User login
First EDition: A-Fib Management Pathway in the ED, more
Atrial Fibrillation Management Pathway in the ED May Lower Hospital Admissions
TED BOSWORTH
FRONTLINE MEDICAL NEWS
An atrial fibrillation (AF) treatment pathway designed specifically to reduce the proportion of patients with this complaint who are admitted to the hospital from the ED was remarkably effective, according to a pilot study presented at the annual International AF Symposium.
“In this single-center observational study, a multidisciplinary AF pathway was associated with 5-fold reduction in admission rate and 2.5-fold reduction in length-of-stay [LOS] for those who were admitted,” reported Jeremy N. Ruskin, MD.
Relative to many other countries, admission rates for AF in the United States are “extremely high,” according to Dr Ruskin, director of the cardiac arrhythmia service at Massachusetts General Hospital, Boston. Citing 2013 figures from the Nationwide Emergency Department Sample (NEDS) database, rates ranged between 60% and 80% by geographic region, with an average of about 66%. In contrast, and as an example of lower rates elsewhere, fewer than 40% of AF patients with similar characteristics presenting at EDs in Ontario, Canada were admitted. Similarly low admission rates have been reported in Europe.
The AF pathway tested in the study at Massachusetts General was developed through collaboration between electrophysiologists and emergency physicians (EPs). It is suitable for patients presenting with a primary complaint of AF without concomitant diseases, such as sepsis or myocardial infarction. Patients were entered into this study after it was shown that AF was the chief complaint. The first step was to determine whether participants were best suited to a rhythm-control or rate-control strategy.
“The rhythm-control group was anticoagulated and then underwent expedited cardioversion with TEE [transesophageal echocardiogram] if necessary. The rate-control group was anticoagulated and then given appropriate pharmacologic therapy,” Dr Ruskin explained. Once patients were on treatment, an electrophysiologist and an EP evaluated the patients’ response. For both groups, stable patients were discharged and unstable patients were admitted.
In this nonrandomized observational study conducted over a 1-year period, 94 patients were managed with the AF pathway. Admissions and outcomes in this group were compared with 265 patients who received usual care.
Only 16% of those managed through the AF pathway were admitted versus 80% (P < .001) in the usual care group. Among those admitted, LOS was shorter in patients managed along the AF pathway relative to usual care (32 vs 85 hours; P = .002). Dr Ruskin reported that both the cardioversion rate and the proportion of patients discharged on novel oral anticoagulation drugs were higher in the AF pathway group.
The reductions in hospital admissions would be expected to translate into large reductions in costs, particularly as follow-up showed no difference in return visits to the hospital between those entered into the AF pathway relative to those who received routine care, according to Dr Ruskin. Emphasizing the cost burden of AF admissions, he noted that the estimated charges for the more than 300,000 AF admissions in US hospitals in 2013 exceeded $7 billion.
Currently, there are no uniform guidelines for managing AF in the ED, and there is wide variation in practice among centers, according to Dr Ruskin. He provided data from the NEDS database demonstrating highly significant variations in rates of admission by geographic region (eg, rates were >10% higher in the northeast vs the west) and hospital type (eg, rates were twice as high in metropolitan than nonmetropolitan hospitals).
In the NEDS database, various patient characteristics were associated with increased odds ratios (ORs) for admission. These included hypertension (OR, 2.3), valvular disease (OR, 3.6), and congestive heart failure (OR, 3.7). However, Dr Ruskin indicated that patients with these or other characteristics associated with increased likelihood of admission, such as older age, have better outcomes with hospitalization.
The data from this initial observational study were recently published, and a larger prospective study of this AF pathway is already underway at both Massachusetts General and at Brigham and Women’s Hospital, Boston. If the data confirm that AF admissions can be safely reduced through this pathway, Dr Ruskin anticipates that implementation will be adopted at other hospitals in the Harvard system.
Ptaszek LM, White B, Lubitz SA, et al. Effect of a multidisciplinary approach for the management of patients with atrial fibrillation in the emergency department on hospital admission rate and length of stay. Am J Cardiol. 2016;118(1):64-71. doi:10.1016/j.amjcard.2016.04.014.
Understanding SSTI Admission, Treatment Crucial to Reducing Disease Burden
DEEPAK CHITNIS
FRONTLINE MEDICAL NEWS
Decreasing the burden of treating skin and soft tissue infections (SSTIs) is critical to improving care and reducing the costs that SSTIs place on health care facilities, according to a study published in Hospital Practice.
“Despite expert panel recommendations and treatment guidelines, there is no widely accepted classification system for grading SSTIs to outcomes,” wrote the study’s lead author, Kristin E. Linder, PharmD, of Hartford (Connecticut) Hospital. “This leads to a considerable variation in treatment approach on initial presentation when deciding which patients should be admitted to receive intravenous antibiotic therapy or treated as outpatients.”
Dr Linder and her coinvestigators conducted a single-center retrospective cohort study with the primary objective of determining rates of admission and re-presentation, along with average LOS and cost of care for both inpatients and outpatients with SSTIs. Patients aged 18 years and older who received a primary diagnosis of an SSTI during May and June 2015 at Hartford Hospital were screened; 446 were deemed eligible, with 357 ultimately selected for inclusion.
“Patients were categorized into two groups based on disposition of care, inpatient or outpatient, on index presentation,” the authors explained. “Economic data were collected using reports from hospital finance databases and included reports of total billed costs.”
Of the 357 patients included for analysis, 106 (29.7%) were admitted as inpatients while the remaining 251 (70.3%) were treated as outpatients. However, there were no significant differences found in re-presentation rates, either overall (22.6% for inpatients and 28.3% for outpatients; P > .05) or for SSTI-related re-presentation (10.4% for inpatients and 15.1% for outpatients; P > .05). For those patients who were admitted, the mean LOS was 7.3 days.
Patients who presented with a Charlson Comorbidity Index (CCI) score of 0 were admitted at a rate of 14.1%, compared to 30.1% of those with a CCI score of 1, and 60.9% of those with a CCI score of 2 or higher. The biggest disparity, however, was in terms of cost of care; while outpatient care cost an average of $413 per patient, inpatient care cost an average of $13,313 per patient.
Wound and abscess cultures that were tested found methicillin-susceptible Staphylococcus aureus to be the most prevalent gram-positive organism (37.1%) found in inpatients, while for outpatients, methicillin-resistant S aureus (MRSA) was the most common (66.7%). According to the investigators, gram-negative bacteria were not isolated in every case, so “prevalent use of combination therapy in this setting may not be warranted.
“Understanding how and where patients with SSTI are treated and their re-presentation rate is important to understand to direct resources for this high-frequency disease,” the authors concluded. “This study demonstrated that approximately 70% of patients presenting to the ED with SSTI were treated as outpatients [and] while 30-day re-presentation was similar for inpatient and outpatients, readmission was more likely in those previously admitted.”
Linder KE, Nicolau DP, Nailor MD. Epidemiology, treatment, and economics of patients presenting to the emergency department for skin and soft tissue infections. Hosp Pract (1995). 2017;16:1-7. doi:10.1080/21548331.2017.1279519.
Adolescents, Boys, Black Children Most Likely To Be Hospitalized for SJS and TEN
WHITNEY MCKNIGHT
FRONTLINE MEDICAL NEWS
Annual hospitalization rates in the United States for Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) were shown to be higher in adolescents, boys, and black children, in a cross-sectional analysis of discharge records from more than 4,100 hospitals.
Using relevant ICD-9 codes, researchers at Harvard University identified 1,571 patients hospitalized for SJS, TEN, or both in 2009 and 2012, as listed in the Kids Inpatient Database from the Agency for Healthcare Research and Quality. The highest hospitalization rates per 100,000 in each year were for adolescents between ages 15 and 19 years (P = .01), boys (P = .03), and black children (P = .82). The overall risk of death from these conditions was 1.5% in 2009 and 0.3% in 2012. The data were published online in a brief report.
Although the difference in the number of hospitalizations for black children was not significant when compared with other ethnic and racial groups, at 1.03 hospitalizations per 100,000 children (95% confidence interval [CI], 0.80-1.31) in 2009 and 1.06 hospitalizations per 100,000 children (95% CI, 0.86-1.30) in 2012, the rate was greatest in this group. The next highest ratio was in white children at 0.82 hospitalizations per 100,000 (95% CI, 0.74-0.91) in 2009, and 0.95 hospitalizations per 100,000 (95% CI, 0.86-1.05) in 2012.
With the number of SJS- and TEN-related hospitalizations between 0.1 and 1.0 per 100,000, lead author Yusuke Okubo, MD, MPH, and colleagues wrote that their data aligned with previous studies; however, regarding the emphasis on demographic differences, theirs was, to the best of their knowledge, “the first study to reveal these disparities.” Compared with adults, they added, mortality was “remarkably lower” in children.
Okubo Y, Nochioka K, Testa MA. Nationwide survey of Stevens-Johnson syndrome and toxic epidermal necrolysis in children in the United States. Pediatr Dermatol. 2016 Dec 19. doi:10.1111/pde.13050. [Epub ahead of print]
Guidelines Released for Diagnosing TB in Adults, Children
MARY ANN MOON
FRONTLINE MEDICAL NEWS
A clinical practice guideline for diagnosing pulmonary, extrapulmonary, and latent tuberculosis (TB) in adults and children has been released jointly by the American Thoracic Society, the Centers for Disease Control and Prevention, and the Infectious Diseases Society of America.
The American Academy of Pediatrics also provided input to the guideline, which includes 23 evidence-based recommendations. The document is intended to assist clinicians in high-resource countries with a low incidence of TB disease and latent TB infection, such as the United States, said David M. Lewinsohn, MD, PhD, and his associates on the joint task force that wrote the guideline.
There were 9,412 cases of TB disease reported in the United States in 2014, the most recent year for which data are available. This translates to a rate of 3.0 cases per 100,000 persons. Two-thirds of the cases in the United States developed in foreign-born persons. “The rate of disease was 13.4 times higher in foreign-born persons than in US-born individuals [15.3 vs 1.1 per 100,000, respectively],” wrote Dr Lewinsohn of pulmonary and critical care medicine, Oregon Health & Science University, Portland, and colleagues.
Even though the case rate is relatively low in the United States and has declined in recent years, “an estimated 11 million persons are infected with Mycobacterium tuberculosis. Thus…there remains a large reservoir of individuals who are infected. Without the application of improved diagnosis and effective treatment for latent [disease], new cases of TB will develop from within this group,” they noted.
Among the guideline’s strongest recommendations are the following:
- Acid-fast bacilli smear microscopy should be performed in all patients suspected of having pulmonary TB, using at least three sputum samples. A sputum volume of at least 3 mL is needed, but 5 to 10 mL would be better.
- Both liquid and solid mycobacterial cultures should be performed on every specimen from patients suspected of having TB disease, rather than either type alone.
- A diagnostic nucleic acid amplification test should be performed on the initial specimen from patients suspected of having pulmonary TB.
- Rapid molecular drug-susceptibility testing of respiratory specimens is advised for certain patients, with a focus on testing for rifampin susceptibility with or without isoniazid.
- Patients suspected of having extrapulmonary TB also should have mycobacterial cultures performed on all specimens.
- For all mycobacterial cultures that are positive for TB, a culture isolate should be submitted for genotyping to a regional genotyping laboratory.
- For patients aged 5 and older who are suspected of having latent TB infection, an interferon-gamma release assay (IGRA) is advised rather than a tuberculin skin test, especially if the patient is not likely to return to have the test result read. A tuberculin skin test is an acceptable alternative if IGRA is not available, is too expensive, or is too burdensome.
The guideline also addresses bronchoscopic sampling, cell counts and chemistries from fluid specimens collected from sites suspected of harboring extrapulmonary TB (such as pleural, cerebrospinal, ascetic, or joint fluids), and measurement of adenosine deaminase levels.
Lewinsohn DM, Leonard MK, LoBue PA, et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017;64(2):e1-e33. doi:10.1093/cid/ciw694.
Atrial Fibrillation Management Pathway in the ED May Lower Hospital Admissions
TED BOSWORTH
FRONTLINE MEDICAL NEWS
An atrial fibrillation (AF) treatment pathway designed specifically to reduce the proportion of patients with this complaint who are admitted to the hospital from the ED was remarkably effective, according to a pilot study presented at the annual International AF Symposium.
“In this single-center observational study, a multidisciplinary AF pathway was associated with 5-fold reduction in admission rate and 2.5-fold reduction in length-of-stay [LOS] for those who were admitted,” reported Jeremy N. Ruskin, MD.
Relative to many other countries, admission rates for AF in the United States are “extremely high,” according to Dr Ruskin, director of the cardiac arrhythmia service at Massachusetts General Hospital, Boston. Citing 2013 figures from the Nationwide Emergency Department Sample (NEDS) database, rates ranged between 60% and 80% by geographic region, with an average of about 66%. In contrast, and as an example of lower rates elsewhere, fewer than 40% of AF patients with similar characteristics presenting at EDs in Ontario, Canada were admitted. Similarly low admission rates have been reported in Europe.
The AF pathway tested in the study at Massachusetts General was developed through collaboration between electrophysiologists and emergency physicians (EPs). It is suitable for patients presenting with a primary complaint of AF without concomitant diseases, such as sepsis or myocardial infarction. Patients were entered into this study after it was shown that AF was the chief complaint. The first step was to determine whether participants were best suited to a rhythm-control or rate-control strategy.
“The rhythm-control group was anticoagulated and then underwent expedited cardioversion with TEE [transesophageal echocardiogram] if necessary. The rate-control group was anticoagulated and then given appropriate pharmacologic therapy,” Dr Ruskin explained. Once patients were on treatment, an electrophysiologist and an EP evaluated the patients’ response. For both groups, stable patients were discharged and unstable patients were admitted.
In this nonrandomized observational study conducted over a 1-year period, 94 patients were managed with the AF pathway. Admissions and outcomes in this group were compared with 265 patients who received usual care.
Only 16% of those managed through the AF pathway were admitted versus 80% (P < .001) in the usual care group. Among those admitted, LOS was shorter in patients managed along the AF pathway relative to usual care (32 vs 85 hours; P = .002). Dr Ruskin reported that both the cardioversion rate and the proportion of patients discharged on novel oral anticoagulation drugs were higher in the AF pathway group.
The reductions in hospital admissions would be expected to translate into large reductions in costs, particularly as follow-up showed no difference in return visits to the hospital between those entered into the AF pathway relative to those who received routine care, according to Dr Ruskin. Emphasizing the cost burden of AF admissions, he noted that the estimated charges for the more than 300,000 AF admissions in US hospitals in 2013 exceeded $7 billion.
Currently, there are no uniform guidelines for managing AF in the ED, and there is wide variation in practice among centers, according to Dr Ruskin. He provided data from the NEDS database demonstrating highly significant variations in rates of admission by geographic region (eg, rates were >10% higher in the northeast vs the west) and hospital type (eg, rates were twice as high in metropolitan than nonmetropolitan hospitals).
In the NEDS database, various patient characteristics were associated with increased odds ratios (ORs) for admission. These included hypertension (OR, 2.3), valvular disease (OR, 3.6), and congestive heart failure (OR, 3.7). However, Dr Ruskin indicated that patients with these or other characteristics associated with increased likelihood of admission, such as older age, have better outcomes with hospitalization.
The data from this initial observational study were recently published, and a larger prospective study of this AF pathway is already underway at both Massachusetts General and at Brigham and Women’s Hospital, Boston. If the data confirm that AF admissions can be safely reduced through this pathway, Dr Ruskin anticipates that implementation will be adopted at other hospitals in the Harvard system.
Ptaszek LM, White B, Lubitz SA, et al. Effect of a multidisciplinary approach for the management of patients with atrial fibrillation in the emergency department on hospital admission rate and length of stay. Am J Cardiol. 2016;118(1):64-71. doi:10.1016/j.amjcard.2016.04.014.
Understanding SSTI Admission, Treatment Crucial to Reducing Disease Burden
DEEPAK CHITNIS
FRONTLINE MEDICAL NEWS
Decreasing the burden of treating skin and soft tissue infections (SSTIs) is critical to improving care and reducing the costs that SSTIs place on health care facilities, according to a study published in Hospital Practice.
“Despite expert panel recommendations and treatment guidelines, there is no widely accepted classification system for grading SSTIs to outcomes,” wrote the study’s lead author, Kristin E. Linder, PharmD, of Hartford (Connecticut) Hospital. “This leads to a considerable variation in treatment approach on initial presentation when deciding which patients should be admitted to receive intravenous antibiotic therapy or treated as outpatients.”
Dr Linder and her coinvestigators conducted a single-center retrospective cohort study with the primary objective of determining rates of admission and re-presentation, along with average LOS and cost of care for both inpatients and outpatients with SSTIs. Patients aged 18 years and older who received a primary diagnosis of an SSTI during May and June 2015 at Hartford Hospital were screened; 446 were deemed eligible, with 357 ultimately selected for inclusion.
“Patients were categorized into two groups based on disposition of care, inpatient or outpatient, on index presentation,” the authors explained. “Economic data were collected using reports from hospital finance databases and included reports of total billed costs.”
Of the 357 patients included for analysis, 106 (29.7%) were admitted as inpatients while the remaining 251 (70.3%) were treated as outpatients. However, there were no significant differences found in re-presentation rates, either overall (22.6% for inpatients and 28.3% for outpatients; P > .05) or for SSTI-related re-presentation (10.4% for inpatients and 15.1% for outpatients; P > .05). For those patients who were admitted, the mean LOS was 7.3 days.
Patients who presented with a Charlson Comorbidity Index (CCI) score of 0 were admitted at a rate of 14.1%, compared to 30.1% of those with a CCI score of 1, and 60.9% of those with a CCI score of 2 or higher. The biggest disparity, however, was in terms of cost of care; while outpatient care cost an average of $413 per patient, inpatient care cost an average of $13,313 per patient.
Wound and abscess cultures that were tested found methicillin-susceptible Staphylococcus aureus to be the most prevalent gram-positive organism (37.1%) found in inpatients, while for outpatients, methicillin-resistant S aureus (MRSA) was the most common (66.7%). According to the investigators, gram-negative bacteria were not isolated in every case, so “prevalent use of combination therapy in this setting may not be warranted.
“Understanding how and where patients with SSTI are treated and their re-presentation rate is important to understand to direct resources for this high-frequency disease,” the authors concluded. “This study demonstrated that approximately 70% of patients presenting to the ED with SSTI were treated as outpatients [and] while 30-day re-presentation was similar for inpatient and outpatients, readmission was more likely in those previously admitted.”
Linder KE, Nicolau DP, Nailor MD. Epidemiology, treatment, and economics of patients presenting to the emergency department for skin and soft tissue infections. Hosp Pract (1995). 2017;16:1-7. doi:10.1080/21548331.2017.1279519.
Adolescents, Boys, Black Children Most Likely To Be Hospitalized for SJS and TEN
WHITNEY MCKNIGHT
FRONTLINE MEDICAL NEWS
Annual hospitalization rates in the United States for Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) were shown to be higher in adolescents, boys, and black children, in a cross-sectional analysis of discharge records from more than 4,100 hospitals.
Using relevant ICD-9 codes, researchers at Harvard University identified 1,571 patients hospitalized for SJS, TEN, or both in 2009 and 2012, as listed in the Kids Inpatient Database from the Agency for Healthcare Research and Quality. The highest hospitalization rates per 100,000 in each year were for adolescents between ages 15 and 19 years (P = .01), boys (P = .03), and black children (P = .82). The overall risk of death from these conditions was 1.5% in 2009 and 0.3% in 2012. The data were published online in a brief report.
Although the difference in the number of hospitalizations for black children was not significant when compared with other ethnic and racial groups, at 1.03 hospitalizations per 100,000 children (95% confidence interval [CI], 0.80-1.31) in 2009 and 1.06 hospitalizations per 100,000 children (95% CI, 0.86-1.30) in 2012, the rate was greatest in this group. The next highest ratio was in white children at 0.82 hospitalizations per 100,000 (95% CI, 0.74-0.91) in 2009, and 0.95 hospitalizations per 100,000 (95% CI, 0.86-1.05) in 2012.
With the number of SJS- and TEN-related hospitalizations between 0.1 and 1.0 per 100,000, lead author Yusuke Okubo, MD, MPH, and colleagues wrote that their data aligned with previous studies; however, regarding the emphasis on demographic differences, theirs was, to the best of their knowledge, “the first study to reveal these disparities.” Compared with adults, they added, mortality was “remarkably lower” in children.
Okubo Y, Nochioka K, Testa MA. Nationwide survey of Stevens-Johnson syndrome and toxic epidermal necrolysis in children in the United States. Pediatr Dermatol. 2016 Dec 19. doi:10.1111/pde.13050. [Epub ahead of print]
Guidelines Released for Diagnosing TB in Adults, Children
MARY ANN MOON
FRONTLINE MEDICAL NEWS
A clinical practice guideline for diagnosing pulmonary, extrapulmonary, and latent tuberculosis (TB) in adults and children has been released jointly by the American Thoracic Society, the Centers for Disease Control and Prevention, and the Infectious Diseases Society of America.
The American Academy of Pediatrics also provided input to the guideline, which includes 23 evidence-based recommendations. The document is intended to assist clinicians in high-resource countries with a low incidence of TB disease and latent TB infection, such as the United States, said David M. Lewinsohn, MD, PhD, and his associates on the joint task force that wrote the guideline.
There were 9,412 cases of TB disease reported in the United States in 2014, the most recent year for which data are available. This translates to a rate of 3.0 cases per 100,000 persons. Two-thirds of the cases in the United States developed in foreign-born persons. “The rate of disease was 13.4 times higher in foreign-born persons than in US-born individuals [15.3 vs 1.1 per 100,000, respectively],” wrote Dr Lewinsohn of pulmonary and critical care medicine, Oregon Health & Science University, Portland, and colleagues.
Even though the case rate is relatively low in the United States and has declined in recent years, “an estimated 11 million persons are infected with Mycobacterium tuberculosis. Thus…there remains a large reservoir of individuals who are infected. Without the application of improved diagnosis and effective treatment for latent [disease], new cases of TB will develop from within this group,” they noted.
Among the guideline’s strongest recommendations are the following:
- Acid-fast bacilli smear microscopy should be performed in all patients suspected of having pulmonary TB, using at least three sputum samples. A sputum volume of at least 3 mL is needed, but 5 to 10 mL would be better.
- Both liquid and solid mycobacterial cultures should be performed on every specimen from patients suspected of having TB disease, rather than either type alone.
- A diagnostic nucleic acid amplification test should be performed on the initial specimen from patients suspected of having pulmonary TB.
- Rapid molecular drug-susceptibility testing of respiratory specimens is advised for certain patients, with a focus on testing for rifampin susceptibility with or without isoniazid.
- Patients suspected of having extrapulmonary TB also should have mycobacterial cultures performed on all specimens.
- For all mycobacterial cultures that are positive for TB, a culture isolate should be submitted for genotyping to a regional genotyping laboratory.
- For patients aged 5 and older who are suspected of having latent TB infection, an interferon-gamma release assay (IGRA) is advised rather than a tuberculin skin test, especially if the patient is not likely to return to have the test result read. A tuberculin skin test is an acceptable alternative if IGRA is not available, is too expensive, or is too burdensome.
The guideline also addresses bronchoscopic sampling, cell counts and chemistries from fluid specimens collected from sites suspected of harboring extrapulmonary TB (such as pleural, cerebrospinal, ascetic, or joint fluids), and measurement of adenosine deaminase levels.
Lewinsohn DM, Leonard MK, LoBue PA, et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017;64(2):e1-e33. doi:10.1093/cid/ciw694.
Atrial Fibrillation Management Pathway in the ED May Lower Hospital Admissions
TED BOSWORTH
FRONTLINE MEDICAL NEWS
An atrial fibrillation (AF) treatment pathway designed specifically to reduce the proportion of patients with this complaint who are admitted to the hospital from the ED was remarkably effective, according to a pilot study presented at the annual International AF Symposium.
“In this single-center observational study, a multidisciplinary AF pathway was associated with 5-fold reduction in admission rate and 2.5-fold reduction in length-of-stay [LOS] for those who were admitted,” reported Jeremy N. Ruskin, MD.
Relative to many other countries, admission rates for AF in the United States are “extremely high,” according to Dr Ruskin, director of the cardiac arrhythmia service at Massachusetts General Hospital, Boston. Citing 2013 figures from the Nationwide Emergency Department Sample (NEDS) database, rates ranged between 60% and 80% by geographic region, with an average of about 66%. In contrast, and as an example of lower rates elsewhere, fewer than 40% of AF patients with similar characteristics presenting at EDs in Ontario, Canada were admitted. Similarly low admission rates have been reported in Europe.
The AF pathway tested in the study at Massachusetts General was developed through collaboration between electrophysiologists and emergency physicians (EPs). It is suitable for patients presenting with a primary complaint of AF without concomitant diseases, such as sepsis or myocardial infarction. Patients were entered into this study after it was shown that AF was the chief complaint. The first step was to determine whether participants were best suited to a rhythm-control or rate-control strategy.
“The rhythm-control group was anticoagulated and then underwent expedited cardioversion with TEE [transesophageal echocardiogram] if necessary. The rate-control group was anticoagulated and then given appropriate pharmacologic therapy,” Dr Ruskin explained. Once patients were on treatment, an electrophysiologist and an EP evaluated the patients’ response. For both groups, stable patients were discharged and unstable patients were admitted.
In this nonrandomized observational study conducted over a 1-year period, 94 patients were managed with the AF pathway. Admissions and outcomes in this group were compared with 265 patients who received usual care.
Only 16% of those managed through the AF pathway were admitted versus 80% (P < .001) in the usual care group. Among those admitted, LOS was shorter in patients managed along the AF pathway relative to usual care (32 vs 85 hours; P = .002). Dr Ruskin reported that both the cardioversion rate and the proportion of patients discharged on novel oral anticoagulation drugs were higher in the AF pathway group.
The reductions in hospital admissions would be expected to translate into large reductions in costs, particularly as follow-up showed no difference in return visits to the hospital between those entered into the AF pathway relative to those who received routine care, according to Dr Ruskin. Emphasizing the cost burden of AF admissions, he noted that the estimated charges for the more than 300,000 AF admissions in US hospitals in 2013 exceeded $7 billion.
Currently, there are no uniform guidelines for managing AF in the ED, and there is wide variation in practice among centers, according to Dr Ruskin. He provided data from the NEDS database demonstrating highly significant variations in rates of admission by geographic region (eg, rates were >10% higher in the northeast vs the west) and hospital type (eg, rates were twice as high in metropolitan than nonmetropolitan hospitals).
In the NEDS database, various patient characteristics were associated with increased odds ratios (ORs) for admission. These included hypertension (OR, 2.3), valvular disease (OR, 3.6), and congestive heart failure (OR, 3.7). However, Dr Ruskin indicated that patients with these or other characteristics associated with increased likelihood of admission, such as older age, have better outcomes with hospitalization.
The data from this initial observational study were recently published, and a larger prospective study of this AF pathway is already underway at both Massachusetts General and at Brigham and Women’s Hospital, Boston. If the data confirm that AF admissions can be safely reduced through this pathway, Dr Ruskin anticipates that implementation will be adopted at other hospitals in the Harvard system.
Ptaszek LM, White B, Lubitz SA, et al. Effect of a multidisciplinary approach for the management of patients with atrial fibrillation in the emergency department on hospital admission rate and length of stay. Am J Cardiol. 2016;118(1):64-71. doi:10.1016/j.amjcard.2016.04.014.
Understanding SSTI Admission, Treatment Crucial to Reducing Disease Burden
DEEPAK CHITNIS
FRONTLINE MEDICAL NEWS
Decreasing the burden of treating skin and soft tissue infections (SSTIs) is critical to improving care and reducing the costs that SSTIs place on health care facilities, according to a study published in Hospital Practice.
“Despite expert panel recommendations and treatment guidelines, there is no widely accepted classification system for grading SSTIs to outcomes,” wrote the study’s lead author, Kristin E. Linder, PharmD, of Hartford (Connecticut) Hospital. “This leads to a considerable variation in treatment approach on initial presentation when deciding which patients should be admitted to receive intravenous antibiotic therapy or treated as outpatients.”
Dr Linder and her coinvestigators conducted a single-center retrospective cohort study with the primary objective of determining rates of admission and re-presentation, along with average LOS and cost of care for both inpatients and outpatients with SSTIs. Patients aged 18 years and older who received a primary diagnosis of an SSTI during May and June 2015 at Hartford Hospital were screened; 446 were deemed eligible, with 357 ultimately selected for inclusion.
“Patients were categorized into two groups based on disposition of care, inpatient or outpatient, on index presentation,” the authors explained. “Economic data were collected using reports from hospital finance databases and included reports of total billed costs.”
Of the 357 patients included for analysis, 106 (29.7%) were admitted as inpatients while the remaining 251 (70.3%) were treated as outpatients. However, there were no significant differences found in re-presentation rates, either overall (22.6% for inpatients and 28.3% for outpatients; P > .05) or for SSTI-related re-presentation (10.4% for inpatients and 15.1% for outpatients; P > .05). For those patients who were admitted, the mean LOS was 7.3 days.
Patients who presented with a Charlson Comorbidity Index (CCI) score of 0 were admitted at a rate of 14.1%, compared to 30.1% of those with a CCI score of 1, and 60.9% of those with a CCI score of 2 or higher. The biggest disparity, however, was in terms of cost of care; while outpatient care cost an average of $413 per patient, inpatient care cost an average of $13,313 per patient.
Wound and abscess cultures that were tested found methicillin-susceptible Staphylococcus aureus to be the most prevalent gram-positive organism (37.1%) found in inpatients, while for outpatients, methicillin-resistant S aureus (MRSA) was the most common (66.7%). According to the investigators, gram-negative bacteria were not isolated in every case, so “prevalent use of combination therapy in this setting may not be warranted.
“Understanding how and where patients with SSTI are treated and their re-presentation rate is important to understand to direct resources for this high-frequency disease,” the authors concluded. “This study demonstrated that approximately 70% of patients presenting to the ED with SSTI were treated as outpatients [and] while 30-day re-presentation was similar for inpatient and outpatients, readmission was more likely in those previously admitted.”
Linder KE, Nicolau DP, Nailor MD. Epidemiology, treatment, and economics of patients presenting to the emergency department for skin and soft tissue infections. Hosp Pract (1995). 2017;16:1-7. doi:10.1080/21548331.2017.1279519.
Adolescents, Boys, Black Children Most Likely To Be Hospitalized for SJS and TEN
WHITNEY MCKNIGHT
FRONTLINE MEDICAL NEWS
Annual hospitalization rates in the United States for Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) were shown to be higher in adolescents, boys, and black children, in a cross-sectional analysis of discharge records from more than 4,100 hospitals.
Using relevant ICD-9 codes, researchers at Harvard University identified 1,571 patients hospitalized for SJS, TEN, or both in 2009 and 2012, as listed in the Kids Inpatient Database from the Agency for Healthcare Research and Quality. The highest hospitalization rates per 100,000 in each year were for adolescents between ages 15 and 19 years (P = .01), boys (P = .03), and black children (P = .82). The overall risk of death from these conditions was 1.5% in 2009 and 0.3% in 2012. The data were published online in a brief report.
Although the difference in the number of hospitalizations for black children was not significant when compared with other ethnic and racial groups, at 1.03 hospitalizations per 100,000 children (95% confidence interval [CI], 0.80-1.31) in 2009 and 1.06 hospitalizations per 100,000 children (95% CI, 0.86-1.30) in 2012, the rate was greatest in this group. The next highest ratio was in white children at 0.82 hospitalizations per 100,000 (95% CI, 0.74-0.91) in 2009, and 0.95 hospitalizations per 100,000 (95% CI, 0.86-1.05) in 2012.
With the number of SJS- and TEN-related hospitalizations between 0.1 and 1.0 per 100,000, lead author Yusuke Okubo, MD, MPH, and colleagues wrote that their data aligned with previous studies; however, regarding the emphasis on demographic differences, theirs was, to the best of their knowledge, “the first study to reveal these disparities.” Compared with adults, they added, mortality was “remarkably lower” in children.
Okubo Y, Nochioka K, Testa MA. Nationwide survey of Stevens-Johnson syndrome and toxic epidermal necrolysis in children in the United States. Pediatr Dermatol. 2016 Dec 19. doi:10.1111/pde.13050. [Epub ahead of print]
Guidelines Released for Diagnosing TB in Adults, Children
MARY ANN MOON
FRONTLINE MEDICAL NEWS
A clinical practice guideline for diagnosing pulmonary, extrapulmonary, and latent tuberculosis (TB) in adults and children has been released jointly by the American Thoracic Society, the Centers for Disease Control and Prevention, and the Infectious Diseases Society of America.
The American Academy of Pediatrics also provided input to the guideline, which includes 23 evidence-based recommendations. The document is intended to assist clinicians in high-resource countries with a low incidence of TB disease and latent TB infection, such as the United States, said David M. Lewinsohn, MD, PhD, and his associates on the joint task force that wrote the guideline.
There were 9,412 cases of TB disease reported in the United States in 2014, the most recent year for which data are available. This translates to a rate of 3.0 cases per 100,000 persons. Two-thirds of the cases in the United States developed in foreign-born persons. “The rate of disease was 13.4 times higher in foreign-born persons than in US-born individuals [15.3 vs 1.1 per 100,000, respectively],” wrote Dr Lewinsohn of pulmonary and critical care medicine, Oregon Health & Science University, Portland, and colleagues.
Even though the case rate is relatively low in the United States and has declined in recent years, “an estimated 11 million persons are infected with Mycobacterium tuberculosis. Thus…there remains a large reservoir of individuals who are infected. Without the application of improved diagnosis and effective treatment for latent [disease], new cases of TB will develop from within this group,” they noted.
Among the guideline’s strongest recommendations are the following:
- Acid-fast bacilli smear microscopy should be performed in all patients suspected of having pulmonary TB, using at least three sputum samples. A sputum volume of at least 3 mL is needed, but 5 to 10 mL would be better.
- Both liquid and solid mycobacterial cultures should be performed on every specimen from patients suspected of having TB disease, rather than either type alone.
- A diagnostic nucleic acid amplification test should be performed on the initial specimen from patients suspected of having pulmonary TB.
- Rapid molecular drug-susceptibility testing of respiratory specimens is advised for certain patients, with a focus on testing for rifampin susceptibility with or without isoniazid.
- Patients suspected of having extrapulmonary TB also should have mycobacterial cultures performed on all specimens.
- For all mycobacterial cultures that are positive for TB, a culture isolate should be submitted for genotyping to a regional genotyping laboratory.
- For patients aged 5 and older who are suspected of having latent TB infection, an interferon-gamma release assay (IGRA) is advised rather than a tuberculin skin test, especially if the patient is not likely to return to have the test result read. A tuberculin skin test is an acceptable alternative if IGRA is not available, is too expensive, or is too burdensome.
The guideline also addresses bronchoscopic sampling, cell counts and chemistries from fluid specimens collected from sites suspected of harboring extrapulmonary TB (such as pleural, cerebrospinal, ascetic, or joint fluids), and measurement of adenosine deaminase levels.
Lewinsohn DM, Leonard MK, LoBue PA, et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017;64(2):e1-e33. doi:10.1093/cid/ciw694.
2017 Update on fertility
Zika virus is a serious problem. Education and infection prevention are critical to effective management, and why we chose to include Zika virus as a topic for this year’s Update. We also discuss obesity’s effects on reproduction—a very relevant concern for all ObGyns and patients alike as about half of reproductive-age women are obese. Finally, subclinical hypothyroidism can present unique management challenges, such as determining when it is present and when treatment is indicated.
Read about counseling patients about Zika virus
Managing attempted pregnancy in the era of Zika virus
Oduyebo T, Igbinosa I, Petersen EE, et al. Update: interim guidance for health care providers caring for pregnant women with possible Zika virus exposure--United States, July 2016. MMWR Morb Mortal Wkly Rep. 2016;65(29):739-744.
Petersen EE, Meaney-Delman D, Neblett-Fanfair R, et al. Update: interim guidance for preconception counseling and prevention of sexual transmission of Zika virus for persons with possible Zika virus exposure--United States, September 2016. MMWR Morb Mortal Wkly Rep. 2016;65(39):1077-1081.
US Food and Drug Administration. Donor Screening Recommendations to Reduce the Risk of Transmission of Zika Virus by Human Cells, Tissues, and Cellular and Tissue-Based Products. http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Tissue/UCM488582.pdf. Published March 2016. Accessed January 12, 2017.
National Institutes of Health. Zika: Overview. https://www.nichd.nih.gov/health/topics/zika/Pages/default.aspx. Accessed January 12, 2017.
World Health Organization. Prevention of sexual transmission of Zika virus interim guidance. WHO reference number: WHO/ZIKV/MOC/16. 1 Rev. 3, September 6, 2016.
Zika Virus Guidance Task Force of the American Society for Reproductive Medicine. Rev. 13, September 2016.
Zika virus presents unique challenges to physicians managing the care of patients attempting pregnancy, with or without fertility treatment. Neonatal Zika virus infection sequelae only recently have been appreciated; microcephaly was associated with Zika virus in October 2015, followed by other neurologic conditions including brain abnormalities, neural tube defects, and eye abnormalities. Results of recent studies involving the US Zika Pregnancy Registry show that 6% of women with Zika at any time in pregnancy had affected babies, but 11% of those who contracted the disease in the first trimester were affected.
Diagnosis is difficult because symptoms are generally mild, with 80% of affected patients asymptomatic. Possible Zika virus exposure is defined as travel to or residence in an area of active Zika virus transmission, or sex without a condom with a partner who traveled to or lived in an area of active transmission. Much is unknown about the interval from exposure to symptoms. Testing availability is limited and variable, and much is unknown about sensitivity and specificity of direct viral RNA testing, appearance and disappearance of detectable immunoglobulin (Ig) M and IgG antibodies that affect false positive and false negative test results, duration of infectious phase, risk of transmission, and numerous other factors.
Positive serum viral testing likely indicates virus in semen or other bodily fluids, but a negative serum viral test cannot definitively preclude virus in other bodily fluids. Zika virus likely can be passed from any combination of semen and vaginal and cervical fluids, but validating tests for these fluids are not yet available. It is not known if sperm preparation and assisted reproductive technology (ART) procedures that minimize risk of HIV transmission are effective against Zika virus or whether or not cryopreservation can destroy the virus.
Pregnancy timing
The Centers for Disease Control and Prevention now recommends that all men with possible Zika virus exposure who are considering attempting pregnancy with their partner wait to get pregnant until at least 6 months after symptom onset (if symptomatic) or last possible Zika virus exposure (if asymptomatic). Women with possible Zika virus exposure are recommended to wait to get pregnant until at least 8 weeks after symptom onset (if symptomatic) or last possible Zika virus exposure (if asymptomatic).
Women and men with possible exposure to Zika virus but without clinical symptoms of illness should consider testing for Zika viral RNA within 2 weeks of suspected exposure and wait at least 8 weeks after the last date of exposure before being re-tested. If direct viral testing (using rRT-PCR) results initially are negative, ideally, antibody testing would be obtained, if available, at 8 weeks. However, no testing paradigm will absolutely guarantee lack of Zika virus infectivity.
Virus management problems are dramatically compounded in areas endemic for Zika. Women and men who have had Zika virus disease should wait at least 6 months after illness onset to attempt reproduction. The temporal relationship between the presence of viral RNA and infectivity is not known definitively, and so the absolute duration of time to wait before attempting pregnancy is unknown. Male and female partners who become infected should avoid all forms of intimate sexual conduct or use condoms for the same 6 months. There is no evidence Zika will cause congenital infection in pregnancies initiated after resolution of maternal Zika viremia. However, any testing performed at a time other than the time of treatment might not reflect true viral status, particularly in areas of active Zika virus transmission.
Prevention
Women and men, especially those residing in areas of active Zika virus transmission, should talk with their physicians regarding pregnancy plans and avoid mosquito bites using the usual precautions: avoid mosquito areas, drain standing water, use mosquito repellent containing DEET, and use mosquito netting. Some people have gone so far as to relocate to nonendemic areas.
Those contemplating pregnancy should be advised to consider what they would do if they become exposed to or have suspected or confirmed Zika virus during pregnancy. Additional considerations are gamete or embryo cryopreservation and quarantine until a subsequent rRT-PCR test result is negative in both the male and female and at least 8 weeks have passed from gamete collection.

Patient counseling essentials
Counsel patients considering reproduction about:
- Zika virus as a new reproductive hazard
- the significance of the hazard to the fetus if infected
- the areas of active transmission, and that they are constantly changing
- avoidance of Zika areas if possible
- methods of transmission through mosquito bites or sex
- avoidance of mosquito bites
- symptoms of Zika infection
- safe sex practices
- testing limitations and knowledge deficiency about Zika.
Not uncommonly, clinical situations require complex individualized management decisions regarding trade-offs of risks, especially in older patients with decreased ovarian reserve. Consultation with infectious disease and reproductive specialists should be obtained when complicated and consequential decisions have to be made.
All practitioners should inform their patients, especially those undergoing fertility treatments, about Zika, and develop language in their informed consent that conveys the gap in knowledge to these patients.
Read how obesity specifically affects reproduction in an adverse way
Obesity adversely affects reproduction, but how specifically?
Practice Committee of the American Society for Reproductive Medicine. Obesity and Reproduction: A committee opinion. Fertil Steril. 2015;104(5):1116-1126.
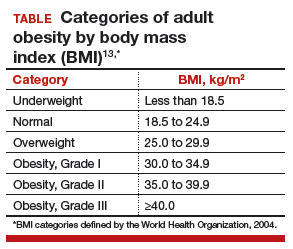
The prevalence of obesity has increased substantially over the past 2 decades. Almost two-thirds of women and three-fourths of men in the United States are overweight or obese (defined as a body mass index [BMI] ≥25 kg/m2 and BMI ≥30 kg/m2, respectively; TABLE). Nearly 50% of reproductive-age women are obese.
A disease of excess body fat and insulin resistance, obesity increases the risks of hypertension, diabetes, dyslipidemia, cardiovascular disease, sleep apnea, respiratory problems, and cancer as well as other serious health problems. While not all individuals with obesity will have infertility, obesity is associated with impaired reproduction in both women and men, adverse obstetric outcomes, and health problems in offspring. The American Society for Reproductive Medicine (ASRM) reviewed this important issue in a recent practice committee opinion.
Menstrual cycle and ovulatory dysfunction
Menstrual cycle abnormalities are more common in women with obesity. Elevated levels of insulin in obese women suppress sex hormone−binding globulin (SHBG) which in turn reduces gonadotropin secretion due to increased production of estrogen from conversion of androgens by adipose aromatase.1 Adipose tissue produces adipokines, which directly can suppress ovarian function.2
Ovulatory dysfunction is common among obese women; the relative risk of such dysfunction is 3.1 (95% confidence interval [CI], 2.2−4.4) among women with BMI levels >27 kg/m2 versus BMI levels 20.0 to 24.9 kg/m2.3,4 Obesity decreases fecundity even in women with normal menstrual cycles.5 This may in part be due to altered ovulatory dynamics with reduced early follicular luteinizing hormone pulse amplitude accompanied by prolonged folliculogenesis and reduced luteal progesterone levels.6
Compared with normal-weight women, obese women have a lower chance of conception within 1 year of stopping contraception; about 66% of obese women conceive within 1 year of stopping contraception, compared with about 81% of women with normal weight.7 Results of a Dutch study of 3,029 women with regular ovulation, at least one patent tube, and a partner with a normal semen analysis indicated a direct correlation between obesity and delayed conception, with a 4% lower spontaneous pregnancy rate per kg/m2 increase in women with a BMI >29 kg/m2 versus a BMI of 21 to 29 kg/m2 (hazard ratio, 0.96; 95% CI, 0.91−0.99).8
Assisted reproduction
Assisted reproduction in women with obesity is associated with lower success rates than in women with normal weight. A systematic review of 27 in vitro fertilization (IVF) studies (23 of which were retrospective) reveals 10% lower live-birth rate in overweight (BMI >25 kg/m2) versus normal-weight women (BMI <25 kg/m2) undergoing IVF (odds ratio [OR], 0.90; 95% CI, 0.82−1.0).9 Data from a meta-analysis of 33 IVF studies, including 47,967 cycles, show that, compared with women with a BMI <25 kg/m2, overweight or obese women have significantly reduced rates of clinical pregnancy (relative risk [RR], 0.90; P<.0001) and live birth (RR, 0.84; P = .0002).10
Results of a retrospective study of 4,609 women undergoing first IVF or IVF/intracytoplasmic sperm injection cycles revealed impaired embryo implantation (controlling for embryo quality and transfer day), reducing the age-adjusted odds of live birth in a BMI-dependent manner by 37% (BMI, 30.0−34.9 kg/m2), 61% (BMI, 35.0−39.9 kg/m2), and 68% (BMI, >40 kg/m2) compared with women with a BMI of 18.5 to 24.9 kg/m2.11 In a study of 12,566 Danish couples undergoing assisted reproduction, overweight and obese ovulatory women had a 12% (95% CI, 0.79−0.99) and 25% (95% CI, 0.63−0.90) reduction in IVF-related live birth rate, respectively (referent BMI, 18.5−24.9 kg/m2), with a 2% (95% CI, 0.97−0.99) decrease in live-birth rate for every one-unit increase in BMI.12 Putative mechanisms for these findings include altered oocyte morphology and reduced fertilization in eggs from obese women,13 and impaired embryo quality in women less than age 35.14 Oocytes from women with a BMI >25 kg/m2 are smaller and less likely to complete development postfertilization, with embryos arrested prior to blastulation containing more triglyceride than those forming blastocysts.15
Blastocysts developed from oocytes of high-BMI women are smaller, contain fewer cells and have a higher content of triglycerides, lower glucose consumption, and altered amino acid metabolism compared with embryos of normal-weight women (BMI <24.9 kg/m2).15 Obesity may alter endometrial receptivity during IVF given the finding that third-party surrogate women with a BMI >35 kg/m2 have a lower live-birth rate (25%) compared with women with a BMI <35 kg/m2 (49%; P<.05).16
Pregnancy outcomes
Obesity is linked to an increased risk of miscarriage. Results of a meta-analysis of 33 IVF studies including 47,967 cycles indicated that overweight or obese women have a higher rate of miscarriage (RR, 1.31; P<.0001) than normal-weight women (BMI <25 kg/m2).17 Maternal and perinatal morbid obesity are strongly associated with obstetric and perinatal complications, including gestational diabetes, hypertension, preeclampsia, preterm delivery, shoulder dystocia, fetal distress, early neonatal death, and small- as well as large-for-gestational age infants.
Obese women who conceive by IVF are at increased risk for preeclampsia, gestational diabetes, preterm delivery, and cesarean delivery.13 Authors of a meta-analysis of 18 observational studies concluded that obese mothers were at increased odds of pregnancies affected by such birth defects as neural tube defects, cardiovascular anomalies, and cleft lip and palate, among others.18
In addition to being the cause of these fetal abnormalities, maternal metabolic dysfunction is linked to promoting obesity in offspring, thereby perpetuating a cycle of obesity and adverse health outcomes that include an increased risk of premature death in adult offspring in subsequent generations.13
Treatment for obesity
Lifestyle modification is the first-line treatment for obesity.
Pre-fertility therapy and pregnancy goals. Targets for pregnancy should include:
- preconception weight loss to a BMI of 35 kg/m2
- prevention of excess weight gain in pregnancy
- long-term reduction in weight.
For all obese individuals, lifestyle modifications should include a weight loss of 7% of body weight and increased physical activity to at least 150 minutes of moderate activity, such as walking, per week. Calorie restriction should be emphasized. A 500 to 1,000 kcal/day decrease from usual dietary intake is expected to result in a 1- to 2-lb weight loss per week. A low-calorie diet of 1,000 to 1,200 kcal/day can lead to an average 10% decrease in total body weight over 6 months.
Adjunct supervised medical therapy or bariatric surgery can play an important role in successful weight loss prepregnancy but are not appropriate for women actively attempting conception. Importantly, pregnancy should be deferred for a minimum of 1 year after bariatric surgery. The decision to postpone pregnancy to achieve weight loss must be balanced against the risk of declining fertility with advancing age of the woman.
Read about when to treat subclinical hypothyroidism
Optimal management of subclinical hypothyroidism in women with infertility
Practice Committee of the American Society for Reproductive Medicine. Subclinical hypothyroidism in the infertile female population: a guideline. Fertil Steril. 2015;104(3):545-553.
Thyroid disorders long have been associated with the potential for adverse reproductive outcomes. While overt hypothyroidism has been linked to infertility, increased miscarriage risk, and poor maternal and fetal outcomes, controversy has existed regarding the association between subclinical hypothyroidism (SCH) and reproductive problems. The ASRM recently published a guideline on the role of SCH in the infertile female population.
How is subclinical hypothyroidism defined?
SCH is classically defined as a thyrotropin (TSH) level above the upper limit of normal range (4.5−5.0 mIU/L) with normal free thyroxine (FT4) levels. The National Health and Nutrition Examination Survey (NHANES III) population has been used to establish normative data for TSH for a disease-free population. These include a median serum level for TSH of 1.5 mIU/L, with the corresponding 2.5 and 97.5 percentiles of 0.41 and 6.10, respectively.19 Data from the National Academy of Clinical Biochemistry, however, reveal that 95% of individuals without evidence of thyroid disease have a TSH level <2.5 mIU/L, and that the normal reference range is skewed to the right.20 Adjusting the upper limit of the normal range to 2.5 mIU/L would result in an additional 11.8% to 14.2% of the United States population (22 to 28 million individuals) being diagnosed with hypothyroidism.
This information raises several important questions.
1. Should nonpregnant women be treated for SCH?
No. There is no benefit from the standpoint of lipid profile or alteration of cardiovascular risk in the treatment of TSH levels between 5 and 10 mIU/L and, therefore, treatment of individuals with TSH <5 mIU/L is questionable. Furthermore, the risk of overtreatment resulting in bone loss is a concern. The Endocrine Society does not recommend changing the current normal TSH range for nonpregnant women.
2. What are normal TSH levels in pregnant women?
Because human chorionic gonadotropin (hCG) can bind to and affect the TSH receptor, thereby influencing TSH values, the normal range for TSH is modified in pregnancy. The Endocrine Society recommends the following pregnancy trimester guidelines for TSH levels: 2.5 mIU/L is the recommended upper limit of normal in the first trimester, 3.0 mIU/L in the second trimester, and 3.5 mIU/L in the third trimester.
3. Is untreated SCH associated with miscarriage?
There is fair evidence that SCH, defined as a TSH level >4 mIU/L during pregnancy, is associated with miscarriage, but there is insufficient evidence that TSH levels between 2.5 and 4 mIU/L are associated with miscarriage.
4. Is untreated SCH associated with infertility?
Limited data are available to assess the effect of SCH on infertility. While a few studies show an association between SCH on unexplained infertility and ovulatory disorders, SCH does not appear to be increased in other causes of infertility.
5. Is SCH associated with adverse obstetric outcomes?
Available data reveal that SCH with TSH levels outside the normal pregnancy range are associated with an increased risk of such obstetric complications as placental abruption, preterm birth, fetal death, and preterm premature rupture of membranes (PPROM). However, it is unclear if prepregnancy TSH levels between 2.5 and 4 mIU/L are associated with adverse obstetric outcomes.
