User login
Can lifestyle modifications delay or prevent Alzheimer’s disease?
Clinicians have devoted strenuous efforts to secondary prevention of Alzheimer’s disease (AD) by diagnosing and treating patients as early as possible. Unfortunately, there is no cure for AD, and the field has witnessed recurrent failures of several pharmacotherapy candidates with either symptomatic or disease-modifying properties.1 An estimated one-third of AD cases can be attributed to modifiable risk factors.2 Thus, implementing primary prevention measures by addressing modifiable risk factors thought to contribute to the disease, with the goal of reducing the risk of developing AD, or at least delaying its onset, is a crucial public health strategy.
Cardiovascular risk factors, such as hypertension, hyperlipidemia, diabetes, hyperhomocysteinemia, obesity, and smoking, have emerged as substantive risk factors for AD.3 Optimal management of these major risk factors, especially in mid-life, may be a preventive approach against AD. Although detailing the evidence on the impact of managing cardiovascular risk factors to delay or prevent AD is beyond the scope of this article, it is becoming clear that “what is good for the heart is good for the brain.”
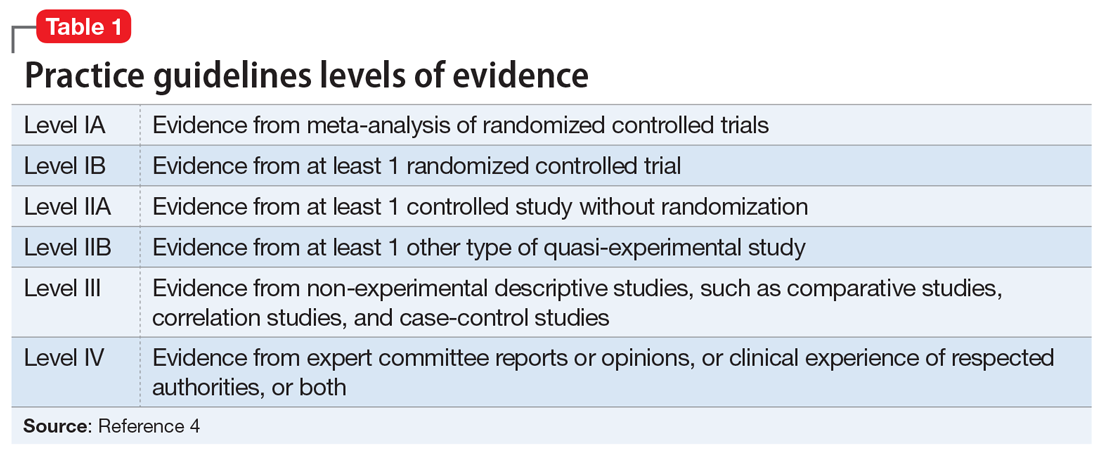
Additional modifiable risk factors are related to lifestyle habits, such as physical exercise, mental and social activity, meditation/spiritual activity, and diet. This article reviews the importance of pursuing a healthy lifestyle in delaying AD, with the corresponding levels of evidence that support each specific lifestyle modification. The levels of evidence are defined in Table 1.4
Physical exercise
Twenty-one percent of AD cases in the United States are attributable to physical inactivity.5 In addition to its beneficial effect on metabolic syndrome, in animal and human research, regular exercise has been shown to have direct neuroprotective effects. High levels of physical activity increase hippocampal neurogenesis and neuroplasticity, increase vascular circulation in the brain regions implicated in AD, and modulate inflammatory mediators as well as brain growth factors such as brain-derived neurotrophic factor (BDNF) and insulin-like growth factor-1 (IGF-1).6
The definition of regular physical exercise varies across the literature, but usually implies aerobic exercise—an ongoing activity sufficient to increase the heart rate and the need for oxygen, sustained for 20 to 30 minutes per session.7 Modalities include household activities and leisure-time activities. In a large prospective cohort study, Scarmeas et al8 categorized leisure-time activities into 3 types:
- light (walking, dancing, calisthenics, golfing, bowling, gardening, horseback riding)
- moderate (bicycling, swimming, hiking, playing tennis)
- vigorous (aerobic dancing, jogging, playing handball).
These types of physical exercise were weighed by the frequency of participation per week. Compared with being physically inactive, low levels of weekly physical activity (0.1 hours of vigorous, 0.8 hours of moderate, or 1.3 hours of light exercise) were associated with a 29% to 41% lower risk of developing AD, while higher weekly physical activity (1.3 hours of vigorous, 2.3 hours of moderate, or 3.8 hours of light exercise) were associated with a 37% to 50% lower risk (level III).8
In another 20-year cohort study, engaging in leisure-time physical activity at least twice a week in mid-life was significantly associated with a reduced risk of AD, after adjusting for age, sex, education, follow-up time, locomotor disorders, apolipoprotein E (ApoE) genotype, vascular disorders, smoking, and alcohol intake (level III).9 Moreover, a systematic review of 29 randomized controlled trials (RCTs) showed that aerobic exercise training, such as brisk walking, jogging, and biking, was associated with improvements in attention, processing speed, executive function, and memory among healthy older adults and those with mild cognitive impairment (MCI; level IA).10
Continue to: From a pathophysiological standpoint...
From a pathophysiological standpoint, higher levels of physical exercise in cognitively intact older adults have been associated with reduced brain amyloid beta deposits, especially in ApoE4 carriers.11 This inverse relationship also has been demonstrated in patients who are presymptomatic who carry 1 of the 3 known autosomal dominant mutations for the familial forms of AD.12
Overall, physicians should recommend that patients—especially those with cardiovascular risk factors that increase their risk for AD—exercise regularly by following the guidelines of the American Heart Association or the American College of Sports Medicine.13 These include muscle-strengthening activities (legs, hips, back, abdomen, shoulders, and arms) at least 2 days/week, in addition to either 30 minutes/day of moderate-intensity aerobic activity such as brisk walking, 5 days/week; or 25 minutes of vigorous aerobic activity such as jogging and running, 3 days/week14 (level IA evidence for overall improvement in cognitive function; level III evidence for AD delay/risk reduction). Neuromotor exercise, such as yoga and tai chi, and flexibility exercise such as muscle stretching, especially after a hot bath, 2 to 3 days/week are also recommended (level III).15
Mental activity
Nineteen percent of AD cases worldwide and 7% in the United States. can be attributed to low educational attainment, which is associated with low brain cognitive reserve.5 Cognitive resilience in later life may be enhanced by building brain reserves through intellectual stimulation, which affects neuronal branching and plasticity.16 Higher levels of complex mental activities measured across the lifespan, such as education, occupation, reading, and writing, are correlated with significantly less hippocampal volume shrinkage over time.17 Frequent participation in mentally stimulating activities—such as listening to the radio; reading newspapers, magazines, or books; playing games (cards, checkers, crosswords or other puzzles); and visiting museums—was associated with an up to 64% reduction in the odds of developing AD in a cohort of cognitively intact older adults followed for 4 years.18 The correlation between mental activity and AD was found to be independent of physical activity, social activity, or baseline cognitive function.19
In a large cohort of cognitively intact older adults (mean age 70), engaging in a mentally stimulating activity (craft activities, computer use, or going to the theater/movies) once to twice a week was significantly associated with a reduced incidence of amnestic MCI.20 Another prospective 21-year study demonstrated a significant reduction in AD risk in community-dwelling cognitively intact older adults (age 75 to 85) who participated in cognitively stimulating activities, such as reading books or newspapers, writing for pleasure, doing crossword puzzles, playing board games or cards, or playing musical instruments, several times/week.21
Growing scientific evidence also suggests that lifelong multilingualism can delay AD onset by 4 to 5 years.22 Multilingualism is associated with greater cognitive reserve, gray matter volume, functional connectivity and white matter density.23
Continue to: Physicians should encourage their patients...
Physicians should encourage their patients to engage in intellectually stimulating activities and creative leisure-time activities several times/week to enhance their cognitive reserves and delay AD onset (level III evidence with respect to AD risk reduction/delay).
Social activity
Social engagement may be an additional protective factor against AD. In a large 4-year prospective study, increased loneliness in cognitively intact older adults doubled the risk of AD.24 Data from the large French cohort PAQUID (Personnes Agées QUID) emphasized the importance of a patient’s social network as a protective factor against AD. In this cohort, the perception of reciprocity in relationships with others (the perception that a person had received more than he or she had given) was associated with a 53% reduction in AD risk (level III).25 In another longitudinal cohort study, social activity was found to decrease the incidence of subjective cognitive decline, which is a prodromal syndrome for MCI and AD (level III).26
A major confounder in studies assessing for social activity is the uncertainty if social withdrawal is a modifiable risk factor or an early manifestation of AD, since apathetic patients with AD tend to be socially withdrawn.27 Another limitation of measuring the impact of social activity relative to AD risk is the difficulty in isolating social activities from activities that have physical and mental activity components, such as leisure-time activities.28
Meditation/spiritual activity
Chronic psychological stress is believed to compromise limbic structures that regulate stress-related behaviors and the memory network, which might explain how being prone to psychological distress may be associated with MCI or AD.29 Cognitive stress may increase the oxidative stress and telomere shortening implicated in the neurodegenerative processes of AD.30 In one study, participants who were highly prone to psychological distress were found to be at 3 times increased risk for developing AD, after adjusting for depression symptoms and physical and mental activities (level III).31 By reducing chronic psychological stress, meditation techniques offer a promising preventive option against AD.
Mindfulness-based interventions (MBI) have gained increased attention in the past decade. They entail directing one’s attention towards the present moment, thereby decreasing ruminative thoughts and stress arousal.32 Recent RCTs have shown that MBI may promote brain health in older adults not only by improving psychological well-being but also by improving attentional control33 and functional connectivity in brain regions implicated in executive functioning,34 as well as by modulating inflammatory processes implicated in AD.35 Furthermore, an RCT of patients diagnosed with MCI found that compared with memory enhancement training, a weekly 60-minute yoga session improved memory and executive functioning.36
Continue to: Kirtan Kriya is a medication technique...
Kirtan Kriya is a meditation technique that is easy to learn and practice by older adults and can improve memory in patients at risk for developing AD.37 However, more rigorous RCTs conducted in larger samples of older adults are needed to better evaluate the effect of all meditation techniques for delaying or preventing AD (level IB with respect to improvement in cognitive functioning/level III for AD delay/risk reduction).38
Spiritual activities, such as going to places of worship or religious meditation, have been associated with a lower prevalence of AD. Attending religious services, gatherings, or retreats involves a social component because these activities often are practiced in groups. They also confer a method of dealing with psychological distress and depression. Additionally, frequent readings of religious texts represents a mentally stimulating activity that may also contribute to delaying/preventing AD (level III).39
Diet
In the past decade, a growing body of evidence has linked diet to cognition. Individuals with a higher intake of calories and fat are at higher risk for developing AD.40 The incidence of AD rose in Japan after the country transitioned to a more Westernized diet.41 A modern Western diet rich in saturated fatty acids and simple carbohydrates may negatively impact hippocampus-mediated functions such as memory and learning, and is associated with an increased risk of AD.42 In contrast with high-glycemic and fatty diets, a “healthy diet” is associated with a decrease in beta-amyloid burden, inflammation, and oxidative stress.43,44
Studies focusing on dietary patterns rather than a single nutrient for delaying or preventing AD have yielded more robust and consistent results.45 In a recent meta-analysis, adhering to a Mediterranean diet—which is rich in fruits and vegetables, whole grains, olive oil, and fish; moderate in some dairy products and wine; and low in red meat—was associated with a decreased risk of AD; this evidence was derived mostly from epidemiologic studies.46 Scarmeas et al8 found that high adherence to the Mediterranean diet was associated with 32% to 40% reduced risk of AD. Combining this diet with physical exercise was associated with an up to 67% reduced risk (level III). The Dietary Approaches to Stop Hypertension (DASH) diet, which is rich in total grains, fruits, vegetables, and dairy products, but low in sodium and sweets, correlated with neurocognitive improvement in patients with hypertension.47 Both the Mediterranean and DASH diets have been associated with better cognitive function48 and slower cognitive decline.49 Thus, an attempt to combine the neuroprotective components from both diets led to the creation of the MIND (Mediterranean-DASH Intervention for Neurodegenerative Delay) diet, which also has been associated with a lower incidence of AD.50
Besides specific diets, some food groups have also been found to promote brain health and may help delay or prevent AD. Berries have the highest amount of antioxidants of all fruit. Among vegetables, tomatoes and green leafy vegetables have the highest amount of nutrients for the brain. Nuts, such as walnuts, which are rich in omega-3 fatty acids, are also considered “power foods” for the brain; however, they should be consumed in moderation because they are also rich in fat. Monounsaturated fatty acids, which are found in olives and olive oil, are also beneficial for the brain. Among the 3 types of omega-3 fatty acids, the most important for cognition is docosahexaenoic acid (DHA) because it constitutes 40% of all fatty acids in the brain. Mainly found in oily fish, DHA has antioxidant and anti-inflammatory properties that may delay or prevent AD. Low levels of DHA have been found in patients with AD.51
Continue to: Curcumin, which is derived from...
Curcumin, which is derived from the curry spice turmeric, is a polyphenol with anti-inflammatory, antioxidant, and anti-amyloid properties that may have a promising role in preventing AD in cognitively intact individuals. Initial trials with curcumin have yielded mixed results on cognition, which was partly related to the low solubility and bioavailability of its formulation.52 However, a recent 18-month double-blind randomized placebo-controlled trial found positive effects on memory and attention, as well as reduction of amyloid plaques and tau tangles deposition in the brain, in non-demented older adults age 51 to 84 who took Theracumin, a highly absorptive oral form of curcumin dispersed with colloidal nanoparticles.53 A longer follow-up is required to determine if curcumin can delay or prevent AD.
Alcohol
The role of alcohol in AD prevention is controversial. Overall, data from prospective studies has shown that low to moderate alcohol consumption may be associated with a reduced risk of AD (level III).54 Alcohol drinking in mid-life showed a U-shaped relationship with cognitive impairment; both abstainers and heavy drinkers had an increased risk of cognitive decline compared with light to moderate drinkers (level III).55 Binge drinking significantly increased the odds of cognitive decline, even after controlling for total alcohol consumption per week.55
The definition of low-to-moderate drinking varies substantially among countries. In addition, the size and amount of alcohol contained in a standard drink may differ.56 According to the National Institute on Alcohol Abuse and Alcoholism (NIAAA),57 moderate drinking is defined as up to 1 drink daily for women and 2 drinks daily for men. Binge drinking involves drinking >4 drinks for women and >5 drinks for men, in approximately 2 hours, at least monthly. In the United States, one standard drink contains 14 grams of pure alcohol, which is usually found in 12 ounces of regular beer, 5 ounces of wine, and 1.5 ounces of distilled spirits (vodka or whiskey).58
In a 5-year prospective Canadian study, having 1 drink weekly (especially wine) was associated with an up to 50% reduced risk of AD (level III).59 In the French cohort PAQUID, mild drinkers (<1 to 2 drinks/day) and moderate drinkers (3 to 4 drinks daily) had a reduced incidence of AD compared with non-drinkers. Wine was the most frequently consumed beverage in this study.60 Other studies have found cognitive benefits from mild to moderate drinking regardless of beverage type.54 However, a recent study that included a 30-year follow-up failed to find a significant protective effect of light drinking over abstinence in terms of hippocampal atrophy.61 Atrophy of the hippocampus was correlated with increasing alcohol amounts in a dose-dependent manner, starting at 7 to 14 drinks/week (level III).61
Research has shown that moderate and heavy alcohol use or misuse can directly induce microglial activation and inflammatory mediators’ release, which induce amyloid beta pathology and leads to brain atrophy.62 Hence, non-drinkers should not be advised to begin drinking, because of the lack of RCTs and the concern that beginning to drink may lead to heavy drinking. All drinkers should be advised to adhere to the NIAAA recommendations.13
Continue to: Coffee/tea
Coffee/tea
Although studies of caffeinated coffee have been heterogeneous and yielded mixed results (beneficial effect vs no effect on delaying cognitive decline), systematic reviews and meta-analyses of cross-sectional, case-control, and longitudinal cohort studies have found a general trend towards a favorable preventive role (level III).63-65 Caffeine exhibits its neuroprotective effect by increasing brain serotonin and acetylcholine, and by stabilizing blood-brain-barrier integrity.66 Moreover, in an animal study, mice given caffeine in their drinking water from young adulthood into older age had lower amyloid beta plasma levels compared with those given decaffeinated water.67 These findings suggest that in humans, 5 cups of regular caffeinated coffee daily, equivalent to 500 mg of caffeine,
An Italian study showed that older adults who don’t or rarely drink coffee (<1 cup daily) and those who recently increased their consumption pattern to >1 cup daily had a higher incidence of MCI than those who habitually consumed 1 to 2 cups daily.69 Therefore, it is not recommended to advise a change in coffee drinking pattern in old age. Older adults who are coffee drinkers should, however, be educated about the association between heavier caffeine intake and anxiety, insomnia, and cardiac arrhythmias.70
Despite its more modest caffeine levels, green tea is rich in polyphenols, which belong to the family of catechins and are characterized by antioxidant and anti-inflammatory properties.71 In a Japanese cohort, higher green tea consumption (up to 1 cup daily) was associated with a decreased incidence of MCI in older adults.72 More studies are needed to confirm its potential preventative role in AD.
Which lifestyle change is the most important?
Focusing on a single lifestyle change may be insufficient, especially because the bulk of evidence for individual interventions comes from population-based cohort studies (level III), rather than strong RCTs with a long follow-up. There is increasing evidence that combining multiple lifestyle modifications may yield better outcomes in maintaining or improving cognition.73
The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER), a large, 2-year RCT that included community-dwelling older adults (age 60 to 77) with no diagnosis of major neurocognitive disorder, found that compared with regular health advice, multi-domain interventions reduced cognitive decline and improved overall cognition, executive functioning, and processing speed. The interventions evaluated in this study combined the following 4 modalities74:
- a healthy diet according to the Finnish nutrition recommendations (eating vegetables, fruits, and berries [minimum: 500 g/d], whole grain cereals [several times a day], and fish [2 to 3 times/week]; using low-salt products; consuming fat-free or low-fat milk products; and limiting red meat consumption to <500 g/week
- regular physical exercise tailored for improving muscle strength (1 to 3 times/week) coupled with aerobic exercise (2 to 5 times/week)
- cognitive training, including group sessions that have a social activity component and computer-based individual sessions 3 times/week that target episodic and working memory and executive functioning
- optimal management of cardiovascular risk factors.
Continue to: This multi-domain approach...
This multi-domain approach for lifestyle modification should be strongly recommended to cognitively intact older patients (level IB).
Modeled after the FINGER study, the Alzheimer’s Association U.S. Study to Protect Brain Health Through Lifestyle Intervention to Reduce Risk (U.S. POINTER) is a 2-year, multicenter, controlled clinical trial aimed at testing the ability of a multidimensional lifestyle intervention to prevent AD in at-risk older adults (age 60 to 79, with established metabolic and cardiovascular risk factors). Interventions include a combination of physical exercise, nutritional counseling and management, cognitive and social stimulation, and improved management of cardiovascular risk factors. Recruitment for this large-scale trial was estimated to begin in January 2019 (NCT03688126).75
On a practical basis, Desai et al13 have proposed a checklist (Table 213) that physicians can use in their routine consultations to improve primary prevention of AD among their older patients.
Bottom Line
Advise patients that pursuing a healthy lifestyle is a key to delaying or preventing Alzheimer’s disease. This involves managing cardiovascular risk factors and a combination of staying physically, mentally, socially, and spiritually active, in addition to adhering to a healthy diet such as the Mediterranean diet.
Related Resources
- Anderson K, Grossberg GT. Brain games to slow cognitive decline in Alzheimer’s disease. J Am Med Dir Assoc. 2014;15(8):536-537.
- Small G, Vorgan G. The memory prescription: Dr. Garry Small’s 14-day plan to keep your brain and body young. New York, NY: Hyperion; 2004.
- Small G, Vorgan G. The Alzheimer’s prevention program; keep your brain healthy for the rest of your life. New York, NY: Workman Publishing Company, Inc.; 2012.
Drug Brand Name
Curcumin • Theracurmin
1. Mehta D, Jackson R, Paul G, et al. Why do trials for Alzheimer’s disease drugs keep failing? A discontinued drug perspective for 2010-2015. Expert Opin Investig Drugs. 2017;26(6):735-739.
2. Norton S, Matthews FE, Barnes DE, et al. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13(8):788-794.
3. Meng XF, Yu JT, Wang HF, et al. Midlife vascular risk factors and the risk of Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis. 2014;42(4):1295-1310.
4. Shekelle PG, Woolf SH, Eccles M, et al. Developing clinical guidelines. West J Med. 1999;170(6):348-351.
5. Barnes DE, Yaffe Y. The projected impact of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10(9):819-828.
6. Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30(9):464-472.
7. Ahlskog JE, Geda YE, Graff-Radford NR, et al. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clin Proc. 2011;86(9):876-884.
8. Scarmeas N, Luchsinger JA, Schupf N, et al. Physical activity, diet, and risk of Alzheimer Disease. JAMA. 2009;302(6):627-637.
9. Rovio S, Kåreholt I, Helkala EL, et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol. 2005;4(11):705-711.
10. Smith PJ et al. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med. 2010;72(3):239-252.
11. Brown BM, Peiffer JJ, Taddei K, et al. Physical activity and amyloid-beta plasma and brain levels: results from the Australian imaging, biomarkers and lifestyle study of ageing. Mol Psychiatry. 2013;18(8):875-881.
12. Brown BM, Sohrabi HR, Taddei K, et al. Habitual exercise levels are associated with cerebral amyloid load in presymptomatic autosomal dominant Alzheimer’s disease. Alzheimers Dement. 2017;13(11):1197-1206.
13. Desai AK, Grossberg GT, Chibnall JT. Healthy brain aging: a road map. Clin Geriatr Med. 2010;26(1):1-16.
14. Centers for Disease Control and Prevention. Physical activity: how much physical activity do older adults need?
15. Garber CE, Blissmer B, Deschenes MR, et al; American College of Sports Medicine. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334-1359.
16. Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113);2673-2734.
17. Valenzuela MJ, Sachdev P, Wen W, et al. Lifespan mental activity predicts diminished rate of hippocampal atrophy. PLoS One. 2008;3(7):e2598. doi.org/10.1371/journal.pone.0002598.
18. Wilson RS, Bennett DA, Bienias JL, et al. Cognitive activity and incident AD in a population-based sample of older persons. Neurology. 2002;59(12):1910-1914.
19. Wilson RS, Scherr PA, Schneider JA, et al. Relation of cognitive activity to risk of developing Alzheimer disease. Neurology. 2007;69(20):1911-1920.
20. Krell-Roesch J, Vemuri P, Pink A, et al. Association between mentally stimulating activities in late life and the outcome of incident mild cognitive impairment, with an analysis of the apoe ε4 genotype. JAMA Neurol. 2017;74(3):332-338.
21. Verghese J, Lipton RB, Katz MJ, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med. 2003;348(25):2508-2516.
22. Klein RM, Christie J, Parkvall M. Does multilingualism affect the incidence of Alzheimer’s disease?: a worldwide analysis by country. SSM Popul Health. 2016;2:463-467.
23. Grundy JG, Anderson JAE, Bialystok E. Neural correlates of cognitive processing in monolinguals and bilinguals. Ann N Y Acad Sci. 2017;1396(1):183-201.
24. Wilson RS, Krueger KR, Arnold SE, et al. Loneliness and risk of Alzheimer disease. Arch Gen Psychiatry. 2007;64(2):234-240.
25. Amieva H, Stoykova R, Matharan F, et al. What aspects of social network are protective for dementia? Not the quantity but the quality of social interactions is protective up to 15 years later. Psychosom Med. 2010;72(9):905-911.
26. Kuiper JS, Oude Voshaar RC, Zuidema SU, et al. The relationship between social functioning and subjective memory complaints in older persons: a population-based longitudinal cohort study. Int J Geriatr Psychiatry. 2017;32(10):1059-1071.
27. Robert P, Onyike CU, Leentjens AF, et al. Proposed diagnostic criteria for apathy in Alzheimer’s disease and other neuropsychiatric disorders. Eur Psychiatry. 2009;24(2):98-104.
28. Marioni RE, Proust-Lima C, Amieva H, et al. Social activity, cognitive decline and dementia risk: a 20-year prospective cohort study. BMC Public Health. 2015;15:1089.
29. Wilson RS, Schneider JA, Boyle PA, et al. Chronic distress and incidence of mild cognitive impairment. Neurology. 2007;68(24):2085-2092.
30. Cai Z, Yan LJ, Ratka A. Telomere shortening and Alzheimer’s disease. Neuromolecular Med. 2013;15(1):25-48.
31. Wilson RS, Arnold SE, Schneider JA, et al. Chronic psychological distress and risk of Alzheimer’s disease in old age. Neuroepidemiology. 2006;27(3):143-153.
32. Epel E, Daubenmier J, Moskowitz JT, et al. Can meditation slow rate of cellular aging? Cognitive stress, mindfulness, and telomeres. Ann N Y Acad Sci. 2009;1172:34-53.
33. Malinowski P, Moore AW, Mead Br, et al. Mindful aging: the effects of regular brief mindfulness practice on electrophysiological markers of cognitive and affective processing in older adults. Mindfulness (N Y). 2017;8(1):78-94.
34. Taren AA, Gianaros PJ, Greco CM, et al. Mindfulness meditation training and executive control network resting state functional connectivity: a randomized controlled trial. Psychosom Med. 2017;79(6):674-683.
35. Fountain-Zaragoza S, Prakash RS. Mindfulness training for healthy aging: impact on attention, well-being, and inflammation. Front in Aging Neurosci. 2017;9:11.
36. Eyre HA, Siddarth P, Acevedo B, et al. A randomized controlled trial of Kundalini yoga in mild cognitive impairment. Int Psychogeriatr. 2017;29(4):557-567.
37. Khalsa DS. Stress, meditation, and Alzheimer’s disease prevention: where the evidence stands. J Alzheimers Dis. 2015;48(1):1-12.
38. Berk L, van Boxtel M, van Os J. Can mindfulness-based interventions influence cognitive functioning in older adults? A review and considerations for future research. Aging Ment Health. 2017;21(11):1113-1120.
39. Hosseini S, Chaurasia A, Oremus M. The effect of religion and spirituality on cognitive function: a systematic review. Gerontologist. 2017. doi: 10.1093/geront/gnx024.
40. Luchsinger JA, Tang MX, Shea S, et al. Caloric intake and the risk of Alzheimer disease. Arch Neurol. 2002;59(8):1258-1263.
41. Grant WB. Trends in diet and Alzheimer’s disease during the nutrition transition in Japan and developing countries. J Alzheimers Dis. 2014;38(3):611-620.
42. Kanoski SE, Davidson TL. Western diet consumption and cognitive impairment: links to hippocampal dysfunction and obesity. Physiol Behav. 2011;103(1):59-68.
43. Hu N, Yu JT, Tan L, et al. Nutrition and the risk of Alzheimer’s disease. Biomed Res Int. 2013;2013:524820. doi: 10.1155/2013/524820.
44. Taylor MK, Sullivan DK, Swerdlow RH, et al. A high-glycemic diet is associated with cerebral amyloid burden in cognitively normal older adults. Am J Clin Nutr. 2017;106(6):1463-1470.
45. van de Rest O, Berendsen AM, Haveman-Nies A, et al. Dietary patterns, cognitive decline, and dementia: a systematic review. Adv Nutr. 2015;6(2):154-168.
46. Petersson SD, Philippou E. Mediterranean diet, cognitive function, and dementia: a systematic review of the evidence. Adv Nutr. 2016;7(5):889-904.
47. Smith PJ, Blumenthal JA, Babyak MA, et al. Effects of the dietary approaches to stop hypertension diet, exercise, and caloric restriction on neurocognition in overweight adults with high blood pressure. Hypertension. 2010;55(6):1331-1338.
48. Wengreen H, Munger RG, Cutler A, et al. Prospective study of dietary approaches to stop hypertension- and Mediterranean-style dietary patterns and age-related cognitive change: the Cache County study on memory, health and aging. Am J Clin Nutr. 2013;98(5):1263-1271.
49. Tangney CC, Li H, Wang Y, et al. Relation of DASH- and Mediterranean-like dietary patterns to cognitive decline in older persons. Neurology. 2014;83(16):1410-1416.
50. Morris MC, Tangney CC, Wang Y, et al. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement. 2015;11(9):1007-1014.
51. Desai AK, Rush J, Naveen L, et al. Nutrition and nutritional supplements to promote brain health. In: Hartman-Stein PE, Rue AL, eds. Enhancing cognitive fitness in adults: a guide to the use and development of community-based programs. New York, NY: Springer; 2011:249-269.
52. Goozee KG, Shah TM, Sohrabi HR, et al. Examining the potential clinical value of curcumin in the prevention and diagnosis of Alzheimer’s disease. Br J Nutr. 2016;115(3):449-465.
53. Small GW, Siddarth P, Li Z, et al. Memory and brain amyloid and tau effects of a bioavailable form of curcumin in non-demented adults: a double-blind, placebo-controlled 18-month trial. Am J Geriatr Psychiatry. 2018;26(3):266-277.
54. Kim JW, Lee DY, Lee BC, et al. Alcohol and cognition in the elderly: a review. Psychiatry Investig. 2012;9(1):8-16.
55. Virtaa JJ, Järvenpää T, Heikkilä K, et al. Midlife alcohol consumption and later risk of cognitive impairment: a twin follow-up study. J Alzheimers Dis. 2010;22(3):939-948.
56. Kerr WC, Stockwell T. Understanding standard drinks and drinking guidelines. Drug and Alcohol Rev. 2012;31(2):200-205.
57. National Institute on Alcohol Abuse and Alcoholism. Drinking levels defined. https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking. Accessed December 9, 2017.
58. National Institute on Alcohol Abuse and Alcoholism. What is a standard drink? https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/what-standard-drink. Accessed November 9, 2017.
59. Lindsay J, Laurin D, Verreault R, et al. Risk factors for Alzheimer’s disease: a prospective analysis from the Canadian study of health and aging. Am J Epidemiol. 2002;156(5):445-453.
60. Orgogozo JM, Dartigues JF, Lafont S, et al. Wine consumption and dementia in the elderly: a prospective community study in the Bordeaux area. Rev Neurol (Paris). 1997;153(3):185-192.
61. Topiwala A, Allan CL, Valkanova V, et al. Moderate alcohol consumption as risk factor for adverse brain outcomes and cognitive decline: longitudinal cohort study. BMJ. 2017;357.
62. Venkataraman A, Kalk N, Sewell G, et al. Alcohol and Alzheimer’s disease-does alcohol dependence contribute to beta-amyloid deposition, neuroinflammation and neurodegeneration in Alzheimer’s Disease? Alcohol Alcohol. 2017;52(2):151-158.
63. Ma QP, Huang C, Cui QY, et al. Meta-analysis of the association between tea intake and the risk of cognitive disorders. PLoS One. 2016;11(11):e0165861. doi: 10.1371/journal.pone.0165861.
64. Santos C, Costa J, Santos J, et al. Caffeine intake and dementia: systematic review and meta-analysis. J Alzheimers Dis. 2010;20(Suppl 1):S187-204.
65. Panza F, Solfrizzi V, Barulli MR, et al. Coffee, tea, and caffeine consumption and prevention of late-life cognitive decline and dementia: a systematic review. J Nutr Health Aging. 2015;19(3):313-328.
66. Wierzejska R. Can coffee consumption lower the risk of Alzheimer’s disease and Parkinson’s disease? A literature review. Arch Med Sci. 2017;13(3):507-514.
67. Arendash GW, Cao C. Caffeine and coffee as therapeutics against Alzheimer’s disease. J Alzheimers Dis. 2010;20 (Suppl 1):S117-S126.
68. Eskelinen MH, Ngandu T, Tuomilehto J, et al. Midlife coffee and tea drinking and the risk of late-life dementia: a population-based CAIDE study. J Alzheimers Dis. 2009;16(1):85-91.
69. Solfrizzi V, Panza F, Imbimbo BP, et al. Coffee consumption habits and the risk of mild cognitive impairment: the Italian longitudinal study on aging. J Alzheimers Dis. 2015;47(4):889-899.
70. Vittoria Mattioli. Beverages of daily life: impact of caffeine on atrial fibrillation. J Atr Fibrillation. 2014;7(2):1133.
71. Chacko SM, Thambi PT, Kuttan R, et al. Beneficial effects of green tea: a literature review. Chin Med. 2010;5:13.
72. Noguchi-Shinohara M, Yuki S, Dohmoto C, et al. Consumption of green tea, but not black tea or coffee, is associated with reduced risk of cognitive decline. PLoS One. 2014;9(5):e96013. doi: 10.1371/journal.pone.0096013.
73. Schneider N, Yvon C. A review of multidomain interventions to support healthy cognitive ageing. J Nutr Health Aging. 2013;17(3):252-257.
74. Ngandu T, Lehitsalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255-2263.
75. U.S. National Library of Medicing. ClinicalTrials.gov. U.S. study to protect brain health through lifestyle intervention to reduce risk (POINTER). https://clinicaltrials.gov/ct2/show/NCT03688126?term=pointer&cond=Alzheimer+Disease&rank=1. Published September 28, 2018. Accessed November 3, 2018.
Clinicians have devoted strenuous efforts to secondary prevention of Alzheimer’s disease (AD) by diagnosing and treating patients as early as possible. Unfortunately, there is no cure for AD, and the field has witnessed recurrent failures of several pharmacotherapy candidates with either symptomatic or disease-modifying properties.1 An estimated one-third of AD cases can be attributed to modifiable risk factors.2 Thus, implementing primary prevention measures by addressing modifiable risk factors thought to contribute to the disease, with the goal of reducing the risk of developing AD, or at least delaying its onset, is a crucial public health strategy.
Cardiovascular risk factors, such as hypertension, hyperlipidemia, diabetes, hyperhomocysteinemia, obesity, and smoking, have emerged as substantive risk factors for AD.3 Optimal management of these major risk factors, especially in mid-life, may be a preventive approach against AD. Although detailing the evidence on the impact of managing cardiovascular risk factors to delay or prevent AD is beyond the scope of this article, it is becoming clear that “what is good for the heart is good for the brain.”
Additional modifiable risk factors are related to lifestyle habits, such as physical exercise, mental and social activity, meditation/spiritual activity, and diet. This article reviews the importance of pursuing a healthy lifestyle in delaying AD, with the corresponding levels of evidence that support each specific lifestyle modification. The levels of evidence are defined in Table 1.4
Physical exercise
Twenty-one percent of AD cases in the United States are attributable to physical inactivity.5 In addition to its beneficial effect on metabolic syndrome, in animal and human research, regular exercise has been shown to have direct neuroprotective effects. High levels of physical activity increase hippocampal neurogenesis and neuroplasticity, increase vascular circulation in the brain regions implicated in AD, and modulate inflammatory mediators as well as brain growth factors such as brain-derived neurotrophic factor (BDNF) and insulin-like growth factor-1 (IGF-1).6
The definition of regular physical exercise varies across the literature, but usually implies aerobic exercise—an ongoing activity sufficient to increase the heart rate and the need for oxygen, sustained for 20 to 30 minutes per session.7 Modalities include household activities and leisure-time activities. In a large prospective cohort study, Scarmeas et al8 categorized leisure-time activities into 3 types:
- light (walking, dancing, calisthenics, golfing, bowling, gardening, horseback riding)
- moderate (bicycling, swimming, hiking, playing tennis)
- vigorous (aerobic dancing, jogging, playing handball).
These types of physical exercise were weighed by the frequency of participation per week. Compared with being physically inactive, low levels of weekly physical activity (0.1 hours of vigorous, 0.8 hours of moderate, or 1.3 hours of light exercise) were associated with a 29% to 41% lower risk of developing AD, while higher weekly physical activity (1.3 hours of vigorous, 2.3 hours of moderate, or 3.8 hours of light exercise) were associated with a 37% to 50% lower risk (level III).8
In another 20-year cohort study, engaging in leisure-time physical activity at least twice a week in mid-life was significantly associated with a reduced risk of AD, after adjusting for age, sex, education, follow-up time, locomotor disorders, apolipoprotein E (ApoE) genotype, vascular disorders, smoking, and alcohol intake (level III).9 Moreover, a systematic review of 29 randomized controlled trials (RCTs) showed that aerobic exercise training, such as brisk walking, jogging, and biking, was associated with improvements in attention, processing speed, executive function, and memory among healthy older adults and those with mild cognitive impairment (MCI; level IA).10
Continue to: From a pathophysiological standpoint...
From a pathophysiological standpoint, higher levels of physical exercise in cognitively intact older adults have been associated with reduced brain amyloid beta deposits, especially in ApoE4 carriers.11 This inverse relationship also has been demonstrated in patients who are presymptomatic who carry 1 of the 3 known autosomal dominant mutations for the familial forms of AD.12
Overall, physicians should recommend that patients—especially those with cardiovascular risk factors that increase their risk for AD—exercise regularly by following the guidelines of the American Heart Association or the American College of Sports Medicine.13 These include muscle-strengthening activities (legs, hips, back, abdomen, shoulders, and arms) at least 2 days/week, in addition to either 30 minutes/day of moderate-intensity aerobic activity such as brisk walking, 5 days/week; or 25 minutes of vigorous aerobic activity such as jogging and running, 3 days/week14 (level IA evidence for overall improvement in cognitive function; level III evidence for AD delay/risk reduction). Neuromotor exercise, such as yoga and tai chi, and flexibility exercise such as muscle stretching, especially after a hot bath, 2 to 3 days/week are also recommended (level III).15
Mental activity
Nineteen percent of AD cases worldwide and 7% in the United States. can be attributed to low educational attainment, which is associated with low brain cognitive reserve.5 Cognitive resilience in later life may be enhanced by building brain reserves through intellectual stimulation, which affects neuronal branching and plasticity.16 Higher levels of complex mental activities measured across the lifespan, such as education, occupation, reading, and writing, are correlated with significantly less hippocampal volume shrinkage over time.17 Frequent participation in mentally stimulating activities—such as listening to the radio; reading newspapers, magazines, or books; playing games (cards, checkers, crosswords or other puzzles); and visiting museums—was associated with an up to 64% reduction in the odds of developing AD in a cohort of cognitively intact older adults followed for 4 years.18 The correlation between mental activity and AD was found to be independent of physical activity, social activity, or baseline cognitive function.19
In a large cohort of cognitively intact older adults (mean age 70), engaging in a mentally stimulating activity (craft activities, computer use, or going to the theater/movies) once to twice a week was significantly associated with a reduced incidence of amnestic MCI.20 Another prospective 21-year study demonstrated a significant reduction in AD risk in community-dwelling cognitively intact older adults (age 75 to 85) who participated in cognitively stimulating activities, such as reading books or newspapers, writing for pleasure, doing crossword puzzles, playing board games or cards, or playing musical instruments, several times/week.21
Growing scientific evidence also suggests that lifelong multilingualism can delay AD onset by 4 to 5 years.22 Multilingualism is associated with greater cognitive reserve, gray matter volume, functional connectivity and white matter density.23
Continue to: Physicians should encourage their patients...
Physicians should encourage their patients to engage in intellectually stimulating activities and creative leisure-time activities several times/week to enhance their cognitive reserves and delay AD onset (level III evidence with respect to AD risk reduction/delay).
Social activity
Social engagement may be an additional protective factor against AD. In a large 4-year prospective study, increased loneliness in cognitively intact older adults doubled the risk of AD.24 Data from the large French cohort PAQUID (Personnes Agées QUID) emphasized the importance of a patient’s social network as a protective factor against AD. In this cohort, the perception of reciprocity in relationships with others (the perception that a person had received more than he or she had given) was associated with a 53% reduction in AD risk (level III).25 In another longitudinal cohort study, social activity was found to decrease the incidence of subjective cognitive decline, which is a prodromal syndrome for MCI and AD (level III).26
A major confounder in studies assessing for social activity is the uncertainty if social withdrawal is a modifiable risk factor or an early manifestation of AD, since apathetic patients with AD tend to be socially withdrawn.27 Another limitation of measuring the impact of social activity relative to AD risk is the difficulty in isolating social activities from activities that have physical and mental activity components, such as leisure-time activities.28
Meditation/spiritual activity
Chronic psychological stress is believed to compromise limbic structures that regulate stress-related behaviors and the memory network, which might explain how being prone to psychological distress may be associated with MCI or AD.29 Cognitive stress may increase the oxidative stress and telomere shortening implicated in the neurodegenerative processes of AD.30 In one study, participants who were highly prone to psychological distress were found to be at 3 times increased risk for developing AD, after adjusting for depression symptoms and physical and mental activities (level III).31 By reducing chronic psychological stress, meditation techniques offer a promising preventive option against AD.
Mindfulness-based interventions (MBI) have gained increased attention in the past decade. They entail directing one’s attention towards the present moment, thereby decreasing ruminative thoughts and stress arousal.32 Recent RCTs have shown that MBI may promote brain health in older adults not only by improving psychological well-being but also by improving attentional control33 and functional connectivity in brain regions implicated in executive functioning,34 as well as by modulating inflammatory processes implicated in AD.35 Furthermore, an RCT of patients diagnosed with MCI found that compared with memory enhancement training, a weekly 60-minute yoga session improved memory and executive functioning.36
Continue to: Kirtan Kriya is a medication technique...
Kirtan Kriya is a meditation technique that is easy to learn and practice by older adults and can improve memory in patients at risk for developing AD.37 However, more rigorous RCTs conducted in larger samples of older adults are needed to better evaluate the effect of all meditation techniques for delaying or preventing AD (level IB with respect to improvement in cognitive functioning/level III for AD delay/risk reduction).38
Spiritual activities, such as going to places of worship or religious meditation, have been associated with a lower prevalence of AD. Attending religious services, gatherings, or retreats involves a social component because these activities often are practiced in groups. They also confer a method of dealing with psychological distress and depression. Additionally, frequent readings of religious texts represents a mentally stimulating activity that may also contribute to delaying/preventing AD (level III).39
Diet
In the past decade, a growing body of evidence has linked diet to cognition. Individuals with a higher intake of calories and fat are at higher risk for developing AD.40 The incidence of AD rose in Japan after the country transitioned to a more Westernized diet.41 A modern Western diet rich in saturated fatty acids and simple carbohydrates may negatively impact hippocampus-mediated functions such as memory and learning, and is associated with an increased risk of AD.42 In contrast with high-glycemic and fatty diets, a “healthy diet” is associated with a decrease in beta-amyloid burden, inflammation, and oxidative stress.43,44
Studies focusing on dietary patterns rather than a single nutrient for delaying or preventing AD have yielded more robust and consistent results.45 In a recent meta-analysis, adhering to a Mediterranean diet—which is rich in fruits and vegetables, whole grains, olive oil, and fish; moderate in some dairy products and wine; and low in red meat—was associated with a decreased risk of AD; this evidence was derived mostly from epidemiologic studies.46 Scarmeas et al8 found that high adherence to the Mediterranean diet was associated with 32% to 40% reduced risk of AD. Combining this diet with physical exercise was associated with an up to 67% reduced risk (level III). The Dietary Approaches to Stop Hypertension (DASH) diet, which is rich in total grains, fruits, vegetables, and dairy products, but low in sodium and sweets, correlated with neurocognitive improvement in patients with hypertension.47 Both the Mediterranean and DASH diets have been associated with better cognitive function48 and slower cognitive decline.49 Thus, an attempt to combine the neuroprotective components from both diets led to the creation of the MIND (Mediterranean-DASH Intervention for Neurodegenerative Delay) diet, which also has been associated with a lower incidence of AD.50
Besides specific diets, some food groups have also been found to promote brain health and may help delay or prevent AD. Berries have the highest amount of antioxidants of all fruit. Among vegetables, tomatoes and green leafy vegetables have the highest amount of nutrients for the brain. Nuts, such as walnuts, which are rich in omega-3 fatty acids, are also considered “power foods” for the brain; however, they should be consumed in moderation because they are also rich in fat. Monounsaturated fatty acids, which are found in olives and olive oil, are also beneficial for the brain. Among the 3 types of omega-3 fatty acids, the most important for cognition is docosahexaenoic acid (DHA) because it constitutes 40% of all fatty acids in the brain. Mainly found in oily fish, DHA has antioxidant and anti-inflammatory properties that may delay or prevent AD. Low levels of DHA have been found in patients with AD.51
Continue to: Curcumin, which is derived from...
Curcumin, which is derived from the curry spice turmeric, is a polyphenol with anti-inflammatory, antioxidant, and anti-amyloid properties that may have a promising role in preventing AD in cognitively intact individuals. Initial trials with curcumin have yielded mixed results on cognition, which was partly related to the low solubility and bioavailability of its formulation.52 However, a recent 18-month double-blind randomized placebo-controlled trial found positive effects on memory and attention, as well as reduction of amyloid plaques and tau tangles deposition in the brain, in non-demented older adults age 51 to 84 who took Theracumin, a highly absorptive oral form of curcumin dispersed with colloidal nanoparticles.53 A longer follow-up is required to determine if curcumin can delay or prevent AD.
Alcohol
The role of alcohol in AD prevention is controversial. Overall, data from prospective studies has shown that low to moderate alcohol consumption may be associated with a reduced risk of AD (level III).54 Alcohol drinking in mid-life showed a U-shaped relationship with cognitive impairment; both abstainers and heavy drinkers had an increased risk of cognitive decline compared with light to moderate drinkers (level III).55 Binge drinking significantly increased the odds of cognitive decline, even after controlling for total alcohol consumption per week.55
The definition of low-to-moderate drinking varies substantially among countries. In addition, the size and amount of alcohol contained in a standard drink may differ.56 According to the National Institute on Alcohol Abuse and Alcoholism (NIAAA),57 moderate drinking is defined as up to 1 drink daily for women and 2 drinks daily for men. Binge drinking involves drinking >4 drinks for women and >5 drinks for men, in approximately 2 hours, at least monthly. In the United States, one standard drink contains 14 grams of pure alcohol, which is usually found in 12 ounces of regular beer, 5 ounces of wine, and 1.5 ounces of distilled spirits (vodka or whiskey).58
In a 5-year prospective Canadian study, having 1 drink weekly (especially wine) was associated with an up to 50% reduced risk of AD (level III).59 In the French cohort PAQUID, mild drinkers (<1 to 2 drinks/day) and moderate drinkers (3 to 4 drinks daily) had a reduced incidence of AD compared with non-drinkers. Wine was the most frequently consumed beverage in this study.60 Other studies have found cognitive benefits from mild to moderate drinking regardless of beverage type.54 However, a recent study that included a 30-year follow-up failed to find a significant protective effect of light drinking over abstinence in terms of hippocampal atrophy.61 Atrophy of the hippocampus was correlated with increasing alcohol amounts in a dose-dependent manner, starting at 7 to 14 drinks/week (level III).61
Research has shown that moderate and heavy alcohol use or misuse can directly induce microglial activation and inflammatory mediators’ release, which induce amyloid beta pathology and leads to brain atrophy.62 Hence, non-drinkers should not be advised to begin drinking, because of the lack of RCTs and the concern that beginning to drink may lead to heavy drinking. All drinkers should be advised to adhere to the NIAAA recommendations.13
Continue to: Coffee/tea
Coffee/tea
Although studies of caffeinated coffee have been heterogeneous and yielded mixed results (beneficial effect vs no effect on delaying cognitive decline), systematic reviews and meta-analyses of cross-sectional, case-control, and longitudinal cohort studies have found a general trend towards a favorable preventive role (level III).63-65 Caffeine exhibits its neuroprotective effect by increasing brain serotonin and acetylcholine, and by stabilizing blood-brain-barrier integrity.66 Moreover, in an animal study, mice given caffeine in their drinking water from young adulthood into older age had lower amyloid beta plasma levels compared with those given decaffeinated water.67 These findings suggest that in humans, 5 cups of regular caffeinated coffee daily, equivalent to 500 mg of caffeine,
An Italian study showed that older adults who don’t or rarely drink coffee (<1 cup daily) and those who recently increased their consumption pattern to >1 cup daily had a higher incidence of MCI than those who habitually consumed 1 to 2 cups daily.69 Therefore, it is not recommended to advise a change in coffee drinking pattern in old age. Older adults who are coffee drinkers should, however, be educated about the association between heavier caffeine intake and anxiety, insomnia, and cardiac arrhythmias.70
Despite its more modest caffeine levels, green tea is rich in polyphenols, which belong to the family of catechins and are characterized by antioxidant and anti-inflammatory properties.71 In a Japanese cohort, higher green tea consumption (up to 1 cup daily) was associated with a decreased incidence of MCI in older adults.72 More studies are needed to confirm its potential preventative role in AD.
Which lifestyle change is the most important?
Focusing on a single lifestyle change may be insufficient, especially because the bulk of evidence for individual interventions comes from population-based cohort studies (level III), rather than strong RCTs with a long follow-up. There is increasing evidence that combining multiple lifestyle modifications may yield better outcomes in maintaining or improving cognition.73
The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER), a large, 2-year RCT that included community-dwelling older adults (age 60 to 77) with no diagnosis of major neurocognitive disorder, found that compared with regular health advice, multi-domain interventions reduced cognitive decline and improved overall cognition, executive functioning, and processing speed. The interventions evaluated in this study combined the following 4 modalities74:
- a healthy diet according to the Finnish nutrition recommendations (eating vegetables, fruits, and berries [minimum: 500 g/d], whole grain cereals [several times a day], and fish [2 to 3 times/week]; using low-salt products; consuming fat-free or low-fat milk products; and limiting red meat consumption to <500 g/week
- regular physical exercise tailored for improving muscle strength (1 to 3 times/week) coupled with aerobic exercise (2 to 5 times/week)
- cognitive training, including group sessions that have a social activity component and computer-based individual sessions 3 times/week that target episodic and working memory and executive functioning
- optimal management of cardiovascular risk factors.
Continue to: This multi-domain approach...
This multi-domain approach for lifestyle modification should be strongly recommended to cognitively intact older patients (level IB).
Modeled after the FINGER study, the Alzheimer’s Association U.S. Study to Protect Brain Health Through Lifestyle Intervention to Reduce Risk (U.S. POINTER) is a 2-year, multicenter, controlled clinical trial aimed at testing the ability of a multidimensional lifestyle intervention to prevent AD in at-risk older adults (age 60 to 79, with established metabolic and cardiovascular risk factors). Interventions include a combination of physical exercise, nutritional counseling and management, cognitive and social stimulation, and improved management of cardiovascular risk factors. Recruitment for this large-scale trial was estimated to begin in January 2019 (NCT03688126).75
On a practical basis, Desai et al13 have proposed a checklist (Table 213) that physicians can use in their routine consultations to improve primary prevention of AD among their older patients.
Bottom Line
Advise patients that pursuing a healthy lifestyle is a key to delaying or preventing Alzheimer’s disease. This involves managing cardiovascular risk factors and a combination of staying physically, mentally, socially, and spiritually active, in addition to adhering to a healthy diet such as the Mediterranean diet.
Related Resources
- Anderson K, Grossberg GT. Brain games to slow cognitive decline in Alzheimer’s disease. J Am Med Dir Assoc. 2014;15(8):536-537.
- Small G, Vorgan G. The memory prescription: Dr. Garry Small’s 14-day plan to keep your brain and body young. New York, NY: Hyperion; 2004.
- Small G, Vorgan G. The Alzheimer’s prevention program; keep your brain healthy for the rest of your life. New York, NY: Workman Publishing Company, Inc.; 2012.
Drug Brand Name
Curcumin • Theracurmin
Clinicians have devoted strenuous efforts to secondary prevention of Alzheimer’s disease (AD) by diagnosing and treating patients as early as possible. Unfortunately, there is no cure for AD, and the field has witnessed recurrent failures of several pharmacotherapy candidates with either symptomatic or disease-modifying properties.1 An estimated one-third of AD cases can be attributed to modifiable risk factors.2 Thus, implementing primary prevention measures by addressing modifiable risk factors thought to contribute to the disease, with the goal of reducing the risk of developing AD, or at least delaying its onset, is a crucial public health strategy.
Cardiovascular risk factors, such as hypertension, hyperlipidemia, diabetes, hyperhomocysteinemia, obesity, and smoking, have emerged as substantive risk factors for AD.3 Optimal management of these major risk factors, especially in mid-life, may be a preventive approach against AD. Although detailing the evidence on the impact of managing cardiovascular risk factors to delay or prevent AD is beyond the scope of this article, it is becoming clear that “what is good for the heart is good for the brain.”
Additional modifiable risk factors are related to lifestyle habits, such as physical exercise, mental and social activity, meditation/spiritual activity, and diet. This article reviews the importance of pursuing a healthy lifestyle in delaying AD, with the corresponding levels of evidence that support each specific lifestyle modification. The levels of evidence are defined in Table 1.4
Physical exercise
Twenty-one percent of AD cases in the United States are attributable to physical inactivity.5 In addition to its beneficial effect on metabolic syndrome, in animal and human research, regular exercise has been shown to have direct neuroprotective effects. High levels of physical activity increase hippocampal neurogenesis and neuroplasticity, increase vascular circulation in the brain regions implicated in AD, and modulate inflammatory mediators as well as brain growth factors such as brain-derived neurotrophic factor (BDNF) and insulin-like growth factor-1 (IGF-1).6
The definition of regular physical exercise varies across the literature, but usually implies aerobic exercise—an ongoing activity sufficient to increase the heart rate and the need for oxygen, sustained for 20 to 30 minutes per session.7 Modalities include household activities and leisure-time activities. In a large prospective cohort study, Scarmeas et al8 categorized leisure-time activities into 3 types:
- light (walking, dancing, calisthenics, golfing, bowling, gardening, horseback riding)
- moderate (bicycling, swimming, hiking, playing tennis)
- vigorous (aerobic dancing, jogging, playing handball).
These types of physical exercise were weighed by the frequency of participation per week. Compared with being physically inactive, low levels of weekly physical activity (0.1 hours of vigorous, 0.8 hours of moderate, or 1.3 hours of light exercise) were associated with a 29% to 41% lower risk of developing AD, while higher weekly physical activity (1.3 hours of vigorous, 2.3 hours of moderate, or 3.8 hours of light exercise) were associated with a 37% to 50% lower risk (level III).8
In another 20-year cohort study, engaging in leisure-time physical activity at least twice a week in mid-life was significantly associated with a reduced risk of AD, after adjusting for age, sex, education, follow-up time, locomotor disorders, apolipoprotein E (ApoE) genotype, vascular disorders, smoking, and alcohol intake (level III).9 Moreover, a systematic review of 29 randomized controlled trials (RCTs) showed that aerobic exercise training, such as brisk walking, jogging, and biking, was associated with improvements in attention, processing speed, executive function, and memory among healthy older adults and those with mild cognitive impairment (MCI; level IA).10
Continue to: From a pathophysiological standpoint...
From a pathophysiological standpoint, higher levels of physical exercise in cognitively intact older adults have been associated with reduced brain amyloid beta deposits, especially in ApoE4 carriers.11 This inverse relationship also has been demonstrated in patients who are presymptomatic who carry 1 of the 3 known autosomal dominant mutations for the familial forms of AD.12
Overall, physicians should recommend that patients—especially those with cardiovascular risk factors that increase their risk for AD—exercise regularly by following the guidelines of the American Heart Association or the American College of Sports Medicine.13 These include muscle-strengthening activities (legs, hips, back, abdomen, shoulders, and arms) at least 2 days/week, in addition to either 30 minutes/day of moderate-intensity aerobic activity such as brisk walking, 5 days/week; or 25 minutes of vigorous aerobic activity such as jogging and running, 3 days/week14 (level IA evidence for overall improvement in cognitive function; level III evidence for AD delay/risk reduction). Neuromotor exercise, such as yoga and tai chi, and flexibility exercise such as muscle stretching, especially after a hot bath, 2 to 3 days/week are also recommended (level III).15
Mental activity
Nineteen percent of AD cases worldwide and 7% in the United States. can be attributed to low educational attainment, which is associated with low brain cognitive reserve.5 Cognitive resilience in later life may be enhanced by building brain reserves through intellectual stimulation, which affects neuronal branching and plasticity.16 Higher levels of complex mental activities measured across the lifespan, such as education, occupation, reading, and writing, are correlated with significantly less hippocampal volume shrinkage over time.17 Frequent participation in mentally stimulating activities—such as listening to the radio; reading newspapers, magazines, or books; playing games (cards, checkers, crosswords or other puzzles); and visiting museums—was associated with an up to 64% reduction in the odds of developing AD in a cohort of cognitively intact older adults followed for 4 years.18 The correlation between mental activity and AD was found to be independent of physical activity, social activity, or baseline cognitive function.19
In a large cohort of cognitively intact older adults (mean age 70), engaging in a mentally stimulating activity (craft activities, computer use, or going to the theater/movies) once to twice a week was significantly associated with a reduced incidence of amnestic MCI.20 Another prospective 21-year study demonstrated a significant reduction in AD risk in community-dwelling cognitively intact older adults (age 75 to 85) who participated in cognitively stimulating activities, such as reading books or newspapers, writing for pleasure, doing crossword puzzles, playing board games or cards, or playing musical instruments, several times/week.21
Growing scientific evidence also suggests that lifelong multilingualism can delay AD onset by 4 to 5 years.22 Multilingualism is associated with greater cognitive reserve, gray matter volume, functional connectivity and white matter density.23
Continue to: Physicians should encourage their patients...
Physicians should encourage their patients to engage in intellectually stimulating activities and creative leisure-time activities several times/week to enhance their cognitive reserves and delay AD onset (level III evidence with respect to AD risk reduction/delay).
Social activity
Social engagement may be an additional protective factor against AD. In a large 4-year prospective study, increased loneliness in cognitively intact older adults doubled the risk of AD.24 Data from the large French cohort PAQUID (Personnes Agées QUID) emphasized the importance of a patient’s social network as a protective factor against AD. In this cohort, the perception of reciprocity in relationships with others (the perception that a person had received more than he or she had given) was associated with a 53% reduction in AD risk (level III).25 In another longitudinal cohort study, social activity was found to decrease the incidence of subjective cognitive decline, which is a prodromal syndrome for MCI and AD (level III).26
A major confounder in studies assessing for social activity is the uncertainty if social withdrawal is a modifiable risk factor or an early manifestation of AD, since apathetic patients with AD tend to be socially withdrawn.27 Another limitation of measuring the impact of social activity relative to AD risk is the difficulty in isolating social activities from activities that have physical and mental activity components, such as leisure-time activities.28
Meditation/spiritual activity
Chronic psychological stress is believed to compromise limbic structures that regulate stress-related behaviors and the memory network, which might explain how being prone to psychological distress may be associated with MCI or AD.29 Cognitive stress may increase the oxidative stress and telomere shortening implicated in the neurodegenerative processes of AD.30 In one study, participants who were highly prone to psychological distress were found to be at 3 times increased risk for developing AD, after adjusting for depression symptoms and physical and mental activities (level III).31 By reducing chronic psychological stress, meditation techniques offer a promising preventive option against AD.
Mindfulness-based interventions (MBI) have gained increased attention in the past decade. They entail directing one’s attention towards the present moment, thereby decreasing ruminative thoughts and stress arousal.32 Recent RCTs have shown that MBI may promote brain health in older adults not only by improving psychological well-being but also by improving attentional control33 and functional connectivity in brain regions implicated in executive functioning,34 as well as by modulating inflammatory processes implicated in AD.35 Furthermore, an RCT of patients diagnosed with MCI found that compared with memory enhancement training, a weekly 60-minute yoga session improved memory and executive functioning.36
Continue to: Kirtan Kriya is a medication technique...
Kirtan Kriya is a meditation technique that is easy to learn and practice by older adults and can improve memory in patients at risk for developing AD.37 However, more rigorous RCTs conducted in larger samples of older adults are needed to better evaluate the effect of all meditation techniques for delaying or preventing AD (level IB with respect to improvement in cognitive functioning/level III for AD delay/risk reduction).38
Spiritual activities, such as going to places of worship or religious meditation, have been associated with a lower prevalence of AD. Attending religious services, gatherings, or retreats involves a social component because these activities often are practiced in groups. They also confer a method of dealing with psychological distress and depression. Additionally, frequent readings of religious texts represents a mentally stimulating activity that may also contribute to delaying/preventing AD (level III).39
Diet
In the past decade, a growing body of evidence has linked diet to cognition. Individuals with a higher intake of calories and fat are at higher risk for developing AD.40 The incidence of AD rose in Japan after the country transitioned to a more Westernized diet.41 A modern Western diet rich in saturated fatty acids and simple carbohydrates may negatively impact hippocampus-mediated functions such as memory and learning, and is associated with an increased risk of AD.42 In contrast with high-glycemic and fatty diets, a “healthy diet” is associated with a decrease in beta-amyloid burden, inflammation, and oxidative stress.43,44
Studies focusing on dietary patterns rather than a single nutrient for delaying or preventing AD have yielded more robust and consistent results.45 In a recent meta-analysis, adhering to a Mediterranean diet—which is rich in fruits and vegetables, whole grains, olive oil, and fish; moderate in some dairy products and wine; and low in red meat—was associated with a decreased risk of AD; this evidence was derived mostly from epidemiologic studies.46 Scarmeas et al8 found that high adherence to the Mediterranean diet was associated with 32% to 40% reduced risk of AD. Combining this diet with physical exercise was associated with an up to 67% reduced risk (level III). The Dietary Approaches to Stop Hypertension (DASH) diet, which is rich in total grains, fruits, vegetables, and dairy products, but low in sodium and sweets, correlated with neurocognitive improvement in patients with hypertension.47 Both the Mediterranean and DASH diets have been associated with better cognitive function48 and slower cognitive decline.49 Thus, an attempt to combine the neuroprotective components from both diets led to the creation of the MIND (Mediterranean-DASH Intervention for Neurodegenerative Delay) diet, which also has been associated with a lower incidence of AD.50
Besides specific diets, some food groups have also been found to promote brain health and may help delay or prevent AD. Berries have the highest amount of antioxidants of all fruit. Among vegetables, tomatoes and green leafy vegetables have the highest amount of nutrients for the brain. Nuts, such as walnuts, which are rich in omega-3 fatty acids, are also considered “power foods” for the brain; however, they should be consumed in moderation because they are also rich in fat. Monounsaturated fatty acids, which are found in olives and olive oil, are also beneficial for the brain. Among the 3 types of omega-3 fatty acids, the most important for cognition is docosahexaenoic acid (DHA) because it constitutes 40% of all fatty acids in the brain. Mainly found in oily fish, DHA has antioxidant and anti-inflammatory properties that may delay or prevent AD. Low levels of DHA have been found in patients with AD.51
Continue to: Curcumin, which is derived from...
Curcumin, which is derived from the curry spice turmeric, is a polyphenol with anti-inflammatory, antioxidant, and anti-amyloid properties that may have a promising role in preventing AD in cognitively intact individuals. Initial trials with curcumin have yielded mixed results on cognition, which was partly related to the low solubility and bioavailability of its formulation.52 However, a recent 18-month double-blind randomized placebo-controlled trial found positive effects on memory and attention, as well as reduction of amyloid plaques and tau tangles deposition in the brain, in non-demented older adults age 51 to 84 who took Theracumin, a highly absorptive oral form of curcumin dispersed with colloidal nanoparticles.53 A longer follow-up is required to determine if curcumin can delay or prevent AD.
Alcohol
The role of alcohol in AD prevention is controversial. Overall, data from prospective studies has shown that low to moderate alcohol consumption may be associated with a reduced risk of AD (level III).54 Alcohol drinking in mid-life showed a U-shaped relationship with cognitive impairment; both abstainers and heavy drinkers had an increased risk of cognitive decline compared with light to moderate drinkers (level III).55 Binge drinking significantly increased the odds of cognitive decline, even after controlling for total alcohol consumption per week.55
The definition of low-to-moderate drinking varies substantially among countries. In addition, the size and amount of alcohol contained in a standard drink may differ.56 According to the National Institute on Alcohol Abuse and Alcoholism (NIAAA),57 moderate drinking is defined as up to 1 drink daily for women and 2 drinks daily for men. Binge drinking involves drinking >4 drinks for women and >5 drinks for men, in approximately 2 hours, at least monthly. In the United States, one standard drink contains 14 grams of pure alcohol, which is usually found in 12 ounces of regular beer, 5 ounces of wine, and 1.5 ounces of distilled spirits (vodka or whiskey).58
In a 5-year prospective Canadian study, having 1 drink weekly (especially wine) was associated with an up to 50% reduced risk of AD (level III).59 In the French cohort PAQUID, mild drinkers (<1 to 2 drinks/day) and moderate drinkers (3 to 4 drinks daily) had a reduced incidence of AD compared with non-drinkers. Wine was the most frequently consumed beverage in this study.60 Other studies have found cognitive benefits from mild to moderate drinking regardless of beverage type.54 However, a recent study that included a 30-year follow-up failed to find a significant protective effect of light drinking over abstinence in terms of hippocampal atrophy.61 Atrophy of the hippocampus was correlated with increasing alcohol amounts in a dose-dependent manner, starting at 7 to 14 drinks/week (level III).61
Research has shown that moderate and heavy alcohol use or misuse can directly induce microglial activation and inflammatory mediators’ release, which induce amyloid beta pathology and leads to brain atrophy.62 Hence, non-drinkers should not be advised to begin drinking, because of the lack of RCTs and the concern that beginning to drink may lead to heavy drinking. All drinkers should be advised to adhere to the NIAAA recommendations.13
Continue to: Coffee/tea
Coffee/tea
Although studies of caffeinated coffee have been heterogeneous and yielded mixed results (beneficial effect vs no effect on delaying cognitive decline), systematic reviews and meta-analyses of cross-sectional, case-control, and longitudinal cohort studies have found a general trend towards a favorable preventive role (level III).63-65 Caffeine exhibits its neuroprotective effect by increasing brain serotonin and acetylcholine, and by stabilizing blood-brain-barrier integrity.66 Moreover, in an animal study, mice given caffeine in their drinking water from young adulthood into older age had lower amyloid beta plasma levels compared with those given decaffeinated water.67 These findings suggest that in humans, 5 cups of regular caffeinated coffee daily, equivalent to 500 mg of caffeine,
An Italian study showed that older adults who don’t or rarely drink coffee (<1 cup daily) and those who recently increased their consumption pattern to >1 cup daily had a higher incidence of MCI than those who habitually consumed 1 to 2 cups daily.69 Therefore, it is not recommended to advise a change in coffee drinking pattern in old age. Older adults who are coffee drinkers should, however, be educated about the association between heavier caffeine intake and anxiety, insomnia, and cardiac arrhythmias.70
Despite its more modest caffeine levels, green tea is rich in polyphenols, which belong to the family of catechins and are characterized by antioxidant and anti-inflammatory properties.71 In a Japanese cohort, higher green tea consumption (up to 1 cup daily) was associated with a decreased incidence of MCI in older adults.72 More studies are needed to confirm its potential preventative role in AD.
Which lifestyle change is the most important?
Focusing on a single lifestyle change may be insufficient, especially because the bulk of evidence for individual interventions comes from population-based cohort studies (level III), rather than strong RCTs with a long follow-up. There is increasing evidence that combining multiple lifestyle modifications may yield better outcomes in maintaining or improving cognition.73
The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER), a large, 2-year RCT that included community-dwelling older adults (age 60 to 77) with no diagnosis of major neurocognitive disorder, found that compared with regular health advice, multi-domain interventions reduced cognitive decline and improved overall cognition, executive functioning, and processing speed. The interventions evaluated in this study combined the following 4 modalities74:
- a healthy diet according to the Finnish nutrition recommendations (eating vegetables, fruits, and berries [minimum: 500 g/d], whole grain cereals [several times a day], and fish [2 to 3 times/week]; using low-salt products; consuming fat-free or low-fat milk products; and limiting red meat consumption to <500 g/week
- regular physical exercise tailored for improving muscle strength (1 to 3 times/week) coupled with aerobic exercise (2 to 5 times/week)
- cognitive training, including group sessions that have a social activity component and computer-based individual sessions 3 times/week that target episodic and working memory and executive functioning
- optimal management of cardiovascular risk factors.
Continue to: This multi-domain approach...
This multi-domain approach for lifestyle modification should be strongly recommended to cognitively intact older patients (level IB).
Modeled after the FINGER study, the Alzheimer’s Association U.S. Study to Protect Brain Health Through Lifestyle Intervention to Reduce Risk (U.S. POINTER) is a 2-year, multicenter, controlled clinical trial aimed at testing the ability of a multidimensional lifestyle intervention to prevent AD in at-risk older adults (age 60 to 79, with established metabolic and cardiovascular risk factors). Interventions include a combination of physical exercise, nutritional counseling and management, cognitive and social stimulation, and improved management of cardiovascular risk factors. Recruitment for this large-scale trial was estimated to begin in January 2019 (NCT03688126).75
On a practical basis, Desai et al13 have proposed a checklist (Table 213) that physicians can use in their routine consultations to improve primary prevention of AD among their older patients.
Bottom Line
Advise patients that pursuing a healthy lifestyle is a key to delaying or preventing Alzheimer’s disease. This involves managing cardiovascular risk factors and a combination of staying physically, mentally, socially, and spiritually active, in addition to adhering to a healthy diet such as the Mediterranean diet.
Related Resources
- Anderson K, Grossberg GT. Brain games to slow cognitive decline in Alzheimer’s disease. J Am Med Dir Assoc. 2014;15(8):536-537.
- Small G, Vorgan G. The memory prescription: Dr. Garry Small’s 14-day plan to keep your brain and body young. New York, NY: Hyperion; 2004.
- Small G, Vorgan G. The Alzheimer’s prevention program; keep your brain healthy for the rest of your life. New York, NY: Workman Publishing Company, Inc.; 2012.
Drug Brand Name
Curcumin • Theracurmin
1. Mehta D, Jackson R, Paul G, et al. Why do trials for Alzheimer’s disease drugs keep failing? A discontinued drug perspective for 2010-2015. Expert Opin Investig Drugs. 2017;26(6):735-739.
2. Norton S, Matthews FE, Barnes DE, et al. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13(8):788-794.
3. Meng XF, Yu JT, Wang HF, et al. Midlife vascular risk factors and the risk of Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis. 2014;42(4):1295-1310.
4. Shekelle PG, Woolf SH, Eccles M, et al. Developing clinical guidelines. West J Med. 1999;170(6):348-351.
5. Barnes DE, Yaffe Y. The projected impact of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10(9):819-828.
6. Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30(9):464-472.
7. Ahlskog JE, Geda YE, Graff-Radford NR, et al. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clin Proc. 2011;86(9):876-884.
8. Scarmeas N, Luchsinger JA, Schupf N, et al. Physical activity, diet, and risk of Alzheimer Disease. JAMA. 2009;302(6):627-637.
9. Rovio S, Kåreholt I, Helkala EL, et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol. 2005;4(11):705-711.
10. Smith PJ et al. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med. 2010;72(3):239-252.
11. Brown BM, Peiffer JJ, Taddei K, et al. Physical activity and amyloid-beta plasma and brain levels: results from the Australian imaging, biomarkers and lifestyle study of ageing. Mol Psychiatry. 2013;18(8):875-881.
12. Brown BM, Sohrabi HR, Taddei K, et al. Habitual exercise levels are associated with cerebral amyloid load in presymptomatic autosomal dominant Alzheimer’s disease. Alzheimers Dement. 2017;13(11):1197-1206.
13. Desai AK, Grossberg GT, Chibnall JT. Healthy brain aging: a road map. Clin Geriatr Med. 2010;26(1):1-16.
14. Centers for Disease Control and Prevention. Physical activity: how much physical activity do older adults need?
15. Garber CE, Blissmer B, Deschenes MR, et al; American College of Sports Medicine. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334-1359.
16. Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113);2673-2734.
17. Valenzuela MJ, Sachdev P, Wen W, et al. Lifespan mental activity predicts diminished rate of hippocampal atrophy. PLoS One. 2008;3(7):e2598. doi.org/10.1371/journal.pone.0002598.
18. Wilson RS, Bennett DA, Bienias JL, et al. Cognitive activity and incident AD in a population-based sample of older persons. Neurology. 2002;59(12):1910-1914.
19. Wilson RS, Scherr PA, Schneider JA, et al. Relation of cognitive activity to risk of developing Alzheimer disease. Neurology. 2007;69(20):1911-1920.
20. Krell-Roesch J, Vemuri P, Pink A, et al. Association between mentally stimulating activities in late life and the outcome of incident mild cognitive impairment, with an analysis of the apoe ε4 genotype. JAMA Neurol. 2017;74(3):332-338.
21. Verghese J, Lipton RB, Katz MJ, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med. 2003;348(25):2508-2516.
22. Klein RM, Christie J, Parkvall M. Does multilingualism affect the incidence of Alzheimer’s disease?: a worldwide analysis by country. SSM Popul Health. 2016;2:463-467.
23. Grundy JG, Anderson JAE, Bialystok E. Neural correlates of cognitive processing in monolinguals and bilinguals. Ann N Y Acad Sci. 2017;1396(1):183-201.
24. Wilson RS, Krueger KR, Arnold SE, et al. Loneliness and risk of Alzheimer disease. Arch Gen Psychiatry. 2007;64(2):234-240.
25. Amieva H, Stoykova R, Matharan F, et al. What aspects of social network are protective for dementia? Not the quantity but the quality of social interactions is protective up to 15 years later. Psychosom Med. 2010;72(9):905-911.
26. Kuiper JS, Oude Voshaar RC, Zuidema SU, et al. The relationship between social functioning and subjective memory complaints in older persons: a population-based longitudinal cohort study. Int J Geriatr Psychiatry. 2017;32(10):1059-1071.
27. Robert P, Onyike CU, Leentjens AF, et al. Proposed diagnostic criteria for apathy in Alzheimer’s disease and other neuropsychiatric disorders. Eur Psychiatry. 2009;24(2):98-104.
28. Marioni RE, Proust-Lima C, Amieva H, et al. Social activity, cognitive decline and dementia risk: a 20-year prospective cohort study. BMC Public Health. 2015;15:1089.
29. Wilson RS, Schneider JA, Boyle PA, et al. Chronic distress and incidence of mild cognitive impairment. Neurology. 2007;68(24):2085-2092.
30. Cai Z, Yan LJ, Ratka A. Telomere shortening and Alzheimer’s disease. Neuromolecular Med. 2013;15(1):25-48.
31. Wilson RS, Arnold SE, Schneider JA, et al. Chronic psychological distress and risk of Alzheimer’s disease in old age. Neuroepidemiology. 2006;27(3):143-153.
32. Epel E, Daubenmier J, Moskowitz JT, et al. Can meditation slow rate of cellular aging? Cognitive stress, mindfulness, and telomeres. Ann N Y Acad Sci. 2009;1172:34-53.
33. Malinowski P, Moore AW, Mead Br, et al. Mindful aging: the effects of regular brief mindfulness practice on electrophysiological markers of cognitive and affective processing in older adults. Mindfulness (N Y). 2017;8(1):78-94.
34. Taren AA, Gianaros PJ, Greco CM, et al. Mindfulness meditation training and executive control network resting state functional connectivity: a randomized controlled trial. Psychosom Med. 2017;79(6):674-683.
35. Fountain-Zaragoza S, Prakash RS. Mindfulness training for healthy aging: impact on attention, well-being, and inflammation. Front in Aging Neurosci. 2017;9:11.
36. Eyre HA, Siddarth P, Acevedo B, et al. A randomized controlled trial of Kundalini yoga in mild cognitive impairment. Int Psychogeriatr. 2017;29(4):557-567.
37. Khalsa DS. Stress, meditation, and Alzheimer’s disease prevention: where the evidence stands. J Alzheimers Dis. 2015;48(1):1-12.
38. Berk L, van Boxtel M, van Os J. Can mindfulness-based interventions influence cognitive functioning in older adults? A review and considerations for future research. Aging Ment Health. 2017;21(11):1113-1120.
39. Hosseini S, Chaurasia A, Oremus M. The effect of religion and spirituality on cognitive function: a systematic review. Gerontologist. 2017. doi: 10.1093/geront/gnx024.
40. Luchsinger JA, Tang MX, Shea S, et al. Caloric intake and the risk of Alzheimer disease. Arch Neurol. 2002;59(8):1258-1263.
41. Grant WB. Trends in diet and Alzheimer’s disease during the nutrition transition in Japan and developing countries. J Alzheimers Dis. 2014;38(3):611-620.
42. Kanoski SE, Davidson TL. Western diet consumption and cognitive impairment: links to hippocampal dysfunction and obesity. Physiol Behav. 2011;103(1):59-68.
43. Hu N, Yu JT, Tan L, et al. Nutrition and the risk of Alzheimer’s disease. Biomed Res Int. 2013;2013:524820. doi: 10.1155/2013/524820.
44. Taylor MK, Sullivan DK, Swerdlow RH, et al. A high-glycemic diet is associated with cerebral amyloid burden in cognitively normal older adults. Am J Clin Nutr. 2017;106(6):1463-1470.
45. van de Rest O, Berendsen AM, Haveman-Nies A, et al. Dietary patterns, cognitive decline, and dementia: a systematic review. Adv Nutr. 2015;6(2):154-168.
46. Petersson SD, Philippou E. Mediterranean diet, cognitive function, and dementia: a systematic review of the evidence. Adv Nutr. 2016;7(5):889-904.
47. Smith PJ, Blumenthal JA, Babyak MA, et al. Effects of the dietary approaches to stop hypertension diet, exercise, and caloric restriction on neurocognition in overweight adults with high blood pressure. Hypertension. 2010;55(6):1331-1338.
48. Wengreen H, Munger RG, Cutler A, et al. Prospective study of dietary approaches to stop hypertension- and Mediterranean-style dietary patterns and age-related cognitive change: the Cache County study on memory, health and aging. Am J Clin Nutr. 2013;98(5):1263-1271.
49. Tangney CC, Li H, Wang Y, et al. Relation of DASH- and Mediterranean-like dietary patterns to cognitive decline in older persons. Neurology. 2014;83(16):1410-1416.
50. Morris MC, Tangney CC, Wang Y, et al. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement. 2015;11(9):1007-1014.
51. Desai AK, Rush J, Naveen L, et al. Nutrition and nutritional supplements to promote brain health. In: Hartman-Stein PE, Rue AL, eds. Enhancing cognitive fitness in adults: a guide to the use and development of community-based programs. New York, NY: Springer; 2011:249-269.
52. Goozee KG, Shah TM, Sohrabi HR, et al. Examining the potential clinical value of curcumin in the prevention and diagnosis of Alzheimer’s disease. Br J Nutr. 2016;115(3):449-465.
53. Small GW, Siddarth P, Li Z, et al. Memory and brain amyloid and tau effects of a bioavailable form of curcumin in non-demented adults: a double-blind, placebo-controlled 18-month trial. Am J Geriatr Psychiatry. 2018;26(3):266-277.
54. Kim JW, Lee DY, Lee BC, et al. Alcohol and cognition in the elderly: a review. Psychiatry Investig. 2012;9(1):8-16.
55. Virtaa JJ, Järvenpää T, Heikkilä K, et al. Midlife alcohol consumption and later risk of cognitive impairment: a twin follow-up study. J Alzheimers Dis. 2010;22(3):939-948.
56. Kerr WC, Stockwell T. Understanding standard drinks and drinking guidelines. Drug and Alcohol Rev. 2012;31(2):200-205.
57. National Institute on Alcohol Abuse and Alcoholism. Drinking levels defined. https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking. Accessed December 9, 2017.
58. National Institute on Alcohol Abuse and Alcoholism. What is a standard drink? https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/what-standard-drink. Accessed November 9, 2017.
59. Lindsay J, Laurin D, Verreault R, et al. Risk factors for Alzheimer’s disease: a prospective analysis from the Canadian study of health and aging. Am J Epidemiol. 2002;156(5):445-453.
60. Orgogozo JM, Dartigues JF, Lafont S, et al. Wine consumption and dementia in the elderly: a prospective community study in the Bordeaux area. Rev Neurol (Paris). 1997;153(3):185-192.
61. Topiwala A, Allan CL, Valkanova V, et al. Moderate alcohol consumption as risk factor for adverse brain outcomes and cognitive decline: longitudinal cohort study. BMJ. 2017;357.
62. Venkataraman A, Kalk N, Sewell G, et al. Alcohol and Alzheimer’s disease-does alcohol dependence contribute to beta-amyloid deposition, neuroinflammation and neurodegeneration in Alzheimer’s Disease? Alcohol Alcohol. 2017;52(2):151-158.
63. Ma QP, Huang C, Cui QY, et al. Meta-analysis of the association between tea intake and the risk of cognitive disorders. PLoS One. 2016;11(11):e0165861. doi: 10.1371/journal.pone.0165861.
64. Santos C, Costa J, Santos J, et al. Caffeine intake and dementia: systematic review and meta-analysis. J Alzheimers Dis. 2010;20(Suppl 1):S187-204.
65. Panza F, Solfrizzi V, Barulli MR, et al. Coffee, tea, and caffeine consumption and prevention of late-life cognitive decline and dementia: a systematic review. J Nutr Health Aging. 2015;19(3):313-328.
