User login
Adenomyosis: Why we need to reassess our understanding of this condition
CASE Painful, heavy menstruation and recurrent pregnancy loss
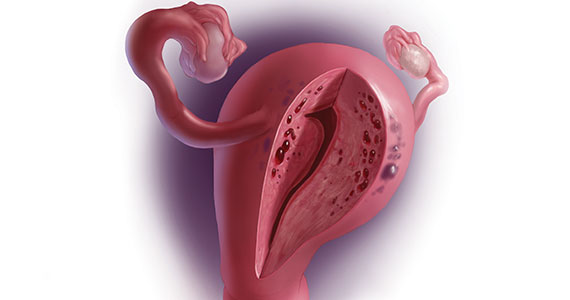
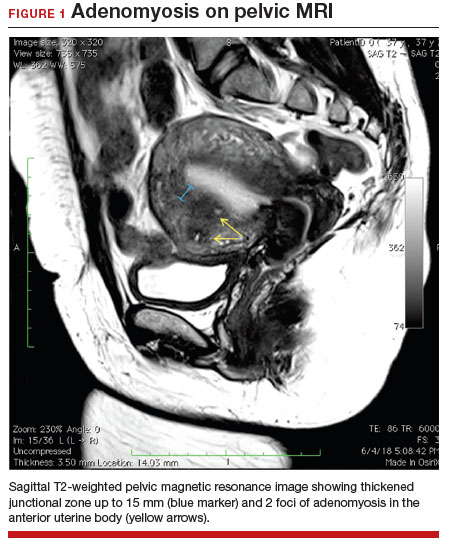
A 37-year-old woman (G3P0030) with a history of recurrent pregnancy loss presents for evaluation. She had 3 losses—most recently a miscarriage at 22 weeks with a cerclage in place. She did not undergo any surgical procedures for these losses. Hormonal and thrombophilia workup is negative and semen analysis is normal. She reports a history of painful, heavy periods for many years, as well as dyspareunia and occasional post-coital bleeding. Past medical history was otherwise unremarkable. Pelvic magnetic resonance imaging (MRI) revealed focal thickening of the junctional zone up to 15 mm with 2 foci of T2 hyperintensities suggesting adenomyosis (FIGURE 1).
How do you counsel this patient regarding the MRI findings and their impact on her fertility?
Adenomyosis is a condition in which endometrial glands and stroma are abnormally present in the uterine myometrium, resulting in smooth muscle hypertrophy and abnormal uterine contractility. Traditional teaching describes a woman in her 40s with heavy and painful menses, a “boggy uterus” on examination, who has completed childbearing and desires definitive treatment. Histologic diagnosis of adenomyosis is made from the uterine specimen at the time of hysterectomy, invariably confounding our understanding of the epidemiology of adenomyosis.
More recently, however, we are beginning to learn that this narrative is misguided. Imaging changes of adenomyosis can be seen in women who desire future fertility and in adolescents with severe dysmenorrhea, suggesting an earlier age of incidence.1 In a recent systematic review, prevalence estimates ranged from 15% to 67%, owing to varying diagnostic methods and patient inclusion criteria.2 It is increasingly being recognized as a primary contributor to infertility, with one study estimating a 30% prevalence of infertility in women with adenomyosis.3 Moreover, treatment with gonadotropin-releasing hormone agonists and/or surgical excision may improve fertility outcomes.4
As we learn more about this prevalent and life-altering condition, we owe it to our patients to consider this diagnosis when counseling on dysmenorrhea, heavy menstrual bleeding, or infertility.
Anatomy of the myometrium
The myometrium is composed of the inner and outer myometrium: the inner myometrium (IM) and endometrium are of Müllerian origin, and the outer myometrium (OM) is of mesenchymal origin. The IM thickens in response to steroid hormones during the menstrual cycle with metaplasia of endometrial stromal cells into myocytes and back again, whereas the OM is not responsive to hormones.5 Emerging literature suggests the OM is further divided into a middle and outer section based on different histologic morphologies, though the clinical implications of this are not understood.6 The term “junctional zone” (JZ) refers to the imaging appearance of what is thought to be the IM. Interestingly it cannot be identified on traditional hematoxylin and eosin staining. When the JZ is thickened or demonstrates irregular borders, it is used as a diagnostic marker for adenomyosis and is postulated to play an important role in adenomyosis pathophysiology, particularly heavy menstrual bleeding and infertility.7
Continue to: Subtypes of adenomyosis...
Subtypes of adenomyosis
While various disease classifications have been suggested for adenomyosis, to date there is no international consensus. Adenomyosis is typically described in 3 forms: diffuse, focal, or adenomyoma.8 As implied, the term focal adenomyosis refers to discrete lesions surrounded by normal myometrium, whereas abnormal glandular changes are pervasive throughout the myometrium in diffuse disease. Adenomyomas are a subgroup of focal adenomyosis that are thought to be surrounded by leiomyomatous smooth muscle and may be well demarcated on imaging.9
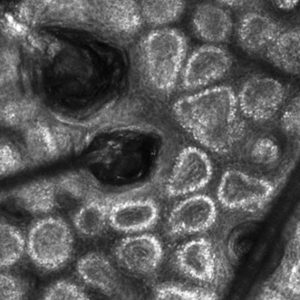
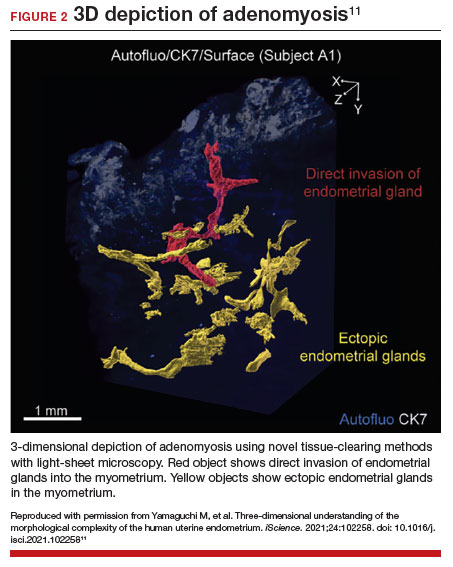
Recent research uses novel histologic imaging techniques to explore adenomyotic growth patterns in 3-dimensional (3D) reconstructions. Combining tissue-clearing methods with light-sheet fluorescence microscopy enables highly detailed 3D representations of the protein and nucleic acid structure of organs.10 For example, Yamaguchi and colleagues used this technology to explore the 3D morphological features of adenomyotic tissue and observed direct invasion of the endometrial glands into the myometrium and an “ant colony ̶ like network” of ectopic endometrial glands in the myometrium (FIGURE 2).11 These abnormal glandular networks have been visualized beyond the IM, which may not be captured on ultrasonography or MRI. While this work is still in its infancy, it has the potential to provide important insight into disease pathogenesis and to inform future therapy.
Pathogenesis
Proposed mechanisms for the development of adenomyosis include endometrial invasion, tissue injury and repair (TIAR) mechanisms, and the stem cell theory.12 According to the endometrial invasion theory, glandular epithelial cells from the basalis layer invaginate through an altered IM, slipping through weak muscle fibers and attracted by certain growth factors. In the TIAR mechanism theory, micro- or macro-trauma to the IM (whether from pregnancy, surgery, or infection) results in chronic proliferation and inflammation leading to the development of adenomyosis. Finally, the stem cell theory proposes that adenomyosis might develop from de novo ectopic endometrial tissue.
While the exact pathogenesis of adenomyosis is largely unknown, it has been associated with predictable molecular changes in the endometrium and surrounding myometrium.12 Myometrial hypercontractility is seen in patients with adenomyosis and dysmenorrhea, whereas neovascularization, high microvessel density, and abnormal uterine contractility are seen in those with abnormal uterine bleeding.13 In patients with infertility, increased inflammation, abnormal endometrial receptivity, and alterations in the myometrial architecture have been suggested to impair contractility and sperm transport.12,14
Differential growth factor expression and abnormal estrogen and progesterone signaling pathways have been observed in the IM in patients with adenomyosis, along with dysregulation of immune factors and increased inflammatory oxidative stress.12 This in turn results in myometrial hypertrophy and fibrosis, impairing normal uterine contractility patterns. This abnormal contractility may alter sperm transport and embryo implantation, and animal models that target pathways leading to fibrosis may improve endometrial receptivity.14,15 Further research is needed to elucidate specific molecular pathways and their complex interplay in this disease.
Continue to: Diagnosis...
Diagnosis
The gold standard for diagnosis of adenomyosis is histopathology from hysterectomy specimens, but specific definitions vary. Published criteria include endometrial glands within the myometrial layer greater than 0.5 to 1 low power field from the basal layer of the endometrium, endometrial glands extending deeper than 25% of the myometrial thickness, or endometrial glands a certain distance (ranging from 1-3 mm) from the basalis layer of the endometrium.16 Various methods of non-hysterectomy tissue sampling have been proposed for diagnosis, including needle, hysteroscopic, or laparoscopic sampling, but the sensitivity of these methods is poor.17 Limiting the diagnosis of adenomyosis to specimen pathology relies on invasive methods and clearly we cannot confirm the diagnosis by hysterectomy in patients with a desire for future fertility. It is for this reason that the prevalence of the disease is widely unknown.
The alternative to pathologic diagnosis is to identify radiologic changes that are associated with adenomyosis via either transvaginal ultrasound (TVUS) or MRI. Features suggestive of adenomyosis on MRI overlap with TVUS features, including uterine enlargement, anteroposterior myometrial asymmetry, T1- or T2-intense myometrial cysts or foci, and a thickened JZ.18 A JZ thicker than 12 mm has been thought to be predictive of adenomyosis, whereas a thickness of less than 8 mm is predictive of its absence, although the JZ may vary in thickness with the menstrual cycle.19,20 A 2021 systematic review and meta-analysis comparing MRI diagnosis with histopathologic findings reported a pooled sensitivity and specificity of 60% and 96%, respectively.21 The reported range for sensitivity and specificity is wide: 70% to 93% for sensitivity and 67% to 93% for specificity.22-24
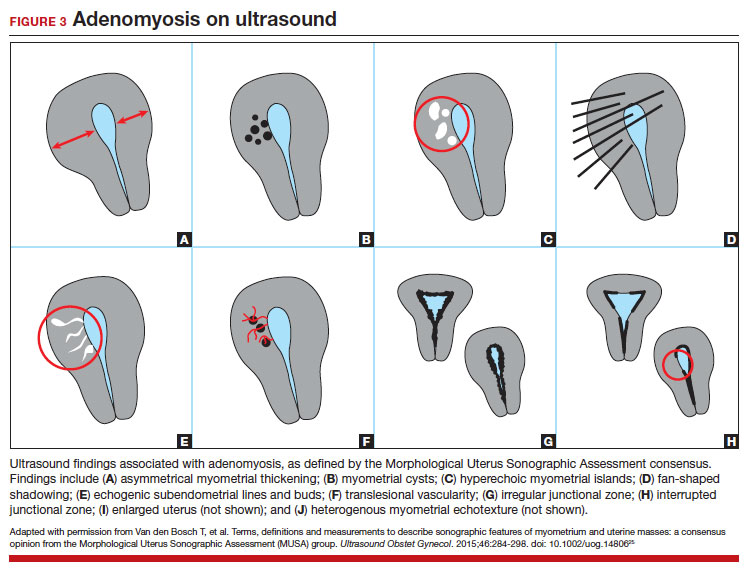
Key TVUS features associated with adenomyosis were defined in 2015 in a consensus statement released by the Morphological Uterus Sonographic Assessment (MUSA) group.25 These include a globally enlarged uterus, anteroposterior myometrial asymmetry, myometrial cysts, fan-shaped shadowing, mixed myometrial echogenicity, translesional vascularity, echogenic subendometrial lines and buds, and a thickened, irregular or discontinuous JZ (FIGURES 3 and 4).25 The accuracy of ultrasonographic diagnosis of adenomyosis using these features has been investigated in multiple systematic reviews and meta-analyses, most recently by Liu and colleagues who found a pooled sensitivity of TVUS of 81% and pooled specificity of 87%.23 The range for ultrasonographic sensitivity and specificity is wide, however, ranging from 33% to 84% for sensitivity and 64% to 100% for specificity.22 Consensus is lacking as to which TVUS features are most predictive of adenomyosis, but in general, the combination of multiple MUSA criteria (particularly myometrial cysts and irregular JZ on 3D imaging) appears to be more accurate than any one feature alone.23 The presence of fibroids may decrease the sensitivity of TVUS, and one study suggested elastography may increase the accuracy of TVUS.24,26 Moreover, given that most radiologists receive limited training on the MUSA criteria, it behooves gynecologists to become familiar with these sonographic features to be able to identify adenomyosis in our patients.

Adenomyosis also may be suspected based on hysteroscopic findings, although a normal hysteroscopy cannot rule out the disease and data are lacking to support these markers as diagnostic. Visual findings can include a “strawberry” pattern, mucosal elevation, cystic hemorrhagic lesions, localized vascularity, or endometrial defects.27 Hysteroscopy may be effective in the treatment of localized lesions, although that discussion is beyond the scope of this review.
Clinical presentation
While many women who are later diagnosed with adenomyosis are asymptomatic, the disease can present with heavy menstrual bleeding and dysmenorrhea, which occur in 50% and 30% of patients, respectively.28 Other symptoms include dyspareunia and infertility. Symptoms were previously reported to develop between the ages of 40 and 50 years; however, this is biased by diagnosis at the time of hysterectomy and the fact that younger patients are less likely to undergo definitive surgery. When using imaging criteria for diagnosis, adenomyosis might be more responsible for dysmenorrhea and chronic pelvic pain in younger patients than previously appreciated.1,29 In a recent study reviewing TVUS in 270 adolescents for any reason, adenomyosis was present in 5% of cases and this increased up to 44% in the presence of endometriosis.30
Adenomyosis often co-exists and shares similar clinical presentations with other gynecologic pathologies such as endometriosis and fibroids, making diagnosis on symptomatology alone challenging. Concurrent adenomyosis has been found in up to 73% and 57% of patients with suspected or diagnosed endometriosis and fibroids, respectively.31,32 Accumulating evidence suggests that pelvic pain previously attributed to endometriosis may in fact be a result of adenomyosis; for example, persistent pelvic pain after optimal resection of endometriosis may be confounded by the presence of adenomyosis.29 In one study of 155 patients with complete resection of deep infiltrating endometriosis, persistent pelvic pain was significantly associated with the presence of adenomyosis on imaging.33
Adenomyosis is increasingly being recognized at the time of infertility evaluation with an estimated prevalence of 30% in women with infertility.3 Among women with infertility, adenomyosis has been associated with a lower clinical pregnancy rate, higher miscarriage rate, and lower live birth rate, as well as obstetric complications such as abnormal placentation.34-36 A study of 37 baboons found the histologic diagnosis of adenomyosis alone at necropsy was associated with a 20-fold increased risk of lifelong infertility (odds ratio [OR], 20.1; 95% CI, 2.1-921), whereas presence of endometriosis was associated with a nonsignificant 3-fold risk of lifelong infertility (OR, 3.6; 95% CI, 0.9-15.8).37
In women with endometriosis and infertility, co-existing adenomyosis portends worse fertility outcomes. In a retrospective study of 244 women who underwent endometriosis surgery, more than five features of adenomyosis on imaging was associated with higher rates of infertility, in vitro fertilization treatments, and a higher number of in vitro fertilization cycles.31 Moreover, in women who underwent surgery for deep infiltrating endometriosis, the presence of adenomyosis on imaging was associated with a 68% reduction in likelihood of pregnancy after surgery.38
Conclusion
As we begin to learn about adenomyosis, our misconceptions become more evident. The notion that it largely affects women at the end of their reproductive lives is biased by using histopathology at hysterectomy as the gold standard for diagnosis. Lack of definitive histologic or imaging criteria and biopsy techniques add to the diagnostic challenge. This in turn leads to inaccurate estimates of incidence and prevalence, as we assume patients’ symptoms must be attributable to what we can see at the time of surgery (for example, Stage I or II endometriosis), rather than what we cannot see. We now know that adenomyosis is present in women of all ages, including adolescents, and can significantly contribute to reduced fertility and quality of life. We owe it to our patients to consider this condition in the differential diagnosis of dysmenorrhea, heavy menstrual bleeding, dyspareunia, and infertility.
CASE Resolved
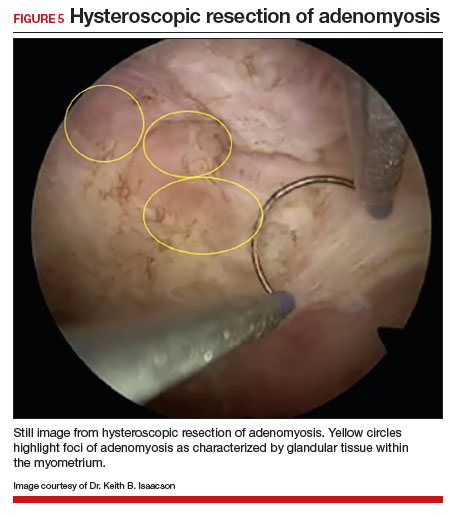
The patient underwent targeted hysteroscopic resection of adenomyosis (FIGURE 5) and conceived spontaneously the following year. ●
- Exacoustos C, Lazzeri L, Martire FG, et al. Ultrasound findings of adenomyosis in adolescents: type and grade of the disease. J Minim Invasive Gynecol. 2021;29:291.e1-299.e1. doi: 10.1016/j.jmig.2021.08.023
- Loring M, Chen TY, Isaacson KB. A systematic review of adenomyosis: it is time to reassess what we thought we knew about the disease. J Minim Invasive Gynecol. 2021;28:644655. doi: 10.1016/j.jmig.2020.10.012
- Bourdon M, Santulli P, Oliveira J, et al. Focal adenomyosis is associated with primary infertility. Fertil Steril. 2020;114:1271-1277. doi: 10.1016/j.fertnstert.2020.06.018
- Lan J, Wu Y, Wu Z, et al. Ultra-long GnRH agonist protocol during IVF/ICSI improves pregnancy outcomes in women with adenomyosis: a retrospective cohort study. Front Endocrinol (Lausanne). 2021;12:609771. doi: 10.3389 /fendo.2021.609771
- Gnecco JS, Brown AT, Kan EL, et al. Physiomimetic models of adenomyosis. Semin Reprod Med. 2020;38:179-196. doi: 10.1055/s-0040-1719084
- Harmsen MJ, Trommelen LM, de Leeuw RA, et al. Uterine junctional zone and adenomyosis: comparison of MRI, transvaginal ultrasound and histology. Ultrasound Obstet Gynecol. 2023;62:42-60. doi: 10.1002/uog.26117
- Xie T, Xu X, Yang Y, et al. The role of abnormal uterine junction zone in the occurrence and development of adenomyosis. Reprod Sci. 2022;29:2719-2730. doi: 10.1007/s43032-021 -00684-2
- Lazzeri L, Morosetti G, Centini G, et al. A sonographic classification of adenomyosis: interobserver reproducibility in the evaluation of type and degree of the myometrial involvement. Fertil Steril. 2018;110:1154-1161.e3. doi: 10.1016 /j.fertnstert.2018.06.031
- Tahlan A, Nanda A, Mohan H. Uterine adenomyoma: a clinicopathologic review of 26 cases and a review of the literature. Int J Gynecol Pathol. 2006;25:361-365. doi: 10.1097/01.pgp.0000209570.08716.b3
- Chung K, Wallace J, Kim S-Y, et al. Structural and molecular interrogation of intact biological systems. Nature. 2013;497:332-337. doi: 10.1038/nature12107
- Yamaguchi M, Yoshihara K, Suda K, et al. Three-dimensional understanding of the morphological complexity of the human uterine endometrium. iScience. 2021;24:102258. doi: 10.1016/j.isci.2021.102258
- Vannuccini S, Tosti C, Carmona F, et al. Pathogenesis of adenomyosis: an update on molecular mechanisms. Reprod Biomed Online. 2017;35:592-601. doi: 10.1016 /j.rbmo.2017.06.016
- Zhai J, Vannuccini S, Petraglia F, et al. Adenomyosis: mechanisms and pathogenesis. Semin Reprod Med. 2020;38:129-143. doi: 10.1055/s-0040-1716687
- Munro MG. Uterine polyps, adenomyosis, leiomyomas, and endometrial receptivity. Fertil Steril. 2019;111:629-640. doi: 10.1016/j.fertnstert.2019.02.008
- Kay N, Huang C-Y, Shiu L-Y, et al. TGF-β1 neutralization improves pregnancy outcomes by restoring endometrial receptivity in mice with adenomyosis. Reprod Sci. 2021;28:877-887. doi: 10.1007/s43032-020-00308-1
- Habiba M, Benagiano G. Classifying adenomyosis: progress and challenges. Int J Environ Res Public Health. 2021;18:12386. doi: 10.3390/ijerph182312386
- Movilla P, Morris S, Isaacson K. A systematic review of tissue sampling techniques for the diagnosis of adenomyosis. J Minim Invasive Gynecol. 2020;27:344-351. doi: 10.1016 /j.jmig.2019.09.001
- Agostinho L, Cruz R, Osório F, et al. MRI for adenomyosis: a pictorial review. Insights Imaging. 2017;8:549-556. doi: 10.1007/s13244-017-0576-z
- Bazot M, Cortez A, Darai E, et al. Ultrasonography compared with magnetic resonance imaging for the diagnosis of adenomyosis: correlation with histopathology. Hum Reprod. 2001;16:2427-2433. doi: 10.1093/humrep/16.11.2427
- Reinhold C, Tafazoli F, Mehio A, et al. Uterine adenomyosis: endovaginal US and MR imaging features with histopathologic correlation. Radiographics. 1999;19:S147-S160. doi: 10.1148 /radiographics.19.suppl_1.g99oc13s147
- Rees CO, Nederend J, Mischi M, et al. Objective measures of adenomyosis on MRI and their diagnostic accuracy—a systematic review & meta-analysis. Acta Obstet Gynecol Scand. 2021;100:1377-1391.
- Chapron C, Vannuccini S, Santulli P, et al. Diagnosing adenomyosis: an integrated clinical and imaging approach. Hum Reprod Update. 2020;26:392-411. doi: 10.1093 /humupd/dmz049
- Liu L, Li W, Leonardi M, et al. Diagnostic accuracy of transvaginal ultrasound and magnetic resonance imaging for adenomyosis: systematic review and meta-analysis and review of sonographic diagnostic criteria. J Ultrasound Med. 2021;40:2289-2306. doi: 10.1002/jum.15635
- Bazot M, Daraï E. Role of transvaginal sonography and magnetic resonance imaging in the diagnosis of uterine adenomyosis. Fertil Steril. 2018;109:389-397. doi: 10.1016 /j.fertnstert.2018.01.024
- Van den Bosch T, Dueholm M, Leone FPG, et al. Terms, definitions and measurements to describe sonographic features of myometrium and uterine masses: a consensus opinion from the Morphological Uterus Sonographic Assessment (MUSA) group. Ultrasound Obstet Gynecol. 2015;46:284-298. doi: 10.1002/uog.14806
- Săsăran V, Turdean S, Gliga M, et al. Value of strainratio elastography in the diagnosis and differentiation of uterine fibroids and adenomyosis. J Pers Med. 2021;11:824. doi: 10.3390/jpm11080824
- Di Spiezio Sardo A, Calagna G, Santangelo F, et al. The role of hysteroscopy in the diagnosis and treatment of adenomyosis. Biomed Res Int. 2017;2017:2518396. doi: 10.1155/2017/2518396
- Azzi R. Adenomyosis: current perspectives. Obstet Gynecol Clin North Am. 1989;16:221-235.
- Parker JD, Leondires M, Sinaii N, et al. Persistence of dysmenorrhea and nonmenstrual pain after optimal endometriosis surgery may indicate adenomyosis. Fertil Steril. 2006;86:711-715. doi: 10.1016/j.fertnstert.2006.01.030
- Martire FG, Lazzeri L, Conway F, et al. Adolescence and endometriosis: symptoms, ultrasound signs and early diagnosis. Fertil Steril. 2020;114:1049-1057. doi: 10.1016 /j.fertnstert.2020.06.012
- Decter D, Arbib N, Markovitz H, et al. Sonographic signs of adenomyosis in women with endometriosis are associated with infertility. J Clin Med. 2021;10:2355. doi: 10.3390 /jcm10112355
- Brucker SY, Huebner M, Wallwiener M, et al. Clinical characteristics indicating adenomyosis coexisting with leiomyomas: a retrospective, questionnaire-based study. Fertil Steril. 2014;101:237-241.e1. doi: 10.1016 /j.fertnstert.2013.09.038
- Perelló MF, Martínez-Zamora MÁ, Torres X, et al. Endometriotic pain is associated with adenomyosis but not with the compartments affected by deep infiltrating endometriosis. Gynecol Obstet Invest. 2017;82:240-246. doi: 10.1159/000447633
- Younes G, Tulandi T. Effects of adenomyosis on in vitro fertilization treatment outcomes: a metaanalysis. Fertil Steril. 2017;108:483-490.e3. doi: 10.1016 /j.fertnstert.2017.06.025
- Nirgianakis K, Kalaitzopoulos DR, Schwartz ASK, et al. Fertility, pregnancy and neonatal outcomes of patients with adenomyosis: a systematic review and meta-analysis. Reprod BioMed Online. 2021;42:185-206. doi: 10.1016 /j.rbmo.2020.09.023
- Ono Y, Ota H, Takimoto K, et al. Perinatal outcomes associated with the positional relationship between the placenta and the adenomyosis lesion. J Gynecol Obstet Hum Reprod. 2021;50:102114. doi: 10.1016/j.jogoh.2021.102114
- Barrier BF, Malinowski MJ, Dick EJ Jr, et al. Adenomyosis in the baboon is associated with primary infertility. Fertil Steril. 2004;82(suppl 3):1091-1094. doi: 10.1016 /j.fertnstert.2003.11.065
- Vercellini P, Consonni D, Barbara G, et al. Adenomyosis and reproductive performance after surgery for rectovaginal and colorectal endometriosis: a systematic review and meta-analysis. Reprod Biomed Online. 2014;28:704-713. doi: 10.1016/j.rbmo.2014.02.006

CASE Painful, heavy menstruation and recurrent pregnancy loss
A 37-year-old woman (G3P0030) with a history of recurrent pregnancy loss presents for evaluation. She had 3 losses—most recently a miscarriage at 22 weeks with a cerclage in place. She did not undergo any surgical procedures for these losses. Hormonal and thrombophilia workup is negative and semen analysis is normal. She reports a history of painful, heavy periods for many years, as well as dyspareunia and occasional post-coital bleeding. Past medical history was otherwise unremarkable. Pelvic magnetic resonance imaging (MRI) revealed focal thickening of the junctional zone up to 15 mm with 2 foci of T2 hyperintensities suggesting adenomyosis (FIGURE 1).
How do you counsel this patient regarding the MRI findings and their impact on her fertility?

Adenomyosis is a condition in which endometrial glands and stroma are abnormally present in the uterine myometrium, resulting in smooth muscle hypertrophy and abnormal uterine contractility. Traditional teaching describes a woman in her 40s with heavy and painful menses, a “boggy uterus” on examination, who has completed childbearing and desires definitive treatment. Histologic diagnosis of adenomyosis is made from the uterine specimen at the time of hysterectomy, invariably confounding our understanding of the epidemiology of adenomyosis.
More recently, however, we are beginning to learn that this narrative is misguided. Imaging changes of adenomyosis can be seen in women who desire future fertility and in adolescents with severe dysmenorrhea, suggesting an earlier age of incidence.1 In a recent systematic review, prevalence estimates ranged from 15% to 67%, owing to varying diagnostic methods and patient inclusion criteria.2 It is increasingly being recognized as a primary contributor to infertility, with one study estimating a 30% prevalence of infertility in women with adenomyosis.3 Moreover, treatment with gonadotropin-releasing hormone agonists and/or surgical excision may improve fertility outcomes.4
As we learn more about this prevalent and life-altering condition, we owe it to our patients to consider this diagnosis when counseling on dysmenorrhea, heavy menstrual bleeding, or infertility.
Anatomy of the myometrium
The myometrium is composed of the inner and outer myometrium: the inner myometrium (IM) and endometrium are of Müllerian origin, and the outer myometrium (OM) is of mesenchymal origin. The IM thickens in response to steroid hormones during the menstrual cycle with metaplasia of endometrial stromal cells into myocytes and back again, whereas the OM is not responsive to hormones.5 Emerging literature suggests the OM is further divided into a middle and outer section based on different histologic morphologies, though the clinical implications of this are not understood.6 The term “junctional zone” (JZ) refers to the imaging appearance of what is thought to be the IM. Interestingly it cannot be identified on traditional hematoxylin and eosin staining. When the JZ is thickened or demonstrates irregular borders, it is used as a diagnostic marker for adenomyosis and is postulated to play an important role in adenomyosis pathophysiology, particularly heavy menstrual bleeding and infertility.7
Continue to: Subtypes of adenomyosis...
Subtypes of adenomyosis
While various disease classifications have been suggested for adenomyosis, to date there is no international consensus. Adenomyosis is typically described in 3 forms: diffuse, focal, or adenomyoma.8 As implied, the term focal adenomyosis refers to discrete lesions surrounded by normal myometrium, whereas abnormal glandular changes are pervasive throughout the myometrium in diffuse disease. Adenomyomas are a subgroup of focal adenomyosis that are thought to be surrounded by leiomyomatous smooth muscle and may be well demarcated on imaging.9
Recent research uses novel histologic imaging techniques to explore adenomyotic growth patterns in 3-dimensional (3D) reconstructions. Combining tissue-clearing methods with light-sheet fluorescence microscopy enables highly detailed 3D representations of the protein and nucleic acid structure of organs.10 For example, Yamaguchi and colleagues used this technology to explore the 3D morphological features of adenomyotic tissue and observed direct invasion of the endometrial glands into the myometrium and an “ant colony ̶ like network” of ectopic endometrial glands in the myometrium (FIGURE 2).11 These abnormal glandular networks have been visualized beyond the IM, which may not be captured on ultrasonography or MRI. While this work is still in its infancy, it has the potential to provide important insight into disease pathogenesis and to inform future therapy.

Pathogenesis
Proposed mechanisms for the development of adenomyosis include endometrial invasion, tissue injury and repair (TIAR) mechanisms, and the stem cell theory.12 According to the endometrial invasion theory, glandular epithelial cells from the basalis layer invaginate through an altered IM, slipping through weak muscle fibers and attracted by certain growth factors. In the TIAR mechanism theory, micro- or macro-trauma to the IM (whether from pregnancy, surgery, or infection) results in chronic proliferation and inflammation leading to the development of adenomyosis. Finally, the stem cell theory proposes that adenomyosis might develop from de novo ectopic endometrial tissue.
While the exact pathogenesis of adenomyosis is largely unknown, it has been associated with predictable molecular changes in the endometrium and surrounding myometrium.12 Myometrial hypercontractility is seen in patients with adenomyosis and dysmenorrhea, whereas neovascularization, high microvessel density, and abnormal uterine contractility are seen in those with abnormal uterine bleeding.13 In patients with infertility, increased inflammation, abnormal endometrial receptivity, and alterations in the myometrial architecture have been suggested to impair contractility and sperm transport.12,14
Differential growth factor expression and abnormal estrogen and progesterone signaling pathways have been observed in the IM in patients with adenomyosis, along with dysregulation of immune factors and increased inflammatory oxidative stress.12 This in turn results in myometrial hypertrophy and fibrosis, impairing normal uterine contractility patterns. This abnormal contractility may alter sperm transport and embryo implantation, and animal models that target pathways leading to fibrosis may improve endometrial receptivity.14,15 Further research is needed to elucidate specific molecular pathways and their complex interplay in this disease.
Continue to: Diagnosis...
Diagnosis
The gold standard for diagnosis of adenomyosis is histopathology from hysterectomy specimens, but specific definitions vary. Published criteria include endometrial glands within the myometrial layer greater than 0.5 to 1 low power field from the basal layer of the endometrium, endometrial glands extending deeper than 25% of the myometrial thickness, or endometrial glands a certain distance (ranging from 1-3 mm) from the basalis layer of the endometrium.16 Various methods of non-hysterectomy tissue sampling have been proposed for diagnosis, including needle, hysteroscopic, or laparoscopic sampling, but the sensitivity of these methods is poor.17 Limiting the diagnosis of adenomyosis to specimen pathology relies on invasive methods and clearly we cannot confirm the diagnosis by hysterectomy in patients with a desire for future fertility. It is for this reason that the prevalence of the disease is widely unknown.
The alternative to pathologic diagnosis is to identify radiologic changes that are associated with adenomyosis via either transvaginal ultrasound (TVUS) or MRI. Features suggestive of adenomyosis on MRI overlap with TVUS features, including uterine enlargement, anteroposterior myometrial asymmetry, T1- or T2-intense myometrial cysts or foci, and a thickened JZ.18 A JZ thicker than 12 mm has been thought to be predictive of adenomyosis, whereas a thickness of less than 8 mm is predictive of its absence, although the JZ may vary in thickness with the menstrual cycle.19,20 A 2021 systematic review and meta-analysis comparing MRI diagnosis with histopathologic findings reported a pooled sensitivity and specificity of 60% and 96%, respectively.21 The reported range for sensitivity and specificity is wide: 70% to 93% for sensitivity and 67% to 93% for specificity.22-24
Key TVUS features associated with adenomyosis were defined in 2015 in a consensus statement released by the Morphological Uterus Sonographic Assessment (MUSA) group.25 These include a globally enlarged uterus, anteroposterior myometrial asymmetry, myometrial cysts, fan-shaped shadowing, mixed myometrial echogenicity, translesional vascularity, echogenic subendometrial lines and buds, and a thickened, irregular or discontinuous JZ (FIGURES 3 and 4).25 The accuracy of ultrasonographic diagnosis of adenomyosis using these features has been investigated in multiple systematic reviews and meta-analyses, most recently by Liu and colleagues who found a pooled sensitivity of TVUS of 81% and pooled specificity of 87%.23 The range for ultrasonographic sensitivity and specificity is wide, however, ranging from 33% to 84% for sensitivity and 64% to 100% for specificity.22 Consensus is lacking as to which TVUS features are most predictive of adenomyosis, but in general, the combination of multiple MUSA criteria (particularly myometrial cysts and irregular JZ on 3D imaging) appears to be more accurate than any one feature alone.23 The presence of fibroids may decrease the sensitivity of TVUS, and one study suggested elastography may increase the accuracy of TVUS.24,26 Moreover, given that most radiologists receive limited training on the MUSA criteria, it behooves gynecologists to become familiar with these sonographic features to be able to identify adenomyosis in our patients.


Adenomyosis also may be suspected based on hysteroscopic findings, although a normal hysteroscopy cannot rule out the disease and data are lacking to support these markers as diagnostic. Visual findings can include a “strawberry” pattern, mucosal elevation, cystic hemorrhagic lesions, localized vascularity, or endometrial defects.27 Hysteroscopy may be effective in the treatment of localized lesions, although that discussion is beyond the scope of this review.
Clinical presentation
While many women who are later diagnosed with adenomyosis are asymptomatic, the disease can present with heavy menstrual bleeding and dysmenorrhea, which occur in 50% and 30% of patients, respectively.28 Other symptoms include dyspareunia and infertility. Symptoms were previously reported to develop between the ages of 40 and 50 years; however, this is biased by diagnosis at the time of hysterectomy and the fact that younger patients are less likely to undergo definitive surgery. When using imaging criteria for diagnosis, adenomyosis might be more responsible for dysmenorrhea and chronic pelvic pain in younger patients than previously appreciated.1,29 In a recent study reviewing TVUS in 270 adolescents for any reason, adenomyosis was present in 5% of cases and this increased up to 44% in the presence of endometriosis.30
Adenomyosis often co-exists and shares similar clinical presentations with other gynecologic pathologies such as endometriosis and fibroids, making diagnosis on symptomatology alone challenging. Concurrent adenomyosis has been found in up to 73% and 57% of patients with suspected or diagnosed endometriosis and fibroids, respectively.31,32 Accumulating evidence suggests that pelvic pain previously attributed to endometriosis may in fact be a result of adenomyosis; for example, persistent pelvic pain after optimal resection of endometriosis may be confounded by the presence of adenomyosis.29 In one study of 155 patients with complete resection of deep infiltrating endometriosis, persistent pelvic pain was significantly associated with the presence of adenomyosis on imaging.33
Adenomyosis is increasingly being recognized at the time of infertility evaluation with an estimated prevalence of 30% in women with infertility.3 Among women with infertility, adenomyosis has been associated with a lower clinical pregnancy rate, higher miscarriage rate, and lower live birth rate, as well as obstetric complications such as abnormal placentation.34-36 A study of 37 baboons found the histologic diagnosis of adenomyosis alone at necropsy was associated with a 20-fold increased risk of lifelong infertility (odds ratio [OR], 20.1; 95% CI, 2.1-921), whereas presence of endometriosis was associated with a nonsignificant 3-fold risk of lifelong infertility (OR, 3.6; 95% CI, 0.9-15.8).37
In women with endometriosis and infertility, co-existing adenomyosis portends worse fertility outcomes. In a retrospective study of 244 women who underwent endometriosis surgery, more than five features of adenomyosis on imaging was associated with higher rates of infertility, in vitro fertilization treatments, and a higher number of in vitro fertilization cycles.31 Moreover, in women who underwent surgery for deep infiltrating endometriosis, the presence of adenomyosis on imaging was associated with a 68% reduction in likelihood of pregnancy after surgery.38
Conclusion
As we begin to learn about adenomyosis, our misconceptions become more evident. The notion that it largely affects women at the end of their reproductive lives is biased by using histopathology at hysterectomy as the gold standard for diagnosis. Lack of definitive histologic or imaging criteria and biopsy techniques add to the diagnostic challenge. This in turn leads to inaccurate estimates of incidence and prevalence, as we assume patients’ symptoms must be attributable to what we can see at the time of surgery (for example, Stage I or II endometriosis), rather than what we cannot see. We now know that adenomyosis is present in women of all ages, including adolescents, and can significantly contribute to reduced fertility and quality of life. We owe it to our patients to consider this condition in the differential diagnosis of dysmenorrhea, heavy menstrual bleeding, dyspareunia, and infertility.
CASE Resolved
The patient underwent targeted hysteroscopic resection of adenomyosis (FIGURE 5) and conceived spontaneously the following year. ●


CASE Painful, heavy menstruation and recurrent pregnancy loss
A 37-year-old woman (G3P0030) with a history of recurrent pregnancy loss presents for evaluation. She had 3 losses—most recently a miscarriage at 22 weeks with a cerclage in place. She did not undergo any surgical procedures for these losses. Hormonal and thrombophilia workup is negative and semen analysis is normal. She reports a history of painful, heavy periods for many years, as well as dyspareunia and occasional post-coital bleeding. Past medical history was otherwise unremarkable. Pelvic magnetic resonance imaging (MRI) revealed focal thickening of the junctional zone up to 15 mm with 2 foci of T2 hyperintensities suggesting adenomyosis (FIGURE 1).
How do you counsel this patient regarding the MRI findings and their impact on her fertility?

Adenomyosis is a condition in which endometrial glands and stroma are abnormally present in the uterine myometrium, resulting in smooth muscle hypertrophy and abnormal uterine contractility. Traditional teaching describes a woman in her 40s with heavy and painful menses, a “boggy uterus” on examination, who has completed childbearing and desires definitive treatment. Histologic diagnosis of adenomyosis is made from the uterine specimen at the time of hysterectomy, invariably confounding our understanding of the epidemiology of adenomyosis.
More recently, however, we are beginning to learn that this narrative is misguided. Imaging changes of adenomyosis can be seen in women who desire future fertility and in adolescents with severe dysmenorrhea, suggesting an earlier age of incidence.1 In a recent systematic review, prevalence estimates ranged from 15% to 67%, owing to varying diagnostic methods and patient inclusion criteria.2 It is increasingly being recognized as a primary contributor to infertility, with one study estimating a 30% prevalence of infertility in women with adenomyosis.3 Moreover, treatment with gonadotropin-releasing hormone agonists and/or surgical excision may improve fertility outcomes.4
As we learn more about this prevalent and life-altering condition, we owe it to our patients to consider this diagnosis when counseling on dysmenorrhea, heavy menstrual bleeding, or infertility.
Anatomy of the myometrium
The myometrium is composed of the inner and outer myometrium: the inner myometrium (IM) and endometrium are of Müllerian origin, and the outer myometrium (OM) is of mesenchymal origin. The IM thickens in response to steroid hormones during the menstrual cycle with metaplasia of endometrial stromal cells into myocytes and back again, whereas the OM is not responsive to hormones.5 Emerging literature suggests the OM is further divided into a middle and outer section based on different histologic morphologies, though the clinical implications of this are not understood.6 The term “junctional zone” (JZ) refers to the imaging appearance of what is thought to be the IM. Interestingly it cannot be identified on traditional hematoxylin and eosin staining. When the JZ is thickened or demonstrates irregular borders, it is used as a diagnostic marker for adenomyosis and is postulated to play an important role in adenomyosis pathophysiology, particularly heavy menstrual bleeding and infertility.7
Continue to: Subtypes of adenomyosis...
Subtypes of adenomyosis
While various disease classifications have been suggested for adenomyosis, to date there is no international consensus. Adenomyosis is typically described in 3 forms: diffuse, focal, or adenomyoma.8 As implied, the term focal adenomyosis refers to discrete lesions surrounded by normal myometrium, whereas abnormal glandular changes are pervasive throughout the myometrium in diffuse disease. Adenomyomas are a subgroup of focal adenomyosis that are thought to be surrounded by leiomyomatous smooth muscle and may be well demarcated on imaging.9
Recent research uses novel histologic imaging techniques to explore adenomyotic growth patterns in 3-dimensional (3D) reconstructions. Combining tissue-clearing methods with light-sheet fluorescence microscopy enables highly detailed 3D representations of the protein and nucleic acid structure of organs.10 For example, Yamaguchi and colleagues used this technology to explore the 3D morphological features of adenomyotic tissue and observed direct invasion of the endometrial glands into the myometrium and an “ant colony ̶ like network” of ectopic endometrial glands in the myometrium (FIGURE 2).11 These abnormal glandular networks have been visualized beyond the IM, which may not be captured on ultrasonography or MRI. While this work is still in its infancy, it has the potential to provide important insight into disease pathogenesis and to inform future therapy.

Pathogenesis
Proposed mechanisms for the development of adenomyosis include endometrial invasion, tissue injury and repair (TIAR) mechanisms, and the stem cell theory.12 According to the endometrial invasion theory, glandular epithelial cells from the basalis layer invaginate through an altered IM, slipping through weak muscle fibers and attracted by certain growth factors. In the TIAR mechanism theory, micro- or macro-trauma to the IM (whether from pregnancy, surgery, or infection) results in chronic proliferation and inflammation leading to the development of adenomyosis. Finally, the stem cell theory proposes that adenomyosis might develop from de novo ectopic endometrial tissue.
While the exact pathogenesis of adenomyosis is largely unknown, it has been associated with predictable molecular changes in the endometrium and surrounding myometrium.12 Myometrial hypercontractility is seen in patients with adenomyosis and dysmenorrhea, whereas neovascularization, high microvessel density, and abnormal uterine contractility are seen in those with abnormal uterine bleeding.13 In patients with infertility, increased inflammation, abnormal endometrial receptivity, and alterations in the myometrial architecture have been suggested to impair contractility and sperm transport.12,14
Differential growth factor expression and abnormal estrogen and progesterone signaling pathways have been observed in the IM in patients with adenomyosis, along with dysregulation of immune factors and increased inflammatory oxidative stress.12 This in turn results in myometrial hypertrophy and fibrosis, impairing normal uterine contractility patterns. This abnormal contractility may alter sperm transport and embryo implantation, and animal models that target pathways leading to fibrosis may improve endometrial receptivity.14,15 Further research is needed to elucidate specific molecular pathways and their complex interplay in this disease.
Continue to: Diagnosis...
Diagnosis
The gold standard for diagnosis of adenomyosis is histopathology from hysterectomy specimens, but specific definitions vary. Published criteria include endometrial glands within the myometrial layer greater than 0.5 to 1 low power field from the basal layer of the endometrium, endometrial glands extending deeper than 25% of the myometrial thickness, or endometrial glands a certain distance (ranging from 1-3 mm) from the basalis layer of the endometrium.16 Various methods of non-hysterectomy tissue sampling have been proposed for diagnosis, including needle, hysteroscopic, or laparoscopic sampling, but the sensitivity of these methods is poor.17 Limiting the diagnosis of adenomyosis to specimen pathology relies on invasive methods and clearly we cannot confirm the diagnosis by hysterectomy in patients with a desire for future fertility. It is for this reason that the prevalence of the disease is widely unknown.
The alternative to pathologic diagnosis is to identify radiologic changes that are associated with adenomyosis via either transvaginal ultrasound (TVUS) or MRI. Features suggestive of adenomyosis on MRI overlap with TVUS features, including uterine enlargement, anteroposterior myometrial asymmetry, T1- or T2-intense myometrial cysts or foci, and a thickened JZ.18 A JZ thicker than 12 mm has been thought to be predictive of adenomyosis, whereas a thickness of less than 8 mm is predictive of its absence, although the JZ may vary in thickness with the menstrual cycle.19,20 A 2021 systematic review and meta-analysis comparing MRI diagnosis with histopathologic findings reported a pooled sensitivity and specificity of 60% and 96%, respectively.21 The reported range for sensitivity and specificity is wide: 70% to 93% for sensitivity and 67% to 93% for specificity.22-24
Key TVUS features associated with adenomyosis were defined in 2015 in a consensus statement released by the Morphological Uterus Sonographic Assessment (MUSA) group.25 These include a globally enlarged uterus, anteroposterior myometrial asymmetry, myometrial cysts, fan-shaped shadowing, mixed myometrial echogenicity, translesional vascularity, echogenic subendometrial lines and buds, and a thickened, irregular or discontinuous JZ (FIGURES 3 and 4).25 The accuracy of ultrasonographic diagnosis of adenomyosis using these features has been investigated in multiple systematic reviews and meta-analyses, most recently by Liu and colleagues who found a pooled sensitivity of TVUS of 81% and pooled specificity of 87%.23 The range for ultrasonographic sensitivity and specificity is wide, however, ranging from 33% to 84% for sensitivity and 64% to 100% for specificity.22 Consensus is lacking as to which TVUS features are most predictive of adenomyosis, but in general, the combination of multiple MUSA criteria (particularly myometrial cysts and irregular JZ on 3D imaging) appears to be more accurate than any one feature alone.23 The presence of fibroids may decrease the sensitivity of TVUS, and one study suggested elastography may increase the accuracy of TVUS.24,26 Moreover, given that most radiologists receive limited training on the MUSA criteria, it behooves gynecologists to become familiar with these sonographic features to be able to identify adenomyosis in our patients.