6. Does untreated SCH affect developmental outcomes in children?
The fetus is solely dependent on maternal thyroid hormone in early pregnancy because the fetal thyroid does not produce thyroid hormone before 10 to 13 weeks of gestation. Significant evidence has associated untreated maternal hypothyroidism with delayed fetal neurologic development, impaired school performance, and lower intelligence quotient (IQ) among offspring.21 There is fair evidence that SCH diagnosed in pregnancy is associated with adverse neurologic development. There is no evidence that SCH prior to pregnancy is associated with adverse neurodevelopmental outcomes. It should be noted that only one study has examined whether treatment of SCH improves developmental outcomes (measured by IQ scored at age 3 years) and no significant differences were observed in women with SCH who were treated with levothyroxine versus those who were not.22
7. Does treatment of SCH improve miscarriage rates, live-birth rates, and/or clinical pregnancy rates?
Small randomized controlled studies of women undergoing infertility treatment and a few observational studies in the general population yield good evidence that levothyroxine treatment in women with SCH defined as TSH >4.0 mIU/L is associated with improvement in pregnancy, live birth, and miscarriage rates. There are no randomized trials assessing whether levothyroxine treatment in women with TSH levels between 2.5 and 4 mIU/L would yield similar benefits to those observed in women with TSH levels above 4 mIU/L.
8. Are thyroid antibodies associated with infertility or adverse reproductive outcomes?
There is good evidence that the thyroid autoimmunity, or the presence of TPO-Ab, is associated with miscarriage and fair evidence that it is associated with infertility. Treatment with levothyroxine may improve pregnancy outcomes especially if the TSH level is above 2.5 mIU/L.
9. Should there be universal screening for hypothyroidism in the first trimester of pregnancy?
Current evidence does not reveal a benefit of universal screening at this time. The American College of Obstetricians and Gynecologists does not recommend routine screening for hypothyroidism in pregnancy unless women have risk factors for thyroid disease, including a personal or family history of thyroid disease, physical findings or symptoms of goiter or hypothyroidism, type 1 diabetes mellitus, infertility, history of miscarriage or preterm delivery, and/or personal or family history of autoimmune disease.
The bottom line
SCH, defined as a TSH level greater than the upper limit of normal range (4.5−5.0 mIU/L)with normal FT4 levels, is associated with adverse reproductive outcomes including miscarriage, pregnancy complications, and delayed fetal neurodevelopment. Thyroid supplementation is beneficial; however, treatment has not been shown to improve long-term neurologic developmental outcomes in offspring. Data are limited on whether TSH values between 2.5 mIU/L and the upper range of normal are associated with adverse pregnancy outcomes and therefore treatment in this group remains controversial. Although available evidence is weak, there may be a benefit in some subgroups, and because risk is minimal, it may be reasonable to treat or to monitor levels and treat above nonpregnant and pregnancy ranges. There is fair evidence that thyroid autoimmunity (positive thyroid antibody) is associated with miscarriage and infertility. Levothyroxine therapy may improve pregnancy outcomes especially if the TSH level is above 2.5 mIU/L. While universal screening of thyroid function in pregnancy is not recommended, women at high risk for thyroid disease should be screened.23
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Pasquali R, Pelusi C, Genghini S, Cacciari M, Gambineri A. Obesity and reproductive disorders in women. Hum Reprod Update. 2003;9(4):359-372.
- Greisen S, Ledet T, Møller N, et al. Effects of leptin on basal and FSH stimulated steroidogenesis in human granulosa luteal cells. Acta Obstet Gynecol Scand. 2000;79(11):931-935.
- Rich-Edwards JW, Goldman MB, Willett WC, et al. Adolescent body mass index and infertility caused by ovulatory disorder. Am J Obstet Gynecol. 1994;171(1):171-177.
- Grodstein F, Goldman MB, Cramer DW. Body mass index and ovulatory infertility. Epidemiology. 1994;5(2):247-250.
- Gesink Law DC, Maclehose RF, Longnecker MP. Obesity and time to pregnancy. Hum Reprod. 2007;22(2):414-420.
- Jain A, Polotsky AJ, Rochester D, et al. Pulsatile luteinizing hormone amplitude and progesterone metabolite excretion are reduced in obese women. J Clin Endocrinol Metab. 2007;92(7):2468-2473.
- Lake JK, Power C, Cole TJ. Women's reproductive health: the role of body mass index in early and adult life. Int J Obes Relat Metab Disord. 1997;21(6):432-438.
- van der Steeg JW, Steures P, Eijkemans MJ, et al. Obesity affects spontaneous pregnancy chances in subfertile, ovulatory women. Hum Reprod. 2008;23(2):324-328.
- Koning AM, Mutsaerts MA, Kuchenbecker WK, et al. Complications and outcome of assisted reproduction technologies in overweight and obese women [Published correction appears in Hum Reprod. 2012;27(8):2570.] Hum Reprod. 2012;27(2):457-467.
- Rittenberg V, Seshadri S, Sunkara SK, Sobaleva S, Oteng-Ntim E, El-Toukhy T. Effect of body mass index on IVF treatment outcome: an updated systematic review and meta-analysis. Reprod Biomed Online. 2011;23(4):421-439.
- Moragianni VA, Jones SM, Ryley DA. The effect of body mass index on the outcomes of first assisted reproductive technology cycles. Fertil Steril. 2012;98(1):102-108.
- Petersen GL, Schmidt L, Pinborg A, Kamper-Jørgensen M. The influence of female and male body mass index on live births after assisted reproductive technology treatment: a nationwide register-based cohort study. Fertil Steril. 2013;99(6):1654-1662.
- Practice Committee of the American Society for Reproductive Medicine. Obesity and Reproduction: A committee opinion. Fertil Steril. 2015;104(5):1116-1126.
- Metwally M, Cutting R, Tipton A, Skull J, Ledger WL, Li TC. Effect of increased body mass index on oocyte and embryo quality in IVF patients. Reprod Biomed Online. 2007;15(5):532-538.
- Leary C, Leese HJ, Sturmey RG. Human embryos from overweight and obese women display phenotypic and metabolic abnormalities. Hum Reprod. 2015;30(1):122-132.
- Deugarte D, Deugarte C, Sahakian V. Surrogate obesity negatively impacts pregnancy rates in third-party reproduction. Fertil Steril. 2010;93(3):1008-1010.
- Rittenberg V, Seshadri S, Sunkara SK, Sobaleva S, Oteng-Ntim E, El-Toukhy T. Effect of body mass index on IVF treatment outcome: an updated systematic review and meta-analysis. Reprod Biomed Online. 2011;23(4):421-439.
- Stothard KJ, Tennant PWG, Bell R, Rankin J. Maternal overweight and obesity and the risk of congenital anomalies: a systematic review and meta-analysis. JAMA. 2009;301(6):636-650.
- Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489-499.
- Baloch Z, Carayon P, Conte-Devolx B, et al. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid. 2003;13(1):3-126.
- Pop VJ, Kuijpens JL, van Baar AL, et al. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin Endocrinol (Oxf). 1999;50(2):149-155.
- Lazarus JH, Bestwick JP, Channon S, et al. Antenatal thyroid screening and childhood cognitive function. N Engl J Med. 2012;366(17):493-501.
- Practice Committee of the American Society for Reproductive Medicine. Subclinical hypothyroidism in the infertile female population: a guideline. Fertil Steril. 2015;104(3):545-553.
Zika virus is a serious problem. Education and infection prevention are critical to effective management, and why we chose to include Zika virus as a topic for this year’s Update. We also discuss obesity’s effects on reproduction—a very relevant concern for all ObGyns and patients alike as about half of reproductive-age women are obese. Finally, subclinical hypothyroidism can present unique management challenges, such as determining when it is present and when treatment is indicated.
Read about counseling patients about Zika virus
Managing attempted pregnancy in the era of Zika virus
Oduyebo T, Igbinosa I, Petersen EE, et al. Update: interim guidance for health care providers caring for pregnant women with possible Zika virus exposure--United States, July 2016. MMWR Morb Mortal Wkly Rep. 2016;65(29):739-744.
Petersen EE, Meaney-Delman D, Neblett-Fanfair R, et al. Update: interim guidance for preconception counseling and prevention of sexual transmission of Zika virus for persons with possible Zika virus exposure--United States, September 2016. MMWR Morb Mortal Wkly Rep. 2016;65(39):1077-1081.
US Food and Drug Administration. Donor Screening Recommendations to Reduce the Risk of Transmission of Zika Virus by Human Cells, Tissues, and Cellular and Tissue-Based Products. http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Tissue/UCM488582.pdf. Published March 2016. Accessed January 12, 2017.
National Institutes of Health. Zika: Overview. https://www.nichd.nih.gov/health/topics/zika/Pages/default.aspx. Accessed January 12, 2017.
World Health Organization. Prevention of sexual transmission of Zika virus interim guidance. WHO reference number: WHO/ZIKV/MOC/16. 1 Rev. 3, September 6, 2016.
Zika Virus Guidance Task Force of the American Society for Reproductive Medicine. Rev. 13, September 2016.
Zika virus presents unique challenges to physicians managing the care of patients attempting pregnancy, with or without fertility treatment. Neonatal Zika virus infection sequelae only recently have been appreciated; microcephaly was associated with Zika virus in October 2015, followed by other neurologic conditions including brain abnormalities, neural tube defects, and eye abnormalities. Results of recent studies involving the US Zika Pregnancy Registry show that 6% of women with Zika at any time in pregnancy had affected babies, but 11% of those who contracted the disease in the first trimester were affected.
Diagnosis is difficult because symptoms are generally mild, with 80% of affected patients asymptomatic. Possible Zika virus exposure is defined as travel to or residence in an area of active Zika virus transmission, or sex without a condom with a partner who traveled to or lived in an area of active transmission. Much is unknown about the interval from exposure to symptoms. Testing availability is limited and variable, and much is unknown about sensitivity and specificity of direct viral RNA testing, appearance and disappearance of detectable immunoglobulin (Ig) M and IgG antibodies that affect false positive and false negative test results, duration of infectious phase, risk of transmission, and numerous other factors.
Positive serum viral testing likely indicates virus in semen or other bodily fluids, but a negative serum viral test cannot definitively preclude virus in other bodily fluids. Zika virus likely can be passed from any combination of semen and vaginal and cervical fluids, but validating tests for these fluids are not yet available. It is not known if sperm preparation and assisted reproductive technology (ART) procedures that minimize risk of HIV transmission are effective against Zika virus or whether or not cryopreservation can destroy the virus.
Pregnancy timing
The Centers for Disease Control and Prevention now recommends that all men with possible Zika virus exposure who are considering attempting pregnancy with their partner wait to get pregnant until at least 6 months after symptom onset (if symptomatic) or last possible Zika virus exposure (if asymptomatic). Women with possible Zika virus exposure are recommended to wait to get pregnant until at least 8 weeks after symptom onset (if symptomatic) or last possible Zika virus exposure (if asymptomatic).
Women and men with possible exposure to Zika virus but without clinical symptoms of illness should consider testing for Zika viral RNA within 2 weeks of suspected exposure and wait at least 8 weeks after the last date of exposure before being re-tested. If direct viral testing (using rRT-PCR) results initially are negative, ideally, antibody testing would be obtained, if available, at 8 weeks. However, no testing paradigm will absolutely guarantee lack of Zika virus infectivity.
Virus management problems are dramatically compounded in areas endemic for Zika. Women and men who have had Zika virus disease should wait at least 6 months after illness onset to attempt reproduction. The temporal relationship between the presence of viral RNA and infectivity is not known definitively, and so the absolute duration of time to wait before attempting pregnancy is unknown. Male and female partners who become infected should avoid all forms of intimate sexual conduct or use condoms for the same 6 months. There is no evidence Zika will cause congenital infection in pregnancies initiated after resolution of maternal Zika viremia. However, any testing performed at a time other than the time of treatment might not reflect true viral status, particularly in areas of active Zika virus transmission.
Prevention
Women and men, especially those residing in areas of active Zika virus transmission, should talk with their physicians regarding pregnancy plans and avoid mosquito bites using the usual precautions: avoid mosquito areas, drain standing water, use mosquito repellent containing DEET, and use mosquito netting. Some people have gone so far as to relocate to nonendemic areas.
Those contemplating pregnancy should be advised to consider what they would do if they become exposed to or have suspected or confirmed Zika virus during pregnancy. Additional considerations are gamete or embryo cryopreservation and quarantine until a subsequent rRT-PCR test result is negative in both the male and female and at least 8 weeks have passed from gamete collection.

Patient counseling essentials
Counsel patients considering reproduction about:
- Zika virus as a new reproductive hazard
- the significance of the hazard to the fetus if infected
- the areas of active transmission, and that they are constantly changing
- avoidance of Zika areas if possible
- methods of transmission through mosquito bites or sex
- avoidance of mosquito bites
- symptoms of Zika infection
- safe sex practices
- testing limitations and knowledge deficiency about Zika.
Not uncommonly, clinical situations require complex individualized management decisions regarding trade-offs of risks, especially in older patients with decreased ovarian reserve. Consultation with infectious disease and reproductive specialists should be obtained when complicated and consequential decisions have to be made.
All practitioners should inform their patients, especially those undergoing fertility treatments, about Zika, and develop language in their informed consent that conveys the gap in knowledge to these patients.
Read how obesity specifically affects reproduction in an adverse way
Obesity adversely affects reproduction, but how specifically?
Practice Committee of the American Society for Reproductive Medicine. Obesity and Reproduction: A committee opinion. Fertil Steril. 2015;104(5):1116-1126.
The prevalence of obesity has increased substantially over the past 2 decades. Almost two-thirds of women and three-fourths of men in the United States are overweight or obese (defined as a body mass index [BMI] ≥25 kg/m2 and BMI ≥30 kg/m2, respectively; TABLE). Nearly 50% of reproductive-age women are obese.

A disease of excess body fat and insulin resistance, obesity increases the risks of hypertension, diabetes, dyslipidemia, cardiovascular disease, sleep apnea, respiratory problems, and cancer as well as other serious health problems. While not all individuals with obesity will have infertility, obesity is associated with impaired reproduction in both women and men, adverse obstetric outcomes, and health problems in offspring. The American Society for Reproductive Medicine (ASRM) reviewed this important issue in a recent practice committee opinion.
Menstrual cycle and ovulatory dysfunction
Menstrual cycle abnormalities are more common in women with obesity. Elevated levels of insulin in obese women suppress sex hormone−binding globulin (SHBG) which in turn reduces gonadotropin secretion due to increased production of estrogen from conversion of androgens by adipose aromatase.1 Adipose tissue produces adipokines, which directly can suppress ovarian function.2
Ovulatory dysfunction is common among obese women; the relative risk of such dysfunction is 3.1 (95% confidence interval [CI], 2.2−4.4) among women with BMI levels >27 kg/m2 versus BMI levels 20.0 to 24.9 kg/m2.3,4 Obesity decreases fecundity even in women with normal menstrual cycles.5 This may in part be due to altered ovulatory dynamics with reduced early follicular luteinizing hormone pulse amplitude accompanied by prolonged folliculogenesis and reduced luteal progesterone levels.6
Compared with normal-weight women, obese women have a lower chance of conception within 1 year of stopping contraception; about 66% of obese women conceive within 1 year of stopping contraception, compared with about 81% of women with normal weight.7 Results of a Dutch study of 3,029 women with regular ovulation, at least one patent tube, and a partner with a normal semen analysis indicated a direct correlation between obesity and delayed conception, with a 4% lower spontaneous pregnancy rate per kg/m2 increase in women with a BMI >29 kg/m2 versus a BMI of 21 to 29 kg/m2 (hazard ratio, 0.96; 95% CI, 0.91−0.99).8
Assisted reproduction
Assisted reproduction in women with obesity is associated with lower success rates than in women with normal weight. A systematic review of 27 in vitro fertilization (IVF) studies (23 of which were retrospective) reveals 10% lower live-birth rate in overweight (BMI >25 kg/m2) versus normal-weight women (BMI <25 kg/m2) undergoing IVF (odds ratio [OR], 0.90; 95% CI, 0.82−1.0).9 Data from a meta-analysis of 33 IVF studies, including 47,967 cycles, show that, compared with women with a BMI <25 kg/m2, overweight or obese women have significantly reduced rates of clinical pregnancy (relative risk [RR], 0.90; P<.0001) and live birth (RR, 0.84; P = .0002).10
Results of a retrospective study of 4,609 women undergoing first IVF or IVF/intracytoplasmic sperm injection cycles revealed impaired embryo implantation (controlling for embryo quality and transfer day), reducing the age-adjusted odds of live birth in a BMI-dependent manner by 37% (BMI, 30.0−34.9 kg/m2), 61% (BMI, 35.0−39.9 kg/m2), and 68% (BMI, >40 kg/m2) compared with women with a BMI of 18.5 to 24.9 kg/m2.11 In a study of 12,566 Danish couples undergoing assisted reproduction, overweight and obese ovulatory women had a 12% (95% CI, 0.79−0.99) and 25% (95% CI, 0.63−0.90) reduction in IVF-related live birth rate, respectively (referent BMI, 18.5−24.9 kg/m2), with a 2% (95% CI, 0.97−0.99) decrease in live-birth rate for every one-unit increase in BMI.12 Putative mechanisms for these findings include altered oocyte morphology and reduced fertilization in eggs from obese women,13 and impaired embryo quality in women less than age 35.14 Oocytes from women with a BMI >25 kg/m2 are smaller and less likely to complete development postfertilization, with embryos arrested prior to blastulation containing more triglyceride than those forming blastocysts.15
Blastocysts developed from oocytes of high-BMI women are smaller, contain fewer cells and have a higher content of triglycerides, lower glucose consumption, and altered amino acid metabolism compared with embryos of normal-weight women (BMI <24.9 kg/m2).15 Obesity may alter endometrial receptivity during IVF given the finding that third-party surrogate women with a BMI >35 kg/m2 have a lower live-birth rate (25%) compared with women with a BMI <35 kg/m2 (49%; P<.05).16
Pregnancy outcomes
Obesity is linked to an increased risk of miscarriage. Results of a meta-analysis of 33 IVF studies including 47,967 cycles indicated that overweight or obese women have a higher rate of miscarriage (RR, 1.31; P<.0001) than normal-weight women (BMI <25 kg/m2).17 Maternal and perinatal morbid obesity are strongly associated with obstetric and perinatal complications, including gestational diabetes, hypertension, preeclampsia, preterm delivery, shoulder dystocia, fetal distress, early neonatal death, and small- as well as large-for-gestational age infants.
Obese women who conceive by IVF are at increased risk for preeclampsia, gestational diabetes, preterm delivery, and cesarean delivery.13 Authors of a meta-analysis of 18 observational studies concluded that obese mothers were at increased odds of pregnancies affected by such birth defects as neural tube defects, cardiovascular anomalies, and cleft lip and palate, among others.18
In addition to being the cause of these fetal abnormalities, maternal metabolic dysfunction is linked to promoting obesity in offspring, thereby perpetuating a cycle of obesity and adverse health outcomes that include an increased risk of premature death in adult offspring in subsequent generations.13
Treatment for obesity
Lifestyle modification is the first-line treatment for obesity.
Pre-fertility therapy and pregnancy goals. Targets for pregnancy should include:
- preconception weight loss to a BMI of 35 kg/m2
- prevention of excess weight gain in pregnancy
- long-term reduction in weight.
For all obese individuals, lifestyle modifications should include a weight loss of 7% of body weight and increased physical activity to at least 150 minutes of moderate activity, such as walking, per week. Calorie restriction should be emphasized. A 500 to 1,000 kcal/day decrease from usual dietary intake is expected to result in a 1- to 2-lb weight loss per week. A low-calorie diet of 1,000 to 1,200 kcal/day can lead to an average 10% decrease in total body weight over 6 months.
Adjunct supervised medical therapy or bariatric surgery can play an important role in successful weight loss prepregnancy but are not appropriate for women actively attempting conception. Importantly, pregnancy should be deferred for a minimum of 1 year after bariatric surgery. The decision to postpone pregnancy to achieve weight loss must be balanced against the risk of declining fertility with advancing age of the woman.
Read about when to treat subclinical hypothyroidism
Optimal management of subclinical hypothyroidism in women with infertility
Practice Committee of the American Society for Reproductive Medicine. Subclinical hypothyroidism in the infertile female population: a guideline. Fertil Steril. 2015;104(3):545-553.
Thyroid disorders long have been associated with the potential for adverse reproductive outcomes. While overt hypothyroidism has been linked to infertility, increased miscarriage risk, and poor maternal and fetal outcomes, controversy has existed regarding the association between subclinical hypothyroidism (SCH) and reproductive problems. The ASRM recently published a guideline on the role of SCH in the infertile female population.
How is subclinical hypothyroidism defined?
SCH is classically defined as a thyrotropin (TSH) level above the upper limit of normal range (4.5−5.0 mIU/L) with normal free thyroxine (FT4) levels. The National Health and Nutrition Examination Survey (NHANES III) population has been used to establish normative data for TSH for a disease-free population. These include a median serum level for TSH of 1.5 mIU/L, with the corresponding 2.5 and 97.5 percentiles of 0.41 and 6.10, respectively.19 Data from the National Academy of Clinical Biochemistry, however, reveal that 95% of individuals without evidence of thyroid disease have a TSH level <2.5 mIU/L, and that the normal reference range is skewed to the right.20 Adjusting the upper limit of the normal range to 2.5 mIU/L would result in an additional 11.8% to 14.2% of the United States population (22 to 28 million individuals) being diagnosed with hypothyroidism.
This information raises several important questions.
1. Should nonpregnant women be treated for SCH?
No. There is no benefit from the standpoint of lipid profile or alteration of cardiovascular risk in the treatment of TSH levels between 5 and 10 mIU/L and, therefore, treatment of individuals with TSH <5 mIU/L is questionable. Furthermore, the risk of overtreatment resulting in bone loss is a concern. The Endocrine Society does not recommend changing the current normal TSH range for nonpregnant women.
2. What are normal TSH levels in pregnant women?
Because human chorionic gonadotropin (hCG) can bind to and affect the TSH receptor, thereby influencing TSH values, the normal range for TSH is modified in pregnancy. The Endocrine Society recommends the following pregnancy trimester guidelines for TSH levels: 2.5 mIU/L is the recommended upper limit of normal in the first trimester, 3.0 mIU/L in the second trimester, and 3.5 mIU/L in the third trimester.
3. Is untreated SCH associated with miscarriage?
There is fair evidence that SCH, defined as a TSH level >4 mIU/L during pregnancy, is associated with miscarriage, but there is insufficient evidence that TSH levels between 2.5 and 4 mIU/L are associated with miscarriage.
4. Is untreated SCH associated with infertility?
Limited data are available to assess the effect of SCH on infertility. While a few studies show an association between SCH on unexplained infertility and ovulatory disorders, SCH does not appear to be increased in other causes of infertility.
5. Is SCH associated with adverse obstetric outcomes?
Available data reveal that SCH with TSH levels outside the normal pregnancy range are associated with an increased risk of such obstetric complications as placental abruption, preterm birth, fetal death, and preterm premature rupture of membranes (PPROM). However, it is unclear if prepregnancy TSH levels between 2.5 and 4 mIU/L are associated with adverse obstetric outcomes.
6. Does untreated SCH affect developmental outcomes in children?
The fetus is solely dependent on maternal thyroid hormone in early pregnancy because the fetal thyroid does not produce thyroid hormone before 10 to 13 weeks of gestation. Significant evidence has associated untreated maternal hypothyroidism with delayed fetal neurologic development, impaired school performance, and lower intelligence quotient (IQ) among offspring.21 There is fair evidence that SCH diagnosed in pregnancy is associated with adverse neurologic development. There is no evidence that SCH prior to pregnancy is associated with adverse neurodevelopmental outcomes. It should be noted that only one study has examined whether treatment of SCH improves developmental outcomes (measured by IQ scored at age 3 years) and no significant differences were observed in women with SCH who were treated with levothyroxine versus those who were not.22
7. Does treatment of SCH improve miscarriage rates, live-birth rates, and/or clinical pregnancy rates?
Small randomized controlled studies of women undergoing infertility treatment and a few observational studies in the general population yield good evidence that levothyroxine treatment in women with SCH defined as TSH >4.0 mIU/L is associated with improvement in pregnancy, live birth, and miscarriage rates. There are no randomized trials assessing whether levothyroxine treatment in women with TSH levels between 2.5 and 4 mIU/L would yield similar benefits to those observed in women with TSH levels above 4 mIU/L.
8. Are thyroid antibodies associated with infertility or adverse reproductive outcomes?
There is good evidence that the thyroid autoimmunity, or the presence of TPO-Ab, is associated with miscarriage and fair evidence that it is associated with infertility. Treatment with levothyroxine may improve pregnancy outcomes especially if the TSH level is above 2.5 mIU/L.
9. Should there be universal screening for hypothyroidism in the first trimester of pregnancy?
Current evidence does not reveal a benefit of universal screening at this time. The American College of Obstetricians and Gynecologists does not recommend routine screening for hypothyroidism in pregnancy unless women have risk factors for thyroid disease, including a personal or family history of thyroid disease, physical findings or symptoms of goiter or hypothyroidism, type 1 diabetes mellitus, infertility, history of miscarriage or preterm delivery, and/or personal or family history of autoimmune disease.
The bottom line
SCH, defined as a TSH level greater than the upper limit of normal range (4.5−5.0 mIU/L)with normal FT4 levels, is associated with adverse reproductive outcomes including miscarriage, pregnancy complications, and delayed fetal neurodevelopment. Thyroid supplementation is beneficial; however, treatment has not been shown to improve long-term neurologic developmental outcomes in offspring. Data are limited on whether TSH values between 2.5 mIU/L and the upper range of normal are associated with adverse pregnancy outcomes and therefore treatment in this group remains controversial. Although available evidence is weak, there may be a benefit in some subgroups, and because risk is minimal, it may be reasonable to treat or to monitor levels and treat above nonpregnant and pregnancy ranges. There is fair evidence that thyroid autoimmunity (positive thyroid antibody) is associated with miscarriage and infertility. Levothyroxine therapy may improve pregnancy outcomes especially if the TSH level is above 2.5 mIU/L. While universal screening of thyroid function in pregnancy is not recommended, women at high risk for thyroid disease should be screened.23
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Zika virus is a serious problem. Education and infection prevention are critical to effective management, and why we chose to include Zika virus as a topic for this year’s Update. We also discuss obesity’s effects on reproduction—a very relevant concern for all ObGyns and patients alike as about half of reproductive-age women are obese. Finally, subclinical hypothyroidism can present unique management challenges, such as determining when it is present and when treatment is indicated.
Read about counseling patients about Zika virus
Managing attempted pregnancy in the era of Zika virus
Oduyebo T, Igbinosa I, Petersen EE, et al. Update: interim guidance for health care providers caring for pregnant women with possible Zika virus exposure--United States, July 2016. MMWR Morb Mortal Wkly Rep. 2016;65(29):739-744.
Petersen EE, Meaney-Delman D, Neblett-Fanfair R, et al. Update: interim guidance for preconception counseling and prevention of sexual transmission of Zika virus for persons with possible Zika virus exposure--United States, September 2016. MMWR Morb Mortal Wkly Rep. 2016;65(39):1077-1081.
US Food and Drug Administration. Donor Screening Recommendations to Reduce the Risk of Transmission of Zika Virus by Human Cells, Tissues, and Cellular and Tissue-Based Products. http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Tissue/UCM488582.pdf. Published March 2016. Accessed January 12, 2017.
National Institutes of Health. Zika: Overview. https://www.nichd.nih.gov/health/topics/zika/Pages/default.aspx. Accessed January 12, 2017.
World Health Organization. Prevention of sexual transmission of Zika virus interim guidance. WHO reference number: WHO/ZIKV/MOC/16. 1 Rev. 3, September 6, 2016.
Zika Virus Guidance Task Force of the American Society for Reproductive Medicine. Rev. 13, September 2016.
Zika virus presents unique challenges to physicians managing the care of patients attempting pregnancy, with or without fertility treatment. Neonatal Zika virus infection sequelae only recently have been appreciated; microcephaly was associated with Zika virus in October 2015, followed by other neurologic conditions including brain abnormalities, neural tube defects, and eye abnormalities. Results of recent studies involving the US Zika Pregnancy Registry show that 6% of women with Zika at any time in pregnancy had affected babies, but 11% of those who contracted the disease in the first trimester were affected.
Diagnosis is difficult because symptoms are generally mild, with 80% of affected patients asymptomatic. Possible Zika virus exposure is defined as travel to or residence in an area of active Zika virus transmission, or sex without a condom with a partner who traveled to or lived in an area of active transmission. Much is unknown about the interval from exposure to symptoms. Testing availability is limited and variable, and much is unknown about sensitivity and specificity of direct viral RNA testing, appearance and disappearance of detectable immunoglobulin (Ig) M and IgG antibodies that affect false positive and false negative test results, duration of infectious phase, risk of transmission, and numerous other factors.
Positive serum viral testing likely indicates virus in semen or other bodily fluids, but a negative serum viral test cannot definitively preclude virus in other bodily fluids. Zika virus likely can be passed from any combination of semen and vaginal and cervical fluids, but validating tests for these fluids are not yet available. It is not known if sperm preparation and assisted reproductive technology (ART) procedures that minimize risk of HIV transmission are effective against Zika virus or whether or not cryopreservation can destroy the virus.
Pregnancy timing
The Centers for Disease Control and Prevention now recommends that all men with possible Zika virus exposure who are considering attempting pregnancy with their partner wait to get pregnant until at least 6 months after symptom onset (if symptomatic) or last possible Zika virus exposure (if asymptomatic). Women with possible Zika virus exposure are recommended to wait to get pregnant until at least 8 weeks after symptom onset (if symptomatic) or last possible Zika virus exposure (if asymptomatic).
Women and men with possible exposure to Zika virus but without clinical symptoms of illness should consider testing for Zika viral RNA within 2 weeks of suspected exposure and wait at least 8 weeks after the last date of exposure before being re-tested. If direct viral testing (using rRT-PCR) results initially are negative, ideally, antibody testing would be obtained, if available, at 8 weeks. However, no testing paradigm will absolutely guarantee lack of Zika virus infectivity.
Virus management problems are dramatically compounded in areas endemic for Zika. Women and men who have had Zika virus disease should wait at least 6 months after illness onset to attempt reproduction. The temporal relationship between the presence of viral RNA and infectivity is not known definitively, and so the absolute duration of time to wait before attempting pregnancy is unknown. Male and female partners who become infected should avoid all forms of intimate sexual conduct or use condoms for the same 6 months. There is no evidence Zika will cause congenital infection in pregnancies initiated after resolution of maternal Zika viremia. However, any testing performed at a time other than the time of treatment might not reflect true viral status, particularly in areas of active Zika virus transmission.
Prevention
Women and men, especially those residing in areas of active Zika virus transmission, should talk with their physicians regarding pregnancy plans and avoid mosquito bites using the usual precautions: avoid mosquito areas, drain standing water, use mosquito repellent containing DEET, and use mosquito netting. Some people have gone so far as to relocate to nonendemic areas.
Those contemplating pregnancy should be advised to consider what they would do if they become exposed to or have suspected or confirmed Zika virus during pregnancy. Additional considerations are gamete or embryo cryopreservation and quarantine until a subsequent rRT-PCR test result is negative in both the male and female and at least 8 weeks have passed from gamete collection.

Patient counseling essentials
Counsel patients considering reproduction about:
- Zika virus as a new reproductive hazard
- the significance of the hazard to the fetus if infected
- the areas of active transmission, and that they are constantly changing
- avoidance of Zika areas if possible
- methods of transmission through mosquito bites or sex
- avoidance of mosquito bites
- symptoms of Zika infection
- safe sex practices
- testing limitations and knowledge deficiency about Zika.
Not uncommonly, clinical situations require complex individualized management decisions regarding trade-offs of risks, especially in older patients with decreased ovarian reserve. Consultation with infectious disease and reproductive specialists should be obtained when complicated and consequential decisions have to be made.
All practitioners should inform their patients, especially those undergoing fertility treatments, about Zika, and develop language in their informed consent that conveys the gap in knowledge to these patients.
Read how obesity specifically affects reproduction in an adverse way
Obesity adversely affects reproduction, but how specifically?
Practice Committee of the American Society for Reproductive Medicine. Obesity and Reproduction: A committee opinion. Fertil Steril. 2015;104(5):1116-1126.
The prevalence of obesity has increased substantially over the past 2 decades. Almost two-thirds of women and three-fourths of men in the United States are overweight or obese (defined as a body mass index [BMI] ≥25 kg/m2 and BMI ≥30 kg/m2, respectively; TABLE). Nearly 50% of reproductive-age women are obese.

A disease of excess body fat and insulin resistance, obesity increases the risks of hypertension, diabetes, dyslipidemia, cardiovascular disease, sleep apnea, respiratory problems, and cancer as well as other serious health problems. While not all individuals with obesity will have infertility, obesity is associated with impaired reproduction in both women and men, adverse obstetric outcomes, and health problems in offspring. The American Society for Reproductive Medicine (ASRM) reviewed this important issue in a recent practice committee opinion.
Menstrual cycle and ovulatory dysfunction
Menstrual cycle abnormalities are more common in women with obesity. Elevated levels of insulin in obese women suppress sex hormone−binding globulin (SHBG) which in turn reduces gonadotropin secretion due to increased production of estrogen from conversion of androgens by adipose aromatase.1 Adipose tissue produces adipokines, which directly can suppress ovarian function.2
Ovulatory dysfunction is common among obese women; the relative risk of such dysfunction is 3.1 (95% confidence interval [CI], 2.2−4.4) among women with BMI levels >27 kg/m2 versus BMI levels 20.0 to 24.9 kg/m2.3,4 Obesity decreases fecundity even in women with normal menstrual cycles.5 This may in part be due to altered ovulatory dynamics with reduced early follicular luteinizing hormone pulse amplitude accompanied by prolonged folliculogenesis and reduced luteal progesterone levels.6
Compared with normal-weight women, obese women have a lower chance of conception within 1 year of stopping contraception; about 66% of obese women conceive within 1 year of stopping contraception, compared with about 81% of women with normal weight.7 Results of a Dutch study of 3,029 women with regular ovulation, at least one patent tube, and a partner with a normal semen analysis indicated a direct correlation between obesity and delayed conception, with a 4% lower spontaneous pregnancy rate per kg/m2 increase in women with a BMI >29 kg/m2 versus a BMI of 21 to 29 kg/m2 (hazard ratio, 0.96; 95% CI, 0.91−0.99).8
Assisted reproduction
Assisted reproduction in women with obesity is associated with lower success rates than in women with normal weight. A systematic review of 27 in vitro fertilization (IVF) studies (23 of which were retrospective) reveals 10% lower live-birth rate in overweight (BMI >25 kg/m2) versus normal-weight women (BMI <25 kg/m2) undergoing IVF (odds ratio [OR], 0.90; 95% CI, 0.82−1.0).9 Data from a meta-analysis of 33 IVF studies, including 47,967 cycles, show that, compared with women with a BMI <25 kg/m2, overweight or obese women have significantly reduced rates of clinical pregnancy (relative risk [RR], 0.90; P<.0001) and live birth (RR, 0.84; P = .0002).10
Results of a retrospective study of 4,609 women undergoing first IVF or IVF/intracytoplasmic sperm injection cycles revealed impaired embryo implantation (controlling for embryo quality and transfer day), reducing the age-adjusted odds of live birth in a BMI-dependent manner by 37% (BMI, 30.0−34.9 kg/m2), 61% (BMI, 35.0−39.9 kg/m2), and 68% (BMI, >40 kg/m2) compared with women with a BMI of 18.5 to 24.9 kg/m2.11 In a study of 12,566 Danish couples undergoing assisted reproduction, overweight and obese ovulatory women had a 12% (95% CI, 0.79−0.99) and 25% (95% CI, 0.63−0.90) reduction in IVF-related live birth rate, respectively (referent BMI, 18.5−24.9 kg/m2), with a 2% (95% CI, 0.97−0.99) decrease in live-birth rate for every one-unit increase in BMI.12 Putative mechanisms for these findings include altered oocyte morphology and reduced fertilization in eggs from obese women,13 and impaired embryo quality in women less than age 35.14 Oocytes from women with a BMI >25 kg/m2 are smaller and less likely to complete development postfertilization, with embryos arrested prior to blastulation containing more triglyceride than those forming blastocysts.15
Blastocysts developed from oocytes of high-BMI women are smaller, contain fewer cells and have a higher content of triglycerides, lower glucose consumption, and altered amino acid metabolism compared with embryos of normal-weight women (BMI <24.9 kg/m2).15 Obesity may alter endometrial receptivity during IVF given the finding that third-party surrogate women with a BMI >35 kg/m2 have a lower live-birth rate (25%) compared with women with a BMI <35 kg/m2 (49%; P<.05).16
Pregnancy outcomes
Obesity is linked to an increased risk of miscarriage. Results of a meta-analysis of 33 IVF studies including 47,967 cycles indicated that overweight or obese women have a higher rate of miscarriage (RR, 1.31; P<.0001) than normal-weight women (BMI <25 kg/m2).17 Maternal and perinatal morbid obesity are strongly associated with obstetric and perinatal complications, including gestational diabetes, hypertension, preeclampsia, preterm delivery, shoulder dystocia, fetal distress, early neonatal death, and small- as well as large-for-gestational age infants.
Obese women who conceive by IVF are at increased risk for preeclampsia, gestational diabetes, preterm delivery, and cesarean delivery.13 Authors of a meta-analysis of 18 observational studies concluded that obese mothers were at increased odds of pregnancies affected by such birth defects as neural tube defects, cardiovascular anomalies, and cleft lip and palate, among others.18
In addition to being the cause of these fetal abnormalities, maternal metabolic dysfunction is linked to promoting obesity in offspring, thereby perpetuating a cycle of obesity and adverse health outcomes that include an increased risk of premature death in adult offspring in subsequent generations.13
Treatment for obesity
Lifestyle modification is the first-line treatment for obesity.
Pre-fertility therapy and pregnancy goals. Targets for pregnancy should include:
- preconception weight loss to a BMI of 35 kg/m2
- prevention of excess weight gain in pregnancy
- long-term reduction in weight.
For all obese individuals, lifestyle modifications should include a weight loss of 7% of body weight and increased physical activity to at least 150 minutes of moderate activity, such as walking, per week. Calorie restriction should be emphasized. A 500 to 1,000 kcal/day decrease from usual dietary intake is expected to result in a 1- to 2-lb weight loss per week. A low-calorie diet of 1,000 to 1,200 kcal/day can lead to an average 10% decrease in total body weight over 6 months.
Adjunct supervised medical therapy or bariatric surgery can play an important role in successful weight loss prepregnancy but are not appropriate for women actively attempting conception. Importantly, pregnancy should be deferred for a minimum of 1 year after bariatric surgery. The decision to postpone pregnancy to achieve weight loss must be balanced against the risk of declining fertility with advancing age of the woman.
Read about when to treat subclinical hypothyroidism
Optimal management of subclinical hypothyroidism in women with infertility
Practice Committee of the American Society for Reproductive Medicine. Subclinical hypothyroidism in the infertile female population: a guideline. Fertil Steril. 2015;104(3):545-553.
Thyroid disorders long have been associated with the potential for adverse reproductive outcomes. While overt hypothyroidism has been linked to infertility, increased miscarriage risk, and poor maternal and fetal outcomes, controversy has existed regarding the association between subclinical hypothyroidism (SCH) and reproductive problems. The ASRM recently published a guideline on the role of SCH in the infertile female population.
How is subclinical hypothyroidism defined?
SCH is classically defined as a thyrotropin (TSH) level above the upper limit of normal range (4.5−5.0 mIU/L) with normal free thyroxine (FT4) levels. The National Health and Nutrition Examination Survey (NHANES III) population has been used to establish normative data for TSH for a disease-free population. These include a median serum level for TSH of 1.5 mIU/L, with the corresponding 2.5 and 97.5 percentiles of 0.41 and 6.10, respectively.19 Data from the National Academy of Clinical Biochemistry, however, reveal that 95% of individuals without evidence of thyroid disease have a TSH level <2.5 mIU/L, and that the normal reference range is skewed to the right.20 Adjusting the upper limit of the normal range to 2.5 mIU/L would result in an additional 11.8% to 14.2% of the United States population (22 to 28 million individuals) being diagnosed with hypothyroidism.
This information raises several important questions.
1. Should nonpregnant women be treated for SCH?
No. There is no benefit from the standpoint of lipid profile or alteration of cardiovascular risk in the treatment of TSH levels between 5 and 10 mIU/L and, therefore, treatment of individuals with TSH <5 mIU/L is questionable. Furthermore, the risk of overtreatment resulting in bone loss is a concern. The Endocrine Society does not recommend changing the current normal TSH range for nonpregnant women.
2. What are normal TSH levels in pregnant women?
Because human chorionic gonadotropin (hCG) can bind to and affect the TSH receptor, thereby influencing TSH values, the normal range for TSH is modified in pregnancy. The Endocrine Society recommends the following pregnancy trimester guidelines for TSH levels: 2.5 mIU/L is the recommended upper limit of normal in the first trimester, 3.0 mIU/L in the second trimester, and 3.5 mIU/L in the third trimester.
3. Is untreated SCH associated with miscarriage?
There is fair evidence that SCH, defined as a TSH level >4 mIU/L during pregnancy, is associated with miscarriage, but there is insufficient evidence that TSH levels between 2.5 and 4 mIU/L are associated with miscarriage.
4. Is untreated SCH associated with infertility?
Limited data are available to assess the effect of SCH on infertility. While a few studies show an association between SCH on unexplained infertility and ovulatory disorders, SCH does not appear to be increased in other causes of infertility.
5. Is SCH associated with adverse obstetric outcomes?
Available data reveal that SCH with TSH levels outside the normal pregnancy range are associated with an increased risk of such obstetric complications as placental abruption, preterm birth, fetal death, and preterm premature rupture of membranes (PPROM). However, it is unclear if prepregnancy TSH levels between 2.5 and 4 mIU/L are associated with adverse obstetric outcomes.
6. Does untreated SCH affect developmental outcomes in children?
The fetus is solely dependent on maternal thyroid hormone in early pregnancy because the fetal thyroid does not produce thyroid hormone before 10 to 13 weeks of gestation. Significant evidence has associated untreated maternal hypothyroidism with delayed fetal neurologic development, impaired school performance, and lower intelligence quotient (IQ) among offspring.21 There is fair evidence that SCH diagnosed in pregnancy is associated with adverse neurologic development. There is no evidence that SCH prior to pregnancy is associated with adverse neurodevelopmental outcomes. It should be noted that only one study has examined whether treatment of SCH improves developmental outcomes (measured by IQ scored at age 3 years) and no significant differences were observed in women with SCH who were treated with levothyroxine versus those who were not.22
7. Does treatment of SCH improve miscarriage rates, live-birth rates, and/or clinical pregnancy rates?
Small randomized controlled studies of women undergoing infertility treatment and a few observational studies in the general population yield good evidence that levothyroxine treatment in women with SCH defined as TSH >4.0 mIU/L is associated with improvement in pregnancy, live birth, and miscarriage rates. There are no randomized trials assessing whether levothyroxine treatment in women with TSH levels between 2.5 and 4 mIU/L would yield similar benefits to those observed in women with TSH levels above 4 mIU/L.
8. Are thyroid antibodies associated with infertility or adverse reproductive outcomes?
There is good evidence that the thyroid autoimmunity, or the presence of TPO-Ab, is associated with miscarriage and fair evidence that it is associated with infertility. Treatment with levothyroxine may improve pregnancy outcomes especially if the TSH level is above 2.5 mIU/L.
9. Should there be universal screening for hypothyroidism in the first trimester of pregnancy?
Current evidence does not reveal a benefit of universal screening at this time. The American College of Obstetricians and Gynecologists does not recommend routine screening for hypothyroidism in pregnancy unless women have risk factors for thyroid disease, including a personal or family history of thyroid disease, physical findings or symptoms of goiter or hypothyroidism, type 1 diabetes mellitus, infertility, history of miscarriage or preterm delivery, and/or personal or family history of autoimmune disease.
The bottom line
SCH, defined as a TSH level greater than the upper limit of normal range (4.5−5.0 mIU/L)with normal FT4 levels, is associated with adverse reproductive outcomes including miscarriage, pregnancy complications, and delayed fetal neurodevelopment. Thyroid supplementation is beneficial; however, treatment has not been shown to improve long-term neurologic developmental outcomes in offspring. Data are limited on whether TSH values between 2.5 mIU/L and the upper range of normal are associated with adverse pregnancy outcomes and therefore treatment in this group remains controversial. Although available evidence is weak, there may be a benefit in some subgroups, and because risk is minimal, it may be reasonable to treat or to monitor levels and treat above nonpregnant and pregnancy ranges. There is fair evidence that thyroid autoimmunity (positive thyroid antibody) is associated with miscarriage and infertility. Levothyroxine therapy may improve pregnancy outcomes especially if the TSH level is above 2.5 mIU/L. While universal screening of thyroid function in pregnancy is not recommended, women at high risk for thyroid disease should be screened.23
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Pasquali R, Pelusi C, Genghini S, Cacciari M, Gambineri A. Obesity and reproductive disorders in women. Hum Reprod Update. 2003;9(4):359-372.
- Greisen S, Ledet T, Møller N, et al. Effects of leptin on basal and FSH stimulated steroidogenesis in human granulosa luteal cells. Acta Obstet Gynecol Scand. 2000;79(11):931-935.
- Rich-Edwards JW, Goldman MB, Willett WC, et al. Adolescent body mass index and infertility caused by ovulatory disorder. Am J Obstet Gynecol. 1994;171(1):171-177.
- Grodstein F, Goldman MB, Cramer DW. Body mass index and ovulatory infertility. Epidemiology. 1994;5(2):247-250.
- Gesink Law DC, Maclehose RF, Longnecker MP. Obesity and time to pregnancy. Hum Reprod. 2007;22(2):414-420.
- Jain A, Polotsky AJ, Rochester D, et al. Pulsatile luteinizing hormone amplitude and progesterone metabolite excretion are reduced in obese women. J Clin Endocrinol Metab. 2007;92(7):2468-2473.
- Lake JK, Power C, Cole TJ. Women's reproductive health: the role of body mass index in early and adult life. Int J Obes Relat Metab Disord. 1997;21(6):432-438.
- van der Steeg JW, Steures P, Eijkemans MJ, et al. Obesity affects spontaneous pregnancy chances in subfertile, ovulatory women. Hum Reprod. 2008;23(2):324-328.
- Koning AM, Mutsaerts MA, Kuchenbecker WK, et al. Complications and outcome of assisted reproduction technologies in overweight and obese women [Published correction appears in Hum Reprod. 2012;27(8):2570.] Hum Reprod. 2012;27(2):457-467.
- Rittenberg V, Seshadri S, Sunkara SK, Sobaleva S, Oteng-Ntim E, El-Toukhy T. Effect of body mass index on IVF treatment outcome: an updated systematic review and meta-analysis. Reprod Biomed Online. 2011;23(4):421-439.
- Moragianni VA, Jones SM, Ryley DA. The effect of body mass index on the outcomes of first assisted reproductive technology cycles. Fertil Steril. 2012;98(1):102-108.
- Petersen GL, Schmidt L, Pinborg A, Kamper-Jørgensen M. The influence of female and male body mass index on live births after assisted reproductive technology treatment: a nationwide register-based cohort study. Fertil Steril. 2013;99(6):1654-1662.
- Practice Committee of the American Society for Reproductive Medicine. Obesity and Reproduction: A committee opinion. Fertil Steril. 2015;104(5):1116-1126.
- Metwally M, Cutting R, Tipton A, Skull J, Ledger WL, Li TC. Effect of increased body mass index on oocyte and embryo quality in IVF patients. Reprod Biomed Online. 2007;15(5):532-538.
- Leary C, Leese HJ, Sturmey RG. Human embryos from overweight and obese women display phenotypic and metabolic abnormalities. Hum Reprod. 2015;30(1):122-132.
- Deugarte D, Deugarte C, Sahakian V. Surrogate obesity negatively impacts pregnancy rates in third-party reproduction. Fertil Steril. 2010;93(3):1008-1010.
- Rittenberg V, Seshadri S, Sunkara SK, Sobaleva S, Oteng-Ntim E, El-Toukhy T. Effect of body mass index on IVF treatment outcome: an updated systematic review and meta-analysis. Reprod Biomed Online. 2011;23(4):421-439.
- Stothard KJ, Tennant PWG, Bell R, Rankin J. Maternal overweight and obesity and the risk of congenital anomalies: a systematic review and meta-analysis. JAMA. 2009;301(6):636-650.
- Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489-499.
- Baloch Z, Carayon P, Conte-Devolx B, et al. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid. 2003;13(1):3-126.
- Pop VJ, Kuijpens JL, van Baar AL, et al. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin Endocrinol (Oxf). 1999;50(2):149-155.
- Lazarus JH, Bestwick JP, Channon S, et al. Antenatal thyroid screening and childhood cognitive function. N Engl J Med. 2012;366(17):493-501.
- Practice Committee of the American Society for Reproductive Medicine. Subclinical hypothyroidism in the infertile female population: a guideline. Fertil Steril. 2015;104(3):545-553.
- Pasquali R, Pelusi C, Genghini S, Cacciari M, Gambineri A. Obesity and reproductive disorders in women. Hum Reprod Update. 2003;9(4):359-372.
- Greisen S, Ledet T, Møller N, et al. Effects of leptin on basal and FSH stimulated steroidogenesis in human granulosa luteal cells. Acta Obstet Gynecol Scand. 2000;79(11):931-935.
- Rich-Edwards JW, Goldman MB, Willett WC, et al. Adolescent body mass index and infertility caused by ovulatory disorder. Am J Obstet Gynecol. 1994;171(1):171-177.
- Grodstein F, Goldman MB, Cramer DW. Body mass index and ovulatory infertility. Epidemiology. 1994;5(2):247-250.
- Gesink Law DC, Maclehose RF, Longnecker MP. Obesity and time to pregnancy. Hum Reprod. 2007;22(2):414-420.
- Jain A, Polotsky AJ, Rochester D, et al. Pulsatile luteinizing hormone amplitude and progesterone metabolite excretion are reduced in obese women. J Clin Endocrinol Metab. 2007;92(7):2468-2473.
- Lake JK, Power C, Cole TJ. Women's reproductive health: the role of body mass index in early and adult life. Int J Obes Relat Metab Disord. 1997;21(6):432-438.
- van der Steeg JW, Steures P, Eijkemans MJ, et al. Obesity affects spontaneous pregnancy chances in subfertile, ovulatory women. Hum Reprod. 2008;23(2):324-328.
- Koning AM, Mutsaerts MA, Kuchenbecker WK, et al. Complications and outcome of assisted reproduction technologies in overweight and obese women [Published correction appears in Hum Reprod. 2012;27(8):2570.] Hum Reprod. 2012;27(2):457-467.
- Rittenberg V, Seshadri S, Sunkara SK, Sobaleva S, Oteng-Ntim E, El-Toukhy T. Effect of body mass index on IVF treatment outcome: an updated systematic review and meta-analysis. Reprod Biomed Online. 2011;23(4):421-439.
- Moragianni VA, Jones SM, Ryley DA. The effect of body mass index on the outcomes of first assisted reproductive technology cycles. Fertil Steril. 2012;98(1):102-108.
- Petersen GL, Schmidt L, Pinborg A, Kamper-Jørgensen M. The influence of female and male body mass index on live births after assisted reproductive technology treatment: a nationwide register-based cohort study. Fertil Steril. 2013;99(6):1654-1662.
- Practice Committee of the American Society for Reproductive Medicine. Obesity and Reproduction: A committee opinion. Fertil Steril. 2015;104(5):1116-1126.
- Metwally M, Cutting R, Tipton A, Skull J, Ledger WL, Li TC. Effect of increased body mass index on oocyte and embryo quality in IVF patients. Reprod Biomed Online. 2007;15(5):532-538.
