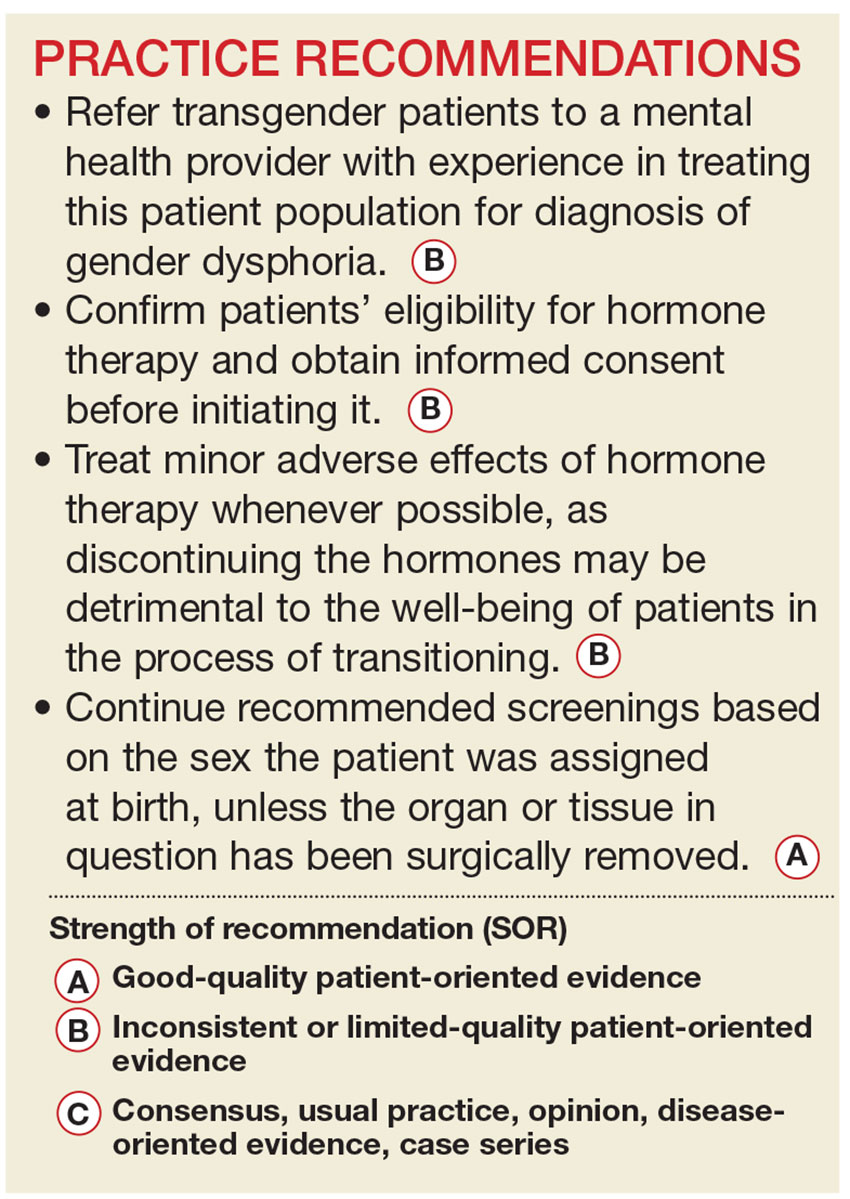
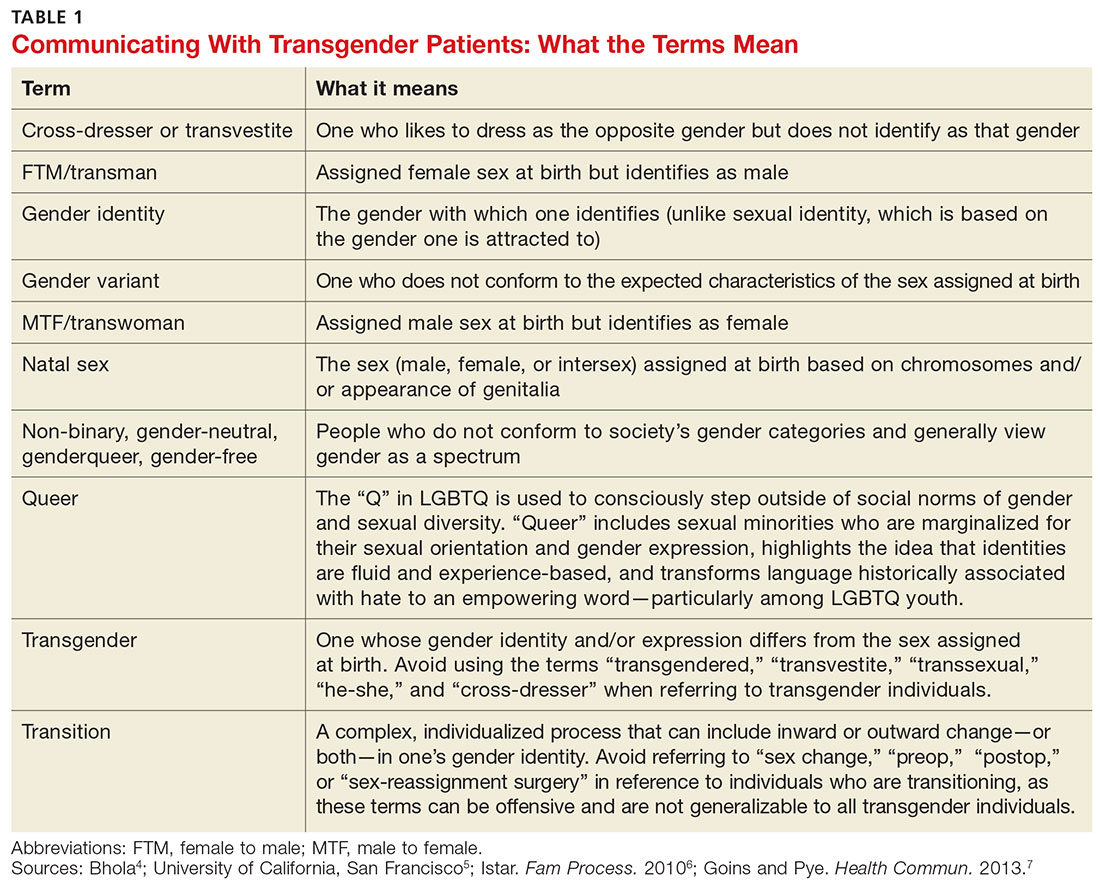
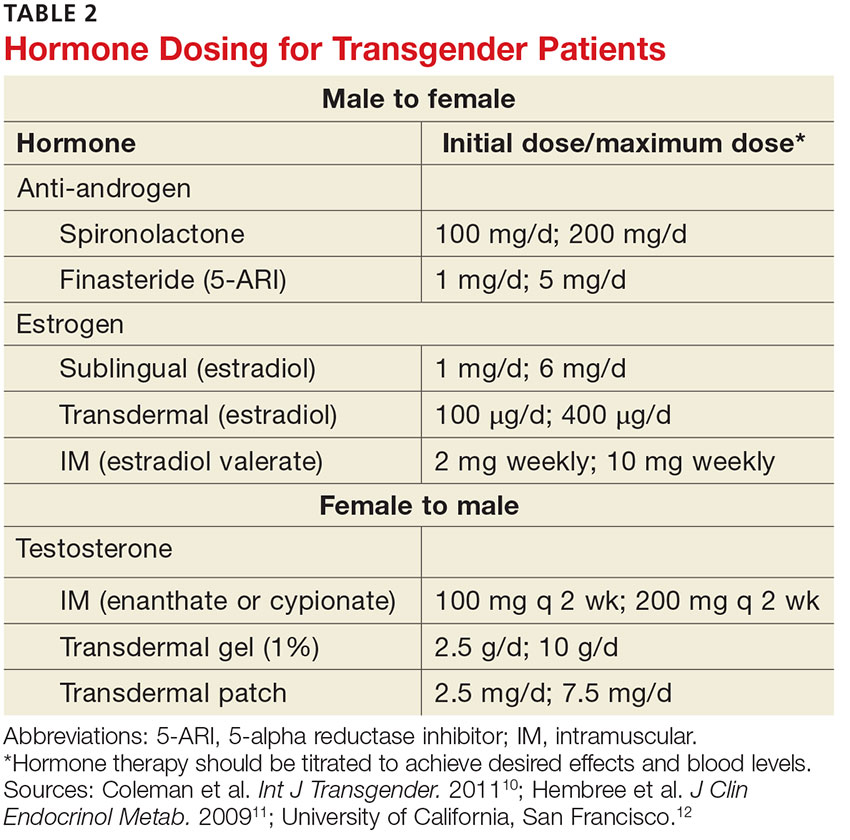
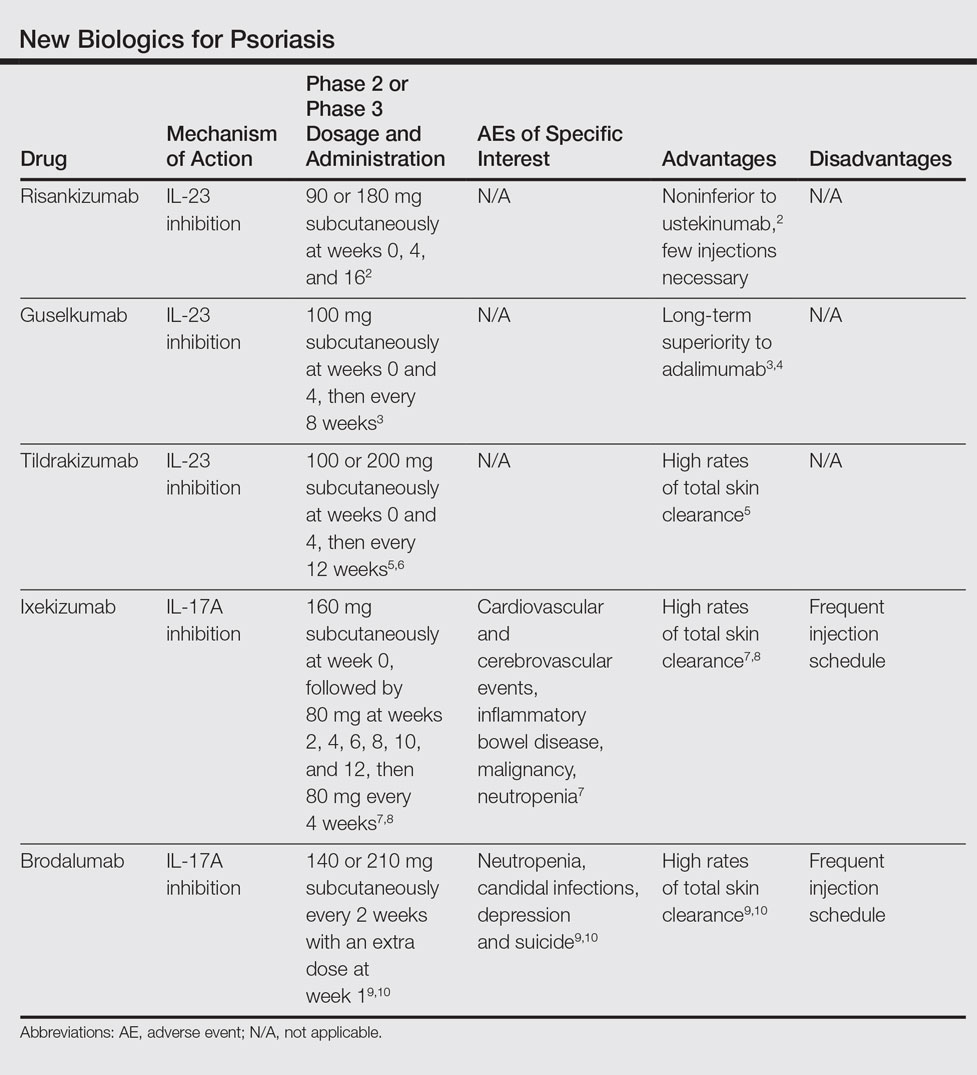
User login
Anticoagulation Management Outcomes in Veterans: Office vs Telephone Visits
Oral anticoagulation with warfarin is used for the treatment and prevention of a variety of thrombotic disorders, including deep venous thrombosis (DVT), pulmonary embolism (PE), stroke prevention in atrial fibrillation (AF) and atrial flutter, and other hypercoagulable conditions. Although a mainstay in the treatment for these conditions, warfarin requires close monitoring due to its narrow therapeutic range, extensive drug and dietary interactions, and dosage variability among patients.1 Patients outside the therapeutic range are at risk of having a thrombotic or bleeding event that could lead to hospitalization or fatality.1 To reduce the risk of these events, patients on warfarin are managed by dose adjustment based on the international normalized ratio (INR). Research has shown that patients on warfarin in pharmacist-managed specialty anticoagulation clinics have more consistent monitoring and lower rates of adverse events (AEs) compared with traditional physician or nurse clinics.2-6 Management through these clinics can be achieved through office visits or telephone visits.
There are advantages and disadvantages to each model of anticoagulation management for patients.Telephone clinics provide time and cost savings, increased access to care, and convenience. However, disadvantages include missed phone calls or inability to contact the patient, difficulty for the patient to hear the provider’s instructions over the phone, and patient unavailability when a critical INR is of concern. Office visits are beneficial in that providers can provide both written and verbal instruction to patients, perform visual or physical patient assessments, and provide timely care if needed. Disadvantages of office visits may include long wait times and inconvenience for patients who live far away.
Telephone anticoagulation clinics have been evaluated for their efficacy and cost-effectiveness in several studies.5,7,8 However, few studies are available that compare patient outcomes between office visits and telephone visits. Two prior studies comparing groups of anticoagulation patients managed by telephone or by office visit concluded that there is no difference in outcomes between the 2 management models.9,10 However, a retrospective study by Stoudenmire and colleagues examined extreme INR values (≤ 1.5 or ≥ 4.5) in each management model and found that telephone clinic patients have a significant increase in extreme INR values but no difference in AEs between the 2 management models.11
The VA North Texas Health Care System (VANTHCS) includes a major medical center, 3 outlying medical facilities, and 5 community-based outpatient clinics (CBOCs). A centralized pharmacist-managed anticoagulation clinic is used to manage more than 2,500 VANTHCS anticoagulation patients. To meet the National Patient Safety Goal measures and provide consistent management across the system, all anticoagulation patients from CBOCs and medical facilities are enrolled in the clinic.12 To facilitate access to care, many patients transitioned from office visits to telephone visits. It was essential to evaluate the transition of patients from office to telephone visits to ensure continued stability and continuity of care across both models. The objective of this study was to determine whether a difference in anticoagulation outcomes exists when patients are transitioned from office to telephone visits.
Methods
The VANTHCS anticoagulation clinic policy for office visits requires that patients arrive at the Dallas VAMC 2 hours before their appointment for INR lab draw. During the office visit, the anticoagulation pharmacist evaluates the INR and pertinent changes since the previous visit. The patient is provided verbal instructions and a written dosage adjustment card. Telephone clinic protocol is similar to office visits with a few exceptions. Any patient, regardless of INR stability, may be enrolled in the telephone clinic as long as the patient provides consent and has a working telephone with voice mail. Patients enrolled in the telephone clinic access blood draws at the nearest VA facility and are given a questionnaire that includes pertinent questions asked during an office visit. Anticoagulation pharmacists evaluate the questionnaire and INR then contact the patient within 1 business day to provide the patient with instructions. If a patient fails to answer the telephone, the anticoagulation pharmacist leaves a voicemail message.
Study Design
This retrospective study was conducted by chart review using Computerized Patient Record System (CPRS) at VANTHCS on patients who met inclusion criteria between January 1, 2011 and May 31, 2014, and it was approved by the institutional review board and research and development committee. The study included patients aged ≥ 18 years on warfarin therapy managed by the VANTHCS anticoagulation clinic who were previously managed in office visits for ≥ 180 days before the telephone transition, then in telephone visits for another ≥ 180 days. Only INR values obtained through the VANTHCS anticoagulation clinic were assessed.
Patients were excluded from the study if they were not managed by the VANTHCS anticoagulation clinic or received direct oral anticoagulants (DOACs). The INR values were excluded if they were nonclinic related INR values (ie, results reported that do not reflect management by the anticoagulation clinic), the first INR after hospitalization, or INRs obtained during the first month of initial warfarin treatment for a patient.
For all patients included in the study, demographic information, goal INR range (2 to 3 or 2.5 to 3.5), indication for warfarin therapy, and duration of warfarin therapy (defined as the first prescription filled for warfarin at the VA) were obtained. Individual INR values were obtained for each patient during the period of investigation and type of visit (office or telephone) for each INR drawn was specified. Any major bleeding or thrombotic events (bleed requiring an emergency department [ED] visit, hospitalization, vitamin K administration, blood transfusion, and/or warfarin therapy hold/discontinuation) were documented. Procedures and number of hospitalizations also during the investigation were recorded.
The primary outcomes measures evaluated INRs for time in therapeutic range (TTR) using the Rosendaal method and percentage of INRs within range.13 The therapeutic range was either 2 to 3 or 2.5 to 3.5 (the “strict range” for INR management). Because many patients fluctuate around the strict range and it is common to avoid therapy adjustment based on slightly elevated or lower values, a “nonstrict” range (1.8 to 3.2 or 2.3 to 3.7) also was evaluated.14 The secondary outcomes examined differences between the 2 management models in rates of major AEs, including thrombosis and major bleeding events as defined earlier.Frequencies, percentages, and other descriptive statistics were used to describe nominal data. A paired t test was used to compare TTR of patients transitioned from office to telephone visits. A P value of < .05 was used for statistical significance.
Results
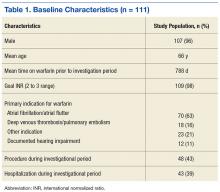
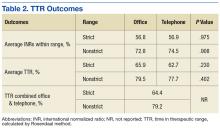
A total of 111 patients met inclusion criteria (Table 1). Most patients were elderly males with AF or atrial flutter as their primary indication for warfarin therapy. No statistically significant difference was found for percentage INRs in strict range (56.8% in office vs 56.9% in telephone, P = .98) or TTR (65.9% in office vs 62.72% in telephone, P = .23) for patients who transitioned from office to telephone visits (Table 2). Similar results were found within the nonstrict range.
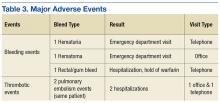
In examining safety, 5 major AEs occurred. One patient had 2 thrombotic pulmonary embolism events. This patient had a history of nonadherence with warfarin therapy. Three major bleeding events occurred (2 in the telephone group and 1 in the office group). Two bleeding events led to ED visits, and 1 event led to hospitalization. Although 43% of patients had a procedure during the study period, only a portion of patients received bridging with low-molecular-weight heparin (LMWH). None of the 3 reported bleeding events discovered during the study were associated with recent LMWH use. No events were fatal (Table 3).
Discussion
This study demonstrates that patients transitioned from office to telephone visits for warfarin management will have no significant change in their TTR. Additionally, patients had similar rates of major AEs before and after transition, although there were few events overall.
Previous research comparing anticoagulation outcomes in telephone vs office visits also has described outcomes to be similar between these 2 management models. Wittkowsky and colleagues examined 2 university-affiliated clinics to evaluate warfarin outcomes and AEs in patients in each management model (office vs telephone) and found no difference in outcomes between the 2 management models.9
Staresinic and colleagues designed a prospective study of 192 patients to evaluate TTR and AEs of the 2 management models at the William S. Middleton Memorial Veterans Hospital in Madison, Wisconsin.10 This study found no difference between the 2 groups in percentage of time maintained within INR range or AEs and concluded that the telephone model was effective for anticoagulant management.
A retrospective study by Stoudenmire and colleagues evaluated office vs telephone management effects on extreme INR values (≤ 1.5 or ≥ 4.5), TTR, and AEs.11 This study found overall TTR and AEs to be similar between groups, but the telephone clinic had a 2-fold increase in extreme INR values compared with the office clinic.11
The current study differs from the previously discussed studies in that it evaluated outcomes for the same patients before and after the transition to telephone. This study did not exclude specific patients from telephone clinic. In the Wittkowsky study, patients were enrolled in the telephone clinic based on criteria such as patient disability or living long distances from the clinic.9 Additionally, in the current study, patients transitioned to telephone visits did not have scheduled office visits for anticoagulation management. In contrast, patients in the Staresinic study had routine anticoagulation office visits every 3 months, thus it was not a true telephone-only clinic.10
This study’s findings support prior studies’ findings that telephone clinics are acceptable for anticoagulation management. Furthermore, safety does not seem to be affected when transitioning patients, although there were few AEs to review. Providers can use telephone clinics to potentially decrease cost and facilitate access to care for patients.
Limitations
Patients were required to be in office and telephone for a sequential 6 months, and this may have produced selection biases toward patients who adhered to appointments and who were on long-term warfarin therapy. Many patients that were excluded from the study transitioned back and forth between the 2 management models. Due to the retrospective nature of this study, the authors were unable to control for all confounding variables. Patients also were not randomly assigned to be transitioned from office to telephone. Although a strength of this study was the limited telephone clinic selection criteria, there may be a few individual situations in which the pharmacist’s clinical judgment influenced the transition to the telephone clinic, creating selection bias.
There may be time bias present as clinical guidelines, providers, and clinic population size differed over the study period and might have influenced management. The population of VA patients was mainly elderly males; therefore, the study results may not be applicable to other populations. Last, the results of the study are reflective of the VANTHCS clinic structure and may not be applicable to other clinic designs.
Conclusion
Veterans in a pharmacist-managed anticoagulation clinic experienced the same outcomes in terms of TTR and major AEs when transitioned from the traditional face-to-face office visits to telephone visits. The study supports the safety and efficacy of transitioning patients from a pharmacist-managed anticoagulation office clinic to telephone clinic.
1. Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G; American College of Chest Physicians. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest. 2008;133(suppl 6):160S-198S.
2. Rudd KM, Dier JG. Comparison of two different models of anticoagulation management services with usual medical care. Pharmacotherapy. 2010;30(4):330-338.
3. Bungard TJ, Gardner L, Archer SL, et al. Evaluation of a pharmacist-managed anticoagulation clinic: improving patient care. Open Med. 2009;3(1):e16-e21.
4. Chiquette E, Amato MG, Bussey HI. Comparison of an anticoagulation clinic with usual medical care: anticoagulation control, patient outcomes, and health care costs. Arch Intern Med. 1998;158(15):1641-1647.
5. Waterman AD, Banet G, Milligan PE, et al. Patient and physician satisfaction with a telephone-based anticoagulation service. J Gen Intern Med. 2001;16(7):460-463.
6. Hasan SS, Shamala R, Syed IA, et al. Factors affecting warfarin-related knowledge and INR control of patients attending physician- and pharmacist-managed anticoagulation clinics. J Pharm Pract. 2011;24(5):485-493.
7. Hassan S, Naboush A, Radbel J, et al. Telephone-based anticoagulation management in the homebound setting: a retrospective observational study. Int J Gen Med. 2013;6:869-875.
8. Moherman LJ, Kolar MM. Complication rates for a telephone-based anticoagulation service. Am J Health Syst Pharm. 1999;56(15):1540-1542.
9. Wittkowsky AK, Nutescu EA, Blackburn J, et al. Outcomes of oral anticoagulant therapy managed by telephone vs in-office visits in an anticoagulation clinic setting. Chest. 2006;130(5):1385-1389.
10. Staresinic AG, Sorkness CA, Goodman BM, Pigarelli DW. Comparison of outcomes using 2 delivery models of anticoagulation care. Arch Intern Med. 2006;166(9):997-1002.
11. Stoudenmire LG, DeRemer CE, Elewa H. Telephone versus office-based management of warfarin: impact on international normalized ratios and outcomes. Int J Hematol. 2014;100(2):119-124.
12. The Joint Commission. National Patient Safety Goals Effective January 1, 2015. http://www.jointcommission.org/assets/1/6/2015_NPSG_AHC1.PDF. Published 2014. Accessed November 23, 2016.
13. Rosendaal FR, Cannegieter SC, van der Meer FJ, Briët E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69(3):236-239.
14. Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuünemann HJ; American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(suppl 2):7S-47S.
Oral anticoagulation with warfarin is used for the treatment and prevention of a variety of thrombotic disorders, including deep venous thrombosis (DVT), pulmonary embolism (PE), stroke prevention in atrial fibrillation (AF) and atrial flutter, and other hypercoagulable conditions. Although a mainstay in the treatment for these conditions, warfarin requires close monitoring due to its narrow therapeutic range, extensive drug and dietary interactions, and dosage variability among patients.1 Patients outside the therapeutic range are at risk of having a thrombotic or bleeding event that could lead to hospitalization or fatality.1 To reduce the risk of these events, patients on warfarin are managed by dose adjustment based on the international normalized ratio (INR). Research has shown that patients on warfarin in pharmacist-managed specialty anticoagulation clinics have more consistent monitoring and lower rates of adverse events (AEs) compared with traditional physician or nurse clinics.2-6 Management through these clinics can be achieved through office visits or telephone visits.
There are advantages and disadvantages to each model of anticoagulation management for patients.Telephone clinics provide time and cost savings, increased access to care, and convenience. However, disadvantages include missed phone calls or inability to contact the patient, difficulty for the patient to hear the provider’s instructions over the phone, and patient unavailability when a critical INR is of concern. Office visits are beneficial in that providers can provide both written and verbal instruction to patients, perform visual or physical patient assessments, and provide timely care if needed. Disadvantages of office visits may include long wait times and inconvenience for patients who live far away.
Telephone anticoagulation clinics have been evaluated for their efficacy and cost-effectiveness in several studies.5,7,8 However, few studies are available that compare patient outcomes between office visits and telephone visits. Two prior studies comparing groups of anticoagulation patients managed by telephone or by office visit concluded that there is no difference in outcomes between the 2 management models.9,10 However, a retrospective study by Stoudenmire and colleagues examined extreme INR values (≤ 1.5 or ≥ 4.5) in each management model and found that telephone clinic patients have a significant increase in extreme INR values but no difference in AEs between the 2 management models.11
The VA North Texas Health Care System (VANTHCS) includes a major medical center, 3 outlying medical facilities, and 5 community-based outpatient clinics (CBOCs). A centralized pharmacist-managed anticoagulation clinic is used to manage more than 2,500 VANTHCS anticoagulation patients. To meet the National Patient Safety Goal measures and provide consistent management across the system, all anticoagulation patients from CBOCs and medical facilities are enrolled in the clinic.12 To facilitate access to care, many patients transitioned from office visits to telephone visits. It was essential to evaluate the transition of patients from office to telephone visits to ensure continued stability and continuity of care across both models. The objective of this study was to determine whether a difference in anticoagulation outcomes exists when patients are transitioned from office to telephone visits.
Methods
The VANTHCS anticoagulation clinic policy for office visits requires that patients arrive at the Dallas VAMC 2 hours before their appointment for INR lab draw. During the office visit, the anticoagulation pharmacist evaluates the INR and pertinent changes since the previous visit. The patient is provided verbal instructions and a written dosage adjustment card. Telephone clinic protocol is similar to office visits with a few exceptions. Any patient, regardless of INR stability, may be enrolled in the telephone clinic as long as the patient provides consent and has a working telephone with voice mail. Patients enrolled in the telephone clinic access blood draws at the nearest VA facility and are given a questionnaire that includes pertinent questions asked during an office visit. Anticoagulation pharmacists evaluate the questionnaire and INR then contact the patient within 1 business day to provide the patient with instructions. If a patient fails to answer the telephone, the anticoagulation pharmacist leaves a voicemail message.
Study Design
This retrospective study was conducted by chart review using Computerized Patient Record System (CPRS) at VANTHCS on patients who met inclusion criteria between January 1, 2011 and May 31, 2014, and it was approved by the institutional review board and research and development committee. The study included patients aged ≥ 18 years on warfarin therapy managed by the VANTHCS anticoagulation clinic who were previously managed in office visits for ≥ 180 days before the telephone transition, then in telephone visits for another ≥ 180 days. Only INR values obtained through the VANTHCS anticoagulation clinic were assessed.
Patients were excluded from the study if they were not managed by the VANTHCS anticoagulation clinic or received direct oral anticoagulants (DOACs). The INR values were excluded if they were nonclinic related INR values (ie, results reported that do not reflect management by the anticoagulation clinic), the first INR after hospitalization, or INRs obtained during the first month of initial warfarin treatment for a patient.
For all patients included in the study, demographic information, goal INR range (2 to 3 or 2.5 to 3.5), indication for warfarin therapy, and duration of warfarin therapy (defined as the first prescription filled for warfarin at the VA) were obtained. Individual INR values were obtained for each patient during the period of investigation and type of visit (office or telephone) for each INR drawn was specified. Any major bleeding or thrombotic events (bleed requiring an emergency department [ED] visit, hospitalization, vitamin K administration, blood transfusion, and/or warfarin therapy hold/discontinuation) were documented. Procedures and number of hospitalizations also during the investigation were recorded.
The primary outcomes measures evaluated INRs for time in therapeutic range (TTR) using the Rosendaal method and percentage of INRs within range.13 The therapeutic range was either 2 to 3 or 2.5 to 3.5 (the “strict range” for INR management). Because many patients fluctuate around the strict range and it is common to avoid therapy adjustment based on slightly elevated or lower values, a “nonstrict” range (1.8 to 3.2 or 2.3 to 3.7) also was evaluated.14 The secondary outcomes examined differences between the 2 management models in rates of major AEs, including thrombosis and major bleeding events as defined earlier.Frequencies, percentages, and other descriptive statistics were used to describe nominal data. A paired t test was used to compare TTR of patients transitioned from office to telephone visits. A P value of < .05 was used for statistical significance.
Results
A total of 111 patients met inclusion criteria (Table 1). Most patients were elderly males with AF or atrial flutter as their primary indication for warfarin therapy. No statistically significant difference was found for percentage INRs in strict range (56.8% in office vs 56.9% in telephone, P = .98) or TTR (65.9% in office vs 62.72% in telephone, P = .23) for patients who transitioned from office to telephone visits (Table 2). Similar results were found within the nonstrict range.
In examining safety, 5 major AEs occurred. One patient had 2 thrombotic pulmonary embolism events. This patient had a history of nonadherence with warfarin therapy. Three major bleeding events occurred (2 in the telephone group and 1 in the office group). Two bleeding events led to ED visits, and 1 event led to hospitalization. Although 43% of patients had a procedure during the study period, only a portion of patients received bridging with low-molecular-weight heparin (LMWH). None of the 3 reported bleeding events discovered during the study were associated with recent LMWH use. No events were fatal (Table 3).
Discussion
This study demonstrates that patients transitioned from office to telephone visits for warfarin management will have no significant change in their TTR. Additionally, patients had similar rates of major AEs before and after transition, although there were few events overall.
Previous research comparing anticoagulation outcomes in telephone vs office visits also has described outcomes to be similar between these 2 management models. Wittkowsky and colleagues examined 2 university-affiliated clinics to evaluate warfarin outcomes and AEs in patients in each management model (office vs telephone) and found no difference in outcomes between the 2 management models.9
Staresinic and colleagues designed a prospective study of 192 patients to evaluate TTR and AEs of the 2 management models at the William S. Middleton Memorial Veterans Hospital in Madison, Wisconsin.10 This study found no difference between the 2 groups in percentage of time maintained within INR range or AEs and concluded that the telephone model was effective for anticoagulant management.
A retrospective study by Stoudenmire and colleagues evaluated office vs telephone management effects on extreme INR values (≤ 1.5 or ≥ 4.5), TTR, and AEs.11 This study found overall TTR and AEs to be similar between groups, but the telephone clinic had a 2-fold increase in extreme INR values compared with the office clinic.11
The current study differs from the previously discussed studies in that it evaluated outcomes for the same patients before and after the transition to telephone. This study did not exclude specific patients from telephone clinic. In the Wittkowsky study, patients were enrolled in the telephone clinic based on criteria such as patient disability or living long distances from the clinic.9 Additionally, in the current study, patients transitioned to telephone visits did not have scheduled office visits for anticoagulation management. In contrast, patients in the Staresinic study had routine anticoagulation office visits every 3 months, thus it was not a true telephone-only clinic.10
This study’s findings support prior studies’ findings that telephone clinics are acceptable for anticoagulation management. Furthermore, safety does not seem to be affected when transitioning patients, although there were few AEs to review. Providers can use telephone clinics to potentially decrease cost and facilitate access to care for patients.
Limitations
Patients were required to be in office and telephone for a sequential 6 months, and this may have produced selection biases toward patients who adhered to appointments and who were on long-term warfarin therapy. Many patients that were excluded from the study transitioned back and forth between the 2 management models. Due to the retrospective nature of this study, the authors were unable to control for all confounding variables. Patients also were not randomly assigned to be transitioned from office to telephone. Although a strength of this study was the limited telephone clinic selection criteria, there may be a few individual situations in which the pharmacist’s clinical judgment influenced the transition to the telephone clinic, creating selection bias.
There may be time bias present as clinical guidelines, providers, and clinic population size differed over the study period and might have influenced management. The population of VA patients was mainly elderly males; therefore, the study results may not be applicable to other populations. Last, the results of the study are reflective of the VANTHCS clinic structure and may not be applicable to other clinic designs.
Conclusion
Veterans in a pharmacist-managed anticoagulation clinic experienced the same outcomes in terms of TTR and major AEs when transitioned from the traditional face-to-face office visits to telephone visits. The study supports the safety and efficacy of transitioning patients from a pharmacist-managed anticoagulation office clinic to telephone clinic.
Oral anticoagulation with warfarin is used for the treatment and prevention of a variety of thrombotic disorders, including deep venous thrombosis (DVT), pulmonary embolism (PE), stroke prevention in atrial fibrillation (AF) and atrial flutter, and other hypercoagulable conditions. Although a mainstay in the treatment for these conditions, warfarin requires close monitoring due to its narrow therapeutic range, extensive drug and dietary interactions, and dosage variability among patients.1 Patients outside the therapeutic range are at risk of having a thrombotic or bleeding event that could lead to hospitalization or fatality.1 To reduce the risk of these events, patients on warfarin are managed by dose adjustment based on the international normalized ratio (INR). Research has shown that patients on warfarin in pharmacist-managed specialty anticoagulation clinics have more consistent monitoring and lower rates of adverse events (AEs) compared with traditional physician or nurse clinics.2-6 Management through these clinics can be achieved through office visits or telephone visits.
There are advantages and disadvantages to each model of anticoagulation management for patients.Telephone clinics provide time and cost savings, increased access to care, and convenience. However, disadvantages include missed phone calls or inability to contact the patient, difficulty for the patient to hear the provider’s instructions over the phone, and patient unavailability when a critical INR is of concern. Office visits are beneficial in that providers can provide both written and verbal instruction to patients, perform visual or physical patient assessments, and provide timely care if needed. Disadvantages of office visits may include long wait times and inconvenience for patients who live far away.
Telephone anticoagulation clinics have been evaluated for their efficacy and cost-effectiveness in several studies.5,7,8 However, few studies are available that compare patient outcomes between office visits and telephone visits. Two prior studies comparing groups of anticoagulation patients managed by telephone or by office visit concluded that there is no difference in outcomes between the 2 management models.9,10 However, a retrospective study by Stoudenmire and colleagues examined extreme INR values (≤ 1.5 or ≥ 4.5) in each management model and found that telephone clinic patients have a significant increase in extreme INR values but no difference in AEs between the 2 management models.11
The VA North Texas Health Care System (VANTHCS) includes a major medical center, 3 outlying medical facilities, and 5 community-based outpatient clinics (CBOCs). A centralized pharmacist-managed anticoagulation clinic is used to manage more than 2,500 VANTHCS anticoagulation patients. To meet the National Patient Safety Goal measures and provide consistent management across the system, all anticoagulation patients from CBOCs and medical facilities are enrolled in the clinic.12 To facilitate access to care, many patients transitioned from office visits to telephone visits. It was essential to evaluate the transition of patients from office to telephone visits to ensure continued stability and continuity of care across both models. The objective of this study was to determine whether a difference in anticoagulation outcomes exists when patients are transitioned from office to telephone visits.
Methods
The VANTHCS anticoagulation clinic policy for office visits requires that patients arrive at the Dallas VAMC 2 hours before their appointment for INR lab draw. During the office visit, the anticoagulation pharmacist evaluates the INR and pertinent changes since the previous visit. The patient is provided verbal instructions and a written dosage adjustment card. Telephone clinic protocol is similar to office visits with a few exceptions. Any patient, regardless of INR stability, may be enrolled in the telephone clinic as long as the patient provides consent and has a working telephone with voice mail. Patients enrolled in the telephone clinic access blood draws at the nearest VA facility and are given a questionnaire that includes pertinent questions asked during an office visit. Anticoagulation pharmacists evaluate the questionnaire and INR then contact the patient within 1 business day to provide the patient with instructions. If a patient fails to answer the telephone, the anticoagulation pharmacist leaves a voicemail message.
Study Design
This retrospective study was conducted by chart review using Computerized Patient Record System (CPRS) at VANTHCS on patients who met inclusion criteria between January 1, 2011 and May 31, 2014, and it was approved by the institutional review board and research and development committee. The study included patients aged ≥ 18 years on warfarin therapy managed by the VANTHCS anticoagulation clinic who were previously managed in office visits for ≥ 180 days before the telephone transition, then in telephone visits for another ≥ 180 days. Only INR values obtained through the VANTHCS anticoagulation clinic were assessed.
Patients were excluded from the study if they were not managed by the VANTHCS anticoagulation clinic or received direct oral anticoagulants (DOACs). The INR values were excluded if they were nonclinic related INR values (ie, results reported that do not reflect management by the anticoagulation clinic), the first INR after hospitalization, or INRs obtained during the first month of initial warfarin treatment for a patient.
For all patients included in the study, demographic information, goal INR range (2 to 3 or 2.5 to 3.5), indication for warfarin therapy, and duration of warfarin therapy (defined as the first prescription filled for warfarin at the VA) were obtained. Individual INR values were obtained for each patient during the period of investigation and type of visit (office or telephone) for each INR drawn was specified. Any major bleeding or thrombotic events (bleed requiring an emergency department [ED] visit, hospitalization, vitamin K administration, blood transfusion, and/or warfarin therapy hold/discontinuation) were documented. Procedures and number of hospitalizations also during the investigation were recorded.
The primary outcomes measures evaluated INRs for time in therapeutic range (TTR) using the Rosendaal method and percentage of INRs within range.13 The therapeutic range was either 2 to 3 or 2.5 to 3.5 (the “strict range” for INR management). Because many patients fluctuate around the strict range and it is common to avoid therapy adjustment based on slightly elevated or lower values, a “nonstrict” range (1.8 to 3.2 or 2.3 to 3.7) also was evaluated.14 The secondary outcomes examined differences between the 2 management models in rates of major AEs, including thrombosis and major bleeding events as defined earlier.Frequencies, percentages, and other descriptive statistics were used to describe nominal data. A paired t test was used to compare TTR of patients transitioned from office to telephone visits. A P value of < .05 was used for statistical significance.
Results
A total of 111 patients met inclusion criteria (Table 1). Most patients were elderly males with AF or atrial flutter as their primary indication for warfarin therapy. No statistically significant difference was found for percentage INRs in strict range (56.8% in office vs 56.9% in telephone, P = .98) or TTR (65.9% in office vs 62.72% in telephone, P = .23) for patients who transitioned from office to telephone visits (Table 2). Similar results were found within the nonstrict range.
In examining safety, 5 major AEs occurred. One patient had 2 thrombotic pulmonary embolism events. This patient had a history of nonadherence with warfarin therapy. Three major bleeding events occurred (2 in the telephone group and 1 in the office group). Two bleeding events led to ED visits, and 1 event led to hospitalization. Although 43% of patients had a procedure during the study period, only a portion of patients received bridging with low-molecular-weight heparin (LMWH). None of the 3 reported bleeding events discovered during the study were associated with recent LMWH use. No events were fatal (Table 3).
Discussion
This study demonstrates that patients transitioned from office to telephone visits for warfarin management will have no significant change in their TTR. Additionally, patients had similar rates of major AEs before and after transition, although there were few events overall.
Previous research comparing anticoagulation outcomes in telephone vs office visits also has described outcomes to be similar between these 2 management models. Wittkowsky and colleagues examined 2 university-affiliated clinics to evaluate warfarin outcomes and AEs in patients in each management model (office vs telephone) and found no difference in outcomes between the 2 management models.9
Staresinic and colleagues designed a prospective study of 192 patients to evaluate TTR and AEs of the 2 management models at the William S. Middleton Memorial Veterans Hospital in Madison, Wisconsin.10 This study found no difference between the 2 groups in percentage of time maintained within INR range or AEs and concluded that the telephone model was effective for anticoagulant management.
A retrospective study by Stoudenmire and colleagues evaluated office vs telephone management effects on extreme INR values (≤ 1.5 or ≥ 4.5), TTR, and AEs.11 This study found overall TTR and AEs to be similar between groups, but the telephone clinic had a 2-fold increase in extreme INR values compared with the office clinic.11
The current study differs from the previously discussed studies in that it evaluated outcomes for the same patients before and after the transition to telephone. This study did not exclude specific patients from telephone clinic. In the Wittkowsky study, patients were enrolled in the telephone clinic based on criteria such as patient disability or living long distances from the clinic.9 Additionally, in the current study, patients transitioned to telephone visits did not have scheduled office visits for anticoagulation management. In contrast, patients in the Staresinic study had routine anticoagulation office visits every 3 months, thus it was not a true telephone-only clinic.10
This study’s findings support prior studies’ findings that telephone clinics are acceptable for anticoagulation management. Furthermore, safety does not seem to be affected when transitioning patients, although there were few AEs to review. Providers can use telephone clinics to potentially decrease cost and facilitate access to care for patients.
Limitations
Patients were required to be in office and telephone for a sequential 6 months, and this may have produced selection biases toward patients who adhered to appointments and who were on long-term warfarin therapy. Many patients that were excluded from the study transitioned back and forth between the 2 management models. Due to the retrospective nature of this study, the authors were unable to control for all confounding variables. Patients also were not randomly assigned to be transitioned from office to telephone. Although a strength of this study was the limited telephone clinic selection criteria, there may be a few individual situations in which the pharmacist’s clinical judgment influenced the transition to the telephone clinic, creating selection bias.
There may be time bias present as clinical guidelines, providers, and clinic population size differed over the study period and might have influenced management. The population of VA patients was mainly elderly males; therefore, the study results may not be applicable to other populations. Last, the results of the study are reflective of the VANTHCS clinic structure and may not be applicable to other clinic designs.
Conclusion
Veterans in a pharmacist-managed anticoagulation clinic experienced the same outcomes in terms of TTR and major AEs when transitioned from the traditional face-to-face office visits to telephone visits. The study supports the safety and efficacy of transitioning patients from a pharmacist-managed anticoagulation office clinic to telephone clinic.
1. Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G; American College of Chest Physicians. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest. 2008;133(suppl 6):160S-198S.
2. Rudd KM, Dier JG. Comparison of two different models of anticoagulation management services with usual medical care. Pharmacotherapy. 2010;30(4):330-338.
3. Bungard TJ, Gardner L, Archer SL, et al. Evaluation of a pharmacist-managed anticoagulation clinic: improving patient care. Open Med. 2009;3(1):e16-e21.
4. Chiquette E, Amato MG, Bussey HI. Comparison of an anticoagulation clinic with usual medical care: anticoagulation control, patient outcomes, and health care costs. Arch Intern Med. 1998;158(15):1641-1647.
5. Waterman AD, Banet G, Milligan PE, et al. Patient and physician satisfaction with a telephone-based anticoagulation service. J Gen Intern Med. 2001;16(7):460-463.
6. Hasan SS, Shamala R, Syed IA, et al. Factors affecting warfarin-related knowledge and INR control of patients attending physician- and pharmacist-managed anticoagulation clinics. J Pharm Pract. 2011;24(5):485-493.
7. Hassan S, Naboush A, Radbel J, et al. Telephone-based anticoagulation management in the homebound setting: a retrospective observational study. Int J Gen Med. 2013;6:869-875.
8. Moherman LJ, Kolar MM. Complication rates for a telephone-based anticoagulation service. Am J Health Syst Pharm. 1999;56(15):1540-1542.
9. Wittkowsky AK, Nutescu EA, Blackburn J, et al. Outcomes of oral anticoagulant therapy managed by telephone vs in-office visits in an anticoagulation clinic setting. Chest. 2006;130(5):1385-1389.
10. Staresinic AG, Sorkness CA, Goodman BM, Pigarelli DW. Comparison of outcomes using 2 delivery models of anticoagulation care. Arch Intern Med. 2006;166(9):997-1002.
11. Stoudenmire LG, DeRemer CE, Elewa H. Telephone versus office-based management of warfarin: impact on international normalized ratios and outcomes. Int J Hematol. 2014;100(2):119-124.
12. The Joint Commission. National Patient Safety Goals Effective January 1, 2015. http://www.jointcommission.org/assets/1/6/2015_NPSG_AHC1.PDF. Published 2014. Accessed November 23, 2016.
13. Rosendaal FR, Cannegieter SC, van der Meer FJ, Briët E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69(3):236-239.
14. Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuünemann HJ; American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(suppl 2):7S-47S.
1. Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G; American College of Chest Physicians. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest. 2008;133(suppl 6):160S-198S.
2. Rudd KM, Dier JG. Comparison of two different models of anticoagulation management services with usual medical care. Pharmacotherapy. 2010;30(4):330-338.
3. Bungard TJ, Gardner L, Archer SL, et al. Evaluation of a pharmacist-managed anticoagulation clinic: improving patient care. Open Med. 2009;3(1):e16-e21.
4. Chiquette E, Amato MG, Bussey HI. Comparison of an anticoagulation clinic with usual medical care: anticoagulation control, patient outcomes, and health care costs. Arch Intern Med. 1998;158(15):1641-1647.
5. Waterman AD, Banet G, Milligan PE, et al. Patient and physician satisfaction with a telephone-based anticoagulation service. J Gen Intern Med. 2001;16(7):460-463.
6. Hasan SS, Shamala R, Syed IA, et al. Factors affecting warfarin-related knowledge and INR control of patients attending physician- and pharmacist-managed anticoagulation clinics. J Pharm Pract. 2011;24(5):485-493.
7. Hassan S, Naboush A, Radbel J, et al. Telephone-based anticoagulation management in the homebound setting: a retrospective observational study. Int J Gen Med. 2013;6:869-875.
8. Moherman LJ, Kolar MM. Complication rates for a telephone-based anticoagulation service. Am J Health Syst Pharm. 1999;56(15):1540-1542.
9. Wittkowsky AK, Nutescu EA, Blackburn J, et al. Outcomes of oral anticoagulant therapy managed by telephone vs in-office visits in an anticoagulation clinic setting. Chest. 2006;130(5):1385-1389.
10. Staresinic AG, Sorkness CA, Goodman BM, Pigarelli DW. Comparison of outcomes using 2 delivery models of anticoagulation care. Arch Intern Med. 2006;166(9):997-1002.
11. Stoudenmire LG, DeRemer CE, Elewa H. Telephone versus office-based management of warfarin: impact on international normalized ratios and outcomes. Int J Hematol. 2014;100(2):119-124.
12. The Joint Commission. National Patient Safety Goals Effective January 1, 2015. http://www.jointcommission.org/assets/1/6/2015_NPSG_AHC1.PDF. Published 2014. Accessed November 23, 2016.
13. Rosendaal FR, Cannegieter SC, van der Meer FJ, Briët E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69(3):236-239.
14. Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuünemann HJ; American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(suppl 2):7S-47S.
Management of Proximal Biceps Pathology in Overhead Athletes: What Is the Role of Biceps Tenodesis?
Take Home Points
- Outcomes after SLAP repair remain guarded.
- Physical examination is key in determining proper management of biceps pathology.
- When performing SLAP repair, knotless technology may prevent future cartilage or rotator cuff injury.
- Revision of SLAP repair is best handled with biceps tenodesis.
- Subpectoral biceps tenodesis avoids residual groove pain.
In recent decades, the long head of the biceps (LHB) tendon has been recognized as a pain generator in the shoulder of throwing athletes. The LHB muscle and its role in glenohumeral kinematics remains largely in question. The LHB tendon varies in size but most commonly is 5 mm to 6mm in diameter and about 9 cm in length, inserting on the superior labrum and supraglenoid tubercle after traveling through the bicipital groove.1 The many conditions that can develop along the course of the biceps tendon include overall biceps tendonitis, biceps tendon subluxation or instability, and injuries to the superior anterior to posterior area of the labrum.
These injuries can occur in young overhead athletes as well as manual laborers and older overhead recreational athletes. Pitching is the most common activity that leads to proximal biceps tendon disorders. The 6 phases of the pitch are linked in a kinetic chain that generates energy that is then translated to high velocity. The amount of force that is exerted on the shoulder during pitching and especially after ball release is impressive, and the athlete’s shoulder changes in many ways as it adapts to the motion.2-5 The late-cocking and deceleration phases are most commonly associated with proximal biceps pathology and the “peel-back” phenomenon. Other common activities that lead to biceps tendon issues in a young population are volleyball, baseball, tennis, softball, swimming, and cricket. Shoulder arthroscopies performed in older patients show degenerative biceps and labrum tears, which should be treated appropriately but perhaps different from how they are treated in overhead athletes.6-8 Further, many professional athletes have asymptomatic superior labrum anterior-posterior (SLAP) tears.9
Mechanism of Injury
Overhead throwing is commonly thought to be the mechanism by which lesions are created in the biceps–labrum complex (BLC). Pitching in particular generates incredible force and torque within the shoulder. In professional pitchers, the resulting throwing speed creates forces regularly in excess of 1000 N.3 These forces effect internal compensatory changes and internal derangement of the BLC. These changes often involve internal rotation deficits and alterations in the rotator cuff, which may contribute to glenohumeral instability and altered joint kinematics.10
Repetitive overhead activity is largely considered the mechanism of injury in this population, though more specific mechanisms have been described, including the peel-back mechanism11 and the posterior superior glenoid impingement. There is little evidence that preventive programs have any effect on decreasing the incidence of SLAP tears in overhead athletes.
Preoperative Evaluation
Preoperative evaluation is arguably the most important step in treating a patient with persistent or recurrent symptoms consistent with a SLAP tear. Evaluation includes thorough history, physical examination, and review of any prior injuries or surgical procedures. The physical examination should focus on maneuvers that define where the problem is occurring. Although SLAP tears are most common in this population, disorders of the biceps tendon within the groove, including inflammation and instability, should be ruled out with physical examination and advanced imaging. Palpation for groove tenderness, impingement-type complaints, internal rotation loss, and SLAP provocative testing are crucial in the diagnosis.12,13 The cause of symptoms may be multifactorial and include the often encountered concomitant pathology of rotator cuff tears, internal impingement, and instability.
Standard radiographs (Grashey anteroposterior, scapular/lateral, axillary lateral) and magnetic resonance imaging (MRI) with or without arthrography can be helpful in identifying and characterizing most SLAP tears as well as failed SLAP tear repairs. However, MRI is often positive for SLAP tears in asymptomatic patients, and diagnosing SLAP tears with MRI is often a challenge.14 MRI can help in determining concomitant pathology, including rotator cuff injury and cysts causing nerve compression. Correlation with clinical examination and patient history is most crucial. Conservative treatment (rest, activity modification, use of oral anti-inflammatory medications) typically is attempted and coordinated with respect to the athlete’s season of play.15,16
Classification
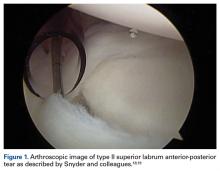
In overhead throwing athletes, SLAP tears typically are associated with anterior shoulder pain. Associated shoulder instability and significant glenohumeral dysfunction are not uncommon in athletes with lesions of the BLC. In 1985, Andrews and colleagues17 were the first to describe SLAP tears in overhead athletes (73 patients). Later, Snyder and colleagues18,19 further classified these lesions into 4 types based on tear stability and location, and they coined the acronym SLAP (Figure 1).
Type I lesions typically are described as fraying at the inner margin of the labrum and are common in throwers, even asymptomatic throwers. Type II lesions, separations of the biceps and labrum from the superior glenoid (≥5 mm of excursion), are the most commonly occurring and treated variant in throwing athletes.20-22 Intraoperative evaluation for a peel-back lesion (placing the arm in abduction with external rotation), rather than for a sulcus of 1 mm to 2 mm, may confirm a type II SLAP tear.20,23,24 It is often important to consider the direction of tear propagation as well. Type III lesions include those with an intact BLC (but with a bucket-handle tear of the superior labral complex and an intact biceps tendon), whereas type IV lesions involve additional extension of the tear into the biceps tendon.18,19The classification systems are well defined. Nevertheless, management of SLAP lesions remains controversial.
Options for Surgical Treatment
SLAP Tear Repair—Outcomes
The incidence of SLAP tear repairs has increased dramatically in recent years.6,25 There are various SLAP tear repair methods, but the most common consists of repairing the labrum and biceps anchor. Management of type II SLAP lesions remains controversial. Several prospective studies have found overall improvement after SLAP tear repair.26-31 Other series have reported less encouraging outcomes, including dissatisfaction with persistent pain and inability to return to throwing.28,32 A 2010 systematic review found that the percentage of patients who returned to their preinjury level of play was only 64%, and outcomes for overhead throwing athletes were even worse—only 22% to 60% of these patients returned to their previous level.33 The right surgery for SLAP tears in this population continues to be an area of uncertainty for many surgeons.
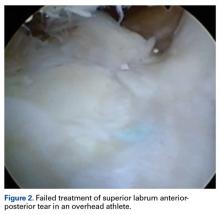
Failed SLAP tear repairs (poor outcomes) have become common in overhead throwing athletes. The reasons for these failed repairs are unclear, but several possible explanations have been offered. One is that labral repair may result in permanent alterations in pitching biomechanics, which may lead to an inability to regain velocity and command.3 Another is that the athlete’s shoulder may remain unstable even after repair.10Hardware complications are a significant concern in this high-level population. Suture anchor pullout or iatrogenic cartilage damage may occur during instrumentation or as a result of suture anchor reactive changes. In addition, there are several reports of glenoid osteochondrolysis (Figure 2) caused by prominent hardware or prominent knots.34-39
Stiffness after SLAP tear repair is a significant problem, with most patients taking up to 6 months to regain full motion.26,48 Overtensioning of the labrum and the glenohumeral ligaments may be the cause, and the solution may be to place anchors posterior (vs anterior) to the biceps insertion. In a large prospective military study, mean forward flexion and external rotation were reduced at final follow-up.31 These outcomes are less acceptable to overhead throwing athletes, who rely on motion for high-end throwing activities.
Primary Biceps Tenodesis—Outcomes
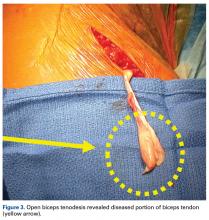
A 2015 database study found a 1.7-fold increase in biceps tenodesis over the preceding 5 years.49 However, relatively few procedures included in the study were performed in patients age younger than 30 years. For many older non-overhead throwers with type II tears, SLAP tear repair has become less popular as a treatment option.32 There is a dearth of knowledge about the outcomes of subpectoral biceps tenodesis as a primary treatment for biceps tendonitis and an associated SLAP tear. Although type I tears historically have been treated with débridement, débridement is seldom used for concomitant biceps tendonitis. It should be coupled with careful clinical examination.
In recent years, biceps tenodesis has been proposed as an alternative to repair for SLAP tears, particularly in older patients.24,44 For obvious reasons, however, there has been some trepidation about performing biceps tenodesis in throwing athletes. Some authors have proposed biceps tenodesis as primary treatment for isolated SLAP tears. Boileau and colleagues44 compared the outcomes of treatment of isolated type II SLAP lesions in 25 consecutive patients. For 10 patients, repair involved suture anchors; for the other 15, arthroscopic biceps tenodesis was performed with an absorbable interference screw. Six of the 10 suture anchor patients were disappointed with their outcome (persistent pain or inability to return to sport), whereas 14 of the 15 biceps tenodesis patients were satisfied. The authors concluded that arthroscopic biceps tenodesis is an effective alternative to repair for type II SLAP lesions, though their study was not isolated to overhead athletes (tenodesis group mean age, 52 years).
In a 2014 series of cases, Ek and colleagues50 reported good outcomes of SLAP tear repair and biceps tenodesis. Again, though, tenodesis was used in older patients, and repair in younger, more active patients, with no high-level athletes in either group. There was no difference in return to sport between groups. In a study of patients who underwent primary biceps tenodesis, Gupta and colleagues51 found 80% excellent outcomes (improved shoulder outcome scores) in select SLAP tear patients, including 8 athletes, 88% of whom were overhead athletes. Gottschalk and colleagues52 reported on differences in prospectively collected outcome data (age, sex, SLAP lesion type II or IV) for primary biceps tenodesis in a series of 33 patients. Twenty-six of the 29 patients who completed follow-up returned to their previous level of activity. These studies suggest that primary biceps tenodesis may be an alternative with lower failure rates in the treatment of SLAP tears in middle-aged patients, and in overhead athletes, though additional specific studies are needed to focus on overhead athletes on a larger scale.
Revision SLAP Tear Repair Versus Biceps Tenodesis
Failed arthroscopic SLAP tear repairs, which are increasingly common, present a unique treatment challenge. In a 2013 prospective cohort series, Gupta and colleagues46 found excellent clinical outcomes of subpectoral biceps tenodesis for failed type II SLAP tears. The authors reported a postoperative SANE (Single Assessment Numeric Evaluation) score of 70.4%, an SST (Simple Shoulder Test) score of 9.33, and an ASES (American Shoulder and Elbow Surgeons) score of 77.96, along with reasonable health-related quality-of-life scores. Werner and colleagues53 evaluated 2-year outcomes of biceps tenodesis performed after SLAP tear repair in 24 patients and found a return to almost normal range of motion as well as good clinical outcome scores. Significantly worse outcomes were found for patients with open worker’s compensation claims.
McCormick and colleagues26 prospectively evaluated the efficacy of biceps tenodesis for failed type II SLAP tear repair in 46 patients. Improvement was noted across all outcome assessments during follow-up (mean, 3.6 years). From these findings, we might conclude that biceps tenodesis is a more predictable option for failed SLAP tear repair, and that it has a relatively low complication rate. However, most investigators have used a heterogeneous patient population, as opposed to overhead athletes specifically. To our knowledge, no one has evaluated the specific population of overhead throwers with failed SLAP tear repairs. In addition, no one has conducted randomized controlled trials comparing débridement, biceps tenodesis, and repair for failed SLAP tear repairs.
Postoperative Considerations
When overhead athletes and their surgeons are considering surgical options, they must take rehabilitation and return to play into account. Many surgeons think the possible marginal clinical benefit of SLAP tear repair may not be worth the protracted rehabilitation. In most practices, rehabilitation after biceps tenodesis is less involved. Discussing the advantages and disadvantages of these 2 procedures can be helpful in decision making.
Dein and colleagues54 reported the case of a middle-aged pitcher who sustained a fracture after biceps tenodesis with an interference screw. Cases like this are concerning. Surgeons should consider altering the rehabilitation regimen when planning postoperative care in cases of biceps tenodesis in throwers. Other reported complications of open tenodesis are deep infection, thrombosis, postoperative stiffness, and nerve injury.55-58
Consequences for Overhead Throwers
The unknown role of the BLC leaves surgeons wary when considering biceps tenodesis for elite athletes. Some have postulated that removing the intra-articular portion of the LHB may cause microinstability and alter joint kinematics.10,59-61 Others have suggested the biceps is desynchronized from the other musculature and is not functionally important.62 Disruption of one portion of the superior labrum may result in instability on the opposite side of the glenoid.10,61 Biomechanical studies, both cadaveric and in vivo, have tried to create proper loads to the LHB and evaluate the kinematics of the shoulder before and after biceps tenodesis and SLAP tear repair.59,60 Using a cadaveric model, Strauss and colleagues63 found that type II SLAP lesions resulted in increased glenohumeral translation compared with baseline. Biceps tenodesis did not restore normal translation, but this did not negatively affect stability in the presence of a SLAP lesion. The consensus is that the role of the biceps is controversial at best.