Adenomyosis also may be suspected based on hysteroscopic findings, although a normal hysteroscopy cannot rule out the disease and data are lacking to support these markers as diagnostic. Visual findings can include a “strawberry” pattern, mucosal elevation, cystic hemorrhagic lesions, localized vascularity, or endometrial defects.27 Hysteroscopy may be effective in the treatment of localized lesions, although that discussion is beyond the scope of this review.
Clinical presentation
While many women who are later diagnosed with adenomyosis are asymptomatic, the disease can present with heavy menstrual bleeding and dysmenorrhea, which occur in 50% and 30% of patients, respectively.28 Other symptoms include dyspareunia and infertility. Symptoms were previously reported to develop between the ages of 40 and 50 years; however, this is biased by diagnosis at the time of hysterectomy and the fact that younger patients are less likely to undergo definitive surgery. When using imaging criteria for diagnosis, adenomyosis might be more responsible for dysmenorrhea and chronic pelvic pain in younger patients than previously appreciated.1,29 In a recent study reviewing TVUS in 270 adolescents for any reason, adenomyosis was present in 5% of cases and this increased up to 44% in the presence of endometriosis.30
Adenomyosis often co-exists and shares similar clinical presentations with other gynecologic pathologies such as endometriosis and fibroids, making diagnosis on symptomatology alone challenging. Concurrent adenomyosis has been found in up to 73% and 57% of patients with suspected or diagnosed endometriosis and fibroids, respectively.31,32 Accumulating evidence suggests that pelvic pain previously attributed to endometriosis may in fact be a result of adenomyosis; for example, persistent pelvic pain after optimal resection of endometriosis may be confounded by the presence of adenomyosis.29 In one study of 155 patients with complete resection of deep infiltrating endometriosis, persistent pelvic pain was significantly associated with the presence of adenomyosis on imaging.33
Adenomyosis is increasingly being recognized at the time of infertility evaluation with an estimated prevalence of 30% in women with infertility.3 Among women with infertility, adenomyosis has been associated with a lower clinical pregnancy rate, higher miscarriage rate, and lower live birth rate, as well as obstetric complications such as abnormal placentation.34-36 A study of 37 baboons found the histologic diagnosis of adenomyosis alone at necropsy was associated with a 20-fold increased risk of lifelong infertility (odds ratio [OR], 20.1; 95% CI, 2.1-921), whereas presence of endometriosis was associated with a nonsignificant 3-fold risk of lifelong infertility (OR, 3.6; 95% CI, 0.9-15.8).37
In women with endometriosis and infertility, co-existing adenomyosis portends worse fertility outcomes. In a retrospective study of 244 women who underwent endometriosis surgery, more than five features of adenomyosis on imaging was associated with higher rates of infertility, in vitro fertilization treatments, and a higher number of in vitro fertilization cycles.31 Moreover, in women who underwent surgery for deep infiltrating endometriosis, the presence of adenomyosis on imaging was associated with a 68% reduction in likelihood of pregnancy after surgery.38
Conclusion
As we begin to learn about adenomyosis, our misconceptions become more evident. The notion that it largely affects women at the end of their reproductive lives is biased by using histopathology at hysterectomy as the gold standard for diagnosis. Lack of definitive histologic or imaging criteria and biopsy techniques add to the diagnostic challenge. This in turn leads to inaccurate estimates of incidence and prevalence, as we assume patients’ symptoms must be attributable to what we can see at the time of surgery (for example, Stage I or II endometriosis), rather than what we cannot see. We now know that adenomyosis is present in women of all ages, including adolescents, and can significantly contribute to reduced fertility and quality of life. We owe it to our patients to consider this condition in the differential diagnosis of dysmenorrhea, heavy menstrual bleeding, dyspareunia, and infertility.
CASE Resolved
The patient underwent targeted hysteroscopic resection of adenomyosis (FIGURE 5) and conceived spontaneously the following year. ●

- Exacoustos C, Lazzeri L, Martire FG, et al. Ultrasound findings of adenomyosis in adolescents: type and grade of the disease. J Minim Invasive Gynecol. 2021;29:291.e1-299.e1. doi: 10.1016/j.jmig.2021.08.023
- Loring M, Chen TY, Isaacson KB. A systematic review of adenomyosis: it is time to reassess what we thought we knew about the disease. J Minim Invasive Gynecol. 2021;28:644655. doi: 10.1016/j.jmig.2020.10.012
- Bourdon M, Santulli P, Oliveira J, et al. Focal adenomyosis is associated with primary infertility. Fertil Steril. 2020;114:1271-1277. doi: 10.1016/j.fertnstert.2020.06.018
- Lan J, Wu Y, Wu Z, et al. Ultra-long GnRH agonist protocol during IVF/ICSI improves pregnancy outcomes in women with adenomyosis: a retrospective cohort study. Front Endocrinol (Lausanne). 2021;12:609771. doi: 10.3389 /fendo.2021.609771
- Gnecco JS, Brown AT, Kan EL, et al. Physiomimetic models of adenomyosis. Semin Reprod Med. 2020;38:179-196. doi: 10.1055/s-0040-1719084
- Harmsen MJ, Trommelen LM, de Leeuw RA, et al. Uterine junctional zone and adenomyosis: comparison of MRI, transvaginal ultrasound and histology. Ultrasound Obstet Gynecol. 2023;62:42-60. doi: 10.1002/uog.26117
- Xie T, Xu X, Yang Y, et al. The role of abnormal uterine junction zone in the occurrence and development of adenomyosis. Reprod Sci. 2022;29:2719-2730. doi: 10.1007/s43032-021 -00684-2
- Lazzeri L, Morosetti G, Centini G, et al. A sonographic classification of adenomyosis: interobserver reproducibility in the evaluation of type and degree of the myometrial involvement. Fertil Steril. 2018;110:1154-1161.e3. doi: 10.1016 /j.fertnstert.2018.06.031
- Tahlan A, Nanda A, Mohan H. Uterine adenomyoma: a clinicopathologic review of 26 cases and a review of the literature. Int J Gynecol Pathol. 2006;25:361-365. doi: 10.1097/01.pgp.0000209570.08716.b3
- Chung K, Wallace J, Kim S-Y, et al. Structural and molecular interrogation of intact biological systems. Nature. 2013;497:332-337. doi: 10.1038/nature12107
- Yamaguchi M, Yoshihara K, Suda K, et al. Three-dimensional understanding of the morphological complexity of the human uterine endometrium. iScience. 2021;24:102258. doi: 10.1016/j.isci.2021.102258
- Vannuccini S, Tosti C, Carmona F, et al. Pathogenesis of adenomyosis: an update on molecular mechanisms. Reprod Biomed Online. 2017;35:592-601. doi: 10.1016 /j.rbmo.2017.06.016
- Zhai J, Vannuccini S, Petraglia F, et al. Adenomyosis: mechanisms and pathogenesis. Semin Reprod Med. 2020;38:129-143. doi: 10.1055/s-0040-1716687
- Munro MG. Uterine polyps, adenomyosis, leiomyomas, and endometrial receptivity. Fertil Steril. 2019;111:629-640. doi: 10.1016/j.fertnstert.2019.02.008
- Kay N, Huang C-Y, Shiu L-Y, et al. TGF-β1 neutralization improves pregnancy outcomes by restoring endometrial receptivity in mice with adenomyosis. Reprod Sci. 2021;28:877-887. doi: 10.1007/s43032-020-00308-1
- Habiba M, Benagiano G. Classifying adenomyosis: progress and challenges. Int J Environ Res Public Health. 2021;18:12386. doi: 10.3390/ijerph182312386
- Movilla P, Morris S, Isaacson K. A systematic review of tissue sampling techniques for the diagnosis of adenomyosis. J Minim Invasive Gynecol. 2020;27:344-351. doi: 10.1016 /j.jmig.2019.09.001
- Agostinho L, Cruz R, Osório F, et al. MRI for adenomyosis: a pictorial review. Insights Imaging. 2017;8:549-556. doi: 10.1007/s13244-017-0576-z
- Bazot M, Cortez A, Darai E, et al. Ultrasonography compared with magnetic resonance imaging for the diagnosis of adenomyosis: correlation with histopathology. Hum Reprod. 2001;16:2427-2433. doi: 10.1093/humrep/16.11.2427
- Reinhold C, Tafazoli F, Mehio A, et al. Uterine adenomyosis: endovaginal US and MR imaging features with histopathologic correlation. Radiographics. 1999;19:S147-S160. doi: 10.1148 /radiographics.19.suppl_1.g99oc13s147
- Rees CO, Nederend J, Mischi M, et al. Objective measures of adenomyosis on MRI and their diagnostic accuracy—a systematic review & meta-analysis. Acta Obstet Gynecol Scand. 2021;100:1377-1391.
- Chapron C, Vannuccini S, Santulli P, et al. Diagnosing adenomyosis: an integrated clinical and imaging approach. Hum Reprod Update. 2020;26:392-411. doi: 10.1093 /humupd/dmz049
- Liu L, Li W, Leonardi M, et al. Diagnostic accuracy of transvaginal ultrasound and magnetic resonance imaging for adenomyosis: systematic review and meta-analysis and review of sonographic diagnostic criteria. J Ultrasound Med. 2021;40:2289-2306. doi: 10.1002/jum.15635
- Bazot M, Daraï E. Role of transvaginal sonography and magnetic resonance imaging in the diagnosis of uterine adenomyosis. Fertil Steril. 2018;109:389-397. doi: 10.1016 /j.fertnstert.2018.01.024
- Van den Bosch T, Dueholm M, Leone FPG, et al. Terms, definitions and measurements to describe sonographic features of myometrium and uterine masses: a consensus opinion from the Morphological Uterus Sonographic Assessment (MUSA) group. Ultrasound Obstet Gynecol. 2015;46:284-298. doi: 10.1002/uog.14806
- Săsăran V, Turdean S, Gliga M, et al. Value of strainratio elastography in the diagnosis and differentiation of uterine fibroids and adenomyosis. J Pers Med. 2021;11:824. doi: 10.3390/jpm11080824
- Di Spiezio Sardo A, Calagna G, Santangelo F, et al. The role of hysteroscopy in the diagnosis and treatment of adenomyosis. Biomed Res Int. 2017;2017:2518396. doi: 10.1155/2017/2518396
- Azzi R. Adenomyosis: current perspectives. Obstet Gynecol Clin North Am. 1989;16:221-235.
- Parker JD, Leondires M, Sinaii N, et al. Persistence of dysmenorrhea and nonmenstrual pain after optimal endometriosis surgery may indicate adenomyosis. Fertil Steril. 2006;86:711-715. doi: 10.1016/j.fertnstert.2006.01.030
- Martire FG, Lazzeri L, Conway F, et al. Adolescence and endometriosis: symptoms, ultrasound signs and early diagnosis. Fertil Steril. 2020;114:1049-1057. doi: 10.1016 /j.fertnstert.2020.06.012
- Decter D, Arbib N, Markovitz H, et al. Sonographic signs of adenomyosis in women with endometriosis are associated with infertility. J Clin Med. 2021;10:2355. doi: 10.3390 /jcm10112355
- Brucker SY, Huebner M, Wallwiener M, et al. Clinical characteristics indicating adenomyosis coexisting with leiomyomas: a retrospective, questionnaire-based study. Fertil Steril. 2014;101:237-241.e1. doi: 10.1016 /j.fertnstert.2013.09.038
- Perelló MF, Martínez-Zamora MÁ, Torres X, et al. Endometriotic pain is associated with adenomyosis but not with the compartments affected by deep infiltrating endometriosis. Gynecol Obstet Invest. 2017;82:240-246. doi: 10.1159/000447633
- Younes G, Tulandi T. Effects of adenomyosis on in vitro fertilization treatment outcomes: a metaanalysis. Fertil Steril. 2017;108:483-490.e3. doi: 10.1016 /j.fertnstert.2017.06.025
- Nirgianakis K, Kalaitzopoulos DR, Schwartz ASK, et al. Fertility, pregnancy and neonatal outcomes of patients with adenomyosis: a systematic review and meta-analysis. Reprod BioMed Online. 2021;42:185-206. doi: 10.1016 /j.rbmo.2020.09.023
- Ono Y, Ota H, Takimoto K, et al. Perinatal outcomes associated with the positional relationship between the placenta and the adenomyosis lesion. J Gynecol Obstet Hum Reprod. 2021;50:102114. doi: 10.1016/j.jogoh.2021.102114
- Barrier BF, Malinowski MJ, Dick EJ Jr, et al. Adenomyosis in the baboon is associated with primary infertility. Fertil Steril. 2004;82(suppl 3):1091-1094. doi: 10.1016 /j.fertnstert.2003.11.065
- Vercellini P, Consonni D, Barbara G, et al. Adenomyosis and reproductive performance after surgery for rectovaginal and colorectal endometriosis: a systematic review and meta-analysis. Reprod Biomed Online. 2014;28:704-713. doi: 10.1016/j.rbmo.2014.02.006
- Exacoustos C, Lazzeri L, Martire FG, et al. Ultrasound findings of adenomyosis in adolescents: type and grade of the disease. J Minim Invasive Gynecol. 2021;29:291.e1-299.e1. doi: 10.1016/j.jmig.2021.08.023
- Loring M, Chen TY, Isaacson KB. A systematic review of adenomyosis: it is time to reassess what we thought we knew about the disease. J Minim Invasive Gynecol. 2021;28:644655. doi: 10.1016/j.jmig.2020.10.012
- Bourdon M, Santulli P, Oliveira J, et al. Focal adenomyosis is associated with primary infertility. Fertil Steril. 2020;114:1271-1277. doi: 10.1016/j.fertnstert.2020.06.018
- Lan J, Wu Y, Wu Z, et al. Ultra-long GnRH agonist protocol during IVF/ICSI improves pregnancy outcomes in women with adenomyosis: a retrospective cohort study. Front Endocrinol (Lausanne). 2021;12:609771. doi: 10.3389 /fendo.2021.609771
- Gnecco JS, Brown AT, Kan EL, et al. Physiomimetic models of adenomyosis. Semin Reprod Med. 2020;38:179-196. doi: 10.1055/s-0040-1719084
- Harmsen MJ, Trommelen LM, de Leeuw RA, et al. Uterine junctional zone and adenomyosis: comparison of MRI, transvaginal ultrasound and histology. Ultrasound Obstet Gynecol. 2023;62:42-60. doi: 10.1002/uog.26117
- Xie T, Xu X, Yang Y, et al. The role of abnormal uterine junction zone in the occurrence and development of adenomyosis. Reprod Sci. 2022;29:2719-2730. doi: 10.1007/s43032-021 -00684-2
- Lazzeri L, Morosetti G, Centini G, et al. A sonographic classification of adenomyosis: interobserver reproducibility in the evaluation of type and degree of the myometrial involvement. Fertil Steril. 2018;110:1154-1161.e3. doi: 10.1016 /j.fertnstert.2018.06.031
- Tahlan A, Nanda A, Mohan H. Uterine adenomyoma: a clinicopathologic review of 26 cases and a review of the literature. Int J Gynecol Pathol. 2006;25:361-365. doi: 10.1097/01.pgp.0000209570.08716.b3
- Chung K, Wallace J, Kim S-Y, et al. Structural and molecular interrogation of intact biological systems. Nature. 2013;497:332-337. doi: 10.1038/nature12107
- Yamaguchi M, Yoshihara K, Suda K, et al. Three-dimensional understanding of the morphological complexity of the human uterine endometrium. iScience. 2021;24:102258. doi: 10.1016/j.isci.2021.102258
- Vannuccini S, Tosti C, Carmona F, et al. Pathogenesis of adenomyosis: an update on molecular mechanisms. Reprod Biomed Online. 2017;35:592-601. doi: 10.1016 /j.rbmo.2017.06.016
- Zhai J, Vannuccini S, Petraglia F, et al. Adenomyosis: mechanisms and pathogenesis. Semin Reprod Med. 2020;38:129-143. doi: 10.1055/s-0040-1716687
- Munro MG. Uterine polyps, adenomyosis, leiomyomas, and endometrial receptivity. Fertil Steril. 2019;111:629-640. doi: 10.1016/j.fertnstert.2019.02.008
- Kay N, Huang C-Y, Shiu L-Y, et al. TGF-β1 neutralization improves pregnancy outcomes by restoring endometrial receptivity in mice with adenomyosis. Reprod Sci. 2021;28:877-887. doi: 10.1007/s43032-020-00308-1
- Habiba M, Benagiano G. Classifying adenomyosis: progress and challenges. Int J Environ Res Public Health. 2021;18:12386. doi: 10.3390/ijerph182312386
- Movilla P, Morris S, Isaacson K. A systematic review of tissue sampling techniques for the diagnosis of adenomyosis. J Minim Invasive Gynecol. 2020;27:344-351. doi: 10.1016 /j.jmig.2019.09.001
- Agostinho L, Cruz R, Osório F, et al. MRI for adenomyosis: a pictorial review. Insights Imaging. 2017;8:549-556. doi: 10.1007/s13244-017-0576-z
- Bazot M, Cortez A, Darai E, et al. Ultrasonography compared with magnetic resonance imaging for the diagnosis of adenomyosis: correlation with histopathology. Hum Reprod. 2001;16:2427-2433. doi: 10.1093/humrep/16.11.2427
- Reinhold C, Tafazoli F, Mehio A, et al. Uterine adenomyosis: endovaginal US and MR imaging features with histopathologic correlation. Radiographics. 1999;19:S147-S160. doi: 10.1148 /radiographics.19.suppl_1.g99oc13s147
- Rees CO, Nederend J, Mischi M, et al. Objective measures of adenomyosis on MRI and their diagnostic accuracy—a systematic review & meta-analysis. Acta Obstet Gynecol Scand. 2021;100:1377-1391.
- Chapron C, Vannuccini S, Santulli P, et al. Diagnosing adenomyosis: an integrated clinical and imaging approach. Hum Reprod Update. 2020;26:392-411. doi: 10.1093 /humupd/dmz049
- Liu L, Li W, Leonardi M, et al. Diagnostic accuracy of transvaginal ultrasound and magnetic resonance imaging for adenomyosis: systematic review and meta-analysis and review of sonographic diagnostic criteria. J Ultrasound Med. 2021;40:2289-2306. doi: 10.1002/jum.15635
- Bazot M, Daraï E. Role of transvaginal sonography and magnetic resonance imaging in the diagnosis of uterine adenomyosis. Fertil Steril. 2018;109:389-397. doi: 10.1016 /j.fertnstert.2018.01.024
- Van den Bosch T, Dueholm M, Leone FPG, et al. Terms, definitions and measurements to describe sonographic features of myometrium and uterine masses: a consensus opinion from the Morphological Uterus Sonographic Assessment (MUSA) group. Ultrasound Obstet Gynecol. 2015;46:284-298. doi: 10.1002/uog.14806
- Săsăran V, Turdean S, Gliga M, et al. Value of strainratio elastography in the diagnosis and differentiation of uterine fibroids and adenomyosis. J Pers Med. 2021;11:824. doi: 10.3390/jpm11080824
- Di Spiezio Sardo A, Calagna G, Santangelo F, et al. The role of hysteroscopy in the diagnosis and treatment of adenomyosis. Biomed Res Int. 2017;2017:2518396. doi: 10.1155/2017/2518396
- Azzi R. Adenomyosis: current perspectives. Obstet Gynecol Clin North Am. 1989;16:221-235.
- Parker JD, Leondires M, Sinaii N, et al. Persistence of dysmenorrhea and nonmenstrual pain after optimal endometriosis surgery may indicate adenomyosis. Fertil Steril. 2006;86:711-715. doi: 10.1016/j.fertnstert.2006.01.030
- Martire FG, Lazzeri L, Conway F, et al. Adolescence and endometriosis: symptoms, ultrasound signs and early diagnosis. Fertil Steril. 2020;114:1049-1057. doi: 10.1016 /j.fertnstert.2020.06.012
- Decter D, Arbib N, Markovitz H, et al. Sonographic signs of adenomyosis in women with endometriosis are associated with infertility. J Clin Med. 2021;10:2355. doi: 10.3390 /jcm10112355
- Brucker SY, Huebner M, Wallwiener M, et al. Clinical characteristics indicating adenomyosis coexisting with leiomyomas: a retrospective, questionnaire-based study. Fertil Steril. 2014;101:237-241.e1. doi: 10.1016 /j.fertnstert.2013.09.038
- Perelló MF, Martínez-Zamora MÁ, Torres X, et al. Endometriotic pain is associated with adenomyosis but not with the compartments affected by deep infiltrating endometriosis. Gynecol Obstet Invest. 2017;82:240-246. doi: 10.1159/000447633
- Younes G, Tulandi T. Effects of adenomyosis on in vitro fertilization treatment outcomes: a metaanalysis. Fertil Steril. 2017;108:483-490.e3. doi: 10.1016 /j.fertnstert.2017.06.025
- Nirgianakis K, Kalaitzopoulos DR, Schwartz ASK, et al. Fertility, pregnancy and neonatal outcomes of patients with adenomyosis: a systematic review and meta-analysis. Reprod BioMed Online. 2021;42:185-206. doi: 10.1016 /j.rbmo.2020.09.023
- Ono Y, Ota H, Takimoto K, et al. Perinatal outcomes associated with the positional relationship between the placenta and the adenomyosis lesion. J Gynecol Obstet Hum Reprod. 2021;50:102114. doi: 10.1016/j.jogoh.2021.102114
- Barrier BF, Malinowski MJ, Dick EJ Jr, et al. Adenomyosis in the baboon is associated with primary infertility. Fertil Steril. 2004;82(suppl 3):1091-1094. doi: 10.1016 /j.fertnstert.2003.11.065
- Vercellini P, Consonni D, Barbara G, et al. Adenomyosis and reproductive performance after surgery for rectovaginal and colorectal endometriosis: a systematic review and meta-analysis. Reprod Biomed Online. 2014;28:704-713. doi: 10.1016/j.rbmo.2014.02.006
Managing intrahepatic cholestasis of pregnancy
CASE Pregnant woman with intense itching
A 28-year-old woman (G1P0) is seen for a routine prenatal visit at 32 3/7 weeks’ gestation. She reports having generalized intense itching, including on her palms and soles, that is most intense at night and has been present for approximately 1 week. Her pregnancy is otherwise uncomplicated to date. Physical exam is within normal limits, with no evidence of a skin rash. Cholestasis of pregnancy is suspected, and laboratory tests are ordered, including bile acids and liver transaminases. Test results show that her aspartate transaminase (AST) and alanine transaminase (ALT) levels are mildly elevated at 55 IU/L and 41 IU/L, respectively, and several days later her bile acid level result is 21 µmol/L.
How should this patient be managed?
Intrahepatic cholestasis of pregnancy (ICP) affects 0.5% to 0.7% of pregnant individuals and results in maternal pruritus and elevated serum bile acid levels.1-3 Pruritus in ICP typically is generalized, including occurrence on the palms of the hands and soles of the feet, and it often is reported to be worse at night.4 Up to 25% of pregnant women will develop pruritus during pregnancy but the majority will not have ICP.2,5 Patients with ICP have no associated rash, but clinicians may note excoriations on exam. ICP typically presents in the third trimester of pregnancy but has been reported to occur earlier in gestation.6
Making a diagnosis of ICP
The presence of maternal pruritus in the absence of a skin condition along with elevated levels of serum bile acids are required for the diagnosis of ICP.7 Thus, a thorough history and physical exam is recommended to rule out another skin condition that could potentially explain the patient’s pruritus.
Some controversy exists regarding the bile acid level cutoff that should be used to make a diagnosis of ICP.8 It has been noted that nonfasting serum bile acid levels in pregnancy are considerably higher than those in in the nonpregnant state, and an upper limit of 18 µmol/L has been proposed as a cutoff in pregnancy.9 However, nonfasting total serum bile acids also have been shown to vary considerably by race, with levels 25.8% higher in Black women compared with those in White women and 24.3% higher in Black women compared with those in south Asian women.9 This raises the question of whether we should be using race-specific bile acid values to make a diagnosis of ICP.
Bile acid levels also vary based on whether a patient is in a fasting or postprandial state.10 Despite this variation, most guidelines do not recommend testing fasting bile acid levels as the postprandial state effect overall is small.7,9,11 The Society for Maternal-Fetal Medicine (SMFM) recommends that if a pregnancy-specific bile acid range is available from the laboratory, then the upper limit of normal for pregnancy should be used when making a diagnosis of ICP.7 The SMFM guidelines also acknowledge, however, that pregnancy-specific values rarely are available, and in this case, levels above the upper limit of normal—often 10 µmol/L should be considered diagnostic for ICP until further evidence regarding optimal bile acid cutoff levels in pregnancy becomes available.7
For patients with suspected ICP, liver transaminase levels should be measured in addition to nonfasting serum bile acid levels.7 A thorough history should include assessment for additional symptoms of liver disease, such as changes in weight, appetite, jaundice, excessive fatigue, malaise, and abdominal pain.7 Elevated transaminases levels may be associated with ICP, but they are not necessary for diagnosis. In the absence of additional clinical symptoms that suggest underlying liver disease or severe early onset ICP, additional evaluation beyond nonfasting serum bile acids and liver transaminase levels, such as liver ultrasonography or evaluation for viral or autoimmune hepatitis, is not recommended.7 Obstetric care clinicians should be aware that there is an increased incidence of preeclampsia among patients with ICP, although no specific guidance regarding further recommendations for screening is provided.7

Continue to: Risks associated with ICP...
Risks associated with ICP
For both patients and clinicians, the greatest concern among patients with ICP is the increased risk of stillbirth. Stillbirth risk in ICP appears to be related to serum bile acid levels and has been reported to be highest in patients with bile acid levels greater than 100 µmol/L. A systematic review and meta-analysis of ICP studies demonstrated no increased risk of stillbirth among patients with bile acid levels less than 100 µmol/L.12 These results, however, must be interpreted with extreme caution as the majority of studies included patients with ICP who were actively managed with attempts to mitigate the risk of stillbirth.7
In the absence of additional pregnancy risk factors, the risk of stillbirth among patients with ICP and serum bile acid levels between 19 and 39 µmol/L does not appear to be elevated above their baseline risk.11 The same is true for pregnant individuals with ICP and no additional pregnancy risk factors with serum bile acid levels between 40 and 99 µmol/L until approximately 38 weeks’ gestation, when the risk of stillbirth is elevated.11 The risk of stillbirth is elevated in ICP with peak bile acid levels greater than 100 µmol/L, with an absolute risk of 3.44%.11
Management of patients with ICP
Laboratory evaluation
There is no consensus on the need for repeat testing of bile acid levels in patients with ICP. SMFM advises that follow-up testing of bile acid levels may help to guide delivery timing, especially in cases of severe ICP, but the society recommends against serial testing.7 By contrast, the Royal College of Obstetricians and Gynaecologists (RCOG) provides a detailed algorithm regarding time intervals between serum bile acid level testing to guide delivery timing.11 The TABLE lists the strategy for reassessment of serum bile acid levels in ICP as recommended by the RCOG.11

In the United States, bile acid testing traditionally takes several days as the testing is commonly performed at reference laboratories. We therefore suggest that clinicians consider repeating bile acid level testing in situations in which the timing of delivery may be altered if further elevations of bile acid levels were noted. This is particularly relevant for patients diagnosed with ICP early in the third trimester when repeat bile acid levels would still allow for an adjustment in delivery timing.
Antepartum fetal surveillance
Unfortunately, antepartum fetal testing for pregnant patients with ICP does not appear to reliably predict or prevent stillbirth as several studies have reported stillbirths within days of normal fetal testing.13-16 It is therefore important to counsel pregnant patients regarding monitoring of fetal movements and advise them to present for evaluation if concerns arise.
Currently, SMFM recommends that patients with ICP should begin antenatal fetal surveillance at a gestational age when abnormal fetal testing would result in delivery.7 Patients should be counseled, however, regarding the unpredictability of stillbirth with ICP in the setting of a low absolute risk of such.
Medications
While SMFM recommends a starting dose of ursodeoxycholic acid 10 to 15 mg/kg per day divided into 2 or 3 daily doses as first-line therapy for the treatment of maternal symptoms of ICP, it is important to acknowledge that the goal of treatment is to alleviate maternal symptoms as there is no evidence that ursodeoxycholic acid improves either maternal serum bile acid levels or perinatal outcomes.7,17,18 Since publication of the guidelines, ursodeoxycholic acid’s lack of benefit has been further confirmed in a meta-analysis, and thus discontinuation is not unreasonable in the absence of any improvement in maternal symptoms.18
Timing of delivery
The optimal management of ICP remains unknown. SMFM recommends delivery based on peak serum bile acid levels. Delivery is recommended at 36 weeks’ gestation with ICP and total bile acid levels greater than 100 µmol/L as these patients have the greatest risk of stillbirth.7 For patients with ICP and bile acid levels less than 100 µmol/L, delivery is recommended between 36 0/7 and 39 0/7 weeks’ gestation.7 This is a wide gestational age window for clinicians to consider timing of delivery, and certainly the risks of stillbirth should be carefully balanced with the morbidity associated with a preterm or early term delivery.
For patients with ICP who have bile acid levels greater than 40 µmol/L, it is reasonable to consider delivery earlier in the gestational age window, given an evidence of increased risk of stillbirth after 38 weeks.7,12 For patients with ICP who have bile acid levels less than 40 µmol/L, delivery closer to 39 weeks’ gestation is recommended, as the risk of stillbirth does not appear to be increased above the baseline risk.7,12 Clinicians should be aware that the presence of concomitant morbidities, such as preeclampsia and gestational diabetes, are associated with an increased risk of stillbirth and should be considered for delivery planning.19
Postpartum follow-up
Routine laboratory evaluation following delivery is not recommended.7 However, in the presence of persistent pruritus or other signs and symptoms of hepatobiliary disease, liver function tests should be repeated with referral to hepatology if results are persistently abnormal 4 to 6 weeks postpartum.7
CASE Patient follow-up and outcomes
- Abedin P, Weaver JB, Egginton E. Intrahepatic cholestasis of pregnancy: prevalence and ethnic distribution. Ethn Health. 1999;4:35-37.
- Kenyon AP, Tribe RM, Nelson-Piercy C, et al. Pruritus in pregnancy: a study of anatomical distribution and prevalence in relation to the development of obstetric cholestasis. Obstet Med. 2010;3:25-29.
- Wikstrom Shemer E, Marschall HU, Ludvigsson JF, et al. Intrahepatic cholestasis of pregnancy and associated adverse pregnancy and fetal outcomes: a 12-year population-based cohort study. BJOG. 2013;120:717-723.
- Ambros-Rudolph CM, Glatz M, Trauner M, et al. The importance of serum bile acid level analysis and treatment with ursodeoxycholic acid in intrahepatic cholestasis of pregnancy: a case series from central Europe. Arch Dermatol. 2007;143:757-762.
- Szczech J, Wiatrowski A, Hirnle L, et al. Prevalence and relevance of pruritus in pregnancy. Biomed Res Int. 2017;2017:4238139.
- Geenes V, Williamson C. Intrahepatic cholestasis of pregnancy. World J Gastroenterol. 2009;15:2049-2066.
- Society for Maternal-Fetal Medicine; Lee RH, Greenberg M, Metz TD, et al. Society for Maternal-Fetal Medicine Consult Series #53: intrahepatic cholestasis of pregnancy: replaces Consult #13, April 2011. Am J Obstet Gynecol. 2021;224:B2-B9.
- Horgan R, Bitas C, Abuhamad A. Intrahepatic cholestasis of pregnancy: a comparison of Society for Maternal-Fetal Medicine and the Royal College of Obstetricians and Gynaecologists’ guidelines. Am J Obstet Gynecol MFM. 2023;5:100838.
- Mitchell AL, Ovadia C, Syngelaki A, et al. Re-evaluating diagnostic thresholds for intrahepatic cholestasis of pregnancy: case-control and cohort study. BJOG. 2021;128:1635-1644.
- Adams A, Jacobs K, Vogel RI, et al. Bile acid determination after standardized glucose load in pregnant women. AJP Rep. 2015;5:e168-e171.
- Girling J, Knight CL, Chappell L; Royal College of Obstetricians and Gynaecologists. Intrahepatic cholestasis of pregnancy: Green-top guideline no. 43, June 2022. BJOG. 2022;129:e95-e114.
- Ovadia C, Seed PT, Sklavounos A, et al. Association of adverse perinatal outcomes of intrahepatic cholestasis of pregnancy with biochemical markers: results of aggregate and individual patient data meta-analyses. Lancet. 2019;393:899-909.
- Alsulyman OM, Ouzounian JG, Ames-Castro M, et al. Intrahepatic cholestasis of pregnancy: perinatal outcome associated with expectant management. Am J Obstet Gynecol. 1996;175:957-960.
- Herrera CA, Manuck TA, Stoddard GJ, et al. Perinatal outcomes associated with intrahepatic cholestasis of pregnancy. J Matern Fetal Neonatal Med. 2018;31:1913-1920.
- Lee RH, Incerpi MH, Miller DA, et al. Sudden fetal death in intrahepatic cholestasis of pregnancy. Obstet Gynecol. 2009;113:528-531.
- Sentilhes L, Verspyck E, Pia P, et al. Fetal death in a patient with intrahepatic cholestasis of pregnancy. Obstet Gynecol. 2006;107:458-460.
- Chappell LC, Bell JL, Smith A, et al; PITCHES Study Group. Ursodeoxycholic acid versus placebo in women with intrahepatic cholestasis of pregnancy (PITCHES): a randomised controlled trial. Lancet. 2019;394:849-860.
- Ovadia C, Sajous J, Seed PT, et al. Ursodeoxycholic acid in intrahepatic cholestasis of pregnancy: a systematic review and individual participant data meta-analysis. Lancet Gastroenterol Hepatol. 2021;6:547-558.
- Geenes V, Chappell LC, Seed PT, et al. Association of severe intrahepatic cholestasis of pregnancy with adverse pregnancy outcomes: a prospective population-based case-control study. Hepatology. 2014;59:1482-1491.
CASE Pregnant woman with intense itching
A 28-year-old woman (G1P0) is seen for a routine prenatal visit at 32 3/7 weeks’ gestation. She reports having generalized intense itching, including on her palms and soles, that is most intense at night and has been present for approximately 1 week. Her pregnancy is otherwise uncomplicated to date. Physical exam is within normal limits, with no evidence of a skin rash. Cholestasis of pregnancy is suspected, and laboratory tests are ordered, including bile acids and liver transaminases. Test results show that her aspartate transaminase (AST) and alanine transaminase (ALT) levels are mildly elevated at 55 IU/L and 41 IU/L, respectively, and several days later her bile acid level result is 21 µmol/L.
How should this patient be managed?
Intrahepatic cholestasis of pregnancy (ICP) affects 0.5% to 0.7% of pregnant individuals and results in maternal pruritus and elevated serum bile acid levels.1-3 Pruritus in ICP typically is generalized, including occurrence on the palms of the hands and soles of the feet, and it often is reported to be worse at night.4 Up to 25% of pregnant women will develop pruritus during pregnancy but the majority will not have ICP.2,5 Patients with ICP have no associated rash, but clinicians may note excoriations on exam. ICP typically presents in the third trimester of pregnancy but has been reported to occur earlier in gestation.6
Making a diagnosis of ICP
The presence of maternal pruritus in the absence of a skin condition along with elevated levels of serum bile acids are required for the diagnosis of ICP.7 Thus, a thorough history and physical exam is recommended to rule out another skin condition that could potentially explain the patient’s pruritus.
Some controversy exists regarding the bile acid level cutoff that should be used to make a diagnosis of ICP.8 It has been noted that nonfasting serum bile acid levels in pregnancy are considerably higher than those in in the nonpregnant state, and an upper limit of 18 µmol/L has been proposed as a cutoff in pregnancy.9 However, nonfasting total serum bile acids also have been shown to vary considerably by race, with levels 25.8% higher in Black women compared with those in White women and 24.3% higher in Black women compared with those in south Asian women.9 This raises the question of whether we should be using race-specific bile acid values to make a diagnosis of ICP.
Bile acid levels also vary based on whether a patient is in a fasting or postprandial state.10 Despite this variation, most guidelines do not recommend testing fasting bile acid levels as the postprandial state effect overall is small.7,9,11 The Society for Maternal-Fetal Medicine (SMFM) recommends that if a pregnancy-specific bile acid range is available from the laboratory, then the upper limit of normal for pregnancy should be used when making a diagnosis of ICP.7 The SMFM guidelines also acknowledge, however, that pregnancy-specific values rarely are available, and in this case, levels above the upper limit of normal—often 10 µmol/L should be considered diagnostic for ICP until further evidence regarding optimal bile acid cutoff levels in pregnancy becomes available.7
For patients with suspected ICP, liver transaminase levels should be measured in addition to nonfasting serum bile acid levels.7 A thorough history should include assessment for additional symptoms of liver disease, such as changes in weight, appetite, jaundice, excessive fatigue, malaise, and abdominal pain.7 Elevated transaminases levels may be associated with ICP, but they are not necessary for diagnosis. In the absence of additional clinical symptoms that suggest underlying liver disease or severe early onset ICP, additional evaluation beyond nonfasting serum bile acids and liver transaminase levels, such as liver ultrasonography or evaluation for viral or autoimmune hepatitis, is not recommended.7 Obstetric care clinicians should be aware that there is an increased incidence of preeclampsia among patients with ICP, although no specific guidance regarding further recommendations for screening is provided.7

Continue to: Risks associated with ICP...
Risks associated with ICP
For both patients and clinicians, the greatest concern among patients with ICP is the increased risk of stillbirth. Stillbirth risk in ICP appears to be related to serum bile acid levels and has been reported to be highest in patients with bile acid levels greater than 100 µmol/L. A systematic review and meta-analysis of ICP studies demonstrated no increased risk of stillbirth among patients with bile acid levels less than 100 µmol/L.12 These results, however, must be interpreted with extreme caution as the majority of studies included patients with ICP who were actively managed with attempts to mitigate the risk of stillbirth.7
In the absence of additional pregnancy risk factors, the risk of stillbirth among patients with ICP and serum bile acid levels between 19 and 39 µmol/L does not appear to be elevated above their baseline risk.11 The same is true for pregnant individuals with ICP and no additional pregnancy risk factors with serum bile acid levels between 40 and 99 µmol/L until approximately 38 weeks’ gestation, when the risk of stillbirth is elevated.11 The risk of stillbirth is elevated in ICP with peak bile acid levels greater than 100 µmol/L, with an absolute risk of 3.44%.11
Management of patients with ICP
Laboratory evaluation
There is no consensus on the need for repeat testing of bile acid levels in patients with ICP. SMFM advises that follow-up testing of bile acid levels may help to guide delivery timing, especially in cases of severe ICP, but the society recommends against serial testing.7 By contrast, the Royal College of Obstetricians and Gynaecologists (RCOG) provides a detailed algorithm regarding time intervals between serum bile acid level testing to guide delivery timing.11 The TABLE lists the strategy for reassessment of serum bile acid levels in ICP as recommended by the RCOG.11

In the United States, bile acid testing traditionally takes several days as the testing is commonly performed at reference laboratories. We therefore suggest that clinicians consider repeating bile acid level testing in situations in which the timing of delivery may be altered if further elevations of bile acid levels were noted. This is particularly relevant for patients diagnosed with ICP early in the third trimester when repeat bile acid levels would still allow for an adjustment in delivery timing.
Antepartum fetal surveillance
Unfortunately, antepartum fetal testing for pregnant patients with ICP does not appear to reliably predict or prevent stillbirth as several studies have reported stillbirths within days of normal fetal testing.13-16 It is therefore important to counsel pregnant patients regarding monitoring of fetal movements and advise them to present for evaluation if concerns arise.
Currently, SMFM recommends that patients with ICP should begin antenatal fetal surveillance at a gestational age when abnormal fetal testing would result in delivery.7 Patients should be counseled, however, regarding the unpredictability of stillbirth with ICP in the setting of a low absolute risk of such.
Medications
While SMFM recommends a starting dose of ursodeoxycholic acid 10 to 15 mg/kg per day divided into 2 or 3 daily doses as first-line therapy for the treatment of maternal symptoms of ICP, it is important to acknowledge that the goal of treatment is to alleviate maternal symptoms as there is no evidence that ursodeoxycholic acid improves either maternal serum bile acid levels or perinatal outcomes.7,17,18 Since publication of the guidelines, ursodeoxycholic acid’s lack of benefit has been further confirmed in a meta-analysis, and thus discontinuation is not unreasonable in the absence of any improvement in maternal symptoms.18
Timing of delivery
The optimal management of ICP remains unknown. SMFM recommends delivery based on peak serum bile acid levels. Delivery is recommended at 36 weeks’ gestation with ICP and total bile acid levels greater than 100 µmol/L as these patients have the greatest risk of stillbirth.7 For patients with ICP and bile acid levels less than 100 µmol/L, delivery is recommended between 36 0/7 and 39 0/7 weeks’ gestation.7 This is a wide gestational age window for clinicians to consider timing of delivery, and certainly the risks of stillbirth should be carefully balanced with the morbidity associated with a preterm or early term delivery.
For patients with ICP who have bile acid levels greater than 40 µmol/L, it is reasonable to consider delivery earlier in the gestational age window, given an evidence of increased risk of stillbirth after 38 weeks.7,12 For patients with ICP who have bile acid levels less than 40 µmol/L, delivery closer to 39 weeks’ gestation is recommended, as the risk of stillbirth does not appear to be increased above the baseline risk.7,12 Clinicians should be aware that the presence of concomitant morbidities, such as preeclampsia and gestational diabetes, are associated with an increased risk of stillbirth and should be considered for delivery planning.19
Postpartum follow-up
Routine laboratory evaluation following delivery is not recommended.7 However, in the presence of persistent pruritus or other signs and symptoms of hepatobiliary disease, liver function tests should be repeated with referral to hepatology if results are persistently abnormal 4 to 6 weeks postpartum.7
CASE Patient follow-up and outcomes
CASE Pregnant woman with intense itching
A 28-year-old woman (G1P0) is seen for a routine prenatal visit at 32 3/7 weeks’ gestation. She reports having generalized intense itching, including on her palms and soles, that is most intense at night and has been present for approximately 1 week. Her pregnancy is otherwise uncomplicated to date. Physical exam is within normal limits, with no evidence of a skin rash. Cholestasis of pregnancy is suspected, and laboratory tests are ordered, including bile acids and liver transaminases. Test results show that her aspartate transaminase (AST) and alanine transaminase (ALT) levels are mildly elevated at 55 IU/L and 41 IU/L, respectively, and several days later her bile acid level result is 21 µmol/L.
How should this patient be managed?
Intrahepatic cholestasis of pregnancy (ICP) affects 0.5% to 0.7% of pregnant individuals and results in maternal pruritus and elevated serum bile acid levels.1-3 Pruritus in ICP typically is generalized, including occurrence on the palms of the hands and soles of the feet, and it often is reported to be worse at night.4 Up to 25% of pregnant women will develop pruritus during pregnancy but the majority will not have ICP.2,5 Patients with ICP have no associated rash, but clinicians may note excoriations on exam. ICP typically presents in the third trimester of pregnancy but has been reported to occur earlier in gestation.6
Making a diagnosis of ICP
The presence of maternal pruritus in the absence of a skin condition along with elevated levels of serum bile acids are required for the diagnosis of ICP.7 Thus, a thorough history and physical exam is recommended to rule out another skin condition that could potentially explain the patient’s pruritus.
Some controversy exists regarding the bile acid level cutoff that should be used to make a diagnosis of ICP.8 It has been noted that nonfasting serum bile acid levels in pregnancy are considerably higher than those in in the nonpregnant state, and an upper limit of 18 µmol/L has been proposed as a cutoff in pregnancy.9 However, nonfasting total serum bile acids also have been shown to vary considerably by race, with levels 25.8% higher in Black women compared with those in White women and 24.3% higher in Black women compared with those in south Asian women.9 This raises the question of whether we should be using race-specific bile acid values to make a diagnosis of ICP.
Bile acid levels also vary based on whether a patient is in a fasting or postprandial state.10 Despite this variation, most guidelines do not recommend testing fasting bile acid levels as the postprandial state effect overall is small.7,9,11 The Society for Maternal-Fetal Medicine (SMFM) recommends that if a pregnancy-specific bile acid range is available from the laboratory, then the upper limit of normal for pregnancy should be used when making a diagnosis of ICP.7 The SMFM guidelines also acknowledge, however, that pregnancy-specific values rarely are available, and in this case, levels above the upper limit of normal—often 10 µmol/L should be considered diagnostic for ICP until further evidence regarding optimal bile acid cutoff levels in pregnancy becomes available.7
For patients with suspected ICP, liver transaminase levels should be measured in addition to nonfasting serum bile acid levels.7 A thorough history should include assessment for additional symptoms of liver disease, such as changes in weight, appetite, jaundice, excessive fatigue, malaise, and abdominal pain.7 Elevated transaminases levels may be associated with ICP, but they are not necessary for diagnosis. In the absence of additional clinical symptoms that suggest underlying liver disease or severe early onset ICP, additional evaluation beyond nonfasting serum bile acids and liver transaminase levels, such as liver ultrasonography or evaluation for viral or autoimmune hepatitis, is not recommended.7 Obstetric care clinicians should be aware that there is an increased incidence of preeclampsia among patients with ICP, although no specific guidance regarding further recommendations for screening is provided.7

Continue to: Risks associated with ICP...
Risks associated with ICP
For both patients and clinicians, the greatest concern among patients with ICP is the increased risk of stillbirth. Stillbirth risk in ICP appears to be related to serum bile acid levels and has been reported to be highest in patients with bile acid levels greater than 100 µmol/L. A systematic review and meta-analysis of ICP studies demonstrated no increased risk of stillbirth among patients with bile acid levels less than 100 µmol/L.12 These results, however, must be interpreted with extreme caution as the majority of studies included patients with ICP who were actively managed with attempts to mitigate the risk of stillbirth.7
In the absence of additional pregnancy risk factors, the risk of stillbirth among patients with ICP and serum bile acid levels between 19 and 39 µmol/L does not appear to be elevated above their baseline risk.11 The same is true for pregnant individuals with ICP and no additional pregnancy risk factors with serum bile acid levels between 40 and 99 µmol/L until approximately 38 weeks’ gestation, when the risk of stillbirth is elevated.11 The risk of stillbirth is elevated in ICP with peak bile acid levels greater than 100 µmol/L, with an absolute risk of 3.44%.11
Management of patients with ICP
Laboratory evaluation
There is no consensus on the need for repeat testing of bile acid levels in patients with ICP. SMFM advises that follow-up testing of bile acid levels may help to guide delivery timing, especially in cases of severe ICP, but the society recommends against serial testing.7 By contrast, the Royal College of Obstetricians and Gynaecologists (RCOG) provides a detailed algorithm regarding time intervals between serum bile acid level testing to guide delivery timing.11 The TABLE lists the strategy for reassessment of serum bile acid levels in ICP as recommended by the RCOG.11

