User login
Automobile Injury: A Common Familiar Risk for Presenting and Comparing Risks in Dermatology
Numerous highly efficacious treatment modalities exist in dermatology, yet patients may be highly wary of their possible adverse events, even when those risks are rare.1,2 Such fears can lead to poor medication adherence and treatment refusal. A key determinant in successful patient-provider care is to effectively communicate risk. The communication of risk is hampered by the lack of any common currency for comparing risks. The development of a standardized unit of risk could help facilitate risk comparisons, allowing physicians and patients to put risk levels into better perspective.
One easily relatable event is the risk of injury in an automobile crash. Driving, whether to the dermatology clinic for a monitoring visit or to the supermarket for weekly groceries, is associated with risk of injury and death. The risk of automobile-related injury warranting a visit to the emergency department could provide a comparator that physicians can use to give patients a more objective sense of treatment risks or to introduce the justification of a monitoring visit. The objective of this study was to develop a standard risk unit based on the lifetime risk (LTR) of automobile injury and to compare this unit of risk to various risks of dermatologic treatments.
Methods
Literature Review
We first identified common risks in dermatology that would be illustrative and then identified keywords. PubMed searches for articles indexed for MEDLINE from November 1996 to February 2017 were performed combining the following terms: (relative risk, odds ratio, lifetime risk) and (isotretinoin, IBD; melanoma, SCC, transplantation; indoor tanning, BCC, SCC; transplant and SCC; biologics and tuberculosis; hydroxychloroquine retinal toxicity; psoriasis and psoriatic arthritis). An additional search was performed in June 2018 including the term blindness and injectable fillers. Our search combined these terms in numerous ways. Results were focused on meta-analyses and observational studies.
The references of relevant studies were included. Articles not focused on meta-analyses but rather on observational studies were individually analyzed for quality and bias using the 9-point Newcastle-Ottawa Scale, with a score of 7 or more as a cutoff for inclusion.
Determination of Risk Comparators
Data from the 2016 National Safety Council’s Injury Facts report were searched for nonmedical-related risk comparators, such as the risk of death by dog attack, by lightning, and by fire or smoke.3 Data from the 2015 US Department of Transportation Traffic Safety Facts were searched for relatable risk comparators, such as the LTR of automobile death and injury.4
Definitions
Automobile injury was defined as an injury warranting a visit to the emergency department.5 Automobile was defined as a road vehicle with 4 wheels and powered by an internal combustion engine or electric motor.6 This definition excluded light trucks, large trucks, and motorcycles.
LTR Calculation
Lifetime risk was used as the comparative measure. Lifetime risk is a type of absolute risk that depicts the probability that a specific disease or event will occur in an individual’s lifespan. The LTRof developing a disease or adverse event due to a dermatologic therapy or interventionwas denoted as LTRadverse event and calculated by the following equation7,8:

In this equation, LTRgeneral population is the LTR of developing the disease or adverse event without being subject to the therapy or intervention, and RRintervention is the relative risk (RR) from previously published RR data (relating to the development of the disease in question or an adverse event of the intervention). The use of equation (1) holds true only when the absolute risk of developing the disease or adverse event (LTRgeneral population) is low.7 Although the calculation of an LTR using a constant lifetime RR may require major approximations, studies evaluating the variation of RR over time are sparse.7,9 The Newcastle-Ottawa Scale was used to control such variance; only high-quality, nonrandomized studies were included. Although the use of residual LTR would be preferable, as LTR depends on age, such epidemiological data do not exist for complex diseases.
When not available, the LTRgeneral population was calculated from the rate of disease (cases per 100,000 individuals per year) multiplied by the average lifespan of an American (78.8 years)10:
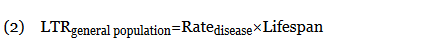
When an odds ratio (OR) was presented, its conversion to RR followed11:
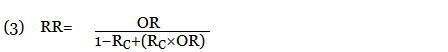
In this equation, RC is the absolute risk in the unexposed group. If the prevalence of the disease was considered low, the rare disease assumption was implemented as the following11,12:

The use of this approximation overestimates the LTR of an event. From a patient perspective, this approach is conservative. If prior LTR values were available, such as the LTR of automobile injury, automobile death, or other intervention, they were used without the need for calculation.
Unit Comparator
The LTRs of all adverse events were normalized to a unit comparator, using the LTR of an automobile injury as reference point, denoted as 1 risk unit (RU):

This equation allows for quick comparison of the magnitude of LTRs between events. Events with an RU less than 1 are less likely to occur than the risk of automobile injury; events with an RU greater than 1 are more likely than the risk of automobile injury. All RR, LTR, and unit comparators were presented as a single pooled estimate of their respective upper-limit CIs. The use of the upper-limit CI conservatively overestimates the LTR of an event.
Results
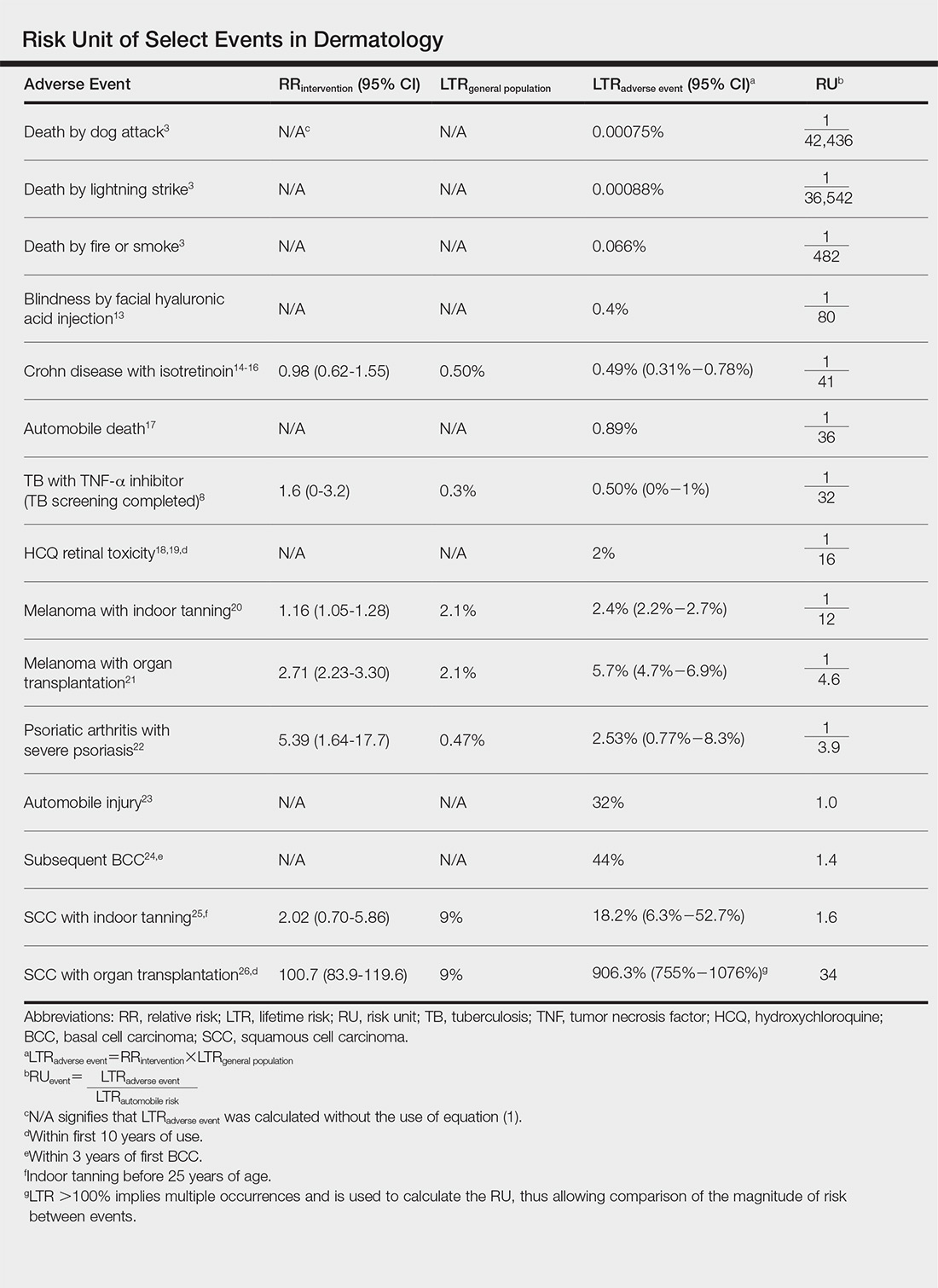
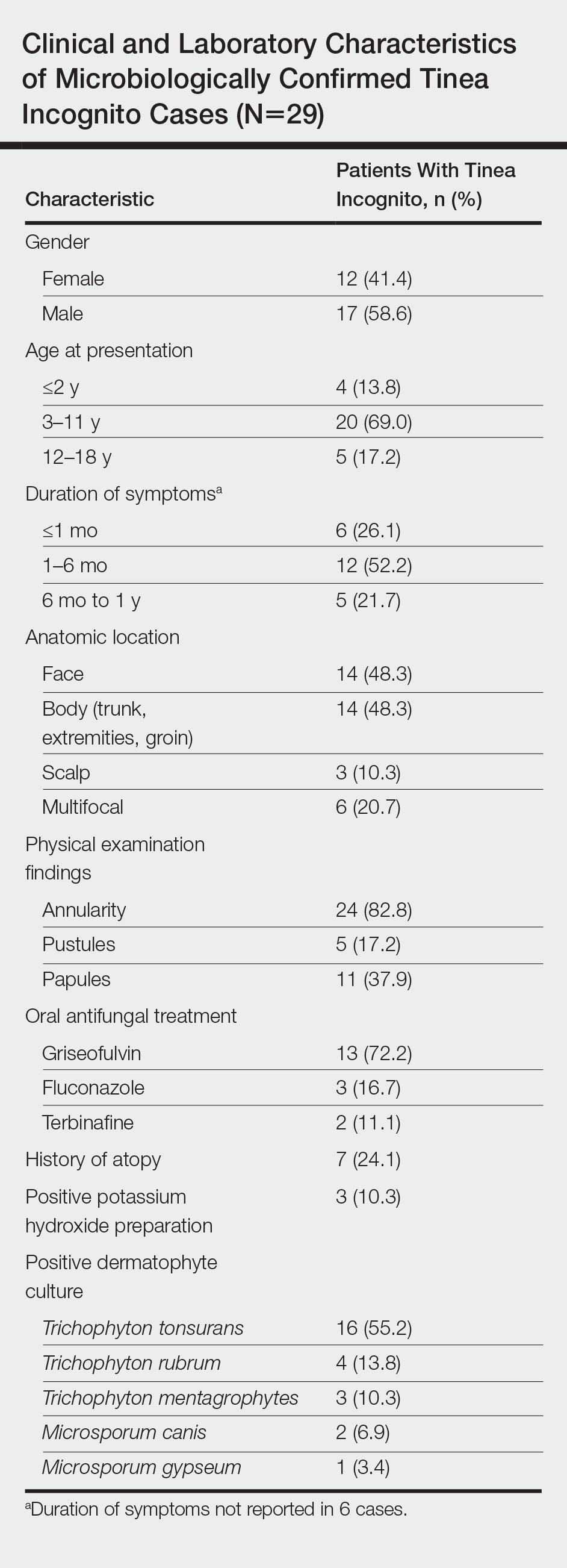
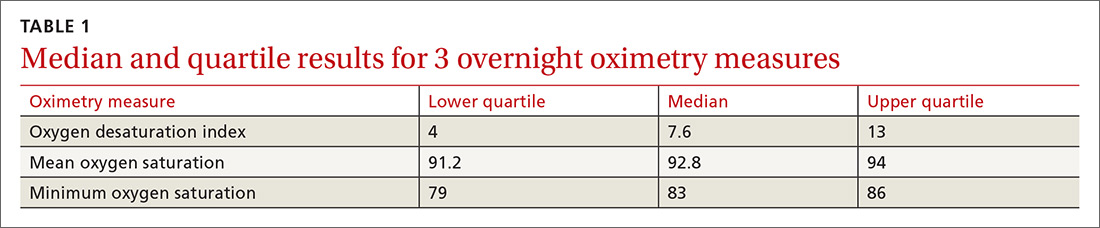
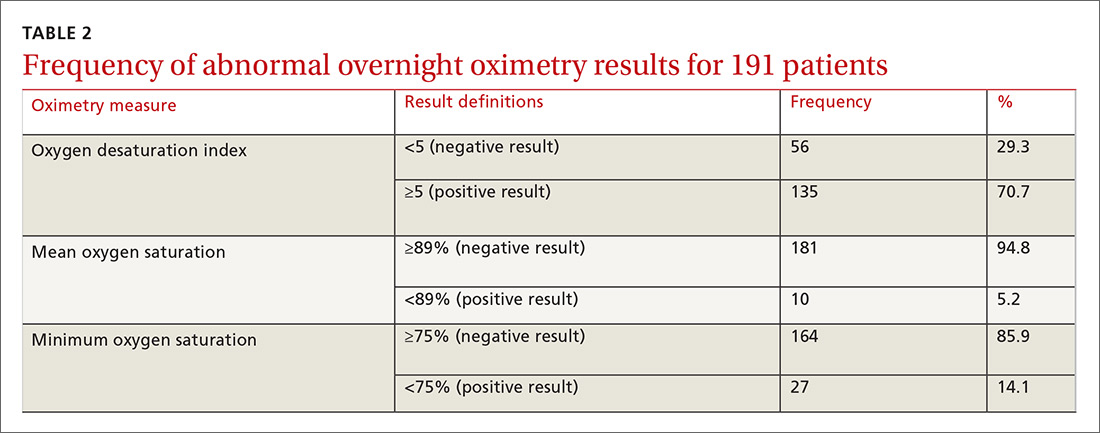
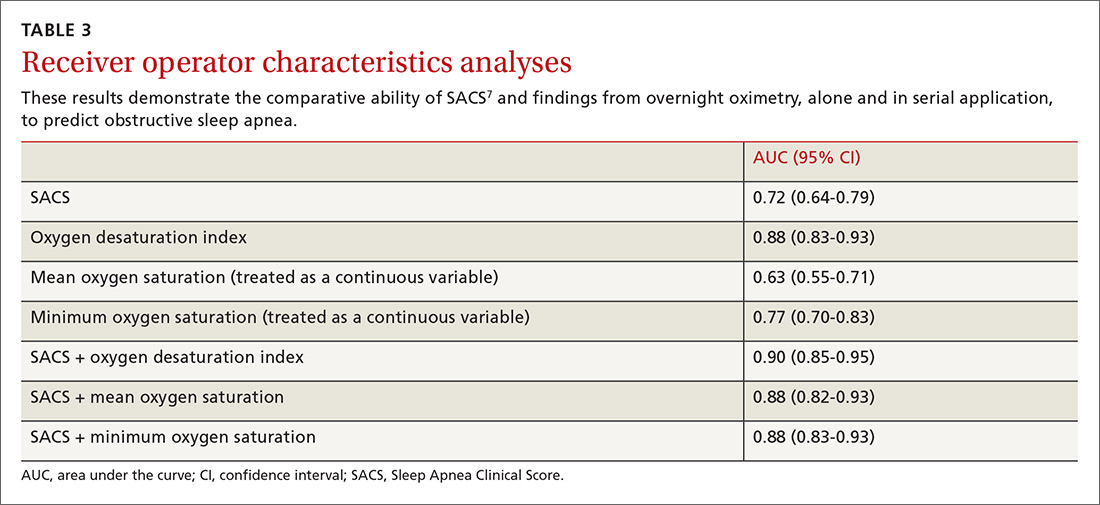
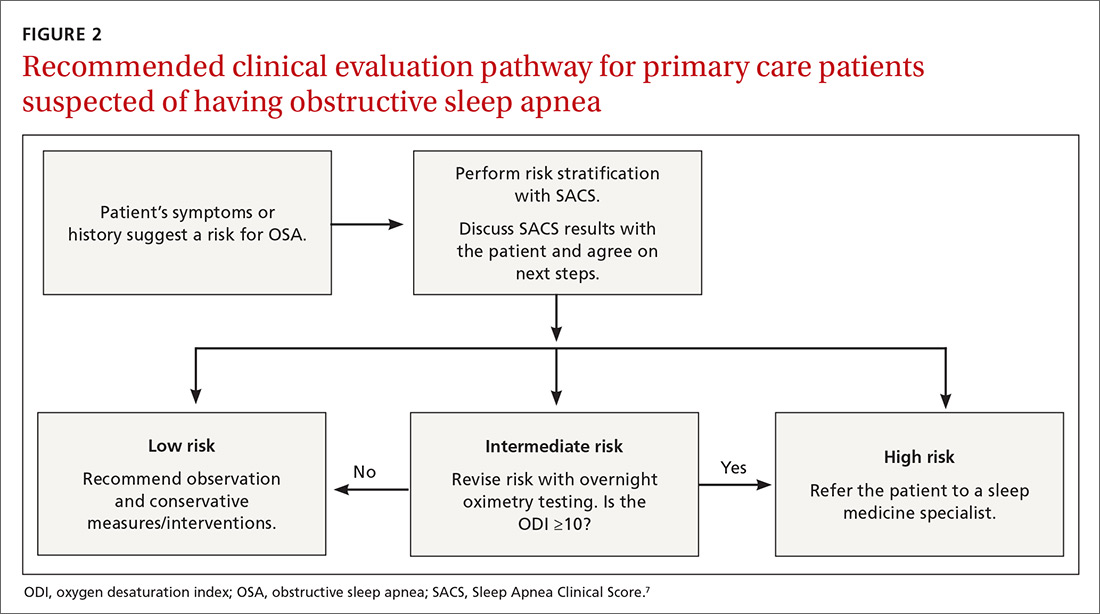
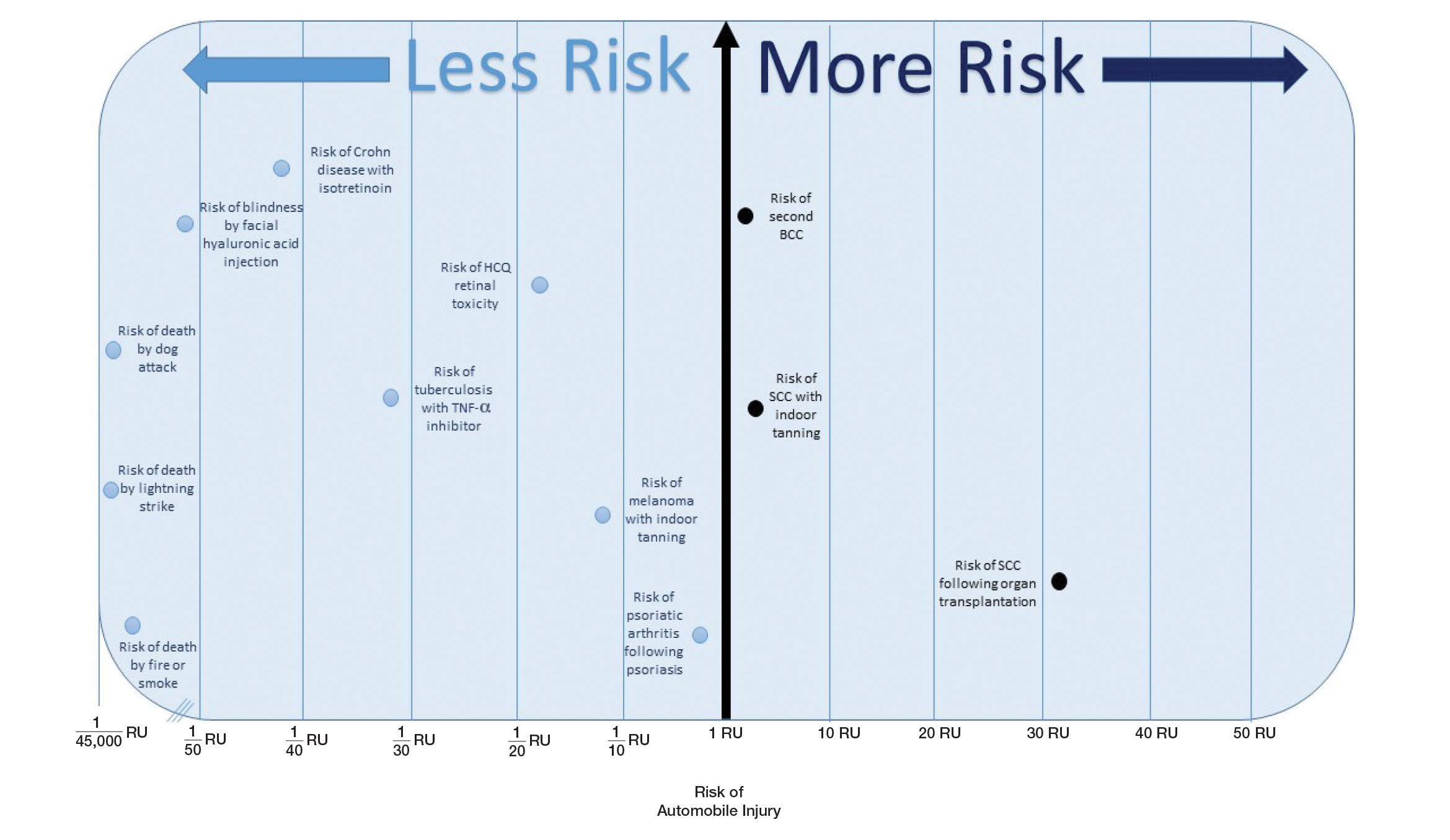
Ten dermatologic interventions were identified as illustrative, to be presented alongside the risk of automobile injury and death. The LTR of automobile injury was 32%, defined as 1.0 RU. The LTR of automobile death was 0.89% (1/36 RU).
Two events had LTRs roughly similar to automobile injury: development of a subsequent basal cell carcinoma within 3 years (1.4 RU) and development of a squamous cell carcinoma (SCC) secondary to indoor tanning (1.6 RU). Development of SCC following organ transplantation (34 RU) was considerably more likely than automobile injury. All other identified events had lower RUs than automobile injury (Table). Three events with small RUs included tuberculosis development with a tumor necrosis factor α inhibitor (1/32 RU), Crohn disease development with isotretinoin (1/41 RU), and blindness following facial hyaluronic acid injection (1/80 RU). The LTR of death by dog attack (1/42,436 RU) and death by lightning strike (1/36,542 RU) also had small RUs.
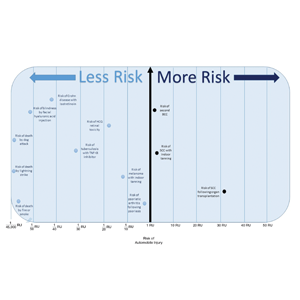
The unit comparators from the Table were adapted into graphic form to depict risk relative to the risk of automobile injury (Figure).
Comment
Numerous interventions in dermatology offer much less risk of an adverse event than the LTR of automobile injury. However, this concept of risk includes only the likelihood of development of an event, not the severity of the measured event, as our numerical and visual tool objectively captures the related risks using an RU comparator. Such use of a standardized RU demonstrates the essence of risk; “zero risk” does not exist, and each intervention or treatment, albeit how small, must be justified in concordance with other types of risk, such as the automobile.
The development of adverse events secondary to dermatologic intervention or therapy, for which monitoring visits are utilized, were used as important comparators to the risk of automobile injury. The continuous practice of monitoring visits may increase patient’s fears regarding possible adverse events secondary to therapy. Hydroxychloroquine retinal toxicity (1/16 RU) and psoriatic arthritis development following severe psoriasis (1/3.9 RU) were less likely to occur than automobile injury. The development of abnormal blood counts or blood tests secondary to therapy or intervention could not be formatted into an RU. The use of equation (1) for the calculation of LTRadverse eventholds true only when the absolute risk of developing the adverse event in the general population—in this case, abnormal blood counts or blood tests—is low.7
Although the unit comparator allows for the comparison of different dermatologic risk, a limitation of the RU model and its visual tool are a dependence on RR, a value that changes following publication of new studies. A solution was the use of a single pooled estimate to represent the upper-limit CIs of LTR. This practice overestimates risk. As with RR, new automobile injury rates are published annually.10 In the last 5 years, the LTR of automobile injury has stayed relatively constant: between 32% and 33%.4 Although the RU calculations and Figure included a wide variety of interventions in dermatology, select clinical situations were not included. It is beyond the scope of this article to systematically review all risk in dermatology but rather introduce the concept of the RU founded on automobile-associated risks. With the introduction of a methodical framework, the reader is invited to calculate RUs pertinent to their clinical interests.
Any intervention or treatment in dermatology is accompanied by risk. The use of a unit comparator using an easily relatable event—the LTR of automobile injury—allows the patient to easily compare risk and internally justify the practice of monitoring visits. Inclusion of a visual tool, such as the Figure, might alleviate many irrational fears that accompany some of the highly effective treatments and interventions used in dermatology and thus lead to better patient outcomes.
Acknowledgment
We thank Taranjeet Singh, MS (Dunn, North Carolina), for her comments on an earlier version of the manuscript.
- Rosen AB, Tsai JS, Downs SM. Variations in risk attitude across race, gender, and education. Med Decis Making. 2003;23:511-517.
- Sandoval LF, Pierce A, Feldman SR. Systemic therapies for psoriasis: an evidence-based update. Am J Clin Dermatol. 2014;15:165-180.
- National Safety Council. Odds of dying. Injury Facts website. http://injuryfacts.nsc.org/all-injuries/preventable-death-overview/odds-of-dying/. Accessed November 4, 2018.
- National Center for Statistics and Analysis (NCSA) motor vehicle traffic crash data resource page. National Highway Traffic Safety Administration website. https://crashstats.nhtsa.dot.gov/#/. Accessed November 4, 2018.
- CDC report shows motor vehicle crash injuries are frequent and costly. Centers for Disease Control and Prevention website. http://www.cdc.gov/media/releases/2014/p1007-crash-injuries.html. Published October 7, 2014. Accessed November 4, 2018.
- Automobile. Business Dictionary website. http://www.businessdictionary.com/definition/automobile.html. Accessed November 4, 2018.
- Dupont WD, Plummer WD Jr. Understanding the relationship between relative and absolute risk. Cancer. 1996;77:2193-2199.
- Kaminska E, Patel I, Dabade TS, et al. Comparing the lifetime risks of TNF-alpha inhibitor use to common benchmarks of risk. J Dermatolog Treat. 2011;24:101-106.
- Dupont WD. Converting relative risks to absolute risks: a graphical approach. Stat Med. 1989;8:641-651.
- Kochanek KD, Murphy SL, Xu J, et al. Deaths: final data for 2014. Natl Vital Stat Rep. 2016;65:1-122.
- Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. The Cochrane Collaboration website. http://handbook.cochrane.org. Updated March 2011. Accessed November 15, 2018.
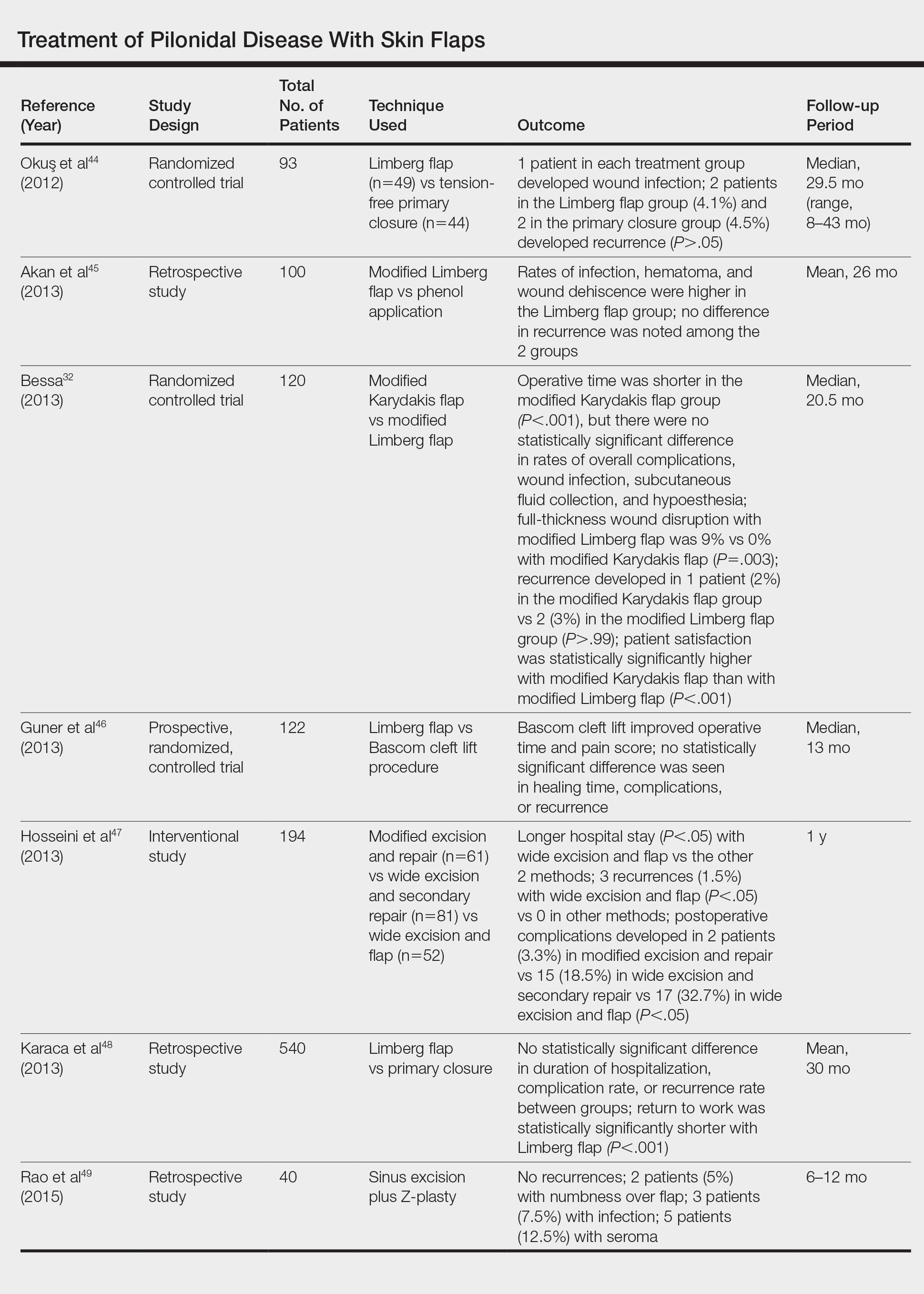
- Katz KA. The (relative) risks of using odds ratios. Arch Dermatol. 2006;142:761-764.
- Rayess HM, Svider PF, Hanba C, et al. A cross-sectional analysis of adverse events and litigation for injectable fillers. JAMA Facial Plast Surg. 2018;20:207-214.
- Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007;5:1424-1429.
- Loftus EV Jr. Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504-1517.
- Lee SY, Jamal MM, Nguyen ET, et al. Does exposure to isotretinoin increase the risk for the development of inflammatory bowel disease? A meta-analysis. Eur J Gastroenterol Hepatol. 2016;28:210-216.
- Injury Facts, 2017. Itasca, IL: National Safety Council; 2017.
- Marmor MF, Kellner U, Lai TY, et al. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision). Ophthalmology. 2016;123:1386-1394.
- Melles RB, Marmor MF. The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmol. 2014;132:1453-1460.
- Colantonio S, Bracken MB, Beecker J. The association of indoor tanning and melanoma in adults: systematic review and meta-analysis. J Am Acad Dermatol. 2014;70:847-857.e1-18.
- Green AC, Olsen CM. Increased risk of melanoma in organ transplant recipients: systematic review and meta-analysis of cohort studies. Acta Derm Venereol. 2015;95:923-927.
- Eder L, Haddad A, Rosen CF, et al. The incidence and risk factors for psoriatic arthritis in patients with psoriasis: a prospective cohort study. Arthritis Rheumatol. 2016;68:915-923.
- National Highway Traffic Safety Administration (NHTSA). Traffic Safety Facts 2015. Washington, DC: US Department of Transportation; 2015.
- Marcil I, Stern RS. Risk of developing a subsequent nonmelanoma skin cancer in patients with a history of nonmelanoma skin cancer: a critical review of the literature and meta-analysis. Arch Dermatol. 2000;136:1524-1530.
- Wehner MR, Shive ML, Chren MM, et al. Indoor tanning and non-melanoma skin cancer: systematic review and meta-analysis. BMJ. 2012;345:E5909.
- Lindelöf B, Sigurgeirsson B, Gäbel H, et al. Incidence of skin cancer in 5356 patients following organ transplantation. Br J Dermatol. 2000;143:513-519.
Numerous highly efficacious treatment modalities exist in dermatology, yet patients may be highly wary of their possible adverse events, even when those risks are rare.1,2 Such fears can lead to poor medication adherence and treatment refusal. A key determinant in successful patient-provider care is to effectively communicate risk. The communication of risk is hampered by the lack of any common currency for comparing risks. The development of a standardized unit of risk could help facilitate risk comparisons, allowing physicians and patients to put risk levels into better perspective.
One easily relatable event is the risk of injury in an automobile crash. Driving, whether to the dermatology clinic for a monitoring visit or to the supermarket for weekly groceries, is associated with risk of injury and death. The risk of automobile-related injury warranting a visit to the emergency department could provide a comparator that physicians can use to give patients a more objective sense of treatment risks or to introduce the justification of a monitoring visit. The objective of this study was to develop a standard risk unit based on the lifetime risk (LTR) of automobile injury and to compare this unit of risk to various risks of dermatologic treatments.
Methods
Literature Review
We first identified common risks in dermatology that would be illustrative and then identified keywords. PubMed searches for articles indexed for MEDLINE from November 1996 to February 2017 were performed combining the following terms: (relative risk, odds ratio, lifetime risk) and (isotretinoin, IBD; melanoma, SCC, transplantation; indoor tanning, BCC, SCC; transplant and SCC; biologics and tuberculosis; hydroxychloroquine retinal toxicity; psoriasis and psoriatic arthritis). An additional search was performed in June 2018 including the term blindness and injectable fillers. Our search combined these terms in numerous ways. Results were focused on meta-analyses and observational studies.
The references of relevant studies were included. Articles not focused on meta-analyses but rather on observational studies were individually analyzed for quality and bias using the 9-point Newcastle-Ottawa Scale, with a score of 7 or more as a cutoff for inclusion.
Determination of Risk Comparators
Data from the 2016 National Safety Council’s Injury Facts report were searched for nonmedical-related risk comparators, such as the risk of death by dog attack, by lightning, and by fire or smoke.3 Data from the 2015 US Department of Transportation Traffic Safety Facts were searched for relatable risk comparators, such as the LTR of automobile death and injury.4
Definitions
Automobile injury was defined as an injury warranting a visit to the emergency department.5 Automobile was defined as a road vehicle with 4 wheels and powered by an internal combustion engine or electric motor.6 This definition excluded light trucks, large trucks, and motorcycles.
LTR Calculation
Lifetime risk was used as the comparative measure. Lifetime risk is a type of absolute risk that depicts the probability that a specific disease or event will occur in an individual’s lifespan. The LTRof developing a disease or adverse event due to a dermatologic therapy or interventionwas denoted as LTRadverse event and calculated by the following equation7,8:

In this equation, LTRgeneral population is the LTR of developing the disease or adverse event without being subject to the therapy or intervention, and RRintervention is the relative risk (RR) from previously published RR data (relating to the development of the disease in question or an adverse event of the intervention). The use of equation (1) holds true only when the absolute risk of developing the disease or adverse event (LTRgeneral population) is low.7 Although the calculation of an LTR using a constant lifetime RR may require major approximations, studies evaluating the variation of RR over time are sparse.7,9 The Newcastle-Ottawa Scale was used to control such variance; only high-quality, nonrandomized studies were included. Although the use of residual LTR would be preferable, as LTR depends on age, such epidemiological data do not exist for complex diseases.
When not available, the LTRgeneral population was calculated from the rate of disease (cases per 100,000 individuals per year) multiplied by the average lifespan of an American (78.8 years)10:
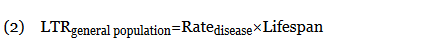
When an odds ratio (OR) was presented, its conversion to RR followed11:
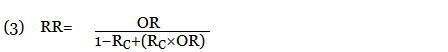
In this equation, RC is the absolute risk in the unexposed group. If the prevalence of the disease was considered low, the rare disease assumption was implemented as the following11,12:

The use of this approximation overestimates the LTR of an event. From a patient perspective, this approach is conservative. If prior LTR values were available, such as the LTR of automobile injury, automobile death, or other intervention, they were used without the need for calculation.
Unit Comparator
The LTRs of all adverse events were normalized to a unit comparator, using the LTR of an automobile injury as reference point, denoted as 1 risk unit (RU):

This equation allows for quick comparison of the magnitude of LTRs between events. Events with an RU less than 1 are less likely to occur than the risk of automobile injury; events with an RU greater than 1 are more likely than the risk of automobile injury. All RR, LTR, and unit comparators were presented as a single pooled estimate of their respective upper-limit CIs. The use of the upper-limit CI conservatively overestimates the LTR of an event.
Results
Ten dermatologic interventions were identified as illustrative, to be presented alongside the risk of automobile injury and death. The LTR of automobile injury was 32%, defined as 1.0 RU. The LTR of automobile death was 0.89% (1/36 RU).
Two events had LTRs roughly similar to automobile injury: development of a subsequent basal cell carcinoma within 3 years (1.4 RU) and development of a squamous cell carcinoma (SCC) secondary to indoor tanning (1.6 RU). Development of SCC following organ transplantation (34 RU) was considerably more likely than automobile injury. All other identified events had lower RUs than automobile injury (Table). Three events with small RUs included tuberculosis development with a tumor necrosis factor α inhibitor (1/32 RU), Crohn disease development with isotretinoin (1/41 RU), and blindness following facial hyaluronic acid injection (1/80 RU). The LTR of death by dog attack (1/42,436 RU) and death by lightning strike (1/36,542 RU) also had small RUs.
The unit comparators from the Table were adapted into graphic form to depict risk relative to the risk of automobile injury (Figure).

Comment
Numerous interventions in dermatology offer much less risk of an adverse event than the LTR of automobile injury. However, this concept of risk includes only the likelihood of development of an event, not the severity of the measured event, as our numerical and visual tool objectively captures the related risks using an RU comparator. Such use of a standardized RU demonstrates the essence of risk; “zero risk” does not exist, and each intervention or treatment, albeit how small, must be justified in concordance with other types of risk, such as the automobile.
The development of adverse events secondary to dermatologic intervention or therapy, for which monitoring visits are utilized, were used as important comparators to the risk of automobile injury. The continuous practice of monitoring visits may increase patient’s fears regarding possible adverse events secondary to therapy. Hydroxychloroquine retinal toxicity (1/16 RU) and psoriatic arthritis development following severe psoriasis (1/3.9 RU) were less likely to occur than automobile injury. The development of abnormal blood counts or blood tests secondary to therapy or intervention could not be formatted into an RU. The use of equation (1) for the calculation of LTRadverse eventholds true only when the absolute risk of developing the adverse event in the general population—in this case, abnormal blood counts or blood tests—is low.7
Although the unit comparator allows for the comparison of different dermatologic risk, a limitation of the RU model and its visual tool are a dependence on RR, a value that changes following publication of new studies. A solution was the use of a single pooled estimate to represent the upper-limit CIs of LTR. This practice overestimates risk. As with RR, new automobile injury rates are published annually.10 In the last 5 years, the LTR of automobile injury has stayed relatively constant: between 32% and 33%.4 Although the RU calculations and Figure included a wide variety of interventions in dermatology, select clinical situations were not included. It is beyond the scope of this article to systematically review all risk in dermatology but rather introduce the concept of the RU founded on automobile-associated risks. With the introduction of a methodical framework, the reader is invited to calculate RUs pertinent to their clinical interests.
Any intervention or treatment in dermatology is accompanied by risk. The use of a unit comparator using an easily relatable event—the LTR of automobile injury—allows the patient to easily compare risk and internally justify the practice of monitoring visits. Inclusion of a visual tool, such as the Figure, might alleviate many irrational fears that accompany some of the highly effective treatments and interventions used in dermatology and thus lead to better patient outcomes.
Acknowledgment
We thank Taranjeet Singh, MS (Dunn, North Carolina), for her comments on an earlier version of the manuscript.
Numerous highly efficacious treatment modalities exist in dermatology, yet patients may be highly wary of their possible adverse events, even when those risks are rare.1,2 Such fears can lead to poor medication adherence and treatment refusal. A key determinant in successful patient-provider care is to effectively communicate risk. The communication of risk is hampered by the lack of any common currency for comparing risks. The development of a standardized unit of risk could help facilitate risk comparisons, allowing physicians and patients to put risk levels into better perspective.
One easily relatable event is the risk of injury in an automobile crash. Driving, whether to the dermatology clinic for a monitoring visit or to the supermarket for weekly groceries, is associated with risk of injury and death. The risk of automobile-related injury warranting a visit to the emergency department could provide a comparator that physicians can use to give patients a more objective sense of treatment risks or to introduce the justification of a monitoring visit. The objective of this study was to develop a standard risk unit based on the lifetime risk (LTR) of automobile injury and to compare this unit of risk to various risks of dermatologic treatments.
Methods
Literature Review
We first identified common risks in dermatology that would be illustrative and then identified keywords. PubMed searches for articles indexed for MEDLINE from November 1996 to February 2017 were performed combining the following terms: (relative risk, odds ratio, lifetime risk) and (isotretinoin, IBD; melanoma, SCC, transplantation; indoor tanning, BCC, SCC; transplant and SCC; biologics and tuberculosis; hydroxychloroquine retinal toxicity; psoriasis and psoriatic arthritis). An additional search was performed in June 2018 including the term blindness and injectable fillers. Our search combined these terms in numerous ways. Results were focused on meta-analyses and observational studies.
The references of relevant studies were included. Articles not focused on meta-analyses but rather on observational studies were individually analyzed for quality and bias using the 9-point Newcastle-Ottawa Scale, with a score of 7 or more as a cutoff for inclusion.
Determination of Risk Comparators
Data from the 2016 National Safety Council’s Injury Facts report were searched for nonmedical-related risk comparators, such as the risk of death by dog attack, by lightning, and by fire or smoke.3 Data from the 2015 US Department of Transportation Traffic Safety Facts were searched for relatable risk comparators, such as the LTR of automobile death and injury.4
Definitions
Automobile injury was defined as an injury warranting a visit to the emergency department.5 Automobile was defined as a road vehicle with 4 wheels and powered by an internal combustion engine or electric motor.6 This definition excluded light trucks, large trucks, and motorcycles.
LTR Calculation
Lifetime risk was used as the comparative measure. Lifetime risk is a type of absolute risk that depicts the probability that a specific disease or event will occur in an individual’s lifespan. The LTRof developing a disease or adverse event due to a dermatologic therapy or interventionwas denoted as LTRadverse event and calculated by the following equation7,8:

In this equation, LTRgeneral population is the LTR of developing the disease or adverse event without being subject to the therapy or intervention, and RRintervention is the relative risk (RR) from previously published RR data (relating to the development of the disease in question or an adverse event of the intervention). The use of equation (1) holds true only when the absolute risk of developing the disease or adverse event (LTRgeneral population) is low.7 Although the calculation of an LTR using a constant lifetime RR may require major approximations, studies evaluating the variation of RR over time are sparse.7,9 The Newcastle-Ottawa Scale was used to control such variance; only high-quality, nonrandomized studies were included. Although the use of residual LTR would be preferable, as LTR depends on age, such epidemiological data do not exist for complex diseases.
When not available, the LTRgeneral population was calculated from the rate of disease (cases per 100,000 individuals per year) multiplied by the average lifespan of an American (78.8 years)10:
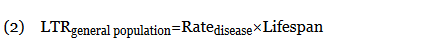
When an odds ratio (OR) was presented, its conversion to RR followed11:
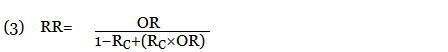
In this equation, RC is the absolute risk in the unexposed group. If the prevalence of the disease was considered low, the rare disease assumption was implemented as the following11,12:

The use of this approximation overestimates the LTR of an event. From a patient perspective, this approach is conservative. If prior LTR values were available, such as the LTR of automobile injury, automobile death, or other intervention, they were used without the need for calculation.
Unit Comparator
The LTRs of all adverse events were normalized to a unit comparator, using the LTR of an automobile injury as reference point, denoted as 1 risk unit (RU):

This equation allows for quick comparison of the magnitude of LTRs between events. Events with an RU less than 1 are less likely to occur than the risk of automobile injury; events with an RU greater than 1 are more likely than the risk of automobile injury. All RR, LTR, and unit comparators were presented as a single pooled estimate of their respective upper-limit CIs. The use of the upper-limit CI conservatively overestimates the LTR of an event.
Results
Ten dermatologic interventions were identified as illustrative, to be presented alongside the risk of automobile injury and death. The LTR of automobile injury was 32%, defined as 1.0 RU. The LTR of automobile death was 0.89% (1/36 RU).
Two events had LTRs roughly similar to automobile injury: development of a subsequent basal cell carcinoma within 3 years (1.4 RU) and development of a squamous cell carcinoma (SCC) secondary to indoor tanning (1.6 RU). Development of SCC following organ transplantation (34 RU) was considerably more likely than automobile injury. All other identified events had lower RUs than automobile injury (Table). Three events with small RUs included tuberculosis development with a tumor necrosis factor α inhibitor (1/32 RU), Crohn disease development with isotretinoin (1/41 RU), and blindness following facial hyaluronic acid injection (1/80 RU). The LTR of death by dog attack (1/42,436 RU) and death by lightning strike (1/36,542 RU) also had small RUs.
The unit comparators from the Table were adapted into graphic form to depict risk relative to the risk of automobile injury (Figure).