Several studies have used electromyography (EMG) to evaluate LHB functioning. In 2014, Chalmers and colleagues59 used surface EMG and motion analysis to evaluate 18 pitchers: 6 underwent SLAP tear repair, 5 underwent biceps tenodesis, and 7 were uninjured controls. There were no significant differences in the activity of the LHB muscle, the short head of the biceps muscle, the deltoid, the infraspinatus, or the latissimus among the 3 groups. Motion analysis showed that the normal pattern of muscular activation within the LHB muscle was more closely restored by biceps tenodesis than by SLAP tear repair. In addition, thoracic rotation patterns were significantly more altered in the SLAP tear repair patients than in the uninjured controls. As the authors noted, given the low frequency with which biceps tenodesis is performed in overhead athletes, it is unlikely that larger scale studies will be conducted without a multicenter effort.
Recommendations and Our Preferred Technique
Which surgical option is best for treating symptomatic SLAP lesions in overhead athletes remains unclear. Many athletes struggle to return to high-level play after SLAP tear repair. Whether the same is true after biceps tenodesis is yet to be determined because of the low frequency with which biceps tenodesis is performed in high-level overhead athletes. The options for fixation, technique, and fixation location are equally broad. In this section, we outline our general line of thinking for cases of proximal biceps pathology.
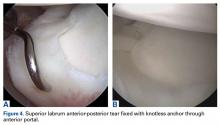
In each case, we perform glenohumeral arthroscopy to evaluate the BLC and identify any other pathology. For overhead athletes who are younger than 30 years and lack bicipital groove pain or signs of gross tendinopathy, we favor arthroscopic SLAP tear repair. Repair is usually performed through an anterior working portal for suture passage and a Wilmington portal for anchor placement. We use knotless technology to achieve stable fixation and stay posterior to the biceps anchor insertion.
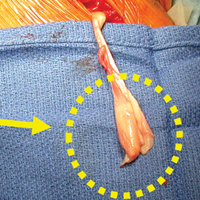
For the prevention of any potential pain from the bicipital groove in carefully selected patients—recreational overhead athletes and patients who want a less involved surgical recovery—we favor open subpectoral biceps tenodesis rather than arthroscopic tenodesis. The outcomes of biceps tenodesis are consistent, according to the literature.47,57,64 Moreover, the open approach is favored for the incidence of postoperative stiffness in the arthroscopic population.65 Tendons can be fixed with multiple procedures, including soft-tissue tenodesis, interference screw fixation, and surface anchors. We favor using a tenodesis screw in the subpectoral location, as outlined by Mazzocca and colleagues.64Our algorithm for SLAP lesions is evolving with our understanding of this complex disease process. For young overhead throwers with type II SLAP lesions, we favor arthroscopic SLAP tear repair with knotless technology. For older recreational overhead athletes, we favor biceps tenodesis in the subpectoral region after diagnostic arthroscopy plus biceps tenotomy with or without additional SLAP tear fixation, depending on the stability of the biceps anchor (Figures 4A, 4B).
Conclusion
Overhead athletes who present with symptomatic SLAP lesions often provide a treatment dilemma. Although SLAP tear repair historically has been standard treatment, biceps tenodesis represents a consistent surgical option with low complication rates and low revision rates. It is likely that, as additional data on glenohumeral kinematics and outcomes in young athletes become available, improved decision-making algorithms will follow.
Am J Orthop. 2017;46(1):E71-E78. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Elser F, Braun S, Dewing CB, Giphart JE, Millett PJ. Anatomy, function, injuries, and treatment of the long head of the biceps brachii tendon. Arthroscopy. 2011;27(4):581-592.
2. Fedoriw WW, Ramkumar P, McCulloch PC, Lintner DM. Return to play after treatment of superior labral tears in professional baseball players. Am J Sports Med. 2014;42(5):1155-1160.
3. Fleisig GS, Andrews JR, Dillman CJ, Escamilla RF. Kinetics of baseball pitching with implications about injury mechanisms. Am J Sports Med. 1995;23(2):233-239.
4. Aydin N, Sirin E, Arya A. Superior labrum anterior to posterior lesions of the shoulder: diagnosis and arthroscopic management. World J Orthop. 2014;5(3):344-350.
5. Barber A, Field LD, Ryu R. Biceps tendon and superior labrum injuries: decision-marking. J Bone Joint Surg Am. 2007;89(8):1844-1855.
6. Onyekwelu I, Khatib O, Zuckerman JD, Rokito AS, Kwon YW. The rising incidence of arthroscopic superior labrum anterior and posterior (SLAP) repairs. J Shoulder Elbow Surg. 2012;21(6):728-731.
7. Patterson BM, Creighton RA, Spang JT, Roberson JR, Kamath GV. Surgical trends in the treatment of superior labrum anterior and posterior lesions of the shoulder: analysis of data from the American Board of Orthopaedic Surgery Certification Examination Database. Am J Sports Med. 2014;42(8):1904-1910.
8. Walton DM, Sadi J. Identifying SLAP lesions: a meta-analysis of clinical tests and exercise in clinical reasoning. Phys Ther Sport. 2008;9(4):167-176.
9. Lesniak BP, Baraga MG, Jose J, Smith MK, Cunningham S, Kaplan LD. Glenohumeral findings on magnetic resonance imaging correlate with innings pitched in asymptomatic pitchers. Am J Sports Med. 2013;41(9):2022-2027.
10. Mihata T, McGarry MH, Tibone JE, Fitzpatrick MJ, Kinoshita M, Lee TQ. Biomechanical assessment of type II superior labral anterior-posterior (SLAP) lesions associated with anterior shoulder capsular laxity as seen in throwers: a cadaveric study. Am J Sports Med. 2008;36(8):1604-1610.
11. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology. Part II: evaluation and treatment of SLAP lesions in throwers. Arthroscopy. 2003;19(5):531-539.
12. Meserve BB, Cleland JA, Boucher TR. A meta-analysis examining clinical test utility for assessing superior labral anterior posterior lesions. Am J Sports Med. 2009;37(11):2252-2258.
13. Pandya NK, Colton A, Webner D, Sennett B, Huffman GR. Physical examination and magnetic resonance imaging in the diagnosis of superior labrum anterior-posterior lesions of the shoulder: a sensitivity analysis. Arthroscopy. 2008;24(3):311-317.
14. Amin MF, Youssef AO. The diagnostic value of magnetic resonance arthrography of the shoulder in detection and grading of SLAP lesions: comparison with arthroscopic findings. Eur J Radiol. 2012;81(9):2343-2347.
15. Cook C, Beaty S, Kissenberth MJ, Siffri P, Pill SG, Hawkins RJ. Diagnostic accuracy of five orthopedic clinical tests for diagnosis of superior labrum anterior posterior (SLAP) lesions. J Shoulder Elbow Surg. 2012;21(1):13-22.
16. Edwards SL, Lee JA, Bell JE, et al. Nonoperative treatment of superior labrum anterior posterior tears: improvements in pain, function, and quality of life. Am J Sports Med. 2010;38(7):1456-1461.
17. Andrews JR, Carson WG Jr, McLeod WD. Glenoid labrum tears related to the long head of the biceps. Am J Sports Med. 1985;13(5):337-341.
18. Snyder SJ, Karzel RP, Del Pizzo W, Ferkel RD, Friedman MJ. SLAP lesions of the shoulder. Arthroscopy. 1990;6(4):274-279.
19. Snyder SJ, Banas MP, Karzel RP. An analysis of 140 injuries to the superior glenoid labrum. J Shoulder Elbow Surg. 1995;4(4):243-248.
20. Morgan CD, Burkhart SS, Palmeri M, Gillespie M. Type II SLAP lesions: three subtypes and their relationships to superior instability and rotator cuff tears. Arthroscopy. 1998;14(6):553-565.
21. Weber SC, Martin DF, Seiler JG 3rd, Harrast JJ. Superior labrum anterior and posterior lesions of the shoulder: incidence rates, complications, and outcomes as reported by American Board of Orthopedic Surgery. Part II candidates. Am J Sports Med. 2012;40(7):1538-1543.
22. Keener JD, Brophy RH. Superior labral tears of the shoulder: pathogenesis, evaluation, and treatment. J Am Acad Orthop Surg. 2009;17(10):627-637.
23. Chen CH, Hsu KY, Chen WJ, Shih CH. Incidence and severity of biceps long head tendon lesion in patients with complete rotator cuff tears. J Trauma. 2005;58(6):1189-1193.
24. Nho SJ, Strauss EJ, Lenart BA, et al. Long head of the biceps tendinopathy: diagnosis and management. J Am Acad Orthop Surg. 2010;18(11):645-656.
25. Zhang AL, Kreulen C, Ngo SS, Hame SL, Wang JC, Gamradt SC. Demographic trends in arthroscopic SLAP repair in the United States. Am J Sports Med. 2012;40(5):1144-1147.
26. McCormick F, Bhatia S, Chalmers P, Gupta A, Verma N, Romeo AA. The management of type II superior labral anterior to posterior injuries. Orthop Clin North Am. 2014;45(1):121-128.
27. Brockmeier SF, Voos JE, Williams RJ 3rd, Altchek DW, Cordasco FA, Allen AA; Hospital for Special Surgery Sports Medicine and Shoulder Service. Outcomes after arthroscopic repair of type-II SLAP lesions. J Bone Joint Surg Am. 2009;91(7):1595-1603.
28. Boileau P, Parratte S, Chuinard C, Roussanne Y, Shia D, Bicknell R. Arthroscopic treatment of isolated type II SLAP lesions: biceps tenodesis as an alternative to reinsertion. Am J Sports Med. 2009;37(5):929-936.
29. Denard PJ, Lädermann A, Burkhart SS. Long-term outcome after arthroscopic repair of type II SLAP lesions: results according to age and workers’ compensation status. Arthroscopy. 2012;28(4):451-457.
30. Friel NA, Karas V, Slabaugh MA, Cole BJ. Outcomes of type II superior labrum, anterior to posterior (SLAP) repair: prospective evaluation at a minimum two-year follow-up. J Shoulder Elbow Surg. 2010;19(6):859-867.
31. Provencher MT, McCormick F, Dewing C, McIntire S, Solomon D. A prospective analysis of 179 type 2 superior labrum anterior and posterior repairs: outcomes and factors associated with success and failure. Am J Sports Med. 2013;41(4):880-886.
32. Gupta AK, Chalmers PN, Klosterman EL, et al. Subpectoral biceps tenodesis for bicipital tendonitis with SLAP tear. Orthopedics. 2015;38(1):e48-e53.
33. Gorantla K, Gill C, Wright RW. The outcome of type II SLAP repair: a systematic review. Arthroscopy. 2010;26(4):537-545.
34. Katz LM, Hsu S, Miller SL, et al. Poor outcomes after SLAP repair: descriptive analysis and prognosis. Arthroscopy. 2009;25(8):849-855.
35. Park MJ, Hsu JE, Harper C, Sennett BJ, Huffman GR. Poly-L/D-lactic acid anchors are associated with reoperation and failure of SLAP repairs. Arthroscopy. 2011;27(10):1335-1340.
36. Sassmannshausen G, Sukay M, Mair SD. Broken or dislodged poly-L-lactic acid bioabsorbable tacks in patients after SLAP lesion surgery. Arthroscopy. 2006;22(6):615-619.
37. Uggen C, Wei A, Glousman RE, et al. Biomechanical comparison of knotless anchor repair versus simple suture repair for type II SLAP lesions. Arthroscopy. 2009;25(10):1085-1092.
38. Weber SC. Surgical management of the failed SLAP repair. Sports Med Arthrosc. 2010;18(3):162-166.
39. Wilkerson JP, Zvijac JE, Uribe JW, Schürhoff MR, Green JB. Failure of polymerized lactic acid tacks in shoulder surgery. J Shoulder Elbow Surg. 2003;12(2):117-121.
40. Weber S. Surgical management of the failed SLAP lesion. Arthroscopy. 2008;24(suppl):e8-e9.
41. Schrøder CP, Skare O, Gjengedal E, Uppheim G, Reikerås O, Brox JI. Long-term results after SLAP repair: a 5-year follow-up study of 107 patients with comparison of patients aged over and under 40 years. Arthroscopy. 2012;28(11):1601-1607.
42. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
43. Mazzocca AD, McCarthy MB, Ledgard FA, et al. Histomorphologic changes of the long head of the biceps tendon in common shoulder pathologies. Arthroscopy. 2013;29(6):972-981.
44. Boileau P, Parratte S, Chuinard C, Roussanne Y, Shia D, Bicknell R. Arthroscopic treatment of isolated type II SLAP lesions: biceps tenodesis as an alternative to reinsertion. Am J Sports Med. 2009;37(5):929-936.
45. Boileau P, Krishnan SG, Coste JS, Walch G. Arthroscopic biceps tenodesis: a new technique using bioabsorbable interference screw fixation. Arthroscopy. 2002;18(9):1002-1012.
46. Gupta AK, Bruce B, Klosterman EL, McCormick F, Harris J, Romeo AA. Subpectoral biceps tenodesis for failed type II SLAP repair. Orthopedics. 2013;36(6):e723-e728.
47. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc. 2008;16(3):170-176.
48. McCarty LP 3rd, Buss DD, Datta MW, Freehill MQ, Giveans MR. Complications observed following labral or rotator cuff repair with use of poly-L-lactic acid implants. J Bone Joint Surg Am. 2013;95(6):507-511.
49. Werner BC, Brockmeier SF, Gwathmey FW. Trends in long head biceps tenodesis. Am J Sports Med. 2015;43(3):570-578.
50. Ek ET, Shi LL, Tompson JD, Freehill MT, Warner JJ. Surgical treatment of isolated type II superior labrum anterior-posterior (SLAP) lesions: repair versus biceps tenodesis. J Shoulder Elbow Surg. 2014;23(7):1059-1065.
51. Gupta AK, Chalmers PN, Klosterman EL, et al. Subpectoral biceps tenodesis for bicipital tendonitis with SLAP tear. Orthopedics. 2015;38(1):e48-e53.
52. Gottschalk MB, Karas SG, Ghattas TN, Burdette R. Subpectoral biceps tenodesis for the treatment of type II and IV superior labral anterior and posterior lesions. Am J Sports Med. 2014;42(9):2128-2135.
53. Werner BC, Pehlivan HC, Hart JM, et al. Biceps tenodesis is a viable option for salvage of failed SLAP repair. J Shoulder Elbow Surg. 2014;23(8):e179-e184.
54. Dein EJ, Huri G, Gordon JC, McFarland EG. A humerus fracture in a baseball pitcher after biceps tenodesis [published correction appears in Am J Sports Med. 2014;42(6):NP39]. Am J Sports Med. 2014;42(4):877-879.
55. Nho SJ, Reiff SN, Verma NN, Slabaugh MA, Mazzocca AD, Romeo AA. Complications associated with subpectoral biceps tenodesis: low rates of incidence following surgery. J Shoulder Elbow Surg. 2010;19(5):764-768.
56. Osbahr DC, Diamond AB, Speer KP. The cosmetic appearance of the biceps muscle after long-head tenotomy versus tenodesis. Arthroscopy. 2002;18(5):483-487.
57. Romeo AA, Mazzocca AD, Tauro JC. Arthroscopic biceps tenodesis. Arthroscopy. 2004;20(2):206-213.
58. Ma H, Van Heest A, Glisson C, Patel S. Musculocutaneous nerve entrapment: an unusual complication after biceps tenodesis. Am J Sports Med. 2009;37(12):2467-2469.
59. Chalmers PN, Trombley R, Cip J, et al. Postoperative restoration of upper extremity motion and neuromuscular control during the overhand pitch: evaluation of tenodesis and repair for superior labral anterior-posterior tears. Am J Sports Med. 2014;42(12):2825-2836.
60. Giphart JE, Elser F, Dewing CB, Torry MR, Millett PJ. The long head of the biceps tendon has minimal effect on in vivo glenohumeral kinematics: a biplane fluoroscopy study. Am J Sports Med. 2012;40(1):202-212.
61. Grossman MG, Tibone JE, McGarry MH, Schneider DJ, Veneziani S, Lee TQ. A cadaveric model of the throwing shoulder: a possible etiology of superior labrum anterior-to-posterior lesions. J Bone Joint Surg Am. 2005;87(4):824-831.
62. Hawkes DH, Alizadehkhaiyat O, Fisher AC, Kemp GJ, Roebuck MM, Frostick SP. Normal shoulder muscular activation and co-ordination during a shoulder elevation task based on activities of daily living: an electromyographic study. J Orthop Res. 2012;30(1):53-60.
63. Strauss EJ, Salata MJ, Sershon RA, et al. Role of the superior labrum after biceps tenodesis in glenohumeral stability. J Shoulder Elbow Surg. 2014;23(4):485-491.
64. Mazzocca AD, Bicos J, Santangelo S, Romeo AA, Arciero RA. The biomechanical evaluation of four fixation techniques for proximal biceps tenodesis. Arthroscopy. 2005;21(11):1296-1306.
65. Werner BC, Pehlivan HC, Hart JM, et al. Increased incidence of postoperative stiffness after arthroscopic compared with open biceps tenodesis. Arthroscopy. 2014;30(9):1075-1084.
66. Denard PJ, Dai X, Hanypsiak BT, Burkhart SS. Anatomy of the biceps tendon: implications for restoring physiological length–tension relation during biceps tenodesis with interference screw fixation. Arthroscopy. 2012;28(10):1352-1358.
67. Mazzocca AD, Rios CG, Romeo AA, Arciero RA. Subpectoral biceps tenodesis with interference screw fixation. Arthroscopy. 2005;21(7):896.
Take Home Points
- Outcomes after SLAP repair remain guarded.
- Physical examination is key in determining proper management of biceps pathology.
- When performing SLAP repair, knotless technology may prevent future cartilage or rotator cuff injury.
- Revision of SLAP repair is best handled with biceps tenodesis.
- Subpectoral biceps tenodesis avoids residual groove pain.
In recent decades, the long head of the biceps (LHB) tendon has been recognized as a pain generator in the shoulder of throwing athletes. The LHB muscle and its role in glenohumeral kinematics remains largely in question. The LHB tendon varies in size but most commonly is 5 mm to 6mm in diameter and about 9 cm in length, inserting on the superior labrum and supraglenoid tubercle after traveling through the bicipital groove.1 The many conditions that can develop along the course of the biceps tendon include overall biceps tendonitis, biceps tendon subluxation or instability, and injuries to the superior anterior to posterior area of the labrum.
These injuries can occur in young overhead athletes as well as manual laborers and older overhead recreational athletes. Pitching is the most common activity that leads to proximal biceps tendon disorders. The 6 phases of the pitch are linked in a kinetic chain that generates energy that is then translated to high velocity. The amount of force that is exerted on the shoulder during pitching and especially after ball release is impressive, and the athlete’s shoulder changes in many ways as it adapts to the motion.2-5 The late-cocking and deceleration phases are most commonly associated with proximal biceps pathology and the “peel-back” phenomenon. Other common activities that lead to biceps tendon issues in a young population are volleyball, baseball, tennis, softball, swimming, and cricket. Shoulder arthroscopies performed in older patients show degenerative biceps and labrum tears, which should be treated appropriately but perhaps different from how they are treated in overhead athletes.6-8 Further, many professional athletes have asymptomatic superior labrum anterior-posterior (SLAP) tears.9
Mechanism of Injury
Overhead throwing is commonly thought to be the mechanism by which lesions are created in the biceps–labrum complex (BLC). Pitching in particular generates incredible force and torque within the shoulder. In professional pitchers, the resulting throwing speed creates forces regularly in excess of 1000 N.3 These forces effect internal compensatory changes and internal derangement of the BLC. These changes often involve internal rotation deficits and alterations in the rotator cuff, which may contribute to glenohumeral instability and altered joint kinematics.10
Repetitive overhead activity is largely considered the mechanism of injury in this population, though more specific mechanisms have been described, including the peel-back mechanism11 and the posterior superior glenoid impingement. There is little evidence that preventive programs have any effect on decreasing the incidence of SLAP tears in overhead athletes.
Preoperative Evaluation
Preoperative evaluation is arguably the most important step in treating a patient with persistent or recurrent symptoms consistent with a SLAP tear. Evaluation includes thorough history, physical examination, and review of any prior injuries or surgical procedures. The physical examination should focus on maneuvers that define where the problem is occurring. Although SLAP tears are most common in this population, disorders of the biceps tendon within the groove, including inflammation and instability, should be ruled out with physical examination and advanced imaging. Palpation for groove tenderness, impingement-type complaints, internal rotation loss, and SLAP provocative testing are crucial in the diagnosis.12,13 The cause of symptoms may be multifactorial and include the often encountered concomitant pathology of rotator cuff tears, internal impingement, and instability.
Standard radiographs (Grashey anteroposterior, scapular/lateral, axillary lateral) and magnetic resonance imaging (MRI) with or without arthrography can be helpful in identifying and characterizing most SLAP tears as well as failed SLAP tear repairs. However, MRI is often positive for SLAP tears in asymptomatic patients, and diagnosing SLAP tears with MRI is often a challenge.14 MRI can help in determining concomitant pathology, including rotator cuff injury and cysts causing nerve compression. Correlation with clinical examination and patient history is most crucial. Conservative treatment (rest, activity modification, use of oral anti-inflammatory medications) typically is attempted and coordinated with respect to the athlete’s season of play.15,16
Classification
In overhead throwing athletes, SLAP tears typically are associated with anterior shoulder pain. Associated shoulder instability and significant glenohumeral dysfunction are not uncommon in athletes with lesions of the BLC. In 1985, Andrews and colleagues17 were the first to describe SLAP tears in overhead athletes (73 patients). Later, Snyder and colleagues18,19 further classified these lesions into 4 types based on tear stability and location, and they coined the acronym SLAP (Figure 1).
Type I lesions typically are described as fraying at the inner margin of the labrum and are common in throwers, even asymptomatic throwers. Type II lesions, separations of the biceps and labrum from the superior glenoid (≥5 mm of excursion), are the most commonly occurring and treated variant in throwing athletes.20-22 Intraoperative evaluation for a peel-back lesion (placing the arm in abduction with external rotation), rather than for a sulcus of 1 mm to 2 mm, may confirm a type II SLAP tear.20,23,24 It is often important to consider the direction of tear propagation as well. Type III lesions include those with an intact BLC (but with a bucket-handle tear of the superior labral complex and an intact biceps tendon), whereas type IV lesions involve additional extension of the tear into the biceps tendon.18,19The classification systems are well defined. Nevertheless, management of SLAP lesions remains controversial.
Options for Surgical Treatment
SLAP Tear Repair—Outcomes
The incidence of SLAP tear repairs has increased dramatically in recent years.6,25 There are various SLAP tear repair methods, but the most common consists of repairing the labrum and biceps anchor. Management of type II SLAP lesions remains controversial. Several prospective studies have found overall improvement after SLAP tear repair.26-31 Other series have reported less encouraging outcomes, including dissatisfaction with persistent pain and inability to return to throwing.28,32 A 2010 systematic review found that the percentage of patients who returned to their preinjury level of play was only 64%, and outcomes for overhead throwing athletes were even worse—only 22% to 60% of these patients returned to their previous level.33 The right surgery for SLAP tears in this population continues to be an area of uncertainty for many surgeons.
Failed SLAP tear repairs (poor outcomes) have become common in overhead throwing athletes. The reasons for these failed repairs are unclear, but several possible explanations have been offered. One is that labral repair may result in permanent alterations in pitching biomechanics, which may lead to an inability to regain velocity and command.3 Another is that the athlete’s shoulder may remain unstable even after repair.10Hardware complications are a significant concern in this high-level population. Suture anchor pullout or iatrogenic cartilage damage may occur during instrumentation or as a result of suture anchor reactive changes. In addition, there are several reports of glenoid osteochondrolysis (Figure 2) caused by prominent hardware or prominent knots.34-39
Stiffness after SLAP tear repair is a significant problem, with most patients taking up to 6 months to regain full motion.26,48 Overtensioning of the labrum and the glenohumeral ligaments may be the cause, and the solution may be to place anchors posterior (vs anterior) to the biceps insertion. In a large prospective military study, mean forward flexion and external rotation were reduced at final follow-up.31 These outcomes are less acceptable to overhead throwing athletes, who rely on motion for high-end throwing activities.
Primary Biceps Tenodesis—Outcomes
A 2015 database study found a 1.7-fold increase in biceps tenodesis over the preceding 5 years.49 However, relatively few procedures included in the study were performed in patients age younger than 30 years. For many older non-overhead throwers with type II tears, SLAP tear repair has become less popular as a treatment option.32 There is a dearth of knowledge about the outcomes of subpectoral biceps tenodesis as a primary treatment for biceps tendonitis and an associated SLAP tear. Although type I tears historically have been treated with débridement, débridement is seldom used for concomitant biceps tendonitis. It should be coupled with careful clinical examination.
In recent years, biceps tenodesis has been proposed as an alternative to repair for SLAP tears, particularly in older patients.24,44 For obvious reasons, however, there has been some trepidation about performing biceps tenodesis in throwing athletes. Some authors have proposed biceps tenodesis as primary treatment for isolated SLAP tears. Boileau and colleagues44 compared the outcomes of treatment of isolated type II SLAP lesions in 25 consecutive patients. For 10 patients, repair involved suture anchors; for the other 15, arthroscopic biceps tenodesis was performed with an absorbable interference screw. Six of the 10 suture anchor patients were disappointed with their outcome (persistent pain or inability to return to sport), whereas 14 of the 15 biceps tenodesis patients were satisfied. The authors concluded that arthroscopic biceps tenodesis is an effective alternative to repair for type II SLAP lesions, though their study was not isolated to overhead athletes (tenodesis group mean age, 52 years).
In a 2014 series of cases, Ek and colleagues50 reported good outcomes of SLAP tear repair and biceps tenodesis. Again, though, tenodesis was used in older patients, and repair in younger, more active patients, with no high-level athletes in either group. There was no difference in return to sport between groups. In a study of patients who underwent primary biceps tenodesis, Gupta and colleagues51 found 80% excellent outcomes (improved shoulder outcome scores) in select SLAP tear patients, including 8 athletes, 88% of whom were overhead athletes. Gottschalk and colleagues52 reported on differences in prospectively collected outcome data (age, sex, SLAP lesion type II or IV) for primary biceps tenodesis in a series of 33 patients. Twenty-six of the 29 patients who completed follow-up returned to their previous level of activity. These studies suggest that primary biceps tenodesis may be an alternative with lower failure rates in the treatment of SLAP tears in middle-aged patients, and in overhead athletes, though additional specific studies are needed to focus on overhead athletes on a larger scale.
Revision SLAP Tear Repair Versus Biceps Tenodesis
Failed arthroscopic SLAP tear repairs, which are increasingly common, present a unique treatment challenge. In a 2013 prospective cohort series, Gupta and colleagues46 found excellent clinical outcomes of subpectoral biceps tenodesis for failed type II SLAP tears. The authors reported a postoperative SANE (Single Assessment Numeric Evaluation) score of 70.4%, an SST (Simple Shoulder Test) score of 9.33, and an ASES (American Shoulder and Elbow Surgeons) score of 77.96, along with reasonable health-related quality-of-life scores. Werner and colleagues53 evaluated 2-year outcomes of biceps tenodesis performed after SLAP tear repair in 24 patients and found a return to almost normal range of motion as well as good clinical outcome scores. Significantly worse outcomes were found for patients with open worker’s compensation claims.
McCormick and colleagues26 prospectively evaluated the efficacy of biceps tenodesis for failed type II SLAP tear repair in 46 patients. Improvement was noted across all outcome assessments during follow-up (mean, 3.6 years). From these findings, we might conclude that biceps tenodesis is a more predictable option for failed SLAP tear repair, and that it has a relatively low complication rate. However, most investigators have used a heterogeneous patient population, as opposed to overhead athletes specifically. To our knowledge, no one has evaluated the specific population of overhead throwers with failed SLAP tear repairs. In addition, no one has conducted randomized controlled trials comparing débridement, biceps tenodesis, and repair for failed SLAP tear repairs.
Postoperative Considerations
When overhead athletes and their surgeons are considering surgical options, they must take rehabilitation and return to play into account. Many surgeons think the possible marginal clinical benefit of SLAP tear repair may not be worth the protracted rehabilitation. In most practices, rehabilitation after biceps tenodesis is less involved. Discussing the advantages and disadvantages of these 2 procedures can be helpful in decision making.
Dein and colleagues54 reported the case of a middle-aged pitcher who sustained a fracture after biceps tenodesis with an interference screw. Cases like this are concerning. Surgeons should consider altering the rehabilitation regimen when planning postoperative care in cases of biceps tenodesis in throwers. Other reported complications of open tenodesis are deep infection, thrombosis, postoperative stiffness, and nerve injury.55-58
Consequences for Overhead Throwers
The unknown role of the BLC leaves surgeons wary when considering biceps tenodesis for elite athletes. Some have postulated that removing the intra-articular portion of the LHB may cause microinstability and alter joint kinematics.10,59-61 Others have suggested the biceps is desynchronized from the other musculature and is not functionally important.62 Disruption of one portion of the superior labrum may result in instability on the opposite side of the glenoid.10,61 Biomechanical studies, both cadaveric and in vivo, have tried to create proper loads to the LHB and evaluate the kinematics of the shoulder before and after biceps tenodesis and SLAP tear repair.59,60 Using a cadaveric model, Strauss and colleagues63 found that type II SLAP lesions resulted in increased glenohumeral translation compared with baseline. Biceps tenodesis did not restore normal translation, but this did not negatively affect stability in the presence of a SLAP lesion. The consensus is that the role of the biceps is controversial at best.
Several studies have used electromyography (EMG) to evaluate LHB functioning. In 2014, Chalmers and colleagues59 used surface EMG and motion analysis to evaluate 18 pitchers: 6 underwent SLAP tear repair, 5 underwent biceps tenodesis, and 7 were uninjured controls. There were no significant differences in the activity of the LHB muscle, the short head of the biceps muscle, the deltoid, the infraspinatus, or the latissimus among the 3 groups. Motion analysis showed that the normal pattern of muscular activation within the LHB muscle was more closely restored by biceps tenodesis than by SLAP tear repair. In addition, thoracic rotation patterns were significantly more altered in the SLAP tear repair patients than in the uninjured controls. As the authors noted, given the low frequency with which biceps tenodesis is performed in overhead athletes, it is unlikely that larger scale studies will be conducted without a multicenter effort.
Recommendations and Our Preferred Technique
Which surgical option is best for treating symptomatic SLAP lesions in overhead athletes remains unclear. Many athletes struggle to return to high-level play after SLAP tear repair. Whether the same is true after biceps tenodesis is yet to be determined because of the low frequency with which biceps tenodesis is performed in high-level overhead athletes. The options for fixation, technique, and fixation location are equally broad. In this section, we outline our general line of thinking for cases of proximal biceps pathology.
In each case, we perform glenohumeral arthroscopy to evaluate the BLC and identify any other pathology. For overhead athletes who are younger than 30 years and lack bicipital groove pain or signs of gross tendinopathy, we favor arthroscopic SLAP tear repair. Repair is usually performed through an anterior working portal for suture passage and a Wilmington portal for anchor placement. We use knotless technology to achieve stable fixation and stay posterior to the biceps anchor insertion.
For the prevention of any potential pain from the bicipital groove in carefully selected patients—recreational overhead athletes and patients who want a less involved surgical recovery—we favor open subpectoral biceps tenodesis rather than arthroscopic tenodesis. The outcomes of biceps tenodesis are consistent, according to the literature.47,57,64 Moreover, the open approach is favored for the incidence of postoperative stiffness in the arthroscopic population.65 Tendons can be fixed with multiple procedures, including soft-tissue tenodesis, interference screw fixation, and surface anchors. We favor using a tenodesis screw in the subpectoral location, as outlined by Mazzocca and colleagues.64Our algorithm for SLAP lesions is evolving with our understanding of this complex disease process. For young overhead throwers with type II SLAP lesions, we favor arthroscopic SLAP tear repair with knotless technology. For older recreational overhead athletes, we favor biceps tenodesis in the subpectoral region after diagnostic arthroscopy plus biceps tenotomy with or without additional SLAP tear fixation, depending on the stability of the biceps anchor (Figures 4A, 4B).
Conclusion
Overhead athletes who present with symptomatic SLAP lesions often provide a treatment dilemma. Although SLAP tear repair historically has been standard treatment, biceps tenodesis represents a consistent surgical option with low complication rates and low revision rates. It is likely that, as additional data on glenohumeral kinematics and outcomes in young athletes become available, improved decision-making algorithms will follow.
Am J Orthop. 2017;46(1):E71-E78. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Take Home Points
- Outcomes after SLAP repair remain guarded.
- Physical examination is key in determining proper management of biceps pathology.
- When performing SLAP repair, knotless technology may prevent future cartilage or rotator cuff injury.
- Revision of SLAP repair is best handled with biceps tenodesis.
- Subpectoral biceps tenodesis avoids residual groove pain.
In recent decades, the long head of the biceps (LHB) tendon has been recognized as a pain generator in the shoulder of throwing athletes. The LHB muscle and its role in glenohumeral kinematics remains largely in question. The LHB tendon varies in size but most commonly is 5 mm to 6mm in diameter and about 9 cm in length, inserting on the superior labrum and supraglenoid tubercle after traveling through the bicipital groove.1 The many conditions that can develop along the course of the biceps tendon include overall biceps tendonitis, biceps tendon subluxation or instability, and injuries to the superior anterior to posterior area of the labrum.
These injuries can occur in young overhead athletes as well as manual laborers and older overhead recreational athletes. Pitching is the most common activity that leads to proximal biceps tendon disorders. The 6 phases of the pitch are linked in a kinetic chain that generates energy that is then translated to high velocity. The amount of force that is exerted on the shoulder during pitching and especially after ball release is impressive, and the athlete’s shoulder changes in many ways as it adapts to the motion.2-5 The late-cocking and deceleration phases are most commonly associated with proximal biceps pathology and the “peel-back” phenomenon. Other common activities that lead to biceps tendon issues in a young population are volleyball, baseball, tennis, softball, swimming, and cricket. Shoulder arthroscopies performed in older patients show degenerative biceps and labrum tears, which should be treated appropriately but perhaps different from how they are treated in overhead athletes.6-8 Further, many professional athletes have asymptomatic superior labrum anterior-posterior (SLAP) tears.9
Mechanism of Injury
Overhead throwing is commonly thought to be the mechanism by which lesions are created in the biceps–labrum complex (BLC). Pitching in particular generates incredible force and torque within the shoulder. In professional pitchers, the resulting throwing speed creates forces regularly in excess of 1000 N.3 These forces effect internal compensatory changes and internal derangement of the BLC. These changes often involve internal rotation deficits and alterations in the rotator cuff, which may contribute to glenohumeral instability and altered joint kinematics.10
Repetitive overhead activity is largely considered the mechanism of injury in this population, though more specific mechanisms have been described, including the peel-back mechanism11 and the posterior superior glenoid impingement. There is little evidence that preventive programs have any effect on decreasing the incidence of SLAP tears in overhead athletes.
Preoperative Evaluation
Preoperative evaluation is arguably the most important step in treating a patient with persistent or recurrent symptoms consistent with a SLAP tear. Evaluation includes thorough history, physical examination, and review of any prior injuries or surgical procedures. The physical examination should focus on maneuvers that define where the problem is occurring. Although SLAP tears are most common in this population, disorders of the biceps tendon within the groove, including inflammation and instability, should be ruled out with physical examination and advanced imaging. Palpation for groove tenderness, impingement-type complaints, internal rotation loss, and SLAP provocative testing are crucial in the diagnosis.12,13 The cause of symptoms may be multifactorial and include the often encountered concomitant pathology of rotator cuff tears, internal impingement, and instability.
Standard radiographs (Grashey anteroposterior, scapular/lateral, axillary lateral) and magnetic resonance imaging (MRI) with or without arthrography can be helpful in identifying and characterizing most SLAP tears as well as failed SLAP tear repairs. However, MRI is often positive for SLAP tears in asymptomatic patients, and diagnosing SLAP tears with MRI is often a challenge.14 MRI can help in determining concomitant pathology, including rotator cuff injury and cysts causing nerve compression. Correlation with clinical examination and patient history is most crucial. Conservative treatment (rest, activity modification, use of oral anti-inflammatory medications) typically is attempted and coordinated with respect to the athlete’s season of play.15,16
Classification
In overhead throwing athletes, SLAP tears typically are associated with anterior shoulder pain. Associated shoulder instability and significant glenohumeral dysfunction are not uncommon in athletes with lesions of the BLC. In 1985, Andrews and colleagues17 were the first to describe SLAP tears in overhead athletes (73 patients). Later, Snyder and colleagues18,19 further classified these lesions into 4 types based on tear stability and location, and they coined the acronym SLAP (Figure 1).
Type I lesions typically are described as fraying at the inner margin of the labrum and are common in throwers, even asymptomatic throwers. Type II lesions, separations of the biceps and labrum from the superior glenoid (≥5 mm of excursion), are the most commonly occurring and treated variant in throwing athletes.20-22 Intraoperative evaluation for a peel-back lesion (placing the arm in abduction with external rotation), rather than for a sulcus of 1 mm to 2 mm, may confirm a type II SLAP tear.20,23,24 It is often important to consider the direction of tear propagation as well. Type III lesions include those with an intact BLC (but with a bucket-handle tear of the superior labral complex and an intact biceps tendon), whereas type IV lesions involve additional extension of the tear into the biceps tendon.18,19The classification systems are well defined. Nevertheless, management of SLAP lesions remains controversial.
Options for Surgical Treatment
SLAP Tear Repair—Outcomes
The incidence of SLAP tear repairs has increased dramatically in recent years.6,25 There are various SLAP tear repair methods, but the most common consists of repairing the labrum and biceps anchor. Management of type II SLAP lesions remains controversial. Several prospective studies have found overall improvement after SLAP tear repair.26-31 Other series have reported less encouraging outcomes, including dissatisfaction with persistent pain and inability to return to throwing.28,32 A 2010 systematic review found that the percentage of patients who returned to their preinjury level of play was only 64%, and outcomes for overhead throwing athletes were even worse—only 22% to 60% of these patients returned to their previous level.33 The right surgery for SLAP tears in this population continues to be an area of uncertainty for many surgeons.
Failed SLAP tear repairs (poor outcomes) have become common in overhead throwing athletes. The reasons for these failed repairs are unclear, but several possible explanations have been offered. One is that labral repair may result in permanent alterations in pitching biomechanics, which may lead to an inability to regain velocity and command.3 Another is that the athlete’s shoulder may remain unstable even after repair.10Hardware complications are a significant concern in this high-level population. Suture anchor pullout or iatrogenic cartilage damage may occur during instrumentation or as a result of suture anchor reactive changes. In addition, there are several reports of glenoid osteochondrolysis (Figure 2) caused by prominent hardware or prominent knots.34-39
Stiffness after SLAP tear repair is a significant problem, with most patients taking up to 6 months to regain full motion.26,48 Overtensioning of the labrum and the glenohumeral ligaments may be the cause, and the solution may be to place anchors posterior (vs anterior) to the biceps insertion. In a large prospective military study, mean forward flexion and external rotation were reduced at final follow-up.31 These outcomes are less acceptable to overhead throwing athletes, who rely on motion for high-end throwing activities.
Primary Biceps Tenodesis—Outcomes
A 2015 database study found a 1.7-fold increase in biceps tenodesis over the preceding 5 years.49 However, relatively few procedures included in the study were performed in patients age younger than 30 years. For many older non-overhead throwers with type II tears, SLAP tear repair has become less popular as a treatment option.32 There is a dearth of knowledge about the outcomes of subpectoral biceps tenodesis as a primary treatment for biceps tendonitis and an associated SLAP tear. Although type I tears historically have been treated with débridement, débridement is seldom used for concomitant biceps tendonitis. It should be coupled with careful clinical examination.
In recent years, biceps tenodesis has been proposed as an alternative to repair for SLAP tears, particularly in older patients.24,44 For obvious reasons, however, there has been some trepidation about performing biceps tenodesis in throwing athletes. Some authors have proposed biceps tenodesis as primary treatment for isolated SLAP tears. Boileau and colleagues44 compared the outcomes of treatment of isolated type II SLAP lesions in 25 consecutive patients. For 10 patients, repair involved suture anchors; for the other 15, arthroscopic biceps tenodesis was performed with an absorbable interference screw. Six of the 10 suture anchor patients were disappointed with their outcome (persistent pain or inability to return to sport), whereas 14 of the 15 biceps tenodesis patients were satisfied. The authors concluded that arthroscopic biceps tenodesis is an effective alternative to repair for type II SLAP lesions, though their study was not isolated to overhead athletes (tenodesis group mean age, 52 years).
In a 2014 series of cases, Ek and colleagues50 reported good outcomes of SLAP tear repair and biceps tenodesis. Again, though, tenodesis was used in older patients, and repair in younger, more active patients, with no high-level athletes in either group. There was no difference in return to sport between groups. In a study of patients who underwent primary biceps tenodesis, Gupta and colleagues51 found 80% excellent outcomes (improved shoulder outcome scores) in select SLAP tear patients, including 8 athletes, 88% of whom were overhead athletes. Gottschalk and colleagues52 reported on differences in prospectively collected outcome data (age, sex, SLAP lesion type II or IV) for primary biceps tenodesis in a series of 33 patients. Twenty-six of the 29 patients who completed follow-up returned to their previous level of activity. These studies suggest that primary biceps tenodesis may be an alternative with lower failure rates in the treatment of SLAP tears in middle-aged patients, and in overhead athletes, though additional specific studies are needed to focus on overhead athletes on a larger scale.
Revision SLAP Tear Repair Versus Biceps Tenodesis
Failed arthroscopic SLAP tear repairs, which are increasingly common, present a unique treatment challenge. In a 2013 prospective cohort series, Gupta and colleagues46 found excellent clinical outcomes of subpectoral biceps tenodesis for failed type II SLAP tears. The authors reported a postoperative SANE (Single Assessment Numeric Evaluation) score of 70.4%, an SST (Simple Shoulder Test) score of 9.33, and an ASES (American Shoulder and Elbow Surgeons) score of 77.96, along with reasonable health-related quality-of-life scores. Werner and colleagues53 evaluated 2-year outcomes of biceps tenodesis performed after SLAP tear repair in 24 patients and found a return to almost normal range of motion as well as good clinical outcome scores. Significantly worse outcomes were found for patients with open worker’s compensation claims.
McCormick and colleagues26 prospectively evaluated the efficacy of biceps tenodesis for failed type II SLAP tear repair in 46 patients. Improvement was noted across all outcome assessments during follow-up (mean, 3.6 years). From these findings, we might conclude that biceps tenodesis is a more predictable option for failed SLAP tear repair, and that it has a relatively low complication rate. However, most investigators have used a heterogeneous patient population, as opposed to overhead athletes specifically. To our knowledge, no one has evaluated the specific population of overhead throwers with failed SLAP tear repairs. In addition, no one has conducted randomized controlled trials comparing débridement, biceps tenodesis, and repair for failed SLAP tear repairs.
Postoperative Considerations
When overhead athletes and their surgeons are considering surgical options, they must take rehabilitation and return to play into account. Many surgeons think the possible marginal clinical benefit of SLAP tear repair may not be worth the protracted rehabilitation. In most practices, rehabilitation after biceps tenodesis is less involved. Discussing the advantages and disadvantages of these 2 procedures can be helpful in decision making.
Dein and colleagues54 reported the case of a middle-aged pitcher who sustained a fracture after biceps tenodesis with an interference screw. Cases like this are concerning. Surgeons should consider altering the rehabilitation regimen when planning postoperative care in cases of biceps tenodesis in throwers. Other reported complications of open tenodesis are deep infection, thrombosis, postoperative stiffness, and nerve injury.55-58
Consequences for Overhead Throwers
The unknown role of the BLC leaves surgeons wary when considering biceps tenodesis for elite athletes. Some have postulated that removing the intra-articular portion of the LHB may cause microinstability and alter joint kinematics.10,59-61 Others have suggested the biceps is desynchronized from the other musculature and is not functionally important.62 Disruption of one portion of the superior labrum may result in instability on the opposite side of the glenoid.10,61 Biomechanical studies, both cadaveric and in vivo, have tried to create proper loads to the LHB and evaluate the kinematics of the shoulder before and after biceps tenodesis and SLAP tear repair.59,60 Using a cadaveric model, Strauss and colleagues63 found that type II SLAP lesions resulted in increased glenohumeral translation compared with baseline. Biceps tenodesis did not restore normal translation, but this did not negatively affect stability in the presence of a SLAP lesion. The consensus is that the role of the biceps is controversial at best.
Several studies have used electromyography (EMG) to evaluate LHB functioning. In 2014, Chalmers and colleagues59 used surface EMG and motion analysis to evaluate 18 pitchers: 6 underwent SLAP tear repair, 5 underwent biceps tenodesis, and 7 were uninjured controls. There were no significant differences in the activity of the LHB muscle, the short head of the biceps muscle, the deltoid, the infraspinatus, or the latissimus among the 3 groups. Motion analysis showed that the normal pattern of muscular activation within the LHB muscle was more closely restored by biceps tenodesis than by SLAP tear repair. In addition, thoracic rotation patterns were significantly more altered in the SLAP tear repair patients than in the uninjured controls. As the authors noted, given the low frequency with which biceps tenodesis is performed in overhead athletes, it is unlikely that larger scale studies will be conducted without a multicenter effort.
Recommendations and Our Preferred Technique
Which surgical option is best for treating symptomatic SLAP lesions in overhead athletes remains unclear. Many athletes struggle to return to high-level play after SLAP tear repair. Whether the same is true after biceps tenodesis is yet to be determined because of the low frequency with which biceps tenodesis is performed in high-level overhead athletes. The options for fixation, technique, and fixation location are equally broad. In this section, we outline our general line of thinking for cases of proximal biceps pathology.
In each case, we perform glenohumeral arthroscopy to evaluate the BLC and identify any other pathology. For overhead athletes who are younger than 30 years and lack bicipital groove pain or signs of gross tendinopathy, we favor arthroscopic SLAP tear repair. Repair is usually performed through an anterior working portal for suture passage and a Wilmington portal for anchor placement. We use knotless technology to achieve stable fixation and stay posterior to the biceps anchor insertion.
For the prevention of any potential pain from the bicipital groove in carefully selected patients—recreational overhead athletes and patients who want a less involved surgical recovery—we favor open subpectoral biceps tenodesis rather than arthroscopic tenodesis. The outcomes of biceps tenodesis are consistent, according to the literature.47,57,64 Moreover, the open approach is favored for the incidence of postoperative stiffness in the arthroscopic population.65 Tendons can be fixed with multiple procedures, including soft-tissue tenodesis, interference screw fixation, and surface anchors. We favor using a tenodesis screw in the subpectoral location, as outlined by Mazzocca and colleagues.64Our algorithm for SLAP lesions is evolving with our understanding of this complex disease process. For young overhead throwers with type II SLAP lesions, we favor arthroscopic SLAP tear repair with knotless technology. For older recreational overhead athletes, we favor biceps tenodesis in the subpectoral region after diagnostic arthroscopy plus biceps tenotomy with or without additional SLAP tear fixation, depending on the stability of the biceps anchor (Figures 4A, 4B).
Conclusion
Overhead athletes who present with symptomatic SLAP lesions often provide a treatment dilemma. Although SLAP tear repair historically has been standard treatment, biceps tenodesis represents a consistent surgical option with low complication rates and low revision rates. It is likely that, as additional data on glenohumeral kinematics and outcomes in young athletes become available, improved decision-making algorithms will follow.
Am J Orthop. 2017;46(1):E71-E78. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Elser F, Braun S, Dewing CB, Giphart JE, Millett PJ. Anatomy, function, injuries, and treatment of the long head of the biceps brachii tendon. Arthroscopy. 2011;27(4):581-592.
2. Fedoriw WW, Ramkumar P, McCulloch PC, Lintner DM. Return to play after treatment of superior labral tears in professional baseball players. Am J Sports Med. 2014;42(5):1155-1160.
3. Fleisig GS, Andrews JR, Dillman CJ, Escamilla RF. Kinetics of baseball pitching with implications about injury mechanisms. Am J Sports Med. 1995;23(2):233-239.
4. Aydin N, Sirin E, Arya A. Superior labrum anterior to posterior lesions of the shoulder: diagnosis and arthroscopic management. World J Orthop. 2014;5(3):344-350.
5. Barber A, Field LD, Ryu R. Biceps tendon and superior labrum injuries: decision-marking. J Bone Joint Surg Am. 2007;89(8):1844-1855.
6. Onyekwelu I, Khatib O, Zuckerman JD, Rokito AS, Kwon YW. The rising incidence of arthroscopic superior labrum anterior and posterior (SLAP) repairs. J Shoulder Elbow Surg. 2012;21(6):728-731.
7. Patterson BM, Creighton RA, Spang JT, Roberson JR, Kamath GV. Surgical trends in the treatment of superior labrum anterior and posterior lesions of the shoulder: analysis of data from the American Board of Orthopaedic Surgery Certification Examination Database. Am J Sports Med. 2014;42(8):1904-1910.
8. Walton DM, Sadi J. Identifying SLAP lesions: a meta-analysis of clinical tests and exercise in clinical reasoning. Phys Ther Sport. 2008;9(4):167-176.
9. Lesniak BP, Baraga MG, Jose J, Smith MK, Cunningham S, Kaplan LD. Glenohumeral findings on magnetic resonance imaging correlate with innings pitched in asymptomatic pitchers. Am J Sports Med. 2013;41(9):2022-2027.
10. Mihata T, McGarry MH, Tibone JE, Fitzpatrick MJ, Kinoshita M, Lee TQ. Biomechanical assessment of type II superior labral anterior-posterior (SLAP) lesions associated with anterior shoulder capsular laxity as seen in throwers: a cadaveric study. Am J Sports Med. 2008;36(8):1604-1610.
11. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology. Part II: evaluation and treatment of SLAP lesions in throwers. Arthroscopy. 2003;19(5):531-539.
12. Meserve BB, Cleland JA, Boucher TR. A meta-analysis examining clinical test utility for assessing superior labral anterior posterior lesions. Am J Sports Med. 2009;37(11):2252-2258.
13. Pandya NK, Colton A, Webner D, Sennett B, Huffman GR. Physical examination and magnetic resonance imaging in the diagnosis of superior labrum anterior-posterior lesions of the shoulder: a sensitivity analysis. Arthroscopy. 2008;24(3):311-317.
14. Amin MF, Youssef AO. The diagnostic value of magnetic resonance arthrography of the shoulder in detection and grading of SLAP lesions: comparison with arthroscopic findings. Eur J Radiol. 2012;81(9):2343-2347.
15. Cook C, Beaty S, Kissenberth MJ, Siffri P, Pill SG, Hawkins RJ. Diagnostic accuracy of five orthopedic clinical tests for diagnosis of superior labrum anterior posterior (SLAP) lesions. J Shoulder Elbow Surg. 2012;21(1):13-22.
16. Edwards SL, Lee JA, Bell JE, et al. Nonoperative treatment of superior labrum anterior posterior tears: improvements in pain, function, and quality of life. Am J Sports Med. 2010;38(7):1456-1461.
17. Andrews JR, Carson WG Jr, McLeod WD. Glenoid labrum tears related to the long head of the biceps. Am J Sports Med. 1985;13(5):337-341.
18. Snyder SJ, Karzel RP, Del Pizzo W, Ferkel RD, Friedman MJ. SLAP lesions of the shoulder. Arthroscopy. 1990;6(4):274-279.
19. Snyder SJ, Banas MP, Karzel RP. An analysis of 140 injuries to the superior glenoid labrum. J Shoulder Elbow Surg. 1995;4(4):243-248.
20. Morgan CD, Burkhart SS, Palmeri M, Gillespie M. Type II SLAP lesions: three subtypes and their relationships to superior instability and rotator cuff tears. Arthroscopy. 1998;14(6):553-565.
21. Weber SC, Martin DF, Seiler JG 3rd, Harrast JJ. Superior labrum anterior and posterior lesions of the shoulder: incidence rates, complications, and outcomes as reported by American Board of Orthopedic Surgery. Part II candidates. Am J Sports Med. 2012;40(7):1538-1543.
22. Keener JD, Brophy RH. Superior labral tears of the shoulder: pathogenesis, evaluation, and treatment. J Am Acad Orthop Surg. 2009;17(10):627-637.
23. Chen CH, Hsu KY, Chen WJ, Shih CH. Incidence and severity of biceps long head tendon lesion in patients with complete rotator cuff tears. J Trauma. 2005;58(6):1189-1193.
24. Nho SJ, Strauss EJ, Lenart BA, et al. Long head of the biceps tendinopathy: diagnosis and management. J Am Acad Orthop Surg. 2010;18(11):645-656.
25. Zhang AL, Kreulen C, Ngo SS, Hame SL, Wang JC, Gamradt SC. Demographic trends in arthroscopic SLAP repair in the United States. Am J Sports Med. 2012;40(5):1144-1147.
26. McCormick F, Bhatia S, Chalmers P, Gupta A, Verma N, Romeo AA. The management of type II superior labral anterior to posterior injuries. Orthop Clin North Am. 2014;45(1):121-128.
27. Brockmeier SF, Voos JE, Williams RJ 3rd, Altchek DW, Cordasco FA, Allen AA; Hospital for Special Surgery Sports Medicine and Shoulder Service. Outcomes after arthroscopic repair of type-II SLAP lesions. J Bone Joint Surg Am. 2009;91(7):1595-1603.
28. Boileau P, Parratte S, Chuinard C, Roussanne Y, Shia D, Bicknell R. Arthroscopic treatment of isolated type II SLAP lesions: biceps tenodesis as an alternative to reinsertion. Am J Sports Med. 2009;37(5):929-936.
29. Denard PJ, Lädermann A, Burkhart SS. Long-term outcome after arthroscopic repair of type II SLAP lesions: results according to age and workers’ compensation status. Arthroscopy. 2012;28(4):451-457.
30. Friel NA, Karas V, Slabaugh MA, Cole BJ. Outcomes of type II superior labrum, anterior to posterior (SLAP) repair: prospective evaluation at a minimum two-year follow-up. J Shoulder Elbow Surg. 2010;19(6):859-867.
31. Provencher MT, McCormick F, Dewing C, McIntire S, Solomon D. A prospective analysis of 179 type 2 superior labrum anterior and posterior repairs: outcomes and factors associated with success and failure. Am J Sports Med. 2013;41(4):880-886.
32. Gupta AK, Chalmers PN, Klosterman EL, et al. Subpectoral biceps tenodesis for bicipital tendonitis with SLAP tear. Orthopedics. 2015;38(1):e48-e53.
33. Gorantla K, Gill C, Wright RW. The outcome of type II SLAP repair: a systematic review. Arthroscopy. 2010;26(4):537-545.
34. Katz LM, Hsu S, Miller SL, et al. Poor outcomes after SLAP repair: descriptive analysis and prognosis. Arthroscopy. 2009;25(8):849-855.
35. Park MJ, Hsu JE, Harper C, Sennett BJ, Huffman GR. Poly-L/D-lactic acid anchors are associated with reoperation and failure of SLAP repairs. Arthroscopy. 2011;27(10):1335-1340.
36. Sassmannshausen G, Sukay M, Mair SD. Broken or dislodged poly-L-lactic acid bioabsorbable tacks in patients after SLAP lesion surgery. Arthroscopy. 2006;22(6):615-619.
37. Uggen C, Wei A, Glousman RE, et al. Biomechanical comparison of knotless anchor repair versus simple suture repair for type II SLAP lesions. Arthroscopy. 2009;25(10):1085-1092.
38. Weber SC. Surgical management of the failed SLAP repair. Sports Med Arthrosc. 2010;18(3):162-166.
39. Wilkerson JP, Zvijac JE, Uribe JW, Schürhoff MR, Green JB. Failure of polymerized lactic acid tacks in shoulder surgery. J Shoulder Elbow Surg. 2003;12(2):117-121.
40. Weber S. Surgical management of the failed SLAP lesion. Arthroscopy. 2008;24(suppl):e8-e9.
41. Schrøder CP, Skare O, Gjengedal E, Uppheim G, Reikerås O, Brox JI. Long-term results after SLAP repair: a 5-year follow-up study of 107 patients with comparison of patients aged over and under 40 years. Arthroscopy. 2012;28(11):1601-1607.
42. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
43. Mazzocca AD, McCarthy MB, Ledgard FA, et al. Histomorphologic changes of the long head of the biceps tendon in common shoulder pathologies. Arthroscopy. 2013;29(6):972-981.
44. Boileau P, Parratte S, Chuinard C, Roussanne Y, Shia D, Bicknell R. Arthroscopic treatment of isolated type II SLAP lesions: biceps tenodesis as an alternative to reinsertion. Am J Sports Med. 2009;37(5):929-936.
45. Boileau P, Krishnan SG, Coste JS, Walch G. Arthroscopic biceps tenodesis: a new technique using bioabsorbable interference screw fixation. Arthroscopy. 2002;18(9):1002-1012.
46. Gupta AK, Bruce B, Klosterman EL, McCormick F, Harris J, Romeo AA. Subpectoral biceps tenodesis for failed type II SLAP repair. Orthopedics. 2013;36(6):e723-e728.
47. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc. 2008;16(3):170-176.
48. McCarty LP 3rd, Buss DD, Datta MW, Freehill MQ, Giveans MR. Complications observed following labral or rotator cuff repair with use of poly-L-lactic acid implants. J Bone Joint Surg Am. 2013;95(6):507-511.
49. Werner BC, Brockmeier SF, Gwathmey FW. Trends in long head biceps tenodesis. Am J Sports Med. 2015;43(3):570-578.
50. Ek ET, Shi LL, Tompson JD, Freehill MT, Warner JJ. Surgical treatment of isolated type II superior labrum anterior-posterior (SLAP) lesions: repair versus biceps tenodesis. J Shoulder Elbow Surg. 2014;23(7):1059-1065.
51. Gupta AK, Chalmers PN, Klosterman EL, et al. Subpectoral biceps tenodesis for bicipital tendonitis with SLAP tear. Orthopedics. 2015;38(1):e48-e53.
52. Gottschalk MB, Karas SG, Ghattas TN, Burdette R. Subpectoral biceps tenodesis for the treatment of type II and IV superior labral anterior and posterior lesions. Am J Sports Med. 2014;42(9):2128-2135.
53. Werner BC, Pehlivan HC, Hart JM, et al. Biceps tenodesis is a viable option for salvage of failed SLAP repair. J Shoulder Elbow Surg. 2014;23(8):e179-e184.
54. Dein EJ, Huri G, Gordon JC, McFarland EG. A humerus fracture in a baseball pitcher after biceps tenodesis [published correction appears in Am J Sports Med. 2014;42(6):NP39]. Am J Sports Med. 2014;42(4):877-879.
55. Nho SJ, Reiff SN, Verma NN, Slabaugh MA, Mazzocca AD, Romeo AA. Complications associated with subpectoral biceps tenodesis: low rates of incidence following surgery. J Shoulder Elbow Surg. 2010;19(5):764-768.
56. Osbahr DC, Diamond AB, Speer KP. The cosmetic appearance of the biceps muscle after long-head tenotomy versus tenodesis. Arthroscopy. 2002;18(5):483-487.
57. Romeo AA, Mazzocca AD, Tauro JC. Arthroscopic biceps tenodesis. Arthroscopy. 2004;20(2):206-213.
58. Ma H, Van Heest A, Glisson C, Patel S. Musculocutaneous nerve entrapment: an unusual complication after biceps tenodesis. Am J Sports Med. 2009;37(12):2467-2469.
59. Chalmers PN, Trombley R, Cip J, et al. Postoperative restoration of upper extremity motion and neuromuscular control during the overhand pitch: evaluation of tenodesis and repair for superior labral anterior-posterior tears. Am J Sports Med. 2014;42(12):2825-2836.
60. Giphart JE, Elser F, Dewing CB, Torry MR, Millett PJ. The long head of the biceps tendon has minimal effect on in vivo glenohumeral kinematics: a biplane fluoroscopy study. Am J Sports Med. 2012;40(1):202-212.