In the United States, bile acid testing traditionally takes several days as the testing is commonly performed at reference laboratories. We therefore suggest that clinicians consider repeating bile acid level testing in situations in which the timing of delivery may be altered if further elevations of bile acid levels were noted. This is particularly relevant for patients diagnosed with ICP early in the third trimester when repeat bile acid levels would still allow for an adjustment in delivery timing.
Antepartum fetal surveillance
Unfortunately, antepartum fetal testing for pregnant patients with ICP does not appear to reliably predict or prevent stillbirth as several studies have reported stillbirths within days of normal fetal testing.13-16 It is therefore important to counsel pregnant patients regarding monitoring of fetal movements and advise them to present for evaluation if concerns arise.
Currently, SMFM recommends that patients with ICP should begin antenatal fetal surveillance at a gestational age when abnormal fetal testing would result in delivery.7 Patients should be counseled, however, regarding the unpredictability of stillbirth with ICP in the setting of a low absolute risk of such.
Medications
While SMFM recommends a starting dose of ursodeoxycholic acid 10 to 15 mg/kg per day divided into 2 or 3 daily doses as first-line therapy for the treatment of maternal symptoms of ICP, it is important to acknowledge that the goal of treatment is to alleviate maternal symptoms as there is no evidence that ursodeoxycholic acid improves either maternal serum bile acid levels or perinatal outcomes.7,17,18 Since publication of the guidelines, ursodeoxycholic acid’s lack of benefit has been further confirmed in a meta-analysis, and thus discontinuation is not unreasonable in the absence of any improvement in maternal symptoms.18
Timing of delivery
The optimal management of ICP remains unknown. SMFM recommends delivery based on peak serum bile acid levels. Delivery is recommended at 36 weeks’ gestation with ICP and total bile acid levels greater than 100 µmol/L as these patients have the greatest risk of stillbirth.7 For patients with ICP and bile acid levels less than 100 µmol/L, delivery is recommended between 36 0/7 and 39 0/7 weeks’ gestation.7 This is a wide gestational age window for clinicians to consider timing of delivery, and certainly the risks of stillbirth should be carefully balanced with the morbidity associated with a preterm or early term delivery.
For patients with ICP who have bile acid levels greater than 40 µmol/L, it is reasonable to consider delivery earlier in the gestational age window, given an evidence of increased risk of stillbirth after 38 weeks.7,12 For patients with ICP who have bile acid levels less than 40 µmol/L, delivery closer to 39 weeks’ gestation is recommended, as the risk of stillbirth does not appear to be increased above the baseline risk.7,12 Clinicians should be aware that the presence of concomitant morbidities, such as preeclampsia and gestational diabetes, are associated with an increased risk of stillbirth and should be considered for delivery planning.19
Postpartum follow-up
Routine laboratory evaluation following delivery is not recommended.7 However, in the presence of persistent pruritus or other signs and symptoms of hepatobiliary disease, liver function tests should be repeated with referral to hepatology if results are persistently abnormal 4 to 6 weeks postpartum.7
CASE Patient follow-up and outcomes
- Abedin P, Weaver JB, Egginton E. Intrahepatic cholestasis of pregnancy: prevalence and ethnic distribution. Ethn Health. 1999;4:35-37.
- Kenyon AP, Tribe RM, Nelson-Piercy C, et al. Pruritus in pregnancy: a study of anatomical distribution and prevalence in relation to the development of obstetric cholestasis. Obstet Med. 2010;3:25-29.
- Wikstrom Shemer E, Marschall HU, Ludvigsson JF, et al. Intrahepatic cholestasis of pregnancy and associated adverse pregnancy and fetal outcomes: a 12-year population-based cohort study. BJOG. 2013;120:717-723.
- Ambros-Rudolph CM, Glatz M, Trauner M, et al. The importance of serum bile acid level analysis and treatment with ursodeoxycholic acid in intrahepatic cholestasis of pregnancy: a case series from central Europe. Arch Dermatol. 2007;143:757-762.
- Szczech J, Wiatrowski A, Hirnle L, et al. Prevalence and relevance of pruritus in pregnancy. Biomed Res Int. 2017;2017:4238139.
- Geenes V, Williamson C. Intrahepatic cholestasis of pregnancy. World J Gastroenterol. 2009;15:2049-2066.
- Society for Maternal-Fetal Medicine; Lee RH, Greenberg M, Metz TD, et al. Society for Maternal-Fetal Medicine Consult Series #53: intrahepatic cholestasis of pregnancy: replaces Consult #13, April 2011. Am J Obstet Gynecol. 2021;224:B2-B9.
- Horgan R, Bitas C, Abuhamad A. Intrahepatic cholestasis of pregnancy: a comparison of Society for Maternal-Fetal Medicine and the Royal College of Obstetricians and Gynaecologists’ guidelines. Am J Obstet Gynecol MFM. 2023;5:100838.
- Mitchell AL, Ovadia C, Syngelaki A, et al. Re-evaluating diagnostic thresholds for intrahepatic cholestasis of pregnancy: case-control and cohort study. BJOG. 2021;128:1635-1644.
- Adams A, Jacobs K, Vogel RI, et al. Bile acid determination after standardized glucose load in pregnant women. AJP Rep. 2015;5:e168-e171.
- Girling J, Knight CL, Chappell L; Royal College of Obstetricians and Gynaecologists. Intrahepatic cholestasis of pregnancy: Green-top guideline no. 43, June 2022. BJOG. 2022;129:e95-e114.
- Ovadia C, Seed PT, Sklavounos A, et al. Association of adverse perinatal outcomes of intrahepatic cholestasis of pregnancy with biochemical markers: results of aggregate and individual patient data meta-analyses. Lancet. 2019;393:899-909.
- Alsulyman OM, Ouzounian JG, Ames-Castro M, et al. Intrahepatic cholestasis of pregnancy: perinatal outcome associated with expectant management. Am J Obstet Gynecol. 1996;175:957-960.
- Herrera CA, Manuck TA, Stoddard GJ, et al. Perinatal outcomes associated with intrahepatic cholestasis of pregnancy. J Matern Fetal Neonatal Med. 2018;31:1913-1920.
- Lee RH, Incerpi MH, Miller DA, et al. Sudden fetal death in intrahepatic cholestasis of pregnancy. Obstet Gynecol. 2009;113:528-531.
- Sentilhes L, Verspyck E, Pia P, et al. Fetal death in a patient with intrahepatic cholestasis of pregnancy. Obstet Gynecol. 2006;107:458-460.
- Chappell LC, Bell JL, Smith A, et al; PITCHES Study Group. Ursodeoxycholic acid versus placebo in women with intrahepatic cholestasis of pregnancy (PITCHES): a randomised controlled trial. Lancet. 2019;394:849-860.
- Ovadia C, Sajous J, Seed PT, et al. Ursodeoxycholic acid in intrahepatic cholestasis of pregnancy: a systematic review and individual participant data meta-analysis. Lancet Gastroenterol Hepatol. 2021;6:547-558.
- Geenes V, Chappell LC, Seed PT, et al. Association of severe intrahepatic cholestasis of pregnancy with adverse pregnancy outcomes: a prospective population-based case-control study. Hepatology. 2014;59:1482-1491.
- Abedin P, Weaver JB, Egginton E. Intrahepatic cholestasis of pregnancy: prevalence and ethnic distribution. Ethn Health. 1999;4:35-37.
- Kenyon AP, Tribe RM, Nelson-Piercy C, et al. Pruritus in pregnancy: a study of anatomical distribution and prevalence in relation to the development of obstetric cholestasis. Obstet Med. 2010;3:25-29.
- Wikstrom Shemer E, Marschall HU, Ludvigsson JF, et al. Intrahepatic cholestasis of pregnancy and associated adverse pregnancy and fetal outcomes: a 12-year population-based cohort study. BJOG. 2013;120:717-723.
- Ambros-Rudolph CM, Glatz M, Trauner M, et al. The importance of serum bile acid level analysis and treatment with ursodeoxycholic acid in intrahepatic cholestasis of pregnancy: a case series from central Europe. Arch Dermatol. 2007;143:757-762.
- Szczech J, Wiatrowski A, Hirnle L, et al. Prevalence and relevance of pruritus in pregnancy. Biomed Res Int. 2017;2017:4238139.
- Geenes V, Williamson C. Intrahepatic cholestasis of pregnancy. World J Gastroenterol. 2009;15:2049-2066.
- Society for Maternal-Fetal Medicine; Lee RH, Greenberg M, Metz TD, et al. Society for Maternal-Fetal Medicine Consult Series #53: intrahepatic cholestasis of pregnancy: replaces Consult #13, April 2011. Am J Obstet Gynecol. 2021;224:B2-B9.
- Horgan R, Bitas C, Abuhamad A. Intrahepatic cholestasis of pregnancy: a comparison of Society for Maternal-Fetal Medicine and the Royal College of Obstetricians and Gynaecologists’ guidelines. Am J Obstet Gynecol MFM. 2023;5:100838.
- Mitchell AL, Ovadia C, Syngelaki A, et al. Re-evaluating diagnostic thresholds for intrahepatic cholestasis of pregnancy: case-control and cohort study. BJOG. 2021;128:1635-1644.
- Adams A, Jacobs K, Vogel RI, et al. Bile acid determination after standardized glucose load in pregnant women. AJP Rep. 2015;5:e168-e171.
- Girling J, Knight CL, Chappell L; Royal College of Obstetricians and Gynaecologists. Intrahepatic cholestasis of pregnancy: Green-top guideline no. 43, June 2022. BJOG. 2022;129:e95-e114.
- Ovadia C, Seed PT, Sklavounos A, et al. Association of adverse perinatal outcomes of intrahepatic cholestasis of pregnancy with biochemical markers: results of aggregate and individual patient data meta-analyses. Lancet. 2019;393:899-909.
- Alsulyman OM, Ouzounian JG, Ames-Castro M, et al. Intrahepatic cholestasis of pregnancy: perinatal outcome associated with expectant management. Am J Obstet Gynecol. 1996;175:957-960.
- Herrera CA, Manuck TA, Stoddard GJ, et al. Perinatal outcomes associated with intrahepatic cholestasis of pregnancy. J Matern Fetal Neonatal Med. 2018;31:1913-1920.
- Lee RH, Incerpi MH, Miller DA, et al. Sudden fetal death in intrahepatic cholestasis of pregnancy. Obstet Gynecol. 2009;113:528-531.
- Sentilhes L, Verspyck E, Pia P, et al. Fetal death in a patient with intrahepatic cholestasis of pregnancy. Obstet Gynecol. 2006;107:458-460.
- Chappell LC, Bell JL, Smith A, et al; PITCHES Study Group. Ursodeoxycholic acid versus placebo in women with intrahepatic cholestasis of pregnancy (PITCHES): a randomised controlled trial. Lancet. 2019;394:849-860.
- Ovadia C, Sajous J, Seed PT, et al. Ursodeoxycholic acid in intrahepatic cholestasis of pregnancy: a systematic review and individual participant data meta-analysis. Lancet Gastroenterol Hepatol. 2021;6:547-558.
- Geenes V, Chappell LC, Seed PT, et al. Association of severe intrahepatic cholestasis of pregnancy with adverse pregnancy outcomes: a prospective population-based case-control study. Hepatology. 2014;59:1482-1491.
Managing clinician burnout: Challenges and opportunities
Physicians have some of the highest rates of burnout among all professions.1 Complicating matters is that clinicians (including residents)2 may avoid seeking treatment out of fear it will affect their license or privileges.3 In this article, we consider burnout in greater detail, as well as ways of successfully addressing the level of burnout in the profession (FIGURE 1), including steps individual practitioners, health care entities, and regulators should consider to reduce burnout and its harmful effects.
How burnout becomes a problem
Six general factors are commonly identified as leading to clinician career dissatisfaction and burnout:4
1. work overload
2. lack of autonomy and control
3. inadequate rewards, financial and otherwise
4. work-home schedules
5. perception of lack of fairness
6. values conflict between the clinician and employer (including a breakdown of professional community).
At the top of the list of causes of burnout is often “administrative and bureaucratic headaches.”5 More specifically, electronic health records (EHRs), including computerized order entry, is commonly cited as a major cause of burnout.6,7 According to some studies, clinicians spend as much as 49% of working time doing clerical work,8 and studies found the extension of work into home life.9
Increased measurement of performance metrics in health care services are a significant contributor to physician burnout.10 These include pressure to see more patients, perform more procedures, and respond quickly to patient requests (eg, through email).7 As we will see, medical malpractice cases, or the risk of such cases, have also played a role in burnout in some medical specialties.11 The pandemic also contributed, at least temporarily, to burnout.12,13
Rates of burnout among physicians are notably higher than among the general population14 or other professions.6 Although physicians have generally entered clinical practice with lower rates of burnout than the general population,15 The American College of Obstetricians and Gynecologists (ACOG) reports that 40% to 75% of ObGyns “experience some form of professional burnout.”16,17 Other source(s) cite that 53% of ObGyns report burnout (TABLE 1).
Code QD85
Burnout is a syndrome conceptualized as resulting from chronic workplace stress that has not been successfully managed. It is characterized by 3 dimensions:
- feelings of energy depletion or exhaustion
- increased mental distance from one’s job, or feelings of negativism or cynicism related to one’s job
- a sense of ineffectiveness and lack of accomplishment. Burn-out refers specifically to phenomena in the occupational context and should not be applied to describe experiences in other areas of life. Exclusions to burnout diagnosis include adjustment disorder, disorders specifically associated with stress, anxiety or fear-related disorders, and mood disorders.
Reference
1. International Classification of Diseases Eleventh Revision (ICD-11). Geneva, Switzerland: World Health Organization; 2022.
Burnout undoubtedly contributes to professionals leaving practice, leading to a significant shortage of ObGyns.18 It also raises several significant legal concerns. Despite the enormity and seriousness of the problem, there is considerable optimism and assurance that the epidemic of burnout is solvable on the individual, specialty, and profession-wide levels. ACOG and other organizations have made suggestions for physicians, the profession, and to health care institutions for reducing burnout.19 This is not to say that solutions are simple or easy for individual professionals or institutions, but they are within the reach of the profession (FIGURE 2).
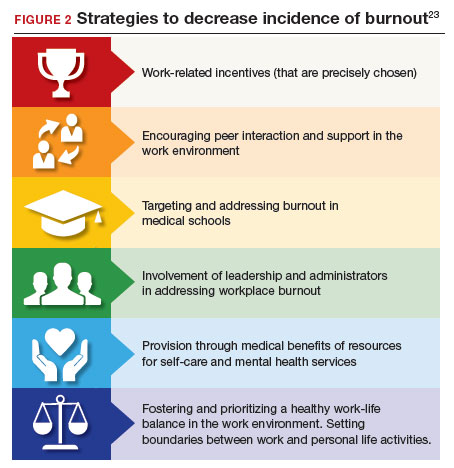
Suicide among health care professionals is one other concern (TABLE 2)20 and theoretically can stem from burnout, depression, and other psychosocial concerns.
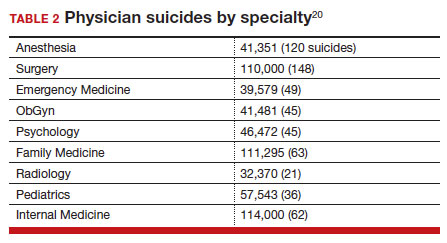
Costs of clinician burnout
Burnout is endemic among health care providers, with numerous studies detailing the professional, emotional, and financial costs. Prior to the pandemic, one analysis of nationwide fiscal costs associated with burnout estimated an annual cost of $4.6B due to physician turnover and reduced clinical hours.21 The COVID-19 epidemic has by all accounts worsened rates of health care worker burnout, particularly for those in high patient-contact positions.22
Female clinicians appear to be differentially affected; in one recent study women reported symptoms of burnout at twice the rate of their male counterparts.23 Whether burnout rates will return to pre-pandemic levels remains an open question, but since burnout is frequently related to one’s own assessment of work-life balance, it is possible that a longer term shift in burnout rates associated with post-pandemic occupational attitudes will be observed.
Combining factors contribute to burnout
Burnout is a universal occupational hazard, but extant data suggest that physicians and other health care providers may be at higher risk. Among physicians, younger age, female gender, and front-line specialty status appear associated with higher burnout rates.24 Given that ObGyn physicians are overwhelmingly female (60% of physicians and 86% of residents),25,26 gender-related burnout factors exist alongside other specific occupational burnout risks. While gender parity has been achieved among health care providers, gender disparities persist in terms of those in leadership positions, compensation, and other factors.22
The smattering of evidence suggesting that ObGyns have higher rates of burnout than many other specialties is understandable given the unique legal challenges confronting ObGyn practice. This may be of special significance because ObGyn malpractice insurance rates are among the highest of all specialties.27 The overall shortage of ObGyns has been exacerbated by the demonstrated negative effects on training and workforce representation stemming from recent legislation that has the effect of criminalizing certain aspects of ObGyn practice;28 for instance, uncertainty regarding abortion regulations.
These negative effects are particularly heightened in states in which the law is in flux or where there are continuing efforts to substantially limit access to abortion. The efforts to increase civil and even criminal penalties related to abortion care challenge ObGyns’ professional practices, as legal rules are frequently changing. In some states, ObGyns may face additional workloads secondary to a flight of ObGyns from restrictive jurisdictions in addition to legal and professional repercussions. In a small study of 19 genetic counselors dealing with restrictive legislation in the state of Ohio,29 increased stress and burnout rates were identified as a consequence of practice uncertainties under this legislation. It is certain that other professionals working in reproductive health care are similarly affected.30
The programs provide individual resources to providers in distress, periodically survey initiatives at Stanford to assess burnout at the organizational level, and provide input designed to spur organizational change to reduce the burden of burnout. Ways that they build community and connections include:
- Live Story Rounds events (as told by Stanford Medicine physicians)
- Commensality Groups (facilitated small discussion groups built around tested evidence)
- Aim to increase sense of connection and collegiality among physicians and build comradery at work
- CME-accredited physician wellness forum, including annual doctor’s day events
Continue to: Assessment of burnout...
Assessment of burnout
Numerous scales for the assessment of burnout exist. Of these, the 22-item Maslach Burnout Inventory (MBI) is the best studied. The MBI is a well-investigated tool for assessing burnout. The MBI consists of 3 major subscales measuring overall burnout, emotional exhaustion, depersonalization, and low personal accomplishment. It exists in numerous forms. For instance, the MBI-HSS (MP), adapted for medical personnel, is available. However, the most commonly used form for assessing burnout in clinicians is the MBI-HHS (Human Services Survey); approximately 85% of all burnout studies examined in a recent meta-analysis used this survey version.31 As those authors commented, while burnout is a recognized phenomenon, a great deal of variability in study design, interpretation of subscale scores, and sample selection makes generalizations regarding burnout difficult to assess.
The MBI in various forms has been extensively used over the past 40 years to assess burnout amongst physicians and physicians in training. While not the only instrument designed to measure such factors, it is by far the most prevalent. Williamson and colleagues32 compared the MBI with several other measures of quality of life and found good correlation between the various instruments used, a finding replicated by other studies.33 Brady and colleagues compared item responses to the Stanford Professional Fulfillment Index and the Min-Z Single-item Burnout scale (a 1-item screening measure) to MBI’s Emotional Exhaustion and Depersonalization subscales. Basing their findings on a survey of more than 1,300 physicians, they found that all analyzed scales were significantly correlated with such adverse outcomes as depression, distress, or intent to leave the profession.
It is important to note that most surveys of clinician burnout were conducted prior to the pandemic. While the psychometric analyses of the MBI and other scales are likely still germane, observed rates of clinician burnout have likely increased. Thus, comparisons of pre- and post-pandemic studies should factor in an increase in the incidence and prevalence of burnout.
Management strategies
In general, there are several interventions for managing burnout34:
- individual-focused (including self-care and communications-skills workshops)
- mindfulness training
- yoga
- meditation
- organizational/structural (workload reduction, schedule realignment, teamwork training, and group-delivered stress management interventions)
- combination(s) of the above.
There is little evidence to suggest that any particular individual intervention (whether delivered in individual or group-based formats) is superior to any other in treating clinician burnout. A recent analysis of 24 studies employing mindfulness-based interventions demonstrated generally positive results for such interventions.35 Other studies have also found general support for mindfulness-based interventions, although mindfulness is often integrated with other stress-reduction techniques, such as meditation, yoga, and communication skills. Such interventions are nonspecific but generally effective.
An accumulation of evidence to date suggests that a combination of individual and organizational interventions is most effective in combatting clinician burnout. No individual intervention can be successful without addressing root causes, such as overscheduling, lack of organizational support, and the effect of restrictive legislation on practice.
Several large teaching hospitals have established programs to address physician and health care provider burnout. Notable among these is the Stanford University School of Medicine’s WellMD and WellPhD programs (https://wellmd.stanford.edu/about.html). These programs were described by Olson and colleagues36 as using a model focused on practice efficiency, organizational culture, and personal resilience to enhance physicians’ well-being. (See “Aspects of the WellMD and WellPhD programs from Stanford University.”)
A growing number of institutions have established burnout programs to support physicians experiencing work/life imbalances and other aspects of burnout.37 In general, these share common features of assessment, individual and/or group intervention, and organizational change. Fear of repercussion may be one factor preventing physicians from seeking individual treatment for burnout.38 Importantly, they emphasize the need for professional confidentiality when offering treatment to patients within organizational settings. Those authors also reported that a focus on organizational engagement may be an important factor in addressing burnout in female physicians, as they tend to report lower levels of organizational engagement.
Continue to: Legal considerations...
Legal considerations
Until recently, physician burnout “received little notice in the legal literature.”39 Although there have been burnout legal consequences in the past, the legal issues are now becoming more visible.40
Medical malpractice
A well-documented consequence of burnout is an increase in errors.14 Medical errors, of course, are at the heart of malpractice claims. Technically, malpractice is medical or professional negligence. It is the breach of a duty owed by the physician, or other provider, or organization (defendant) to the patient, which causes injury to the plaintiff/patient.41
“Medical error” is generally a meaningful deviation from the “standard of care” or accepted medical practice.42 Many medical errors do not cause injury to the patient; in those cases, the negligence does not result in liability. In instances in which the negligence causes harm, the clinician and health care facility may be subject to liability for that injury. Fortunately, however, for a variety of reasons, most harmful medical errors do not result in a medical malpractice claim or lawsuit. The absence of a good clinician-patient relationship is likely associated with an increased inclination of a patient to file a malpractice action.43Clinician burnout may, therefore, contribute to increased malpractice claims in two ways. First, burnout likely leads to increased medical errors, perhaps because burnout is associated with lower concentration, inattention, reduced cognitive vigilance, and fatigue.8,44 It may also lead to less time with patients, reduced patient empathy, and lower patient rapport, which may make injured patients more likely to file a claim or lawsuit.45 Because the relationshipbetween burnout and medical error is bidirectional, malpractice claims tend to increase burnout, which increases error. Given the time it takes to resolve most malpractice claims, the uncertainty of medical malpractice may be especially stressful for health care providers.46,47
Burnout is not a mitigating factor in malpractice. Our sympathies may go out to a professional suffering from burnout, but it does not excuse or reduce liability—it may, indeed, be an aggravating factor. Clinicians who can diagnose burnout and know its negative consequences but fail to deal with their own burnout may be demonstrating negligence if there has been harm to a patient related to the burnout.48
Institutional or corporate liability to patients
Health care institutions have obligations to avoid injury to patients. Just as poorly maintained medical equipment may harm patients, so may burned-out professionals. Therefore, institutions have some obligation to supervise and avoid the increased risks to patients posed by professionals suffering from burnout.
Respondeat superior and institutional negligence. Institutional liability may arise in two ways, the first through agency, or respondeat superior. That is, if the physician or other professional is an employee (or similar agent) of the health care institution, that institution is generally responsible for the physician’s negligence during the employment.49 Even if the physician is not an employee (for example, an independent contractor providing care or using the hospital facilities), the health care facility may be liable for the physician’s negligence.50 Liability may occur, for example, if the health care facility was aware that the physician was engaged in careless practice or was otherwise a risk to patients but the facility did not take steps to avoid those risks.51 The basis for liability is that the health care organization owes a duty to patients to take reasonable care to ensure that its facilities are not used to injure patients negligently.52 Just as it must take care that unqualified physicians are not granted privileges to practice, it also must take reasonable steps to protect patients when it is aware (through nurses or other agents) of a physician’s negligent practice.
In one case, for example, the court found liability where a staff member had “severe” burnout in a physician’s office and failed to read fetal monitoring strips. The physician was found negligent for relying on the staff member who was obviously making errors in interpretation of fetal distress.53
Continue to: Legal obligations of health care organizations to physicians and others...
Legal obligations of health care organizations to physicians and others
In addition to obligations to patients, health care organizations may have obligations to employees (and others) at risk for injury. For example, assume a patient is diagnosed with a highly contagious disease. The health care organization would be obligated to warn, and take reasonable steps to protect, the staff (employees and independent contractors) from being harmed from exposure to the disease. This principle may apply to coworkers of employees with significant burnout, thereby presenting a danger in the workplace. The liability issue is more difficult for employees experiencing job-related burnout themselves. Organizations generally compensate injured employees through no-fault workers’ compensation (an insurance-like system); for independent contractors, the liability is usually through a tort claim (negligence).54
In modern times, a focus has been on preventing those injuries, not just providing compensation after injuries have occurred. Notably, federal and state occupational health and safety laws (particularly the Occupational Safety and Health Administration [OSHA]) require most organizations (including those employing health care providers) to take steps to mitigate various kinds of worker injuries.55
Although these worker protections have commonly been applied to hospitals and other health care providers, burnout has not traditionally been a significant concern in federal or state OSHA enforcement. For example, no formal federal OSHA regulations govern work-related burnout. Regulators, including OSHA, are increasingly interested in burnout that may affect many employees. OSHA has several recommendations for reducing health care work burnout.56 The Surgeon General has expressed similar concerns.57 The federal government recently allocated $103 million from the American Rescue Plan to address burnout among health care workers.58 Also, OSHA appears to be increasing its oversight of healthcare-institution-worker injuries.55
Is burnout a “disability”?
The federal Americans with Disabilities Act (ADA) and similar state laws prohibit discrimination based on disability.59 A disability is defined as a “physical or mental impairment that substantially limits one or more major life activities” or “perceived as having such an impairment.”60 The initial issue is whether burnout is a “mental impairment.” As noted earlier, it is not officially a “medical condition.”61 To date, the United Nations has classified it as an “occupational phenomenon.”62 It may, therefore, not qualify under the ADA, even if it “interferes with a major life activity.” There is, however, some movement toward defining burnout as a mental condition. Even if defined as a disability, there would still be legal issues of how severe it must be to qualify as a disability and the proper accommodation. Apart from the legal definition of an ADA disability, as a practical matter it likely is in the best interest of health care facilities to provide accommodations that reduce burnout. A number of strategies to decrease the incidence of burnout include the role of health care systems (FIGURE 2).
In conclusion we look at several things that can be done to “treat” or reduce burnout. That effort requires the cooperation of physicians and other providers, health care facilities, training programs, licensing authorities, and professional organizations. See suggestions below.
Conclusion
There are many excellent suggestions for reducing burnout and improving patient care and practitioner satisfaction.63-65 We conclude with a summary of some of these suggestions for individual practitioners, health care organizations, the profession, and licensing. It is worth remembering, however, that it will require the efforts of each area to reduce burnout substantially.
For practitioners:
- Engage in quality coaching/therapy on mindfulness and stress management.
- Practice self-care, including exercise and relaxation techniques.
- Make work-life balance a priority.
- Take opportunities for collegial social and professional discussions.
- Prioritize (and periodically assess) your own professional satisfaction and burnout risk.
- Smile—enjoy a sense of humor (endorphins and cortisol).
For health care organizations:
- Urgently work with vendors and regulators to revise electronic health records to reduce their substantial impact on burnout.
- Reduce physicians’ time on clerical and administrative tasks (eg, by enhancing the use of quality AI, scribes, and automated notes from appointments. (This may increase the time they spend with patients.) Eliminate “pajama-time” charting.
- Provide various kinds of confidential professional counseling, therapy, and support related to burnout prevention and treatment, and avoid any penalty or stigma related to their use.
- Provide reasonable flexibility in scheduling.
- Routinely provide employees with information about burnout prevention and services.
- Appoint a wellness officer with authority to ensure the organization maximizes its prevention and treatment services.
- Constantly seek input from practitioners on how to improve the atmosphere for practice to maximize patient care and practitioner satisfaction.
- Provide ample professional and social opportunities for discussing and learning about work-life balance, resilience, intellectual stimulation, and career development.
For regulators, licensors, and professional organizations:
- Work with health care organizations and EHR vendors to substantially reduce the complexity, physician effort, and stress associated with those record systems. Streamlining should, in the future, be part of formally certifying EHR systems.
- Reduce the administrative burden on physicians by modifying complex regulations and using AI and other technology to the extent possible to obtain necessary reimbursement information.
- Eliminate unnecessary data gathering that requires practitioner time or attention.
- Licensing, educational, and certifying bodies should eliminate any questions regarding the diagnosis or treatment of mental health and focus on current (or very recent) impairments.
- Seek funding for research on burnout prevention and treatment.
Dr. H is a 58-year-old ObGyn who, after completing residency, went into solo practice. The practice grew, and Dr. H found it increasingly more challenging to cover, especially the obstetrics sector. Dr. H then merged the practice with a group of 3 other ObGyns. Their practice expanded, and began recruiting recent residency graduates. In time, the practice was bought out by the local hospital health care system. Dr. H was faced with complying with the rules and regulations of that health care system. The electronic health record (EHR) component proved challenging, as did the restrictions on staff hiring (and firing), but Dr. H did receive a paycheck each month and complied with it all. The health care system administrators had clear financial targets Dr. H was to meet each quarter, which created additional pressure. Dr. H used to love being an OB and providing excellent care for every patient, but that sense of accomplishment was being lost.
Dr. H increasingly found it difficult to focus because of mind wandering, especially in the operating room (OR). Thoughts occurred about retirement, the current challenges imposed by “the new way of practicing medicine” (more focused on financial productivity restraints and reimbursement), and EHR challenges. Then Dr. H’s attention would return to the OR case at hand. All of this resulted in considerable stress and emotional exhaustion, and sometimes a sense of being disconnected. A few times, colleagues or nurses had asked Dr. H if everything was “okay,” or if a break would help. Dr. H made more small errors than usual, but Dr. H’s self-assessment was “doing an adequate job.” Patient satisfaction scores (collected routinely by the health care system) declined over the last 9 months.
Six months ago, Dr. H finished doing a laparoscopic total hysterectomy and bilateral salpingo-oophorectomy and got into the right uterine artery. The estimated blood loss was 3,500 mL. Using minimally invasive techniques, Dr. H identified the bleeder and, with monopolar current, got everything under control. The patient went to the post-anesthesia care unit, and all appeared to be in order. Her vital signs were stable, and she was discharged home the same day.
The patient presented 1 week later with lower abdominal and right flank pain. Dr. H addressed the problem in the emergency department and admitted the patient for further evaluation and urology consultation. The right ureter was damaged and obstructed; ultimately, the urologist performed a psoas bladder hitch. The patient recovered slowly, lost several weeks of work, experienced significant pain, and had other disruptions and costs. Additional medical care related to the surgery is ongoing. A health care system committee asked Dr. H to explain the problem. Over the last 6 months, Dr. H’s frustration with practice and being tired and disconnected have increased.
Dr. H has received a letter from a law firm saying that he and the health care system are being sued for malpractice focused on an iatrogenic ureter injury. The letter names two very reputable experts who are prepared to testify that the patient’s injury resulted from clear negligence. Dr. H has told the malpractice carrier absolutely not to settle this case—it is “a sham— without merit.” The health care system has asked Dr. H to take a “burnout test.”
Legal considerations
Dr. H exhibits relatively clear signs of professional burnout. The fact that there was a bad outcome while Dr. H was experiencing burnout is not proof of negligence (or, breach of duty of care to the patient). Nor is it a defense or mitigation to any malpractice that occurred.
In the malpractice case, the plaintiff will have the burden of proving that Dr. H’s treatment was negligent in that it fell below the standard of care. Even if it was a medical error, the question is whether it was negligence. If the patient/plaintiff, using expert witnesses, can prove that Dr. H fell below the standard of care that caused injury, Dr. H may be liable for the resulting extra costs, loss of income, and pain and suffering resulting from the negligent care.
The health care system likely will also be responsible for Dr. H’s negligence, either through respondeat superior (for example, if Dr. H is an employee) or for its own negligence. The case for its negligence is that the nurses and assistants had repeatedly seen him making errors and becoming disengaged (to the extent that they asked Dr. H if “everything is okay” or if a break would help). Furthermore, Dr. H’s patient satisfaction scores have been declining for several months. The plaintiff will argue that Dr. H exhibited classic burnout symptoms with the attendant risks of medical errors. However, the health care system did not take action to protect patients or to assist Dr. H. In short, one way or another, there is some likelihood that the health care system may also be liable if patient injuries are found to have been caused by negligence.
At this point, the health care system also faces the question of how to work with Dr. H in the future. The most pressing question is whether or not to allow Dr. H to continue practicing. If, as it appears, Dr. H is dealing with burnout, the pressure of the malpractice claim could well increase the probability of other medical mistakes. The institution has asked Dr. H to take a burnout test, but it is unclear where things go if the test (as likely) demonstrates significant burnout. This is a counseling and human relations question, at least as much as a legal issue, and the institution should probably proceed in that way—which is, trying to understand and support Dr. H and determining what can be done to address the burnout. At the same time, the system must reasonably assess Dr. H’s fitness to continue practicing as the matters are resolved. Almost everyone shares the goal to provide every individual and corporate opportunity for Dr. H to deal with burnout issues and return to successful practice.
Dr. H will be represented in the malpractice case by counsel provided through the insurance carrier. However, Dr. H would be well advised to retain a trusted and knowledgeable personal attorney. For example, the instruction not to consider settlement is likely misguided, but Dr. H needs to talk with an attorney that Dr. H has chosen and trusts. In addition, the attorney can help guide Dr. H through a rational process of dealing with the health care system, putting the practice in order, and considering the options for the future.
The health care system should reconsider its processes to deal with burnout to ensure the quality of care, patient satisfaction, professional retention, and economic stability. Several burnoutresponse programs have had success in achieving these goals.
What’s the Verdict?
Dr. H received good mental health, legal, and professional advice. As a result, an out of court settlement was reached following pretrial discovery. Dr. H has continued consultation regarding burnout and has returned to productive practice.
- Shanafelt TD, West CP, Sinsky C, et al. Changes in burnout and satisfaction with work-life integration in physicians and the general US working population between 2011 and 2017. Mayo Clinic Proceed. 2019;94:1681-1694.
- Smith R, Rayburn W. Burnout in obstetrician-gynecologists. Its prevalence, identification, prevention, and reversal. Obstet Gynecol Clin North Am. 2021;48:231-245. https://doi. org/10.1016/j.ogc.2021.06.003
- Patti MG, Schlottmann F, Sarr MG. The problem of burnout among surgeons. JAMA Surg. 2018;153:403-404. doi:10.1001 /jamasurg.2018.0047
- Carrau D, Janis JE. Physician burnout: solutions for individuals and organizations. Plastic and Reconstructive Surgery Global Open. 2021;91-97.
- Southwick R. The key to fixing physician burnout is the workplace not the worker. Contemporary Ob/Gyn. March 13, 2023.
- Patel RS, Bachu R, Adikey A, et al. Factors related to physician burnout and its consequences: a review. Behav Sciences. 2018;8:98.
- Melnick ER, Dyrbye LN, Sinsky CA, et al. The association between perceived electronic health record usability and professional burnout among US physicians. Mayo Clinic Proceed. 2020;95:476-487.
- Shanafelt TD, Dyrbye LN, West CP. Addressing physician burnout: the way forward. JAMA. 2017;317:901-902. doi:10.1001/jama.2017.0076
- Ommaya AK, Cipriano PF, Hoyt DB, et al. Care-centered clinical documentation in the digital environment: Solutions to alleviate burnout. National Academy of Medicine Perspectives. 2018.
- Hartzband P, Groopman J. Physician burnout, interrupted. N Engl J Med. 2020;382:2485-2487. Discussion Paper, National Academy of Medicine. Accessed July 21, 2023. https://nam .edu/care
- Ji YD, Robertson FC, Patel NA, et al. Assessment of risk factors for suicide among US health care professionals. JAMA Surg. 2020;155:713-721. centered-clinical-documentation-digital -environment-solutions-alleviate-burnout/
- Shanafelt TD, West CP, Dyrbye LN, et al. Changes in burnout and satisfaction with work-life integration in physicians during the first 2 years of the COVID-19 pandemic. Mayo Clinic Proceed. 2022;97:2248-2258.
- Herber-Valdez C, Kupesic-Plavsic S. Satisfaction and shortfall of OB-GYN physicians and radiologists. J. Ultrasound Obstet Gynecol. 2021;15:387-392.
- Dyrbye LN, Shanafelt TD, Sinsky CA, et al. Burnout among health care professionals: a call to explore and address this underrecognized threat to safe, high-quality care. National Academy of Medicine Perspectives. Accessed July 5, 2017. https://iuhcpe.org/file_manager/1501524077-Burnout -Among-Health-Care-Professionals-A-Call-to-Explore-and -Address-This-Underrecognized-Threat.pdf
- Olson KD. Physician burnout—a leading indicator of health system performance? Mayo Clinic Proceed. 2017;92: 1608-1611.
- American College of Obstetricians and Gynecologists. Why obgyns are burning out. October 28, 2019. Accessed July 21, 2023. https://www.acog.org/news/news-articles/2019/10/why-ob -gyns-are-burning-out#:~:text=A%202017%20report%20 by%20the,exhaustion%20or%20lack%20of%20motivation
- Peckham C. National physician burnout & depression report 2018. Medscape. January 17, 2018. https://nap. nationalacademies.org/catalog/25521/taking-action -against-clinician-burnout-a-systems-approach-to -professional
- Marsa L. Labor pains: The OB-GYN shortage. AAMC News. Nov. 15, 2018. Accessed July 21, 2023. https://www.aamc.org /news-insights/labor-pains-ob-gyn-shortage
- American College of Obstetricians and Gynecologists. Coping with the stress of medical professional liability litigation. ACOG Committee Opinion. February 2005;309:453454. Accessed July 21, 2023. https://www.acog.org/clinical /clinical-guidance/committee-opinion/articles/2013/01 /coping-with-the-stress-of-medical-professional-liability -litigation
- Reith TP. Burnout in United States healthcare professionals: a narrative review. Cureus. 2018;10:e3681. doi: 10.7759 /cureus.3681
- Han S, Shanafelt TD, Sinsky CA, et al. Estimating the attributable cost of physician burnout in the United States. Ann Intern Med. 2019;4:784-790.
- Sullivan D, Sullivan V, Weatherspoon D, et al. Comparison of nurse burnout, before and during the COVID-19 pandemic. Nurs Clin North Am. 2022;57:79-99. doi: 10.1016 /j.cnur.2021.11.006
- Chandawarkar A, Chaparro JD. Burnout in clinicians. Curr Prob Pediatr Adolesc Health Care. 2021;51:101-104. https ://doi.org/10.1016/j.cppeds.2021.101104
- Brady KJS, Sheldrick RC, Ni P, et al. Examining the measurement equivalence of the Maslach Burnout Inventory across age, gender, and specialty groups in US physicians. J Patient-Reported Outcomes. 2021;5.
- Association of American Medical Colleges. Physician Specialty Data Report—Active Physicians by Sex and Specialty, 2021. Accessed June 19, 2023. https://www.aamc .org/data-reports/workforce/data/active-physicians-sex -specialty-2021
- Association of American Medical Colleges. Physician Specialty Data Report—ACGME Residents and Fellows by Sex and Specialty, 2021. Accessed June 19, 2023. https://www .aamc.org/data-reports/workforce/data/acgme-residents -fellows-sex-and-specialty-2021
- Painter LM, Biggans KA, Turner CT. Risk managementobstetrics and gynecology perspective. Clin Obstet Gynecol. 2023;66:331-341. DOI:10.1097/GRF.0000000000000775
- Darney BG, Boniface E, Liberty A. Assessing the effect of abortion restrictions. Obstetr Gynecol. 2023;141:233-235.
- Heuerman AC, Bessett D, Antommaria AHM, et al. Experiences of reproductive genetic counselors with abortion regulations in Ohio. J Genet Counseling. 2022;31:641-652.
- Brandi K, Gill P. Abortion restrictions threaten all reproductive health care clinicians. Am J Public Health. 2023;113:384-385.
- Rotenstein LS, Torre M, Ramos MA, et al. Prevalence of burnout among physicians: a systematic review. JAMA. 2018;320:1131-1150. doi: 10.1001/jama.2018.1277
- Williamson K, Lank PM, Cheema N, et al. Comparing the Maslach Burnout Inventory to other well-being instruments in emergency medicine residents. J Graduate Med Education. 2018;532-536. DOI: http://dx.doi.org/10.4300 /JGME-D-18-00155.1
- Brady KJS, Sheldrick RC, Ni P, et al. Establishing crosswalks between common measures of burnout in US physicians. J Gen Intern Med. 2022;37:777-784.
- Zhang X, Song Y, Jiang T, et al. Interventions to reduce burnout of physicians and nurses: an overview of systematic reviews and meta-analyses. Medicine (Baltimore). 2020;26:e20992. DOI: 10.1097/MD.0000000000020992
- Scheepers RA, Emke H, Ronald M, et al. The impact of mindfulness-based interventions on doctors’ well-being and performance: a systematic review. Med Education. 2020;54:138-149. https://doi.org/10.1111/medu.14020
- Olson K, Marchalik D, Farley H, et al. Organizational strategies to reduce physician burnout and improve professional fulfillment. Curr Prob Pediatr Adolesc Health Care. 2019;49:12. https://doi.org/10.1016/j.cppeds.2019.100664
- Berry LL, Awdish RLA, Swensen SJ. 5 ways to restore depleted health care workers. Harvard Business Rev. February 11, 2022.
- Sullivan AB, Hersh CM, Rensel M, et al. Leadership inequity, burnout, and lower engagement of women in medicine. J Health Serv Psychol. 2023;49:33-39.
- Hoffman S. Healing the healers: legal remedies for physician burnout. Yale J Health Policy Law Ethics. 2018;18:56-113.
- Federation of State Medical Boards. Physician wellness and burnout: report and recommendations of the workgroup on physician wellness and burnout. (Policy adopted by FSMB). April 2018. Accessed July 21, 2023. https://www.fsmb.org /siteassets/advocacy/policies/policy-on-wellness-and -burnout.pdf
- Robinson C, Kettering C, Sanfilippo JS. Medical malpractice lawsuits. Clin Obstet Gynecol. 2023;66:256-260. DOI: https ://doi.org/10.1097/GRF.0000000000000777
- Gittler GJ, Goldstein EJ. The elements of medical malpractice: an overview. Clin Infect Dis. 1996;23:1152-1155.
- Bal BS. An introduction to medical malpractice in the United States. Clin Orthop Relat Res. 2009;467:339-347.
- Tawfik DS, Profit J, Morgenthaler TI, et al. Physician burnout, well-being, and work unit safety grades in relationship to reported medical errors. Mayo Clinic Proceed. 2018;93: 1571-1580.
- Sundholm B. Elevating physician-patient relationships in the shadow of metric mania. Drexel L Rev. 2020;12:287-330.
- Ghaith S, Campbell RL, Pollock JR, et al. Medical malpractice lawsuits involving trainees in obstetrics and gynecology in the USA. Healthcare. 2022;10:1328.
- Muller TM, Warsi S. Litigation culture causing burnout in American physicians. Trauma Mental Health Report. April 9, 2021.
- Levine AS. Legal 101: Tort law and medical malpractice for physicians. Contemp OBGYN. 2015:60;26-28, 30.
- Regan JJ, Regan WM. Medical malpractice and respondeat superior. Southern Med J. 2002;95.5:545-549. DOI 10.1097/00007611-200295050-00018
- Levin H. Hospital vicarious liability for negligence by independent contractor physicians: new rule for new times. Univ Illinois Law Rev. 2005:1291-1332.
- Darling v Charleston Hospital, 33 Ill. 2d 326, 211 N.E.2d 253 (Ill. 1965).
- Dangel R. Hospital liability for physician malpractice. Ohio State Law J. 1986;47:1077-1098.
- Reffitt v Hajjar, 892 S.W.2d 599, 605 (Ky. Ct. App. 1994).
- McMichael BJ. Malpractice. In Laws of Medicine: Core Legal Aspects for the Healthcare Professional. New York, NY: Springer International; 2022:129-150.
- Occupational Safety and Health Administration. Worker safety in hospitals: caring for our caregivers. Accessed June 8, 2023. https://www.osha.gov/hospitals
- Occupational Safety and Health Administration. Workplace stress. Accessed June 8, 2023. https://www.osha.gov /workplace-stress/understanding-the-problem
- U.S. Surgeon General’s Advisory on Building a Thriving Health Workforce. Addressing health worker burnout. Accessed July 21, 2023. https://www.hhs.gov/sites/default/files/health -worker-wellbeing-advisory.pdf
- Department of Health & Human Services. Biden-Harris administration awards $103 Million in American Rescue Plan funds to reduce burnout and promote mental health and wellness among health care workforce. January 20, 2022. Accessed July 24, 2023. https://www.hhs.gov/about /news/2022/01/20/biden-harris-administration-awards -103-million-american-rescue-plan-funds-reduce-burnout -promote-mental-health-wellness-among-health-care -workforce.html
- Rothstein LF, Irzyk J. Disabilities and the Law. 4th ed. Toronto, Canada: Thompson Reuters; 2023.
- Department of Labor. Guide to disability rights laws. February 28, 2020. Accessed July 24, 2023. https://www .ada.gov/resources/disability-rights-guide/#:~:text=An%20 individual%20with%20a%20disability%20is%20defined%20 by%20the%20ADA,as%20having%20such%20an%20 impairment
- Nadon L, De Beer LT, Morin AJS. Should burnout be conceptualized as a mental disorder? Behavioral Sci. 2022;12:82.
- World Health Organization. Burn-out an “occupational phenomenon”: International Classification of Diseases. May 28, 2019. Accessed July 21, 2023. https://www.who.int/news /item/28-05-2019-burn-out-an-occupational-phenomenon -international-classification-of-diseases
- Hoffman S. Physician burnout: why legal and regulatory systems may need to step in. The Conversation. July 9, 2019. https://theconversation.com/physician-burnout-why-legal -and-regulatory-systems-may-need-to-step-in-119705
- Jha A, Iliff A, Chaoi A, et al. A crisis in healthcare: a call to action on physician burnout. Harvard Global Health Institute. 2019. Accessed July 21, 2023. https://www.massmed.org /Publications/Research,-Studies,-and-Reports/Physician -Burnout-Report-2018/
- Arnsten AF, Shanafelt T. Physician distress and burnout: the neurobiological perspective. Mayo Clin Proceed. 2021;96:763-769.
Physicians have some of the highest rates of burnout among all professions.1 Complicating matters is that clinicians (including residents)2 may avoid seeking treatment out of fear it will affect their license or privileges.3 In this article, we consider burnout in greater detail, as well as ways of successfully addressing the level of burnout in the profession (FIGURE 1), including steps individual practitioners, health care entities, and regulators should consider to reduce burnout and its harmful effects.

How burnout becomes a problem
Six general factors are commonly identified as leading to clinician career dissatisfaction and burnout:4
1. work overload
2. lack of autonomy and control
3. inadequate rewards, financial and otherwise
4. work-home schedules
5. perception of lack of fairness
6. values conflict between the clinician and employer (including a breakdown of professional community).
At the top of the list of causes of burnout is often “administrative and bureaucratic headaches.”5 More specifically, electronic health records (EHRs), including computerized order entry, is commonly cited as a major cause of burnout.6,7 According to some studies, clinicians spend as much as 49% of working time doing clerical work,8 and studies found the extension of work into home life.9
Increased measurement of performance metrics in health care services are a significant contributor to physician burnout.10 These include pressure to see more patients, perform more procedures, and respond quickly to patient requests (eg, through email).7 As we will see, medical malpractice cases, or the risk of such cases, have also played a role in burnout in some medical specialties.11 The pandemic also contributed, at least temporarily, to burnout.12,13
Rates of burnout among physicians are notably higher than among the general population14 or other professions.6 Although physicians have generally entered clinical practice with lower rates of burnout than the general population,15 The American College of Obstetricians and Gynecologists (ACOG) reports that 40% to 75% of ObGyns “experience some form of professional burnout.”16,17 Other source(s) cite that 53% of ObGyns report burnout (TABLE 1).
Code QD85
Burnout is a syndrome conceptualized as resulting from chronic workplace stress that has not been successfully managed. It is characterized by 3 dimensions:
- feelings of energy depletion or exhaustion
- increased mental distance from one’s job, or feelings of negativism or cynicism related to one’s job
- a sense of ineffectiveness and lack of accomplishment. Burn-out refers specifically to phenomena in the occupational context and should not be applied to describe experiences in other areas of life. Exclusions to burnout diagnosis include adjustment disorder, disorders specifically associated with stress, anxiety or fear-related disorders, and mood disorders.
Reference
1. International Classification of Diseases Eleventh Revision (ICD-11). Geneva, Switzerland: World Health Organization; 2022.

Burnout undoubtedly contributes to professionals leaving practice, leading to a significant shortage of ObGyns.18 It also raises several significant legal concerns. Despite the enormity and seriousness of the problem, there is considerable optimism and assurance that the epidemic of burnout is solvable on the individual, specialty, and profession-wide levels. ACOG and other organizations have made suggestions for physicians, the profession, and to health care institutions for reducing burnout.19 This is not to say that solutions are simple or easy for individual professionals or institutions, but they are within the reach of the profession (FIGURE 2).

Suicide among health care professionals is one other concern (TABLE 2)20 and theoretically can stem from burnout, depression, and other psychosocial concerns.