Comment
Numerous interventions in dermatology offer much less risk of an adverse event than the LTR of automobile injury. However, this concept of risk includes only the likelihood of development of an event, not the severity of the measured event, as our numerical and visual tool objectively captures the related risks using an RU comparator. Such use of a standardized RU demonstrates the essence of risk; “zero risk” does not exist, and each intervention or treatment, albeit how small, must be justified in concordance with other types of risk, such as the automobile.
The development of adverse events secondary to dermatologic intervention or therapy, for which monitoring visits are utilized, were used as important comparators to the risk of automobile injury. The continuous practice of monitoring visits may increase patient’s fears regarding possible adverse events secondary to therapy. Hydroxychloroquine retinal toxicity (1/16 RU) and psoriatic arthritis development following severe psoriasis (1/3.9 RU) were less likely to occur than automobile injury. The development of abnormal blood counts or blood tests secondary to therapy or intervention could not be formatted into an RU. The use of equation (1) for the calculation of LTRadverse eventholds true only when the absolute risk of developing the adverse event in the general population—in this case, abnormal blood counts or blood tests—is low.7
Although the unit comparator allows for the comparison of different dermatologic risk, a limitation of the RU model and its visual tool are a dependence on RR, a value that changes following publication of new studies. A solution was the use of a single pooled estimate to represent the upper-limit CIs of LTR. This practice overestimates risk. As with RR, new automobile injury rates are published annually.10 In the last 5 years, the LTR of automobile injury has stayed relatively constant: between 32% and 33%.4 Although the RU calculations and Figure included a wide variety of interventions in dermatology, select clinical situations were not included. It is beyond the scope of this article to systematically review all risk in dermatology but rather introduce the concept of the RU founded on automobile-associated risks. With the introduction of a methodical framework, the reader is invited to calculate RUs pertinent to their clinical interests.
Any intervention or treatment in dermatology is accompanied by risk. The use of a unit comparator using an easily relatable event—the LTR of automobile injury—allows the patient to easily compare risk and internally justify the practice of monitoring visits. Inclusion of a visual tool, such as the Figure, might alleviate many irrational fears that accompany some of the highly effective treatments and interventions used in dermatology and thus lead to better patient outcomes.
Acknowledgment
We thank Taranjeet Singh, MS (Dunn, North Carolina), for her comments on an earlier version of the manuscript.
- Rosen AB, Tsai JS, Downs SM. Variations in risk attitude across race, gender, and education. Med Decis Making. 2003;23:511-517.
- Sandoval LF, Pierce A, Feldman SR. Systemic therapies for psoriasis: an evidence-based update. Am J Clin Dermatol. 2014;15:165-180.
- National Safety Council. Odds of dying. Injury Facts website. http://injuryfacts.nsc.org/all-injuries/preventable-death-overview/odds-of-dying/. Accessed November 4, 2018.
- National Center for Statistics and Analysis (NCSA) motor vehicle traffic crash data resource page. National Highway Traffic Safety Administration website. https://crashstats.nhtsa.dot.gov/#/. Accessed November 4, 2018.
- CDC report shows motor vehicle crash injuries are frequent and costly. Centers for Disease Control and Prevention website. http://www.cdc.gov/media/releases/2014/p1007-crash-injuries.html. Published October 7, 2014. Accessed November 4, 2018.
- Automobile. Business Dictionary website. http://www.businessdictionary.com/definition/automobile.html. Accessed November 4, 2018.
- Dupont WD, Plummer WD Jr. Understanding the relationship between relative and absolute risk. Cancer. 1996;77:2193-2199.
- Kaminska E, Patel I, Dabade TS, et al. Comparing the lifetime risks of TNF-alpha inhibitor use to common benchmarks of risk. J Dermatolog Treat. 2011;24:101-106.
- Dupont WD. Converting relative risks to absolute risks: a graphical approach. Stat Med. 1989;8:641-651.
- Kochanek KD, Murphy SL, Xu J, et al. Deaths: final data for 2014. Natl Vital Stat Rep. 2016;65:1-122.
- Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. The Cochrane Collaboration website. http://handbook.cochrane.org. Updated March 2011. Accessed November 15, 2018.
- Katz KA. The (relative) risks of using odds ratios. Arch Dermatol. 2006;142:761-764.
- Rayess HM, Svider PF, Hanba C, et al. A cross-sectional analysis of adverse events and litigation for injectable fillers. JAMA Facial Plast Surg. 2018;20:207-214.
- Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007;5:1424-1429.
- Loftus EV Jr. Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504-1517.
- Lee SY, Jamal MM, Nguyen ET, et al. Does exposure to isotretinoin increase the risk for the development of inflammatory bowel disease? A meta-analysis. Eur J Gastroenterol Hepatol. 2016;28:210-216.
- Injury Facts, 2017. Itasca, IL: National Safety Council; 2017.
- Marmor MF, Kellner U, Lai TY, et al. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision). Ophthalmology. 2016;123:1386-1394.
- Melles RB, Marmor MF. The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmol. 2014;132:1453-1460.
- Colantonio S, Bracken MB, Beecker J. The association of indoor tanning and melanoma in adults: systematic review and meta-analysis. J Am Acad Dermatol. 2014;70:847-857.e1-18.
- Green AC, Olsen CM. Increased risk of melanoma in organ transplant recipients: systematic review and meta-analysis of cohort studies. Acta Derm Venereol. 2015;95:923-927.
- Eder L, Haddad A, Rosen CF, et al. The incidence and risk factors for psoriatic arthritis in patients with psoriasis: a prospective cohort study. Arthritis Rheumatol. 2016;68:915-923.
- National Highway Traffic Safety Administration (NHTSA). Traffic Safety Facts 2015. Washington, DC: US Department of Transportation; 2015.
- Marcil I, Stern RS. Risk of developing a subsequent nonmelanoma skin cancer in patients with a history of nonmelanoma skin cancer: a critical review of the literature and meta-analysis. Arch Dermatol. 2000;136:1524-1530.
- Wehner MR, Shive ML, Chren MM, et al. Indoor tanning and non-melanoma skin cancer: systematic review and meta-analysis. BMJ. 2012;345:E5909.
- Lindelöf B, Sigurgeirsson B, Gäbel H, et al. Incidence of skin cancer in 5356 patients following organ transplantation. Br J Dermatol. 2000;143:513-519.
- Rosen AB, Tsai JS, Downs SM. Variations in risk attitude across race, gender, and education. Med Decis Making. 2003;23:511-517.
- Sandoval LF, Pierce A, Feldman SR. Systemic therapies for psoriasis: an evidence-based update. Am J Clin Dermatol. 2014;15:165-180.
- National Safety Council. Odds of dying. Injury Facts website. http://injuryfacts.nsc.org/all-injuries/preventable-death-overview/odds-of-dying/. Accessed November 4, 2018.
- National Center for Statistics and Analysis (NCSA) motor vehicle traffic crash data resource page. National Highway Traffic Safety Administration website. https://crashstats.nhtsa.dot.gov/#/. Accessed November 4, 2018.
- CDC report shows motor vehicle crash injuries are frequent and costly. Centers for Disease Control and Prevention website. http://www.cdc.gov/media/releases/2014/p1007-crash-injuries.html. Published October 7, 2014. Accessed November 4, 2018.
- Automobile. Business Dictionary website. http://www.businessdictionary.com/definition/automobile.html. Accessed November 4, 2018.
- Dupont WD, Plummer WD Jr. Understanding the relationship between relative and absolute risk. Cancer. 1996;77:2193-2199.
- Kaminska E, Patel I, Dabade TS, et al. Comparing the lifetime risks of TNF-alpha inhibitor use to common benchmarks of risk. J Dermatolog Treat. 2011;24:101-106.
- Dupont WD. Converting relative risks to absolute risks: a graphical approach. Stat Med. 1989;8:641-651.
- Kochanek KD, Murphy SL, Xu J, et al. Deaths: final data for 2014. Natl Vital Stat Rep. 2016;65:1-122.
- Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. The Cochrane Collaboration website. http://handbook.cochrane.org. Updated March 2011. Accessed November 15, 2018.
- Katz KA. The (relative) risks of using odds ratios. Arch Dermatol. 2006;142:761-764.
- Rayess HM, Svider PF, Hanba C, et al. A cross-sectional analysis of adverse events and litigation for injectable fillers. JAMA Facial Plast Surg. 2018;20:207-214.
- Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007;5:1424-1429.
- Loftus EV Jr. Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504-1517.
- Lee SY, Jamal MM, Nguyen ET, et al. Does exposure to isotretinoin increase the risk for the development of inflammatory bowel disease? A meta-analysis. Eur J Gastroenterol Hepatol. 2016;28:210-216.
- Injury Facts, 2017. Itasca, IL: National Safety Council; 2017.
- Marmor MF, Kellner U, Lai TY, et al. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision). Ophthalmology. 2016;123:1386-1394.
- Melles RB, Marmor MF. The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmol. 2014;132:1453-1460.
- Colantonio S, Bracken MB, Beecker J. The association of indoor tanning and melanoma in adults: systematic review and meta-analysis. J Am Acad Dermatol. 2014;70:847-857.e1-18.
- Green AC, Olsen CM. Increased risk of melanoma in organ transplant recipients: systematic review and meta-analysis of cohort studies. Acta Derm Venereol. 2015;95:923-927.
- Eder L, Haddad A, Rosen CF, et al. The incidence and risk factors for psoriatic arthritis in patients with psoriasis: a prospective cohort study. Arthritis Rheumatol. 2016;68:915-923.
- National Highway Traffic Safety Administration (NHTSA). Traffic Safety Facts 2015. Washington, DC: US Department of Transportation; 2015.
- Marcil I, Stern RS. Risk of developing a subsequent nonmelanoma skin cancer in patients with a history of nonmelanoma skin cancer: a critical review of the literature and meta-analysis. Arch Dermatol. 2000;136:1524-1530.
- Wehner MR, Shive ML, Chren MM, et al. Indoor tanning and non-melanoma skin cancer: systematic review and meta-analysis. BMJ. 2012;345:E5909.
- Lindelöf B, Sigurgeirsson B, Gäbel H, et al. Incidence of skin cancer in 5356 patients following organ transplantation. Br J Dermatol. 2000;143:513-519.
Practice Points
- Using common identifiable risks may help patients put the risk of certain dermatologic interventions into perspective.
- Numerous interventions in dermatology offer much less risk of an adverse event than the lifetime risk of automobile injury.
Treatment Options for Pilonidal Sinus
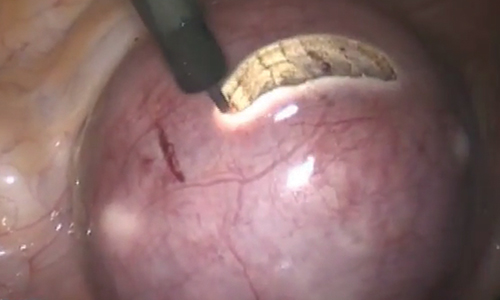
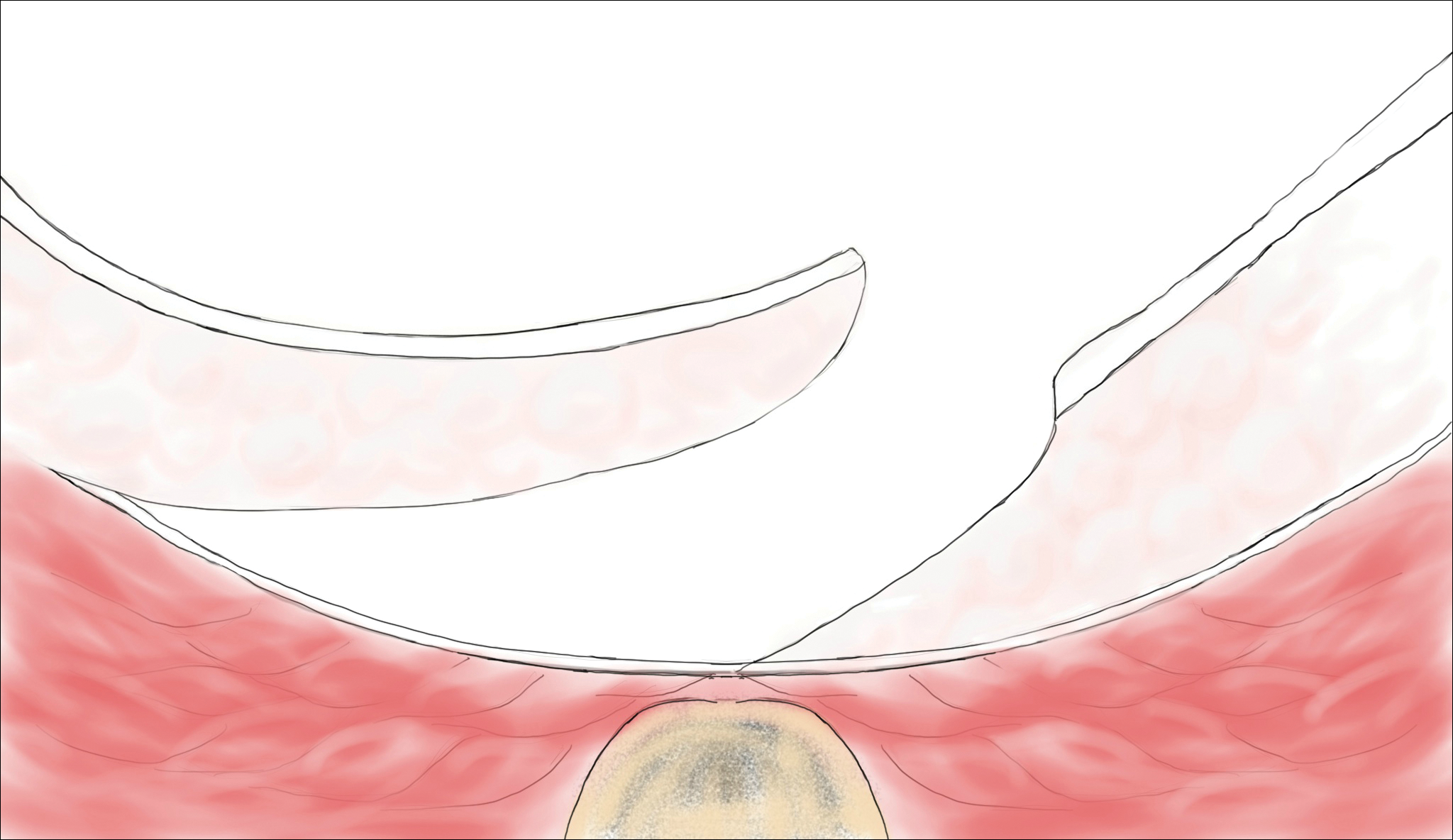
Pilonidal disease was first described by Mayo1 in 1833 who hypothesized that the underlying etiology is incomplete separation of the mesoderm and ectoderm layers during embryogenesis. In 1880, Hodges2 coined the term pilonidal sinus; he postulated that sinus formation was incited by hair.2 Today, Hodges theory is known as the acquired theory: hair induces a foreign body response in surrounding tissue, leading to sinus formation. Although pilonidal cysts can occur anywhere on the body, they most commonly extend cephalad in the sacrococcygeal and upper gluteal cleft (Figure 1).3,4 An acute pilonidal cyst typically presents with pain, tenderness, and swelling, similar to the presentation of a superficial abscess in other locations; however, a clue to the diagnosis is the presence of cutaneous pits along the midline of the gluteal cleft.5 Chronic pilonidal disease varies based on the extent of inflammation and scarring; the underlying cavity communicates with the overlying skin through sinuses and often drains with pressure.6
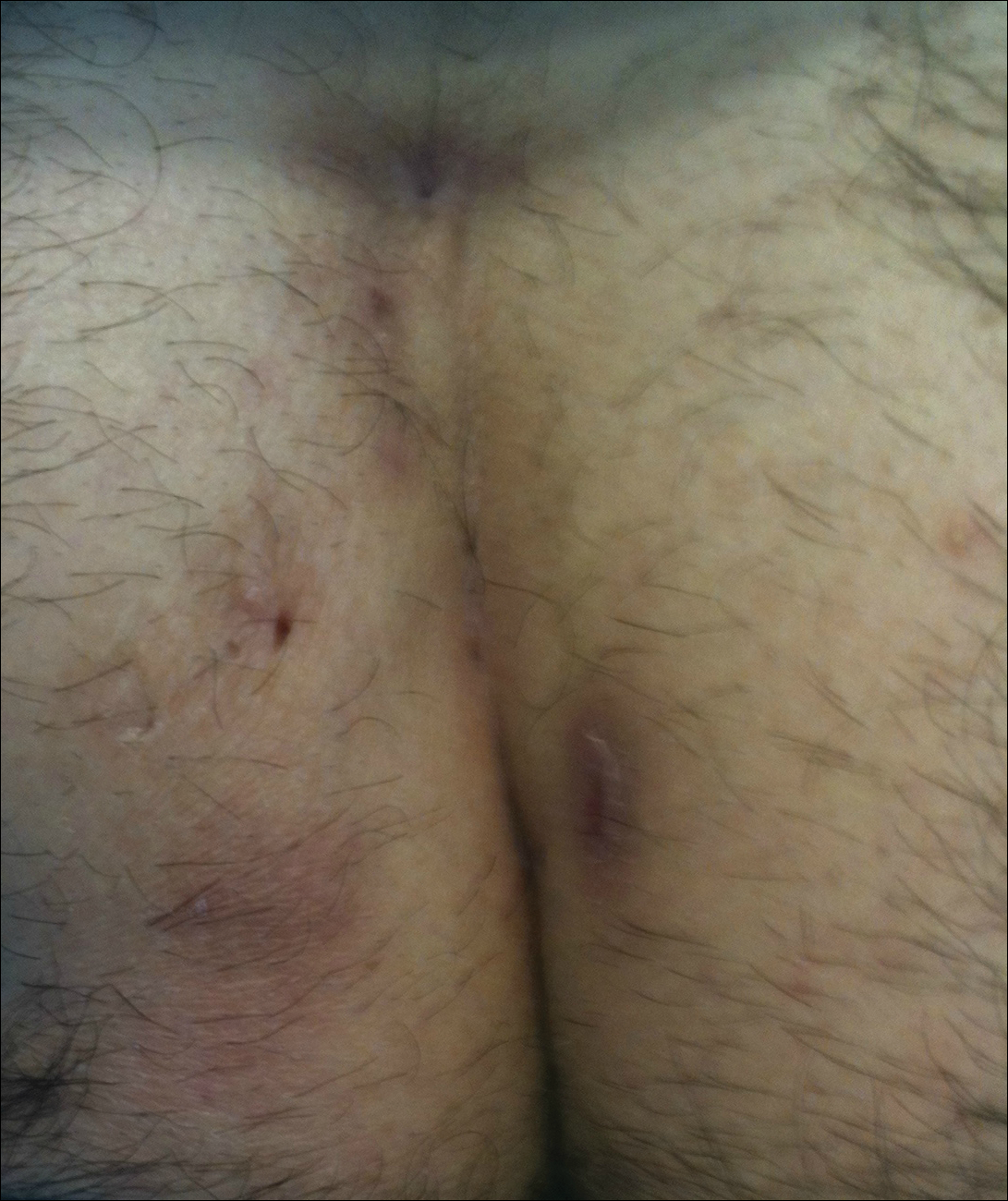
Pilonidal sinuses are rare before puberty or after 40 years of age7 and occur primarily in hirsute men. The ratio of men to women affected is between 3:1 and 4:1.8 Although pilonidal sinuses account for only 15% of anal suppurations, complications arising from pilonidal sinuses are a considerable cause of morbidity, resulting in loss of productivity in otherwise healthy individuals.9 Complications include chronic nonhealing wounds,10 as recurrent pilonidal sinuses tend to become colonized with gram-positive and facultative anaerobic bacteria, whereas primary pilonidal cysts more commonly become infected with anaerobic and gram-negative bacteria.11 Long-standing disease increases the risk of squamous cell carcinoma arising within sinus tracts.10,12
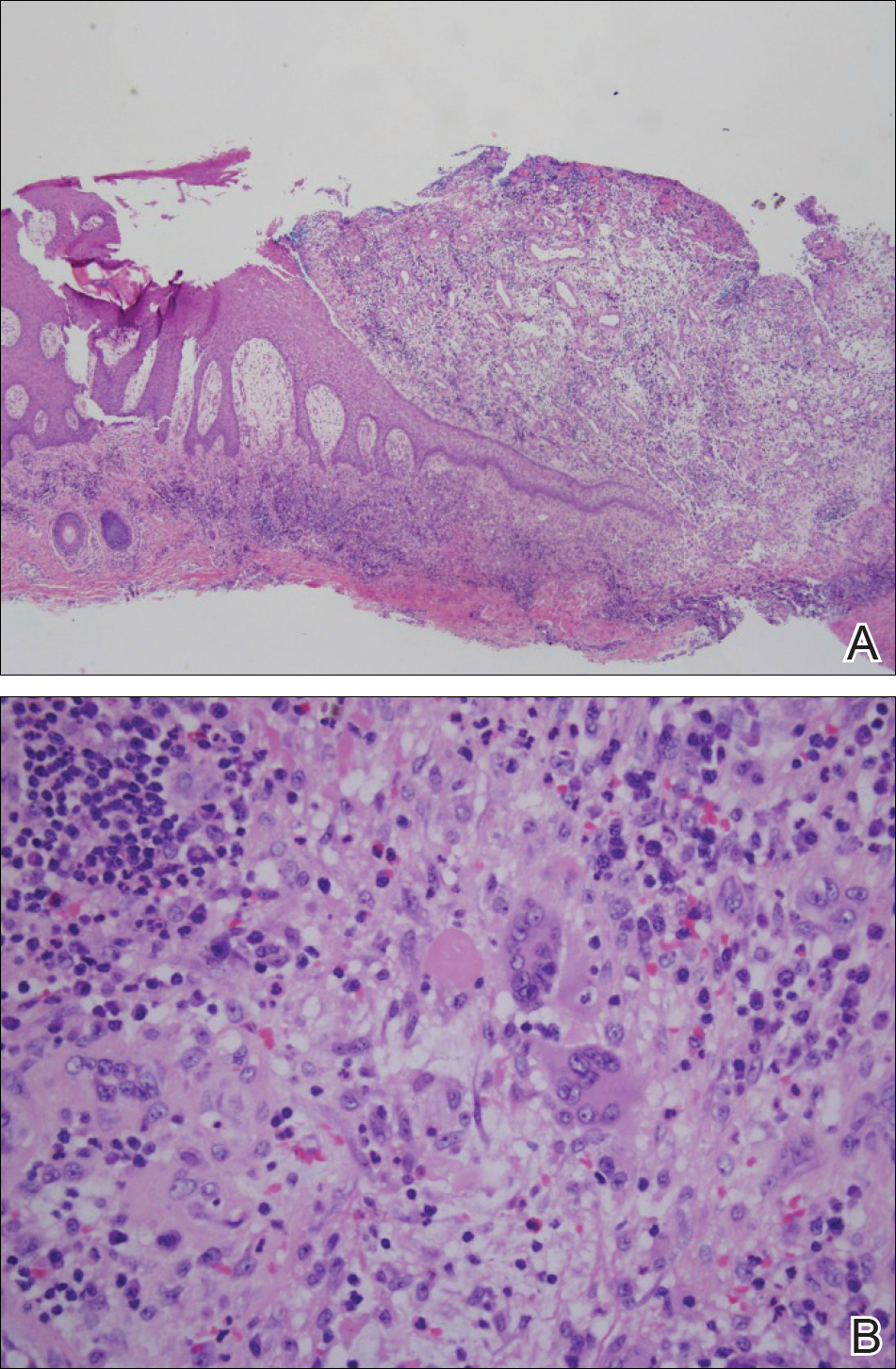
Histopathologically, pilonidal cysts are not true cysts because they lack an epithelial lining. Examination of the cavity commonly reveals hair, debris, and granulation tissue with surrounding foreign-body giant cells (Figure 2).5
The preferred treatment of pilonidal cysts continues to be debated. In this article, we review evidence supporting current modalities including conservative and surgical techniques as well as novel laser therapy for the treatment of pilonidal disease.
Conservative Management Techniques
Phenol Injections
Liquid or crystallized phenol injections have been used for treatment of mild to moderate pilonidal cysts.13 Excess debris is removed by curettage, and phenol is administered through the existing orifices or pits without pressure. The phenol remains in the cavity for 1 to 3 minutes before aspiration. Remaining cyst contents are removed through tissue manipulation, and the sinus is washed with saline. Mean healing time is 20 days (range, +/−14 days).13
Classically, phenol injections have a failure rate of 30% to 40%, especially with multiple sinuses and suppurative disease6; however, the success rate improves with limited disease (ie, no more than 1–3 sinus pits).3 With multiple treatment sessions, a recurrence rate as low as 2% over 25 months has been reported.14 Phenol injection also has been proposed as an adjuvant therapy to pit excision to minimize the need for extensive surgery.15
Simple Incision and Drainage
Simple incision and drainage has a crucial role in the treatment of acute pilonidal disease to decrease pain and relieve tension. Off-midline incisions have been recommended for because the resulting closures fared better against sheer forces applied by the gluteal muscles on the cleft.6 Therefore, the incision often is made off-midline from the gluteal cleft even when the cyst lies directly on the gluteal cleft.
Rates of healing vary widely after incision and drainage, ranging from 45% to 82%.6 Primary pilonidal cysts may respond well, particularly if the cavity is abraded; in one series, 79% (58/73) of patients did not have a recurrence at the average follow-up of 60 months.16
Excision and Unroofing
Techniques for excision and unroofing without primary closure include 2 variants: wide and limited. The wide technique consists of an inwardly slanted excision that is deepest in the center of the cavity. The inward sloping angle of the incision aids in healing because it allows granulation to progress evenly from the base of the wound upward. The depth of the incision should spare the fascia and leave as much fatty tissue as possible while still resecting the entire cavity and associated pits.6 Limited incision techniques aim to shorten the healing period by making smaller incisions into the sinuses, pits, and secondary tracts, and they are frequently supplemented with curettage.6 Noteworthy disadvantages include prolonged healing time, need for professional wound management, and extended medical observation.5 The average duration of wound healing in a study of 300 patients was 5.4 weeks (range, +/−1.1 weeks),17 and the recurrence rate has ranged from 5% to 13%.18,19 Care must be taken to respond to numerous possible complications, including excessive exudation and granulation, superinfection, and walling off.6
Although the cost of treatment varies by hospital, location, and a patient’s insurance coverage, patient reports to the Pilonidal Support Alliance indicate that the cost of conservative management ranges from $500 to $2000.20
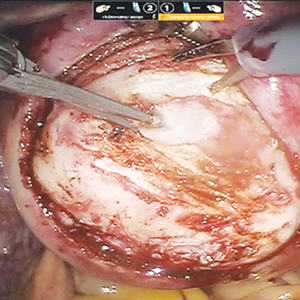
Excision and Primary Closure
An elliptical excision that includes some of the lateral margin is excised down to the level of the fascia. Adjacent lateral tracts may be excised by expanding the incision. To close the wound, edges are approximated with placement of deep and superficial sutures. Wound healing typically occurs faster than secondary granulation, as seen in one randomized controlled trial with a mean of 10 days for primary closure compared to 13 weeks for secondary intention.21 However, as with any surgical procedure, postoperative complications can delay wound healing.19 The recurrence rate after primary closure varies considerably, ranging from 10% to 38%.18,21-23 The average cost of an excision ranges from $3000 to $6000.20
A
Surgical Techniques
For severe or recurrent pilonidal disease, skin flaps often are required. Several flaps have been developed, including advancement, Bascom cleft lift, Karydakis, and modified Limberg flap. Flaps require a vascular pedicle but allow for closure without tension.26 The cost of a flap procedure, ranging from $10,000 to $30,000, is greater than the cost of excision or other conservative therapy20; however, with a lower recurrence rate of pilonidal disease following flap procedures compared to other treatments, patients may save more on treatment over the long-term.
Advancement Flaps
The most commonly used advancement flaps are the V-Y advancement flap and Z-plasty. The V-Y advancement flap creates a full-thickness V-shaped incision down to gluteal fascia that is closed to form a postrepair suture line in the shape of a Y.5 Depending on the size of the defect, the flaps may be utilized unilaterally or bilaterally. A defect as large as 8 to 10 cm can be covered unilaterally; however, defects larger than 10 cm commonly require a bilateral flap.26 The V-Y advancement flap failed to show superiority to primary closure techniques based on complications, recurrence, and patient satisfaction in a large randomized controlled trial.27
Performing a Z-plasty requires excision of diseased tissue with recruitment of lateral flaps incised down to the level of the fascia. The lateral edges are transposed to increase transverse length.26 No statistically significant difference in infection or recurrence rates was noted between excision alone and excision plus Z-plasty; however, wounds were reported to heal faster in patients receiving excision plus Z-plasty (41 vs 15 days).28
Cleft Lift Closure
In 1987, Bascom29 introduced the cleft lift closure for recurrent pilonidal disease. This technique aims to reduce or eliminate lateral gluteal forces on the wounds by filling the gluteal cleft.5 The sinus tracts are excised and a full-thickness skin flap is extended across the cleft and closed off-midline. The adipose tissue fills in the previous space of the gluteal cleft. In the initial study, no recurrences were reported in 30 patients who underwent this procedure at 2-year follow-up; similarly, in another case series of 26 patients who underwent the procedure, no recurrences were noted at a median follow-up of 3 years.30 Compared to excision with secondary wound healing and primary closure on the midline, the Bascom cleft lift demonstrated a decrease in wound healing time (62, 52, and 29 days, respectively).31
The classic Karydakis flap consists of an oblique elliptical excision of diseased tissue with fixation of the flap base to the sacral fascia (Figures 4 and 5). The flap is closed by suturing the edge off-midline.32 This technique prevents a midline wound and aims to remodel and flatten the natal cleft. Karydakis33 performed the most important study for treatment of pilonidal disease with the Karydakis flap, which included more than 5000 patients. The results showed a 0.9% recurrence rate and an 8.5% wound complication rate over a 2- to 20-year follow-up.33 These results have been substantiated by more recent studies, which produced similar results: a 1.8% to 5.3% infection rate and a recurrence rate of 0.9% to 4.4%.34,35
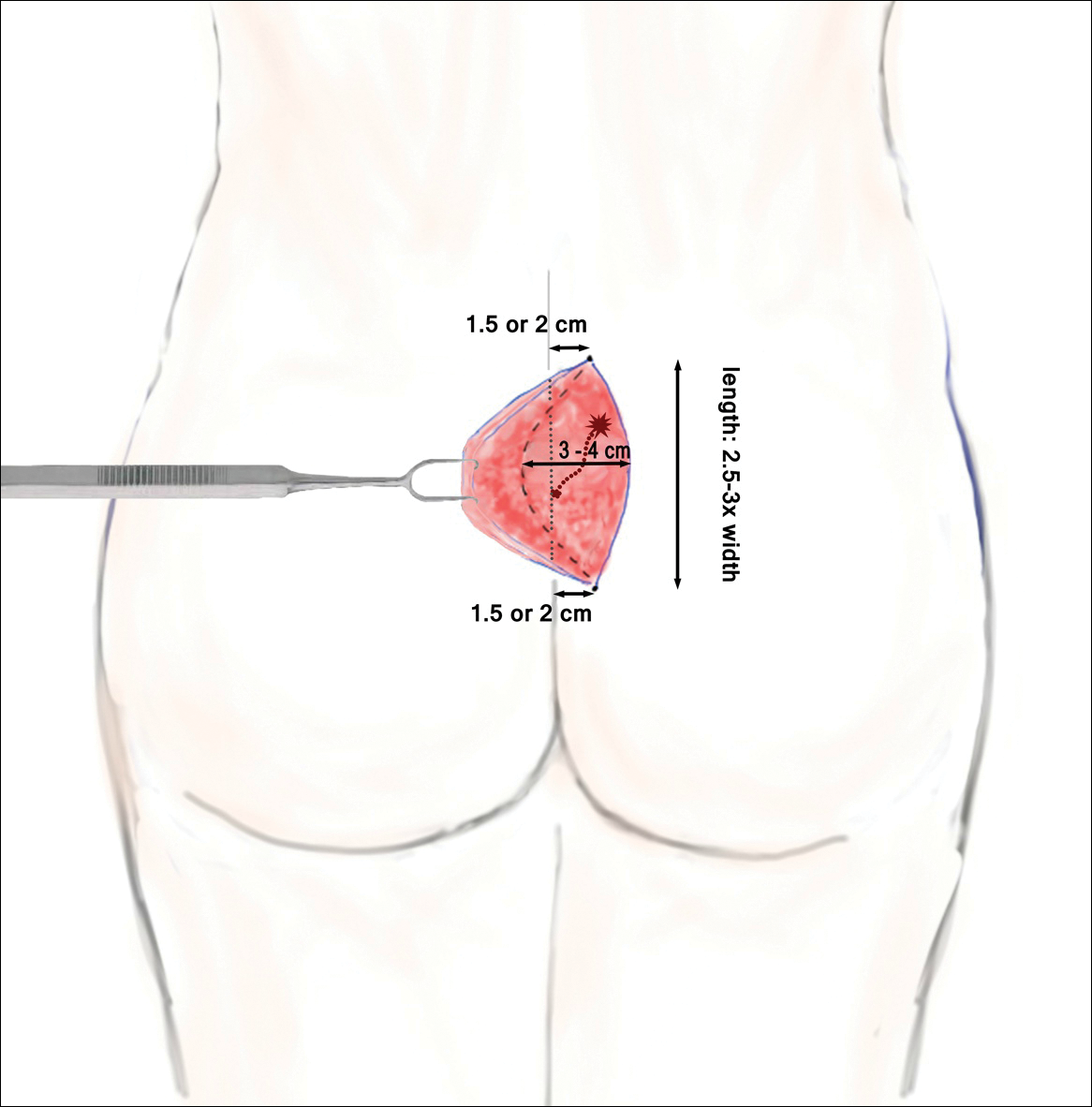
In the modified Karydakis flap, the same excision and closure is performed without tacking the flap to the sacral fascia, aiming to prevent formation of a new vulnerable raphe by flattening the natal cleft. The infection rate was similar to the classic Karydakis flap, and no recurrences were noted during a 20-month follow-up.36
Limberg Flap
The Limberg flap is derived from a rhomboid flap. In the classic Limberg flap, a midline rhomboid incision to the presacral fascia including the sinus is performed. The flap gains mobility by extending the excision laterally to the fascia of the gluteus maximus muscle. A variant of the original flap includes the modified Limberg flap, which lateralizes the midline sutures and flattens the intergluteal sulcus. Compared to the traditional Limberg approach, the modified Limberg flap was associated with a lower failure rate at both early and late time points and a lower rate of infection37,38; however, based on the data it is unclear when primary closure should be favored over a Limberg flap. Several studies show the recurrence rate to be identical; however, hospital stay and pain were reduced in the Limberg flap group compared to primary closure.39,40
Results from randomized controlled trials comparing the modified Limberg flap to the Karydakis flap vary. One of the largest prospective, randomized, controlled trials comparing the 2 flaps included 269 patients.Results showed a lower postoperative complication rate, lower pain scores, shorter operation time, and shorter hospital stay with the Karydakis flap compared to the Limberg flap, though no difference in recurrence was noted between the 2 groups.41
Tw
Overall, larger prospective trials are needed to clarify the differences in outcomes between flap techniques. In
Laser Therapy
Lasers are emerging as primary and adjuvant treatment options for pilonidal sinuses. Depilation with alexandrite, diode, and Nd:YAG lasers has demonstrated the most consistent evidence.50-54 Th
Large randomized controlled trials are needed to fully determine the utility of laser therapy as a primary or adjuvant treatment in pilonidal disease; however, given that laser therapies address the core pathogenesis of pilonidal disease and generally are well tolerated, their use may be strongly considered.
Conclusion
With mild pilonidal disease, more conservative measures can be employed; however, in cases of recurrent or suppurative disease or extensive scarring, excision with flap closure typically is required. Although no single surgical procedure has been identified as superior, one review demonstrated that off-midline procedures are statistically superior to midline closure in healing time, surgical site infection, and recurrence rate.24 Novel techniques continue to emerge in the management of pilonidal disease, including laser therapy. This modality shows promise as either a primary or adjuvant treatment; however, large randomized controlled trials are needed to confirm early findings.
Given that pilonidal disease most commonly occurs in the actively employed population, we recommend that dermatologic surgeons discuss treatment options with patients who have pilonidal disease, taking into consideration cost, length of hospital stay, and recovery time when deciding on a treatment course.
- Mayo OH. Observations on Injuries and Diseases of the Rectum. London, England: Burgess and Hill; 1833.
- Hodges RM. Pilonidal sinus. Boston Med Surg J. 1880;103:485-486.
- Eryilmaz R, Okan I, Ozkan OV, et al. Interdigital pilonidal sinus: a case report and literature review. Dermatol Surg. 2012;38:1400-1403.
- Stone MS. Cysts with a lining of stratified epithelium. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Amsterdam, Netherlands: Elsevier Limited; 2012:1917-1929.
- Khanna A, Rombeau JL. Pilonidal disease. Clin Colon Rectal Surg. 2011;24:46-53.
- de Parades V, Bouchard D, Janier M, et al. Pilonidal sinus disease. J Visc Surg. 2013;150:237-247.
- Harris CL, Laforet K, Sibbald RG, et al. Twelve common mistakes in pilonidal sinus care. Adv Skin Wound Care. 2012;25:325-332.
- Lindholt-Jensen C, Lindholt J, Beyer M, et al. Nd-YAG laser treatment of primary and recurrent pilonidal sinus. Lasers Med Sci. 2012;27:505-508.
- Oueidat D, Rizkallah A, Dirani M, et al. 25 years’ experience in the management of pilonidal sinus disease. Open J Gastro. 2014;4:1-5.
- Gordon P, Grant L, Irwin T. Recurrent pilonidal sepsis. Ulster Med J. 2014;83:10-12.
- Ardelt M, Dittmar Y, Kocijan R, et al. Microbiology of the infected recurrent sacrococcygeal pilonidal sinus. Int Wound J. 2016;13:231-237.
- Eryilmaz R, Bilecik T, Okan I, et al. Recurrent squamous cell carcinoma arising in a neglected pilonidal sinus: report of a case and literature review. Int J Clin Exp Med. 2014;7:446-450.
- Kayaalp C, Aydin C. Review of phenol treatment in sacrococcygeal pilonidal disease. Tech Coloproctol. 2009;13:189-193.
- Dag A, Colak T, Turkmenoglu O, et al. Phenol procedure for pilonidal sinus disease and risk factors for treatment failure. Surgery. 2012;151:113-117.
- Olmez A, Kayaalp C, Aydin C. Treatment of pilonidal disease by combination of pit excision and phenol application. Tech Coloproctol. 2013;17:201-206.
- Jensen SL, Harling H. Prognosis after simple incision and drainage for a first-episode acute pilonidal abscess. Br J Surg. 1988;75:60-61.
- Kepenekci I, Demirkan A, Celasin H, et al. Unroofing and curettage for the treatment of acute and chronic pilonidal disease. World J Surg. 2010;34:153-157.
- Søndenaa K, Nesvik I, Anderson E, et al. Recurrent pilonidal sinus after excision with closed or open treatment: final results of a randomized trial. Eur J Surg. 1996;162:237-240.
- Spivak H, Brooks VL, Nussbaum M, et al. Treatment of chronic pilonidal disease. Dis Colon Rectum. 1996;39:1136-1139.
- Pilonidal surgery costs. Pilonidal Support Alliance website. https://www.pilonidal.org/treatments/surgical-costs/. Updated January 30, 2016. Accessed October 14, 2018.21. al-Hassan HK, Francis IM, Neglén P. Primary closure or secondary granulation after excision of pilonidal sinus? Acta Chir Scand. 1990;156:695-699.
- Khaira HS, Brown JH. Excision and primary suture of pilonidal sinus. Ann R Coll Surg Engl. 1995;77:242-244.
- Clothier PR, Haywood IR. The natural history of the post anal (pilonidal) sinus. Ann R Coll Surg Engl. 1984;66:201-203.
- Al-Khamis A, McCallum I, King PM, et al. Healing by primary versus secondary intention after surgical treatment for pilonidal sinus. Cochrane Database Syst Rev. 2010;1:CD006213.
- McCallum I, King PM, Bruce J. Healing by primary closure versus open healing after surgery for pilonidal sinus: systematic review and meta-analysis. BMJ. 2008;336:868-871.
- Lee PJ, Raniga S, Biyani DK, et al. Sacrococcygeal pilonidal disease. Colorect Dis. 2008;10:639-650.
- Nursal TZ, Ezer A, Calişkan K, et al. Prospective randomized controlled trial comparing V-Y advancement flaps with primary suture methods in pilonidal disease. Am J Surg. 2010;199:170-177.
- Fazeli MS, Adel MG, Lebaschi AH. Comparison of outcomes in Z-plasty and delayed healing by secondary intention of the wound after excision in the sacral pilonidal sinus: results of a randomized, clinical trial. Dis Col Rectum. 2006;49:1831-1836.
- Bascom JU. Repeat pilonidal operations. Am J Surg. 1987;154:118-122.
- Nordon IM, Senapati A, Cripps NP. A prospective randomized controlled trial of simple Bascom’s technique versus Bascom’s cleft closure in the treatment of chronic pilonidal disease. Am J Surg. 2009;197:189-192.
- Dudnik R, Veldkamp J, Nienhujis S, et al. Secondary healing versus midline closure and modified Bascom natal cleft lift for pilonidal sinus disease. Scand J Surg. 2011;100:110-113.
- Bessa SS. Comparison of short-term results between the modified Karydakis flap and the modified Limberg flap in the management of pilonidal sinus disease: a randomized controlled study. Dis Colon Rectum. 2013;56:491-498.
- Karydakis GE. Easy and successful treatment of pilonidal sinus after explanation of its causative process. Aust N Z J Surg. 1992;62:385-389.
- Kitchen PR. Pilonidal sinus: excision and primary closure with a lateralised wound - the Karydakis operation. Aust N Z J Surg. 1982;52:302-305.
- Akinci OF, Coskun A, Uzunköy A. Simple and effective surgical treatment of pilonidal sinus: asymmetric excision and primary closure using suction drain and subcuticular skin closure. Dis Colon Rectum. 2000;43:701-706.
- Bessa SS. Results of the lateral advancing flap operation (modified Karydakis procedure) for the management of pilonidal sinus disease. Dis Colon Rectum. 2007;50:1935-1940.
- Mentes BB, Leventoglu S, Chin A, et al. Modified Limberg transposition flap for sacrococcygeal pilonidal sinus. Surg Today. 2004;34:419-423.
- Cihan A, Ucan BH, Comert M, et al. Superiority of asymmetric modified Limberg flap for surgical treatment of pilonidal cyst disease. Dis Colon Rectum. 2006;49:244-249.
- Muzi MG, Milito G, Cadeddu F, et al. Randomized comparison of Limberg flap versus modified primary closure for treatment of pilonidal disease. Am J Surg. 2010;200:9-14.
- Tavassoli A, Noorshafiee S, Nazarzadeh R. Comparison of excision with primary repair versus Limberg flap. Int J Surg. 2011;9:343-346.
- Ates M, Dirican A, Sarac M, et al. Short and long-term results of the Karydakis flap versus the Limberg flap for treating pilonidal sinus disease: a prospective randomized study. Am J Surg. 2011;202:568-573.
- Can MF, Sevinc MM, Hancerliogullari O, et al. Multicenter prospective randomized trial comparing modified Limberg flap transposition and Karydakis flap reconstruction in patients with saccrococcygeal pilonidal disease. Am J Surg. 2010;200:318-327.
- Ersoy E, Devay AO, Aktimur R, et al. Comparison of short-term results after Limberg and Karydakis procedures for pilonidal disease: randomized prospective analysis of 100 patients. Colorectal Dis. 2009;11:705-710.
- Okuş A, Sevinç B, Karahan O, et al. Comparison of Limberg flap and tension-free primary closure during pilonidal sinus surgery. World J Surg. 2012;36:431-435.
- Akan K, Tihan D, Duman U, et al. Comparison of surgical Limberg flap technique and crystallized phenol application in the treatment of pilonidal sinus disease: a retrospective study. Ulus Cerrahi Derg. 2013;29:162-166.
- Guner A, Boz A, Ozkan OF, et al. Limberg flap versus Bascom cleft lift techniques for sacrococcygeal pilonidal sinus: prospective, randomized trial. World J Surg. 2013;37:2074-2080.
- Hosseini H, Heidari A, Jafarnejad B. Comparison of three surgical methods in treatment of patients with pilonidal sinus: modified excision and repair/wide excision/wide excision and flap in RASOUL, OMID and SADR hospitals (2004-2007). Indian J Surg. 2013;75:395-400.
- Karaca AS, Ali R, Capar M, et al. Comparison of Limberg flap and excision and primary closure of pilonidal sinus disease, in terms of quality of life and complications. J Korean Surg Soc. 2013;85:236-239.
- Rao J, Deora H, Mandia R. A retrospective study of 50 cases of pilonidal sinus with excision of tract and Z-plasty as treatment of choice for both primary and recurrent cases. Indian J Surg. 2015;77(suppl 2):691-693.
- Landa N, Aller O, Landa-Gundin N, et al. Successful treatment of recurrent pilonidal sinus with laser epilation. Dermatol Surg. 2005;31:726-728.
- Oram Y, Kahraman D, Karincaoğlu Y, et al. Evaluation of 60 patients with pilonidal sinus treated with laser epilation after surgery. Dermatol Surg. 2010;36:88-91.
- Benedetto AV, Lewis AT. Pilonidal sinus disease treated by depilation using an 800 nm diode laser and review of the literature. Dermatol Surg. 2005;31:587-591.
- Lindholt-Jensen CS, Lindholt JS, Beyer M, et al. Nd-YAG treatment of primary and recurrent pilonidal sinus. Lasers Med Sci. 2012;27:505-508.
- Jain V, Jain A. Use of lasers for the management of refractory cases of hidradenitis suppurativa and pilonidal sinus. J Cutan Aesthet. 2012;5:190-192.
Pilonidal disease was first described by Mayo1 in 1833 who hypothesized that the underlying etiology is incomplete separation of the mesoderm and ectoderm layers during embryogenesis. In 1880, Hodges2 coined the term pilonidal sinus; he postulated that sinus formation was incited by hair.2 Today, Hodges theory is known as the acquired theory: hair induces a foreign body response in surrounding tissue, leading to sinus formation. Although pilonidal cysts can occur anywhere on the body, they most commonly extend cephalad in the sacrococcygeal and upper gluteal cleft (Figure 1).3,4 An acute pilonidal cyst typically presents with pain, tenderness, and swelling, similar to the presentation of a superficial abscess in other locations; however, a clue to the diagnosis is the presence of cutaneous pits along the midline of the gluteal cleft.5 Chronic pilonidal disease varies based on the extent of inflammation and scarring; the underlying cavity communicates with the overlying skin through sinuses and often drains with pressure.6

Pilonidal sinuses are rare before puberty or after 40 years of age7 and occur primarily in hirsute men. The ratio of men to women affected is between 3:1 and 4:1.8 Although pilonidal sinuses account for only 15% of anal suppurations, complications arising from pilonidal sinuses are a considerable cause of morbidity, resulting in loss of productivity in otherwise healthy individuals.9 Complications include chronic nonhealing wounds,10 as recurrent pilonidal sinuses tend to become colonized with gram-positive and facultative anaerobic bacteria, whereas primary pilonidal cysts more commonly become infected with anaerobic and gram-negative bacteria.11 Long-standing disease increases the risk of squamous cell carcinoma arising within sinus tracts.10,12
Histopathologically, pilonidal cysts are not true cysts because they lack an epithelial lining. Examination of the cavity commonly reveals hair, debris, and granulation tissue with surrounding foreign-body giant cells (Figure 2).5

The preferred treatment of pilonidal cysts continues to be debated. In this article, we review evidence supporting current modalities including conservative and surgical techniques as well as novel laser therapy for the treatment of pilonidal disease.
Conservative Management Techniques
Phenol Injections
Liquid or crystallized phenol injections have been used for treatment of mild to moderate pilonidal cysts.13 Excess debris is removed by curettage, and phenol is administered through the existing orifices or pits without pressure. The phenol remains in the cavity for 1 to 3 minutes before aspiration. Remaining cyst contents are removed through tissue manipulation, and the sinus is washed with saline. Mean healing time is 20 days (range, +/−14 days).13
Classically, phenol injections have a failure rate of 30% to 40%, especially with multiple sinuses and suppurative disease6; however, the success rate improves with limited disease (ie, no more than 1–3 sinus pits).3 With multiple treatment sessions, a recurrence rate as low as 2% over 25 months has been reported.14 Phenol injection also has been proposed as an adjuvant therapy to pit excision to minimize the need for extensive surgery.15
Simple Incision and Drainage
Simple incision and drainage has a crucial role in the treatment of acute pilonidal disease to decrease pain and relieve tension. Off-midline incisions have been recommended for because the resulting closures fared better against sheer forces applied by the gluteal muscles on the cleft.6 Therefore, the incision often is made off-midline from the gluteal cleft even when the cyst lies directly on the gluteal cleft.
Rates of healing vary widely after incision and drainage, ranging from 45% to 82%.6 Primary pilonidal cysts may respond well, particularly if the cavity is abraded; in one series, 79% (58/73) of patients did not have a recurrence at the average follow-up of 60 months.16
Excision and Unroofing
Techniques for excision and unroofing without primary closure include 2 variants: wide and limited. The wide technique consists of an inwardly slanted excision that is deepest in the center of the cavity. The inward sloping angle of the incision aids in healing because it allows granulation to progress evenly from the base of the wound upward. The depth of the incision should spare the fascia and leave as much fatty tissue as possible while still resecting the entire cavity and associated pits.6 Limited incision techniques aim to shorten the healing period by making smaller incisions into the sinuses, pits, and secondary tracts, and they are frequently supplemented with curettage.6 Noteworthy disadvantages include prolonged healing time, need for professional wound management, and extended medical observation.5 The average duration of wound healing in a study of 300 patients was 5.4 weeks (range, +/−1.1 weeks),17 and the recurrence rate has ranged from 5% to 13%.18,19 Care must be taken to respond to numerous possible complications, including excessive exudation and granulation, superinfection, and walling off.6
Although the cost of treatment varies by hospital, location, and a patient’s insurance coverage, patient reports to the Pilonidal Support Alliance indicate that the cost of conservative management ranges from $500 to $2000.20
Excision and Primary Closure
An elliptical excision that includes some of the lateral margin is excised down to the level of the fascia. Adjacent lateral tracts may be excised by expanding the incision. To close the wound, edges are approximated with placement of deep and superficial sutures. Wound healing typically occurs faster than secondary granulation, as seen in one randomized controlled trial with a mean of 10 days for primary closure compared to 13 weeks for secondary intention.21 However, as with any surgical procedure, postoperative complications can delay wound healing.19 The recurrence rate after primary closure varies considerably, ranging from 10% to 38%.18,21-23 The average cost of an excision ranges from $3000 to $6000.20
A

Surgical Techniques
For severe or recurrent pilonidal disease, skin flaps often are required. Several flaps have been developed, including advancement, Bascom cleft lift, Karydakis, and modified Limberg flap. Flaps require a vascular pedicle but allow for closure without tension.26 The cost of a flap procedure, ranging from $10,000 to $30,000, is greater than the cost of excision or other conservative therapy20; however, with a lower recurrence rate of pilonidal disease following flap procedures compared to other treatments, patients may save more on treatment over the long-term.
Advancement Flaps
The most commonly used advancement flaps are the V-Y advancement flap and Z-plasty. The V-Y advancement flap creates a full-thickness V-shaped incision down to gluteal fascia that is closed to form a postrepair suture line in the shape of a Y.5 Depending on the size of the defect, the flaps may be utilized unilaterally or bilaterally. A defect as large as 8 to 10 cm can be covered unilaterally; however, defects larger than 10 cm commonly require a bilateral flap.26 The V-Y advancement flap failed to show superiority to primary closure techniques based on complications, recurrence, and patient satisfaction in a large randomized controlled trial.27
Performing a Z-plasty requires excision of diseased tissue with recruitment of lateral flaps incised down to the level of the fascia. The lateral edges are transposed to increase transverse length.26 No statistically significant difference in infection or recurrence rates was noted between excision alone and excision plus Z-plasty; however, wounds were reported to heal faster in patients receiving excision plus Z-plasty (41 vs 15 days).28
Cleft Lift Closure
In 1987, Bascom29 introduced the cleft lift closure for recurrent pilonidal disease. This technique aims to reduce or eliminate lateral gluteal forces on the wounds by filling the gluteal cleft.5 The sinus tracts are excised and a full-thickness skin flap is extended across the cleft and closed off-midline. The adipose tissue fills in the previous space of the gluteal cleft. In the initial study, no recurrences were reported in 30 patients who underwent this procedure at 2-year follow-up; similarly, in another case series of 26 patients who underwent the procedure, no recurrences were noted at a median follow-up of 3 years.30 Compared to excision with secondary wound healing and primary closure on the midline, the Bascom cleft lift demonstrated a decrease in wound healing time (62, 52, and 29 days, respectively).31
The classic Karydakis flap consists of an oblique elliptical excision of diseased tissue with fixation of the flap base to the sacral fascia (Figures 4 and 5). The flap is closed by suturing the edge off-midline.32 This technique prevents a midline wound and aims to remodel and flatten the natal cleft. Karydakis33 performed the most important study for treatment of pilonidal disease with the Karydakis flap, which included more than 5000 patients. The results showed a 0.9% recurrence rate and an 8.5% wound complication rate over a 2- to 20-year follow-up.33 These results have been substantiated by more recent studies, which produced similar results: a 1.8% to 5.3% infection rate and a recurrence rate of 0.9% to 4.4%.34,35


In the modified Karydakis flap, the same excision and closure is performed without tacking the flap to the sacral fascia, aiming to prevent formation of a new vulnerable raphe by flattening the natal cleft. The infection rate was similar to the classic Karydakis flap, and no recurrences were noted during a 20-month follow-up.36
Limberg Flap
The Limberg flap is derived from a rhomboid flap. In the classic Limberg flap, a midline rhomboid incision to the presacral fascia including the sinus is performed. The flap gains mobility by extending the excision laterally to the fascia of the gluteus maximus muscle. A variant of the original flap includes the modified Limberg flap, which lateralizes the midline sutures and flattens the intergluteal sulcus. Compared to the traditional Limberg approach, the modified Limberg flap was associated with a lower failure rate at both early and late time points and a lower rate of infection37,38; however, based on the data it is unclear when primary closure should be favored over a Limberg flap. Several studies show the recurrence rate to be identical; however, hospital stay and pain were reduced in the Limberg flap group compared to primary closure.39,40
Results from randomized controlled trials comparing the modified Limberg flap to the Karydakis flap vary. One of the largest prospective, randomized, controlled trials comparing the 2 flaps included 269 patients.Results showed a lower postoperative complication rate, lower pain scores, shorter operation time, and shorter hospital stay with the Karydakis flap compared to the Limberg flap, though no difference in recurrence was noted between the 2 groups.41
Tw
Overall, larger prospective trials are needed to clarify the differences in outcomes between flap techniques. In
Laser Therapy
Lasers are emerging as primary and adjuvant treatment options for pilonidal sinuses. Depilation with alexandrite, diode, and Nd:YAG lasers has demonstrated the most consistent evidence.50-54 Th
Large randomized controlled trials are needed to fully determine the utility of laser therapy as a primary or adjuvant treatment in pilonidal disease; however, given that laser therapies address the core pathogenesis of pilonidal disease and generally are well tolerated, their use may be strongly considered.
Conclusion
With mild pilonidal disease, more conservative measures can be employed; however, in cases of recurrent or suppurative disease or extensive scarring, excision with flap closure typically is required. Although no single surgical procedure has been identified as superior, one review demonstrated that off-midline procedures are statistically superior to midline closure in healing time, surgical site infection, and recurrence rate.24 Novel techniques continue to emerge in the management of pilonidal disease, including laser therapy. This modality shows promise as either a primary or adjuvant treatment; however, large randomized controlled trials are needed to confirm early findings.
Given that pilonidal disease most commonly occurs in the actively employed population, we recommend that dermatologic surgeons discuss treatment options with patients who have pilonidal disease, taking into consideration cost, length of hospital stay, and recovery time when deciding on a treatment course.
Pilonidal disease was first described by Mayo1 in 1833 who hypothesized that the underlying etiology is incomplete separation of the mesoderm and ectoderm layers during embryogenesis. In 1880, Hodges2 coined the term pilonidal sinus; he postulated that sinus formation was incited by hair.2 Today, Hodges theory is known as the acquired theory: hair induces a foreign body response in surrounding tissue, leading to sinus formation. Although pilonidal cysts can occur anywhere on the body, they most commonly extend cephalad in the sacrococcygeal and upper gluteal cleft (Figure 1).3,4 An acute pilonidal cyst typically presents with pain, tenderness, and swelling, similar to the presentation of a superficial abscess in other locations; however, a clue to the diagnosis is the presence of cutaneous pits along the midline of the gluteal cleft.5 Chronic pilonidal disease varies based on the extent of inflammation and scarring; the underlying cavity communicates with the overlying skin through sinuses and often drains with pressure.6

Pilonidal sinuses are rare before puberty or after 40 years of age7 and occur primarily in hirsute men. The ratio of men to women affected is between 3:1 and 4:1.8 Although pilonidal sinuses account for only 15% of anal suppurations, complications arising from pilonidal sinuses are a considerable cause of morbidity, resulting in loss of productivity in otherwise healthy individuals.9 Complications include chronic nonhealing wounds,10 as recurrent pilonidal sinuses tend to become colonized with gram-positive and facultative anaerobic bacteria, whereas primary pilonidal cysts more commonly become infected with anaerobic and gram-negative bacteria.11 Long-standing disease increases the risk of squamous cell carcinoma arising within sinus tracts.10,12
Histopathologically, pilonidal cysts are not true cysts because they lack an epithelial lining. Examination of the cavity commonly reveals hair, debris, and granulation tissue with surrounding foreign-body giant cells (Figure 2).5

The preferred treatment of pilonidal cysts continues to be debated. In this article, we review evidence supporting current modalities including conservative and surgical techniques as well as novel laser therapy for the treatment of pilonidal disease.
Conservative Management Techniques
Phenol Injections
Liquid or crystallized phenol injections have been used for treatment of mild to moderate pilonidal cysts.13 Excess debris is removed by curettage, and phenol is administered through the existing orifices or pits without pressure. The phenol remains in the cavity for 1 to 3 minutes before aspiration. Remaining cyst contents are removed through tissue manipulation, and the sinus is washed with saline. Mean healing time is 20 days (range, +/−14 days).13
Classically, phenol injections have a failure rate of 30% to 40%, especially with multiple sinuses and suppurative disease6; however, the success rate improves with limited disease (ie, no more than 1–3 sinus pits).3 With multiple treatment sessions, a recurrence rate as low as 2% over 25 months has been reported.14 Phenol injection also has been proposed as an adjuvant therapy to pit excision to minimize the need for extensive surgery.15
Simple Incision and Drainage
Simple incision and drainage has a crucial role in the treatment of acute pilonidal disease to decrease pain and relieve tension. Off-midline incisions have been recommended for because the resulting closures fared better against sheer forces applied by the gluteal muscles on the cleft.6 Therefore, the incision often is made off-midline from the gluteal cleft even when the cyst lies directly on the gluteal cleft.
Rates of healing vary widely after incision and drainage, ranging from 45% to 82%.6 Primary pilonidal cysts may respond well, particularly if the cavity is abraded; in one series, 79% (58/73) of patients did not have a recurrence at the average follow-up of 60 months.16
Excision and Unroofing
Techniques for excision and unroofing without primary closure include 2 variants: wide and limited. The wide technique consists of an inwardly slanted excision that is deepest in the center of the cavity. The inward sloping angle of the incision aids in healing because it allows granulation to progress evenly from the base of the wound upward. The depth of the incision should spare the fascia and leave as much fatty tissue as possible while still resecting the entire cavity and associated pits.6 Limited incision techniques aim to shorten the healing period by making smaller incisions into the sinuses, pits, and secondary tracts, and they are frequently supplemented with curettage.6 Noteworthy disadvantages include prolonged healing time, need for professional wound management, and extended medical observation.5 The average duration of wound healing in a study of 300 patients was 5.4 weeks (range, +/−1.1 weeks),17 and the recurrence rate has ranged from 5% to 13%.18,19 Care must be taken to respond to numerous possible complications, including excessive exudation and granulation, superinfection, and walling off.6
Although the cost of treatment varies by hospital, location, and a patient’s insurance coverage, patient reports to the Pilonidal Support Alliance indicate that the cost of conservative management ranges from $500 to $2000.20
Excision and Primary Closure
An elliptical excision that includes some of the lateral margin is excised down to the level of the fascia. Adjacent lateral tracts may be excised by expanding the incision. To close the wound, edges are approximated with placement of deep and superficial sutures. Wound healing typically occurs faster than secondary granulation, as seen in one randomized controlled trial with a mean of 10 days for primary closure compared to 13 weeks for secondary intention.21 However, as with any surgical procedure, postoperative complications can delay wound healing.19 The recurrence rate after primary closure varies considerably, ranging from 10% to 38%.18,21-23 The average cost of an excision ranges from $3000 to $6000.20
A

Surgical Techniques
For severe or recurrent pilonidal disease, skin flaps often are required. Several flaps have been developed, including advancement, Bascom cleft lift, Karydakis, and modified Limberg flap. Flaps require a vascular pedicle but allow for closure without tension.26 The cost of a flap procedure, ranging from $10,000 to $30,000, is greater than the cost of excision or other conservative therapy20; however, with a lower recurrence rate of pilonidal disease following flap procedures compared to other treatments, patients may save more on treatment over the long-term.
Advancement Flaps
The most commonly used advancement flaps are the V-Y advancement flap and Z-plasty. The V-Y advancement flap creates a full-thickness V-shaped incision down to gluteal fascia that is closed to form a postrepair suture line in the shape of a Y.5 Depending on the size of the defect, the flaps may be utilized unilaterally or bilaterally. A defect as large as 8 to 10 cm can be covered unilaterally; however, defects larger than 10 cm commonly require a bilateral flap.26 The V-Y advancement flap failed to show superiority to primary closure techniques based on complications, recurrence, and patient satisfaction in a large randomized controlled trial.27
Performing a Z-plasty requires excision of diseased tissue with recruitment of lateral flaps incised down to the level of the fascia. The lateral edges are transposed to increase transverse length.26 No statistically significant difference in infection or recurrence rates was noted between excision alone and excision plus Z-plasty; however, wounds were reported to heal faster in patients receiving excision plus Z-plasty (41 vs 15 days).28
Cleft Lift Closure
In 1987, Bascom29 introduced the cleft lift closure for recurrent pilonidal disease. This technique aims to reduce or eliminate lateral gluteal forces on the wounds by filling the gluteal cleft.5 The sinus tracts are excised and a full-thickness skin flap is extended across the cleft and closed off-midline. The adipose tissue fills in the previous space of the gluteal cleft. In the initial study, no recurrences were reported in 30 patients who underwent this procedure at 2-year follow-up; similarly, in another case series of 26 patients who underwent the procedure, no recurrences were noted at a median follow-up of 3 years.30 Compared to excision with secondary wound healing and primary closure on the midline, the Bascom cleft lift demonstrated a decrease in wound healing time (62, 52, and 29 days, respectively).31
The classic Karydakis flap consists of an oblique elliptical excision of diseased tissue with fixation of the flap base to the sacral fascia (Figures 4 and 5). The flap is closed by suturing the edge off-midline.32 This technique prevents a midline wound and aims to remodel and flatten the natal cleft. Karydakis33 performed the most important study for treatment of pilonidal disease with the Karydakis flap, which included more than 5000 patients. The results showed a 0.9% recurrence rate and an 8.5% wound complication rate over a 2- to 20-year follow-up.33 These results have been substantiated by more recent studies, which produced similar results: a 1.8% to 5.3% infection rate and a recurrence rate of 0.9% to 4.4%.34,35


In the modified Karydakis flap, the same excision and closure is performed without tacking the flap to the sacral fascia, aiming to prevent formation of a new vulnerable raphe by flattening the natal cleft. The infection rate was similar to the classic Karydakis flap, and no recurrences were noted during a 20-month follow-up.36
Limberg Flap
The Limberg flap is derived from a rhomboid flap. In the classic Limberg flap, a midline rhomboid incision to the presacral fascia including the sinus is performed. The flap gains mobility by extending the excision laterally to the fascia of the gluteus maximus muscle. A variant of the original flap includes the modified Limberg flap, which lateralizes the midline sutures and flattens the intergluteal sulcus. Compared to the traditional Limberg approach, the modified Limberg flap was associated with a lower failure rate at both early and late time points and a lower rate of infection37,38; however, based on the data it is unclear when primary closure should be favored over a Limberg flap. Several studies show the recurrence rate to be identical; however, hospital stay and pain were reduced in the Limberg flap group compared to primary closure.39,40
Results from randomized controlled trials comparing the modified Limberg flap to the Karydakis flap vary. One of the largest prospective, randomized, controlled trials comparing the 2 flaps included 269 patients.Results showed a lower postoperative complication rate, lower pain scores, shorter operation time, and shorter hospital stay with the Karydakis flap compared to the Limberg flap, though no difference in recurrence was noted between the 2 groups.41
Tw
Overall, larger prospective trials are needed to clarify the differences in outcomes between flap techniques. In
Laser Therapy
Lasers are emerging as primary and adjuvant treatment options for pilonidal sinuses. Depilation with alexandrite, diode, and Nd:YAG lasers has demonstrated the most consistent evidence.50-54 Th
Large randomized controlled trials are needed to fully determine the utility of laser therapy as a primary or adjuvant treatment in pilonidal disease; however, given that laser therapies address the core pathogenesis of pilonidal disease and generally are well tolerated, their use may be strongly considered.
Conclusion
With mild pilonidal disease, more conservative measures can be employed; however, in cases of recurrent or suppurative disease or extensive scarring, excision with flap closure typically is required. Although no single surgical procedure has been identified as superior, one review demonstrated that off-midline procedures are statistically superior to midline closure in healing time, surgical site infection, and recurrence rate.24 Novel techniques continue to emerge in the management of pilonidal disease, including laser therapy. This modality shows promise as either a primary or adjuvant treatment; however, large randomized controlled trials are needed to confirm early findings.
Given that pilonidal disease most commonly occurs in the actively employed population, we recommend that dermatologic surgeons discuss treatment options with patients who have pilonidal disease, taking into consideration cost, length of hospital stay, and recovery time when deciding on a treatment course.
- Mayo OH. Observations on Injuries and Diseases of the Rectum. London, England: Burgess and Hill; 1833.
- Hodges RM. Pilonidal sinus. Boston Med Surg J. 1880;103:485-486.
- Eryilmaz R, Okan I, Ozkan OV, et al. Interdigital pilonidal sinus: a case report and literature review. Dermatol Surg. 2012;38:1400-1403.
- Stone MS. Cysts with a lining of stratified epithelium. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Amsterdam, Netherlands: Elsevier Limited; 2012:1917-1929.
- Khanna A, Rombeau JL. Pilonidal disease. Clin Colon Rectal Surg. 2011;24:46-53.
- de Parades V, Bouchard D, Janier M, et al. Pilonidal sinus disease. J Visc Surg. 2013;150:237-247.
- Harris CL, Laforet K, Sibbald RG, et al. Twelve common mistakes in pilonidal sinus care. Adv Skin Wound Care. 2012;25:325-332.
- Lindholt-Jensen C, Lindholt J, Beyer M, et al. Nd-YAG laser treatment of primary and recurrent pilonidal sinus. Lasers Med Sci. 2012;27:505-508.
- Oueidat D, Rizkallah A, Dirani M, et al. 25 years’ experience in the management of pilonidal sinus disease. Open J Gastro. 2014;4:1-5.
- Gordon P, Grant L, Irwin T. Recurrent pilonidal sepsis. Ulster Med J. 2014;83:10-12.
- Ardelt M, Dittmar Y, Kocijan R, et al. Microbiology of the infected recurrent sacrococcygeal pilonidal sinus. Int Wound J. 2016;13:231-237.
- Eryilmaz R, Bilecik T, Okan I, et al. Recurrent squamous cell carcinoma arising in a neglected pilonidal sinus: report of a case and literature review. Int J Clin Exp Med. 2014;7:446-450.
- Kayaalp C, Aydin C. Review of phenol treatment in sacrococcygeal pilonidal disease. Tech Coloproctol. 2009;13:189-193.
- Dag A, Colak T, Turkmenoglu O, et al. Phenol procedure for pilonidal sinus disease and risk factors for treatment failure. Surgery. 2012;151:113-117.
- Olmez A, Kayaalp C, Aydin C. Treatment of pilonidal disease by combination of pit excision and phenol application. Tech Coloproctol. 2013;17:201-206.
- Jensen SL, Harling H. Prognosis after simple incision and drainage for a first-episode acute pilonidal abscess. Br J Surg. 1988;75:60-61.
- Kepenekci I, Demirkan A, Celasin H, et al. Unroofing and curettage for the treatment of acute and chronic pilonidal disease. World J Surg. 2010;34:153-157.
- Søndenaa K, Nesvik I, Anderson E, et al. Recurrent pilonidal sinus after excision with closed or open treatment: final results of a randomized trial. Eur J Surg. 1996;162:237-240.
- Spivak H, Brooks VL, Nussbaum M, et al. Treatment of chronic pilonidal disease. Dis Colon Rectum. 1996;39:1136-1139.
- Pilonidal surgery costs. Pilonidal Support Alliance website. https://www.pilonidal.org/treatments/surgical-costs/. Updated January 30, 2016. Accessed October 14, 2018.21. al-Hassan HK, Francis IM, Neglén P. Primary closure or secondary granulation after excision of pilonidal sinus? Acta Chir Scand. 1990;156:695-699.
- Khaira HS, Brown JH. Excision and primary suture of pilonidal sinus. Ann R Coll Surg Engl. 1995;77:242-244.
- Clothier PR, Haywood IR. The natural history of the post anal (pilonidal) sinus. Ann R Coll Surg Engl. 1984;66:201-203.
- Al-Khamis A, McCallum I, King PM, et al. Healing by primary versus secondary intention after surgical treatment for pilonidal sinus. Cochrane Database Syst Rev. 2010;1:CD006213.
- McCallum I, King PM, Bruce J. Healing by primary closure versus open healing after surgery for pilonidal sinus: systematic review and meta-analysis. BMJ. 2008;336:868-871.
- Lee PJ, Raniga S, Biyani DK, et al. Sacrococcygeal pilonidal disease. Colorect Dis. 2008;10:639-650.
- Nursal TZ, Ezer A, Calişkan K, et al. Prospective randomized controlled trial comparing V-Y advancement flaps with primary suture methods in pilonidal disease. Am J Surg. 2010;199:170-177.
- Fazeli MS, Adel MG, Lebaschi AH. Comparison of outcomes in Z-plasty and delayed healing by secondary intention of the wound after excision in the sacral pilonidal sinus: results of a randomized, clinical trial. Dis Col Rectum. 2006;49:1831-1836.
- Bascom JU. Repeat pilonidal operations. Am J Surg. 1987;154:118-122.
- Nordon IM, Senapati A, Cripps NP. A prospective randomized controlled trial of simple Bascom’s technique versus Bascom’s cleft closure in the treatment of chronic pilonidal disease. Am J Surg. 2009;197:189-192.
- Dudnik R, Veldkamp J, Nienhujis S, et al. Secondary healing versus midline closure and modified Bascom natal cleft lift for pilonidal sinus disease. Scand J Surg. 2011;100:110-113.
- Bessa SS. Comparison of short-term results between the modified Karydakis flap and the modified Limberg flap in the management of pilonidal sinus disease: a randomized controlled study. Dis Colon Rectum. 2013;56:491-498.
- Karydakis GE. Easy and successful treatment of pilonidal sinus after explanation of its causative process. Aust N Z J Surg. 1992;62:385-389.
- Kitchen PR. Pilonidal sinus: excision and primary closure with a lateralised wound - the Karydakis operation. Aust N Z J Surg. 1982;52:302-305.
- Akinci OF, Coskun A, Uzunköy A. Simple and effective surgical treatment of pilonidal sinus: asymmetric excision and primary closure using suction drain and subcuticular skin closure. Dis Colon Rectum. 2000;43:701-706.
- Bessa SS. Results of the lateral advancing flap operation (modified Karydakis procedure) for the management of pilonidal sinus disease. Dis Colon Rectum. 2007;50:1935-1940.
- Mentes BB, Leventoglu S, Chin A, et al. Modified Limberg transposition flap for sacrococcygeal pilonidal sinus. Surg Today. 2004;34:419-423.
- Cihan A, Ucan BH, Comert M, et al. Superiority of asymmetric modified Limberg flap for surgical treatment of pilonidal cyst disease. Dis Colon Rectum. 2006;49:244-249.
- Muzi MG, Milito G, Cadeddu F, et al. Randomized comparison of Limberg flap versus modified primary closure for treatment of pilonidal disease. Am J Surg. 2010;200:9-14.
- Tavassoli A, Noorshafiee S, Nazarzadeh R. Comparison of excision with primary repair versus Limberg flap. Int J Surg. 2011;9:343-346.
- Ates M, Dirican A, Sarac M, et al. Short and long-term results of the Karydakis flap versus the Limberg flap for treating pilonidal sinus disease: a prospective randomized study. Am J Surg. 2011;202:568-573.
- Can MF, Sevinc MM, Hancerliogullari O, et al. Multicenter prospective randomized trial comparing modified Limberg flap transposition and Karydakis flap reconstruction in patients with saccrococcygeal pilonidal disease. Am J Surg. 2010;200:318-327.
- Ersoy E, Devay AO, Aktimur R, et al. Comparison of short-term results after Limberg and Karydakis procedures for pilonidal disease: randomized prospective analysis of 100 patients. Colorectal Dis. 2009;11:705-710.
- Okuş A, Sevinç B, Karahan O, et al. Comparison of Limberg flap and tension-free primary closure during pilonidal sinus surgery. World J Surg. 2012;36:431-435.
- Akan K, Tihan D, Duman U, et al. Comparison of surgical Limberg flap technique and crystallized phenol application in the treatment of pilonidal sinus disease: a retrospective study. Ulus Cerrahi Derg. 2013;29:162-166.
- Guner A, Boz A, Ozkan OF, et al. Limberg flap versus Bascom cleft lift techniques for sacrococcygeal pilonidal sinus: prospective, randomized trial. World J Surg. 2013;37:2074-2080.
- Hosseini H, Heidari A, Jafarnejad B. Comparison of three surgical methods in treatment of patients with pilonidal sinus: modified excision and repair/wide excision/wide excision and flap in RASOUL, OMID and SADR hospitals (2004-2007). Indian J Surg. 2013;75:395-400.
- Karaca AS, Ali R, Capar M, et al. Comparison of Limberg flap and excision and primary closure of pilonidal sinus disease, in terms of quality of life and complications. J Korean Surg Soc. 2013;85:236-239.
- Rao J, Deora H, Mandia R. A retrospective study of 50 cases of pilonidal sinus with excision of tract and Z-plasty as treatment of choice for both primary and recurrent cases. Indian J Surg. 2015;77(suppl 2):691-693.
- Landa N, Aller O, Landa-Gundin N, et al. Successful treatment of recurrent pilonidal sinus with laser epilation. Dermatol Surg. 2005;31:726-728.
- Oram Y, Kahraman D, Karincaoğlu Y, et al. Evaluation of 60 patients with pilonidal sinus treated with laser epilation after surgery. Dermatol Surg. 2010;36:88-91.
- Benedetto AV, Lewis AT. Pilonidal sinus disease treated by depilation using an 800 nm diode laser and review of the literature. Dermatol Surg. 2005;31:587-591.
- Lindholt-Jensen CS, Lindholt JS, Beyer M, et al. Nd-YAG treatment of primary and recurrent pilonidal sinus. Lasers Med Sci. 2012;27:505-508.
- Jain V, Jain A. Use of lasers for the management of refractory cases of hidradenitis suppurativa and pilonidal sinus. J Cutan Aesthet. 2012;5:190-192.
- Mayo OH. Observations on Injuries and Diseases of the Rectum. London, England: Burgess and Hill; 1833.
- Hodges RM. Pilonidal sinus. Boston Med Surg J. 1880;103:485-486.
- Eryilmaz R, Okan I, Ozkan OV, et al. Interdigital pilonidal sinus: a case report and literature review. Dermatol Surg. 2012;38:1400-1403.
- Stone MS. Cysts with a lining of stratified epithelium. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Amsterdam, Netherlands: Elsevier Limited; 2012:1917-1929.
- Khanna A, Rombeau JL. Pilonidal disease. Clin Colon Rectal Surg. 2011;24:46-53.
- de Parades V, Bouchard D, Janier M, et al. Pilonidal sinus disease. J Visc Surg. 2013;150:237-247.
- Harris CL, Laforet K, Sibbald RG, et al. Twelve common mistakes in pilonidal sinus care. Adv Skin Wound Care. 2012;25:325-332.
- Lindholt-Jensen C, Lindholt J, Beyer M, et al. Nd-YAG laser treatment of primary and recurrent pilonidal sinus. Lasers Med Sci. 2012;27:505-508.
- Oueidat D, Rizkallah A, Dirani M, et al. 25 years’ experience in the management of pilonidal sinus disease. Open J Gastro. 2014;4:1-5.
- Gordon P, Grant L, Irwin T. Recurrent pilonidal sepsis. Ulster Med J. 2014;83:10-12.
- Ardelt M, Dittmar Y, Kocijan R, et al. Microbiology of the infected recurrent sacrococcygeal pilonidal sinus. Int Wound J. 2016;13:231-237.
- Eryilmaz R, Bilecik T, Okan I, et al. Recurrent squamous cell carcinoma arising in a neglected pilonidal sinus: report of a case and literature review. Int J Clin Exp Med. 2014;7:446-450.
- Kayaalp C, Aydin C. Review of phenol treatment in sacrococcygeal pilonidal disease. Tech Coloproctol. 2009;13:189-193.
- Dag A, Colak T, Turkmenoglu O, et al. Phenol procedure for pilonidal sinus disease and risk factors for treatment failure. Surgery. 2012;151:113-117.
- Olmez A, Kayaalp C, Aydin C. Treatment of pilonidal disease by combination of pit excision and phenol application. Tech Coloproctol. 2013;17:201-206.
- Jensen SL, Harling H. Prognosis after simple incision and drainage for a first-episode acute pilonidal abscess. Br J Surg. 1988;75:60-61.
- Kepenekci I, Demirkan A, Celasin H, et al. Unroofing and curettage for the treatment of acute and chronic pilonidal disease. World J Surg. 2010;34:153-157.
- Søndenaa K, Nesvik I, Anderson E, et al. Recurrent pilonidal sinus after excision with closed or open treatment: final results of a randomized trial. Eur J Surg. 1996;162:237-240.
- Spivak H, Brooks VL, Nussbaum M, et al. Treatment of chronic pilonidal disease. Dis Colon Rectum. 1996;39:1136-1139.
- Pilonidal surgery costs. Pilonidal Support Alliance website. https://www.pilonidal.org/treatments/surgical-costs/. Updated January 30, 2016. Accessed October 14, 2018.21. al-Hassan HK, Francis IM, Neglén P. Primary closure or secondary granulation after excision of pilonidal sinus? Acta Chir Scand. 1990;156:695-699.
- Khaira HS, Brown JH. Excision and primary suture of pilonidal sinus. Ann R Coll Surg Engl. 1995;77:242-244.
- Clothier PR, Haywood IR. The natural history of the post anal (pilonidal) sinus. Ann R Coll Surg Engl. 1984;66:201-203.
- Al-Khamis A, McCallum I, King PM, et al. Healing by primary versus secondary intention after surgical treatment for pilonidal sinus. Cochrane Database Syst Rev. 2010;1:CD006213.
- McCallum I, King PM, Bruce J. Healing by primary closure versus open healing after surgery for pilonidal sinus: systematic review and meta-analysis. BMJ. 2008;336:868-871.
- Lee PJ, Raniga S, Biyani DK, et al. Sacrococcygeal pilonidal disease. Colorect Dis. 2008;10:639-650.
- Nursal TZ, Ezer A, Calişkan K, et al. Prospective randomized controlled trial comparing V-Y advancement flaps with primary suture methods in pilonidal disease. Am J Surg. 2010;199:170-177.
- Fazeli MS, Adel MG, Lebaschi AH. Comparison of outcomes in Z-plasty and delayed healing by secondary intention of the wound after excision in the sacral pilonidal sinus: results of a randomized, clinical trial. Dis Col Rectum. 2006;49:1831-1836.
- Bascom JU. Repeat pilonidal operations. Am J Surg. 1987;154:118-122.
- Nordon IM, Senapati A, Cripps NP. A prospective randomized controlled trial of simple Bascom’s technique versus Bascom’s cleft closure in the treatment of chronic pilonidal disease. Am J Surg. 2009;197:189-192.
- Dudnik R, Veldkamp J, Nienhujis S, et al. Secondary healing versus midline closure and modified Bascom natal cleft lift for pilonidal sinus disease. Scand J Surg. 2011;100:110-113.
- Bessa SS. Comparison of short-term results between the modified Karydakis flap and the modified Limberg flap in the management of pilonidal sinus disease: a randomized controlled study. Dis Colon Rectum. 2013;56:491-498.
- Karydakis GE. Easy and successful treatment of pilonidal sinus after explanation of its causative process. Aust N Z J Surg. 1992;62:385-389.
- Kitchen PR. Pilonidal sinus: excision and primary closure with a lateralised wound - the Karydakis operation. Aust N Z J Surg. 1982;52:302-305.
- Akinci OF, Coskun A, Uzunköy A. Simple and effective surgical treatment of pilonidal sinus: asymmetric excision and primary closure using suction drain and subcuticular skin closure. Dis Colon Rectum. 2000;43:701-706.
- Bessa SS. Results of the lateral advancing flap operation (modified Karydakis procedure) for the management of pilonidal sinus disease. Dis Colon Rectum. 2007;50:1935-1940.
- Mentes BB, Leventoglu S, Chin A, et al. Modified Limberg transposition flap for sacrococcygeal pilonidal sinus. Surg Today. 2004;34:419-423.
- Cihan A, Ucan BH, Comert M, et al. Superiority of asymmetric modified Limberg flap for surgical treatment of pilonidal cyst disease. Dis Colon Rectum. 2006;49:244-249.
- Muzi MG, Milito G, Cadeddu F, et al. Randomized comparison of Limberg flap versus modified primary closure for treatment of pilonidal disease. Am J Surg. 2010;200:9-14.
- Tavassoli A, Noorshafiee S, Nazarzadeh R. Comparison of excision with primary repair versus Limberg flap. Int J Surg. 2011;9:343-346.
- Ates M, Dirican A, Sarac M, et al. Short and long-term results of the Karydakis flap versus the Limberg flap for treating pilonidal sinus disease: a prospective randomized study. Am J Surg. 2011;202:568-573.
- Can MF, Sevinc MM, Hancerliogullari O, et al. Multicenter prospective randomized trial comparing modified Limberg flap transposition and Karydakis flap reconstruction in patients with saccrococcygeal pilonidal disease. Am J Surg. 2010;200:318-327.
- Ersoy E, Devay AO, Aktimur R, et al. Comparison of short-term results after Limberg and Karydakis procedures for pilonidal disease: randomized prospective analysis of 100 patients. Colorectal Dis. 2009;11:705-710.
- Okuş A, Sevinç B, Karahan O, et al. Comparison of Limberg flap and tension-free primary closure during pilonidal sinus surgery. World J Surg. 2012;36:431-435.
- Akan K, Tihan D, Duman U, et al. Comparison of surgical Limberg flap technique and crystallized phenol application in the treatment of pilonidal sinus disease: a retrospective study. Ulus Cerrahi Derg. 2013;29:162-166.
- Guner A, Boz A, Ozkan OF, et al. Limberg flap versus Bascom cleft lift techniques for sacrococcygeal pilonidal sinus: prospective, randomized trial. World J Surg. 2013;37:2074-2080.
- Hosseini H, Heidari A, Jafarnejad B. Comparison of three surgical methods in treatment of patients with pilonidal sinus: modified excision and repair/wide excision/wide excision and flap in RASOUL, OMID and SADR hospitals (2004-2007). Indian J Surg. 2013;75:395-400.
- Karaca AS, Ali R, Capar M, et al. Comparison of Limberg flap and excision and primary closure of pilonidal sinus disease, in terms of quality of life and complications. J Korean Surg Soc. 2013;85:236-239.
- Rao J, Deora H, Mandia R. A retrospective study of 50 cases of pilonidal sinus with excision of tract and Z-plasty as treatment of choice for both primary and recurrent cases. Indian J Surg. 2015;77(suppl 2):691-693.
- Landa N, Aller O, Landa-Gundin N, et al. Successful treatment of recurrent pilonidal sinus with laser epilation. Dermatol Surg. 2005;31:726-728.
- Oram Y, Kahraman D, Karincaoğlu Y, et al. Evaluation of 60 patients with pilonidal sinus treated with laser epilation after surgery. Dermatol Surg. 2010;36:88-91.
- Benedetto AV, Lewis AT. Pilonidal sinus disease treated by depilation using an 800 nm diode laser and review of the literature. Dermatol Surg. 2005;31:587-591.
- Lindholt-Jensen CS, Lindholt JS, Beyer M, et al. Nd-YAG treatment of primary and recurrent pilonidal sinus. Lasers Med Sci. 2012;27:505-508.
- Jain V, Jain A. Use of lasers for the management of refractory cases of hidradenitis suppurativa and pilonidal sinus. J Cutan Aesthet. 2012;5:190-192.
Practice Points
- Mild pilonidal disease can be treated with conservative measures, including phenol injection and simple excision and drainage. Recurrent disease or the presence of extensive scarring or suppurative disease typically necessitates excision with flap closure.
- Off-midline procedures have been shown to be statistically superior to midline closure with regard to healing time, infection at the surgical site, and rate of recurrence.
- Laser excision holds promise as a primary or adjuvant treatment of pilonidal disease; however, large randomized controlled trials are needed to confirm early findings.
Tinea Incognito in an Urban Pediatric Population
Tinea incognito (TI) describes a dermatophytosis with often atypical clinical features attributed to prior use of topical corticosteroids or other immunomodulating agents. Tinea incognito may lack the scale and elevated margin typical of cutaneous dermatophytoses and can be mistaken for other pediatric cutaneous diseases, particularly atopic dermatitis. 1 Given the prevalence of TI and its susceptibility to misdiagnosis, we conducted a retrospective medical record review of cases of pediatric dermatophytosis presenting from 2005 to 2016.
Methods
We reviewed medical records for patients younger than 18 years who had been seen at the Faculty Group Practice of the Ronald O. Perelman Department of Dermatology, New York University School of Medicine (New York, New York), between January 1, 2005, and October 21, 2016, using International Classification of Diseases, Ninth Revision (ICD-9) codes 110.0 (tinea capitis), 110.1 (onychomycosis/tinea unguium), 110.3 (tinea cruris), 110.4 (tinea pedis), 110.5 (tinea corporis), and 110.9 (tinea, unspecified site). Cases were included in this study if there was documentation of dermatophytosis previously treated with topical corticosteroids or calcineurin inhibitors as well as positive potassium hydroxide (KOH) preparation or fungal culture with dermatophyte growth obtained from lesions satisfying the first criterion. This study was approved by the New York University School of Medicine institutional review board (study no. S15-01388).
Statistical analyses were conducted in SPSS 19.0 for Windows. Categorical variables were assessed using the χ2 test for independence and the Fisher exact test.
Results
A total of 464 cases were reviewed. A positive KOH preparation or dermatophyte fungal culture was documented in 83 cases. Of them, 29 (34.9%) were treated with topical steroids and/or calcineurin inhibitors prior to presentation to dermatology (Table). The mean age at presentation was 8 years. Duration of symptoms prior to presentation was recorded for 23 of 29 patients (79.3%). Of them, 6 (26.1%) experienced symptoms for 1 month or less, 12 (52.2%) for 1 to 6 months, and 5 (21.7%) for 6 months to 1 year.
Physical examination findings (Figure) were documented in all 29 cases. Annular lesions were noted in 24 patients (82.8%). Pustules were present in 5 patients (17.2%) and papules in 11 patients (37.9%). Fourteen patients (48.3%) had involvement of the face, 14 (48.3%) of the body (ie, trunk, extremities, or groin), and 3 (10.3%) of the scalp. Six patients (20.7%) demonstrated findings at more than one body site.
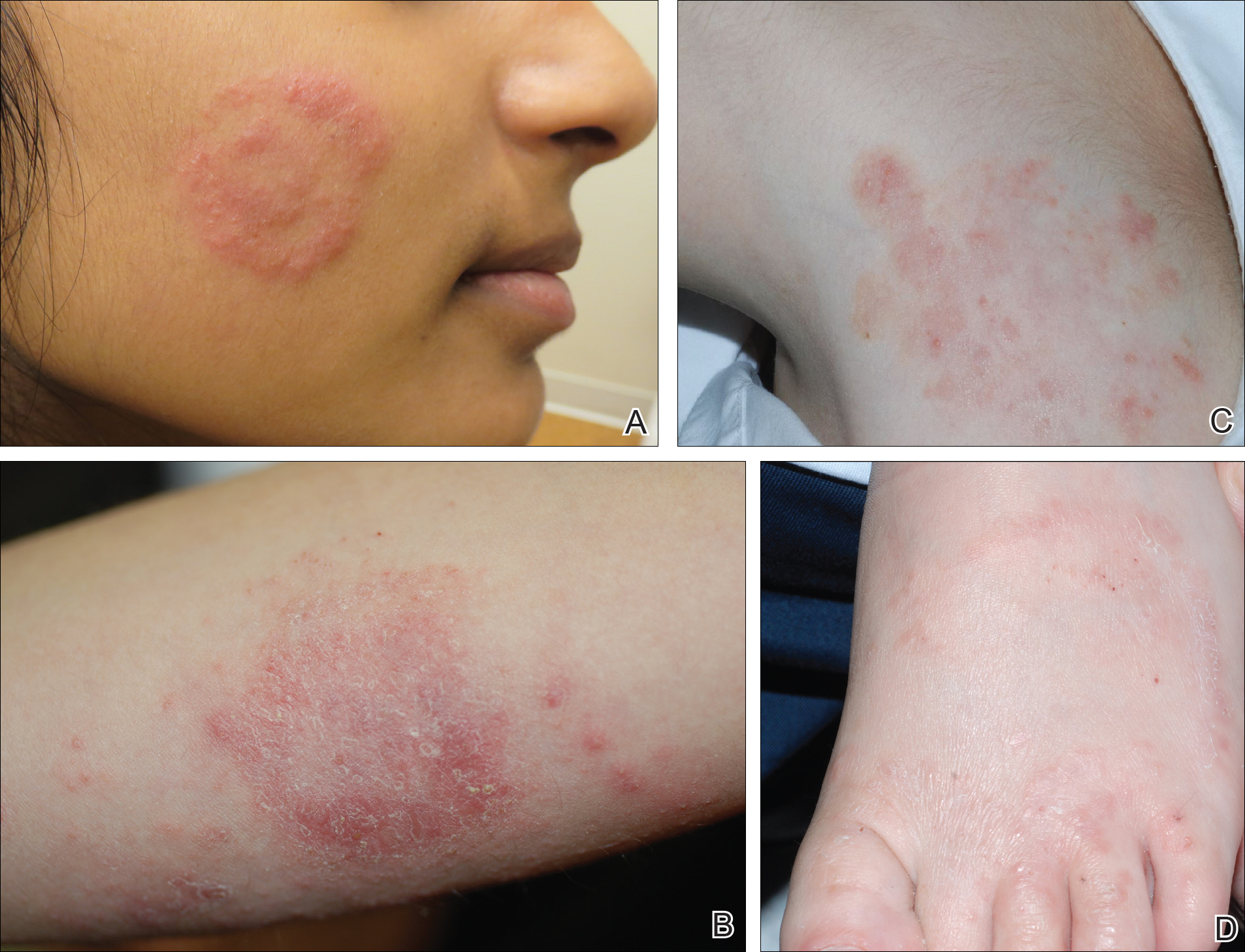
Females were more likely to demonstrate facial lesions (P=.02), while males were more likely to present with body lesions (P=.04). Of 26 patients diagnosed via fungal culture, 16 (55.2%) grew Trichophyton tonsurans, 4 (13.8%) grew Trichophyton rubrum, 3 (10.3%) grew Trichophyton mentagrophytes, 2 (6.9%) grew Microsporum canis, and 1 (3.4%) grew Microsporum gypseum. Treatment entailed oral medication in 18 cases (62.1%). Of them, 13 (72.2%) were treated with griseofulvin, 3 (16.7%) with fluconazole, and 2 (11.1%) with terbinafine. Topical antifungals were prescribed in the remaining 11 cases (37.9%); no further treatment was documented.
Comment
Since the initial description of TI, approximately 60 case reports and small series as well as several larger observational studies describing TI have been published. In our series of pediatric patients, 29 of 83 culture- or KOH-confirmed dermatophytosis cases (34.9%) were considered to be TI due to treatment with topical corticosteroids and/or calcineurin inhibitors prior to presentation. This high prevalence contrasts with the 5.6% prevalence reported in the only prior large case series examining TI in childhood.2 These authors further reported that in their pediatric population, TI was significantly (odds ratio, 8.7; 95% CI, 4.7-16.1) more likely to occur on the face relative to other dermatophytoses and significantly (odds ratio, 0.014; 95% CI, 0.002-0.099) less likely to occur on the scalp.2 We noted a significant association between female gender and facial symptoms as well as between male gender and truncal symptoms. Taken together, these findings suggest an increased likelihood of pediatric tinea faciei to be inappropriately treated, particularly in females.
Although TI treated with topical corticosteroids or calcineurin inhibitors can mimic other skin diseases, a majority of patients in our series demonstrated findings associated with classic tinea, such as annularity and scale. Further, we found that T tonsurans was the causative organism in most cases with T rubrum uncommonly seen, though it is the most prevalent dermatophyte observed worldwide and in 2 large TI case series.3,4 Regional variation in dermatophytes may account for these differences. In our study, griseofulvin was used most frequently in TI treatment, though a systematic review of oral antifungals in tinea capitis supported terbinafine’s greater efficacy in patients infected with T tonsurans.5
Conclusion
Our case series demonstrated a 35% prevalence of TI cases in a population of children with confirmed dermatophytosis presenting to dermatologists at an American academic medical center. We hope that noting the high prevalence and manifold presentations of this disease will aid practitioners in maintaining clinical suspicion for dermatophytosis and thereby facilitate appropriate identification and treatment of TI.
- Paloni G, Valerio E, Berti I, et al. Tinea incognito [published online September 28, 2015]. J Pediatr. 2015;167:1450-e2.
- del Boz J, Crespo V, Rivas‐Ruiz F, et al. Tinea incognito in children: 54 cases. Mycoses. 2011;54:254-258.
- Romano C, Maritati E, Gianni C. Tinea incognito in Italy: a 15-year survey. Mycoses. 2006;49:383-387.
- Kim WJ, Kim TW, Mun JH, et al. Tinea incognito in Korea and itsrisk factors: nine-year multicenter survey. J Korean Med Sci. 2013;28:145-151.
- Chen X, Jiang X, Yang M, et al. Systemic antifungal therapy for tinea capitis in children: an abridged Cochrane review. J Am Acad Dermatol. 2017;76:368-374.
Tinea incognito (TI) describes a dermatophytosis with often atypical clinical features attributed to prior use of topical corticosteroids or other immunomodulating agents. Tinea incognito may lack the scale and elevated margin typical of cutaneous dermatophytoses and can be mistaken for other pediatric cutaneous diseases, particularly atopic dermatitis. 1 Given the prevalence of TI and its susceptibility to misdiagnosis, we conducted a retrospective medical record review of cases of pediatric dermatophytosis presenting from 2005 to 2016.
Methods
We reviewed medical records for patients younger than 18 years who had been seen at the Faculty Group Practice of the Ronald O. Perelman Department of Dermatology, New York University School of Medicine (New York, New York), between January 1, 2005, and October 21, 2016, using International Classification of Diseases, Ninth Revision (ICD-9) codes 110.0 (tinea capitis), 110.1 (onychomycosis/tinea unguium), 110.3 (tinea cruris), 110.4 (tinea pedis), 110.5 (tinea corporis), and 110.9 (tinea, unspecified site). Cases were included in this study if there was documentation of dermatophytosis previously treated with topical corticosteroids or calcineurin inhibitors as well as positive potassium hydroxide (KOH) preparation or fungal culture with dermatophyte growth obtained from lesions satisfying the first criterion. This study was approved by the New York University School of Medicine institutional review board (study no. S15-01388).
Statistical analyses were conducted in SPSS 19.0 for Windows. Categorical variables were assessed using the χ2 test for independence and the Fisher exact test.
Results
A total of 464 cases were reviewed. A positive KOH preparation or dermatophyte fungal culture was documented in 83 cases. Of them, 29 (34.9%) were treated with topical steroids and/or calcineurin inhibitors prior to presentation to dermatology (Table). The mean age at presentation was 8 years. Duration of symptoms prior to presentation was recorded for 23 of 29 patients (79.3%). Of them, 6 (26.1%) experienced symptoms for 1 month or less, 12 (52.2%) for 1 to 6 months, and 5 (21.7%) for 6 months to 1 year.
Physical examination findings (Figure) were documented in all 29 cases. Annular lesions were noted in 24 patients (82.8%). Pustules were present in 5 patients (17.2%) and papules in 11 patients (37.9%). Fourteen patients (48.3%) had involvement of the face, 14 (48.3%) of the body (ie, trunk, extremities, or groin), and 3 (10.3%) of the scalp. Six patients (20.7%) demonstrated findings at more than one body site.

