.
- Tomar S, Sethi R, Lai PS. Specific phenotype semantics facilitate gene prioritization in clinical exome sequencing. Eur J Hum Genet. 2019;27:1389-1397.
- Aarabi M, Sniezek O, Jiang H, et al. Importance of complete phenotyping in prenatal whole exome sequencing. Hum Genet. 2018;137:175-181.
- Hsieh TC, Mensah MA, Pantel JT, et al. PEDIA: prioritization of exome data by image analysis. Genet Med. 2019;21:2807-2814.
As prenatal genetic testing and imaging have advanced, the diagnosis of genetic disorders has moved from the postnatal to the prenatal time frame. This has largely been facilitated by the increasing use of exome sequencing (ES) in the prenatal setting. Two landmark trials published in January 2019 highlighted the overall diagnostic yields of prenatal ES as 8.5% and 10% in fetuses with normal karyotype and microarray.1,2
Although this is a huge step forward in prenatal diagnosis, ES is currently a manually curated, labor-intensive task. The process involves reviewing thousands of sequence variants for any given sample and prioritizing each variant based on bioinformatic data, prediction models, literature review, and specific patient characteristics. The patient characteristics, or phenotypic information, are critically important in prioritizing candidate variants.
To date, prenatal ES has been limited by the use of inconsistent terminology and the lack of well-understood prenatal phenotypes. In this Update, we highlight how recently published work draws attention to these critical gaps in prenatal diagnosis.
Standardizing phenotyping language in the prenatal setting
Tomar S, Sethi R, Lai PS. Specific phenotype semantics facilitate gene prioritization in clinical exome sequencing. Eur J Hum Genet. 2019;27:1389-1397.
Clinical ES in pediatric and adult populations is enhanced by the use of standardized vocabulary to describe disorders. Standardized language ensures that identified variants are filtered correctly and in a systematic fashion based on the patient characteristics that are provided. One commonly used platform is the Human Phenotype Ontology (HPO).
Tomar and colleagues assessed the impact of HPO-based clinical information on the performance of a gene prioritization tool.3 Gene prioritization (or simulation) tools are used for interpretation of ES data to help analysts efficiently sort through the thousands of variants in an individual’s genetic sequence. The performance, or accuracy, of a prioritization tool can be assessed by looking at the location of the disease-causing gene in the suggested gene list.
Continue to: Cohort of diagnosed patients and gene prioritization...
Cohort of diagnosed patients and gene prioritization
In this experimental model, Tomar and colleagues included 50 cases with neuromuscular disorders; all had available sequencing data, fully described phenotypes, and known causal genes. The authors varied the level of available clinical information in the HPO terms used for simulated variant analysis. Using 3 web-based gene prioritization tools on the 50 cases, they varied the HPO input to include a random selection of 10%, 30%, and 50% of HPO terms derived from deep phenotyping.
The 3 prioritization tools ranked input genes based on gene-phenotype associations that were derived from gene-phenotype databases. The authors then assessed the quality of the candidate gene lists by the location of the known causative gene on the generated rank lists. They repeated this analysis 4 times with different randomly selected HPO terms.
Inclusion of more HPO terms allowed for more accurate diagnoses in rare disorders
The authors found that the phenotype input for ES matters. When only 10% and 30% of the HPO terms were used to create a candidate gene list, the causative gene was less likely to be in the top portions of gene lists than when 50% or 100% of the available HPO terms were used.
For well-characterized disorders, use of the top 10% HPO terms performed as well as when all available HPO terms were used. For previously undescribed disease-gene associations, identification of the disease gene suffered with more limited HPO term availability.
What this study contributes
This study was a simulation of previously sequenced patients with neuromuscular disorders. It examined a small sample size for a narrow spectrum of disease. However, it clearly illustrated the principle that completeness of phenotypic information for ES pipelines is relevant for interpretation.
The quantity and quality of phenotype input into ES matters for assessing genetic variants. HPO terms have been developed to represent prenatal sonographic findings, and these have been extended to include gestational age of onset in some cases. Providing as much data as possible about the prenatal phenotype through accepted uniform vocabulary (such as HPO) will increase the likelihood that a prenatal diagnosis can be made.
Detailed description of prenatal findings is essential to diagnosis
Aarabi M, Sniezek O, Jiang H, et al. Importance of complete phenotyping in prenatal whole exome sequencing. Hum Genet. 2018;137:175-181.
In a retrospective cohort study, Aarabi and colleagues evaluated the diagnostic utility and limitations of ES in prenatal cases with structural birth defects.4
A case series study
The investigators included 20 pregnancies with structural birth defects that were referred to their center for prenatal diagnosis between 12 and 20 weeks’ gestation. All pregnancies had normal karyotype and microarray analyses prior to enrollment.
ES was performed on trio samples, which included fetal and parental DNA samples (extracted from peripheral blood). Reports provided by the commercial laboratories were normal for all cases and included no pathogenic or likely pathogenic variants. The laboratory provided the investigators with the FASTQ (genetic sequence) files for reanalysis, which was performed using both prenatal and postnatal detailed phenotypic information.
Continue to: Use of postnatal information facilitated diagnoses...
Use of postnatal information facilitated diagnoses
Reanalysis of ES data using detailed postnatal findings revealed a possible diagnosis in 20% of cases. Each case in which a diagnosis was made, detailed below, highlights an important limitation in our current ability to make prenatal diagnoses.
Case 1. A fetus was diagnosed prenatally with arthrogryposis, plagiocephaly, and club feet. After birth, the infant also was found to have generalized muscle weakness, elevated creatine phosphokinase, and congenital hip dislocation.
Reanalysis of the ES data revealed compound heterozygous missense variants in the nebulin gene (NEB). Although classified as variants of uncertain significance (VUS), these are consistent with the phenotype, the authors argued, and with the diagnosis of autosomal recessive nemaline myopathy 2.
Case 2. Prenatal diagnosis was made of a right limb anomaly, tetralogy of Fallot, intrauterine growth restriction, ambiguous genitalia, and dextrocardia. Postnatal evaluation revealed absent pulmonary valve syndrome, right arm dysplasia, pectus carinatum deformity, and failure to thrive.
In this case, ES with the postnatal information revealed a VUS in the NOTCH1 gene, which has been associated with Adams-Oliver syndrome. Although by strict criteria this variant is also of uncertain significance, Adams-Oliver syndrome is characterized, in part, by transverse limb defects and congenital heart disease, as was found in the proband.
Case 3. Prenatal ultrasonography revealed microcephaly and absence of the septum pellucidum. Postnatal magnetic resonance imaging revealed semi-lobar holoprosencephaly. A holoprosencephaly-specific gene panel revealed a deletion in the ZIC2 gene, which is known to cause holoprosencephaly.
Careful re-examination of the ES data revealed some abnormality in the ZIC2 signal, which might have been studied in greater detail and thereby detected if the prenatal diagnosis of holoprosencephaly had been made.
Case 4. An ultrasound evaluation at 12 weeks’ gestation revealed a cystic hygroma, short long bones, and possible absent hand and fibula. A postnatal fetal autopsy at 14 weeks showed split-hand and split-foot malformations, which were not appreciated on ultrasonography.
In filtering the ES data with this information, a pathogenic variant in the PRCN gene was identified as causal, and the diagnosis of Goltz syndrome was made.
Challenges facing prenatal diagnosis
A case series is inherently limited by its small sample size. Nevertheless, the authors suggest 2 major challenges in our ability to make the above diagnoses in the prenatal setting:
1) the prenatal assessment being limited to major structural abnormalities, and 2) commercial laboratories not having enough experience or volume to interpret the limited information provided by prenatal imaging.
Prenatal genetic diagnosis often is limited by incomplete information about the features seen on ultrasonography. Although not all features are visible prenatally, more diagnoses can be made if laboratories are provided with detailed information about the structural abnormalities that are seen. Furthermore, if ES does not provide a prenatal diagnosis, the data should be reviewed postnatally if more detailed phenotypic information becomes available.
Can AI technology be incorporated to make a genetic diagnosis?
Hsieh TC, Mensah MA, Pantel JT, et al. PEDIA: prioritization of exome data by image analysis. Genet Med. 2019;21:2807-2814.
Increasingly, ES is used in all types of undiagnosed, rare genetic diseases. Although there is a high diagnostic yield in many populations, ES’s clinical utility is limited by the labor-intensive process of interpreting each variant in the context of detailed phenotypic information. The widespread use of HPO would be one step toward standardizing the information that is entered into the analysis of ES data, but even HPO cannot capture certain visual clues.
Hsieh and colleagues attempted to use artificial intelligence (AI) for “next-generation phenotyping” to assess facial dysmorphology and integrate the information into variant classification.5 The authors described their approach of incorporating AI as “prioritization of exome data by image analysis” (PEDIA).
Continue to: Designing dysmorphology machine learning...
Designing dysmorphology machine learning
The cohort included 679 individuals with 105 different genetic disorders. All individuals had a previously confirmed molecular diagnosis that would be detected by ES. Each individual had a frontal facial photograph analyzed and detailed clinical features documented in HPO terms extracted by 2 clinicians.
A facial analysis software called DeepGestalt, trained on 17,000 patient images, was used to create a Gestalt score. Each individual had 4 different predicted gene scoring approaches:
- a molecular deleteriousness score
- facial analysis with the Gestalt score
- a combination of molecular deleteriousness score and HPO-based gene-prioritization tool (termed semantic similarity score)
- the PEDIA score, which included all 3 prior approaches.
A type of machine learning algorithm (support vector machine, or SVM) was applied, validated, and used to prioritize genes based on the combined scores.
AI seemed to improve diagnostic accuracy
Utilizing the combination of machine learning, HPO terms, and facial analysis software greatly improved the accuracy of variant classification predictions over any approach alone.
Using only the sequence variant and molecular deleteriousness score, the causative variant was ranked in the top 10 of all identified variants in less than 45% of cases. Adding the HPO-based gene prioritization tools increased the accuracy to 63% to 94%. Use of the PEDIA score, which incorporated all 3, increased the accuracy to 99% for the top 10 ranking.
Even more impressive improvements were made in the top 1 ranking accuracy rate, which went from 36% to 74% without facial image information to 86% to 89% with inclusion of DeepGestalt scores.
Study strengths and limitations
This study’s innovative application of facial analysis and machine learning, combined with HPO-driven variant classification, showed added benefit. To achieve this with available patient photographs and thorough phenotyping, previously diagnosed patients were used. Because complete ES information was not available for those patients, their known pathogenic variant was inserted into randomly selected exomes from the 1000 Genomes Project (healthy individuals). The authors additionally noted that the PEDIA score performance was diminished for rare disorders in which limited data were available.
The accuracy of gene prediction in pediatric and adult populations is enhanced by the use of computer-assisted image analysis and machine-learning algorithms. These computational methods may be employed to automate variant classification, making it more accurate, efficient, and less laborious. Detailed descriptions or characteristic images of prenatal findings also may allow this technology to be introduced in the prenatal setting.
As prenatal genetic testing and imaging have advanced, the diagnosis of genetic disorders has moved from the postnatal to the prenatal time frame. This has largely been facilitated by the increasing use of exome sequencing (ES) in the prenatal setting. Two landmark trials published in January 2019 highlighted the overall diagnostic yields of prenatal ES as 8.5% and 10% in fetuses with normal karyotype and microarray.1,2
Although this is a huge step forward in prenatal diagnosis, ES is currently a manually curated, labor-intensive task. The process involves reviewing thousands of sequence variants for any given sample and prioritizing each variant based on bioinformatic data, prediction models, literature review, and specific patient characteristics. The patient characteristics, or phenotypic information, are critically important in prioritizing candidate variants.
To date, prenatal ES has been limited by the use of inconsistent terminology and the lack of well-understood prenatal phenotypes. In this Update, we highlight how recently published work draws attention to these critical gaps in prenatal diagnosis.
Standardizing phenotyping language in the prenatal setting
Tomar S, Sethi R, Lai PS. Specific phenotype semantics facilitate gene prioritization in clinical exome sequencing. Eur J Hum Genet. 2019;27:1389-1397.
Clinical ES in pediatric and adult populations is enhanced by the use of standardized vocabulary to describe disorders. Standardized language ensures that identified variants are filtered correctly and in a systematic fashion based on the patient characteristics that are provided. One commonly used platform is the Human Phenotype Ontology (HPO).
Tomar and colleagues assessed the impact of HPO-based clinical information on the performance of a gene prioritization tool.3 Gene prioritization (or simulation) tools are used for interpretation of ES data to help analysts efficiently sort through the thousands of variants in an individual’s genetic sequence. The performance, or accuracy, of a prioritization tool can be assessed by looking at the location of the disease-causing gene in the suggested gene list.
Continue to: Cohort of diagnosed patients and gene prioritization...
Cohort of diagnosed patients and gene prioritization
In this experimental model, Tomar and colleagues included 50 cases with neuromuscular disorders; all had available sequencing data, fully described phenotypes, and known causal genes. The authors varied the level of available clinical information in the HPO terms used for simulated variant analysis. Using 3 web-based gene prioritization tools on the 50 cases, they varied the HPO input to include a random selection of 10%, 30%, and 50% of HPO terms derived from deep phenotyping.
The 3 prioritization tools ranked input genes based on gene-phenotype associations that were derived from gene-phenotype databases. The authors then assessed the quality of the candidate gene lists by the location of the known causative gene on the generated rank lists. They repeated this analysis 4 times with different randomly selected HPO terms.
Inclusion of more HPO terms allowed for more accurate diagnoses in rare disorders
The authors found that the phenotype input for ES matters. When only 10% and 30% of the HPO terms were used to create a candidate gene list, the causative gene was less likely to be in the top portions of gene lists than when 50% or 100% of the available HPO terms were used.
For well-characterized disorders, use of the top 10% HPO terms performed as well as when all available HPO terms were used. For previously undescribed disease-gene associations, identification of the disease gene suffered with more limited HPO term availability.
What this study contributes
This study was a simulation of previously sequenced patients with neuromuscular disorders. It examined a small sample size for a narrow spectrum of disease. However, it clearly illustrated the principle that completeness of phenotypic information for ES pipelines is relevant for interpretation.
The quantity and quality of phenotype input into ES matters for assessing genetic variants. HPO terms have been developed to represent prenatal sonographic findings, and these have been extended to include gestational age of onset in some cases. Providing as much data as possible about the prenatal phenotype through accepted uniform vocabulary (such as HPO) will increase the likelihood that a prenatal diagnosis can be made.
Detailed description of prenatal findings is essential to diagnosis
Aarabi M, Sniezek O, Jiang H, et al. Importance of complete phenotyping in prenatal whole exome sequencing. Hum Genet. 2018;137:175-181.
In a retrospective cohort study, Aarabi and colleagues evaluated the diagnostic utility and limitations of ES in prenatal cases with structural birth defects.4
A case series study
The investigators included 20 pregnancies with structural birth defects that were referred to their center for prenatal diagnosis between 12 and 20 weeks’ gestation. All pregnancies had normal karyotype and microarray analyses prior to enrollment.
ES was performed on trio samples, which included fetal and parental DNA samples (extracted from peripheral blood). Reports provided by the commercial laboratories were normal for all cases and included no pathogenic or likely pathogenic variants. The laboratory provided the investigators with the FASTQ (genetic sequence) files for reanalysis, which was performed using both prenatal and postnatal detailed phenotypic information.
Continue to: Use of postnatal information facilitated diagnoses...
Use of postnatal information facilitated diagnoses
Reanalysis of ES data using detailed postnatal findings revealed a possible diagnosis in 20% of cases. Each case in which a diagnosis was made, detailed below, highlights an important limitation in our current ability to make prenatal diagnoses.
Case 1. A fetus was diagnosed prenatally with arthrogryposis, plagiocephaly, and club feet. After birth, the infant also was found to have generalized muscle weakness, elevated creatine phosphokinase, and congenital hip dislocation.
Reanalysis of the ES data revealed compound heterozygous missense variants in the nebulin gene (NEB). Although classified as variants of uncertain significance (VUS), these are consistent with the phenotype, the authors argued, and with the diagnosis of autosomal recessive nemaline myopathy 2.
Case 2. Prenatal diagnosis was made of a right limb anomaly, tetralogy of Fallot, intrauterine growth restriction, ambiguous genitalia, and dextrocardia. Postnatal evaluation revealed absent pulmonary valve syndrome, right arm dysplasia, pectus carinatum deformity, and failure to thrive.
In this case, ES with the postnatal information revealed a VUS in the NOTCH1 gene, which has been associated with Adams-Oliver syndrome. Although by strict criteria this variant is also of uncertain significance, Adams-Oliver syndrome is characterized, in part, by transverse limb defects and congenital heart disease, as was found in the proband.
Case 3. Prenatal ultrasonography revealed microcephaly and absence of the septum pellucidum. Postnatal magnetic resonance imaging revealed semi-lobar holoprosencephaly. A holoprosencephaly-specific gene panel revealed a deletion in the ZIC2 gene, which is known to cause holoprosencephaly.
Careful re-examination of the ES data revealed some abnormality in the ZIC2 signal, which might have been studied in greater detail and thereby detected if the prenatal diagnosis of holoprosencephaly had been made.
Case 4. An ultrasound evaluation at 12 weeks’ gestation revealed a cystic hygroma, short long bones, and possible absent hand and fibula. A postnatal fetal autopsy at 14 weeks showed split-hand and split-foot malformations, which were not appreciated on ultrasonography.
In filtering the ES data with this information, a pathogenic variant in the PRCN gene was identified as causal, and the diagnosis of Goltz syndrome was made.
Challenges facing prenatal diagnosis
A case series is inherently limited by its small sample size. Nevertheless, the authors suggest 2 major challenges in our ability to make the above diagnoses in the prenatal setting:
1) the prenatal assessment being limited to major structural abnormalities, and 2) commercial laboratories not having enough experience or volume to interpret the limited information provided by prenatal imaging.
Prenatal genetic diagnosis often is limited by incomplete information about the features seen on ultrasonography. Although not all features are visible prenatally, more diagnoses can be made if laboratories are provided with detailed information about the structural abnormalities that are seen. Furthermore, if ES does not provide a prenatal diagnosis, the data should be reviewed postnatally if more detailed phenotypic information becomes available.
Can AI technology be incorporated to make a genetic diagnosis?
Hsieh TC, Mensah MA, Pantel JT, et al. PEDIA: prioritization of exome data by image analysis. Genet Med. 2019;21:2807-2814.
Increasingly, ES is used in all types of undiagnosed, rare genetic diseases. Although there is a high diagnostic yield in many populations, ES’s clinical utility is limited by the labor-intensive process of interpreting each variant in the context of detailed phenotypic information. The widespread use of HPO would be one step toward standardizing the information that is entered into the analysis of ES data, but even HPO cannot capture certain visual clues.
Hsieh and colleagues attempted to use artificial intelligence (AI) for “next-generation phenotyping” to assess facial dysmorphology and integrate the information into variant classification.5 The authors described their approach of incorporating AI as “prioritization of exome data by image analysis” (PEDIA).
Continue to: Designing dysmorphology machine learning...
Designing dysmorphology machine learning
The cohort included 679 individuals with 105 different genetic disorders. All individuals had a previously confirmed molecular diagnosis that would be detected by ES. Each individual had a frontal facial photograph analyzed and detailed clinical features documented in HPO terms extracted by 2 clinicians.
A facial analysis software called DeepGestalt, trained on 17,000 patient images, was used to create a Gestalt score. Each individual had 4 different predicted gene scoring approaches:
- a molecular deleteriousness score
- facial analysis with the Gestalt score
- a combination of molecular deleteriousness score and HPO-based gene-prioritization tool (termed semantic similarity score)
- the PEDIA score, which included all 3 prior approaches.
A type of machine learning algorithm (support vector machine, or SVM) was applied, validated, and used to prioritize genes based on the combined scores.
AI seemed to improve diagnostic accuracy
Utilizing the combination of machine learning, HPO terms, and facial analysis software greatly improved the accuracy of variant classification predictions over any approach alone.
Using only the sequence variant and molecular deleteriousness score, the causative variant was ranked in the top 10 of all identified variants in less than 45% of cases. Adding the HPO-based gene prioritization tools increased the accuracy to 63% to 94%. Use of the PEDIA score, which incorporated all 3, increased the accuracy to 99% for the top 10 ranking.
Even more impressive improvements were made in the top 1 ranking accuracy rate, which went from 36% to 74% without facial image information to 86% to 89% with inclusion of DeepGestalt scores.
Study strengths and limitations
This study’s innovative application of facial analysis and machine learning, combined with HPO-driven variant classification, showed added benefit. To achieve this with available patient photographs and thorough phenotyping, previously diagnosed patients were used. Because complete ES information was not available for those patients, their known pathogenic variant was inserted into randomly selected exomes from the 1000 Genomes Project (healthy individuals). The authors additionally noted that the PEDIA score performance was diminished for rare disorders in which limited data were available.
The accuracy of gene prediction in pediatric and adult populations is enhanced by the use of computer-assisted image analysis and machine-learning algorithms. These computational methods may be employed to automate variant classification, making it more accurate, efficient, and less laborious. Detailed descriptions or characteristic images of prenatal findings also may allow this technology to be introduced in the prenatal setting.
- Lord J, McMullan DJ, Eberhardt RY, et al; for the Prenatal Assessment of Genomes and Exomes Consortium. Prenatal exome sequencing analysis in fetal structural anomalies detected by ultrasonography (PAGE): a cohort study. Lancet. 2019;393:747-757.
- Petrovski S, Aggarwal V, Giordano JL, et al. Whole-exome sequencing in the evaluation of fetal structural anomalies: a prospective cohort study. Lancet. 2019;393:758-767.
- Tomar S, Sethi R, Lai PS. Specific phenotype semantics facilitate gene prioritization in clinical exome sequencing. Eur J Hum Genet. 2019;27:1389-1397.
- Aarabi M, Sniezek O, Jiang H, et al. Importance of complete phenotyping in prenatal whole exome sequencing. Hum Genet. 2018;137:175-181.
- Hsieh TC, Mensah MA, Pantel JT, et al. PEDIA: prioritization of exome data by image analysis. Genet Med. 2019;21:2807-2814.
- Lord J, McMullan DJ, Eberhardt RY, et al; for the Prenatal Assessment of Genomes and Exomes Consortium. Prenatal exome sequencing analysis in fetal structural anomalies detected by ultrasonography (PAGE): a cohort study. Lancet. 2019;393:747-757.
- Petrovski S, Aggarwal V, Giordano JL, et al. Whole-exome sequencing in the evaluation of fetal structural anomalies: a prospective cohort study. Lancet. 2019;393:758-767.
- Tomar S, Sethi R, Lai PS. Specific phenotype semantics facilitate gene prioritization in clinical exome sequencing. Eur J Hum Genet. 2019;27:1389-1397.
- Aarabi M, Sniezek O, Jiang H, et al. Importance of complete phenotyping in prenatal whole exome sequencing. Hum Genet. 2018;137:175-181.
- Hsieh TC, Mensah MA, Pantel JT, et al. PEDIA: prioritization of exome data by image analysis. Genet Med. 2019;21:2807-2814.
Progesterone for preterm delivery prevention
Researchers have been studying the use of exogenous progestins for prevention of preterm delivery (PTD) for almost 60 years, but conflicting results contribute to an ongoing debate. Interpretation of the available data is particularly difficult because different forms and doses of progestins have been used in disparate study populations.
Based on available data, progesterone supplementation is not effective as a primary prevention strategy for PTD in the general low-risk obstetric population. PTD is a complex problem with varied and incompletely elucidated pathogenic pathways, making it unlikely that one interventional approach would be effective for all pregnant women. As a result, emerging indications for the use of progesterone are based on risk factors for PTD (ie, prior PTD and/or short cervix). However, this secondary prevention approach is a limiting factor in itself because 50% of women destined to have a PTD have no identifiable risk factors.1 In addition, researchers have found that progestins are ineffective at delaying delivery for women with multiple gestation, suggesting that a distinct underlying mechanism of early parturition is present in these women, and that this mechanism is unresponsive to progestins.2
The formulations used in the study of progestin supplementation for PTD prevention have been almost exclusively either the synthetic 17 alpha-hydroxyprogesterone caproate (17-OHPC) or natural progesterone administered orally or vaginally. In 2003, the American College of Obstetricians and Gynecologists (ACOG) supported the use of progesterone to reduce the rate of PTD,3 and in 2011, the US Food and Drug Administration (FDA) approved 17-OHPC for use as prophylaxis against recurrent PTD. As a result, in recent years, the perceived standard of care for a majority of practitioners in the United States had been that all women with a previous preterm birth should be offered 17-OHPC. It may be interesting to note that in other parts of the world, the same enthusiastic adoption did not occur. For example, in Australia and New Zealand in 2007, only 5% of practitioners were using progesterone for this indication.4 Further, 17-OHPC is not recommended by professional guidelines in the United Kingdom and has remained unavailable in Germany.
The publication in 2019 of the PROLONG trial called into question the use of 17-OHPC for the prevention of PTD.5 In the December 2019 issue of OBG Management (“Managing preterm birth in those at risk: Expert strategies”), I expressed the opinion that with only rare exceptions, 17-OHPC is no longer a viable option for recurrent PTD prevention.6 In light of these developments, what scientific evidence is relevant and applicable to the care of women at risk for PTD?
Continue to: Case 1 Previous spontaneous PTD at 31 weeks...
Case 1 Previous spontaneous PTD at 31 weeks
MC is an asymptomatic 32-year-old woman with a singleton pregnancy at 13 weeks’ gestation. You see her for a maternal-fetal medicine consultation because 2 years ago she had a spontaneous PTD at 31 weeks’ gestation. What management recommendations can you make to decrease her risk of recurrent PTD?
Cervical length measurement narrows in on risk
The indication “previous preterm birth” is largely meaningless because of the heterogeneity in preterm birth pathways (preterm birth as a syndrome7) and inadequate risk characterization. Among women who experience a spontaneous PTD, 70% to 80% do not deliver prematurely in subsequent pregnancies.8 To better characterize the risk of PTD recurrence, ultrasound assessment of cervical length should be used. Research has shown that among women with a prior spontaneous PTD who maintain a normal cervical length until 24 weeks’ gestation, more than 90% will deliver at 35 weeks or after without intervention.9 Such an approach not only identifies the subgroup of women at significantly increased risk of recurrence but also eliminates unnecessary interventions.
Cervical ultrasound surveillance should be initiated at 16 weeks’ gestation. A short cervix before 16 weeks is not associated with a statistically significant increase in risk for PTD.10 Shortening of the cervix begins approximately 10 weeks before delivery in any gestational age group.11 Therefore, ultrasound assessment of the cervix at 28 weeks and after is irrelevant. In addition, after 28 weeks, cervical length varies greatly leading to loss in the predictive power of the cervical measurement.12 Based on these considerations, cervical surveillance may be extended up to 26 weeks. Although cervical cerclage is not an option in the United States in cases in which a short cervix is detected between 24 and 26 weeks, vaginal progesterone supplementation may still be considered.
Case 1 Continued
MC was started on ultrasound cervical surveillance at 16 weeks’ gestation. Her cervical length was initially normal (> 2.5 cm), but at 18 weeks the measurement was 2.2 cm. What is your recommendation?
The value of vaginal progesterone
There appears to be increasing consensus on the value of vaginal progesterone for women with a midtrimester short cervix on sonography, with or without a history of PTD. An individual patient data meta-analysis demonstrated the benefits of vaginal progesterone.13 Although there was no evidence of an effect on PTD at less than 37 weeks, the rates of PTD at less than 36 weeks and spontaneous PTD at less than 34 weeks were significantly reduced (by 20% and 28%, respectively). Also, there was a significant reduction in the risk of respiratory distress syndrome (53%) and composite neonatal morbidity and mortality (41%), with no significant impact on infant development up to the second year of life.13
The lack of generalizable evidence of benefit on childhood outcomes, combined with considerable uncertainty about the exact role and mechanism of action of exogenous progestins, contribute to the ongoing debate. Vaginal progesterone dosage regimens have been based on extrapolations from experience with progesterone in nonpregnant women, and recent pharmacokinetic studies have revealed how precarious such extrapolations may be. As an example, in nonpregnant women, the bioavailability of oral and vaginal progesterone is similar.14 In pregnancy, however, while daily oral progesterone doubles a pregnant woman’s serum progesterone level,15 daily vaginal administration of progesterone results in only a modest rise in serum progesterone, with a coefficient of variation among individuals that is double that outside of pregnancy.16 It is, therefore, considered that vaginal progesterone in pregnancy may have a local action secondary to the uterine first-pass effect. The uterine first-pass effect for vaginal progesterone was described in nonpregnant women and is only assumed to occur in pregnancy as well. 17
After evaluating the data from the largest available study of vaginal progesterone,18 the FDA concluded in 2012 that the study did not meet the statistical significance generally expected to support the approval of a new product. However, according to a more comprehensive evidence review developed in 2019 by the National Guideline Alliance in the United Kingdom, women with a history of PTD and women with a short cervix derive an important benefit from the use of vaginal progesterone; thus, this intervention should be offered to them.19 At this time, a short cervix and PTD prevention are not considered FDA-approved indications for progesterone supplementation in pregnancy. However, vaginal progesterone is FDA approved for use in pregnant women with a history of infertility.
Continue to: Case 1 Continued...
Case 1 Continued
MC initiated treatment with daily vaginal progesterone at 18 weeks’ gestation and returned for ultrasound cervical length examination weekly instead of every other week. At 20 weeks’ gestation, cervical length was 2.0 cm; the following week it was 1.4 cm. What would you recommend at this point?
When to consider cerclage
If cervical shortening progresses to about 1.5 cm while a woman is being treated with vaginal progesterone, cerclage may be considered. The benefit of cerclage in patients with prior PTD and a short cervix was highlighted in a 2018 Cochrane Review.20 In this stepwise management approach to a short cervix, waiting for a cervix to be less than 1.5 cm may be unadvisable. Under conditions of a very short cervix that is frequently dilated with exposure of fetal membranes, ascending subclinical intra-amniotic infection may already be present, reducing the efficacy of any preventive measures. Preferential consideration for cerclage from the start over initial vaginal progesterone also may be appropriate when there is a history of 2 spontaneous PTDs or mid-trimester losses, a history of a successful cerclage, or with a very short cervix (< 1.0 cm) at the initial evaluation. As for the latter, a 2018 individual patient data meta-analysis of vaginal progesterone found no benefit when the cervix was less than 1.0 cm.13
Progesterone plus cerclage likely to add benefit
The results of an adjusted indirect comparison meta-analysis suggest that both interventions—vaginal progesterone and cerclage—are equally effective.21 Assuming that there is no clinically meaningful difference in benefit associated with these 2 treatments, the next logical question is whether combining the 2 therapies provides any added benefit; limited observational data seem to suggest that it does. In a retrospective cohort of 86 consecutive singleton pregnancies among women who underwent ultrasound-indicated cerclage, those who used vaginal progesterone after cerclage (n = 45) had a lower rate of PTD.22 Also, a small (66 cases) case-control study demonstrated the benefit of administration of vaginal progesterone as a rescue intervention in women with cerclage and progressive cervical shortening despite cerclage.23
Case 2 Woman experiences adverse effects from vaginal progesterone
MS is a 25-year-old G2P0101 who was started on vaginal progesterone as prophylaxis for recurrent PTD. She is now at 20 weeks’ gestation, with a stable remnant cervical length of 2.0 cm. She is reporting an increasing vaginal burning sensation and vaginal discharge caused by the nightly vaginal progesterone applications, to the point that she is unwilling to continue the treatment. She asks if any alternatives to vaginal progesterone are available to decrease her risk of PTD.
Continue to: Is oral progesterone an option?...
Is oral progesterone an option?
In the 1980s and 1990s, oral micronized progesterone was widely used in France at doses of 900 to 1,200 mg/d for women at risk for PTD. The practice was stopped when secondary hepatic effects, including cholestasis of pregnancy, were reported at a higher rate in treated women.24 A rise in the serum concentration of progesterone metabolites has been associated with impaired biliary excretion and subsequent accumulation of bile acids.25 In other reports, elevated serum transaminase activity was found in pregnant women treated with oral micronized progesterone, and withdrawal of treatment frequently has led to improvement in transaminase levels.26 The synthesis of endogenous progesterone during normal pregnancy is between 250 and 500 mg/d,26 and experts have expressed concern that exogenous progesterone supplementation may impose an additional load on the hepatic transport of sulfated metabolites. Unlike orally administered progesterone, progestins given by the vaginal route avoid the hepatic first-pass effect. For this reason, they may be associated with less hepatic dysfunction.
Although not recommended by professional guidelines, oral progesterone administration for the prevention of PTD has been used in the United States. A 2015 survey of Wisconsin prenatal care providers found that of those who prescribed any progesterone for PTD prevention, oral progesterone was prescribed by 13.1% of obstetricians, 24.4% of midwives, and 40.7% of family medicine practitioners.27
Some limited recent evidence from a meta-analysis of 3 trials investigating oral progesterone versus placebo suggests effectiveness in the prevention of recurrent PTD and reduction in perinatal morbidity and mortality.15 However, the number of cases included in the meta-analysis (386) was too small to support definitive clinical recommendations. Furthermore, questions have been raised in the literature about the reliability of the largest trial included in that meta-analysis.28
Case 3 Two previous spontaneous PTDs
A 29-year-old G3P0201 presents for her first prenatal appointment at 10 weeks’ gestation. With her first pregnancy she had a spontaneous PTD at 23 weeks, and the neonate did not survive. In her second pregnancy, she was treated with 17-OHPC from 16 weeks’ gestation. She had a spontaneous PTD at 29 weeks, and that child is developing normally by her report. She believes that 17-OHPC helped her in her last pregnancy and is anxious about the risk for still another PTD. Consistent with the concept of shared decision-making, you inform her of the results of the recent PROLONG trial and statements on the subject released by professional organizations such as ACOG and the Society for Maternal-Fetal Medicine (SMFM). What options does she have?
17-OHPC may be a possibility in very high-risk women
According to a SMFM statement released in the wake of the PROLONG trial publication, “. . . SMFM believes that it is reasonable for providers to use 17-OHPC in women with a profile more representative of the very high-risk population reported in the Meis trial”.29 Only a few women will have a recurrence risk of PTD over 50%, as was the background event rate in the Meis trial.30 Such a risk level may be suspected, as an example, in women with 2 or more prior early (before 34 weeks) PTDs without intervening term deliveries. Even in those cases, if treatment with 17-OHPC is decided upon, ultrasound cervical surveillance should be added as an additional safety measure. ●
- Iams JD, Goldenberg RL, Mercer BM, et al. The preterm prediction study: can low-risk women destined for spontaneous preterm birth be identified? Am J Obstet Gynecol. 2001;184:652-655.
- Murray SR, Stock SJ, Cowan S, et al. Spontaneous preterm birth prevention in multiple pregnancy. Obstet Gynecol. 2018;20:57-63.
- American College of Obstetricians and Gynecologists. ACOG committee opinion. Use of progesterone to reduce preterm birth. Obstet Gynecol. 2003;102:1115-1116.
- Dodd JM, Ashwood P, Flenady V, et al. A survey of clinician and patient attitudes towards the use of progesterone for women at risk of preterm birth. Aust N Z J Obstet Gynaecol. 2007;47:106-109.
- Blackwell SC, Gyamfi -Bannerman C, Biggio JR, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2020;37:127-136.
- Duff P, Vidaeff AC, Ross MG, Norwitz ER. Managing preterm birth in those at risk: expert strategies. OBG Manag. 2019;31:39-42.
- Romero R, Mazor M, Munoz H, et al. The preterm labor syndrome. Ann N Y Acad Sci. 1994;734:414-429.
- Phillips C, Velji Z, Hanly C, et al. Risk of recurrent spontaneous preterm birth: a systematic review and meta-analysis. BMJ Open. 2017;7:e015402.
- Berghella V, Seibel-Seamon J. Contemporary use of cervical cerclage. Clin Obstet Gynecol. 2007;50:468-477.
- Naim A, Haberman S, Burgess T, et al. Changes in cervical length and the risk of preterm labor. Am J Obstet Gynecol. 2002;186:887-889.
- Zilianti M, Azuaga A, Calderon F, et al. Monitoring the effacement of the uterine cervix by transperineal sonography: a new perspective. J Ultrasound Med. 1995;14:719-724.
- Goldenberg RL, Iams JD, Miodovnik M, et al. The preterm prediction study: risk factors in twin gestation. Am J Obstet Gynecol. 1996;175:1047-1053.
- Romero R, Conde-Agudelo A, Da Fonseca E, et al. Vaginal progesterone for preventing preterm birth and adverse perinatal outcomes in singleton gestations with a short cervix: a meta-analysis of individual patient data. Am J Obstet Gynecol. 2018;218:161-180.
- Norman T, Morse C, Dennerstein L. Comparative bioavailability of orally and vaginally administered progesterone. Fertil Steril. 1991;56:1034-1039.
- Boelig RC, Della Corte L, Ashoush S, et al. Oral progesterone for the prevention of recurrent preterm birth: systematic review and metaanalysis. Am J Obstet Gynecol MFM. 2019;1:50-62.
- Boelig RC, Zuppa AF, Kraft WK, et al. Pharmacokinetics of vaginal progesterone in pregnancy. Am J Obstet Gynecol. 2019;221:263.e1-7.
- Bulletti C, de Ziegler D, Flamigni C, et al. Targeted drug delivery in gynaecology: the first uterine pass effect. Hum Reprod. 1997;12:1073-1079.
- Hassan SS, Romero R, Vidyadhari D, et al. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double-blind, placebocontrolled trial. Ultrasound Obstet Gynecol. 2011;38:18-31.
- Preterm labour and birth. Evidence review for clinical effectiveness of prophylactic progesterone in preventing preterm labour. London: National Institute for Health and Care Excellence (UK); August 2019.
- Alfirevic Z, Stampalija T, Medley N. Cervical stitch (cerclage) for preventing preterm birth in singleton pregnancy. Cochrane Database Syst Rev. 2017;6:CD008991.
- Conde-Agudelo A, Romero R, Da Fonseca E, et al. Vaginal progesterone is as effective as cervical cerclage to prevent preterm birth in women with a singleton gestation, previous spontaneous preterm birth, and a short cervix: updated indirect comparison meta-analysis. Am J Obstet Gynecol. 2018;219:10-25.
- Park JY, Jung YM, Kook S-Y, et al. The effect of postoperative vaginal progesterone in ultrasound-indicated cerclage to prevent preterm birth. J Matern Fetal Neonatal Med. 2019:1-8.
- Roman AR, Da Silva Costa F, et al. Rescue adjuvant vaginal progesterone may improve outcomes in cervical cerclage failure. Geburt Frauen. 2018;78:785-790.
- Benifle JL, Dumont M, Levardon M, et al. Effects of natural micronized progesterone on the liver in the third trimester of pregnancy. Contracept Fertil Sex. 1997;25:165-169.
- Vallejo M, Briz O, Serrano MA, et al. Potential role of transinhibition of the bile salt export pump by progesterone metabolites in the etiopathogenesis of intrahepatic cholestasis of pregnancy. J Hepatol. 2006;44:1150-1157.
- Bacq Y, Sapey T, Bréchot MC, et al. Intrahepatic cholestasis of pregnancy: a French prospective study. Hepatology. 1997;26:358-364.
- Hoppe K, Kramer RD, Ha B, et al. Progesterone supplementation for the prevention of preterm birth: provider practice in Wisconsin. WMJ. 2019;118:126-131.
- Katsanevakis E, Mol BW, Thornton J. A question about the reliability of a recent trial of progesterone for preterm birth prevention, published in Acta. Acta Obstet Gynecol Scand. 2020;99:426.
- Society for Maternal-Fetal Medicine (SMFM) Publications Committee. SMFM Statement: use of 17-alpha hydroxyprogesterone caproate for prevention of recurrent preterm birth. https://www.smfm.org/publications/280smfm-statement-use-of-17-alpha-hydroxyprogesteronecaproate-for-prevention-of-recurrent-preterm-birth. Accessed March 23, 2020.
- Meis PJ, Klebanoff M, Thom E, et al. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;384:2379-2385.
Researchers have been studying the use of exogenous progestins for prevention of preterm delivery (PTD) for almost 60 years, but conflicting results contribute to an ongoing debate. Interpretation of the available data is particularly difficult because different forms and doses of progestins have been used in disparate study populations.
Based on available data, progesterone supplementation is not effective as a primary prevention strategy for PTD in the general low-risk obstetric population. PTD is a complex problem with varied and incompletely elucidated pathogenic pathways, making it unlikely that one interventional approach would be effective for all pregnant women. As a result, emerging indications for the use of progesterone are based on risk factors for PTD (ie, prior PTD and/or short cervix). However, this secondary prevention approach is a limiting factor in itself because 50% of women destined to have a PTD have no identifiable risk factors.1 In addition, researchers have found that progestins are ineffective at delaying delivery for women with multiple gestation, suggesting that a distinct underlying mechanism of early parturition is present in these women, and that this mechanism is unresponsive to progestins.2
The formulations used in the study of progestin supplementation for PTD prevention have been almost exclusively either the synthetic 17 alpha-hydroxyprogesterone caproate (17-OHPC) or natural progesterone administered orally or vaginally. In 2003, the American College of Obstetricians and Gynecologists (ACOG) supported the use of progesterone to reduce the rate of PTD,3 and in 2011, the US Food and Drug Administration (FDA) approved 17-OHPC for use as prophylaxis against recurrent PTD. As a result, in recent years, the perceived standard of care for a majority of practitioners in the United States had been that all women with a previous preterm birth should be offered 17-OHPC. It may be interesting to note that in other parts of the world, the same enthusiastic adoption did not occur. For example, in Australia and New Zealand in 2007, only 5% of practitioners were using progesterone for this indication.4 Further, 17-OHPC is not recommended by professional guidelines in the United Kingdom and has remained unavailable in Germany.
The publication in 2019 of the PROLONG trial called into question the use of 17-OHPC for the prevention of PTD.5 In the December 2019 issue of OBG Management (“Managing preterm birth in those at risk: Expert strategies”), I expressed the opinion that with only rare exceptions, 17-OHPC is no longer a viable option for recurrent PTD prevention.6 In light of these developments, what scientific evidence is relevant and applicable to the care of women at risk for PTD?
Continue to: Case 1 Previous spontaneous PTD at 31 weeks...
Case 1 Previous spontaneous PTD at 31 weeks
MC is an asymptomatic 32-year-old woman with a singleton pregnancy at 13 weeks’ gestation. You see her for a maternal-fetal medicine consultation because 2 years ago she had a spontaneous PTD at 31 weeks’ gestation. What management recommendations can you make to decrease her risk of recurrent PTD?
Cervical length measurement narrows in on risk
The indication “previous preterm birth” is largely meaningless because of the heterogeneity in preterm birth pathways (preterm birth as a syndrome7) and inadequate risk characterization. Among women who experience a spontaneous PTD, 70% to 80% do not deliver prematurely in subsequent pregnancies.8 To better characterize the risk of PTD recurrence, ultrasound assessment of cervical length should be used. Research has shown that among women with a prior spontaneous PTD who maintain a normal cervical length until 24 weeks’ gestation, more than 90% will deliver at 35 weeks or after without intervention.9 Such an approach not only identifies the subgroup of women at significantly increased risk of recurrence but also eliminates unnecessary interventions.
Cervical ultrasound surveillance should be initiated at 16 weeks’ gestation. A short cervix before 16 weeks is not associated with a statistically significant increase in risk for PTD.10 Shortening of the cervix begins approximately 10 weeks before delivery in any gestational age group.11 Therefore, ultrasound assessment of the cervix at 28 weeks and after is irrelevant. In addition, after 28 weeks, cervical length varies greatly leading to loss in the predictive power of the cervical measurement.12 Based on these considerations, cervical surveillance may be extended up to 26 weeks. Although cervical cerclage is not an option in the United States in cases in which a short cervix is detected between 24 and 26 weeks, vaginal progesterone supplementation may still be considered.
Case 1 Continued
MC was started on ultrasound cervical surveillance at 16 weeks’ gestation. Her cervical length was initially normal (> 2.5 cm), but at 18 weeks the measurement was 2.2 cm. What is your recommendation?
The value of vaginal progesterone
There appears to be increasing consensus on the value of vaginal progesterone for women with a midtrimester short cervix on sonography, with or without a history of PTD. An individual patient data meta-analysis demonstrated the benefits of vaginal progesterone.13 Although there was no evidence of an effect on PTD at less than 37 weeks, the rates of PTD at less than 36 weeks and spontaneous PTD at less than 34 weeks were significantly reduced (by 20% and 28%, respectively). Also, there was a significant reduction in the risk of respiratory distress syndrome (53%) and composite neonatal morbidity and mortality (41%), with no significant impact on infant development up to the second year of life.13
The lack of generalizable evidence of benefit on childhood outcomes, combined with considerable uncertainty about the exact role and mechanism of action of exogenous progestins, contribute to the ongoing debate. Vaginal progesterone dosage regimens have been based on extrapolations from experience with progesterone in nonpregnant women, and recent pharmacokinetic studies have revealed how precarious such extrapolations may be. As an example, in nonpregnant women, the bioavailability of oral and vaginal progesterone is similar.14 In pregnancy, however, while daily oral progesterone doubles a pregnant woman’s serum progesterone level,15 daily vaginal administration of progesterone results in only a modest rise in serum progesterone, with a coefficient of variation among individuals that is double that outside of pregnancy.16 It is, therefore, considered that vaginal progesterone in pregnancy may have a local action secondary to the uterine first-pass effect. The uterine first-pass effect for vaginal progesterone was described in nonpregnant women and is only assumed to occur in pregnancy as well. 17
After evaluating the data from the largest available study of vaginal progesterone,18 the FDA concluded in 2012 that the study did not meet the statistical significance generally expected to support the approval of a new product. However, according to a more comprehensive evidence review developed in 2019 by the National Guideline Alliance in the United Kingdom, women with a history of PTD and women with a short cervix derive an important benefit from the use of vaginal progesterone; thus, this intervention should be offered to them.19 At this time, a short cervix and PTD prevention are not considered FDA-approved indications for progesterone supplementation in pregnancy. However, vaginal progesterone is FDA approved for use in pregnant women with a history of infertility.
Continue to: Case 1 Continued...
Case 1 Continued
MC initiated treatment with daily vaginal progesterone at 18 weeks’ gestation and returned for ultrasound cervical length examination weekly instead of every other week. At 20 weeks’ gestation, cervical length was 2.0 cm; the following week it was 1.4 cm. What would you recommend at this point?
When to consider cerclage
If cervical shortening progresses to about 1.5 cm while a woman is being treated with vaginal progesterone, cerclage may be considered. The benefit of cerclage in patients with prior PTD and a short cervix was highlighted in a 2018 Cochrane Review.20 In this stepwise management approach to a short cervix, waiting for a cervix to be less than 1.5 cm may be unadvisable. Under conditions of a very short cervix that is frequently dilated with exposure of fetal membranes, ascending subclinical intra-amniotic infection may already be present, reducing the efficacy of any preventive measures. Preferential consideration for cerclage from the start over initial vaginal progesterone also may be appropriate when there is a history of 2 spontaneous PTDs or mid-trimester losses, a history of a successful cerclage, or with a very short cervix (< 1.0 cm) at the initial evaluation. As for the latter, a 2018 individual patient data meta-analysis of vaginal progesterone found no benefit when the cervix was less than 1.0 cm.13
Progesterone plus cerclage likely to add benefit
The results of an adjusted indirect comparison meta-analysis suggest that both interventions—vaginal progesterone and cerclage—are equally effective.21 Assuming that there is no clinically meaningful difference in benefit associated with these 2 treatments, the next logical question is whether combining the 2 therapies provides any added benefit; limited observational data seem to suggest that it does. In a retrospective cohort of 86 consecutive singleton pregnancies among women who underwent ultrasound-indicated cerclage, those who used vaginal progesterone after cerclage (n = 45) had a lower rate of PTD.22 Also, a small (66 cases) case-control study demonstrated the benefit of administration of vaginal progesterone as a rescue intervention in women with cerclage and progressive cervical shortening despite cerclage.23
Case 2 Woman experiences adverse effects from vaginal progesterone
MS is a 25-year-old G2P0101 who was started on vaginal progesterone as prophylaxis for recurrent PTD. She is now at 20 weeks’ gestation, with a stable remnant cervical length of 2.0 cm. She is reporting an increasing vaginal burning sensation and vaginal discharge caused by the nightly vaginal progesterone applications, to the point that she is unwilling to continue the treatment. She asks if any alternatives to vaginal progesterone are available to decrease her risk of PTD.
Continue to: Is oral progesterone an option?...
Is oral progesterone an option?
In the 1980s and 1990s, oral micronized progesterone was widely used in France at doses of 900 to 1,200 mg/d for women at risk for PTD. The practice was stopped when secondary hepatic effects, including cholestasis of pregnancy, were reported at a higher rate in treated women.24 A rise in the serum concentration of progesterone metabolites has been associated with impaired biliary excretion and subsequent accumulation of bile acids.25 In other reports, elevated serum transaminase activity was found in pregnant women treated with oral micronized progesterone, and withdrawal of treatment frequently has led to improvement in transaminase levels.26 The synthesis of endogenous progesterone during normal pregnancy is between 250 and 500 mg/d,26 and experts have expressed concern that exogenous progesterone supplementation may impose an additional load on the hepatic transport of sulfated metabolites. Unlike orally administered progesterone, progestins given by the vaginal route avoid the hepatic first-pass effect. For this reason, they may be associated with less hepatic dysfunction.
Although not recommended by professional guidelines, oral progesterone administration for the prevention of PTD has been used in the United States. A 2015 survey of Wisconsin prenatal care providers found that of those who prescribed any progesterone for PTD prevention, oral progesterone was prescribed by 13.1% of obstetricians, 24.4% of midwives, and 40.7% of family medicine practitioners.27
Some limited recent evidence from a meta-analysis of 3 trials investigating oral progesterone versus placebo suggests effectiveness in the prevention of recurrent PTD and reduction in perinatal morbidity and mortality.15 However, the number of cases included in the meta-analysis (386) was too small to support definitive clinical recommendations. Furthermore, questions have been raised in the literature about the reliability of the largest trial included in that meta-analysis.28
Case 3 Two previous spontaneous PTDs
A 29-year-old G3P0201 presents for her first prenatal appointment at 10 weeks’ gestation. With her first pregnancy she had a spontaneous PTD at 23 weeks, and the neonate did not survive. In her second pregnancy, she was treated with 17-OHPC from 16 weeks’ gestation. She had a spontaneous PTD at 29 weeks, and that child is developing normally by her report. She believes that 17-OHPC helped her in her last pregnancy and is anxious about the risk for still another PTD. Consistent with the concept of shared decision-making, you inform her of the results of the recent PROLONG trial and statements on the subject released by professional organizations such as ACOG and the Society for Maternal-Fetal Medicine (SMFM). What options does she have?
17-OHPC may be a possibility in very high-risk women
According to a SMFM statement released in the wake of the PROLONG trial publication, “. . . SMFM believes that it is reasonable for providers to use 17-OHPC in women with a profile more representative of the very high-risk population reported in the Meis trial”.29 Only a few women will have a recurrence risk of PTD over 50%, as was the background event rate in the Meis trial.30 Such a risk level may be suspected, as an example, in women with 2 or more prior early (before 34 weeks) PTDs without intervening term deliveries. Even in those cases, if treatment with 17-OHPC is decided upon, ultrasound cervical surveillance should be added as an additional safety measure. ●
Researchers have been studying the use of exogenous progestins for prevention of preterm delivery (PTD) for almost 60 years, but conflicting results contribute to an ongoing debate. Interpretation of the available data is particularly difficult because different forms and doses of progestins have been used in disparate study populations.
Based on available data, progesterone supplementation is not effective as a primary prevention strategy for PTD in the general low-risk obstetric population. PTD is a complex problem with varied and incompletely elucidated pathogenic pathways, making it unlikely that one interventional approach would be effective for all pregnant women. As a result, emerging indications for the use of progesterone are based on risk factors for PTD (ie, prior PTD and/or short cervix). However, this secondary prevention approach is a limiting factor in itself because 50% of women destined to have a PTD have no identifiable risk factors.1 In addition, researchers have found that progestins are ineffective at delaying delivery for women with multiple gestation, suggesting that a distinct underlying mechanism of early parturition is present in these women, and that this mechanism is unresponsive to progestins.2
The formulations used in the study of progestin supplementation for PTD prevention have been almost exclusively either the synthetic 17 alpha-hydroxyprogesterone caproate (17-OHPC) or natural progesterone administered orally or vaginally. In 2003, the American College of Obstetricians and Gynecologists (ACOG) supported the use of progesterone to reduce the rate of PTD,3 and in 2011, the US Food and Drug Administration (FDA) approved 17-OHPC for use as prophylaxis against recurrent PTD. As a result, in recent years, the perceived standard of care for a majority of practitioners in the United States had been that all women with a previous preterm birth should be offered 17-OHPC. It may be interesting to note that in other parts of the world, the same enthusiastic adoption did not occur. For example, in Australia and New Zealand in 2007, only 5% of practitioners were using progesterone for this indication.4 Further, 17-OHPC is not recommended by professional guidelines in the United Kingdom and has remained unavailable in Germany.
The publication in 2019 of the PROLONG trial called into question the use of 17-OHPC for the prevention of PTD.5 In the December 2019 issue of OBG Management (“Managing preterm birth in those at risk: Expert strategies”), I expressed the opinion that with only rare exceptions, 17-OHPC is no longer a viable option for recurrent PTD prevention.6 In light of these developments, what scientific evidence is relevant and applicable to the care of women at risk for PTD?
Continue to: Case 1 Previous spontaneous PTD at 31 weeks...
Case 1 Previous spontaneous PTD at 31 weeks
MC is an asymptomatic 32-year-old woman with a singleton pregnancy at 13 weeks’ gestation. You see her for a maternal-fetal medicine consultation because 2 years ago she had a spontaneous PTD at 31 weeks’ gestation. What management recommendations can you make to decrease her risk of recurrent PTD?
Cervical length measurement narrows in on risk
The indication “previous preterm birth” is largely meaningless because of the heterogeneity in preterm birth pathways (preterm birth as a syndrome7) and inadequate risk characterization. Among women who experience a spontaneous PTD, 70% to 80% do not deliver prematurely in subsequent pregnancies.8 To better characterize the risk of PTD recurrence, ultrasound assessment of cervical length should be used. Research has shown that among women with a prior spontaneous PTD who maintain a normal cervical length until 24 weeks’ gestation, more than 90% will deliver at 35 weeks or after without intervention.9 Such an approach not only identifies the subgroup of women at significantly increased risk of recurrence but also eliminates unnecessary interventions.
Cervical ultrasound surveillance should be initiated at 16 weeks’ gestation. A short cervix before 16 weeks is not associated with a statistically significant increase in risk for PTD.10 Shortening of the cervix begins approximately 10 weeks before delivery in any gestational age group.11 Therefore, ultrasound assessment of the cervix at 28 weeks and after is irrelevant. In addition, after 28 weeks, cervical length varies greatly leading to loss in the predictive power of the cervical measurement.12 Based on these considerations, cervical surveillance may be extended up to 26 weeks. Although cervical cerclage is not an option in the United States in cases in which a short cervix is detected between 24 and 26 weeks, vaginal progesterone supplementation may still be considered.
Case 1 Continued
MC was started on ultrasound cervical surveillance at 16 weeks’ gestation. Her cervical length was initially normal (> 2.5 cm), but at 18 weeks the measurement was 2.2 cm. What is your recommendation?
The value of vaginal progesterone
There appears to be increasing consensus on the value of vaginal progesterone for women with a midtrimester short cervix on sonography, with or without a history of PTD. An individual patient data meta-analysis demonstrated the benefits of vaginal progesterone.13 Although there was no evidence of an effect on PTD at less than 37 weeks, the rates of PTD at less than 36 weeks and spontaneous PTD at less than 34 weeks were significantly reduced (by 20% and 28%, respectively). Also, there was a significant reduction in the risk of respiratory distress syndrome (53%) and composite neonatal morbidity and mortality (41%), with no significant impact on infant development up to the second year of life.13
The lack of generalizable evidence of benefit on childhood outcomes, combined with considerable uncertainty about the exact role and mechanism of action of exogenous progestins, contribute to the ongoing debate. Vaginal progesterone dosage regimens have been based on extrapolations from experience with progesterone in nonpregnant women, and recent pharmacokinetic studies have revealed how precarious such extrapolations may be. As an example, in nonpregnant women, the bioavailability of oral and vaginal progesterone is similar.14 In pregnancy, however, while daily oral progesterone doubles a pregnant woman’s serum progesterone level,15 daily vaginal administration of progesterone results in only a modest rise in serum progesterone, with a coefficient of variation among individuals that is double that outside of pregnancy.16 It is, therefore, considered that vaginal progesterone in pregnancy may have a local action secondary to the uterine first-pass effect. The uterine first-pass effect for vaginal progesterone was described in nonpregnant women and is only assumed to occur in pregnancy as well. 17
After evaluating the data from the largest available study of vaginal progesterone,18 the FDA concluded in 2012 that the study did not meet the statistical significance generally expected to support the approval of a new product. However, according to a more comprehensive evidence review developed in 2019 by the National Guideline Alliance in the United Kingdom, women with a history of PTD and women with a short cervix derive an important benefit from the use of vaginal progesterone; thus, this intervention should be offered to them.19 At this time, a short cervix and PTD prevention are not considered FDA-approved indications for progesterone supplementation in pregnancy. However, vaginal progesterone is FDA approved for use in pregnant women with a history of infertility.
Continue to: Case 1 Continued...
Case 1 Continued
MC initiated treatment with daily vaginal progesterone at 18 weeks’ gestation and returned for ultrasound cervical length examination weekly instead of every other week. At 20 weeks’ gestation, cervical length was 2.0 cm; the following week it was 1.4 cm. What would you recommend at this point?
When to consider cerclage
If cervical shortening progresses to about 1.5 cm while a woman is being treated with vaginal progesterone, cerclage may be considered. The benefit of cerclage in patients with prior PTD and a short cervix was highlighted in a 2018 Cochrane Review.20 In this stepwise management approach to a short cervix, waiting for a cervix to be less than 1.5 cm may be unadvisable. Under conditions of a very short cervix that is frequently dilated with exposure of fetal membranes, ascending subclinical intra-amniotic infection may already be present, reducing the efficacy of any preventive measures. Preferential consideration for cerclage from the start over initial vaginal progesterone also may be appropriate when there is a history of 2 spontaneous PTDs or mid-trimester losses, a history of a successful cerclage, or with a very short cervix (< 1.0 cm) at the initial evaluation. As for the latter, a 2018 individual patient data meta-analysis of vaginal progesterone found no benefit when the cervix was less than 1.0 cm.13
Progesterone plus cerclage likely to add benefit
The results of an adjusted indirect comparison meta-analysis suggest that both interventions—vaginal progesterone and cerclage—are equally effective.21 Assuming that there is no clinically meaningful difference in benefit associated with these 2 treatments, the next logical question is whether combining the 2 therapies provides any added benefit; limited observational data seem to suggest that it does. In a retrospective cohort of 86 consecutive singleton pregnancies among women who underwent ultrasound-indicated cerclage, those who used vaginal progesterone after cerclage (n = 45) had a lower rate of PTD.22 Also, a small (66 cases) case-control study demonstrated the benefit of administration of vaginal progesterone as a rescue intervention in women with cerclage and progressive cervical shortening despite cerclage.23
Case 2 Woman experiences adverse effects from vaginal progesterone
MS is a 25-year-old G2P0101 who was started on vaginal progesterone as prophylaxis for recurrent PTD. She is now at 20 weeks’ gestation, with a stable remnant cervical length of 2.0 cm. She is reporting an increasing vaginal burning sensation and vaginal discharge caused by the nightly vaginal progesterone applications, to the point that she is unwilling to continue the treatment. She asks if any alternatives to vaginal progesterone are available to decrease her risk of PTD.
Continue to: Is oral progesterone an option?...
Is oral progesterone an option?
In the 1980s and 1990s, oral micronized progesterone was widely used in France at doses of 900 to 1,200 mg/d for women at risk for PTD. The practice was stopped when secondary hepatic effects, including cholestasis of pregnancy, were reported at a higher rate in treated women.24 A rise in the serum concentration of progesterone metabolites has been associated with impaired biliary excretion and subsequent accumulation of bile acids.25 In other reports, elevated serum transaminase activity was found in pregnant women treated with oral micronized progesterone, and withdrawal of treatment frequently has led to improvement in transaminase levels.26 The synthesis of endogenous progesterone during normal pregnancy is between 250 and 500 mg/d,26 and experts have expressed concern that exogenous progesterone supplementation may impose an additional load on the hepatic transport of sulfated metabolites. Unlike orally administered progesterone, progestins given by the vaginal route avoid the hepatic first-pass effect. For this reason, they may be associated with less hepatic dysfunction.
Although not recommended by professional guidelines, oral progesterone administration for the prevention of PTD has been used in the United States. A 2015 survey of Wisconsin prenatal care providers found that of those who prescribed any progesterone for PTD prevention, oral progesterone was prescribed by 13.1% of obstetricians, 24.4% of midwives, and 40.7% of family medicine practitioners.27
Some limited recent evidence from a meta-analysis of 3 trials investigating oral progesterone versus placebo suggests effectiveness in the prevention of recurrent PTD and reduction in perinatal morbidity and mortality.15 However, the number of cases included in the meta-analysis (386) was too small to support definitive clinical recommendations. Furthermore, questions have been raised in the literature about the reliability of the largest trial included in that meta-analysis.28
Case 3 Two previous spontaneous PTDs
A 29-year-old G3P0201 presents for her first prenatal appointment at 10 weeks’ gestation. With her first pregnancy she had a spontaneous PTD at 23 weeks, and the neonate did not survive. In her second pregnancy, she was treated with 17-OHPC from 16 weeks’ gestation. She had a spontaneous PTD at 29 weeks, and that child is developing normally by her report. She believes that 17-OHPC helped her in her last pregnancy and is anxious about the risk for still another PTD. Consistent with the concept of shared decision-making, you inform her of the results of the recent PROLONG trial and statements on the subject released by professional organizations such as ACOG and the Society for Maternal-Fetal Medicine (SMFM). What options does she have?
17-OHPC may be a possibility in very high-risk women
According to a SMFM statement released in the wake of the PROLONG trial publication, “. . . SMFM believes that it is reasonable for providers to use 17-OHPC in women with a profile more representative of the very high-risk population reported in the Meis trial”.29 Only a few women will have a recurrence risk of PTD over 50%, as was the background event rate in the Meis trial.30 Such a risk level may be suspected, as an example, in women with 2 or more prior early (before 34 weeks) PTDs without intervening term deliveries. Even in those cases, if treatment with 17-OHPC is decided upon, ultrasound cervical surveillance should be added as an additional safety measure. ●
- Iams JD, Goldenberg RL, Mercer BM, et al. The preterm prediction study: can low-risk women destined for spontaneous preterm birth be identified? Am J Obstet Gynecol. 2001;184:652-655.
- Murray SR, Stock SJ, Cowan S, et al. Spontaneous preterm birth prevention in multiple pregnancy. Obstet Gynecol. 2018;20:57-63.
- American College of Obstetricians and Gynecologists. ACOG committee opinion. Use of progesterone to reduce preterm birth. Obstet Gynecol. 2003;102:1115-1116.
- Dodd JM, Ashwood P, Flenady V, et al. A survey of clinician and patient attitudes towards the use of progesterone for women at risk of preterm birth. Aust N Z J Obstet Gynaecol. 2007;47:106-109.
- Blackwell SC, Gyamfi -Bannerman C, Biggio JR, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2020;37:127-136.
- Duff P, Vidaeff AC, Ross MG, Norwitz ER. Managing preterm birth in those at risk: expert strategies. OBG Manag. 2019;31:39-42.
- Romero R, Mazor M, Munoz H, et al. The preterm labor syndrome. Ann N Y Acad Sci. 1994;734:414-429.
- Phillips C, Velji Z, Hanly C, et al. Risk of recurrent spontaneous preterm birth: a systematic review and meta-analysis. BMJ Open. 2017;7:e015402.
- Berghella V, Seibel-Seamon J. Contemporary use of cervical cerclage. Clin Obstet Gynecol. 2007;50:468-477.
- Naim A, Haberman S, Burgess T, et al. Changes in cervical length and the risk of preterm labor. Am J Obstet Gynecol. 2002;186:887-889.
- Zilianti M, Azuaga A, Calderon F, et al. Monitoring the effacement of the uterine cervix by transperineal sonography: a new perspective. J Ultrasound Med. 1995;14:719-724.
- Goldenberg RL, Iams JD, Miodovnik M, et al. The preterm prediction study: risk factors in twin gestation. Am J Obstet Gynecol. 1996;175:1047-1053.
- Romero R, Conde-Agudelo A, Da Fonseca E, et al. Vaginal progesterone for preventing preterm birth and adverse perinatal outcomes in singleton gestations with a short cervix: a meta-analysis of individual patient data. Am J Obstet Gynecol. 2018;218:161-180.
- Norman T, Morse C, Dennerstein L. Comparative bioavailability of orally and vaginally administered progesterone. Fertil Steril. 1991;56:1034-1039.
- Boelig RC, Della Corte L, Ashoush S, et al. Oral progesterone for the prevention of recurrent preterm birth: systematic review and metaanalysis. Am J Obstet Gynecol MFM. 2019;1:50-62.
- Boelig RC, Zuppa AF, Kraft WK, et al. Pharmacokinetics of vaginal progesterone in pregnancy. Am J Obstet Gynecol. 2019;221:263.e1-7.
- Bulletti C, de Ziegler D, Flamigni C, et al. Targeted drug delivery in gynaecology: the first uterine pass effect. Hum Reprod. 1997;12:1073-1079.
- Hassan SS, Romero R, Vidyadhari D, et al. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double-blind, placebocontrolled trial. Ultrasound Obstet Gynecol. 2011;38:18-31.
- Preterm labour and birth. Evidence review for clinical effectiveness of prophylactic progesterone in preventing preterm labour. London: National Institute for Health and Care Excellence (UK); August 2019.
- Alfirevic Z, Stampalija T, Medley N. Cervical stitch (cerclage) for preventing preterm birth in singleton pregnancy. Cochrane Database Syst Rev. 2017;6:CD008991.
- Conde-Agudelo A, Romero R, Da Fonseca E, et al. Vaginal progesterone is as effective as cervical cerclage to prevent preterm birth in women with a singleton gestation, previous spontaneous preterm birth, and a short cervix: updated indirect comparison meta-analysis. Am J Obstet Gynecol. 2018;219:10-25.
- Park JY, Jung YM, Kook S-Y, et al. The effect of postoperative vaginal progesterone in ultrasound-indicated cerclage to prevent preterm birth. J Matern Fetal Neonatal Med. 2019:1-8.
- Roman AR, Da Silva Costa F, et al. Rescue adjuvant vaginal progesterone may improve outcomes in cervical cerclage failure. Geburt Frauen. 2018;78:785-790.
- Benifle JL, Dumont M, Levardon M, et al. Effects of natural micronized progesterone on the liver in the third trimester of pregnancy. Contracept Fertil Sex. 1997;25:165-169.
- Vallejo M, Briz O, Serrano MA, et al. Potential role of transinhibition of the bile salt export pump by progesterone metabolites in the etiopathogenesis of intrahepatic cholestasis of pregnancy. J Hepatol. 2006;44:1150-1157.
- Bacq Y, Sapey T, Bréchot MC, et al. Intrahepatic cholestasis of pregnancy: a French prospective study. Hepatology. 1997;26:358-364.
- Hoppe K, Kramer RD, Ha B, et al. Progesterone supplementation for the prevention of preterm birth: provider practice in Wisconsin. WMJ. 2019;118:126-131.
- Katsanevakis E, Mol BW, Thornton J. A question about the reliability of a recent trial of progesterone for preterm birth prevention, published in Acta. Acta Obstet Gynecol Scand. 2020;99:426.
- Society for Maternal-Fetal Medicine (SMFM) Publications Committee. SMFM Statement: use of 17-alpha hydroxyprogesterone caproate for prevention of recurrent preterm birth. https://www.smfm.org/publications/280smfm-statement-use-of-17-alpha-hydroxyprogesteronecaproate-for-prevention-of-recurrent-preterm-birth. Accessed March 23, 2020.
- Meis PJ, Klebanoff M, Thom E, et al. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;384:2379-2385.
- Iams JD, Goldenberg RL, Mercer BM, et al. The preterm prediction study: can low-risk women destined for spontaneous preterm birth be identified? Am J Obstet Gynecol. 2001;184:652-655.
- Murray SR, Stock SJ, Cowan S, et al. Spontaneous preterm birth prevention in multiple pregnancy. Obstet Gynecol. 2018;20:57-63.
- American College of Obstetricians and Gynecologists. ACOG committee opinion. Use of progesterone to reduce preterm birth. Obstet Gynecol. 2003;102:1115-1116.
- Dodd JM, Ashwood P, Flenady V, et al. A survey of clinician and patient attitudes towards the use of progesterone for women at risk of preterm birth. Aust N Z J Obstet Gynaecol. 2007;47:106-109.
- Blackwell SC, Gyamfi -Bannerman C, Biggio JR, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2020;37:127-136.
- Duff P, Vidaeff AC, Ross MG, Norwitz ER. Managing preterm birth in those at risk: expert strategies. OBG Manag. 2019;31:39-42.
- Romero R, Mazor M, Munoz H, et al. The preterm labor syndrome. Ann N Y Acad Sci. 1994;734:414-429.
- Phillips C, Velji Z, Hanly C, et al. Risk of recurrent spontaneous preterm birth: a systematic review and meta-analysis. BMJ Open. 2017;7:e015402.
- Berghella V, Seibel-Seamon J. Contemporary use of cervical cerclage. Clin Obstet Gynecol. 2007;50:468-477.
- Naim A, Haberman S, Burgess T, et al. Changes in cervical length and the risk of preterm labor. Am J Obstet Gynecol. 2002;186:887-889.
- Zilianti M, Azuaga A, Calderon F, et al. Monitoring the effacement of the uterine cervix by transperineal sonography: a new perspective. J Ultrasound Med. 1995;14:719-724.
- Goldenberg RL, Iams JD, Miodovnik M, et al. The preterm prediction study: risk factors in twin gestation. Am J Obstet Gynecol. 1996;175:1047-1053.
- Romero R, Conde-Agudelo A, Da Fonseca E, et al. Vaginal progesterone for preventing preterm birth and adverse perinatal outcomes in singleton gestations with a short cervix: a meta-analysis of individual patient data. Am J Obstet Gynecol. 2018;218:161-180.
- Norman T, Morse C, Dennerstein L. Comparative bioavailability of orally and vaginally administered progesterone. Fertil Steril. 1991;56:1034-1039.
- Boelig RC, Della Corte L, Ashoush S, et al. Oral progesterone for the prevention of recurrent preterm birth: systematic review and metaanalysis. Am J Obstet Gynecol MFM. 2019;1:50-62.
- Boelig RC, Zuppa AF, Kraft WK, et al. Pharmacokinetics of vaginal progesterone in pregnancy. Am J Obstet Gynecol. 2019;221:263.e1-7.
- Bulletti C, de Ziegler D, Flamigni C, et al. Targeted drug delivery in gynaecology: the first uterine pass effect. Hum Reprod. 1997;12:1073-1079.
- Hassan SS, Romero R, Vidyadhari D, et al. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double-blind, placebocontrolled trial. Ultrasound Obstet Gynecol. 2011;38:18-31.
- Preterm labour and birth. Evidence review for clinical effectiveness of prophylactic progesterone in preventing preterm labour. London: National Institute for Health and Care Excellence (UK); August 2019.
- Alfirevic Z, Stampalija T, Medley N. Cervical stitch (cerclage) for preventing preterm birth in singleton pregnancy. Cochrane Database Syst Rev. 2017;6:CD008991.
- Conde-Agudelo A, Romero R, Da Fonseca E, et al. Vaginal progesterone is as effective as cervical cerclage to prevent preterm birth in women with a singleton gestation, previous spontaneous preterm birth, and a short cervix: updated indirect comparison meta-analysis. Am J Obstet Gynecol. 2018;219:10-25.
- Park JY, Jung YM, Kook S-Y, et al. The effect of postoperative vaginal progesterone in ultrasound-indicated cerclage to prevent preterm birth. J Matern Fetal Neonatal Med. 2019:1-8.
- Roman AR, Da Silva Costa F, et al. Rescue adjuvant vaginal progesterone may improve outcomes in cervical cerclage failure. Geburt Frauen. 2018;78:785-790.
- Benifle JL, Dumont M, Levardon M, et al. Effects of natural micronized progesterone on the liver in the third trimester of pregnancy. Contracept Fertil Sex. 1997;25:165-169.
- Vallejo M, Briz O, Serrano MA, et al. Potential role of transinhibition of the bile salt export pump by progesterone metabolites in the etiopathogenesis of intrahepatic cholestasis of pregnancy. J Hepatol. 2006;44:1150-1157.
- Bacq Y, Sapey T, Bréchot MC, et al. Intrahepatic cholestasis of pregnancy: a French prospective study. Hepatology. 1997;26:358-364.
- Hoppe K, Kramer RD, Ha B, et al. Progesterone supplementation for the prevention of preterm birth: provider practice in Wisconsin. WMJ. 2019;118:126-131.
- Katsanevakis E, Mol BW, Thornton J. A question about the reliability of a recent trial of progesterone for preterm birth prevention, published in Acta. Acta Obstet Gynecol Scand. 2020;99:426.
- Society for Maternal-Fetal Medicine (SMFM) Publications Committee. SMFM Statement: use of 17-alpha hydroxyprogesterone caproate for prevention of recurrent preterm birth. https://www.smfm.org/publications/280smfm-statement-use-of-17-alpha-hydroxyprogesteronecaproate-for-prevention-of-recurrent-preterm-birth. Accessed March 23, 2020.
- Meis PJ, Klebanoff M, Thom E, et al. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;384:2379-2385.
Systemic Treatment for Advanced Hepatocellular Carcinoma
From the University of Alabama at Birmingham, Division of Hematology Oncology, Birmingham, AL, and the University of South Alabama, Division of Hematology Oncology, Mobile, AL. Dr. Paluri and Dr. Hatic contributed equally to this article.
Abstract
- Objective: To review systemic treatment options for patients with locally advanced unresectable hepatocellular carcinoma (HCC).
- Methods: Review of the literature.
- Results: The paradigm of what constitutes first-line treatment for advanced HCC is shifting. Until recently, many patients with advanced HCC were treated with repeated locoregional therapies, such as transartertial embolization (TACE). However, retrospective studies suggest that continuing TACE after refractoriness or failure may not be beneficial and may delay subsequent treatments because of deterioration of liver function or declines in performance status. With recent approvals of several systemic therapy options, including immunotherapy, it is vital to conduct a risk-benefit assessment prior to repeating TACE after failure, so that patients are not denied the use of available systemic therapeutic options due to declined performance status or organ function from these procedures. The optimal timing and the sequence of systemic and locoregional therapy must be carefully evaluated by a multidisciplinary team.
- Conclusion: Randomized clinical trials to improve patient selection and determine the proper sequence of treatments are needed. Given the heterogeneity of HCC, molecular profiling of the tumor to differentiate responders from nonresponders may elucidate potential biomarkers to effectively guide treatment recommendations.
Keywords: liver cancer; molecular therapy; immunotherapy.
Hepatocellular carcinoma (HCC) represents 90% of primary liver malignancies. It is the fifth most common malignancy in males and the ninth most common in females worldwide.1 In contrast to other major cancers (colon, breast, prostate), the incidence of and mortality from HCC has increased over the past decade, following a brief decline between 1999 and 2004.2 The epidemiology and incidence of HCC is closely linked to chronic liver disease and conditions predisposing to liver cirrhosis. Worldwide, hepatitis B virus infection is the leading cause of liver cirrhosis and, hence, HCC. In the United States, 50% of HCC cases are linked to hepatitis C virus (HCV) infection. Diabetes mellitus and alcoholic and nonalcoholic steatohepatitis are the other major etiologies of HCC. Indeed, the metabolic syndrome, independent of other factors, is associated with a 2-fold increase in the risk of HCC.3
Although most cases of HCC are predated by liver cirrhosis, in about 20% of patients HCC occurs without liver cirrhosis.4 Similar to other malignancies, surgery in the form of resection (for isolated lesions in the context of good liver function) or liver transplant (for low-volume disease with mildly impaired liver function) provides the best chance of a cure. Locoregional therapies involving hepatic artery–directed therapy are offered for patients with more advanced disease that is limited to the liver, while systemic therapy is offered for advanced unresectable disease that involves portal vein invasion, lymph nodes, and distant metastasis. The
Molecular Pathogenesis
Similar to other malignancies, a multistep process of carcinogenesis, with accumulation of genomic alterations at the molecular and cellular levels, is recognized in HCC. In about 80% of cases, repeated and chronic injury, inflammation, and repair lead to a distortion of normal liver architecture and development of cirrhotic nodules. Exome sequencing of HCC tissues has identified risk factor–specific mutational signatures, including those related to the tumor microenvironment, and defined the extensive landscape of altered genes and pathways in HCC (eg, angiogenic and MET pathways).7 In the Schulze et al study, the frequency of alterations that could be targeted by available Food and Drug Administration (FDA)–approved drugs comprised either amplifications or mutations of FLTs (6%), FGF3 or 4 or 19 (4%), PDGFRs (3%), VEGFA (1%), HGF (3%), MTOR (2%), EGFR (1%), FGFRs (1%), and MET (1%).7 Epigenetic modification of growth-factor expression, particularly insulin-like growth factor 2 and transforming growth factor alpha, and structural alterations that lead to loss of heterozygosity are early events that cause hepatocyte proliferation and progression of dysplastic nodules.8,9 Advances in whole-exome sequencing have identified TERT promoter mutations, leading to activation of telomerase, as an early event in HCC pathogenesis. Other commonly altered genes include CTNNB1 (B-Catenin) and TP53. As a group, alterations in the MAP kinase pathway genes occur in about 40% of HCC cases.
Actionable oncogenic driver alterations are not as common as tumor suppressor pathway alterations in HCC, making targeted drug development challenging.10,11 The FGFR (fibroblast growth factor receptor) pathway, which plays a critical role in carcinogenesis-related cell growth, survival, neo-angiogenesis, and acquired resistance to other cancer treatments, is being explored as a treatment target.12 The molecular characterization of HCC may help with identifying new biomarkers and present opportunities for developing therapeutic targets.
CASE PRESENTATION
A 61-year-old man with a history of chronic hepatitis C and hypertension presents to his primary care physician due to right upper quadrant pain. Laboratory evaluation shows transaminases elevated 2 times the upper limit of normal. This leads to an ultrasound and follow-up magnetic resonance imaging. Imaging shows diffuse cirrhotic changes, with a 6-cm, well-circumscribed lesion within the left lobe of the liver that shows rapid arterial enhancement with venous washout. These vascular characteristics are consistent with HCC. In addition, 2 satellite lesions in the left lobe and sonographic evidence of invasion into the portal vein are present. Periportal lymph nodes are pathologically enlarged.
The physical examination is unremarkable, except for mild tenderness over the right upper quadrant of the abdomen. Serum bilirubin, albumin, platelets, and international normalized ratio are normal, and alpha fetoprotein (AFP) is elevated at 1769 ng/mL. The patient’s family history is unremarkable for any major illness or cancer. Computed tomography scan of the chest and pelvis shows no evidence of other lesions. His liver disease is classified as Child–Pugh A. Due to locally advanced presentation, the tumor is deemed to be nontransplantable and unresectable, and is staged as BCLC-C. The patient continues to work and his performance status is ECOG (
What systemic treatment would you recommend for this patient with locally advanced unresectable HCC with nodal metastasis?
First-Line Therapeutic Options
Systemic treatment of HCC is challenging because of the underlying liver cirrhosis and hepatic dysfunction present in most patients. Overall prognosis is therefore dependent on the disease biology and burden and on the degree of hepatic dysfunction. These factors must be considered in patients with advanced disease who present for systemic therapy. As such, patients with BCLC class D HCC with poor performance status and impaired liver function are better off with best supportive care and hospice services (Figure). Table 1 and Table 2 outline the landmark trials that led to the approval of agents for advanced HCC treatment.
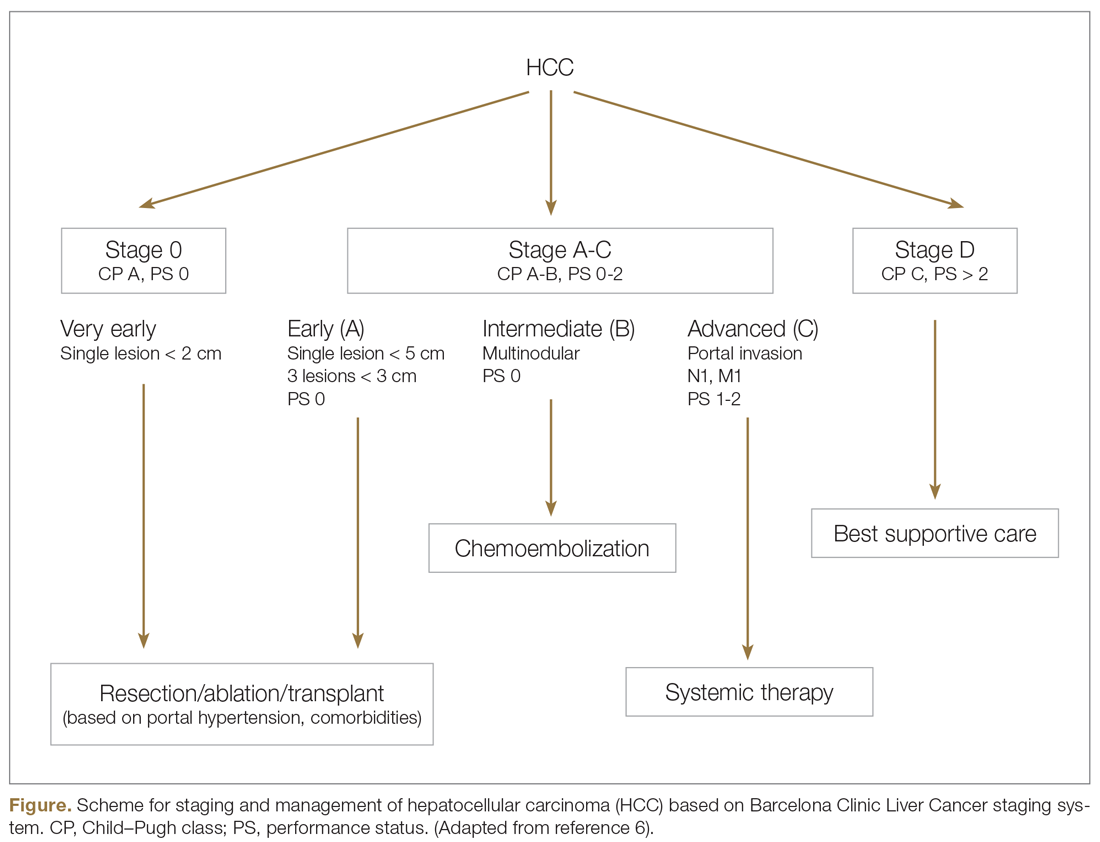
Sorafenib
In the patient with BCLC class C HCC who has preserved liver function (traditionally based on a Child–Pugh score of ≤ 6 and a decent functional status [ECOG performance status 1-2]), sorafenib is the first FDA-approved first-line treatment. Sorafenib is a small-molecule tyrosine kinase inhibitor that targets vascular endothelial growth factor receptor (VEGFR) kinase signaling, in addition to many other tyrosine kinase pathways (including the platelet-derived growth factor and Raf-Ras pathways). Evidence for the clinical benefit of sorafenib comes from the SHARP trial.13 This was a multinational, but primarily European, randomized phase 3 study that compared sorafenib to best supportive care for advanced HCC in patients with a Child–Pugh score ≤ 6A and a robust performance status (ECOG 0 and 1). Overall survival (OS) with placebo and sorafenib was 7.9 months and 10.7 months, respectively. There was no difference in time to radiologic progression, and the progression-free survival (PFS) at 4 months was 62% with sorafenib and 42% with placebo. Patients with HCV-associated HCC appeared to derive a more substantial benefit from sorafenib.14 In a smaller randomized study of sorafenib in Asian patients with predominantly hepatitis B–associated HCC, OS in the sorafenib and best supportive care arms was lower than that reported in the SHARP study (6.5 months vs 4.2 months), although OS still was longer in the sorafenib group.15
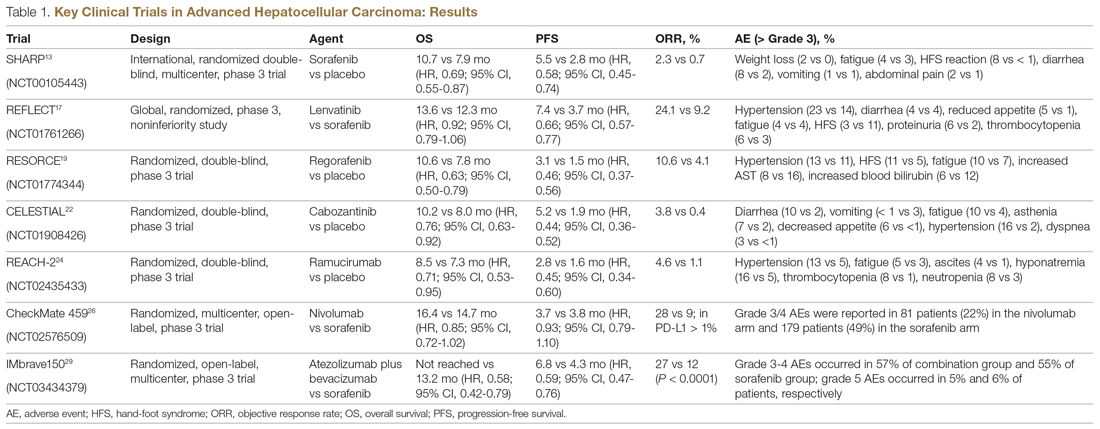
Significant adverse events reported with sorafenib include fatigue (30%), hand and foot syndrome (30%), diarrhea (15%), and mucositis (10%). Major proportions of patients in the clinical setting have not tolerated the standard dose of 400 mg twice daily. Dose-adjusted administration of sorafenib has been advocated in patients with more impaired liver function (Child–Pugh class 7B) and bilirubin of 1.5 to 3 times the upper limit of normal, although it is unclear whether these patients are deriving any benefit from sorafenib.16 At this time, in a patient with preserved liver function, starting with 400 mg twice daily, followed by dose reduction based on toxicity, remains standard.
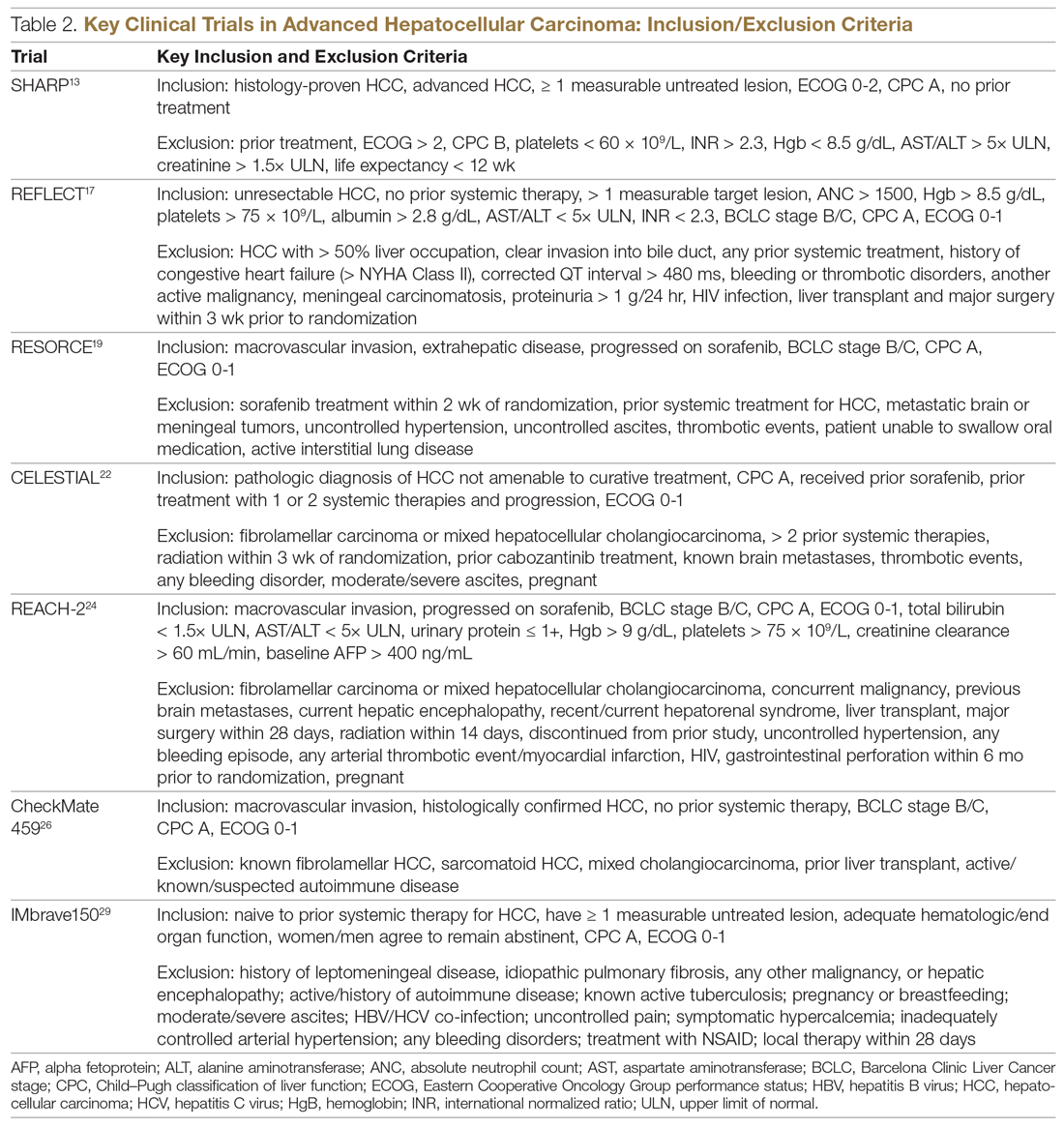
Lenvatinib
After multiple attempts to develop newer first-line treatments for HCC,
Second-Line Therapeutic Options
Following the sorafenib approval, clinical studies of several other agents did not meet their primary endpoint and failed to show improvement in clinical outcomes compared to sorafenib. However, over the past years the treatment landscape for advanced HCC has been changed with the approval of several agents in the second line. The overall response rate (ORR) has become the new theme for management of advanced disease. With multiple therapeutic options available, optimal sequencing now plays a critical role, especially for transitioning from locoregional to systemic therapy. Five drugs are now indicated for second-line treatment of patients who progressed on or were intolerant to sorafenib: regorafenib, cabozantinib, ramucirumab, nivolumab, and pembrolizumab.
Regorafenib
Regorafenib was evaluated in the advanced HCC setting in a single-arm, phase 2 trial involving 36 patients with Child–Pugh class A liver disease who had progressed on prior sorafenib.18 Patients received regorafenib 160 mg orally once daily for 3 weeks on/1 week off cycles. Disease control was achieved in 72% of patients, with stable disease in 25 patients (69%). Based on these results, regorafenib was further evaluated in the multicenter, phase 3, 2:1 randomized, double-blind, placebo-controlled RESORCE study, which enrolled 573 patients.19 Due to the overlapping safety profiles of sorafenib and regorafenib, the inclusion criteria required patients to have tolerated a sorafenib dose of at least 400 mg daily for 20 of the past 28 days of treatment prior to enrollment. The primary endpoint of the study, OS, was met (median OS of 10.6 months in regorafenib arm versus 7.8 months in placebo arm; hazard ratio [HR], 0.63; P < 0.0001).
Cabozantinib
CELESTIAL was a phase 3, double-blind study that assessed the efficacy of cabozantinib versus placebo in patients with advanced HCC who had received prior sorafenib.22 In this study, 707 patients with Child–Pugh class A liver disease who progressed on at least 1 prior systemic therapy were randomized in a 2:1 ratio to treatment with cabozantinib at 60 mg daily or placebo. Patients treated with cabozantinib had a longer OS (10.2 months vs 8.0 months), resulting in a 24% reduction in the risk of death (HR, 0.76), and a longer median PFS (5.2 months versus 1.9 months). The disease control rate was higher with cabozantinib (64% vs 33%) as well. The incidence of high‐grade adverse events in the cabozantinib group was twice that of the placebo group. Common adverse events reported with cabozantinib included HFSR (17%), hypertension (16%), increased aspartate aminotransferase (12%), fatigue (10%), and diarrhea (10%).
Ramucirumab
REACH was a phase 3 study exploring the efficacy of ramucirumab that did not meet its primary endpoint; however, the subgroup analysis in AFP-high patients showed an OS improvement with ramucirumab.23 This led to the phase 3 REACH-2 trial, a multicenter, randomized, double-blind biomarker study in patients with advanced HCC who either progressed on or were intolerant to sorafenib and had an AFP level ≥ 400 ng/mL.24 Patients were randomized to ramucirumab 8 mg/kg every 2 weeks or placebo. The study met its primary endpoint, showing improved OS (8.5 months vs 7.3 months; P = 0.0059). The most common treatment-related adverse events in the ramucirumab group were ascites (5%), hypertension (12%), asthenia (5%), malignant neoplasm progression (6%), increased aspartate aminotransferase concentration (5%), and thrombocytopenia.
Immunotherapy
HCC is considered an inflammation-induced cancer, which renders immunotherapeutic strategies more appealing. The PD-L1/PD-1 pathway is the critical immune checkpoint mechanism and is an important target for treatment. HCC uses a complex, overlapping set of mechanisms to evade cancer-specific immunity and to suppress the immune system. Initial efforts to develop immunotherapies for HCC focused on anti-PD-1 and anti-PD-L1 antibodies. CheckMate 040 evaluated nivolumab in 262 sorafenib-naïve and -treated patients with advanced HCC (98% with Child–Pugh scores of 5 or 6), with a median follow-up of 12.9 months.25 In sorafenib-naïve patients (n = 80), the ORR was 23%, and the disease control rate was 63%. In sorafenib-treated patients (n = 182), the ORR was 18%. Response was not associated with PD-L1 expression. Durable objective responses, a manageable safety profile, and promising efficacy led the FDA to grant accelerated approval of nivolumab for the treatment of patients with HCC who have been previously treated with sorafenib. Based on this, the phase 3 randomized CheckMate-459 trial evaluated the efficacy of nivolumab versus sorafenib in the first-line. Median OS and ORR were better with nivolumab (16.4 months vs 14.7 months; HR 0.85; P = 0.752; and 15% [with 5 complete responses] vs 7%), as was the safety profile (22% vs 49% reporting grade 3 and 4 adverse events). 26
The KEYNOTE-224 study27 evaluated pembrolizumab in 104 patients with previously treated advanced HCC. This study showed an ORR of 17%, with 1 complete response and 17 partial responses. One-third of the patients had progressive disease, while 46 had stable disease. Among those who responded, 56% maintained a durable response for more than 1 year. Subsequently, in KEYNOTE 240, pembrolizumab showed an improvement in OS (13.9 months vs 10.6 months; HR, 0.78; P = 0.0238) and PFS (3.0 months versus 2.8 months; HR, 0.78; P = 0.0186) compared with placebo.28 The ORR for pembrolizumab was 16.9% (95% confidence interval [CI], 12.7%-21.8%) versus 2.2% (95% CI, 0.5%-6.4%; P = 0.00001) for placebo. Mean duration of response was 13.8 months.
In the IMbrave150 trial, atezolizumab/bevacizumab combination, compared to sorafenib, had better OS (not estimable vs 13.2 months; P = 0.0006), PFS (6.8 months vs 4.5 months, P < 0.0001), and ORR (33% vs 13%, P < 0.0001), but grade 3-4 events were similar.29 This combination has potential for first-line approval. The COSMIC–312 study is comparing the combination of cabozantinib and atezolizumab to sorafenib monotherapy and cabozantinib monotherapy in advanced HCC.
Resistance to immunotherapy can be extrinsic, associated with activation mechanisms of T-cells, or intrinsic, related to immune recognition, gene expression, and cell-signaling pathways.30 Tumor-immune heterogeneity and antigen presentation contribute to complex resistance mechanisms.31,32 Although clinical outcomes have improved with immune checkpoint inhibitors, the response rate is low and responses are inconsistent, likely due to an immunosuppressive tumor microenvironment.33 Therefore, several novel combinations of checkpoint inhibitors and targeted drugs are being evaluated to bypass some of the resistance mechanisms (Table 3).
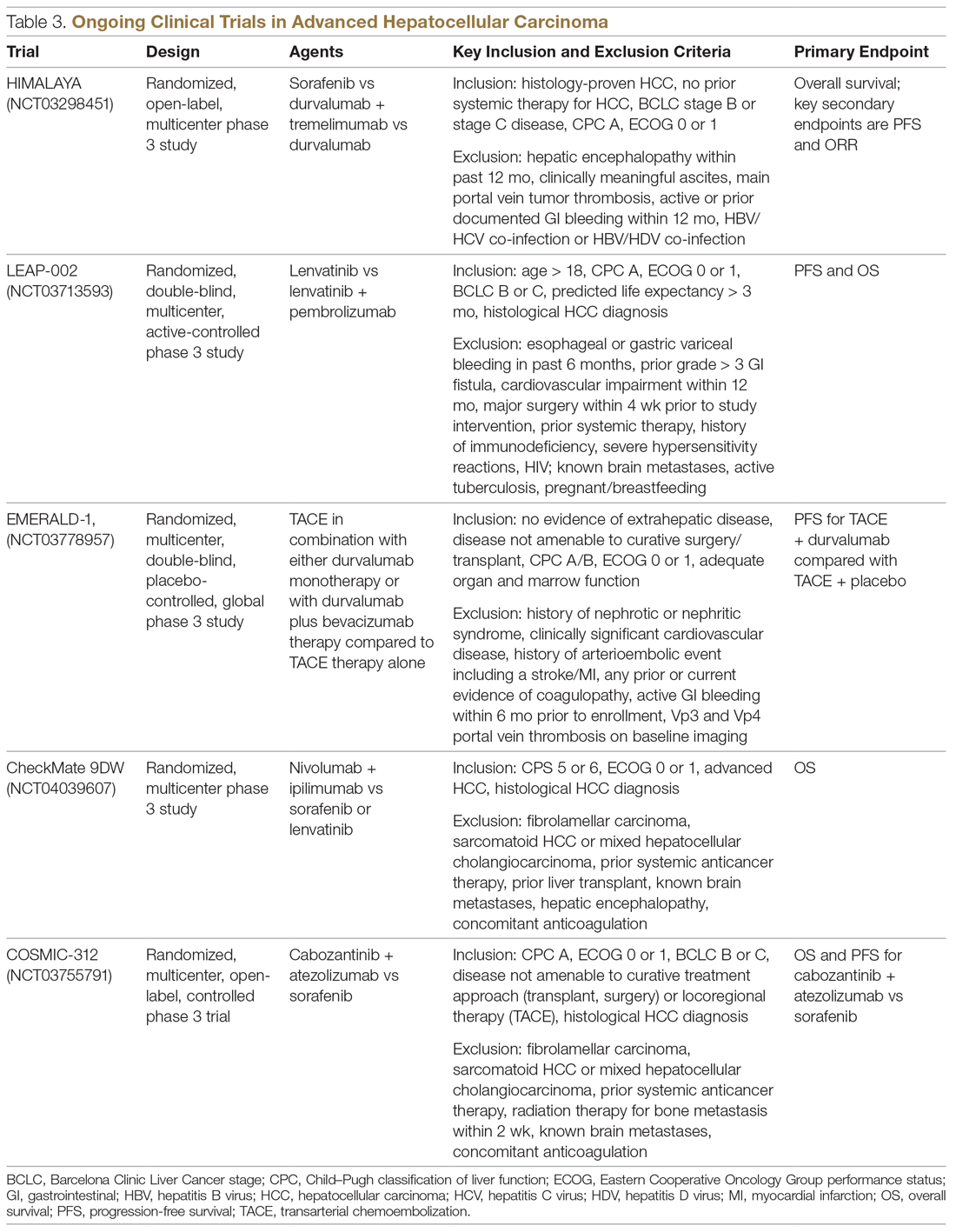
Chemotherapy
Multiple combinations of cytotoxic regimens have been evaluated, but efficacy has been modest, suggesting the limited role for traditional chemotherapy in the systemic management of advanced HCC. Response rates to chemotherapy are low and responses are not durable. Gemcitabine- and doxorubicin-based treatment and FOLFOX (5-fluorouracil, leucovorin, oxaliplatin) are some regimens that have been studied, with a median OS of less than 1 year for these regimens.34-36 FOLFOX had a higher response rate (8.15% vs 2.67%; P = 0.02) and longer median OS (6.40 months versus 4.97 months; HR, 0.80; 95% CI, 0.63-1.02; P = 0.07) than doxorubicin.34 With the gemcitabine/oxaliplatin combination, ORR was 18%, with stable disease in 58% of patients, and median PFS and OS were 6.3 months and 11.5 months, respectively.35 In a study that compared doxorubicin and PIAF (cisplatin/interferon a-2b/doxorubicin/5-fluorouracil), median OS was 6.83 months and 8.67 months, respectively (P = 0.83). The hazard ratio for death from any cause in the PIAF group compared with the doxorubicin group was 0.97 (95% CI, 0.71-1.32). PIAF had a higher ORR (20.9%; 95% CI, 12.5%-29.2%) than doxorubicin (10.5%; 95% CI, 3.9%-16.9%).
The phase 3 ALLIANCE study evaluated the combination of sorafenib and doxorubicin in treatment-naïve HCC patients with Child–Pugh class A liver disease, and did not demonstrate superiority with the addition of cytotoxic chemotherapy.37 Indeed, the combination of chemotherapy with sorafenib appears harmful in terms of lower OS (9.3 months vs 10.6 months; HR, 1.06; 95% CI, 0.8-1.4) and worse toxicity. Patients treated with the combination experienced more hematologic (37.8% vs 8.1%) and nonhematologic adverse events (63.6% vs 61.5%).
Locoregional Therapy
The role of locoregional therapy in advanced HCC remains the subject of intense debate. Patients with BCLC stage C HCC with metastatic disease and those with lymph node involvement are candidates for systemic therapy. The optimal candidate for locoregional therapy is the patient with localized intermediate-stage disease, particularly hepatic artery–delivered therapeutic interventions. However, the presence of a solitary large tumor or portal vein involvement constitutes gray areas regarding which therapy to deliver directly to the tumor via the hepatic artery, and increasingly stereotactic body radiation therapy is being offered.
Transarterial Chemoembolization
Transarterial chemoembolization (TACE), with or without chemotherapy, is the most widely adopted locoregional therapy in the management of HCC. TACE exploits the differential vascular supply to the HCC and normal liver parenchyma. Normal liver receives only one-fourth of its blood supply from the hepatic artery (three-fourths from the portal vein), whereas HCC is mainly supplied by the hepatic artery. A survival benefit for TACE compared to best supportive care is widely acknowledged for intermediate-stage HCC, and transarterial embolization (TAE) with gelatin sponge or microspheres is noninferior to TACE.38,39 Overall safety profile and efficacy inform therapy selection in advanced HCC, although the evidence for TACE in advanced HCC is less robust. Although single-institution experiences suggest survival numbers similar to and even superior to sorafenib,40,41 there is a scarcity of large randomized clinical trial data to back this up. Based on this, patients with advanced HCC should only be offered liver-directed therapy within a clinical trial or on a case-by-case basis under multidisciplinary tumor board consensus.
A serious adverse effect of TACE is post-embolization syndrome, which occurs in about 30% of patients and may be associated with poor prognosis.42 The syndrome consists of right upper quadrant abdominal pain, malaise, and worsening nausea/vomiting following the embolization procedure. Laboratory abnormalities and other complications may persist for up to 30 days after the procedure. This is a concern, because post-embolization syndrome may affect the ability to deliver systemic therapy.
Transarterial Radioembolization
In the past few years, there has been an uptick in the utilization of transarterial radioembolization (TARE), which instead of delivering glass beads, as done in TAE, or chemotherapy-infused beads, as done in TACE, delivers the radioisotope Y-90 to the tumor via the hepatic artery. TARE is able to administer larger doses of radiation to the tumor while avoiding normal liver tissue, as compared to external-beam radiation. There has been no head-to-head comparison of these different intra-arterial therapy approaches, but TARE with Y-90 has been shown to be safe in patients with portal vein thrombosis. A recent multicenter retrospective study of TARE demonstrated a median OS of 8.8 to 10.8 months in patients with BCLC C HCC,43 and in a large randomized study of Y-90 compared to sorafenib in advanced and previously treated intermediate HCC, there was no difference in median OS between the treatment modalities (8 months for selective internal radiotherapy, 9 months for sorafenib; P = 0.18). Treatment with Y-90 was better tolerated.44 A major impediment to the adoption of TARE is the time it takes to order, plan, and deliver Y-90 to patients. Radio-embolization-induced liver disease, similar to post-embolization syndrome, is characterized by jaundice and ascites, which may occur 4 to 8 weeks postprocedure and is more common in patients with HCC who do not have cirrhosis. Compared to TACE, TARE may offer a better adverse effect profile, with improvement in quality of life.
Combination of Systemic and Locoregional Therapy
Even in carefully selected patients with intermediate- and advanced-stage HCC, locoregional therapy is not curative. Tumor embolization may promote more angiogenesis, and hence tumor progression, by causing hypoxia and upregulation of hypoxia-inducible factor.45 This upregulation of angiogenesis as a resistance mechanism to tumor embolization provides a rationale for combining systemic therapy (typically based on abrogating angiogenesis) with TACE/TAE. Most of the experience has been with sorafenib in intermediate-stage disease, and the results have been disappointing. The administration of sorafenib after at least a partial response with TACE did not provide additional benefit in terms of time to progression.46 Similarly, in the SPACE trial, concurrent therapy with TACE-doxorubicin-eluting beads and sorafenib compared to TACE-doxorubicin-eluting beads and placebo yielded similar time to progression numbers for both treatment modalities.47 While the data have been disappointing in intermediate-stage disease, as described earlier, registry data suggest that patients with advanced-stage disease may benefit from this approach.48
In the phase 2 TACTICS trial, 156 patients with unresectable HCC were randomized to receive TACE alone or sorafenib plus TACE, with a novel endpoint, time to untreatable progression (TTUP) and/or progression to TACE refractoriness.49 Treatment with sorafenib following TACE was continued until TTUP, decline in liver function to Child–Pugh class C, or the development of vascular invasion or extrahepatic spread. Development of new lesions while on sorafenib was not considered as progressive disease as long as the lesions were amenable to TACE. In this study, PFS was longer with sorafenib-TACE compared to TACE alone (26.7 months vs 20.6 months; P = 0.02). However, the TTUP endpoint needs further validation, and we are still awaiting the survival outcomes of this study. At this time, there are insufficient data to recommend the combination of liver-directed locoregional therapy and sorafenib or other systemic therapy options outside of a clinical trial setting.
Current Treatment Approach for Advanced HCC (BCLC-C)
Although progress is being made in the development of effective therapies, advanced HCC is generally incurable. These patients experience significant symptom burden throughout the course of the disease. Therefore, the optimal treatment plan must focus on improving or maintaining quality of life, in addition to overall efficacy. It is important to actively involve patients in treatment decisions for an individualized treatment plan, and to discuss the best strategy for incorporating current advances in targeted and immunotherapies. The paradigm of what constitutes first-line treatment for advanced HCC is shifting due to the recent systemic therapy approvals. Prior to the availability of these therapies, many patients with advanced HCC were treated with repeated locoregional therapies. For instance, TACE was often used to treat unresectable HCC multiple times beyond progression. There was no consensus on the definition of TACE failure, and hence it was used in broader, unselected populations. Retrospective studies suggest that continuing TACE after refractoriness or failure may not be beneficial, and may delay subsequent treatments because of deterioration of liver function or declines in performance status. With recent approvals of several systemic therapy options, including immunotherapy, it is vital to conduct a risk-benefit assessment prior to repeating TACE after failure, so that patients are not denied the use of available systemic therapeutic options due to declined performance status or organ function from these procedures. The optimal timing and the sequence of systemic and locoregional therapy must be carefully evaluated by a multidisciplinary team.
CASE CONCLUSION
An important part of evaluating a new patient with HCC is to determine whether they are a candidate for curative therapies, such as transplant or surgical resection. These are no longer an option for patients with intermediate disease. For patients with advanced disease characteristics, such as vascular invasion or systemic metastasis, the evidence supports using systemic therapy with sorafenib or lenvatinib. Lenvatinib, with a better tolerance profile and response rate, is the treatment of choice for the patient described in the case scenario. Lenvatinib is also indicated for first-line treatment of advanced HCC, and is useful in very aggressive tumors, such as those with an AFP level exceeding 200 ng/mL.
Future Directions
The emerging role of novel systemic therapeutics, including immunotherapy, has drastically changed the treatment landscape for hepatocellular cancers, with 6 new drugs for treating advanced hepatocellular cancers approved recently. While these systemic drugs have improved survival in advanced HCC in the past decade, patient selection and treatment sequencing remain a challenge, due to a lack of biomarkers capable of predicting antitumor responses. In addition, there is an unmet need for treatment options for patients with Child–Pugh class B7 and C liver disease and poor performance status.
The goal of future management should be to achieve personalized care aimed at improved safety and efficacy by targeting multiple cancer pathways in the HCC cascade with combination treatments. Randomized clinical trials to improve patient selection and determine the proper sequence of treatments are needed. Given the heterogeneity of HCC, molecular profiling of the tumor to differentiate responders from nonresponders may elucidate potential biomarkers to effectively guide treatment recommendations.
1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424.
2. Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485-1491.
3. Welzel TM, Graubard BI, Zeuzem S, et al. Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-Medicare database. Hepatology. 2011;54:463-471.
4. Schutte K, Schulz C, Poranzke J, et al. Characterization and prognosis of patients with hepatocellular carcinoma (HCC) in the non-cirrhotic liver. BMC Gastroenterol. 2014;14:117.
5. Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338.
6. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314.
7. Schulze K, Imbeaud S, Letouzé E, et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet. 2015;47:505-511.
8. Thorgeirsson SS, Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet. 2002;31:339-346.
9. Dhanasekaran R, Bandoh S, Roberts LR. Molecular pathogenesis of hepatocellular carcinoma and impact of therapeutic advances. F1000Res. 2016;5.
10. Schulze K, Imbeaud S, Letouze E, et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet. 2015;47:505-511.
11. Cancer Genome Atlas Research Network. Electronic address: [email protected]; Cancer Genome Atlas Research Network. Comprehensive and integrative genomic characterization of hepatocellular carcinoma. Cell. 2017;169:1327-1134.
12. Chae YK, Ranganath K, Hammerman PS, et al: Inhibition of the fibroblast growth factor receptor (FGFR) pathway: the current landscape and barriers to clinical application. Oncotarget. 2016;8:16052-16074.
13. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390.
14. Jackson R, Psarelli EE, Berhane S, et al. Impact of viral status on survival in patients receiving sorafenib for advanced hepatocellular cancer: a meta-analysis of randomized phase III trials. J Clin Oncol. 2017;35:622-628.
15. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34.
16. Da Fonseca LG, Barroso-Sousa R, Bento AD, et al. Safety and efficacy of sorafenib in patients with Child-Pugh B advanced hepatocellular carcinoma. Mol Clin Oncol. 2015;3:793-796.
17. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173.
18. Bruix J, Tak W-Y, Gasbarrini A, et al. Regorafenib as second-line therapy for intermediate or advanced hepatocellular carcinoma: Multicentre, open-label, phase II safety study. Eur J Cancer. 2013;49:3412-3419.
19. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56-66.
20. Bruix J, Merle P, Granito A, et al. Hand-foot skin reaction (HFSR) and overall survival (OS) in the phase 3 RESORCE trial of regorafenib for treatment of hepatocellular carcinoma (HCC) progressing on sorafenib. J Clin Oncol. 2018;36:412-412.
21. Finn RS, Merle P, Granito A, et al. Outcomes of sequential treatment with sorafenib followed by regorafenib for HCC: Additional analyses from the phase III RESORCE trial. J Hepatol. 2018;69:353-358.
22. Abou-Alfa GK, Meyer T, Cheng A-L, et al. Cabozantinib (C) versus placebo (P) in patients (pts) with advanced hepatocellular carcinoma (HCC) who have received prior sorafenib: Results from the randomized phase III CELESTIAL trial. J Clin Oncol. 2018;36:207-207.
23. Zhu AX, Park JO, Ryoo B-Y, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncology. 2015;16:859-870.
24. Zhu AX, Kang Y-K, Yen C-J, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased αfetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncology. 2019;20:282-296.
25. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492-2502.
26. Yau T, Park JW, Finn RS, et al. CheckMate 459: A randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Ann Oncol. 2020;30:v874-v875.
27. Zhu AX, Finn RS, Cattan S, et al. KEYNOTE-224: Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib. J Clin Oncol. 2018;36:942-952.
28. Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38:193-202.
29. Cheng A-L, Qin S, Ikeda M, et al. IMbrave150: efficacy and safety results from a ph III study evaluating atezolizumab (atezo) + bevacizumab (bev) vs sorafenib (sor) as first treatment (tx) for patients (pts) with unresectable hepatocellular carcinoma (HCC). Ann Oncol. 2019;30 (suppl_9):ix183-ix202.
30. Jiang Y, Han Q-J, Zhang J. Hepatocellular carcinoma: Mechanisms of progression and immunotherapy. World J Gastroenterol. 2019;25:3151-3167.
31. Xu F, Jin T, Zhu Y, et al. Immune checkpoint therapy in liver cancer. J Exp Clin Cancer Res. 2018;37:110.
32. Koyama S, Akbay EA, Li YY, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. 2016;7:10501.
33. Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2015;12:681-700.
34. Qin S, Bai Y, Lim HY, et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol. 2013;31:3501-3508.
35. Louafi S, Boige V, Ducreux M, et al. Gemcitabine plus oxaliplatin (GEMOX) in patients with advanced hepatocellular carcinoma (HCC). Cancer. 2007;109:1384-1390.
36. Tang A, Chan AT, Zee B, et al. A randomized phase iii study of doxorubicin versus cisplatin/interferon α-2b/doxorubicin/fluorouracil (PIAF) combination chemotherapy for unresectable hepatocellular carcinoma. J Natl Cancer Inst. 2005;97:1532-1538.
37. Abou-Alfa GK, Niedzwieski D, Knox JJ, et al. Phase III randomized study of sorafenib plus doxorubicin versus sorafenib in patients with advanced hepatocellular carcinoma (HCC): CALGB 80802 (Alliance). J Clin Oncol. 2016;34:192.
38. Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739.
39. Brown KT, Do RK, Gonen M, et al. randomized trial of hepatic artery embolization for hepatocellular carcinoma using doxorubicin-eluting microspheres compared with embolization with microspheres alone. J Clin Oncol. 2016;34:2046-2053.
40. Kirstein MM, Voigtlander T, Schweitzer N, et al. Transarterial chemoembolization versus sorafenib in patients with hepatocellular carcinoma and extrahepatic disease. United European Gastroenterol J. 2018;6:238-246.
41. Pinter M, Hucke F, Graziadei I, et al. Advanced-stage hepatocellular carcinoma: transarterial chemoembolization versus sorafenib. Radiology. 2012;263:590-599.
42. Mason MC, Massarweh NN, Salami A, et al. Post-embolization syndrome as an early predictor of overall survival after transarterial chemoembolization for hepatocellular carcinoma. HPB (Oxford). 2015;17:1137-1144.
43. Sangro B, Maini CL, Ettorre GM, et al. Radioembolisation in patients with hepatocellular carcinoma that have previously received liver-directed therapies. Eur J Nucl Med Mol Imaging. 2018;45:1721-1730.
44. Vilgrain V, Pereira H, Assenat E, et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2017;18:1624-1636.
45. Sergio A, Cristofori C, Cardin R, et al. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol. 2008;103:914-921.
46. Kudo M, Imanaka K, Chida N, et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer. 2011;47:2117-2127.
47. Lencioni R, Llovet JM, Han G, et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: The SPACE trial. J Hepatol. 2016;64:1090-1098.
48. Geschwind JF, Chapiro J. Sorafenib in combination with transarterial chemoembolization for the treatment of hepatocellular carcinoma. Clin Adv Hematol Oncol. 2016;14:585-587.
49. Kudo M, Ueshima K, Ikeda M, et al. Randomized, open label, multicenter, phase II trial comparing transarterial chemoembolization (TACE) plus sorafenib with TACE alone in patients with hepatocellular carcinoma (HCC): TACTICS trial. J Clin Oncol. 2018;36:206.
From the University of Alabama at Birmingham, Division of Hematology Oncology, Birmingham, AL, and the University of South Alabama, Division of Hematology Oncology, Mobile, AL. Dr. Paluri and Dr. Hatic contributed equally to this article.
Abstract
- Objective: To review systemic treatment options for patients with locally advanced unresectable hepatocellular carcinoma (HCC).
- Methods: Review of the literature.
- Results: The paradigm of what constitutes first-line treatment for advanced HCC is shifting. Until recently, many patients with advanced HCC were treated with repeated locoregional therapies, such as transartertial embolization (TACE). However, retrospective studies suggest that continuing TACE after refractoriness or failure may not be beneficial and may delay subsequent treatments because of deterioration of liver function or declines in performance status. With recent approvals of several systemic therapy options, including immunotherapy, it is vital to conduct a risk-benefit assessment prior to repeating TACE after failure, so that patients are not denied the use of available systemic therapeutic options due to declined performance status or organ function from these procedures. The optimal timing and the sequence of systemic and locoregional therapy must be carefully evaluated by a multidisciplinary team.
- Conclusion: Randomized clinical trials to improve patient selection and determine the proper sequence of treatments are needed. Given the heterogeneity of HCC, molecular profiling of the tumor to differentiate responders from nonresponders may elucidate potential biomarkers to effectively guide treatment recommendations.
Keywords: liver cancer; molecular therapy; immunotherapy.
Hepatocellular carcinoma (HCC) represents 90% of primary liver malignancies. It is the fifth most common malignancy in males and the ninth most common in females worldwide.1 In contrast to other major cancers (colon, breast, prostate), the incidence of and mortality from HCC has increased over the past decade, following a brief decline between 1999 and 2004.2 The epidemiology and incidence of HCC is closely linked to chronic liver disease and conditions predisposing to liver cirrhosis. Worldwide, hepatitis B virus infection is the leading cause of liver cirrhosis and, hence, HCC. In the United States, 50% of HCC cases are linked to hepatitis C virus (HCV) infection. Diabetes mellitus and alcoholic and nonalcoholic steatohepatitis are the other major etiologies of HCC. Indeed, the metabolic syndrome, independent of other factors, is associated with a 2-fold increase in the risk of HCC.3
Although most cases of HCC are predated by liver cirrhosis, in about 20% of patients HCC occurs without liver cirrhosis.4 Similar to other malignancies, surgery in the form of resection (for isolated lesions in the context of good liver function) or liver transplant (for low-volume disease with mildly impaired liver function) provides the best chance of a cure. Locoregional therapies involving hepatic artery–directed therapy are offered for patients with more advanced disease that is limited to the liver, while systemic therapy is offered for advanced unresectable disease that involves portal vein invasion, lymph nodes, and distant metastasis. The
Molecular Pathogenesis
Similar to other malignancies, a multistep process of carcinogenesis, with accumulation of genomic alterations at the molecular and cellular levels, is recognized in HCC. In about 80% of cases, repeated and chronic injury, inflammation, and repair lead to a distortion of normal liver architecture and development of cirrhotic nodules. Exome sequencing of HCC tissues has identified risk factor–specific mutational signatures, including those related to the tumor microenvironment, and defined the extensive landscape of altered genes and pathways in HCC (eg, angiogenic and MET pathways).7 In the Schulze et al study, the frequency of alterations that could be targeted by available Food and Drug Administration (FDA)–approved drugs comprised either amplifications or mutations of FLTs (6%), FGF3 or 4 or 19 (4%), PDGFRs (3%), VEGFA (1%), HGF (3%), MTOR (2%), EGFR (1%), FGFRs (1%), and MET (1%).7 Epigenetic modification of growth-factor expression, particularly insulin-like growth factor 2 and transforming growth factor alpha, and structural alterations that lead to loss of heterozygosity are early events that cause hepatocyte proliferation and progression of dysplastic nodules.8,9 Advances in whole-exome sequencing have identified TERT promoter mutations, leading to activation of telomerase, as an early event in HCC pathogenesis. Other commonly altered genes include CTNNB1 (B-Catenin) and TP53. As a group, alterations in the MAP kinase pathway genes occur in about 40% of HCC cases.
Actionable oncogenic driver alterations are not as common as tumor suppressor pathway alterations in HCC, making targeted drug development challenging.10,11 The FGFR (fibroblast growth factor receptor) pathway, which plays a critical role in carcinogenesis-related cell growth, survival, neo-angiogenesis, and acquired resistance to other cancer treatments, is being explored as a treatment target.12 The molecular characterization of HCC may help with identifying new biomarkers and present opportunities for developing therapeutic targets.
CASE PRESENTATION
A 61-year-old man with a history of chronic hepatitis C and hypertension presents to his primary care physician due to right upper quadrant pain. Laboratory evaluation shows transaminases elevated 2 times the upper limit of normal. This leads to an ultrasound and follow-up magnetic resonance imaging. Imaging shows diffuse cirrhotic changes, with a 6-cm, well-circumscribed lesion within the left lobe of the liver that shows rapid arterial enhancement with venous washout. These vascular characteristics are consistent with HCC. In addition, 2 satellite lesions in the left lobe and sonographic evidence of invasion into the portal vein are present. Periportal lymph nodes are pathologically enlarged.
The physical examination is unremarkable, except for mild tenderness over the right upper quadrant of the abdomen. Serum bilirubin, albumin, platelets, and international normalized ratio are normal, and alpha fetoprotein (AFP) is elevated at 1769 ng/mL. The patient’s family history is unremarkable for any major illness or cancer. Computed tomography scan of the chest and pelvis shows no evidence of other lesions. His liver disease is classified as Child–Pugh A. Due to locally advanced presentation, the tumor is deemed to be nontransplantable and unresectable, and is staged as BCLC-C. The patient continues to work and his performance status is ECOG (
What systemic treatment would you recommend for this patient with locally advanced unresectable HCC with nodal metastasis?
First-Line Therapeutic Options
Systemic treatment of HCC is challenging because of the underlying liver cirrhosis and hepatic dysfunction present in most patients. Overall prognosis is therefore dependent on the disease biology and burden and on the degree of hepatic dysfunction. These factors must be considered in patients with advanced disease who present for systemic therapy. As such, patients with BCLC class D HCC with poor performance status and impaired liver function are better off with best supportive care and hospice services (Figure). Table 1 and Table 2 outline the landmark trials that led to the approval of agents for advanced HCC treatment.