61. Grossman MG, Tibone JE, McGarry MH, Schneider DJ, Veneziani S, Lee TQ. A cadaveric model of the throwing shoulder: a possible etiology of superior labrum anterior-to-posterior lesions. J Bone Joint Surg Am. 2005;87(4):824-831.
62. Hawkes DH, Alizadehkhaiyat O, Fisher AC, Kemp GJ, Roebuck MM, Frostick SP. Normal shoulder muscular activation and co-ordination during a shoulder elevation task based on activities of daily living: an electromyographic study. J Orthop Res. 2012;30(1):53-60.
63. Strauss EJ, Salata MJ, Sershon RA, et al. Role of the superior labrum after biceps tenodesis in glenohumeral stability. J Shoulder Elbow Surg. 2014;23(4):485-491.
64. Mazzocca AD, Bicos J, Santangelo S, Romeo AA, Arciero RA. The biomechanical evaluation of four fixation techniques for proximal biceps tenodesis. Arthroscopy. 2005;21(11):1296-1306.
65. Werner BC, Pehlivan HC, Hart JM, et al. Increased incidence of postoperative stiffness after arthroscopic compared with open biceps tenodesis. Arthroscopy. 2014;30(9):1075-1084.
66. Denard PJ, Dai X, Hanypsiak BT, Burkhart SS. Anatomy of the biceps tendon: implications for restoring physiological length–tension relation during biceps tenodesis with interference screw fixation. Arthroscopy. 2012;28(10):1352-1358.
67. Mazzocca AD, Rios CG, Romeo AA, Arciero RA. Subpectoral biceps tenodesis with interference screw fixation. Arthroscopy. 2005;21(7):896.
1. Elser F, Braun S, Dewing CB, Giphart JE, Millett PJ. Anatomy, function, injuries, and treatment of the long head of the biceps brachii tendon. Arthroscopy. 2011;27(4):581-592.
2. Fedoriw WW, Ramkumar P, McCulloch PC, Lintner DM. Return to play after treatment of superior labral tears in professional baseball players. Am J Sports Med. 2014;42(5):1155-1160.
3. Fleisig GS, Andrews JR, Dillman CJ, Escamilla RF. Kinetics of baseball pitching with implications about injury mechanisms. Am J Sports Med. 1995;23(2):233-239.
4. Aydin N, Sirin E, Arya A. Superior labrum anterior to posterior lesions of the shoulder: diagnosis and arthroscopic management. World J Orthop. 2014;5(3):344-350.
5. Barber A, Field LD, Ryu R. Biceps tendon and superior labrum injuries: decision-marking. J Bone Joint Surg Am. 2007;89(8):1844-1855.
6. Onyekwelu I, Khatib O, Zuckerman JD, Rokito AS, Kwon YW. The rising incidence of arthroscopic superior labrum anterior and posterior (SLAP) repairs. J Shoulder Elbow Surg. 2012;21(6):728-731.
7. Patterson BM, Creighton RA, Spang JT, Roberson JR, Kamath GV. Surgical trends in the treatment of superior labrum anterior and posterior lesions of the shoulder: analysis of data from the American Board of Orthopaedic Surgery Certification Examination Database. Am J Sports Med. 2014;42(8):1904-1910.
8. Walton DM, Sadi J. Identifying SLAP lesions: a meta-analysis of clinical tests and exercise in clinical reasoning. Phys Ther Sport. 2008;9(4):167-176.
9. Lesniak BP, Baraga MG, Jose J, Smith MK, Cunningham S, Kaplan LD. Glenohumeral findings on magnetic resonance imaging correlate with innings pitched in asymptomatic pitchers. Am J Sports Med. 2013;41(9):2022-2027.
10. Mihata T, McGarry MH, Tibone JE, Fitzpatrick MJ, Kinoshita M, Lee TQ. Biomechanical assessment of type II superior labral anterior-posterior (SLAP) lesions associated with anterior shoulder capsular laxity as seen in throwers: a cadaveric study. Am J Sports Med. 2008;36(8):1604-1610.
11. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology. Part II: evaluation and treatment of SLAP lesions in throwers. Arthroscopy. 2003;19(5):531-539.
12. Meserve BB, Cleland JA, Boucher TR. A meta-analysis examining clinical test utility for assessing superior labral anterior posterior lesions. Am J Sports Med. 2009;37(11):2252-2258.
13. Pandya NK, Colton A, Webner D, Sennett B, Huffman GR. Physical examination and magnetic resonance imaging in the diagnosis of superior labrum anterior-posterior lesions of the shoulder: a sensitivity analysis. Arthroscopy. 2008;24(3):311-317.
14. Amin MF, Youssef AO. The diagnostic value of magnetic resonance arthrography of the shoulder in detection and grading of SLAP lesions: comparison with arthroscopic findings. Eur J Radiol. 2012;81(9):2343-2347.
15. Cook C, Beaty S, Kissenberth MJ, Siffri P, Pill SG, Hawkins RJ. Diagnostic accuracy of five orthopedic clinical tests for diagnosis of superior labrum anterior posterior (SLAP) lesions. J Shoulder Elbow Surg. 2012;21(1):13-22.
16. Edwards SL, Lee JA, Bell JE, et al. Nonoperative treatment of superior labrum anterior posterior tears: improvements in pain, function, and quality of life. Am J Sports Med. 2010;38(7):1456-1461.
17. Andrews JR, Carson WG Jr, McLeod WD. Glenoid labrum tears related to the long head of the biceps. Am J Sports Med. 1985;13(5):337-341.
18. Snyder SJ, Karzel RP, Del Pizzo W, Ferkel RD, Friedman MJ. SLAP lesions of the shoulder. Arthroscopy. 1990;6(4):274-279.
19. Snyder SJ, Banas MP, Karzel RP. An analysis of 140 injuries to the superior glenoid labrum. J Shoulder Elbow Surg. 1995;4(4):243-248.
20. Morgan CD, Burkhart SS, Palmeri M, Gillespie M. Type II SLAP lesions: three subtypes and their relationships to superior instability and rotator cuff tears. Arthroscopy. 1998;14(6):553-565.
21. Weber SC, Martin DF, Seiler JG 3rd, Harrast JJ. Superior labrum anterior and posterior lesions of the shoulder: incidence rates, complications, and outcomes as reported by American Board of Orthopedic Surgery. Part II candidates. Am J Sports Med. 2012;40(7):1538-1543.
22. Keener JD, Brophy RH. Superior labral tears of the shoulder: pathogenesis, evaluation, and treatment. J Am Acad Orthop Surg. 2009;17(10):627-637.
23. Chen CH, Hsu KY, Chen WJ, Shih CH. Incidence and severity of biceps long head tendon lesion in patients with complete rotator cuff tears. J Trauma. 2005;58(6):1189-1193.
24. Nho SJ, Strauss EJ, Lenart BA, et al. Long head of the biceps tendinopathy: diagnosis and management. J Am Acad Orthop Surg. 2010;18(11):645-656.
25. Zhang AL, Kreulen C, Ngo SS, Hame SL, Wang JC, Gamradt SC. Demographic trends in arthroscopic SLAP repair in the United States. Am J Sports Med. 2012;40(5):1144-1147.
26. McCormick F, Bhatia S, Chalmers P, Gupta A, Verma N, Romeo AA. The management of type II superior labral anterior to posterior injuries. Orthop Clin North Am. 2014;45(1):121-128.
27. Brockmeier SF, Voos JE, Williams RJ 3rd, Altchek DW, Cordasco FA, Allen AA; Hospital for Special Surgery Sports Medicine and Shoulder Service. Outcomes after arthroscopic repair of type-II SLAP lesions. J Bone Joint Surg Am. 2009;91(7):1595-1603.
28. Boileau P, Parratte S, Chuinard C, Roussanne Y, Shia D, Bicknell R. Arthroscopic treatment of isolated type II SLAP lesions: biceps tenodesis as an alternative to reinsertion. Am J Sports Med. 2009;37(5):929-936.
29. Denard PJ, Lädermann A, Burkhart SS. Long-term outcome after arthroscopic repair of type II SLAP lesions: results according to age and workers’ compensation status. Arthroscopy. 2012;28(4):451-457.
30. Friel NA, Karas V, Slabaugh MA, Cole BJ. Outcomes of type II superior labrum, anterior to posterior (SLAP) repair: prospective evaluation at a minimum two-year follow-up. J Shoulder Elbow Surg. 2010;19(6):859-867.
31. Provencher MT, McCormick F, Dewing C, McIntire S, Solomon D. A prospective analysis of 179 type 2 superior labrum anterior and posterior repairs: outcomes and factors associated with success and failure. Am J Sports Med. 2013;41(4):880-886.
32. Gupta AK, Chalmers PN, Klosterman EL, et al. Subpectoral biceps tenodesis for bicipital tendonitis with SLAP tear. Orthopedics. 2015;38(1):e48-e53.
33. Gorantla K, Gill C, Wright RW. The outcome of type II SLAP repair: a systematic review. Arthroscopy. 2010;26(4):537-545.
34. Katz LM, Hsu S, Miller SL, et al. Poor outcomes after SLAP repair: descriptive analysis and prognosis. Arthroscopy. 2009;25(8):849-855.
35. Park MJ, Hsu JE, Harper C, Sennett BJ, Huffman GR. Poly-L/D-lactic acid anchors are associated with reoperation and failure of SLAP repairs. Arthroscopy. 2011;27(10):1335-1340.
36. Sassmannshausen G, Sukay M, Mair SD. Broken or dislodged poly-L-lactic acid bioabsorbable tacks in patients after SLAP lesion surgery. Arthroscopy. 2006;22(6):615-619.
37. Uggen C, Wei A, Glousman RE, et al. Biomechanical comparison of knotless anchor repair versus simple suture repair for type II SLAP lesions. Arthroscopy. 2009;25(10):1085-1092.
38. Weber SC. Surgical management of the failed SLAP repair. Sports Med Arthrosc. 2010;18(3):162-166.
39. Wilkerson JP, Zvijac JE, Uribe JW, Schürhoff MR, Green JB. Failure of polymerized lactic acid tacks in shoulder surgery. J Shoulder Elbow Surg. 2003;12(2):117-121.
40. Weber S. Surgical management of the failed SLAP lesion. Arthroscopy. 2008;24(suppl):e8-e9.
41. Schrøder CP, Skare O, Gjengedal E, Uppheim G, Reikerås O, Brox JI. Long-term results after SLAP repair: a 5-year follow-up study of 107 patients with comparison of patients aged over and under 40 years. Arthroscopy. 2012;28(11):1601-1607.
42. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
43. Mazzocca AD, McCarthy MB, Ledgard FA, et al. Histomorphologic changes of the long head of the biceps tendon in common shoulder pathologies. Arthroscopy. 2013;29(6):972-981.
44. Boileau P, Parratte S, Chuinard C, Roussanne Y, Shia D, Bicknell R. Arthroscopic treatment of isolated type II SLAP lesions: biceps tenodesis as an alternative to reinsertion. Am J Sports Med. 2009;37(5):929-936.
45. Boileau P, Krishnan SG, Coste JS, Walch G. Arthroscopic biceps tenodesis: a new technique using bioabsorbable interference screw fixation. Arthroscopy. 2002;18(9):1002-1012.
46. Gupta AK, Bruce B, Klosterman EL, McCormick F, Harris J, Romeo AA. Subpectoral biceps tenodesis for failed type II SLAP repair. Orthopedics. 2013;36(6):e723-e728.
47. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc. 2008;16(3):170-176.
48. McCarty LP 3rd, Buss DD, Datta MW, Freehill MQ, Giveans MR. Complications observed following labral or rotator cuff repair with use of poly-L-lactic acid implants. J Bone Joint Surg Am. 2013;95(6):507-511.
49. Werner BC, Brockmeier SF, Gwathmey FW. Trends in long head biceps tenodesis. Am J Sports Med. 2015;43(3):570-578.
50. Ek ET, Shi LL, Tompson JD, Freehill MT, Warner JJ. Surgical treatment of isolated type II superior labrum anterior-posterior (SLAP) lesions: repair versus biceps tenodesis. J Shoulder Elbow Surg. 2014;23(7):1059-1065.
51. Gupta AK, Chalmers PN, Klosterman EL, et al. Subpectoral biceps tenodesis for bicipital tendonitis with SLAP tear. Orthopedics. 2015;38(1):e48-e53.
52. Gottschalk MB, Karas SG, Ghattas TN, Burdette R. Subpectoral biceps tenodesis for the treatment of type II and IV superior labral anterior and posterior lesions. Am J Sports Med. 2014;42(9):2128-2135.
53. Werner BC, Pehlivan HC, Hart JM, et al. Biceps tenodesis is a viable option for salvage of failed SLAP repair. J Shoulder Elbow Surg. 2014;23(8):e179-e184.
54. Dein EJ, Huri G, Gordon JC, McFarland EG. A humerus fracture in a baseball pitcher after biceps tenodesis [published correction appears in Am J Sports Med. 2014;42(6):NP39]. Am J Sports Med. 2014;42(4):877-879.
55. Nho SJ, Reiff SN, Verma NN, Slabaugh MA, Mazzocca AD, Romeo AA. Complications associated with subpectoral biceps tenodesis: low rates of incidence following surgery. J Shoulder Elbow Surg. 2010;19(5):764-768.
56. Osbahr DC, Diamond AB, Speer KP. The cosmetic appearance of the biceps muscle after long-head tenotomy versus tenodesis. Arthroscopy. 2002;18(5):483-487.
57. Romeo AA, Mazzocca AD, Tauro JC. Arthroscopic biceps tenodesis. Arthroscopy. 2004;20(2):206-213.
58. Ma H, Van Heest A, Glisson C, Patel S. Musculocutaneous nerve entrapment: an unusual complication after biceps tenodesis. Am J Sports Med. 2009;37(12):2467-2469.
59. Chalmers PN, Trombley R, Cip J, et al. Postoperative restoration of upper extremity motion and neuromuscular control during the overhand pitch: evaluation of tenodesis and repair for superior labral anterior-posterior tears. Am J Sports Med. 2014;42(12):2825-2836.
60. Giphart JE, Elser F, Dewing CB, Torry MR, Millett PJ. The long head of the biceps tendon has minimal effect on in vivo glenohumeral kinematics: a biplane fluoroscopy study. Am J Sports Med. 2012;40(1):202-212.
61. Grossman MG, Tibone JE, McGarry MH, Schneider DJ, Veneziani S, Lee TQ. A cadaveric model of the throwing shoulder: a possible etiology of superior labrum anterior-to-posterior lesions. J Bone Joint Surg Am. 2005;87(4):824-831.
62. Hawkes DH, Alizadehkhaiyat O, Fisher AC, Kemp GJ, Roebuck MM, Frostick SP. Normal shoulder muscular activation and co-ordination during a shoulder elevation task based on activities of daily living: an electromyographic study. J Orthop Res. 2012;30(1):53-60.
63. Strauss EJ, Salata MJ, Sershon RA, et al. Role of the superior labrum after biceps tenodesis in glenohumeral stability. J Shoulder Elbow Surg. 2014;23(4):485-491.
64. Mazzocca AD, Bicos J, Santangelo S, Romeo AA, Arciero RA. The biomechanical evaluation of four fixation techniques for proximal biceps tenodesis. Arthroscopy. 2005;21(11):1296-1306.
65. Werner BC, Pehlivan HC, Hart JM, et al. Increased incidence of postoperative stiffness after arthroscopic compared with open biceps tenodesis. Arthroscopy. 2014;30(9):1075-1084.
66. Denard PJ, Dai X, Hanypsiak BT, Burkhart SS. Anatomy of the biceps tendon: implications for restoring physiological length–tension relation during biceps tenodesis with interference screw fixation. Arthroscopy. 2012;28(10):1352-1358.
67. Mazzocca AD, Rios CG, Romeo AA, Arciero RA. Subpectoral biceps tenodesis with interference screw fixation. Arthroscopy. 2005;21(7):896.
Actinomycetoma: An Update on Diagnosis and Treatment
Mycetoma is a subcutaneous disease that can be caused by aerobic bacteria (actinomycetoma) or fungi (eumycetoma). Diagnosis is based on clinical manifestations, including swelling and deformity of affected areas, as well as the presence of granulation tissue, scars, abscesses, sinus tracts, and a purulent exudate that contains the microorganisms.
The worldwide proportion of mycetomas is 60% actinomycetomas and 40% eumycetomas.1 The disease is endemic in tropical, subtropical, and temperate regions, predominating between latitudes 30°N and 15°S. Most cases occur in Africa, especially Sudan, Mauritania, and Senegal; India; Yemen; and Pakistan. In the Americas, the countries with the most reported cases are Mexico and Venezuela.1
Although mycetoma is rare in developed countries, migration of patients from endemic areas makes knowledge of this condition crucial for dermatologists worldwide. We present a review of the current concepts in the epidemiology, clinical presentation, diagnosis, and treatment of actinomycetoma.
Epidemiology
Actinomycetoma is more common in Latin America, with Mexico having the highest incidence. At last count, there were 2631 cases reported in Mexico.2 The majority of cases of mycetoma in Mexico are actinomycetoma (98%), including Nocardia (86%) and Actinomadura madurae (10%). Eumycetoma is rare in Mexico, constituting only 2% of cases.2 Worldwide, men are affected more commonly than women, which is thought to be related to a higher occupational risk during agricultural labor.
Clinical Features
Mycetoma can affect the skin, subcutaneous tissue, bones, and occasionally the internal organs. It is characterized by swelling, deformation of the affected area, and fistulae that drain serosanguineous or purulent exudates.
In Mexico, 60% of cases of mycetoma affect the lower extremities; the feet are the most commonly affected area, followed by the trunk (back and chest), arms, forearms, legs, knees, and thighs.1 Other sites include the hands, shoulders, and abdominal wall. The head and neck area are seldom affected.3 Mycetoma lesions grow and disseminate locally. Bone lesions are possible depending on the osteophilic affinity of the etiological agent and on the interactions between the fungus and the host’s immune system. In severe advanced cases of mycetoma, the lesions may involve tendons and nerves. Dissemination via blood or lymphatics is extremely rare.4
Diagnosis
Diagnosis of actinomycetoma is suspected based on clinical features and confirmed by direct examination of exudates with Lugol iodine or saline solution. On direct microscopy, actinomycetes are recognized by the production of filaments with a width of 0.5 to 1 μm. On hematoxylin and eosin stain, the small grains of Nocardia appear eosinophilic with a blue center and pink filaments. On Gram stain, actinomycetoma grains show positive branching filaments. Culture of grains recovered from aspirated material or biopsy specimens provides specific etiologic diagnosis. Cultures should be held for at least 4 weeks. Additionally, there are some enzymatic, molecular, and serologic tests available for diagnosis.5-7 Serologic diagnosis is available in a few centers in Mexico and can be helpful in some cases for diagnosis or follow-up during treatment. Antibodies can be determined via enzyme-linked immunosorbent assay, Western blot analysis, immunodiffusion, or counterimmunoelectrophoresis.8
The causative agents of actinomycetoma can be isolated in Sabouraud dextrose agar. Deep wedge biopsies (or puncture aspiration) are useful in observing the diagnostic grains, which can be identified adequately with Gram stain. Grains usually are surrounded and/or infiltrated by neutrophils. The size, form, and color of grains can identify the causative agent.1 The granules of Nocardia are small (80–130 mm) and reniform or wormlike, with club structures in their periphery (Figure 1). Actinomadura madurae is characterized by large, white-yellow granules that can be seen with the naked eye (1–3 mm). On microscopic examination with hematoxylin and eosin stain, these grains are purple and exhibit peripheral pink pseudofilaments (Figure 2).2 The grains of Actinomadura pelletieri are large (1–3 mm) and red or violaceous. They fragment or break easily, giving the appearance of a broken dish (Figure 3). Streptomyces somaliensis forms round grains approximately 0.5 to 1 cm in diameter. These grains stain poorly and are extremely hard. Cutting the grains during processing results in striation, giving them the appearance of a potato chip (Figure 4).2

Treatment of Actinomycetoma
Precise identification of the etiologic agent is essential to provide effective treatment of actinomycetoma. Without treatment, or in resistant cases, progressive osseous and visceral involvement is inevitable.9 Actinomycetoma without osseous involvement usually responds well to medical treatment.
The treatment of choice for actinomycetoma involving Nocardia brasiliensis is a combination of dapsone 100 to 200 mg once daily and trimethoprim-sulfamethoxazole (TMP-SMX) 80/400 to 160/800 mg once daily for 2 to 3 years.10 Other treatments have included the following: (1) amikacin 15 mg/kg or 500 mg intramuscularly twice daily for 3 weeks plus dapsone 100 to 200 mg once daily plus TMP-SMX 80/400 to 160/800 mg daily for 2 to 3 years (amikacin, however, is expensive and potentially toxic [nephrotoxicity and ototoxicity] and therefore is used only in resistant cases); (2) dapsone 100 to 200 mg once daily or TMP-SMX 80/400 to 160/800 mg daily for 2 to 3 years plus intramuscular kanamycin 15 mg/kg once daily for 2 weeks at the beginning of treatment, alternating with rest periods to reduce the risk for nephrotoxicity and ototoxicity10; (3) dapsone 1.5 mg/kg orally twice daily plus phosphomycin 500 mg once daily; (4) dapsone 1.5 mg/kg orally twice daily plus streptomycin 1 g once daily (14 mg/kg/d) for 1 month, then the same dose every other day for 1 to 2 months monitoring for ototoxicity; and (5) TMP-SMX 80/400 to 160/800 mg once daily for 2 to 3 years or rifampicin (15–20 mg/kg/d) plus streptomycin 1 g once daily (14 mg/kg/d) for 1 month at the beginning of treatment, then the same dose every other day for 2 to 3 months until a total dose of 60 g is administered, monitoring for ototoxicity.11 Audiometric tests and creatinine levels must be performed every 5 weeks during the treatment to monitor toxicity.10
The best results for infections with A pelletieri, A madurae, and S somaliensis have been with streptomycin (1 g once daily in adults; 20 mg/kg once daily in children) until a total dose of 50 g is reached in combination with TMP-SMX or dapsone12 (Figure 5). Alternatives for A madurae infections include streptomycin plus oral clofazimine (100 mg once daily), oral rifampicin (300 mg twice daily), oral tetracycline (1 g once daily), oral isoniazid (300–600 mg once daily), or oral minocycline (100 mg twice daily; also effective for A pelletieri).

More recently, other drugs have been used such as carbapenems (eg, imipenem, meropenem), which have wide-spectrum efficacy and are resistant to β-lactamases. Patients should be hospitalized to receive intravenous therapy with imipenem.2 Carbapenems are effective against gram-positive and gram-negative as well as Nocardia species.13,14 Mycetoma that is resistant, severe, or has visceral involvement can be treated with a combination of amikacin and imipenem.15,16 Meropenem is a similar drug that is available as an oral formulation. Both imipenem and meropenem are recommended in cases with bone involvement.17,18 Alternatives for resistant cases include amoxicillin–clavulanic acid 500/125 mg orally 3 times daily for 3 to 6 months or intravenous cefotaxime 1 g every 8 hours plus intramuscular amikacin 500 mg twice daily plus oral levamisole 300 mg once weekly for 4 weeks.19-23
For resistant cases associated with Nocardia species, clindamycin plus quinolones (eg, ciprofloxacin, moxifloxacin, garenoxacin) at a dose of 25 mg/kg once daily for at least 3 months has been suggested in in vivo studies.23
Overall, the cure rate for actinomycetoma treated with any of the prior therapies ranges from 60% to 90%. Treatment must be modified or stopped if there is clinical or laboratory evidence of drug toxicity.13,24 Surgical treatment of actinomycetoma is contraindicated, as it may cause hematogenous dissemination.
Prognosis
Actinomycetomas of a few months’ duration and without bone involvement respond well to therapy. If no therapy is provided or if there is resistance, the functional and cosmetic prognosis is poor, mainly for the feet. There is a risk for spine involvement with mycetoma on the back and posterior head. Thoracic lesions may penetrate into the lungs. The muscular fascia impedes the penetration of abdominal lesions, but the inguinal canals can offer a path for intra-abdominal dissemination.4 Advanced cases lead to a poor general condition of patients, difficulty in using affected extremities, and in extreme cases even death.
The criteria used to guide the discontinuation of initial therapy for any mycetoma include a decrease in the volume of the lesion, closure of fistulae, 3 consecutive negative monthly cultures, imaging studies showing bone regeneration, lack of echoes and cavities on echography, and absence of grains on examination of fine-needle aspirates.11 After the initial treatment protocol is finished, most experts recommend continuing treatment with dapsone 100 to 300 mg once daily for several years to prevent recurrence.12
Prevention
Mycetoma is a disease associated with poverty. It could be prevented by improving living conditions and by regular use of shoes in rural populations.2
Conclusion
Mycetoma is a chronic infection that develops after traumatic inoculation of the skin with either true fungi or aerobic actinomycetes. The resultant infections are known as eumycetoma or actinomycetoma, respectively. The etiologic agents can be found in the so-called grains. Black grains suggest a fungal infection, minute white grains suggest Nocardia, and red grains are due to A pelletieri. Larger white grains or yellow-white grains may be fungal or actinomycotic in origin.
Specific diagnosis requires direct examination, culture, and biopsy. The treatment of choice for actinomycetoma by N brasiliensis is a combination of dapsone 100 to 200 mg once daily and TMP-SMX 80/400 to 160/800 mg once daily for 2 to 3 years. Other effective treatments include aminoglycosides (eg, amikacine, streptomycin) and quinolones. More recently, some other agents have been used such as carbapenems and natural products of Streptomyces cattleya (imipenem), which have wide-spectrum efficacy and are resistant to β-lactamases.
- Welsh O, Vera-Cabrera L, Welsh E, et al. Actinomycetoma and advances in its treatment. Clin Dermatol. 2012;30:372-381.
- Arenas R. Micología Medica Ilustrada. 4th ed. Mexico City, Mexico: McGraw-Hill Interamericana; 2011:125-146.
- McGinnis MR. Mycetoma. Dermatol Clin. 1996;14:97-104.
- Fahal AH. Mycetoma: Clinico-pathological Monograph. Khartoum, Sudan: University of Khartoum Press; 2006:20-23, 81-82.
- Estrada-Chavez GE, Vega-Memije ME, Arenas R, et al. Eumycotic mycetoma caused by Madurella mycetomatis successfully treated with antifungals, surgery, and topical negative pressure therapy. Int J Dermatol. 2009;48:401-403.
- Chávez G, Arenas R, Pérez-Polito A, et al. Eumycetic mycetoma due to Madurella mycetomatis. report of six cases. Rev Iberoam Micol. 1998;15:90-93.
- Vasquez del Mercado E, Arenas R, Moreno G. Sequelae and long-term consequences of systemic and subcutaneous mycoses. In: Fratamico PM, Smith JL, Brogden KA, eds. Sequelae and Long-term Consequences of Infectious Diseases. Washington, DC: ASM Press; 2009:415-420.
- Mancini N, Ossi CM, Perotti M, et al. Molecular mycological diagnosis and correct antimycotic treatments. J Clin Microbiol. 2005;43:3584-3585.
- Arenas R, Lavalle P. Micetoma (madura foot). In: Arenas R, Estrada R, eds. Tropical Dermatology. Austin, TX: Landes Bioscience; 2001:51-61.
- Welsh O, Sauceda E, González J, et al. Amikacin alone and in combination with trimethoprim-sulfamethoxazole in the treatment of actinomycotic mycetoma. J Am Acad Dermatol. 1987;17:443-448.
- Fahal AH. Mycetoma: clinico-pathological monograph. In: Fahal AH. Evidence Based Guidelines for the Management of Mycetoma Patients. Khartoum, Sudan: University of Khartoum Press; 2002:5-15.
- Welsh O, Salinas MC, Rodríguez MA. Treatment of eumycetoma and actinomycetoma. Curr Top Med Mycol. 1995;6:47-71.
- Valle ACF, Welsh O, Vera-Cabrera L. Subcutaneous mycoses—mycetoma. In: Tyring SK, Lupi O, Hengge UR, eds. Tropical Dermatology. Philadelphia, PA: Elsevier Churchill Livingstone; 2006:197-200.
- Fuentes A, Arenas R, Reyes M, et al. Actinomicetoma por Nocardia sp. Informe de cinco casos tratados con imipenem solo o combinado con amikacina. Gac Med Mex. 2006;142:247-252.
- Gombert ME, Aulicino TM, DuBouchet L, et al. Therapy of experimental cerebral nocardiosis with imipenem, amikacin, trimethoprim-sulfamethoxazole, and minocylina. Antimicrob Agents Chemother. 1986;30:270-273.
- Calandra GB, Ricci FM, Wang C, et al. Safety and tolerance comparison of imipenem-cilastatin to cephalotin and cefazolin. J Antimicrob Chemother. 1983;12:125-131.
- Ameen M, Arenas R, Vasquez del Mercado E, et al. Efficacy of imipenem therapy for Nocardia actinomycetomas refractory to sulfonamides. J Am Acad Dermatol. 2010;62:239-246.
- Ameen M, Vargas F, Vasquez del Mercado E, et al. Successful treatment of Nocardia actinomycetoma with meropenem and amikacin combination therapy. Int J Dermatol. 2011;50:443-445.
- Ameen M, Arenas R. Emerging therapeutic regimes for the management of mycetomas. Expert Opin Pharmacother. 2008;9:2077-2085.
- Vera-Cabrera L, Daw-Garza A, Said-Fernández S, et al. Therapeutic effect of a novel oxazolidinone, DA-7867 in BALB/c mice infected with Nocardia brasiliensis. PloS Negl Trop Dis. 2008;2:e289.
- Gómez A, Saúl A, Bonifaz A. Amoxicillin and clavulanic acid in the treatment of actinomicetoma. Int J Dermatol. 1993;32:218-220.
- Méndez-Tovar L, Serrano-Jaen L, Almeida-Arvizu VM. Cefotaxima mas amikacina asociadas a inmunomodulación en el tratamiento de actinomicetoma resistente a tratamiento convencional. Gac Med Mex. 1999;135:517-521.
- Chacon-Moreno BE, Welsh O, Cavazos-Rocha N, et al. Efficacy of ciprofloxacin and moxifloxacin against Nocardia brasiliensis in vitro in an experimental model of actinomycetoma in BALB/c mice. Antimicrob Agents Chemother. 2009;53:295-297.
- Welsh O. Treatment of actinomycetoma. Arch Med Res. 1993;24:413-415.
Mycetoma is a subcutaneous disease that can be caused by aerobic bacteria (actinomycetoma) or fungi (eumycetoma). Diagnosis is based on clinical manifestations, including swelling and deformity of affected areas, as well as the presence of granulation tissue, scars, abscesses, sinus tracts, and a purulent exudate that contains the microorganisms.
The worldwide proportion of mycetomas is 60% actinomycetomas and 40% eumycetomas.1 The disease is endemic in tropical, subtropical, and temperate regions, predominating between latitudes 30°N and 15°S. Most cases occur in Africa, especially Sudan, Mauritania, and Senegal; India; Yemen; and Pakistan. In the Americas, the countries with the most reported cases are Mexico and Venezuela.1
Although mycetoma is rare in developed countries, migration of patients from endemic areas makes knowledge of this condition crucial for dermatologists worldwide. We present a review of the current concepts in the epidemiology, clinical presentation, diagnosis, and treatment of actinomycetoma.
Epidemiology
Actinomycetoma is more common in Latin America, with Mexico having the highest incidence. At last count, there were 2631 cases reported in Mexico.2 The majority of cases of mycetoma in Mexico are actinomycetoma (98%), including Nocardia (86%) and Actinomadura madurae (10%). Eumycetoma is rare in Mexico, constituting only 2% of cases.2 Worldwide, men are affected more commonly than women, which is thought to be related to a higher occupational risk during agricultural labor.
Clinical Features
Mycetoma can affect the skin, subcutaneous tissue, bones, and occasionally the internal organs. It is characterized by swelling, deformation of the affected area, and fistulae that drain serosanguineous or purulent exudates.
In Mexico, 60% of cases of mycetoma affect the lower extremities; the feet are the most commonly affected area, followed by the trunk (back and chest), arms, forearms, legs, knees, and thighs.1 Other sites include the hands, shoulders, and abdominal wall. The head and neck area are seldom affected.3 Mycetoma lesions grow and disseminate locally. Bone lesions are possible depending on the osteophilic affinity of the etiological agent and on the interactions between the fungus and the host’s immune system. In severe advanced cases of mycetoma, the lesions may involve tendons and nerves. Dissemination via blood or lymphatics is extremely rare.4
Diagnosis
Diagnosis of actinomycetoma is suspected based on clinical features and confirmed by direct examination of exudates with Lugol iodine or saline solution. On direct microscopy, actinomycetes are recognized by the production of filaments with a width of 0.5 to 1 μm. On hematoxylin and eosin stain, the small grains of Nocardia appear eosinophilic with a blue center and pink filaments. On Gram stain, actinomycetoma grains show positive branching filaments. Culture of grains recovered from aspirated material or biopsy specimens provides specific etiologic diagnosis. Cultures should be held for at least 4 weeks. Additionally, there are some enzymatic, molecular, and serologic tests available for diagnosis.5-7 Serologic diagnosis is available in a few centers in Mexico and can be helpful in some cases for diagnosis or follow-up during treatment. Antibodies can be determined via enzyme-linked immunosorbent assay, Western blot analysis, immunodiffusion, or counterimmunoelectrophoresis.8
The causative agents of actinomycetoma can be isolated in Sabouraud dextrose agar. Deep wedge biopsies (or puncture aspiration) are useful in observing the diagnostic grains, which can be identified adequately with Gram stain. Grains usually are surrounded and/or infiltrated by neutrophils. The size, form, and color of grains can identify the causative agent.1 The granules of Nocardia are small (80–130 mm) and reniform or wormlike, with club structures in their periphery (Figure 1). Actinomadura madurae is characterized by large, white-yellow granules that can be seen with the naked eye (1–3 mm). On microscopic examination with hematoxylin and eosin stain, these grains are purple and exhibit peripheral pink pseudofilaments (Figure 2).2 The grains of Actinomadura pelletieri are large (1–3 mm) and red or violaceous. They fragment or break easily, giving the appearance of a broken dish (Figure 3). Streptomyces somaliensis forms round grains approximately 0.5 to 1 cm in diameter. These grains stain poorly and are extremely hard. Cutting the grains during processing results in striation, giving them the appearance of a potato chip (Figure 4).2




Treatment of Actinomycetoma
Precise identification of the etiologic agent is essential to provide effective treatment of actinomycetoma. Without treatment, or in resistant cases, progressive osseous and visceral involvement is inevitable.9 Actinomycetoma without osseous involvement usually responds well to medical treatment.
The treatment of choice for actinomycetoma involving Nocardia brasiliensis is a combination of dapsone 100 to 200 mg once daily and trimethoprim-sulfamethoxazole (TMP-SMX) 80/400 to 160/800 mg once daily for 2 to 3 years.10 Other treatments have included the following: (1) amikacin 15 mg/kg or 500 mg intramuscularly twice daily for 3 weeks plus dapsone 100 to 200 mg once daily plus TMP-SMX 80/400 to 160/800 mg daily for 2 to 3 years (amikacin, however, is expensive and potentially toxic [nephrotoxicity and ototoxicity] and therefore is used only in resistant cases); (2) dapsone 100 to 200 mg once daily or TMP-SMX 80/400 to 160/800 mg daily for 2 to 3 years plus intramuscular kanamycin 15 mg/kg once daily for 2 weeks at the beginning of treatment, alternating with rest periods to reduce the risk for nephrotoxicity and ototoxicity10; (3) dapsone 1.5 mg/kg orally twice daily plus phosphomycin 500 mg once daily; (4) dapsone 1.5 mg/kg orally twice daily plus streptomycin 1 g once daily (14 mg/kg/d) for 1 month, then the same dose every other day for 1 to 2 months monitoring for ototoxicity; and (5) TMP-SMX 80/400 to 160/800 mg once daily for 2 to 3 years or rifampicin (15–20 mg/kg/d) plus streptomycin 1 g once daily (14 mg/kg/d) for 1 month at the beginning of treatment, then the same dose every other day for 2 to 3 months until a total dose of 60 g is administered, monitoring for ototoxicity.11 Audiometric tests and creatinine levels must be performed every 5 weeks during the treatment to monitor toxicity.10
The best results for infections with A pelletieri, A madurae, and S somaliensis have been with streptomycin (1 g once daily in adults; 20 mg/kg once daily in children) until a total dose of 50 g is reached in combination with TMP-SMX or dapsone12 (Figure 5). Alternatives for A madurae infections include streptomycin plus oral clofazimine (100 mg once daily), oral rifampicin (300 mg twice daily), oral tetracycline (1 g once daily), oral isoniazid (300–600 mg once daily), or oral minocycline (100 mg twice daily; also effective for A pelletieri).

More recently, other drugs have been used such as carbapenems (eg, imipenem, meropenem), which have wide-spectrum efficacy and are resistant to β-lactamases. Patients should be hospitalized to receive intravenous therapy with imipenem.2 Carbapenems are effective against gram-positive and gram-negative as well as Nocardia species.13,14 Mycetoma that is resistant, severe, or has visceral involvement can be treated with a combination of amikacin and imipenem.15,16 Meropenem is a similar drug that is available as an oral formulation. Both imipenem and meropenem are recommended in cases with bone involvement.17,18 Alternatives for resistant cases include amoxicillin–clavulanic acid 500/125 mg orally 3 times daily for 3 to 6 months or intravenous cefotaxime 1 g every 8 hours plus intramuscular amikacin 500 mg twice daily plus oral levamisole 300 mg once weekly for 4 weeks.19-23
For resistant cases associated with Nocardia species, clindamycin plus quinolones (eg, ciprofloxacin, moxifloxacin, garenoxacin) at a dose of 25 mg/kg once daily for at least 3 months has been suggested in in vivo studies.23
Overall, the cure rate for actinomycetoma treated with any of the prior therapies ranges from 60% to 90%. Treatment must be modified or stopped if there is clinical or laboratory evidence of drug toxicity.13,24 Surgical treatment of actinomycetoma is contraindicated, as it may cause hematogenous dissemination.
Prognosis
Actinomycetomas of a few months’ duration and without bone involvement respond well to therapy. If no therapy is provided or if there is resistance, the functional and cosmetic prognosis is poor, mainly for the feet. There is a risk for spine involvement with mycetoma on the back and posterior head. Thoracic lesions may penetrate into the lungs. The muscular fascia impedes the penetration of abdominal lesions, but the inguinal canals can offer a path for intra-abdominal dissemination.4 Advanced cases lead to a poor general condition of patients, difficulty in using affected extremities, and in extreme cases even death.
The criteria used to guide the discontinuation of initial therapy for any mycetoma include a decrease in the volume of the lesion, closure of fistulae, 3 consecutive negative monthly cultures, imaging studies showing bone regeneration, lack of echoes and cavities on echography, and absence of grains on examination of fine-needle aspirates.11 After the initial treatment protocol is finished, most experts recommend continuing treatment with dapsone 100 to 300 mg once daily for several years to prevent recurrence.12
Prevention
Mycetoma is a disease associated with poverty. It could be prevented by improving living conditions and by regular use of shoes in rural populations.2
Conclusion
Mycetoma is a chronic infection that develops after traumatic inoculation of the skin with either true fungi or aerobic actinomycetes. The resultant infections are known as eumycetoma or actinomycetoma, respectively. The etiologic agents can be found in the so-called grains. Black grains suggest a fungal infection, minute white grains suggest Nocardia, and red grains are due to A pelletieri. Larger white grains or yellow-white grains may be fungal or actinomycotic in origin.
Specific diagnosis requires direct examination, culture, and biopsy. The treatment of choice for actinomycetoma by N brasiliensis is a combination of dapsone 100 to 200 mg once daily and TMP-SMX 80/400 to 160/800 mg once daily for 2 to 3 years. Other effective treatments include aminoglycosides (eg, amikacine, streptomycin) and quinolones. More recently, some other agents have been used such as carbapenems and natural products of Streptomyces cattleya (imipenem), which have wide-spectrum efficacy and are resistant to β-lactamases.
Mycetoma is a subcutaneous disease that can be caused by aerobic bacteria (actinomycetoma) or fungi (eumycetoma). Diagnosis is based on clinical manifestations, including swelling and deformity of affected areas, as well as the presence of granulation tissue, scars, abscesses, sinus tracts, and a purulent exudate that contains the microorganisms.
The worldwide proportion of mycetomas is 60% actinomycetomas and 40% eumycetomas.1 The disease is endemic in tropical, subtropical, and temperate regions, predominating between latitudes 30°N and 15°S. Most cases occur in Africa, especially Sudan, Mauritania, and Senegal; India; Yemen; and Pakistan. In the Americas, the countries with the most reported cases are Mexico and Venezuela.1
Although mycetoma is rare in developed countries, migration of patients from endemic areas makes knowledge of this condition crucial for dermatologists worldwide. We present a review of the current concepts in the epidemiology, clinical presentation, diagnosis, and treatment of actinomycetoma.
Epidemiology
Actinomycetoma is more common in Latin America, with Mexico having the highest incidence. At last count, there were 2631 cases reported in Mexico.2 The majority of cases of mycetoma in Mexico are actinomycetoma (98%), including Nocardia (86%) and Actinomadura madurae (10%). Eumycetoma is rare in Mexico, constituting only 2% of cases.2 Worldwide, men are affected more commonly than women, which is thought to be related to a higher occupational risk during agricultural labor.
Clinical Features
Mycetoma can affect the skin, subcutaneous tissue, bones, and occasionally the internal organs. It is characterized by swelling, deformation of the affected area, and fistulae that drain serosanguineous or purulent exudates.
In Mexico, 60% of cases of mycetoma affect the lower extremities; the feet are the most commonly affected area, followed by the trunk (back and chest), arms, forearms, legs, knees, and thighs.1 Other sites include the hands, shoulders, and abdominal wall. The head and neck area are seldom affected.3 Mycetoma lesions grow and disseminate locally. Bone lesions are possible depending on the osteophilic affinity of the etiological agent and on the interactions between the fungus and the host’s immune system. In severe advanced cases of mycetoma, the lesions may involve tendons and nerves. Dissemination via blood or lymphatics is extremely rare.4
Diagnosis
Diagnosis of actinomycetoma is suspected based on clinical features and confirmed by direct examination of exudates with Lugol iodine or saline solution. On direct microscopy, actinomycetes are recognized by the production of filaments with a width of 0.5 to 1 μm. On hematoxylin and eosin stain, the small grains of Nocardia appear eosinophilic with a blue center and pink filaments. On Gram stain, actinomycetoma grains show positive branching filaments. Culture of grains recovered from aspirated material or biopsy specimens provides specific etiologic diagnosis. Cultures should be held for at least 4 weeks. Additionally, there are some enzymatic, molecular, and serologic tests available for diagnosis.5-7 Serologic diagnosis is available in a few centers in Mexico and can be helpful in some cases for diagnosis or follow-up during treatment. Antibodies can be determined via enzyme-linked immunosorbent assay, Western blot analysis, immunodiffusion, or counterimmunoelectrophoresis.8
The causative agents of actinomycetoma can be isolated in Sabouraud dextrose agar. Deep wedge biopsies (or puncture aspiration) are useful in observing the diagnostic grains, which can be identified adequately with Gram stain. Grains usually are surrounded and/or infiltrated by neutrophils. The size, form, and color of grains can identify the causative agent.1 The granules of Nocardia are small (80–130 mm) and reniform or wormlike, with club structures in their periphery (Figure 1). Actinomadura madurae is characterized by large, white-yellow granules that can be seen with the naked eye (1–3 mm). On microscopic examination with hematoxylin and eosin stain, these grains are purple and exhibit peripheral pink pseudofilaments (Figure 2).2 The grains of Actinomadura pelletieri are large (1–3 mm) and red or violaceous. They fragment or break easily, giving the appearance of a broken dish (Figure 3). Streptomyces somaliensis forms round grains approximately 0.5 to 1 cm in diameter. These grains stain poorly and are extremely hard. Cutting the grains during processing results in striation, giving them the appearance of a potato chip (Figure 4).2




Treatment of Actinomycetoma
Precise identification of the etiologic agent is essential to provide effective treatment of actinomycetoma. Without treatment, or in resistant cases, progressive osseous and visceral involvement is inevitable.9 Actinomycetoma without osseous involvement usually responds well to medical treatment.
The treatment of choice for actinomycetoma involving Nocardia brasiliensis is a combination of dapsone 100 to 200 mg once daily and trimethoprim-sulfamethoxazole (TMP-SMX) 80/400 to 160/800 mg once daily for 2 to 3 years.10 Other treatments have included the following: (1) amikacin 15 mg/kg or 500 mg intramuscularly twice daily for 3 weeks plus dapsone 100 to 200 mg once daily plus TMP-SMX 80/400 to 160/800 mg daily for 2 to 3 years (amikacin, however, is expensive and potentially toxic [nephrotoxicity and ototoxicity] and therefore is used only in resistant cases); (2) dapsone 100 to 200 mg once daily or TMP-SMX 80/400 to 160/800 mg daily for 2 to 3 years plus intramuscular kanamycin 15 mg/kg once daily for 2 weeks at the beginning of treatment, alternating with rest periods to reduce the risk for nephrotoxicity and ototoxicity10; (3) dapsone 1.5 mg/kg orally twice daily plus phosphomycin 500 mg once daily; (4) dapsone 1.5 mg/kg orally twice daily plus streptomycin 1 g once daily (14 mg/kg/d) for 1 month, then the same dose every other day for 1 to 2 months monitoring for ototoxicity; and (5) TMP-SMX 80/400 to 160/800 mg once daily for 2 to 3 years or rifampicin (15–20 mg/kg/d) plus streptomycin 1 g once daily (14 mg/kg/d) for 1 month at the beginning of treatment, then the same dose every other day for 2 to 3 months until a total dose of 60 g is administered, monitoring for ototoxicity.11 Audiometric tests and creatinine levels must be performed every 5 weeks during the treatment to monitor toxicity.10
The best results for infections with A pelletieri, A madurae, and S somaliensis have been with streptomycin (1 g once daily in adults; 20 mg/kg once daily in children) until a total dose of 50 g is reached in combination with TMP-SMX or dapsone12 (Figure 5). Alternatives for A madurae infections include streptomycin plus oral clofazimine (100 mg once daily), oral rifampicin (300 mg twice daily), oral tetracycline (1 g once daily), oral isoniazid (300–600 mg once daily), or oral minocycline (100 mg twice daily; also effective for A pelletieri).

More recently, other drugs have been used such as carbapenems (eg, imipenem, meropenem), which have wide-spectrum efficacy and are resistant to β-lactamases. Patients should be hospitalized to receive intravenous therapy with imipenem.2 Carbapenems are effective against gram-positive and gram-negative as well as Nocardia species.13,14 Mycetoma that is resistant, severe, or has visceral involvement can be treated with a combination of amikacin and imipenem.15,16 Meropenem is a similar drug that is available as an oral formulation. Both imipenem and meropenem are recommended in cases with bone involvement.17,18 Alternatives for resistant cases include amoxicillin–clavulanic acid 500/125 mg orally 3 times daily for 3 to 6 months or intravenous cefotaxime 1 g every 8 hours plus intramuscular amikacin 500 mg twice daily plus oral levamisole 300 mg once weekly for 4 weeks.19-23
For resistant cases associated with Nocardia species, clindamycin plus quinolones (eg, ciprofloxacin, moxifloxacin, garenoxacin) at a dose of 25 mg/kg once daily for at least 3 months has been suggested in in vivo studies.23
Overall, the cure rate for actinomycetoma treated with any of the prior therapies ranges from 60% to 90%. Treatment must be modified or stopped if there is clinical or laboratory evidence of drug toxicity.13,24 Surgical treatment of actinomycetoma is contraindicated, as it may cause hematogenous dissemination.
Prognosis
Actinomycetomas of a few months’ duration and without bone involvement respond well to therapy. If no therapy is provided or if there is resistance, the functional and cosmetic prognosis is poor, mainly for the feet. There is a risk for spine involvement with mycetoma on the back and posterior head. Thoracic lesions may penetrate into the lungs. The muscular fascia impedes the penetration of abdominal lesions, but the inguinal canals can offer a path for intra-abdominal dissemination.4 Advanced cases lead to a poor general condition of patients, difficulty in using affected extremities, and in extreme cases even death.
The criteria used to guide the discontinuation of initial therapy for any mycetoma include a decrease in the volume of the lesion, closure of fistulae, 3 consecutive negative monthly cultures, imaging studies showing bone regeneration, lack of echoes and cavities on echography, and absence of grains on examination of fine-needle aspirates.11 After the initial treatment protocol is finished, most experts recommend continuing treatment with dapsone 100 to 300 mg once daily for several years to prevent recurrence.12
Prevention
Mycetoma is a disease associated with poverty. It could be prevented by improving living conditions and by regular use of shoes in rural populations.2
Conclusion
Mycetoma is a chronic infection that develops after traumatic inoculation of the skin with either true fungi or aerobic actinomycetes. The resultant infections are known as eumycetoma or actinomycetoma, respectively. The etiologic agents can be found in the so-called grains. Black grains suggest a fungal infection, minute white grains suggest Nocardia, and red grains are due to A pelletieri. Larger white grains or yellow-white grains may be fungal or actinomycotic in origin.
Specific diagnosis requires direct examination, culture, and biopsy. The treatment of choice for actinomycetoma by N brasiliensis is a combination of dapsone 100 to 200 mg once daily and TMP-SMX 80/400 to 160/800 mg once daily for 2 to 3 years. Other effective treatments include aminoglycosides (eg, amikacine, streptomycin) and quinolones. More recently, some other agents have been used such as carbapenems and natural products of Streptomyces cattleya (imipenem), which have wide-spectrum efficacy and are resistant to β-lactamases.
- Welsh O, Vera-Cabrera L, Welsh E, et al. Actinomycetoma and advances in its treatment. Clin Dermatol. 2012;30:372-381.
- Arenas R. Micología Medica Ilustrada. 4th ed. Mexico City, Mexico: McGraw-Hill Interamericana; 2011:125-146.
- McGinnis MR. Mycetoma. Dermatol Clin. 1996;14:97-104.
- Fahal AH. Mycetoma: Clinico-pathological Monograph. Khartoum, Sudan: University of Khartoum Press; 2006:20-23, 81-82.
- Estrada-Chavez GE, Vega-Memije ME, Arenas R, et al. Eumycotic mycetoma caused by Madurella mycetomatis successfully treated with antifungals, surgery, and topical negative pressure therapy. Int J Dermatol. 2009;48:401-403.
- Chávez G, Arenas R, Pérez-Polito A, et al. Eumycetic mycetoma due to Madurella mycetomatis. report of six cases. Rev Iberoam Micol. 1998;15:90-93.
- Vasquez del Mercado E, Arenas R, Moreno G. Sequelae and long-term consequences of systemic and subcutaneous mycoses. In: Fratamico PM, Smith JL, Brogden KA, eds. Sequelae and Long-term Consequences of Infectious Diseases. Washington, DC: ASM Press; 2009:415-420.
- Mancini N, Ossi CM, Perotti M, et al. Molecular mycological diagnosis and correct antimycotic treatments. J Clin Microbiol. 2005;43:3584-3585.
- Arenas R, Lavalle P. Micetoma (madura foot). In: Arenas R, Estrada R, eds. Tropical Dermatology. Austin, TX: Landes Bioscience; 2001:51-61.
- Welsh O, Sauceda E, González J, et al. Amikacin alone and in combination with trimethoprim-sulfamethoxazole in the treatment of actinomycotic mycetoma. J Am Acad Dermatol. 1987;17:443-448.
- Fahal AH. Mycetoma: clinico-pathological monograph. In: Fahal AH. Evidence Based Guidelines for the Management of Mycetoma Patients. Khartoum, Sudan: University of Khartoum Press; 2002:5-15.
- Welsh O, Salinas MC, Rodríguez MA. Treatment of eumycetoma and actinomycetoma. Curr Top Med Mycol. 1995;6:47-71.
- Valle ACF, Welsh O, Vera-Cabrera L. Subcutaneous mycoses—mycetoma. In: Tyring SK, Lupi O, Hengge UR, eds. Tropical Dermatology. Philadelphia, PA: Elsevier Churchill Livingstone; 2006:197-200.
- Fuentes A, Arenas R, Reyes M, et al. Actinomicetoma por Nocardia sp. Informe de cinco casos tratados con imipenem solo o combinado con amikacina. Gac Med Mex. 2006;142:247-252.
- Gombert ME, Aulicino TM, DuBouchet L, et al. Therapy of experimental cerebral nocardiosis with imipenem, amikacin, trimethoprim-sulfamethoxazole, and minocylina. Antimicrob Agents Chemother. 1986;30:270-273.
- Calandra GB, Ricci FM, Wang C, et al. Safety and tolerance comparison of imipenem-cilastatin to cephalotin and cefazolin. J Antimicrob Chemother. 1983;12:125-131.
- Ameen M, Arenas R, Vasquez del Mercado E, et al. Efficacy of imipenem therapy for Nocardia actinomycetomas refractory to sulfonamides. J Am Acad Dermatol. 2010;62:239-246.
- Ameen M, Vargas F, Vasquez del Mercado E, et al. Successful treatment of Nocardia actinomycetoma with meropenem and amikacin combination therapy. Int J Dermatol. 2011;50:443-445.
- Ameen M, Arenas R. Emerging therapeutic regimes for the management of mycetomas. Expert Opin Pharmacother. 2008;9:2077-2085.
- Vera-Cabrera L, Daw-Garza A, Said-Fernández S, et al. Therapeutic effect of a novel oxazolidinone, DA-7867 in BALB/c mice infected with Nocardia brasiliensis. PloS Negl Trop Dis. 2008;2:e289.
- Gómez A, Saúl A, Bonifaz A. Amoxicillin and clavulanic acid in the treatment of actinomicetoma. Int J Dermatol. 1993;32:218-220.
- Méndez-Tovar L, Serrano-Jaen L, Almeida-Arvizu VM. Cefotaxima mas amikacina asociadas a inmunomodulación en el tratamiento de actinomicetoma resistente a tratamiento convencional. Gac Med Mex. 1999;135:517-521.
- Chacon-Moreno BE, Welsh O, Cavazos-Rocha N, et al. Efficacy of ciprofloxacin and moxifloxacin against Nocardia brasiliensis in vitro in an experimental model of actinomycetoma in BALB/c mice. Antimicrob Agents Chemother. 2009;53:295-297.
- Welsh O. Treatment of actinomycetoma. Arch Med Res. 1993;24:413-415.
- Welsh O, Vera-Cabrera L, Welsh E, et al. Actinomycetoma and advances in its treatment. Clin Dermatol. 2012;30:372-381.
- Arenas R. Micología Medica Ilustrada. 4th ed. Mexico City, Mexico: McGraw-Hill Interamericana; 2011:125-146.
- McGinnis MR. Mycetoma. Dermatol Clin. 1996;14:97-104.
- Fahal AH. Mycetoma: Clinico-pathological Monograph. Khartoum, Sudan: University of Khartoum Press; 2006:20-23, 81-82.
- Estrada-Chavez GE, Vega-Memije ME, Arenas R, et al. Eumycotic mycetoma caused by Madurella mycetomatis successfully treated with antifungals, surgery, and topical negative pressure therapy. Int J Dermatol. 2009;48:401-403.
- Chávez G, Arenas R, Pérez-Polito A, et al. Eumycetic mycetoma due to Madurella mycetomatis. report of six cases. Rev Iberoam Micol. 1998;15:90-93.
- Vasquez del Mercado E, Arenas R, Moreno G. Sequelae and long-term consequences of systemic and subcutaneous mycoses. In: Fratamico PM, Smith JL, Brogden KA, eds. Sequelae and Long-term Consequences of Infectious Diseases. Washington, DC: ASM Press; 2009:415-420.
- Mancini N, Ossi CM, Perotti M, et al. Molecular mycological diagnosis and correct antimycotic treatments. J Clin Microbiol. 2005;43:3584-3585.
- Arenas R, Lavalle P. Micetoma (madura foot). In: Arenas R, Estrada R, eds. Tropical Dermatology. Austin, TX: Landes Bioscience; 2001:51-61.
- Welsh O, Sauceda E, González J, et al. Amikacin alone and in combination with trimethoprim-sulfamethoxazole in the treatment of actinomycotic mycetoma. J Am Acad Dermatol. 1987;17:443-448.
- Fahal AH. Mycetoma: clinico-pathological monograph. In: Fahal AH. Evidence Based Guidelines for the Management of Mycetoma Patients. Khartoum, Sudan: University of Khartoum Press; 2002:5-15.
- Welsh O, Salinas MC, Rodríguez MA. Treatment of eumycetoma and actinomycetoma. Curr Top Med Mycol. 1995;6:47-71.
- Valle ACF, Welsh O, Vera-Cabrera L. Subcutaneous mycoses—mycetoma. In: Tyring SK, Lupi O, Hengge UR, eds. Tropical Dermatology. Philadelphia, PA: Elsevier Churchill Livingstone; 2006:197-200.
- Fuentes A, Arenas R, Reyes M, et al. Actinomicetoma por Nocardia sp. Informe de cinco casos tratados con imipenem solo o combinado con amikacina. Gac Med Mex. 2006;142:247-252.
- Gombert ME, Aulicino TM, DuBouchet L, et al. Therapy of experimental cerebral nocardiosis with imipenem, amikacin, trimethoprim-sulfamethoxazole, and minocylina. Antimicrob Agents Chemother. 1986;30:270-273.
- Calandra GB, Ricci FM, Wang C, et al. Safety and tolerance comparison of imipenem-cilastatin to cephalotin and cefazolin. J Antimicrob Chemother. 1983;12:125-131.
- Ameen M, Arenas R, Vasquez del Mercado E, et al. Efficacy of imipenem therapy for Nocardia actinomycetomas refractory to sulfonamides. J Am Acad Dermatol. 2010;62:239-246.
- Ameen M, Vargas F, Vasquez del Mercado E, et al. Successful treatment of Nocardia actinomycetoma with meropenem and amikacin combination therapy. Int J Dermatol. 2011;50:443-445.
- Ameen M, Arenas R. Emerging therapeutic regimes for the management of mycetomas. Expert Opin Pharmacother. 2008;9:2077-2085.
- Vera-Cabrera L, Daw-Garza A, Said-Fernández S, et al. Therapeutic effect of a novel oxazolidinone, DA-7867 in BALB/c mice infected with Nocardia brasiliensis. PloS Negl Trop Dis. 2008;2:e289.
- Gómez A, Saúl A, Bonifaz A. Amoxicillin and clavulanic acid in the treatment of actinomicetoma. Int J Dermatol. 1993;32:218-220.
- Méndez-Tovar L, Serrano-Jaen L, Almeida-Arvizu VM. Cefotaxima mas amikacina asociadas a inmunomodulación en el tratamiento de actinomicetoma resistente a tratamiento convencional. Gac Med Mex. 1999;135:517-521.
- Chacon-Moreno BE, Welsh O, Cavazos-Rocha N, et al. Efficacy of ciprofloxacin and moxifloxacin against Nocardia brasiliensis in vitro in an experimental model of actinomycetoma in BALB/c mice. Antimicrob Agents Chemother. 2009;53:295-297.
- Welsh O. Treatment of actinomycetoma. Arch Med Res. 1993;24:413-415.
Practice Points
- Diagnosis of actinomycetoma is based on clinical manifestations including increased swelling and deformity of affected areas, presence of granulation tissue, scars, abscesses, sinus tracts, and a purulent exudate containing microorganisms.
- The feet are the most commonly affected location, followed by the trunk (back and chest), arms, forearms, legs, knees, and thighs.
- Specific diagnosis of actinomycetoma requires clinical examination as well as direct examination of culture and biopsy results.
- Overall, the cure rate for actinomycetoma ranges from 60% to 90%.