Females were more likely to demonstrate facial lesions (P=.02), while males were more likely to present with body lesions (P=.04). Of 26 patients diagnosed via fungal culture, 16 (55.2%) grew Trichophyton tonsurans, 4 (13.8%) grew Trichophyton rubrum, 3 (10.3%) grew Trichophyton mentagrophytes, 2 (6.9%) grew Microsporum canis, and 1 (3.4%) grew Microsporum gypseum. Treatment entailed oral medication in 18 cases (62.1%). Of them, 13 (72.2%) were treated with griseofulvin, 3 (16.7%) with fluconazole, and 2 (11.1%) with terbinafine. Topical antifungals were prescribed in the remaining 11 cases (37.9%); no further treatment was documented.
Comment
Since the initial description of TI, approximately 60 case reports and small series as well as several larger observational studies describing TI have been published. In our series of pediatric patients, 29 of 83 culture- or KOH-confirmed dermatophytosis cases (34.9%) were considered to be TI due to treatment with topical corticosteroids and/or calcineurin inhibitors prior to presentation. This high prevalence contrasts with the 5.6% prevalence reported in the only prior large case series examining TI in childhood.2 These authors further reported that in their pediatric population, TI was significantly (odds ratio, 8.7; 95% CI, 4.7-16.1) more likely to occur on the face relative to other dermatophytoses and significantly (odds ratio, 0.014; 95% CI, 0.002-0.099) less likely to occur on the scalp.2 We noted a significant association between female gender and facial symptoms as well as between male gender and truncal symptoms. Taken together, these findings suggest an increased likelihood of pediatric tinea faciei to be inappropriately treated, particularly in females.
Although TI treated with topical corticosteroids or calcineurin inhibitors can mimic other skin diseases, a majority of patients in our series demonstrated findings associated with classic tinea, such as annularity and scale. Further, we found that T tonsurans was the causative organism in most cases with T rubrum uncommonly seen, though it is the most prevalent dermatophyte observed worldwide and in 2 large TI case series.3,4 Regional variation in dermatophytes may account for these differences. In our study, griseofulvin was used most frequently in TI treatment, though a systematic review of oral antifungals in tinea capitis supported terbinafine’s greater efficacy in patients infected with T tonsurans.5
Conclusion
Our case series demonstrated a 35% prevalence of TI cases in a population of children with confirmed dermatophytosis presenting to dermatologists at an American academic medical center. We hope that noting the high prevalence and manifold presentations of this disease will aid practitioners in maintaining clinical suspicion for dermatophytosis and thereby facilitate appropriate identification and treatment of TI.
Tinea incognito (TI) describes a dermatophytosis with often atypical clinical features attributed to prior use of topical corticosteroids or other immunomodulating agents. Tinea incognito may lack the scale and elevated margin typical of cutaneous dermatophytoses and can be mistaken for other pediatric cutaneous diseases, particularly atopic dermatitis. 1 Given the prevalence of TI and its susceptibility to misdiagnosis, we conducted a retrospective medical record review of cases of pediatric dermatophytosis presenting from 2005 to 2016.
Methods
We reviewed medical records for patients younger than 18 years who had been seen at the Faculty Group Practice of the Ronald O. Perelman Department of Dermatology, New York University School of Medicine (New York, New York), between January 1, 2005, and October 21, 2016, using International Classification of Diseases, Ninth Revision (ICD-9) codes 110.0 (tinea capitis), 110.1 (onychomycosis/tinea unguium), 110.3 (tinea cruris), 110.4 (tinea pedis), 110.5 (tinea corporis), and 110.9 (tinea, unspecified site). Cases were included in this study if there was documentation of dermatophytosis previously treated with topical corticosteroids or calcineurin inhibitors as well as positive potassium hydroxide (KOH) preparation or fungal culture with dermatophyte growth obtained from lesions satisfying the first criterion. This study was approved by the New York University School of Medicine institutional review board (study no. S15-01388).
Statistical analyses were conducted in SPSS 19.0 for Windows. Categorical variables were assessed using the χ2 test for independence and the Fisher exact test.
Results
A total of 464 cases were reviewed. A positive KOH preparation or dermatophyte fungal culture was documented in 83 cases. Of them, 29 (34.9%) were treated with topical steroids and/or calcineurin inhibitors prior to presentation to dermatology (Table). The mean age at presentation was 8 years. Duration of symptoms prior to presentation was recorded for 23 of 29 patients (79.3%). Of them, 6 (26.1%) experienced symptoms for 1 month or less, 12 (52.2%) for 1 to 6 months, and 5 (21.7%) for 6 months to 1 year.
Physical examination findings (Figure) were documented in all 29 cases. Annular lesions were noted in 24 patients (82.8%). Pustules were present in 5 patients (17.2%) and papules in 11 patients (37.9%). Fourteen patients (48.3%) had involvement of the face, 14 (48.3%) of the body (ie, trunk, extremities, or groin), and 3 (10.3%) of the scalp. Six patients (20.7%) demonstrated findings at more than one body site.

Females were more likely to demonstrate facial lesions (P=.02), while males were more likely to present with body lesions (P=.04). Of 26 patients diagnosed via fungal culture, 16 (55.2%) grew Trichophyton tonsurans, 4 (13.8%) grew Trichophyton rubrum, 3 (10.3%) grew Trichophyton mentagrophytes, 2 (6.9%) grew Microsporum canis, and 1 (3.4%) grew Microsporum gypseum. Treatment entailed oral medication in 18 cases (62.1%). Of them, 13 (72.2%) were treated with griseofulvin, 3 (16.7%) with fluconazole, and 2 (11.1%) with terbinafine. Topical antifungals were prescribed in the remaining 11 cases (37.9%); no further treatment was documented.
Comment
Since the initial description of TI, approximately 60 case reports and small series as well as several larger observational studies describing TI have been published. In our series of pediatric patients, 29 of 83 culture- or KOH-confirmed dermatophytosis cases (34.9%) were considered to be TI due to treatment with topical corticosteroids and/or calcineurin inhibitors prior to presentation. This high prevalence contrasts with the 5.6% prevalence reported in the only prior large case series examining TI in childhood.2 These authors further reported that in their pediatric population, TI was significantly (odds ratio, 8.7; 95% CI, 4.7-16.1) more likely to occur on the face relative to other dermatophytoses and significantly (odds ratio, 0.014; 95% CI, 0.002-0.099) less likely to occur on the scalp.2 We noted a significant association between female gender and facial symptoms as well as between male gender and truncal symptoms. Taken together, these findings suggest an increased likelihood of pediatric tinea faciei to be inappropriately treated, particularly in females.
Although TI treated with topical corticosteroids or calcineurin inhibitors can mimic other skin diseases, a majority of patients in our series demonstrated findings associated with classic tinea, such as annularity and scale. Further, we found that T tonsurans was the causative organism in most cases with T rubrum uncommonly seen, though it is the most prevalent dermatophyte observed worldwide and in 2 large TI case series.3,4 Regional variation in dermatophytes may account for these differences. In our study, griseofulvin was used most frequently in TI treatment, though a systematic review of oral antifungals in tinea capitis supported terbinafine’s greater efficacy in patients infected with T tonsurans.5
Conclusion
Our case series demonstrated a 35% prevalence of TI cases in a population of children with confirmed dermatophytosis presenting to dermatologists at an American academic medical center. We hope that noting the high prevalence and manifold presentations of this disease will aid practitioners in maintaining clinical suspicion for dermatophytosis and thereby facilitate appropriate identification and treatment of TI.
- Paloni G, Valerio E, Berti I, et al. Tinea incognito [published online September 28, 2015]. J Pediatr. 2015;167:1450-e2.
- del Boz J, Crespo V, Rivas‐Ruiz F, et al. Tinea incognito in children: 54 cases. Mycoses. 2011;54:254-258.
- Romano C, Maritati E, Gianni C. Tinea incognito in Italy: a 15-year survey. Mycoses. 2006;49:383-387.
- Kim WJ, Kim TW, Mun JH, et al. Tinea incognito in Korea and itsrisk factors: nine-year multicenter survey. J Korean Med Sci. 2013;28:145-151.
- Chen X, Jiang X, Yang M, et al. Systemic antifungal therapy for tinea capitis in children: an abridged Cochrane review. J Am Acad Dermatol. 2017;76:368-374.
- Paloni G, Valerio E, Berti I, et al. Tinea incognito [published online September 28, 2015]. J Pediatr. 2015;167:1450-e2.
- del Boz J, Crespo V, Rivas‐Ruiz F, et al. Tinea incognito in children: 54 cases. Mycoses. 2011;54:254-258.
- Romano C, Maritati E, Gianni C. Tinea incognito in Italy: a 15-year survey. Mycoses. 2006;49:383-387.
- Kim WJ, Kim TW, Mun JH, et al. Tinea incognito in Korea and itsrisk factors: nine-year multicenter survey. J Korean Med Sci. 2013;28:145-151.
- Chen X, Jiang X, Yang M, et al. Systemic antifungal therapy for tinea capitis in children: an abridged Cochrane review. J Am Acad Dermatol. 2017;76:368-374.
Practice Points
- Within our pediatric study population of microbiologically confirmed tinea cases at an American academic center, we found a 35% prevalence of tinea incognito (TI).
- Unlike investigations of TI in other countries, Trichophyton tonsurans was found to be the most common causative dermatophyte.
- Our data suggest that facial tinea may be more likely to be improperly treated in females and likewise tinea of the trunk or extremities in males.
Hand-foot-and-mouth Disease Caused by Coxsackievirus A6 on the Rise
Hand-foot-and-mouth disease (HFMD) is a viral illness caused by several enteroviruses, most commonly coxsackievirus A16 (CVA16) and enterovirus 71 (EV71). The disease is generally seen in children younger than 5 years, characterized by lesions of the oral mucosa, palms, and soles, usually lasting 7 to 10 days. Other coxsackie type A viruses, including CVA6, CVA9, and CVA10, also are associated with HFMD.1-5 Although CVA16 has traditionally been the primary strain causing HFMD, CVA6 has become a major cause of HFMD outbreaks in the United States and worldwide in recent years.6-12 Interestingly, CVA6 also has been found to be associated with adult HFMD, which has increased in incidence. The CVA6 strain was first identified in association with the disease during HFMD outbreaks in Finland and Singapore in 2008,13,14 with similar strains detected in subsequent outbreaks in Taiwan, Japan, Spain, France, China, India, and the United States.12,15-25 Most cases took place in warmer months, with one winter outbreak in Massachusetts in 2012.24
Herein, we review the incidence of CVA6, as well as its atypical presentation, diagnosis, and treatment to aid dermatologists. Given the increasing incidence of HFMD caused by CVA6 and its often atypical presentation, it is important for dermatologists to be aware of this increasingly notable disease state and its viral cause.
Incidence of CVA6
Coxsackievirus A6 has been identified as the cause of many reported outbreaks of HFMD since it was first identified in 2008, and it is known to cause both pediatric and adult outbreaks.7-12 It may even be surpassing other strains in frequency in certain areas. In Tianjin, China, for example, EV71 and CVA16 were the most common serotypes causing HFMD from 2008 to 2012; however, in 2013, CVA6 was the most prevalent strain.26
The incidence of CVA6 also has been increasing in other areas.28
In 2015, an outbreak of HFMD took place at Lackland Air Force Base in Texas during a basic military training. Eight cases were confirmed and 45 cases were suspected. The rate of infection was 0.4% (50/12,270) among trainees and 0.3% (2/602) among instructors.7 Eight of 12 nasopharyngeal swabs tested positive for EV by way of local real-time reverse transcription–polymerase chain reaction (RT-PCR). Four nasopharyngeal swabs were sent to the CDC for evaluation and all were positive for CVA6.7
Presentation
Because the prevalence of CVA6 has increased, it is important to be able to identify the presentation of HFMD caused by this strain. Coxsackievirus A6 has been found to affect a broader demographic and cause more severe cases of HFMD with its unique constellation of findings compared to other known strains. Patients present with flulike symptoms; higher fever than present in typical HFMD; and a longer duration of disease, typically lasting 2 weeks. Patients also may present with more severe skin disease compared to classic HFMD, not only including vesicles but also large bullae, erosions, and ulcers on the dorsal and plantar feet (Figure 1).
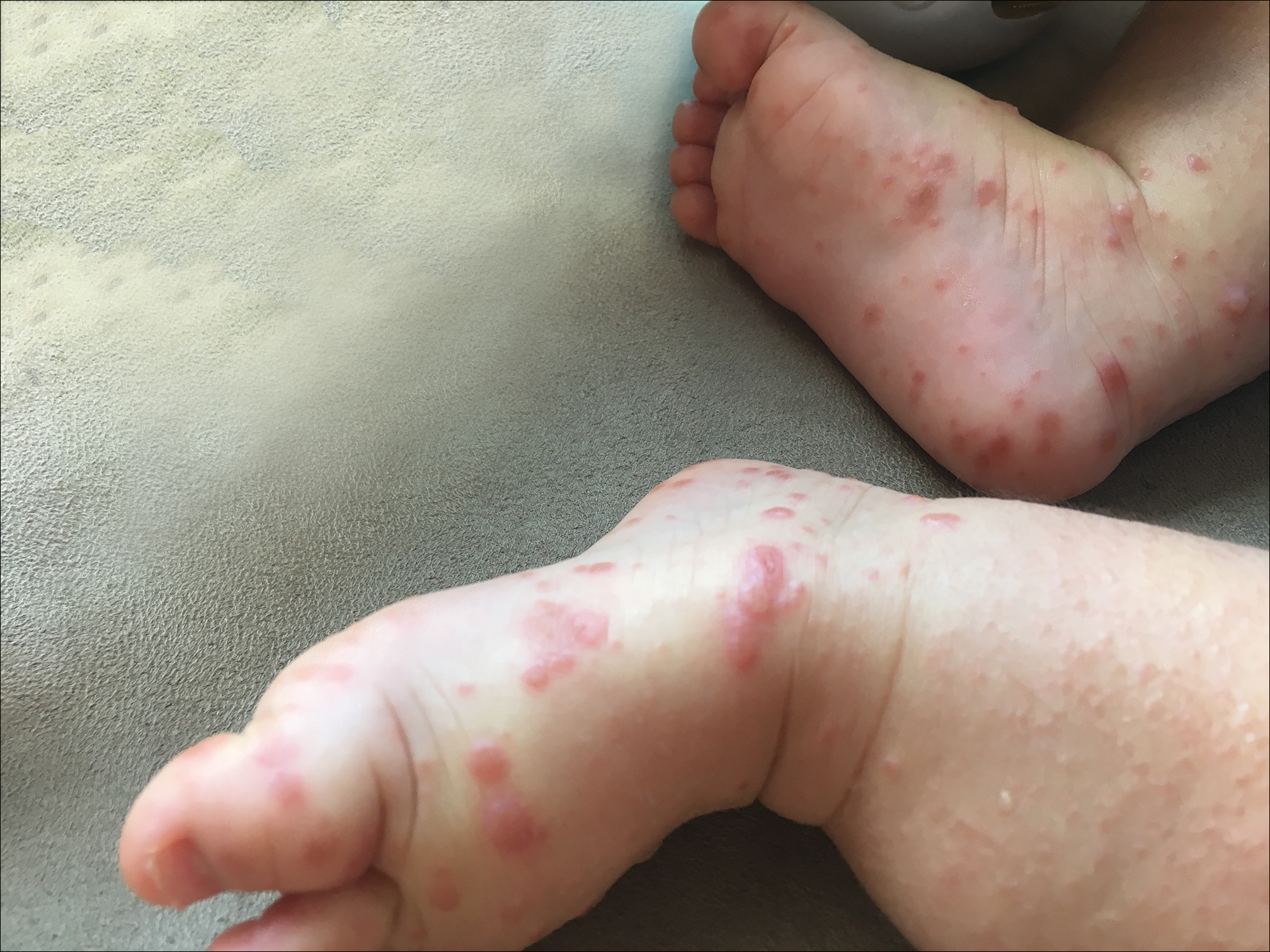
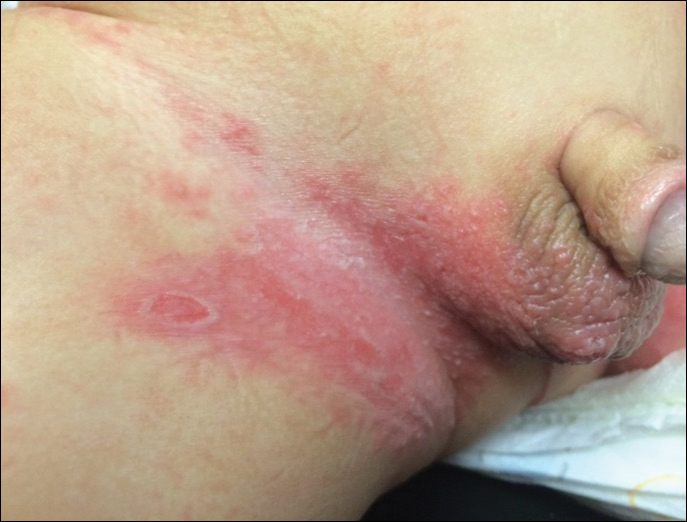
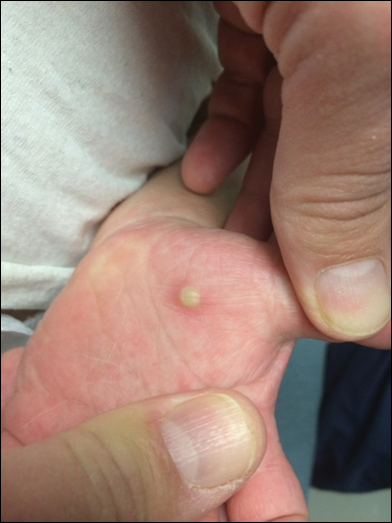
In patients with atopic dermatitis, CVA6 also shows a predilection to appear in areas of skin disease, such as the flexural regions of the arms and legs, and is referred to as eczema coxsackium.24,38,39 It can mimic eczema herpeticum or varicella superinfection, which are important considerations to include in the differential diagnosis. Additionally, CVA6-induced lesions often show up in previously irritated or traumatized areas such as sunburns, fungal infections, and diaper dermatitis in children. Lesions have been described to sometimes mimic Gianotti-Crosti syndrome, with involvement of the extensor surfaces, buttocks, and cheeks, and sparing of the trunk.24
Clinical Diagnosis
Because HFMD is uncommon and atypical in adults, skin biopsies may be used in the initial workup and evaluation of patients. It is important to understand the histologic features associated with HFMD, including spongiosis with exocytosis of neutrophils as well as keratinocyte necrosis and pallor with associated shadow cells.6 In one series, the most extensively involved areas of keratinocyte necrosis were the stratum granulosum and upper half of the stratum spinosum.40 In the dermis, vascular involvement may be present on a spectrum with the extravasation of red blood cells and leukocytoclasis or true leukocytoclastic vasculitis.6,40 Vesicular lesions show severe dermal edema and inflammatory infiltrate.6,41 CD3+ and CD8+ lymphocytes predominate. Cytotoxic T lymphocytes are present and express granzyme B and granulysin, both important mediators of apoptosis in virally infected keratinocytes.6
Adult HFMD primarily is a clinical diagnosis, and histopathologic analysis can be a useful tool in certain cases. Coxsackievirus A6 does not grow well on culture and is not detected by standard serologic testing laboratories, necessitating the use of quantitative RT-PCR analysis.41,42 In one study, culture was able to detect only 14% to 16% of samples that tested positive by quantitative RT-PCR.43 This form of PCR identifies viral subtype through amplification of enterovirus viral protein 1 capsid gene sequence.24 Unfortunately, this testing often is not offered in most readily available laboratories and often necessitates being sent out to more well-equipped laboratories.2,24
Treatment
Hand-foot-and-mouth disease is a self-limited illness and requires only supportive care with a focus on hydration and pain management. Lesions heal without scarring but may leave notable postinflammatory pigment alteration that may last months to years, depending on extent of disease and skin type. Secondarily infected individuals should be treated with appropriate antibiotics or antivirals depending on the infectious agent. Hand hygiene is of great importance, and hospitalized patients should be put on strict contact precautions. It also is important to isolate patients from vulnerable individuals, especially pregnant women, as coxsackievirus has been linked to intrauterine infections and loss of pregnancy.24
Genetic Analysis
Genetic studies of the virus have suggested that nonstructural genes may be playing an interesting role in clinical phenotypes and outcomes of CVA6 infection.44 These genetic studies also are being implemented into the understanding of the virus’ evolution as well as the construction of vaccinations.27,44
Conclusion
With the increasing prevalence of CVA6-associated HFMD, it is important to understand the clinical presentation and histologic findings associated with this atypical presentation of the disease as well as the changing epidemiology of the viral strains causing HFMD.
- Galen WK. Cutaneous manifestations of enterovirus infections. In: Tyring SK, ed. Mucocutaneous Manifestations of Viral Diseases. New York, NY: Marcel Dekker; 2002:455-467.
- Ramirez-Fort M, Downing C, Doan H, et al. Coxsackievirus A6 associated hand, foot and mouth disease in adults: clinical presentation and review of the literature. J Clin Virol. 2014;60:381-386.
- Khetsuriani N, Lamonte-Fowlkes A, Oberst S, et al. Enterovirus surveillance—United States, 1970-2005. MMWR Surveill Summ. 2006;55:1-20.
- Yang F, Zhang T, Hu Y, et al. Survey of enterovirus infections from hand, foot and mouth disease outbreak in China, 2009. Virol J. 2011;8:508.
- Ho M, Chen ER, Hsu KH, et al. An epidemic of enterovirus 71 infection in Taiwan. Taiwan Enterovirus Epidemic Working Group. N Engl J Med. 1999;341:929-935.
- Second J, Velter C, Calès S, et al. Clinicopathologic analysis of atypical hand, foot, and mouth disease in adult patients. J Am Acad Dermatol. 2016;76:722-729.
- Banta J, Lenz B, Pawlak M, et al. Notes from the field: outbreak of hand, foot, and mouth disease caused by coxsackievirus A6 among basic military trainees—Texas, 2015. MMWR Morb Mortal Wkly Rep. 2016;65.26:678-680.
- Bian L, Wang Y, Yao X, et al. Coxsackievirus A6: a new emerging pathogen causing hand, foot and mouth disease outbreaks worldwide. Expert Rev Anti Infect Ther. 2015;13:1061-1071.
- Buttery VW, Kenyon C, Grunewald S, et al. Notes from the field: atypical presentations of hand, foot, and mouth disease caused by coxsackievirus A6—Minnesota, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:805.
- Puenpa J, Chieochansin T, Linsuwanon P, et al. Hand, foot, and mouth disease caused by coxsackievirus A6, Thailand, 2012. Emerg Infect Dis. 2013;19:641-643.
- Flett K, Youngster I, Huang J, et al. Hand, foot, and mouth disease caused by coxsackievirus A6. Emerg Infect Dis. 2012;18:1702-1704.
- Centers for Disease Control and Prevention. Notes from the field: severe hand, foot, and mouth disease associated with coxsackievirus A6—Alabama, Connecticut, California, and Nevada, November 2011-February 2012. MMWR Morb Mortal Wkly Rep. 2012;61:213-214.
- Blomqvist S, Klemola P, Kaijalainen S, et al. Co-circulation of coxsackieviruses A6 and A10 in hand, foot and mouth disease outbreak in Finland. J Clin Virol. 2010;48:49-54.
- Osterback R, Vuorinen T, Linna M, et al. Coxsackievirus A6 and hand, foot, and mouth disease, Finland. Emerg Infect Dis. 2009;15:1485-1488.
- Zeng H, Lu J, Zheng H, et al. The epidemiological study of coxsackievirus A6 revealing hand, foot and mouth disease epidemic patterns in Guandong, China. Sci Rep. 2015;5:10550.
- Mirand A, Henquell C, Archimbaud C, et al. Outbreak of hand, foot and mouth disease/herpangina associated with coxsackievirus A6 andA10 infections in 2010, France: a large citywide, prospective observational study. Clin Microbiol Infect. 2012;18:E110-E118.
- Wei SH, Huang YP, Liu MC, et al. An outbreak of coxsackievirus A6 hand, foot, and mouth disease associated with onychomadesis in Taiwan, 2010. BMC Infect Dis. 2011;11:346.
- Fujimoto T, Iizuka S, Enomoto M, et al. Hand, foot, and mouth disease caused by coxsackievirus A6, Japan, 2011. Emerg Infect Dis. 2012;18:337-339.
- Bracho MA, Gonzalez-Candelas F, Valero A, et al. Enterovirus co-infections and onychomadesis after hand, foot, and mouth disease, Spain, 2008. Emerg Infect Dis. 2011;17:2223-2231.
- Gopalkrishna V, Patil PR, Patil GP, et al. Circulation of multiple enterovirus serotypes causing hand, foot and mouth disease in India. J Med Microbiol. 2012;61:420-425.
- Lo SH, Huang YC, Huang CG, et al. Clinical and epidemiologic features of coxsackievirus A6 infection in children in northern Taiwan between 2004 and 2009. J Microbiol Immunol Infect. 2011;44:252-257.
- Lu QB, Zhang XA, Wo Y, et al. Circulation of coxsackievirus A10 and A6 in hand-foot-mouth disease in China, 2009-2011. PLoS One. 2012;7:E52073.
- Wu Y, Yeo A, Phoon MC, et al. The largest outbreak of hand; foot and mouth disease in Singapore in 2008: the role of enterovirus 71 and coxsackievirus A strains. Int J Infect Dis. 2010;14:E1076-E1081.
- Ventarola D, Bordone L, Silverberg N. Update on hand-foot-and-mouth disease. Clin Dermatol. 2015;33:340-346.
- Li Y, Chang Z, Wu P, et al. Emerging enteroviruses causing hand, foot and mouth disease, China. 2010-2016. Emerg Infect Dis. 2018;24:1902-1906.
- Tan X, Li L, Zhang B, et al. Molecular epidemiology of coxsackievirus A6 associated with outbreaks of hand, foot, and mouth disease in Tianjin, China, in 2013. Arch Virol. 2015;160:1097-1104.
- Li Y, Bao H, Zhang X, et al. Epidemiological and genetic analysis concerning the non-enterovirus 71 and non-coxsackievirus A16 causative agents related to hand, foot and mouth disease in Anyang City, Henan Province, China, from 2011 to 2015. J Med Virol. 2017;89:1749-1758.
- Guan H, Wang J, Wang C, et al. Etiology of multiple non-EV71 and non-CVA16 enteroviruses associated with hand, foot, and mouth disease in Jinan, China, 2009-2013. PLoS One. 2015;10:E0142733.
- Cabrerizo M, Tarrago´ D, Muñoz-Almagro C, et al. Mollecular epidemiology of enterovirus 71, coxsackievirus A16 and A6 associated with hand, foot and mouth disease in Spain. Clin Microbiol Infect. 2014;20:O150-O156.
- Lønnberg A, Elberling J, Fischer T, et al. Two cases of hand, foot, and mouth disease involving the scalp. Acta Derm Venereol. 2013;93:467-468.
- Lott JP, Liu K, Landry ML, et al. Atypical hand-foot-and-mouth disease associated with coxsackievirus A6 infection. J Am Acad Dermatol. 2013;69:736-741.
- Kaminska K, Martinetti G, Lucchini R, et al. Coxsackievirus A6 and hand, foot and mouth disease: three case reports of familial child-to-immunocompetent adult transmission and a literature review. Case Rep Dermatol. 2013;5:203-209.
- Shin JU, Oh SH, Lee JH. A case of hand-foot-mouth disease in an immunocompetent adult. Ann Dermatol. 2010;22:216-218.
- Osterback R, Vuorinen T, Linna M, et al. Coxsackievirus A6 and hand, foot, and mouth disease, Finland. Emerg Infect Dis. 2009;15:1485-1488.
- Feder HM, Bennett N, Modlin JF. Atypical hand, foot, and mouth disease: a vesiculobullous eruption caused by coxsackie virus A6. Lancet Infect Dis. 2014;14:83-86.
- Wei SH, Huang YP, Liu MC, et al. An outbreak of coxsackievirus A6 hand, foot, and mouth disease associated with onychomadesis in Taiwan, 2010. BMC Infect Dis. 2011;11:346.
- Kim M, Kim B, Byun S, et al. Beau’s lines and onychomadesis after hand-foot-mouth disease. Clin Pediatr Dermatol. 2015;1:1.
- Mathes EF, Oza V, Frieden IJ, et al. “Eczema coxsackium” and unusual cutaneous findings in an enterovirus outbreak. Pediatrics. 2013;132:E149-E157.
- Lynch M, Sears A, Cookson H, et al. Disseminated coxsackievirus A6 affecting children with atopic dermatitis. Clin Exp Dermatol. 2015;40:525-528.
- Laga A, Shroba S, Hanna J. Atypical hand, foot and mouth disease in adults associated with coxsackievirus A6: a clinicopathologic study. J Cutan Pathol. 2016;43:940-945.
- Schmidt NJ, Ho HH, Lennette EH. Propagation and isolation of group A coxsackieviruses in RD cells. J Clin Microbiol. 1975;2:183-185.
- Oberste MS, Penaranda S, Rogers SL, et al. Comparative evaluation of Taqman real-time PCR and semi-nested VP1 PCR for detection of enteroviruses in clinical specimens. J Clin Virol. 2010;49:73-74.
- Lee MK, Chan PK, Ho II, et al. Enterovirus infection among patients admitted to hospital in Hong Kong in 2010: epidemiology, clinical characteristics, and importance of molecular diagnosis. J Med Virol. 2013;85:1811-1817.
- Yee PTI, Laa Poh C. Impact of genetic changes, pathogenicity and antigenicity on enterovirus A71 vaccine development. Virology. 2017;506:121-129.
Hand-foot-and-mouth disease (HFMD) is a viral illness caused by several enteroviruses, most commonly coxsackievirus A16 (CVA16) and enterovirus 71 (EV71). The disease is generally seen in children younger than 5 years, characterized by lesions of the oral mucosa, palms, and soles, usually lasting 7 to 10 days. Other coxsackie type A viruses, including CVA6, CVA9, and CVA10, also are associated with HFMD.1-5 Although CVA16 has traditionally been the primary strain causing HFMD, CVA6 has become a major cause of HFMD outbreaks in the United States and worldwide in recent years.6-12 Interestingly, CVA6 also has been found to be associated with adult HFMD, which has increased in incidence. The CVA6 strain was first identified in association with the disease during HFMD outbreaks in Finland and Singapore in 2008,13,14 with similar strains detected in subsequent outbreaks in Taiwan, Japan, Spain, France, China, India, and the United States.12,15-25 Most cases took place in warmer months, with one winter outbreak in Massachusetts in 2012.24
Herein, we review the incidence of CVA6, as well as its atypical presentation, diagnosis, and treatment to aid dermatologists. Given the increasing incidence of HFMD caused by CVA6 and its often atypical presentation, it is important for dermatologists to be aware of this increasingly notable disease state and its viral cause.
Incidence of CVA6
Coxsackievirus A6 has been identified as the cause of many reported outbreaks of HFMD since it was first identified in 2008, and it is known to cause both pediatric and adult outbreaks.7-12 It may even be surpassing other strains in frequency in certain areas. In Tianjin, China, for example, EV71 and CVA16 were the most common serotypes causing HFMD from 2008 to 2012; however, in 2013, CVA6 was the most prevalent strain.26
The incidence of CVA6 also has been increasing in other areas.28
In 2015, an outbreak of HFMD took place at Lackland Air Force Base in Texas during a basic military training. Eight cases were confirmed and 45 cases were suspected. The rate of infection was 0.4% (50/12,270) among trainees and 0.3% (2/602) among instructors.7 Eight of 12 nasopharyngeal swabs tested positive for EV by way of local real-time reverse transcription–polymerase chain reaction (RT-PCR). Four nasopharyngeal swabs were sent to the CDC for evaluation and all were positive for CVA6.7
Presentation
Because the prevalence of CVA6 has increased, it is important to be able to identify the presentation of HFMD caused by this strain. Coxsackievirus A6 has been found to affect a broader demographic and cause more severe cases of HFMD with its unique constellation of findings compared to other known strains. Patients present with flulike symptoms; higher fever than present in typical HFMD; and a longer duration of disease, typically lasting 2 weeks. Patients also may present with more severe skin disease compared to classic HFMD, not only including vesicles but also large bullae, erosions, and ulcers on the dorsal and plantar feet (Figure 1).



In patients with atopic dermatitis, CVA6 also shows a predilection to appear in areas of skin disease, such as the flexural regions of the arms and legs, and is referred to as eczema coxsackium.24,38,39 It can mimic eczema herpeticum or varicella superinfection, which are important considerations to include in the differential diagnosis. Additionally, CVA6-induced lesions often show up in previously irritated or traumatized areas such as sunburns, fungal infections, and diaper dermatitis in children. Lesions have been described to sometimes mimic Gianotti-Crosti syndrome, with involvement of the extensor surfaces, buttocks, and cheeks, and sparing of the trunk.24
Clinical Diagnosis
Because HFMD is uncommon and atypical in adults, skin biopsies may be used in the initial workup and evaluation of patients. It is important to understand the histologic features associated with HFMD, including spongiosis with exocytosis of neutrophils as well as keratinocyte necrosis and pallor with associated shadow cells.6 In one series, the most extensively involved areas of keratinocyte necrosis were the stratum granulosum and upper half of the stratum spinosum.40 In the dermis, vascular involvement may be present on a spectrum with the extravasation of red blood cells and leukocytoclasis or true leukocytoclastic vasculitis.6,40 Vesicular lesions show severe dermal edema and inflammatory infiltrate.6,41 CD3+ and CD8+ lymphocytes predominate. Cytotoxic T lymphocytes are present and express granzyme B and granulysin, both important mediators of apoptosis in virally infected keratinocytes.6
Adult HFMD primarily is a clinical diagnosis, and histopathologic analysis can be a useful tool in certain cases. Coxsackievirus A6 does not grow well on culture and is not detected by standard serologic testing laboratories, necessitating the use of quantitative RT-PCR analysis.41,42 In one study, culture was able to detect only 14% to 16% of samples that tested positive by quantitative RT-PCR.43 This form of PCR identifies viral subtype through amplification of enterovirus viral protein 1 capsid gene sequence.24 Unfortunately, this testing often is not offered in most readily available laboratories and often necessitates being sent out to more well-equipped laboratories.2,24
Treatment
Hand-foot-and-mouth disease is a self-limited illness and requires only supportive care with a focus on hydration and pain management. Lesions heal without scarring but may leave notable postinflammatory pigment alteration that may last months to years, depending on extent of disease and skin type. Secondarily infected individuals should be treated with appropriate antibiotics or antivirals depending on the infectious agent. Hand hygiene is of great importance, and hospitalized patients should be put on strict contact precautions. It also is important to isolate patients from vulnerable individuals, especially pregnant women, as coxsackievirus has been linked to intrauterine infections and loss of pregnancy.24
Genetic Analysis
Genetic studies of the virus have suggested that nonstructural genes may be playing an interesting role in clinical phenotypes and outcomes of CVA6 infection.44 These genetic studies also are being implemented into the understanding of the virus’ evolution as well as the construction of vaccinations.27,44
Conclusion
With the increasing prevalence of CVA6-associated HFMD, it is important to understand the clinical presentation and histologic findings associated with this atypical presentation of the disease as well as the changing epidemiology of the viral strains causing HFMD.
Hand-foot-and-mouth disease (HFMD) is a viral illness caused by several enteroviruses, most commonly coxsackievirus A16 (CVA16) and enterovirus 71 (EV71). The disease is generally seen in children younger than 5 years, characterized by lesions of the oral mucosa, palms, and soles, usually lasting 7 to 10 days. Other coxsackie type A viruses, including CVA6, CVA9, and CVA10, also are associated with HFMD.1-5 Although CVA16 has traditionally been the primary strain causing HFMD, CVA6 has become a major cause of HFMD outbreaks in the United States and worldwide in recent years.6-12 Interestingly, CVA6 also has been found to be associated with adult HFMD, which has increased in incidence. The CVA6 strain was first identified in association with the disease during HFMD outbreaks in Finland and Singapore in 2008,13,14 with similar strains detected in subsequent outbreaks in Taiwan, Japan, Spain, France, China, India, and the United States.12,15-25 Most cases took place in warmer months, with one winter outbreak in Massachusetts in 2012.24
Herein, we review the incidence of CVA6, as well as its atypical presentation, diagnosis, and treatment to aid dermatologists. Given the increasing incidence of HFMD caused by CVA6 and its often atypical presentation, it is important for dermatologists to be aware of this increasingly notable disease state and its viral cause.
Incidence of CVA6
Coxsackievirus A6 has been identified as the cause of many reported outbreaks of HFMD since it was first identified in 2008, and it is known to cause both pediatric and adult outbreaks.7-12 It may even be surpassing other strains in frequency in certain areas. In Tianjin, China, for example, EV71 and CVA16 were the most common serotypes causing HFMD from 2008 to 2012; however, in 2013, CVA6 was the most prevalent strain.26
The incidence of CVA6 also has been increasing in other areas.28
In 2015, an outbreak of HFMD took place at Lackland Air Force Base in Texas during a basic military training. Eight cases were confirmed and 45 cases were suspected. The rate of infection was 0.4% (50/12,270) among trainees and 0.3% (2/602) among instructors.7 Eight of 12 nasopharyngeal swabs tested positive for EV by way of local real-time reverse transcription–polymerase chain reaction (RT-PCR). Four nasopharyngeal swabs were sent to the CDC for evaluation and all were positive for CVA6.7
Presentation
Because the prevalence of CVA6 has increased, it is important to be able to identify the presentation of HFMD caused by this strain. Coxsackievirus A6 has been found to affect a broader demographic and cause more severe cases of HFMD with its unique constellation of findings compared to other known strains. Patients present with flulike symptoms; higher fever than present in typical HFMD; and a longer duration of disease, typically lasting 2 weeks. Patients also may present with more severe skin disease compared to classic HFMD, not only including vesicles but also large bullae, erosions, and ulcers on the dorsal and plantar feet (Figure 1).