Sorafenib
In the patient with BCLC class C HCC who has preserved liver function (traditionally based on a Child–Pugh score of ≤ 6 and a decent functional status [ECOG performance status 1-2]), sorafenib is the first FDA-approved first-line treatment. Sorafenib is a small-molecule tyrosine kinase inhibitor that targets vascular endothelial growth factor receptor (VEGFR) kinase signaling, in addition to many other tyrosine kinase pathways (including the platelet-derived growth factor and Raf-Ras pathways). Evidence for the clinical benefit of sorafenib comes from the SHARP trial.13 This was a multinational, but primarily European, randomized phase 3 study that compared sorafenib to best supportive care for advanced HCC in patients with a Child–Pugh score ≤ 6A and a robust performance status (ECOG 0 and 1). Overall survival (OS) with placebo and sorafenib was 7.9 months and 10.7 months, respectively. There was no difference in time to radiologic progression, and the progression-free survival (PFS) at 4 months was 62% with sorafenib and 42% with placebo. Patients with HCV-associated HCC appeared to derive a more substantial benefit from sorafenib.14 In a smaller randomized study of sorafenib in Asian patients with predominantly hepatitis B–associated HCC, OS in the sorafenib and best supportive care arms was lower than that reported in the SHARP study (6.5 months vs 4.2 months), although OS still was longer in the sorafenib group.15