- Leary C, Leese HJ, Sturmey RG. Human embryos from overweight and obese women display phenotypic and metabolic abnormalities. Hum Reprod. 2015;30(1):122-132.
- Deugarte D, Deugarte C, Sahakian V. Surrogate obesity negatively impacts pregnancy rates in third-party reproduction. Fertil Steril. 2010;93(3):1008-1010.
- Rittenberg V, Seshadri S, Sunkara SK, Sobaleva S, Oteng-Ntim E, El-Toukhy T. Effect of body mass index on IVF treatment outcome: an updated systematic review and meta-analysis. Reprod Biomed Online. 2011;23(4):421-439.
- Stothard KJ, Tennant PWG, Bell R, Rankin J. Maternal overweight and obesity and the risk of congenital anomalies: a systematic review and meta-analysis. JAMA. 2009;301(6):636-650.
- Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489-499.
- Baloch Z, Carayon P, Conte-Devolx B, et al. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid. 2003;13(1):3-126.
- Pop VJ, Kuijpens JL, van Baar AL, et al. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin Endocrinol (Oxf). 1999;50(2):149-155.
- Lazarus JH, Bestwick JP, Channon S, et al. Antenatal thyroid screening and childhood cognitive function. N Engl J Med. 2012;366(17):493-501.
- Practice Committee of the American Society for Reproductive Medicine. Subclinical hypothyroidism in the infertile female population: a guideline. Fertil Steril. 2015;104(3):545-553.
First EDition: Liquid Nicotine Risks, more
Risks of Electronic Cigarettes Include Unintentional Ingestion of Liquid Nicotine
BY JEFF BAUER
A recent case report of a 6-year-old girl who developed severe toxicity and required intubation after an unintentional exposure to liquid nicotine emphasizes a potential danger of commercially available liquid nicotine, which is highly concentrated, unreliably packaged, and poorly regulated.
Liquid nicotine is commonly sold in concentrated “refill” solutions intended for electronic cigarette users to dilute themselves. Previous studies have found that these refill products have unreliable commercial labeling, and that the actual nicotine concentration of these solutions can vary widely from the advertised concentration.
In this case report, the girl’s mother had purchased a concentrated nicotine solution online and had used an empty ibuprofen bottle, which she relabeled as “NIC,” to dilute the solution. Afterward, the patient’s father gave his daughter a 10-mL dose of the liquid from the repurposed bottle, believing it to be ibuprofen. Immediately upon consumption, the girl experienced a burning sensation in her mouth and throat. When the father tasted the liquid, he realized it contained the nicotine solution.
Within 5 minutes of the ingestion, the patient’s father called the regional poison control center and emergency medical services, while the girl’s mother attempted to manually induce vomiting, which produced only a small amount of emesis. When the paramedics arrived, the girl was conscious and breathing spontaneously, but she did not respond to questions or follow commands. The only intervention the paramedics performed was insertion of a peripheral intravenous line.
The girl arrived at the ED approximately 25 minutes after having ingested the nicotine. Her vital signs were: temperature, 95.4°F; heart rate (HR), 140 to 150 beats/min; and blood pressure, 93/70 mm Hg. Oxygen saturation was 95% on room air. She was alternately agitated and unresponsive. Her HR decreased to 60 beats/min, and she developed vomiting, diaphoresis, fasciculations, obtundation, and copious secretions. She was given ondansetron (0.1 mg/kg) and lorazepam (0.05 mg/kg), and within 6 minutes from arrival, she was sedated and intubated. Activated charcoal (25 g) was administered via nasogastric tube, and she was admitted to the pediatric intensive care unit.
Laboratory results from blood drawn upon the girl’s arrival at the ED indicated elevated lactate, creatinine, and potassium levels. A serum sample obtained 60 minutes after the girl had ingested the liquid was notable for elevated levels of nicotine (348 ng/mL). With the parents’ permission, the liquid in the ibuprofen container was analyzed and found to contain nicotine, 70.3 mg/mL, which meant the girl had consumed 703 mg of nicotine, or 35 mg/kg. A recent review suggested a fatal nicotine dose of 500 to 1,000 mg in adults. Assuming the mother had correctly diluted the liquid nicotine by half as she had intended to, the original product’s nicotine concentration was 140.6 mg/mL, or 234% of the amount listed on the package (60 mg/mL).
The girl remained sedated and intubated overnight without requiring additional medication or treatment. She was extubated the next morning. Her lactate, creatinine, and potassium levels returned to normal, and electrocardiography and chest radiography results were normal. She was discharged home in stable condition. The Department of Human Services conducted a brief investigation, which they closed when the patient was discharged.
The authors of this case report concluded that emergency physicians (EPs) should be aware of the widespread availability of liquid nicotine products, and the potential of severe toxicity from ingestion of liquid nicotine.
Noble MJ, Longstreet B, Hendrickson RG, Gerona R. Unintentional pediatric ingestion of electronic cigarette nicotine refill liquid necessitating intubation. Ann Emerg Med. 2017;69(1):94-97. doi:10.1016/j.annemergmed.2016.08.448.
Emergency Radiologists’ Job Satisfaction Tied to How Often They Have to Work Overnight Shifts
BY JEFF BAUER
According to a recent survey of emergency radiologists, those who frequently work overnight shifts are less likely to be satisfied with their job than counterparts who work fewer or no overnight shifts.
Approximately 1,100 emergency radiologists received an e-mail invitation to complete an online survey; 327 did so (29.6% response rate). Seventy-three percent of respondents were male, 69% were age 40 years or older, and 87% practiced full-time. Respondents were asked to rate statements such as “I enjoy my job” and “At times I feel overwhelmed at work” on a Likert scale from “disagree or strongly disagree” to “agree or strongly agree.”
Overall, 81% of respondents reported some measure of job enjoyment. There was an association between the average number of overnight shifts performed per year and job enjoyment. Emergency radiologists who did no overnight shifts were 2.21 times more likely to report enjoying their job than those who worked 17 weeks or more of overnight shifts a year.
Hanna TN, Shekhani H, Lamoureux C, et al. Emergency radiology practice patterns: shifts, schedules, and job satisfaction. J Am Coll Radiol. 2016. Dec 4. [Epub ahead of print]. doi:10.1016/j.jacr.2016.09.018.
Discharging Select Diverticulitis Patients From the ED May Be Acceptable
DOUG BRUNK
FRONTLINE MEDICAL NEWS
Among patients diagnosed with diverticulitis via computed tomography (CT) scan in the ED who were discharged home, only 13% required a return visit to the hospital, results from a long-term retrospective analysis demonstrated.
“In select patients whose assessment includes a CT scan, discharge to home from the emergency department with treatment for diverticulitis is safe,” study author Anne-Marie Sirany, MD, said at the annual meeting of the Western Surgical Association.
According to Dr Sirany, a general surgery resident at Hennepin County Medical Center, Minneapolis, diverticulitis accounts for about 150,000 hospital admissions per year in the United States, and only 15% of these patients require surgical intervention. However, between 2006 and 2011, ED visits for diverticulitis increased by 21%, and the annual direct medical cost related to the condition is estimated to exceed $1.8 billion. At the same time, medical literature regarding uncomplicated diverticulitis is scarce. “Most of the literature focuses on complicated diverticulitis, which includes episodes associated with extraluminal air, free perforation, abscess, fistula, obstruction, and stricture,” Dr Sirany said.
A few years ago, researchers conducted a randomized trial to evaluate the treatment of uncomplicated diverticulitis. Patients were diagnosed with diverticulitis in the ED and randomized to either hospital admission or outpatient management at home. The investigators found no significant differences between the readmission rates of the inpatient and outpatient groups, but the health care costs were three times lower in the outpatient group. Dr Sirany and her associates set out to compare the outcomes of patients diagnosed with and treated for diverticulitis in the ED who were discharged to home, versus those who were admitted to the hospital. They reviewed the medical records of 240 patients with a primary diagnosis of diverticulitis by CT scan who were evaluated in the ED at one of four hospitals and one academic medical center from September 2010 to January 2012. The primary outcome was hospital readmission or return to the ED within 30 days, while the secondary outcomes were recurrent diverticulitis or surgical resection for diverticulitis.
The mean age of the 240 patients was 59 years, 45% were men, 22% had a Charlson Comorbidity Index (CCI) of >2, and 7.5% were on corticosteroids or immunosuppressant medications. More than half (62%) were admitted to the hospital, while the remaining 38% were discharged home on oral antibiotics. Compared with patients discharged home, those admitted to the hospital were more likely to be older than 65 years (43% vs 24%, respectively; P = .003), have a CCI of 2 or greater (28% vs 13%; P = .007), were more likely to be on immunosuppressant or steroid medications (11% vs 1%; P = .003), show extraluminal air on CT (30% vs 7%; P < .001), or show abscess on CT (19% vs 1%; P < .001). “Of note: We did not have any patients who had CT scan findings of pneumoperitoneum who were discharged home, and 48% of patients admitted to the hospital had uncomplicated diverticulitis,” she said.
After a median follow-up of 37 months, no significant differences were observed between patients discharged to home and those admitted to the hospital in readmission or return to the ED (13% vs 14%), recurrent diverticulitis (23% in each group), or in colon resection at subsequent encounter (16% vs 19%). “Among patients discharged to home, only one patient required emergency surgery, and this was 20 months after their index admission,” Dr Sirany said. “We think that the low rate of readmission in patients discharged home demonstrates that this is a safe approach to management of patients with diverticulitis, when using information from the CT scan.”
Closer analysis of patients who were discharged home revealed that six patients had extraluminal air on CT scan, three of whom returned to the ED or were admitted to the hospital. In addition, 11% of those with uncomplicated diverticulitis returned to the ED or were admitted to the hospital.
Dr Sirany acknowledged certain limitations of the study, including its retrospective design, a lack of complete follow-up for all patients, and the fact that it included patients with recurrent diverticulitis. “Despite the limitations, we recommend that young, relatively healthy patients with uncomplicated findings on CT scan can be discharged to home and managed as an outpatient,” she said. “In an era where there’s increasing attention to health care costs, we need to think more critically about which patients need to be admitted for management of uncomplicated diverticulitis.”
Microsensor Perfectly Distinguished Coagulopathy Patients From Controls
AMY KARON
FRONTLINE MEDICAL NEWS
Using less than a drop of blood, a portable microsensor provided a comprehensive coagulation profile in <15 minutes and perfectly distinguished various coagulopathies from normal blood samples—handily beating the results from both activated partial thromboplastin time (aPTT) and prothrombin time (PT).
Dubbed ClotChip, the disposable device detects coagulation factors and platelet activity using dielectric spectroscopy, Evi X. Stavrou, MD, said at the annual meeting of the American Society of Hematology. The development points the way for comprehensive, rapid, point-of-care (POC) assessment of critically ill or severely injured patients and those who need ongoing monitoring to evaluate response to anticoagulant therapy, she added.
Existing POC coagulation assays have several shortcomings, Dr Stavrou, of Case Western Reserve University, Cleveland, said during a press briefing at the conference. They are relatively insensitive, fail to measure platelet activity, or are only approved for specific subgroups of patients, such as those on warfarin, she specified.
To develop an alternative, Dr Stavrou and her associates added a parallel-plate capacitive sensing structure to an inexpensive, disposable microfluidic biochip designed to test 9 microliters (less than one drop) of blood. They built the microsensor from biocompatible and chemically inert materials to minimize the chances of artificial contact activation.
To test the device, the researchers used calcium dichloride to induce coagulation in whole blood samples from 11 controls with normal aPTT and PT values. Time curves of output from the microsensor showed that coagulation consistently peaked within 4.5 to 6 minutes.
Next, the investigators tested blood from 12 patients with coagulopathies, including hemophilia A, hemophilia B, acquired von Willebrand factor defect, and congenital hypodysfibrinogenemia. These samples all yielded abnormal curves, with prolonged times to peak that ranged between 7 and 15 minutes—significantly exceeding those of healthy controls (P = .002).
By plotting rates of true positives against rates of true negatives, the researchers obtained areas under the receiver-operating curves of 100% for ClotChip, 78% for aPTT, and 57% for PT. In other words, ClotChip correctly identified all cases and controls in this small patient cohort, while neither aPTT nor PT did.
Finally, the researchers used the microsensor to measure coagulation activity in normal blood samples that they treated with prostaglandin E2 to inhibit platelet aggregation. Normalized permittivity (an electrical measure) was significantly lower than in untreated control samples (P = .03), but time-to-peak values were the same in both groups. This finding confirms the chip can identify abnormal platelet function, Dr Stavrou said. “ClotChip is sensitive to the complete hemostasis process, exhibits better sensitivity and specificity than conventional coagulation assays, and discriminates between coagulation and platelet defects,” she concluded.
The investigators are recruiting volunteers for an expanded round of testing for the device, and are working to optimize construction to further enhance its sensitivity.
Survey: Overprescribing Is the Cause of the Opioid Crisis
M. ALEXANDER OTTO
FRONTLINE MEDICAL NEWS
Almost a third of doctors blamed overprescribing as the cause of the opioid crisis, according to a survey of 225 US primary care, emergency medicine, and pain management physicians by InCrowd, an online physician survey company.
Respondents said their and other physicians’ overprescribing is the single biggest factor fueling the leap in opioid abuse over the past 5 years.
“We were told…that [opioids] wouldn’t be addictive in the great majority of patients. This was obviously wrong,” said a Utah EP in practice for 38 years. Meanwhile, 24% of the respondents cited aggressive patient drug-seeking as the primary cause, and 18% blamed drug dealers.
In short, the survey pointed out what front-line doctors think needs to be fixed as the nation combats prescription opioid abuse and the subsequent heroin epidemic. Their insights “should be a rallying cry” for changes in 2017, said epidemiologist Diane Hayes, PhD, president and cofounder of InCrowd.
Making pain the “fifth vital sign” and allowing patients to downgrade doctors on surveys if they don’t prescribe or refill opioid prescriptions compounded the situation. Lengthy waits for specialists with better pain options, many of whom are not covered by Medicaid or the Affordable Care Act, also added to the problem, survey respondents said.
“We’re caught in the middle” between the Joint Commission on Accreditation of Healthcare Organization’s fifth vital sign and overprescribing, a primary care physician (PCP) said.
Seventy-three percent of survey respondents said that they want opioid alternatives, noting exasperation with nonsteroidal anti-inflammatory drugs, physical therapy, and exercise. About half recommend behavioral health interventions, while 20% recommend vitamin and herbal supplements. Only 10% recommend medical marijuana, probably because it is inaccessible to most US patients. Meanwhile, the respondents said they want opioid prescribing “hemmed in.” Almost two-thirds wanted refill limits and more frequent refill evaluations, and many agreed that there needs to be a weaning protocol before the drugs are even started. Some wanted to limit advertising.
Easton Jackson, MD, a PCP in West Valley City, Utah, who answered the survey, helped make the answers real by sharing his thoughts.
“We need to recognize that…people don’t set out to get addicted to opioids….We need to educate [patients] and assist them with their expectations. They need to understand that they’re going to have pain from surgery and injuries. Our goal isn’t to make them pain-free. It’s to manage their pain,” he said.
“We as physicians need to write for fewer pills and in lower doses. We need to see our patients back sooner. If it’s not working, stop increasing the dose and instead taper the patient off the medication. We need to be familiar with the adjuvant therapies. As easy as it is to say, ‘send them all to the pain specialist,’ there simply aren’t enough of them around,” Dr Jackson said.
Physician respondents to InCrowd’s opioid survey have practiced an average of 25 years, and were scattered around the United States. They filled out the four-question survey during October 27 to 28, 2016. They signed up to receive and answer InCrowd’s questions, and were paid nominally for their time.
Half (50%) of respondents estimated that they prescribed opioids to <10% of their patients; 38% said they prescribed to less than half of their patients; and 12% estimated they prescribed opioids to more than half of their patients.
Adding Respiratory Rate to Triage Criteria Improves Accurate Staging of Chest Trauma Patients
MICHELE G. SULLIVAN
FRONTLINE MEDICAL NEWS
Adding respiratory rate (RR) and suspected blunt chest injury to a trauma assessment in the field significantly improved the appropriate triaging of level III trauma patients.
When the assessment specifically evaluated for tachypnea in the setting of blunt chest injury, undertriaging improved by 1.2%, John Yonge, MD, said at the annual clinical congress of the American College of Surgeons.
“When we applied this new criteria to our 10-year study, we identified 661 patients who should have been activated as a level I or level II,” but instead were assessed as less critically injured, Dr Yonge said in an interview. This initial misstep significantly extended the time before patients could have critical surgical procedures and was related to higher mortality among them.
Dr Yonge, a surgical fellow at Oregon Health & Science University (OHSU), Portland, and his mentor Martin Schreiber, MD, conducted the retrospective study of 7,880 trauma patients admitted at level III activation from 2004 to 2014. The OHSU trauma system has three activation levels.
- Level I activations are reserved for the most critically injured patients; attending trauma surgeon and anesthesiologist presence is mandatory.
- Level II activations capture moderate-to-severe injuries; trauma surgeon and respiratory therapist presence is mandated.
- Level III activations are designed to capture patients who do not require an immediate lifesaving intervention; the presence of the trauma surgery chief resident and attending emergency medicine physician is mandatory.
Patients were considered undertriaged if they were admitted as level III activations, but then required a critical intervention (chest tube placement, intubation, needle thoracostomy, or intracranial pressure monitoring) in the ED or ultimately met level I or II activation criteria.
Among all the level III patients, 466 (6%) were undertriaged: 390 were undertriaged based on the existing level I or II activation criteria, and 76 were considered undertriaged based on the need for a critical intervention.
Most of the undertriaged patients (65%) met criteria for level I activation; the rest should have been triaged as level II patients. Compared with appropriately staged level III patients, mortality among the undertriaged patients was significantly higher (3.2% vs 0.6%). Undertriaged patients also experienced longer delays before initiation of major emergency surgery: a mean of 147 minutes, compared with 106 minutes for appropriately triaged level I patients and 62 minutes for appropriately triaged level II patients.
Dr Yonge then looked for clinical measures that would improve triage. Tachypnea (RR >20 breaths/min) in the field stood out as a significant factor. Tachypneic patients who had a suspected chest injury were 70% more likely to be undertriaged than were those with a normal RR. Tachypnea was significantly associated with a diagnosis of flail chest, ED intubation, and chest-tube placement.
The team then constructed a new triage criterion for patients with suspected chest injury—tachypnea combined with suspected blunt thoracic injury. By applying that model to their study population of level III patients, they determined that the level III undertriage rate would be reduced by 1.2%.
Tying the physiological marker of tachypnea to a suspected clinical diagnosis is a key factor, Dr Yonge noted. “Just adding tachypnea doesn’t help us. In fact, it would overwhelm us, because a trauma patient could very well be tachypneic because he’s experiencing panic. But tying it to a suspected clinical diagnosis gives us a meaningful result.”
He confirmed this linkage with an additional analysis. “We looked to see how severely injured these patients were and found that 71% of them had an Abbreviated Injury Score (AIS) to the chest of 3 or more, indicating a severe chest injury. Only 29% had an AIS of 2 or less. So this proves that respiratory rate is a valid triage criterion and can be used to identify patients who need a higher level of trauma care.”
The challenge now, Dr Yonge said, is incorporating the marker into clinical practice. “It doesn’t matter how many statistics you do, if you can’t educate the prehospital providers in this, it’s useless. They are the crux of the trauma system.”
Although national guidelines do recommend assessing RR as part of field triage, it often isn’t recorded or is only estimated, Dr Yonge said. That’s one reason he used the 20 breaths/min cutoff rate. “It doesn’t even take a full minute to assess this, but it can make a big improvement in care.”
Risks of Electronic Cigarettes Include Unintentional Ingestion of Liquid Nicotine
BY JEFF BAUER
A recent case report of a 6-year-old girl who developed severe toxicity and required intubation after an unintentional exposure to liquid nicotine emphasizes a potential danger of commercially available liquid nicotine, which is highly concentrated, unreliably packaged, and poorly regulated.
Liquid nicotine is commonly sold in concentrated “refill” solutions intended for electronic cigarette users to dilute themselves. Previous studies have found that these refill products have unreliable commercial labeling, and that the actual nicotine concentration of these solutions can vary widely from the advertised concentration.
In this case report, the girl’s mother had purchased a concentrated nicotine solution online and had used an empty ibuprofen bottle, which she relabeled as “NIC,” to dilute the solution. Afterward, the patient’s father gave his daughter a 10-mL dose of the liquid from the repurposed bottle, believing it to be ibuprofen. Immediately upon consumption, the girl experienced a burning sensation in her mouth and throat. When the father tasted the liquid, he realized it contained the nicotine solution.
Within 5 minutes of the ingestion, the patient’s father called the regional poison control center and emergency medical services, while the girl’s mother attempted to manually induce vomiting, which produced only a small amount of emesis. When the paramedics arrived, the girl was conscious and breathing spontaneously, but she did not respond to questions or follow commands. The only intervention the paramedics performed was insertion of a peripheral intravenous line.
The girl arrived at the ED approximately 25 minutes after having ingested the nicotine. Her vital signs were: temperature, 95.4°F; heart rate (HR), 140 to 150 beats/min; and blood pressure, 93/70 mm Hg. Oxygen saturation was 95% on room air. She was alternately agitated and unresponsive. Her HR decreased to 60 beats/min, and she developed vomiting, diaphoresis, fasciculations, obtundation, and copious secretions. She was given ondansetron (0.1 mg/kg) and lorazepam (0.05 mg/kg), and within 6 minutes from arrival, she was sedated and intubated. Activated charcoal (25 g) was administered via nasogastric tube, and she was admitted to the pediatric intensive care unit.
Laboratory results from blood drawn upon the girl’s arrival at the ED indicated elevated lactate, creatinine, and potassium levels. A serum sample obtained 60 minutes after the girl had ingested the liquid was notable for elevated levels of nicotine (348 ng/mL). With the parents’ permission, the liquid in the ibuprofen container was analyzed and found to contain nicotine, 70.3 mg/mL, which meant the girl had consumed 703 mg of nicotine, or 35 mg/kg. A recent review suggested a fatal nicotine dose of 500 to 1,000 mg in adults. Assuming the mother had correctly diluted the liquid nicotine by half as she had intended to, the original product’s nicotine concentration was 140.6 mg/mL, or 234% of the amount listed on the package (60 mg/mL).
The girl remained sedated and intubated overnight without requiring additional medication or treatment. She was extubated the next morning. Her lactate, creatinine, and potassium levels returned to normal, and electrocardiography and chest radiography results were normal. She was discharged home in stable condition. The Department of Human Services conducted a brief investigation, which they closed when the patient was discharged.
The authors of this case report concluded that emergency physicians (EPs) should be aware of the widespread availability of liquid nicotine products, and the potential of severe toxicity from ingestion of liquid nicotine.
Noble MJ, Longstreet B, Hendrickson RG, Gerona R. Unintentional pediatric ingestion of electronic cigarette nicotine refill liquid necessitating intubation. Ann Emerg Med. 2017;69(1):94-97. doi:10.1016/j.annemergmed.2016.08.448.
Emergency Radiologists’ Job Satisfaction Tied to How Often They Have to Work Overnight Shifts
BY JEFF BAUER
According to a recent survey of emergency radiologists, those who frequently work overnight shifts are less likely to be satisfied with their job than counterparts who work fewer or no overnight shifts.
Approximately 1,100 emergency radiologists received an e-mail invitation to complete an online survey; 327 did so (29.6% response rate). Seventy-three percent of respondents were male, 69% were age 40 years or older, and 87% practiced full-time. Respondents were asked to rate statements such as “I enjoy my job” and “At times I feel overwhelmed at work” on a Likert scale from “disagree or strongly disagree” to “agree or strongly agree.”
Overall, 81% of respondents reported some measure of job enjoyment. There was an association between the average number of overnight shifts performed per year and job enjoyment. Emergency radiologists who did no overnight shifts were 2.21 times more likely to report enjoying their job than those who worked 17 weeks or more of overnight shifts a year.
Hanna TN, Shekhani H, Lamoureux C, et al. Emergency radiology practice patterns: shifts, schedules, and job satisfaction. J Am Coll Radiol. 2016. Dec 4. [Epub ahead of print]. doi:10.1016/j.jacr.2016.09.018.
Discharging Select Diverticulitis Patients From the ED May Be Acceptable
DOUG BRUNK
FRONTLINE MEDICAL NEWS
Among patients diagnosed with diverticulitis via computed tomography (CT) scan in the ED who were discharged home, only 13% required a return visit to the hospital, results from a long-term retrospective analysis demonstrated.
“In select patients whose assessment includes a CT scan, discharge to home from the emergency department with treatment for diverticulitis is safe,” study author Anne-Marie Sirany, MD, said at the annual meeting of the Western Surgical Association.
According to Dr Sirany, a general surgery resident at Hennepin County Medical Center, Minneapolis, diverticulitis accounts for about 150,000 hospital admissions per year in the United States, and only 15% of these patients require surgical intervention. However, between 2006 and 2011, ED visits for diverticulitis increased by 21%, and the annual direct medical cost related to the condition is estimated to exceed $1.8 billion. At the same time, medical literature regarding uncomplicated diverticulitis is scarce. “Most of the literature focuses on complicated diverticulitis, which includes episodes associated with extraluminal air, free perforation, abscess, fistula, obstruction, and stricture,” Dr Sirany said.
A few years ago, researchers conducted a randomized trial to evaluate the treatment of uncomplicated diverticulitis. Patients were diagnosed with diverticulitis in the ED and randomized to either hospital admission or outpatient management at home. The investigators found no significant differences between the readmission rates of the inpatient and outpatient groups, but the health care costs were three times lower in the outpatient group. Dr Sirany and her associates set out to compare the outcomes of patients diagnosed with and treated for diverticulitis in the ED who were discharged to home, versus those who were admitted to the hospital. They reviewed the medical records of 240 patients with a primary diagnosis of diverticulitis by CT scan who were evaluated in the ED at one of four hospitals and one academic medical center from September 2010 to January 2012. The primary outcome was hospital readmission or return to the ED within 30 days, while the secondary outcomes were recurrent diverticulitis or surgical resection for diverticulitis.
The mean age of the 240 patients was 59 years, 45% were men, 22% had a Charlson Comorbidity Index (CCI) of >2, and 7.5% were on corticosteroids or immunosuppressant medications. More than half (62%) were admitted to the hospital, while the remaining 38% were discharged home on oral antibiotics. Compared with patients discharged home, those admitted to the hospital were more likely to be older than 65 years (43% vs 24%, respectively; P = .003), have a CCI of 2 or greater (28% vs 13%; P = .007), were more likely to be on immunosuppressant or steroid medications (11% vs 1%; P = .003), show extraluminal air on CT (30% vs 7%; P < .001), or show abscess on CT (19% vs 1%; P < .001). “Of note: We did not have any patients who had CT scan findings of pneumoperitoneum who were discharged home, and 48% of patients admitted to the hospital had uncomplicated diverticulitis,” she said.
After a median follow-up of 37 months, no significant differences were observed between patients discharged to home and those admitted to the hospital in readmission or return to the ED (13% vs 14%), recurrent diverticulitis (23% in each group), or in colon resection at subsequent encounter (16% vs 19%). “Among patients discharged to home, only one patient required emergency surgery, and this was 20 months after their index admission,” Dr Sirany said. “We think that the low rate of readmission in patients discharged home demonstrates that this is a safe approach to management of patients with diverticulitis, when using information from the CT scan.”
Closer analysis of patients who were discharged home revealed that six patients had extraluminal air on CT scan, three of whom returned to the ED or were admitted to the hospital. In addition, 11% of those with uncomplicated diverticulitis returned to the ED or were admitted to the hospital.
Dr Sirany acknowledged certain limitations of the study, including its retrospective design, a lack of complete follow-up for all patients, and the fact that it included patients with recurrent diverticulitis. “Despite the limitations, we recommend that young, relatively healthy patients with uncomplicated findings on CT scan can be discharged to home and managed as an outpatient,” she said. “In an era where there’s increasing attention to health care costs, we need to think more critically about which patients need to be admitted for management of uncomplicated diverticulitis.”
Microsensor Perfectly Distinguished Coagulopathy Patients From Controls
AMY KARON
FRONTLINE MEDICAL NEWS
Using less than a drop of blood, a portable microsensor provided a comprehensive coagulation profile in <15 minutes and perfectly distinguished various coagulopathies from normal blood samples—handily beating the results from both activated partial thromboplastin time (aPTT) and prothrombin time (PT).
Dubbed ClotChip, the disposable device detects coagulation factors and platelet activity using dielectric spectroscopy, Evi X. Stavrou, MD, said at the annual meeting of the American Society of Hematology. The development points the way for comprehensive, rapid, point-of-care (POC) assessment of critically ill or severely injured patients and those who need ongoing monitoring to evaluate response to anticoagulant therapy, she added.
Existing POC coagulation assays have several shortcomings, Dr Stavrou, of Case Western Reserve University, Cleveland, said during a press briefing at the conference. They are relatively insensitive, fail to measure platelet activity, or are only approved for specific subgroups of patients, such as those on warfarin, she specified.
To develop an alternative, Dr Stavrou and her associates added a parallel-plate capacitive sensing structure to an inexpensive, disposable microfluidic biochip designed to test 9 microliters (less than one drop) of blood. They built the microsensor from biocompatible and chemically inert materials to minimize the chances of artificial contact activation.
To test the device, the researchers used calcium dichloride to induce coagulation in whole blood samples from 11 controls with normal aPTT and PT values. Time curves of output from the microsensor showed that coagulation consistently peaked within 4.5 to 6 minutes.
Next, the investigators tested blood from 12 patients with coagulopathies, including hemophilia A, hemophilia B, acquired von Willebrand factor defect, and congenital hypodysfibrinogenemia. These samples all yielded abnormal curves, with prolonged times to peak that ranged between 7 and 15 minutes—significantly exceeding those of healthy controls (P = .002).
By plotting rates of true positives against rates of true negatives, the researchers obtained areas under the receiver-operating curves of 100% for ClotChip, 78% for aPTT, and 57% for PT. In other words, ClotChip correctly identified all cases and controls in this small patient cohort, while neither aPTT nor PT did.
Finally, the researchers used the microsensor to measure coagulation activity in normal blood samples that they treated with prostaglandin E2 to inhibit platelet aggregation. Normalized permittivity (an electrical measure) was significantly lower than in untreated control samples (P = .03), but time-to-peak values were the same in both groups. This finding confirms the chip can identify abnormal platelet function, Dr Stavrou said. “ClotChip is sensitive to the complete hemostasis process, exhibits better sensitivity and specificity than conventional coagulation assays, and discriminates between coagulation and platelet defects,” she concluded.
The investigators are recruiting volunteers for an expanded round of testing for the device, and are working to optimize construction to further enhance its sensitivity.
Survey: Overprescribing Is the Cause of the Opioid Crisis
M. ALEXANDER OTTO
FRONTLINE MEDICAL NEWS
Almost a third of doctors blamed overprescribing as the cause of the opioid crisis, according to a survey of 225 US primary care, emergency medicine, and pain management physicians by InCrowd, an online physician survey company.
Respondents said their and other physicians’ overprescribing is the single biggest factor fueling the leap in opioid abuse over the past 5 years.
“We were told…that [opioids] wouldn’t be addictive in the great majority of patients. This was obviously wrong,” said a Utah EP in practice for 38 years. Meanwhile, 24% of the respondents cited aggressive patient drug-seeking as the primary cause, and 18% blamed drug dealers.
In short, the survey pointed out what front-line doctors think needs to be fixed as the nation combats prescription opioid abuse and the subsequent heroin epidemic. Their insights “should be a rallying cry” for changes in 2017, said epidemiologist Diane Hayes, PhD, president and cofounder of InCrowd.
Making pain the “fifth vital sign” and allowing patients to downgrade doctors on surveys if they don’t prescribe or refill opioid prescriptions compounded the situation. Lengthy waits for specialists with better pain options, many of whom are not covered by Medicaid or the Affordable Care Act, also added to the problem, survey respondents said.
“We’re caught in the middle” between the Joint Commission on Accreditation of Healthcare Organization’s fifth vital sign and overprescribing, a primary care physician (PCP) said.
Seventy-three percent of survey respondents said that they want opioid alternatives, noting exasperation with nonsteroidal anti-inflammatory drugs, physical therapy, and exercise. About half recommend behavioral health interventions, while 20% recommend vitamin and herbal supplements. Only 10% recommend medical marijuana, probably because it is inaccessible to most US patients. Meanwhile, the respondents said they want opioid prescribing “hemmed in.” Almost two-thirds wanted refill limits and more frequent refill evaluations, and many agreed that there needs to be a weaning protocol before the drugs are even started. Some wanted to limit advertising.
Easton Jackson, MD, a PCP in West Valley City, Utah, who answered the survey, helped make the answers real by sharing his thoughts.
“We need to recognize that…people don’t set out to get addicted to opioids….We need to educate [patients] and assist them with their expectations. They need to understand that they’re going to have pain from surgery and injuries. Our goal isn’t to make them pain-free. It’s to manage their pain,” he said.
“We as physicians need to write for fewer pills and in lower doses. We need to see our patients back sooner. If it’s not working, stop increasing the dose and instead taper the patient off the medication. We need to be familiar with the adjuvant therapies. As easy as it is to say, ‘send them all to the pain specialist,’ there simply aren’t enough of them around,” Dr Jackson said.
Physician respondents to InCrowd’s opioid survey have practiced an average of 25 years, and were scattered around the United States. They filled out the four-question survey during October 27 to 28, 2016. They signed up to receive and answer InCrowd’s questions, and were paid nominally for their time.
Half (50%) of respondents estimated that they prescribed opioids to <10% of their patients; 38% said they prescribed to less than half of their patients; and 12% estimated they prescribed opioids to more than half of their patients.
Adding Respiratory Rate to Triage Criteria Improves Accurate Staging of Chest Trauma Patients
MICHELE G. SULLIVAN
FRONTLINE MEDICAL NEWS
Adding respiratory rate (RR) and suspected blunt chest injury to a trauma assessment in the field significantly improved the appropriate triaging of level III trauma patients.
When the assessment specifically evaluated for tachypnea in the setting of blunt chest injury, undertriaging improved by 1.2%, John Yonge, MD, said at the annual clinical congress of the American College of Surgeons.
“When we applied this new criteria to our 10-year study, we identified 661 patients who should have been activated as a level I or level II,” but instead were assessed as less critically injured, Dr Yonge said in an interview. This initial misstep significantly extended the time before patients could have critical surgical procedures and was related to higher mortality among them.
Dr Yonge, a surgical fellow at Oregon Health & Science University (OHSU), Portland, and his mentor Martin Schreiber, MD, conducted the retrospective study of 7,880 trauma patients admitted at level III activation from 2004 to 2014. The OHSU trauma system has three activation levels.
- Level I activations are reserved for the most critically injured patients; attending trauma surgeon and anesthesiologist presence is mandatory.
- Level II activations capture moderate-to-severe injuries; trauma surgeon and respiratory therapist presence is mandated.
- Level III activations are designed to capture patients who do not require an immediate lifesaving intervention; the presence of the trauma surgery chief resident and attending emergency medicine physician is mandatory.
Patients were considered undertriaged if they were admitted as level III activations, but then required a critical intervention (chest tube placement, intubation, needle thoracostomy, or intracranial pressure monitoring) in the ED or ultimately met level I or II activation criteria.
Among all the level III patients, 466 (6%) were undertriaged: 390 were undertriaged based on the existing level I or II activation criteria, and 76 were considered undertriaged based on the need for a critical intervention.
Most of the undertriaged patients (65%) met criteria for level I activation; the rest should have been triaged as level II patients. Compared with appropriately staged level III patients, mortality among the undertriaged patients was significantly higher (3.2% vs 0.6%). Undertriaged patients also experienced longer delays before initiation of major emergency surgery: a mean of 147 minutes, compared with 106 minutes for appropriately triaged level I patients and 62 minutes for appropriately triaged level II patients.
Dr Yonge then looked for clinical measures that would improve triage. Tachypnea (RR >20 breaths/min) in the field stood out as a significant factor. Tachypneic patients who had a suspected chest injury were 70% more likely to be undertriaged than were those with a normal RR. Tachypnea was significantly associated with a diagnosis of flail chest, ED intubation, and chest-tube placement.
The team then constructed a new triage criterion for patients with suspected chest injury—tachypnea combined with suspected blunt thoracic injury. By applying that model to their study population of level III patients, they determined that the level III undertriage rate would be reduced by 1.2%.
Tying the physiological marker of tachypnea to a suspected clinical diagnosis is a key factor, Dr Yonge noted. “Just adding tachypnea doesn’t help us. In fact, it would overwhelm us, because a trauma patient could very well be tachypneic because he’s experiencing panic. But tying it to a suspected clinical diagnosis gives us a meaningful result.”
He confirmed this linkage with an additional analysis. “We looked to see how severely injured these patients were and found that 71% of them had an Abbreviated Injury Score (AIS) to the chest of 3 or more, indicating a severe chest injury. Only 29% had an AIS of 2 or less. So this proves that respiratory rate is a valid triage criterion and can be used to identify patients who need a higher level of trauma care.”
The challenge now, Dr Yonge said, is incorporating the marker into clinical practice. “It doesn’t matter how many statistics you do, if you can’t educate the prehospital providers in this, it’s useless. They are the crux of the trauma system.”
Although national guidelines do recommend assessing RR as part of field triage, it often isn’t recorded or is only estimated, Dr Yonge said. That’s one reason he used the 20 breaths/min cutoff rate. “It doesn’t even take a full minute to assess this, but it can make a big improvement in care.”
Risks of Electronic Cigarettes Include Unintentional Ingestion of Liquid Nicotine
BY JEFF BAUER
A recent case report of a 6-year-old girl who developed severe toxicity and required intubation after an unintentional exposure to liquid nicotine emphasizes a potential danger of commercially available liquid nicotine, which is highly concentrated, unreliably packaged, and poorly regulated.
Liquid nicotine is commonly sold in concentrated “refill” solutions intended for electronic cigarette users to dilute themselves. Previous studies have found that these refill products have unreliable commercial labeling, and that the actual nicotine concentration of these solutions can vary widely from the advertised concentration.
In this case report, the girl’s mother had purchased a concentrated nicotine solution online and had used an empty ibuprofen bottle, which she relabeled as “NIC,” to dilute the solution. Afterward, the patient’s father gave his daughter a 10-mL dose of the liquid from the repurposed bottle, believing it to be ibuprofen. Immediately upon consumption, the girl experienced a burning sensation in her mouth and throat. When the father tasted the liquid, he realized it contained the nicotine solution.
Within 5 minutes of the ingestion, the patient’s father called the regional poison control center and emergency medical services, while the girl’s mother attempted to manually induce vomiting, which produced only a small amount of emesis. When the paramedics arrived, the girl was conscious and breathing spontaneously, but she did not respond to questions or follow commands. The only intervention the paramedics performed was insertion of a peripheral intravenous line.
The girl arrived at the ED approximately 25 minutes after having ingested the nicotine. Her vital signs were: temperature, 95.4°F; heart rate (HR), 140 to 150 beats/min; and blood pressure, 93/70 mm Hg. Oxygen saturation was 95% on room air. She was alternately agitated and unresponsive. Her HR decreased to 60 beats/min, and she developed vomiting, diaphoresis, fasciculations, obtundation, and copious secretions. She was given ondansetron (0.1 mg/kg) and lorazepam (0.05 mg/kg), and within 6 minutes from arrival, she was sedated and intubated. Activated charcoal (25 g) was administered via nasogastric tube, and she was admitted to the pediatric intensive care unit.
Laboratory results from blood drawn upon the girl’s arrival at the ED indicated elevated lactate, creatinine, and potassium levels. A serum sample obtained 60 minutes after the girl had ingested the liquid was notable for elevated levels of nicotine (348 ng/mL). With the parents’ permission, the liquid in the ibuprofen container was analyzed and found to contain nicotine, 70.3 mg/mL, which meant the girl had consumed 703 mg of nicotine, or 35 mg/kg. A recent review suggested a fatal nicotine dose of 500 to 1,000 mg in adults. Assuming the mother had correctly diluted the liquid nicotine by half as she had intended to, the original product’s nicotine concentration was 140.6 mg/mL, or 234% of the amount listed on the package (60 mg/mL).
The girl remained sedated and intubated overnight without requiring additional medication or treatment. She was extubated the next morning. Her lactate, creatinine, and potassium levels returned to normal, and electrocardiography and chest radiography results were normal. She was discharged home in stable condition. The Department of Human Services conducted a brief investigation, which they closed when the patient was discharged.
The authors of this case report concluded that emergency physicians (EPs) should be aware of the widespread availability of liquid nicotine products, and the potential of severe toxicity from ingestion of liquid nicotine.
Noble MJ, Longstreet B, Hendrickson RG, Gerona R. Unintentional pediatric ingestion of electronic cigarette nicotine refill liquid necessitating intubation. Ann Emerg Med. 2017;69(1):94-97. doi:10.1016/j.annemergmed.2016.08.448.
Emergency Radiologists’ Job Satisfaction Tied to How Often They Have to Work Overnight Shifts
BY JEFF BAUER
According to a recent survey of emergency radiologists, those who frequently work overnight shifts are less likely to be satisfied with their job than counterparts who work fewer or no overnight shifts.
Approximately 1,100 emergency radiologists received an e-mail invitation to complete an online survey; 327 did so (29.6% response rate). Seventy-three percent of respondents were male, 69% were age 40 years or older, and 87% practiced full-time. Respondents were asked to rate statements such as “I enjoy my job” and “At times I feel overwhelmed at work” on a Likert scale from “disagree or strongly disagree” to “agree or strongly agree.”
Overall, 81% of respondents reported some measure of job enjoyment. There was an association between the average number of overnight shifts performed per year and job enjoyment. Emergency radiologists who did no overnight shifts were 2.21 times more likely to report enjoying their job than those who worked 17 weeks or more of overnight shifts a year.
Hanna TN, Shekhani H, Lamoureux C, et al. Emergency radiology practice patterns: shifts, schedules, and job satisfaction. J Am Coll Radiol. 2016. Dec 4. [Epub ahead of print]. doi:10.1016/j.jacr.2016.09.018.
Discharging Select Diverticulitis Patients From the ED May Be Acceptable
DOUG BRUNK
FRONTLINE MEDICAL NEWS
Among patients diagnosed with diverticulitis via computed tomography (CT) scan in the ED who were discharged home, only 13% required a return visit to the hospital, results from a long-term retrospective analysis demonstrated.
“In select patients whose assessment includes a CT scan, discharge to home from the emergency department with treatment for diverticulitis is safe,” study author Anne-Marie Sirany, MD, said at the annual meeting of the Western Surgical Association.
According to Dr Sirany, a general surgery resident at Hennepin County Medical Center, Minneapolis, diverticulitis accounts for about 150,000 hospital admissions per year in the United States, and only 15% of these patients require surgical intervention. However, between 2006 and 2011, ED visits for diverticulitis increased by 21%, and the annual direct medical cost related to the condition is estimated to exceed $1.8 billion. At the same time, medical literature regarding uncomplicated diverticulitis is scarce. “Most of the literature focuses on complicated diverticulitis, which includes episodes associated with extraluminal air, free perforation, abscess, fistula, obstruction, and stricture,” Dr Sirany said.
A few years ago, researchers conducted a randomized trial to evaluate the treatment of uncomplicated diverticulitis. Patients were diagnosed with diverticulitis in the ED and randomized to either hospital admission or outpatient management at home. The investigators found no significant differences between the readmission rates of the inpatient and outpatient groups, but the health care costs were three times lower in the outpatient group. Dr Sirany and her associates set out to compare the outcomes of patients diagnosed with and treated for diverticulitis in the ED who were discharged to home, versus those who were admitted to the hospital. They reviewed the medical records of 240 patients with a primary diagnosis of diverticulitis by CT scan who were evaluated in the ED at one of four hospitals and one academic medical center from September 2010 to January 2012. The primary outcome was hospital readmission or return to the ED within 30 days, while the secondary outcomes were recurrent diverticulitis or surgical resection for diverticulitis.
The mean age of the 240 patients was 59 years, 45% were men, 22% had a Charlson Comorbidity Index (CCI) of >2, and 7.5% were on corticosteroids or immunosuppressant medications. More than half (62%) were admitted to the hospital, while the remaining 38% were discharged home on oral antibiotics. Compared with patients discharged home, those admitted to the hospital were more likely to be older than 65 years (43% vs 24%, respectively; P = .003), have a CCI of 2 or greater (28% vs 13%; P = .007), were more likely to be on immunosuppressant or steroid medications (11% vs 1%; P = .003), show extraluminal air on CT (30% vs 7%; P < .001), or show abscess on CT (19% vs 1%; P < .001). “Of note: We did not have any patients who had CT scan findings of pneumoperitoneum who were discharged home, and 48% of patients admitted to the hospital had uncomplicated diverticulitis,” she said.
After a median follow-up of 37 months, no significant differences were observed between patients discharged to home and those admitted to the hospital in readmission or return to the ED (13% vs 14%), recurrent diverticulitis (23% in each group), or in colon resection at subsequent encounter (16% vs 19%). “Among patients discharged to home, only one patient required emergency surgery, and this was 20 months after their index admission,” Dr Sirany said. “We think that the low rate of readmission in patients discharged home demonstrates that this is a safe approach to management of patients with diverticulitis, when using information from the CT scan.”
Closer analysis of patients who were discharged home revealed that six patients had extraluminal air on CT scan, three of whom returned to the ED or were admitted to the hospital. In addition, 11% of those with uncomplicated diverticulitis returned to the ED or were admitted to the hospital.
Dr Sirany acknowledged certain limitations of the study, including its retrospective design, a lack of complete follow-up for all patients, and the fact that it included patients with recurrent diverticulitis. “Despite the limitations, we recommend that young, relatively healthy patients with uncomplicated findings on CT scan can be discharged to home and managed as an outpatient,” she said. “In an era where there’s increasing attention to health care costs, we need to think more critically about which patients need to be admitted for management of uncomplicated diverticulitis.”
Microsensor Perfectly Distinguished Coagulopathy Patients From Controls
AMY KARON
FRONTLINE MEDICAL NEWS
Using less than a drop of blood, a portable microsensor provided a comprehensive coagulation profile in <15 minutes and perfectly distinguished various coagulopathies from normal blood samples—handily beating the results from both activated partial thromboplastin time (aPTT) and prothrombin time (PT).
Dubbed ClotChip, the disposable device detects coagulation factors and platelet activity using dielectric spectroscopy, Evi X. Stavrou, MD, said at the annual meeting of the American Society of Hematology. The development points the way for comprehensive, rapid, point-of-care (POC) assessment of critically ill or severely injured patients and those who need ongoing monitoring to evaluate response to anticoagulant therapy, she added.
Existing POC coagulation assays have several shortcomings, Dr Stavrou, of Case Western Reserve University, Cleveland, said during a press briefing at the conference. They are relatively insensitive, fail to measure platelet activity, or are only approved for specific subgroups of patients, such as those on warfarin, she specified.
To develop an alternative, Dr Stavrou and her associates added a parallel-plate capacitive sensing structure to an inexpensive, disposable microfluidic biochip designed to test 9 microliters (less than one drop) of blood. They built the microsensor from biocompatible and chemically inert materials to minimize the chances of artificial contact activation.
To test the device, the researchers used calcium dichloride to induce coagulation in whole blood samples from 11 controls with normal aPTT and PT values. Time curves of output from the microsensor showed that coagulation consistently peaked within 4.5 to 6 minutes.
Next, the investigators tested blood from 12 patients with coagulopathies, including hemophilia A, hemophilia B, acquired von Willebrand factor defect, and congenital hypodysfibrinogenemia. These samples all yielded abnormal curves, with prolonged times to peak that ranged between 7 and 15 minutes—significantly exceeding those of healthy controls (P = .002).
By plotting rates of true positives against rates of true negatives, the researchers obtained areas under the receiver-operating curves of 100% for ClotChip, 78% for aPTT, and 57% for PT. In other words, ClotChip correctly identified all cases and controls in this small patient cohort, while neither aPTT nor PT did.
Finally, the researchers used the microsensor to measure coagulation activity in normal blood samples that they treated with prostaglandin E2 to inhibit platelet aggregation. Normalized permittivity (an electrical measure) was significantly lower than in untreated control samples (P = .03), but time-to-peak values were the same in both groups. This finding confirms the chip can identify abnormal platelet function, Dr Stavrou said. “ClotChip is sensitive to the complete hemostasis process, exhibits better sensitivity and specificity than conventional coagulation assays, and discriminates between coagulation and platelet defects,” she concluded.
The investigators are recruiting volunteers for an expanded round of testing for the device, and are working to optimize construction to further enhance its sensitivity.
Survey: Overprescribing Is the Cause of the Opioid Crisis
M. ALEXANDER OTTO
FRONTLINE MEDICAL NEWS
Almost a third of doctors blamed overprescribing as the cause of the opioid crisis, according to a survey of 225 US primary care, emergency medicine, and pain management physicians by InCrowd, an online physician survey company.
Respondents said their and other physicians’ overprescribing is the single biggest factor fueling the leap in opioid abuse over the past 5 years.
“We were told…that [opioids] wouldn’t be addictive in the great majority of patients. This was obviously wrong,” said a Utah EP in practice for 38 years. Meanwhile, 24% of the respondents cited aggressive patient drug-seeking as the primary cause, and 18% blamed drug dealers.
In short, the survey pointed out what front-line doctors think needs to be fixed as the nation combats prescription opioid abuse and the subsequent heroin epidemic. Their insights “should be a rallying cry” for changes in 2017, said epidemiologist Diane Hayes, PhD, president and cofounder of InCrowd.
Making pain the “fifth vital sign” and allowing patients to downgrade doctors on surveys if they don’t prescribe or refill opioid prescriptions compounded the situation. Lengthy waits for specialists with better pain options, many of whom are not covered by Medicaid or the Affordable Care Act, also added to the problem, survey respondents said.
“We’re caught in the middle” between the Joint Commission on Accreditation of Healthcare Organization’s fifth vital sign and overprescribing, a primary care physician (PCP) said.
Seventy-three percent of survey respondents said that they want opioid alternatives, noting exasperation with nonsteroidal anti-inflammatory drugs, physical therapy, and exercise. About half recommend behavioral health interventions, while 20% recommend vitamin and herbal supplements. Only 10% recommend medical marijuana, probably because it is inaccessible to most US patients. Meanwhile, the respondents said they want opioid prescribing “hemmed in.” Almost two-thirds wanted refill limits and more frequent refill evaluations, and many agreed that there needs to be a weaning protocol before the drugs are even started. Some wanted to limit advertising.
Easton Jackson, MD, a PCP in West Valley City, Utah, who answered the survey, helped make the answers real by sharing his thoughts.
“We need to recognize that…people don’t set out to get addicted to opioids….We need to educate [patients] and assist them with their expectations. They need to understand that they’re going to have pain from surgery and injuries. Our goal isn’t to make them pain-free. It’s to manage their pain,” he said.
“We as physicians need to write for fewer pills and in lower doses. We need to see our patients back sooner. If it’s not working, stop increasing the dose and instead taper the patient off the medication. We need to be familiar with the adjuvant therapies. As easy as it is to say, ‘send them all to the pain specialist,’ there simply aren’t enough of them around,” Dr Jackson said.
Physician respondents to InCrowd’s opioid survey have practiced an average of 25 years, and were scattered around the United States. They filled out the four-question survey during October 27 to 28, 2016. They signed up to receive and answer InCrowd’s questions, and were paid nominally for their time.
Half (50%) of respondents estimated that they prescribed opioids to <10% of their patients; 38% said they prescribed to less than half of their patients; and 12% estimated they prescribed opioids to more than half of their patients.