In patients with atopic dermatitis, CVA6 also shows a predilection to appear in areas of skin disease, such as the flexural regions of the arms and legs, and is referred to as eczema coxsackium.24,38,39 It can mimic eczema herpeticum or varicella superinfection, which are important considerations to include in the differential diagnosis. Additionally, CVA6-induced lesions often show up in previously irritated or traumatized areas such as sunburns, fungal infections, and diaper dermatitis in children. Lesions have been described to sometimes mimic Gianotti-Crosti syndrome, with involvement of the extensor surfaces, buttocks, and cheeks, and sparing of the trunk.24
Clinical Diagnosis
Because HFMD is uncommon and atypical in adults, skin biopsies may be used in the initial workup and evaluation of patients. It is important to understand the histologic features associated with HFMD, including spongiosis with exocytosis of neutrophils as well as keratinocyte necrosis and pallor with associated shadow cells.6 In one series, the most extensively involved areas of keratinocyte necrosis were the stratum granulosum and upper half of the stratum spinosum.40 In the dermis, vascular involvement may be present on a spectrum with the extravasation of red blood cells and leukocytoclasis or true leukocytoclastic vasculitis.6,40 Vesicular lesions show severe dermal edema and inflammatory infiltrate.6,41 CD3+ and CD8+ lymphocytes predominate. Cytotoxic T lymphocytes are present and express granzyme B and granulysin, both important mediators of apoptosis in virally infected keratinocytes.6
Adult HFMD primarily is a clinical diagnosis, and histopathologic analysis can be a useful tool in certain cases. Coxsackievirus A6 does not grow well on culture and is not detected by standard serologic testing laboratories, necessitating the use of quantitative RT-PCR analysis.41,42 In one study, culture was able to detect only 14% to 16% of samples that tested positive by quantitative RT-PCR.43 This form of PCR identifies viral subtype through amplification of enterovirus viral protein 1 capsid gene sequence.24 Unfortunately, this testing often is not offered in most readily available laboratories and often necessitates being sent out to more well-equipped laboratories.2,24
Treatment
Hand-foot-and-mouth disease is a self-limited illness and requires only supportive care with a focus on hydration and pain management. Lesions heal without scarring but may leave notable postinflammatory pigment alteration that may last months to years, depending on extent of disease and skin type. Secondarily infected individuals should be treated with appropriate antibiotics or antivirals depending on the infectious agent. Hand hygiene is of great importance, and hospitalized patients should be put on strict contact precautions. It also is important to isolate patients from vulnerable individuals, especially pregnant women, as coxsackievirus has been linked to intrauterine infections and loss of pregnancy.24
Genetic Analysis
Genetic studies of the virus have suggested that nonstructural genes may be playing an interesting role in clinical phenotypes and outcomes of CVA6 infection.44 These genetic studies also are being implemented into the understanding of the virus’ evolution as well as the construction of vaccinations.27,44
Conclusion
With the increasing prevalence of CVA6-associated HFMD, it is important to understand the clinical presentation and histologic findings associated with this atypical presentation of the disease as well as the changing epidemiology of the viral strains causing HFMD.
- Galen WK. Cutaneous manifestations of enterovirus infections. In: Tyring SK, ed. Mucocutaneous Manifestations of Viral Diseases. New York, NY: Marcel Dekker; 2002:455-467.
- Ramirez-Fort M, Downing C, Doan H, et al. Coxsackievirus A6 associated hand, foot and mouth disease in adults: clinical presentation and review of the literature. J Clin Virol. 2014;60:381-386.
- Khetsuriani N, Lamonte-Fowlkes A, Oberst S, et al. Enterovirus surveillance—United States, 1970-2005. MMWR Surveill Summ. 2006;55:1-20.
- Yang F, Zhang T, Hu Y, et al. Survey of enterovirus infections from hand, foot and mouth disease outbreak in China, 2009. Virol J. 2011;8:508.
- Ho M, Chen ER, Hsu KH, et al. An epidemic of enterovirus 71 infection in Taiwan. Taiwan Enterovirus Epidemic Working Group. N Engl J Med. 1999;341:929-935.
- Second J, Velter C, Calès S, et al. Clinicopathologic analysis of atypical hand, foot, and mouth disease in adult patients. J Am Acad Dermatol. 2016;76:722-729.
- Banta J, Lenz B, Pawlak M, et al. Notes from the field: outbreak of hand, foot, and mouth disease caused by coxsackievirus A6 among basic military trainees—Texas, 2015. MMWR Morb Mortal Wkly Rep. 2016;65.26:678-680.
- Bian L, Wang Y, Yao X, et al. Coxsackievirus A6: a new emerging pathogen causing hand, foot and mouth disease outbreaks worldwide. Expert Rev Anti Infect Ther. 2015;13:1061-1071.
- Buttery VW, Kenyon C, Grunewald S, et al. Notes from the field: atypical presentations of hand, foot, and mouth disease caused by coxsackievirus A6—Minnesota, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:805.
- Puenpa J, Chieochansin T, Linsuwanon P, et al. Hand, foot, and mouth disease caused by coxsackievirus A6, Thailand, 2012. Emerg Infect Dis. 2013;19:641-643.
- Flett K, Youngster I, Huang J, et al. Hand, foot, and mouth disease caused by coxsackievirus A6. Emerg Infect Dis. 2012;18:1702-1704.
- Centers for Disease Control and Prevention. Notes from the field: severe hand, foot, and mouth disease associated with coxsackievirus A6—Alabama, Connecticut, California, and Nevada, November 2011-February 2012. MMWR Morb Mortal Wkly Rep. 2012;61:213-214.
- Blomqvist S, Klemola P, Kaijalainen S, et al. Co-circulation of coxsackieviruses A6 and A10 in hand, foot and mouth disease outbreak in Finland. J Clin Virol. 2010;48:49-54.
- Osterback R, Vuorinen T, Linna M, et al. Coxsackievirus A6 and hand, foot, and mouth disease, Finland. Emerg Infect Dis. 2009;15:1485-1488.
- Zeng H, Lu J, Zheng H, et al. The epidemiological study of coxsackievirus A6 revealing hand, foot and mouth disease epidemic patterns in Guandong, China. Sci Rep. 2015;5:10550.
- Mirand A, Henquell C, Archimbaud C, et al. Outbreak of hand, foot and mouth disease/herpangina associated with coxsackievirus A6 andA10 infections in 2010, France: a large citywide, prospective observational study. Clin Microbiol Infect. 2012;18:E110-E118.
- Wei SH, Huang YP, Liu MC, et al. An outbreak of coxsackievirus A6 hand, foot, and mouth disease associated with onychomadesis in Taiwan, 2010. BMC Infect Dis. 2011;11:346.
- Fujimoto T, Iizuka S, Enomoto M, et al. Hand, foot, and mouth disease caused by coxsackievirus A6, Japan, 2011. Emerg Infect Dis. 2012;18:337-339.
- Bracho MA, Gonzalez-Candelas F, Valero A, et al. Enterovirus co-infections and onychomadesis after hand, foot, and mouth disease, Spain, 2008. Emerg Infect Dis. 2011;17:2223-2231.
- Gopalkrishna V, Patil PR, Patil GP, et al. Circulation of multiple enterovirus serotypes causing hand, foot and mouth disease in India. J Med Microbiol. 2012;61:420-425.
- Lo SH, Huang YC, Huang CG, et al. Clinical and epidemiologic features of coxsackievirus A6 infection in children in northern Taiwan between 2004 and 2009. J Microbiol Immunol Infect. 2011;44:252-257.
- Lu QB, Zhang XA, Wo Y, et al. Circulation of coxsackievirus A10 and A6 in hand-foot-mouth disease in China, 2009-2011. PLoS One. 2012;7:E52073.
- Wu Y, Yeo A, Phoon MC, et al. The largest outbreak of hand; foot and mouth disease in Singapore in 2008: the role of enterovirus 71 and coxsackievirus A strains. Int J Infect Dis. 2010;14:E1076-E1081.
- Ventarola D, Bordone L, Silverberg N. Update on hand-foot-and-mouth disease. Clin Dermatol. 2015;33:340-346.
- Li Y, Chang Z, Wu P, et al. Emerging enteroviruses causing hand, foot and mouth disease, China. 2010-2016. Emerg Infect Dis. 2018;24:1902-1906.
- Tan X, Li L, Zhang B, et al. Molecular epidemiology of coxsackievirus A6 associated with outbreaks of hand, foot, and mouth disease in Tianjin, China, in 2013. Arch Virol. 2015;160:1097-1104.
- Li Y, Bao H, Zhang X, et al. Epidemiological and genetic analysis concerning the non-enterovirus 71 and non-coxsackievirus A16 causative agents related to hand, foot and mouth disease in Anyang City, Henan Province, China, from 2011 to 2015. J Med Virol. 2017;89:1749-1758.
- Guan H, Wang J, Wang C, et al. Etiology of multiple non-EV71 and non-CVA16 enteroviruses associated with hand, foot, and mouth disease in Jinan, China, 2009-2013. PLoS One. 2015;10:E0142733.
- Cabrerizo M, Tarrago´ D, Muñoz-Almagro C, et al. Mollecular epidemiology of enterovirus 71, coxsackievirus A16 and A6 associated with hand, foot and mouth disease in Spain. Clin Microbiol Infect. 2014;20:O150-O156.
- Lønnberg A, Elberling J, Fischer T, et al. Two cases of hand, foot, and mouth disease involving the scalp. Acta Derm Venereol. 2013;93:467-468.
- Lott JP, Liu K, Landry ML, et al. Atypical hand-foot-and-mouth disease associated with coxsackievirus A6 infection. J Am Acad Dermatol. 2013;69:736-741.
- Kaminska K, Martinetti G, Lucchini R, et al. Coxsackievirus A6 and hand, foot and mouth disease: three case reports of familial child-to-immunocompetent adult transmission and a literature review. Case Rep Dermatol. 2013;5:203-209.
- Shin JU, Oh SH, Lee JH. A case of hand-foot-mouth disease in an immunocompetent adult. Ann Dermatol. 2010;22:216-218.
- Osterback R, Vuorinen T, Linna M, et al. Coxsackievirus A6 and hand, foot, and mouth disease, Finland. Emerg Infect Dis. 2009;15:1485-1488.
- Feder HM, Bennett N, Modlin JF. Atypical hand, foot, and mouth disease: a vesiculobullous eruption caused by coxsackie virus A6. Lancet Infect Dis. 2014;14:83-86.
- Wei SH, Huang YP, Liu MC, et al. An outbreak of coxsackievirus A6 hand, foot, and mouth disease associated with onychomadesis in Taiwan, 2010. BMC Infect Dis. 2011;11:346.
- Kim M, Kim B, Byun S, et al. Beau’s lines and onychomadesis after hand-foot-mouth disease. Clin Pediatr Dermatol. 2015;1:1.
- Mathes EF, Oza V, Frieden IJ, et al. “Eczema coxsackium” and unusual cutaneous findings in an enterovirus outbreak. Pediatrics. 2013;132:E149-E157.
- Lynch M, Sears A, Cookson H, et al. Disseminated coxsackievirus A6 affecting children with atopic dermatitis. Clin Exp Dermatol. 2015;40:525-528.
- Laga A, Shroba S, Hanna J. Atypical hand, foot and mouth disease in adults associated with coxsackievirus A6: a clinicopathologic study. J Cutan Pathol. 2016;43:940-945.
- Schmidt NJ, Ho HH, Lennette EH. Propagation and isolation of group A coxsackieviruses in RD cells. J Clin Microbiol. 1975;2:183-185.
- Oberste MS, Penaranda S, Rogers SL, et al. Comparative evaluation of Taqman real-time PCR and semi-nested VP1 PCR for detection of enteroviruses in clinical specimens. J Clin Virol. 2010;49:73-74.
- Lee MK, Chan PK, Ho II, et al. Enterovirus infection among patients admitted to hospital in Hong Kong in 2010: epidemiology, clinical characteristics, and importance of molecular diagnosis. J Med Virol. 2013;85:1811-1817.
- Yee PTI, Laa Poh C. Impact of genetic changes, pathogenicity and antigenicity on enterovirus A71 vaccine development. Virology. 2017;506:121-129.
- Galen WK. Cutaneous manifestations of enterovirus infections. In: Tyring SK, ed. Mucocutaneous Manifestations of Viral Diseases. New York, NY: Marcel Dekker; 2002:455-467.
- Ramirez-Fort M, Downing C, Doan H, et al. Coxsackievirus A6 associated hand, foot and mouth disease in adults: clinical presentation and review of the literature. J Clin Virol. 2014;60:381-386.
- Khetsuriani N, Lamonte-Fowlkes A, Oberst S, et al. Enterovirus surveillance—United States, 1970-2005. MMWR Surveill Summ. 2006;55:1-20.
- Yang F, Zhang T, Hu Y, et al. Survey of enterovirus infections from hand, foot and mouth disease outbreak in China, 2009. Virol J. 2011;8:508.
- Ho M, Chen ER, Hsu KH, et al. An epidemic of enterovirus 71 infection in Taiwan. Taiwan Enterovirus Epidemic Working Group. N Engl J Med. 1999;341:929-935.
- Second J, Velter C, Calès S, et al. Clinicopathologic analysis of atypical hand, foot, and mouth disease in adult patients. J Am Acad Dermatol. 2016;76:722-729.
- Banta J, Lenz B, Pawlak M, et al. Notes from the field: outbreak of hand, foot, and mouth disease caused by coxsackievirus A6 among basic military trainees—Texas, 2015. MMWR Morb Mortal Wkly Rep. 2016;65.26:678-680.
- Bian L, Wang Y, Yao X, et al. Coxsackievirus A6: a new emerging pathogen causing hand, foot and mouth disease outbreaks worldwide. Expert Rev Anti Infect Ther. 2015;13:1061-1071.
- Buttery VW, Kenyon C, Grunewald S, et al. Notes from the field: atypical presentations of hand, foot, and mouth disease caused by coxsackievirus A6—Minnesota, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:805.
- Puenpa J, Chieochansin T, Linsuwanon P, et al. Hand, foot, and mouth disease caused by coxsackievirus A6, Thailand, 2012. Emerg Infect Dis. 2013;19:641-643.
- Flett K, Youngster I, Huang J, et al. Hand, foot, and mouth disease caused by coxsackievirus A6. Emerg Infect Dis. 2012;18:1702-1704.
- Centers for Disease Control and Prevention. Notes from the field: severe hand, foot, and mouth disease associated with coxsackievirus A6—Alabama, Connecticut, California, and Nevada, November 2011-February 2012. MMWR Morb Mortal Wkly Rep. 2012;61:213-214.
- Blomqvist S, Klemola P, Kaijalainen S, et al. Co-circulation of coxsackieviruses A6 and A10 in hand, foot and mouth disease outbreak in Finland. J Clin Virol. 2010;48:49-54.
- Osterback R, Vuorinen T, Linna M, et al. Coxsackievirus A6 and hand, foot, and mouth disease, Finland. Emerg Infect Dis. 2009;15:1485-1488.
- Zeng H, Lu J, Zheng H, et al. The epidemiological study of coxsackievirus A6 revealing hand, foot and mouth disease epidemic patterns in Guandong, China. Sci Rep. 2015;5:10550.
- Mirand A, Henquell C, Archimbaud C, et al. Outbreak of hand, foot and mouth disease/herpangina associated with coxsackievirus A6 andA10 infections in 2010, France: a large citywide, prospective observational study. Clin Microbiol Infect. 2012;18:E110-E118.
- Wei SH, Huang YP, Liu MC, et al. An outbreak of coxsackievirus A6 hand, foot, and mouth disease associated with onychomadesis in Taiwan, 2010. BMC Infect Dis. 2011;11:346.
- Fujimoto T, Iizuka S, Enomoto M, et al. Hand, foot, and mouth disease caused by coxsackievirus A6, Japan, 2011. Emerg Infect Dis. 2012;18:337-339.
- Bracho MA, Gonzalez-Candelas F, Valero A, et al. Enterovirus co-infections and onychomadesis after hand, foot, and mouth disease, Spain, 2008. Emerg Infect Dis. 2011;17:2223-2231.
- Gopalkrishna V, Patil PR, Patil GP, et al. Circulation of multiple enterovirus serotypes causing hand, foot and mouth disease in India. J Med Microbiol. 2012;61:420-425.
- Lo SH, Huang YC, Huang CG, et al. Clinical and epidemiologic features of coxsackievirus A6 infection in children in northern Taiwan between 2004 and 2009. J Microbiol Immunol Infect. 2011;44:252-257.
- Lu QB, Zhang XA, Wo Y, et al. Circulation of coxsackievirus A10 and A6 in hand-foot-mouth disease in China, 2009-2011. PLoS One. 2012;7:E52073.
- Wu Y, Yeo A, Phoon MC, et al. The largest outbreak of hand; foot and mouth disease in Singapore in 2008: the role of enterovirus 71 and coxsackievirus A strains. Int J Infect Dis. 2010;14:E1076-E1081.
- Ventarola D, Bordone L, Silverberg N. Update on hand-foot-and-mouth disease. Clin Dermatol. 2015;33:340-346.
- Li Y, Chang Z, Wu P, et al. Emerging enteroviruses causing hand, foot and mouth disease, China. 2010-2016. Emerg Infect Dis. 2018;24:1902-1906.
- Tan X, Li L, Zhang B, et al. Molecular epidemiology of coxsackievirus A6 associated with outbreaks of hand, foot, and mouth disease in Tianjin, China, in 2013. Arch Virol. 2015;160:1097-1104.
- Li Y, Bao H, Zhang X, et al. Epidemiological and genetic analysis concerning the non-enterovirus 71 and non-coxsackievirus A16 causative agents related to hand, foot and mouth disease in Anyang City, Henan Province, China, from 2011 to 2015. J Med Virol. 2017;89:1749-1758.
- Guan H, Wang J, Wang C, et al. Etiology of multiple non-EV71 and non-CVA16 enteroviruses associated with hand, foot, and mouth disease in Jinan, China, 2009-2013. PLoS One. 2015;10:E0142733.
- Cabrerizo M, Tarrago´ D, Muñoz-Almagro C, et al. Mollecular epidemiology of enterovirus 71, coxsackievirus A16 and A6 associated with hand, foot and mouth disease in Spain. Clin Microbiol Infect. 2014;20:O150-O156.
- Lønnberg A, Elberling J, Fischer T, et al. Two cases of hand, foot, and mouth disease involving the scalp. Acta Derm Venereol. 2013;93:467-468.
- Lott JP, Liu K, Landry ML, et al. Atypical hand-foot-and-mouth disease associated with coxsackievirus A6 infection. J Am Acad Dermatol. 2013;69:736-741.
- Kaminska K, Martinetti G, Lucchini R, et al. Coxsackievirus A6 and hand, foot and mouth disease: three case reports of familial child-to-immunocompetent adult transmission and a literature review. Case Rep Dermatol. 2013;5:203-209.
- Shin JU, Oh SH, Lee JH. A case of hand-foot-mouth disease in an immunocompetent adult. Ann Dermatol. 2010;22:216-218.
- Osterback R, Vuorinen T, Linna M, et al. Coxsackievirus A6 and hand, foot, and mouth disease, Finland. Emerg Infect Dis. 2009;15:1485-1488.
- Feder HM, Bennett N, Modlin JF. Atypical hand, foot, and mouth disease: a vesiculobullous eruption caused by coxsackie virus A6. Lancet Infect Dis. 2014;14:83-86.
- Wei SH, Huang YP, Liu MC, et al. An outbreak of coxsackievirus A6 hand, foot, and mouth disease associated with onychomadesis in Taiwan, 2010. BMC Infect Dis. 2011;11:346.
- Kim M, Kim B, Byun S, et al. Beau’s lines and onychomadesis after hand-foot-mouth disease. Clin Pediatr Dermatol. 2015;1:1.
- Mathes EF, Oza V, Frieden IJ, et al. “Eczema coxsackium” and unusual cutaneous findings in an enterovirus outbreak. Pediatrics. 2013;132:E149-E157.
- Lynch M, Sears A, Cookson H, et al. Disseminated coxsackievirus A6 affecting children with atopic dermatitis. Clin Exp Dermatol. 2015;40:525-528.
- Laga A, Shroba S, Hanna J. Atypical hand, foot and mouth disease in adults associated with coxsackievirus A6: a clinicopathologic study. J Cutan Pathol. 2016;43:940-945.
- Schmidt NJ, Ho HH, Lennette EH. Propagation and isolation of group A coxsackieviruses in RD cells. J Clin Microbiol. 1975;2:183-185.
- Oberste MS, Penaranda S, Rogers SL, et al. Comparative evaluation of Taqman real-time PCR and semi-nested VP1 PCR for detection of enteroviruses in clinical specimens. J Clin Virol. 2010;49:73-74.
- Lee MK, Chan PK, Ho II, et al. Enterovirus infection among patients admitted to hospital in Hong Kong in 2010: epidemiology, clinical characteristics, and importance of molecular diagnosis. J Med Virol. 2013;85:1811-1817.
- Yee PTI, Laa Poh C. Impact of genetic changes, pathogenicity and antigenicity on enterovirus A71 vaccine development. Virology. 2017;506:121-129.
Practice Points
- Coxsackievirus A6 is an increasingly more common cause of hand-foot-and-mouth disease (HFMD), often with atypical presentation, more severe disease, and association with HFMD in adults.
- Coxsackievirus A6 has become a major cause of HFMD outbreak in the United States and worldwide.
Obstructive sleep apnea: A better Dx model for primary care
ABSTRACT
Purpose To derive a predictive model for obstructive sleep apnea (OSA) in primary care practice, using home-based overnight oximetry results to refine posttest probability (PTP) of disease after initial risk stratification with the Sleep Apnea Clinical Score (SACS).
Methods We performed secondary analyses on data from a SACS validation cohort, to compare the diagnostic accuracy of 3 overnight oximetry measurements (oxygen desaturation index [ODI], mean saturation, and minimum saturation) in predicting OSA. Receiver operator characteristics (ROC) were computed for each measurement independently and sequentially after risk stratifying with SACS. We examined the implications of oximetry results for OSA PTP for participants categorized as intermediate risk (SACS 6-14; 66/191 participants [35%]; OSA probability 41%). We calculated positive likelihood ratios (LR) for multiple ODI results and determined which ones allowed recalibration to high- or low-risk PTP.
Results Among the 3 oximetry findings, ODI best predicted OSA (area under the curve [AUC], 0.88; 95% confidence interval [CI], 0.83-0.93). An ODI ≥8.4 (likelihood ratio [LR], 4.19; 95% CI, 2.87-6.10) created a PTP of 77%, while an ODI of 0 to <8.4 (LR, 0.19, 95% CI, 0.12-0.33) created a 14% PTP. Sequential application of SACS and ODI results yielded an AUC result of 0.90 (95% CI, 0.85-0.95).
Conclusions SACS risk stratification provides an advantage over clinical gestalt. In those at intermediate risk, ODI results provide a simple and clinically useful way to further refine diagnostic prediction. Sequential use of SACS and selectively employed overnight oximetry may limit unnecessary polysomnography. Oximetry testing should be avoided in patients deemed low or high risk by SACS, as positive results do not substantially recalibrate risk.
Obstructive sleep apnea (OSA) is a prevalent and underdiagnosed condition. The National Sleep Foundation estimates that 18 million Americans have OSA.1 Primary care practice may be the best setting in which to identify OSA, as many of our patients have conditions frequently associated with apnea (eg, hypertension, obesity, diabetes, arrhythmia, and neurologic illness). Up to a third of patients in primary care practice may be at increased risk.2,3
Clinical guidelines of the American Academy of Sleep Medicine (AASM) recommend obtaining a sleep history to evaluate for possible OSA in 3 instances: as part of a routine health maintenance examination, during evaluation of specific complaints associated with OSA (eg, snoring, apnea, daytime sleepiness), and during comprehensive evaluations for individuals with high-risk conditions (ie, obesity, congestive heart failure, refractory hypertension, diabetes, stroke history).4
The American College of Physicians (ACP) Clinical Practice Guideline suggests assessing individuals who have unexplained daytime sleepiness.5 The ACP considers this assessment “High-Value Care,” as “evidence shows that before diagnosis, patients with OSA have higher rates of health care use, more frequent and longer hospital stays, and higher health care costs than after diagnosis.”5
Continue to: We recently validated the diagnostic accuracy...
We recently validated the diagnostic accuracy of the Sleep Apnea Clinical Score (SACS) for use in a primary care patient population suspected of having OSA.6 SACS uses historical and clinical data to derive a score that identifies a patient’s risk level.7 However, as an alternative to the 2 levels described in Flemons’ SACS,7 we propose creating 3 risk strata (FIGURE 17,8). We believe that patients at high risk (SACS ≥15) should be encouraged to undergo sleep evaluations as their posttest probability (PTP) of OSA is 75% to 80%. Individuals at low risk (SACS ≤5; PTP <20%) could receive lifestyle advice and simple clinical interventions that decrease symptoms (eg, weight loss, increased physical activity, sleeping on one’s side). For low-risk patients, clinical observation and reevaluation could take place over time with their primary care provider, without additional testing or referral to specialists.
What about patients at intermediate risk? Many patients suspected of having OSA will be assigned to intermediate risk (SACS 6-14), and their PTP of OSA remains at 40% to 45%, the pre-test level most commonly encountered in suspected OSA. As polysomnography is a limited and expensive clinical resource, intermediate-risk patients would benefit from recalibration of their SACS-based risk assessment using an additional surrogate test such as home-based overnight oximetry. Our internal OSA practice guidelines recommend referral for sleep medicine consultation when oximetry results are abnormal—specifically, an oxygen desaturation index (ODI) of ≥5, a mean saturation less than 89%, and a minimum saturation of 75% or less.
Our objectives in this study were to compare the diagnostic implications of these 3 measurements from home-based overnight oximetry reports and use the most relevant result to derive a predictive model further refining PTP of OSA in a primary care patient population first stratified to intermediate risk by SACS.
METHODS
Subjects
We performed secondary analyses on data obtained from our SACS validation cohort.6 In brief, these were patients suspected of having OSA based on the presence of signs, symptoms, or associated risk factors. One hundred ninety-one patients completed all assessments. Sixty-six of 191 patients (35%) were categorized as intermediate risk (SACS 6-14; OSA probability 41% [27/66]).
Data collection and analyses
Participants completed home-based overnight oximetry using Nonin Model 2500 oximeters (Nonin Medical Inc., Plymouth, Minn). We transferred oximetry results from the sleep lab database to a statistical program for analyses of ODI, mean saturation, and minimal saturation. ODI was defined as the number of 4% drops in saturation from baseline divided by the number of hours of recording time. Although the AASM states that a diagnosis of OSA is confirmed if the number of obstructive events is more than 15 per hour or more than 5 per hour in a patient who reports related symptoms,4 we defined OSA as an apnea-hypopnea index (AHI) of >10 based on polysomnography (as this was the threshold used in the derivation cohort for SACS).7 We demonstrated the predictive ability of SACS at various AHI definitions of OSA in our validation cohort.6 The use of SACS in our validation cohort showed a statistically similar ability to predict OSA at both an AHI of 10 and 20, compared with the derivation cohort.
Continue to: We entered additional information...
We entered additional information reported directly by patients and obtained from their sleep studies into a REDCap database and transferred that to our statistical program. We used descriptive statistics to determine ranges and central tendencies of oximetry results. Receiver operator characteristic (ROC) analyses described the predictive abilities for each oximetry result individually and in serial application with prior SACS determinations. For comparison, we used the area under the ROC curve (AUC) from logistic regression to model the probability of OSA.
We calculated positive likelihood ratios (LR) and 95% confidence intervals (CI) to determine the degree of oximetry abnormality that would recalibrate risk either to a high PTP of OSA (>75%) or a low PTP (<25%). We sorted intermediate-risk SACS scores into quintiles based on ODI results to compare the resulting PTPs of OSA. We applied the PTP of OSA from our previous work (using the SACS score to compute the LR) as the new PTP, estimated the LR based on ODI, and computed an updated PTP of OSA. We also used ROC analysis to determine the optimal cutoff value of the ODI.
Finally, in accordance with our internal clinical practice recommendations, we examined the predictive ability of a “positive” ODI result of ≥5 to recalibrate risk prediction for OSA for patients in the low-risk group. We performed analyses using SAS 9.4 (SAS Institute, Cary, NC).
RESULTS
One hundred ninety-one subjects completed assessments. The median and quartile results for ODI, mean saturation, and minimum saturation are found in TABLE 1. TABLE 2 shows the distribution of patients with positive oximetry results. An ODI of 5 or greater was the most frequent abnormal result (135/191; 70.7%).
We used the AUC to measure the comparative abilities of SACS and the 3 overnight oximetry results in predicting OSA (TABLE 3). ODI results demonstrated the best ability to predict OSA, compared with polysomnography as the relative gold standard (AUC, 0.88; 95% confidence interval [CI], 0.83-0.93). Serial application of SACS and ODI yielded even better diagnostic results (AUC, 0.90; 95% CI, 0.85-0.95).
Continue to: As ODI was found to be the strongest predictor of OSA...
As ODI was found to be the strongest predictor of OSA, we grouped these results in quintiles and calculated positive LRs. TABLE 4 shows their effect on PTP of disease among patients with intermediate risk. An ODI result >10 effected an upward recalibration of disease probability (LR, 2.33; 95% CI, 1.27-4.26). The optimal cutoff of ODI to discriminate between those with and without OSA was determined by ROC analysis. An ODI greater than 8.4 created a PTP of disease of approximately 73% to 77%.
Our internal clinical guidelines recommend referring patients with an ODI of 5 or greater for sleep medicine consultation. We examined the ability of this ODI result to recalibrate disease suspicion for a patient at low risk (SACS ≤5). The LR for ODI of 5 or greater is 2.1, but this only results in a recalibration of risk from 24% pretest probability in our validation cohort to 41% PTP (95% CI, 33-49). This low cutoff for a positive test creates false-positive results more than 40% of the time due to low specificity (0.58). This is insufficient to change the suspicion of disease, resulting only in a shift to intermediate OSA risk.
DISCUSSION
Among 3 different oximetry measurements, an ODI ≥10 best predicts OSA, both independently and when used sequentially after the SACS. ODI was by far the most frequent abnormality on oximetry in our cohort, thereby increasing its utility in clinical decision making. For those subjects at intermediate risk, a cutoff of 10 for the ODI result may be a simple and clinically effective way to recalibrate risk and aid in making referral decisions. (This may also be simpler and more easily remembered by clinicians than the 8.4 ODI results from the ROC analyses.)
Assessment is inadequate without a clinical prediction rule. Unfortunately, providers cannot simply rely on clinical gestalt in diagnosing OSA. In their derivation cohort, Flemens et al examined the LRs created by SACS and by clinician prediction based on history and physical exam.7 The SACS LRs ranged from 5.17 to 0.25, a 20-fold range. This reflected superior diagnostic information compared with subjective physician impression, where LRs ranged from 3.7 to 0.52, a seven-fold range. Myers et al prepared a meta-analysis of 4 different trials that examined physicians’ ability to predict OSA.9 Despite the researchers’ use of experienced sleep medicine doctors, the overall diagnostic accuracy of clinical impression was modest (summary positive LR, 1.7; 95% CI, 1.5-2; I2 = 0%; summary negative LR, 0.67; 95% CI, 0.60-0.74; I2 = 10%; sensitivity, 58%; specificity, 67%). This is similar to reliance on a single clinical sign or symptom to predict OSA.
Wise use of oximetry augments SACS calculation. To limit unnecessary oximetry testing in low- and high-risk groups and to avoid polysomnography in cases of a low PTP of disease, we advocate limiting oximetry testing to individuals in the SACS intermediate-risk group (FIGURE 2) wherein ODI results can potentially recalibrate risk assessment up or down. (Those in the high- risk group should be referred to a sleep medicine specialist.) Our institutional recommendation of using an ODI result of ≥5 as a threshold to increase suspicion of disease requires a caveat for the low-risk group. “Positive” results at that low diagnostic threshold are frequently false.
Continue to: Multiple benefits of SACS
Multiple benefits of SACS. We believe using the SACS calculation during clinical encounters with patients potentially at risk for OSA would increase diagnostic accuracy. Performing risk stratification with SACS should not be an undue burden on providers, and the increased time spent with patients has its own benefits, including helping them better understand their risk. Using this standardized process—augmented, as needed, with overnight ODI assessment—might also encourage more patients to follow through on subsequent recommendations, as their risk is further quantified objectively. Lastly, unnecessary testing with polysomnography could be avoided.
Limitations of our study. This study’s findings were derived from a patient population in a single institution. Replication of the findings from other settings would be helpful.
Looking forward. It is yet unclear if clinicians will embrace these strategies in real-world primary care practice. We have designed an implementation-and-dissemination trial to assess whether family physicians will use the SACS clinical predication rule in everyday practice and whether our evidence-based recommendations about overnight oximetry will be followed. Underlying our suggested clinical evaluation pathway (FIGURE 2) is the belief that there is value gained from sharing the decision-making process with patients. Although we provide new evidence that informs these conversations, the patient’s values and preferences are important when determining the best direction to proceed in the evaluation for suspected OSA. These recommendations are intended to aid, not replace, good clinical judgment.
Home-based sleep testing has become more widely available, is convenient for patients, and is less expensive than lab-based polysomnography. Our study did not directly address the appropriate circumstances for home studies in clinical evaluation. We rely on the expertise of our sleep medicine colleagues to determine which patients are appropriate candidates for home-based studies.
The AASM states that “portable monitors (PM) for the diagnosis of OSA should be [used] only in conjunction with a comprehensive sleep evaluation. Clinical sleep evaluations using PM must be supervised by a practitioner with board certification in sleep medicine or an individual who fulfills the eligibility criteria for the sleep medicine certification examination.”4 Additionally, the group recommends that PM “may be used in the unattended setting as an alternative to polysomnography for the diagnosis of OSA in patients with a high pretest probability of moderate to severe OSA and no comorbid sleep disorder or major comorbid medical disorders.”4
Continue to: GRANT SUPPORT
GRANT SUPPORT
The use of the REDCap database is supported by grant UL1 TR000135. This work was supported by a Mayo Foundation CR-20 grant awarded to Dr. Mookadam as Principal investigator and Dr. Grover as Coinvestigator.
Statistical analyses were supported, in part, by the Department of Family Medicine, Mayo Clinic, Scottsdale, Ariz.
CORRESPONDENCE
Michael Grover, DO, Mayo Clinic Thunderbird Primary Care Center-Family Medicine, 13737 N 92nd Street, Scottsdale, AZ 85260; [email protected]
1. National Sleep Foundation. Sleep apnea. https://sleepfoundation.org/sleep-disorders-problems/sleep-apnea. Accessed September 14, 2018.
2. Grover M, Mookadam M, Armas D, et al. Identifying patients at risk for obstructive sleep apnea in a primary care practice. J Am Board Fam Med. 2011;24:152-160.
3. Mold JW, Quattlebaum C, Schinnerer E, et al. Identification by primary care clinicians of patients with obstructive sleep apnea: a practice-based research network (PBRN) study. J Am Board Fam Med. 2011;24:138-145.
4. Epstein LJ, Kristo D, Strollo PJ, Jr., et al; Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263-276.
5. Qaseem A, Dallas P, Owens DK, et al. Diagnosis of obstructive sleep apnea in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2014;161:210-220.
6. Grover M, Mookadam M, Chang Y-H, et al. Validating the Sleep Apnea Clinical Score for use in primary care populations. Mayo Clin Proc. 2016;91:469-476.
7. Flemons WW, Whitelaw WA, Brant R, et al. Likelihood ratios for a sleep apnea clinical prediction rule. Am J Respir Crit Care Med. 1994;150:1279-1285.
8. Gali B, Whalen FX, Gay PC, et al. Management plan to reduce risks in perioperative care of patients with presumed obstructive sleep apnea syndrome. J Clin Sleep Med. 2007;3:582-588.
9. Myers KA, Mrkobrada M, Simel DL. Does this patient have obstructive sleep apnea?: The rational clinical examination systematic review. JAMA. 2013;310(7):731-741.
ABSTRACT
Purpose To derive a predictive model for obstructive sleep apnea (OSA) in primary care practice, using home-based overnight oximetry results to refine posttest probability (PTP) of disease after initial risk stratification with the Sleep Apnea Clinical Score (SACS).
Methods We performed secondary analyses on data from a SACS validation cohort, to compare the diagnostic accuracy of 3 overnight oximetry measurements (oxygen desaturation index [ODI], mean saturation, and minimum saturation) in predicting OSA. Receiver operator characteristics (ROC) were computed for each measurement independently and sequentially after risk stratifying with SACS. We examined the implications of oximetry results for OSA PTP for participants categorized as intermediate risk (SACS 6-14; 66/191 participants [35%]; OSA probability 41%). We calculated positive likelihood ratios (LR) for multiple ODI results and determined which ones allowed recalibration to high- or low-risk PTP.
Results Among the 3 oximetry findings, ODI best predicted OSA (area under the curve [AUC], 0.88; 95% confidence interval [CI], 0.83-0.93). An ODI ≥8.4 (likelihood ratio [LR], 4.19; 95% CI, 2.87-6.10) created a PTP of 77%, while an ODI of 0 to <8.4 (LR, 0.19, 95% CI, 0.12-0.33) created a 14% PTP. Sequential application of SACS and ODI results yielded an AUC result of 0.90 (95% CI, 0.85-0.95).
Conclusions SACS risk stratification provides an advantage over clinical gestalt. In those at intermediate risk, ODI results provide a simple and clinically useful way to further refine diagnostic prediction. Sequential use of SACS and selectively employed overnight oximetry may limit unnecessary polysomnography. Oximetry testing should be avoided in patients deemed low or high risk by SACS, as positive results do not substantially recalibrate risk.
Obstructive sleep apnea (OSA) is a prevalent and underdiagnosed condition. The National Sleep Foundation estimates that 18 million Americans have OSA.1 Primary care practice may be the best setting in which to identify OSA, as many of our patients have conditions frequently associated with apnea (eg, hypertension, obesity, diabetes, arrhythmia, and neurologic illness). Up to a third of patients in primary care practice may be at increased risk.2,3
Clinical guidelines of the American Academy of Sleep Medicine (AASM) recommend obtaining a sleep history to evaluate for possible OSA in 3 instances: as part of a routine health maintenance examination, during evaluation of specific complaints associated with OSA (eg, snoring, apnea, daytime sleepiness), and during comprehensive evaluations for individuals with high-risk conditions (ie, obesity, congestive heart failure, refractory hypertension, diabetes, stroke history).4
The American College of Physicians (ACP) Clinical Practice Guideline suggests assessing individuals who have unexplained daytime sleepiness.5 The ACP considers this assessment “High-Value Care,” as “evidence shows that before diagnosis, patients with OSA have higher rates of health care use, more frequent and longer hospital stays, and higher health care costs than after diagnosis.”5
Continue to: We recently validated the diagnostic accuracy...
We recently validated the diagnostic accuracy of the Sleep Apnea Clinical Score (SACS) for use in a primary care patient population suspected of having OSA.6 SACS uses historical and clinical data to derive a score that identifies a patient’s risk level.7 However, as an alternative to the 2 levels described in Flemons’ SACS,7 we propose creating 3 risk strata (FIGURE 17,8). We believe that patients at high risk (SACS ≥15) should be encouraged to undergo sleep evaluations as their posttest probability (PTP) of OSA is 75% to 80%. Individuals at low risk (SACS ≤5; PTP <20%) could receive lifestyle advice and simple clinical interventions that decrease symptoms (eg, weight loss, increased physical activity, sleeping on one’s side). For low-risk patients, clinical observation and reevaluation could take place over time with their primary care provider, without additional testing or referral to specialists.
What about patients at intermediate risk? Many patients suspected of having OSA will be assigned to intermediate risk (SACS 6-14), and their PTP of OSA remains at 40% to 45%, the pre-test level most commonly encountered in suspected OSA. As polysomnography is a limited and expensive clinical resource, intermediate-risk patients would benefit from recalibration of their SACS-based risk assessment using an additional surrogate test such as home-based overnight oximetry. Our internal OSA practice guidelines recommend referral for sleep medicine consultation when oximetry results are abnormal—specifically, an oxygen desaturation index (ODI) of ≥5, a mean saturation less than 89%, and a minimum saturation of 75% or less.
Our objectives in this study were to compare the diagnostic implications of these 3 measurements from home-based overnight oximetry reports and use the most relevant result to derive a predictive model further refining PTP of OSA in a primary care patient population first stratified to intermediate risk by SACS.
METHODS
Subjects
We performed secondary analyses on data obtained from our SACS validation cohort.6 In brief, these were patients suspected of having OSA based on the presence of signs, symptoms, or associated risk factors. One hundred ninety-one patients completed all assessments. Sixty-six of 191 patients (35%) were categorized as intermediate risk (SACS 6-14; OSA probability 41% [27/66]).
Data collection and analyses
Participants completed home-based overnight oximetry using Nonin Model 2500 oximeters (Nonin Medical Inc., Plymouth, Minn). We transferred oximetry results from the sleep lab database to a statistical program for analyses of ODI, mean saturation, and minimal saturation. ODI was defined as the number of 4% drops in saturation from baseline divided by the number of hours of recording time. Although the AASM states that a diagnosis of OSA is confirmed if the number of obstructive events is more than 15 per hour or more than 5 per hour in a patient who reports related symptoms,4 we defined OSA as an apnea-hypopnea index (AHI) of >10 based on polysomnography (as this was the threshold used in the derivation cohort for SACS).7 We demonstrated the predictive ability of SACS at various AHI definitions of OSA in our validation cohort.6 The use of SACS in our validation cohort showed a statistically similar ability to predict OSA at both an AHI of 10 and 20, compared with the derivation cohort.
Continue to: We entered additional information...
We entered additional information reported directly by patients and obtained from their sleep studies into a REDCap database and transferred that to our statistical program. We used descriptive statistics to determine ranges and central tendencies of oximetry results. Receiver operator characteristic (ROC) analyses described the predictive abilities for each oximetry result individually and in serial application with prior SACS determinations. For comparison, we used the area under the ROC curve (AUC) from logistic regression to model the probability of OSA.
We calculated positive likelihood ratios (LR) and 95% confidence intervals (CI) to determine the degree of oximetry abnormality that would recalibrate risk either to a high PTP of OSA (>75%) or a low PTP (<25%). We sorted intermediate-risk SACS scores into quintiles based on ODI results to compare the resulting PTPs of OSA. We applied the PTP of OSA from our previous work (using the SACS score to compute the LR) as the new PTP, estimated the LR based on ODI, and computed an updated PTP of OSA. We also used ROC analysis to determine the optimal cutoff value of the ODI.
Finally, in accordance with our internal clinical practice recommendations, we examined the predictive ability of a “positive” ODI result of ≥5 to recalibrate risk prediction for OSA for patients in the low-risk group. We performed analyses using SAS 9.4 (SAS Institute, Cary, NC).
RESULTS
One hundred ninety-one subjects completed assessments. The median and quartile results for ODI, mean saturation, and minimum saturation are found in TABLE 1. TABLE 2 shows the distribution of patients with positive oximetry results. An ODI of 5 or greater was the most frequent abnormal result (135/191; 70.7%).
We used the AUC to measure the comparative abilities of SACS and the 3 overnight oximetry results in predicting OSA (TABLE 3). ODI results demonstrated the best ability to predict OSA, compared with polysomnography as the relative gold standard (AUC, 0.88; 95% confidence interval [CI], 0.83-0.93). Serial application of SACS and ODI yielded even better diagnostic results (AUC, 0.90; 95% CI, 0.85-0.95).
Continue to: As ODI was found to be the strongest predictor of OSA...
As ODI was found to be the strongest predictor of OSA, we grouped these results in quintiles and calculated positive LRs. TABLE 4 shows their effect on PTP of disease among patients with intermediate risk. An ODI result >10 effected an upward recalibration of disease probability (LR, 2.33; 95% CI, 1.27-4.26). The optimal cutoff of ODI to discriminate between those with and without OSA was determined by ROC analysis. An ODI greater than 8.4 created a PTP of disease of approximately 73% to 77%.
Our internal clinical guidelines recommend referring patients with an ODI of 5 or greater for sleep medicine consultation. We examined the ability of this ODI result to recalibrate disease suspicion for a patient at low risk (SACS ≤5). The LR for ODI of 5 or greater is 2.1, but this only results in a recalibration of risk from 24% pretest probability in our validation cohort to 41% PTP (95% CI, 33-49). This low cutoff for a positive test creates false-positive results more than 40% of the time due to low specificity (0.58). This is insufficient to change the suspicion of disease, resulting only in a shift to intermediate OSA risk.
DISCUSSION
Among 3 different oximetry measurements, an ODI ≥10 best predicts OSA, both independently and when used sequentially after the SACS. ODI was by far the most frequent abnormality on oximetry in our cohort, thereby increasing its utility in clinical decision making. For those subjects at intermediate risk, a cutoff of 10 for the ODI result may be a simple and clinically effective way to recalibrate risk and aid in making referral decisions. (This may also be simpler and more easily remembered by clinicians than the 8.4 ODI results from the ROC analyses.)
Assessment is inadequate without a clinical prediction rule. Unfortunately, providers cannot simply rely on clinical gestalt in diagnosing OSA. In their derivation cohort, Flemens et al examined the LRs created by SACS and by clinician prediction based on history and physical exam.7 The SACS LRs ranged from 5.17 to 0.25, a 20-fold range. This reflected superior diagnostic information compared with subjective physician impression, where LRs ranged from 3.7 to 0.52, a seven-fold range. Myers et al prepared a meta-analysis of 4 different trials that examined physicians’ ability to predict OSA.9 Despite the researchers’ use of experienced sleep medicine doctors, the overall diagnostic accuracy of clinical impression was modest (summary positive LR, 1.7; 95% CI, 1.5-2; I2 = 0%; summary negative LR, 0.67; 95% CI, 0.60-0.74; I2 = 10%; sensitivity, 58%; specificity, 67%). This is similar to reliance on a single clinical sign or symptom to predict OSA.
Wise use of oximetry augments SACS calculation. To limit unnecessary oximetry testing in low- and high-risk groups and to avoid polysomnography in cases of a low PTP of disease, we advocate limiting oximetry testing to individuals in the SACS intermediate-risk group (FIGURE 2) wherein ODI results can potentially recalibrate risk assessment up or down. (Those in the high- risk group should be referred to a sleep medicine specialist.) Our institutional recommendation of using an ODI result of ≥5 as a threshold to increase suspicion of disease requires a caveat for the low-risk group. “Positive” results at that low diagnostic threshold are frequently false.
Continue to: Multiple benefits of SACS
Multiple benefits of SACS. We believe using the SACS calculation during clinical encounters with patients potentially at risk for OSA would increase diagnostic accuracy. Performing risk stratification with SACS should not be an undue burden on providers, and the increased time spent with patients has its own benefits, including helping them better understand their risk. Using this standardized process—augmented, as needed, with overnight ODI assessment—might also encourage more patients to follow through on subsequent recommendations, as their risk is further quantified objectively. Lastly, unnecessary testing with polysomnography could be avoided.
Limitations of our study. This study’s findings were derived from a patient population in a single institution. Replication of the findings from other settings would be helpful.
Looking forward. It is yet unclear if clinicians will embrace these strategies in real-world primary care practice. We have designed an implementation-and-dissemination trial to assess whether family physicians will use the SACS clinical predication rule in everyday practice and whether our evidence-based recommendations about overnight oximetry will be followed. Underlying our suggested clinical evaluation pathway (FIGURE 2) is the belief that there is value gained from sharing the decision-making process with patients. Although we provide new evidence that informs these conversations, the patient’s values and preferences are important when determining the best direction to proceed in the evaluation for suspected OSA. These recommendations are intended to aid, not replace, good clinical judgment.
Home-based sleep testing has become more widely available, is convenient for patients, and is less expensive than lab-based polysomnography. Our study did not directly address the appropriate circumstances for home studies in clinical evaluation. We rely on the expertise of our sleep medicine colleagues to determine which patients are appropriate candidates for home-based studies.
The AASM states that “portable monitors (PM) for the diagnosis of OSA should be [used] only in conjunction with a comprehensive sleep evaluation. Clinical sleep evaluations using PM must be supervised by a practitioner with board certification in sleep medicine or an individual who fulfills the eligibility criteria for the sleep medicine certification examination.”4 Additionally, the group recommends that PM “may be used in the unattended setting as an alternative to polysomnography for the diagnosis of OSA in patients with a high pretest probability of moderate to severe OSA and no comorbid sleep disorder or major comorbid medical disorders.”4
Continue to: GRANT SUPPORT
GRANT SUPPORT
The use of the REDCap database is supported by grant UL1 TR000135. This work was supported by a Mayo Foundation CR-20 grant awarded to Dr. Mookadam as Principal investigator and Dr. Grover as Coinvestigator.
Statistical analyses were supported, in part, by the Department of Family Medicine, Mayo Clinic, Scottsdale, Ariz.
CORRESPONDENCE
Michael Grover, DO, Mayo Clinic Thunderbird Primary Care Center-Family Medicine, 13737 N 92nd Street, Scottsdale, AZ 85260; [email protected]
ABSTRACT
Purpose To derive a predictive model for obstructive sleep apnea (OSA) in primary care practice, using home-based overnight oximetry results to refine posttest probability (PTP) of disease after initial risk stratification with the Sleep Apnea Clinical Score (SACS).
Methods We performed secondary analyses on data from a SACS validation cohort, to compare the diagnostic accuracy of 3 overnight oximetry measurements (oxygen desaturation index [ODI], mean saturation, and minimum saturation) in predicting OSA. Receiver operator characteristics (ROC) were computed for each measurement independently and sequentially after risk stratifying with SACS. We examined the implications of oximetry results for OSA PTP for participants categorized as intermediate risk (SACS 6-14; 66/191 participants [35%]; OSA probability 41%). We calculated positive likelihood ratios (LR) for multiple ODI results and determined which ones allowed recalibration to high- or low-risk PTP.
Results Among the 3 oximetry findings, ODI best predicted OSA (area under the curve [AUC], 0.88; 95% confidence interval [CI], 0.83-0.93). An ODI ≥8.4 (likelihood ratio [LR], 4.19; 95% CI, 2.87-6.10) created a PTP of 77%, while an ODI of 0 to <8.4 (LR, 0.19, 95% CI, 0.12-0.33) created a 14% PTP. Sequential application of SACS and ODI results yielded an AUC result of 0.90 (95% CI, 0.85-0.95).
Conclusions SACS risk stratification provides an advantage over clinical gestalt. In those at intermediate risk, ODI results provide a simple and clinically useful way to further refine diagnostic prediction. Sequential use of SACS and selectively employed overnight oximetry may limit unnecessary polysomnography. Oximetry testing should be avoided in patients deemed low or high risk by SACS, as positive results do not substantially recalibrate risk.
Obstructive sleep apnea (OSA) is a prevalent and underdiagnosed condition. The National Sleep Foundation estimates that 18 million Americans have OSA.1 Primary care practice may be the best setting in which to identify OSA, as many of our patients have conditions frequently associated with apnea (eg, hypertension, obesity, diabetes, arrhythmia, and neurologic illness). Up to a third of patients in primary care practice may be at increased risk.2,3
Clinical guidelines of the American Academy of Sleep Medicine (AASM) recommend obtaining a sleep history to evaluate for possible OSA in 3 instances: as part of a routine health maintenance examination, during evaluation of specific complaints associated with OSA (eg, snoring, apnea, daytime sleepiness), and during comprehensive evaluations for individuals with high-risk conditions (ie, obesity, congestive heart failure, refractory hypertension, diabetes, stroke history).4
The American College of Physicians (ACP) Clinical Practice Guideline suggests assessing individuals who have unexplained daytime sleepiness.5 The ACP considers this assessment “High-Value Care,” as “evidence shows that before diagnosis, patients with OSA have higher rates of health care use, more frequent and longer hospital stays, and higher health care costs than after diagnosis.”5
Continue to: We recently validated the diagnostic accuracy...
We recently validated the diagnostic accuracy of the Sleep Apnea Clinical Score (SACS) for use in a primary care patient population suspected of having OSA.6 SACS uses historical and clinical data to derive a score that identifies a patient’s risk level.7 However, as an alternative to the 2 levels described in Flemons’ SACS,7 we propose creating 3 risk strata (FIGURE 17,8). We believe that patients at high risk (SACS ≥15) should be encouraged to undergo sleep evaluations as their posttest probability (PTP) of OSA is 75% to 80%. Individuals at low risk (SACS ≤5; PTP <20%) could receive lifestyle advice and simple clinical interventions that decrease symptoms (eg, weight loss, increased physical activity, sleeping on one’s side). For low-risk patients, clinical observation and reevaluation could take place over time with their primary care provider, without additional testing or referral to specialists.
What about patients at intermediate risk? Many patients suspected of having OSA will be assigned to intermediate risk (SACS 6-14), and their PTP of OSA remains at 40% to 45%, the pre-test level most commonly encountered in suspected OSA. As polysomnography is a limited and expensive clinical resource, intermediate-risk patients would benefit from recalibration of their SACS-based risk assessment using an additional surrogate test such as home-based overnight oximetry. Our internal OSA practice guidelines recommend referral for sleep medicine consultation when oximetry results are abnormal—specifically, an oxygen desaturation index (ODI) of ≥5, a mean saturation less than 89%, and a minimum saturation of 75% or less.
Our objectives in this study were to compare the diagnostic implications of these 3 measurements from home-based overnight oximetry reports and use the most relevant result to derive a predictive model further refining PTP of OSA in a primary care patient population first stratified to intermediate risk by SACS.
METHODS
Subjects
We performed secondary analyses on data obtained from our SACS validation cohort.6 In brief, these were patients suspected of having OSA based on the presence of signs, symptoms, or associated risk factors. One hundred ninety-one patients completed all assessments. Sixty-six of 191 patients (35%) were categorized as intermediate risk (SACS 6-14; OSA probability 41% [27/66]).
Data collection and analyses
Participants completed home-based overnight oximetry using Nonin Model 2500 oximeters (Nonin Medical Inc., Plymouth, Minn). We transferred oximetry results from the sleep lab database to a statistical program for analyses of ODI, mean saturation, and minimal saturation. ODI was defined as the number of 4% drops in saturation from baseline divided by the number of hours of recording time. Although the AASM states that a diagnosis of OSA is confirmed if the number of obstructive events is more than 15 per hour or more than 5 per hour in a patient who reports related symptoms,4 we defined OSA as an apnea-hypopnea index (AHI) of >10 based on polysomnography (as this was the threshold used in the derivation cohort for SACS).7 We demonstrated the predictive ability of SACS at various AHI definitions of OSA in our validation cohort.6 The use of SACS in our validation cohort showed a statistically similar ability to predict OSA at both an AHI of 10 and 20, compared with the derivation cohort.
Continue to: We entered additional information...
We entered additional information reported directly by patients and obtained from their sleep studies into a REDCap database and transferred that to our statistical program. We used descriptive statistics to determine ranges and central tendencies of oximetry results. Receiver operator characteristic (ROC) analyses described the predictive abilities for each oximetry result individually and in serial application with prior SACS determinations. For comparison, we used the area under the ROC curve (AUC) from logistic regression to model the probability of OSA.
We calculated positive likelihood ratios (LR) and 95% confidence intervals (CI) to determine the degree of oximetry abnormality that would recalibrate risk either to a high PTP of OSA (>75%) or a low PTP (<25%). We sorted intermediate-risk SACS scores into quintiles based on ODI results to compare the resulting PTPs of OSA. We applied the PTP of OSA from our previous work (using the SACS score to compute the LR) as the new PTP, estimated the LR based on ODI, and computed an updated PTP of OSA. We also used ROC analysis to determine the optimal cutoff value of the ODI.
Finally, in accordance with our internal clinical practice recommendations, we examined the predictive ability of a “positive” ODI result of ≥5 to recalibrate risk prediction for OSA for patients in the low-risk group. We performed analyses using SAS 9.4 (SAS Institute, Cary, NC).
RESULTS
One hundred ninety-one subjects completed assessments. The median and quartile results for ODI, mean saturation, and minimum saturation are found in TABLE 1. TABLE 2 shows the distribution of patients with positive oximetry results. An ODI of 5 or greater was the most frequent abnormal result (135/191; 70.7%).
We used the AUC to measure the comparative abilities of SACS and the 3 overnight oximetry results in predicting OSA (TABLE 3). ODI results demonstrated the best ability to predict OSA, compared with polysomnography as the relative gold standard (AUC, 0.88; 95% confidence interval [CI], 0.83-0.93). Serial application of SACS and ODI yielded even better diagnostic results (AUC, 0.90; 95% CI, 0.85-0.95).
Continue to: As ODI was found to be the strongest predictor of OSA...
As ODI was found to be the strongest predictor of OSA, we grouped these results in quintiles and calculated positive LRs. TABLE 4 shows their effect on PTP of disease among patients with intermediate risk. An ODI result >10 effected an upward recalibration of disease probability (LR, 2.33; 95% CI, 1.27-4.26). The optimal cutoff of ODI to discriminate between those with and without OSA was determined by ROC analysis. An ODI greater than 8.4 created a PTP of disease of approximately 73% to 77%.
Our internal clinical guidelines recommend referring patients with an ODI of 5 or greater for sleep medicine consultation. We examined the ability of this ODI result to recalibrate disease suspicion for a patient at low risk (SACS ≤5). The LR for ODI of 5 or greater is 2.1, but this only results in a recalibration of risk from 24% pretest probability in our validation cohort to 41% PTP (95% CI, 33-49). This low cutoff for a positive test creates false-positive results more than 40% of the time due to low specificity (0.58). This is insufficient to change the suspicion of disease, resulting only in a shift to intermediate OSA risk.
DISCUSSION
Among 3 different oximetry measurements, an ODI ≥10 best predicts OSA, both independently and when used sequentially after the SACS. ODI was by far the most frequent abnormality on oximetry in our cohort, thereby increasing its utility in clinical decision making. For those subjects at intermediate risk, a cutoff of 10 for the ODI result may be a simple and clinically effective way to recalibrate risk and aid in making referral decisions. (This may also be simpler and more easily remembered by clinicians than the 8.4 ODI results from the ROC analyses.)
Assessment is inadequate without a clinical prediction rule. Unfortunately, providers cannot simply rely on clinical gestalt in diagnosing OSA. In their derivation cohort, Flemens et al examined the LRs created by SACS and by clinician prediction based on history and physical exam.7 The SACS LRs ranged from 5.17 to 0.25, a 20-fold range. This reflected superior diagnostic information compared with subjective physician impression, where LRs ranged from 3.7 to 0.52, a seven-fold range. Myers et al prepared a meta-analysis of 4 different trials that examined physicians’ ability to predict OSA.9 Despite the researchers’ use of experienced sleep medicine doctors, the overall diagnostic accuracy of clinical impression was modest (summary positive LR, 1.7; 95% CI, 1.5-2; I2 = 0%; summary negative LR, 0.67; 95% CI, 0.60-0.74; I2 = 10%; sensitivity, 58%; specificity, 67%). This is similar to reliance on a single clinical sign or symptom to predict OSA.
Wise use of oximetry augments SACS calculation. To limit unnecessary oximetry testing in low- and high-risk groups and to avoid polysomnography in cases of a low PTP of disease, we advocate limiting oximetry testing to individuals in the SACS intermediate-risk group (FIGURE 2) wherein ODI results can potentially recalibrate risk assessment up or down. (Those in the high- risk group should be referred to a sleep medicine specialist.) Our institutional recommendation of using an ODI result of ≥5 as a threshold to increase suspicion of disease requires a caveat for the low-risk group. “Positive” results at that low diagnostic threshold are frequently false.
Continue to: Multiple benefits of SACS
Multiple benefits of SACS. We believe using the SACS calculation during clinical encounters with patients potentially at risk for OSA would increase diagnostic accuracy. Performing risk stratification with SACS should not be an undue burden on providers, and the increased time spent with patients has its own benefits, including helping them better understand their risk. Using this standardized process—augmented, as needed, with overnight ODI assessment—might also encourage more patients to follow through on subsequent recommendations, as their risk is further quantified objectively. Lastly, unnecessary testing with polysomnography could be avoided.
Limitations of our study. This study’s findings were derived from a patient population in a single institution. Replication of the findings from other settings would be helpful.
Looking forward. It is yet unclear if clinicians will embrace these strategies in real-world primary care practice. We have designed an implementation-and-dissemination trial to assess whether family physicians will use the SACS clinical predication rule in everyday practice and whether our evidence-based recommendations about overnight oximetry will be followed. Underlying our suggested clinical evaluation pathway (FIGURE 2) is the belief that there is value gained from sharing the decision-making process with patients. Although we provide new evidence that informs these conversations, the patient’s values and preferences are important when determining the best direction to proceed in the evaluation for suspected OSA. These recommendations are intended to aid, not replace, good clinical judgment.
Home-based sleep testing has become more widely available, is convenient for patients, and is less expensive than lab-based polysomnography. Our study did not directly address the appropriate circumstances for home studies in clinical evaluation. We rely on the expertise of our sleep medicine colleagues to determine which patients are appropriate candidates for home-based studies.
The AASM states that “portable monitors (PM) for the diagnosis of OSA should be [used] only in conjunction with a comprehensive sleep evaluation. Clinical sleep evaluations using PM must be supervised by a practitioner with board certification in sleep medicine or an individual who fulfills the eligibility criteria for the sleep medicine certification examination.”4 Additionally, the group recommends that PM “may be used in the unattended setting as an alternative to polysomnography for the diagnosis of OSA in patients with a high pretest probability of moderate to severe OSA and no comorbid sleep disorder or major comorbid medical disorders.”4
Continue to: GRANT SUPPORT
GRANT SUPPORT
The use of the REDCap database is supported by grant UL1 TR000135. This work was supported by a Mayo Foundation CR-20 grant awarded to Dr. Mookadam as Principal investigator and Dr. Grover as Coinvestigator.
Statistical analyses were supported, in part, by the Department of Family Medicine, Mayo Clinic, Scottsdale, Ariz.
CORRESPONDENCE
Michael Grover, DO, Mayo Clinic Thunderbird Primary Care Center-Family Medicine, 13737 N 92nd Street, Scottsdale, AZ 85260; [email protected]
1. National Sleep Foundation. Sleep apnea. https://sleepfoundation.org/sleep-disorders-problems/sleep-apnea. Accessed September 14, 2018.
2. Grover M, Mookadam M, Armas D, et al. Identifying patients at risk for obstructive sleep apnea in a primary care practice. J Am Board Fam Med. 2011;24:152-160.
3. Mold JW, Quattlebaum C, Schinnerer E, et al. Identification by primary care clinicians of patients with obstructive sleep apnea: a practice-based research network (PBRN) study. J Am Board Fam Med. 2011;24:138-145.
4. Epstein LJ, Kristo D, Strollo PJ, Jr., et al; Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263-276.
5. Qaseem A, Dallas P, Owens DK, et al. Diagnosis of obstructive sleep apnea in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2014;161:210-220.
6. Grover M, Mookadam M, Chang Y-H, et al. Validating the Sleep Apnea Clinical Score for use in primary care populations. Mayo Clin Proc. 2016;91:469-476.
7. Flemons WW, Whitelaw WA, Brant R, et al. Likelihood ratios for a sleep apnea clinical prediction rule. Am J Respir Crit Care Med. 1994;150:1279-1285.
8. Gali B, Whalen FX, Gay PC, et al. Management plan to reduce risks in perioperative care of patients with presumed obstructive sleep apnea syndrome. J Clin Sleep Med. 2007;3:582-588.
9. Myers KA, Mrkobrada M, Simel DL. Does this patient have obstructive sleep apnea?: The rational clinical examination systematic review. JAMA. 2013;310(7):731-741.
1. National Sleep Foundation. Sleep apnea. https://sleepfoundation.org/sleep-disorders-problems/sleep-apnea. Accessed September 14, 2018.
2. Grover M, Mookadam M, Armas D, et al. Identifying patients at risk for obstructive sleep apnea in a primary care practice. J Am Board Fam Med. 2011;24:152-160.
3. Mold JW, Quattlebaum C, Schinnerer E, et al. Identification by primary care clinicians of patients with obstructive sleep apnea: a practice-based research network (PBRN) study. J Am Board Fam Med. 2011;24:138-145.
4. Epstein LJ, Kristo D, Strollo PJ, Jr., et al; Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263-276.
5. Qaseem A, Dallas P, Owens DK, et al. Diagnosis of obstructive sleep apnea in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2014;161:210-220.
6. Grover M, Mookadam M, Chang Y-H, et al. Validating the Sleep Apnea Clinical Score for use in primary care populations. Mayo Clin Proc. 2016;91:469-476.
7. Flemons WW, Whitelaw WA, Brant R, et al. Likelihood ratios for a sleep apnea clinical prediction rule. Am J Respir Crit Care Med. 1994;150:1279-1285.
8. Gali B, Whalen FX, Gay PC, et al. Management plan to reduce risks in perioperative care of patients with presumed obstructive sleep apnea syndrome. J Clin Sleep Med. 2007;3:582-588.
9. Myers KA, Mrkobrada M, Simel DL. Does this patient have obstructive sleep apnea?: The rational clinical examination systematic review. JAMA. 2013;310(7):731-741.
Barriers to Self-Management in African American Adolescents with Asthma
From Wayne State University, Detroit, MI (Dr. Gibson-Scipio), and the University of Texas Rio Grande Valley, Edinburg, TX (Dr. Krouse).
Abstract
- Objective: To review the literature on barriers to asthma self-management amo
ng African American adolescents. - Methods: Review of the literature.
- Results: Asthma self-management barriers experienced by African American adolescents are often related to developmental needs, lack of knowledge, and personal perspectives and experiences. Adolescents find managing their symptoms and adhering to prescriptive therapies a burden and desire to be more like healthy peers. As they struggle to identify with peers, they may engage in risky behaviors such as ignoring symptoms and delaying treatment, thus leading to poorer asthma control and health outcomes. African American adolescents struggle with perceptions of racial biases from health care providers and teachers that interfere with self-management behaviors. They also describe the influence of culturally based practices learned from caregivers that contribute to their misconceptions and inadequate skills in effectively managing their asthma.
- Conclusion: Researchers should seek to develop interventions to address the unique contextual and culturally based needs of African American adolescents that support the development of effective asthma self-management behaviors. This may include making use of family members (especially mothers) and extended support for self-management during this period of rapid growth and transition. Health care providers should consider a team-based approach to the adolescent patient. Such an approach should be grounded in recommendations from national guidelines that suggest a patient-centered approach to care that includes a partnership between the patient and the provider to address unique barriers to effective self-management.
Keywords: youth; caregiver; drug-therapy; self-efficacy; disease-management; patient-centered care.
Effective asthma self-management by urban African American adolescents is a critical aspect of care that should be addressed with vigilance due to the persistent disparities in disease prevalence, morbidity, and mortality compared to Caucasians.1-3 The overarching goal of asthma self-management is to achieve symptom control, maintain normal activity levels, and minimize future risk of exacerbations and medication side effects.4,5 Best practices for asthma self-management begin with a partnership between health care providers and clients (including parent/caregiver). This relationship should help affected individuals gain asthma control based on knowledge of their disease and treatment options, confidence and skills in trigger avoidance, medication administration, and management of acute exacerbations.4,5
Among youth aged 18 years and younger, African Americans have the highest asthma prevalence rates of all racial and ethnic groups, and between 2001 and 2009 asthma prevalence rates rose by 50% among African American youth.6 As of 2015, prevalence rates for asthma among African American youth were 13.4%, as compared to 7.4% for white youth.7 African American youth have been found to have more frequent asthma exacerbations and related school absences than white youth.8 Furthermore, African American youth younger than 18 years are more likely to be admitted to the hospital for asthma and are 10 times more likely to die from asthma compared to non-Hispanic white children.6
Urban African American adolescents with asthma are particularly vulnerable to poor asthma self-management due to the complexity of the disease in this population.3 African American youth must deal with multiple adverse environmental conditions, lack of knowledge or disbelief concerning effective disease self-management strategies, variable access and quality of care, and the psychosocial dynamics of being young while having a chronic disease.2,3,9-11 It is important to understand and address barriers to successful asthma self-management during adolescence, as behaviors developed during this stage of life often persist into adulthood.9 In this article, we review the literature on barriers to asthma self-management among African American adolescents and offer suggestions on clinical strategies for improving self-management in this vulnerable population.
Methods
The initial search strategy was developed in collaboration with an experienced librarian. Keywords, MeSH terms, and potential databases were identified. Keywords included urban, African American, adolescent, asthma, self-management, and barriers. These terms were expanded based on search results and a review of abstracts that fit the intent of our review. The search was limited to U.S. studies published between 2005 and 2017. Excluded from the search were conference abstracts, doctoral dissertations, master’s theses, meta-analyses, systematic reviews, and studies conducted outside of the United States. Additional articles for the review were identified during the review process from the reference lists of the publications.
Abstracts were reviewed for articles that reported a study population inclusive of African American adolescents with asthma and that were related to self-management. Studies that used qualitative and other descriptive methods and cohort and randomized control trials were reviewed. Due to the limited number of articles found that exclusively focused on African Americans, the authors set a threshold for African American participants at 40% or greater for inclusion in this review.
Full papers were retrieved that met the inclusion criteria for a full review. Each author initially independently reviewed a selected number of papers and abstracted the study purpose, sample, study design, results, conclusions, and limitations. Subsequently, both authors reviewed in tandem and then discussed each selected manuscript to assure the appropriateness for inclusion. The subject matter was considered the priority for inclusion in the review. Study methods, sample size, and noted limitations were categorized but were not considered as a basis for exclusion. Thematic analysis was used to identify common themes across studies.
Results
We identified 23 papers that met our criteria (Table). Five common themes were found that related to barriers in disease self-management for African American adolescents: (a) knowledge and skills, (b) beliefs and attitudes, (c) personal/emotional factors, (d) caregivers, and (e) schools.
Knowledge and Skills
Adequate knowledge of the elements of asthma self-management is critical for achieving control of this condition. Asthma knowledge includes a basic understanding of the disease process and treatment strategies, an awareness of early signs and symptoms of worsening asthma, and an understanding of how to manage environmental triggers.4,5 Sin and colleagues conducted one of the earlier studies to examine the influence of asthma knowledge on asthma self-management in African American adolescents and found a significant positive association between knowledge and asthma self-management behaviors.12
Adherence to an asthma medication, especially inhaled corticosteroids (ICS), is one of the cornerstones to successful self-management of asthma.13,14 Consistent use of ICS therapy to control asthma symptoms and disease progression is often suboptimal in African American adolescents and tends to worsen as they age;15 studies have found lower adherence levels were more prominent in older African American adolescents and males.13,16 In a recent study of adolescents with persistent asthma who were prescribed daily ICS, youth with greater ICS knowledge as assessed using a standardized instrument demonstrated significantly higher adherence rates.13 Proper technique in the use of an inhaler is also important in medication administration. Asthma ICS medication delivery devices vary significantly and require different techniques for medication administration. However, inhaler device skills have been found to be very inadequate in high-risk African American adolescents.17 Thus, knowledge related to ICS therapy and proper skills in the use of inhaler devices is an important aspect of asthma self-management that have been found to be inadequate in African American Adolescents.
Interventions and programs geared to improving education may lead to improved self-management. Multisystemic Therapy-Health Care (MST-HC) is a tailored home-based intervention that includes knowledge and skill-building components. In a study of African American youth with poorly controlled asthma, the program was found to improve illness management.18 In addition, adolescents who complete formal asthma education programs demonstrate significantly higher scores in self-management than those youth who do not participate in these programs.13,19 Unfortunately, few African American teens report participation in an asthma education program.19 In a study of a motivational interviewing intervention to improve controller medication adherence for African American adolescents,14 youth reported gaining more knowledge about their asthma medications and were significantly more motivated to take their controller medications after participating in the intervention; however, while adherence to controller medications was greater than baseline, it was not significantly different.14 This study demonstrated the value of asthma education and the feasibility of a motivational intervention to support controller medication adherence. However, this study also demonstrated the complexity of medication adherence in that neither knowledge or motivation led to significant changes in medication adherence among African American adolescents.
Low health literacy can also act as a barrier to asthma self-management. Health literacy requires skills and knowledge that enable an individual to communicate, process, and understand basic health information that informs health decisions.20 Health literacy was found to be associated with indicators of poor disease self-management among urban African American adolescents in grades 9 through 12.21 In this study, health literacy was established using questions about confidence in filling out medical forms, self-reported problems with learning about the youth’s medical condition, and the need for assistance in reading hospital materials. Adolescents with poor health literacy scores were more likely to reside in a household with the following characteristics: mother with less than a high school education, Medicaid health insurance, family members with a body mass index exceeding the 85th percentile, and lack of rescue medication. Poor health literacy was most common among younger adolescents (ie, ninth graders). Some youth with poor health literacy also reported more emergency department visits, hospitalizations, and lower overall quality of life.21
Beliefs and Attitudes
Beliefs and attitudes towards taking asthma medications can act as barriers to adherence in the adolescent. African American adolescents often report the belief that ICS are not helpful or necessary.16,22-25 These beliefs have been correlated with a lack of understanding of the inflammatory mechanisms of asthma, reports of asthma attacks despite use of controller medications, fear of addiction to medications, and a belief that nontraditional interventions (eg, exercise) will work better to get rid of asthma or abate symptoms.16-19,22-24 African American adolescents also report beliefs that asthma will go away or get better as they age, and they are willing to forgo the use of controller medications based on these beliefs.24
African American adolescents often engage in asthma self-management independent of caregivers. These youth describe asthma self-management activities an annoyance and of low priority in part due to competing tasks and negative interactions with caregivers.25 During early adolescence asthma self-management is often suboptimal, and as youth age they become less observant regarding their asthma and are less likely to seek help.26 Adolescents’ beliefs and low prioritization of asthma self-management may contribute to forgetfulness and loss of inhalers, which are common reasons reported for poor adherence to ICS.16,23-26 Further, the role of caregivers during this period has often been overlooked. Caregivers of African American adolescents have been found to be stressed and overwhelmed with personal responsibilities and neighborhood conditions, leaving them little time to attend to the asthma self-management behavior of youth. Due to these contextual factors, interactions with chronically ill youth may be strained, resulting in negative interactions with youth related to asthma self-management. However, in an intervention study that used multisystemic therapy (an approach that targets the affected individual, family, and community), improvement in positive parenting behaviors related to asthma self-management contributed to improved ICS adherence by adolescents.27
Adolescents can perceive traditional asthma self-management as conflicting with their own personal and/or cultural beliefs. They may seek options beyond the use of medicine and have voiced preferences for behaviors that they believe will “strengthen their lungs” more naturally.24 An appreciation of how youth might use complementary/alternative medicine (CAM) as an adjunctive therapy or in place of evidence-based asthma care is important to understanding the potential effect on morbidity and mortality. Behaviors and beliefs about the use of CAM have not been well studied among urban African American adolescents with asthma. Only one study was found that assessed the use of CAM among a primarily urban African American adolescent population. In that study, 71% of the population reported using some form of CAM during the past 30 days.28 Prayer and relaxation were the most frequently used strategies in the management of asthma symptoms. Perceived efficacy of relaxation and prayer among teens who engaged in this form of CAM was 87% and 85%, respectively. Other CAM strategies included yoga, meditation, guided imagery, and biofeedback. When adolescents were asked if they shared their use of CAM in asthma management with a health care provider, most reported sharing the use of yoga and dietary changes but were least likely to share their use of prayer and guided imagery.28
Personal/Emotional Factors
African American adolescents have reported asthma as a limiting factor in terms of both physical and social activities. They perceive asthma as a burden to themselves and others (eg, peers, family, coaches).9,25 The burden of asthma is further exemplified in the emotional response to the symptoms of the disease and the self-management responsibilities. The need to prevent and respond to asthma symptoms is associated with being embarrassed, frustrated, angry, annoyed, worried, lonely, and isolated.9,11,25 Negative coping strategies by youth in response to psychosocial experiences include decisions to disregard or give minimal attention to asthma symptoms and to delay or not take prescribed medications. Students report ignoring asthma symptom management while engaging in physical activities to maintain a sense of normalcy among peers and as a way of dealing with perceptions by coaches or teachers that they are weak or in need of being protected.24,25
Negative thoughts and experiences can result in depressive disorders and poor quality of life. Depression is a common finding among urban youth with asthma.29,30 Youth diagnosed with asthma who have comorbid depression may benefit from interventions to improve self-management. In a secondary analysis from a Web-based asthma management intervention targeting African American adolescents, depression was found to have a modifying effect on the emotional domain of quality of life for youth in the intervention arm of the study. This finding indicates that participants who were depressed and who reported low levels of emotional quality of life benefited from the Web-based interventions that targeted self-management.31
Caregivers
Caregivers (especially moms) are a common source of support for the development and implementation of asthma self-management behaviors in adolescents.32 Caregivers sometimes hold beliefs similar to those of youth and believe the urban environment can act as a barrier to asthma management.9,25,32 They describe the complexity of asthma treatment plans, a lack of understanding of the disease process, and insensitivity of health care providers to their expressed needs along with the providers’ limited cultural awareness in the development of self-management plans.9,22,33 Caregivers describe how family finances, insurance gaps, access to care, and their own familial/cultural beliefs influence their decisions and ability to support their child’s asthma management.33 When faced with the cost of care they report instances of having to decide between necessities such as food and housing or co-pays for medications and office visits.22,33 They also report concerns about visits with multiple providers due to an inability to access their primary care provider, which can lead to delays in their child being diagnosed with asthma.22
Caregivers report a need to include culturally based practices, past experiences, and personal beliefs into the adolescents’ asthma management plan.22,32,33 In a small interview-based study of caregivers residing in 3 New Jersey public housing communities, caregivers reported preferring “familial” methods of controlling asthma (eg, restriction of activities; use of showers, steam, vaporizers, and nebulizers) over evidence-based recommendations. Many caregivers were confused or lacked knowledge about asthma action plans.33 Caregivers have also been found to lack adequate or accurate knowledge related to asthma medications and factors that improved or worsened asthma. While caregivers report a desire to help educate their teens by passing on what they know, their lack of adequate asthma knowledge may hamper their efforts and potentially worsen the teens’ asthma self-management.32
While African American caregivers often describe themselves as hypervigilant concerning their child’s asthma, they may report different information than their adolescent when both are questioned about asthma symptom experiences and functional status.34 Factors increasing the level of congruence between caregiver and teen asthma symptom reports were found to be related to the adolescents’ age and asthma disease classification. Symptom questionnaire responses of older teens and those with mild intermittent asthma were more likely to be similar to caregiver reports. The researchers concluded that clinicians and researchers may obtain reliable asthma symptom and functional status reports by asking the adolescent directly.34
Schools
Caregivers and adolescents describe schools as a threat to self-management and the overall health of youth with asthma.9,32 They perceive that a lack of knowledge by staff, teachers, and coaches contributes to inattentiveness or disbelief in the credibility of reported asthma symptoms by youth.11,23 These misperceptions and the lack of attentiveness by adults in the school may pose safety and health issues for African American youth.9,25,33,34 For example, adolescents report pressure from teacher, coaches, and peers in school settings to partake in sports and/or gym classes. Youth want to identify with healthy peers and thus often choose not to take asthma medications during such activities or opt to continue participating while being compromised by airway obstruction. Of great concern were reports by caregivers and teens of not being allowed to call a parent for support or retrieve their medications when needed for asthma symptoms.32
Future Research and Practice Implications
In this review, we identified 5 common themes around barriers to asthma self-management for African American adolescents (knowledge and skills, beliefs and attitudes, personal/emotional factors, caregivers, and schools). Caregivers, especially mothers, play a pivotal role in the development of effective asthma self-management behaviors. Depsite good intentions, there is evidence of caregivers passing on ineffective experiential and culturally based beliefs and practices to their adolescents that can negatively influence self-care behaviors.13,28,38 Studies are needed to further investigate these findings among caregivers as their beliefs and practices for asthma self-management have been found to coexist among adolescents. Studies that investigate how to incorporate caregiver asthma knowledge, cultural beliefs and behaviors in developing self-management interventions have the potential to positively influence asthma outcomes among African American adolescents.27 The unique cultural beliefs, contextual environmental, and social disparities faced by African American caregivers should not be neglected.
African American adolescents, like adolescents in other racial or ethnic groups, desire to be autonomous in their asthma self-management. However, as adolescents age their adherence behaviors often decline. This may suggest a need for a longer transition period to self-management that extends into emerging adulthood (18-25 years). While youth want to feel supported, there appears to be a fine line between receiving needed support and what youth describe as “nagging” behaviors by adults. Additional investigations into how asthma responsibilities are transitioned from the parent to youth and how best to support the development and maintenance of related behaviors and skills are warranted. In addition, teens described problems related to communicating with health care providers, noting a lack of clarity in explanations received about how to manage their asthma. Some teens believed the communication challenges were based on beliefs and biases held by providers that African American youth had limited capacities for self-management.9 There is a need to better understand interactions among African American adolescents, parents, and clinicians so that communication and transitioning asthma care to the youth will produce optimal health outcomes.
According to asthma guidelines, the patient-provider relationship is essential to effective asthma self-management.4,5 However, there is little mention in the literature of team-based care. Clinicians such as physicians, physician assistants, and nurse practitioners provide direct care to adolescents in terms of disease management and the overall effectiveness of treatment plans. African American youth demonstrate a need for asthma education that is comprehensive and that is contextualized to their daily lives. A team-based approach to care that includes social workers and community health workers may help to extend the reach of clinicians. Follow-up times with families and youth between office visits can be used to support adolescents to develop asthma self-management and allow them a safe space to describe frustrations and other emotions that contribute to their desire to be disease-free.
Summary
Asthma is a chronic disease that is often more severe and difficult to manage in African American adolescents. While African American adolescents describe developmental needs like those of other youth, cultural beliefs and contextual experiences influence their self-care management in unique ways. Opportunities exist to better understand the needs of African American adolescents and to help them successfully gain the knowledge, skills, and behaviors needed to effectively engage in self-management of their asthma.
Corresponding author: Wanda Gibson-Scipio, PhD, FNP-BC, FAANP, 5557 Cass Ave., 346 Cohn Building, Detroit, MI 48324; [email protected].
Financial disclosures: None.
1. Akinbami LJ, Moorman JE, Bailey C, et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001-2010. NCHS Data Brief. 2012:1-8.
2. Bruzzese JM, Bonner S, Vincent EJ, et al. Asthma education: the adolescent experience. Patient Educ Couns. 2004;55:396-406.
3. Bryant-Stephens T. Asthma disparities in urban environments. J Allergy Clin Immunol. 2009;123:1199-1206.
4. National Heart, Lung, and Blood Institute. National Asthma Education and Prevention Program. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Heart, Lung, and Blood Institute; 2007.
5. GINA. Global strategy for asthma management and prevention. 2017. www.ginaasthma.org. Accessed Dec 15, 2017.
6. Centers for Disease Control and Prevention. Vital signs. 2011. https://www.cdc.gov/vitalsigns/asthma/index.html. Accessed December 15, 2017.
7. Centers for Disease Control and Prevention. 2015 National Health Interview Survey (NHIS) Data. National Center for Environmental Health, 2017. https://www.cdc.gov/asthma/nhis/2015/table4-1.htm. Accessed December 15, 2017.
8. Gupta RS, Carrión-Carire V, Weiss KB. The widening black/white gap in asthma hospitalizations and mortality. J Allergy Clin Immunol. 2006;117:351-358.
9. Evans-Agnew R. Asthma management disparities: a photovoice investigation with African American youth. J Sch Nurs. 2016;32:99-111.
10. Naar-King S, Ellis, D, Kolmodin, K. Feasibility of adapting multisystemic therapy to improve illness management behaviors and reduce asthma morbidity in high risk African American youth: a case series. J Child Fam Stud. 2009;18:564-573.
11. Rhee H, Wenzel J, Steeves RH. Adolescents’ psychosocial experiences living with asthma: a focus group study. J Pediatr Health Care. 2007;21:99-107.
12. Sin MK, Kang DH, Weaver M. Relationships of asthma knowledge, self-management, and social support in African American adolescents with asthma. Int J Nurs Stud. 2005;42:307-313.
13. Mosnaim G, Li H, Martin M, et al. Factors associated with levels of adherence to inhaled corticosteroids in minority adolescents with asthma. Ann Allergy Asthma Immunol. 2014;112:116-120.
14. Riekert KA, Borrelli B, Bilderback A, Rand CS. The development of a motivational interviewing intervention to promote medication adherence among inner-city, African American adolescents with asthma. Patient Educ Couns. 2011;82:117-122.
15. Bruzzese JM, Stepney C, Fiorino EK, et al. Asthma self-management is sub-optimal in urban Hispanic and African American/black early adolescents with uncontrolled persistent asthma. J Asthma. 2012;49:90-97.
16. Naimi DR, Freedman TG, Ginsburg KR, et al. Adolescents and asthma: why bother with our meds? J Allergy Clin Immunol. 2009;123:1335-1341.
17. Naar-King S, Lam P, Ellis D, et al. Asthma medication device skills in high-risk African American adolescents. J Asthma. 2013;50:579-582.
18. Ellis DA, King P, Naar-King S. Mediators of treatment effects in a randomized clinical trial of multisystemic therapy-health care in adolescents with poorly controlled asthma: disease knowledge and device use skills. J Pediatr Psychol. 2016;41:522-530.
19. Crowder SJ, Hanna KM, Carpenter JS, Broome ME. Factors associated with asthma self-management in African American adolescents. J Pediatric Nurs. 2015;30:e35-e43.
20. U.S. Department of Health and Human Services. Healthy people 2010: understanding and improving health. 2nd ed. Washington (DC): U.S. Government Printing Office; November 2000.
21. Valerio MA, Peterson EL, Wittich AR, Joseph CLM. Examining health literacy among urban African-American adolescents with asthma. J Asthma. 2016;53:1041-1047.
22. Laster N, Holsey CN, Shendell DG, et al. Barriers to asthma management among urban families: caregiver and child perspectives. J Asthma. 2009;46:731-739.
23. Ayala GX, Miller D, Zagami E, et al. Asthma in middle schools: what students have to say about their asthma. J Sch Health. 2006;76:208-214.
24. Gibson-Scipio W, Gourdin D, Krouse, HJ. Asthma self-management goals, beliefs and behaviors of urban African American adolescents prior to transitioning to adult health care. J Pediatric Nurs. 2015;30:e53-e61.
25. Blaakman SW, Cohen A, Fagnano M, Halterman JS. Asthma medication adherence among urban teens: a qualitative analysis of barriers, facilitators and experiences with school-based care. J Asthma. 2014;51:522-529.
26. Bruzzese JM, Idalski Carcone A, Lam P, et al. Adherence to asthma medication regimens in urban African American adolescents: application of self-determination theory. Health Psychol. 2014;33:461-464.
27. Ellis DA, King P, Naar-King S, et al. Effects of family treatment on parenting beliefs among caregivers of youth with poorly controlled asthma. J Dev Behav Pediatr. 2014;35:486-493.
28. Cotton S, Luberto CM, Yi MS, Tsevat J. Complementary and alternative medicine behaviors and beliefs in urban adolescents with asthma. J Asthma. 2011;48:531-538.
29. Bahreinian S, Ball GDC, Colman I, et al. Depression is more common in girls with nonatopic asthma. Chest. 2011;140:1138-1145.
30. Bender BG. Risk taking, depression, adherence, and symptom control in adolescents and young adults with asthma. Am J Respir Crit Care Med. 2006;173:953-957.
31. Guglani L, Havstad SL, Johnson CC, et al. Effect of depressive symptoms on asthma intervention in urban teens. Ann Allergy Asthma Immunol. 2012;109:237-242.
32. Gibson-Scipio W, Krouse HJ. Goals, beliefs, and concerns of urban caregivers of middle and older adolescents with asthma. J Asthma. 2013;50:242-249.
33. Wagner F, Steefel L. Beliefs regarding asthma management relating to asthma action plans (AAPs) of African American caregivers residing in Newark, New Jersey public housing communities. J Pediatr Nurs. 2017;36:92-97.
34. Houle CR, Joseph CL, Caldwell CH, et al. Congruence between urban adolescent and caregiver responses to questions about the adolescent’s asthma. J Urban Health. 2011;88:30-40.
From Wayne State University, Detroit, MI (Dr. Gibson-Scipio), and the University of Texas Rio Grande Valley, Edinburg, TX (Dr. Krouse).
Abstract
- Objective: To review the literature on barriers to asthma self-management amo
ng African American adolescents. - Methods: Review of the literature.
- Results: Asthma self-management barriers experienced by African American adolescents are often related to developmental needs, lack of knowledge, and personal perspectives and experiences. Adolescents find managing their symptoms and adhering to prescriptive therapies a burden and desire to be more like healthy peers. As they struggle to identify with peers, they may engage in risky behaviors such as ignoring symptoms and delaying treatment, thus leading to poorer asthma control and health outcomes. African American adolescents struggle with perceptions of racial biases from health care providers and teachers that interfere with self-management behaviors. They also describe the influence of culturally based practices learned from caregivers that contribute to their misconceptions and inadequate skills in effectively managing their asthma.
- Conclusion: Researchers should seek to develop interventions to address the unique contextual and culturally based needs of African American adolescents that support the development of effective asthma self-management behaviors. This may include making use of family members (especially mothers) and extended support for self-management during this period of rapid growth and transition. Health care providers should consider a team-based approach to the adolescent patient. Such an approach should be grounded in recommendations from national guidelines that suggest a patient-centered approach to care that includes a partnership between the patient and the provider to address unique barriers to effective self-management.
Keywords: youth; caregiver; drug-therapy; self-efficacy; disease-management; patient-centered care.
Effective asthma self-management by urban African American adolescents is a critical aspect of care that should be addressed with vigilance due to the persistent disparities in disease prevalence, morbidity, and mortality compared to Caucasians.1-3 The overarching goal of asthma self-management is to achieve symptom control, maintain normal activity levels, and minimize future risk of exacerbations and medication side effects.4,5 Best practices for asthma self-management begin with a partnership between health care providers and clients (including parent/caregiver). This relationship should help affected individuals gain asthma control based on knowledge of their disease and treatment options, confidence and skills in trigger avoidance, medication administration, and management of acute exacerbations.4,5
Among youth aged 18 years and younger, African Americans have the highest asthma prevalence rates of all racial and ethnic groups, and between 2001 and 2009 asthma prevalence rates rose by 50% among African American youth.6 As of 2015, prevalence rates for asthma among African American youth were 13.4%, as compared to 7.4% for white youth.7 African American youth have been found to have more frequent asthma exacerbations and related school absences than white youth.8 Furthermore, African American youth younger than 18 years are more likely to be admitted to the hospital for asthma and are 10 times more likely to die from asthma compared to non-Hispanic white children.6
Urban African American adolescents with asthma are particularly vulnerable to poor asthma self-management due to the complexity of the disease in this population.3 African American youth must deal with multiple adverse environmental conditions, lack of knowledge or disbelief concerning effective disease self-management strategies, variable access and quality of care, and the psychosocial dynamics of being young while having a chronic disease.2,3,9-11 It is important to understand and address barriers to successful asthma self-management during adolescence, as behaviors developed during this stage of life often persist into adulthood.9 In this article, we review the literature on barriers to asthma self-management among African American adolescents and offer suggestions on clinical strategies for improving self-management in this vulnerable population.
Methods
The initial search strategy was developed in collaboration with an experienced librarian. Keywords, MeSH terms, and potential databases were identified. Keywords included urban, African American, adolescent, asthma, self-management, and barriers. These terms were expanded based on search results and a review of abstracts that fit the intent of our review. The search was limited to U.S. studies published between 2005 and 2017. Excluded from the search were conference abstracts, doctoral dissertations, master’s theses, meta-analyses, systematic reviews, and studies conducted outside of the United States. Additional articles for the review were identified during the review process from the reference lists of the publications.
Abstracts were reviewed for articles that reported a study population inclusive of African American adolescents with asthma and that were related to self-management. Studies that used qualitative and other descriptive methods and cohort and randomized control trials were reviewed. Due to the limited number of articles found that exclusively focused on African Americans, the authors set a threshold for African American participants at 40% or greater for inclusion in this review.
Full papers were retrieved that met the inclusion criteria for a full review. Each author initially independently reviewed a selected number of papers and abstracted the study purpose, sample, study design, results, conclusions, and limitations. Subsequently, both authors reviewed in tandem and then discussed each selected manuscript to assure the appropriateness for inclusion. The subject matter was considered the priority for inclusion in the review. Study methods, sample size, and noted limitations were categorized but were not considered as a basis for exclusion. Thematic analysis was used to identify common themes across studies.
Results
We identified 23 papers that met our criteria (Table). Five common themes were found that related to barriers in disease self-management for African American adolescents: (a) knowledge and skills, (b) beliefs and attitudes, (c) personal/emotional factors, (d) caregivers, and (e) schools.
Knowledge and Skills
Adequate knowledge of the elements of asthma self-management is critical for achieving control of this condition. Asthma knowledge includes a basic understanding of the disease process and treatment strategies, an awareness of early signs and symptoms of worsening asthma, and an understanding of how to manage environmental triggers.4,5 Sin and colleagues conducted one of the earlier studies to examine the influence of asthma knowledge on asthma self-management in African American adolescents and found a significant positive association between knowledge and asthma self-management behaviors.12
Adherence to an asthma medication, especially inhaled corticosteroids (ICS), is one of the cornerstones to successful self-management of asthma.13,14 Consistent use of ICS therapy to control asthma symptoms and disease progression is often suboptimal in African American adolescents and tends to worsen as they age;15 studies have found lower adherence levels were more prominent in older African American adolescents and males.13,16 In a recent study of adolescents with persistent asthma who were prescribed daily ICS, youth with greater ICS knowledge as assessed using a standardized instrument demonstrated significantly higher adherence rates.13 Proper technique in the use of an inhaler is also important in medication administration. Asthma ICS medication delivery devices vary significantly and require different techniques for medication administration. However, inhaler device skills have been found to be very inadequate in high-risk African American adolescents.17 Thus, knowledge related to ICS therapy and proper skills in the use of inhaler devices is an important aspect of asthma self-management that have been found to be inadequate in African American Adolescents.
Interventions and programs geared to improving education may lead to improved self-management. Multisystemic Therapy-Health Care (MST-HC) is a tailored home-based intervention that includes knowledge and skill-building components. In a study of African American youth with poorly controlled asthma, the program was found to improve illness management.18 In addition, adolescents who complete formal asthma education programs demonstrate significantly higher scores in self-management than those youth who do not participate in these programs.13,19 Unfortunately, few African American teens report participation in an asthma education program.19 In a study of a motivational interviewing intervention to improve controller medication adherence for African American adolescents,14 youth reported gaining more knowledge about their asthma medications and were significantly more motivated to take their controller medications after participating in the intervention; however, while adherence to controller medications was greater than baseline, it was not significantly different.14 This study demonstrated the value of asthma education and the feasibility of a motivational intervention to support controller medication adherence. However, this study also demonstrated the complexity of medication adherence in that neither knowledge or motivation led to significant changes in medication adherence among African American adolescents.
Low health literacy can also act as a barrier to asthma self-management. Health literacy requires skills and knowledge that enable an individual to communicate, process, and understand basic health information that informs health decisions.20 Health literacy was found to be associated with indicators of poor disease self-management among urban African American adolescents in grades 9 through 12.21 In this study, health literacy was established using questions about confidence in filling out medical forms, self-reported problems with learning about the youth’s medical condition, and the need for assistance in reading hospital materials. Adolescents with poor health literacy scores were more likely to reside in a household with the following characteristics: mother with less than a high school education, Medicaid health insurance, family members with a body mass index exceeding the 85th percentile, and lack of rescue medication. Poor health literacy was most common among younger adolescents (ie, ninth graders). Some youth with poor health literacy also reported more emergency department visits, hospitalizations, and lower overall quality of life.21
Beliefs and Attitudes
Beliefs and attitudes towards taking asthma medications can act as barriers to adherence in the adolescent. African American adolescents often report the belief that ICS are not helpful or necessary.16,22-25 These beliefs have been correlated with a lack of understanding of the inflammatory mechanisms of asthma, reports of asthma attacks despite use of controller medications, fear of addiction to medications, and a belief that nontraditional interventions (eg, exercise) will work better to get rid of asthma or abate symptoms.16-19,22-24 African American adolescents also report beliefs that asthma will go away or get better as they age, and they are willing to forgo the use of controller medications based on these beliefs.24
African American adolescents often engage in asthma self-management independent of caregivers. These youth describe asthma self-management activities an annoyance and of low priority in part due to competing tasks and negative interactions with caregivers.25 During early adolescence asthma self-management is often suboptimal, and as youth age they become less observant regarding their asthma and are less likely to seek help.26 Adolescents’ beliefs and low prioritization of asthma self-management may contribute to forgetfulness and loss of inhalers, which are common reasons reported for poor adherence to ICS.16,23-26 Further, the role of caregivers during this period has often been overlooked. Caregivers of African American adolescents have been found to be stressed and overwhelmed with personal responsibilities and neighborhood conditions, leaving them little time to attend to the asthma self-management behavior of youth. Due to these contextual factors, interactions with chronically ill youth may be strained, resulting in negative interactions with youth related to asthma self-management. However, in an intervention study that used multisystemic therapy (an approach that targets the affected individual, family, and community), improvement in positive parenting behaviors related to asthma self-management contributed to improved ICS adherence by adolescents.27
Adolescents can perceive traditional asthma self-management as conflicting with their own personal and/or cultural beliefs. They may seek options beyond the use of medicine and have voiced preferences for behaviors that they believe will “strengthen their lungs” more naturally.24 An appreciation of how youth might use complementary/alternative medicine (CAM) as an adjunctive therapy or in place of evidence-based asthma care is important to understanding the potential effect on morbidity and mortality. Behaviors and beliefs about the use of CAM have not been well studied among urban African American adolescents with asthma. Only one study was found that assessed the use of CAM among a primarily urban African American adolescent population. In that study, 71% of the population reported using some form of CAM during the past 30 days.28 Prayer and relaxation were the most frequently used strategies in the management of asthma symptoms. Perceived efficacy of relaxation and prayer among teens who engaged in this form of CAM was 87% and 85%, respectively. Other CAM strategies included yoga, meditation, guided imagery, and biofeedback. When adolescents were asked if they shared their use of CAM in asthma management with a health care provider, most reported sharing the use of yoga and dietary changes but were least likely to share their use of prayer and guided imagery.28
Personal/Emotional Factors
African American adolescents have reported asthma as a limiting factor in terms of both physical and social activities. They perceive asthma as a burden to themselves and others (eg, peers, family, coaches).9,25 The burden of asthma is further exemplified in the emotional response to the symptoms of the disease and the self-management responsibilities. The need to prevent and respond to asthma symptoms is associated with being embarrassed, frustrated, angry, annoyed, worried, lonely, and isolated.9,11,25 Negative coping strategies by youth in response to psychosocial experiences include decisions to disregard or give minimal attention to asthma symptoms and to delay or not take prescribed medications. Students report ignoring asthma symptom management while engaging in physical activities to maintain a sense of normalcy among peers and as a way of dealing with perceptions by coaches or teachers that they are weak or in need of being protected.24,25
Negative thoughts and experiences can result in depressive disorders and poor quality of life. Depression is a common finding among urban youth with asthma.29,30 Youth diagnosed with asthma who have comorbid depression may benefit from interventions to improve self-management. In a secondary analysis from a Web-based asthma management intervention targeting African American adolescents, depression was found to have a modifying effect on the emotional domain of quality of life for youth in the intervention arm of the study. This finding indicates that participants who were depressed and who reported low levels of emotional quality of life benefited from the Web-based interventions that targeted self-management.31
Caregivers
Caregivers (especially moms) are a common source of support for the development and implementation of asthma self-management behaviors in adolescents.32 Caregivers sometimes hold beliefs similar to those of youth and believe the urban environment can act as a barrier to asthma management.9,25,32 They describe the complexity of asthma treatment plans, a lack of understanding of the disease process, and insensitivity of health care providers to their expressed needs along with the providers’ limited cultural awareness in the development of self-management plans.9,22,33 Caregivers describe how family finances, insurance gaps, access to care, and their own familial/cultural beliefs influence their decisions and ability to support their child’s asthma management.33 When faced with the cost of care they report instances of having to decide between necessities such as food and housing or co-pays for medications and office visits.22,33 They also report concerns about visits with multiple providers due to an inability to access their primary care provider, which can lead to delays in their child being diagnosed with asthma.22
Caregivers report a need to include culturally based practices, past experiences, and personal beliefs into the adolescents’ asthma management plan.22,32,33 In a small interview-based study of caregivers residing in 3 New Jersey public housing communities, caregivers reported preferring “familial” methods of controlling asthma (eg, restriction of activities; use of showers, steam, vaporizers, and nebulizers) over evidence-based recommendations. Many caregivers were confused or lacked knowledge about asthma action plans.33 Caregivers have also been found to lack adequate or accurate knowledge related to asthma medications and factors that improved or worsened asthma. While caregivers report a desire to help educate their teens by passing on what they know, their lack of adequate asthma knowledge may hamper their efforts and potentially worsen the teens’ asthma self-management.32
While African American caregivers often describe themselves as hypervigilant concerning their child’s asthma, they may report different information than their adolescent when both are questioned about asthma symptom experiences and functional status.34 Factors increasing the level of congruence between caregiver and teen asthma symptom reports were found to be related to the adolescents’ age and asthma disease classification. Symptom questionnaire responses of older teens and those with mild intermittent asthma were more likely to be similar to caregiver reports. The researchers concluded that clinicians and researchers may obtain reliable asthma symptom and functional status reports by asking the adolescent directly.34
Schools
Caregivers and adolescents describe schools as a threat to self-management and the overall health of youth with asthma.9,32 They perceive that a lack of knowledge by staff, teachers, and coaches contributes to inattentiveness or disbelief in the credibility of reported asthma symptoms by youth.11,23 These misperceptions and the lack of attentiveness by adults in the school may pose safety and health issues for African American youth.9,25,33,34 For example, adolescents report pressure from teacher, coaches, and peers in school settings to partake in sports and/or gym classes. Youth want to identify with healthy peers and thus often choose not to take asthma medications during such activities or opt to continue participating while being compromised by airway obstruction. Of great concern were reports by caregivers and teens of not being allowed to call a parent for support or retrieve their medications when needed for asthma symptoms.32
Future Research and Practice Implications
In this review, we identified 5 common themes around barriers to asthma self-management for African American adolescents (knowledge and skills, beliefs and attitudes, personal/emotional factors, caregivers, and schools). Caregivers, especially mothers, play a pivotal role in the development of effective asthma self-management behaviors. Depsite good intentions, there is evidence of caregivers passing on ineffective experiential and culturally based beliefs and practices to their adolescents that can negatively influence self-care behaviors.13,28,38 Studies are needed to further investigate these findings among caregivers as their beliefs and practices for asthma self-management have been found to coexist among adolescents. Studies that investigate how to incorporate caregiver asthma knowledge, cultural beliefs and behaviors in developing self-management interventions have the potential to positively influence asthma outcomes among African American adolescents.27 The unique cultural beliefs, contextual environmental, and social disparities faced by African American caregivers should not be neglected.
African American adolescents, like adolescents in other racial or ethnic groups, desire to be autonomous in their asthma self-management. However, as adolescents age their adherence behaviors often decline. This may suggest a need for a longer transition period to self-management that extends into emerging adulthood (18-25 years). While youth want to feel supported, there appears to be a fine line between receiving needed support and what youth describe as “nagging” behaviors by adults. Additional investigations into how asthma responsibilities are transitioned from the parent to youth and how best to support the development and maintenance of related behaviors and skills are warranted. In addition, teens described problems related to communicating with health care providers, noting a lack of clarity in explanations received about how to manage their asthma. Some teens believed the communication challenges were based on beliefs and biases held by providers that African American youth had limited capacities for self-management.9 There is a need to better understand interactions among African American adolescents, parents, and clinicians so that communication and transitioning asthma care to the youth will produce optimal health outcomes.
According to asthma guidelines, the patient-provider relationship is essential to effective asthma self-management.4,5 However, there is little mention in the literature of team-based care. Clinicians such as physicians, physician assistants, and nurse practitioners provide direct care to adolescents in terms of disease management and the overall effectiveness of treatment plans. African American youth demonstrate a need for asthma education that is comprehensive and that is contextualized to their daily lives. A team-based approach to care that includes social workers and community health workers may help to extend the reach of clinicians. Follow-up times with families and youth between office visits can be used to support adolescents to develop asthma self-management and allow them a safe space to describe frustrations and other emotions that contribute to their desire to be disease-free.
Summary
Asthma is a chronic disease that is often more severe and difficult to manage in African American adolescents. While African American adolescents describe developmental needs like those of other youth, cultural beliefs and contextual experiences influence their self-care management in unique ways. Opportunities exist to better understand the needs of African American adolescents and to help them successfully gain the knowledge, skills, and behaviors needed to effectively engage in self-management of their asthma.
Corresponding author: Wanda Gibson-Scipio, PhD, FNP-BC, FAANP, 5557 Cass Ave., 346 Cohn Building, Detroit, MI 48324; [email protected].
Financial disclosures: None.
From Wayne State University, Detroit, MI (Dr. Gibson-Scipio), and the University of Texas Rio Grande Valley, Edinburg, TX (Dr. Krouse).
Abstract
- Objective: To review the literature on barriers to asthma self-management amo
ng African American adolescents. - Methods: Review of the literature.
- Results: Asthma self-management barriers experienced by African American adolescents are often related to developmental needs, lack of knowledge, and personal perspectives and experiences. Adolescents find managing their symptoms and adhering to prescriptive therapies a burden and desire to be more like healthy peers. As they struggle to identify with peers, they may engage in risky behaviors such as ignoring symptoms and delaying treatment, thus leading to poorer asthma control and health outcomes. African American adolescents struggle with perceptions of racial biases from health care providers and teachers that interfere with self-management behaviors. They also describe the influence of culturally based practices learned from caregivers that contribute to their misconceptions and inadequate skills in effectively managing their asthma.
- Conclusion: Researchers should seek to develop interventions to address the unique contextual and culturally based needs of African American adolescents that support the development of effective asthma self-management behaviors. This may include making use of family members (especially mothers) and extended support for self-management during this period of rapid growth and transition. Health care providers should consider a team-based approach to the adolescent patient. Such an approach should be grounded in recommendations from national guidelines that suggest a patient-centered approach to care that includes a partnership between the patient and the provider to address unique barriers to effective self-management.
Keywords: youth; caregiver; drug-therapy; self-efficacy; disease-management; patient-centered care.
Effective asthma self-management by urban African American adolescents is a critical aspect of care that should be addressed with vigilance due to the persistent disparities in disease prevalence, morbidity, and mortality compared to Caucasians.1-3 The overarching goal of asthma self-management is to achieve symptom control, maintain normal activity levels, and minimize future risk of exacerbations and medication side effects.4,5 Best practices for asthma self-management begin with a partnership between health care providers and clients (including parent/caregiver). This relationship should help affected individuals gain asthma control based on knowledge of their disease and treatment options, confidence and skills in trigger avoidance, medication administration, and management of acute exacerbations.4,5
Among youth aged 18 years and younger, African Americans have the highest asthma prevalence rates of all racial and ethnic groups, and between 2001 and 2009 asthma prevalence rates rose by 50% among African American youth.6 As of 2015, prevalence rates for asthma among African American youth were 13.4%, as compared to 7.4% for white youth.7 African American youth have been found to have more frequent asthma exacerbations and related school absences than white youth.8 Furthermore, African American youth younger than 18 years are more likely to be admitted to the hospital for asthma and are 10 times more likely to die from asthma compared to non-Hispanic white children.6
Urban African American adolescents with asthma are particularly vulnerable to poor asthma self-management due to the complexity of the disease in this population.3 African American youth must deal with multiple adverse environmental conditions, lack of knowledge or disbelief concerning effective disease self-management strategies, variable access and quality of care, and the psychosocial dynamics of being young while having a chronic disease.2,3,9-11 It is important to understand and address barriers to successful asthma self-management during adolescence, as behaviors developed during this stage of life often persist into adulthood.9 In this article, we review the literature on barriers to asthma self-management among African American adolescents and offer suggestions on clinical strategies for improving self-management in this vulnerable population.
Methods
The initial search strategy was developed in collaboration with an experienced librarian. Keywords, MeSH terms, and potential databases were identified. Keywords included urban, African American, adolescent, asthma, self-management, and barriers. These terms were expanded based on search results and a review of abstracts that fit the intent of our review. The search was limited to U.S. studies published between 2005 and 2017. Excluded from the search were conference abstracts, doctoral dissertations, master’s theses, meta-analyses, systematic reviews, and studies conducted outside of the United States. Additional articles for the review were identified during the review process from the reference lists of the publications.
Abstracts were reviewed for articles that reported a study population inclusive of African American adolescents with asthma and that were related to self-management. Studies that used qualitative and other descriptive methods and cohort and randomized control trials were reviewed. Due to the limited number of articles found that exclusively focused on African Americans, the authors set a threshold for African American participants at 40% or greater for inclusion in this review.
Full papers were retrieved that met the inclusion criteria for a full review. Each author initially independently reviewed a selected number of papers and abstracted the study purpose, sample, study design, results, conclusions, and limitations. Subsequently, both authors reviewed in tandem and then discussed each selected manuscript to assure the appropriateness for inclusion. The subject matter was considered the priority for inclusion in the review. Study methods, sample size, and noted limitations were categorized but were not considered as a basis for exclusion. Thematic analysis was used to identify common themes across studies.
Results
We identified 23 papers that met our criteria (Table). Five common themes were found that related to barriers in disease self-management for African American adolescents: (a) knowledge and skills, (b) beliefs and attitudes, (c) personal/emotional factors, (d) caregivers, and (e) schools.
Knowledge and Skills
Adequate knowledge of the elements of asthma self-management is critical for achieving control of this condition. Asthma knowledge includes a basic understanding of the disease process and treatment strategies, an awareness of early signs and symptoms of worsening asthma, and an understanding of how to manage environmental triggers.4,5 Sin and colleagues conducted one of the earlier studies to examine the influence of asthma knowledge on asthma self-management in African American adolescents and found a significant positive association between knowledge and asthma self-management behaviors.12
Adherence to an asthma medication, especially inhaled corticosteroids (ICS), is one of the cornerstones to successful self-management of asthma.13,14 Consistent use of ICS therapy to control asthma symptoms and disease progression is often suboptimal in African American adolescents and tends to worsen as they age;15 studies have found lower adherence levels were more prominent in older African American adolescents and males.13,16 In a recent study of adolescents with persistent asthma who were prescribed daily ICS, youth with greater ICS knowledge as assessed using a standardized instrument demonstrated significantly higher adherence rates.13 Proper technique in the use of an inhaler is also important in medication administration. Asthma ICS medication delivery devices vary significantly and require different techniques for medication administration. However, inhaler device skills have been found to be very inadequate in high-risk African American adolescents.17 Thus, knowledge related to ICS therapy and proper skills in the use of inhaler devices is an important aspect of asthma self-management that have been found to be inadequate in African American Adolescents.
Interventions and programs geared to improving education may lead to improved self-management. Multisystemic Therapy-Health Care (MST-HC) is a tailored home-based intervention that includes knowledge and skill-building components. In a study of African American youth with poorly controlled asthma, the program was found to improve illness management.18 In addition, adolescents who complete formal asthma education programs demonstrate significantly higher scores in self-management than those youth who do not participate in these programs.13,19 Unfortunately, few African American teens report participation in an asthma education program.19 In a study of a motivational interviewing intervention to improve controller medication adherence for African American adolescents,14 youth reported gaining more knowledge about their asthma medications and were significantly more motivated to take their controller medications after participating in the intervention; however, while adherence to controller medications was greater than baseline, it was not significantly different.14 This study demonstrated the value of asthma education and the feasibility of a motivational intervention to support controller medication adherence. However, this study also demonstrated the complexity of medication adherence in that neither knowledge or motivation led to significant changes in medication adherence among African American adolescents.
Low health literacy can also act as a barrier to asthma self-management. Health literacy requires skills and knowledge that enable an individual to communicate, process, and understand basic health information that informs health decisions.20 Health literacy was found to be associated with indicators of poor disease self-management among urban African American adolescents in grades 9 through 12.21 In this study, health literacy was established using questions about confidence in filling out medical forms, self-reported problems with learning about the youth’s medical condition, and the need for assistance in reading hospital materials. Adolescents with poor health literacy scores were more likely to reside in a household with the following characteristics: mother with less than a high school education, Medicaid health insurance, family members with a body mass index exceeding the 85th percentile, and lack of rescue medication. Poor health literacy was most common among younger adolescents (ie, ninth graders). Some youth with poor health literacy also reported more emergency department visits, hospitalizations, and lower overall quality of life.21
Beliefs and Attitudes
Beliefs and attitudes towards taking asthma medications can act as barriers to adherence in the adolescent. African American adolescents often report the belief that ICS are not helpful or necessary.16,22-25 These beliefs have been correlated with a lack of understanding of the inflammatory mechanisms of asthma, reports of asthma attacks despite use of controller medications, fear of addiction to medications, and a belief that nontraditional interventions (eg, exercise) will work better to get rid of asthma or abate symptoms.16-19,22-24 African American adolescents also report beliefs that asthma will go away or get better as they age, and they are willing to forgo the use of controller medications based on these beliefs.24
African American adolescents often engage in asthma self-management independent of caregivers. These youth describe asthma self-management activities an annoyance and of low priority in part due to competing tasks and negative interactions with caregivers.25 During early adolescence asthma self-management is often suboptimal, and as youth age they become less observant regarding their asthma and are less likely to seek help.26 Adolescents’ beliefs and low prioritization of asthma self-management may contribute to forgetfulness and loss of inhalers, which are common reasons reported for poor adherence to ICS.16,23-26 Further, the role of caregivers during this period has often been overlooked. Caregivers of African American adolescents have been found to be stressed and overwhelmed with personal responsibilities and neighborhood conditions, leaving them little time to attend to the asthma self-management behavior of youth. Due to these contextual factors, interactions with chronically ill youth may be strained, resulting in negative interactions with youth related to asthma self-management. However, in an intervention study that used multisystemic therapy (an approach that targets the affected individual, family, and community), improvement in positive parenting behaviors related to asthma self-management contributed to improved ICS adherence by adolescents.27
Adolescents can perceive traditional asthma self-management as conflicting with their own personal and/or cultural beliefs. They may seek options beyond the use of medicine and have voiced preferences for behaviors that they believe will “strengthen their lungs” more naturally.24 An appreciation of how youth might use complementary/alternative medicine (CAM) as an adjunctive therapy or in place of evidence-based asthma care is important to understanding the potential effect on morbidity and mortality. Behaviors and beliefs about the use of CAM have not been well studied among urban African American adolescents with asthma. Only one study was found that assessed the use of CAM among a primarily urban African American adolescent population. In that study, 71% of the population reported using some form of CAM during the past 30 days.28 Prayer and relaxation were the most frequently used strategies in the management of asthma symptoms. Perceived efficacy of relaxation and prayer among teens who engaged in this form of CAM was 87% and 85%, respectively. Other CAM strategies included yoga, meditation, guided imagery, and biofeedback. When adolescents were asked if they shared their use of CAM in asthma management with a health care provider, most reported sharing the use of yoga and dietary changes but were least likely to share their use of prayer and guided imagery.28
Personal/Emotional Factors
African American adolescents have reported asthma as a limiting factor in terms of both physical and social activities. They perceive asthma as a burden to themselves and others (eg, peers, family, coaches).9,25 The burden of asthma is further exemplified in the emotional response to the symptoms of the disease and the self-management responsibilities. The need to prevent and respond to asthma symptoms is associated with being embarrassed, frustrated, angry, annoyed, worried, lonely, and isolated.9,11,25 Negative coping strategies by youth in response to psychosocial experiences include decisions to disregard or give minimal attention to asthma symptoms and to delay or not take prescribed medications. Students report ignoring asthma symptom management while engaging in physical activities to maintain a sense of normalcy among peers and as a way of dealing with perceptions by coaches or teachers that they are weak or in need of being protected.24,25
Negative thoughts and experiences can result in depressive disorders and poor quality of life. Depression is a common finding among urban youth with asthma.29,30 Youth diagnosed with asthma who have comorbid depression may benefit from interventions to improve self-management. In a secondary analysis from a Web-based asthma management intervention targeting African American adolescents, depression was found to have a modifying effect on the emotional domain of quality of life for youth in the intervention arm of the study. This finding indicates that participants who were depressed and who reported low levels of emotional quality of life benefited from the Web-based interventions that targeted self-management.31
Caregivers
Caregivers (especially moms) are a common source of support for the development and implementation of asthma self-management behaviors in adolescents.32 Caregivers sometimes hold beliefs similar to those of youth and believe the urban environment can act as a barrier to asthma management.9,25,32 They describe the complexity of asthma treatment plans, a lack of understanding of the disease process, and insensitivity of health care providers to their expressed needs along with the providers’ limited cultural awareness in the development of self-management plans.9,22,33 Caregivers describe how family finances, insurance gaps, access to care, and their own familial/cultural beliefs influence their decisions and ability to support their child’s asthma management.33 When faced with the cost of care they report instances of having to decide between necessities such as food and housing or co-pays for medications and office visits.22,33 They also report concerns about visits with multiple providers due to an inability to access their primary care provider, which can lead to delays in their child being diagnosed with asthma.22
Caregivers report a need to include culturally based practices, past experiences, and personal beliefs into the adolescents’ asthma management plan.22,32,33 In a small interview-based study of caregivers residing in 3 New Jersey public housing communities, caregivers reported preferring “familial” methods of controlling asthma (eg, restriction of activities; use of showers, steam, vaporizers, and nebulizers) over evidence-based recommendations. Many caregivers were confused or lacked knowledge about asthma action plans.33 Caregivers have also been found to lack adequate or accurate knowledge related to asthma medications and factors that improved or worsened asthma. While caregivers report a desire to help educate their teens by passing on what they know, their lack of adequate asthma knowledge may hamper their efforts and potentially worsen the teens’ asthma self-management.32
While African American caregivers often describe themselves as hypervigilant concerning their child’s asthma, they may report different information than their adolescent when both are questioned about asthma symptom experiences and functional status.34 Factors increasing the level of congruence between caregiver and teen asthma symptom reports were found to be related to the adolescents’ age and asthma disease classification. Symptom questionnaire responses of older teens and those with mild intermittent asthma were more likely to be similar to caregiver reports. The researchers concluded that clinicians and researchers may obtain reliable asthma symptom and functional status reports by asking the adolescent directly.34
Schools
Caregivers and adolescents describe schools as a threat to self-management and the overall health of youth with asthma.9,32 They perceive that a lack of knowledge by staff, teachers, and coaches contributes to inattentiveness or disbelief in the credibility of reported asthma symptoms by youth.11,23 These misperceptions and the lack of attentiveness by adults in the school may pose safety and health issues for African American youth.9,25,33,34 For example, adolescents report pressure from teacher, coaches, and peers in school settings to partake in sports and/or gym classes. Youth want to identify with healthy peers and thus often choose not to take asthma medications during such activities or opt to continue participating while being compromised by airway obstruction. Of great concern were reports by caregivers and teens of not being allowed to call a parent for support or retrieve their medications when needed for asthma symptoms.32
Future Research and Practice Implications
In this review, we identified 5 common themes around barriers to asthma self-management for African American adolescents (knowledge and skills, beliefs and attitudes, personal/emotional factors, caregivers, and schools). Caregivers, especially mothers, play a pivotal role in the development of effective asthma self-management behaviors. Depsite good intentions, there is evidence of caregivers passing on ineffective experiential and culturally based beliefs and practices to their adolescents that can negatively influence self-care behaviors.13,28,38 Studies are needed to further investigate these findings among caregivers as their beliefs and practices for asthma self-management have been found to coexist among adolescents. Studies that investigate how to incorporate caregiver asthma knowledge, cultural beliefs and behaviors in developing self-management interventions have the potential to positively influence asthma outcomes among African American adolescents.27 The unique cultural beliefs, contextual environmental, and social disparities faced by African American caregivers should not be neglected.
African American adolescents, like adolescents in other racial or ethnic groups, desire to be autonomous in their asthma self-management. However, as adolescents age their adherence behaviors often decline. This may suggest a need for a longer transition period to self-management that extends into emerging adulthood (18-25 years). While youth want to feel supported, there appears to be a fine line between receiving needed support and what youth describe as “nagging” behaviors by adults. Additional investigations into how asthma responsibilities are transitioned from the parent to youth and how best to support the development and maintenance of related behaviors and skills are warranted. In addition, teens described problems related to communicating with health care providers, noting a lack of clarity in explanations received about how to manage their asthma. Some teens believed the communication challenges were based on beliefs and biases held by providers that African American youth had limited capacities for self-management.9 There is a need to better understand interactions among African American adolescents, parents, and clinicians so that communication and transitioning asthma care to the youth will produce optimal health outcomes.
According to asthma guidelines, the patient-provider relationship is essential to effective asthma self-management.4,5 However, there is little mention in the literature of team-based care. Clinicians such as physicians, physician assistants, and nurse practitioners provide direct care to adolescents in terms of disease management and the overall effectiveness of treatment plans. African American youth demonstrate a need for asthma education that is comprehensive and that is contextualized to their daily lives. A team-based approach to care that includes social workers and community health workers may help to extend the reach of clinicians. Follow-up times with families and youth between office visits can be used to support adolescents to develop asthma self-management and allow them a safe space to describe frustrations and other emotions that contribute to their desire to be disease-free.
Summary
Asthma is a chronic disease that is often more severe and difficult to manage in African American adolescents. While African American adolescents describe developmental needs like those of other youth, cultural beliefs and contextual experiences influence their self-care management in unique ways. Opportunities exist to better understand the needs of African American adolescents and to help them successfully gain the knowledge, skills, and behaviors needed to effectively engage in self-management of their asthma.
Corresponding author: Wanda Gibson-Scipio, PhD, FNP-BC, FAANP, 5557 Cass Ave., 346 Cohn Building, Detroit, MI 48324; [email protected].
Financial disclosures: None.
1. Akinbami LJ, Moorman JE, Bailey C, et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001-2010. NCHS Data Brief. 2012:1-8.
2. Bruzzese JM, Bonner S, Vincent EJ, et al. Asthma education: the adolescent experience. Patient Educ Couns. 2004;55:396-406.
3. Bryant-Stephens T. Asthma disparities in urban environments. J Allergy Clin Immunol. 2009;123:1199-1206.
4. National Heart, Lung, and Blood Institute. National Asthma Education and Prevention Program. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Heart, Lung, and Blood Institute; 2007.
5. GINA. Global strategy for asthma management and prevention. 2017. www.ginaasthma.org. Accessed Dec 15, 2017.
6. Centers for Disease Control and Prevention. Vital signs. 2011. https://www.cdc.gov/vitalsigns/asthma/index.html. Accessed December 15, 2017.
7. Centers for Disease Control and Prevention. 2015 National Health Interview Survey (NHIS) Data. National Center for Environmental Health, 2017. https://www.cdc.gov/asthma/nhis/2015/table4-1.htm. Accessed December 15, 2017.
8. Gupta RS, Carrión-Carire V, Weiss KB. The widening black/white gap in asthma hospitalizations and mortality. J Allergy Clin Immunol. 2006;117:351-358.
9. Evans-Agnew R. Asthma management disparities: a photovoice investigation with African American youth. J Sch Nurs. 2016;32:99-111.
10. Naar-King S, Ellis, D, Kolmodin, K. Feasibility of adapting multisystemic therapy to improve illness management behaviors and reduce asthma morbidity in high risk African American youth: a case series. J Child Fam Stud. 2009;18:564-573.
11. Rhee H, Wenzel J, Steeves RH. Adolescents’ psychosocial experiences living with asthma: a focus group study. J Pediatr Health Care. 2007;21:99-107.
12. Sin MK, Kang DH, Weaver M. Relationships of asthma knowledge, self-management, and social support in African American adolescents with asthma. Int J Nurs Stud. 2005;42:307-313.
13. Mosnaim G, Li H, Martin M, et al. Factors associated with levels of adherence to inhaled corticosteroids in minority adolescents with asthma. Ann Allergy Asthma Immunol. 2014;112:116-120.
14. Riekert KA, Borrelli B, Bilderback A, Rand CS. The development of a motivational interviewing intervention to promote medication adherence among inner-city, African American adolescents with asthma. Patient Educ Couns. 2011;82:117-122.
15. Bruzzese JM, Stepney C, Fiorino EK, et al. Asthma self-management is sub-optimal in urban Hispanic and African American/black early adolescents with uncontrolled persistent asthma. J Asthma. 2012;49:90-97.
16. Naimi DR, Freedman TG, Ginsburg KR, et al. Adolescents and asthma: why bother with our meds? J Allergy Clin Immunol. 2009;123:1335-1341.
17. Naar-King S, Lam P, Ellis D, et al. Asthma medication device skills in high-risk African American adolescents. J Asthma. 2013;50:579-582.
18. Ellis DA, King P, Naar-King S. Mediators of treatment effects in a randomized clinical trial of multisystemic therapy-health care in adolescents with poorly controlled asthma: disease knowledge and device use skills. J Pediatr Psychol. 2016;41:522-530.
19. Crowder SJ, Hanna KM, Carpenter JS, Broome ME. Factors associated with asthma self-management in African American adolescents. J Pediatric Nurs. 2015;30:e35-e43.
20. U.S. Department of Health and Human Services. Healthy people 2010: understanding and improving health. 2nd ed. Washington (DC): U.S. Government Printing Office; November 2000.
21. Valerio MA, Peterson EL, Wittich AR, Joseph CLM. Examining health literacy among urban African-American adolescents with asthma. J Asthma. 2016;53:1041-1047.
22. Laster N, Holsey CN, Shendell DG, et al. Barriers to asthma management among urban families: caregiver and child perspectives. J Asthma. 2009;46:731-739.
23. Ayala GX, Miller D, Zagami E, et al. Asthma in middle schools: what students have to say about their asthma. J Sch Health. 2006;76:208-214.
24. Gibson-Scipio W, Gourdin D, Krouse, HJ. Asthma self-management goals, beliefs and behaviors of urban African American adolescents prior to transitioning to adult health care. J Pediatric Nurs. 2015;30:e53-e61.
25. Blaakman SW, Cohen A, Fagnano M, Halterman JS. Asthma medication adherence among urban teens: a qualitative analysis of barriers, facilitators and experiences with school-based care. J Asthma. 2014;51:522-529.
26. Bruzzese JM, Idalski Carcone A, Lam P, et al. Adherence to asthma medication regimens in urban African American adolescents: application of self-determination theory. Health Psychol. 2014;33:461-464.
27. Ellis DA, King P, Naar-King S, et al. Effects of family treatment on parenting beliefs among caregivers of youth with poorly controlled asthma. J Dev Behav Pediatr. 2014;35:486-493.
28. Cotton S, Luberto CM, Yi MS, Tsevat J. Complementary and alternative medicine behaviors and beliefs in urban adolescents with asthma. J Asthma. 2011;48:531-538.
29. Bahreinian S, Ball GDC, Colman I, et al. Depression is more common in girls with nonatopic asthma. Chest. 2011;140:1138-1145.
30. Bender BG. Risk taking, depression, adherence, and symptom control in adolescents and young adults with asthma. Am J Respir Crit Care Med. 2006;173:953-957.
31. Guglani L, Havstad SL, Johnson CC, et al. Effect of depressive symptoms on asthma intervention in urban teens. Ann Allergy Asthma Immunol. 2012;109:237-242.
32. Gibson-Scipio W, Krouse HJ. Goals, beliefs, and concerns of urban caregivers of middle and older adolescents with asthma. J Asthma. 2013;50:242-249.
33. Wagner F, Steefel L. Beliefs regarding asthma management relating to asthma action plans (AAPs) of African American caregivers residing in Newark, New Jersey public housing communities. J Pediatr Nurs. 2017;36:92-97.
34. Houle CR, Joseph CL, Caldwell CH, et al. Congruence between urban adolescent and caregiver responses to questions about the adolescent’s asthma. J Urban Health. 2011;88:30-40.
1. Akinbami LJ, Moorman JE, Bailey C, et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001-2010. NCHS Data Brief. 2012:1-8.
2. Bruzzese JM, Bonner S, Vincent EJ, et al. Asthma education: the adolescent experience. Patient Educ Couns. 2004;55:396-406.
3. Bryant-Stephens T. Asthma disparities in urban environments. J Allergy Clin Immunol. 2009;123:1199-1206.
4. National Heart, Lung, and Blood Institute. National Asthma Education and Prevention Program. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Heart, Lung, and Blood Institute; 2007.
5. GINA. Global strategy for asthma management and prevention. 2017. www.ginaasthma.org. Accessed Dec 15, 2017.
6. Centers for Disease Control and Prevention. Vital signs. 2011. https://www.cdc.gov/vitalsigns/asthma/index.html. Accessed December 15, 2017.
7. Centers for Disease Control and Prevention. 2015 National Health Interview Survey (NHIS) Data. National Center for Environmental Health, 2017. https://www.cdc.gov/asthma/nhis/2015/table4-1.htm. Accessed December 15, 2017.
8. Gupta RS, Carrión-Carire V, Weiss KB. The widening black/white gap in asthma hospitalizations and mortality. J Allergy Clin Immunol. 2006;117:351-358.
9. Evans-Agnew R. Asthma management disparities: a photovoice investigation with African American youth. J Sch Nurs. 2016;32:99-111.
10. Naar-King S, Ellis, D, Kolmodin, K. Feasibility of adapting multisystemic therapy to improve illness management behaviors and reduce asthma morbidity in high risk African American youth: a case series. J Child Fam Stud. 2009;18:564-573.
11. Rhee H, Wenzel J, Steeves RH. Adolescents’ psychosocial experiences living with asthma: a focus group study. J Pediatr Health Care. 2007;21:99-107.
12. Sin MK, Kang DH, Weaver M. Relationships of asthma knowledge, self-management, and social support in African American adolescents with asthma. Int J Nurs Stud. 2005;42:307-313.
13. Mosnaim G, Li H, Martin M, et al. Factors associated with levels of adherence to inhaled corticosteroids in minority adolescents with asthma. Ann Allergy Asthma Immunol. 2014;112:116-120.
14. Riekert KA, Borrelli B, Bilderback A, Rand CS. The development of a motivational interviewing intervention to promote medication adherence among inner-city, African American adolescents with asthma. Patient Educ Couns. 2011;82:117-122.
15. Bruzzese JM, Stepney C, Fiorino EK, et al. Asthma self-management is sub-optimal in urban Hispanic and African American/black early adolescents with uncontrolled persistent asthma. J Asthma. 2012;49:90-97.
16. Naimi DR, Freedman TG, Ginsburg KR, et al. Adolescents and asthma: why bother with our meds? J Allergy Clin Immunol. 2009;123:1335-1341.
17. Naar-King S, Lam P, Ellis D, et al. Asthma medication device skills in high-risk African American adolescents. J Asthma. 2013;50:579-582.
18. Ellis DA, King P, Naar-King S. Mediators of treatment effects in a randomized clinical trial of multisystemic therapy-health care in adolescents with poorly controlled asthma: disease knowledge and device use skills. J Pediatr Psychol. 2016;41:522-530.
19. Crowder SJ, Hanna KM, Carpenter JS, Broome ME. Factors associated with asthma self-management in African American adolescents. J Pediatric Nurs. 2015;30:e35-e43.
20. U.S. Department of Health and Human Services. Healthy people 2010: understanding and improving health. 2nd ed. Washington (DC): U.S. Government Printing Office; November 2000.
21. Valerio MA, Peterson EL, Wittich AR, Joseph CLM. Examining health literacy among urban African-American adolescents with asthma. J Asthma. 2016;53:1041-1047.
22. Laster N, Holsey CN, Shendell DG, et al. Barriers to asthma management among urban families: caregiver and child perspectives. J Asthma. 2009;46:731-739.
23. Ayala GX, Miller D, Zagami E, et al. Asthma in middle schools: what students have to say about their asthma. J Sch Health. 2006;76:208-214.
24. Gibson-Scipio W, Gourdin D, Krouse, HJ. Asthma self-management goals, beliefs and behaviors of urban African American adolescents prior to transitioning to adult health care. J Pediatric Nurs. 2015;30:e53-e61.
25. Blaakman SW, Cohen A, Fagnano M, Halterman JS. Asthma medication adherence among urban teens: a qualitative analysis of barriers, facilitators and experiences with school-based care. J Asthma. 2014;51:522-529.
26. Bruzzese JM, Idalski Carcone A, Lam P, et al. Adherence to asthma medication regimens in urban African American adolescents: application of self-determination theory. Health Psychol. 2014;33:461-464.
27. Ellis DA, King P, Naar-King S, et al. Effects of family treatment on parenting beliefs among caregivers of youth with poorly controlled asthma. J Dev Behav Pediatr. 2014;35:486-493.
28. Cotton S, Luberto CM, Yi MS, Tsevat J. Complementary and alternative medicine behaviors and beliefs in urban adolescents with asthma. J Asthma. 2011;48:531-538.
29. Bahreinian S, Ball GDC, Colman I, et al. Depression is more common in girls with nonatopic asthma. Chest. 2011;140:1138-1145.
30. Bender BG. Risk taking, depression, adherence, and symptom control in adolescents and young adults with asthma. Am J Respir Crit Care Med. 2006;173:953-957.
31. Guglani L, Havstad SL, Johnson CC, et al. Effect of depressive symptoms on asthma intervention in urban teens. Ann Allergy Asthma Immunol. 2012;109:237-242.
32. Gibson-Scipio W, Krouse HJ. Goals, beliefs, and concerns of urban caregivers of middle and older adolescents with asthma. J Asthma. 2013;50:242-249.
33. Wagner F, Steefel L. Beliefs regarding asthma management relating to asthma action plans (AAPs) of African American caregivers residing in Newark, New Jersey public housing communities. J Pediatr Nurs. 2017;36:92-97.
34. Houle CR, Joseph CL, Caldwell CH, et al. Congruence between urban adolescent and caregiver responses to questions about the adolescent’s asthma. J Urban Health. 2011;88:30-40.
Health Care–Associated Urinary Tract Infections: Prevention and Management
From the University of Arizona College of Medicine, Tucson, AZ (Dr. Beatty), and the Baylor College of Medicine, Houston, TX (Dr. Mohajer).
Abstract
- Objective: To review management issues regarding health care–associated urinary tract infections (UTIs) commonly encountered by practicing clinicians.
- Methods: Review of the literature.
- Results: Because urinary catheter (UC) placement plays a major role in the development of catheter-associated UTIs (CA-UTI), clinicians should be aware of the appropriate and inappropriate uses of UCs and their association with CA-UTI development. Removal of a UC when no longer necessary is key to preventing CA-UTI. Treatment of asymptomatic bacteriuria is generally not indicated. Percutaneous nephrostomy and ureteral stenting need close monitoring, and early removal should be performed if infection is suspected. Candiduria rarely leads to symptoms unless it is related to an ascending process. Proper urine collection is crucial in determining whether contamination, colonization, or infection is present. Fluconazole is recommended in most cases of Candida UTI, while intravenous amphotericin B is recommended for fluconazole-resistant Candida species.
- Conclusion: Continued use of evidence-based strategies for preventing and managing health care–associated UTI should lead to further improvements in patient outcomes and overall decreased rates of infection.
Keywords: bacteriuria; catheter-associated UTI; catheterization; percutaneous nephrostomy; candiduria.
Health care–associated urinary tract infections (UTIs) are estimated to be the most common adverse infectious event in U.S. hospitals, occurring in 1 of 10 admitted patients.1-3 Approximately 32% of all health care–associated infections are UTIs.1 Furthermore, urinary catheters (UCs) are associated with 8% to 21% of health care–associated infections that occur in the intensive care unit.4 The most important predisposing factor for nosocomial UTI is urinary catheterization.5 Genitourinary manipulation and/or implementation also play a major role in the development of nosocomial UTIs.
In 2008, the U.S. Centers for Medicare & Medicaid Services instituted a new policy that reduced reimbursement rates for hospitalizations linked to health care–associated infections.6 Indwelling UCs are among the most overused health care devices in the hospital setting. They are placed in an estimated 15% to 25% of all hospitalized patients,7,8 and are often inserted in the emergency department (ED) without a physician order or appropriate indication.9 Intermittent straight catheterization, male or female condom catheterization, and/or placement of an indwelling UC are the most common causes of catheter-associated asymptomatic bacteriuria (CA-ASB) and catheter-associated UTIs (CA-UTI).5 Prevention and management of CA-ASB and CA-UTI can be challenging and require an evidence-based approach. Furthermore, guidelines for the management of UTIs in the setting of active percutaneous nephrostomy (PCN) drainage and/or ureteral stenting are not established.5 This may leave clinicians with little mainstream data to aid in management decisions.
In 2009 the Centers for Disease Control and Prevention provided a guideline for the appropriate and inappropriate use of indwelling UCs to help promote their proper use.10 In the time since the guideline’s initiatives were instituted around the United States, published data have shown some improvement in the use of UCs,11,12 but other recent reports indicate that rates of UC use have remained unchanged.13 This review discusses management issues regarding health care–associated UTIs that are commonly encountered by practicing clinicians, with a focus on current guidelines and evidence.
Catheter-Associated UTI
CA-UTI is defined as the presence of signs or symptoms of UTI with no other explainable infectious source along with ≥ 1000 colony-forming units (cfu) of ≥ 1 bacterial species per milliliter in a urine specimen from a catheter that has been changed within 48 hours of collection of the urine specimen.5 Signs and symptoms of CA-UTI include, but are not limited to: new-onset or worsening fever, chills, altered sensorium from baseline, lethargy, malaise, flank pain, pelvic pain, costovertebral angle tenderness, and acute hematuria.5 New-onset “foul-smelling” (odorous)urine and “cloudy” urine are neither sensitive nor specific when assessing for CA-UTI, and do not have significant clinical relevance when found alone.14,15 Patients who have removed or exchanged the UC during this event and then experience dysuria, increased frequency, urgency, or suprapubic pain are likely having symptoms of CA-UTI.5
What is the recommended method for collecting urine samples when CA-UTI is suspected?
In a patient with an indwelling catheter that has been in place for more than 2 weeks at the onset of a suspected CA-UTI, the catheter should be replaced (if still indicated) or removed to accelerate resolution of symptoms and to reduce the risk of subsequent catheter-associated bacteriuria and CA-UTI. The urine culture should be acquired from the freshly placed UC.5
When should a patient be empirically treated?
A patient presenting with evidence of sepsis should be empirically treated with antimicrobials. Empiric coverage should be based on risk factors for multidrug-resistant organisms and data pertaining to local antimicrobial resistance patterns. A urine specimen for urinalysis and possible culture should be sent prior to administering empiric antibiotics (if possible) in a symptomatic patient.5
What bacteria are commonly associated with CA-UTI?
The bacteria most commonly associated with CA-UTI are found in or around the gastrointestinal and genitourinary tracts and also are part of the normal skin flora. The introduction and/or facilitated ascension of these microorganisms is believed to occur during UC insertion.16,17 Two-thirds of all isolated uropathogens in those with indwelling UCs are extraluminally acquired (via ascension along the catheter-urethral mucosa interface), and one-third are believed to be intraluminally acquired.18
The most commonly isolated bacteria in CA-UTI are Enterobacteriaceae, which include Escherichia coli (most common), Klebsiella species (K. oxytoca, K. pneumoniae), Serratia species (S. marcescens), Citrobacter species (C. koseri), Enterobacter species (E. cloacae), and Proteus species; non-Enterobacteriaceae such as Pseudomonas species; and gram-positive cocci, which include coagulase-negative staphylococci (S. saprophyticus), Staphylococcus aureus, group B streptococci, and Enterococcus species (E. faecalis, E. faecium).19-21 Coagulase-negative staphylococci and Enterococcus species can lead to CA-UTI but are usually avirulent and more commonly isolated from asymptomatic individuals.19 Also, coagulase-negative staphylococci such as S. epidermidis and S. lugdunensis are usually the manifestation of contamination during the collection process and their presence should prompt a repeat sample collection under sterile techniques. Monomicrobial infection is usually seen in those with short-term catheter use and CA-UTI. In contrast, polymicrobial infection is more common in those with long-term indwelling UCs and CA-UTI.19 Providencia stuartii, Proteus mirabilis, S. aureus, and Morganella morganii have all been associated with CA-UTI in those with long-term indwelling UCs.
Growth of S. aureus in the urine should prompt further investigation with blood cultures to explore the possibility of hematogenous dissemination to the urinary tract. Organisms leading to bacteremia due to CA-UTI are most commonly gram-negative bacilli (E. coli, Klebsiella species, Pseudomonas aeruginosa) and E. faecalis.21
What is the difference between CA-ASB and CA-UTI?
CA-ASB is defined as the presence of ≥ 1 bacteria species growing on urine culture at ≥ 100,000 cfu/mL in a patient with a history of urinary catheterization and/or indwelling UC who lacks signs or symptoms of UTI. In a man with a condom catheter, CA-ASB is defined using the same criteria, but the urine sample is collected after a fresh condom catheter is applied.5 The difference between CA-ASB and CA-UTI is simply the presence or absence of signs and symptoms related to UTI. Currently, there is no standard definition for significant bacteriuria in a catheterized patient.5 Pyuria found on urinalysis is indicative of genitourinary inflammation and can be present in both CA-ASB and CA-UTI. The absence, presence, and/or degree of pyuria in catheterized patients does not accurately differentiate between CA-ASB and CA-UTI.5,22,23 On the other hand, the absence of pyuria in a symptomatic catheterized patient suggests an etiology other than CA-UTI.5
How can CA-UTI be prevented in patients with a short-term indwelling urinary catheter?
If a short-term UC is essential, the most important approach to preventing CA-UTI is limiting the duration of time it will be used. Strategies such as computer-based order entry and care maps with automated discontinuation of UCs have been shown to decrease catheter usage.19 Using closed-systems for UC collection with ports in the distal catheter for needle aspiration of urine has also been shown to decrease the incidence of CA-UTI.5 Securing the UC to avoid urethral trauma, aseptic techniques for insertion and repositioning, and placement of the tubing and collection bag below the level of the bladder to prevent reflux will likely also prevent CA-UTI, but these strategies have not been evaluated thoroughly.19
When should you screen for and treat CA-ASB?
The 2009 Infectious Diseases Society of America (IDSA) guidelines recommend that the only patients who should be screened and treated for CA-ASB are pregnant women and those who will undergo a potentially traumatic urologic procedure for which mucosal breaching may occur, causing bleeding. Routinely screening or treating patients for CA-ASB in not recommended in any other group of patients and will lead to unnecessary antibiotic use and antibiotic resistance.5
UTI Associated with Percutaneous Nephrostomy and Ureteral Stenting
Similar systemic symptoms of infection (fever, rigors, malaise, shock) are present in patients with and without percutaneous nephrostomy (PCN) and/or ureteral stent placement. Dysuria is not commonly present in those with PCN. The first signs of CA-UTI may be decreased urine output and pericatheter leakage due to an obstructive process resulting from the encrustation.24-27 The most common complaint among patients with either acute or chronic ureteral stenting is discomfort, which has been described as “urinary symptoms” and “body pain.”28 This discomfort can be related to ureteral hyperperistalsis after placement of the stent and is usually self-limiting. Ureteral stent migration, usually at the distal end, can also lead to discomfort, but is easily rectified with cystoscopy.25 Body pain and/or urinary symptoms in the setting of ureteral stenting are not indicative of infection alone.
What is urinary catheter and/or ureteral stent encrustation?
Encrustation is the formation of a conditioning film that develops on the surface of the UC or ureteral stent. The exact mechanism is not well understood, but it is believed to involve electrostatic interactions of urinary proteins that stimulate binding onto the stent or UC surface.25 Encrustation increases exponentially with the dwell time. Among patients with ureteral stents placed due to urolithiasis, encrustation occurred in 9.2% of stents removed prior to 6 weeks, 47.5% of stents removed at 6 weeks, and 76.3% of stents removed at 12 weeks.26 Encrustation is most common at the proximal and distal ends (pigtails) of the ureteral stent and usually spares or presents last within the lumen.29 Attempts have been made to prevent ureteral stent encrustation through the development of biodegradable, drug-eluting, and tissue-engineered substrates. These developments are promising, but currently there is limited observational data from large randomized trials to suggest that these new modalities decrease rates of encrustation.25 Encrustation is highly associated with certain microorganisms, especially those that create biofilms.30 Urease-producing bacteria, most commonly P. mirabilis, play a role in encrustation formation.31 Bacteria most commonly associated with encrustation include Proteus species (P. mirabilis is most common), P. aeruginosa, K. pneumoniae, Providencia species (P. stuartii is most common), and M. morganii.
Does ureteral stent bacterial colonization correlate with UTI?
Ureteral stent colonization with bacteria increases with dwell time and is found in 40% to 98.5% of stents placed.32-35 If UTI is suspected in a patient with an active indwelling ureteral stent, a sample of urine should be cultured while the stent is in place.25 Typically, genitourinary and normal skin flora pathogens are found when the ureteral stent is cultured. The top 3 organisms cultured from ureteral stents are S. aureus, P. aeruginosa, and E. faecalis.34 Urine culture usually does not correlate with stent culture results, which has brought up the debate of how bacterial colonization occurs. It has been postulated that colonization is actually a manifestation of contamination during the insertion procedure, but this has yet to be validated.25 In patients with symptoms of UTI in the setting of an indwelling ureteral stent, a positive culture has low sensitivity, with estimates between 21% and 40%.35 Therefore, a negative urine culture does not rule out UTI alone in a symptomatic patient. Multiple studies have suggested that colonization of the ureteral stent does not correlate strongly with developing a UTI.25,32-34
How can UTI be prevented in those receiving PCN or ureteral stent placement?
Antibiotic prophylaxis has been recommended to prevent UTI in patients who will undergo PCN or ureteral stent placement. The American Urological Association recommends empiric treatment even in the absence of signs and symptoms of UTI,36 but substantial evidence is lacking that this approach prevents infection.37 Ciprofloxacin or trimethoprim/sulfamethoxazole has been recommended by some for empiric coverage for enteric gram-negative bacilli and enterococcus in those undergoing genitourinary manipulation or instrumentation.32,38 Most patients who develop CA-UTI and pyelonephritis do so within the first 2 to 6 weeks after placement.37,39 Bacteriuria, candiduria, and/or pyuria are present in all patients approximately within 9 weeks even when sterile urine is confirmed prior to PCN placement.39 Data on the effectiveness of antibiotic prophylaxis to prevent CA-UTI in those with PCN or ureteral stenting is limited. Currently, there are no recommendations from the IDSA on how to prevent infection in these situations.5 Early or frequent stent removal or exchanges has been proven to reduce UTI in those with ureteral stenting.33 Patients with diabetes mellitus and chronic renal failure are at high-risk for UTI when ureteral stents are in place. This population should undergo close monitoring for UTI development and may warrant more frequent stent exchanges.27,40
What is the treatment of CA-UTI associated with PCN and/or ureteral stenting?
The IDSA guidelines do not apply to patients with PCN and/or ureteral stenting.5 There is no treatment protocol for UTI related to these processes. Generally speaking, they are considered “complicated UTI” by most experts. Broad-spectrum, empiric antibiotic administration along with prompt removal of the PCN and/or ureteral stent is the gold standard of therapy.27 The recommended duration of targeted antibiotic therapy is generally between 5 and 14 days.19 Most clinicians will treat this complicated UTI for at least 10 to 14 days. Antibiotic administration should be continued even after removal of the catheter and/or stent to complete the full course. Repeat urinalysis and culture is not indicated at the end of therapy if the patient is clinically improving or has remission of symptoms.
What is the exchange rate for those who require chronic PCN and/or ureteral stent use?
On average a PCN or ureteral stent should be exchanged every 2 to 3 months in patients who require chronic usage.24,27 Some patients with persistent complications may require more frequent exchanges (< 10 weeks).27 Encrustation and bacterial colonization become more prevalent the longer the devices are in place. This process is estimated to begin within the first 2 weeks after placement.27,33,34 A “forgotten stent” is one that has been left in place after the patient is lost to follow-up. This unfortunate event can lead to massive encrustation, UTI, stent fracturing, and complete ureteral obstruction.24 As noted, patients with diabetes mellitus, chronic renal failure, and frequent UTI may warrant more frequent exchanges, but this should be determined on a case-by-case basis.
Catheter-Associated UTI in Patients with Spinal Cord Injury
Spinal cord injury (SCI) at any level can cause neurogenic bladder. This process ultimately leads to urinary stasis and colonization of the bladder with bacteria. According to the IDSA, the acceptable indications for UC insertion are: clinically significant urinary retention (if medical therapy is not effective), urinary incontinence, accurate urine output monitoring required, and patient is unable and/or unwilling to collect urine (Table 1).5 Recently, further guidelines were published regarding appropriate and inappropriate indwelling UC placement in hospitalized medical patients (Table 2 and Table 3), expanding upon the earlier acceptable criteria provided by the IDSA.41 According to the IDSA and Ann Arbor Criteria for Appropriate Urinary Catheter Use, patients with SCI and subsequent neurogenic bladder without obstruction where intermittent bladder straight catheterization for the drainage of urine is not feasible will likely need an indwelling UC.41 SCI patients often experience decubitus ulcers, and an indwelling UC can be used if needed to help with wound healing if other urinary management alternatives have been attempted. Other such situations in which an indwelling UC can used before attempting alternative approaches would be in a patient who is actively dying and is pursuing comfort care and/or hospice.41
Which indwelling UCs should be used in patients with SCI?
Efforts to reduce the likelihood of infection in patients with SCI have led to several advances in the design and manufacturing of UCs. UCs are made of either latex, plastic, silicone, or polytetrafluoroethylene (Teflon). None of these substrates is free of complications, but of them latex UCs have been studied the most in regards to their associated complications. Aside from being allergenic in nature, latex UCs have an increased propensity to allow bacteria to adhere to their surface due to microscopic planes of unevenness.42 Silicone UCs are less frequently associated with infection but are more rigid, leading to increased discomfort.43,44 Hydrophilic and silver-hydrogel coatings are innovative methods that have been developed to increase comfort and reduce the likelihood of infection. Hydrophilic-coated UCs are associated with reduced microbial adherence, decreased encrustation, and better patient satisfaction.45,46 In SCI patients, these UCs have demonstrated lower complication rates, including UTI; fewer episodes of post-, intra-, and inter-catheterization bleeding; and decreased rates of antibiotic-resistant bacteria.45,46 Silver-hydrogel-coated UCs are less well studied but have also demonstrated reduced UTI rates in SCI patients; however, their efficacy over the long term has yet to be determined. Antibiotic-impregnated UCs are not currently recommended for either short- or long-term indwelling UC use.5
How can CA-UTI be prevented in a patient who will require a long-term indwelling catheter?
At this time, the data is insufficient to make a recommendation on routine UC exchange (eg, every 2 to 4 weeks) in patients who require long-term indwelling urethral or suprapubic catheters in an attempt to reduce the risk of CA-ASB or CA-UTI. This is also true for those who experience even repeated early catheter blockage from encrustation.5 Thus, the rate at which these exchanges occur can be controversial, but typically around every 4 weeks is a common approach. Some would argue that if the patient has repeated CA-UTI, an exchange rate of every 2 weeks might be needed, but data is currently lacking to support this practice.5 If intermittent urinary catheterization is feasible, it should be done at least every 6 hours and before bedtime. In general, when the volume of urine in the bladder reaches approximately 400 mL, the patient should undergo bladder catheterization to prevent stasis and infection.47 A closed drainage system is recommended in all patients who require long-term indwelling UC use.48,49 Placement of the collection bag above the catheter or above the level of the bladder and a breach in the closed drainage system have been shown to result in higher rates of catheter-associated bacteriuria.48,50 Proper hand hygiene and sterile and/or clean techniques should be used when placing or exchanging a UC. However, in one study there was no difference in bacteremia or UTIs when using sterile versus clean techniques.51
Asymptomatic bacteriuria should not be treated in patients with long-term indwelling UCs, and prophylactic antibiotics have led to the emergence of resistance.52 At this time it is not clear whether prophylactic weekly oral cyclic antibiotic administration can effectively reduce the frequency of CA-UTI in patients with SCI and chronic indwelling UC use.
What are the signs and symptoms of CA-UTI in a patient with SCI?
Subjective and objective findings may be limited when a patient with SCI presents with CA-UTI. These patients often lack the usual symptoms of UTI (dysuria, suprapubic discomfort, urgency, increased frequency) and pyelonephritis (flank pain, costovertebral angle tenderness). Caregivers and health care providers should be aware that nonspecific symptoms such as foul-smelling urine, pyuria, increased residual volume of urine in the bladder, change in voiding habits, worsening detrusor spasticity, and aggravation of autonomic dysreflexia can be the only initial presenting symptoms.53
What is the duration of therapy for CA-UTI in SCI, and how can antibiotic stewardship principles be applied in this patient population?
Antibiotic therapy is indicated for a duration of 7 days if there is prompt resolution of symptoms, or for a total of 10 to 14 days if the response is delayed, regardless of whether the patient remains with a UC.5 Antibiotic stewardship is very important to reduce the risk for developing drug resistance in this high-risk population. Methods such as prescriber education practices, institution protocols, guideline implementation, auditing and feedback, restriction and reauthorization practices, computer-assisted programs, de-escalation or streamlining, and antibiotic cycling or dosage optimization have all been shown to assist in antibiotic stewardship in UTIs.54
Candida UTI
What is the initial evaluation for a patient with candiduria?
The workup for candiduria hinges on determining whether the candiduria likely represents contamination, colonization, or infection; certain predisposing risk factors are associated with Candida UTI (Table 4).55-57 The most important aspect to candiduria is the patient’s clinical status and comorbid conditions.58 Among funguria, Candida species are the most common and represent 95% of organisms isolated on urine cultures (Table 5).59 Candiduria is usually present in those with significant comorbidities and rarely is associated with healthy individuals.59,60 Candiduria is increasing in prevalence among hospitalized patients, representing 22% to 40% of all nosocomial UTIs.59,60 Markers in the urine (leukocyte esterase, colony count of culture growth, presence or absence of Candida casts and pseudohyphae) cannot alone differentiate colonization from infection.55 When candiduria is discovered in a patient with symptoms related to UTI in the setting of predisposing risk factors, it should be considered a real infection until proven otherwise.
In a situation where an asymptomatic patient without an indwelling UC has Candida species isolated from a urine culture, a repeat culture (clean-catch midstream sample) should be performed to assess for a likely contaminated collection.55 If the patient has an indwelling UC then it should be exchanged and urine collected from the fresh catheter.61 When candiduria is found in healthy asymptomatic adults, it is most commonly associated with poor collection techniques or postcollection contamination.59 If candiduria persists in an asymptomatic patient, the patient should be assessed for predisposing factors. This includes checking hemoglobin A1C for developing diabetes and renal ultrasound looking for urolithiasis, renal abscess, hydronephrosis, and fungus ball. Postvoid residual urinary retention should also be ruled out with bladder ultrasound. Treatment of predisposing factors can lead to resolution of candiduria without antifungal treatment, and a urine culture should be repeated (1 to 2 weeks later).61 Asymptomatic patients lacking any predisposing factors can be observed with repeat urine cultures in 1 to 3 months.61
Candiduria may actually represent candidemia in those patients who have a predisposing risk factor for disseminated candidiasis. These risk factors include central venous catheters, administration of total parental nutrition, antibiotic use (especially broad spectrum), critical illness, recent surgical intervention (especially intra-abdominal), acute renal failure, nasogastric tube use, and active gastric acid suppression (ie, proton pump inhibitors).62,63 The hematogenous spread of Candida can lead to the detection of candiduria in 46% to 80% of persons who are experiencing candidemia.59 If the patient is at risk for candidemia, then blood fungal cultures should be drawn. It is not unreasonable to also order a serum-D-glucan assay if suspicions are high. A thorough skin assessment should be completed and ophthalmology consulted for a detailed eye exam in the event that the patient has candidemia. Candiduria is highly prevalent among those who are candidemic, but overall candidemia is encountered in less than 5% of patients in most intensive care units.59 Thus, most patients with candiduria do not have disseminated candidiasis.
Candiduria rarely leads to symptoms of UTI,58 unless the pathogenesis is related to an ascending process.56 Symptoms of Candida UTI are no different from those experienced from a bacterial etiology. Some patients may complain of pneumaturia and/or endorse seeing particulate matter in their urine.55 Patients showing signs of sepsis (fever, chills, flank pain) should be investigated for possible Candida pyelonephritis in the setting of candiduria.64
When should asymptomatic candiduria be treated?
In adult patients with asymptomatic candiduria, there are 2 situations in which antifungal therapy is recommended. A patient undergoing a traumatic urologic procedure would be treated to avoid the risk for candidemia caused by the procedure. Also, in neutropenic patients empiric antifungal therapy should be administered because there is a high likelihood that this candiduria may actually represent hematogenous spread from candidemia.61,65
What is the treatment for symptomatic Candida cystitis?
Empiric treatment with oral fluconazole 200 to 400 mg daily for a total of 2 weeks is recommended in patients with persisting candiduria and symptoms of cystitis.65 Identifying the species is a crucial step in the treatment of Candida UTI. Several species (C. glabrata, C. krusei) are known to be resistant to fluconazole. Species identification and antifungal sensitivities should be done and therapy directed after obtaining these results.55
What is the recommended treatment for Candida pyelonephritis?
Treatment for pyelonephritis caused by fluconazole-susceptible Candida species is oral fluconazole 200 to 400 mg (3-6 mg/kg) for a total of 2 weeks.65 A fluconazole-resistant organism should be suspected when a non-albicans Candida species is isolated, such as C. krusei or C. glabrata. In this circumstance, in vitro antifungal susceptibility testing should be done. Echinocandins are not a good option in this situation because they do not reach adequate urine concentration and treatment failure is well documented.66-68 Amphotericin B deoxycholate (AmB) 0.3 to 0.6 mg/kg daily for 1 to 7 days, with or without oral flucytosine (25 mg/kg) 4 times daily, is recommended by the IDSA for the treatment of fluconazole-resistant isolates of C. glabrata and C. krusei.65 Further imaging with ultrasound, CT, or magnetic resonance should be done to rule out urinary tract obstruction and/or “fungus ball” formation. Emphysematous pyelonephritis and necrosis can occur and usually require nephrectomy. Perinephric abscess will need drainage, which can be accomplished through interventional radiological techniques.55
If a “fungus ball” is suspected in the kidney, how does the management change in a patient with Candida pyelonephritis?
A fungus ball must be treated with both antifungals and surgical intervention. Antifungal therapy should be continued during the surgical removal process to avoid fungemia. Interventional radiology should be consulted and is usually the best option for removal. Fungus ball(s) can and often do cause urinary obstruction. Temporary nephrostomy tube placement may be warranted in these situations to relieve the obstruction.55,65 AmB can be infused through the nephrostomy tube to increase local concentrations. This route of administration is not known to be nephrotoxic.55 Fluconazole infusion through a nephrostomy tube has also been used in the successful treatment of a fungus ball.69
Summary
Health care–associated UTIs are the most common nosocomial infection in the United States. UC placement and genitourinary manipulation or instrumentation play a major role in the development of CA-UTI. Clinicians should be aware of the appropriate and inappropriate use of UCs and their association with CA-UTI development. Removal of a UC when no longer necessary is key in prevention of CA-UTI. Treatment of asymptomatic bacteriuria is generally not indicated. A multidisciplinary approach is essential when managing chronic indwelling UCs in SCI. PCN and ureteral stenting need close monitoring, and early removal should be performed if infection is suspected.
Candiduria is an emerging nosocomial source of UTI but rarely leads to symptoms unless related to an ascending process. Proper urine collection is crucial in determining whether you are dealing with contamination, colonization, or infection. If candiduria persists in an asymptomatic individual, then further investigations should be done in regards to possible predisposing risks factors. Fluconazole is recommended for treatment of most patients with Candida UTI, while intravenous AmB is the treatment of choice for fluconazole-resistant Candida species. As we continue to take an evidence-based approach to the prevention and management of health care-associated UTI, we will likely see continued improvement in patient outcomes and overall decreased rates of infection.
Corresponding author: Norman Beatty, MD, 1501 N. Campbell Ave., Tucson, AZ 85724; [email protected].
Financial disclosures: None.
1. Klevens RM, Edwards JR, Richards CL Jr. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122:160-166.
2. Burke JP. Infection control—a problem for patient safety. N Engl J Med. 2003;348:651-656.
3. Scott RD. The direct medical costs of healthcare-associated infections in U.S. hospitals and the benefits of prevention. Division of Healthcare Quality Promotion; National Center for Preparedness Detection and Control of Infectious Diseases; Coordinating Center for Infectious Diseases; Centers for Disease Control and Prevention. March 2009. Contract No.: CS200891-A. www.cdc.gov/HAI/pdfs/hai/Scott_CostPaper.pdf.
4. Eriksen HM, Iverson BG, Aavitsland P. Prevalence of nosocomial infections in hospitals in Norway, 2002 and 2003. J Hosp Infect. 2005;60:40-45.
5. Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:625-663.
6. Mattie AS, Webster BL. Centers for Medicare and Medicaid Services’ “never events”: an analysis and recommendations to hospitals. Health Care Manag. 2008;27:338-349.
7. Warren JW. Catheter-associated urinary tract infections. Int J Antimicrob Agents. 2001;17:299-303.
8. Weinstein JW, Mazon D, Pantelick E, et al. A decade of prevalence surveys in a tertiary-care center: trends in nosocomial infection rates, device utilization, and patient acuity. Infect Control Hosp Epidemiol. 1999;20:543-548.
9. Fakih MG, Pena ME, Shemes S, et al. Effect of establishing guidelines on appropriate urinary catheter placement. Acad Emerg Med. 2010;17:337-340.
10. Gould CV, Umscheid CA, Agarwal RK, et al. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31:319-326.
11. Fakih MG, Rey JE, Pena ME, et al. Sustained reductions in urinary catheter use over 5 years: bedside nurses view themselves responsible for evaluation of catheter necessity. Am J Infect Control. 2013;41:236-239.
12. Meddings J, Rogers MA, Krein SL, et al. Reducing unnecessary urinary catheter use and other strategies to prevent catheter-associated urinary tract infections: an integrative review. BMJ Qual Saf. 2014;23:277-289.
13. Gould C. Catheter-associated urinary tract infection: the national perspective. In: Essential Hospitals Engagement Network. Patient Harm Series II: new tools to prevent CAUTI webinar. April 16, 2014. http://bit.ly/1UWndRA.
14. Nicolle LE. Consequences of asymptomatic bacteriuria in the elderly. Int J Antimicrob Agents. 1994;4:107-111.
15. Nicolle LE. Urinary tract infections in long-term-care facilities. Infect Control Hosp Epidemiol. 2001;22:167-175
16. Cohen A. A microbiological comparison of a povidone-iodine lubricating gel and a control as catheter lubricants. J Hosp Infect. 1985;6(Suppl A):155-161.
17. Daifuku R, Stamm WE. Bacterial adherence to bladder uroepithelial cells in catheter-associated urinary tract infection. N Engl J Med. 1986;314:1208-1213.
18. Tambyah PA, Halvorson KT, Maki DG. A prospective study of pathogenesis of catheter-associated urinary tract infections. Mayo Clin Proc. 1999;74:131-136.
19. Nicolle LE. Catheter-related urinary tract infection. Drugs Aging. 2005;22:627-639.
20. Redder JD, Leth RA, Møller JK. Incidence rates of hospital-acquired urinary tract and bloodstream infections generated by automated compilation of electronically available healthcare data. J Hosp Infect. 2015;91:231-236.
21. Ortega M, Marco F, Soriano A, et al. Epidemiology and prognostic determinants of bacteraemic catheter-acquired urinary tract infection in a single institution from 1991 to 2010. J Infect. 2013;67:282-287.
22. Tambyah PA, Maki DG. The relationship between pyuria and infection in patients with indwelling urinary catheters: a prospective study of 761 patients. Arch Intern Med. 2000;160:673-677.
23. Musher DM, Thorsteinsson SB, Airola VM, II. Quantitative urinalysis:diagnosing urinary tract infection in men. JAMA. 1976;236:2069-2072.
24. Hausegger KA, Portugaller HR. Percutaneous nephrostomy and antegrade ureteral stenting: technique-indications-complications. Eur Radiol. 2006;16:2016-2030.
25. Lange D, Bidnur S, Hoag N, et al. Ureteral stent-associated complications--where we are and where we are going. Nat Rev Urol. 2015;12:17-25.
26. el-Faqih SR, Shamsuddin AB, Chakrabarti A, et al. Polyurethane internal ureteral stents in treatment of stone patients: morbidity related to indwelling times. J Urol. 1991;146:1487-1491.
27. Adamo R, Saad WE, Brown DB. Management of nephrostomy drains and ureteral stents. Tech Vasc Interv Radiol. 2009;12:193-204.
28. Joshi HB, Newns N, Stainthorpe A, et al. Ureteral Stent Symptom Questionnaire: development and validation of a multidimensional quality of life measure. J Urol. 2003;169:1060-1064.
29. Singh I, Gupta NP, Hemal AK, et al. Severely encrusted polyurethane ureteral stents: management and analysis of potential risk factors. Urology. 2001;58:526-531.
30. Saint S, Chenoweth CE. Biofilms and catheter-associated urinary tract infections. Infect Dis Clin North Am. 2003;17:411-432.
31. Mobley HL, Warren JW. Urease-positive bacteriuria and obstruction of long-term urinary catheters. J Clin Microbiol. 1987;25:2216-2217.
32. Kehinde EO, Rotimi VO, Al-Hunayan A, et al. Bacteriology of urinary tract infection associated with indwelling J ureteral stents. J Endourol. 2004;18:891-896.
33. Paick SH, Park HK, Oh SJ, Kim HH. Characteristics of bacterial colonization and urinary tract infection after indwelling of double-J ureteral stent. Urology. 2003;62:214-217.
34. Klis R, Korczak-Kozakiewicz E, Denys A, et al. Relationship between urinary tract infection and self-retaining double-J catheter colonization. J Endourol. 2009;23:1015-1019.
35. Farsi HM, Mosli HA, Al-Zemaity M, et al. Bacteriuria and colonization of double-pigtail ureteral stents: long-term experience with 237 patients. J Endourol. 1995;9:469-472.
36. Wolfe JS Jr, Bennet CJ, Dmochowski RR, et al. Best practice policy statement on urologic surgery antimicrobial prophylaxis. J Urol. 2008;179:1379-1390.
37. Bahu R, Chaftari AM, Hachem RY, et al. Nephrostomy tube related pyelonephritis in patients with cancer: epidemiology, infection rate and risk factors. J Urol. 2013;189:130-135.
38. Bratzler DW, Dellinger EP, Olsen KM, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Surg Infect (Larchmt). 2013;14:73-156.
39. Cronan JJ, Horn DL, Marcello A, et al: Antibiotics and nephrostomy tube care: preliminary observations. Part II. Bacteremia. Radiology. 1989;172(3 Pt 2):1043-1045.
40. Akay AF, Aflay U, Gedik A, et al. Risk factors for lower urinary tract infection and bacterial stent colonization in patients with a double J ureteral stent. Int Urol Nephrol. 2007;39:95-98.
41. Meddings J, Saint S, Fowler KE, et al. The Ann Arbor criteria for appropriate urinary catheter use in hospitalized medical patients: results obtained by using the RAND/ UCLA appropriateness method. Ann Intern Med. 2015;162(9 Suppl):S1-34.
42. Stickler D, Young R, Jones G, et al. Why are Foley catheters so vulnerable to encrustation and blockage by crystalline bacterial biofilm? Urol Res. 2003;31:306-311.
43. Denstedt J, Wollin T, Reid G. Biomaterials used in urology: current issues of biocompatibility, infection, and encrustation. J Endourol. 1998;12:493-500.
44. Morris N, Stickler D, Winters C. Which indwelling urethral catheters resist encrustation by Proteus mirabilis biofilms? Br J Urol. 1997;80:58-63.
45. Cardenas D, Moore K, Dannels-McClure A, et al. Intermittent catheterization with a hydrophilic-coated catheter delays urinary tract infections in acute spinal cord injury: a prospective, randomized, multicenter trial. PMR. 2011;3:408-417.
46. Spinu A, Onose G, Daia C, et al. Intermittent catheterization in the management of post spinal cord injury (SCI) neurogenic bladder using new hydrophilic, with lubrication in close circuit devices-our own preliminary results. J Med Life. 2012;5:21-28.
47. Shekelle P, Morton S, Clark K, et al. Systematic review of risk factors for urinary tract infection in adults with spinal cord dysfunction. J Spinal Cord Med. 1998;22:258-272.
48. Siddiq D, Darouiche R. New strategies to prevent catheter-associated urinary tract infections. Nat Rev Urol. 2012;9:305-314.
49. Gould C, Umscheid C, Agarwal R, et al. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31:319-326.
50. Maki D, Tambyah P. Engineering out the risk for infection with urinary catheters. Emerg Infect Dis. 2001;7:342-347.
51. Munasinghe R, Yazdani H, Siddique M, et al. Appropriateness of use of indwelling urinary catheters in patients admitted to the medical service. Infect Control Hosp Epidemiol. 2001;22:647-649.
52. Nicolle L, Bradley S, Colgan R, et al; Infectious Diseases Society of America; American Society of Nephrology; American Geriatric Society. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40: 643-654.
53. Linsenmeyer T, Oakley A. Accuracy of individuals with spinal cord injury at predicting urinary tract infections based on their symptoms. J Spinal Cord Med. 2002;26:352-357.
54. Abbo LM, Hooton TM. Antimicrobial stewardship and urinary tract infections. Antibiotics. 2014;3:174-192.
55. Kauffman CA. Diagnosis and management of fungal urinary tract infection. Infect Dis Clin North Am. 2014;28:61-74.
56. Alvarez-Lerma F, Nolla-Salas J, Leon C, et al. Candiduria in critically ill patients admitted to intensive care medical units. Intensive Care Med. 2003;29:1069-1076.
57. Colodner R, Nuri Y, Chazan B, et al. Community-acquired and hospital-acquired candiduria: comparison of prevalence and clinical characteristics. Eur J Clin Microbiol Infect Dis. 2008;27:301-305.
58. Kauffman CA, Vazquez JA, Sobel JD, et al. Prospective multicenter surveillance study of funguria in hospitalized patients. Clin Infect Dis. 2000;30:14-18.
59. Sobel JD, Fisher JF, Kauffman CA, et al. Candida urinary tract infections—epidemiology. Clin Infect Dis. 2011;52(suppl 6): S433-436.
60. Richards MJ, Edwards JR, Culver DH, et al. Nosocomial infections in combined medical-surgical intensive care units in the United States. Infect Control Hosp Epidemiol. 2000;21:510-515.
61. Fisher JF, Sobel JD, Kauffman CA, et al. Candida urinary tract infections—treatment. Clin Infect Dis. 2011;52(suppl 6):S457-466.
62. Blumberg HM, Jarvis WR, Soucie JM, et al. Risk factors for candidal bloodstream infections in surgical intensive care unit patients: the NEMIS prospective multicenter study. Clin Infect Dis. 2001;33:177-186.
63. Puzniak LP, Teutsch S, Powderly W, et al. Has the epidemiology of nosocomial candidemia changed? Infect Control Hosp Epidemiol. 2004;25:628-633.
64. Siddique MS, Gayed N, McGuire N, et al. Salient features of Candida pyelonephritis in adults. Infect Dis Clin Pract. 1992;1:239-245
65. Pappas PG, Kauffman CA, Andes DR, et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:e1-e50.
66. Sobel JD, Bradshaw SK, Lipka CJ, et al. Caspofungin in the treatment of symptomatic candiduria. Clin Infect Dis. 2007;44:e46-9.
67. Malani AN. Failure of caspofungin for treatment of Candida glabrata candiduria. Case report and review of the literature. Infect Dis Clin Pract. 2010;18:271-272.
68. Schelenz S, Ross CN. Limitations of caspofugin in the treatment of obstructive pyelonephrosis due to Candida glabrata infection. BMC Infect Dis. 2006;56:126-130.
69. Chung BH, Chang SY, Kim SI, et al. Successfully treated renal fungal ball with continuous irrigation of fluconazole. J Urol. 2001;166:1835-1836.
From the University of Arizona College of Medicine, Tucson, AZ (Dr. Beatty), and the Baylor College of Medicine, Houston, TX (Dr. Mohajer).
Abstract
- Objective: To review management issues regarding health care–associated urinary tract infections (UTIs) commonly encountered by practicing clinicians.
- Methods: Review of the literature.
- Results: Because urinary catheter (UC) placement plays a major role in the development of catheter-associated UTIs (CA-UTI), clinicians should be aware of the appropriate and inappropriate uses of UCs and their association with CA-UTI development. Removal of a UC when no longer necessary is key to preventing CA-UTI. Treatment of asymptomatic bacteriuria is generally not indicated. Percutaneous nephrostomy and ureteral stenting need close monitoring, and early removal should be performed if infection is suspected. Candiduria rarely leads to symptoms unless it is related to an ascending process. Proper urine collection is crucial in determining whether contamination, colonization, or infection is present. Fluconazole is recommended in most cases of Candida UTI, while intravenous amphotericin B is recommended for fluconazole-resistant Candida species.
- Conclusion: Continued use of evidence-based strategies for preventing and managing health care–associated UTI should lead to further improvements in patient outcomes and overall decreased rates of infection.
Keywords: bacteriuria; catheter-associated UTI; catheterization; percutaneous nephrostomy; candiduria.
Health care–associated urinary tract infections (UTIs) are estimated to be the most common adverse infectious event in U.S. hospitals, occurring in 1 of 10 admitted patients.1-3 Approximately 32% of all health care–associated infections are UTIs.1 Furthermore, urinary catheters (UCs) are associated with 8% to 21% of health care–associated infections that occur in the intensive care unit.4 The most important predisposing factor for nosocomial UTI is urinary catheterization.5 Genitourinary manipulation and/or implementation also play a major role in the development of nosocomial UTIs.
In 2008, the U.S. Centers for Medicare & Medicaid Services instituted a new policy that reduced reimbursement rates for hospitalizations linked to health care–associated infections.6 Indwelling UCs are among the most overused health care devices in the hospital setting. They are placed in an estimated 15% to 25% of all hospitalized patients,7,8 and are often inserted in the emergency department (ED) without a physician order or appropriate indication.9 Intermittent straight catheterization, male or female condom catheterization, and/or placement of an indwelling UC are the most common causes of catheter-associated asymptomatic bacteriuria (CA-ASB) and catheter-associated UTIs (CA-UTI).5 Prevention and management of CA-ASB and CA-UTI can be challenging and require an evidence-based approach. Furthermore, guidelines for the management of UTIs in the setting of active percutaneous nephrostomy (PCN) drainage and/or ureteral stenting are not established.5 This may leave clinicians with little mainstream data to aid in management decisions.
In 2009 the Centers for Disease Control and Prevention provided a guideline for the appropriate and inappropriate use of indwelling UCs to help promote their proper use.10 In the time since the guideline’s initiatives were instituted around the United States, published data have shown some improvement in the use of UCs,11,12 but other recent reports indicate that rates of UC use have remained unchanged.13 This review discusses management issues regarding health care–associated UTIs that are commonly encountered by practicing clinicians, with a focus on current guidelines and evidence.
Catheter-Associated UTI
CA-UTI is defined as the presence of signs or symptoms of UTI with no other explainable infectious source along with ≥ 1000 colony-forming units (cfu) of ≥ 1 bacterial species per milliliter in a urine specimen from a catheter that has been changed within 48 hours of collection of the urine specimen.5 Signs and symptoms of CA-UTI include, but are not limited to: new-onset or worsening fever, chills, altered sensorium from baseline, lethargy, malaise, flank pain, pelvic pain, costovertebral angle tenderness, and acute hematuria.5 New-onset “foul-smelling” (odorous)urine and “cloudy” urine are neither sensitive nor specific when assessing for CA-UTI, and do not have significant clinical relevance when found alone.14,15 Patients who have removed or exchanged the UC during this event and then experience dysuria, increased frequency, urgency, or suprapubic pain are likely having symptoms of CA-UTI.5
What is the recommended method for collecting urine samples when CA-UTI is suspected?
In a patient with an indwelling catheter that has been in place for more than 2 weeks at the onset of a suspected CA-UTI, the catheter should be replaced (if still indicated) or removed to accelerate resolution of symptoms and to reduce the risk of subsequent catheter-associated bacteriuria and CA-UTI. The urine culture should be acquired from the freshly placed UC.5
When should a patient be empirically treated?
A patient presenting with evidence of sepsis should be empirically treated with antimicrobials. Empiric coverage should be based on risk factors for multidrug-resistant organisms and data pertaining to local antimicrobial resistance patterns. A urine specimen for urinalysis and possible culture should be sent prior to administering empiric antibiotics (if possible) in a symptomatic patient.5
What bacteria are commonly associated with CA-UTI?
The bacteria most commonly associated with CA-UTI are found in or around the gastrointestinal and genitourinary tracts and also are part of the normal skin flora. The introduction and/or facilitated ascension of these microorganisms is believed to occur during UC insertion.16,17 Two-thirds of all isolated uropathogens in those with indwelling UCs are extraluminally acquired (via ascension along the catheter-urethral mucosa interface), and one-third are believed to be intraluminally acquired.18
The most commonly isolated bacteria in CA-UTI are Enterobacteriaceae, which include Escherichia coli (most common), Klebsiella species (K. oxytoca, K. pneumoniae), Serratia species (S. marcescens), Citrobacter species (C. koseri), Enterobacter species (E. cloacae), and Proteus species; non-Enterobacteriaceae such as Pseudomonas species; and gram-positive cocci, which include coagulase-negative staphylococci (S. saprophyticus), Staphylococcus aureus, group B streptococci, and Enterococcus species (E. faecalis, E. faecium).19-21 Coagulase-negative staphylococci and Enterococcus species can lead to CA-UTI but are usually avirulent and more commonly isolated from asymptomatic individuals.19 Also, coagulase-negative staphylococci such as S. epidermidis and S. lugdunensis are usually the manifestation of contamination during the collection process and their presence should prompt a repeat sample collection under sterile techniques. Monomicrobial infection is usually seen in those with short-term catheter use and CA-UTI. In contrast, polymicrobial infection is more common in those with long-term indwelling UCs and CA-UTI.19 Providencia stuartii, Proteus mirabilis, S. aureus, and Morganella morganii have all been associated with CA-UTI in those with long-term indwelling UCs.
Growth of S. aureus in the urine should prompt further investigation with blood cultures to explore the possibility of hematogenous dissemination to the urinary tract. Organisms leading to bacteremia due to CA-UTI are most commonly gram-negative bacilli (E. coli, Klebsiella species, Pseudomonas aeruginosa) and E. faecalis.21
What is the difference between CA-ASB and CA-UTI?
CA-ASB is defined as the presence of ≥ 1 bacteria species growing on urine culture at ≥ 100,000 cfu/mL in a patient with a history of urinary catheterization and/or indwelling UC who lacks signs or symptoms of UTI. In a man with a condom catheter, CA-ASB is defined using the same criteria, but the urine sample is collected after a fresh condom catheter is applied.5 The difference between CA-ASB and CA-UTI is simply the presence or absence of signs and symptoms related to UTI. Currently, there is no standard definition for significant bacteriuria in a catheterized patient.5 Pyuria found on urinalysis is indicative of genitourinary inflammation and can be present in both CA-ASB and CA-UTI. The absence, presence, and/or degree of pyuria in catheterized patients does not accurately differentiate between CA-ASB and CA-UTI.5,22,23 On the other hand, the absence of pyuria in a symptomatic catheterized patient suggests an etiology other than CA-UTI.5
How can CA-UTI be prevented in patients with a short-term indwelling urinary catheter?
If a short-term UC is essential, the most important approach to preventing CA-UTI is limiting the duration of time it will be used. Strategies such as computer-based order entry and care maps with automated discontinuation of UCs have been shown to decrease catheter usage.19 Using closed-systems for UC collection with ports in the distal catheter for needle aspiration of urine has also been shown to decrease the incidence of CA-UTI.5 Securing the UC to avoid urethral trauma, aseptic techniques for insertion and repositioning, and placement of the tubing and collection bag below the level of the bladder to prevent reflux will likely also prevent CA-UTI, but these strategies have not been evaluated thoroughly.19
When should you screen for and treat CA-ASB?
The 2009 Infectious Diseases Society of America (IDSA) guidelines recommend that the only patients who should be screened and treated for CA-ASB are pregnant women and those who will undergo a potentially traumatic urologic procedure for which mucosal breaching may occur, causing bleeding. Routinely screening or treating patients for CA-ASB in not recommended in any other group of patients and will lead to unnecessary antibiotic use and antibiotic resistance.5
UTI Associated with Percutaneous Nephrostomy and Ureteral Stenting
Similar systemic symptoms of infection (fever, rigors, malaise, shock) are present in patients with and without percutaneous nephrostomy (PCN) and/or ureteral stent placement. Dysuria is not commonly present in those with PCN. The first signs of CA-UTI may be decreased urine output and pericatheter leakage due to an obstructive process resulting from the encrustation.24-27 The most common complaint among patients with either acute or chronic ureteral stenting is discomfort, which has been described as “urinary symptoms” and “body pain.”28 This discomfort can be related to ureteral hyperperistalsis after placement of the stent and is usually self-limiting. Ureteral stent migration, usually at the distal end, can also lead to discomfort, but is easily rectified with cystoscopy.25 Body pain and/or urinary symptoms in the setting of ureteral stenting are not indicative of infection alone.
What is urinary catheter and/or ureteral stent encrustation?
Encrustation is the formation of a conditioning film that develops on the surface of the UC or ureteral stent. The exact mechanism is not well understood, but it is believed to involve electrostatic interactions of urinary proteins that stimulate binding onto the stent or UC surface.25 Encrustation increases exponentially with the dwell time. Among patients with ureteral stents placed due to urolithiasis, encrustation occurred in 9.2% of stents removed prior to 6 weeks, 47.5% of stents removed at 6 weeks, and 76.3% of stents removed at 12 weeks.26 Encrustation is most common at the proximal and distal ends (pigtails) of the ureteral stent and usually spares or presents last within the lumen.29 Attempts have been made to prevent ureteral stent encrustation through the development of biodegradable, drug-eluting, and tissue-engineered substrates. These developments are promising, but currently there is limited observational data from large randomized trials to suggest that these new modalities decrease rates of encrustation.25 Encrustation is highly associated with certain microorganisms, especially those that create biofilms.30 Urease-producing bacteria, most commonly P. mirabilis, play a role in encrustation formation.31 Bacteria most commonly associated with encrustation include Proteus species (P. mirabilis is most common), P. aeruginosa, K. pneumoniae, Providencia species (P. stuartii is most common), and M. morganii.
Does ureteral stent bacterial colonization correlate with UTI?
Ureteral stent colonization with bacteria increases with dwell time and is found in 40% to 98.5% of stents placed.32-35 If UTI is suspected in a patient with an active indwelling ureteral stent, a sample of urine should be cultured while the stent is in place.25 Typically, genitourinary and normal skin flora pathogens are found when the ureteral stent is cultured. The top 3 organisms cultured from ureteral stents are S. aureus, P. aeruginosa, and E. faecalis.34 Urine culture usually does not correlate with stent culture results, which has brought up the debate of how bacterial colonization occurs. It has been postulated that colonization is actually a manifestation of contamination during the insertion procedure, but this has yet to be validated.25 In patients with symptoms of UTI in the setting of an indwelling ureteral stent, a positive culture has low sensitivity, with estimates between 21% and 40%.35 Therefore, a negative urine culture does not rule out UTI alone in a symptomatic patient. Multiple studies have suggested that colonization of the ureteral stent does not correlate strongly with developing a UTI.25,32-34
How can UTI be prevented in those receiving PCN or ureteral stent placement?
Antibiotic prophylaxis has been recommended to prevent UTI in patients who will undergo PCN or ureteral stent placement. The American Urological Association recommends empiric treatment even in the absence of signs and symptoms of UTI,36 but substantial evidence is lacking that this approach prevents infection.37 Ciprofloxacin or trimethoprim/sulfamethoxazole has been recommended by some for empiric coverage for enteric gram-negative bacilli and enterococcus in those undergoing genitourinary manipulation or instrumentation.32,38 Most patients who develop CA-UTI and pyelonephritis do so within the first 2 to 6 weeks after placement.37,39 Bacteriuria, candiduria, and/or pyuria are present in all patients approximately within 9 weeks even when sterile urine is confirmed prior to PCN placement.39 Data on the effectiveness of antibiotic prophylaxis to prevent CA-UTI in those with PCN or ureteral stenting is limited. Currently, there are no recommendations from the IDSA on how to prevent infection in these situations.5 Early or frequent stent removal or exchanges has been proven to reduce UTI in those with ureteral stenting.33 Patients with diabetes mellitus and chronic renal failure are at high-risk for UTI when ureteral stents are in place. This population should undergo close monitoring for UTI development and may warrant more frequent stent exchanges.27,40
What is the treatment of CA-UTI associated with PCN and/or ureteral stenting?
The IDSA guidelines do not apply to patients with PCN and/or ureteral stenting.5 There is no treatment protocol for UTI related to these processes. Generally speaking, they are considered “complicated UTI” by most experts. Broad-spectrum, empiric antibiotic administration along with prompt removal of the PCN and/or ureteral stent is the gold standard of therapy.27 The recommended duration of targeted antibiotic therapy is generally between 5 and 14 days.19 Most clinicians will treat this complicated UTI for at least 10 to 14 days. Antibiotic administration should be continued even after removal of the catheter and/or stent to complete the full course. Repeat urinalysis and culture is not indicated at the end of therapy if the patient is clinically improving or has remission of symptoms.
What is the exchange rate for those who require chronic PCN and/or ureteral stent use?
On average a PCN or ureteral stent should be exchanged every 2 to 3 months in patients who require chronic usage.24,27 Some patients with persistent complications may require more frequent exchanges (< 10 weeks).27 Encrustation and bacterial colonization become more prevalent the longer the devices are in place. This process is estimated to begin within the first 2 weeks after placement.27,33,34 A “forgotten stent” is one that has been left in place after the patient is lost to follow-up. This unfortunate event can lead to massive encrustation, UTI, stent fracturing, and complete ureteral obstruction.24 As noted, patients with diabetes mellitus, chronic renal failure, and frequent UTI may warrant more frequent exchanges, but this should be determined on a case-by-case basis.
Catheter-Associated UTI in Patients with Spinal Cord Injury
Spinal cord injury (SCI) at any level can cause neurogenic bladder. This process ultimately leads to urinary stasis and colonization of the bladder with bacteria. According to the IDSA, the acceptable indications for UC insertion are: clinically significant urinary retention (if medical therapy is not effective), urinary incontinence, accurate urine output monitoring required, and patient is unable and/or unwilling to collect urine (Table 1).5 Recently, further guidelines were published regarding appropriate and inappropriate indwelling UC placement in hospitalized medical patients (Table 2 and Table 3), expanding upon the earlier acceptable criteria provided by the IDSA.41 According to the IDSA and Ann Arbor Criteria for Appropriate Urinary Catheter Use, patients with SCI and subsequent neurogenic bladder without obstruction where intermittent bladder straight catheterization for the drainage of urine is not feasible will likely need an indwelling UC.41 SCI patients often experience decubitus ulcers, and an indwelling UC can be used if needed to help with wound healing if other urinary management alternatives have been attempted. Other such situations in which an indwelling UC can used before attempting alternative approaches would be in a patient who is actively dying and is pursuing comfort care and/or hospice.41
Which indwelling UCs should be used in patients with SCI?
Efforts to reduce the likelihood of infection in patients with SCI have led to several advances in the design and manufacturing of UCs. UCs are made of either latex, plastic, silicone, or polytetrafluoroethylene (Teflon). None of these substrates is free of complications, but of them latex UCs have been studied the most in regards to their associated complications. Aside from being allergenic in nature, latex UCs have an increased propensity to allow bacteria to adhere to their surface due to microscopic planes of unevenness.42 Silicone UCs are less frequently associated with infection but are more rigid, leading to increased discomfort.43,44 Hydrophilic and silver-hydrogel coatings are innovative methods that have been developed to increase comfort and reduce the likelihood of infection. Hydrophilic-coated UCs are associated with reduced microbial adherence, decreased encrustation, and better patient satisfaction.45,46 In SCI patients, these UCs have demonstrated lower complication rates, including UTI; fewer episodes of post-, intra-, and inter-catheterization bleeding; and decreased rates of antibiotic-resistant bacteria.45,46 Silver-hydrogel-coated UCs are less well studied but have also demonstrated reduced UTI rates in SCI patients; however, their efficacy over the long term has yet to be determined. Antibiotic-impregnated UCs are not currently recommended for either short- or long-term indwelling UC use.5
How can CA-UTI be prevented in a patient who will require a long-term indwelling catheter?
At this time, the data is insufficient to make a recommendation on routine UC exchange (eg, every 2 to 4 weeks) in patients who require long-term indwelling urethral or suprapubic catheters in an attempt to reduce the risk of CA-ASB or CA-UTI. This is also true for those who experience even repeated early catheter blockage from encrustation.5 Thus, the rate at which these exchanges occur can be controversial, but typically around every 4 weeks is a common approach. Some would argue that if the patient has repeated CA-UTI, an exchange rate of every 2 weeks might be needed, but data is currently lacking to support this practice.5 If intermittent urinary catheterization is feasible, it should be done at least every 6 hours and before bedtime. In general, when the volume of urine in the bladder reaches approximately 400 mL, the patient should undergo bladder catheterization to prevent stasis and infection.47 A closed drainage system is recommended in all patients who require long-term indwelling UC use.48,49 Placement of the collection bag above the catheter or above the level of the bladder and a breach in the closed drainage system have been shown to result in higher rates of catheter-associated bacteriuria.48,50 Proper hand hygiene and sterile and/or clean techniques should be used when placing or exchanging a UC. However, in one study there was no difference in bacteremia or UTIs when using sterile versus clean techniques.51
Asymptomatic bacteriuria should not be treated in patients with long-term indwelling UCs, and prophylactic antibiotics have led to the emergence of resistance.52 At this time it is not clear whether prophylactic weekly oral cyclic antibiotic administration can effectively reduce the frequency of CA-UTI in patients with SCI and chronic indwelling UC use.
What are the signs and symptoms of CA-UTI in a patient with SCI?
Subjective and objective findings may be limited when a patient with SCI presents with CA-UTI. These patients often lack the usual symptoms of UTI (dysuria, suprapubic discomfort, urgency, increased frequency) and pyelonephritis (flank pain, costovertebral angle tenderness). Caregivers and health care providers should be aware that nonspecific symptoms such as foul-smelling urine, pyuria, increased residual volume of urine in the bladder, change in voiding habits, worsening detrusor spasticity, and aggravation of autonomic dysreflexia can be the only initial presenting symptoms.53
What is the duration of therapy for CA-UTI in SCI, and how can antibiotic stewardship principles be applied in this patient population?
Antibiotic therapy is indicated for a duration of 7 days if there is prompt resolution of symptoms, or for a total of 10 to 14 days if the response is delayed, regardless of whether the patient remains with a UC.5 Antibiotic stewardship is very important to reduce the risk for developing drug resistance in this high-risk population. Methods such as prescriber education practices, institution protocols, guideline implementation, auditing and feedback, restriction and reauthorization practices, computer-assisted programs, de-escalation or streamlining, and antibiotic cycling or dosage optimization have all been shown to assist in antibiotic stewardship in UTIs.54
Candida UTI
What is the initial evaluation for a patient with candiduria?
The workup for candiduria hinges on determining whether the candiduria likely represents contamination, colonization, or infection; certain predisposing risk factors are associated with Candida UTI (Table 4).55-57 The most important aspect to candiduria is the patient’s clinical status and comorbid conditions.58 Among funguria, Candida species are the most common and represent 95% of organisms isolated on urine cultures (Table 5).59 Candiduria is usually present in those with significant comorbidities and rarely is associated with healthy individuals.59,60 Candiduria is increasing in prevalence among hospitalized patients, representing 22% to 40% of all nosocomial UTIs.59,60 Markers in the urine (leukocyte esterase, colony count of culture growth, presence or absence of Candida casts and pseudohyphae) cannot alone differentiate colonization from infection.55 When candiduria is discovered in a patient with symptoms related to UTI in the setting of predisposing risk factors, it should be considered a real infection until proven otherwise.
In a situation where an asymptomatic patient without an indwelling UC has Candida species isolated from a urine culture, a repeat culture (clean-catch midstream sample) should be performed to assess for a likely contaminated collection.55 If the patient has an indwelling UC then it should be exchanged and urine collected from the fresh catheter.61 When candiduria is found in healthy asymptomatic adults, it is most commonly associated with poor collection techniques or postcollection contamination.59 If candiduria persists in an asymptomatic patient, the patient should be assessed for predisposing factors. This includes checking hemoglobin A1C for developing diabetes and renal ultrasound looking for urolithiasis, renal abscess, hydronephrosis, and fungus ball. Postvoid residual urinary retention should also be ruled out with bladder ultrasound. Treatment of predisposing factors can lead to resolution of candiduria without antifungal treatment, and a urine culture should be repeated (1 to 2 weeks later).61 Asymptomatic patients lacking any predisposing factors can be observed with repeat urine cultures in 1 to 3 months.61
Candiduria may actually represent candidemia in those patients who have a predisposing risk factor for disseminated candidiasis. These risk factors include central venous catheters, administration of total parental nutrition, antibiotic use (especially broad spectrum), critical illness, recent surgical intervention (especially intra-abdominal), acute renal failure, nasogastric tube use, and active gastric acid suppression (ie, proton pump inhibitors).62,63 The hematogenous spread of Candida can lead to the detection of candiduria in 46% to 80% of persons who are experiencing candidemia.59 If the patient is at risk for candidemia, then blood fungal cultures should be drawn. It is not unreasonable to also order a serum-D-glucan assay if suspicions are high. A thorough skin assessment should be completed and ophthalmology consulted for a detailed eye exam in the event that the patient has candidemia. Candiduria is highly prevalent among those who are candidemic, but overall candidemia is encountered in less than 5% of patients in most intensive care units.59 Thus, most patients with candiduria do not have disseminated candidiasis.
Candiduria rarely leads to symptoms of UTI,58 unless the pathogenesis is related to an ascending process.56 Symptoms of Candida UTI are no different from those experienced from a bacterial etiology. Some patients may complain of pneumaturia and/or endorse seeing particulate matter in their urine.55 Patients showing signs of sepsis (fever, chills, flank pain) should be investigated for possible Candida pyelonephritis in the setting of candiduria.64
When should asymptomatic candiduria be treated?
In adult patients with asymptomatic candiduria, there are 2 situations in which antifungal therapy is recommended. A patient undergoing a traumatic urologic procedure would be treated to avoid the risk for candidemia caused by the procedure. Also, in neutropenic patients empiric antifungal therapy should be administered because there is a high likelihood that this candiduria may actually represent hematogenous spread from candidemia.61,65
What is the treatment for symptomatic Candida cystitis?
Empiric treatment with oral fluconazole 200 to 400 mg daily for a total of 2 weeks is recommended in patients with persisting candiduria and symptoms of cystitis.65 Identifying the species is a crucial step in the treatment of Candida UTI. Several species (C. glabrata, C. krusei) are known to be resistant to fluconazole. Species identification and antifungal sensitivities should be done and therapy directed after obtaining these results.55
What is the recommended treatment for Candida pyelonephritis?
Treatment for pyelonephritis caused by fluconazole-susceptible Candida species is oral fluconazole 200 to 400 mg (3-6 mg/kg) for a total of 2 weeks.65 A fluconazole-resistant organism should be suspected when a non-albicans Candida species is isolated, such as C. krusei or C. glabrata. In this circumstance, in vitro antifungal susceptibility testing should be done. Echinocandins are not a good option in this situation because they do not reach adequate urine concentration and treatment failure is well documented.66-68 Amphotericin B deoxycholate (AmB) 0.3 to 0.6 mg/kg daily for 1 to 7 days, with or without oral flucytosine (25 mg/kg) 4 times daily, is recommended by the IDSA for the treatment of fluconazole-resistant isolates of C. glabrata and C. krusei.65 Further imaging with ultrasound, CT, or magnetic resonance should be done to rule out urinary tract obstruction and/or “fungus ball” formation. Emphysematous pyelonephritis and necrosis can occur and usually require nephrectomy. Perinephric abscess will need drainage, which can be accomplished through interventional radiological techniques.55
If a “fungus ball” is suspected in the kidney, how does the management change in a patient with Candida pyelonephritis?
A fungus ball must be treated with both antifungals and surgical intervention. Antifungal therapy should be continued during the surgical removal process to avoid fungemia. Interventional radiology should be consulted and is usually the best option for removal. Fungus ball(s) can and often do cause urinary obstruction. Temporary nephrostomy tube placement may be warranted in these situations to relieve the obstruction.55,65 AmB can be infused through the nephrostomy tube to increase local concentrations. This route of administration is not known to be nephrotoxic.55 Fluconazole infusion through a nephrostomy tube has also been used in the successful treatment of a fungus ball.69
Summary
Health care–associated UTIs are the most common nosocomial infection in the United States. UC placement and genitourinary manipulation or instrumentation play a major role in the development of CA-UTI. Clinicians should be aware of the appropriate and inappropriate use of UCs and their association with CA-UTI development. Removal of a UC when no longer necessary is key in prevention of CA-UTI. Treatment of asymptomatic bacteriuria is generally not indicated. A multidisciplinary approach is essential when managing chronic indwelling UCs in SCI. PCN and ureteral stenting need close monitoring, and early removal should be performed if infection is suspected.
Candiduria is an emerging nosocomial source of UTI but rarely leads to symptoms unless related to an ascending process. Proper urine collection is crucial in determining whether you are dealing with contamination, colonization, or infection. If candiduria persists in an asymptomatic individual, then further investigations should be done in regards to possible predisposing risks factors. Fluconazole is recommended for treatment of most patients with Candida UTI, while intravenous AmB is the treatment of choice for fluconazole-resistant Candida species. As we continue to take an evidence-based approach to the prevention and management of health care-associated UTI, we will likely see continued improvement in patient outcomes and overall decreased rates of infection.
Corresponding author: Norman Beatty, MD, 1501 N. Campbell Ave., Tucson, AZ 85724; [email protected].
Financial disclosures: None.
From the University of Arizona College of Medicine, Tucson, AZ (Dr. Beatty), and the Baylor College of Medicine, Houston, TX (Dr. Mohajer).
Abstract
- Objective: To review management issues regarding health care–associated urinary tract infections (UTIs) commonly encountered by practicing clinicians.
- Methods: Review of the literature.
- Results: Because urinary catheter (UC) placement plays a major role in the development of catheter-associated UTIs (CA-UTI), clinicians should be aware of the appropriate and inappropriate uses of UCs and their association with CA-UTI development. Removal of a UC when no longer necessary is key to preventing CA-UTI. Treatment of asymptomatic bacteriuria is generally not indicated. Percutaneous nephrostomy and ureteral stenting need close monitoring, and early removal should be performed if infection is suspected. Candiduria rarely leads to symptoms unless it is related to an ascending process. Proper urine collection is crucial in determining whether contamination, colonization, or infection is present. Fluconazole is recommended in most cases of Candida UTI, while intravenous amphotericin B is recommended for fluconazole-resistant Candida species.
- Conclusion: Continued use of evidence-based strategies for preventing and managing health care–associated UTI should lead to further improvements in patient outcomes and overall decreased rates of infection.
Keywords: bacteriuria; catheter-associated UTI; catheterization; percutaneous nephrostomy; candiduria.
Health care–associated urinary tract infections (UTIs) are estimated to be the most common adverse infectious event in U.S. hospitals, occurring in 1 of 10 admitted patients.1-3 Approximately 32% of all health care–associated infections are UTIs.1 Furthermore, urinary catheters (UCs) are associated with 8% to 21% of health care–associated infections that occur in the intensive care unit.4 The most important predisposing factor for nosocomial UTI is urinary catheterization.5 Genitourinary manipulation and/or implementation also play a major role in the development of nosocomial UTIs.
In 2008, the U.S. Centers for Medicare & Medicaid Services instituted a new policy that reduced reimbursement rates for hospitalizations linked to health care–associated infections.6 Indwelling UCs are among the most overused health care devices in the hospital setting. They are placed in an estimated 15% to 25% of all hospitalized patients,7,8 and are often inserted in the emergency department (ED) without a physician order or appropriate indication.9 Intermittent straight catheterization, male or female condom catheterization, and/or placement of an indwelling UC are the most common causes of catheter-associated asymptomatic bacteriuria (CA-ASB) and catheter-associated UTIs (CA-UTI).5 Prevention and management of CA-ASB and CA-UTI can be challenging and require an evidence-based approach. Furthermore, guidelines for the management of UTIs in the setting of active percutaneous nephrostomy (PCN) drainage and/or ureteral stenting are not established.5 This may leave clinicians with little mainstream data to aid in management decisions.
In 2009 the Centers for Disease Control and Prevention provided a guideline for the appropriate and inappropriate use of indwelling UCs to help promote their proper use.10 In the time since the guideline’s initiatives were instituted around the United States, published data have shown some improvement in the use of UCs,11,12 but other recent reports indicate that rates of UC use have remained unchanged.13 This review discusses management issues regarding health care–associated UTIs that are commonly encountered by practicing clinicians, with a focus on current guidelines and evidence.
Catheter-Associated UTI
CA-UTI is defined as the presence of signs or symptoms of UTI with no other explainable infectious source along with ≥ 1000 colony-forming units (cfu) of ≥ 1 bacterial species per milliliter in a urine specimen from a catheter that has been changed within 48 hours of collection of the urine specimen.5 Signs and symptoms of CA-UTI include, but are not limited to: new-onset or worsening fever, chills, altered sensorium from baseline, lethargy, malaise, flank pain, pelvic pain, costovertebral angle tenderness, and acute hematuria.5 New-onset “foul-smelling” (odorous)urine and “cloudy” urine are neither sensitive nor specific when assessing for CA-UTI, and do not have significant clinical relevance when found alone.14,15 Patients who have removed or exchanged the UC during this event and then experience dysuria, increased frequency, urgency, or suprapubic pain are likely having symptoms of CA-UTI.5
What is the recommended method for collecting urine samples when CA-UTI is suspected?
In a patient with an indwelling catheter that has been in place for more than 2 weeks at the onset of a suspected CA-UTI, the catheter should be replaced (if still indicated) or removed to accelerate resolution of symptoms and to reduce the risk of subsequent catheter-associated bacteriuria and CA-UTI. The urine culture should be acquired from the freshly placed UC.5
When should a patient be empirically treated?
A patient presenting with evidence of sepsis should be empirically treated with antimicrobials. Empiric coverage should be based on risk factors for multidrug-resistant organisms and data pertaining to local antimicrobial resistance patterns. A urine specimen for urinalysis and possible culture should be sent prior to administering empiric antibiotics (if possible) in a symptomatic patient.5
What bacteria are commonly associated with CA-UTI?
The bacteria most commonly associated with CA-UTI are found in or around the gastrointestinal and genitourinary tracts and also are part of the normal skin flora. The introduction and/or facilitated ascension of these microorganisms is believed to occur during UC insertion.16,17 Two-thirds of all isolated uropathogens in those with indwelling UCs are extraluminally acquired (via ascension along the catheter-urethral mucosa interface), and one-third are believed to be intraluminally acquired.18
The most commonly isolated bacteria in CA-UTI are Enterobacteriaceae, which include Escherichia coli (most common), Klebsiella species (K. oxytoca, K. pneumoniae), Serratia species (S. marcescens), Citrobacter species (C. koseri), Enterobacter species (E. cloacae), and Proteus species; non-Enterobacteriaceae such as Pseudomonas species; and gram-positive cocci, which include coagulase-negative staphylococci (S. saprophyticus), Staphylococcus aureus, group B streptococci, and Enterococcus species (E. faecalis, E. faecium).19-21 Coagulase-negative staphylococci and Enterococcus species can lead to CA-UTI but are usually avirulent and more commonly isolated from asymptomatic individuals.19 Also, coagulase-negative staphylococci such as S. epidermidis and S. lugdunensis are usually the manifestation of contamination during the collection process and their presence should prompt a repeat sample collection under sterile techniques. Monomicrobial infection is usually seen in those with short-term catheter use and CA-UTI. In contrast, polymicrobial infection is more common in those with long-term indwelling UCs and CA-UTI.19 Providencia stuartii, Proteus mirabilis, S. aureus, and Morganella morganii have all been associated with CA-UTI in those with long-term indwelling UCs.
Growth of S. aureus in the urine should prompt further investigation with blood cultures to explore the possibility of hematogenous dissemination to the urinary tract. Organisms leading to bacteremia due to CA-UTI are most commonly gram-negative bacilli (E. coli, Klebsiella species, Pseudomonas aeruginosa) and E. faecalis.21
What is the difference between CA-ASB and CA-UTI?
CA-ASB is defined as the presence of ≥ 1 bacteria species growing on urine culture at ≥ 100,000 cfu/mL in a patient with a history of urinary catheterization and/or indwelling UC who lacks signs or symptoms of UTI. In a man with a condom catheter, CA-ASB is defined using the same criteria, but the urine sample is collected after a fresh condom catheter is applied.5 The difference between CA-ASB and CA-UTI is simply the presence or absence of signs and symptoms related to UTI. Currently, there is no standard definition for significant bacteriuria in a catheterized patient.5 Pyuria found on urinalysis is indicative of genitourinary inflammation and can be present in both CA-ASB and CA-UTI. The absence, presence, and/or degree of pyuria in catheterized patients does not accurately differentiate between CA-ASB and CA-UTI.5,22,23 On the other hand, the absence of pyuria in a symptomatic catheterized patient suggests an etiology other than CA-UTI.5
How can CA-UTI be prevented in patients with a short-term indwelling urinary catheter?
If a short-term UC is essential, the most important approach to preventing CA-UTI is limiting the duration of time it will be used. Strategies such as computer-based order entry and care maps with automated discontinuation of UCs have been shown to decrease catheter usage.19 Using closed-systems for UC collection with ports in the distal catheter for needle aspiration of urine has also been shown to decrease the incidence of CA-UTI.5 Securing the UC to avoid urethral trauma, aseptic techniques for insertion and repositioning, and placement of the tubing and collection bag below the level of the bladder to prevent reflux will likely also prevent CA-UTI, but these strategies have not been evaluated thoroughly.19
When should you screen for and treat CA-ASB?
The 2009 Infectious Diseases Society of America (IDSA) guidelines recommend that the only patients who should be screened and treated for CA-ASB are pregnant women and those who will undergo a potentially traumatic urologic procedure for which mucosal breaching may occur, causing bleeding. Routinely screening or treating patients for CA-ASB in not recommended in any other group of patients and will lead to unnecessary antibiotic use and antibiotic resistance.5
UTI Associated with Percutaneous Nephrostomy and Ureteral Stenting
Similar systemic symptoms of infection (fever, rigors, malaise, shock) are present in patients with and without percutaneous nephrostomy (PCN) and/or ureteral stent placement. Dysuria is not commonly present in those with PCN. The first signs of CA-UTI may be decreased urine output and pericatheter leakage due to an obstructive process resulting from the encrustation.24-27 The most common complaint among patients with either acute or chronic ureteral stenting is discomfort, which has been described as “urinary symptoms” and “body pain.”28 This discomfort can be related to ureteral hyperperistalsis after placement of the stent and is usually self-limiting. Ureteral stent migration, usually at the distal end, can also lead to discomfort, but is easily rectified with cystoscopy.25 Body pain and/or urinary symptoms in the setting of ureteral stenting are not indicative of infection alone.
What is urinary catheter and/or ureteral stent encrustation?
Encrustation is the formation of a conditioning film that develops on the surface of the UC or ureteral stent. The exact mechanism is not well understood, but it is believed to involve electrostatic interactions of urinary proteins that stimulate binding onto the stent or UC surface.25 Encrustation increases exponentially with the dwell time. Among patients with ureteral stents placed due to urolithiasis, encrustation occurred in 9.2% of stents removed prior to 6 weeks, 47.5% of stents removed at 6 weeks, and 76.3% of stents removed at 12 weeks.26 Encrustation is most common at the proximal and distal ends (pigtails) of the ureteral stent and usually spares or presents last within the lumen.29 Attempts have been made to prevent ureteral stent encrustation through the development of biodegradable, drug-eluting, and tissue-engineered substrates. These developments are promising, but currently there is limited observational data from large randomized trials to suggest that these new modalities decrease rates of encrustation.25 Encrustation is highly associated with certain microorganisms, especially those that create biofilms.30 Urease-producing bacteria, most commonly P. mirabilis, play a role in encrustation formation.31 Bacteria most commonly associated with encrustation include Proteus species (P. mirabilis is most common), P. aeruginosa, K. pneumoniae, Providencia species (P. stuartii is most common), and M. morganii.
Does ureteral stent bacterial colonization correlate with UTI?
Ureteral stent colonization with bacteria increases with dwell time and is found in 40% to 98.5% of stents placed.32-35 If UTI is suspected in a patient with an active indwelling ureteral stent, a sample of urine should be cultured while the stent is in place.25 Typically, genitourinary and normal skin flora pathogens are found when the ureteral stent is cultured. The top 3 organisms cultured from ureteral stents are S. aureus, P. aeruginosa, and E. faecalis.34 Urine culture usually does not correlate with stent culture results, which has brought up the debate of how bacterial colonization occurs. It has been postulated that colonization is actually a manifestation of contamination during the insertion procedure, but this has yet to be validated.25 In patients with symptoms of UTI in the setting of an indwelling ureteral stent, a positive culture has low sensitivity, with estimates between 21% and 40%.35 Therefore, a negative urine culture does not rule out UTI alone in a symptomatic patient. Multiple studies have suggested that colonization of the ureteral stent does not correlate strongly with developing a UTI.25,32-34
How can UTI be prevented in those receiving PCN or ureteral stent placement?
Antibiotic prophylaxis has been recommended to prevent UTI in patients who will undergo PCN or ureteral stent placement. The American Urological Association recommends empiric treatment even in the absence of signs and symptoms of UTI,36 but substantial evidence is lacking that this approach prevents infection.37 Ciprofloxacin or trimethoprim/sulfamethoxazole has been recommended by some for empiric coverage for enteric gram-negative bacilli and enterococcus in those undergoing genitourinary manipulation or instrumentation.32,38 Most patients who develop CA-UTI and pyelonephritis do so within the first 2 to 6 weeks after placement.37,39 Bacteriuria, candiduria, and/or pyuria are present in all patients approximately within 9 weeks even when sterile urine is confirmed prior to PCN placement.39 Data on the effectiveness of antibiotic prophylaxis to prevent CA-UTI in those with PCN or ureteral stenting is limited. Currently, there are no recommendations from the IDSA on how to prevent infection in these situations.5 Early or frequent stent removal or exchanges has been proven to reduce UTI in those with ureteral stenting.33 Patients with diabetes mellitus and chronic renal failure are at high-risk for UTI when ureteral stents are in place. This population should undergo close monitoring for UTI development and may warrant more frequent stent exchanges.27,40
What is the treatment of CA-UTI associated with PCN and/or ureteral stenting?
The IDSA guidelines do not apply to patients with PCN and/or ureteral stenting.5 There is no treatment protocol for UTI related to these processes. Generally speaking, they are considered “complicated UTI” by most experts. Broad-spectrum, empiric antibiotic administration along with prompt removal of the PCN and/or ureteral stent is the gold standard of therapy.27 The recommended duration of targeted antibiotic therapy is generally between 5 and 14 days.19 Most clinicians will treat this complicated UTI for at least 10 to 14 days. Antibiotic administration should be continued even after removal of the catheter and/or stent to complete the full course. Repeat urinalysis and culture is not indicated at the end of therapy if the patient is clinically improving or has remission of symptoms.
What is the exchange rate for those who require chronic PCN and/or ureteral stent use?
On average a PCN or ureteral stent should be exchanged every 2 to 3 months in patients who require chronic usage.24,27 Some patients with persistent complications may require more frequent exchanges (< 10 weeks).27 Encrustation and bacterial colonization become more prevalent the longer the devices are in place. This process is estimated to begin within the first 2 weeks after placement.27,33,34 A “forgotten stent” is one that has been left in place after the patient is lost to follow-up. This unfortunate event can lead to massive encrustation, UTI, stent fracturing, and complete ureteral obstruction.24 As noted, patients with diabetes mellitus, chronic renal failure, and frequent UTI may warrant more frequent exchanges, but this should be determined on a case-by-case basis.
Catheter-Associated UTI in Patients with Spinal Cord Injury
Spinal cord injury (SCI) at any level can cause neurogenic bladder. This process ultimately leads to urinary stasis and colonization of the bladder with bacteria. According to the IDSA, the acceptable indications for UC insertion are: clinically significant urinary retention (if medical therapy is not effective), urinary incontinence, accurate urine output monitoring required, and patient is unable and/or unwilling to collect urine (Table 1).5 Recently, further guidelines were published regarding appropriate and inappropriate indwelling UC placement in hospitalized medical patients (Table 2 and Table 3), expanding upon the earlier acceptable criteria provided by the IDSA.41 According to the IDSA and Ann Arbor Criteria for Appropriate Urinary Catheter Use, patients with SCI and subsequent neurogenic bladder without obstruction where intermittent bladder straight catheterization for the drainage of urine is not feasible will likely need an indwelling UC.41 SCI patients often experience decubitus ulcers, and an indwelling UC can be used if needed to help with wound healing if other urinary management alternatives have been attempted. Other such situations in which an indwelling UC can used before attempting alternative approaches would be in a patient who is actively dying and is pursuing comfort care and/or hospice.41
Which indwelling UCs should be used in patients with SCI?
Efforts to reduce the likelihood of infection in patients with SCI have led to several advances in the design and manufacturing of UCs. UCs are made of either latex, plastic, silicone, or polytetrafluoroethylene (Teflon). None of these substrates is free of complications, but of them latex UCs have been studied the most in regards to their associated complications. Aside from being allergenic in nature, latex UCs have an increased propensity to allow bacteria to adhere to their surface due to microscopic planes of unevenness.42 Silicone UCs are less frequently associated with infection but are more rigid, leading to increased discomfort.43,44 Hydrophilic and silver-hydrogel coatings are innovative methods that have been developed to increase comfort and reduce the likelihood of infection. Hydrophilic-coated UCs are associated with reduced microbial adherence, decreased encrustation, and better patient satisfaction.45,46 In SCI patients, these UCs have demonstrated lower complication rates, including UTI; fewer episodes of post-, intra-, and inter-catheterization bleeding; and decreased rates of antibiotic-resistant bacteria.45,46 Silver-hydrogel-coated UCs are less well studied but have also demonstrated reduced UTI rates in SCI patients; however, their efficacy over the long term has yet to be determined. Antibiotic-impregnated UCs are not currently recommended for either short- or long-term indwelling UC use.5
How can CA-UTI be prevented in a patient who will require a long-term indwelling catheter?
At this time, the data is insufficient to make a recommendation on routine UC exchange (eg, every 2 to 4 weeks) in patients who require long-term indwelling urethral or suprapubic catheters in an attempt to reduce the risk of CA-ASB or CA-UTI. This is also true for those who experience even repeated early catheter blockage from encrustation.5 Thus, the rate at which these exchanges occur can be controversial, but typically around every 4 weeks is a common approach. Some would argue that if the patient has repeated CA-UTI, an exchange rate of every 2 weeks might be needed, but data is currently lacking to support this practice.5 If intermittent urinary catheterization is feasible, it should be done at least every 6 hours and before bedtime. In general, when the volume of urine in the bladder reaches approximately 400 mL, the patient should undergo bladder catheterization to prevent stasis and infection.47 A closed drainage system is recommended in all patients who require long-term indwelling UC use.48,49 Placement of the collection bag above the catheter or above the level of the bladder and a breach in the closed drainage system have been shown to result in higher rates of catheter-associated bacteriuria.48,50 Proper hand hygiene and sterile and/or clean techniques should be used when placing or exchanging a UC. However, in one study there was no difference in bacteremia or UTIs when using sterile versus clean techniques.51
Asymptomatic bacteriuria should not be treated in patients with long-term indwelling UCs, and prophylactic antibiotics have led to the emergence of resistance.52 At this time it is not clear whether prophylactic weekly oral cyclic antibiotic administration can effectively reduce the frequency of CA-UTI in patients with SCI and chronic indwelling UC use.
What are the signs and symptoms of CA-UTI in a patient with SCI?
Subjective and objective findings may be limited when a patient with SCI presents with CA-UTI. These patients often lack the usual symptoms of UTI (dysuria, suprapubic discomfort, urgency, increased frequency) and pyelonephritis (flank pain, costovertebral angle tenderness). Caregivers and health care providers should be aware that nonspecific symptoms such as foul-smelling urine, pyuria, increased residual volume of urine in the bladder, change in voiding habits, worsening detrusor spasticity, and aggravation of autonomic dysreflexia can be the only initial presenting symptoms.53
What is the duration of therapy for CA-UTI in SCI, and how can antibiotic stewardship principles be applied in this patient population?
Antibiotic therapy is indicated for a duration of 7 days if there is prompt resolution of symptoms, or for a total of 10 to 14 days if the response is delayed, regardless of whether the patient remains with a UC.5 Antibiotic stewardship is very important to reduce the risk for developing drug resistance in this high-risk population. Methods such as prescriber education practices, institution protocols, guideline implementation, auditing and feedback, restriction and reauthorization practices, computer-assisted programs, de-escalation or streamlining, and antibiotic cycling or dosage optimization have all been shown to assist in antibiotic stewardship in UTIs.54
Candida UTI
What is the initial evaluation for a patient with candiduria?
The workup for candiduria hinges on determining whether the candiduria likely represents contamination, colonization, or infection; certain predisposing risk factors are associated with Candida UTI (Table 4).55-57 The most important aspect to candiduria is the patient’s clinical status and comorbid conditions.58 Among funguria, Candida species are the most common and represent 95% of organisms isolated on urine cultures (Table 5).59 Candiduria is usually present in those with significant comorbidities and rarely is associated with healthy individuals.59,60 Candiduria is increasing in prevalence among hospitalized patients, representing 22% to 40% of all nosocomial UTIs.59,60 Markers in the urine (leukocyte esterase, colony count of culture growth, presence or absence of Candida casts and pseudohyphae) cannot alone differentiate colonization from infection.55 When candiduria is discovered in a patient with symptoms related to UTI in the setting of predisposing risk factors, it should be considered a real infection until proven otherwise.
In a situation where an asymptomatic patient without an indwelling UC has Candida species isolated from a urine culture, a repeat culture (clean-catch midstream sample) should be performed to assess for a likely contaminated collection.55 If the patient has an indwelling UC then it should be exchanged and urine collected from the fresh catheter.61 When candiduria is found in healthy asymptomatic adults, it is most commonly associated with poor collection techniques or postcollection contamination.59 If candiduria persists in an asymptomatic patient, the patient should be assessed for predisposing factors. This includes checking hemoglobin A1C for developing diabetes and renal ultrasound looking for urolithiasis, renal abscess, hydronephrosis, and fungus ball. Postvoid residual urinary retention should also be ruled out with bladder ultrasound. Treatment of predisposing factors can lead to resolution of candiduria without antifungal treatment, and a urine culture should be repeated (1 to 2 weeks later).61 Asymptomatic patients lacking any predisposing factors can be observed with repeat urine cultures in 1 to 3 months.61
Candiduria may actually represent candidemia in those patients who have a predisposing risk factor for disseminated candidiasis. These risk factors include central venous catheters, administration of total parental nutrition, antibiotic use (especially broad spectrum), critical illness, recent surgical intervention (especially intra-abdominal), acute renal failure, nasogastric tube use, and active gastric acid suppression (ie, proton pump inhibitors).62,63 The hematogenous spread of Candida can lead to the detection of candiduria in 46% to 80% of persons who are experiencing candidemia.59 If the patient is at risk for candidemia, then blood fungal cultures should be drawn. It is not unreasonable to also order a serum-D-glucan assay if suspicions are high. A thorough skin assessment should be completed and ophthalmology consulted for a detailed eye exam in the event that the patient has candidemia. Candiduria is highly prevalent among those who are candidemic, but overall candidemia is encountered in less than 5% of patients in most intensive care units.59 Thus, most patients with candiduria do not have disseminated candidiasis.
Candiduria rarely leads to symptoms of UTI,58 unless the pathogenesis is related to an ascending process.56 Symptoms of Candida UTI are no different from those experienced from a bacterial etiology. Some patients may complain of pneumaturia and/or endorse seeing particulate matter in their urine.55 Patients showing signs of sepsis (fever, chills, flank pain) should be investigated for possible Candida pyelonephritis in the setting of candiduria.64
When should asymptomatic candiduria be treated?
In adult patients with asymptomatic candiduria, there are 2 situations in which antifungal therapy is recommended. A patient undergoing a traumatic urologic procedure would be treated to avoid the risk for candidemia caused by the procedure. Also, in neutropenic patients empiric antifungal therapy should be administered because there is a high likelihood that this candiduria may actually represent hematogenous spread from candidemia.61,65
What is the treatment for symptomatic Candida cystitis?
Empiric treatment with oral fluconazole 200 to 400 mg daily for a total of 2 weeks is recommended in patients with persisting candiduria and symptoms of cystitis.65 Identifying the species is a crucial step in the treatment of Candida UTI. Several species (C. glabrata, C. krusei) are known to be resistant to fluconazole. Species identification and antifungal sensitivities should be done and therapy directed after obtaining these results.55
What is the recommended treatment for Candida pyelonephritis?
Treatment for pyelonephritis caused by fluconazole-susceptible Candida species is oral fluconazole 200 to 400 mg (3-6 mg/kg) for a total of 2 weeks.65 A fluconazole-resistant organism should be suspected when a non-albicans Candida species is isolated, such as C. krusei or C. glabrata. In this circumstance, in vitro antifungal susceptibility testing should be done. Echinocandins are not a good option in this situation because they do not reach adequate urine concentration and treatment failure is well documented.66-68 Amphotericin B deoxycholate (AmB) 0.3 to 0.6 mg/kg daily for 1 to 7 days, with or without oral flucytosine (25 mg/kg) 4 times daily, is recommended by the IDSA for the treatment of fluconazole-resistant isolates of C. glabrata and C. krusei.65 Further imaging with ultrasound, CT, or magnetic resonance should be done to rule out urinary tract obstruction and/or “fungus ball” formation. Emphysematous pyelonephritis and necrosis can occur and usually require nephrectomy. Perinephric abscess will need drainage, which can be accomplished through interventional radiological techniques.55
If a “fungus ball” is suspected in the kidney, how does the management change in a patient with Candida pyelonephritis?
A fungus ball must be treated with both antifungals and surgical intervention. Antifungal therapy should be continued during the surgical removal process to avoid fungemia. Interventional radiology should be consulted and is usually the best option for removal. Fungus ball(s) can and often do cause urinary obstruction. Temporary nephrostomy tube placement may be warranted in these situations to relieve the obstruction.55,65 AmB can be infused through the nephrostomy tube to increase local concentrations. This route of administration is not known to be nephrotoxic.55 Fluconazole infusion through a nephrostomy tube has also been used in the successful treatment of a fungus ball.69
Summary
Health care–associated UTIs are the most common nosocomial infection in the United States. UC placement and genitourinary manipulation or instrumentation play a major role in the development of CA-UTI. Clinicians should be aware of the appropriate and inappropriate use of UCs and their association with CA-UTI development. Removal of a UC when no longer necessary is key in prevention of CA-UTI. Treatment of asymptomatic bacteriuria is generally not indicated. A multidisciplinary approach is essential when managing chronic indwelling UCs in SCI. PCN and ureteral stenting need close monitoring, and early removal should be performed if infection is suspected.
Candiduria is an emerging nosocomial source of UTI but rarely leads to symptoms unless related to an ascending process. Proper urine collection is crucial in determining whether you are dealing with contamination, colonization, or infection. If candiduria persists in an asymptomatic individual, then further investigations should be done in regards to possible predisposing risks factors. Fluconazole is recommended for treatment of most patients with Candida UTI, while intravenous AmB is the treatment of choice for fluconazole-resistant Candida species. As we continue to take an evidence-based approach to the prevention and management of health care-associated UTI, we will likely see continued improvement in patient outcomes and overall decreased rates of infection.
Corresponding author: Norman Beatty, MD, 1501 N. Campbell Ave., Tucson, AZ 85724; [email protected].
Financial disclosures: None.
1. Klevens RM, Edwards JR, Richards CL Jr. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122:160-166.
2. Burke JP. Infection control—a problem for patient safety. N Engl J Med. 2003;348:651-656.
3. Scott RD. The direct medical costs of healthcare-associated infections in U.S. hospitals and the benefits of prevention. Division of Healthcare Quality Promotion; National Center for Preparedness Detection and Control of Infectious Diseases; Coordinating Center for Infectious Diseases; Centers for Disease Control and Prevention. March 2009. Contract No.: CS200891-A. www.cdc.gov/HAI/pdfs/hai/Scott_CostPaper.pdf.
4. Eriksen HM, Iverson BG, Aavitsland P. Prevalence of nosocomial infections in hospitals in Norway, 2002 and 2003. J Hosp Infect. 2005;60:40-45.
5. Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:625-663.
6. Mattie AS, Webster BL. Centers for Medicare and Medicaid Services’ “never events”: an analysis and recommendations to hospitals. Health Care Manag. 2008;27:338-349.
7. Warren JW. Catheter-associated urinary tract infections. Int J Antimicrob Agents. 2001;17:299-303.
8. Weinstein JW, Mazon D, Pantelick E, et al. A decade of prevalence surveys in a tertiary-care center: trends in nosocomial infection rates, device utilization, and patient acuity. Infect Control Hosp Epidemiol. 1999;20:543-548.
9. Fakih MG, Pena ME, Shemes S, et al. Effect of establishing guidelines on appropriate urinary catheter placement. Acad Emerg Med. 2010;17:337-340.
10. Gould CV, Umscheid CA, Agarwal RK, et al. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31:319-326.
11. Fakih MG, Rey JE, Pena ME, et al. Sustained reductions in urinary catheter use over 5 years: bedside nurses view themselves responsible for evaluation of catheter necessity. Am J Infect Control. 2013;41:236-239.
12. Meddings J, Rogers MA, Krein SL, et al. Reducing unnecessary urinary catheter use and other strategies to prevent catheter-associated urinary tract infections: an integrative review. BMJ Qual Saf. 2014;23:277-289.
13. Gould C. Catheter-associated urinary tract infection: the national perspective. In: Essential Hospitals Engagement Network. Patient Harm Series II: new tools to prevent CAUTI webinar. April 16, 2014. http://bit.ly/1UWndRA.
14. Nicolle LE. Consequences of asymptomatic bacteriuria in the elderly. Int J Antimicrob Agents. 1994;4:107-111.
15. Nicolle LE. Urinary tract infections in long-term-care facilities. Infect Control Hosp Epidemiol. 2001;22:167-175
16. Cohen A. A microbiological comparison of a povidone-iodine lubricating gel and a control as catheter lubricants. J Hosp Infect. 1985;6(Suppl A):155-161.
17. Daifuku R, Stamm WE. Bacterial adherence to bladder uroepithelial cells in catheter-associated urinary tract infection. N Engl J Med. 1986;314:1208-1213.
18. Tambyah PA, Halvorson KT, Maki DG. A prospective study of pathogenesis of catheter-associated urinary tract infections. Mayo Clin Proc. 1999;74:131-136.
19. Nicolle LE. Catheter-related urinary tract infection. Drugs Aging. 2005;22:627-639.
20. Redder JD, Leth RA, Møller JK. Incidence rates of hospital-acquired urinary tract and bloodstream infections generated by automated compilation of electronically available healthcare data. J Hosp Infect. 2015;91:231-236.
21. Ortega M, Marco F, Soriano A, et al. Epidemiology and prognostic determinants of bacteraemic catheter-acquired urinary tract infection in a single institution from 1991 to 2010. J Infect. 2013;67:282-287.
22. Tambyah PA, Maki DG. The relationship between pyuria and infection in patients with indwelling urinary catheters: a prospective study of 761 patients. Arch Intern Med. 2000;160:673-677.
23. Musher DM, Thorsteinsson SB, Airola VM, II. Quantitative urinalysis:diagnosing urinary tract infection in men. JAMA. 1976;236:2069-2072.
24. Hausegger KA, Portugaller HR. Percutaneous nephrostomy and antegrade ureteral stenting: technique-indications-complications. Eur Radiol. 2006;16:2016-2030.
25. Lange D, Bidnur S, Hoag N, et al. Ureteral stent-associated complications--where we are and where we are going. Nat Rev Urol. 2015;12:17-25.
26. el-Faqih SR, Shamsuddin AB, Chakrabarti A, et al. Polyurethane internal ureteral stents in treatment of stone patients: morbidity related to indwelling times. J Urol. 1991;146:1487-1491.
27. Adamo R, Saad WE, Brown DB. Management of nephrostomy drains and ureteral stents. Tech Vasc Interv Radiol. 2009;12:193-204.
28. Joshi HB, Newns N, Stainthorpe A, et al. Ureteral Stent Symptom Questionnaire: development and validation of a multidimensional quality of life measure. J Urol. 2003;169:1060-1064.
29. Singh I, Gupta NP, Hemal AK, et al. Severely encrusted polyurethane ureteral stents: management and analysis of potential risk factors. Urology. 2001;58:526-531.
30. Saint S, Chenoweth CE. Biofilms and catheter-associated urinary tract infections. Infect Dis Clin North Am. 2003;17:411-432.
31. Mobley HL, Warren JW. Urease-positive bacteriuria and obstruction of long-term urinary catheters. J Clin Microbiol. 1987;25:2216-2217.
32. Kehinde EO, Rotimi VO, Al-Hunayan A, et al. Bacteriology of urinary tract infection associated with indwelling J ureteral stents. J Endourol. 2004;18:891-896.
33. Paick SH, Park HK, Oh SJ, Kim HH. Characteristics of bacterial colonization and urinary tract infection after indwelling of double-J ureteral stent. Urology. 2003;62:214-217.
34. Klis R, Korczak-Kozakiewicz E, Denys A, et al. Relationship between urinary tract infection and self-retaining double-J catheter colonization. J Endourol. 2009;23:1015-1019.
35. Farsi HM, Mosli HA, Al-Zemaity M, et al. Bacteriuria and colonization of double-pigtail ureteral stents: long-term experience with 237 patients. J Endourol. 1995;9:469-472.
36. Wolfe JS Jr, Bennet CJ, Dmochowski RR, et al. Best practice policy statement on urologic surgery antimicrobial prophylaxis. J Urol. 2008;179:1379-1390.
37. Bahu R, Chaftari AM, Hachem RY, et al. Nephrostomy tube related pyelonephritis in patients with cancer: epidemiology, infection rate and risk factors. J Urol. 2013;189:130-135.
38. Bratzler DW, Dellinger EP, Olsen KM, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Surg Infect (Larchmt). 2013;14:73-156.
39. Cronan JJ, Horn DL, Marcello A, et al: Antibiotics and nephrostomy tube care: preliminary observations. Part II. Bacteremia. Radiology. 1989;172(3 Pt 2):1043-1045.
40. Akay AF, Aflay U, Gedik A, et al. Risk factors for lower urinary tract infection and bacterial stent colonization in patients with a double J ureteral stent. Int Urol Nephrol. 2007;39:95-98.
41. Meddings J, Saint S, Fowler KE, et al. The Ann Arbor criteria for appropriate urinary catheter use in hospitalized medical patients: results obtained by using the RAND/ UCLA appropriateness method. Ann Intern Med. 2015;162(9 Suppl):S1-34.
42. Stickler D, Young R, Jones G, et al. Why are Foley catheters so vulnerable to encrustation and blockage by crystalline bacterial biofilm? Urol Res. 2003;31:306-311.
43. Denstedt J, Wollin T, Reid G. Biomaterials used in urology: current issues of biocompatibility, infection, and encrustation. J Endourol. 1998;12:493-500.
44. Morris N, Stickler D, Winters C. Which indwelling urethral catheters resist encrustation by Proteus mirabilis biofilms? Br J Urol. 1997;80:58-63.
45. Cardenas D, Moore K, Dannels-McClure A, et al. Intermittent catheterization with a hydrophilic-coated catheter delays urinary tract infections in acute spinal cord injury: a prospective, randomized, multicenter trial. PMR. 2011;3:408-417.
46. Spinu A, Onose G, Daia C, et al. Intermittent catheterization in the management of post spinal cord injury (SCI) neurogenic bladder using new hydrophilic, with lubrication in close circuit devices-our own preliminary results. J Med Life. 2012;5:21-28.
47. Shekelle P, Morton S, Clark K, et al. Systematic review of risk factors for urinary tract infection in adults with spinal cord dysfunction. J Spinal Cord Med. 1998;22:258-272.
48. Siddiq D, Darouiche R. New strategies to prevent catheter-associated urinary tract infections. Nat Rev Urol. 2012;9:305-314.
49. Gould C, Umscheid C, Agarwal R, et al. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31:319-326.
50. Maki D, Tambyah P. Engineering out the risk for infection with urinary catheters. Emerg Infect Dis. 2001;7:342-347.
51. Munasinghe R, Yazdani H, Siddique M, et al. Appropriateness of use of indwelling urinary catheters in patients admitted to the medical service. Infect Control Hosp Epidemiol. 2001;22:647-649.
52. Nicolle L, Bradley S, Colgan R, et al; Infectious Diseases Society of America; American Society of Nephrology; American Geriatric Society. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40: 643-654.
53. Linsenmeyer T, Oakley A. Accuracy of individuals with spinal cord injury at predicting urinary tract infections based on their symptoms. J Spinal Cord Med. 2002;26:352-357.
54. Abbo LM, Hooton TM. Antimicrobial stewardship and urinary tract infections. Antibiotics. 2014;3:174-192.
55. Kauffman CA. Diagnosis and management of fungal urinary tract infection. Infect Dis Clin North Am. 2014;28:61-74.
56. Alvarez-Lerma F, Nolla-Salas J, Leon C, et al. Candiduria in critically ill patients admitted to intensive care medical units. Intensive Care Med. 2003;29:1069-1076.
57. Colodner R, Nuri Y, Chazan B, et al. Community-acquired and hospital-acquired candiduria: comparison of prevalence and clinical characteristics. Eur J Clin Microbiol Infect Dis. 2008;27:301-305.
58. Kauffman CA, Vazquez JA, Sobel JD, et al. Prospective multicenter surveillance study of funguria in hospitalized patients. Clin Infect Dis. 2000;30:14-18.
59. Sobel JD, Fisher JF, Kauffman CA, et al. Candida urinary tract infections—epidemiology. Clin Infect Dis. 2011;52(suppl 6): S433-436.
60. Richards MJ, Edwards JR, Culver DH, et al. Nosocomial infections in combined medical-surgical intensive care units in the United States. Infect Control Hosp Epidemiol. 2000;21:510-515.
61. Fisher JF, Sobel JD, Kauffman CA, et al. Candida urinary tract infections—treatment. Clin Infect Dis. 2011;52(suppl 6):S457-466.
62. Blumberg HM, Jarvis WR, Soucie JM, et al. Risk factors for candidal bloodstream infections in surgical intensive care unit patients: the NEMIS prospective multicenter study. Clin Infect Dis. 2001;33:177-186.
63. Puzniak LP, Teutsch S, Powderly W, et al. Has the epidemiology of nosocomial candidemia changed? Infect Control Hosp Epidemiol. 2004;25:628-633.
64. Siddique MS, Gayed N, McGuire N, et al. Salient features of Candida pyelonephritis in adults. Infect Dis Clin Pract. 1992;1:239-245
65. Pappas PG, Kauffman CA, Andes DR, et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:e1-e50.
66. Sobel JD, Bradshaw SK, Lipka CJ, et al. Caspofungin in the treatment of symptomatic candiduria. Clin Infect Dis. 2007;44:e46-9.
67. Malani AN. Failure of caspofungin for treatment of Candida glabrata candiduria. Case report and review of the literature. Infect Dis Clin Pract. 2010;18:271-272.
68. Schelenz S, Ross CN. Limitations of caspofugin in the treatment of obstructive pyelonephrosis due to Candida glabrata infection. BMC Infect Dis. 2006;56:126-130.
69. Chung BH, Chang SY, Kim SI, et al. Successfully treated renal fungal ball with continuous irrigation of fluconazole. J Urol. 2001;166:1835-1836.
1. Klevens RM, Edwards JR, Richards CL Jr. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122:160-166.
2. Burke JP. Infection control—a problem for patient safety. N Engl J Med. 2003;348:651-656.
3. Scott RD. The direct medical costs of healthcare-associated infections in U.S. hospitals and the benefits of prevention. Division of Healthcare Quality Promotion; National Center for Preparedness Detection and Control of Infectious Diseases; Coordinating Center for Infectious Diseases; Centers for Disease Control and Prevention. March 2009. Contract No.: CS200891-A. www.cdc.gov/HAI/pdfs/hai/Scott_CostPaper.pdf.
4. Eriksen HM, Iverson BG, Aavitsland P. Prevalence of nosocomial infections in hospitals in Norway, 2002 and 2003. J Hosp Infect. 2005;60:40-45.
5. Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:625-663.
6. Mattie AS, Webster BL. Centers for Medicare and Medicaid Services’ “never events”: an analysis and recommendations to hospitals. Health Care Manag. 2008;27:338-349.
7. Warren JW. Catheter-associated urinary tract infections. Int J Antimicrob Agents. 2001;17:299-303.
8. Weinstein JW, Mazon D, Pantelick E, et al. A decade of prevalence surveys in a tertiary-care center: trends in nosocomial infection rates, device utilization, and patient acuity. Infect Control Hosp Epidemiol. 1999;20:543-548.
9. Fakih MG, Pena ME, Shemes S, et al. Effect of establishing guidelines on appropriate urinary catheter placement. Acad Emerg Med. 2010;17:337-340.
10. Gould CV, Umscheid CA, Agarwal RK, et al. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31:319-326.
11. Fakih MG, Rey JE, Pena ME, et al. Sustained reductions in urinary catheter use over 5 years: bedside nurses view themselves responsible for evaluation of catheter necessity. Am J Infect Control. 2013;41:236-239.
12. Meddings J, Rogers MA, Krein SL, et al. Reducing unnecessary urinary catheter use and other strategies to prevent catheter-associated urinary tract infections: an integrative review. BMJ Qual Saf. 2014;23:277-289.
13. Gould C. Catheter-associated urinary tract infection: the national perspective. In: Essential Hospitals Engagement Network. Patient Harm Series II: new tools to prevent CAUTI webinar. April 16, 2014. http://bit.ly/1UWndRA.
14. Nicolle LE. Consequences of asymptomatic bacteriuria in the elderly. Int J Antimicrob Agents. 1994;4:107-111.
15. Nicolle LE. Urinary tract infections in long-term-care facilities. Infect Control Hosp Epidemiol. 2001;22:167-175
16. Cohen A. A microbiological comparison of a povidone-iodine lubricating gel and a control as catheter lubricants. J Hosp Infect. 1985;6(Suppl A):155-161.
17. Daifuku R, Stamm WE. Bacterial adherence to bladder uroepithelial cells in catheter-associated urinary tract infection. N Engl J Med. 1986;314:1208-1213.
18. Tambyah PA, Halvorson KT, Maki DG. A prospective study of pathogenesis of catheter-associated urinary tract infections. Mayo Clin Proc. 1999;74:131-136.
19. Nicolle LE. Catheter-related urinary tract infection. Drugs Aging. 2005;22:627-639.
20. Redder JD, Leth RA, Møller JK. Incidence rates of hospital-acquired urinary tract and bloodstream infections generated by automated compilation of electronically available healthcare data. J Hosp Infect. 2015;91:231-236.
21. Ortega M, Marco F, Soriano A, et al. Epidemiology and prognostic determinants of bacteraemic catheter-acquired urinary tract infection in a single institution from 1991 to 2010. J Infect. 2013;67:282-287.
22. Tambyah PA, Maki DG. The relationship between pyuria and infection in patients with indwelling urinary catheters: a prospective study of 761 patients. Arch Intern Med. 2000;160:673-677.
23. Musher DM, Thorsteinsson SB, Airola VM, II. Quantitative urinalysis:diagnosing urinary tract infection in men. JAMA. 1976;236:2069-2072.
24. Hausegger KA, Portugaller HR. Percutaneous nephrostomy and antegrade ureteral stenting: technique-indications-complications. Eur Radiol. 2006;16:2016-2030.
25. Lange D, Bidnur S, Hoag N, et al. Ureteral stent-associated complications--where we are and where we are going. Nat Rev Urol. 2015;12:17-25.
26. el-Faqih SR, Shamsuddin AB, Chakrabarti A, et al. Polyurethane internal ureteral stents in treatment of stone patients: morbidity related to indwelling times. J Urol. 1991;146:1487-1491.
27. Adamo R, Saad WE, Brown DB. Management of nephrostomy drains and ureteral stents. Tech Vasc Interv Radiol. 2009;12:193-204.
28. Joshi HB, Newns N, Stainthorpe A, et al. Ureteral Stent Symptom Questionnaire: development and validation of a multidimensional quality of life measure. J Urol. 2003;169:1060-1064.
29. Singh I, Gupta NP, Hemal AK, et al. Severely encrusted polyurethane ureteral stents: management and analysis of potential risk factors. Urology. 2001;58:526-531.
30. Saint S, Chenoweth CE. Biofilms and catheter-associated urinary tract infections. Infect Dis Clin North Am. 2003;17:411-432.
31. Mobley HL, Warren JW. Urease-positive bacteriuria and obstruction of long-term urinary catheters. J Clin Microbiol. 1987;25:2216-2217.
32. Kehinde EO, Rotimi VO, Al-Hunayan A, et al. Bacteriology of urinary tract infection associated with indwelling J ureteral stents. J Endourol. 2004;18:891-896.
33. Paick SH, Park HK, Oh SJ, Kim HH. Characteristics of bacterial colonization and urinary tract infection after indwelling of double-J ureteral stent. Urology. 2003;62:214-217.
34. Klis R, Korczak-Kozakiewicz E, Denys A, et al. Relationship between urinary tract infection and self-retaining double-J catheter colonization. J Endourol. 2009;23:1015-1019.
35. Farsi HM, Mosli HA, Al-Zemaity M, et al. Bacteriuria and colonization of double-pigtail ureteral stents: long-term experience with 237 patients. J Endourol. 1995;9:469-472.
36. Wolfe JS Jr, Bennet CJ, Dmochowski RR, et al. Best practice policy statement on urologic surgery antimicrobial prophylaxis. J Urol. 2008;179:1379-1390.
37. Bahu R, Chaftari AM, Hachem RY, et al. Nephrostomy tube related pyelonephritis in patients with cancer: epidemiology, infection rate and risk factors. J Urol. 2013;189:130-135.
38. Bratzler DW, Dellinger EP, Olsen KM, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Surg Infect (Larchmt). 2013;14:73-156.
39. Cronan JJ, Horn DL, Marcello A, et al: Antibiotics and nephrostomy tube care: preliminary observations. Part II. Bacteremia. Radiology. 1989;172(3 Pt 2):1043-1045.
40. Akay AF, Aflay U, Gedik A, et al. Risk factors for lower urinary tract infection and bacterial stent colonization in patients with a double J ureteral stent. Int Urol Nephrol. 2007;39:95-98.
41. Meddings J, Saint S, Fowler KE, et al. The Ann Arbor criteria for appropriate urinary catheter use in hospitalized medical patients: results obtained by using the RAND/ UCLA appropriateness method. Ann Intern Med. 2015;162(9 Suppl):S1-34.
42. Stickler D, Young R, Jones G, et al. Why are Foley catheters so vulnerable to encrustation and blockage by crystalline bacterial biofilm? Urol Res. 2003;31:306-311.
43. Denstedt J, Wollin T, Reid G. Biomaterials used in urology: current issues of biocompatibility, infection, and encrustation. J Endourol. 1998;12:493-500.
44. Morris N, Stickler D, Winters C. Which indwelling urethral catheters resist encrustation by Proteus mirabilis biofilms? Br J Urol. 1997;80:58-63.
45. Cardenas D, Moore K, Dannels-McClure A, et al. Intermittent catheterization with a hydrophilic-coated catheter delays urinary tract infections in acute spinal cord injury: a prospective, randomized, multicenter trial. PMR. 2011;3:408-417.
46. Spinu A, Onose G, Daia C, et al. Intermittent catheterization in the management of post spinal cord injury (SCI) neurogenic bladder using new hydrophilic, with lubrication in close circuit devices-our own preliminary results. J Med Life. 2012;5:21-28.
47. Shekelle P, Morton S, Clark K, et al. Systematic review of risk factors for urinary tract infection in adults with spinal cord dysfunction. J Spinal Cord Med. 1998;22:258-272.
48. Siddiq D, Darouiche R. New strategies to prevent catheter-associated urinary tract infections. Nat Rev Urol. 2012;9:305-314.
49. Gould C, Umscheid C, Agarwal R, et al. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31:319-326.
50. Maki D, Tambyah P. Engineering out the risk for infection with urinary catheters. Emerg Infect Dis. 2001;7:342-347.
51. Munasinghe R, Yazdani H, Siddique M, et al. Appropriateness of use of indwelling urinary catheters in patients admitted to the medical service. Infect Control Hosp Epidemiol. 2001;22:647-649.
52. Nicolle L, Bradley S, Colgan R, et al; Infectious Diseases Society of America; American Society of Nephrology; American Geriatric Society. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40: 643-654.
53. Linsenmeyer T, Oakley A. Accuracy of individuals with spinal cord injury at predicting urinary tract infections based on their symptoms. J Spinal Cord Med. 2002;26:352-357.
54. Abbo LM, Hooton TM. Antimicrobial stewardship and urinary tract infections. Antibiotics. 2014;3:174-192.
55. Kauffman CA. Diagnosis and management of fungal urinary tract infection. Infect Dis Clin North Am. 2014;28:61-74.
56. Alvarez-Lerma F, Nolla-Salas J, Leon C, et al. Candiduria in critically ill patients admitted to intensive care medical units. Intensive Care Med. 2003;29:1069-1076.
57. Colodner R, Nuri Y, Chazan B, et al. Community-acquired and hospital-acquired candiduria: comparison of prevalence and clinical characteristics. Eur J Clin Microbiol Infect Dis. 2008;27:301-305.
58. Kauffman CA, Vazquez JA, Sobel JD, et al. Prospective multicenter surveillance study of funguria in hospitalized patients. Clin Infect Dis. 2000;30:14-18.
59. Sobel JD, Fisher JF, Kauffman CA, et al. Candida urinary tract infections—epidemiology. Clin Infect Dis. 2011;52(suppl 6): S433-436.
60. Richards MJ, Edwards JR, Culver DH, et al. Nosocomial infections in combined medical-surgical intensive care units in the United States. Infect Control Hosp Epidemiol. 2000;21:510-515.
61. Fisher JF, Sobel JD, Kauffman CA, et al. Candida urinary tract infections—treatment. Clin Infect Dis. 2011;52(suppl 6):S457-466.
62. Blumberg HM, Jarvis WR, Soucie JM, et al. Risk factors for candidal bloodstream infections in surgical intensive care unit patients: the NEMIS prospective multicenter study. Clin Infect Dis. 2001;33:177-186.
63. Puzniak LP, Teutsch S, Powderly W, et al. Has the epidemiology of nosocomial candidemia changed? Infect Control Hosp Epidemiol. 2004;25:628-633.
64. Siddique MS, Gayed N, McGuire N, et al. Salient features of Candida pyelonephritis in adults. Infect Dis Clin Pract. 1992;1:239-245
65. Pappas PG, Kauffman CA, Andes DR, et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:e1-e50.
66. Sobel JD, Bradshaw SK, Lipka CJ, et al. Caspofungin in the treatment of symptomatic candiduria. Clin Infect Dis. 2007;44:e46-9.
67. Malani AN. Failure of caspofungin for treatment of Candida glabrata candiduria. Case report and review of the literature. Infect Dis Clin Pract. 2010;18:271-272.
68. Schelenz S, Ross CN. Limitations of caspofugin in the treatment of obstructive pyelonephrosis due to Candida glabrata infection. BMC Infect Dis. 2006;56:126-130.
69. Chung BH, Chang SY, Kim SI, et al. Successfully treated renal fungal ball with continuous irrigation of fluconazole. J Urol. 2001;166:1835-1836.
Low sexual desire: Appropriate use of testosterone in menopausal women
CASE Midlife woman with low libido causing distress
At her annual gynecologic visit, a 55-year-old woman notes that she has almost no interest in sex. In the past, her libido was good and relations were pleasurable. Since her mid-40s, she has noticed a gradual decline in libido and orgasmic response. Sexual frequency has declined from once or twice weekly to just a few times per month. She has been married for 25 years and describes the relationship as caring and strong. Her husband is healthy with a good libido; his intermittent erectile dysfunction is treated with a phosphodiesterase-5 inhibitor. The patient’s low libido is distressing, as the decline in sexual frequency is causing some conflict for the couple. She requests that her testosterone level be checked because she heard that treatment with testosterone cream will solve this problem.
Evaluating and treating low libido in menopausal women
Low libido is a very common sexual problem for women. When sexual problems are accompanied by distress, they are classified as sexual dysfunctions. Although ObGyns should discuss sexual concerns at every comprehensive visit, if the patient has no associated distress, treatment is not necessarily indicated. A woman with low libido or anorgasmia who is satisfied with her sex life and is not bothered by these issues does not require any intervention.
Currently, the only indication for testosterone therapy that is supported by clinical trial evidence is low sexual desire with associated distress, known as hypoactive sexual desire disorder (HSDD). Although other sexual problems also commonly occur in menopausal women, such as disorders of orgasm and pain, testosterone is not recommended for these problems. In addition, testosterone is not approved by the US Food and Drug Administration (FDA) for the treatment of female sexual dysfunction.
Routinely inquire about sexual functioning
Ask your patients about sexual concerns at every comprehensive visit. You can easily incorporate into the review of systems a general question, such as, “Do you have any sexual concerns?” If the patient does mention a sexual problem, schedule a separate visit (given appointment time constraints) to address it. History and physical examination information you gather during the comprehensive visit will be helpful in the subsequent problem-focused visit.
Taking a thorough history is key when addressing a patient’s sexual problems, since identifying possible etiologies guides treatment. Often, the cause of female sexual dysfunction is multifactorial and includes physiologic, psychologic, and relationship issues.
- Evidence supports low-dose transdermal testosterone in carefully selected menopausal women with HSDD and no other identifiable reason for the sexual dysfunction
- Inform women considering testosterone for HSDD of the limited effectiveness and high placebo responses seen in clinical trials
- Women also must be informed that treatment is off-label (no testosterone formulations are FDA approved for women)
- Review with patients the limitations of compounded medications, and discuss possible adverse effects of androgens. Long-term safety is unknown and, as androgens are converted to estrogens
Explore potential causes, recommend standard therapies
Common causes of low libido in menopausal women include vasomotor symptoms, insomnia, urinary incontinence, cancer or another major medical problem, weight gain, poor body image, genitourinary syndrome of menopause (GSM) with dyspareunia, fatigue, stress, aging, relationship duration, lack of novelty, relationship conflict, and a partner’s sexual problems. Other common etiologies include depression, anxiety, and substance use disorders, as well as medications used to treat these disorders, including selective serotonin reuptake inhibitors (SSRIs).
Continue to: There are many effective therapies...
There are many effective therapies for low sexual desire to consider prior to initiating a trial of testosterone, which should be considered for HSDD only if the disorder persists after addressing all other possible contributing factors (TABLE 1).
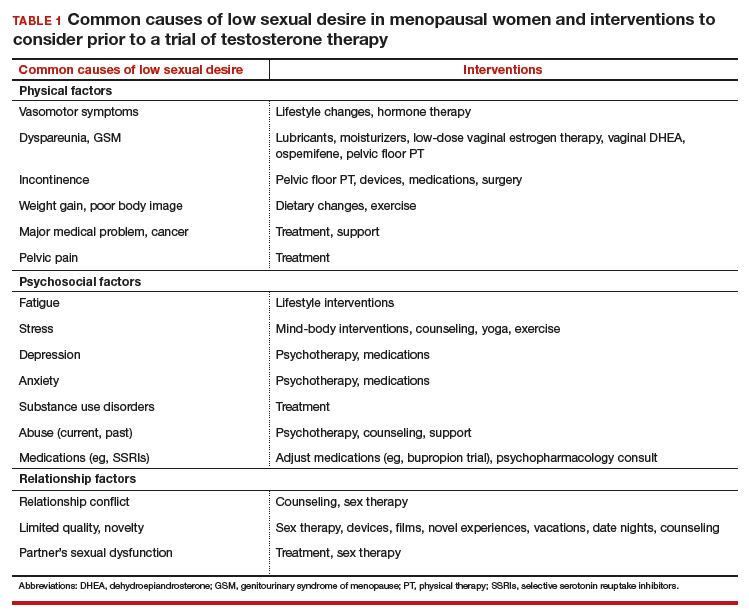
Sex therapy, for example, provides information on sexual functioning and helps improve communication and mutual pleasure and satisfaction. Strongly encourage—if not require—a consultation with a sex therapist before prescribing testosterone for low libido. Any testosterone-derived improvement in sexual functioning will be enhanced by improved communication and additional strategies to achieve mutual pleasure.
Hormone therapy. Vasomotor symptoms, with their associated sleep disruption, fatigue, and reduced quality of life (QOL), often adversely impact sexual desire. Estrogen therapy does not appear to improve libido in otherwise asymptomatic women; however, in women with bothersome vasomotor symptoms treated with estrogen, sexual interest may increase as a result of improved sleep, fatigue, and overall QOL. The benefits of systemic hormone therapy generally outweigh its risks for most healthy women younger than age 60 who have bothersome hot flashes and night sweats.1
Nonhormonal and other therapies. GSM with dyspareunia is a principal cause of sexual dysfunction in older women.2 Many safe and effective treatments are available, including low-dose vaginal estrogen therapy, nonhormonal moisturizers and lubricants, ospemifene, vaginal dehydroepiandrosterone, and pelvic floor physical therapy.3 Urinary incontinence commonly occurs in midlife women and contributes to low libido.4
Lifestyle approaches. Address fatigue and stress by having the patient adjust her work and sleep schedules, obtain help with housework and meals, and engage in mind-body interventions, counseling, or yoga. Sexual function may benefit from yoga practice, likely as a result of the patient experiencing reduced stress and enhanced body image. Improving overall health and body image with regular exercise, optimal diet, and weight management may contribute to a more satisfying sex life after the onset of menopause.
Relationship refresh. Women’s sexual interest often declines with relationship duration, and both men and women who are in new relationships generally have increased libido, affirming the importance of novelty over the long term. Couples will benefit from “date nights,” weekends away from home, and trying novel positions, locations, and times for sex. Couple’s counseling may address relationship conflict.
Expert referral. Depression, anxiety, and substance use disorders are prevalent in menopausal women and contribute to sexual dysfunction. Effective therapy is available, although some pharmacologic treatments (including SSRIs) may be an additional cause of sexual dysfunction. In addition to recommending appropriate counseling and support, referring the patient to a psychopharmacologist with expertise in managing sexual adverse effects of medications may optimize care.
Continue to: Sexual function improves, but patient still wants to try testosterone
CASE Sexual function improves, but patient still wants to try testosterone
The patient returns for follow-up visits scheduled specifically to address her sexual concerns. Sex is more comfortable and pleasurable since initiating low-dose vaginal estrogen therapy. Having been on an SSRI since her mid-40s for mild depression, the patient switched to bupropion and notes improved libido and orgasmic response. She is exercising more regularly and working with a nutritionist to address a 15-lb weight gain after menopause. The couple saw a sex therapist and is communicating better about sex with more novelty in their repertoire. They are enjoying a regular date night. Although the patient’s sex life has improved with these interventions, she is still very interested in trying testosterone.
Testosterone’s effects on HSDD in menopausal women
After addressing the many factors that contribute to sexual disinterest, a trial of testosterone may be appropriate for a menopausal woman who continues to experience low libido with associated distress.
Testosterone levels decrease with aging in both men and women. Although testosterone levels decline by approximately 50% with bilateral oophorectomy, there is no decline in androgen levels with natural menopause.5 Testosterone circulates tightly bound to sex hormone–binding globulin (SHBG), so free or active testosterone will be reduced by oral estrogens, which increase SHBG levels.6 As most menopausal women will have a low testosterone level due to aging, measuring the testosterone level does not provide information about the etiology of the sexual problem.
Although some studies have identified an association between endogenous androgen levels and sexual function, the associations are modest and are of uncertain clinical significance.7-9 Not surprisingly, other factors, such as physical and psychologic health and the quality of the relationship, often are reported as more important predictors of sexual satisfaction than androgen levels.10
While endogenous testosterone levels may not correlate with sexual function, clinical trials of carefully selected menopausal women with HSDD have shown that androgen treatment generally results in improved sexual function.11 Studies demonstrate substantial improvements in sexual desire, orgasmic response, and frequency in menopausal women treated with high doses of intramuscular testosterone, which result in supraphysiologic androgen levels.12,13 While it is interesting that women with testosterone levels in the male low range have sizeable increases in sexual desire and response, long-term use of high-dose testosterone would result in unacceptable androgenic adverse effects and risks.
Continue to: Testosterone in low doses...
Testosterone in low doses. It is more relevant to consider the impact on female sexual function of low doses of testosterone, which raise the reduced testosterone levels seen in older women to the higher levels seen in reproductive-aged women.
A series of double-blind, multicenter, randomized, placebo-controlled trials in menopausal women with HSDD examined the impact on sexual function of a transdermal testosterone patch (300 μg) that increased blood testosterone levels to the upper limit of normal for young women.14-17 In these studies, compared with placebo, women using testosterone reported significant improvements in sexual desire, arousal, orgasmic response, frequency, and sexually related distress. Findings were consistent in surgically and naturally menopausal women, with and without the use of concurrent estrogen therapy. Improvements were clinically limited, however. On average, testosterone-treated women experienced 1 to 1.5 additional satisfying sexual events in a 4-week period compared with those treated with placebo. The percentage of women reporting a clinically meaningful benefit from treatment was significantly greater in women treated with testosterone (52%) compared with the placebo-treated women (31%).18 An appreciable placebo response was seen, typical of most studies of therapies for sexual dysfunction.
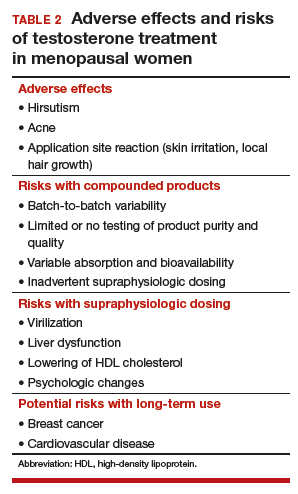
Safety concerns