Review of the Long-Term Effects of Proton Pump Inhibitors
Proton pump inhibitors (PPIs) are one of the most frequently used drug classes, given that they are readily accessible over-the-counter as well as via prescription. About 100 million PPI prescriptions dispensed an
The human stomach uses 3 primary neurotransmitters that regulate gastric acid secretion: acetylcholine (ACh), histamine (H), and gastrin (G). The interactions between these neurotransmitters promote and inhibit hydrogen ion (H+) generation. Stimulation of their corresponding receptors draws H+ into parietal cells that line the stomach. Once in the cell, a H+-K+-ATPase (more commonly known as the proton pump) actively transports H+ into the lumen of the stomach. The H+ bind with chlorine ions to form hydrochloric acid, which increases stomach acidity.5 Histamine receptors were thought to be responsible for the greatest degree of stimulation. Hence, histamine type 2-receptor antagonists (H2RAs) became a novel means of therapy to reduce stomach acidity. While utilizing H2RAs was effective, it was theorized the downstream inhibition of the action of all 3 neurotransmitters would serve as a more successful therapy. Therefore, PPIs were developed to target the H+-K+-ATPase Over the past decade, many studies have evaluated the long-term PPI adverse effects (AEs). These include calcium and magnesium malabsorption, vitamin B12 deficiency, Clostridium difficile (C difficile) associated disease (CDAD), and community-acquired pneumonia (CAP). Within the past year, data have become available linking PPI use to dementia and chronic kidney disease (CKD).3,4 The following article reviews literature on the safety of long-term PPI use and proposes recommendations for proper use for their most common indications.
Malabsorption
Calcium & Long-Term Fracture Risk
Calcium is an essential component in bone health and formation. In fact, 99% of all calcium found in the body is stored in bones.6 The primary source of calcium is through diet and oral supplements. After it is ingested, calcium is absorbed from the stomach into the blood in a pH dependent manner. If the pH of the stomach is too high (ie, too basic) calcium is not absorbed into blood and remains in the gastrointestinal (GI) tract for fecal excretion. Without sufficient calcium, the body’s osteoclasts and osteoblasts remain inactive, which hinders proper bone turnover.7
The decrease in acidity leads to calcium malabsorption and increases fracture risk long- term.8 Khalili and colleagues surveyed 80,000 postmenopausal women to measure the incidence of hip fracture in women taking PPIs. The study found that there was a 35% increase in risk of hip fracture among women who regularly used PPIs for at least 2 years (age-adjusted hazard ratio [HR] 1.35; 95% confidence interval [CI], 1.13 -1.62). Adjusted HRs for 4-year and 6- to 8-year use of a PPI was 1.42 (95% CI, 1.05-1.93) and 1.55 (95% CI, 1.03-2.32), respectively, indicating that the longer women were on PPI therapy, the higher the risk of hip fracture. The study also evaluated the time since stopping PPI and the risk of hip fracture. Women who stopped PPI use more than 2 years prior had a similar risk to that of women who never used a PPI, indicating that the effect was reversible.9
Magnesium
Magnesium is an important intracellular ion that has a number of key functions in metabolism and ion transport in the human body. Once ingested, magnesium is absorbed into the bloodstream from the small and large intestines via passive and active transport. Transient receptor potential melastatin 6 (TRPM6) is one of the essential proteins that serve as a transporter for magnesium.10 The high affinity for magnesium of these transporters allows them to maintain adequate levels of magnesium in the blood. In states of low magnesium (hypomagnesemia), the body is at risk for many AEs including seizures, arrhythmias, tetany, and hypotension.11
Proton pump inhibitors have been linked to hypomagnesemia, and recent evaluation has clarified a potential mechanism.12 TRPM6 activity is increased in an acidic environment. When a PPI increases the pH of the stomach, TRPM6 and magnesium levels decrease.12 Luk and colleagues identified 66,102 subjects experiencing AEs while taking a PPI. Hypomagnesemia had a prevalence rate of 1% in these patients. According to the researchers, PPIs were associated with hypomagnesemia and that pantoprazole had the highest incidence among all other PPIs studied (OR, 4.3; 95% CI, 3.3 – 5.7; P < .001).13
Vitamin B12
In recent years, vitamin B12 has been the subject of many studies. An area of concern is vitamin B12’s neurologic effect, as it has been successfully demonstrated that vitamin B12 is essential for proper cognitive function.14 Some data suggest that degeneration is present in parts of the spinal column in patients with cognitive decline or neurologic problems. These lesions are due to improper myelin formation and are specific to vitamin B12 deficiency.15 In 2013 the CDC published the Healthy Brain Initiative, which stated cognitive impairment can be caused by vitamin B12 deficiency.16
Similar to calcium, vitamin B12 needs an acidic environment to be digested and absorbed.17 Vitamin B12 is released from food proteins via gastric acid and pepsin. Once free, the vitamin B12 pairs with R-binders secreted in the stomach. Pancreatic enzymes then degrade this complex into a form that can be absorbed into circulation by the intestine. Given that PPIs reduce the acidity of the stomach, they also reduce the body’s ability to release vitamin B12 from food proteins and be paired with the R-binders.18
In 2013, Lam and colleagues evaluated the association between vitamin B12 deficiency and the use of PPIs and H2RAs. An extensive evaluation was performed on 25,956 patients with a diagnosis of vitamin B12 deficiency and 184,199 patients without. About 12% of patients with vitamin B12 deficiency had received more than a 2-year supply of a PPI, whereas only 7.2% of the patients without vitamin B12 deficiency received a 2-year supply of a PPI. Four point 3 percent of patients with vitamin B12 deficiency received more than a 2-year supply of an H2RA. Only 3.2% of patients without vitamin B12 deficiency received more than a 2-year supply of H2RA. The study concluded that a 2-year or greater history of PPI (OR, 1.65; 95% CI, 1.58-1.73) or H2RA (OR, 1.25; 95% CI, 1.17-1.34) use was associated with vitamin B12 deficiency.19
PPIs and Infections
Clostridium difficile-associated disease
Nationwide CDAD has become a prevalent infection nationwide. In 2011, C difficile caused nearly 500,000 infections and was associated with 29,000 deaths in the U.S.20 One study stated that C difficile is the third most common cause of infectious diarrhea in people aged >75 years.21
C difficile is part of the body’s normal flora in the large intestine. It grows and colonizes in an environment of low acidity. Therefore, in the stomach, where the pH is relatively low, C difficile is unable to colonize.22 When a PPI is introduced, the increased gastric pH increases the risk for CDAD.
Dial and colleagues conducted a multicenter case control study to determine whether gastric acid suppression increases the risk of CDAD. Compared with patients who did not take a gastric acid suppressant, those taking a PPI had a 2.9-fold increase in developing CDAD (95% CI, 2.4-3.4). Comparatively, H2RAs had a 2.0-fold increase for CDAD (95% CI, 1.6 to 2.7). These results correlated with the fact that PPIs have a greater impact on gastric pH than do H2RAs.23
Community-Acquired Pneumonia
Community-acquired pneumonia (CAP) has become a growing concern in the U.S. According to the Infectious Disease Society of America (IDSA) and American Thoracic clinical consensus guidelines, CAP remains one of the top reasons for hospitalizations in the U.S., and about 10% of patients admitted to the hospital for CAP end up in the intensive care unit (ICU).24 In the past, PPIs have been linked to patients’ predisposal for developingCAP.25 Although controversial, available evidence suggests a direct association. In 2008 Sarker and colleagues theorized a mechanism that the acid reduction of the gastric lumen allows for increased bacterial colonization in the upper part of the GI tract.26 Since the acidity of the stomach serves as a defense mechanism against many ingested bacteria, many pathogens will be able to survive in the more basic environment.25
Sarkar and colleagues went on to evaluate 80,000 cases over 15 years. The objective was to examine the association between PPI use and the date of diagnosis of the CAP infection, known as the index date. The study demonstrated that PPI use was not associated with increased CAP risk in the long-term (adjusted odds ratio (OR), 1.02; 95% CI, 0.97-1.08). The study did find a strong increase in the risk of CAP if a PPI was started within 2 days (adjusted OR, 6.53; 95% CI, 3.95-10.80), 7 days (adjusted OR, 3.79; 95% CI, 2.66-5.42), and 14 days (adjusted OR, 3.21; 95% CI, 2.46-4.18) of the index date.26
Four years later, de Jagar and colleagues examined the differences in microbial etiology in CAP patients with and without an active PPI. Over a 4-year study period, 463 individuals were selected with clinical suspicion of CAP. The microbial etiology could be determined in 70% of those patients. The remaining 30% were excluded due to an alternative diagnosis. One of the most likely pathogens to cause a CAP infection is Streptococcus pneumoniae (S pneumonia).27 Patients prescribed a PPI were significantly more likely to be infected with S pneumoniae than those not prescribed a PPI (28% vs 11%). The study concluded that the risk of S pneumoniae in patients taking a PPI was 2.23 times more likely (95% CI, 1.28-3.75).28
Dementia
In 2040, it is estimated that more than 80 million people will have from dementia.29 This is expected to become a large fiscal burden on the health care system. In 2010, about $604 billion was spent on therapy for dementia worldwide.30 Although no cure for dementia exists, it is more feasible than in previous years to prevent its occurrence. However, many medications, including PPIs, are associated with the development of dementia; therefore, it is important to minimize their use when possible.
As noted earlier vitamin B12 deficiency may lead to cognitive decline. Due to the malabsorption of vitamin B12 that results from PPI use, it is hypothesized that PPIs may be associated with incidence of dementia. Badiola and colleagues discovered that in the brains of mice given a PPI, levels of β-amyloid increased significantly affecting enzymes responsible for cognition.31 In a February 2016, JAMA article, researchers conducted a prospective cohort study evaluating 73,679 patients aged ≥75 years with no dementia at baseline. They went on to assess regular use of a PPI, defined as at least 1 PPI prescription every 3 months, and the incidence of dementia. Patients with regular use of a PPI (≥ 1 PPI prescription every 3 months) had a 44% increase risk of incident dementia (HR, 1.44; 95% CI, 1.36-1.52; P < .001).3 Therefore, it is theorized that avoiding PPI use in the elderly may prevent the development of dementia.
Chronic Kidney Disease
The prevalence of CKD has drastically increased in recent decades. It is estimated that up to 13% of people in the U.S. are affected by CKD.32 Some studies suggest that dosing errors occur at much higher rates in patients with declined glomerular filtration rate (GFR).33 The correct utilization use of medications becomes especially pertinent to this population. Several studies have already linked PPI use to acute interstitial nephritis (AIN) and acute kidney injury (AKI).34-36
Lazarus and colleagues evaluated the association between PPI use and the incidence of CKD. Their analysis was performed in a long-term running population-based cohort and replicated in a separate health care system. In the running cohort, patients receiving a PPI had a 1.45-fold greater chance of developing CKD (95% CI, 1.11-1.90; P = .006). In that same cohort, patients on a PPI had a 1.72-fold increase risk of AKI (95% CI, 1.28-2.30; P < .001).4 Similar outcomes were seen in the replicated cohort. However, the replicated cohort did observe that twice daily dosing of a PPI (adjusted HR, 1.46; CI, 1.28-1.67; P < .001) had a stronger association with CKD than once- daily dosing (adjusted HR, 1.15; 95% CI, 1.09-1.21; P < .001). H2RAs exhibited no association with CKD in the running cohort (HR, 1.15; 97% CI, 0.98-1.36; P = .10) or the replication cohort (HR, 0.93; 95% CI, 0.88-0.99; P = .03).4
Clinical PPI Recommendations
There are several FDA-approved and unapproved indications that warrant PPI therapy. Proton pump inhibitor indications include gastroesophageal reflux disease (GERD), peptic ulcer disease (PUD), Helicobacter pylori, and ulcers associated with the use of nonsteroidal anti-inflammatory drugs (NSAIDs).
GERD Recommendations
Optimal dosing and duration is important with all medications to maximize efficacy and minimize toxicity. In the case of PPIs, dosing and duration are of particularly concern due to the aforementioned AEs. Table illustrates manufacturer-recommended dosing and duration for the most commonly prescribed PPIs. Although these dosing regimens are based on clinical studies, PPIs are commonly prescribed at higher doses and for longer durations. By extending the duration of therapy, the risk of potential long-term AEs increases dramatically. If durations are limited to the recommended window, risk of AEs can be reduced.
Alternative Therapies
There are several strategies that exist to limit the use of PPIs, including lifestyle modifications to prevent GERD, supplementation of an alternative agent to prevent high doses of the PPI, or discontinuing PPI therapy all together. Lifestyle modifications provide additional benefit as monotherapy or to supplement a pharmacologic regimen.
The American Journal of Gastroenterology promoted lifestyle modifications that include:
- Weight loss for patients with GERD who are overweight and had a recent weight gain;
- Elevation of the head of the bed (if nighttime symptoms present);
- Elimination of dietary triggers;
- Fatty foods, caffeine, chocolate, spicy food, food with high fat content, carbonated beverages, and peppermint;
- Avoiding tight fitting garments to prevent increase in gastric pressure;
- Promote salivation through oral lozenges or chewing gum to neutralize refluxed acid;
- Avoidance of tobacco and alcohol; and
- Abdominal breathing exercise to strengthen the barrier of the lower esophageal sphincter.37
The above modifications may reduce the need for pharmacologic therapy, thereby reducing possible of long-term AEs.
If lifestyle modifications alone are not enough, it is reasonable to use a H2RA for acute symptom relief or reduce high doses and frequencies of a PPI. H2RAs are well studied and effective in the management of GERD. According to the American College of Gastroenterology 2013 clinical practice guidelines, H2RAs can serve as an effective maintenance medication to relieve heartburn in patients without erosive disease. The guideline also states that a bedtime H2RA can be used to supplement a once- daily daytime PPI if nighttime reflux exists. This can eliminate the need to exceed manufacturer-recommended doses.37
One of the final challenges to overcome is a patient that has been maintained on chronic PPI therapy. However, caution should be exercised if choosing to discontinue a PPI. In a study by Niklesson and colleagues, after a 4-week course of pantoprazole given to healthy volunteers, those patients with no preexisting symptoms developed dyspeptic symptoms of GERD, such as heartburn, indigestion, and stomach discomfort. This correlation suggests that a rebound hypersecretion occurs after prolonged suppression of the proton pump, and therefore a gradual taper should be used.38 Although no definitive national recommendations on how to taper a patient off of a PPI exist, one suggestion is a 2- to 3-week taper by using a half-dose once daily or full dose on alternate days.39 This strategy has exhibited moderate success rates when used. Oral and written education on symptom management and the administration of H2RAs for infrequent breakthrough symptoms supplemented the reduction of the PPI.
Conclusion
Proton pump inhibitors have become a popular and effective drug class for a multitude of indications. However, it is crucial to recognize the risk of long-term use. It is important to properly assess the need for a PPI and to use appropriate dosing and duration, since prolonged durations and doses above the manufacturer’s recommendations is a primary contributor to long-term consequences. Both package inserts and clinical guidelines serve as valuable resources to help balance the risks and benefits of this medication class and can help guide therapeutic decisions.
1. U.S. Food and Drug Administration. FDA Drug Safety Communication: Low magnesium levels can be associated with long-term use of Proton Pump Inhibitor drugs (PPIs). http://www.fda.gov/Drugs/DrugSafety/ucm245011.htm. Updated April 7, 2016. Accessed January 12, 2017.
2. Forgacs I. Overprescribing proton pump inhibitors. BMJ. 2008;336(7634):2-3.
3. Gomm W, von Holt K, Thome F, et al. Association of proton pump inhibitors with risk of dementia. JAMA Neurol. 2016;73(4):410-416.
4. Lazarus B, Chen Y, Wilson FP, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med. 2016;176(2):238-246.
5. Wolfe MM, Soll AH. The physiology of gastric acid secretion. N Engl J Med. 1988;319(26):1707-1715.
6. Flynn A. The role of dietary calcium in bone health. Proc Nutr Soc. 2003;62(4):851-858.
7. Mizunashi K, Furukawa Y, Katano K, Abe K. Effect of omeprazole, an inhibitor of H+, K(+)-ATPase, on bone resorption in humans. Calcif Tissue Int. 1993;53(1):21-25.
8. O’Connell MB, Darren DM, Murray AM, Heaney RP, Kerzner LJ. Effects of proton pump inhibitors on calcium carbonate absorption in women: a randomized crossover trial. Am J Med. 2005;118(7):778-781.
9. Khalili H, Huang ES, Jacobson BC, Camargo CA Jr, Feskanich D, Chan AT. Use of proton pump inhibitors and risk of hip fracture in relation to dietary and lifestyle factors: a prospective cohort study. BMJ. 2012;344:e372.
10. Schweigel M, Martens H. Magnesium transport in the gastrointestinal tract. Front Biosci. 2000;5:D666-D677.
11. Hess MW, Hoenderop JG, Bindels RJ, Drenth JP. Systematic review: hypomagnesaemia induced by proton pump inhibition. Aliment Pharmacol Ther. 2012;36(5):405-413.
12. William JH, Danziger J. Proton-pump inhibitor-induced hypomagnesemia: current research and proposed mechanisms. World J Nephrol. 2016;5(2):152-157.
13. Luk CP, Parsons R, Lee YP, Hughes JD. Proton pump inhibitor-associated hypomagnesemia: what do FDA data tell us? Ann Pharmacother. 2013;47(6):773-780.
14. Health Quality Ontario. Vitamin B12 and cognitive function: an evidence-based analysis. Ont Health Technol Assess Ser. 2013;13(23):1-45.
15. Green R, Kinsella LJ. Current concepts in the diagnosis of cobalamin deficiency. Neurology. 1995;45(8):1435-1440.
16. Centers for Disease Control and Prevention. The Healthy Brain Initiative. https://www.cdc.gov/aging/pdf/2013-healthy-brain-initiative.pdf. Accessed January 17, 2017.
17. Toh BH, van Driel IR, Gleeson PA. Pernicious anemia. N Engl J Med. 1997;337(20):1441-1448.
18. Tefferi A, Pruthi RK. The biochemical basis of cobalamin deficiency. Mayo Clin Proc. 1994;69(2):181-186.
19. Lam JR, Schneider JL, Zhao W, Corley DA. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA. 2013;310(22):2435-2442.
20. Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372(9):825-834.
21. National Clostridium difficile Standards Group. National Clostridium difficile Standards Group: report to the Department of Health. J Hosp Infect. 2004;56(suppl 1):1-38.
22. Thorens J, Frohlich F, Schwizer W, et al. Bacterial overgrowth during treatment with omeprazole compared with cimetidine. Gut. 1996;39(1):54-59.
23. Dial S, Delaney JAC, Barkun AN, et al. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA. 2005;294(23):2989-2995
24. Mandell LA, Wunderink RG, Anzueto A, et al; Infectious Diseases Society of America; and American Thoracic Society. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27-S72.
25. Laheij RJ, Sturkenboom MC, Hassing RJ, Dieleman J, Stricker BH, Jansen JB. Risk of community-acquired pneumonia and use of gastric acid-suppressive drugs. JAMA. 2004;292(16):1955-1960.
26. Sarkar M, Hennessy S, Yang Y. Proton-pump inhibitor use and the risk for community-acquired pneumonia. Ann Intern Med. 2008;149(6):391-398.
27. Waterer GW, Wunderink RG. The influence of the severity of community-acquired pneumonia on the usefulness of blood cultures. Respir Med. 2001;95(1):78-82.
28. de Jagar CP, Wever PC, Gemen EF, et al. Proton pump inhibitor therapy predisposes to community-acquired Streptococcus pneumoniae pneumonia. Aliment Pharmacol Ther. 2012;36(10):941-949.
29. Reitz C, Brayne C, Mayeux R. Epidemiology of Alzheimer disease. Nat Rev Neurol. 2011;7(3):137-152.
30. Wimo A, Jönsson L, Bond J, Prince M, Winblad B; Alzheimer Disease International. The worldwide economic impact of dementia 2010. Alzheimers Dement. 2013;9(1):1-11.
31. Badiola N, Alcalde V, Pujol A, et al. The proton-pump inhibitor lansoprazole enhances amyloid beta production. PLoS One. 2013;8(3):e58537.
32. Stevens LA, Li S, Wang C, et al. Prevalence of CKD and comorbid illness in elderly patients in the United States: results from the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis. 2010;55(3)(suppl 2):S23-S33.
33. Weir MR, Fink JC. Safety of medical therapy in patients with chronic kidney disease and end-stage renal disease. Curr Opin Nephrol Hypertens 2014;23(3):306-313.
34. Blank ML, Parkin L, Paul C, Herbison P. A nationwide nested case-control study indicates an increased risk of acute interstitial nephritis with proton pump inhibitor use. Kidney Int. 2014;86(4):837-844.
35. Antoniou T, Macdonald EM, Holland S, et al. Proton pump inhibitors and the risk of acute kidney injury in older patients: a population-based cohort study. CMAJ Open. 2015;3(2):E166-E171.
36. Klepser DG, Collier DS, Cochran GL. Proton pump inhibitors and acute kidney injury: a nested case-control study. BMC Nephrol. 2013;14:150.
37. Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013;108(3):308-328.
38. Niklasson A, Lindström L, Simrén M, Lindberg G, Björnsson E. Dyspeptic symptom development after discontinuation of a proton pump inhibitor: a double-blind placebo-controlled trial. Am J Gastroenterol. 2010;105(7):1531-1537.
39. Haastrup P, Paulsen MS, Begtrup LM, Hansen JM, Jarbøl DE. Strategies for discontinuation of proton pump inhibitors: a systematic review. Fam Pract. 2014;31(6):625-630.
40. Nexium [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals; 2012.
41. Prevacid [package insert]. Deerfield, IL: Takeda Pharmaceuticals; 2012.
42. Prilosec [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals; 2012.
43. Protonix [package insert]. Konstanz, Germany: Pfizer; 2012.
Proton pump inhibitors (PPIs) are one of the most frequently used drug classes, given that they are readily accessible over-the-counter as well as via prescription. About 100 million PPI prescriptions dispensed an
The human stomach uses 3 primary neurotransmitters that regulate gastric acid secretion: acetylcholine (ACh), histamine (H), and gastrin (G). The interactions between these neurotransmitters promote and inhibit hydrogen ion (H+) generation. Stimulation of their corresponding receptors draws H+ into parietal cells that line the stomach. Once in the cell, a H+-K+-ATPase (more commonly known as the proton pump) actively transports H+ into the lumen of the stomach. The H+ bind with chlorine ions to form hydrochloric acid, which increases stomach acidity.5 Histamine receptors were thought to be responsible for the greatest degree of stimulation. Hence, histamine type 2-receptor antagonists (H2RAs) became a novel means of therapy to reduce stomach acidity. While utilizing H2RAs was effective, it was theorized the downstream inhibition of the action of all 3 neurotransmitters would serve as a more successful therapy. Therefore, PPIs were developed to target the H+-K+-ATPase Over the past decade, many studies have evaluated the long-term PPI adverse effects (AEs). These include calcium and magnesium malabsorption, vitamin B12 deficiency, Clostridium difficile (C difficile) associated disease (CDAD), and community-acquired pneumonia (CAP). Within the past year, data have become available linking PPI use to dementia and chronic kidney disease (CKD).3,4 The following article reviews literature on the safety of long-term PPI use and proposes recommendations for proper use for their most common indications.
Malabsorption
Calcium & Long-Term Fracture Risk
Calcium is an essential component in bone health and formation. In fact, 99% of all calcium found in the body is stored in bones.6 The primary source of calcium is through diet and oral supplements. After it is ingested, calcium is absorbed from the stomach into the blood in a pH dependent manner. If the pH of the stomach is too high (ie, too basic) calcium is not absorbed into blood and remains in the gastrointestinal (GI) tract for fecal excretion. Without sufficient calcium, the body’s osteoclasts and osteoblasts remain inactive, which hinders proper bone turnover.7
The decrease in acidity leads to calcium malabsorption and increases fracture risk long- term.8 Khalili and colleagues surveyed 80,000 postmenopausal women to measure the incidence of hip fracture in women taking PPIs. The study found that there was a 35% increase in risk of hip fracture among women who regularly used PPIs for at least 2 years (age-adjusted hazard ratio [HR] 1.35; 95% confidence interval [CI], 1.13 -1.62). Adjusted HRs for 4-year and 6- to 8-year use of a PPI was 1.42 (95% CI, 1.05-1.93) and 1.55 (95% CI, 1.03-2.32), respectively, indicating that the longer women were on PPI therapy, the higher the risk of hip fracture. The study also evaluated the time since stopping PPI and the risk of hip fracture. Women who stopped PPI use more than 2 years prior had a similar risk to that of women who never used a PPI, indicating that the effect was reversible.9
Magnesium
Magnesium is an important intracellular ion that has a number of key functions in metabolism and ion transport in the human body. Once ingested, magnesium is absorbed into the bloodstream from the small and large intestines via passive and active transport. Transient receptor potential melastatin 6 (TRPM6) is one of the essential proteins that serve as a transporter for magnesium.10 The high affinity for magnesium of these transporters allows them to maintain adequate levels of magnesium in the blood. In states of low magnesium (hypomagnesemia), the body is at risk for many AEs including seizures, arrhythmias, tetany, and hypotension.11
Proton pump inhibitors have been linked to hypomagnesemia, and recent evaluation has clarified a potential mechanism.12 TRPM6 activity is increased in an acidic environment. When a PPI increases the pH of the stomach, TRPM6 and magnesium levels decrease.12 Luk and colleagues identified 66,102 subjects experiencing AEs while taking a PPI. Hypomagnesemia had a prevalence rate of 1% in these patients. According to the researchers, PPIs were associated with hypomagnesemia and that pantoprazole had the highest incidence among all other PPIs studied (OR, 4.3; 95% CI, 3.3 – 5.7; P < .001).13
Vitamin B12
In recent years, vitamin B12 has been the subject of many studies. An area of concern is vitamin B12’s neurologic effect, as it has been successfully demonstrated that vitamin B12 is essential for proper cognitive function.14 Some data suggest that degeneration is present in parts of the spinal column in patients with cognitive decline or neurologic problems. These lesions are due to improper myelin formation and are specific to vitamin B12 deficiency.15 In 2013 the CDC published the Healthy Brain Initiative, which stated cognitive impairment can be caused by vitamin B12 deficiency.16
Similar to calcium, vitamin B12 needs an acidic environment to be digested and absorbed.17 Vitamin B12 is released from food proteins via gastric acid and pepsin. Once free, the vitamin B12 pairs with R-binders secreted in the stomach. Pancreatic enzymes then degrade this complex into a form that can be absorbed into circulation by the intestine. Given that PPIs reduce the acidity of the stomach, they also reduce the body’s ability to release vitamin B12 from food proteins and be paired with the R-binders.18
In 2013, Lam and colleagues evaluated the association between vitamin B12 deficiency and the use of PPIs and H2RAs. An extensive evaluation was performed on 25,956 patients with a diagnosis of vitamin B12 deficiency and 184,199 patients without. About 12% of patients with vitamin B12 deficiency had received more than a 2-year supply of a PPI, whereas only 7.2% of the patients without vitamin B12 deficiency received a 2-year supply of a PPI. Four point 3 percent of patients with vitamin B12 deficiency received more than a 2-year supply of an H2RA. Only 3.2% of patients without vitamin B12 deficiency received more than a 2-year supply of H2RA. The study concluded that a 2-year or greater history of PPI (OR, 1.65; 95% CI, 1.58-1.73) or H2RA (OR, 1.25; 95% CI, 1.17-1.34) use was associated with vitamin B12 deficiency.19
PPIs and Infections
Clostridium difficile-associated disease
Nationwide CDAD has become a prevalent infection nationwide. In 2011, C difficile caused nearly 500,000 infections and was associated with 29,000 deaths in the U.S.20 One study stated that C difficile is the third most common cause of infectious diarrhea in people aged >75 years.21
C difficile is part of the body’s normal flora in the large intestine. It grows and colonizes in an environment of low acidity. Therefore, in the stomach, where the pH is relatively low, C difficile is unable to colonize.22 When a PPI is introduced, the increased gastric pH increases the risk for CDAD.
Dial and colleagues conducted a multicenter case control study to determine whether gastric acid suppression increases the risk of CDAD. Compared with patients who did not take a gastric acid suppressant, those taking a PPI had a 2.9-fold increase in developing CDAD (95% CI, 2.4-3.4). Comparatively, H2RAs had a 2.0-fold increase for CDAD (95% CI, 1.6 to 2.7). These results correlated with the fact that PPIs have a greater impact on gastric pH than do H2RAs.23
Community-Acquired Pneumonia
Community-acquired pneumonia (CAP) has become a growing concern in the U.S. According to the Infectious Disease Society of America (IDSA) and American Thoracic clinical consensus guidelines, CAP remains one of the top reasons for hospitalizations in the U.S., and about 10% of patients admitted to the hospital for CAP end up in the intensive care unit (ICU).24 In the past, PPIs have been linked to patients’ predisposal for developingCAP.25 Although controversial, available evidence suggests a direct association. In 2008 Sarker and colleagues theorized a mechanism that the acid reduction of the gastric lumen allows for increased bacterial colonization in the upper part of the GI tract.26 Since the acidity of the stomach serves as a defense mechanism against many ingested bacteria, many pathogens will be able to survive in the more basic environment.25
Sarkar and colleagues went on to evaluate 80,000 cases over 15 years. The objective was to examine the association between PPI use and the date of diagnosis of the CAP infection, known as the index date. The study demonstrated that PPI use was not associated with increased CAP risk in the long-term (adjusted odds ratio (OR), 1.02; 95% CI, 0.97-1.08). The study did find a strong increase in the risk of CAP if a PPI was started within 2 days (adjusted OR, 6.53; 95% CI, 3.95-10.80), 7 days (adjusted OR, 3.79; 95% CI, 2.66-5.42), and 14 days (adjusted OR, 3.21; 95% CI, 2.46-4.18) of the index date.26
Four years later, de Jagar and colleagues examined the differences in microbial etiology in CAP patients with and without an active PPI. Over a 4-year study period, 463 individuals were selected with clinical suspicion of CAP. The microbial etiology could be determined in 70% of those patients. The remaining 30% were excluded due to an alternative diagnosis. One of the most likely pathogens to cause a CAP infection is Streptococcus pneumoniae (S pneumonia).27 Patients prescribed a PPI were significantly more likely to be infected with S pneumoniae than those not prescribed a PPI (28% vs 11%). The study concluded that the risk of S pneumoniae in patients taking a PPI was 2.23 times more likely (95% CI, 1.28-3.75).28
Dementia
In 2040, it is estimated that more than 80 million people will have from dementia.29 This is expected to become a large fiscal burden on the health care system. In 2010, about $604 billion was spent on therapy for dementia worldwide.30 Although no cure for dementia exists, it is more feasible than in previous years to prevent its occurrence. However, many medications, including PPIs, are associated with the development of dementia; therefore, it is important to minimize their use when possible.
As noted earlier vitamin B12 deficiency may lead to cognitive decline. Due to the malabsorption of vitamin B12 that results from PPI use, it is hypothesized that PPIs may be associated with incidence of dementia. Badiola and colleagues discovered that in the brains of mice given a PPI, levels of β-amyloid increased significantly affecting enzymes responsible for cognition.31 In a February 2016, JAMA article, researchers conducted a prospective cohort study evaluating 73,679 patients aged ≥75 years with no dementia at baseline. They went on to assess regular use of a PPI, defined as at least 1 PPI prescription every 3 months, and the incidence of dementia. Patients with regular use of a PPI (≥ 1 PPI prescription every 3 months) had a 44% increase risk of incident dementia (HR, 1.44; 95% CI, 1.36-1.52; P < .001).3 Therefore, it is theorized that avoiding PPI use in the elderly may prevent the development of dementia.
Chronic Kidney Disease
The prevalence of CKD has drastically increased in recent decades. It is estimated that up to 13% of people in the U.S. are affected by CKD.32 Some studies suggest that dosing errors occur at much higher rates in patients with declined glomerular filtration rate (GFR).33 The correct utilization use of medications becomes especially pertinent to this population. Several studies have already linked PPI use to acute interstitial nephritis (AIN) and acute kidney injury (AKI).34-36
Lazarus and colleagues evaluated the association between PPI use and the incidence of CKD. Their analysis was performed in a long-term running population-based cohort and replicated in a separate health care system. In the running cohort, patients receiving a PPI had a 1.45-fold greater chance of developing CKD (95% CI, 1.11-1.90; P = .006). In that same cohort, patients on a PPI had a 1.72-fold increase risk of AKI (95% CI, 1.28-2.30; P < .001).4 Similar outcomes were seen in the replicated cohort. However, the replicated cohort did observe that twice daily dosing of a PPI (adjusted HR, 1.46; CI, 1.28-1.67; P < .001) had a stronger association with CKD than once- daily dosing (adjusted HR, 1.15; 95% CI, 1.09-1.21; P < .001). H2RAs exhibited no association with CKD in the running cohort (HR, 1.15; 97% CI, 0.98-1.36; P = .10) or the replication cohort (HR, 0.93; 95% CI, 0.88-0.99; P = .03).4
Clinical PPI Recommendations
There are several FDA-approved and unapproved indications that warrant PPI therapy. Proton pump inhibitor indications include gastroesophageal reflux disease (GERD), peptic ulcer disease (PUD), Helicobacter pylori, and ulcers associated with the use of nonsteroidal anti-inflammatory drugs (NSAIDs).
GERD Recommendations
Optimal dosing and duration is important with all medications to maximize efficacy and minimize toxicity. In the case of PPIs, dosing and duration are of particularly concern due to the aforementioned AEs. Table illustrates manufacturer-recommended dosing and duration for the most commonly prescribed PPIs. Although these dosing regimens are based on clinical studies, PPIs are commonly prescribed at higher doses and for longer durations. By extending the duration of therapy, the risk of potential long-term AEs increases dramatically. If durations are limited to the recommended window, risk of AEs can be reduced.
Alternative Therapies
There are several strategies that exist to limit the use of PPIs, including lifestyle modifications to prevent GERD, supplementation of an alternative agent to prevent high doses of the PPI, or discontinuing PPI therapy all together. Lifestyle modifications provide additional benefit as monotherapy or to supplement a pharmacologic regimen.
The American Journal of Gastroenterology promoted lifestyle modifications that include:
- Weight loss for patients with GERD who are overweight and had a recent weight gain;
- Elevation of the head of the bed (if nighttime symptoms present);
- Elimination of dietary triggers;
- Fatty foods, caffeine, chocolate, spicy food, food with high fat content, carbonated beverages, and peppermint;
- Avoiding tight fitting garments to prevent increase in gastric pressure;
- Promote salivation through oral lozenges or chewing gum to neutralize refluxed acid;
- Avoidance of tobacco and alcohol; and
- Abdominal breathing exercise to strengthen the barrier of the lower esophageal sphincter.37
The above modifications may reduce the need for pharmacologic therapy, thereby reducing possible of long-term AEs.
If lifestyle modifications alone are not enough, it is reasonable to use a H2RA for acute symptom relief or reduce high doses and frequencies of a PPI. H2RAs are well studied and effective in the management of GERD. According to the American College of Gastroenterology 2013 clinical practice guidelines, H2RAs can serve as an effective maintenance medication to relieve heartburn in patients without erosive disease. The guideline also states that a bedtime H2RA can be used to supplement a once- daily daytime PPI if nighttime reflux exists. This can eliminate the need to exceed manufacturer-recommended doses.37
One of the final challenges to overcome is a patient that has been maintained on chronic PPI therapy. However, caution should be exercised if choosing to discontinue a PPI. In a study by Niklesson and colleagues, after a 4-week course of pantoprazole given to healthy volunteers, those patients with no preexisting symptoms developed dyspeptic symptoms of GERD, such as heartburn, indigestion, and stomach discomfort. This correlation suggests that a rebound hypersecretion occurs after prolonged suppression of the proton pump, and therefore a gradual taper should be used.38 Although no definitive national recommendations on how to taper a patient off of a PPI exist, one suggestion is a 2- to 3-week taper by using a half-dose once daily or full dose on alternate days.39 This strategy has exhibited moderate success rates when used. Oral and written education on symptom management and the administration of H2RAs for infrequent breakthrough symptoms supplemented the reduction of the PPI.
Conclusion
Proton pump inhibitors have become a popular and effective drug class for a multitude of indications. However, it is crucial to recognize the risk of long-term use. It is important to properly assess the need for a PPI and to use appropriate dosing and duration, since prolonged durations and doses above the manufacturer’s recommendations is a primary contributor to long-term consequences. Both package inserts and clinical guidelines serve as valuable resources to help balance the risks and benefits of this medication class and can help guide therapeutic decisions.
Proton pump inhibitors (PPIs) are one of the most frequently used drug classes, given that they are readily accessible over-the-counter as well as via prescription. About 100 million PPI prescriptions dispensed an
The human stomach uses 3 primary neurotransmitters that regulate gastric acid secretion: acetylcholine (ACh), histamine (H), and gastrin (G). The interactions between these neurotransmitters promote and inhibit hydrogen ion (H+) generation. Stimulation of their corresponding receptors draws H+ into parietal cells that line the stomach. Once in the cell, a H+-K+-ATPase (more commonly known as the proton pump) actively transports H+ into the lumen of the stomach. The H+ bind with chlorine ions to form hydrochloric acid, which increases stomach acidity.5 Histamine receptors were thought to be responsible for the greatest degree of stimulation. Hence, histamine type 2-receptor antagonists (H2RAs) became a novel means of therapy to reduce stomach acidity. While utilizing H2RAs was effective, it was theorized the downstream inhibition of the action of all 3 neurotransmitters would serve as a more successful therapy. Therefore, PPIs were developed to target the H+-K+-ATPase Over the past decade, many studies have evaluated the long-term PPI adverse effects (AEs). These include calcium and magnesium malabsorption, vitamin B12 deficiency, Clostridium difficile (C difficile) associated disease (CDAD), and community-acquired pneumonia (CAP). Within the past year, data have become available linking PPI use to dementia and chronic kidney disease (CKD).3,4 The following article reviews literature on the safety of long-term PPI use and proposes recommendations for proper use for their most common indications.
Malabsorption
Calcium & Long-Term Fracture Risk
Calcium is an essential component in bone health and formation. In fact, 99% of all calcium found in the body is stored in bones.6 The primary source of calcium is through diet and oral supplements. After it is ingested, calcium is absorbed from the stomach into the blood in a pH dependent manner. If the pH of the stomach is too high (ie, too basic) calcium is not absorbed into blood and remains in the gastrointestinal (GI) tract for fecal excretion. Without sufficient calcium, the body’s osteoclasts and osteoblasts remain inactive, which hinders proper bone turnover.7
The decrease in acidity leads to calcium malabsorption and increases fracture risk long- term.8 Khalili and colleagues surveyed 80,000 postmenopausal women to measure the incidence of hip fracture in women taking PPIs. The study found that there was a 35% increase in risk of hip fracture among women who regularly used PPIs for at least 2 years (age-adjusted hazard ratio [HR] 1.35; 95% confidence interval [CI], 1.13 -1.62). Adjusted HRs for 4-year and 6- to 8-year use of a PPI was 1.42 (95% CI, 1.05-1.93) and 1.55 (95% CI, 1.03-2.32), respectively, indicating that the longer women were on PPI therapy, the higher the risk of hip fracture. The study also evaluated the time since stopping PPI and the risk of hip fracture. Women who stopped PPI use more than 2 years prior had a similar risk to that of women who never used a PPI, indicating that the effect was reversible.9
Magnesium
Magnesium is an important intracellular ion that has a number of key functions in metabolism and ion transport in the human body. Once ingested, magnesium is absorbed into the bloodstream from the small and large intestines via passive and active transport. Transient receptor potential melastatin 6 (TRPM6) is one of the essential proteins that serve as a transporter for magnesium.10 The high affinity for magnesium of these transporters allows them to maintain adequate levels of magnesium in the blood. In states of low magnesium (hypomagnesemia), the body is at risk for many AEs including seizures, arrhythmias, tetany, and hypotension.11
Proton pump inhibitors have been linked to hypomagnesemia, and recent evaluation has clarified a potential mechanism.12 TRPM6 activity is increased in an acidic environment. When a PPI increases the pH of the stomach, TRPM6 and magnesium levels decrease.12 Luk and colleagues identified 66,102 subjects experiencing AEs while taking a PPI. Hypomagnesemia had a prevalence rate of 1% in these patients. According to the researchers, PPIs were associated with hypomagnesemia and that pantoprazole had the highest incidence among all other PPIs studied (OR, 4.3; 95% CI, 3.3 – 5.7; P < .001).13
Vitamin B12
In recent years, vitamin B12 has been the subject of many studies. An area of concern is vitamin B12’s neurologic effect, as it has been successfully demonstrated that vitamin B12 is essential for proper cognitive function.14 Some data suggest that degeneration is present in parts of the spinal column in patients with cognitive decline or neurologic problems. These lesions are due to improper myelin formation and are specific to vitamin B12 deficiency.15 In 2013 the CDC published the Healthy Brain Initiative, which stated cognitive impairment can be caused by vitamin B12 deficiency.16
Similar to calcium, vitamin B12 needs an acidic environment to be digested and absorbed.17 Vitamin B12 is released from food proteins via gastric acid and pepsin. Once free, the vitamin B12 pairs with R-binders secreted in the stomach. Pancreatic enzymes then degrade this complex into a form that can be absorbed into circulation by the intestine. Given that PPIs reduce the acidity of the stomach, they also reduce the body’s ability to release vitamin B12 from food proteins and be paired with the R-binders.18
In 2013, Lam and colleagues evaluated the association between vitamin B12 deficiency and the use of PPIs and H2RAs. An extensive evaluation was performed on 25,956 patients with a diagnosis of vitamin B12 deficiency and 184,199 patients without. About 12% of patients with vitamin B12 deficiency had received more than a 2-year supply of a PPI, whereas only 7.2% of the patients without vitamin B12 deficiency received a 2-year supply of a PPI. Four point 3 percent of patients with vitamin B12 deficiency received more than a 2-year supply of an H2RA. Only 3.2% of patients without vitamin B12 deficiency received more than a 2-year supply of H2RA. The study concluded that a 2-year or greater history of PPI (OR, 1.65; 95% CI, 1.58-1.73) or H2RA (OR, 1.25; 95% CI, 1.17-1.34) use was associated with vitamin B12 deficiency.19
PPIs and Infections
Clostridium difficile-associated disease
Nationwide CDAD has become a prevalent infection nationwide. In 2011, C difficile caused nearly 500,000 infections and was associated with 29,000 deaths in the U.S.20 One study stated that C difficile is the third most common cause of infectious diarrhea in people aged >75 years.21
C difficile is part of the body’s normal flora in the large intestine. It grows and colonizes in an environment of low acidity. Therefore, in the stomach, where the pH is relatively low, C difficile is unable to colonize.22 When a PPI is introduced, the increased gastric pH increases the risk for CDAD.
Dial and colleagues conducted a multicenter case control study to determine whether gastric acid suppression increases the risk of CDAD. Compared with patients who did not take a gastric acid suppressant, those taking a PPI had a 2.9-fold increase in developing CDAD (95% CI, 2.4-3.4). Comparatively, H2RAs had a 2.0-fold increase for CDAD (95% CI, 1.6 to 2.7). These results correlated with the fact that PPIs have a greater impact on gastric pH than do H2RAs.23
Community-Acquired Pneumonia
Community-acquired pneumonia (CAP) has become a growing concern in the U.S. According to the Infectious Disease Society of America (IDSA) and American Thoracic clinical consensus guidelines, CAP remains one of the top reasons for hospitalizations in the U.S., and about 10% of patients admitted to the hospital for CAP end up in the intensive care unit (ICU).24 In the past, PPIs have been linked to patients’ predisposal for developingCAP.25 Although controversial, available evidence suggests a direct association. In 2008 Sarker and colleagues theorized a mechanism that the acid reduction of the gastric lumen allows for increased bacterial colonization in the upper part of the GI tract.26 Since the acidity of the stomach serves as a defense mechanism against many ingested bacteria, many pathogens will be able to survive in the more basic environment.25
Sarkar and colleagues went on to evaluate 80,000 cases over 15 years. The objective was to examine the association between PPI use and the date of diagnosis of the CAP infection, known as the index date. The study demonstrated that PPI use was not associated with increased CAP risk in the long-term (adjusted odds ratio (OR), 1.02; 95% CI, 0.97-1.08). The study did find a strong increase in the risk of CAP if a PPI was started within 2 days (adjusted OR, 6.53; 95% CI, 3.95-10.80), 7 days (adjusted OR, 3.79; 95% CI, 2.66-5.42), and 14 days (adjusted OR, 3.21; 95% CI, 2.46-4.18) of the index date.26
Four years later, de Jagar and colleagues examined the differences in microbial etiology in CAP patients with and without an active PPI. Over a 4-year study period, 463 individuals were selected with clinical suspicion of CAP. The microbial etiology could be determined in 70% of those patients. The remaining 30% were excluded due to an alternative diagnosis. One of the most likely pathogens to cause a CAP infection is Streptococcus pneumoniae (S pneumonia).27 Patients prescribed a PPI were significantly more likely to be infected with S pneumoniae than those not prescribed a PPI (28% vs 11%). The study concluded that the risk of S pneumoniae in patients taking a PPI was 2.23 times more likely (95% CI, 1.28-3.75).28
Dementia
In 2040, it is estimated that more than 80 million people will have from dementia.29 This is expected to become a large fiscal burden on the health care system. In 2010, about $604 billion was spent on therapy for dementia worldwide.30 Although no cure for dementia exists, it is more feasible than in previous years to prevent its occurrence. However, many medications, including PPIs, are associated with the development of dementia; therefore, it is important to minimize their use when possible.
As noted earlier vitamin B12 deficiency may lead to cognitive decline. Due to the malabsorption of vitamin B12 that results from PPI use, it is hypothesized that PPIs may be associated with incidence of dementia. Badiola and colleagues discovered that in the brains of mice given a PPI, levels of β-amyloid increased significantly affecting enzymes responsible for cognition.31 In a February 2016, JAMA article, researchers conducted a prospective cohort study evaluating 73,679 patients aged ≥75 years with no dementia at baseline. They went on to assess regular use of a PPI, defined as at least 1 PPI prescription every 3 months, and the incidence of dementia. Patients with regular use of a PPI (≥ 1 PPI prescription every 3 months) had a 44% increase risk of incident dementia (HR, 1.44; 95% CI, 1.36-1.52; P < .001).3 Therefore, it is theorized that avoiding PPI use in the elderly may prevent the development of dementia.
Chronic Kidney Disease
The prevalence of CKD has drastically increased in recent decades. It is estimated that up to 13% of people in the U.S. are affected by CKD.32 Some studies suggest that dosing errors occur at much higher rates in patients with declined glomerular filtration rate (GFR).33 The correct utilization use of medications becomes especially pertinent to this population. Several studies have already linked PPI use to acute interstitial nephritis (AIN) and acute kidney injury (AKI).34-36
Lazarus and colleagues evaluated the association between PPI use and the incidence of CKD. Their analysis was performed in a long-term running population-based cohort and replicated in a separate health care system. In the running cohort, patients receiving a PPI had a 1.45-fold greater chance of developing CKD (95% CI, 1.11-1.90; P = .006). In that same cohort, patients on a PPI had a 1.72-fold increase risk of AKI (95% CI, 1.28-2.30; P < .001).4 Similar outcomes were seen in the replicated cohort. However, the replicated cohort did observe that twice daily dosing of a PPI (adjusted HR, 1.46; CI, 1.28-1.67; P < .001) had a stronger association with CKD than once- daily dosing (adjusted HR, 1.15; 95% CI, 1.09-1.21; P < .001). H2RAs exhibited no association with CKD in the running cohort (HR, 1.15; 97% CI, 0.98-1.36; P = .10) or the replication cohort (HR, 0.93; 95% CI, 0.88-0.99; P = .03).4
Clinical PPI Recommendations
There are several FDA-approved and unapproved indications that warrant PPI therapy. Proton pump inhibitor indications include gastroesophageal reflux disease (GERD), peptic ulcer disease (PUD), Helicobacter pylori, and ulcers associated with the use of nonsteroidal anti-inflammatory drugs (NSAIDs).
GERD Recommendations
Optimal dosing and duration is important with all medications to maximize efficacy and minimize toxicity. In the case of PPIs, dosing and duration are of particularly concern due to the aforementioned AEs. Table illustrates manufacturer-recommended dosing and duration for the most commonly prescribed PPIs. Although these dosing regimens are based on clinical studies, PPIs are commonly prescribed at higher doses and for longer durations. By extending the duration of therapy, the risk of potential long-term AEs increases dramatically. If durations are limited to the recommended window, risk of AEs can be reduced.
Alternative Therapies
There are several strategies that exist to limit the use of PPIs, including lifestyle modifications to prevent GERD, supplementation of an alternative agent to prevent high doses of the PPI, or discontinuing PPI therapy all together. Lifestyle modifications provide additional benefit as monotherapy or to supplement a pharmacologic regimen.
The American Journal of Gastroenterology promoted lifestyle modifications that include:
- Weight loss for patients with GERD who are overweight and had a recent weight gain;
- Elevation of the head of the bed (if nighttime symptoms present);
- Elimination of dietary triggers;
- Fatty foods, caffeine, chocolate, spicy food, food with high fat content, carbonated beverages, and peppermint;
- Avoiding tight fitting garments to prevent increase in gastric pressure;
- Promote salivation through oral lozenges or chewing gum to neutralize refluxed acid;
- Avoidance of tobacco and alcohol; and
- Abdominal breathing exercise to strengthen the barrier of the lower esophageal sphincter.37
The above modifications may reduce the need for pharmacologic therapy, thereby reducing possible of long-term AEs.
If lifestyle modifications alone are not enough, it is reasonable to use a H2RA for acute symptom relief or reduce high doses and frequencies of a PPI. H2RAs are well studied and effective in the management of GERD. According to the American College of Gastroenterology 2013 clinical practice guidelines, H2RAs can serve as an effective maintenance medication to relieve heartburn in patients without erosive disease. The guideline also states that a bedtime H2RA can be used to supplement a once- daily daytime PPI if nighttime reflux exists. This can eliminate the need to exceed manufacturer-recommended doses.37
One of the final challenges to overcome is a patient that has been maintained on chronic PPI therapy. However, caution should be exercised if choosing to discontinue a PPI. In a study by Niklesson and colleagues, after a 4-week course of pantoprazole given to healthy volunteers, those patients with no preexisting symptoms developed dyspeptic symptoms of GERD, such as heartburn, indigestion, and stomach discomfort. This correlation suggests that a rebound hypersecretion occurs after prolonged suppression of the proton pump, and therefore a gradual taper should be used.38 Although no definitive national recommendations on how to taper a patient off of a PPI exist, one suggestion is a 2- to 3-week taper by using a half-dose once daily or full dose on alternate days.39 This strategy has exhibited moderate success rates when used. Oral and written education on symptom management and the administration of H2RAs for infrequent breakthrough symptoms supplemented the reduction of the PPI.
Conclusion
Proton pump inhibitors have become a popular and effective drug class for a multitude of indications. However, it is crucial to recognize the risk of long-term use. It is important to properly assess the need for a PPI and to use appropriate dosing and duration, since prolonged durations and doses above the manufacturer’s recommendations is a primary contributor to long-term consequences. Both package inserts and clinical guidelines serve as valuable resources to help balance the risks and benefits of this medication class and can help guide therapeutic decisions.
1. U.S. Food and Drug Administration. FDA Drug Safety Communication: Low magnesium levels can be associated with long-term use of Proton Pump Inhibitor drugs (PPIs). http://www.fda.gov/Drugs/DrugSafety/ucm245011.htm. Updated April 7, 2016. Accessed January 12, 2017.
2. Forgacs I. Overprescribing proton pump inhibitors. BMJ. 2008;336(7634):2-3.
3. Gomm W, von Holt K, Thome F, et al. Association of proton pump inhibitors with risk of dementia. JAMA Neurol. 2016;73(4):410-416.
4. Lazarus B, Chen Y, Wilson FP, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med. 2016;176(2):238-246.
5. Wolfe MM, Soll AH. The physiology of gastric acid secretion. N Engl J Med. 1988;319(26):1707-1715.
6. Flynn A. The role of dietary calcium in bone health. Proc Nutr Soc. 2003;62(4):851-858.
7. Mizunashi K, Furukawa Y, Katano K, Abe K. Effect of omeprazole, an inhibitor of H+, K(+)-ATPase, on bone resorption in humans. Calcif Tissue Int. 1993;53(1):21-25.
8. O’Connell MB, Darren DM, Murray AM, Heaney RP, Kerzner LJ. Effects of proton pump inhibitors on calcium carbonate absorption in women: a randomized crossover trial. Am J Med. 2005;118(7):778-781.
9. Khalili H, Huang ES, Jacobson BC, Camargo CA Jr, Feskanich D, Chan AT. Use of proton pump inhibitors and risk of hip fracture in relation to dietary and lifestyle factors: a prospective cohort study. BMJ. 2012;344:e372.
10. Schweigel M, Martens H. Magnesium transport in the gastrointestinal tract. Front Biosci. 2000;5:D666-D677.
11. Hess MW, Hoenderop JG, Bindels RJ, Drenth JP. Systematic review: hypomagnesaemia induced by proton pump inhibition. Aliment Pharmacol Ther. 2012;36(5):405-413.
12. William JH, Danziger J. Proton-pump inhibitor-induced hypomagnesemia: current research and proposed mechanisms. World J Nephrol. 2016;5(2):152-157.
13. Luk CP, Parsons R, Lee YP, Hughes JD. Proton pump inhibitor-associated hypomagnesemia: what do FDA data tell us? Ann Pharmacother. 2013;47(6):773-780.
14. Health Quality Ontario. Vitamin B12 and cognitive function: an evidence-based analysis. Ont Health Technol Assess Ser. 2013;13(23):1-45.
15. Green R, Kinsella LJ. Current concepts in the diagnosis of cobalamin deficiency. Neurology. 1995;45(8):1435-1440.
16. Centers for Disease Control and Prevention. The Healthy Brain Initiative. https://www.cdc.gov/aging/pdf/2013-healthy-brain-initiative.pdf. Accessed January 17, 2017.
17. Toh BH, van Driel IR, Gleeson PA. Pernicious anemia. N Engl J Med. 1997;337(20):1441-1448.
18. Tefferi A, Pruthi RK. The biochemical basis of cobalamin deficiency. Mayo Clin Proc. 1994;69(2):181-186.
19. Lam JR, Schneider JL, Zhao W, Corley DA. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA. 2013;310(22):2435-2442.
20. Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372(9):825-834.
21. National Clostridium difficile Standards Group. National Clostridium difficile Standards Group: report to the Department of Health. J Hosp Infect. 2004;56(suppl 1):1-38.
22. Thorens J, Frohlich F, Schwizer W, et al. Bacterial overgrowth during treatment with omeprazole compared with cimetidine. Gut. 1996;39(1):54-59.
23. Dial S, Delaney JAC, Barkun AN, et al. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA. 2005;294(23):2989-2995
24. Mandell LA, Wunderink RG, Anzueto A, et al; Infectious Diseases Society of America; and American Thoracic Society. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27-S72.
25. Laheij RJ, Sturkenboom MC, Hassing RJ, Dieleman J, Stricker BH, Jansen JB. Risk of community-acquired pneumonia and use of gastric acid-suppressive drugs. JAMA. 2004;292(16):1955-1960.
26. Sarkar M, Hennessy S, Yang Y. Proton-pump inhibitor use and the risk for community-acquired pneumonia. Ann Intern Med. 2008;149(6):391-398.
27. Waterer GW, Wunderink RG. The influence of the severity of community-acquired pneumonia on the usefulness of blood cultures. Respir Med. 2001;95(1):78-82.
28. de Jagar CP, Wever PC, Gemen EF, et al. Proton pump inhibitor therapy predisposes to community-acquired Streptococcus pneumoniae pneumonia. Aliment Pharmacol Ther. 2012;36(10):941-949.
29. Reitz C, Brayne C, Mayeux R. Epidemiology of Alzheimer disease. Nat Rev Neurol. 2011;7(3):137-152.
30. Wimo A, Jönsson L, Bond J, Prince M, Winblad B; Alzheimer Disease International. The worldwide economic impact of dementia 2010. Alzheimers Dement. 2013;9(1):1-11.
31. Badiola N, Alcalde V, Pujol A, et al. The proton-pump inhibitor lansoprazole enhances amyloid beta production. PLoS One. 2013;8(3):e58537.
32. Stevens LA, Li S, Wang C, et al. Prevalence of CKD and comorbid illness in elderly patients in the United States: results from the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis. 2010;55(3)(suppl 2):S23-S33.
33. Weir MR, Fink JC. Safety of medical therapy in patients with chronic kidney disease and end-stage renal disease. Curr Opin Nephrol Hypertens 2014;23(3):306-313.
34. Blank ML, Parkin L, Paul C, Herbison P. A nationwide nested case-control study indicates an increased risk of acute interstitial nephritis with proton pump inhibitor use. Kidney Int. 2014;86(4):837-844.
35. Antoniou T, Macdonald EM, Holland S, et al. Proton pump inhibitors and the risk of acute kidney injury in older patients: a population-based cohort study. CMAJ Open. 2015;3(2):E166-E171.
36. Klepser DG, Collier DS, Cochran GL. Proton pump inhibitors and acute kidney injury: a nested case-control study. BMC Nephrol. 2013;14:150.
37. Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013;108(3):308-328.
38. Niklasson A, Lindström L, Simrén M, Lindberg G, Björnsson E. Dyspeptic symptom development after discontinuation of a proton pump inhibitor: a double-blind placebo-controlled trial. Am J Gastroenterol. 2010;105(7):1531-1537.
39. Haastrup P, Paulsen MS, Begtrup LM, Hansen JM, Jarbøl DE. Strategies for discontinuation of proton pump inhibitors: a systematic review. Fam Pract. 2014;31(6):625-630.
40. Nexium [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals; 2012.
41. Prevacid [package insert]. Deerfield, IL: Takeda Pharmaceuticals; 2012.
42. Prilosec [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals; 2012.
43. Protonix [package insert]. Konstanz, Germany: Pfizer; 2012.
1. U.S. Food and Drug Administration. FDA Drug Safety Communication: Low magnesium levels can be associated with long-term use of Proton Pump Inhibitor drugs (PPIs). http://www.fda.gov/Drugs/DrugSafety/ucm245011.htm. Updated April 7, 2016. Accessed January 12, 2017.
2. Forgacs I. Overprescribing proton pump inhibitors. BMJ. 2008;336(7634):2-3.
3. Gomm W, von Holt K, Thome F, et al. Association of proton pump inhibitors with risk of dementia. JAMA Neurol. 2016;73(4):410-416.
4. Lazarus B, Chen Y, Wilson FP, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med. 2016;176(2):238-246.
5. Wolfe MM, Soll AH. The physiology of gastric acid secretion. N Engl J Med. 1988;319(26):1707-1715.
6. Flynn A. The role of dietary calcium in bone health. Proc Nutr Soc. 2003;62(4):851-858.
7. Mizunashi K, Furukawa Y, Katano K, Abe K. Effect of omeprazole, an inhibitor of H+, K(+)-ATPase, on bone resorption in humans. Calcif Tissue Int. 1993;53(1):21-25.
8. O’Connell MB, Darren DM, Murray AM, Heaney RP, Kerzner LJ. Effects of proton pump inhibitors on calcium carbonate absorption in women: a randomized crossover trial. Am J Med. 2005;118(7):778-781.
9. Khalili H, Huang ES, Jacobson BC, Camargo CA Jr, Feskanich D, Chan AT. Use of proton pump inhibitors and risk of hip fracture in relation to dietary and lifestyle factors: a prospective cohort study. BMJ. 2012;344:e372.
10. Schweigel M, Martens H. Magnesium transport in the gastrointestinal tract. Front Biosci. 2000;5:D666-D677.
11. Hess MW, Hoenderop JG, Bindels RJ, Drenth JP. Systematic review: hypomagnesaemia induced by proton pump inhibition. Aliment Pharmacol Ther. 2012;36(5):405-413.
12. William JH, Danziger J. Proton-pump inhibitor-induced hypomagnesemia: current research and proposed mechanisms. World J Nephrol. 2016;5(2):152-157.