Significant adverse events reported with sorafenib include fatigue (30%), hand and foot syndrome (30%), diarrhea (15%), and mucositis (10%). Major proportions of patients in the clinical setting have not tolerated the standard dose of 400 mg twice daily. Dose-adjusted administration of sorafenib has been advocated in patients with more impaired liver function (Child–Pugh class 7B) and bilirubin of 1.5 to 3 times the upper limit of normal, although it is unclear whether these patients are deriving any benefit from sorafenib.16 At this time, in a patient with preserved liver function, starting with 400 mg twice daily, followed by dose reduction based on toxicity, remains standard.

Lenvatinib
After multiple attempts to develop newer first-line treatments for HCC,
Second-Line Therapeutic Options
Following the sorafenib approval, clinical studies of several other agents did not meet their primary endpoint and failed to show improvement in clinical outcomes compared to sorafenib. However, over the past years the treatment landscape for advanced HCC has been changed with the approval of several agents in the second line. The overall response rate (ORR) has become the new theme for management of advanced disease. With multiple therapeutic options available, optimal sequencing now plays a critical role, especially for transitioning from locoregional to systemic therapy. Five drugs are now indicated for second-line treatment of patients who progressed on or were intolerant to sorafenib: regorafenib, cabozantinib, ramucirumab, nivolumab, and pembrolizumab.
Regorafenib
Regorafenib was evaluated in the advanced HCC setting in a single-arm, phase 2 trial involving 36 patients with Child–Pugh class A liver disease who had progressed on prior sorafenib.18 Patients received regorafenib 160 mg orally once daily for 3 weeks on/1 week off cycles. Disease control was achieved in 72% of patients, with stable disease in 25 patients (69%). Based on these results, regorafenib was further evaluated in the multicenter, phase 3, 2:1 randomized, double-blind, placebo-controlled RESORCE study, which enrolled 573 patients.19 Due to the overlapping safety profiles of sorafenib and regorafenib, the inclusion criteria required patients to have tolerated a sorafenib dose of at least 400 mg daily for 20 of the past 28 days of treatment prior to enrollment. The primary endpoint of the study, OS, was met (median OS of 10.6 months in regorafenib arm versus 7.8 months in placebo arm; hazard ratio [HR], 0.63; P < 0.0001).
Cabozantinib
CELESTIAL was a phase 3, double-blind study that assessed the efficacy of cabozantinib versus placebo in patients with advanced HCC who had received prior sorafenib.22 In this study, 707 patients with Child–Pugh class A liver disease who progressed on at least 1 prior systemic therapy were randomized in a 2:1 ratio to treatment with cabozantinib at 60 mg daily or placebo. Patients treated with cabozantinib had a longer OS (10.2 months vs 8.0 months), resulting in a 24% reduction in the risk of death (HR, 0.76), and a longer median PFS (5.2 months versus 1.9 months). The disease control rate was higher with cabozantinib (64% vs 33%) as well. The incidence of high‐grade adverse events in the cabozantinib group was twice that of the placebo group. Common adverse events reported with cabozantinib included HFSR (17%), hypertension (16%), increased aspartate aminotransferase (12%), fatigue (10%), and diarrhea (10%).
Ramucirumab
REACH was a phase 3 study exploring the efficacy of ramucirumab that did not meet its primary endpoint; however, the subgroup analysis in AFP-high patients showed an OS improvement with ramucirumab.23 This led to the phase 3 REACH-2 trial, a multicenter, randomized, double-blind biomarker study in patients with advanced HCC who either progressed on or were intolerant to sorafenib and had an AFP level ≥ 400 ng/mL.24 Patients were randomized to ramucirumab 8 mg/kg every 2 weeks or placebo. The study met its primary endpoint, showing improved OS (8.5 months vs 7.3 months; P = 0.0059). The most common treatment-related adverse events in the ramucirumab group were ascites (5%), hypertension (12%), asthenia (5%), malignant neoplasm progression (6%), increased aspartate aminotransferase concentration (5%), and thrombocytopenia.
Immunotherapy
HCC is considered an inflammation-induced cancer, which renders immunotherapeutic strategies more appealing. The PD-L1/PD-1 pathway is the critical immune checkpoint mechanism and is an important target for treatment. HCC uses a complex, overlapping set of mechanisms to evade cancer-specific immunity and to suppress the immune system. Initial efforts to develop immunotherapies for HCC focused on anti-PD-1 and anti-PD-L1 antibodies. CheckMate 040 evaluated nivolumab in 262 sorafenib-naïve and -treated patients with advanced HCC (98% with Child–Pugh scores of 5 or 6), with a median follow-up of 12.9 months.25 In sorafenib-naïve patients (n = 80), the ORR was 23%, and the disease control rate was 63%. In sorafenib-treated patients (n = 182), the ORR was 18%. Response was not associated with PD-L1 expression. Durable objective responses, a manageable safety profile, and promising efficacy led the FDA to grant accelerated approval of nivolumab for the treatment of patients with HCC who have been previously treated with sorafenib. Based on this, the phase 3 randomized CheckMate-459 trial evaluated the efficacy of nivolumab versus sorafenib in the first-line. Median OS and ORR were better with nivolumab (16.4 months vs 14.7 months; HR 0.85; P = 0.752; and 15% [with 5 complete responses] vs 7%), as was the safety profile (22% vs 49% reporting grade 3 and 4 adverse events). 26
The KEYNOTE-224 study27 evaluated pembrolizumab in 104 patients with previously treated advanced HCC. This study showed an ORR of 17%, with 1 complete response and 17 partial responses. One-third of the patients had progressive disease, while 46 had stable disease. Among those who responded, 56% maintained a durable response for more than 1 year. Subsequently, in KEYNOTE 240, pembrolizumab showed an improvement in OS (13.9 months vs 10.6 months; HR, 0.78; P = 0.0238) and PFS (3.0 months versus 2.8 months; HR, 0.78; P = 0.0186) compared with placebo.28 The ORR for pembrolizumab was 16.9% (95% confidence interval [CI], 12.7%-21.8%) versus 2.2% (95% CI, 0.5%-6.4%; P = 0.00001) for placebo. Mean duration of response was 13.8 months.
In the IMbrave150 trial, atezolizumab/bevacizumab combination, compared to sorafenib, had better OS (not estimable vs 13.2 months; P = 0.0006), PFS (6.8 months vs 4.5 months, P < 0.0001), and ORR (33% vs 13%, P < 0.0001), but grade 3-4 events were similar.29 This combination has potential for first-line approval. The COSMIC–312 study is comparing the combination of cabozantinib and atezolizumab to sorafenib monotherapy and cabozantinib monotherapy in advanced HCC.
Resistance to immunotherapy can be extrinsic, associated with activation mechanisms of T-cells, or intrinsic, related to immune recognition, gene expression, and cell-signaling pathways.30 Tumor-immune heterogeneity and antigen presentation contribute to complex resistance mechanisms.31,32 Although clinical outcomes have improved with immune checkpoint inhibitors, the response rate is low and responses are inconsistent, likely due to an immunosuppressive tumor microenvironment.33 Therefore, several novel combinations of checkpoint inhibitors and targeted drugs are being evaluated to bypass some of the resistance mechanisms (Table 3).