Potential risks of testosterone treatment include acne, hirsutism, irreversible deepening of the voice, and adverse changes in lipids and liver function (TABLE 2).19 Adverse effects are dose dependent and are unlikely with physiologically dosed testosterone.
A 1-year study of testosterone patches in approximately 800 menopausal women with HSDD (with a subgroup of women followed for an additional year) provides the most comprehensive safety data available.17 Unwanted hair growth occurred more often in women receiving testosterone, without significant differences in blood biochemistry,hematologic parameters, carbohydrate metabolism, or lipids. Breast cancer was diagnosed in more women receiving testosterone than placebo. Although this finding may have been due to chance, the investigators concluded that long-term effects of testosterone treatment remain uncertain.
The FDA reviewed the data from the testosterone patch studies and determined that testosterone patches were effective for the treatment of HSDD in menopausal women, but more information was needed on long-term safety before approval could be granted. Another company then developed a testosterone gel product that produced similar blood levels as the testosterone patch. It was presumed that there would be similar efficacy; the principal goal of these studies was to examine long-term safety, particularly with respect to breast cancer and cardiovascular disease. Unexpectedly, although it raised testosterone blood levels to the upper limit of normal for young women, the testosterone gel product was no more effective than placebo.20 The clinical trial was ended, with safety data never published.
Continue to: Availability of testosterone formulations
Availability of testosterone formulations
Currently, no androgen therapies are FDA approved for the treatment of female sexual dysfunction. Although the best evidence regarding testosterone efficacy and safety involves the use of testosterone patches (300 μg), appropriately dosed for women, these patches are not currently available. FDA-approved testosterone patches are approved for the treatment of male hypogonadism, but use of these patches in women is not recommended since they would result in very high circulating testosterone levels.
Testosterone subcutaneous implants, pellets, and intramuscular injections also are not recommended for women because of the risk of excessive dosing. Small trials of menopausal women taking oral estrogen with low sexual desire found that oral formulations of testosterone improved libido in this study population.21 The combination of esterified estrogens (0.625 mg) and methyltestosterone (1.25 mg) is available as a compounded, non-FDA approved product. Oral androgen formulations generally are not advised, due to potential adverse effects on lipids and liver function.22
Compounded testosterone products. Ointments and creams may be compounded by prescription (TABLE 3). Product purity, dose, bioavailability, and quality typically are untested, and substantial variability exists between formulations and batches.23 Applying 1% testosterone cream or gel (0.5 g/day) topically to the thigh or lower abdomen should increase the low testosterone levels typically seen in menopausal women to the higher levels seen in younger women.24,25 Application to the vulva or vagina is not advised, as it may cause local irritation and is unpredictably absorbed.