Adding Respiratory Rate to Triage Criteria Improves Accurate Staging of Chest Trauma Patients
MICHELE G. SULLIVAN
FRONTLINE MEDICAL NEWS
Adding respiratory rate (RR) and suspected blunt chest injury to a trauma assessment in the field significantly improved the appropriate triaging of level III trauma patients.
When the assessment specifically evaluated for tachypnea in the setting of blunt chest injury, undertriaging improved by 1.2%, John Yonge, MD, said at the annual clinical congress of the American College of Surgeons.
“When we applied this new criteria to our 10-year study, we identified 661 patients who should have been activated as a level I or level II,” but instead were assessed as less critically injured, Dr Yonge said in an interview. This initial misstep significantly extended the time before patients could have critical surgical procedures and was related to higher mortality among them.
Dr Yonge, a surgical fellow at Oregon Health & Science University (OHSU), Portland, and his mentor Martin Schreiber, MD, conducted the retrospective study of 7,880 trauma patients admitted at level III activation from 2004 to 2014. The OHSU trauma system has three activation levels.
- Level I activations are reserved for the most critically injured patients; attending trauma surgeon and anesthesiologist presence is mandatory.
- Level II activations capture moderate-to-severe injuries; trauma surgeon and respiratory therapist presence is mandated.
- Level III activations are designed to capture patients who do not require an immediate lifesaving intervention; the presence of the trauma surgery chief resident and attending emergency medicine physician is mandatory.
Patients were considered undertriaged if they were admitted as level III activations, but then required a critical intervention (chest tube placement, intubation, needle thoracostomy, or intracranial pressure monitoring) in the ED or ultimately met level I or II activation criteria.
Among all the level III patients, 466 (6%) were undertriaged: 390 were undertriaged based on the existing level I or II activation criteria, and 76 were considered undertriaged based on the need for a critical intervention.
Most of the undertriaged patients (65%) met criteria for level I activation; the rest should have been triaged as level II patients. Compared with appropriately staged level III patients, mortality among the undertriaged patients was significantly higher (3.2% vs 0.6%). Undertriaged patients also experienced longer delays before initiation of major emergency surgery: a mean of 147 minutes, compared with 106 minutes for appropriately triaged level I patients and 62 minutes for appropriately triaged level II patients.
Dr Yonge then looked for clinical measures that would improve triage. Tachypnea (RR >20 breaths/min) in the field stood out as a significant factor. Tachypneic patients who had a suspected chest injury were 70% more likely to be undertriaged than were those with a normal RR. Tachypnea was significantly associated with a diagnosis of flail chest, ED intubation, and chest-tube placement.
The team then constructed a new triage criterion for patients with suspected chest injury—tachypnea combined with suspected blunt thoracic injury. By applying that model to their study population of level III patients, they determined that the level III undertriage rate would be reduced by 1.2%.
Tying the physiological marker of tachypnea to a suspected clinical diagnosis is a key factor, Dr Yonge noted. “Just adding tachypnea doesn’t help us. In fact, it would overwhelm us, because a trauma patient could very well be tachypneic because he’s experiencing panic. But tying it to a suspected clinical diagnosis gives us a meaningful result.”
He confirmed this linkage with an additional analysis. “We looked to see how severely injured these patients were and found that 71% of them had an Abbreviated Injury Score (AIS) to the chest of 3 or more, indicating a severe chest injury. Only 29% had an AIS of 2 or less. So this proves that respiratory rate is a valid triage criterion and can be used to identify patients who need a higher level of trauma care.”
The challenge now, Dr Yonge said, is incorporating the marker into clinical practice. “It doesn’t matter how many statistics you do, if you can’t educate the prehospital providers in this, it’s useless. They are the crux of the trauma system.”
Although national guidelines do recommend assessing RR as part of field triage, it often isn’t recorded or is only estimated, Dr Yonge said. That’s one reason he used the 20 breaths/min cutoff rate. “It doesn’t even take a full minute to assess this, but it can make a big improvement in care.”
Racial Differences in Adherence to Prescribed Analgesia in Cancer Patients: An Integrated Review of Quantitative Research
From the University of Pennsylvania School of Nursing, Philadelphia, PA.
Abstract
- Background: Racial/ethnic disparities in analgesic treatment for pain have been widely documented in the United States. However, the connection between race/ethnicity and adherence to prescribed analgesics has not been described.
- Objectives: To review and synthesize quantitative research documenting racial/ethnic differences in adherence to prescribed analgesia in cancer patients.
- Methods: We performed a systematic search of quantitative, primary studies in Scopus, CINAHL, PubMed, Ovid, PsychInfo, and EMBASE. The title and abstract of each article was reviewed for relevance and whether inclusion criteria were met. Evidence was examined for relevant outcomes, data collection methods, variables studied in relation to adherence, and the magnitude of association between race/ethnicity and adherence.
- Results: Seven studies met inclusion criteria. Reported rates of adherence varied in studies among Hispanic/Latinos, African Americans, Asians, and whites based on variation in measurement tools, research questions, populations from which participants were recruited, and predictive variables analyzed. Most existing studies of analgesic adherence used self-report to measure adherence. Only 1 study used a validated, real-time electronic instrument to monitor prescribed opioid adherence and had a longitudinal study design.
- Conclusion: Limited research has examined relationships between adherence to prescribed analgesic regimens and racial disparities. Existing studies point to the clinical and socioeconomic factors that may interact with race/ethnicity in explaining analgesic and opioid adherence outcomes in cancer patients.
Key words: race, ethnicity, adherence, opiates, analgesics, pain management, cancer, pain treatment disparities.
The ongoing opioid epidemic and recent development of the Centers for Disease Control and Prevention (CDC) guidelines for chronic pain management have shaped a national conversation on opioid prescription and utilization [1]. The CDC delineates provider recommendations for opioid prescription. This focus on prescribed medication regimens is inadequate without an understanding of how patients take or adhere to prescribed medications. Cancer patients are a unique group. Moderate to severe pain in cancer patients is usually treated with opioids, and adherence to analgesia has been conceptualized a key mediator of cancer pain outcomes. For instance, a recent study found that patterns of analgesic adherence, specifically, inconsistent adherence to strong opioids (World Health Organization step 3), is one of the strongest predictors of health care utilization among outpatients with cancer pain [2]. Approximately 67% to 77% of cancer patients experience pain that requires management with analgesia [3], especially in the absence of access to nonpharmacologic pain treatments [2]. Thus, barriers in relation to adequate pain management can result in poor pain treatment outcomes and impaired quality of life for cancer patients.
Insufficient pain management has been found to have a negative impact on the quality of life and physical and mental functions of patients with cancer [4]. Patients who experience severe cancer pain are significantly more likely to experience multiple other symptoms such as depression, fatigue, and insomnia, resulting in diminished physical function [5], social role function [6], and greater out of pocket cost of managing pain and asso-ciated symptoms [7]. Minority populations, however, disproportionately carry the burden of undertreated pain [4,8–11,13–16]. Evidence suggests that blacks/African Americans are more likely to experience unrelieved cancer pain [4,8–11,13–16]. They are also less likely than their white counterparts to receive analgesic treatment for cancer pain [8–11,13,15,16]. Little is known, however, about racial disparities in relation to adherence to analgesia for cancer pain when providers prescribe analgesics.
The purpose of this paper is to review the published literature that has addressed the associations between disparities and adherence to analgesia among cancer patients. Evidence was examined for outcomes studied, data collection methods, variables studied in relation to adherence, and the magnitude of association based on race and adherence.
Methods
We performed a systematic search of studies published between 1990 and the present in Scopus, CINAHL, PubMed, Ovid, PsychInfo, and the EMBASE databases. The inclusion criteria consisted of published articles in the aforementioned databases that were (1) set in the United States, (2) primary studies, (3) employed quantitative design, (4) assessed adherence or compliance to analgesics or adequacy of pain management using the Pain Management Index (PMI), (5) sample was exclusively minority or may have had a comparative group. The title and abstract of each article in the the search results was reviewed for relevance to study aims and inclusion and exclusion criteria, and any duplicates were eliminated. A total of 6 studies were found using this method (Table 1), and an additional study was found in the reference list of 1 of these 6.
Results
The 7 included studies were observational in nature; 4 were cross-sectional [4,12,15,16], 2 were retrospective [3,14], and 1 was prospective and used objective measures of analgesic adherence [13] (Table 2).
Defining and Operationalizing Adherence
Meghani and Bruner [16] point out that analgesic adherence is a “heterogeneous construct that lends itself to varied results and interpretations depending on the measurements used or dimensions studied.” Adherence to analgesia was explicitly defined in all 7 studies (Table 3). One study reported an adherence rate that was the total dose over 24 hours divided by the dose prescribed then multiplied by 100 [4]. The total dose over 24 hours was used in another study but was converted to an equianalgesic calculation [12]. Another set of studies used a similar definition but specified percentages based on medication or type of prescription, such as an around-the-clock(ATC) regimen [13,15,16]. In 2 studies, adherence was measured based on chart review of yes/no questions posed about whether or not patients had taken medications as prescribed [3,15].
The measurements of adherence differed between studies. Four studies [4,12,14,16] used adherence as a primary outcome and the rest employed adherence as a facet of pain management [3,13,15]. The most frequent measure of adherence was self-report. The widely validated Morisky Medication Adherence Scale (MMAS) instrument was used in 3 of 7 studies [12,13,15]. Meghani and Bruner [15] utilized the modified MMAS plus a previously validated visual analog scale for doses of medication to assess adherence over week- and month-long intervals. One study used patient interviews to capture self-reporting of opioid prescription and opioid use. Additionally, the study used MMAS to further characterize the adherence measurements [12]. Using a more objective method, Meghani et al [13] employed a microprocessor in the medication cap to determine the percentage of the total number of prescribed doses that were actually taken [13]. The processor sensed when the bottle was open, which served as a proxy for taking medications at appropriate times.
Analgesic Adherence Rate
To report the analgesic adherence rates, 6 studies presented a percentage [3,4,12,13,15] and all but 1 highlighted the barriers associated with poor adherence [3,4,12,13,15,16].
The results of a pilot study exploring intentional and unintentional adherence revealed that 85.5% of patients took the prescribed medications in the previous week. Further analysis using visual analogue scale for dose adherence found that that 51% took up to 60% of the prescribed medications [15]. In an exclusively African-American sample, the adherence rate was reported as 46% [4]. Another study by Meghani et al compared adherence to prescribed ATC analgesics between African Americans and whites with cancer-related pain using an electronic monitoring system [13]. The overall adherence rate for African Americans was 53% and 74% for whites [13]. The authors concluded that there was a significant difference between the analgesic adherence rates between African Americans and whites in this study. On sub-analysis, analgesic adherence rates for African Americans were much lower for weak opioids (34%) and higher for long-acting opioids (63%).
In a study of individuals from an outpatient supportive care center with a majority white sample (74% Caucasian), overall 9.6% of patients deviated from the opioid regimen, while approximately 90% reported high adherence [12]. It is important to note that a convenience sample was used here. Of the total 19 patients that deviated from the regimen, 11 used less opioids than prescribed and 8 used higher doses. Upon analysis, the opioid deviation was more frequent in males and non-whites. However, statistical analyses of the magnitude of deviation from prescribed dose and non-white racial/ethnic background were not reported. Within the “non-whites” category, the race/ethnicity is defined as African American (16%, n = 32) and “other” (9%, n = 18). The authors contend that this strong adherence resulted from a strong understanding of the regimen as evidenced by a high agreement between the prescribed dose and the patient reported prescription [12]. Nguyen et al [12] argue that the literature shows that lower adherence rates for minority patients may be explained by the presence of comorbidities and lack of insurance.
Two other studies reported adherence rates for separate insurance cohorts [3,14]. The Medicaid cohort was younger and had a higher percentage of African-American individuals. However, in the self-pay/charity care group, the majority was Hispanic [3]. In the pilot study, the differences between the groups on adherence with prescribed medication regimens did not achieve statistical significance. The data were summarized to suggest that nonadherence was more likely in the self-pay/charity care group and more follow-up visits occurred after discharge [3]. During the larger retrospective study there was no difference in number of patients adhering to the regimen at each follow-up visit in each benefit group. The study concluded that the long-acting opiate adherence was influenced only by the benefits of use and that race/ethnicity was not a statistically significant predictor [14].
Factors Associated with Adherence
Multiple studies investigated factors underlying reported analgesic adherence rates for the ethnic and racial groups studied. Both clinical and sociodemographic variables were associated with analgesic adherence (Table 4). These included cancer type and disease stage [3,4,13,14], pain intensity [3,4,13–16], side effects [13,15], type of analgesic prescribed [3,4,13–16], income/socioeconomic status [3,13,14], behavioral history [3,12,13], gender [3,4,12–16], and perceived barriers [3,4,13,15,16].
Cancer Type and Stage
Most studies did not find significant associations between analgesic adherence rates and cancer type and stage [3,12,14]. However, 1 study that sought to identify unique factors underlying analgesic adherence for African Americans and whites found that whites reported higher analgesic adherence in relation to “time since cancer diagnosis,” possibly indicating disease severity and progression [13]. In another study that involved a majority of African-American patients, individuals with colon and rectal cancer had lower adherence rates [4]. In this study, patients with colon and rectal cancer had more analgesic prescriptions (2.5 +/– 2.3 analgesics) compared to patients with other cancer diagnoses. The authors concluded that an increased medication burden might have contributed to a decreased adherence rate. Overall, other cancer types did not correlate with adherence rates [4].
Pain Intensity
Six studies examined pain intensity and duration [3,4,13–16]. Three studies found a difference in reported pain intensity between racial/ethnic groups [3,13,16], 1 found no correlation between pain intensity and race/ethnicity [14], and 3 concluded that pain intensity was a significant predictor of adherence rates [3,13,15].
Meghani and Bruner’s pilot study explored possible correlates associated with intentional and unintentional nonadherence [15]. Overall, individuals were more likely to report forgetfulness (unintentional nonadherence) and to stop taking pain medicine when feeling “worse” (intentional nonadherence) if they believed that it was easier to deal with pain than with the side effects of analgesia [15]. Further, forgetfulness was negatively associated with the need for “stronger” pain medication. Concern about using too much pain medication was positively correlated with both forgetfulness and carelessness. The need for stronger pain medication was also correlated with significantly higher pain levels and lower pain relief [15].
In a comparative study of African Americans and whites, African Americans reported greater cancer pain and lower pain relief on the Brief Pain Inventory (BPI) and had a negative PMI. The PMI measure is a simple index linking the usual severity of cancer pain with the category of medication prescribed to treat it. PMI is calculated by subtracting patient’s pain levels (“pain worst” score from the BPI coded as mild, moderate, or severe) from the most potent analgesia prescribed. A negative PMI implies inadequate analgesic prescription relative to the reported pain level. Pain intensity was a significant factor related to increased adherence in whites but not African Americans. For African Americans, analgesic adherence was predicted by socioeconomic status, provider communication factors, and side effects. Similarly, in another study that compared African Americans and Hispanics, African Americans were more likely to have a negative PMI than Hispanics and were less likely to report that pain medication relieved pain [16]. In a pilot study that compared Medicaid recipients to self-pay/charity care patients, African-American participants had lower reported pain scores than Hispanics and Caucasians [3]. In the larger follow-up study, however, ethnicity did not prove a significant predictor for pain levels [14].
In a study with exclusively African-American patients, a significant correlation was found between pain intensity and adherence; specifically, as intensity increased, adherence increased [4]. Results for the entire African-American cohort indicated that 90% of patients had analgesic prescriptions for cancer-related pain, but 86% continued to report having moderate to severe worst pain [4]. A study that compared African Americans and whites showed that lower pain relief with analgesics was associated with lower adherence to analgesia for cancer pain among whites [13]. For every unit increase in “least pain” scores (indicating lower pain relief) on the BPI item, dose adherence decreased by 2.88%. Pain levels and relief did not explain adherence rates among African Americans. Whites were also more likely to make decisions on analgesic use based on the amount of relief anticipated from the use of analgesics [13] whereas African Americans were more likely to make analgesic use decisions based on analgesic side effects.
Side Effects
In a pilot study that explored the intricacies of adherence, some individuals felt it was easier to deal with pain than with the side effects of pain medications. These individuals were also more likely to report forgetfulness and to stop taking medications if feeling “worse” [15]. One study, which included African-American and white cohorts, found that an increase in the severity of side effects was associated with lower adherence to analgesia for African Americans but not whites. Furthermore, African Americans reported a greater number of analgesic side effects at baseline. African Americans were also more likely to make analgesic decisions based on side effects in comparison to whites participants, who made decisions based on expectation of pain relief [13]. In a study with exclusively African-American patients, patients with concerns about pain medication possibly causing confusion were more likely to have poor adherence [4].
Type of Analgesic Prescribed
In the analyses, 3 studies found a difference between analgesic prescriptions among ethnic groups [12,13,16], 3 found that there was a statistical significance between type of prescription and adherence [4,13,16], and 2 studies [3,14] found no statistical correlation between type of analgesic prescribed and adherence.
In a study of African Americans and Hispanics, both groups took analgesics on an “as-needed” basis despite the guidelines for cancer pain management [16]. However, African Americans reported taking analgesics less than twice daily. Overall, only a small percentage of patients took sustained-release analgesics that require fewer doses per day [16]. Similarly, in another study that compared adherence between African Americans and whites, the overall analgesic adherence rate was different on sub-analysis for specific analgesic prescriptions. The analgesic adherence rates for African Americans ranged from 34% for weak opioids to 63% for long-acting opioids. In comparison, the analgesic adherence rates for whites ranged from 55% for weak opioids to 78% for long-acting opioids [13]. In conclusion, patients on long-acting opioids were more likely to have higher adherence. Adherence rates for African Americans were found in another study. The adherence rate for adjuvant analgesics was highest at 65%, step 2 opioids at 44% and step 3 opioids at 43% [4].
In a study with exclusively African-American patients, poor adherence was significantly correlated with step 3 opioids [4]. Another study that explored the correlation between type of analgesic and adherence found that intentional nonadherence was less likely in individuals that were prescribed step 3 opioids [15]. Specifically, individuals with this behavior were also more likely to report lower pain levels and chose to stop the use of analgesics when feeling better [15].
Within a pilot study that compared benefit programs and payor groups, the differences in the prescription of long-acting opiates did not reach statistical significance [3]. However, in the larger, definitive study, the comparison revealed that patients in the self-pay/charity care group were less likely to receive a prescription for long-acting opiates. The data further revealed that Hispanic and Asian patients were prescribed long-acting opiates at a lower rate compared to the larger sample. Further, African Americans and Caucasians were prescribed long-acting opiates at a higher rate than the larger sample. In another analysis, with benefits and race/ethnicity, benefits were the only statistically significant predictor. While statistically controlling for race/ethnicity, Medicaid patients were 2.4 times more likely to receive a prescription for long-acting opioids than the self-pay/charity care patients [14].
Income/Socioeconomic Status
Three studies in this analysis [3,13,14] found that income and socioeconomic status were significant predictors of analgesic adherence for cancer pain. In a comparison between African Americans and whites, income was the strongest predictor of analgesic adherence for cancer pain in African Americans [13]; specifically, individuals with a household income of less than $10,000 a year had a 41.83% lower percentage of dose adherence. Among whites, income did not have a significant correlation with analgesic rates [13].
A pilot study and larger definitive study [3,14] were conducted to compare the effects of prescription benefits. The prescription benefits included were Medicaid and self-pay/charity care. Through comparison, none of the Medicaid patients reported financial barriers but the self-pay/charity care patients were more likely to report financial barriers to adherence [3]. In the larger study, the findings indicated that there was significant association of adherence by benefits and race/ethnicity. As mentioned above, benefits were a dominant predictor of long acting opiate use and further adherence [14].
Gender
Apart from ethnicity or race as a variable associated with adherence, association of analgesic adherence and gender were observed in 4 studies [3,13–15] and evaluated in 2 studies. One study [4] found that a patient’s gender and education level did not correlate with adherence rates. However, in another study [12] men were more likely to deviate from the prescribed dose. Overall, within the entire cohort [12] men and minority patients were most likely to deviate from the prescribed dosing regimen in comparison to all other patient demographic factors.
Attitudes and Barriers
Five of the 7 studies investigated perceived barriers to analgesic adherence [3,4,13,15,16]. Four used the Barriers Questionnaire II (BQ-II) [18] to further understand patients’ beliefs about cancer pain management [3,12,13,15]. Using this validated tool, 1 study found that non-white individuals had higher scores on the BQ-II than white patients [12]. Within the non-white group in the above study, the mean score on the BQ-II for African Americans was 1.76 (± 0.81) and the mean score for “other” was 2.16 (± 0.93) [12]. Further, low MMAS scores were significantly associated with higher BQ-II scores. Similarly, higher BQ-II scores correlated with opioid deviation toward higher than prescribed dose [12].
Another study with a primarily African-American cohort did not use the BQ-II but asked specific questions in regards to perceived barriers to analgesics. Within the cohort, 87% reported a fear of addiction to pain medicine. Further, 77% had a fear of injection, 75% were concerned about a tolerance for analgesics, and side effects were a major concern. Overall, nausea was the greatest reported concern followed by potential for confusion, which was negatively associated with taking analgesics. Distracting the doctor from curing their illness was a predictor of improved adherence; however, individuals were more likely to take Tylenol for pain relief. Similarly, no significant barrier items affected adherence to NSAIDs. In relation to step 2 opioids, patients who felt it was important to be strong by not talking about pain were more likely to have better adherence [4]. Similar results with African Americans were identified in another study [13]. In the comparison between African Americans and whites, African Americans had more subjective barriers compared to whites. Particularly for African Americans, each unit increase in concern about distracting the doctor from curing the disease, the percentage of dose adherence decreased by 7.44 [13].
In a study that compared payer groups, a questionnaire elicited reasons for nonadherence [3]. Similar reasons for nonadherence emerged including financial, fear of addiction or increased medication use, and running out medication.
Behavioral History
Only 1 study used CAGE (Cut down, Annoyed, Guilty, and Eye-opener), an alcohol-screening questionnaire, to determine a possible relationship with analgesic adherence. In this study, there were 19 cases of opioid deviation, 16% of which were CAGE positive and had severe deviation toward less than the prescribed doses [12]. In further analysis, no association was found between CAGE positively and opioid deviation to higher intake [12]. Two other studies gathered data on history of depression, substance use, and alcohol use but no significant correlation was found [3,13].
Discussion
Previous literature has reported overall analgesic adherence rates among oncology patients ranging from 62% to 72% [23]. Factors at the provider and system level have been considered in past research, but the patient perspective is poorly represented in the literature [13]. A majority of studies on analgesic adherence have been completed with cohorts made up predominantly of white individuals [13,23,24], while others focus on racially homogenous and/or ethnically different populations in other countries [21,25,26].
This review confirms that there is a paucity of well-designed studies that describe the associations between racial and ethnic disparities and adherence to opioids among patients with cancer pain. This is despite the fact that moderate to severe cancer pain in the U.S. is managed mainly with analgesics and specifically with opioids [19]. In addition, cancer patients with health insurance have both more pharmacy claims as well as more claims for higher doses of opioids [20] compared to noncancer patients. The lack of attention to analgesic and opioid adherence among cancer patients is surprising in the light of the recent high-profile initiatives to reduce opioid misuse [31].
Multiple studies highlighted the importance of pain management education and adequate pain assessment for effective analgesic use [4,16]. In the study in the palliative care setting, the authors concluded that patients who are educated, counseled, and monitored by a palliative or supportive care team have less episodes of opioid deviation and trends toward lower opioid use [12]. A systematic review and meta-analysis confirmed findings that educational interventions for patients improved knowledge about cancer pain management, however, most did not improve reported adherence to analgesics [27,28]. These findings emphasize the need for further research on interventions to improve racial/ethnic disparities in analgesic adherence for cancer pain.
Limitations
The findings of this review should be evaluated in the context of the following limitations. First, adherence to a prescribed regimen is a difficult outcome to measure and a majority of studies in this review used subjective measures to assess analgesic adherence for cancer pain. Of note, self-report was the primary measurement employed. Studies in non–cancer pain settings that have evaluated various methodological approaches to adherence measurement found that patients are likely to over-report adherence when using self-report or a diary format in comparison to an electronic monitoring system. Only 1 study in this review used an objective measure of adherence [13]. Some previous studies contend that self-report in comparison to other, objective measurements of medication adherence are accurate [23]. Further research is needed to determine the most accurate measurement of analgesic adherence in cancer patients.
Also, invariably the studies employed an English-speaking sample, which excludes an understanding of analgesic adherence for cancer pain in linguistically diverse Americans. In addition, most studies included patients who were either white Americans or African Americans and some studies lumped several racial ethnic minority subgroups as “nonwhites” or “other.”
A majority of studies were cross-sectional [4,12,15,16]. For instance, studies used a 24-hour time period to assess ATC medication as well as as-needed regimens, which may not capture the information needed to understand adherence to as-needed regimens [4]. With longitudinal studies, a greater understanding of adherence can be determined. However, there is potential bias with studies that track patients primarily at follow-up appointments. Individuals who are compliant with follow-up appointments may present with different analgesic adherence compared to those who do not attend follow-up appointments. This potential bias should be evaluated in longitudinal studies with various sensitivity analyses or using tools that identify healthy user bias.
Most studies recruited patients from outpatient oncology clinics, however, 1 study was conducted with a sample from an outpatient supportive care center managed by a palliative care team [12]. Due to the goals of palliative care, which include specialized treatment for individuals with serious illness and a focus on symptom management and relief, patients in this setting may have a different attitude toward using opioids.
Conclusion
Although data remain limited, our review suggests that while overuse of opioids has been a well-cited concern in patients with chronic non-cancer pain [21,33], cancer patients demonstrate considerable underuse and inconsistent use of prescribed analgesics. This is important as a recent study found that inconsistent adherence to prescribed around-the-clock analgesics, specifically the interaction of strong opioids and inconsistent adherence, is a strong risk factor for hospitalization among cancer outpatients who are prescribed analgesics for pain [1]. Of note, adherence to opioids in patients with cancer may be driven by a unique set of factors and these factors may differ for minorities and non-minority patients. For instance, studies in this review indicate that income is a strong predictor of analgesic adherence for African Americans but not for whites. This is because race and socioeconomic status frequently overlap in the United States [29]. In addition, like cancer pain, analgesic side effects may also be poorly managed among African Americans and other minorities. For example, in 1 study, Meghani et al used a trade-off analysis technique (conjoint analysis) to understand trade-offs African Americans and whites employ in using analgesics for cancer pain [30]. The authors found that African Americans were more likely to make analgesic adherence decisions based on side effects whereas whites were more likely to make adherence decisions based on pain relief [30]. In subsequent analysis, these authors showed that the race effect found in their previous studies was mediated by the type of analgesics prescribed to African Americans vs. whites [31]. African Americans with cancer pain were prescribed analgesics that had a worse side effect profile after statistically adjusting for insurance type and clinical risks such as renal insufficiency [31].
Together, the available evidence indicates that both patients’ socioeconomic status and clinician treatment bias contributes to racial and ethnic disparities in analgesic adherence for cancer pain and subsequent cancer pain outcomes. Thus, future research should investigate interventions for improving analgesic adherence among low-income minorities. Also, there is a need for clinician-level interventions focusing on cognitive bias modification related to cancer pain and side effects management, which appears to relate to analgesic nonadherence among racial/ethnic minorities. In addition, further research is needed to (1) rigorously describe analgesic and opioid adherence for cancer pain, (2) elucidate racial/ethnic and other socioeconomic and clinical disparities in analgesic and opioid adherence for cancer pain; (3) and clarify the role of analgesic and opioid adherence for cancer patients including outcomes for the patients and the health care system.
Corresponding author: Salimah H. Meghani, PhD, MBE, RN, University of Pennsylvania School of Nursing, Room 337, Fagin Hall, 418 Curie Blvd, Philadelphia, PA 19104, [email protected].
Financial disclosures: None.
1. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain - United States, 2016. JAMA 2016;315:1624–45.
2. Meghani SH, Knafl GJ. Patterns of analgesic adherence predict health care utilization among outpatients with cancer pain. Patient Prefer Adher 2016;10:81–98.
3. Bryan M, De La Rosa N, Hill AM, et al. Influence of prescription benefits on reported pain in cancer patients. Pain Med 2008;9:1148–57.
4. Rhee YO, Kim E, Kim B. Assessment of pain and analgesic use in African American cancer patients: Factors related to adherence to analgesics. J Immigr Minor Health 2012;14:1045–51.
5. Laird BJ, Scott AC, Colvin LA, et al. Pain, depression, and fatigue as a symptom cluster in advanced cancer. J Pain Symptom Manage 2011;42:1–11.
6. Ferreira KA, Kimura M, Teixeira MJ, et al. Impact of cancer-related symptom synergisms on health-related quality of life and performance status. J Pain Symptom Manage 2008;35:604–16.
7. Craig BM, Strassels SA. Out-of-pocket prices of opioid analgesics in the United States, 1999-2004. Pain Med 2010;11:240–47.
8. Institute of Medicine: Relieving pain in america: a blueprint for transforming prevention, care, education, and research. Washington, DC: National Academies Press; 2011.
9. Institutes of Medicine. Unequal treatment: confronting racial and ethnic disparities in health care. Washington, DC: National Academies Press; 2003.
10. Meghani SH, Byun E, Gallagher RM. Time to take stock: A meta-analysis and systematic review of analgesic treatment disparities for pain in the United States. Pain Med 2012;13:150–74.
11. Cleeland CS, Gonin R, Baez L, Loehrer P, Pandya KJ. Pain and treatment of pain in minority patients with cancer. The Eastern Cooperative Oncology Group Minority Outpatient Pain Study. Ann Intern Med 1997;127:813–6.
12. Nguyen LMT, Rhondali W, De la Cruz M, et al. Frequency and predictors of patient deviation from prescribed opioids and barriers to opioid pain management in patients with advanced cancer. J Pain Symptom Manage 2013;45:506–16.
13. Meghani SH, Thompson AML, Chittams J, et al. Adherence to analgesics for cancer pain: A comparative study of African Americans and whites using an electronic monitoring device. J Pain 2015;16:825–35.
14. Weider R, DeLaRosa N, Bryan M, et al. Prescription coverage in indigent patients affects the use of long acting opioids in management of cancer pain. Pain Med 2014;15:42–51.
15. Meghani SH, Brune DW. A pilot study to identify correlates of intentional versus unintentional nonadherence. Pain Manag Nurs 2013;14:e22-30.
16. Anderson KO, Mendoza TR, Valero V, et al. Minority cancer patients and their providers: pain management attitudes and practice. Cancer 2000;88:1929–38.
17. Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998;52:377–84.
18. Ward SE, Goldberg N, Miller-McCauley V, et al. Patient-related barriers to management of cancer pain. Pain 1993;52:319–24.
19. Glare PA, Davies PS, Finlay E, et al. Pain in cancer survivors. J Clin Oncol 2014;32:1739–47.
20. van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, et al. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol 2007;18:1437–49.
21. Jacobsen R, Samsanaviciene J, Liubarskiene Z, et al. Barriers to cancer pain management in Danish and Lithuanian patients treated in pain and palliative care units. Pain Manag Nurs 2014; 15:51–8.
22. National Institutes of Health. Pathways to prevention: the role of opioids in the treatment of chronic pain. September 29–30, 2014. Executive summary: final report. Accessed 10 Sep 2015 at https://prevention.nih.gov/docs/programs/p2p/ODPPainPanelStatementFinal_10-02-14.pdf.
23. Miaskowski C, Dodd MJ, West C, et al. Lack of adherence with the analgesic regimen: A significant barrier to effective cancer pain management. J Clin Oncol 2001;19:4275–79.
24. Yoong J, Traeger LN, Gallagher ER, et al. A pilot study to investigate adherence to long-acting opioids among patients with advanced lung cancer. J Palliat Med 2013;16:391–6.
25. Lai YH, Keefe FJ, Sun WZ, et al. Relationship between pain-specific beliefs and adherence to analgesic regimens in Taiwanese cancer patients: A preliminary study. J Pain Symptom Manage 2002;24:415–22.
26. Cohen MZ, Musgrave CF, McGuire DB, et al. The cancer pain experience of Israeli adults 65 years and older: the influence of pain interference, symptom severity, and knowledge and attitudes on pain and pain control. Support Care Cancer 2005;13:708–14.
27. Bennett MI, Bagnall AM, Jose Closs S. How effective are patient-based educational interventions in the management of cancer pain? Systematic review and meta analysis. Pain 2009;143:192–9.
28. Oldenmenger WH, Sillevis Smitt PA, van Dooren S, et al. A systematic review on barriers hindering adequate cancer pain management and interventions to reduce them: a critical appraisal. Eur J Cancer 2009;45:1370–80.
29. Meghani SH, Chittams J. Controlling for socioeconomic status in pain disparities research: all-else-equal analysis when “all else” is not equal. Pain Med 2015;16:2222–5.
30. Meghani SH, Chittams J, Hanlon A, Curry J. Measuring preferences for analgesic treatment for cancer pain: how do African Americans and whites perform on choice-based conjoint analysis experiments? BMC Med Inform Decis Mak 2013;13:118.
31. Meghani SH, Kang Y, Chittams J, et al. African Americans with cancer pain are more likely to receive an analgesic with toxic metabolite despite clinical risks: a mediation analysis study. J Clin Oncol 2014;32:2773–9.
32. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids forchronic pain - United States, 2016. MMWR Recomm Rep 2016 Mar 18;65:1–49.
33. Chapman CR, Lipschitz DL, Angst MS, et al. Opioid pharmacotherapy for chronic non-cancer pain in the United States: a research guideline for developing an evidence-base. J Pain 2010;11:807–29.
From the University of Pennsylvania School of Nursing, Philadelphia, PA.
Abstract
- Background: Racial/ethnic disparities in analgesic treatment for pain have been widely documented in the United States. However, the connection between race/ethnicity and adherence to prescribed analgesics has not been described.
- Objectives: To review and synthesize quantitative research documenting racial/ethnic differences in adherence to prescribed analgesia in cancer patients.
- Methods: We performed a systematic search of quantitative, primary studies in Scopus, CINAHL, PubMed, Ovid, PsychInfo, and EMBASE. The title and abstract of each article was reviewed for relevance and whether inclusion criteria were met. Evidence was examined for relevant outcomes, data collection methods, variables studied in relation to adherence, and the magnitude of association between race/ethnicity and adherence.
- Results: Seven studies met inclusion criteria. Reported rates of adherence varied in studies among Hispanic/Latinos, African Americans, Asians, and whites based on variation in measurement tools, research questions, populations from which participants were recruited, and predictive variables analyzed. Most existing studies of analgesic adherence used self-report to measure adherence. Only 1 study used a validated, real-time electronic instrument to monitor prescribed opioid adherence and had a longitudinal study design.
- Conclusion: Limited research has examined relationships between adherence to prescribed analgesic regimens and racial disparities. Existing studies point to the clinical and socioeconomic factors that may interact with race/ethnicity in explaining analgesic and opioid adherence outcomes in cancer patients.
Key words: race, ethnicity, adherence, opiates, analgesics, pain management, cancer, pain treatment disparities.
The ongoing opioid epidemic and recent development of the Centers for Disease Control and Prevention (CDC) guidelines for chronic pain management have shaped a national conversation on opioid prescription and utilization [1]. The CDC delineates provider recommendations for opioid prescription. This focus on prescribed medication regimens is inadequate without an understanding of how patients take or adhere to prescribed medications. Cancer patients are a unique group. Moderate to severe pain in cancer patients is usually treated with opioids, and adherence to analgesia has been conceptualized a key mediator of cancer pain outcomes. For instance, a recent study found that patterns of analgesic adherence, specifically, inconsistent adherence to strong opioids (World Health Organization step 3), is one of the strongest predictors of health care utilization among outpatients with cancer pain [2]. Approximately 67% to 77% of cancer patients experience pain that requires management with analgesia [3], especially in the absence of access to nonpharmacologic pain treatments [2]. Thus, barriers in relation to adequate pain management can result in poor pain treatment outcomes and impaired quality of life for cancer patients.
Insufficient pain management has been found to have a negative impact on the quality of life and physical and mental functions of patients with cancer [4]. Patients who experience severe cancer pain are significantly more likely to experience multiple other symptoms such as depression, fatigue, and insomnia, resulting in diminished physical function [5], social role function [6], and greater out of pocket cost of managing pain and asso-ciated symptoms [7]. Minority populations, however, disproportionately carry the burden of undertreated pain [4,8–11,13–16]. Evidence suggests that blacks/African Americans are more likely to experience unrelieved cancer pain [4,8–11,13–16]. They are also less likely than their white counterparts to receive analgesic treatment for cancer pain [8–11,13,15,16]. Little is known, however, about racial disparities in relation to adherence to analgesia for cancer pain when providers prescribe analgesics.
The purpose of this paper is to review the published literature that has addressed the associations between disparities and adherence to analgesia among cancer patients. Evidence was examined for outcomes studied, data collection methods, variables studied in relation to adherence, and the magnitude of association based on race and adherence.
Methods
We performed a systematic search of studies published between 1990 and the present in Scopus, CINAHL, PubMed, Ovid, PsychInfo, and the EMBASE databases. The inclusion criteria consisted of published articles in the aforementioned databases that were (1) set in the United States, (2) primary studies, (3) employed quantitative design, (4) assessed adherence or compliance to analgesics or adequacy of pain management using the Pain Management Index (PMI), (5) sample was exclusively minority or may have had a comparative group. The title and abstract of each article in the the search results was reviewed for relevance to study aims and inclusion and exclusion criteria, and any duplicates were eliminated. A total of 6 studies were found using this method (Table 1), and an additional study was found in the reference list of 1 of these 6.
Results
The 7 included studies were observational in nature; 4 were cross-sectional [4,12,15,16], 2 were retrospective [3,14], and 1 was prospective and used objective measures of analgesic adherence [13] (Table 2).
Defining and Operationalizing Adherence
Meghani and Bruner [16] point out that analgesic adherence is a “heterogeneous construct that lends itself to varied results and interpretations depending on the measurements used or dimensions studied.” Adherence to analgesia was explicitly defined in all 7 studies (Table 3). One study reported an adherence rate that was the total dose over 24 hours divided by the dose prescribed then multiplied by 100 [4]. The total dose over 24 hours was used in another study but was converted to an equianalgesic calculation [12]. Another set of studies used a similar definition but specified percentages based on medication or type of prescription, such as an around-the-clock(ATC) regimen [13,15,16]. In 2 studies, adherence was measured based on chart review of yes/no questions posed about whether or not patients had taken medications as prescribed [3,15].
The measurements of adherence differed between studies. Four studies [4,12,14,16] used adherence as a primary outcome and the rest employed adherence as a facet of pain management [3,13,15]. The most frequent measure of adherence was self-report. The widely validated Morisky Medication Adherence Scale (MMAS) instrument was used in 3 of 7 studies [12,13,15]. Meghani and Bruner [15] utilized the modified MMAS plus a previously validated visual analog scale for doses of medication to assess adherence over week- and month-long intervals. One study used patient interviews to capture self-reporting of opioid prescription and opioid use. Additionally, the study used MMAS to further characterize the adherence measurements [12]. Using a more objective method, Meghani et al [13] employed a microprocessor in the medication cap to determine the percentage of the total number of prescribed doses that were actually taken [13]. The processor sensed when the bottle was open, which served as a proxy for taking medications at appropriate times.
Analgesic Adherence Rate
To report the analgesic adherence rates, 6 studies presented a percentage [3,4,12,13,15] and all but 1 highlighted the barriers associated with poor adherence [3,4,12,13,15,16].
The results of a pilot study exploring intentional and unintentional adherence revealed that 85.5% of patients took the prescribed medications in the previous week. Further analysis using visual analogue scale for dose adherence found that that 51% took up to 60% of the prescribed medications [15]. In an exclusively African-American sample, the adherence rate was reported as 46% [4]. Another study by Meghani et al compared adherence to prescribed ATC analgesics between African Americans and whites with cancer-related pain using an electronic monitoring system [13]. The overall adherence rate for African Americans was 53% and 74% for whites [13]. The authors concluded that there was a significant difference between the analgesic adherence rates between African Americans and whites in this study. On sub-analysis, analgesic adherence rates for African Americans were much lower for weak opioids (34%) and higher for long-acting opioids (63%).
In a study of individuals from an outpatient supportive care center with a majority white sample (74% Caucasian), overall 9.6% of patients deviated from the opioid regimen, while approximately 90% reported high adherence [12]. It is important to note that a convenience sample was used here. Of the total 19 patients that deviated from the regimen, 11 used less opioids than prescribed and 8 used higher doses. Upon analysis, the opioid deviation was more frequent in males and non-whites. However, statistical analyses of the magnitude of deviation from prescribed dose and non-white racial/ethnic background were not reported. Within the “non-whites” category, the race/ethnicity is defined as African American (16%, n = 32) and “other” (9%, n = 18). The authors contend that this strong adherence resulted from a strong understanding of the regimen as evidenced by a high agreement between the prescribed dose and the patient reported prescription [12]. Nguyen et al [12] argue that the literature shows that lower adherence rates for minority patients may be explained by the presence of comorbidities and lack of insurance.
Two other studies reported adherence rates for separate insurance cohorts [3,14]. The Medicaid cohort was younger and had a higher percentage of African-American individuals. However, in the self-pay/charity care group, the majority was Hispanic [3]. In the pilot study, the differences between the groups on adherence with prescribed medication regimens did not achieve statistical significance. The data were summarized to suggest that nonadherence was more likely in the self-pay/charity care group and more follow-up visits occurred after discharge [3]. During the larger retrospective study there was no difference in number of patients adhering to the regimen at each follow-up visit in each benefit group. The study concluded that the long-acting opiate adherence was influenced only by the benefits of use and that race/ethnicity was not a statistically significant predictor [14].
Factors Associated with Adherence
Multiple studies investigated factors underlying reported analgesic adherence rates for the ethnic and racial groups studied. Both clinical and sociodemographic variables were associated with analgesic adherence (Table 4). These included cancer type and disease stage [3,4,13,14], pain intensity [3,4,13–16], side effects [13,15], type of analgesic prescribed [3,4,13–16], income/socioeconomic status [3,13,14], behavioral history [3,12,13], gender [3,4,12–16], and perceived barriers [3,4,13,15,16].
Cancer Type and Stage
Most studies did not find significant associations between analgesic adherence rates and cancer type and stage [3,12,14]. However, 1 study that sought to identify unique factors underlying analgesic adherence for African Americans and whites found that whites reported higher analgesic adherence in relation to “time since cancer diagnosis,” possibly indicating disease severity and progression [13]. In another study that involved a majority of African-American patients, individuals with colon and rectal cancer had lower adherence rates [4]. In this study, patients with colon and rectal cancer had more analgesic prescriptions (2.5 +/– 2.3 analgesics) compared to patients with other cancer diagnoses. The authors concluded that an increased medication burden might have contributed to a decreased adherence rate. Overall, other cancer types did not correlate with adherence rates [4].
Pain Intensity
Six studies examined pain intensity and duration [3,4,13–16]. Three studies found a difference in reported pain intensity between racial/ethnic groups [3,13,16], 1 found no correlation between pain intensity and race/ethnicity [14], and 3 concluded that pain intensity was a significant predictor of adherence rates [3,13,15].
Meghani and Bruner’s pilot study explored possible correlates associated with intentional and unintentional nonadherence [15]. Overall, individuals were more likely to report forgetfulness (unintentional nonadherence) and to stop taking pain medicine when feeling “worse” (intentional nonadherence) if they believed that it was easier to deal with pain than with the side effects of analgesia [15]. Further, forgetfulness was negatively associated with the need for “stronger” pain medication. Concern about using too much pain medication was positively correlated with both forgetfulness and carelessness. The need for stronger pain medication was also correlated with significantly higher pain levels and lower pain relief [15].
In a comparative study of African Americans and whites, African Americans reported greater cancer pain and lower pain relief on the Brief Pain Inventory (BPI) and had a negative PMI. The PMI measure is a simple index linking the usual severity of cancer pain with the category of medication prescribed to treat it. PMI is calculated by subtracting patient’s pain levels (“pain worst” score from the BPI coded as mild, moderate, or severe) from the most potent analgesia prescribed. A negative PMI implies inadequate analgesic prescription relative to the reported pain level. Pain intensity was a significant factor related to increased adherence in whites but not African Americans. For African Americans, analgesic adherence was predicted by socioeconomic status, provider communication factors, and side effects. Similarly, in another study that compared African Americans and Hispanics, African Americans were more likely to have a negative PMI than Hispanics and were less likely to report that pain medication relieved pain [16]. In a pilot study that compared Medicaid recipients to self-pay/charity care patients, African-American participants had lower reported pain scores than Hispanics and Caucasians [3]. In the larger follow-up study, however, ethnicity did not prove a significant predictor for pain levels [14].
In a study with exclusively African-American patients, a significant correlation was found between pain intensity and adherence; specifically, as intensity increased, adherence increased [4]. Results for the entire African-American cohort indicated that 90% of patients had analgesic prescriptions for cancer-related pain, but 86% continued to report having moderate to severe worst pain [4]. A study that compared African Americans and whites showed that lower pain relief with analgesics was associated with lower adherence to analgesia for cancer pain among whites [13]. For every unit increase in “least pain” scores (indicating lower pain relief) on the BPI item, dose adherence decreased by 2.88%. Pain levels and relief did not explain adherence rates among African Americans. Whites were also more likely to make decisions on analgesic use based on the amount of relief anticipated from the use of analgesics [13] whereas African Americans were more likely to make analgesic use decisions based on analgesic side effects.
Side Effects
In a pilot study that explored the intricacies of adherence, some individuals felt it was easier to deal with pain than with the side effects of pain medications. These individuals were also more likely to report forgetfulness and to stop taking medications if feeling “worse” [15]. One study, which included African-American and white cohorts, found that an increase in the severity of side effects was associated with lower adherence to analgesia for African Americans but not whites. Furthermore, African Americans reported a greater number of analgesic side effects at baseline. African Americans were also more likely to make analgesic decisions based on side effects in comparison to whites participants, who made decisions based on expectation of pain relief [13]. In a study with exclusively African-American patients, patients with concerns about pain medication possibly causing confusion were more likely to have poor adherence [4].
Type of Analgesic Prescribed
In the analyses, 3 studies found a difference between analgesic prescriptions among ethnic groups [12,13,16], 3 found that there was a statistical significance between type of prescription and adherence [4,13,16], and 2 studies [3,14] found no statistical correlation between type of analgesic prescribed and adherence.
In a study of African Americans and Hispanics, both groups took analgesics on an “as-needed” basis despite the guidelines for cancer pain management [16]. However, African Americans reported taking analgesics less than twice daily. Overall, only a small percentage of patients took sustained-release analgesics that require fewer doses per day [16]. Similarly, in another study that compared adherence between African Americans and whites, the overall analgesic adherence rate was different on sub-analysis for specific analgesic prescriptions. The analgesic adherence rates for African Americans ranged from 34% for weak opioids to 63% for long-acting opioids. In comparison, the analgesic adherence rates for whites ranged from 55% for weak opioids to 78% for long-acting opioids [13]. In conclusion, patients on long-acting opioids were more likely to have higher adherence. Adherence rates for African Americans were found in another study. The adherence rate for adjuvant analgesics was highest at 65%, step 2 opioids at 44% and step 3 opioids at 43% [4].
In a study with exclusively African-American patients, poor adherence was significantly correlated with step 3 opioids [4]. Another study that explored the correlation between type of analgesic and adherence found that intentional nonadherence was less likely in individuals that were prescribed step 3 opioids [15]. Specifically, individuals with this behavior were also more likely to report lower pain levels and chose to stop the use of analgesics when feeling better [15].
Within a pilot study that compared benefit programs and payor groups, the differences in the prescription of long-acting opiates did not reach statistical significance [3]. However, in the larger, definitive study, the comparison revealed that patients in the self-pay/charity care group were less likely to receive a prescription for long-acting opiates. The data further revealed that Hispanic and Asian patients were prescribed long-acting opiates at a lower rate compared to the larger sample. Further, African Americans and Caucasians were prescribed long-acting opiates at a higher rate than the larger sample. In another analysis, with benefits and race/ethnicity, benefits were the only statistically significant predictor. While statistically controlling for race/ethnicity, Medicaid patients were 2.4 times more likely to receive a prescription for long-acting opioids than the self-pay/charity care patients [14].
Income/Socioeconomic Status
Three studies in this analysis [3,13,14] found that income and socioeconomic status were significant predictors of analgesic adherence for cancer pain. In a comparison between African Americans and whites, income was the strongest predictor of analgesic adherence for cancer pain in African Americans [13]; specifically, individuals with a household income of less than $10,000 a year had a 41.83% lower percentage of dose adherence. Among whites, income did not have a significant correlation with analgesic rates [13].
A pilot study and larger definitive study [3,14] were conducted to compare the effects of prescription benefits. The prescription benefits included were Medicaid and self-pay/charity care. Through comparison, none of the Medicaid patients reported financial barriers but the self-pay/charity care patients were more likely to report financial barriers to adherence [3]. In the larger study, the findings indicated that there was significant association of adherence by benefits and race/ethnicity. As mentioned above, benefits were a dominant predictor of long acting opiate use and further adherence [14].
Gender
Apart from ethnicity or race as a variable associated with adherence, association of analgesic adherence and gender were observed in 4 studies [3,13–15] and evaluated in 2 studies. One study [4] found that a patient’s gender and education level did not correlate with adherence rates. However, in another study [12] men were more likely to deviate from the prescribed dose. Overall, within the entire cohort [12] men and minority patients were most likely to deviate from the prescribed dosing regimen in comparison to all other patient demographic factors.
Attitudes and Barriers
Five of the 7 studies investigated perceived barriers to analgesic adherence [3,4,13,15,16]. Four used the Barriers Questionnaire II (BQ-II) [18] to further understand patients’ beliefs about cancer pain management [3,12,13,15]. Using this validated tool, 1 study found that non-white individuals had higher scores on the BQ-II than white patients [12]. Within the non-white group in the above study, the mean score on the BQ-II for African Americans was 1.76 (± 0.81) and the mean score for “other” was 2.16 (± 0.93) [12]. Further, low MMAS scores were significantly associated with higher BQ-II scores. Similarly, higher BQ-II scores correlated with opioid deviation toward higher than prescribed dose [12].
Another study with a primarily African-American cohort did not use the BQ-II but asked specific questions in regards to perceived barriers to analgesics. Within the cohort, 87% reported a fear of addiction to pain medicine. Further, 77% had a fear of injection, 75% were concerned about a tolerance for analgesics, and side effects were a major concern. Overall, nausea was the greatest reported concern followed by potential for confusion, which was negatively associated with taking analgesics. Distracting the doctor from curing their illness was a predictor of improved adherence; however, individuals were more likely to take Tylenol for pain relief. Similarly, no significant barrier items affected adherence to NSAIDs. In relation to step 2 opioids, patients who felt it was important to be strong by not talking about pain were more likely to have better adherence [4]. Similar results with African Americans were identified in another study [13]. In the comparison between African Americans and whites, African Americans had more subjective barriers compared to whites. Particularly for African Americans, each unit increase in concern about distracting the doctor from curing the disease, the percentage of dose adherence decreased by 7.44 [13].
In a study that compared payer groups, a questionnaire elicited reasons for nonadherence [3]. Similar reasons for nonadherence emerged including financial, fear of addiction or increased medication use, and running out medication.