Chemotherapy
Multiple combinations of cytotoxic regimens have been evaluated, but efficacy has been modest, suggesting the limited role for traditional chemotherapy in the systemic management of advanced HCC. Response rates to chemotherapy are low and responses are not durable. Gemcitabine- and doxorubicin-based treatment and FOLFOX (5-fluorouracil, leucovorin, oxaliplatin) are some regimens that have been studied, with a median OS of less than 1 year for these regimens.34-36 FOLFOX had a higher response rate (8.15% vs 2.67%; P = 0.02) and longer median OS (6.40 months versus 4.97 months; HR, 0.80; 95% CI, 0.63-1.02; P = 0.07) than doxorubicin.34 With the gemcitabine/oxaliplatin combination, ORR was 18%, with stable disease in 58% of patients, and median PFS and OS were 6.3 months and 11.5 months, respectively.35 In a study that compared doxorubicin and PIAF (cisplatin/interferon a-2b/doxorubicin/5-fluorouracil), median OS was 6.83 months and 8.67 months, respectively (P = 0.83). The hazard ratio for death from any cause in the PIAF group compared with the doxorubicin group was 0.97 (95% CI, 0.71-1.32). PIAF had a higher ORR (20.9%; 95% CI, 12.5%-29.2%) than doxorubicin (10.5%; 95% CI, 3.9%-16.9%).
The phase 3 ALLIANCE study evaluated the combination of sorafenib and doxorubicin in treatment-naïve HCC patients with Child–Pugh class A liver disease, and did not demonstrate superiority with the addition of cytotoxic chemotherapy.37 Indeed, the combination of chemotherapy with sorafenib appears harmful in terms of lower OS (9.3 months vs 10.6 months; HR, 1.06; 95% CI, 0.8-1.4) and worse toxicity. Patients treated with the combination experienced more hematologic (37.8% vs 8.1%) and nonhematologic adverse events (63.6% vs 61.5%).
Locoregional Therapy
The role of locoregional therapy in advanced HCC remains the subject of intense debate. Patients with BCLC stage C HCC with metastatic disease and those with lymph node involvement are candidates for systemic therapy. The optimal candidate for locoregional therapy is the patient with localized intermediate-stage disease, particularly hepatic artery–delivered therapeutic interventions. However, the presence of a solitary large tumor or portal vein involvement constitutes gray areas regarding which therapy to deliver directly to the tumor via the hepatic artery, and increasingly stereotactic body radiation therapy is being offered.
Transarterial Chemoembolization
Transarterial chemoembolization (TACE), with or without chemotherapy, is the most widely adopted locoregional therapy in the management of HCC. TACE exploits the differential vascular supply to the HCC and normal liver parenchyma. Normal liver receives only one-fourth of its blood supply from the hepatic artery (three-fourths from the portal vein), whereas HCC is mainly supplied by the hepatic artery. A survival benefit for TACE compared to best supportive care is widely acknowledged for intermediate-stage HCC, and transarterial embolization (TAE) with gelatin sponge or microspheres is noninferior to TACE.38,39 Overall safety profile and efficacy inform therapy selection in advanced HCC, although the evidence for TACE in advanced HCC is less robust. Although single-institution experiences suggest survival numbers similar to and even superior to sorafenib,40,41 there is a scarcity of large randomized clinical trial data to back this up. Based on this, patients with advanced HCC should only be offered liver-directed therapy within a clinical trial or on a case-by-case basis under multidisciplinary tumor board consensus.
A serious adverse effect of TACE is post-embolization syndrome, which occurs in about 30% of patients and may be associated with poor prognosis.42 The syndrome consists of right upper quadrant abdominal pain, malaise, and worsening nausea/vomiting following the embolization procedure. Laboratory abnormalities and other complications may persist for up to 30 days after the procedure. This is a concern, because post-embolization syndrome may affect the ability to deliver systemic therapy.
Transarterial Radioembolization
In the past few years, there has been an uptick in the utilization of transarterial radioembolization (TARE), which instead of delivering glass beads, as done in TAE, or chemotherapy-infused beads, as done in TACE, delivers the radioisotope Y-90 to the tumor via the hepatic artery. TARE is able to administer larger doses of radiation to the tumor while avoiding normal liver tissue, as compared to external-beam radiation. There has been no head-to-head comparison of these different intra-arterial therapy approaches, but TARE with Y-90 has been shown to be safe in patients with portal vein thrombosis. A recent multicenter retrospective study of TARE demonstrated a median OS of 8.8 to 10.8 months in patients with BCLC C HCC,43 and in a large randomized study of Y-90 compared to sorafenib in advanced and previously treated intermediate HCC, there was no difference in median OS between the treatment modalities (8 months for selective internal radiotherapy, 9 months for sorafenib; P = 0.18). Treatment with Y-90 was better tolerated.44 A major impediment to the adoption of TARE is the time it takes to order, plan, and deliver Y-90 to patients. Radio-embolization-induced liver disease, similar to post-embolization syndrome, is characterized by jaundice and ascites, which may occur 4 to 8 weeks postprocedure and is more common in patients with HCC who do not have cirrhosis. Compared to TACE, TARE may offer a better adverse effect profile, with improvement in quality of life.
Combination of Systemic and Locoregional Therapy
Even in carefully selected patients with intermediate- and advanced-stage HCC, locoregional therapy is not curative. Tumor embolization may promote more angiogenesis, and hence tumor progression, by causing hypoxia and upregulation of hypoxia-inducible factor.45 This upregulation of angiogenesis as a resistance mechanism to tumor embolization provides a rationale for combining systemic therapy (typically based on abrogating angiogenesis) with TACE/TAE. Most of the experience has been with sorafenib in intermediate-stage disease, and the results have been disappointing. The administration of sorafenib after at least a partial response with TACE did not provide additional benefit in terms of time to progression.46 Similarly, in the SPACE trial, concurrent therapy with TACE-doxorubicin-eluting beads and sorafenib compared to TACE-doxorubicin-eluting beads and placebo yielded similar time to progression numbers for both treatment modalities.47 While the data have been disappointing in intermediate-stage disease, as described earlier, registry data suggest that patients with advanced-stage disease may benefit from this approach.48
In the phase 2 TACTICS trial, 156 patients with unresectable HCC were randomized to receive TACE alone or sorafenib plus TACE, with a novel endpoint, time to untreatable progression (TTUP) and/or progression to TACE refractoriness.49 Treatment with sorafenib following TACE was continued until TTUP, decline in liver function to Child–Pugh class C, or the development of vascular invasion or extrahepatic spread. Development of new lesions while on sorafenib was not considered as progressive disease as long as the lesions were amenable to TACE. In this study, PFS was longer with sorafenib-TACE compared to TACE alone (26.7 months vs 20.6 months; P = 0.02). However, the TTUP endpoint needs further validation, and we are still awaiting the survival outcomes of this study. At this time, there are insufficient data to recommend the combination of liver-directed locoregional therapy and sorafenib or other systemic therapy options outside of a clinical trial setting.
Current Treatment Approach for Advanced HCC (BCLC-C)
Although progress is being made in the development of effective therapies, advanced HCC is generally incurable. These patients experience significant symptom burden throughout the course of the disease. Therefore, the optimal treatment plan must focus on improving or maintaining quality of life, in addition to overall efficacy. It is important to actively involve patients in treatment decisions for an individualized treatment plan, and to discuss the best strategy for incorporating current advances in targeted and immunotherapies. The paradigm of what constitutes first-line treatment for advanced HCC is shifting due to the recent systemic therapy approvals. Prior to the availability of these therapies, many patients with advanced HCC were treated with repeated locoregional therapies. For instance, TACE was often used to treat unresectable HCC multiple times beyond progression. There was no consensus on the definition of TACE failure, and hence it was used in broader, unselected populations. Retrospective studies suggest that continuing TACE after refractoriness or failure may not be beneficial, and may delay subsequent treatments because of deterioration of liver function or declines in performance status. With recent approvals of several systemic therapy options, including immunotherapy, it is vital to conduct a risk-benefit assessment prior to repeating TACE after failure, so that patients are not denied the use of available systemic therapeutic options due to declined performance status or organ function from these procedures. The optimal timing and the sequence of systemic and locoregional therapy must be carefully evaluated by a multidisciplinary team.
CASE CONCLUSION
An important part of evaluating a new patient with HCC is to determine whether they are a candidate for curative therapies, such as transplant or surgical resection. These are no longer an option for patients with intermediate disease. For patients with advanced disease characteristics, such as vascular invasion or systemic metastasis, the evidence supports using systemic therapy with sorafenib or lenvatinib. Lenvatinib, with a better tolerance profile and response rate, is the treatment of choice for the patient described in the case scenario. Lenvatinib is also indicated for first-line treatment of advanced HCC, and is useful in very aggressive tumors, such as those with an AFP level exceeding 200 ng/mL.
Future Directions
The emerging role of novel systemic therapeutics, including immunotherapy, has drastically changed the treatment landscape for hepatocellular cancers, with 6 new drugs for treating advanced hepatocellular cancers approved recently. While these systemic drugs have improved survival in advanced HCC in the past decade, patient selection and treatment sequencing remain a challenge, due to a lack of biomarkers capable of predicting antitumor responses. In addition, there is an unmet need for treatment options for patients with Child–Pugh class B7 and C liver disease and poor performance status.
The goal of future management should be to achieve personalized care aimed at improved safety and efficacy by targeting multiple cancer pathways in the HCC cascade with combination treatments. Randomized clinical trials to improve patient selection and determine the proper sequence of treatments are needed. Given the heterogeneity of HCC, molecular profiling of the tumor to differentiate responders from nonresponders may elucidate potential biomarkers to effectively guide treatment recommendations.
From the University of Alabama at Birmingham, Division of Hematology Oncology, Birmingham, AL, and the University of South Alabama, Division of Hematology Oncology, Mobile, AL. Dr. Paluri and Dr. Hatic contributed equally to this article.
Abstract
- Objective: To review systemic treatment options for patients with locally advanced unresectable hepatocellular carcinoma (HCC).
- Methods: Review of the literature.
- Results: The paradigm of what constitutes first-line treatment for advanced HCC is shifting. Until recently, many patients with advanced HCC were treated with repeated locoregional therapies, such as transartertial embolization (TACE). However, retrospective studies suggest that continuing TACE after refractoriness or failure may not be beneficial and may delay subsequent treatments because of deterioration of liver function or declines in performance status. With recent approvals of several systemic therapy options, including immunotherapy, it is vital to conduct a risk-benefit assessment prior to repeating TACE after failure, so that patients are not denied the use of available systemic therapeutic options due to declined performance status or organ function from these procedures. The optimal timing and the sequence of systemic and locoregional therapy must be carefully evaluated by a multidisciplinary team.
- Conclusion: Randomized clinical trials to improve patient selection and determine the proper sequence of treatments are needed. Given the heterogeneity of HCC, molecular profiling of the tumor to differentiate responders from nonresponders may elucidate potential biomarkers to effectively guide treatment recommendations.
Keywords: liver cancer; molecular therapy; immunotherapy.
Hepatocellular carcinoma (HCC) represents 90% of primary liver malignancies. It is the fifth most common malignancy in males and the ninth most common in females worldwide.1 In contrast to other major cancers (colon, breast, prostate), the incidence of and mortality from HCC has increased over the past decade, following a brief decline between 1999 and 2004.2 The epidemiology and incidence of HCC is closely linked to chronic liver disease and conditions predisposing to liver cirrhosis. Worldwide, hepatitis B virus infection is the leading cause of liver cirrhosis and, hence, HCC. In the United States, 50% of HCC cases are linked to hepatitis C virus (HCV) infection. Diabetes mellitus and alcoholic and nonalcoholic steatohepatitis are the other major etiologies of HCC. Indeed, the metabolic syndrome, independent of other factors, is associated with a 2-fold increase in the risk of HCC.3
Although most cases of HCC are predated by liver cirrhosis, in about 20% of patients HCC occurs without liver cirrhosis.4 Similar to other malignancies, surgery in the form of resection (for isolated lesions in the context of good liver function) or liver transplant (for low-volume disease with mildly impaired liver function) provides the best chance of a cure. Locoregional therapies involving hepatic artery–directed therapy are offered for patients with more advanced disease that is limited to the liver, while systemic therapy is offered for advanced unresectable disease that involves portal vein invasion, lymph nodes, and distant metastasis. The
Molecular Pathogenesis
Similar to other malignancies, a multistep process of carcinogenesis, with accumulation of genomic alterations at the molecular and cellular levels, is recognized in HCC. In about 80% of cases, repeated and chronic injury, inflammation, and repair lead to a distortion of normal liver architecture and development of cirrhotic nodules. Exome sequencing of HCC tissues has identified risk factor–specific mutational signatures, including those related to the tumor microenvironment, and defined the extensive landscape of altered genes and pathways in HCC (eg, angiogenic and MET pathways).7 In the Schulze et al study, the frequency of alterations that could be targeted by available Food and Drug Administration (FDA)–approved drugs comprised either amplifications or mutations of FLTs (6%), FGF3 or 4 or 19 (4%), PDGFRs (3%), VEGFA (1%), HGF (3%), MTOR (2%), EGFR (1%), FGFRs (1%), and MET (1%).7 Epigenetic modification of growth-factor expression, particularly insulin-like growth factor 2 and transforming growth factor alpha, and structural alterations that lead to loss of heterozygosity are early events that cause hepatocyte proliferation and progression of dysplastic nodules.8,9 Advances in whole-exome sequencing have identified TERT promoter mutations, leading to activation of telomerase, as an early event in HCC pathogenesis. Other commonly altered genes include CTNNB1 (B-Catenin) and TP53. As a group, alterations in the MAP kinase pathway genes occur in about 40% of HCC cases.
Actionable oncogenic driver alterations are not as common as tumor suppressor pathway alterations in HCC, making targeted drug development challenging.10,11 The FGFR (fibroblast growth factor receptor) pathway, which plays a critical role in carcinogenesis-related cell growth, survival, neo-angiogenesis, and acquired resistance to other cancer treatments, is being explored as a treatment target.12 The molecular characterization of HCC may help with identifying new biomarkers and present opportunities for developing therapeutic targets.
CASE PRESENTATION
A 61-year-old man with a history of chronic hepatitis C and hypertension presents to his primary care physician due to right upper quadrant pain. Laboratory evaluation shows transaminases elevated 2 times the upper limit of normal. This leads to an ultrasound and follow-up magnetic resonance imaging. Imaging shows diffuse cirrhotic changes, with a 6-cm, well-circumscribed lesion within the left lobe of the liver that shows rapid arterial enhancement with venous washout. These vascular characteristics are consistent with HCC. In addition, 2 satellite lesions in the left lobe and sonographic evidence of invasion into the portal vein are present. Periportal lymph nodes are pathologically enlarged.
The physical examination is unremarkable, except for mild tenderness over the right upper quadrant of the abdomen. Serum bilirubin, albumin, platelets, and international normalized ratio are normal, and alpha fetoprotein (AFP) is elevated at 1769 ng/mL. The patient’s family history is unremarkable for any major illness or cancer. Computed tomography scan of the chest and pelvis shows no evidence of other lesions. His liver disease is classified as Child–Pugh A. Due to locally advanced presentation, the tumor is deemed to be nontransplantable and unresectable, and is staged as BCLC-C. The patient continues to work and his performance status is ECOG (
What systemic treatment would you recommend for this patient with locally advanced unresectable HCC with nodal metastasis?
First-Line Therapeutic Options
Systemic treatment of HCC is challenging because of the underlying liver cirrhosis and hepatic dysfunction present in most patients. Overall prognosis is therefore dependent on the disease biology and burden and on the degree of hepatic dysfunction. These factors must be considered in patients with advanced disease who present for systemic therapy. As such, patients with BCLC class D HCC with poor performance status and impaired liver function are better off with best supportive care and hospice services (Figure). Table 1 and Table 2 outline the landmark trials that led to the approval of agents for advanced HCC treatment.

Sorafenib
In the patient with BCLC class C HCC who has preserved liver function (traditionally based on a Child–Pugh score of ≤ 6 and a decent functional status [ECOG performance status 1-2]), sorafenib is the first FDA-approved first-line treatment. Sorafenib is a small-molecule tyrosine kinase inhibitor that targets vascular endothelial growth factor receptor (VEGFR) kinase signaling, in addition to many other tyrosine kinase pathways (including the platelet-derived growth factor and Raf-Ras pathways). Evidence for the clinical benefit of sorafenib comes from the SHARP trial.13 This was a multinational, but primarily European, randomized phase 3 study that compared sorafenib to best supportive care for advanced HCC in patients with a Child–Pugh score ≤ 6A and a robust performance status (ECOG 0 and 1). Overall survival (OS) with placebo and sorafenib was 7.9 months and 10.7 months, respectively. There was no difference in time to radiologic progression, and the progression-free survival (PFS) at 4 months was 62% with sorafenib and 42% with placebo. Patients with HCV-associated HCC appeared to derive a more substantial benefit from sorafenib.14 In a smaller randomized study of sorafenib in Asian patients with predominantly hepatitis B–associated HCC, OS in the sorafenib and best supportive care arms was lower than that reported in the SHARP study (6.5 months vs 4.2 months), although OS still was longer in the sorafenib group.15

Significant adverse events reported with sorafenib include fatigue (30%), hand and foot syndrome (30%), diarrhea (15%), and mucositis (10%). Major proportions of patients in the clinical setting have not tolerated the standard dose of 400 mg twice daily. Dose-adjusted administration of sorafenib has been advocated in patients with more impaired liver function (Child–Pugh class 7B) and bilirubin of 1.5 to 3 times the upper limit of normal, although it is unclear whether these patients are deriving any benefit from sorafenib.16 At this time, in a patient with preserved liver function, starting with 400 mg twice daily, followed by dose reduction based on toxicity, remains standard.

Lenvatinib
After multiple attempts to develop newer first-line treatments for HCC,
Second-Line Therapeutic Options
Following the sorafenib approval, clinical studies of several other agents did not meet their primary endpoint and failed to show improvement in clinical outcomes compared to sorafenib. However, over the past years the treatment landscape for advanced HCC has been changed with the approval of several agents in the second line. The overall response rate (ORR) has become the new theme for management of advanced disease. With multiple therapeutic options available, optimal sequencing now plays a critical role, especially for transitioning from locoregional to systemic therapy. Five drugs are now indicated for second-line treatment of patients who progressed on or were intolerant to sorafenib: regorafenib, cabozantinib, ramucirumab, nivolumab, and pembrolizumab.
Regorafenib
Regorafenib was evaluated in the advanced HCC setting in a single-arm, phase 2 trial involving 36 patients with Child–Pugh class A liver disease who had progressed on prior sorafenib.18 Patients received regorafenib 160 mg orally once daily for 3 weeks on/1 week off cycles. Disease control was achieved in 72% of patients, with stable disease in 25 patients (69%). Based on these results, regorafenib was further evaluated in the multicenter, phase 3, 2:1 randomized, double-blind, placebo-controlled RESORCE study, which enrolled 573 patients.19 Due to the overlapping safety profiles of sorafenib and regorafenib, the inclusion criteria required patients to have tolerated a sorafenib dose of at least 400 mg daily for 20 of the past 28 days of treatment prior to enrollment. The primary endpoint of the study, OS, was met (median OS of 10.6 months in regorafenib arm versus 7.8 months in placebo arm; hazard ratio [HR], 0.63; P < 0.0001).
Cabozantinib
CELESTIAL was a phase 3, double-blind study that assessed the efficacy of cabozantinib versus placebo in patients with advanced HCC who had received prior sorafenib.22 In this study, 707 patients with Child–Pugh class A liver disease who progressed on at least 1 prior systemic therapy were randomized in a 2:1 ratio to treatment with cabozantinib at 60 mg daily or placebo. Patients treated with cabozantinib had a longer OS (10.2 months vs 8.0 months), resulting in a 24% reduction in the risk of death (HR, 0.76), and a longer median PFS (5.2 months versus 1.9 months). The disease control rate was higher with cabozantinib (64% vs 33%) as well. The incidence of high‐grade adverse events in the cabozantinib group was twice that of the placebo group. Common adverse events reported with cabozantinib included HFSR (17%), hypertension (16%), increased aspartate aminotransferase (12%), fatigue (10%), and diarrhea (10%).
Ramucirumab
REACH was a phase 3 study exploring the efficacy of ramucirumab that did not meet its primary endpoint; however, the subgroup analysis in AFP-high patients showed an OS improvement with ramucirumab.23 This led to the phase 3 REACH-2 trial, a multicenter, randomized, double-blind biomarker study in patients with advanced HCC who either progressed on or were intolerant to sorafenib and had an AFP level ≥ 400 ng/mL.24 Patients were randomized to ramucirumab 8 mg/kg every 2 weeks or placebo. The study met its primary endpoint, showing improved OS (8.5 months vs 7.3 months; P = 0.0059). The most common treatment-related adverse events in the ramucirumab group were ascites (5%), hypertension (12%), asthenia (5%), malignant neoplasm progression (6%), increased aspartate aminotransferase concentration (5%), and thrombocytopenia.
Immunotherapy
HCC is considered an inflammation-induced cancer, which renders immunotherapeutic strategies more appealing. The PD-L1/PD-1 pathway is the critical immune checkpoint mechanism and is an important target for treatment. HCC uses a complex, overlapping set of mechanisms to evade cancer-specific immunity and to suppress the immune system. Initial efforts to develop immunotherapies for HCC focused on anti-PD-1 and anti-PD-L1 antibodies. CheckMate 040 evaluated nivolumab in 262 sorafenib-naïve and -treated patients with advanced HCC (98% with Child–Pugh scores of 5 or 6), with a median follow-up of 12.9 months.25 In sorafenib-naïve patients (n = 80), the ORR was 23%, and the disease control rate was 63%. In sorafenib-treated patients (n = 182), the ORR was 18%. Response was not associated with PD-L1 expression. Durable objective responses, a manageable safety profile, and promising efficacy led the FDA to grant accelerated approval of nivolumab for the treatment of patients with HCC who have been previously treated with sorafenib. Based on this, the phase 3 randomized CheckMate-459 trial evaluated the efficacy of nivolumab versus sorafenib in the first-line. Median OS and ORR were better with nivolumab (16.4 months vs 14.7 months; HR 0.85; P = 0.752; and 15% [with 5 complete responses] vs 7%), as was the safety profile (22% vs 49% reporting grade 3 and 4 adverse events). 26
The KEYNOTE-224 study27 evaluated pembrolizumab in 104 patients with previously treated advanced HCC. This study showed an ORR of 17%, with 1 complete response and 17 partial responses. One-third of the patients had progressive disease, while 46 had stable disease. Among those who responded, 56% maintained a durable response for more than 1 year. Subsequently, in KEYNOTE 240, pembrolizumab showed an improvement in OS (13.9 months vs 10.6 months; HR, 0.78; P = 0.0238) and PFS (3.0 months versus 2.8 months; HR, 0.78; P = 0.0186) compared with placebo.28 The ORR for pembrolizumab was 16.9% (95% confidence interval [CI], 12.7%-21.8%) versus 2.2% (95% CI, 0.5%-6.4%; P = 0.00001) for placebo. Mean duration of response was 13.8 months.
In the IMbrave150 trial, atezolizumab/bevacizumab combination, compared to sorafenib, had better OS (not estimable vs 13.2 months; P = 0.0006), PFS (6.8 months vs 4.5 months, P < 0.0001), and ORR (33% vs 13%, P < 0.0001), but grade 3-4 events were similar.29 This combination has potential for first-line approval. The COSMIC–312 study is comparing the combination of cabozantinib and atezolizumab to sorafenib monotherapy and cabozantinib monotherapy in advanced HCC.
Resistance to immunotherapy can be extrinsic, associated with activation mechanisms of T-cells, or intrinsic, related to immune recognition, gene expression, and cell-signaling pathways.30 Tumor-immune heterogeneity and antigen presentation contribute to complex resistance mechanisms.31,32 Although clinical outcomes have improved with immune checkpoint inhibitors, the response rate is low and responses are inconsistent, likely due to an immunosuppressive tumor microenvironment.33 Therefore, several novel combinations of checkpoint inhibitors and targeted drugs are being evaluated to bypass some of the resistance mechanisms (Table 3).