66. Wierzejska R. Can coffee consumption lower the risk of Alzheimer’s disease and Parkinson’s disease? A literature review. Arch Med Sci. 2017;13(3):507-514.
67. Arendash GW, Cao C. Caffeine and coffee as therapeutics against Alzheimer’s disease. J Alzheimers Dis. 2010;20 (Suppl 1):S117-S126.
68. Eskelinen MH, Ngandu T, Tuomilehto J, et al. Midlife coffee and tea drinking and the risk of late-life dementia: a population-based CAIDE study. J Alzheimers Dis. 2009;16(1):85-91.
69. Solfrizzi V, Panza F, Imbimbo BP, et al. Coffee consumption habits and the risk of mild cognitive impairment: the Italian longitudinal study on aging. J Alzheimers Dis. 2015;47(4):889-899.
70. Vittoria Mattioli. Beverages of daily life: impact of caffeine on atrial fibrillation. J Atr Fibrillation. 2014;7(2):1133.
71. Chacko SM, Thambi PT, Kuttan R, et al. Beneficial effects of green tea: a literature review. Chin Med. 2010;5:13.
72. Noguchi-Shinohara M, Yuki S, Dohmoto C, et al. Consumption of green tea, but not black tea or coffee, is associated with reduced risk of cognitive decline. PLoS One. 2014;9(5):e96013. doi: 10.1371/journal.pone.0096013.
73. Schneider N, Yvon C. A review of multidomain interventions to support healthy cognitive ageing. J Nutr Health Aging. 2013;17(3):252-257.
74. Ngandu T, Lehitsalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255-2263.
75. U.S. National Library of Medicing. ClinicalTrials.gov. U.S. study to protect brain health through lifestyle intervention to reduce risk (POINTER). https://clinicaltrials.gov/ct2/show/NCT03688126?term=pointer&cond=Alzheimer+Disease&rank=1. Published September 28, 2018. Accessed November 3, 2018.
1. Mehta D, Jackson R, Paul G, et al. Why do trials for Alzheimer’s disease drugs keep failing? A discontinued drug perspective for 2010-2015. Expert Opin Investig Drugs. 2017;26(6):735-739.
2. Norton S, Matthews FE, Barnes DE, et al. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13(8):788-794.
3. Meng XF, Yu JT, Wang HF, et al. Midlife vascular risk factors and the risk of Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis. 2014;42(4):1295-1310.
4. Shekelle PG, Woolf SH, Eccles M, et al. Developing clinical guidelines. West J Med. 1999;170(6):348-351.
5. Barnes DE, Yaffe Y. The projected impact of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10(9):819-828.
6. Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30(9):464-472.
7. Ahlskog JE, Geda YE, Graff-Radford NR, et al. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clin Proc. 2011;86(9):876-884.
8. Scarmeas N, Luchsinger JA, Schupf N, et al. Physical activity, diet, and risk of Alzheimer Disease. JAMA. 2009;302(6):627-637.
9. Rovio S, Kåreholt I, Helkala EL, et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol. 2005;4(11):705-711.
10. Smith PJ et al. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med. 2010;72(3):239-252.
11. Brown BM, Peiffer JJ, Taddei K, et al. Physical activity and amyloid-beta plasma and brain levels: results from the Australian imaging, biomarkers and lifestyle study of ageing. Mol Psychiatry. 2013;18(8):875-881.
12. Brown BM, Sohrabi HR, Taddei K, et al. Habitual exercise levels are associated with cerebral amyloid load in presymptomatic autosomal dominant Alzheimer’s disease. Alzheimers Dement. 2017;13(11):1197-1206.
13. Desai AK, Grossberg GT, Chibnall JT. Healthy brain aging: a road map. Clin Geriatr Med. 2010;26(1):1-16.
14. Centers for Disease Control and Prevention. Physical activity: how much physical activity do older adults need?
15. Garber CE, Blissmer B, Deschenes MR, et al; American College of Sports Medicine. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334-1359.
16. Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113);2673-2734.
17. Valenzuela MJ, Sachdev P, Wen W, et al. Lifespan mental activity predicts diminished rate of hippocampal atrophy. PLoS One. 2008;3(7):e2598. doi.org/10.1371/journal.pone.0002598.
18. Wilson RS, Bennett DA, Bienias JL, et al. Cognitive activity and incident AD in a population-based sample of older persons. Neurology. 2002;59(12):1910-1914.
19. Wilson RS, Scherr PA, Schneider JA, et al. Relation of cognitive activity to risk of developing Alzheimer disease. Neurology. 2007;69(20):1911-1920.
20. Krell-Roesch J, Vemuri P, Pink A, et al. Association between mentally stimulating activities in late life and the outcome of incident mild cognitive impairment, with an analysis of the apoe ε4 genotype. JAMA Neurol. 2017;74(3):332-338.
21. Verghese J, Lipton RB, Katz MJ, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med. 2003;348(25):2508-2516.
22. Klein RM, Christie J, Parkvall M. Does multilingualism affect the incidence of Alzheimer’s disease?: a worldwide analysis by country. SSM Popul Health. 2016;2:463-467.
23. Grundy JG, Anderson JAE, Bialystok E. Neural correlates of cognitive processing in monolinguals and bilinguals. Ann N Y Acad Sci. 2017;1396(1):183-201.
24. Wilson RS, Krueger KR, Arnold SE, et al. Loneliness and risk of Alzheimer disease. Arch Gen Psychiatry. 2007;64(2):234-240.
25. Amieva H, Stoykova R, Matharan F, et al. What aspects of social network are protective for dementia? Not the quantity but the quality of social interactions is protective up to 15 years later. Psychosom Med. 2010;72(9):905-911.
26. Kuiper JS, Oude Voshaar RC, Zuidema SU, et al. The relationship between social functioning and subjective memory complaints in older persons: a population-based longitudinal cohort study. Int J Geriatr Psychiatry. 2017;32(10):1059-1071.
27. Robert P, Onyike CU, Leentjens AF, et al. Proposed diagnostic criteria for apathy in Alzheimer’s disease and other neuropsychiatric disorders. Eur Psychiatry. 2009;24(2):98-104.
28. Marioni RE, Proust-Lima C, Amieva H, et al. Social activity, cognitive decline and dementia risk: a 20-year prospective cohort study. BMC Public Health. 2015;15:1089.
29. Wilson RS, Schneider JA, Boyle PA, et al. Chronic distress and incidence of mild cognitive impairment. Neurology. 2007;68(24):2085-2092.
30. Cai Z, Yan LJ, Ratka A. Telomere shortening and Alzheimer’s disease. Neuromolecular Med. 2013;15(1):25-48.
31. Wilson RS, Arnold SE, Schneider JA, et al. Chronic psychological distress and risk of Alzheimer’s disease in old age. Neuroepidemiology. 2006;27(3):143-153.
32. Epel E, Daubenmier J, Moskowitz JT, et al. Can meditation slow rate of cellular aging? Cognitive stress, mindfulness, and telomeres. Ann N Y Acad Sci. 2009;1172:34-53.
33. Malinowski P, Moore AW, Mead Br, et al. Mindful aging: the effects of regular brief mindfulness practice on electrophysiological markers of cognitive and affective processing in older adults. Mindfulness (N Y). 2017;8(1):78-94.
34. Taren AA, Gianaros PJ, Greco CM, et al. Mindfulness meditation training and executive control network resting state functional connectivity: a randomized controlled trial. Psychosom Med. 2017;79(6):674-683.
35. Fountain-Zaragoza S, Prakash RS. Mindfulness training for healthy aging: impact on attention, well-being, and inflammation. Front in Aging Neurosci. 2017;9:11.
36. Eyre HA, Siddarth P, Acevedo B, et al. A randomized controlled trial of Kundalini yoga in mild cognitive impairment. Int Psychogeriatr. 2017;29(4):557-567.
37. Khalsa DS. Stress, meditation, and Alzheimer’s disease prevention: where the evidence stands. J Alzheimers Dis. 2015;48(1):1-12.
38. Berk L, van Boxtel M, van Os J. Can mindfulness-based interventions influence cognitive functioning in older adults? A review and considerations for future research. Aging Ment Health. 2017;21(11):1113-1120.
39. Hosseini S, Chaurasia A, Oremus M. The effect of religion and spirituality on cognitive function: a systematic review. Gerontologist. 2017. doi: 10.1093/geront/gnx024.
40. Luchsinger JA, Tang MX, Shea S, et al. Caloric intake and the risk of Alzheimer disease. Arch Neurol. 2002;59(8):1258-1263.
41. Grant WB. Trends in diet and Alzheimer’s disease during the nutrition transition in Japan and developing countries. J Alzheimers Dis. 2014;38(3):611-620.
42. Kanoski SE, Davidson TL. Western diet consumption and cognitive impairment: links to hippocampal dysfunction and obesity. Physiol Behav. 2011;103(1):59-68.
43. Hu N, Yu JT, Tan L, et al. Nutrition and the risk of Alzheimer’s disease. Biomed Res Int. 2013;2013:524820. doi: 10.1155/2013/524820.
44. Taylor MK, Sullivan DK, Swerdlow RH, et al. A high-glycemic diet is associated with cerebral amyloid burden in cognitively normal older adults. Am J Clin Nutr. 2017;106(6):1463-1470.
45. van de Rest O, Berendsen AM, Haveman-Nies A, et al. Dietary patterns, cognitive decline, and dementia: a systematic review. Adv Nutr. 2015;6(2):154-168.
46. Petersson SD, Philippou E. Mediterranean diet, cognitive function, and dementia: a systematic review of the evidence. Adv Nutr. 2016;7(5):889-904.
47. Smith PJ, Blumenthal JA, Babyak MA, et al. Effects of the dietary approaches to stop hypertension diet, exercise, and caloric restriction on neurocognition in overweight adults with high blood pressure. Hypertension. 2010;55(6):1331-1338.
48. Wengreen H, Munger RG, Cutler A, et al. Prospective study of dietary approaches to stop hypertension- and Mediterranean-style dietary patterns and age-related cognitive change: the Cache County study on memory, health and aging. Am J Clin Nutr. 2013;98(5):1263-1271.
49. Tangney CC, Li H, Wang Y, et al. Relation of DASH- and Mediterranean-like dietary patterns to cognitive decline in older persons. Neurology. 2014;83(16):1410-1416.
50. Morris MC, Tangney CC, Wang Y, et al. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement. 2015;11(9):1007-1014.
51. Desai AK, Rush J, Naveen L, et al. Nutrition and nutritional supplements to promote brain health. In: Hartman-Stein PE, Rue AL, eds. Enhancing cognitive fitness in adults: a guide to the use and development of community-based programs. New York, NY: Springer; 2011:249-269.
52. Goozee KG, Shah TM, Sohrabi HR, et al. Examining the potential clinical value of curcumin in the prevention and diagnosis of Alzheimer’s disease. Br J Nutr. 2016;115(3):449-465.
53. Small GW, Siddarth P, Li Z, et al. Memory and brain amyloid and tau effects of a bioavailable form of curcumin in non-demented adults: a double-blind, placebo-controlled 18-month trial. Am J Geriatr Psychiatry. 2018;26(3):266-277.
54. Kim JW, Lee DY, Lee BC, et al. Alcohol and cognition in the elderly: a review. Psychiatry Investig. 2012;9(1):8-16.
55. Virtaa JJ, Järvenpää T, Heikkilä K, et al. Midlife alcohol consumption and later risk of cognitive impairment: a twin follow-up study. J Alzheimers Dis. 2010;22(3):939-948.
56. Kerr WC, Stockwell T. Understanding standard drinks and drinking guidelines. Drug and Alcohol Rev. 2012;31(2):200-205.
57. National Institute on Alcohol Abuse and Alcoholism. Drinking levels defined. https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking. Accessed December 9, 2017.
58. National Institute on Alcohol Abuse and Alcoholism. What is a standard drink? https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/what-standard-drink. Accessed November 9, 2017.
59. Lindsay J, Laurin D, Verreault R, et al. Risk factors for Alzheimer’s disease: a prospective analysis from the Canadian study of health and aging. Am J Epidemiol. 2002;156(5):445-453.
60. Orgogozo JM, Dartigues JF, Lafont S, et al. Wine consumption and dementia in the elderly: a prospective community study in the Bordeaux area. Rev Neurol (Paris). 1997;153(3):185-192.
61. Topiwala A, Allan CL, Valkanova V, et al. Moderate alcohol consumption as risk factor for adverse brain outcomes and cognitive decline: longitudinal cohort study. BMJ. 2017;357.
62. Venkataraman A, Kalk N, Sewell G, et al. Alcohol and Alzheimer’s disease-does alcohol dependence contribute to beta-amyloid deposition, neuroinflammation and neurodegeneration in Alzheimer’s Disease? Alcohol Alcohol. 2017;52(2):151-158.
63. Ma QP, Huang C, Cui QY, et al. Meta-analysis of the association between tea intake and the risk of cognitive disorders. PLoS One. 2016;11(11):e0165861. doi: 10.1371/journal.pone.0165861.
64. Santos C, Costa J, Santos J, et al. Caffeine intake and dementia: systematic review and meta-analysis. J Alzheimers Dis. 2010;20(Suppl 1):S187-204.
65. Panza F, Solfrizzi V, Barulli MR, et al. Coffee, tea, and caffeine consumption and prevention of late-life cognitive decline and dementia: a systematic review. J Nutr Health Aging. 2015;19(3):313-328.
66. Wierzejska R. Can coffee consumption lower the risk of Alzheimer’s disease and Parkinson’s disease? A literature review. Arch Med Sci. 2017;13(3):507-514.
67. Arendash GW, Cao C. Caffeine and coffee as therapeutics against Alzheimer’s disease. J Alzheimers Dis. 2010;20 (Suppl 1):S117-S126.
68. Eskelinen MH, Ngandu T, Tuomilehto J, et al. Midlife coffee and tea drinking and the risk of late-life dementia: a population-based CAIDE study. J Alzheimers Dis. 2009;16(1):85-91.
69. Solfrizzi V, Panza F, Imbimbo BP, et al. Coffee consumption habits and the risk of mild cognitive impairment: the Italian longitudinal study on aging. J Alzheimers Dis. 2015;47(4):889-899.
70. Vittoria Mattioli. Beverages of daily life: impact of caffeine on atrial fibrillation. J Atr Fibrillation. 2014;7(2):1133.
71. Chacko SM, Thambi PT, Kuttan R, et al. Beneficial effects of green tea: a literature review. Chin Med. 2010;5:13.
72. Noguchi-Shinohara M, Yuki S, Dohmoto C, et al. Consumption of green tea, but not black tea or coffee, is associated with reduced risk of cognitive decline. PLoS One. 2014;9(5):e96013. doi: 10.1371/journal.pone.0096013.
73. Schneider N, Yvon C. A review of multidomain interventions to support healthy cognitive ageing. J Nutr Health Aging. 2013;17(3):252-257.
74. Ngandu T, Lehitsalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255-2263.
75. U.S. National Library of Medicing. ClinicalTrials.gov. U.S. study to protect brain health through lifestyle intervention to reduce risk (POINTER). https://clinicaltrials.gov/ct2/show/NCT03688126?term=pointer&cond=Alzheimer+Disease&rank=1. Published September 28, 2018. Accessed November 3, 2018.
Abuse of psychiatric medications: Not just stimulants and benzodiazepines
While some classes of medications used to treat psychiatric disorders, such as stimulants and benzodiazepines, are well-recognized as controlled substances and drugs of abuse, clinicians may be less familiar with the potential misuse/abuse of other psychiatric medications. This article reviews the evidence related to the misuse/abuse of anticholinergics, antidepressants, antipsychotics, and gabapentinoids.
The terms “misuse,” “abuse,” and “addiction” are used variably in the literature without standardized definitions. For this review, “misuse/abuse (M/A)” will be used to collectively describe self-administration that is recreational or otherwise inconsistent with legal or medical guidelines, unless a specific distinction is made. Whether or not the medications reviewed are truly “addictive” will be briefly discussed for each drug class, but the focus will be on clinically relevant aspects of M/A, including:
- excessive self-administration
- self-administration by non-oral routes
- co-administration with other drugs of abuse
- malingering of psychiatric symptoms to obtain prescriptions
- diversion for sale to third parties
- toxicity from overdose.
Anticholinergic medications
The first case describing the deliberate M/A of an anticholinergic medication for its euphoric effects was published in 1960.Further reportsfollowed in Europe before the M/A potential of prescription anticholinergic medications among psychiatric patients with an overdose syndrome characterized by atropinism and toxic psychosis was more widely recognized in the United States in the 1970s. Most reported cases of M/A to date have occurred among patients with psychiatric illness because anticholinergic medications, including trihexyphenidyl, benztropine, biperiden, procyclidine, and orphenadrine, were commonly prescribed for the management of first-generation and high dopamine D2-affinity antipsychotic-induced extrapyramidal symptoms (EPS). For example, one study of 234 consecutively hospitalized patients with schizophrenia noted an anticholinergic M/A incidence of 6.5%.1
However, anticholinergic M/A is not limited to individuals with psychotic disorders. A UK study of 154 admissions to an inpatient unit specializing in behavioral disturbances found a 12-month trihexyphenidyl M/A incidence of 17%; the most common diagnosis among abusers was antisocial personality disorder.2 Anticholinergic M/A has also been reported among patients with a primary diagnosis of substance use disorders (SUDs)3 as well as more indiscriminately in prison settings,4 with some inmates exchanging trihexyphenidyl as currency and using it recreationally by crushing it into powder and smoking it with tobacco.5 Others have noted that abusers sometimes take anticholinergics with alcohol in order to “potentiate” the effects of each substance.6,7 Pullen et al8 described individuals with and without psychiatric illness who stole anticholinergic medications, purchased them from other patients, or bought them “on the street.” Malingering EPS in order to obtain anticholinergic medications has also been well documented.9 Clearly, anticholinergic M/A can occur in psychiatric and non-psychiatric populations, both within and outside of clinical settings. Although anticholinergic M/A appears to be less frequent in the United States now that second-generation antipsychotics (SGAs) are more frequently prescribed, M/A remains common in some settings outside of the United States.7
Among the various anticholinergic medications prescribed for EPS, trihexyphenidyl has been reported to have the greatest M/A potential, which has been attributed to its potency,10 its stimulating effects (whereas benztropine is more sedating),11 and its former popularity among prescribers.8 Marken et al11 published a review of 110 reports of M/A occurring in patients receiving anticholinergic medications as part of psychiatric treatment in which 69% of cases involved taking trihexyphenidyl 15 to 60 mg at a time (recommended dosing is 6 to 10 mg/d in divided doses).Most of these patients were prescribed anticholinergic medications for diagnostically appropriate reasons—only 7% were described as “true abusers” with no medical indication. Anticholinergic M/A was typically driven by a desire for euphoric and psychedelic/hallucinogenic effects, although in some cases, anticholinergic M/A was attributed to self-medication of EPS and depressive symptoms. These findings illustrate the blurred distinction between recreational use and perceived subjective benefit, and match those of a subsequent study of 50 psychiatric patients who reported anticholinergic M/A not only to “get high,” but to “decrease depression,” “increase energy,” and decrease antipsychotic adverse effects.12 Once again, trihexyphenidyl was the most frequently misused anticholinergic in this sample.
Table 12,3,7,8,10-15 outlines the subjective effects sought and experienced by anticholinergic abusers as well as potential toxic effects; there is the potential for overlap. Several authors have also described physiologic dependence with long-term trihexyphenidyl use, including tolerance and a withdrawal/abstinence syndrome.7,16 In addition, there have been several reports of coma13 and death in the setting of intended suicide by overdose of anticholinergic medications.14,15
Although anticholinergic M/A in the United States now appears to be less common, clinicians should remain aware of the M/A potential of anticholinergic medications prescribed for EPS. Management of M/A involves:
- detection
- reducing anticholinergic exposure by managing EPS with alternative strategies, such as switching or reducing the dose of the antipsychotic medication
- gradual tapering of anticholinergic medications to minimize withdrawal.11
Continue to: Antidepressants
Antidepressants
Haddad17 published a review of 21 English-language case reports from 1966 to 1998 describing antidepressant use in which individuals met DSM-IV criteria for substance dependence to the medication. An additional 14 cases of antidepressant M/A were excluded based on insufficient details to support a diagnosis of dependence. The 21 reported cases involved:
- tranylcypromine (a monoamine oxidase inhibitor [MAOI])
- amitriptyline (a tricyclic antidepressant [TCA])
- fluoxetine (a selective serotonin reuptake inhibitor [SSRI])
- amineptine (a TCA previously available in France but removed from the market in 1999 in part due to its abuse potential)
- nomifensine (a norepinephrine/dopamine reuptake inhibitor previously available in the United Kingdom but removed in 1986 due to hemolytic anemia).
In 95% of cases, the antidepressants were prescribed for treatment of an affective disorder but were abused for stimulant effects or the perceived ability to lift mood, cause euphoria or a “high,” or to improve functioning. Two-thirds of cases involved patients with preexisting substance misuse. Placing the case reports in the context of the millions of patients prescribed antidepressants during this period, Haddad concluded the “incidence of [antidepressant] addiction [is] so low as to be clinically irrelevant.”17
Despite this conclusion, Haddad singled out amineptine and tranylcypromine as antidepressants with some evidence of true addictive potential.17,18 A more recent case series described 14 patients who met DSM-IV criteria for substance abuse of tertiary amine TCAs (which have strong anticholinergic activity) and concluded that “misuse of [TCAs] is more common than generally appreciated.”19 In keeping with that claim, a study of 54 outpatients taking unspecified antidepressants found that up to 15% met DSM-III-R criteria for substance dependence (for the antidepressant) in the past year, although that rate was much lower than the rate of benzodiazepine dependence (47%) in a comparative sample.20 Finally, a comprehensive review by Evans and Sullivan21 found anecdotal reports published before 2014 that detailed misuse, abuse, and dependence with MAOIs, TCAs, fluoxetine, venlafaxine, bupropion, tianeptine, and amineptine. Taken together, existing evidence indicates that select individuals—typically those with other SUD comorbidity—sometimes misuse antidepressants in a way that suggests addiction.
Still, while it is well known that abrupt cessation of antidepressants can result in a discontinuation syndrome characterized by flu-like symptoms, nausea, and dizziness,22 physiologic withdrawal effects must be distinguished from historical definitions of substance “abuse” and the broader concept of psychological “addiction” or drug dependence18,23 now incorporated into the DSM-5 definition of SUDs.24 Indeed, although withdrawal symptoms were reported by more than half of those who took antidepressants and responded to a recent online survey,25 evidence to support the existence of significant antidepressant tolerance, craving, or compulsive use is lacking.17,18 Antidepressants as a class do not appear to be significantly rewarding or reinforcing and, on the contrary, discontinuation by patients is common in clinical practice.26 The popular claim that some individuals taking antidepressants “can’t quit”27 must also be disentangled from loss of therapeutic effects upon cessation.
Bupropion. A more convincing argument for antidepressant addiction can be made for bupropion, a weak norepinephrine and dopamine reuptake inhibitor with an otherwise unclear mechanism of action.28 In 2002, the first report of recreational bupropion M/A described a 13-year-old girl who took 2,400 mg orally (recommended maximum dose is 450 mg/d in divided doses) after being told it would give her “a better high than amphetamine.”29 This was followed in the same year by the first report of recreational M/A of bupropion via nasal insufflation (snorting), resulting in a seizure,30 and in 2013 by the first published case of M/A by IV self-administration.31
Continue to: The M/A potential of bupropion...
The M/A potential of bupropion, most commonly via intranasal administration, is now broadly recognized based on several case reports describing desired effects that include a euphoric high and a stimulating “buzz” similar to that of cocaine or methamphetamine but less intense.29-36 Among recreational users, bupropion tablets are referred to as “welbys,” “wellies,” “dubs,” or “barnies.”37 Media coverage of a 2013 outbreak of bupropion M/A in Toronto detailed administration by snorting, smoking, and injection, and described bupropion as “poor man’s cocaine.”38 Between 2003 and 2016, 2,232 cases of bupropion misuse/abuse/dependence adverse drug reactions were reported to the European Monitoring Agency.37 A review of intentional bupropion M/A reported to US Poison Control Centers between 2000 to 2013 found 975 such cases, with the yearly number tripling between 2000 and 2012.39 In this sample, nearly half (45%) of the users were age 13 to 19, and 76% of cases involved oral ingestion. In addition to bupropion M/A among younger people, individuals who misuse bupropion often include those with existing SUDs but limited access to illicit stimulants and those trying to evade detection by urine toxicology screening.33 For example, widespread use and diversion has been well documented within correctional settings, and as a result, many facilities have removed bupropion from their formularies.21,28,33,34,40
Beyond desired effects, the most common adverse events associated with bupropion M/A are listed in Table 2,28,30,32-34,36,39 along with their incidence based on cases brought to the attention of US Poison Control Centers.39 With relatively little evidence of a significant bupropion withdrawal syndrome,37 the argument in favor of modeling bupropion as a truly addictive drug is limited to anecdotal reports of cravings and compulsive self-administration35 and pro-dopaminergic activity (reuptake inhibition) that might provide a mechanism for potential rewarding and reinforcing effects.40 While early preclinical studies of bupropion failed to provide evidence of amphetamine-like abuse potential,41,42 non-oral administration in amounts well beyond therapeutic dosing could account for euphoric effects and a greater risk of psychological dependence and addiction.21,28,40
Bupropion also has an FDA indication as an aid to smoking cessation treatment, and the medication demonstrated early promise in the pharmacologic treatment of psychostimulant use disorders, with reported improvements in cravings and other SUD outcomes.43-45 However, subsequent randomized controlled trials (RCTs) failed to demonstrate a clear therapeutic role for bupropion in the treatment of cocaine46,47 and methamphetamine use disorders (although some secondary analyses suggest possible therapeutic effects among non-daily stimulant users who are able to maintain good adherence with bupropion).48-51 Given these overall discouraging results, the additive seizure risk of bupropion use with concomitant psychostimulant use, and the potential for M/A and diversion of bupropion (particularly among those with existing SUDs), the use of bupropion for the off-label treatment of stimulant use disorders is not advised.
Antipsychotics
As dopamine antagonists, antipsychotics are typically considered to have low potential for rewarding or reinforcing effects. Indeed, misuse of antipsychotics was a rarity in the first-generation era, with only a few published reports of haloperidol M/A within a small cluster of naïve young people who developed acute EPS,52 and a report of diversion in a prison with the “sadistic” intent of inflicting dystonic reactions on others.53 A more recent report described 2additional cases of M/A involving haloperidol and trifluoperazine.54 Some authors have described occasional drug-seeking behavior for low-potency D2 blockers such as chlorpromazine, presumably based on their M/A as anticholinergic medications.55
The potential for antipsychotic M/A has gained wider recognition since the advent of the SGAs. Three cases of prescription olanzapine M/A have been published to date. One involved a man who malingered manic symptoms to obtain olanzapine, taking ≥40 mg at a time (beyond his prescribed dose of 20 mg twice daily) to get a “buzz,” and combining it with alcohol and benzodiazepines for additive effects or to “come down” from cocaine.56 This patient noted that olanzapine was “a popular drug at parties” and was bought, sold, or traded among users, and occasionally administered intravenously. Two other cases described women who self-administered olanzapine, 40 to 50 mg/d, for euphoric and anxiolytic effects.57,58 James et al59 detailed a sample of 28 adults who reported “non-medical use” of olanzapine for anxiolytic effects, as a sleep aid, or to “escape from worries.”
Continue to: Quetiapine
Quetiapine. In contrast to some reports of olanzapine M/A in which the line between M/A and “self-medication” was blurred, quetiapine has become a more convincing example of clear recreational antipsychotic M/A. Since the first report of oral and intranasal quetiapine M/A in the Los Angeles County Jail published in 2004,55 subsequent cases have detailed other novel methods of recreational self-administration60-68 (Table 355,60-68), and additional reports have been published in non-English language journals.69,70 Collectively, these case reports have detailed that quetiapine is:
- misused for primary subjective effects as well as to mitigate the unpleasant effects of other drugs60,67
- referred to as “quell,”“Q,” “Susie-Q,” “squirrel,” and “baby heroin”55,71,72
- often obtained by malingering psychiatric symptoms55,61,63,65
- diverted/sold with “street value” both within and outside of psychiatric facilities and correctional settings.55,60-62,67,68,73
These anecdotal accounts of quetiapine M/A have since been corroborated on a larger scale based on several retrospective studies. Although early reports of quetiapine M/A occurring in correctional settings have resulted in formulary removal,71,74 quetiapine M/A is by no means limited to forensic populations and is especially common among those with comorbid SUDs. A survey of 74 patients enrolled in a Canadian methadone program reported that nearly 60% had misused quetiapine at some point.75 Among an Australian sample of 868 individuals with active IV drug abuse, 31% reported having misused quetiapine.76 Finally, within a small sample of patients with SUDs admitted to a detoxification unit in New York City, 17% reported M/A of SGAs.77 In this study, SGAs were often taken in conjunction with other drugs of abuse in order to “recover” from or “enhance” the effects of other drugs or to “experiment.” Quetiapine was by far the most frequently abused SGA, reported in 96% of the sample; the most frequently reported SGA/drug combinations were quetiapine/alcohol/opioids, quetiapine/cocaine, and quetiapine/opioids.
Looking more broadly at poison center data, reports to the US National Poison Data System (NPDS) from 2005 to 2011 included 3,116 cases of quetiapine abuse (37.5%, defined as intentional recreational use in order to obtain a “high”) or misuse (62.5%, defined as improper use or dosing for non-recreational purposes).78 A more recent analysis of NPDS reports from 2003 to 2013 found 2,118 cases of quetiapine abuse, representing 61% of all cases of reported SGA abuse.79 An analysis of the European Medicines Agency Adverse Drug Database yielded 18,112 reports of quetiapine misuse, abuse, dependence, and withdrawal for quetiapine (from 2005 to 2016) compared with 4,178 for olanzapine (from 2004 to 2016).80 These reports identified 368 fatalities associated with quetiapine.
The rate of quetiapine M/A appears to be increasing sharply. Reports of quetiapine M/A to poison centers in Australia increased nearly 7-fold from 2006 to 2016.81 Based on reports to the Drug Abuse Warning System, US emergency department visits for M/A of quetiapine increased from 19,195 in 2005 to 32,024 in 2011 (an average of 27,114 visits/year), with 75% of cases involving quetiapine taken in combination with other prescription drugs, alcohol, or illicit drugs.82 Consistent with poison center data, M/A was reported for other antipsychotics, but none nearly as frequently as for quetiapine.
With increasingly frequent quetiapine M/A, clinicians should be vigilant in monitoring for medical morbidity related to quetiapine and cumulative toxicity with other drugs. The most frequent adverse events associated with quetiapine M/A reported to US Poison Control Centers are presented in Table 4.78,79
Continue to: Unlike bupropion...
Unlike bupropion, quetiapine’s dopamine antagonism makes it unlikely to be a truly addictive drug, although this mechanism of action could mediate an increase in concurrent psychostimulant use.83 A few case reports have described a quetiapine discontinuation syndrome similar to that of antidepressants,60,65,84-88 but withdrawal symptoms suggestive of physiologic dependence may be mediated by non-dopaminergic effects through histamine and serotonin receptors.84,89 Evidence for quetiapine misuse being associated with craving and compulsive use is lacking, and true quetiapine addiction is probably rare.
Similar to bupropion, preliminary findings have suggested promise for quetiapine as a putative therapy for other SUDs.90-93 However, subsequent RCTs have failed to demonstrate a therapeutic effect for alcohol and cocaine use disorders.94-96 Given these negative results and the clear M/A potential of quetiapine, off-label use of quetiapine for the treatment of SUDs and psychiatric symptoms among those with SUDs must be considered judiciously, with an eye towards possible diversion and avoiding the substitution of one drug of abuse for another.
Gabapentinoids
In 1997, the first published case report of gabapentin M/A described a woman who self-administered her husband’s gabapentin to reduce cravings for and withdrawal from cocaine.97 The authors highlighted the possible therapeutic benefit of gabapentin in this regard rather than raising concerns about diversion and M/A. By 2004, however, reports of recreational gabapentin M/A emerged among inmates incarcerated within Florida correctional facilities who self-administered intranasal gabapentin to achieve a “high” that was “reminiscent of prior effects from intranasal ingestion of cocaine powder.”98 In 2007, a single case of gabapentin misuse up to 7,200 mg/d (recommended dosing is ≤3,600 mg/d) was reported, with documentation of both tolerance and withdrawal symptoms.99 As of 2017, a total of 36 cases of gabapentin M/A and 19 cases of pregabalin M/A have been published.100
In the past decade, anecdotal reports have given way to larger-scale epidemiologic data painting a clear picture of the now-widespread M/A of gabapentin and other gabapentinoids. For example, a study of online descriptions of gabapentin and pregabalin M/A from 2008 to 2010 documented:
- oral and IM use (gabapentin)
- IV and rectal (“plugging”) use (pregabalin)
- “parachuting” (emptying the contents of capsules for a larger dose) (pregabalin)
- euphoric, entactogenic, stimulant, calming/anxiolytic, and dissociative subjective effects (gabapentin/pregabalin)
- rapid development of tolerance to euphoric effects leading to self-administration of increasing doses (gabapentin/pregabalin)
- frequent co-administration with other drugs of abuse, including alcohol, benzodiazepines, cannabis, stimulants, opiates, hallucinogens, gamma-hydroxybutyrate, mephedrone, and Salvia divinorum (gabapentin/pregabalin)101
Several systematic reviews of both anecdotal reports and epidemiologic studies published in the past few years provide additional evidence of the above, such as:
- excessive dosing with self-administration
- intranasal and inhaled routes of administration
- diversion and “street value”
- greater M/A potential of pregabalin than gabapentin
- the presence of gabapentinoids in postmortem toxicology analyses, suggesting a role in overdose fatalities when combined with other drugs.100,102,103
Continue to: The European Medicine Agency's EudraVigilance database...
The European Medicine Agency’s EudraVigilance database included 4,301 reports of gabapentin misuse, abuse, or dependence, and 7,639 such reports for pregabalin, from 2006 to 2015 (rising sharply after 2012), with 86 gabapentin-related and 27 pregabalin-related fatalities.104 Data from the Drug Diversion Program of the Researched Abuse, Diversion, and Addiction-Related Surveillance System from 2002 to 2015 have likewise revealed that gabapentin diversion increased significantly in 2013.105
While the prevalence of gabapentinoid M/A is not known, rates appear to be significantly lower than for traditional drugs of abuse such as cannabis, cocaine, 3,4-methylenedioxymethamphetamine (MDMA), and opioids.106,107 However, gabapentin and pregabalin M/A appears to be increasingly common among individuals with SUDs and in particular among those with opioid use disorders (OUDs). For example, a 2015 report indicated that 15% of an adult cohort in Appalachian Kentucky with nonmedical use of diverted prescription opioids reported gabapentin M/A, an increase of nearly 3,000% since 2008.108 Based on data from a US insurance enrollment and claims database, researchers found that the rate of gabapentin overuse among those also overusing opioids was 12% compared with only 2% for those using gabapentin alone.109 It has also been reported that gabapentin is sometimes used as a “cutting agent” for heroin.110
Those who use gabapentinoids together with opioids report that gabapentin and pregabalin potentiate the euphoric effects of methadone111 and endorse specific beliefs that pregabalin increases both the desired effects of heroin as well as negative effects such as “blackouts,” loss of control, and risk of overdose.112 Indeed, sustained M/A of gabapentin and opioids together has been found to increase emergency department utilization, drug-related hospitalization, and respiratory depression.113 Based on a case-control study of opioid users in Canada, co-prescription of gabapentin and opioids was associated with a 50% increase in death from opioid-related causes compared with prescription of opioids alone.114
Case reports documenting tolerance, withdrawal, craving, and loss of control suggest a true addictive potential for gabapentinoids, but Bonnet and Sherbaum100 concluded that while there is robust evidence of abusers “liking” gabapentin and pregabalin (eg, reward), evidence of “wanting” them (eg, psychological dependence) in the absence of other SUDs has been limited to only a few anecdotal reports with pregabalin. Accordingly, the risk of true addiction to gabapentinoids by those without preexisting SUDs appears to be low. Nonetheless, the M/A potential of both gabapentin and pregabalin is clear and in the context of a nationwide opioid epidemic, the increased morbidity/mortality risk related to combined use of gabapentinoids and opioids is both striking and concerning. Consequently, the state of Kentucky recently recognized the M/A potential of gabapentin by designating it a Schedule V controlled substance (pregabalin is already a Schedule V drug according to the US Drug Enforcement Agency),103,113 and several other states now mandate the reporting of gabapentin prescriptions to prescription drug monitoring programs.115
Following a similar pattern to antidepressants and antipsychotics, a potential role for gabapentin in the treatment of cocaine use disorders was supported in preliminary studies,116-118 but not in subsequent RCTs.119-121 However, there is evidence from RCTs to support the use of gabapentin and pregabalin in the treatment of alcohol use disorders.122-124 Gabapentin was also found to significantly reduce cannabis use and withdrawal symptoms in patients compared with placebo in an RCT of individuals with cannabis use disorders.125 The perceived safety of gabapentinoids by clinicians, their subjective desirability by patients with SUDs, and efficacy data supporting a therapeutic role in SUDs must be balanced with recognition that approximately 80% of gabapentin prescriptions are written for off-label indications for which there is little supporting evidence,109 such as low back pain.126 Clinicians considering prescribing gabapentinoids to manage psychiatric symptoms, such as anxiety and insomnia, should carefully consider the risk of M/A and other potential morbidities, especially in the setting of SUDs and OUD in particular.
Continue to: Problematic, even if not addictive
Problematic, even if not addictive
It is sometimes claimed that “addiction” to psychiatric medications is not limited to stimulants and benzodiazepines.27,127 Although anticholinergics, antidepressants, antipsychotics, and gabapentinoids can be drugs of abuse, with some users reporting physiologic withdrawal upon discontinuation, there is only limited evidence that the M/A of these psychiatric medications is associated with the characteristic features of a more complete definition of “addiction,” which may include:
- inability to consistently abstain
- impairment in behavioral control
- diminished recognition of significant problems associated with use
- a dysfunctional emotional response to chronic use.128
Nonetheless, the literature documenting anticholinergic, antidepressant, antipsychotic, and gabapentinoid M/A includes several common features, including:
- initial reports among those with limited access to illicit drugs (eg, young people and incarcerated individuals) and subsequent spread to a wider population with more unconventional routes of administration
- use for recreational purposes and other subjective pseudo-therapeutic effects, often in combination with alcohol and illicit drugs
- greater M/A potential of certain medications within each of these drug classes (eg, trihexyphenidyl, bupropion, quetiapine)
- malingering psychiatric symptoms in order to obtain medications from prescribers and diversion for black market sale
- observations that medications might constitute therapy for SUDs that were not supported in subsequent RCTs (with the exception of gabapentin for alcohol and cannabis use disorders)
- increasing evidence of toxicity related to M/A, which suggests that prescription by clinicians has limited benefit and high risk for patients with SUDs.
Bottom Line
Some psychiatric medications are taken as drugs of abuse. Clinicians should be particularly aware of the misuse/abuse potential of anticholinergics, antidepressants, antipsychotics, and gabapentinoids, and use them cautiously, if at all, when treating patients with existing substance use disorders.
Related Resources
- Substance Abuse and Mental Health Services Administration. Prescription drug misuse and abuse. https://www.samhsa.gov/topics/prescription-drug-misuse-abuse.
- Substance Abuse and Mental Health Services Administration. Types of commonly misused or abused drugs. https://www.samhsa.gov/prescription-drug-misuse-abuse/types.
- National Institute on Drug Abuse. Misuse of prescription drugs. https://www.drugabuse.gov/publications/research-reports/misuse-prescription-drugs/summary.
- National Institute on Drug Abuse. New clinician screening tool available for substance use. https://www.drugabuse.gov/news-events/news-releases/2018/06/newclinician-screening-tool-available-substance-use.
Drug Brand Names
Amitriptyline • Elavil, Endep
Benztropine • Cogentin
Biperiden • Akineton
Bupropion • Wellbutrin, Zyban
Chlorpromazine • Thorzine
Fluoxetine • Prozac
Haloperidol • Haldol
Olanzapine • Zyprexa
Orphenadrine • Disipal, Norflex
Pregabalin • Lyrica, Lyrica CR
Procyclidine • Kemadrin
Quetiapine • Seroquel
Tianeptine • Coaxil, Stablon
Tranylcypromine • Parnate
Trifluoperazine • Stelazine
Trihexyphenidyl • Artane, Tremin
Venlafaxine • Effexor
1. Zemishlany Z, Aizenberg D, Weiner Z, et al. Trihexyphenidyl (Artane) abuse in schizophrenic patients. Int Clin Psychopharmacol. 1996;11(3):199-202.
2. Crawshaw JA, Mullen PE. A study of benzhexol abuse. Brit J Psychiatry. 1984;145:300-303.
3. Woody GE, O’Brien CP. Anticholinergic toxic psychosis in drug abusers treated with benztropine. Comp Psychiatry. 1974;15(5):439-442.
4. Lowry TP. Trihexyphenidyl abuse. Am J Psychiatry. 1977;134(11):1315.
5. Rouchell AM, Dixon SP. Trihexyphenidyl abuse. Am J Psychiatry. 1977;134(11):1315.
6. Kaminer Y, Munitz H, Wijsenbeek H. Trihexyphenidyl (Artane) abuse: euphoriant and anxiolytic. Brit J Psychiatry. 1982;140(5):473-474.
7. Nappo SA, de Oliviera LG, Sanchez Zv, et al. Trihexyphenidyl (Artane): a Brazilian study of its abuse. Subst Use Misuse. 2005;40(4):473-482.
8. Pullen GP, Best NR, Macguire J. Anticholinergic drug abuse: a common problem? Brit Med J (Clin Res Ed). 1984;289(6445):612-613.
9. Rubinstein JS. Abuse of antiparkinsonian drugs: feigning of extrapyramidal symptoms to obtain trihexyphenidyl. JAMA. 1978;239(22):2365-2366.
10. Mohan D, Mohandas E, Dube S. Trihexyphenidyl abuse. Brit J Addiction. 1981:76(2);195-197.
11. Marken PA, Stoner SC, Bunker MT. Anticholinergic drug abuse and misuse. CNS Drugs. 1996;5(3):190-199.
12. Buhrich N, Weller A, Kevans P. Misuse of anticholinergic drugs by people with serious mental illness. Psychiatric Serv. 2000;51(7):928-929.
13. Goldstein MR, Kasper R. Hyperpyrexia and coma due to overdose of benztropine. South Med J. 1968;61(9):984.
14. Petkovi
15. McIntyre IM, Mallett P, Burton CG, et al. Acute benztropine intoxication and fatality. J Forensic Sci. 2014;59(6):1675-1678.
16. Dilsaver SC. Antimuscarinic agents as substances of abuse: A review. J Clin Psychopharmacol. 1988:8(1):14-22.
17. Haddad P. Do antidepressants have any potential to cause addiction? J Psychopharmacol. 1999;13(3):300-307.
18. Haddad PM. Do antidepressants cause dependence? Epidemiol Psichiatr Soc. 2005;14(2):58-62.
19. Shenouda R, Desan PH. Abuse of tricyclic antidepressant drugs: a case series. J Clin Psychopharmacol. 2013;33(3):440-442.
20. van Broekhoven F, Kan CC, Zitman FG. Dependence potential of antidepressants compared to benzodiazepines. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(5):939-943.
21. Evans EA, Sullivan MA. Abuse and misuse of antidepressants. Subst Abuse Rehabil. 2014;5:107-120.
22. Warner CH, Bobo W, Warner C, et al. Antidepressant discontinuation syndrome. Am Fam Physician. 2006;74(3):449-456.
23. Lichtigfeld FJ, Gillman MA. Antidepressants are not drugs of abuse or dependence. Postgrad Med J. 1998;74(875):529-532.
24. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
25. Read J, Cartwright C, Gibson K. Adverse emotional and interpersonal effects reported by 1829 New Zealanders while taking antidepressants. Psychiatry Res. 2014;216(1):67-73.
26. Haddad P, Anderson I. Antidepressants aren’t addictive: clinicians have depended on them for years. J Psychopharmacol. 1999;13(3):291-292.
27. Carey B, Gebeloff R. Many people taking antidepressants discover they cannot quit. New York Times. https://www.nytimes.com/2018/04/07/health/antidepressants-withdrawal-prozac-cymbalta.html. Published April 7, 2018. Accessed December 11, 2018.
28. Kim D, Steinhart B. Seizures induced by recreational abuse of bupropion tablets via nasal insufflation. CJEM. 2010;12(2):158-161.
29. McCormick J. Recreational bupropion in a teenager. Br J Clin Pharmacol. 2002;53(2):214.
30. Welsh C, Doyon S. Seizure induced by insufflation of bupropion. N Engl J Med. 2002; 347(2):951.
31. Baribeau D, Araki KF. Intravenous bupropion: A previously undocumented method of abuse of a commonly prescribed antidepressant agent. J Addict Med. 2013;7(3):216-217.
32. Hill SH, Sikand H, Lee J. A case report of seizure induced by bupropion nasal insufflation. Prim Care Companion J Clin Psych. 2007;9(1):67-69.
33. Yoon G, Westermeyer J. Intranasal bupropion abuse. Am J Addict. 2013;22(2):180.
34. Reeves RR, Ladner ME. Additional evidence of the abuse potential of bupropion. J Clin Psychopharmacol. 2013;33(4):584-585.
35. Oppek K, Koller G, Zwergal A, et al. Intravenous administration and abuse of bupropion: a case report and a review of the literature. J Addict Med. 2014;8(4):290-293.
36. Strike M, Hatcher S. Bupropion injection resulting in tissue necrosis and psychosis: previously undocumented complications of intravenous bupropion use disorder. J Addict Med. 2015;9(3):246-250.
37. Schifano F, Chiappini S. Is there a potential of misuse for venlafaxine and bupropion? Front Pharmacol. 2018;9:239.
38. Tryon J, Logan N. Antidepressant Wellbutrin becomes ‘poor man’s cocaine’ on Toronto streets. Global News. https://globalnews.ca/news/846576/antidepressant-wellbutrin-becomes-poor-mans-cocaine-on-toronto-streets/. Published September 18, 2013. Accessed December 11, 2018.
39. Stassinos GL, Klein-Schwartz W. Bupropion “abuse” reported to US Poison Centers. J Addict Med. 2016;10(5):357-362.
40. Hilliard WT, Barloon L, Farley P, et al. Bupropion diversion and misuse in the correctional facility. J Correct Health Care. 2013;19(3):211-217.
41. Griffith JD, Carranza J, Griffith C, et al. Bupropion clinical assay for amphetamine-like abuse potential. J Clin Psychiatry.1983;44(5 Pt 2):206-208.
42. Miller L, Griffith J. A comparison of bupropion, dextroamphetamine, and placebo in mixed-substance abusers. Psychopharmacol (Berl). 1983;80(3):199-205.
43. Berigan TR, Russell ML. Treatment of methamphetamine cravings with bupropion: A case report. Prim Care Companion J Clin Psychiatry. 2001;3(6):267-268.
44. Tardieu T, Poirier Y, Micallef J, et al. Amphetamine-like stimulant cessation in an abusing patient treated with bupropion. Acta Psychiatr Scand. 2004;109(1):75-78.
45. Newton TF, Roache JD, De La Garza R, et al. Bupropion reduces methamphetamine-induced subjective effects and cue-induced cravings. Neuropsychopharmacology. 2006;31(7):1537-1544.
46. Margolin A, Kosten TR, Avants SK, et al. A multicenter trial for cocaine dependence in methadone-maintained patients. Drug Alcohol Depend. 1995;40(2):125-131.
47. Shoptaw S, Heinzerling KG, Rotheram-Fuller E, et al. Bupropion hydrochloride versus placebo, in combination with cognitive behavioral therapy, for the treatment of cocaine abuse/dependence. J Addict Dis. 2008;27(1):13-23.
48. Anderson AL, Li S, Markova D, et al. Bupropion for the treatment of methamphetamine dependence in non-daily users: a randomized, double-blind placebo-controlled trial. Drug Alcohol Depend. 2015;150:170-174.
49. Shoptaw S, Heinzerling KG, Rotheram-Fuller E, et al. Randomized, placebo-controlled trial of bupropion for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2008;96(3):222-232.
50. Elkashef AM, Rawson RA, Anderson AL, et al. Bupropion for the treatment of methamphetamine dependence. Neuropsychopharmacology. 2008;33(5):1162-1170.
51. Heinzerling KG, Swanson A, Hall TM, et al. Randomized, placebo-controlled trial of bupropion in methamphetamine-dependent participants with less than daily methamphetamine use. Addiction. 2014;109(11):1878-1886.
52. Doenecke AL, Heuerman RC. Treatment of haloperidol abuse with diphenhydramine. Am J Psychiatry. 1980;137(4):487-488.
53. Weddington WW, Leventhal BL. Sadistic abuse of haloperidol. Am J Psychiatry. 1982;139:132-133.
54. Basu D, Marudkar M, Khurana H. Abuse of neuroleptic drugs by psychiatric patients. Indian J Med Sci. 2000;54(2):59-62.
55. Pierre JM, Shnayder I, Wirshing DA, et al. Intranasal quetiapine abuse. Am J Psychiatry 2004;161(9):1718.
56. Reeves RR. Abuse of olanzapine by substance abusers. J Psychoactive Drugs. 2007;39(3):297-299.
57. Kumsar NA, Erol A. Olanzapine abuse. Subst Abus. 2013;34(1):73-74.
58. Lai C. Olanzapine abuse was relieved after switching to aripiprazole in a patient with psychotic depression. Prog Neuropsychpharmacol Biol Psychiatry. 2010;34(7):1363-1364.
59. James PD, Fida AS, Konovalov P, et al. Non-medical use of olanzapine by people on methadone treatment. BJPsych Bull. 2016;40(6):314-317.
60. Reeves RR, Brister JC. Additional evidence of the abuse potential of quetiapine. South Med J. 2007;100(8):834-836.
61. Murphy D, Bailey K, Stone M, et al. Addictive potential of quetiapine. Am J Psychiatry. 2008;165(7):918.
62. Paparrigopoulos T, Karaiskos D, Liappas J. Quetiapine: another drug with potential for misuse? J Clin Psychiatry. 2008;69(1):162-163.
63. Reeves RR, Burke RS. Abuse of the combination of gabapentin and quetiapine. Prim Care Companion CNS Disord. 2014;16(5): doi: 10.4088/PCC.14l01660.
64. Morin AK. Possible intranasal quetiapine misuse. Am J Health Syst Pharm. 2007;64(7):723-725.
65. Caniato RN, Gundabawady A, Baune BT, et al. Malingered psychotic symptoms and quetiapine abuse in a forensic setting. J Forens Psychiatr Psychol. 2009;20(6):928-935.
66. Hussain MZ, Waheed W, Hussain S. Intravenous quetiapine abuse. Am J Psychiatry. 2005; 162(9):1755-1756.
67. Waters BM, Joshi KG. Intravenous quetiapine-cocaine use (“Q-ball”). Am J Psychiatry. 2007;164(1):173-174.
68. Haridas A, Kushon D, Gurmu S, et al. Smoking quetiapine: a “Maq ball?” Prim Psychiatry. 2010;17:38-39.
69. Cubala WJ, Springer J. Quetiapine abuse and dependence in psychiatric patients: a systematic review of 25 case reports in the literature. J Subs Use. 2014;19(5):388-393.
70. Piróg-Balcerzak A, Habrat B, Mierzejewski P. Misuse and abuse of quetiapine [in Polish]. Psychiatr Pol. 2015;49(1):81-93.
71. Pinta ER, Taylor RE. Quetiapine addiction? Am J Psychiatry. 2007;164(1):174.
72. Tamburello AC, Lieberman JA, Baum RM, et al. Successful removal of quetiapine from a correctional formulary. J Amer Acad Psychiatr Law. 2012;40(4):502-508.
73. Tarasoff G, Osti K. Black-market value of antipsychotics, antidepressants, and hypnotics in Las Vegas, Nevada. Am J Psychiatry. 2007;164(2):350.
74. Reccoppa L. Less abuse potential with XR formulation of quetiapine. Am J Addiction. 2010;20(2):178.
75. McLarnon ME, Fulton HG, MacIsaac C, et al. Characteristics of quetiapine misuse among clients of a community-based methadone maintenance program. J Clin Psychopharmacol. 2012;32(5):721-723.
76. Reddel SE, Bruno R, Burns L, et al. Prevalence and associations of quetiapine fumarate misuse among an Australian national city sample of people who regularly inject drugs. Addiction. 2013;109(2):295-302.
77. Malekshahi T, Tioleco N, Ahmed N, et al. Misuse of atypical antipsychotics in conjunction with alcohol and other drugs of abuse. J Subs Abuse Treat. 2015;48(1):8-12.
78. Klein-Schwartz W, Schwartz EK, Anderson BD. Evaluation of quetiapine abuse and misuse reported to poison centers. J Addict Med. 2014;8(3):195-198.
79. Klein L, Bangh S, Cole JB. Intentional recreational abuse of quetiapine compared to other second-generation antipsychotics. West J Emerg Med. 2017;18(2):243-250.
80. Chiappini S, Schifano F. Is there a potential of misuse for quetiapine?: Literature review and analysis of the European Medicines Agency/European Medicines Agency Adverse Drug Reactions’ Database. J Clin Psychopharmacol. 2018;38(1):72-79.
81. Lee J, Pilgrim J, Gerostamoulos D, et al. Increasing rates of quetiapine overdose, misuse, and mortality in Victoria, Australia. Drug Alcohol Depend. 2018;187:95-99.
82. Mattson ME, Albright VA, Yoon J, et al. Emergency department visits involving misuse and abuse of the antipsychotic quetiapine: Results from the
83. Brutcher RE, Nader SH, Nader MA. Evaluation of the reinforcing effect of quetiapine, alone and in combination with cocaine, in rhesus monkeys. J Pharmacol Exp Ther. 2016;356(2):244-250.
84. Kim DR, Staab JP. Quetiapine discontinuation syndrome. Am J Psychiatry. 2005;162(5):1020.
85. Thurstone CC, Alahi P. A possible case of quetiapine withdrawal syndrome. J Clin Psychiatry. 2000;61(8):602-603.
86. Kohen I, Kremen N. A case report of quetiapine withdrawal syndrome in a geriatric patient. World J Biol Psychiatry. 2009;10(4 pt 3):985-986.
87. Yargic I, Caferov C. Quetiapine dependence and withdrawal: a case report. Subst Abus. 2011;32(3):168-169.
88. Koch HJ. Severe quetiapine withdrawal syndrome with nausea and vomiting in a 65-year-old patient with psychotic depression. Therapie. 2015;70(6):537-538.
89. Fischer BA, Boggs DL. The role of antihistaminic effects in the misuse of quetiapine: a case report and review of the literature. Neurosci Biobehav Rev. 2010;34(4):555-558.
90. Longoria J, Brown ES, Perantie DC, et al. Quetiapine for alcohol use and craving in bipolar disorder. J Clin Psychopharmacol. 2004;24(1):101-102.
91. Monnelly EP, Ciraulo DA, Knapp C, et al. Quetiapine for treatment of alcohol dependence. J Clin Psychopharmacol. 2004;24(5):532-535.
92. Kennedy A, Wood AE, Saxon AJ, et al. Quetiapine for the treatment of cocaine dependence: an open-label trial. J Clin Psychopharmacol. 2008;28(2):221-224.
93. Mariani JJ, Pavlicova M, Mamczur A, et al. Open-label pilot study of quetiapine treatment for cannabis dependence. Am J Drug Alcohol Abuse. 2014;40(4):280-284.
94. Guardia J, Roncero C, Galan J, et al. A double-blind, placebo-controlled, randomized pilot study comparing quetiapine with placebo, associated to naltrexone, in the treatment of alcohol-dependent patients. Addict Behav. 2011;36(3):265-269.
95. Litten RZ, Fertig JB, Falk DE, et al; NCIG 001 Study Group. A double-blind, placebo-controlled trial to assess the efficacy of quetiapine fumarate XR in very heavy-drinking alcohol-dependent patients. Alcohol Clin Exp Res. 2012;36(3):406-416.
96. Tapp A, Wood AE, Kennedy A, et al. Quetiapine for the treatment of cocaine use disorder. Drug Alcohol Depend. 2015;149:18-24.
97. Markowitz JS, Finkenbine R, Myrick H, et al. Gabapentin abuse in a cocaine user: Implications for treatment. J Clin Psychopharmacol. 1997;17(5):423-424.
98. Reccoppa L, Malcolm R, Ware M. Gabapentin abuse in inmates with prior history of cocaine dependence. Am J Addict. 2004;13(3):321-323.
99. Victorri-Vigneau C, Guelais M, Jolliet P. Abuse, dependency and withdrawal with gabapentin: a first case report. Pharmacopsychiatry. 2007;40(1):43-44.
100. Bonnet U, Sherbaum N. How addictive are gabapentin and pregabalin? A systematic review. Eur Neuropsychopharmacol. 2017;27(12):1185-1215.
101. Schifano F, D’Offizi S, Piccione M, et al. Is there a recreational misuse potential for pregabalin? Analysis of anecdotal online reports in comparison with related gabapentin and clonazepam data. Psychother Psychosom. 2011;80(2):118-122.
102. Evoy KE, Morrison MD, Saklad SR. Abuse and misuse of pregabalin and gabapentin. Drugs. 2017;77(4):403-426.
103. Smith RV, Havens JR, Walsh SL. Gabapentin misuse, abuse and diversion: a systematic review. Addiction. 2016;111(7):1160-1174.
104. Chiappini S, Shifano F. A decade of gabapentinoid misuse: an analysis of the European Medicines Agency’s ‘suspected adverse drug reactions’ database. CNS Drugs. 2016;30(7):647-654.
105. Buttram ME, Kurtz SP, Dart R, et al. Law enforcement-derived data on gabapentin diversion and misuse, 2002-2015: diversion rates and qualitative research findings. Pharmacoepidemiol Drug Saf. 2017;26(9):1083-1086.
106. Kapil V, Green JL, Le Lait M, et al. Misuse of the y-aminobutyric acid analogues baclofen, gabapentin and pregabalin in the UK. Br J Clin Pharmacol. 2013;78(1):190-191.
107. Peckham AM, Fairman KA, Sclar DA. Prevalence of gabapentin abuse: comparison with agents with known abuse potential in a commercially insured US population. Clin Drug Invest. 2017;37(8):763-773.
108. Smith RV, Lofwall MR, Havens JR. Abuse and diversion of gabapentin among nonmedical prescription opioid users in Appalachian Kentucky. Am J Psychiatry. 2015;172(5):487-488.
109. Peckham AM, Evoy KE, Covvey JR, et al. Predictors of gabapentin overuse with or without concomitant opioids in a commercially insured U.S. population. Pharmacotherapy. 2018;38(4):436-443.
110. Smith BH, Higgins C, Baldacchino A, et al. Substance misuse of gabapentin. Br J Gen Pract. 2012;62(601):401-407.
111. Baird CRW, Fox P, Colvin LA. Gabapentinoid abuse in order to potentiate the effect of methadone: a survey among substance misusers. Eur Addict Res. 2014;20(3):115-118.
112. Lyndon A, Audrey S, Wells C, et al. Risk to heroin users of polydrug use of pregabalin or gabapentin. Addiction. 2017;112(9):1580-1589.
113. Peckham AM, Fairman KA, Sclar DA. All-cause and drug-related medical events associated with overuse of gabapentin and/or opioid medications: a retrospective cohort analysis of a commercially insured US population. Drug Saf. 2018;41(2):213-228.
114. Gomes T, Juurlink DN, Antoniou T, et al. Gabapentin, opioids, and the risk of opioid-related death: a population-based nested case-control study. PLoS Med. 2017;14(10):e10022396. doi: 10.1371/journal.pmed.1002396.
115. Peckham AM, Fairman K, Sclar DA. Policies to mitigate nonmedical use of prescription medications: how should emerging evidence of gabapentin misuse be addressed? Exp Opin Drug Saf. 2018;17(5):519-523.
116. Raby WN. Gabapentin for cocaine cravings. Am J Psychiatry. 2000;157(12):2058-2059.
117. Myrick H, Henderson S, Brady KT, et al. Gabapentin in the treatment of cocaine dependence: a case series. J CLin Psychiatry. 2001;62(1):19-23.
118. Raby WN, Coomaraswamy S. Gabapentin reduces cocaine use among addicts from a community clinic sample. J Clin Psychiatry. 2004;65(1):84-86.
119. Hart CL, Ward AS, Collins ED, et al. Gabapentin maintenance decreases smoked cocaine-related subjective effects, but not self-administration by humans. Drug Alcohol Depend. 2004;73(3):279-287.
120. Bisaga A, Aharonovich E, Garawi F, et al. A randomized placebo-controlled trial of gabapentin for cocaine dependence. Drug Alc Depend. 2006;81(3):267-274.
121. Hart CL, Haney M, Collins ED, et al. Smoked cocaine self-administration by humans is not reduced by large gabapentin maintenance doses. Drug Alcohol Depend. 2007;86(2-3):274-277.
122. Furieri FA, Nakamura-Palacios EM. Gabapentin reduces alcohol consumption and craving: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2007;68(11):1691-1700.
123. Mason BJ, Quello S, Goodell V, et al. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77.
124. Martinotti G, Di Nicola M, Tedeschi D, et al. Pregabalin versus naltrexone in alcohol dependence: a randomised, double-blind, comparison trial. J Psychopharmacol. 2010;24(9):1367-1374.
125. Mason BJ, Crean R, Goodell V, et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychpharmacology. 2012;27(7):1689-1698.
126. Enke O, New HA, New CH, et al. Anticonvulsants in the treatment of low back pain and lumbar radicular pain: a systematic review and meta-analysis. CMAJ. 2018;190(26):E786-E793.
127. Cartwright C, Gibson K, Read J, et al. Long-term antidepressant use: patient perspectives of benefits and adverse effects. Patient Prefer Adherence. 2016;10:1401-1407.
128. American Society of Addiction Medicine. Public policy statement: definition of addiction. https://www.asam.org/docs/default-source/public-policy-statements/1definition_of_addiction_long_4-11.pdf?sfvrsn=a8f64512_4. Published August 15, 2011. Accessed July 23, 2018.
While some classes of medications used to treat psychiatric disorders, such as stimulants and benzodiazepines, are well-recognized as controlled substances and drugs of abuse, clinicians may be less familiar with the potential misuse/abuse of other psychiatric medications. This article reviews the evidence related to the misuse/abuse of anticholinergics, antidepressants, antipsychotics, and gabapentinoids.
The terms “misuse,” “abuse,” and “addiction” are used variably in the literature without standardized definitions. For this review, “misuse/abuse (M/A)” will be used to collectively describe self-administration that is recreational or otherwise inconsistent with legal or medical guidelines, unless a specific distinction is made. Whether or not the medications reviewed are truly “addictive” will be briefly discussed for each drug class, but the focus will be on clinically relevant aspects of M/A, including:
- excessive self-administration
- self-administration by non-oral routes
- co-administration with other drugs of abuse
- malingering of psychiatric symptoms to obtain prescriptions
- diversion for sale to third parties
- toxicity from overdose.
Anticholinergic medications
The first case describing the deliberate M/A of an anticholinergic medication for its euphoric effects was published in 1960.Further reportsfollowed in Europe before the M/A potential of prescription anticholinergic medications among psychiatric patients with an overdose syndrome characterized by atropinism and toxic psychosis was more widely recognized in the United States in the 1970s. Most reported cases of M/A to date have occurred among patients with psychiatric illness because anticholinergic medications, including trihexyphenidyl, benztropine, biperiden, procyclidine, and orphenadrine, were commonly prescribed for the management of first-generation and high dopamine D2-affinity antipsychotic-induced extrapyramidal symptoms (EPS). For example, one study of 234 consecutively hospitalized patients with schizophrenia noted an anticholinergic M/A incidence of 6.5%.1
However, anticholinergic M/A is not limited to individuals with psychotic disorders. A UK study of 154 admissions to an inpatient unit specializing in behavioral disturbances found a 12-month trihexyphenidyl M/A incidence of 17%; the most common diagnosis among abusers was antisocial personality disorder.2 Anticholinergic M/A has also been reported among patients with a primary diagnosis of substance use disorders (SUDs)3 as well as more indiscriminately in prison settings,4 with some inmates exchanging trihexyphenidyl as currency and using it recreationally by crushing it into powder and smoking it with tobacco.5 Others have noted that abusers sometimes take anticholinergics with alcohol in order to “potentiate” the effects of each substance.6,7 Pullen et al8 described individuals with and without psychiatric illness who stole anticholinergic medications, purchased them from other patients, or bought them “on the street.” Malingering EPS in order to obtain anticholinergic medications has also been well documented.9 Clearly, anticholinergic M/A can occur in psychiatric and non-psychiatric populations, both within and outside of clinical settings. Although anticholinergic M/A appears to be less frequent in the United States now that second-generation antipsychotics (SGAs) are more frequently prescribed, M/A remains common in some settings outside of the United States.7
Among the various anticholinergic medications prescribed for EPS, trihexyphenidyl has been reported to have the greatest M/A potential, which has been attributed to its potency,10 its stimulating effects (whereas benztropine is more sedating),11 and its former popularity among prescribers.8 Marken et al11 published a review of 110 reports of M/A occurring in patients receiving anticholinergic medications as part of psychiatric treatment in which 69% of cases involved taking trihexyphenidyl 15 to 60 mg at a time (recommended dosing is 6 to 10 mg/d in divided doses).Most of these patients were prescribed anticholinergic medications for diagnostically appropriate reasons—only 7% were described as “true abusers” with no medical indication. Anticholinergic M/A was typically driven by a desire for euphoric and psychedelic/hallucinogenic effects, although in some cases, anticholinergic M/A was attributed to self-medication of EPS and depressive symptoms. These findings illustrate the blurred distinction between recreational use and perceived subjective benefit, and match those of a subsequent study of 50 psychiatric patients who reported anticholinergic M/A not only to “get high,” but to “decrease depression,” “increase energy,” and decrease antipsychotic adverse effects.12 Once again, trihexyphenidyl was the most frequently misused anticholinergic in this sample.
Table 12,3,7,8,10-15 outlines the subjective effects sought and experienced by anticholinergic abusers as well as potential toxic effects; there is the potential for overlap. Several authors have also described physiologic dependence with long-term trihexyphenidyl use, including tolerance and a withdrawal/abstinence syndrome.7,16 In addition, there have been several reports of coma13 and death in the setting of intended suicide by overdose of anticholinergic medications.14,15
Although anticholinergic M/A in the United States now appears to be less common, clinicians should remain aware of the M/A potential of anticholinergic medications prescribed for EPS. Management of M/A involves:
- detection
- reducing anticholinergic exposure by managing EPS with alternative strategies, such as switching or reducing the dose of the antipsychotic medication
- gradual tapering of anticholinergic medications to minimize withdrawal.11
Continue to: Antidepressants
Antidepressants
Haddad17 published a review of 21 English-language case reports from 1966 to 1998 describing antidepressant use in which individuals met DSM-IV criteria for substance dependence to the medication. An additional 14 cases of antidepressant M/A were excluded based on insufficient details to support a diagnosis of dependence. The 21 reported cases involved:
- tranylcypromine (a monoamine oxidase inhibitor [MAOI])
- amitriptyline (a tricyclic antidepressant [TCA])
- fluoxetine (a selective serotonin reuptake inhibitor [SSRI])
- amineptine (a TCA previously available in France but removed from the market in 1999 in part due to its abuse potential)
- nomifensine (a norepinephrine/dopamine reuptake inhibitor previously available in the United Kingdom but removed in 1986 due to hemolytic anemia).
In 95% of cases, the antidepressants were prescribed for treatment of an affective disorder but were abused for stimulant effects or the perceived ability to lift mood, cause euphoria or a “high,” or to improve functioning. Two-thirds of cases involved patients with preexisting substance misuse. Placing the case reports in the context of the millions of patients prescribed antidepressants during this period, Haddad concluded the “incidence of [antidepressant] addiction [is] so low as to be clinically irrelevant.”17
Despite this conclusion, Haddad singled out amineptine and tranylcypromine as antidepressants with some evidence of true addictive potential.17,18 A more recent case series described 14 patients who met DSM-IV criteria for substance abuse of tertiary amine TCAs (which have strong anticholinergic activity) and concluded that “misuse of [TCAs] is more common than generally appreciated.”19 In keeping with that claim, a study of 54 outpatients taking unspecified antidepressants found that up to 15% met DSM-III-R criteria for substance dependence (for the antidepressant) in the past year, although that rate was much lower than the rate of benzodiazepine dependence (47%) in a comparative sample.20 Finally, a comprehensive review by Evans and Sullivan21 found anecdotal reports published before 2014 that detailed misuse, abuse, and dependence with MAOIs, TCAs, fluoxetine, venlafaxine, bupropion, tianeptine, and amineptine. Taken together, existing evidence indicates that select individuals—typically those with other SUD comorbidity—sometimes misuse antidepressants in a way that suggests addiction.
Still, while it is well known that abrupt cessation of antidepressants can result in a discontinuation syndrome characterized by flu-like symptoms, nausea, and dizziness,22 physiologic withdrawal effects must be distinguished from historical definitions of substance “abuse” and the broader concept of psychological “addiction” or drug dependence18,23 now incorporated into the DSM-5 definition of SUDs.24 Indeed, although withdrawal symptoms were reported by more than half of those who took antidepressants and responded to a recent online survey,25 evidence to support the existence of significant antidepressant tolerance, craving, or compulsive use is lacking.17,18 Antidepressants as a class do not appear to be significantly rewarding or reinforcing and, on the contrary, discontinuation by patients is common in clinical practice.26 The popular claim that some individuals taking antidepressants “can’t quit”27 must also be disentangled from loss of therapeutic effects upon cessation.
Bupropion. A more convincing argument for antidepressant addiction can be made for bupropion, a weak norepinephrine and dopamine reuptake inhibitor with an otherwise unclear mechanism of action.28 In 2002, the first report of recreational bupropion M/A described a 13-year-old girl who took 2,400 mg orally (recommended maximum dose is 450 mg/d in divided doses) after being told it would give her “a better high than amphetamine.”29 This was followed in the same year by the first report of recreational M/A of bupropion via nasal insufflation (snorting), resulting in a seizure,30 and in 2013 by the first published case of M/A by IV self-administration.31
Continue to: The M/A potential of bupropion...
The M/A potential of bupropion, most commonly via intranasal administration, is now broadly recognized based on several case reports describing desired effects that include a euphoric high and a stimulating “buzz” similar to that of cocaine or methamphetamine but less intense.29-36 Among recreational users, bupropion tablets are referred to as “welbys,” “wellies,” “dubs,” or “barnies.”37 Media coverage of a 2013 outbreak of bupropion M/A in Toronto detailed administration by snorting, smoking, and injection, and described bupropion as “poor man’s cocaine.”38 Between 2003 and 2016, 2,232 cases of bupropion misuse/abuse/dependence adverse drug reactions were reported to the European Monitoring Agency.37 A review of intentional bupropion M/A reported to US Poison Control Centers between 2000 to 2013 found 975 such cases, with the yearly number tripling between 2000 and 2012.39 In this sample, nearly half (45%) of the users were age 13 to 19, and 76% of cases involved oral ingestion. In addition to bupropion M/A among younger people, individuals who misuse bupropion often include those with existing SUDs but limited access to illicit stimulants and those trying to evade detection by urine toxicology screening.33 For example, widespread use and diversion has been well documented within correctional settings, and as a result, many facilities have removed bupropion from their formularies.21,28,33,34,40
Beyond desired effects, the most common adverse events associated with bupropion M/A are listed in Table 2,28,30,32-34,36,39 along with their incidence based on cases brought to the attention of US Poison Control Centers.39 With relatively little evidence of a significant bupropion withdrawal syndrome,37 the argument in favor of modeling bupropion as a truly addictive drug is limited to anecdotal reports of cravings and compulsive self-administration35 and pro-dopaminergic activity (reuptake inhibition) that might provide a mechanism for potential rewarding and reinforcing effects.40 While early preclinical studies of bupropion failed to provide evidence of amphetamine-like abuse potential,41,42 non-oral administration in amounts well beyond therapeutic dosing could account for euphoric effects and a greater risk of psychological dependence and addiction.21,28,40
Bupropion also has an FDA indication as an aid to smoking cessation treatment, and the medication demonstrated early promise in the pharmacologic treatment of psychostimulant use disorders, with reported improvements in cravings and other SUD outcomes.43-45 However, subsequent randomized controlled trials (RCTs) failed to demonstrate a clear therapeutic role for bupropion in the treatment of cocaine46,47 and methamphetamine use disorders (although some secondary analyses suggest possible therapeutic effects among non-daily stimulant users who are able to maintain good adherence with bupropion).48-51 Given these overall discouraging results, the additive seizure risk of bupropion use with concomitant psychostimulant use, and the potential for M/A and diversion of bupropion (particularly among those with existing SUDs), the use of bupropion for the off-label treatment of stimulant use disorders is not advised.
Antipsychotics
As dopamine antagonists, antipsychotics are typically considered to have low potential for rewarding or reinforcing effects. Indeed, misuse of antipsychotics was a rarity in the first-generation era, with only a few published reports of haloperidol M/A within a small cluster of naïve young people who developed acute EPS,52 and a report of diversion in a prison with the “sadistic” intent of inflicting dystonic reactions on others.53 A more recent report described 2additional cases of M/A involving haloperidol and trifluoperazine.54 Some authors have described occasional drug-seeking behavior for low-potency D2 blockers such as chlorpromazine, presumably based on their M/A as anticholinergic medications.55
The potential for antipsychotic M/A has gained wider recognition since the advent of the SGAs. Three cases of prescription olanzapine M/A have been published to date. One involved a man who malingered manic symptoms to obtain olanzapine, taking ≥40 mg at a time (beyond his prescribed dose of 20 mg twice daily) to get a “buzz,” and combining it with alcohol and benzodiazepines for additive effects or to “come down” from cocaine.56 This patient noted that olanzapine was “a popular drug at parties” and was bought, sold, or traded among users, and occasionally administered intravenously. Two other cases described women who self-administered olanzapine, 40 to 50 mg/d, for euphoric and anxiolytic effects.57,58 James et al59 detailed a sample of 28 adults who reported “non-medical use” of olanzapine for anxiolytic effects, as a sleep aid, or to “escape from worries.”
Continue to: Quetiapine
Quetiapine. In contrast to some reports of olanzapine M/A in which the line between M/A and “self-medication” was blurred, quetiapine has become a more convincing example of clear recreational antipsychotic M/A. Since the first report of oral and intranasal quetiapine M/A in the Los Angeles County Jail published in 2004,55 subsequent cases have detailed other novel methods of recreational self-administration60-68 (Table 355,60-68), and additional reports have been published in non-English language journals.69,70 Collectively, these case reports have detailed that quetiapine is:
- misused for primary subjective effects as well as to mitigate the unpleasant effects of other drugs60,67
- referred to as “quell,”“Q,” “Susie-Q,” “squirrel,” and “baby heroin”55,71,72
- often obtained by malingering psychiatric symptoms55,61,63,65
- diverted/sold with “street value” both within and outside of psychiatric facilities and correctional settings.55,60-62,67,68,73
These anecdotal accounts of quetiapine M/A have since been corroborated on a larger scale based on several retrospective studies. Although early reports of quetiapine M/A occurring in correctional settings have resulted in formulary removal,71,74 quetiapine M/A is by no means limited to forensic populations and is especially common among those with comorbid SUDs. A survey of 74 patients enrolled in a Canadian methadone program reported that nearly 60% had misused quetiapine at some point.75 Among an Australian sample of 868 individuals with active IV drug abuse, 31% reported having misused quetiapine.76 Finally, within a small sample of patients with SUDs admitted to a detoxification unit in New York City, 17% reported M/A of SGAs.77 In this study, SGAs were often taken in conjunction with other drugs of abuse in order to “recover” from or “enhance” the effects of other drugs or to “experiment.” Quetiapine was by far the most frequently abused SGA, reported in 96% of the sample; the most frequently reported SGA/drug combinations were quetiapine/alcohol/opioids, quetiapine/cocaine, and quetiapine/opioids.
Looking more broadly at poison center data, reports to the US National Poison Data System (NPDS) from 2005 to 2011 included 3,116 cases of quetiapine abuse (37.5%, defined as intentional recreational use in order to obtain a “high”) or misuse (62.5%, defined as improper use or dosing for non-recreational purposes).78 A more recent analysis of NPDS reports from 2003 to 2013 found 2,118 cases of quetiapine abuse, representing 61% of all cases of reported SGA abuse.79 An analysis of the European Medicines Agency Adverse Drug Database yielded 18,112 reports of quetiapine misuse, abuse, dependence, and withdrawal for quetiapine (from 2005 to 2016) compared with 4,178 for olanzapine (from 2004 to 2016).80 These reports identified 368 fatalities associated with quetiapine.
The rate of quetiapine M/A appears to be increasing sharply. Reports of quetiapine M/A to poison centers in Australia increased nearly 7-fold from 2006 to 2016.81 Based on reports to the Drug Abuse Warning System, US emergency department visits for M/A of quetiapine increased from 19,195 in 2005 to 32,024 in 2011 (an average of 27,114 visits/year), with 75% of cases involving quetiapine taken in combination with other prescription drugs, alcohol, or illicit drugs.82 Consistent with poison center data, M/A was reported for other antipsychotics, but none nearly as frequently as for quetiapine.
With increasingly frequent quetiapine M/A, clinicians should be vigilant in monitoring for medical morbidity related to quetiapine and cumulative toxicity with other drugs. The most frequent adverse events associated with quetiapine M/A reported to US Poison Control Centers are presented in Table 4.78,79
Continue to: Unlike bupropion...
Unlike bupropion, quetiapine’s dopamine antagonism makes it unlikely to be a truly addictive drug, although this mechanism of action could mediate an increase in concurrent psychostimulant use.83 A few case reports have described a quetiapine discontinuation syndrome similar to that of antidepressants,60,65,84-88 but withdrawal symptoms suggestive of physiologic dependence may be mediated by non-dopaminergic effects through histamine and serotonin receptors.84,89 Evidence for quetiapine misuse being associated with craving and compulsive use is lacking, and true quetiapine addiction is probably rare.
Similar to bupropion, preliminary findings have suggested promise for quetiapine as a putative therapy for other SUDs.90-93 However, subsequent RCTs have failed to demonstrate a therapeutic effect for alcohol and cocaine use disorders.94-96 Given these negative results and the clear M/A potential of quetiapine, off-label use of quetiapine for the treatment of SUDs and psychiatric symptoms among those with SUDs must be considered judiciously, with an eye towards possible diversion and avoiding the substitution of one drug of abuse for another.
Gabapentinoids
In 1997, the first published case report of gabapentin M/A described a woman who self-administered her husband’s gabapentin to reduce cravings for and withdrawal from cocaine.97 The authors highlighted the possible therapeutic benefit of gabapentin in this regard rather than raising concerns about diversion and M/A. By 2004, however, reports of recreational gabapentin M/A emerged among inmates incarcerated within Florida correctional facilities who self-administered intranasal gabapentin to achieve a “high” that was “reminiscent of prior effects from intranasal ingestion of cocaine powder.”98 In 2007, a single case of gabapentin misuse up to 7,200 mg/d (recommended dosing is ≤3,600 mg/d) was reported, with documentation of both tolerance and withdrawal symptoms.99 As of 2017, a total of 36 cases of gabapentin M/A and 19 cases of pregabalin M/A have been published.100
In the past decade, anecdotal reports have given way to larger-scale epidemiologic data painting a clear picture of the now-widespread M/A of gabapentin and other gabapentinoids. For example, a study of online descriptions of gabapentin and pregabalin M/A from 2008 to 2010 documented:
- oral and IM use (gabapentin)
- IV and rectal (“plugging”) use (pregabalin)
- “parachuting” (emptying the contents of capsules for a larger dose) (pregabalin)
- euphoric, entactogenic, stimulant, calming/anxiolytic, and dissociative subjective effects (gabapentin/pregabalin)
- rapid development of tolerance to euphoric effects leading to self-administration of increasing doses (gabapentin/pregabalin)
- frequent co-administration with other drugs of abuse, including alcohol, benzodiazepines, cannabis, stimulants, opiates, hallucinogens, gamma-hydroxybutyrate, mephedrone, and Salvia divinorum (gabapentin/pregabalin)101
Several systematic reviews of both anecdotal reports and epidemiologic studies published in the past few years provide additional evidence of the above, such as:
- excessive dosing with self-administration
- intranasal and inhaled routes of administration
- diversion and “street value”
- greater M/A potential of pregabalin than gabapentin
- the presence of gabapentinoids in postmortem toxicology analyses, suggesting a role in overdose fatalities when combined with other drugs.100,102,103
Continue to: The European Medicine Agency's EudraVigilance database...