Costs of clinician burnout
Burnout is endemic among health care providers, with numerous studies detailing the professional, emotional, and financial costs. Prior to the pandemic, one analysis of nationwide fiscal costs associated with burnout estimated an annual cost of $4.6B due to physician turnover and reduced clinical hours.21 The COVID-19 epidemic has by all accounts worsened rates of health care worker burnout, particularly for those in high patient-contact positions.22
Female clinicians appear to be differentially affected; in one recent study women reported symptoms of burnout at twice the rate of their male counterparts.23 Whether burnout rates will return to pre-pandemic levels remains an open question, but since burnout is frequently related to one’s own assessment of work-life balance, it is possible that a longer term shift in burnout rates associated with post-pandemic occupational attitudes will be observed.
Combining factors contribute to burnout
Burnout is a universal occupational hazard, but extant data suggest that physicians and other health care providers may be at higher risk. Among physicians, younger age, female gender, and front-line specialty status appear associated with higher burnout rates.24 Given that ObGyn physicians are overwhelmingly female (60% of physicians and 86% of residents),25,26 gender-related burnout factors exist alongside other specific occupational burnout risks. While gender parity has been achieved among health care providers, gender disparities persist in terms of those in leadership positions, compensation, and other factors.22
The smattering of evidence suggesting that ObGyns have higher rates of burnout than many other specialties is understandable given the unique legal challenges confronting ObGyn practice. This may be of special significance because ObGyn malpractice insurance rates are among the highest of all specialties.27 The overall shortage of ObGyns has been exacerbated by the demonstrated negative effects on training and workforce representation stemming from recent legislation that has the effect of criminalizing certain aspects of ObGyn practice;28 for instance, uncertainty regarding abortion regulations.
These negative effects are particularly heightened in states in which the law is in flux or where there are continuing efforts to substantially limit access to abortion. The efforts to increase civil and even criminal penalties related to abortion care challenge ObGyns’ professional practices, as legal rules are frequently changing. In some states, ObGyns may face additional workloads secondary to a flight of ObGyns from restrictive jurisdictions in addition to legal and professional repercussions. In a small study of 19 genetic counselors dealing with restrictive legislation in the state of Ohio,29 increased stress and burnout rates were identified as a consequence of practice uncertainties under this legislation. It is certain that other professionals working in reproductive health care are similarly affected.30
The programs provide individual resources to providers in distress, periodically survey initiatives at Stanford to assess burnout at the organizational level, and provide input designed to spur organizational change to reduce the burden of burnout. Ways that they build community and connections include:
- Live Story Rounds events (as told by Stanford Medicine physicians)
- Commensality Groups (facilitated small discussion groups built around tested evidence)
- Aim to increase sense of connection and collegiality among physicians and build comradery at work
- CME-accredited physician wellness forum, including annual doctor’s day events
Continue to: Assessment of burnout...
Assessment of burnout
Numerous scales for the assessment of burnout exist. Of these, the 22-item Maslach Burnout Inventory (MBI) is the best studied. The MBI is a well-investigated tool for assessing burnout. The MBI consists of 3 major subscales measuring overall burnout, emotional exhaustion, depersonalization, and low personal accomplishment. It exists in numerous forms. For instance, the MBI-HSS (MP), adapted for medical personnel, is available. However, the most commonly used form for assessing burnout in clinicians is the MBI-HHS (Human Services Survey); approximately 85% of all burnout studies examined in a recent meta-analysis used this survey version.31 As those authors commented, while burnout is a recognized phenomenon, a great deal of variability in study design, interpretation of subscale scores, and sample selection makes generalizations regarding burnout difficult to assess.
The MBI in various forms has been extensively used over the past 40 years to assess burnout amongst physicians and physicians in training. While not the only instrument designed to measure such factors, it is by far the most prevalent. Williamson and colleagues32 compared the MBI with several other measures of quality of life and found good correlation between the various instruments used, a finding replicated by other studies.33 Brady and colleagues compared item responses to the Stanford Professional Fulfillment Index and the Min-Z Single-item Burnout scale (a 1-item screening measure) to MBI’s Emotional Exhaustion and Depersonalization subscales. Basing their findings on a survey of more than 1,300 physicians, they found that all analyzed scales were significantly correlated with such adverse outcomes as depression, distress, or intent to leave the profession.
It is important to note that most surveys of clinician burnout were conducted prior to the pandemic. While the psychometric analyses of the MBI and other scales are likely still germane, observed rates of clinician burnout have likely increased. Thus, comparisons of pre- and post-pandemic studies should factor in an increase in the incidence and prevalence of burnout.
Management strategies
In general, there are several interventions for managing burnout34:
- individual-focused (including self-care and communications-skills workshops)
- mindfulness training
- yoga
- meditation
- organizational/structural (workload reduction, schedule realignment, teamwork training, and group-delivered stress management interventions)
- combination(s) of the above.
There is little evidence to suggest that any particular individual intervention (whether delivered in individual or group-based formats) is superior to any other in treating clinician burnout. A recent analysis of 24 studies employing mindfulness-based interventions demonstrated generally positive results for such interventions.35 Other studies have also found general support for mindfulness-based interventions, although mindfulness is often integrated with other stress-reduction techniques, such as meditation, yoga, and communication skills. Such interventions are nonspecific but generally effective.
An accumulation of evidence to date suggests that a combination of individual and organizational interventions is most effective in combatting clinician burnout. No individual intervention can be successful without addressing root causes, such as overscheduling, lack of organizational support, and the effect of restrictive legislation on practice.
Several large teaching hospitals have established programs to address physician and health care provider burnout. Notable among these is the Stanford University School of Medicine’s WellMD and WellPhD programs (https://wellmd.stanford.edu/about.html). These programs were described by Olson and colleagues36 as using a model focused on practice efficiency, organizational culture, and personal resilience to enhance physicians’ well-being. (See “Aspects of the WellMD and WellPhD programs from Stanford University.”)
A growing number of institutions have established burnout programs to support physicians experiencing work/life imbalances and other aspects of burnout.37 In general, these share common features of assessment, individual and/or group intervention, and organizational change. Fear of repercussion may be one factor preventing physicians from seeking individual treatment for burnout.38 Importantly, they emphasize the need for professional confidentiality when offering treatment to patients within organizational settings. Those authors also reported that a focus on organizational engagement may be an important factor in addressing burnout in female physicians, as they tend to report lower levels of organizational engagement.
Continue to: Legal considerations...
Legal considerations
Until recently, physician burnout “received little notice in the legal literature.”39 Although there have been burnout legal consequences in the past, the legal issues are now becoming more visible.40
Medical malpractice
A well-documented consequence of burnout is an increase in errors.14 Medical errors, of course, are at the heart of malpractice claims. Technically, malpractice is medical or professional negligence. It is the breach of a duty owed by the physician, or other provider, or organization (defendant) to the patient, which causes injury to the plaintiff/patient.41
“Medical error” is generally a meaningful deviation from the “standard of care” or accepted medical practice.42 Many medical errors do not cause injury to the patient; in those cases, the negligence does not result in liability. In instances in which the negligence causes harm, the clinician and health care facility may be subject to liability for that injury. Fortunately, however, for a variety of reasons, most harmful medical errors do not result in a medical malpractice claim or lawsuit. The absence of a good clinician-patient relationship is likely associated with an increased inclination of a patient to file a malpractice action.43Clinician burnout may, therefore, contribute to increased malpractice claims in two ways. First, burnout likely leads to increased medical errors, perhaps because burnout is associated with lower concentration, inattention, reduced cognitive vigilance, and fatigue.8,44 It may also lead to less time with patients, reduced patient empathy, and lower patient rapport, which may make injured patients more likely to file a claim or lawsuit.45 Because the relationshipbetween burnout and medical error is bidirectional, malpractice claims tend to increase burnout, which increases error. Given the time it takes to resolve most malpractice claims, the uncertainty of medical malpractice may be especially stressful for health care providers.46,47
Burnout is not a mitigating factor in malpractice. Our sympathies may go out to a professional suffering from burnout, but it does not excuse or reduce liability—it may, indeed, be an aggravating factor. Clinicians who can diagnose burnout and know its negative consequences but fail to deal with their own burnout may be demonstrating negligence if there has been harm to a patient related to the burnout.48
Institutional or corporate liability to patients
Health care institutions have obligations to avoid injury to patients. Just as poorly maintained medical equipment may harm patients, so may burned-out professionals. Therefore, institutions have some obligation to supervise and avoid the increased risks to patients posed by professionals suffering from burnout.
Respondeat superior and institutional negligence. Institutional liability may arise in two ways, the first through agency, or respondeat superior. That is, if the physician or other professional is an employee (or similar agent) of the health care institution, that institution is generally responsible for the physician’s negligence during the employment.49 Even if the physician is not an employee (for example, an independent contractor providing care or using the hospital facilities), the health care facility may be liable for the physician’s negligence.50 Liability may occur, for example, if the health care facility was aware that the physician was engaged in careless practice or was otherwise a risk to patients but the facility did not take steps to avoid those risks.51 The basis for liability is that the health care organization owes a duty to patients to take reasonable care to ensure that its facilities are not used to injure patients negligently.52 Just as it must take care that unqualified physicians are not granted privileges to practice, it also must take reasonable steps to protect patients when it is aware (through nurses or other agents) of a physician’s negligent practice.
In one case, for example, the court found liability where a staff member had “severe” burnout in a physician’s office and failed to read fetal monitoring strips. The physician was found negligent for relying on the staff member who was obviously making errors in interpretation of fetal distress.53
Continue to: Legal obligations of health care organizations to physicians and others...
Legal obligations of health care organizations to physicians and others
In addition to obligations to patients, health care organizations may have obligations to employees (and others) at risk for injury. For example, assume a patient is diagnosed with a highly contagious disease. The health care organization would be obligated to warn, and take reasonable steps to protect, the staff (employees and independent contractors) from being harmed from exposure to the disease. This principle may apply to coworkers of employees with significant burnout, thereby presenting a danger in the workplace. The liability issue is more difficult for employees experiencing job-related burnout themselves. Organizations generally compensate injured employees through no-fault workers’ compensation (an insurance-like system); for independent contractors, the liability is usually through a tort claim (negligence).54
In modern times, a focus has been on preventing those injuries, not just providing compensation after injuries have occurred. Notably, federal and state occupational health and safety laws (particularly the Occupational Safety and Health Administration [OSHA]) require most organizations (including those employing health care providers) to take steps to mitigate various kinds of worker injuries.55
Although these worker protections have commonly been applied to hospitals and other health care providers, burnout has not traditionally been a significant concern in federal or state OSHA enforcement. For example, no formal federal OSHA regulations govern work-related burnout. Regulators, including OSHA, are increasingly interested in burnout that may affect many employees. OSHA has several recommendations for reducing health care work burnout.56 The Surgeon General has expressed similar concerns.57 The federal government recently allocated $103 million from the American Rescue Plan to address burnout among health care workers.58 Also, OSHA appears to be increasing its oversight of healthcare-institution-worker injuries.55
Is burnout a “disability”?
The federal Americans with Disabilities Act (ADA) and similar state laws prohibit discrimination based on disability.59 A disability is defined as a “physical or mental impairment that substantially limits one or more major life activities” or “perceived as having such an impairment.”60 The initial issue is whether burnout is a “mental impairment.” As noted earlier, it is not officially a “medical condition.”61 To date, the United Nations has classified it as an “occupational phenomenon.”62 It may, therefore, not qualify under the ADA, even if it “interferes with a major life activity.” There is, however, some movement toward defining burnout as a mental condition. Even if defined as a disability, there would still be legal issues of how severe it must be to qualify as a disability and the proper accommodation. Apart from the legal definition of an ADA disability, as a practical matter it likely is in the best interest of health care facilities to provide accommodations that reduce burnout. A number of strategies to decrease the incidence of burnout include the role of health care systems (FIGURE 2).
In conclusion we look at several things that can be done to “treat” or reduce burnout. That effort requires the cooperation of physicians and other providers, health care facilities, training programs, licensing authorities, and professional organizations. See suggestions below.
Conclusion
There are many excellent suggestions for reducing burnout and improving patient care and practitioner satisfaction.63-65 We conclude with a summary of some of these suggestions for individual practitioners, health care organizations, the profession, and licensing. It is worth remembering, however, that it will require the efforts of each area to reduce burnout substantially.
For practitioners:
- Engage in quality coaching/therapy on mindfulness and stress management.
- Practice self-care, including exercise and relaxation techniques.
- Make work-life balance a priority.
- Take opportunities for collegial social and professional discussions.
- Prioritize (and periodically assess) your own professional satisfaction and burnout risk.
- Smile—enjoy a sense of humor (endorphins and cortisol).
For health care organizations:
- Urgently work with vendors and regulators to revise electronic health records to reduce their substantial impact on burnout.
- Reduce physicians’ time on clerical and administrative tasks (eg, by enhancing the use of quality AI, scribes, and automated notes from appointments. (This may increase the time they spend with patients.) Eliminate “pajama-time” charting.
- Provide various kinds of confidential professional counseling, therapy, and support related to burnout prevention and treatment, and avoid any penalty or stigma related to their use.
- Provide reasonable flexibility in scheduling.
- Routinely provide employees with information about burnout prevention and services.
- Appoint a wellness officer with authority to ensure the organization maximizes its prevention and treatment services.
- Constantly seek input from practitioners on how to improve the atmosphere for practice to maximize patient care and practitioner satisfaction.
- Provide ample professional and social opportunities for discussing and learning about work-life balance, resilience, intellectual stimulation, and career development.
For regulators, licensors, and professional organizations:
- Work with health care organizations and EHR vendors to substantially reduce the complexity, physician effort, and stress associated with those record systems. Streamlining should, in the future, be part of formally certifying EHR systems.
- Reduce the administrative burden on physicians by modifying complex regulations and using AI and other technology to the extent possible to obtain necessary reimbursement information.
- Eliminate unnecessary data gathering that requires practitioner time or attention.
- Licensing, educational, and certifying bodies should eliminate any questions regarding the diagnosis or treatment of mental health and focus on current (or very recent) impairments.
- Seek funding for research on burnout prevention and treatment.
Dr. H is a 58-year-old ObGyn who, after completing residency, went into solo practice. The practice grew, and Dr. H found it increasingly more challenging to cover, especially the obstetrics sector. Dr. H then merged the practice with a group of 3 other ObGyns. Their practice expanded, and began recruiting recent residency graduates. In time, the practice was bought out by the local hospital health care system. Dr. H was faced with complying with the rules and regulations of that health care system. The electronic health record (EHR) component proved challenging, as did the restrictions on staff hiring (and firing), but Dr. H did receive a paycheck each month and complied with it all. The health care system administrators had clear financial targets Dr. H was to meet each quarter, which created additional pressure. Dr. H used to love being an OB and providing excellent care for every patient, but that sense of accomplishment was being lost.
Dr. H increasingly found it difficult to focus because of mind wandering, especially in the operating room (OR). Thoughts occurred about retirement, the current challenges imposed by “the new way of practicing medicine” (more focused on financial productivity restraints and reimbursement), and EHR challenges. Then Dr. H’s attention would return to the OR case at hand. All of this resulted in considerable stress and emotional exhaustion, and sometimes a sense of being disconnected. A few times, colleagues or nurses had asked Dr. H if everything was “okay,” or if a break would help. Dr. H made more small errors than usual, but Dr. H’s self-assessment was “doing an adequate job.” Patient satisfaction scores (collected routinely by the health care system) declined over the last 9 months.
Six months ago, Dr. H finished doing a laparoscopic total hysterectomy and bilateral salpingo-oophorectomy and got into the right uterine artery. The estimated blood loss was 3,500 mL. Using minimally invasive techniques, Dr. H identified the bleeder and, with monopolar current, got everything under control. The patient went to the post-anesthesia care unit, and all appeared to be in order. Her vital signs were stable, and she was discharged home the same day.
The patient presented 1 week later with lower abdominal and right flank pain. Dr. H addressed the problem in the emergency department and admitted the patient for further evaluation and urology consultation. The right ureter was damaged and obstructed; ultimately, the urologist performed a psoas bladder hitch. The patient recovered slowly, lost several weeks of work, experienced significant pain, and had other disruptions and costs. Additional medical care related to the surgery is ongoing. A health care system committee asked Dr. H to explain the problem. Over the last 6 months, Dr. H’s frustration with practice and being tired and disconnected have increased.
Dr. H has received a letter from a law firm saying that he and the health care system are being sued for malpractice focused on an iatrogenic ureter injury. The letter names two very reputable experts who are prepared to testify that the patient’s injury resulted from clear negligence. Dr. H has told the malpractice carrier absolutely not to settle this case—it is “a sham— without merit.” The health care system has asked Dr. H to take a “burnout test.”
Legal considerations
Dr. H exhibits relatively clear signs of professional burnout. The fact that there was a bad outcome while Dr. H was experiencing burnout is not proof of negligence (or, breach of duty of care to the patient). Nor is it a defense or mitigation to any malpractice that occurred.
In the malpractice case, the plaintiff will have the burden of proving that Dr. H’s treatment was negligent in that it fell below the standard of care. Even if it was a medical error, the question is whether it was negligence. If the patient/plaintiff, using expert witnesses, can prove that Dr. H fell below the standard of care that caused injury, Dr. H may be liable for the resulting extra costs, loss of income, and pain and suffering resulting from the negligent care.
The health care system likely will also be responsible for Dr. H’s negligence, either through respondeat superior (for example, if Dr. H is an employee) or for its own negligence. The case for its negligence is that the nurses and assistants had repeatedly seen him making errors and becoming disengaged (to the extent that they asked Dr. H if “everything is okay” or if a break would help). Furthermore, Dr. H’s patient satisfaction scores have been declining for several months. The plaintiff will argue that Dr. H exhibited classic burnout symptoms with the attendant risks of medical errors. However, the health care system did not take action to protect patients or to assist Dr. H. In short, one way or another, there is some likelihood that the health care system may also be liable if patient injuries are found to have been caused by negligence.
At this point, the health care system also faces the question of how to work with Dr. H in the future. The most pressing question is whether or not to allow Dr. H to continue practicing. If, as it appears, Dr. H is dealing with burnout, the pressure of the malpractice claim could well increase the probability of other medical mistakes. The institution has asked Dr. H to take a burnout test, but it is unclear where things go if the test (as likely) demonstrates significant burnout. This is a counseling and human relations question, at least as much as a legal issue, and the institution should probably proceed in that way—which is, trying to understand and support Dr. H and determining what can be done to address the burnout. At the same time, the system must reasonably assess Dr. H’s fitness to continue practicing as the matters are resolved. Almost everyone shares the goal to provide every individual and corporate opportunity for Dr. H to deal with burnout issues and return to successful practice.
Dr. H will be represented in the malpractice case by counsel provided through the insurance carrier. However, Dr. H would be well advised to retain a trusted and knowledgeable personal attorney. For example, the instruction not to consider settlement is likely misguided, but Dr. H needs to talk with an attorney that Dr. H has chosen and trusts. In addition, the attorney can help guide Dr. H through a rational process of dealing with the health care system, putting the practice in order, and considering the options for the future.
The health care system should reconsider its processes to deal with burnout to ensure the quality of care, patient satisfaction, professional retention, and economic stability. Several burnoutresponse programs have had success in achieving these goals.
What’s the Verdict?
Dr. H received good mental health, legal, and professional advice. As a result, an out of court settlement was reached following pretrial discovery. Dr. H has continued consultation regarding burnout and has returned to productive practice.
Physicians have some of the highest rates of burnout among all professions.1 Complicating matters is that clinicians (including residents)2 may avoid seeking treatment out of fear it will affect their license or privileges.3 In this article, we consider burnout in greater detail, as well as ways of successfully addressing the level of burnout in the profession (FIGURE 1), including steps individual practitioners, health care entities, and regulators should consider to reduce burnout and its harmful effects.

How burnout becomes a problem
Six general factors are commonly identified as leading to clinician career dissatisfaction and burnout:4
1. work overload
2. lack of autonomy and control
3. inadequate rewards, financial and otherwise
4. work-home schedules
5. perception of lack of fairness
6. values conflict between the clinician and employer (including a breakdown of professional community).
At the top of the list of causes of burnout is often “administrative and bureaucratic headaches.”5 More specifically, electronic health records (EHRs), including computerized order entry, is commonly cited as a major cause of burnout.6,7 According to some studies, clinicians spend as much as 49% of working time doing clerical work,8 and studies found the extension of work into home life.9
Increased measurement of performance metrics in health care services are a significant contributor to physician burnout.10 These include pressure to see more patients, perform more procedures, and respond quickly to patient requests (eg, through email).7 As we will see, medical malpractice cases, or the risk of such cases, have also played a role in burnout in some medical specialties.11 The pandemic also contributed, at least temporarily, to burnout.12,13
Rates of burnout among physicians are notably higher than among the general population14 or other professions.6 Although physicians have generally entered clinical practice with lower rates of burnout than the general population,15 The American College of Obstetricians and Gynecologists (ACOG) reports that 40% to 75% of ObGyns “experience some form of professional burnout.”16,17 Other source(s) cite that 53% of ObGyns report burnout (TABLE 1).
Code QD85
Burnout is a syndrome conceptualized as resulting from chronic workplace stress that has not been successfully managed. It is characterized by 3 dimensions:
- feelings of energy depletion or exhaustion
- increased mental distance from one’s job, or feelings of negativism or cynicism related to one’s job
- a sense of ineffectiveness and lack of accomplishment. Burn-out refers specifically to phenomena in the occupational context and should not be applied to describe experiences in other areas of life. Exclusions to burnout diagnosis include adjustment disorder, disorders specifically associated with stress, anxiety or fear-related disorders, and mood disorders.
Reference
1. International Classification of Diseases Eleventh Revision (ICD-11). Geneva, Switzerland: World Health Organization; 2022.

Burnout undoubtedly contributes to professionals leaving practice, leading to a significant shortage of ObGyns.18 It also raises several significant legal concerns. Despite the enormity and seriousness of the problem, there is considerable optimism and assurance that the epidemic of burnout is solvable on the individual, specialty, and profession-wide levels. ACOG and other organizations have made suggestions for physicians, the profession, and to health care institutions for reducing burnout.19 This is not to say that solutions are simple or easy for individual professionals or institutions, but they are within the reach of the profession (FIGURE 2).

Suicide among health care professionals is one other concern (TABLE 2)20 and theoretically can stem from burnout, depression, and other psychosocial concerns.

Costs of clinician burnout
Burnout is endemic among health care providers, with numerous studies detailing the professional, emotional, and financial costs. Prior to the pandemic, one analysis of nationwide fiscal costs associated with burnout estimated an annual cost of $4.6B due to physician turnover and reduced clinical hours.21 The COVID-19 epidemic has by all accounts worsened rates of health care worker burnout, particularly for those in high patient-contact positions.22
Female clinicians appear to be differentially affected; in one recent study women reported symptoms of burnout at twice the rate of their male counterparts.23 Whether burnout rates will return to pre-pandemic levels remains an open question, but since burnout is frequently related to one’s own assessment of work-life balance, it is possible that a longer term shift in burnout rates associated with post-pandemic occupational attitudes will be observed.
Combining factors contribute to burnout
Burnout is a universal occupational hazard, but extant data suggest that physicians and other health care providers may be at higher risk. Among physicians, younger age, female gender, and front-line specialty status appear associated with higher burnout rates.24 Given that ObGyn physicians are overwhelmingly female (60% of physicians and 86% of residents),25,26 gender-related burnout factors exist alongside other specific occupational burnout risks. While gender parity has been achieved among health care providers, gender disparities persist in terms of those in leadership positions, compensation, and other factors.22
The smattering of evidence suggesting that ObGyns have higher rates of burnout than many other specialties is understandable given the unique legal challenges confronting ObGyn practice. This may be of special significance because ObGyn malpractice insurance rates are among the highest of all specialties.27 The overall shortage of ObGyns has been exacerbated by the demonstrated negative effects on training and workforce representation stemming from recent legislation that has the effect of criminalizing certain aspects of ObGyn practice;28 for instance, uncertainty regarding abortion regulations.
These negative effects are particularly heightened in states in which the law is in flux or where there are continuing efforts to substantially limit access to abortion. The efforts to increase civil and even criminal penalties related to abortion care challenge ObGyns’ professional practices, as legal rules are frequently changing. In some states, ObGyns may face additional workloads secondary to a flight of ObGyns from restrictive jurisdictions in addition to legal and professional repercussions. In a small study of 19 genetic counselors dealing with restrictive legislation in the state of Ohio,29 increased stress and burnout rates were identified as a consequence of practice uncertainties under this legislation. It is certain that other professionals working in reproductive health care are similarly affected.30
The programs provide individual resources to providers in distress, periodically survey initiatives at Stanford to assess burnout at the organizational level, and provide input designed to spur organizational change to reduce the burden of burnout. Ways that they build community and connections include:
- Live Story Rounds events (as told by Stanford Medicine physicians)
- Commensality Groups (facilitated small discussion groups built around tested evidence)
- Aim to increase sense of connection and collegiality among physicians and build comradery at work
- CME-accredited physician wellness forum, including annual doctor’s day events
Continue to: Assessment of burnout...
Assessment of burnout
Numerous scales for the assessment of burnout exist. Of these, the 22-item Maslach Burnout Inventory (MBI) is the best studied. The MBI is a well-investigated tool for assessing burnout. The MBI consists of 3 major subscales measuring overall burnout, emotional exhaustion, depersonalization, and low personal accomplishment. It exists in numerous forms. For instance, the MBI-HSS (MP), adapted for medical personnel, is available. However, the most commonly used form for assessing burnout in clinicians is the MBI-HHS (Human Services Survey); approximately 85% of all burnout studies examined in a recent meta-analysis used this survey version.31 As those authors commented, while burnout is a recognized phenomenon, a great deal of variability in study design, interpretation of subscale scores, and sample selection makes generalizations regarding burnout difficult to assess.
The MBI in various forms has been extensively used over the past 40 years to assess burnout amongst physicians and physicians in training. While not the only instrument designed to measure such factors, it is by far the most prevalent. Williamson and colleagues32 compared the MBI with several other measures of quality of life and found good correlation between the various instruments used, a finding replicated by other studies.33 Brady and colleagues compared item responses to the Stanford Professional Fulfillment Index and the Min-Z Single-item Burnout scale (a 1-item screening measure) to MBI’s Emotional Exhaustion and Depersonalization subscales. Basing their findings on a survey of more than 1,300 physicians, they found that all analyzed scales were significantly correlated with such adverse outcomes as depression, distress, or intent to leave the profession.
It is important to note that most surveys of clinician burnout were conducted prior to the pandemic. While the psychometric analyses of the MBI and other scales are likely still germane, observed rates of clinician burnout have likely increased. Thus, comparisons of pre- and post-pandemic studies should factor in an increase in the incidence and prevalence of burnout.
Management strategies
In general, there are several interventions for managing burnout34:
- individual-focused (including self-care and communications-skills workshops)
- mindfulness training
- yoga
- meditation
- organizational/structural (workload reduction, schedule realignment, teamwork training, and group-delivered stress management interventions)
- combination(s) of the above.
There is little evidence to suggest that any particular individual intervention (whether delivered in individual or group-based formats) is superior to any other in treating clinician burnout. A recent analysis of 24 studies employing mindfulness-based interventions demonstrated generally positive results for such interventions.35 Other studies have also found general support for mindfulness-based interventions, although mindfulness is often integrated with other stress-reduction techniques, such as meditation, yoga, and communication skills. Such interventions are nonspecific but generally effective.
An accumulation of evidence to date suggests that a combination of individual and organizational interventions is most effective in combatting clinician burnout. No individual intervention can be successful without addressing root causes, such as overscheduling, lack of organizational support, and the effect of restrictive legislation on practice.
Several large teaching hospitals have established programs to address physician and health care provider burnout. Notable among these is the Stanford University School of Medicine’s WellMD and WellPhD programs (https://wellmd.stanford.edu/about.html). These programs were described by Olson and colleagues36 as using a model focused on practice efficiency, organizational culture, and personal resilience to enhance physicians’ well-being. (See “Aspects of the WellMD and WellPhD programs from Stanford University.”)
A growing number of institutions have established burnout programs to support physicians experiencing work/life imbalances and other aspects of burnout.37 In general, these share common features of assessment, individual and/or group intervention, and organizational change. Fear of repercussion may be one factor preventing physicians from seeking individual treatment for burnout.38 Importantly, they emphasize the need for professional confidentiality when offering treatment to patients within organizational settings. Those authors also reported that a focus on organizational engagement may be an important factor in addressing burnout in female physicians, as they tend to report lower levels of organizational engagement.
Continue to: Legal considerations...
Legal considerations
Until recently, physician burnout “received little notice in the legal literature.”39 Although there have been burnout legal consequences in the past, the legal issues are now becoming more visible.40
Medical malpractice
A well-documented consequence of burnout is an increase in errors.14 Medical errors, of course, are at the heart of malpractice claims. Technically, malpractice is medical or professional negligence. It is the breach of a duty owed by the physician, or other provider, or organization (defendant) to the patient, which causes injury to the plaintiff/patient.41
“Medical error” is generally a meaningful deviation from the “standard of care” or accepted medical practice.42 Many medical errors do not cause injury to the patient; in those cases, the negligence does not result in liability. In instances in which the negligence causes harm, the clinician and health care facility may be subject to liability for that injury. Fortunately, however, for a variety of reasons, most harmful medical errors do not result in a medical malpractice claim or lawsuit. The absence of a good clinician-patient relationship is likely associated with an increased inclination of a patient to file a malpractice action.43Clinician burnout may, therefore, contribute to increased malpractice claims in two ways. First, burnout likely leads to increased medical errors, perhaps because burnout is associated with lower concentration, inattention, reduced cognitive vigilance, and fatigue.8,44 It may also lead to less time with patients, reduced patient empathy, and lower patient rapport, which may make injured patients more likely to file a claim or lawsuit.45 Because the relationshipbetween burnout and medical error is bidirectional, malpractice claims tend to increase burnout, which increases error. Given the time it takes to resolve most malpractice claims, the uncertainty of medical malpractice may be especially stressful for health care providers.46,47
Burnout is not a mitigating factor in malpractice. Our sympathies may go out to a professional suffering from burnout, but it does not excuse or reduce liability—it may, indeed, be an aggravating factor. Clinicians who can diagnose burnout and know its negative consequences but fail to deal with their own burnout may be demonstrating negligence if there has been harm to a patient related to the burnout.48
Institutional or corporate liability to patients
Health care institutions have obligations to avoid injury to patients. Just as poorly maintained medical equipment may harm patients, so may burned-out professionals. Therefore, institutions have some obligation to supervise and avoid the increased risks to patients posed by professionals suffering from burnout.
Respondeat superior and institutional negligence. Institutional liability may arise in two ways, the first through agency, or respondeat superior. That is, if the physician or other professional is an employee (or similar agent) of the health care institution, that institution is generally responsible for the physician’s negligence during the employment.49 Even if the physician is not an employee (for example, an independent contractor providing care or using the hospital facilities), the health care facility may be liable for the physician’s negligence.50 Liability may occur, for example, if the health care facility was aware that the physician was engaged in careless practice or was otherwise a risk to patients but the facility did not take steps to avoid those risks.51 The basis for liability is that the health care organization owes a duty to patients to take reasonable care to ensure that its facilities are not used to injure patients negligently.52 Just as it must take care that unqualified physicians are not granted privileges to practice, it also must take reasonable steps to protect patients when it is aware (through nurses or other agents) of a physician’s negligent practice.
In one case, for example, the court found liability where a staff member had “severe” burnout in a physician’s office and failed to read fetal monitoring strips. The physician was found negligent for relying on the staff member who was obviously making errors in interpretation of fetal distress.53
Continue to: Legal obligations of health care organizations to physicians and others...
Legal obligations of health care organizations to physicians and others
In addition to obligations to patients, health care organizations may have obligations to employees (and others) at risk for injury. For example, assume a patient is diagnosed with a highly contagious disease. The health care organization would be obligated to warn, and take reasonable steps to protect, the staff (employees and independent contractors) from being harmed from exposure to the disease. This principle may apply to coworkers of employees with significant burnout, thereby presenting a danger in the workplace. The liability issue is more difficult for employees experiencing job-related burnout themselves. Organizations generally compensate injured employees through no-fault workers’ compensation (an insurance-like system); for independent contractors, the liability is usually through a tort claim (negligence).54
In modern times, a focus has been on preventing those injuries, not just providing compensation after injuries have occurred. Notably, federal and state occupational health and safety laws (particularly the Occupational Safety and Health Administration [OSHA]) require most organizations (including those employing health care providers) to take steps to mitigate various kinds of worker injuries.55
Although these worker protections have commonly been applied to hospitals and other health care providers, burnout has not traditionally been a significant concern in federal or state OSHA enforcement. For example, no formal federal OSHA regulations govern work-related burnout. Regulators, including OSHA, are increasingly interested in burnout that may affect many employees. OSHA has several recommendations for reducing health care work burnout.56 The Surgeon General has expressed similar concerns.57 The federal government recently allocated $103 million from the American Rescue Plan to address burnout among health care workers.58 Also, OSHA appears to be increasing its oversight of healthcare-institution-worker injuries.55
Is burnout a “disability”?
The federal Americans with Disabilities Act (ADA) and similar state laws prohibit discrimination based on disability.59 A disability is defined as a “physical or mental impairment that substantially limits one or more major life activities” or “perceived as having such an impairment.”60 The initial issue is whether burnout is a “mental impairment.” As noted earlier, it is not officially a “medical condition.”61 To date, the United Nations has classified it as an “occupational phenomenon.”62 It may, therefore, not qualify under the ADA, even if it “interferes with a major life activity.” There is, however, some movement toward defining burnout as a mental condition. Even if defined as a disability, there would still be legal issues of how severe it must be to qualify as a disability and the proper accommodation. Apart from the legal definition of an ADA disability, as a practical matter it likely is in the best interest of health care facilities to provide accommodations that reduce burnout. A number of strategies to decrease the incidence of burnout include the role of health care systems (FIGURE 2).
In conclusion we look at several things that can be done to “treat” or reduce burnout. That effort requires the cooperation of physicians and other providers, health care facilities, training programs, licensing authorities, and professional organizations. See suggestions below.
Conclusion
There are many excellent suggestions for reducing burnout and improving patient care and practitioner satisfaction.63-65 We conclude with a summary of some of these suggestions for individual practitioners, health care organizations, the profession, and licensing. It is worth remembering, however, that it will require the efforts of each area to reduce burnout substantially.
For practitioners:
- Engage in quality coaching/therapy on mindfulness and stress management.
- Practice self-care, including exercise and relaxation techniques.
- Make work-life balance a priority.
- Take opportunities for collegial social and professional discussions.
- Prioritize (and periodically assess) your own professional satisfaction and burnout risk.
- Smile—enjoy a sense of humor (endorphins and cortisol).
For health care organizations:
- Urgently work with vendors and regulators to revise electronic health records to reduce their substantial impact on burnout.
- Reduce physicians’ time on clerical and administrative tasks (eg, by enhancing the use of quality AI, scribes, and automated notes from appointments. (This may increase the time they spend with patients.) Eliminate “pajama-time” charting.
- Provide various kinds of confidential professional counseling, therapy, and support related to burnout prevention and treatment, and avoid any penalty or stigma related to their use.
- Provide reasonable flexibility in scheduling.
- Routinely provide employees with information about burnout prevention and services.
- Appoint a wellness officer with authority to ensure the organization maximizes its prevention and treatment services.
- Constantly seek input from practitioners on how to improve the atmosphere for practice to maximize patient care and practitioner satisfaction.
- Provide ample professional and social opportunities for discussing and learning about work-life balance, resilience, intellectual stimulation, and career development.
For regulators, licensors, and professional organizations:
- Work with health care organizations and EHR vendors to substantially reduce the complexity, physician effort, and stress associated with those record systems. Streamlining should, in the future, be part of formally certifying EHR systems.
- Reduce the administrative burden on physicians by modifying complex regulations and using AI and other technology to the extent possible to obtain necessary reimbursement information.
- Eliminate unnecessary data gathering that requires practitioner time or attention.
- Licensing, educational, and certifying bodies should eliminate any questions regarding the diagnosis or treatment of mental health and focus on current (or very recent) impairments.
- Seek funding for research on burnout prevention and treatment.
Dr. H is a 58-year-old ObGyn who, after completing residency, went into solo practice. The practice grew, and Dr. H found it increasingly more challenging to cover, especially the obstetrics sector. Dr. H then merged the practice with a group of 3 other ObGyns. Their practice expanded, and began recruiting recent residency graduates. In time, the practice was bought out by the local hospital health care system. Dr. H was faced with complying with the rules and regulations of that health care system. The electronic health record (EHR) component proved challenging, as did the restrictions on staff hiring (and firing), but Dr. H did receive a paycheck each month and complied with it all. The health care system administrators had clear financial targets Dr. H was to meet each quarter, which created additional pressure. Dr. H used to love being an OB and providing excellent care for every patient, but that sense of accomplishment was being lost.
Dr. H increasingly found it difficult to focus because of mind wandering, especially in the operating room (OR). Thoughts occurred about retirement, the current challenges imposed by “the new way of practicing medicine” (more focused on financial productivity restraints and reimbursement), and EHR challenges. Then Dr. H’s attention would return to the OR case at hand. All of this resulted in considerable stress and emotional exhaustion, and sometimes a sense of being disconnected. A few times, colleagues or nurses had asked Dr. H if everything was “okay,” or if a break would help. Dr. H made more small errors than usual, but Dr. H’s self-assessment was “doing an adequate job.” Patient satisfaction scores (collected routinely by the health care system) declined over the last 9 months.
Six months ago, Dr. H finished doing a laparoscopic total hysterectomy and bilateral salpingo-oophorectomy and got into the right uterine artery. The estimated blood loss was 3,500 mL. Using minimally invasive techniques, Dr. H identified the bleeder and, with monopolar current, got everything under control. The patient went to the post-anesthesia care unit, and all appeared to be in order. Her vital signs were stable, and she was discharged home the same day.
The patient presented 1 week later with lower abdominal and right flank pain. Dr. H addressed the problem in the emergency department and admitted the patient for further evaluation and urology consultation. The right ureter was damaged and obstructed; ultimately, the urologist performed a psoas bladder hitch. The patient recovered slowly, lost several weeks of work, experienced significant pain, and had other disruptions and costs. Additional medical care related to the surgery is ongoing. A health care system committee asked Dr. H to explain the problem. Over the last 6 months, Dr. H’s frustration with practice and being tired and disconnected have increased.
Dr. H has received a letter from a law firm saying that he and the health care system are being sued for malpractice focused on an iatrogenic ureter injury. The letter names two very reputable experts who are prepared to testify that the patient’s injury resulted from clear negligence. Dr. H has told the malpractice carrier absolutely not to settle this case—it is “a sham— without merit.” The health care system has asked Dr. H to take a “burnout test.”
Legal considerations
Dr. H exhibits relatively clear signs of professional burnout. The fact that there was a bad outcome while Dr. H was experiencing burnout is not proof of negligence (or, breach of duty of care to the patient). Nor is it a defense or mitigation to any malpractice that occurred.
In the malpractice case, the plaintiff will have the burden of proving that Dr. H’s treatment was negligent in that it fell below the standard of care. Even if it was a medical error, the question is whether it was negligence. If the patient/plaintiff, using expert witnesses, can prove that Dr. H fell below the standard of care that caused injury, Dr. H may be liable for the resulting extra costs, loss of income, and pain and suffering resulting from the negligent care.
The health care system likely will also be responsible for Dr. H’s negligence, either through respondeat superior (for example, if Dr. H is an employee) or for its own negligence. The case for its negligence is that the nurses and assistants had repeatedly seen him making errors and becoming disengaged (to the extent that they asked Dr. H if “everything is okay” or if a break would help). Furthermore, Dr. H’s patient satisfaction scores have been declining for several months. The plaintiff will argue that Dr. H exhibited classic burnout symptoms with the attendant risks of medical errors. However, the health care system did not take action to protect patients or to assist Dr. H. In short, one way or another, there is some likelihood that the health care system may also be liable if patient injuries are found to have been caused by negligence.
At this point, the health care system also faces the question of how to work with Dr. H in the future. The most pressing question is whether or not to allow Dr. H to continue practicing. If, as it appears, Dr. H is dealing with burnout, the pressure of the malpractice claim could well increase the probability of other medical mistakes. The institution has asked Dr. H to take a burnout test, but it is unclear where things go if the test (as likely) demonstrates significant burnout. This is a counseling and human relations question, at least as much as a legal issue, and the institution should probably proceed in that way—which is, trying to understand and support Dr. H and determining what can be done to address the burnout. At the same time, the system must reasonably assess Dr. H’s fitness to continue practicing as the matters are resolved. Almost everyone shares the goal to provide every individual and corporate opportunity for Dr. H to deal with burnout issues and return to successful practice.
Dr. H will be represented in the malpractice case by counsel provided through the insurance carrier. However, Dr. H would be well advised to retain a trusted and knowledgeable personal attorney. For example, the instruction not to consider settlement is likely misguided, but Dr. H needs to talk with an attorney that Dr. H has chosen and trusts. In addition, the attorney can help guide Dr. H through a rational process of dealing with the health care system, putting the practice in order, and considering the options for the future.
The health care system should reconsider its processes to deal with burnout to ensure the quality of care, patient satisfaction, professional retention, and economic stability. Several burnoutresponse programs have had success in achieving these goals.
What’s the Verdict?
Dr. H received good mental health, legal, and professional advice. As a result, an out of court settlement was reached following pretrial discovery. Dr. H has continued consultation regarding burnout and has returned to productive practice.
- Shanafelt TD, West CP, Sinsky C, et al. Changes in burnout and satisfaction with work-life integration in physicians and the general US working population between 2011 and 2017. Mayo Clinic Proceed. 2019;94:1681-1694.
- Smith R, Rayburn W. Burnout in obstetrician-gynecologists. Its prevalence, identification, prevention, and reversal. Obstet Gynecol Clin North Am. 2021;48:231-245. https://doi. org/10.1016/j.ogc.2021.06.003
- Patti MG, Schlottmann F, Sarr MG. The problem of burnout among surgeons. JAMA Surg. 2018;153:403-404. doi:10.1001 /jamasurg.2018.0047
- Carrau D, Janis JE. Physician burnout: solutions for individuals and organizations. Plastic and Reconstructive Surgery Global Open. 2021;91-97.
- Southwick R. The key to fixing physician burnout is the workplace not the worker. Contemporary Ob/Gyn. March 13, 2023.
- Patel RS, Bachu R, Adikey A, et al. Factors related to physician burnout and its consequences: a review. Behav Sciences. 2018;8:98.
- Melnick ER, Dyrbye LN, Sinsky CA, et al. The association between perceived electronic health record usability and professional burnout among US physicians. Mayo Clinic Proceed. 2020;95:476-487.
- Shanafelt TD, Dyrbye LN, West CP. Addressing physician burnout: the way forward. JAMA. 2017;317:901-902. doi:10.1001/jama.2017.0076
- Ommaya AK, Cipriano PF, Hoyt DB, et al. Care-centered clinical documentation in the digital environment: Solutions to alleviate burnout. National Academy of Medicine Perspectives. 2018.
- Hartzband P, Groopman J. Physician burnout, interrupted. N Engl J Med. 2020;382:2485-2487. Discussion Paper, National Academy of Medicine. Accessed July 21, 2023. https://nam .edu/care
- Ji YD, Robertson FC, Patel NA, et al. Assessment of risk factors for suicide among US health care professionals. JAMA Surg. 2020;155:713-721. centered-clinical-documentation-digital -environment-solutions-alleviate-burnout/
- Shanafelt TD, West CP, Dyrbye LN, et al. Changes in burnout and satisfaction with work-life integration in physicians during the first 2 years of the COVID-19 pandemic. Mayo Clinic Proceed. 2022;97:2248-2258.
- Herber-Valdez C, Kupesic-Plavsic S. Satisfaction and shortfall of OB-GYN physicians and radiologists. J. Ultrasound Obstet Gynecol. 2021;15:387-392.
- Dyrbye LN, Shanafelt TD, Sinsky CA, et al. Burnout among health care professionals: a call to explore and address this underrecognized threat to safe, high-quality care. National Academy of Medicine Perspectives. Accessed July 5, 2017. https://iuhcpe.org/file_manager/1501524077-Burnout -Among-Health-Care-Professionals-A-Call-to-Explore-and -Address-This-Underrecognized-Threat.pdf
- Olson KD. Physician burnout—a leading indicator of health system performance? Mayo Clinic Proceed. 2017;92: 1608-1611.
- American College of Obstetricians and Gynecologists. Why obgyns are burning out. October 28, 2019. Accessed July 21, 2023. https://www.acog.org/news/news-articles/2019/10/why-ob -gyns-are-burning-out#:~:text=A%202017%20report%20 by%20the,exhaustion%20or%20lack%20of%20motivation
- Peckham C. National physician burnout & depression report 2018. Medscape. January 17, 2018. https://nap. nationalacademies.org/catalog/25521/taking-action -against-clinician-burnout-a-systems-approach-to -professional
- Marsa L. Labor pains: The OB-GYN shortage. AAMC News. Nov. 15, 2018. Accessed July 21, 2023. https://www.aamc.org /news-insights/labor-pains-ob-gyn-shortage
- American College of Obstetricians and Gynecologists. Coping with the stress of medical professional liability litigation. ACOG Committee Opinion. February 2005;309:453454. Accessed July 21, 2023. https://www.acog.org/clinical /clinical-guidance/committee-opinion/articles/2013/01 /coping-with-the-stress-of-medical-professional-liability -litigation
- Reith TP. Burnout in United States healthcare professionals: a narrative review. Cureus. 2018;10:e3681. doi: 10.7759 /cureus.3681
- Han S, Shanafelt TD, Sinsky CA, et al. Estimating the attributable cost of physician burnout in the United States. Ann Intern Med. 2019;4:784-790.
- Sullivan D, Sullivan V, Weatherspoon D, et al. Comparison of nurse burnout, before and during the COVID-19 pandemic. Nurs Clin North Am. 2022;57:79-99. doi: 10.1016 /j.cnur.2021.11.006
- Chandawarkar A, Chaparro JD. Burnout in clinicians. Curr Prob Pediatr Adolesc Health Care. 2021;51:101-104. https ://doi.org/10.1016/j.cppeds.2021.101104
- Brady KJS, Sheldrick RC, Ni P, et al. Examining the measurement equivalence of the Maslach Burnout Inventory across age, gender, and specialty groups in US physicians. J Patient-Reported Outcomes. 2021;5.
- Association of American Medical Colleges. Physician Specialty Data Report—Active Physicians by Sex and Specialty, 2021. Accessed June 19, 2023. https://www.aamc .org/data-reports/workforce/data/active-physicians-sex -specialty-2021
- Association of American Medical Colleges. Physician Specialty Data Report—ACGME Residents and Fellows by Sex and Specialty, 2021. Accessed June 19, 2023. https://www .aamc.org/data-reports/workforce/data/acgme-residents -fellows-sex-and-specialty-2021
- Painter LM, Biggans KA, Turner CT. Risk managementobstetrics and gynecology perspective. Clin Obstet Gynecol. 2023;66:331-341. DOI:10.1097/GRF.0000000000000775
- Darney BG, Boniface E, Liberty A. Assessing the effect of abortion restrictions. Obstetr Gynecol. 2023;141:233-235.
- Heuerman AC, Bessett D, Antommaria AHM, et al. Experiences of reproductive genetic counselors with abortion regulations in Ohio. J Genet Counseling. 2022;31:641-652.
- Brandi K, Gill P. Abortion restrictions threaten all reproductive health care clinicians. Am J Public Health. 2023;113:384-385.
- Rotenstein LS, Torre M, Ramos MA, et al. Prevalence of burnout among physicians: a systematic review. JAMA. 2018;320:1131-1150. doi: 10.1001/jama.2018.1277
- Williamson K, Lank PM, Cheema N, et al. Comparing the Maslach Burnout Inventory to other well-being instruments in emergency medicine residents. J Graduate Med Education. 2018;532-536. DOI: http://dx.doi.org/10.4300 /JGME-D-18-00155.1
- Brady KJS, Sheldrick RC, Ni P, et al. Establishing crosswalks between common measures of burnout in US physicians. J Gen Intern Med. 2022;37:777-784.
- Zhang X, Song Y, Jiang T, et al. Interventions to reduce burnout of physicians and nurses: an overview of systematic reviews and meta-analyses. Medicine (Baltimore). 2020;26:e20992. DOI: 10.1097/MD.0000000000020992
- Scheepers RA, Emke H, Ronald M, et al. The impact of mindfulness-based interventions on doctors’ well-being and performance: a systematic review. Med Education. 2020;54:138-149. https://doi.org/10.1111/medu.14020
- Olson K, Marchalik D, Farley H, et al. Organizational strategies to reduce physician burnout and improve professional fulfillment. Curr Prob Pediatr Adolesc Health Care. 2019;49:12. https://doi.org/10.1016/j.cppeds.2019.100664
- Berry LL, Awdish RLA, Swensen SJ. 5 ways to restore depleted health care workers. Harvard Business Rev. February 11, 2022.
- Sullivan AB, Hersh CM, Rensel M, et al. Leadership inequity, burnout, and lower engagement of women in medicine. J Health Serv Psychol. 2023;49:33-39.
- Hoffman S. Healing the healers: legal remedies for physician burnout. Yale J Health Policy Law Ethics. 2018;18:56-113.
- Federation of State Medical Boards. Physician wellness and burnout: report and recommendations of the workgroup on physician wellness and burnout. (Policy adopted by FSMB). April 2018. Accessed July 21, 2023. https://www.fsmb.org /siteassets/advocacy/policies/policy-on-wellness-and -burnout.pdf
- Robinson C, Kettering C, Sanfilippo JS. Medical malpractice lawsuits. Clin Obstet Gynecol. 2023;66:256-260. DOI: https ://doi.org/10.1097/GRF.0000000000000777
- Gittler GJ, Goldstein EJ. The elements of medical malpractice: an overview. Clin Infect Dis. 1996;23:1152-1155.
- Bal BS. An introduction to medical malpractice in the United States. Clin Orthop Relat Res. 2009;467:339-347.
- Tawfik DS, Profit J, Morgenthaler TI, et al. Physician burnout, well-being, and work unit safety grades in relationship to reported medical errors. Mayo Clinic Proceed. 2018;93: 1571-1580.
- Sundholm B. Elevating physician-patient relationships in the shadow of metric mania. Drexel L Rev. 2020;12:287-330.
- Ghaith S, Campbell RL, Pollock JR, et al. Medical malpractice lawsuits involving trainees in obstetrics and gynecology in the USA. Healthcare. 2022;10:1328.
- Muller TM, Warsi S. Litigation culture causing burnout in American physicians. Trauma Mental Health Report. April 9, 2021.
- Levine AS. Legal 101: Tort law and medical malpractice for physicians. Contemp OBGYN. 2015:60;26-28, 30.
- Regan JJ, Regan WM. Medical malpractice and respondeat superior. Southern Med J. 2002;95.5:545-549. DOI 10.1097/00007611-200295050-00018
- Levin H. Hospital vicarious liability for negligence by independent contractor physicians: new rule for new times. Univ Illinois Law Rev. 2005:1291-1332.
- Darling v Charleston Hospital, 33 Ill. 2d 326, 211 N.E.2d 253 (Ill. 1965).
- Dangel R. Hospital liability for physician malpractice. Ohio State Law J. 1986;47:1077-1098.
- Reffitt v Hajjar, 892 S.W.2d 599, 605 (Ky. Ct. App. 1994).
- McMichael BJ. Malpractice. In Laws of Medicine: Core Legal Aspects for the Healthcare Professional. New York, NY: Springer International; 2022:129-150.
- Occupational Safety and Health Administration. Worker safety in hospitals: caring for our caregivers. Accessed June 8, 2023. https://www.osha.gov/hospitals
- Occupational Safety and Health Administration. Workplace stress. Accessed June 8, 2023. https://www.osha.gov /workplace-stress/understanding-the-problem
- U.S. Surgeon General’s Advisory on Building a Thriving Health Workforce. Addressing health worker burnout. Accessed July 21, 2023. https://www.hhs.gov/sites/default/files/health -worker-wellbeing-advisory.pdf
- Department of Health & Human Services. Biden-Harris administration awards $103 Million in American Rescue Plan funds to reduce burnout and promote mental health and wellness among health care workforce. January 20, 2022. Accessed July 24, 2023. https://www.hhs.gov/about /news/2022/01/20/biden-harris-administration-awards -103-million-american-rescue-plan-funds-reduce-burnout -promote-mental-health-wellness-among-health-care -workforce.html
- Rothstein LF, Irzyk J. Disabilities and the Law. 4th ed. Toronto, Canada: Thompson Reuters; 2023.
- Department of Labor. Guide to disability rights laws. February 28, 2020. Accessed July 24, 2023. https://www .ada.gov/resources/disability-rights-guide/#:~:text=An%20 individual%20with%20a%20disability%20is%20defined%20 by%20the%20ADA,as%20having%20such%20an%20 impairment
- Nadon L, De Beer LT, Morin AJS. Should burnout be conceptualized as a mental disorder? Behavioral Sci. 2022;12:82.
- World Health Organization. Burn-out an “occupational phenomenon”: International Classification of Diseases. May 28, 2019. Accessed July 21, 2023. https://www.who.int/news /item/28-05-2019-burn-out-an-occupational-phenomenon -international-classification-of-diseases
- Hoffman S. Physician burnout: why legal and regulatory systems may need to step in. The Conversation. July 9, 2019. https://theconversation.com/physician-burnout-why-legal -and-regulatory-systems-may-need-to-step-in-119705
- Jha A, Iliff A, Chaoi A, et al. A crisis in healthcare: a call to action on physician burnout. Harvard Global Health Institute. 2019. Accessed July 21, 2023. https://www.massmed.org /Publications/Research,-Studies,-and-Reports/Physician -Burnout-Report-2018/
- Arnsten AF, Shanafelt T. Physician distress and burnout: the neurobiological perspective. Mayo Clin Proceed. 2021;96:763-769.
- Shanafelt TD, West CP, Sinsky C, et al. Changes in burnout and satisfaction with work-life integration in physicians and the general US working population between 2011 and 2017. Mayo Clinic Proceed. 2019;94:1681-1694.
- Smith R, Rayburn W. Burnout in obstetrician-gynecologists. Its prevalence, identification, prevention, and reversal. Obstet Gynecol Clin North Am. 2021;48:231-245. https://doi. org/10.1016/j.ogc.2021.06.003
- Patti MG, Schlottmann F, Sarr MG. The problem of burnout among surgeons. JAMA Surg. 2018;153:403-404. doi:10.1001 /jamasurg.2018.0047
- Carrau D, Janis JE. Physician burnout: solutions for individuals and organizations. Plastic and Reconstructive Surgery Global Open. 2021;91-97.
- Southwick R. The key to fixing physician burnout is the workplace not the worker. Contemporary Ob/Gyn. March 13, 2023.
- Patel RS, Bachu R, Adikey A, et al. Factors related to physician burnout and its consequences: a review. Behav Sciences. 2018;8:98.
- Melnick ER, Dyrbye LN, Sinsky CA, et al. The association between perceived electronic health record usability and professional burnout among US physicians. Mayo Clinic Proceed. 2020;95:476-487.
- Shanafelt TD, Dyrbye LN, West CP. Addressing physician burnout: the way forward. JAMA. 2017;317:901-902. doi:10.1001/jama.2017.0076
- Ommaya AK, Cipriano PF, Hoyt DB, et al. Care-centered clinical documentation in the digital environment: Solutions to alleviate burnout. National Academy of Medicine Perspectives. 2018.
- Hartzband P, Groopman J. Physician burnout, interrupted. N Engl J Med. 2020;382:2485-2487. Discussion Paper, National Academy of Medicine. Accessed July 21, 2023. https://nam .edu/care
- Ji YD, Robertson FC, Patel NA, et al. Assessment of risk factors for suicide among US health care professionals. JAMA Surg. 2020;155:713-721. centered-clinical-documentation-digital -environment-solutions-alleviate-burnout/
- Shanafelt TD, West CP, Dyrbye LN, et al. Changes in burnout and satisfaction with work-life integration in physicians during the first 2 years of the COVID-19 pandemic. Mayo Clinic Proceed. 2022;97:2248-2258.
- Herber-Valdez C, Kupesic-Plavsic S. Satisfaction and shortfall of OB-GYN physicians and radiologists. J. Ultrasound Obstet Gynecol. 2021;15:387-392.
- Dyrbye LN, Shanafelt TD, Sinsky CA, et al. Burnout among health care professionals: a call to explore and address this underrecognized threat to safe, high-quality care. National Academy of Medicine Perspectives. Accessed July 5, 2017. https://iuhcpe.org/file_manager/1501524077-Burnout -Among-Health-Care-Professionals-A-Call-to-Explore-and -Address-This-Underrecognized-Threat.pdf
- Olson KD. Physician burnout—a leading indicator of health system performance? Mayo Clinic Proceed. 2017;92: 1608-1611.
- American College of Obstetricians and Gynecologists. Why obgyns are burning out. October 28, 2019. Accessed July 21, 2023. https://www.acog.org/news/news-articles/2019/10/why-ob -gyns-are-burning-out#:~:text=A%202017%20report%20 by%20the,exhaustion%20or%20lack%20of%20motivation
- Peckham C. National physician burnout & depression report 2018. Medscape. January 17, 2018. https://nap. nationalacademies.org/catalog/25521/taking-action -against-clinician-burnout-a-systems-approach-to -professional
- Marsa L. Labor pains: The OB-GYN shortage. AAMC News. Nov. 15, 2018. Accessed July 21, 2023. https://www.aamc.org /news-insights/labor-pains-ob-gyn-shortage
- American College of Obstetricians and Gynecologists. Coping with the stress of medical professional liability litigation. ACOG Committee Opinion. February 2005;309:453454. Accessed July 21, 2023. https://www.acog.org/clinical /clinical-guidance/committee-opinion/articles/2013/01 /coping-with-the-stress-of-medical-professional-liability -litigation
- Reith TP. Burnout in United States healthcare professionals: a narrative review. Cureus. 2018;10:e3681. doi: 10.7759 /cureus.3681
- Han S, Shanafelt TD, Sinsky CA, et al. Estimating the attributable cost of physician burnout in the United States. Ann Intern Med. 2019;4:784-790.
- Sullivan D, Sullivan V, Weatherspoon D, et al. Comparison of nurse burnout, before and during the COVID-19 pandemic. Nurs Clin North Am. 2022;57:79-99. doi: 10.1016 /j.cnur.2021.11.006
- Chandawarkar A, Chaparro JD. Burnout in clinicians. Curr Prob Pediatr Adolesc Health Care. 2021;51:101-104. https ://doi.org/10.1016/j.cppeds.2021.101104
- Brady KJS, Sheldrick RC, Ni P, et al. Examining the measurement equivalence of the Maslach Burnout Inventory across age, gender, and specialty groups in US physicians. J Patient-Reported Outcomes. 2021;5.
- Association of American Medical Colleges. Physician Specialty Data Report—Active Physicians by Sex and Specialty, 2021. Accessed June 19, 2023. https://www.aamc .org/data-reports/workforce/data/active-physicians-sex -specialty-2021
- Association of American Medical Colleges. Physician Specialty Data Report—ACGME Residents and Fellows by Sex and Specialty, 2021. Accessed June 19, 2023. https://www .aamc.org/data-reports/workforce/data/acgme-residents -fellows-sex-and-specialty-2021
- Painter LM, Biggans KA, Turner CT. Risk managementobstetrics and gynecology perspective. Clin Obstet Gynecol. 2023;66:331-341. DOI:10.1097/GRF.0000000000000775
- Darney BG, Boniface E, Liberty A. Assessing the effect of abortion restrictions. Obstetr Gynecol. 2023;141:233-235.
- Heuerman AC, Bessett D, Antommaria AHM, et al. Experiences of reproductive genetic counselors with abortion regulations in Ohio. J Genet Counseling. 2022;31:641-652.
- Brandi K, Gill P. Abortion restrictions threaten all reproductive health care clinicians. Am J Public Health. 2023;113:384-385.
- Rotenstein LS, Torre M, Ramos MA, et al. Prevalence of burnout among physicians: a systematic review. JAMA. 2018;320:1131-1150. doi: 10.1001/jama.2018.1277
- Williamson K, Lank PM, Cheema N, et al. Comparing the Maslach Burnout Inventory to other well-being instruments in emergency medicine residents. J Graduate Med Education. 2018;532-536. DOI: http://dx.doi.org/10.4300 /JGME-D-18-00155.1
- Brady KJS, Sheldrick RC, Ni P, et al. Establishing crosswalks between common measures of burnout in US physicians. J Gen Intern Med. 2022;37:777-784.
- Zhang X, Song Y, Jiang T, et al. Interventions to reduce burnout of physicians and nurses: an overview of systematic reviews and meta-analyses. Medicine (Baltimore). 2020;26:e20992. DOI: 10.1097/MD.0000000000020992
- Scheepers RA, Emke H, Ronald M, et al. The impact of mindfulness-based interventions on doctors’ well-being and performance: a systematic review. Med Education. 2020;54:138-149. https://doi.org/10.1111/medu.14020
- Olson K, Marchalik D, Farley H, et al. Organizational strategies to reduce physician burnout and improve professional fulfillment. Curr Prob Pediatr Adolesc Health Care. 2019;49:12. https://doi.org/10.1016/j.cppeds.2019.100664
- Berry LL, Awdish RLA, Swensen SJ. 5 ways to restore depleted health care workers. Harvard Business Rev. February 11, 2022.
- Sullivan AB, Hersh CM, Rensel M, et al. Leadership inequity, burnout, and lower engagement of women in medicine. J Health Serv Psychol. 2023;49:33-39.
- Hoffman S. Healing the healers: legal remedies for physician burnout. Yale J Health Policy Law Ethics. 2018;18:56-113.
- Federation of State Medical Boards. Physician wellness and burnout: report and recommendations of the workgroup on physician wellness and burnout. (Policy adopted by FSMB). April 2018. Accessed July 21, 2023. https://www.fsmb.org /siteassets/advocacy/policies/policy-on-wellness-and -burnout.pdf
- Robinson C, Kettering C, Sanfilippo JS. Medical malpractice lawsuits. Clin Obstet Gynecol. 2023;66:256-260. DOI: https ://doi.org/10.1097/GRF.0000000000000777
- Gittler GJ, Goldstein EJ. The elements of medical malpractice: an overview. Clin Infect Dis. 1996;23:1152-1155.
- Bal BS. An introduction to medical malpractice in the United States. Clin Orthop Relat Res. 2009;467:339-347.
- Tawfik DS, Profit J, Morgenthaler TI, et al. Physician burnout, well-being, and work unit safety grades in relationship to reported medical errors. Mayo Clinic Proceed. 2018;93: 1571-1580.
- Sundholm B. Elevating physician-patient relationships in the shadow of metric mania. Drexel L Rev. 2020;12:287-330.
- Ghaith S, Campbell RL, Pollock JR, et al. Medical malpractice lawsuits involving trainees in obstetrics and gynecology in the USA. Healthcare. 2022;10:1328.
- Muller TM, Warsi S. Litigation culture causing burnout in American physicians. Trauma Mental Health Report. April 9, 2021.
- Levine AS. Legal 101: Tort law and medical malpractice for physicians. Contemp OBGYN. 2015:60;26-28, 30.
- Regan JJ, Regan WM. Medical malpractice and respondeat superior. Southern Med J. 2002;95.5:545-549. DOI 10.1097/00007611-200295050-00018
- Levin H. Hospital vicarious liability for negligence by independent contractor physicians: new rule for new times. Univ Illinois Law Rev. 2005:1291-1332.
- Darling v Charleston Hospital, 33 Ill. 2d 326, 211 N.E.2d 253 (Ill. 1965).
- Dangel R. Hospital liability for physician malpractice. Ohio State Law J. 1986;47:1077-1098.
- Reffitt v Hajjar, 892 S.W.2d 599, 605 (Ky. Ct. App. 1994).
- McMichael BJ. Malpractice. In Laws of Medicine: Core Legal Aspects for the Healthcare Professional. New York, NY: Springer International; 2022:129-150.
- Occupational Safety and Health Administration. Worker safety in hospitals: caring for our caregivers. Accessed June 8, 2023. https://www.osha.gov/hospitals
- Occupational Safety and Health Administration. Workplace stress. Accessed June 8, 2023. https://www.osha.gov /workplace-stress/understanding-the-problem
- U.S. Surgeon General’s Advisory on Building a Thriving Health Workforce. Addressing health worker burnout. Accessed July 21, 2023. https://www.hhs.gov/sites/default/files/health -worker-wellbeing-advisory.pdf
- Department of Health & Human Services. Biden-Harris administration awards $103 Million in American Rescue Plan funds to reduce burnout and promote mental health and wellness among health care workforce. January 20, 2022. Accessed July 24, 2023. https://www.hhs.gov/about /news/2022/01/20/biden-harris-administration-awards -103-million-american-rescue-plan-funds-reduce-burnout -promote-mental-health-wellness-among-health-care -workforce.html
- Rothstein LF, Irzyk J. Disabilities and the Law. 4th ed. Toronto, Canada: Thompson Reuters; 2023.
- Department of Labor. Guide to disability rights laws. February 28, 2020. Accessed July 24, 2023. https://www .ada.gov/resources/disability-rights-guide/#:~:text=An%20 individual%20with%20a%20disability%20is%20defined%20 by%20the%20ADA,as%20having%20such%20an%20 impairment
- Nadon L, De Beer LT, Morin AJS. Should burnout be conceptualized as a mental disorder? Behavioral Sci. 2022;12:82.
- World Health Organization. Burn-out an “occupational phenomenon”: International Classification of Diseases. May 28, 2019. Accessed July 21, 2023. https://www.who.int/news /item/28-05-2019-burn-out-an-occupational-phenomenon -international-classification-of-diseases
- Hoffman S. Physician burnout: why legal and regulatory systems may need to step in. The Conversation. July 9, 2019. https://theconversation.com/physician-burnout-why-legal -and-regulatory-systems-may-need-to-step-in-119705
- Jha A, Iliff A, Chaoi A, et al. A crisis in healthcare: a call to action on physician burnout. Harvard Global Health Institute. 2019. Accessed July 21, 2023. https://www.massmed.org /Publications/Research,-Studies,-and-Reports/Physician -Burnout-Report-2018/
- Arnsten AF, Shanafelt T. Physician distress and burnout: the neurobiological perspective. Mayo Clin Proceed. 2021;96:763-769.
Imaging Tools for Noninvasive Hair Assessment
New imaging tools along with adaptations to existing technologies have been emerging in recent years, with the potential to improve hair diagnostics and treatment monitoring. We provide an overview of 4 noninvasive hair imaging technologies: global photography, trichoscopy, reflectance confocal microscopy (RCM), and optical coherence tomography (OCT). For each instrument, we discuss current and future applications in clinical practice and research along with advantages and disadvantages.
Global Photography
Global photography allows for the analysis of hair growth, volume, distribution, and density through serial standardized photographs.1 Global photography was first introduced for hair growth studies in 1987 and soon after was used for hair and scalp assessments in finasteride clinical trials.2
Hair Assessment—Washed, dried, and combed hair, without hair product, are required for accurate imaging; wet conditions increase reflection and promote hair clumping, thus revealing more scalp and depicting the patient as having less hair.1 Headshots are taken from short distances and use stereotactic positioning devices to create 4 global views: vertex, midline, frontal, and temporal.3 Stereotactic positioning involves fixing the patient’s chin and forehead as well as mounting the camera and flash device to ensure proper magnification. These adjustments ensure lighting remains consistent throughout consecutive study visits.4 Various grading scales are available for use in hair growth clinical studies to increase objectivity in the analysis of serial global photographs. A blinded evaluator should assess the before and after photographs to limit experimenter bias. Global photography often is combined with quantitative software analysis for improved detection of hair changes.1
Advancements—Growing interest in improving global photography has resulted in various application-based, artificial intelligence (AI)–mediated tools to simplify photograph collection and analysis. For instance, new hair analysis software utilizes AI algorithms to account for facial features in determining the optimal angle for capturing global photographs (Figure 1), which simplifies the generation of global photography images through smartphone applications and obviates the need for additional stereotactic positioning equipment.5,6