Adapting male testosterone products. High-quality FDA-approved testosterone gel formulations are available for male hypogonadism. However, since women have approximately one-tenth the circulating testosterone levels of men, supraphysiologic dosing is a risk when these products are prescribed for women. Most testosterone products approved for men are provided in pumps or packets, and they are difficult to dose-adjust for women. Applying one-tenth the male dose of 1% testosterone gel (Testim), which comes in a resealable unit-dose tube, is an alternative to compounding. For men, the dose is 1 tube per day, so women should make 1 tube last for 10 days by using 3 to 4 drops of testosterone gel per day. Close physical contact must be avoided immediately after application, as topical hormone creams and gels are easily transferred to others. The safety and efficacy of compounded or dose-adjusted male testosterone products used in women are unknown.
Follow treated women closely. Women who elect to use transdermal testosterone therapy should be seen at 8 to 12 weeks to assess treatment response. Regular follow-up visits are required to assess response, satisfaction, and adverse effects, including acne and hirsutism. Since there may be little correlation between serum testosterone levels and the prescribed dose of a compounded testosterone product, testosterone levels should be measured regularly as a safety measure. The goal is to keep serum testosterone concentrations within the normal range for reproductive-aged women to reduce the likelihood of adverse effects. Testosterone levels should not be tested as an efficacy measure, however, as there is no testosterone level that will assure a satisfactory sex life.
CASE Conclusion
After a thorough discussion of high placebo response rates, potential adverse effects, unknown long-term risks, and off-label nature of testosterone use, the patient elects a trial of compounded 1% testosterone cream. Her clinician informs her of the limitations of compounded formulations and the need for regular testing of testosterone levels to prevent supraphysiologic dosing. At a follow-up visit 8 weeks later, she reports improved sexual desire and elects to continue treatment and monitoring. After using testosterone for 2 years, the patient is uncertain that she still is experiencing a significant benefit, stops testosterone treatment, and remains satisfied with her sex life.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- The North American Menopause Society Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
- Simon JA, Nappi RE, Kingsberg SA, et al. Clarifying Vaginal Atrophy's Impact on Sex and Relationships (CLOSER) survey: emotional and physical impact of vaginal discomfort on North American postmenopausal women and their partners. Menopause. 2014;21:137-142.
- The North American Menopause Society. Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause. 2013;20:888-902.
- Shifren J, Monz B, Russo P, et al. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112:970-978.
- Davison S, Bell R, Donath S, et al. Androgen levels in adult females: changes with age, menopause, and oophorectomy. J Clin Endocrinol Metab. 2005;90:3847-3853.
- Shifren JL, Desindes S, McIlwain M, et al. A randomized, open-label, crossover study comparing the effects of oral versus transdermal estrogen therapy on serum androgens, thyroid hormones, and adrenal hormones in naturally menopausal women. Menopause. 2007;14:985-994.
- Davis SR, Davison SL, Donath S, et al. Circulating androgen levels and self-reported sexual function in women. JAMA. 2005;294:91-96.
- Wahlin-Jacobsen S, Pedersen AT, Kristensen E, et al. Is there a correlation between androgens and sexual desire in women? J Sex Med. 2015;12:358-373.
- Randolph JF Jr, Zheng H, Avis NE, et al. Masturbation frequency and sexual function domains are associated with serum reproductive hormone levels across the menopausal transition. J Clin Endocrinol Metab. 2015;100:258-266.
- Dennerstein L, Lehert P, Burger H. The relative effects of hormones and relationship factors on sexual function of women through the natural menopausal transition. Fertil Steril. 2005;84:174-180.
- Shifren JL, Davis SR. Androgens in postmenopausal women: a review. Menopause. 2017;24:970-979.
- Sherwin BB, Gelfand MM, Brender W. Androgen enhances sexual motivation in females: a prospective, crossover study of sex steroid administration in the surgical menopause. Psychosom Med. 1985;47:339-351.
- Huang G, Basaria S, Travison TG, et al. Testosterone dose-response relationships in hysterectomized women with or without oophorectomy: effects on sexual function, body composition, muscle performance and physical function in a randomized trial. Menopause. 2014;21:612-623.
- Shifren JL, Braunstein GD, Simon JA, et al. Transdermal testosterone treatment in women with impaired sexual function after oophorectomy. N Engl J Med. 2000;343:682-688.
- Simon J, Braunstein G, Nachtigall L, et al. Testosterone patch increases sexual activity and desire in surgically menopausal women with hypoactive sexual desire disorder. J Clin Endocrinol Metab. 2005;90:5226-5233.
- Shifren JL, Davis SR, Moreau M, et al. Testosterone patch for the treatment of hypoactive sexual desire disorder in naturally menopausal women: results from the INTIMATE NM1 study. Menopause. 2006;13:770-779.
- Davis SR, Moreau M, Kroll R, et al; APHRODITE Study Team. Testosterone for low libido in postmenopausal women not taking estrogen. N Engl J Med. 2008;359:2005-2017.
- Kingsberg S, Shifren J, Wekselman K, et al. Evaluation of the clinical relevance of benefits associated with transdermal testosterone treatment in postmenopausal women with hypoactive sexual desire disorder. J Sex Med. 2007;4:1001-1008.
- Wierman ME, Arlt W, Basson R, et al. Androgen therapy in women: a reappraisal: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2014;99:3489-3510.
- Snabes M, Zborowski J, Simes S. Libigel (testosterone gel) does not differentiate from placebo therapy in the treatment of hypoactive sexual desire in postmenopausal women (abstract). J Sex Med. 2012;9(suppl 3):171.
- Lobo RA, Rosen RC, Yang HM, et al. Comparative effects of oral esterified estrogens with and without methyltestosterone on endocrine profiles and dimensions of sexual function in postmenopausal women with hypoactive sexual desire. Fertil Steril. 2003;79:1341-1352.
- Somboonporn W, Davis S, Seif M, et al. Testsoterone for peri- and postmenopausal women. Cochrane Database Syst Rev. 2005;19:CD004509.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and American Society for Reproductive Medicine. Committee opinion 532: compounded bioidentical menopausal hormone therapy. Obstet Gynecol. 2012;(2 pt 1):411-415.
- Fooladi E, Reuter SE, Bell RJ, et al. Pharmacokinetics of a transdermal testosterone cream in healthy postmenopausal women. Menopause. 2015;22:44-49.
- Shifren JL. Testosterone for midlife women: the hormone of desire? Menopause. 2015;22:1147-1149.
CASE Midlife woman with low libido causing distress
At her annual gynecologic visit, a 55-year-old woman notes that she has almost no interest in sex. In the past, her libido was good and relations were pleasurable. Since her mid-40s, she has noticed a gradual decline in libido and orgasmic response. Sexual frequency has declined from once or twice weekly to just a few times per month. She has been married for 25 years and describes the relationship as caring and strong. Her husband is healthy with a good libido; his intermittent erectile dysfunction is treated with a phosphodiesterase-5 inhibitor. The patient’s low libido is distressing, as the decline in sexual frequency is causing some conflict for the couple. She requests that her testosterone level be checked because she heard that treatment with testosterone cream will solve this problem.
Evaluating and treating low libido in menopausal women
Low libido is a very common sexual problem for women. When sexual problems are accompanied by distress, they are classified as sexual dysfunctions. Although ObGyns should discuss sexual concerns at every comprehensive visit, if the patient has no associated distress, treatment is not necessarily indicated. A woman with low libido or anorgasmia who is satisfied with her sex life and is not bothered by these issues does not require any intervention.
Currently, the only indication for testosterone therapy that is supported by clinical trial evidence is low sexual desire with associated distress, known as hypoactive sexual desire disorder (HSDD). Although other sexual problems also commonly occur in menopausal women, such as disorders of orgasm and pain, testosterone is not recommended for these problems. In addition, testosterone is not approved by the US Food and Drug Administration (FDA) for the treatment of female sexual dysfunction.
Routinely inquire about sexual functioning
Ask your patients about sexual concerns at every comprehensive visit. You can easily incorporate into the review of systems a general question, such as, “Do you have any sexual concerns?” If the patient does mention a sexual problem, schedule a separate visit (given appointment time constraints) to address it. History and physical examination information you gather during the comprehensive visit will be helpful in the subsequent problem-focused visit.
Taking a thorough history is key when addressing a patient’s sexual problems, since identifying possible etiologies guides treatment. Often, the cause of female sexual dysfunction is multifactorial and includes physiologic, psychologic, and relationship issues.
- Evidence supports low-dose transdermal testosterone in carefully selected menopausal women with HSDD and no other identifiable reason for the sexual dysfunction
- Inform women considering testosterone for HSDD of the limited effectiveness and high placebo responses seen in clinical trials
- Women also must be informed that treatment is off-label (no testosterone formulations are FDA approved for women)
- Review with patients the limitations of compounded medications, and discuss possible adverse effects of androgens. Long-term safety is unknown and, as androgens are converted to estrogens
Explore potential causes, recommend standard therapies
Common causes of low libido in menopausal women include vasomotor symptoms, insomnia, urinary incontinence, cancer or another major medical problem, weight gain, poor body image, genitourinary syndrome of menopause (GSM) with dyspareunia, fatigue, stress, aging, relationship duration, lack of novelty, relationship conflict, and a partner’s sexual problems. Other common etiologies include depression, anxiety, and substance use disorders, as well as medications used to treat these disorders, including selective serotonin reuptake inhibitors (SSRIs).
Continue to: There are many effective therapies...
There are many effective therapies for low sexual desire to consider prior to initiating a trial of testosterone, which should be considered for HSDD only if the disorder persists after addressing all other possible contributing factors (TABLE 1).