13. Luk CP, Parsons R, Lee YP, Hughes JD. Proton pump inhibitor-associated hypomagnesemia: what do FDA data tell us? Ann Pharmacother. 2013;47(6):773-780.
14. Health Quality Ontario. Vitamin B12 and cognitive function: an evidence-based analysis. Ont Health Technol Assess Ser. 2013;13(23):1-45.
15. Green R, Kinsella LJ. Current concepts in the diagnosis of cobalamin deficiency. Neurology. 1995;45(8):1435-1440.
16. Centers for Disease Control and Prevention. The Healthy Brain Initiative. https://www.cdc.gov/aging/pdf/2013-healthy-brain-initiative.pdf. Accessed January 17, 2017.
17. Toh BH, van Driel IR, Gleeson PA. Pernicious anemia. N Engl J Med. 1997;337(20):1441-1448.
18. Tefferi A, Pruthi RK. The biochemical basis of cobalamin deficiency. Mayo Clin Proc. 1994;69(2):181-186.
19. Lam JR, Schneider JL, Zhao W, Corley DA. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA. 2013;310(22):2435-2442.
20. Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372(9):825-834.
21. National Clostridium difficile Standards Group. National Clostridium difficile Standards Group: report to the Department of Health. J Hosp Infect. 2004;56(suppl 1):1-38.
22. Thorens J, Frohlich F, Schwizer W, et al. Bacterial overgrowth during treatment with omeprazole compared with cimetidine. Gut. 1996;39(1):54-59.
23. Dial S, Delaney JAC, Barkun AN, et al. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA. 2005;294(23):2989-2995
24. Mandell LA, Wunderink RG, Anzueto A, et al; Infectious Diseases Society of America; and American Thoracic Society. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27-S72.
25. Laheij RJ, Sturkenboom MC, Hassing RJ, Dieleman J, Stricker BH, Jansen JB. Risk of community-acquired pneumonia and use of gastric acid-suppressive drugs. JAMA. 2004;292(16):1955-1960.
26. Sarkar M, Hennessy S, Yang Y. Proton-pump inhibitor use and the risk for community-acquired pneumonia. Ann Intern Med. 2008;149(6):391-398.
27. Waterer GW, Wunderink RG. The influence of the severity of community-acquired pneumonia on the usefulness of blood cultures. Respir Med. 2001;95(1):78-82.
28. de Jagar CP, Wever PC, Gemen EF, et al. Proton pump inhibitor therapy predisposes to community-acquired Streptococcus pneumoniae pneumonia. Aliment Pharmacol Ther. 2012;36(10):941-949.
29. Reitz C, Brayne C, Mayeux R. Epidemiology of Alzheimer disease. Nat Rev Neurol. 2011;7(3):137-152.
30. Wimo A, Jönsson L, Bond J, Prince M, Winblad B; Alzheimer Disease International. The worldwide economic impact of dementia 2010. Alzheimers Dement. 2013;9(1):1-11.
31. Badiola N, Alcalde V, Pujol A, et al. The proton-pump inhibitor lansoprazole enhances amyloid beta production. PLoS One. 2013;8(3):e58537.
32. Stevens LA, Li S, Wang C, et al. Prevalence of CKD and comorbid illness in elderly patients in the United States: results from the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis. 2010;55(3)(suppl 2):S23-S33.
33. Weir MR, Fink JC. Safety of medical therapy in patients with chronic kidney disease and end-stage renal disease. Curr Opin Nephrol Hypertens 2014;23(3):306-313.
34. Blank ML, Parkin L, Paul C, Herbison P. A nationwide nested case-control study indicates an increased risk of acute interstitial nephritis with proton pump inhibitor use. Kidney Int. 2014;86(4):837-844.
35. Antoniou T, Macdonald EM, Holland S, et al. Proton pump inhibitors and the risk of acute kidney injury in older patients: a population-based cohort study. CMAJ Open. 2015;3(2):E166-E171.
36. Klepser DG, Collier DS, Cochran GL. Proton pump inhibitors and acute kidney injury: a nested case-control study. BMC Nephrol. 2013;14:150.
37. Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013;108(3):308-328.
38. Niklasson A, Lindström L, Simrén M, Lindberg G, Björnsson E. Dyspeptic symptom development after discontinuation of a proton pump inhibitor: a double-blind placebo-controlled trial. Am J Gastroenterol. 2010;105(7):1531-1537.
39. Haastrup P, Paulsen MS, Begtrup LM, Hansen JM, Jarbøl DE. Strategies for discontinuation of proton pump inhibitors: a systematic review. Fam Pract. 2014;31(6):625-630.
40. Nexium [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals; 2012.
41. Prevacid [package insert]. Deerfield, IL: Takeda Pharmaceuticals; 2012.
42. Prilosec [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals; 2012.
43. Protonix [package insert]. Konstanz, Germany: Pfizer; 2012.
Biosimilars in Psoriasis: The Future or Not?
According to the US Food and Drug Administration (FDA), a biosimilar is “highly similar to an FDA-approved biological product, . . . and has no clinically meaningful differences in terms of safety and effectiveness.”1 The Biologics Price Competition and Innovation (BPCI) Act of 2009 created an expedited pathway for the approval of products shown to be biosimilar to FDA-licensed reference products.2 In 2013, the European Medicines Agency approved the first biosimilar modeled on infliximab (Remsima [formerly known as CT-P13], Celltrion Healthcare Co, Ltd) for the same indications as its reference product.3 In 2016, the FDA approved Inflectra (Hospira, a Pfizer Company), an infliximab biosimilar; Erelzi (Sandoz, a Novartis Division), an etanercept biosimilar; and Amjevita (Amgen Inc), an adalimumab biosimilar, all for numerous clinical indications including plaque psoriasis and psoriatic arthritis.4-6
There has been a substantial amount of distrust surrounding the biosimilars; however, as the patents for the biologic agents expire, new biosimilars will undoubtedly flood the market. In this article, we provide information that will help dermatologists understand the need for and use of these agents.
Biosimilars Versus Generic Drugs
Small-molecule generics can be made in a process that is relatively inexpensive, reproducible, and able to yield identical products with each lot.7 In contrast, biosimilars are large complex proteins made in living cells. They differ from their reference product because of changes that occur during manufacturing (eg, purification system, posttranslational modifications).7-9 Glycosylation is particularly sensitive to manufacturing and can affect the immunogenicity of the product.9 The impact of manufacturing can be substantial; for example, during phase 3 trials for efalizumab, a change in the manufacturing facility affected pharmacokinetic properties to such a degree that the FDA required a repeat of the trials.10
FDA Guidelines on Biosimilarity
The FDA outlines the following approach to demonstrate biosimilarity.2 The first step is structural characterization to evaluate the primary, secondary, tertiary, and quaternary structures and posttranslational modifications. The next step utilizes in vivo and/or in vitro functional assays to compare the biosimilar and reference product. The third step is a focus on toxicity and immunogenicity. The fourth step involves clinical studies to study pharmacokinetic and pharmacodynamic data, immunogenicity, safety, and efficacy. After the biosimilar has been approved, there must be a system in place to monitor postmarketing safety. If a biosimilar is tested in one patient population (eg, patients with plaque psoriasis), a request can be made to approve the drug for all the conditions that the reference product was approved for, such as plaque psoriasis, rheumatoid arthritis, and inflammatory bowel disease, even though clinical trials were not performed in all of these patient populations.2 The BPCI Act leaves it up to the FDA to determine how much and what type of data (eg, in vitro, in vivo, clinical) are required.11
Extrapolation and Interchangeability
Once a biosimilar has been approved, 2 questions must be answered: First, can its use be extrapolated to all indications for the reference product? The infliximab biosimilar approved by the European Medicines Agency and the FDA had only been studied in patients with ankylosing spondylitis12 and rheumatoid arthritis,13 yet it was granted all the indications for infliximab, including severe plaque psoriasis.14 As of now, the various regulatory agencies differ on their policies regarding extrapolation. Extrapolation is not automatically bestowed on a biosimilar in the United States but can be requested by the manufacturer.2
Second, can the biosimilar be seamlessly switched with its reference product at the pharmacy level? The BPCI Act allows for the substitution of biosimilars that are deemed interchangeable without notifying the provider, yet individual states ultimately can pass laws regarding this issue.15,16 An interchangeable agent would “produce the same clinical result as the reference product,” and “the risk in terms of safety or diminished efficacy of alternating or switching between use of the biological product and the reference product is not greater than the risk of using the reference product.”15 Generic drugs are allowed to be substituted without notifying the patient or prescriber16; however, biosimilars that are not deemed interchangeable would require permission from the prescriber before substitution.11
Biosimilars for Psoriasis
In April 2016, an infliximab biosimilar (Inflectra) became the second biosimilar approved by the FDA.4 Inflectra was studied in clinical trials for patients with ankylosing spondylitis17 and rheumatoid arthritis,18 and in both trials the biosimilar was found to have similar efficacy and safety profiles to that of the reference product. In August 2016, an etanercept biosimilar (Erelzi) was approved,5 and in September 2016, an adalimumab biosimilar (Amjevita) was approved.6
The Table summarizes clinical trials (both completed and ongoing) evaluating biosimilars in adults with plaque psoriasis; thus far, there are 2464 participants enrolled across 5 different studies of adalimumab biosimilars (registered at www.clinicaltrials.gov with the identifiers NCT01970488, NCT02016105, NCT02489227, NCT02714322, NCT02581345) and 531 participants in an etanercept biosimilar study (NCT01891864).
A phase 3 double-blind study compared adalimumab to an adalimumab biosimilar (ABP 501) in 350 adults with plaque psoriasis (NCT01970488). Participants received an initial loading dose of adalimumab (n=175) or ABP 501 (n=175) 80 mg subcutaneously on week 1/day 1, followed by 40 mg at week 2 every 2 weeks thereafter. At week 16, participants with psoriasis area and severity index (PASI) 50 or greater remained in the study for up to 52 weeks; those who were receiving adalimumab were re-randomized to receive either ABP 501 or adalimumab. Participants receiving ABP 501 continued to receive the biosimilar. The mean PASI improvement at weeks 16, 32, and 50 was 86.6, 87.6, and 87.2, respectively, in the ABP 501/ABP 501 group (A/A) compared to 88.0, 88.2, and 88.1, respectively, in the adalimumab/adalimumab group (B/B).19 Autoantibodies developed in 68.4% of participants in the A/A group compared to 74.7% in the B/B group. The incidence of treatment-emergent adverse events (TEAEs) was 86.2% in the A/A group and 78.5% in the B/B group. The most common TEAEs were nasopharyngitis, headache, and upper respiratory tract infection. The incidence of serious TEAEs was 4.6% in the A/A group compared to 5.1% in the B/B group. Overall, the efficacy, safety, and immunogenicity of the adalimumab biosimilar was comparable to the reference product.19
A second phase 3 trial (ADACCESS) evaluated the adalimumab biosimilar GP2017 (NCT02016105). Participants received an initial dose of 80 mg subcutaneously of either GP2017 or adalimumab at week 0, followed by 40 mg every other week starting at week 1 and ending at week 51. The study has been completed but results are not yet available.
The third trial is evaluating the adalimumab biosimilar CHS-1420 (NCT02489227). Participants in the experimental arm receive two 40-mg doses of CHS-1420 at week 0/day 0, and then 1 dose every 2 weeks from week 1 for 23 weeks. At week 24, participants continue with an open-label study. Participants in the adalimumab group receive two 40-mg doses at week 0/day 0, and then 1 dose every 2 weeks from week 1 to week 15. At week 16, participants will be re-randomized (1:1) to continue adalimumab or start CHS-1420 at one 40-mg dose every 2 weeks during weeks 17 to 23. At week 24, participants will switch to CHS-1420 open label until the end of the study. Study results are not yet available; the study is ongoing but not recruiting.
The fourth ongoing trial is evaluating the adalimumab biosimilar MYL-1401A (NCT02714322). Participants receive an initial dose of 80 mg subcutaneously of either MYL-1401A or adalimumab (2:1), followed by 40 mg every other week starting 1 week after the initial dose. After the 52-week treatment period, there is an 8-week safety follow-up period. Study results are not yet available; the study is ongoing but not recruiting.
A fifth adalimumab biosimilar, M923, also is currently being tested in clinical trials (NCT02581345). Participants receive either M923, adalimumab, or alternate between the 2 agents. Although the study is still ongoing, data released from the manufacturer state that the proportion of participants who achieved PASI 75 after 16 weeks of treatment was equivalent in the 2 treatment groups. The proportion of participants who achieved PASI 90, as well as the type, frequency, and severity of adverse events, also were comparable.20
The EGALITY trial, completed in March 2015, compared the etanercept biosimilar GP2015 to etanercept over a 52-week period (NCT01891864). Participants received either GP2015 or etanercept 50 mg twice weekly for the first 12 weeks. Participants with at least PASI 50 were then re-randomized into 4 groups: the first 2 groups stayed with their current treatments while the other 2 groups alternated treatments every 6 weeks until week 30. Participants then stayed on their last treatment from week 30 to week 52. The adjusted PASI 75 response rate at week 12 was 73.4% in the group receiving GP2015 and 75.7% in the group receiving etanercept.21 The percentage change in PASI score at all time points was found to be comparable from baseline until week 52. Importantly, the incidence of TEAEs up to week 52 was comparable and no new safety issues were reported. Additionally, switching participants from etanercept to the biosimilar during the subsequent treatment periods did not cause an increase in formation of antidrug antibodies.21
There are 2 upcoming studies involving biosimilars that are not yet recruiting patients. The first (NCT02925338) will analyze the characteristics of patients treated with Inflectra as well as their response to treatment. The second (NCT02762955) will be comparing the efficacy and safety of an adalimumab biosimilar (BCD-057, BIOCAD) to adalimumab.
Economic Advantages of Biosimilars
The annual economic burden of psoriasis in the United States is substantial, with estimates between $35.2 billion22 and $112 billion.23 Biosimilars can be 25% to 30% cheaper than their reference products9,11,24 and have the potential to save the US health care system billions of dollars.25 Furthermore, the developers of biosimilars could offer patient assistance programs.11 That being said, drug developers can extend patents for their branded drugs; for instance, 2 patents for Enbrel (Amgen Inc) could protect the drug until 2029.26,27
Although cost is an important factor in deciding which medications to prescribe for patients, it should never take precedence over safety and efficacy. Manufacturers can develop new drugs with greater efficacy, fewer side effects, or more convenient dosing schedules,26,27 or they could offer co-payment assistance programs.26,28 Physicians also must consider how the biosimilars will be integrated into drug formularies. Would patients be required to use a biosimilar before a branded drug?11,29 Will patients already taking a branded drug be grandfathered in?11 Would they have to pay a premium to continue taking their drug? And finally, could changes in formularies and employer-payer relationships destabilize patient regimens?30
Conclusion
Preliminary results suggest that biosimilars can have similar safety, efficacy, and immunogenicity data compared to their reference products.19,21 Biosimilars have the potential to greatly reduce the cost burden associated with psoriasis. However, how similar is “highly similar”? Although cost is an important consideration in selecting drug therapies, the reason for using a biosimilar should never be based on cost alone.
- Information on biosimilars. US Food and Drug Administration website. http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/TherapeuticBiologicApplications/Biosimilars/. Updated May 10, 2016. Accessed July 5, 2016.
- US Department of Health and Human Services. Scientific Considerations in Demonstrating Biosimilarity to a Reference Product: Guidance for Industry. Silver Spring, MD: US Food and Drug Administration; 2015.
- McKeage K. A review of CT-P13: an infliximab biosimilar. BioDrugs. 2014;28:313-321.
- FDA approves Inflectra, a biosimilar to Remicade [news release]. Silver Spring, MD: US Food and Drug Administration; April 5, 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm494227.htm. Updated April 20, 2016. Accessed January 23, 2017.
- FDA approves Erelzi, a biosimilar to Enbrel [news release]. Silver Spring, MD: US Food and Drug Administration; August 30, 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm518639.htm. Accessed January 23, 2017.
- FDA approves Amjevita, a biosimilar to Humira [news release]. Silver Spring, MD: US Food and Drug Administration; September 23, 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm522243.htm. Accessed January 23, 2017.
- Scott BJ, Klein AV, Wang J. Biosimilar monoclonal antibodies: a Canadian regulatory perspective on the assessment of clinically relevant differences and indication extrapolation [published online June 26, 2014]. J Clin Pharmacol. 2015;55(suppl 3):S123-S132.
- Mellstedt H, Niederwieser D, Ludwig H. The challenge of biosimilars [published online September 14, 2007]. Ann Oncol. 2008;19:411-419.
- Puig L. Biosimilars and reference biologics: decisions on biosimilar interchangeability require the involvement of dermatologists [published online October 2, 2013]. Actas Dermosifiliogr. 2014;105:435-437.
- Strober BE, Armour K, Romiti R, et al. Biopharmaceuticals and biosimilars in psoriasis: what the dermatologist needs to know. J Am Acad Dermatol. 2012;66:317-322.
- Falit BP, Singh SC, Brennan TA. Biosimilar competition in the United States: statutory incentives, payers, and pharmacy benefit managers. Health Aff (Millwood). 2015;34:294-301.
- Park W, Hrycaj P, Jeka S, et al. A randomised, double-blind, multicentre, parallel-group, prospective study comparing the pharmacokinetics, safety, and efficacy of CT-P13 and innovator infliximab in patients with ankylosing spondylitis: the PLANETAS study. Ann Rheum Dis. 2013;72:1605-1612.
- Yoo DH, Hrycaj P, Miranda P, et al. A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study. Ann Rheum Dis. 2013;72:1613-1620.
- Carretero Hernandez G, Puig L. The use of biosimilar drugs in psoriasis: a position paper. Actas Dermosifiliogr. 2015;106:249-251.
- Regulation of Biological Products, 42 USC §262 (2013).
- Ventola CL. Evaluation of biosimilars for formulary inclusion: factors for consideration by P&T committees. P T. 2015;40:680-689.
- Park W, Yoo DH, Jaworski J, et al. Comparable long-term efficacy, as assessed by patient-reported outcomes, safety and pharmacokinetics, of CT-P13 and reference infliximab in patients with ankylosing spondylitis: 54-week results from the randomized, parallel-group PLANETAS study. Arthritis Res Ther. 2016;18:25.
- Yoo DH, Racewicz A, Brzezicki J, et al. A phase III randomized study to evaluate the efficacy and safety of CT-P13 compared with reference infliximab in patients with active rheumatoid arthritis: 54-week results from the PLANETRA study. Arthritis Res Ther. 2015;18:82.
- Strober B, Foley P, Philipp S, et al. Evaluation of efficacy and safety of ABP 501 in a phase 3 study in subjects with moderate to severe plaque psoriasis: 52-week results. J Am Acad Dermatol. 2016;74(5, suppl 1):AB249.
- Momenta Pharmaceuticals announces positive top-line phase 3 results for M923, a proposed Humira (adalimumab) biosimilar [news release]. Cambridge, MA: Momenta Pharmaceuticals, Inc; November 29, 2016. http://ir.momentapharma.com/releasedetail.cfm?ReleaseID=1001255. Accessed January 25, 2017.
- Griffiths CE, Thaci D, Gerdes S, et al. The EGALITY study: a confirmatory, randomised, double-blind study comparing the efficacy, safety and immunogenicity of GP2015, a proposed etanercept biosimilar, versus the originator product in patients with moderate to severe chronic plaque-type psoriasis [published online October 27, 2016]. Br J Dermatol. doi:10.1111/bjd.15152.
- Vanderpuye-Orgle J, Zhao Y, Lu J, et al. Evaluating the economic burden of psoriasis in the United States [published online April 14, 2015]. J Am Acad Dermatol. 2015;72:961-967.
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151:651-658.
- Menter MA, Griffiths CE. Psoriasis: the future. Dermatol Clin. 2015;33:161-166.
- Hackbarth GM, Crosson FJ, Miller ME. Report to the Congress: improving incentives in the Medicare program. Medicare Payment Advisory Commission, Washington, DC; 2009.
- Lovenworth SJ. The new biosimilar era: the basics, the landscape, and the future. Bloomberg website. http://about.bloomberglaw.com/practitioner-contributions/the-new-biosimilar-era-the-basics-the-landscape-and-the-future. Published September 21, 2012. Accessed July 6, 2016.
- Blackstone EA, Joseph PF. The economics of biosimilars. Am Health Drug Benefits. 2013;6:469-478.
- Calvo B, Zuniga L. The US approach to biosimilars: the long-awaited FDA approval pathway. BioDrugs. 2012;26:357-361.
- Lucio SD, Stevenson JG, Hoffman JM. Biosimilars: implications for health-system pharmacists. Am J Health Syst Pharm. 2013;70:2004-2017.
- Barriers to access attributed to formulary changes. Manag Care. 2012;21:41.
According to the US Food and Drug Administration (FDA), a biosimilar is “highly similar to an FDA-approved biological product, . . . and has no clinically meaningful differences in terms of safety and effectiveness.”1 The Biologics Price Competition and Innovation (BPCI) Act of 2009 created an expedited pathway for the approval of products shown to be biosimilar to FDA-licensed reference products.2 In 2013, the European Medicines Agency approved the first biosimilar modeled on infliximab (Remsima [formerly known as CT-P13], Celltrion Healthcare Co, Ltd) for the same indications as its reference product.3 In 2016, the FDA approved Inflectra (Hospira, a Pfizer Company), an infliximab biosimilar; Erelzi (Sandoz, a Novartis Division), an etanercept biosimilar; and Amjevita (Amgen Inc), an adalimumab biosimilar, all for numerous clinical indications including plaque psoriasis and psoriatic arthritis.4-6
There has been a substantial amount of distrust surrounding the biosimilars; however, as the patents for the biologic agents expire, new biosimilars will undoubtedly flood the market. In this article, we provide information that will help dermatologists understand the need for and use of these agents.
Biosimilars Versus Generic Drugs
Small-molecule generics can be made in a process that is relatively inexpensive, reproducible, and able to yield identical products with each lot.7 In contrast, biosimilars are large complex proteins made in living cells. They differ from their reference product because of changes that occur during manufacturing (eg, purification system, posttranslational modifications).7-9 Glycosylation is particularly sensitive to manufacturing and can affect the immunogenicity of the product.9 The impact of manufacturing can be substantial; for example, during phase 3 trials for efalizumab, a change in the manufacturing facility affected pharmacokinetic properties to such a degree that the FDA required a repeat of the trials.10
FDA Guidelines on Biosimilarity
The FDA outlines the following approach to demonstrate biosimilarity.2 The first step is structural characterization to evaluate the primary, secondary, tertiary, and quaternary structures and posttranslational modifications. The next step utilizes in vivo and/or in vitro functional assays to compare the biosimilar and reference product. The third step is a focus on toxicity and immunogenicity. The fourth step involves clinical studies to study pharmacokinetic and pharmacodynamic data, immunogenicity, safety, and efficacy. After the biosimilar has been approved, there must be a system in place to monitor postmarketing safety. If a biosimilar is tested in one patient population (eg, patients with plaque psoriasis), a request can be made to approve the drug for all the conditions that the reference product was approved for, such as plaque psoriasis, rheumatoid arthritis, and inflammatory bowel disease, even though clinical trials were not performed in all of these patient populations.2 The BPCI Act leaves it up to the FDA to determine how much and what type of data (eg, in vitro, in vivo, clinical) are required.11
Extrapolation and Interchangeability
Once a biosimilar has been approved, 2 questions must be answered: First, can its use be extrapolated to all indications for the reference product? The infliximab biosimilar approved by the European Medicines Agency and the FDA had only been studied in patients with ankylosing spondylitis12 and rheumatoid arthritis,13 yet it was granted all the indications for infliximab, including severe plaque psoriasis.14 As of now, the various regulatory agencies differ on their policies regarding extrapolation. Extrapolation is not automatically bestowed on a biosimilar in the United States but can be requested by the manufacturer.2
Second, can the biosimilar be seamlessly switched with its reference product at the pharmacy level? The BPCI Act allows for the substitution of biosimilars that are deemed interchangeable without notifying the provider, yet individual states ultimately can pass laws regarding this issue.15,16 An interchangeable agent would “produce the same clinical result as the reference product,” and “the risk in terms of safety or diminished efficacy of alternating or switching between use of the biological product and the reference product is not greater than the risk of using the reference product.”15 Generic drugs are allowed to be substituted without notifying the patient or prescriber16; however, biosimilars that are not deemed interchangeable would require permission from the prescriber before substitution.11
Biosimilars for Psoriasis
In April 2016, an infliximab biosimilar (Inflectra) became the second biosimilar approved by the FDA.4 Inflectra was studied in clinical trials for patients with ankylosing spondylitis17 and rheumatoid arthritis,18 and in both trials the biosimilar was found to have similar efficacy and safety profiles to that of the reference product. In August 2016, an etanercept biosimilar (Erelzi) was approved,5 and in September 2016, an adalimumab biosimilar (Amjevita) was approved.6
The Table summarizes clinical trials (both completed and ongoing) evaluating biosimilars in adults with plaque psoriasis; thus far, there are 2464 participants enrolled across 5 different studies of adalimumab biosimilars (registered at www.clinicaltrials.gov with the identifiers NCT01970488, NCT02016105, NCT02489227, NCT02714322, NCT02581345) and 531 participants in an etanercept biosimilar study (NCT01891864).
A phase 3 double-blind study compared adalimumab to an adalimumab biosimilar (ABP 501) in 350 adults with plaque psoriasis (NCT01970488). Participants received an initial loading dose of adalimumab (n=175) or ABP 501 (n=175) 80 mg subcutaneously on week 1/day 1, followed by 40 mg at week 2 every 2 weeks thereafter. At week 16, participants with psoriasis area and severity index (PASI) 50 or greater remained in the study for up to 52 weeks; those who were receiving adalimumab were re-randomized to receive either ABP 501 or adalimumab. Participants receiving ABP 501 continued to receive the biosimilar. The mean PASI improvement at weeks 16, 32, and 50 was 86.6, 87.6, and 87.2, respectively, in the ABP 501/ABP 501 group (A/A) compared to 88.0, 88.2, and 88.1, respectively, in the adalimumab/adalimumab group (B/B).19 Autoantibodies developed in 68.4% of participants in the A/A group compared to 74.7% in the B/B group. The incidence of treatment-emergent adverse events (TEAEs) was 86.2% in the A/A group and 78.5% in the B/B group. The most common TEAEs were nasopharyngitis, headache, and upper respiratory tract infection. The incidence of serious TEAEs was 4.6% in the A/A group compared to 5.1% in the B/B group. Overall, the efficacy, safety, and immunogenicity of the adalimumab biosimilar was comparable to the reference product.19
A second phase 3 trial (ADACCESS) evaluated the adalimumab biosimilar GP2017 (NCT02016105). Participants received an initial dose of 80 mg subcutaneously of either GP2017 or adalimumab at week 0, followed by 40 mg every other week starting at week 1 and ending at week 51. The study has been completed but results are not yet available.
The third trial is evaluating the adalimumab biosimilar CHS-1420 (NCT02489227). Participants in the experimental arm receive two 40-mg doses of CHS-1420 at week 0/day 0, and then 1 dose every 2 weeks from week 1 for 23 weeks. At week 24, participants continue with an open-label study. Participants in the adalimumab group receive two 40-mg doses at week 0/day 0, and then 1 dose every 2 weeks from week 1 to week 15. At week 16, participants will be re-randomized (1:1) to continue adalimumab or start CHS-1420 at one 40-mg dose every 2 weeks during weeks 17 to 23. At week 24, participants will switch to CHS-1420 open label until the end of the study. Study results are not yet available; the study is ongoing but not recruiting.
The fourth ongoing trial is evaluating the adalimumab biosimilar MYL-1401A (NCT02714322). Participants receive an initial dose of 80 mg subcutaneously of either MYL-1401A or adalimumab (2:1), followed by 40 mg every other week starting 1 week after the initial dose. After the 52-week treatment period, there is an 8-week safety follow-up period. Study results are not yet available; the study is ongoing but not recruiting.
A fifth adalimumab biosimilar, M923, also is currently being tested in clinical trials (NCT02581345). Participants receive either M923, adalimumab, or alternate between the 2 agents. Although the study is still ongoing, data released from the manufacturer state that the proportion of participants who achieved PASI 75 after 16 weeks of treatment was equivalent in the 2 treatment groups. The proportion of participants who achieved PASI 90, as well as the type, frequency, and severity of adverse events, also were comparable.20
The EGALITY trial, completed in March 2015, compared the etanercept biosimilar GP2015 to etanercept over a 52-week period (NCT01891864). Participants received either GP2015 or etanercept 50 mg twice weekly for the first 12 weeks. Participants with at least PASI 50 were then re-randomized into 4 groups: the first 2 groups stayed with their current treatments while the other 2 groups alternated treatments every 6 weeks until week 30. Participants then stayed on their last treatment from week 30 to week 52. The adjusted PASI 75 response rate at week 12 was 73.4% in the group receiving GP2015 and 75.7% in the group receiving etanercept.21 The percentage change in PASI score at all time points was found to be comparable from baseline until week 52. Importantly, the incidence of TEAEs up to week 52 was comparable and no new safety issues were reported. Additionally, switching participants from etanercept to the biosimilar during the subsequent treatment periods did not cause an increase in formation of antidrug antibodies.21
There are 2 upcoming studies involving biosimilars that are not yet recruiting patients. The first (NCT02925338) will analyze the characteristics of patients treated with Inflectra as well as their response to treatment. The second (NCT02762955) will be comparing the efficacy and safety of an adalimumab biosimilar (BCD-057, BIOCAD) to adalimumab.
Economic Advantages of Biosimilars
The annual economic burden of psoriasis in the United States is substantial, with estimates between $35.2 billion22 and $112 billion.23 Biosimilars can be 25% to 30% cheaper than their reference products9,11,24 and have the potential to save the US health care system billions of dollars.25 Furthermore, the developers of biosimilars could offer patient assistance programs.11 That being said, drug developers can extend patents for their branded drugs; for instance, 2 patents for Enbrel (Amgen Inc) could protect the drug until 2029.26,27
Although cost is an important factor in deciding which medications to prescribe for patients, it should never take precedence over safety and efficacy. Manufacturers can develop new drugs with greater efficacy, fewer side effects, or more convenient dosing schedules,26,27 or they could offer co-payment assistance programs.26,28 Physicians also must consider how the biosimilars will be integrated into drug formularies. Would patients be required to use a biosimilar before a branded drug?11,29 Will patients already taking a branded drug be grandfathered in?11 Would they have to pay a premium to continue taking their drug? And finally, could changes in formularies and employer-payer relationships destabilize patient regimens?30
Conclusion
Preliminary results suggest that biosimilars can have similar safety, efficacy, and immunogenicity data compared to their reference products.19,21 Biosimilars have the potential to greatly reduce the cost burden associated with psoriasis. However, how similar is “highly similar”? Although cost is an important consideration in selecting drug therapies, the reason for using a biosimilar should never be based on cost alone.
According to the US Food and Drug Administration (FDA), a biosimilar is “highly similar to an FDA-approved biological product, . . . and has no clinically meaningful differences in terms of safety and effectiveness.”1 The Biologics Price Competition and Innovation (BPCI) Act of 2009 created an expedited pathway for the approval of products shown to be biosimilar to FDA-licensed reference products.2 In 2013, the European Medicines Agency approved the first biosimilar modeled on infliximab (Remsima [formerly known as CT-P13], Celltrion Healthcare Co, Ltd) for the same indications as its reference product.3 In 2016, the FDA approved Inflectra (Hospira, a Pfizer Company), an infliximab biosimilar; Erelzi (Sandoz, a Novartis Division), an etanercept biosimilar; and Amjevita (Amgen Inc), an adalimumab biosimilar, all for numerous clinical indications including plaque psoriasis and psoriatic arthritis.4-6
There has been a substantial amount of distrust surrounding the biosimilars; however, as the patents for the biologic agents expire, new biosimilars will undoubtedly flood the market. In this article, we provide information that will help dermatologists understand the need for and use of these agents.
Biosimilars Versus Generic Drugs
Small-molecule generics can be made in a process that is relatively inexpensive, reproducible, and able to yield identical products with each lot.7 In contrast, biosimilars are large complex proteins made in living cells. They differ from their reference product because of changes that occur during manufacturing (eg, purification system, posttranslational modifications).7-9 Glycosylation is particularly sensitive to manufacturing and can affect the immunogenicity of the product.9 The impact of manufacturing can be substantial; for example, during phase 3 trials for efalizumab, a change in the manufacturing facility affected pharmacokinetic properties to such a degree that the FDA required a repeat of the trials.10
FDA Guidelines on Biosimilarity
The FDA outlines the following approach to demonstrate biosimilarity.2 The first step is structural characterization to evaluate the primary, secondary, tertiary, and quaternary structures and posttranslational modifications. The next step utilizes in vivo and/or in vitro functional assays to compare the biosimilar and reference product. The third step is a focus on toxicity and immunogenicity. The fourth step involves clinical studies to study pharmacokinetic and pharmacodynamic data, immunogenicity, safety, and efficacy. After the biosimilar has been approved, there must be a system in place to monitor postmarketing safety. If a biosimilar is tested in one patient population (eg, patients with plaque psoriasis), a request can be made to approve the drug for all the conditions that the reference product was approved for, such as plaque psoriasis, rheumatoid arthritis, and inflammatory bowel disease, even though clinical trials were not performed in all of these patient populations.2 The BPCI Act leaves it up to the FDA to determine how much and what type of data (eg, in vitro, in vivo, clinical) are required.11
Extrapolation and Interchangeability
Once a biosimilar has been approved, 2 questions must be answered: First, can its use be extrapolated to all indications for the reference product? The infliximab biosimilar approved by the European Medicines Agency and the FDA had only been studied in patients with ankylosing spondylitis12 and rheumatoid arthritis,13 yet it was granted all the indications for infliximab, including severe plaque psoriasis.14 As of now, the various regulatory agencies differ on their policies regarding extrapolation. Extrapolation is not automatically bestowed on a biosimilar in the United States but can be requested by the manufacturer.2
Second, can the biosimilar be seamlessly switched with its reference product at the pharmacy level? The BPCI Act allows for the substitution of biosimilars that are deemed interchangeable without notifying the provider, yet individual states ultimately can pass laws regarding this issue.15,16 An interchangeable agent would “produce the same clinical result as the reference product,” and “the risk in terms of safety or diminished efficacy of alternating or switching between use of the biological product and the reference product is not greater than the risk of using the reference product.”15 Generic drugs are allowed to be substituted without notifying the patient or prescriber16; however, biosimilars that are not deemed interchangeable would require permission from the prescriber before substitution.11
Biosimilars for Psoriasis
In April 2016, an infliximab biosimilar (Inflectra) became the second biosimilar approved by the FDA.4 Inflectra was studied in clinical trials for patients with ankylosing spondylitis17 and rheumatoid arthritis,18 and in both trials the biosimilar was found to have similar efficacy and safety profiles to that of the reference product. In August 2016, an etanercept biosimilar (Erelzi) was approved,5 and in September 2016, an adalimumab biosimilar (Amjevita) was approved.6
The Table summarizes clinical trials (both completed and ongoing) evaluating biosimilars in adults with plaque psoriasis; thus far, there are 2464 participants enrolled across 5 different studies of adalimumab biosimilars (registered at www.clinicaltrials.gov with the identifiers NCT01970488, NCT02016105, NCT02489227, NCT02714322, NCT02581345) and 531 participants in an etanercept biosimilar study (NCT01891864).
A phase 3 double-blind study compared adalimumab to an adalimumab biosimilar (ABP 501) in 350 adults with plaque psoriasis (NCT01970488). Participants received an initial loading dose of adalimumab (n=175) or ABP 501 (n=175) 80 mg subcutaneously on week 1/day 1, followed by 40 mg at week 2 every 2 weeks thereafter. At week 16, participants with psoriasis area and severity index (PASI) 50 or greater remained in the study for up to 52 weeks; those who were receiving adalimumab were re-randomized to receive either ABP 501 or adalimumab. Participants receiving ABP 501 continued to receive the biosimilar. The mean PASI improvement at weeks 16, 32, and 50 was 86.6, 87.6, and 87.2, respectively, in the ABP 501/ABP 501 group (A/A) compared to 88.0, 88.2, and 88.1, respectively, in the adalimumab/adalimumab group (B/B).19 Autoantibodies developed in 68.4% of participants in the A/A group compared to 74.7% in the B/B group. The incidence of treatment-emergent adverse events (TEAEs) was 86.2% in the A/A group and 78.5% in the B/B group. The most common TEAEs were nasopharyngitis, headache, and upper respiratory tract infection. The incidence of serious TEAEs was 4.6% in the A/A group compared to 5.1% in the B/B group. Overall, the efficacy, safety, and immunogenicity of the adalimumab biosimilar was comparable to the reference product.19
A second phase 3 trial (ADACCESS) evaluated the adalimumab biosimilar GP2017 (NCT02016105). Participants received an initial dose of 80 mg subcutaneously of either GP2017 or adalimumab at week 0, followed by 40 mg every other week starting at week 1 and ending at week 51. The study has been completed but results are not yet available.
The third trial is evaluating the adalimumab biosimilar CHS-1420 (NCT02489227). Participants in the experimental arm receive two 40-mg doses of CHS-1420 at week 0/day 0, and then 1 dose every 2 weeks from week 1 for 23 weeks. At week 24, participants continue with an open-label study. Participants in the adalimumab group receive two 40-mg doses at week 0/day 0, and then 1 dose every 2 weeks from week 1 to week 15. At week 16, participants will be re-randomized (1:1) to continue adalimumab or start CHS-1420 at one 40-mg dose every 2 weeks during weeks 17 to 23. At week 24, participants will switch to CHS-1420 open label until the end of the study. Study results are not yet available; the study is ongoing but not recruiting.
The fourth ongoing trial is evaluating the adalimumab biosimilar MYL-1401A (NCT02714322). Participants receive an initial dose of 80 mg subcutaneously of either MYL-1401A or adalimumab (2:1), followed by 40 mg every other week starting 1 week after the initial dose. After the 52-week treatment period, there is an 8-week safety follow-up period. Study results are not yet available; the study is ongoing but not recruiting.
A fifth adalimumab biosimilar, M923, also is currently being tested in clinical trials (NCT02581345). Participants receive either M923, adalimumab, or alternate between the 2 agents. Although the study is still ongoing, data released from the manufacturer state that the proportion of participants who achieved PASI 75 after 16 weeks of treatment was equivalent in the 2 treatment groups. The proportion of participants who achieved PASI 90, as well as the type, frequency, and severity of adverse events, also were comparable.20
The EGALITY trial, completed in March 2015, compared the etanercept biosimilar GP2015 to etanercept over a 52-week period (NCT01891864). Participants received either GP2015 or etanercept 50 mg twice weekly for the first 12 weeks. Participants with at least PASI 50 were then re-randomized into 4 groups: the first 2 groups stayed with their current treatments while the other 2 groups alternated treatments every 6 weeks until week 30. Participants then stayed on their last treatment from week 30 to week 52. The adjusted PASI 75 response rate at week 12 was 73.4% in the group receiving GP2015 and 75.7% in the group receiving etanercept.21 The percentage change in PASI score at all time points was found to be comparable from baseline until week 52. Importantly, the incidence of TEAEs up to week 52 was comparable and no new safety issues were reported. Additionally, switching participants from etanercept to the biosimilar during the subsequent treatment periods did not cause an increase in formation of antidrug antibodies.21
There are 2 upcoming studies involving biosimilars that are not yet recruiting patients. The first (NCT02925338) will analyze the characteristics of patients treated with Inflectra as well as their response to treatment. The second (NCT02762955) will be comparing the efficacy and safety of an adalimumab biosimilar (BCD-057, BIOCAD) to adalimumab.
Economic Advantages of Biosimilars
The annual economic burden of psoriasis in the United States is substantial, with estimates between $35.2 billion22 and $112 billion.23 Biosimilars can be 25% to 30% cheaper than their reference products9,11,24 and have the potential to save the US health care system billions of dollars.25 Furthermore, the developers of biosimilars could offer patient assistance programs.11 That being said, drug developers can extend patents for their branded drugs; for instance, 2 patents for Enbrel (Amgen Inc) could protect the drug until 2029.26,27
Although cost is an important factor in deciding which medications to prescribe for patients, it should never take precedence over safety and efficacy. Manufacturers can develop new drugs with greater efficacy, fewer side effects, or more convenient dosing schedules,26,27 or they could offer co-payment assistance programs.26,28 Physicians also must consider how the biosimilars will be integrated into drug formularies. Would patients be required to use a biosimilar before a branded drug?11,29 Will patients already taking a branded drug be grandfathered in?11 Would they have to pay a premium to continue taking their drug? And finally, could changes in formularies and employer-payer relationships destabilize patient regimens?30
Conclusion
Preliminary results suggest that biosimilars can have similar safety, efficacy, and immunogenicity data compared to their reference products.19,21 Biosimilars have the potential to greatly reduce the cost burden associated with psoriasis. However, how similar is “highly similar”? Although cost is an important consideration in selecting drug therapies, the reason for using a biosimilar should never be based on cost alone.
- Information on biosimilars. US Food and Drug Administration website. http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/TherapeuticBiologicApplications/Biosimilars/. Updated May 10, 2016. Accessed July 5, 2016.
- US Department of Health and Human Services. Scientific Considerations in Demonstrating Biosimilarity to a Reference Product: Guidance for Industry. Silver Spring, MD: US Food and Drug Administration; 2015.
- McKeage K. A review of CT-P13: an infliximab biosimilar. BioDrugs. 2014;28:313-321.
- FDA approves Inflectra, a biosimilar to Remicade [news release]. Silver Spring, MD: US Food and Drug Administration; April 5, 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm494227.htm. Updated April 20, 2016. Accessed January 23, 2017.
- FDA approves Erelzi, a biosimilar to Enbrel [news release]. Silver Spring, MD: US Food and Drug Administration; August 30, 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm518639.htm. Accessed January 23, 2017.
- FDA approves Amjevita, a biosimilar to Humira [news release]. Silver Spring, MD: US Food and Drug Administration; September 23, 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm522243.htm. Accessed January 23, 2017.
- Scott BJ, Klein AV, Wang J. Biosimilar monoclonal antibodies: a Canadian regulatory perspective on the assessment of clinically relevant differences and indication extrapolation [published online June 26, 2014]. J Clin Pharmacol. 2015;55(suppl 3):S123-S132.
- Mellstedt H, Niederwieser D, Ludwig H. The challenge of biosimilars [published online September 14, 2007]. Ann Oncol. 2008;19:411-419.
- Puig L. Biosimilars and reference biologics: decisions on biosimilar interchangeability require the involvement of dermatologists [published online October 2, 2013]. Actas Dermosifiliogr. 2014;105:435-437.
- Strober BE, Armour K, Romiti R, et al. Biopharmaceuticals and biosimilars in psoriasis: what the dermatologist needs to know. J Am Acad Dermatol. 2012;66:317-322.
- Falit BP, Singh SC, Brennan TA. Biosimilar competition in the United States: statutory incentives, payers, and pharmacy benefit managers. Health Aff (Millwood). 2015;34:294-301.
- Park W, Hrycaj P, Jeka S, et al. A randomised, double-blind, multicentre, parallel-group, prospective study comparing the pharmacokinetics, safety, and efficacy of CT-P13 and innovator infliximab in patients with ankylosing spondylitis: the PLANETAS study. Ann Rheum Dis. 2013;72:1605-1612.
- Yoo DH, Hrycaj P, Miranda P, et al. A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study. Ann Rheum Dis. 2013;72:1613-1620.
- Carretero Hernandez G, Puig L. The use of biosimilar drugs in psoriasis: a position paper. Actas Dermosifiliogr. 2015;106:249-251.
- Regulation of Biological Products, 42 USC §262 (2013).
- Ventola CL. Evaluation of biosimilars for formulary inclusion: factors for consideration by P&T committees. P T. 2015;40:680-689.
- Park W, Yoo DH, Jaworski J, et al. Comparable long-term efficacy, as assessed by patient-reported outcomes, safety and pharmacokinetics, of CT-P13 and reference infliximab in patients with ankylosing spondylitis: 54-week results from the randomized, parallel-group PLANETAS study. Arthritis Res Ther. 2016;18:25.
- Yoo DH, Racewicz A, Brzezicki J, et al. A phase III randomized study to evaluate the efficacy and safety of CT-P13 compared with reference infliximab in patients with active rheumatoid arthritis: 54-week results from the PLANETRA study. Arthritis Res Ther. 2015;18:82.
- Strober B, Foley P, Philipp S, et al. Evaluation of efficacy and safety of ABP 501 in a phase 3 study in subjects with moderate to severe plaque psoriasis: 52-week results. J Am Acad Dermatol. 2016;74(5, suppl 1):AB249.
- Momenta Pharmaceuticals announces positive top-line phase 3 results for M923, a proposed Humira (adalimumab) biosimilar [news release]. Cambridge, MA: Momenta Pharmaceuticals, Inc; November 29, 2016. http://ir.momentapharma.com/releasedetail.cfm?ReleaseID=1001255. Accessed January 25, 2017.
- Griffiths CE, Thaci D, Gerdes S, et al. The EGALITY study: a confirmatory, randomised, double-blind study comparing the efficacy, safety and immunogenicity of GP2015, a proposed etanercept biosimilar, versus the originator product in patients with moderate to severe chronic plaque-type psoriasis [published online October 27, 2016]. Br J Dermatol. doi:10.1111/bjd.15152.
- Vanderpuye-Orgle J, Zhao Y, Lu J, et al. Evaluating the economic burden of psoriasis in the United States [published online April 14, 2015]. J Am Acad Dermatol. 2015;72:961-967.
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151:651-658.
- Menter MA, Griffiths CE. Psoriasis: the future. Dermatol Clin. 2015;33:161-166.
- Hackbarth GM, Crosson FJ, Miller ME. Report to the Congress: improving incentives in the Medicare program. Medicare Payment Advisory Commission, Washington, DC; 2009.
- Lovenworth SJ. The new biosimilar era: the basics, the landscape, and the future. Bloomberg website. http://about.bloomberglaw.com/practitioner-contributions/the-new-biosimilar-era-the-basics-the-landscape-and-the-future. Published September 21, 2012. Accessed July 6, 2016.
- Blackstone EA, Joseph PF. The economics of biosimilars. Am Health Drug Benefits. 2013;6:469-478.
- Calvo B, Zuniga L. The US approach to biosimilars: the long-awaited FDA approval pathway. BioDrugs. 2012;26:357-361.
- Lucio SD, Stevenson JG, Hoffman JM. Biosimilars: implications for health-system pharmacists. Am J Health Syst Pharm. 2013;70:2004-2017.
- Barriers to access attributed to formulary changes. Manag Care. 2012;21:41.
- Information on biosimilars. US Food and Drug Administration website. http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/TherapeuticBiologicApplications/Biosimilars/. Updated May 10, 2016. Accessed July 5, 2016.
- US Department of Health and Human Services. Scientific Considerations in Demonstrating Biosimilarity to a Reference Product: Guidance for Industry. Silver Spring, MD: US Food and Drug Administration; 2015.
- McKeage K. A review of CT-P13: an infliximab biosimilar. BioDrugs. 2014;28:313-321.
- FDA approves Inflectra, a biosimilar to Remicade [news release]. Silver Spring, MD: US Food and Drug Administration; April 5, 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm494227.htm. Updated April 20, 2016. Accessed January 23, 2017.
- FDA approves Erelzi, a biosimilar to Enbrel [news release]. Silver Spring, MD: US Food and Drug Administration; August 30, 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm518639.htm. Accessed January 23, 2017.
- FDA approves Amjevita, a biosimilar to Humira [news release]. Silver Spring, MD: US Food and Drug Administration; September 23, 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm522243.htm. Accessed January 23, 2017.
- Scott BJ, Klein AV, Wang J. Biosimilar monoclonal antibodies: a Canadian regulatory perspective on the assessment of clinically relevant differences and indication extrapolation [published online June 26, 2014]. J Clin Pharmacol. 2015;55(suppl 3):S123-S132.
- Mellstedt H, Niederwieser D, Ludwig H. The challenge of biosimilars [published online September 14, 2007]. Ann Oncol. 2008;19:411-419.
- Puig L. Biosimilars and reference biologics: decisions on biosimilar interchangeability require the involvement of dermatologists [published online October 2, 2013]. Actas Dermosifiliogr. 2014;105:435-437.
- Strober BE, Armour K, Romiti R, et al. Biopharmaceuticals and biosimilars in psoriasis: what the dermatologist needs to know. J Am Acad Dermatol. 2012;66:317-322.
- Falit BP, Singh SC, Brennan TA. Biosimilar competition in the United States: statutory incentives, payers, and pharmacy benefit managers. Health Aff (Millwood). 2015;34:294-301.
- Park W, Hrycaj P, Jeka S, et al. A randomised, double-blind, multicentre, parallel-group, prospective study comparing the pharmacokinetics, safety, and efficacy of CT-P13 and innovator infliximab in patients with ankylosing spondylitis: the PLANETAS study. Ann Rheum Dis. 2013;72:1605-1612.
- Yoo DH, Hrycaj P, Miranda P, et al. A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study. Ann Rheum Dis. 2013;72:1613-1620.
- Carretero Hernandez G, Puig L. The use of biosimilar drugs in psoriasis: a position paper. Actas Dermosifiliogr. 2015;106:249-251.
- Regulation of Biological Products, 42 USC §262 (2013).
- Ventola CL. Evaluation of biosimilars for formulary inclusion: factors for consideration by P&T committees. P T. 2015;40:680-689.
- Park W, Yoo DH, Jaworski J, et al. Comparable long-term efficacy, as assessed by patient-reported outcomes, safety and pharmacokinetics, of CT-P13 and reference infliximab in patients with ankylosing spondylitis: 54-week results from the randomized, parallel-group PLANETAS study. Arthritis Res Ther. 2016;18:25.
- Yoo DH, Racewicz A, Brzezicki J, et al. A phase III randomized study to evaluate the efficacy and safety of CT-P13 compared with reference infliximab in patients with active rheumatoid arthritis: 54-week results from the PLANETRA study. Arthritis Res Ther. 2015;18:82.
- Strober B, Foley P, Philipp S, et al. Evaluation of efficacy and safety of ABP 501 in a phase 3 study in subjects with moderate to severe plaque psoriasis: 52-week results. J Am Acad Dermatol. 2016;74(5, suppl 1):AB249.
- Momenta Pharmaceuticals announces positive top-line phase 3 results for M923, a proposed Humira (adalimumab) biosimilar [news release]. Cambridge, MA: Momenta Pharmaceuticals, Inc; November 29, 2016. http://ir.momentapharma.com/releasedetail.cfm?ReleaseID=1001255. Accessed January 25, 2017.
- Griffiths CE, Thaci D, Gerdes S, et al. The EGALITY study: a confirmatory, randomised, double-blind study comparing the efficacy, safety and immunogenicity of GP2015, a proposed etanercept biosimilar, versus the originator product in patients with moderate to severe chronic plaque-type psoriasis [published online October 27, 2016]. Br J Dermatol. doi:10.1111/bjd.15152.
- Vanderpuye-Orgle J, Zhao Y, Lu J, et al. Evaluating the economic burden of psoriasis in the United States [published online April 14, 2015]. J Am Acad Dermatol. 2015;72:961-967.
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151:651-658.
- Menter MA, Griffiths CE. Psoriasis: the future. Dermatol Clin. 2015;33:161-166.
- Hackbarth GM, Crosson FJ, Miller ME. Report to the Congress: improving incentives in the Medicare program. Medicare Payment Advisory Commission, Washington, DC; 2009.
- Lovenworth SJ. The new biosimilar era: the basics, the landscape, and the future. Bloomberg website. http://about.bloomberglaw.com/practitioner-contributions/the-new-biosimilar-era-the-basics-the-landscape-and-the-future. Published September 21, 2012. Accessed July 6, 2016.
- Blackstone EA, Joseph PF. The economics of biosimilars. Am Health Drug Benefits. 2013;6:469-478.
- Calvo B, Zuniga L. The US approach to biosimilars: the long-awaited FDA approval pathway. BioDrugs. 2012;26:357-361.
- Lucio SD, Stevenson JG, Hoffman JM. Biosimilars: implications for health-system pharmacists. Am J Health Syst Pharm. 2013;70:2004-2017.
- Barriers to access attributed to formulary changes. Manag Care. 2012;21:41.
Practice Points
- Three biosimilars have been approved by the US Food and Drug Administration to treat adult patients with plaque psoriasis and psoriatic arthritis.
- By virtue of their production, biosimilars are not identical to their reference products, and we must ensure that their safety is comparable.
Transgender Patients: Providing Sensitive Care
Civil rights for the lesbian, gay, bisexual, and transgender population have advanced markedly in the past decade, and the medical community has gradually begun to address more of their health concerns. More recently, media attention to transgender individuals has encouraged many more to openly seek care.1,2
It is estimated that anywhere from 0.3% to 5% of the US population identifies as transgender.1-3 While awareness of this population has slowly increased, there is a paucity of research on the hormone treatment that is often essential to patients’ well-being. Studies of surgical options for transgender patients have been minimal, as well.
Primary care providers are uniquely positioned to coordinate medical services and ensure continuity of care for transgender patients as they strive to become their authentic selves. Our goal in writing this article is to equip you with the tools to provide this patient population with sensitive, high-quality care (see Table 1).4-7 Our focus is on the diagnosis of gender dysphoria (GD) and its medical and hormonal management—the realm of primary care providers. We briefly discuss surgical management of GD, as well.
UNDERSTANDING AND DIAGNOSING GENDER DYSPHORIA
Two classification systems are used for diagnoses related to GD: the Diagnostic and Statistical Manual of Mental Disorders, Fifth Ed (DSM-5)8 and the International Classification of Diseases, 10th Rev (ICD-10).9
ICD-10 criteria use the term gender identity disorder; DSM-5 refers to gender dysphoria instead. It is important to emphasize that these classification systems represent an attempt to categorize a group of signs and symptoms that lead to distress for the patient and are not meant to suggest that being transgender is pathological. In fact, in DSM-5—released in 2013—the American Psychiatric Association revised the terminology to emphasize that such individuals are not “disordered” by the nature of their identity, but rather by the distress that being transgender causes.8
For a diagnosis of GD in children, DSM-5 criteria include characteristics perceived to be incongruent between the child’s sex at birth and the self-identified gender based on preferred activities or dislike of his or her own sexual anatomy. The child must meet six or more of the following for at least six months
- A repeatedly stated desire to be, or insistence that he or she is, of the other gender
- In boys, a preference for cross-dressing or simulating female attire; in girls, insistence on wearing only stereotypical masculine clothing
- Strong and persistent preferences for cross-gender roles in make-believe play or fantasy
- A strong rejection of toys/games typically associated with the child’s sex
- Intense desire to participate in stereotypical games and pastimes of the other gender
- Strong preference for playmates of the other gender
- A strong dislike of one’s sexual anatomy
- A strong desire for the primary (eg, penis or vagina) or secondary (eg, menstruation) sex characteristics of the other gender.8
Adolescents and adults must meet two or more of the following for at least six months
- A noticeable incongruence between the gender that the patient sees themselves as and their sex characteristics
- An intense need to do away with (or prevent) his or her primary or secondary sex features
- An intense desire to have the primary and/or secondary sex features of the other gender
- A deep desire to transform into another gender
- A profound need for society to treat them as someone of the other gender
- A powerful assurance of having the characteristic feelings and responses of the other gender.8
For children as well as adolescents and adults, the condition should cause the patient significant distress or significantly affect him or her socially, at work or school, and in other important areas of life.8
Is the patient a candidate for hormone therapy?
Two primary sources—Standards of Care for the Health of Transsexual, Transgender, and Gender-Nonconforming People, Version 7, issued by the World Professional Association for Transgender Health (WPATH)10 and Endocrine Treatment of Transsexual Persons11 by the Endocrine Society—offer clinical practice guidance based on evidence and expert opinion.
WPATH recommends that a mental health professional (MHP) experienced in transgender care diagnose GD to ensure that it is not mistaken for a psychiatric condition manifesting as altered gender identity. However, if no one with such experience is available or accessible in the region, it is reasonable for a primary care provider to make the diagnosis and consider initiating hormone therapy without a mental health referral,12 as the expected benefits outweigh the risks of nontreatment.13
Whether or not an MHP confirms a diagnosis of GD, it is still up to the treating provider to confirm the patient’s eligibility and readiness for hormone therapy: He or she should meet DSM-5 or ICD-10 criteria for GD, have no psychiatric comorbidity (eg, schizophrenia, body dysmorphic disorder, or uncontrolled bipolar disorder) likely to interfere with treatment, understand the expected outcomes and the social benefits and risks, and have indicated a willingness to take the hormones responsibly.
Historically, patients were required to have a documented real-life experience, defined as having fully adopted the new gender role in everyday life for at least three months.10,11 This model has fallen out of favor, however, as it is unsupported by evidence and may place transgender individuals at physical and emotional risk. Instead, readiness is confirmed by obtaining informed consent.12
Puberty may be suppressed with a gonadotropin-releasing hormone (GnRH) agonist in adolescents who have a GD diagnosis and are at Tanner stage 2 to 3 of puberty until age 16. At that point, hormone therapy consistent with their gender identification may be initiated (see “How to Help Transgender Teens”).11
Beginning the transition
The transitioning process is a complex and individualized journey that can include inward or outward change, or both.
For patients interested in medical interventions, possible therapies include cross-sex hormone administration and gender-affirming surgery. Both are aimed at making the physical and the psychologic more congruent. Hormone treatment (see Table 2) is often essential to reduce the distress of individuals with GD and to help them feel comfortable in their own body.10,11,21 Psychologic conditions, such as depression, tend to improve as the transitioning process gets underway.22
FEMALE-TO-MALE TRANSITION
CASE 1 Jennie R, a 55-year-old postmenopausal patient, comes to your office for an annual exam. Although you’ve been her primary care provider for several years, she confides for the first time that she has never been comfortable as a woman. “I’ve always felt that my body didn’t belong to me,” the patient admits, and goes on to say that for the past several years she has been living as a man. Jennie R says she is ready to start hormone therapy to assist with the gender transition and asks about the process, the benefits and risks, and how quickly she can expect to achieve the desired results.
If Jennie R were your patient, how would you respond?
Masculinizing hormone treatment
As you would explain to a patient like Jennie R, the goal of hormone therapy is to suppress the effects of the sex assigned at birth and replace them with those of the desired gender. In the case of a female transitioning to a male (known as a transman), masculinizing hormones would promote growth of facial and body hair, cessation of menses, increased muscle mass, deepening of the voice, and clitoral enlargement.
Physical changes induced by masculinizing hormone therapy have an expected onset of one to six months and achieve maximum effect in approximately two to five years.10,11 Although there have been no controlled clinical trials evaluating the safety or efficacy of any transitional hormone regimen, WPATH and the Center of Excellence for Transgender Health at the University of California, San Francisco, suggest initiating intramuscular or transdermal testosterone at increasing doses until normal physiologic male testosterone levels between 350 and 700 ng/dL are achieved, or until cessation of menses.13,25-28 The dose at which either, or both, occur should be continued as long-term maintenance therapy. Medroxyprogesterone can be added, if necessary for menstrual cessation, and a GnRH agonist or endometrial ablation can be used for refractory uterine bleeding.29,30
Testosterone is not a contraceptive. It is important to emphasize to transmen like Jennie that they remain at risk for pregnancy if they are having sex with fertile males. Caution patients not to assume that the possibility of pregnancy ends when menses stop.
Treat minor adverse effects. Adverse effects of masculinizing hormones include vaginal atrophy, fat redistribution and weight gain, polycythemia, acne, scalp hair loss, sleep apnea, elevated liver enzymes, hyperlipidemia, cardiovascular disease, diabetes, and bone density loss. Increased risk for cancer of the female organs has not been proven.10,11 It is reasonable to treat minor adverse effects after reviewing the risks/benefits of doing so, as discontinuing hormone therapy could be detrimental to the well-being of transitioning patients.11
There are absolute contraindications to masculinizing hormone therapy, however, including pregnancy, unstable coronary artery disease, and untreated polycythemia with a hematocrit > 55%.10
Monitoring is essential. Patients receiving masculinizing hormone therapy should be monitored every three months during the first year and once or twice a year thereafter, with a focused history (including mood symptoms), physical exam (including weight and blood pressure), and labs (including complete blood count, liver function, renal function, and lipids) at each visit.11,23 Some clinicians also check estradiol levels until they fall below 50 pg/mL,23,27 while others take the cessation of uterine bleeding for > 6 months as an indicator of estrogen suppression.