Behavioral History
Only 1 study used CAGE (Cut down, Annoyed, Guilty, and Eye-opener), an alcohol-screening questionnaire, to determine a possible relationship with analgesic adherence. In this study, there were 19 cases of opioid deviation, 16% of which were CAGE positive and had severe deviation toward less than the prescribed doses [12]. In further analysis, no association was found between CAGE positively and opioid deviation to higher intake [12]. Two other studies gathered data on history of depression, substance use, and alcohol use but no significant correlation was found [3,13].
Discussion
Previous literature has reported overall analgesic adherence rates among oncology patients ranging from 62% to 72% [23]. Factors at the provider and system level have been considered in past research, but the patient perspective is poorly represented in the literature [13]. A majority of studies on analgesic adherence have been completed with cohorts made up predominantly of white individuals [13,23,24], while others focus on racially homogenous and/or ethnically different populations in other countries [21,25,26].
This review confirms that there is a paucity of well-designed studies that describe the associations between racial and ethnic disparities and adherence to opioids among patients with cancer pain. This is despite the fact that moderate to severe cancer pain in the U.S. is managed mainly with analgesics and specifically with opioids [19]. In addition, cancer patients with health insurance have both more pharmacy claims as well as more claims for higher doses of opioids [20] compared to noncancer patients. The lack of attention to analgesic and opioid adherence among cancer patients is surprising in the light of the recent high-profile initiatives to reduce opioid misuse [31].
Multiple studies highlighted the importance of pain management education and adequate pain assessment for effective analgesic use [4,16]. In the study in the palliative care setting, the authors concluded that patients who are educated, counseled, and monitored by a palliative or supportive care team have less episodes of opioid deviation and trends toward lower opioid use [12]. A systematic review and meta-analysis confirmed findings that educational interventions for patients improved knowledge about cancer pain management, however, most did not improve reported adherence to analgesics [27,28]. These findings emphasize the need for further research on interventions to improve racial/ethnic disparities in analgesic adherence for cancer pain.
Limitations
The findings of this review should be evaluated in the context of the following limitations. First, adherence to a prescribed regimen is a difficult outcome to measure and a majority of studies in this review used subjective measures to assess analgesic adherence for cancer pain. Of note, self-report was the primary measurement employed. Studies in non–cancer pain settings that have evaluated various methodological approaches to adherence measurement found that patients are likely to over-report adherence when using self-report or a diary format in comparison to an electronic monitoring system. Only 1 study in this review used an objective measure of adherence [13]. Some previous studies contend that self-report in comparison to other, objective measurements of medication adherence are accurate [23]. Further research is needed to determine the most accurate measurement of analgesic adherence in cancer patients.
Also, invariably the studies employed an English-speaking sample, which excludes an understanding of analgesic adherence for cancer pain in linguistically diverse Americans. In addition, most studies included patients who were either white Americans or African Americans and some studies lumped several racial ethnic minority subgroups as “nonwhites” or “other.”
A majority of studies were cross-sectional [4,12,15,16]. For instance, studies used a 24-hour time period to assess ATC medication as well as as-needed regimens, which may not capture the information needed to understand adherence to as-needed regimens [4]. With longitudinal studies, a greater understanding of adherence can be determined. However, there is potential bias with studies that track patients primarily at follow-up appointments. Individuals who are compliant with follow-up appointments may present with different analgesic adherence compared to those who do not attend follow-up appointments. This potential bias should be evaluated in longitudinal studies with various sensitivity analyses or using tools that identify healthy user bias.
Most studies recruited patients from outpatient oncology clinics, however, 1 study was conducted with a sample from an outpatient supportive care center managed by a palliative care team [12]. Due to the goals of palliative care, which include specialized treatment for individuals with serious illness and a focus on symptom management and relief, patients in this setting may have a different attitude toward using opioids.
Conclusion
Although data remain limited, our review suggests that while overuse of opioids has been a well-cited concern in patients with chronic non-cancer pain [21,33], cancer patients demonstrate considerable underuse and inconsistent use of prescribed analgesics. This is important as a recent study found that inconsistent adherence to prescribed around-the-clock analgesics, specifically the interaction of strong opioids and inconsistent adherence, is a strong risk factor for hospitalization among cancer outpatients who are prescribed analgesics for pain [1]. Of note, adherence to opioids in patients with cancer may be driven by a unique set of factors and these factors may differ for minorities and non-minority patients. For instance, studies in this review indicate that income is a strong predictor of analgesic adherence for African Americans but not for whites. This is because race and socioeconomic status frequently overlap in the United States [29]. In addition, like cancer pain, analgesic side effects may also be poorly managed among African Americans and other minorities. For example, in 1 study, Meghani et al used a trade-off analysis technique (conjoint analysis) to understand trade-offs African Americans and whites employ in using analgesics for cancer pain [30]. The authors found that African Americans were more likely to make analgesic adherence decisions based on side effects whereas whites were more likely to make adherence decisions based on pain relief [30]. In subsequent analysis, these authors showed that the race effect found in their previous studies was mediated by the type of analgesics prescribed to African Americans vs. whites [31]. African Americans with cancer pain were prescribed analgesics that had a worse side effect profile after statistically adjusting for insurance type and clinical risks such as renal insufficiency [31].
Together, the available evidence indicates that both patients’ socioeconomic status and clinician treatment bias contributes to racial and ethnic disparities in analgesic adherence for cancer pain and subsequent cancer pain outcomes. Thus, future research should investigate interventions for improving analgesic adherence among low-income minorities. Also, there is a need for clinician-level interventions focusing on cognitive bias modification related to cancer pain and side effects management, which appears to relate to analgesic nonadherence among racial/ethnic minorities. In addition, further research is needed to (1) rigorously describe analgesic and opioid adherence for cancer pain, (2) elucidate racial/ethnic and other socioeconomic and clinical disparities in analgesic and opioid adherence for cancer pain; (3) and clarify the role of analgesic and opioid adherence for cancer patients including outcomes for the patients and the health care system.
Corresponding author: Salimah H. Meghani, PhD, MBE, RN, University of Pennsylvania School of Nursing, Room 337, Fagin Hall, 418 Curie Blvd, Philadelphia, PA 19104, [email protected].
Financial disclosures: None.
From the University of Pennsylvania School of Nursing, Philadelphia, PA.
Abstract
- Background: Racial/ethnic disparities in analgesic treatment for pain have been widely documented in the United States. However, the connection between race/ethnicity and adherence to prescribed analgesics has not been described.
- Objectives: To review and synthesize quantitative research documenting racial/ethnic differences in adherence to prescribed analgesia in cancer patients.
- Methods: We performed a systematic search of quantitative, primary studies in Scopus, CINAHL, PubMed, Ovid, PsychInfo, and EMBASE. The title and abstract of each article was reviewed for relevance and whether inclusion criteria were met. Evidence was examined for relevant outcomes, data collection methods, variables studied in relation to adherence, and the magnitude of association between race/ethnicity and adherence.
- Results: Seven studies met inclusion criteria. Reported rates of adherence varied in studies among Hispanic/Latinos, African Americans, Asians, and whites based on variation in measurement tools, research questions, populations from which participants were recruited, and predictive variables analyzed. Most existing studies of analgesic adherence used self-report to measure adherence. Only 1 study used a validated, real-time electronic instrument to monitor prescribed opioid adherence and had a longitudinal study design.
- Conclusion: Limited research has examined relationships between adherence to prescribed analgesic regimens and racial disparities. Existing studies point to the clinical and socioeconomic factors that may interact with race/ethnicity in explaining analgesic and opioid adherence outcomes in cancer patients.
Key words: race, ethnicity, adherence, opiates, analgesics, pain management, cancer, pain treatment disparities.
The ongoing opioid epidemic and recent development of the Centers for Disease Control and Prevention (CDC) guidelines for chronic pain management have shaped a national conversation on opioid prescription and utilization [1]. The CDC delineates provider recommendations for opioid prescription. This focus on prescribed medication regimens is inadequate without an understanding of how patients take or adhere to prescribed medications. Cancer patients are a unique group. Moderate to severe pain in cancer patients is usually treated with opioids, and adherence to analgesia has been conceptualized a key mediator of cancer pain outcomes. For instance, a recent study found that patterns of analgesic adherence, specifically, inconsistent adherence to strong opioids (World Health Organization step 3), is one of the strongest predictors of health care utilization among outpatients with cancer pain [2]. Approximately 67% to 77% of cancer patients experience pain that requires management with analgesia [3], especially in the absence of access to nonpharmacologic pain treatments [2]. Thus, barriers in relation to adequate pain management can result in poor pain treatment outcomes and impaired quality of life for cancer patients.
Insufficient pain management has been found to have a negative impact on the quality of life and physical and mental functions of patients with cancer [4]. Patients who experience severe cancer pain are significantly more likely to experience multiple other symptoms such as depression, fatigue, and insomnia, resulting in diminished physical function [5], social role function [6], and greater out of pocket cost of managing pain and asso-ciated symptoms [7]. Minority populations, however, disproportionately carry the burden of undertreated pain [4,8–11,13–16]. Evidence suggests that blacks/African Americans are more likely to experience unrelieved cancer pain [4,8–11,13–16]. They are also less likely than their white counterparts to receive analgesic treatment for cancer pain [8–11,13,15,16]. Little is known, however, about racial disparities in relation to adherence to analgesia for cancer pain when providers prescribe analgesics.
The purpose of this paper is to review the published literature that has addressed the associations between disparities and adherence to analgesia among cancer patients. Evidence was examined for outcomes studied, data collection methods, variables studied in relation to adherence, and the magnitude of association based on race and adherence.
Methods
We performed a systematic search of studies published between 1990 and the present in Scopus, CINAHL, PubMed, Ovid, PsychInfo, and the EMBASE databases. The inclusion criteria consisted of published articles in the aforementioned databases that were (1) set in the United States, (2) primary studies, (3) employed quantitative design, (4) assessed adherence or compliance to analgesics or adequacy of pain management using the Pain Management Index (PMI), (5) sample was exclusively minority or may have had a comparative group. The title and abstract of each article in the the search results was reviewed for relevance to study aims and inclusion and exclusion criteria, and any duplicates were eliminated. A total of 6 studies were found using this method (Table 1), and an additional study was found in the reference list of 1 of these 6.
Results
The 7 included studies were observational in nature; 4 were cross-sectional [4,12,15,16], 2 were retrospective [3,14], and 1 was prospective and used objective measures of analgesic adherence [13] (Table 2).
Defining and Operationalizing Adherence
Meghani and Bruner [16] point out that analgesic adherence is a “heterogeneous construct that lends itself to varied results and interpretations depending on the measurements used or dimensions studied.” Adherence to analgesia was explicitly defined in all 7 studies (Table 3). One study reported an adherence rate that was the total dose over 24 hours divided by the dose prescribed then multiplied by 100 [4]. The total dose over 24 hours was used in another study but was converted to an equianalgesic calculation [12]. Another set of studies used a similar definition but specified percentages based on medication or type of prescription, such as an around-the-clock(ATC) regimen [13,15,16]. In 2 studies, adherence was measured based on chart review of yes/no questions posed about whether or not patients had taken medications as prescribed [3,15].
The measurements of adherence differed between studies. Four studies [4,12,14,16] used adherence as a primary outcome and the rest employed adherence as a facet of pain management [3,13,15]. The most frequent measure of adherence was self-report. The widely validated Morisky Medication Adherence Scale (MMAS) instrument was used in 3 of 7 studies [12,13,15]. Meghani and Bruner [15] utilized the modified MMAS plus a previously validated visual analog scale for doses of medication to assess adherence over week- and month-long intervals. One study used patient interviews to capture self-reporting of opioid prescription and opioid use. Additionally, the study used MMAS to further characterize the adherence measurements [12]. Using a more objective method, Meghani et al [13] employed a microprocessor in the medication cap to determine the percentage of the total number of prescribed doses that were actually taken [13]. The processor sensed when the bottle was open, which served as a proxy for taking medications at appropriate times.
Analgesic Adherence Rate
To report the analgesic adherence rates, 6 studies presented a percentage [3,4,12,13,15] and all but 1 highlighted the barriers associated with poor adherence [3,4,12,13,15,16].
The results of a pilot study exploring intentional and unintentional adherence revealed that 85.5% of patients took the prescribed medications in the previous week. Further analysis using visual analogue scale for dose adherence found that that 51% took up to 60% of the prescribed medications [15]. In an exclusively African-American sample, the adherence rate was reported as 46% [4]. Another study by Meghani et al compared adherence to prescribed ATC analgesics between African Americans and whites with cancer-related pain using an electronic monitoring system [13]. The overall adherence rate for African Americans was 53% and 74% for whites [13]. The authors concluded that there was a significant difference between the analgesic adherence rates between African Americans and whites in this study. On sub-analysis, analgesic adherence rates for African Americans were much lower for weak opioids (34%) and higher for long-acting opioids (63%).
In a study of individuals from an outpatient supportive care center with a majority white sample (74% Caucasian), overall 9.6% of patients deviated from the opioid regimen, while approximately 90% reported high adherence [12]. It is important to note that a convenience sample was used here. Of the total 19 patients that deviated from the regimen, 11 used less opioids than prescribed and 8 used higher doses. Upon analysis, the opioid deviation was more frequent in males and non-whites. However, statistical analyses of the magnitude of deviation from prescribed dose and non-white racial/ethnic background were not reported. Within the “non-whites” category, the race/ethnicity is defined as African American (16%, n = 32) and “other” (9%, n = 18). The authors contend that this strong adherence resulted from a strong understanding of the regimen as evidenced by a high agreement between the prescribed dose and the patient reported prescription [12]. Nguyen et al [12] argue that the literature shows that lower adherence rates for minority patients may be explained by the presence of comorbidities and lack of insurance.
Two other studies reported adherence rates for separate insurance cohorts [3,14]. The Medicaid cohort was younger and had a higher percentage of African-American individuals. However, in the self-pay/charity care group, the majority was Hispanic [3]. In the pilot study, the differences between the groups on adherence with prescribed medication regimens did not achieve statistical significance. The data were summarized to suggest that nonadherence was more likely in the self-pay/charity care group and more follow-up visits occurred after discharge [3]. During the larger retrospective study there was no difference in number of patients adhering to the regimen at each follow-up visit in each benefit group. The study concluded that the long-acting opiate adherence was influenced only by the benefits of use and that race/ethnicity was not a statistically significant predictor [14].
Factors Associated with Adherence
Multiple studies investigated factors underlying reported analgesic adherence rates for the ethnic and racial groups studied. Both clinical and sociodemographic variables were associated with analgesic adherence (Table 4). These included cancer type and disease stage [3,4,13,14], pain intensity [3,4,13–16], side effects [13,15], type of analgesic prescribed [3,4,13–16], income/socioeconomic status [3,13,14], behavioral history [3,12,13], gender [3,4,12–16], and perceived barriers [3,4,13,15,16].
Cancer Type and Stage
Most studies did not find significant associations between analgesic adherence rates and cancer type and stage [3,12,14]. However, 1 study that sought to identify unique factors underlying analgesic adherence for African Americans and whites found that whites reported higher analgesic adherence in relation to “time since cancer diagnosis,” possibly indicating disease severity and progression [13]. In another study that involved a majority of African-American patients, individuals with colon and rectal cancer had lower adherence rates [4]. In this study, patients with colon and rectal cancer had more analgesic prescriptions (2.5 +/– 2.3 analgesics) compared to patients with other cancer diagnoses. The authors concluded that an increased medication burden might have contributed to a decreased adherence rate. Overall, other cancer types did not correlate with adherence rates [4].
Pain Intensity
Six studies examined pain intensity and duration [3,4,13–16]. Three studies found a difference in reported pain intensity between racial/ethnic groups [3,13,16], 1 found no correlation between pain intensity and race/ethnicity [14], and 3 concluded that pain intensity was a significant predictor of adherence rates [3,13,15].
Meghani and Bruner’s pilot study explored possible correlates associated with intentional and unintentional nonadherence [15]. Overall, individuals were more likely to report forgetfulness (unintentional nonadherence) and to stop taking pain medicine when feeling “worse” (intentional nonadherence) if they believed that it was easier to deal with pain than with the side effects of analgesia [15]. Further, forgetfulness was negatively associated with the need for “stronger” pain medication. Concern about using too much pain medication was positively correlated with both forgetfulness and carelessness. The need for stronger pain medication was also correlated with significantly higher pain levels and lower pain relief [15].
In a comparative study of African Americans and whites, African Americans reported greater cancer pain and lower pain relief on the Brief Pain Inventory (BPI) and had a negative PMI. The PMI measure is a simple index linking the usual severity of cancer pain with the category of medication prescribed to treat it. PMI is calculated by subtracting patient’s pain levels (“pain worst” score from the BPI coded as mild, moderate, or severe) from the most potent analgesia prescribed. A negative PMI implies inadequate analgesic prescription relative to the reported pain level. Pain intensity was a significant factor related to increased adherence in whites but not African Americans. For African Americans, analgesic adherence was predicted by socioeconomic status, provider communication factors, and side effects. Similarly, in another study that compared African Americans and Hispanics, African Americans were more likely to have a negative PMI than Hispanics and were less likely to report that pain medication relieved pain [16]. In a pilot study that compared Medicaid recipients to self-pay/charity care patients, African-American participants had lower reported pain scores than Hispanics and Caucasians [3]. In the larger follow-up study, however, ethnicity did not prove a significant predictor for pain levels [14].
In a study with exclusively African-American patients, a significant correlation was found between pain intensity and adherence; specifically, as intensity increased, adherence increased [4]. Results for the entire African-American cohort indicated that 90% of patients had analgesic prescriptions for cancer-related pain, but 86% continued to report having moderate to severe worst pain [4]. A study that compared African Americans and whites showed that lower pain relief with analgesics was associated with lower adherence to analgesia for cancer pain among whites [13]. For every unit increase in “least pain” scores (indicating lower pain relief) on the BPI item, dose adherence decreased by 2.88%. Pain levels and relief did not explain adherence rates among African Americans. Whites were also more likely to make decisions on analgesic use based on the amount of relief anticipated from the use of analgesics [13] whereas African Americans were more likely to make analgesic use decisions based on analgesic side effects.
Side Effects
In a pilot study that explored the intricacies of adherence, some individuals felt it was easier to deal with pain than with the side effects of pain medications. These individuals were also more likely to report forgetfulness and to stop taking medications if feeling “worse” [15]. One study, which included African-American and white cohorts, found that an increase in the severity of side effects was associated with lower adherence to analgesia for African Americans but not whites. Furthermore, African Americans reported a greater number of analgesic side effects at baseline. African Americans were also more likely to make analgesic decisions based on side effects in comparison to whites participants, who made decisions based on expectation of pain relief [13]. In a study with exclusively African-American patients, patients with concerns about pain medication possibly causing confusion were more likely to have poor adherence [4].
Type of Analgesic Prescribed
In the analyses, 3 studies found a difference between analgesic prescriptions among ethnic groups [12,13,16], 3 found that there was a statistical significance between type of prescription and adherence [4,13,16], and 2 studies [3,14] found no statistical correlation between type of analgesic prescribed and adherence.
In a study of African Americans and Hispanics, both groups took analgesics on an “as-needed” basis despite the guidelines for cancer pain management [16]. However, African Americans reported taking analgesics less than twice daily. Overall, only a small percentage of patients took sustained-release analgesics that require fewer doses per day [16]. Similarly, in another study that compared adherence between African Americans and whites, the overall analgesic adherence rate was different on sub-analysis for specific analgesic prescriptions. The analgesic adherence rates for African Americans ranged from 34% for weak opioids to 63% for long-acting opioids. In comparison, the analgesic adherence rates for whites ranged from 55% for weak opioids to 78% for long-acting opioids [13]. In conclusion, patients on long-acting opioids were more likely to have higher adherence. Adherence rates for African Americans were found in another study. The adherence rate for adjuvant analgesics was highest at 65%, step 2 opioids at 44% and step 3 opioids at 43% [4].
In a study with exclusively African-American patients, poor adherence was significantly correlated with step 3 opioids [4]. Another study that explored the correlation between type of analgesic and adherence found that intentional nonadherence was less likely in individuals that were prescribed step 3 opioids [15]. Specifically, individuals with this behavior were also more likely to report lower pain levels and chose to stop the use of analgesics when feeling better [15].
Within a pilot study that compared benefit programs and payor groups, the differences in the prescription of long-acting opiates did not reach statistical significance [3]. However, in the larger, definitive study, the comparison revealed that patients in the self-pay/charity care group were less likely to receive a prescription for long-acting opiates. The data further revealed that Hispanic and Asian patients were prescribed long-acting opiates at a lower rate compared to the larger sample. Further, African Americans and Caucasians were prescribed long-acting opiates at a higher rate than the larger sample. In another analysis, with benefits and race/ethnicity, benefits were the only statistically significant predictor. While statistically controlling for race/ethnicity, Medicaid patients were 2.4 times more likely to receive a prescription for long-acting opioids than the self-pay/charity care patients [14].
Income/Socioeconomic Status
Three studies in this analysis [3,13,14] found that income and socioeconomic status were significant predictors of analgesic adherence for cancer pain. In a comparison between African Americans and whites, income was the strongest predictor of analgesic adherence for cancer pain in African Americans [13]; specifically, individuals with a household income of less than $10,000 a year had a 41.83% lower percentage of dose adherence. Among whites, income did not have a significant correlation with analgesic rates [13].
A pilot study and larger definitive study [3,14] were conducted to compare the effects of prescription benefits. The prescription benefits included were Medicaid and self-pay/charity care. Through comparison, none of the Medicaid patients reported financial barriers but the self-pay/charity care patients were more likely to report financial barriers to adherence [3]. In the larger study, the findings indicated that there was significant association of adherence by benefits and race/ethnicity. As mentioned above, benefits were a dominant predictor of long acting opiate use and further adherence [14].
Gender
Apart from ethnicity or race as a variable associated with adherence, association of analgesic adherence and gender were observed in 4 studies [3,13–15] and evaluated in 2 studies. One study [4] found that a patient’s gender and education level did not correlate with adherence rates. However, in another study [12] men were more likely to deviate from the prescribed dose. Overall, within the entire cohort [12] men and minority patients were most likely to deviate from the prescribed dosing regimen in comparison to all other patient demographic factors.
Attitudes and Barriers
Five of the 7 studies investigated perceived barriers to analgesic adherence [3,4,13,15,16]. Four used the Barriers Questionnaire II (BQ-II) [18] to further understand patients’ beliefs about cancer pain management [3,12,13,15]. Using this validated tool, 1 study found that non-white individuals had higher scores on the BQ-II than white patients [12]. Within the non-white group in the above study, the mean score on the BQ-II for African Americans was 1.76 (± 0.81) and the mean score for “other” was 2.16 (± 0.93) [12]. Further, low MMAS scores were significantly associated with higher BQ-II scores. Similarly, higher BQ-II scores correlated with opioid deviation toward higher than prescribed dose [12].
Another study with a primarily African-American cohort did not use the BQ-II but asked specific questions in regards to perceived barriers to analgesics. Within the cohort, 87% reported a fear of addiction to pain medicine. Further, 77% had a fear of injection, 75% were concerned about a tolerance for analgesics, and side effects were a major concern. Overall, nausea was the greatest reported concern followed by potential for confusion, which was negatively associated with taking analgesics. Distracting the doctor from curing their illness was a predictor of improved adherence; however, individuals were more likely to take Tylenol for pain relief. Similarly, no significant barrier items affected adherence to NSAIDs. In relation to step 2 opioids, patients who felt it was important to be strong by not talking about pain were more likely to have better adherence [4]. Similar results with African Americans were identified in another study [13]. In the comparison between African Americans and whites, African Americans had more subjective barriers compared to whites. Particularly for African Americans, each unit increase in concern about distracting the doctor from curing the disease, the percentage of dose adherence decreased by 7.44 [13].
In a study that compared payer groups, a questionnaire elicited reasons for nonadherence [3]. Similar reasons for nonadherence emerged including financial, fear of addiction or increased medication use, and running out medication.
Behavioral History
Only 1 study used CAGE (Cut down, Annoyed, Guilty, and Eye-opener), an alcohol-screening questionnaire, to determine a possible relationship with analgesic adherence. In this study, there were 19 cases of opioid deviation, 16% of which were CAGE positive and had severe deviation toward less than the prescribed doses [12]. In further analysis, no association was found between CAGE positively and opioid deviation to higher intake [12]. Two other studies gathered data on history of depression, substance use, and alcohol use but no significant correlation was found [3,13].
Discussion
Previous literature has reported overall analgesic adherence rates among oncology patients ranging from 62% to 72% [23]. Factors at the provider and system level have been considered in past research, but the patient perspective is poorly represented in the literature [13]. A majority of studies on analgesic adherence have been completed with cohorts made up predominantly of white individuals [13,23,24], while others focus on racially homogenous and/or ethnically different populations in other countries [21,25,26].
This review confirms that there is a paucity of well-designed studies that describe the associations between racial and ethnic disparities and adherence to opioids among patients with cancer pain. This is despite the fact that moderate to severe cancer pain in the U.S. is managed mainly with analgesics and specifically with opioids [19]. In addition, cancer patients with health insurance have both more pharmacy claims as well as more claims for higher doses of opioids [20] compared to noncancer patients. The lack of attention to analgesic and opioid adherence among cancer patients is surprising in the light of the recent high-profile initiatives to reduce opioid misuse [31].
Multiple studies highlighted the importance of pain management education and adequate pain assessment for effective analgesic use [4,16]. In the study in the palliative care setting, the authors concluded that patients who are educated, counseled, and monitored by a palliative or supportive care team have less episodes of opioid deviation and trends toward lower opioid use [12]. A systematic review and meta-analysis confirmed findings that educational interventions for patients improved knowledge about cancer pain management, however, most did not improve reported adherence to analgesics [27,28]. These findings emphasize the need for further research on interventions to improve racial/ethnic disparities in analgesic adherence for cancer pain.
Limitations
The findings of this review should be evaluated in the context of the following limitations. First, adherence to a prescribed regimen is a difficult outcome to measure and a majority of studies in this review used subjective measures to assess analgesic adherence for cancer pain. Of note, self-report was the primary measurement employed. Studies in non–cancer pain settings that have evaluated various methodological approaches to adherence measurement found that patients are likely to over-report adherence when using self-report or a diary format in comparison to an electronic monitoring system. Only 1 study in this review used an objective measure of adherence [13]. Some previous studies contend that self-report in comparison to other, objective measurements of medication adherence are accurate [23]. Further research is needed to determine the most accurate measurement of analgesic adherence in cancer patients.
Also, invariably the studies employed an English-speaking sample, which excludes an understanding of analgesic adherence for cancer pain in linguistically diverse Americans. In addition, most studies included patients who were either white Americans or African Americans and some studies lumped several racial ethnic minority subgroups as “nonwhites” or “other.”
A majority of studies were cross-sectional [4,12,15,16]. For instance, studies used a 24-hour time period to assess ATC medication as well as as-needed regimens, which may not capture the information needed to understand adherence to as-needed regimens [4]. With longitudinal studies, a greater understanding of adherence can be determined. However, there is potential bias with studies that track patients primarily at follow-up appointments. Individuals who are compliant with follow-up appointments may present with different analgesic adherence compared to those who do not attend follow-up appointments. This potential bias should be evaluated in longitudinal studies with various sensitivity analyses or using tools that identify healthy user bias.
Most studies recruited patients from outpatient oncology clinics, however, 1 study was conducted with a sample from an outpatient supportive care center managed by a palliative care team [12]. Due to the goals of palliative care, which include specialized treatment for individuals with serious illness and a focus on symptom management and relief, patients in this setting may have a different attitude toward using opioids.
Conclusion
Although data remain limited, our review suggests that while overuse of opioids has been a well-cited concern in patients with chronic non-cancer pain [21,33], cancer patients demonstrate considerable underuse and inconsistent use of prescribed analgesics. This is important as a recent study found that inconsistent adherence to prescribed around-the-clock analgesics, specifically the interaction of strong opioids and inconsistent adherence, is a strong risk factor for hospitalization among cancer outpatients who are prescribed analgesics for pain [1]. Of note, adherence to opioids in patients with cancer may be driven by a unique set of factors and these factors may differ for minorities and non-minority patients. For instance, studies in this review indicate that income is a strong predictor of analgesic adherence for African Americans but not for whites. This is because race and socioeconomic status frequently overlap in the United States [29]. In addition, like cancer pain, analgesic side effects may also be poorly managed among African Americans and other minorities. For example, in 1 study, Meghani et al used a trade-off analysis technique (conjoint analysis) to understand trade-offs African Americans and whites employ in using analgesics for cancer pain [30]. The authors found that African Americans were more likely to make analgesic adherence decisions based on side effects whereas whites were more likely to make adherence decisions based on pain relief [30]. In subsequent analysis, these authors showed that the race effect found in their previous studies was mediated by the type of analgesics prescribed to African Americans vs. whites [31]. African Americans with cancer pain were prescribed analgesics that had a worse side effect profile after statistically adjusting for insurance type and clinical risks such as renal insufficiency [31].
Together, the available evidence indicates that both patients’ socioeconomic status and clinician treatment bias contributes to racial and ethnic disparities in analgesic adherence for cancer pain and subsequent cancer pain outcomes. Thus, future research should investigate interventions for improving analgesic adherence among low-income minorities. Also, there is a need for clinician-level interventions focusing on cognitive bias modification related to cancer pain and side effects management, which appears to relate to analgesic nonadherence among racial/ethnic minorities. In addition, further research is needed to (1) rigorously describe analgesic and opioid adherence for cancer pain, (2) elucidate racial/ethnic and other socioeconomic and clinical disparities in analgesic and opioid adherence for cancer pain; (3) and clarify the role of analgesic and opioid adherence for cancer patients including outcomes for the patients and the health care system.
Corresponding author: Salimah H. Meghani, PhD, MBE, RN, University of Pennsylvania School of Nursing, Room 337, Fagin Hall, 418 Curie Blvd, Philadelphia, PA 19104, [email protected].
Financial disclosures: None.
1. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain - United States, 2016. JAMA 2016;315:1624–45.
2. Meghani SH, Knafl GJ. Patterns of analgesic adherence predict health care utilization among outpatients with cancer pain. Patient Prefer Adher 2016;10:81–98.
3. Bryan M, De La Rosa N, Hill AM, et al. Influence of prescription benefits on reported pain in cancer patients. Pain Med 2008;9:1148–57.
4. Rhee YO, Kim E, Kim B. Assessment of pain and analgesic use in African American cancer patients: Factors related to adherence to analgesics. J Immigr Minor Health 2012;14:1045–51.
5. Laird BJ, Scott AC, Colvin LA, et al. Pain, depression, and fatigue as a symptom cluster in advanced cancer. J Pain Symptom Manage 2011;42:1–11.
6. Ferreira KA, Kimura M, Teixeira MJ, et al. Impact of cancer-related symptom synergisms on health-related quality of life and performance status. J Pain Symptom Manage 2008;35:604–16.
7. Craig BM, Strassels SA. Out-of-pocket prices of opioid analgesics in the United States, 1999-2004. Pain Med 2010;11:240–47.
8. Institute of Medicine: Relieving pain in america: a blueprint for transforming prevention, care, education, and research. Washington, DC: National Academies Press; 2011.
9. Institutes of Medicine. Unequal treatment: confronting racial and ethnic disparities in health care. Washington, DC: National Academies Press; 2003.
10. Meghani SH, Byun E, Gallagher RM. Time to take stock: A meta-analysis and systematic review of analgesic treatment disparities for pain in the United States. Pain Med 2012;13:150–74.
11. Cleeland CS, Gonin R, Baez L, Loehrer P, Pandya KJ. Pain and treatment of pain in minority patients with cancer. The Eastern Cooperative Oncology Group Minority Outpatient Pain Study. Ann Intern Med 1997;127:813–6.
12. Nguyen LMT, Rhondali W, De la Cruz M, et al. Frequency and predictors of patient deviation from prescribed opioids and barriers to opioid pain management in patients with advanced cancer. J Pain Symptom Manage 2013;45:506–16.
13. Meghani SH, Thompson AML, Chittams J, et al. Adherence to analgesics for cancer pain: A comparative study of African Americans and whites using an electronic monitoring device. J Pain 2015;16:825–35.
14. Weider R, DeLaRosa N, Bryan M, et al. Prescription coverage in indigent patients affects the use of long acting opioids in management of cancer pain. Pain Med 2014;15:42–51.
15. Meghani SH, Brune DW. A pilot study to identify correlates of intentional versus unintentional nonadherence. Pain Manag Nurs 2013;14:e22-30.
16. Anderson KO, Mendoza TR, Valero V, et al. Minority cancer patients and their providers: pain management attitudes and practice. Cancer 2000;88:1929–38.
17. Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998;52:377–84.
18. Ward SE, Goldberg N, Miller-McCauley V, et al. Patient-related barriers to management of cancer pain. Pain 1993;52:319–24.
19. Glare PA, Davies PS, Finlay E, et al. Pain in cancer survivors. J Clin Oncol 2014;32:1739–47.
20. van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, et al. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol 2007;18:1437–49.
21. Jacobsen R, Samsanaviciene J, Liubarskiene Z, et al. Barriers to cancer pain management in Danish and Lithuanian patients treated in pain and palliative care units. Pain Manag Nurs 2014; 15:51–8.
22. National Institutes of Health. Pathways to prevention: the role of opioids in the treatment of chronic pain. September 29–30, 2014. Executive summary: final report. Accessed 10 Sep 2015 at https://prevention.nih.gov/docs/programs/p2p/ODPPainPanelStatementFinal_10-02-14.pdf.
23. Miaskowski C, Dodd MJ, West C, et al. Lack of adherence with the analgesic regimen: A significant barrier to effective cancer pain management. J Clin Oncol 2001;19:4275–79.
24. Yoong J, Traeger LN, Gallagher ER, et al. A pilot study to investigate adherence to long-acting opioids among patients with advanced lung cancer. J Palliat Med 2013;16:391–6.
25. Lai YH, Keefe FJ, Sun WZ, et al. Relationship between pain-specific beliefs and adherence to analgesic regimens in Taiwanese cancer patients: A preliminary study. J Pain Symptom Manage 2002;24:415–22.
26. Cohen MZ, Musgrave CF, McGuire DB, et al. The cancer pain experience of Israeli adults 65 years and older: the influence of pain interference, symptom severity, and knowledge and attitudes on pain and pain control. Support Care Cancer 2005;13:708–14.
27. Bennett MI, Bagnall AM, Jose Closs S. How effective are patient-based educational interventions in the management of cancer pain? Systematic review and meta analysis. Pain 2009;143:192–9.
28. Oldenmenger WH, Sillevis Smitt PA, van Dooren S, et al. A systematic review on barriers hindering adequate cancer pain management and interventions to reduce them: a critical appraisal. Eur J Cancer 2009;45:1370–80.
29. Meghani SH, Chittams J. Controlling for socioeconomic status in pain disparities research: all-else-equal analysis when “all else” is not equal. Pain Med 2015;16:2222–5.
30. Meghani SH, Chittams J, Hanlon A, Curry J. Measuring preferences for analgesic treatment for cancer pain: how do African Americans and whites perform on choice-based conjoint analysis experiments? BMC Med Inform Decis Mak 2013;13:118.
31. Meghani SH, Kang Y, Chittams J, et al. African Americans with cancer pain are more likely to receive an analgesic with toxic metabolite despite clinical risks: a mediation analysis study. J Clin Oncol 2014;32:2773–9.
32. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids forchronic pain - United States, 2016. MMWR Recomm Rep 2016 Mar 18;65:1–49.
33. Chapman CR, Lipschitz DL, Angst MS, et al. Opioid pharmacotherapy for chronic non-cancer pain in the United States: a research guideline for developing an evidence-base. J Pain 2010;11:807–29.
1. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain - United States, 2016. JAMA 2016;315:1624–45.
2. Meghani SH, Knafl GJ. Patterns of analgesic adherence predict health care utilization among outpatients with cancer pain. Patient Prefer Adher 2016;10:81–98.
3. Bryan M, De La Rosa N, Hill AM, et al. Influence of prescription benefits on reported pain in cancer patients. Pain Med 2008;9:1148–57.
4. Rhee YO, Kim E, Kim B. Assessment of pain and analgesic use in African American cancer patients: Factors related to adherence to analgesics. J Immigr Minor Health 2012;14:1045–51.
5. Laird BJ, Scott AC, Colvin LA, et al. Pain, depression, and fatigue as a symptom cluster in advanced cancer. J Pain Symptom Manage 2011;42:1–11.
6. Ferreira KA, Kimura M, Teixeira MJ, et al. Impact of cancer-related symptom synergisms on health-related quality of life and performance status. J Pain Symptom Manage 2008;35:604–16.
7. Craig BM, Strassels SA. Out-of-pocket prices of opioid analgesics in the United States, 1999-2004. Pain Med 2010;11:240–47.
8. Institute of Medicine: Relieving pain in america: a blueprint for transforming prevention, care, education, and research. Washington, DC: National Academies Press; 2011.
9. Institutes of Medicine. Unequal treatment: confronting racial and ethnic disparities in health care. Washington, DC: National Academies Press; 2003.
10. Meghani SH, Byun E, Gallagher RM. Time to take stock: A meta-analysis and systematic review of analgesic treatment disparities for pain in the United States. Pain Med 2012;13:150–74.
11. Cleeland CS, Gonin R, Baez L, Loehrer P, Pandya KJ. Pain and treatment of pain in minority patients with cancer. The Eastern Cooperative Oncology Group Minority Outpatient Pain Study. Ann Intern Med 1997;127:813–6.
12. Nguyen LMT, Rhondali W, De la Cruz M, et al. Frequency and predictors of patient deviation from prescribed opioids and barriers to opioid pain management in patients with advanced cancer. J Pain Symptom Manage 2013;45:506–16.
13. Meghani SH, Thompson AML, Chittams J, et al. Adherence to analgesics for cancer pain: A comparative study of African Americans and whites using an electronic monitoring device. J Pain 2015;16:825–35.
14. Weider R, DeLaRosa N, Bryan M, et al. Prescription coverage in indigent patients affects the use of long acting opioids in management of cancer pain. Pain Med 2014;15:42–51.
15. Meghani SH, Brune DW. A pilot study to identify correlates of intentional versus unintentional nonadherence. Pain Manag Nurs 2013;14:e22-30.
16. Anderson KO, Mendoza TR, Valero V, et al. Minority cancer patients and their providers: pain management attitudes and practice. Cancer 2000;88:1929–38.
17. Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998;52:377–84.
18. Ward SE, Goldberg N, Miller-McCauley V, et al. Patient-related barriers to management of cancer pain. Pain 1993;52:319–24.
19. Glare PA, Davies PS, Finlay E, et al. Pain in cancer survivors. J Clin Oncol 2014;32:1739–47.
20. van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, et al. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol 2007;18:1437–49.
21. Jacobsen R, Samsanaviciene J, Liubarskiene Z, et al. Barriers to cancer pain management in Danish and Lithuanian patients treated in pain and palliative care units. Pain Manag Nurs 2014; 15:51–8.
22. National Institutes of Health. Pathways to prevention: the role of opioids in the treatment of chronic pain. September 29–30, 2014. Executive summary: final report. Accessed 10 Sep 2015 at https://prevention.nih.gov/docs/programs/p2p/ODPPainPanelStatementFinal_10-02-14.pdf.
23. Miaskowski C, Dodd MJ, West C, et al. Lack of adherence with the analgesic regimen: A significant barrier to effective cancer pain management. J Clin Oncol 2001;19:4275–79.
24. Yoong J, Traeger LN, Gallagher ER, et al. A pilot study to investigate adherence to long-acting opioids among patients with advanced lung cancer. J Palliat Med 2013;16:391–6.
25. Lai YH, Keefe FJ, Sun WZ, et al. Relationship between pain-specific beliefs and adherence to analgesic regimens in Taiwanese cancer patients: A preliminary study. J Pain Symptom Manage 2002;24:415–22.
26. Cohen MZ, Musgrave CF, McGuire DB, et al. The cancer pain experience of Israeli adults 65 years and older: the influence of pain interference, symptom severity, and knowledge and attitudes on pain and pain control. Support Care Cancer 2005;13:708–14.
27. Bennett MI, Bagnall AM, Jose Closs S. How effective are patient-based educational interventions in the management of cancer pain? Systematic review and meta analysis. Pain 2009;143:192–9.
28. Oldenmenger WH, Sillevis Smitt PA, van Dooren S, et al. A systematic review on barriers hindering adequate cancer pain management and interventions to reduce them: a critical appraisal. Eur J Cancer 2009;45:1370–80.
29. Meghani SH, Chittams J. Controlling for socioeconomic status in pain disparities research: all-else-equal analysis when “all else” is not equal. Pain Med 2015;16:2222–5.
30. Meghani SH, Chittams J, Hanlon A, Curry J. Measuring preferences for analgesic treatment for cancer pain: how do African Americans and whites perform on choice-based conjoint analysis experiments? BMC Med Inform Decis Mak 2013;13:118.
31. Meghani SH, Kang Y, Chittams J, et al. African Americans with cancer pain are more likely to receive an analgesic with toxic metabolite despite clinical risks: a mediation analysis study. J Clin Oncol 2014;32:2773–9.
32. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids forchronic pain - United States, 2016. MMWR Recomm Rep 2016 Mar 18;65:1–49.
33. Chapman CR, Lipschitz DL, Angst MS, et al. Opioid pharmacotherapy for chronic non-cancer pain in the United States: a research guideline for developing an evidence-base. J Pain 2010;11:807–29.
2017 Update on obstetrics
In this Update we discuss several exciting new recommendations for preventive treatments in pregnancy and prenatal diagnostic tests. Our A-to-Z coverage includes:
- antenatal steroids in late preterm pregnancy
- expanded list of high-risk conditions warranting low-dose aspirin for preeclampsia prevention
- chromosomal microarray analysis versus karyotype for specific clinical situations
- Zika virus infection evolving information.
Next: New recommendation for timing of late preterm antenatal steroids
New recommendation offered for timing of late preterm antenatal steroids
Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al; for the NICHD Maternal-Fetal Medicine Units Network. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;374(14):1311-1320.
American College of Obstetricians and Gynecologists. Committee Opinion No. 677. Antenatal corticosteroidtherapy for fetal maturation. Obstet Gynecol. 2016;128(4):e187-e194.
Kamath-Rayne BD, Rozance PJ, Goldenberg RL, Jobe AH. Antenatal corticosteroids beyond 34 weeks gestation: what do we do now? Am J Obstet Gynecol. 2016;215(4):423-430.
A dramatic recommendation for obstetric practice change occurred in 2016: the option of administering antenatal steroids for fetal lung maturity after 34 weeks. In the Antenatal Late Preterm Steroids (ALPS) trial of betamethasone in the late preterm period in patients at "high risk" of imminent delivery, Gyamfi-Bannerman and colleagues demonstrated that the treated group had a significant decrease in the rate of neonatal respiratory complications.
The primary outcome, a composite of respiratory morbidities (including transient tachypnea of the newborn, surfactant use, and need for resuscitation at birth) within the first 72 hours of life, had significant differences between groups, occurring in 165 of 1,427 infants (11.6%) in the betamethasone-treated group and 202 of 1,400 (14.4%) in the placebo group (relative risk in the betamethasone group, 0.80; 95% confidence interval, 0.66-0.97; P = .02). However, there was no statistically significant difference in respiratory distress syndrome, apnea, or pneumonia between groups, and the significant difference noted in bronchopulmonary dysplasia was based on a total number of 11 cases.
In response to these findings, both the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine (SMFM) released practice advisories and interim updates, culminating in a final recommendation for a single course of betamethasone in patients at high risk of preterm delivery between 34 and 36 6/7 weeks who have not received a previous course.
Related article:
When could use of antenatal corticosteroids in the late preterm birth period be beneficial?
In a thorough review of the literature on antenatal steroid use, Kamath-Rayne and colleagues highlighted several factors that should be considered before adopting universal use of steroids at >34 weeks. These include:
- The definition of "high risk of imminent delivery" as preterm labor with at least 3-cm dilation or 75% effacement, or spontaneous rupture of membranes. The effect of less stringent inclusion criteria in real-world clinical practice is not known, and many patients who will go on to deliver at term will receive steroids unnecessarily.
- Multiple gestation, patients with pre-existing diabetes, women who had previously received a course of steroids, and fetuses with anomalies were excluded from the ALPS study. Use of antenatal steroids in these groups at >34 weeks should be evaluated before universal adoption.
Related article:
What is the ideal gestational age for twin delivery to minimize perinatal deaths?
- The incidence of neonatal hypoglycemia in the treated group was significantly increased. This affects our colleagues in pediatrics considerably from a systems standpoint (need for changes to newborn protocols and communication between services).
- The long-term outcomes of patients exposed to steroids in the late preterm period are yet to be delineated, specifically, the potential neurodevelopmental effects of a medication known to alter preterm brain development as well as cardiovascular and metabolic consequences.
Next: Low-dose aspirin for reducing preeclampsia risk
Low-dose aspirin clearly is effective for reducing the risk of preeclampsia
American College of Obstetricians and Gynecologists. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122-1131.
Henderson JT, Whitlock EP, O'Connor E, Senger CA, Thompson JH, Rowland MG. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: a systematic evidence review for the US Preventive Services Task Force. Ann Intern Med. 2014;160(10):695-703.
LeFevre ML; US Preventive Services Task Force. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161(11):819-826.
American College of Obstetricians and Gynecologists. Practice advisory on low-dose aspirin and prevention of preeclampsia: updated recommendations. http://www.acog.org/About-ACOG/News-Room/Practice-Advisories/Practice-Advisory-Low-Dose-Aspirin-and-Prevention-of-Preeclampsia-Updated-Recommendations. Published July 11, 2016. Accessed December 6, 2016.
In the 2013 ACOG Task Force on Hypertension in Pregnancy report, low-dose aspirin (60-80 mg) was recommended to be initiated in the late first trimester to reduce preeclampsia risk for women with:
- prior early onset preeclampsia with preterm delivery at <34 weeks' gestation, or
- preeclampsia in more than one prior pregnancy.
This recommendation was based on several meta-analyses that demonstrated a 10% to 17% reduction in risk with no increase in bleeding, placental abruption, or other adverse events.
In 2014, the US Preventive Services Task Force (USPSTF) conducted a systematic evidence review of low-dose aspirin use for prevention of morbidity and mortality from preeclampsia. That report revealed a 24% risk reduction of preeclampsia in high-risk women treated with low-dose aspirin, as well as a 14% reduction in preterm birth and a 20% reduction in fetal growth restriction. A final statement from the USPSTF in 2014 recommended low-dose aspirin (60-150 mg) starting between 12 and 28 weeks' gestation for women at "high" risk who have:
- a history of preeclampsia, especially if accompanied by an adverse outcome
- multifetal gestation
- chronic hypertension
- diabetes (type 1 or type 2)
- renal disease
- autoimmune disease (such as systematic lupus erythematosus, antiphospholipid syndrome).
Related article:
Start offering aspirin to pregnant women at high risk for preeclampsia
As of July 11, 2016, ACOG supports this expanded list of high-risk conditions. Additionally, the USPSTF identified a "moderate" risk group in which low-dose aspirin may be considered if a patient has several risk factors, such as obesity, nulliparity, family history of preeclampsia, age 35 years or older, or another poor pregnancy outcome. ACOG notes, however, that the evidence supporting this practice is uncertain and does not make a recommendation regarding aspirin use in this population. Further study should be conducted to determine the benefit of low-dose aspirin in these patients as well as the long-term effects of treatment on maternal and child outcomes.
Next: CMA for prenatal genetic diagnosis
Chromosomal microarray analysis is preferable to karyotype in certain situations
Pauli JM, Repke JT. Update on obstetrics. OBG Manag. 2013;25(1):28-32.
Society for Maternal-Fetal Medicine (SMFM), Dugoff L, Norton ME, Kuller JA. The use of chromosomal microarray for prenatal diagnosis. Am J Obstet Gynecol. 2016;215(4):B2-B9.
American College of Obstetricians and Gynecologists. Committee Opinion No. 682. Microarrays and next- generation sequencing technology: the use of advanced genetic diagnostic tools in obstetrics and gynecology.Obstet Gynecol. 2016;128(6):e262-e268.
We previously addressed the use of chromosomal microarray analysis (CMA) for prenatal diagnosis in our 2013 "Update on obstetrics," specifically, the question of whether CMA could replace karyotype. The main differences between karyotype and CMA are that 1) only karyotype can detect balanced translocations/inversions and 2) only CMA can detect copy number variants (CNV). There are some differences in the technology and capabilities of the 2 types of CMA currently available as well.
In our 2013 article we concluded that "The total costs of such an approach--test, interpretation, counseling, and long-term follow-up of uncertain results--are unknown at this time and may prove to be unaffordable on a population-wide basis." Today, the cost of CMA is still higher than karyotype, but it is expected to decrease and insurance coverage for this test is expected to increase.
Related article:
Cell-free DNA screening for women at low risk for fetal aneuploidy
Both SMFM and ACOG released recommendations in 2016 regarding the use of CMA in prenatal genetic diagnosis, summarized as follows:
- CMA is recommended over karyotype for fetuses with structural abnormalities on ultrasound
- The detection rate for clinically relevant abnormal CNVs in this population is about 6%
- CMA is recommended for diagnosis for stillbirth specimens
- CMA does not require dividing cells and may be a quicker and more reliable test in this population
- Karotype or fluorescence in situ hybridization (FISH) is recommended for fetuses with ultrasound findings suggestive of aneuploidy
- If it is negative, then CMA is recommended
- Karyotype or CMA is recommended for patients desiring prenatal diagnostic testing with a normal fetal ultrasound
- The detection rate for clinically relevant CNVs in this population (advanced maternal age, abnormal serum screening, prior aneuploidy, parental anxiety) is about 1%
- Pretest and posttest counseling about the limitations of CMA and a 2% risk of detection of variants of unknown significance (VUS) should be performed by a provider who has expertise in CMA and who has access to databases with genotype/phenotype information for VUS
- This counseling should also include the possibility of diagnosis of nonpaternity, consanguinity, and adult-onset disease
- Karyotype is recommended for couples with recurrent pregnancy loss
- The identification of balanced translocations in this population is most relevant in this patient population
- Prenatal diagnosis with routine use of whole-genome or whole-exome sequencing is not recommended.
Next: Zika virus: Check for updates
Zika virus infection: Check often for the latest updates
American College of Obstetricians and Gynecologists, Society for Maternal-Fetal Medicine. Practice advisory on Zika virus. http://www.acog.org/About-ACOG/News-Room/Practice-Advisories/Practice-Advisory-Interim-Guidance-for-Care-of-Obstetric-Patients- During-a-Zika-Virus-Outbreak. Published December 5, 2016. Accessed December 6, 2016.
Centers for Disease Control and Prevention. Zika virus. http://www.cdc.gov/zika/pregnancy/index.html. Updated August 22, 2016. Accessed December 6, 2016.
Petersen EE, Meaney-Delman D, Neblett-Fanfair R, et al. Update: interim guidance for preconception counseling and prevention of sexual transmission of Zika virus for persons with possible Zika virus exposure--United States, September 2016. MMWR Morbid Mortal Wkly Rep. 2016;65(39):1077-1081.
A yearly update on obstetrics would be remiss without mention of the Zika virus and its impact on pregnancy and reproduction. That being said, any recommendations we offer may be out of date by the time this article is published given the rapidly changing picture of Zika virus since it first dominated the headlines in 2016. Here are the basics as summarized from ACOG and the Centers for Disease Control and Prevention (CDC):
Viral spread. Zika virus may be spread in several ways: by an infected Aedes species mosquito, mother to fetus, sexual contact, blood transfusion, or laboratory exposure.
Symptoms of infection include conjunctivitis, fever, rash, and arthralgia, but most patients (4/5) are asymptomatic.