The European Medicine Agency’s EudraVigilance database included 4,301 reports of gabapentin misuse, abuse, or dependence, and 7,639 such reports for pregabalin, from 2006 to 2015 (rising sharply after 2012), with 86 gabapentin-related and 27 pregabalin-related fatalities.104 Data from the Drug Diversion Program of the Researched Abuse, Diversion, and Addiction-Related Surveillance System from 2002 to 2015 have likewise revealed that gabapentin diversion increased significantly in 2013.105
While the prevalence of gabapentinoid M/A is not known, rates appear to be significantly lower than for traditional drugs of abuse such as cannabis, cocaine, 3,4-methylenedioxymethamphetamine (MDMA), and opioids.106,107 However, gabapentin and pregabalin M/A appears to be increasingly common among individuals with SUDs and in particular among those with opioid use disorders (OUDs). For example, a 2015 report indicated that 15% of an adult cohort in Appalachian Kentucky with nonmedical use of diverted prescription opioids reported gabapentin M/A, an increase of nearly 3,000% since 2008.108 Based on data from a US insurance enrollment and claims database, researchers found that the rate of gabapentin overuse among those also overusing opioids was 12% compared with only 2% for those using gabapentin alone.109 It has also been reported that gabapentin is sometimes used as a “cutting agent” for heroin.110
Those who use gabapentinoids together with opioids report that gabapentin and pregabalin potentiate the euphoric effects of methadone111 and endorse specific beliefs that pregabalin increases both the desired effects of heroin as well as negative effects such as “blackouts,” loss of control, and risk of overdose.112 Indeed, sustained M/A of gabapentin and opioids together has been found to increase emergency department utilization, drug-related hospitalization, and respiratory depression.113 Based on a case-control study of opioid users in Canada, co-prescription of gabapentin and opioids was associated with a 50% increase in death from opioid-related causes compared with prescription of opioids alone.114
Case reports documenting tolerance, withdrawal, craving, and loss of control suggest a true addictive potential for gabapentinoids, but Bonnet and Sherbaum100 concluded that while there is robust evidence of abusers “liking” gabapentin and pregabalin (eg, reward), evidence of “wanting” them (eg, psychological dependence) in the absence of other SUDs has been limited to only a few anecdotal reports with pregabalin. Accordingly, the risk of true addiction to gabapentinoids by those without preexisting SUDs appears to be low. Nonetheless, the M/A potential of both gabapentin and pregabalin is clear and in the context of a nationwide opioid epidemic, the increased morbidity/mortality risk related to combined use of gabapentinoids and opioids is both striking and concerning. Consequently, the state of Kentucky recently recognized the M/A potential of gabapentin by designating it a Schedule V controlled substance (pregabalin is already a Schedule V drug according to the US Drug Enforcement Agency),103,113 and several other states now mandate the reporting of gabapentin prescriptions to prescription drug monitoring programs.115
Following a similar pattern to antidepressants and antipsychotics, a potential role for gabapentin in the treatment of cocaine use disorders was supported in preliminary studies,116-118 but not in subsequent RCTs.119-121 However, there is evidence from RCTs to support the use of gabapentin and pregabalin in the treatment of alcohol use disorders.122-124 Gabapentin was also found to significantly reduce cannabis use and withdrawal symptoms in patients compared with placebo in an RCT of individuals with cannabis use disorders.125 The perceived safety of gabapentinoids by clinicians, their subjective desirability by patients with SUDs, and efficacy data supporting a therapeutic role in SUDs must be balanced with recognition that approximately 80% of gabapentin prescriptions are written for off-label indications for which there is little supporting evidence,109 such as low back pain.126 Clinicians considering prescribing gabapentinoids to manage psychiatric symptoms, such as anxiety and insomnia, should carefully consider the risk of M/A and other potential morbidities, especially in the setting of SUDs and OUD in particular.
Continue to: Problematic, even if not addictive
Problematic, even if not addictive
It is sometimes claimed that “addiction” to psychiatric medications is not limited to stimulants and benzodiazepines.27,127 Although anticholinergics, antidepressants, antipsychotics, and gabapentinoids can be drugs of abuse, with some users reporting physiologic withdrawal upon discontinuation, there is only limited evidence that the M/A of these psychiatric medications is associated with the characteristic features of a more complete definition of “addiction,” which may include:
- inability to consistently abstain
- impairment in behavioral control
- diminished recognition of significant problems associated with use
- a dysfunctional emotional response to chronic use.128
Nonetheless, the literature documenting anticholinergic, antidepressant, antipsychotic, and gabapentinoid M/A includes several common features, including:
- initial reports among those with limited access to illicit drugs (eg, young people and incarcerated individuals) and subsequent spread to a wider population with more unconventional routes of administration
- use for recreational purposes and other subjective pseudo-therapeutic effects, often in combination with alcohol and illicit drugs
- greater M/A potential of certain medications within each of these drug classes (eg, trihexyphenidyl, bupropion, quetiapine)
- malingering psychiatric symptoms in order to obtain medications from prescribers and diversion for black market sale
- observations that medications might constitute therapy for SUDs that were not supported in subsequent RCTs (with the exception of gabapentin for alcohol and cannabis use disorders)
- increasing evidence of toxicity related to M/A, which suggests that prescription by clinicians has limited benefit and high risk for patients with SUDs.
Bottom Line
Some psychiatric medications are taken as drugs of abuse. Clinicians should be particularly aware of the misuse/abuse potential of anticholinergics, antidepressants, antipsychotics, and gabapentinoids, and use them cautiously, if at all, when treating patients with existing substance use disorders.
Related Resources
- Substance Abuse and Mental Health Services Administration. Prescription drug misuse and abuse. https://www.samhsa.gov/topics/prescription-drug-misuse-abuse.
- Substance Abuse and Mental Health Services Administration. Types of commonly misused or abused drugs. https://www.samhsa.gov/prescription-drug-misuse-abuse/types.
- National Institute on Drug Abuse. Misuse of prescription drugs. https://www.drugabuse.gov/publications/research-reports/misuse-prescription-drugs/summary.
- National Institute on Drug Abuse. New clinician screening tool available for substance use. https://www.drugabuse.gov/news-events/news-releases/2018/06/newclinician-screening-tool-available-substance-use.
Drug Brand Names
Amitriptyline • Elavil, Endep
Benztropine • Cogentin
Biperiden • Akineton
Bupropion • Wellbutrin, Zyban
Chlorpromazine • Thorzine
Fluoxetine • Prozac
Haloperidol • Haldol
Olanzapine • Zyprexa
Orphenadrine • Disipal, Norflex
Pregabalin • Lyrica, Lyrica CR
Procyclidine • Kemadrin
Quetiapine • Seroquel
Tianeptine • Coaxil, Stablon
Tranylcypromine • Parnate
Trifluoperazine • Stelazine
Trihexyphenidyl • Artane, Tremin
Venlafaxine • Effexor
While some classes of medications used to treat psychiatric disorders, such as stimulants and benzodiazepines, are well-recognized as controlled substances and drugs of abuse, clinicians may be less familiar with the potential misuse/abuse of other psychiatric medications. This article reviews the evidence related to the misuse/abuse of anticholinergics, antidepressants, antipsychotics, and gabapentinoids.
The terms “misuse,” “abuse,” and “addiction” are used variably in the literature without standardized definitions. For this review, “misuse/abuse (M/A)” will be used to collectively describe self-administration that is recreational or otherwise inconsistent with legal or medical guidelines, unless a specific distinction is made. Whether or not the medications reviewed are truly “addictive” will be briefly discussed for each drug class, but the focus will be on clinically relevant aspects of M/A, including:
- excessive self-administration
- self-administration by non-oral routes
- co-administration with other drugs of abuse
- malingering of psychiatric symptoms to obtain prescriptions
- diversion for sale to third parties
- toxicity from overdose.
Anticholinergic medications
The first case describing the deliberate M/A of an anticholinergic medication for its euphoric effects was published in 1960.Further reportsfollowed in Europe before the M/A potential of prescription anticholinergic medications among psychiatric patients with an overdose syndrome characterized by atropinism and toxic psychosis was more widely recognized in the United States in the 1970s. Most reported cases of M/A to date have occurred among patients with psychiatric illness because anticholinergic medications, including trihexyphenidyl, benztropine, biperiden, procyclidine, and orphenadrine, were commonly prescribed for the management of first-generation and high dopamine D2-affinity antipsychotic-induced extrapyramidal symptoms (EPS). For example, one study of 234 consecutively hospitalized patients with schizophrenia noted an anticholinergic M/A incidence of 6.5%.1
However, anticholinergic M/A is not limited to individuals with psychotic disorders. A UK study of 154 admissions to an inpatient unit specializing in behavioral disturbances found a 12-month trihexyphenidyl M/A incidence of 17%; the most common diagnosis among abusers was antisocial personality disorder.2 Anticholinergic M/A has also been reported among patients with a primary diagnosis of substance use disorders (SUDs)3 as well as more indiscriminately in prison settings,4 with some inmates exchanging trihexyphenidyl as currency and using it recreationally by crushing it into powder and smoking it with tobacco.5 Others have noted that abusers sometimes take anticholinergics with alcohol in order to “potentiate” the effects of each substance.6,7 Pullen et al8 described individuals with and without psychiatric illness who stole anticholinergic medications, purchased them from other patients, or bought them “on the street.” Malingering EPS in order to obtain anticholinergic medications has also been well documented.9 Clearly, anticholinergic M/A can occur in psychiatric and non-psychiatric populations, both within and outside of clinical settings. Although anticholinergic M/A appears to be less frequent in the United States now that second-generation antipsychotics (SGAs) are more frequently prescribed, M/A remains common in some settings outside of the United States.7
Among the various anticholinergic medications prescribed for EPS, trihexyphenidyl has been reported to have the greatest M/A potential, which has been attributed to its potency,10 its stimulating effects (whereas benztropine is more sedating),11 and its former popularity among prescribers.8 Marken et al11 published a review of 110 reports of M/A occurring in patients receiving anticholinergic medications as part of psychiatric treatment in which 69% of cases involved taking trihexyphenidyl 15 to 60 mg at a time (recommended dosing is 6 to 10 mg/d in divided doses).Most of these patients were prescribed anticholinergic medications for diagnostically appropriate reasons—only 7% were described as “true abusers” with no medical indication. Anticholinergic M/A was typically driven by a desire for euphoric and psychedelic/hallucinogenic effects, although in some cases, anticholinergic M/A was attributed to self-medication of EPS and depressive symptoms. These findings illustrate the blurred distinction between recreational use and perceived subjective benefit, and match those of a subsequent study of 50 psychiatric patients who reported anticholinergic M/A not only to “get high,” but to “decrease depression,” “increase energy,” and decrease antipsychotic adverse effects.12 Once again, trihexyphenidyl was the most frequently misused anticholinergic in this sample.
Table 12,3,7,8,10-15 outlines the subjective effects sought and experienced by anticholinergic abusers as well as potential toxic effects; there is the potential for overlap. Several authors have also described physiologic dependence with long-term trihexyphenidyl use, including tolerance and a withdrawal/abstinence syndrome.7,16 In addition, there have been several reports of coma13 and death in the setting of intended suicide by overdose of anticholinergic medications.14,15
Although anticholinergic M/A in the United States now appears to be less common, clinicians should remain aware of the M/A potential of anticholinergic medications prescribed for EPS. Management of M/A involves:
- detection
- reducing anticholinergic exposure by managing EPS with alternative strategies, such as switching or reducing the dose of the antipsychotic medication
- gradual tapering of anticholinergic medications to minimize withdrawal.11
Continue to: Antidepressants
Antidepressants
Haddad17 published a review of 21 English-language case reports from 1966 to 1998 describing antidepressant use in which individuals met DSM-IV criteria for substance dependence to the medication. An additional 14 cases of antidepressant M/A were excluded based on insufficient details to support a diagnosis of dependence. The 21 reported cases involved:
- tranylcypromine (a monoamine oxidase inhibitor [MAOI])
- amitriptyline (a tricyclic antidepressant [TCA])
- fluoxetine (a selective serotonin reuptake inhibitor [SSRI])
- amineptine (a TCA previously available in France but removed from the market in 1999 in part due to its abuse potential)
- nomifensine (a norepinephrine/dopamine reuptake inhibitor previously available in the United Kingdom but removed in 1986 due to hemolytic anemia).
In 95% of cases, the antidepressants were prescribed for treatment of an affective disorder but were abused for stimulant effects or the perceived ability to lift mood, cause euphoria or a “high,” or to improve functioning. Two-thirds of cases involved patients with preexisting substance misuse. Placing the case reports in the context of the millions of patients prescribed antidepressants during this period, Haddad concluded the “incidence of [antidepressant] addiction [is] so low as to be clinically irrelevant.”17
Despite this conclusion, Haddad singled out amineptine and tranylcypromine as antidepressants with some evidence of true addictive potential.17,18 A more recent case series described 14 patients who met DSM-IV criteria for substance abuse of tertiary amine TCAs (which have strong anticholinergic activity) and concluded that “misuse of [TCAs] is more common than generally appreciated.”19 In keeping with that claim, a study of 54 outpatients taking unspecified antidepressants found that up to 15% met DSM-III-R criteria for substance dependence (for the antidepressant) in the past year, although that rate was much lower than the rate of benzodiazepine dependence (47%) in a comparative sample.20 Finally, a comprehensive review by Evans and Sullivan21 found anecdotal reports published before 2014 that detailed misuse, abuse, and dependence with MAOIs, TCAs, fluoxetine, venlafaxine, bupropion, tianeptine, and amineptine. Taken together, existing evidence indicates that select individuals—typically those with other SUD comorbidity—sometimes misuse antidepressants in a way that suggests addiction.
Still, while it is well known that abrupt cessation of antidepressants can result in a discontinuation syndrome characterized by flu-like symptoms, nausea, and dizziness,22 physiologic withdrawal effects must be distinguished from historical definitions of substance “abuse” and the broader concept of psychological “addiction” or drug dependence18,23 now incorporated into the DSM-5 definition of SUDs.24 Indeed, although withdrawal symptoms were reported by more than half of those who took antidepressants and responded to a recent online survey,25 evidence to support the existence of significant antidepressant tolerance, craving, or compulsive use is lacking.17,18 Antidepressants as a class do not appear to be significantly rewarding or reinforcing and, on the contrary, discontinuation by patients is common in clinical practice.26 The popular claim that some individuals taking antidepressants “can’t quit”27 must also be disentangled from loss of therapeutic effects upon cessation.
Bupropion. A more convincing argument for antidepressant addiction can be made for bupropion, a weak norepinephrine and dopamine reuptake inhibitor with an otherwise unclear mechanism of action.28 In 2002, the first report of recreational bupropion M/A described a 13-year-old girl who took 2,400 mg orally (recommended maximum dose is 450 mg/d in divided doses) after being told it would give her “a better high than amphetamine.”29 This was followed in the same year by the first report of recreational M/A of bupropion via nasal insufflation (snorting), resulting in a seizure,30 and in 2013 by the first published case of M/A by IV self-administration.31
Continue to: The M/A potential of bupropion...
The M/A potential of bupropion, most commonly via intranasal administration, is now broadly recognized based on several case reports describing desired effects that include a euphoric high and a stimulating “buzz” similar to that of cocaine or methamphetamine but less intense.29-36 Among recreational users, bupropion tablets are referred to as “welbys,” “wellies,” “dubs,” or “barnies.”37 Media coverage of a 2013 outbreak of bupropion M/A in Toronto detailed administration by snorting, smoking, and injection, and described bupropion as “poor man’s cocaine.”38 Between 2003 and 2016, 2,232 cases of bupropion misuse/abuse/dependence adverse drug reactions were reported to the European Monitoring Agency.37 A review of intentional bupropion M/A reported to US Poison Control Centers between 2000 to 2013 found 975 such cases, with the yearly number tripling between 2000 and 2012.39 In this sample, nearly half (45%) of the users were age 13 to 19, and 76% of cases involved oral ingestion. In addition to bupropion M/A among younger people, individuals who misuse bupropion often include those with existing SUDs but limited access to illicit stimulants and those trying to evade detection by urine toxicology screening.33 For example, widespread use and diversion has been well documented within correctional settings, and as a result, many facilities have removed bupropion from their formularies.21,28,33,34,40
Beyond desired effects, the most common adverse events associated with bupropion M/A are listed in Table 2,28,30,32-34,36,39 along with their incidence based on cases brought to the attention of US Poison Control Centers.39 With relatively little evidence of a significant bupropion withdrawal syndrome,37 the argument in favor of modeling bupropion as a truly addictive drug is limited to anecdotal reports of cravings and compulsive self-administration35 and pro-dopaminergic activity (reuptake inhibition) that might provide a mechanism for potential rewarding and reinforcing effects.40 While early preclinical studies of bupropion failed to provide evidence of amphetamine-like abuse potential,41,42 non-oral administration in amounts well beyond therapeutic dosing could account for euphoric effects and a greater risk of psychological dependence and addiction.21,28,40
Bupropion also has an FDA indication as an aid to smoking cessation treatment, and the medication demonstrated early promise in the pharmacologic treatment of psychostimulant use disorders, with reported improvements in cravings and other SUD outcomes.43-45 However, subsequent randomized controlled trials (RCTs) failed to demonstrate a clear therapeutic role for bupropion in the treatment of cocaine46,47 and methamphetamine use disorders (although some secondary analyses suggest possible therapeutic effects among non-daily stimulant users who are able to maintain good adherence with bupropion).48-51 Given these overall discouraging results, the additive seizure risk of bupropion use with concomitant psychostimulant use, and the potential for M/A and diversion of bupropion (particularly among those with existing SUDs), the use of bupropion for the off-label treatment of stimulant use disorders is not advised.
Antipsychotics
As dopamine antagonists, antipsychotics are typically considered to have low potential for rewarding or reinforcing effects. Indeed, misuse of antipsychotics was a rarity in the first-generation era, with only a few published reports of haloperidol M/A within a small cluster of naïve young people who developed acute EPS,52 and a report of diversion in a prison with the “sadistic” intent of inflicting dystonic reactions on others.53 A more recent report described 2additional cases of M/A involving haloperidol and trifluoperazine.54 Some authors have described occasional drug-seeking behavior for low-potency D2 blockers such as chlorpromazine, presumably based on their M/A as anticholinergic medications.55
The potential for antipsychotic M/A has gained wider recognition since the advent of the SGAs. Three cases of prescription olanzapine M/A have been published to date. One involved a man who malingered manic symptoms to obtain olanzapine, taking ≥40 mg at a time (beyond his prescribed dose of 20 mg twice daily) to get a “buzz,” and combining it with alcohol and benzodiazepines for additive effects or to “come down” from cocaine.56 This patient noted that olanzapine was “a popular drug at parties” and was bought, sold, or traded among users, and occasionally administered intravenously. Two other cases described women who self-administered olanzapine, 40 to 50 mg/d, for euphoric and anxiolytic effects.57,58 James et al59 detailed a sample of 28 adults who reported “non-medical use” of olanzapine for anxiolytic effects, as a sleep aid, or to “escape from worries.”
Continue to: Quetiapine
Quetiapine. In contrast to some reports of olanzapine M/A in which the line between M/A and “self-medication” was blurred, quetiapine has become a more convincing example of clear recreational antipsychotic M/A. Since the first report of oral and intranasal quetiapine M/A in the Los Angeles County Jail published in 2004,55 subsequent cases have detailed other novel methods of recreational self-administration60-68 (Table 355,60-68), and additional reports have been published in non-English language journals.69,70 Collectively, these case reports have detailed that quetiapine is:
- misused for primary subjective effects as well as to mitigate the unpleasant effects of other drugs60,67
- referred to as “quell,”“Q,” “Susie-Q,” “squirrel,” and “baby heroin”55,71,72
- often obtained by malingering psychiatric symptoms55,61,63,65
- diverted/sold with “street value” both within and outside of psychiatric facilities and correctional settings.55,60-62,67,68,73
These anecdotal accounts of quetiapine M/A have since been corroborated on a larger scale based on several retrospective studies. Although early reports of quetiapine M/A occurring in correctional settings have resulted in formulary removal,71,74 quetiapine M/A is by no means limited to forensic populations and is especially common among those with comorbid SUDs. A survey of 74 patients enrolled in a Canadian methadone program reported that nearly 60% had misused quetiapine at some point.75 Among an Australian sample of 868 individuals with active IV drug abuse, 31% reported having misused quetiapine.76 Finally, within a small sample of patients with SUDs admitted to a detoxification unit in New York City, 17% reported M/A of SGAs.77 In this study, SGAs were often taken in conjunction with other drugs of abuse in order to “recover” from or “enhance” the effects of other drugs or to “experiment.” Quetiapine was by far the most frequently abused SGA, reported in 96% of the sample; the most frequently reported SGA/drug combinations were quetiapine/alcohol/opioids, quetiapine/cocaine, and quetiapine/opioids.
Looking more broadly at poison center data, reports to the US National Poison Data System (NPDS) from 2005 to 2011 included 3,116 cases of quetiapine abuse (37.5%, defined as intentional recreational use in order to obtain a “high”) or misuse (62.5%, defined as improper use or dosing for non-recreational purposes).78 A more recent analysis of NPDS reports from 2003 to 2013 found 2,118 cases of quetiapine abuse, representing 61% of all cases of reported SGA abuse.79 An analysis of the European Medicines Agency Adverse Drug Database yielded 18,112 reports of quetiapine misuse, abuse, dependence, and withdrawal for quetiapine (from 2005 to 2016) compared with 4,178 for olanzapine (from 2004 to 2016).80 These reports identified 368 fatalities associated with quetiapine.
The rate of quetiapine M/A appears to be increasing sharply. Reports of quetiapine M/A to poison centers in Australia increased nearly 7-fold from 2006 to 2016.81 Based on reports to the Drug Abuse Warning System, US emergency department visits for M/A of quetiapine increased from 19,195 in 2005 to 32,024 in 2011 (an average of 27,114 visits/year), with 75% of cases involving quetiapine taken in combination with other prescription drugs, alcohol, or illicit drugs.82 Consistent with poison center data, M/A was reported for other antipsychotics, but none nearly as frequently as for quetiapine.
With increasingly frequent quetiapine M/A, clinicians should be vigilant in monitoring for medical morbidity related to quetiapine and cumulative toxicity with other drugs. The most frequent adverse events associated with quetiapine M/A reported to US Poison Control Centers are presented in Table 4.78,79
Continue to: Unlike bupropion...
Unlike bupropion, quetiapine’s dopamine antagonism makes it unlikely to be a truly addictive drug, although this mechanism of action could mediate an increase in concurrent psychostimulant use.83 A few case reports have described a quetiapine discontinuation syndrome similar to that of antidepressants,60,65,84-88 but withdrawal symptoms suggestive of physiologic dependence may be mediated by non-dopaminergic effects through histamine and serotonin receptors.84,89 Evidence for quetiapine misuse being associated with craving and compulsive use is lacking, and true quetiapine addiction is probably rare.
Similar to bupropion, preliminary findings have suggested promise for quetiapine as a putative therapy for other SUDs.90-93 However, subsequent RCTs have failed to demonstrate a therapeutic effect for alcohol and cocaine use disorders.94-96 Given these negative results and the clear M/A potential of quetiapine, off-label use of quetiapine for the treatment of SUDs and psychiatric symptoms among those with SUDs must be considered judiciously, with an eye towards possible diversion and avoiding the substitution of one drug of abuse for another.
Gabapentinoids
In 1997, the first published case report of gabapentin M/A described a woman who self-administered her husband’s gabapentin to reduce cravings for and withdrawal from cocaine.97 The authors highlighted the possible therapeutic benefit of gabapentin in this regard rather than raising concerns about diversion and M/A. By 2004, however, reports of recreational gabapentin M/A emerged among inmates incarcerated within Florida correctional facilities who self-administered intranasal gabapentin to achieve a “high” that was “reminiscent of prior effects from intranasal ingestion of cocaine powder.”98 In 2007, a single case of gabapentin misuse up to 7,200 mg/d (recommended dosing is ≤3,600 mg/d) was reported, with documentation of both tolerance and withdrawal symptoms.99 As of 2017, a total of 36 cases of gabapentin M/A and 19 cases of pregabalin M/A have been published.100
In the past decade, anecdotal reports have given way to larger-scale epidemiologic data painting a clear picture of the now-widespread M/A of gabapentin and other gabapentinoids. For example, a study of online descriptions of gabapentin and pregabalin M/A from 2008 to 2010 documented:
- oral and IM use (gabapentin)
- IV and rectal (“plugging”) use (pregabalin)
- “parachuting” (emptying the contents of capsules for a larger dose) (pregabalin)
- euphoric, entactogenic, stimulant, calming/anxiolytic, and dissociative subjective effects (gabapentin/pregabalin)
- rapid development of tolerance to euphoric effects leading to self-administration of increasing doses (gabapentin/pregabalin)
- frequent co-administration with other drugs of abuse, including alcohol, benzodiazepines, cannabis, stimulants, opiates, hallucinogens, gamma-hydroxybutyrate, mephedrone, and Salvia divinorum (gabapentin/pregabalin)101
Several systematic reviews of both anecdotal reports and epidemiologic studies published in the past few years provide additional evidence of the above, such as:
- excessive dosing with self-administration
- intranasal and inhaled routes of administration
- diversion and “street value”
- greater M/A potential of pregabalin than gabapentin
- the presence of gabapentinoids in postmortem toxicology analyses, suggesting a role in overdose fatalities when combined with other drugs.100,102,103
Continue to: The European Medicine Agency's EudraVigilance database...
The European Medicine Agency’s EudraVigilance database included 4,301 reports of gabapentin misuse, abuse, or dependence, and 7,639 such reports for pregabalin, from 2006 to 2015 (rising sharply after 2012), with 86 gabapentin-related and 27 pregabalin-related fatalities.104 Data from the Drug Diversion Program of the Researched Abuse, Diversion, and Addiction-Related Surveillance System from 2002 to 2015 have likewise revealed that gabapentin diversion increased significantly in 2013.105
While the prevalence of gabapentinoid M/A is not known, rates appear to be significantly lower than for traditional drugs of abuse such as cannabis, cocaine, 3,4-methylenedioxymethamphetamine (MDMA), and opioids.106,107 However, gabapentin and pregabalin M/A appears to be increasingly common among individuals with SUDs and in particular among those with opioid use disorders (OUDs). For example, a 2015 report indicated that 15% of an adult cohort in Appalachian Kentucky with nonmedical use of diverted prescription opioids reported gabapentin M/A, an increase of nearly 3,000% since 2008.108 Based on data from a US insurance enrollment and claims database, researchers found that the rate of gabapentin overuse among those also overusing opioids was 12% compared with only 2% for those using gabapentin alone.109 It has also been reported that gabapentin is sometimes used as a “cutting agent” for heroin.110
Those who use gabapentinoids together with opioids report that gabapentin and pregabalin potentiate the euphoric effects of methadone111 and endorse specific beliefs that pregabalin increases both the desired effects of heroin as well as negative effects such as “blackouts,” loss of control, and risk of overdose.112 Indeed, sustained M/A of gabapentin and opioids together has been found to increase emergency department utilization, drug-related hospitalization, and respiratory depression.113 Based on a case-control study of opioid users in Canada, co-prescription of gabapentin and opioids was associated with a 50% increase in death from opioid-related causes compared with prescription of opioids alone.114
Case reports documenting tolerance, withdrawal, craving, and loss of control suggest a true addictive potential for gabapentinoids, but Bonnet and Sherbaum100 concluded that while there is robust evidence of abusers “liking” gabapentin and pregabalin (eg, reward), evidence of “wanting” them (eg, psychological dependence) in the absence of other SUDs has been limited to only a few anecdotal reports with pregabalin. Accordingly, the risk of true addiction to gabapentinoids by those without preexisting SUDs appears to be low. Nonetheless, the M/A potential of both gabapentin and pregabalin is clear and in the context of a nationwide opioid epidemic, the increased morbidity/mortality risk related to combined use of gabapentinoids and opioids is both striking and concerning. Consequently, the state of Kentucky recently recognized the M/A potential of gabapentin by designating it a Schedule V controlled substance (pregabalin is already a Schedule V drug according to the US Drug Enforcement Agency),103,113 and several other states now mandate the reporting of gabapentin prescriptions to prescription drug monitoring programs.115
Following a similar pattern to antidepressants and antipsychotics, a potential role for gabapentin in the treatment of cocaine use disorders was supported in preliminary studies,116-118 but not in subsequent RCTs.119-121 However, there is evidence from RCTs to support the use of gabapentin and pregabalin in the treatment of alcohol use disorders.122-124 Gabapentin was also found to significantly reduce cannabis use and withdrawal symptoms in patients compared with placebo in an RCT of individuals with cannabis use disorders.125 The perceived safety of gabapentinoids by clinicians, their subjective desirability by patients with SUDs, and efficacy data supporting a therapeutic role in SUDs must be balanced with recognition that approximately 80% of gabapentin prescriptions are written for off-label indications for which there is little supporting evidence,109 such as low back pain.126 Clinicians considering prescribing gabapentinoids to manage psychiatric symptoms, such as anxiety and insomnia, should carefully consider the risk of M/A and other potential morbidities, especially in the setting of SUDs and OUD in particular.
Continue to: Problematic, even if not addictive
Problematic, even if not addictive
It is sometimes claimed that “addiction” to psychiatric medications is not limited to stimulants and benzodiazepines.27,127 Although anticholinergics, antidepressants, antipsychotics, and gabapentinoids can be drugs of abuse, with some users reporting physiologic withdrawal upon discontinuation, there is only limited evidence that the M/A of these psychiatric medications is associated with the characteristic features of a more complete definition of “addiction,” which may include:
- inability to consistently abstain
- impairment in behavioral control
- diminished recognition of significant problems associated with use
- a dysfunctional emotional response to chronic use.128
Nonetheless, the literature documenting anticholinergic, antidepressant, antipsychotic, and gabapentinoid M/A includes several common features, including:
- initial reports among those with limited access to illicit drugs (eg, young people and incarcerated individuals) and subsequent spread to a wider population with more unconventional routes of administration
- use for recreational purposes and other subjective pseudo-therapeutic effects, often in combination with alcohol and illicit drugs
- greater M/A potential of certain medications within each of these drug classes (eg, trihexyphenidyl, bupropion, quetiapine)
- malingering psychiatric symptoms in order to obtain medications from prescribers and diversion for black market sale
- observations that medications might constitute therapy for SUDs that were not supported in subsequent RCTs (with the exception of gabapentin for alcohol and cannabis use disorders)
- increasing evidence of toxicity related to M/A, which suggests that prescription by clinicians has limited benefit and high risk for patients with SUDs.
Bottom Line
Some psychiatric medications are taken as drugs of abuse. Clinicians should be particularly aware of the misuse/abuse potential of anticholinergics, antidepressants, antipsychotics, and gabapentinoids, and use them cautiously, if at all, when treating patients with existing substance use disorders.
Related Resources
- Substance Abuse and Mental Health Services Administration. Prescription drug misuse and abuse. https://www.samhsa.gov/topics/prescription-drug-misuse-abuse.
- Substance Abuse and Mental Health Services Administration. Types of commonly misused or abused drugs. https://www.samhsa.gov/prescription-drug-misuse-abuse/types.
- National Institute on Drug Abuse. Misuse of prescription drugs. https://www.drugabuse.gov/publications/research-reports/misuse-prescription-drugs/summary.
- National Institute on Drug Abuse. New clinician screening tool available for substance use. https://www.drugabuse.gov/news-events/news-releases/2018/06/newclinician-screening-tool-available-substance-use.
Drug Brand Names
Amitriptyline • Elavil, Endep
Benztropine • Cogentin
Biperiden • Akineton
Bupropion • Wellbutrin, Zyban
Chlorpromazine • Thorzine
Fluoxetine • Prozac
Haloperidol • Haldol
Olanzapine • Zyprexa
Orphenadrine • Disipal, Norflex
Pregabalin • Lyrica, Lyrica CR
Procyclidine • Kemadrin
Quetiapine • Seroquel
Tianeptine • Coaxil, Stablon
Tranylcypromine • Parnate
Trifluoperazine • Stelazine
Trihexyphenidyl • Artane, Tremin
Venlafaxine • Effexor
1. Zemishlany Z, Aizenberg D, Weiner Z, et al. Trihexyphenidyl (Artane) abuse in schizophrenic patients. Int Clin Psychopharmacol. 1996;11(3):199-202.
2. Crawshaw JA, Mullen PE. A study of benzhexol abuse. Brit J Psychiatry. 1984;145:300-303.
3. Woody GE, O’Brien CP. Anticholinergic toxic psychosis in drug abusers treated with benztropine. Comp Psychiatry. 1974;15(5):439-442.
4. Lowry TP. Trihexyphenidyl abuse. Am J Psychiatry. 1977;134(11):1315.
5. Rouchell AM, Dixon SP. Trihexyphenidyl abuse. Am J Psychiatry. 1977;134(11):1315.
6. Kaminer Y, Munitz H, Wijsenbeek H. Trihexyphenidyl (Artane) abuse: euphoriant and anxiolytic. Brit J Psychiatry. 1982;140(5):473-474.
7. Nappo SA, de Oliviera LG, Sanchez Zv, et al. Trihexyphenidyl (Artane): a Brazilian study of its abuse. Subst Use Misuse. 2005;40(4):473-482.
8. Pullen GP, Best NR, Macguire J. Anticholinergic drug abuse: a common problem? Brit Med J (Clin Res Ed). 1984;289(6445):612-613.
9. Rubinstein JS. Abuse of antiparkinsonian drugs: feigning of extrapyramidal symptoms to obtain trihexyphenidyl. JAMA. 1978;239(22):2365-2366.
10. Mohan D, Mohandas E, Dube S. Trihexyphenidyl abuse. Brit J Addiction. 1981:76(2);195-197.
11. Marken PA, Stoner SC, Bunker MT. Anticholinergic drug abuse and misuse. CNS Drugs. 1996;5(3):190-199.
12. Buhrich N, Weller A, Kevans P. Misuse of anticholinergic drugs by people with serious mental illness. Psychiatric Serv. 2000;51(7):928-929.
13. Goldstein MR, Kasper R. Hyperpyrexia and coma due to overdose of benztropine. South Med J. 1968;61(9):984.
14. Petkovi
15. McIntyre IM, Mallett P, Burton CG, et al. Acute benztropine intoxication and fatality. J Forensic Sci. 2014;59(6):1675-1678.
16. Dilsaver SC. Antimuscarinic agents as substances of abuse: A review. J Clin Psychopharmacol. 1988:8(1):14-22.
17. Haddad P. Do antidepressants have any potential to cause addiction? J Psychopharmacol. 1999;13(3):300-307.
18. Haddad PM. Do antidepressants cause dependence? Epidemiol Psichiatr Soc. 2005;14(2):58-62.
19. Shenouda R, Desan PH. Abuse of tricyclic antidepressant drugs: a case series. J Clin Psychopharmacol. 2013;33(3):440-442.
20. van Broekhoven F, Kan CC, Zitman FG. Dependence potential of antidepressants compared to benzodiazepines. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(5):939-943.
21. Evans EA, Sullivan MA. Abuse and misuse of antidepressants. Subst Abuse Rehabil. 2014;5:107-120.
22. Warner CH, Bobo W, Warner C, et al. Antidepressant discontinuation syndrome. Am Fam Physician. 2006;74(3):449-456.
23. Lichtigfeld FJ, Gillman MA. Antidepressants are not drugs of abuse or dependence. Postgrad Med J. 1998;74(875):529-532.
24. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
25. Read J, Cartwright C, Gibson K. Adverse emotional and interpersonal effects reported by 1829 New Zealanders while taking antidepressants. Psychiatry Res. 2014;216(1):67-73.
26. Haddad P, Anderson I. Antidepressants aren’t addictive: clinicians have depended on them for years. J Psychopharmacol. 1999;13(3):291-292.
27. Carey B, Gebeloff R. Many people taking antidepressants discover they cannot quit. New York Times. https://www.nytimes.com/2018/04/07/health/antidepressants-withdrawal-prozac-cymbalta.html. Published April 7, 2018. Accessed December 11, 2018.
28. Kim D, Steinhart B. Seizures induced by recreational abuse of bupropion tablets via nasal insufflation. CJEM. 2010;12(2):158-161.
29. McCormick J. Recreational bupropion in a teenager. Br J Clin Pharmacol. 2002;53(2):214.
30. Welsh C, Doyon S. Seizure induced by insufflation of bupropion. N Engl J Med. 2002; 347(2):951.
31. Baribeau D, Araki KF. Intravenous bupropion: A previously undocumented method of abuse of a commonly prescribed antidepressant agent. J Addict Med. 2013;7(3):216-217.
32. Hill SH, Sikand H, Lee J. A case report of seizure induced by bupropion nasal insufflation. Prim Care Companion J Clin Psych. 2007;9(1):67-69.
33. Yoon G, Westermeyer J. Intranasal bupropion abuse. Am J Addict. 2013;22(2):180.
34. Reeves RR, Ladner ME. Additional evidence of the abuse potential of bupropion. J Clin Psychopharmacol. 2013;33(4):584-585.
35. Oppek K, Koller G, Zwergal A, et al. Intravenous administration and abuse of bupropion: a case report and a review of the literature. J Addict Med. 2014;8(4):290-293.
36. Strike M, Hatcher S. Bupropion injection resulting in tissue necrosis and psychosis: previously undocumented complications of intravenous bupropion use disorder. J Addict Med. 2015;9(3):246-250.
37. Schifano F, Chiappini S. Is there a potential of misuse for venlafaxine and bupropion? Front Pharmacol. 2018;9:239.
38. Tryon J, Logan N. Antidepressant Wellbutrin becomes ‘poor man’s cocaine’ on Toronto streets. Global News. https://globalnews.ca/news/846576/antidepressant-wellbutrin-becomes-poor-mans-cocaine-on-toronto-streets/. Published September 18, 2013. Accessed December 11, 2018.
39. Stassinos GL, Klein-Schwartz W. Bupropion “abuse” reported to US Poison Centers. J Addict Med. 2016;10(5):357-362.
40. Hilliard WT, Barloon L, Farley P, et al. Bupropion diversion and misuse in the correctional facility. J Correct Health Care. 2013;19(3):211-217.
41. Griffith JD, Carranza J, Griffith C, et al. Bupropion clinical assay for amphetamine-like abuse potential. J Clin Psychiatry.1983;44(5 Pt 2):206-208.
42. Miller L, Griffith J. A comparison of bupropion, dextroamphetamine, and placebo in mixed-substance abusers. Psychopharmacol (Berl). 1983;80(3):199-205.
43. Berigan TR, Russell ML. Treatment of methamphetamine cravings with bupropion: A case report. Prim Care Companion J Clin Psychiatry. 2001;3(6):267-268.
44. Tardieu T, Poirier Y, Micallef J, et al. Amphetamine-like stimulant cessation in an abusing patient treated with bupropion. Acta Psychiatr Scand. 2004;109(1):75-78.
45. Newton TF, Roache JD, De La Garza R, et al. Bupropion reduces methamphetamine-induced subjective effects and cue-induced cravings. Neuropsychopharmacology. 2006;31(7):1537-1544.
46. Margolin A, Kosten TR, Avants SK, et al. A multicenter trial for cocaine dependence in methadone-maintained patients. Drug Alcohol Depend. 1995;40(2):125-131.
47. Shoptaw S, Heinzerling KG, Rotheram-Fuller E, et al. Bupropion hydrochloride versus placebo, in combination with cognitive behavioral therapy, for the treatment of cocaine abuse/dependence. J Addict Dis. 2008;27(1):13-23.
48. Anderson AL, Li S, Markova D, et al. Bupropion for the treatment of methamphetamine dependence in non-daily users: a randomized, double-blind placebo-controlled trial. Drug Alcohol Depend. 2015;150:170-174.
49. Shoptaw S, Heinzerling KG, Rotheram-Fuller E, et al. Randomized, placebo-controlled trial of bupropion for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2008;96(3):222-232.
50. Elkashef AM, Rawson RA, Anderson AL, et al. Bupropion for the treatment of methamphetamine dependence. Neuropsychopharmacology. 2008;33(5):1162-1170.
51. Heinzerling KG, Swanson A, Hall TM, et al. Randomized, placebo-controlled trial of bupropion in methamphetamine-dependent participants with less than daily methamphetamine use. Addiction. 2014;109(11):1878-1886.
52. Doenecke AL, Heuerman RC. Treatment of haloperidol abuse with diphenhydramine. Am J Psychiatry. 1980;137(4):487-488.
53. Weddington WW, Leventhal BL. Sadistic abuse of haloperidol. Am J Psychiatry. 1982;139:132-133.
54. Basu D, Marudkar M, Khurana H. Abuse of neuroleptic drugs by psychiatric patients. Indian J Med Sci. 2000;54(2):59-62.
55. Pierre JM, Shnayder I, Wirshing DA, et al. Intranasal quetiapine abuse. Am J Psychiatry 2004;161(9):1718.
56. Reeves RR. Abuse of olanzapine by substance abusers. J Psychoactive Drugs. 2007;39(3):297-299.
57. Kumsar NA, Erol A. Olanzapine abuse. Subst Abus. 2013;34(1):73-74.
58. Lai C. Olanzapine abuse was relieved after switching to aripiprazole in a patient with psychotic depression. Prog Neuropsychpharmacol Biol Psychiatry. 2010;34(7):1363-1364.
59. James PD, Fida AS, Konovalov P, et al. Non-medical use of olanzapine by people on methadone treatment. BJPsych Bull. 2016;40(6):314-317.
60. Reeves RR, Brister JC. Additional evidence of the abuse potential of quetiapine. South Med J. 2007;100(8):834-836.
61. Murphy D, Bailey K, Stone M, et al. Addictive potential of quetiapine. Am J Psychiatry. 2008;165(7):918.
62. Paparrigopoulos T, Karaiskos D, Liappas J. Quetiapine: another drug with potential for misuse? J Clin Psychiatry. 2008;69(1):162-163.
63. Reeves RR, Burke RS. Abuse of the combination of gabapentin and quetiapine. Prim Care Companion CNS Disord. 2014;16(5): doi: 10.4088/PCC.14l01660.
64. Morin AK. Possible intranasal quetiapine misuse. Am J Health Syst Pharm. 2007;64(7):723-725.
65. Caniato RN, Gundabawady A, Baune BT, et al. Malingered psychotic symptoms and quetiapine abuse in a forensic setting. J Forens Psychiatr Psychol. 2009;20(6):928-935.
66. Hussain MZ, Waheed W, Hussain S. Intravenous quetiapine abuse. Am J Psychiatry. 2005; 162(9):1755-1756.
67. Waters BM, Joshi KG. Intravenous quetiapine-cocaine use (“Q-ball”). Am J Psychiatry. 2007;164(1):173-174.
68. Haridas A, Kushon D, Gurmu S, et al. Smoking quetiapine: a “Maq ball?” Prim Psychiatry. 2010;17:38-39.
69. Cubala WJ, Springer J. Quetiapine abuse and dependence in psychiatric patients: a systematic review of 25 case reports in the literature. J Subs Use. 2014;19(5):388-393.
70. Piróg-Balcerzak A, Habrat B, Mierzejewski P. Misuse and abuse of quetiapine [in Polish]. Psychiatr Pol. 2015;49(1):81-93.
71. Pinta ER, Taylor RE. Quetiapine addiction? Am J Psychiatry. 2007;164(1):174.
72. Tamburello AC, Lieberman JA, Baum RM, et al. Successful removal of quetiapine from a correctional formulary. J Amer Acad Psychiatr Law. 2012;40(4):502-508.
73. Tarasoff G, Osti K. Black-market value of antipsychotics, antidepressants, and hypnotics in Las Vegas, Nevada. Am J Psychiatry. 2007;164(2):350.
74. Reccoppa L. Less abuse potential with XR formulation of quetiapine. Am J Addiction. 2010;20(2):178.
75. McLarnon ME, Fulton HG, MacIsaac C, et al. Characteristics of quetiapine misuse among clients of a community-based methadone maintenance program. J Clin Psychopharmacol. 2012;32(5):721-723.
76. Reddel SE, Bruno R, Burns L, et al. Prevalence and associations of quetiapine fumarate misuse among an Australian national city sample of people who regularly inject drugs. Addiction. 2013;109(2):295-302.
77. Malekshahi T, Tioleco N, Ahmed N, et al. Misuse of atypical antipsychotics in conjunction with alcohol and other drugs of abuse. J Subs Abuse Treat. 2015;48(1):8-12.
78. Klein-Schwartz W, Schwartz EK, Anderson BD. Evaluation of quetiapine abuse and misuse reported to poison centers. J Addict Med. 2014;8(3):195-198.
79. Klein L, Bangh S, Cole JB. Intentional recreational abuse of quetiapine compared to other second-generation antipsychotics. West J Emerg Med. 2017;18(2):243-250.
80. Chiappini S, Schifano F. Is there a potential of misuse for quetiapine?: Literature review and analysis of the European Medicines Agency/European Medicines Agency Adverse Drug Reactions’ Database. J Clin Psychopharmacol. 2018;38(1):72-79.
81. Lee J, Pilgrim J, Gerostamoulos D, et al. Increasing rates of quetiapine overdose, misuse, and mortality in Victoria, Australia. Drug Alcohol Depend. 2018;187:95-99.
82. Mattson ME, Albright VA, Yoon J, et al. Emergency department visits involving misuse and abuse of the antipsychotic quetiapine: Results from the
83. Brutcher RE, Nader SH, Nader MA. Evaluation of the reinforcing effect of quetiapine, alone and in combination with cocaine, in rhesus monkeys. J Pharmacol Exp Ther. 2016;356(2):244-250.
84. Kim DR, Staab JP. Quetiapine discontinuation syndrome. Am J Psychiatry. 2005;162(5):1020.
85. Thurstone CC, Alahi P. A possible case of quetiapine withdrawal syndrome. J Clin Psychiatry. 2000;61(8):602-603.
86. Kohen I, Kremen N. A case report of quetiapine withdrawal syndrome in a geriatric patient. World J Biol Psychiatry. 2009;10(4 pt 3):985-986.
87. Yargic I, Caferov C. Quetiapine dependence and withdrawal: a case report. Subst Abus. 2011;32(3):168-169.
88. Koch HJ. Severe quetiapine withdrawal syndrome with nausea and vomiting in a 65-year-old patient with psychotic depression. Therapie. 2015;70(6):537-538.
89. Fischer BA, Boggs DL. The role of antihistaminic effects in the misuse of quetiapine: a case report and review of the literature. Neurosci Biobehav Rev. 2010;34(4):555-558.
90. Longoria J, Brown ES, Perantie DC, et al. Quetiapine for alcohol use and craving in bipolar disorder. J Clin Psychopharmacol. 2004;24(1):101-102.
91. Monnelly EP, Ciraulo DA, Knapp C, et al. Quetiapine for treatment of alcohol dependence. J Clin Psychopharmacol. 2004;24(5):532-535.
92. Kennedy A, Wood AE, Saxon AJ, et al. Quetiapine for the treatment of cocaine dependence: an open-label trial. J Clin Psychopharmacol. 2008;28(2):221-224.
93. Mariani JJ, Pavlicova M, Mamczur A, et al. Open-label pilot study of quetiapine treatment for cannabis dependence. Am J Drug Alcohol Abuse. 2014;40(4):280-284.
94. Guardia J, Roncero C, Galan J, et al. A double-blind, placebo-controlled, randomized pilot study comparing quetiapine with placebo, associated to naltrexone, in the treatment of alcohol-dependent patients. Addict Behav. 2011;36(3):265-269.
95. Litten RZ, Fertig JB, Falk DE, et al; NCIG 001 Study Group. A double-blind, placebo-controlled trial to assess the efficacy of quetiapine fumarate XR in very heavy-drinking alcohol-dependent patients. Alcohol Clin Exp Res. 2012;36(3):406-416.
96. Tapp A, Wood AE, Kennedy A, et al. Quetiapine for the treatment of cocaine use disorder. Drug Alcohol Depend. 2015;149:18-24.
97. Markowitz JS, Finkenbine R, Myrick H, et al. Gabapentin abuse in a cocaine user: Implications for treatment. J Clin Psychopharmacol. 1997;17(5):423-424.
98. Reccoppa L, Malcolm R, Ware M. Gabapentin abuse in inmates with prior history of cocaine dependence. Am J Addict. 2004;13(3):321-323.
99. Victorri-Vigneau C, Guelais M, Jolliet P. Abuse, dependency and withdrawal with gabapentin: a first case report. Pharmacopsychiatry. 2007;40(1):43-44.
100. Bonnet U, Sherbaum N. How addictive are gabapentin and pregabalin? A systematic review. Eur Neuropsychopharmacol. 2017;27(12):1185-1215.
101. Schifano F, D’Offizi S, Piccione M, et al. Is there a recreational misuse potential for pregabalin? Analysis of anecdotal online reports in comparison with related gabapentin and clonazepam data. Psychother Psychosom. 2011;80(2):118-122.
102. Evoy KE, Morrison MD, Saklad SR. Abuse and misuse of pregabalin and gabapentin. Drugs. 2017;77(4):403-426.
103. Smith RV, Havens JR, Walsh SL. Gabapentin misuse, abuse and diversion: a systematic review. Addiction. 2016;111(7):1160-1174.
104. Chiappini S, Shifano F. A decade of gabapentinoid misuse: an analysis of the European Medicines Agency’s ‘suspected adverse drug reactions’ database. CNS Drugs. 2016;30(7):647-654.
105. Buttram ME, Kurtz SP, Dart R, et al. Law enforcement-derived data on gabapentin diversion and misuse, 2002-2015: diversion rates and qualitative research findings. Pharmacoepidemiol Drug Saf. 2017;26(9):1083-1086.
106. Kapil V, Green JL, Le Lait M, et al. Misuse of the y-aminobutyric acid analogues baclofen, gabapentin and pregabalin in the UK. Br J Clin Pharmacol. 2013;78(1):190-191.
107. Peckham AM, Fairman KA, Sclar DA. Prevalence of gabapentin abuse: comparison with agents with known abuse potential in a commercially insured US population. Clin Drug Invest. 2017;37(8):763-773.
108. Smith RV, Lofwall MR, Havens JR. Abuse and diversion of gabapentin among nonmedical prescription opioid users in Appalachian Kentucky. Am J Psychiatry. 2015;172(5):487-488.
109. Peckham AM, Evoy KE, Covvey JR, et al. Predictors of gabapentin overuse with or without concomitant opioids in a commercially insured U.S. population. Pharmacotherapy. 2018;38(4):436-443.
110. Smith BH, Higgins C, Baldacchino A, et al. Substance misuse of gabapentin. Br J Gen Pract. 2012;62(601):401-407.
111. Baird CRW, Fox P, Colvin LA. Gabapentinoid abuse in order to potentiate the effect of methadone: a survey among substance misusers. Eur Addict Res. 2014;20(3):115-118.
112. Lyndon A, Audrey S, Wells C, et al. Risk to heroin users of polydrug use of pregabalin or gabapentin. Addiction. 2017;112(9):1580-1589.
113. Peckham AM, Fairman KA, Sclar DA. All-cause and drug-related medical events associated with overuse of gabapentin and/or opioid medications: a retrospective cohort analysis of a commercially insured US population. Drug Saf. 2018;41(2):213-228.
114. Gomes T, Juurlink DN, Antoniou T, et al. Gabapentin, opioids, and the risk of opioid-related death: a population-based nested case-control study. PLoS Med. 2017;14(10):e10022396. doi: 10.1371/journal.pmed.1002396.
115. Peckham AM, Fairman K, Sclar DA. Policies to mitigate nonmedical use of prescription medications: how should emerging evidence of gabapentin misuse be addressed? Exp Opin Drug Saf. 2018;17(5):519-523.
116. Raby WN. Gabapentin for cocaine cravings. Am J Psychiatry. 2000;157(12):2058-2059.
117. Myrick H, Henderson S, Brady KT, et al. Gabapentin in the treatment of cocaine dependence: a case series. J CLin Psychiatry. 2001;62(1):19-23.
118. Raby WN, Coomaraswamy S. Gabapentin reduces cocaine use among addicts from a community clinic sample. J Clin Psychiatry. 2004;65(1):84-86.
119. Hart CL, Ward AS, Collins ED, et al. Gabapentin maintenance decreases smoked cocaine-related subjective effects, but not self-administration by humans. Drug Alcohol Depend. 2004;73(3):279-287.
120. Bisaga A, Aharonovich E, Garawi F, et al. A randomized placebo-controlled trial of gabapentin for cocaine dependence. Drug Alc Depend. 2006;81(3):267-274.
121. Hart CL, Haney M, Collins ED, et al. Smoked cocaine self-administration by humans is not reduced by large gabapentin maintenance doses. Drug Alcohol Depend. 2007;86(2-3):274-277.
122. Furieri FA, Nakamura-Palacios EM. Gabapentin reduces alcohol consumption and craving: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2007;68(11):1691-1700.
123. Mason BJ, Quello S, Goodell V, et al. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77.
124. Martinotti G, Di Nicola M, Tedeschi D, et al. Pregabalin versus naltrexone in alcohol dependence: a randomised, double-blind, comparison trial. J Psychopharmacol. 2010;24(9):1367-1374.
125. Mason BJ, Crean R, Goodell V, et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychpharmacology. 2012;27(7):1689-1698.
126. Enke O, New HA, New CH, et al. Anticonvulsants in the treatment of low back pain and lumbar radicular pain: a systematic review and meta-analysis. CMAJ. 2018;190(26):E786-E793.
127. Cartwright C, Gibson K, Read J, et al. Long-term antidepressant use: patient perspectives of benefits and adverse effects. Patient Prefer Adherence. 2016;10:1401-1407.
128. American Society of Addiction Medicine. Public policy statement: definition of addiction. https://www.asam.org/docs/default-source/public-policy-statements/1definition_of_addiction_long_4-11.pdf?sfvrsn=a8f64512_4. Published August 15, 2011. Accessed July 23, 2018.
1. Zemishlany Z, Aizenberg D, Weiner Z, et al. Trihexyphenidyl (Artane) abuse in schizophrenic patients. Int Clin Psychopharmacol. 1996;11(3):199-202.
2. Crawshaw JA, Mullen PE. A study of benzhexol abuse. Brit J Psychiatry. 1984;145:300-303.
3. Woody GE, O’Brien CP. Anticholinergic toxic psychosis in drug abusers treated with benztropine. Comp Psychiatry. 1974;15(5):439-442.
4. Lowry TP. Trihexyphenidyl abuse. Am J Psychiatry. 1977;134(11):1315.
5. Rouchell AM, Dixon SP. Trihexyphenidyl abuse. Am J Psychiatry. 1977;134(11):1315.
6. Kaminer Y, Munitz H, Wijsenbeek H. Trihexyphenidyl (Artane) abuse: euphoriant and anxiolytic. Brit J Psychiatry. 1982;140(5):473-474.
7. Nappo SA, de Oliviera LG, Sanchez Zv, et al. Trihexyphenidyl (Artane): a Brazilian study of its abuse. Subst Use Misuse. 2005;40(4):473-482.
8. Pullen GP, Best NR, Macguire J. Anticholinergic drug abuse: a common problem? Brit Med J (Clin Res Ed). 1984;289(6445):612-613.
9. Rubinstein JS. Abuse of antiparkinsonian drugs: feigning of extrapyramidal symptoms to obtain trihexyphenidyl. JAMA. 1978;239(22):2365-2366.
10. Mohan D, Mohandas E, Dube S. Trihexyphenidyl abuse. Brit J Addiction. 1981:76(2);195-197.
11. Marken PA, Stoner SC, Bunker MT. Anticholinergic drug abuse and misuse. CNS Drugs. 1996;5(3):190-199.
12. Buhrich N, Weller A, Kevans P. Misuse of anticholinergic drugs by people with serious mental illness. Psychiatric Serv. 2000;51(7):928-929.
13. Goldstein MR, Kasper R. Hyperpyrexia and coma due to overdose of benztropine. South Med J. 1968;61(9):984.
14. Petkovi
15. McIntyre IM, Mallett P, Burton CG, et al. Acute benztropine intoxication and fatality. J Forensic Sci. 2014;59(6):1675-1678.
16. Dilsaver SC. Antimuscarinic agents as substances of abuse: A review. J Clin Psychopharmacol. 1988:8(1):14-22.
17. Haddad P. Do antidepressants have any potential to cause addiction? J Psychopharmacol. 1999;13(3):300-307.
18. Haddad PM. Do antidepressants cause dependence? Epidemiol Psichiatr Soc. 2005;14(2):58-62.
19. Shenouda R, Desan PH. Abuse of tricyclic antidepressant drugs: a case series. J Clin Psychopharmacol. 2013;33(3):440-442.
20. van Broekhoven F, Kan CC, Zitman FG. Dependence potential of antidepressants compared to benzodiazepines. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(5):939-943.
21. Evans EA, Sullivan MA. Abuse and misuse of antidepressants. Subst Abuse Rehabil. 2014;5:107-120.
22. Warner CH, Bobo W, Warner C, et al. Antidepressant discontinuation syndrome. Am Fam Physician. 2006;74(3):449-456.
23. Lichtigfeld FJ, Gillman MA. Antidepressants are not drugs of abuse or dependence. Postgrad Med J. 1998;74(875):529-532.
24. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
25. Read J, Cartwright C, Gibson K. Adverse emotional and interpersonal effects reported by 1829 New Zealanders while taking antidepressants. Psychiatry Res. 2014;216(1):67-73.
26. Haddad P, Anderson I. Antidepressants aren’t addictive: clinicians have depended on them for years. J Psychopharmacol. 1999;13(3):291-292.
27. Carey B, Gebeloff R. Many people taking antidepressants discover they cannot quit. New York Times. https://www.nytimes.com/2018/04/07/health/antidepressants-withdrawal-prozac-cymbalta.html. Published April 7, 2018. Accessed December 11, 2018.
28. Kim D, Steinhart B. Seizures induced by recreational abuse of bupropion tablets via nasal insufflation. CJEM. 2010;12(2):158-161.
29. McCormick J. Recreational bupropion in a teenager. Br J Clin Pharmacol. 2002;53(2):214.
30. Welsh C, Doyon S. Seizure induced by insufflation of bupropion. N Engl J Med. 2002; 347(2):951.
31. Baribeau D, Araki KF. Intravenous bupropion: A previously undocumented method of abuse of a commonly prescribed antidepressant agent. J Addict Med. 2013;7(3):216-217.
32. Hill SH, Sikand H, Lee J. A case report of seizure induced by bupropion nasal insufflation. Prim Care Companion J Clin Psych. 2007;9(1):67-69.
33. Yoon G, Westermeyer J. Intranasal bupropion abuse. Am J Addict. 2013;22(2):180.
34. Reeves RR, Ladner ME. Additional evidence of the abuse potential of bupropion. J Clin Psychopharmacol. 2013;33(4):584-585.
35. Oppek K, Koller G, Zwergal A, et al. Intravenous administration and abuse of bupropion: a case report and a review of the literature. J Addict Med. 2014;8(4):290-293.
36. Strike M, Hatcher S. Bupropion injection resulting in tissue necrosis and psychosis: previously undocumented complications of intravenous bupropion use disorder. J Addict Med. 2015;9(3):246-250.
37. Schifano F, Chiappini S. Is there a potential of misuse for venlafaxine and bupropion? Front Pharmacol. 2018;9:239.
38. Tryon J, Logan N. Antidepressant Wellbutrin becomes ‘poor man’s cocaine’ on Toronto streets. Global News. https://globalnews.ca/news/846576/antidepressant-wellbutrin-becomes-poor-mans-cocaine-on-toronto-streets/. Published September 18, 2013. Accessed December 11, 2018.
39. Stassinos GL, Klein-Schwartz W. Bupropion “abuse” reported to US Poison Centers. J Addict Med. 2016;10(5):357-362.
40. Hilliard WT, Barloon L, Farley P, et al. Bupropion diversion and misuse in the correctional facility. J Correct Health Care. 2013;19(3):211-217.
41. Griffith JD, Carranza J, Griffith C, et al. Bupropion clinical assay for amphetamine-like abuse potential. J Clin Psychiatry.1983;44(5 Pt 2):206-208.
42. Miller L, Griffith J. A comparison of bupropion, dextroamphetamine, and placebo in mixed-substance abusers. Psychopharmacol (Berl). 1983;80(3):199-205.
43. Berigan TR, Russell ML. Treatment of methamphetamine cravings with bupropion: A case report. Prim Care Companion J Clin Psychiatry. 2001;3(6):267-268.
44. Tardieu T, Poirier Y, Micallef J, et al. Amphetamine-like stimulant cessation in an abusing patient treated with bupropion. Acta Psychiatr Scand. 2004;109(1):75-78.
45. Newton TF, Roache JD, De La Garza R, et al. Bupropion reduces methamphetamine-induced subjective effects and cue-induced cravings. Neuropsychopharmacology. 2006;31(7):1537-1544.
46. Margolin A, Kosten TR, Avants SK, et al. A multicenter trial for cocaine dependence in methadone-maintained patients. Drug Alcohol Depend. 1995;40(2):125-131.
47. Shoptaw S, Heinzerling KG, Rotheram-Fuller E, et al. Bupropion hydrochloride versus placebo, in combination with cognitive behavioral therapy, for the treatment of cocaine abuse/dependence. J Addict Dis. 2008;27(1):13-23.
48. Anderson AL, Li S, Markova D, et al. Bupropion for the treatment of methamphetamine dependence in non-daily users: a randomized, double-blind placebo-controlled trial. Drug Alcohol Depend. 2015;150:170-174.
49. Shoptaw S, Heinzerling KG, Rotheram-Fuller E, et al. Randomized, placebo-controlled trial of bupropion for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2008;96(3):222-232.
50. Elkashef AM, Rawson RA, Anderson AL, et al. Bupropion for the treatment of methamphetamine dependence. Neuropsychopharmacology. 2008;33(5):1162-1170.
51. Heinzerling KG, Swanson A, Hall TM, et al. Randomized, placebo-controlled trial of bupropion in methamphetamine-dependent participants with less than daily methamphetamine use. Addiction. 2014;109(11):1878-1886.
52. Doenecke AL, Heuerman RC. Treatment of haloperidol abuse with diphenhydramine. Am J Psychiatry. 1980;137(4):487-488.
53. Weddington WW, Leventhal BL. Sadistic abuse of haloperidol. Am J Psychiatry. 1982;139:132-133.
54. Basu D, Marudkar M, Khurana H. Abuse of neuroleptic drugs by psychiatric patients. Indian J Med Sci. 2000;54(2):59-62.
55. Pierre JM, Shnayder I, Wirshing DA, et al. Intranasal quetiapine abuse. Am J Psychiatry 2004;161(9):1718.
56. Reeves RR. Abuse of olanzapine by substance abusers. J Psychoactive Drugs. 2007;39(3):297-299.
57. Kumsar NA, Erol A. Olanzapine abuse. Subst Abus. 2013;34(1):73-74.
58. Lai C. Olanzapine abuse was relieved after switching to aripiprazole in a patient with psychotic depression. Prog Neuropsychpharmacol Biol Psychiatry. 2010;34(7):1363-1364.
59. James PD, Fida AS, Konovalov P, et al. Non-medical use of olanzapine by people on methadone treatment. BJPsych Bull. 2016;40(6):314-317.
60. Reeves RR, Brister JC. Additional evidence of the abuse potential of quetiapine. South Med J. 2007;100(8):834-836.
61. Murphy D, Bailey K, Stone M, et al. Addictive potential of quetiapine. Am J Psychiatry. 2008;165(7):918.
62. Paparrigopoulos T, Karaiskos D, Liappas J. Quetiapine: another drug with potential for misuse? J Clin Psychiatry. 2008;69(1):162-163.
63. Reeves RR, Burke RS. Abuse of the combination of gabapentin and quetiapine. Prim Care Companion CNS Disord. 2014;16(5): doi: 10.4088/PCC.14l01660.
64. Morin AK. Possible intranasal quetiapine misuse. Am J Health Syst Pharm. 2007;64(7):723-725.
65. Caniato RN, Gundabawady A, Baune BT, et al. Malingered psychotic symptoms and quetiapine abuse in a forensic setting. J Forens Psychiatr Psychol. 2009;20(6):928-935.
66. Hussain MZ, Waheed W, Hussain S. Intravenous quetiapine abuse. Am J Psychiatry. 2005; 162(9):1755-1756.
67. Waters BM, Joshi KG. Intravenous quetiapine-cocaine use (“Q-ball”). Am J Psychiatry. 2007;164(1):173-174.
68. Haridas A, Kushon D, Gurmu S, et al. Smoking quetiapine: a “Maq ball?” Prim Psychiatry. 2010;17:38-39.
69. Cubala WJ, Springer J. Quetiapine abuse and dependence in psychiatric patients: a systematic review of 25 case reports in the literature. J Subs Use. 2014;19(5):388-393.
70. Piróg-Balcerzak A, Habrat B, Mierzejewski P. Misuse and abuse of quetiapine [in Polish]. Psychiatr Pol. 2015;49(1):81-93.
71. Pinta ER, Taylor RE. Quetiapine addiction? Am J Psychiatry. 2007;164(1):174.
72. Tamburello AC, Lieberman JA, Baum RM, et al. Successful removal of quetiapine from a correctional formulary. J Amer Acad Psychiatr Law. 2012;40(4):502-508.
73. Tarasoff G, Osti K. Black-market value of antipsychotics, antidepressants, and hypnotics in Las Vegas, Nevada. Am J Psychiatry. 2007;164(2):350.
74. Reccoppa L. Less abuse potential with XR formulation of quetiapine. Am J Addiction. 2010;20(2):178.
75. McLarnon ME, Fulton HG, MacIsaac C, et al. Characteristics of quetiapine misuse among clients of a community-based methadone maintenance program. J Clin Psychopharmacol. 2012;32(5):721-723.
76. Reddel SE, Bruno R, Burns L, et al. Prevalence and associations of quetiapine fumarate misuse among an Australian national city sample of people who regularly inject drugs. Addiction. 2013;109(2):295-302.
77. Malekshahi T, Tioleco N, Ahmed N, et al. Misuse of atypical antipsychotics in conjunction with alcohol and other drugs of abuse. J Subs Abuse Treat. 2015;48(1):8-12.
78. Klein-Schwartz W, Schwartz EK, Anderson BD. Evaluation of quetiapine abuse and misuse reported to poison centers. J Addict Med. 2014;8(3):195-198.
79. Klein L, Bangh S, Cole JB. Intentional recreational abuse of quetiapine compared to other second-generation antipsychotics. West J Emerg Med. 2017;18(2):243-250.
80. Chiappini S, Schifano F. Is there a potential of misuse for quetiapine?: Literature review and analysis of the European Medicines Agency/European Medicines Agency Adverse Drug Reactions’ Database. J Clin Psychopharmacol. 2018;38(1):72-79.
81. Lee J, Pilgrim J, Gerostamoulos D, et al. Increasing rates of quetiapine overdose, misuse, and mortality in Victoria, Australia. Drug Alcohol Depend. 2018;187:95-99.
82. Mattson ME, Albright VA, Yoon J, et al. Emergency department visits involving misuse and abuse of the antipsychotic quetiapine: Results from the
83. Brutcher RE, Nader SH, Nader MA. Evaluation of the reinforcing effect of quetiapine, alone and in combination with cocaine, in rhesus monkeys. J Pharmacol Exp Ther. 2016;356(2):244-250.
84. Kim DR, Staab JP. Quetiapine discontinuation syndrome. Am J Psychiatry. 2005;162(5):1020.
85. Thurstone CC, Alahi P. A possible case of quetiapine withdrawal syndrome. J Clin Psychiatry. 2000;61(8):602-603.
86. Kohen I, Kremen N. A case report of quetiapine withdrawal syndrome in a geriatric patient. World J Biol Psychiatry. 2009;10(4 pt 3):985-986.
87. Yargic I, Caferov C. Quetiapine dependence and withdrawal: a case report. Subst Abus. 2011;32(3):168-169.
88. Koch HJ. Severe quetiapine withdrawal syndrome with nausea and vomiting in a 65-year-old patient with psychotic depression. Therapie. 2015;70(6):537-538.
89. Fischer BA, Boggs DL. The role of antihistaminic effects in the misuse of quetiapine: a case report and review of the literature. Neurosci Biobehav Rev. 2010;34(4):555-558.
90. Longoria J, Brown ES, Perantie DC, et al. Quetiapine for alcohol use and craving in bipolar disorder. J Clin Psychopharmacol. 2004;24(1):101-102.
91. Monnelly EP, Ciraulo DA, Knapp C, et al. Quetiapine for treatment of alcohol dependence. J Clin Psychopharmacol. 2004;24(5):532-535.
92. Kennedy A, Wood AE, Saxon AJ, et al. Quetiapine for the treatment of cocaine dependence: an open-label trial. J Clin Psychopharmacol. 2008;28(2):221-224.
93. Mariani JJ, Pavlicova M, Mamczur A, et al. Open-label pilot study of quetiapine treatment for cannabis dependence. Am J Drug Alcohol Abuse. 2014;40(4):280-284.
94. Guardia J, Roncero C, Galan J, et al. A double-blind, placebo-controlled, randomized pilot study comparing quetiapine with placebo, associated to naltrexone, in the treatment of alcohol-dependent patients. Addict Behav. 2011;36(3):265-269.
95. Litten RZ, Fertig JB, Falk DE, et al; NCIG 001 Study Group. A double-blind, placebo-controlled trial to assess the efficacy of quetiapine fumarate XR in very heavy-drinking alcohol-dependent patients. Alcohol Clin Exp Res. 2012;36(3):406-416.
96. Tapp A, Wood AE, Kennedy A, et al. Quetiapine for the treatment of cocaine use disorder. Drug Alcohol Depend. 2015;149:18-24.
97. Markowitz JS, Finkenbine R, Myrick H, et al. Gabapentin abuse in a cocaine user: Implications for treatment. J Clin Psychopharmacol. 1997;17(5):423-424.
98. Reccoppa L, Malcolm R, Ware M. Gabapentin abuse in inmates with prior history of cocaine dependence. Am J Addict. 2004;13(3):321-323.
99. Victorri-Vigneau C, Guelais M, Jolliet P. Abuse, dependency and withdrawal with gabapentin: a first case report. Pharmacopsychiatry. 2007;40(1):43-44.
100. Bonnet U, Sherbaum N. How addictive are gabapentin and pregabalin? A systematic review. Eur Neuropsychopharmacol. 2017;27(12):1185-1215.
101. Schifano F, D’Offizi S, Piccione M, et al. Is there a recreational misuse potential for pregabalin? Analysis of anecdotal online reports in comparison with related gabapentin and clonazepam data. Psychother Psychosom. 2011;80(2):118-122.
102. Evoy KE, Morrison MD, Saklad SR. Abuse and misuse of pregabalin and gabapentin. Drugs. 2017;77(4):403-426.
103. Smith RV, Havens JR, Walsh SL. Gabapentin misuse, abuse and diversion: a systematic review. Addiction. 2016;111(7):1160-1174.
104. Chiappini S, Shifano F. A decade of gabapentinoid misuse: an analysis of the European Medicines Agency’s ‘suspected adverse drug reactions’ database. CNS Drugs. 2016;30(7):647-654.
105. Buttram ME, Kurtz SP, Dart R, et al. Law enforcement-derived data on gabapentin diversion and misuse, 2002-2015: diversion rates and qualitative research findings. Pharmacoepidemiol Drug Saf. 2017;26(9):1083-1086.
106. Kapil V, Green JL, Le Lait M, et al. Misuse of the y-aminobutyric acid analogues baclofen, gabapentin and pregabalin in the UK. Br J Clin Pharmacol. 2013;78(1):190-191.
107. Peckham AM, Fairman KA, Sclar DA. Prevalence of gabapentin abuse: comparison with agents with known abuse potential in a commercially insured US population. Clin Drug Invest. 2017;37(8):763-773.
108. Smith RV, Lofwall MR, Havens JR. Abuse and diversion of gabapentin among nonmedical prescription opioid users in Appalachian Kentucky. Am J Psychiatry. 2015;172(5):487-488.
109. Peckham AM, Evoy KE, Covvey JR, et al. Predictors of gabapentin overuse with or without concomitant opioids in a commercially insured U.S. population. Pharmacotherapy. 2018;38(4):436-443.
110. Smith BH, Higgins C, Baldacchino A, et al. Substance misuse of gabapentin. Br J Gen Pract. 2012;62(601):401-407.
111. Baird CRW, Fox P, Colvin LA. Gabapentinoid abuse in order to potentiate the effect of methadone: a survey among substance misusers. Eur Addict Res. 2014;20(3):115-118.
112. Lyndon A, Audrey S, Wells C, et al. Risk to heroin users of polydrug use of pregabalin or gabapentin. Addiction. 2017;112(9):1580-1589.
113. Peckham AM, Fairman KA, Sclar DA. All-cause and drug-related medical events associated with overuse of gabapentin and/or opioid medications: a retrospective cohort analysis of a commercially insured US population. Drug Saf. 2018;41(2):213-228.
114. Gomes T, Juurlink DN, Antoniou T, et al. Gabapentin, opioids, and the risk of opioid-related death: a population-based nested case-control study. PLoS Med. 2017;14(10):e10022396. doi: 10.1371/journal.pmed.1002396.
115. Peckham AM, Fairman K, Sclar DA. Policies to mitigate nonmedical use of prescription medications: how should emerging evidence of gabapentin misuse be addressed? Exp Opin Drug Saf. 2018;17(5):519-523.
116. Raby WN. Gabapentin for cocaine cravings. Am J Psychiatry. 2000;157(12):2058-2059.
117. Myrick H, Henderson S, Brady KT, et al. Gabapentin in the treatment of cocaine dependence: a case series. J CLin Psychiatry. 2001;62(1):19-23.
118. Raby WN, Coomaraswamy S. Gabapentin reduces cocaine use among addicts from a community clinic sample. J Clin Psychiatry. 2004;65(1):84-86.
119. Hart CL, Ward AS, Collins ED, et al. Gabapentin maintenance decreases smoked cocaine-related subjective effects, but not self-administration by humans. Drug Alcohol Depend. 2004;73(3):279-287.
120. Bisaga A, Aharonovich E, Garawi F, et al. A randomized placebo-controlled trial of gabapentin for cocaine dependence. Drug Alc Depend. 2006;81(3):267-274.
121. Hart CL, Haney M, Collins ED, et al. Smoked cocaine self-administration by humans is not reduced by large gabapentin maintenance doses. Drug Alcohol Depend. 2007;86(2-3):274-277.
122. Furieri FA, Nakamura-Palacios EM. Gabapentin reduces alcohol consumption and craving: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2007;68(11):1691-1700.
123. Mason BJ, Quello S, Goodell V, et al. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77.
124. Martinotti G, Di Nicola M, Tedeschi D, et al. Pregabalin versus naltrexone in alcohol dependence: a randomised, double-blind, comparison trial. J Psychopharmacol. 2010;24(9):1367-1374.
125. Mason BJ, Crean R, Goodell V, et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychpharmacology. 2012;27(7):1689-1698.
126. Enke O, New HA, New CH, et al. Anticonvulsants in the treatment of low back pain and lumbar radicular pain: a systematic review and meta-analysis. CMAJ. 2018;190(26):E786-E793.
127. Cartwright C, Gibson K, Read J, et al. Long-term antidepressant use: patient perspectives of benefits and adverse effects. Patient Prefer Adherence. 2016;10:1401-1407.
128. American Society of Addiction Medicine. Public policy statement: definition of addiction. https://www.asam.org/docs/default-source/public-policy-statements/1definition_of_addiction_long_4-11.pdf?sfvrsn=a8f64512_4. Published August 15, 2011. Accessed July 23, 2018.
How I Became a Derm Guru (And How You Can, Too)
Many years ago, when I was still in primary care (internal medicine), I thought I knew a bit about the practice of medicine. I was totally comfortable in the hospital (in those days, we saw our own patients twice a day in the hospital), including the ER, the OR, even obstetrics. MIs, shootings, stab wounds, renal failure—I would never say I had mastered them, but I was comfortable with most of what I saw. Deliveries, assisting with C-sections, performing lumbar punctures, performing and interpreting exercise tolerance tests, performing flexible sigmoidoscopies—no problem.
But the one thing that nearly always stopped me in my tracks was … you guessed it: dermatology complaints. Rashes, lesions, or any other skin complaint the least bit out of the ordinary were completely baffling to me. I still remember that feeling after all these years (and I still occasionally experience it!).
I felt like saying to those patients: What in the world would make you think I’d have any idea what that is? But of course, I couldn’t say that, so I’d mumble something, throw some cream at it, then quickly change the subject. Mind you, this was in a setting where a derm referral from us would take 4 to 6 months. And in case you’re wondering, the other providers in my department were as bad at derm as I was.
Long story short, it got to the point that I would scan my schedule every morning, praying I wouldn’t see the word “rash” or “skin.” But, of course, they still came—often just as my hand touched the doorknob to leave: “Oh, by the way, what about this …?” You get the picture. Many of you, if not most, live that picture.
I finally got up the nerve to go to our dermatology department to ask if I could follow one of the docs while he saw patients. Little did I know that practically every provider in the building had already done the same, and had been dismissed with words that essentially meant, “You? A mere PA? You can’t get there from here. Just send ’em to us.”
For a short time, I bought that line—but in the meantime, my patients were not getting the care they needed. So, driven in part by anger at the notion that a mere PA was simply unable to learn dermatology, I bought a decent textbook, Fitzpatrick’s Color Atlas of Dermatology, and started reading it. I also started collecting all the derm articles I could find in the journals, and read about those cases.
I won’t bore you with the grimy details, but what I did differently was work at learning derm (what a concept!). I started going to derm conferences, bought a good camera and started taking pictures with it, and continued to buy books (this was in the pre-computer days of the ’80s) and actually read them.
Continue to: And a funny thing happened...
And a funny thing happened: The more I read, the more diagnoses I recognized on my patients. My colleagues and the clinic schedulers took note of this and began sending me their problem cases. Even the derm department, beleaguered as usual by huge backlogs of patients, started sending patients to me. By 1985, even though I was in the internal medicine department, I had transitioned to doing derm fulltime. And that’s what I’ve been doing since.
Around 1992, I discovered that I was one of 6 dermatology PAs in this country. Last time I checked, our numbers were approaching 4,000. So, yes, derm is indeed difficult, but rocket science it isn’t.
Being the pedantic sort that I am, and finding that whole experience so enlightening, I resolved to make it my mission to foster the use of PAs in dermatology—part of which involves the education of those PAs, by means of taking students but also by writing articles (several hundred at last count) and lecturing at conferences and at PA programs. Nearing retirement, I only practice two days a week, but I write and publish at least 5 clinical articles a month, all of which are based on real cases: my cases, using my photos, doing new research on each case. This keeps my knowledge fresh and my 75-year-old mind sharp, helps ward off burnout, and, most importantly, saves lives while reducing patient discomfort.
What follows are 10 dermatology pearls that I have gleaned along the way. My apologies to my former students and attendees at my lectures who’ve heard all this before:
1 If the treatment for your diagnosis isn’t working, consider another diagnosis. Here’s an example (Figure 1): A man in his 50s was sent to dermatology for psoriasis that wasn’t responding to a biologic. Was it really psoriasis? A KOH prep quickly showed it to be tinea corporis, which cleared completely with a month’s worth of oral terbinafine (250 mg qid).
Continue to: #2...
2 The correct diagnosis dictates correct treatment. This may sound obvious, but in primary care, the emphasis is often on “let’s try this” or “let’s try that,” an understandable approach to a symptomatic patient with an uncertain diagnosis. But by the time he finally gets to dermatology, the patient has tried a whole bag full of prescription and OTC products given for numerous, totally different diagnoses. A better approach might be to expedite an urgent referral to dermatology, when possible.
3 Cutaneous fungal infections (ie, dermatophytosis) are vastly overdiagnosed, especially by novices. If you truly suspect it, ask about a potential source; one doesn’t acquire a fungal infection out of thin air. It must come from a person, animal, or occasionally, the soil. It also helps if the victim has been rendered susceptible by the injudicious use of steroids. Better yet, find the fungus with a microscopic examination (KOH prep) or culture. Finally, remember, not everything round and scaly is fungal (see Figure 2).
4 Remember these ancient words of wisdom regarding skin complaints: (a) A diagnosis is seldom made if not entertained, (b) you won’t entertain it if you’ve never heard of it, (c) you will not see it if you’re not looking for it, and (d) even if you did see it, you would not “see” it because you’re not looking for it. Dermatology is far deeper and wider than most imagine it to be. The trick is to expose yourself to as many different diagnoses as possible, by reading and attending lectures, ahead of the possible sighting. Figures 3 and 4 offer examples of common conditions that are seldom recognized outside dermatology.
5 Skin cancer can present as a rash. Examples abound, such as mammary and extramammary Paget’s disease (Figure 5), mycosis fungoides, metastatic breast cancer (Figure 6), and superficial basal cell carcinoma. A biopsy is usually required to diagnose these, but you wouldn’t think to do that if you’d never heard of the condition.