Sex therapy, for example, provides information on sexual functioning and helps improve communication and mutual pleasure and satisfaction. Strongly encourage—if not require—a consultation with a sex therapist before prescribing testosterone for low libido. Any testosterone-derived improvement in sexual functioning will be enhanced by improved communication and additional strategies to achieve mutual pleasure.
Hormone therapy. Vasomotor symptoms, with their associated sleep disruption, fatigue, and reduced quality of life (QOL), often adversely impact sexual desire. Estrogen therapy does not appear to improve libido in otherwise asymptomatic women; however, in women with bothersome vasomotor symptoms treated with estrogen, sexual interest may increase as a result of improved sleep, fatigue, and overall QOL. The benefits of systemic hormone therapy generally outweigh its risks for most healthy women younger than age 60 who have bothersome hot flashes and night sweats.1
Nonhormonal and other therapies. GSM with dyspareunia is a principal cause of sexual dysfunction in older women.2 Many safe and effective treatments are available, including low-dose vaginal estrogen therapy, nonhormonal moisturizers and lubricants, ospemifene, vaginal dehydroepiandrosterone, and pelvic floor physical therapy.3 Urinary incontinence commonly occurs in midlife women and contributes to low libido.4
Lifestyle approaches. Address fatigue and stress by having the patient adjust her work and sleep schedules, obtain help with housework and meals, and engage in mind-body interventions, counseling, or yoga. Sexual function may benefit from yoga practice, likely as a result of the patient experiencing reduced stress and enhanced body image. Improving overall health and body image with regular exercise, optimal diet, and weight management may contribute to a more satisfying sex life after the onset of menopause.
Relationship refresh. Women’s sexual interest often declines with relationship duration, and both men and women who are in new relationships generally have increased libido, affirming the importance of novelty over the long term. Couples will benefit from “date nights,” weekends away from home, and trying novel positions, locations, and times for sex. Couple’s counseling may address relationship conflict.
Expert referral. Depression, anxiety, and substance use disorders are prevalent in menopausal women and contribute to sexual dysfunction. Effective therapy is available, although some pharmacologic treatments (including SSRIs) may be an additional cause of sexual dysfunction. In addition to recommending appropriate counseling and support, referring the patient to a psychopharmacologist with expertise in managing sexual adverse effects of medications may optimize care.
Continue to: Sexual function improves, but patient still wants to try testosterone
CASE Sexual function improves, but patient still wants to try testosterone
The patient returns for follow-up visits scheduled specifically to address her sexual concerns. Sex is more comfortable and pleasurable since initiating low-dose vaginal estrogen therapy. Having been on an SSRI since her mid-40s for mild depression, the patient switched to bupropion and notes improved libido and orgasmic response. She is exercising more regularly and working with a nutritionist to address a 15-lb weight gain after menopause. The couple saw a sex therapist and is communicating better about sex with more novelty in their repertoire. They are enjoying a regular date night. Although the patient’s sex life has improved with these interventions, she is still very interested in trying testosterone.
Testosterone’s effects on HSDD in menopausal women
After addressing the many factors that contribute to sexual disinterest, a trial of testosterone may be appropriate for a menopausal woman who continues to experience low libido with associated distress.
Testosterone levels decrease with aging in both men and women. Although testosterone levels decline by approximately 50% with bilateral oophorectomy, there is no decline in androgen levels with natural menopause.5 Testosterone circulates tightly bound to sex hormone–binding globulin (SHBG), so free or active testosterone will be reduced by oral estrogens, which increase SHBG levels.6 As most menopausal women will have a low testosterone level due to aging, measuring the testosterone level does not provide information about the etiology of the sexual problem.
Although some studies have identified an association between endogenous androgen levels and sexual function, the associations are modest and are of uncertain clinical significance.7-9 Not surprisingly, other factors, such as physical and psychologic health and the quality of the relationship, often are reported as more important predictors of sexual satisfaction than androgen levels.10
While endogenous testosterone levels may not correlate with sexual function, clinical trials of carefully selected menopausal women with HSDD have shown that androgen treatment generally results in improved sexual function.11 Studies demonstrate substantial improvements in sexual desire, orgasmic response, and frequency in menopausal women treated with high doses of intramuscular testosterone, which result in supraphysiologic androgen levels.12,13 While it is interesting that women with testosterone levels in the male low range have sizeable increases in sexual desire and response, long-term use of high-dose testosterone would result in unacceptable androgenic adverse effects and risks.
Continue to: Testosterone in low doses...
Testosterone in low doses. It is more relevant to consider the impact on female sexual function of low doses of testosterone, which raise the reduced testosterone levels seen in older women to the higher levels seen in reproductive-aged women.
A series of double-blind, multicenter, randomized, placebo-controlled trials in menopausal women with HSDD examined the impact on sexual function of a transdermal testosterone patch (300 μg) that increased blood testosterone levels to the upper limit of normal for young women.14-17 In these studies, compared with placebo, women using testosterone reported significant improvements in sexual desire, arousal, orgasmic response, frequency, and sexually related distress. Findings were consistent in surgically and naturally menopausal women, with and without the use of concurrent estrogen therapy. Improvements were clinically limited, however. On average, testosterone-treated women experienced 1 to 1.5 additional satisfying sexual events in a 4-week period compared with those treated with placebo. The percentage of women reporting a clinically meaningful benefit from treatment was significantly greater in women treated with testosterone (52%) compared with the placebo-treated women (31%).18 An appreciable placebo response was seen, typical of most studies of therapies for sexual dysfunction.

Safety concerns

Potential risks of testosterone treatment include acne, hirsutism, irreversible deepening of the voice, and adverse changes in lipids and liver function (TABLE 2).19 Adverse effects are dose dependent and are unlikely with physiologically dosed testosterone.
A 1-year study of testosterone patches in approximately 800 menopausal women with HSDD (with a subgroup of women followed for an additional year) provides the most comprehensive safety data available.17 Unwanted hair growth occurred more often in women receiving testosterone, without significant differences in blood biochemistry,hematologic parameters, carbohydrate metabolism, or lipids. Breast cancer was diagnosed in more women receiving testosterone than placebo. Although this finding may have been due to chance, the investigators concluded that long-term effects of testosterone treatment remain uncertain.
The FDA reviewed the data from the testosterone patch studies and determined that testosterone patches were effective for the treatment of HSDD in menopausal women, but more information was needed on long-term safety before approval could be granted. Another company then developed a testosterone gel product that produced similar blood levels as the testosterone patch. It was presumed that there would be similar efficacy; the principal goal of these studies was to examine long-term safety, particularly with respect to breast cancer and cardiovascular disease. Unexpectedly, although it raised testosterone blood levels to the upper limit of normal for young women, the testosterone gel product was no more effective than placebo.20 The clinical trial was ended, with safety data never published.
Continue to: Availability of testosterone formulations
Availability of testosterone formulations
Currently, no androgen therapies are FDA approved for the treatment of female sexual dysfunction. Although the best evidence regarding testosterone efficacy and safety involves the use of testosterone patches (300 μg), appropriately dosed for women, these patches are not currently available. FDA-approved testosterone patches are approved for the treatment of male hypogonadism, but use of these patches in women is not recommended since they would result in very high circulating testosterone levels.
Testosterone subcutaneous implants, pellets, and intramuscular injections also are not recommended for women because of the risk of excessive dosing. Small trials of menopausal women taking oral estrogen with low sexual desire found that oral formulations of testosterone improved libido in this study population.21 The combination of esterified estrogens (0.625 mg) and methyltestosterone (1.25 mg) is available as a compounded, non-FDA approved product. Oral androgen formulations generally are not advised, due to potential adverse effects on lipids and liver function.22
Compounded testosterone products. Ointments and creams may be compounded by prescription (TABLE 3). Product purity, dose, bioavailability, and quality typically are untested, and substantial variability exists between formulations and batches.23 Applying 1% testosterone cream or gel (0.5 g/day) topically to the thigh or lower abdomen should increase the low testosterone levels typically seen in menopausal women to the higher levels seen in younger women.24,25 Application to the vulva or vagina is not advised, as it may cause local irritation and is unpredictably absorbed.

Adapting male testosterone products. High-quality FDA-approved testosterone gel formulations are available for male hypogonadism. However, since women have approximately one-tenth the circulating testosterone levels of men, supraphysiologic dosing is a risk when these products are prescribed for women. Most testosterone products approved for men are provided in pumps or packets, and they are difficult to dose-adjust for women. Applying one-tenth the male dose of 1% testosterone gel (Testim), which comes in a resealable unit-dose tube, is an alternative to compounding. For men, the dose is 1 tube per day, so women should make 1 tube last for 10 days by using 3 to 4 drops of testosterone gel per day. Close physical contact must be avoided immediately after application, as topical hormone creams and gels are easily transferred to others. The safety and efficacy of compounded or dose-adjusted male testosterone products used in women are unknown.
Follow treated women closely. Women who elect to use transdermal testosterone therapy should be seen at 8 to 12 weeks to assess treatment response. Regular follow-up visits are required to assess response, satisfaction, and adverse effects, including acne and hirsutism. Since there may be little correlation between serum testosterone levels and the prescribed dose of a compounded testosterone product, testosterone levels should be measured regularly as a safety measure. The goal is to keep serum testosterone concentrations within the normal range for reproductive-aged women to reduce the likelihood of adverse effects. Testosterone levels should not be tested as an efficacy measure, however, as there is no testosterone level that will assure a satisfactory sex life.
CASE Conclusion
After a thorough discussion of high placebo response rates, potential adverse effects, unknown long-term risks, and off-label nature of testosterone use, the patient elects a trial of compounded 1% testosterone cream. Her clinician informs her of the limitations of compounded formulations and the need for regular testing of testosterone levels to prevent supraphysiologic dosing. At a follow-up visit 8 weeks later, she reports improved sexual desire and elects to continue treatment and monitoring. After using testosterone for 2 years, the patient is uncertain that she still is experiencing a significant benefit, stops testosterone treatment, and remains satisfied with her sex life.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
CASE Midlife woman with low libido causing distress
At her annual gynecologic visit, a 55-year-old woman notes that she has almost no interest in sex. In the past, her libido was good and relations were pleasurable. Since her mid-40s, she has noticed a gradual decline in libido and orgasmic response. Sexual frequency has declined from once or twice weekly to just a few times per month. She has been married for 25 years and describes the relationship as caring and strong. Her husband is healthy with a good libido; his intermittent erectile dysfunction is treated with a phosphodiesterase-5 inhibitor. The patient’s low libido is distressing, as the decline in sexual frequency is causing some conflict for the couple. She requests that her testosterone level be checked because she heard that treatment with testosterone cream will solve this problem.
Evaluating and treating low libido in menopausal women
Low libido is a very common sexual problem for women. When sexual problems are accompanied by distress, they are classified as sexual dysfunctions. Although ObGyns should discuss sexual concerns at every comprehensive visit, if the patient has no associated distress, treatment is not necessarily indicated. A woman with low libido or anorgasmia who is satisfied with her sex life and is not bothered by these issues does not require any intervention.
Currently, the only indication for testosterone therapy that is supported by clinical trial evidence is low sexual desire with associated distress, known as hypoactive sexual desire disorder (HSDD). Although other sexual problems also commonly occur in menopausal women, such as disorders of orgasm and pain, testosterone is not recommended for these problems. In addition, testosterone is not approved by the US Food and Drug Administration (FDA) for the treatment of female sexual dysfunction.
Routinely inquire about sexual functioning
Ask your patients about sexual concerns at every comprehensive visit. You can easily incorporate into the review of systems a general question, such as, “Do you have any sexual concerns?” If the patient does mention a sexual problem, schedule a separate visit (given appointment time constraints) to address it. History and physical examination information you gather during the comprehensive visit will be helpful in the subsequent problem-focused visit.
Taking a thorough history is key when addressing a patient’s sexual problems, since identifying possible etiologies guides treatment. Often, the cause of female sexual dysfunction is multifactorial and includes physiologic, psychologic, and relationship issues.
- Evidence supports low-dose transdermal testosterone in carefully selected menopausal women with HSDD and no other identifiable reason for the sexual dysfunction
- Inform women considering testosterone for HSDD of the limited effectiveness and high placebo responses seen in clinical trials
- Women also must be informed that treatment is off-label (no testosterone formulations are FDA approved for women)
- Review with patients the limitations of compounded medications, and discuss possible adverse effects of androgens. Long-term safety is unknown and, as androgens are converted to estrogens
Explore potential causes, recommend standard therapies
Common causes of low libido in menopausal women include vasomotor symptoms, insomnia, urinary incontinence, cancer or another major medical problem, weight gain, poor body image, genitourinary syndrome of menopause (GSM) with dyspareunia, fatigue, stress, aging, relationship duration, lack of novelty, relationship conflict, and a partner’s sexual problems. Other common etiologies include depression, anxiety, and substance use disorders, as well as medications used to treat these disorders, including selective serotonin reuptake inhibitors (SSRIs).
Continue to: There are many effective therapies...
There are many effective therapies for low sexual desire to consider prior to initiating a trial of testosterone, which should be considered for HSDD only if the disorder persists after addressing all other possible contributing factors (TABLE 1).

Sex therapy, for example, provides information on sexual functioning and helps improve communication and mutual pleasure and satisfaction. Strongly encourage—if not require—a consultation with a sex therapist before prescribing testosterone for low libido. Any testosterone-derived improvement in sexual functioning will be enhanced by improved communication and additional strategies to achieve mutual pleasure.
Hormone therapy. Vasomotor symptoms, with their associated sleep disruption, fatigue, and reduced quality of life (QOL), often adversely impact sexual desire. Estrogen therapy does not appear to improve libido in otherwise asymptomatic women; however, in women with bothersome vasomotor symptoms treated with estrogen, sexual interest may increase as a result of improved sleep, fatigue, and overall QOL. The benefits of systemic hormone therapy generally outweigh its risks for most healthy women younger than age 60 who have bothersome hot flashes and night sweats.1
Nonhormonal and other therapies. GSM with dyspareunia is a principal cause of sexual dysfunction in older women.2 Many safe and effective treatments are available, including low-dose vaginal estrogen therapy, nonhormonal moisturizers and lubricants, ospemifene, vaginal dehydroepiandrosterone, and pelvic floor physical therapy.3 Urinary incontinence commonly occurs in midlife women and contributes to low libido.4
Lifestyle approaches. Address fatigue and stress by having the patient adjust her work and sleep schedules, obtain help with housework and meals, and engage in mind-body interventions, counseling, or yoga. Sexual function may benefit from yoga practice, likely as a result of the patient experiencing reduced stress and enhanced body image. Improving overall health and body image with regular exercise, optimal diet, and weight management may contribute to a more satisfying sex life after the onset of menopause.
Relationship refresh. Women’s sexual interest often declines with relationship duration, and both men and women who are in new relationships generally have increased libido, affirming the importance of novelty over the long term. Couples will benefit from “date nights,” weekends away from home, and trying novel positions, locations, and times for sex. Couple’s counseling may address relationship conflict.
Expert referral. Depression, anxiety, and substance use disorders are prevalent in menopausal women and contribute to sexual dysfunction. Effective therapy is available, although some pharmacologic treatments (including SSRIs) may be an additional cause of sexual dysfunction. In addition to recommending appropriate counseling and support, referring the patient to a psychopharmacologist with expertise in managing sexual adverse effects of medications may optimize care.
Continue to: Sexual function improves, but patient still wants to try testosterone
CASE Sexual function improves, but patient still wants to try testosterone
The patient returns for follow-up visits scheduled specifically to address her sexual concerns. Sex is more comfortable and pleasurable since initiating low-dose vaginal estrogen therapy. Having been on an SSRI since her mid-40s for mild depression, the patient switched to bupropion and notes improved libido and orgasmic response. She is exercising more regularly and working with a nutritionist to address a 15-lb weight gain after menopause. The couple saw a sex therapist and is communicating better about sex with more novelty in their repertoire. They are enjoying a regular date night. Although the patient’s sex life has improved with these interventions, she is still very interested in trying testosterone.
Testosterone’s effects on HSDD in menopausal women
After addressing the many factors that contribute to sexual disinterest, a trial of testosterone may be appropriate for a menopausal woman who continues to experience low libido with associated distress.
Testosterone levels decrease with aging in both men and women. Although testosterone levels decline by approximately 50% with bilateral oophorectomy, there is no decline in androgen levels with natural menopause.5 Testosterone circulates tightly bound to sex hormone–binding globulin (SHBG), so free or active testosterone will be reduced by oral estrogens, which increase SHBG levels.6 As most menopausal women will have a low testosterone level due to aging, measuring the testosterone level does not provide information about the etiology of the sexual problem.
Although some studies have identified an association between endogenous androgen levels and sexual function, the associations are modest and are of uncertain clinical significance.7-9 Not surprisingly, other factors, such as physical and psychologic health and the quality of the relationship, often are reported as more important predictors of sexual satisfaction than androgen levels.10
While endogenous testosterone levels may not correlate with sexual function, clinical trials of carefully selected menopausal women with HSDD have shown that androgen treatment generally results in improved sexual function.11 Studies demonstrate substantial improvements in sexual desire, orgasmic response, and frequency in menopausal women treated with high doses of intramuscular testosterone, which result in supraphysiologic androgen levels.12,13 While it is interesting that women with testosterone levels in the male low range have sizeable increases in sexual desire and response, long-term use of high-dose testosterone would result in unacceptable androgenic adverse effects and risks.
Continue to: Testosterone in low doses...
Testosterone in low doses. It is more relevant to consider the impact on female sexual function of low doses of testosterone, which raise the reduced testosterone levels seen in older women to the higher levels seen in reproductive-aged women.
A series of double-blind, multicenter, randomized, placebo-controlled trials in menopausal women with HSDD examined the impact on sexual function of a transdermal testosterone patch (300 μg) that increased blood testosterone levels to the upper limit of normal for young women.14-17 In these studies, compared with placebo, women using testosterone reported significant improvements in sexual desire, arousal, orgasmic response, frequency, and sexually related distress. Findings were consistent in surgically and naturally menopausal women, with and without the use of concurrent estrogen therapy. Improvements were clinically limited, however. On average, testosterone-treated women experienced 1 to 1.5 additional satisfying sexual events in a 4-week period compared with those treated with placebo. The percentage of women reporting a clinically meaningful benefit from treatment was significantly greater in women treated with testosterone (52%) compared with the placebo-treated women (31%).18 An appreciable placebo response was seen, typical of most studies of therapies for sexual dysfunction.

Safety concerns

Potential risks of testosterone treatment include acne, hirsutism, irreversible deepening of the voice, and adverse changes in lipids and liver function (TABLE 2).19 Adverse effects are dose dependent and are unlikely with physiologically dosed testosterone.
A 1-year study of testosterone patches in approximately 800 menopausal women with HSDD (with a subgroup of women followed for an additional year) provides the most comprehensive safety data available.17 Unwanted hair growth occurred more often in women receiving testosterone, without significant differences in blood biochemistry,hematologic parameters, carbohydrate metabolism, or lipids. Breast cancer was diagnosed in more women receiving testosterone than placebo. Although this finding may have been due to chance, the investigators concluded that long-term effects of testosterone treatment remain uncertain.
The FDA reviewed the data from the testosterone patch studies and determined that testosterone patches were effective for the treatment of HSDD in menopausal women, but more information was needed on long-term safety before approval could be granted. Another company then developed a testosterone gel product that produced similar blood levels as the testosterone patch. It was presumed that there would be similar efficacy; the principal goal of these studies was to examine long-term safety, particularly with respect to breast cancer and cardiovascular disease. Unexpectedly, although it raised testosterone blood levels to the upper limit of normal for young women, the testosterone gel product was no more effective than placebo.20 The clinical trial was ended, with safety data never published.
Continue to: Availability of testosterone formulations
Availability of testosterone formulations
Currently, no androgen therapies are FDA approved for the treatment of female sexual dysfunction. Although the best evidence regarding testosterone efficacy and safety involves the use of testosterone patches (300 μg), appropriately dosed for women, these patches are not currently available. FDA-approved testosterone patches are approved for the treatment of male hypogonadism, but use of these patches in women is not recommended since they would result in very high circulating testosterone levels.
Testosterone subcutaneous implants, pellets, and intramuscular injections also are not recommended for women because of the risk of excessive dosing. Small trials of menopausal women taking oral estrogen with low sexual desire found that oral formulations of testosterone improved libido in this study population.21 The combination of esterified estrogens (0.625 mg) and methyltestosterone (1.25 mg) is available as a compounded, non-FDA approved product. Oral androgen formulations generally are not advised, due to potential adverse effects on lipids and liver function.22
Compounded testosterone products. Ointments and creams may be compounded by prescription (TABLE 3). Product purity, dose, bioavailability, and quality typically are untested, and substantial variability exists between formulations and batches.23 Applying 1% testosterone cream or gel (0.5 g/day) topically to the thigh or lower abdomen should increase the low testosterone levels typically seen in menopausal women to the higher levels seen in younger women.24,25 Application to the vulva or vagina is not advised, as it may cause local irritation and is unpredictably absorbed.