Chemotherapy
Multiple combinations of cytotoxic regimens have been evaluated, but efficacy has been modest, suggesting the limited role for traditional chemotherapy in the systemic management of advanced HCC. Response rates to chemotherapy are low and responses are not durable. Gemcitabine- and doxorubicin-based treatment and FOLFOX (5-fluorouracil, leucovorin, oxaliplatin) are some regimens that have been studied, with a median OS of less than 1 year for these regimens.34-36 FOLFOX had a higher response rate (8.15% vs 2.67%; P = 0.02) and longer median OS (6.40 months versus 4.97 months; HR, 0.80; 95% CI, 0.63-1.02; P = 0.07) than doxorubicin.34 With the gemcitabine/oxaliplatin combination, ORR was 18%, with stable disease in 58% of patients, and median PFS and OS were 6.3 months and 11.5 months, respectively.35 In a study that compared doxorubicin and PIAF (cisplatin/interferon a-2b/doxorubicin/5-fluorouracil), median OS was 6.83 months and 8.67 months, respectively (P = 0.83). The hazard ratio for death from any cause in the PIAF group compared with the doxorubicin group was 0.97 (95% CI, 0.71-1.32). PIAF had a higher ORR (20.9%; 95% CI, 12.5%-29.2%) than doxorubicin (10.5%; 95% CI, 3.9%-16.9%).
The phase 3 ALLIANCE study evaluated the combination of sorafenib and doxorubicin in treatment-naïve HCC patients with Child–Pugh class A liver disease, and did not demonstrate superiority with the addition of cytotoxic chemotherapy.37 Indeed, the combination of chemotherapy with sorafenib appears harmful in terms of lower OS (9.3 months vs 10.6 months; HR, 1.06; 95% CI, 0.8-1.4) and worse toxicity. Patients treated with the combination experienced more hematologic (37.8% vs 8.1%) and nonhematologic adverse events (63.6% vs 61.5%).
Locoregional Therapy
The role of locoregional therapy in advanced HCC remains the subject of intense debate. Patients with BCLC stage C HCC with metastatic disease and those with lymph node involvement are candidates for systemic therapy. The optimal candidate for locoregional therapy is the patient with localized intermediate-stage disease, particularly hepatic artery–delivered therapeutic interventions. However, the presence of a solitary large tumor or portal vein involvement constitutes gray areas regarding which therapy to deliver directly to the tumor via the hepatic artery, and increasingly stereotactic body radiation therapy is being offered.
Transarterial Chemoembolization
Transarterial chemoembolization (TACE), with or without chemotherapy, is the most widely adopted locoregional therapy in the management of HCC. TACE exploits the differential vascular supply to the HCC and normal liver parenchyma. Normal liver receives only one-fourth of its blood supply from the hepatic artery (three-fourths from the portal vein), whereas HCC is mainly supplied by the hepatic artery. A survival benefit for TACE compared to best supportive care is widely acknowledged for intermediate-stage HCC, and transarterial embolization (TAE) with gelatin sponge or microspheres is noninferior to TACE.38,39 Overall safety profile and efficacy inform therapy selection in advanced HCC, although the evidence for TACE in advanced HCC is less robust. Although single-institution experiences suggest survival numbers similar to and even superior to sorafenib,40,41 there is a scarcity of large randomized clinical trial data to back this up. Based on this, patients with advanced HCC should only be offered liver-directed therapy within a clinical trial or on a case-by-case basis under multidisciplinary tumor board consensus.
A serious adverse effect of TACE is post-embolization syndrome, which occurs in about 30% of patients and may be associated with poor prognosis.42 The syndrome consists of right upper quadrant abdominal pain, malaise, and worsening nausea/vomiting following the embolization procedure. Laboratory abnormalities and other complications may persist for up to 30 days after the procedure. This is a concern, because post-embolization syndrome may affect the ability to deliver systemic therapy.
Transarterial Radioembolization
In the past few years, there has been an uptick in the utilization of transarterial radioembolization (TARE), which instead of delivering glass beads, as done in TAE, or chemotherapy-infused beads, as done in TACE, delivers the radioisotope Y-90 to the tumor via the hepatic artery. TARE is able to administer larger doses of radiation to the tumor while avoiding normal liver tissue, as compared to external-beam radiation. There has been no head-to-head comparison of these different intra-arterial therapy approaches, but TARE with Y-90 has been shown to be safe in patients with portal vein thrombosis. A recent multicenter retrospective study of TARE demonstrated a median OS of 8.8 to 10.8 months in patients with BCLC C HCC,43 and in a large randomized study of Y-90 compared to sorafenib in advanced and previously treated intermediate HCC, there was no difference in median OS between the treatment modalities (8 months for selective internal radiotherapy, 9 months for sorafenib; P = 0.18). Treatment with Y-90 was better tolerated.44 A major impediment to the adoption of TARE is the time it takes to order, plan, and deliver Y-90 to patients. Radio-embolization-induced liver disease, similar to post-embolization syndrome, is characterized by jaundice and ascites, which may occur 4 to 8 weeks postprocedure and is more common in patients with HCC who do not have cirrhosis. Compared to TACE, TARE may offer a better adverse effect profile, with improvement in quality of life.
Combination of Systemic and Locoregional Therapy
Even in carefully selected patients with intermediate- and advanced-stage HCC, locoregional therapy is not curative. Tumor embolization may promote more angiogenesis, and hence tumor progression, by causing hypoxia and upregulation of hypoxia-inducible factor.45 This upregulation of angiogenesis as a resistance mechanism to tumor embolization provides a rationale for combining systemic therapy (typically based on abrogating angiogenesis) with TACE/TAE. Most of the experience has been with sorafenib in intermediate-stage disease, and the results have been disappointing. The administration of sorafenib after at least a partial response with TACE did not provide additional benefit in terms of time to progression.46 Similarly, in the SPACE trial, concurrent therapy with TACE-doxorubicin-eluting beads and sorafenib compared to TACE-doxorubicin-eluting beads and placebo yielded similar time to progression numbers for both treatment modalities.47 While the data have been disappointing in intermediate-stage disease, as described earlier, registry data suggest that patients with advanced-stage disease may benefit from this approach.48
In the phase 2 TACTICS trial, 156 patients with unresectable HCC were randomized to receive TACE alone or sorafenib plus TACE, with a novel endpoint, time to untreatable progression (TTUP) and/or progression to TACE refractoriness.49 Treatment with sorafenib following TACE was continued until TTUP, decline in liver function to Child–Pugh class C, or the development of vascular invasion or extrahepatic spread. Development of new lesions while on sorafenib was not considered as progressive disease as long as the lesions were amenable to TACE. In this study, PFS was longer with sorafenib-TACE compared to TACE alone (26.7 months vs 20.6 months; P = 0.02). However, the TTUP endpoint needs further validation, and we are still awaiting the survival outcomes of this study. At this time, there are insufficient data to recommend the combination of liver-directed locoregional therapy and sorafenib or other systemic therapy options outside of a clinical trial setting.
Current Treatment Approach for Advanced HCC (BCLC-C)
Although progress is being made in the development of effective therapies, advanced HCC is generally incurable. These patients experience significant symptom burden throughout the course of the disease. Therefore, the optimal treatment plan must focus on improving or maintaining quality of life, in addition to overall efficacy. It is important to actively involve patients in treatment decisions for an individualized treatment plan, and to discuss the best strategy for incorporating current advances in targeted and immunotherapies. The paradigm of what constitutes first-line treatment for advanced HCC is shifting due to the recent systemic therapy approvals. Prior to the availability of these therapies, many patients with advanced HCC were treated with repeated locoregional therapies. For instance, TACE was often used to treat unresectable HCC multiple times beyond progression. There was no consensus on the definition of TACE failure, and hence it was used in broader, unselected populations. Retrospective studies suggest that continuing TACE after refractoriness or failure may not be beneficial, and may delay subsequent treatments because of deterioration of liver function or declines in performance status. With recent approvals of several systemic therapy options, including immunotherapy, it is vital to conduct a risk-benefit assessment prior to repeating TACE after failure, so that patients are not denied the use of available systemic therapeutic options due to declined performance status or organ function from these procedures. The optimal timing and the sequence of systemic and locoregional therapy must be carefully evaluated by a multidisciplinary team.
CASE CONCLUSION
An important part of evaluating a new patient with HCC is to determine whether they are a candidate for curative therapies, such as transplant or surgical resection. These are no longer an option for patients with intermediate disease. For patients with advanced disease characteristics, such as vascular invasion or systemic metastasis, the evidence supports using systemic therapy with sorafenib or lenvatinib. Lenvatinib, with a better tolerance profile and response rate, is the treatment of choice for the patient described in the case scenario. Lenvatinib is also indicated for first-line treatment of advanced HCC, and is useful in very aggressive tumors, such as those with an AFP level exceeding 200 ng/mL.
Future Directions
The emerging role of novel systemic therapeutics, including immunotherapy, has drastically changed the treatment landscape for hepatocellular cancers, with 6 new drugs for treating advanced hepatocellular cancers approved recently. While these systemic drugs have improved survival in advanced HCC in the past decade, patient selection and treatment sequencing remain a challenge, due to a lack of biomarkers capable of predicting antitumor responses. In addition, there is an unmet need for treatment options for patients with Child–Pugh class B7 and C liver disease and poor performance status.
The goal of future management should be to achieve personalized care aimed at improved safety and efficacy by targeting multiple cancer pathways in the HCC cascade with combination treatments. Randomized clinical trials to improve patient selection and determine the proper sequence of treatments are needed. Given the heterogeneity of HCC, molecular profiling of the tumor to differentiate responders from nonresponders may elucidate potential biomarkers to effectively guide treatment recommendations.
1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424.
2. Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485-1491.
3. Welzel TM, Graubard BI, Zeuzem S, et al. Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-Medicare database. Hepatology. 2011;54:463-471.
4. Schutte K, Schulz C, Poranzke J, et al. Characterization and prognosis of patients with hepatocellular carcinoma (HCC) in the non-cirrhotic liver. BMC Gastroenterol. 2014;14:117.
5. Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338.
6. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314.
7. Schulze K, Imbeaud S, Letouzé E, et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet. 2015;47:505-511.
8. Thorgeirsson SS, Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet. 2002;31:339-346.
9. Dhanasekaran R, Bandoh S, Roberts LR. Molecular pathogenesis of hepatocellular carcinoma and impact of therapeutic advances. F1000Res. 2016;5.
10. Schulze K, Imbeaud S, Letouze E, et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet. 2015;47:505-511.
11. Cancer Genome Atlas Research Network. Electronic address: [email protected]; Cancer Genome Atlas Research Network. Comprehensive and integrative genomic characterization of hepatocellular carcinoma. Cell. 2017;169:1327-1134.
12. Chae YK, Ranganath K, Hammerman PS, et al: Inhibition of the fibroblast growth factor receptor (FGFR) pathway: the current landscape and barriers to clinical application. Oncotarget. 2016;8:16052-16074.
13. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390.
14. Jackson R, Psarelli EE, Berhane S, et al. Impact of viral status on survival in patients receiving sorafenib for advanced hepatocellular cancer: a meta-analysis of randomized phase III trials. J Clin Oncol. 2017;35:622-628.
15. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34.
16. Da Fonseca LG, Barroso-Sousa R, Bento AD, et al. Safety and efficacy of sorafenib in patients with Child-Pugh B advanced hepatocellular carcinoma. Mol Clin Oncol. 2015;3:793-796.
17. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173.
18. Bruix J, Tak W-Y, Gasbarrini A, et al. Regorafenib as second-line therapy for intermediate or advanced hepatocellular carcinoma: Multicentre, open-label, phase II safety study. Eur J Cancer. 2013;49:3412-3419.
19. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56-66.
20. Bruix J, Merle P, Granito A, et al. Hand-foot skin reaction (HFSR) and overall survival (OS) in the phase 3 RESORCE trial of regorafenib for treatment of hepatocellular carcinoma (HCC) progressing on sorafenib. J Clin Oncol. 2018;36:412-412.
21. Finn RS, Merle P, Granito A, et al. Outcomes of sequential treatment with sorafenib followed by regorafenib for HCC: Additional analyses from the phase III RESORCE trial. J Hepatol. 2018;69:353-358.
22. Abou-Alfa GK, Meyer T, Cheng A-L, et al. Cabozantinib (C) versus placebo (P) in patients (pts) with advanced hepatocellular carcinoma (HCC) who have received prior sorafenib: Results from the randomized phase III CELESTIAL trial. J Clin Oncol. 2018;36:207-207.
23. Zhu AX, Park JO, Ryoo B-Y, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncology. 2015;16:859-870.
24. Zhu AX, Kang Y-K, Yen C-J, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased αfetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncology. 2019;20:282-296.
25. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492-2502.
26. Yau T, Park JW, Finn RS, et al. CheckMate 459: A randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Ann Oncol. 2020;30:v874-v875.
27. Zhu AX, Finn RS, Cattan S, et al. KEYNOTE-224: Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib. J Clin Oncol. 2018;36:942-952.
28. Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38:193-202.
29. Cheng A-L, Qin S, Ikeda M, et al. IMbrave150: efficacy and safety results from a ph III study evaluating atezolizumab (atezo) + bevacizumab (bev) vs sorafenib (sor) as first treatment (tx) for patients (pts) with unresectable hepatocellular carcinoma (HCC). Ann Oncol. 2019;30 (suppl_9):ix183-ix202.
30. Jiang Y, Han Q-J, Zhang J. Hepatocellular carcinoma: Mechanisms of progression and immunotherapy. World J Gastroenterol. 2019;25:3151-3167.
31. Xu F, Jin T, Zhu Y, et al. Immune checkpoint therapy in liver cancer. J Exp Clin Cancer Res. 2018;37:110.
32. Koyama S, Akbay EA, Li YY, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. 2016;7:10501.
33. Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2015;12:681-700.
34. Qin S, Bai Y, Lim HY, et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol. 2013;31:3501-3508.
35. Louafi S, Boige V, Ducreux M, et al. Gemcitabine plus oxaliplatin (GEMOX) in patients with advanced hepatocellular carcinoma (HCC). Cancer. 2007;109:1384-1390.
36. Tang A, Chan AT, Zee B, et al. A randomized phase iii study of doxorubicin versus cisplatin/interferon α-2b/doxorubicin/fluorouracil (PIAF) combination chemotherapy for unresectable hepatocellular carcinoma. J Natl Cancer Inst. 2005;97:1532-1538.
37. Abou-Alfa GK, Niedzwieski D, Knox JJ, et al. Phase III randomized study of sorafenib plus doxorubicin versus sorafenib in patients with advanced hepatocellular carcinoma (HCC): CALGB 80802 (Alliance). J Clin Oncol. 2016;34:192.
38. Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739.
39. Brown KT, Do RK, Gonen M, et al. randomized trial of hepatic artery embolization for hepatocellular carcinoma using doxorubicin-eluting microspheres compared with embolization with microspheres alone. J Clin Oncol. 2016;34:2046-2053.
40. Kirstein MM, Voigtlander T, Schweitzer N, et al. Transarterial chemoembolization versus sorafenib in patients with hepatocellular carcinoma and extrahepatic disease. United European Gastroenterol J. 2018;6:238-246.
41. Pinter M, Hucke F, Graziadei I, et al. Advanced-stage hepatocellular carcinoma: transarterial chemoembolization versus sorafenib. Radiology. 2012;263:590-599.
42. Mason MC, Massarweh NN, Salami A, et al. Post-embolization syndrome as an early predictor of overall survival after transarterial chemoembolization for hepatocellular carcinoma. HPB (Oxford). 2015;17:1137-1144.
43. Sangro B, Maini CL, Ettorre GM, et al. Radioembolisation in patients with hepatocellular carcinoma that have previously received liver-directed therapies. Eur J Nucl Med Mol Imaging. 2018;45:1721-1730.
44. Vilgrain V, Pereira H, Assenat E, et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2017;18:1624-1636.
45. Sergio A, Cristofori C, Cardin R, et al. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol. 2008;103:914-921.
46. Kudo M, Imanaka K, Chida N, et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer. 2011;47:2117-2127.
47. Lencioni R, Llovet JM, Han G, et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: The SPACE trial. J Hepatol. 2016;64:1090-1098.
48. Geschwind JF, Chapiro J. Sorafenib in combination with transarterial chemoembolization for the treatment of hepatocellular carcinoma. Clin Adv Hematol Oncol. 2016;14:585-587.
49. Kudo M, Ueshima K, Ikeda M, et al. Randomized, open label, multicenter, phase II trial comparing transarterial chemoembolization (TACE) plus sorafenib with TACE alone in patients with hepatocellular carcinoma (HCC): TACTICS trial. J Clin Oncol. 2018;36:206.
1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424.
2. Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485-1491.
3. Welzel TM, Graubard BI, Zeuzem S, et al. Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-Medicare database. Hepatology. 2011;54:463-471.
4. Schutte K, Schulz C, Poranzke J, et al. Characterization and prognosis of patients with hepatocellular carcinoma (HCC) in the non-cirrhotic liver. BMC Gastroenterol. 2014;14:117.
5. Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338.
6. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314.
7. Schulze K, Imbeaud S, Letouzé E, et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet. 2015;47:505-511.
8. Thorgeirsson SS, Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet. 2002;31:339-346.
9. Dhanasekaran R, Bandoh S, Roberts LR. Molecular pathogenesis of hepatocellular carcinoma and impact of therapeutic advances. F1000Res. 2016;5.
10. Schulze K, Imbeaud S, Letouze E, et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet. 2015;47:505-511.
11. Cancer Genome Atlas Research Network. Electronic address: [email protected]; Cancer Genome Atlas Research Network. Comprehensive and integrative genomic characterization of hepatocellular carcinoma. Cell. 2017;169:1327-1134.
12. Chae YK, Ranganath K, Hammerman PS, et al: Inhibition of the fibroblast growth factor receptor (FGFR) pathway: the current landscape and barriers to clinical application. Oncotarget. 2016;8:16052-16074.
13. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390.
14. Jackson R, Psarelli EE, Berhane S, et al. Impact of viral status on survival in patients receiving sorafenib for advanced hepatocellular cancer: a meta-analysis of randomized phase III trials. J Clin Oncol. 2017;35:622-628.
15. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34.
16. Da Fonseca LG, Barroso-Sousa R, Bento AD, et al. Safety and efficacy of sorafenib in patients with Child-Pugh B advanced hepatocellular carcinoma. Mol Clin Oncol. 2015;3:793-796.
17. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173.
18. Bruix J, Tak W-Y, Gasbarrini A, et al. Regorafenib as second-line therapy for intermediate or advanced hepatocellular carcinoma: Multicentre, open-label, phase II safety study. Eur J Cancer. 2013;49:3412-3419.
19. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56-66.
20. Bruix J, Merle P, Granito A, et al. Hand-foot skin reaction (HFSR) and overall survival (OS) in the phase 3 RESORCE trial of regorafenib for treatment of hepatocellular carcinoma (HCC) progressing on sorafenib. J Clin Oncol. 2018;36:412-412.
21. Finn RS, Merle P, Granito A, et al. Outcomes of sequential treatment with sorafenib followed by regorafenib for HCC: Additional analyses from the phase III RESORCE trial. J Hepatol. 2018;69:353-358.
22. Abou-Alfa GK, Meyer T, Cheng A-L, et al. Cabozantinib (C) versus placebo (P) in patients (pts) with advanced hepatocellular carcinoma (HCC) who have received prior sorafenib: Results from the randomized phase III CELESTIAL trial. J Clin Oncol. 2018;36:207-207.
23. Zhu AX, Park JO, Ryoo B-Y, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncology. 2015;16:859-870.
24. Zhu AX, Kang Y-K, Yen C-J, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased αfetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncology. 2019;20:282-296.
25. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492-2502.
26. Yau T, Park JW, Finn RS, et al. CheckMate 459: A randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Ann Oncol. 2020;30:v874-v875.
27. Zhu AX, Finn RS, Cattan S, et al. KEYNOTE-224: Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib. J Clin Oncol. 2018;36:942-952.
28. Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38:193-202.
29. Cheng A-L, Qin S, Ikeda M, et al. IMbrave150: efficacy and safety results from a ph III study evaluating atezolizumab (atezo) + bevacizumab (bev) vs sorafenib (sor) as first treatment (tx) for patients (pts) with unresectable hepatocellular carcinoma (HCC). Ann Oncol. 2019;30 (suppl_9):ix183-ix202.
30. Jiang Y, Han Q-J, Zhang J. Hepatocellular carcinoma: Mechanisms of progression and immunotherapy. World J Gastroenterol. 2019;25:3151-3167.
31. Xu F, Jin T, Zhu Y, et al. Immune checkpoint therapy in liver cancer. J Exp Clin Cancer Res. 2018;37:110.
32. Koyama S, Akbay EA, Li YY, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. 2016;7:10501.
33. Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2015;12:681-700.
34. Qin S, Bai Y, Lim HY, et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol. 2013;31:3501-3508.
35. Louafi S, Boige V, Ducreux M, et al. Gemcitabine plus oxaliplatin (GEMOX) in patients with advanced hepatocellular carcinoma (HCC). Cancer. 2007;109:1384-1390.
36. Tang A, Chan AT, Zee B, et al. A randomized phase iii study of doxorubicin versus cisplatin/interferon α-2b/doxorubicin/fluorouracil (PIAF) combination chemotherapy for unresectable hepatocellular carcinoma. J Natl Cancer Inst. 2005;97:1532-1538.
37. Abou-Alfa GK, Niedzwieski D, Knox JJ, et al. Phase III randomized study of sorafenib plus doxorubicin versus sorafenib in patients with advanced hepatocellular carcinoma (HCC): CALGB 80802 (Alliance). J Clin Oncol. 2016;34:192.
38. Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739.
39. Brown KT, Do RK, Gonen M, et al. randomized trial of hepatic artery embolization for hepatocellular carcinoma using doxorubicin-eluting microspheres compared with embolization with microspheres alone. J Clin Oncol. 2016;34:2046-2053.
40. Kirstein MM, Voigtlander T, Schweitzer N, et al. Transarterial chemoembolization versus sorafenib in patients with hepatocellular carcinoma and extrahepatic disease. United European Gastroenterol J. 2018;6:238-246.
41. Pinter M, Hucke F, Graziadei I, et al. Advanced-stage hepatocellular carcinoma: transarterial chemoembolization versus sorafenib. Radiology. 2012;263:590-599.
42. Mason MC, Massarweh NN, Salami A, et al. Post-embolization syndrome as an early predictor of overall survival after transarterial chemoembolization for hepatocellular carcinoma. HPB (Oxford). 2015;17:1137-1144.
43. Sangro B, Maini CL, Ettorre GM, et al. Radioembolisation in patients with hepatocellular carcinoma that have previously received liver-directed therapies. Eur J Nucl Med Mol Imaging. 2018;45:1721-1730.
44. Vilgrain V, Pereira H, Assenat E, et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2017;18:1624-1636.
45. Sergio A, Cristofori C, Cardin R, et al. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol. 2008;103:914-921.
46. Kudo M, Imanaka K, Chida N, et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer. 2011;47:2117-2127.
47. Lencioni R, Llovet JM, Han G, et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: The SPACE trial. J Hepatol. 2016;64:1090-1098.
48. Geschwind JF, Chapiro J. Sorafenib in combination with transarterial chemoembolization for the treatment of hepatocellular carcinoma. Clin Adv Hematol Oncol. 2016;14:585-587.
49. Kudo M, Ueshima K, Ikeda M, et al. Randomized, open label, multicenter, phase II trial comparing transarterial chemoembolization (TACE) plus sorafenib with TACE alone in patients with hepatocellular carcinoma (HCC): TACTICS trial. J Clin Oncol. 2018;36:206.
Chlamydia trachomatis infections
CASE Pregnant woman with symptoms of genital infection
A 23-year-old primigravid woman at 15 weeks and 2 days’ gestation reported having a 2-week history of increased urinary frequency and vaginal discharge. She said she experienced similar symptoms 6 weeks previously that resolved within a week. The patient has had 3 sexual partners in the past year. Her current partner was experiencing a yellow urethral discharge and dysuria. On the patient’s speculum examination, the clinician noted a yellow-green discharge emanating from the cervix as well as cervical motion tenderness.
What is the most likely diagnosis, and how would you treat this patient?
The culprit was chlamydia
Chlamydia trachomatis is an obligate intracellular bacterium that does not stain with Gram staining. A rigid cell wall encloses its intracellular component. C trachomatis infection begins when the chlamydial elementary body enters a susceptible host cell.
Once ingested, the organism’s surface antigens (major outer membrane protein and lipopolysaccharide antigens) provide intracellular sanctuary for the bacterium by inhibiting phagolysosomal fusion. Subsequently, the elementary body morphs into a reticular body, which replicates through adenosine triphosphate (ATP)–dependent binary fission. After approximately 48 hours of replication, the organism again morphs into an elementary body and is released to infect additional cells and acquire new ATP stores for further replication.
Chlamydia can be transmitted horizontally during oral, vaginal, or anal intercourse or vertically to the infant during vaginal delivery.
The US’s most common notifiable disease
According to the Centers for Disease Control and Prevention (CDC), the incidence of chlamydia infection in the United States increased considerably in recent years: from 976,455 cases in 2005 to 1,758,668 cases in 2018.1 In 2018, rates of chlamydia infection in women were nearly double the rates in men, with an incidence of 688.2 versus 377.5 per 100,000 cases, and a prevalence of 1,150,672 versus 612,020.1
Young adults have a higher frequency of chlamydia infection than any other age group. From 2017 to 2018, reported cases in women aged 15–19 years increased by 1.3%, to 3,306.8 per 100,000; in women aged 20–24 years, cases increased by 0.8%, to 4,064.6 per 100,000. In young men in the same age ranges, reported cases increased by 3.7%, to 959.0 cases per 100,000, and by 3.3%, to 1,784.5 per 100,000 cases, respectively.1
Both the incidence and prevalence of chlamydia infection are higher in African Americans than in whites, while Asians have the lowest rates.1 The prevalence of infection also is increased with incarceration, lower socioeconomic status, and residence in the southern United States.
The prevalence of chlamydia infection in pregnant women is approximately 2% to 3%, but it may be as high as 30% in high-risk populations, such as women who are unmarried, have multiple sex partners, are coinfected with another sexually transmitted disease (STD), have partners with nongonococcal urethritis, have mucopurulent discharge, have acute urethral syndrome, and have late or no prenatal care.2 Since chlamydia infection often is asymptomatic and some infections resolve spontaneously, the true prevalence of infection probably is underreported.
Continue to: Chlamydia infection can cause serious clinical manifestations...
Chlamydia infection can cause serious clinical manifestations
The 15 serotypes of C trachomatis are grouped into 3 categories according to clinical manifestations:
- Serotypes A, B, Ba, and C cause endemic trachoma, characterized by bilateral irritation of the eyelids that progresses to eyelid thickening and scarring, eventually leading to corneal abrasion and blindness.
- Serotypes D–K manifest as conjunctivitis and pneumonia in newborns, proctitis in men (especially in men who have sex with men), and genitourinary infections in women. Reactive arthritis and inclusion conjunctivitis also can occur with D–K serotypes.
- Serotypes L1–L3 cause lymphogranuloma venereum.
About 70% of women with chlamydia infection are asymptomatic. Those who have symptoms often present with endocervicitis or acute urethral syndrome (acute urethritis). Manifestations of these 2 conditions include a frothy yellow-green vaginal and/or urethral discharge, dysuria, and frequency. Women who engage in rectal intercourse also may notice a purulent discharge from the anus. Untreated, C trachomatis organisms may ascend the reproductive tract, causing both endometritis and pelvic inflammatory disease (PID).
While a single episode of PID increases tubal infertility risk by 10%, a second episode increases the risk by 40%.3 Over time, recurrent and/or chronic PID causes scarring and adhesion formation, which may result in chronic pelvic pain. In addition, chronic infection is the single most important risk factor for ectopic pregnancy. Finally, chlamydia infection is a risk factor for Fitz-Hugh-Cutis syndrome (perihepatitis). In this condition, organisms ascend from the site of pelvic infection along the pericolic gutter to ultimately infect the liver capsule.
Specific complications in pregnancy
Chlamydia infection in pregnant women is associated with preterm delivery and preterm premature rupture of membranes. Infants born to mothers with untreated chlamydia infection are at risk for pneumonia, conjunctivitis, and even perinatal death.2 Acquisition of infection occurs at the time of delivery rather than in the antepartum period.
The significant morbidity associated with chlamydia infection underscores the importance of regular screening, especially in pregnant women. The current United States Preventive Service Task Force guidelines recommend annual screening of all sexually active women who are 24 years of age or younger, as well as of older, high-risk women.
The CDC recommends routine screening of all pregnant women for chlamydia at the first prenatal visit. Repeat screening is recommended in the third trimester for all pregnant women younger than 25 years, those at increased risk, and those infected within the past 3 to 6 months or during the first trimester. Those who test positive should be retested 3 weeks after completion of treatment.1
Chlamydia screening strategies
Historically, a chlamydia diagnosis was made by isolating the organisms in tissue culture. In the 1990s, however, that extremely time-consuming and resource-intensive procedure was replaced by nucleic acid amplification testing (NAAT).
NAAT methodology. NAAT is the gold standard for diagnosing C trachomatis infection; this methodology utilizes various assays, including polymerase chain reaction, ligase chain reaction, and transcription-mediated amplification.
Continue to: Compared with previous culture and antigen detection techniques...
Compared with previous culture and antigen detection techniques, NAAT’s advantages include excellent sensitivity and specificity (>90% and ≥99%, respectively), enabling detection of a low inoculum of organisms in a sample obtained by noninvasive methods, such as first-void urine collection or vaginal swab.2,4,5 Furthermore, NAAT does not impose any specific storage regulations on collected specimens, is cost effective, and can jointly test for Neisseria gonorrhoeae, which commonly co-infects with C trachomatis.6
Screening in pregnancy. In 2012, Blatt and colleagues examined testing patterns in nearly 1.3 million obstetric patients and found that only 59% (761,315) of women were tested for chlamydia at least once in pregnancy.7 Only 1 in 3 women were tested during the first prenatal visit, as CDC guidelines recommend. Testing rates declined with increasing age. Of women screened, 3.5% tested positive for chlamydia.7 Of these, 3 of 4 were retested at least once, with almost 20% having at least 1 subsequent positive result.7
Of note, in a study of women who reported receptive anal intercourse (n = 2,818), 292 women tested positive for chlamydia; 10.4% tested positive in genital-only sites, 58.6% in genital and rectal sites, and 20.5% at the rectal site only.8
It is alarming that only 59% of pregnant women are screened for chlamydia given the significant perinatal complications associated with this infection. Barriers to screening pregnant women may include clinician discomfort in discussing STDs and patient refusal of screening. Furthermore, clinicians should routinely ask women about receptive anal sex. Women who report this risk factor should be tested for chlamydia in both the endocervix and rectum.
Retesting and follow-up. After the initial diagnosis of chlamydia, a test of cure 3 weeks after treatment is an important aspect of care. Thus, identifying and overcoming barriers to retesting is important. Clinicians should educate patients about the importance of follow-up. Also consider incorporating the use of home-based, self-obtained vaginal swabs for retesting. Results from 2 randomized trials showed that eliminating a patient’s transportation barriers and providing a home-based alternative to a follow-up visit significantly increased rescreening rates by 33% in STD clinic patients and by 59.2% in family planning clinic patients.9
Reinfection risk. The rate of venereal chlamydia transmission in heterosexual partners is 70%. Since sexually active chlamydia-positive patients are at risk for reinfection by their partner after treatment completion, clinicians should refer the sex partners for evaluation. If the sex partners are reluctant to have testing, it is reasonable to provide empiric antibiotic treatment to decrease the risk of re-infection in the patient.7 Before doing so, however, make certain that state law permits this practice, and be sure to document the prescribed treatment in the patient’s record.
Treatment options
Prompt treatment of C trachomatis infection is essential to decrease the risk of disease sequelae. Nonpregnant adults can be treated with oral doxycycline 100 mg twice daily for 7 days.
In a head-to-head study performed in a controlled environment that ensured treatment adherence, 97% efficacy was achieved with one oral dose of azithromycin (1 g) compared with 100% efficacy with doxycycline.10 However, in the real-world setting, imperfect adherence to the multi-day doxycycline regimen is associated with treatment failures. Thus, a single dose of azithromycin is preferable for patients with questionable compliance.11
In obstetric patients, azithromycin and amoxicillin are preferred as first-line agents for treatment of C trachomatis due to their improved safety profile in this demographic. Amoxicillin 500 mg orally 3 times daily for 7 days has 95% efficacy.2
Women allergic to these agents may be treated with an alternative regimen of erythromycin base, 500 mg orally 4 times daily for 7 days, or erythromycin ethylsuccinate, 800 mg orally 4 times daily for 7 days. Erythromycin should be reserved for second-line therapy because of its lower efficacy (64%) and frequent gastrointestinal adverse effects.2 Doxycycline is contraindicated in pregnancy because of possible teratogenic effects on the teeth and bone of the fetus.
- Centers for Disease Control and Prevention Division of STD Prevention. Sexually transmitted disease surveillance 2018. October 2019. https://www.cdc.gov/std/stats18/default.htm. 2019. Accessed January 4, 2020.
- Duff P. Maternal and fetal infections. In: Creasy RK, Resnik R, Iams JD, et al, eds. Creasy and Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Philadelphia, PA: Elsevier Saunders; 2019:869.
- Ljubin-Sternak S, Meštrović T. Chlamydia trachomatis and genital mycoplasmas: pathogens with an impact on human reproductive health. J Pathog. 2014. doi: 10.1155/2014/183167.
- Meyer T. Diagnostic procedures to detect Chlamydia trachomatis infections. Microorganisms. 2016:4(3).
- Centers for Disease Control and Prevention.. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae. MMWR Recomm Rep. 2014;63:1-19.
- Wiesenfeld HC. Screening for Chlamydia trachomatis infections in women. N Engl J Med. 2017;376:765-773.
- Blatt AJ, Lieberman JM, Hoover DR, et al. Chlamydial and gonococcal testing during pregnancy in the United States. Am J Obstet Gynecol. 2012;207:55.e1-8.
- Llata E, Braxton J, Asbel L, et al. Rectal Chlamydia trachomatis and Neisseria gonorrhoeae infections among women reporting anal intercourse. Obstet Gynecol. 2018;132:692-697.
- Xu F, Stoner BP, Taylor SN, et al. Use of home-obtained vaginal swabs to facilitate rescreening for Chlamydia trachomatis infections: two randomized controlled trials. Obstet Gynecol. 2011;118(2 pt 1):231-239.
- Geisler WM, Uniyal A, Lee JY, et al. Azithromycin versus doxycycline for urogenital Chlamydia trachomatis infection. N Engl J Med. 2015;373:2512-2521.
- Quinn TC, Gaydos CA. Treatment for chlamydia infection—doxycycline versus azithromycin. N Engl J Med. 2015;373:2573-2575.
CASE Pregnant woman with symptoms of genital infection
A 23-year-old primigravid woman at 15 weeks and 2 days’ gestation reported having a 2-week history of increased urinary frequency and vaginal discharge. She said she experienced similar symptoms 6 weeks previously that resolved within a week. The patient has had 3 sexual partners in the past year. Her current partner was experiencing a yellow urethral discharge and dysuria. On the patient’s speculum examination, the clinician noted a yellow-green discharge emanating from the cervix as well as cervical motion tenderness.
What is the most likely diagnosis, and how would you treat this patient?
The culprit was chlamydia
Chlamydia trachomatis is an obligate intracellular bacterium that does not stain with Gram staining. A rigid cell wall encloses its intracellular component. C trachomatis infection begins when the chlamydial elementary body enters a susceptible host cell.
Once ingested, the organism’s surface antigens (major outer membrane protein and lipopolysaccharide antigens) provide intracellular sanctuary for the bacterium by inhibiting phagolysosomal fusion. Subsequently, the elementary body morphs into a reticular body, which replicates through adenosine triphosphate (ATP)–dependent binary fission. After approximately 48 hours of replication, the organism again morphs into an elementary body and is released to infect additional cells and acquire new ATP stores for further replication.
Chlamydia can be transmitted horizontally during oral, vaginal, or anal intercourse or vertically to the infant during vaginal delivery.
The US’s most common notifiable disease
According to the Centers for Disease Control and Prevention (CDC), the incidence of chlamydia infection in the United States increased considerably in recent years: from 976,455 cases in 2005 to 1,758,668 cases in 2018.1 In 2018, rates of chlamydia infection in women were nearly double the rates in men, with an incidence of 688.2 versus 377.5 per 100,000 cases, and a prevalence of 1,150,672 versus 612,020.1
Young adults have a higher frequency of chlamydia infection than any other age group. From 2017 to 2018, reported cases in women aged 15–19 years increased by 1.3%, to 3,306.8 per 100,000; in women aged 20–24 years, cases increased by 0.8%, to 4,064.6 per 100,000. In young men in the same age ranges, reported cases increased by 3.7%, to 959.0 cases per 100,000, and by 3.3%, to 1,784.5 per 100,000 cases, respectively.1
Both the incidence and prevalence of chlamydia infection are higher in African Americans than in whites, while Asians have the lowest rates.1 The prevalence of infection also is increased with incarceration, lower socioeconomic status, and residence in the southern United States.
The prevalence of chlamydia infection in pregnant women is approximately 2% to 3%, but it may be as high as 30% in high-risk populations, such as women who are unmarried, have multiple sex partners, are coinfected with another sexually transmitted disease (STD), have partners with nongonococcal urethritis, have mucopurulent discharge, have acute urethral syndrome, and have late or no prenatal care.2 Since chlamydia infection often is asymptomatic and some infections resolve spontaneously, the true prevalence of infection probably is underreported.
Continue to: Chlamydia infection can cause serious clinical manifestations...
Chlamydia infection can cause serious clinical manifestations
The 15 serotypes of C trachomatis are grouped into 3 categories according to clinical manifestations:
- Serotypes A, B, Ba, and C cause endemic trachoma, characterized by bilateral irritation of the eyelids that progresses to eyelid thickening and scarring, eventually leading to corneal abrasion and blindness.
- Serotypes D–K manifest as conjunctivitis and pneumonia in newborns, proctitis in men (especially in men who have sex with men), and genitourinary infections in women. Reactive arthritis and inclusion conjunctivitis also can occur with D–K serotypes.
- Serotypes L1–L3 cause lymphogranuloma venereum.
About 70% of women with chlamydia infection are asymptomatic. Those who have symptoms often present with endocervicitis or acute urethral syndrome (acute urethritis). Manifestations of these 2 conditions include a frothy yellow-green vaginal and/or urethral discharge, dysuria, and frequency. Women who engage in rectal intercourse also may notice a purulent discharge from the anus. Untreated, C trachomatis organisms may ascend the reproductive tract, causing both endometritis and pelvic inflammatory disease (PID).
While a single episode of PID increases tubal infertility risk by 10%, a second episode increases the risk by 40%.3 Over time, recurrent and/or chronic PID causes scarring and adhesion formation, which may result in chronic pelvic pain. In addition, chronic infection is the single most important risk factor for ectopic pregnancy. Finally, chlamydia infection is a risk factor for Fitz-Hugh-Cutis syndrome (perihepatitis). In this condition, organisms ascend from the site of pelvic infection along the pericolic gutter to ultimately infect the liver capsule.
Specific complications in pregnancy
Chlamydia infection in pregnant women is associated with preterm delivery and preterm premature rupture of membranes. Infants born to mothers with untreated chlamydia infection are at risk for pneumonia, conjunctivitis, and even perinatal death.2 Acquisition of infection occurs at the time of delivery rather than in the antepartum period.
The significant morbidity associated with chlamydia infection underscores the importance of regular screening, especially in pregnant women. The current United States Preventive Service Task Force guidelines recommend annual screening of all sexually active women who are 24 years of age or younger, as well as of older, high-risk women.
The CDC recommends routine screening of all pregnant women for chlamydia at the first prenatal visit. Repeat screening is recommended in the third trimester for all pregnant women younger than 25 years, those at increased risk, and those infected within the past 3 to 6 months or during the first trimester. Those who test positive should be retested 3 weeks after completion of treatment.1
Chlamydia screening strategies
Historically, a chlamydia diagnosis was made by isolating the organisms in tissue culture. In the 1990s, however, that extremely time-consuming and resource-intensive procedure was replaced by nucleic acid amplification testing (NAAT).
NAAT methodology. NAAT is the gold standard for diagnosing C trachomatis infection; this methodology utilizes various assays, including polymerase chain reaction, ligase chain reaction, and transcription-mediated amplification.
Continue to: Compared with previous culture and antigen detection techniques...
Compared with previous culture and antigen detection techniques, NAAT’s advantages include excellent sensitivity and specificity (>90% and ≥99%, respectively), enabling detection of a low inoculum of organisms in a sample obtained by noninvasive methods, such as first-void urine collection or vaginal swab.2,4,5 Furthermore, NAAT does not impose any specific storage regulations on collected specimens, is cost effective, and can jointly test for Neisseria gonorrhoeae, which commonly co-infects with C trachomatis.6
Screening in pregnancy. In 2012, Blatt and colleagues examined testing patterns in nearly 1.3 million obstetric patients and found that only 59% (761,315) of women were tested for chlamydia at least once in pregnancy.7 Only 1 in 3 women were tested during the first prenatal visit, as CDC guidelines recommend. Testing rates declined with increasing age. Of women screened, 3.5% tested positive for chlamydia.7 Of these, 3 of 4 were retested at least once, with almost 20% having at least 1 subsequent positive result.7
Of note, in a study of women who reported receptive anal intercourse (n = 2,818), 292 women tested positive for chlamydia; 10.4% tested positive in genital-only sites, 58.6% in genital and rectal sites, and 20.5% at the rectal site only.8
It is alarming that only 59% of pregnant women are screened for chlamydia given the significant perinatal complications associated with this infection. Barriers to screening pregnant women may include clinician discomfort in discussing STDs and patient refusal of screening. Furthermore, clinicians should routinely ask women about receptive anal sex. Women who report this risk factor should be tested for chlamydia in both the endocervix and rectum.
Retesting and follow-up. After the initial diagnosis of chlamydia, a test of cure 3 weeks after treatment is an important aspect of care. Thus, identifying and overcoming barriers to retesting is important. Clinicians should educate patients about the importance of follow-up. Also consider incorporating the use of home-based, self-obtained vaginal swabs for retesting. Results from 2 randomized trials showed that eliminating a patient’s transportation barriers and providing a home-based alternative to a follow-up visit significantly increased rescreening rates by 33% in STD clinic patients and by 59.2% in family planning clinic patients.9
Reinfection risk. The rate of venereal chlamydia transmission in heterosexual partners is 70%. Since sexually active chlamydia-positive patients are at risk for reinfection by their partner after treatment completion, clinicians should refer the sex partners for evaluation. If the sex partners are reluctant to have testing, it is reasonable to provide empiric antibiotic treatment to decrease the risk of re-infection in the patient.7 Before doing so, however, make certain that state law permits this practice, and be sure to document the prescribed treatment in the patient’s record.
Treatment options
Prompt treatment of C trachomatis infection is essential to decrease the risk of disease sequelae. Nonpregnant adults can be treated with oral doxycycline 100 mg twice daily for 7 days.
In a head-to-head study performed in a controlled environment that ensured treatment adherence, 97% efficacy was achieved with one oral dose of azithromycin (1 g) compared with 100% efficacy with doxycycline.10 However, in the real-world setting, imperfect adherence to the multi-day doxycycline regimen is associated with treatment failures. Thus, a single dose of azithromycin is preferable for patients with questionable compliance.11
In obstetric patients, azithromycin and amoxicillin are preferred as first-line agents for treatment of C trachomatis due to their improved safety profile in this demographic. Amoxicillin 500 mg orally 3 times daily for 7 days has 95% efficacy.2
Women allergic to these agents may be treated with an alternative regimen of erythromycin base, 500 mg orally 4 times daily for 7 days, or erythromycin ethylsuccinate, 800 mg orally 4 times daily for 7 days. Erythromycin should be reserved for second-line therapy because of its lower efficacy (64%) and frequent gastrointestinal adverse effects.2 Doxycycline is contraindicated in pregnancy because of possible teratogenic effects on the teeth and bone of the fetus.
CASE Pregnant woman with symptoms of genital infection
A 23-year-old primigravid woman at 15 weeks and 2 days’ gestation reported having a 2-week history of increased urinary frequency and vaginal discharge. She said she experienced similar symptoms 6 weeks previously that resolved within a week. The patient has had 3 sexual partners in the past year. Her current partner was experiencing a yellow urethral discharge and dysuria. On the patient’s speculum examination, the clinician noted a yellow-green discharge emanating from the cervix as well as cervical motion tenderness.
What is the most likely diagnosis, and how would you treat this patient?
The culprit was chlamydia
Chlamydia trachomatis is an obligate intracellular bacterium that does not stain with Gram staining. A rigid cell wall encloses its intracellular component. C trachomatis infection begins when the chlamydial elementary body enters a susceptible host cell.
Once ingested, the organism’s surface antigens (major outer membrane protein and lipopolysaccharide antigens) provide intracellular sanctuary for the bacterium by inhibiting phagolysosomal fusion. Subsequently, the elementary body morphs into a reticular body, which replicates through adenosine triphosphate (ATP)–dependent binary fission. After approximately 48 hours of replication, the organism again morphs into an elementary body and is released to infect additional cells and acquire new ATP stores for further replication.
Chlamydia can be transmitted horizontally during oral, vaginal, or anal intercourse or vertically to the infant during vaginal delivery.
The US’s most common notifiable disease
According to the Centers for Disease Control and Prevention (CDC), the incidence of chlamydia infection in the United States increased considerably in recent years: from 976,455 cases in 2005 to 1,758,668 cases in 2018.1 In 2018, rates of chlamydia infection in women were nearly double the rates in men, with an incidence of 688.2 versus 377.5 per 100,000 cases, and a prevalence of 1,150,672 versus 612,020.1
Young adults have a higher frequency of chlamydia infection than any other age group. From 2017 to 2018, reported cases in women aged 15–19 years increased by 1.3%, to 3,306.8 per 100,000; in women aged 20–24 years, cases increased by 0.8%, to 4,064.6 per 100,000. In young men in the same age ranges, reported cases increased by 3.7%, to 959.0 cases per 100,000, and by 3.3%, to 1,784.5 per 100,000 cases, respectively.1
Both the incidence and prevalence of chlamydia infection are higher in African Americans than in whites, while Asians have the lowest rates.1 The prevalence of infection also is increased with incarceration, lower socioeconomic status, and residence in the southern United States.
The prevalence of chlamydia infection in pregnant women is approximately 2% to 3%, but it may be as high as 30% in high-risk populations, such as women who are unmarried, have multiple sex partners, are coinfected with another sexually transmitted disease (STD), have partners with nongonococcal urethritis, have mucopurulent discharge, have acute urethral syndrome, and have late or no prenatal care.2 Since chlamydia infection often is asymptomatic and some infections resolve spontaneously, the true prevalence of infection probably is underreported.
Continue to: Chlamydia infection can cause serious clinical manifestations...
Chlamydia infection can cause serious clinical manifestations
The 15 serotypes of C trachomatis are grouped into 3 categories according to clinical manifestations:
- Serotypes A, B, Ba, and C cause endemic trachoma, characterized by bilateral irritation of the eyelids that progresses to eyelid thickening and scarring, eventually leading to corneal abrasion and blindness.
- Serotypes D–K manifest as conjunctivitis and pneumonia in newborns, proctitis in men (especially in men who have sex with men), and genitourinary infections in women. Reactive arthritis and inclusion conjunctivitis also can occur with D–K serotypes.
- Serotypes L1–L3 cause lymphogranuloma venereum.
About 70% of women with chlamydia infection are asymptomatic. Those who have symptoms often present with endocervicitis or acute urethral syndrome (acute urethritis). Manifestations of these 2 conditions include a frothy yellow-green vaginal and/or urethral discharge, dysuria, and frequency. Women who engage in rectal intercourse also may notice a purulent discharge from the anus. Untreated, C trachomatis organisms may ascend the reproductive tract, causing both endometritis and pelvic inflammatory disease (PID).
While a single episode of PID increases tubal infertility risk by 10%, a second episode increases the risk by 40%.3 Over time, recurrent and/or chronic PID causes scarring and adhesion formation, which may result in chronic pelvic pain. In addition, chronic infection is the single most important risk factor for ectopic pregnancy. Finally, chlamydia infection is a risk factor for Fitz-Hugh-Cutis syndrome (perihepatitis). In this condition, organisms ascend from the site of pelvic infection along the pericolic gutter to ultimately infect the liver capsule.
Specific complications in pregnancy
Chlamydia infection in pregnant women is associated with preterm delivery and preterm premature rupture of membranes. Infants born to mothers with untreated chlamydia infection are at risk for pneumonia, conjunctivitis, and even perinatal death.2 Acquisition of infection occurs at the time of delivery rather than in the antepartum period.
The significant morbidity associated with chlamydia infection underscores the importance of regular screening, especially in pregnant women. The current United States Preventive Service Task Force guidelines recommend annual screening of all sexually active women who are 24 years of age or younger, as well as of older, high-risk women.
The CDC recommends routine screening of all pregnant women for chlamydia at the first prenatal visit. Repeat screening is recommended in the third trimester for all pregnant women younger than 25 years, those at increased risk, and those infected within the past 3 to 6 months or during the first trimester. Those who test positive should be retested 3 weeks after completion of treatment.1
Chlamydia screening strategies
Historically, a chlamydia diagnosis was made by isolating the organisms in tissue culture. In the 1990s, however, that extremely time-consuming and resource-intensive procedure was replaced by nucleic acid amplification testing (NAAT).
NAAT methodology. NAAT is the gold standard for diagnosing C trachomatis infection; this methodology utilizes various assays, including polymerase chain reaction, ligase chain reaction, and transcription-mediated amplification.
Continue to: Compared with previous culture and antigen detection techniques...
Compared with previous culture and antigen detection techniques, NAAT’s advantages include excellent sensitivity and specificity (>90% and ≥99%, respectively), enabling detection of a low inoculum of organisms in a sample obtained by noninvasive methods, such as first-void urine collection or vaginal swab.2,4,5 Furthermore, NAAT does not impose any specific storage regulations on collected specimens, is cost effective, and can jointly test for Neisseria gonorrhoeae, which commonly co-infects with C trachomatis.6
Screening in pregnancy. In 2012, Blatt and colleagues examined testing patterns in nearly 1.3 million obstetric patients and found that only 59% (761,315) of women were tested for chlamydia at least once in pregnancy.7 Only 1 in 3 women were tested during the first prenatal visit, as CDC guidelines recommend. Testing rates declined with increasing age. Of women screened, 3.5% tested positive for chlamydia.7 Of these, 3 of 4 were retested at least once, with almost 20% having at least 1 subsequent positive result.7
Of note, in a study of women who reported receptive anal intercourse (n = 2,818), 292 women tested positive for chlamydia; 10.4% tested positive in genital-only sites, 58.6% in genital and rectal sites, and 20.5% at the rectal site only.8
It is alarming that only 59% of pregnant women are screened for chlamydia given the significant perinatal complications associated with this infection. Barriers to screening pregnant women may include clinician discomfort in discussing STDs and patient refusal of screening. Furthermore, clinicians should routinely ask women about receptive anal sex. Women who report this risk factor should be tested for chlamydia in both the endocervix and rectum.
Retesting and follow-up. After the initial diagnosis of chlamydia, a test of cure 3 weeks after treatment is an important aspect of care. Thus, identifying and overcoming barriers to retesting is important. Clinicians should educate patients about the importance of follow-up. Also consider incorporating the use of home-based, self-obtained vaginal swabs for retesting. Results from 2 randomized trials showed that eliminating a patient’s transportation barriers and providing a home-based alternative to a follow-up visit significantly increased rescreening rates by 33% in STD clinic patients and by 59.2% in family planning clinic patients.9
Reinfection risk. The rate of venereal chlamydia transmission in heterosexual partners is 70%. Since sexually active chlamydia-positive patients are at risk for reinfection by their partner after treatment completion, clinicians should refer the sex partners for evaluation. If the sex partners are reluctant to have testing, it is reasonable to provide empiric antibiotic treatment to decrease the risk of re-infection in the patient.7 Before doing so, however, make certain that state law permits this practice, and be sure to document the prescribed treatment in the patient’s record.
Treatment options
Prompt treatment of C trachomatis infection is essential to decrease the risk of disease sequelae. Nonpregnant adults can be treated with oral doxycycline 100 mg twice daily for 7 days.
In a head-to-head study performed in a controlled environment that ensured treatment adherence, 97% efficacy was achieved with one oral dose of azithromycin (1 g) compared with 100% efficacy with doxycycline.10 However, in the real-world setting, imperfect adherence to the multi-day doxycycline regimen is associated with treatment failures. Thus, a single dose of azithromycin is preferable for patients with questionable compliance.11
In obstetric patients, azithromycin and amoxicillin are preferred as first-line agents for treatment of C trachomatis due to their improved safety profile in this demographic. Amoxicillin 500 mg orally 3 times daily for 7 days has 95% efficacy.2
Women allergic to these agents may be treated with an alternative regimen of erythromycin base, 500 mg orally 4 times daily for 7 days, or erythromycin ethylsuccinate, 800 mg orally 4 times daily for 7 days. Erythromycin should be reserved for second-line therapy because of its lower efficacy (64%) and frequent gastrointestinal adverse effects.2 Doxycycline is contraindicated in pregnancy because of possible teratogenic effects on the teeth and bone of the fetus.
- Centers for Disease Control and Prevention Division of STD Prevention. Sexually transmitted disease surveillance 2018. October 2019. https://www.cdc.gov/std/stats18/default.htm. 2019. Accessed January 4, 2020.
- Duff P. Maternal and fetal infections. In: Creasy RK, Resnik R, Iams JD, et al, eds. Creasy and Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Philadelphia, PA: Elsevier Saunders; 2019:869.
- Ljubin-Sternak S, Meštrović T. Chlamydia trachomatis and genital mycoplasmas: pathogens with an impact on human reproductive health. J Pathog. 2014. doi: 10.1155/2014/183167.
- Meyer T. Diagnostic procedures to detect Chlamydia trachomatis infections. Microorganisms. 2016:4(3).
- Centers for Disease Control and Prevention.. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae. MMWR Recomm Rep. 2014;63:1-19.
- Wiesenfeld HC. Screening for Chlamydia trachomatis infections in women. N Engl J Med. 2017;376:765-773.
- Blatt AJ, Lieberman JM, Hoover DR, et al. Chlamydial and gonococcal testing during pregnancy in the United States. Am J Obstet Gynecol. 2012;207:55.e1-8.
- Llata E, Braxton J, Asbel L, et al. Rectal Chlamydia trachomatis and Neisseria gonorrhoeae infections among women reporting anal intercourse. Obstet Gynecol. 2018;132:692-697.
- Xu F, Stoner BP, Taylor SN, et al. Use of home-obtained vaginal swabs to facilitate rescreening for Chlamydia trachomatis infections: two randomized controlled trials. Obstet Gynecol. 2011;118(2 pt 1):231-239.
- Geisler WM, Uniyal A, Lee JY, et al. Azithromycin versus doxycycline for urogenital Chlamydia trachomatis infection. N Engl J Med. 2015;373:2512-2521.
- Quinn TC, Gaydos CA. Treatment for chlamydia infection—doxycycline versus azithromycin. N Engl J Med. 2015;373:2573-2575.
- Centers for Disease Control and Prevention Division of STD Prevention. Sexually transmitted disease surveillance 2018. October 2019. https://www.cdc.gov/std/stats18/default.htm. 2019. Accessed January 4, 2020.
- Duff P. Maternal and fetal infections. In: Creasy RK, Resnik R, Iams JD, et al, eds. Creasy and Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Philadelphia, PA: Elsevier Saunders; 2019:869.
- Ljubin-Sternak S, Meštrović T. Chlamydia trachomatis and genital mycoplasmas: pathogens with an impact on human reproductive health. J Pathog. 2014. doi: 10.1155/2014/183167.
- Meyer T. Diagnostic procedures to detect Chlamydia trachomatis infections. Microorganisms. 2016:4(3).
- Centers for Disease Control and Prevention.. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae. MMWR Recomm Rep. 2014;63:1-19.
- Wiesenfeld HC. Screening for Chlamydia trachomatis infections in women. N Engl J Med. 2017;376:765-773.
- Blatt AJ, Lieberman JM, Hoover DR, et al. Chlamydial and gonococcal testing during pregnancy in the United States. Am J Obstet Gynecol. 2012;207:55.e1-8.
- Llata E, Braxton J, Asbel L, et al. Rectal Chlamydia trachomatis and Neisseria gonorrhoeae infections among women reporting anal intercourse. Obstet Gynecol. 2018;132:692-697.
- Xu F, Stoner BP, Taylor SN, et al. Use of home-obtained vaginal swabs to facilitate rescreening for Chlamydia trachomatis infections: two randomized controlled trials. Obstet Gynecol. 2011;118(2 pt 1):231-239.
- Geisler WM, Uniyal A, Lee JY, et al. Azithromycin versus doxycycline for urogenital Chlamydia trachomatis infection. N Engl J Med. 2015;373:2512-2521.
- Quinn TC, Gaydos CA. Treatment for chlamydia infection—doxycycline versus azithromycin. N Engl J Med. 2015;373:2573-2575.
COVID-19 during pregnancy: How would you proceed in this case of a novel and ominous emerging pathogen?
CASE Pregnant patient with fever who has travel history to Italy
A 28-year-old primigravid woman at 12 weeks’ gestation just returned from a 2-week vacation in Italy. She requests medical evaluation because of malaise; fever; chills; rhinorrhea; mild dyspnea; a dry, nonproductive cough; and diarrhea. On physical examination, her temperature is 38.6° C (101.5° F), pulse 104 bpm, respirations 22/minute, and blood pressure 100/70 mm Hg. Auscultation of the lungs demonstrates scattered rales, rhonchi, and expiratory wheezes in both posterior lung fields. The fetal heart rate is 168 bpm. What are the most likely diagnoses? What diagnostic tests are indicated? And what clinical treatment is indicated?
In the presented case scenario, the patient’s symptoms are consistent with a viral influenza. Her recent travel history certainly makes coronavirus disease 2019 (COVID-19) the most likely diagnosis.
COVID-19, caused by a novel new coronavirus, has evolved with lightning speed since it was first identified in early December 2019.1 The disease originated in Wuhan, China. Its epicenter is now in Europe, and over 100 countries and regions have reported cases. New cases in the United States are being identified daily, and there is no clear end to the outbreak. Several areas of the United States have been particularly hard hit by this disease: Seattle, New Orleans, and New York City.
COVID-19 has provoked widespread unsettledness in many populations and an extraordinary response from public health officials, large corporations, professional organizations, and financial markets. We are learning more about somewhat unfamiliar public health concepts such as quarantine, containment, mitigation, reproduction number (R), and “flattening the curve.” Disneyland and Walt Disney World are now temporarily closed. Professional and collegiate sports organizations have cancelled or suspended games and tournaments. Scientific and trade association meetings have been postponed or cancelled. Broadway, Carnegie Hall, and the Metropolitan Museum of Art have now “turned out the lights.” The Centers for Disease Control and Prevention has recommended that everyone avoid gatherings that include more than 10 other persons.
This article will review the evolving epidemiology of COVID-19, describe the usual clinical manifestations of the disease, highlight the key diagnostic tests, and present guidelines for treatment. It will review the limited information currently available about the impact of COVID-19 in pregnant women. The review will conclude by describing measures that individuals can employ to prevent acquisition or transmission of infection and then by highlighting key “unanswered questions” about this new and ominous pathogen (TABLE).
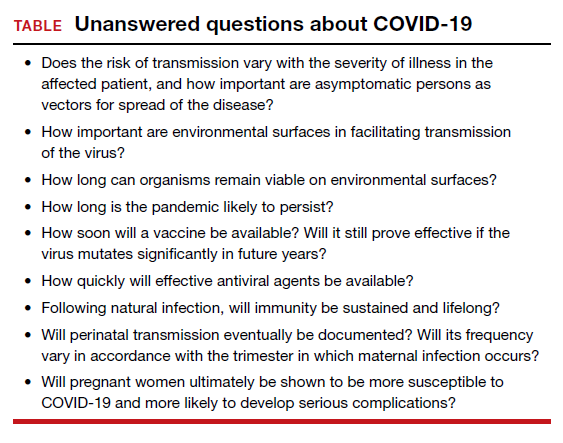
Continue to: What we know about epidemiology...
What we know about epidemiology
COVID-19 is caused by a novel new coronavirus that shares some genetic overlap with the viruses that caused Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS).2 The first case of COVID-19 was reported on December 1, 2019, from Wuhan, China.1 Within a very short period of time the disease has spread throughout the world, and on March 11, 2020, the World Health Organization (WHO) declared the infection to be a true pandemic. The countries with the highest prevalence of COVID-19 include China, South Korea, Iran, Italy, France, Spain, and the United States. However, more than 100 other countries and regions have reported cases. As of the first week of April, approximately 1 million persons in the world have been diagnosed with COVID-19. Of those infected, slightly more than 50,000 deaths have occurred. At the time of this writing, 234,483 cases have been documented in the United States, and current estimates indicate that approximately 7% of the population in the country could become infected.1,3,4
The virus responsible for COVID-19 is a single-stranded, enveloped RNA virus. Like its counterparts that caused SARS and MERS, this virus originates in animals, primarily bats. The early cases seem to have resulted from patient contact with exotic animals displayed in the Huanan Seafood Wholesale Market.1
The virus is transmitted directly by respiratory droplets and by close surface-to-hand contact with infected respiratory secretions. The virus appears to remain viable on environmental surfaces for 1 to 3 days, although the degree of infectivity over time is not well delineated. With direct exposure to respiratory droplets, the infectivity is relatively high; approximately 2 to 3 individuals become infected as the result of contact with an infected patient. By contrast, the “reproduction number (R)” for influenza is closer to 1.2,5
Certain persons appear to be at increased risk for developing infection and becoming seriously ill2,6:
- persons older than age 60
- persons with underlying medical illness
- persons who are immunosuppressed.
The reported range in the case fatality rate (CFR) varies from 1% to 13%, with the higher rates concentrated in older patients with comorbidities.3 These initial reports of high CFRs may be misleading because in the initial phases of this pandemic many patients with mild or no symptoms were not tested, and, thus, the overall prevalence of infection is not clear. By way of comparison, the CRF for influenza A and B is about 0.1%.2
Of note, the number of reported cases in the pediatric population is low, and the outcomes in these individuals are much better than in the older population.2,3,6 At present, there are only two reports of COVID-19 in pregnancy; these two studies include 18 women and 19 infants.7,8 The frequency of preterm delivery was 50% in these reports. Sixteen of the 18 patients were delivered by cesarean delivery; at least 6 of these procedures were performed for a non-reassuring fetal heart rate tracing. No maternal deaths were identified, and no cases of vertical transmission occurred.
We must remember that the number of patients described in these two reports is very small. Although the initial reports are favorable, in other influenza epidemics, pregnant women have not fared so well and have experienced disproportionately higher rates of morbidity and mortality.2
Reported clinical manifestations
The incubation period of COVID-19 ranges from 2 to 14 days; the median is 5.2 days. Many patients with proven COVID-19 infection are asymptomatic. When clinical findings are present, they usually are relatively mild and include low-grade fever, myalgias, arthralgias, sore throat, mild dyspnea, and a dry nonproductive cough. Some patients also may experience diarrhea. Of course, these findings are also consistent with influenza A or B or atypical pneumonia. One key to differentiation is the patient’s history of recent travel to an area of high COVID-19 prevalence or contact with a person who has been in one of these areas and who is clinically ill.2,3,9,10
In some patients, notably those who are older than 65 years of age and/or who have underlying medical illnesses, the respiratory manifestations are more prominent.6 These patients may develop severe dyspnea, pneumonia, adult respiratory distress syndrome (ARDS), multiorgan failure, and septic shock. Interestingly, the more severe manifestations tend to occur during the second week of the illness. In this group of more severely ill patients requiring hospitalization, 17% to 29% develop ARDS, and 23% to 32% require admission to the intensive care unit.2,6
Pregnant patients who become severely ill may be at risk for spontaneous miscarriage and preterm labor. With profound maternal hypoxia, fetal heart rate abnormalities may become apparent. To date, no clearly proven cases of vertical transmission of infection to the newborn have been identified. However, as noted above, current reports only include 18 pregnancies and 19 infants.2,3,7,8,11
Continue to: Diagnostic testing...
Diagnostic testing
Infected patients may have a decreased peripheral white blood cell count, with a specific decrease in the number of lymphocytes. Thrombocytopenia may be present, as well as an elevation in the hepatic transaminase enzymes (ALT, AST).2
X-ray, chest CT, and RT-PCR. The three most important diagnostic tests are chest x-ray, chest computed tomography (CT) scan, and real-time PCR (RT-PCR) or nucleic acid amplification test (NAAT).2,6 Specimens for RT-PCR or NAAT should be obtained from the oropharynx and nasopharynx using a synthetic-tipped applicator with an aluminum shaft. Patients who are intubated should have specimens obtained by broncho-alveolar lavage. The virus also has been recovered from blood and stool, but not yet from urine, amniotic fluid, placenta, cord blood, or breast milk.2
CT and chest x-ray show characteristic ground-glass opacities in both lung fields, combined with multiple areas of consolidation. Chest imaging is particularly helpful when the patient has all the major clinical manifestations, but the initial RT-PCR or NAAT is negative.
Treatment
Fortunately, most infected persons can be treated as outpatients. Because this condition may be confused with influenza A or B, initial treatment with a drug such as oseltamivir 75 mg orally twice daily for five days is very reasonable.9 Supportive therapy is critically important in this clinical setting. Acetaminophen, up to 3,000 mg/d in divided doses, or ibuprofen, up to 2,400 mg/d in divided doses, can be used to reduce fever and relieve myalgias and arthralgias. The latter drug, of course, should not be used in pregnant women. The patient should be encouraged to rest and to stay well hydrated. Loperamide can be used to treat diarrhea, 4 mg orally initially, then 2 mg orally after each loose stool up to a maximum of 16 mg/d. Pregnant patients should be cautioned to watch for signs of preterm labor.9,12 Patients should remain in relative isolation at home until they are free of signs of illness and they test negative for COVID-19.
For patients who are more severely ill at initial evaluation or who deteriorate while undergoing outpatient management, hospitalization is indicated.2,6 Patients should be placed in rooms that provide protection against aerosolized infection. They should receive supplemental oxygen and be observed closely for signs of superimposed bacterial infection. Depending upon the suspected bacterial pathogen, appropriate antibiotics may include ceftriaxone, which targets Streptococcus pneumoniae, Hemophilus influenzae, and Moraxella catarrhalis; azithromycin, which targets mycoplasmas; and vancomycin, which specifically covers Staphylococcus aureus. Health care workers should wear appropriate personal protective equipment when interacting with these patients, including cap, N95 mask, face shield, gloves, gown, and shoe covers. If a woman with COVID-19 has delivered, and the pediatrician permits rooming in, the isolette should be positioned at least 6 feet away from the mother. The mother should use a mechanical breast pump to obtain milk and then have another family member feed the baby until the mother tests negative for the virus. The breast pump needs to be cleaned meticulously after each use. The number of visitors to the mother’s room should be strictly limited.3,9
At the present time, there is no specific antiviral drug approved by the US Food and Drug Administration for treatment of COVID-19. The National Institutes of Health is currently conducting a trial of remdesivir for affected patients.13 The drug is also available from the manufacturer outside of this trial on a “compassionate use” basis. Another treatment regimen receiving extensive publicity is the combination of azithromycin and hydroxychloroquine. Its effectiveness has not been confirmed in a properly designed randomized trial.
Prevention hinges on commonsense precautions
Although vaccine trials are underway, public health authorities estimate that a vaccine will not be commercially available for at least 12 to 18 months. Therefore, independent of “community/organizational” mitigation programs, individuals should observe the following commonsense precautions to minimize their risk of contracting or transmitting COVID-192,3,5,14:
- Eliminate any nonessential travel, particularly by plane or cruise ship.
- Avoid events that draw large crowds, such as concerts, theater performances, movies, and even religious services.
- When out in public, try to maintain a distance of 6 feet from others
- Remain at home if you feel ill, particularly if you have respiratory symptoms.
- Cough or sneeze into your sleeve rather than your bare hand.
- Avoid handshakes.
- Wash your hands frequently in warm soapy water for at least 20 seconds, particularly after touching environmental surfaces such as counter tops and handrails.
- If you use hand sanitizers, they should have an alcohol content of at least 60%.
- Clean environmental surfaces frequently with a dilute bleach solution.
CASE Resolved
The clinical manifestations displayed by this patient are consistent with viral influenza. The recent travel history to one of the European epicenters makes COVID-19 the most likely diagnosis. The patient should have a chest CT scan and a RT-PCR or NAAT to confirm the diagnosis. If the diagnosis is confirmed, she and her close contacts should be self-quarantined at home for 14 days. She should receive appropriate supportive care with anti-pyretics, analgesics, and anti-diarrhea agents. If she develops signs of serious respiratory compromise, she should be admitted to an isolation room in the hospital for intensive respiratory therapy and close observation for superimposed bacterial pneumonia.

- Holshue ML, DeBolt C, Lindquist S, et al; Washington State 2019-nCoV Case Investigation Team. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929-936.
- Rasmussen SA, Smulian JC, Lednicky JA, et al. Coronavirus disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am J Obstet Gynecol. February 24, 2020. doi: 10.1016/j.ajog.2020.02.017.
- Rasmussen SA, Jamieson DJ. Coronavirus disease 2019 (COVID-19) and pregnancy: responding to a rapidly evolving situation [in press]. Obstet Gynecol. 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019: Cases in US. CDC website. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed March 18, 2020.
- Wang H, Wang Z, Dong Y, et al. Phase-adjusted estimation of the number of Coronavirus Disease 2019 cases in Wuhan, China. Cell Discov. 2020;6:10.
- Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727-733.
- Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395;809-815.
- Lei D, Wang C, Li C, et al. Clinical characteristics of pregnancy with the 2019 novel coronavirus disease (COVID-19) infection. Chin J Perinat Med. 2020:23.
- Dotters-Katz S, Hughes BL. Coronavirus (COVID-19) and pregnancy: what maternal-fetal medicine subspecialists need to know. Society for Maternal-Fetal Medicine. March 17, 2020. https://s3.amazonaws.com/cdn.smfm.org/media/2267/COVID19-_updated_3-17-20_PDF.pdf. Accessed March 17, 2020.
- Perlman S. Another decade, another coronavirus. N Engl J Med. 2020;382:760-762.
- Yang H, Wang C, Poon LC. Novel coronavirus infection and pregnancy. Ultrasound Obstet Gynecol. March 5, 2020. doi:10.1002/uog.22006.
- American College of Obstetricians and Gynecologists. Practice Advisory: novel coronavirus 2019 (COVID-19). March 13, 2020. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Practice-Advisory-Novel-Coronavirus2019?IsMobileSet=false. Accessed March 17, 2020.
- National Institutes of Health. NIH clinical trial of remdesivir to treat COVID-19 begins. February 25, 2020. https://www.nih.gov/news-events/news-releases/nih-clinical-trial-remdesivir-treat-covid-19-begins. Accessed March 17, 2020.
- Munster VJ, Koopmans M, Van Doremalen N, et al. A novel coronavirus emerging in China – key questions for impact assessment. N Engl J Med. 2020;382:692-694.
CASE Pregnant patient with fever who has travel history to Italy
A 28-year-old primigravid woman at 12 weeks’ gestation just returned from a 2-week vacation in Italy. She requests medical evaluation because of malaise; fever; chills; rhinorrhea; mild dyspnea; a dry, nonproductive cough; and diarrhea. On physical examination, her temperature is 38.6° C (101.5° F), pulse 104 bpm, respirations 22/minute, and blood pressure 100/70 mm Hg. Auscultation of the lungs demonstrates scattered rales, rhonchi, and expiratory wheezes in both posterior lung fields. The fetal heart rate is 168 bpm. What are the most likely diagnoses? What diagnostic tests are indicated? And what clinical treatment is indicated?
In the presented case scenario, the patient’s symptoms are consistent with a viral influenza. Her recent travel history certainly makes coronavirus disease 2019 (COVID-19) the most likely diagnosis.
COVID-19, caused by a novel new coronavirus, has evolved with lightning speed since it was first identified in early December 2019.1 The disease originated in Wuhan, China. Its epicenter is now in Europe, and over 100 countries and regions have reported cases. New cases in the United States are being identified daily, and there is no clear end to the outbreak. Several areas of the United States have been particularly hard hit by this disease: Seattle, New Orleans, and New York City.
COVID-19 has provoked widespread unsettledness in many populations and an extraordinary response from public health officials, large corporations, professional organizations, and financial markets. We are learning more about somewhat unfamiliar public health concepts such as quarantine, containment, mitigation, reproduction number (R), and “flattening the curve.” Disneyland and Walt Disney World are now temporarily closed. Professional and collegiate sports organizations have cancelled or suspended games and tournaments. Scientific and trade association meetings have been postponed or cancelled. Broadway, Carnegie Hall, and the Metropolitan Museum of Art have now “turned out the lights.” The Centers for Disease Control and Prevention has recommended that everyone avoid gatherings that include more than 10 other persons.
This article will review the evolving epidemiology of COVID-19, describe the usual clinical manifestations of the disease, highlight the key diagnostic tests, and present guidelines for treatment. It will review the limited information currently available about the impact of COVID-19 in pregnant women. The review will conclude by describing measures that individuals can employ to prevent acquisition or transmission of infection and then by highlighting key “unanswered questions” about this new and ominous pathogen (TABLE).