6 Melanoma doesn’t typically arise from a mole or other pre-existing lesion. Far more often, it arises “de novo,” out of nothing. So, in general, we’re not worried about “moles” (nevi) unless there’s a history of change (see Figure 7).
Continue to: #7...
7 When looking for skin cancer, pay as much attention to the owner as to the lesion. The most common skin cancers—basal cell and squamous cell carcinoma—usually occur on sun-damaged, fair-skinned, blue-eyed older patients. Though there are certainly exceptions to this paradigm, it pays to be generally suspicious of any odd lesion seen on these patients (Figure 8).
8 It’s practically impossible to overstate the role of atopy when evaluating pediatric skin complaints. These children—20% of all newborns!—are born with thin, dry, sensitive, overreactive skin that is prone to eczema and urticaria. They will also have a marked tendency to develop seasonal allergies, allergic rhinitis, and asthma. Parents find it difficult to accept the genetic basis for atopic dermatitis (Figure 9), preferring instead to blame everything on laundry detergent or food. Education (of oneself first!) is the key.
9 “Infections” are not always what they seem
10 Overcome your fear of steroids by educating yourself about their safe use. Glucocorticoids (eg, triamcinolone, prednisone, betamethasone) are extremely useful in treating common derm conditions. We see patients every day who are so frightened of steroids, they won’t even consider using them because some well-meaning medical provider scared them to death. The proper use of these miraculous products could easily be the subject of an entire article. For now, I’ll advise you to read about their safe use in any number of dermatology texts (including online publications).
Many years ago, when I was still in primary care (internal medicine), I thought I knew a bit about the practice of medicine. I was totally comfortable in the hospital (in those days, we saw our own patients twice a day in the hospital), including the ER, the OR, even obstetrics. MIs, shootings, stab wounds, renal failure—I would never say I had mastered them, but I was comfortable with most of what I saw. Deliveries, assisting with C-sections, performing lumbar punctures, performing and interpreting exercise tolerance tests, performing flexible sigmoidoscopies—no problem.
But the one thing that nearly always stopped me in my tracks was … you guessed it: dermatology complaints. Rashes, lesions, or any other skin complaint the least bit out of the ordinary were completely baffling to me. I still remember that feeling after all these years (and I still occasionally experience it!).
I felt like saying to those patients: What in the world would make you think I’d have any idea what that is? But of course, I couldn’t say that, so I’d mumble something, throw some cream at it, then quickly change the subject. Mind you, this was in a setting where a derm referral from us would take 4 to 6 months. And in case you’re wondering, the other providers in my department were as bad at derm as I was.
Long story short, it got to the point that I would scan my schedule every morning, praying I wouldn’t see the word “rash” or “skin.” But, of course, they still came—often just as my hand touched the doorknob to leave: “Oh, by the way, what about this …?” You get the picture. Many of you, if not most, live that picture.
I finally got up the nerve to go to our dermatology department to ask if I could follow one of the docs while he saw patients. Little did I know that practically every provider in the building had already done the same, and had been dismissed with words that essentially meant, “You? A mere PA? You can’t get there from here. Just send ’em to us.”
For a short time, I bought that line—but in the meantime, my patients were not getting the care they needed. So, driven in part by anger at the notion that a mere PA was simply unable to learn dermatology, I bought a decent textbook, Fitzpatrick’s Color Atlas of Dermatology, and started reading it. I also started collecting all the derm articles I could find in the journals, and read about those cases.
I won’t bore you with the grimy details, but what I did differently was work at learning derm (what a concept!). I started going to derm conferences, bought a good camera and started taking pictures with it, and continued to buy books (this was in the pre-computer days of the ’80s) and actually read them.
Continue to: And a funny thing happened...
And a funny thing happened: The more I read, the more diagnoses I recognized on my patients. My colleagues and the clinic schedulers took note of this and began sending me their problem cases. Even the derm department, beleaguered as usual by huge backlogs of patients, started sending patients to me. By 1985, even though I was in the internal medicine department, I had transitioned to doing derm fulltime. And that’s what I’ve been doing since.
Around 1992, I discovered that I was one of 6 dermatology PAs in this country. Last time I checked, our numbers were approaching 4,000. So, yes, derm is indeed difficult, but rocket science it isn’t.
Being the pedantic sort that I am, and finding that whole experience so enlightening, I resolved to make it my mission to foster the use of PAs in dermatology—part of which involves the education of those PAs, by means of taking students but also by writing articles (several hundred at last count) and lecturing at conferences and at PA programs. Nearing retirement, I only practice two days a week, but I write and publish at least 5 clinical articles a month, all of which are based on real cases: my cases, using my photos, doing new research on each case. This keeps my knowledge fresh and my 75-year-old mind sharp, helps ward off burnout, and, most importantly, saves lives while reducing patient discomfort.
What follows are 10 dermatology pearls that I have gleaned along the way. My apologies to my former students and attendees at my lectures who’ve heard all this before:
1 If the treatment for your diagnosis isn’t working, consider another diagnosis. Here’s an example (Figure 1): A man in his 50s was sent to dermatology for psoriasis that wasn’t responding to a biologic. Was it really psoriasis? A KOH prep quickly showed it to be tinea corporis, which cleared completely with a month’s worth of oral terbinafine (250 mg qid).
Continue to: #2...
2 The correct diagnosis dictates correct treatment. This may sound obvious, but in primary care, the emphasis is often on “let’s try this” or “let’s try that,” an understandable approach to a symptomatic patient with an uncertain diagnosis. But by the time he finally gets to dermatology, the patient has tried a whole bag full of prescription and OTC products given for numerous, totally different diagnoses. A better approach might be to expedite an urgent referral to dermatology, when possible.
3 Cutaneous fungal infections (ie, dermatophytosis) are vastly overdiagnosed, especially by novices. If you truly suspect it, ask about a potential source; one doesn’t acquire a fungal infection out of thin air. It must come from a person, animal, or occasionally, the soil. It also helps if the victim has been rendered susceptible by the injudicious use of steroids. Better yet, find the fungus with a microscopic examination (KOH prep) or culture. Finally, remember, not everything round and scaly is fungal (see Figure 2).
4 Remember these ancient words of wisdom regarding skin complaints: (a) A diagnosis is seldom made if not entertained, (b) you won’t entertain it if you’ve never heard of it, (c) you will not see it if you’re not looking for it, and (d) even if you did see it, you would not “see” it because you’re not looking for it. Dermatology is far deeper and wider than most imagine it to be. The trick is to expose yourself to as many different diagnoses as possible, by reading and attending lectures, ahead of the possible sighting. Figures 3 and 4 offer examples of common conditions that are seldom recognized outside dermatology.
5 Skin cancer can present as a rash. Examples abound, such as mammary and extramammary Paget’s disease (Figure 5), mycosis fungoides, metastatic breast cancer (Figure 6), and superficial basal cell carcinoma. A biopsy is usually required to diagnose these, but you wouldn’t think to do that if you’d never heard of the condition.
6 Melanoma doesn’t typically arise from a mole or other pre-existing lesion. Far more often, it arises “de novo,” out of nothing. So, in general, we’re not worried about “moles” (nevi) unless there’s a history of change (see Figure 7).
Continue to: #7...
7 When looking for skin cancer, pay as much attention to the owner as to the lesion. The most common skin cancers—basal cell and squamous cell carcinoma—usually occur on sun-damaged, fair-skinned, blue-eyed older patients. Though there are certainly exceptions to this paradigm, it pays to be generally suspicious of any odd lesion seen on these patients (Figure 8).
8 It’s practically impossible to overstate the role of atopy when evaluating pediatric skin complaints. These children—20% of all newborns!—are born with thin, dry, sensitive, overreactive skin that is prone to eczema and urticaria. They will also have a marked tendency to develop seasonal allergies, allergic rhinitis, and asthma. Parents find it difficult to accept the genetic basis for atopic dermatitis (Figure 9), preferring instead to blame everything on laundry detergent or food. Education (of oneself first!) is the key.
9 “Infections” are not always what they seem
10 Overcome your fear of steroids by educating yourself about their safe use. Glucocorticoids (eg, triamcinolone, prednisone, betamethasone) are extremely useful in treating common derm conditions. We see patients every day who are so frightened of steroids, they won’t even consider using them because some well-meaning medical provider scared them to death. The proper use of these miraculous products could easily be the subject of an entire article. For now, I’ll advise you to read about their safe use in any number of dermatology texts (including online publications).
Many years ago, when I was still in primary care (internal medicine), I thought I knew a bit about the practice of medicine. I was totally comfortable in the hospital (in those days, we saw our own patients twice a day in the hospital), including the ER, the OR, even obstetrics. MIs, shootings, stab wounds, renal failure—I would never say I had mastered them, but I was comfortable with most of what I saw. Deliveries, assisting with C-sections, performing lumbar punctures, performing and interpreting exercise tolerance tests, performing flexible sigmoidoscopies—no problem.
But the one thing that nearly always stopped me in my tracks was … you guessed it: dermatology complaints. Rashes, lesions, or any other skin complaint the least bit out of the ordinary were completely baffling to me. I still remember that feeling after all these years (and I still occasionally experience it!).
I felt like saying to those patients: What in the world would make you think I’d have any idea what that is? But of course, I couldn’t say that, so I’d mumble something, throw some cream at it, then quickly change the subject. Mind you, this was in a setting where a derm referral from us would take 4 to 6 months. And in case you’re wondering, the other providers in my department were as bad at derm as I was.
Long story short, it got to the point that I would scan my schedule every morning, praying I wouldn’t see the word “rash” or “skin.” But, of course, they still came—often just as my hand touched the doorknob to leave: “Oh, by the way, what about this …?” You get the picture. Many of you, if not most, live that picture.
I finally got up the nerve to go to our dermatology department to ask if I could follow one of the docs while he saw patients. Little did I know that practically every provider in the building had already done the same, and had been dismissed with words that essentially meant, “You? A mere PA? You can’t get there from here. Just send ’em to us.”
For a short time, I bought that line—but in the meantime, my patients were not getting the care they needed. So, driven in part by anger at the notion that a mere PA was simply unable to learn dermatology, I bought a decent textbook, Fitzpatrick’s Color Atlas of Dermatology, and started reading it. I also started collecting all the derm articles I could find in the journals, and read about those cases.
I won’t bore you with the grimy details, but what I did differently was work at learning derm (what a concept!). I started going to derm conferences, bought a good camera and started taking pictures with it, and continued to buy books (this was in the pre-computer days of the ’80s) and actually read them.
Continue to: And a funny thing happened...
And a funny thing happened: The more I read, the more diagnoses I recognized on my patients. My colleagues and the clinic schedulers took note of this and began sending me their problem cases. Even the derm department, beleaguered as usual by huge backlogs of patients, started sending patients to me. By 1985, even though I was in the internal medicine department, I had transitioned to doing derm fulltime. And that’s what I’ve been doing since.
Around 1992, I discovered that I was one of 6 dermatology PAs in this country. Last time I checked, our numbers were approaching 4,000. So, yes, derm is indeed difficult, but rocket science it isn’t.
Being the pedantic sort that I am, and finding that whole experience so enlightening, I resolved to make it my mission to foster the use of PAs in dermatology—part of which involves the education of those PAs, by means of taking students but also by writing articles (several hundred at last count) and lecturing at conferences and at PA programs. Nearing retirement, I only practice two days a week, but I write and publish at least 5 clinical articles a month, all of which are based on real cases: my cases, using my photos, doing new research on each case. This keeps my knowledge fresh and my 75-year-old mind sharp, helps ward off burnout, and, most importantly, saves lives while reducing patient discomfort.
What follows are 10 dermatology pearls that I have gleaned along the way. My apologies to my former students and attendees at my lectures who’ve heard all this before:
1 If the treatment for your diagnosis isn’t working, consider another diagnosis. Here’s an example (Figure 1): A man in his 50s was sent to dermatology for psoriasis that wasn’t responding to a biologic. Was it really psoriasis? A KOH prep quickly showed it to be tinea corporis, which cleared completely with a month’s worth of oral terbinafine (250 mg qid).
Continue to: #2...
2 The correct diagnosis dictates correct treatment. This may sound obvious, but in primary care, the emphasis is often on “let’s try this” or “let’s try that,” an understandable approach to a symptomatic patient with an uncertain diagnosis. But by the time he finally gets to dermatology, the patient has tried a whole bag full of prescription and OTC products given for numerous, totally different diagnoses. A better approach might be to expedite an urgent referral to dermatology, when possible.
3 Cutaneous fungal infections (ie, dermatophytosis) are vastly overdiagnosed, especially by novices. If you truly suspect it, ask about a potential source; one doesn’t acquire a fungal infection out of thin air. It must come from a person, animal, or occasionally, the soil. It also helps if the victim has been rendered susceptible by the injudicious use of steroids. Better yet, find the fungus with a microscopic examination (KOH prep) or culture. Finally, remember, not everything round and scaly is fungal (see Figure 2).
4 Remember these ancient words of wisdom regarding skin complaints: (a) A diagnosis is seldom made if not entertained, (b) you won’t entertain it if you’ve never heard of it, (c) you will not see it if you’re not looking for it, and (d) even if you did see it, you would not “see” it because you’re not looking for it. Dermatology is far deeper and wider than most imagine it to be. The trick is to expose yourself to as many different diagnoses as possible, by reading and attending lectures, ahead of the possible sighting. Figures 3 and 4 offer examples of common conditions that are seldom recognized outside dermatology.
5 Skin cancer can present as a rash. Examples abound, such as mammary and extramammary Paget’s disease (Figure 5), mycosis fungoides, metastatic breast cancer (Figure 6), and superficial basal cell carcinoma. A biopsy is usually required to diagnose these, but you wouldn’t think to do that if you’d never heard of the condition.
6 Melanoma doesn’t typically arise from a mole or other pre-existing lesion. Far more often, it arises “de novo,” out of nothing. So, in general, we’re not worried about “moles” (nevi) unless there’s a history of change (see Figure 7).
Continue to: #7...
7 When looking for skin cancer, pay as much attention to the owner as to the lesion. The most common skin cancers—basal cell and squamous cell carcinoma—usually occur on sun-damaged, fair-skinned, blue-eyed older patients. Though there are certainly exceptions to this paradigm, it pays to be generally suspicious of any odd lesion seen on these patients (Figure 8).
8 It’s practically impossible to overstate the role of atopy when evaluating pediatric skin complaints. These children—20% of all newborns!—are born with thin, dry, sensitive, overreactive skin that is prone to eczema and urticaria. They will also have a marked tendency to develop seasonal allergies, allergic rhinitis, and asthma. Parents find it difficult to accept the genetic basis for atopic dermatitis (Figure 9), preferring instead to blame everything on laundry detergent or food. Education (of oneself first!) is the key.
9 “Infections” are not always what they seem
10 Overcome your fear of steroids by educating yourself about their safe use. Glucocorticoids (eg, triamcinolone, prednisone, betamethasone) are extremely useful in treating common derm conditions. We see patients every day who are so frightened of steroids, they won’t even consider using them because some well-meaning medical provider scared them to death. The proper use of these miraculous products could easily be the subject of an entire article. For now, I’ll advise you to read about their safe use in any number of dermatology texts (including online publications).
Health Apps Every Primary Care Provider Should Know About
We live in an ever-changing, fast-paced, and transitional world, and our health care system is no different. It’s hardly surprising, then, that digital health apps are becoming more commonplace in clinical practice. Need a useful tool to help you manage or monitor your patient’s chronic condition or educate them on preventive health and wellness measures? There’s an app for that.
If you question or lament this continual digital creep—or think it has no bearing on your patient population—you may be surprised to know that 77% of Americans have a smartphone with texting and/or mobile application abilities, creating innovative opportunities for health care providers to incorporate health apps into patient care.1 And the benefits are not just a sales pitch on the part of manufacturers—in fact, the American Diabetes Association’s 2018 Standards of Care include a recommendation for use of mobile apps for the prevention and delayed progression of type 2 diabetes.2 Of course, research shows that clinicians are more likely to adopt digital health tools if those tools improve practice efficiency, increase patient safety, improve diagnostic ability, help reduce burnout, and enhance patient-provider relationships.3
So maybe you see a role for apps in patient care. But the sheer volume and continuous proliferation of apps present an obstacle to effective evaluation and recommendation. With more than 318,000 health apps on the market and another 200 added every day, how do you know which ones are clinically sound and useful for your patients?4 Fortunately, there are two strategies that can help you integrate digital health apps into patient care.
1 HEALTH APPS AS MEDICATIONS
Viewing health apps as if they were medications can be helpful. Think about the process we as clinicians use when we’re thinking about prescribing a medication to a particular patient: we evaluate, manage, and prescribe.
Evaluate: As clinicians, we learn about the newest biopharmaceutical agents on the market to effectively govern our personal repertoire of medications and provide the best care for our patients. In this process, we evaluate clinical efficacy, safety, costs, benefits, barriers, contextual elements, caregiver impact, clinical studies, and more. This type of vetting process is also an effective approach to selecting and recommending health apps for your patients.
Manage: We each have a personal catalog of medications with which we become well versed, and comfortable, to effectively manage and help our patients with a multitude of medical conditions. This registry of medications represents our very special and individual “favorites,” per se. So, create a personal repertoire of health apps to improve and manage patient care.
Prescribe: Similar to medications, many digital health apps have demonstrated impressive patient outcomes with supporting clinical evidence. So why not get comfortable with prescribing digital health applications for behavior modifications or common medical conditions, just as you would with a medication?
Continue to: 2 BUILD YOUR PERSONAL APP LIBRARY
2 BUILD YOUR PERSONAL APP LIBRARY
Another strategy—touched upon in the “Manage” section earlier—is to create a personal library of highly regarded, well-vetted health apps to address common patient care matters. These could be recommended to a broad audience and will form the cornerstone of your digital compendium.
To get you started, Table 1 outlines a handful of health apps every primary care clinician should know about. These apps are supported by clinical research, endorsed or ranked by health care/industry expert organizations, and come recommended by clinical colleagues, students, or myself. The presented health apps are easily accessible via the App Store or Google Play and offer free versions, so you can assess and recommend them to your patients at no cost.
I hope you find these apps helpful with your future patient care efforts.
The author would like to acknowledge The Pace-Lenox Hill Hospital PA Program Class of 2019 for their research, evaluation, and feedback on a variety of digital health apps, and Jean Covino, DHSc, MPA , PA-C , for her encouragement to write and teach about my passion for health innovation.
1. Pew Research Center. Internet and technology: mobile fact sheet. www.pewinternet.org/fact-sheet/mobile/. Accessed November 9, 2018.
2. American Diabetes Association. Standards of Medical Care in Diabetes—2018. Diabetes Care . 2018;41(1):S51-S54.
3. American Medical Association. Digital Health Study: Physicians’ motivations and requirements for adopting digital clinical tools. www.ama-assn.org/sites/default/files/media-browser/specialty%20group/washington/ama-digital-health-report923.pdf. Accessed November 9, 2018.
4. IQVIA TM Institute for Human Data Science. The growing value of digital health: evidence and impact on human health and the healthcare system. www.iqvia.com/institute/reports/the-growing-value-of-digital-health. Accessed November 9, 2018.
We live in an ever-changing, fast-paced, and transitional world, and our health care system is no different. It’s hardly surprising, then, that digital health apps are becoming more commonplace in clinical practice. Need a useful tool to help you manage or monitor your patient’s chronic condition or educate them on preventive health and wellness measures? There’s an app for that.
If you question or lament this continual digital creep—or think it has no bearing on your patient population—you may be surprised to know that 77% of Americans have a smartphone with texting and/or mobile application abilities, creating innovative opportunities for health care providers to incorporate health apps into patient care.1 And the benefits are not just a sales pitch on the part of manufacturers—in fact, the American Diabetes Association’s 2018 Standards of Care include a recommendation for use of mobile apps for the prevention and delayed progression of type 2 diabetes.2 Of course, research shows that clinicians are more likely to adopt digital health tools if those tools improve practice efficiency, increase patient safety, improve diagnostic ability, help reduce burnout, and enhance patient-provider relationships.3
So maybe you see a role for apps in patient care. But the sheer volume and continuous proliferation of apps present an obstacle to effective evaluation and recommendation. With more than 318,000 health apps on the market and another 200 added every day, how do you know which ones are clinically sound and useful for your patients?4 Fortunately, there are two strategies that can help you integrate digital health apps into patient care.
1 HEALTH APPS AS MEDICATIONS
Viewing health apps as if they were medications can be helpful. Think about the process we as clinicians use when we’re thinking about prescribing a medication to a particular patient: we evaluate, manage, and prescribe.
Evaluate: As clinicians, we learn about the newest biopharmaceutical agents on the market to effectively govern our personal repertoire of medications and provide the best care for our patients. In this process, we evaluate clinical efficacy, safety, costs, benefits, barriers, contextual elements, caregiver impact, clinical studies, and more. This type of vetting process is also an effective approach to selecting and recommending health apps for your patients.
Manage: We each have a personal catalog of medications with which we become well versed, and comfortable, to effectively manage and help our patients with a multitude of medical conditions. This registry of medications represents our very special and individual “favorites,” per se. So, create a personal repertoire of health apps to improve and manage patient care.
Prescribe: Similar to medications, many digital health apps have demonstrated impressive patient outcomes with supporting clinical evidence. So why not get comfortable with prescribing digital health applications for behavior modifications or common medical conditions, just as you would with a medication?
Continue to: 2 BUILD YOUR PERSONAL APP LIBRARY
2 BUILD YOUR PERSONAL APP LIBRARY
Another strategy—touched upon in the “Manage” section earlier—is to create a personal library of highly regarded, well-vetted health apps to address common patient care matters. These could be recommended to a broad audience and will form the cornerstone of your digital compendium.
To get you started, Table 1 outlines a handful of health apps every primary care clinician should know about. These apps are supported by clinical research, endorsed or ranked by health care/industry expert organizations, and come recommended by clinical colleagues, students, or myself. The presented health apps are easily accessible via the App Store or Google Play and offer free versions, so you can assess and recommend them to your patients at no cost.
I hope you find these apps helpful with your future patient care efforts.
The author would like to acknowledge The Pace-Lenox Hill Hospital PA Program Class of 2019 for their research, evaluation, and feedback on a variety of digital health apps, and Jean Covino, DHSc, MPA , PA-C , for her encouragement to write and teach about my passion for health innovation.
We live in an ever-changing, fast-paced, and transitional world, and our health care system is no different. It’s hardly surprising, then, that digital health apps are becoming more commonplace in clinical practice. Need a useful tool to help you manage or monitor your patient’s chronic condition or educate them on preventive health and wellness measures? There’s an app for that.
If you question or lament this continual digital creep—or think it has no bearing on your patient population—you may be surprised to know that 77% of Americans have a smartphone with texting and/or mobile application abilities, creating innovative opportunities for health care providers to incorporate health apps into patient care.1 And the benefits are not just a sales pitch on the part of manufacturers—in fact, the American Diabetes Association’s 2018 Standards of Care include a recommendation for use of mobile apps for the prevention and delayed progression of type 2 diabetes.2 Of course, research shows that clinicians are more likely to adopt digital health tools if those tools improve practice efficiency, increase patient safety, improve diagnostic ability, help reduce burnout, and enhance patient-provider relationships.3
So maybe you see a role for apps in patient care. But the sheer volume and continuous proliferation of apps present an obstacle to effective evaluation and recommendation. With more than 318,000 health apps on the market and another 200 added every day, how do you know which ones are clinically sound and useful for your patients?4 Fortunately, there are two strategies that can help you integrate digital health apps into patient care.
1 HEALTH APPS AS MEDICATIONS
Viewing health apps as if they were medications can be helpful. Think about the process we as clinicians use when we’re thinking about prescribing a medication to a particular patient: we evaluate, manage, and prescribe.
Evaluate: As clinicians, we learn about the newest biopharmaceutical agents on the market to effectively govern our personal repertoire of medications and provide the best care for our patients. In this process, we evaluate clinical efficacy, safety, costs, benefits, barriers, contextual elements, caregiver impact, clinical studies, and more. This type of vetting process is also an effective approach to selecting and recommending health apps for your patients.
Manage: We each have a personal catalog of medications with which we become well versed, and comfortable, to effectively manage and help our patients with a multitude of medical conditions. This registry of medications represents our very special and individual “favorites,” per se. So, create a personal repertoire of health apps to improve and manage patient care.
Prescribe: Similar to medications, many digital health apps have demonstrated impressive patient outcomes with supporting clinical evidence. So why not get comfortable with prescribing digital health applications for behavior modifications or common medical conditions, just as you would with a medication?
Continue to: 2 BUILD YOUR PERSONAL APP LIBRARY
2 BUILD YOUR PERSONAL APP LIBRARY
Another strategy—touched upon in the “Manage” section earlier—is to create a personal library of highly regarded, well-vetted health apps to address common patient care matters. These could be recommended to a broad audience and will form the cornerstone of your digital compendium.
To get you started, Table 1 outlines a handful of health apps every primary care clinician should know about. These apps are supported by clinical research, endorsed or ranked by health care/industry expert organizations, and come recommended by clinical colleagues, students, or myself. The presented health apps are easily accessible via the App Store or Google Play and offer free versions, so you can assess and recommend them to your patients at no cost.
I hope you find these apps helpful with your future patient care efforts.
The author would like to acknowledge The Pace-Lenox Hill Hospital PA Program Class of 2019 for their research, evaluation, and feedback on a variety of digital health apps, and Jean Covino, DHSc, MPA , PA-C , for her encouragement to write and teach about my passion for health innovation.
1. Pew Research Center. Internet and technology: mobile fact sheet. www.pewinternet.org/fact-sheet/mobile/. Accessed November 9, 2018.
2. American Diabetes Association. Standards of Medical Care in Diabetes—2018. Diabetes Care . 2018;41(1):S51-S54.
3. American Medical Association. Digital Health Study: Physicians’ motivations and requirements for adopting digital clinical tools. www.ama-assn.org/sites/default/files/media-browser/specialty%20group/washington/ama-digital-health-report923.pdf. Accessed November 9, 2018.
4. IQVIA TM Institute for Human Data Science. The growing value of digital health: evidence and impact on human health and the healthcare system. www.iqvia.com/institute/reports/the-growing-value-of-digital-health. Accessed November 9, 2018.
1. Pew Research Center. Internet and technology: mobile fact sheet. www.pewinternet.org/fact-sheet/mobile/. Accessed November 9, 2018.
2. American Diabetes Association. Standards of Medical Care in Diabetes—2018. Diabetes Care . 2018;41(1):S51-S54.
3. American Medical Association. Digital Health Study: Physicians’ motivations and requirements for adopting digital clinical tools. www.ama-assn.org/sites/default/files/media-browser/specialty%20group/washington/ama-digital-health-report923.pdf. Accessed November 9, 2018.
4. IQVIA TM Institute for Human Data Science. The growing value of digital health: evidence and impact on human health and the healthcare system. www.iqvia.com/institute/reports/the-growing-value-of-digital-health. Accessed November 9, 2018.
Meaningful endometriosis treatment requires a holistic approach and an understanding of chronic pain
Although it has been more than 100 years since endometriosis was first described in the literature, deciphering the mechanisms that cause pain in women with this enigmatic disease is an ongoing pursuit.
Pain is the most debilitating symptom of endometriosis.1,2 In many cases, it has a profoundly negative impact on a patient’s quality of life, and contributes significantly to disease burden, as well as to personal and societal costs from lost productivity.3,4 Women with endometriosis often experience chronic pelvic pain, deep dyspareunia, dysmenorrhea, and subfertility.5 The majority of women with the disease also have one or more comorbidities, including adenomyosis, adhesive disease, and other pelvic pain conditions such as interstitial cystitis, irritable bowel disease, inflammatory bowel disease, and pelvic floor myalgia.6-8
Recent studies have yielded new insights into the development of endometriosis-associated pelvic pain. The role of peritoneal inflammation, de novo innervation of endometriosis implants, and changes in the central nervous system are becoming increasingly clear.5,9,10 These discoveries have important treatment implications.
In this article, Andrea J. Rapkin, MD, Professor of Obstetrics and Gynecology at the University of California, Los Angeles, and Founder and Director of the UCLA Pelvic Pain Center, offers her expert opinion on the findings of key studies and their clinical implications, including the importance of a multidisciplinary treatment approach that focuses on the whole patient.
Q What mechanisms underlie the chronic pain that many women with endometriosis feel?
Although pain is the primary symptom experienced by women with endometriosis, the disease burden and symptom severity do not often correlate.11,12 “This was the first conundrum presented to clinicians,” noted Dr. Rapkin. “In fact, we do not know the true prevalence of endometriosis because women with endometriosis only come to diagnosis either based on pain or infertility. When infertility is the problem, very often we are surprised by how much disease is present in an individual with either no pain or minimal pain. Conversely, in other individuals with very severe pain, upon laparoscopic surgery, have minimal or mild endometriosis.”
Efforts to solve this clinical puzzle began decades ago. “Dr. Michael Vernon discovered that the small, red, endometriosis implants that looked like petechial hemorrhages produced more prostaglandin E2 (PGE2) in vitro than the older black-brown lesions. PGE2 is a pain-producing (algesic) chemical produced after cytokines stimulation,” said Dr. Rapkin. “This was the first evidence that, yes, there is a reason for pain in many individuals with lower-stage disease.”
“Prostaglandins are known to be a major cause of dysmenorrhea. Prostaglandins induce uterine cramping, sensitize nerve endings, and promote other inflammatory factors responsible for attracting monocytes that become macrophages, further contributing to inflammation,” Dr. Rapkin continued. “PGE2 also stimulates the enzyme aromatase, which allows androgens to be converted to estrogen, which promotes growth of endometriotic lesions. This is a self-feeding aspect of endometriosis.”
Continue to: These discoveries were followed by the realization that deeply infiltrating endometriosis...
These discoveries were followed by the realization that deeply infiltrating endometriosis (defined by disease infiltration of more than 5 mm, often in the uterosacral ligaments) was more likely to be painful than superficial disease, said Dr. Rapkin. “In some women with endometriosis, the disease we see laparoscopically is really the tip of the iceberg.”
In 2005, landmark studies performed by Karen J. Berkley, PhD, were summarized in a paper coauthored by Dr. Berkley, Dr. Rapkin, and Raymond E. Papka, PhD.13 “In a rodent model where endometriosis was developed by suturing pieces of endometrium in the mesentery, the endometriosis implants developed a vascular supply and a nerve supply. These nerves were not just functioning to govern the dilation and contraction of the blood vessels (in other words the sympathetic type nerves), but these nerves stained for neurotransmitters associated with pain (algesic agents, such as substance P and CGRP),” said Dr. Rapkin. “At UCLA, we acquired tissue from women with endometriosis and analyzed in Dr. Papka’s lab. Those tissues also showed nerves staining for pain-producing chemicals.” Other studies performed worldwide also demonstrated nerve endings with neurotrophic and algesic chemicals in endometriotic tissues. In addition to prostaglandins and cytokines, increased expression of various neuropeptides, neurotrophins, and alterations in ion channels contribute to hypersensitivity and pain.
Q What other chronic pain conditions might women with endometriosis experience?
Overlapping chronic pain conditions are common in women with endometriosis. “There is a very high co-occurrence of interstitial cystitis/painful bladder syndrome,” said Dr. Rapkin. “Irritable bowel syndrome is more common in women with endometriosis, as is vulvodynia. Fibromyalgia, migraine headache, temporo-mandibular joint pain (TMJ), anxiety, and depression also commonly co-occur in women with endometriosis.”
“Two concepts may be relevant to why these overlapping pain conditions develop,” Dr. Rapkin continued. “First, visceral sensitization: If one organ or tissue is inflamed and becomes hyperalgesic then other organs in the adjacent region with shared thoracolumbar and sacral innervation can become sensitized through shared cell bodies in the spinal cord, cross-sensitization in the cord, or at higher regions of the CNS. In addition, visceral somatic conversion occurs, whereby somatic tissues such as muscles and subcutaneous tissues with the same nerve supply as the affected organs become sensitized. This process may explain why abdominal wall and pelvic floor muscles become painful. The involvement of surrounding musculature is an important contributor to the pain in many women with endometriosis.”
“Finally, genetic studies of alterations in genes that encode for chemicals affecting the sensitivity and perception of pain are shedding light on the development of chronic pain. Ultimately these studies will advance our understanding of pain related to endometriosis.”
Continue to: Q How have new understandings about the pain mechanisms...
Q How have new understandings about the pain mechanisms involved with endometriosis-caused pelvic pain improved treatment?
According to Dr. Rapkin, the increased understanding of the mechanisms involved in endometriosis-associated pain gained from these key studies led to a paradigm shift, with endometriosis being viewed not just as a condition with mechanical hypersensitivity due to altered anatomy and inflammation but also as a neurologic condition, or a nerve pain condition with peripheral and central sensitization. “This means there is upregulation or hyperactivity both in the periphery (in the pelvis) and centrally (in the spinal cord and brain),” said Dr. Rapkin.
“In the periphery, the endometriotic lesions develop an afferent sensory innervation and communicate with the brain. Stimulation of these nerves by the inflammatory milieu contributes to pain.” Dr. Rapkin noted research by Maria Adele Giamberardino, which demonstrated that women with endometriosis and pain have a lower threshold for feeling pain in the tissues overlying the pelvis (the abdominal wall and back).14 This also has been shown by Dr. Berkley in rodents given endometriosis.
“The muscles develop trigger points and tender hyperalgesic points as part of the sensitization process. In addition, distant sensitization develops—women with pelvic pain and endometriosis have a lower threshold for sensing experimental pain in areas outside the pelvis, for example the back, leg, or shoulder. These discoveries clearly reflect up regulation for pain processing in the central nervous system.”
Dr. Rapkin also pointed to research published in 2016 by Sawson As-Sanie, MD, MPH, that showed an association between endometriosis-associated pelvic pain and altered brain chemistry and function.16 “Dr. As-Sanie demonstrated a decrease in gray matter volume in key neural pain processing areas in the brain in women with pain with endometriosis. This was not found in women with endometriosis who did not have pain,” she said. “Altered connectivity in brain areas related to perception and inhibition of pain is important in maintaining pain. Dr. As-Sanie’s studies also found that these changes are correlated with anxiety, depression, and pain intensity in patients with endometriosis and chronic pain.”
Continue to: Q What are some newer treatment approaches to chronic pain with endometriosis?
Q What are some newer treatment approaches to chronic pain with endometriosis?
“Multidisciplinary approaches to endometriosis-related pain are important,” said Dr. Rapkin. “Although it is important to excise or cauterize endometriosis lesions, or debulk as much as can safely be removed during laparoscopic surgery, it is now standard of care that medical therapy, not surgery, is the first approach to treatment. Endometriosis is a chronic condition. Inflammatory factors will continue to proliferate in patients who menstruate and produce high levels of estrogen with ovulation. The goal of medical therapy is to decrease the levels of estrogen that contribute to maintenance and proliferation of the implants. We want to suppress estrogen in a way that is compatible with long-term quality of life for our patients. Wiping out estrogen and placing patients into a chemical or surgical menopause for most of their reproductive years is not desirable.”
Approaches to hormonally modulate endometriosis include combined hormonal contraceptives and progestin-only medications, such as the levonogestrol-containing IUD, progestin-containing contraceptive implants, injections, or tablets. Second-line medical therapy consists of gonadotropin-releasing hormone agonists and antagonists that can be used for 6 months to 2 years and allows for further lowering of estrogen levels. These may not provide sufficient pain relief for some patients. “There is some evidence from Dr. Giamberadino’s studies that after women with dysmenorrhea were treated with oral contraceptives, the abdominal wall hyperalgesia decreased,” said Dr. Rapkin. “The question is, why don’t we see this in all patients? We come to the realization that endometriosis has to be treated as a neurologically mediated disorder. We have to treat the peripheral and central sensitization in a multidisciplinary way.”
A holistic approach to endometriosis is a new and exciting area for the field, said Dr. Rapkin. “We have to treat ‘bottom-up’, and ‘top-down.’ Bottom-up means we are addressing the peripheral factors that contribute to pain: endometriotic lesions, other pelvic organ pain, myofascial pain, trigger points, the tender points, and the muscle dysfunction in the abdominal wall, the back, and the pelvic floor. Pelvic floor physical therapists help women with pain and endometriosis. Often, women with endometriosis have myofascial pain and pain related to the other comorbid pain conditions they may have developed. Peripheral nerve blocks and medications used for neuropathic pain that alter nerve firing can be helpful in many situations. Pain can be augmented by cognitions and beliefs about pain, and by anxiety and depression. So the top-down approach addresses the cognitions, depression, and anxiety. We do not consider endometriosis a psychosomatic condition, but we know that if you do not address the central upregulation, including anxiety and depression, we may not get anywhere.”
“Interestingly, neurotransmitters and brain regions governing mood contribute to nerve pain. Medications such as tricyclic antidepressants, serotonin norepinephrine reuptake inhibitors, anticonvulsants, and calcium channel blocking agents may prove fruitful. Cognitive behavioral therapy is another approach—to stimulate the prefrontal cortex, the area that is involved in pain inhibition, and other areas of the brain that may produce endogenous opioids to help with inhibiting pain. Bringing in complementary approaches is very important—for example, mindfulness-based meditation or yoga. There is growing evidence for acupuncture as well. Physical therapists, pain psychologists, anesthesiologists, or gynecologists who are facile with nerve blocks, to help tone down hyperalgesic tissues, in addition to medical and surgical therapy, have the possibility of really improving the lives of women with endometriosis.”
Q What key pearls would you like to share with readers?
“It is important to evaluate the entire individual,” she said. “Do not just viscerally focus on the uterus, the ovaries, fallopian tubes, and the peritoneum; investigate the adjacent organs and somatic tissues. Think about the abdominal wall, think about the pelvic floor. Learn how to evaluate these structures. There are simple evaluation techniques that gynecologists can learn and should include with every patient with pelvic pain, whether or not they are suspected of having endometriosis. You also want to get a complete history to determine if there are other co-occurring pain conditions. If there are, it is already a sign that there may be central sensitization.”
“Very often, it is necessary to bring in a pain psychologist—not because the disease is psychosomatic but because therapy can help the patient to learn how to use their brain to erase pain memory, and of course to address the concomitant anxiety, depression, and social isolation that happens with pain.”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Olive DL, Lindheim SR, Pritts EA. New medical treatments for endometriosis. Best Pract Res Clin Obstet Gynaecol. 2004;18(2):319-328.
- Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364(9447): 1789-1799.
- Nnoaham KE, Hummelshoj L, Webster P, et al; World Endometriosis Research Foundation Global Study of Women’s Health consortium. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril. 2011;96(2):366-373.e8.
- Simoens S, Dunselman G, Dirksen C, et al. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod. 2012;27(5):1292–1299.
- Bruner-Tran KL, Mokshagundam S, Herington JL. Rodent models of experimental endometriosis: identifying mechanisms of disease and therapeutic targets. Curr Womens Health Rev. 2018;14(2):173-188.
- Sinaii N, Cleary SD, Ballweg ML, Nieman LK, Stratton P. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod. 2002;17(10):2715-2724.
- Struble J, Reid S, Bedaiwy MA. Adenomyosis: a clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol. 2016;23(2):164-185.
- Tirlapur SA, Kuhrt K, Chaliha C. The ‘evil twin syndrome’ in chronic pelvic pain: a systematic review of prevalence studies of bladder pain syndrome and endometriosis. Int J Surg. 2013;11(3):233-237.
- Coxon L, Horne AW, Vincent K. Pathophysiology of endometriosis-associated pain: a review of pelvic and central nervous system mechanisms. Best Pract Res Clin Obstet Gynaecol. 2018 Feb 15. pii: S1521-6934(18)30032-4. doi: 10.1016/j.bpobgyn.2018.01.014. [Epub ahead of print]
- Yan D, Liu X, Guo SW. Nerve fibers and endometriotic lesions: partners in crime in inflicting pains in women with endometriosis. Eur J Obstet Gynecol Reprod Biol. 2017;209:14-24.
- Vercellini P, Fedele L, Aimi G, Pietropaolo G, Consonni D, Crosignani PG. Association between endometriosis stage, lesion type, patient characteristics and severity of pelvic pain symptoms: a multivariate analysis of over 1000 patients. Hum Reprod. 2007;22(1):266-271.
- Fedele L, Parazzini F, Bianchi S. Stage and localization of pelvic endometriosis and pain. Fertil Steril. 1990;53(1):155-158.
- Berkley KJ, Rapkin AJ, Papka RE. The pains of endometriosis. Science. 2005;308(5728):1587-1589.
- Giamberardino MA, Tana C, Costantini R. Pain thresholds in women with chronic pelvic pain. Curr Opin Obstet Gynecol. 2014;26(4):253-259.
- Giamberardino MA, Berkley KJ, Affaitati G. Influence of endometriosis on pain behaviors and muscle hyperalgesia induced by a ureteral calculosis in female rats. Pain. 2002;95(3):247-257.
- As-Sanie S, Kim J, Schmidt-Wilcke T. Functional connectivity is associated with altered brain chemistry in women with endometriosis-associated chronic pelvic pain. J Pain. 2016;17(1):1-13.
Although it has been more than 100 years since endometriosis was first described in the literature, deciphering the mechanisms that cause pain in women with this enigmatic disease is an ongoing pursuit.
Pain is the most debilitating symptom of endometriosis.1,2 In many cases, it has a profoundly negative impact on a patient’s quality of life, and contributes significantly to disease burden, as well as to personal and societal costs from lost productivity.3,4 Women with endometriosis often experience chronic pelvic pain, deep dyspareunia, dysmenorrhea, and subfertility.5 The majority of women with the disease also have one or more comorbidities, including adenomyosis, adhesive disease, and other pelvic pain conditions such as interstitial cystitis, irritable bowel disease, inflammatory bowel disease, and pelvic floor myalgia.6-8
Recent studies have yielded new insights into the development of endometriosis-associated pelvic pain. The role of peritoneal inflammation, de novo innervation of endometriosis implants, and changes in the central nervous system are becoming increasingly clear.5,9,10 These discoveries have important treatment implications.
In this article, Andrea J. Rapkin, MD, Professor of Obstetrics and Gynecology at the University of California, Los Angeles, and Founder and Director of the UCLA Pelvic Pain Center, offers her expert opinion on the findings of key studies and their clinical implications, including the importance of a multidisciplinary treatment approach that focuses on the whole patient.
Q What mechanisms underlie the chronic pain that many women with endometriosis feel?
Although pain is the primary symptom experienced by women with endometriosis, the disease burden and symptom severity do not often correlate.11,12 “This was the first conundrum presented to clinicians,” noted Dr. Rapkin. “In fact, we do not know the true prevalence of endometriosis because women with endometriosis only come to diagnosis either based on pain or infertility. When infertility is the problem, very often we are surprised by how much disease is present in an individual with either no pain or minimal pain. Conversely, in other individuals with very severe pain, upon laparoscopic surgery, have minimal or mild endometriosis.”
Efforts to solve this clinical puzzle began decades ago. “Dr. Michael Vernon discovered that the small, red, endometriosis implants that looked like petechial hemorrhages produced more prostaglandin E2 (PGE2) in vitro than the older black-brown lesions. PGE2 is a pain-producing (algesic) chemical produced after cytokines stimulation,” said Dr. Rapkin. “This was the first evidence that, yes, there is a reason for pain in many individuals with lower-stage disease.”
“Prostaglandins are known to be a major cause of dysmenorrhea. Prostaglandins induce uterine cramping, sensitize nerve endings, and promote other inflammatory factors responsible for attracting monocytes that become macrophages, further contributing to inflammation,” Dr. Rapkin continued. “PGE2 also stimulates the enzyme aromatase, which allows androgens to be converted to estrogen, which promotes growth of endometriotic lesions. This is a self-feeding aspect of endometriosis.”
Continue to: These discoveries were followed by the realization that deeply infiltrating endometriosis...
These discoveries were followed by the realization that deeply infiltrating endometriosis (defined by disease infiltration of more than 5 mm, often in the uterosacral ligaments) was more likely to be painful than superficial disease, said Dr. Rapkin. “In some women with endometriosis, the disease we see laparoscopically is really the tip of the iceberg.”
In 2005, landmark studies performed by Karen J. Berkley, PhD, were summarized in a paper coauthored by Dr. Berkley, Dr. Rapkin, and Raymond E. Papka, PhD.13 “In a rodent model where endometriosis was developed by suturing pieces of endometrium in the mesentery, the endometriosis implants developed a vascular supply and a nerve supply. These nerves were not just functioning to govern the dilation and contraction of the blood vessels (in other words the sympathetic type nerves), but these nerves stained for neurotransmitters associated with pain (algesic agents, such as substance P and CGRP),” said Dr. Rapkin. “At UCLA, we acquired tissue from women with endometriosis and analyzed in Dr. Papka’s lab. Those tissues also showed nerves staining for pain-producing chemicals.” Other studies performed worldwide also demonstrated nerve endings with neurotrophic and algesic chemicals in endometriotic tissues. In addition to prostaglandins and cytokines, increased expression of various neuropeptides, neurotrophins, and alterations in ion channels contribute to hypersensitivity and pain.
Q What other chronic pain conditions might women with endometriosis experience?
Overlapping chronic pain conditions are common in women with endometriosis. “There is a very high co-occurrence of interstitial cystitis/painful bladder syndrome,” said Dr. Rapkin. “Irritable bowel syndrome is more common in women with endometriosis, as is vulvodynia. Fibromyalgia, migraine headache, temporo-mandibular joint pain (TMJ), anxiety, and depression also commonly co-occur in women with endometriosis.”
“Two concepts may be relevant to why these overlapping pain conditions develop,” Dr. Rapkin continued. “First, visceral sensitization: If one organ or tissue is inflamed and becomes hyperalgesic then other organs in the adjacent region with shared thoracolumbar and sacral innervation can become sensitized through shared cell bodies in the spinal cord, cross-sensitization in the cord, or at higher regions of the CNS. In addition, visceral somatic conversion occurs, whereby somatic tissues such as muscles and subcutaneous tissues with the same nerve supply as the affected organs become sensitized. This process may explain why abdominal wall and pelvic floor muscles become painful. The involvement of surrounding musculature is an important contributor to the pain in many women with endometriosis.”
“Finally, genetic studies of alterations in genes that encode for chemicals affecting the sensitivity and perception of pain are shedding light on the development of chronic pain. Ultimately these studies will advance our understanding of pain related to endometriosis.”
Continue to: Q How have new understandings about the pain mechanisms...
Q How have new understandings about the pain mechanisms involved with endometriosis-caused pelvic pain improved treatment?
According to Dr. Rapkin, the increased understanding of the mechanisms involved in endometriosis-associated pain gained from these key studies led to a paradigm shift, with endometriosis being viewed not just as a condition with mechanical hypersensitivity due to altered anatomy and inflammation but also as a neurologic condition, or a nerve pain condition with peripheral and central sensitization. “This means there is upregulation or hyperactivity both in the periphery (in the pelvis) and centrally (in the spinal cord and brain),” said Dr. Rapkin.
“In the periphery, the endometriotic lesions develop an afferent sensory innervation and communicate with the brain. Stimulation of these nerves by the inflammatory milieu contributes to pain.” Dr. Rapkin noted research by Maria Adele Giamberardino, which demonstrated that women with endometriosis and pain have a lower threshold for feeling pain in the tissues overlying the pelvis (the abdominal wall and back).14 This also has been shown by Dr. Berkley in rodents given endometriosis.
“The muscles develop trigger points and tender hyperalgesic points as part of the sensitization process. In addition, distant sensitization develops—women with pelvic pain and endometriosis have a lower threshold for sensing experimental pain in areas outside the pelvis, for example the back, leg, or shoulder. These discoveries clearly reflect up regulation for pain processing in the central nervous system.”
Dr. Rapkin also pointed to research published in 2016 by Sawson As-Sanie, MD, MPH, that showed an association between endometriosis-associated pelvic pain and altered brain chemistry and function.16 “Dr. As-Sanie demonstrated a decrease in gray matter volume in key neural pain processing areas in the brain in women with pain with endometriosis. This was not found in women with endometriosis who did not have pain,” she said. “Altered connectivity in brain areas related to perception and inhibition of pain is important in maintaining pain. Dr. As-Sanie’s studies also found that these changes are correlated with anxiety, depression, and pain intensity in patients with endometriosis and chronic pain.”
Continue to: Q What are some newer treatment approaches to chronic pain with endometriosis?
Q What are some newer treatment approaches to chronic pain with endometriosis?
“Multidisciplinary approaches to endometriosis-related pain are important,” said Dr. Rapkin. “Although it is important to excise or cauterize endometriosis lesions, or debulk as much as can safely be removed during laparoscopic surgery, it is now standard of care that medical therapy, not surgery, is the first approach to treatment. Endometriosis is a chronic condition. Inflammatory factors will continue to proliferate in patients who menstruate and produce high levels of estrogen with ovulation. The goal of medical therapy is to decrease the levels of estrogen that contribute to maintenance and proliferation of the implants. We want to suppress estrogen in a way that is compatible with long-term quality of life for our patients. Wiping out estrogen and placing patients into a chemical or surgical menopause for most of their reproductive years is not desirable.”
Approaches to hormonally modulate endometriosis include combined hormonal contraceptives and progestin-only medications, such as the levonogestrol-containing IUD, progestin-containing contraceptive implants, injections, or tablets. Second-line medical therapy consists of gonadotropin-releasing hormone agonists and antagonists that can be used for 6 months to 2 years and allows for further lowering of estrogen levels. These may not provide sufficient pain relief for some patients. “There is some evidence from Dr. Giamberadino’s studies that after women with dysmenorrhea were treated with oral contraceptives, the abdominal wall hyperalgesia decreased,” said Dr. Rapkin. “The question is, why don’t we see this in all patients? We come to the realization that endometriosis has to be treated as a neurologically mediated disorder. We have to treat the peripheral and central sensitization in a multidisciplinary way.”
A holistic approach to endometriosis is a new and exciting area for the field, said Dr. Rapkin. “We have to treat ‘bottom-up’, and ‘top-down.’ Bottom-up means we are addressing the peripheral factors that contribute to pain: endometriotic lesions, other pelvic organ pain, myofascial pain, trigger points, the tender points, and the muscle dysfunction in the abdominal wall, the back, and the pelvic floor. Pelvic floor physical therapists help women with pain and endometriosis. Often, women with endometriosis have myofascial pain and pain related to the other comorbid pain conditions they may have developed. Peripheral nerve blocks and medications used for neuropathic pain that alter nerve firing can be helpful in many situations. Pain can be augmented by cognitions and beliefs about pain, and by anxiety and depression. So the top-down approach addresses the cognitions, depression, and anxiety. We do not consider endometriosis a psychosomatic condition, but we know that if you do not address the central upregulation, including anxiety and depression, we may not get anywhere.”
“Interestingly, neurotransmitters and brain regions governing mood contribute to nerve pain. Medications such as tricyclic antidepressants, serotonin norepinephrine reuptake inhibitors, anticonvulsants, and calcium channel blocking agents may prove fruitful. Cognitive behavioral therapy is another approach—to stimulate the prefrontal cortex, the area that is involved in pain inhibition, and other areas of the brain that may produce endogenous opioids to help with inhibiting pain. Bringing in complementary approaches is very important—for example, mindfulness-based meditation or yoga. There is growing evidence for acupuncture as well. Physical therapists, pain psychologists, anesthesiologists, or gynecologists who are facile with nerve blocks, to help tone down hyperalgesic tissues, in addition to medical and surgical therapy, have the possibility of really improving the lives of women with endometriosis.”
Q What key pearls would you like to share with readers?
“It is important to evaluate the entire individual,” she said. “Do not just viscerally focus on the uterus, the ovaries, fallopian tubes, and the peritoneum; investigate the adjacent organs and somatic tissues. Think about the abdominal wall, think about the pelvic floor. Learn how to evaluate these structures. There are simple evaluation techniques that gynecologists can learn and should include with every patient with pelvic pain, whether or not they are suspected of having endometriosis. You also want to get a complete history to determine if there are other co-occurring pain conditions. If there are, it is already a sign that there may be central sensitization.”
“Very often, it is necessary to bring in a pain psychologist—not because the disease is psychosomatic but because therapy can help the patient to learn how to use their brain to erase pain memory, and of course to address the concomitant anxiety, depression, and social isolation that happens with pain.”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Although it has been more than 100 years since endometriosis was first described in the literature, deciphering the mechanisms that cause pain in women with this enigmatic disease is an ongoing pursuit.
Pain is the most debilitating symptom of endometriosis.1,2 In many cases, it has a profoundly negative impact on a patient’s quality of life, and contributes significantly to disease burden, as well as to personal and societal costs from lost productivity.3,4 Women with endometriosis often experience chronic pelvic pain, deep dyspareunia, dysmenorrhea, and subfertility.5 The majority of women with the disease also have one or more comorbidities, including adenomyosis, adhesive disease, and other pelvic pain conditions such as interstitial cystitis, irritable bowel disease, inflammatory bowel disease, and pelvic floor myalgia.6-8
Recent studies have yielded new insights into the development of endometriosis-associated pelvic pain. The role of peritoneal inflammation, de novo innervation of endometriosis implants, and changes in the central nervous system are becoming increasingly clear.5,9,10 These discoveries have important treatment implications.
In this article, Andrea J. Rapkin, MD, Professor of Obstetrics and Gynecology at the University of California, Los Angeles, and Founder and Director of the UCLA Pelvic Pain Center, offers her expert opinion on the findings of key studies and their clinical implications, including the importance of a multidisciplinary treatment approach that focuses on the whole patient.
Q What mechanisms underlie the chronic pain that many women with endometriosis feel?
Although pain is the primary symptom experienced by women with endometriosis, the disease burden and symptom severity do not often correlate.11,12 “This was the first conundrum presented to clinicians,” noted Dr. Rapkin. “In fact, we do not know the true prevalence of endometriosis because women with endometriosis only come to diagnosis either based on pain or infertility. When infertility is the problem, very often we are surprised by how much disease is present in an individual with either no pain or minimal pain. Conversely, in other individuals with very severe pain, upon laparoscopic surgery, have minimal or mild endometriosis.”
Efforts to solve this clinical puzzle began decades ago. “Dr. Michael Vernon discovered that the small, red, endometriosis implants that looked like petechial hemorrhages produced more prostaglandin E2 (PGE2) in vitro than the older black-brown lesions. PGE2 is a pain-producing (algesic) chemical produced after cytokines stimulation,” said Dr. Rapkin. “This was the first evidence that, yes, there is a reason for pain in many individuals with lower-stage disease.”
“Prostaglandins are known to be a major cause of dysmenorrhea. Prostaglandins induce uterine cramping, sensitize nerve endings, and promote other inflammatory factors responsible for attracting monocytes that become macrophages, further contributing to inflammation,” Dr. Rapkin continued. “PGE2 also stimulates the enzyme aromatase, which allows androgens to be converted to estrogen, which promotes growth of endometriotic lesions. This is a self-feeding aspect of endometriosis.”
Continue to: These discoveries were followed by the realization that deeply infiltrating endometriosis...
These discoveries were followed by the realization that deeply infiltrating endometriosis (defined by disease infiltration of more than 5 mm, often in the uterosacral ligaments) was more likely to be painful than superficial disease, said Dr. Rapkin. “In some women with endometriosis, the disease we see laparoscopically is really the tip of the iceberg.”
In 2005, landmark studies performed by Karen J. Berkley, PhD, were summarized in a paper coauthored by Dr. Berkley, Dr. Rapkin, and Raymond E. Papka, PhD.13 “In a rodent model where endometriosis was developed by suturing pieces of endometrium in the mesentery, the endometriosis implants developed a vascular supply and a nerve supply. These nerves were not just functioning to govern the dilation and contraction of the blood vessels (in other words the sympathetic type nerves), but these nerves stained for neurotransmitters associated with pain (algesic agents, such as substance P and CGRP),” said Dr. Rapkin. “At UCLA, we acquired tissue from women with endometriosis and analyzed in Dr. Papka’s lab. Those tissues also showed nerves staining for pain-producing chemicals.” Other studies performed worldwide also demonstrated nerve endings with neurotrophic and algesic chemicals in endometriotic tissues. In addition to prostaglandins and cytokines, increased expression of various neuropeptides, neurotrophins, and alterations in ion channels contribute to hypersensitivity and pain.
Q What other chronic pain conditions might women with endometriosis experience?
Overlapping chronic pain conditions are common in women with endometriosis. “There is a very high co-occurrence of interstitial cystitis/painful bladder syndrome,” said Dr. Rapkin. “Irritable bowel syndrome is more common in women with endometriosis, as is vulvodynia. Fibromyalgia, migraine headache, temporo-mandibular joint pain (TMJ), anxiety, and depression also commonly co-occur in women with endometriosis.”
“Two concepts may be relevant to why these overlapping pain conditions develop,” Dr. Rapkin continued. “First, visceral sensitization: If one organ or tissue is inflamed and becomes hyperalgesic then other organs in the adjacent region with shared thoracolumbar and sacral innervation can become sensitized through shared cell bodies in the spinal cord, cross-sensitization in the cord, or at higher regions of the CNS. In addition, visceral somatic conversion occurs, whereby somatic tissues such as muscles and subcutaneous tissues with the same nerve supply as the affected organs become sensitized. This process may explain why abdominal wall and pelvic floor muscles become painful. The involvement of surrounding musculature is an important contributor to the pain in many women with endometriosis.”
“Finally, genetic studies of alterations in genes that encode for chemicals affecting the sensitivity and perception of pain are shedding light on the development of chronic pain. Ultimately these studies will advance our understanding of pain related to endometriosis.”
Continue to: Q How have new understandings about the pain mechanisms...
Q How have new understandings about the pain mechanisms involved with endometriosis-caused pelvic pain improved treatment?
According to Dr. Rapkin, the increased understanding of the mechanisms involved in endometriosis-associated pain gained from these key studies led to a paradigm shift, with endometriosis being viewed not just as a condition with mechanical hypersensitivity due to altered anatomy and inflammation but also as a neurologic condition, or a nerve pain condition with peripheral and central sensitization. “This means there is upregulation or hyperactivity both in the periphery (in the pelvis) and centrally (in the spinal cord and brain),” said Dr. Rapkin.
“In the periphery, the endometriotic lesions develop an afferent sensory innervation and communicate with the brain. Stimulation of these nerves by the inflammatory milieu contributes to pain.” Dr. Rapkin noted research by Maria Adele Giamberardino, which demonstrated that women with endometriosis and pain have a lower threshold for feeling pain in the tissues overlying the pelvis (the abdominal wall and back).14 This also has been shown by Dr. Berkley in rodents given endometriosis.
“The muscles develop trigger points and tender hyperalgesic points as part of the sensitization process. In addition, distant sensitization develops—women with pelvic pain and endometriosis have a lower threshold for sensing experimental pain in areas outside the pelvis, for example the back, leg, or shoulder. These discoveries clearly reflect up regulation for pain processing in the central nervous system.”
Dr. Rapkin also pointed to research published in 2016 by Sawson As-Sanie, MD, MPH, that showed an association between endometriosis-associated pelvic pain and altered brain chemistry and function.16 “Dr. As-Sanie demonstrated a decrease in gray matter volume in key neural pain processing areas in the brain in women with pain with endometriosis. This was not found in women with endometriosis who did not have pain,” she said. “Altered connectivity in brain areas related to perception and inhibition of pain is important in maintaining pain. Dr. As-Sanie’s studies also found that these changes are correlated with anxiety, depression, and pain intensity in patients with endometriosis and chronic pain.”
Continue to: Q What are some newer treatment approaches to chronic pain with endometriosis?
Q What are some newer treatment approaches to chronic pain with endometriosis?
“Multidisciplinary approaches to endometriosis-related pain are important,” said Dr. Rapkin. “Although it is important to excise or cauterize endometriosis lesions, or debulk as much as can safely be removed during laparoscopic surgery, it is now standard of care that medical therapy, not surgery, is the first approach to treatment. Endometriosis is a chronic condition. Inflammatory factors will continue to proliferate in patients who menstruate and produce high levels of estrogen with ovulation. The goal of medical therapy is to decrease the levels of estrogen that contribute to maintenance and proliferation of the implants. We want to suppress estrogen in a way that is compatible with long-term quality of life for our patients. Wiping out estrogen and placing patients into a chemical or surgical menopause for most of their reproductive years is not desirable.”
Approaches to hormonally modulate endometriosis include combined hormonal contraceptives and progestin-only medications, such as the levonogestrol-containing IUD, progestin-containing contraceptive implants, injections, or tablets. Second-line medical therapy consists of gonadotropin-releasing hormone agonists and antagonists that can be used for 6 months to 2 years and allows for further lowering of estrogen levels. These may not provide sufficient pain relief for some patients. “There is some evidence from Dr. Giamberadino’s studies that after women with dysmenorrhea were treated with oral contraceptives, the abdominal wall hyperalgesia decreased,” said Dr. Rapkin. “The question is, why don’t we see this in all patients? We come to the realization that endometriosis has to be treated as a neurologically mediated disorder. We have to treat the peripheral and central sensitization in a multidisciplinary way.”
A holistic approach to endometriosis is a new and exciting area for the field, said Dr. Rapkin. “We have to treat ‘bottom-up’, and ‘top-down.’ Bottom-up means we are addressing the peripheral factors that contribute to pain: endometriotic lesions, other pelvic organ pain, myofascial pain, trigger points, the tender points, and the muscle dysfunction in the abdominal wall, the back, and the pelvic floor. Pelvic floor physical therapists help women with pain and endometriosis. Often, women with endometriosis have myofascial pain and pain related to the other comorbid pain conditions they may have developed. Peripheral nerve blocks and medications used for neuropathic pain that alter nerve firing can be helpful in many situations. Pain can be augmented by cognitions and beliefs about pain, and by anxiety and depression. So the top-down approach addresses the cognitions, depression, and anxiety. We do not consider endometriosis a psychosomatic condition, but we know that if you do not address the central upregulation, including anxiety and depression, we may not get anywhere.”
“Interestingly, neurotransmitters and brain regions governing mood contribute to nerve pain. Medications such as tricyclic antidepressants, serotonin norepinephrine reuptake inhibitors, anticonvulsants, and calcium channel blocking agents may prove fruitful. Cognitive behavioral therapy is another approach—to stimulate the prefrontal cortex, the area that is involved in pain inhibition, and other areas of the brain that may produce endogenous opioids to help with inhibiting pain. Bringing in complementary approaches is very important—for example, mindfulness-based meditation or yoga. There is growing evidence for acupuncture as well. Physical therapists, pain psychologists, anesthesiologists, or gynecologists who are facile with nerve blocks, to help tone down hyperalgesic tissues, in addition to medical and surgical therapy, have the possibility of really improving the lives of women with endometriosis.”
Q What key pearls would you like to share with readers?
“It is important to evaluate the entire individual,” she said. “Do not just viscerally focus on the uterus, the ovaries, fallopian tubes, and the peritoneum; investigate the adjacent organs and somatic tissues. Think about the abdominal wall, think about the pelvic floor. Learn how to evaluate these structures. There are simple evaluation techniques that gynecologists can learn and should include with every patient with pelvic pain, whether or not they are suspected of having endometriosis. You also want to get a complete history to determine if there are other co-occurring pain conditions. If there are, it is already a sign that there may be central sensitization.”
“Very often, it is necessary to bring in a pain psychologist—not because the disease is psychosomatic but because therapy can help the patient to learn how to use their brain to erase pain memory, and of course to address the concomitant anxiety, depression, and social isolation that happens with pain.”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Olive DL, Lindheim SR, Pritts EA. New medical treatments for endometriosis. Best Pract Res Clin Obstet Gynaecol. 2004;18(2):319-328.
- Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364(9447): 1789-1799.
- Nnoaham KE, Hummelshoj L, Webster P, et al; World Endometriosis Research Foundation Global Study of Women’s Health consortium. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril. 2011;96(2):366-373.e8.
- Simoens S, Dunselman G, Dirksen C, et al. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod. 2012;27(5):1292–1299.
- Bruner-Tran KL, Mokshagundam S, Herington JL. Rodent models of experimental endometriosis: identifying mechanisms of disease and therapeutic targets. Curr Womens Health Rev. 2018;14(2):173-188.
- Sinaii N, Cleary SD, Ballweg ML, Nieman LK, Stratton P. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod. 2002;17(10):2715-2724.
- Struble J, Reid S, Bedaiwy MA. Adenomyosis: a clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol. 2016;23(2):164-185.
- Tirlapur SA, Kuhrt K, Chaliha C. The ‘evil twin syndrome’ in chronic pelvic pain: a systematic review of prevalence studies of bladder pain syndrome and endometriosis. Int J Surg. 2013;11(3):233-237.
- Coxon L, Horne AW, Vincent K. Pathophysiology of endometriosis-associated pain: a review of pelvic and central nervous system mechanisms. Best Pract Res Clin Obstet Gynaecol. 2018 Feb 15. pii: S1521-6934(18)30032-4. doi: 10.1016/j.bpobgyn.2018.01.014. [Epub ahead of print]
- Yan D, Liu X, Guo SW. Nerve fibers and endometriotic lesions: partners in crime in inflicting pains in women with endometriosis. Eur J Obstet Gynecol Reprod Biol. 2017;209:14-24.
- Vercellini P, Fedele L, Aimi G, Pietropaolo G, Consonni D, Crosignani PG. Association between endometriosis stage, lesion type, patient characteristics and severity of pelvic pain symptoms: a multivariate analysis of over 1000 patients. Hum Reprod. 2007;22(1):266-271.
- Fedele L, Parazzini F, Bianchi S. Stage and localization of pelvic endometriosis and pain. Fertil Steril. 1990;53(1):155-158.
- Berkley KJ, Rapkin AJ, Papka RE. The pains of endometriosis. Science. 2005;308(5728):1587-1589.
- Giamberardino MA, Tana C, Costantini R. Pain thresholds in women with chronic pelvic pain. Curr Opin Obstet Gynecol. 2014;26(4):253-259.
- Giamberardino MA, Berkley KJ, Affaitati G. Influence of endometriosis on pain behaviors and muscle hyperalgesia induced by a ureteral calculosis in female rats. Pain. 2002;95(3):247-257.
- As-Sanie S, Kim J, Schmidt-Wilcke T. Functional connectivity is associated with altered brain chemistry in women with endometriosis-associated chronic pelvic pain. J Pain. 2016;17(1):1-13.
- Olive DL, Lindheim SR, Pritts EA. New medical treatments for endometriosis. Best Pract Res Clin Obstet Gynaecol. 2004;18(2):319-328.
- Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364(9447): 1789-1799.
- Nnoaham KE, Hummelshoj L, Webster P, et al; World Endometriosis Research Foundation Global Study of Women’s Health consortium. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril. 2011;96(2):366-373.e8.
- Simoens S, Dunselman G, Dirksen C, et al. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod. 2012;27(5):1292–1299.
- Bruner-Tran KL, Mokshagundam S, Herington JL. Rodent models of experimental endometriosis: identifying mechanisms of disease and therapeutic targets. Curr Womens Health Rev. 2018;14(2):173-188.
- Sinaii N, Cleary SD, Ballweg ML, Nieman LK, Stratton P. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod. 2002;17(10):2715-2724.
- Struble J, Reid S, Bedaiwy MA. Adenomyosis: a clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol. 2016;23(2):164-185.
- Tirlapur SA, Kuhrt K, Chaliha C. The ‘evil twin syndrome’ in chronic pelvic pain: a systematic review of prevalence studies of bladder pain syndrome and endometriosis. Int J Surg. 2013;11(3):233-237.
- Coxon L, Horne AW, Vincent K. Pathophysiology of endometriosis-associated pain: a review of pelvic and central nervous system mechanisms. Best Pract Res Clin Obstet Gynaecol. 2018 Feb 15. pii: S1521-6934(18)30032-4. doi: 10.1016/j.bpobgyn.2018.01.014. [Epub ahead of print]
- Yan D, Liu X, Guo SW. Nerve fibers and endometriotic lesions: partners in crime in inflicting pains in women with endometriosis. Eur J Obstet Gynecol Reprod Biol. 2017;209:14-24.
- Vercellini P, Fedele L, Aimi G, Pietropaolo G, Consonni D, Crosignani PG. Association between endometriosis stage, lesion type, patient characteristics and severity of pelvic pain symptoms: a multivariate analysis of over 1000 patients. Hum Reprod. 2007;22(1):266-271.
- Fedele L, Parazzini F, Bianchi S. Stage and localization of pelvic endometriosis and pain. Fertil Steril. 1990;53(1):155-158.
- Berkley KJ, Rapkin AJ, Papka RE. The pains of endometriosis. Science. 2005;308(5728):1587-1589.
- Giamberardino MA, Tana C, Costantini R. Pain thresholds in women with chronic pelvic pain. Curr Opin Obstet Gynecol. 2014;26(4):253-259.
- Giamberardino MA, Berkley KJ, Affaitati G. Influence of endometriosis on pain behaviors and muscle hyperalgesia induced by a ureteral calculosis in female rats. Pain. 2002;95(3):247-257.
- As-Sanie S, Kim J, Schmidt-Wilcke T. Functional connectivity is associated with altered brain chemistry in women with endometriosis-associated chronic pelvic pain. J Pain. 2016;17(1):1-13.
2018 Update on bone health
As ObGyns, we are the first-line health care providers for our menopausal patients in terms of identifying, preventing, and initiating treatment for women at risk for fragility fractures. Osteoporosis is probably the most important risk factor for bone health, although sarcopenia, frailty, poor eyesight, and falls also play a significant role in bone health and fragility fracture.
In 2005, more than 2 million incident fractures were reported in the United States, with a total cost of $17 billion.1 By 2025, annual fractures and costs are expected to rise by almost 50%. People who are 65 to 74 years of age will likely experience the largest increase in fracture—greater than 87%.1
Findings from the Women’s Health Initiative study showed that the number of women who had a clinical fracture in 1 year exceeded all the cases of myocardial infarction, stroke, and breast cancer combined.2 Furthermore, the morbidity and mortality rates for fractures are staggering. Thirty percent of women with a hip fracture will be dead within 1 year.3 So, although many patients fear developing breast cancer, and cardiovascular disease remains the number 1 cause of death, the impact of maintaining and protecting bone health cannot be emphasized enough.

_
WHI incidental findings: Hormone-treated menopausal women had decreased hip fracture rate
Manson JE, Aragaki AK, Rossouw JE, et al; WHI Investigators. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318:927-938.
Manson and colleagues examined the total and cause-specific cumulative mortality of the 2 Women’s Health Initiative (WHI) hormone therapy trials. This was an observational follow-up of US multiethnic postmenopausal women aged 50 to 79 years (mean age at baseline, 63.4 years) enrolled in 2 randomized clinical trials between 1993 and 1998 and followed up through December 31, 2014. A total of 27,347 women were randomly assigned to treatment.
Treatment groups
Depending on the presence or absence of a uterus, women received conjugated equine estrogens (CEE, 0.625 mg/d) plus medroxyprogesterone acetate (MPA, 2.5 mg/d) (n = 8,506) or placebo (n = 8,102) for a median of 5.6 years or CEE alone (n = 5,310) versus placebo (n = 5,429) for a median of 7.2 years. All-cause mortality (the primary outcome) and cause-specific mortality (cardiovascular disease mortality, cancer mortality, and other major causes of mortality) were analyzed in the 2 trials pooled and in each trial individually.
All-cause and cause-specific mortality findings
Mortality follow-up was available for more than 98% of participants. During the cumulative 18-year follow-up, 7,489 deaths occurred. In the overall pooled cohort, all-cause mortality in the hormone therapy group was 27.1% compared with 27.6% in the placebo group (hazard ratio [HR], 0.99 [95% confidence interval (CI), 0.94–1.03]). In the CEE plus MPA group, the HR was 1.02 (95% CI, 0.96–1.08). For those in the CEE-alone group, the HR was 0.94 (95% CI, 0.88–1.01).
In the pooled cohort for cardiovascular mortality, the HR was 1.00 (95% CI, 0.92–1.08 [8.9% with hormone therapy vs 9.0% with placebo]). For total cancer mortality, the HR was 1.03 (95% CI, 0.95–1.12 [8.2% with hormone therapy vs 8.0% with placebo]). For other causes, the HR was 0.95 (95% CI, 0.88–1.02 [10.0% with hormone therapy vs 10.7% with placebo]). Results did not differ significantly between trials.
Key takeaway
The study authors concluded that among postmenopausal women, hormone therapy with CEE plus MPA for a median of 5.6 years or with CEE alone for a median of 7.2 years was not associated with risk of all-cause, cardiovascular, or cancer mortality during a cumulative follow-up of 18 years.
Postmenopausal hormone therapy is arguably the most effective “bone drug” available. While all other antiresorptive agents show hip fracture efficacy only in subgroup analyses of the highest-risk patients (women with established osteoporosis, who often already have pre-existing vertebral fractures), the hormone-treated women in the WHI—who were not chosen for having low bone mass (in fact, dual-energy x-ray absorptiometry [DXA] scores were not even recorded)—still had a statistically significant decrease in hip fracture as an adverse event when compared with placebo-treated women. Increasing data on the long-term safety of hormone therapy in menopausal patients will perhaps encourage its greater use from a bone health perspective.
Continue to: Appropriate to defer DXA testing to age 65...
Appropriate to defer DXA testing to age 65 when baseline FRAX score is below treatment level
Gourlay ML, Overman RA, Fine JP, et al; Women’s Health Initiative Investigators. Time to clinically relevant fracture risk scores in postmenopausal women. Am J Med. 2017;130:862.e15-862.e23.
Gourlay ML, Fine JP, Preisser JS, et al; Study of Osteoporotic Fractures Research Group. Bone-density testing interval and transition to osteoporosis in older women. N Engl J Med. 2012;366:225-233.
Many clinicians used to (and still do) order bone mineral density (BMD) testing at 23-month intervals because that was what insurance would allow. Gourlay and colleagues previously published a study on BMD testing intervals and the time it takes to develop osteoporosis. I covered that information in previous Updates.4,5
To recap, Gourlay and colleagues studied 4,957 women, 67 years of age or older, with normal BMD or osteopenia and with no history of hip or clinical vertebral fracture or of treatment for osteoporosis; the women were followed prospectively for up to 15 years. The estimated time for 10% of women to make the transition to osteoporosis was 16.8 years for those with normal BMD, 4.7 years for those with moderate osteopenia, and 1.1 years for women with advanced osteopenia.
Today, FRAX is recommended to assess need for treatment
Older treatment recommendations involved determining various osteopenic BMD levels and the presence or absence of certain risk factors. More recently, the National Osteoporosis Foundation and many medical societies, including the American College of Obstetricians and Gynecologists, have recommended using the FRAX fracture prediction algorithm (available at https://www.sheffield.ac.uk/FRAX/) instead of T-scores to consider initiating pharmacotherapy.
The FRAX calculation tool uses information such as the country where the patient lives, age, sex, height, weight, history of previous fracture, parental fracture, current smoking, glucocorticoid use, rheumatoid arthritis, secondary osteoporosis, alcohol use of 3 or more units per day, and, if available, BMD determination at the femoral neck. It then yields the 10-year absolute risk of hip fracture and any major osteoporotic fracture for that individual or, more precisely, for an individual like that.
In the United States, accepted levels for cost-effective pharmacotherapy are a 10-year absolute risk of hip fracture of 3% or major osteoporotic fracture of 20%.
Continue to: Age also is a key factor in fracture risk assessment
Age also is a key factor in fracture risk assessment
Gourlay and colleagues more recently conducted a retrospective analysis of new occurrence of treatment-level fracture risk scores in postmenopausal women (50 years of age and older) before they received pharmacologic treatment and before they experienced a first hip or clinical vertebral fracture.
In 54,280 postmenopausal women aged 50 to 64 without a BMD test, the time for 10% to develop a treatment-level FRAX score could not be estimated accurately because of the rarity of treatment-level scores. In 6,096 women who had FRAX scores calculated with their BMD score, the estimated time to treatment-level FRAX was 7.6 years for those 65 to 69 and 5.1 years for 75 to 79 year olds. Furthermore, of 17,967 women aged 50 to 64 with a screening-level FRAX at baseline, only 100 (0.6%) experienced a hip or clinical vertebral fracture by age 65.
The investigators concluded that, “Postmenopausal women with sub-threshold fracture risk scores at baseline were unlikely to develop a treatment-level FRAX score between ages 50 and 64 years. After age 65, the increased incidence of treatment-level fracture risk scores, osteoporosis, and major osteoporotic fracture supports more frequent consideration of FRAX and bone mineral density testing.”
Many health care providers begin BMD testing early in menopause. Bone mass results may motivate patients to initiate healthy lifestyle choices, such as adequate dietary calcium, vitamin D supplementation, exercise, moderate alcohol use, smoking cessation, and fall prevention strategies. However, providers and their patients should be aware that if the fracture risk is beneath the threshold score at baseline, the risk of experiencing an osteoporotic fracture prior to age 65 is extremely low, and this should be taken into account before prescribing pharmacotherapy. Furthermore, as stated, FRAX can be performed without a DXA score. When the result is beneath a treatment level in a woman under 65, DXA testing may be deferred until age 65.
Continue to: USPSTF offers updated recommendations for osteoporosis screening
USPSTF offers updated recommendations for osteoporosis screening
US Preventive Services Task Force, Curry SJ, Krist AH, Owens DK, et al. Screening for osteoporosis to prevent fractures: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:2521-2531.
The 2018 updated osteoporosis screening recommendations from the United States Preventative Services Task Force (USPSTF) may seem contradictory to the conclusions of Gourlay and colleagues discussed above. They are not.
The USPSTF authors point out that by 2020, about 12.3 million US individuals older than 50 years are expected to have osteoporosis. Osteoporotic fractures (especially hip fractures) are associated with limitations in ambulation, chronic pain and disability, loss of independence, and decreased quality of life. In fact, 21% to 30% of people who sustain a hip fracture die within 1 year. As the US population continues to age, the potential preventable burden will likely increase.
_
Evidence on bone measurement tests, risk assessment tools, and drug therapy efficacy
The USPSTF conducted an evidence review on screening for and treatment of osteoporotic fractures in women as well as risk assessment tools. The task force found the evidence convincing that bone measurement tests are accurate for detecting osteoporosis and predicting osteoporotic fractures. In addition, there is adequate evidence that clinical risk assessment tools are moderately accurate in identifying risk of osteoporosis and osteoporotic fractures. Furthermore, there is convincing evidence that drug therapies reduce subsequent fracture rates in postmenopausal women.
The USPSTF recommends the following:
- For women aged 65 and older, screen for osteoporosis with bone measurement testing to prevent osteoporotic fractures.
- For women younger than 65 who are at increased risk for osteoporosis based on formal clinical risk assessment tools, screen for osteoporosis with bone measurement testing to prevent osteoporotic fractures.
We all agree that women older than 65 years of age should be screened with DXA measurements of bone mass. The USPSTF says that in women under 65, a fracture assessment tool like FRAX, which does not require bone density testing to yield an individual’s absolute 10-year fracture risk, should be used to determine if bone mass measurement by DXA is, in fact, warranted. This recommendation is further supported by the article by Gourlay and colleagues, in which women aged 50 to 64 with subthreshold FRAX scores had a very low risk of fracture prior to age 65.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Burge R, Dawson-Hughes B, Solomon DH, et al. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22:465-475.
- Cauley JA, Wampler NS, Barnhart JM, et al; Women’s Health Initiative Observational Study. Incidence of fractures compared to cardiovascular disease and breast cancer: the Women’s Health Initiative Observational Study. Osteoporos Int. 2008;19:1717-1723.
- Brauer CA, Coca-Perraillon M, Cutler DM, et al. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302:1573-1579.
- Goldstein SR. Update on osteoporosis. OBG Manag. 2012;24:16-21.
- Goldstein SR. 2017 update on bone health. OBG Manag. 2017;29-32, 48.
As ObGyns, we are the first-line health care providers for our menopausal patients in terms of identifying, preventing, and initiating treatment for women at risk for fragility fractures. Osteoporosis is probably the most important risk factor for bone health, although sarcopenia, frailty, poor eyesight, and falls also play a significant role in bone health and fragility fracture.
In 2005, more than 2 million incident fractures were reported in the United States, with a total cost of $17 billion.1 By 2025, annual fractures and costs are expected to rise by almost 50%. People who are 65 to 74 years of age will likely experience the largest increase in fracture—greater than 87%.1
Findings from the Women’s Health Initiative study showed that the number of women who had a clinical fracture in 1 year exceeded all the cases of myocardial infarction, stroke, and breast cancer combined.2 Furthermore, the morbidity and mortality rates for fractures are staggering. Thirty percent of women with a hip fracture will be dead within 1 year.3 So, although many patients fear developing breast cancer, and cardiovascular disease remains the number 1 cause of death, the impact of maintaining and protecting bone health cannot be emphasized enough.

_
WHI incidental findings: Hormone-treated menopausal women had decreased hip fracture rate
Manson JE, Aragaki AK, Rossouw JE, et al; WHI Investigators. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318:927-938.