Sequelae. Zika virus infection during pregnancy is believed to cause fetal and neonatal microcephaly, intracranial calcifications, and brain and eye abnormalities. The rate of these findings in infected individuals, as well as the rate of vertical transmission, is not known.
Travel advisory. Pregnant women should not travel to areas with active Zika infection (the CDC website regularly updates these restricted areas).
Preventive measures. If traveling to an area of active Zika infection, pregnant women should take preventative measures day and night against mosquito bites, such as use of insect repellents approved by the Environmental Protection Agency, clothing that covers exposed skin, and staying indoors.
Safe sex. Abstinence or consistent condom use is recommended for pregnant women with partners who travel to or live in areas of active Zika infection.
Delay conception. Conception should be postponed for at least 6 months in men with Zika infection and at least 8 weeks in women with Zika infection.
Testing recommendations. Pregnant women with Zika virus exposure should be tested, regardless of symptoms. Symptomatic exposed nonpregnant women and all men should be tested.
Prenatal surveillance. High-risk consultation and serial ultrasounds for fetal anatomy and growth should be considered in patients with Zika virus infection during pregnancy. Amniocentesis can be considered on a case-by-case basis.
Related article:
Zika virus update: A rapidly moving target
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In this Update we discuss several exciting new recommendations for preventive treatments in pregnancy and prenatal diagnostic tests. Our A-to-Z coverage includes:
- antenatal steroids in late preterm pregnancy
- expanded list of high-risk conditions warranting low-dose aspirin for preeclampsia prevention
- chromosomal microarray analysis versus karyotype for specific clinical situations
- Zika virus infection evolving information.
Next: New recommendation for timing of late preterm antenatal steroids
New recommendation offered for timing of late preterm antenatal steroids
Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al; for the NICHD Maternal-Fetal Medicine Units Network. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;374(14):1311-1320.
American College of Obstetricians and Gynecologists. Committee Opinion No. 677. Antenatal corticosteroidtherapy for fetal maturation. Obstet Gynecol. 2016;128(4):e187-e194.
Kamath-Rayne BD, Rozance PJ, Goldenberg RL, Jobe AH. Antenatal corticosteroids beyond 34 weeks gestation: what do we do now? Am J Obstet Gynecol. 2016;215(4):423-430.
A dramatic recommendation for obstetric practice change occurred in 2016: the option of administering antenatal steroids for fetal lung maturity after 34 weeks. In the Antenatal Late Preterm Steroids (ALPS) trial of betamethasone in the late preterm period in patients at "high risk" of imminent delivery, Gyamfi-Bannerman and colleagues demonstrated that the treated group had a significant decrease in the rate of neonatal respiratory complications.
The primary outcome, a composite of respiratory morbidities (including transient tachypnea of the newborn, surfactant use, and need for resuscitation at birth) within the first 72 hours of life, had significant differences between groups, occurring in 165 of 1,427 infants (11.6%) in the betamethasone-treated group and 202 of 1,400 (14.4%) in the placebo group (relative risk in the betamethasone group, 0.80; 95% confidence interval, 0.66-0.97; P = .02). However, there was no statistically significant difference in respiratory distress syndrome, apnea, or pneumonia between groups, and the significant difference noted in bronchopulmonary dysplasia was based on a total number of 11 cases.
In response to these findings, both the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine (SMFM) released practice advisories and interim updates, culminating in a final recommendation for a single course of betamethasone in patients at high risk of preterm delivery between 34 and 36 6/7 weeks who have not received a previous course.
Related article:
When could use of antenatal corticosteroids in the late preterm birth period be beneficial?
In a thorough review of the literature on antenatal steroid use, Kamath-Rayne and colleagues highlighted several factors that should be considered before adopting universal use of steroids at >34 weeks. These include:
- The definition of "high risk of imminent delivery" as preterm labor with at least 3-cm dilation or 75% effacement, or spontaneous rupture of membranes. The effect of less stringent inclusion criteria in real-world clinical practice is not known, and many patients who will go on to deliver at term will receive steroids unnecessarily.
- Multiple gestation, patients with pre-existing diabetes, women who had previously received a course of steroids, and fetuses with anomalies were excluded from the ALPS study. Use of antenatal steroids in these groups at >34 weeks should be evaluated before universal adoption.
Related article:
What is the ideal gestational age for twin delivery to minimize perinatal deaths?
- The incidence of neonatal hypoglycemia in the treated group was significantly increased. This affects our colleagues in pediatrics considerably from a systems standpoint (need for changes to newborn protocols and communication between services).
- The long-term outcomes of patients exposed to steroids in the late preterm period are yet to be delineated, specifically, the potential neurodevelopmental effects of a medication known to alter preterm brain development as well as cardiovascular and metabolic consequences.
Next: Low-dose aspirin for reducing preeclampsia risk
Low-dose aspirin clearly is effective for reducing the risk of preeclampsia
American College of Obstetricians and Gynecologists. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122-1131.
Henderson JT, Whitlock EP, O'Connor E, Senger CA, Thompson JH, Rowland MG. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: a systematic evidence review for the US Preventive Services Task Force. Ann Intern Med. 2014;160(10):695-703.
LeFevre ML; US Preventive Services Task Force. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161(11):819-826.
American College of Obstetricians and Gynecologists. Practice advisory on low-dose aspirin and prevention of preeclampsia: updated recommendations. http://www.acog.org/About-ACOG/News-Room/Practice-Advisories/Practice-Advisory-Low-Dose-Aspirin-and-Prevention-of-Preeclampsia-Updated-Recommendations. Published July 11, 2016. Accessed December 6, 2016.
In the 2013 ACOG Task Force on Hypertension in Pregnancy report, low-dose aspirin (60-80 mg) was recommended to be initiated in the late first trimester to reduce preeclampsia risk for women with:
- prior early onset preeclampsia with preterm delivery at <34 weeks' gestation, or
- preeclampsia in more than one prior pregnancy.
This recommendation was based on several meta-analyses that demonstrated a 10% to 17% reduction in risk with no increase in bleeding, placental abruption, or other adverse events.
In 2014, the US Preventive Services Task Force (USPSTF) conducted a systematic evidence review of low-dose aspirin use for prevention of morbidity and mortality from preeclampsia. That report revealed a 24% risk reduction of preeclampsia in high-risk women treated with low-dose aspirin, as well as a 14% reduction in preterm birth and a 20% reduction in fetal growth restriction. A final statement from the USPSTF in 2014 recommended low-dose aspirin (60-150 mg) starting between 12 and 28 weeks' gestation for women at "high" risk who have:
- a history of preeclampsia, especially if accompanied by an adverse outcome
- multifetal gestation
- chronic hypertension
- diabetes (type 1 or type 2)
- renal disease
- autoimmune disease (such as systematic lupus erythematosus, antiphospholipid syndrome).
Related article:
Start offering aspirin to pregnant women at high risk for preeclampsia
As of July 11, 2016, ACOG supports this expanded list of high-risk conditions. Additionally, the USPSTF identified a "moderate" risk group in which low-dose aspirin may be considered if a patient has several risk factors, such as obesity, nulliparity, family history of preeclampsia, age 35 years or older, or another poor pregnancy outcome. ACOG notes, however, that the evidence supporting this practice is uncertain and does not make a recommendation regarding aspirin use in this population. Further study should be conducted to determine the benefit of low-dose aspirin in these patients as well as the long-term effects of treatment on maternal and child outcomes.
Next: CMA for prenatal genetic diagnosis
Chromosomal microarray analysis is preferable to karyotype in certain situations
Pauli JM, Repke JT. Update on obstetrics. OBG Manag. 2013;25(1):28-32.
Society for Maternal-Fetal Medicine (SMFM), Dugoff L, Norton ME, Kuller JA. The use of chromosomal microarray for prenatal diagnosis. Am J Obstet Gynecol. 2016;215(4):B2-B9.
American College of Obstetricians and Gynecologists. Committee Opinion No. 682. Microarrays and next- generation sequencing technology: the use of advanced genetic diagnostic tools in obstetrics and gynecology.Obstet Gynecol. 2016;128(6):e262-e268.
We previously addressed the use of chromosomal microarray analysis (CMA) for prenatal diagnosis in our 2013 "Update on obstetrics," specifically, the question of whether CMA could replace karyotype. The main differences between karyotype and CMA are that 1) only karyotype can detect balanced translocations/inversions and 2) only CMA can detect copy number variants (CNV). There are some differences in the technology and capabilities of the 2 types of CMA currently available as well.
In our 2013 article we concluded that "The total costs of such an approach--test, interpretation, counseling, and long-term follow-up of uncertain results--are unknown at this time and may prove to be unaffordable on a population-wide basis." Today, the cost of CMA is still higher than karyotype, but it is expected to decrease and insurance coverage for this test is expected to increase.
Related article:
Cell-free DNA screening for women at low risk for fetal aneuploidy
Both SMFM and ACOG released recommendations in 2016 regarding the use of CMA in prenatal genetic diagnosis, summarized as follows:
- CMA is recommended over karyotype for fetuses with structural abnormalities on ultrasound
- The detection rate for clinically relevant abnormal CNVs in this population is about 6%
- CMA is recommended for diagnosis for stillbirth specimens
- CMA does not require dividing cells and may be a quicker and more reliable test in this population
- Karotype or fluorescence in situ hybridization (FISH) is recommended for fetuses with ultrasound findings suggestive of aneuploidy
- If it is negative, then CMA is recommended
- Karyotype or CMA is recommended for patients desiring prenatal diagnostic testing with a normal fetal ultrasound
- The detection rate for clinically relevant CNVs in this population (advanced maternal age, abnormal serum screening, prior aneuploidy, parental anxiety) is about 1%
- Pretest and posttest counseling about the limitations of CMA and a 2% risk of detection of variants of unknown significance (VUS) should be performed by a provider who has expertise in CMA and who has access to databases with genotype/phenotype information for VUS
- This counseling should also include the possibility of diagnosis of nonpaternity, consanguinity, and adult-onset disease
- Karyotype is recommended for couples with recurrent pregnancy loss
- The identification of balanced translocations in this population is most relevant in this patient population
- Prenatal diagnosis with routine use of whole-genome or whole-exome sequencing is not recommended.
Next: Zika virus: Check for updates
Zika virus infection: Check often for the latest updates
American College of Obstetricians and Gynecologists, Society for Maternal-Fetal Medicine. Practice advisory on Zika virus. http://www.acog.org/About-ACOG/News-Room/Practice-Advisories/Practice-Advisory-Interim-Guidance-for-Care-of-Obstetric-Patients- During-a-Zika-Virus-Outbreak. Published December 5, 2016. Accessed December 6, 2016.
Centers for Disease Control and Prevention. Zika virus. http://www.cdc.gov/zika/pregnancy/index.html. Updated August 22, 2016. Accessed December 6, 2016.
Petersen EE, Meaney-Delman D, Neblett-Fanfair R, et al. Update: interim guidance for preconception counseling and prevention of sexual transmission of Zika virus for persons with possible Zika virus exposure--United States, September 2016. MMWR Morbid Mortal Wkly Rep. 2016;65(39):1077-1081.
A yearly update on obstetrics would be remiss without mention of the Zika virus and its impact on pregnancy and reproduction. That being said, any recommendations we offer may be out of date by the time this article is published given the rapidly changing picture of Zika virus since it first dominated the headlines in 2016. Here are the basics as summarized from ACOG and the Centers for Disease Control and Prevention (CDC):
Viral spread. Zika virus may be spread in several ways: by an infected Aedes species mosquito, mother to fetus, sexual contact, blood transfusion, or laboratory exposure.
Symptoms of infection include conjunctivitis, fever, rash, and arthralgia, but most patients (4/5) are asymptomatic.
Sequelae. Zika virus infection during pregnancy is believed to cause fetal and neonatal microcephaly, intracranial calcifications, and brain and eye abnormalities. The rate of these findings in infected individuals, as well as the rate of vertical transmission, is not known.
Travel advisory. Pregnant women should not travel to areas with active Zika infection (the CDC website regularly updates these restricted areas).
Preventive measures. If traveling to an area of active Zika infection, pregnant women should take preventative measures day and night against mosquito bites, such as use of insect repellents approved by the Environmental Protection Agency, clothing that covers exposed skin, and staying indoors.
Safe sex. Abstinence or consistent condom use is recommended for pregnant women with partners who travel to or live in areas of active Zika infection.
Delay conception. Conception should be postponed for at least 6 months in men with Zika infection and at least 8 weeks in women with Zika infection.
Testing recommendations. Pregnant women with Zika virus exposure should be tested, regardless of symptoms. Symptomatic exposed nonpregnant women and all men should be tested.
Prenatal surveillance. High-risk consultation and serial ultrasounds for fetal anatomy and growth should be considered in patients with Zika virus infection during pregnancy. Amniocentesis can be considered on a case-by-case basis.
Related article:
Zika virus update: A rapidly moving target
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In this Update we discuss several exciting new recommendations for preventive treatments in pregnancy and prenatal diagnostic tests. Our A-to-Z coverage includes:
- antenatal steroids in late preterm pregnancy
- expanded list of high-risk conditions warranting low-dose aspirin for preeclampsia prevention
- chromosomal microarray analysis versus karyotype for specific clinical situations
- Zika virus infection evolving information.
Next: New recommendation for timing of late preterm antenatal steroids
New recommendation offered for timing of late preterm antenatal steroids
Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al; for the NICHD Maternal-Fetal Medicine Units Network. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;374(14):1311-1320.
American College of Obstetricians and Gynecologists. Committee Opinion No. 677. Antenatal corticosteroidtherapy for fetal maturation. Obstet Gynecol. 2016;128(4):e187-e194.
Kamath-Rayne BD, Rozance PJ, Goldenberg RL, Jobe AH. Antenatal corticosteroids beyond 34 weeks gestation: what do we do now? Am J Obstet Gynecol. 2016;215(4):423-430.
A dramatic recommendation for obstetric practice change occurred in 2016: the option of administering antenatal steroids for fetal lung maturity after 34 weeks. In the Antenatal Late Preterm Steroids (ALPS) trial of betamethasone in the late preterm period in patients at "high risk" of imminent delivery, Gyamfi-Bannerman and colleagues demonstrated that the treated group had a significant decrease in the rate of neonatal respiratory complications.
The primary outcome, a composite of respiratory morbidities (including transient tachypnea of the newborn, surfactant use, and need for resuscitation at birth) within the first 72 hours of life, had significant differences between groups, occurring in 165 of 1,427 infants (11.6%) in the betamethasone-treated group and 202 of 1,400 (14.4%) in the placebo group (relative risk in the betamethasone group, 0.80; 95% confidence interval, 0.66-0.97; P = .02). However, there was no statistically significant difference in respiratory distress syndrome, apnea, or pneumonia between groups, and the significant difference noted in bronchopulmonary dysplasia was based on a total number of 11 cases.
In response to these findings, both the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine (SMFM) released practice advisories and interim updates, culminating in a final recommendation for a single course of betamethasone in patients at high risk of preterm delivery between 34 and 36 6/7 weeks who have not received a previous course.
Related article:
When could use of antenatal corticosteroids in the late preterm birth period be beneficial?
In a thorough review of the literature on antenatal steroid use, Kamath-Rayne and colleagues highlighted several factors that should be considered before adopting universal use of steroids at >34 weeks. These include:
- The definition of "high risk of imminent delivery" as preterm labor with at least 3-cm dilation or 75% effacement, or spontaneous rupture of membranes. The effect of less stringent inclusion criteria in real-world clinical practice is not known, and many patients who will go on to deliver at term will receive steroids unnecessarily.
- Multiple gestation, patients with pre-existing diabetes, women who had previously received a course of steroids, and fetuses with anomalies were excluded from the ALPS study. Use of antenatal steroids in these groups at >34 weeks should be evaluated before universal adoption.
Related article:
What is the ideal gestational age for twin delivery to minimize perinatal deaths?
- The incidence of neonatal hypoglycemia in the treated group was significantly increased. This affects our colleagues in pediatrics considerably from a systems standpoint (need for changes to newborn protocols and communication between services).
- The long-term outcomes of patients exposed to steroids in the late preterm period are yet to be delineated, specifically, the potential neurodevelopmental effects of a medication known to alter preterm brain development as well as cardiovascular and metabolic consequences.
Next: Low-dose aspirin for reducing preeclampsia risk
Low-dose aspirin clearly is effective for reducing the risk of preeclampsia
American College of Obstetricians and Gynecologists. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122-1131.
Henderson JT, Whitlock EP, O'Connor E, Senger CA, Thompson JH, Rowland MG. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: a systematic evidence review for the US Preventive Services Task Force. Ann Intern Med. 2014;160(10):695-703.
LeFevre ML; US Preventive Services Task Force. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161(11):819-826.
American College of Obstetricians and Gynecologists. Practice advisory on low-dose aspirin and prevention of preeclampsia: updated recommendations. http://www.acog.org/About-ACOG/News-Room/Practice-Advisories/Practice-Advisory-Low-Dose-Aspirin-and-Prevention-of-Preeclampsia-Updated-Recommendations. Published July 11, 2016. Accessed December 6, 2016.
In the 2013 ACOG Task Force on Hypertension in Pregnancy report, low-dose aspirin (60-80 mg) was recommended to be initiated in the late first trimester to reduce preeclampsia risk for women with:
- prior early onset preeclampsia with preterm delivery at <34 weeks' gestation, or
- preeclampsia in more than one prior pregnancy.
This recommendation was based on several meta-analyses that demonstrated a 10% to 17% reduction in risk with no increase in bleeding, placental abruption, or other adverse events.
In 2014, the US Preventive Services Task Force (USPSTF) conducted a systematic evidence review of low-dose aspirin use for prevention of morbidity and mortality from preeclampsia. That report revealed a 24% risk reduction of preeclampsia in high-risk women treated with low-dose aspirin, as well as a 14% reduction in preterm birth and a 20% reduction in fetal growth restriction. A final statement from the USPSTF in 2014 recommended low-dose aspirin (60-150 mg) starting between 12 and 28 weeks' gestation for women at "high" risk who have:
- a history of preeclampsia, especially if accompanied by an adverse outcome
- multifetal gestation
- chronic hypertension
- diabetes (type 1 or type 2)
- renal disease
- autoimmune disease (such as systematic lupus erythematosus, antiphospholipid syndrome).
Related article:
Start offering aspirin to pregnant women at high risk for preeclampsia
As of July 11, 2016, ACOG supports this expanded list of high-risk conditions. Additionally, the USPSTF identified a "moderate" risk group in which low-dose aspirin may be considered if a patient has several risk factors, such as obesity, nulliparity, family history of preeclampsia, age 35 years or older, or another poor pregnancy outcome. ACOG notes, however, that the evidence supporting this practice is uncertain and does not make a recommendation regarding aspirin use in this population. Further study should be conducted to determine the benefit of low-dose aspirin in these patients as well as the long-term effects of treatment on maternal and child outcomes.
Next: CMA for prenatal genetic diagnosis
Chromosomal microarray analysis is preferable to karyotype in certain situations
Pauli JM, Repke JT. Update on obstetrics. OBG Manag. 2013;25(1):28-32.
Society for Maternal-Fetal Medicine (SMFM), Dugoff L, Norton ME, Kuller JA. The use of chromosomal microarray for prenatal diagnosis. Am J Obstet Gynecol. 2016;215(4):B2-B9.
American College of Obstetricians and Gynecologists. Committee Opinion No. 682. Microarrays and next- generation sequencing technology: the use of advanced genetic diagnostic tools in obstetrics and gynecology.Obstet Gynecol. 2016;128(6):e262-e268.
We previously addressed the use of chromosomal microarray analysis (CMA) for prenatal diagnosis in our 2013 "Update on obstetrics," specifically, the question of whether CMA could replace karyotype. The main differences between karyotype and CMA are that 1) only karyotype can detect balanced translocations/inversions and 2) only CMA can detect copy number variants (CNV). There are some differences in the technology and capabilities of the 2 types of CMA currently available as well.
In our 2013 article we concluded that "The total costs of such an approach--test, interpretation, counseling, and long-term follow-up of uncertain results--are unknown at this time and may prove to be unaffordable on a population-wide basis." Today, the cost of CMA is still higher than karyotype, but it is expected to decrease and insurance coverage for this test is expected to increase.
Related article:
Cell-free DNA screening for women at low risk for fetal aneuploidy
Both SMFM and ACOG released recommendations in 2016 regarding the use of CMA in prenatal genetic diagnosis, summarized as follows:
- CMA is recommended over karyotype for fetuses with structural abnormalities on ultrasound
- The detection rate for clinically relevant abnormal CNVs in this population is about 6%
- CMA is recommended for diagnosis for stillbirth specimens
- CMA does not require dividing cells and may be a quicker and more reliable test in this population
- Karotype or fluorescence in situ hybridization (FISH) is recommended for fetuses with ultrasound findings suggestive of aneuploidy
- If it is negative, then CMA is recommended
- Karyotype or CMA is recommended for patients desiring prenatal diagnostic testing with a normal fetal ultrasound
- The detection rate for clinically relevant CNVs in this population (advanced maternal age, abnormal serum screening, prior aneuploidy, parental anxiety) is about 1%
- Pretest and posttest counseling about the limitations of CMA and a 2% risk of detection of variants of unknown significance (VUS) should be performed by a provider who has expertise in CMA and who has access to databases with genotype/phenotype information for VUS
- This counseling should also include the possibility of diagnosis of nonpaternity, consanguinity, and adult-onset disease
- Karyotype is recommended for couples with recurrent pregnancy loss
- The identification of balanced translocations in this population is most relevant in this patient population
- Prenatal diagnosis with routine use of whole-genome or whole-exome sequencing is not recommended.
Next: Zika virus: Check for updates
Zika virus infection: Check often for the latest updates
American College of Obstetricians and Gynecologists, Society for Maternal-Fetal Medicine. Practice advisory on Zika virus. http://www.acog.org/About-ACOG/News-Room/Practice-Advisories/Practice-Advisory-Interim-Guidance-for-Care-of-Obstetric-Patients- During-a-Zika-Virus-Outbreak. Published December 5, 2016. Accessed December 6, 2016.
Centers for Disease Control and Prevention. Zika virus. http://www.cdc.gov/zika/pregnancy/index.html. Updated August 22, 2016. Accessed December 6, 2016.
Petersen EE, Meaney-Delman D, Neblett-Fanfair R, et al. Update: interim guidance for preconception counseling and prevention of sexual transmission of Zika virus for persons with possible Zika virus exposure--United States, September 2016. MMWR Morbid Mortal Wkly Rep. 2016;65(39):1077-1081.
A yearly update on obstetrics would be remiss without mention of the Zika virus and its impact on pregnancy and reproduction. That being said, any recommendations we offer may be out of date by the time this article is published given the rapidly changing picture of Zika virus since it first dominated the headlines in 2016. Here are the basics as summarized from ACOG and the Centers for Disease Control and Prevention (CDC):
Viral spread. Zika virus may be spread in several ways: by an infected Aedes species mosquito, mother to fetus, sexual contact, blood transfusion, or laboratory exposure.
Symptoms of infection include conjunctivitis, fever, rash, and arthralgia, but most patients (4/5) are asymptomatic.
Sequelae. Zika virus infection during pregnancy is believed to cause fetal and neonatal microcephaly, intracranial calcifications, and brain and eye abnormalities. The rate of these findings in infected individuals, as well as the rate of vertical transmission, is not known.
Travel advisory. Pregnant women should not travel to areas with active Zika infection (the CDC website regularly updates these restricted areas).
Preventive measures. If traveling to an area of active Zika infection, pregnant women should take preventative measures day and night against mosquito bites, such as use of insect repellents approved by the Environmental Protection Agency, clothing that covers exposed skin, and staying indoors.
Safe sex. Abstinence or consistent condom use is recommended for pregnant women with partners who travel to or live in areas of active Zika infection.
Delay conception. Conception should be postponed for at least 6 months in men with Zika infection and at least 8 weeks in women with Zika infection.
Testing recommendations. Pregnant women with Zika virus exposure should be tested, regardless of symptoms. Symptomatic exposed nonpregnant women and all men should be tested.
Prenatal surveillance. High-risk consultation and serial ultrasounds for fetal anatomy and growth should be considered in patients with Zika virus infection during pregnancy. Amniocentesis can be considered on a case-by-case basis.
Related article:
Zika virus update: A rapidly moving target
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Overcoming LARC complications: 7 case challenges
The use of long-acting reversible contraceptive (LARC) methods has shown a steady increase in the United States. The major factors for increasing acceptance include high efficacy, ease of use, and an acceptable adverse effect profile. Since these methods require placement under the skin (implantable device) or into the uterus (intrauterine devices [IUDs]), unique management issues arise during their usage. Recently, the American College of Obstetricians and Gynecologists (ACOG) released a committee opinion addressing several of these clinical challenges—among them: pain with insertion, what to do when the IUD strings are not visualized, and the plan of action for a nonpalpable IUD or contraceptive implant.1 In this article we present 7 cases, and successful management approaches, that reflect ACOG’s recent recommendations and our extensive clinical experience.
Read the first CHALLENGE: Pain with IUD insertion
CHALLENGE 1: Pain with IUD insertion
CASE First-time, nulliparous IUD user apprehensive about insertion pain
A 21-year-old woman (G0) presents for placement of a 52-mg levonorgestrel IUD for contraception and treatment of dysmenorrhea. Her medical and surgical histories are unremarkable. She has heard that IUD insertion “is more painful if you haven’t had a baby yet” and she asks what treatments are available to aid in pain relief.
What can you offer her?
A number of approaches have been used to reduce IUD insertion pain, including:
- placing lidocaine gel into or on the cervix
- lidocaine paracervical block
- preinsertion use of misoprostol or nonsteroidal anti-inflammatory drugs.
Authors of a recent Cochrane review2 indicated that none of these approaches were particularly effective at reducing insertion pain for nulliparous women. Naproxen sodium 550 mg or tramadol 50 mg taken 1 hour prior to IUD insertion have been found to decrease IUD insertion pain in multiparous patients.3 Misoprostol, apart from being ineffective in reducing insertion pain, also requires use for a number of hours before insertion and can cause painful uterine cramping, upset stomach, and diarrhea.2 Some studies do suggest that use of a paracervical block does reduce the pain associated with tenaculum placement but not the IUD insertion itself.
Related article:
Benefit of self-administered vaginal lidocaine gel in IUD placement
A reasonable pain management strategy for nulliparous patients. Given these data, there is not an evidence-based IUD insertion pain management strategy that can be used for the nulliparous case patient. A practical approach for nulliparous patients is to offer naproxen sodium or tramadol, which have been found to be beneficial in multiparous patients, to a nulliparous patient. Additionally, lidocaine gel applied to the cervix or tenaculum-site injection can be considered for tenaculum-associated pain, although it does not appear to help significantly with IUD insertion pain. Misoprostol should be avoided as it does not alleviate the pain of insertion and it can cause bothersome adverse effects.
Read CHALLENGE 2: IUD strings not visualized
CHALLENGE 2: IUD strings not visualized
CASE No strings palpated 6 weeks after postpartum IUD placement
A 26-year-old woman (G2P2) presents to your office for a postpartum visit 6 weeks after an uncomplicated cesarean delivery at term. She had requested that a 52-mg levonorgestrel IUD be placed at the time of delivery, and the delivery report describes an uneventful placement. The patient has not been able to feel the IUD strings using her fingers and you do not find them on examination. She does not remember the IUD falling out.
What are the next steps in her management?
Failure to palpate the IUD strings by the user or failure to visualize the strings is a fairly common occurrence. This is especially true when an IUD is placed immediatelypostpartum, as in this patient’s case.
When the strings cannot be palpated, it is important to exclude pregnancy and recommend a form of backup contraception, such as condoms and emergency contraception if appropriate, until evaluation can be completed.
Steps to locate a device. In the office setting, the strings often can be located by inserting a cytobrush into the endocervical canal to extract them. If that maneuver fails to locate them, an ultrasound should be completed to determine if the device is in the uterus. If the ultrasound does not detect the device in the uterus, obtain an anteroposterior (AP) x-ray encompassing the entire abdomen and pelvis. All IUDs used in the United States are radiopaque and will be observed on x-ray if present. If the IUD is identified, operative removal is indicated.
Related article:
How to identify and localize IUDs on ultrasound
Intraperitoneal location. If an IUD is found in this location, it is usually the result of a perforation that occurred at the time of insertion. In general, the device can be removed via laparoscopy. Occasionally, laparotomy is needed if there is significant pelvic infection, possible bowel perforation, or if there is an inability to locate the device at laparoscopy.4 The copper IUD is more inflammatory than the levonorgestrel IUDs.
Abdominal location. No matter the IUD type, operative removal of intra-abdominal IUDs should take place expeditiously after they are discovered.
In the case of expulsion. If the IUD is not seen on x-ray, expulsion is the likely cause. Expulsion tends to be more common among5:
- parous users
- those younger than age 20
- placements that immediately follow a delivery or second-trimester abortion.
Nulliparity and type of device are not associated with increased risk of expulsion.
Read CHALLENGE 3: Difficult IUD removal
CHALLENGE 3: Difficult IUD removal
CASE Strings not palpated in a patient with history of LEEP
A 37-year-old woman (G3P2) presents to your office for IUD removal. She underwent a loop electrosurgical excision procedure 2 years ago for cervical intraepithelial neoplasia (CIN) 2 and since then has not been able to feel the IUD strings. On pelvic examination, you do not palpate or visualize the IUD strings after speculum placement.
How can you achieve IUD removal for your patient?
When a patient requests that her IUD be removed, but the strings are not visible and the woman is not pregnant, employ ultrasonography to confirm the IUD remains intrauterine and to rule out expulsion or perforation.
Employ alligator forceps or an IUD hook. Once intrauterine position is confirmed, use an alligator forceps of suitable length and with a small diameter to extract the device (FIGURE 1). It is useful to utilize ultrasonography for guidance during the removal procedure. The alligator forceps will grasp both the IUD device itself and IUD strings well, so either can be targeted during removal.
A second useful tool for IUD removal is an IUD hook (FIGURE 2). In a similar way that a curette is used for endometrial sampling, IUD hooks can be used to drag the IUD from the uterus.

Anesthesia is not usually necessary for IUD removal with alligator forceps or an IUD hook, although it may be appropriate in select patients. Data are limited with regard to the utility of paracervical blocks in this situation.
Related article:
Surgical removal of malpositioned IUDs
Hysteroscopy is an option. If removal with an alligator forceps or IUD hook is unsuccessful, or if preferred by the clinician, hysteroscopic-guided removal is a management option. Hysteroscopic removal may be required if the IUD has become embedded in the uterine wall.
Read CHALLENGE 4: Nonfundal IUD location
CHALLENGE 4: Nonfundal IUD location
CASE Copper IUD found in lower uterine segment
A 31-year-old woman (G1P1) calls your office to report that she thinks her copper IUD strings are longer than before. Office examination confirms that the strings are noticeably longer than is typical. Pelvic ultrasonography shows the copper IUD in the lower uterine segment.
What is the appropriate course of action?
Occasionally, IUDs are noted to be located in the lower uterine segment (FIGURE 3) or cervix. With malposition, users may be experiencing cramping or abnormal bleeding.
Cervical malposition calls for removal. ACOG advises that, regardless of a patient’s presenting symptoms, clinicians should remove IUDs located in the cervix (ie, the stem below the internal os) due to an increased risk of pregnancy and address the woman’s contraceptive needs.
Related article:
STOP relying on 2D ultrasound for IUD localization
Lower-uterine-segment malposition man‑agement less clear. If the patient is symptomatic, remove the device and initiate some form of contraception. If the woman is asymptomatic, the woman should be given the option of having the device removed or left in place. The mechanisms of action of both the copper and levonorgestrel-releasing IUDs suggest that this lower location is unlikely to be associated with a significant decrease in efficacy.
Unfortunately, it is difficult to estimate the risk of pregnancy for a patient whose device is located in the lower uterine segment. Braaten and Goldberg discussed case-controlled data in their 2012 article that suggest malposition may be more important to the efficacy of copper IUDs than of levonorgestrel IUDs.6,7 As unintended pregnancy is an important risk to avoid, ultimately, it is the woman’s decision as to whether she wants removal or continued IUD use.
Read CHALLENGE 5: Pregnancy in an IUD user
CHALLENGE 5: Pregnancy in an IUD user
CASE 3-year copper IUD user with positive pregnancy test
A 25-year-old woman (G3P2) presents to your office because of missed menses and a positive home pregnancy test. Her last menstrual period was 6 weeks ago. She has had a copper IUD in place for 3 years and can feel the strings herself. She has experienced light cramping but no bleeding. Office examination is notable for the IUD stem present at the external cervical os. While the pregnancy is unplanned, the patient desires that it continue.
Should you remove the IUD?
The pregnancy rate among IUD users is less than 1%—a rate that is equivalent to that experienced by women undergoing tubal sterilization. Although there is an overall low risk of pregnancy, a higher proportion of pregnancies among IUD users compared with nonusers are ectopic. Therefore, subsequent management of pregnancy in an IUD user needs to be determined by, using ultrasound, both the location of the pregnancy and whether the IUD is in place.
If an ectopic pregnancy is found, it may be managed medically or surgically with the IUD left in place if desired. If you find an intrauterine pregnancy that is undesired, the IUD can be removed at the time of a surgical abortion or before the initiation of a medical abortion.
If you fail to locate the IUD either before or after the abortion procedure, use an AP x-ray of the entire abdomen and pelvis to determine whether the IUD is in the peritoneal cavity or whether it was likely expelled prior to the pregnancy.
Related article:
In which clinical situations can the use of the 52-mg levonorgestrel-releasing IUD (Mirena) and the TCu380A copper-IUD (ParaGard) be extended?
With a desired pregnancy, if the strings are visible, remove the IUD with gentle traction. If the IUD is left in place, the risk of spontaneous abortion is significantly increased. If the strings are not seen, but the device was noted to be in the cervix by ultrasound, remove the device if the stem is below the internal cervical os. For IUDs that are located above the cervix, removal should not be attempted; counsel the patient about the increased risk of spontaneous abortion, infection, and preterm delivery.
Read CHALLENGE 6: Pregnancy in an implant user
CHALLENGE 6: Pregnancy in an implant user
CASE 3-week implant user with positive pregnancy test
Your 21-year-old patient who received a contraceptive implant 3 weeks earlier now pre‑sents with nausea and abdominal cramping. Her last menstrual period was 6 weeks ago. She has regular cycles that are 28 days in length. Results of urine pregnancy testing are positive. Prior to using the implant, the patient inconsistently used condoms.
How should you counsel your patient?
The rate of pregnancy among implant users is very low; it is estimated at 5 pregnancies per 10,000 implant users per year.8 As in this case, apparent “failures” of the contraceptive implant actually may represent placements that occurred before a very early pregnancy was recognized. Similar to IUDs, the proportion of pregnancies that are ectopic among implant users compared to nonusers may be higher.
With a pregnancy that is ectopic or that is intrauterine and undesired, the device may be left in and use continued after the pregnancy has been terminated. Although the effectiveness of medication abortion with pre-existing contraceptive implant in situ is not well known, researchers have demonstrated that medication abortion initiated at the same time as contraceptive implant insertion does not influence success of the medication abortion.9
Related article:
2016 Update on contraception
For women with desired intrauterine pregnancies, remove the device as soon as feasible and counsel the woman that there is no known teratogenic risk associated with the contraceptive implant.
Read CHALLENGE 7: Nonpalpable contraceptive implant
CHALLENGE 7: Nonpalpable contraceptive implant
CASE Patient requests device removal to attempt conception
A 30-year-old woman (G2P2) presents for contraceptive implant removal because she would like to have another child. The device was placed 30 months ago in the patient’s left arm. The insertion note in the patient’s medical record is unremarkable, and standard insertion technique was used. On physical examination, you cannot palpate the device.
What is your next course of action?
Nonpalpable implants, particularly if removal is desired, present a significant clinical challenge. Do not attempt removing a nonpalpable implant before trying to locate the device through past medical records or radiography. Records that describe the original insertion, particularly the location and type of device, are helpful.
Related article:
2015 Update on contraception
Appropriate imaging assistance. Ultrasonography with a high frequency linear array transducer (10 MHz or greater) may allow an experienced radiologist to identify the implant—including earlier versions without barium (Implanon) and later ones with barium (Nexplanon). Magnetic resonance imaging (MRI), computed tomography scan, or plain x-ray also can be used to detect a barium-containing device; MRI can be used to locate a non−barium-containing implant.
Carry out removal using ultrasonographic guidance. If a deep insertion is felt to be close to a neurovascular bundle, device removal should be carried out in an operating room by a surgeon familiar with the anatomy of the upper arm.
When an implant cannot be located despite radiography. This is an infrequent occurrence. Merck, the manufacturer of the etonorgestrel implant, provides advice and support in this circumstance. (Visit https://www.merckconnect.com/nexplanon/over view.html.)
Recently, published case reports detail episodes of implants inserted into the venous system with migration to the heart or lungs.10 While this phenomenon is considered rare, the manufacturer has recommended that insertion of the contraceptive implant avoid the sulcus between the biceps and triceps muscles.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- American College of Obstetricians and Gynecologists. Committee Opinion No. 672: clinical challenges of long-acting reversible contraceptive methods. Obstet Gynecol. 2016;128(3):e69−e77.
- Lopez LM, Bernholc A, Zeng Y, et al. Interventions for pain with intrauterine device insertion. Cochrane Database Syst Rev. 2015;(7):CD007373.
- Karabayirli S, Ayrim AA, Muslu B. Comparison of the analgesic effects of oral tramadol and naproxen sodium on pain relief during IUD insertion. J Minim Invasive Gynecol. 2012;19(5):581−584.
- Kho KA, Chamsy DJ. Perforated intraperitoneal intrauterine contraceptive devices: diagnosis, management, and clinical outcomes. J Minim Invasive Gynecol. 2014;21(4):596−601.
- Madden T, McNicholas C, Zhao Q, Secura GM, Eisenberg DL, Peipert JF. Association of age and parity with intrauterine device expulsion. Obstet Gynecol. 2014;124(4):718−726.
- Patil E, Bednarek PH. Immediate intrauterine device insertion following surgical abortion. Obstet Gynecol Clin North Am. 2015;42(4):583−546.
- Braaten and Goldberg. OBG Manag. Malpositioned IUDs: When you should intervene (and when you should not). OBG Manag. 2012;24(8):38−46.
- Trussell J. Contraceptive failure in the United States. Contraception. 2011;83(5):397−404.
- Raymond EG, Weaver MA, Tan YL, et al. Effect of immediate compared with delayed insertion of etonogestrel implants on medical abortion efficacy and repeat pregnancy: a randomized controlled trial. Obstet Gynecol. 2017;127(2):306−312.
- Rowlands S, Mansour D, Walling M. Intravascular migration of contraceptive implants: two more cases. Contraception. 2016. In press.
The use of long-acting reversible contraceptive (LARC) methods has shown a steady increase in the United States. The major factors for increasing acceptance include high efficacy, ease of use, and an acceptable adverse effect profile. Since these methods require placement under the skin (implantable device) or into the uterus (intrauterine devices [IUDs]), unique management issues arise during their usage. Recently, the American College of Obstetricians and Gynecologists (ACOG) released a committee opinion addressing several of these clinical challenges—among them: pain with insertion, what to do when the IUD strings are not visualized, and the plan of action for a nonpalpable IUD or contraceptive implant.1 In this article we present 7 cases, and successful management approaches, that reflect ACOG’s recent recommendations and our extensive clinical experience.
Read the first CHALLENGE: Pain with IUD insertion
CHALLENGE 1: Pain with IUD insertion
CASE First-time, nulliparous IUD user apprehensive about insertion pain
A 21-year-old woman (G0) presents for placement of a 52-mg levonorgestrel IUD for contraception and treatment of dysmenorrhea. Her medical and surgical histories are unremarkable. She has heard that IUD insertion “is more painful if you haven’t had a baby yet” and she asks what treatments are available to aid in pain relief.
What can you offer her?
A number of approaches have been used to reduce IUD insertion pain, including:
- placing lidocaine gel into or on the cervix
- lidocaine paracervical block
- preinsertion use of misoprostol or nonsteroidal anti-inflammatory drugs.
Authors of a recent Cochrane review2 indicated that none of these approaches were particularly effective at reducing insertion pain for nulliparous women. Naproxen sodium 550 mg or tramadol 50 mg taken 1 hour prior to IUD insertion have been found to decrease IUD insertion pain in multiparous patients.3 Misoprostol, apart from being ineffective in reducing insertion pain, also requires use for a number of hours before insertion and can cause painful uterine cramping, upset stomach, and diarrhea.2 Some studies do suggest that use of a paracervical block does reduce the pain associated with tenaculum placement but not the IUD insertion itself.
Related article:
Benefit of self-administered vaginal lidocaine gel in IUD placement
A reasonable pain management strategy for nulliparous patients. Given these data, there is not an evidence-based IUD insertion pain management strategy that can be used for the nulliparous case patient. A practical approach for nulliparous patients is to offer naproxen sodium or tramadol, which have been found to be beneficial in multiparous patients, to a nulliparous patient. Additionally, lidocaine gel applied to the cervix or tenaculum-site injection can be considered for tenaculum-associated pain, although it does not appear to help significantly with IUD insertion pain. Misoprostol should be avoided as it does not alleviate the pain of insertion and it can cause bothersome adverse effects.
Read CHALLENGE 2: IUD strings not visualized
CHALLENGE 2: IUD strings not visualized
CASE No strings palpated 6 weeks after postpartum IUD placement
A 26-year-old woman (G2P2) presents to your office for a postpartum visit 6 weeks after an uncomplicated cesarean delivery at term. She had requested that a 52-mg levonorgestrel IUD be placed at the time of delivery, and the delivery report describes an uneventful placement. The patient has not been able to feel the IUD strings using her fingers and you do not find them on examination. She does not remember the IUD falling out.
What are the next steps in her management?
Failure to palpate the IUD strings by the user or failure to visualize the strings is a fairly common occurrence. This is especially true when an IUD is placed immediatelypostpartum, as in this patient’s case.
When the strings cannot be palpated, it is important to exclude pregnancy and recommend a form of backup contraception, such as condoms and emergency contraception if appropriate, until evaluation can be completed.
Steps to locate a device. In the office setting, the strings often can be located by inserting a cytobrush into the endocervical canal to extract them. If that maneuver fails to locate them, an ultrasound should be completed to determine if the device is in the uterus. If the ultrasound does not detect the device in the uterus, obtain an anteroposterior (AP) x-ray encompassing the entire abdomen and pelvis. All IUDs used in the United States are radiopaque and will be observed on x-ray if present. If the IUD is identified, operative removal is indicated.
Related article:
How to identify and localize IUDs on ultrasound
Intraperitoneal location. If an IUD is found in this location, it is usually the result of a perforation that occurred at the time of insertion. In general, the device can be removed via laparoscopy. Occasionally, laparotomy is needed if there is significant pelvic infection, possible bowel perforation, or if there is an inability to locate the device at laparoscopy.4 The copper IUD is more inflammatory than the levonorgestrel IUDs.
Abdominal location. No matter the IUD type, operative removal of intra-abdominal IUDs should take place expeditiously after they are discovered.
In the case of expulsion. If the IUD is not seen on x-ray, expulsion is the likely cause. Expulsion tends to be more common among5:
- parous users
- those younger than age 20
- placements that immediately follow a delivery or second-trimester abortion.
Nulliparity and type of device are not associated with increased risk of expulsion.
Read CHALLENGE 3: Difficult IUD removal
CHALLENGE 3: Difficult IUD removal
CASE Strings not palpated in a patient with history of LEEP
A 37-year-old woman (G3P2) presents to your office for IUD removal. She underwent a loop electrosurgical excision procedure 2 years ago for cervical intraepithelial neoplasia (CIN) 2 and since then has not been able to feel the IUD strings. On pelvic examination, you do not palpate or visualize the IUD strings after speculum placement.
How can you achieve IUD removal for your patient?
When a patient requests that her IUD be removed, but the strings are not visible and the woman is not pregnant, employ ultrasonography to confirm the IUD remains intrauterine and to rule out expulsion or perforation.
Employ alligator forceps or an IUD hook. Once intrauterine position is confirmed, use an alligator forceps of suitable length and with a small diameter to extract the device (FIGURE 1). It is useful to utilize ultrasonography for guidance during the removal procedure. The alligator forceps will grasp both the IUD device itself and IUD strings well, so either can be targeted during removal.

A second useful tool for IUD removal is an IUD hook (FIGURE 2). In a similar way that a curette is used for endometrial sampling, IUD hooks can be used to drag the IUD from the uterus.

Anesthesia is not usually necessary for IUD removal with alligator forceps or an IUD hook, although it may be appropriate in select patients. Data are limited with regard to the utility of paracervical blocks in this situation.
Related article:
Surgical removal of malpositioned IUDs
Hysteroscopy is an option. If removal with an alligator forceps or IUD hook is unsuccessful, or if preferred by the clinician, hysteroscopic-guided removal is a management option. Hysteroscopic removal may be required if the IUD has become embedded in the uterine wall.
Read CHALLENGE 4: Nonfundal IUD location
CHALLENGE 4: Nonfundal IUD location
CASE Copper IUD found in lower uterine segment
A 31-year-old woman (G1P1) calls your office to report that she thinks her copper IUD strings are longer than before. Office examination confirms that the strings are noticeably longer than is typical. Pelvic ultrasonography shows the copper IUD in the lower uterine segment.
What is the appropriate course of action?
Occasionally, IUDs are noted to be located in the lower uterine segment (FIGURE 3) or cervix. With malposition, users may be experiencing cramping or abnormal bleeding.

Cervical malposition calls for removal. ACOG advises that, regardless of a patient’s presenting symptoms, clinicians should remove IUDs located in the cervix (ie, the stem below the internal os) due to an increased risk of pregnancy and address the woman’s contraceptive needs.
Related article:
STOP relying on 2D ultrasound for IUD localization
Lower-uterine-segment malposition man‑agement less clear. If the patient is symptomatic, remove the device and initiate some form of contraception. If the woman is asymptomatic, the woman should be given the option of having the device removed or left in place. The mechanisms of action of both the copper and levonorgestrel-releasing IUDs suggest that this lower location is unlikely to be associated with a significant decrease in efficacy.
Unfortunately, it is difficult to estimate the risk of pregnancy for a patient whose device is located in the lower uterine segment. Braaten and Goldberg discussed case-controlled data in their 2012 article that suggest malposition may be more important to the efficacy of copper IUDs than of levonorgestrel IUDs.6,7 As unintended pregnancy is an important risk to avoid, ultimately, it is the woman’s decision as to whether she wants removal or continued IUD use.
Read CHALLENGE 5: Pregnancy in an IUD user
CHALLENGE 5: Pregnancy in an IUD user
CASE 3-year copper IUD user with positive pregnancy test
A 25-year-old woman (G3P2) presents to your office because of missed menses and a positive home pregnancy test. Her last menstrual period was 6 weeks ago. She has had a copper IUD in place for 3 years and can feel the strings herself. She has experienced light cramping but no bleeding. Office examination is notable for the IUD stem present at the external cervical os. While the pregnancy is unplanned, the patient desires that it continue.
Should you remove the IUD?
The pregnancy rate among IUD users is less than 1%—a rate that is equivalent to that experienced by women undergoing tubal sterilization. Although there is an overall low risk of pregnancy, a higher proportion of pregnancies among IUD users compared with nonusers are ectopic. Therefore, subsequent management of pregnancy in an IUD user needs to be determined by, using ultrasound, both the location of the pregnancy and whether the IUD is in place.
If an ectopic pregnancy is found, it may be managed medically or surgically with the IUD left in place if desired. If you find an intrauterine pregnancy that is undesired, the IUD can be removed at the time of a surgical abortion or before the initiation of a medical abortion.
If you fail to locate the IUD either before or after the abortion procedure, use an AP x-ray of the entire abdomen and pelvis to determine whether the IUD is in the peritoneal cavity or whether it was likely expelled prior to the pregnancy.
Related article:
In which clinical situations can the use of the 52-mg levonorgestrel-releasing IUD (Mirena) and the TCu380A copper-IUD (ParaGard) be extended?
With a desired pregnancy, if the strings are visible, remove the IUD with gentle traction. If the IUD is left in place, the risk of spontaneous abortion is significantly increased. If the strings are not seen, but the device was noted to be in the cervix by ultrasound, remove the device if the stem is below the internal cervical os. For IUDs that are located above the cervix, removal should not be attempted; counsel the patient about the increased risk of spontaneous abortion, infection, and preterm delivery.
Read CHALLENGE 6: Pregnancy in an implant user
CHALLENGE 6: Pregnancy in an implant user
CASE 3-week implant user with positive pregnancy test
Your 21-year-old patient who received a contraceptive implant 3 weeks earlier now pre‑sents with nausea and abdominal cramping. Her last menstrual period was 6 weeks ago. She has regular cycles that are 28 days in length. Results of urine pregnancy testing are positive. Prior to using the implant, the patient inconsistently used condoms.
How should you counsel your patient?
The rate of pregnancy among implant users is very low; it is estimated at 5 pregnancies per 10,000 implant users per year.8 As in this case, apparent “failures” of the contraceptive implant actually may represent placements that occurred before a very early pregnancy was recognized. Similar to IUDs, the proportion of pregnancies that are ectopic among implant users compared to nonusers may be higher.
With a pregnancy that is ectopic or that is intrauterine and undesired, the device may be left in and use continued after the pregnancy has been terminated. Although the effectiveness of medication abortion with pre-existing contraceptive implant in situ is not well known, researchers have demonstrated that medication abortion initiated at the same time as contraceptive implant insertion does not influence success of the medication abortion.9
Related article:
2016 Update on contraception
For women with desired intrauterine pregnancies, remove the device as soon as feasible and counsel the woman that there is no known teratogenic risk associated with the contraceptive implant.
Read CHALLENGE 7: Nonpalpable contraceptive implant
CHALLENGE 7: Nonpalpable contraceptive implant
CASE Patient requests device removal to attempt conception
A 30-year-old woman (G2P2) presents for contraceptive implant removal because she would like to have another child. The device was placed 30 months ago in the patient’s left arm. The insertion note in the patient’s medical record is unremarkable, and standard insertion technique was used. On physical examination, you cannot palpate the device.
What is your next course of action?
Nonpalpable implants, particularly if removal is desired, present a significant clinical challenge. Do not attempt removing a nonpalpable implant before trying to locate the device through past medical records or radiography. Records that describe the original insertion, particularly the location and type of device, are helpful.
Related article:
2015 Update on contraception
Appropriate imaging assistance. Ultrasonography with a high frequency linear array transducer (10 MHz or greater) may allow an experienced radiologist to identify the implant—including earlier versions without barium (Implanon) and later ones with barium (Nexplanon). Magnetic resonance imaging (MRI), computed tomography scan, or plain x-ray also can be used to detect a barium-containing device; MRI can be used to locate a non−barium-containing implant.
Carry out removal using ultrasonographic guidance. If a deep insertion is felt to be close to a neurovascular bundle, device removal should be carried out in an operating room by a surgeon familiar with the anatomy of the upper arm.
When an implant cannot be located despite radiography. This is an infrequent occurrence. Merck, the manufacturer of the etonorgestrel implant, provides advice and support in this circumstance. (Visit https://www.merckconnect.com/nexplanon/over view.html.)