Adapting male testosterone products. High-quality FDA-approved testosterone gel formulations are available for male hypogonadism. However, since women have approximately one-tenth the circulating testosterone levels of men, supraphysiologic dosing is a risk when these products are prescribed for women. Most testosterone products approved for men are provided in pumps or packets, and they are difficult to dose-adjust for women. Applying one-tenth the male dose of 1% testosterone gel (Testim), which comes in a resealable unit-dose tube, is an alternative to compounding. For men, the dose is 1 tube per day, so women should make 1 tube last for 10 days by using 3 to 4 drops of testosterone gel per day. Close physical contact must be avoided immediately after application, as topical hormone creams and gels are easily transferred to others. The safety and efficacy of compounded or dose-adjusted male testosterone products used in women are unknown.
Follow treated women closely. Women who elect to use transdermal testosterone therapy should be seen at 8 to 12 weeks to assess treatment response. Regular follow-up visits are required to assess response, satisfaction, and adverse effects, including acne and hirsutism. Since there may be little correlation between serum testosterone levels and the prescribed dose of a compounded testosterone product, testosterone levels should be measured regularly as a safety measure. The goal is to keep serum testosterone concentrations within the normal range for reproductive-aged women to reduce the likelihood of adverse effects. Testosterone levels should not be tested as an efficacy measure, however, as there is no testosterone level that will assure a satisfactory sex life.
CASE Conclusion
After a thorough discussion of high placebo response rates, potential adverse effects, unknown long-term risks, and off-label nature of testosterone use, the patient elects a trial of compounded 1% testosterone cream. Her clinician informs her of the limitations of compounded formulations and the need for regular testing of testosterone levels to prevent supraphysiologic dosing. At a follow-up visit 8 weeks later, she reports improved sexual desire and elects to continue treatment and monitoring. After using testosterone for 2 years, the patient is uncertain that she still is experiencing a significant benefit, stops testosterone treatment, and remains satisfied with her sex life.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- The North American Menopause Society Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
- Simon JA, Nappi RE, Kingsberg SA, et al. Clarifying Vaginal Atrophy's Impact on Sex and Relationships (CLOSER) survey: emotional and physical impact of vaginal discomfort on North American postmenopausal women and their partners. Menopause. 2014;21:137-142.
- The North American Menopause Society. Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause. 2013;20:888-902.
- Shifren J, Monz B, Russo P, et al. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112:970-978.
- Davison S, Bell R, Donath S, et al. Androgen levels in adult females: changes with age, menopause, and oophorectomy. J Clin Endocrinol Metab. 2005;90:3847-3853.
- Shifren JL, Desindes S, McIlwain M, et al. A randomized, open-label, crossover study comparing the effects of oral versus transdermal estrogen therapy on serum androgens, thyroid hormones, and adrenal hormones in naturally menopausal women. Menopause. 2007;14:985-994.
- Davis SR, Davison SL, Donath S, et al. Circulating androgen levels and self-reported sexual function in women. JAMA. 2005;294:91-96.
- Wahlin-Jacobsen S, Pedersen AT, Kristensen E, et al. Is there a correlation between androgens and sexual desire in women? J Sex Med. 2015;12:358-373.
- Randolph JF Jr, Zheng H, Avis NE, et al. Masturbation frequency and sexual function domains are associated with serum reproductive hormone levels across the menopausal transition. J Clin Endocrinol Metab. 2015;100:258-266.
- Dennerstein L, Lehert P, Burger H. The relative effects of hormones and relationship factors on sexual function of women through the natural menopausal transition. Fertil Steril. 2005;84:174-180.
- Shifren JL, Davis SR. Androgens in postmenopausal women: a review. Menopause. 2017;24:970-979.
- Sherwin BB, Gelfand MM, Brender W. Androgen enhances sexual motivation in females: a prospective, crossover study of sex steroid administration in the surgical menopause. Psychosom Med. 1985;47:339-351.
- Huang G, Basaria S, Travison TG, et al. Testosterone dose-response relationships in hysterectomized women with or without oophorectomy: effects on sexual function, body composition, muscle performance and physical function in a randomized trial. Menopause. 2014;21:612-623.
- Shifren JL, Braunstein GD, Simon JA, et al. Transdermal testosterone treatment in women with impaired sexual function after oophorectomy. N Engl J Med. 2000;343:682-688.
- Simon J, Braunstein G, Nachtigall L, et al. Testosterone patch increases sexual activity and desire in surgically menopausal women with hypoactive sexual desire disorder. J Clin Endocrinol Metab. 2005;90:5226-5233.
- Shifren JL, Davis SR, Moreau M, et al. Testosterone patch for the treatment of hypoactive sexual desire disorder in naturally menopausal women: results from the INTIMATE NM1 study. Menopause. 2006;13:770-779.
- Davis SR, Moreau M, Kroll R, et al; APHRODITE Study Team. Testosterone for low libido in postmenopausal women not taking estrogen. N Engl J Med. 2008;359:2005-2017.
- Kingsberg S, Shifren J, Wekselman K, et al. Evaluation of the clinical relevance of benefits associated with transdermal testosterone treatment in postmenopausal women with hypoactive sexual desire disorder. J Sex Med. 2007;4:1001-1008.
- Wierman ME, Arlt W, Basson R, et al. Androgen therapy in women: a reappraisal: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2014;99:3489-3510.
- Snabes M, Zborowski J, Simes S. Libigel (testosterone gel) does not differentiate from placebo therapy in the treatment of hypoactive sexual desire in postmenopausal women (abstract). J Sex Med. 2012;9(suppl 3):171.
- Lobo RA, Rosen RC, Yang HM, et al. Comparative effects of oral esterified estrogens with and without methyltestosterone on endocrine profiles and dimensions of sexual function in postmenopausal women with hypoactive sexual desire. Fertil Steril. 2003;79:1341-1352.
- Somboonporn W, Davis S, Seif M, et al. Testsoterone for peri- and postmenopausal women. Cochrane Database Syst Rev. 2005;19:CD004509.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and American Society for Reproductive Medicine. Committee opinion 532: compounded bioidentical menopausal hormone therapy. Obstet Gynecol. 2012;(2 pt 1):411-415.
- Fooladi E, Reuter SE, Bell RJ, et al. Pharmacokinetics of a transdermal testosterone cream in healthy postmenopausal women. Menopause. 2015;22:44-49.
- Shifren JL. Testosterone for midlife women: the hormone of desire? Menopause. 2015;22:1147-1149.
- The North American Menopause Society Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
- Simon JA, Nappi RE, Kingsberg SA, et al. Clarifying Vaginal Atrophy's Impact on Sex and Relationships (CLOSER) survey: emotional and physical impact of vaginal discomfort on North American postmenopausal women and their partners. Menopause. 2014;21:137-142.
- The North American Menopause Society. Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause. 2013;20:888-902.
- Shifren J, Monz B, Russo P, et al. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112:970-978.
- Davison S, Bell R, Donath S, et al. Androgen levels in adult females: changes with age, menopause, and oophorectomy. J Clin Endocrinol Metab. 2005;90:3847-3853.
- Shifren JL, Desindes S, McIlwain M, et al. A randomized, open-label, crossover study comparing the effects of oral versus transdermal estrogen therapy on serum androgens, thyroid hormones, and adrenal hormones in naturally menopausal women. Menopause. 2007;14:985-994.
- Davis SR, Davison SL, Donath S, et al. Circulating androgen levels and self-reported sexual function in women. JAMA. 2005;294:91-96.
- Wahlin-Jacobsen S, Pedersen AT, Kristensen E, et al. Is there a correlation between androgens and sexual desire in women? J Sex Med. 2015;12:358-373.
- Randolph JF Jr, Zheng H, Avis NE, et al. Masturbation frequency and sexual function domains are associated with serum reproductive hormone levels across the menopausal transition. J Clin Endocrinol Metab. 2015;100:258-266.
- Dennerstein L, Lehert P, Burger H. The relative effects of hormones and relationship factors on sexual function of women through the natural menopausal transition. Fertil Steril. 2005;84:174-180.
- Shifren JL, Davis SR. Androgens in postmenopausal women: a review. Menopause. 2017;24:970-979.
- Sherwin BB, Gelfand MM, Brender W. Androgen enhances sexual motivation in females: a prospective, crossover study of sex steroid administration in the surgical menopause. Psychosom Med. 1985;47:339-351.
- Huang G, Basaria S, Travison TG, et al. Testosterone dose-response relationships in hysterectomized women with or without oophorectomy: effects on sexual function, body composition, muscle performance and physical function in a randomized trial. Menopause. 2014;21:612-623.
- Shifren JL, Braunstein GD, Simon JA, et al. Transdermal testosterone treatment in women with impaired sexual function after oophorectomy. N Engl J Med. 2000;343:682-688.
- Simon J, Braunstein G, Nachtigall L, et al. Testosterone patch increases sexual activity and desire in surgically menopausal women with hypoactive sexual desire disorder. J Clin Endocrinol Metab. 2005;90:5226-5233.
- Shifren JL, Davis SR, Moreau M, et al. Testosterone patch for the treatment of hypoactive sexual desire disorder in naturally menopausal women: results from the INTIMATE NM1 study. Menopause. 2006;13:770-779.
- Davis SR, Moreau M, Kroll R, et al; APHRODITE Study Team. Testosterone for low libido in postmenopausal women not taking estrogen. N Engl J Med. 2008;359:2005-2017.
- Kingsberg S, Shifren J, Wekselman K, et al. Evaluation of the clinical relevance of benefits associated with transdermal testosterone treatment in postmenopausal women with hypoactive sexual desire disorder. J Sex Med. 2007;4:1001-1008.
- Wierman ME, Arlt W, Basson R, et al. Androgen therapy in women: a reappraisal: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2014;99:3489-3510.
- Snabes M, Zborowski J, Simes S. Libigel (testosterone gel) does not differentiate from placebo therapy in the treatment of hypoactive sexual desire in postmenopausal women (abstract). J Sex Med. 2012;9(suppl 3):171.
- Lobo RA, Rosen RC, Yang HM, et al. Comparative effects of oral esterified estrogens with and without methyltestosterone on endocrine profiles and dimensions of sexual function in postmenopausal women with hypoactive sexual desire. Fertil Steril. 2003;79:1341-1352.
- Somboonporn W, Davis S, Seif M, et al. Testsoterone for peri- and postmenopausal women. Cochrane Database Syst Rev. 2005;19:CD004509.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and American Society for Reproductive Medicine. Committee opinion 532: compounded bioidentical menopausal hormone therapy. Obstet Gynecol. 2012;(2 pt 1):411-415.
- Fooladi E, Reuter SE, Bell RJ, et al. Pharmacokinetics of a transdermal testosterone cream in healthy postmenopausal women. Menopause. 2015;22:44-49.
- Shifren JL. Testosterone for midlife women: the hormone of desire? Menopause. 2015;22:1147-1149.
2018 Update on minimally invasive gynecologic surgery
Uterine fibroids are the most common solid pelvic tumor in women and a leading indication for hysterectomy in the United States.1 As a result, they represent significant morbidity for many women and are a major public health problem. By age 50, 70% of white women and 80% of black women have fibroids.2
Although fibroids are sometimes asymptomatic, the symptoms most commonly reported are abnormal uterine bleeding (AUB) with resultant anemia and bulk/pressure symptoms. Uterine fibroids also are associated with reproductive dysfunction, such as recurrent pregnancy loss, and even infertility.3
The clinical diagnosis of uterine fibroids is made based on a combination of physical examination and imaging studies, including pelvic ultrasonography, saline infusion sonography, and magnetic resonance imaging (MRI). When medical management, such as combination oral contraceptive pills, fails in patients with AUB and/or bulk predominant symptoms or patients present with compromised fertility, the only option for conservative surgical management is a myomectomy.4
The route of myomectomy—hysteroscopy, laparotomy, conventional laparoscopic myomectomy (LM), or robot-assisted laparoscopic myomectomy (RALM)—depends on the size, number, location, and consistency of the uterine fibroids and, to a certain extent, the indication for the myomectomy. In some cases, multiple routes must be used to achieve optimal results, and sometimes these procedures have to be staged. In this literature review and technical summary, we focus on conventional LM and RALM approaches.
Literature review: In the right hands, LM and RALM have clear benefits
In the past, laparotomy was the surgical route of choice for fibroid removal. This surgery was associated with a long hospital stay, a high rate of blood transfusions, postoperative pain, and a lengthy recovery period. As minimally invasive surgery gained popularity, conventional LM became more commonly performed and was accepted by many as the gold standard approach for myomectomy.5
LM has considerable advantages over laparotomy
Compared with the traditional, more invasive route, the conventional LM approach has many benefits. These include less blood loss, decreased postoperative pain, shorter recovery time, shorter hospitalization stay, and decreased perioperative complications.6 LM should be considered the first-line approach unless the size of an intramural myoma exceeds 10 to 12 cm or multiple myomas (consensus, approximately 4 or more) are present and necessitate several incisions according to their varying locations within the uterus.7,8 While this is a recommendation, reports have been published on the successful laparoscopic approach to myomas larger than 20 cm, demonstrating that a skilled, experienced surgeon can perform this procedure safely.9-11
Many studies comparing LM with the abdominal approach showed that LM is associated with decreased blood loss, less postoperative pain, shorter hospital stay, and quicker recovery.12-14 Unfortunately, myomectomy via conventional laparoscopy can be technically challenging, thereby limiting patient accessibility to this approach. Major challenges with conventional LM include enucleation of the fibroid along the correct plane and a multilayered hysterotomy closure.15 The obvious concern with the latter is the potential risk for uterine rupture when improperly performed as a result of deficient suturing skills. Accordingly, several cases of uterine rupture in the second and third trimester of pregnancy after LM led to recommendations for stricter selection criteria, which excluded patients with fibroids larger than 5 cm, multiple fibroids, and deep intramural fibroids.16
Continue to: The RALM approach
The RALM approach
RALM was developed as a surgical alternative and to help overcome conventional laparoscopy challenges, such as suturing, as well as to offer minimally invasive options to a broader patient pool. In 2004, Advincula and colleagues reported the first case series of 35 women who underwent RALM.17 Since that report was published, multiple retrospective studies have confirmed RALM’s safety, feasibility, and efficacy.
How RALM stacks up against laparotomy. Compared with traditional abdominal myomectomy (AM), RALM has been associated with less blood loss, shorter hospital stay, quicker recovery time, fewer complications, and higher costs.18 In a comparative analysis of surgical outcomes and costs of RALM versus AM, Nash and colleagues found that RALM patients required less intravenous narcotics, had shorter hospital stays, and had equivalent clinical outcomes compared with AM-treated patients.19 In addition, the authors observed a correlation between increased specimen size and decreased operative efficiency with RALM. Retrospective cohort studies by Mansour and colleagues and Sangha and colleagues echoed similar conclusions.20,21
RALM versus conventional LM. The comparisons between conventional LM and RALM are not as clear-cut, and although evidence strongly suggests a role for RALM, more comparative studies are needed.
In 2013, Pundir and colleagues completed a meta-analysis and systematic review comparing RALM with AM and LM.22 They reviewed 10 observational studies; 7 compared RALM with AM, 4 compared RALM with LM, and 1 study compared RALM with AM and LM (this was included in both groups). In the comparison between RALM and AM, estimated blood loss, blood transfusion, and length of hospital stay were significantly lower with RALM, risk of complication was similar, and operating time and costs were significantly higher. The cost findings were not too dissimilar to conclusions drawn by Advincula and colleagues in an earlier study.18
Further, when Pundir and colleagues compared RALM with LM, blood transfusion risk and costs were higher with RALM, but no significant differences were noted in estimated blood loss, operating time, length of hospital stay, and complications.22 In this analysis, RALM showed significant short-term benefits when compared with AM but no benefit when compared with LM.
Continue to: Benefits after RALM over time
Benefits after RALM over time
Long-term benefits from RALM, such as symptom recurrence rates and fertility outcomes, have been demonstrated. In 2015, Pitter and colleagues published the first paper on symptom recurrence after RALM.23 In this retrospective survey, 426 women underwent RALM for symptom relief or infertility across 3 practice sites; 62.9% reported being symptom free after 3 years. In addition, 80% of symptom-free women who had undergone RALM to improve fertility outcomes conceived after 3 years. The mean (SD) time to pregnancy was 7.9 (9.4) months. Overall, pregnancy rates improved and symptom recurrence increased with the interval of time since surgery.23
In another study, Pitter and colleagues reported on pregnancy outcomes in greater detail.24 They evaluated 872 women who underwent RALM between October 2005 and November 2010 at 3 centers. Of these women, 107 conceived, resulting in 127 pregnancies and 92 deliveries through 2011. The means (SD) for age at myomectomy, number of myomas removed, and myoma size were 34.8 (4.5) years, 3.9 (3.2), and 7.5 (3.0) cm (weight, 191.7 [144.8] g), respectively. Overall, the pregnancy outcomes in this study were comparable to those reported in the literature for conventional LM.
Cela and colleagues reported similar outcomes based on their review of 48 patients who underwent RALM between 2007 and 2011.25 Seven women became pregnant (8 pregnancies). There were no spontaneous abortions or uterine ruptures. Following suit, Kang and colleagues reported outcomes in 100 women who underwent RALM for deep intramural fibroids (FIGO type 2 to 5).26 The average (SD) number of fibroids was 3.8 (3.5) with a mean (SD) size of 7.5 (2.1) cm. All patients recovered without major complications, and 75% of those pursuing pregnancy conceived.
The importance of LM and RALM
After this brief review of the data on conventional LM and RALM, it is fair to conclude that both surgical options are a game changer for the minimally invasive management of uterine fibroids. Despite strong evidence that suggests laparoscopy is superior to laparotomy for myomectomy, the technical demands required for performing conventional LM may explain why it is underutilized and why the advantages of robotic surgery—with its 3-dimensional imaging and articulated instruments—make this approach an attractive alternative.
The myomectomy technique we prefer at our institution
At our medical center, we approach the majority of abdominal myomectomies via conventional LM or RALM. We carefully select candidates with the goal of ensuring a successful procedure and minimizing the risk of conversion. When selecting candidates, we consider these factors:
- size, number, location, and consistency of the fibroids
- patient’s body habitus, and
- relative size of the uterus to the length of the patient’s torso.
Additionally, any concerns raised during the preoperative workup regarding a suspected risk of occult leiomyosarcoma preclude a minimally invasive approach. Otherwise, deciding between
conventional LM and RALM is based on surgeon preference.
View these surgical techniques on the multimedia channel
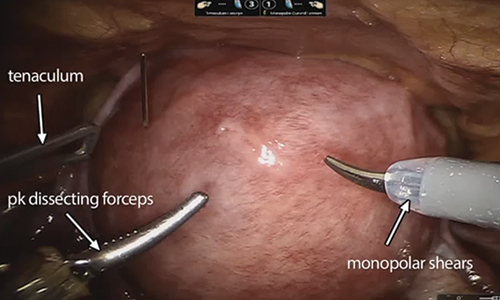
Robot-assisted laparoscopic myomectomy
Arnold P. Advincula, MD, Victoria M. Fratto, MD, and Caroline Key
A systematic approach to surgery in a 39-year-old woman with heavy menstrual bleeding who desires future fertility. Features include robot-specific techniques that facilitate fibroid enucleation and hysterotomy repair and demonstration of the ExCITE technique for tissue extraction.
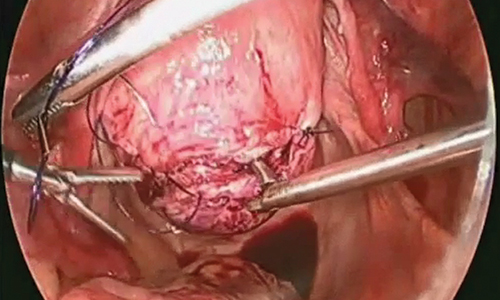
Laparoscopic myomectomy technique
William H. Parker, MD
A step-by-step demonstration of the laparoscopic myomectomy technique used to resect a 7-cm posterior fibroid in a 44-year-old woman.
Laparoscopic myomectomy with enclosed transvaginal tissue extraction
Ceana Nezhat, MD, and Erica Dun, MD, MPH
A surgical case of a 41-yearold woman with radiating lower abdominal pain and menorrhagia who desired removal of symptomatic myomas. Preoperative transvaginal ultrasonography revealed a 4-cm posterior pedunculated myoma and a 5-cm fundal intramural myoma.
Continue to: Preoperative MRI guides surgical approach
Preoperative MRI guides surgical approach
An MRI scan is a critical component of the patient’s preoperative evaluation. It helps to define the uterine architecture as it relates to fibroids and to rule out the presence of adenomyosis. In general, we do not offer RALM to patients who have more than 15 myomas, a single myoma that is larger than 12 to 15 cm, or when the uterus is more than 2 fingerbreadths above the umbilicus (unless the patient’s torso allows for an adequate insufflated workspace). We also try to avoid preoperative treatment with a gonadotropin–releasing hormone agonist to minimize softening of the myoma and blurring of the dissection planes.
Steps in the procedure

Once the patient is intubated, properly positioned, prepped, and draped, we turn our attention toward peritoneal entry. Factors that influence entry include the patient’s surgical history, radiologic imaging, physical examination (particularly the exam under anesthesia), and surgeon preference for optimizing access. Quite often we use a left upper quadrant entry via Palmer’s point, with subsequent port placement individualized to the patient’s pathology and abdominal topography. Three or more incisions are required to accommodate the camera and at least 2 to 3 operative instruments. Port sizes vary from 5 to 12 mm depending on the desired equipment and surgeon preference (conventional LM versus RALM [FIGURE 1]).
A uterine manipulator is a crucial tool used when performing LM.27 This instrument enables elevation of the uterus to allow for adequate visualization of the targeted myomas, traction-countertraction during enucleation, and strategic positioning during hysterotomy repair. We also use a bedside-mounted electric uterine positioning system that provides static orientation of the uterus by interfacing with the uterine manipulator, thereby obviating the need for a bedside assistant to provide that service (FIGURE 2).

To minimize blood loss during the course of the myomectomy, we inject a dilute concentration of vasopressin (20 U in 50 mL of saline) via a 7-inch, 22-gauge spinal needle into the myometrium surrounding the targeted myomas (FIGURE 3). Additional methods for mitigating blood loss include the use of vascular clamps, clips, or ties (both permanent and temporary) on the bilateral uterine arteries; intravaginal prostaglandins; intravenous tranexamic acid; gelatin-thrombin matrices; and cell salvage systems.28
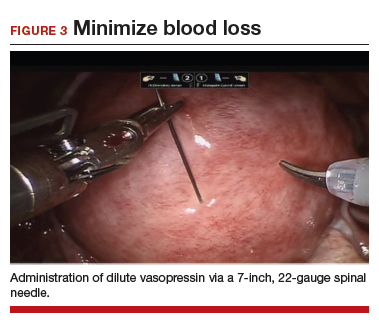
Once we observe adequate myometrial blanching from the vasopressin administration, we make a strategic hysterotomy incision (preferably transverse) to allow the surgeon to more ergonomically close the defect. We then identify the pseudocapsule so that the surgeon can circumferentially enucleate the myoma and dissect it from its fibrous attachments to the surrounding myometrium.
Continue to: The energy devices used to perform the hysterotomy...
The energy devices used to perform the hysterotomy and enucleation are selected largely based on surgeon preference, but various instruments can be used to accomplish these steps, including an ultrasonically activated scalpel or such electrosurgical instruments as monopolar scissors or hooks.
A reliable tenaculum is critical to the success of any enucleation, whether the approach is conventional LM or RALM, in order to provide adequate traction on the myoma (FIGURE 4). We try to minimize the number of hysterotomy incisions not only to reduce further blood loss, as the majority of bleeding ensues from the surrounding myometrium, but also to minimize compromise of myometrial integrity. Additionally, we take care to avoid entry into the endometrial cavity.
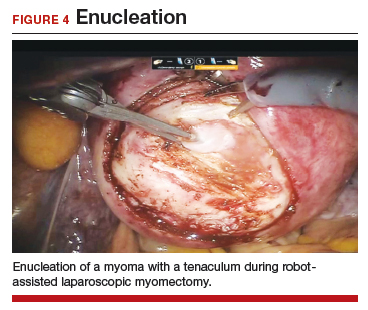
As we enucleate a myoma, we place it in either the anterior or posterior cul de sac. Most important, if we enucleate multiple myomas, we keep careful track of their number. We string the myomas together with suture until we extract them to ensure this.
While hysterotomy closure can be performed with either barbed or nonbarbed sutures in a single- or a multi-layered fashion, we prefer to use a barbed suture.29,30 Just as enucleation requires appropriate instruments, suturing requires proper needle drivers (FIGURE 5). We advise judicious use of energy to minimize thermal effects and maintain the viability of the surrounding myometrium. Once we have sutured the myometrium closed, we place an adhesion barrier.
Although discussion of tissue extraction is beyond the scope of this Update, any surgeon embarking on either conventional LM or RALM must have a strategy for safe contained tissue extraction given the recent concerns over uncontained power morcellation.31,32
Surgical skill and careful patient selection are key to optimal outcomes
Patients seeking conservative surgical management of their uterine fibroids should be considered candidates for either a conventional LM or RALM. Both the scientific literature and technologic advances make these approaches viable options, especially when the surgeon’s skill is appropriate and the patient’s candidacy is adequately vetted. A well thought out surgical strategy from start to finish will ensure the chances for successful completion and optimized outcomes.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Matchar DB, Myers ER, Barber MW, et al. Management of uterine fibroids: summary. AHRQ Evidence Report Summaries. Rockville, MD; Agency for Healthcare Research and Quality; 2001. AHRQ Publication No. 01-E051.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Stewart EA. Uterine fibroids. Lancet. 2001;357:293-298.
- Nash K, Feinglass J, Zei C, et al. Robotic-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparative analysis of surgical outcomes and costs. Arch Gynecol Obstet. 2012;285:435-440.
- Herrmann A, De Wilde RL. Laparoscopic myomectomy—the gold standard. Gynecol Minim Invasive Ther. 2014;3:31-38.
- Stoica RA, Bistriceanu I, Sima R, et al. Laparoscopic myomectomy. J Med Life. 2014;7:522-524.
- Donnez J, Dolmans MM. Uterine fibroid management: from the present to the future. Hum Reprod Update. 2016;22:665-686.
- Holub Z. Laparoscopic myomectomy: indications and limits. Ceska Gynekol. 2007;72:64-68.
- Sinha R, Hegde A, Mahajan C, et al. Laparoscopic myomectomy: do size, number, and location of the myomas form limiting factors for laparoscopic myomectomy? J Minim Invasive Gynecol. 2008;15:292-300.
- Aksoy H, Aydin T, Ozdamar O, et al. Successful use of laparoscopic myomectomy to remove a giant uterine myoma: a case report. J Med Case Rep. 2015;9:286.
- Damiani A, Melgrati L, Marziali M, et al. Laparoscopic myomectomy for very large myomas using an isobaric (gasless) technique. JSLS. 2005;9:434-438.
- Holzer A, Jirecek ST, Illievich UM, et al. Laparoscopic versus open myomectomy: a double-blind study to evaluate postoperative pain. Anesth Analg. 2006;102:1480-1484.
- Mais V, Ajossa S, Guerriero S, et al. Laparoscopic versus abdominal myomectomy: a prospective, randomized trial to evaluate benefits in early outcome. Am J Obstet Gynecol. 1996;174:654-658.
- Jin C, Hu Y, Chen XC, et al. Laparoscopic versus open myomectomy—a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2009;145:14-21.
- Pluchino N, Litta P, Freschi L, et al. Comparison of the initial surgical experience with robotic and laparoscopic myomectomy. Int J Med Robot. 2014;10:208-212.
- Parker WH, Iacampo K, Long T. Uterine rupture after laparoscopic removal of a pedunculated myoma. J Minim Invasive Gynecol. 2007;14:362-364.
- Advincula AP, Song A, Burke W, et al. Preliminary experience with robot-assisted laparoscopic myomectomy. J Am Assoc Gynecol Laparosc. 2004;11:511-518.
- Advincula AP, Xu X, Goudeau S 4th, et al. Robot-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparison of short-term surgical outcomes and immediate costs. J Minim Invasive Gynecol. 2007;14:698-705.
- Nash K, Feinglass J, Zei C, et al. Robotic-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparative analysis of surgical outcomes and costs. Arch Gynecol Obstet. 2012;285:435-440.
- Mansour FW, Kives S, Urbach DR, et al. Robotically assisted laparoscopic myomectomy: a Canadian experience. J Obstet Gynaecol Canada. 2012;34:353-358.
- Sangha R, Eisenstein D, George A, et al. Comparison of surgical outcomes for robotic assisted laparoscopic myomectomy compared to abdominal myomectomy (abstract 373). J Minim Invasive Gynecol. 2010;17(suppl):S90-S108.
- Pundir J, Pundir V, Walavalkar R, et al. Robotic-assisted laparoscopic vs abdominal and laparoscopic myomectomy: systematic review and meta-analysis. J Minim Invasive Gynecol. 2013; 20:335–345.
- Pitter MC, Srouji SS, Gargiulo AR, et al. Fertility and symptom relief following robot-assisted laparoscopic myomectomy. Obstet Gynecol Int. 2015. doi:10.1155/2015/967568.
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013; 28:99-108.
- Cela V, Freschi L, Simi G, et al. Fertility and endocrine outcome after robot-assisted laparoscopic myomectomy (RALM). Gynecol Endocrinol. 2013;29:79-82.
- Kang SY, Jeung IC, Chung YJ, et al. Robot-assisted laparoscopic myomectomy for deep intramural myomas. Int J Med Robot. 2017;13. doi:10.1002/rcs.1742.
- van den Haak L, Alleblas C, Nieboer TE, et al. Efficacy and safety of uterine manipulators in laparoscopic surgery: a review. Arch Gynecol Obstet. 2015;292:1003-1011.
- Hickman LC, Kotlyar A, Shue S, et al. Hemostatic techniques for myomectomy: an evidence-based approach. J Minim Invasive Gynecol. 2016;23:497-504.
- Tulandi T, Einarsson JI. The use of barbed suture for laparoscopic hysterectomy and myomectomy: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2014;21:210-216.
- Alessandri F, Remorgida V, Venturini PL, et al. Unidirectional barbed suture versus continuous suture with intracorporeal knots in laparoscopic myomectomy: a randomized study. J Minim Invasive Gynecol. 2010;17:725-729.
- AAGL Advancing Minimally Invasive Gynecology Worldwide. AAGL practice report: morcellation during uterine tissue extraction. J Minim Invasive Gynecol. 2014;21:517-530.
- Meurs EA, Brito LG, Ajao MO, et al. Comparison of morcellation techniques at the time of laparoscopic hysterectomy and myomectomy. J Minim Invasive Gynecol. 2017;24:843-849.
Uterine fibroids are the most common solid pelvic tumor in women and a leading indication for hysterectomy in the United States.1 As a result, they represent significant morbidity for many women and are a major public health problem. By age 50, 70% of white women and 80% of black women have fibroids.2
Although fibroids are sometimes asymptomatic, the symptoms most commonly reported are abnormal uterine bleeding (AUB) with resultant anemia and bulk/pressure symptoms. Uterine fibroids also are associated with reproductive dysfunction, such as recurrent pregnancy loss, and even infertility.3
The clinical diagnosis of uterine fibroids is made based on a combination of physical examination and imaging studies, including pelvic ultrasonography, saline infusion sonography, and magnetic resonance imaging (MRI). When medical management, such as combination oral contraceptive pills, fails in patients with AUB and/or bulk predominant symptoms or patients present with compromised fertility, the only option for conservative surgical management is a myomectomy.4
The route of myomectomy—hysteroscopy, laparotomy, conventional laparoscopic myomectomy (LM), or robot-assisted laparoscopic myomectomy (RALM)—depends on the size, number, location, and consistency of the uterine fibroids and, to a certain extent, the indication for the myomectomy. In some cases, multiple routes must be used to achieve optimal results, and sometimes these procedures have to be staged. In this literature review and technical summary, we focus on conventional LM and RALM approaches.
Literature review: In the right hands, LM and RALM have clear benefits
In the past, laparotomy was the surgical route of choice for fibroid removal. This surgery was associated with a long hospital stay, a high rate of blood transfusions, postoperative pain, and a lengthy recovery period. As minimally invasive surgery gained popularity, conventional LM became more commonly performed and was accepted by many as the gold standard approach for myomectomy.5
LM has considerable advantages over laparotomy
Compared with the traditional, more invasive route, the conventional LM approach has many benefits. These include less blood loss, decreased postoperative pain, shorter recovery time, shorter hospitalization stay, and decreased perioperative complications.6 LM should be considered the first-line approach unless the size of an intramural myoma exceeds 10 to 12 cm or multiple myomas (consensus, approximately 4 or more) are present and necessitate several incisions according to their varying locations within the uterus.7,8 While this is a recommendation, reports have been published on the successful laparoscopic approach to myomas larger than 20 cm, demonstrating that a skilled, experienced surgeon can perform this procedure safely.9-11
Many studies comparing LM with the abdominal approach showed that LM is associated with decreased blood loss, less postoperative pain, shorter hospital stay, and quicker recovery.12-14 Unfortunately, myomectomy via conventional laparoscopy can be technically challenging, thereby limiting patient accessibility to this approach. Major challenges with conventional LM include enucleation of the fibroid along the correct plane and a multilayered hysterotomy closure.15 The obvious concern with the latter is the potential risk for uterine rupture when improperly performed as a result of deficient suturing skills. Accordingly, several cases of uterine rupture in the second and third trimester of pregnancy after LM led to recommendations for stricter selection criteria, which excluded patients with fibroids larger than 5 cm, multiple fibroids, and deep intramural fibroids.16
Continue to: The RALM approach
The RALM approach
RALM was developed as a surgical alternative and to help overcome conventional laparoscopy challenges, such as suturing, as well as to offer minimally invasive options to a broader patient pool. In 2004, Advincula and colleagues reported the first case series of 35 women who underwent RALM.17 Since that report was published, multiple retrospective studies have confirmed RALM’s safety, feasibility, and efficacy.
How RALM stacks up against laparotomy. Compared with traditional abdominal myomectomy (AM), RALM has been associated with less blood loss, shorter hospital stay, quicker recovery time, fewer complications, and higher costs.18 In a comparative analysis of surgical outcomes and costs of RALM versus AM, Nash and colleagues found that RALM patients required less intravenous narcotics, had shorter hospital stays, and had equivalent clinical outcomes compared with AM-treated patients.19 In addition, the authors observed a correlation between increased specimen size and decreased operative efficiency with RALM. Retrospective cohort studies by Mansour and colleagues and Sangha and colleagues echoed similar conclusions.20,21
RALM versus conventional LM. The comparisons between conventional LM and RALM are not as clear-cut, and although evidence strongly suggests a role for RALM, more comparative studies are needed.
In 2013, Pundir and colleagues completed a meta-analysis and systematic review comparing RALM with AM and LM.22 They reviewed 10 observational studies; 7 compared RALM with AM, 4 compared RALM with LM, and 1 study compared RALM with AM and LM (this was included in both groups). In the comparison between RALM and AM, estimated blood loss, blood transfusion, and length of hospital stay were significantly lower with RALM, risk of complication was similar, and operating time and costs were significantly higher. The cost findings were not too dissimilar to conclusions drawn by Advincula and colleagues in an earlier study.18
Further, when Pundir and colleagues compared RALM with LM, blood transfusion risk and costs were higher with RALM, but no significant differences were noted in estimated blood loss, operating time, length of hospital stay, and complications.22 In this analysis, RALM showed significant short-term benefits when compared with AM but no benefit when compared with LM.
Continue to: Benefits after RALM over time
Benefits after RALM over time
Long-term benefits from RALM, such as symptom recurrence rates and fertility outcomes, have been demonstrated. In 2015, Pitter and colleagues published the first paper on symptom recurrence after RALM.23 In this retrospective survey, 426 women underwent RALM for symptom relief or infertility across 3 practice sites; 62.9% reported being symptom free after 3 years. In addition, 80% of symptom-free women who had undergone RALM to improve fertility outcomes conceived after 3 years. The mean (SD) time to pregnancy was 7.9 (9.4) months. Overall, pregnancy rates improved and symptom recurrence increased with the interval of time since surgery.23
In another study, Pitter and colleagues reported on pregnancy outcomes in greater detail.24 They evaluated 872 women who underwent RALM between October 2005 and November 2010 at 3 centers. Of these women, 107 conceived, resulting in 127 pregnancies and 92 deliveries through 2011. The means (SD) for age at myomectomy, number of myomas removed, and myoma size were 34.8 (4.5) years, 3.9 (3.2), and 7.5 (3.0) cm (weight, 191.7 [144.8] g), respectively. Overall, the pregnancy outcomes in this study were comparable to those reported in the literature for conventional LM.
Cela and colleagues reported similar outcomes based on their review of 48 patients who underwent RALM between 2007 and 2011.25 Seven women became pregnant (8 pregnancies). There were no spontaneous abortions or uterine ruptures. Following suit, Kang and colleagues reported outcomes in 100 women who underwent RALM for deep intramural fibroids (FIGO type 2 to 5).26 The average (SD) number of fibroids was 3.8 (3.5) with a mean (SD) size of 7.5 (2.1) cm. All patients recovered without major complications, and 75% of those pursuing pregnancy conceived.
The importance of LM and RALM
After this brief review of the data on conventional LM and RALM, it is fair to conclude that both surgical options are a game changer for the minimally invasive management of uterine fibroids. Despite strong evidence that suggests laparoscopy is superior to laparotomy for myomectomy, the technical demands required for performing conventional LM may explain why it is underutilized and why the advantages of robotic surgery—with its 3-dimensional imaging and articulated instruments—make this approach an attractive alternative.
The myomectomy technique we prefer at our institution
At our medical center, we approach the majority of abdominal myomectomies via conventional LM or RALM. We carefully select candidates with the goal of ensuring a successful procedure and minimizing the risk of conversion. When selecting candidates, we consider these factors:
- size, number, location, and consistency of the fibroids
- patient’s body habitus, and
- relative size of the uterus to the length of the patient’s torso.
Additionally, any concerns raised during the preoperative workup regarding a suspected risk of occult leiomyosarcoma preclude a minimally invasive approach. Otherwise, deciding between
conventional LM and RALM is based on surgeon preference.
View these surgical techniques on the multimedia channel

Robot-assisted laparoscopic myomectomy
Arnold P. Advincula, MD, Victoria M. Fratto, MD, and Caroline Key
A systematic approach to surgery in a 39-year-old woman with heavy menstrual bleeding who desires future fertility. Features include robot-specific techniques that facilitate fibroid enucleation and hysterotomy repair and demonstration of the ExCITE technique for tissue extraction.

Laparoscopic myomectomy technique
William H. Parker, MD
A step-by-step demonstration of the laparoscopic myomectomy technique used to resect a 7-cm posterior fibroid in a 44-year-old woman.
Laparoscopic myomectomy with enclosed transvaginal tissue extraction
Ceana Nezhat, MD, and Erica Dun, MD, MPH
A surgical case of a 41-yearold woman with radiating lower abdominal pain and menorrhagia who desired removal of symptomatic myomas. Preoperative transvaginal ultrasonography revealed a 4-cm posterior pedunculated myoma and a 5-cm fundal intramural myoma.
Continue to: Preoperative MRI guides surgical approach
Preoperative MRI guides surgical approach
An MRI scan is a critical component of the patient’s preoperative evaluation. It helps to define the uterine architecture as it relates to fibroids and to rule out the presence of adenomyosis. In general, we do not offer RALM to patients who have more than 15 myomas, a single myoma that is larger than 12 to 15 cm, or when the uterus is more than 2 fingerbreadths above the umbilicus (unless the patient’s torso allows for an adequate insufflated workspace). We also try to avoid preoperative treatment with a gonadotropin–releasing hormone agonist to minimize softening of the myoma and blurring of the dissection planes.

Steps in the procedure

Once the patient is intubated, properly positioned, prepped, and draped, we turn our attention toward peritoneal entry. Factors that influence entry include the patient’s surgical history, radiologic imaging, physical examination (particularly the exam under anesthesia), and surgeon preference for optimizing access. Quite often we use a left upper quadrant entry via Palmer’s point, with subsequent port placement individualized to the patient’s pathology and abdominal topography. Three or more incisions are required to accommodate the camera and at least 2 to 3 operative instruments. Port sizes vary from 5 to 12 mm depending on the desired equipment and surgeon preference (conventional LM versus RALM [FIGURE 1]).
A uterine manipulator is a crucial tool used when performing LM.27 This instrument enables elevation of the uterus to allow for adequate visualization of the targeted myomas, traction-countertraction during enucleation, and strategic positioning during hysterotomy repair. We also use a bedside-mounted electric uterine positioning system that provides static orientation of the uterus by interfacing with the uterine manipulator, thereby obviating the need for a bedside assistant to provide that service (FIGURE 2).

To minimize blood loss during the course of the myomectomy, we inject a dilute concentration of vasopressin (20 U in 50 mL of saline) via a 7-inch, 22-gauge spinal needle into the myometrium surrounding the targeted myomas (FIGURE 3). Additional methods for mitigating blood loss include the use of vascular clamps, clips, or ties (both permanent and temporary) on the bilateral uterine arteries; intravaginal prostaglandins; intravenous tranexamic acid; gelatin-thrombin matrices; and cell salvage systems.28

Once we observe adequate myometrial blanching from the vasopressin administration, we make a strategic hysterotomy incision (preferably transverse) to allow the surgeon to more ergonomically close the defect. We then identify the pseudocapsule so that the surgeon can circumferentially enucleate the myoma and dissect it from its fibrous attachments to the surrounding myometrium.
Continue to: The energy devices used to perform the hysterotomy...
The energy devices used to perform the hysterotomy and enucleation are selected largely based on surgeon preference, but various instruments can be used to accomplish these steps, including an ultrasonically activated scalpel or such electrosurgical instruments as monopolar scissors or hooks.
A reliable tenaculum is critical to the success of any enucleation, whether the approach is conventional LM or RALM, in order to provide adequate traction on the myoma (FIGURE 4). We try to minimize the number of hysterotomy incisions not only to reduce further blood loss, as the majority of bleeding ensues from the surrounding myometrium, but also to minimize compromise of myometrial integrity. Additionally, we take care to avoid entry into the endometrial cavity.

As we enucleate a myoma, we place it in either the anterior or posterior cul de sac. Most important, if we enucleate multiple myomas, we keep careful track of their number. We string the myomas together with suture until we extract them to ensure this.
While hysterotomy closure can be performed with either barbed or nonbarbed sutures in a single- or a multi-layered fashion, we prefer to use a barbed suture.29,30 Just as enucleation requires appropriate instruments, suturing requires proper needle drivers (FIGURE 5). We advise judicious use of energy to minimize thermal effects and maintain the viability of the surrounding myometrium. Once we have sutured the myometrium closed, we place an adhesion barrier.

Although discussion of tissue extraction is beyond the scope of this Update, any surgeon embarking on either conventional LM or RALM must have a strategy for safe contained tissue extraction given the recent concerns over uncontained power morcellation.31,32
Surgical skill and careful patient selection are key to optimal outcomes
Patients seeking conservative surgical management of their uterine fibroids should be considered candidates for either a conventional LM or RALM. Both the scientific literature and technologic advances make these approaches viable options, especially when the surgeon’s skill is appropriate and the patient’s candidacy is adequately vetted. A well thought out surgical strategy from start to finish will ensure the chances for successful completion and optimized outcomes.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Uterine fibroids are the most common solid pelvic tumor in women and a leading indication for hysterectomy in the United States.1 As a result, they represent significant morbidity for many women and are a major public health problem. By age 50, 70% of white women and 80% of black women have fibroids.2
Although fibroids are sometimes asymptomatic, the symptoms most commonly reported are abnormal uterine bleeding (AUB) with resultant anemia and bulk/pressure symptoms. Uterine fibroids also are associated with reproductive dysfunction, such as recurrent pregnancy loss, and even infertility.3
The clinical diagnosis of uterine fibroids is made based on a combination of physical examination and imaging studies, including pelvic ultrasonography, saline infusion sonography, and magnetic resonance imaging (MRI). When medical management, such as combination oral contraceptive pills, fails in patients with AUB and/or bulk predominant symptoms or patients present with compromised fertility, the only option for conservative surgical management is a myomectomy.4
The route of myomectomy—hysteroscopy, laparotomy, conventional laparoscopic myomectomy (LM), or robot-assisted laparoscopic myomectomy (RALM)—depends on the size, number, location, and consistency of the uterine fibroids and, to a certain extent, the indication for the myomectomy. In some cases, multiple routes must be used to achieve optimal results, and sometimes these procedures have to be staged. In this literature review and technical summary, we focus on conventional LM and RALM approaches.
Literature review: In the right hands, LM and RALM have clear benefits
In the past, laparotomy was the surgical route of choice for fibroid removal. This surgery was associated with a long hospital stay, a high rate of blood transfusions, postoperative pain, and a lengthy recovery period. As minimally invasive surgery gained popularity, conventional LM became more commonly performed and was accepted by many as the gold standard approach for myomectomy.5
LM has considerable advantages over laparotomy
Compared with the traditional, more invasive route, the conventional LM approach has many benefits. These include less blood loss, decreased postoperative pain, shorter recovery time, shorter hospitalization stay, and decreased perioperative complications.6 LM should be considered the first-line approach unless the size of an intramural myoma exceeds 10 to 12 cm or multiple myomas (consensus, approximately 4 or more) are present and necessitate several incisions according to their varying locations within the uterus.7,8 While this is a recommendation, reports have been published on the successful laparoscopic approach to myomas larger than 20 cm, demonstrating that a skilled, experienced surgeon can perform this procedure safely.9-11
Many studies comparing LM with the abdominal approach showed that LM is associated with decreased blood loss, less postoperative pain, shorter hospital stay, and quicker recovery.12-14 Unfortunately, myomectomy via conventional laparoscopy can be technically challenging, thereby limiting patient accessibility to this approach. Major challenges with conventional LM include enucleation of the fibroid along the correct plane and a multilayered hysterotomy closure.15 The obvious concern with the latter is the potential risk for uterine rupture when improperly performed as a result of deficient suturing skills. Accordingly, several cases of uterine rupture in the second and third trimester of pregnancy after LM led to recommendations for stricter selection criteria, which excluded patients with fibroids larger than 5 cm, multiple fibroids, and deep intramural fibroids.16
Continue to: The RALM approach
The RALM approach
RALM was developed as a surgical alternative and to help overcome conventional laparoscopy challenges, such as suturing, as well as to offer minimally invasive options to a broader patient pool. In 2004, Advincula and colleagues reported the first case series of 35 women who underwent RALM.17 Since that report was published, multiple retrospective studies have confirmed RALM’s safety, feasibility, and efficacy.
How RALM stacks up against laparotomy. Compared with traditional abdominal myomectomy (AM), RALM has been associated with less blood loss, shorter hospital stay, quicker recovery time, fewer complications, and higher costs.18 In a comparative analysis of surgical outcomes and costs of RALM versus AM, Nash and colleagues found that RALM patients required less intravenous narcotics, had shorter hospital stays, and had equivalent clinical outcomes compared with AM-treated patients.19 In addition, the authors observed a correlation between increased specimen size and decreased operative efficiency with RALM. Retrospective cohort studies by Mansour and colleagues and Sangha and colleagues echoed similar conclusions.20,21
RALM versus conventional LM. The comparisons between conventional LM and RALM are not as clear-cut, and although evidence strongly suggests a role for RALM, more comparative studies are needed.
In 2013, Pundir and colleagues completed a meta-analysis and systematic review comparing RALM with AM and LM.22 They reviewed 10 observational studies; 7 compared RALM with AM, 4 compared RALM with LM, and 1 study compared RALM with AM and LM (this was included in both groups). In the comparison between RALM and AM, estimated blood loss, blood transfusion, and length of hospital stay were significantly lower with RALM, risk of complication was similar, and operating time and costs were significantly higher. The cost findings were not too dissimilar to conclusions drawn by Advincula and colleagues in an earlier study.18
Further, when Pundir and colleagues compared RALM with LM, blood transfusion risk and costs were higher with RALM, but no significant differences were noted in estimated blood loss, operating time, length of hospital stay, and complications.22 In this analysis, RALM showed significant short-term benefits when compared with AM but no benefit when compared with LM.
Continue to: Benefits after RALM over time
Benefits after RALM over time
Long-term benefits from RALM, such as symptom recurrence rates and fertility outcomes, have been demonstrated. In 2015, Pitter and colleagues published the first paper on symptom recurrence after RALM.23 In this retrospective survey, 426 women underwent RALM for symptom relief or infertility across 3 practice sites; 62.9% reported being symptom free after 3 years. In addition, 80% of symptom-free women who had undergone RALM to improve fertility outcomes conceived after 3 years. The mean (SD) time to pregnancy was 7.9 (9.4) months. Overall, pregnancy rates improved and symptom recurrence increased with the interval of time since surgery.23
In another study, Pitter and colleagues reported on pregnancy outcomes in greater detail.24 They evaluated 872 women who underwent RALM between October 2005 and November 2010 at 3 centers. Of these women, 107 conceived, resulting in 127 pregnancies and 92 deliveries through 2011. The means (SD) for age at myomectomy, number of myomas removed, and myoma size were 34.8 (4.5) years, 3.9 (3.2), and 7.5 (3.0) cm (weight, 191.7 [144.8] g), respectively. Overall, the pregnancy outcomes in this study were comparable to those reported in the literature for conventional LM.
Cela and colleagues reported similar outcomes based on their review of 48 patients who underwent RALM between 2007 and 2011.25 Seven women became pregnant (8 pregnancies). There were no spontaneous abortions or uterine ruptures. Following suit, Kang and colleagues reported outcomes in 100 women who underwent RALM for deep intramural fibroids (FIGO type 2 to 5).26 The average (SD) number of fibroids was 3.8 (3.5) with a mean (SD) size of 7.5 (2.1) cm. All patients recovered without major complications, and 75% of those pursuing pregnancy conceived.
The importance of LM and RALM
After this brief review of the data on conventional LM and RALM, it is fair to conclude that both surgical options are a game changer for the minimally invasive management of uterine fibroids. Despite strong evidence that suggests laparoscopy is superior to laparotomy for myomectomy, the technical demands required for performing conventional LM may explain why it is underutilized and why the advantages of robotic surgery—with its 3-dimensional imaging and articulated instruments—make this approach an attractive alternative.
The myomectomy technique we prefer at our institution
At our medical center, we approach the majority of abdominal myomectomies via conventional LM or RALM. We carefully select candidates with the goal of ensuring a successful procedure and minimizing the risk of conversion. When selecting candidates, we consider these factors:
- size, number, location, and consistency of the fibroids
- patient’s body habitus, and
- relative size of the uterus to the length of the patient’s torso.
Additionally, any concerns raised during the preoperative workup regarding a suspected risk of occult leiomyosarcoma preclude a minimally invasive approach. Otherwise, deciding between
conventional LM and RALM is based on surgeon preference.
View these surgical techniques on the multimedia channel

Robot-assisted laparoscopic myomectomy
Arnold P. Advincula, MD, Victoria M. Fratto, MD, and Caroline Key
A systematic approach to surgery in a 39-year-old woman with heavy menstrual bleeding who desires future fertility. Features include robot-specific techniques that facilitate fibroid enucleation and hysterotomy repair and demonstration of the ExCITE technique for tissue extraction.

Laparoscopic myomectomy technique
William H. Parker, MD
A step-by-step demonstration of the laparoscopic myomectomy technique used to resect a 7-cm posterior fibroid in a 44-year-old woman.
Laparoscopic myomectomy with enclosed transvaginal tissue extraction
Ceana Nezhat, MD, and Erica Dun, MD, MPH
A surgical case of a 41-yearold woman with radiating lower abdominal pain and menorrhagia who desired removal of symptomatic myomas. Preoperative transvaginal ultrasonography revealed a 4-cm posterior pedunculated myoma and a 5-cm fundal intramural myoma.
Continue to: Preoperative MRI guides surgical approach
Preoperative MRI guides surgical approach
An MRI scan is a critical component of the patient’s preoperative evaluation. It helps to define the uterine architecture as it relates to fibroids and to rule out the presence of adenomyosis. In general, we do not offer RALM to patients who have more than 15 myomas, a single myoma that is larger than 12 to 15 cm, or when the uterus is more than 2 fingerbreadths above the umbilicus (unless the patient’s torso allows for an adequate insufflated workspace). We also try to avoid preoperative treatment with a gonadotropin–releasing hormone agonist to minimize softening of the myoma and blurring of the dissection planes.

Steps in the procedure

Once the patient is intubated, properly positioned, prepped, and draped, we turn our attention toward peritoneal entry. Factors that influence entry include the patient’s surgical history, radiologic imaging, physical examination (particularly the exam under anesthesia), and surgeon preference for optimizing access. Quite often we use a left upper quadrant entry via Palmer’s point, with subsequent port placement individualized to the patient’s pathology and abdominal topography. Three or more incisions are required to accommodate the camera and at least 2 to 3 operative instruments. Port sizes vary from 5 to 12 mm depending on the desired equipment and surgeon preference (conventional LM versus RALM [FIGURE 1]).
A uterine manipulator is a crucial tool used when performing LM.27 This instrument enables elevation of the uterus to allow for adequate visualization of the targeted myomas, traction-countertraction during enucleation, and strategic positioning during hysterotomy repair. We also use a bedside-mounted electric uterine positioning system that provides static orientation of the uterus by interfacing with the uterine manipulator, thereby obviating the need for a bedside assistant to provide that service (FIGURE 2).

To minimize blood loss during the course of the myomectomy, we inject a dilute concentration of vasopressin (20 U in 50 mL of saline) via a 7-inch, 22-gauge spinal needle into the myometrium surrounding the targeted myomas (FIGURE 3). Additional methods for mitigating blood loss include the use of vascular clamps, clips, or ties (both permanent and temporary) on the bilateral uterine arteries; intravaginal prostaglandins; intravenous tranexamic acid; gelatin-thrombin matrices; and cell salvage systems.28

Once we observe adequate myometrial blanching from the vasopressin administration, we make a strategic hysterotomy incision (preferably transverse) to allow the surgeon to more ergonomically close the defect. We then identify the pseudocapsule so that the surgeon can circumferentially enucleate the myoma and dissect it from its fibrous attachments to the surrounding myometrium.
Continue to: The energy devices used to perform the hysterotomy...
The energy devices used to perform the hysterotomy and enucleation are selected largely based on surgeon preference, but various instruments can be used to accomplish these steps, including an ultrasonically activated scalpel or such electrosurgical instruments as monopolar scissors or hooks.
A reliable tenaculum is critical to the success of any enucleation, whether the approach is conventional LM or RALM, in order to provide adequate traction on the myoma (FIGURE 4). We try to minimize the number of hysterotomy incisions not only to reduce further blood loss, as the majority of bleeding ensues from the surrounding myometrium, but also to minimize compromise of myometrial integrity. Additionally, we take care to avoid entry into the endometrial cavity.

As we enucleate a myoma, we place it in either the anterior or posterior cul de sac. Most important, if we enucleate multiple myomas, we keep careful track of their number. We string the myomas together with suture until we extract them to ensure this.
While hysterotomy closure can be performed with either barbed or nonbarbed sutures in a single- or a multi-layered fashion, we prefer to use a barbed suture.29,30 Just as enucleation requires appropriate instruments, suturing requires proper needle drivers (FIGURE 5). We advise judicious use of energy to minimize thermal effects and maintain the viability of the surrounding myometrium. Once we have sutured the myometrium closed, we place an adhesion barrier.

Although discussion of tissue extraction is beyond the scope of this Update, any surgeon embarking on either conventional LM or RALM must have a strategy for safe contained tissue extraction given the recent concerns over uncontained power morcellation.31,32
Surgical skill and careful patient selection are key to optimal outcomes
Patients seeking conservative surgical management of their uterine fibroids should be considered candidates for either a conventional LM or RALM. Both the scientific literature and technologic advances make these approaches viable options, especially when the surgeon’s skill is appropriate and the patient’s candidacy is adequately vetted. A well thought out surgical strategy from start to finish will ensure the chances for successful completion and optimized outcomes.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Matchar DB, Myers ER, Barber MW, et al. Management of uterine fibroids: summary. AHRQ Evidence Report Summaries. Rockville, MD; Agency for Healthcare Research and Quality; 2001. AHRQ Publication No. 01-E051.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Stewart EA. Uterine fibroids. Lancet. 2001;357:293-298.
- Nash K, Feinglass J, Zei C, et al. Robotic-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparative analysis of surgical outcomes and costs. Arch Gynecol Obstet. 2012;285:435-440.
- Herrmann A, De Wilde RL. Laparoscopic myomectomy—the gold standard. Gynecol Minim Invasive Ther. 2014;3:31-38.
- Stoica RA, Bistriceanu I, Sima R, et al. Laparoscopic myomectomy. J Med Life. 2014;7:522-524.
- Donnez J, Dolmans MM. Uterine fibroid management: from the present to the future. Hum Reprod Update. 2016;22:665-686.
- Holub Z. Laparoscopic myomectomy: indications and limits. Ceska Gynekol. 2007;72:64-68.
- Sinha R, Hegde A, Mahajan C, et al. Laparoscopic myomectomy: do size, number, and location of the myomas form limiting factors for laparoscopic myomectomy? J Minim Invasive Gynecol. 2008;15:292-300.
- Aksoy H, Aydin T, Ozdamar O, et al. Successful use of laparoscopic myomectomy to remove a giant uterine myoma: a case report. J Med Case Rep. 2015;9:286.
- Damiani A, Melgrati L, Marziali M, et al. Laparoscopic myomectomy for very large myomas using an isobaric (gasless) technique. JSLS. 2005;9:434-438.
- Holzer A, Jirecek ST, Illievich UM, et al. Laparoscopic versus open myomectomy: a double-blind study to evaluate postoperative pain. Anesth Analg. 2006;102:1480-1484.
- Mais V, Ajossa S, Guerriero S, et al. Laparoscopic versus abdominal myomectomy: a prospective, randomized trial to evaluate benefits in early outcome. Am J Obstet Gynecol. 1996;174:654-658.
- Jin C, Hu Y, Chen XC, et al. Laparoscopic versus open myomectomy—a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2009;145:14-21.
- Pluchino N, Litta P, Freschi L, et al. Comparison of the initial surgical experience with robotic and laparoscopic myomectomy. Int J Med Robot. 2014;10:208-212.
- Parker WH, Iacampo K, Long T. Uterine rupture after laparoscopic removal of a pedunculated myoma. J Minim Invasive Gynecol. 2007;14:362-364.
- Advincula AP, Song A, Burke W, et al. Preliminary experience with robot-assisted laparoscopic myomectomy. J Am Assoc Gynecol Laparosc. 2004;11:511-518.
- Advincula AP, Xu X, Goudeau S 4th, et al. Robot-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparison of short-term surgical outcomes and immediate costs. J Minim Invasive Gynecol. 2007;14:698-705.
- Nash K, Feinglass J, Zei C, et al. Robotic-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparative analysis of surgical outcomes and costs. Arch Gynecol Obstet. 2012;285:435-440.
- Mansour FW, Kives S, Urbach DR, et al. Robotically assisted laparoscopic myomectomy: a Canadian experience. J Obstet Gynaecol Canada. 2012;34:353-358.
- Sangha R, Eisenstein D, George A, et al. Comparison of surgical outcomes for robotic assisted laparoscopic myomectomy compared to abdominal myomectomy (abstract 373). J Minim Invasive Gynecol. 2010;17(suppl):S90-S108.
- Pundir J, Pundir V, Walavalkar R, et al. Robotic-assisted laparoscopic vs abdominal and laparoscopic myomectomy: systematic review and meta-analysis. J Minim Invasive Gynecol. 2013; 20:335–345.
- Pitter MC, Srouji SS, Gargiulo AR, et al. Fertility and symptom relief following robot-assisted laparoscopic myomectomy. Obstet Gynecol Int. 2015. doi:10.1155/2015/967568.
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013; 28:99-108.
- Cela V, Freschi L, Simi G, et al. Fertility and endocrine outcome after robot-assisted laparoscopic myomectomy (RALM). Gynecol Endocrinol. 2013;29:79-82.
- Kang SY, Jeung IC, Chung YJ, et al. Robot-assisted laparoscopic myomectomy for deep intramural myomas. Int J Med Robot. 2017;13. doi:10.1002/rcs.1742.
- van den Haak L, Alleblas C, Nieboer TE, et al. Efficacy and safety of uterine manipulators in laparoscopic surgery: a review. Arch Gynecol Obstet. 2015;292:1003-1011.
- Hickman LC, Kotlyar A, Shue S, et al. Hemostatic techniques for myomectomy: an evidence-based approach. J Minim Invasive Gynecol. 2016;23:497-504.
- Tulandi T, Einarsson JI. The use of barbed suture for laparoscopic hysterectomy and myomectomy: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2014;21:210-216.
- Alessandri F, Remorgida V, Venturini PL, et al. Unidirectional barbed suture versus continuous suture with intracorporeal knots in laparoscopic myomectomy: a randomized study. J Minim Invasive Gynecol. 2010;17:725-729.
- AAGL Advancing Minimally Invasive Gynecology Worldwide. AAGL practice report: morcellation during uterine tissue extraction. J Minim Invasive Gynecol. 2014;21:517-530.
- Meurs EA, Brito LG, Ajao MO, et al. Comparison of morcellation techniques at the time of laparoscopic hysterectomy and myomectomy. J Minim Invasive Gynecol. 2017;24:843-849.
- Matchar DB, Myers ER, Barber MW, et al. Management of uterine fibroids: summary. AHRQ Evidence Report Summaries. Rockville, MD; Agency for Healthcare Research and Quality; 2001. AHRQ Publication No. 01-E051.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Stewart EA. Uterine fibroids. Lancet. 2001;357:293-298.
- Nash K, Feinglass J, Zei C, et al. Robotic-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparative analysis of surgical outcomes and costs. Arch Gynecol Obstet. 2012;285:435-440.
- Herrmann A, De Wilde RL. Laparoscopic myomectomy—the gold standard. Gynecol Minim Invasive Ther. 2014;3:31-38.
- Stoica RA, Bistriceanu I, Sima R, et al. Laparoscopic myomectomy. J Med Life. 2014;7:522-524.
- Donnez J, Dolmans MM. Uterine fibroid management: from the present to the future. Hum Reprod Update. 2016;22:665-686.
- Holub Z. Laparoscopic myomectomy: indications and limits. Ceska Gynekol. 2007;72:64-68.
- Sinha R, Hegde A, Mahajan C, et al. Laparoscopic myomectomy: do size, number, and location of the myomas form limiting factors for laparoscopic myomectomy? J Minim Invasive Gynecol. 2008;15:292-300.
- Aksoy H, Aydin T, Ozdamar O, et al. Successful use of laparoscopic myomectomy to remove a giant uterine myoma: a case report. J Med Case Rep. 2015;9:286.
- Damiani A, Melgrati L, Marziali M, et al. Laparoscopic myomectomy for very large myomas using an isobaric (gasless) technique. JSLS. 2005;9:434-438.
- Holzer A, Jirecek ST, Illievich UM, et al. Laparoscopic versus open myomectomy: a double-blind study to evaluate postoperative pain. Anesth Analg. 2006;102:1480-1484.
- Mais V, Ajossa S, Guerriero S, et al. Laparoscopic versus abdominal myomectomy: a prospective, randomized trial to evaluate benefits in early outcome. Am J Obstet Gynecol. 1996;174:654-658.
- Jin C, Hu Y, Chen XC, et al. Laparoscopic versus open myomectomy—a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2009;145:14-21.
- Pluchino N, Litta P, Freschi L, et al. Comparison of the initial surgical experience with robotic and laparoscopic myomectomy. Int J Med Robot. 2014;10:208-212.
- Parker WH, Iacampo K, Long T. Uterine rupture after laparoscopic removal of a pedunculated myoma. J Minim Invasive Gynecol. 2007;14:362-364.
- Advincula AP, Song A, Burke W, et al. Preliminary experience with robot-assisted laparoscopic myomectomy. J Am Assoc Gynecol Laparosc. 2004;11:511-518.
- Advincula AP, Xu X, Goudeau S 4th, et al. Robot-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparison of short-term surgical outcomes and immediate costs. J Minim Invasive Gynecol. 2007;14:698-705.
- Nash K, Feinglass J, Zei C, et al. Robotic-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparative analysis of surgical outcomes and costs. Arch Gynecol Obstet. 2012;285:435-440.
- Mansour FW, Kives S, Urbach DR, et al. Robotically assisted laparoscopic myomectomy: a Canadian experience. J Obstet Gynaecol Canada. 2012;34:353-358.
- Sangha R, Eisenstein D, George A, et al. Comparison of surgical outcomes for robotic assisted laparoscopic myomectomy compared to abdominal myomectomy (abstract 373). J Minim Invasive Gynecol. 2010;17(suppl):S90-S108.
- Pundir J, Pundir V, Walavalkar R, et al. Robotic-assisted laparoscopic vs abdominal and laparoscopic myomectomy: systematic review and meta-analysis. J Minim Invasive Gynecol. 2013; 20:335–345.
- Pitter MC, Srouji SS, Gargiulo AR, et al. Fertility and symptom relief following robot-assisted laparoscopic myomectomy. Obstet Gynecol Int. 2015. doi:10.1155/2015/967568.
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013; 28:99-108.
- Cela V, Freschi L, Simi G, et al. Fertility and endocrine outcome after robot-assisted laparoscopic myomectomy (RALM). Gynecol Endocrinol. 2013;29:79-82.
- Kang SY, Jeung IC, Chung YJ, et al. Robot-assisted laparoscopic myomectomy for deep intramural myomas. Int J Med Robot. 2017;13. doi:10.1002/rcs.1742.
- van den Haak L, Alleblas C, Nieboer TE, et al. Efficacy and safety of uterine manipulators in laparoscopic surgery: a review. Arch Gynecol Obstet. 2015;292:1003-1011.
- Hickman LC, Kotlyar A, Shue S, et al. Hemostatic techniques for myomectomy: an evidence-based approach. J Minim Invasive Gynecol. 2016;23:497-504.
- Tulandi T, Einarsson JI. The use of barbed suture for laparoscopic hysterectomy and myomectomy: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2014;21:210-216.
- Alessandri F, Remorgida V, Venturini PL, et al. Unidirectional barbed suture versus continuous suture with intracorporeal knots in laparoscopic myomectomy: a randomized study. J Minim Invasive Gynecol. 2010;17:725-729.
- AAGL Advancing Minimally Invasive Gynecology Worldwide. AAGL practice report: morcellation during uterine tissue extraction. J Minim Invasive Gynecol. 2014;21:517-530.
- Meurs EA, Brito LG, Ajao MO, et al. Comparison of morcellation techniques at the time of laparoscopic hysterectomy and myomectomy. J Minim Invasive Gynecol. 2017;24:843-849.