Manson and colleagues examined the total and cause-specific cumulative mortality of the 2 Women’s Health Initiative (WHI) hormone therapy trials. This was an observational follow-up of US multiethnic postmenopausal women aged 50 to 79 years (mean age at baseline, 63.4 years) enrolled in 2 randomized clinical trials between 1993 and 1998 and followed up through December 31, 2014. A total of 27,347 women were randomly assigned to treatment.
Treatment groups
Depending on the presence or absence of a uterus, women received conjugated equine estrogens (CEE, 0.625 mg/d) plus medroxyprogesterone acetate (MPA, 2.5 mg/d) (n = 8,506) or placebo (n = 8,102) for a median of 5.6 years or CEE alone (n = 5,310) versus placebo (n = 5,429) for a median of 7.2 years. All-cause mortality (the primary outcome) and cause-specific mortality (cardiovascular disease mortality, cancer mortality, and other major causes of mortality) were analyzed in the 2 trials pooled and in each trial individually.
All-cause and cause-specific mortality findings
Mortality follow-up was available for more than 98% of participants. During the cumulative 18-year follow-up, 7,489 deaths occurred. In the overall pooled cohort, all-cause mortality in the hormone therapy group was 27.1% compared with 27.6% in the placebo group (hazard ratio [HR], 0.99 [95% confidence interval (CI), 0.94–1.03]). In the CEE plus MPA group, the HR was 1.02 (95% CI, 0.96–1.08). For those in the CEE-alone group, the HR was 0.94 (95% CI, 0.88–1.01).
In the pooled cohort for cardiovascular mortality, the HR was 1.00 (95% CI, 0.92–1.08 [8.9% with hormone therapy vs 9.0% with placebo]). For total cancer mortality, the HR was 1.03 (95% CI, 0.95–1.12 [8.2% with hormone therapy vs 8.0% with placebo]). For other causes, the HR was 0.95 (95% CI, 0.88–1.02 [10.0% with hormone therapy vs 10.7% with placebo]). Results did not differ significantly between trials.
Key takeaway
The study authors concluded that among postmenopausal women, hormone therapy with CEE plus MPA for a median of 5.6 years or with CEE alone for a median of 7.2 years was not associated with risk of all-cause, cardiovascular, or cancer mortality during a cumulative follow-up of 18 years.
Postmenopausal hormone therapy is arguably the most effective “bone drug” available. While all other antiresorptive agents show hip fracture efficacy only in subgroup analyses of the highest-risk patients (women with established osteoporosis, who often already have pre-existing vertebral fractures), the hormone-treated women in the WHI—who were not chosen for having low bone mass (in fact, dual-energy x-ray absorptiometry [DXA] scores were not even recorded)—still had a statistically significant decrease in hip fracture as an adverse event when compared with placebo-treated women. Increasing data on the long-term safety of hormone therapy in menopausal patients will perhaps encourage its greater use from a bone health perspective.
Continue to: Appropriate to defer DXA testing to age 65...
Appropriate to defer DXA testing to age 65 when baseline FRAX score is below treatment level
Gourlay ML, Overman RA, Fine JP, et al; Women’s Health Initiative Investigators. Time to clinically relevant fracture risk scores in postmenopausal women. Am J Med. 2017;130:862.e15-862.e23.
Gourlay ML, Fine JP, Preisser JS, et al; Study of Osteoporotic Fractures Research Group. Bone-density testing interval and transition to osteoporosis in older women. N Engl J Med. 2012;366:225-233.
Many clinicians used to (and still do) order bone mineral density (BMD) testing at 23-month intervals because that was what insurance would allow. Gourlay and colleagues previously published a study on BMD testing intervals and the time it takes to develop osteoporosis. I covered that information in previous Updates.4,5
To recap, Gourlay and colleagues studied 4,957 women, 67 years of age or older, with normal BMD or osteopenia and with no history of hip or clinical vertebral fracture or of treatment for osteoporosis; the women were followed prospectively for up to 15 years. The estimated time for 10% of women to make the transition to osteoporosis was 16.8 years for those with normal BMD, 4.7 years for those with moderate osteopenia, and 1.1 years for women with advanced osteopenia.
Today, FRAX is recommended to assess need for treatment
Older treatment recommendations involved determining various osteopenic BMD levels and the presence or absence of certain risk factors. More recently, the National Osteoporosis Foundation and many medical societies, including the American College of Obstetricians and Gynecologists, have recommended using the FRAX fracture prediction algorithm (available at https://www.sheffield.ac.uk/FRAX/) instead of T-scores to consider initiating pharmacotherapy.
The FRAX calculation tool uses information such as the country where the patient lives, age, sex, height, weight, history of previous fracture, parental fracture, current smoking, glucocorticoid use, rheumatoid arthritis, secondary osteoporosis, alcohol use of 3 or more units per day, and, if available, BMD determination at the femoral neck. It then yields the 10-year absolute risk of hip fracture and any major osteoporotic fracture for that individual or, more precisely, for an individual like that.
In the United States, accepted levels for cost-effective pharmacotherapy are a 10-year absolute risk of hip fracture of 3% or major osteoporotic fracture of 20%.
Continue to: Age also is a key factor in fracture risk assessment
Age also is a key factor in fracture risk assessment
Gourlay and colleagues more recently conducted a retrospective analysis of new occurrence of treatment-level fracture risk scores in postmenopausal women (50 years of age and older) before they received pharmacologic treatment and before they experienced a first hip or clinical vertebral fracture.
In 54,280 postmenopausal women aged 50 to 64 without a BMD test, the time for 10% to develop a treatment-level FRAX score could not be estimated accurately because of the rarity of treatment-level scores. In 6,096 women who had FRAX scores calculated with their BMD score, the estimated time to treatment-level FRAX was 7.6 years for those 65 to 69 and 5.1 years for 75 to 79 year olds. Furthermore, of 17,967 women aged 50 to 64 with a screening-level FRAX at baseline, only 100 (0.6%) experienced a hip or clinical vertebral fracture by age 65.
The investigators concluded that, “Postmenopausal women with sub-threshold fracture risk scores at baseline were unlikely to develop a treatment-level FRAX score between ages 50 and 64 years. After age 65, the increased incidence of treatment-level fracture risk scores, osteoporosis, and major osteoporotic fracture supports more frequent consideration of FRAX and bone mineral density testing.”
Many health care providers begin BMD testing early in menopause. Bone mass results may motivate patients to initiate healthy lifestyle choices, such as adequate dietary calcium, vitamin D supplementation, exercise, moderate alcohol use, smoking cessation, and fall prevention strategies. However, providers and their patients should be aware that if the fracture risk is beneath the threshold score at baseline, the risk of experiencing an osteoporotic fracture prior to age 65 is extremely low, and this should be taken into account before prescribing pharmacotherapy. Furthermore, as stated, FRAX can be performed without a DXA score. When the result is beneath a treatment level in a woman under 65, DXA testing may be deferred until age 65.
Continue to: USPSTF offers updated recommendations for osteoporosis screening
USPSTF offers updated recommendations for osteoporosis screening
US Preventive Services Task Force, Curry SJ, Krist AH, Owens DK, et al. Screening for osteoporosis to prevent fractures: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:2521-2531.
The 2018 updated osteoporosis screening recommendations from the United States Preventative Services Task Force (USPSTF) may seem contradictory to the conclusions of Gourlay and colleagues discussed above. They are not.
The USPSTF authors point out that by 2020, about 12.3 million US individuals older than 50 years are expected to have osteoporosis. Osteoporotic fractures (especially hip fractures) are associated with limitations in ambulation, chronic pain and disability, loss of independence, and decreased quality of life. In fact, 21% to 30% of people who sustain a hip fracture die within 1 year. As the US population continues to age, the potential preventable burden will likely increase.
_
Evidence on bone measurement tests, risk assessment tools, and drug therapy efficacy
The USPSTF conducted an evidence review on screening for and treatment of osteoporotic fractures in women as well as risk assessment tools. The task force found the evidence convincing that bone measurement tests are accurate for detecting osteoporosis and predicting osteoporotic fractures. In addition, there is adequate evidence that clinical risk assessment tools are moderately accurate in identifying risk of osteoporosis and osteoporotic fractures. Furthermore, there is convincing evidence that drug therapies reduce subsequent fracture rates in postmenopausal women.
The USPSTF recommends the following:
- For women aged 65 and older, screen for osteoporosis with bone measurement testing to prevent osteoporotic fractures.
- For women younger than 65 who are at increased risk for osteoporosis based on formal clinical risk assessment tools, screen for osteoporosis with bone measurement testing to prevent osteoporotic fractures.
We all agree that women older than 65 years of age should be screened with DXA measurements of bone mass. The USPSTF says that in women under 65, a fracture assessment tool like FRAX, which does not require bone density testing to yield an individual’s absolute 10-year fracture risk, should be used to determine if bone mass measurement by DXA is, in fact, warranted. This recommendation is further supported by the article by Gourlay and colleagues, in which women aged 50 to 64 with subthreshold FRAX scores had a very low risk of fracture prior to age 65.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
As ObGyns, we are the first-line health care providers for our menopausal patients in terms of identifying, preventing, and initiating treatment for women at risk for fragility fractures. Osteoporosis is probably the most important risk factor for bone health, although sarcopenia, frailty, poor eyesight, and falls also play a significant role in bone health and fragility fracture.
In 2005, more than 2 million incident fractures were reported in the United States, with a total cost of $17 billion.1 By 2025, annual fractures and costs are expected to rise by almost 50%. People who are 65 to 74 years of age will likely experience the largest increase in fracture—greater than 87%.1
Findings from the Women’s Health Initiative study showed that the number of women who had a clinical fracture in 1 year exceeded all the cases of myocardial infarction, stroke, and breast cancer combined.2 Furthermore, the morbidity and mortality rates for fractures are staggering. Thirty percent of women with a hip fracture will be dead within 1 year.3 So, although many patients fear developing breast cancer, and cardiovascular disease remains the number 1 cause of death, the impact of maintaining and protecting bone health cannot be emphasized enough.

_
WHI incidental findings: Hormone-treated menopausal women had decreased hip fracture rate
Manson JE, Aragaki AK, Rossouw JE, et al; WHI Investigators. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318:927-938.
Manson and colleagues examined the total and cause-specific cumulative mortality of the 2 Women’s Health Initiative (WHI) hormone therapy trials. This was an observational follow-up of US multiethnic postmenopausal women aged 50 to 79 years (mean age at baseline, 63.4 years) enrolled in 2 randomized clinical trials between 1993 and 1998 and followed up through December 31, 2014. A total of 27,347 women were randomly assigned to treatment.
Treatment groups
Depending on the presence or absence of a uterus, women received conjugated equine estrogens (CEE, 0.625 mg/d) plus medroxyprogesterone acetate (MPA, 2.5 mg/d) (n = 8,506) or placebo (n = 8,102) for a median of 5.6 years or CEE alone (n = 5,310) versus placebo (n = 5,429) for a median of 7.2 years. All-cause mortality (the primary outcome) and cause-specific mortality (cardiovascular disease mortality, cancer mortality, and other major causes of mortality) were analyzed in the 2 trials pooled and in each trial individually.
All-cause and cause-specific mortality findings
Mortality follow-up was available for more than 98% of participants. During the cumulative 18-year follow-up, 7,489 deaths occurred. In the overall pooled cohort, all-cause mortality in the hormone therapy group was 27.1% compared with 27.6% in the placebo group (hazard ratio [HR], 0.99 [95% confidence interval (CI), 0.94–1.03]). In the CEE plus MPA group, the HR was 1.02 (95% CI, 0.96–1.08). For those in the CEE-alone group, the HR was 0.94 (95% CI, 0.88–1.01).
In the pooled cohort for cardiovascular mortality, the HR was 1.00 (95% CI, 0.92–1.08 [8.9% with hormone therapy vs 9.0% with placebo]). For total cancer mortality, the HR was 1.03 (95% CI, 0.95–1.12 [8.2% with hormone therapy vs 8.0% with placebo]). For other causes, the HR was 0.95 (95% CI, 0.88–1.02 [10.0% with hormone therapy vs 10.7% with placebo]). Results did not differ significantly between trials.
Key takeaway
The study authors concluded that among postmenopausal women, hormone therapy with CEE plus MPA for a median of 5.6 years or with CEE alone for a median of 7.2 years was not associated with risk of all-cause, cardiovascular, or cancer mortality during a cumulative follow-up of 18 years.
Postmenopausal hormone therapy is arguably the most effective “bone drug” available. While all other antiresorptive agents show hip fracture efficacy only in subgroup analyses of the highest-risk patients (women with established osteoporosis, who often already have pre-existing vertebral fractures), the hormone-treated women in the WHI—who were not chosen for having low bone mass (in fact, dual-energy x-ray absorptiometry [DXA] scores were not even recorded)—still had a statistically significant decrease in hip fracture as an adverse event when compared with placebo-treated women. Increasing data on the long-term safety of hormone therapy in menopausal patients will perhaps encourage its greater use from a bone health perspective.
Continue to: Appropriate to defer DXA testing to age 65...
Appropriate to defer DXA testing to age 65 when baseline FRAX score is below treatment level
Gourlay ML, Overman RA, Fine JP, et al; Women’s Health Initiative Investigators. Time to clinically relevant fracture risk scores in postmenopausal women. Am J Med. 2017;130:862.e15-862.e23.
Gourlay ML, Fine JP, Preisser JS, et al; Study of Osteoporotic Fractures Research Group. Bone-density testing interval and transition to osteoporosis in older women. N Engl J Med. 2012;366:225-233.
Many clinicians used to (and still do) order bone mineral density (BMD) testing at 23-month intervals because that was what insurance would allow. Gourlay and colleagues previously published a study on BMD testing intervals and the time it takes to develop osteoporosis. I covered that information in previous Updates.4,5
To recap, Gourlay and colleagues studied 4,957 women, 67 years of age or older, with normal BMD or osteopenia and with no history of hip or clinical vertebral fracture or of treatment for osteoporosis; the women were followed prospectively for up to 15 years. The estimated time for 10% of women to make the transition to osteoporosis was 16.8 years for those with normal BMD, 4.7 years for those with moderate osteopenia, and 1.1 years for women with advanced osteopenia.
Today, FRAX is recommended to assess need for treatment
Older treatment recommendations involved determining various osteopenic BMD levels and the presence or absence of certain risk factors. More recently, the National Osteoporosis Foundation and many medical societies, including the American College of Obstetricians and Gynecologists, have recommended using the FRAX fracture prediction algorithm (available at https://www.sheffield.ac.uk/FRAX/) instead of T-scores to consider initiating pharmacotherapy.
The FRAX calculation tool uses information such as the country where the patient lives, age, sex, height, weight, history of previous fracture, parental fracture, current smoking, glucocorticoid use, rheumatoid arthritis, secondary osteoporosis, alcohol use of 3 or more units per day, and, if available, BMD determination at the femoral neck. It then yields the 10-year absolute risk of hip fracture and any major osteoporotic fracture for that individual or, more precisely, for an individual like that.
In the United States, accepted levels for cost-effective pharmacotherapy are a 10-year absolute risk of hip fracture of 3% or major osteoporotic fracture of 20%.
Continue to: Age also is a key factor in fracture risk assessment
Age also is a key factor in fracture risk assessment
Gourlay and colleagues more recently conducted a retrospective analysis of new occurrence of treatment-level fracture risk scores in postmenopausal women (50 years of age and older) before they received pharmacologic treatment and before they experienced a first hip or clinical vertebral fracture.
In 54,280 postmenopausal women aged 50 to 64 without a BMD test, the time for 10% to develop a treatment-level FRAX score could not be estimated accurately because of the rarity of treatment-level scores. In 6,096 women who had FRAX scores calculated with their BMD score, the estimated time to treatment-level FRAX was 7.6 years for those 65 to 69 and 5.1 years for 75 to 79 year olds. Furthermore, of 17,967 women aged 50 to 64 with a screening-level FRAX at baseline, only 100 (0.6%) experienced a hip or clinical vertebral fracture by age 65.
The investigators concluded that, “Postmenopausal women with sub-threshold fracture risk scores at baseline were unlikely to develop a treatment-level FRAX score between ages 50 and 64 years. After age 65, the increased incidence of treatment-level fracture risk scores, osteoporosis, and major osteoporotic fracture supports more frequent consideration of FRAX and bone mineral density testing.”
Many health care providers begin BMD testing early in menopause. Bone mass results may motivate patients to initiate healthy lifestyle choices, such as adequate dietary calcium, vitamin D supplementation, exercise, moderate alcohol use, smoking cessation, and fall prevention strategies. However, providers and their patients should be aware that if the fracture risk is beneath the threshold score at baseline, the risk of experiencing an osteoporotic fracture prior to age 65 is extremely low, and this should be taken into account before prescribing pharmacotherapy. Furthermore, as stated, FRAX can be performed without a DXA score. When the result is beneath a treatment level in a woman under 65, DXA testing may be deferred until age 65.
Continue to: USPSTF offers updated recommendations for osteoporosis screening
USPSTF offers updated recommendations for osteoporosis screening
US Preventive Services Task Force, Curry SJ, Krist AH, Owens DK, et al. Screening for osteoporosis to prevent fractures: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:2521-2531.
The 2018 updated osteoporosis screening recommendations from the United States Preventative Services Task Force (USPSTF) may seem contradictory to the conclusions of Gourlay and colleagues discussed above. They are not.
The USPSTF authors point out that by 2020, about 12.3 million US individuals older than 50 years are expected to have osteoporosis. Osteoporotic fractures (especially hip fractures) are associated with limitations in ambulation, chronic pain and disability, loss of independence, and decreased quality of life. In fact, 21% to 30% of people who sustain a hip fracture die within 1 year. As the US population continues to age, the potential preventable burden will likely increase.
_
Evidence on bone measurement tests, risk assessment tools, and drug therapy efficacy
The USPSTF conducted an evidence review on screening for and treatment of osteoporotic fractures in women as well as risk assessment tools. The task force found the evidence convincing that bone measurement tests are accurate for detecting osteoporosis and predicting osteoporotic fractures. In addition, there is adequate evidence that clinical risk assessment tools are moderately accurate in identifying risk of osteoporosis and osteoporotic fractures. Furthermore, there is convincing evidence that drug therapies reduce subsequent fracture rates in postmenopausal women.
The USPSTF recommends the following:
- For women aged 65 and older, screen for osteoporosis with bone measurement testing to prevent osteoporotic fractures.
- For women younger than 65 who are at increased risk for osteoporosis based on formal clinical risk assessment tools, screen for osteoporosis with bone measurement testing to prevent osteoporotic fractures.
We all agree that women older than 65 years of age should be screened with DXA measurements of bone mass. The USPSTF says that in women under 65, a fracture assessment tool like FRAX, which does not require bone density testing to yield an individual’s absolute 10-year fracture risk, should be used to determine if bone mass measurement by DXA is, in fact, warranted. This recommendation is further supported by the article by Gourlay and colleagues, in which women aged 50 to 64 with subthreshold FRAX scores had a very low risk of fracture prior to age 65.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Burge R, Dawson-Hughes B, Solomon DH, et al. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22:465-475.
- Cauley JA, Wampler NS, Barnhart JM, et al; Women’s Health Initiative Observational Study. Incidence of fractures compared to cardiovascular disease and breast cancer: the Women’s Health Initiative Observational Study. Osteoporos Int. 2008;19:1717-1723.
- Brauer CA, Coca-Perraillon M, Cutler DM, et al. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302:1573-1579.
- Goldstein SR. Update on osteoporosis. OBG Manag. 2012;24:16-21.
- Goldstein SR. 2017 update on bone health. OBG Manag. 2017;29-32, 48.
- Burge R, Dawson-Hughes B, Solomon DH, et al. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22:465-475.
- Cauley JA, Wampler NS, Barnhart JM, et al; Women’s Health Initiative Observational Study. Incidence of fractures compared to cardiovascular disease and breast cancer: the Women’s Health Initiative Observational Study. Osteoporos Int. 2008;19:1717-1723.
- Brauer CA, Coca-Perraillon M, Cutler DM, et al. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302:1573-1579.
- Goldstein SR. Update on osteoporosis. OBG Manag. 2012;24:16-21.
- Goldstein SR. 2017 update on bone health. OBG Manag. 2017;29-32, 48.
Adults with Congenital Heart Disease: The Critical Transition from Pediatric to Adult Care
From the Greenville Health System, Greenville, SC.
Abstract
- Objective: To review the management of patients with congenital heart disease (CHD) transitioning from pediatric to adult care.
- Methods: Review of the literature.
- Results: Persons with CHD require close monitoring and evaluation throughout life to address the physiologic consequences of acquired cardiopulmonary, gastrointestinal, and renal disease in the setting of underlying congenital heart lesions. During the transition from pediatric to adult cardiology, a high proportion of patients are lost to follow up or have long gaps in care after leaving pediatric cardiology, which can lead to poor outcomes. Care of the adult with CHD requires close coordination between the patient’s primary care physician), cardiologist, adult CHD specialist, and other specialists. The transition process for CHD patients begin at 12 years of age, with a goal of discussing future expectations of the child’s education, employment, and independent living. Successful transition programs use a systematic approach to addressing the medical, psychosocial, and educational/vocational needs of the adolescent as he or she moves from the family-centered pediatric to the patient-centered adult health care system.
- Conclusion: The transition from pediatric to adult care in ACHD patients is best provided through a comprehensive transition program that begins in early adolescence and enables patients to take charge of their disease process in adulthood, allowing them to maximize their quality of life and societal contributions.
Keywords: adult; congenital heart defects; complications; disease management; patient care team.
The population of adults with congenital heart disease (CHD) in developed countries has grown at an exponential rate in the past 4 decades. With advances in medical care and surgical interventions, the proportion of pediatric patients reaching adulthood has increased from 15% in the 1930s-60s to more than 95% for patients with mild to moderate complexity CHD. The rate of survival to adulthood for patients with severely complex CHD remains lower at around 56%.1
There are now more adult than pediatric patients with CHD in the United States. Because adult CHD (ACHD) patients have increased morbidity and mortality in their young adult years, it is imperative for all providers to understand and address the long-terms needs of this population. Unfortunately, adults with CHD do not always receive adequate health care, frequently because they are lost to follow-up, particularly during their adolescent years when they are expected to gain independence in their medical management. As will be discussed, CHD is a chronic illness fraught with numerous expected and unexpected complications that require close monitoring and re-interventions. Effectively anticipating and addressing these complications requires a standardized and comprehensive process of transition from the pediatric to the adult population to ensure maximal quality of life.
Epidemiology
The actual prevalence of ACHD in the United States is unknown, as a national database of persons with CHD has not been established.2 In contrast, Europe and China have maintained databases that enable ongoing monitoring of the evolving CHD epidemiology in those regions.3,4 The best estimates of the U.S. incidence and prevalence of ACHD stem from extrapolations from Canadian data. According to this data, there were more than 1.2 million adults with ACHD in the United States in 2012, with an anticipated 5% annual increase.1,5 However, the limitations of such extrapolations must be noted, as the Canadian population does not perfectly mirror that of the United States. Canada has lower infant mortality and adult obesity rates, and the United States has larger African American and Hispanic populations.6 Also, the juxtaposition of universal access to health care in Canada and the socioeconomic class–dependent access in the United States causes variations in care and outcomes of ACHD between the 2 populations. These differing genetic and social backgrounds may change the incidence of CHD by affecting maternal-fetal health.7
The 32nd Bethesda Conference on “Care of the Adult with Congenital Heart Disease” in 2000 was tasked with characterizing the ACHD population in the United States. This project found a prevalence similar to that of the Canadian extrapolation and showed that among persons with ACHD in the United States, 45% have mild disease, 37% moderate disease, and 13% severe disease.8
Characterizing the true incidence of CHD in the United States also has proven difficult because of variations in the definitions and methods used to detect lesions across the multiple studies that have looked at this matter. The estimated incidence of CHD, grouped according to severity, is 2.5 to 3 per 1000 live births for severe CHD, and from 3 to 13 per 1000 live births for moderately severe forms.9 When all forms are considered, including minor CHD (which includes tiny muscular ventricular septal defect [VSDs] present at birth and other trivial lesions), the total incidence of CHD rises to 75 per 1000 live births.9 CHD is one of the most common chronic illnesses in young adults with special health care needs.
Complications in Adulthood
The ACHD population represents a diverse population in terms of severity of CHD, history of surgical/catheter-based interventions, and socioeconomic status. However, a unifying clinical concern for these patients is their increased risk for morbidity and mortality in the young adult years. Despite the tremendous advances in the field over previous decades, mortality in this population in adulthood is estimated to be up to 7 times higher compared to age-matched peers.10,11 For many patients, palliative CHD interventions result in a significant drop in early morbidity and mortality but frequently lead to delayed morbidity from secondary multi-organ complications as these patients transition from pediatric to adult care. For example, due to the chronic low flow and low cardiac output state created by Fontan palliations, patients are at risk for diastolic dysfunction, arrhythmias, thrombotic events, protein-losing enteropathy, and cirrhosis/congestive hepatopathy, among other chronic conditions. These patients require frequent follow up and management by a multidisciplinary team including a primary care provider and various specialty groups.
Cardiac Disease
The most common causes of death in ACHD patients are heart failure (27%) and sudden cardiac death (19%), which occur at mean ages of 48 years and 39 years, respectively.10 The form of heart failure in ACHD patients is related to subsystemic right ventricle (RV) dysfunction, coronary under-perfusion, residual shunts, and residual progressive valve regurgitation. One of the more common examples of this is seen in palliated Tetralogy of Fallot patients who have undergone a transannular patch as a neonate. These patients are frequently left with significant pulmonary regurgitation leading to RV dilation, RV failure, and subsequent left ventricle (LV) failure. Another common example is the patient with dextro-transposition of the great arteries (DTGA) status post atrial switch who has a subsystemic morphologic RV. These patients will often develop significant RV dysfunction related to the chronic high pressures associated with systemic circulation.
Arrhythmias are a major contributor to morbidity and mortality in this population and are the most common reason patients present back into care. Difficult to control, multifocal intra-atrial re-entrant tachycardia is extremely common in ACHD, with an estimated 50% of all patients developing atrial arrhythmia by age 55. A recent study determined that the risk of atrial fibrillation in individuals with CHD was 22 times higher than that in age-matched controls, with the highest risk being seen in patients with conotruncal defects. Furthermore 10% of these patients develop heart failure.12 The risk for, incidence of, and type of arrhythmia is associated with the severity of the congenital heart lesions, as well as the type and timing of surgical interventions. Later age of repair has been associated with an increased likelihood of arrhythmias.13 Tetralogy of Fallot is an example of a moderately complex congenital heart lesion and is the most common cyanotic congenital heart lesion. In these individuals, the risk for atrial tachycardias, ventricular tachycardias, and need for a pacemaker is much higher than in age-matched peers.14 This includes an increased risk of sudden cardiac death, with many of these patients requiring placement of an implantable cardioverter defibrillator.
Pulmonary Disease
There is a 44% to 56% prevalence of restrictive pulmonary disease in the ACHD population, compared to 9% in the general non-CHD adult population. The incidence of pulmonary hypertension is also significantly higher in the ACHD population. The etiology for development of pulmonary hypertension is multifactorial, including chronic thromboembolic disease, left-sided heart disease, longstanding left to right shunts, and obstructive sleep apnea. These conditions have a significant impact on survival, as moderate/severe lung function impairment is an independent predictor of survival. Patients with shunt lesions are at risk of developing pulmonary arterial hypertension later in life,1 which quadruples the risk of all-cause mortality and more than triples the risk of cardiovascular mortality.7
Liver Disease
Hepatic morbidity associated with palliated CHD is often related to prior surgical interventions. The most common morbidities include chronic hepatitis C and liver failure from chronic under-perfusion and passive congestion, especially following Fontan palliation. Long term, these complications can lead to cirrhosis and hepatocellular carcinoma.15-18 Unfortunately, hepatic morbidity often precludes patients from having a surgical intervention, complicating the management of a population with baseline significantly increased need for surgical re-intervention.
Renal Disease
Approximately 50% of the ACHD population has some degree of renal dysfunction, with a higher incidence in cyanotic CHD.19 The American College of Cardiology/American Heart Association (ACC/AHA) recommends routine assessment of renal function in all adults with moderate and severe CHD due to its association with a poor prognosis in the ACHD population.1 In the immediate cardiac postoperative period, acute kidney injury leads to an eightfold increase in mortality.20 Over the longer term, there is a fivefold increase in mortality with moderate to severe renal impairment and a twofold increase with mild renal impairment compared to those with normal renal function.21
Acquired Cardiovascular Disease
As the ACHD patient ages, acquired cardiovascular disease becomes a significant issue. Approximately 80% of adults with CHD have at least 1 cardiovascular risk factor,22 though overall there is a relative lack of specific data regarding the U.S. population. Surveillance of the Canadian CHD population older than 65 years shows a 47% prevalence of hypertension,23 with increased risk in certain conditions such as aortic coarctation and renal disease associated with CHD. Although studies on the increased risk of diabetes mellitus in the ACHD population have yielded conflicting results,22,24 there is evidence of abnormal glucose metabolism in ACHD patients, which is a predictor of cardiac morbidity and mortality.25,26 The incidence of hyperlipidemia in U.S. ACHD patients is estimated to be at least as high as that of the general population.1 These factors combine with abnormalities in the myocardial substrate, hemodynamic abnormalities, arrhythmias, and sequelae of surgical repairs to confer an increased risk of ischemic heart disease and cerebrovascular disease in the ACHD population.15,27 One large case-control cohort study showed that the risk for ischemic heart disease was 16.5 times higher in patients with CHD as compared with non-CHD patients, with the highest incidence being in those with conotruncal defects and severe non-conotruncal defects. Interestingly, hypertension and diabetes were less common among CHD patients with ischemic heart disease than among non-CHD patients with ischemic heart disease.28
Adults with CHD have an increased risk for cerebrovascular disease compared with the general population, and cerebrovascular disease appears to occur at a younger age.29 The risk of ischemic stroke in individuals with ACHD younger than 55 years is 9 to 12 times higher than that in the general population. As in the general population, the incidence of ischemic stroke in ACHD patients increases with age, and in those older than 55 years, the incidence remains 2 to 4 times higher than in the general population.30,31
Clearly, complications arising from therapeutic interventions in CHD patients contribute significantly to morbidity/mortality in adult life, which underscores the need for life-long follow up and prevention of lapses in care.
The Transition from Pediatric to Adult Care
The monitoring and evaluation of CHD patients throughout life requires close coordination between the patient’s primary care physician, cardiologist, ACHD specialist, and other specialists, as appropriate. The timing of routine follow-up appointments is largely dependent on the severity of the congenital heart lesion and clinical status of the individual patient. Routine surveillance often includes cardiac imaging, preconception/genetic counseling, Holter screenings for arrhythmia, laboratory testing, and titration of medication. Unfortunately, only 30% of adults with CHD receive the recommended cardiac care.32
Children with chronic conditions transitioning to adulthood frequently experience a drop off in coordinated services as they transition from pediatric to adult medicine. Adult institutions often have less multidisciplinary support staff in the form of social workers and case management.33 Furthermore, a recent systematic review of articles that outlined the transition process from pediatric to adult cardiology in the CHD population showed that a high proportion of patients were either lost to follow up or had long gaps in care after leaving pediatric cardiology, with the first lapse in care commonly occurring at approximately age 19 years.28,34 A 2004 study showed that only 48% of adolescents with CHD underwent successful transition.35 A multicenter study of 922 ACHD patients found a gap in care lasting longer than 3 years in 42%, with 8% having gaps exceeding 10 years.36 Another study showed that lapses exceeding 2 years occurred in 63% of patients, with a median duration of lapse of medical care of 10 years. The most common reasons for lapse in care were: being told that cardiac follow up was not required (33%); being discharged from a children’s hospital without appropriate follow up plans in place (23%); being aware of need for follow up but having no symptoms (19%); lack of insurance (18%); and ignoring follow up recommendations for fear of receiving bad news (7%).37 Moreover, living independently from one’s parents was independently associated with a lapse in care, and patients with moderate complexity defects were more likely to experience a lapse than those with high complexity defects.
In the absence of a structured transition program, there is often delayed or inadequate care, which can result in significant emotional and financial stress on families and increased stress on the health care system.38 Inadequate, incomplete, or nonexistent transition and transfer for care has been shown to lead to poor health outcomes. Patients who experienced a lapse in care were 3 times more likely to require urgent cardiac intervention and to have an adverse outcome.37 The urgent interventions required by these patients included pulmonary valve replacement, mitral and tricuspid valve repair/replacement, VSD closure, pulmonary artery stenting, Fontan revision, and pacemaker/defibrillator placement.37 Clearly, there is significant room for improvement in the transition process of patients with CHD.
Best Practices in Transitioning CHD Patients to Adulthood
The overarching goal of pediatric to adult care CHD transition programs is to empower the patient and their support system to assume ownership of the disease process in order to maximize quality of life, life expectancy, and productivity.39 This involves ensuring that the patient has a thorough understanding of their diagnosis, heart anatomy, prior cardiac interventions, limitations imposed upon them by their condition, and the frequency of their anticipated follow-up care. The components of a successful transition program include a systematic approach to addressing the medical, psychosocial, and educational/vocational needs of the adolescent as he or she moves from the family-centered pediatric to the patient-centered adult health care system.40 The visits during the transition period are also an opportunity to discuss reproductive issues and the need for planning pregnancies for women with CHD. The goal is to encourage autonomy and promote ownership of their medical condition to the best of their social-cognitive ability. Adolescents should be encouraged to speak alone with their doctor to foster independence and self-management in their disease process; this has been shown to be protective against failure in transition.32 They should be encouraged to start calling their doctors, requesting refills, and making appointments.
The ACC/AHA appropriately recommend that the transition process for CHD patients begin at 12 years of age, with a goal of discussing future expectations of the child’s education, employment, and independent living.41 As part of this process, it is important that the practitioner educate the child and the family of the need for lifelong surveillance. The exact timing of the transition process is heavily influenced by a number of factors, including the degree of dependence of the child on their guardians, the severity of the congenital heart lesion, and the anticipated short- and long-term prognosis. However, regardless of these circumstances a reasonable age of transition into adult services should be established early on so that an expectation remains in place and the family is adequately prepared.
The challenge of learning how to navigate the adult health care system is as daunting for the transitioning patient as the medical consequences of their disease process. It is critical for patients to have easy access to social workers and case managers, ideally in the setting of a medical home, to connect them to community resources as needed. It is incredibly important that patients consider vocational options and training along with planning their insurance and/or disability qualifications as they move into adulthood. Establishing guardianship is also an important consideration for young adults with CHD who have remained dependent on their guardians.
Towards this end, the AHA/ACC has developed a curriculum that outlines the core principles that should be addressed before the patient moves to the ACHD clinic.27 The transition program should be flexible to accommodate for the patient’s degree of development, and the transfer should not occur before the adolescent has demonstrated the ability to independently manage their own health care to the greatest possible extent.
The ideal transition occurs through the auspices of a medical home that can coordinate the multiple subspecialists involved in the patient’s care. However, what often occurs is that a patient transitions from the pediatric cardiologist’s care before transitioning from pediatric to adult primary care. Prior to transition, the pediatric cardiologist should identify a cardiac destination at an ACHD center. This must be done in conjunction with the pediatrician, who will help identify an internist to take over the patient’s primary care and continue the coordination via the medical home. Information regarding the patient’s complete medical history, medication lists, exercise prescriptions, dietary restrictions, anesthetic issues, functional status, diagnostic studies, and comorbidities should be compiled in a health summary.40 To aid the process of transitioning, the ACC has developed several tools that may be used during the transition process, including self-knowledge assessments and medical summary templates.42
The Primary Care Provider’s Role and the Medical Home
Ensuring adequate care during the transition period requires close coordination between the patient’s various subspecialists. It is vital to avoid multiple subspecialists providing care without knowledge of each other’s treatments, as the treatment course for each ACHD patient is dependent on their unique history of prior therapies.27 The role of the primary care physician in establishing a “medical home” in this setting, as defined by the American Academy of Pediatrics Policy Statement, is exceedingly important.43 In this structure, the primary care physician maintains an easily accessible, centralized, and comprehensive record of the patient’s entire medical history, including surgical and medical treatments of both cardiac and noncardiac issues. Establishing the medical home framework is crucial, as it has been shown to lead to better outcomes in transitioning youth with special health care needs.44
With the establishment of this centralized care, the primary care physician must be able to negotiate the various medications prescribed by subspecialists and monitor for drug levels, adverse effects, and drug-drug interactions. ACHD patients also need regular monitoring and care aside from the care related to their chronic disease. Medical issues of particular importance to the ACHD patient include vaccinations, cholesterol and hypertension screening, cancer screening, and nutritional counseling. The primary care physician is responsible for addressing both the cardiac and noncardiac needs of the patient, ensuring that the patient truly receives comprehensive care. Thorough knowledge of a patient’s unique medical/surgical history will enable the primary care physician to adequately triage and appropriately refer for the development of a new symptom in an ACHD patient. On the other end of the spectrum, the patient’s subspecialists must maintain accurate and up to date information regarding their patient and transmit this to the patient’s medical home.
ACHD Centers
ACHD centers are an important part of any ACHD patient’s clinical team. Regardless of the complexity of the heart defect, there is tremendous value in the education and anticipatory guidance ACHD centers provide for their patients. The providers at these centers are often board-certified ACHD physicians who will work within a multidisciplinary team that includes mid-level practitioners, electrophysiology physicians, high-risk obstetrics/gynecology physicians, pulmonologists, and hepatologists. Each center differs in terms of their on-site interventional capacity and experience. However, the ACHD provider community is highly capable in directing patients who require interventions to centers of excellence, where there is proven quality in congenital surgical and interventional outcomes. ACHD centers often serve as the portals of reentry into care and are critical for providing and coordinating the complex care of each patient. Regular follow-up at these centers will ensure that patients receive adequate management of complications as they arise and preventive care against acquired heart disease.
The timing of follow-up at ACHD centers varies according to the complexity of heart disease. Individuals with simple CHD should be evaluated at an ACHD center at least once to determine the need for further follow-up. Patients with moderate and complex CHD must be monitored at a minimum of every 12 to 24 months, whereas very complex CHD should be monitored every 6 to 12 months.23 The frequency with which the young adult population moves may hinder adequate continuity of care and long-term follow up; a searchable directory of ACHD clinics in the United States and Canada can be found at www.achaheart.org/your-heart/clinic-directory/clinic-listings/.
Managing Specific Issues in the Transitioning Patient
Arrhythmias and Heart Failure
As mentioned, arrhythmias in the ACHD population are extremely common, the most frequent being atrial arrhythmias, especially in patients who have undergone single-ventricle repairs. Patients with late repair of an atrial septal defect have a high incidence of supraventricular tachycardia, which can be treated with catheter ablation procedures.45,46 Pacemaker implantation is another therapeutic option, especially in those who have undergone atrial surgery (ie, Mustard or Senning repairs). In these individuals, particularly in adolescents, abdominal implantation of a pacemaker generator may lessen the psychological impact of the external appearance of the pacemaker. In this population avoiding blunt contact sports (ie, tackle football, wrestling) is also important.28 It is critical that adult and pediatric electrophysiologists work together in the care and management of these complex, recurrent arrhythmias.
As noted above, many ACHD patients will require surgical or catheter-based interventions (as high as 40% in 1 study),47 and many encounter late-onset morbidity as a sequela of interventions earlier in life or as a result of failure of these interventions. The key for adult cardiologists and ACHD providers is delineating the reversible causes (eg, residual shunts, progressive valve regurgitation, and recoarctation) through routine intermittent surveillance, including echocardiograms, magnetic resonance imaging, and cardiac catherization, so that heart failure and arrhythmias in these patients can be identified, treated, and even prevented.
Pregnancy
Pregnancy is the most common reason for women to reenter care. Pregnancy is associated with significant hemodynamic changes, resulting in an increase in cardiac output to up to 150% of pre-pregnancy levels at 32 weeks, and up to 180% during labor. The outcome of pregnancy in patients with CHD is favorable in most instances provided that functional class systemic ventricular function is good. Accordingly, pregnancy is contraindicated in instances of severe pulmonary arterial hypertension (eg, Eisenmenger’s physiology), systemic ventricular dysfunction, and severe left-sided obstructions (eg, aortic or mitral stenosis). It is therefore imperative for health care providers to address the risks of pregnancy and the need for contraception with women who have CHD and are of reproductive age. The AHA advises beginning this conversation at 12 years of age and recommends that counseling be provided by health care providers knowledgeable in both CHD and adolescent health.27 Given the thrombotic potential of estrogen-containing contraception, the selection of contraception for women with ACHD who are seeking birth control requires discussion between the health care provider and patient. Though there have been limited studies performed on the use of contraception in women with CHD, a British working group has developed a consensus statement regarding contraceptive use in women with heart disease based on the World Health Organization format.48,49
Surgical Procedures
The need for operative interventions and re-interventions, both cardiac and noncardiac, in many CHD populations is considerable. Regardless of the type of procedure, these patients should receive a comprehensive preoperative risk assessment as well as appropriate intraoperative and postoperative management, ideally at a center equipped to meet their unique needs. Approaching the surgical procedure under the guidance of an interdisciplinary team that includes an ACHD specialist, anesthesiologist, and surgeon ensures that critical issues for appropriate management are not overlooked.
The preoperative risk assessment should be aimed at identifying and minimizing major risk factors. Historical factors to consider include the congenital lesion, outcomes of prior surgeries, history of syncope or arrhythmias, and the presence of pulmonary disease, among others.27 If the patient has a pacemaker or defibrillator, this should be interrogated prior to the planned procedure to ensure proper functioning. The preoperative evaluation should include consultation with a cardiologist experienced in the care of adolescents with CHD. Cardiac medications should be continued until the time of surgery and restarted as soon after the procedure as possible. Periods without anticoagulation should be minimized if indicated at baseline, and may require substituting warfarin with heparin in the preoperative period. The need for endocarditis prophylaxis must be considered as well; antibacterial prophylaxis prior to dental surgery, respiratory tract procedures, and procedures on infected skin and musculoskeletal structures is recommended in individuals with prosthetic heart valves, previous infective endocarditis, unrepaired CHD, repaired CHD with prosthetic material for the first 6 months after surgery, repaired CHD with residual defects, and valvulopathy after cardiac transplantation.50
Fluid management is important intraoperatively and post procedure, particularly in individuals who are preload dependent at baseline (eg, patients who have had Fontan palliation). Mechanical ventilation strategies with high positive end-expiratory pressure and tidal volume may decrease systemic venous return and should be monitored closely. Early mobilization and pulmonary toilet post extubation is advised to avoid pulmonary infection.
Exercise Capacity and Restrictions
The ability to exercise is an important factor in the quality of life of ACHD patients, especially in the adolescent period when participation in school and recreational athletics oftentimes functions as a social institution. Exercise ability is influenced by both real limitations imposed by limited cardiopulmonary reserve as a result of underlying pathology and by misconceptions of and anxiety about their ability to safely participate in these activities. There is evidence of diminished aerobic activity in all groups with CHD. However, symptomatic restrictions account for only approximately 30% of all barriers to exercise,51 and some studies have shown that exercise training programs can improve functional capacity and some standards of quality of life in CHD patients, in addition to the general health benefits associated with obesity prevention.52
Recommendations regarding exercise capacity are often addressed at primary care visits, and should be reinforced by the patient’s cardiologist. In general, most patients with repaired or mild defects can engage in moderate- to high-intensity exercise; those with more complex defects, cyanosis, or arrhythmias should be evaluated by an ACHD specialist to determine an appropriate level of activity.27 The “exercise prescription” provided to the patient should include type of exercise tolerated as well as heart rate goals and limits. In patients with extremely limited exercise capacity, a cardiac rehabilitation program can be beneficial. The presence of significant pulmonary hypertension, cyanosis or aortic stenosis, symptomatic arrhythmias, or evidence of myocardial dysfunction usually restricts the degree of exercise; full recommendations by activity and lesion type can be found in the guidelines proposed by the 36th Bethesda Conference.53 The importance of serial and regular evaluations is emphasized in these guidelines due to changing hemodynamic status of the patient over time as their cardiac lesions evolve and new complications arise.
Social and Psychological Impact of Chronic Illness
Living with a chronic disease can have a psychological impact on the child and transitioning adolescent. Frequent hospitalizations, physician visits, medical tests, and management of medical emergencies take a toll on the patient’s self-image and self-esteem, particularly during their formative adolescent years. Adolescents with CHD often feel “different” from their peers due to their condition,54 causing them to withhold disclosures about their heart disease to others out of fear of its impact on personal and professional relationships. Recent studies have shown that children and adolescents with CHD are at risk of internalizing problems and exhibiting behavior problems;55 they are also more likely to have impaired quality of life secondary to their increased incidence of psychosocial difficulties.56 The social and physical debility often experienced by patients with ACHD leads to a higher incidence of depression and anxiety in this population.57 Studies have shown that ACHD patients are interested in psychological treatment and peer support of their mood and anxiety disorders.58
At least some degree of the mental health issues ACHD patients experience is thought to have a physiological basis and be related to early cyanosis and neonatal surgical bypass duration. Prolonged duration of deep hypothermic circulatory arrest (DHCA) during corrective surgery is associated with reduced social competence, and has been found to be an independent risk factor for anxiety, depression, aggressive behavior, and attention deficiencies.59 In other studies, DHCA has been associated with decreased intellectual ability and worse fine motor skills, memory, and visuospatial skills, among other neurodevelopmental outcomes.60-62 Psychiatric disorders have also been associated with genetic syndromes like DiGeorge syndrome.63 This impacts executive function, leading to missed appointments, delay in clinical visits, and medication noncompliance. Given the potential for worse outcomes and risk of transition failure, primary care providers should routinely evaluate CHD patients for mood disorders and neurocognitive delay.
Social Determinants of Health and Medical Legal Partnerships
Social determinants of health and workplace discrimination play a large role in determining the ability of individuals with CHD to achieve adequate health care and maintain gainful employment. Individuals with CHD often face significant challenges as they prepare to enter the workforce, including discrimination within the workplace and maintaining employment through medical emergencies. Studies have shown that while educational milestones are similar between patients with and without CHD, those with CHD are much less likely to be employed.64 Challenges facing adolescents as they enter the workforce include hiring discrimination, physical challenges imposed by functional limitations, and misunderstanding of disease process and actual functional capacity. Career counseling is therefore an integral part of the transitioning process and should be started in early adolescence to allow for full assessment of mental, physical, and social abilities.65
Medical-legal partnerships (MLPs) can be extremely beneficial to the CHD population adversely affected by social determinants of health and workplace discrimination. These partnerships integrate lawyers into health care to address legal problems that create and perpetuate poor health; on a broader scale, these partnerships can advance and support public policy changes that improve population health.66
The major social determinants of health addressed by MLPs are income supports/insurance, housing/utilities, employment/education, legal status, and personal/family stability (summarized in the mnemonic I-HELP).67 Some of the more specific areas in which MLPs may assist in the delivery of care to CHD patients include case management, translation services, health literacy, and legal aid/legal services. ACHD patients also often experience a significant loss of services, including physical, occupational, and speech therapy and nutrition services, as adult clinics may not be prepared to provide these services. While physicians can best address the individual patient’s health, members of the legal system can address the systemic ailments that propagate that patient’s recurrent hospitalizations and other use of medical resources. Members of the legal system are present onsite in health care settings and participate in clinical meetings, which allows a coordinated and comprehensive screening for social needs that may harm a patient’s health.
Loss of insurance coverage is a major issue for transitioning patients; while adolescents with complex medical conditions are eligible for Medicaid to help cover the significant cost of their health care that goes beyond the abilities of private insurance, this eligibility ends when the patient turns 21. Additionally, the Social Security Administration re-determines supplemental security income (SSI) eligibility when the patient turns 18, and about one-third of patients lose their SSI benefits. Without appropriate guidance in navigating the nuances of insurance, many patients are at risk of losing coverage for their health care expenditures as they transition. Uninsured adults with a chronic condition are 8 times more likely to have unmet medical needs and 6 times more likely to have no access to routine care than insured young adults, with a 35% likelihood of the unmet medical need being due to cost.68 Undoubtedly, linability to pay for health care contributes to the lack of follow-up in the adult population, and MLPs may be a valuable tool to aid in ameliorating this problem.
Studies have shown that when legal services are used to address the social determinants of health, patients with chronic illnesses such as asthma and sickle cell disease have reduced hospital admissions.69,70 Other studies have shown utilization of MLPs has reduced spending on the care of high-need, high-use patients.71 According to a 2016 national survey of health care organizations conducted by the National Center for Medical-Legal Partnership, 39% clinicians reported improved compliance with medical treatment and 66% reported improved health outcomes after their patients received MLP services.72 Families referred to MLPs have shown increased access to health care, food, and income resources, and two-thirds reported improved child health and well-being.73 Given the numerous challenges faced by patients with CHD, involving MLPs as a part of both the transition process and the patient-centered medical home benefits these patients greatly and allows them to maximize their quality of life.
Conclusion
As more patients are living to adulthood with CHD, there is an increasing need for long-term care and adequate follow up, especially regarding the need for re-intervention and management of physiologic consequences of acquired cardiopulmonary, gastrointestinal, and renal disease in the setting of underlying congenital heart lesions. Beyond the purely medical aspects of the individual’s long-term management, psychosocial issues must be addressed, including preparing the individual for future employment and family counseling. Crucial to this process is the implementation of a comprehensive transition that begins in early adolescence and enables patients to take charge of their disease process in adulthood and ultimately enables them to maximize their quality of life and societal contributions. Towards this end, the role of MLPs may be important in ensuring that local, state, and federal policies that promote health harming norms are addressed.
Acknowledgments: We thank Dr. Frances ‘Kitty’ O’Hare and Bobbie Lewis for inviting us to submit this review; Dr. Russ Kolarik, Current Med-Peds Residency Program Director and Former President of the National Med-Peds Program Directors Association; and Dr. Peter Tilkemeier, Chairman, Department of Internal Medicine at Greenville Health System, for his unending support of our ACHD program. We also thank our patients, whose resounding resilience in the face of ongoing medical and psychosocial challenges remains our daily inspiration.
Corresponding author: Manisha S. Patel, MD, Department of Medicine and Pediatrics, Division of Cardiology, University of South Carolina School of Medicine, Columbia, SC; [email protected].
Financial disclosures: None.
1. Bhatt AB, Foster E, Kuehl K, et al. Congenital heart disease in the older adult: a scientific Statement from the American Heart Association. Circulation. 2015;26;131:1884-1931.
2. Gilboa SM, Devine OJ, Kucik JE, et al. Congenital heart defects in the United States: estimating the magnitude of the affected population in 2010. Circulation. 2016;134:101-109.
3. Dolk H, Loane M, Garne E. Congenital heart defects in Europe. Circulation. 2011;123:841-849.
4. Qu Y, Liu X, Zhuang J, et al. Incidence of congenital heart disease: the 9-year experience of the Guangdog Registry of Congenital Heart Disease, China. PloS One. 2016;11:e0159257.
5. Brickner ME, Hillis LD, Lange RA. Congenital heart disease in adults. N Engl J Med.
6. McGee Banks C. Variations in diversity in the United States and Canada. 2010. http://www.canadianstudies.isp.msu.edu/docs/Cherry%20McGee%20Banks.pdf.
7. van der Linde D, Konings EE, Slager MA, et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;58:2241-2247.
8. Warnes CA, Liberthson R, Danielson GK, et al. Task force 1: the changing profile of congenital heart disease in adult life. J Am Coll Cardiol. 2001;37:1170-1175.
9. Hoffman JIE, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39;1890-1900.
10. Verheugt CL, Uiterwaal CS, van der Velde ET, et al. Mortality in adult congenital heart disease. Eur Heart J. 2010;31:1220-1229.
11. Greutmann M, Tobler D, Kovacs AH, et al. Increasing mortality burden among adults with complex congenital heart disease. Congenit Heart Dis. 2015;10:117-127.
12. Mandalenakis Z, Rosengren A, Lappas G, et al. Atrial fibrillation burden in young patients with congenital heart disease. Circulation. 2018;137:928-937.
13. Fishberger SB, Wernovsky G, Gentles TL, et al. Factors that influence the development of atrial flutter after the Fontan operation. J Thorac Cardiovasc Surg. 1997;113:80–86.
14. Khairy P, Aboulhosn J, Gurvitz M; AARC. Arrhythmia burden in adults with surgically repaired tetralogy of Fallot. Circulation. 2010;122:868-875.
15. Ewe SH, Tan JL. Hepatotocellular carcinoma—a rare complication post Fontan operation. Congenit Heart Dis. 2009;4:103-106.
16. Ghaferi AA, Hutchins GM. Progression of liver pathology in patients undergoing the Fontan procedure: Chronic passive congestion, cardiac cirrhosis, hepatic adenoma, and hepatocellular carcinoma. J Thorac Cardiovasc Surg. 2005;129:1348-1352.
17. Saliba T, Dorkhom S, O’Reilly EM, et al. Hepatocellular carcinoma in two patients with cardiac cirrhosis. Eur J Gastroenterol Hepatol. 2010;22:889-891.
18. Wang A, Book W, McConnell M, et al. Prevalence of hepatitis C infection in adult patients who underwent congenital heart surgery prior to screening in 1992. Am J Cardiol. 2006;100:1307-1309.
19. Dimopoulos K, Diller GP, Koltsida E, et al. Prevalence, predictors, and prognostic value of renal dysfunction in adults with congenital heart disease. Circulation. 2008;117:2320-2328.
20. Karkouti K, Wijeysundera DN, Yau TM, et al. Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation. 2009;119:495-502.
21. Dimopoulos K, Diller GP, Koltsida E, et al. Prevalence, predictors, and prognostic value of renal dysfunction in adults with congenital heart disease. Circulation. 2008;117:2320-2328.
22. Moons P, Van Deyk K, Dedroog D, et al. Prevalence of cardiovascular risk factors in adults with congenital heart disease. Eur J Cardiovasc Prev Rehabil. 2006;13:612-616.
23. Afilalo J, Therrien J, Pilote L, et al. Geriatric congenital heart disease: burden of disease and predictors of mortality. J Am Coll Cardiol. 2011;58:1509-1515.
24. Billett J, Cowie MR, Gatzoulis MA, et al. Comorbidity, healthcare utilisation and process of care measures in patients with congenital heart disease in the UK: cross-sectional, population-based study with case-control analysis. Heart. 2008;94:1194-1199.
25. Hait G, Corpus M, Lamarre FR, et al. Alteration of glucose and insulin metabolism in congenital heart disease. Circulation. 1972;46:333-346.
26. Ohuchi H, Miyamoto Y, Yamamoto M, et al. High prevalence of abnormal glucose metabolism in young adult patients with complex congenital heart disease. Am Heart J. 2009;158:30-39.
27. Lui GK, Fernandes S, McElhinney DB. Management of cardiovascular risk factors in adults with congenital heart disease. J Am Heart Assoc. 2014;3(6):e001076.
28. Fedchenko M, Mandalenakis Z, Rosegren A, et al. Ischemic heart disease in children and young adults with congenital heart disease in Sweden. Int J Cardiol. 2017;248:143-148.
29. Mandalenakis Z, Rosengren A, Lappas G, et al. Ischemic stroke in children and young adults with congenital heart disease. J Am Heart Assoc. 2016. 23;5(2):e003071..
30. Hoffmann A, Chockalingam P, Balint OH, et al. Cerebrovascular accidents in adult patients with congenital heart disease. Heart. 2010;96:1223-1226.
31. Lanz J, Brophy JM, Therrien J, et al. Stroke in adults with congenital heart disease: incidence, cumulative risk, and predictors. Circulation. 2015;132:2385-2394
32. Sable C, Foster E, Uzark K, et al; on behalf of the American Heart Association Congenital Heart Defects Committee of the Council on Cardiovascular Disease in the Young, Council on Cardiovascular Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease. Best practices in managing transition to adulthood for adolescents with congenital heart disease: the transition process and medical and psychosocial issues: a scientific statement from the American Heart Association. Circulation. 2011;123:1454-1485.
33. Steinway C, Gable J, Jan S. Transitioning to adult care: supporting youth with special health care needs. Children’s Hospital of Philadelphia: Policylab Evidence to Action in Brief. Spring 2017.
34. Heery E, Sheehan AM, While AE, Coyne I. Experiences and outcomes of transition from pediatric to adult health care services for young people with congenital heart disease: a systematic review. Congenit Heart Dis. 2015;10413-427.
35. Reid GJ, Irvine MJ, McCrindle BW, et al. Prevalence and correlates of successful transfer from pediatric to adult health care among a cohort of young adults with complex congenital heart defects. Pediatrics. 2004;113(part 1):e197-e205.
36. Gurvitz M, Valente AM, Broberg C, et al; AARCC. Prevalence and predictors of gaps in care among adult congenital heart disease patients (The Health, Education and Access Research Trial). J Am Coll Cardiol. 2013;61:2180-2184.
37. Yeung E, Kay J, Roosevelt GE, et al. Lapse of care as a predictor for morbidity in adults in congenital heart disease. Int J Cardiol. 2008;125:62-65.
38. Meadows AK, Bosco V, Tong E, et al. Transition and transfer from pediatric to adult care of young adults with complex congenital heart disease. Current Cardiol Rep. 2009; 11;4;291-297.
39. lum RW, Garell D, Hodgman CH, et al. Transition from child-centered to adult health-care systems for adolescents with chronic conditions: a position paper of the Society for Adolescent Medicine. J Adolesc Health. 1993;14:570-576.
40. Rosen DS, Blum RW, Britto M, et al; Society for Adolescent Medicine. Transition to adult health care for adolescents and young adults with chronic conditions: position paper of the Society for Adolescent Medicine. J Adolesc Health. 2003;33:309-311.
41. Reiss JG, Gibson RW, Walker LR. Health care transition: youth, family, and provider perspectives. Pediatrics. 2005;115:112-120.
42. Congenital heart disease transition tools. American College of Cardiology. www.acc.org/membership/sections-and-councils/adult-congenital-and-pediatric-cardiology-section/resources/chdtransitiontools. Accessed November 1, 2018.
43. American Academy of Pediatrics Medical Home Initiatives for Children With Special Needs Project Advisory Committee. Policy statement: organizational principles to guide and define the child health care system and/or improve the health of all children. Pediatrics. 2004;113(suppl):1545-1547.
44. Lotstein DS, McPherson M, Strickland B, Newacheck PW. Transition planning for youth with special health care needs: results from the National Survey of Children with Special Health Care Needs. Pediatrics. 2005;115:1562-1568.
45. Gatzoulis MA, Freeman MA, Siu SC, et al. Atrial arrhythmia after surgical closure of atrial septal defects in adults. N Engl J Med. 1999;340:839-846.
46. Triedman JK, Bergau DM, Saul JP, et al. Efficacy of radiofrequency ablation for control of intraatrial reentrant tachycardia in patients with congenital heart disease. J Am Coll Cardiol. 1997;30:1032-1038.
47. Zomer AC, Verheugt CL, Vaartjes I, et al. Surgery in adults with congenital heart disease. Circulation. 2011;124:2195-2201.
48. Silversides CK, Sermer M, Siu SC. Choosing the best contraceptive method for the adult with congenital heart disease. Curr Cardiol Rep. 2009;11:298-305.
49. Thorne S, Nelson-Piercy C, MacGregor A, et al. Pregnancy and contraception in heart disease and pulmonary arterial hypertension. J Fam Plann Reprod Health Care. 2006;32:75-81.
50. Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group [published correction appears in Circulation. 2007;116:e376–e377]. Circulation. 2007;116:1736-1754.
51. Warnes CA, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Adults With Congenital Heart Disease): developed in collaboration with the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2008;118:2395-2451.
52. Curran TJ, Rhodes J. Cardiac rehabilitation improves heart rate recovery following peak exercise in children with repaired congenital heart disease. Pediatr Cardiol. 2007;28:276-279.
53. Graham TP Jr, Driscoll DJ, Gersony WM, et al Task force 2: congenital heart disease. J Am Coll Cardiol. 2005;45:1326-1333.
54. Tong EM, Sparacino PS, Messias DK, et al. Growing up with congenital heart disease: the dilemmas of adolescents and young adults. Cardiol Young. 1998;8:303-309.
55. Karsdorp PA, Everaerd W, Kindt M, Mulder BJ. Psychological and cognitive functioning in children and adolescents with congenital heart disease: a meta-analysis. J Pediatr Psychol. 2007;32:527-541.
56. Kovacs AH, Moons P. Psychosocial functioning and quality of life in adults with congenital heart disease and heart failure. Heart Fail Clin. 2014;10:35-42.
57. Bromberg JI, Beasley PJ, D’Angelo EJ, et al. Depression and anxiety in adults with congenital heart disease: a pilot study. Heart Lung. 2003;32:105–10.
58. Kovacs AH, Bendell KL, Colman J, et al. Adults with congenital heart disease: psychological needs and treatment preferences. Congenit Heart Dis. 2009;4:139-146
59. Hovels-Gurich HH, Konrad K, Wiesner M, et al. Long term behavioural outcome after neonatal arterial switch operation for transposition of the great arteries. Arch Dis Child. 2002;87:506–510.
60. Forbess JM, Visconti KJ, Hancock-Friesen C, et al. Neurodevelopmental outcome after congenital heart surgery: results from an institutional registry. Circulation. 2002;106:195-102.
61. Bellinger DC, Wypij D, duPlessis AJ, et al. Neurodevelopmental status at eight years in children with dextro-transposition of the great arteries: the Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg. 2003;126:1385-1396.
62. Wypij D, Newburger JW, Rappaport LA, et al. The effect of duration of deep hypothermic circulatory arrest in infant heart surgery on late neurodevelopment: the Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg. 2003;126:1397-1403.
63. Tang SX, Yi JJ, Calkins ME, et al. Psychiatric disorders in 22q11.2 deletion syndrome are prevalent but undertreated. Pscychol Med 2017;44:1267-1277.
64. Simko LC, McGinnis KA, Schembri J. Educational needs of adults with congenital heart disease. J Cardiovasc Nurs. 2006;21:85-94.
65. Foster E, Graham TP Jr, Driscoll DJ, et al. Task force 2: special health care needs of adults with congenital heart disease. J Am Coll Cardiol. 2001;37:1176-1183.
66. Sandel M, Hansen M, Kahn R, et al. Medical-legal partnerships: transforming primary care by addressing the legal needs of vulnerable populations. Health Aff. 2010;29:1697-1705.
67. The National Center for Medical-Legal Partnership. medical-legalpartnership.org. Accessed November 1, 2018.
68. Callahan ST, Cooper WO. Access to health care for young adults with disabling chronic conditions. Arch Pediatr Adolesc Med. 2006;160:178-182.
69. Pettignano R, Caley SB, Bliss LR. Medical-legal partnership: impact on patients with sickle cell disease. Pediatrics. 2011;128:1482-1488.
70. Klein MD, Beck AF, Henize AW, et al. Doctors and lawyers collaborating to HeLP children--outcomes from a successful partnership between professions. J Health Care Poor Underserved. 2013;24:1063-1073.
71. Martin J, Martin A, Schultz C, Sandel M. Embedding civil legal aid in care of high-utilizing patients using medical-legal partnership. Health Affairs blog. 22 April 2015. www.healthaffairs.org/do/10.1377/hblog20150422.047143/full. Accessed November 1, 2018.
72. Regenstein M, Sharac J, Williamson A. The state of the medical legal partnership field: findings from the 2016 National Center for Medical-Legal Partnership Surveys. August 2017.
73. Weintraub D, Rodgers MA, Botcheva L, et al. Pilot study of medical-legal partnership to address social and legal needs of patients. J Health Care Poor Underserved. 2010;21(2 Suppl):157-168.
From the Greenville Health System, Greenville, SC.
Abstract
- Objective: To review the management of patients with congenital heart disease (CHD) transitioning from pediatric to adult care.
- Methods: Review of the literature.
- Results: Persons with CHD require close monitoring and evaluation throughout life to address the physiologic consequences of acquired cardiopulmonary, gastrointestinal, and renal disease in the setting of underlying congenital heart lesions. During the transition from pediatric to adult cardiology, a high proportion of patients are lost to follow up or have long gaps in care after leaving pediatric cardiology, which can lead to poor outcomes. Care of the adult with CHD requires close coordination between the patient’s primary care physician), cardiologist, adult CHD specialist, and other specialists. The transition process for CHD patients begin at 12 years of age, with a goal of discussing future expectations of the child’s education, employment, and independent living. Successful transition programs use a systematic approach to addressing the medical, psychosocial, and educational/vocational needs of the adolescent as he or she moves from the family-centered pediatric to the patient-centered adult health care system.
- Conclusion: The transition from pediatric to adult care in ACHD patients is best provided through a comprehensive transition program that begins in early adolescence and enables patients to take charge of their disease process in adulthood, allowing them to maximize their quality of life and societal contributions.
Keywords: adult; congenital heart defects; complications; disease management; patient care team.
The population of adults with congenital heart disease (CHD) in developed countries has grown at an exponential rate in the past 4 decades. With advances in medical care and surgical interventions, the proportion of pediatric patients reaching adulthood has increased from 15% in the 1930s-60s to more than 95% for patients with mild to moderate complexity CHD. The rate of survival to adulthood for patients with severely complex CHD remains lower at around 56%.1
There are now more adult than pediatric patients with CHD in the United States. Because adult CHD (ACHD) patients have increased morbidity and mortality in their young adult years, it is imperative for all providers to understand and address the long-terms needs of this population. Unfortunately, adults with CHD do not always receive adequate health care, frequently because they are lost to follow-up, particularly during their adolescent years when they are expected to gain independence in their medical management. As will be discussed, CHD is a chronic illness fraught with numerous expected and unexpected complications that require close monitoring and re-interventions. Effectively anticipating and addressing these complications requires a standardized and comprehensive process of transition from the pediatric to the adult population to ensure maximal quality of life.
Epidemiology
The actual prevalence of ACHD in the United States is unknown, as a national database of persons with CHD has not been established.2 In contrast, Europe and China have maintained databases that enable ongoing monitoring of the evolving CHD epidemiology in those regions.3,4 The best estimates of the U.S. incidence and prevalence of ACHD stem from extrapolations from Canadian data. According to this data, there were more than 1.2 million adults with ACHD in the United States in 2012, with an anticipated 5% annual increase.1,5 However, the limitations of such extrapolations must be noted, as the Canadian population does not perfectly mirror that of the United States. Canada has lower infant mortality and adult obesity rates, and the United States has larger African American and Hispanic populations.6 Also, the juxtaposition of universal access to health care in Canada and the socioeconomic class–dependent access in the United States causes variations in care and outcomes of ACHD between the 2 populations. These differing genetic and social backgrounds may change the incidence of CHD by affecting maternal-fetal health.7
The 32nd Bethesda Conference on “Care of the Adult with Congenital Heart Disease” in 2000 was tasked with characterizing the ACHD population in the United States. This project found a prevalence similar to that of the Canadian extrapolation and showed that among persons with ACHD in the United States, 45% have mild disease, 37% moderate disease, and 13% severe disease.8
Characterizing the true incidence of CHD in the United States also has proven difficult because of variations in the definitions and methods used to detect lesions across the multiple studies that have looked at this matter. The estimated incidence of CHD, grouped according to severity, is 2.5 to 3 per 1000 live births for severe CHD, and from 3 to 13 per 1000 live births for moderately severe forms.9 When all forms are considered, including minor CHD (which includes tiny muscular ventricular septal defect [VSDs] present at birth and other trivial lesions), the total incidence of CHD rises to 75 per 1000 live births.9 CHD is one of the most common chronic illnesses in young adults with special health care needs.
Complications in Adulthood
The ACHD population represents a diverse population in terms of severity of CHD, history of surgical/catheter-based interventions, and socioeconomic status. However, a unifying clinical concern for these patients is their increased risk for morbidity and mortality in the young adult years. Despite the tremendous advances in the field over previous decades, mortality in this population in adulthood is estimated to be up to 7 times higher compared to age-matched peers.10,11 For many patients, palliative CHD interventions result in a significant drop in early morbidity and mortality but frequently lead to delayed morbidity from secondary multi-organ complications as these patients transition from pediatric to adult care. For example, due to the chronic low flow and low cardiac output state created by Fontan palliations, patients are at risk for diastolic dysfunction, arrhythmias, thrombotic events, protein-losing enteropathy, and cirrhosis/congestive hepatopathy, among other chronic conditions. These patients require frequent follow up and management by a multidisciplinary team including a primary care provider and various specialty groups.
Cardiac Disease
The most common causes of death in ACHD patients are heart failure (27%) and sudden cardiac death (19%), which occur at mean ages of 48 years and 39 years, respectively.10 The form of heart failure in ACHD patients is related to subsystemic right ventricle (RV) dysfunction, coronary under-perfusion, residual shunts, and residual progressive valve regurgitation. One of the more common examples of this is seen in palliated Tetralogy of Fallot patients who have undergone a transannular patch as a neonate. These patients are frequently left with significant pulmonary regurgitation leading to RV dilation, RV failure, and subsequent left ventricle (LV) failure. Another common example is the patient with dextro-transposition of the great arteries (DTGA) status post atrial switch who has a subsystemic morphologic RV. These patients will often develop significant RV dysfunction related to the chronic high pressures associated with systemic circulation.
Arrhythmias are a major contributor to morbidity and mortality in this population and are the most common reason patients present back into care. Difficult to control, multifocal intra-atrial re-entrant tachycardia is extremely common in ACHD, with an estimated 50% of all patients developing atrial arrhythmia by age 55. A recent study determined that the risk of atrial fibrillation in individuals with CHD was 22 times higher than that in age-matched controls, with the highest risk being seen in patients with conotruncal defects. Furthermore 10% of these patients develop heart failure.12 The risk for, incidence of, and type of arrhythmia is associated with the severity of the congenital heart lesions, as well as the type and timing of surgical interventions. Later age of repair has been associated with an increased likelihood of arrhythmias.13 Tetralogy of Fallot is an example of a moderately complex congenital heart lesion and is the most common cyanotic congenital heart lesion. In these individuals, the risk for atrial tachycardias, ventricular tachycardias, and need for a pacemaker is much higher than in age-matched peers.14 This includes an increased risk of sudden cardiac death, with many of these patients requiring placement of an implantable cardioverter defibrillator.
Pulmonary Disease
There is a 44% to 56% prevalence of restrictive pulmonary disease in the ACHD population, compared to 9% in the general non-CHD adult population. The incidence of pulmonary hypertension is also significantly higher in the ACHD population. The etiology for development of pulmonary hypertension is multifactorial, including chronic thromboembolic disease, left-sided heart disease, longstanding left to right shunts, and obstructive sleep apnea. These conditions have a significant impact on survival, as moderate/severe lung function impairment is an independent predictor of survival. Patients with shunt lesions are at risk of developing pulmonary arterial hypertension later in life,1 which quadruples the risk of all-cause mortality and more than triples the risk of cardiovascular mortality.7
Liver Disease
Hepatic morbidity associated with palliated CHD is often related to prior surgical interventions. The most common morbidities include chronic hepatitis C and liver failure from chronic under-perfusion and passive congestion, especially following Fontan palliation. Long term, these complications can lead to cirrhosis and hepatocellular carcinoma.15-18 Unfortunately, hepatic morbidity often precludes patients from having a surgical intervention, complicating the management of a population with baseline significantly increased need for surgical re-intervention.
Renal Disease
Approximately 50% of the ACHD population has some degree of renal dysfunction, with a higher incidence in cyanotic CHD.19 The American College of Cardiology/American Heart Association (ACC/AHA) recommends routine assessment of renal function in all adults with moderate and severe CHD due to its association with a poor prognosis in the ACHD population.1 In the immediate cardiac postoperative period, acute kidney injury leads to an eightfold increase in mortality.20 Over the longer term, there is a fivefold increase in mortality with moderate to severe renal impairment and a twofold increase with mild renal impairment compared to those with normal renal function.21
Acquired Cardiovascular Disease
As the ACHD patient ages, acquired cardiovascular disease becomes a significant issue. Approximately 80% of adults with CHD have at least 1 cardiovascular risk factor,22 though overall there is a relative lack of specific data regarding the U.S. population. Surveillance of the Canadian CHD population older than 65 years shows a 47% prevalence of hypertension,23 with increased risk in certain conditions such as aortic coarctation and renal disease associated with CHD. Although studies on the increased risk of diabetes mellitus in the ACHD population have yielded conflicting results,22,24 there is evidence of abnormal glucose metabolism in ACHD patients, which is a predictor of cardiac morbidity and mortality.25,26 The incidence of hyperlipidemia in U.S. ACHD patients is estimated to be at least as high as that of the general population.1 These factors combine with abnormalities in the myocardial substrate, hemodynamic abnormalities, arrhythmias, and sequelae of surgical repairs to confer an increased risk of ischemic heart disease and cerebrovascular disease in the ACHD population.15,27 One large case-control cohort study showed that the risk for ischemic heart disease was 16.5 times higher in patients with CHD as compared with non-CHD patients, with the highest incidence being in those with conotruncal defects and severe non-conotruncal defects. Interestingly, hypertension and diabetes were less common among CHD patients with ischemic heart disease than among non-CHD patients with ischemic heart disease.28
Adults with CHD have an increased risk for cerebrovascular disease compared with the general population, and cerebrovascular disease appears to occur at a younger age.29 The risk of ischemic stroke in individuals with ACHD younger than 55 years is 9 to 12 times higher than that in the general population. As in the general population, the incidence of ischemic stroke in ACHD patients increases with age, and in those older than 55 years, the incidence remains 2 to 4 times higher than in the general population.30,31
Clearly, complications arising from therapeutic interventions in CHD patients contribute significantly to morbidity/mortality in adult life, which underscores the need for life-long follow up and prevention of lapses in care.
The Transition from Pediatric to Adult Care
The monitoring and evaluation of CHD patients throughout life requires close coordination between the patient’s primary care physician, cardiologist, ACHD specialist, and other specialists, as appropriate. The timing of routine follow-up appointments is largely dependent on the severity of the congenital heart lesion and clinical status of the individual patient. Routine surveillance often includes cardiac imaging, preconception/genetic counseling, Holter screenings for arrhythmia, laboratory testing, and titration of medication. Unfortunately, only 30% of adults with CHD receive the recommended cardiac care.32
Children with chronic conditions transitioning to adulthood frequently experience a drop off in coordinated services as they transition from pediatric to adult medicine. Adult institutions often have less multidisciplinary support staff in the form of social workers and case management.33 Furthermore, a recent systematic review of articles that outlined the transition process from pediatric to adult cardiology in the CHD population showed that a high proportion of patients were either lost to follow up or had long gaps in care after leaving pediatric cardiology, with the first lapse in care commonly occurring at approximately age 19 years.28,34 A 2004 study showed that only 48% of adolescents with CHD underwent successful transition.35 A multicenter study of 922 ACHD patients found a gap in care lasting longer than 3 years in 42%, with 8% having gaps exceeding 10 years.36 Another study showed that lapses exceeding 2 years occurred in 63% of patients, with a median duration of lapse of medical care of 10 years. The most common reasons for lapse in care were: being told that cardiac follow up was not required (33%); being discharged from a children’s hospital without appropriate follow up plans in place (23%); being aware of need for follow up but having no symptoms (19%); lack of insurance (18%); and ignoring follow up recommendations for fear of receiving bad news (7%).37 Moreover, living independently from one’s parents was independently associated with a lapse in care, and patients with moderate complexity defects were more likely to experience a lapse than those with high complexity defects.
In the absence of a structured transition program, there is often delayed or inadequate care, which can result in significant emotional and financial stress on families and increased stress on the health care system.38 Inadequate, incomplete, or nonexistent transition and transfer for care has been shown to lead to poor health outcomes. Patients who experienced a lapse in care were 3 times more likely to require urgent cardiac intervention and to have an adverse outcome.37 The urgent interventions required by these patients included pulmonary valve replacement, mitral and tricuspid valve repair/replacement, VSD closure, pulmonary artery stenting, Fontan revision, and pacemaker/defibrillator placement.37 Clearly, there is significant room for improvement in the transition process of patients with CHD.
Best Practices in Transitioning CHD Patients to Adulthood
The overarching goal of pediatric to adult care CHD transition programs is to empower the patient and their support system to assume ownership of the disease process in order to maximize quality of life, life expectancy, and productivity.39 This involves ensuring that the patient has a thorough understanding of their diagnosis, heart anatomy, prior cardiac interventions, limitations imposed upon them by their condition, and the frequency of their anticipated follow-up care. The components of a successful transition program include a systematic approach to addressing the medical, psychosocial, and educational/vocational needs of the adolescent as he or she moves from the family-centered pediatric to the patient-centered adult health care system.40 The visits during the transition period are also an opportunity to discuss reproductive issues and the need for planning pregnancies for women with CHD. The goal is to encourage autonomy and promote ownership of their medical condition to the best of their social-cognitive ability. Adolescents should be encouraged to speak alone with their doctor to foster independence and self-management in their disease process; this has been shown to be protective against failure in transition.32 They should be encouraged to start calling their doctors, requesting refills, and making appointments.
The ACC/AHA appropriately recommend that the transition process for CHD patients begin at 12 years of age, with a goal of discussing future expectations of the child’s education, employment, and independent living.41 As part of this process, it is important that the practitioner educate the child and the family of the need for lifelong surveillance. The exact timing of the transition process is heavily influenced by a number of factors, including the degree of dependence of the child on their guardians, the severity of the congenital heart lesion, and the anticipated short- and long-term prognosis. However, regardless of these circumstances a reasonable age of transition into adult services should be established early on so that an expectation remains in place and the family is adequately prepared.
The challenge of learning how to navigate the adult health care system is as daunting for the transitioning patient as the medical consequences of their disease process. It is critical for patients to have easy access to social workers and case managers, ideally in the setting of a medical home, to connect them to community resources as needed. It is incredibly important that patients consider vocational options and training along with planning their insurance and/or disability qualifications as they move into adulthood. Establishing guardianship is also an important consideration for young adults with CHD who have remained dependent on their guardians.
Towards this end, the AHA/ACC has developed a curriculum that outlines the core principles that should be addressed before the patient moves to the ACHD clinic.27 The transition program should be flexible to accommodate for the patient’s degree of development, and the transfer should not occur before the adolescent has demonstrated the ability to independently manage their own health care to the greatest possible extent.
The ideal transition occurs through the auspices of a medical home that can coordinate the multiple subspecialists involved in the patient’s care. However, what often occurs is that a patient transitions from the pediatric cardiologist’s care before transitioning from pediatric to adult primary care. Prior to transition, the pediatric cardiologist should identify a cardiac destination at an ACHD center. This must be done in conjunction with the pediatrician, who will help identify an internist to take over the patient’s primary care and continue the coordination via the medical home. Information regarding the patient’s complete medical history, medication lists, exercise prescriptions, dietary restrictions, anesthetic issues, functional status, diagnostic studies, and comorbidities should be compiled in a health summary.40 To aid the process of transitioning, the ACC has developed several tools that may be used during the transition process, including self-knowledge assessments and medical summary templates.42
The Primary Care Provider’s Role and the Medical Home
Ensuring adequate care during the transition period requires close coordination between the patient’s various subspecialists. It is vital to avoid multiple subspecialists providing care without knowledge of each other’s treatments, as the treatment course for each ACHD patient is dependent on their unique history of prior therapies.27 The role of the primary care physician in establishing a “medical home” in this setting, as defined by the American Academy of Pediatrics Policy Statement, is exceedingly important.43 In this structure, the primary care physician maintains an easily accessible, centralized, and comprehensive record of the patient’s entire medical history, including surgical and medical treatments of both cardiac and noncardiac issues. Establishing the medical home framework is crucial, as it has been shown to lead to better outcomes in transitioning youth with special health care needs.44
With the establishment of this centralized care, the primary care physician must be able to negotiate the various medications prescribed by subspecialists and monitor for drug levels, adverse effects, and drug-drug interactions. ACHD patients also need regular monitoring and care aside from the care related to their chronic disease. Medical issues of particular importance to the ACHD patient include vaccinations, cholesterol and hypertension screening, cancer screening, and nutritional counseling. The primary care physician is responsible for addressing both the cardiac and noncardiac needs of the patient, ensuring that the patient truly receives comprehensive care. Thorough knowledge of a patient’s unique medical/surgical history will enable the primary care physician to adequately triage and appropriately refer for the development of a new symptom in an ACHD patient. On the other end of the spectrum, the patient’s subspecialists must maintain accurate and up to date information regarding their patient and transmit this to the patient’s medical home.
ACHD Centers
ACHD centers are an important part of any ACHD patient’s clinical team. Regardless of the complexity of the heart defect, there is tremendous value in the education and anticipatory guidance ACHD centers provide for their patients. The providers at these centers are often board-certified ACHD physicians who will work within a multidisciplinary team that includes mid-level practitioners, electrophysiology physicians, high-risk obstetrics/gynecology physicians, pulmonologists, and hepatologists. Each center differs in terms of their on-site interventional capacity and experience. However, the ACHD provider community is highly capable in directing patients who require interventions to centers of excellence, where there is proven quality in congenital surgical and interventional outcomes. ACHD centers often serve as the portals of reentry into care and are critical for providing and coordinating the complex care of each patient. Regular follow-up at these centers will ensure that patients receive adequate management of complications as they arise and preventive care against acquired heart disease.