Recently, published case reports detail episodes of implants inserted into the venous system with migration to the heart or lungs.10 While this phenomenon is considered rare, the manufacturer has recommended that insertion of the contraceptive implant avoid the sulcus between the biceps and triceps muscles.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The use of long-acting reversible contraceptive (LARC) methods has shown a steady increase in the United States. The major factors for increasing acceptance include high efficacy, ease of use, and an acceptable adverse effect profile. Since these methods require placement under the skin (implantable device) or into the uterus (intrauterine devices [IUDs]), unique management issues arise during their usage. Recently, the American College of Obstetricians and Gynecologists (ACOG) released a committee opinion addressing several of these clinical challenges—among them: pain with insertion, what to do when the IUD strings are not visualized, and the plan of action for a nonpalpable IUD or contraceptive implant.1 In this article we present 7 cases, and successful management approaches, that reflect ACOG’s recent recommendations and our extensive clinical experience.
Read the first CHALLENGE: Pain with IUD insertion
CHALLENGE 1: Pain with IUD insertion
CASE First-time, nulliparous IUD user apprehensive about insertion pain
A 21-year-old woman (G0) presents for placement of a 52-mg levonorgestrel IUD for contraception and treatment of dysmenorrhea. Her medical and surgical histories are unremarkable. She has heard that IUD insertion “is more painful if you haven’t had a baby yet” and she asks what treatments are available to aid in pain relief.
What can you offer her?
A number of approaches have been used to reduce IUD insertion pain, including:
- placing lidocaine gel into or on the cervix
- lidocaine paracervical block
- preinsertion use of misoprostol or nonsteroidal anti-inflammatory drugs.
Authors of a recent Cochrane review2 indicated that none of these approaches were particularly effective at reducing insertion pain for nulliparous women. Naproxen sodium 550 mg or tramadol 50 mg taken 1 hour prior to IUD insertion have been found to decrease IUD insertion pain in multiparous patients.3 Misoprostol, apart from being ineffective in reducing insertion pain, also requires use for a number of hours before insertion and can cause painful uterine cramping, upset stomach, and diarrhea.2 Some studies do suggest that use of a paracervical block does reduce the pain associated with tenaculum placement but not the IUD insertion itself.
Related article:
Benefit of self-administered vaginal lidocaine gel in IUD placement
A reasonable pain management strategy for nulliparous patients. Given these data, there is not an evidence-based IUD insertion pain management strategy that can be used for the nulliparous case patient. A practical approach for nulliparous patients is to offer naproxen sodium or tramadol, which have been found to be beneficial in multiparous patients, to a nulliparous patient. Additionally, lidocaine gel applied to the cervix or tenaculum-site injection can be considered for tenaculum-associated pain, although it does not appear to help significantly with IUD insertion pain. Misoprostol should be avoided as it does not alleviate the pain of insertion and it can cause bothersome adverse effects.
Read CHALLENGE 2: IUD strings not visualized
CHALLENGE 2: IUD strings not visualized
CASE No strings palpated 6 weeks after postpartum IUD placement
A 26-year-old woman (G2P2) presents to your office for a postpartum visit 6 weeks after an uncomplicated cesarean delivery at term. She had requested that a 52-mg levonorgestrel IUD be placed at the time of delivery, and the delivery report describes an uneventful placement. The patient has not been able to feel the IUD strings using her fingers and you do not find them on examination. She does not remember the IUD falling out.
What are the next steps in her management?
Failure to palpate the IUD strings by the user or failure to visualize the strings is a fairly common occurrence. This is especially true when an IUD is placed immediatelypostpartum, as in this patient’s case.
When the strings cannot be palpated, it is important to exclude pregnancy and recommend a form of backup contraception, such as condoms and emergency contraception if appropriate, until evaluation can be completed.
Steps to locate a device. In the office setting, the strings often can be located by inserting a cytobrush into the endocervical canal to extract them. If that maneuver fails to locate them, an ultrasound should be completed to determine if the device is in the uterus. If the ultrasound does not detect the device in the uterus, obtain an anteroposterior (AP) x-ray encompassing the entire abdomen and pelvis. All IUDs used in the United States are radiopaque and will be observed on x-ray if present. If the IUD is identified, operative removal is indicated.
Related article:
How to identify and localize IUDs on ultrasound
Intraperitoneal location. If an IUD is found in this location, it is usually the result of a perforation that occurred at the time of insertion. In general, the device can be removed via laparoscopy. Occasionally, laparotomy is needed if there is significant pelvic infection, possible bowel perforation, or if there is an inability to locate the device at laparoscopy.4 The copper IUD is more inflammatory than the levonorgestrel IUDs.
Abdominal location. No matter the IUD type, operative removal of intra-abdominal IUDs should take place expeditiously after they are discovered.
In the case of expulsion. If the IUD is not seen on x-ray, expulsion is the likely cause. Expulsion tends to be more common among5:
- parous users
- those younger than age 20
- placements that immediately follow a delivery or second-trimester abortion.
Nulliparity and type of device are not associated with increased risk of expulsion.
Read CHALLENGE 3: Difficult IUD removal
CHALLENGE 3: Difficult IUD removal
CASE Strings not palpated in a patient with history of LEEP
A 37-year-old woman (G3P2) presents to your office for IUD removal. She underwent a loop electrosurgical excision procedure 2 years ago for cervical intraepithelial neoplasia (CIN) 2 and since then has not been able to feel the IUD strings. On pelvic examination, you do not palpate or visualize the IUD strings after speculum placement.
How can you achieve IUD removal for your patient?
When a patient requests that her IUD be removed, but the strings are not visible and the woman is not pregnant, employ ultrasonography to confirm the IUD remains intrauterine and to rule out expulsion or perforation.
Employ alligator forceps or an IUD hook. Once intrauterine position is confirmed, use an alligator forceps of suitable length and with a small diameter to extract the device (FIGURE 1). It is useful to utilize ultrasonography for guidance during the removal procedure. The alligator forceps will grasp both the IUD device itself and IUD strings well, so either can be targeted during removal.

A second useful tool for IUD removal is an IUD hook (FIGURE 2). In a similar way that a curette is used for endometrial sampling, IUD hooks can be used to drag the IUD from the uterus.

Anesthesia is not usually necessary for IUD removal with alligator forceps or an IUD hook, although it may be appropriate in select patients. Data are limited with regard to the utility of paracervical blocks in this situation.
Related article:
Surgical removal of malpositioned IUDs
Hysteroscopy is an option. If removal with an alligator forceps or IUD hook is unsuccessful, or if preferred by the clinician, hysteroscopic-guided removal is a management option. Hysteroscopic removal may be required if the IUD has become embedded in the uterine wall.
Read CHALLENGE 4: Nonfundal IUD location
CHALLENGE 4: Nonfundal IUD location
CASE Copper IUD found in lower uterine segment
A 31-year-old woman (G1P1) calls your office to report that she thinks her copper IUD strings are longer than before. Office examination confirms that the strings are noticeably longer than is typical. Pelvic ultrasonography shows the copper IUD in the lower uterine segment.
What is the appropriate course of action?
Occasionally, IUDs are noted to be located in the lower uterine segment (FIGURE 3) or cervix. With malposition, users may be experiencing cramping or abnormal bleeding.

Cervical malposition calls for removal. ACOG advises that, regardless of a patient’s presenting symptoms, clinicians should remove IUDs located in the cervix (ie, the stem below the internal os) due to an increased risk of pregnancy and address the woman’s contraceptive needs.
Related article:
STOP relying on 2D ultrasound for IUD localization
Lower-uterine-segment malposition man‑agement less clear. If the patient is symptomatic, remove the device and initiate some form of contraception. If the woman is asymptomatic, the woman should be given the option of having the device removed or left in place. The mechanisms of action of both the copper and levonorgestrel-releasing IUDs suggest that this lower location is unlikely to be associated with a significant decrease in efficacy.
Unfortunately, it is difficult to estimate the risk of pregnancy for a patient whose device is located in the lower uterine segment. Braaten and Goldberg discussed case-controlled data in their 2012 article that suggest malposition may be more important to the efficacy of copper IUDs than of levonorgestrel IUDs.6,7 As unintended pregnancy is an important risk to avoid, ultimately, it is the woman’s decision as to whether she wants removal or continued IUD use.
Read CHALLENGE 5: Pregnancy in an IUD user
CHALLENGE 5: Pregnancy in an IUD user
CASE 3-year copper IUD user with positive pregnancy test
A 25-year-old woman (G3P2) presents to your office because of missed menses and a positive home pregnancy test. Her last menstrual period was 6 weeks ago. She has had a copper IUD in place for 3 years and can feel the strings herself. She has experienced light cramping but no bleeding. Office examination is notable for the IUD stem present at the external cervical os. While the pregnancy is unplanned, the patient desires that it continue.
Should you remove the IUD?
The pregnancy rate among IUD users is less than 1%—a rate that is equivalent to that experienced by women undergoing tubal sterilization. Although there is an overall low risk of pregnancy, a higher proportion of pregnancies among IUD users compared with nonusers are ectopic. Therefore, subsequent management of pregnancy in an IUD user needs to be determined by, using ultrasound, both the location of the pregnancy and whether the IUD is in place.
If an ectopic pregnancy is found, it may be managed medically or surgically with the IUD left in place if desired. If you find an intrauterine pregnancy that is undesired, the IUD can be removed at the time of a surgical abortion or before the initiation of a medical abortion.
If you fail to locate the IUD either before or after the abortion procedure, use an AP x-ray of the entire abdomen and pelvis to determine whether the IUD is in the peritoneal cavity or whether it was likely expelled prior to the pregnancy.
Related article:
In which clinical situations can the use of the 52-mg levonorgestrel-releasing IUD (Mirena) and the TCu380A copper-IUD (ParaGard) be extended?
With a desired pregnancy, if the strings are visible, remove the IUD with gentle traction. If the IUD is left in place, the risk of spontaneous abortion is significantly increased. If the strings are not seen, but the device was noted to be in the cervix by ultrasound, remove the device if the stem is below the internal cervical os. For IUDs that are located above the cervix, removal should not be attempted; counsel the patient about the increased risk of spontaneous abortion, infection, and preterm delivery.
Read CHALLENGE 6: Pregnancy in an implant user
CHALLENGE 6: Pregnancy in an implant user
CASE 3-week implant user with positive pregnancy test
Your 21-year-old patient who received a contraceptive implant 3 weeks earlier now pre‑sents with nausea and abdominal cramping. Her last menstrual period was 6 weeks ago. She has regular cycles that are 28 days in length. Results of urine pregnancy testing are positive. Prior to using the implant, the patient inconsistently used condoms.
How should you counsel your patient?
The rate of pregnancy among implant users is very low; it is estimated at 5 pregnancies per 10,000 implant users per year.8 As in this case, apparent “failures” of the contraceptive implant actually may represent placements that occurred before a very early pregnancy was recognized. Similar to IUDs, the proportion of pregnancies that are ectopic among implant users compared to nonusers may be higher.
With a pregnancy that is ectopic or that is intrauterine and undesired, the device may be left in and use continued after the pregnancy has been terminated. Although the effectiveness of medication abortion with pre-existing contraceptive implant in situ is not well known, researchers have demonstrated that medication abortion initiated at the same time as contraceptive implant insertion does not influence success of the medication abortion.9
Related article:
2016 Update on contraception
For women with desired intrauterine pregnancies, remove the device as soon as feasible and counsel the woman that there is no known teratogenic risk associated with the contraceptive implant.
Read CHALLENGE 7: Nonpalpable contraceptive implant
CHALLENGE 7: Nonpalpable contraceptive implant
CASE Patient requests device removal to attempt conception
A 30-year-old woman (G2P2) presents for contraceptive implant removal because she would like to have another child. The device was placed 30 months ago in the patient’s left arm. The insertion note in the patient’s medical record is unremarkable, and standard insertion technique was used. On physical examination, you cannot palpate the device.
What is your next course of action?
Nonpalpable implants, particularly if removal is desired, present a significant clinical challenge. Do not attempt removing a nonpalpable implant before trying to locate the device through past medical records or radiography. Records that describe the original insertion, particularly the location and type of device, are helpful.
Related article:
2015 Update on contraception
Appropriate imaging assistance. Ultrasonography with a high frequency linear array transducer (10 MHz or greater) may allow an experienced radiologist to identify the implant—including earlier versions without barium (Implanon) and later ones with barium (Nexplanon). Magnetic resonance imaging (MRI), computed tomography scan, or plain x-ray also can be used to detect a barium-containing device; MRI can be used to locate a non−barium-containing implant.
Carry out removal using ultrasonographic guidance. If a deep insertion is felt to be close to a neurovascular bundle, device removal should be carried out in an operating room by a surgeon familiar with the anatomy of the upper arm.
When an implant cannot be located despite radiography. This is an infrequent occurrence. Merck, the manufacturer of the etonorgestrel implant, provides advice and support in this circumstance. (Visit https://www.merckconnect.com/nexplanon/over view.html.)
Recently, published case reports detail episodes of implants inserted into the venous system with migration to the heart or lungs.10 While this phenomenon is considered rare, the manufacturer has recommended that insertion of the contraceptive implant avoid the sulcus between the biceps and triceps muscles.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- American College of Obstetricians and Gynecologists. Committee Opinion No. 672: clinical challenges of long-acting reversible contraceptive methods. Obstet Gynecol. 2016;128(3):e69−e77.
- Lopez LM, Bernholc A, Zeng Y, et al. Interventions for pain with intrauterine device insertion. Cochrane Database Syst Rev. 2015;(7):CD007373.
- Karabayirli S, Ayrim AA, Muslu B. Comparison of the analgesic effects of oral tramadol and naproxen sodium on pain relief during IUD insertion. J Minim Invasive Gynecol. 2012;19(5):581−584.
- Kho KA, Chamsy DJ. Perforated intraperitoneal intrauterine contraceptive devices: diagnosis, management, and clinical outcomes. J Minim Invasive Gynecol. 2014;21(4):596−601.
- Madden T, McNicholas C, Zhao Q, Secura GM, Eisenberg DL, Peipert JF. Association of age and parity with intrauterine device expulsion. Obstet Gynecol. 2014;124(4):718−726.
- Patil E, Bednarek PH. Immediate intrauterine device insertion following surgical abortion. Obstet Gynecol Clin North Am. 2015;42(4):583−546.
- Braaten and Goldberg. OBG Manag. Malpositioned IUDs: When you should intervene (and when you should not). OBG Manag. 2012;24(8):38−46.
- Trussell J. Contraceptive failure in the United States. Contraception. 2011;83(5):397−404.
- Raymond EG, Weaver MA, Tan YL, et al. Effect of immediate compared with delayed insertion of etonogestrel implants on medical abortion efficacy and repeat pregnancy: a randomized controlled trial. Obstet Gynecol. 2017;127(2):306−312.
- Rowlands S, Mansour D, Walling M. Intravascular migration of contraceptive implants: two more cases. Contraception. 2016. In press.
- American College of Obstetricians and Gynecologists. Committee Opinion No. 672: clinical challenges of long-acting reversible contraceptive methods. Obstet Gynecol. 2016;128(3):e69−e77.
- Lopez LM, Bernholc A, Zeng Y, et al. Interventions for pain with intrauterine device insertion. Cochrane Database Syst Rev. 2015;(7):CD007373.
- Karabayirli S, Ayrim AA, Muslu B. Comparison of the analgesic effects of oral tramadol and naproxen sodium on pain relief during IUD insertion. J Minim Invasive Gynecol. 2012;19(5):581−584.
- Kho KA, Chamsy DJ. Perforated intraperitoneal intrauterine contraceptive devices: diagnosis, management, and clinical outcomes. J Minim Invasive Gynecol. 2014;21(4):596−601.
- Madden T, McNicholas C, Zhao Q, Secura GM, Eisenberg DL, Peipert JF. Association of age and parity with intrauterine device expulsion. Obstet Gynecol. 2014;124(4):718−726.
- Patil E, Bednarek PH. Immediate intrauterine device insertion following surgical abortion. Obstet Gynecol Clin North Am. 2015;42(4):583−546.
- Braaten and Goldberg. OBG Manag. Malpositioned IUDs: When you should intervene (and when you should not). OBG Manag. 2012;24(8):38−46.
- Trussell J. Contraceptive failure in the United States. Contraception. 2011;83(5):397−404.
- Raymond EG, Weaver MA, Tan YL, et al. Effect of immediate compared with delayed insertion of etonogestrel implants on medical abortion efficacy and repeat pregnancy: a randomized controlled trial. Obstet Gynecol. 2017;127(2):306−312.
- Rowlands S, Mansour D, Walling M. Intravascular migration of contraceptive implants: two more cases. Contraception. 2016. In press.
Three good apps for calculating the date of delivery
Technology has changed--and continues to change--the practice of medicine. Health care providers access word processing programs, e-mail, and electronic medical records using desktop and laptop computers. Now, providers are accessing these same tools with handheld devices such as smartphones, tablets, and "phablets" (a class of mobile devices designed to combine the form of a smartphone and a tablet).
Critical to the popularity and functionality of handheld devices are mobile applications, also known as "apps." An app is a self-contained program or piece of software designed to run on handheld devices to perform a specific purpose. App overload and app inaccuracy, however, are major problems. Health care providers do not have the time to search through thousands of medical apps in app stores to find specialty-related apps that might be useful in their practice--or to check the accuracy of those apps.
Vetted apps for ObGyns
My team's research has focused on identifying apps for ObGyns to use in clinical practice.1 In the process, we have developed the APPLICATIONS scoring system, which contains objective and subjective components to help differentiate among the accurate apps.2 This new quarterly "App review" series in OBG Management will showcase recommended apps for the busy ObGyn in the hope of improving work efficiency and the provider-patient relationship.
First up: Apps for calculating the date of delivery. This first app review focuses on pregnancy wheels, or due date calculators. Calculator apps are preferred over other types of apps such as procedure/case documentation apps, as providers use smartphones at point of care to allow rapid decision making.3 Calculating the estimated date of delivery (EDD) and gestational age (GA) is an important, vital task for providers of obstetric care. In fact, new guidelines for calculating EDD were recently developed by the American College of Obstetricians and Gynecologists (ACOG), the American Institute of Ultrasound in Medicine (AIUM), and the Society for Maternal-Fetal Medicine (SMFM).4 Notably, pregnancy wheel apps are more accurate than paper wheels.5 My team checked the accuracy of the pregnancy wheel apps by applying strict criteria to ensure the correct EDD and GA and then scored them in a systematic, nonbiased, conflict-of-interest-free manner.2
Related article:
Elective induction of labor at 39 (vs 41) weeks: Caveats and considerations
The TABLE below lists the top 3 recommended pregnancy wheel or due date calculator apps vetted by our research. The apps are listed alphabetically, and details for each app are provided based on a shortened version of the APPLICATIONS scoring system, APPLI--app comprehensiveness, price, platform, literature use, and important special features.
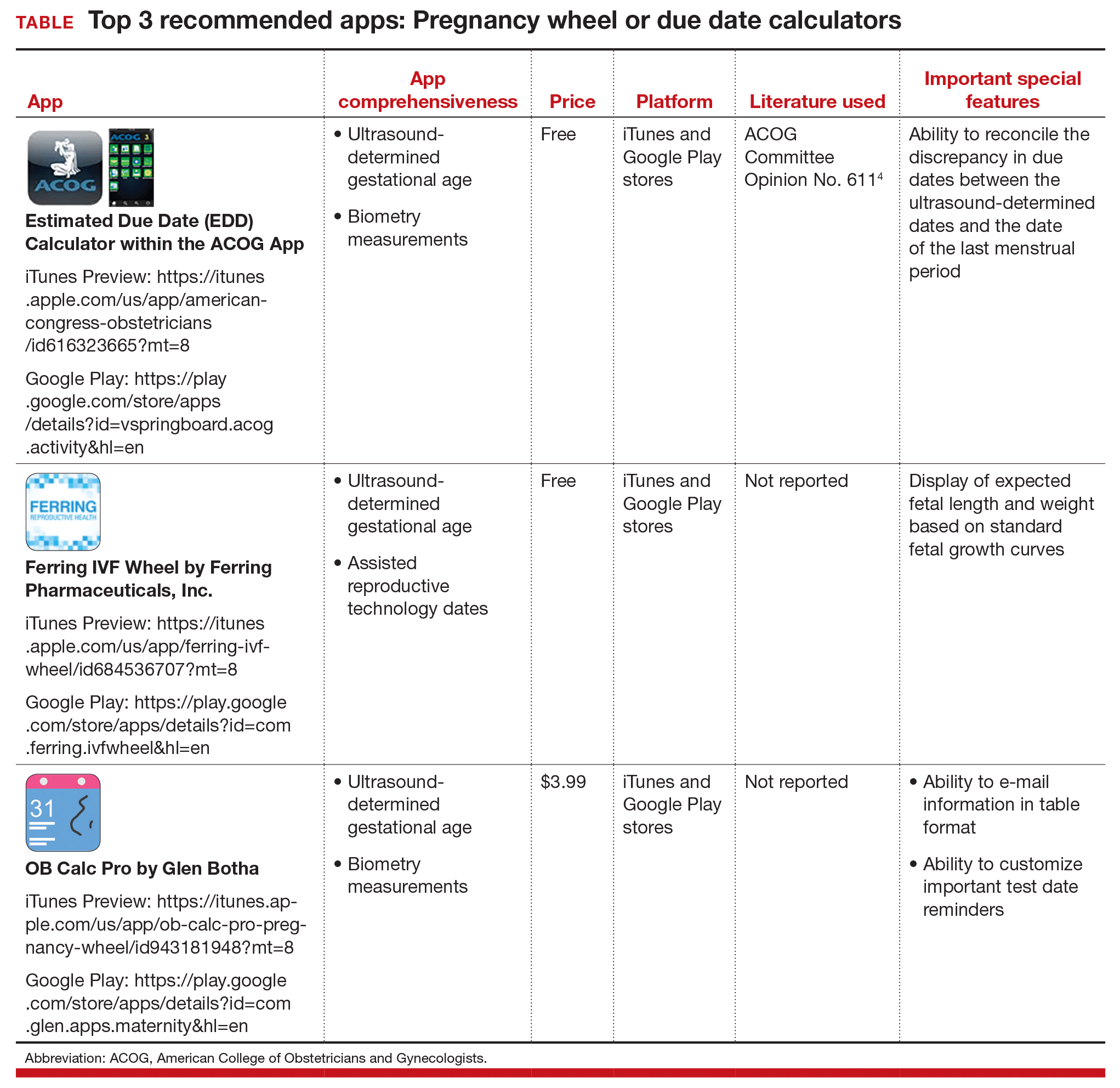
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Farag S, Chyjek K, Chen KT. Identification of iPhone and iPad applications for obstetrics and gynecology providers. Obstet Gynecol. 2014;124(5):941-945.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125(6):1478-1483.
- Payne KB, Wharrad H, Watts K. Smartphone and medical related App use among medical students and junior doctors in the United Kingdom (UK): a regional survey. BMC Inform Decis Mak. 2012;12:121.
- American College of Obstetricians and Gynecologists. Committee Opinion No. 611. Method for estimating due date. Obstet Gynecol. 2014;124(4):863-866.
- Chambliss LR, Clark SL. Paper gestational age wheels are generally inaccurate. Am J Obstet Gynecol. 2014;210(2):145.e1-e4.
Technology has changed--and continues to change--the practice of medicine. Health care providers access word processing programs, e-mail, and electronic medical records using desktop and laptop computers. Now, providers are accessing these same tools with handheld devices such as smartphones, tablets, and "phablets" (a class of mobile devices designed to combine the form of a smartphone and a tablet).
Critical to the popularity and functionality of handheld devices are mobile applications, also known as "apps." An app is a self-contained program or piece of software designed to run on handheld devices to perform a specific purpose. App overload and app inaccuracy, however, are major problems. Health care providers do not have the time to search through thousands of medical apps in app stores to find specialty-related apps that might be useful in their practice--or to check the accuracy of those apps.
Vetted apps for ObGyns
My team's research has focused on identifying apps for ObGyns to use in clinical practice.1 In the process, we have developed the APPLICATIONS scoring system, which contains objective and subjective components to help differentiate among the accurate apps.2 This new quarterly "App review" series in OBG Management will showcase recommended apps for the busy ObGyn in the hope of improving work efficiency and the provider-patient relationship.
First up: Apps for calculating the date of delivery. This first app review focuses on pregnancy wheels, or due date calculators. Calculator apps are preferred over other types of apps such as procedure/case documentation apps, as providers use smartphones at point of care to allow rapid decision making.3 Calculating the estimated date of delivery (EDD) and gestational age (GA) is an important, vital task for providers of obstetric care. In fact, new guidelines for calculating EDD were recently developed by the American College of Obstetricians and Gynecologists (ACOG), the American Institute of Ultrasound in Medicine (AIUM), and the Society for Maternal-Fetal Medicine (SMFM).4 Notably, pregnancy wheel apps are more accurate than paper wheels.5 My team checked the accuracy of the pregnancy wheel apps by applying strict criteria to ensure the correct EDD and GA and then scored them in a systematic, nonbiased, conflict-of-interest-free manner.2
Related article:
Elective induction of labor at 39 (vs 41) weeks: Caveats and considerations
The TABLE below lists the top 3 recommended pregnancy wheel or due date calculator apps vetted by our research. The apps are listed alphabetically, and details for each app are provided based on a shortened version of the APPLICATIONS scoring system, APPLI--app comprehensiveness, price, platform, literature use, and important special features.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Technology has changed--and continues to change--the practice of medicine. Health care providers access word processing programs, e-mail, and electronic medical records using desktop and laptop computers. Now, providers are accessing these same tools with handheld devices such as smartphones, tablets, and "phablets" (a class of mobile devices designed to combine the form of a smartphone and a tablet).
Critical to the popularity and functionality of handheld devices are mobile applications, also known as "apps." An app is a self-contained program or piece of software designed to run on handheld devices to perform a specific purpose. App overload and app inaccuracy, however, are major problems. Health care providers do not have the time to search through thousands of medical apps in app stores to find specialty-related apps that might be useful in their practice--or to check the accuracy of those apps.
Vetted apps for ObGyns
My team's research has focused on identifying apps for ObGyns to use in clinical practice.1 In the process, we have developed the APPLICATIONS scoring system, which contains objective and subjective components to help differentiate among the accurate apps.2 This new quarterly "App review" series in OBG Management will showcase recommended apps for the busy ObGyn in the hope of improving work efficiency and the provider-patient relationship.
First up: Apps for calculating the date of delivery. This first app review focuses on pregnancy wheels, or due date calculators. Calculator apps are preferred over other types of apps such as procedure/case documentation apps, as providers use smartphones at point of care to allow rapid decision making.3 Calculating the estimated date of delivery (EDD) and gestational age (GA) is an important, vital task for providers of obstetric care. In fact, new guidelines for calculating EDD were recently developed by the American College of Obstetricians and Gynecologists (ACOG), the American Institute of Ultrasound in Medicine (AIUM), and the Society for Maternal-Fetal Medicine (SMFM).4 Notably, pregnancy wheel apps are more accurate than paper wheels.5 My team checked the accuracy of the pregnancy wheel apps by applying strict criteria to ensure the correct EDD and GA and then scored them in a systematic, nonbiased, conflict-of-interest-free manner.2
Related article:
Elective induction of labor at 39 (vs 41) weeks: Caveats and considerations
The TABLE below lists the top 3 recommended pregnancy wheel or due date calculator apps vetted by our research. The apps are listed alphabetically, and details for each app are provided based on a shortened version of the APPLICATIONS scoring system, APPLI--app comprehensiveness, price, platform, literature use, and important special features.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Farag S, Chyjek K, Chen KT. Identification of iPhone and iPad applications for obstetrics and gynecology providers. Obstet Gynecol. 2014;124(5):941-945.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125(6):1478-1483.
- Payne KB, Wharrad H, Watts K. Smartphone and medical related App use among medical students and junior doctors in the United Kingdom (UK): a regional survey. BMC Inform Decis Mak. 2012;12:121.
- American College of Obstetricians and Gynecologists. Committee Opinion No. 611. Method for estimating due date. Obstet Gynecol. 2014;124(4):863-866.
- Chambliss LR, Clark SL. Paper gestational age wheels are generally inaccurate. Am J Obstet Gynecol. 2014;210(2):145.e1-e4.
- Farag S, Chyjek K, Chen KT. Identification of iPhone and iPad applications for obstetrics and gynecology providers. Obstet Gynecol. 2014;124(5):941-945.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125(6):1478-1483.
- Payne KB, Wharrad H, Watts K. Smartphone and medical related App use among medical students and junior doctors in the United Kingdom (UK): a regional survey. BMC Inform Decis Mak. 2012;12:121.
- American College of Obstetricians and Gynecologists. Committee Opinion No. 611. Method for estimating due date. Obstet Gynecol. 2014;124(4):863-866.
- Chambliss LR, Clark SL. Paper gestational age wheels are generally inaccurate. Am J Obstet Gynecol. 2014;210(2):145.e1-e4.
Epilepsy Raises Risks for Veterans
Iraq and Afghanistan veterans (IAV) with epilepsy have more than twice the risk of dying than do those without epilepsy, according to VA researchers.
In the study of 320,583 veterans, 2,187 met the epilepsy criteria. About 5 times more veterans with epilepsy had died by the end of follow-up compared with those without epilepsy. Veterans with epilepsy also are more likely to have mental and physical comorbidities, such as posttraumatic stress disorder, depression, traumatic brain injury, substance use disorder, and hypertension.
Related: Providing Quality Epilepsy Care for Veterans
Before their study, which is the first examining mortality in IAV with epilepsy, the researchers say little information existed about comorbidities and mortality. Epilepsy in veterans usually develops during or after service. People with epilepsy usually are excluded from military service (DoD standards require a 5-year period without seizures or treatment for seizures prior to enlistment). The age-adjusted prevalence of seizure disorder in IAV is 6.1 per 1,000 compared with 7.1 to 10 per 1,000 in the general population.
In response to the higher risk of epilepsy in IAV with traumatic brain injury, the VA established the Epilepsy Centers of Excellence, the researchers note, to increase access to comprehensive multidisciplinary epilepsy specialty care. However, the significantly higher prevalence of comorbidities in this population suggests that “closer integration of primary care, epilepsy specialty care, and mental health care might be needed to reduce excess mortality.”
Related: VA to Reexamine 24,000 Veterans for TBI
The researchers suggest that public health agencies, including the VA, implement evidence-based, chronic disease self-management programs and supports that target physical and psychiatric comorbidity, study long-term outcomes, and ensure links to appropriate clinical and community health care facilities and social service providers.
Iraq and Afghanistan veterans (IAV) with epilepsy have more than twice the risk of dying than do those without epilepsy, according to VA researchers.
In the study of 320,583 veterans, 2,187 met the epilepsy criteria. About 5 times more veterans with epilepsy had died by the end of follow-up compared with those without epilepsy. Veterans with epilepsy also are more likely to have mental and physical comorbidities, such as posttraumatic stress disorder, depression, traumatic brain injury, substance use disorder, and hypertension.
Related: Providing Quality Epilepsy Care for Veterans
Before their study, which is the first examining mortality in IAV with epilepsy, the researchers say little information existed about comorbidities and mortality. Epilepsy in veterans usually develops during or after service. People with epilepsy usually are excluded from military service (DoD standards require a 5-year period without seizures or treatment for seizures prior to enlistment). The age-adjusted prevalence of seizure disorder in IAV is 6.1 per 1,000 compared with 7.1 to 10 per 1,000 in the general population.
In response to the higher risk of epilepsy in IAV with traumatic brain injury, the VA established the Epilepsy Centers of Excellence, the researchers note, to increase access to comprehensive multidisciplinary epilepsy specialty care. However, the significantly higher prevalence of comorbidities in this population suggests that “closer integration of primary care, epilepsy specialty care, and mental health care might be needed to reduce excess mortality.”
Related: VA to Reexamine 24,000 Veterans for TBI
The researchers suggest that public health agencies, including the VA, implement evidence-based, chronic disease self-management programs and supports that target physical and psychiatric comorbidity, study long-term outcomes, and ensure links to appropriate clinical and community health care facilities and social service providers.
Iraq and Afghanistan veterans (IAV) with epilepsy have more than twice the risk of dying than do those without epilepsy, according to VA researchers.
In the study of 320,583 veterans, 2,187 met the epilepsy criteria. About 5 times more veterans with epilepsy had died by the end of follow-up compared with those without epilepsy. Veterans with epilepsy also are more likely to have mental and physical comorbidities, such as posttraumatic stress disorder, depression, traumatic brain injury, substance use disorder, and hypertension.
Related: Providing Quality Epilepsy Care for Veterans
Before their study, which is the first examining mortality in IAV with epilepsy, the researchers say little information existed about comorbidities and mortality. Epilepsy in veterans usually develops during or after service. People with epilepsy usually are excluded from military service (DoD standards require a 5-year period without seizures or treatment for seizures prior to enlistment). The age-adjusted prevalence of seizure disorder in IAV is 6.1 per 1,000 compared with 7.1 to 10 per 1,000 in the general population.
In response to the higher risk of epilepsy in IAV with traumatic brain injury, the VA established the Epilepsy Centers of Excellence, the researchers note, to increase access to comprehensive multidisciplinary epilepsy specialty care. However, the significantly higher prevalence of comorbidities in this population suggests that “closer integration of primary care, epilepsy specialty care, and mental health care might be needed to reduce excess mortality.”
Related: VA to Reexamine 24,000 Veterans for TBI
The researchers suggest that public health agencies, including the VA, implement evidence-based, chronic disease self-management programs and supports that target physical and psychiatric comorbidity, study long-term outcomes, and ensure links to appropriate clinical and community health care facilities and social service providers.
Patch Testing for Adverse Drug Reactions
Adverse drug reactions account for 3% to 6% of hospital admissions in the United States and occur in 10% to 15% of hospitalized patients.1,2 The most common culprits are antibiotics and nonsteroidal anti-inflammatory drugs (NSAIDs).3-12 In most cases, diagnoses are made clinically without diagnostic testing. To identify drug allergies associated with diagnostic testing, one center selected patients with suspected cutaneous drug reactions (2006-2010) for further evaluation.13 Of 612 patients who were evaluated, 141 had a high suspicion of drug allergy and were included in the analysis. The excluded patients had pseudoallergic reactions, reactive exanthemas due to infection, histopathologic exclusion of drug allergy, angioedema, or other dermatological conditions such as contact dermatitis and eczema. Of the included patients, 107 were diagnosed with drug reactions, while the remainder had non–drug-related exanthemas or unknown etiology after testing. Identified culprit drugs were predominantly antibiotics (39.8%) and NSAIDs (21.2%); contrast media, anticoagulants, anticonvulsants, antimalarials, antifungals, glucocorticoids, antihypertensives, and proton pump inhibitors also were implicated. They were identified with skin prick, intradermal, and patch tests (62.6%); lymphocyte transformation test (17.7%); oral rechallenge (5.6%); or without skin testing (6.5%). One quarter of patients with a high suspicion for drug allergy did not have a confirmed drug eruption in this study. Another study found that 10% to 20% of patients with reported penicillin allergy had confirmation via skin prick testing.14 These findings suggest that confirmation of suspected drug allergy may require more than one diagnostic test.
Tests for Adverse Drug Reactions
The following tests have been shown to aid in the identification of cutaneous drug eruptions: (1) patch tests15-21; (2) intradermal tests14,15,19,20; (3) drug provocation tests15,20; and (4) lymphocyte transformation tests.20 Intradermal or skin prick tests are most useful in urticarial eruptions but can be considered in nonurticarial eruptions with delayed inspection of test sites up to 1 week after testing. Drug provocation tests are considered the gold standard but involve patient risk. Lymphocyte transformation tests use the principle that T lymphocytes proliferate in the presence of drugs to which the patient is sensitized. Patch tests will be discussed in greater detail below. Immunohistochemistry can determine immunologic mechanisms of eruptions but cannot identify causative agents.16,17,22
A retrospective study of patients referred for evaluation of adverse drug reactions between 1996 and 2006 found the collective negative predictive value (NPV)—the percentage of truly negative skin tests based on provocation or substitution testing—of cutaneous drug tests including patch, prick, and intradermal tests to be 89.6% (95% confidence interval, 85.9%-93.3%).23 The NPVs of each test were not reported. Patients with negative cutaneous tests had subsequent oral rechallenge or substitution testing with medication from the same drug class.23 Another study16 found the NPV of patch testing to be at least 79% after review of data from other studies using patch and provocation testing.16,24 These studies suggest that cutaneous testing can be useful, albeit imperfect, in the evaluation and diagnosis of drug allergy.
Review of the Patch Test
Patch tests can be helpful in diagnosis of delayed hypersensitivities.18 Patch testing is most commonly and effectively used to diagnose allergic contact dermatitis, but its utility in other applications, such as diagnosis of cutaneous drug eruptions, has not been extensively studied.
The development of patch tests to diagnose systemic drug allergies is inhibited by the uncertainty of percutaneous drug penetration, a dearth of studies to determine the best test concentrations of active drug in the patch test, and the potential for nonimmunologic contact urticaria upon skin exposure. Furthermore, cutaneous metabolism of many antigens is well documented, but correlation to systemic metabolism often is unknown, which can confound patch test results and lead to false-negative results when the skin’s metabolic capacity does not match the body’s capacity to generate antigens capable of eliciting immunogenic responses.21 Additionally, the method used to suspend and disperse drugs in patch test vehicles is unfamiliar to most pharmacists, and standardized concentrations and vehicles are available only for some medications.25 Studies sufficient to obtain US Food and Drug Administration approval of patch tests for systemic drug eruptions would be costly and therefore prohibitive to investigators. The majority of the literature consists of case reports and data extrapolated from reviews. Patch test results of many drugs have been reported in the literature, with the highest frequencies of positive results associated with anticonvulsants,26 antibiotics, corticosteroids, calcium channel blockers, and benzodiazepines.21
Patch test placement affects the diagnostic value of the test. Placing patch tests on previously involved sites of fixed drug eruptions improves yield over placement on uninvolved skin.27 Placing patch tests on previously involved sites of other drug eruptions such as toxic epidermal necrolysis also may aid in diagnosis, though the literature is sparse.25,26,28
Patch Testing in Drug Eruptions
Morbilliform eruptions account for 48% to 91% of patients with adverse drug reactions.4-6 Other drug eruptions include urticarial eruptions, acute generalized exanthematous pustulosis (AGEP), drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, toxic epidermal necrolysis, Stevens-Johnson syndrome, lichenoid drug eruption, symmetric drug-related intertriginous and flexural exanthema (SDRIFE), erythema multiforme (EM), and systemic contact dermatitis. The Table summarizes reports of positive patch tests with various medications for these drug eruptions.
In general, antimicrobials and NSAIDs were the most implicated drugs with positive patch test results in AGEP, DRESS syndrome, EM, fixed drug eruptions, and morbilliform eruptions. In AGEP, positive results also were reported for other drugs, including terbinafine and morphine.29-38 In fixed drug eruptions, patch testing on involved skin showed positive results to NSAIDs, analgesics, platelet inhibitors, and antimicrobials.27,52-55 Patch testing in DRESS syndrome has shown many positive reactions to antiepileptics and antipsychotics.39-43 One study used patch tests in SDRIFE, reporting positive results with antimicrobials, antineoplastics, decongestants, and glucocorticoids.61 Nonsteroidal anti-inflammatory drugs, antimicrobials, calcium channel blockers, and histamine antagonists were implicated in EM.47-51 Positive patch tests were seen in morbilliform eruptions with selective serotonin reuptake inhibitors, antiepileptics/benzodiazepines, NSAIDs, and antimicrobials.28,57-60 In toxic epidermal necrolysis, diagnosis with patch testing was made using patches placed on previously involved skin with sulfamethoxazole.62
Systemic Contact Dermatitis
Drugs historically recognized as causing allergic contact dermatitis (eg, topical gentamycin) can cause systemic contact dermatitis, which can be patch tested. In these situations, systemic contact dermatitis may be due to either the active drug or excipients in the medication formulation. Excipients are inactive ingredients in medications that provide a suitable consistency, appearance, or form. Often overlooked as culprits of drug hypersensitivity because they are theoretically inert, excipients are increasingly implicated in drug allergy. Swerlick and Campbell63 described 11 cases in which chronic unexplained pruritus responded to medication changes to avoid coloring agents. The most common culprits were FD&C Blue No. 1 and FD&C Blue No. 2. Patch testing for allergies to dyes can be clinically useful, though a lack of commercially available patch tests makes diagnosis difficult.64
Other excipients can cause cutaneous reactions. Propylene glycol, commonly implicated in allergic contact dermatitis, also can cause cutaneous eruptions upon systemic exposure.65 Corticosteroid-induced systemic contact dermatitis has been reported, though it is less prevalent than allergic contact dermatitis.66 These reactions usually are due to nonmethylated and nonhalogenated corticosteroids including budesonide, cortisone, hydrocortisone, prednisolone, and methylprednisolone.67,68 Patch testing in these situations is complicated by the possibility of false-negative results due to the anti-inflammatory effects of the corticosteroids. Therefore, patch testing should be performed using standardized and not treatment concentrations.
In our clinic, we have anecdotally observed several patients with chronic dermatitis and suspected NSAID allergies have positive patch test results with propylene glycol and not the suspected drug. Excipients encountered in multiple drugs and foods are more likely to present as chronic dermatitis, while active drug ingredients started in hospital settings more often present as acute dermatitis.
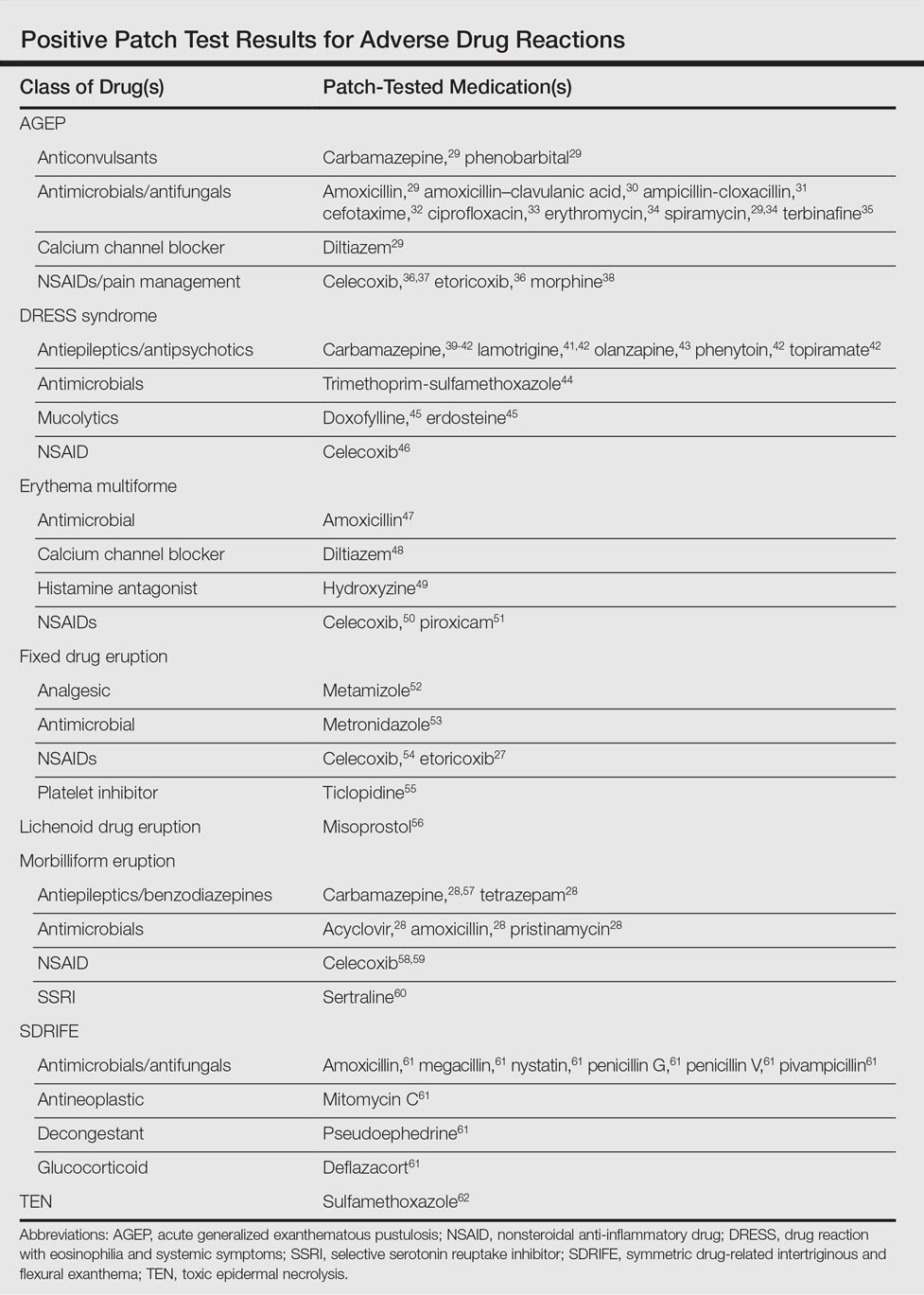
Our Experience
We have patch tested a handful of patients with suspected drug eruptions (University Hospitals Cleveland Medical Center institutional review board #07-12-27). Medications, excipients, and their concentrations (in % weight per weight) and vehicles that were tested include ibuprofen (10% petrolatum), aspirin (10% petrolatum), hydrochlorothiazide (10% petrolatum), captopril (5% petrolatum), and propylene glycol (30% water or 5% petrolatum). Patch tests were read at 48 and 72 hours and scored according to the International Contact Dermatitis Research Group patch test scoring guidelines.69 Two patients tested for ibuprofen reacted positively only to propylene glycol; the 3 other patients did not react to aspirin, hydrochlorothiazide, and captopril. Overall, we observed no positive patch tests to medications and 2 positive tests to propylene glycol in 5 patients tested (unpublished data).
Areas of Uncertainty
Although tests for immediate-type hypersensitivity reactions to drugs exist as skin prick tests, diagnostic testing for the majority of drug reactions does not exist. Drug allergy diagnosis is made with history and temporality, potentially resulting in unnecessary avoidance of helpful medications. Ideal patch test concentrations and vehicles as well as the sensitivity and specificity of these tests are unknown.
Guidelines From Professional Societies
Drug allergy testing guidelines are available from the British Society for Allergy and Clinical Immunology70 and American Academy of Allergy, Asthma and Immunology.71 The guidelines recommend diagnosis by history and temporality, and it is stated that patch testing is potentially useful in maculopapular rashes, AGEP, fixed drug eruptions, and DRESS syndrome.
Conclusion
Case reports in the literature suggest the utility of patch testing in some drug allergies. We suggest testing excipients such as propylene glycol and benzoic acid to rule out systemic contact dermatitis when patch testing with active drugs to confirm cause of suspected adverse cutaneous reactions to medications.
- Arndt KA, Jick H. Rates of cutaneous reactions to drugs. a report from the Boston Collaborative Drug Surveillance Program. JAMA. 1976;235:918-922.
- Bigby M, Jick S, Jick H, et al. Drug-induced cutaneous reactions. a report from the Boston Collaborative Drug Surveillance Program on 15,483 consecutive inpatients, 1975 to 1982. JAMA. 1986;256:3358-3363.
- Fiszenson-Albala F, Auzerie V, Mahe E, et al. A 6-month prospective survey of cutaneous drug reactions in a hospital setting. Br J Dermatol. 2003;149:1018-1022.
- Thong BY, Leong KP, Tang CY, et al. Drug allergy in a general hospital: results of a novel prospective inpatient reporting system. Ann Allergy Asthma Immunol. 2003;90:342-347.
- Hunziker T, Kunzi UP, Braunschweig S, et al. Comprehensive hospital drug monitoring (CHDM): adverse skin reactions, a 20-year survey. Allergy. 1997;52:388-393.
- Swanbeck G, Dahlberg E. Cutaneous drug reactions. an attempt to quantitative estimation. Arch Dermatol Res. 1992;284:215-218.
- Naldi L, Conforti A, Venegoni M, et al. Cutaneous reactions to drugs. an analysis of spontaneous reports in four Italian regions. Br J Clin Pharmacol. 1999;48:839-846.
- French LE, Prins C. Erythema multiforme, Stevens-Johnson syndrome and toxic epidermal necrolysis. In: Bolognia, JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012:319-333.
- Vasconcelos C, Magina S, Quirino P, et al. Cutaneous drug reactions to piroxicam. Contact Dermatitis. 1998;39:145.
- Gerber D. Adverse reactions of piroxicam. Drug Intell Clin Pharm. 1987;21:707-710.
- Revuz J, Valeyrie-Allanore L. Drug reactions. In: Bolognia, JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012:335-356.
- Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: part II. management and therapeutics. J Am Acad Dermatol. 2013;68:709.e1-709.e9; quiz 718-720.
- Heinzerling LM, Tomsitz D, Anliker MD. Is drug allergy less prevalent than previously assumed? a 5-year analysis. Br J Dermatol. 2012;166:107-114.
- Salkind AR, Cuddy PG. Is this patient allergic to penicillin?: an evidence-based analysis of the likelihood of penicillin allergy. JAMA. 2001;285:2498-2505.
- Torres MJ, Gomez F, Doña I, et al. Diagnostic evaluation of patients with nonimmediate cutaneous hypersensitivity reactions to iodinated contrast media. Allergy. 2012;67:929-935.
- Cham PM, Warshaw EM. Patch testing for evaluating drug reactions due to systemic antibiotics. Dermatitis. 2007;18:63-77.
- Andrade P, Brinca A, Gonçalo M. Patch testing in fixed drug eruptions—a 20-year review. Contact Dermatitis. 2011;65:195-201.
- Romano A, Viola M, Gaeta F, et al. Patch testing in non-immediate drug eruptions. Allergy Asthma Clin Immunol. 2008;4:66-74.
- Rosso R, Mattiacci G, Bernardi ML, et al. Very delayed reactions to beta-lactam antibiotics. Contact Dermatitis. 2000;42:293-295.
- Romano A, Torres MJ, Castells M, et al. Diagnosis and management of drug hypersensitivity reactions. J Allergy Clin Immunol. 2011;127(3 suppl):S67-S73.
- Friedmann PS, Ardern-Jones M. Patch testing in drug allergy. Curr Opin Allergy Clin Immunol. 2010;10:291-296.
- Torres MJ, Mayorga C, Blanca M. Nonimmediate allergic reactions induced by drugs: pathogenesis and diagnostic tests. J Investig Allergol Clin Immunol. 2009;19:80-90.
- Waton J, Tréchot P, Loss-Ayay C, et al. Negative predictive value of drug skin tests in investigating cutaneous adverse drug reactions. Br J Dermatol. 2009;160:786-794.
- Romano A, Viola M, Mondino C, et al. Diagnosing nonimmediate reactions to penicillins by in vivo tests. Int Arch Allergy Immunol. 2002;129:169-174.
- De Groot AC. Patch Testing. Test Concentrations and Vehicles for 4350 Chemicals. 3rd ed. Wapserveen, Netherlands: acdegroot publishing; 2008.
- Elzagallaai AA, Knowles SR, Rieder MJ, et al. Patch testing for the diagnosis of anticonvulsant hypersensitivity syndrome: a systematic review. Drug Saf. 2009;32:391-408.
- Andrade P, Gonçalo M. Fixed drug eruption caused by etoricoxib—2 cases confirmed by patch testing. Contact Dermatitis. 2011;64:118-120.
- Barbaud A, Reichert-Penetrat S, Tréchot P, et al. The use of skin testing in the investigation of cutaneous adverse drug reactions. Br J Dermatol. 1998;139:49-58.
- Wolkenstein P, Chosidow O, Fléchet ML, et al. Patch testing in severe cutaneous adverse drug reactions, including Stevens-Johnson syndrome and toxic epidermal necrolysis. Contact Dermatitis. 1996;35:234-236.
- Harries MJ, McIntyre SJ, Kingston TP. Co-amoxiclav-induced acute generalized exanthematous pustulosis confirmed by patch testing. Contact Dermatitis. 2006;55:372.