Preventive health measures continue. Routine screening should continue, based on the patient’s assigned sex at birth. Thus, a transman who has not had a hysterectomy still needs Pap smears, mammograms if the patient has not had a double mastectomy, and bone mineral density (BMD) testing to screen for osteoporosis.31,32 Some experts recommend starting to test BMD at age 50 for patients receiving masculinizing hormones, given the unknown effect of testosterone on bone density.11,31,32
CASE 1 The first question for a transgender patient is about his or her current gender identity, but Jennie R has already reported living as a man. So you start by asking “What name do you prefer to use?” and “Do you prefer to be referred to with male or female pronouns?”
The patient tells you that he sees himself as a man, he wants to be called Jeff, and he prefers male pronouns. You explain that you believe he has gender dysphoria and would benefit from hormone therapy, but it is important to confirm this diagnosis with an MHP. You explain that testosterone can be prescribed for masculinizing effects, and describe the expected effects—more facial and body hair, a deeper voice, and greater muscle mass, among others—and review the likely time frame.
You also discuss the risks of masculinizing hormones (hyperlipidemia, cardiovascular disease, diabetes, and loss of bone density) that will need to be monitored. Before he leaves, you give him the name of an MHP who is experienced in transgender care and tell him to make a follow-up appointment with you after he has seen her. At the conclusion of the visit, you make a note of the patient’s name and gender identity in the chart and inform the staff of the changes.
MALE-TO-FEMALE TRANSITION
CASE 2 Before heading into your office to talk to a new patient named Carl S, you glance at his chart and see that he is a healthy 21-year-old who has come in for a routine physical. When you enter the room, you find Carl wearing a dress, heels, and make-up. After confirming that you have the right patient, you ask, “What is your current gender identity?” “Female,” says Carl, who indicates that she now goes by Carol. The patient has no medical problems, surgical history, or significant family history but reports that she has been taking spironolactone and estrogen for the past three years. Carol also says she has a new female partner and is having unprotected sexual activity.
Feminizing hormone treatment
The desired effects of feminizing hormones include voice change, decreased hair growth, breast growth, body fat redistribution, decreased muscle mass, skin softening, decreased oiliness of skin and hair, and a decrease in spontaneous erections, testicular volume, and sperm production.10,11 The onset of feminizing effects ranges from one month to one year and the expected maximum effect occurs anywhere between three months and five years.10,11 Regimens usually include anti-androgen agents and estrogen.13,26-28
The medications that have been most studied with anti-androgenic effects include spironolactone and 5-α reductase inhibitors (5-ARIs) such as finasteride. Spironolactone inhibits testosterone secretion and inhibits androgen binding to androgen receptors; 5-ARIs block the conversion of testosterone to 5-α-dihydrotestosterone, the more active form.
Estrogen can be administered via oral, sublingual, transdermal, or intramuscular route, but parenteral formulations are preferred to avoid first-pass metabolism. The serum estradiol target is similar to the mean daily level of premenopausal women (< 200 pg/mL) and the level of testosterone should be in the normal female range (< 55 ng/dL).13,26-28
The selection of medications should be individualized for each patient. Comorbidities must be considered, as well as the risk for adverse effects, which include venous thromboembolism, elevated liver enzymes, breast cancer, cardiovascular disease, diabetes, hyperprolactinemia, weight gain, gallstones, cerebrovascular disease, and severe migraine headaches.10,11 Estrogen therapy is not reported to induce hypertrophy or premalignant changes in the prostate.33 As is the case for masculinizing hormones, feminizing hormone therapy should be continued indefinitely for long-term effects.
Frequent monitoring is recommended. Patients taking feminizing hormones (transwomen) should be seen every two to three months in the first year and monitored once or twice a year thereafter. Serum testosterone and estradiol levels should initially be monitored every three months; serum electrolytes, specifically potassium, should be monitored every two to three months in the first year until stable.
CASE 2 You recommend that Carol S be screened annually for sexually transmitted diseases, as you would for any 21-year-old patient. You point out, too, that while estrogen and androgen-suppressing therapy decrease sperm production, there is a possibility that the patient could impregnate a female partner and recommend that contraception be used if the couple is not trying to conceive.
You also discuss the risks and benefits of hormone therapy and reasonable expectations of continued treatment. You ask Carol to schedule a follow-up visit in six months, as her hormone regimen is stable. Finally, if the patient remains on hormone therapy, you mention that the only screening unique to men transitioning to women is for breast cancer, which should begin at age 40 to 50 (as it should for all women).
Gender-affirming surgical options
Surgical management of transgender patients is not within the scope of family medicine. But it is essential to know what procedures are available, as you may have occasion to advocate for patients during the surgical referral process and possibly to provide postoperative care.
For transmen, surgical options include chest reconstruction, hysterectomy/oophorectomy, metoidioplasty (using the clitoris to surgically approximate a penis), phalloplasty, scrotoplasty, urethroplasty, and vaginectomy.10,34 The surgeries available for transwomen are orchiectomy, vaginoplasty, penectomy, breast augmentation, thyroid chondroplasty and voice surgery, and facial feminization.10,34 Keep in mind that not all transgender individuals desire surgery as part of the transitioning process.
The authors would like to acknowledge the assistance of Michelle Forcier, MD, MPH, and Karen S. Bernstein, MD, MPH, in the preparation of this manuscript.
1. Pew Research Center. A survey of LGBT Americans: attitudes, experiences and values in changing times. www.pewsocialtrends.org/2013/06/13/a-survey-of-lgbt-americans. Accessed January 13, 2017.
2. Gates GJ. How many people are lesbian, gay, bisexual and transgender? http://williamsinstitute.law.ucla.edu/wp-content/uploads/Gates-How-Many-People-LGBT-Apr-2011.pdf. Accessed January 13, 2017.
3. van Kesteren PJ, Gooren LJ, Megens JA. An epidemiological and demographic study of transsexuals in The Netherlands. Arch Sex Behav. 1996;25:589-600.
4. Bhola S. An ally’s guide to terminology: talking about LGBT people & equality. www.glaad.org/2011/07/28/an-allys-guide-to-terminology-talking-about-lgbt-people-equality. Accessed January 13, 2017.
5. University of California, San Francisco. Transgender terminology. UCSF Center of Excellence for Transgender Health. http://transhealth.ucsf.edu/tcoe?page=protocol-terminology. Accessed January 13, 2017.
6. Istar A. How queer! The development of gender identity and sexual orientation in LGBTQ-headed families. Fam Process. 2010;49:268-290.
7. Goins ES, Pye D. Check the box that best describes you: reflexively managing theory and praxis in LGBTQ health communication research. Health Commun. 2013;28:397-407.
8. American Psychiatric Association. Gender dysphoria. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013: 451-459.
9. World Health Organization. The International Classification of Diseases, 10th rev. Classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines. 1992; Geneva.
10. Coleman E, Bockting W, Botzer M, et al; World Professional Association for Transgender Health. Standards of Care for the Health of Transsexual, Transgender, and Gender-Nonconforming People, Version 7. Int J Transgender. 2011; 13:165-232.
11. Hembree WC, Cohen-Kettenis P, Delemarre-van de Waal HA, et al. Endocrine treatment of transsexual persons: an Endocrine Society clinical practice guideline. J Clin Endo Metabol. 2009;94:3132-3154.
12. University of California, San Francisco. Assessing readiness for hormones. UCSF Center of Excellence for Transgender Health. http://transhealth.ucsf.edu/tcoe?page=protocol-hormone-ready. Accessed January 13, 2017.
13. Gooren L. Hormone treatment of the adult transsexual patient. Horm Res. 2005;64(suppl 2):S31-S36.
14. Hembree WC. Guidelines for pubertal suspension and gender reassignment for transgender adolescents. Child Adolesc Psychiatr Clin N Am. 2011;20:725-732.
15. Gay, Lesbian, and Straight Education Network (GLSEN). Harsh realities. The experiences of transgender youth in our nation’s schools. www.glsen.org/sites/default/files/Harsh%20Realities.pdf. Accessed January 13, 2017.
16. Berman M, Balingit M. Eleven states sue Obama administration over bathroom guidance for transgender students. May 25, 2016. Washington Post. www.washingtonpost.com/news/post-nation/wp/2016/05/25/texas-governor-says-state-will-sue-obama-administration-over-bathroom-directive/. Accessed January 13, 2017.
17. de Vries AL, Cohen-Kettenis PT, Delemarre-van de Waal H. Clinical management of gender dysphoria in adolescents. 2006. Vancouver Coastal Health - Transgender Health Program. www.amsa.org/wp-content/uploads/2015/04/CaringForTransgenderAdolescents.pdf. Accessed January 13, 2017.
18. TransYouth Family Allies. Empowering transgender youth & families. www.imatyfa.org/. Accessed January 13, 2017.
19. Human Rights Campaign. On our own: a survival guide for independent LGBTQ youth. www.hrc.org/resources/on-our-own-a-survival-guide-for-independent-lgbtq-youth. Accessed January 13, 2017.
20. Gay, Lesbian, Bisexual, and Transgender National Help Center. www.glbthotline.org. Accessed January 13, 2017.
21. University of California, San Francisco. Hormone administration. UCSF Center of Excellence for Transgender Health. http://transhealth.ucsf.edu/trans?page=protocol-hormones. Accessed January 13, 2017.
22. Gorin-Lazard A, Baumstarck K, Boyer L, et al. Hormonal therapy is associated with better self-esteem, mood, and quality of life in transsexuals. J Nerv Ment Dis. 2013;201:996-1000.
23. Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in adult men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2006;91:1995-2010.
24. Boloña ER, Uraga MV, Haddad RM, et al. Testosterone use in men with sexual dysfunction: a systematic review and meta-analysis of randomized placebo-controlled trials. Mayo Clin Proc. 2007;82:20-28.
25. Gooren LJ, Giltay EJ. Review of studies of androgen treatment of female-to-male transsexuals: effects and risks of administration of androgens to females. J Sex Med. 2008; 5:765-776.
26. Levy A, Crown A, Reid R. Endocrine intervention for transsexuals. Clin Endocrinol (Oxf). 2003;59:409-418.
27. Moore E, Wisniewski A, Dobs A. Endocrine treatment of transsexual people: a review of treatment regimens, outcomes, and adverse effects. J Clin Endocrinol Metab. 2003;88:3467-3473.
28. Tangpricha V, Ducharme SH, Barber TW, et al. Endocrinologic treatment of gender identity disorders. Endocr Pract. 2003;9:12-21.
29. Dickersin K, Munro MG, Clark M, et al. Hysterectomy compared with endometrial ablation for dysfunctional uterine bleeding: a randomized controlled trial. Obstet Gynecol. 2007;110:1279-1289.
30. Prasad P, Powell MC. Prospective observational study of Thermablate Endometrial Ablation System as an outpatient procedure. J Minim Invasive Gynecol. 2008;15:476-479.
31. University of California, San Francisco. General prevention and screening. UCSF Center of Excellence for Transgender Health. http://transhealth.ucsf.edu/trans?page=protocol-screening. Accessed January 13, 2017.
32. Ganly I, Taylor EW. Breast cancer in a trans-sexual man receiving hormone replacement therapy. Br J Surg. 1995; 82:341.
33. Meriggiola MC, Gava G. Endocrine care of transpeople part II: a review of cross-sex hormonal treatments, outcomes and adverse effects in transwomen. Clin Endocrinol (Oxf). 2015;83:607-615.
34. University of California, San Francisco. Surgical options. UCSF Center of Excellence for Transgender Health. http://transhealth.ucsf.edu/trans?page=protocol-surgery. Accessed January 13, 2017.
Civil rights for the lesbian, gay, bisexual, and transgender population have advanced markedly in the past decade, and the medical community has gradually begun to address more of their health concerns. More recently, media attention to transgender individuals has encouraged many more to openly seek care.1,2
It is estimated that anywhere from 0.3% to 5% of the US population identifies as transgender.1-3 While awareness of this population has slowly increased, there is a paucity of research on the hormone treatment that is often essential to patients’ well-being. Studies of surgical options for transgender patients have been minimal, as well.
Primary care providers are uniquely positioned to coordinate medical services and ensure continuity of care for transgender patients as they strive to become their authentic selves. Our goal in writing this article is to equip you with the tools to provide this patient population with sensitive, high-quality care (see Table 1).4-7 Our focus is on the diagnosis of gender dysphoria (GD) and its medical and hormonal management—the realm of primary care providers. We briefly discuss surgical management of GD, as well.
UNDERSTANDING AND DIAGNOSING GENDER DYSPHORIA
Two classification systems are used for diagnoses related to GD: the Diagnostic and Statistical Manual of Mental Disorders, Fifth Ed (DSM-5)8 and the International Classification of Diseases, 10th Rev (ICD-10).9
ICD-10 criteria use the term gender identity disorder; DSM-5 refers to gender dysphoria instead. It is important to emphasize that these classification systems represent an attempt to categorize a group of signs and symptoms that lead to distress for the patient and are not meant to suggest that being transgender is pathological. In fact, in DSM-5—released in 2013—the American Psychiatric Association revised the terminology to emphasize that such individuals are not “disordered” by the nature of their identity, but rather by the distress that being transgender causes.8
For a diagnosis of GD in children, DSM-5 criteria include characteristics perceived to be incongruent between the child’s sex at birth and the self-identified gender based on preferred activities or dislike of his or her own sexual anatomy. The child must meet six or more of the following for at least six months
- A repeatedly stated desire to be, or insistence that he or she is, of the other gender
- In boys, a preference for cross-dressing or simulating female attire; in girls, insistence on wearing only stereotypical masculine clothing
- Strong and persistent preferences for cross-gender roles in make-believe play or fantasy
- A strong rejection of toys/games typically associated with the child’s sex
- Intense desire to participate in stereotypical games and pastimes of the other gender
- Strong preference for playmates of the other gender
- A strong dislike of one’s sexual anatomy
- A strong desire for the primary (eg, penis or vagina) or secondary (eg, menstruation) sex characteristics of the other gender.8
Adolescents and adults must meet two or more of the following for at least six months
- A noticeable incongruence between the gender that the patient sees themselves as and their sex characteristics
- An intense need to do away with (or prevent) his or her primary or secondary sex features
- An intense desire to have the primary and/or secondary sex features of the other gender
- A deep desire to transform into another gender
- A profound need for society to treat them as someone of the other gender
- A powerful assurance of having the characteristic feelings and responses of the other gender.8
For children as well as adolescents and adults, the condition should cause the patient significant distress or significantly affect him or her socially, at work or school, and in other important areas of life.8
Is the patient a candidate for hormone therapy?
Two primary sources—Standards of Care for the Health of Transsexual, Transgender, and Gender-Nonconforming People, Version 7, issued by the World Professional Association for Transgender Health (WPATH)10 and Endocrine Treatment of Transsexual Persons11 by the Endocrine Society—offer clinical practice guidance based on evidence and expert opinion.
WPATH recommends that a mental health professional (MHP) experienced in transgender care diagnose GD to ensure that it is not mistaken for a psychiatric condition manifesting as altered gender identity. However, if no one with such experience is available or accessible in the region, it is reasonable for a primary care provider to make the diagnosis and consider initiating hormone therapy without a mental health referral,12 as the expected benefits outweigh the risks of nontreatment.13
Whether or not an MHP confirms a diagnosis of GD, it is still up to the treating provider to confirm the patient’s eligibility and readiness for hormone therapy: He or she should meet DSM-5 or ICD-10 criteria for GD, have no psychiatric comorbidity (eg, schizophrenia, body dysmorphic disorder, or uncontrolled bipolar disorder) likely to interfere with treatment, understand the expected outcomes and the social benefits and risks, and have indicated a willingness to take the hormones responsibly.
Historically, patients were required to have a documented real-life experience, defined as having fully adopted the new gender role in everyday life for at least three months.10,11 This model has fallen out of favor, however, as it is unsupported by evidence and may place transgender individuals at physical and emotional risk. Instead, readiness is confirmed by obtaining informed consent.12
Puberty may be suppressed with a gonadotropin-releasing hormone (GnRH) agonist in adolescents who have a GD diagnosis and are at Tanner stage 2 to 3 of puberty until age 16. At that point, hormone therapy consistent with their gender identification may be initiated (see “How to Help Transgender Teens”).11
Beginning the transition
The transitioning process is a complex and individualized journey that can include inward or outward change, or both.
For patients interested in medical interventions, possible therapies include cross-sex hormone administration and gender-affirming surgery. Both are aimed at making the physical and the psychologic more congruent. Hormone treatment (see Table 2) is often essential to reduce the distress of individuals with GD and to help them feel comfortable in their own body.10,11,21 Psychologic conditions, such as depression, tend to improve as the transitioning process gets underway.22
FEMALE-TO-MALE TRANSITION
CASE 1 Jennie R, a 55-year-old postmenopausal patient, comes to your office for an annual exam. Although you’ve been her primary care provider for several years, she confides for the first time that she has never been comfortable as a woman. “I’ve always felt that my body didn’t belong to me,” the patient admits, and goes on to say that for the past several years she has been living as a man. Jennie R says she is ready to start hormone therapy to assist with the gender transition and asks about the process, the benefits and risks, and how quickly she can expect to achieve the desired results.
If Jennie R were your patient, how would you respond?
Masculinizing hormone treatment
As you would explain to a patient like Jennie R, the goal of hormone therapy is to suppress the effects of the sex assigned at birth and replace them with those of the desired gender. In the case of a female transitioning to a male (known as a transman), masculinizing hormones would promote growth of facial and body hair, cessation of menses, increased muscle mass, deepening of the voice, and clitoral enlargement.
Physical changes induced by masculinizing hormone therapy have an expected onset of one to six months and achieve maximum effect in approximately two to five years.10,11 Although there have been no controlled clinical trials evaluating the safety or efficacy of any transitional hormone regimen, WPATH and the Center of Excellence for Transgender Health at the University of California, San Francisco, suggest initiating intramuscular or transdermal testosterone at increasing doses until normal physiologic male testosterone levels between 350 and 700 ng/dL are achieved, or until cessation of menses.13,25-28 The dose at which either, or both, occur should be continued as long-term maintenance therapy. Medroxyprogesterone can be added, if necessary for menstrual cessation, and a GnRH agonist or endometrial ablation can be used for refractory uterine bleeding.29,30
Testosterone is not a contraceptive. It is important to emphasize to transmen like Jennie that they remain at risk for pregnancy if they are having sex with fertile males. Caution patients not to assume that the possibility of pregnancy ends when menses stop.
Treat minor adverse effects. Adverse effects of masculinizing hormones include vaginal atrophy, fat redistribution and weight gain, polycythemia, acne, scalp hair loss, sleep apnea, elevated liver enzymes, hyperlipidemia, cardiovascular disease, diabetes, and bone density loss. Increased risk for cancer of the female organs has not been proven.10,11 It is reasonable to treat minor adverse effects after reviewing the risks/benefits of doing so, as discontinuing hormone therapy could be detrimental to the well-being of transitioning patients.11
There are absolute contraindications to masculinizing hormone therapy, however, including pregnancy, unstable coronary artery disease, and untreated polycythemia with a hematocrit > 55%.10
Monitoring is essential. Patients receiving masculinizing hormone therapy should be monitored every three months during the first year and once or twice a year thereafter, with a focused history (including mood symptoms), physical exam (including weight and blood pressure), and labs (including complete blood count, liver function, renal function, and lipids) at each visit.11,23 Some clinicians also check estradiol levels until they fall below 50 pg/mL,23,27 while others take the cessation of uterine bleeding for > 6 months as an indicator of estrogen suppression.
Preventive health measures continue. Routine screening should continue, based on the patient’s assigned sex at birth. Thus, a transman who has not had a hysterectomy still needs Pap smears, mammograms if the patient has not had a double mastectomy, and bone mineral density (BMD) testing to screen for osteoporosis.31,32 Some experts recommend starting to test BMD at age 50 for patients receiving masculinizing hormones, given the unknown effect of testosterone on bone density.11,31,32
CASE 1 The first question for a transgender patient is about his or her current gender identity, but Jennie R has already reported living as a man. So you start by asking “What name do you prefer to use?” and “Do you prefer to be referred to with male or female pronouns?”
The patient tells you that he sees himself as a man, he wants to be called Jeff, and he prefers male pronouns. You explain that you believe he has gender dysphoria and would benefit from hormone therapy, but it is important to confirm this diagnosis with an MHP. You explain that testosterone can be prescribed for masculinizing effects, and describe the expected effects—more facial and body hair, a deeper voice, and greater muscle mass, among others—and review the likely time frame.
You also discuss the risks of masculinizing hormones (hyperlipidemia, cardiovascular disease, diabetes, and loss of bone density) that will need to be monitored. Before he leaves, you give him the name of an MHP who is experienced in transgender care and tell him to make a follow-up appointment with you after he has seen her. At the conclusion of the visit, you make a note of the patient’s name and gender identity in the chart and inform the staff of the changes.
MALE-TO-FEMALE TRANSITION
CASE 2 Before heading into your office to talk to a new patient named Carl S, you glance at his chart and see that he is a healthy 21-year-old who has come in for a routine physical. When you enter the room, you find Carl wearing a dress, heels, and make-up. After confirming that you have the right patient, you ask, “What is your current gender identity?” “Female,” says Carl, who indicates that she now goes by Carol. The patient has no medical problems, surgical history, or significant family history but reports that she has been taking spironolactone and estrogen for the past three years. Carol also says she has a new female partner and is having unprotected sexual activity.
Feminizing hormone treatment
The desired effects of feminizing hormones include voice change, decreased hair growth, breast growth, body fat redistribution, decreased muscle mass, skin softening, decreased oiliness of skin and hair, and a decrease in spontaneous erections, testicular volume, and sperm production.10,11 The onset of feminizing effects ranges from one month to one year and the expected maximum effect occurs anywhere between three months and five years.10,11 Regimens usually include anti-androgen agents and estrogen.13,26-28
The medications that have been most studied with anti-androgenic effects include spironolactone and 5-α reductase inhibitors (5-ARIs) such as finasteride. Spironolactone inhibits testosterone secretion and inhibits androgen binding to androgen receptors; 5-ARIs block the conversion of testosterone to 5-α-dihydrotestosterone, the more active form.
Estrogen can be administered via oral, sublingual, transdermal, or intramuscular route, but parenteral formulations are preferred to avoid first-pass metabolism. The serum estradiol target is similar to the mean daily level of premenopausal women (< 200 pg/mL) and the level of testosterone should be in the normal female range (< 55 ng/dL).13,26-28
The selection of medications should be individualized for each patient. Comorbidities must be considered, as well as the risk for adverse effects, which include venous thromboembolism, elevated liver enzymes, breast cancer, cardiovascular disease, diabetes, hyperprolactinemia, weight gain, gallstones, cerebrovascular disease, and severe migraine headaches.10,11 Estrogen therapy is not reported to induce hypertrophy or premalignant changes in the prostate.33 As is the case for masculinizing hormones, feminizing hormone therapy should be continued indefinitely for long-term effects.
Frequent monitoring is recommended. Patients taking feminizing hormones (transwomen) should be seen every two to three months in the first year and monitored once or twice a year thereafter. Serum testosterone and estradiol levels should initially be monitored every three months; serum electrolytes, specifically potassium, should be monitored every two to three months in the first year until stable.
CASE 2 You recommend that Carol S be screened annually for sexually transmitted diseases, as you would for any 21-year-old patient. You point out, too, that while estrogen and androgen-suppressing therapy decrease sperm production, there is a possibility that the patient could impregnate a female partner and recommend that contraception be used if the couple is not trying to conceive.
You also discuss the risks and benefits of hormone therapy and reasonable expectations of continued treatment. You ask Carol to schedule a follow-up visit in six months, as her hormone regimen is stable. Finally, if the patient remains on hormone therapy, you mention that the only screening unique to men transitioning to women is for breast cancer, which should begin at age 40 to 50 (as it should for all women).
Gender-affirming surgical options
Surgical management of transgender patients is not within the scope of family medicine. But it is essential to know what procedures are available, as you may have occasion to advocate for patients during the surgical referral process and possibly to provide postoperative care.
For transmen, surgical options include chest reconstruction, hysterectomy/oophorectomy, metoidioplasty (using the clitoris to surgically approximate a penis), phalloplasty, scrotoplasty, urethroplasty, and vaginectomy.10,34 The surgeries available for transwomen are orchiectomy, vaginoplasty, penectomy, breast augmentation, thyroid chondroplasty and voice surgery, and facial feminization.10,34 Keep in mind that not all transgender individuals desire surgery as part of the transitioning process.
The authors would like to acknowledge the assistance of Michelle Forcier, MD, MPH, and Karen S. Bernstein, MD, MPH, in the preparation of this manuscript.
Civil rights for the lesbian, gay, bisexual, and transgender population have advanced markedly in the past decade, and the medical community has gradually begun to address more of their health concerns. More recently, media attention to transgender individuals has encouraged many more to openly seek care.1,2
It is estimated that anywhere from 0.3% to 5% of the US population identifies as transgender.1-3 While awareness of this population has slowly increased, there is a paucity of research on the hormone treatment that is often essential to patients’ well-being. Studies of surgical options for transgender patients have been minimal, as well.
Primary care providers are uniquely positioned to coordinate medical services and ensure continuity of care for transgender patients as they strive to become their authentic selves. Our goal in writing this article is to equip you with the tools to provide this patient population with sensitive, high-quality care (see Table 1).4-7 Our focus is on the diagnosis of gender dysphoria (GD) and its medical and hormonal management—the realm of primary care providers. We briefly discuss surgical management of GD, as well.
UNDERSTANDING AND DIAGNOSING GENDER DYSPHORIA
Two classification systems are used for diagnoses related to GD: the Diagnostic and Statistical Manual of Mental Disorders, Fifth Ed (DSM-5)8 and the International Classification of Diseases, 10th Rev (ICD-10).9
ICD-10 criteria use the term gender identity disorder; DSM-5 refers to gender dysphoria instead. It is important to emphasize that these classification systems represent an attempt to categorize a group of signs and symptoms that lead to distress for the patient and are not meant to suggest that being transgender is pathological. In fact, in DSM-5—released in 2013—the American Psychiatric Association revised the terminology to emphasize that such individuals are not “disordered” by the nature of their identity, but rather by the distress that being transgender causes.8
For a diagnosis of GD in children, DSM-5 criteria include characteristics perceived to be incongruent between the child’s sex at birth and the self-identified gender based on preferred activities or dislike of his or her own sexual anatomy. The child must meet six or more of the following for at least six months
- A repeatedly stated desire to be, or insistence that he or she is, of the other gender
- In boys, a preference for cross-dressing or simulating female attire; in girls, insistence on wearing only stereotypical masculine clothing
- Strong and persistent preferences for cross-gender roles in make-believe play or fantasy
- A strong rejection of toys/games typically associated with the child’s sex
- Intense desire to participate in stereotypical games and pastimes of the other gender
- Strong preference for playmates of the other gender
- A strong dislike of one’s sexual anatomy
- A strong desire for the primary (eg, penis or vagina) or secondary (eg, menstruation) sex characteristics of the other gender.8
Adolescents and adults must meet two or more of the following for at least six months
- A noticeable incongruence between the gender that the patient sees themselves as and their sex characteristics
- An intense need to do away with (or prevent) his or her primary or secondary sex features
- An intense desire to have the primary and/or secondary sex features of the other gender
- A deep desire to transform into another gender
- A profound need for society to treat them as someone of the other gender
- A powerful assurance of having the characteristic feelings and responses of the other gender.8
For children as well as adolescents and adults, the condition should cause the patient significant distress or significantly affect him or her socially, at work or school, and in other important areas of life.8
Is the patient a candidate for hormone therapy?
Two primary sources—Standards of Care for the Health of Transsexual, Transgender, and Gender-Nonconforming People, Version 7, issued by the World Professional Association for Transgender Health (WPATH)10 and Endocrine Treatment of Transsexual Persons11 by the Endocrine Society—offer clinical practice guidance based on evidence and expert opinion.
WPATH recommends that a mental health professional (MHP) experienced in transgender care diagnose GD to ensure that it is not mistaken for a psychiatric condition manifesting as altered gender identity. However, if no one with such experience is available or accessible in the region, it is reasonable for a primary care provider to make the diagnosis and consider initiating hormone therapy without a mental health referral,12 as the expected benefits outweigh the risks of nontreatment.13
Whether or not an MHP confirms a diagnosis of GD, it is still up to the treating provider to confirm the patient’s eligibility and readiness for hormone therapy: He or she should meet DSM-5 or ICD-10 criteria for GD, have no psychiatric comorbidity (eg, schizophrenia, body dysmorphic disorder, or uncontrolled bipolar disorder) likely to interfere with treatment, understand the expected outcomes and the social benefits and risks, and have indicated a willingness to take the hormones responsibly.
Historically, patients were required to have a documented real-life experience, defined as having fully adopted the new gender role in everyday life for at least three months.10,11 This model has fallen out of favor, however, as it is unsupported by evidence and may place transgender individuals at physical and emotional risk. Instead, readiness is confirmed by obtaining informed consent.12
Puberty may be suppressed with a gonadotropin-releasing hormone (GnRH) agonist in adolescents who have a GD diagnosis and are at Tanner stage 2 to 3 of puberty until age 16. At that point, hormone therapy consistent with their gender identification may be initiated (see “How to Help Transgender Teens”).11
Beginning the transition
The transitioning process is a complex and individualized journey that can include inward or outward change, or both.
For patients interested in medical interventions, possible therapies include cross-sex hormone administration and gender-affirming surgery. Both are aimed at making the physical and the psychologic more congruent. Hormone treatment (see Table 2) is often essential to reduce the distress of individuals with GD and to help them feel comfortable in their own body.10,11,21 Psychologic conditions, such as depression, tend to improve as the transitioning process gets underway.22
FEMALE-TO-MALE TRANSITION
CASE 1 Jennie R, a 55-year-old postmenopausal patient, comes to your office for an annual exam. Although you’ve been her primary care provider for several years, she confides for the first time that she has never been comfortable as a woman. “I’ve always felt that my body didn’t belong to me,” the patient admits, and goes on to say that for the past several years she has been living as a man. Jennie R says she is ready to start hormone therapy to assist with the gender transition and asks about the process, the benefits and risks, and how quickly she can expect to achieve the desired results.
If Jennie R were your patient, how would you respond?
Masculinizing hormone treatment
As you would explain to a patient like Jennie R, the goal of hormone therapy is to suppress the effects of the sex assigned at birth and replace them with those of the desired gender. In the case of a female transitioning to a male (known as a transman), masculinizing hormones would promote growth of facial and body hair, cessation of menses, increased muscle mass, deepening of the voice, and clitoral enlargement.
Physical changes induced by masculinizing hormone therapy have an expected onset of one to six months and achieve maximum effect in approximately two to five years.10,11 Although there have been no controlled clinical trials evaluating the safety or efficacy of any transitional hormone regimen, WPATH and the Center of Excellence for Transgender Health at the University of California, San Francisco, suggest initiating intramuscular or transdermal testosterone at increasing doses until normal physiologic male testosterone levels between 350 and 700 ng/dL are achieved, or until cessation of menses.13,25-28 The dose at which either, or both, occur should be continued as long-term maintenance therapy. Medroxyprogesterone can be added, if necessary for menstrual cessation, and a GnRH agonist or endometrial ablation can be used for refractory uterine bleeding.29,30
Testosterone is not a contraceptive. It is important to emphasize to transmen like Jennie that they remain at risk for pregnancy if they are having sex with fertile males. Caution patients not to assume that the possibility of pregnancy ends when menses stop.
Treat minor adverse effects. Adverse effects of masculinizing hormones include vaginal atrophy, fat redistribution and weight gain, polycythemia, acne, scalp hair loss, sleep apnea, elevated liver enzymes, hyperlipidemia, cardiovascular disease, diabetes, and bone density loss. Increased risk for cancer of the female organs has not been proven.10,11 It is reasonable to treat minor adverse effects after reviewing the risks/benefits of doing so, as discontinuing hormone therapy could be detrimental to the well-being of transitioning patients.11
There are absolute contraindications to masculinizing hormone therapy, however, including pregnancy, unstable coronary artery disease, and untreated polycythemia with a hematocrit > 55%.10
Monitoring is essential. Patients receiving masculinizing hormone therapy should be monitored every three months during the first year and once or twice a year thereafter, with a focused history (including mood symptoms), physical exam (including weight and blood pressure), and labs (including complete blood count, liver function, renal function, and lipids) at each visit.11,23 Some clinicians also check estradiol levels until they fall below 50 pg/mL,23,27 while others take the cessation of uterine bleeding for > 6 months as an indicator of estrogen suppression.
Preventive health measures continue. Routine screening should continue, based on the patient’s assigned sex at birth. Thus, a transman who has not had a hysterectomy still needs Pap smears, mammograms if the patient has not had a double mastectomy, and bone mineral density (BMD) testing to screen for osteoporosis.31,32 Some experts recommend starting to test BMD at age 50 for patients receiving masculinizing hormones, given the unknown effect of testosterone on bone density.11,31,32
CASE 1 The first question for a transgender patient is about his or her current gender identity, but Jennie R has already reported living as a man. So you start by asking “What name do you prefer to use?” and “Do you prefer to be referred to with male or female pronouns?”
The patient tells you that he sees himself as a man, he wants to be called Jeff, and he prefers male pronouns. You explain that you believe he has gender dysphoria and would benefit from hormone therapy, but it is important to confirm this diagnosis with an MHP. You explain that testosterone can be prescribed for masculinizing effects, and describe the expected effects—more facial and body hair, a deeper voice, and greater muscle mass, among others—and review the likely time frame.
You also discuss the risks of masculinizing hormones (hyperlipidemia, cardiovascular disease, diabetes, and loss of bone density) that will need to be monitored. Before he leaves, you give him the name of an MHP who is experienced in transgender care and tell him to make a follow-up appointment with you after he has seen her. At the conclusion of the visit, you make a note of the patient’s name and gender identity in the chart and inform the staff of the changes.
MALE-TO-FEMALE TRANSITION
CASE 2 Before heading into your office to talk to a new patient named Carl S, you glance at his chart and see that he is a healthy 21-year-old who has come in for a routine physical. When you enter the room, you find Carl wearing a dress, heels, and make-up. After confirming that you have the right patient, you ask, “What is your current gender identity?” “Female,” says Carl, who indicates that she now goes by Carol. The patient has no medical problems, surgical history, or significant family history but reports that she has been taking spironolactone and estrogen for the past three years. Carol also says she has a new female partner and is having unprotected sexual activity.
Feminizing hormone treatment
The desired effects of feminizing hormones include voice change, decreased hair growth, breast growth, body fat redistribution, decreased muscle mass, skin softening, decreased oiliness of skin and hair, and a decrease in spontaneous erections, testicular volume, and sperm production.10,11 The onset of feminizing effects ranges from one month to one year and the expected maximum effect occurs anywhere between three months and five years.10,11 Regimens usually include anti-androgen agents and estrogen.13,26-28
The medications that have been most studied with anti-androgenic effects include spironolactone and 5-α reductase inhibitors (5-ARIs) such as finasteride. Spironolactone inhibits testosterone secretion and inhibits androgen binding to androgen receptors; 5-ARIs block the conversion of testosterone to 5-α-dihydrotestosterone, the more active form.
Estrogen can be administered via oral, sublingual, transdermal, or intramuscular route, but parenteral formulations are preferred to avoid first-pass metabolism. The serum estradiol target is similar to the mean daily level of premenopausal women (< 200 pg/mL) and the level of testosterone should be in the normal female range (< 55 ng/dL).13,26-28
The selection of medications should be individualized for each patient. Comorbidities must be considered, as well as the risk for adverse effects, which include venous thromboembolism, elevated liver enzymes, breast cancer, cardiovascular disease, diabetes, hyperprolactinemia, weight gain, gallstones, cerebrovascular disease, and severe migraine headaches.10,11 Estrogen therapy is not reported to induce hypertrophy or premalignant changes in the prostate.33 As is the case for masculinizing hormones, feminizing hormone therapy should be continued indefinitely for long-term effects.
Frequent monitoring is recommended. Patients taking feminizing hormones (transwomen) should be seen every two to three months in the first year and monitored once or twice a year thereafter. Serum testosterone and estradiol levels should initially be monitored every three months; serum electrolytes, specifically potassium, should be monitored every two to three months in the first year until stable.
CASE 2 You recommend that Carol S be screened annually for sexually transmitted diseases, as you would for any 21-year-old patient. You point out, too, that while estrogen and androgen-suppressing therapy decrease sperm production, there is a possibility that the patient could impregnate a female partner and recommend that contraception be used if the couple is not trying to conceive.
You also discuss the risks and benefits of hormone therapy and reasonable expectations of continued treatment. You ask Carol to schedule a follow-up visit in six months, as her hormone regimen is stable. Finally, if the patient remains on hormone therapy, you mention that the only screening unique to men transitioning to women is for breast cancer, which should begin at age 40 to 50 (as it should for all women).
Gender-affirming surgical options
Surgical management of transgender patients is not within the scope of family medicine. But it is essential to know what procedures are available, as you may have occasion to advocate for patients during the surgical referral process and possibly to provide postoperative care.
For transmen, surgical options include chest reconstruction, hysterectomy/oophorectomy, metoidioplasty (using the clitoris to surgically approximate a penis), phalloplasty, scrotoplasty, urethroplasty, and vaginectomy.10,34 The surgeries available for transwomen are orchiectomy, vaginoplasty, penectomy, breast augmentation, thyroid chondroplasty and voice surgery, and facial feminization.10,34 Keep in mind that not all transgender individuals desire surgery as part of the transitioning process.
The authors would like to acknowledge the assistance of Michelle Forcier, MD, MPH, and Karen S. Bernstein, MD, MPH, in the preparation of this manuscript.
1. Pew Research Center. A survey of LGBT Americans: attitudes, experiences and values in changing times. www.pewsocialtrends.org/2013/06/13/a-survey-of-lgbt-americans. Accessed January 13, 2017.
2. Gates GJ. How many people are lesbian, gay, bisexual and transgender? http://williamsinstitute.law.ucla.edu/wp-content/uploads/Gates-How-Many-People-LGBT-Apr-2011.pdf. Accessed January 13, 2017.
3. van Kesteren PJ, Gooren LJ, Megens JA. An epidemiological and demographic study of transsexuals in The Netherlands. Arch Sex Behav. 1996;25:589-600.
4. Bhola S. An ally’s guide to terminology: talking about LGBT people & equality. www.glaad.org/2011/07/28/an-allys-guide-to-terminology-talking-about-lgbt-people-equality. Accessed January 13, 2017.
5. University of California, San Francisco. Transgender terminology. UCSF Center of Excellence for Transgender Health. http://transhealth.ucsf.edu/tcoe?page=protocol-terminology. Accessed January 13, 2017.
6. Istar A. How queer! The development of gender identity and sexual orientation in LGBTQ-headed families. Fam Process. 2010;49:268-290.
7. Goins ES, Pye D. Check the box that best describes you: reflexively managing theory and praxis in LGBTQ health communication research. Health Commun. 2013;28:397-407.
8. American Psychiatric Association. Gender dysphoria. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013: 451-459.
9. World Health Organization. The International Classification of Diseases, 10th rev. Classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines. 1992; Geneva.
10. Coleman E, Bockting W, Botzer M, et al; World Professional Association for Transgender Health. Standards of Care for the Health of Transsexual, Transgender, and Gender-Nonconforming People, Version 7. Int J Transgender. 2011; 13:165-232.
11. Hembree WC, Cohen-Kettenis P, Delemarre-van de Waal HA, et al. Endocrine treatment of transsexual persons: an Endocrine Society clinical practice guideline. J Clin Endo Metabol. 2009;94:3132-3154.
12. University of California, San Francisco. Assessing readiness for hormones. UCSF Center of Excellence for Transgender Health. http://transhealth.ucsf.edu/tcoe?page=protocol-hormone-ready. Accessed January 13, 2017.
13. Gooren L. Hormone treatment of the adult transsexual patient. Horm Res. 2005;64(suppl 2):S31-S36.
14. Hembree WC. Guidelines for pubertal suspension and gender reassignment for transgender adolescents. Child Adolesc Psychiatr Clin N Am. 2011;20:725-732.
15. Gay, Lesbian, and Straight Education Network (GLSEN). Harsh realities. The experiences of transgender youth in our nation’s schools. www.glsen.org/sites/default/files/Harsh%20Realities.pdf. Accessed January 13, 2017.
16. Berman M, Balingit M. Eleven states sue Obama administration over bathroom guidance for transgender students. May 25, 2016. Washington Post. www.washingtonpost.com/news/post-nation/wp/2016/05/25/texas-governor-says-state-will-sue-obama-administration-over-bathroom-directive/. Accessed January 13, 2017.
17. de Vries AL, Cohen-Kettenis PT, Delemarre-van de Waal H. Clinical management of gender dysphoria in adolescents. 2006. Vancouver Coastal Health - Transgender Health Program. www.amsa.org/wp-content/uploads/2015/04/CaringForTransgenderAdolescents.pdf. Accessed January 13, 2017.
18. TransYouth Family Allies. Empowering transgender youth & families. www.imatyfa.org/. Accessed January 13, 2017.
19. Human Rights Campaign. On our own: a survival guide for independent LGBTQ youth. www.hrc.org/resources/on-our-own-a-survival-guide-for-independent-lgbtq-youth. Accessed January 13, 2017.
20. Gay, Lesbian, Bisexual, and Transgender National Help Center. www.glbthotline.org. Accessed January 13, 2017.
21. University of California, San Francisco. Hormone administration. UCSF Center of Excellence for Transgender Health. http://transhealth.ucsf.edu/trans?page=protocol-hormones. Accessed January 13, 2017.
22. Gorin-Lazard A, Baumstarck K, Boyer L, et al. Hormonal therapy is associated with better self-esteem, mood, and quality of life in transsexuals. J Nerv Ment Dis. 2013;201:996-1000.
23. Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in adult men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2006;91:1995-2010.
24. Boloña ER, Uraga MV, Haddad RM, et al. Testosterone use in men with sexual dysfunction: a systematic review and meta-analysis of randomized placebo-controlled trials. Mayo Clin Proc. 2007;82:20-28.
25. Gooren LJ, Giltay EJ. Review of studies of androgen treatment of female-to-male transsexuals: effects and risks of administration of androgens to females. J Sex Med. 2008; 5:765-776.
26. Levy A, Crown A, Reid R. Endocrine intervention for transsexuals. Clin Endocrinol (Oxf). 2003;59:409-418.
27. Moore E, Wisniewski A, Dobs A. Endocrine treatment of transsexual people: a review of treatment regimens, outcomes, and adverse effects. J Clin Endocrinol Metab. 2003;88:3467-3473.
28. Tangpricha V, Ducharme SH, Barber TW, et al. Endocrinologic treatment of gender identity disorders. Endocr Pract. 2003;9:12-21.
29. Dickersin K, Munro MG, Clark M, et al. Hysterectomy compared with endometrial ablation for dysfunctional uterine bleeding: a randomized controlled trial. Obstet Gynecol. 2007;110:1279-1289.
30. Prasad P, Powell MC. Prospective observational study of Thermablate Endometrial Ablation System as an outpatient procedure. J Minim Invasive Gynecol. 2008;15:476-479.
31. University of California, San Francisco. General prevention and screening. UCSF Center of Excellence for Transgender Health. http://transhealth.ucsf.edu/trans?page=protocol-screening. Accessed January 13, 2017.
32. Ganly I, Taylor EW. Breast cancer in a trans-sexual man receiving hormone replacement therapy. Br J Surg. 1995; 82:341.
33. Meriggiola MC, Gava G. Endocrine care of transpeople part II: a review of cross-sex hormonal treatments, outcomes and adverse effects in transwomen. Clin Endocrinol (Oxf). 2015;83:607-615.
34. University of California, San Francisco. Surgical options. UCSF Center of Excellence for Transgender Health. http://transhealth.ucsf.edu/trans?page=protocol-surgery. Accessed January 13, 2017.
1. Pew Research Center. A survey of LGBT Americans: attitudes, experiences and values in changing times. www.pewsocialtrends.org/2013/06/13/a-survey-of-lgbt-americans. Accessed January 13, 2017.
2. Gates GJ. How many people are lesbian, gay, bisexual and transgender? http://williamsinstitute.law.ucla.edu/wp-content/uploads/Gates-How-Many-People-LGBT-Apr-2011.pdf. Accessed January 13, 2017.
3. van Kesteren PJ, Gooren LJ, Megens JA. An epidemiological and demographic study of transsexuals in The Netherlands. Arch Sex Behav. 1996;25:589-600.
4. Bhola S. An ally’s guide to terminology: talking about LGBT people & equality. www.glaad.org/2011/07/28/an-allys-guide-to-terminology-talking-about-lgbt-people-equality. Accessed January 13, 2017.
5. University of California, San Francisco. Transgender terminology. UCSF Center of Excellence for Transgender Health. http://transhealth.ucsf.edu/tcoe?page=protocol-terminology. Accessed January 13, 2017.
6. Istar A. How queer! The development of gender identity and sexual orientation in LGBTQ-headed families. Fam Process. 2010;49:268-290.
7. Goins ES, Pye D. Check the box that best describes you: reflexively managing theory and praxis in LGBTQ health communication research. Health Commun. 2013;28:397-407.
8. American Psychiatric Association. Gender dysphoria. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013: 451-459.
9. World Health Organization. The International Classification of Diseases, 10th rev. Classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines. 1992; Geneva.
10. Coleman E, Bockting W, Botzer M, et al; World Professional Association for Transgender Health. Standards of Care for the Health of Transsexual, Transgender, and Gender-Nonconforming People, Version 7. Int J Transgender. 2011; 13:165-232.
11. Hembree WC, Cohen-Kettenis P, Delemarre-van de Waal HA, et al. Endocrine treatment of transsexual persons: an Endocrine Society clinical practice guideline. J Clin Endo Metabol. 2009;94:3132-3154.
12. University of California, San Francisco. Assessing readiness for hormones. UCSF Center of Excellence for Transgender Health. http://transhealth.ucsf.edu/tcoe?page=protocol-hormone-ready. Accessed January 13, 2017.
13. Gooren L. Hormone treatment of the adult transsexual patient. Horm Res. 2005;64(suppl 2):S31-S36.
14. Hembree WC. Guidelines for pubertal suspension and gender reassignment for transgender adolescents. Child Adolesc Psychiatr Clin N Am. 2011;20:725-732.
15. Gay, Lesbian, and Straight Education Network (GLSEN). Harsh realities. The experiences of transgender youth in our nation’s schools. www.glsen.org/sites/default/files/Harsh%20Realities.pdf. Accessed January 13, 2017.
16. Berman M, Balingit M. Eleven states sue Obama administration over bathroom guidance for transgender students. May 25, 2016. Washington Post. www.washingtonpost.com/news/post-nation/wp/2016/05/25/texas-governor-says-state-will-sue-obama-administration-over-bathroom-directive/. Accessed January 13, 2017.
17. de Vries AL, Cohen-Kettenis PT, Delemarre-van de Waal H. Clinical management of gender dysphoria in adolescents. 2006. Vancouver Coastal Health - Transgender Health Program. www.amsa.org/wp-content/uploads/2015/04/CaringForTransgenderAdolescents.pdf. Accessed January 13, 2017.
18. TransYouth Family Allies. Empowering transgender youth & families. www.imatyfa.org/. Accessed January 13, 2017.
19. Human Rights Campaign. On our own: a survival guide for independent LGBTQ youth. www.hrc.org/resources/on-our-own-a-survival-guide-for-independent-lgbtq-youth. Accessed January 13, 2017.
20. Gay, Lesbian, Bisexual, and Transgender National Help Center. www.glbthotline.org. Accessed January 13, 2017.
21. University of California, San Francisco. Hormone administration. UCSF Center of Excellence for Transgender Health. http://transhealth.ucsf.edu/trans?page=protocol-hormones. Accessed January 13, 2017.
22. Gorin-Lazard A, Baumstarck K, Boyer L, et al. Hormonal therapy is associated with better self-esteem, mood, and quality of life in transsexuals. J Nerv Ment Dis. 2013;201:996-1000.
23. Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in adult men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2006;91:1995-2010.
24. Boloña ER, Uraga MV, Haddad RM, et al. Testosterone use in men with sexual dysfunction: a systematic review and meta-analysis of randomized placebo-controlled trials. Mayo Clin Proc. 2007;82:20-28.
25. Gooren LJ, Giltay EJ. Review of studies of androgen treatment of female-to-male transsexuals: effects and risks of administration of androgens to females. J Sex Med. 2008; 5:765-776.
26. Levy A, Crown A, Reid R. Endocrine intervention for transsexuals. Clin Endocrinol (Oxf). 2003;59:409-418.
27. Moore E, Wisniewski A, Dobs A. Endocrine treatment of transsexual people: a review of treatment regimens, outcomes, and adverse effects. J Clin Endocrinol Metab. 2003;88:3467-3473.
28. Tangpricha V, Ducharme SH, Barber TW, et al. Endocrinologic treatment of gender identity disorders. Endocr Pract. 2003;9:12-21.
29. Dickersin K, Munro MG, Clark M, et al. Hysterectomy compared with endometrial ablation for dysfunctional uterine bleeding: a randomized controlled trial. Obstet Gynecol. 2007;110:1279-1289.
30. Prasad P, Powell MC. Prospective observational study of Thermablate Endometrial Ablation System as an outpatient procedure. J Minim Invasive Gynecol. 2008;15:476-479.
31. University of California, San Francisco. General prevention and screening. UCSF Center of Excellence for Transgender Health. http://transhealth.ucsf.edu/trans?page=protocol-screening. Accessed January 13, 2017.
32. Ganly I, Taylor EW. Breast cancer in a trans-sexual man receiving hormone replacement therapy. Br J Surg. 1995; 82:341.
33. Meriggiola MC, Gava G. Endocrine care of transpeople part II: a review of cross-sex hormonal treatments, outcomes and adverse effects in transwomen. Clin Endocrinol (Oxf). 2015;83:607-615.
34. University of California, San Francisco. Surgical options. UCSF Center of Excellence for Transgender Health. http://transhealth.ucsf.edu/trans?page=protocol-surgery. Accessed January 13, 2017.
New Biologics in Psoriasis: An Update on IL-23 and IL-17 Inhibitors
The role of current biologic therapies in psoriasis predicates on the pathogenic role of upregulated, immune-related mechanisms that result in the activation of myeloid dendritic cells, which release IL-17, IL-23, and other cytokines to activate T cells, including helper T cell TH17. Along with other immune cells, TH17 produces IL-17. This proinflammatory cascade results in keratinocyte proliferation, angiogenesis, and migration of immune cells toward psoriatic lesions.1 Thus, the newest classes of biologics target IL-12, IL-23, and IL-17 to disrupt this inflammatory cascade.
We provide an updated review of the most recent clinical efficacy and safety data on the newest IL-23 and IL-17 inhibitors in the pipeline or approved for psoriasis, including risankizumab, guselkumab, tildrakizumab, ixekizumab, and brodalumab (Table). Ustekinumab and adalimumab, which have been previously approved by the US Food and Drug Administration (FDA), will be discussed here only as comparators.
IL-23 Inhibitors
Risankizumab
Risankizumab (formerly known as BI 655066)(Boehringer Ingelheim) is a selective human monoclonal antibody targeting the p19 subunit of IL-23 and currently is undergoing phase 3 trials for psoriasis. A proof-of-concept phase 1 study of 39 participants demonstrated efficacy after 12 weeks of treatment at varying subcutaneous and intravenous doses with placebo control.11 At week 12, 87% (27/31)(P<.001) of all risankizumab-treated participants achieved 75% reduction in psoriasis area and severity index (PASI) score compared to 0% of 8 placebo-treated participants. Common adverse effects (AEs) occurred in 65% (20/31) of risankizumab-treated participants, including non–dose-dependent upper respiratory tract infections, nasopharyngitis, and headache. Serious adverse events (SAEs) that occurred were considered unrelated to the study medication.11
A phase 2 trial of 166 participants compared 3 dosing regimens of subcutaneous risankizumab (single 18-mg dose at week 0; single 90-mg dose at weeks 0, 4, and 16; or single 180-mg dose at weeks 0, 4, and 16) and ustekinumab (weight-based single 45- or 90-mg dose at weeks 0, 4, and 16), demonstrating noninferiority at higher doses of risankizumab.2 Preliminary primary end point results at week 12 showed PASI 90 in 32.6% (P=.4667), 73.2% (P=.0013), 81.0% (P<.0001), and 40.0% of the treatment groups, respectively. Participants in the 180-mg risankizumab group achieved PASI 90 eight weeks faster than those on ustekinumab, lasting more than 2 months longer. Adverse effects were similar across all treatment groups and SAEs were unrelated to the study medications.2
Guselkumab
Guselkumab (Janssen Biotech, Inc) is a selective human monoclonal antibody against the p19 subunit of IL-23. The 52-week phase 2 X-PLORE trial compared dose-ranging subcutaneous guselkumab (5 mg at weeks 0 and 4, then every 12 weeks; 15 mg every 8 weeks; 50 mg at weeks 0 and 4, then every 12 weeks; 100 mg every 8 weeks; or 200 mg at weeks 0 and 4, then every 12 weeks), adalimumab (80-mg loading dose, followed by 40 mg at week 1, then every other week), and placebo in 293 randomized participants.4 At week 16, 34% (P=.002) of participants in the 5-mg guselkumab group, 61% (P<.001) in the 15-mg group, 79% (P<.001) in the 50-mg group, 86% (P<.001) in the 100-mg group, 83% (P<.001) in the 200-mg group, and 58% (P<.001) in the adalimumab group achieved physician global assessment (PGA) scores of 0 (clear) or 1 (minimal psoriasis) compared to 7% of the placebo group. Achievement of PASI 75 similarly favored the guselkumab (44% [P<.001]; 76% [no P value given]; 81% [P<.001]; 79% [P<.001]; and 81% [P<.001], respectively) and adalimumab treatment arms (70% [P<.001]) compared to 5% in the placebo group. In longer-term comparisons to week 40, participants in the 50-, 100-, and 200-mg guselkumab groups showed significantly greater remission of psoriatic lesions, measured by a PGA score of 0 or 1, than participants in the adalimumab group (71% [P=.05]; 77% [P=.005]; 81% [P=.01]; and 49%, respectively).4
Preliminary results from VOYAGE 1 (N=837), the first of several phase 3 trials, further demonstrate the superiority of guselkumab 100 mg at weeks 0 and 4 and then every 8 weeks over adalimumab (standard dosing) and placebo; at week 16, 73.3% (P<.001 for both comparisons) versus 49.7% and 2.9% of participants, respectively, achieved PASI 90, with sustained superiority of skin clearance in guselkumab-treated participants compared to adalimumab and placebo through week 48.3
Long-term safety data showed no dose dependence or trend from 0 to 16 weeks and 16 to 52 weeks of treatment regarding rates of AEs, SAEs, or serious infections.4 Between weeks 16 and 52, 48.9% of all guselkumab-treated participants exhibited AEs compared to 60.5% of adalimumab-treated participants and 51.3% of placebo participants. Overall infection rates also were lowest in the guselkumab group at 29.8% compared to 36.8% and 35.9%, respectively. Three participants treated with guselkumab had major cardiovascular events, including a fatal myocardial infarction. No cases of tuberculosis or serious opportunistic infections were reported.4
Tildrakizumab
Tildrakizumab (formerly known as MK-3222)(Sun Pharmaceutical Industries Ltd) is a human monoclonal antibody also targeting the p19 subunit of IL-23. In a phase 2 study of 355 participants with chronic plaque psoriasis, participants received 5-, 25-, 100-, or 200-mg subcutaneous tildrakizumab or placebo at weeks 0 and 4 and then every 12 weeks for a total of 52 weeks.6 At week 16, PASI 75 results were 33.3%, 64.4%, 66.3%, 74.4%, and 4.4%, respectively (P<.001 for each comparison). Improvement began within the first month of treatment, with median times to PASI 75 of 57 days at 200-mg dosing and 84 days at 100-mg dosing. Of those participants achieving PASI 75 by drug discontinuation at week 52, 96% of the 100-mg group and 93% of the 200-mg group maintained PASI 75 through week 72, suggesting low relapse rates after treatment cessation.6
In October 2016, the efficacy results of 2 pivotal phase 3 trials (reSURFACE 1 and reSURFACE 2) involving more than 1800 participants combined revealed PASI 90 achievement in an average of 54% of participants on tildrakizumab 100 mg and 59% of participants on tildrakizumab 200 mg at week 28.5 Achievement of PASI 100 occurred in 24% and 30% of participants at week 28, respectively. The second of these trials included an etanercept comparison group and demonstrated head-to-head superiority of 100 and 200 mg subcutaneous tildrakizumab at week 12 by end point measures.5
Treatment-related AEs occurred at rates of 25% in tildrakizumab-treated participants and 22% in placebo-treated participants, most frequently nasopharyngitis and headache.6 At least 1 AE occurred in 64% of tildrakizumab-treated participants without dose dependence compared to 69% of placebo-treated participants. Severe AEs thought to be drug treatment related were bacterial arthritis, lymphedema, melanoma, stroke, and epiglottitis.6
IL-17 Inhibitors
Ixekizumab
Ixekizumab (Eli Lilly and Company), a monoclonal inhibitor of IL-17A, is the most recently approved psoriasis biologic on the market and has been cleared for use in adults with moderate to severe plaque psoriasis. Recommended dosing is 160 mg (given in two 80-mg subcutaneous injections via an autoinjector or prefilled syringe) at week 0, followed by an 80-mg injection at weeks 2, 4, 6, 8, 10, and 12, and then 80 mg every 4 weeks thereafter. The FDA approved ixekizumab in March 2016 following favorable results of several phase 3 trials: UNCOVER-1, UNCOVER-2, and UNCOVER-3.7,8
In UNCOVER-1, 1296 participants were randomized to 1 of 2 ixekizumab treatment arms—160 mg starting dose at week 0, 80 mg every 2 or 4 weeks thereafter—or placebo.7 At week 12, 89.1%, 82.6%, and 3.9% achieved PASI 75, respectively (P<.001 for both). Importantly, high numbers of participants also achieved PASI 90 (70.9% in the 2-week group and 64.6% in the 4-week group vs 0.5% in the placebo group [P<.001]) and PASI 100 (35.3% and 33.6% vs 0%, respectively [P<.001]), suggesting high rates of disease clearance.7
UNCOVER-2 (N=1224) and UNCOVER-3 (N=1346) investigated the same 2 dosing regimens of ixekizumab compared to etanercept 50 mg biweekly and placebo.8 At week 12, the percentage of participants achieving PASI 90 in UNCOVER-2 was 70.7%, 59.7%, 18.7%, and 0.6%, respectively, and 68.1%, 65.3%, 25.7%, and 3.1%, respectively, in UNCOVER-3 (P<.0001 for all comparisons to placebo and etanercept). At week 12, PASI 100 results also showed striking superiority, with 40.5%, 30.8%, 5.3%, and 0.6% of participants, respectively, in UNCOVER-2, and 37.7%, 35%, 7.3%, and 0%, respectively, in UNCOVER-3, achieving complete clearance of disease (P<.0001 for all comparisons to placebo and etanercept). Responses to ixekizumab were observed as early as weeks 1 and 2, while no participants in the etanercept and placebo treatment groups achieved comparative efficiency.8
In an extension of UNCOVER-3, efficacy increased from week 12 to week 60 according to PASI 90 (68%–73% in the 2-week group; 65%–72% in the 4-week group) and PASI 100 measures (38%–55% in the 2-week group; 35%–52% in the 4-week group).7
The most common AEs associated with ixekizumab treatment from weeks 0 to 12 occurred at higher rates in the 2-week and 4-week ixekizumab groups compared to placebo, including nasopharyngitis (9.5% and 9% vs 8.7%, respectively), upper respiratory tract infection (4.4% and 3.9% vs 3.5%, respectively), injection-site reaction (10% and 7.7% vs 1%, respectively), arthralgia (4.4% and 4.3% vs 2.9%, respectively), and headache (2.5% and 1.9% vs 2.1%, respectively). Infections, including candidal, oral, vulvovaginal, and cutaneous, occurred in 27% of the 2-week dosing group and 27.4% of the 4-week dosing group compared to 22.9% of the placebo group during weeks 0 to 12, with candidal infections in particular occurring more frequently in the active treatment groups and exhibiting dose dependence. Other AEs of special interest that occurred among all ixekizumab-treated participants (n=3736) from weeks 0 to 60 were cardiovascular and cerebrovascular events (22 [0.6%]), inflammatory bowel disease (11 [0.3%]), non–skin cancer malignancy (14 [0.4%]), and nonmelanoma skin cancer (20 [0.5%]). Neutropenia occurred at higher rates in ixekizumab-treated participants (9.3% in the 2-week group and 8.6% in the 4-week group) compared to placebo (3.3%) and occurred in 11.5% of all ixekizumab participants over 60 weeks.7
Brodalumab
Brodalumab (Valeant Pharmaceuticals International, Inc) is a human monoclonal antibody targeting the IL-17A receptor currently under review for FDA approval after undergoing phase 3 trials. The first of these trials, AMAGINE-1, showed efficacy of subcutaneous brodalumab (140 or 210 mg administered every 2 weeks with an extra dose at week 1) compared to placebo in 661 participants.9 At week 12, 60%, 83%, and 3%, respectively, achieved PASI 75; 43%, 70%, and 1%, respectively, achieved PASI 90; and 23%, 42%, and 1%, respectively, achieved PASI 100 (P<.001 for all respective comparisons to placebo). These effects were retained through 52 weeks of treatment. The median time to complete disease clearance in participants reaching PASI 100 was 12 weeks. Conversely, participants who were re-randomized to placebo after week 12 of brodalumab treatment relapsed within weeks to months.9
AMAGINE-2 and AMAGINE-3 further demonstrated the efficacy of brodalumab (140 or 210 mg every 2 weeks with extra dose at week 1) compared to ustekinumab (45 or 90 mg weight-based standard dosing) and placebo in 1831 participants, respectively.10 In AMAGINE-2, 49% of participants in the 140-mg group (P<.001 vs placebo), 70% in the 210-mg group (P<.001 vs placebo), 47% in the ustekinumab group, and 3% in the placebo group achieved PASI 90 at week 12. Similarly, in AMAGINE-3, 52% of participants in the 140-mg group (P<.001), 69% in the 210-mg group (P<.001), 48% in the ustekinumab group, and 2% in the placebo group achieved PASI 90. Impressively, complete clearance (PASI 100) at week 12 occurred in 26% of the 140-mg group (P<.001 vs placebo), 44% of the 210-mg group (P<.001 vs placebo), and 22% of the ustekinumab group compared to 2% of the placebo group in AMAGINE-2, with similar rates in AMAGINE-3. Brodalumab was significantly superior to ustekinumab at the 210-mg dose by PASI 90 measures (P<.001) in both studies and at the 140-mg dose by PASI 100 measures (P=.007) in AMAGINE-3 only.10
Common AEs were nasopharyngitis, upper respiratory tract infection, headache, and arthralgia, all occurring at grossly similar rates (49%–60%) across all experimental groups in AMAGINE-1, AMAGINE-2, and AMAGINE-3 during the first 12-week treatment period.9,10 Brodalumab treatment groups had high rates of specific interest AEs compared to ustekinumab and placebo groups, including neutropenia (0.8%, 1.1%, 0.3%, and 0%, respectively) and candidal infections (0.8%, 1.3%, 0.3%, and 0.3%, respectively). Induction phase (weeks 0–12) depression rates were concerning, with 6 cases each in AMAGINE-2 (4 [0.7%] in the 140-mg group, 2 [0.3%] in the 210-mg group) and AMAGINE-3 (4 [0.6%] in the 140-mg group, 2 [0.3%] in the 210-mg group). Cases of neutropenia were mild, were not associated with major infection, and were transient or reversible. Depression rates after 52 weeks of treatment were 1.7% (23/1567) of brodalumab participants in AMAGINE-2 and 1.8% (21/1613) in AMAGINE-3. Three participants, all on constant 210-mg dosing through week 52, attempted suicide with 1 completion10; however, because no other IL-17 inhibitors were associated with depression or suicide in other trials, it has been suggested that these cases were incidental and not treatment related.12 An FDA advisory panel recommended approval of brodalumab in July 2016 despite ongoing concerns of depression and suicide.13
Conclusion
The robust investigation into IL-23 and IL-17 inhibitors to treat plaque psoriasis has yielded promising results, including the unprecedented rates of PASI 100 achievement with these new biologics. Risankizumab, ixekizumab, and brodalumab have demonstrated superior efficacy in trials compared to ustekinumab. Tildrakizumab has shown low disease relapse after drug cessation. Ixekizumab and brodalumab have shown high rates of total disease clearance. Thus far, safety findings for these pipeline biologics have been consistent with those of ustekinumab. With ixekizumab approved in 2016 and brodalumab under review, new options in biologic therapy will offer patients and clinicians greater choices in treating severe and recalcitrant psoriasis.