Limitations—Clinicians should be aware of global photography’s requirements for consistency in lighting, camera settings, film, and image processing, which can limit the accuracy of hair assessment over time if not replicated correctly.7,8 Emerging global photography software has helped to overcome some of these limitations.
Global photography is less precise when a patient’s hair loss is less than 50%, as it is difficult to discern subtle hair changes. Thus, global photography provides limited utility in assessing minimal to moderate hair loss.9 Currently, global photography largely functions as an adjunct tool for other hair analysis methods rather than as a stand-alone tool.
Trichoscopy
Trichoscopy (also known as dermoscopy of the hair and scalp) may be performed with a manual dermoscope (with 10× magnification) or a digital videodermatoscope (up to 1000× magnification).10-12 Unlike global photography, trichoscopy provides a detailed structural analysis of hair shafts, follicular openings, and perifollicular and interfollicular areas.13 Kinoshita-Ise and Sachdeva13 provided an in-depth, updated review of trichoscopy terminology with their definitions and associated conditions (with prevalence), which should be referenced when performing trichoscopic examination.
Hair Assessment—Trichoscopic assessment begins with inspection of follicular openings (also referred to as “dots”), which vary in color depending on the material filling them—degrading keratinocytes, keratin, sebaceous debris, melanin, or fractured hairs.13 The structure of hair shafts also is examined, showing broken hairs, short vellus hairs, and comma hairs, among others. Perifollicular areas are examined for scale, erythema, blue-gray dots, and whitish halos. Interfollicular areas are examined for pigment pattern as well as vascularization, which often presents in a looping configuration under dermoscopy. A combination of dot colorization, hair shaft structure, and perifollicular and interfollicular findings inform diagnostic algorithms of hair and scalp conditions. For example, central centrifugal cicatricial alopecia, the most common alopecia seen in Black women, has been associated with a combination of honeycomb pigment pattern, perifollicular whitish halo, pinpoint white dots, white patches, and perifollicular erythema.13
Advantages—Perhaps the most useful feature of trichoscopy is its ability to translate visualized features into simple diagnostic algorithms. For instance, if the clinician has diagnosed the patient with noncicatricial alopecia, they would next focus on dot colors. With black dots, the next step would be to determine whether the hairs are tapered or coiled, and so on. This systematic approach enables the clinician to narrow possible diagnoses.2 An additional advantage of trichoscopy is that it examines large surface areas noninvasively as compared to hair-pull tests and scalp biopsy.14,15 Trichoscopy allows temporal comparisons of the same area for disease and treatment monitoring with more diagnostic detail than global photography.16 Trichoscopy also is useful in selecting biopsy locations by discerning and avoiding areas of scar tissue.17
Limitations—Diagnosis via the trichoscopy algorithm is limiting because it is not comprehensive of all hair and scalp disease.18 Additionally, many pathologies exhibit overlapping follicular and interfollicular patterning. For example, almost all subtypes of scarring alopecia present with hair loss and scarred follicles once they have progressed to advanced stages. Further studies should identify more specific patterns of hair and scalp pathologies, which could then be incorporated into a diagnostic algorithm.13
Advancements—The advent of hair analysis software has expanded the role of videodermoscopy by rapidly quantifying hair growth parameters such as hair count, follicular density, and follicular diameter, as well as interfollicular distances (Figure 2).14,17 Vellus and terminal hairs are differentiated according to their thickness and length.17 Moreover, the software can analyze the same area of the scalp over time by either virtual tattoos, semipermanent markings, or precise location measurements, increasing intra- and interclass correlation. The rate of hair growth, hair shedding, and parameters of anagen and telogen hairs can be studied by a method termed phototrichogram whereby a transitional area of hair loss and normal hair growth is identified and trimmed to less than 1 mm from the skin surface.19 A baseline photograph is taken using videodermoscopy. After approximately 3 days, the identical region is photographed and compared with the initial image to observe changes in the hair. Software programs can distinguish the growing hair as anagen and nongrowing hair as telogen, calculating the anagen-to-telogen ratio as well as hair growth rate, which are essential measurements in hair research and clinical studies. Software programs have replaced laborious and time-consuming manual hair counts and have rapidly grown in popularity in evaluating patterned hair loss.
Reflectance Confocal Microscopy
Reflectance confocal microscopy is a noninvasive imaging tool that visualizes skin and its appendages at near-histologic resolution (lateral resolution of 0.5–1 μm). It produces grayscale horizontal images that can be taken at levels ranging from the stratum corneum to the superficial papillary dermis, corresponding to a depth of approximately 100 to 150 µm. Thus, a hair follicle can be imaged starting from the follicular ostia down to the reachable papillary dermis (Figure 3).20 Image contrast is provided by differences in the size and refractive indices of cellular organelles.21,22 There are 2 commercially available RCM devices: VivaScope 1500 and VivaScope 3000 (Caliber Imaging & Diagnostics, Inc).
VivaScope 1500, a wide-probe microscope, requires the attachment of a plastic window to the desired imaging area. The plastic window is lined with medical adhesive tape to prevent movement during imaging. The adhesive tape can pull on hair upon removal, which is not ideal for patients with existing hair loss. Additionally, the image quality of VivaSope 1500 is best in flat areas and areas where hair is shaved.20,23,24 Despite these disadvantages, VivaScope 1500 has successfully shown utility in research studies, which suggests that these obstacles can be overcome by experienced users. The handheld VivaScope 3000 is ergonomically designed and suitable for curved surfaces such as the scalp, with the advantage of not requiring any adhesive. However, the images acquired from the VivaScope 3000 cover a smaller surface area.
Structures Visualized—Structures distinguished with RCM include keratinocytes, melanocytes, inflammatory cells, hair follicles, hair shafts, adnexal infundibular epithelium, blood vessels, fibroblasts, and collagen.23 Real-time visualization of blood flow also can be seen.
Applications of RCM—Reflectance confocal microscopy has been used to study scalp discoid lupus, lichen planopilaris, frontal fibrosing alopecia, folliculitis decalvans, chemotherapy-induced alopecia (CIA), alopecia areata, and androgenetic alopecia. Diagnostic RCM criteria for such alopecias have been developed based on their correspondence to histopathology. An RCM study of classic lichen planopilaris and frontal fibrosing alopecia identified features of epidermal disarray, infundibular hyperkeratosis, inflammatory cells, pigment incontinence, perifollicular fibrosis, bandlike scarring, melanophages in the dermis, dilated blood vessels, basal layer vacuolar degeneration, and necrotic keratinocytes.25 Pigment incontinence in the superficial epidermis, perifollicular lichenoid inflammation, and hyperkeratosis were characteristic RCM features of early-stage lichen planopilaris, while perifollicular fibrosis and dilated blood vessels were characteristic RCM features of late-stage disease. The ability of RCM features to distinguish different stages of lichen planopilaris shows its potential in treating early disease and preventing irreversible hair loss.
Differentiating between scarring and nonscarring alopecia also is possible through RCM. The presence of periadnexal, epidermal, and dermal inflammatory cells, in addition to periadnexal sclerosis, are defining RCM features of scarring alopecia.26 These features are absent in nonscarring alopecias. Reflectance confocal microscopy additionally has been shown to be useful in the treatment monitoring of lichen planopilaris and discoid lupus erythematosus.20 Independent reviewers, blinded to the patients’ identities, were able to characterize and follow features of these scarring alopecias by RCM. The assessed RCM features were comparable to those observed by histopathologic evaluation: epidermal disarray, spongiosis, exocytosis of inflammatory cells in the epidermis, interface dermatitis, peri- and intra-adnexal infiltration of inflammatory cells, dilated vessels in the dermis, dermal infiltration of inflammatory cells and melanophages, and dermal sclerosis. A reduction in inflammatory cells across multiple skin layers and at the level of the adnexal epithelium correlated with clinical response to treatment. Reflectance confocal microscopy also was able to detect recurrence of inflammation in cases where treatment had been interrupted before clinical signs of disease recurrence were evident. The authors thus concluded that RCM’s sensitivity can guide timing of treatment and avoid delays in starting or restarting treatment.20
Reflectance confocal microscopy also has served as a learning tool for new subclinical understandings of alopecia. In a study of CIA, the disease was found to be a dynamic process that could be categorized into 4 distinct phases distinguishable by combined confocal and dermoscopic features. This study also identified a new feature observable on RCM images—a CIA dot—defined as a dilated follicular infundibulum containing mashed, malted, nonhomogeneous material and normal or fragmented hair. This dot is thought to represent the initial microscopic sign of direct toxicity of chemotherapy on the hair follicle. Chemotherapy-induced alopecia dots persist throughout chemotherapy and subsequently disappear after chemotherapy ends.27
Limitations and Advantages—Currently, subtypes of cicatricial alopecias cannot be characterized on RCM because inflammatory cell types are not distinguished from each other (eg, eosinophils vs neutrophils). Another limitation of RCM is the loss of resolution below the superficial papillary dermis (a depth of approximately 150 µm); thus, deeper structures, such as the hair bulb, cannot be visualized.
Unlike global photography and trichoscopy, which are low-cost methods, RCM is much more costly, ranging upwards of several thousand dollars, and it may require additional technical support fees, making it less accessible for clinical practice. However, RCM imaging continues to be recommended as an intermediate step between trichoscopy and histology for the diagnosis and management of hair disease.26 If a biopsy is required, RCM can aid in the selection of a biopsy site, as areas with active inflammation are more informative than atrophic and fibrosed areas.23 The role of RCM in trichoscopy can be expanded by designing a more cost-effective and ergonomically suited scope for hair and scalp assessment.
Optical Coherence Tomography
Optical coherence tomography is a noninvasive handheld device that emits low-power infrared light to visualize the skin and adnexal structures. Optical coherence tomography relies on the principle of interferometry to detect phase differences in optical backscattering at varying tissue depths.28,29 It allows visualization up to 2 mm, which is 2 to 5 times deeper than RCM.36 Unlike RCM, which has cellular resolution, OCT has an axial resolution of 3 to 15 μm, which allows only for the detection of structural boundaries.30 There are various OCT modalities that differ in lateral and axial resolutions and maximum depth. Commercial software is available that measures changes in vascular density by depth, epidermal thickness, skin surface texture, and optical attenuation—the latter being an indirect measurement of collagen density and skin hydration.
Structures Visualized—Hair follicles can be well distinguished on OCT images, and as such, OCT is recognized as a diagnostic tool in trichology (Figure 4).31 Follicular openings, interfollicular collagen, and outlines of the hair shafts are visible; however, detailed components of the follicular unit cannot be visualized by OCT. Keratin hyperrefractivity identifies the hair shaft. Additionally, the hair matrix is denoted by a slightly granular texture in the dermis. Dynamic OCT produces colorized images that visualize blood flow within vessels.
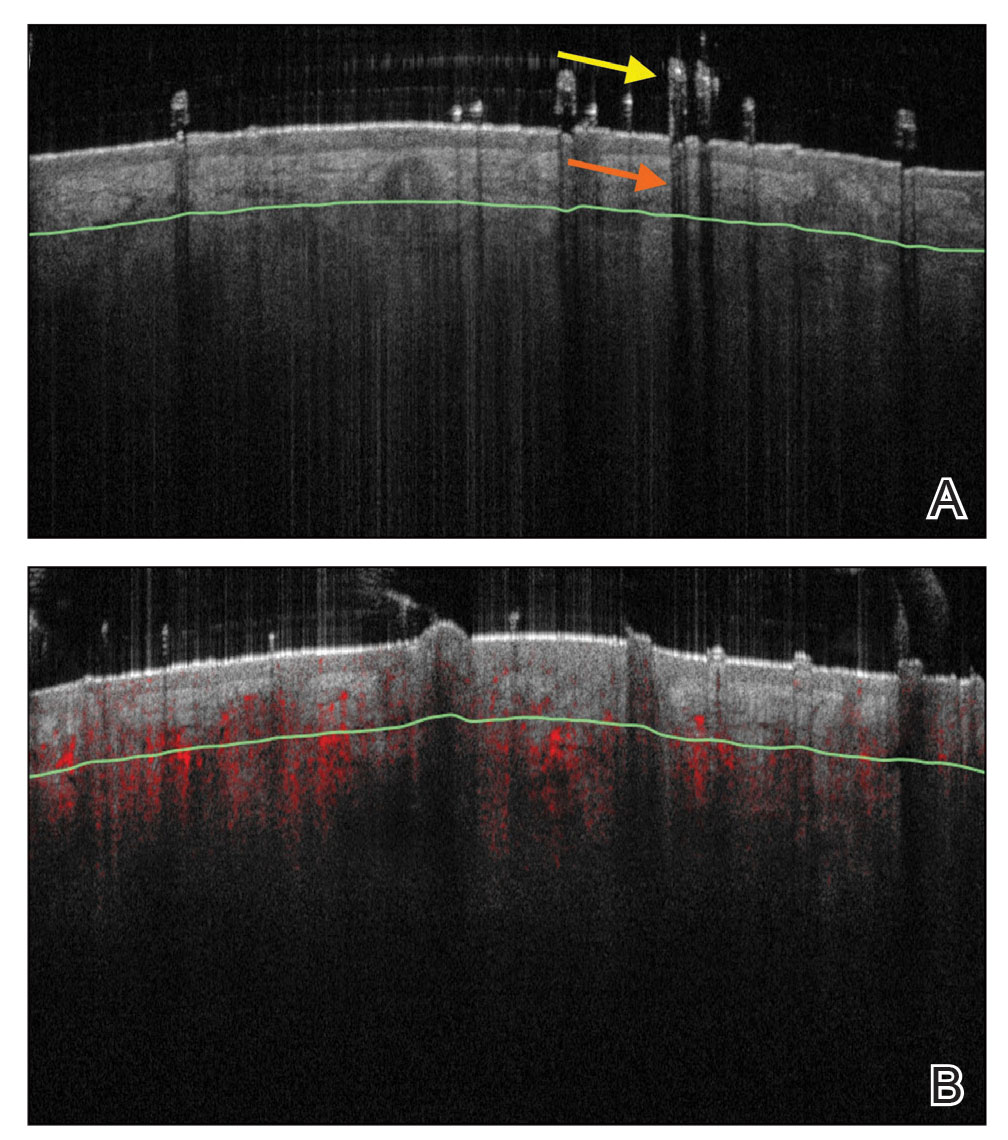
Applications of OCT—Optical coherence tomography is utilized in investigative trichology because it provides highly reproducible measurements of hair shaft diameters, cross-sectional surface areas, and form factor, which is a surrogate parameter for hair shape. The cross-section of hair shafts provides insight into local metabolism and perifollicular inflammation. Cross-sections of hair shafts in areas of alopecia areata were found to be smaller than cross-sections in the unaffected scalp within the same individual.32 Follicular density can be manually quantified on OCT images, but there also is promise for automated quantification. A recent study by Urban et al33 described training a convolutional neural network to automatically count hair as well as hair-bearing and non–hair-bearing follicles in OCT scans. These investigators also were able to color-code hair according to height, resulting in the creation of a “height” map.
Optical coherence tomography has furthered our understanding of the pathophysiology of cicatricial and nonscarring alopecias. Vazquez-Herrera et al34 assessed the inflammatory and cicatricial stages of frontal fibrosing alopecia by OCT imaging. Inflammatory hairlines, which are seen in the early stages of frontal fibrosing alopecia, exhibited a thickened dermis, irregular distribution of collagen, and increased vascularity in both the superficial and deep dermal layers compared to cicatricial and healthy scalp. Conversely, late-stage cicatricial areas exhibited a thin dermis and collagen that appeared in a hyperreflective, concentric, onion-shaped pattern around remnant follicular openings. Vascular flow was reduced in the superficial dermis of a cicatricial scalp but increased in the deep dermal layers compared with a healthy scalp. The attenuation coefficients of these disease stages also were assessed. The attenuation coefficient of the inflammatory hairline was higher compared with normal skin, likely as a reflection of inflammatory infiltrate and edema, whereas the attenuation coefficient of cicatricial scalp was lower compared with normal skin, likely reflecting the reduced water content of atrophic skin.34 This differentiation of early- and late-stage cicatricial alopecias has implications for early treatment and improved prognosis. Additionally, there is potential for OCT to assist in the differentiation of alopecia subtypes, as it can measure the epidermal thickness and follicular density and was previously used to compare scarring and nonscarring alopecia.35
Advantages and Limitations—Similar to RCM, OCT may be cost prohibitive for some clinicians. In addition, OCT cannot visualize the follicular unit in cellular detail. However, the extent of OCT’s capabilities may not be fully realized. Dynamic OCT is a new angiographic type of OCT that shows potential in monitoring early subclinical responses to novel alopecia therapies, such as platelet-rich plasminogen, which is hypothesized to stimulate hair growth through angiogenesis. Additionally, OCT may improve outcomes of hair transplantation procedures by allowing for visualization of the subcutaneous angle of hair follicles. Blind extraction of hair follicles in follicular unit extraction procedures can result in inadvertent transection and damage to the hair follicle; OCT could help identify good candidates for follicular unit extraction, such as patients with hair follicles in parallel arrangement, who are predicted to have better results.36
Conclusion
The field of trichology will continue to evolve with the emergence of noninvasive imaging technologies that diagnose hair disease in early stages and enable treatment monitoring with quantification of hair parameters. As discussed in this review, global photography, trichoscopy, RCM, and OCT have furthered our understanding of alopecia pathophysiology and provided objective methods of treatment evaluation. The capabilities of these tools will continue to expand with advancements in add-on software and AI algorithms.
- Canfield D. Photographic documentation of hair growth in androgenetic alopecia. Dermatol Clin. 1996;14:713-721.
- Peytavi U, Hillmann K, Guarrera M. Hair growth assessment techniques. In: Peytavi U, Hillmann K, Guarrera M, eds. Hair Growth and Disorders. 4th ed. Springer; 2008:140-144.
- Chamberlain AJ, Dawber RP. Methods of evaluating hair growth. Australas J Dermatol. 2003;44:10-18.
- Dhurat R, Saraogi P. Hair evaluation methods: merits and demerits. Int J Trichology. 2009;1:108-119.
- Kaufman KD, Olsen EA, Whiting D, et al. Finasteride in the treatment of men with androgenetic alopecia. J Am Acad Dermatol. 1998;39:578-579.
- Capily Institute. Artificial intelligence (A.I.) powered hair growth tracking. Accessed July 31, 2023. https://tss-aesthetics.com/capily-hair-tracking-syst
- Dinh Q, Sinclair R. Female pattern hair loss: current treatment concepts. Clin Interv Aging. 2007;2:189-199.
- Dhurat R, Saraogi P. Hair evaluation methods: merits and demerits. Int J Trichology. 2009;1:108-119.
- Wikramanayake TC, Mauro LM, Tabas IA, et al. Cross-section trichometry: a clinical tool for assessing the progression and treatment response of alopecia. Int J Trichology. 2012;4:259-264.
- Alessandrini A, Bruni F, Piraccini BM, et al. Common causes of hair loss—clinical manifestations, trichoscopy and therapy. J Eur Acad Dermatol Venereol. 2021;35:629-640.
- Ashique K, Kaliyadan F. Clinical photography for trichology practice: tips and tricks. Int J Trichology. 2011;3:7-13.
- Rudnicka L, Olszewska M, Rakowska A, et al. Trichoscopy: a new method for diagnosing hair loss. J Drugs Dermatol. 2008;7:651-654.
- Kinoshita-Ise M, Sachdeva M. Update on trichoscopy: integration of the terminology by systematic approach and a proposal of a diagnostic flowchart. J Dermatol. 2022;49:4-18. doi:10.1111/1346-8138.16233
- Van Neste D, Trüeb RM. Critical study of hair growth analysis with computer-assisted methods. J Eur Acad Dermatol Venereol. 2006;20:578-583.
- Romero J, Grimalt R. Trichoscopy: essentials for the dermatologist. World J Dermatol. 2015;4:63-68.
- Trichoscopy: a new frontier for the diagnosis of hair diseases. Exp Rev Dermatol. 2012;7:429-437.
- Lee B, Chan J, Monselise A, et al. Assessment of hair density and caliber in Caucasian and Asian female subjects with female pattern hair loss by using the Folliscope. J Am Acad Dermatol. 2012;66:166-167.
- Inui S. Trichoscopy for common hair loss diseases: algorithmic method for diagnosis. J Dermatol. 2010;38:71-75.
- Dhurat R. Phototrichogram. Indian J Dermatol Venereol Leprol. 2006;72:242-244.
- Agozzino M, Tosti A, Barbieri L, et al. Confocal microscopic features of scarring alopecia: preliminary report. Br J Dermatol. 2011;165:534-540.
- Kuck M, Schanzer S, Ulrich M, et al. Analysis of the efficiency of hair removal by different optical methods: comparison of Trichoscan, reflectance confocal microscopy, and optical coherence tomography. J Biomed Opt. 2012;17:101504.
- Levine A, Markowitz O. Introduction to reflectance confocal microscopy and its use in clinical practice. JAAD Case Rep. 2018;4:1014-1023.
- Agozzino M, Ardigò M. Scalp confocal microscopy. In: Humbert P, Maibach H, Fanian F, et al, eds. Agache’s Measuring the Skin: Non-invasive Investigations, Physiology, Normal Constants. 2nd ed. Springer International Publishing; 2016:311-326.
- Rudnicka L, Olszewska M, Rakowska A. In vivo reflectance confocal microscopy: usefulness for diagnosing hair diseases. J Dermatol Case Rep. 2008;2:55-59.
- Kurzeja M, Czuwara J, Walecka I, et al. Features of classic lichen planopilaris and frontal fibrosing alopecia in reflectance confocal microscopy: a preliminary study. Skin Res Technol. 2021;27:266-271.
- Ardigò M, Agozzino M, Franceschini C, et al. Reflectance confocal microscopy for scarring and non-scarring alopecia real-time assessment. Arch Dermatol Res. 2016;308:309-318.
- Franceschini C, Garelli V, Persechino F, et al. Dermoscopy and confocal microscopy for different chemotherapy-induced alopecia (CIA) phases characterization: preliminary study. Skin Res Technol. 2020;26:269-276.
- Martinez-Velasco MA, Perper M, Maddy AJ, et al. In vitro determination of Mexican Mestizo hair shaft diameter using optical coherence tomography. Skin Res Technol. 2018;24;274-277.
- Srivastava R, Manfredini M, Rao BK. Noninvasive imaging tools in dermatology. Cutis. 2019;104:108-113.
- Wan B, Ganier C, Du-Harpur X, et al. Applications and future directions for optical coherence tomography in dermatology. Br J Dermatol. 2021;184:1014-1022.
- Blume-Peytavi U, Vieten J, Knuttel A et al. Optical coherent tomography (OCT): a new method for online-measurement of hair shaft thickness. J Dtsch Dermatol Ges. 2004;2:546.
- Garcia Bartels N, Jahnke I, Patzelt A, et al. Hair shaft abnormalities in alopecia areata evaluated by optical coherence tomography. Skin Res Technol. 2011;17:201-205.
- Urban G, Feil N, Csuka E, et al. Combining deep learning with optical coherence tomography imaging to determine scalp hair and follicle counts. Lasers Surg Med. 2021;53:171-178.
- Vazquez-Herrera NE, Eber AE, Martinez-Velasco MA, et al. Optical coherence tomography for the investigation of frontal fibrosing alopecia. J Eur Acad Dermatol Venereol. 2018;32:318-322.
- Ekelem C, Feil N, Csuka E, et al. Optical coherence tomography in the evaluation of the scalp and hair: common features and clinical utility. Lasers Surg Med. 2021;53:129-140.
- Schicho K, Seemann R, Binder M, et al. Optical coherence tomography for planning of follicular unit extraction. Dermatol Surg. 2015;41:358-363.
New imaging tools along with adaptations to existing technologies have been emerging in recent years, with the potential to improve hair diagnostics and treatment monitoring. We provide an overview of 4 noninvasive hair imaging technologies: global photography, trichoscopy, reflectance confocal microscopy (RCM), and optical coherence tomography (OCT). For each instrument, we discuss current and future applications in clinical practice and research along with advantages and disadvantages.
Global Photography
Global photography allows for the analysis of hair growth, volume, distribution, and density through serial standardized photographs.1 Global photography was first introduced for hair growth studies in 1987 and soon after was used for hair and scalp assessments in finasteride clinical trials.2
Hair Assessment—Washed, dried, and combed hair, without hair product, are required for accurate imaging; wet conditions increase reflection and promote hair clumping, thus revealing more scalp and depicting the patient as having less hair.1 Headshots are taken from short distances and use stereotactic positioning devices to create 4 global views: vertex, midline, frontal, and temporal.3 Stereotactic positioning involves fixing the patient’s chin and forehead as well as mounting the camera and flash device to ensure proper magnification. These adjustments ensure lighting remains consistent throughout consecutive study visits.4 Various grading scales are available for use in hair growth clinical studies to increase objectivity in the analysis of serial global photographs. A blinded evaluator should assess the before and after photographs to limit experimenter bias. Global photography often is combined with quantitative software analysis for improved detection of hair changes.1
Advancements—Growing interest in improving global photography has resulted in various application-based, artificial intelligence (AI)–mediated tools to simplify photograph collection and analysis. For instance, new hair analysis software utilizes AI algorithms to account for facial features in determining the optimal angle for capturing global photographs (Figure 1), which simplifies the generation of global photography images through smartphone applications and obviates the need for additional stereotactic positioning equipment.5,6

Limitations—Clinicians should be aware of global photography’s requirements for consistency in lighting, camera settings, film, and image processing, which can limit the accuracy of hair assessment over time if not replicated correctly.7,8 Emerging global photography software has helped to overcome some of these limitations.
Global photography is less precise when a patient’s hair loss is less than 50%, as it is difficult to discern subtle hair changes. Thus, global photography provides limited utility in assessing minimal to moderate hair loss.9 Currently, global photography largely functions as an adjunct tool for other hair analysis methods rather than as a stand-alone tool.
Trichoscopy
Trichoscopy (also known as dermoscopy of the hair and scalp) may be performed with a manual dermoscope (with 10× magnification) or a digital videodermatoscope (up to 1000× magnification).10-12 Unlike global photography, trichoscopy provides a detailed structural analysis of hair shafts, follicular openings, and perifollicular and interfollicular areas.13 Kinoshita-Ise and Sachdeva13 provided an in-depth, updated review of trichoscopy terminology with their definitions and associated conditions (with prevalence), which should be referenced when performing trichoscopic examination.
Hair Assessment—Trichoscopic assessment begins with inspection of follicular openings (also referred to as “dots”), which vary in color depending on the material filling them—degrading keratinocytes, keratin, sebaceous debris, melanin, or fractured hairs.13 The structure of hair shafts also is examined, showing broken hairs, short vellus hairs, and comma hairs, among others. Perifollicular areas are examined for scale, erythema, blue-gray dots, and whitish halos. Interfollicular areas are examined for pigment pattern as well as vascularization, which often presents in a looping configuration under dermoscopy. A combination of dot colorization, hair shaft structure, and perifollicular and interfollicular findings inform diagnostic algorithms of hair and scalp conditions. For example, central centrifugal cicatricial alopecia, the most common alopecia seen in Black women, has been associated with a combination of honeycomb pigment pattern, perifollicular whitish halo, pinpoint white dots, white patches, and perifollicular erythema.13
Advantages—Perhaps the most useful feature of trichoscopy is its ability to translate visualized features into simple diagnostic algorithms. For instance, if the clinician has diagnosed the patient with noncicatricial alopecia, they would next focus on dot colors. With black dots, the next step would be to determine whether the hairs are tapered or coiled, and so on. This systematic approach enables the clinician to narrow possible diagnoses.2 An additional advantage of trichoscopy is that it examines large surface areas noninvasively as compared to hair-pull tests and scalp biopsy.14,15 Trichoscopy allows temporal comparisons of the same area for disease and treatment monitoring with more diagnostic detail than global photography.16 Trichoscopy also is useful in selecting biopsy locations by discerning and avoiding areas of scar tissue.17
Limitations—Diagnosis via the trichoscopy algorithm is limiting because it is not comprehensive of all hair and scalp disease.18 Additionally, many pathologies exhibit overlapping follicular and interfollicular patterning. For example, almost all subtypes of scarring alopecia present with hair loss and scarred follicles once they have progressed to advanced stages. Further studies should identify more specific patterns of hair and scalp pathologies, which could then be incorporated into a diagnostic algorithm.13
Advancements—The advent of hair analysis software has expanded the role of videodermoscopy by rapidly quantifying hair growth parameters such as hair count, follicular density, and follicular diameter, as well as interfollicular distances (Figure 2).14,17 Vellus and terminal hairs are differentiated according to their thickness and length.17 Moreover, the software can analyze the same area of the scalp over time by either virtual tattoos, semipermanent markings, or precise location measurements, increasing intra- and interclass correlation. The rate of hair growth, hair shedding, and parameters of anagen and telogen hairs can be studied by a method termed phototrichogram whereby a transitional area of hair loss and normal hair growth is identified and trimmed to less than 1 mm from the skin surface.19 A baseline photograph is taken using videodermoscopy. After approximately 3 days, the identical region is photographed and compared with the initial image to observe changes in the hair. Software programs can distinguish the growing hair as anagen and nongrowing hair as telogen, calculating the anagen-to-telogen ratio as well as hair growth rate, which are essential measurements in hair research and clinical studies. Software programs have replaced laborious and time-consuming manual hair counts and have rapidly grown in popularity in evaluating patterned hair loss.

Reflectance Confocal Microscopy
Reflectance confocal microscopy is a noninvasive imaging tool that visualizes skin and its appendages at near-histologic resolution (lateral resolution of 0.5–1 μm). It produces grayscale horizontal images that can be taken at levels ranging from the stratum corneum to the superficial papillary dermis, corresponding to a depth of approximately 100 to 150 µm. Thus, a hair follicle can be imaged starting from the follicular ostia down to the reachable papillary dermis (Figure 3).20 Image contrast is provided by differences in the size and refractive indices of cellular organelles.21,22 There are 2 commercially available RCM devices: VivaScope 1500 and VivaScope 3000 (Caliber Imaging & Diagnostics, Inc).