- Matsumoto Y, Okubo Y, Yamamoto T, et al. Case of acute generalized exanthematous pustulosis caused by ampicillin/cloxacillin sodium in a pregnant woman. J Dermatol. 2008;35:362-364.
- Chaabane A, Aouam K, Gassab L, et al. Acute generalized exanthematous pustulosis (AGEP) induced by cefotaxime. Fundam Clin Pharmacol. 2010;24:429-432.
- Hausermann P, Scherer K, Weber M, et al. Ciprofloxacin-induced acute generalized exanthematous pustulosis mimicking bullous drug eruption confirmed by a positive patch test. Dermatology. 2005;211:277-280.
- Moreau A, Dompmartin A, Castel B, et al. Drug-induced acute generalized exanthematous pustulosis with positive patch tests. Int J Dermatol. 1995;34:263-266.
- Kempinaire A, De Raevea L, Merckx M, et al. Terbinafine-induced acute generalized exanthematous pustulosis confirmed by a positive patch-test result. J Am Acad Dermatol. 1997;37:653-655.
- Mäkelä L, Lammintausta K. Etoricoxib-induced acute generalized exanthematous pustulosis. Acta Derm Venereol. 2008;88:200-201.
- Yang CC, Lee JY, Chen WC. Acute generalized exanthematous pustulosis caused by celecoxib. J Formos Med Assoc. 2004;103:555-557.
- Kardaun SH, de Monchy JG. Acute generalized exanthematous pustulosis caused by morphine, confirmed by positive patch test and lymphocyte transformation test. J Am Acad Dermatol. 2006;55(2 suppl):S21-S23.
- Inadomi T. Drug rash with eosinophilia and systemic symptoms (DRESS): changing carbamazepine to phenobarbital controlled epilepsy without the recurrence of DRESS. Eur J Dermatol. 2010;20:220-222.
- Buyuktiryaki AB, Bezirganoglu H, Sahiner UM, et al. Patch testing is an effective method for the diagnosis of carbamazepine-induced drug reaction, eosinophilia and systemic symptoms (DRESS) syndrome in an 8-year-old girl. Australas J Dermatol. 2012;53:274-277.
- Aouam K, Ben Romdhane F, Loussaief C, et al. Hypersensitivity syndrome induced by anticonvulsants: possible cross-reactivity between carbamazepine and lamotrigine. J Clin Pharmacol. 2009;49:1488-1491.
- Santiago F, Gonçalo M, Vieira R, et al. Epicutaneous patch testing in drug hypersensitivity syndrome (DRESS). Contact Dermatitis. 2010;62:47-53.
- Prevost P, Bédry R, Lacoste D, et al. Hypersensitivity syndrome with olanzapine confirmed by patch tests. Eur J Dermatol. 2012;22:126-127.
- Hubiche T, Milpied B, Cazeau C, et al. Association of immunologically confirmed delayed drug reaction and human herpesvirus 6 viremia in a pediatric case of drug-induced hypersensitivity syndrome. Dermatology. 2011;222:140-141.
- Song WJ, Shim EJ, Kang MG, et al. Severe drug hypersensitivity induced by erdosteine and doxofylline as confirmed by patch and lymphocyte transformation tests: a case report. J Investig Allergol Clin Immunol. 2012;22:230-232.
- Lee JH, Park HK, Heo J, et al. Drug rash with eosinophilia and systemic symptoms (DRESS) syndrome induced by celecoxib and anti-tuberculosis drugs. J Korean Med Sci. 2008;23:521-525.
- González-Delgado P, Blanes M, Soriano V, et al. Erythema multiforme to amoxicillin with concurrent infection by Epstein-Barr virus. Allergol Immunopathol. 2006;34:76-78.
- Gonzalo Garijo MA, Pérez Calderón R, de Argila Fernández-Durán D, et al. Cutaneous reactions due to diltiazem and cross reactivity with other calcium channel blockers. Allergol Immunopathol (Madr). 2005;33:238-240.
- Peña AL, Henriquezsantana A, Gonzalez-Seco E, et al. Exudative erythema multiforme induced by hydroxyzine. Eur J Dermatol. 2008;18:194-195.
- Arakawa Y, Nakai N, Katoh N. Celecoxib-induced erythema multiforme-type drug eruption with a positive patch test. J Dermatol. 2011;38:1185-1188.
- Prieto A, De barrio M, Pérez C, et al. Piroxicam-induced erythema multiforme. Contact Dermatitis. 2004;50:263.
- Dalmau J, Serra-baldrich E, Roé E, et al. Use of patch test in fixed drug eruption due to metamizole (Nolotil). Contact Dermatitis. 2006;54:127-128.
- Gastaminza G, Anda M, Audicana MT, et al. Fixed-drug eruption due to metronidazole with positive topical provocation. Contact Dermatitis. 2001;44:36.
- Bellini V, Stingeni L, Lisi P. Multifocal fixed drug eruption due to celecoxib. Dermatitis. 2009;20:174-176.
- García CM, Carmena R, García R, et al. Fixed drug eruption from ticlopidine, with positive lesional patch test. Contact Dermatitis. 2001;44:40-41.
- Cruz MJ, Duarte AF, Baudrier T, et al. Lichenoid drug eruption induced by misoprostol. Contact Dermatitis. 2009;61:240-242.
- Alanko K. Patch testing in cutaneous reactions caused by carbamazepine. Contact Dermatitis. 1993;29:254-257.
- Grob M, Scheidegger P, Wüthrich B. Allergic skin reaction to celecoxib. Dermatology. 2000;201:383.
- Alonso JC, Ortega JD, Gonzalo MJ. Cutaneous reaction to oral celecoxib with positive patch test. Contact Dermatitis. 2004;50:48-49.
- Fernandes B, Brites M, Gonçalo M, et al. Maculopapular eruption from sertraline with positive patch tests. Contact Dermatitis. 2000;42:287.
- Häusermann P, Harr T, Bircher AJ. Baboon syndrome resulting from systemic drugs: is there strife between SDRIFE and allergic contact dermatitis syndrome? Contact Dermatitis. 2004;51:297-310.
- Klein CE, Trautmann A, Zillikens D, et al. Patch testing in an unusual case of toxic epidermal necrolysis. Contact Dermatitis. 1996;35:175-176.
- Swerlick RA, Campbell CF. Medication dyes as a source of drug allergy. J Drugs Dermatol. 2013;12:99-102.
- Guin JD. Patch testing to FD&C and D&C dyes. Contact Dermatitis. 2003;49:217-218.
- Lowther A, McCormick T, Nedorost S. Systemic contact dermatitis from propylene glycol. Dermatitis. 2008;19:105-108.
- Baeck M, Goossens A. Systemic contact dermatitis to corticosteroids. Allergy. 2012;67:1580-1585.
- Baeck M, Goossens A. Immediate and delayed allergic hypersensitivity to corticosteroids: practical guidelines. Contact Dermatitis. 2012;66:38-45.
- Basedow S, Eigelshoven S, Homey B. Immediate and delayed hypersensitivity to corticosteroids. J Dtsch Dermatol Ges. 2011;9:885-888.
- Johansen JD, Aalto-korte K, Agner T, et al. European Society of Contact Dermatitis guideline for diagnostic patch testing—recommendations on best practice. Contact Dermatitis. 2015;73:195-221.
- Mirakian R, Ewan PW, Durham SR, et al. BSACI guidelines for the management of drug allergy. Clin Exp Allergy. 2009;39:43-61.
- Joint Task Force on Practice Parameters; American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105:259-273.
Adverse drug reactions account for 3% to 6% of hospital admissions in the United States and occur in 10% to 15% of hospitalized patients.1,2 The most common culprits are antibiotics and nonsteroidal anti-inflammatory drugs (NSAIDs).3-12 In most cases, diagnoses are made clinically without diagnostic testing. To identify drug allergies associated with diagnostic testing, one center selected patients with suspected cutaneous drug reactions (2006-2010) for further evaluation.13 Of 612 patients who were evaluated, 141 had a high suspicion of drug allergy and were included in the analysis. The excluded patients had pseudoallergic reactions, reactive exanthemas due to infection, histopathologic exclusion of drug allergy, angioedema, or other dermatological conditions such as contact dermatitis and eczema. Of the included patients, 107 were diagnosed with drug reactions, while the remainder had non–drug-related exanthemas or unknown etiology after testing. Identified culprit drugs were predominantly antibiotics (39.8%) and NSAIDs (21.2%); contrast media, anticoagulants, anticonvulsants, antimalarials, antifungals, glucocorticoids, antihypertensives, and proton pump inhibitors also were implicated. They were identified with skin prick, intradermal, and patch tests (62.6%); lymphocyte transformation test (17.7%); oral rechallenge (5.6%); or without skin testing (6.5%). One quarter of patients with a high suspicion for drug allergy did not have a confirmed drug eruption in this study. Another study found that 10% to 20% of patients with reported penicillin allergy had confirmation via skin prick testing.14 These findings suggest that confirmation of suspected drug allergy may require more than one diagnostic test.
Tests for Adverse Drug Reactions
The following tests have been shown to aid in the identification of cutaneous drug eruptions: (1) patch tests15-21; (2) intradermal tests14,15,19,20; (3) drug provocation tests15,20; and (4) lymphocyte transformation tests.20 Intradermal or skin prick tests are most useful in urticarial eruptions but can be considered in nonurticarial eruptions with delayed inspection of test sites up to 1 week after testing. Drug provocation tests are considered the gold standard but involve patient risk. Lymphocyte transformation tests use the principle that T lymphocytes proliferate in the presence of drugs to which the patient is sensitized. Patch tests will be discussed in greater detail below. Immunohistochemistry can determine immunologic mechanisms of eruptions but cannot identify causative agents.16,17,22
A retrospective study of patients referred for evaluation of adverse drug reactions between 1996 and 2006 found the collective negative predictive value (NPV)—the percentage of truly negative skin tests based on provocation or substitution testing—of cutaneous drug tests including patch, prick, and intradermal tests to be 89.6% (95% confidence interval, 85.9%-93.3%).23 The NPVs of each test were not reported. Patients with negative cutaneous tests had subsequent oral rechallenge or substitution testing with medication from the same drug class.23 Another study16 found the NPV of patch testing to be at least 79% after review of data from other studies using patch and provocation testing.16,24 These studies suggest that cutaneous testing can be useful, albeit imperfect, in the evaluation and diagnosis of drug allergy.
Review of the Patch Test
Patch tests can be helpful in diagnosis of delayed hypersensitivities.18 Patch testing is most commonly and effectively used to diagnose allergic contact dermatitis, but its utility in other applications, such as diagnosis of cutaneous drug eruptions, has not been extensively studied.
The development of patch tests to diagnose systemic drug allergies is inhibited by the uncertainty of percutaneous drug penetration, a dearth of studies to determine the best test concentrations of active drug in the patch test, and the potential for nonimmunologic contact urticaria upon skin exposure. Furthermore, cutaneous metabolism of many antigens is well documented, but correlation to systemic metabolism often is unknown, which can confound patch test results and lead to false-negative results when the skin’s metabolic capacity does not match the body’s capacity to generate antigens capable of eliciting immunogenic responses.21 Additionally, the method used to suspend and disperse drugs in patch test vehicles is unfamiliar to most pharmacists, and standardized concentrations and vehicles are available only for some medications.25 Studies sufficient to obtain US Food and Drug Administration approval of patch tests for systemic drug eruptions would be costly and therefore prohibitive to investigators. The majority of the literature consists of case reports and data extrapolated from reviews. Patch test results of many drugs have been reported in the literature, with the highest frequencies of positive results associated with anticonvulsants,26 antibiotics, corticosteroids, calcium channel blockers, and benzodiazepines.21
Patch test placement affects the diagnostic value of the test. Placing patch tests on previously involved sites of fixed drug eruptions improves yield over placement on uninvolved skin.27 Placing patch tests on previously involved sites of other drug eruptions such as toxic epidermal necrolysis also may aid in diagnosis, though the literature is sparse.25,26,28
Patch Testing in Drug Eruptions
Morbilliform eruptions account for 48% to 91% of patients with adverse drug reactions.4-6 Other drug eruptions include urticarial eruptions, acute generalized exanthematous pustulosis (AGEP), drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, toxic epidermal necrolysis, Stevens-Johnson syndrome, lichenoid drug eruption, symmetric drug-related intertriginous and flexural exanthema (SDRIFE), erythema multiforme (EM), and systemic contact dermatitis. The Table summarizes reports of positive patch tests with various medications for these drug eruptions.
In general, antimicrobials and NSAIDs were the most implicated drugs with positive patch test results in AGEP, DRESS syndrome, EM, fixed drug eruptions, and morbilliform eruptions. In AGEP, positive results also were reported for other drugs, including terbinafine and morphine.29-38 In fixed drug eruptions, patch testing on involved skin showed positive results to NSAIDs, analgesics, platelet inhibitors, and antimicrobials.27,52-55 Patch testing in DRESS syndrome has shown many positive reactions to antiepileptics and antipsychotics.39-43 One study used patch tests in SDRIFE, reporting positive results with antimicrobials, antineoplastics, decongestants, and glucocorticoids.61 Nonsteroidal anti-inflammatory drugs, antimicrobials, calcium channel blockers, and histamine antagonists were implicated in EM.47-51 Positive patch tests were seen in morbilliform eruptions with selective serotonin reuptake inhibitors, antiepileptics/benzodiazepines, NSAIDs, and antimicrobials.28,57-60 In toxic epidermal necrolysis, diagnosis with patch testing was made using patches placed on previously involved skin with sulfamethoxazole.62
Systemic Contact Dermatitis
Drugs historically recognized as causing allergic contact dermatitis (eg, topical gentamycin) can cause systemic contact dermatitis, which can be patch tested. In these situations, systemic contact dermatitis may be due to either the active drug or excipients in the medication formulation. Excipients are inactive ingredients in medications that provide a suitable consistency, appearance, or form. Often overlooked as culprits of drug hypersensitivity because they are theoretically inert, excipients are increasingly implicated in drug allergy. Swerlick and Campbell63 described 11 cases in which chronic unexplained pruritus responded to medication changes to avoid coloring agents. The most common culprits were FD&C Blue No. 1 and FD&C Blue No. 2. Patch testing for allergies to dyes can be clinically useful, though a lack of commercially available patch tests makes diagnosis difficult.64
Other excipients can cause cutaneous reactions. Propylene glycol, commonly implicated in allergic contact dermatitis, also can cause cutaneous eruptions upon systemic exposure.65 Corticosteroid-induced systemic contact dermatitis has been reported, though it is less prevalent than allergic contact dermatitis.66 These reactions usually are due to nonmethylated and nonhalogenated corticosteroids including budesonide, cortisone, hydrocortisone, prednisolone, and methylprednisolone.67,68 Patch testing in these situations is complicated by the possibility of false-negative results due to the anti-inflammatory effects of the corticosteroids. Therefore, patch testing should be performed using standardized and not treatment concentrations.
In our clinic, we have anecdotally observed several patients with chronic dermatitis and suspected NSAID allergies have positive patch test results with propylene glycol and not the suspected drug. Excipients encountered in multiple drugs and foods are more likely to present as chronic dermatitis, while active drug ingredients started in hospital settings more often present as acute dermatitis.

Our Experience
We have patch tested a handful of patients with suspected drug eruptions (University Hospitals Cleveland Medical Center institutional review board #07-12-27). Medications, excipients, and their concentrations (in % weight per weight) and vehicles that were tested include ibuprofen (10% petrolatum), aspirin (10% petrolatum), hydrochlorothiazide (10% petrolatum), captopril (5% petrolatum), and propylene glycol (30% water or 5% petrolatum). Patch tests were read at 48 and 72 hours and scored according to the International Contact Dermatitis Research Group patch test scoring guidelines.69 Two patients tested for ibuprofen reacted positively only to propylene glycol; the 3 other patients did not react to aspirin, hydrochlorothiazide, and captopril. Overall, we observed no positive patch tests to medications and 2 positive tests to propylene glycol in 5 patients tested (unpublished data).
Areas of Uncertainty
Although tests for immediate-type hypersensitivity reactions to drugs exist as skin prick tests, diagnostic testing for the majority of drug reactions does not exist. Drug allergy diagnosis is made with history and temporality, potentially resulting in unnecessary avoidance of helpful medications. Ideal patch test concentrations and vehicles as well as the sensitivity and specificity of these tests are unknown.
Guidelines From Professional Societies
Drug allergy testing guidelines are available from the British Society for Allergy and Clinical Immunology70 and American Academy of Allergy, Asthma and Immunology.71 The guidelines recommend diagnosis by history and temporality, and it is stated that patch testing is potentially useful in maculopapular rashes, AGEP, fixed drug eruptions, and DRESS syndrome.
Conclusion
Case reports in the literature suggest the utility of patch testing in some drug allergies. We suggest testing excipients such as propylene glycol and benzoic acid to rule out systemic contact dermatitis when patch testing with active drugs to confirm cause of suspected adverse cutaneous reactions to medications.
Adverse drug reactions account for 3% to 6% of hospital admissions in the United States and occur in 10% to 15% of hospitalized patients.1,2 The most common culprits are antibiotics and nonsteroidal anti-inflammatory drugs (NSAIDs).3-12 In most cases, diagnoses are made clinically without diagnostic testing. To identify drug allergies associated with diagnostic testing, one center selected patients with suspected cutaneous drug reactions (2006-2010) for further evaluation.13 Of 612 patients who were evaluated, 141 had a high suspicion of drug allergy and were included in the analysis. The excluded patients had pseudoallergic reactions, reactive exanthemas due to infection, histopathologic exclusion of drug allergy, angioedema, or other dermatological conditions such as contact dermatitis and eczema. Of the included patients, 107 were diagnosed with drug reactions, while the remainder had non–drug-related exanthemas or unknown etiology after testing. Identified culprit drugs were predominantly antibiotics (39.8%) and NSAIDs (21.2%); contrast media, anticoagulants, anticonvulsants, antimalarials, antifungals, glucocorticoids, antihypertensives, and proton pump inhibitors also were implicated. They were identified with skin prick, intradermal, and patch tests (62.6%); lymphocyte transformation test (17.7%); oral rechallenge (5.6%); or without skin testing (6.5%). One quarter of patients with a high suspicion for drug allergy did not have a confirmed drug eruption in this study. Another study found that 10% to 20% of patients with reported penicillin allergy had confirmation via skin prick testing.14 These findings suggest that confirmation of suspected drug allergy may require more than one diagnostic test.
Tests for Adverse Drug Reactions
The following tests have been shown to aid in the identification of cutaneous drug eruptions: (1) patch tests15-21; (2) intradermal tests14,15,19,20; (3) drug provocation tests15,20; and (4) lymphocyte transformation tests.20 Intradermal or skin prick tests are most useful in urticarial eruptions but can be considered in nonurticarial eruptions with delayed inspection of test sites up to 1 week after testing. Drug provocation tests are considered the gold standard but involve patient risk. Lymphocyte transformation tests use the principle that T lymphocytes proliferate in the presence of drugs to which the patient is sensitized. Patch tests will be discussed in greater detail below. Immunohistochemistry can determine immunologic mechanisms of eruptions but cannot identify causative agents.16,17,22
A retrospective study of patients referred for evaluation of adverse drug reactions between 1996 and 2006 found the collective negative predictive value (NPV)—the percentage of truly negative skin tests based on provocation or substitution testing—of cutaneous drug tests including patch, prick, and intradermal tests to be 89.6% (95% confidence interval, 85.9%-93.3%).23 The NPVs of each test were not reported. Patients with negative cutaneous tests had subsequent oral rechallenge or substitution testing with medication from the same drug class.23 Another study16 found the NPV of patch testing to be at least 79% after review of data from other studies using patch and provocation testing.16,24 These studies suggest that cutaneous testing can be useful, albeit imperfect, in the evaluation and diagnosis of drug allergy.
Review of the Patch Test
Patch tests can be helpful in diagnosis of delayed hypersensitivities.18 Patch testing is most commonly and effectively used to diagnose allergic contact dermatitis, but its utility in other applications, such as diagnosis of cutaneous drug eruptions, has not been extensively studied.
The development of patch tests to diagnose systemic drug allergies is inhibited by the uncertainty of percutaneous drug penetration, a dearth of studies to determine the best test concentrations of active drug in the patch test, and the potential for nonimmunologic contact urticaria upon skin exposure. Furthermore, cutaneous metabolism of many antigens is well documented, but correlation to systemic metabolism often is unknown, which can confound patch test results and lead to false-negative results when the skin’s metabolic capacity does not match the body’s capacity to generate antigens capable of eliciting immunogenic responses.21 Additionally, the method used to suspend and disperse drugs in patch test vehicles is unfamiliar to most pharmacists, and standardized concentrations and vehicles are available only for some medications.25 Studies sufficient to obtain US Food and Drug Administration approval of patch tests for systemic drug eruptions would be costly and therefore prohibitive to investigators. The majority of the literature consists of case reports and data extrapolated from reviews. Patch test results of many drugs have been reported in the literature, with the highest frequencies of positive results associated with anticonvulsants,26 antibiotics, corticosteroids, calcium channel blockers, and benzodiazepines.21
Patch test placement affects the diagnostic value of the test. Placing patch tests on previously involved sites of fixed drug eruptions improves yield over placement on uninvolved skin.27 Placing patch tests on previously involved sites of other drug eruptions such as toxic epidermal necrolysis also may aid in diagnosis, though the literature is sparse.25,26,28
Patch Testing in Drug Eruptions
Morbilliform eruptions account for 48% to 91% of patients with adverse drug reactions.4-6 Other drug eruptions include urticarial eruptions, acute generalized exanthematous pustulosis (AGEP), drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, toxic epidermal necrolysis, Stevens-Johnson syndrome, lichenoid drug eruption, symmetric drug-related intertriginous and flexural exanthema (SDRIFE), erythema multiforme (EM), and systemic contact dermatitis. The Table summarizes reports of positive patch tests with various medications for these drug eruptions.
In general, antimicrobials and NSAIDs were the most implicated drugs with positive patch test results in AGEP, DRESS syndrome, EM, fixed drug eruptions, and morbilliform eruptions. In AGEP, positive results also were reported for other drugs, including terbinafine and morphine.29-38 In fixed drug eruptions, patch testing on involved skin showed positive results to NSAIDs, analgesics, platelet inhibitors, and antimicrobials.27,52-55 Patch testing in DRESS syndrome has shown many positive reactions to antiepileptics and antipsychotics.39-43 One study used patch tests in SDRIFE, reporting positive results with antimicrobials, antineoplastics, decongestants, and glucocorticoids.61 Nonsteroidal anti-inflammatory drugs, antimicrobials, calcium channel blockers, and histamine antagonists were implicated in EM.47-51 Positive patch tests were seen in morbilliform eruptions with selective serotonin reuptake inhibitors, antiepileptics/benzodiazepines, NSAIDs, and antimicrobials.28,57-60 In toxic epidermal necrolysis, diagnosis with patch testing was made using patches placed on previously involved skin with sulfamethoxazole.62
Systemic Contact Dermatitis
Drugs historically recognized as causing allergic contact dermatitis (eg, topical gentamycin) can cause systemic contact dermatitis, which can be patch tested. In these situations, systemic contact dermatitis may be due to either the active drug or excipients in the medication formulation. Excipients are inactive ingredients in medications that provide a suitable consistency, appearance, or form. Often overlooked as culprits of drug hypersensitivity because they are theoretically inert, excipients are increasingly implicated in drug allergy. Swerlick and Campbell63 described 11 cases in which chronic unexplained pruritus responded to medication changes to avoid coloring agents. The most common culprits were FD&C Blue No. 1 and FD&C Blue No. 2. Patch testing for allergies to dyes can be clinically useful, though a lack of commercially available patch tests makes diagnosis difficult.64
Other excipients can cause cutaneous reactions. Propylene glycol, commonly implicated in allergic contact dermatitis, also can cause cutaneous eruptions upon systemic exposure.65 Corticosteroid-induced systemic contact dermatitis has been reported, though it is less prevalent than allergic contact dermatitis.66 These reactions usually are due to nonmethylated and nonhalogenated corticosteroids including budesonide, cortisone, hydrocortisone, prednisolone, and methylprednisolone.67,68 Patch testing in these situations is complicated by the possibility of false-negative results due to the anti-inflammatory effects of the corticosteroids. Therefore, patch testing should be performed using standardized and not treatment concentrations.
In our clinic, we have anecdotally observed several patients with chronic dermatitis and suspected NSAID allergies have positive patch test results with propylene glycol and not the suspected drug. Excipients encountered in multiple drugs and foods are more likely to present as chronic dermatitis, while active drug ingredients started in hospital settings more often present as acute dermatitis.

Our Experience
We have patch tested a handful of patients with suspected drug eruptions (University Hospitals Cleveland Medical Center institutional review board #07-12-27). Medications, excipients, and their concentrations (in % weight per weight) and vehicles that were tested include ibuprofen (10% petrolatum), aspirin (10% petrolatum), hydrochlorothiazide (10% petrolatum), captopril (5% petrolatum), and propylene glycol (30% water or 5% petrolatum). Patch tests were read at 48 and 72 hours and scored according to the International Contact Dermatitis Research Group patch test scoring guidelines.69 Two patients tested for ibuprofen reacted positively only to propylene glycol; the 3 other patients did not react to aspirin, hydrochlorothiazide, and captopril. Overall, we observed no positive patch tests to medications and 2 positive tests to propylene glycol in 5 patients tested (unpublished data).
Areas of Uncertainty
Although tests for immediate-type hypersensitivity reactions to drugs exist as skin prick tests, diagnostic testing for the majority of drug reactions does not exist. Drug allergy diagnosis is made with history and temporality, potentially resulting in unnecessary avoidance of helpful medications. Ideal patch test concentrations and vehicles as well as the sensitivity and specificity of these tests are unknown.
Guidelines From Professional Societies
Drug allergy testing guidelines are available from the British Society for Allergy and Clinical Immunology70 and American Academy of Allergy, Asthma and Immunology.71 The guidelines recommend diagnosis by history and temporality, and it is stated that patch testing is potentially useful in maculopapular rashes, AGEP, fixed drug eruptions, and DRESS syndrome.
Conclusion
Case reports in the literature suggest the utility of patch testing in some drug allergies. We suggest testing excipients such as propylene glycol and benzoic acid to rule out systemic contact dermatitis when patch testing with active drugs to confirm cause of suspected adverse cutaneous reactions to medications.
- Arndt KA, Jick H. Rates of cutaneous reactions to drugs. a report from the Boston Collaborative Drug Surveillance Program. JAMA. 1976;235:918-922.
- Bigby M, Jick S, Jick H, et al. Drug-induced cutaneous reactions. a report from the Boston Collaborative Drug Surveillance Program on 15,483 consecutive inpatients, 1975 to 1982. JAMA. 1986;256:3358-3363.
- Fiszenson-Albala F, Auzerie V, Mahe E, et al. A 6-month prospective survey of cutaneous drug reactions in a hospital setting. Br J Dermatol. 2003;149:1018-1022.
- Thong BY, Leong KP, Tang CY, et al. Drug allergy in a general hospital: results of a novel prospective inpatient reporting system. Ann Allergy Asthma Immunol. 2003;90:342-347.
- Hunziker T, Kunzi UP, Braunschweig S, et al. Comprehensive hospital drug monitoring (CHDM): adverse skin reactions, a 20-year survey. Allergy. 1997;52:388-393.
- Swanbeck G, Dahlberg E. Cutaneous drug reactions. an attempt to quantitative estimation. Arch Dermatol Res. 1992;284:215-218.
- Naldi L, Conforti A, Venegoni M, et al. Cutaneous reactions to drugs. an analysis of spontaneous reports in four Italian regions. Br J Clin Pharmacol. 1999;48:839-846.
- French LE, Prins C. Erythema multiforme, Stevens-Johnson syndrome and toxic epidermal necrolysis. In: Bolognia, JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012:319-333.
- Vasconcelos C, Magina S, Quirino P, et al. Cutaneous drug reactions to piroxicam. Contact Dermatitis. 1998;39:145.
- Gerber D. Adverse reactions of piroxicam. Drug Intell Clin Pharm. 1987;21:707-710.
- Revuz J, Valeyrie-Allanore L. Drug reactions. In: Bolognia, JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012:335-356.
- Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: part II. management and therapeutics. J Am Acad Dermatol. 2013;68:709.e1-709.e9; quiz 718-720.
- Heinzerling LM, Tomsitz D, Anliker MD. Is drug allergy less prevalent than previously assumed? a 5-year analysis. Br J Dermatol. 2012;166:107-114.
- Salkind AR, Cuddy PG. Is this patient allergic to penicillin?: an evidence-based analysis of the likelihood of penicillin allergy. JAMA. 2001;285:2498-2505.
- Torres MJ, Gomez F, Doña I, et al. Diagnostic evaluation of patients with nonimmediate cutaneous hypersensitivity reactions to iodinated contrast media. Allergy. 2012;67:929-935.
- Cham PM, Warshaw EM. Patch testing for evaluating drug reactions due to systemic antibiotics. Dermatitis. 2007;18:63-77.
- Andrade P, Brinca A, Gonçalo M. Patch testing in fixed drug eruptions—a 20-year review. Contact Dermatitis. 2011;65:195-201.
- Romano A, Viola M, Gaeta F, et al. Patch testing in non-immediate drug eruptions. Allergy Asthma Clin Immunol. 2008;4:66-74.
- Rosso R, Mattiacci G, Bernardi ML, et al. Very delayed reactions to beta-lactam antibiotics. Contact Dermatitis. 2000;42:293-295.
- Romano A, Torres MJ, Castells M, et al. Diagnosis and management of drug hypersensitivity reactions. J Allergy Clin Immunol. 2011;127(3 suppl):S67-S73.
- Friedmann PS, Ardern-Jones M. Patch testing in drug allergy. Curr Opin Allergy Clin Immunol. 2010;10:291-296.
- Torres MJ, Mayorga C, Blanca M. Nonimmediate allergic reactions induced by drugs: pathogenesis and diagnostic tests. J Investig Allergol Clin Immunol. 2009;19:80-90.
- Waton J, Tréchot P, Loss-Ayay C, et al. Negative predictive value of drug skin tests in investigating cutaneous adverse drug reactions. Br J Dermatol. 2009;160:786-794.
- Romano A, Viola M, Mondino C, et al. Diagnosing nonimmediate reactions to penicillins by in vivo tests. Int Arch Allergy Immunol. 2002;129:169-174.
- De Groot AC. Patch Testing. Test Concentrations and Vehicles for 4350 Chemicals. 3rd ed. Wapserveen, Netherlands: acdegroot publishing; 2008.
- Elzagallaai AA, Knowles SR, Rieder MJ, et al. Patch testing for the diagnosis of anticonvulsant hypersensitivity syndrome: a systematic review. Drug Saf. 2009;32:391-408.
- Andrade P, Gonçalo M. Fixed drug eruption caused by etoricoxib—2 cases confirmed by patch testing. Contact Dermatitis. 2011;64:118-120.
- Barbaud A, Reichert-Penetrat S, Tréchot P, et al. The use of skin testing in the investigation of cutaneous adverse drug reactions. Br J Dermatol. 1998;139:49-58.
- Wolkenstein P, Chosidow O, Fléchet ML, et al. Patch testing in severe cutaneous adverse drug reactions, including Stevens-Johnson syndrome and toxic epidermal necrolysis. Contact Dermatitis. 1996;35:234-236.
- Harries MJ, McIntyre SJ, Kingston TP. Co-amoxiclav-induced acute generalized exanthematous pustulosis confirmed by patch testing. Contact Dermatitis. 2006;55:372.
- Matsumoto Y, Okubo Y, Yamamoto T, et al. Case of acute generalized exanthematous pustulosis caused by ampicillin/cloxacillin sodium in a pregnant woman. J Dermatol. 2008;35:362-364.
- Chaabane A, Aouam K, Gassab L, et al. Acute generalized exanthematous pustulosis (AGEP) induced by cefotaxime. Fundam Clin Pharmacol. 2010;24:429-432.
- Hausermann P, Scherer K, Weber M, et al. Ciprofloxacin-induced acute generalized exanthematous pustulosis mimicking bullous drug eruption confirmed by a positive patch test. Dermatology. 2005;211:277-280.
- Moreau A, Dompmartin A, Castel B, et al. Drug-induced acute generalized exanthematous pustulosis with positive patch tests. Int J Dermatol. 1995;34:263-266.
- Kempinaire A, De Raevea L, Merckx M, et al. Terbinafine-induced acute generalized exanthematous pustulosis confirmed by a positive patch-test result. J Am Acad Dermatol. 1997;37:653-655.
- Mäkelä L, Lammintausta K. Etoricoxib-induced acute generalized exanthematous pustulosis. Acta Derm Venereol. 2008;88:200-201.
- Yang CC, Lee JY, Chen WC. Acute generalized exanthematous pustulosis caused by celecoxib. J Formos Med Assoc. 2004;103:555-557.
- Kardaun SH, de Monchy JG. Acute generalized exanthematous pustulosis caused by morphine, confirmed by positive patch test and lymphocyte transformation test. J Am Acad Dermatol. 2006;55(2 suppl):S21-S23.
- Inadomi T. Drug rash with eosinophilia and systemic symptoms (DRESS): changing carbamazepine to phenobarbital controlled epilepsy without the recurrence of DRESS. Eur J Dermatol. 2010;20:220-222.
- Buyuktiryaki AB, Bezirganoglu H, Sahiner UM, et al. Patch testing is an effective method for the diagnosis of carbamazepine-induced drug reaction, eosinophilia and systemic symptoms (DRESS) syndrome in an 8-year-old girl. Australas J Dermatol. 2012;53:274-277.
- Aouam K, Ben Romdhane F, Loussaief C, et al. Hypersensitivity syndrome induced by anticonvulsants: possible cross-reactivity between carbamazepine and lamotrigine. J Clin Pharmacol. 2009;49:1488-1491.
- Santiago F, Gonçalo M, Vieira R, et al. Epicutaneous patch testing in drug hypersensitivity syndrome (DRESS). Contact Dermatitis. 2010;62:47-53.
- Prevost P, Bédry R, Lacoste D, et al. Hypersensitivity syndrome with olanzapine confirmed by patch tests. Eur J Dermatol. 2012;22:126-127.
- Hubiche T, Milpied B, Cazeau C, et al. Association of immunologically confirmed delayed drug reaction and human herpesvirus 6 viremia in a pediatric case of drug-induced hypersensitivity syndrome. Dermatology. 2011;222:140-141.
- Song WJ, Shim EJ, Kang MG, et al. Severe drug hypersensitivity induced by erdosteine and doxofylline as confirmed by patch and lymphocyte transformation tests: a case report. J Investig Allergol Clin Immunol. 2012;22:230-232.
- Lee JH, Park HK, Heo J, et al. Drug rash with eosinophilia and systemic symptoms (DRESS) syndrome induced by celecoxib and anti-tuberculosis drugs. J Korean Med Sci. 2008;23:521-525.
- González-Delgado P, Blanes M, Soriano V, et al. Erythema multiforme to amoxicillin with concurrent infection by Epstein-Barr virus. Allergol Immunopathol. 2006;34:76-78.
- Gonzalo Garijo MA, Pérez Calderón R, de Argila Fernández-Durán D, et al. Cutaneous reactions due to diltiazem and cross reactivity with other calcium channel blockers. Allergol Immunopathol (Madr). 2005;33:238-240.
- Peña AL, Henriquezsantana A, Gonzalez-Seco E, et al. Exudative erythema multiforme induced by hydroxyzine. Eur J Dermatol. 2008;18:194-195.
- Arakawa Y, Nakai N, Katoh N. Celecoxib-induced erythema multiforme-type drug eruption with a positive patch test. J Dermatol. 2011;38:1185-1188.
- Prieto A, De barrio M, Pérez C, et al. Piroxicam-induced erythema multiforme. Contact Dermatitis. 2004;50:263.
- Dalmau J, Serra-baldrich E, Roé E, et al. Use of patch test in fixed drug eruption due to metamizole (Nolotil). Contact Dermatitis. 2006;54:127-128.
- Gastaminza G, Anda M, Audicana MT, et al. Fixed-drug eruption due to metronidazole with positive topical provocation. Contact Dermatitis. 2001;44:36.
- Bellini V, Stingeni L, Lisi P. Multifocal fixed drug eruption due to celecoxib. Dermatitis. 2009;20:174-176.
- García CM, Carmena R, García R, et al. Fixed drug eruption from ticlopidine, with positive lesional patch test. Contact Dermatitis. 2001;44:40-41.
- Cruz MJ, Duarte AF, Baudrier T, et al. Lichenoid drug eruption induced by misoprostol. Contact Dermatitis. 2009;61:240-242.
- Alanko K. Patch testing in cutaneous reactions caused by carbamazepine. Contact Dermatitis. 1993;29:254-257.
- Grob M, Scheidegger P, Wüthrich B. Allergic skin reaction to celecoxib. Dermatology. 2000;201:383.
- Alonso JC, Ortega JD, Gonzalo MJ. Cutaneous reaction to oral celecoxib with positive patch test. Contact Dermatitis. 2004;50:48-49.
- Fernandes B, Brites M, Gonçalo M, et al. Maculopapular eruption from sertraline with positive patch tests. Contact Dermatitis. 2000;42:287.
- Häusermann P, Harr T, Bircher AJ. Baboon syndrome resulting from systemic drugs: is there strife between SDRIFE and allergic contact dermatitis syndrome? Contact Dermatitis. 2004;51:297-310.
- Klein CE, Trautmann A, Zillikens D, et al. Patch testing in an unusual case of toxic epidermal necrolysis. Contact Dermatitis. 1996;35:175-176.
- Swerlick RA, Campbell CF. Medication dyes as a source of drug allergy. J Drugs Dermatol. 2013;12:99-102.
- Guin JD. Patch testing to FD&C and D&C dyes. Contact Dermatitis. 2003;49:217-218.
- Lowther A, McCormick T, Nedorost S. Systemic contact dermatitis from propylene glycol. Dermatitis. 2008;19:105-108.
- Baeck M, Goossens A. Systemic contact dermatitis to corticosteroids. Allergy. 2012;67:1580-1585.
- Baeck M, Goossens A. Immediate and delayed allergic hypersensitivity to corticosteroids: practical guidelines. Contact Dermatitis. 2012;66:38-45.
- Basedow S, Eigelshoven S, Homey B. Immediate and delayed hypersensitivity to corticosteroids. J Dtsch Dermatol Ges. 2011;9:885-888.
- Johansen JD, Aalto-korte K, Agner T, et al. European Society of Contact Dermatitis guideline for diagnostic patch testing—recommendations on best practice. Contact Dermatitis. 2015;73:195-221.
- Mirakian R, Ewan PW, Durham SR, et al. BSACI guidelines for the management of drug allergy. Clin Exp Allergy. 2009;39:43-61.
- Joint Task Force on Practice Parameters; American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105:259-273.
- Arndt KA, Jick H. Rates of cutaneous reactions to drugs. a report from the Boston Collaborative Drug Surveillance Program. JAMA. 1976;235:918-922.
- Bigby M, Jick S, Jick H, et al. Drug-induced cutaneous reactions. a report from the Boston Collaborative Drug Surveillance Program on 15,483 consecutive inpatients, 1975 to 1982. JAMA. 1986;256:3358-3363.
- Fiszenson-Albala F, Auzerie V, Mahe E, et al. A 6-month prospective survey of cutaneous drug reactions in a hospital setting. Br J Dermatol. 2003;149:1018-1022.
- Thong BY, Leong KP, Tang CY, et al. Drug allergy in a general hospital: results of a novel prospective inpatient reporting system. Ann Allergy Asthma Immunol. 2003;90:342-347.
- Hunziker T, Kunzi UP, Braunschweig S, et al. Comprehensive hospital drug monitoring (CHDM): adverse skin reactions, a 20-year survey. Allergy. 1997;52:388-393.
- Swanbeck G, Dahlberg E. Cutaneous drug reactions. an attempt to quantitative estimation. Arch Dermatol Res. 1992;284:215-218.
- Naldi L, Conforti A, Venegoni M, et al. Cutaneous reactions to drugs. an analysis of spontaneous reports in four Italian regions. Br J Clin Pharmacol. 1999;48:839-846.
- French LE, Prins C. Erythema multiforme, Stevens-Johnson syndrome and toxic epidermal necrolysis. In: Bolognia, JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012:319-333.
- Vasconcelos C, Magina S, Quirino P, et al. Cutaneous drug reactions to piroxicam. Contact Dermatitis. 1998;39:145.
- Gerber D. Adverse reactions of piroxicam. Drug Intell Clin Pharm. 1987;21:707-710.
- Revuz J, Valeyrie-Allanore L. Drug reactions. In: Bolognia, JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012:335-356.
- Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: part II. management and therapeutics. J Am Acad Dermatol. 2013;68:709.e1-709.e9; quiz 718-720.
- Heinzerling LM, Tomsitz D, Anliker MD. Is drug allergy less prevalent than previously assumed? a 5-year analysis. Br J Dermatol. 2012;166:107-114.
- Salkind AR, Cuddy PG. Is this patient allergic to penicillin?: an evidence-based analysis of the likelihood of penicillin allergy. JAMA. 2001;285:2498-2505.
- Torres MJ, Gomez F, Doña I, et al. Diagnostic evaluation of patients with nonimmediate cutaneous hypersensitivity reactions to iodinated contrast media. Allergy. 2012;67:929-935.
- Cham PM, Warshaw EM. Patch testing for evaluating drug reactions due to systemic antibiotics. Dermatitis. 2007;18:63-77.
- Andrade P, Brinca A, Gonçalo M. Patch testing in fixed drug eruptions—a 20-year review. Contact Dermatitis. 2011;65:195-201.
- Romano A, Viola M, Gaeta F, et al. Patch testing in non-immediate drug eruptions. Allergy Asthma Clin Immunol. 2008;4:66-74.
- Rosso R, Mattiacci G, Bernardi ML, et al. Very delayed reactions to beta-lactam antibiotics. Contact Dermatitis. 2000;42:293-295.
- Romano A, Torres MJ, Castells M, et al. Diagnosis and management of drug hypersensitivity reactions. J Allergy Clin Immunol. 2011;127(3 suppl):S67-S73.
- Friedmann PS, Ardern-Jones M. Patch testing in drug allergy. Curr Opin Allergy Clin Immunol. 2010;10:291-296.
- Torres MJ, Mayorga C, Blanca M. Nonimmediate allergic reactions induced by drugs: pathogenesis and diagnostic tests. J Investig Allergol Clin Immunol. 2009;19:80-90.
- Waton J, Tréchot P, Loss-Ayay C, et al. Negative predictive value of drug skin tests in investigating cutaneous adverse drug reactions. Br J Dermatol. 2009;160:786-794.
- Romano A, Viola M, Mondino C, et al. Diagnosing nonimmediate reactions to penicillins by in vivo tests. Int Arch Allergy Immunol. 2002;129:169-174.
- De Groot AC. Patch Testing. Test Concentrations and Vehicles for 4350 Chemicals. 3rd ed. Wapserveen, Netherlands: acdegroot publishing; 2008.
- Elzagallaai AA, Knowles SR, Rieder MJ, et al. Patch testing for the diagnosis of anticonvulsant hypersensitivity syndrome: a systematic review. Drug Saf. 2009;32:391-408.
- Andrade P, Gonçalo M. Fixed drug eruption caused by etoricoxib—2 cases confirmed by patch testing. Contact Dermatitis. 2011;64:118-120.
- Barbaud A, Reichert-Penetrat S, Tréchot P, et al. The use of skin testing in the investigation of cutaneous adverse drug reactions. Br J Dermatol. 1998;139:49-58.
- Wolkenstein P, Chosidow O, Fléchet ML, et al. Patch testing in severe cutaneous adverse drug reactions, including Stevens-Johnson syndrome and toxic epidermal necrolysis. Contact Dermatitis. 1996;35:234-236.
- Harries MJ, McIntyre SJ, Kingston TP. Co-amoxiclav-induced acute generalized exanthematous pustulosis confirmed by patch testing. Contact Dermatitis. 2006;55:372.
- Matsumoto Y, Okubo Y, Yamamoto T, et al. Case of acute generalized exanthematous pustulosis caused by ampicillin/cloxacillin sodium in a pregnant woman. J Dermatol. 2008;35:362-364.
- Chaabane A, Aouam K, Gassab L, et al. Acute generalized exanthematous pustulosis (AGEP) induced by cefotaxime. Fundam Clin Pharmacol. 2010;24:429-432.
- Hausermann P, Scherer K, Weber M, et al. Ciprofloxacin-induced acute generalized exanthematous pustulosis mimicking bullous drug eruption confirmed by a positive patch test. Dermatology. 2005;211:277-280.
- Moreau A, Dompmartin A, Castel B, et al. Drug-induced acute generalized exanthematous pustulosis with positive patch tests. Int J Dermatol. 1995;34:263-266.
- Kempinaire A, De Raevea L, Merckx M, et al. Terbinafine-induced acute generalized exanthematous pustulosis confirmed by a positive patch-test result. J Am Acad Dermatol. 1997;37:653-655.
- Mäkelä L, Lammintausta K. Etoricoxib-induced acute generalized exanthematous pustulosis. Acta Derm Venereol. 2008;88:200-201.
- Yang CC, Lee JY, Chen WC. Acute generalized exanthematous pustulosis caused by celecoxib. J Formos Med Assoc. 2004;103:555-557.
- Kardaun SH, de Monchy JG. Acute generalized exanthematous pustulosis caused by morphine, confirmed by positive patch test and lymphocyte transformation test. J Am Acad Dermatol. 2006;55(2 suppl):S21-S23.
- Inadomi T. Drug rash with eosinophilia and systemic symptoms (DRESS): changing carbamazepine to phenobarbital controlled epilepsy without the recurrence of DRESS. Eur J Dermatol. 2010;20:220-222.
- Buyuktiryaki AB, Bezirganoglu H, Sahiner UM, et al. Patch testing is an effective method for the diagnosis of carbamazepine-induced drug reaction, eosinophilia and systemic symptoms (DRESS) syndrome in an 8-year-old girl. Australas J Dermatol. 2012;53:274-277.
- Aouam K, Ben Romdhane F, Loussaief C, et al. Hypersensitivity syndrome induced by anticonvulsants: possible cross-reactivity between carbamazepine and lamotrigine. J Clin Pharmacol. 2009;49:1488-1491.
- Santiago F, Gonçalo M, Vieira R, et al. Epicutaneous patch testing in drug hypersensitivity syndrome (DRESS). Contact Dermatitis. 2010;62:47-53.
- Prevost P, Bédry R, Lacoste D, et al. Hypersensitivity syndrome with olanzapine confirmed by patch tests. Eur J Dermatol. 2012;22:126-127.
- Hubiche T, Milpied B, Cazeau C, et al. Association of immunologically confirmed delayed drug reaction and human herpesvirus 6 viremia in a pediatric case of drug-induced hypersensitivity syndrome. Dermatology. 2011;222:140-141.
- Song WJ, Shim EJ, Kang MG, et al. Severe drug hypersensitivity induced by erdosteine and doxofylline as confirmed by patch and lymphocyte transformation tests: a case report. J Investig Allergol Clin Immunol. 2012;22:230-232.
- Lee JH, Park HK, Heo J, et al. Drug rash with eosinophilia and systemic symptoms (DRESS) syndrome induced by celecoxib and anti-tuberculosis drugs. J Korean Med Sci. 2008;23:521-525.
- González-Delgado P, Blanes M, Soriano V, et al. Erythema multiforme to amoxicillin with concurrent infection by Epstein-Barr virus. Allergol Immunopathol. 2006;34:76-78.
- Gonzalo Garijo MA, Pérez Calderón R, de Argila Fernández-Durán D, et al. Cutaneous reactions due to diltiazem and cross reactivity with other calcium channel blockers. Allergol Immunopathol (Madr). 2005;33:238-240.
- Peña AL, Henriquezsantana A, Gonzalez-Seco E, et al. Exudative erythema multiforme induced by hydroxyzine. Eur J Dermatol. 2008;18:194-195.
- Arakawa Y, Nakai N, Katoh N. Celecoxib-induced erythema multiforme-type drug eruption with a positive patch test. J Dermatol. 2011;38:1185-1188.
- Prieto A, De barrio M, Pérez C, et al. Piroxicam-induced erythema multiforme. Contact Dermatitis. 2004;50:263.
- Dalmau J, Serra-baldrich E, Roé E, et al. Use of patch test in fixed drug eruption due to metamizole (Nolotil). Contact Dermatitis. 2006;54:127-128.
- Gastaminza G, Anda M, Audicana MT, et al. Fixed-drug eruption due to metronidazole with positive topical provocation. Contact Dermatitis. 2001;44:36.
- Bellini V, Stingeni L, Lisi P. Multifocal fixed drug eruption due to celecoxib. Dermatitis. 2009;20:174-176.
- García CM, Carmena R, García R, et al. Fixed drug eruption from ticlopidine, with positive lesional patch test. Contact Dermatitis. 2001;44:40-41.
- Cruz MJ, Duarte AF, Baudrier T, et al. Lichenoid drug eruption induced by misoprostol. Contact Dermatitis. 2009;61:240-242.
- Alanko K. Patch testing in cutaneous reactions caused by carbamazepine. Contact Dermatitis. 1993;29:254-257.
- Grob M, Scheidegger P, Wüthrich B. Allergic skin reaction to celecoxib. Dermatology. 2000;201:383.
- Alonso JC, Ortega JD, Gonzalo MJ. Cutaneous reaction to oral celecoxib with positive patch test. Contact Dermatitis. 2004;50:48-49.
- Fernandes B, Brites M, Gonçalo M, et al. Maculopapular eruption from sertraline with positive patch tests. Contact Dermatitis. 2000;42:287.
- Häusermann P, Harr T, Bircher AJ. Baboon syndrome resulting from systemic drugs: is there strife between SDRIFE and allergic contact dermatitis syndrome? Contact Dermatitis. 2004;51:297-310.
- Klein CE, Trautmann A, Zillikens D, et al. Patch testing in an unusual case of toxic epidermal necrolysis. Contact Dermatitis. 1996;35:175-176.
- Swerlick RA, Campbell CF. Medication dyes as a source of drug allergy. J Drugs Dermatol. 2013;12:99-102.
- Guin JD. Patch testing to FD&C and D&C dyes. Contact Dermatitis. 2003;49:217-218.
- Lowther A, McCormick T, Nedorost S. Systemic contact dermatitis from propylene glycol. Dermatitis. 2008;19:105-108.
- Baeck M, Goossens A. Systemic contact dermatitis to corticosteroids. Allergy. 2012;67:1580-1585.
- Baeck M, Goossens A. Immediate and delayed allergic hypersensitivity to corticosteroids: practical guidelines. Contact Dermatitis. 2012;66:38-45.
- Basedow S, Eigelshoven S, Homey B. Immediate and delayed hypersensitivity to corticosteroids. J Dtsch Dermatol Ges. 2011;9:885-888.
- Johansen JD, Aalto-korte K, Agner T, et al. European Society of Contact Dermatitis guideline for diagnostic patch testing—recommendations on best practice. Contact Dermatitis. 2015;73:195-221.
- Mirakian R, Ewan PW, Durham SR, et al. BSACI guidelines for the management of drug allergy. Clin Exp Allergy. 2009;39:43-61.
- Joint Task Force on Practice Parameters; American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105:259-273.
Practice Points
- Consider patch testing in suspected eczematous drug rashes and fixed drug eruption.
- Patch test to inactive excipients as well as active ingredients.
- Caution patients that sensitivity of patch testing for systemic drug reactions is unknown and likely lower than specificity.