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509.
- Papp K, Menter A, Sofen H, et al. Efficacy and safety of different dose regimens of a selective IL-23p19 inhibitor (BI 655066) compared with ustekinumab in patients with moderate-to-severe plaque psoriasis with and without psoriatic arthritis. Paper presented at: 2015 American College of Rheumatology/Association of Rheumatology Health Professionals Annual Meeting; November 6-11, 2015; San Francisco, CA.
- New phase 3 data show significant efficacy versus placebo and superiority of guselkumab versus Humira in treatment of moderate to severe plaque psoriasis [press release]. Vienna, Austria; Janssen Research & Development, LLC: October 1, 2016.
- Gordon KB, Duffin KC, Bissonnette R, et al. A phase 2 trial of guselkumab versus adalimumab for plaque psoriasis. N Engl J Med. 2015;373:136-144.
- Sun Pharma to announce late-breaking results for investigational IL-23p19 inhibitor, Tildrakizumab, achieves primary end point in both phase-3 studies in patients with moderate-to-severe plaque psoriasis [press release]. Mumbai, India; Sun Pharmaceutical Industries Ltd: October 1, 2016.
- Papp K, Thaci D, Reich K, et al. Tildrakizumab (MK-3222), an anti-interleukin-23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo-controlled trial. Br J Dermatol. 2015;173:930-939.
- Gordon KB, Blauvelt A, Papp KA, et al; UNCOVER-1 Study Group, UNCOVER-2 Study Group, UNCOVER-3 Study Group. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:345-356.
- Griffiths CE, Reich K, Lebwohl M, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386:541-551.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis [published online June 23, 2016]. Br J Dermatol. 2016;175:273-286.
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373:1318-1328.
- Krueger JG, Ferris LK, Menter A, et al. Anti-IL-23A mAb BI 655066 for treatment of moderate-to-severe psoriasis: safety, efficacy, pharmacokinetics, and biomarker results of a single-rising-dose, randomized, double-blind, placebo-controlled trial [published online March 1, 2015]. J Allergy Clin Immunol. 2015;136:116-124.e7.
- Chiricozzi A, Romanelli M, Saraceno R, et al. No meaningful association between suicidal behavior and the use of IL-17A-neutralizing or IL-17RA-blocking agents [published online August 31, 2016]. Expert Opin Drug Saf. 2016;15:1653-1659.
- FDA advisory committee recommends approval of brodalumab for treatment of moderate-to-severe plaque psoriasis [news release]. Laval, Quebec: Valeant Pharmaceuticals International, Inc; July 19, 2016.
The role of current biologic therapies in psoriasis predicates on the pathogenic role of upregulated, immune-related mechanisms that result in the activation of myeloid dendritic cells, which release IL-17, IL-23, and other cytokines to activate T cells, including helper T cell TH17. Along with other immune cells, TH17 produces IL-17. This proinflammatory cascade results in keratinocyte proliferation, angiogenesis, and migration of immune cells toward psoriatic lesions.1 Thus, the newest classes of biologics target IL-12, IL-23, and IL-17 to disrupt this inflammatory cascade.
We provide an updated review of the most recent clinical efficacy and safety data on the newest IL-23 and IL-17 inhibitors in the pipeline or approved for psoriasis, including risankizumab, guselkumab, tildrakizumab, ixekizumab, and brodalumab (Table). Ustekinumab and adalimumab, which have been previously approved by the US Food and Drug Administration (FDA), will be discussed here only as comparators.
IL-23 Inhibitors
Risankizumab
Risankizumab (formerly known as BI 655066)(Boehringer Ingelheim) is a selective human monoclonal antibody targeting the p19 subunit of IL-23 and currently is undergoing phase 3 trials for psoriasis. A proof-of-concept phase 1 study of 39 participants demonstrated efficacy after 12 weeks of treatment at varying subcutaneous and intravenous doses with placebo control.11 At week 12, 87% (27/31)(P<.001) of all risankizumab-treated participants achieved 75% reduction in psoriasis area and severity index (PASI) score compared to 0% of 8 placebo-treated participants. Common adverse effects (AEs) occurred in 65% (20/31) of risankizumab-treated participants, including non–dose-dependent upper respiratory tract infections, nasopharyngitis, and headache. Serious adverse events (SAEs) that occurred were considered unrelated to the study medication.11
A phase 2 trial of 166 participants compared 3 dosing regimens of subcutaneous risankizumab (single 18-mg dose at week 0; single 90-mg dose at weeks 0, 4, and 16; or single 180-mg dose at weeks 0, 4, and 16) and ustekinumab (weight-based single 45- or 90-mg dose at weeks 0, 4, and 16), demonstrating noninferiority at higher doses of risankizumab.2 Preliminary primary end point results at week 12 showed PASI 90 in 32.6% (P=.4667), 73.2% (P=.0013), 81.0% (P<.0001), and 40.0% of the treatment groups, respectively. Participants in the 180-mg risankizumab group achieved PASI 90 eight weeks faster than those on ustekinumab, lasting more than 2 months longer. Adverse effects were similar across all treatment groups and SAEs were unrelated to the study medications.2
Guselkumab
Guselkumab (Janssen Biotech, Inc) is a selective human monoclonal antibody against the p19 subunit of IL-23. The 52-week phase 2 X-PLORE trial compared dose-ranging subcutaneous guselkumab (5 mg at weeks 0 and 4, then every 12 weeks; 15 mg every 8 weeks; 50 mg at weeks 0 and 4, then every 12 weeks; 100 mg every 8 weeks; or 200 mg at weeks 0 and 4, then every 12 weeks), adalimumab (80-mg loading dose, followed by 40 mg at week 1, then every other week), and placebo in 293 randomized participants.4 At week 16, 34% (P=.002) of participants in the 5-mg guselkumab group, 61% (P<.001) in the 15-mg group, 79% (P<.001) in the 50-mg group, 86% (P<.001) in the 100-mg group, 83% (P<.001) in the 200-mg group, and 58% (P<.001) in the adalimumab group achieved physician global assessment (PGA) scores of 0 (clear) or 1 (minimal psoriasis) compared to 7% of the placebo group. Achievement of PASI 75 similarly favored the guselkumab (44% [P<.001]; 76% [no P value given]; 81% [P<.001]; 79% [P<.001]; and 81% [P<.001], respectively) and adalimumab treatment arms (70% [P<.001]) compared to 5% in the placebo group. In longer-term comparisons to week 40, participants in the 50-, 100-, and 200-mg guselkumab groups showed significantly greater remission of psoriatic lesions, measured by a PGA score of 0 or 1, than participants in the adalimumab group (71% [P=.05]; 77% [P=.005]; 81% [P=.01]; and 49%, respectively).4
Preliminary results from VOYAGE 1 (N=837), the first of several phase 3 trials, further demonstrate the superiority of guselkumab 100 mg at weeks 0 and 4 and then every 8 weeks over adalimumab (standard dosing) and placebo; at week 16, 73.3% (P<.001 for both comparisons) versus 49.7% and 2.9% of participants, respectively, achieved PASI 90, with sustained superiority of skin clearance in guselkumab-treated participants compared to adalimumab and placebo through week 48.3
Long-term safety data showed no dose dependence or trend from 0 to 16 weeks and 16 to 52 weeks of treatment regarding rates of AEs, SAEs, or serious infections.4 Between weeks 16 and 52, 48.9% of all guselkumab-treated participants exhibited AEs compared to 60.5% of adalimumab-treated participants and 51.3% of placebo participants. Overall infection rates also were lowest in the guselkumab group at 29.8% compared to 36.8% and 35.9%, respectively. Three participants treated with guselkumab had major cardiovascular events, including a fatal myocardial infarction. No cases of tuberculosis or serious opportunistic infections were reported.4
Tildrakizumab
Tildrakizumab (formerly known as MK-3222)(Sun Pharmaceutical Industries Ltd) is a human monoclonal antibody also targeting the p19 subunit of IL-23. In a phase 2 study of 355 participants with chronic plaque psoriasis, participants received 5-, 25-, 100-, or 200-mg subcutaneous tildrakizumab or placebo at weeks 0 and 4 and then every 12 weeks for a total of 52 weeks.6 At week 16, PASI 75 results were 33.3%, 64.4%, 66.3%, 74.4%, and 4.4%, respectively (P<.001 for each comparison). Improvement began within the first month of treatment, with median times to PASI 75 of 57 days at 200-mg dosing and 84 days at 100-mg dosing. Of those participants achieving PASI 75 by drug discontinuation at week 52, 96% of the 100-mg group and 93% of the 200-mg group maintained PASI 75 through week 72, suggesting low relapse rates after treatment cessation.6
In October 2016, the efficacy results of 2 pivotal phase 3 trials (reSURFACE 1 and reSURFACE 2) involving more than 1800 participants combined revealed PASI 90 achievement in an average of 54% of participants on tildrakizumab 100 mg and 59% of participants on tildrakizumab 200 mg at week 28.5 Achievement of PASI 100 occurred in 24% and 30% of participants at week 28, respectively. The second of these trials included an etanercept comparison group and demonstrated head-to-head superiority of 100 and 200 mg subcutaneous tildrakizumab at week 12 by end point measures.5
Treatment-related AEs occurred at rates of 25% in tildrakizumab-treated participants and 22% in placebo-treated participants, most frequently nasopharyngitis and headache.6 At least 1 AE occurred in 64% of tildrakizumab-treated participants without dose dependence compared to 69% of placebo-treated participants. Severe AEs thought to be drug treatment related were bacterial arthritis, lymphedema, melanoma, stroke, and epiglottitis.6
IL-17 Inhibitors
Ixekizumab
Ixekizumab (Eli Lilly and Company), a monoclonal inhibitor of IL-17A, is the most recently approved psoriasis biologic on the market and has been cleared for use in adults with moderate to severe plaque psoriasis. Recommended dosing is 160 mg (given in two 80-mg subcutaneous injections via an autoinjector or prefilled syringe) at week 0, followed by an 80-mg injection at weeks 2, 4, 6, 8, 10, and 12, and then 80 mg every 4 weeks thereafter. The FDA approved ixekizumab in March 2016 following favorable results of several phase 3 trials: UNCOVER-1, UNCOVER-2, and UNCOVER-3.7,8
In UNCOVER-1, 1296 participants were randomized to 1 of 2 ixekizumab treatment arms—160 mg starting dose at week 0, 80 mg every 2 or 4 weeks thereafter—or placebo.7 At week 12, 89.1%, 82.6%, and 3.9% achieved PASI 75, respectively (P<.001 for both). Importantly, high numbers of participants also achieved PASI 90 (70.9% in the 2-week group and 64.6% in the 4-week group vs 0.5% in the placebo group [P<.001]) and PASI 100 (35.3% and 33.6% vs 0%, respectively [P<.001]), suggesting high rates of disease clearance.7
UNCOVER-2 (N=1224) and UNCOVER-3 (N=1346) investigated the same 2 dosing regimens of ixekizumab compared to etanercept 50 mg biweekly and placebo.8 At week 12, the percentage of participants achieving PASI 90 in UNCOVER-2 was 70.7%, 59.7%, 18.7%, and 0.6%, respectively, and 68.1%, 65.3%, 25.7%, and 3.1%, respectively, in UNCOVER-3 (P<.0001 for all comparisons to placebo and etanercept). At week 12, PASI 100 results also showed striking superiority, with 40.5%, 30.8%, 5.3%, and 0.6% of participants, respectively, in UNCOVER-2, and 37.7%, 35%, 7.3%, and 0%, respectively, in UNCOVER-3, achieving complete clearance of disease (P<.0001 for all comparisons to placebo and etanercept). Responses to ixekizumab were observed as early as weeks 1 and 2, while no participants in the etanercept and placebo treatment groups achieved comparative efficiency.8
In an extension of UNCOVER-3, efficacy increased from week 12 to week 60 according to PASI 90 (68%–73% in the 2-week group; 65%–72% in the 4-week group) and PASI 100 measures (38%–55% in the 2-week group; 35%–52% in the 4-week group).7
The most common AEs associated with ixekizumab treatment from weeks 0 to 12 occurred at higher rates in the 2-week and 4-week ixekizumab groups compared to placebo, including nasopharyngitis (9.5% and 9% vs 8.7%, respectively), upper respiratory tract infection (4.4% and 3.9% vs 3.5%, respectively), injection-site reaction (10% and 7.7% vs 1%, respectively), arthralgia (4.4% and 4.3% vs 2.9%, respectively), and headache (2.5% and 1.9% vs 2.1%, respectively). Infections, including candidal, oral, vulvovaginal, and cutaneous, occurred in 27% of the 2-week dosing group and 27.4% of the 4-week dosing group compared to 22.9% of the placebo group during weeks 0 to 12, with candidal infections in particular occurring more frequently in the active treatment groups and exhibiting dose dependence. Other AEs of special interest that occurred among all ixekizumab-treated participants (n=3736) from weeks 0 to 60 were cardiovascular and cerebrovascular events (22 [0.6%]), inflammatory bowel disease (11 [0.3%]), non–skin cancer malignancy (14 [0.4%]), and nonmelanoma skin cancer (20 [0.5%]). Neutropenia occurred at higher rates in ixekizumab-treated participants (9.3% in the 2-week group and 8.6% in the 4-week group) compared to placebo (3.3%) and occurred in 11.5% of all ixekizumab participants over 60 weeks.7
Brodalumab
Brodalumab (Valeant Pharmaceuticals International, Inc) is a human monoclonal antibody targeting the IL-17A receptor currently under review for FDA approval after undergoing phase 3 trials. The first of these trials, AMAGINE-1, showed efficacy of subcutaneous brodalumab (140 or 210 mg administered every 2 weeks with an extra dose at week 1) compared to placebo in 661 participants.9 At week 12, 60%, 83%, and 3%, respectively, achieved PASI 75; 43%, 70%, and 1%, respectively, achieved PASI 90; and 23%, 42%, and 1%, respectively, achieved PASI 100 (P<.001 for all respective comparisons to placebo). These effects were retained through 52 weeks of treatment. The median time to complete disease clearance in participants reaching PASI 100 was 12 weeks. Conversely, participants who were re-randomized to placebo after week 12 of brodalumab treatment relapsed within weeks to months.9
AMAGINE-2 and AMAGINE-3 further demonstrated the efficacy of brodalumab (140 or 210 mg every 2 weeks with extra dose at week 1) compared to ustekinumab (45 or 90 mg weight-based standard dosing) and placebo in 1831 participants, respectively.10 In AMAGINE-2, 49% of participants in the 140-mg group (P<.001 vs placebo), 70% in the 210-mg group (P<.001 vs placebo), 47% in the ustekinumab group, and 3% in the placebo group achieved PASI 90 at week 12. Similarly, in AMAGINE-3, 52% of participants in the 140-mg group (P<.001), 69% in the 210-mg group (P<.001), 48% in the ustekinumab group, and 2% in the placebo group achieved PASI 90. Impressively, complete clearance (PASI 100) at week 12 occurred in 26% of the 140-mg group (P<.001 vs placebo), 44% of the 210-mg group (P<.001 vs placebo), and 22% of the ustekinumab group compared to 2% of the placebo group in AMAGINE-2, with similar rates in AMAGINE-3. Brodalumab was significantly superior to ustekinumab at the 210-mg dose by PASI 90 measures (P<.001) in both studies and at the 140-mg dose by PASI 100 measures (P=.007) in AMAGINE-3 only.10
Common AEs were nasopharyngitis, upper respiratory tract infection, headache, and arthralgia, all occurring at grossly similar rates (49%–60%) across all experimental groups in AMAGINE-1, AMAGINE-2, and AMAGINE-3 during the first 12-week treatment period.9,10 Brodalumab treatment groups had high rates of specific interest AEs compared to ustekinumab and placebo groups, including neutropenia (0.8%, 1.1%, 0.3%, and 0%, respectively) and candidal infections (0.8%, 1.3%, 0.3%, and 0.3%, respectively). Induction phase (weeks 0–12) depression rates were concerning, with 6 cases each in AMAGINE-2 (4 [0.7%] in the 140-mg group, 2 [0.3%] in the 210-mg group) and AMAGINE-3 (4 [0.6%] in the 140-mg group, 2 [0.3%] in the 210-mg group). Cases of neutropenia were mild, were not associated with major infection, and were transient or reversible. Depression rates after 52 weeks of treatment were 1.7% (23/1567) of brodalumab participants in AMAGINE-2 and 1.8% (21/1613) in AMAGINE-3. Three participants, all on constant 210-mg dosing through week 52, attempted suicide with 1 completion10; however, because no other IL-17 inhibitors were associated with depression or suicide in other trials, it has been suggested that these cases were incidental and not treatment related.12 An FDA advisory panel recommended approval of brodalumab in July 2016 despite ongoing concerns of depression and suicide.13
Conclusion
The robust investigation into IL-23 and IL-17 inhibitors to treat plaque psoriasis has yielded promising results, including the unprecedented rates of PASI 100 achievement with these new biologics. Risankizumab, ixekizumab, and brodalumab have demonstrated superior efficacy in trials compared to ustekinumab. Tildrakizumab has shown low disease relapse after drug cessation. Ixekizumab and brodalumab have shown high rates of total disease clearance. Thus far, safety findings for these pipeline biologics have been consistent with those of ustekinumab. With ixekizumab approved in 2016 and brodalumab under review, new options in biologic therapy will offer patients and clinicians greater choices in treating severe and recalcitrant psoriasis.
The role of current biologic therapies in psoriasis predicates on the pathogenic role of upregulated, immune-related mechanisms that result in the activation of myeloid dendritic cells, which release IL-17, IL-23, and other cytokines to activate T cells, including helper T cell TH17. Along with other immune cells, TH17 produces IL-17. This proinflammatory cascade results in keratinocyte proliferation, angiogenesis, and migration of immune cells toward psoriatic lesions.1 Thus, the newest classes of biologics target IL-12, IL-23, and IL-17 to disrupt this inflammatory cascade.
We provide an updated review of the most recent clinical efficacy and safety data on the newest IL-23 and IL-17 inhibitors in the pipeline or approved for psoriasis, including risankizumab, guselkumab, tildrakizumab, ixekizumab, and brodalumab (Table). Ustekinumab and adalimumab, which have been previously approved by the US Food and Drug Administration (FDA), will be discussed here only as comparators.
IL-23 Inhibitors
Risankizumab
Risankizumab (formerly known as BI 655066)(Boehringer Ingelheim) is a selective human monoclonal antibody targeting the p19 subunit of IL-23 and currently is undergoing phase 3 trials for psoriasis. A proof-of-concept phase 1 study of 39 participants demonstrated efficacy after 12 weeks of treatment at varying subcutaneous and intravenous doses with placebo control.11 At week 12, 87% (27/31)(P<.001) of all risankizumab-treated participants achieved 75% reduction in psoriasis area and severity index (PASI) score compared to 0% of 8 placebo-treated participants. Common adverse effects (AEs) occurred in 65% (20/31) of risankizumab-treated participants, including non–dose-dependent upper respiratory tract infections, nasopharyngitis, and headache. Serious adverse events (SAEs) that occurred were considered unrelated to the study medication.11
A phase 2 trial of 166 participants compared 3 dosing regimens of subcutaneous risankizumab (single 18-mg dose at week 0; single 90-mg dose at weeks 0, 4, and 16; or single 180-mg dose at weeks 0, 4, and 16) and ustekinumab (weight-based single 45- or 90-mg dose at weeks 0, 4, and 16), demonstrating noninferiority at higher doses of risankizumab.2 Preliminary primary end point results at week 12 showed PASI 90 in 32.6% (P=.4667), 73.2% (P=.0013), 81.0% (P<.0001), and 40.0% of the treatment groups, respectively. Participants in the 180-mg risankizumab group achieved PASI 90 eight weeks faster than those on ustekinumab, lasting more than 2 months longer. Adverse effects were similar across all treatment groups and SAEs were unrelated to the study medications.2
Guselkumab
Guselkumab (Janssen Biotech, Inc) is a selective human monoclonal antibody against the p19 subunit of IL-23. The 52-week phase 2 X-PLORE trial compared dose-ranging subcutaneous guselkumab (5 mg at weeks 0 and 4, then every 12 weeks; 15 mg every 8 weeks; 50 mg at weeks 0 and 4, then every 12 weeks; 100 mg every 8 weeks; or 200 mg at weeks 0 and 4, then every 12 weeks), adalimumab (80-mg loading dose, followed by 40 mg at week 1, then every other week), and placebo in 293 randomized participants.4 At week 16, 34% (P=.002) of participants in the 5-mg guselkumab group, 61% (P<.001) in the 15-mg group, 79% (P<.001) in the 50-mg group, 86% (P<.001) in the 100-mg group, 83% (P<.001) in the 200-mg group, and 58% (P<.001) in the adalimumab group achieved physician global assessment (PGA) scores of 0 (clear) or 1 (minimal psoriasis) compared to 7% of the placebo group. Achievement of PASI 75 similarly favored the guselkumab (44% [P<.001]; 76% [no P value given]; 81% [P<.001]; 79% [P<.001]; and 81% [P<.001], respectively) and adalimumab treatment arms (70% [P<.001]) compared to 5% in the placebo group. In longer-term comparisons to week 40, participants in the 50-, 100-, and 200-mg guselkumab groups showed significantly greater remission of psoriatic lesions, measured by a PGA score of 0 or 1, than participants in the adalimumab group (71% [P=.05]; 77% [P=.005]; 81% [P=.01]; and 49%, respectively).4
Preliminary results from VOYAGE 1 (N=837), the first of several phase 3 trials, further demonstrate the superiority of guselkumab 100 mg at weeks 0 and 4 and then every 8 weeks over adalimumab (standard dosing) and placebo; at week 16, 73.3% (P<.001 for both comparisons) versus 49.7% and 2.9% of participants, respectively, achieved PASI 90, with sustained superiority of skin clearance in guselkumab-treated participants compared to adalimumab and placebo through week 48.3
Long-term safety data showed no dose dependence or trend from 0 to 16 weeks and 16 to 52 weeks of treatment regarding rates of AEs, SAEs, or serious infections.4 Between weeks 16 and 52, 48.9% of all guselkumab-treated participants exhibited AEs compared to 60.5% of adalimumab-treated participants and 51.3% of placebo participants. Overall infection rates also were lowest in the guselkumab group at 29.8% compared to 36.8% and 35.9%, respectively. Three participants treated with guselkumab had major cardiovascular events, including a fatal myocardial infarction. No cases of tuberculosis or serious opportunistic infections were reported.4
Tildrakizumab
Tildrakizumab (formerly known as MK-3222)(Sun Pharmaceutical Industries Ltd) is a human monoclonal antibody also targeting the p19 subunit of IL-23. In a phase 2 study of 355 participants with chronic plaque psoriasis, participants received 5-, 25-, 100-, or 200-mg subcutaneous tildrakizumab or placebo at weeks 0 and 4 and then every 12 weeks for a total of 52 weeks.6 At week 16, PASI 75 results were 33.3%, 64.4%, 66.3%, 74.4%, and 4.4%, respectively (P<.001 for each comparison). Improvement began within the first month of treatment, with median times to PASI 75 of 57 days at 200-mg dosing and 84 days at 100-mg dosing. Of those participants achieving PASI 75 by drug discontinuation at week 52, 96% of the 100-mg group and 93% of the 200-mg group maintained PASI 75 through week 72, suggesting low relapse rates after treatment cessation.6
In October 2016, the efficacy results of 2 pivotal phase 3 trials (reSURFACE 1 and reSURFACE 2) involving more than 1800 participants combined revealed PASI 90 achievement in an average of 54% of participants on tildrakizumab 100 mg and 59% of participants on tildrakizumab 200 mg at week 28.5 Achievement of PASI 100 occurred in 24% and 30% of participants at week 28, respectively. The second of these trials included an etanercept comparison group and demonstrated head-to-head superiority of 100 and 200 mg subcutaneous tildrakizumab at week 12 by end point measures.5
Treatment-related AEs occurred at rates of 25% in tildrakizumab-treated participants and 22% in placebo-treated participants, most frequently nasopharyngitis and headache.6 At least 1 AE occurred in 64% of tildrakizumab-treated participants without dose dependence compared to 69% of placebo-treated participants. Severe AEs thought to be drug treatment related were bacterial arthritis, lymphedema, melanoma, stroke, and epiglottitis.6
IL-17 Inhibitors
Ixekizumab
Ixekizumab (Eli Lilly and Company), a monoclonal inhibitor of IL-17A, is the most recently approved psoriasis biologic on the market and has been cleared for use in adults with moderate to severe plaque psoriasis. Recommended dosing is 160 mg (given in two 80-mg subcutaneous injections via an autoinjector or prefilled syringe) at week 0, followed by an 80-mg injection at weeks 2, 4, 6, 8, 10, and 12, and then 80 mg every 4 weeks thereafter. The FDA approved ixekizumab in March 2016 following favorable results of several phase 3 trials: UNCOVER-1, UNCOVER-2, and UNCOVER-3.7,8
In UNCOVER-1, 1296 participants were randomized to 1 of 2 ixekizumab treatment arms—160 mg starting dose at week 0, 80 mg every 2 or 4 weeks thereafter—or placebo.7 At week 12, 89.1%, 82.6%, and 3.9% achieved PASI 75, respectively (P<.001 for both). Importantly, high numbers of participants also achieved PASI 90 (70.9% in the 2-week group and 64.6% in the 4-week group vs 0.5% in the placebo group [P<.001]) and PASI 100 (35.3% and 33.6% vs 0%, respectively [P<.001]), suggesting high rates of disease clearance.7
UNCOVER-2 (N=1224) and UNCOVER-3 (N=1346) investigated the same 2 dosing regimens of ixekizumab compared to etanercept 50 mg biweekly and placebo.8 At week 12, the percentage of participants achieving PASI 90 in UNCOVER-2 was 70.7%, 59.7%, 18.7%, and 0.6%, respectively, and 68.1%, 65.3%, 25.7%, and 3.1%, respectively, in UNCOVER-3 (P<.0001 for all comparisons to placebo and etanercept). At week 12, PASI 100 results also showed striking superiority, with 40.5%, 30.8%, 5.3%, and 0.6% of participants, respectively, in UNCOVER-2, and 37.7%, 35%, 7.3%, and 0%, respectively, in UNCOVER-3, achieving complete clearance of disease (P<.0001 for all comparisons to placebo and etanercept). Responses to ixekizumab were observed as early as weeks 1 and 2, while no participants in the etanercept and placebo treatment groups achieved comparative efficiency.8
In an extension of UNCOVER-3, efficacy increased from week 12 to week 60 according to PASI 90 (68%–73% in the 2-week group; 65%–72% in the 4-week group) and PASI 100 measures (38%–55% in the 2-week group; 35%–52% in the 4-week group).7
The most common AEs associated with ixekizumab treatment from weeks 0 to 12 occurred at higher rates in the 2-week and 4-week ixekizumab groups compared to placebo, including nasopharyngitis (9.5% and 9% vs 8.7%, respectively), upper respiratory tract infection (4.4% and 3.9% vs 3.5%, respectively), injection-site reaction (10% and 7.7% vs 1%, respectively), arthralgia (4.4% and 4.3% vs 2.9%, respectively), and headache (2.5% and 1.9% vs 2.1%, respectively). Infections, including candidal, oral, vulvovaginal, and cutaneous, occurred in 27% of the 2-week dosing group and 27.4% of the 4-week dosing group compared to 22.9% of the placebo group during weeks 0 to 12, with candidal infections in particular occurring more frequently in the active treatment groups and exhibiting dose dependence. Other AEs of special interest that occurred among all ixekizumab-treated participants (n=3736) from weeks 0 to 60 were cardiovascular and cerebrovascular events (22 [0.6%]), inflammatory bowel disease (11 [0.3%]), non–skin cancer malignancy (14 [0.4%]), and nonmelanoma skin cancer (20 [0.5%]). Neutropenia occurred at higher rates in ixekizumab-treated participants (9.3% in the 2-week group and 8.6% in the 4-week group) compared to placebo (3.3%) and occurred in 11.5% of all ixekizumab participants over 60 weeks.7
Brodalumab
Brodalumab (Valeant Pharmaceuticals International, Inc) is a human monoclonal antibody targeting the IL-17A receptor currently under review for FDA approval after undergoing phase 3 trials. The first of these trials, AMAGINE-1, showed efficacy of subcutaneous brodalumab (140 or 210 mg administered every 2 weeks with an extra dose at week 1) compared to placebo in 661 participants.9 At week 12, 60%, 83%, and 3%, respectively, achieved PASI 75; 43%, 70%, and 1%, respectively, achieved PASI 90; and 23%, 42%, and 1%, respectively, achieved PASI 100 (P<.001 for all respective comparisons to placebo). These effects were retained through 52 weeks of treatment. The median time to complete disease clearance in participants reaching PASI 100 was 12 weeks. Conversely, participants who were re-randomized to placebo after week 12 of brodalumab treatment relapsed within weeks to months.9
AMAGINE-2 and AMAGINE-3 further demonstrated the efficacy of brodalumab (140 or 210 mg every 2 weeks with extra dose at week 1) compared to ustekinumab (45 or 90 mg weight-based standard dosing) and placebo in 1831 participants, respectively.10 In AMAGINE-2, 49% of participants in the 140-mg group (P<.001 vs placebo), 70% in the 210-mg group (P<.001 vs placebo), 47% in the ustekinumab group, and 3% in the placebo group achieved PASI 90 at week 12. Similarly, in AMAGINE-3, 52% of participants in the 140-mg group (P<.001), 69% in the 210-mg group (P<.001), 48% in the ustekinumab group, and 2% in the placebo group achieved PASI 90. Impressively, complete clearance (PASI 100) at week 12 occurred in 26% of the 140-mg group (P<.001 vs placebo), 44% of the 210-mg group (P<.001 vs placebo), and 22% of the ustekinumab group compared to 2% of the placebo group in AMAGINE-2, with similar rates in AMAGINE-3. Brodalumab was significantly superior to ustekinumab at the 210-mg dose by PASI 90 measures (P<.001) in both studies and at the 140-mg dose by PASI 100 measures (P=.007) in AMAGINE-3 only.10
Common AEs were nasopharyngitis, upper respiratory tract infection, headache, and arthralgia, all occurring at grossly similar rates (49%–60%) across all experimental groups in AMAGINE-1, AMAGINE-2, and AMAGINE-3 during the first 12-week treatment period.9,10 Brodalumab treatment groups had high rates of specific interest AEs compared to ustekinumab and placebo groups, including neutropenia (0.8%, 1.1%, 0.3%, and 0%, respectively) and candidal infections (0.8%, 1.3%, 0.3%, and 0.3%, respectively). Induction phase (weeks 0–12) depression rates were concerning, with 6 cases each in AMAGINE-2 (4 [0.7%] in the 140-mg group, 2 [0.3%] in the 210-mg group) and AMAGINE-3 (4 [0.6%] in the 140-mg group, 2 [0.3%] in the 210-mg group). Cases of neutropenia were mild, were not associated with major infection, and were transient or reversible. Depression rates after 52 weeks of treatment were 1.7% (23/1567) of brodalumab participants in AMAGINE-2 and 1.8% (21/1613) in AMAGINE-3. Three participants, all on constant 210-mg dosing through week 52, attempted suicide with 1 completion10; however, because no other IL-17 inhibitors were associated with depression or suicide in other trials, it has been suggested that these cases were incidental and not treatment related.12 An FDA advisory panel recommended approval of brodalumab in July 2016 despite ongoing concerns of depression and suicide.13
Conclusion
The robust investigation into IL-23 and IL-17 inhibitors to treat plaque psoriasis has yielded promising results, including the unprecedented rates of PASI 100 achievement with these new biologics. Risankizumab, ixekizumab, and brodalumab have demonstrated superior efficacy in trials compared to ustekinumab. Tildrakizumab has shown low disease relapse after drug cessation. Ixekizumab and brodalumab have shown high rates of total disease clearance. Thus far, safety findings for these pipeline biologics have been consistent with those of ustekinumab. With ixekizumab approved in 2016 and brodalumab under review, new options in biologic therapy will offer patients and clinicians greater choices in treating severe and recalcitrant psoriasis.
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509.
- Papp K, Menter A, Sofen H, et al. Efficacy and safety of different dose regimens of a selective IL-23p19 inhibitor (BI 655066) compared with ustekinumab in patients with moderate-to-severe plaque psoriasis with and without psoriatic arthritis. Paper presented at: 2015 American College of Rheumatology/Association of Rheumatology Health Professionals Annual Meeting; November 6-11, 2015; San Francisco, CA.
- New phase 3 data show significant efficacy versus placebo and superiority of guselkumab versus Humira in treatment of moderate to severe plaque psoriasis [press release]. Vienna, Austria; Janssen Research & Development, LLC: October 1, 2016.
- Gordon KB, Duffin KC, Bissonnette R, et al. A phase 2 trial of guselkumab versus adalimumab for plaque psoriasis. N Engl J Med. 2015;373:136-144.
- Sun Pharma to announce late-breaking results for investigational IL-23p19 inhibitor, Tildrakizumab, achieves primary end point in both phase-3 studies in patients with moderate-to-severe plaque psoriasis [press release]. Mumbai, India; Sun Pharmaceutical Industries Ltd: October 1, 2016.
- Papp K, Thaci D, Reich K, et al. Tildrakizumab (MK-3222), an anti-interleukin-23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo-controlled trial. Br J Dermatol. 2015;173:930-939.
- Gordon KB, Blauvelt A, Papp KA, et al; UNCOVER-1 Study Group, UNCOVER-2 Study Group, UNCOVER-3 Study Group. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:345-356.
- Griffiths CE, Reich K, Lebwohl M, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386:541-551.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis [published online June 23, 2016]. Br J Dermatol. 2016;175:273-286.
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373:1318-1328.
- Krueger JG, Ferris LK, Menter A, et al. Anti-IL-23A mAb BI 655066 for treatment of moderate-to-severe psoriasis: safety, efficacy, pharmacokinetics, and biomarker results of a single-rising-dose, randomized, double-blind, placebo-controlled trial [published online March 1, 2015]. J Allergy Clin Immunol. 2015;136:116-124.e7.
- Chiricozzi A, Romanelli M, Saraceno R, et al. No meaningful association between suicidal behavior and the use of IL-17A-neutralizing or IL-17RA-blocking agents [published online August 31, 2016]. Expert Opin Drug Saf. 2016;15:1653-1659.
- FDA advisory committee recommends approval of brodalumab for treatment of moderate-to-severe plaque psoriasis [news release]. Laval, Quebec: Valeant Pharmaceuticals International, Inc; July 19, 2016.
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509.
- Papp K, Menter A, Sofen H, et al. Efficacy and safety of different dose regimens of a selective IL-23p19 inhibitor (BI 655066) compared with ustekinumab in patients with moderate-to-severe plaque psoriasis with and without psoriatic arthritis. Paper presented at: 2015 American College of Rheumatology/Association of Rheumatology Health Professionals Annual Meeting; November 6-11, 2015; San Francisco, CA.
- New phase 3 data show significant efficacy versus placebo and superiority of guselkumab versus Humira in treatment of moderate to severe plaque psoriasis [press release]. Vienna, Austria; Janssen Research & Development, LLC: October 1, 2016.
- Gordon KB, Duffin KC, Bissonnette R, et al. A phase 2 trial of guselkumab versus adalimumab for plaque psoriasis. N Engl J Med. 2015;373:136-144.
- Sun Pharma to announce late-breaking results for investigational IL-23p19 inhibitor, Tildrakizumab, achieves primary end point in both phase-3 studies in patients with moderate-to-severe plaque psoriasis [press release]. Mumbai, India; Sun Pharmaceutical Industries Ltd: October 1, 2016.
- Papp K, Thaci D, Reich K, et al. Tildrakizumab (MK-3222), an anti-interleukin-23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo-controlled trial. Br J Dermatol. 2015;173:930-939.
- Gordon KB, Blauvelt A, Papp KA, et al; UNCOVER-1 Study Group, UNCOVER-2 Study Group, UNCOVER-3 Study Group. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:345-356.
- Griffiths CE, Reich K, Lebwohl M, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386:541-551.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis [published online June 23, 2016]. Br J Dermatol. 2016;175:273-286.
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373:1318-1328.
- Krueger JG, Ferris LK, Menter A, et al. Anti-IL-23A mAb BI 655066 for treatment of moderate-to-severe psoriasis: safety, efficacy, pharmacokinetics, and biomarker results of a single-rising-dose, randomized, double-blind, placebo-controlled trial [published online March 1, 2015]. J Allergy Clin Immunol. 2015;136:116-124.e7.
- Chiricozzi A, Romanelli M, Saraceno R, et al. No meaningful association between suicidal behavior and the use of IL-17A-neutralizing or IL-17RA-blocking agents [published online August 31, 2016]. Expert Opin Drug Saf. 2016;15:1653-1659.
- FDA advisory committee recommends approval of brodalumab for treatment of moderate-to-severe plaque psoriasis [news release]. Laval, Quebec: Valeant Pharmaceuticals International, Inc; July 19, 2016.
Practice Points
- The newest biologics for treatment of moderate to severe plaque psoriasis are IL-23 and IL-17 inhibitors with unprecedented efficacy of complete skin clearance compared to older biologics.
- Risankizumab, guselkumab, and tildrakizumab are new IL-23 inhibitors currently in phase 3 trials with promising early efficacy and safety results.
- Ixekizumab, which recently was approved, and brodalumab, which is pending US Food and Drug Administration review, are new IL-17 inhibitors that achieved total skin clearance in more than one-quarter of phase 3 participants after 12 weeks of treatment.
The New Opioid Epidemic: Prescriptions, Synthetics, and Street Drugs
Opioid misuse, which often is the result of a prescription written for a very painful condition, has created an epidemic of opioid abuse, addiction, and fatalities across the United States. To reduce the risks from prescribed opioids, regulators and public health authorities have implemented intensive risk mitigation programs, prescription-monitoring programs, and prescribing guidelines.
Clinicians have been encouraged to manage acute and chronic pain more comprehensively. Concurrently, pharmaceutical companies have introduced tamper-resistant formulations, also known as abuse-deterrent formulations, intended to limit manipulation of the contents for insufflation or injection. Although some of these formulations have made tampering difficult, overall they have not effectively reduced inappropriate use or abuse.
All of these interventions have resulted in a reduction in the availability of affordable, commercially available pharmaceutical opioids (Table 1). Simultaneously, other prescription opioid users have found that the analgesic or euphoric effects of their prescription opioids were no longer sufficient, due to opioid tolerance and hyperalgesia. Both of these forces are driving opioid users to seek more potent opioid products and higher doses to achieve the desired psychoactive and pain-relieving effects.
For these reasons, many opioid users turned to less expensive, readily available, illicitly produced heroin and potent synthetic opioids—mainly fentanyl derivatives. The increased use of heroin and synthetic opioids has resulted in a sharp rise in overdoses and deaths, which continue to be a daily presentation in EDs throughout the country.
This review describes the emergence of the new synthetic opioids, and the steps emergency physicians (EPs) can take to identify and manage ED patients who have been exposed to these agents.
Case
A 34-year-old woman with a history of opioid-use disorder was found unresponsive by a family member who immediately called emergency medical services (EMS). Upon arrival, the emergency medical technicians noted the patient’s agonal respiration and pinpoint pupils. They immediately provided assisted ventilations via a bag-valve-mask (BVM) and administered 2 mg of intranasal naloxone prior to transport. The patient remained unresponsive, with no improvement in her respiratory status.
Upon arrival at the ED, the patient was still comatose, and her pupils remained pinpoint. Vital signs at presentation were: heart rate, 48 beats/min; blood pressure, 70/40 mm Hg; agonal respiration; and temperature, 98.2°F. Oxygen saturation was 86% while receiving assisted ventilation through BVM. An intravenous (IV) line was established.
What is the differential diagnosis of this toxidrome in the current era of emerging drugs of abuse?
The differential diagnosis of a patient with pinpoint pupils and respiratory depression who does not respond to naloxone typically includes overdose with gamma-hydroxybutyrate, clonidine, or the combined use of sedative-hypnotic agents with ethanol (organophosphate exposure and pontine strokes are two other causes). Naloxone administration may help diagnose opioids as a cause, and, in the past, a lack of response to naloxone was used to help exclude opioids as a cause. However, opioid poisoning should no longer be excluded from consideration in the differential diagnosis when patients are nonresponsive to naloxone. Patients who combine the use of opioids with another sedative hypnotic or who develop hypoxic encephalopathy following opioid overdose may not respond to naloxone with arousal. Most important, the emergence of ultra-potent synthetic opioid use raises the possibility that a patient may appear to be resistant to naloxone due to the extreme potency of these drugs, but may respond to extremely large doses of naloxone. These new opioids pose a grave public health threat and have already resulted in hundreds, if not thousands, of deaths.1
What are novel synthetic opioids?
Unlike heroin, which requires harvesting of plant-derived opium, the novel synthetic opioids are synthesized in laboratories, primarily in China, and shipped to the United States through commercial channels (eg, US Postal Service).2,3 Over the past few years, novel synthetic opioids have been supplementing or replacing heroin sold on the illicit market.1 Most of these novel synthetic opioids are fentanyl analogs (Table 2) that are purchased in bulk on the “Darknet”—an area hidden deep in the Internet (not discoverable by the common major search engines) that allows users to engage in questionable, even illegal, activities utilizing nontraceable currencies such as Bitcoin.4
At the local level, dealers may seek to attract heroin users by adulterating, or even replacing, heroin with fentanyl or novel synthetic opioids, marketing it as a “high-quality” heroin offering more rapid, intense effects. These fentanyl analogs are often hundreds of times more potent than fentanyl, and therefore thousands of times more potent than heroin. Only a miniscule amount increases the perceived potency of the “heroin,” allowing dealers to increase their profit margins.
Selling and using novel synthetic opioids leave little room for error, and small dosing miscalculations have resulted in profound overdoses and deaths. Obviously, the quality control, contents, and dose uniformity of illicitly traded products are poor, adding to the risks of use. In some cases, the novel synthetic opioids are pressed into tablets and marketed as diverted prescription opioids or benzodiazepines. In many, if not most, circumstances, intermediary dealers, as well as users, may be unaware of the product’s contents.5,6 Carfentanil, used as a large-animal tranquilizer, is reportedly 10,000 times more potent than morphine and has recently been implicated in a cluster of deaths of opioid users in the Midwest.7,8 Other synthetic opioids coming to market were initially developed for laboratory research, including W18, which was identified in Canada; and U47700, an opioid identified on autopsy of the musician Prince3,9 (Table 2).
Novel synthetic opioids can be identified only by specific, specialized assays not available in clinical settings. Because their molecular structures differ substantially from morphine, these compounds skirt identification by standard urine “opiate” drug screens. With the exception of fentanyl, pharmacokinetic data for the use of the majority of these agents in humans is unknown.
How are patients who present to EDs with an opioid toxidrome managed in practice today?
Classic teaching for the management of opioid-induced respiratory depression in adults is to provide ventilatory support (ie, BVM or intubation) or administer a low dose of naloxone (0.04 mg IV every 2-5 minutes, up to 2 mg) until adequate respirations are restored. This approach is reasonable for patients exposed to heroin or fentanyl, and provides safer reversal in the ED than administration of a large bolus dose of 0.4 or 2 mg naloxone in opioid-dependent patients.
However, patients exposed to novel synthetic opioids may ultimately require higher than usual doses of naloxone to achieve reversal—reportedly IV doses as high as 6 to 10 mg or more.10 It is not yet fully understood if the need for high-dose naloxone is due to the binding affinity of the opioid or the relatively high dose of opioid administered.
Because the clinical effects of the novel synthetic opioids are generally indistinguishable from those of other opioids, providing respiratory support in the ED remains a critical intervention while awaiting the effect of titrated doses of naloxone. Of concern, though, is that these opioids are so potent that they may cause immediate respiratory arrest, resulting in a more rapid progression to cardiac arrest, limiting the ability to administer rescue breathing or antidote.
In the “bystander” setting, administration of a larger initial dose of naloxone may be reasonable, given the lack of advanced medical supportive care. However, the ability to provide larger doses in these settings is hampered by the accessibility of the antidote. In addition, prehospital-care providers need to consider the possibility of precipitating opioid withdrawal in patients with opioid dependence, which itself can carry significant consequences (eg, aspiration, agitated delirium), as well as the subsequent uncooperativeness of the victim, who may attempt to leave the scene and self-administer an additional dose of opioid or develop recurrent respiratory depression when the naloxone wanes. Since many patients with life-threatening opioid intoxication will suffer long-term consequences if reversal is delayed, the risk of administering high-dose naloxone in the bystander setting generally is worthwhile. However, the risks and benefits of naloxone must still be thoughtfully considered by prehospital-care providers who can provide alternative supportive therapies.
In the ED, the EP must decide whether to intubate the patient directly or first give a brief trial of low-dose naloxone. If a trial of naloxone is unsuccessful at reversing the respiratory depression, dose escalation can be tried while supporting oxygenation and ventilation noninvasively. Administration of naloxone postintubation is not usually necessary or even desired, since respiratory depression, the primary mechanism of death, has been addressed.
Are any special precautions required for health care workers?
Some of the ultra-potent synthetic opioids are available as powders or sprays that can be inadvertently absorbed through the skin (after dissolution in skin moisture) or inhaled.8 The safety of health care providers and law enforcement personnel who may be exposed to synthetic opioids in this manner is currently unknown, though some law enforcement and public health agencies have published warnings in an effort to be proactively cautious.8
While it is highly unlikely that the handling of body fluids of opioid-intoxicated patients poses any health threats, universal safety precautions of wearing disposable gloves should be utilized. As noted, contact with the actual substances may be more concerning, particularly when airborne; in such situations, a particulate mask should also be utilized. Although fentanyl in liquid formulation can slowly enter the skin transdermally (eg, fentanyl patch), there are very limited data to either support or refute the ability of the newer potent opioids to do so. Until more data on these opioid analogs become available, those entering grossly contaminated areas, in which dermal or inhalational exposure is high, should employ a higher level of personal safety precautions.11 In addition, naloxone should be readily available.
How can we detect novel opioid use?
As noted, there is no ability to specifically detect the use of novel potent opioids in the clinical setting (eg, hospital laboratory); therefore, clinicians must maintain a high level of suspicion and provide care empirically. The ability to make a specific diagnosis is further clouded because a patient who has used a synthetic opioid may have also used certain prescription opioids or heroin, which can be detected by standard testing.
Blood and urine samples obtained early in care and sent to specialized laboratories may provide specific identification. Such testing is typically only done by reference laboratories, health departments, or law enforcement agencies. The information obtained from these analyses may help to understand the epidemiology of novel opioid abuse, prevent others from succumbing to addiction, and determine the cause of related deaths.
Which patients can be safely discharged from the ED after an opioid overdose?
Patients who survive reversal of an opioid overdose, whether from a conventional or novel opioid, are at extremely high risk of subsequent death from continued use, as well as from the initial exposure to a long-acting opioid that outlasts the reversal effects of naloxone. Such patients should undergo a sufficient observation period after the last dose of naloxone has been administered to allow its effects to dissipate. This is likely at least 2 hours, but may be longer in certain individuals. Attempts at establishing a link for the patient to long-term treatment or (where available) providing a naloxone rescue kit and training to patients and their families are worthwhile. Although some data support releasing responsive patients after a short, but safe interval after naloxone administration, the changing landscape of opioid use should prompt reconsideration of such practices.12
To whom should suspected opioid overdose patients be reported?
While most EPs are familiar with the management of patients with opioid-induced respiratory depression, atypical cases (eg, patients less responsive to naloxone, those who suffer cardiac arrest) or clusters of suspected cases should always be reported to a regional poison control center (PCC) or health department. The PCC is typically engaged in surveillance and works cooperatively with area EDs and public health officials to track and notify physicians of emerging trends. The epidemiological data derived from reports from a variety of hospitals allow health officials to effectively engage resources for public warnings, facilitate forensic identification of circulating products, and determine any unique clinical information that can then be broadly disseminated.
Case Conclusion
The patient was supported with BVM ventilations. Despite additional titrated IV naloxone (up to a total of 4 mg) the patient was nonresponsive and unarousable. She was intubated, and awoke several hours later. She fully recovered and subsequently was referred to both a harm-reduction and an opioid detoxification program. Analysis of her blood and urine, available several weeks later, confirmed an exposure to U47700.
1. Centers for Disease Control and Prevention. Health Alert Network. Increases in fentanyl drug confiscations and fentanyl-related overdose fatalities. https://emergency.cdc.gov/han/han00384.asp. Updated October 26, 2015. Accessed January 10, 2017.
2. MacQuarrie B. Synthetic opioids are getting into US by mail. Boston Globe. December 27, 2016. http://www.bostonglobe.com/metro/2016/12/26/synthetic-opioids-slipping-into-via-mail-security-experts-say/23TCEuIES8aEQYAWWHKCiI/story.html. Accessed January 10, 2017.
3. Lucyk SN, Nelson LS. Novel synthetic opioids: an opioid epidemic within an opioid epidemic. Ann Emerg Med. 2017;69(1):91-93. doi:10.1016/j.annemergmed.2016.08.445.
4. Mounteney J, Bo A, Oteo A; OteoEuropean Monitoring Centre for Drugs and Drug Addiction project group. The Internet and Drug Markets. Publications Office of the European Union, Luxembourg, Luxembourg; 2016:1-136. http://www.emcdda.europa.eu/system/files/publications/2155/TDXD16001ENN_FINAL. pdf. doi:10.2810/324608. Accessed January 17, 2017.
5. Associated Press. ‘Norco’ fentanyl overdose deaths rise to 14; problem spreads to Bay Area. Los Angeles Times. April 26, 2016. http://www.latimes.com/local/lanow/la-me-ln-norco-fentanyl-overdose-deaths-rise-to-14-problem-spreads-to-bay-area-20160426-story.html.
6. Centers for Disease Control and Prevention. Health Alert Network. Influx of fentanyl-laced counterfeit pills and toxic fentanyl-related compounds further increases risk of fentanyl-related overdose and fatalities. https://emergency.cdc.gov/han/han00395.asp. Accessed January 10, 2017.
7. Sandy E. Cleveland Scene. 236 heroin overdoses in Akron in 3 weeks; heroin being cut with elephant sedative. http://www.clevescene.com/scene-and-heard/archives/2016/07/14/akron-police-chief-heroin-being-cut-with-elephant-sedative-88-overdoses-since-july-5. Accessed January 10, 2017.
8. DEA issues carfentanil warning to police and public [news release]. Washington, DC: United States Drug Enforcement Administration; September 22, 2016. https://www.dea.gov/divisions/hq/2016/hq092216.shtml. Accessed January 10, 2017.
9. Armenian P, Olson A, Anaya A, Kurtz A, Ruegner R, Gerona RR. Fentanyl and a novel synthetic opioid U-47700 masquerading as street “Norco” in Central California: a case report. Ann Emerg Med. 2017;69(1):87-90. doi:10.1016/j.annemergmed.2016.06.014.
10. Schumann H, Erickson T, Thompson TM, Zautcke JL, Denton JS. Fentanyl epidemic in Chicago, Illinois and surrounding Cook County. Clin Toxicol (Phila). 2008;46(6):501-506. doi:10.1080/15563650701877374.
11. George AV, Lu JJ, Pisano MV, Metz J, Erickson TB. Carfentanil—an ultra potent opioid. Am J Emerg Med. 2010;28(4):530-532. doi:10.1016/j.ajem.2010.03.003.
12. Kolinsky D, Keim SM, Cohn BG, Schwarz ES, Yealy DM. Is a prehospital treat and release protocol for opioid overdose safe? J Emerg Med. 2017;52(1):52-58. doi:10.1016/j.jemermed.2016.09.015.
Opioid misuse, which often is the result of a prescription written for a very painful condition, has created an epidemic of opioid abuse, addiction, and fatalities across the United States. To reduce the risks from prescribed opioids, regulators and public health authorities have implemented intensive risk mitigation programs, prescription-monitoring programs, and prescribing guidelines.
Clinicians have been encouraged to manage acute and chronic pain more comprehensively. Concurrently, pharmaceutical companies have introduced tamper-resistant formulations, also known as abuse-deterrent formulations, intended to limit manipulation of the contents for insufflation or injection. Although some of these formulations have made tampering difficult, overall they have not effectively reduced inappropriate use or abuse.
All of these interventions have resulted in a reduction in the availability of affordable, commercially available pharmaceutical opioids (Table 1). Simultaneously, other prescription opioid users have found that the analgesic or euphoric effects of their prescription opioids were no longer sufficient, due to opioid tolerance and hyperalgesia. Both of these forces are driving opioid users to seek more potent opioid products and higher doses to achieve the desired psychoactive and pain-relieving effects.
For these reasons, many opioid users turned to less expensive, readily available, illicitly produced heroin and potent synthetic opioids—mainly fentanyl derivatives. The increased use of heroin and synthetic opioids has resulted in a sharp rise in overdoses and deaths, which continue to be a daily presentation in EDs throughout the country.
This review describes the emergence of the new synthetic opioids, and the steps emergency physicians (EPs) can take to identify and manage ED patients who have been exposed to these agents.
Case
A 34-year-old woman with a history of opioid-use disorder was found unresponsive by a family member who immediately called emergency medical services (EMS). Upon arrival, the emergency medical technicians noted the patient’s agonal respiration and pinpoint pupils. They immediately provided assisted ventilations via a bag-valve-mask (BVM) and administered 2 mg of intranasal naloxone prior to transport. The patient remained unresponsive, with no improvement in her respiratory status.
Upon arrival at the ED, the patient was still comatose, and her pupils remained pinpoint. Vital signs at presentation were: heart rate, 48 beats/min; blood pressure, 70/40 mm Hg; agonal respiration; and temperature, 98.2°F. Oxygen saturation was 86% while receiving assisted ventilation through BVM. An intravenous (IV) line was established.
What is the differential diagnosis of this toxidrome in the current era of emerging drugs of abuse?
The differential diagnosis of a patient with pinpoint pupils and respiratory depression who does not respond to naloxone typically includes overdose with gamma-hydroxybutyrate, clonidine, or the combined use of sedative-hypnotic agents with ethanol (organophosphate exposure and pontine strokes are two other causes). Naloxone administration may help diagnose opioids as a cause, and, in the past, a lack of response to naloxone was used to help exclude opioids as a cause. However, opioid poisoning should no longer be excluded from consideration in the differential diagnosis when patients are nonresponsive to naloxone. Patients who combine the use of opioids with another sedative hypnotic or who develop hypoxic encephalopathy following opioid overdose may not respond to naloxone with arousal. Most important, the emergence of ultra-potent synthetic opioid use raises the possibility that a patient may appear to be resistant to naloxone due to the extreme potency of these drugs, but may respond to extremely large doses of naloxone. These new opioids pose a grave public health threat and have already resulted in hundreds, if not thousands, of deaths.1
What are novel synthetic opioids?