VivaScope 1500, a wide-probe microscope, requires the attachment of a plastic window to the desired imaging area. The plastic window is lined with medical adhesive tape to prevent movement during imaging. The adhesive tape can pull on hair upon removal, which is not ideal for patients with existing hair loss. Additionally, the image quality of VivaSope 1500 is best in flat areas and areas where hair is shaved.20,23,24 Despite these disadvantages, VivaScope 1500 has successfully shown utility in research studies, which suggests that these obstacles can be overcome by experienced users. The handheld VivaScope 3000 is ergonomically designed and suitable for curved surfaces such as the scalp, with the advantage of not requiring any adhesive. However, the images acquired from the VivaScope 3000 cover a smaller surface area.
Structures Visualized—Structures distinguished with RCM include keratinocytes, melanocytes, inflammatory cells, hair follicles, hair shafts, adnexal infundibular epithelium, blood vessels, fibroblasts, and collagen.23 Real-time visualization of blood flow also can be seen.
Applications of RCM—Reflectance confocal microscopy has been used to study scalp discoid lupus, lichen planopilaris, frontal fibrosing alopecia, folliculitis decalvans, chemotherapy-induced alopecia (CIA), alopecia areata, and androgenetic alopecia. Diagnostic RCM criteria for such alopecias have been developed based on their correspondence to histopathology. An RCM study of classic lichen planopilaris and frontal fibrosing alopecia identified features of epidermal disarray, infundibular hyperkeratosis, inflammatory cells, pigment incontinence, perifollicular fibrosis, bandlike scarring, melanophages in the dermis, dilated blood vessels, basal layer vacuolar degeneration, and necrotic keratinocytes.25 Pigment incontinence in the superficial epidermis, perifollicular lichenoid inflammation, and hyperkeratosis were characteristic RCM features of early-stage lichen planopilaris, while perifollicular fibrosis and dilated blood vessels were characteristic RCM features of late-stage disease. The ability of RCM features to distinguish different stages of lichen planopilaris shows its potential in treating early disease and preventing irreversible hair loss.
Differentiating between scarring and nonscarring alopecia also is possible through RCM. The presence of periadnexal, epidermal, and dermal inflammatory cells, in addition to periadnexal sclerosis, are defining RCM features of scarring alopecia.26 These features are absent in nonscarring alopecias. Reflectance confocal microscopy additionally has been shown to be useful in the treatment monitoring of lichen planopilaris and discoid lupus erythematosus.20 Independent reviewers, blinded to the patients’ identities, were able to characterize and follow features of these scarring alopecias by RCM. The assessed RCM features were comparable to those observed by histopathologic evaluation: epidermal disarray, spongiosis, exocytosis of inflammatory cells in the epidermis, interface dermatitis, peri- and intra-adnexal infiltration of inflammatory cells, dilated vessels in the dermis, dermal infiltration of inflammatory cells and melanophages, and dermal sclerosis. A reduction in inflammatory cells across multiple skin layers and at the level of the adnexal epithelium correlated with clinical response to treatment. Reflectance confocal microscopy also was able to detect recurrence of inflammation in cases where treatment had been interrupted before clinical signs of disease recurrence were evident. The authors thus concluded that RCM’s sensitivity can guide timing of treatment and avoid delays in starting or restarting treatment.20
Reflectance confocal microscopy also has served as a learning tool for new subclinical understandings of alopecia. In a study of CIA, the disease was found to be a dynamic process that could be categorized into 4 distinct phases distinguishable by combined confocal and dermoscopic features. This study also identified a new feature observable on RCM images—a CIA dot—defined as a dilated follicular infundibulum containing mashed, malted, nonhomogeneous material and normal or fragmented hair. This dot is thought to represent the initial microscopic sign of direct toxicity of chemotherapy on the hair follicle. Chemotherapy-induced alopecia dots persist throughout chemotherapy and subsequently disappear after chemotherapy ends.27
Limitations and Advantages—Currently, subtypes of cicatricial alopecias cannot be characterized on RCM because inflammatory cell types are not distinguished from each other (eg, eosinophils vs neutrophils). Another limitation of RCM is the loss of resolution below the superficial papillary dermis (a depth of approximately 150 µm); thus, deeper structures, such as the hair bulb, cannot be visualized.
Unlike global photography and trichoscopy, which are low-cost methods, RCM is much more costly, ranging upwards of several thousand dollars, and it may require additional technical support fees, making it less accessible for clinical practice. However, RCM imaging continues to be recommended as an intermediate step between trichoscopy and histology for the diagnosis and management of hair disease.26 If a biopsy is required, RCM can aid in the selection of a biopsy site, as areas with active inflammation are more informative than atrophic and fibrosed areas.23 The role of RCM in trichoscopy can be expanded by designing a more cost-effective and ergonomically suited scope for hair and scalp assessment.
Optical Coherence Tomography
Optical coherence tomography is a noninvasive handheld device that emits low-power infrared light to visualize the skin and adnexal structures. Optical coherence tomography relies on the principle of interferometry to detect phase differences in optical backscattering at varying tissue depths.28,29 It allows visualization up to 2 mm, which is 2 to 5 times deeper than RCM.36 Unlike RCM, which has cellular resolution, OCT has an axial resolution of 3 to 15 μm, which allows only for the detection of structural boundaries.30 There are various OCT modalities that differ in lateral and axial resolutions and maximum depth. Commercial software is available that measures changes in vascular density by depth, epidermal thickness, skin surface texture, and optical attenuation—the latter being an indirect measurement of collagen density and skin hydration.
Structures Visualized—Hair follicles can be well distinguished on OCT images, and as such, OCT is recognized as a diagnostic tool in trichology (Figure 4).31 Follicular openings, interfollicular collagen, and outlines of the hair shafts are visible; however, detailed components of the follicular unit cannot be visualized by OCT. Keratin hyperrefractivity identifies the hair shaft. Additionally, the hair matrix is denoted by a slightly granular texture in the dermis. Dynamic OCT produces colorized images that visualize blood flow within vessels.

Applications of OCT—Optical coherence tomography is utilized in investigative trichology because it provides highly reproducible measurements of hair shaft diameters, cross-sectional surface areas, and form factor, which is a surrogate parameter for hair shape. The cross-section of hair shafts provides insight into local metabolism and perifollicular inflammation. Cross-sections of hair shafts in areas of alopecia areata were found to be smaller than cross-sections in the unaffected scalp within the same individual.32 Follicular density can be manually quantified on OCT images, but there also is promise for automated quantification. A recent study by Urban et al33 described training a convolutional neural network to automatically count hair as well as hair-bearing and non–hair-bearing follicles in OCT scans. These investigators also were able to color-code hair according to height, resulting in the creation of a “height” map.
Optical coherence tomography has furthered our understanding of the pathophysiology of cicatricial and nonscarring alopecias. Vazquez-Herrera et al34 assessed the inflammatory and cicatricial stages of frontal fibrosing alopecia by OCT imaging. Inflammatory hairlines, which are seen in the early stages of frontal fibrosing alopecia, exhibited a thickened dermis, irregular distribution of collagen, and increased vascularity in both the superficial and deep dermal layers compared to cicatricial and healthy scalp. Conversely, late-stage cicatricial areas exhibited a thin dermis and collagen that appeared in a hyperreflective, concentric, onion-shaped pattern around remnant follicular openings. Vascular flow was reduced in the superficial dermis of a cicatricial scalp but increased in the deep dermal layers compared with a healthy scalp. The attenuation coefficients of these disease stages also were assessed. The attenuation coefficient of the inflammatory hairline was higher compared with normal skin, likely as a reflection of inflammatory infiltrate and edema, whereas the attenuation coefficient of cicatricial scalp was lower compared with normal skin, likely reflecting the reduced water content of atrophic skin.34 This differentiation of early- and late-stage cicatricial alopecias has implications for early treatment and improved prognosis. Additionally, there is potential for OCT to assist in the differentiation of alopecia subtypes, as it can measure the epidermal thickness and follicular density and was previously used to compare scarring and nonscarring alopecia.35
Advantages and Limitations—Similar to RCM, OCT may be cost prohibitive for some clinicians. In addition, OCT cannot visualize the follicular unit in cellular detail. However, the extent of OCT’s capabilities may not be fully realized. Dynamic OCT is a new angiographic type of OCT that shows potential in monitoring early subclinical responses to novel alopecia therapies, such as platelet-rich plasminogen, which is hypothesized to stimulate hair growth through angiogenesis. Additionally, OCT may improve outcomes of hair transplantation procedures by allowing for visualization of the subcutaneous angle of hair follicles. Blind extraction of hair follicles in follicular unit extraction procedures can result in inadvertent transection and damage to the hair follicle; OCT could help identify good candidates for follicular unit extraction, such as patients with hair follicles in parallel arrangement, who are predicted to have better results.36
Conclusion
The field of trichology will continue to evolve with the emergence of noninvasive imaging technologies that diagnose hair disease in early stages and enable treatment monitoring with quantification of hair parameters. As discussed in this review, global photography, trichoscopy, RCM, and OCT have furthered our understanding of alopecia pathophysiology and provided objective methods of treatment evaluation. The capabilities of these tools will continue to expand with advancements in add-on software and AI algorithms.
New imaging tools along with adaptations to existing technologies have been emerging in recent years, with the potential to improve hair diagnostics and treatment monitoring. We provide an overview of 4 noninvasive hair imaging technologies: global photography, trichoscopy, reflectance confocal microscopy (RCM), and optical coherence tomography (OCT). For each instrument, we discuss current and future applications in clinical practice and research along with advantages and disadvantages.
Global Photography
Global photography allows for the analysis of hair growth, volume, distribution, and density through serial standardized photographs.1 Global photography was first introduced for hair growth studies in 1987 and soon after was used for hair and scalp assessments in finasteride clinical trials.2
Hair Assessment—Washed, dried, and combed hair, without hair product, are required for accurate imaging; wet conditions increase reflection and promote hair clumping, thus revealing more scalp and depicting the patient as having less hair.1 Headshots are taken from short distances and use stereotactic positioning devices to create 4 global views: vertex, midline, frontal, and temporal.3 Stereotactic positioning involves fixing the patient’s chin and forehead as well as mounting the camera and flash device to ensure proper magnification. These adjustments ensure lighting remains consistent throughout consecutive study visits.4 Various grading scales are available for use in hair growth clinical studies to increase objectivity in the analysis of serial global photographs. A blinded evaluator should assess the before and after photographs to limit experimenter bias. Global photography often is combined with quantitative software analysis for improved detection of hair changes.1
Advancements—Growing interest in improving global photography has resulted in various application-based, artificial intelligence (AI)–mediated tools to simplify photograph collection and analysis. For instance, new hair analysis software utilizes AI algorithms to account for facial features in determining the optimal angle for capturing global photographs (Figure 1), which simplifies the generation of global photography images through smartphone applications and obviates the need for additional stereotactic positioning equipment.5,6

Limitations—Clinicians should be aware of global photography’s requirements for consistency in lighting, camera settings, film, and image processing, which can limit the accuracy of hair assessment over time if not replicated correctly.7,8 Emerging global photography software has helped to overcome some of these limitations.
Global photography is less precise when a patient’s hair loss is less than 50%, as it is difficult to discern subtle hair changes. Thus, global photography provides limited utility in assessing minimal to moderate hair loss.9 Currently, global photography largely functions as an adjunct tool for other hair analysis methods rather than as a stand-alone tool.
Trichoscopy
Trichoscopy (also known as dermoscopy of the hair and scalp) may be performed with a manual dermoscope (with 10× magnification) or a digital videodermatoscope (up to 1000× magnification).10-12 Unlike global photography, trichoscopy provides a detailed structural analysis of hair shafts, follicular openings, and perifollicular and interfollicular areas.13 Kinoshita-Ise and Sachdeva13 provided an in-depth, updated review of trichoscopy terminology with their definitions and associated conditions (with prevalence), which should be referenced when performing trichoscopic examination.
Hair Assessment—Trichoscopic assessment begins with inspection of follicular openings (also referred to as “dots”), which vary in color depending on the material filling them—degrading keratinocytes, keratin, sebaceous debris, melanin, or fractured hairs.13 The structure of hair shafts also is examined, showing broken hairs, short vellus hairs, and comma hairs, among others. Perifollicular areas are examined for scale, erythema, blue-gray dots, and whitish halos. Interfollicular areas are examined for pigment pattern as well as vascularization, which often presents in a looping configuration under dermoscopy. A combination of dot colorization, hair shaft structure, and perifollicular and interfollicular findings inform diagnostic algorithms of hair and scalp conditions. For example, central centrifugal cicatricial alopecia, the most common alopecia seen in Black women, has been associated with a combination of honeycomb pigment pattern, perifollicular whitish halo, pinpoint white dots, white patches, and perifollicular erythema.13
Advantages—Perhaps the most useful feature of trichoscopy is its ability to translate visualized features into simple diagnostic algorithms. For instance, if the clinician has diagnosed the patient with noncicatricial alopecia, they would next focus on dot colors. With black dots, the next step would be to determine whether the hairs are tapered or coiled, and so on. This systematic approach enables the clinician to narrow possible diagnoses.2 An additional advantage of trichoscopy is that it examines large surface areas noninvasively as compared to hair-pull tests and scalp biopsy.14,15 Trichoscopy allows temporal comparisons of the same area for disease and treatment monitoring with more diagnostic detail than global photography.16 Trichoscopy also is useful in selecting biopsy locations by discerning and avoiding areas of scar tissue.17
Limitations—Diagnosis via the trichoscopy algorithm is limiting because it is not comprehensive of all hair and scalp disease.18 Additionally, many pathologies exhibit overlapping follicular and interfollicular patterning. For example, almost all subtypes of scarring alopecia present with hair loss and scarred follicles once they have progressed to advanced stages. Further studies should identify more specific patterns of hair and scalp pathologies, which could then be incorporated into a diagnostic algorithm.13
Advancements—The advent of hair analysis software has expanded the role of videodermoscopy by rapidly quantifying hair growth parameters such as hair count, follicular density, and follicular diameter, as well as interfollicular distances (Figure 2).14,17 Vellus and terminal hairs are differentiated according to their thickness and length.17 Moreover, the software can analyze the same area of the scalp over time by either virtual tattoos, semipermanent markings, or precise location measurements, increasing intra- and interclass correlation. The rate of hair growth, hair shedding, and parameters of anagen and telogen hairs can be studied by a method termed phototrichogram whereby a transitional area of hair loss and normal hair growth is identified and trimmed to less than 1 mm from the skin surface.19 A baseline photograph is taken using videodermoscopy. After approximately 3 days, the identical region is photographed and compared with the initial image to observe changes in the hair. Software programs can distinguish the growing hair as anagen and nongrowing hair as telogen, calculating the anagen-to-telogen ratio as well as hair growth rate, which are essential measurements in hair research and clinical studies. Software programs have replaced laborious and time-consuming manual hair counts and have rapidly grown in popularity in evaluating patterned hair loss.

Reflectance Confocal Microscopy
Reflectance confocal microscopy is a noninvasive imaging tool that visualizes skin and its appendages at near-histologic resolution (lateral resolution of 0.5–1 μm). It produces grayscale horizontal images that can be taken at levels ranging from the stratum corneum to the superficial papillary dermis, corresponding to a depth of approximately 100 to 150 µm. Thus, a hair follicle can be imaged starting from the follicular ostia down to the reachable papillary dermis (Figure 3).20 Image contrast is provided by differences in the size and refractive indices of cellular organelles.21,22 There are 2 commercially available RCM devices: VivaScope 1500 and VivaScope 3000 (Caliber Imaging & Diagnostics, Inc).

VivaScope 1500, a wide-probe microscope, requires the attachment of a plastic window to the desired imaging area. The plastic window is lined with medical adhesive tape to prevent movement during imaging. The adhesive tape can pull on hair upon removal, which is not ideal for patients with existing hair loss. Additionally, the image quality of VivaSope 1500 is best in flat areas and areas where hair is shaved.20,23,24 Despite these disadvantages, VivaScope 1500 has successfully shown utility in research studies, which suggests that these obstacles can be overcome by experienced users. The handheld VivaScope 3000 is ergonomically designed and suitable for curved surfaces such as the scalp, with the advantage of not requiring any adhesive. However, the images acquired from the VivaScope 3000 cover a smaller surface area.
Structures Visualized—Structures distinguished with RCM include keratinocytes, melanocytes, inflammatory cells, hair follicles, hair shafts, adnexal infundibular epithelium, blood vessels, fibroblasts, and collagen.23 Real-time visualization of blood flow also can be seen.
Applications of RCM—Reflectance confocal microscopy has been used to study scalp discoid lupus, lichen planopilaris, frontal fibrosing alopecia, folliculitis decalvans, chemotherapy-induced alopecia (CIA), alopecia areata, and androgenetic alopecia. Diagnostic RCM criteria for such alopecias have been developed based on their correspondence to histopathology. An RCM study of classic lichen planopilaris and frontal fibrosing alopecia identified features of epidermal disarray, infundibular hyperkeratosis, inflammatory cells, pigment incontinence, perifollicular fibrosis, bandlike scarring, melanophages in the dermis, dilated blood vessels, basal layer vacuolar degeneration, and necrotic keratinocytes.25 Pigment incontinence in the superficial epidermis, perifollicular lichenoid inflammation, and hyperkeratosis were characteristic RCM features of early-stage lichen planopilaris, while perifollicular fibrosis and dilated blood vessels were characteristic RCM features of late-stage disease. The ability of RCM features to distinguish different stages of lichen planopilaris shows its potential in treating early disease and preventing irreversible hair loss.
Differentiating between scarring and nonscarring alopecia also is possible through RCM. The presence of periadnexal, epidermal, and dermal inflammatory cells, in addition to periadnexal sclerosis, are defining RCM features of scarring alopecia.26 These features are absent in nonscarring alopecias. Reflectance confocal microscopy additionally has been shown to be useful in the treatment monitoring of lichen planopilaris and discoid lupus erythematosus.20 Independent reviewers, blinded to the patients’ identities, were able to characterize and follow features of these scarring alopecias by RCM. The assessed RCM features were comparable to those observed by histopathologic evaluation: epidermal disarray, spongiosis, exocytosis of inflammatory cells in the epidermis, interface dermatitis, peri- and intra-adnexal infiltration of inflammatory cells, dilated vessels in the dermis, dermal infiltration of inflammatory cells and melanophages, and dermal sclerosis. A reduction in inflammatory cells across multiple skin layers and at the level of the adnexal epithelium correlated with clinical response to treatment. Reflectance confocal microscopy also was able to detect recurrence of inflammation in cases where treatment had been interrupted before clinical signs of disease recurrence were evident. The authors thus concluded that RCM’s sensitivity can guide timing of treatment and avoid delays in starting or restarting treatment.20
Reflectance confocal microscopy also has served as a learning tool for new subclinical understandings of alopecia. In a study of CIA, the disease was found to be a dynamic process that could be categorized into 4 distinct phases distinguishable by combined confocal and dermoscopic features. This study also identified a new feature observable on RCM images—a CIA dot—defined as a dilated follicular infundibulum containing mashed, malted, nonhomogeneous material and normal or fragmented hair. This dot is thought to represent the initial microscopic sign of direct toxicity of chemotherapy on the hair follicle. Chemotherapy-induced alopecia dots persist throughout chemotherapy and subsequently disappear after chemotherapy ends.27
Limitations and Advantages—Currently, subtypes of cicatricial alopecias cannot be characterized on RCM because inflammatory cell types are not distinguished from each other (eg, eosinophils vs neutrophils). Another limitation of RCM is the loss of resolution below the superficial papillary dermis (a depth of approximately 150 µm); thus, deeper structures, such as the hair bulb, cannot be visualized.
Unlike global photography and trichoscopy, which are low-cost methods, RCM is much more costly, ranging upwards of several thousand dollars, and it may require additional technical support fees, making it less accessible for clinical practice. However, RCM imaging continues to be recommended as an intermediate step between trichoscopy and histology for the diagnosis and management of hair disease.26 If a biopsy is required, RCM can aid in the selection of a biopsy site, as areas with active inflammation are more informative than atrophic and fibrosed areas.23 The role of RCM in trichoscopy can be expanded by designing a more cost-effective and ergonomically suited scope for hair and scalp assessment.
Optical Coherence Tomography
Optical coherence tomography is a noninvasive handheld device that emits low-power infrared light to visualize the skin and adnexal structures. Optical coherence tomography relies on the principle of interferometry to detect phase differences in optical backscattering at varying tissue depths.28,29 It allows visualization up to 2 mm, which is 2 to 5 times deeper than RCM.36 Unlike RCM, which has cellular resolution, OCT has an axial resolution of 3 to 15 μm, which allows only for the detection of structural boundaries.30 There are various OCT modalities that differ in lateral and axial resolutions and maximum depth. Commercial software is available that measures changes in vascular density by depth, epidermal thickness, skin surface texture, and optical attenuation—the latter being an indirect measurement of collagen density and skin hydration.
Structures Visualized—Hair follicles can be well distinguished on OCT images, and as such, OCT is recognized as a diagnostic tool in trichology (Figure 4).31 Follicular openings, interfollicular collagen, and outlines of the hair shafts are visible; however, detailed components of the follicular unit cannot be visualized by OCT. Keratin hyperrefractivity identifies the hair shaft. Additionally, the hair matrix is denoted by a slightly granular texture in the dermis. Dynamic OCT produces colorized images that visualize blood flow within vessels.

Applications of OCT—Optical coherence tomography is utilized in investigative trichology because it provides highly reproducible measurements of hair shaft diameters, cross-sectional surface areas, and form factor, which is a surrogate parameter for hair shape. The cross-section of hair shafts provides insight into local metabolism and perifollicular inflammation. Cross-sections of hair shafts in areas of alopecia areata were found to be smaller than cross-sections in the unaffected scalp within the same individual.32 Follicular density can be manually quantified on OCT images, but there also is promise for automated quantification. A recent study by Urban et al33 described training a convolutional neural network to automatically count hair as well as hair-bearing and non–hair-bearing follicles in OCT scans. These investigators also were able to color-code hair according to height, resulting in the creation of a “height” map.
Optical coherence tomography has furthered our understanding of the pathophysiology of cicatricial and nonscarring alopecias. Vazquez-Herrera et al34 assessed the inflammatory and cicatricial stages of frontal fibrosing alopecia by OCT imaging. Inflammatory hairlines, which are seen in the early stages of frontal fibrosing alopecia, exhibited a thickened dermis, irregular distribution of collagen, and increased vascularity in both the superficial and deep dermal layers compared to cicatricial and healthy scalp. Conversely, late-stage cicatricial areas exhibited a thin dermis and collagen that appeared in a hyperreflective, concentric, onion-shaped pattern around remnant follicular openings. Vascular flow was reduced in the superficial dermis of a cicatricial scalp but increased in the deep dermal layers compared with a healthy scalp. The attenuation coefficients of these disease stages also were assessed. The attenuation coefficient of the inflammatory hairline was higher compared with normal skin, likely as a reflection of inflammatory infiltrate and edema, whereas the attenuation coefficient of cicatricial scalp was lower compared with normal skin, likely reflecting the reduced water content of atrophic skin.34 This differentiation of early- and late-stage cicatricial alopecias has implications for early treatment and improved prognosis. Additionally, there is potential for OCT to assist in the differentiation of alopecia subtypes, as it can measure the epidermal thickness and follicular density and was previously used to compare scarring and nonscarring alopecia.35
Advantages and Limitations—Similar to RCM, OCT may be cost prohibitive for some clinicians. In addition, OCT cannot visualize the follicular unit in cellular detail. However, the extent of OCT’s capabilities may not be fully realized. Dynamic OCT is a new angiographic type of OCT that shows potential in monitoring early subclinical responses to novel alopecia therapies, such as platelet-rich plasminogen, which is hypothesized to stimulate hair growth through angiogenesis. Additionally, OCT may improve outcomes of hair transplantation procedures by allowing for visualization of the subcutaneous angle of hair follicles. Blind extraction of hair follicles in follicular unit extraction procedures can result in inadvertent transection and damage to the hair follicle; OCT could help identify good candidates for follicular unit extraction, such as patients with hair follicles in parallel arrangement, who are predicted to have better results.36
Conclusion
The field of trichology will continue to evolve with the emergence of noninvasive imaging technologies that diagnose hair disease in early stages and enable treatment monitoring with quantification of hair parameters. As discussed in this review, global photography, trichoscopy, RCM, and OCT have furthered our understanding of alopecia pathophysiology and provided objective methods of treatment evaluation. The capabilities of these tools will continue to expand with advancements in add-on software and AI algorithms.
- Canfield D. Photographic documentation of hair growth in androgenetic alopecia. Dermatol Clin. 1996;14:713-721.
- Peytavi U, Hillmann K, Guarrera M. Hair growth assessment techniques. In: Peytavi U, Hillmann K, Guarrera M, eds. Hair Growth and Disorders. 4th ed. Springer; 2008:140-144.
- Chamberlain AJ, Dawber RP. Methods of evaluating hair growth. Australas J Dermatol. 2003;44:10-18.
- Dhurat R, Saraogi P. Hair evaluation methods: merits and demerits. Int J Trichology. 2009;1:108-119.
- Kaufman KD, Olsen EA, Whiting D, et al. Finasteride in the treatment of men with androgenetic alopecia. J Am Acad Dermatol. 1998;39:578-579.
- Capily Institute. Artificial intelligence (A.I.) powered hair growth tracking. Accessed July 31, 2023. https://tss-aesthetics.com/capily-hair-tracking-syst
- Dinh Q, Sinclair R. Female pattern hair loss: current treatment concepts. Clin Interv Aging. 2007;2:189-199.
- Dhurat R, Saraogi P. Hair evaluation methods: merits and demerits. Int J Trichology. 2009;1:108-119.
- Wikramanayake TC, Mauro LM, Tabas IA, et al. Cross-section trichometry: a clinical tool for assessing the progression and treatment response of alopecia. Int J Trichology. 2012;4:259-264.
- Alessandrini A, Bruni F, Piraccini BM, et al. Common causes of hair loss—clinical manifestations, trichoscopy and therapy. J Eur Acad Dermatol Venereol. 2021;35:629-640.
- Ashique K, Kaliyadan F. Clinical photography for trichology practice: tips and tricks. Int J Trichology. 2011;3:7-13.
- Rudnicka L, Olszewska M, Rakowska A, et al. Trichoscopy: a new method for diagnosing hair loss. J Drugs Dermatol. 2008;7:651-654.
- Kinoshita-Ise M, Sachdeva M. Update on trichoscopy: integration of the terminology by systematic approach and a proposal of a diagnostic flowchart. J Dermatol. 2022;49:4-18. doi:10.1111/1346-8138.16233
- Van Neste D, Trüeb RM. Critical study of hair growth analysis with computer-assisted methods. J Eur Acad Dermatol Venereol. 2006;20:578-583.
- Romero J, Grimalt R. Trichoscopy: essentials for the dermatologist. World J Dermatol. 2015;4:63-68.
- Trichoscopy: a new frontier for the diagnosis of hair diseases. Exp Rev Dermatol. 2012;7:429-437.
- Lee B, Chan J, Monselise A, et al. Assessment of hair density and caliber in Caucasian and Asian female subjects with female pattern hair loss by using the Folliscope. J Am Acad Dermatol. 2012;66:166-167.
- Inui S. Trichoscopy for common hair loss diseases: algorithmic method for diagnosis. J Dermatol. 2010;38:71-75.
- Dhurat R. Phototrichogram. Indian J Dermatol Venereol Leprol. 2006;72:242-244.
- Agozzino M, Tosti A, Barbieri L, et al. Confocal microscopic features of scarring alopecia: preliminary report. Br J Dermatol. 2011;165:534-540.
- Kuck M, Schanzer S, Ulrich M, et al. Analysis of the efficiency of hair removal by different optical methods: comparison of Trichoscan, reflectance confocal microscopy, and optical coherence tomography. J Biomed Opt. 2012;17:101504.
- Levine A, Markowitz O. Introduction to reflectance confocal microscopy and its use in clinical practice. JAAD Case Rep. 2018;4:1014-1023.
- Agozzino M, Ardigò M. Scalp confocal microscopy. In: Humbert P, Maibach H, Fanian F, et al, eds. Agache’s Measuring the Skin: Non-invasive Investigations, Physiology, Normal Constants. 2nd ed. Springer International Publishing; 2016:311-326.
- Rudnicka L, Olszewska M, Rakowska A. In vivo reflectance confocal microscopy: usefulness for diagnosing hair diseases. J Dermatol Case Rep. 2008;2:55-59.
- Kurzeja M, Czuwara J, Walecka I, et al. Features of classic lichen planopilaris and frontal fibrosing alopecia in reflectance confocal microscopy: a preliminary study. Skin Res Technol. 2021;27:266-271.
- Ardigò M, Agozzino M, Franceschini C, et al. Reflectance confocal microscopy for scarring and non-scarring alopecia real-time assessment. Arch Dermatol Res. 2016;308:309-318.
- Franceschini C, Garelli V, Persechino F, et al. Dermoscopy and confocal microscopy for different chemotherapy-induced alopecia (CIA) phases characterization: preliminary study. Skin Res Technol. 2020;26:269-276.
- Martinez-Velasco MA, Perper M, Maddy AJ, et al. In vitro determination of Mexican Mestizo hair shaft diameter using optical coherence tomography. Skin Res Technol. 2018;24;274-277.
- Srivastava R, Manfredini M, Rao BK. Noninvasive imaging tools in dermatology. Cutis. 2019;104:108-113.
- Wan B, Ganier C, Du-Harpur X, et al. Applications and future directions for optical coherence tomography in dermatology. Br J Dermatol. 2021;184:1014-1022.
- Blume-Peytavi U, Vieten J, Knuttel A et al. Optical coherent tomography (OCT): a new method for online-measurement of hair shaft thickness. J Dtsch Dermatol Ges. 2004;2:546.
- Garcia Bartels N, Jahnke I, Patzelt A, et al. Hair shaft abnormalities in alopecia areata evaluated by optical coherence tomography. Skin Res Technol. 2011;17:201-205.
- Urban G, Feil N, Csuka E, et al. Combining deep learning with optical coherence tomography imaging to determine scalp hair and follicle counts. Lasers Surg Med. 2021;53:171-178.
- Vazquez-Herrera NE, Eber AE, Martinez-Velasco MA, et al. Optical coherence tomography for the investigation of frontal fibrosing alopecia. J Eur Acad Dermatol Venereol. 2018;32:318-322.
- Ekelem C, Feil N, Csuka E, et al. Optical coherence tomography in the evaluation of the scalp and hair: common features and clinical utility. Lasers Surg Med. 2021;53:129-140.
- Schicho K, Seemann R, Binder M, et al. Optical coherence tomography for planning of follicular unit extraction. Dermatol Surg. 2015;41:358-363.
- Canfield D. Photographic documentation of hair growth in androgenetic alopecia. Dermatol Clin. 1996;14:713-721.
- Peytavi U, Hillmann K, Guarrera M. Hair growth assessment techniques. In: Peytavi U, Hillmann K, Guarrera M, eds. Hair Growth and Disorders. 4th ed. Springer; 2008:140-144.
- Chamberlain AJ, Dawber RP. Methods of evaluating hair growth. Australas J Dermatol. 2003;44:10-18.
- Dhurat R, Saraogi P. Hair evaluation methods: merits and demerits. Int J Trichology. 2009;1:108-119.
- Kaufman KD, Olsen EA, Whiting D, et al. Finasteride in the treatment of men with androgenetic alopecia. J Am Acad Dermatol. 1998;39:578-579.
- Capily Institute. Artificial intelligence (A.I.) powered hair growth tracking. Accessed July 31, 2023. https://tss-aesthetics.com/capily-hair-tracking-syst
- Dinh Q, Sinclair R. Female pattern hair loss: current treatment concepts. Clin Interv Aging. 2007;2:189-199.
- Dhurat R, Saraogi P. Hair evaluation methods: merits and demerits. Int J Trichology. 2009;1:108-119.
- Wikramanayake TC, Mauro LM, Tabas IA, et al. Cross-section trichometry: a clinical tool for assessing the progression and treatment response of alopecia. Int J Trichology. 2012;4:259-264.
- Alessandrini A, Bruni F, Piraccini BM, et al. Common causes of hair loss—clinical manifestations, trichoscopy and therapy. J Eur Acad Dermatol Venereol. 2021;35:629-640.
- Ashique K, Kaliyadan F. Clinical photography for trichology practice: tips and tricks. Int J Trichology. 2011;3:7-13.
- Rudnicka L, Olszewska M, Rakowska A, et al. Trichoscopy: a new method for diagnosing hair loss. J Drugs Dermatol. 2008;7:651-654.
- Kinoshita-Ise M, Sachdeva M. Update on trichoscopy: integration of the terminology by systematic approach and a proposal of a diagnostic flowchart. J Dermatol. 2022;49:4-18. doi:10.1111/1346-8138.16233
- Van Neste D, Trüeb RM. Critical study of hair growth analysis with computer-assisted methods. J Eur Acad Dermatol Venereol. 2006;20:578-583.
- Romero J, Grimalt R. Trichoscopy: essentials for the dermatologist. World J Dermatol. 2015;4:63-68.
- Trichoscopy: a new frontier for the diagnosis of hair diseases. Exp Rev Dermatol. 2012;7:429-437.
- Lee B, Chan J, Monselise A, et al. Assessment of hair density and caliber in Caucasian and Asian female subjects with female pattern hair loss by using the Folliscope. J Am Acad Dermatol. 2012;66:166-167.
- Inui S. Trichoscopy for common hair loss diseases: algorithmic method for diagnosis. J Dermatol. 2010;38:71-75.
- Dhurat R. Phototrichogram. Indian J Dermatol Venereol Leprol. 2006;72:242-244.
- Agozzino M, Tosti A, Barbieri L, et al. Confocal microscopic features of scarring alopecia: preliminary report. Br J Dermatol. 2011;165:534-540.
- Kuck M, Schanzer S, Ulrich M, et al. Analysis of the efficiency of hair removal by different optical methods: comparison of Trichoscan, reflectance confocal microscopy, and optical coherence tomography. J Biomed Opt. 2012;17:101504.
- Levine A, Markowitz O. Introduction to reflectance confocal microscopy and its use in clinical practice. JAAD Case Rep. 2018;4:1014-1023.
- Agozzino M, Ardigò M. Scalp confocal microscopy. In: Humbert P, Maibach H, Fanian F, et al, eds. Agache’s Measuring the Skin: Non-invasive Investigations, Physiology, Normal Constants. 2nd ed. Springer International Publishing; 2016:311-326.
- Rudnicka L, Olszewska M, Rakowska A. In vivo reflectance confocal microscopy: usefulness for diagnosing hair diseases. J Dermatol Case Rep. 2008;2:55-59.
- Kurzeja M, Czuwara J, Walecka I, et al. Features of classic lichen planopilaris and frontal fibrosing alopecia in reflectance confocal microscopy: a preliminary study. Skin Res Technol. 2021;27:266-271.
- Ardigò M, Agozzino M, Franceschini C, et al. Reflectance confocal microscopy for scarring and non-scarring alopecia real-time assessment. Arch Dermatol Res. 2016;308:309-318.
- Franceschini C, Garelli V, Persechino F, et al. Dermoscopy and confocal microscopy for different chemotherapy-induced alopecia (CIA) phases characterization: preliminary study. Skin Res Technol. 2020;26:269-276.
- Martinez-Velasco MA, Perper M, Maddy AJ, et al. In vitro determination of Mexican Mestizo hair shaft diameter using optical coherence tomography. Skin Res Technol. 2018;24;274-277.
- Srivastava R, Manfredini M, Rao BK. Noninvasive imaging tools in dermatology. Cutis. 2019;104:108-113.
- Wan B, Ganier C, Du-Harpur X, et al. Applications and future directions for optical coherence tomography in dermatology. Br J Dermatol. 2021;184:1014-1022.
- Blume-Peytavi U, Vieten J, Knuttel A et al. Optical coherent tomography (OCT): a new method for online-measurement of hair shaft thickness. J Dtsch Dermatol Ges. 2004;2:546.
- Garcia Bartels N, Jahnke I, Patzelt A, et al. Hair shaft abnormalities in alopecia areata evaluated by optical coherence tomography. Skin Res Technol. 2011;17:201-205.
- Urban G, Feil N, Csuka E, et al. Combining deep learning with optical coherence tomography imaging to determine scalp hair and follicle counts. Lasers Surg Med. 2021;53:171-178.
- Vazquez-Herrera NE, Eber AE, Martinez-Velasco MA, et al. Optical coherence tomography for the investigation of frontal fibrosing alopecia. J Eur Acad Dermatol Venereol. 2018;32:318-322.
- Ekelem C, Feil N, Csuka E, et al. Optical coherence tomography in the evaluation of the scalp and hair: common features and clinical utility. Lasers Surg Med. 2021;53:129-140.
- Schicho K, Seemann R, Binder M, et al. Optical coherence tomography for planning of follicular unit extraction. Dermatol Surg. 2015;41:358-363.
Practice Points
- Reflectance confocal microscopy (RCM) imaging can be taken at levels from the stratum corneum to the papillary dermis and can be used to study scalp discoid lupus, lichen planopilaris, frontal fibrosing alopecia, alopecia areata, and androgenetic alopecia.
- Because of its ability to distinguish different stages of disease, RCM can be recommended as an intermediate step between trichoscopy and histology for the diagnosis and management of hair disease.
- Optical coherence tomography has the potential to monitor early subclinical responses to alopecia therapies while also improving hair transplantation outcomes by allowing for visualization of the subcutaneous angle of hair follicles.
- Software development paired with trichoscopy has the ability to quantify hair growth parameters such as hair count, density, and diameter.
Brachioradial Pruritus: An Etiologic Review and Treatment Summary
Brachioradial pruritus (BRP) is a neuropathic condition typically characterized by localized dysesthesia of the dorsolateral arms.1 This dysesthesia has been described as a persistent painful itching, burning, tingling, or stinging sensation2-4 and has a median duration of expression of 24 months.5,6 The condition may be unilateral or bilateral in nature but tends to have a predilection for a bilateral distribution along the C5 to C6 dermatomes.1,7,8 There are no primary skin lesions associated with BRP; however, excoriations, prurigo nodules, and lichenification may arise secondary to scratching of the irritated skin.1,4,5,9 Brachioradial pruritus tends to have a predilection for adult females (3:1 ratio) with lighter skin. The mean age at diagnosis is 59 years, but cases have been reported in patients aged 12 to 84 years.1,5 The diagnosis of BRP is based on clinical signs and symptoms, though the ice-pack sign tends to be pathognomonic for the diagnosis.10,11 Although there is no clear evidence on the exact cause of BRP, there are 2 prevalent theories: cervical radiculopathy secondary to cervical spine pathology and/or excessive exposure to UV radiation (UVR) in the summer months.3-5,12 Brachioradial pruritus remains poorly described in the literature, and even its origin is under debate. As such, the clinician may have difficulty deciding on the best course of management. The goal of this article is to identify and discuss known treatment options for BRP (Table).
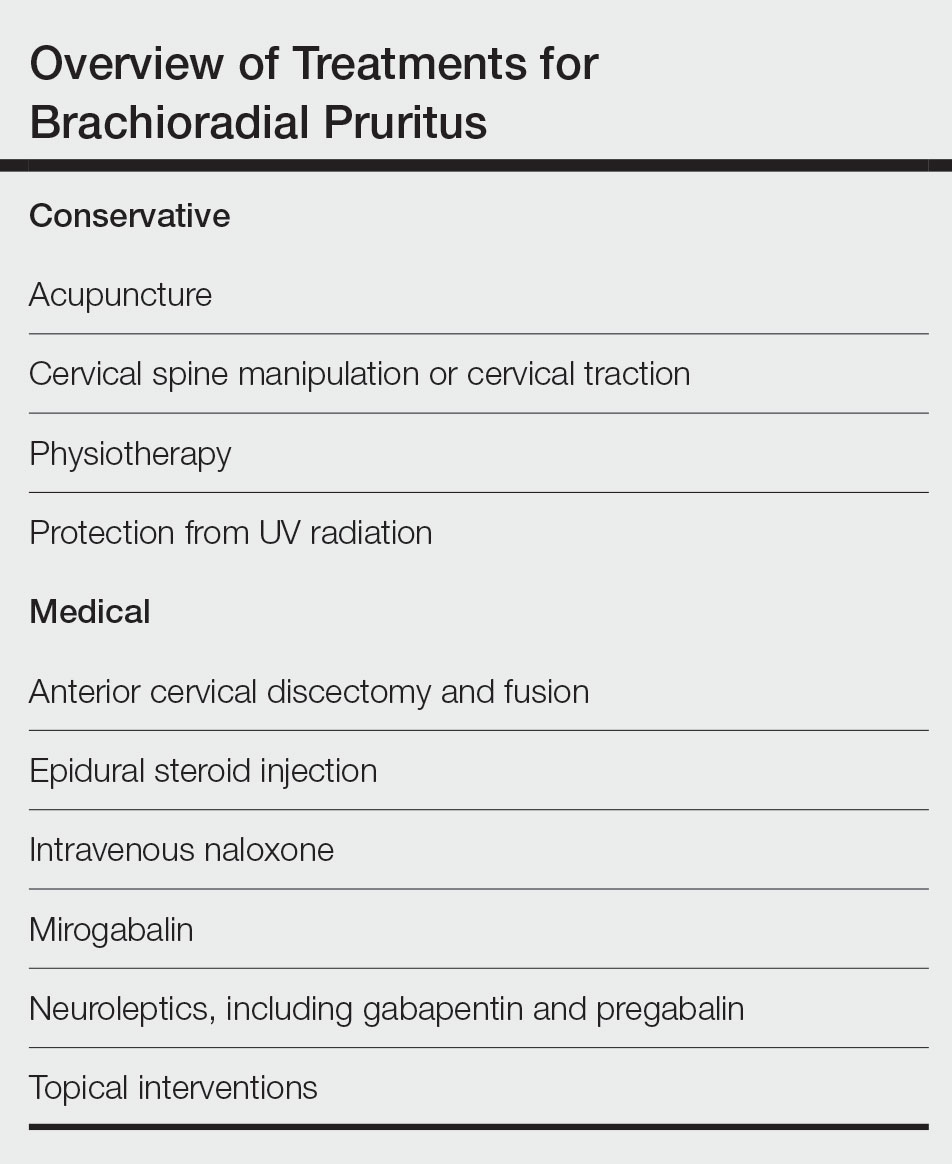
Etiology
Cervical Spine Pathology—A correlation appears to exist between BRP and cervical spine changes seen on plain film radiographs at the levels of C3 to C7, with increased incidence at the C5 to C6 levels. These plain film radiographs typically show degenerative joint disease and neural foraminal stenosis at levels that correlate to the dermatomal distribution of BRP.1,7,10,12-14 In addition to plain film radiography, some studies have utilized magnetic resonance imaging to view the cervical spine and have documented evidence of intervertebral disc protrusion/bulging, central canal stenosis, neuroforaminal stenosis, and spondylosis at the affected regions.5,15-17 Moreover, supporting the theory that the cervical spine is responsible for the emergence of BRP, Marziniak et al17 investigated 41 patients with BRP utilizing magnetic resonance tomography to find that 33 patients (80.5%) had changes in nerve compression, and 8 patients (19.5%) had degenerative changes. In addition to these findings, they found that there was a significant correlation (P<.01) between the dermatomal expression of BRP and the location of cervical anatomical changes.17 Further validating the relationship between cervical spine pathology and BRP is a case study of a patient who saw rapid and complete resolution of the pruritus following spinal decompression surgery.10 Another case study described an intramedullary tumor found in a patient with BRP that was diagnosed as an ependymoma after magnetic resonance imaging revealed an intramedullary lesion within the spinal cord between C4 and C7. The location of the tumor and dermatomal pattern of the neuropathic itch pointed to a possible association between nerve compression and BRP.14 Electromyography studies performed on individuals with BRP have shown an increase in polyphasic units, decreased motor units, and/or denervation changes along the C5/C6 or C6 nerve roots, which provides additional support for the theory of cervical spine pathology as a causative factor for BRP.16
UVR Exposure—Another etiologic theory for BRP is that UVR exposure may be responsible for the genesis of pruritus. Previously known as solar pruritus, BRP was deemed a clinical condition, as there was increased prevalence in patients living in warmer climates, such as Florida.9 Wallengren and Dahlbäck18 reported that sun exposure is a notable factor in the onset of BRP, as they saw an increase in symptoms during the late summer and a decrease in symptoms over the winter months.To further support the theory that UVR is linked to BRP, several studies have shown that the utilization of sun protection is linked to a reduction of symptoms, specifically in patients who showed seasonal variations of their symptoms.9,12,19 Additionally, a study by Mirzoyev and Davis5 retrospectively reviewed 111 patients diagnosed with BRP. Of these patients, 84 (75.7%) presented with bilateral symptoms, and 54 (48.6%) reported prolonged sun exposure. Both of these findings demonstrate correlation between UVR and BRP.5 Interestingly, UV light exposure is known to release β-endorphin in the skin and may theoretically provide an area of exploration between UVR and cervical spine theories.
Conservative Treatment
Chiropractic Manipulation—Because one etiologic theory includes disease of the cervical spine, there is evidence that targeting this region with treatment is beneficial.7 Two case reports found in the literature noted that cervical spine manipulation and cervical traction yielded positive results.20,21 It has been established that pain generated by disc lesions can be the result of local nociceptive fiber activation, direct mechanical compression of the nerve roots, or inflammatory mediators.22 There are several postulated models describing the hypoalgesic effects of spinal manipulation, which contains both biomechanical and neurophysiological mechanisms. Biomechanical changes theorized to elicit analgesia include restoration of faulty biomechanical movement patterns, breaking up of periarticular adhesions, and reflexogenic muscle inhibition of hypertonic musculature. Hypothesized neurophysiological effects of joint manipulation include an increase in afferent information overwhelming the nociceptive input, reduction of temporal summation, and autonomic activation leading to non–opioid-induced hypoalgesia.23 Cervical traction is another plausible treatment for BRP, wherein the physiological effects of traction allow for a separation of vertebral bodies and expansion of the intervertebral foramen circumference, thus decreasing compression of the nerve roots.24
Acupuncture—Neurogenic pruritus, including BRP, is a group of conditions that have been treated using acupuncture. Acupuncture treatment consists of intramuscular needle stimulation and has been found to alleviate itching in patients with neurogenic pruritus. In 1 retrospective case series, acupuncture was used to treat 16 patients who were identified as having segmental pruritus. Acupuncture targeted the spasmed paravertebral muscles of the affected dermatomal levels as well as other regions of the body, and it was found that 12 patients (75%) experienced full resolution of symptoms. However, relapse did occur in 6 patients (37%) within 1 to 12 months following treatment.25 Multiple theories exist as to why acupuncture may help. One is that it relieves muscle spasms, which in turn relieves neural irritation of the spinal nerves as they traverse the respective paraspinal musculature. Another is that acupuncture decreases nociception by stimulating release of opioid peptides in the dorsal horn.26 A third proposed theory is that acupuncture acts on the afferent nerve fibers responsible for transmitting pain—Aδ and C fibers—activating these afferent nerves to produce an analgesic effect.27
Physiotherapy—The literature suggests that possible first-line therapies for neurogenic pruritus, including BRP and notalgia paresthetica, consist of noninvasive nondermatologic treatments that target cervical spine disease. Notalgia paresthetica and BRP have similar proposed mechanisms of nerve impingement; therefore, they often are grouped together when discussing proposed manual treatment options. Physiotherapy treatment includes cervical muscle strengthening, increased range of motion, application of cervical soft collars, massage, transcutaneous electronic nerve stimulation, and cervical traction.7 A study of 12 patients by Raison-Peyron et al28 in 1999 discussed the use of spinal and paraspinal ultrasound or radiation physiotherapy. Six patients underwent this treatment, and the symptoms subsided in 4 cases.28 Another study by Fleischer et al29 in 2011 discussed improvement in 2 patients with notalgia paresthetica by exercise involving active range of motion and strengthening.
Photoprotection—Avoidance of UVR exposure has been beneficial to some patients to reduce symptoms. Use of sunscreen and long-sleeved UV-protective clothing during outdoor activities or the warmer summer months may be beneficial.1
Medical Treatment
Medication—Because of the nonspecific clinical presentation of BRP, initial treatment often involves prescription of first-line antipruritic agents, including steroid creams and systemic antihistamines, both of which generally fail to provide symptom relief.1,30 Medications with neurologic mechanisms of action appear to provide potentially superior outcomes.
Topical interventions for BRP and related neurogenic pruritus have shown limited success. A case series evaluating capsaicin for pruritus offered only transient relief, likely because of its temporary hyperstimulatory and desensitizing effect on neuropeptides.7,33 In small populations, the use of topical antidepressants has yielded cutaneous and pathological relief for BRP. A case study of a 70-year-old woman evaluated the efficacy of a combination cream of ketamine and amitriptyline (a tricyclic antidepressant) yielding moderate pruritus improvement and notable improvement of secondary brachial skin lesions.34 Oral steroids also have shown success in the treatment of chronic pruritus; however, limited research is available on the efficacy of such medications for BRP, and the long-term use of oral steroids is limited by many side effects.30
Interventional Pain Procedure—A 2018 case series investigated 3 patients with a clinical diagnosis of BRP who were treated between 2010 and 2016 with
Surgery—There are multiple case studies in the literature that discuss
Conclusion
The pathogenesis of BRP continues to be an area of debate—it may be secondary to cervical spine disease or UVR. This review found there is more research pointing to cervical spine disease. There is an abundance of literature discussing both conservative and invasive treatment strategies, both of which carry benefits. Further research is needed to better establish the etiology of BRP so that formal treatment guidelines may be established.
Neuropathic itch can be a frustrating condition for providers and patients, and many treatment modalities often are tried before arriving at a helpful treatment for a particular patient. Clinicians who may encounter BRP in practice benefit from up-to-date literature reviews that provide a summary of management strategies.
- Robbins BA, Schmieder GJ. Brachioradial pruritus. StatPearls Publishing; 2020. Updated September 12, 2022. Accessed July 25, 2023. https://www.ncbi.nlm.nih.gov/books/NBK459321/
- Crevits L. Brachioradial pruritus—a peculiar neuropathic disorder. Clin Neurol Neurosurg. 2006;108:803-805.
- Lane J, McKenzie J, Spiegel J. Brachioradial pruritus: a case report and review of the literature. Cutis. 2008;81:37-40.
- Wallengren J. Brachioradial pruritus: a recurrent solar dermopathy. J Am Acad Dermatol. 1998;39:803-806.
- Mirzoyev S, Davis M. Brachioradial pruritus: Mayo Clinic experience over the past decade. Br J Dermatol. 2013;169:1007-1015.
- Pinto AC, Wachholz PA, Masuda PY, et al. Clinical, epidemiological and therapeutic profile of patients with brachioradial pruritus in a reference service in dermatology. An Bras Dermatol. 2016;91:549-551. doi:10.1590/abd1806-4841.201644767
- Alai NN, Skinner HB. Concurrent notalgia paresthetica and brachioradial pruritus associated with cervical degenerative disc disease. Cutis. 2018;102:185, 186, 189, 190.
- Atis¸ G, Bilir Kaya B. Pregabalin treatment of three cases with brachioradial pruritus. Dermatol Ther. 2017;30:e12459.
- Waisman M. Solar pruritus of the elbows (brachioradial summer pruritus). Arch Dermatol. 1968;98:481-485.
- Binder A, Fölster-Holst R, Sahan G, et al. A case of neuropathic brachioradial pruritus caused by cervical disc herniation. Nat Clin Pract Neurol. 2008;4:338-342.
- Bernhard JD, Bordeaux JS. Medical pearl: the ice-pack sign in brachioradial pruritus. J Am Acad Dermatol. 2005;52:1073.
- Veien N, Laurberg G. Brachioradial pruritus: a follow-up of 76 patients. Acta Derm Venereol. 2011;91:183-185.
- Mataix J, Silvestre JF, Climent JM, et al. Brachioradial pruritus as a symptom of cervical radiculopathy. Article in Spanish. Actas Dermosifiliogr. 2008;99:719-722.
- Kavak A, Dosoglu M. Can a spinal cord tumor cause brachioradial pruritus? J Am Acad Dermatol. 2002;46:437-440.
- Zeidler C, Pereira MP, Ständer S. Brachioradial pruritus successfully treated with intravenous naloxone. J Eur Acad Dermatol Venereol. 2023;37:e87-e89. doi:10.1111/jdv.18553
- Shields LB, Iyer VG, Zhang Y, et al. Brachioradial pruritus: clinical, electromyographic, and cervical MRI features in nine patients. Cureus. 2022;14:e21811. doi:10.7759/cureus.21811
- Marziniak M, Phan NQ, Raap U, et al. Brachioradial pruritus as a result of cervical spine pathology: the results of a magneticresonance tomography study. J Am Acad Dermatol. 2011;65:756-762. doi:10.1016/j.jaad.2010.07.036
- Wallengren J, Dahlbäck K. Familial brachioradial pruritus. Br J Dermatol. 2005;153:1016-1018.
- Salzmann SN, Okano I, Shue J, et al. Disabling pruritus in a patient with cervical stenosis. J Am Acad Orthop Surg Glob Res Rev. 2020;4:e19.00178. doi:10.5435/JAAOSGlobal-D-19-00178
- Golden KJ, Diana RM. A case of brachioradial pruritus treated with chiropractic and acupuncture. Case Rep Dermatol. 2022;14:93-97. doi:10.1159/000524054
- Tait CP, Grigg E, Quirk CJ. Brachioradial pruritus and cervical spine manipulation. Australas J Dermatol. 1998;39:168-170. doi:10.1111/j.1440-0960.1998.tb01274.x
- Freynhagen R, Baron R. The evaluation of neuropathic components in low back pain. Curr Pain Headache Rep. 2009;13:185-190. doi:10.1007/s11916-009-0032-y
- Gyer G, Michael J, Inklebarger J, et al. Spinal manipulation therapy: is it all about the brain? A current review of the neurophysiological effects of manipulation. J Integr Med. 2019;17:328-337. doi:10.1016/j.joim.2019.05.004
- Graham N, Gross A, Goldsmith CH, et al. Mechanical traction for neck pain with or without radiculopathy. Cochrane Database Syst Rev. 2008:CD006408. doi:10.1002/14651858.CD006408.pub2
- Stellon A. Neurogenic pruritus: an unrecognised problem? A retrospective case series of treatment by acupuncture. Acupunct Med. 2002;20:186-190. doi:10.1136/aim.20.4.186
- Bowsher D. Mechanisms of acupuncture. In: Filshie J, White A, eds. Medical Acupuncture: A Western Scientific Approach. Churchill Livingstone; 1998:69-82.
- Lim TK, Ma Y, Berger F, et al. Acupuncture and neural mechanism in the management of low back pain-an update. Medicines (Basel). 2018;5:63.
- Raison-Peyron N, Meunier L, Acevedo M, et al. Notalgia paresthetica: clinical, physiopathological and therapeutic aspects. a study of 12 cases. J Eur Acad Dermatol Venereol. 1999;12:215-221.
- Fleischer AB, Meade TJ, Fleischer AB. Notalgia paresthetica: successful treatment with exercises. Acta Derm Venereol. 2011;91:356-357. doi:10.2340/00015555-1039
- Kouwenhoven TA, van de Kerkhof PCM, Kamsteeg M. Use of oral antidepressants in patients with chronic pruritus: a systematic review. J Am Acad Dermatol. 2017;77:1068-1073.e7. doi:10.1016/j.jaad.2017.08.025
- Matsuda KM, Sharma D, Schonfeld AR, et al. Gabapentin and pregabalin for the treatment of chronic pruritus. J Am Acad Dermatol. 2016;75:619-625.e6. doi:10.1016/j.jaad.2016.02.1237
- Okuno S, Hashimoto T, Satoh T. Case of neuropathic itch-associated prurigo nodules on the bilateral upper arms after unilateral herpes zoster in a patient with cervical herniated discs: successful treatment with mirogabalin. J Dermatol. 2021;48:e585-e586.
- Papoiu AD, Yosipovitch G. Topical capsaicin. The fire of a ‘hot’ medicine is reignited. Expert Opin Pharmacother. 2010;11:1359-1371. doi:10.1517/14656566.2010.481670
- Magazin M, Daze RP, Okeson N. Treatment refractory brachioradial pruritus treated with topical amitriptyline and ketamine. Cureus. 2019;11:e5117. doi:10.7759/cureus.5117
- Weinberg BD, Amans M, Deviren S, et al. Brachioradial pruritus treated with computed tomography-guided cervical nerve root block: a case series. JAAD Case Rep. 2018;4:640-644. doi:10.1016/j.jdcr.2018.03.025
- De Ridder D, Hans G, Pals P, et al. A C-fiber-mediated neuropathic brachioradial pruritus. J Neurosurg. 2010;113:118-121. doi:10.3171/2009.9.JNS09620
- Morosanu CO, Etim G, Alalade AF. Brachioradial pruritus secondary to cervical disc protrusion—a case report. J Surg Case Rep. 2022:rjac277. doi:10.1093/jscr/rjac277
Brachioradial pruritus (BRP) is a neuropathic condition typically characterized by localized dysesthesia of the dorsolateral arms.1 This dysesthesia has been described as a persistent painful itching, burning, tingling, or stinging sensation2-4 and has a median duration of expression of 24 months.5,6 The condition may be unilateral or bilateral in nature but tends to have a predilection for a bilateral distribution along the C5 to C6 dermatomes.1,7,8 There are no primary skin lesions associated with BRP; however, excoriations, prurigo nodules, and lichenification may arise secondary to scratching of the irritated skin.1,4,5,9 Brachioradial pruritus tends to have a predilection for adult females (3:1 ratio) with lighter skin. The mean age at diagnosis is 59 years, but cases have been reported in patients aged 12 to 84 years.1,5 The diagnosis of BRP is based on clinical signs and symptoms, though the ice-pack sign tends to be pathognomonic for the diagnosis.10,11 Although there is no clear evidence on the exact cause of BRP, there are 2 prevalent theories: cervical radiculopathy secondary to cervical spine pathology and/or excessive exposure to UV radiation (UVR) in the summer months.3-5,12 Brachioradial pruritus remains poorly described in the literature, and even its origin is under debate. As such, the clinician may have difficulty deciding on the best course of management. The goal of this article is to identify and discuss known treatment options for BRP (Table).