Continue to: What we know about epidemiology...
What we know about epidemiology
COVID-19 is caused by a novel new coronavirus that shares some genetic overlap with the viruses that caused Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS).2 The first case of COVID-19 was reported on December 1, 2019, from Wuhan, China.1 Within a very short period of time the disease has spread throughout the world, and on March 11, 2020, the World Health Organization (WHO) declared the infection to be a true pandemic. The countries with the highest prevalence of COVID-19 include China, South Korea, Iran, Italy, France, Spain, and the United States. However, more than 100 other countries and regions have reported cases. As of the first week of April, approximately 1 million persons in the world have been diagnosed with COVID-19. Of those infected, slightly more than 50,000 deaths have occurred. At the time of this writing, 234,483 cases have been documented in the United States, and current estimates indicate that approximately 7% of the population in the country could become infected.1,3,4
The virus responsible for COVID-19 is a single-stranded, enveloped RNA virus. Like its counterparts that caused SARS and MERS, this virus originates in animals, primarily bats. The early cases seem to have resulted from patient contact with exotic animals displayed in the Huanan Seafood Wholesale Market.1
The virus is transmitted directly by respiratory droplets and by close surface-to-hand contact with infected respiratory secretions. The virus appears to remain viable on environmental surfaces for 1 to 3 days, although the degree of infectivity over time is not well delineated. With direct exposure to respiratory droplets, the infectivity is relatively high; approximately 2 to 3 individuals become infected as the result of contact with an infected patient. By contrast, the “reproduction number (R)” for influenza is closer to 1.2,5
Certain persons appear to be at increased risk for developing infection and becoming seriously ill2,6:
- persons older than age 60
- persons with underlying medical illness
- persons who are immunosuppressed.
The reported range in the case fatality rate (CFR) varies from 1% to 13%, with the higher rates concentrated in older patients with comorbidities.3 These initial reports of high CFRs may be misleading because in the initial phases of this pandemic many patients with mild or no symptoms were not tested, and, thus, the overall prevalence of infection is not clear. By way of comparison, the CRF for influenza A and B is about 0.1%.2
Of note, the number of reported cases in the pediatric population is low, and the outcomes in these individuals are much better than in the older population.2,3,6 At present, there are only two reports of COVID-19 in pregnancy; these two studies include 18 women and 19 infants.7,8 The frequency of preterm delivery was 50% in these reports. Sixteen of the 18 patients were delivered by cesarean delivery; at least 6 of these procedures were performed for a non-reassuring fetal heart rate tracing. No maternal deaths were identified, and no cases of vertical transmission occurred.
We must remember that the number of patients described in these two reports is very small. Although the initial reports are favorable, in other influenza epidemics, pregnant women have not fared so well and have experienced disproportionately higher rates of morbidity and mortality.2
Reported clinical manifestations
The incubation period of COVID-19 ranges from 2 to 14 days; the median is 5.2 days. Many patients with proven COVID-19 infection are asymptomatic. When clinical findings are present, they usually are relatively mild and include low-grade fever, myalgias, arthralgias, sore throat, mild dyspnea, and a dry nonproductive cough. Some patients also may experience diarrhea. Of course, these findings are also consistent with influenza A or B or atypical pneumonia. One key to differentiation is the patient’s history of recent travel to an area of high COVID-19 prevalence or contact with a person who has been in one of these areas and who is clinically ill.2,3,9,10
In some patients, notably those who are older than 65 years of age and/or who have underlying medical illnesses, the respiratory manifestations are more prominent.6 These patients may develop severe dyspnea, pneumonia, adult respiratory distress syndrome (ARDS), multiorgan failure, and septic shock. Interestingly, the more severe manifestations tend to occur during the second week of the illness. In this group of more severely ill patients requiring hospitalization, 17% to 29% develop ARDS, and 23% to 32% require admission to the intensive care unit.2,6
Pregnant patients who become severely ill may be at risk for spontaneous miscarriage and preterm labor. With profound maternal hypoxia, fetal heart rate abnormalities may become apparent. To date, no clearly proven cases of vertical transmission of infection to the newborn have been identified. However, as noted above, current reports only include 18 pregnancies and 19 infants.2,3,7,8,11
Continue to: Diagnostic testing...
Diagnostic testing
Infected patients may have a decreased peripheral white blood cell count, with a specific decrease in the number of lymphocytes. Thrombocytopenia may be present, as well as an elevation in the hepatic transaminase enzymes (ALT, AST).2
X-ray, chest CT, and RT-PCR. The three most important diagnostic tests are chest x-ray, chest computed tomography (CT) scan, and real-time PCR (RT-PCR) or nucleic acid amplification test (NAAT).2,6 Specimens for RT-PCR or NAAT should be obtained from the oropharynx and nasopharynx using a synthetic-tipped applicator with an aluminum shaft. Patients who are intubated should have specimens obtained by broncho-alveolar lavage. The virus also has been recovered from blood and stool, but not yet from urine, amniotic fluid, placenta, cord blood, or breast milk.2
CT and chest x-ray show characteristic ground-glass opacities in both lung fields, combined with multiple areas of consolidation. Chest imaging is particularly helpful when the patient has all the major clinical manifestations, but the initial RT-PCR or NAAT is negative.
Treatment
Fortunately, most infected persons can be treated as outpatients. Because this condition may be confused with influenza A or B, initial treatment with a drug such as oseltamivir 75 mg orally twice daily for five days is very reasonable.9 Supportive therapy is critically important in this clinical setting. Acetaminophen, up to 3,000 mg/d in divided doses, or ibuprofen, up to 2,400 mg/d in divided doses, can be used to reduce fever and relieve myalgias and arthralgias. The latter drug, of course, should not be used in pregnant women. The patient should be encouraged to rest and to stay well hydrated. Loperamide can be used to treat diarrhea, 4 mg orally initially, then 2 mg orally after each loose stool up to a maximum of 16 mg/d. Pregnant patients should be cautioned to watch for signs of preterm labor.9,12 Patients should remain in relative isolation at home until they are free of signs of illness and they test negative for COVID-19.
For patients who are more severely ill at initial evaluation or who deteriorate while undergoing outpatient management, hospitalization is indicated.2,6 Patients should be placed in rooms that provide protection against aerosolized infection. They should receive supplemental oxygen and be observed closely for signs of superimposed bacterial infection. Depending upon the suspected bacterial pathogen, appropriate antibiotics may include ceftriaxone, which targets Streptococcus pneumoniae, Hemophilus influenzae, and Moraxella catarrhalis; azithromycin, which targets mycoplasmas; and vancomycin, which specifically covers Staphylococcus aureus. Health care workers should wear appropriate personal protective equipment when interacting with these patients, including cap, N95 mask, face shield, gloves, gown, and shoe covers. If a woman with COVID-19 has delivered, and the pediatrician permits rooming in, the isolette should be positioned at least 6 feet away from the mother. The mother should use a mechanical breast pump to obtain milk and then have another family member feed the baby until the mother tests negative for the virus. The breast pump needs to be cleaned meticulously after each use. The number of visitors to the mother’s room should be strictly limited.3,9
At the present time, there is no specific antiviral drug approved by the US Food and Drug Administration for treatment of COVID-19. The National Institutes of Health is currently conducting a trial of remdesivir for affected patients.13 The drug is also available from the manufacturer outside of this trial on a “compassionate use” basis. Another treatment regimen receiving extensive publicity is the combination of azithromycin and hydroxychloroquine. Its effectiveness has not been confirmed in a properly designed randomized trial.
Prevention hinges on commonsense precautions
Although vaccine trials are underway, public health authorities estimate that a vaccine will not be commercially available for at least 12 to 18 months. Therefore, independent of “community/organizational” mitigation programs, individuals should observe the following commonsense precautions to minimize their risk of contracting or transmitting COVID-192,3,5,14:
- Eliminate any nonessential travel, particularly by plane or cruise ship.
- Avoid events that draw large crowds, such as concerts, theater performances, movies, and even religious services.
- When out in public, try to maintain a distance of 6 feet from others
- Remain at home if you feel ill, particularly if you have respiratory symptoms.
- Cough or sneeze into your sleeve rather than your bare hand.
- Avoid handshakes.
- Wash your hands frequently in warm soapy water for at least 20 seconds, particularly after touching environmental surfaces such as counter tops and handrails.
- If you use hand sanitizers, they should have an alcohol content of at least 60%.
- Clean environmental surfaces frequently with a dilute bleach solution.
CASE Resolved
The clinical manifestations displayed by this patient are consistent with viral influenza. The recent travel history to one of the European epicenters makes COVID-19 the most likely diagnosis. The patient should have a chest CT scan and a RT-PCR or NAAT to confirm the diagnosis. If the diagnosis is confirmed, she and her close contacts should be self-quarantined at home for 14 days. She should receive appropriate supportive care with anti-pyretics, analgesics, and anti-diarrhea agents. If she develops signs of serious respiratory compromise, she should be admitted to an isolation room in the hospital for intensive respiratory therapy and close observation for superimposed bacterial pneumonia.

CASE Pregnant patient with fever who has travel history to Italy
A 28-year-old primigravid woman at 12 weeks’ gestation just returned from a 2-week vacation in Italy. She requests medical evaluation because of malaise; fever; chills; rhinorrhea; mild dyspnea; a dry, nonproductive cough; and diarrhea. On physical examination, her temperature is 38.6° C (101.5° F), pulse 104 bpm, respirations 22/minute, and blood pressure 100/70 mm Hg. Auscultation of the lungs demonstrates scattered rales, rhonchi, and expiratory wheezes in both posterior lung fields. The fetal heart rate is 168 bpm. What are the most likely diagnoses? What diagnostic tests are indicated? And what clinical treatment is indicated?
In the presented case scenario, the patient’s symptoms are consistent with a viral influenza. Her recent travel history certainly makes coronavirus disease 2019 (COVID-19) the most likely diagnosis.
COVID-19, caused by a novel new coronavirus, has evolved with lightning speed since it was first identified in early December 2019.1 The disease originated in Wuhan, China. Its epicenter is now in Europe, and over 100 countries and regions have reported cases. New cases in the United States are being identified daily, and there is no clear end to the outbreak. Several areas of the United States have been particularly hard hit by this disease: Seattle, New Orleans, and New York City.
COVID-19 has provoked widespread unsettledness in many populations and an extraordinary response from public health officials, large corporations, professional organizations, and financial markets. We are learning more about somewhat unfamiliar public health concepts such as quarantine, containment, mitigation, reproduction number (R), and “flattening the curve.” Disneyland and Walt Disney World are now temporarily closed. Professional and collegiate sports organizations have cancelled or suspended games and tournaments. Scientific and trade association meetings have been postponed or cancelled. Broadway, Carnegie Hall, and the Metropolitan Museum of Art have now “turned out the lights.” The Centers for Disease Control and Prevention has recommended that everyone avoid gatherings that include more than 10 other persons.
This article will review the evolving epidemiology of COVID-19, describe the usual clinical manifestations of the disease, highlight the key diagnostic tests, and present guidelines for treatment. It will review the limited information currently available about the impact of COVID-19 in pregnant women. The review will conclude by describing measures that individuals can employ to prevent acquisition or transmission of infection and then by highlighting key “unanswered questions” about this new and ominous pathogen (TABLE).

Continue to: What we know about epidemiology...
What we know about epidemiology
COVID-19 is caused by a novel new coronavirus that shares some genetic overlap with the viruses that caused Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS).2 The first case of COVID-19 was reported on December 1, 2019, from Wuhan, China.1 Within a very short period of time the disease has spread throughout the world, and on March 11, 2020, the World Health Organization (WHO) declared the infection to be a true pandemic. The countries with the highest prevalence of COVID-19 include China, South Korea, Iran, Italy, France, Spain, and the United States. However, more than 100 other countries and regions have reported cases. As of the first week of April, approximately 1 million persons in the world have been diagnosed with COVID-19. Of those infected, slightly more than 50,000 deaths have occurred. At the time of this writing, 234,483 cases have been documented in the United States, and current estimates indicate that approximately 7% of the population in the country could become infected.1,3,4
The virus responsible for COVID-19 is a single-stranded, enveloped RNA virus. Like its counterparts that caused SARS and MERS, this virus originates in animals, primarily bats. The early cases seem to have resulted from patient contact with exotic animals displayed in the Huanan Seafood Wholesale Market.1
The virus is transmitted directly by respiratory droplets and by close surface-to-hand contact with infected respiratory secretions. The virus appears to remain viable on environmental surfaces for 1 to 3 days, although the degree of infectivity over time is not well delineated. With direct exposure to respiratory droplets, the infectivity is relatively high; approximately 2 to 3 individuals become infected as the result of contact with an infected patient. By contrast, the “reproduction number (R)” for influenza is closer to 1.2,5
Certain persons appear to be at increased risk for developing infection and becoming seriously ill2,6:
- persons older than age 60
- persons with underlying medical illness
- persons who are immunosuppressed.
The reported range in the case fatality rate (CFR) varies from 1% to 13%, with the higher rates concentrated in older patients with comorbidities.3 These initial reports of high CFRs may be misleading because in the initial phases of this pandemic many patients with mild or no symptoms were not tested, and, thus, the overall prevalence of infection is not clear. By way of comparison, the CRF for influenza A and B is about 0.1%.2
Of note, the number of reported cases in the pediatric population is low, and the outcomes in these individuals are much better than in the older population.2,3,6 At present, there are only two reports of COVID-19 in pregnancy; these two studies include 18 women and 19 infants.7,8 The frequency of preterm delivery was 50% in these reports. Sixteen of the 18 patients were delivered by cesarean delivery; at least 6 of these procedures were performed for a non-reassuring fetal heart rate tracing. No maternal deaths were identified, and no cases of vertical transmission occurred.
We must remember that the number of patients described in these two reports is very small. Although the initial reports are favorable, in other influenza epidemics, pregnant women have not fared so well and have experienced disproportionately higher rates of morbidity and mortality.2
Reported clinical manifestations
The incubation period of COVID-19 ranges from 2 to 14 days; the median is 5.2 days. Many patients with proven COVID-19 infection are asymptomatic. When clinical findings are present, they usually are relatively mild and include low-grade fever, myalgias, arthralgias, sore throat, mild dyspnea, and a dry nonproductive cough. Some patients also may experience diarrhea. Of course, these findings are also consistent with influenza A or B or atypical pneumonia. One key to differentiation is the patient’s history of recent travel to an area of high COVID-19 prevalence or contact with a person who has been in one of these areas and who is clinically ill.2,3,9,10
In some patients, notably those who are older than 65 years of age and/or who have underlying medical illnesses, the respiratory manifestations are more prominent.6 These patients may develop severe dyspnea, pneumonia, adult respiratory distress syndrome (ARDS), multiorgan failure, and septic shock. Interestingly, the more severe manifestations tend to occur during the second week of the illness. In this group of more severely ill patients requiring hospitalization, 17% to 29% develop ARDS, and 23% to 32% require admission to the intensive care unit.2,6
Pregnant patients who become severely ill may be at risk for spontaneous miscarriage and preterm labor. With profound maternal hypoxia, fetal heart rate abnormalities may become apparent. To date, no clearly proven cases of vertical transmission of infection to the newborn have been identified. However, as noted above, current reports only include 18 pregnancies and 19 infants.2,3,7,8,11
Continue to: Diagnostic testing...
Diagnostic testing
Infected patients may have a decreased peripheral white blood cell count, with a specific decrease in the number of lymphocytes. Thrombocytopenia may be present, as well as an elevation in the hepatic transaminase enzymes (ALT, AST).2
X-ray, chest CT, and RT-PCR. The three most important diagnostic tests are chest x-ray, chest computed tomography (CT) scan, and real-time PCR (RT-PCR) or nucleic acid amplification test (NAAT).2,6 Specimens for RT-PCR or NAAT should be obtained from the oropharynx and nasopharynx using a synthetic-tipped applicator with an aluminum shaft. Patients who are intubated should have specimens obtained by broncho-alveolar lavage. The virus also has been recovered from blood and stool, but not yet from urine, amniotic fluid, placenta, cord blood, or breast milk.2
CT and chest x-ray show characteristic ground-glass opacities in both lung fields, combined with multiple areas of consolidation. Chest imaging is particularly helpful when the patient has all the major clinical manifestations, but the initial RT-PCR or NAAT is negative.
Treatment
Fortunately, most infected persons can be treated as outpatients. Because this condition may be confused with influenza A or B, initial treatment with a drug such as oseltamivir 75 mg orally twice daily for five days is very reasonable.9 Supportive therapy is critically important in this clinical setting. Acetaminophen, up to 3,000 mg/d in divided doses, or ibuprofen, up to 2,400 mg/d in divided doses, can be used to reduce fever and relieve myalgias and arthralgias. The latter drug, of course, should not be used in pregnant women. The patient should be encouraged to rest and to stay well hydrated. Loperamide can be used to treat diarrhea, 4 mg orally initially, then 2 mg orally after each loose stool up to a maximum of 16 mg/d. Pregnant patients should be cautioned to watch for signs of preterm labor.9,12 Patients should remain in relative isolation at home until they are free of signs of illness and they test negative for COVID-19.
For patients who are more severely ill at initial evaluation or who deteriorate while undergoing outpatient management, hospitalization is indicated.2,6 Patients should be placed in rooms that provide protection against aerosolized infection. They should receive supplemental oxygen and be observed closely for signs of superimposed bacterial infection. Depending upon the suspected bacterial pathogen, appropriate antibiotics may include ceftriaxone, which targets Streptococcus pneumoniae, Hemophilus influenzae, and Moraxella catarrhalis; azithromycin, which targets mycoplasmas; and vancomycin, which specifically covers Staphylococcus aureus. Health care workers should wear appropriate personal protective equipment when interacting with these patients, including cap, N95 mask, face shield, gloves, gown, and shoe covers. If a woman with COVID-19 has delivered, and the pediatrician permits rooming in, the isolette should be positioned at least 6 feet away from the mother. The mother should use a mechanical breast pump to obtain milk and then have another family member feed the baby until the mother tests negative for the virus. The breast pump needs to be cleaned meticulously after each use. The number of visitors to the mother’s room should be strictly limited.3,9
At the present time, there is no specific antiviral drug approved by the US Food and Drug Administration for treatment of COVID-19. The National Institutes of Health is currently conducting a trial of remdesivir for affected patients.13 The drug is also available from the manufacturer outside of this trial on a “compassionate use” basis. Another treatment regimen receiving extensive publicity is the combination of azithromycin and hydroxychloroquine. Its effectiveness has not been confirmed in a properly designed randomized trial.
Prevention hinges on commonsense precautions
Although vaccine trials are underway, public health authorities estimate that a vaccine will not be commercially available for at least 12 to 18 months. Therefore, independent of “community/organizational” mitigation programs, individuals should observe the following commonsense precautions to minimize their risk of contracting or transmitting COVID-192,3,5,14:
- Eliminate any nonessential travel, particularly by plane or cruise ship.
- Avoid events that draw large crowds, such as concerts, theater performances, movies, and even religious services.
- When out in public, try to maintain a distance of 6 feet from others
- Remain at home if you feel ill, particularly if you have respiratory symptoms.
- Cough or sneeze into your sleeve rather than your bare hand.
- Avoid handshakes.
- Wash your hands frequently in warm soapy water for at least 20 seconds, particularly after touching environmental surfaces such as counter tops and handrails.
- If you use hand sanitizers, they should have an alcohol content of at least 60%.
- Clean environmental surfaces frequently with a dilute bleach solution.
CASE Resolved
The clinical manifestations displayed by this patient are consistent with viral influenza. The recent travel history to one of the European epicenters makes COVID-19 the most likely diagnosis. The patient should have a chest CT scan and a RT-PCR or NAAT to confirm the diagnosis. If the diagnosis is confirmed, she and her close contacts should be self-quarantined at home for 14 days. She should receive appropriate supportive care with anti-pyretics, analgesics, and anti-diarrhea agents. If she develops signs of serious respiratory compromise, she should be admitted to an isolation room in the hospital for intensive respiratory therapy and close observation for superimposed bacterial pneumonia.

- Holshue ML, DeBolt C, Lindquist S, et al; Washington State 2019-nCoV Case Investigation Team. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929-936.
- Rasmussen SA, Smulian JC, Lednicky JA, et al. Coronavirus disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am J Obstet Gynecol. February 24, 2020. doi: 10.1016/j.ajog.2020.02.017.
- Rasmussen SA, Jamieson DJ. Coronavirus disease 2019 (COVID-19) and pregnancy: responding to a rapidly evolving situation [in press]. Obstet Gynecol. 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019: Cases in US. CDC website. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed March 18, 2020.
- Wang H, Wang Z, Dong Y, et al. Phase-adjusted estimation of the number of Coronavirus Disease 2019 cases in Wuhan, China. Cell Discov. 2020;6:10.
- Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727-733.
- Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395;809-815.
- Lei D, Wang C, Li C, et al. Clinical characteristics of pregnancy with the 2019 novel coronavirus disease (COVID-19) infection. Chin J Perinat Med. 2020:23.
- Dotters-Katz S, Hughes BL. Coronavirus (COVID-19) and pregnancy: what maternal-fetal medicine subspecialists need to know. Society for Maternal-Fetal Medicine. March 17, 2020. https://s3.amazonaws.com/cdn.smfm.org/media/2267/COVID19-_updated_3-17-20_PDF.pdf. Accessed March 17, 2020.
- Perlman S. Another decade, another coronavirus. N Engl J Med. 2020;382:760-762.
- Yang H, Wang C, Poon LC. Novel coronavirus infection and pregnancy. Ultrasound Obstet Gynecol. March 5, 2020. doi:10.1002/uog.22006.
- American College of Obstetricians and Gynecologists. Practice Advisory: novel coronavirus 2019 (COVID-19). March 13, 2020. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Practice-Advisory-Novel-Coronavirus2019?IsMobileSet=false. Accessed March 17, 2020.
- National Institutes of Health. NIH clinical trial of remdesivir to treat COVID-19 begins. February 25, 2020. https://www.nih.gov/news-events/news-releases/nih-clinical-trial-remdesivir-treat-covid-19-begins. Accessed March 17, 2020.
- Munster VJ, Koopmans M, Van Doremalen N, et al. A novel coronavirus emerging in China – key questions for impact assessment. N Engl J Med. 2020;382:692-694.
- Holshue ML, DeBolt C, Lindquist S, et al; Washington State 2019-nCoV Case Investigation Team. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929-936.
- Rasmussen SA, Smulian JC, Lednicky JA, et al. Coronavirus disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am J Obstet Gynecol. February 24, 2020. doi: 10.1016/j.ajog.2020.02.017.
- Rasmussen SA, Jamieson DJ. Coronavirus disease 2019 (COVID-19) and pregnancy: responding to a rapidly evolving situation [in press]. Obstet Gynecol. 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019: Cases in US. CDC website. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed March 18, 2020.
- Wang H, Wang Z, Dong Y, et al. Phase-adjusted estimation of the number of Coronavirus Disease 2019 cases in Wuhan, China. Cell Discov. 2020;6:10.
- Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727-733.
- Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395;809-815.
- Lei D, Wang C, Li C, et al. Clinical characteristics of pregnancy with the 2019 novel coronavirus disease (COVID-19) infection. Chin J Perinat Med. 2020:23.
- Dotters-Katz S, Hughes BL. Coronavirus (COVID-19) and pregnancy: what maternal-fetal medicine subspecialists need to know. Society for Maternal-Fetal Medicine. March 17, 2020. https://s3.amazonaws.com/cdn.smfm.org/media/2267/COVID19-_updated_3-17-20_PDF.pdf. Accessed March 17, 2020.
- Perlman S. Another decade, another coronavirus. N Engl J Med. 2020;382:760-762.
- Yang H, Wang C, Poon LC. Novel coronavirus infection and pregnancy. Ultrasound Obstet Gynecol. March 5, 2020. doi:10.1002/uog.22006.
- American College of Obstetricians and Gynecologists. Practice Advisory: novel coronavirus 2019 (COVID-19). March 13, 2020. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Practice-Advisory-Novel-Coronavirus2019?IsMobileSet=false. Accessed March 17, 2020.
- National Institutes of Health. NIH clinical trial of remdesivir to treat COVID-19 begins. February 25, 2020. https://www.nih.gov/news-events/news-releases/nih-clinical-trial-remdesivir-treat-covid-19-begins. Accessed March 17, 2020.
- Munster VJ, Koopmans M, Van Doremalen N, et al. A novel coronavirus emerging in China – key questions for impact assessment. N Engl J Med. 2020;382:692-694.
2020 Update on gynecologic cancer
Over the past year, major strides have been made in the treatment of gynecologic malignancies. In this Update, we highlight 3 notable studies. The first is a phase 3, multicenter, international, randomized clinical trial that demonstrated a significant improvement in both overall and failure-free survival with the use of adjuvant chemoradiation versus radiotherapy alone in patients with stage III or high-risk uterine cancer. Additionally, we describe the results of 2 phase 3, multicenter, international, randomized clinical trials in ovarian cancer treatment: use of poly(adenosine diphosphate-ribose) polymerase (PARP) inhibitors in combination with platinum and taxane-based chemotherapy followed by the PARP inhibitor as maintenance therapy, and secondary cytoreductive surgery in platinum-sensitive, recurrent ovarian cancer.
We provide a brief overview of current treatment strategies, summarize the key findings of these trials, and establish how these findings have changed our management of these gynecologic malignancies.
Adjuvant chemotherapy and radiotherapy improves survival in women with high-risk endometrial cancer
de Boer SM, Powell ME, Mileshkin L, et al; on behalf of the PORTEC Study Group. Adjuvant chemoradiotherapy versus radiotherapy alone in women with high-risk endometrial cancer (PORTEC-3): patterns of recurrence and post-hoc survival analysis of a randomised phase 3 trial. Lancet Oncol. 2019;1273-1285.
In the United States, it is estimated that more than 61,000 women were diagnosed with endometrial cancer in 2019.1 Women with endometrial cancer usually have a favorable prognosis; more than 65% are diagnosed with early-stage disease, which is associated with a 95% 5-year survival rate.1 However, 15% to 20% of patients have disease with high-risk features, including advanced stage (stage II-IV), high tumor grade, lymphovascular space invasion, deep myometrial invasion, or nonendometrioid histologic subtypes (serous or clear cell).2 The presence of these high-risk disease features is associated with an increased incidence of distant metastases and cancer-related death.
Adjuvant therapy in high-risk endometrial cancer
To date, the optimal adjuvant therapy for patients with high-risk endometrial cancer remains controversial. Prior data from Gynecologic Oncology Group (GOG) protocol 122 demonstrated that chemotherapy significantly improved progression-free survival and overall survival when compared with radiotherapy in patients with advanced-stage endometrial cancer.3 As such, chemotherapy now is frequently used in this population, often in combination with radiation, although data describing the benefit of chemoradiation are limited.4 For women with earlier-stage disease with high-risk features, the value of chemotherapy plus radiation is uncertain.5,6
Continue to: Benefit observed with adjuvant chemoradiotherapy...
Benefit observed with adjuvant chemoradiotherapy
In a multicenter, international, randomized phase 3 trial, known as the PORTEC-3 trial, de Boer and colleagues sought to determine if combined adjuvant chemoradiation improved overall survival (OS) and failure-free survival when compared with external-beam radiation therapy (EBRT) alone in the treatment of women with high-risk endometrial cancer.7 Women were eligible for the study if they had histologically confirmed stage I, grade 3 endometrioid endometrial cancer with deep invasion and/or lymphovascular space invasion, stage II or III disease, or stage I-III disease with serous or clear cell histology.
Participants were randomly assigned in a 1:1 ratio; 330 women received adjuvant EBRT alone (total dose of 48.6 Gy administered in 27 fractions), and 330 received adjuvant chemotherapy during and after radiation therapy (CTRT) (2 cycles of cisplatin 50 mg/m2 IV given on days 1 and 22 of EBRT followed by 4 cycles of carboplatin AUC 5 and paclitaxel 175 mg/m2 IV every 3 weeks).
At a median follow-up of 73 months, treatment with adjuvant CTRT, compared with adjuvant EBRT alone, was associated with a significant improvement in both overall survival (5-year OS: 81.4% vs 76.1%, P = .034 [FIGURE]) and failure-free survival (5-year failure-free survival: 76.5% vs 69.1%, P = .016).

The greatest absolute benefit of adjuvant CTRT, compared with EBRT alone, in survival was among women with stage III endometrial cancer (5-year OS: 78.5% vs 68.5%, P = .043) or serous cancers (19% absolute improvement in 5-year OS), or both. Significant differences in 5-year OS and failure-free survival in women with stage I-II cancer were not observed with adjuvant CTRT when compared with adjuvant EBRT alone. At 5 years, significantly more adverse events of grade 2 or worse were reported in the adjuvant CTRT arm.
Results from similar trials
Since the publication of results from the updated analysis of PORTEC-3, results from 2 pertinent trials have been published.8,9 In the GOG 249 trial, women with stage I-II endometrial cancer with high-risk features were randomly assigned to receive 3 cycles of carboplatin-paclitaxel chemotherapy with vaginal brachytherapy or EBRT.8 There was no difference in survival, but a significant increase in both pelvic and para-aortic recurrences were seen after the combination of chemotherapy and vaginal brachytherapy.8
In GOG 258, women with stage III-IVA endometrial cancer were randomly assigned to receive chemotherapy alone (carboplatin-paclitaxel) or adjuvant chemotherapy after EBRT.9 No differences in recurrence-free or overall survival were noted, but there was a significant increase in the number of vaginal and pelvic or para-aortic recurrences in patients in the chemotherapy-only arm.9
The conflicting data regarding the ideal adjuvant therapy for endometrial cancer suggests that treatment decisions should be individualized. Pelvic EBRT with concurrent adjuvant chemotherapy should be considered in women with stage III endometrial cancer or serous cancers as combination therapy improves survival, although dual modality treatment is associated with increased toxicity. Chemoradiation appears to have less benefit for women with stage I–II cancers with other pathologic risk factors.
Role for PARP inhibitor plus first-line chemotherapy, and as maintenance therapy, in ovarian cancer treatment
Coleman RL, Fleming GF, Brady MF, et al. Veliparib with first-line chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med. 2019;381:2403-2415.
Ovarian cancer is the leading cause of gynecologic cancer-related deaths among women in the United States.10 Treatment consists of cytoreductive surgery combined with platinum and taxane-based chemotherapy.11 Despite favorable initial responses, more than 80% of patients experience a recurrence, with an 18-month median time to progression.12 As a result, recent efforts have focused on finding novel therapeutic approaches to improve treatment outcomes and mitigate the risk of disease recurrence.
Continue to: PARP inhibitors are changing the face of treatment...
PARP inhibitors are changing the face of treatment
Poly(adenosine diphosphate-ribose) polymerases (PARPs) are a family of enzymes that play a critical role in DNA damage repair. These enzymes promote DNA repair by recruiting proteins involved in repairing single-strand and double-strand DNA breaks and in protecting and restarting stalled DNA replication forks.13 The predominant mechanisms of action of PARP inhibitors in cells with homologous-recombination deficiency (HRD) include inhibiting repair of single-strand DNA breaks and trapping PARP-DNA complexes at stalled DNA replication forks.14
Germline or somatic BRCA1/2 mutations and genetic alterations resulting in HRD are present in about 20% and 30% of ovarian carcinomas, respectively, and increase the susceptibility of tumors to platinum-based agents and PARP inhibitors.15,16 Based on multiple clinical trials that demonstrated the efficacy of single-agent PARP in the treatment of recurrent ovarian carcinoma and as maintenance therapy after an initial response to platinum-based therapy, the US Food and Drug Administration approved olaparib, niraparib, and rucaparib for the treatment of high-grade epithelial ovarian cancer.17-19 Only olaparib is approved for maintenance therapy after initial adjuvant therapy in patients with BRCA mutations.20
Given the robust response to PARP inhibitors, there has been great interest in using these agents earlier in the disease course in combination with chemotherapy.
Efficacy of veliparib with chemotherapy and as maintenance monotherapy
In a randomized, double-blind, placebo-controlled phase 3 trial, Coleman and colleagues sought to determine the efficacy of the PARP inhibitor veliparib when administered with first-line carboplatin and paclitaxel induction chemotherapy and subsequently continued as maintenance monotherapy.21
Women with stage III or IV high-grade epithelial ovarian, fallopian tube, or primary peritoneal carcinoma were eligible for the study. Cytoreductive surgery could be performed prior to the initiation of trial treatment or after 3 cycles of chemotherapy.
Participants were randomized in a 1:1:1 ratio: 371 women received carboplatin and paclitaxel plus placebo followed by placebo maintenance (control arm); 376 received chemotherapy plus veliparib followed by placebo maintenance (veliparib combination-only arm); and 377 received chemotherapy plus veliparib followed by veliparib maintenance therapy (veliparib-throughout arm). Combination chemotherapy consisted of 6 cycles, and maintenance therapy was an additional 30 cycles.
Progression-free survival extended
At a median follow-up of 28 months, investigators observed a significant improvement in progression-free survival in the veliparib-throughout (initial and maintenance therapy) arm compared with the control arm in 3 cohorts: the BRCA-mutation cohort, the HRD cohort, and the intention-to-treat population (all participants undergoing randomization).
In the BRCA-mutation cohort, the median progression-free survival was 12.7 months longer in the veliparib-throughout arm than in the control arm. Similarly, in the HRD cohort, the median progression-free survival was 11.4 months longer in the veliparib-throughout arm than in the control group. In the intention-to-treat population, the median progression-free survival increased from 17.3 to 23.5 months in the veliparib-throughout arm compared with the control arm.
Women who received veliparib experienced increased rates of nausea, anemia, and fatigue and were more likely to require dose reductions and treatment interruptions. Myelodysplastic syndrome was reported in 1 patient (BRCA1 positive) in the veliparib combination-only arm.
For women with newly diagnosed, previously untreated stage III or IV high-grade serous ovarian carcinoma, carboplatin, paclitaxel, and veliparib induction therapy followed by single-agent veliparib maintenance therapy resulted in a significant improvement in median progression-free survival compared with induction chemotherapy alone. However, veliparib use was also associated with a higher incidence of adverse effects that required dose reduction and/or interruption during both the combination and maintenance phases of treatment.
Secondary cytoreductive surgery or chemotherapy alone for platinum-sensitive recurrent ovarian carcinoma?
Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
Primary surgical cytoreduction combined with platinum and taxane-based chemotherapy remains the mainstay of ovarian cancer treatment.11 The role of surgery for women with recurrent ovarian cancer, so-called secondary cytoreduction, remains controversial.22
Data have shown that among women who undergo secondary surgery, those with little or no postoperative residual disease benefit the most from a secondary debulking.23-26 Prior work largely is based on small retrospective reports and is limited by substantial bias in the selection of patients undergoing surgery. Additionally, with the availability of targeted therapies such as bevacizumab and PARP inhibitors as maintenance—medical interventions with a demonstrated benefit in progression-free survival17-19,27—the role of secondary cytoreduction in the treatment of ovarian carcinoma needs to be clarified.
Continue to: Overall survival after secondary cytoreduction followed by chemotherapy...
Overall survival after secondary cytoreduction followed by chemotherapy
Coleman and colleagues conducted a prospective, multicenter, international, randomized phase 3 trial to assess whether secondary cytoreductive surgery followed by chemotherapy would improve overall survival versus chemotherapy alone among women with resectable platinum-sensitive, recurrent ovarian cancer.22 Platinum sensitivity was defined as a disease-free interval of at least 6 months after the last cycle of platinum-based chemotherapy.
All women had recurrent epithelial ovarian carcinoma considered to be amenable to complete gross surgical resection by the investigator and a history of complete response to at least 3 cycles of platinum-based chemotherapy as determined by a normal CA-125 value or negative imaging studies (if obtained).
Participants were randomly assigned 1:1, with 240 women assigned to secondary surgical cytoreduction followed by platinum-based chemotherapy, and 245 assigned to chemotherapy alone. The type of adjuvant chemotherapy used (carboplatin-paclitaxel or carboplatin-gemcitabine) and whether or not bevacizumab was administered were at the investigators' discretion.
Shorter survival, decline in quality of life
Among the participants assigned to and who underwent surgery, complete gross resection was achieved in 67%. Eighty-four percent of the entire study population received platinum-based chemotherapy with bevacizumab followed by bevacizumab maintenance therapy, which was equally distributed between the 2 study arms.
At a median follow-up of 48.1 months, median overall survival was 50.6 months in the surgery arm compared with 64.7 months in the chemotherapy arm, corresponding to a hazard ratio (HR) for death of 1.29 (95% confidence interval [CI], 0.97-1.72; P = .08). This effect was unchanged after adjusting for platinum-free interval, chemotherapy choice, and restricting the analysis to women who had a complete gross resection.
Similarly, the adjusted HR for disease progression or death was 0.82 (95% CI, 0.66-1.01) and corresponded to a median progression-free survival of 18.9 months for the surgery group and 16.2 months for the chemotherapy group. Surgical morbidity was reported in 9% of patients who underwent surgery, and 1 patient (0.4%) died from postoperative complications.
While a significant decline in both quality of life and patient-reported outcomes was reported immediately after surgery, significant differences were not noted between the 2 groups after the initial postoperative recovery period.
For women with platinum-sensitive, recurrent ovarian cancer, a secondary cytoreductive surgery followed by chemotherapy was not associated with an improvement in overall survival when compared with chemotherapy alone. Secondary cytoreductive surgery should not be used routinely in women with recurrent ovarian cancer.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;20:7-34.
- Colombo N, Creutzberg C, Amant F, et al; ESMO-ESGOESTRO Endometrial Consensus Conference Working Group. ESMO-ESGO-ESTRO consensus conference on endometrial cancer: diagnosis, treatment and follow-up. Ann Oncol. 2016;27:16-41.
- Randall ME, Filiaci VL, Muss H, et al; Gynecologic Oncology Group Study. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2006;24:36-44.
- Syeda S, Chen L, Hou JY, et al. Chemotherapy, radiation, or combination therapy for stage III uterine cancer. Obstet Gynecol. 2019;134:17-29.
- Maggi R, Lissoni A, Spina F, et al. Adjuvant chemotherapy vs radiotherapy in high-risk endometrial carcinoma: results of a randomised trial. Br J Cancer. 2006;95:266-271.
- Susumu N, Sagae S, Udagawa Y, et al; Japanese Gynecologic Oncology Group. Randomized phase III trial of pelvic radiotherapy versus cisplatin-based combined chemotherapy in patients with intermediate- and high-risk endometrial cancer: a Japanese Gynecologic Oncology Group study. Gynecol Oncol. 2008;108:226-233.
- de Boer SM, Powell ME, Mileshkin L, et al; PORTEC Study Group. Adjuvant chemoradiotherapy versus radiotherapy alone in women with high-risk endometrial cancer (PORTEC-3): patterns of recurrence and post-hoc survival analysis of a randomised phase 3 trial. Lancet Oncol. 2019;20:1273-1285.
- Randall ME, Filiaci V, McMeekin DS, et al. Phase III trial: adjuvant pelvic radiation therapy versus vaginal brachytherapy plus paclitaxel/carboplatin in high-intermediate and high-risk early stage endometrial cancer. J Clin Oncol. 2019;37:1810-1818.
- Matei D, Filiaci V, Randall ME, et al. Adjuvant chemotherapy plus radiation for locally advanced endometrial cancer. N Engl J Med. 2019;380:2317-2326.
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424.
- Armstrong DK, Alvarez RD, Bakkum-Gamez JN, et al. NCCN guidelines insights: ovarian cancer, version 1.2019. J Natl Compr Canc Netw. 2019;17:896-909.
- Ledermann JA, Raja FA, Fotopoulou C, et al. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(suppl 6):vi24-vi32.
- Moore KN, Mirza MR, Matulonis UA. The poly (ADP ribose) polymerase inhibitor niraparib: management of toxicities. Gynecol Oncol. 2018;149:214-220.
- Konstantinopoulos PA, Matulonis UA. PARP inhibitors in ovarian cancer: a trailblazing and transformative journey. Clin Cancer Res. 2018;24:4062-4065.
- Pennington KP, Walsh T, Harrell MI, et al. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer Res. 2014;20:764-775.
- Mukhopadhyay A, Plummer ER, Elattar A, et al. Clinicopathological features of homologous recombination-deficient epithelial ovarian cancers: sensitivity to PARP inhibitors, platinum, and survival. Cancer Res. 2012;72:5675-5682.
- Mirza MR, Monk BJ, Herrstedt J, et al; ENGOT-OV16/ NOVA Investigators. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375:2154-2164.
- Pujade-Lauraine E, Ledermann JA, Selle F, et al; SOLO2/ ENGOT-Ov21 Investigators. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1274-1284.
- Coleman RL, Oza AM, Lorusso D, et al; ARIEL3 Investigators. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:1949-1961.
- Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505.
- Coleman RL, Fleming GF, Brady MF, et al. Veliparib with firstline chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med. 2019;381:2403-2415.
- Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
- Bommert M, Harter P, Heitz F, et al. When should surgery be used for recurrent ovarian carcinoma? Clin Oncol (R Coll Radiol). 2018;30:493-497.
- Santillan A, Karam AK, Li AJ, et al. Secondary cytoreductive surgery for isolated nodal recurrence in patients with epithelial ovarian cancer. Gynecol Oncol. 2007;104:686-690.
- Zang RY, Harter P, Chi DS, et al. Predictors of survival in patients with recurrent ovarian cancer undergoing secondary cytoreductive surgery based on the pooled analysis of an international collaborative cohort. Br J Cancer. 2011;105:890-896.
- Chi DS, McCaughty K, Diaz JP, et al. Guidelines and selection criteria for secondary cytoreductive surgery in patients with recurrent, platinum-sensitive epithelial ovarian carcinoma. Cancer. 2006;106:1933-1939.
- Aghajanian C, Blank SV, Goff BA, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012;30: 2039-2045.
Over the past year, major strides have been made in the treatment of gynecologic malignancies. In this Update, we highlight 3 notable studies. The first is a phase 3, multicenter, international, randomized clinical trial that demonstrated a significant improvement in both overall and failure-free survival with the use of adjuvant chemoradiation versus radiotherapy alone in patients with stage III or high-risk uterine cancer. Additionally, we describe the results of 2 phase 3, multicenter, international, randomized clinical trials in ovarian cancer treatment: use of poly(adenosine diphosphate-ribose) polymerase (PARP) inhibitors in combination with platinum and taxane-based chemotherapy followed by the PARP inhibitor as maintenance therapy, and secondary cytoreductive surgery in platinum-sensitive, recurrent ovarian cancer.
We provide a brief overview of current treatment strategies, summarize the key findings of these trials, and establish how these findings have changed our management of these gynecologic malignancies.
Adjuvant chemotherapy and radiotherapy improves survival in women with high-risk endometrial cancer
de Boer SM, Powell ME, Mileshkin L, et al; on behalf of the PORTEC Study Group. Adjuvant chemoradiotherapy versus radiotherapy alone in women with high-risk endometrial cancer (PORTEC-3): patterns of recurrence and post-hoc survival analysis of a randomised phase 3 trial. Lancet Oncol. 2019;1273-1285.
In the United States, it is estimated that more than 61,000 women were diagnosed with endometrial cancer in 2019.1 Women with endometrial cancer usually have a favorable prognosis; more than 65% are diagnosed with early-stage disease, which is associated with a 95% 5-year survival rate.1 However, 15% to 20% of patients have disease with high-risk features, including advanced stage (stage II-IV), high tumor grade, lymphovascular space invasion, deep myometrial invasion, or nonendometrioid histologic subtypes (serous or clear cell).2 The presence of these high-risk disease features is associated with an increased incidence of distant metastases and cancer-related death.
Adjuvant therapy in high-risk endometrial cancer
To date, the optimal adjuvant therapy for patients with high-risk endometrial cancer remains controversial. Prior data from Gynecologic Oncology Group (GOG) protocol 122 demonstrated that chemotherapy significantly improved progression-free survival and overall survival when compared with radiotherapy in patients with advanced-stage endometrial cancer.3 As such, chemotherapy now is frequently used in this population, often in combination with radiation, although data describing the benefit of chemoradiation are limited.4 For women with earlier-stage disease with high-risk features, the value of chemotherapy plus radiation is uncertain.5,6
Continue to: Benefit observed with adjuvant chemoradiotherapy...
Benefit observed with adjuvant chemoradiotherapy
In a multicenter, international, randomized phase 3 trial, known as the PORTEC-3 trial, de Boer and colleagues sought to determine if combined adjuvant chemoradiation improved overall survival (OS) and failure-free survival when compared with external-beam radiation therapy (EBRT) alone in the treatment of women with high-risk endometrial cancer.7 Women were eligible for the study if they had histologically confirmed stage I, grade 3 endometrioid endometrial cancer with deep invasion and/or lymphovascular space invasion, stage II or III disease, or stage I-III disease with serous or clear cell histology.
Participants were randomly assigned in a 1:1 ratio; 330 women received adjuvant EBRT alone (total dose of 48.6 Gy administered in 27 fractions), and 330 received adjuvant chemotherapy during and after radiation therapy (CTRT) (2 cycles of cisplatin 50 mg/m2 IV given on days 1 and 22 of EBRT followed by 4 cycles of carboplatin AUC 5 and paclitaxel 175 mg/m2 IV every 3 weeks).
At a median follow-up of 73 months, treatment with adjuvant CTRT, compared with adjuvant EBRT alone, was associated with a significant improvement in both overall survival (5-year OS: 81.4% vs 76.1%, P = .034 [FIGURE]) and failure-free survival (5-year failure-free survival: 76.5% vs 69.1%, P = .016).