Unlike heroin, which requires harvesting of plant-derived opium, the novel synthetic opioids are synthesized in laboratories, primarily in China, and shipped to the United States through commercial channels (eg, US Postal Service).2,3 Over the past few years, novel synthetic opioids have been supplementing or replacing heroin sold on the illicit market.1 Most of these novel synthetic opioids are fentanyl analogs (Table 2) that are purchased in bulk on the “Darknet”—an area hidden deep in the Internet (not discoverable by the common major search engines) that allows users to engage in questionable, even illegal, activities utilizing nontraceable currencies such as Bitcoin.4
At the local level, dealers may seek to attract heroin users by adulterating, or even replacing, heroin with fentanyl or novel synthetic opioids, marketing it as a “high-quality” heroin offering more rapid, intense effects. These fentanyl analogs are often hundreds of times more potent than fentanyl, and therefore thousands of times more potent than heroin. Only a miniscule amount increases the perceived potency of the “heroin,” allowing dealers to increase their profit margins.
Selling and using novel synthetic opioids leave little room for error, and small dosing miscalculations have resulted in profound overdoses and deaths. Obviously, the quality control, contents, and dose uniformity of illicitly traded products are poor, adding to the risks of use. In some cases, the novel synthetic opioids are pressed into tablets and marketed as diverted prescription opioids or benzodiazepines. In many, if not most, circumstances, intermediary dealers, as well as users, may be unaware of the product’s contents.5,6 Carfentanil, used as a large-animal tranquilizer, is reportedly 10,000 times more potent than morphine and has recently been implicated in a cluster of deaths of opioid users in the Midwest.7,8 Other synthetic opioids coming to market were initially developed for laboratory research, including W18, which was identified in Canada; and U47700, an opioid identified on autopsy of the musician Prince3,9 (Table 2).
Novel synthetic opioids can be identified only by specific, specialized assays not available in clinical settings. Because their molecular structures differ substantially from morphine, these compounds skirt identification by standard urine “opiate” drug screens. With the exception of fentanyl, pharmacokinetic data for the use of the majority of these agents in humans is unknown.
How are patients who present to EDs with an opioid toxidrome managed in practice today?
Classic teaching for the management of opioid-induced respiratory depression in adults is to provide ventilatory support (ie, BVM or intubation) or administer a low dose of naloxone (0.04 mg IV every 2-5 minutes, up to 2 mg) until adequate respirations are restored. This approach is reasonable for patients exposed to heroin or fentanyl, and provides safer reversal in the ED than administration of a large bolus dose of 0.4 or 2 mg naloxone in opioid-dependent patients.
However, patients exposed to novel synthetic opioids may ultimately require higher than usual doses of naloxone to achieve reversal—reportedly IV doses as high as 6 to 10 mg or more.10 It is not yet fully understood if the need for high-dose naloxone is due to the binding affinity of the opioid or the relatively high dose of opioid administered.
Because the clinical effects of the novel synthetic opioids are generally indistinguishable from those of other opioids, providing respiratory support in the ED remains a critical intervention while awaiting the effect of titrated doses of naloxone. Of concern, though, is that these opioids are so potent that they may cause immediate respiratory arrest, resulting in a more rapid progression to cardiac arrest, limiting the ability to administer rescue breathing or antidote.
In the “bystander” setting, administration of a larger initial dose of naloxone may be reasonable, given the lack of advanced medical supportive care. However, the ability to provide larger doses in these settings is hampered by the accessibility of the antidote. In addition, prehospital-care providers need to consider the possibility of precipitating opioid withdrawal in patients with opioid dependence, which itself can carry significant consequences (eg, aspiration, agitated delirium), as well as the subsequent uncooperativeness of the victim, who may attempt to leave the scene and self-administer an additional dose of opioid or develop recurrent respiratory depression when the naloxone wanes. Since many patients with life-threatening opioid intoxication will suffer long-term consequences if reversal is delayed, the risk of administering high-dose naloxone in the bystander setting generally is worthwhile. However, the risks and benefits of naloxone must still be thoughtfully considered by prehospital-care providers who can provide alternative supportive therapies.
In the ED, the EP must decide whether to intubate the patient directly or first give a brief trial of low-dose naloxone. If a trial of naloxone is unsuccessful at reversing the respiratory depression, dose escalation can be tried while supporting oxygenation and ventilation noninvasively. Administration of naloxone postintubation is not usually necessary or even desired, since respiratory depression, the primary mechanism of death, has been addressed.
Are any special precautions required for health care workers?
Some of the ultra-potent synthetic opioids are available as powders or sprays that can be inadvertently absorbed through the skin (after dissolution in skin moisture) or inhaled.8 The safety of health care providers and law enforcement personnel who may be exposed to synthetic opioids in this manner is currently unknown, though some law enforcement and public health agencies have published warnings in an effort to be proactively cautious.8
While it is highly unlikely that the handling of body fluids of opioid-intoxicated patients poses any health threats, universal safety precautions of wearing disposable gloves should be utilized. As noted, contact with the actual substances may be more concerning, particularly when airborne; in such situations, a particulate mask should also be utilized. Although fentanyl in liquid formulation can slowly enter the skin transdermally (eg, fentanyl patch), there are very limited data to either support or refute the ability of the newer potent opioids to do so. Until more data on these opioid analogs become available, those entering grossly contaminated areas, in which dermal or inhalational exposure is high, should employ a higher level of personal safety precautions.11 In addition, naloxone should be readily available.
How can we detect novel opioid use?
As noted, there is no ability to specifically detect the use of novel potent opioids in the clinical setting (eg, hospital laboratory); therefore, clinicians must maintain a high level of suspicion and provide care empirically. The ability to make a specific diagnosis is further clouded because a patient who has used a synthetic opioid may have also used certain prescription opioids or heroin, which can be detected by standard testing.
Blood and urine samples obtained early in care and sent to specialized laboratories may provide specific identification. Such testing is typically only done by reference laboratories, health departments, or law enforcement agencies. The information obtained from these analyses may help to understand the epidemiology of novel opioid abuse, prevent others from succumbing to addiction, and determine the cause of related deaths.
Which patients can be safely discharged from the ED after an opioid overdose?
Patients who survive reversal of an opioid overdose, whether from a conventional or novel opioid, are at extremely high risk of subsequent death from continued use, as well as from the initial exposure to a long-acting opioid that outlasts the reversal effects of naloxone. Such patients should undergo a sufficient observation period after the last dose of naloxone has been administered to allow its effects to dissipate. This is likely at least 2 hours, but may be longer in certain individuals. Attempts at establishing a link for the patient to long-term treatment or (where available) providing a naloxone rescue kit and training to patients and their families are worthwhile. Although some data support releasing responsive patients after a short, but safe interval after naloxone administration, the changing landscape of opioid use should prompt reconsideration of such practices.12
To whom should suspected opioid overdose patients be reported?
While most EPs are familiar with the management of patients with opioid-induced respiratory depression, atypical cases (eg, patients less responsive to naloxone, those who suffer cardiac arrest) or clusters of suspected cases should always be reported to a regional poison control center (PCC) or health department. The PCC is typically engaged in surveillance and works cooperatively with area EDs and public health officials to track and notify physicians of emerging trends. The epidemiological data derived from reports from a variety of hospitals allow health officials to effectively engage resources for public warnings, facilitate forensic identification of circulating products, and determine any unique clinical information that can then be broadly disseminated.
Case Conclusion
The patient was supported with BVM ventilations. Despite additional titrated IV naloxone (up to a total of 4 mg) the patient was nonresponsive and unarousable. She was intubated, and awoke several hours later. She fully recovered and subsequently was referred to both a harm-reduction and an opioid detoxification program. Analysis of her blood and urine, available several weeks later, confirmed an exposure to U47700.
Opioid misuse, which often is the result of a prescription written for a very painful condition, has created an epidemic of opioid abuse, addiction, and fatalities across the United States. To reduce the risks from prescribed opioids, regulators and public health authorities have implemented intensive risk mitigation programs, prescription-monitoring programs, and prescribing guidelines.
Clinicians have been encouraged to manage acute and chronic pain more comprehensively. Concurrently, pharmaceutical companies have introduced tamper-resistant formulations, also known as abuse-deterrent formulations, intended to limit manipulation of the contents for insufflation or injection. Although some of these formulations have made tampering difficult, overall they have not effectively reduced inappropriate use or abuse.
All of these interventions have resulted in a reduction in the availability of affordable, commercially available pharmaceutical opioids (Table 1). Simultaneously, other prescription opioid users have found that the analgesic or euphoric effects of their prescription opioids were no longer sufficient, due to opioid tolerance and hyperalgesia. Both of these forces are driving opioid users to seek more potent opioid products and higher doses to achieve the desired psychoactive and pain-relieving effects.
For these reasons, many opioid users turned to less expensive, readily available, illicitly produced heroin and potent synthetic opioids—mainly fentanyl derivatives. The increased use of heroin and synthetic opioids has resulted in a sharp rise in overdoses and deaths, which continue to be a daily presentation in EDs throughout the country.
This review describes the emergence of the new synthetic opioids, and the steps emergency physicians (EPs) can take to identify and manage ED patients who have been exposed to these agents.
Case
A 34-year-old woman with a history of opioid-use disorder was found unresponsive by a family member who immediately called emergency medical services (EMS). Upon arrival, the emergency medical technicians noted the patient’s agonal respiration and pinpoint pupils. They immediately provided assisted ventilations via a bag-valve-mask (BVM) and administered 2 mg of intranasal naloxone prior to transport. The patient remained unresponsive, with no improvement in her respiratory status.
Upon arrival at the ED, the patient was still comatose, and her pupils remained pinpoint. Vital signs at presentation were: heart rate, 48 beats/min; blood pressure, 70/40 mm Hg; agonal respiration; and temperature, 98.2°F. Oxygen saturation was 86% while receiving assisted ventilation through BVM. An intravenous (IV) line was established.
What is the differential diagnosis of this toxidrome in the current era of emerging drugs of abuse?
The differential diagnosis of a patient with pinpoint pupils and respiratory depression who does not respond to naloxone typically includes overdose with gamma-hydroxybutyrate, clonidine, or the combined use of sedative-hypnotic agents with ethanol (organophosphate exposure and pontine strokes are two other causes). Naloxone administration may help diagnose opioids as a cause, and, in the past, a lack of response to naloxone was used to help exclude opioids as a cause. However, opioid poisoning should no longer be excluded from consideration in the differential diagnosis when patients are nonresponsive to naloxone. Patients who combine the use of opioids with another sedative hypnotic or who develop hypoxic encephalopathy following opioid overdose may not respond to naloxone with arousal. Most important, the emergence of ultra-potent synthetic opioid use raises the possibility that a patient may appear to be resistant to naloxone due to the extreme potency of these drugs, but may respond to extremely large doses of naloxone. These new opioids pose a grave public health threat and have already resulted in hundreds, if not thousands, of deaths.1
What are novel synthetic opioids?
Unlike heroin, which requires harvesting of plant-derived opium, the novel synthetic opioids are synthesized in laboratories, primarily in China, and shipped to the United States through commercial channels (eg, US Postal Service).2,3 Over the past few years, novel synthetic opioids have been supplementing or replacing heroin sold on the illicit market.1 Most of these novel synthetic opioids are fentanyl analogs (Table 2) that are purchased in bulk on the “Darknet”—an area hidden deep in the Internet (not discoverable by the common major search engines) that allows users to engage in questionable, even illegal, activities utilizing nontraceable currencies such as Bitcoin.4
At the local level, dealers may seek to attract heroin users by adulterating, or even replacing, heroin with fentanyl or novel synthetic opioids, marketing it as a “high-quality” heroin offering more rapid, intense effects. These fentanyl analogs are often hundreds of times more potent than fentanyl, and therefore thousands of times more potent than heroin. Only a miniscule amount increases the perceived potency of the “heroin,” allowing dealers to increase their profit margins.
Selling and using novel synthetic opioids leave little room for error, and small dosing miscalculations have resulted in profound overdoses and deaths. Obviously, the quality control, contents, and dose uniformity of illicitly traded products are poor, adding to the risks of use. In some cases, the novel synthetic opioids are pressed into tablets and marketed as diverted prescription opioids or benzodiazepines. In many, if not most, circumstances, intermediary dealers, as well as users, may be unaware of the product’s contents.5,6 Carfentanil, used as a large-animal tranquilizer, is reportedly 10,000 times more potent than morphine and has recently been implicated in a cluster of deaths of opioid users in the Midwest.7,8 Other synthetic opioids coming to market were initially developed for laboratory research, including W18, which was identified in Canada; and U47700, an opioid identified on autopsy of the musician Prince3,9 (Table 2).
Novel synthetic opioids can be identified only by specific, specialized assays not available in clinical settings. Because their molecular structures differ substantially from morphine, these compounds skirt identification by standard urine “opiate” drug screens. With the exception of fentanyl, pharmacokinetic data for the use of the majority of these agents in humans is unknown.
How are patients who present to EDs with an opioid toxidrome managed in practice today?
Classic teaching for the management of opioid-induced respiratory depression in adults is to provide ventilatory support (ie, BVM or intubation) or administer a low dose of naloxone (0.04 mg IV every 2-5 minutes, up to 2 mg) until adequate respirations are restored. This approach is reasonable for patients exposed to heroin or fentanyl, and provides safer reversal in the ED than administration of a large bolus dose of 0.4 or 2 mg naloxone in opioid-dependent patients.
However, patients exposed to novel synthetic opioids may ultimately require higher than usual doses of naloxone to achieve reversal—reportedly IV doses as high as 6 to 10 mg or more.10 It is not yet fully understood if the need for high-dose naloxone is due to the binding affinity of the opioid or the relatively high dose of opioid administered.
Because the clinical effects of the novel synthetic opioids are generally indistinguishable from those of other opioids, providing respiratory support in the ED remains a critical intervention while awaiting the effect of titrated doses of naloxone. Of concern, though, is that these opioids are so potent that they may cause immediate respiratory arrest, resulting in a more rapid progression to cardiac arrest, limiting the ability to administer rescue breathing or antidote.
In the “bystander” setting, administration of a larger initial dose of naloxone may be reasonable, given the lack of advanced medical supportive care. However, the ability to provide larger doses in these settings is hampered by the accessibility of the antidote. In addition, prehospital-care providers need to consider the possibility of precipitating opioid withdrawal in patients with opioid dependence, which itself can carry significant consequences (eg, aspiration, agitated delirium), as well as the subsequent uncooperativeness of the victim, who may attempt to leave the scene and self-administer an additional dose of opioid or develop recurrent respiratory depression when the naloxone wanes. Since many patients with life-threatening opioid intoxication will suffer long-term consequences if reversal is delayed, the risk of administering high-dose naloxone in the bystander setting generally is worthwhile. However, the risks and benefits of naloxone must still be thoughtfully considered by prehospital-care providers who can provide alternative supportive therapies.
In the ED, the EP must decide whether to intubate the patient directly or first give a brief trial of low-dose naloxone. If a trial of naloxone is unsuccessful at reversing the respiratory depression, dose escalation can be tried while supporting oxygenation and ventilation noninvasively. Administration of naloxone postintubation is not usually necessary or even desired, since respiratory depression, the primary mechanism of death, has been addressed.
Are any special precautions required for health care workers?
Some of the ultra-potent synthetic opioids are available as powders or sprays that can be inadvertently absorbed through the skin (after dissolution in skin moisture) or inhaled.8 The safety of health care providers and law enforcement personnel who may be exposed to synthetic opioids in this manner is currently unknown, though some law enforcement and public health agencies have published warnings in an effort to be proactively cautious.8
While it is highly unlikely that the handling of body fluids of opioid-intoxicated patients poses any health threats, universal safety precautions of wearing disposable gloves should be utilized. As noted, contact with the actual substances may be more concerning, particularly when airborne; in such situations, a particulate mask should also be utilized. Although fentanyl in liquid formulation can slowly enter the skin transdermally (eg, fentanyl patch), there are very limited data to either support or refute the ability of the newer potent opioids to do so. Until more data on these opioid analogs become available, those entering grossly contaminated areas, in which dermal or inhalational exposure is high, should employ a higher level of personal safety precautions.11 In addition, naloxone should be readily available.
How can we detect novel opioid use?
As noted, there is no ability to specifically detect the use of novel potent opioids in the clinical setting (eg, hospital laboratory); therefore, clinicians must maintain a high level of suspicion and provide care empirically. The ability to make a specific diagnosis is further clouded because a patient who has used a synthetic opioid may have also used certain prescription opioids or heroin, which can be detected by standard testing.
Blood and urine samples obtained early in care and sent to specialized laboratories may provide specific identification. Such testing is typically only done by reference laboratories, health departments, or law enforcement agencies. The information obtained from these analyses may help to understand the epidemiology of novel opioid abuse, prevent others from succumbing to addiction, and determine the cause of related deaths.
Which patients can be safely discharged from the ED after an opioid overdose?
Patients who survive reversal of an opioid overdose, whether from a conventional or novel opioid, are at extremely high risk of subsequent death from continued use, as well as from the initial exposure to a long-acting opioid that outlasts the reversal effects of naloxone. Such patients should undergo a sufficient observation period after the last dose of naloxone has been administered to allow its effects to dissipate. This is likely at least 2 hours, but may be longer in certain individuals. Attempts at establishing a link for the patient to long-term treatment or (where available) providing a naloxone rescue kit and training to patients and their families are worthwhile. Although some data support releasing responsive patients after a short, but safe interval after naloxone administration, the changing landscape of opioid use should prompt reconsideration of such practices.12
To whom should suspected opioid overdose patients be reported?
While most EPs are familiar with the management of patients with opioid-induced respiratory depression, atypical cases (eg, patients less responsive to naloxone, those who suffer cardiac arrest) or clusters of suspected cases should always be reported to a regional poison control center (PCC) or health department. The PCC is typically engaged in surveillance and works cooperatively with area EDs and public health officials to track and notify physicians of emerging trends. The epidemiological data derived from reports from a variety of hospitals allow health officials to effectively engage resources for public warnings, facilitate forensic identification of circulating products, and determine any unique clinical information that can then be broadly disseminated.
Case Conclusion
The patient was supported with BVM ventilations. Despite additional titrated IV naloxone (up to a total of 4 mg) the patient was nonresponsive and unarousable. She was intubated, and awoke several hours later. She fully recovered and subsequently was referred to both a harm-reduction and an opioid detoxification program. Analysis of her blood and urine, available several weeks later, confirmed an exposure to U47700.
1. Centers for Disease Control and Prevention. Health Alert Network. Increases in fentanyl drug confiscations and fentanyl-related overdose fatalities. https://emergency.cdc.gov/han/han00384.asp. Updated October 26, 2015. Accessed January 10, 2017.
2. MacQuarrie B. Synthetic opioids are getting into US by mail. Boston Globe. December 27, 2016. http://www.bostonglobe.com/metro/2016/12/26/synthetic-opioids-slipping-into-via-mail-security-experts-say/23TCEuIES8aEQYAWWHKCiI/story.html. Accessed January 10, 2017.
3. Lucyk SN, Nelson LS. Novel synthetic opioids: an opioid epidemic within an opioid epidemic. Ann Emerg Med. 2017;69(1):91-93. doi:10.1016/j.annemergmed.2016.08.445.
4. Mounteney J, Bo A, Oteo A; OteoEuropean Monitoring Centre for Drugs and Drug Addiction project group. The Internet and Drug Markets. Publications Office of the European Union, Luxembourg, Luxembourg; 2016:1-136. http://www.emcdda.europa.eu/system/files/publications/2155/TDXD16001ENN_FINAL. pdf. doi:10.2810/324608. Accessed January 17, 2017.
5. Associated Press. ‘Norco’ fentanyl overdose deaths rise to 14; problem spreads to Bay Area. Los Angeles Times. April 26, 2016. http://www.latimes.com/local/lanow/la-me-ln-norco-fentanyl-overdose-deaths-rise-to-14-problem-spreads-to-bay-area-20160426-story.html.
6. Centers for Disease Control and Prevention. Health Alert Network. Influx of fentanyl-laced counterfeit pills and toxic fentanyl-related compounds further increases risk of fentanyl-related overdose and fatalities. https://emergency.cdc.gov/han/han00395.asp. Accessed January 10, 2017.
7. Sandy E. Cleveland Scene. 236 heroin overdoses in Akron in 3 weeks; heroin being cut with elephant sedative. http://www.clevescene.com/scene-and-heard/archives/2016/07/14/akron-police-chief-heroin-being-cut-with-elephant-sedative-88-overdoses-since-july-5. Accessed January 10, 2017.
8. DEA issues carfentanil warning to police and public [news release]. Washington, DC: United States Drug Enforcement Administration; September 22, 2016. https://www.dea.gov/divisions/hq/2016/hq092216.shtml. Accessed January 10, 2017.
9. Armenian P, Olson A, Anaya A, Kurtz A, Ruegner R, Gerona RR. Fentanyl and a novel synthetic opioid U-47700 masquerading as street “Norco” in Central California: a case report. Ann Emerg Med. 2017;69(1):87-90. doi:10.1016/j.annemergmed.2016.06.014.
10. Schumann H, Erickson T, Thompson TM, Zautcke JL, Denton JS. Fentanyl epidemic in Chicago, Illinois and surrounding Cook County. Clin Toxicol (Phila). 2008;46(6):501-506. doi:10.1080/15563650701877374.
11. George AV, Lu JJ, Pisano MV, Metz J, Erickson TB. Carfentanil—an ultra potent opioid. Am J Emerg Med. 2010;28(4):530-532. doi:10.1016/j.ajem.2010.03.003.
12. Kolinsky D, Keim SM, Cohn BG, Schwarz ES, Yealy DM. Is a prehospital treat and release protocol for opioid overdose safe? J Emerg Med. 2017;52(1):52-58. doi:10.1016/j.jemermed.2016.09.015.
1. Centers for Disease Control and Prevention. Health Alert Network. Increases in fentanyl drug confiscations and fentanyl-related overdose fatalities. https://emergency.cdc.gov/han/han00384.asp. Updated October 26, 2015. Accessed January 10, 2017.
2. MacQuarrie B. Synthetic opioids are getting into US by mail. Boston Globe. December 27, 2016. http://www.bostonglobe.com/metro/2016/12/26/synthetic-opioids-slipping-into-via-mail-security-experts-say/23TCEuIES8aEQYAWWHKCiI/story.html. Accessed January 10, 2017.
3. Lucyk SN, Nelson LS. Novel synthetic opioids: an opioid epidemic within an opioid epidemic. Ann Emerg Med. 2017;69(1):91-93. doi:10.1016/j.annemergmed.2016.08.445.
4. Mounteney J, Bo A, Oteo A; OteoEuropean Monitoring Centre for Drugs and Drug Addiction project group. The Internet and Drug Markets. Publications Office of the European Union, Luxembourg, Luxembourg; 2016:1-136. http://www.emcdda.europa.eu/system/files/publications/2155/TDXD16001ENN_FINAL. pdf. doi:10.2810/324608. Accessed January 17, 2017.
5. Associated Press. ‘Norco’ fentanyl overdose deaths rise to 14; problem spreads to Bay Area. Los Angeles Times. April 26, 2016. http://www.latimes.com/local/lanow/la-me-ln-norco-fentanyl-overdose-deaths-rise-to-14-problem-spreads-to-bay-area-20160426-story.html.
6. Centers for Disease Control and Prevention. Health Alert Network. Influx of fentanyl-laced counterfeit pills and toxic fentanyl-related compounds further increases risk of fentanyl-related overdose and fatalities. https://emergency.cdc.gov/han/han00395.asp. Accessed January 10, 2017.
7. Sandy E. Cleveland Scene. 236 heroin overdoses in Akron in 3 weeks; heroin being cut with elephant sedative. http://www.clevescene.com/scene-and-heard/archives/2016/07/14/akron-police-chief-heroin-being-cut-with-elephant-sedative-88-overdoses-since-july-5. Accessed January 10, 2017.
8. DEA issues carfentanil warning to police and public [news release]. Washington, DC: United States Drug Enforcement Administration; September 22, 2016. https://www.dea.gov/divisions/hq/2016/hq092216.shtml. Accessed January 10, 2017.
9. Armenian P, Olson A, Anaya A, Kurtz A, Ruegner R, Gerona RR. Fentanyl and a novel synthetic opioid U-47700 masquerading as street “Norco” in Central California: a case report. Ann Emerg Med. 2017;69(1):87-90. doi:10.1016/j.annemergmed.2016.06.014.
10. Schumann H, Erickson T, Thompson TM, Zautcke JL, Denton JS. Fentanyl epidemic in Chicago, Illinois and surrounding Cook County. Clin Toxicol (Phila). 2008;46(6):501-506. doi:10.1080/15563650701877374.
11. George AV, Lu JJ, Pisano MV, Metz J, Erickson TB. Carfentanil—an ultra potent opioid. Am J Emerg Med. 2010;28(4):530-532. doi:10.1016/j.ajem.2010.03.003.
12. Kolinsky D, Keim SM, Cohn BG, Schwarz ES, Yealy DM. Is a prehospital treat and release protocol for opioid overdose safe? J Emerg Med. 2017;52(1):52-58. doi:10.1016/j.jemermed.2016.09.015.
First EDition: A-Fib Management Pathway in the ED, more
Atrial Fibrillation Management Pathway in the ED May Lower Hospital Admissions
TED BOSWORTH
FRONTLINE MEDICAL NEWS
An atrial fibrillation (AF) treatment pathway designed specifically to reduce the proportion of patients with this complaint who are admitted to the hospital from the ED was remarkably effective, according to a pilot study presented at the annual International AF Symposium.
“In this single-center observational study, a multidisciplinary AF pathway was associated with 5-fold reduction in admission rate and 2.5-fold reduction in length-of-stay [LOS] for those who were admitted,” reported Jeremy N. Ruskin, MD.
Relative to many other countries, admission rates for AF in the United States are “extremely high,” according to Dr Ruskin, director of the cardiac arrhythmia service at Massachusetts General Hospital, Boston. Citing 2013 figures from the Nationwide Emergency Department Sample (NEDS) database, rates ranged between 60% and 80% by geographic region, with an average of about 66%. In contrast, and as an example of lower rates elsewhere, fewer than 40% of AF patients with similar characteristics presenting at EDs in Ontario, Canada were admitted. Similarly low admission rates have been reported in Europe.
The AF pathway tested in the study at Massachusetts General was developed through collaboration between electrophysiologists and emergency physicians (EPs). It is suitable for patients presenting with a primary complaint of AF without concomitant diseases, such as sepsis or myocardial infarction. Patients were entered into this study after it was shown that AF was the chief complaint. The first step was to determine whether participants were best suited to a rhythm-control or rate-control strategy.
“The rhythm-control group was anticoagulated and then underwent expedited cardioversion with TEE [transesophageal echocardiogram] if necessary. The rate-control group was anticoagulated and then given appropriate pharmacologic therapy,” Dr Ruskin explained. Once patients were on treatment, an electrophysiologist and an EP evaluated the patients’ response. For both groups, stable patients were discharged and unstable patients were admitted.
In this nonrandomized observational study conducted over a 1-year period, 94 patients were managed with the AF pathway. Admissions and outcomes in this group were compared with 265 patients who received usual care.
Only 16% of those managed through the AF pathway were admitted versus 80% (P < .001) in the usual care group. Among those admitted, LOS was shorter in patients managed along the AF pathway relative to usual care (32 vs 85 hours; P = .002). Dr Ruskin reported that both the cardioversion rate and the proportion of patients discharged on novel oral anticoagulation drugs were higher in the AF pathway group.
The reductions in hospital admissions would be expected to translate into large reductions in costs, particularly as follow-up showed no difference in return visits to the hospital between those entered into the AF pathway relative to those who received routine care, according to Dr Ruskin. Emphasizing the cost burden of AF admissions, he noted that the estimated charges for the more than 300,000 AF admissions in US hospitals in 2013 exceeded $7 billion.
Currently, there are no uniform guidelines for managing AF in the ED, and there is wide variation in practice among centers, according to Dr Ruskin. He provided data from the NEDS database demonstrating highly significant variations in rates of admission by geographic region (eg, rates were >10% higher in the northeast vs the west) and hospital type (eg, rates were twice as high in metropolitan than nonmetropolitan hospitals).
In the NEDS database, various patient characteristics were associated with increased odds ratios (ORs) for admission. These included hypertension (OR, 2.3), valvular disease (OR, 3.6), and congestive heart failure (OR, 3.7). However, Dr Ruskin indicated that patients with these or other characteristics associated with increased likelihood of admission, such as older age, have better outcomes with hospitalization.
The data from this initial observational study were recently published, and a larger prospective study of this AF pathway is already underway at both Massachusetts General and at Brigham and Women’s Hospital, Boston. If the data confirm that AF admissions can be safely reduced through this pathway, Dr Ruskin anticipates that implementation will be adopted at other hospitals in the Harvard system.
Ptaszek LM, White B, Lubitz SA, et al. Effect of a multidisciplinary approach for the management of patients with atrial fibrillation in the emergency department on hospital admission rate and length of stay. Am J Cardiol. 2016;118(1):64-71. doi:10.1016/j.amjcard.2016.04.014.
Understanding SSTI Admission, Treatment Crucial to Reducing Disease Burden
DEEPAK CHITNIS
FRONTLINE MEDICAL NEWS
Decreasing the burden of treating skin and soft tissue infections (SSTIs) is critical to improving care and reducing the costs that SSTIs place on health care facilities, according to a study published in Hospital Practice.
“Despite expert panel recommendations and treatment guidelines, there is no widely accepted classification system for grading SSTIs to outcomes,” wrote the study’s lead author, Kristin E. Linder, PharmD, of Hartford (Connecticut) Hospital. “This leads to a considerable variation in treatment approach on initial presentation when deciding which patients should be admitted to receive intravenous antibiotic therapy or treated as outpatients.”
Dr Linder and her coinvestigators conducted a single-center retrospective cohort study with the primary objective of determining rates of admission and re-presentation, along with average LOS and cost of care for both inpatients and outpatients with SSTIs. Patients aged 18 years and older who received a primary diagnosis of an SSTI during May and June 2015 at Hartford Hospital were screened; 446 were deemed eligible, with 357 ultimately selected for inclusion.
“Patients were categorized into two groups based on disposition of care, inpatient or outpatient, on index presentation,” the authors explained. “Economic data were collected using reports from hospital finance databases and included reports of total billed costs.”
Of the 357 patients included for analysis, 106 (29.7%) were admitted as inpatients while the remaining 251 (70.3%) were treated as outpatients. However, there were no significant differences found in re-presentation rates, either overall (22.6% for inpatients and 28.3% for outpatients; P > .05) or for SSTI-related re-presentation (10.4% for inpatients and 15.1% for outpatients; P > .05). For those patients who were admitted, the mean LOS was 7.3 days.
Patients who presented with a Charlson Comorbidity Index (CCI) score of 0 were admitted at a rate of 14.1%, compared to 30.1% of those with a CCI score of 1, and 60.9% of those with a CCI score of 2 or higher. The biggest disparity, however, was in terms of cost of care; while outpatient care cost an average of $413 per patient, inpatient care cost an average of $13,313 per patient.
Wound and abscess cultures that were tested found methicillin-susceptible Staphylococcus aureus to be the most prevalent gram-positive organism (37.1%) found in inpatients, while for outpatients, methicillin-resistant S aureus (MRSA) was the most common (66.7%). According to the investigators, gram-negative bacteria were not isolated in every case, so “prevalent use of combination therapy in this setting may not be warranted.
“Understanding how and where patients with SSTI are treated and their re-presentation rate is important to understand to direct resources for this high-frequency disease,” the authors concluded. “This study demonstrated that approximately 70% of patients presenting to the ED with SSTI were treated as outpatients [and] while 30-day re-presentation was similar for inpatient and outpatients, readmission was more likely in those previously admitted.”
Linder KE, Nicolau DP, Nailor MD. Epidemiology, treatment, and economics of patients presenting to the emergency department for skin and soft tissue infections. Hosp Pract (1995). 2017;16:1-7. doi:10.1080/21548331.2017.1279519.
Adolescents, Boys, Black Children Most Likely To Be Hospitalized for SJS and TEN
WHITNEY MCKNIGHT
FRONTLINE MEDICAL NEWS
Annual hospitalization rates in the United States for Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) were shown to be higher in adolescents, boys, and black children, in a cross-sectional analysis of discharge records from more than 4,100 hospitals.
Using relevant ICD-9 codes, researchers at Harvard University identified 1,571 patients hospitalized for SJS, TEN, or both in 2009 and 2012, as listed in the Kids Inpatient Database from the Agency for Healthcare Research and Quality. The highest hospitalization rates per 100,000 in each year were for adolescents between ages 15 and 19 years (P = .01), boys (P = .03), and black children (P = .82). The overall risk of death from these conditions was 1.5% in 2009 and 0.3% in 2012. The data were published online in a brief report.
Although the difference in the number of hospitalizations for black children was not significant when compared with other ethnic and racial groups, at 1.03 hospitalizations per 100,000 children (95% confidence interval [CI], 0.80-1.31) in 2009 and 1.06 hospitalizations per 100,000 children (95% CI, 0.86-1.30) in 2012, the rate was greatest in this group. The next highest ratio was in white children at 0.82 hospitalizations per 100,000 (95% CI, 0.74-0.91) in 2009, and 0.95 hospitalizations per 100,000 (95% CI, 0.86-1.05) in 2012.
With the number of SJS- and TEN-related hospitalizations between 0.1 and 1.0 per 100,000, lead author Yusuke Okubo, MD, MPH, and colleagues wrote that their data aligned with previous studies; however, regarding the emphasis on demographic differences, theirs was, to the best of their knowledge, “the first study to reveal these disparities.” Compared with adults, they added, mortality was “remarkably lower” in children.
Okubo Y, Nochioka K, Testa MA. Nationwide survey of Stevens-Johnson syndrome and toxic epidermal necrolysis in children in the United States. Pediatr Dermatol. 2016 Dec 19. doi:10.1111/pde.13050. [Epub ahead of print]
Guidelines Released for Diagnosing TB in Adults, Children
MARY ANN MOON
FRONTLINE MEDICAL NEWS
A clinical practice guideline for diagnosing pulmonary, extrapulmonary, and latent tuberculosis (TB) in adults and children has been released jointly by the American Thoracic Society, the Centers for Disease Control and Prevention, and the Infectious Diseases Society of America.
The American Academy of Pediatrics also provided input to the guideline, which includes 23 evidence-based recommendations. The document is intended to assist clinicians in high-resource countries with a low incidence of TB disease and latent TB infection, such as the United States, said David M. Lewinsohn, MD, PhD, and his associates on the joint task force that wrote the guideline.
There were 9,412 cases of TB disease reported in the United States in 2014, the most recent year for which data are available. This translates to a rate of 3.0 cases per 100,000 persons. Two-thirds of the cases in the United States developed in foreign-born persons. “The rate of disease was 13.4 times higher in foreign-born persons than in US-born individuals [15.3 vs 1.1 per 100,000, respectively],” wrote Dr Lewinsohn of pulmonary and critical care medicine, Oregon Health & Science University, Portland, and colleagues.
Even though the case rate is relatively low in the United States and has declined in recent years, “an estimated 11 million persons are infected with Mycobacterium tuberculosis. Thus…there remains a large reservoir of individuals who are infected. Without the application of improved diagnosis and effective treatment for latent [disease], new cases of TB will develop from within this group,” they noted.
Among the guideline’s strongest recommendations are the following:
- Acid-fast bacilli smear microscopy should be performed in all patients suspected of having pulmonary TB, using at least three sputum samples. A sputum volume of at least 3 mL is needed, but 5 to 10 mL would be better.
- Both liquid and solid mycobacterial cultures should be performed on every specimen from patients suspected of having TB disease, rather than either type alone.
- A diagnostic nucleic acid amplification test should be performed on the initial specimen from patients suspected of having pulmonary TB.
- Rapid molecular drug-susceptibility testing of respiratory specimens is advised for certain patients, with a focus on testing for rifampin susceptibility with or without isoniazid.
- Patients suspected of having extrapulmonary TB also should have mycobacterial cultures performed on all specimens.
- For all mycobacterial cultures that are positive for TB, a culture isolate should be submitted for genotyping to a regional genotyping laboratory.
- For patients aged 5 and older who are suspected of having latent TB infection, an interferon-gamma release assay (IGRA) is advised rather than a tuberculin skin test, especially if the patient is not likely to return to have the test result read. A tuberculin skin test is an acceptable alternative if IGRA is not available, is too expensive, or is too burdensome.
The guideline also addresses bronchoscopic sampling, cell counts and chemistries from fluid specimens collected from sites suspected of harboring extrapulmonary TB (such as pleural, cerebrospinal, ascetic, or joint fluids), and measurement of adenosine deaminase levels.
Lewinsohn DM, Leonard MK, LoBue PA, et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017;64(2):e1-e33. doi:10.1093/cid/ciw694.
Atrial Fibrillation Management Pathway in the ED May Lower Hospital Admissions
TED BOSWORTH
FRONTLINE MEDICAL NEWS
An atrial fibrillation (AF) treatment pathway designed specifically to reduce the proportion of patients with this complaint who are admitted to the hospital from the ED was remarkably effective, according to a pilot study presented at the annual International AF Symposium.
“In this single-center observational study, a multidisciplinary AF pathway was associated with 5-fold reduction in admission rate and 2.5-fold reduction in length-of-stay [LOS] for those who were admitted,” reported Jeremy N. Ruskin, MD.
Relative to many other countries, admission rates for AF in the United States are “extremely high,” according to Dr Ruskin, director of the cardiac arrhythmia service at Massachusetts General Hospital, Boston. Citing 2013 figures from the Nationwide Emergency Department Sample (NEDS) database, rates ranged between 60% and 80% by geographic region, with an average of about 66%. In contrast, and as an example of lower rates elsewhere, fewer than 40% of AF patients with similar characteristics presenting at EDs in Ontario, Canada were admitted. Similarly low admission rates have been reported in Europe.
The AF pathway tested in the study at Massachusetts General was developed through collaboration between electrophysiologists and emergency physicians (EPs). It is suitable for patients presenting with a primary complaint of AF without concomitant diseases, such as sepsis or myocardial infarction. Patients were entered into this study after it was shown that AF was the chief complaint. The first step was to determine whether participants were best suited to a rhythm-control or rate-control strategy.
“The rhythm-control group was anticoagulated and then underwent expedited cardioversion with TEE [transesophageal echocardiogram] if necessary. The rate-control group was anticoagulated and then given appropriate pharmacologic therapy,” Dr Ruskin explained. Once patients were on treatment, an electrophysiologist and an EP evaluated the patients’ response. For both groups, stable patients were discharged and unstable patients were admitted.
In this nonrandomized observational study conducted over a 1-year period, 94 patients were managed with the AF pathway. Admissions and outcomes in this group were compared with 265 patients who received usual care.
Only 16% of those managed through the AF pathway were admitted versus 80% (P < .001) in the usual care group. Among those admitted, LOS was shorter in patients managed along the AF pathway relative to usual care (32 vs 85 hours; P = .002). Dr Ruskin reported that both the cardioversion rate and the proportion of patients discharged on novel oral anticoagulation drugs were higher in the AF pathway group.
The reductions in hospital admissions would be expected to translate into large reductions in costs, particularly as follow-up showed no difference in return visits to the hospital between those entered into the AF pathway relative to those who received routine care, according to Dr Ruskin. Emphasizing the cost burden of AF admissions, he noted that the estimated charges for the more than 300,000 AF admissions in US hospitals in 2013 exceeded $7 billion.
Currently, there are no uniform guidelines for managing AF in the ED, and there is wide variation in practice among centers, according to Dr Ruskin. He provided data from the NEDS database demonstrating highly significant variations in rates of admission by geographic region (eg, rates were >10% higher in the northeast vs the west) and hospital type (eg, rates were twice as high in metropolitan than nonmetropolitan hospitals).
In the NEDS database, various patient characteristics were associated with increased odds ratios (ORs) for admission. These included hypertension (OR, 2.3), valvular disease (OR, 3.6), and congestive heart failure (OR, 3.7). However, Dr Ruskin indicated that patients with these or other characteristics associated with increased likelihood of admission, such as older age, have better outcomes with hospitalization.
The data from this initial observational study were recently published, and a larger prospective study of this AF pathway is already underway at both Massachusetts General and at Brigham and Women’s Hospital, Boston. If the data confirm that AF admissions can be safely reduced through this pathway, Dr Ruskin anticipates that implementation will be adopted at other hospitals in the Harvard system.
Ptaszek LM, White B, Lubitz SA, et al. Effect of a multidisciplinary approach for the management of patients with atrial fibrillation in the emergency department on hospital admission rate and length of stay. Am J Cardiol. 2016;118(1):64-71. doi:10.1016/j.amjcard.2016.04.014.
Understanding SSTI Admission, Treatment Crucial to Reducing Disease Burden
DEEPAK CHITNIS
FRONTLINE MEDICAL NEWS
Decreasing the burden of treating skin and soft tissue infections (SSTIs) is critical to improving care and reducing the costs that SSTIs place on health care facilities, according to a study published in Hospital Practice.
“Despite expert panel recommendations and treatment guidelines, there is no widely accepted classification system for grading SSTIs to outcomes,” wrote the study’s lead author, Kristin E. Linder, PharmD, of Hartford (Connecticut) Hospital. “This leads to a considerable variation in treatment approach on initial presentation when deciding which patients should be admitted to receive intravenous antibiotic therapy or treated as outpatients.”
Dr Linder and her coinvestigators conducted a single-center retrospective cohort study with the primary objective of determining rates of admission and re-presentation, along with average LOS and cost of care for both inpatients and outpatients with SSTIs. Patients aged 18 years and older who received a primary diagnosis of an SSTI during May and June 2015 at Hartford Hospital were screened; 446 were deemed eligible, with 357 ultimately selected for inclusion.
“Patients were categorized into two groups based on disposition of care, inpatient or outpatient, on index presentation,” the authors explained. “Economic data were collected using reports from hospital finance databases and included reports of total billed costs.”
Of the 357 patients included for analysis, 106 (29.7%) were admitted as inpatients while the remaining 251 (70.3%) were treated as outpatients. However, there were no significant differences found in re-presentation rates, either overall (22.6% for inpatients and 28.3% for outpatients; P > .05) or for SSTI-related re-presentation (10.4% for inpatients and 15.1% for outpatients; P > .05). For those patients who were admitted, the mean LOS was 7.3 days.
Patients who presented with a Charlson Comorbidity Index (CCI) score of 0 were admitted at a rate of 14.1%, compared to 30.1% of those with a CCI score of 1, and 60.9% of those with a CCI score of 2 or higher. The biggest disparity, however, was in terms of cost of care; while outpatient care cost an average of $413 per patient, inpatient care cost an average of $13,313 per patient.
Wound and abscess cultures that were tested found methicillin-susceptible Staphylococcus aureus to be the most prevalent gram-positive organism (37.1%) found in inpatients, while for outpatients, methicillin-resistant S aureus (MRSA) was the most common (66.7%). According to the investigators, gram-negative bacteria were not isolated in every case, so “prevalent use of combination therapy in this setting may not be warranted.
“Understanding how and where patients with SSTI are treated and their re-presentation rate is important to understand to direct resources for this high-frequency disease,” the authors concluded. “This study demonstrated that approximately 70% of patients presenting to the ED with SSTI were treated as outpatients [and] while 30-day re-presentation was similar for inpatient and outpatients, readmission was more likely in those previously admitted.”
Linder KE, Nicolau DP, Nailor MD. Epidemiology, treatment, and economics of patients presenting to the emergency department for skin and soft tissue infections. Hosp Pract (1995). 2017;16:1-7. doi:10.1080/21548331.2017.1279519.
Adolescents, Boys, Black Children Most Likely To Be Hospitalized for SJS and TEN
WHITNEY MCKNIGHT
FRONTLINE MEDICAL NEWS
Annual hospitalization rates in the United States for Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) were shown to be higher in adolescents, boys, and black children, in a cross-sectional analysis of discharge records from more than 4,100 hospitals.
Using relevant ICD-9 codes, researchers at Harvard University identified 1,571 patients hospitalized for SJS, TEN, or both in 2009 and 2012, as listed in the Kids Inpatient Database from the Agency for Healthcare Research and Quality. The highest hospitalization rates per 100,000 in each year were for adolescents between ages 15 and 19 years (P = .01), boys (P = .03), and black children (P = .82). The overall risk of death from these conditions was 1.5% in 2009 and 0.3% in 2012. The data were published online in a brief report.
Although the difference in the number of hospitalizations for black children was not significant when compared with other ethnic and racial groups, at 1.03 hospitalizations per 100,000 children (95% confidence interval [CI], 0.80-1.31) in 2009 and 1.06 hospitalizations per 100,000 children (95% CI, 0.86-1.30) in 2012, the rate was greatest in this group. The next highest ratio was in white children at 0.82 hospitalizations per 100,000 (95% CI, 0.74-0.91) in 2009, and 0.95 hospitalizations per 100,000 (95% CI, 0.86-1.05) in 2012.
With the number of SJS- and TEN-related hospitalizations between 0.1 and 1.0 per 100,000, lead author Yusuke Okubo, MD, MPH, and colleagues wrote that their data aligned with previous studies; however, regarding the emphasis on demographic differences, theirs was, to the best of their knowledge, “the first study to reveal these disparities.” Compared with adults, they added, mortality was “remarkably lower” in children.
Okubo Y, Nochioka K, Testa MA. Nationwide survey of Stevens-Johnson syndrome and toxic epidermal necrolysis in children in the United States. Pediatr Dermatol. 2016 Dec 19. doi:10.1111/pde.13050. [Epub ahead of print]
Guidelines Released for Diagnosing TB in Adults, Children
MARY ANN MOON
FRONTLINE MEDICAL NEWS
A clinical practice guideline for diagnosing pulmonary, extrapulmonary, and latent tuberculosis (TB) in adults and children has been released jointly by the American Thoracic Society, the Centers for Disease Control and Prevention, and the Infectious Diseases Society of America.
The American Academy of Pediatrics also provided input to the guideline, which includes 23 evidence-based recommendations. The document is intended to assist clinicians in high-resource countries with a low incidence of TB disease and latent TB infection, such as the United States, said David M. Lewinsohn, MD, PhD, and his associates on the joint task force that wrote the guideline.
There were 9,412 cases of TB disease reported in the United States in 2014, the most recent year for which data are available. This translates to a rate of 3.0 cases per 100,000 persons. Two-thirds of the cases in the United States developed in foreign-born persons. “The rate of disease was 13.4 times higher in foreign-born persons than in US-born individuals [15.3 vs 1.1 per 100,000, respectively],” wrote Dr Lewinsohn of pulmonary and critical care medicine, Oregon Health & Science University, Portland, and colleagues.
Even though the case rate is relatively low in the United States and has declined in recent years, “an estimated 11 million persons are infected with Mycobacterium tuberculosis. Thus…there remains a large reservoir of individuals who are infected. Without the application of improved diagnosis and effective treatment for latent [disease], new cases of TB will develop from within this group,” they noted.
Among the guideline’s strongest recommendations are the following:
- Acid-fast bacilli smear microscopy should be performed in all patients suspected of having pulmonary TB, using at least three sputum samples. A sputum volume of at least 3 mL is needed, but 5 to 10 mL would be better.
- Both liquid and solid mycobacterial cultures should be performed on every specimen from patients suspected of having TB disease, rather than either type alone.
- A diagnostic nucleic acid amplification test should be performed on the initial specimen from patients suspected of having pulmonary TB.
- Rapid molecular drug-susceptibility testing of respiratory specimens is advised for certain patients, with a focus on testing for rifampin susceptibility with or without isoniazid.
- Patients suspected of having extrapulmonary TB also should have mycobacterial cultures performed on all specimens.
- For all mycobacterial cultures that are positive for TB, a culture isolate should be submitted for genotyping to a regional genotyping laboratory.
- For patients aged 5 and older who are suspected of having latent TB infection, an interferon-gamma release assay (IGRA) is advised rather than a tuberculin skin test, especially if the patient is not likely to return to have the test result read. A tuberculin skin test is an acceptable alternative if IGRA is not available, is too expensive, or is too burdensome.
The guideline also addresses bronchoscopic sampling, cell counts and chemistries from fluid specimens collected from sites suspected of harboring extrapulmonary TB (such as pleural, cerebrospinal, ascetic, or joint fluids), and measurement of adenosine deaminase levels.
Lewinsohn DM, Leonard MK, LoBue PA, et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017;64(2):e1-e33. doi:10.1093/cid/ciw694.
Atrial Fibrillation Management Pathway in the ED May Lower Hospital Admissions
TED BOSWORTH
FRONTLINE MEDICAL NEWS
An atrial fibrillation (AF) treatment pathway designed specifically to reduce the proportion of patients with this complaint who are admitted to the hospital from the ED was remarkably effective, according to a pilot study presented at the annual International AF Symposium.
“In this single-center observational study, a multidisciplinary AF pathway was associated with 5-fold reduction in admission rate and 2.5-fold reduction in length-of-stay [LOS] for those who were admitted,” reported Jeremy N. Ruskin, MD.
Relative to many other countries, admission rates for AF in the United States are “extremely high,” according to Dr Ruskin, director of the cardiac arrhythmia service at Massachusetts General Hospital, Boston. Citing 2013 figures from the Nationwide Emergency Department Sample (NEDS) database, rates ranged between 60% and 80% by geographic region, with an average of about 66%. In contrast, and as an example of lower rates elsewhere, fewer than 40% of AF patients with similar characteristics presenting at EDs in Ontario, Canada were admitted. Similarly low admission rates have been reported in Europe.
The AF pathway tested in the study at Massachusetts General was developed through collaboration between electrophysiologists and emergency physicians (EPs). It is suitable for patients presenting with a primary complaint of AF without concomitant diseases, such as sepsis or myocardial infarction. Patients were entered into this study after it was shown that AF was the chief complaint. The first step was to determine whether participants were best suited to a rhythm-control or rate-control strategy.
“The rhythm-control group was anticoagulated and then underwent expedited cardioversion with TEE [transesophageal echocardiogram] if necessary. The rate-control group was anticoagulated and then given appropriate pharmacologic therapy,” Dr Ruskin explained. Once patients were on treatment, an electrophysiologist and an EP evaluated the patients’ response. For both groups, stable patients were discharged and unstable patients were admitted.
In this nonrandomized observational study conducted over a 1-year period, 94 patients were managed with the AF pathway. Admissions and outcomes in this group were compared with 265 patients who received usual care.
Only 16% of those managed through the AF pathway were admitted versus 80% (P < .001) in the usual care group. Among those admitted, LOS was shorter in patients managed along the AF pathway relative to usual care (32 vs 85 hours; P = .002). Dr Ruskin reported that both the cardioversion rate and the proportion of patients discharged on novel oral anticoagulation drugs were higher in the AF pathway group.
The reductions in hospital admissions would be expected to translate into large reductions in costs, particularly as follow-up showed no difference in return visits to the hospital between those entered into the AF pathway relative to those who received routine care, according to Dr Ruskin. Emphasizing the cost burden of AF admissions, he noted that the estimated charges for the more than 300,000 AF admissions in US hospitals in 2013 exceeded $7 billion.
Currently, there are no uniform guidelines for managing AF in the ED, and there is wide variation in practice among centers, according to Dr Ruskin. He provided data from the NEDS database demonstrating highly significant variations in rates of admission by geographic region (eg, rates were >10% higher in the northeast vs the west) and hospital type (eg, rates were twice as high in metropolitan than nonmetropolitan hospitals).
In the NEDS database, various patient characteristics were associated with increased odds ratios (ORs) for admission. These included hypertension (OR, 2.3), valvular disease (OR, 3.6), and congestive heart failure (OR, 3.7). However, Dr Ruskin indicated that patients with these or other characteristics associated with increased likelihood of admission, such as older age, have better outcomes with hospitalization.
The data from this initial observational study were recently published, and a larger prospective study of this AF pathway is already underway at both Massachusetts General and at Brigham and Women’s Hospital, Boston. If the data confirm that AF admissions can be safely reduced through this pathway, Dr Ruskin anticipates that implementation will be adopted at other hospitals in the Harvard system.
Ptaszek LM, White B, Lubitz SA, et al. Effect of a multidisciplinary approach for the management of patients with atrial fibrillation in the emergency department on hospital admission rate and length of stay. Am J Cardiol. 2016;118(1):64-71. doi:10.1016/j.amjcard.2016.04.014.
Understanding SSTI Admission, Treatment Crucial to Reducing Disease Burden
DEEPAK CHITNIS
FRONTLINE MEDICAL NEWS
Decreasing the burden of treating skin and soft tissue infections (SSTIs) is critical to improving care and reducing the costs that SSTIs place on health care facilities, according to a study published in Hospital Practice.
“Despite expert panel recommendations and treatment guidelines, there is no widely accepted classification system for grading SSTIs to outcomes,” wrote the study’s lead author, Kristin E. Linder, PharmD, of Hartford (Connecticut) Hospital. “This leads to a considerable variation in treatment approach on initial presentation when deciding which patients should be admitted to receive intravenous antibiotic therapy or treated as outpatients.”
Dr Linder and her coinvestigators conducted a single-center retrospective cohort study with the primary objective of determining rates of admission and re-presentation, along with average LOS and cost of care for both inpatients and outpatients with SSTIs. Patients aged 18 years and older who received a primary diagnosis of an SSTI during May and June 2015 at Hartford Hospital were screened; 446 were deemed eligible, with 357 ultimately selected for inclusion.
“Patients were categorized into two groups based on disposition of care, inpatient or outpatient, on index presentation,” the authors explained. “Economic data were collected using reports from hospital finance databases and included reports of total billed costs.”
Of the 357 patients included for analysis, 106 (29.7%) were admitted as inpatients while the remaining 251 (70.3%) were treated as outpatients. However, there were no significant differences found in re-presentation rates, either overall (22.6% for inpatients and 28.3% for outpatients; P > .05) or for SSTI-related re-presentation (10.4% for inpatients and 15.1% for outpatients; P > .05). For those patients who were admitted, the mean LOS was 7.3 days.
Patients who presented with a Charlson Comorbidity Index (CCI) score of 0 were admitted at a rate of 14.1%, compared to 30.1% of those with a CCI score of 1, and 60.9% of those with a CCI score of 2 or higher. The biggest disparity, however, was in terms of cost of care; while outpatient care cost an average of $413 per patient, inpatient care cost an average of $13,313 per patient.
Wound and abscess cultures that were tested found methicillin-susceptible Staphylococcus aureus to be the most prevalent gram-positive organism (37.1%) found in inpatients, while for outpatients, methicillin-resistant S aureus (MRSA) was the most common (66.7%). According to the investigators, gram-negative bacteria were not isolated in every case, so “prevalent use of combination therapy in this setting may not be warranted.
“Understanding how and where patients with SSTI are treated and their re-presentation rate is important to understand to direct resources for this high-frequency disease,” the authors concluded. “This study demonstrated that approximately 70% of patients presenting to the ED with SSTI were treated as outpatients [and] while 30-day re-presentation was similar for inpatient and outpatients, readmission was more likely in those previously admitted.”
Linder KE, Nicolau DP, Nailor MD. Epidemiology, treatment, and economics of patients presenting to the emergency department for skin and soft tissue infections. Hosp Pract (1995). 2017;16:1-7. doi:10.1080/21548331.2017.1279519.
Adolescents, Boys, Black Children Most Likely To Be Hospitalized for SJS and TEN
WHITNEY MCKNIGHT
FRONTLINE MEDICAL NEWS
Annual hospitalization rates in the United States for Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) were shown to be higher in adolescents, boys, and black children, in a cross-sectional analysis of discharge records from more than 4,100 hospitals.
Using relevant ICD-9 codes, researchers at Harvard University identified 1,571 patients hospitalized for SJS, TEN, or both in 2009 and 2012, as listed in the Kids Inpatient Database from the Agency for Healthcare Research and Quality. The highest hospitalization rates per 100,000 in each year were for adolescents between ages 15 and 19 years (P = .01), boys (P = .03), and black children (P = .82). The overall risk of death from these conditions was 1.5% in 2009 and 0.3% in 2012. The data were published online in a brief report.
Although the difference in the number of hospitalizations for black children was not significant when compared with other ethnic and racial groups, at 1.03 hospitalizations per 100,000 children (95% confidence interval [CI], 0.80-1.31) in 2009 and 1.06 hospitalizations per 100,000 children (95% CI, 0.86-1.30) in 2012, the rate was greatest in this group. The next highest ratio was in white children at 0.82 hospitalizations per 100,000 (95% CI, 0.74-0.91) in 2009, and 0.95 hospitalizations per 100,000 (95% CI, 0.86-1.05) in 2012.
With the number of SJS- and TEN-related hospitalizations between 0.1 and 1.0 per 100,000, lead author Yusuke Okubo, MD, MPH, and colleagues wrote that their data aligned with previous studies; however, regarding the emphasis on demographic differences, theirs was, to the best of their knowledge, “the first study to reveal these disparities.” Compared with adults, they added, mortality was “remarkably lower” in children.
Okubo Y, Nochioka K, Testa MA. Nationwide survey of Stevens-Johnson syndrome and toxic epidermal necrolysis in children in the United States. Pediatr Dermatol. 2016 Dec 19. doi:10.1111/pde.13050. [Epub ahead of print]
Guidelines Released for Diagnosing TB in Adults, Children
MARY ANN MOON
FRONTLINE MEDICAL NEWS
A clinical practice guideline for diagnosing pulmonary, extrapulmonary, and latent tuberculosis (TB) in adults and children has been released jointly by the American Thoracic Society, the Centers for Disease Control and Prevention, and the Infectious Diseases Society of America.
The American Academy of Pediatrics also provided input to the guideline, which includes 23 evidence-based recommendations. The document is intended to assist clinicians in high-resource countries with a low incidence of TB disease and latent TB infection, such as the United States, said David M. Lewinsohn, MD, PhD, and his associates on the joint task force that wrote the guideline.
There were 9,412 cases of TB disease reported in the United States in 2014, the most recent year for which data are available. This translates to a rate of 3.0 cases per 100,000 persons. Two-thirds of the cases in the United States developed in foreign-born persons. “The rate of disease was 13.4 times higher in foreign-born persons than in US-born individuals [15.3 vs 1.1 per 100,000, respectively],” wrote Dr Lewinsohn of pulmonary and critical care medicine, Oregon Health & Science University, Portland, and colleagues.
Even though the case rate is relatively low in the United States and has declined in recent years, “an estimated 11 million persons are infected with Mycobacterium tuberculosis. Thus…there remains a large reservoir of individuals who are infected. Without the application of improved diagnosis and effective treatment for latent [disease], new cases of TB will develop from within this group,” they noted.
Among the guideline’s strongest recommendations are the following:
- Acid-fast bacilli smear microscopy should be performed in all patients suspected of having pulmonary TB, using at least three sputum samples. A sputum volume of at least 3 mL is needed, but 5 to 10 mL would be better.
- Both liquid and solid mycobacterial cultures should be performed on every specimen from patients suspected of having TB disease, rather than either type alone.
- A diagnostic nucleic acid amplification test should be performed on the initial specimen from patients suspected of having pulmonary TB.
- Rapid molecular drug-susceptibility testing of respiratory specimens is advised for certain patients, with a focus on testing for rifampin susceptibility with or without isoniazid.
- Patients suspected of having extrapulmonary TB also should have mycobacterial cultures performed on all specimens.
- For all mycobacterial cultures that are positive for TB, a culture isolate should be submitted for genotyping to a regional genotyping laboratory.
- For patients aged 5 and older who are suspected of having latent TB infection, an interferon-gamma release assay (IGRA) is advised rather than a tuberculin skin test, especially if the patient is not likely to return to have the test result read. A tuberculin skin test is an acceptable alternative if IGRA is not available, is too expensive, or is too burdensome.
The guideline also addresses bronchoscopic sampling, cell counts and chemistries from fluid specimens collected from sites suspected of harboring extrapulmonary TB (such as pleural, cerebrospinal, ascetic, or joint fluids), and measurement of adenosine deaminase levels.
Lewinsohn DM, Leonard MK, LoBue PA, et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017;64(2):e1-e33. doi:10.1093/cid/ciw694.