The timing of follow-up at ACHD centers varies according to the complexity of heart disease. Individuals with simple CHD should be evaluated at an ACHD center at least once to determine the need for further follow-up. Patients with moderate and complex CHD must be monitored at a minimum of every 12 to 24 months, whereas very complex CHD should be monitored every 6 to 12 months.23 The frequency with which the young adult population moves may hinder adequate continuity of care and long-term follow up; a searchable directory of ACHD clinics in the United States and Canada can be found at www.achaheart.org/your-heart/clinic-directory/clinic-listings/.
Managing Specific Issues in the Transitioning Patient
Arrhythmias and Heart Failure
As mentioned, arrhythmias in the ACHD population are extremely common, the most frequent being atrial arrhythmias, especially in patients who have undergone single-ventricle repairs. Patients with late repair of an atrial septal defect have a high incidence of supraventricular tachycardia, which can be treated with catheter ablation procedures.45,46 Pacemaker implantation is another therapeutic option, especially in those who have undergone atrial surgery (ie, Mustard or Senning repairs). In these individuals, particularly in adolescents, abdominal implantation of a pacemaker generator may lessen the psychological impact of the external appearance of the pacemaker. In this population avoiding blunt contact sports (ie, tackle football, wrestling) is also important.28 It is critical that adult and pediatric electrophysiologists work together in the care and management of these complex, recurrent arrhythmias.
As noted above, many ACHD patients will require surgical or catheter-based interventions (as high as 40% in 1 study),47 and many encounter late-onset morbidity as a sequela of interventions earlier in life or as a result of failure of these interventions. The key for adult cardiologists and ACHD providers is delineating the reversible causes (eg, residual shunts, progressive valve regurgitation, and recoarctation) through routine intermittent surveillance, including echocardiograms, magnetic resonance imaging, and cardiac catherization, so that heart failure and arrhythmias in these patients can be identified, treated, and even prevented.
Pregnancy
Pregnancy is the most common reason for women to reenter care. Pregnancy is associated with significant hemodynamic changes, resulting in an increase in cardiac output to up to 150% of pre-pregnancy levels at 32 weeks, and up to 180% during labor. The outcome of pregnancy in patients with CHD is favorable in most instances provided that functional class systemic ventricular function is good. Accordingly, pregnancy is contraindicated in instances of severe pulmonary arterial hypertension (eg, Eisenmenger’s physiology), systemic ventricular dysfunction, and severe left-sided obstructions (eg, aortic or mitral stenosis). It is therefore imperative for health care providers to address the risks of pregnancy and the need for contraception with women who have CHD and are of reproductive age. The AHA advises beginning this conversation at 12 years of age and recommends that counseling be provided by health care providers knowledgeable in both CHD and adolescent health.27 Given the thrombotic potential of estrogen-containing contraception, the selection of contraception for women with ACHD who are seeking birth control requires discussion between the health care provider and patient. Though there have been limited studies performed on the use of contraception in women with CHD, a British working group has developed a consensus statement regarding contraceptive use in women with heart disease based on the World Health Organization format.48,49
Surgical Procedures
The need for operative interventions and re-interventions, both cardiac and noncardiac, in many CHD populations is considerable. Regardless of the type of procedure, these patients should receive a comprehensive preoperative risk assessment as well as appropriate intraoperative and postoperative management, ideally at a center equipped to meet their unique needs. Approaching the surgical procedure under the guidance of an interdisciplinary team that includes an ACHD specialist, anesthesiologist, and surgeon ensures that critical issues for appropriate management are not overlooked.
The preoperative risk assessment should be aimed at identifying and minimizing major risk factors. Historical factors to consider include the congenital lesion, outcomes of prior surgeries, history of syncope or arrhythmias, and the presence of pulmonary disease, among others.27 If the patient has a pacemaker or defibrillator, this should be interrogated prior to the planned procedure to ensure proper functioning. The preoperative evaluation should include consultation with a cardiologist experienced in the care of adolescents with CHD. Cardiac medications should be continued until the time of surgery and restarted as soon after the procedure as possible. Periods without anticoagulation should be minimized if indicated at baseline, and may require substituting warfarin with heparin in the preoperative period. The need for endocarditis prophylaxis must be considered as well; antibacterial prophylaxis prior to dental surgery, respiratory tract procedures, and procedures on infected skin and musculoskeletal structures is recommended in individuals with prosthetic heart valves, previous infective endocarditis, unrepaired CHD, repaired CHD with prosthetic material for the first 6 months after surgery, repaired CHD with residual defects, and valvulopathy after cardiac transplantation.50
Fluid management is important intraoperatively and post procedure, particularly in individuals who are preload dependent at baseline (eg, patients who have had Fontan palliation). Mechanical ventilation strategies with high positive end-expiratory pressure and tidal volume may decrease systemic venous return and should be monitored closely. Early mobilization and pulmonary toilet post extubation is advised to avoid pulmonary infection.
Exercise Capacity and Restrictions
The ability to exercise is an important factor in the quality of life of ACHD patients, especially in the adolescent period when participation in school and recreational athletics oftentimes functions as a social institution. Exercise ability is influenced by both real limitations imposed by limited cardiopulmonary reserve as a result of underlying pathology and by misconceptions of and anxiety about their ability to safely participate in these activities. There is evidence of diminished aerobic activity in all groups with CHD. However, symptomatic restrictions account for only approximately 30% of all barriers to exercise,51 and some studies have shown that exercise training programs can improve functional capacity and some standards of quality of life in CHD patients, in addition to the general health benefits associated with obesity prevention.52
Recommendations regarding exercise capacity are often addressed at primary care visits, and should be reinforced by the patient’s cardiologist. In general, most patients with repaired or mild defects can engage in moderate- to high-intensity exercise; those with more complex defects, cyanosis, or arrhythmias should be evaluated by an ACHD specialist to determine an appropriate level of activity.27 The “exercise prescription” provided to the patient should include type of exercise tolerated as well as heart rate goals and limits. In patients with extremely limited exercise capacity, a cardiac rehabilitation program can be beneficial. The presence of significant pulmonary hypertension, cyanosis or aortic stenosis, symptomatic arrhythmias, or evidence of myocardial dysfunction usually restricts the degree of exercise; full recommendations by activity and lesion type can be found in the guidelines proposed by the 36th Bethesda Conference.53 The importance of serial and regular evaluations is emphasized in these guidelines due to changing hemodynamic status of the patient over time as their cardiac lesions evolve and new complications arise.
Social and Psychological Impact of Chronic Illness
Living with a chronic disease can have a psychological impact on the child and transitioning adolescent. Frequent hospitalizations, physician visits, medical tests, and management of medical emergencies take a toll on the patient’s self-image and self-esteem, particularly during their formative adolescent years. Adolescents with CHD often feel “different” from their peers due to their condition,54 causing them to withhold disclosures about their heart disease to others out of fear of its impact on personal and professional relationships. Recent studies have shown that children and adolescents with CHD are at risk of internalizing problems and exhibiting behavior problems;55 they are also more likely to have impaired quality of life secondary to their increased incidence of psychosocial difficulties.56 The social and physical debility often experienced by patients with ACHD leads to a higher incidence of depression and anxiety in this population.57 Studies have shown that ACHD patients are interested in psychological treatment and peer support of their mood and anxiety disorders.58
At least some degree of the mental health issues ACHD patients experience is thought to have a physiological basis and be related to early cyanosis and neonatal surgical bypass duration. Prolonged duration of deep hypothermic circulatory arrest (DHCA) during corrective surgery is associated with reduced social competence, and has been found to be an independent risk factor for anxiety, depression, aggressive behavior, and attention deficiencies.59 In other studies, DHCA has been associated with decreased intellectual ability and worse fine motor skills, memory, and visuospatial skills, among other neurodevelopmental outcomes.60-62 Psychiatric disorders have also been associated with genetic syndromes like DiGeorge syndrome.63 This impacts executive function, leading to missed appointments, delay in clinical visits, and medication noncompliance. Given the potential for worse outcomes and risk of transition failure, primary care providers should routinely evaluate CHD patients for mood disorders and neurocognitive delay.
Social Determinants of Health and Medical Legal Partnerships
Social determinants of health and workplace discrimination play a large role in determining the ability of individuals with CHD to achieve adequate health care and maintain gainful employment. Individuals with CHD often face significant challenges as they prepare to enter the workforce, including discrimination within the workplace and maintaining employment through medical emergencies. Studies have shown that while educational milestones are similar between patients with and without CHD, those with CHD are much less likely to be employed.64 Challenges facing adolescents as they enter the workforce include hiring discrimination, physical challenges imposed by functional limitations, and misunderstanding of disease process and actual functional capacity. Career counseling is therefore an integral part of the transitioning process and should be started in early adolescence to allow for full assessment of mental, physical, and social abilities.65
Medical-legal partnerships (MLPs) can be extremely beneficial to the CHD population adversely affected by social determinants of health and workplace discrimination. These partnerships integrate lawyers into health care to address legal problems that create and perpetuate poor health; on a broader scale, these partnerships can advance and support public policy changes that improve population health.66
The major social determinants of health addressed by MLPs are income supports/insurance, housing/utilities, employment/education, legal status, and personal/family stability (summarized in the mnemonic I-HELP).67 Some of the more specific areas in which MLPs may assist in the delivery of care to CHD patients include case management, translation services, health literacy, and legal aid/legal services. ACHD patients also often experience a significant loss of services, including physical, occupational, and speech therapy and nutrition services, as adult clinics may not be prepared to provide these services. While physicians can best address the individual patient’s health, members of the legal system can address the systemic ailments that propagate that patient’s recurrent hospitalizations and other use of medical resources. Members of the legal system are present onsite in health care settings and participate in clinical meetings, which allows a coordinated and comprehensive screening for social needs that may harm a patient’s health.
Loss of insurance coverage is a major issue for transitioning patients; while adolescents with complex medical conditions are eligible for Medicaid to help cover the significant cost of their health care that goes beyond the abilities of private insurance, this eligibility ends when the patient turns 21. Additionally, the Social Security Administration re-determines supplemental security income (SSI) eligibility when the patient turns 18, and about one-third of patients lose their SSI benefits. Without appropriate guidance in navigating the nuances of insurance, many patients are at risk of losing coverage for their health care expenditures as they transition. Uninsured adults with a chronic condition are 8 times more likely to have unmet medical needs and 6 times more likely to have no access to routine care than insured young adults, with a 35% likelihood of the unmet medical need being due to cost.68 Undoubtedly, linability to pay for health care contributes to the lack of follow-up in the adult population, and MLPs may be a valuable tool to aid in ameliorating this problem.
Studies have shown that when legal services are used to address the social determinants of health, patients with chronic illnesses such as asthma and sickle cell disease have reduced hospital admissions.69,70 Other studies have shown utilization of MLPs has reduced spending on the care of high-need, high-use patients.71 According to a 2016 national survey of health care organizations conducted by the National Center for Medical-Legal Partnership, 39% clinicians reported improved compliance with medical treatment and 66% reported improved health outcomes after their patients received MLP services.72 Families referred to MLPs have shown increased access to health care, food, and income resources, and two-thirds reported improved child health and well-being.73 Given the numerous challenges faced by patients with CHD, involving MLPs as a part of both the transition process and the patient-centered medical home benefits these patients greatly and allows them to maximize their quality of life.
Conclusion
As more patients are living to adulthood with CHD, there is an increasing need for long-term care and adequate follow up, especially regarding the need for re-intervention and management of physiologic consequences of acquired cardiopulmonary, gastrointestinal, and renal disease in the setting of underlying congenital heart lesions. Beyond the purely medical aspects of the individual’s long-term management, psychosocial issues must be addressed, including preparing the individual for future employment and family counseling. Crucial to this process is the implementation of a comprehensive transition that begins in early adolescence and enables patients to take charge of their disease process in adulthood and ultimately enables them to maximize their quality of life and societal contributions. Towards this end, the role of MLPs may be important in ensuring that local, state, and federal policies that promote health harming norms are addressed.
Acknowledgments: We thank Dr. Frances ‘Kitty’ O’Hare and Bobbie Lewis for inviting us to submit this review; Dr. Russ Kolarik, Current Med-Peds Residency Program Director and Former President of the National Med-Peds Program Directors Association; and Dr. Peter Tilkemeier, Chairman, Department of Internal Medicine at Greenville Health System, for his unending support of our ACHD program. We also thank our patients, whose resounding resilience in the face of ongoing medical and psychosocial challenges remains our daily inspiration.
Corresponding author: Manisha S. Patel, MD, Department of Medicine and Pediatrics, Division of Cardiology, University of South Carolina School of Medicine, Columbia, SC; [email protected].
Financial disclosures: None.
From the Greenville Health System, Greenville, SC.
Abstract
- Objective: To review the management of patients with congenital heart disease (CHD) transitioning from pediatric to adult care.
- Methods: Review of the literature.
- Results: Persons with CHD require close monitoring and evaluation throughout life to address the physiologic consequences of acquired cardiopulmonary, gastrointestinal, and renal disease in the setting of underlying congenital heart lesions. During the transition from pediatric to adult cardiology, a high proportion of patients are lost to follow up or have long gaps in care after leaving pediatric cardiology, which can lead to poor outcomes. Care of the adult with CHD requires close coordination between the patient’s primary care physician), cardiologist, adult CHD specialist, and other specialists. The transition process for CHD patients begin at 12 years of age, with a goal of discussing future expectations of the child’s education, employment, and independent living. Successful transition programs use a systematic approach to addressing the medical, psychosocial, and educational/vocational needs of the adolescent as he or she moves from the family-centered pediatric to the patient-centered adult health care system.
- Conclusion: The transition from pediatric to adult care in ACHD patients is best provided through a comprehensive transition program that begins in early adolescence and enables patients to take charge of their disease process in adulthood, allowing them to maximize their quality of life and societal contributions.
Keywords: adult; congenital heart defects; complications; disease management; patient care team.
The population of adults with congenital heart disease (CHD) in developed countries has grown at an exponential rate in the past 4 decades. With advances in medical care and surgical interventions, the proportion of pediatric patients reaching adulthood has increased from 15% in the 1930s-60s to more than 95% for patients with mild to moderate complexity CHD. The rate of survival to adulthood for patients with severely complex CHD remains lower at around 56%.1
There are now more adult than pediatric patients with CHD in the United States. Because adult CHD (ACHD) patients have increased morbidity and mortality in their young adult years, it is imperative for all providers to understand and address the long-terms needs of this population. Unfortunately, adults with CHD do not always receive adequate health care, frequently because they are lost to follow-up, particularly during their adolescent years when they are expected to gain independence in their medical management. As will be discussed, CHD is a chronic illness fraught with numerous expected and unexpected complications that require close monitoring and re-interventions. Effectively anticipating and addressing these complications requires a standardized and comprehensive process of transition from the pediatric to the adult population to ensure maximal quality of life.
Epidemiology
The actual prevalence of ACHD in the United States is unknown, as a national database of persons with CHD has not been established.2 In contrast, Europe and China have maintained databases that enable ongoing monitoring of the evolving CHD epidemiology in those regions.3,4 The best estimates of the U.S. incidence and prevalence of ACHD stem from extrapolations from Canadian data. According to this data, there were more than 1.2 million adults with ACHD in the United States in 2012, with an anticipated 5% annual increase.1,5 However, the limitations of such extrapolations must be noted, as the Canadian population does not perfectly mirror that of the United States. Canada has lower infant mortality and adult obesity rates, and the United States has larger African American and Hispanic populations.6 Also, the juxtaposition of universal access to health care in Canada and the socioeconomic class–dependent access in the United States causes variations in care and outcomes of ACHD between the 2 populations. These differing genetic and social backgrounds may change the incidence of CHD by affecting maternal-fetal health.7
The 32nd Bethesda Conference on “Care of the Adult with Congenital Heart Disease” in 2000 was tasked with characterizing the ACHD population in the United States. This project found a prevalence similar to that of the Canadian extrapolation and showed that among persons with ACHD in the United States, 45% have mild disease, 37% moderate disease, and 13% severe disease.8
Characterizing the true incidence of CHD in the United States also has proven difficult because of variations in the definitions and methods used to detect lesions across the multiple studies that have looked at this matter. The estimated incidence of CHD, grouped according to severity, is 2.5 to 3 per 1000 live births for severe CHD, and from 3 to 13 per 1000 live births for moderately severe forms.9 When all forms are considered, including minor CHD (which includes tiny muscular ventricular septal defect [VSDs] present at birth and other trivial lesions), the total incidence of CHD rises to 75 per 1000 live births.9 CHD is one of the most common chronic illnesses in young adults with special health care needs.
Complications in Adulthood
The ACHD population represents a diverse population in terms of severity of CHD, history of surgical/catheter-based interventions, and socioeconomic status. However, a unifying clinical concern for these patients is their increased risk for morbidity and mortality in the young adult years. Despite the tremendous advances in the field over previous decades, mortality in this population in adulthood is estimated to be up to 7 times higher compared to age-matched peers.10,11 For many patients, palliative CHD interventions result in a significant drop in early morbidity and mortality but frequently lead to delayed morbidity from secondary multi-organ complications as these patients transition from pediatric to adult care. For example, due to the chronic low flow and low cardiac output state created by Fontan palliations, patients are at risk for diastolic dysfunction, arrhythmias, thrombotic events, protein-losing enteropathy, and cirrhosis/congestive hepatopathy, among other chronic conditions. These patients require frequent follow up and management by a multidisciplinary team including a primary care provider and various specialty groups.
Cardiac Disease
The most common causes of death in ACHD patients are heart failure (27%) and sudden cardiac death (19%), which occur at mean ages of 48 years and 39 years, respectively.10 The form of heart failure in ACHD patients is related to subsystemic right ventricle (RV) dysfunction, coronary under-perfusion, residual shunts, and residual progressive valve regurgitation. One of the more common examples of this is seen in palliated Tetralogy of Fallot patients who have undergone a transannular patch as a neonate. These patients are frequently left with significant pulmonary regurgitation leading to RV dilation, RV failure, and subsequent left ventricle (LV) failure. Another common example is the patient with dextro-transposition of the great arteries (DTGA) status post atrial switch who has a subsystemic morphologic RV. These patients will often develop significant RV dysfunction related to the chronic high pressures associated with systemic circulation.
Arrhythmias are a major contributor to morbidity and mortality in this population and are the most common reason patients present back into care. Difficult to control, multifocal intra-atrial re-entrant tachycardia is extremely common in ACHD, with an estimated 50% of all patients developing atrial arrhythmia by age 55. A recent study determined that the risk of atrial fibrillation in individuals with CHD was 22 times higher than that in age-matched controls, with the highest risk being seen in patients with conotruncal defects. Furthermore 10% of these patients develop heart failure.12 The risk for, incidence of, and type of arrhythmia is associated with the severity of the congenital heart lesions, as well as the type and timing of surgical interventions. Later age of repair has been associated with an increased likelihood of arrhythmias.13 Tetralogy of Fallot is an example of a moderately complex congenital heart lesion and is the most common cyanotic congenital heart lesion. In these individuals, the risk for atrial tachycardias, ventricular tachycardias, and need for a pacemaker is much higher than in age-matched peers.14 This includes an increased risk of sudden cardiac death, with many of these patients requiring placement of an implantable cardioverter defibrillator.
Pulmonary Disease
There is a 44% to 56% prevalence of restrictive pulmonary disease in the ACHD population, compared to 9% in the general non-CHD adult population. The incidence of pulmonary hypertension is also significantly higher in the ACHD population. The etiology for development of pulmonary hypertension is multifactorial, including chronic thromboembolic disease, left-sided heart disease, longstanding left to right shunts, and obstructive sleep apnea. These conditions have a significant impact on survival, as moderate/severe lung function impairment is an independent predictor of survival. Patients with shunt lesions are at risk of developing pulmonary arterial hypertension later in life,1 which quadruples the risk of all-cause mortality and more than triples the risk of cardiovascular mortality.7
Liver Disease
Hepatic morbidity associated with palliated CHD is often related to prior surgical interventions. The most common morbidities include chronic hepatitis C and liver failure from chronic under-perfusion and passive congestion, especially following Fontan palliation. Long term, these complications can lead to cirrhosis and hepatocellular carcinoma.15-18 Unfortunately, hepatic morbidity often precludes patients from having a surgical intervention, complicating the management of a population with baseline significantly increased need for surgical re-intervention.
Renal Disease
Approximately 50% of the ACHD population has some degree of renal dysfunction, with a higher incidence in cyanotic CHD.19 The American College of Cardiology/American Heart Association (ACC/AHA) recommends routine assessment of renal function in all adults with moderate and severe CHD due to its association with a poor prognosis in the ACHD population.1 In the immediate cardiac postoperative period, acute kidney injury leads to an eightfold increase in mortality.20 Over the longer term, there is a fivefold increase in mortality with moderate to severe renal impairment and a twofold increase with mild renal impairment compared to those with normal renal function.21
Acquired Cardiovascular Disease
As the ACHD patient ages, acquired cardiovascular disease becomes a significant issue. Approximately 80% of adults with CHD have at least 1 cardiovascular risk factor,22 though overall there is a relative lack of specific data regarding the U.S. population. Surveillance of the Canadian CHD population older than 65 years shows a 47% prevalence of hypertension,23 with increased risk in certain conditions such as aortic coarctation and renal disease associated with CHD. Although studies on the increased risk of diabetes mellitus in the ACHD population have yielded conflicting results,22,24 there is evidence of abnormal glucose metabolism in ACHD patients, which is a predictor of cardiac morbidity and mortality.25,26 The incidence of hyperlipidemia in U.S. ACHD patients is estimated to be at least as high as that of the general population.1 These factors combine with abnormalities in the myocardial substrate, hemodynamic abnormalities, arrhythmias, and sequelae of surgical repairs to confer an increased risk of ischemic heart disease and cerebrovascular disease in the ACHD population.15,27 One large case-control cohort study showed that the risk for ischemic heart disease was 16.5 times higher in patients with CHD as compared with non-CHD patients, with the highest incidence being in those with conotruncal defects and severe non-conotruncal defects. Interestingly, hypertension and diabetes were less common among CHD patients with ischemic heart disease than among non-CHD patients with ischemic heart disease.28
Adults with CHD have an increased risk for cerebrovascular disease compared with the general population, and cerebrovascular disease appears to occur at a younger age.29 The risk of ischemic stroke in individuals with ACHD younger than 55 years is 9 to 12 times higher than that in the general population. As in the general population, the incidence of ischemic stroke in ACHD patients increases with age, and in those older than 55 years, the incidence remains 2 to 4 times higher than in the general population.30,31
Clearly, complications arising from therapeutic interventions in CHD patients contribute significantly to morbidity/mortality in adult life, which underscores the need for life-long follow up and prevention of lapses in care.
The Transition from Pediatric to Adult Care
The monitoring and evaluation of CHD patients throughout life requires close coordination between the patient’s primary care physician, cardiologist, ACHD specialist, and other specialists, as appropriate. The timing of routine follow-up appointments is largely dependent on the severity of the congenital heart lesion and clinical status of the individual patient. Routine surveillance often includes cardiac imaging, preconception/genetic counseling, Holter screenings for arrhythmia, laboratory testing, and titration of medication. Unfortunately, only 30% of adults with CHD receive the recommended cardiac care.32
Children with chronic conditions transitioning to adulthood frequently experience a drop off in coordinated services as they transition from pediatric to adult medicine. Adult institutions often have less multidisciplinary support staff in the form of social workers and case management.33 Furthermore, a recent systematic review of articles that outlined the transition process from pediatric to adult cardiology in the CHD population showed that a high proportion of patients were either lost to follow up or had long gaps in care after leaving pediatric cardiology, with the first lapse in care commonly occurring at approximately age 19 years.28,34 A 2004 study showed that only 48% of adolescents with CHD underwent successful transition.35 A multicenter study of 922 ACHD patients found a gap in care lasting longer than 3 years in 42%, with 8% having gaps exceeding 10 years.36 Another study showed that lapses exceeding 2 years occurred in 63% of patients, with a median duration of lapse of medical care of 10 years. The most common reasons for lapse in care were: being told that cardiac follow up was not required (33%); being discharged from a children’s hospital without appropriate follow up plans in place (23%); being aware of need for follow up but having no symptoms (19%); lack of insurance (18%); and ignoring follow up recommendations for fear of receiving bad news (7%).37 Moreover, living independently from one’s parents was independently associated with a lapse in care, and patients with moderate complexity defects were more likely to experience a lapse than those with high complexity defects.
In the absence of a structured transition program, there is often delayed or inadequate care, which can result in significant emotional and financial stress on families and increased stress on the health care system.38 Inadequate, incomplete, or nonexistent transition and transfer for care has been shown to lead to poor health outcomes. Patients who experienced a lapse in care were 3 times more likely to require urgent cardiac intervention and to have an adverse outcome.37 The urgent interventions required by these patients included pulmonary valve replacement, mitral and tricuspid valve repair/replacement, VSD closure, pulmonary artery stenting, Fontan revision, and pacemaker/defibrillator placement.37 Clearly, there is significant room for improvement in the transition process of patients with CHD.
Best Practices in Transitioning CHD Patients to Adulthood
The overarching goal of pediatric to adult care CHD transition programs is to empower the patient and their support system to assume ownership of the disease process in order to maximize quality of life, life expectancy, and productivity.39 This involves ensuring that the patient has a thorough understanding of their diagnosis, heart anatomy, prior cardiac interventions, limitations imposed upon them by their condition, and the frequency of their anticipated follow-up care. The components of a successful transition program include a systematic approach to addressing the medical, psychosocial, and educational/vocational needs of the adolescent as he or she moves from the family-centered pediatric to the patient-centered adult health care system.40 The visits during the transition period are also an opportunity to discuss reproductive issues and the need for planning pregnancies for women with CHD. The goal is to encourage autonomy and promote ownership of their medical condition to the best of their social-cognitive ability. Adolescents should be encouraged to speak alone with their doctor to foster independence and self-management in their disease process; this has been shown to be protective against failure in transition.32 They should be encouraged to start calling their doctors, requesting refills, and making appointments.
The ACC/AHA appropriately recommend that the transition process for CHD patients begin at 12 years of age, with a goal of discussing future expectations of the child’s education, employment, and independent living.41 As part of this process, it is important that the practitioner educate the child and the family of the need for lifelong surveillance. The exact timing of the transition process is heavily influenced by a number of factors, including the degree of dependence of the child on their guardians, the severity of the congenital heart lesion, and the anticipated short- and long-term prognosis. However, regardless of these circumstances a reasonable age of transition into adult services should be established early on so that an expectation remains in place and the family is adequately prepared.
The challenge of learning how to navigate the adult health care system is as daunting for the transitioning patient as the medical consequences of their disease process. It is critical for patients to have easy access to social workers and case managers, ideally in the setting of a medical home, to connect them to community resources as needed. It is incredibly important that patients consider vocational options and training along with planning their insurance and/or disability qualifications as they move into adulthood. Establishing guardianship is also an important consideration for young adults with CHD who have remained dependent on their guardians.
Towards this end, the AHA/ACC has developed a curriculum that outlines the core principles that should be addressed before the patient moves to the ACHD clinic.27 The transition program should be flexible to accommodate for the patient’s degree of development, and the transfer should not occur before the adolescent has demonstrated the ability to independently manage their own health care to the greatest possible extent.
The ideal transition occurs through the auspices of a medical home that can coordinate the multiple subspecialists involved in the patient’s care. However, what often occurs is that a patient transitions from the pediatric cardiologist’s care before transitioning from pediatric to adult primary care. Prior to transition, the pediatric cardiologist should identify a cardiac destination at an ACHD center. This must be done in conjunction with the pediatrician, who will help identify an internist to take over the patient’s primary care and continue the coordination via the medical home. Information regarding the patient’s complete medical history, medication lists, exercise prescriptions, dietary restrictions, anesthetic issues, functional status, diagnostic studies, and comorbidities should be compiled in a health summary.40 To aid the process of transitioning, the ACC has developed several tools that may be used during the transition process, including self-knowledge assessments and medical summary templates.42
The Primary Care Provider’s Role and the Medical Home
Ensuring adequate care during the transition period requires close coordination between the patient’s various subspecialists. It is vital to avoid multiple subspecialists providing care without knowledge of each other’s treatments, as the treatment course for each ACHD patient is dependent on their unique history of prior therapies.27 The role of the primary care physician in establishing a “medical home” in this setting, as defined by the American Academy of Pediatrics Policy Statement, is exceedingly important.43 In this structure, the primary care physician maintains an easily accessible, centralized, and comprehensive record of the patient’s entire medical history, including surgical and medical treatments of both cardiac and noncardiac issues. Establishing the medical home framework is crucial, as it has been shown to lead to better outcomes in transitioning youth with special health care needs.44
With the establishment of this centralized care, the primary care physician must be able to negotiate the various medications prescribed by subspecialists and monitor for drug levels, adverse effects, and drug-drug interactions. ACHD patients also need regular monitoring and care aside from the care related to their chronic disease. Medical issues of particular importance to the ACHD patient include vaccinations, cholesterol and hypertension screening, cancer screening, and nutritional counseling. The primary care physician is responsible for addressing both the cardiac and noncardiac needs of the patient, ensuring that the patient truly receives comprehensive care. Thorough knowledge of a patient’s unique medical/surgical history will enable the primary care physician to adequately triage and appropriately refer for the development of a new symptom in an ACHD patient. On the other end of the spectrum, the patient’s subspecialists must maintain accurate and up to date information regarding their patient and transmit this to the patient’s medical home.
ACHD Centers
ACHD centers are an important part of any ACHD patient’s clinical team. Regardless of the complexity of the heart defect, there is tremendous value in the education and anticipatory guidance ACHD centers provide for their patients. The providers at these centers are often board-certified ACHD physicians who will work within a multidisciplinary team that includes mid-level practitioners, electrophysiology physicians, high-risk obstetrics/gynecology physicians, pulmonologists, and hepatologists. Each center differs in terms of their on-site interventional capacity and experience. However, the ACHD provider community is highly capable in directing patients who require interventions to centers of excellence, where there is proven quality in congenital surgical and interventional outcomes. ACHD centers often serve as the portals of reentry into care and are critical for providing and coordinating the complex care of each patient. Regular follow-up at these centers will ensure that patients receive adequate management of complications as they arise and preventive care against acquired heart disease.
The timing of follow-up at ACHD centers varies according to the complexity of heart disease. Individuals with simple CHD should be evaluated at an ACHD center at least once to determine the need for further follow-up. Patients with moderate and complex CHD must be monitored at a minimum of every 12 to 24 months, whereas very complex CHD should be monitored every 6 to 12 months.23 The frequency with which the young adult population moves may hinder adequate continuity of care and long-term follow up; a searchable directory of ACHD clinics in the United States and Canada can be found at www.achaheart.org/your-heart/clinic-directory/clinic-listings/.
Managing Specific Issues in the Transitioning Patient
Arrhythmias and Heart Failure
As mentioned, arrhythmias in the ACHD population are extremely common, the most frequent being atrial arrhythmias, especially in patients who have undergone single-ventricle repairs. Patients with late repair of an atrial septal defect have a high incidence of supraventricular tachycardia, which can be treated with catheter ablation procedures.45,46 Pacemaker implantation is another therapeutic option, especially in those who have undergone atrial surgery (ie, Mustard or Senning repairs). In these individuals, particularly in adolescents, abdominal implantation of a pacemaker generator may lessen the psychological impact of the external appearance of the pacemaker. In this population avoiding blunt contact sports (ie, tackle football, wrestling) is also important.28 It is critical that adult and pediatric electrophysiologists work together in the care and management of these complex, recurrent arrhythmias.
As noted above, many ACHD patients will require surgical or catheter-based interventions (as high as 40% in 1 study),47 and many encounter late-onset morbidity as a sequela of interventions earlier in life or as a result of failure of these interventions. The key for adult cardiologists and ACHD providers is delineating the reversible causes (eg, residual shunts, progressive valve regurgitation, and recoarctation) through routine intermittent surveillance, including echocardiograms, magnetic resonance imaging, and cardiac catherization, so that heart failure and arrhythmias in these patients can be identified, treated, and even prevented.
Pregnancy
Pregnancy is the most common reason for women to reenter care. Pregnancy is associated with significant hemodynamic changes, resulting in an increase in cardiac output to up to 150% of pre-pregnancy levels at 32 weeks, and up to 180% during labor. The outcome of pregnancy in patients with CHD is favorable in most instances provided that functional class systemic ventricular function is good. Accordingly, pregnancy is contraindicated in instances of severe pulmonary arterial hypertension (eg, Eisenmenger’s physiology), systemic ventricular dysfunction, and severe left-sided obstructions (eg, aortic or mitral stenosis). It is therefore imperative for health care providers to address the risks of pregnancy and the need for contraception with women who have CHD and are of reproductive age. The AHA advises beginning this conversation at 12 years of age and recommends that counseling be provided by health care providers knowledgeable in both CHD and adolescent health.27 Given the thrombotic potential of estrogen-containing contraception, the selection of contraception for women with ACHD who are seeking birth control requires discussion between the health care provider and patient. Though there have been limited studies performed on the use of contraception in women with CHD, a British working group has developed a consensus statement regarding contraceptive use in women with heart disease based on the World Health Organization format.48,49
Surgical Procedures
The need for operative interventions and re-interventions, both cardiac and noncardiac, in many CHD populations is considerable. Regardless of the type of procedure, these patients should receive a comprehensive preoperative risk assessment as well as appropriate intraoperative and postoperative management, ideally at a center equipped to meet their unique needs. Approaching the surgical procedure under the guidance of an interdisciplinary team that includes an ACHD specialist, anesthesiologist, and surgeon ensures that critical issues for appropriate management are not overlooked.
The preoperative risk assessment should be aimed at identifying and minimizing major risk factors. Historical factors to consider include the congenital lesion, outcomes of prior surgeries, history of syncope or arrhythmias, and the presence of pulmonary disease, among others.27 If the patient has a pacemaker or defibrillator, this should be interrogated prior to the planned procedure to ensure proper functioning. The preoperative evaluation should include consultation with a cardiologist experienced in the care of adolescents with CHD. Cardiac medications should be continued until the time of surgery and restarted as soon after the procedure as possible. Periods without anticoagulation should be minimized if indicated at baseline, and may require substituting warfarin with heparin in the preoperative period. The need for endocarditis prophylaxis must be considered as well; antibacterial prophylaxis prior to dental surgery, respiratory tract procedures, and procedures on infected skin and musculoskeletal structures is recommended in individuals with prosthetic heart valves, previous infective endocarditis, unrepaired CHD, repaired CHD with prosthetic material for the first 6 months after surgery, repaired CHD with residual defects, and valvulopathy after cardiac transplantation.50
Fluid management is important intraoperatively and post procedure, particularly in individuals who are preload dependent at baseline (eg, patients who have had Fontan palliation). Mechanical ventilation strategies with high positive end-expiratory pressure and tidal volume may decrease systemic venous return and should be monitored closely. Early mobilization and pulmonary toilet post extubation is advised to avoid pulmonary infection.
Exercise Capacity and Restrictions
The ability to exercise is an important factor in the quality of life of ACHD patients, especially in the adolescent period when participation in school and recreational athletics oftentimes functions as a social institution. Exercise ability is influenced by both real limitations imposed by limited cardiopulmonary reserve as a result of underlying pathology and by misconceptions of and anxiety about their ability to safely participate in these activities. There is evidence of diminished aerobic activity in all groups with CHD. However, symptomatic restrictions account for only approximately 30% of all barriers to exercise,51 and some studies have shown that exercise training programs can improve functional capacity and some standards of quality of life in CHD patients, in addition to the general health benefits associated with obesity prevention.52
Recommendations regarding exercise capacity are often addressed at primary care visits, and should be reinforced by the patient’s cardiologist. In general, most patients with repaired or mild defects can engage in moderate- to high-intensity exercise; those with more complex defects, cyanosis, or arrhythmias should be evaluated by an ACHD specialist to determine an appropriate level of activity.27 The “exercise prescription” provided to the patient should include type of exercise tolerated as well as heart rate goals and limits. In patients with extremely limited exercise capacity, a cardiac rehabilitation program can be beneficial. The presence of significant pulmonary hypertension, cyanosis or aortic stenosis, symptomatic arrhythmias, or evidence of myocardial dysfunction usually restricts the degree of exercise; full recommendations by activity and lesion type can be found in the guidelines proposed by the 36th Bethesda Conference.53 The importance of serial and regular evaluations is emphasized in these guidelines due to changing hemodynamic status of the patient over time as their cardiac lesions evolve and new complications arise.
Social and Psychological Impact of Chronic Illness
Living with a chronic disease can have a psychological impact on the child and transitioning adolescent. Frequent hospitalizations, physician visits, medical tests, and management of medical emergencies take a toll on the patient’s self-image and self-esteem, particularly during their formative adolescent years. Adolescents with CHD often feel “different” from their peers due to their condition,54 causing them to withhold disclosures about their heart disease to others out of fear of its impact on personal and professional relationships. Recent studies have shown that children and adolescents with CHD are at risk of internalizing problems and exhibiting behavior problems;55 they are also more likely to have impaired quality of life secondary to their increased incidence of psychosocial difficulties.56 The social and physical debility often experienced by patients with ACHD leads to a higher incidence of depression and anxiety in this population.57 Studies have shown that ACHD patients are interested in psychological treatment and peer support of their mood and anxiety disorders.58
At least some degree of the mental health issues ACHD patients experience is thought to have a physiological basis and be related to early cyanosis and neonatal surgical bypass duration. Prolonged duration of deep hypothermic circulatory arrest (DHCA) during corrective surgery is associated with reduced social competence, and has been found to be an independent risk factor for anxiety, depression, aggressive behavior, and attention deficiencies.59 In other studies, DHCA has been associated with decreased intellectual ability and worse fine motor skills, memory, and visuospatial skills, among other neurodevelopmental outcomes.60-62 Psychiatric disorders have also been associated with genetic syndromes like DiGeorge syndrome.63 This impacts executive function, leading to missed appointments, delay in clinical visits, and medication noncompliance. Given the potential for worse outcomes and risk of transition failure, primary care providers should routinely evaluate CHD patients for mood disorders and neurocognitive delay.
Social Determinants of Health and Medical Legal Partnerships
Social determinants of health and workplace discrimination play a large role in determining the ability of individuals with CHD to achieve adequate health care and maintain gainful employment. Individuals with CHD often face significant challenges as they prepare to enter the workforce, including discrimination within the workplace and maintaining employment through medical emergencies. Studies have shown that while educational milestones are similar between patients with and without CHD, those with CHD are much less likely to be employed.64 Challenges facing adolescents as they enter the workforce include hiring discrimination, physical challenges imposed by functional limitations, and misunderstanding of disease process and actual functional capacity. Career counseling is therefore an integral part of the transitioning process and should be started in early adolescence to allow for full assessment of mental, physical, and social abilities.65
Medical-legal partnerships (MLPs) can be extremely beneficial to the CHD population adversely affected by social determinants of health and workplace discrimination. These partnerships integrate lawyers into health care to address legal problems that create and perpetuate poor health; on a broader scale, these partnerships can advance and support public policy changes that improve population health.66
The major social determinants of health addressed by MLPs are income supports/insurance, housing/utilities, employment/education, legal status, and personal/family stability (summarized in the mnemonic I-HELP).67 Some of the more specific areas in which MLPs may assist in the delivery of care to CHD patients include case management, translation services, health literacy, and legal aid/legal services. ACHD patients also often experience a significant loss of services, including physical, occupational, and speech therapy and nutrition services, as adult clinics may not be prepared to provide these services. While physicians can best address the individual patient’s health, members of the legal system can address the systemic ailments that propagate that patient’s recurrent hospitalizations and other use of medical resources. Members of the legal system are present onsite in health care settings and participate in clinical meetings, which allows a coordinated and comprehensive screening for social needs that may harm a patient’s health.
Loss of insurance coverage is a major issue for transitioning patients; while adolescents with complex medical conditions are eligible for Medicaid to help cover the significant cost of their health care that goes beyond the abilities of private insurance, this eligibility ends when the patient turns 21. Additionally, the Social Security Administration re-determines supplemental security income (SSI) eligibility when the patient turns 18, and about one-third of patients lose their SSI benefits. Without appropriate guidance in navigating the nuances of insurance, many patients are at risk of losing coverage for their health care expenditures as they transition. Uninsured adults with a chronic condition are 8 times more likely to have unmet medical needs and 6 times more likely to have no access to routine care than insured young adults, with a 35% likelihood of the unmet medical need being due to cost.68 Undoubtedly, linability to pay for health care contributes to the lack of follow-up in the adult population, and MLPs may be a valuable tool to aid in ameliorating this problem.
Studies have shown that when legal services are used to address the social determinants of health, patients with chronic illnesses such as asthma and sickle cell disease have reduced hospital admissions.69,70 Other studies have shown utilization of MLPs has reduced spending on the care of high-need, high-use patients.71 According to a 2016 national survey of health care organizations conducted by the National Center for Medical-Legal Partnership, 39% clinicians reported improved compliance with medical treatment and 66% reported improved health outcomes after their patients received MLP services.72 Families referred to MLPs have shown increased access to health care, food, and income resources, and two-thirds reported improved child health and well-being.73 Given the numerous challenges faced by patients with CHD, involving MLPs as a part of both the transition process and the patient-centered medical home benefits these patients greatly and allows them to maximize their quality of life.
Conclusion
As more patients are living to adulthood with CHD, there is an increasing need for long-term care and adequate follow up, especially regarding the need for re-intervention and management of physiologic consequences of acquired cardiopulmonary, gastrointestinal, and renal disease in the setting of underlying congenital heart lesions. Beyond the purely medical aspects of the individual’s long-term management, psychosocial issues must be addressed, including preparing the individual for future employment and family counseling. Crucial to this process is the implementation of a comprehensive transition that begins in early adolescence and enables patients to take charge of their disease process in adulthood and ultimately enables them to maximize their quality of life and societal contributions. Towards this end, the role of MLPs may be important in ensuring that local, state, and federal policies that promote health harming norms are addressed.
Acknowledgments: We thank Dr. Frances ‘Kitty’ O’Hare and Bobbie Lewis for inviting us to submit this review; Dr. Russ Kolarik, Current Med-Peds Residency Program Director and Former President of the National Med-Peds Program Directors Association; and Dr. Peter Tilkemeier, Chairman, Department of Internal Medicine at Greenville Health System, for his unending support of our ACHD program. We also thank our patients, whose resounding resilience in the face of ongoing medical and psychosocial challenges remains our daily inspiration.
Corresponding author: Manisha S. Patel, MD, Department of Medicine and Pediatrics, Division of Cardiology, University of South Carolina School of Medicine, Columbia, SC; [email protected].
Financial disclosures: None.
1. Bhatt AB, Foster E, Kuehl K, et al. Congenital heart disease in the older adult: a scientific Statement from the American Heart Association. Circulation. 2015;26;131:1884-1931.
2. Gilboa SM, Devine OJ, Kucik JE, et al. Congenital heart defects in the United States: estimating the magnitude of the affected population in 2010. Circulation. 2016;134:101-109.
3. Dolk H, Loane M, Garne E. Congenital heart defects in Europe. Circulation. 2011;123:841-849.
4. Qu Y, Liu X, Zhuang J, et al. Incidence of congenital heart disease: the 9-year experience of the Guangdog Registry of Congenital Heart Disease, China. PloS One. 2016;11:e0159257.
5. Brickner ME, Hillis LD, Lange RA. Congenital heart disease in adults. N Engl J Med.
6. McGee Banks C. Variations in diversity in the United States and Canada. 2010. http://www.canadianstudies.isp.msu.edu/docs/Cherry%20McGee%20Banks.pdf.
7. van der Linde D, Konings EE, Slager MA, et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;58:2241-2247.
8. Warnes CA, Liberthson R, Danielson GK, et al. Task force 1: the changing profile of congenital heart disease in adult life. J Am Coll Cardiol. 2001;37:1170-1175.
9. Hoffman JIE, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39;1890-1900.
10. Verheugt CL, Uiterwaal CS, van der Velde ET, et al. Mortality in adult congenital heart disease. Eur Heart J. 2010;31:1220-1229.
11. Greutmann M, Tobler D, Kovacs AH, et al. Increasing mortality burden among adults with complex congenital heart disease. Congenit Heart Dis. 2015;10:117-127.
12. Mandalenakis Z, Rosengren A, Lappas G, et al. Atrial fibrillation burden in young patients with congenital heart disease. Circulation. 2018;137:928-937.
13. Fishberger SB, Wernovsky G, Gentles TL, et al. Factors that influence the development of atrial flutter after the Fontan operation. J Thorac Cardiovasc Surg. 1997;113:80–86.
14. Khairy P, Aboulhosn J, Gurvitz M; AARC. Arrhythmia burden in adults with surgically repaired tetralogy of Fallot. Circulation. 2010;122:868-875.
15. Ewe SH, Tan JL. Hepatotocellular carcinoma—a rare complication post Fontan operation. Congenit Heart Dis. 2009;4:103-106.
16. Ghaferi AA, Hutchins GM. Progression of liver pathology in patients undergoing the Fontan procedure: Chronic passive congestion, cardiac cirrhosis, hepatic adenoma, and hepatocellular carcinoma. J Thorac Cardiovasc Surg. 2005;129:1348-1352.
17. Saliba T, Dorkhom S, O’Reilly EM, et al. Hepatocellular carcinoma in two patients with cardiac cirrhosis. Eur J Gastroenterol Hepatol. 2010;22:889-891.
18. Wang A, Book W, McConnell M, et al. Prevalence of hepatitis C infection in adult patients who underwent congenital heart surgery prior to screening in 1992. Am J Cardiol. 2006;100:1307-1309.
19. Dimopoulos K, Diller GP, Koltsida E, et al. Prevalence, predictors, and prognostic value of renal dysfunction in adults with congenital heart disease. Circulation. 2008;117:2320-2328.
20. Karkouti K, Wijeysundera DN, Yau TM, et al. Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation. 2009;119:495-502.
21. Dimopoulos K, Diller GP, Koltsida E, et al. Prevalence, predictors, and prognostic value of renal dysfunction in adults with congenital heart disease. Circulation. 2008;117:2320-2328.
22. Moons P, Van Deyk K, Dedroog D, et al. Prevalence of cardiovascular risk factors in adults with congenital heart disease. Eur J Cardiovasc Prev Rehabil. 2006;13:612-616.
23. Afilalo J, Therrien J, Pilote L, et al. Geriatric congenital heart disease: burden of disease and predictors of mortality. J Am Coll Cardiol. 2011;58:1509-1515.
24. Billett J, Cowie MR, Gatzoulis MA, et al. Comorbidity, healthcare utilisation and process of care measures in patients with congenital heart disease in the UK: cross-sectional, population-based study with case-control analysis. Heart. 2008;94:1194-1199.
25. Hait G, Corpus M, Lamarre FR, et al. Alteration of glucose and insulin metabolism in congenital heart disease. Circulation. 1972;46:333-346.
26. Ohuchi H, Miyamoto Y, Yamamoto M, et al. High prevalence of abnormal glucose metabolism in young adult patients with complex congenital heart disease. Am Heart J. 2009;158:30-39.
27. Lui GK, Fernandes S, McElhinney DB. Management of cardiovascular risk factors in adults with congenital heart disease. J Am Heart Assoc. 2014;3(6):e001076.
28. Fedchenko M, Mandalenakis Z, Rosegren A, et al. Ischemic heart disease in children and young adults with congenital heart disease in Sweden. Int J Cardiol. 2017;248:143-148.
29. Mandalenakis Z, Rosengren A, Lappas G, et al. Ischemic stroke in children and young adults with congenital heart disease. J Am Heart Assoc. 2016. 23;5(2):e003071..
30. Hoffmann A, Chockalingam P, Balint OH, et al. Cerebrovascular accidents in adult patients with congenital heart disease. Heart. 2010;96:1223-1226.
31. Lanz J, Brophy JM, Therrien J, et al. Stroke in adults with congenital heart disease: incidence, cumulative risk, and predictors. Circulation. 2015;132:2385-2394
32. Sable C, Foster E, Uzark K, et al; on behalf of the American Heart Association Congenital Heart Defects Committee of the Council on Cardiovascular Disease in the Young, Council on Cardiovascular Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease. Best practices in managing transition to adulthood for adolescents with congenital heart disease: the transition process and medical and psychosocial issues: a scientific statement from the American Heart Association. Circulation. 2011;123:1454-1485.
33. Steinway C, Gable J, Jan S. Transitioning to adult care: supporting youth with special health care needs. Children’s Hospital of Philadelphia: Policylab Evidence to Action in Brief. Spring 2017.
34. Heery E, Sheehan AM, While AE, Coyne I. Experiences and outcomes of transition from pediatric to adult health care services for young people with congenital heart disease: a systematic review. Congenit Heart Dis. 2015;10413-427.
35. Reid GJ, Irvine MJ, McCrindle BW, et al. Prevalence and correlates of successful transfer from pediatric to adult health care among a cohort of young adults with complex congenital heart defects. Pediatrics. 2004;113(part 1):e197-e205.
36. Gurvitz M, Valente AM, Broberg C, et al; AARCC. Prevalence and predictors of gaps in care among adult congenital heart disease patients (The Health, Education and Access Research Trial). J Am Coll Cardiol. 2013;61:2180-2184.
37. Yeung E, Kay J, Roosevelt GE, et al. Lapse of care as a predictor for morbidity in adults in congenital heart disease. Int J Cardiol. 2008;125:62-65.
38. Meadows AK, Bosco V, Tong E, et al. Transition and transfer from pediatric to adult care of young adults with complex congenital heart disease. Current Cardiol Rep. 2009; 11;4;291-297.
39. lum RW, Garell D, Hodgman CH, et al. Transition from child-centered to adult health-care systems for adolescents with chronic conditions: a position paper of the Society for Adolescent Medicine. J Adolesc Health. 1993;14:570-576.
40. Rosen DS, Blum RW, Britto M, et al; Society for Adolescent Medicine. Transition to adult health care for adolescents and young adults with chronic conditions: position paper of the Society for Adolescent Medicine. J Adolesc Health. 2003;33:309-311.
41. Reiss JG, Gibson RW, Walker LR. Health care transition: youth, family, and provider perspectives. Pediatrics. 2005;115:112-120.
42. Congenital heart disease transition tools. American College of Cardiology. www.acc.org/membership/sections-and-councils/adult-congenital-and-pediatric-cardiology-section/resources/chdtransitiontools. Accessed November 1, 2018.
43. American Academy of Pediatrics Medical Home Initiatives for Children With Special Needs Project Advisory Committee. Policy statement: organizational principles to guide and define the child health care system and/or improve the health of all children. Pediatrics. 2004;113(suppl):1545-1547.
44. Lotstein DS, McPherson M, Strickland B, Newacheck PW. Transition planning for youth with special health care needs: results from the National Survey of Children with Special Health Care Needs. Pediatrics. 2005;115:1562-1568.
45. Gatzoulis MA, Freeman MA, Siu SC, et al. Atrial arrhythmia after surgical closure of atrial septal defects in adults. N Engl J Med. 1999;340:839-846.
46. Triedman JK, Bergau DM, Saul JP, et al. Efficacy of radiofrequency ablation for control of intraatrial reentrant tachycardia in patients with congenital heart disease. J Am Coll Cardiol. 1997;30:1032-1038.
47. Zomer AC, Verheugt CL, Vaartjes I, et al. Surgery in adults with congenital heart disease. Circulation. 2011;124:2195-2201.
48. Silversides CK, Sermer M, Siu SC. Choosing the best contraceptive method for the adult with congenital heart disease. Curr Cardiol Rep. 2009;11:298-305.
49. Thorne S, Nelson-Piercy C, MacGregor A, et al. Pregnancy and contraception in heart disease and pulmonary arterial hypertension. J Fam Plann Reprod Health Care. 2006;32:75-81.
50. Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group [published correction appears in Circulation. 2007;116:e376–e377]. Circulation. 2007;116:1736-1754.
51. Warnes CA, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Adults With Congenital Heart Disease): developed in collaboration with the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2008;118:2395-2451.
52. Curran TJ, Rhodes J. Cardiac rehabilitation improves heart rate recovery following peak exercise in children with repaired congenital heart disease. Pediatr Cardiol. 2007;28:276-279.
53. Graham TP Jr, Driscoll DJ, Gersony WM, et al Task force 2: congenital heart disease. J Am Coll Cardiol. 2005;45:1326-1333.
54. Tong EM, Sparacino PS, Messias DK, et al. Growing up with congenital heart disease: the dilemmas of adolescents and young adults. Cardiol Young. 1998;8:303-309.
55. Karsdorp PA, Everaerd W, Kindt M, Mulder BJ. Psychological and cognitive functioning in children and adolescents with congenital heart disease: a meta-analysis. J Pediatr Psychol. 2007;32:527-541.
56. Kovacs AH, Moons P. Psychosocial functioning and quality of life in adults with congenital heart disease and heart failure. Heart Fail Clin. 2014;10:35-42.
57. Bromberg JI, Beasley PJ, D’Angelo EJ, et al. Depression and anxiety in adults with congenital heart disease: a pilot study. Heart Lung. 2003;32:105–10.
58. Kovacs AH, Bendell KL, Colman J, et al. Adults with congenital heart disease: psychological needs and treatment preferences. Congenit Heart Dis. 2009;4:139-146
59. Hovels-Gurich HH, Konrad K, Wiesner M, et al. Long term behavioural outcome after neonatal arterial switch operation for transposition of the great arteries. Arch Dis Child. 2002;87:506–510.
60. Forbess JM, Visconti KJ, Hancock-Friesen C, et al. Neurodevelopmental outcome after congenital heart surgery: results from an institutional registry. Circulation. 2002;106:195-102.
61. Bellinger DC, Wypij D, duPlessis AJ, et al. Neurodevelopmental status at eight years in children with dextro-transposition of the great arteries: the Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg. 2003;126:1385-1396.
62. Wypij D, Newburger JW, Rappaport LA, et al. The effect of duration of deep hypothermic circulatory arrest in infant heart surgery on late neurodevelopment: the Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg. 2003;126:1397-1403.
63. Tang SX, Yi JJ, Calkins ME, et al. Psychiatric disorders in 22q11.2 deletion syndrome are prevalent but undertreated. Pscychol Med 2017;44:1267-1277.
64. Simko LC, McGinnis KA, Schembri J. Educational needs of adults with congenital heart disease. J Cardiovasc Nurs. 2006;21:85-94.
65. Foster E, Graham TP Jr, Driscoll DJ, et al. Task force 2: special health care needs of adults with congenital heart disease. J Am Coll Cardiol. 2001;37:1176-1183.
66. Sandel M, Hansen M, Kahn R, et al. Medical-legal partnerships: transforming primary care by addressing the legal needs of vulnerable populations. Health Aff. 2010;29:1697-1705.
67. The National Center for Medical-Legal Partnership. medical-legalpartnership.org. Accessed November 1, 2018.
68. Callahan ST, Cooper WO. Access to health care for young adults with disabling chronic conditions. Arch Pediatr Adolesc Med. 2006;160:178-182.
69. Pettignano R, Caley SB, Bliss LR. Medical-legal partnership: impact on patients with sickle cell disease. Pediatrics. 2011;128:1482-1488.
70. Klein MD, Beck AF, Henize AW, et al. Doctors and lawyers collaborating to HeLP children--outcomes from a successful partnership between professions. J Health Care Poor Underserved. 2013;24:1063-1073.
71. Martin J, Martin A, Schultz C, Sandel M. Embedding civil legal aid in care of high-utilizing patients using medical-legal partnership. Health Affairs blog. 22 April 2015. www.healthaffairs.org/do/10.1377/hblog20150422.047143/full. Accessed November 1, 2018.
72. Regenstein M, Sharac J, Williamson A. The state of the medical legal partnership field: findings from the 2016 National Center for Medical-Legal Partnership Surveys. August 2017.
73. Weintraub D, Rodgers MA, Botcheva L, et al. Pilot study of medical-legal partnership to address social and legal needs of patients. J Health Care Poor Underserved. 2010;21(2 Suppl):157-168.
1. Bhatt AB, Foster E, Kuehl K, et al. Congenital heart disease in the older adult: a scientific Statement from the American Heart Association. Circulation. 2015;26;131:1884-1931.
2. Gilboa SM, Devine OJ, Kucik JE, et al. Congenital heart defects in the United States: estimating the magnitude of the affected population in 2010. Circulation. 2016;134:101-109.
3. Dolk H, Loane M, Garne E. Congenital heart defects in Europe. Circulation. 2011;123:841-849.
4. Qu Y, Liu X, Zhuang J, et al. Incidence of congenital heart disease: the 9-year experience of the Guangdog Registry of Congenital Heart Disease, China. PloS One. 2016;11:e0159257.
5. Brickner ME, Hillis LD, Lange RA. Congenital heart disease in adults. N Engl J Med.
6. McGee Banks C. Variations in diversity in the United States and Canada. 2010. http://www.canadianstudies.isp.msu.edu/docs/Cherry%20McGee%20Banks.pdf.
7. van der Linde D, Konings EE, Slager MA, et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;58:2241-2247.
8. Warnes CA, Liberthson R, Danielson GK, et al. Task force 1: the changing profile of congenital heart disease in adult life. J Am Coll Cardiol. 2001;37:1170-1175.
9. Hoffman JIE, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39;1890-1900.
10. Verheugt CL, Uiterwaal CS, van der Velde ET, et al. Mortality in adult congenital heart disease. Eur Heart J. 2010;31:1220-1229.
11. Greutmann M, Tobler D, Kovacs AH, et al. Increasing mortality burden among adults with complex congenital heart disease. Congenit Heart Dis. 2015;10:117-127.
12. Mandalenakis Z, Rosengren A, Lappas G, et al. Atrial fibrillation burden in young patients with congenital heart disease. Circulation. 2018;137:928-937.
13. Fishberger SB, Wernovsky G, Gentles TL, et al. Factors that influence the development of atrial flutter after the Fontan operation. J Thorac Cardiovasc Surg. 1997;113:80–86.
14. Khairy P, Aboulhosn J, Gurvitz M; AARC. Arrhythmia burden in adults with surgically repaired tetralogy of Fallot. Circulation. 2010;122:868-875.
15. Ewe SH, Tan JL. Hepatotocellular carcinoma—a rare complication post Fontan operation. Congenit Heart Dis. 2009;4:103-106.
16. Ghaferi AA, Hutchins GM. Progression of liver pathology in patients undergoing the Fontan procedure: Chronic passive congestion, cardiac cirrhosis, hepatic adenoma, and hepatocellular carcinoma. J Thorac Cardiovasc Surg. 2005;129:1348-1352.
17. Saliba T, Dorkhom S, O’Reilly EM, et al. Hepatocellular carcinoma in two patients with cardiac cirrhosis. Eur J Gastroenterol Hepatol. 2010;22:889-891.
18. Wang A, Book W, McConnell M, et al. Prevalence of hepatitis C infection in adult patients who underwent congenital heart surgery prior to screening in 1992. Am J Cardiol. 2006;100:1307-1309.
19. Dimopoulos K, Diller GP, Koltsida E, et al. Prevalence, predictors, and prognostic value of renal dysfunction in adults with congenital heart disease. Circulation. 2008;117:2320-2328.
20. Karkouti K, Wijeysundera DN, Yau TM, et al. Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation. 2009;119:495-502.
21. Dimopoulos K, Diller GP, Koltsida E, et al. Prevalence, predictors, and prognostic value of renal dysfunction in adults with congenital heart disease. Circulation. 2008;117:2320-2328.
22. Moons P, Van Deyk K, Dedroog D, et al. Prevalence of cardiovascular risk factors in adults with congenital heart disease. Eur J Cardiovasc Prev Rehabil. 2006;13:612-616.
23. Afilalo J, Therrien J, Pilote L, et al. Geriatric congenital heart disease: burden of disease and predictors of mortality. J Am Coll Cardiol. 2011;58:1509-1515.
24. Billett J, Cowie MR, Gatzoulis MA, et al. Comorbidity, healthcare utilisation and process of care measures in patients with congenital heart disease in the UK: cross-sectional, population-based study with case-control analysis. Heart. 2008;94:1194-1199.
25. Hait G, Corpus M, Lamarre FR, et al. Alteration of glucose and insulin metabolism in congenital heart disease. Circulation. 1972;46:333-346.
26. Ohuchi H, Miyamoto Y, Yamamoto M, et al. High prevalence of abnormal glucose metabolism in young adult patients with complex congenital heart disease. Am Heart J. 2009;158:30-39.
27. Lui GK, Fernandes S, McElhinney DB. Management of cardiovascular risk factors in adults with congenital heart disease. J Am Heart Assoc. 2014;3(6):e001076.
28. Fedchenko M, Mandalenakis Z, Rosegren A, et al. Ischemic heart disease in children and young adults with congenital heart disease in Sweden. Int J Cardiol. 2017;248:143-148.
29. Mandalenakis Z, Rosengren A, Lappas G, et al. Ischemic stroke in children and young adults with congenital heart disease. J Am Heart Assoc. 2016. 23;5(2):e003071..
30. Hoffmann A, Chockalingam P, Balint OH, et al. Cerebrovascular accidents in adult patients with congenital heart disease. Heart. 2010;96:1223-1226.
31. Lanz J, Brophy JM, Therrien J, et al. Stroke in adults with congenital heart disease: incidence, cumulative risk, and predictors. Circulation. 2015;132:2385-2394
32. Sable C, Foster E, Uzark K, et al; on behalf of the American Heart Association Congenital Heart Defects Committee of the Council on Cardiovascular Disease in the Young, Council on Cardiovascular Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease. Best practices in managing transition to adulthood for adolescents with congenital heart disease: the transition process and medical and psychosocial issues: a scientific statement from the American Heart Association. Circulation. 2011;123:1454-1485.
33. Steinway C, Gable J, Jan S. Transitioning to adult care: supporting youth with special health care needs. Children’s Hospital of Philadelphia: Policylab Evidence to Action in Brief. Spring 2017.
34. Heery E, Sheehan AM, While AE, Coyne I. Experiences and outcomes of transition from pediatric to adult health care services for young people with congenital heart disease: a systematic review. Congenit Heart Dis. 2015;10413-427.
35. Reid GJ, Irvine MJ, McCrindle BW, et al. Prevalence and correlates of successful transfer from pediatric to adult health care among a cohort of young adults with complex congenital heart defects. Pediatrics. 2004;113(part 1):e197-e205.
36. Gurvitz M, Valente AM, Broberg C, et al; AARCC. Prevalence and predictors of gaps in care among adult congenital heart disease patients (The Health, Education and Access Research Trial). J Am Coll Cardiol. 2013;61:2180-2184.
37. Yeung E, Kay J, Roosevelt GE, et al. Lapse of care as a predictor for morbidity in adults in congenital heart disease. Int J Cardiol. 2008;125:62-65.
38. Meadows AK, Bosco V, Tong E, et al. Transition and transfer from pediatric to adult care of young adults with complex congenital heart disease. Current Cardiol Rep. 2009; 11;4;291-297.
39. lum RW, Garell D, Hodgman CH, et al. Transition from child-centered to adult health-care systems for adolescents with chronic conditions: a position paper of the Society for Adolescent Medicine. J Adolesc Health. 1993;14:570-576.
40. Rosen DS, Blum RW, Britto M, et al; Society for Adolescent Medicine. Transition to adult health care for adolescents and young adults with chronic conditions: position paper of the Society for Adolescent Medicine. J Adolesc Health. 2003;33:309-311.
41. Reiss JG, Gibson RW, Walker LR. Health care transition: youth, family, and provider perspectives. Pediatrics. 2005;115:112-120.
42. Congenital heart disease transition tools. American College of Cardiology. www.acc.org/membership/sections-and-councils/adult-congenital-and-pediatric-cardiology-section/resources/chdtransitiontools. Accessed November 1, 2018.
43. American Academy of Pediatrics Medical Home Initiatives for Children With Special Needs Project Advisory Committee. Policy statement: organizational principles to guide and define the child health care system and/or improve the health of all children. Pediatrics. 2004;113(suppl):1545-1547.
44. Lotstein DS, McPherson M, Strickland B, Newacheck PW. Transition planning for youth with special health care needs: results from the National Survey of Children with Special Health Care Needs. Pediatrics. 2005;115:1562-1568.
45. Gatzoulis MA, Freeman MA, Siu SC, et al. Atrial arrhythmia after surgical closure of atrial septal defects in adults. N Engl J Med. 1999;340:839-846.
46. Triedman JK, Bergau DM, Saul JP, et al. Efficacy of radiofrequency ablation for control of intraatrial reentrant tachycardia in patients with congenital heart disease. J Am Coll Cardiol. 1997;30:1032-1038.
47. Zomer AC, Verheugt CL, Vaartjes I, et al. Surgery in adults with congenital heart disease. Circulation. 2011;124:2195-2201.
48. Silversides CK, Sermer M, Siu SC. Choosing the best contraceptive method for the adult with congenital heart disease. Curr Cardiol Rep. 2009;11:298-305.
49. Thorne S, Nelson-Piercy C, MacGregor A, et al. Pregnancy and contraception in heart disease and pulmonary arterial hypertension. J Fam Plann Reprod Health Care. 2006;32:75-81.
50. Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group [published correction appears in Circulation. 2007;116:e376–e377]. Circulation. 2007;116:1736-1754.
51. Warnes CA, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Adults With Congenital Heart Disease): developed in collaboration with the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2008;118:2395-2451.
52. Curran TJ, Rhodes J. Cardiac rehabilitation improves heart rate recovery following peak exercise in children with repaired congenital heart disease. Pediatr Cardiol. 2007;28:276-279.
53. Graham TP Jr, Driscoll DJ, Gersony WM, et al Task force 2: congenital heart disease. J Am Coll Cardiol. 2005;45:1326-1333.
54. Tong EM, Sparacino PS, Messias DK, et al. Growing up with congenital heart disease: the dilemmas of adolescents and young adults. Cardiol Young. 1998;8:303-309.
55. Karsdorp PA, Everaerd W, Kindt M, Mulder BJ. Psychological and cognitive functioning in children and adolescents with congenital heart disease: a meta-analysis. J Pediatr Psychol. 2007;32:527-541.
56. Kovacs AH, Moons P. Psychosocial functioning and quality of life in adults with congenital heart disease and heart failure. Heart Fail Clin. 2014;10:35-42.
57. Bromberg JI, Beasley PJ, D’Angelo EJ, et al. Depression and anxiety in adults with congenital heart disease: a pilot study. Heart Lung. 2003;32:105–10.
58. Kovacs AH, Bendell KL, Colman J, et al. Adults with congenital heart disease: psychological needs and treatment preferences. Congenit Heart Dis. 2009;4:139-146
59. Hovels-Gurich HH, Konrad K, Wiesner M, et al. Long term behavioural outcome after neonatal arterial switch operation for transposition of the great arteries. Arch Dis Child. 2002;87:506–510.
60. Forbess JM, Visconti KJ, Hancock-Friesen C, et al. Neurodevelopmental outcome after congenital heart surgery: results from an institutional registry. Circulation. 2002;106:195-102.
61. Bellinger DC, Wypij D, duPlessis AJ, et al. Neurodevelopmental status at eight years in children with dextro-transposition of the great arteries: the Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg. 2003;126:1385-1396.
62. Wypij D, Newburger JW, Rappaport LA, et al. The effect of duration of deep hypothermic circulatory arrest in infant heart surgery on late neurodevelopment: the Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg. 2003;126:1397-1403.
63. Tang SX, Yi JJ, Calkins ME, et al. Psychiatric disorders in 22q11.2 deletion syndrome are prevalent but undertreated. Pscychol Med 2017;44:1267-1277.
64. Simko LC, McGinnis KA, Schembri J. Educational needs of adults with congenital heart disease. J Cardiovasc Nurs. 2006;21:85-94.
65. Foster E, Graham TP Jr, Driscoll DJ, et al. Task force 2: special health care needs of adults with congenital heart disease. J Am Coll Cardiol. 2001;37:1176-1183.
66. Sandel M, Hansen M, Kahn R, et al. Medical-legal partnerships: transforming primary care by addressing the legal needs of vulnerable populations. Health Aff. 2010;29:1697-1705.
67. The National Center for Medical-Legal Partnership. medical-legalpartnership.org. Accessed November 1, 2018.
68. Callahan ST, Cooper WO. Access to health care for young adults with disabling chronic conditions. Arch Pediatr Adolesc Med. 2006;160:178-182.
69. Pettignano R, Caley SB, Bliss LR. Medical-legal partnership: impact on patients with sickle cell disease. Pediatrics. 2011;128:1482-1488.
70. Klein MD, Beck AF, Henize AW, et al. Doctors and lawyers collaborating to HeLP children--outcomes from a successful partnership between professions. J Health Care Poor Underserved. 2013;24:1063-1073.
71. Martin J, Martin A, Schultz C, Sandel M. Embedding civil legal aid in care of high-utilizing patients using medical-legal partnership. Health Affairs blog. 22 April 2015. www.healthaffairs.org/do/10.1377/hblog20150422.047143/full. Accessed November 1, 2018.
72. Regenstein M, Sharac J, Williamson A. The state of the medical legal partnership field: findings from the 2016 National Center for Medical-Legal Partnership Surveys. August 2017.
73. Weintraub D, Rodgers MA, Botcheva L, et al. Pilot study of medical-legal partnership to address social and legal needs of patients. J Health Care Poor Underserved. 2010;21(2 Suppl):157-168.
Sexually Transmitted Infections Caused by Mycoplasma genitalium and Neisseria gonorrhoeae: Diagnosis and Treatment
From the Fargo Veterans Affairs Health Care System, Fargo, ND (Dr. Dietz, Dr. Hammer, Dr. Zegarra, and Dr. Lo), and the Queen Elizabeth Hospital, Hong Kong, China (Dr. Cho).
Abstract
- Objective: To review the management of patients with Mycoplasma genitalium and Neisseria gonorrhoeae infections.
- Methods: Review of the literature.
- Results: Mycoplasma genitalium and Neisseria gonorrhoeae are organisms that cause urethritis, cervicitis, and pelvic inflammatory disease. There is increasing antibiotic resistance to both organisms, which poses significant challenges to clinicians. Additionally, diagnostic tests for M. genitalium are not widely available, and commonly used tests for both organisms do not provide antibiotic sensitivity information. The increasing resistance of both M. genitalium and N. gonorrhoeae to currently used antimicrobial agents is alarming and warrants cautious monitoring.
- Conclusion: As the yield of new or effective antibiotic therapies has decreased over the past few years, increasing antibiotic resistance will lead to difficult treatment scenarios for sexually transmitted infections caused by these 2 organisms.
Keywords: Mycoplasma genitalium, Neisseria gonorrhoeae, antibiotic resistance, sexually transmitted infections, STIs.
The World Health Organization (WHO) estimates that more than 1 million cases of sexually transmitted Infections (STIs) are acquired every day worldwide,1 and that the majority of STIs have few or no symptoms, making diagnosis difficult. Two organisms of interest are Mycoplasma genitalium and Neisseria gonorrhoeae. In contrast to Chlamydia trachomatis, which is rarely resistant to treatment regimens, M. genitalium and N. gonorrhoeae are becoming increasingly resistant to antibiotic treatment and pose an impending threat. These bacteria can cause urethritis, cervicitis, and pelvic inflammatory disease (PID). Whereas antibiotic resistance to M. genitalium is emerging, resistance to N. gonorrhea has been a continual problem for decades. Drug resistance, especially for N. gonorrhoeae, is listed as a major threat to efforts to reduce the impact of STIs worldwide.2 In 2013, the U.S. Centers for Disease Control and Prevention (CDC) classified N. gonorrhoeae drug resistance as an urgent threat.3 As the yield of new or effective antibiotic therapies has decreased over the past few years, increasing antibiotic resistance will lead to challenging treatment scenarios for STIs caused by these 2 organisms.
Epidemiology and Pathogenesis
M. genitalium
M. genitalium is an emerging pathogen that is an etiologic agent of upper and lower genital tract STIs, such as urethritis, cervicitis, and PID.4-13 In addition, it is thought to be involved in tubal infertility and acquisition of other sexually transmitted pathogens, including HIV.7,8,13 The prevalence of M. genitalium in the general U.S. population in 2016 was reported to be approximately 17.2% for males and 16.1% for females.14 Infections are more common in patients aged 30 years and younger than in older populations.15 Also, patients self-identifying as black were found to have a higher prevalence of M. genitalium.14 This organism was first reported as being isolated from the urethras of 2 men with non-gonococcal urethritis (NGU) in London in 1980.15,16 It is a significant cause of acute and chronic NGU in males, and is estimated to account for 6% to 50% of cases of NGU.17,18M. genitalium in females has been associated with cervicitis4,9 and PID.8,10 A meta-analysis by Lis et al showed that M. genitalium infection was associated with an increased risk for preterm birth and spontaneous abortion.11 In addition, M. genitalium infections occur frequently in HIV-positive patients.19,20 M. genitalium increases susceptibility for passage of HIV across the epithelium by reducing epithelial barrier integrity.19
Beta lactams are ineffective against M. genitalium because mycoplasmas lack a cell wall and thus cell wall penicillin-binding proteins.21M. genitalium’s abilty to invade host epithelial cells is another mechanism that can protect the bacteria from antibiotic exposure.20 One of the first reports of antibiotic sensitivity testing for M. genitalium, published in 1997, noted that the organism was not susceptible to nalidixic acid, cephalosporins, penicillins, and rifampicin.22 In general, mycoplasmas are normally susceptible to antibiotics that inhibit protein synthesis,23 and initial good sensitivity to doxycycline and erythromycin was noted but this has since decreased. New antibiotics are on the horizon, but they have not been extensively tested in vivo.23
N. gonorrhoeae
Gonorrhea is the second most common STI of bacterial origin following C. trachomatis,24-26 which is rarely resistant to conventional regimens. In 2008, the World Health Organization (WHO) estimated that 106 million cases of N. gonorrhoeae infection were acquired annually and that 36.4 million adults were infected with N. gonorrhoeae.27 In the United States, the CDC estimates that gonorrhea cases are under-reported. An estimated 800,000 or more new cases are reported per year.28
The most common clinical presentations are urethritis in men and cervicitis in women.29 While urethritis is most likely to be symptomatic, only 50% of women with acute gonorrhea are symptomatic.29 In addition to lower urogenital tract infection, N. gonorrhoeae can also cause PID, ectopic pregnancy, infertility in women, and epididymitis in men.29,30 Rare complications can develop from the spread of N. gonorrhoeae to other parts of the body including the joints, eyes, cardiovascular system, and skin.29
N. gonorrhoeae can attach to the columnar epithelium and causes host innate immune-driven inflammation with neutrophil influx.29 It can avoid the immune response by varying its outer membrane protein expression. The organism is also able to acquire DNA from other Neisseria species30 and genera, which results in reduced susceptibility to therapies.
The Gonococcal Isolate Surveillance Project (GISP), established in 1986, is a collaborative project involving the CDC and STI clinics in 26 cities in the United States along with 5 regional laboratories.31 The GISP monitors susceptibilities in N. gonorrhoeae isolates obtained from roughly 6000 symptomatic men each year.31 Data collected from the GISP allows clinicians to treat infections with the correct antibiotic. Just as they observed patterns of fluoroquinolone-resistant N. gonorrhoeae, there has been a geographic progression of decreasing susceptibility to cephalosporins in recent years.31
The ease with which N. gonorrhoeae can develop resistance is particularly alarming. Sulfonamide use began in the 1930s, but resistance developed within approximately 10 years.30,32N. gonorrhoeae has acquired resistance to each therapeutic agent used for treatment over the course of its lifetime. One hypothesis is that use of single-dose therapy to rapidly treat the infection has led to treatment failure and allows for selective pressure where organisms with decreased antibiotic susceptibility are more likely to survive.30 However, there is limited evidence to support monotherapy versus combination therapy in treating N. gonorrhoeae.33,34 It is no exaggeration to say gonorrhea is now at risk of becoming an untreatable disease because of the rapid emergence of multidrug resistant N. gonorrhoeae strains worldwide.35
Diagnosis
Whether the urethritis, cervicitis, or PID is caused by N. gonorrhoeae, M. genitalium, or other non-gonococcal microorganisms (eg, C. trachomatis), no symptoms are specific to any of the microorganisms. Therefore, clinicians rely on laboratory tests to diagnose STIs caused by N. gonorrhoeae or M. genitalium.
M. genitalium
Gram Stain. Because M. genitalium lacks a cell wall, it cannot be identified by routine Gram stain.
Culture. Culturing of this fastidious bacterium might offer the advantage of assessing antibiotic susceptibility;36 however, the procedure is labor intensive and time consuming, and only a few labs in the world have the capability to perform this culture.12 Thus, this testing method is primarily undertaken for research purposes.
Serological Testing. Because of serologic cross-reactions between Mycoplasma pneumoniae and M. genitalium, there are no standardized serological tests for M. genitalium.37
Nucleic Acid Amplification Tests. M. genitalium diagnosis currently is made based exclusively on nucleic acid amplification testing (NAAT) methodology (polymerase chain reaction [PCR] or transcription-mediated amplification [TMA]), which is the only clinically useful method to detect M. genitalium. TMA for M. genitalium is commercially available in an analyte-specific reagent (ASR) format, but this has not been approved by the Food and Drug Administration (FDA).38 A study analyzing urogenital specimens from female patients via this TMA product found a 98.7% true-positive result when confirmed with repeat testing or alternative-target TMA, and only a 0.5% false-negative rate.38 There is evidence that this TMA product can be used to identify M. genitalium in urine, stool, and pharyngeal samples.39 These assays are currently available in some reference labs and large medical centers but are not widely available. Table 1 summarizes the diagnostic methods for M. genitalium.
N. gonorrhoeae
Gonococcal infection can involve the urogenital tract, but can also be extra-urogenital. The method of diagnoses of urogenital infections has expanded from Gram stain of urethral or cervical discharge and the use of selective media culture (usually Thayer-Martin media)40 to molecular methods such as NAATs, which have a higher sensitivity than cultures.41,42
Gram Stain. A Gram stain that shows polymorphonuclear leukocytes with intracellular gram-negative diplococci can be considered diagnostic for N. gonorrhoeae urethritis infection in symptomatic men when samples are obtained from the urethra.43 A retrospective study of 1148 women with gonorrhea revealed that of 1049 cases of cervical gonorrhea, only 6.4% were positive by smear alone; and of 841 cases of urethral gonorrhea, only 5.1% were positive by smear alone; therefore, other diagnostic methods are generally preferred in women.44 Because Gram stain of vaginal specimens is positive in only 50% to 60% of females, its use in women and in suspected extragenital gonococcal infections is not recommended.43-45 When Gram stain was performed in asymptomatic men, the sensitivity was around 80%.39 Thus, in asymptomatic men with a high pre-test probability of having the infection, the use of other additional testing would increase the rate of detection.43
Culture. Urethral swab specimens from males with symptomatic urethritis and cervical swab samples from females with endocervical infection must be inoculated onto both a selective medium (eg, modified Thayer-Martin medium or Martin Lewis medium) and a nonselective medium (eg, chocolate agar). A selective medium is used because it can suppress the growth of contaminating organisms, and a nonselective medium is used because some strains of N. gonorrhoeae are inhibited by the vancomycin present in the selective medium.40 Specimens collected from sterile sites, such as blood, synovial fluid, and cerebrospinal fluid, should be streaked on nonselective medium such as chocolate agar. The material used for collection is critical; the preferred swabs should have plastic or wire shafts and rayon, Dacron, or calcium alginate tips. Materials such as wooden shafts or cotton tips can be toxic to N. gonorrhoeae.40 The specimen should be inoculated immediately onto the appropriate medium and transported rapidly to the laboratory, where it should be incubated at 35º to 37ºC with 5% CO2 and examined at 24 and 48 hours post collection.40 If the specimens cannot be inoculated immediately onto the appropriate medium, the specimen swab should be delivered to the lab in a special transport system that can keep the N. gonorrhoeae viable for up to 48 hours at room temperature.46
The following specimen collection techniques are recommended by the CDC:40
- In males, the cotton swab should be inserted about 2 to 3 cm into the urethral meatus and rotated 360° degrees 2 or 3 times.
- In females, collection of cervical specimens requires inserting the tip of the swab 1 to 2 centimeters into the cervical os and rotating 360° 2 or 3 times.
- Samples obtained outside of the urogenital tract: rectal specimens may be obtained by inserting the swab 3 to 4 cm into the rectal vault. Pharyngeal specimens are to be obtained from the posterior pharynx with a swab.