The greatest absolute benefit of adjuvant CTRT, compared with EBRT alone, in survival was among women with stage III endometrial cancer (5-year OS: 78.5% vs 68.5%, P = .043) or serous cancers (19% absolute improvement in 5-year OS), or both. Significant differences in 5-year OS and failure-free survival in women with stage I-II cancer were not observed with adjuvant CTRT when compared with adjuvant EBRT alone. At 5 years, significantly more adverse events of grade 2 or worse were reported in the adjuvant CTRT arm.
Results from similar trials
Since the publication of results from the updated analysis of PORTEC-3, results from 2 pertinent trials have been published.8,9 In the GOG 249 trial, women with stage I-II endometrial cancer with high-risk features were randomly assigned to receive 3 cycles of carboplatin-paclitaxel chemotherapy with vaginal brachytherapy or EBRT.8 There was no difference in survival, but a significant increase in both pelvic and para-aortic recurrences were seen after the combination of chemotherapy and vaginal brachytherapy.8
In GOG 258, women with stage III-IVA endometrial cancer were randomly assigned to receive chemotherapy alone (carboplatin-paclitaxel) or adjuvant chemotherapy after EBRT.9 No differences in recurrence-free or overall survival were noted, but there was a significant increase in the number of vaginal and pelvic or para-aortic recurrences in patients in the chemotherapy-only arm.9
The conflicting data regarding the ideal adjuvant therapy for endometrial cancer suggests that treatment decisions should be individualized. Pelvic EBRT with concurrent adjuvant chemotherapy should be considered in women with stage III endometrial cancer or serous cancers as combination therapy improves survival, although dual modality treatment is associated with increased toxicity. Chemoradiation appears to have less benefit for women with stage I–II cancers with other pathologic risk factors.
Role for PARP inhibitor plus first-line chemotherapy, and as maintenance therapy, in ovarian cancer treatment
Coleman RL, Fleming GF, Brady MF, et al. Veliparib with first-line chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med. 2019;381:2403-2415.
Ovarian cancer is the leading cause of gynecologic cancer-related deaths among women in the United States.10 Treatment consists of cytoreductive surgery combined with platinum and taxane-based chemotherapy.11 Despite favorable initial responses, more than 80% of patients experience a recurrence, with an 18-month median time to progression.12 As a result, recent efforts have focused on finding novel therapeutic approaches to improve treatment outcomes and mitigate the risk of disease recurrence.
Continue to: PARP inhibitors are changing the face of treatment...
PARP inhibitors are changing the face of treatment
Poly(adenosine diphosphate-ribose) polymerases (PARPs) are a family of enzymes that play a critical role in DNA damage repair. These enzymes promote DNA repair by recruiting proteins involved in repairing single-strand and double-strand DNA breaks and in protecting and restarting stalled DNA replication forks.13 The predominant mechanisms of action of PARP inhibitors in cells with homologous-recombination deficiency (HRD) include inhibiting repair of single-strand DNA breaks and trapping PARP-DNA complexes at stalled DNA replication forks.14
Germline or somatic BRCA1/2 mutations and genetic alterations resulting in HRD are present in about 20% and 30% of ovarian carcinomas, respectively, and increase the susceptibility of tumors to platinum-based agents and PARP inhibitors.15,16 Based on multiple clinical trials that demonstrated the efficacy of single-agent PARP in the treatment of recurrent ovarian carcinoma and as maintenance therapy after an initial response to platinum-based therapy, the US Food and Drug Administration approved olaparib, niraparib, and rucaparib for the treatment of high-grade epithelial ovarian cancer.17-19 Only olaparib is approved for maintenance therapy after initial adjuvant therapy in patients with BRCA mutations.20
Given the robust response to PARP inhibitors, there has been great interest in using these agents earlier in the disease course in combination with chemotherapy.
Efficacy of veliparib with chemotherapy and as maintenance monotherapy
In a randomized, double-blind, placebo-controlled phase 3 trial, Coleman and colleagues sought to determine the efficacy of the PARP inhibitor veliparib when administered with first-line carboplatin and paclitaxel induction chemotherapy and subsequently continued as maintenance monotherapy.21
Women with stage III or IV high-grade epithelial ovarian, fallopian tube, or primary peritoneal carcinoma were eligible for the study. Cytoreductive surgery could be performed prior to the initiation of trial treatment or after 3 cycles of chemotherapy.
Participants were randomized in a 1:1:1 ratio: 371 women received carboplatin and paclitaxel plus placebo followed by placebo maintenance (control arm); 376 received chemotherapy plus veliparib followed by placebo maintenance (veliparib combination-only arm); and 377 received chemotherapy plus veliparib followed by veliparib maintenance therapy (veliparib-throughout arm). Combination chemotherapy consisted of 6 cycles, and maintenance therapy was an additional 30 cycles.
Progression-free survival extended
At a median follow-up of 28 months, investigators observed a significant improvement in progression-free survival in the veliparib-throughout (initial and maintenance therapy) arm compared with the control arm in 3 cohorts: the BRCA-mutation cohort, the HRD cohort, and the intention-to-treat population (all participants undergoing randomization).
In the BRCA-mutation cohort, the median progression-free survival was 12.7 months longer in the veliparib-throughout arm than in the control arm. Similarly, in the HRD cohort, the median progression-free survival was 11.4 months longer in the veliparib-throughout arm than in the control group. In the intention-to-treat population, the median progression-free survival increased from 17.3 to 23.5 months in the veliparib-throughout arm compared with the control arm.
Women who received veliparib experienced increased rates of nausea, anemia, and fatigue and were more likely to require dose reductions and treatment interruptions. Myelodysplastic syndrome was reported in 1 patient (BRCA1 positive) in the veliparib combination-only arm.
For women with newly diagnosed, previously untreated stage III or IV high-grade serous ovarian carcinoma, carboplatin, paclitaxel, and veliparib induction therapy followed by single-agent veliparib maintenance therapy resulted in a significant improvement in median progression-free survival compared with induction chemotherapy alone. However, veliparib use was also associated with a higher incidence of adverse effects that required dose reduction and/or interruption during both the combination and maintenance phases of treatment.
Secondary cytoreductive surgery or chemotherapy alone for platinum-sensitive recurrent ovarian carcinoma?
Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
Primary surgical cytoreduction combined with platinum and taxane-based chemotherapy remains the mainstay of ovarian cancer treatment.11 The role of surgery for women with recurrent ovarian cancer, so-called secondary cytoreduction, remains controversial.22
Data have shown that among women who undergo secondary surgery, those with little or no postoperative residual disease benefit the most from a secondary debulking.23-26 Prior work largely is based on small retrospective reports and is limited by substantial bias in the selection of patients undergoing surgery. Additionally, with the availability of targeted therapies such as bevacizumab and PARP inhibitors as maintenance—medical interventions with a demonstrated benefit in progression-free survival17-19,27—the role of secondary cytoreduction in the treatment of ovarian carcinoma needs to be clarified.
Continue to: Overall survival after secondary cytoreduction followed by chemotherapy...
Overall survival after secondary cytoreduction followed by chemotherapy
Coleman and colleagues conducted a prospective, multicenter, international, randomized phase 3 trial to assess whether secondary cytoreductive surgery followed by chemotherapy would improve overall survival versus chemotherapy alone among women with resectable platinum-sensitive, recurrent ovarian cancer.22 Platinum sensitivity was defined as a disease-free interval of at least 6 months after the last cycle of platinum-based chemotherapy.
All women had recurrent epithelial ovarian carcinoma considered to be amenable to complete gross surgical resection by the investigator and a history of complete response to at least 3 cycles of platinum-based chemotherapy as determined by a normal CA-125 value or negative imaging studies (if obtained).
Participants were randomly assigned 1:1, with 240 women assigned to secondary surgical cytoreduction followed by platinum-based chemotherapy, and 245 assigned to chemotherapy alone. The type of adjuvant chemotherapy used (carboplatin-paclitaxel or carboplatin-gemcitabine) and whether or not bevacizumab was administered were at the investigators' discretion.
Shorter survival, decline in quality of life
Among the participants assigned to and who underwent surgery, complete gross resection was achieved in 67%. Eighty-four percent of the entire study population received platinum-based chemotherapy with bevacizumab followed by bevacizumab maintenance therapy, which was equally distributed between the 2 study arms.
At a median follow-up of 48.1 months, median overall survival was 50.6 months in the surgery arm compared with 64.7 months in the chemotherapy arm, corresponding to a hazard ratio (HR) for death of 1.29 (95% confidence interval [CI], 0.97-1.72; P = .08). This effect was unchanged after adjusting for platinum-free interval, chemotherapy choice, and restricting the analysis to women who had a complete gross resection.
Similarly, the adjusted HR for disease progression or death was 0.82 (95% CI, 0.66-1.01) and corresponded to a median progression-free survival of 18.9 months for the surgery group and 16.2 months for the chemotherapy group. Surgical morbidity was reported in 9% of patients who underwent surgery, and 1 patient (0.4%) died from postoperative complications.
While a significant decline in both quality of life and patient-reported outcomes was reported immediately after surgery, significant differences were not noted between the 2 groups after the initial postoperative recovery period.
For women with platinum-sensitive, recurrent ovarian cancer, a secondary cytoreductive surgery followed by chemotherapy was not associated with an improvement in overall survival when compared with chemotherapy alone. Secondary cytoreductive surgery should not be used routinely in women with recurrent ovarian cancer.
Over the past year, major strides have been made in the treatment of gynecologic malignancies. In this Update, we highlight 3 notable studies. The first is a phase 3, multicenter, international, randomized clinical trial that demonstrated a significant improvement in both overall and failure-free survival with the use of adjuvant chemoradiation versus radiotherapy alone in patients with stage III or high-risk uterine cancer. Additionally, we describe the results of 2 phase 3, multicenter, international, randomized clinical trials in ovarian cancer treatment: use of poly(adenosine diphosphate-ribose) polymerase (PARP) inhibitors in combination with platinum and taxane-based chemotherapy followed by the PARP inhibitor as maintenance therapy, and secondary cytoreductive surgery in platinum-sensitive, recurrent ovarian cancer.
We provide a brief overview of current treatment strategies, summarize the key findings of these trials, and establish how these findings have changed our management of these gynecologic malignancies.
Adjuvant chemotherapy and radiotherapy improves survival in women with high-risk endometrial cancer
de Boer SM, Powell ME, Mileshkin L, et al; on behalf of the PORTEC Study Group. Adjuvant chemoradiotherapy versus radiotherapy alone in women with high-risk endometrial cancer (PORTEC-3): patterns of recurrence and post-hoc survival analysis of a randomised phase 3 trial. Lancet Oncol. 2019;1273-1285.
In the United States, it is estimated that more than 61,000 women were diagnosed with endometrial cancer in 2019.1 Women with endometrial cancer usually have a favorable prognosis; more than 65% are diagnosed with early-stage disease, which is associated with a 95% 5-year survival rate.1 However, 15% to 20% of patients have disease with high-risk features, including advanced stage (stage II-IV), high tumor grade, lymphovascular space invasion, deep myometrial invasion, or nonendometrioid histologic subtypes (serous or clear cell).2 The presence of these high-risk disease features is associated with an increased incidence of distant metastases and cancer-related death.
Adjuvant therapy in high-risk endometrial cancer
To date, the optimal adjuvant therapy for patients with high-risk endometrial cancer remains controversial. Prior data from Gynecologic Oncology Group (GOG) protocol 122 demonstrated that chemotherapy significantly improved progression-free survival and overall survival when compared with radiotherapy in patients with advanced-stage endometrial cancer.3 As such, chemotherapy now is frequently used in this population, often in combination with radiation, although data describing the benefit of chemoradiation are limited.4 For women with earlier-stage disease with high-risk features, the value of chemotherapy plus radiation is uncertain.5,6
Continue to: Benefit observed with adjuvant chemoradiotherapy...
Benefit observed with adjuvant chemoradiotherapy
In a multicenter, international, randomized phase 3 trial, known as the PORTEC-3 trial, de Boer and colleagues sought to determine if combined adjuvant chemoradiation improved overall survival (OS) and failure-free survival when compared with external-beam radiation therapy (EBRT) alone in the treatment of women with high-risk endometrial cancer.7 Women were eligible for the study if they had histologically confirmed stage I, grade 3 endometrioid endometrial cancer with deep invasion and/or lymphovascular space invasion, stage II or III disease, or stage I-III disease with serous or clear cell histology.
Participants were randomly assigned in a 1:1 ratio; 330 women received adjuvant EBRT alone (total dose of 48.6 Gy administered in 27 fractions), and 330 received adjuvant chemotherapy during and after radiation therapy (CTRT) (2 cycles of cisplatin 50 mg/m2 IV given on days 1 and 22 of EBRT followed by 4 cycles of carboplatin AUC 5 and paclitaxel 175 mg/m2 IV every 3 weeks).
At a median follow-up of 73 months, treatment with adjuvant CTRT, compared with adjuvant EBRT alone, was associated with a significant improvement in both overall survival (5-year OS: 81.4% vs 76.1%, P = .034 [FIGURE]) and failure-free survival (5-year failure-free survival: 76.5% vs 69.1%, P = .016).

The greatest absolute benefit of adjuvant CTRT, compared with EBRT alone, in survival was among women with stage III endometrial cancer (5-year OS: 78.5% vs 68.5%, P = .043) or serous cancers (19% absolute improvement in 5-year OS), or both. Significant differences in 5-year OS and failure-free survival in women with stage I-II cancer were not observed with adjuvant CTRT when compared with adjuvant EBRT alone. At 5 years, significantly more adverse events of grade 2 or worse were reported in the adjuvant CTRT arm.
Results from similar trials
Since the publication of results from the updated analysis of PORTEC-3, results from 2 pertinent trials have been published.8,9 In the GOG 249 trial, women with stage I-II endometrial cancer with high-risk features were randomly assigned to receive 3 cycles of carboplatin-paclitaxel chemotherapy with vaginal brachytherapy or EBRT.8 There was no difference in survival, but a significant increase in both pelvic and para-aortic recurrences were seen after the combination of chemotherapy and vaginal brachytherapy.8
In GOG 258, women with stage III-IVA endometrial cancer were randomly assigned to receive chemotherapy alone (carboplatin-paclitaxel) or adjuvant chemotherapy after EBRT.9 No differences in recurrence-free or overall survival were noted, but there was a significant increase in the number of vaginal and pelvic or para-aortic recurrences in patients in the chemotherapy-only arm.9
The conflicting data regarding the ideal adjuvant therapy for endometrial cancer suggests that treatment decisions should be individualized. Pelvic EBRT with concurrent adjuvant chemotherapy should be considered in women with stage III endometrial cancer or serous cancers as combination therapy improves survival, although dual modality treatment is associated with increased toxicity. Chemoradiation appears to have less benefit for women with stage I–II cancers with other pathologic risk factors.
Role for PARP inhibitor plus first-line chemotherapy, and as maintenance therapy, in ovarian cancer treatment
Coleman RL, Fleming GF, Brady MF, et al. Veliparib with first-line chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med. 2019;381:2403-2415.
Ovarian cancer is the leading cause of gynecologic cancer-related deaths among women in the United States.10 Treatment consists of cytoreductive surgery combined with platinum and taxane-based chemotherapy.11 Despite favorable initial responses, more than 80% of patients experience a recurrence, with an 18-month median time to progression.12 As a result, recent efforts have focused on finding novel therapeutic approaches to improve treatment outcomes and mitigate the risk of disease recurrence.
Continue to: PARP inhibitors are changing the face of treatment...
PARP inhibitors are changing the face of treatment
Poly(adenosine diphosphate-ribose) polymerases (PARPs) are a family of enzymes that play a critical role in DNA damage repair. These enzymes promote DNA repair by recruiting proteins involved in repairing single-strand and double-strand DNA breaks and in protecting and restarting stalled DNA replication forks.13 The predominant mechanisms of action of PARP inhibitors in cells with homologous-recombination deficiency (HRD) include inhibiting repair of single-strand DNA breaks and trapping PARP-DNA complexes at stalled DNA replication forks.14
Germline or somatic BRCA1/2 mutations and genetic alterations resulting in HRD are present in about 20% and 30% of ovarian carcinomas, respectively, and increase the susceptibility of tumors to platinum-based agents and PARP inhibitors.15,16 Based on multiple clinical trials that demonstrated the efficacy of single-agent PARP in the treatment of recurrent ovarian carcinoma and as maintenance therapy after an initial response to platinum-based therapy, the US Food and Drug Administration approved olaparib, niraparib, and rucaparib for the treatment of high-grade epithelial ovarian cancer.17-19 Only olaparib is approved for maintenance therapy after initial adjuvant therapy in patients with BRCA mutations.20
Given the robust response to PARP inhibitors, there has been great interest in using these agents earlier in the disease course in combination with chemotherapy.
Efficacy of veliparib with chemotherapy and as maintenance monotherapy
In a randomized, double-blind, placebo-controlled phase 3 trial, Coleman and colleagues sought to determine the efficacy of the PARP inhibitor veliparib when administered with first-line carboplatin and paclitaxel induction chemotherapy and subsequently continued as maintenance monotherapy.21
Women with stage III or IV high-grade epithelial ovarian, fallopian tube, or primary peritoneal carcinoma were eligible for the study. Cytoreductive surgery could be performed prior to the initiation of trial treatment or after 3 cycles of chemotherapy.
Participants were randomized in a 1:1:1 ratio: 371 women received carboplatin and paclitaxel plus placebo followed by placebo maintenance (control arm); 376 received chemotherapy plus veliparib followed by placebo maintenance (veliparib combination-only arm); and 377 received chemotherapy plus veliparib followed by veliparib maintenance therapy (veliparib-throughout arm). Combination chemotherapy consisted of 6 cycles, and maintenance therapy was an additional 30 cycles.
Progression-free survival extended
At a median follow-up of 28 months, investigators observed a significant improvement in progression-free survival in the veliparib-throughout (initial and maintenance therapy) arm compared with the control arm in 3 cohorts: the BRCA-mutation cohort, the HRD cohort, and the intention-to-treat population (all participants undergoing randomization).
In the BRCA-mutation cohort, the median progression-free survival was 12.7 months longer in the veliparib-throughout arm than in the control arm. Similarly, in the HRD cohort, the median progression-free survival was 11.4 months longer in the veliparib-throughout arm than in the control group. In the intention-to-treat population, the median progression-free survival increased from 17.3 to 23.5 months in the veliparib-throughout arm compared with the control arm.
Women who received veliparib experienced increased rates of nausea, anemia, and fatigue and were more likely to require dose reductions and treatment interruptions. Myelodysplastic syndrome was reported in 1 patient (BRCA1 positive) in the veliparib combination-only arm.
For women with newly diagnosed, previously untreated stage III or IV high-grade serous ovarian carcinoma, carboplatin, paclitaxel, and veliparib induction therapy followed by single-agent veliparib maintenance therapy resulted in a significant improvement in median progression-free survival compared with induction chemotherapy alone. However, veliparib use was also associated with a higher incidence of adverse effects that required dose reduction and/or interruption during both the combination and maintenance phases of treatment.
Secondary cytoreductive surgery or chemotherapy alone for platinum-sensitive recurrent ovarian carcinoma?
Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
Primary surgical cytoreduction combined with platinum and taxane-based chemotherapy remains the mainstay of ovarian cancer treatment.11 The role of surgery for women with recurrent ovarian cancer, so-called secondary cytoreduction, remains controversial.22
Data have shown that among women who undergo secondary surgery, those with little or no postoperative residual disease benefit the most from a secondary debulking.23-26 Prior work largely is based on small retrospective reports and is limited by substantial bias in the selection of patients undergoing surgery. Additionally, with the availability of targeted therapies such as bevacizumab and PARP inhibitors as maintenance—medical interventions with a demonstrated benefit in progression-free survival17-19,27—the role of secondary cytoreduction in the treatment of ovarian carcinoma needs to be clarified.
Continue to: Overall survival after secondary cytoreduction followed by chemotherapy...
Overall survival after secondary cytoreduction followed by chemotherapy
Coleman and colleagues conducted a prospective, multicenter, international, randomized phase 3 trial to assess whether secondary cytoreductive surgery followed by chemotherapy would improve overall survival versus chemotherapy alone among women with resectable platinum-sensitive, recurrent ovarian cancer.22 Platinum sensitivity was defined as a disease-free interval of at least 6 months after the last cycle of platinum-based chemotherapy.
All women had recurrent epithelial ovarian carcinoma considered to be amenable to complete gross surgical resection by the investigator and a history of complete response to at least 3 cycles of platinum-based chemotherapy as determined by a normal CA-125 value or negative imaging studies (if obtained).
Participants were randomly assigned 1:1, with 240 women assigned to secondary surgical cytoreduction followed by platinum-based chemotherapy, and 245 assigned to chemotherapy alone. The type of adjuvant chemotherapy used (carboplatin-paclitaxel or carboplatin-gemcitabine) and whether or not bevacizumab was administered were at the investigators' discretion.
Shorter survival, decline in quality of life
Among the participants assigned to and who underwent surgery, complete gross resection was achieved in 67%. Eighty-four percent of the entire study population received platinum-based chemotherapy with bevacizumab followed by bevacizumab maintenance therapy, which was equally distributed between the 2 study arms.
At a median follow-up of 48.1 months, median overall survival was 50.6 months in the surgery arm compared with 64.7 months in the chemotherapy arm, corresponding to a hazard ratio (HR) for death of 1.29 (95% confidence interval [CI], 0.97-1.72; P = .08). This effect was unchanged after adjusting for platinum-free interval, chemotherapy choice, and restricting the analysis to women who had a complete gross resection.
Similarly, the adjusted HR for disease progression or death was 0.82 (95% CI, 0.66-1.01) and corresponded to a median progression-free survival of 18.9 months for the surgery group and 16.2 months for the chemotherapy group. Surgical morbidity was reported in 9% of patients who underwent surgery, and 1 patient (0.4%) died from postoperative complications.
While a significant decline in both quality of life and patient-reported outcomes was reported immediately after surgery, significant differences were not noted between the 2 groups after the initial postoperative recovery period.
For women with platinum-sensitive, recurrent ovarian cancer, a secondary cytoreductive surgery followed by chemotherapy was not associated with an improvement in overall survival when compared with chemotherapy alone. Secondary cytoreductive surgery should not be used routinely in women with recurrent ovarian cancer.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;20:7-34.
- Colombo N, Creutzberg C, Amant F, et al; ESMO-ESGOESTRO Endometrial Consensus Conference Working Group. ESMO-ESGO-ESTRO consensus conference on endometrial cancer: diagnosis, treatment and follow-up. Ann Oncol. 2016;27:16-41.
- Randall ME, Filiaci VL, Muss H, et al; Gynecologic Oncology Group Study. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2006;24:36-44.
- Syeda S, Chen L, Hou JY, et al. Chemotherapy, radiation, or combination therapy for stage III uterine cancer. Obstet Gynecol. 2019;134:17-29.
- Maggi R, Lissoni A, Spina F, et al. Adjuvant chemotherapy vs radiotherapy in high-risk endometrial carcinoma: results of a randomised trial. Br J Cancer. 2006;95:266-271.
- Susumu N, Sagae S, Udagawa Y, et al; Japanese Gynecologic Oncology Group. Randomized phase III trial of pelvic radiotherapy versus cisplatin-based combined chemotherapy in patients with intermediate- and high-risk endometrial cancer: a Japanese Gynecologic Oncology Group study. Gynecol Oncol. 2008;108:226-233.
- de Boer SM, Powell ME, Mileshkin L, et al; PORTEC Study Group. Adjuvant chemoradiotherapy versus radiotherapy alone in women with high-risk endometrial cancer (PORTEC-3): patterns of recurrence and post-hoc survival analysis of a randomised phase 3 trial. Lancet Oncol. 2019;20:1273-1285.
- Randall ME, Filiaci V, McMeekin DS, et al. Phase III trial: adjuvant pelvic radiation therapy versus vaginal brachytherapy plus paclitaxel/carboplatin in high-intermediate and high-risk early stage endometrial cancer. J Clin Oncol. 2019;37:1810-1818.
- Matei D, Filiaci V, Randall ME, et al. Adjuvant chemotherapy plus radiation for locally advanced endometrial cancer. N Engl J Med. 2019;380:2317-2326.
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424.
- Armstrong DK, Alvarez RD, Bakkum-Gamez JN, et al. NCCN guidelines insights: ovarian cancer, version 1.2019. J Natl Compr Canc Netw. 2019;17:896-909.
- Ledermann JA, Raja FA, Fotopoulou C, et al. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(suppl 6):vi24-vi32.
- Moore KN, Mirza MR, Matulonis UA. The poly (ADP ribose) polymerase inhibitor niraparib: management of toxicities. Gynecol Oncol. 2018;149:214-220.
- Konstantinopoulos PA, Matulonis UA. PARP inhibitors in ovarian cancer: a trailblazing and transformative journey. Clin Cancer Res. 2018;24:4062-4065.
- Pennington KP, Walsh T, Harrell MI, et al. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer Res. 2014;20:764-775.
- Mukhopadhyay A, Plummer ER, Elattar A, et al. Clinicopathological features of homologous recombination-deficient epithelial ovarian cancers: sensitivity to PARP inhibitors, platinum, and survival. Cancer Res. 2012;72:5675-5682.
- Mirza MR, Monk BJ, Herrstedt J, et al; ENGOT-OV16/ NOVA Investigators. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375:2154-2164.
- Pujade-Lauraine E, Ledermann JA, Selle F, et al; SOLO2/ ENGOT-Ov21 Investigators. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1274-1284.
- Coleman RL, Oza AM, Lorusso D, et al; ARIEL3 Investigators. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:1949-1961.
- Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505.
- Coleman RL, Fleming GF, Brady MF, et al. Veliparib with firstline chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med. 2019;381:2403-2415.
- Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
- Bommert M, Harter P, Heitz F, et al. When should surgery be used for recurrent ovarian carcinoma? Clin Oncol (R Coll Radiol). 2018;30:493-497.
- Santillan A, Karam AK, Li AJ, et al. Secondary cytoreductive surgery for isolated nodal recurrence in patients with epithelial ovarian cancer. Gynecol Oncol. 2007;104:686-690.
- Zang RY, Harter P, Chi DS, et al. Predictors of survival in patients with recurrent ovarian cancer undergoing secondary cytoreductive surgery based on the pooled analysis of an international collaborative cohort. Br J Cancer. 2011;105:890-896.
- Chi DS, McCaughty K, Diaz JP, et al. Guidelines and selection criteria for secondary cytoreductive surgery in patients with recurrent, platinum-sensitive epithelial ovarian carcinoma. Cancer. 2006;106:1933-1939.
- Aghajanian C, Blank SV, Goff BA, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012;30: 2039-2045.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;20:7-34.
- Colombo N, Creutzberg C, Amant F, et al; ESMO-ESGOESTRO Endometrial Consensus Conference Working Group. ESMO-ESGO-ESTRO consensus conference on endometrial cancer: diagnosis, treatment and follow-up. Ann Oncol. 2016;27:16-41.
- Randall ME, Filiaci VL, Muss H, et al; Gynecologic Oncology Group Study. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2006;24:36-44.
- Syeda S, Chen L, Hou JY, et al. Chemotherapy, radiation, or combination therapy for stage III uterine cancer. Obstet Gynecol. 2019;134:17-29.
- Maggi R, Lissoni A, Spina F, et al. Adjuvant chemotherapy vs radiotherapy in high-risk endometrial carcinoma: results of a randomised trial. Br J Cancer. 2006;95:266-271.
- Susumu N, Sagae S, Udagawa Y, et al; Japanese Gynecologic Oncology Group. Randomized phase III trial of pelvic radiotherapy versus cisplatin-based combined chemotherapy in patients with intermediate- and high-risk endometrial cancer: a Japanese Gynecologic Oncology Group study. Gynecol Oncol. 2008;108:226-233.
- de Boer SM, Powell ME, Mileshkin L, et al; PORTEC Study Group. Adjuvant chemoradiotherapy versus radiotherapy alone in women with high-risk endometrial cancer (PORTEC-3): patterns of recurrence and post-hoc survival analysis of a randomised phase 3 trial. Lancet Oncol. 2019;20:1273-1285.
- Randall ME, Filiaci V, McMeekin DS, et al. Phase III trial: adjuvant pelvic radiation therapy versus vaginal brachytherapy plus paclitaxel/carboplatin in high-intermediate and high-risk early stage endometrial cancer. J Clin Oncol. 2019;37:1810-1818.
- Matei D, Filiaci V, Randall ME, et al. Adjuvant chemotherapy plus radiation for locally advanced endometrial cancer. N Engl J Med. 2019;380:2317-2326.
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424.
- Armstrong DK, Alvarez RD, Bakkum-Gamez JN, et al. NCCN guidelines insights: ovarian cancer, version 1.2019. J Natl Compr Canc Netw. 2019;17:896-909.
- Ledermann JA, Raja FA, Fotopoulou C, et al. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(suppl 6):vi24-vi32.
- Moore KN, Mirza MR, Matulonis UA. The poly (ADP ribose) polymerase inhibitor niraparib: management of toxicities. Gynecol Oncol. 2018;149:214-220.
- Konstantinopoulos PA, Matulonis UA. PARP inhibitors in ovarian cancer: a trailblazing and transformative journey. Clin Cancer Res. 2018;24:4062-4065.
- Pennington KP, Walsh T, Harrell MI, et al. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer Res. 2014;20:764-775.
- Mukhopadhyay A, Plummer ER, Elattar A, et al. Clinicopathological features of homologous recombination-deficient epithelial ovarian cancers: sensitivity to PARP inhibitors, platinum, and survival. Cancer Res. 2012;72:5675-5682.
- Mirza MR, Monk BJ, Herrstedt J, et al; ENGOT-OV16/ NOVA Investigators. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375:2154-2164.
- Pujade-Lauraine E, Ledermann JA, Selle F, et al; SOLO2/ ENGOT-Ov21 Investigators. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1274-1284.
- Coleman RL, Oza AM, Lorusso D, et al; ARIEL3 Investigators. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:1949-1961.
- Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505.
- Coleman RL, Fleming GF, Brady MF, et al. Veliparib with firstline chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med. 2019;381:2403-2415.
- Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
- Bommert M, Harter P, Heitz F, et al. When should surgery be used for recurrent ovarian carcinoma? Clin Oncol (R Coll Radiol). 2018;30:493-497.
- Santillan A, Karam AK, Li AJ, et al. Secondary cytoreductive surgery for isolated nodal recurrence in patients with epithelial ovarian cancer. Gynecol Oncol. 2007;104:686-690.
- Zang RY, Harter P, Chi DS, et al. Predictors of survival in patients with recurrent ovarian cancer undergoing secondary cytoreductive surgery based on the pooled analysis of an international collaborative cohort. Br J Cancer. 2011;105:890-896.
- Chi DS, McCaughty K, Diaz JP, et al. Guidelines and selection criteria for secondary cytoreductive surgery in patients with recurrent, platinum-sensitive epithelial ovarian carcinoma. Cancer. 2006;106:1933-1939.
- Aghajanian C, Blank SV, Goff BA, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012;30: 2039-2045.
The role of hysteroscopy in diagnosing endometrial cancer
For more than 45 years, gynecologists have used hysteroscopy to diagnose endometrial carcinoma and to associate morphologic descriptive terms with visual findings.1 Today, considerably more clinical evidence supports visual pattern recognition to assess the risk for and presence of endometrial carcinoma, improving observer-dependent biopsy of the most suspect lesions (VIDEO 1).
In this article, I discuss the clinical evolution of hysteroscopic pattern recognition of endometrial disease and review the visual findings that correlate with the likelihood of endometrial carcinoma. In addition, I have provided 9 short videos that show hysteroscopic views of various endometrial pathologies in the online version of this article at https://www.mdedge.com/obgyn.
Video 1. Endometrial carcinoma and visually directed biopsy

The negative hysteroscopic view defined
In 1989, Dr. Frank Loffer confirmed the diagnostic superiority of visually directed biopsy. He demonstrated the advantages of using hysteroscopy and directed biopsy in the evaluation of abnormal uterine bleeding (AUB) to obtain a more accurate diagnosis compared with dilation and curettage (D&C) alone (sensitivity, 98% vs 65%, respectively).2
Also derived from this work is the clinical application of the “negative hysteroscopic view” (NHV). Loffer used the following criteria to define the NHV: good visualization of the entire uterine cavity, no structural abnormalities of the cavity, and a uniformly thin, homogeneous-appearing endometrium without variations in thickness (TABLE 1). The last criterion can be expected to occur only in the early proliferative phase or in postmenopausal women.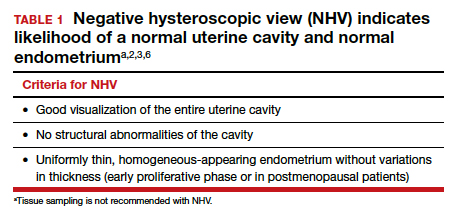
Use of hysteroscopy therefore can predict accurately the absence of intrauterine and endometrial pathology when visual findings are negative and tissue sampling is not warranted (FIGURE 1, VIDEO 2).
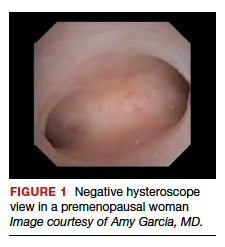
Video 2. Negative hysteroscopic view

Efforts in hysteroscopic classification of endometrial carcinoma
Lesion morphologic characteristics. Sugimoto was among the first to describe the hysteroscopic identification of visual morphologic features that are most likely to be associated with endometrial carcinoma.1 Patients with AUB were evaluated with hysteroscopy as first-line management to describe lesion morphology and confirm biopsy with histopathology. Sugimoto classified endometrial carcinoma as circumscribed or exophytic with distinct forms, such as polypoid, nodular, papillary, and ulcerated (FIGURE 2). Diffuse or endophytic carcinoma is defined by an ulcerated type of lesion that indicates necrosis; this is most likely to represent an undifferentiated tumor. Sugimoto also described abnormal vascularity that often is associated with carcinoma.1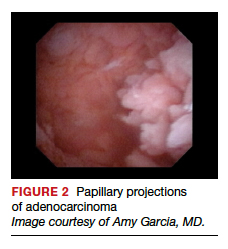
Endometrial features. Valli and Zupi created a nomenclature and classification for hysteroscopic endometrial lesions by prospectively grading 4 features: thickness, surface, vascularization, and color.3 Features were scored based on the degree of abnormality and could be considered to be of low or high risk for the presence of carcinoma. High-risk hysteroscopic features included endometrial thickness greater than 10 mm, polymorphous surface, irregular vascularization, and white-grayish color. The sensitivity for accurately diagnosing endometrial lesions was 86.9% for mild lesions and 96% for severe lesions.3 Also, these investigators confirmed the clinical value of the NHV and associated overall risk of precancer or cancer of the endometrium.
Continue to: Amount of endometrial involvement...
Amount of endometrial involvement. A few years later, Garuti and colleagues retrospectively related the hysteroscopic tumor features of known endometrial adenocarcinoma to stage, grade, and overall survival.4 In this system, they focused on classification of tumor morphology as nodular (bulging), polypoid (thin pedicles), or papillary (numerous dendritic projections), as well as whether the amount of abnormal tissue present was less than or more than half of the endometrium and if the lesion involved the cervix.
Several important findings associated with this system may improve visual diagnosis. First, hysteroscopic evaluation had a 100% negative predictive value for the cervical spread of disease (FIGURE 3, VIDEO 3). Second, the hysteroscopic morphologic tumor type did not relate to surgical stage or pathologic grade. Third, when less than half of the endometrium was involved, stage I disease was found (97%, 33 of 34). Last, when more than half of the endometrium was involved, advanced disease beyond stage I was found (9 of 26, 6 of whom had poorly differentiated disease).4
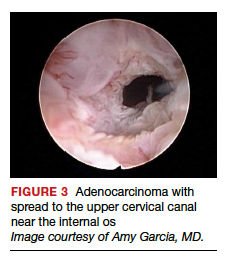
Video 3. Cervical spread of adenocarcinoma and visually directed biopsy

Structured pattern analysis. Recently, Dueholm and co-investigators published a prospective evaluation of women with postmenopausal bleeding and an endometrial thickness of 5 mm or greater.5 They used a structured system of visual pattern analysis during hysteroscopy that they termed the hysteroscopic cancer (HYCA) scoring system. The HYCA scoring system is based on surface outline (uneven, polypoid, and papillary projections), necrosis (cotton candy endometrium [FIGURE 4], whitish-grayish areas without vessels on the surface), and vessel pattern (tortuous S-shaped, loops, irregular caliber, irregular branching, and irregular distribution [FIGURE 5]). Structured pattern analysis predicted cancer with higher accuracy than subjective evaluation.5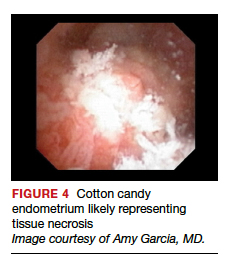
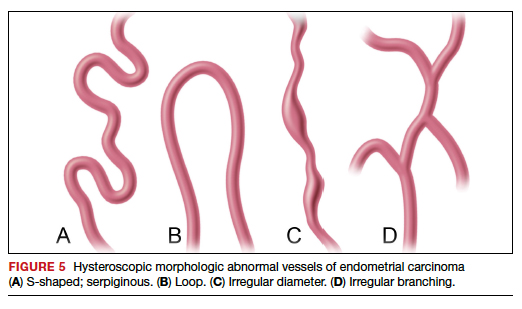
Morphologic variables as indicators. In 2016, Ianieri and colleagues published a retrospective study on a risk scoring system for diagnosing endometrial hyperplasia and adenocarcinoma via hysteroscopy.6 They created a statistical risk model for development of the scoring system. A number of morphologic variables were prognostic indicators of atypical endometrial hyperplasia (AEH) and adenocarcinoma. These included widespread and irregular endometrial thickness, presence of multiple polyps with irregular aspects, dilated glandular orifices, irregular endometrial color (grey, white, or hyperemic), atypical vessels, crumbling of the endometrial neoplasms, and growth of cerebroid and arborescent aspects (VIDEO 4).
Video 4. Endometrial adenocarcinoma

The scoring system for endometrial adenocarcinoma correctly classified 42 of 44 cancers (sensitivity, 95.4%; specificity, 98.2%), and AEH had a sensitivity of 63.3% and a specificity of 90.4%.6 These investigators also showed a high negative predictive value of 99.5% for endometrial adenocarcinoma associated with a negative view at hysteroscopy. Similar to the Dueholm data, Ianieri and colleagues’ morphologic pattern analysis predicted cancer with high accuracy.
Glomerular pattern association. Su and colleagues also showed that pattern recognition could aid in the accurate hysteroscopic diagnosis of endometrial adenocarcinoma.7 They used the hysteroscopic presence of a glomerular pattern to predict the association with endometrial adenocarcinoma. A glomerular pattern was described as polypoid endometrium with a papillary-like feature, containing an abnormal neovascularization feature with “intertwined neovascular vessels covered by a thin layer of endometrial tissue” (FIGURE 6). The presence of a glomerular pattern indicated grade 2 or grade 3 disease in 25 of 26 women (96%; sensitivity, 84.6%, specificity, 81.8%)7 (see video 4).
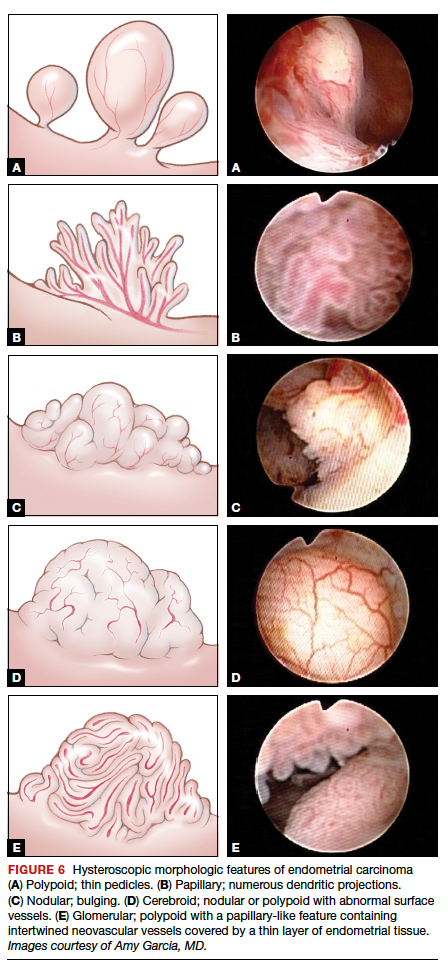
TABLE 2 summarizes significant morphologic findings relating to the presences of endometrial carcinoma.
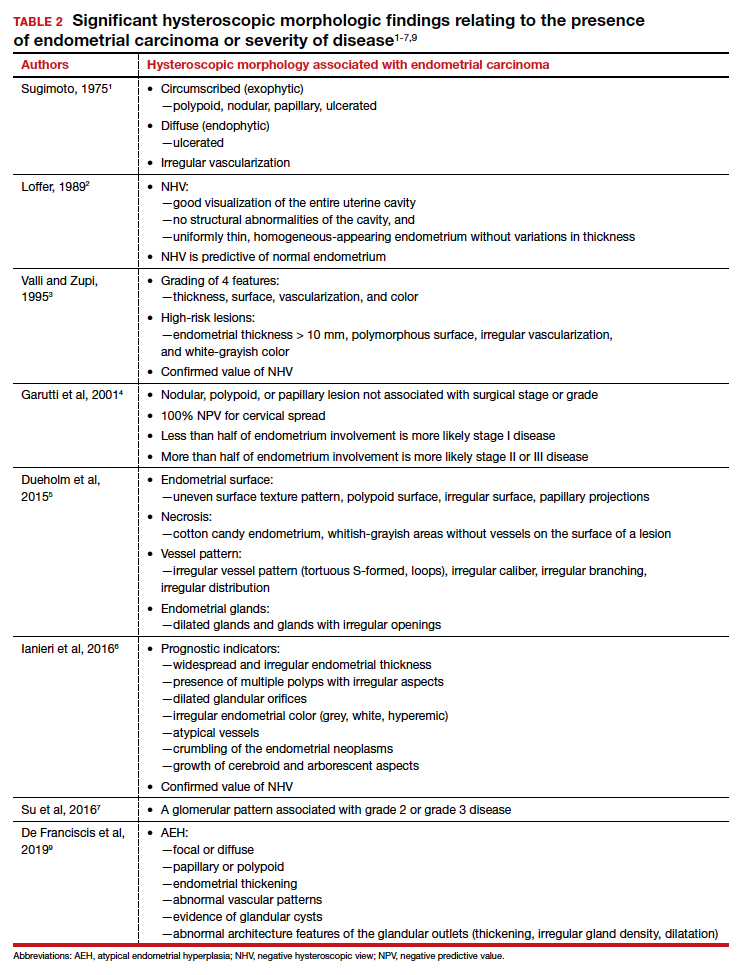
Continue to: Atypical endometrial hyperplasia: A difficult diagnosis...
Atypical endometrial hyperplasia: A difficult diagnosis
The most common type of endometrial cancer is endometrioid adenocarcinoma (type 1 endometrial carcinoma), and it accounts for approximately 75% to 80% of endometrial cancer diagnoses.8 Risk factors include prolonged unopposed estrogen exposure, obesity, diabetes, and age. Type 1 endometrial carcinoma follows a progressive continuum of histopathologic change: from endometrial hyperplasia without atypia to endometrial hyperplasia with atypia (AEH) to well-differentiated endometrial cancer. Therefore, it is possible for endometrial carcinoma to be present simultaneously with AEH. The reported prevalence of concurrent endometrial carcinoma among patients with AEH on biopsy is between 17% and 52%.8 Thus, the clinical consideration is for hysterectomy, especially in the postmenopausal patient with a diagnosis of AEH.
Hysteroscopic diagnosis of AEH, however, is more difficult than identification of endometrial carcinoma because a range of morphologic characteristics exist that resemble normal endometrium as well as more progressive disease (VIDEO 5). De Franciscis and colleagues based a hysteroscopic diagnosis of hyperplasia on one or more of the following findings: focal or diffuse, papillary or polypoid, endometrial thickening; abnormal vascular patterns; evidence of glandular cysts; and abnormal architecture features of the glandular outlets (thickening, irregular gland density, or dilatation)9 (VIDEO 6).
Video 5. Endometrial polyp and atypical hyperplasia

Additional studies, including that from Ianieri and colleagues, also have determined that AEH is difficult to discern visually from normal endometrium and other endometrial pathologies.6 In another investigation, Lasmar and coauthors reported a retrospective analysis of 4,054 hysteroscopic procedures with directed biopsies evaluating for concordance between the hysteroscopic view and histopathology.10 Agreement was 56.3% for AEH versus 94% for endometrial carcinoma. Among those with a histologic diagnosis of AEH, in 35.4% benign disease was suspected; in 2.1%, endometrial carcinoma was suspected; and in 6%, normal findings were presumed.10
Video 6. Nodular, polypoid atypical hyperplasia

Because of the similarities in morphologic features between AEH and endometrial carcinoma, tissue biopsy under direct visualization is warranted to assure sampling of the most significantly abnormal tissue and to confirm visual interpretation of findings.
Techniques for hysteroscopic-directed biopsy
Using a visual assessment of endometrial abnormalities allows the surgeon to examine the entire uterine cavity and to biopsy the most suspicious and concerning lesions. The directed biopsy technique can involve a simple grasping maneuver: With the jaws of a small grasper open, push slightly forward to accumulate tissue within the jaw, close the jaw, and remove the tissue carefully through the cervix (VIDEO 7). The size of the sample may be limited, and multiple samples may be needed, depending on the quantity of the tissue retrieved.
Video 7. Visually directed endometrial biopsy

Another technique involves first creating a plane of tissue to be removed with scissors and subsequently grasping and removing the tissue (see video 1 and video 3). This particular technique will yield more tissue with one pass of the hysteroscope into the cavity. Careful removal of tissue through the cervix is facilitated by withdrawing the sample in the grasper and the hysteroscope together at the same time, without pulling the sample through the operative channel of the hysteroscope. Also, by turning off the inflow port, the stream of saline does not wash the sample off the grasper at hysteroscope removal from the cervix.
Blind biopsy. If visual inspection reveals a diffuse process within the uterine cavity such that no normal endometrium is noted and the abnormality is of equal degree throughout the endometrial surface, a decision can be made to replace directed biopsy with a blind biopsy. In this scenario, the blind biopsy is certain to sample the representative disease process and not potentially miss significant lesions (see video 4 and video 6). Otherwise, the hysteroscope-directed biopsy would be preferable.
Continue to: Potential for intraperitoneal dissemination of endometrial cancer...
Potential for intraperitoneal dissemination of endometrial cancer
There is some concern about intraperitoneal dissemination of endometrial carcinoma at the time of hysteroscopy and effect on disease prognosis. Chang and colleagues conducted a large meta-analysis and found that hysteroscopy performed in the presence of type 1 endometrial carcinoma statistically significantly increased the likelihood of positive intraperitoneal cytology.11 In the included studies that reported survival rates (6 of 19), positive cytology did not alter the clinical outcome. The investigators recommended that hysteroscopy not be avoided for this reason, as it helps in the diagnosis of endometrial carcinoma, especially in the early stages of disease.11
In a recent retrospective analysis, Namazov and colleagues included only stage I endometrial carcinoma (to exclude the adverse effect of advanced stage on survival) and evaluated the assumed isolated effect of hysteroscopy on survival.12 They compared women in whom stage I endometrial carcinoma was diagnosed: 355 by hysteroscopy and 969 by a nonhysteroscopy method (D&C or office endometrial biopsy). Tumors were classified and grouped as low grade (endometrioid grade 1-2 and villoglandular) and high grade, consisting of endometrioid grade 3 and type 2 endometrial carcinoma (serous carcinoma, clear cell carcinoma, and carcinosarcoma) (VIDEOS 8 and 9). Positive intraperitoneal cytology at the time of surgery was 2.3% and 2.1% (P = .832), with an average interval from diagnosis to surgery of 34.6 days (range, 7–43 days).
Video 8. Carcinosarcoma