Etiology
Cervical Spine Pathology—A correlation appears to exist between BRP and cervical spine changes seen on plain film radiographs at the levels of C3 to C7, with increased incidence at the C5 to C6 levels. These plain film radiographs typically show degenerative joint disease and neural foraminal stenosis at levels that correlate to the dermatomal distribution of BRP.1,7,10,12-14 In addition to plain film radiography, some studies have utilized magnetic resonance imaging to view the cervical spine and have documented evidence of intervertebral disc protrusion/bulging, central canal stenosis, neuroforaminal stenosis, and spondylosis at the affected regions.5,15-17 Moreover, supporting the theory that the cervical spine is responsible for the emergence of BRP, Marziniak et al17 investigated 41 patients with BRP utilizing magnetic resonance tomography to find that 33 patients (80.5%) had changes in nerve compression, and 8 patients (19.5%) had degenerative changes. In addition to these findings, they found that there was a significant correlation (P<.01) between the dermatomal expression of BRP and the location of cervical anatomical changes.17 Further validating the relationship between cervical spine pathology and BRP is a case study of a patient who saw rapid and complete resolution of the pruritus following spinal decompression surgery.10 Another case study described an intramedullary tumor found in a patient with BRP that was diagnosed as an ependymoma after magnetic resonance imaging revealed an intramedullary lesion within the spinal cord between C4 and C7. The location of the tumor and dermatomal pattern of the neuropathic itch pointed to a possible association between nerve compression and BRP.14 Electromyography studies performed on individuals with BRP have shown an increase in polyphasic units, decreased motor units, and/or denervation changes along the C5/C6 or C6 nerve roots, which provides additional support for the theory of cervical spine pathology as a causative factor for BRP.16
UVR Exposure—Another etiologic theory for BRP is that UVR exposure may be responsible for the genesis of pruritus. Previously known as solar pruritus, BRP was deemed a clinical condition, as there was increased prevalence in patients living in warmer climates, such as Florida.9 Wallengren and Dahlbäck18 reported that sun exposure is a notable factor in the onset of BRP, as they saw an increase in symptoms during the late summer and a decrease in symptoms over the winter months.To further support the theory that UVR is linked to BRP, several studies have shown that the utilization of sun protection is linked to a reduction of symptoms, specifically in patients who showed seasonal variations of their symptoms.9,12,19 Additionally, a study by Mirzoyev and Davis5 retrospectively reviewed 111 patients diagnosed with BRP. Of these patients, 84 (75.7%) presented with bilateral symptoms, and 54 (48.6%) reported prolonged sun exposure. Both of these findings demonstrate correlation between UVR and BRP.5 Interestingly, UV light exposure is known to release β-endorphin in the skin and may theoretically provide an area of exploration between UVR and cervical spine theories.
Conservative Treatment
Chiropractic Manipulation—Because one etiologic theory includes disease of the cervical spine, there is evidence that targeting this region with treatment is beneficial.7 Two case reports found in the literature noted that cervical spine manipulation and cervical traction yielded positive results.20,21 It has been established that pain generated by disc lesions can be the result of local nociceptive fiber activation, direct mechanical compression of the nerve roots, or inflammatory mediators.22 There are several postulated models describing the hypoalgesic effects of spinal manipulation, which contains both biomechanical and neurophysiological mechanisms. Biomechanical changes theorized to elicit analgesia include restoration of faulty biomechanical movement patterns, breaking up of periarticular adhesions, and reflexogenic muscle inhibition of hypertonic musculature. Hypothesized neurophysiological effects of joint manipulation include an increase in afferent information overwhelming the nociceptive input, reduction of temporal summation, and autonomic activation leading to non–opioid-induced hypoalgesia.23 Cervical traction is another plausible treatment for BRP, wherein the physiological effects of traction allow for a separation of vertebral bodies and expansion of the intervertebral foramen circumference, thus decreasing compression of the nerve roots.24
Acupuncture—Neurogenic pruritus, including BRP, is a group of conditions that have been treated using acupuncture. Acupuncture treatment consists of intramuscular needle stimulation and has been found to alleviate itching in patients with neurogenic pruritus. In 1 retrospective case series, acupuncture was used to treat 16 patients who were identified as having segmental pruritus. Acupuncture targeted the spasmed paravertebral muscles of the affected dermatomal levels as well as other regions of the body, and it was found that 12 patients (75%) experienced full resolution of symptoms. However, relapse did occur in 6 patients (37%) within 1 to 12 months following treatment.25 Multiple theories exist as to why acupuncture may help. One is that it relieves muscle spasms, which in turn relieves neural irritation of the spinal nerves as they traverse the respective paraspinal musculature. Another is that acupuncture decreases nociception by stimulating release of opioid peptides in the dorsal horn.26 A third proposed theory is that acupuncture acts on the afferent nerve fibers responsible for transmitting pain—Aδ and C fibers—activating these afferent nerves to produce an analgesic effect.27
Physiotherapy—The literature suggests that possible first-line therapies for neurogenic pruritus, including BRP and notalgia paresthetica, consist of noninvasive nondermatologic treatments that target cervical spine disease. Notalgia paresthetica and BRP have similar proposed mechanisms of nerve impingement; therefore, they often are grouped together when discussing proposed manual treatment options. Physiotherapy treatment includes cervical muscle strengthening, increased range of motion, application of cervical soft collars, massage, transcutaneous electronic nerve stimulation, and cervical traction.7 A study of 12 patients by Raison-Peyron et al28 in 1999 discussed the use of spinal and paraspinal ultrasound or radiation physiotherapy. Six patients underwent this treatment, and the symptoms subsided in 4 cases.28 Another study by Fleischer et al29 in 2011 discussed improvement in 2 patients with notalgia paresthetica by exercise involving active range of motion and strengthening.
Photoprotection—Avoidance of UVR exposure has been beneficial to some patients to reduce symptoms. Use of sunscreen and long-sleeved UV-protective clothing during outdoor activities or the warmer summer months may be beneficial.1
Medical Treatment
Medication—Because of the nonspecific clinical presentation of BRP, initial treatment often involves prescription of first-line antipruritic agents, including steroid creams and systemic antihistamines, both of which generally fail to provide symptom relief.1,30 Medications with neurologic mechanisms of action appear to provide potentially superior outcomes.
Topical interventions for BRP and related neurogenic pruritus have shown limited success. A case series evaluating capsaicin for pruritus offered only transient relief, likely because of its temporary hyperstimulatory and desensitizing effect on neuropeptides.7,33 In small populations, the use of topical antidepressants has yielded cutaneous and pathological relief for BRP. A case study of a 70-year-old woman evaluated the efficacy of a combination cream of ketamine and amitriptyline (a tricyclic antidepressant) yielding moderate pruritus improvement and notable improvement of secondary brachial skin lesions.34 Oral steroids also have shown success in the treatment of chronic pruritus; however, limited research is available on the efficacy of such medications for BRP, and the long-term use of oral steroids is limited by many side effects.30
Interventional Pain Procedure—A 2018 case series investigated 3 patients with a clinical diagnosis of BRP who were treated between 2010 and 2016 with
Surgery—There are multiple case studies in the literature that discuss
Conclusion
The pathogenesis of BRP continues to be an area of debate—it may be secondary to cervical spine disease or UVR. This review found there is more research pointing to cervical spine disease. There is an abundance of literature discussing both conservative and invasive treatment strategies, both of which carry benefits. Further research is needed to better establish the etiology of BRP so that formal treatment guidelines may be established.
Neuropathic itch can be a frustrating condition for providers and patients, and many treatment modalities often are tried before arriving at a helpful treatment for a particular patient. Clinicians who may encounter BRP in practice benefit from up-to-date literature reviews that provide a summary of management strategies.
Brachioradial pruritus (BRP) is a neuropathic condition typically characterized by localized dysesthesia of the dorsolateral arms.1 This dysesthesia has been described as a persistent painful itching, burning, tingling, or stinging sensation2-4 and has a median duration of expression of 24 months.5,6 The condition may be unilateral or bilateral in nature but tends to have a predilection for a bilateral distribution along the C5 to C6 dermatomes.1,7,8 There are no primary skin lesions associated with BRP; however, excoriations, prurigo nodules, and lichenification may arise secondary to scratching of the irritated skin.1,4,5,9 Brachioradial pruritus tends to have a predilection for adult females (3:1 ratio) with lighter skin. The mean age at diagnosis is 59 years, but cases have been reported in patients aged 12 to 84 years.1,5 The diagnosis of BRP is based on clinical signs and symptoms, though the ice-pack sign tends to be pathognomonic for the diagnosis.10,11 Although there is no clear evidence on the exact cause of BRP, there are 2 prevalent theories: cervical radiculopathy secondary to cervical spine pathology and/or excessive exposure to UV radiation (UVR) in the summer months.3-5,12 Brachioradial pruritus remains poorly described in the literature, and even its origin is under debate. As such, the clinician may have difficulty deciding on the best course of management. The goal of this article is to identify and discuss known treatment options for BRP (Table).

Etiology
Cervical Spine Pathology—A correlation appears to exist between BRP and cervical spine changes seen on plain film radiographs at the levels of C3 to C7, with increased incidence at the C5 to C6 levels. These plain film radiographs typically show degenerative joint disease and neural foraminal stenosis at levels that correlate to the dermatomal distribution of BRP.1,7,10,12-14 In addition to plain film radiography, some studies have utilized magnetic resonance imaging to view the cervical spine and have documented evidence of intervertebral disc protrusion/bulging, central canal stenosis, neuroforaminal stenosis, and spondylosis at the affected regions.5,15-17 Moreover, supporting the theory that the cervical spine is responsible for the emergence of BRP, Marziniak et al17 investigated 41 patients with BRP utilizing magnetic resonance tomography to find that 33 patients (80.5%) had changes in nerve compression, and 8 patients (19.5%) had degenerative changes. In addition to these findings, they found that there was a significant correlation (P<.01) between the dermatomal expression of BRP and the location of cervical anatomical changes.17 Further validating the relationship between cervical spine pathology and BRP is a case study of a patient who saw rapid and complete resolution of the pruritus following spinal decompression surgery.10 Another case study described an intramedullary tumor found in a patient with BRP that was diagnosed as an ependymoma after magnetic resonance imaging revealed an intramedullary lesion within the spinal cord between C4 and C7. The location of the tumor and dermatomal pattern of the neuropathic itch pointed to a possible association between nerve compression and BRP.14 Electromyography studies performed on individuals with BRP have shown an increase in polyphasic units, decreased motor units, and/or denervation changes along the C5/C6 or C6 nerve roots, which provides additional support for the theory of cervical spine pathology as a causative factor for BRP.16
UVR Exposure—Another etiologic theory for BRP is that UVR exposure may be responsible for the genesis of pruritus. Previously known as solar pruritus, BRP was deemed a clinical condition, as there was increased prevalence in patients living in warmer climates, such as Florida.9 Wallengren and Dahlbäck18 reported that sun exposure is a notable factor in the onset of BRP, as they saw an increase in symptoms during the late summer and a decrease in symptoms over the winter months.To further support the theory that UVR is linked to BRP, several studies have shown that the utilization of sun protection is linked to a reduction of symptoms, specifically in patients who showed seasonal variations of their symptoms.9,12,19 Additionally, a study by Mirzoyev and Davis5 retrospectively reviewed 111 patients diagnosed with BRP. Of these patients, 84 (75.7%) presented with bilateral symptoms, and 54 (48.6%) reported prolonged sun exposure. Both of these findings demonstrate correlation between UVR and BRP.5 Interestingly, UV light exposure is known to release β-endorphin in the skin and may theoretically provide an area of exploration between UVR and cervical spine theories.
Conservative Treatment
Chiropractic Manipulation—Because one etiologic theory includes disease of the cervical spine, there is evidence that targeting this region with treatment is beneficial.7 Two case reports found in the literature noted that cervical spine manipulation and cervical traction yielded positive results.20,21 It has been established that pain generated by disc lesions can be the result of local nociceptive fiber activation, direct mechanical compression of the nerve roots, or inflammatory mediators.22 There are several postulated models describing the hypoalgesic effects of spinal manipulation, which contains both biomechanical and neurophysiological mechanisms. Biomechanical changes theorized to elicit analgesia include restoration of faulty biomechanical movement patterns, breaking up of periarticular adhesions, and reflexogenic muscle inhibition of hypertonic musculature. Hypothesized neurophysiological effects of joint manipulation include an increase in afferent information overwhelming the nociceptive input, reduction of temporal summation, and autonomic activation leading to non–opioid-induced hypoalgesia.23 Cervical traction is another plausible treatment for BRP, wherein the physiological effects of traction allow for a separation of vertebral bodies and expansion of the intervertebral foramen circumference, thus decreasing compression of the nerve roots.24
Acupuncture—Neurogenic pruritus, including BRP, is a group of conditions that have been treated using acupuncture. Acupuncture treatment consists of intramuscular needle stimulation and has been found to alleviate itching in patients with neurogenic pruritus. In 1 retrospective case series, acupuncture was used to treat 16 patients who were identified as having segmental pruritus. Acupuncture targeted the spasmed paravertebral muscles of the affected dermatomal levels as well as other regions of the body, and it was found that 12 patients (75%) experienced full resolution of symptoms. However, relapse did occur in 6 patients (37%) within 1 to 12 months following treatment.25 Multiple theories exist as to why acupuncture may help. One is that it relieves muscle spasms, which in turn relieves neural irritation of the spinal nerves as they traverse the respective paraspinal musculature. Another is that acupuncture decreases nociception by stimulating release of opioid peptides in the dorsal horn.26 A third proposed theory is that acupuncture acts on the afferent nerve fibers responsible for transmitting pain—Aδ and C fibers—activating these afferent nerves to produce an analgesic effect.27
Physiotherapy—The literature suggests that possible first-line therapies for neurogenic pruritus, including BRP and notalgia paresthetica, consist of noninvasive nondermatologic treatments that target cervical spine disease. Notalgia paresthetica and BRP have similar proposed mechanisms of nerve impingement; therefore, they often are grouped together when discussing proposed manual treatment options. Physiotherapy treatment includes cervical muscle strengthening, increased range of motion, application of cervical soft collars, massage, transcutaneous electronic nerve stimulation, and cervical traction.7 A study of 12 patients by Raison-Peyron et al28 in 1999 discussed the use of spinal and paraspinal ultrasound or radiation physiotherapy. Six patients underwent this treatment, and the symptoms subsided in 4 cases.28 Another study by Fleischer et al29 in 2011 discussed improvement in 2 patients with notalgia paresthetica by exercise involving active range of motion and strengthening.
Photoprotection—Avoidance of UVR exposure has been beneficial to some patients to reduce symptoms. Use of sunscreen and long-sleeved UV-protective clothing during outdoor activities or the warmer summer months may be beneficial.1
Medical Treatment
Medication—Because of the nonspecific clinical presentation of BRP, initial treatment often involves prescription of first-line antipruritic agents, including steroid creams and systemic antihistamines, both of which generally fail to provide symptom relief.1,30 Medications with neurologic mechanisms of action appear to provide potentially superior outcomes.
Topical interventions for BRP and related neurogenic pruritus have shown limited success. A case series evaluating capsaicin for pruritus offered only transient relief, likely because of its temporary hyperstimulatory and desensitizing effect on neuropeptides.7,33 In small populations, the use of topical antidepressants has yielded cutaneous and pathological relief for BRP. A case study of a 70-year-old woman evaluated the efficacy of a combination cream of ketamine and amitriptyline (a tricyclic antidepressant) yielding moderate pruritus improvement and notable improvement of secondary brachial skin lesions.34 Oral steroids also have shown success in the treatment of chronic pruritus; however, limited research is available on the efficacy of such medications for BRP, and the long-term use of oral steroids is limited by many side effects.30
Interventional Pain Procedure—A 2018 case series investigated 3 patients with a clinical diagnosis of BRP who were treated between 2010 and 2016 with
Surgery—There are multiple case studies in the literature that discuss
Conclusion
The pathogenesis of BRP continues to be an area of debate—it may be secondary to cervical spine disease or UVR. This review found there is more research pointing to cervical spine disease. There is an abundance of literature discussing both conservative and invasive treatment strategies, both of which carry benefits. Further research is needed to better establish the etiology of BRP so that formal treatment guidelines may be established.
Neuropathic itch can be a frustrating condition for providers and patients, and many treatment modalities often are tried before arriving at a helpful treatment for a particular patient. Clinicians who may encounter BRP in practice benefit from up-to-date literature reviews that provide a summary of management strategies.
- Robbins BA, Schmieder GJ. Brachioradial pruritus. StatPearls Publishing; 2020. Updated September 12, 2022. Accessed July 25, 2023. https://www.ncbi.nlm.nih.gov/books/NBK459321/
- Crevits L. Brachioradial pruritus—a peculiar neuropathic disorder. Clin Neurol Neurosurg. 2006;108:803-805.
- Lane J, McKenzie J, Spiegel J. Brachioradial pruritus: a case report and review of the literature. Cutis. 2008;81:37-40.
- Wallengren J. Brachioradial pruritus: a recurrent solar dermopathy. J Am Acad Dermatol. 1998;39:803-806.
- Mirzoyev S, Davis M. Brachioradial pruritus: Mayo Clinic experience over the past decade. Br J Dermatol. 2013;169:1007-1015.
- Pinto AC, Wachholz PA, Masuda PY, et al. Clinical, epidemiological and therapeutic profile of patients with brachioradial pruritus in a reference service in dermatology. An Bras Dermatol. 2016;91:549-551. doi:10.1590/abd1806-4841.201644767
- Alai NN, Skinner HB. Concurrent notalgia paresthetica and brachioradial pruritus associated with cervical degenerative disc disease. Cutis. 2018;102:185, 186, 189, 190.
- Atis¸ G, Bilir Kaya B. Pregabalin treatment of three cases with brachioradial pruritus. Dermatol Ther. 2017;30:e12459.
- Waisman M. Solar pruritus of the elbows (brachioradial summer pruritus). Arch Dermatol. 1968;98:481-485.
- Binder A, Fölster-Holst R, Sahan G, et al. A case of neuropathic brachioradial pruritus caused by cervical disc herniation. Nat Clin Pract Neurol. 2008;4:338-342.
- Bernhard JD, Bordeaux JS. Medical pearl: the ice-pack sign in brachioradial pruritus. J Am Acad Dermatol. 2005;52:1073.
- Veien N, Laurberg G. Brachioradial pruritus: a follow-up of 76 patients. Acta Derm Venereol. 2011;91:183-185.
- Mataix J, Silvestre JF, Climent JM, et al. Brachioradial pruritus as a symptom of cervical radiculopathy. Article in Spanish. Actas Dermosifiliogr. 2008;99:719-722.
- Kavak A, Dosoglu M. Can a spinal cord tumor cause brachioradial pruritus? J Am Acad Dermatol. 2002;46:437-440.
- Zeidler C, Pereira MP, Ständer S. Brachioradial pruritus successfully treated with intravenous naloxone. J Eur Acad Dermatol Venereol. 2023;37:e87-e89. doi:10.1111/jdv.18553
- Shields LB, Iyer VG, Zhang Y, et al. Brachioradial pruritus: clinical, electromyographic, and cervical MRI features in nine patients. Cureus. 2022;14:e21811. doi:10.7759/cureus.21811
- Marziniak M, Phan NQ, Raap U, et al. Brachioradial pruritus as a result of cervical spine pathology: the results of a magneticresonance tomography study. J Am Acad Dermatol. 2011;65:756-762. doi:10.1016/j.jaad.2010.07.036
- Wallengren J, Dahlbäck K. Familial brachioradial pruritus. Br J Dermatol. 2005;153:1016-1018.
- Salzmann SN, Okano I, Shue J, et al. Disabling pruritus in a patient with cervical stenosis. J Am Acad Orthop Surg Glob Res Rev. 2020;4:e19.00178. doi:10.5435/JAAOSGlobal-D-19-00178
- Golden KJ, Diana RM. A case of brachioradial pruritus treated with chiropractic and acupuncture. Case Rep Dermatol. 2022;14:93-97. doi:10.1159/000524054
- Tait CP, Grigg E, Quirk CJ. Brachioradial pruritus and cervical spine manipulation. Australas J Dermatol. 1998;39:168-170. doi:10.1111/j.1440-0960.1998.tb01274.x
- Freynhagen R, Baron R. The evaluation of neuropathic components in low back pain. Curr Pain Headache Rep. 2009;13:185-190. doi:10.1007/s11916-009-0032-y
- Gyer G, Michael J, Inklebarger J, et al. Spinal manipulation therapy: is it all about the brain? A current review of the neurophysiological effects of manipulation. J Integr Med. 2019;17:328-337. doi:10.1016/j.joim.2019.05.004
- Graham N, Gross A, Goldsmith CH, et al. Mechanical traction for neck pain with or without radiculopathy. Cochrane Database Syst Rev. 2008:CD006408. doi:10.1002/14651858.CD006408.pub2
- Stellon A. Neurogenic pruritus: an unrecognised problem? A retrospective case series of treatment by acupuncture. Acupunct Med. 2002;20:186-190. doi:10.1136/aim.20.4.186
- Bowsher D. Mechanisms of acupuncture. In: Filshie J, White A, eds. Medical Acupuncture: A Western Scientific Approach. Churchill Livingstone; 1998:69-82.
- Lim TK, Ma Y, Berger F, et al. Acupuncture and neural mechanism in the management of low back pain-an update. Medicines (Basel). 2018;5:63.
- Raison-Peyron N, Meunier L, Acevedo M, et al. Notalgia paresthetica: clinical, physiopathological and therapeutic aspects. a study of 12 cases. J Eur Acad Dermatol Venereol. 1999;12:215-221.
- Fleischer AB, Meade TJ, Fleischer AB. Notalgia paresthetica: successful treatment with exercises. Acta Derm Venereol. 2011;91:356-357. doi:10.2340/00015555-1039
- Kouwenhoven TA, van de Kerkhof PCM, Kamsteeg M. Use of oral antidepressants in patients with chronic pruritus: a systematic review. J Am Acad Dermatol. 2017;77:1068-1073.e7. doi:10.1016/j.jaad.2017.08.025
- Matsuda KM, Sharma D, Schonfeld AR, et al. Gabapentin and pregabalin for the treatment of chronic pruritus. J Am Acad Dermatol. 2016;75:619-625.e6. doi:10.1016/j.jaad.2016.02.1237
- Okuno S, Hashimoto T, Satoh T. Case of neuropathic itch-associated prurigo nodules on the bilateral upper arms after unilateral herpes zoster in a patient with cervical herniated discs: successful treatment with mirogabalin. J Dermatol. 2021;48:e585-e586.
- Papoiu AD, Yosipovitch G. Topical capsaicin. The fire of a ‘hot’ medicine is reignited. Expert Opin Pharmacother. 2010;11:1359-1371. doi:10.1517/14656566.2010.481670
- Magazin M, Daze RP, Okeson N. Treatment refractory brachioradial pruritus treated with topical amitriptyline and ketamine. Cureus. 2019;11:e5117. doi:10.7759/cureus.5117
- Weinberg BD, Amans M, Deviren S, et al. Brachioradial pruritus treated with computed tomography-guided cervical nerve root block: a case series. JAAD Case Rep. 2018;4:640-644. doi:10.1016/j.jdcr.2018.03.025
- De Ridder D, Hans G, Pals P, et al. A C-fiber-mediated neuropathic brachioradial pruritus. J Neurosurg. 2010;113:118-121. doi:10.3171/2009.9.JNS09620
- Morosanu CO, Etim G, Alalade AF. Brachioradial pruritus secondary to cervical disc protrusion—a case report. J Surg Case Rep. 2022:rjac277. doi:10.1093/jscr/rjac277
- Robbins BA, Schmieder GJ. Brachioradial pruritus. StatPearls Publishing; 2020. Updated September 12, 2022. Accessed July 25, 2023. https://www.ncbi.nlm.nih.gov/books/NBK459321/
- Crevits L. Brachioradial pruritus—a peculiar neuropathic disorder. Clin Neurol Neurosurg. 2006;108:803-805.
- Lane J, McKenzie J, Spiegel J. Brachioradial pruritus: a case report and review of the literature. Cutis. 2008;81:37-40.
- Wallengren J. Brachioradial pruritus: a recurrent solar dermopathy. J Am Acad Dermatol. 1998;39:803-806.
- Mirzoyev S, Davis M. Brachioradial pruritus: Mayo Clinic experience over the past decade. Br J Dermatol. 2013;169:1007-1015.
- Pinto AC, Wachholz PA, Masuda PY, et al. Clinical, epidemiological and therapeutic profile of patients with brachioradial pruritus in a reference service in dermatology. An Bras Dermatol. 2016;91:549-551. doi:10.1590/abd1806-4841.201644767
- Alai NN, Skinner HB. Concurrent notalgia paresthetica and brachioradial pruritus associated with cervical degenerative disc disease. Cutis. 2018;102:185, 186, 189, 190.
- Atis¸ G, Bilir Kaya B. Pregabalin treatment of three cases with brachioradial pruritus. Dermatol Ther. 2017;30:e12459.
- Waisman M. Solar pruritus of the elbows (brachioradial summer pruritus). Arch Dermatol. 1968;98:481-485.
- Binder A, Fölster-Holst R, Sahan G, et al. A case of neuropathic brachioradial pruritus caused by cervical disc herniation. Nat Clin Pract Neurol. 2008;4:338-342.
- Bernhard JD, Bordeaux JS. Medical pearl: the ice-pack sign in brachioradial pruritus. J Am Acad Dermatol. 2005;52:1073.
- Veien N, Laurberg G. Brachioradial pruritus: a follow-up of 76 patients. Acta Derm Venereol. 2011;91:183-185.
- Mataix J, Silvestre JF, Climent JM, et al. Brachioradial pruritus as a symptom of cervical radiculopathy. Article in Spanish. Actas Dermosifiliogr. 2008;99:719-722.
- Kavak A, Dosoglu M. Can a spinal cord tumor cause brachioradial pruritus? J Am Acad Dermatol. 2002;46:437-440.
- Zeidler C, Pereira MP, Ständer S. Brachioradial pruritus successfully treated with intravenous naloxone. J Eur Acad Dermatol Venereol. 2023;37:e87-e89. doi:10.1111/jdv.18553
- Shields LB, Iyer VG, Zhang Y, et al. Brachioradial pruritus: clinical, electromyographic, and cervical MRI features in nine patients. Cureus. 2022;14:e21811. doi:10.7759/cureus.21811
- Marziniak M, Phan NQ, Raap U, et al. Brachioradial pruritus as a result of cervical spine pathology: the results of a magneticresonance tomography study. J Am Acad Dermatol. 2011;65:756-762. doi:10.1016/j.jaad.2010.07.036
- Wallengren J, Dahlbäck K. Familial brachioradial pruritus. Br J Dermatol. 2005;153:1016-1018.
- Salzmann SN, Okano I, Shue J, et al. Disabling pruritus in a patient with cervical stenosis. J Am Acad Orthop Surg Glob Res Rev. 2020;4:e19.00178. doi:10.5435/JAAOSGlobal-D-19-00178
- Golden KJ, Diana RM. A case of brachioradial pruritus treated with chiropractic and acupuncture. Case Rep Dermatol. 2022;14:93-97. doi:10.1159/000524054
- Tait CP, Grigg E, Quirk CJ. Brachioradial pruritus and cervical spine manipulation. Australas J Dermatol. 1998;39:168-170. doi:10.1111/j.1440-0960.1998.tb01274.x
- Freynhagen R, Baron R. The evaluation of neuropathic components in low back pain. Curr Pain Headache Rep. 2009;13:185-190. doi:10.1007/s11916-009-0032-y
- Gyer G, Michael J, Inklebarger J, et al. Spinal manipulation therapy: is it all about the brain? A current review of the neurophysiological effects of manipulation. J Integr Med. 2019;17:328-337. doi:10.1016/j.joim.2019.05.004
- Graham N, Gross A, Goldsmith CH, et al. Mechanical traction for neck pain with or without radiculopathy. Cochrane Database Syst Rev. 2008:CD006408. doi:10.1002/14651858.CD006408.pub2
- Stellon A. Neurogenic pruritus: an unrecognised problem? A retrospective case series of treatment by acupuncture. Acupunct Med. 2002;20:186-190. doi:10.1136/aim.20.4.186
- Bowsher D. Mechanisms of acupuncture. In: Filshie J, White A, eds. Medical Acupuncture: A Western Scientific Approach. Churchill Livingstone; 1998:69-82.
- Lim TK, Ma Y, Berger F, et al. Acupuncture and neural mechanism in the management of low back pain-an update. Medicines (Basel). 2018;5:63.
- Raison-Peyron N, Meunier L, Acevedo M, et al. Notalgia paresthetica: clinical, physiopathological and therapeutic aspects. a study of 12 cases. J Eur Acad Dermatol Venereol. 1999;12:215-221.
- Fleischer AB, Meade TJ, Fleischer AB. Notalgia paresthetica: successful treatment with exercises. Acta Derm Venereol. 2011;91:356-357. doi:10.2340/00015555-1039
- Kouwenhoven TA, van de Kerkhof PCM, Kamsteeg M. Use of oral antidepressants in patients with chronic pruritus: a systematic review. J Am Acad Dermatol. 2017;77:1068-1073.e7. doi:10.1016/j.jaad.2017.08.025
- Matsuda KM, Sharma D, Schonfeld AR, et al. Gabapentin and pregabalin for the treatment of chronic pruritus. J Am Acad Dermatol. 2016;75:619-625.e6. doi:10.1016/j.jaad.2016.02.1237
- Okuno S, Hashimoto T, Satoh T. Case of neuropathic itch-associated prurigo nodules on the bilateral upper arms after unilateral herpes zoster in a patient with cervical herniated discs: successful treatment with mirogabalin. J Dermatol. 2021;48:e585-e586.
- Papoiu AD, Yosipovitch G. Topical capsaicin. The fire of a ‘hot’ medicine is reignited. Expert Opin Pharmacother. 2010;11:1359-1371. doi:10.1517/14656566.2010.481670
- Magazin M, Daze RP, Okeson N. Treatment refractory brachioradial pruritus treated with topical amitriptyline and ketamine. Cureus. 2019;11:e5117. doi:10.7759/cureus.5117
- Weinberg BD, Amans M, Deviren S, et al. Brachioradial pruritus treated with computed tomography-guided cervical nerve root block: a case series. JAAD Case Rep. 2018;4:640-644. doi:10.1016/j.jdcr.2018.03.025
- De Ridder D, Hans G, Pals P, et al. A C-fiber-mediated neuropathic brachioradial pruritus. J Neurosurg. 2010;113:118-121. doi:10.3171/2009.9.JNS09620
- Morosanu CO, Etim G, Alalade AF. Brachioradial pruritus secondary to cervical disc protrusion—a case report. J Surg Case Rep. 2022:rjac277. doi:10.1093/jscr/rjac277
Practice Points
- The etiology of brachioradial pruritus (BRP) has been associated with cervical spine pathology and/or UV radiation exposure.
- Treatment options for BRP range from conservative to invasive, and clinicians should consider the evidence for all options to decide what is best for each patient.
Pigmenting Purpuric Dermatoses: Striking But Not a Manifestation of COVID-19 Infection
Pigmented purpuric dermatoses (PPDs) are characterized by petechiae, dusky macules representative of postinflammatory hyperpigmentation and dermal hemosiderin, and purpura generally localized to the lower extremities. They typically represent a spectrum of lymphocytic capillaritis, variable erythrocyte extravasation from papillary dermal blood vessels, and deposition of hemosiderin, yielding the classic red to orange to golden-brown findings on gross examination. Clinical overlap exists, but variants include Schamberg disease (SD), Majocchi purpura, Gougerot-Blum purpura, eczematoid purpura of Doucas and Kapetanakis (DK), and lichen aureus.1 Other forms are rarer, including linear, granulomatous, quadrantic, transitory, and familial variants. It remains controversial whether PPD may precede or have an association with cutaneous T-cell lymphoma.2 Dermoscopy usually shows copper-red pigmentation in the background, oval red dots, linear vessels, brown globules, and follicular openings. Although these findings may be useful in PPD diagnosis, they are not applicable in differentiating among the variants.
Pigmented purpuric dermatoses can easily be mistaken for stasis dermatitis or cellulitis, as these may occur concomitantly or in populations at risk for all 3 conditions, such as women older than 50 years with recent trauma or infection in the affected area. Tissue biopsy and clinical laboratory evaluation may be required to differentiate between PPD from leukocytoclastic vasculitis or the myriad causes of retiform purpura. Importantly, clinicians also should differentiate PPD from the purpuric eruptions of the lower extremities associated with COVID-19 infection.
Pigmented Purpuric Dermatoses
Schamberg Disease—In 1901, Jay Frank Schamberg, a distinguished professor of dermatology in Philadelphia, Pennsylvania, described “a peculiar progressive pigmentary disease of the skin” in a 15-year-old adolescent boy.3 Schamberg disease is the most common PPD, characterized by pruritic spots resembling cayenne pepper (Figure 1) with orange-brown pigmented macules on the legs and feet.4 Although platelet dysfunction, coagulation deficiencies, or dermal atrophy may contribute to hemorrhaging that manifests as petechiae or ecchymoses, SD typically is not associated with any laboratory abnormalities, and petechial eruption is not widespread.5 Capillary fragility can be assessed by the tourniquet test, in which pressure is applied to the forearm with a blood pressure cuff inflated between systolic and diastolic blood pressure for 5 to 10 minutes. Upon removing the cuff, a positive test is indicated by 15 or more petechiae in an area 5 cm in diameter due to poor platelet function. A positive result may be seen in SD.6
Histologically, SD is characterized by patchy parakeratosis, mild spongiosis of the stratum Malpighi, and lymphoid capillaritis (Figure 2).7 In addition to CD3+, CD4+, CD8+, CD1a+, and CD36+ lymphocytes, histology also may contain dendritic cells and cellular adhesion molecules (intercellular adhesion molecule 1, epithelial cell adhesion molecule 1) within the superficial perivascular infiltrate.8 There is no definitive therapy, but first-line interventions include emollients, topical steroids, and oral antihistamines. Nonpharmacologic management includes compression or support stockings, elevation of the lower extremities, and avoidance of offending medications (if identifiable).1
Majocchi Purpura—Domenico Majocchi was a renowned Italian dermatologist who described an entity in 1898 that he called purpura annularis telangiectodes, now also known as Majocchi purpura.9 It is more common in females, young adults, and children. Majocchi purpura has rarely been reported in families with a possible autosomal-dominant inheritance.10 Typically, bluish-red annular macules with central atrophy surrounded by hyperpigmentation may be seen on the lower extremities, potentially extending to the upper extremities.1 Treatment of Majocchi purpura remains a challenge but may respond to narrowband UVB phototherapy. Emollients and topical steroids also are used as first-line treatments. Biopsy demonstrates telangiectasia, pericapillary infiltration of mononuclear lymphocytes, and papillary dermal hemosiderin.11
Gougerot-Blum Purpura—In 1925, French dermatologists Henri Gougerot and Paul Blum described a pigmented purpuric lichenoid dermatitis known as Gougerot-Blum purpura,12 a rare PPD characterized by lichenoid papules that eventually coalesce into plaques of various colors, along with red-brown hyperpigmentation.4 As with other PPD variants, the legs are most involved, with rare extension to the trunk or thighs. The plaques may resemble and be mistaken for Kaposi sarcoma, cutaneous vasculitis, traumatic purpura, or mycosis fungoides. Dermoscopic examination reveals small, polygonal or round, red dots underlying brown scaly patches.13 Gougerot-Blum purpura is found more commonly in adult men and rarely affects children.4 Histologically, a lichenoid and superficial perivascular infiltrate composed of lymphocytes and macrophages is seen. Various therapies have been described, including topical steroids, antihistamines, psoralen plus UVA phototherapy, and cyclosporin A.14
Eczematoid Purpura of Doucas and Kapetanakis—In 1949, Greek dermatologists Christopher Doucas and John Kapetanakis observed several cases of purpuric dermatosis similar in form to the “pigmented purpuric lichenoid dermatitis” of Gougerot-Blum purpura12 and to the “progressive pigmentary dermatitis” of Schamberg disease.3 After observing a gradual disappearance of the classic yellow color from hemosiderin deposition, Doucas and Kapetanakis described a new bright red eruption with lichenification.15 Eczematoid purpura of Doucas and Kapetanakis is rare and predominantly seen in middle-aged males. Hyperpigmented or dark brown macules may develop bilaterally on the legs, progressing to the thighs and upper extremities. Unlike the other types of PPD, DK is extensive and severely pruritic.4
Although most PPD can be drug induced, DK has shown the greatest tendency for pruritic erythematous plaques following drug usage including but not limited to amlodipine, aspirin, acetaminophen, thiamine, interferon alfa, chlordiazepoxide, and isotretinoin. Additionally, DK has been associated with a contact allergy to clothing dyes and rubber.4 On histology, epidermal spongiosis may be seen, correlating with the eczematoid clinical findings. Spontaneous remission also is more common compared to the other PPDs. Treatment consists of topical corticosteroids and antihistamines.16
Lichen Aureus—Lichen aureus was first observed by the dermatologist R.H. Martin in 1958.17 It is clinically characterized by closely aggregated purpuric papules with a distinctive golden-brown color more often localized to the lower extremities and sometimes in a dermatomal distribution. Lichen aureus affects males and females equally, and similar to Majocchi purpura can be seen in children.4 Histopathologic examination reveals a prominent lichenoid plus superficial and deep perivascular lymphocytic infiltrate, extravasated erythrocytes, papillary dermal edema, hemosiderophages, and an unaffected epidermis. In rare cases, perineural infiltrates may be seen. Topical steroids usually are ineffective in lichen aureus treatment, but responses to psoralen plus UVA therapy also have been noted.17
Differential Diagnosis
COVID-19–Related Cutaneous Changes—Because COVID-19–related pathology is now a common differential diagnosis for many cutaneous eruptions,one must be mindful of the possibility for patients to have PPD, cutaneous changes from underlying COVID-19, or both.18 The microvascular changes from COVID-19 infection can be variable.19 Besides the presence of erythema along a distal digit, manifestations can include reticulated dusky erythema mimicking livedoid vasculopathy or inflammatory purpura.19
Retiform Purpura—Retiform purpura may occur in the setting of microvascular occlusion and can represent the pattern of underlying dermal vasculature. It is nonblanching and typically stellate or branching.20 The microvascular occlusion may be a result of hypercoagulability or may be secondary to cutaneous vasculitis, resulting in thrombosis and subsequent vascular occlusion.21 There are many reasons for hypercoagulability in retiform purpura, including disseminated intravascular coagulation in the setting of COVID-19 infection.22 The treatment of retiform purpura is aimed at alleviating the underlying cause and providing symptomatic relief. Conversely, the PPDs generally are benign and require minimal workup.
Leukocytoclastic Vasculitis—The hallmark of leukocytoclastic vasculitis is palpable purpura, often appearing as nonblanchable papules, typically in a dependent distribution such as the lower extremities (Figure 3). Although it primarily affects children, Henoch-Schönlein purpura is a type of leukocytoclastic vasculitis with lesions potentially similar in appearance to those of PPD.23 Palpable purpura may be painful and may ulcerate but rarely is pruritic. Leukocytoclastic vasculitis represents perivascular infiltrates composed of neutrophils, lymphocytes, and occasionally eosinophils, along with karyorrhexis, luminal fibrin, and fibrinoid degeneration of blood vessel walls, often resulting from immune complex deposition. Leukocytoclastic vasculitis may affect blood vessels of any size and requires further clinical and laboratory evaluation for infection (including COVID-19), hypercoagulability, autoimmune disease, or medication-related reactions.24
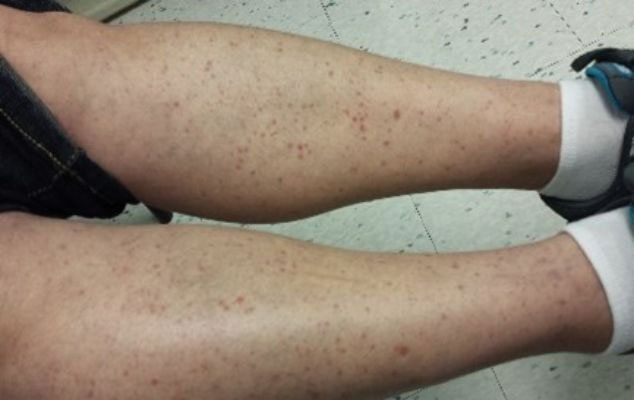
Stasis Dermatitis—Stasis dermatitis, a chronic inflammatory condition stemming from retrograde venous flow due to incompetent venous valves, mimics PPD. Stasis dermatitis initially appears as demarcated erythematous plaques, fissures, and scaling of the lower legs bilaterally, usually involving the medial malleolus.25 With time, the affected region develops overlying brawny hyperpigmentation and fibrosis (Figure 4). Pruritus or pain are common features, while fissures and superficial erosions may heal and recur, leading to lichenification.
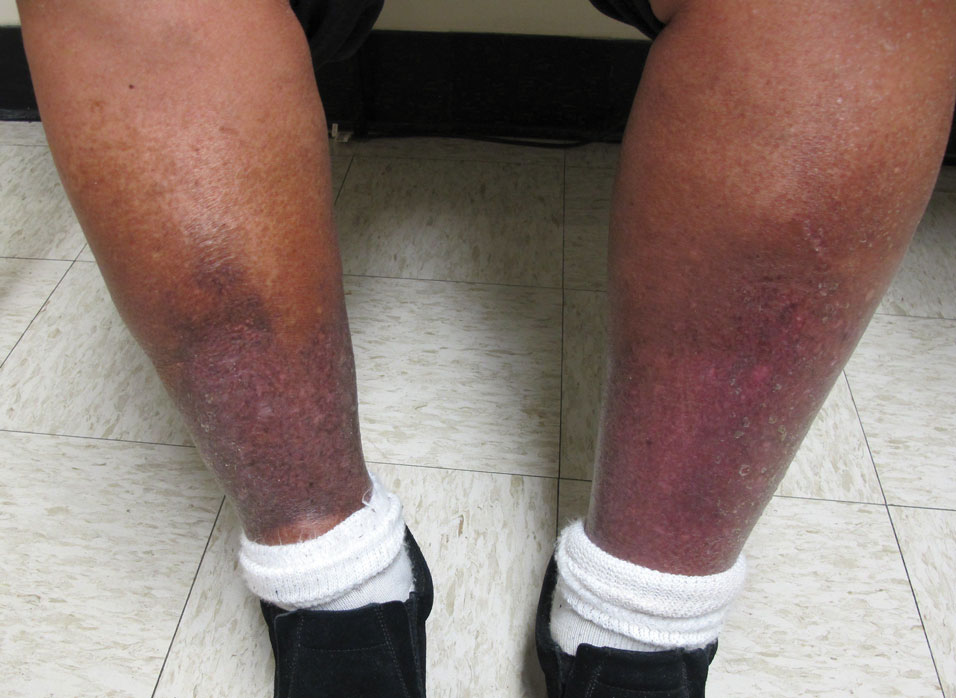
Although both commonly appear on the lower extremities, duplex ultrasonography may be helpful to distinguish PPDs from stasis dermatitis since the latter occurs in the context of chronic venous insufficiency, varicose veins, soft tissue edema, and lymphedema.25 Additionally, pruritus, lichenification, and edema often are not seen in most PPD variants, although stasis dermatitis and PPD may occur in tandem. Conservative treatment involves elevation of the extremities, compression, and topical steroids for symptomatic relief.
Cellulitis—The key characteristics of cellulitis are redness, swelling, warmth, tenderness, fever, and leukocytosis. A history of trauma, such as a prior break in the skin, and pain in the affected area suggest cellulitis. Several skin conditions present similarly to cellulitis, including PPD, and thus approximately 30% of cases are misdiagnosed.26 Cellulitis rarely presents in a bilateral or diffusely scattered pattern as seen in PPDs. Rather, it is unilateral with smooth indistinct borders. Variables suggestive of cellulitis include immunosuppression, rapid progression, and previous occurrences. Hyperpigmented plaques or thickening of the skin are more indicative of a chronic process such as stasis dermatitis or lipodermatosclerosis rather than acute cellulitis. Purpura is not a typical finding in most cases of soft tissue cellulitis. Treatment may be case specific depending on severity, presence or absence of sepsis, findings on blood cultures, or other pathologic evaluation. Antibiotics are directed to the causative organism, typically Streptococcus and Staphylococcus species, although coverage against various gram-negative organisms may be indicated.27
Caution With Teledermatology
COVID-19 has established the value of telemedicine in providing access to health care services for at-risk or underserved individuals. The PPDs are benign, often asymptomatic, and potentially identifiable with teledermatology alone; however, they also can easily be mistaken for COVID-19–related eruptions, vasculitis, other types of purpura, stasis dermatitis, or other complications of lower extremity stasis and lymphedema, especially in an aging population. If tissue biopsy is required, as in the workup of vasculitis, the efficacy of telemedicine becomes more questionable. It is important to delineate the potentially confusing PPDs from other potentially dangerous or life-threatening inflammatory dermatoses.28
- Sardana K, Sarkar R , Sehgal VN. Pigmented purpuric dermatoses: an overview. Int J Dermatol. 2004;43:482-488.
- Çaytemel C, Baykut B, Ag˘ırgöl S¸, et al. Pigmented purpuric dermatosis: ten years of experience in a tertiary hospital and awareness of mycosis fungoides in differential diagnosis. J Cutan Pathol. 2021;48:611-616.
- Schamberg JF. A peculiar progressive pigmentary disease of the skin. Br J Dermatol. 1901;13:1-5.
- Martínez Pallás I, Conejero Del Mazo R, Lezcano Biosca V. Pigmented purpuric dermatosis: a review of the literature. Actas Dermosifiliogr (Engl Ed). 2020;111:196-204.
- Ozkaya DB, Emiroglu N, Su O, et al. Dermatoscopic findings of pigmented purpuric dermatosis. An Bras Dermatol. 2016;91:584-587.
- Lava SAG, Milani GP, Fossali EF, et al. Cutaneous manifestations of small-vessel leukocytoclastic vasculitides in childhood. Clin Rev Allergy Immunol. 2017;53:439-451.
- Bonnet U, Selle C, Isbruch K, et al. Recurrent purpura due to alcohol-related Schamberg’s disease and its association with serum immunoglobulins: a longitudinal observation of a heavy drinker. J Med Case Rep. 2016;10:301.
- Zaldivar Fujigaki JL, Anjum F. Schamberg Disease. StatPearls Publishing; 2021.
- Majocchi J. Purpura annularis telangiectodes. Arch Dermatol Syph. 1898;43:447.
- Sethuraman G, Sugandhan S, Bansal A, et al. Familial pigmented purpuric dermatoses. J Dermatol. 2006;33:639-641.
- Miller K, Fischer M, Kamino H, et al. Purpura annularis telangiectoides. Dermatol Online J. 2012;18:5.
- Coulombe J, Jean SE, Hatami A, et al. Pigmented purpuric dermatosis: clinicopathologic characterization in a pediatric series. Pediatr Dermatol. 2015;32:358-362.
- Park MY, Shim WH, Kim JM, et al. Dermoscopic finding in pigmented purpuric lichenoid dermatosis of Gougerot-Blum: a useful tool for clinical diagnosis. Ann Dermatol. 2018;30:245-247.
- Risikesan J, Sommerlund M, Ramsing M, et al. Successful topical treatment of pigmented purpuric lichenoid dermatitis of Gougerot-Blum in a young patient: a case report and summary of the most common pigmented purpuric dermatoses. Case Rep Dermatol. 2017;9:169-176.
- Doucas C, Kapetanakis J. Eczematid-like purpura. Dermatologica. 1953;106:86-95.
- Kim DH, Seo SH, Ahn HH, et al. Characteristics and clinical manifestations of pigmented purpuric dermatosis. Ann Dermatol. 2015;27:404-410.
- Aung PP, Burns SJ, Bhawan J. Lichen aureus: an unusual histopathological presentation: a case report and a review of literature. Am J Dermatopathol. 2014;36:E1-E4.
- Singh P, Schwartz RA. Disseminated intravascular coagulation: a devastating systemic disorder of special concern with COVID-19. Dermatol Ther. 2020;33:E14053.
- Almutairi N, Schwartz RA. COVID-19 with dermatologic manifestations and implications: an unfolding conundrum. Dermatol Ther. 2020;33:E13544.
- Georgesen C, Fox LP, Harp J. Retiform purpura: a diagnostic approach. J Am Acad Dermatol. 2020;82:783-796.
- Torregrosa Calatayud JL, Garcías Ladaria J, De Unamuno Bustos B, et al. Retiform purpura caused by the use of cocaine, that was probably adulterated with levamisole. Ann Dermatol. 2015;27:117-119.
- Keim CK, Schwartz RA, Kapila R. Levamisole-induced and COVID-19-induced retiform purpura: two overlapping, emerging clinical syndromes. Arch Dermatol Res. 2021;22:1-9.
- González LM, Janniger CK, Schwartz RA. Pediatric Henoch-Schönlein purpura. Int J Dermatol. 2009;48:1157-1165.
- Yıldırım Bay E, Moustafa E, Semiz Y, et al. Leukocytoclastic vasculitis secondary to COVID-19 infection presenting with inclusion bodies: a histopathological correlation. J Cosmet Dermatol. 2022;21:27-29.
- Sundaresan S, Migden MR, Silapunt S. Stasis dermatitis: pathophysiology, evaluation, and management. Am J Clin Dermatol. 2017;18:383-390.
- Hirschmann JV, Raugi GJ. Lower limb cellulitis and its mimics: part I. lower limb cellulitis. J Am Acad Dermatol. 2012;67:163.E1-E12; quiz 75-76.
- Keller EC, Tomecki KJ, Alraies MC. Distinguishing cellulitis from its mimics. Cleveland Clin J Med. 2012;79:547-552.
- Georgesen C, Fox LP, Harp J. Retiform purpura: workup and therapeutic considerations in select conditions. J Am Acad Dermatol. 2020;82:799-816.
Pigmented purpuric dermatoses (PPDs) are characterized by petechiae, dusky macules representative of postinflammatory hyperpigmentation and dermal hemosiderin, and purpura generally localized to the lower extremities. They typically represent a spectrum of lymphocytic capillaritis, variable erythrocyte extravasation from papillary dermal blood vessels, and deposition of hemosiderin, yielding the classic red to orange to golden-brown findings on gross examination. Clinical overlap exists, but variants include Schamberg disease (SD), Majocchi purpura, Gougerot-Blum purpura, eczematoid purpura of Doucas and Kapetanakis (DK), and lichen aureus.1 Other forms are rarer, including linear, granulomatous, quadrantic, transitory, and familial variants. It remains controversial whether PPD may precede or have an association with cutaneous T-cell lymphoma.2 Dermoscopy usually shows copper-red pigmentation in the background, oval red dots, linear vessels, brown globules, and follicular openings. Although these findings may be useful in PPD diagnosis, they are not applicable in differentiating among the variants.
Pigmented purpuric dermatoses can easily be mistaken for stasis dermatitis or cellulitis, as these may occur concomitantly or in populations at risk for all 3 conditions, such as women older than 50 years with recent trauma or infection in the affected area. Tissue biopsy and clinical laboratory evaluation may be required to differentiate between PPD from leukocytoclastic vasculitis or the myriad causes of retiform purpura. Importantly, clinicians also should differentiate PPD from the purpuric eruptions of the lower extremities associated with COVID-19 infection.
Pigmented Purpuric Dermatoses
Schamberg Disease—In 1901, Jay Frank Schamberg, a distinguished professor of dermatology in Philadelphia, Pennsylvania, described “a peculiar progressive pigmentary disease of the skin” in a 15-year-old adolescent boy.3 Schamberg disease is the most common PPD, characterized by pruritic spots resembling cayenne pepper (Figure 1) with orange-brown pigmented macules on the legs and feet.4 Although platelet dysfunction, coagulation deficiencies, or dermal atrophy may contribute to hemorrhaging that manifests as petechiae or ecchymoses, SD typically is not associated with any laboratory abnormalities, and petechial eruption is not widespread.5 Capillary fragility can be assessed by the tourniquet test, in which pressure is applied to the forearm with a blood pressure cuff inflated between systolic and diastolic blood pressure for 5 to 10 minutes. Upon removing the cuff, a positive test is indicated by 15 or more petechiae in an area 5 cm in diameter due to poor platelet function. A positive result may be seen in SD.6