Culture tests allow the clinician to assess antimicrobial susceptibility and are relatively low cost when compared with nucleic acid detection tests. The sensitivity of culture ranges from 72% to 95% for symptomatic patients, but drops to 65% to 85% for asymptomatic patients.45-47 This low sensitivity is a major disadvantage of culture tests when compared to NAATs. Other disadvantages are the need for the specimens to be transported under conditions adequate to maintain the viability of organisms and the fact that 24 to 72 hours is required to report presumptive culture results.42 Antimicrobial sensitivity testing generally is not recommended; however, it is advisable to perform antimicrobial sensitivity in cases of treatment failure or disseminated gonococcal infection.12
Nucleic Acid Amplification Tests. NAATs use techniques that allow the amplification and detection of N. gonorrhoeae DNA or RNA sequences through various methods, which include assays such as PCR (eg, Amplicor; Roche, Nutley, NJ), TMA (eg, APTIMA; Gen-Probe, San Diego, CA), and strand-displacement amplification (SDA; Probe-Tec; Becton Dickinson, Franklin Lake, NJ). While PCR and SDA methods amplify bacterial DNA, TMA amplifies bacterial rRNA.41
The FDA has cleared NAATs to test endocervical, vaginal, and urethral (men) swab specimens and urine for both men and women. There are several NAATs available to test rectal, oropharyngeal, and conjunctival specimens; however, none of them are FDA-cleared. Some local and commercial laboratories have validated the reliability of these extra-urogenital NAATs.12,48 Compared to cultures, NAATs have the advantages of being more sensitive and requiring less strict collection and transport conditions. However, they are costlier than cultures, do not provide any antimicrobial susceptibility information, and have varying specificity.49,50
Rapid Tests. NAAT results are usually available in approximately 1 to 2 days, so there has been significant interest in creating technologies that would allow for a more rapid turnaround time. The GeneXpert CT/NG is a newly developed real-time PCR-based assay that can simultaneously detect C. trachomatis and N. gonorrhoeae. The advantage of this technique is the 90-minute turnaround time and its ability to process more than 90 samples at a time. The specificity of this test for N. gonorrhoeae is similar to that of other NAATs (> 99.3%), suggesting that cross-reactivity is not a significant problem.51 Table 2 summarizes the test methods used for diagnosing N. gonorrhoeae.
Treatment
M. genitalium
M. genitalium, Mycoplasma hominis, and the ureaplasmas (U. urealyticum and U. parvum) are generally transmitted sexually, and the natural habitat of this Mycoplasmataceae family of bacteria is the genitourinary tract. All the mycoplasmas can cause NGU, cervicitis, and PID. Presently, multiple-drug resistant M. hominis and ureaplasmas remain uncommon, but the prevalence of M. genitalium resistant to multiple antibiotics has increased significantly in recent years.23,52
In the 1990s, M. genitalium was highly sensitive to the tetracyclines in vitro,53 and doxycycline was the drug of choice for treating NGU. However, it later became apparent that doxycycline was largely ineffective in treating urethritis caused by M. genitalium.54,55
Subsequently, azithromycin, a macrolide, became popular in treating urethritis in males and cervicitis in females because it was highly active against C. trachomatis54 and M. genitalium56 and it can be given orally as a single 1-g dose, thus increasing patients’ compliance. However, azithromycin-resistant M. genitalium has rapidly emerged and rates of treatment failure with azithromycin as high as 40% have been reported in recent studies.57,58 The resistance was found to be mediated by mutations in the 23S rRNA gene upon exposure of M. genitalium to azithromycin.15,57-59 Multiple studies conducted in various countries (including the United States, Netherlands, England, and France) all found high rates of 23S rRNA gene mutations.15,57-59M. genitalium samples were analyzed using reverse transcription-PCR and Sanger sequencing of the 23S tRNA to assess rates of macrolide resistance markers. The study found that 50.8% of female participants and 42% of male participants harbored mutations indicating macrolide resistance.15
An in vitro study conducted in France showed that the respiratory fluoroquinolone moxifloxacin was highly active against mycoplasmas, including M. genitalium.60 This study and others led to the use of moxifloxacin in treating infections caused by azithromycin-resistant M. genitalium. Moxifloxacin initially was successful in treating previous treatment failure cases.61 Unfortunately, the success has been short-lived, as researchers from Japan and Australia have reported moxifloxacin treament failures.62-64 These treatment failures were related to mutations in the parC and gyrA genes.62
Because M. genitalium exhibits significantly increased resistance to the tetracyclines, macrolides, and fluoroquinolones, leading to treatment failures associated with the resistance, the recently published CDC sexually transmitted diseases guidelines (2015) do not specifically recommend or endorse one class of antibiotics over another to treat M. genitalium infections; this contrasts with their approach for other infections in which they make specific recommendations for treatment.12 The lack of clear recommendations from the CDC makes standardized treatment for this pathogen difficult. The CDC guidelines do identify M. genitalium as an emerging issue, and mention that a single 1-g dose of azithromycin should likely be recommended over doxycycline due to the low cure rate of 31% seen with doxycycline. Moxifloxacin is mentioned as a possible alternative, but it is noted that the medication has not been evaluated in clinical trials and several studies have shown failures.12
Although the existing antibiotics to treat M. genitalium infections are far from desirable, treatment approaches have been recommended:65
- Azithromycin or doxycycline should be considered for empiric treatment without documented M. genitalium infection.
- Azithromycin is suggested as the first choice in documented M. genitalium infections.
- In patients with urethritis, azithromycin is recommended over doxycycline based on multiple studies. A single 1-g dose of azithromycin is preferred to an extended regimen due to increased compliance despite the extended regimen being slightly superior in effectiveness. The single-dose regimen is associated with selection of macrolide-resistant strains.65
- Women with cervicitis and PID with documented M. genitalium infection should receive an azithromycin-containing regimen.
Although the existing antibiotics on the market could not keep up with the rapid mutations of M. genitalium, a few recent studies have provided a glimmer of hope to tackle this wily microorganism. Two recent studies from Japan demonstrated that sitafloxacin, a novel fluoroquinolone, administered 100 mg twice a day to patients with M. genitalium was superior to other older fluoroquinolones.66,67 This fluoroquinolone could turn out to be a promising first-line antibiotic for treatment of STIs caused by M. genitalium. Bissessor and colleagues conducted a prospective cohort study of M. genitalium-infected male and female patients attending a STI clinic in Melbourne, Australia, and found that oral pristinamycin is highly effective in treating the M. genitalium strains that are resistant to azithromycin and moxifloxacin.68 Jensen et al reported on the novel fluoroketolide solithromycin, which demonstrated superior in vitro activity against M. genitalium compared with doxycycline, fluoroquinolones, and other macrolides.69 Solithromycin could potentially become a new antibiotic to treat infection caused by multi-drug resistant M. genitalium.
N. gonorrhoeae
Because of increasing resistance of N. gonorrhoeae to fluoroquinolones in the United States, the CDC recommended against their routine use for all cases of gonorrhea in August 2007.70 In some countries, penicillin-, tetracycline-, and ciprofloxacin-resistance rates could be as high as 100%, and these antibacterial agents are no longer treatment options for gonorrhea. The WHO released new N. gonorrhoeae treatment guidelines in 2016 due to high-level of resistance to previously recommended fluoroquinolones and decreased susceptibility to the third-generation cephalosporins, which were a first-line recommendation in the 2003 guidelines.45 The CDC’s currently recommended regimens for the treatment of uncomplicated and disseminated gonorrheal infections are summarized in Table 3 and Table 4.12 Recommendations from the WHO guidelines are very similar to the CDC recommendations.45
In light of the increasing resistance of N. gonorrhoeae to cephalosporins, 1 g of oral azithromycin should be added to ceftriaxone 250 mg intramuscularly in treating all cases of gonorrhea. The rationale for adding azithromycin to ceftriaxone is that azithromycin is active against N. gonorrhoeae at a different molecular target at a high dose, and it can also cover other co-pathogens.71 Unfortunately, susceptibility to cephalosporins has been decreasing rapidly.72 The greatest concern is the potential worldwide spread of the strain isolated in Kyoto, Japan, in 2009 from a patient with pharyngeal gonorrhea that was highly resistant to ceftriaxone (minimum inhibitory concentration of 2.0 to 4.0 µg/mL).73 At this time, N. gonorrhoeae isolates that are highly resistant to ceftriaxone are still rare globally.
Although cefixime is listed as an alternative treatment if ceftriaxone is not available, the 2015 CDC gonorrhea treatment guidelines note that N. gonorrhoeae is becoming more resistant to this oral third-generation cephalosporin; this increasing resistance is due in part to the genetic exchange between N. gonorrhoeae and other oral commensals actively taking place in the oral cavity, creating more resistant species. Another possible reason for cefixime resistance is that the concentration of cefixime used in treating gonococcal pharyngeal infection is subtherapeutic.74 A recent randomized multicenter trial in the United States compared 2 non-cephalosporin regimens: a single 240-mg dose of intramuscular gentamicin plus a single 2-g dose of oral azithromycin, and a single 320-mg dose of oral gemifloxacin plus a single 2-g dose of oral azithromycin. These combinations achieved 100% and 99.5% microbiological cure rates, respectively, in 401 patients with urogenital gonorrhea.75 Thus, these combination regimens can be considered as alternatives when the N. gonorrhoeae is resistant to cephalosporins or the patient is intolerant or allergic to cephalosporins.
Because N. gonorrhoeae has evolved into a “superbug,” becoming resistant to all currently available antimicrobial agents, it is important to focus on developing new agents with unique mechanisms of action to treat N. gonorrhoeae–related infections. Zoliflodacin (ETX0914), a novel topoisomerase II inhibitor, has the potential to become an effective agent to treat multi-drug resistant N. gonorrhoeae. A recent phase 2 trial demonstrated that a single oral 2000-mg dose of zoliflodacin microbiologically cleared 98% of gonorrhea patients, and some of the trial participants were infected with ciprofloxacin- or azithromycin-resistant strains.76 An additional phase 2 clinical trial compared oral zoliflodacin and intramuscular ceftriaxone. For uncomplicated urogential infections, 96% of patients in the zoliflodacin group achieved microbiologic cure versus 100% in the ceftriaxone group; however, zoliflodacin was less efficacious for pharyngeal infections.77 Gepotidacin (GSK2140944) is another new antimicrobial agent in the pipeline that looks promising. It is a novel first-in-class triazaacenaphthylene that inhibits bacterial DNA replication. A recent phase 2 clinical trial demonstrated that 1.5-g and 3-g single oral doses eradicated urogenital N. gonorrhoeae with microbiological success rates of 97% and 95%, respectively.78
Test of Cure
Because of the decreasing susceptibility of M. genitalium and N. gonorrhoeae to recommended treatment regimens, the European Guidelines consider test of cure essential in STIs caused by these 2 organisms to ensure eradication of infection and identify emerging resistance.79 However, test of cure is not routinely recommended by the CDC for these organisms in asymptomatic patients.12
Sexual Risk-Reduction Counseling
Besides aggressive treatment with appropriate antimicrobial agents, it is also essential that patients and their partners receive counseling to reduce the risk of STI. A recently published systematic review demonstrated that high-intensity counseling could decrease STI incidents in adolescents and adults.80
Conclusion
It is clear that these 2 sexually transmitted ”superbugs” are increasingly resistant to antibiotics and pose an increasing threat. Future epidemiological research and drug development studies need to be devoted to these 2 organisms, as well as to the potential development of a vaccine. This is especially important considering that antimicrobials may no longer be recommended when the prevalence of resistance to a particular antimicrobial reaches 5%, as is the case with WHO and other agencies that set the standard of ≥ 95% effectiveness for an antimicrobial to be considered as a recommended treatment.32 With current resistance rates for penicillin, ciprofloxacin, and tetracycline at close to 100% for N. gonorrhoeae in some countries,30,79 it is important to remain cognizant about current and future treatment options.
Because screening methods for M. genitalium are not available in most countries and there is not an FDA-approved screening method in the United States, M. genitalium poses a significant challenge for clinicians treating urethritis, cervicitis, and PID. Thus, the development of an effective screening method and established screening guidelines for M. genitalium is urgently needed. Better surveillance, prudent use of available antibiotics, and development of novel compounds are necessary to eliminate the impending threat caused by M. genitalium and N. gonorrhoeae.
This article is the result of work supported with resources and the use of facilities at the Fargo VA Health Care System. The contents of this manuscript do not represent the views of the Department of Veterans Affairs or the United States Government.
Corresponding author: Tze Shien Lo, MD, Veterans Affairs Medical Center, 2101 Elm Street N, Fargo, ND 58102.
Financial disclosures: None.
1. World Health Organization. Sexually transmitted infections (STIs). www.who.int/mediacentre/factsheets/fs110/en/. Fact Sheet #110. Updated August 2016. Accessed December 16, 2017.
2. World Health Organization. Growing antibiotic resistance forces updates to recommended treatment for sexually transmitted infections www.who.int/en/news-room/detail/30-08-2016-growing-antibiotic-resistance-forces-updates-to-recommended-treatment-for-sexually-transmitted-infections. Released August 30, 2016.
3. Centers for Disease Control and Prevention. Antibiotic/antimicrobial resistance biggest threats. www.cdc.gov/drugresistance/biggest_threats.html. Released February 27, 2018.
4. Taylor-Robinson D, Jensen JS. Mycoplasma genitalium: From chrysalis to multicolored butterfly. Clin Microbiol Rev. 2011;24:498-514.
5. Jensen JS. Mycoplasma genitalium: The aetiological agent of urethritis and other sexually transmitted diseases. J Eur Acad Dermatol Venereol. 2004;18:1-11.
6. Jaiyeoba O, Lazenby G, Soper DE. Recommendations and rationale for the treatment of pelvic inflammatory disease. Expert Rev Anti Infect Ther. 2011;9:61-70.
7. McGowin CL, Anderson-Smits C. Mycoplasma genitalium: An emerging cause of sexually transmitted disease in women. PLoS Pathog. 2011;7:e1001324.
8. Manhart LE, Broad JM, Golden MR. Mycoplasma genitalium: Should we treat and how? Clin Infect Dis. 2011;53 Suppl 3:S129-42.
9. Gaydos C, Maldeis NE, Hardick A, et al. Mycoplasma genitalium as a contributor to the multiple etiologies of cervicitis in women attending sexually transmitted disease clinics. Sex Transm Dis. 2009;36(1SE0):598-606.
10. Wiesenfeld HC, Hillier SL, Meyn L, et al. O04.6 Mycoplasma genitalium-Is it a pathogen in acute pelvic inflammatory disease (PID)? Sex Transm Infect. 2013 89:A34 http://sti.bmj.com/content/89/Suppl_1/A34.2. Accessed February 1, 2018.
11. Lis R, Rowhani-Rahbar A, Manhart LE. Mycoplasma genitalium infection and female reproductive tract disease: A meta-analysis. Clin Infect Dis. 2015;61:418-426.
12. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1-137.
13. Davies N. Mycoplasma genitalium: The need for testing and emerging diagnostic options. MLO Med Lab Obs. 2015;47:8,10-11.
14. Getman D, Jiang A, O’Donnell M, Cohen S. Mycoplasma genitalium prevalence, coinfection, and macrolide antibiotic resistance frequency in a multicenter clinical study cohort in the United States. J Clin Microbiol. 2016;54:2278-2283.
15. Tully JG, Taylor-Robinson D, Cole RM, Rose DL. A newly discovered mycoplasma in the human urogenital tract. Lancet. 1981;1(8233):1288-1291.
16. Taylor-Robinson D. The Harrison Lecture. The history and role of Mycoplasma genitalium in sexually transmitted diseases. Genitourin Med. 1995;71:1-8.
17. Horner P, Thomas B, Gilroy CB, Egger M, Taylor-Robinson D. Role of Mycoplasma genitalium and ureaplasma urealyticum in acute and chronic nongonococcal urethritis. Clin Infect Dis. 2001;32:995-1003.
18. Horner P, Blee K, O’Mahony C, et al. Clinical Effectiveness Group of the British Association of Sexual Health and HIV. 2015 UK National Guideline on the management of non-gonococcal urethritis. Int J STD AIDS. 2016;27:85-96.
19. Das K, De la Garza G, Siwak EB, et al. Mycoplasma genitalium promotes epithelial crossing and peripheral blood mononuclear cell infection by HIV-1. Int J Infect Dis. 2014;23:31-38.
20. McGowin CL, Annan RS, Quayle AJ, et al. Persistent Mycoplasma genitalium infection of human endocervical epithelial cells elicits chronic inflammatory cytokine secretion. Infect Immun. 2012;80:3842-3849.
21. Salado-Rasmussen K, Jensen JS. Mycoplasma genitalium testing pattern and macrolide resistance: A Danish nationwide retrospective survey. Clin Infect Dis. 2014;59:24-30.
22. Taylor-Robinson D, Bebear C. Antibiotic susceptibilities of mycoplasmas and treatment of mycoplasmal infections. J Antimicrob Chemother. 1997;40:622-630.
23. Taylor-Robinson D. Diagnosis and antimicrobial treatment of Mycoplasma genitalium infection: Sobering thoughts. Expert Rev Anti Infect Ther. 2014;12:715-722.
24. Ison CA. Biology of Neisseria gonorrhoeae and the clinical picture of infection. In: Gross G, Tyring SK, eds. Sexually Transmitted Infections and Sexually Transmitted Diseases.1st ed. Berlin, Heidelberg: Springer-Verlag; 2011:77-90.
25. Criss AK, Seifert HS. A bacterial siren song: Intimate interactions between neisseria and neutrophils. Nat Rev Microbiol. 2012;10:178-190.
26. Urban CF, Lourido S, Zychlinsky A. How do microbes evade neutrophil killing? Cell Microbiol. 2006;8:1687-1696.
27. World Health Organization, Dept. of Reproductive Health and Research. Global incidence and prevalence of selected curable sexually transmitted infections - 2008. www.who.int/reproductivehealth/publications/rtis/stisestimates/en/. Published 2012. Accessed February 6, 2018.
28. Centers for Disease Control and Prevention 2015 sexually transmitted diseases treatment guidelines. www.cdc.gov/std/tg2015/emerging.htm. Updated June 4, 2015.
29. Skerlev M, Culav-Koscak I. Gonorrhea: New challenges. Clin Dermatol. 2014;32:275-281.
30. Kirkcaldy RD, Ballard RC, Dowell D. Gonococcal resistance: Are cephalosporins next? Curr Infect Dis Rep. 2011;13:196-204.
31. Kidd S, Kirkcaldy R, Weinstock H, Bolan G. Tackling multidrug-resistant gonorrhea: How should we prepare for the untreatable? Expert Rev Anti Infect Ther. 2012;10:831-833.
32. Wang SA, Harvey AB, Conner SM, et al. Antimicrobial resistance for Neisseria gonorrhoeae in the United States, 1988 to 2003: The spread of fluoroquinolone resistance. Ann Intern Med. 2007;147:81-88.
33. Barbee LA, Kerani RP, Dombrowski JC, et al. A retrospective comparative study of 2-drug oral and intramuscular cephalosporin treatment regimens for pharyngeal gonorrhea. Clin Infect Dis. 2013;56:1539-434.
34. Sathia L, Ellis B, Phillip S, et al. Pharyngeal gonorrhoea - is dual therapy the way forward? Int J STD AIDS. 2007;18:647–8.
35. Tanaka M. Emergence of multidrug-resistant Neisseria gonorrhoeae strains circulating worldwide. Int J Urol. 2012;19:98-99.
36. Hamasuna R, Osada Y, Jensen JS. Isolation of Mycoplasma genitalium from first-void urine specimens by coculture with vero cells. J Clin Microbiol. 2007;45:847-850.
37. Razin S. Mycoplasma. In: Boricello SP, Murray PR, Funke G, eds. Topley & Wilson’s Microbiology and Microbial Infections. London, UK: Hodder Arnold; 2005:1957-2005.
38. Munson E, Bykowski H, Munson K, et al. Clinical laboratory assessment of Mycoplasma genitalium transcription-medicated ampliflication using primary female urogenital specimens. J Clin Microbiol. 2016;54:432-437.
39. Munson E, Wenten D, Jhansale S, et al. Expansion of comprehensive screening of male-sexually transmitted infection clinic attendees with Mycoplasma genitalium and Trichomonas vaginalis molecule assessment: a restrospective analysis. J Clin Microbiol. 2016;55:321-325.
40. Centers for Disease Control and Prevention. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae--2014. MMWR Recomm Rep. 2014;63(RR-02):1-19.
41. Boyadzhyan B, Yashina T, Yatabe JH, et al. Comparison of the APTIMA CT and GC assays with the APTIMA combo 2 assay, the Abbott LCx assay, and direct fluorescent-antibody and culture assays for detection of Chlamydia trachomatis and Neisseria gonorrhoeae. J Clin Microbiol. 2004;42:3089-3093.
42. Graseck AS, Shih SL, Peipert JF. Home versus clinic-based specimen collection for Chlamydia trachomatis and Neisseria gonorrhoeae. Expert Rev Anti Infect Ther. 2011;9:183-194.
43. Sherrard J, Barlow D. Gonorrhoea in men: Clinical and diagnostic aspects. Genitourin Med. 1996;72:422-426.
44. Goh BT, Varia KB, Ayliffe PF, Lim FK Diagnosis of gonorrhea by gram-stained smears and cultures in men and women: role of the urethral smear. Sex Transm Dis. 1985;12:135-139.
45. World Health Organization. WHO Guidelines for the Treatment of Neisseria gonorrhoeae. www.who.int/reproductivehealth/publications/rtis/gonorrhoea-treatment-guidelines/en/. Published 2016. Accessed December 16, 2017.
46. Arbique JC, Forward KR, LeBlanc J. Evaluation of four commercial transport media for the survival of Neisseria gonorrhoeae. Diagn Microbiol Infect Dis. 2000;36:163-168.
47. Schink JC, Keith LG. Problems in the culture diagnosis of gonorrhea. J Reprod Med. 1985;30(3 Suppl):244-249.
48. Marrazzo JM, Apicella MA. Neisseria gonorrhoeae (gonorrhea). In: Bennett JE, Dolin R, Blaser MJ, eds. Principles and Practice of Infectious Diseases. 8th ed. Philadelphia, PA: Elsevier; 2015:2446-2462.
49. Barry PM, Klausner JD. The use of cephalosporins for gonorrhea: The impending problem of resistance. Expert Opin Pharmacother. 2009;10:555-577.
50. Tabrizi SN, Unemo M, Limnios AE, et al. Evaluation of six commercial nucleic acid amplification tests for detection of Neisseria gonorrhoeae and other Neisseria species. J Clin Microbiol. 2011;49:3610-3615.
51. Goldenberg SD, Finn J, Sedudzi E, et al. Performance of the GeneXpert CT/NG assay compared to that of the Aptima AC2 assay for detection of rectal Chlamydia trachomatis and Neisseria gonorrhoeae by use of residual Aptima Samples. J Clin Microbiol. 2012;50:3867-3869.
52. Martin D. Mycoplasma genitalium, Mycoplasma hominis, and Ureaplasma species. In: Bennet J, Dolin R, Blaser M, eds. Principles and Practice of Infectious Diseases. 8th ed. Philadelphia, PA: Elsevier Sauders; 2015:2190-2193.
53. Hannan PC. Comparative susceptibilities of various AIDS-associated and human urogenital tract mycoplasmas and strains of Mycoplasma pneumoniae to 10 classes of antimicrobial agent in vitro. J Med Microbiol. 1998;47:1115-1122.
54. Mena LA, Mroczkowski TF, Nsuami M, Martin DH. A randomized comparison of azithromycin and doxycycline for the treatment of Mycoplasma genitalium-positive urethritis in men. Clin Infect Dis. 2009;48:1649-1654.
55. Schwebke JR, Rompalo A, Taylor S, et al. Re-evaluating the treatment of nongonococcal urethritis: Emphasizing emerging pathogens--a randomized clinical trial. Clin Infect Dis. 2011;52:163-170.
56. Bjornelius E, Anagrius C, Bojs G, et al. Antibiotic treatment of symptomatic Mycoplasma genitalium infection in Scandinavia: A controlled clinical trial. Sex Transm Infect. 2008;84:72-76.
57. Nijhuis RH, Severs TT, Van der Vegt DS, et al. High levels of macrolide resistance-associated mutations in Mycoplasma genitalium warrant antibiotic susceptibility-guided treatment. J Antimicrob Chemother. 2015;70:2515-2518.
58. Pond MJ, Nori AV, Witney AA, et al. High prevalence of antibiotic-resistant Mycoplasma genitalium in nongonococcal urethritis: The need for routine testing and the inadequacy of current treatment options. Clin Infect Dis. 2014;58:631-637.
59. Touati A, Peuchant O, Jensen JS, et al. Direct detection of macrolide resistance in Mycoplasma genitalium isolates from clinical specimens from France by use of real-time PCR and melting curve analysis. J Clin Microbiol. 2014;52:1549-1555.
60. Bebear CM, de Barbeyrac B, Pereyre S, et al. Activity of moxifloxacin against the urogenital Mycoplasmas ureaplasma spp., Mycoplasma hominis and Mycoplasma genitalium and Chlamydia trachomatis. Clin Microbiol Infect. 2008;14:801-805.
61. Jernberg E, Moghaddam A, Moi H. Azithromycin and moxifloxacin for microbiological cure of Mycoplasma genitalium infection: An open study. Int J STD AIDS. 2008;19:676-679.
62. Tagg KA, Jeoffreys NJ, Couldwell DL, et al. Fluoroquinolone and macrolide resistance-associated mutations in Mycoplasma genitalium. J Clin Microbiol. 2013;51:2245-2249.
63. Couldwell DL, Tagg KA, Jeoffreys NJ, Gilbert GL. Failure of moxifloxacin treatment in Mycoplasma genitalium infections due to macrolide and fluoroquinolone resistance. Int J STD AIDS. 2013;24:822-828.
64. Shimada Y, Deguchi T, Nakane K, et al. Emergence of clinical strains of Mycoplasma genitalium harbouring alterations in ParC associated with fluoroquinolone resistance. Int J Antimicrob Agents. 2010;36:255-258.
65. Mobley V, Seña A. Mycoplasma genitalium infection in men and women. In: UpToDate. www.uptodate.com. Last updated March 8, 2017. Accessed February 13, 2018.
66. Takahashi S, Hamasuna R, Yasuda M, et al. Clinical efficacy of sitafloxacin 100 mg twice daily for 7 days for patients with non-gonococcal urethritis. J Infect Chemother. 2013;19:941-945.
67. Ito S, Yasuda M, Seike K, et al. Clinical and microbiological outcomes in treatment of men with non-gonococcal urethritis with a 100-mg twice-daily dose regimen of sitafloxacin. J Infect Chemother. 2012;18:414-418.
68. Bissessor M, Tabrizi SN, Twin J, et al. Macrolide resistance and azithromycin failure in a Mycoplasma genitalium-infected cohort, and response of azithromycin failures to alternative antibiotic regimens. Clin Infect Dis. 2014;60:1228-1236.
69. Jensen JS, Fernandes P, Unemo M. In vitro activity of the new fluoroketolide solithromycin (CEM-101) against macrolide-resistant and -susceptible Mycoplasma genitalium strains. Antimicrob Agents Chemother. 2014;58:3151-3156.
70. Centers for Disease Control and Prevention (CDC). Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: Fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep. 2007;56:332-336.
71. Sexually transmitted diseases treatment guidelines, 2010. www.cdc.gov/std/treatment/default.htm. Published 2015. Accessed February13, 2016.
72. Centers for Disease Control and Prevention (CDC). Cephalosporin susceptibility among Neisseria gonorrhoeae isolates--United States, 2000-2010. MMWR Morb Mortal Wkly Rep. 2011;60:873-877.
73. Ohnishi M, Saika T, Hoshina S, et al. Ceftriaxone-resistant Neisseria gonorrhoeae, Japan. Emerg Infect Dis. 2011;17:148-149.
74. Centers for Disease Control and Prevention (CDC). Update to CDC’s sexually transmitted diseases treatment guidelines, 2010: Oral cephalosporins no longer a recommended treatment for gonococcal infections. MMWR Morb Mortal Wkly Rep. 2012;61:590-594.
75. Kirkcaldy RD, Weinstock HS, Moore PC, et al. The efficacy and safety of gentamicin plus azithromycin and gemifloxacin plus azithromycin as treatment of uncomplicated gonorrhea. Clin Infect Dis. 2014;59:1083-1091.
76. Seña AC, Taylor SN, Marrazzo J, et al. Microbiological cure rates and antimicrobial susceptibility of Neisseria gonorrhoeae to ETX0914 (AZD0914) in a phase II treatment trial for urogenital gonorrhea. (Poster 1308) Program and Abstract of ID Week 2016. New Orleans, LA, . October 25-30, 2016.
77. Taylor S, Marrazzo J, Batteiger B, et al. Single-dose zoliflodacin (ETX0914) for treatment of urogential gonorrhea. N Engl J Med. 2018;379:1835-1845.
78. Perry C, Dumont E, Raychaudhuri A. O05.3 A phase II, randomised, stdy in adults subjects evaluating the efficacy, safety, and tolerability of single doses of gepotidacin (GSK2140944) for treatment of uncomplicated urogenital gonorrhea. Sex Transm Infect. 2017;93(Suppl 2).
79. Bignell C, Unemo M, European STI Guidelines Editorial Board. 2012 European guideline on the diagnosis and treatment of gonorrhoea in adults. Int J STD AIDS. 2013;24:85-92.
80. O’Connor EA, Lin JS, Burda BU, et al. Behavioral sexual risk-reduction counseling in primary care to prevent sexually transmitted infections: A systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;161:874-883.
From the Fargo Veterans Affairs Health Care System, Fargo, ND (Dr. Dietz, Dr. Hammer, Dr. Zegarra, and Dr. Lo), and the Queen Elizabeth Hospital, Hong Kong, China (Dr. Cho).
Abstract
- Objective: To review the management of patients with Mycoplasma genitalium and Neisseria gonorrhoeae infections.
- Methods: Review of the literature.
- Results: Mycoplasma genitalium and Neisseria gonorrhoeae are organisms that cause urethritis, cervicitis, and pelvic inflammatory disease. There is increasing antibiotic resistance to both organisms, which poses significant challenges to clinicians. Additionally, diagnostic tests for M. genitalium are not widely available, and commonly used tests for both organisms do not provide antibiotic sensitivity information. The increasing resistance of both M. genitalium and N. gonorrhoeae to currently used antimicrobial agents is alarming and warrants cautious monitoring.
- Conclusion: As the yield of new or effective antibiotic therapies has decreased over the past few years, increasing antibiotic resistance will lead to difficult treatment scenarios for sexually transmitted infections caused by these 2 organisms.
Keywords: Mycoplasma genitalium, Neisseria gonorrhoeae, antibiotic resistance, sexually transmitted infections, STIs.
The World Health Organization (WHO) estimates that more than 1 million cases of sexually transmitted Infections (STIs) are acquired every day worldwide,1 and that the majority of STIs have few or no symptoms, making diagnosis difficult. Two organisms of interest are Mycoplasma genitalium and Neisseria gonorrhoeae. In contrast to Chlamydia trachomatis, which is rarely resistant to treatment regimens, M. genitalium and N. gonorrhoeae are becoming increasingly resistant to antibiotic treatment and pose an impending threat. These bacteria can cause urethritis, cervicitis, and pelvic inflammatory disease (PID). Whereas antibiotic resistance to M. genitalium is emerging, resistance to N. gonorrhea has been a continual problem for decades. Drug resistance, especially for N. gonorrhoeae, is listed as a major threat to efforts to reduce the impact of STIs worldwide.2 In 2013, the U.S. Centers for Disease Control and Prevention (CDC) classified N. gonorrhoeae drug resistance as an urgent threat.3 As the yield of new or effective antibiotic therapies has decreased over the past few years, increasing antibiotic resistance will lead to challenging treatment scenarios for STIs caused by these 2 organisms.
Epidemiology and Pathogenesis
M. genitalium
M. genitalium is an emerging pathogen that is an etiologic agent of upper and lower genital tract STIs, such as urethritis, cervicitis, and PID.4-13 In addition, it is thought to be involved in tubal infertility and acquisition of other sexually transmitted pathogens, including HIV.7,8,13 The prevalence of M. genitalium in the general U.S. population in 2016 was reported to be approximately 17.2% for males and 16.1% for females.14 Infections are more common in patients aged 30 years and younger than in older populations.15 Also, patients self-identifying as black were found to have a higher prevalence of M. genitalium.14 This organism was first reported as being isolated from the urethras of 2 men with non-gonococcal urethritis (NGU) in London in 1980.15,16 It is a significant cause of acute and chronic NGU in males, and is estimated to account for 6% to 50% of cases of NGU.17,18M. genitalium in females has been associated with cervicitis4,9 and PID.8,10 A meta-analysis by Lis et al showed that M. genitalium infection was associated with an increased risk for preterm birth and spontaneous abortion.11 In addition, M. genitalium infections occur frequently in HIV-positive patients.19,20 M. genitalium increases susceptibility for passage of HIV across the epithelium by reducing epithelial barrier integrity.19
Beta lactams are ineffective against M. genitalium because mycoplasmas lack a cell wall and thus cell wall penicillin-binding proteins.21M. genitalium’s abilty to invade host epithelial cells is another mechanism that can protect the bacteria from antibiotic exposure.20 One of the first reports of antibiotic sensitivity testing for M. genitalium, published in 1997, noted that the organism was not susceptible to nalidixic acid, cephalosporins, penicillins, and rifampicin.22 In general, mycoplasmas are normally susceptible to antibiotics that inhibit protein synthesis,23 and initial good sensitivity to doxycycline and erythromycin was noted but this has since decreased. New antibiotics are on the horizon, but they have not been extensively tested in vivo.23
N. gonorrhoeae
Gonorrhea is the second most common STI of bacterial origin following C. trachomatis,24-26 which is rarely resistant to conventional regimens. In 2008, the World Health Organization (WHO) estimated that 106 million cases of N. gonorrhoeae infection were acquired annually and that 36.4 million adults were infected with N. gonorrhoeae.27 In the United States, the CDC estimates that gonorrhea cases are under-reported. An estimated 800,000 or more new cases are reported per year.28
The most common clinical presentations are urethritis in men and cervicitis in women.29 While urethritis is most likely to be symptomatic, only 50% of women with acute gonorrhea are symptomatic.29 In addition to lower urogenital tract infection, N. gonorrhoeae can also cause PID, ectopic pregnancy, infertility in women, and epididymitis in men.29,30 Rare complications can develop from the spread of N. gonorrhoeae to other parts of the body including the joints, eyes, cardiovascular system, and skin.29
N. gonorrhoeae can attach to the columnar epithelium and causes host innate immune-driven inflammation with neutrophil influx.29 It can avoid the immune response by varying its outer membrane protein expression. The organism is also able to acquire DNA from other Neisseria species30 and genera, which results in reduced susceptibility to therapies.
The Gonococcal Isolate Surveillance Project (GISP), established in 1986, is a collaborative project involving the CDC and STI clinics in 26 cities in the United States along with 5 regional laboratories.31 The GISP monitors susceptibilities in N. gonorrhoeae isolates obtained from roughly 6000 symptomatic men each year.31 Data collected from the GISP allows clinicians to treat infections with the correct antibiotic. Just as they observed patterns of fluoroquinolone-resistant N. gonorrhoeae, there has been a geographic progression of decreasing susceptibility to cephalosporins in recent years.31
The ease with which N. gonorrhoeae can develop resistance is particularly alarming. Sulfonamide use began in the 1930s, but resistance developed within approximately 10 years.30,32N. gonorrhoeae has acquired resistance to each therapeutic agent used for treatment over the course of its lifetime. One hypothesis is that use of single-dose therapy to rapidly treat the infection has led to treatment failure and allows for selective pressure where organisms with decreased antibiotic susceptibility are more likely to survive.30 However, there is limited evidence to support monotherapy versus combination therapy in treating N. gonorrhoeae.33,34 It is no exaggeration to say gonorrhea is now at risk of becoming an untreatable disease because of the rapid emergence of multidrug resistant N. gonorrhoeae strains worldwide.35
Diagnosis
Whether the urethritis, cervicitis, or PID is caused by N. gonorrhoeae, M. genitalium, or other non-gonococcal microorganisms (eg, C. trachomatis), no symptoms are specific to any of the microorganisms. Therefore, clinicians rely on laboratory tests to diagnose STIs caused by N. gonorrhoeae or M. genitalium.
M. genitalium
Gram Stain. Because M. genitalium lacks a cell wall, it cannot be identified by routine Gram stain.
Culture. Culturing of this fastidious bacterium might offer the advantage of assessing antibiotic susceptibility;36 however, the procedure is labor intensive and time consuming, and only a few labs in the world have the capability to perform this culture.12 Thus, this testing method is primarily undertaken for research purposes.
Serological Testing. Because of serologic cross-reactions between Mycoplasma pneumoniae and M. genitalium, there are no standardized serological tests for M. genitalium.37
Nucleic Acid Amplification Tests. M. genitalium diagnosis currently is made based exclusively on nucleic acid amplification testing (NAAT) methodology (polymerase chain reaction [PCR] or transcription-mediated amplification [TMA]), which is the only clinically useful method to detect M. genitalium. TMA for M. genitalium is commercially available in an analyte-specific reagent (ASR) format, but this has not been approved by the Food and Drug Administration (FDA).38 A study analyzing urogenital specimens from female patients via this TMA product found a 98.7% true-positive result when confirmed with repeat testing or alternative-target TMA, and only a 0.5% false-negative rate.38 There is evidence that this TMA product can be used to identify M. genitalium in urine, stool, and pharyngeal samples.39 These assays are currently available in some reference labs and large medical centers but are not widely available. Table 1 summarizes the diagnostic methods for M. genitalium.
N. gonorrhoeae
Gonococcal infection can involve the urogenital tract, but can also be extra-urogenital. The method of diagnoses of urogenital infections has expanded from Gram stain of urethral or cervical discharge and the use of selective media culture (usually Thayer-Martin media)40 to molecular methods such as NAATs, which have a higher sensitivity than cultures.41,42
Gram Stain. A Gram stain that shows polymorphonuclear leukocytes with intracellular gram-negative diplococci can be considered diagnostic for N. gonorrhoeae urethritis infection in symptomatic men when samples are obtained from the urethra.43 A retrospective study of 1148 women with gonorrhea revealed that of 1049 cases of cervical gonorrhea, only 6.4% were positive by smear alone; and of 841 cases of urethral gonorrhea, only 5.1% were positive by smear alone; therefore, other diagnostic methods are generally preferred in women.44 Because Gram stain of vaginal specimens is positive in only 50% to 60% of females, its use in women and in suspected extragenital gonococcal infections is not recommended.43-45 When Gram stain was performed in asymptomatic men, the sensitivity was around 80%.39 Thus, in asymptomatic men with a high pre-test probability of having the infection, the use of other additional testing would increase the rate of detection.43
Culture. Urethral swab specimens from males with symptomatic urethritis and cervical swab samples from females with endocervical infection must be inoculated onto both a selective medium (eg, modified Thayer-Martin medium or Martin Lewis medium) and a nonselective medium (eg, chocolate agar). A selective medium is used because it can suppress the growth of contaminating organisms, and a nonselective medium is used because some strains of N. gonorrhoeae are inhibited by the vancomycin present in the selective medium.40 Specimens collected from sterile sites, such as blood, synovial fluid, and cerebrospinal fluid, should be streaked on nonselective medium such as chocolate agar. The material used for collection is critical; the preferred swabs should have plastic or wire shafts and rayon, Dacron, or calcium alginate tips. Materials such as wooden shafts or cotton tips can be toxic to N. gonorrhoeae.40 The specimen should be inoculated immediately onto the appropriate medium and transported rapidly to the laboratory, where it should be incubated at 35º to 37ºC with 5% CO2 and examined at 24 and 48 hours post collection.40 If the specimens cannot be inoculated immediately onto the appropriate medium, the specimen swab should be delivered to the lab in a special transport system that can keep the N. gonorrhoeae viable for up to 48 hours at room temperature.46
The following specimen collection techniques are recommended by the CDC:40
- In males, the cotton swab should be inserted about 2 to 3 cm into the urethral meatus and rotated 360° degrees 2 or 3 times.
- In females, collection of cervical specimens requires inserting the tip of the swab 1 to 2 centimeters into the cervical os and rotating 360° 2 or 3 times.
- Samples obtained outside of the urogenital tract: rectal specimens may be obtained by inserting the swab 3 to 4 cm into the rectal vault. Pharyngeal specimens are to be obtained from the posterior pharynx with a swab.
Culture tests allow the clinician to assess antimicrobial susceptibility and are relatively low cost when compared with nucleic acid detection tests. The sensitivity of culture ranges from 72% to 95% for symptomatic patients, but drops to 65% to 85% for asymptomatic patients.45-47 This low sensitivity is a major disadvantage of culture tests when compared to NAATs. Other disadvantages are the need for the specimens to be transported under conditions adequate to maintain the viability of organisms and the fact that 24 to 72 hours is required to report presumptive culture results.42 Antimicrobial sensitivity testing generally is not recommended; however, it is advisable to perform antimicrobial sensitivity in cases of treatment failure or disseminated gonococcal infection.12
Nucleic Acid Amplification Tests. NAATs use techniques that allow the amplification and detection of N. gonorrhoeae DNA or RNA sequences through various methods, which include assays such as PCR (eg, Amplicor; Roche, Nutley, NJ), TMA (eg, APTIMA; Gen-Probe, San Diego, CA), and strand-displacement amplification (SDA; Probe-Tec; Becton Dickinson, Franklin Lake, NJ). While PCR and SDA methods amplify bacterial DNA, TMA amplifies bacterial rRNA.41
The FDA has cleared NAATs to test endocervical, vaginal, and urethral (men) swab specimens and urine for both men and women. There are several NAATs available to test rectal, oropharyngeal, and conjunctival specimens; however, none of them are FDA-cleared. Some local and commercial laboratories have validated the reliability of these extra-urogenital NAATs.12,48 Compared to cultures, NAATs have the advantages of being more sensitive and requiring less strict collection and transport conditions. However, they are costlier than cultures, do not provide any antimicrobial susceptibility information, and have varying specificity.49,50
Rapid Tests. NAAT results are usually available in approximately 1 to 2 days, so there has been significant interest in creating technologies that would allow for a more rapid turnaround time. The GeneXpert CT/NG is a newly developed real-time PCR-based assay that can simultaneously detect C. trachomatis and N. gonorrhoeae. The advantage of this technique is the 90-minute turnaround time and its ability to process more than 90 samples at a time. The specificity of this test for N. gonorrhoeae is similar to that of other NAATs (> 99.3%), suggesting that cross-reactivity is not a significant problem.51 Table 2 summarizes the test methods used for diagnosing N. gonorrhoeae.
Treatment
M. genitalium
M. genitalium, Mycoplasma hominis, and the ureaplasmas (U. urealyticum and U. parvum) are generally transmitted sexually, and the natural habitat of this Mycoplasmataceae family of bacteria is the genitourinary tract. All the mycoplasmas can cause NGU, cervicitis, and PID. Presently, multiple-drug resistant M. hominis and ureaplasmas remain uncommon, but the prevalence of M. genitalium resistant to multiple antibiotics has increased significantly in recent years.23,52
In the 1990s, M. genitalium was highly sensitive to the tetracyclines in vitro,53 and doxycycline was the drug of choice for treating NGU. However, it later became apparent that doxycycline was largely ineffective in treating urethritis caused by M. genitalium.54,55
Subsequently, azithromycin, a macrolide, became popular in treating urethritis in males and cervicitis in females because it was highly active against C. trachomatis54 and M. genitalium56 and it can be given orally as a single 1-g dose, thus increasing patients’ compliance. However, azithromycin-resistant M. genitalium has rapidly emerged and rates of treatment failure with azithromycin as high as 40% have been reported in recent studies.57,58 The resistance was found to be mediated by mutations in the 23S rRNA gene upon exposure of M. genitalium to azithromycin.15,57-59 Multiple studies conducted in various countries (including the United States, Netherlands, England, and France) all found high rates of 23S rRNA gene mutations.15,57-59M. genitalium samples were analyzed using reverse transcription-PCR and Sanger sequencing of the 23S tRNA to assess rates of macrolide resistance markers. The study found that 50.8% of female participants and 42% of male participants harbored mutations indicating macrolide resistance.15
An in vitro study conducted in France showed that the respiratory fluoroquinolone moxifloxacin was highly active against mycoplasmas, including M. genitalium.60 This study and others led to the use of moxifloxacin in treating infections caused by azithromycin-resistant M. genitalium. Moxifloxacin initially was successful in treating previous treatment failure cases.61 Unfortunately, the success has been short-lived, as researchers from Japan and Australia have reported moxifloxacin treament failures.62-64 These treatment failures were related to mutations in the parC and gyrA genes.62
Because M. genitalium exhibits significantly increased resistance to the tetracyclines, macrolides, and fluoroquinolones, leading to treatment failures associated with the resistance, the recently published CDC sexually transmitted diseases guidelines (2015) do not specifically recommend or endorse one class of antibiotics over another to treat M. genitalium infections; this contrasts with their approach for other infections in which they make specific recommendations for treatment.12 The lack of clear recommendations from the CDC makes standardized treatment for this pathogen difficult. The CDC guidelines do identify M. genitalium as an emerging issue, and mention that a single 1-g dose of azithromycin should likely be recommended over doxycycline due to the low cure rate of 31% seen with doxycycline. Moxifloxacin is mentioned as a possible alternative, but it is noted that the medication has not been evaluated in clinical trials and several studies have shown failures.12
Although the existing antibiotics to treat M. genitalium infections are far from desirable, treatment approaches have been recommended:65
- Azithromycin or doxycycline should be considered for empiric treatment without documented M. genitalium infection.
- Azithromycin is suggested as the first choice in documented M. genitalium infections.
- In patients with urethritis, azithromycin is recommended over doxycycline based on multiple studies. A single 1-g dose of azithromycin is preferred to an extended regimen due to increased compliance despite the extended regimen being slightly superior in effectiveness. The single-dose regimen is associated with selection of macrolide-resistant strains.65
- Women with cervicitis and PID with documented M. genitalium infection should receive an azithromycin-containing regimen.
Although the existing antibiotics on the market could not keep up with the rapid mutations of M. genitalium, a few recent studies have provided a glimmer of hope to tackle this wily microorganism. Two recent studies from Japan demonstrated that sitafloxacin, a novel fluoroquinolone, administered 100 mg twice a day to patients with M. genitalium was superior to other older fluoroquinolones.66,67 This fluoroquinolone could turn out to be a promising first-line antibiotic for treatment of STIs caused by M. genitalium. Bissessor and colleagues conducted a prospective cohort study of M. genitalium-infected male and female patients attending a STI clinic in Melbourne, Australia, and found that oral pristinamycin is highly effective in treating the M. genitalium strains that are resistant to azithromycin and moxifloxacin.68 Jensen et al reported on the novel fluoroketolide solithromycin, which demonstrated superior in vitro activity against M. genitalium compared with doxycycline, fluoroquinolones, and other macrolides.69 Solithromycin could potentially become a new antibiotic to treat infection caused by multi-drug resistant M. genitalium.
N. gonorrhoeae
Because of increasing resistance of N. gonorrhoeae to fluoroquinolones in the United States, the CDC recommended against their routine use for all cases of gonorrhea in August 2007.70 In some countries, penicillin-, tetracycline-, and ciprofloxacin-resistance rates could be as high as 100%, and these antibacterial agents are no longer treatment options for gonorrhea. The WHO released new N. gonorrhoeae treatment guidelines in 2016 due to high-level of resistance to previously recommended fluoroquinolones and decreased susceptibility to the third-generation cephalosporins, which were a first-line recommendation in the 2003 guidelines.45 The CDC’s currently recommended regimens for the treatment of uncomplicated and disseminated gonorrheal infections are summarized in Table 3 and Table 4.12 Recommendations from the WHO guidelines are very similar to the CDC recommendations.45
In light of the increasing resistance of N. gonorrhoeae to cephalosporins, 1 g of oral azithromycin should be added to ceftriaxone 250 mg intramuscularly in treating all cases of gonorrhea. The rationale for adding azithromycin to ceftriaxone is that azithromycin is active against N. gonorrhoeae at a different molecular target at a high dose, and it can also cover other co-pathogens.71 Unfortunately, susceptibility to cephalosporins has been decreasing rapidly.72 The greatest concern is the potential worldwide spread of the strain isolated in Kyoto, Japan, in 2009 from a patient with pharyngeal gonorrhea that was highly resistant to ceftriaxone (minimum inhibitory concentration of 2.0 to 4.0 µg/mL).73 At this time, N. gonorrhoeae isolates that are highly resistant to ceftriaxone are still rare globally.
Although cefixime is listed as an alternative treatment if ceftriaxone is not available, the 2015 CDC gonorrhea treatment guidelines note that N. gonorrhoeae is becoming more resistant to this oral third-generation cephalosporin; this increasing resistance is due in part to the genetic exchange between N. gonorrhoeae and other oral commensals actively taking place in the oral cavity, creating more resistant species. Another possible reason for cefixime resistance is that the concentration of cefixime used in treating gonococcal pharyngeal infection is subtherapeutic.74 A recent randomized multicenter trial in the United States compared 2 non-cephalosporin regimens: a single 240-mg dose of intramuscular gentamicin plus a single 2-g dose of oral azithromycin, and a single 320-mg dose of oral gemifloxacin plus a single 2-g dose of oral azithromycin. These combinations achieved 100% and 99.5% microbiological cure rates, respectively, in 401 patients with urogenital gonorrhea.75 Thus, these combination regimens can be considered as alternatives when the N. gonorrhoeae is resistant to cephalosporins or the patient is intolerant or allergic to cephalosporins.
Because N. gonorrhoeae has evolved into a “superbug,” becoming resistant to all currently available antimicrobial agents, it is important to focus on developing new agents with unique mechanisms of action to treat N. gonorrhoeae–related infections. Zoliflodacin (ETX0914), a novel topoisomerase II inhibitor, has the potential to become an effective agent to treat multi-drug resistant N. gonorrhoeae. A recent phase 2 trial demonstrated that a single oral 2000-mg dose of zoliflodacin microbiologically cleared 98% of gonorrhea patients, and some of the trial participants were infected with ciprofloxacin- or azithromycin-resistant strains.76 An additional phase 2 clinical trial compared oral zoliflodacin and intramuscular ceftriaxone. For uncomplicated urogential infections, 96% of patients in the zoliflodacin group achieved microbiologic cure versus 100% in the ceftriaxone group; however, zoliflodacin was less efficacious for pharyngeal infections.77 Gepotidacin (GSK2140944) is another new antimicrobial agent in the pipeline that looks promising. It is a novel first-in-class triazaacenaphthylene that inhibits bacterial DNA replication. A recent phase 2 clinical trial demonstrated that 1.5-g and 3-g single oral doses eradicated urogenital N. gonorrhoeae with microbiological success rates of 97% and 95%, respectively.78
Test of Cure
Because of the decreasing susceptibility of M. genitalium and N. gonorrhoeae to recommended treatment regimens, the European Guidelines consider test of cure essential in STIs caused by these 2 organisms to ensure eradication of infection and identify emerging resistance.79 However, test of cure is not routinely recommended by the CDC for these organisms in asymptomatic patients.12
Sexual Risk-Reduction Counseling
Besides aggressive treatment with appropriate antimicrobial agents, it is also essential that patients and their partners receive counseling to reduce the risk of STI. A recently published systematic review demonstrated that high-intensity counseling could decrease STI incidents in adolescents and adults.80
Conclusion
It is clear that these 2 sexually transmitted ”superbugs” are increasingly resistant to antibiotics and pose an increasing threat. Future epidemiological research and drug development studies need to be devoted to these 2 organisms, as well as to the potential development of a vaccine. This is especially important considering that antimicrobials may no longer be recommended when the prevalence of resistance to a particular antimicrobial reaches 5%, as is the case with WHO and other agencies that set the standard of ≥ 95% effectiveness for an antimicrobial to be considered as a recommended treatment.32 With current resistance rates for penicillin, ciprofloxacin, and tetracycline at close to 100% for N. gonorrhoeae in some countries,30,79 it is important to remain cognizant about current and future treatment options.
Because screening methods for M. genitalium are not available in most countries and there is not an FDA-approved screening method in the United States, M. genitalium poses a significant challenge for clinicians treating urethritis, cervicitis, and PID. Thus, the development of an effective screening method and established screening guidelines for M. genitalium is urgently needed. Better surveillance, prudent use of available antibiotics, and development of novel compounds are necessary to eliminate the impending threat caused by M. genitalium and N. gonorrhoeae.
This article is the result of work supported with resources and the use of facilities at the Fargo VA Health Care System. The contents of this manuscript do not represent the views of the Department of Veterans Affairs or the United States Government.
Corresponding author: Tze Shien Lo, MD, Veterans Affairs Medical Center, 2101 Elm Street N, Fargo, ND 58102.
Financial disclosures: None.
From the Fargo Veterans Affairs Health Care System, Fargo, ND (Dr. Dietz, Dr. Hammer, Dr. Zegarra, and Dr. Lo), and the Queen Elizabeth Hospital, Hong Kong, China (Dr. Cho).
Abstract
- Objective: To review the management of patients with Mycoplasma genitalium and Neisseria gonorrhoeae infections.
- Methods: Review of the literature.
- Results: Mycoplasma genitalium and Neisseria gonorrhoeae are organisms that cause urethritis, cervicitis, and pelvic inflammatory disease. There is increasing antibiotic resistance to both organisms, which poses significant challenges to clinicians. Additionally, diagnostic tests for M. genitalium are not widely available, and commonly used tests for both organisms do not provide antibiotic sensitivity information. The increasing resistance of both M. genitalium and N. gonorrhoeae to currently used antimicrobial agents is alarming and warrants cautious monitoring.
- Conclusion: As the yield of new or effective antibiotic therapies has decreased over the past few years, increasing antibiotic resistance will lead to difficult treatment scenarios for sexually transmitted infections caused by these 2 organisms.
Keywords: Mycoplasma genitalium, Neisseria gonorrhoeae, antibiotic resistance, sexually transmitted infections, STIs.
The World Health Organization (WHO) estimates that more than 1 million cases of sexually transmitted Infections (STIs) are acquired every day worldwide,1 and that the majority of STIs have few or no symptoms, making diagnosis difficult. Two organisms of interest are Mycoplasma genitalium and Neisseria gonorrhoeae. In contrast to Chlamydia trachomatis, which is rarely resistant to treatment regimens, M. genitalium and N. gonorrhoeae are becoming increasingly resistant to antibiotic treatment and pose an impending threat. These bacteria can cause urethritis, cervicitis, and pelvic inflammatory disease (PID). Whereas antibiotic resistance to M. genitalium is emerging, resistance to N. gonorrhea has been a continual problem for decades. Drug resistance, especially for N. gonorrhoeae, is listed as a major threat to efforts to reduce the impact of STIs worldwide.2 In 2013, the U.S. Centers for Disease Control and Prevention (CDC) classified N. gonorrhoeae drug resistance as an urgent threat.3 As the yield of new or effective antibiotic therapies has decreased over the past few years, increasing antibiotic resistance will lead to challenging treatment scenarios for STIs caused by these 2 organisms.
Epidemiology and Pathogenesis
M. genitalium
M. genitalium is an emerging pathogen that is an etiologic agent of upper and lower genital tract STIs, such as urethritis, cervicitis, and PID.4-13 In addition, it is thought to be involved in tubal infertility and acquisition of other sexually transmitted pathogens, including HIV.7,8,13 The prevalence of M. genitalium in the general U.S. population in 2016 was reported to be approximately 17.2% for males and 16.1% for females.14 Infections are more common in patients aged 30 years and younger than in older populations.15 Also, patients self-identifying as black were found to have a higher prevalence of M. genitalium.14 This organism was first reported as being isolated from the urethras of 2 men with non-gonococcal urethritis (NGU) in London in 1980.15,16 It is a significant cause of acute and chronic NGU in males, and is estimated to account for 6% to 50% of cases of NGU.17,18M. genitalium in females has been associated with cervicitis4,9 and PID.8,10 A meta-analysis by Lis et al showed that M. genitalium infection was associated with an increased risk for preterm birth and spontaneous abortion.11 In addition, M. genitalium infections occur frequently in HIV-positive patients.19,20 M. genitalium increases susceptibility for passage of HIV across the epithelium by reducing epithelial barrier integrity.19
Beta lactams are ineffective against M. genitalium because mycoplasmas lack a cell wall and thus cell wall penicillin-binding proteins.21M. genitalium’s abilty to invade host epithelial cells is another mechanism that can protect the bacteria from antibiotic exposure.20 One of the first reports of antibiotic sensitivity testing for M. genitalium, published in 1997, noted that the organism was not susceptible to nalidixic acid, cephalosporins, penicillins, and rifampicin.22 In general, mycoplasmas are normally susceptible to antibiotics that inhibit protein synthesis,23 and initial good sensitivity to doxycycline and erythromycin was noted but this has since decreased. New antibiotics are on the horizon, but they have not been extensively tested in vivo.23
N. gonorrhoeae
Gonorrhea is the second most common STI of bacterial origin following C. trachomatis,24-26 which is rarely resistant to conventional regimens. In 2008, the World Health Organization (WHO) estimated that 106 million cases of N. gonorrhoeae infection were acquired annually and that 36.4 million adults were infected with N. gonorrhoeae.27 In the United States, the CDC estimates that gonorrhea cases are under-reported. An estimated 800,000 or more new cases are reported per year.28
The most common clinical presentations are urethritis in men and cervicitis in women.29 While urethritis is most likely to be symptomatic, only 50% of women with acute gonorrhea are symptomatic.29 In addition to lower urogenital tract infection, N. gonorrhoeae can also cause PID, ectopic pregnancy, infertility in women, and epididymitis in men.29,30 Rare complications can develop from the spread of N. gonorrhoeae to other parts of the body including the joints, eyes, cardiovascular system, and skin.29
N. gonorrhoeae can attach to the columnar epithelium and causes host innate immune-driven inflammation with neutrophil influx.29 It can avoid the immune response by varying its outer membrane protein expression. The organism is also able to acquire DNA from other Neisseria species30 and genera, which results in reduced susceptibility to therapies.
The Gonococcal Isolate Surveillance Project (GISP), established in 1986, is a collaborative project involving the CDC and STI clinics in 26 cities in the United States along with 5 regional laboratories.31 The GISP monitors susceptibilities in N. gonorrhoeae isolates obtained from roughly 6000 symptomatic men each year.31 Data collected from the GISP allows clinicians to treat infections with the correct antibiotic. Just as they observed patterns of fluoroquinolone-resistant N. gonorrhoeae, there has been a geographic progression of decreasing susceptibility to cephalosporins in recent years.31
The ease with which N. gonorrhoeae can develop resistance is particularly alarming. Sulfonamide use began in the 1930s, but resistance developed within approximately 10 years.30,32N. gonorrhoeae has acquired resistance to each therapeutic agent used for treatment over the course of its lifetime. One hypothesis is that use of single-dose therapy to rapidly treat the infection has led to treatment failure and allows for selective pressure where organisms with decreased antibiotic susceptibility are more likely to survive.30 However, there is limited evidence to support monotherapy versus combination therapy in treating N. gonorrhoeae.33,34 It is no exaggeration to say gonorrhea is now at risk of becoming an untreatable disease because of the rapid emergence of multidrug resistant N. gonorrhoeae strains worldwide.35
Diagnosis
Whether the urethritis, cervicitis, or PID is caused by N. gonorrhoeae, M. genitalium, or other non-gonococcal microorganisms (eg, C. trachomatis), no symptoms are specific to any of the microorganisms. Therefore, clinicians rely on laboratory tests to diagnose STIs caused by N. gonorrhoeae or M. genitalium.
M. genitalium
Gram Stain. Because M. genitalium lacks a cell wall, it cannot be identified by routine Gram stain.
Culture. Culturing of this fastidious bacterium might offer the advantage of assessing antibiotic susceptibility;36 however, the procedure is labor intensive and time consuming, and only a few labs in the world have the capability to perform this culture.12 Thus, this testing method is primarily undertaken for research purposes.
Serological Testing. Because of serologic cross-reactions between Mycoplasma pneumoniae and M. genitalium, there are no standardized serological tests for M. genitalium.37
Nucleic Acid Amplification Tests. M. genitalium diagnosis currently is made based exclusively on nucleic acid amplification testing (NAAT) methodology (polymerase chain reaction [PCR] or transcription-mediated amplification [TMA]), which is the only clinically useful method to detect M. genitalium. TMA for M. genitalium is commercially available in an analyte-specific reagent (ASR) format, but this has not been approved by the Food and Drug Administration (FDA).38 A study analyzing urogenital specimens from female patients via this TMA product found a 98.7% true-positive result when confirmed with repeat testing or alternative-target TMA, and only a 0.5% false-negative rate.38 There is evidence that this TMA product can be used to identify M. genitalium in urine, stool, and pharyngeal samples.39 These assays are currently available in some reference labs and large medical centers but are not widely available. Table 1 summarizes the diagnostic methods for M. genitalium.
N. gonorrhoeae
Gonococcal infection can involve the urogenital tract, but can also be extra-urogenital. The method of diagnoses of urogenital infections has expanded from Gram stain of urethral or cervical discharge and the use of selective media culture (usually Thayer-Martin media)40 to molecular methods such as NAATs, which have a higher sensitivity than cultures.41,42
Gram Stain. A Gram stain that shows polymorphonuclear leukocytes with intracellular gram-negative diplococci can be considered diagnostic for N. gonorrhoeae urethritis infection in symptomatic men when samples are obtained from the urethra.43 A retrospective study of 1148 women with gonorrhea revealed that of 1049 cases of cervical gonorrhea, only 6.4% were positive by smear alone; and of 841 cases of urethral gonorrhea, only 5.1% were positive by smear alone; therefore, other diagnostic methods are generally preferred in women.44 Because Gram stain of vaginal specimens is positive in only 50% to 60% of females, its use in women and in suspected extragenital gonococcal infections is not recommended.43-45 When Gram stain was performed in asymptomatic men, the sensitivity was around 80%.39 Thus, in asymptomatic men with a high pre-test probability of having the infection, the use of other additional testing would increase the rate of detection.43
Culture. Urethral swab specimens from males with symptomatic urethritis and cervical swab samples from females with endocervical infection must be inoculated onto both a selective medium (eg, modified Thayer-Martin medium or Martin Lewis medium) and a nonselective medium (eg, chocolate agar). A selective medium is used because it can suppress the growth of contaminating organisms, and a nonselective medium is used because some strains of N. gonorrhoeae are inhibited by the vancomycin present in the selective medium.40 Specimens collected from sterile sites, such as blood, synovial fluid, and cerebrospinal fluid, should be streaked on nonselective medium such as chocolate agar. The material used for collection is critical; the preferred swabs should have plastic or wire shafts and rayon, Dacron, or calcium alginate tips. Materials such as wooden shafts or cotton tips can be toxic to N. gonorrhoeae.40 The specimen should be inoculated immediately onto the appropriate medium and transported rapidly to the laboratory, where it should be incubated at 35º to 37ºC with 5% CO2 and examined at 24 and 48 hours post collection.40 If the specimens cannot be inoculated immediately onto the appropriate medium, the specimen swab should be delivered to the lab in a special transport system that can keep the N. gonorrhoeae viable for up to 48 hours at room temperature.46
The following specimen collection techniques are recommended by the CDC:40
- In males, the cotton swab should be inserted about 2 to 3 cm into the urethral meatus and rotated 360° degrees 2 or 3 times.
- In females, collection of cervical specimens requires inserting the tip of the swab 1 to 2 centimeters into the cervical os and rotating 360° 2 or 3 times.
- Samples obtained outside of the urogenital tract: rectal specimens may be obtained by inserting the swab 3 to 4 cm into the rectal vault. Pharyngeal specimens are to be obtained from the posterior pharynx with a swab.
Culture tests allow the clinician to assess antimicrobial susceptibility and are relatively low cost when compared with nucleic acid detection tests. The sensitivity of culture ranges from 72% to 95% for symptomatic patients, but drops to 65% to 85% for asymptomatic patients.45-47 This low sensitivity is a major disadvantage of culture tests when compared to NAATs. Other disadvantages are the need for the specimens to be transported under conditions adequate to maintain the viability of organisms and the fact that 24 to 72 hours is required to report presumptive culture results.42 Antimicrobial sensitivity testing generally is not recommended; however, it is advisable to perform antimicrobial sensitivity in cases of treatment failure or disseminated gonococcal infection.12
Nucleic Acid Amplification Tests. NAATs use techniques that allow the amplification and detection of N. gonorrhoeae DNA or RNA sequences through various methods, which include assays such as PCR (eg, Amplicor; Roche, Nutley, NJ), TMA (eg, APTIMA; Gen-Probe, San Diego, CA), and strand-displacement amplification (SDA; Probe-Tec; Becton Dickinson, Franklin Lake, NJ). While PCR and SDA methods amplify bacterial DNA, TMA amplifies bacterial rRNA.41
The FDA has cleared NAATs to test endocervical, vaginal, and urethral (men) swab specimens and urine for both men and women. There are several NAATs available to test rectal, oropharyngeal, and conjunctival specimens; however, none of them are FDA-cleared. Some local and commercial laboratories have validated the reliability of these extra-urogenital NAATs.12,48 Compared to cultures, NAATs have the advantages of being more sensitive and requiring less strict collection and transport conditions. However, they are costlier than cultures, do not provide any antimicrobial susceptibility information, and have varying specificity.49,50
Rapid Tests. NAAT results are usually available in approximately 1 to 2 days, so there has been significant interest in creating technologies that would allow for a more rapid turnaround time. The GeneXpert CT/NG is a newly developed real-time PCR-based assay that can simultaneously detect C. trachomatis and N. gonorrhoeae. The advantage of this technique is the 90-minute turnaround time and its ability to process more than 90 samples at a time. The specificity of this test for N. gonorrhoeae is similar to that of other NAATs (> 99.3%), suggesting that cross-reactivity is not a significant problem.51 Table 2 summarizes the test methods used for diagnosing N. gonorrhoeae.
Treatment
M. genitalium
M. genitalium, Mycoplasma hominis, and the ureaplasmas (U. urealyticum and U. parvum) are generally transmitted sexually, and the natural habitat of this Mycoplasmataceae family of bacteria is the genitourinary tract. All the mycoplasmas can cause NGU, cervicitis, and PID. Presently, multiple-drug resistant M. hominis and ureaplasmas remain uncommon, but the prevalence of M. genitalium resistant to multiple antibiotics has increased significantly in recent years.23,52
In the 1990s, M. genitalium was highly sensitive to the tetracyclines in vitro,53 and doxycycline was the drug of choice for treating NGU. However, it later became apparent that doxycycline was largely ineffective in treating urethritis caused by M. genitalium.54,55
Subsequently, azithromycin, a macrolide, became popular in treating urethritis in males and cervicitis in females because it was highly active against C. trachomatis54 and M. genitalium56 and it can be given orally as a single 1-g dose, thus increasing patients’ compliance. However, azithromycin-resistant M. genitalium has rapidly emerged and rates of treatment failure with azithromycin as high as 40% have been reported in recent studies.57,58 The resistance was found to be mediated by mutations in the 23S rRNA gene upon exposure of M. genitalium to azithromycin.15,57-59 Multiple studies conducted in various countries (including the United States, Netherlands, England, and France) all found high rates of 23S rRNA gene mutations.15,57-59M. genitalium samples were analyzed using reverse transcription-PCR and Sanger sequencing of the 23S tRNA to assess rates of macrolide resistance markers. The study found that 50.8% of female participants and 42% of male participants harbored mutations indicating macrolide resistance.15
An in vitro study conducted in France showed that the respiratory fluoroquinolone moxifloxacin was highly active against mycoplasmas, including M. genitalium.60 This study and others led to the use of moxifloxacin in treating infections caused by azithromycin-resistant M. genitalium. Moxifloxacin initially was successful in treating previous treatment failure cases.61 Unfortunately, the success has been short-lived, as researchers from Japan and Australia have reported moxifloxacin treament failures.62-64 These treatment failures were related to mutations in the parC and gyrA genes.62
Because M. genitalium exhibits significantly increased resistance to the tetracyclines, macrolides, and fluoroquinolones, leading to treatment failures associated with the resistance, the recently published CDC sexually transmitted diseases guidelines (2015) do not specifically recommend or endorse one class of antibiotics over another to treat M. genitalium infections; this contrasts with their approach for other infections in which they make specific recommendations for treatment.12 The lack of clear recommendations from the CDC makes standardized treatment for this pathogen difficult. The CDC guidelines do identify M. genitalium as an emerging issue, and mention that a single 1-g dose of azithromycin should likely be recommended over doxycycline due to the low cure rate of 31% seen with doxycycline. Moxifloxacin is mentioned as a possible alternative, but it is noted that the medication has not been evaluated in clinical trials and several studies have shown failures.12
Although the existing antibiotics to treat M. genitalium infections are far from desirable, treatment approaches have been recommended:65
- Azithromycin or doxycycline should be considered for empiric treatment without documented M. genitalium infection.
- Azithromycin is suggested as the first choice in documented M. genitalium infections.
- In patients with urethritis, azithromycin is recommended over doxycycline based on multiple studies. A single 1-g dose of azithromycin is preferred to an extended regimen due to increased compliance despite the extended regimen being slightly superior in effectiveness. The single-dose regimen is associated with selection of macrolide-resistant strains.65
- Women with cervicitis and PID with documented M. genitalium infection should receive an azithromycin-containing regimen.
Although the existing antibiotics on the market could not keep up with the rapid mutations of M. genitalium, a few recent studies have provided a glimmer of hope to tackle this wily microorganism. Two recent studies from Japan demonstrated that sitafloxacin, a novel fluoroquinolone, administered 100 mg twice a day to patients with M. genitalium was superior to other older fluoroquinolones.66,67 This fluoroquinolone could turn out to be a promising first-line antibiotic for treatment of STIs caused by M. genitalium. Bissessor and colleagues conducted a prospective cohort study of M. genitalium-infected male and female patients attending a STI clinic in Melbourne, Australia, and found that oral pristinamycin is highly effective in treating the M. genitalium strains that are resistant to azithromycin and moxifloxacin.68 Jensen et al reported on the novel fluoroketolide solithromycin, which demonstrated superior in vitro activity against M. genitalium compared with doxycycline, fluoroquinolones, and other macrolides.69 Solithromycin could potentially become a new antibiotic to treat infection caused by multi-drug resistant M. genitalium.
N. gonorrhoeae
Because of increasing resistance of N. gonorrhoeae to fluoroquinolones in the United States, the CDC recommended against their routine use for all cases of gonorrhea in August 2007.70 In some countries, penicillin-, tetracycline-, and ciprofloxacin-resistance rates could be as high as 100%, and these antibacterial agents are no longer treatment options for gonorrhea. The WHO released new N. gonorrhoeae treatment guidelines in 2016 due to high-level of resistance to previously recommended fluoroquinolones and decreased susceptibility to the third-generation cephalosporins, which were a first-line recommendation in the 2003 guidelines.45 The CDC’s currently recommended regimens for the treatment of uncomplicated and disseminated gonorrheal infections are summarized in Table 3 and Table 4.12 Recommendations from the WHO guidelines are very similar to the CDC recommendations.45
In light of the increasing resistance of N. gonorrhoeae to cephalosporins, 1 g of oral azithromycin should be added to ceftriaxone 250 mg intramuscularly in treating all cases of gonorrhea. The rationale for adding azithromycin to ceftriaxone is that azithromycin is active against N. gonorrhoeae at a different molecular target at a high dose, and it can also cover other co-pathogens.71 Unfortunately, susceptibility to cephalosporins has been decreasing rapidly.72 The greatest concern is the potential worldwide spread of the strain isolated in Kyoto, Japan, in 2009 from a patient with pharyngeal gonorrhea that was highly resistant to ceftriaxone (minimum inhibitory concentration of 2.0 to 4.0 µg/mL).73 At this time, N. gonorrhoeae isolates that are highly resistant to ceftriaxone are still rare globally.
Although cefixime is listed as an alternative treatment if ceftriaxone is not available, the 2015 CDC gonorrhea treatment guidelines note that N. gonorrhoeae is becoming more resistant to this oral third-generation cephalosporin; this increasing resistance is due in part to the genetic exchange between N. gonorrhoeae and other oral commensals actively taking place in the oral cavity, creating more resistant species. Another possible reason for cefixime resistance is that the concentration of cefixime used in treating gonococcal pharyngeal infection is subtherapeutic.74 A recent randomized multicenter trial in the United States compared 2 non-cephalosporin regimens: a single 240-mg dose of intramuscular gentamicin plus a single 2-g dose of oral azithromycin, and a single 320-mg dose of oral gemifloxacin plus a single 2-g dose of oral azithromycin. These combinations achieved 100% and 99.5% microbiological cure rates, respectively, in 401 patients with urogenital gonorrhea.75 Thus, these combination regimens can be considered as alternatives when the N. gonorrhoeae is resistant to cephalosporins or the patient is intolerant or allergic to cephalosporins.
Because N. gonorrhoeae has evolved into a “superbug,” becoming resistant to all currently available antimicrobial agents, it is important to focus on developing new agents with unique mechanisms of action to treat N. gonorrhoeae–related infections. Zoliflodacin (ETX0914), a novel topoisomerase II inhibitor, has the potential to become an effective agent to treat multi-drug resistant N. gonorrhoeae. A recent phase 2 trial demonstrated that a single oral 2000-mg dose of zoliflodacin microbiologically cleared 98% of gonorrhea patients, and some of the trial participants were infected with ciprofloxacin- or azithromycin-resistant strains.76 An additional phase 2 clinical trial compared oral zoliflodacin and intramuscular ceftriaxone. For uncomplicated urogential infections, 96% of patients in the zoliflodacin group achieved microbiologic cure versus 100% in the ceftriaxone group; however, zoliflodacin was less efficacious for pharyngeal infections.77 Gepotidacin (GSK2140944) is another new antimicrobial agent in the pipeline that looks promising. It is a novel first-in-class triazaacenaphthylene that inhibits bacterial DNA replication. A recent phase 2 clinical trial demonstrated that 1.5-g and 3-g single oral doses eradicated urogenital N. gonorrhoeae with microbiological success rates of 97% and 95%, respectively.78
Test of Cure
Because of the decreasing susceptibility of M. genitalium and N. gonorrhoeae to recommended treatment regimens, the European Guidelines consider test of cure essential in STIs caused by these 2 organisms to ensure eradication of infection and identify emerging resistance.79 However, test of cure is not routinely recommended by the CDC for these organisms in asymptomatic patients.12
Sexual Risk-Reduction Counseling
Besides aggressive treatment with appropriate antimicrobial agents, it is also essential that patients and their partners receive counseling to reduce the risk of STI. A recently published systematic review demonstrated that high-intensity counseling could decrease STI incidents in adolescents and adults.80
Conclusion
It is clear that these 2 sexually transmitted ”superbugs” are increasingly resistant to antibiotics and pose an increasing threat. Future epidemiological research and drug development studies need to be devoted to these 2 organisms, as well as to the potential development of a vaccine. This is especially important considering that antimicrobials may no longer be recommended when the prevalence of resistance to a particular antimicrobial reaches 5%, as is the case with WHO and other agencies that set the standard of ≥ 95% effectiveness for an antimicrobial to be considered as a recommended treatment.32 With current resistance rates for penicillin, ciprofloxacin, and tetracycline at close to 100% for N. gonorrhoeae in some countries,30,79 it is important to remain cognizant about current and future treatment options.
Because screening methods for M. genitalium are not available in most countries and there is not an FDA-approved screening method in the United States, M. genitalium poses a significant challenge for clinicians treating urethritis, cervicitis, and PID. Thus, the development of an effective screening method and established screening guidelines for M. genitalium is urgently needed. Better surveillance, prudent use of available antibiotics, and development of novel compounds are necessary to eliminate the impending threat caused by M. genitalium and N. gonorrhoeae.
This article is the result of work supported with resources and the use of facilities at the Fargo VA Health Care System. The contents of this manuscript do not represent the views of the Department of Veterans Affairs or the United States Government.
Corresponding author: Tze Shien Lo, MD, Veterans Affairs Medical Center, 2101 Elm Street N, Fargo, ND 58102.
Financial disclosures: None.
1. World Health Organization. Sexually transmitted infections (STIs). www.who.int/mediacentre/factsheets/fs110/en/. Fact Sheet #110. Updated August 2016. Accessed December 16, 2017.
2. World Health Organization. Growing antibiotic resistance forces updates to recommended treatment for sexually transmitted infections www.who.int/en/news-room/detail/30-08-2016-growing-antibiotic-resistance-forces-updates-to-recommended-treatment-for-sexually-transmitted-infections. Released August 30, 2016.
3. Centers for Disease Control and Prevention. Antibiotic/antimicrobial resistance biggest threats. www.cdc.gov/drugresistance/biggest_threats.html. Released February 27, 2018.
4. Taylor-Robinson D, Jensen JS. Mycoplasma genitalium: From chrysalis to multicolored butterfly. Clin Microbiol Rev. 2011;24:498-514.
5. Jensen JS. Mycoplasma genitalium: The aetiological agent of urethritis and other sexually transmitted diseases. J Eur Acad Dermatol Venereol. 2004;18:1-11.
6. Jaiyeoba O, Lazenby G, Soper DE. Recommendations and rationale for the treatment of pelvic inflammatory disease. Expert Rev Anti Infect Ther. 2011;9:61-70.
7. McGowin CL, Anderson-Smits C. Mycoplasma genitalium: An emerging cause of sexually transmitted disease in women. PLoS Pathog. 2011;7:e1001324.
8. Manhart LE, Broad JM, Golden MR. Mycoplasma genitalium: Should we treat and how? Clin Infect Dis. 2011;53 Suppl 3:S129-42.
9. Gaydos C, Maldeis NE, Hardick A, et al. Mycoplasma genitalium as a contributor to the multiple etiologies of cervicitis in women attending sexually transmitted disease clinics. Sex Transm Dis. 2009;36(1SE0):598-606.
10. Wiesenfeld HC, Hillier SL, Meyn L, et al. O04.6 Mycoplasma genitalium-Is it a pathogen in acute pelvic inflammatory disease (PID)? Sex Transm Infect. 2013 89:A34 http://sti.bmj.com/content/89/Suppl_1/A34.2. Accessed February 1, 2018.
11. Lis R, Rowhani-Rahbar A, Manhart LE. Mycoplasma genitalium infection and female reproductive tract disease: A meta-analysis. Clin Infect Dis. 2015;61:418-426.
12. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1-137.
13. Davies N. Mycoplasma genitalium: The need for testing and emerging diagnostic options. MLO Med Lab Obs. 2015;47:8,10-11.
14. Getman D, Jiang A, O’Donnell M, Cohen S. Mycoplasma genitalium prevalence, coinfection, and macrolide antibiotic resistance frequency in a multicenter clinical study cohort in the United States. J Clin Microbiol. 2016;54:2278-2283.
15. Tully JG, Taylor-Robinson D, Cole RM, Rose DL. A newly discovered mycoplasma in the human urogenital tract. Lancet. 1981;1(8233):1288-1291.
16. Taylor-Robinson D. The Harrison Lecture. The history and role of Mycoplasma genitalium in sexually transmitted diseases. Genitourin Med. 1995;71:1-8.
17. Horner P, Thomas B, Gilroy CB, Egger M, Taylor-Robinson D. Role of Mycoplasma genitalium and ureaplasma urealyticum in acute and chronic nongonococcal urethritis. Clin Infect Dis. 2001;32:995-1003.
18. Horner P, Blee K, O’Mahony C, et al. Clinical Effectiveness Group of the British Association of Sexual Health and HIV. 2015 UK National Guideline on the management of non-gonococcal urethritis. Int J STD AIDS. 2016;27:85-96.
19. Das K, De la Garza G, Siwak EB, et al. Mycoplasma genitalium promotes epithelial crossing and peripheral blood mononuclear cell infection by HIV-1. Int J Infect Dis. 2014;23:31-38.
20. McGowin CL, Annan RS, Quayle AJ, et al. Persistent Mycoplasma genitalium infection of human endocervical epithelial cells elicits chronic inflammatory cytokine secretion. Infect Immun. 2012;80:3842-3849.
21. Salado-Rasmussen K, Jensen JS. Mycoplasma genitalium testing pattern and macrolide resistance: A Danish nationwide retrospective survey. Clin Infect Dis. 2014;59:24-30.
22. Taylor-Robinson D, Bebear C. Antibiotic susceptibilities of mycoplasmas and treatment of mycoplasmal infections. J Antimicrob Chemother. 1997;40:622-630.
23. Taylor-Robinson D. Diagnosis and antimicrobial treatment of Mycoplasma genitalium infection: Sobering thoughts. Expert Rev Anti Infect Ther. 2014;12:715-722.
24. Ison CA. Biology of Neisseria gonorrhoeae and the clinical picture of infection. In: Gross G, Tyring SK, eds. Sexually Transmitted Infections and Sexually Transmitted Diseases.1st ed. Berlin, Heidelberg: Springer-Verlag; 2011:77-90.
25. Criss AK, Seifert HS. A bacterial siren song: Intimate interactions between neisseria and neutrophils. Nat Rev Microbiol. 2012;10:178-190.
26. Urban CF, Lourido S, Zychlinsky A. How do microbes evade neutrophil killing? Cell Microbiol. 2006;8:1687-1696.
27. World Health Organization, Dept. of Reproductive Health and Research. Global incidence and prevalence of selected curable sexually transmitted infections - 2008. www.who.int/reproductivehealth/publications/rtis/stisestimates/en/. Published 2012. Accessed February 6, 2018.