The authors proposed several explanations for the low rate of intraperitoneal cytology with hysteroscopy. One possibility is having lower mean intrauterine pressure below 100 mm Hg for saline uterine distension, although this was not standardized for all surgeons in the study but rather was a custom of the institution. In addition, the length of time between hysteroscopy and surgery may allow the immune-reactive peritoneum to respond to the cellular insult, thus decreasing the biologic burden at the time of surgery. The median follow-up was 52 months (range, 12–120 months), and there were no differences between the hysteroscopy and the nonhysteroscopy groups in the 5-year recurrence-free survival (90.2% vs 88.2%; P = .53), disease-specific survival (93.4% vs 91.7%; P = .5), and overall survival (86.2% vs 80.6%; P = .22). The authors concluded that hysteroscopy does not compromise the survival of patients with early-stage endometrial cancer.12
Video 9. Carcinosarcoma

Retrospective data from Chen and colleagues regarding type 2 endometrial carcinoma indicated a statistically significant increase in positive intraperitoneal cytology for carcinomas evaluated by hysteroscopy versus D&C (30% vs 12%; P = .008).13 Among the patients who died, there was no difference in disease-specific survival (53 months for hysteroscopy and 63.5 months for D&C; P = .34), and there was no difference in overall recurrence rates.13 Compared with type 1 endometrial carcinoma, type 2 endometrial carcinoma behaves more aggressively, with a higher incidence of extrauterine disease and an increased propensity for recurrence and poor outcome even in the early stages of the disease. This makes it difficult to determine the role of hysteroscopy in the prognosis of these carcinomas, especially in this study where most patients were diagnosed at a later stage.
Key takeaways
Hysteroscopy and directed biopsy are highly effective for visual and histopathologic diagnosis of atypical endometrial hyperplasia and endometrial carcinoma, and they are recommended in the evaluation of AUB, especially in the postmenopausal woman. When the hysteroscopic view is negative, there is a high correlation with the absence of uterine cavity and endometrial pathology. Hysteroscopic diagnostic accuracy is improved with structured use of visual grading scales, well-defined descriptors of endometrial pathology, and hysteroscopist experience.
Low operating intrauterine pressure may decrease the intraperitoneal spread of carcinoma cells during hysteroscopy, and current evidence suggests that there is no change in type 1 endometrial carcinoma prognosis and overall outcomes. Type 2 endometrial carcinoma is more aggressive and is associated with poor outcomes even in early stages, and the effect on disease progression by intraperitoneal spread of carcinoma cells at hysteroscopy is not yet known. Hysteroscopic evaluation of the uterine cavity and directed biopsy is easily and safely performed in the office and adds significantly to the evaluation and management of endometrial carcinoma.
Access them in the article online at mdedge.com/obgyn
Video 1. Endometrial carcinoma and visually directed biopsy
Nodular endometrioid adenocarcinoma grade 1 (type 1 endometrial carcinoma), benign endometrial polyps, and endometrial atrophy in a postmenopausal woman with bleeding. This video demonstrates visually directed biopsy to assure sampling of the most significant lesion.
Video 2. Negative hysteroscopic view
Digital flexible diagnostic hysteroscopy showing a negative hysteroscopic view in a premenopausal woman.
Video 3. Cervical spread of adenocarcinoma and visually directed biopsy
Diffuse endometrioid adenocarcinoma spread to the upper cervical canal near the internal cervical os. Hysteroscopic directed biopsy is performed.
Video 4. Endometrial adenocarcinoma
Fiberoptic flexible diagnostic hysteroscopy demonstrating diffuse endometrioid adenocarcinoma grade 3 with multiple morphologic features: polypoid, nodular, papillary, and glomerular with areas of necrosis.
Video 5. Endometrial polyp and atypical hyperplasia
Large benign endometrial polyp in an asymptomatic postmenopausal woman with enlarged endometrial stripe on pelvic ultrasound. The endometrium is atrophic except for a small whitish area on the anterior wall, which is atypical hyperplasia. This video highlights the need for visually directed biopsy to assure sampling of the most significant lesion.
Video 6. Nodular, polypoid atypical hyperplasia
Fiberoptic flexible diagnostic hysteroscopy showing diffuse nodular and polypoid atypical hyperplasia with abnormal glandular openings in a postmenopausal woman. Hysterectomy was performed secondary to the significant likelihood of concomitant endometrial carcinoma.
Video 7. Visually directed endometrial biopsy
Hysteroscopic-directed biopsy showing the technique of grasping and removing tissue of a benign adenomyosis cyst and proliferative endometrium.
Video 8. Carcinosarcoma
Carcinosarcoma (type 2 endometrial carcinoma) presents as a large intracavitary mass with soft, polypoid-like tissue in a symptomatic postmenopausal woman with bleeding.
Video 9. Carcinosarcoma
Carcinosarcoma (type 2 endometrial carcinoma) presents as a dense mass in a symptomatic postmenopausal woman with bleeding. This video shows the mass is nodular. These cancers typically grow into a spherical mass within the cavity
- Sugimoto O. Hysteroscopic diagnosis of endometrial carcinoma. A report of fifty-three cases examined at the Women’s Clinic of Kyoto University Hospital. Am J Obstet Gynecol. 1975;121:105-113.
- Loffer FD. Hysteroscopy with selective endometrial sampling compared with D&C for abnormal uterine bleeding: the value of a negative hysteroscopic view. Obstet Gynecol. 1989;73:16-20.
- Valli E, Zupi E. A new hysteroscopic classification of and nomenclature for endometrial lesions. J Am Assoc Gynecol Laparosc. 1995;2:279-283.
- Garuti G, De Giorgi O, Sambruni I, et al. Prognostic significance of hysteroscopic imaging in endometrioid endometrial adenocarcinoma. Gynecol Oncol. 2001;81: 408-413.
- Dueholm M, Hjorth IMD, Secher P, et al. Structured hysteroscopic evaluation of endometrium in women with postmenopausal bleeding. J Minim Invasive Gynecol. 2015;22:1215-1224.
- Ianieri MM, Staniscia T, Pontrelli G, et al. A new hysteroscopic risk scoring system for diagnosing endometrial hyperplasia and adenocarcinoma. J Minim Invasive Gynecol. 2016;23: 712-718.
- Su H, Pandey D, Liu L-Y, et al. Pattern recognition to prognosticate endometrial cancer: the science behind the art of office hysteroscopy—a retrospective study. Int J Gynecol Cancer. 2016;26:705-710.
- Trimble CL, Kauderer J, Zaino R, et al. Concurrent endometrial carcinoma in women with a biopsy diagnosis of atypical endometrial hyperplasia: a Gynecologic Oncology Group study. Cancer. 2006;106:812-819.
- De Franciscis P, Riemma G, Schiattarella A, et al. Concordance between the hysteroscopic diagnosis of endometrial hyperplasia and histopathological examination. Diagnostics (Basel). 2019;9(4).
- Lasmar RB, Barrozo PRM, de Oliveira MAP, et al. Validation of hysteroscopic view in cases of endometrial hyperplasia and cancer in patients with abnormal uterine bleeding. J Minim Invasive Gynecol. 2006;13:409-412.
- Chang Y-N, Zhang Y, Wang Y-J, et al. Effect of hysteroscopy on the peritoneal dissemination of endometrial cancer cells: a meta-analysis. Fertil Steril. 2011;96:957-961.
- Namazov A, Gemer O, Helpman L, et al. The oncological safety of hysteroscopy in the diagnosis of early-stage endometrial cancer: an Israel Gynecologic Oncology Group study. Eur J Obstet Gynecol Reprod Biol. 2019;243:120-124.
- Chen J, Clark LH, Kong W-M, et al. Does hysteroscopy worsen prognosis in women with type II endometrial carcinoma? PLoS One. 2017;12(3):e0174226.
For more than 45 years, gynecologists have used hysteroscopy to diagnose endometrial carcinoma and to associate morphologic descriptive terms with visual findings.1 Today, considerably more clinical evidence supports visual pattern recognition to assess the risk for and presence of endometrial carcinoma, improving observer-dependent biopsy of the most suspect lesions (VIDEO 1).
In this article, I discuss the clinical evolution of hysteroscopic pattern recognition of endometrial disease and review the visual findings that correlate with the likelihood of endometrial carcinoma. In addition, I have provided 9 short videos that show hysteroscopic views of various endometrial pathologies in the online version of this article at https://www.mdedge.com/obgyn.
Video 1. Endometrial carcinoma and visually directed biopsy

The negative hysteroscopic view defined
In 1989, Dr. Frank Loffer confirmed the diagnostic superiority of visually directed biopsy. He demonstrated the advantages of using hysteroscopy and directed biopsy in the evaluation of abnormal uterine bleeding (AUB) to obtain a more accurate diagnosis compared with dilation and curettage (D&C) alone (sensitivity, 98% vs 65%, respectively).2
Also derived from this work is the clinical application of the “negative hysteroscopic view” (NHV). Loffer used the following criteria to define the NHV: good visualization of the entire uterine cavity, no structural abnormalities of the cavity, and a uniformly thin, homogeneous-appearing endometrium without variations in thickness (TABLE 1). The last criterion can be expected to occur only in the early proliferative phase or in postmenopausal women.
Use of hysteroscopy therefore can predict accurately the absence of intrauterine and endometrial pathology when visual findings are negative and tissue sampling is not warranted (FIGURE 1, VIDEO 2).

Video 2. Negative hysteroscopic view

Efforts in hysteroscopic classification of endometrial carcinoma
Lesion morphologic characteristics. Sugimoto was among the first to describe the hysteroscopic identification of visual morphologic features that are most likely to be associated with endometrial carcinoma.1 Patients with AUB were evaluated with hysteroscopy as first-line management to describe lesion morphology and confirm biopsy with histopathology. Sugimoto classified endometrial carcinoma as circumscribed or exophytic with distinct forms, such as polypoid, nodular, papillary, and ulcerated (FIGURE 2). Diffuse or endophytic carcinoma is defined by an ulcerated type of lesion that indicates necrosis; this is most likely to represent an undifferentiated tumor. Sugimoto also described abnormal vascularity that often is associated with carcinoma.1
Endometrial features. Valli and Zupi created a nomenclature and classification for hysteroscopic endometrial lesions by prospectively grading 4 features: thickness, surface, vascularization, and color.3 Features were scored based on the degree of abnormality and could be considered to be of low or high risk for the presence of carcinoma. High-risk hysteroscopic features included endometrial thickness greater than 10 mm, polymorphous surface, irregular vascularization, and white-grayish color. The sensitivity for accurately diagnosing endometrial lesions was 86.9% for mild lesions and 96% for severe lesions.3 Also, these investigators confirmed the clinical value of the NHV and associated overall risk of precancer or cancer of the endometrium.
Continue to: Amount of endometrial involvement...
Amount of endometrial involvement. A few years later, Garuti and colleagues retrospectively related the hysteroscopic tumor features of known endometrial adenocarcinoma to stage, grade, and overall survival.4 In this system, they focused on classification of tumor morphology as nodular (bulging), polypoid (thin pedicles), or papillary (numerous dendritic projections), as well as whether the amount of abnormal tissue present was less than or more than half of the endometrium and if the lesion involved the cervix.
Several important findings associated with this system may improve visual diagnosis. First, hysteroscopic evaluation had a 100% negative predictive value for the cervical spread of disease (FIGURE 3, VIDEO 3). Second, the hysteroscopic morphologic tumor type did not relate to surgical stage or pathologic grade. Third, when less than half of the endometrium was involved, stage I disease was found (97%, 33 of 34). Last, when more than half of the endometrium was involved, advanced disease beyond stage I was found (9 of 26, 6 of whom had poorly differentiated disease).4

Video 3. Cervical spread of adenocarcinoma and visually directed biopsy

Structured pattern analysis. Recently, Dueholm and co-investigators published a prospective evaluation of women with postmenopausal bleeding and an endometrial thickness of 5 mm or greater.5 They used a structured system of visual pattern analysis during hysteroscopy that they termed the hysteroscopic cancer (HYCA) scoring system. The HYCA scoring system is based on surface outline (uneven, polypoid, and papillary projections), necrosis (cotton candy endometrium [FIGURE 4], whitish-grayish areas without vessels on the surface), and vessel pattern (tortuous S-shaped, loops, irregular caliber, irregular branching, and irregular distribution [FIGURE 5]). Structured pattern analysis predicted cancer with higher accuracy than subjective evaluation.5

Morphologic variables as indicators. In 2016, Ianieri and colleagues published a retrospective study on a risk scoring system for diagnosing endometrial hyperplasia and adenocarcinoma via hysteroscopy.6 They created a statistical risk model for development of the scoring system. A number of morphologic variables were prognostic indicators of atypical endometrial hyperplasia (AEH) and adenocarcinoma. These included widespread and irregular endometrial thickness, presence of multiple polyps with irregular aspects, dilated glandular orifices, irregular endometrial color (grey, white, or hyperemic), atypical vessels, crumbling of the endometrial neoplasms, and growth of cerebroid and arborescent aspects (VIDEO 4).
Video 4. Endometrial adenocarcinoma

The scoring system for endometrial adenocarcinoma correctly classified 42 of 44 cancers (sensitivity, 95.4%; specificity, 98.2%), and AEH had a sensitivity of 63.3% and a specificity of 90.4%.6 These investigators also showed a high negative predictive value of 99.5% for endometrial adenocarcinoma associated with a negative view at hysteroscopy. Similar to the Dueholm data, Ianieri and colleagues’ morphologic pattern analysis predicted cancer with high accuracy.
Glomerular pattern association. Su and colleagues also showed that pattern recognition could aid in the accurate hysteroscopic diagnosis of endometrial adenocarcinoma.7 They used the hysteroscopic presence of a glomerular pattern to predict the association with endometrial adenocarcinoma. A glomerular pattern was described as polypoid endometrium with a papillary-like feature, containing an abnormal neovascularization feature with “intertwined neovascular vessels covered by a thin layer of endometrial tissue” (FIGURE 6). The presence of a glomerular pattern indicated grade 2 or grade 3 disease in 25 of 26 women (96%; sensitivity, 84.6%, specificity, 81.8%)7 (see video 4).

TABLE 2 summarizes significant morphologic findings relating to the presences of endometrial carcinoma.

Continue to: Atypical endometrial hyperplasia: A difficult diagnosis...
Atypical endometrial hyperplasia: A difficult diagnosis
The most common type of endometrial cancer is endometrioid adenocarcinoma (type 1 endometrial carcinoma), and it accounts for approximately 75% to 80% of endometrial cancer diagnoses.8 Risk factors include prolonged unopposed estrogen exposure, obesity, diabetes, and age. Type 1 endometrial carcinoma follows a progressive continuum of histopathologic change: from endometrial hyperplasia without atypia to endometrial hyperplasia with atypia (AEH) to well-differentiated endometrial cancer. Therefore, it is possible for endometrial carcinoma to be present simultaneously with AEH. The reported prevalence of concurrent endometrial carcinoma among patients with AEH on biopsy is between 17% and 52%.8 Thus, the clinical consideration is for hysterectomy, especially in the postmenopausal patient with a diagnosis of AEH.
Hysteroscopic diagnosis of AEH, however, is more difficult than identification of endometrial carcinoma because a range of morphologic characteristics exist that resemble normal endometrium as well as more progressive disease (VIDEO 5). De Franciscis and colleagues based a hysteroscopic diagnosis of hyperplasia on one or more of the following findings: focal or diffuse, papillary or polypoid, endometrial thickening; abnormal vascular patterns; evidence of glandular cysts; and abnormal architecture features of the glandular outlets (thickening, irregular gland density, or dilatation)9 (VIDEO 6).
Video 5. Endometrial polyp and atypical hyperplasia

Additional studies, including that from Ianieri and colleagues, also have determined that AEH is difficult to discern visually from normal endometrium and other endometrial pathologies.6 In another investigation, Lasmar and coauthors reported a retrospective analysis of 4,054 hysteroscopic procedures with directed biopsies evaluating for concordance between the hysteroscopic view and histopathology.10 Agreement was 56.3% for AEH versus 94% for endometrial carcinoma. Among those with a histologic diagnosis of AEH, in 35.4% benign disease was suspected; in 2.1%, endometrial carcinoma was suspected; and in 6%, normal findings were presumed.10
Video 6. Nodular, polypoid atypical hyperplasia

Because of the similarities in morphologic features between AEH and endometrial carcinoma, tissue biopsy under direct visualization is warranted to assure sampling of the most significantly abnormal tissue and to confirm visual interpretation of findings.
Techniques for hysteroscopic-directed biopsy
Using a visual assessment of endometrial abnormalities allows the surgeon to examine the entire uterine cavity and to biopsy the most suspicious and concerning lesions. The directed biopsy technique can involve a simple grasping maneuver: With the jaws of a small grasper open, push slightly forward to accumulate tissue within the jaw, close the jaw, and remove the tissue carefully through the cervix (VIDEO 7). The size of the sample may be limited, and multiple samples may be needed, depending on the quantity of the tissue retrieved.
Video 7. Visually directed endometrial biopsy

Another technique involves first creating a plane of tissue to be removed with scissors and subsequently grasping and removing the tissue (see video 1 and video 3). This particular technique will yield more tissue with one pass of the hysteroscope into the cavity. Careful removal of tissue through the cervix is facilitated by withdrawing the sample in the grasper and the hysteroscope together at the same time, without pulling the sample through the operative channel of the hysteroscope. Also, by turning off the inflow port, the stream of saline does not wash the sample off the grasper at hysteroscope removal from the cervix.
Blind biopsy. If visual inspection reveals a diffuse process within the uterine cavity such that no normal endometrium is noted and the abnormality is of equal degree throughout the endometrial surface, a decision can be made to replace directed biopsy with a blind biopsy. In this scenario, the blind biopsy is certain to sample the representative disease process and not potentially miss significant lesions (see video 4 and video 6). Otherwise, the hysteroscope-directed biopsy would be preferable.
Continue to: Potential for intraperitoneal dissemination of endometrial cancer...
Potential for intraperitoneal dissemination of endometrial cancer
There is some concern about intraperitoneal dissemination of endometrial carcinoma at the time of hysteroscopy and effect on disease prognosis. Chang and colleagues conducted a large meta-analysis and found that hysteroscopy performed in the presence of type 1 endometrial carcinoma statistically significantly increased the likelihood of positive intraperitoneal cytology.11 In the included studies that reported survival rates (6 of 19), positive cytology did not alter the clinical outcome. The investigators recommended that hysteroscopy not be avoided for this reason, as it helps in the diagnosis of endometrial carcinoma, especially in the early stages of disease.11
In a recent retrospective analysis, Namazov and colleagues included only stage I endometrial carcinoma (to exclude the adverse effect of advanced stage on survival) and evaluated the assumed isolated effect of hysteroscopy on survival.12 They compared women in whom stage I endometrial carcinoma was diagnosed: 355 by hysteroscopy and 969 by a nonhysteroscopy method (D&C or office endometrial biopsy). Tumors were classified and grouped as low grade (endometrioid grade 1-2 and villoglandular) and high grade, consisting of endometrioid grade 3 and type 2 endometrial carcinoma (serous carcinoma, clear cell carcinoma, and carcinosarcoma) (VIDEOS 8 and 9). Positive intraperitoneal cytology at the time of surgery was 2.3% and 2.1% (P = .832), with an average interval from diagnosis to surgery of 34.6 days (range, 7–43 days).
Video 8. Carcinosarcoma

The authors proposed several explanations for the low rate of intraperitoneal cytology with hysteroscopy. One possibility is having lower mean intrauterine pressure below 100 mm Hg for saline uterine distension, although this was not standardized for all surgeons in the study but rather was a custom of the institution. In addition, the length of time between hysteroscopy and surgery may allow the immune-reactive peritoneum to respond to the cellular insult, thus decreasing the biologic burden at the time of surgery. The median follow-up was 52 months (range, 12–120 months), and there were no differences between the hysteroscopy and the nonhysteroscopy groups in the 5-year recurrence-free survival (90.2% vs 88.2%; P = .53), disease-specific survival (93.4% vs 91.7%; P = .5), and overall survival (86.2% vs 80.6%; P = .22). The authors concluded that hysteroscopy does not compromise the survival of patients with early-stage endometrial cancer.12
Video 9. Carcinosarcoma

Retrospective data from Chen and colleagues regarding type 2 endometrial carcinoma indicated a statistically significant increase in positive intraperitoneal cytology for carcinomas evaluated by hysteroscopy versus D&C (30% vs 12%; P = .008).13 Among the patients who died, there was no difference in disease-specific survival (53 months for hysteroscopy and 63.5 months for D&C; P = .34), and there was no difference in overall recurrence rates.13 Compared with type 1 endometrial carcinoma, type 2 endometrial carcinoma behaves more aggressively, with a higher incidence of extrauterine disease and an increased propensity for recurrence and poor outcome even in the early stages of the disease. This makes it difficult to determine the role of hysteroscopy in the prognosis of these carcinomas, especially in this study where most patients were diagnosed at a later stage.
Key takeaways
Hysteroscopy and directed biopsy are highly effective for visual and histopathologic diagnosis of atypical endometrial hyperplasia and endometrial carcinoma, and they are recommended in the evaluation of AUB, especially in the postmenopausal woman. When the hysteroscopic view is negative, there is a high correlation with the absence of uterine cavity and endometrial pathology. Hysteroscopic diagnostic accuracy is improved with structured use of visual grading scales, well-defined descriptors of endometrial pathology, and hysteroscopist experience.
Low operating intrauterine pressure may decrease the intraperitoneal spread of carcinoma cells during hysteroscopy, and current evidence suggests that there is no change in type 1 endometrial carcinoma prognosis and overall outcomes. Type 2 endometrial carcinoma is more aggressive and is associated with poor outcomes even in early stages, and the effect on disease progression by intraperitoneal spread of carcinoma cells at hysteroscopy is not yet known. Hysteroscopic evaluation of the uterine cavity and directed biopsy is easily and safely performed in the office and adds significantly to the evaluation and management of endometrial carcinoma.
Access them in the article online at mdedge.com/obgyn
Video 1. Endometrial carcinoma and visually directed biopsy
Nodular endometrioid adenocarcinoma grade 1 (type 1 endometrial carcinoma), benign endometrial polyps, and endometrial atrophy in a postmenopausal woman with bleeding. This video demonstrates visually directed biopsy to assure sampling of the most significant lesion.
Video 2. Negative hysteroscopic view
Digital flexible diagnostic hysteroscopy showing a negative hysteroscopic view in a premenopausal woman.
Video 3. Cervical spread of adenocarcinoma and visually directed biopsy
Diffuse endometrioid adenocarcinoma spread to the upper cervical canal near the internal cervical os. Hysteroscopic directed biopsy is performed.
Video 4. Endometrial adenocarcinoma
Fiberoptic flexible diagnostic hysteroscopy demonstrating diffuse endometrioid adenocarcinoma grade 3 with multiple morphologic features: polypoid, nodular, papillary, and glomerular with areas of necrosis.
Video 5. Endometrial polyp and atypical hyperplasia
Large benign endometrial polyp in an asymptomatic postmenopausal woman with enlarged endometrial stripe on pelvic ultrasound. The endometrium is atrophic except for a small whitish area on the anterior wall, which is atypical hyperplasia. This video highlights the need for visually directed biopsy to assure sampling of the most significant lesion.
Video 6. Nodular, polypoid atypical hyperplasia
Fiberoptic flexible diagnostic hysteroscopy showing diffuse nodular and polypoid atypical hyperplasia with abnormal glandular openings in a postmenopausal woman. Hysterectomy was performed secondary to the significant likelihood of concomitant endometrial carcinoma.
Video 7. Visually directed endometrial biopsy
Hysteroscopic-directed biopsy showing the technique of grasping and removing tissue of a benign adenomyosis cyst and proliferative endometrium.
Video 8. Carcinosarcoma
Carcinosarcoma (type 2 endometrial carcinoma) presents as a large intracavitary mass with soft, polypoid-like tissue in a symptomatic postmenopausal woman with bleeding.
Video 9. Carcinosarcoma
Carcinosarcoma (type 2 endometrial carcinoma) presents as a dense mass in a symptomatic postmenopausal woman with bleeding. This video shows the mass is nodular. These cancers typically grow into a spherical mass within the cavity
For more than 45 years, gynecologists have used hysteroscopy to diagnose endometrial carcinoma and to associate morphologic descriptive terms with visual findings.1 Today, considerably more clinical evidence supports visual pattern recognition to assess the risk for and presence of endometrial carcinoma, improving observer-dependent biopsy of the most suspect lesions (VIDEO 1).
In this article, I discuss the clinical evolution of hysteroscopic pattern recognition of endometrial disease and review the visual findings that correlate with the likelihood of endometrial carcinoma. In addition, I have provided 9 short videos that show hysteroscopic views of various endometrial pathologies in the online version of this article at https://www.mdedge.com/obgyn.
Video 1. Endometrial carcinoma and visually directed biopsy

The negative hysteroscopic view defined
In 1989, Dr. Frank Loffer confirmed the diagnostic superiority of visually directed biopsy. He demonstrated the advantages of using hysteroscopy and directed biopsy in the evaluation of abnormal uterine bleeding (AUB) to obtain a more accurate diagnosis compared with dilation and curettage (D&C) alone (sensitivity, 98% vs 65%, respectively).2
Also derived from this work is the clinical application of the “negative hysteroscopic view” (NHV). Loffer used the following criteria to define the NHV: good visualization of the entire uterine cavity, no structural abnormalities of the cavity, and a uniformly thin, homogeneous-appearing endometrium without variations in thickness (TABLE 1). The last criterion can be expected to occur only in the early proliferative phase or in postmenopausal women.
Use of hysteroscopy therefore can predict accurately the absence of intrauterine and endometrial pathology when visual findings are negative and tissue sampling is not warranted (FIGURE 1, VIDEO 2).

Video 2. Negative hysteroscopic view

Efforts in hysteroscopic classification of endometrial carcinoma
Lesion morphologic characteristics. Sugimoto was among the first to describe the hysteroscopic identification of visual morphologic features that are most likely to be associated with endometrial carcinoma.1 Patients with AUB were evaluated with hysteroscopy as first-line management to describe lesion morphology and confirm biopsy with histopathology. Sugimoto classified endometrial carcinoma as circumscribed or exophytic with distinct forms, such as polypoid, nodular, papillary, and ulcerated (FIGURE 2). Diffuse or endophytic carcinoma is defined by an ulcerated type of lesion that indicates necrosis; this is most likely to represent an undifferentiated tumor. Sugimoto also described abnormal vascularity that often is associated with carcinoma.1
Endometrial features. Valli and Zupi created a nomenclature and classification for hysteroscopic endometrial lesions by prospectively grading 4 features: thickness, surface, vascularization, and color.3 Features were scored based on the degree of abnormality and could be considered to be of low or high risk for the presence of carcinoma. High-risk hysteroscopic features included endometrial thickness greater than 10 mm, polymorphous surface, irregular vascularization, and white-grayish color. The sensitivity for accurately diagnosing endometrial lesions was 86.9% for mild lesions and 96% for severe lesions.3 Also, these investigators confirmed the clinical value of the NHV and associated overall risk of precancer or cancer of the endometrium.
Continue to: Amount of endometrial involvement...
Amount of endometrial involvement. A few years later, Garuti and colleagues retrospectively related the hysteroscopic tumor features of known endometrial adenocarcinoma to stage, grade, and overall survival.4 In this system, they focused on classification of tumor morphology as nodular (bulging), polypoid (thin pedicles), or papillary (numerous dendritic projections), as well as whether the amount of abnormal tissue present was less than or more than half of the endometrium and if the lesion involved the cervix.
Several important findings associated with this system may improve visual diagnosis. First, hysteroscopic evaluation had a 100% negative predictive value for the cervical spread of disease (FIGURE 3, VIDEO 3). Second, the hysteroscopic morphologic tumor type did not relate to surgical stage or pathologic grade. Third, when less than half of the endometrium was involved, stage I disease was found (97%, 33 of 34). Last, when more than half of the endometrium was involved, advanced disease beyond stage I was found (9 of 26, 6 of whom had poorly differentiated disease).4

Video 3. Cervical spread of adenocarcinoma and visually directed biopsy

Structured pattern analysis. Recently, Dueholm and co-investigators published a prospective evaluation of women with postmenopausal bleeding and an endometrial thickness of 5 mm or greater.5 They used a structured system of visual pattern analysis during hysteroscopy that they termed the hysteroscopic cancer (HYCA) scoring system. The HYCA scoring system is based on surface outline (uneven, polypoid, and papillary projections), necrosis (cotton candy endometrium [FIGURE 4], whitish-grayish areas without vessels on the surface), and vessel pattern (tortuous S-shaped, loops, irregular caliber, irregular branching, and irregular distribution [FIGURE 5]). Structured pattern analysis predicted cancer with higher accuracy than subjective evaluation.5

Morphologic variables as indicators. In 2016, Ianieri and colleagues published a retrospective study on a risk scoring system for diagnosing endometrial hyperplasia and adenocarcinoma via hysteroscopy.6 They created a statistical risk model for development of the scoring system. A number of morphologic variables were prognostic indicators of atypical endometrial hyperplasia (AEH) and adenocarcinoma. These included widespread and irregular endometrial thickness, presence of multiple polyps with irregular aspects, dilated glandular orifices, irregular endometrial color (grey, white, or hyperemic), atypical vessels, crumbling of the endometrial neoplasms, and growth of cerebroid and arborescent aspects (VIDEO 4).
Video 4. Endometrial adenocarcinoma

The scoring system for endometrial adenocarcinoma correctly classified 42 of 44 cancers (sensitivity, 95.4%; specificity, 98.2%), and AEH had a sensitivity of 63.3% and a specificity of 90.4%.6 These investigators also showed a high negative predictive value of 99.5% for endometrial adenocarcinoma associated with a negative view at hysteroscopy. Similar to the Dueholm data, Ianieri and colleagues’ morphologic pattern analysis predicted cancer with high accuracy.
Glomerular pattern association. Su and colleagues also showed that pattern recognition could aid in the accurate hysteroscopic diagnosis of endometrial adenocarcinoma.7 They used the hysteroscopic presence of a glomerular pattern to predict the association with endometrial adenocarcinoma. A glomerular pattern was described as polypoid endometrium with a papillary-like feature, containing an abnormal neovascularization feature with “intertwined neovascular vessels covered by a thin layer of endometrial tissue” (FIGURE 6). The presence of a glomerular pattern indicated grade 2 or grade 3 disease in 25 of 26 women (96%; sensitivity, 84.6%, specificity, 81.8%)7 (see video 4).

TABLE 2 summarizes significant morphologic findings relating to the presences of endometrial carcinoma.

Continue to: Atypical endometrial hyperplasia: A difficult diagnosis...
Atypical endometrial hyperplasia: A difficult diagnosis
The most common type of endometrial cancer is endometrioid adenocarcinoma (type 1 endometrial carcinoma), and it accounts for approximately 75% to 80% of endometrial cancer diagnoses.8 Risk factors include prolonged unopposed estrogen exposure, obesity, diabetes, and age. Type 1 endometrial carcinoma follows a progressive continuum of histopathologic change: from endometrial hyperplasia without atypia to endometrial hyperplasia with atypia (AEH) to well-differentiated endometrial cancer. Therefore, it is possible for endometrial carcinoma to be present simultaneously with AEH. The reported prevalence of concurrent endometrial carcinoma among patients with AEH on biopsy is between 17% and 52%.8 Thus, the clinical consideration is for hysterectomy, especially in the postmenopausal patient with a diagnosis of AEH.
Hysteroscopic diagnosis of AEH, however, is more difficult than identification of endometrial carcinoma because a range of morphologic characteristics exist that resemble normal endometrium as well as more progressive disease (VIDEO 5). De Franciscis and colleagues based a hysteroscopic diagnosis of hyperplasia on one or more of the following findings: focal or diffuse, papillary or polypoid, endometrial thickening; abnormal vascular patterns; evidence of glandular cysts; and abnormal architecture features of the glandular outlets (thickening, irregular gland density, or dilatation)9 (VIDEO 6).
Video 5. Endometrial polyp and atypical hyperplasia

Additional studies, including that from Ianieri and colleagues, also have determined that AEH is difficult to discern visually from normal endometrium and other endometrial pathologies.6 In another investigation, Lasmar and coauthors reported a retrospective analysis of 4,054 hysteroscopic procedures with directed biopsies evaluating for concordance between the hysteroscopic view and histopathology.10 Agreement was 56.3% for AEH versus 94% for endometrial carcinoma. Among those with a histologic diagnosis of AEH, in 35.4% benign disease was suspected; in 2.1%, endometrial carcinoma was suspected; and in 6%, normal findings were presumed.10
Video 6. Nodular, polypoid atypical hyperplasia

Because of the similarities in morphologic features between AEH and endometrial carcinoma, tissue biopsy under direct visualization is warranted to assure sampling of the most significantly abnormal tissue and to confirm visual interpretation of findings.
Techniques for hysteroscopic-directed biopsy
Using a visual assessment of endometrial abnormalities allows the surgeon to examine the entire uterine cavity and to biopsy the most suspicious and concerning lesions. The directed biopsy technique can involve a simple grasping maneuver: With the jaws of a small grasper open, push slightly forward to accumulate tissue within the jaw, close the jaw, and remove the tissue carefully through the cervix (VIDEO 7). The size of the sample may be limited, and multiple samples may be needed, depending on the quantity of the tissue retrieved.
Video 7. Visually directed endometrial biopsy

Another technique involves first creating a plane of tissue to be removed with scissors and subsequently grasping and removing the tissue (see video 1 and video 3). This particular technique will yield more tissue with one pass of the hysteroscope into the cavity. Careful removal of tissue through the cervix is facilitated by withdrawing the sample in the grasper and the hysteroscope together at the same time, without pulling the sample through the operative channel of the hysteroscope. Also, by turning off the inflow port, the stream of saline does not wash the sample off the grasper at hysteroscope removal from the cervix.
Blind biopsy. If visual inspection reveals a diffuse process within the uterine cavity such that no normal endometrium is noted and the abnormality is of equal degree throughout the endometrial surface, a decision can be made to replace directed biopsy with a blind biopsy. In this scenario, the blind biopsy is certain to sample the representative disease process and not potentially miss significant lesions (see video 4 and video 6). Otherwise, the hysteroscope-directed biopsy would be preferable.
Continue to: Potential for intraperitoneal dissemination of endometrial cancer...
Potential for intraperitoneal dissemination of endometrial cancer
There is some concern about intraperitoneal dissemination of endometrial carcinoma at the time of hysteroscopy and effect on disease prognosis. Chang and colleagues conducted a large meta-analysis and found that hysteroscopy performed in the presence of type 1 endometrial carcinoma statistically significantly increased the likelihood of positive intraperitoneal cytology.11 In the included studies that reported survival rates (6 of 19), positive cytology did not alter the clinical outcome. The investigators recommended that hysteroscopy not be avoided for this reason, as it helps in the diagnosis of endometrial carcinoma, especially in the early stages of disease.11
In a recent retrospective analysis, Namazov and colleagues included only stage I endometrial carcinoma (to exclude the adverse effect of advanced stage on survival) and evaluated the assumed isolated effect of hysteroscopy on survival.12 They compared women in whom stage I endometrial carcinoma was diagnosed: 355 by hysteroscopy and 969 by a nonhysteroscopy method (D&C or office endometrial biopsy). Tumors were classified and grouped as low grade (endometrioid grade 1-2 and villoglandular) and high grade, consisting of endometrioid grade 3 and type 2 endometrial carcinoma (serous carcinoma, clear cell carcinoma, and carcinosarcoma) (VIDEOS 8 and 9). Positive intraperitoneal cytology at the time of surgery was 2.3% and 2.1% (P = .832), with an average interval from diagnosis to surgery of 34.6 days (range, 7–43 days).
Video 8. Carcinosarcoma

The authors proposed several explanations for the low rate of intraperitoneal cytology with hysteroscopy. One possibility is having lower mean intrauterine pressure below 100 mm Hg for saline uterine distension, although this was not standardized for all surgeons in the study but rather was a custom of the institution. In addition, the length of time between hysteroscopy and surgery may allow the immune-reactive peritoneum to respond to the cellular insult, thus decreasing the biologic burden at the time of surgery. The median follow-up was 52 months (range, 12–120 months), and there were no differences between the hysteroscopy and the nonhysteroscopy groups in the 5-year recurrence-free survival (90.2% vs 88.2%; P = .53), disease-specific survival (93.4% vs 91.7%; P = .5), and overall survival (86.2% vs 80.6%; P = .22). The authors concluded that hysteroscopy does not compromise the survival of patients with early-stage endometrial cancer.12
Video 9. Carcinosarcoma

Retrospective data from Chen and colleagues regarding type 2 endometrial carcinoma indicated a statistically significant increase in positive intraperitoneal cytology for carcinomas evaluated by hysteroscopy versus D&C (30% vs 12%; P = .008).13 Among the patients who died, there was no difference in disease-specific survival (53 months for hysteroscopy and 63.5 months for D&C; P = .34), and there was no difference in overall recurrence rates.13 Compared with type 1 endometrial carcinoma, type 2 endometrial carcinoma behaves more aggressively, with a higher incidence of extrauterine disease and an increased propensity for recurrence and poor outcome even in the early stages of the disease. This makes it difficult to determine the role of hysteroscopy in the prognosis of these carcinomas, especially in this study where most patients were diagnosed at a later stage.
Key takeaways
Hysteroscopy and directed biopsy are highly effective for visual and histopathologic diagnosis of atypical endometrial hyperplasia and endometrial carcinoma, and they are recommended in the evaluation of AUB, especially in the postmenopausal woman. When the hysteroscopic view is negative, there is a high correlation with the absence of uterine cavity and endometrial pathology. Hysteroscopic diagnostic accuracy is improved with structured use of visual grading scales, well-defined descriptors of endometrial pathology, and hysteroscopist experience.
Low operating intrauterine pressure may decrease the intraperitoneal spread of carcinoma cells during hysteroscopy, and current evidence suggests that there is no change in type 1 endometrial carcinoma prognosis and overall outcomes. Type 2 endometrial carcinoma is more aggressive and is associated with poor outcomes even in early stages, and the effect on disease progression by intraperitoneal spread of carcinoma cells at hysteroscopy is not yet known. Hysteroscopic evaluation of the uterine cavity and directed biopsy is easily and safely performed in the office and adds significantly to the evaluation and management of endometrial carcinoma.
Access them in the article online at mdedge.com/obgyn
Video 1. Endometrial carcinoma and visually directed biopsy
Nodular endometrioid adenocarcinoma grade 1 (type 1 endometrial carcinoma), benign endometrial polyps, and endometrial atrophy in a postmenopausal woman with bleeding. This video demonstrates visually directed biopsy to assure sampling of the most significant lesion.
Video 2. Negative hysteroscopic view
Digital flexible diagnostic hysteroscopy showing a negative hysteroscopic view in a premenopausal woman.
Video 3. Cervical spread of adenocarcinoma and visually directed biopsy
Diffuse endometrioid adenocarcinoma spread to the upper cervical canal near the internal cervical os. Hysteroscopic directed biopsy is performed.
Video 4. Endometrial adenocarcinoma
Fiberoptic flexible diagnostic hysteroscopy demonstrating diffuse endometrioid adenocarcinoma grade 3 with multiple morphologic features: polypoid, nodular, papillary, and glomerular with areas of necrosis.
Video 5. Endometrial polyp and atypical hyperplasia
Large benign endometrial polyp in an asymptomatic postmenopausal woman with enlarged endometrial stripe on pelvic ultrasound. The endometrium is atrophic except for a small whitish area on the anterior wall, which is atypical hyperplasia. This video highlights the need for visually directed biopsy to assure sampling of the most significant lesion.
Video 6. Nodular, polypoid atypical hyperplasia
Fiberoptic flexible diagnostic hysteroscopy showing diffuse nodular and polypoid atypical hyperplasia with abnormal glandular openings in a postmenopausal woman. Hysterectomy was performed secondary to the significant likelihood of concomitant endometrial carcinoma.
Video 7. Visually directed endometrial biopsy
Hysteroscopic-directed biopsy showing the technique of grasping and removing tissue of a benign adenomyosis cyst and proliferative endometrium.
Video 8. Carcinosarcoma
Carcinosarcoma (type 2 endometrial carcinoma) presents as a large intracavitary mass with soft, polypoid-like tissue in a symptomatic postmenopausal woman with bleeding.
Video 9. Carcinosarcoma
Carcinosarcoma (type 2 endometrial carcinoma) presents as a dense mass in a symptomatic postmenopausal woman with bleeding. This video shows the mass is nodular. These cancers typically grow into a spherical mass within the cavity
- Sugimoto O. Hysteroscopic diagnosis of endometrial carcinoma. A report of fifty-three cases examined at the Women’s Clinic of Kyoto University Hospital. Am J Obstet Gynecol. 1975;121:105-113.
- Loffer FD. Hysteroscopy with selective endometrial sampling compared with D&C for abnormal uterine bleeding: the value of a negative hysteroscopic view. Obstet Gynecol. 1989;73:16-20.
- Valli E, Zupi E. A new hysteroscopic classification of and nomenclature for endometrial lesions. J Am Assoc Gynecol Laparosc. 1995;2:279-283.
- Garuti G, De Giorgi O, Sambruni I, et al. Prognostic significance of hysteroscopic imaging in endometrioid endometrial adenocarcinoma. Gynecol Oncol. 2001;81: 408-413.
- Dueholm M, Hjorth IMD, Secher P, et al. Structured hysteroscopic evaluation of endometrium in women with postmenopausal bleeding. J Minim Invasive Gynecol. 2015;22:1215-1224.
- Ianieri MM, Staniscia T, Pontrelli G, et al. A new hysteroscopic risk scoring system for diagnosing endometrial hyperplasia and adenocarcinoma. J Minim Invasive Gynecol. 2016;23: 712-718.
- Su H, Pandey D, Liu L-Y, et al. Pattern recognition to prognosticate endometrial cancer: the science behind the art of office hysteroscopy—a retrospective study. Int J Gynecol Cancer. 2016;26:705-710.
- Trimble CL, Kauderer J, Zaino R, et al. Concurrent endometrial carcinoma in women with a biopsy diagnosis of atypical endometrial hyperplasia: a Gynecologic Oncology Group study. Cancer. 2006;106:812-819.
- De Franciscis P, Riemma G, Schiattarella A, et al. Concordance between the hysteroscopic diagnosis of endometrial hyperplasia and histopathological examination. Diagnostics (Basel). 2019;9(4).
- Lasmar RB, Barrozo PRM, de Oliveira MAP, et al. Validation of hysteroscopic view in cases of endometrial hyperplasia and cancer in patients with abnormal uterine bleeding. J Minim Invasive Gynecol. 2006;13:409-412.
- Chang Y-N, Zhang Y, Wang Y-J, et al. Effect of hysteroscopy on the peritoneal dissemination of endometrial cancer cells: a meta-analysis. Fertil Steril. 2011;96:957-961.
- Namazov A, Gemer O, Helpman L, et al. The oncological safety of hysteroscopy in the diagnosis of early-stage endometrial cancer: an Israel Gynecologic Oncology Group study. Eur J Obstet Gynecol Reprod Biol. 2019;243:120-124.
- Chen J, Clark LH, Kong W-M, et al. Does hysteroscopy worsen prognosis in women with type II endometrial carcinoma? PLoS One. 2017;12(3):e0174226.
- Sugimoto O. Hysteroscopic diagnosis of endometrial carcinoma. A report of fifty-three cases examined at the Women’s Clinic of Kyoto University Hospital. Am J Obstet Gynecol. 1975;121:105-113.
- Loffer FD. Hysteroscopy with selective endometrial sampling compared with D&C for abnormal uterine bleeding: the value of a negative hysteroscopic view. Obstet Gynecol. 1989;73:16-20.
- Valli E, Zupi E. A new hysteroscopic classification of and nomenclature for endometrial lesions. J Am Assoc Gynecol Laparosc. 1995;2:279-283.
- Garuti G, De Giorgi O, Sambruni I, et al. Prognostic significance of hysteroscopic imaging in endometrioid endometrial adenocarcinoma. Gynecol Oncol. 2001;81: 408-413.
- Dueholm M, Hjorth IMD, Secher P, et al. Structured hysteroscopic evaluation of endometrium in women with postmenopausal bleeding. J Minim Invasive Gynecol. 2015;22:1215-1224.
- Ianieri MM, Staniscia T, Pontrelli G, et al. A new hysteroscopic risk scoring system for diagnosing endometrial hyperplasia and adenocarcinoma. J Minim Invasive Gynecol. 2016;23: 712-718.
- Su H, Pandey D, Liu L-Y, et al. Pattern recognition to prognosticate endometrial cancer: the science behind the art of office hysteroscopy—a retrospective study. Int J Gynecol Cancer. 2016;26:705-710.
- Trimble CL, Kauderer J, Zaino R, et al. Concurrent endometrial carcinoma in women with a biopsy diagnosis of atypical endometrial hyperplasia: a Gynecologic Oncology Group study. Cancer. 2006;106:812-819.
- De Franciscis P, Riemma G, Schiattarella A, et al. Concordance between the hysteroscopic diagnosis of endometrial hyperplasia and histopathological examination. Diagnostics (Basel). 2019;9(4).
- Lasmar RB, Barrozo PRM, de Oliveira MAP, et al. Validation of hysteroscopic view in cases of endometrial hyperplasia and cancer in patients with abnormal uterine bleeding. J Minim Invasive Gynecol. 2006;13:409-412.
- Chang Y-N, Zhang Y, Wang Y-J, et al. Effect of hysteroscopy on the peritoneal dissemination of endometrial cancer cells: a meta-analysis. Fertil Steril. 2011;96:957-961.
- Namazov A, Gemer O, Helpman L, et al. The oncological safety of hysteroscopy in the diagnosis of early-stage endometrial cancer: an Israel Gynecologic Oncology Group study. Eur J Obstet Gynecol Reprod Biol. 2019;243:120-124.
- Chen J, Clark LH, Kong W-M, et al. Does hysteroscopy worsen prognosis in women with type II endometrial carcinoma? PLoS One. 2017;12(3):e0174226.