28. Centers for Disease Control and Prevention 2015 sexually transmitted diseases treatment guidelines. www.cdc.gov/std/tg2015/emerging.htm. Updated June 4, 2015.
29. Skerlev M, Culav-Koscak I. Gonorrhea: New challenges. Clin Dermatol. 2014;32:275-281.
30. Kirkcaldy RD, Ballard RC, Dowell D. Gonococcal resistance: Are cephalosporins next? Curr Infect Dis Rep. 2011;13:196-204.
31. Kidd S, Kirkcaldy R, Weinstock H, Bolan G. Tackling multidrug-resistant gonorrhea: How should we prepare for the untreatable? Expert Rev Anti Infect Ther. 2012;10:831-833.
32. Wang SA, Harvey AB, Conner SM, et al. Antimicrobial resistance for Neisseria gonorrhoeae in the United States, 1988 to 2003: The spread of fluoroquinolone resistance. Ann Intern Med. 2007;147:81-88.
33. Barbee LA, Kerani RP, Dombrowski JC, et al. A retrospective comparative study of 2-drug oral and intramuscular cephalosporin treatment regimens for pharyngeal gonorrhea. Clin Infect Dis. 2013;56:1539-434.
34. Sathia L, Ellis B, Phillip S, et al. Pharyngeal gonorrhoea - is dual therapy the way forward? Int J STD AIDS. 2007;18:647–8.
35. Tanaka M. Emergence of multidrug-resistant Neisseria gonorrhoeae strains circulating worldwide. Int J Urol. 2012;19:98-99.
36. Hamasuna R, Osada Y, Jensen JS. Isolation of Mycoplasma genitalium from first-void urine specimens by coculture with vero cells. J Clin Microbiol. 2007;45:847-850.
37. Razin S. Mycoplasma. In: Boricello SP, Murray PR, Funke G, eds. Topley & Wilson’s Microbiology and Microbial Infections. London, UK: Hodder Arnold; 2005:1957-2005.
38. Munson E, Bykowski H, Munson K, et al. Clinical laboratory assessment of Mycoplasma genitalium transcription-medicated ampliflication using primary female urogenital specimens. J Clin Microbiol. 2016;54:432-437.
39. Munson E, Wenten D, Jhansale S, et al. Expansion of comprehensive screening of male-sexually transmitted infection clinic attendees with Mycoplasma genitalium and Trichomonas vaginalis molecule assessment: a restrospective analysis. J Clin Microbiol. 2016;55:321-325.
40. Centers for Disease Control and Prevention. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae--2014. MMWR Recomm Rep. 2014;63(RR-02):1-19.
41. Boyadzhyan B, Yashina T, Yatabe JH, et al. Comparison of the APTIMA CT and GC assays with the APTIMA combo 2 assay, the Abbott LCx assay, and direct fluorescent-antibody and culture assays for detection of Chlamydia trachomatis and Neisseria gonorrhoeae. J Clin Microbiol. 2004;42:3089-3093.
42. Graseck AS, Shih SL, Peipert JF. Home versus clinic-based specimen collection for Chlamydia trachomatis and Neisseria gonorrhoeae. Expert Rev Anti Infect Ther. 2011;9:183-194.
43. Sherrard J, Barlow D. Gonorrhoea in men: Clinical and diagnostic aspects. Genitourin Med. 1996;72:422-426.
44. Goh BT, Varia KB, Ayliffe PF, Lim FK Diagnosis of gonorrhea by gram-stained smears and cultures in men and women: role of the urethral smear. Sex Transm Dis. 1985;12:135-139.
45. World Health Organization. WHO Guidelines for the Treatment of Neisseria gonorrhoeae. www.who.int/reproductivehealth/publications/rtis/gonorrhoea-treatment-guidelines/en/. Published 2016. Accessed December 16, 2017.
46. Arbique JC, Forward KR, LeBlanc J. Evaluation of four commercial transport media for the survival of Neisseria gonorrhoeae. Diagn Microbiol Infect Dis. 2000;36:163-168.
47. Schink JC, Keith LG. Problems in the culture diagnosis of gonorrhea. J Reprod Med. 1985;30(3 Suppl):244-249.
48. Marrazzo JM, Apicella MA. Neisseria gonorrhoeae (gonorrhea). In: Bennett JE, Dolin R, Blaser MJ, eds. Principles and Practice of Infectious Diseases. 8th ed. Philadelphia, PA: Elsevier; 2015:2446-2462.
49. Barry PM, Klausner JD. The use of cephalosporins for gonorrhea: The impending problem of resistance. Expert Opin Pharmacother. 2009;10:555-577.
50. Tabrizi SN, Unemo M, Limnios AE, et al. Evaluation of six commercial nucleic acid amplification tests for detection of Neisseria gonorrhoeae and other Neisseria species. J Clin Microbiol. 2011;49:3610-3615.
51. Goldenberg SD, Finn J, Sedudzi E, et al. Performance of the GeneXpert CT/NG assay compared to that of the Aptima AC2 assay for detection of rectal Chlamydia trachomatis and Neisseria gonorrhoeae by use of residual Aptima Samples. J Clin Microbiol. 2012;50:3867-3869.
52. Martin D. Mycoplasma genitalium, Mycoplasma hominis, and Ureaplasma species. In: Bennet J, Dolin R, Blaser M, eds. Principles and Practice of Infectious Diseases. 8th ed. Philadelphia, PA: Elsevier Sauders; 2015:2190-2193.
53. Hannan PC. Comparative susceptibilities of various AIDS-associated and human urogenital tract mycoplasmas and strains of Mycoplasma pneumoniae to 10 classes of antimicrobial agent in vitro. J Med Microbiol. 1998;47:1115-1122.
54. Mena LA, Mroczkowski TF, Nsuami M, Martin DH. A randomized comparison of azithromycin and doxycycline for the treatment of Mycoplasma genitalium-positive urethritis in men. Clin Infect Dis. 2009;48:1649-1654.
55. Schwebke JR, Rompalo A, Taylor S, et al. Re-evaluating the treatment of nongonococcal urethritis: Emphasizing emerging pathogens--a randomized clinical trial. Clin Infect Dis. 2011;52:163-170.
56. Bjornelius E, Anagrius C, Bojs G, et al. Antibiotic treatment of symptomatic Mycoplasma genitalium infection in Scandinavia: A controlled clinical trial. Sex Transm Infect. 2008;84:72-76.
57. Nijhuis RH, Severs TT, Van der Vegt DS, et al. High levels of macrolide resistance-associated mutations in Mycoplasma genitalium warrant antibiotic susceptibility-guided treatment. J Antimicrob Chemother. 2015;70:2515-2518.
58. Pond MJ, Nori AV, Witney AA, et al. High prevalence of antibiotic-resistant Mycoplasma genitalium in nongonococcal urethritis: The need for routine testing and the inadequacy of current treatment options. Clin Infect Dis. 2014;58:631-637.
59. Touati A, Peuchant O, Jensen JS, et al. Direct detection of macrolide resistance in Mycoplasma genitalium isolates from clinical specimens from France by use of real-time PCR and melting curve analysis. J Clin Microbiol. 2014;52:1549-1555.
60. Bebear CM, de Barbeyrac B, Pereyre S, et al. Activity of moxifloxacin against the urogenital Mycoplasmas ureaplasma spp., Mycoplasma hominis and Mycoplasma genitalium and Chlamydia trachomatis. Clin Microbiol Infect. 2008;14:801-805.
61. Jernberg E, Moghaddam A, Moi H. Azithromycin and moxifloxacin for microbiological cure of Mycoplasma genitalium infection: An open study. Int J STD AIDS. 2008;19:676-679.
62. Tagg KA, Jeoffreys NJ, Couldwell DL, et al. Fluoroquinolone and macrolide resistance-associated mutations in Mycoplasma genitalium. J Clin Microbiol. 2013;51:2245-2249.
63. Couldwell DL, Tagg KA, Jeoffreys NJ, Gilbert GL. Failure of moxifloxacin treatment in Mycoplasma genitalium infections due to macrolide and fluoroquinolone resistance. Int J STD AIDS. 2013;24:822-828.
64. Shimada Y, Deguchi T, Nakane K, et al. Emergence of clinical strains of Mycoplasma genitalium harbouring alterations in ParC associated with fluoroquinolone resistance. Int J Antimicrob Agents. 2010;36:255-258.
65. Mobley V, Seña A. Mycoplasma genitalium infection in men and women. In: UpToDate. www.uptodate.com. Last updated March 8, 2017. Accessed February 13, 2018.
66. Takahashi S, Hamasuna R, Yasuda M, et al. Clinical efficacy of sitafloxacin 100 mg twice daily for 7 days for patients with non-gonococcal urethritis. J Infect Chemother. 2013;19:941-945.
67. Ito S, Yasuda M, Seike K, et al. Clinical and microbiological outcomes in treatment of men with non-gonococcal urethritis with a 100-mg twice-daily dose regimen of sitafloxacin. J Infect Chemother. 2012;18:414-418.
68. Bissessor M, Tabrizi SN, Twin J, et al. Macrolide resistance and azithromycin failure in a Mycoplasma genitalium-infected cohort, and response of azithromycin failures to alternative antibiotic regimens. Clin Infect Dis. 2014;60:1228-1236.
69. Jensen JS, Fernandes P, Unemo M. In vitro activity of the new fluoroketolide solithromycin (CEM-101) against macrolide-resistant and -susceptible Mycoplasma genitalium strains. Antimicrob Agents Chemother. 2014;58:3151-3156.
70. Centers for Disease Control and Prevention (CDC). Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: Fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep. 2007;56:332-336.
71. Sexually transmitted diseases treatment guidelines, 2010. www.cdc.gov/std/treatment/default.htm. Published 2015. Accessed February13, 2016.
72. Centers for Disease Control and Prevention (CDC). Cephalosporin susceptibility among Neisseria gonorrhoeae isolates--United States, 2000-2010. MMWR Morb Mortal Wkly Rep. 2011;60:873-877.
73. Ohnishi M, Saika T, Hoshina S, et al. Ceftriaxone-resistant Neisseria gonorrhoeae, Japan. Emerg Infect Dis. 2011;17:148-149.
74. Centers for Disease Control and Prevention (CDC). Update to CDC’s sexually transmitted diseases treatment guidelines, 2010: Oral cephalosporins no longer a recommended treatment for gonococcal infections. MMWR Morb Mortal Wkly Rep. 2012;61:590-594.
75. Kirkcaldy RD, Weinstock HS, Moore PC, et al. The efficacy and safety of gentamicin plus azithromycin and gemifloxacin plus azithromycin as treatment of uncomplicated gonorrhea. Clin Infect Dis. 2014;59:1083-1091.
76. Seña AC, Taylor SN, Marrazzo J, et al. Microbiological cure rates and antimicrobial susceptibility of Neisseria gonorrhoeae to ETX0914 (AZD0914) in a phase II treatment trial for urogenital gonorrhea. (Poster 1308) Program and Abstract of ID Week 2016. New Orleans, LA, . October 25-30, 2016.
77. Taylor S, Marrazzo J, Batteiger B, et al. Single-dose zoliflodacin (ETX0914) for treatment of urogential gonorrhea. N Engl J Med. 2018;379:1835-1845.
78. Perry C, Dumont E, Raychaudhuri A. O05.3 A phase II, randomised, stdy in adults subjects evaluating the efficacy, safety, and tolerability of single doses of gepotidacin (GSK2140944) for treatment of uncomplicated urogenital gonorrhea. Sex Transm Infect. 2017;93(Suppl 2).
79. Bignell C, Unemo M, European STI Guidelines Editorial Board. 2012 European guideline on the diagnosis and treatment of gonorrhoea in adults. Int J STD AIDS. 2013;24:85-92.
80. O’Connor EA, Lin JS, Burda BU, et al. Behavioral sexual risk-reduction counseling in primary care to prevent sexually transmitted infections: A systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;161:874-883.
1. World Health Organization. Sexually transmitted infections (STIs). www.who.int/mediacentre/factsheets/fs110/en/. Fact Sheet #110. Updated August 2016. Accessed December 16, 2017.
2. World Health Organization. Growing antibiotic resistance forces updates to recommended treatment for sexually transmitted infections www.who.int/en/news-room/detail/30-08-2016-growing-antibiotic-resistance-forces-updates-to-recommended-treatment-for-sexually-transmitted-infections. Released August 30, 2016.
3. Centers for Disease Control and Prevention. Antibiotic/antimicrobial resistance biggest threats. www.cdc.gov/drugresistance/biggest_threats.html. Released February 27, 2018.
4. Taylor-Robinson D, Jensen JS. Mycoplasma genitalium: From chrysalis to multicolored butterfly. Clin Microbiol Rev. 2011;24:498-514.
5. Jensen JS. Mycoplasma genitalium: The aetiological agent of urethritis and other sexually transmitted diseases. J Eur Acad Dermatol Venereol. 2004;18:1-11.
6. Jaiyeoba O, Lazenby G, Soper DE. Recommendations and rationale for the treatment of pelvic inflammatory disease. Expert Rev Anti Infect Ther. 2011;9:61-70.
7. McGowin CL, Anderson-Smits C. Mycoplasma genitalium: An emerging cause of sexually transmitted disease in women. PLoS Pathog. 2011;7:e1001324.
8. Manhart LE, Broad JM, Golden MR. Mycoplasma genitalium: Should we treat and how? Clin Infect Dis. 2011;53 Suppl 3:S129-42.
9. Gaydos C, Maldeis NE, Hardick A, et al. Mycoplasma genitalium as a contributor to the multiple etiologies of cervicitis in women attending sexually transmitted disease clinics. Sex Transm Dis. 2009;36(1SE0):598-606.
10. Wiesenfeld HC, Hillier SL, Meyn L, et al. O04.6 Mycoplasma genitalium-Is it a pathogen in acute pelvic inflammatory disease (PID)? Sex Transm Infect. 2013 89:A34 http://sti.bmj.com/content/89/Suppl_1/A34.2. Accessed February 1, 2018.
11. Lis R, Rowhani-Rahbar A, Manhart LE. Mycoplasma genitalium infection and female reproductive tract disease: A meta-analysis. Clin Infect Dis. 2015;61:418-426.
12. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1-137.
13. Davies N. Mycoplasma genitalium: The need for testing and emerging diagnostic options. MLO Med Lab Obs. 2015;47:8,10-11.
14. Getman D, Jiang A, O’Donnell M, Cohen S. Mycoplasma genitalium prevalence, coinfection, and macrolide antibiotic resistance frequency in a multicenter clinical study cohort in the United States. J Clin Microbiol. 2016;54:2278-2283.
15. Tully JG, Taylor-Robinson D, Cole RM, Rose DL. A newly discovered mycoplasma in the human urogenital tract. Lancet. 1981;1(8233):1288-1291.
16. Taylor-Robinson D. The Harrison Lecture. The history and role of Mycoplasma genitalium in sexually transmitted diseases. Genitourin Med. 1995;71:1-8.
17. Horner P, Thomas B, Gilroy CB, Egger M, Taylor-Robinson D. Role of Mycoplasma genitalium and ureaplasma urealyticum in acute and chronic nongonococcal urethritis. Clin Infect Dis. 2001;32:995-1003.
18. Horner P, Blee K, O’Mahony C, et al. Clinical Effectiveness Group of the British Association of Sexual Health and HIV. 2015 UK National Guideline on the management of non-gonococcal urethritis. Int J STD AIDS. 2016;27:85-96.
19. Das K, De la Garza G, Siwak EB, et al. Mycoplasma genitalium promotes epithelial crossing and peripheral blood mononuclear cell infection by HIV-1. Int J Infect Dis. 2014;23:31-38.
20. McGowin CL, Annan RS, Quayle AJ, et al. Persistent Mycoplasma genitalium infection of human endocervical epithelial cells elicits chronic inflammatory cytokine secretion. Infect Immun. 2012;80:3842-3849.
21. Salado-Rasmussen K, Jensen JS. Mycoplasma genitalium testing pattern and macrolide resistance: A Danish nationwide retrospective survey. Clin Infect Dis. 2014;59:24-30.
22. Taylor-Robinson D, Bebear C. Antibiotic susceptibilities of mycoplasmas and treatment of mycoplasmal infections. J Antimicrob Chemother. 1997;40:622-630.
23. Taylor-Robinson D. Diagnosis and antimicrobial treatment of Mycoplasma genitalium infection: Sobering thoughts. Expert Rev Anti Infect Ther. 2014;12:715-722.
24. Ison CA. Biology of Neisseria gonorrhoeae and the clinical picture of infection. In: Gross G, Tyring SK, eds. Sexually Transmitted Infections and Sexually Transmitted Diseases.1st ed. Berlin, Heidelberg: Springer-Verlag; 2011:77-90.
25. Criss AK, Seifert HS. A bacterial siren song: Intimate interactions between neisseria and neutrophils. Nat Rev Microbiol. 2012;10:178-190.
26. Urban CF, Lourido S, Zychlinsky A. How do microbes evade neutrophil killing? Cell Microbiol. 2006;8:1687-1696.
27. World Health Organization, Dept. of Reproductive Health and Research. Global incidence and prevalence of selected curable sexually transmitted infections - 2008. www.who.int/reproductivehealth/publications/rtis/stisestimates/en/. Published 2012. Accessed February 6, 2018.
28. Centers for Disease Control and Prevention 2015 sexually transmitted diseases treatment guidelines. www.cdc.gov/std/tg2015/emerging.htm. Updated June 4, 2015.
29. Skerlev M, Culav-Koscak I. Gonorrhea: New challenges. Clin Dermatol. 2014;32:275-281.
30. Kirkcaldy RD, Ballard RC, Dowell D. Gonococcal resistance: Are cephalosporins next? Curr Infect Dis Rep. 2011;13:196-204.
31. Kidd S, Kirkcaldy R, Weinstock H, Bolan G. Tackling multidrug-resistant gonorrhea: How should we prepare for the untreatable? Expert Rev Anti Infect Ther. 2012;10:831-833.
32. Wang SA, Harvey AB, Conner SM, et al. Antimicrobial resistance for Neisseria gonorrhoeae in the United States, 1988 to 2003: The spread of fluoroquinolone resistance. Ann Intern Med. 2007;147:81-88.
33. Barbee LA, Kerani RP, Dombrowski JC, et al. A retrospective comparative study of 2-drug oral and intramuscular cephalosporin treatment regimens for pharyngeal gonorrhea. Clin Infect Dis. 2013;56:1539-434.
34. Sathia L, Ellis B, Phillip S, et al. Pharyngeal gonorrhoea - is dual therapy the way forward? Int J STD AIDS. 2007;18:647–8.
35. Tanaka M. Emergence of multidrug-resistant Neisseria gonorrhoeae strains circulating worldwide. Int J Urol. 2012;19:98-99.
36. Hamasuna R, Osada Y, Jensen JS. Isolation of Mycoplasma genitalium from first-void urine specimens by coculture with vero cells. J Clin Microbiol. 2007;45:847-850.
37. Razin S. Mycoplasma. In: Boricello SP, Murray PR, Funke G, eds. Topley & Wilson’s Microbiology and Microbial Infections. London, UK: Hodder Arnold; 2005:1957-2005.
38. Munson E, Bykowski H, Munson K, et al. Clinical laboratory assessment of Mycoplasma genitalium transcription-medicated ampliflication using primary female urogenital specimens. J Clin Microbiol. 2016;54:432-437.
39. Munson E, Wenten D, Jhansale S, et al. Expansion of comprehensive screening of male-sexually transmitted infection clinic attendees with Mycoplasma genitalium and Trichomonas vaginalis molecule assessment: a restrospective analysis. J Clin Microbiol. 2016;55:321-325.
40. Centers for Disease Control and Prevention. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae--2014. MMWR Recomm Rep. 2014;63(RR-02):1-19.
41. Boyadzhyan B, Yashina T, Yatabe JH, et al. Comparison of the APTIMA CT and GC assays with the APTIMA combo 2 assay, the Abbott LCx assay, and direct fluorescent-antibody and culture assays for detection of Chlamydia trachomatis and Neisseria gonorrhoeae. J Clin Microbiol. 2004;42:3089-3093.
42. Graseck AS, Shih SL, Peipert JF. Home versus clinic-based specimen collection for Chlamydia trachomatis and Neisseria gonorrhoeae. Expert Rev Anti Infect Ther. 2011;9:183-194.
43. Sherrard J, Barlow D. Gonorrhoea in men: Clinical and diagnostic aspects. Genitourin Med. 1996;72:422-426.
44. Goh BT, Varia KB, Ayliffe PF, Lim FK Diagnosis of gonorrhea by gram-stained smears and cultures in men and women: role of the urethral smear. Sex Transm Dis. 1985;12:135-139.
45. World Health Organization. WHO Guidelines for the Treatment of Neisseria gonorrhoeae. www.who.int/reproductivehealth/publications/rtis/gonorrhoea-treatment-guidelines/en/. Published 2016. Accessed December 16, 2017.
46. Arbique JC, Forward KR, LeBlanc J. Evaluation of four commercial transport media for the survival of Neisseria gonorrhoeae. Diagn Microbiol Infect Dis. 2000;36:163-168.
47. Schink JC, Keith LG. Problems in the culture diagnosis of gonorrhea. J Reprod Med. 1985;30(3 Suppl):244-249.
48. Marrazzo JM, Apicella MA. Neisseria gonorrhoeae (gonorrhea). In: Bennett JE, Dolin R, Blaser MJ, eds. Principles and Practice of Infectious Diseases. 8th ed. Philadelphia, PA: Elsevier; 2015:2446-2462.
49. Barry PM, Klausner JD. The use of cephalosporins for gonorrhea: The impending problem of resistance. Expert Opin Pharmacother. 2009;10:555-577.
50. Tabrizi SN, Unemo M, Limnios AE, et al. Evaluation of six commercial nucleic acid amplification tests for detection of Neisseria gonorrhoeae and other Neisseria species. J Clin Microbiol. 2011;49:3610-3615.
51. Goldenberg SD, Finn J, Sedudzi E, et al. Performance of the GeneXpert CT/NG assay compared to that of the Aptima AC2 assay for detection of rectal Chlamydia trachomatis and Neisseria gonorrhoeae by use of residual Aptima Samples. J Clin Microbiol. 2012;50:3867-3869.
52. Martin D. Mycoplasma genitalium, Mycoplasma hominis, and Ureaplasma species. In: Bennet J, Dolin R, Blaser M, eds. Principles and Practice of Infectious Diseases. 8th ed. Philadelphia, PA: Elsevier Sauders; 2015:2190-2193.
53. Hannan PC. Comparative susceptibilities of various AIDS-associated and human urogenital tract mycoplasmas and strains of Mycoplasma pneumoniae to 10 classes of antimicrobial agent in vitro. J Med Microbiol. 1998;47:1115-1122.
54. Mena LA, Mroczkowski TF, Nsuami M, Martin DH. A randomized comparison of azithromycin and doxycycline for the treatment of Mycoplasma genitalium-positive urethritis in men. Clin Infect Dis. 2009;48:1649-1654.
55. Schwebke JR, Rompalo A, Taylor S, et al. Re-evaluating the treatment of nongonococcal urethritis: Emphasizing emerging pathogens--a randomized clinical trial. Clin Infect Dis. 2011;52:163-170.
56. Bjornelius E, Anagrius C, Bojs G, et al. Antibiotic treatment of symptomatic Mycoplasma genitalium infection in Scandinavia: A controlled clinical trial. Sex Transm Infect. 2008;84:72-76.
57. Nijhuis RH, Severs TT, Van der Vegt DS, et al. High levels of macrolide resistance-associated mutations in Mycoplasma genitalium warrant antibiotic susceptibility-guided treatment. J Antimicrob Chemother. 2015;70:2515-2518.
58. Pond MJ, Nori AV, Witney AA, et al. High prevalence of antibiotic-resistant Mycoplasma genitalium in nongonococcal urethritis: The need for routine testing and the inadequacy of current treatment options. Clin Infect Dis. 2014;58:631-637.
59. Touati A, Peuchant O, Jensen JS, et al. Direct detection of macrolide resistance in Mycoplasma genitalium isolates from clinical specimens from France by use of real-time PCR and melting curve analysis. J Clin Microbiol. 2014;52:1549-1555.
60. Bebear CM, de Barbeyrac B, Pereyre S, et al. Activity of moxifloxacin against the urogenital Mycoplasmas ureaplasma spp., Mycoplasma hominis and Mycoplasma genitalium and Chlamydia trachomatis. Clin Microbiol Infect. 2008;14:801-805.
61. Jernberg E, Moghaddam A, Moi H. Azithromycin and moxifloxacin for microbiological cure of Mycoplasma genitalium infection: An open study. Int J STD AIDS. 2008;19:676-679.
62. Tagg KA, Jeoffreys NJ, Couldwell DL, et al. Fluoroquinolone and macrolide resistance-associated mutations in Mycoplasma genitalium. J Clin Microbiol. 2013;51:2245-2249.
63. Couldwell DL, Tagg KA, Jeoffreys NJ, Gilbert GL. Failure of moxifloxacin treatment in Mycoplasma genitalium infections due to macrolide and fluoroquinolone resistance. Int J STD AIDS. 2013;24:822-828.
64. Shimada Y, Deguchi T, Nakane K, et al. Emergence of clinical strains of Mycoplasma genitalium harbouring alterations in ParC associated with fluoroquinolone resistance. Int J Antimicrob Agents. 2010;36:255-258.
65. Mobley V, Seña A. Mycoplasma genitalium infection in men and women. In: UpToDate. www.uptodate.com. Last updated March 8, 2017. Accessed February 13, 2018.
66. Takahashi S, Hamasuna R, Yasuda M, et al. Clinical efficacy of sitafloxacin 100 mg twice daily for 7 days for patients with non-gonococcal urethritis. J Infect Chemother. 2013;19:941-945.
67. Ito S, Yasuda M, Seike K, et al. Clinical and microbiological outcomes in treatment of men with non-gonococcal urethritis with a 100-mg twice-daily dose regimen of sitafloxacin. J Infect Chemother. 2012;18:414-418.
68. Bissessor M, Tabrizi SN, Twin J, et al. Macrolide resistance and azithromycin failure in a Mycoplasma genitalium-infected cohort, and response of azithromycin failures to alternative antibiotic regimens. Clin Infect Dis. 2014;60:1228-1236.
69. Jensen JS, Fernandes P, Unemo M. In vitro activity of the new fluoroketolide solithromycin (CEM-101) against macrolide-resistant and -susceptible Mycoplasma genitalium strains. Antimicrob Agents Chemother. 2014;58:3151-3156.
70. Centers for Disease Control and Prevention (CDC). Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: Fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep. 2007;56:332-336.
71. Sexually transmitted diseases treatment guidelines, 2010. www.cdc.gov/std/treatment/default.htm. Published 2015. Accessed February13, 2016.
72. Centers for Disease Control and Prevention (CDC). Cephalosporin susceptibility among Neisseria gonorrhoeae isolates--United States, 2000-2010. MMWR Morb Mortal Wkly Rep. 2011;60:873-877.
73. Ohnishi M, Saika T, Hoshina S, et al. Ceftriaxone-resistant Neisseria gonorrhoeae, Japan. Emerg Infect Dis. 2011;17:148-149.
74. Centers for Disease Control and Prevention (CDC). Update to CDC’s sexually transmitted diseases treatment guidelines, 2010: Oral cephalosporins no longer a recommended treatment for gonococcal infections. MMWR Morb Mortal Wkly Rep. 2012;61:590-594.
75. Kirkcaldy RD, Weinstock HS, Moore PC, et al. The efficacy and safety of gentamicin plus azithromycin and gemifloxacin plus azithromycin as treatment of uncomplicated gonorrhea. Clin Infect Dis. 2014;59:1083-1091.
76. Seña AC, Taylor SN, Marrazzo J, et al. Microbiological cure rates and antimicrobial susceptibility of Neisseria gonorrhoeae to ETX0914 (AZD0914) in a phase II treatment trial for urogenital gonorrhea. (Poster 1308) Program and Abstract of ID Week 2016. New Orleans, LA, . October 25-30, 2016.
77. Taylor S, Marrazzo J, Batteiger B, et al. Single-dose zoliflodacin (ETX0914) for treatment of urogential gonorrhea. N Engl J Med. 2018;379:1835-1845.
78. Perry C, Dumont E, Raychaudhuri A. O05.3 A phase II, randomised, stdy in adults subjects evaluating the efficacy, safety, and tolerability of single doses of gepotidacin (GSK2140944) for treatment of uncomplicated urogenital gonorrhea. Sex Transm Infect. 2017;93(Suppl 2).
79. Bignell C, Unemo M, European STI Guidelines Editorial Board. 2012 European guideline on the diagnosis and treatment of gonorrhoea in adults. Int J STD AIDS. 2013;24:85-92.
80. O’Connor EA, Lin JS, Burda BU, et al. Behavioral sexual risk-reduction counseling in primary care to prevent sexually transmitted infections: A systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;161:874-883.
Treating negative symptoms of schizophrenia
In schizophrenia, negative symptoms such as social withdrawal, avoidance, lack of spontaneity and flow of conversation, reduced initiative, anhedonia, and blunted affect are among the most challenging to treat. These symptoms commonly persist after positive symptoms such as hallucinations, delusions, and paranoia have subsided. In an analysis of 20 pivotal placebo-controlled trials of second-generation antipsychotics (SGAs), almost 45% of patients who completed 6 weeks of treatment still had at least 1 residual negative symptom of at least moderate severity, and approximately 25% had 2 or more.1 Negative symptoms are viewed as being intrinsic to schizophrenia, and also as the result of extrapyramidal symptoms, depression, and psychosis.
Nearly a decade ago, the Schizophrenia Patient Outcomes Research Team (PORT) published its recommendations for psychopharmacologic and psychosocial treatments of schizophrenia. Unfortunately, due to insufficient evidence, there is still no proven treatment for negative symptoms.2-4 This is particularly problematic because negative symptoms are a major determinant of the poor social and vocational abilities of patients with schizophrenia.
This review focuses on treatments for negative symptoms of schizophrenia that have been evaluated since the PORT treatment recommendations were published and highlights those approaches that show promise.
_
The limitations of antipsychotics
Antipsychotics can both worsen and alleviate negative symptoms by reducing psychotic symptoms. Double-blind, placebo-controlled trials have found that most, if not all, antipsychotics are superior to placebo for treating negative symptoms in patients with acute psychosis.4 However, because these improvements occur in the early stages of treatment, concomitantly with improvement of psychotic symptoms, antipsychotics generally are not viewed as being very effective in the treatment of primary negative symptoms.4 Indeed, an examination of patients with prominent negative symptoms without prominent positive symptoms in the NEWMEDS cohort, which was extracted from 20 pivotal placebo-controlled trials of SGAs, revealed no clinically meaningful treatment effect on negative symptoms.1
There is evidence that antipsychotics can contribute to the development of apathy, flat affect, and other negative symptoms.5 Dopamine (D2)-blocking antipsychotics produce secondary negative symptoms that are not always easy to distinguish from primary negative symptoms.6 In a double-blind, placebo-controlled trial of single doses of risperidone, haloperidol, or placebo in healthy participants, the antipsychotics increased negative symptoms, particularly avolition/apathy.7 Another study found that chronic treatment with antipsychotics did not necessarily affect motivation in patients with schizophrenia.8
Adverse effects, such as anhedonia, often produce and enhance negative symptoms and therefore can limit the use of pharmacologic treatment options. Other adverse effects associated with specific antipsychotics include extrapyramidal symptoms, sedation, increased prolactin secretion, weight gain, and other metabolic abnormalities.
Continue to: Seeking new pharmacologic options
Seeking new pharmacologic options
The years since the PORT review have been filled with initial promise, a series of bitter disappointments, and a renewed spark of hope in the quest to treat negative symptoms in schizophrenia.
Compounds that have been abandoned. Since PORT, researchers have evaluated 5 major compounds that mainly targeted cognition and negative symptoms in patients with schizophrenia (Box9-17). Unfortunately, 4 of them failed to provide significant superiority over placebo, and 1 was withdrawn due to safety concerns.
Box
Since the Schizophrenia Patient Outcomes Research Team (PORT) treatment recommendations were published in 2010, many compounds have been investigated for treating negative symptoms of schizophrenia. Based on the findings of early research, further development of 5 of these has been abandoned.
Encenicline and TC-56199 were both α-7 nicotinic acetylcholine receptor agonists10; bitopertin and AMG 74711 were glycine reuptake inhibitors12; and pomaglumetad methionil13 was an amino acid analog drug that acts as a highly selective agonist for the metabotropic glutamate receptor.
Encenicline showed a treatment effect on negative symptoms in an add-on phase II study,14 but not in 2 subsequent phase III trials (NCT01716975, NCT01714661). TC-5619 showed a treatment effect in a 12-week phase II study of participants with persistent negative symptoms,15 but then failed in a subsequent study.9 Bitopertin showed a treatment effect on negative symptoms in 1 clinical trial,16 but the results were not replicated in a subsequent large multi-center trial.17 The AMG 747 development program was halted due to safety concerns.11 Finally, pomaglumetad methionil failed to meet its primary endpoint in a study of prominent negative symptoms and to show a treatment effect on psychotic symptoms in 2 pivotal phase III trials.13
Initial favorable results. Registered, robust trials of other compounds have had some initial favorable results that need to be replicated. These agents include:
- MIN-101 is a novel cyclic amide derivative.18 In a phase IIb 12-week study of MIN-101 monotherapy (32 mg, n = 78; 64 mg, n = 83) vs placebo (n = 83), both dose groups had significantly more improvement on the Positive and Negative Syndrome Scale (PANSS) negative factor score, which was the primary outcome measure, than placebo (32 mg/d; effect size = .45, P < .02, 64 mg/d; effect size = .57, P < .004) as well as on PANSS negative symptom score and other measures of negative symptoms.18
- Cariprazine is a D2 and D3 receptor partial agonist with high selectivity towards the D3 receptor19
- Minocycline is a broad-spectrum tetracyclic antibiotic displaying neuroprotective properties18,20,21
- Raloxifene is a selective estrogen receptor modulator for postmenopausal women22,23
- Pimavanserin, which was FDA-approved in 2016 for the treatment of Parkinson’s disease psychosis, is being tested in a large trial for adjunctive treatment of patients with negative symptoms of schizophrenia. This medication is a nondopaminergic antipsychotic that acts as a selective serotonin inverse agonist that preferentially targets 5-HT2A receptors while avoiding activity at common targets such as dopamine.24
All of these compounds except MIN-101 are currently available in the U.S. but have not been approved for the treatment of negative symptoms in patients with schizophrenia. MIN-101 is in phase III testing (NCT03397134).
Continue to: Nonpharmacologic treatments
Nonpharmacologic treatments
Recent studies of nonpharmacologic treatments for negative symptoms, including psychosocial approaches and noninvasive electromagnetic neurostimulation, have also been performed. The major psychosocial approaches that have been studied include social skills training (SST), cognitive-behavioral therapy (CBT) for psychosis, cognitive remediation, and family intervention. Some positive findings have been reported. A recent review of psychosocial treatments for negative symptoms in schizophrenia concluded that CBT and SST have the most empirical support, with some evidence even suggesting that gains from CBT are maintained as long as 6 months after treatment.25 Another review found that CBT was significantly more efficacious for reducing positive symptoms and SST in reducing negative symptoms.26
It remains unclear if a combined treatment approach provides improvements above and beyond those associated with each individual treatment modality. Motivation and Enhancement therapy (MOVE) is a potentially promising approach that combines environmental support, CBT, skills training, and other components in an attempt to address all domains of negative symptoms.27 Preliminary results from a randomized controlled trial examining 51 patients with clinically meaningful negative symptoms suggested that MOVE improves negative symptoms. However, the group differences were not significant until after 9 months of treatment and not for all negative symptom scales. A follow-up study has been completed, but the results are not yet available.28
Some small studies have suggested improvement of negative symptoms after noninvasive electromagnetic neurostimulation,29-31 but this has not been replicated in larger studies.32 In the last few years, there were several studies underway that could help clarify if there is a role for noninvasive electromagnetic neurostimulation in the treatment of negative symptoms in schizophrenia; however, results have not been reported at this time.33-35
_
Social skills training and combined interventions
Taken together, the data suggest that treating negative symptoms in schizophrenia remains a major challenge. Patients with negative symptoms are difficult to engage and motivate for treatment and there are no well-supported treatment options. Given the lack of evidence, it is not possible to synthesize this data into clear treatment recommendations. Because many of the negative symptoms are social in nature, it is perhaps not surprising that some evidence has emerged supporting the role of psychosocial approaches. Studies have pointed to the potential role of SST. It is believed to be beneficial as it targets participants’ social functioning by training verbal and nonverbal communication alongside perception and responses to social cues.36 Some evidence suggests that treatment packages that combine several psychosocial interventions (eg, family psychoeducation and skill training) achieve better outcomes than standalone interventions.37 Thus, psychosocial approaches appear to be potentially effective for the treatment of negative symptoms in patients with schizophrenia. In addition, because some antipsychotics has been shown to be associated with fewer negative symptoms than others, another treatment strategy could be to attempt the use of a different antipsychotic, or to revisit whether continued antipsychotic treatment is needed in the absence of positive symptoms.
Bottom Line
Treating negative symptoms in schizophrenia remains a major challenge. There is a lack of evidence for pharmacologic treatments; psychosocial approaches may be beneficial due to the social nature of many negative symptoms. Further, some evidence suggests that treatment packages that combine several psychosocial interventions may achieve better outcomes than standalone interventions.
Related Resource
Tandon R, Jibson M. Negative symptoms of schizophrenia: How to treat them most effectively. Current Psychiatry. 2002;1(9):36-42.
Drug Brand Names
Cariprazine • Vraylar
Haloperidol • Haldol
Minocycline • Dynacin, Minocin
Pimavanserin • Nuplazid
Raloxifene • Evista
Risperidone • Risperdal
1. Rabinowitz J, Werbeloff N, Caers I, et al. Negative symptoms in schizophrenia--the remarkable impact of inclusion definitions in clinical trials and their consequences. Schizophr Res. 2013;150(2-3):334-338.
2. Kreyenbuhl J, Buchanan RW, Dickerson FB, et al. The schizophrenia patient outcomes research team (PORT): updated treatment recommendations 2009. Schizophrenia bulletin. 2010;36(1):94-103.
3. Veerman SRT, Schulte PFJ, de Haan L. Treatment for negative symptoms in schizophrenia: a comprehensive review. Drugs. 2017.
4. Aleman A, Lincoln TM, Bruggeman R, et al. Treatment of negative symptoms: Where do we stand, and where do we go? Schizophr Res. 2017;186:55-62.
5. Awad AG. Subjective tolerability of antipsychotic medications and the emerging science of subjective tolerability disorders. Expert Rev Pharmacoecon Outcomes Res. 2010;10(1):1-4.
6. Kirkpatrick B. Recognizing primary vs secondary negative symptoms and apathy vs expression domains. J Clin Psychiatry. 2014;75(4):e09.
7. Artaloytia JF, Arango C, Lahti A, et al. Negative signs and symptoms secondary to antipsychotics: a double-blind, randomized trial of a single dose of placebo, haloperidol, and risperidone in healthy volunteers. Am J Psychiatry. 2006;163(3):488-493.
8. Fervaha G, Takeuchi H, Lee J, et al. Antipsychotics and amotivation. Neuropsychopharmacology. 2015;40(6):1539-1548.
9. Walling D, Marder SR, Kane J, et al. Phase 2 Trial of an alpha-7 nicotinic receptor agonist (TC-5619) in negative and cognitive symptoms of schizophrenia. Schizophr Bull. 2016;42(2):335-343.
10. Haig GM, Bain EE, Robieson WZ, et al. A randomized trial to assess the efficacy and safety of ABT-126, a selective alpha7 nicotinic acetylcholine receptor agonist, in the treatment of cognitive impairment in schizophrenia. Am J Psychiatry. 2016;173(8):827-835.
11. U.S. National Library of Medicing. ClinicalTrials.gov. 20110165: Study to evaluate the effect of AMG 747 on schizophrenia negative symptoms (study 165). https://clinicaltrials.gov/ct2/show/NCT01568229. Accessed July 1, 2017.
12. Bugarski-Kirola D, Blaettler T, Arango C, et al. Bitopertin in negative symptoms of schizophrenia-results from the phase III FlashLyte and DayLyte studies. Biol Psychiatry. 2017;82(1):8-16.
13. Stauffer VL, Millen BA, Andersen S, et al. Pomaglumetad methionil: no significant difference as an adjunctive treatment for patients with prominent negative symptoms of schizophrenia compared to placebo. Schizophr Res. 2013;150(2-3):434-441.
14. Keefe RS, Meltzer HA, Dgetluck N, et al. Randomized, double-blind, placebo-controlled study of encenicline, an alpha7 nicotinic acetylcholine receptor agonist, as a treatment for cognitive impairment in schizophrenia. Neuropsychopharmacology. 2015;40(13):3053-3060.
15. Lieberman JA, Dunbar G, Segreti AC, et al. A randomized exploratory trial of an alpha-7 nicotinic receptor agonist (TC-5619) for cognitive enhancement in schizophrenia. Neuropsychopharmacology. 2013;38(6):968-975.
16. Umbricht D, Alberati D, Martin-Facklam M, et al. Effect of bitopertin, a glycine reuptake inhibitor, on negative symptoms of schizophrenia: a randomized, double-blind, proof-of-concept study. JAMA Psychiatry. 2014;71(6):637-646.
17. Kingwell K. Schizophrenia drug gets negative results for negative symptoms. Nat Rev Drug Discov. 2014;13(4):244-245.
18. Davidson M, Saoud J, Staner C, et al. Efficacy and safety of MIN-101: a 12-week randomized, double-blind, placebo-controlled trial of a new drug in development for the treatment of negative symptoms in schizophrenia. Am J Psychiatry. 2017;172(12):1195-1202.
19. Nemeth G, Laszlovszky I, Czobor P, et al. Cariprazine versus risperidone monotherapy for treatment of predominant negative symptoms in patients with schizophrenia: a randomised, double-blind, controlled trial. Lancet. 2017;389(10074):1103-1113.
20. Levkovitz Y, Mendlovich S, Riwkes S, et al. A double-blind, randomized study of minocycline for the treatment of negative and cognitive symptoms in early-phase schizophrenia. J Clin Psychiatry. 2010;71(2):138-149.
21. Chaudhry IB, Hallak J, Husain N, et al. Minocycline benefits negative symptoms in early schizophrenia: a randomised double-blind placebo-controlled clinical trial in patients on standard treatment. J Psychopharmacology. 2012;26(9):1185-1193.
22. Usall J, Huerta-Ramos E, Labad J, et al. Raloxifene as an adjunctive treatment for postmenopausal women with schizophrenia: a 24-week double-blind, randomized, parallel, placebo-controlled trial. Schizophr Bull. 2016;42(2):309-317.
23. Usall J, Huerta-Ramos E, Iniesta R, et al. Raloxifene as an adjunctive treatment for postmenopausal women with schizophrenia: a double-blind, randomized, placebo-controlled trial. J Clin Psychiatry. 2011;72(11):1552-1557.
24. Acadia Pharmaceuticals. Pimavanserin - schizophrenia negative symptoms. http://www.acadia-pharm.com/pipeline/pimavanserin-schizophrenia-negative-symptoms/. Accessed July 23, 2017.
25. Elis O, Caponigro JM, Kring AM. Psychosocial treatments for negative symptoms in schizophrenia: current practices and future directions. Clin Psychol Rev. 2013;33(8):914-928.
26. Turner DT, van der Gaag M, Karyotaki E, et al. Psychological interventions for psychosis: a meta-analysis of comparative outcome studies. Am J Psychiatry. 2014;171(5):523-538.
27. Velligan DI, Roberts D, Mintz J, et al. A randomized pilot study of MOtiVation and Enhancement (MOVE) Training for negative symptoms in schizophrenia. Schizophr Res. 2015;165(2-3):175-180.
28. U.S. National Library of Medicing. ClinicalTrials.gov. Treatment Development Targeting Severe and Persistent Negative Symptoms (MOVE). https://clinicaltrials.gov/ct2/show/NCT01550666. Accessed July 20, 2017.
29. Rabany L, Deutsch L, Levkovitz Y. Double-blind, randomized sham controlled study of deep-TMS add-on treatment for negative symptoms and cognitive deficits in schizophrenia. J Psychopharmacology. 2014;28(7):686-690.
30. Bation R, Brunelin J, Saoud M, et al. Intermittent theta burst stimulation of the left dorsolateral prefrontal cortex for the treatment of persistent negative symptoms in schizophrenia. European Neuropsychopharmacology. 2015;25:S329-S30.
31. Li Z, Yin M, Lyu XL, et al. Delayed effect of repetitive transcranial magnetic stimulation (rTMS) on negative symptoms of schizophrenia: findings from a randomized controlled trial. Psychiatry Res. 2016;240:333-335.
32. Wobrock T, Guse B, Cordes J, et al. Left prefrontal high-frequency repetitive transcranial magnetic stimulation for the treatment of schizophrenia with predominant negative symptoms: a sham-controlled, randomized multicenter trial. Biol Psychiatry. 2015;77(11):979-988.
33. U.S. National Library of Medicing. ClinicalTrials.gov. Repetitive transcranial magnetic stimulation and intermittent theta burst (iTBS) in schizophrenia phase 2. https://clinicaltrials.gov/ct2/show/NCT01315587. Accessed July 18, 2017.
34. Treatment of Negative Symptoms and Schizophrenia (STICCS) Phase 1/2. https://clinicaltrials.gov/ct2/show/NCT02204787. Accessed July 15, 2017.
35. U.S. National Library of Medicing. ClinicalTrials.gov. Schizophrenia TreAtment With electRic Transcranial Stimulation (STARTS). https://clinicaltrials.gov/ct2/show/NCT02535676. Accessed July 10, 2017.
36. Bellack AS, Mueser KT, Gingerich S, Agresta J. Social skills training for schizophrenia. A step-by-step guide. New York, NY: Guilford Press; 1997:20-30.
37. Hogarty GE, Anderson CM, Reiss DJ, et al. Family psychoeducation, social skills training, and maintenance chemotherapy in the aftercare treatment of schizophrenia. I. one-year effects of a controlled study on relapse and expressed emotion. Arch Gen Psychiatry. 1986;43(7):633-642.
In schizophrenia, negative symptoms such as social withdrawal, avoidance, lack of spontaneity and flow of conversation, reduced initiative, anhedonia, and blunted affect are among the most challenging to treat. These symptoms commonly persist after positive symptoms such as hallucinations, delusions, and paranoia have subsided. In an analysis of 20 pivotal placebo-controlled trials of second-generation antipsychotics (SGAs), almost 45% of patients who completed 6 weeks of treatment still had at least 1 residual negative symptom of at least moderate severity, and approximately 25% had 2 or more.1 Negative symptoms are viewed as being intrinsic to schizophrenia, and also as the result of extrapyramidal symptoms, depression, and psychosis.
Nearly a decade ago, the Schizophrenia Patient Outcomes Research Team (PORT) published its recommendations for psychopharmacologic and psychosocial treatments of schizophrenia. Unfortunately, due to insufficient evidence, there is still no proven treatment for negative symptoms.2-4 This is particularly problematic because negative symptoms are a major determinant of the poor social and vocational abilities of patients with schizophrenia.
This review focuses on treatments for negative symptoms of schizophrenia that have been evaluated since the PORT treatment recommendations were published and highlights those approaches that show promise.
_
The limitations of antipsychotics
Antipsychotics can both worsen and alleviate negative symptoms by reducing psychotic symptoms. Double-blind, placebo-controlled trials have found that most, if not all, antipsychotics are superior to placebo for treating negative symptoms in patients with acute psychosis.4 However, because these improvements occur in the early stages of treatment, concomitantly with improvement of psychotic symptoms, antipsychotics generally are not viewed as being very effective in the treatment of primary negative symptoms.4 Indeed, an examination of patients with prominent negative symptoms without prominent positive symptoms in the NEWMEDS cohort, which was extracted from 20 pivotal placebo-controlled trials of SGAs, revealed no clinically meaningful treatment effect on negative symptoms.1
There is evidence that antipsychotics can contribute to the development of apathy, flat affect, and other negative symptoms.5 Dopamine (D2)-blocking antipsychotics produce secondary negative symptoms that are not always easy to distinguish from primary negative symptoms.6 In a double-blind, placebo-controlled trial of single doses of risperidone, haloperidol, or placebo in healthy participants, the antipsychotics increased negative symptoms, particularly avolition/apathy.7 Another study found that chronic treatment with antipsychotics did not necessarily affect motivation in patients with schizophrenia.8
Adverse effects, such as anhedonia, often produce and enhance negative symptoms and therefore can limit the use of pharmacologic treatment options. Other adverse effects associated with specific antipsychotics include extrapyramidal symptoms, sedation, increased prolactin secretion, weight gain, and other metabolic abnormalities.
Continue to: Seeking new pharmacologic options
Seeking new pharmacologic options
The years since the PORT review have been filled with initial promise, a series of bitter disappointments, and a renewed spark of hope in the quest to treat negative symptoms in schizophrenia.
Compounds that have been abandoned. Since PORT, researchers have evaluated 5 major compounds that mainly targeted cognition and negative symptoms in patients with schizophrenia (Box9-17). Unfortunately, 4 of them failed to provide significant superiority over placebo, and 1 was withdrawn due to safety concerns.
Box
Since the Schizophrenia Patient Outcomes Research Team (PORT) treatment recommendations were published in 2010, many compounds have been investigated for treating negative symptoms of schizophrenia. Based on the findings of early research, further development of 5 of these has been abandoned.
Encenicline and TC-56199 were both α-7 nicotinic acetylcholine receptor agonists10; bitopertin and AMG 74711 were glycine reuptake inhibitors12; and pomaglumetad methionil13 was an amino acid analog drug that acts as a highly selective agonist for the metabotropic glutamate receptor.
Encenicline showed a treatment effect on negative symptoms in an add-on phase II study,14 but not in 2 subsequent phase III trials (NCT01716975, NCT01714661). TC-5619 showed a treatment effect in a 12-week phase II study of participants with persistent negative symptoms,15 but then failed in a subsequent study.9 Bitopertin showed a treatment effect on negative symptoms in 1 clinical trial,16 but the results were not replicated in a subsequent large multi-center trial.17 The AMG 747 development program was halted due to safety concerns.11 Finally, pomaglumetad methionil failed to meet its primary endpoint in a study of prominent negative symptoms and to show a treatment effect on psychotic symptoms in 2 pivotal phase III trials.13
Initial favorable results. Registered, robust trials of other compounds have had some initial favorable results that need to be replicated. These agents include:
- MIN-101 is a novel cyclic amide derivative.18 In a phase IIb 12-week study of MIN-101 monotherapy (32 mg, n = 78; 64 mg, n = 83) vs placebo (n = 83), both dose groups had significantly more improvement on the Positive and Negative Syndrome Scale (PANSS) negative factor score, which was the primary outcome measure, than placebo (32 mg/d; effect size = .45, P < .02, 64 mg/d; effect size = .57, P < .004) as well as on PANSS negative symptom score and other measures of negative symptoms.18
- Cariprazine is a D2 and D3 receptor partial agonist with high selectivity towards the D3 receptor19
- Minocycline is a broad-spectrum tetracyclic antibiotic displaying neuroprotective properties18,20,21
- Raloxifene is a selective estrogen receptor modulator for postmenopausal women22,23
- Pimavanserin, which was FDA-approved in 2016 for the treatment of Parkinson’s disease psychosis, is being tested in a large trial for adjunctive treatment of patients with negative symptoms of schizophrenia. This medication is a nondopaminergic antipsychotic that acts as a selective serotonin inverse agonist that preferentially targets 5-HT2A receptors while avoiding activity at common targets such as dopamine.24
All of these compounds except MIN-101 are currently available in the U.S. but have not been approved for the treatment of negative symptoms in patients with schizophrenia. MIN-101 is in phase III testing (NCT03397134).
Continue to: Nonpharmacologic treatments
Nonpharmacologic treatments
Recent studies of nonpharmacologic treatments for negative symptoms, including psychosocial approaches and noninvasive electromagnetic neurostimulation, have also been performed. The major psychosocial approaches that have been studied include social skills training (SST), cognitive-behavioral therapy (CBT) for psychosis, cognitive remediation, and family intervention. Some positive findings have been reported. A recent review of psychosocial treatments for negative symptoms in schizophrenia concluded that CBT and SST have the most empirical support, with some evidence even suggesting that gains from CBT are maintained as long as 6 months after treatment.25 Another review found that CBT was significantly more efficacious for reducing positive symptoms and SST in reducing negative symptoms.26
It remains unclear if a combined treatment approach provides improvements above and beyond those associated with each individual treatment modality. Motivation and Enhancement therapy (MOVE) is a potentially promising approach that combines environmental support, CBT, skills training, and other components in an attempt to address all domains of negative symptoms.27 Preliminary results from a randomized controlled trial examining 51 patients with clinically meaningful negative symptoms suggested that MOVE improves negative symptoms. However, the group differences were not significant until after 9 months of treatment and not for all negative symptom scales. A follow-up study has been completed, but the results are not yet available.28
Some small studies have suggested improvement of negative symptoms after noninvasive electromagnetic neurostimulation,29-31 but this has not been replicated in larger studies.32 In the last few years, there were several studies underway that could help clarify if there is a role for noninvasive electromagnetic neurostimulation in the treatment of negative symptoms in schizophrenia; however, results have not been reported at this time.33-35
_
Social skills training and combined interventions
Taken together, the data suggest that treating negative symptoms in schizophrenia remains a major challenge. Patients with negative symptoms are difficult to engage and motivate for treatment and there are no well-supported treatment options. Given the lack of evidence, it is not possible to synthesize this data into clear treatment recommendations. Because many of the negative symptoms are social in nature, it is perhaps not surprising that some evidence has emerged supporting the role of psychosocial approaches. Studies have pointed to the potential role of SST. It is believed to be beneficial as it targets participants’ social functioning by training verbal and nonverbal communication alongside perception and responses to social cues.36 Some evidence suggests that treatment packages that combine several psychosocial interventions (eg, family psychoeducation and skill training) achieve better outcomes than standalone interventions.37 Thus, psychosocial approaches appear to be potentially effective for the treatment of negative symptoms in patients with schizophrenia. In addition, because some antipsychotics has been shown to be associated with fewer negative symptoms than others, another treatment strategy could be to attempt the use of a different antipsychotic, or to revisit whether continued antipsychotic treatment is needed in the absence of positive symptoms.
Bottom Line
Treating negative symptoms in schizophrenia remains a major challenge. There is a lack of evidence for pharmacologic treatments; psychosocial approaches may be beneficial due to the social nature of many negative symptoms. Further, some evidence suggests that treatment packages that combine several psychosocial interventions may achieve better outcomes than standalone interventions.
Related Resource
Tandon R, Jibson M. Negative symptoms of schizophrenia: How to treat them most effectively. Current Psychiatry. 2002;1(9):36-42.
Drug Brand Names
Cariprazine • Vraylar
Haloperidol • Haldol
Minocycline • Dynacin, Minocin
Pimavanserin • Nuplazid
Raloxifene • Evista
Risperidone • Risperdal
In schizophrenia, negative symptoms such as social withdrawal, avoidance, lack of spontaneity and flow of conversation, reduced initiative, anhedonia, and blunted affect are among the most challenging to treat. These symptoms commonly persist after positive symptoms such as hallucinations, delusions, and paranoia have subsided. In an analysis of 20 pivotal placebo-controlled trials of second-generation antipsychotics (SGAs), almost 45% of patients who completed 6 weeks of treatment still had at least 1 residual negative symptom of at least moderate severity, and approximately 25% had 2 or more.1 Negative symptoms are viewed as being intrinsic to schizophrenia, and also as the result of extrapyramidal symptoms, depression, and psychosis.
Nearly a decade ago, the Schizophrenia Patient Outcomes Research Team (PORT) published its recommendations for psychopharmacologic and psychosocial treatments of schizophrenia. Unfortunately, due to insufficient evidence, there is still no proven treatment for negative symptoms.2-4 This is particularly problematic because negative symptoms are a major determinant of the poor social and vocational abilities of patients with schizophrenia.
This review focuses on treatments for negative symptoms of schizophrenia that have been evaluated since the PORT treatment recommendations were published and highlights those approaches that show promise.
_
The limitations of antipsychotics
Antipsychotics can both worsen and alleviate negative symptoms by reducing psychotic symptoms. Double-blind, placebo-controlled trials have found that most, if not all, antipsychotics are superior to placebo for treating negative symptoms in patients with acute psychosis.4 However, because these improvements occur in the early stages of treatment, concomitantly with improvement of psychotic symptoms, antipsychotics generally are not viewed as being very effective in the treatment of primary negative symptoms.4 Indeed, an examination of patients with prominent negative symptoms without prominent positive symptoms in the NEWMEDS cohort, which was extracted from 20 pivotal placebo-controlled trials of SGAs, revealed no clinically meaningful treatment effect on negative symptoms.1
There is evidence that antipsychotics can contribute to the development of apathy, flat affect, and other negative symptoms.5 Dopamine (D2)-blocking antipsychotics produce secondary negative symptoms that are not always easy to distinguish from primary negative symptoms.6 In a double-blind, placebo-controlled trial of single doses of risperidone, haloperidol, or placebo in healthy participants, the antipsychotics increased negative symptoms, particularly avolition/apathy.7 Another study found that chronic treatment with antipsychotics did not necessarily affect motivation in patients with schizophrenia.8
Adverse effects, such as anhedonia, often produce and enhance negative symptoms and therefore can limit the use of pharmacologic treatment options. Other adverse effects associated with specific antipsychotics include extrapyramidal symptoms, sedation, increased prolactin secretion, weight gain, and other metabolic abnormalities.
Continue to: Seeking new pharmacologic options
Seeking new pharmacologic options
The years since the PORT review have been filled with initial promise, a series of bitter disappointments, and a renewed spark of hope in the quest to treat negative symptoms in schizophrenia.
Compounds that have been abandoned. Since PORT, researchers have evaluated 5 major compounds that mainly targeted cognition and negative symptoms in patients with schizophrenia (Box9-17). Unfortunately, 4 of them failed to provide significant superiority over placebo, and 1 was withdrawn due to safety concerns.
Box
Since the Schizophrenia Patient Outcomes Research Team (PORT) treatment recommendations were published in 2010, many compounds have been investigated for treating negative symptoms of schizophrenia. Based on the findings of early research, further development of 5 of these has been abandoned.
Encenicline and TC-56199 were both α-7 nicotinic acetylcholine receptor agonists10; bitopertin and AMG 74711 were glycine reuptake inhibitors12; and pomaglumetad methionil13 was an amino acid analog drug that acts as a highly selective agonist for the metabotropic glutamate receptor.
Encenicline showed a treatment effect on negative symptoms in an add-on phase II study,14 but not in 2 subsequent phase III trials (NCT01716975, NCT01714661). TC-5619 showed a treatment effect in a 12-week phase II study of participants with persistent negative symptoms,15 but then failed in a subsequent study.9 Bitopertin showed a treatment effect on negative symptoms in 1 clinical trial,16 but the results were not replicated in a subsequent large multi-center trial.17 The AMG 747 development program was halted due to safety concerns.11 Finally, pomaglumetad methionil failed to meet its primary endpoint in a study of prominent negative symptoms and to show a treatment effect on psychotic symptoms in 2 pivotal phase III trials.13
Initial favorable results. Registered, robust trials of other compounds have had some initial favorable results that need to be replicated. These agents include:
- MIN-101 is a novel cyclic amide derivative.18 In a phase IIb 12-week study of MIN-101 monotherapy (32 mg, n = 78; 64 mg, n = 83) vs placebo (n = 83), both dose groups had significantly more improvement on the Positive and Negative Syndrome Scale (PANSS) negative factor score, which was the primary outcome measure, than placebo (32 mg/d; effect size = .45, P < .02, 64 mg/d; effect size = .57, P < .004) as well as on PANSS negative symptom score and other measures of negative symptoms.18
- Cariprazine is a D2 and D3 receptor partial agonist with high selectivity towards the D3 receptor19
- Minocycline is a broad-spectrum tetracyclic antibiotic displaying neuroprotective properties18,20,21
- Raloxifene is a selective estrogen receptor modulator for postmenopausal women22,23
- Pimavanserin, which was FDA-approved in 2016 for the treatment of Parkinson’s disease psychosis, is being tested in a large trial for adjunctive treatment of patients with negative symptoms of schizophrenia. This medication is a nondopaminergic antipsychotic that acts as a selective serotonin inverse agonist that preferentially targets 5-HT2A receptors while avoiding activity at common targets such as dopamine.24
All of these compounds except MIN-101 are currently available in the U.S. but have not been approved for the treatment of negative symptoms in patients with schizophrenia. MIN-101 is in phase III testing (NCT03397134).
Continue to: Nonpharmacologic treatments
Nonpharmacologic treatments
Recent studies of nonpharmacologic treatments for negative symptoms, including psychosocial approaches and noninvasive electromagnetic neurostimulation, have also been performed. The major psychosocial approaches that have been studied include social skills training (SST), cognitive-behavioral therapy (CBT) for psychosis, cognitive remediation, and family intervention. Some positive findings have been reported. A recent review of psychosocial treatments for negative symptoms in schizophrenia concluded that CBT and SST have the most empirical support, with some evidence even suggesting that gains from CBT are maintained as long as 6 months after treatment.25 Another review found that CBT was significantly more efficacious for reducing positive symptoms and SST in reducing negative symptoms.26
It remains unclear if a combined treatment approach provides improvements above and beyond those associated with each individual treatment modality. Motivation and Enhancement therapy (MOVE) is a potentially promising approach that combines environmental support, CBT, skills training, and other components in an attempt to address all domains of negative symptoms.27 Preliminary results from a randomized controlled trial examining 51 patients with clinically meaningful negative symptoms suggested that MOVE improves negative symptoms. However, the group differences were not significant until after 9 months of treatment and not for all negative symptom scales. A follow-up study has been completed, but the results are not yet available.28
Some small studies have suggested improvement of negative symptoms after noninvasive electromagnetic neurostimulation,29-31 but this has not been replicated in larger studies.32 In the last few years, there were several studies underway that could help clarify if there is a role for noninvasive electromagnetic neurostimulation in the treatment of negative symptoms in schizophrenia; however, results have not been reported at this time.33-35
_
Social skills training and combined interventions
Taken together, the data suggest that treating negative symptoms in schizophrenia remains a major challenge. Patients with negative symptoms are difficult to engage and motivate for treatment and there are no well-supported treatment options. Given the lack of evidence, it is not possible to synthesize this data into clear treatment recommendations. Because many of the negative symptoms are social in nature, it is perhaps not surprising that some evidence has emerged supporting the role of psychosocial approaches. Studies have pointed to the potential role of SST. It is believed to be beneficial as it targets participants’ social functioning by training verbal and nonverbal communication alongside perception and responses to social cues.36 Some evidence suggests that treatment packages that combine several psychosocial interventions (eg, family psychoeducation and skill training) achieve better outcomes than standalone interventions.37 Thus, psychosocial approaches appear to be potentially effective for the treatment of negative symptoms in patients with schizophrenia. In addition, because some antipsychotics has been shown to be associated with fewer negative symptoms than others, another treatment strategy could be to attempt the use of a different antipsychotic, or to revisit whether continued antipsychotic treatment is needed in the absence of positive symptoms.
Bottom Line
Treating negative symptoms in schizophrenia remains a major challenge. There is a lack of evidence for pharmacologic treatments; psychosocial approaches may be beneficial due to the social nature of many negative symptoms. Further, some evidence suggests that treatment packages that combine several psychosocial interventions may achieve better outcomes than standalone interventions.
Related Resource
Tandon R, Jibson M. Negative symptoms of schizophrenia: How to treat them most effectively. Current Psychiatry. 2002;1(9):36-42.
Drug Brand Names
Cariprazine • Vraylar
Haloperidol • Haldol
Minocycline • Dynacin, Minocin
Pimavanserin • Nuplazid
Raloxifene • Evista
Risperidone • Risperdal
1. Rabinowitz J, Werbeloff N, Caers I, et al. Negative symptoms in schizophrenia--the remarkable impact of inclusion definitions in clinical trials and their consequences. Schizophr Res. 2013;150(2-3):334-338.
2. Kreyenbuhl J, Buchanan RW, Dickerson FB, et al. The schizophrenia patient outcomes research team (PORT): updated treatment recommendations 2009. Schizophrenia bulletin. 2010;36(1):94-103.
3. Veerman SRT, Schulte PFJ, de Haan L. Treatment for negative symptoms in schizophrenia: a comprehensive review. Drugs. 2017.
4. Aleman A, Lincoln TM, Bruggeman R, et al. Treatment of negative symptoms: Where do we stand, and where do we go? Schizophr Res. 2017;186:55-62.
5. Awad AG. Subjective tolerability of antipsychotic medications and the emerging science of subjective tolerability disorders. Expert Rev Pharmacoecon Outcomes Res. 2010;10(1):1-4.
6. Kirkpatrick B. Recognizing primary vs secondary negative symptoms and apathy vs expression domains. J Clin Psychiatry. 2014;75(4):e09.
7. Artaloytia JF, Arango C, Lahti A, et al. Negative signs and symptoms secondary to antipsychotics: a double-blind, randomized trial of a single dose of placebo, haloperidol, and risperidone in healthy volunteers. Am J Psychiatry. 2006;163(3):488-493.
8. Fervaha G, Takeuchi H, Lee J, et al. Antipsychotics and amotivation. Neuropsychopharmacology. 2015;40(6):1539-1548.
9. Walling D, Marder SR, Kane J, et al. Phase 2 Trial of an alpha-7 nicotinic receptor agonist (TC-5619) in negative and cognitive symptoms of schizophrenia. Schizophr Bull. 2016;42(2):335-343.
10. Haig GM, Bain EE, Robieson WZ, et al. A randomized trial to assess the efficacy and safety of ABT-126, a selective alpha7 nicotinic acetylcholine receptor agonist, in the treatment of cognitive impairment in schizophrenia. Am J Psychiatry. 2016;173(8):827-835.
11. U.S. National Library of Medicing. ClinicalTrials.gov. 20110165: Study to evaluate the effect of AMG 747 on schizophrenia negative symptoms (study 165). https://clinicaltrials.gov/ct2/show/NCT01568229. Accessed July 1, 2017.
12. Bugarski-Kirola D, Blaettler T, Arango C, et al. Bitopertin in negative symptoms of schizophrenia-results from the phase III FlashLyte and DayLyte studies. Biol Psychiatry. 2017;82(1):8-16.
13. Stauffer VL, Millen BA, Andersen S, et al. Pomaglumetad methionil: no significant difference as an adjunctive treatment for patients with prominent negative symptoms of schizophrenia compared to placebo. Schizophr Res. 2013;150(2-3):434-441.
14. Keefe RS, Meltzer HA, Dgetluck N, et al. Randomized, double-blind, placebo-controlled study of encenicline, an alpha7 nicotinic acetylcholine receptor agonist, as a treatment for cognitive impairment in schizophrenia. Neuropsychopharmacology. 2015;40(13):3053-3060.
15. Lieberman JA, Dunbar G, Segreti AC, et al. A randomized exploratory trial of an alpha-7 nicotinic receptor agonist (TC-5619) for cognitive enhancement in schizophrenia. Neuropsychopharmacology. 2013;38(6):968-975.
16. Umbricht D, Alberati D, Martin-Facklam M, et al. Effect of bitopertin, a glycine reuptake inhibitor, on negative symptoms of schizophrenia: a randomized, double-blind, proof-of-concept study. JAMA Psychiatry. 2014;71(6):637-646.
17. Kingwell K. Schizophrenia drug gets negative results for negative symptoms. Nat Rev Drug Discov. 2014;13(4):244-245.
18. Davidson M, Saoud J, Staner C, et al. Efficacy and safety of MIN-101: a 12-week randomized, double-blind, placebo-controlled trial of a new drug in development for the treatment of negative symptoms in schizophrenia. Am J Psychiatry. 2017;172(12):1195-1202.
19. Nemeth G, Laszlovszky I, Czobor P, et al. Cariprazine versus risperidone monotherapy for treatment of predominant negative symptoms in patients with schizophrenia: a randomised, double-blind, controlled trial. Lancet. 2017;389(10074):1103-1113.
20. Levkovitz Y, Mendlovich S, Riwkes S, et al. A double-blind, randomized study of minocycline for the treatment of negative and cognitive symptoms in early-phase schizophrenia. J Clin Psychiatry. 2010;71(2):138-149.
21. Chaudhry IB, Hallak J, Husain N, et al. Minocycline benefits negative symptoms in early schizophrenia: a randomised double-blind placebo-controlled clinical trial in patients on standard treatment. J Psychopharmacology. 2012;26(9):1185-1193.
22. Usall J, Huerta-Ramos E, Labad J, et al. Raloxifene as an adjunctive treatment for postmenopausal women with schizophrenia: a 24-week double-blind, randomized, parallel, placebo-controlled trial. Schizophr Bull. 2016;42(2):309-317.
23. Usall J, Huerta-Ramos E, Iniesta R, et al. Raloxifene as an adjunctive treatment for postmenopausal women with schizophrenia: a double-blind, randomized, placebo-controlled trial. J Clin Psychiatry. 2011;72(11):1552-1557.
24. Acadia Pharmaceuticals. Pimavanserin - schizophrenia negative symptoms. http://www.acadia-pharm.com/pipeline/pimavanserin-schizophrenia-negative-symptoms/. Accessed July 23, 2017.
25. Elis O, Caponigro JM, Kring AM. Psychosocial treatments for negative symptoms in schizophrenia: current practices and future directions. Clin Psychol Rev. 2013;33(8):914-928.
26. Turner DT, van der Gaag M, Karyotaki E, et al. Psychological interventions for psychosis: a meta-analysis of comparative outcome studies. Am J Psychiatry. 2014;171(5):523-538.
27. Velligan DI, Roberts D, Mintz J, et al. A randomized pilot study of MOtiVation and Enhancement (MOVE) Training for negative symptoms in schizophrenia. Schizophr Res. 2015;165(2-3):175-180.
28. U.S. National Library of Medicing. ClinicalTrials.gov. Treatment Development Targeting Severe and Persistent Negative Symptoms (MOVE). https://clinicaltrials.gov/ct2/show/NCT01550666. Accessed July 20, 2017.
29. Rabany L, Deutsch L, Levkovitz Y. Double-blind, randomized sham controlled study of deep-TMS add-on treatment for negative symptoms and cognitive deficits in schizophrenia. J Psychopharmacology. 2014;28(7):686-690.
30. Bation R, Brunelin J, Saoud M, et al. Intermittent theta burst stimulation of the left dorsolateral prefrontal cortex for the treatment of persistent negative symptoms in schizophrenia. European Neuropsychopharmacology. 2015;25:S329-S30.
31. Li Z, Yin M, Lyu XL, et al. Delayed effect of repetitive transcranial magnetic stimulation (rTMS) on negative symptoms of schizophrenia: findings from a randomized controlled trial. Psychiatry Res. 2016;240:333-335.
32. Wobrock T, Guse B, Cordes J, et al. Left prefrontal high-frequency repetitive transcranial magnetic stimulation for the treatment of schizophrenia with predominant negative symptoms: a sham-controlled, randomized multicenter trial. Biol Psychiatry. 2015;77(11):979-988.
33. U.S. National Library of Medicing. ClinicalTrials.gov. Repetitive transcranial magnetic stimulation and intermittent theta burst (iTBS) in schizophrenia phase 2. https://clinicaltrials.gov/ct2/show/NCT01315587. Accessed July 18, 2017.
34. Treatment of Negative Symptoms and Schizophrenia (STICCS) Phase 1/2. https://clinicaltrials.gov/ct2/show/NCT02204787. Accessed July 15, 2017.
35. U.S. National Library of Medicing. ClinicalTrials.gov. Schizophrenia TreAtment With electRic Transcranial Stimulation (STARTS). https://clinicaltrials.gov/ct2/show/NCT02535676. Accessed July 10, 2017.
36. Bellack AS, Mueser KT, Gingerich S, Agresta J. Social skills training for schizophrenia. A step-by-step guide. New York, NY: Guilford Press; 1997:20-30.
37. Hogarty GE, Anderson CM, Reiss DJ, et al. Family psychoeducation, social skills training, and maintenance chemotherapy in the aftercare treatment of schizophrenia. I. one-year effects of a controlled study on relapse and expressed emotion. Arch Gen Psychiatry. 1986;43(7):633-642.
1. Rabinowitz J, Werbeloff N, Caers I, et al. Negative symptoms in schizophrenia--the remarkable impact of inclusion definitions in clinical trials and their consequences. Schizophr Res. 2013;150(2-3):334-338.
2. Kreyenbuhl J, Buchanan RW, Dickerson FB, et al. The schizophrenia patient outcomes research team (PORT): updated treatment recommendations 2009. Schizophrenia bulletin. 2010;36(1):94-103.
3. Veerman SRT, Schulte PFJ, de Haan L. Treatment for negative symptoms in schizophrenia: a comprehensive review. Drugs. 2017.
4. Aleman A, Lincoln TM, Bruggeman R, et al. Treatment of negative symptoms: Where do we stand, and where do we go? Schizophr Res. 2017;186:55-62.
5. Awad AG. Subjective tolerability of antipsychotic medications and the emerging science of subjective tolerability disorders. Expert Rev Pharmacoecon Outcomes Res. 2010;10(1):1-4.
6. Kirkpatrick B. Recognizing primary vs secondary negative symptoms and apathy vs expression domains. J Clin Psychiatry. 2014;75(4):e09.
7. Artaloytia JF, Arango C, Lahti A, et al. Negative signs and symptoms secondary to antipsychotics: a double-blind, randomized trial of a single dose of placebo, haloperidol, and risperidone in healthy volunteers. Am J Psychiatry. 2006;163(3):488-493.
8. Fervaha G, Takeuchi H, Lee J, et al. Antipsychotics and amotivation. Neuropsychopharmacology. 2015;40(6):1539-1548.
9. Walling D, Marder SR, Kane J, et al. Phase 2 Trial of an alpha-7 nicotinic receptor agonist (TC-5619) in negative and cognitive symptoms of schizophrenia. Schizophr Bull. 2016;42(2):335-343.
10. Haig GM, Bain EE, Robieson WZ, et al. A randomized trial to assess the efficacy and safety of ABT-126, a selective alpha7 nicotinic acetylcholine receptor agonist, in the treatment of cognitive impairment in schizophrenia. Am J Psychiatry. 2016;173(8):827-835.
11. U.S. National Library of Medicing. ClinicalTrials.gov. 20110165: Study to evaluate the effect of AMG 747 on schizophrenia negative symptoms (study 165). https://clinicaltrials.gov/ct2/show/NCT01568229. Accessed July 1, 2017.
12. Bugarski-Kirola D, Blaettler T, Arango C, et al. Bitopertin in negative symptoms of schizophrenia-results from the phase III FlashLyte and DayLyte studies. Biol Psychiatry. 2017;82(1):8-16.
13. Stauffer VL, Millen BA, Andersen S, et al. Pomaglumetad methionil: no significant difference as an adjunctive treatment for patients with prominent negative symptoms of schizophrenia compared to placebo. Schizophr Res. 2013;150(2-3):434-441.
14. Keefe RS, Meltzer HA, Dgetluck N, et al. Randomized, double-blind, placebo-controlled study of encenicline, an alpha7 nicotinic acetylcholine receptor agonist, as a treatment for cognitive impairment in schizophrenia. Neuropsychopharmacology. 2015;40(13):3053-3060.
15. Lieberman JA, Dunbar G, Segreti AC, et al. A randomized exploratory trial of an alpha-7 nicotinic receptor agonist (TC-5619) for cognitive enhancement in schizophrenia. Neuropsychopharmacology. 2013;38(6):968-975.
16. Umbricht D, Alberati D, Martin-Facklam M, et al. Effect of bitopertin, a glycine reuptake inhibitor, on negative symptoms of schizophrenia: a randomized, double-blind, proof-of-concept study. JAMA Psychiatry. 2014;71(6):637-646.
17. Kingwell K. Schizophrenia drug gets negative results for negative symptoms. Nat Rev Drug Discov. 2014;13(4):244-245.
18. Davidson M, Saoud J, Staner C, et al. Efficacy and safety of MIN-101: a 12-week randomized, double-blind, placebo-controlled trial of a new drug in development for the treatment of negative symptoms in schizophrenia. Am J Psychiatry. 2017;172(12):1195-1202.
19. Nemeth G, Laszlovszky I, Czobor P, et al. Cariprazine versus risperidone monotherapy for treatment of predominant negative symptoms in patients with schizophrenia: a randomised, double-blind, controlled trial. Lancet. 2017;389(10074):1103-1113.
20. Levkovitz Y, Mendlovich S, Riwkes S, et al. A double-blind, randomized study of minocycline for the treatment of negative and cognitive symptoms in early-phase schizophrenia. J Clin Psychiatry. 2010;71(2):138-149.
21. Chaudhry IB, Hallak J, Husain N, et al. Minocycline benefits negative symptoms in early schizophrenia: a randomised double-blind placebo-controlled clinical trial in patients on standard treatment. J Psychopharmacology. 2012;26(9):1185-1193.
22. Usall J, Huerta-Ramos E, Labad J, et al. Raloxifene as an adjunctive treatment for postmenopausal women with schizophrenia: a 24-week double-blind, randomized, parallel, placebo-controlled trial. Schizophr Bull. 2016;42(2):309-317.
23. Usall J, Huerta-Ramos E, Iniesta R, et al. Raloxifene as an adjunctive treatment for postmenopausal women with schizophrenia: a double-blind, randomized, placebo-controlled trial. J Clin Psychiatry. 2011;72(11):1552-1557.
24. Acadia Pharmaceuticals. Pimavanserin - schizophrenia negative symptoms. http://www.acadia-pharm.com/pipeline/pimavanserin-schizophrenia-negative-symptoms/. Accessed July 23, 2017.
25. Elis O, Caponigro JM, Kring AM. Psychosocial treatments for negative symptoms in schizophrenia: current practices and future directions. Clin Psychol Rev. 2013;33(8):914-928.
26. Turner DT, van der Gaag M, Karyotaki E, et al. Psychological interventions for psychosis: a meta-analysis of comparative outcome studies. Am J Psychiatry. 2014;171(5):523-538.
27. Velligan DI, Roberts D, Mintz J, et al. A randomized pilot study of MOtiVation and Enhancement (MOVE) Training for negative symptoms in schizophrenia. Schizophr Res. 2015;165(2-3):175-180.
28. U.S. National Library of Medicing. ClinicalTrials.gov. Treatment Development Targeting Severe and Persistent Negative Symptoms (MOVE). https://clinicaltrials.gov/ct2/show/NCT01550666. Accessed July 20, 2017.
29. Rabany L, Deutsch L, Levkovitz Y. Double-blind, randomized sham controlled study of deep-TMS add-on treatment for negative symptoms and cognitive deficits in schizophrenia. J Psychopharmacology. 2014;28(7):686-690.
30. Bation R, Brunelin J, Saoud M, et al. Intermittent theta burst stimulation of the left dorsolateral prefrontal cortex for the treatment of persistent negative symptoms in schizophrenia. European Neuropsychopharmacology. 2015;25:S329-S30.
31. Li Z, Yin M, Lyu XL, et al. Delayed effect of repetitive transcranial magnetic stimulation (rTMS) on negative symptoms of schizophrenia: findings from a randomized controlled trial. Psychiatry Res. 2016;240:333-335.
32. Wobrock T, Guse B, Cordes J, et al. Left prefrontal high-frequency repetitive transcranial magnetic stimulation for the treatment of schizophrenia with predominant negative symptoms: a sham-controlled, randomized multicenter trial. Biol Psychiatry. 2015;77(11):979-988.
33. U.S. National Library of Medicing. ClinicalTrials.gov. Repetitive transcranial magnetic stimulation and intermittent theta burst (iTBS) in schizophrenia phase 2. https://clinicaltrials.gov/ct2/show/NCT01315587. Accessed July 18, 2017.
34. Treatment of Negative Symptoms and Schizophrenia (STICCS) Phase 1/2. https://clinicaltrials.gov/ct2/show/NCT02204787. Accessed July 15, 2017.
35. U.S. National Library of Medicing. ClinicalTrials.gov. Schizophrenia TreAtment With electRic Transcranial Stimulation (STARTS). https://clinicaltrials.gov/ct2/show/NCT02535676. Accessed July 10, 2017.
36. Bellack AS, Mueser KT, Gingerich S, Agresta J. Social skills training for schizophrenia. A step-by-step guide. New York, NY: Guilford Press; 1997:20-30.
37. Hogarty GE, Anderson CM, Reiss DJ, et al. Family psychoeducation, social skills training, and maintenance chemotherapy in the aftercare treatment of schizophrenia. I. one-year effects of a controlled study on relapse and expressed emotion. Arch Gen Psychiatry. 1986;43(7):633-642.