Histologically, SD is characterized by patchy parakeratosis, mild spongiosis of the stratum Malpighi, and lymphoid capillaritis (Figure 2).7 In addition to CD3+, CD4+, CD8+, CD1a+, and CD36+ lymphocytes, histology also may contain dendritic cells and cellular adhesion molecules (intercellular adhesion molecule 1, epithelial cell adhesion molecule 1) within the superficial perivascular infiltrate.8 There is no definitive therapy, but first-line interventions include emollients, topical steroids, and oral antihistamines. Nonpharmacologic management includes compression or support stockings, elevation of the lower extremities, and avoidance of offending medications (if identifiable).1

Majocchi Purpura—Domenico Majocchi was a renowned Italian dermatologist who described an entity in 1898 that he called purpura annularis telangiectodes, now also known as Majocchi purpura.9 It is more common in females, young adults, and children. Majocchi purpura has rarely been reported in families with a possible autosomal-dominant inheritance.10 Typically, bluish-red annular macules with central atrophy surrounded by hyperpigmentation may be seen on the lower extremities, potentially extending to the upper extremities.1 Treatment of Majocchi purpura remains a challenge but may respond to narrowband UVB phototherapy. Emollients and topical steroids also are used as first-line treatments. Biopsy demonstrates telangiectasia, pericapillary infiltration of mononuclear lymphocytes, and papillary dermal hemosiderin.11
Gougerot-Blum Purpura—In 1925, French dermatologists Henri Gougerot and Paul Blum described a pigmented purpuric lichenoid dermatitis known as Gougerot-Blum purpura,12 a rare PPD characterized by lichenoid papules that eventually coalesce into plaques of various colors, along with red-brown hyperpigmentation.4 As with other PPD variants, the legs are most involved, with rare extension to the trunk or thighs. The plaques may resemble and be mistaken for Kaposi sarcoma, cutaneous vasculitis, traumatic purpura, or mycosis fungoides. Dermoscopic examination reveals small, polygonal or round, red dots underlying brown scaly patches.13 Gougerot-Blum purpura is found more commonly in adult men and rarely affects children.4 Histologically, a lichenoid and superficial perivascular infiltrate composed of lymphocytes and macrophages is seen. Various therapies have been described, including topical steroids, antihistamines, psoralen plus UVA phototherapy, and cyclosporin A.14
Eczematoid Purpura of Doucas and Kapetanakis—In 1949, Greek dermatologists Christopher Doucas and John Kapetanakis observed several cases of purpuric dermatosis similar in form to the “pigmented purpuric lichenoid dermatitis” of Gougerot-Blum purpura12 and to the “progressive pigmentary dermatitis” of Schamberg disease.3 After observing a gradual disappearance of the classic yellow color from hemosiderin deposition, Doucas and Kapetanakis described a new bright red eruption with lichenification.15 Eczematoid purpura of Doucas and Kapetanakis is rare and predominantly seen in middle-aged males. Hyperpigmented or dark brown macules may develop bilaterally on the legs, progressing to the thighs and upper extremities. Unlike the other types of PPD, DK is extensive and severely pruritic.4
Although most PPD can be drug induced, DK has shown the greatest tendency for pruritic erythematous plaques following drug usage including but not limited to amlodipine, aspirin, acetaminophen, thiamine, interferon alfa, chlordiazepoxide, and isotretinoin. Additionally, DK has been associated with a contact allergy to clothing dyes and rubber.4 On histology, epidermal spongiosis may be seen, correlating with the eczematoid clinical findings. Spontaneous remission also is more common compared to the other PPDs. Treatment consists of topical corticosteroids and antihistamines.16
Lichen Aureus—Lichen aureus was first observed by the dermatologist R.H. Martin in 1958.17 It is clinically characterized by closely aggregated purpuric papules with a distinctive golden-brown color more often localized to the lower extremities and sometimes in a dermatomal distribution. Lichen aureus affects males and females equally, and similar to Majocchi purpura can be seen in children.4 Histopathologic examination reveals a prominent lichenoid plus superficial and deep perivascular lymphocytic infiltrate, extravasated erythrocytes, papillary dermal edema, hemosiderophages, and an unaffected epidermis. In rare cases, perineural infiltrates may be seen. Topical steroids usually are ineffective in lichen aureus treatment, but responses to psoralen plus UVA therapy also have been noted.17
Differential Diagnosis
COVID-19–Related Cutaneous Changes—Because COVID-19–related pathology is now a common differential diagnosis for many cutaneous eruptions,one must be mindful of the possibility for patients to have PPD, cutaneous changes from underlying COVID-19, or both.18 The microvascular changes from COVID-19 infection can be variable.19 Besides the presence of erythema along a distal digit, manifestations can include reticulated dusky erythema mimicking livedoid vasculopathy or inflammatory purpura.19
Retiform Purpura—Retiform purpura may occur in the setting of microvascular occlusion and can represent the pattern of underlying dermal vasculature. It is nonblanching and typically stellate or branching.20 The microvascular occlusion may be a result of hypercoagulability or may be secondary to cutaneous vasculitis, resulting in thrombosis and subsequent vascular occlusion.21 There are many reasons for hypercoagulability in retiform purpura, including disseminated intravascular coagulation in the setting of COVID-19 infection.22 The treatment of retiform purpura is aimed at alleviating the underlying cause and providing symptomatic relief. Conversely, the PPDs generally are benign and require minimal workup.
Leukocytoclastic Vasculitis—The hallmark of leukocytoclastic vasculitis is palpable purpura, often appearing as nonblanchable papules, typically in a dependent distribution such as the lower extremities (Figure 3). Although it primarily affects children, Henoch-Schönlein purpura is a type of leukocytoclastic vasculitis with lesions potentially similar in appearance to those of PPD.23 Palpable purpura may be painful and may ulcerate but rarely is pruritic. Leukocytoclastic vasculitis represents perivascular infiltrates composed of neutrophils, lymphocytes, and occasionally eosinophils, along with karyorrhexis, luminal fibrin, and fibrinoid degeneration of blood vessel walls, often resulting from immune complex deposition. Leukocytoclastic vasculitis may affect blood vessels of any size and requires further clinical and laboratory evaluation for infection (including COVID-19), hypercoagulability, autoimmune disease, or medication-related reactions.24

Stasis Dermatitis—Stasis dermatitis, a chronic inflammatory condition stemming from retrograde venous flow due to incompetent venous valves, mimics PPD. Stasis dermatitis initially appears as demarcated erythematous plaques, fissures, and scaling of the lower legs bilaterally, usually involving the medial malleolus.25 With time, the affected region develops overlying brawny hyperpigmentation and fibrosis (Figure 4). Pruritus or pain are common features, while fissures and superficial erosions may heal and recur, leading to lichenification.

Although both commonly appear on the lower extremities, duplex ultrasonography may be helpful to distinguish PPDs from stasis dermatitis since the latter occurs in the context of chronic venous insufficiency, varicose veins, soft tissue edema, and lymphedema.25 Additionally, pruritus, lichenification, and edema often are not seen in most PPD variants, although stasis dermatitis and PPD may occur in tandem. Conservative treatment involves elevation of the extremities, compression, and topical steroids for symptomatic relief.
Cellulitis—The key characteristics of cellulitis are redness, swelling, warmth, tenderness, fever, and leukocytosis. A history of trauma, such as a prior break in the skin, and pain in the affected area suggest cellulitis. Several skin conditions present similarly to cellulitis, including PPD, and thus approximately 30% of cases are misdiagnosed.26 Cellulitis rarely presents in a bilateral or diffusely scattered pattern as seen in PPDs. Rather, it is unilateral with smooth indistinct borders. Variables suggestive of cellulitis include immunosuppression, rapid progression, and previous occurrences. Hyperpigmented plaques or thickening of the skin are more indicative of a chronic process such as stasis dermatitis or lipodermatosclerosis rather than acute cellulitis. Purpura is not a typical finding in most cases of soft tissue cellulitis. Treatment may be case specific depending on severity, presence or absence of sepsis, findings on blood cultures, or other pathologic evaluation. Antibiotics are directed to the causative organism, typically Streptococcus and Staphylococcus species, although coverage against various gram-negative organisms may be indicated.27
Caution With Teledermatology
COVID-19 has established the value of telemedicine in providing access to health care services for at-risk or underserved individuals. The PPDs are benign, often asymptomatic, and potentially identifiable with teledermatology alone; however, they also can easily be mistaken for COVID-19–related eruptions, vasculitis, other types of purpura, stasis dermatitis, or other complications of lower extremity stasis and lymphedema, especially in an aging population. If tissue biopsy is required, as in the workup of vasculitis, the efficacy of telemedicine becomes more questionable. It is important to delineate the potentially confusing PPDs from other potentially dangerous or life-threatening inflammatory dermatoses.28
Pigmented purpuric dermatoses (PPDs) are characterized by petechiae, dusky macules representative of postinflammatory hyperpigmentation and dermal hemosiderin, and purpura generally localized to the lower extremities. They typically represent a spectrum of lymphocytic capillaritis, variable erythrocyte extravasation from papillary dermal blood vessels, and deposition of hemosiderin, yielding the classic red to orange to golden-brown findings on gross examination. Clinical overlap exists, but variants include Schamberg disease (SD), Majocchi purpura, Gougerot-Blum purpura, eczematoid purpura of Doucas and Kapetanakis (DK), and lichen aureus.1 Other forms are rarer, including linear, granulomatous, quadrantic, transitory, and familial variants. It remains controversial whether PPD may precede or have an association with cutaneous T-cell lymphoma.2 Dermoscopy usually shows copper-red pigmentation in the background, oval red dots, linear vessels, brown globules, and follicular openings. Although these findings may be useful in PPD diagnosis, they are not applicable in differentiating among the variants.
Pigmented purpuric dermatoses can easily be mistaken for stasis dermatitis or cellulitis, as these may occur concomitantly or in populations at risk for all 3 conditions, such as women older than 50 years with recent trauma or infection in the affected area. Tissue biopsy and clinical laboratory evaluation may be required to differentiate between PPD from leukocytoclastic vasculitis or the myriad causes of retiform purpura. Importantly, clinicians also should differentiate PPD from the purpuric eruptions of the lower extremities associated with COVID-19 infection.
Pigmented Purpuric Dermatoses
Schamberg Disease—In 1901, Jay Frank Schamberg, a distinguished professor of dermatology in Philadelphia, Pennsylvania, described “a peculiar progressive pigmentary disease of the skin” in a 15-year-old adolescent boy.3 Schamberg disease is the most common PPD, characterized by pruritic spots resembling cayenne pepper (Figure 1) with orange-brown pigmented macules on the legs and feet.4 Although platelet dysfunction, coagulation deficiencies, or dermal atrophy may contribute to hemorrhaging that manifests as petechiae or ecchymoses, SD typically is not associated with any laboratory abnormalities, and petechial eruption is not widespread.5 Capillary fragility can be assessed by the tourniquet test, in which pressure is applied to the forearm with a blood pressure cuff inflated between systolic and diastolic blood pressure for 5 to 10 minutes. Upon removing the cuff, a positive test is indicated by 15 or more petechiae in an area 5 cm in diameter due to poor platelet function. A positive result may be seen in SD.6

Histologically, SD is characterized by patchy parakeratosis, mild spongiosis of the stratum Malpighi, and lymphoid capillaritis (Figure 2).7 In addition to CD3+, CD4+, CD8+, CD1a+, and CD36+ lymphocytes, histology also may contain dendritic cells and cellular adhesion molecules (intercellular adhesion molecule 1, epithelial cell adhesion molecule 1) within the superficial perivascular infiltrate.8 There is no definitive therapy, but first-line interventions include emollients, topical steroids, and oral antihistamines. Nonpharmacologic management includes compression or support stockings, elevation of the lower extremities, and avoidance of offending medications (if identifiable).1

Majocchi Purpura—Domenico Majocchi was a renowned Italian dermatologist who described an entity in 1898 that he called purpura annularis telangiectodes, now also known as Majocchi purpura.9 It is more common in females, young adults, and children. Majocchi purpura has rarely been reported in families with a possible autosomal-dominant inheritance.10 Typically, bluish-red annular macules with central atrophy surrounded by hyperpigmentation may be seen on the lower extremities, potentially extending to the upper extremities.1 Treatment of Majocchi purpura remains a challenge but may respond to narrowband UVB phototherapy. Emollients and topical steroids also are used as first-line treatments. Biopsy demonstrates telangiectasia, pericapillary infiltration of mononuclear lymphocytes, and papillary dermal hemosiderin.11
Gougerot-Blum Purpura—In 1925, French dermatologists Henri Gougerot and Paul Blum described a pigmented purpuric lichenoid dermatitis known as Gougerot-Blum purpura,12 a rare PPD characterized by lichenoid papules that eventually coalesce into plaques of various colors, along with red-brown hyperpigmentation.4 As with other PPD variants, the legs are most involved, with rare extension to the trunk or thighs. The plaques may resemble and be mistaken for Kaposi sarcoma, cutaneous vasculitis, traumatic purpura, or mycosis fungoides. Dermoscopic examination reveals small, polygonal or round, red dots underlying brown scaly patches.13 Gougerot-Blum purpura is found more commonly in adult men and rarely affects children.4 Histologically, a lichenoid and superficial perivascular infiltrate composed of lymphocytes and macrophages is seen. Various therapies have been described, including topical steroids, antihistamines, psoralen plus UVA phototherapy, and cyclosporin A.14
Eczematoid Purpura of Doucas and Kapetanakis—In 1949, Greek dermatologists Christopher Doucas and John Kapetanakis observed several cases of purpuric dermatosis similar in form to the “pigmented purpuric lichenoid dermatitis” of Gougerot-Blum purpura12 and to the “progressive pigmentary dermatitis” of Schamberg disease.3 After observing a gradual disappearance of the classic yellow color from hemosiderin deposition, Doucas and Kapetanakis described a new bright red eruption with lichenification.15 Eczematoid purpura of Doucas and Kapetanakis is rare and predominantly seen in middle-aged males. Hyperpigmented or dark brown macules may develop bilaterally on the legs, progressing to the thighs and upper extremities. Unlike the other types of PPD, DK is extensive and severely pruritic.4
Although most PPD can be drug induced, DK has shown the greatest tendency for pruritic erythematous plaques following drug usage including but not limited to amlodipine, aspirin, acetaminophen, thiamine, interferon alfa, chlordiazepoxide, and isotretinoin. Additionally, DK has been associated with a contact allergy to clothing dyes and rubber.4 On histology, epidermal spongiosis may be seen, correlating with the eczematoid clinical findings. Spontaneous remission also is more common compared to the other PPDs. Treatment consists of topical corticosteroids and antihistamines.16
Lichen Aureus—Lichen aureus was first observed by the dermatologist R.H. Martin in 1958.17 It is clinically characterized by closely aggregated purpuric papules with a distinctive golden-brown color more often localized to the lower extremities and sometimes in a dermatomal distribution. Lichen aureus affects males and females equally, and similar to Majocchi purpura can be seen in children.4 Histopathologic examination reveals a prominent lichenoid plus superficial and deep perivascular lymphocytic infiltrate, extravasated erythrocytes, papillary dermal edema, hemosiderophages, and an unaffected epidermis. In rare cases, perineural infiltrates may be seen. Topical steroids usually are ineffective in lichen aureus treatment, but responses to psoralen plus UVA therapy also have been noted.17
Differential Diagnosis
COVID-19–Related Cutaneous Changes—Because COVID-19–related pathology is now a common differential diagnosis for many cutaneous eruptions,one must be mindful of the possibility for patients to have PPD, cutaneous changes from underlying COVID-19, or both.18 The microvascular changes from COVID-19 infection can be variable.19 Besides the presence of erythema along a distal digit, manifestations can include reticulated dusky erythema mimicking livedoid vasculopathy or inflammatory purpura.19
Retiform Purpura—Retiform purpura may occur in the setting of microvascular occlusion and can represent the pattern of underlying dermal vasculature. It is nonblanching and typically stellate or branching.20 The microvascular occlusion may be a result of hypercoagulability or may be secondary to cutaneous vasculitis, resulting in thrombosis and subsequent vascular occlusion.21 There are many reasons for hypercoagulability in retiform purpura, including disseminated intravascular coagulation in the setting of COVID-19 infection.22 The treatment of retiform purpura is aimed at alleviating the underlying cause and providing symptomatic relief. Conversely, the PPDs generally are benign and require minimal workup.
Leukocytoclastic Vasculitis—The hallmark of leukocytoclastic vasculitis is palpable purpura, often appearing as nonblanchable papules, typically in a dependent distribution such as the lower extremities (Figure 3). Although it primarily affects children, Henoch-Schönlein purpura is a type of leukocytoclastic vasculitis with lesions potentially similar in appearance to those of PPD.23 Palpable purpura may be painful and may ulcerate but rarely is pruritic. Leukocytoclastic vasculitis represents perivascular infiltrates composed of neutrophils, lymphocytes, and occasionally eosinophils, along with karyorrhexis, luminal fibrin, and fibrinoid degeneration of blood vessel walls, often resulting from immune complex deposition. Leukocytoclastic vasculitis may affect blood vessels of any size and requires further clinical and laboratory evaluation for infection (including COVID-19), hypercoagulability, autoimmune disease, or medication-related reactions.24

Stasis Dermatitis—Stasis dermatitis, a chronic inflammatory condition stemming from retrograde venous flow due to incompetent venous valves, mimics PPD. Stasis dermatitis initially appears as demarcated erythematous plaques, fissures, and scaling of the lower legs bilaterally, usually involving the medial malleolus.25 With time, the affected region develops overlying brawny hyperpigmentation and fibrosis (Figure 4). Pruritus or pain are common features, while fissures and superficial erosions may heal and recur, leading to lichenification.

Although both commonly appear on the lower extremities, duplex ultrasonography may be helpful to distinguish PPDs from stasis dermatitis since the latter occurs in the context of chronic venous insufficiency, varicose veins, soft tissue edema, and lymphedema.25 Additionally, pruritus, lichenification, and edema often are not seen in most PPD variants, although stasis dermatitis and PPD may occur in tandem. Conservative treatment involves elevation of the extremities, compression, and topical steroids for symptomatic relief.
Cellulitis—The key characteristics of cellulitis are redness, swelling, warmth, tenderness, fever, and leukocytosis. A history of trauma, such as a prior break in the skin, and pain in the affected area suggest cellulitis. Several skin conditions present similarly to cellulitis, including PPD, and thus approximately 30% of cases are misdiagnosed.26 Cellulitis rarely presents in a bilateral or diffusely scattered pattern as seen in PPDs. Rather, it is unilateral with smooth indistinct borders. Variables suggestive of cellulitis include immunosuppression, rapid progression, and previous occurrences. Hyperpigmented plaques or thickening of the skin are more indicative of a chronic process such as stasis dermatitis or lipodermatosclerosis rather than acute cellulitis. Purpura is not a typical finding in most cases of soft tissue cellulitis. Treatment may be case specific depending on severity, presence or absence of sepsis, findings on blood cultures, or other pathologic evaluation. Antibiotics are directed to the causative organism, typically Streptococcus and Staphylococcus species, although coverage against various gram-negative organisms may be indicated.27
Caution With Teledermatology
COVID-19 has established the value of telemedicine in providing access to health care services for at-risk or underserved individuals. The PPDs are benign, often asymptomatic, and potentially identifiable with teledermatology alone; however, they also can easily be mistaken for COVID-19–related eruptions, vasculitis, other types of purpura, stasis dermatitis, or other complications of lower extremity stasis and lymphedema, especially in an aging population. If tissue biopsy is required, as in the workup of vasculitis, the efficacy of telemedicine becomes more questionable. It is important to delineate the potentially confusing PPDs from other potentially dangerous or life-threatening inflammatory dermatoses.28
- Sardana K, Sarkar R , Sehgal VN. Pigmented purpuric dermatoses: an overview. Int J Dermatol. 2004;43:482-488.
- Çaytemel C, Baykut B, Ag˘ırgöl S¸, et al. Pigmented purpuric dermatosis: ten years of experience in a tertiary hospital and awareness of mycosis fungoides in differential diagnosis. J Cutan Pathol. 2021;48:611-616.
- Schamberg JF. A peculiar progressive pigmentary disease of the skin. Br J Dermatol. 1901;13:1-5.
- Martínez Pallás I, Conejero Del Mazo R, Lezcano Biosca V. Pigmented purpuric dermatosis: a review of the literature. Actas Dermosifiliogr (Engl Ed). 2020;111:196-204.
- Ozkaya DB, Emiroglu N, Su O, et al. Dermatoscopic findings of pigmented purpuric dermatosis. An Bras Dermatol. 2016;91:584-587.
- Lava SAG, Milani GP, Fossali EF, et al. Cutaneous manifestations of small-vessel leukocytoclastic vasculitides in childhood. Clin Rev Allergy Immunol. 2017;53:439-451.
- Bonnet U, Selle C, Isbruch K, et al. Recurrent purpura due to alcohol-related Schamberg’s disease and its association with serum immunoglobulins: a longitudinal observation of a heavy drinker. J Med Case Rep. 2016;10:301.
- Zaldivar Fujigaki JL, Anjum F. Schamberg Disease. StatPearls Publishing; 2021.
- Majocchi J. Purpura annularis telangiectodes. Arch Dermatol Syph. 1898;43:447.
- Sethuraman G, Sugandhan S, Bansal A, et al. Familial pigmented purpuric dermatoses. J Dermatol. 2006;33:639-641.
- Miller K, Fischer M, Kamino H, et al. Purpura annularis telangiectoides. Dermatol Online J. 2012;18:5.
- Coulombe J, Jean SE, Hatami A, et al. Pigmented purpuric dermatosis: clinicopathologic characterization in a pediatric series. Pediatr Dermatol. 2015;32:358-362.
- Park MY, Shim WH, Kim JM, et al. Dermoscopic finding in pigmented purpuric lichenoid dermatosis of Gougerot-Blum: a useful tool for clinical diagnosis. Ann Dermatol. 2018;30:245-247.
- Risikesan J, Sommerlund M, Ramsing M, et al. Successful topical treatment of pigmented purpuric lichenoid dermatitis of Gougerot-Blum in a young patient: a case report and summary of the most common pigmented purpuric dermatoses. Case Rep Dermatol. 2017;9:169-176.
- Doucas C, Kapetanakis J. Eczematid-like purpura. Dermatologica. 1953;106:86-95.
- Kim DH, Seo SH, Ahn HH, et al. Characteristics and clinical manifestations of pigmented purpuric dermatosis. Ann Dermatol. 2015;27:404-410.
- Aung PP, Burns SJ, Bhawan J. Lichen aureus: an unusual histopathological presentation: a case report and a review of literature. Am J Dermatopathol. 2014;36:E1-E4.
- Singh P, Schwartz RA. Disseminated intravascular coagulation: a devastating systemic disorder of special concern with COVID-19. Dermatol Ther. 2020;33:E14053.
- Almutairi N, Schwartz RA. COVID-19 with dermatologic manifestations and implications: an unfolding conundrum. Dermatol Ther. 2020;33:E13544.
- Georgesen C, Fox LP, Harp J. Retiform purpura: a diagnostic approach. J Am Acad Dermatol. 2020;82:783-796.
- Torregrosa Calatayud JL, Garcías Ladaria J, De Unamuno Bustos B, et al. Retiform purpura caused by the use of cocaine, that was probably adulterated with levamisole. Ann Dermatol. 2015;27:117-119.
- Keim CK, Schwartz RA, Kapila R. Levamisole-induced and COVID-19-induced retiform purpura: two overlapping, emerging clinical syndromes. Arch Dermatol Res. 2021;22:1-9.
- González LM, Janniger CK, Schwartz RA. Pediatric Henoch-Schönlein purpura. Int J Dermatol. 2009;48:1157-1165.
- Yıldırım Bay E, Moustafa E, Semiz Y, et al. Leukocytoclastic vasculitis secondary to COVID-19 infection presenting with inclusion bodies: a histopathological correlation. J Cosmet Dermatol. 2022;21:27-29.
- Sundaresan S, Migden MR, Silapunt S. Stasis dermatitis: pathophysiology, evaluation, and management. Am J Clin Dermatol. 2017;18:383-390.
- Hirschmann JV, Raugi GJ. Lower limb cellulitis and its mimics: part I. lower limb cellulitis. J Am Acad Dermatol. 2012;67:163.E1-E12; quiz 75-76.
- Keller EC, Tomecki KJ, Alraies MC. Distinguishing cellulitis from its mimics. Cleveland Clin J Med. 2012;79:547-552.
- Georgesen C, Fox LP, Harp J. Retiform purpura: workup and therapeutic considerations in select conditions. J Am Acad Dermatol. 2020;82:799-816.
- Sardana K, Sarkar R , Sehgal VN. Pigmented purpuric dermatoses: an overview. Int J Dermatol. 2004;43:482-488.
- Çaytemel C, Baykut B, Ag˘ırgöl S¸, et al. Pigmented purpuric dermatosis: ten years of experience in a tertiary hospital and awareness of mycosis fungoides in differential diagnosis. J Cutan Pathol. 2021;48:611-616.
- Schamberg JF. A peculiar progressive pigmentary disease of the skin. Br J Dermatol. 1901;13:1-5.
- Martínez Pallás I, Conejero Del Mazo R, Lezcano Biosca V. Pigmented purpuric dermatosis: a review of the literature. Actas Dermosifiliogr (Engl Ed). 2020;111:196-204.
- Ozkaya DB, Emiroglu N, Su O, et al. Dermatoscopic findings of pigmented purpuric dermatosis. An Bras Dermatol. 2016;91:584-587.
- Lava SAG, Milani GP, Fossali EF, et al. Cutaneous manifestations of small-vessel leukocytoclastic vasculitides in childhood. Clin Rev Allergy Immunol. 2017;53:439-451.
- Bonnet U, Selle C, Isbruch K, et al. Recurrent purpura due to alcohol-related Schamberg’s disease and its association with serum immunoglobulins: a longitudinal observation of a heavy drinker. J Med Case Rep. 2016;10:301.
- Zaldivar Fujigaki JL, Anjum F. Schamberg Disease. StatPearls Publishing; 2021.
- Majocchi J. Purpura annularis telangiectodes. Arch Dermatol Syph. 1898;43:447.
- Sethuraman G, Sugandhan S, Bansal A, et al. Familial pigmented purpuric dermatoses. J Dermatol. 2006;33:639-641.
- Miller K, Fischer M, Kamino H, et al. Purpura annularis telangiectoides. Dermatol Online J. 2012;18:5.
- Coulombe J, Jean SE, Hatami A, et al. Pigmented purpuric dermatosis: clinicopathologic characterization in a pediatric series. Pediatr Dermatol. 2015;32:358-362.
- Park MY, Shim WH, Kim JM, et al. Dermoscopic finding in pigmented purpuric lichenoid dermatosis of Gougerot-Blum: a useful tool for clinical diagnosis. Ann Dermatol. 2018;30:245-247.
- Risikesan J, Sommerlund M, Ramsing M, et al. Successful topical treatment of pigmented purpuric lichenoid dermatitis of Gougerot-Blum in a young patient: a case report and summary of the most common pigmented purpuric dermatoses. Case Rep Dermatol. 2017;9:169-176.
- Doucas C, Kapetanakis J. Eczematid-like purpura. Dermatologica. 1953;106:86-95.
- Kim DH, Seo SH, Ahn HH, et al. Characteristics and clinical manifestations of pigmented purpuric dermatosis. Ann Dermatol. 2015;27:404-410.
- Aung PP, Burns SJ, Bhawan J. Lichen aureus: an unusual histopathological presentation: a case report and a review of literature. Am J Dermatopathol. 2014;36:E1-E4.
- Singh P, Schwartz RA. Disseminated intravascular coagulation: a devastating systemic disorder of special concern with COVID-19. Dermatol Ther. 2020;33:E14053.
- Almutairi N, Schwartz RA. COVID-19 with dermatologic manifestations and implications: an unfolding conundrum. Dermatol Ther. 2020;33:E13544.
- Georgesen C, Fox LP, Harp J. Retiform purpura: a diagnostic approach. J Am Acad Dermatol. 2020;82:783-796.
- Torregrosa Calatayud JL, Garcías Ladaria J, De Unamuno Bustos B, et al. Retiform purpura caused by the use of cocaine, that was probably adulterated with levamisole. Ann Dermatol. 2015;27:117-119.
- Keim CK, Schwartz RA, Kapila R. Levamisole-induced and COVID-19-induced retiform purpura: two overlapping, emerging clinical syndromes. Arch Dermatol Res. 2021;22:1-9.
- González LM, Janniger CK, Schwartz RA. Pediatric Henoch-Schönlein purpura. Int J Dermatol. 2009;48:1157-1165.
- Yıldırım Bay E, Moustafa E, Semiz Y, et al. Leukocytoclastic vasculitis secondary to COVID-19 infection presenting with inclusion bodies: a histopathological correlation. J Cosmet Dermatol. 2022;21:27-29.
- Sundaresan S, Migden MR, Silapunt S. Stasis dermatitis: pathophysiology, evaluation, and management. Am J Clin Dermatol. 2017;18:383-390.
- Hirschmann JV, Raugi GJ. Lower limb cellulitis and its mimics: part I. lower limb cellulitis. J Am Acad Dermatol. 2012;67:163.E1-E12; quiz 75-76.
- Keller EC, Tomecki KJ, Alraies MC. Distinguishing cellulitis from its mimics. Cleveland Clin J Med. 2012;79:547-552.
- Georgesen C, Fox LP, Harp J. Retiform purpura: workup and therapeutic considerations in select conditions. J Am Acad Dermatol. 2020;82:799-816.
Practice Points
- Dermatologists should be aware of the clinical presentations of pigmenting purpuric dermatoses (PPDs).
- Certain PPDs may resemble the thromboembolic events seen in COVID-19. Clinicians should especially be aware of how to differentiate these benign pigmentary disorders from other serious conditions.
- Teledermatology is widely utilized, but caution may be prudent when evaluating erythematous or purpuric dermatoses, especially those of the lower extremities.
- Pigmenting purpuric dermatoses generally are benign and do not require immediate treatment.
Gender and racial biases in Press Ganey patient satisfaction surveys

Patient satisfaction questionnaires were developed in the 1980s as part of the movement to better understand the patient’s experience and their perspective of the quality of care. In 1985, the Press Ganey survey—now the most widely used method to assess patient satisfaction—was developed by 2 professors in anthropology and sociology-statistics at Notre Dame. Initially intended for inpatient admissions, the survey was validated based on a few thousand survey results.1 Given the strong interest in improving patient satisfaction at the time, it became widely adopted and quickly expanded into outpatient encounters and ambulatory surgery settings.
Although other surveys have been developed,2 the Press Ganey survey is the most commonly used assessment tool for patient satisfaction metrics in the United States, with approximately 50% of all hospitals and more than 41,000 health care organizations using its services.3,4 The survey consists of 6 domains related to satisfaction with:
1. the care provider
2. the nurse or assistant
3. personal issues
4. overall assessment
5. access
6. moving through the visit.
Survey items are scored using a 5-point Likert scale, with scores ranging from “very poor” (a score of 1) to “very good” (a score of 5). According to the company, because this format is balanced and parallel (unlike a “poor” to “excellent” format), responses can be quantified and used statistically without violating methodologic assumptions. Also, variability in patients’ responses with this format allows for the identification of opportunities to improve, unlike “yes/no” response formats.1 There are limitations to this design, however, which can impact data quality,5 as we will see.
Adoption of the survey as we move toward value-based care
More recently, patients’ satisfaction with their health care has received increased attention as we move to a patient-centered care model and as health care reimbursement models shift toward value-based care. Current trends in health care policy statements include the importance of raising the standard of care and shifting from a “fee-for-service” to a “pay-for-performance” reimbursement model.7,8 As a result, hospitals are establishing systems to measure “performance” that are not nationally standardized or extensively studied with objective measures. The changing standard of health care expectations in the United States is a topic of much public debate.9 And as expectations and new standards are defined, the impact of implementing novel measures of performance should be evaluated prior to widespread adoption and utilization.
Patient satisfaction also has been identified as a driver for hospital finances through loyalty, described as the “likelihood to return to that system for future medical services.”10,11 This measure has contributed to policy changes that reinforce prioritization of patient satisfaction. For example, the Affordable Care Act tied Medicare reimbursement and patient satisfaction together in the Hospital Value-Based Purchasing Program. This program uses measures of clinical processes, efficiency, outcomes, and patient experiences to calculate a total score that results in hospital reimbursement and incentives,12 which creates a direct pathway from patient experience to reimbursement—underscoring hospitals’ desire for ongoing assessment of patient satisfaction.
In 2005, the Centers for Medicare and Medicaid Services and the Agency for Health care Research and Quality developed the Hospital Consumer Assessment of Health care Providers and Systems (HCAHPS) survey in response to criticisms of the Press Ganey survey. The HCAHPS survey consists of 27 questions with 3 broad goals19:
- to produce data about patients’ perspectives of care that allow for objective and meaningful comparisons of hospitals
- to publicly report survey results and create new incentives for hospitals to improve quality of care
- to produce public reports that enhance accountability by increasing transparency.
One difference with the HCAHPS is that it measures frequency, or how often a service was performed (“never”, “sometimes”, “usually”, “always”), whereas Press Ganey measures satisfaction. It also only surveys inpatients and does not address outpatient encounters. Despite the differences, it is a widely used patient satisfaction survey and is subject to similar issues and biases as the Press Ganey survey.
Continue to: Gender, race, and age bias...
Gender, race, and age bias
Although the rationale behind gathering patient input is important, recent data suggest that patient satisfaction surveys are subject to inherent biases.6,13,14 These biases tend to negatively impact women and non-White physicians, adding to the systemic discrimination against women and physicians of color that already exists in health care.
In a single-site retrospective study performed in 2018 by Rogo-Gupta et al, female gynecologists were found to be 47% less likely to receive top patient satisfaction scores than their male counterparts owing to their gender alone, suggesting that gender bias may impact the results of patient satisfaction questionnaires.13 The authors encouraged that the results of patient satisfaction surveys be interpreted with great caution until the impact on female physicians is better understood.
A multi-center study by the same group (Rogo-Gupta et al) assessed the same construct across 5 different geographically diverse institutions.15 This study confirmed that female gynecologists were less likely to receive a top satisfaction score from their patients (19% lower odds when compared with male gynecologists). They also studied the effects of other patient demographics, including age, race/ethnicity, and race concordance. Older patients (aged ≥63 years) had an over-3-fold increase in odds of providing a top satisfaction score than younger patients. Additionally, Asian physicians had significantly lower odds of receiving a top satisfaction score when compared with White physicians, while Asian patients had significantly lower odds of providing a top satisfaction score when compared with White patients. Lastly, in most cases, when underrepresented-in-medicine patients saw an underrepresented-in-medicine physician (race concordance), there was a significant increase in odds of receiving a top satisfaction score. Asian race concordance, however, actually resulted in a lower likelihood of receiving a top satisfaction score.15
Literature from other specialties supports these findings. These results are consistent with emerging data from other medical specialties that also suggest that Press Ganey survey data are subject to inherent biases. For example, data from emergency medicine literature have shown discrepancies between patient satisfaction for providers at tertiary inner-city institutions versus those in affluent suburban populations,16 and that worse mortality is actually correlated with better patient satisfaction scores, and vice versa.17
Another study by Sotto-Santiago in 2019 assessed patient satisfaction scores in multiple specialties at a single institution where quality-related financial incentives were offered based on this metric. They found a significant difference in patient satisfaction scores between underrepresented and White physicians, which suggests a potential bias among patients and institutional practices—ultimately leading to pay inequities through differences in financial incentives.18
Percentile differences reveal small gaps in satisfaction ratings
When examining the difference between raw Press Ganey patient satisfaction data and the percentiles associated with these scores, an interesting finding arises. Looking at the 2023 multicenter study by Rogo-Gupta et al, the difference in the top raw scores between male and female gynecologists appears to be small (3.3%).15 However, in 2020, the difference in top scores separating the top (75th) and bottom (25th) percentile quartiles of physicians was also small, at only 6.9%.
Considering the percentiles, if a provider who scores in the 25th percentile is compared with a colleague who scores in the 75th percentile, they may think the reported satisfaction score differences were quite large. This may potentially invoke feelings of decreased self-worth, negatively impact their professional identity or overall well-being, and they may seek (or be told to seek) improvement opportunities. Now imagine the provider in question realizes the difference between the 25th percentile and 75th percentile is actually only 6.9%. This information may completely change how the results are interpreted and acted upon by administrators. This is further changed with the understanding that 3.3% of the difference may be due to gender alone, narrowing the gap even further. Providers would become understandably frustrated if measures of success such as reimbursement, financial bonus or incentives, promotion, or advancement are linked to these results. It violates the value of fairness and does not offer an equitable starting point.
Evolution of the data distribution. Another consideration, as noted by Robert C. Lloyd, PhD, one of the statisticians who helped develop the percentile statistical analysis mapping in 1985, is that it was based on a classic bell-shaped distribution of patient satisfaction survey scores.19 Because hospitals, medical groups, and physicians have been working these past 20 years to achieve higher Press Ganey scores, the data no longer have a bell-shaped distribution. Rather, there are significant clusters of raw scores at the high end with a very narrow response range. When these data are mapped to the percentile spectrum, they are highly inaccurate.19
Impact of sample size. According to Press Ganey, a minimum of 30 survey responses collected over the designated time period is necessary to draw meaningful conclusions of the data for a specific individual, program, or hospital. Despite this requirement to achieve statistical significance, Sullivan and DeLucia found that the firm often provides comparative data about hospital departments and individual physicians based on a smaller sample size that may create an unacceptably large margin of error.20 Sullivan, for example, said his department may only have 8 to 10 Press Ganey survey responses per month and yet still receives monthly reports from the company analyzing the data. Because of the small sample size, 1 month his department ranked in the 1st percentile and 2 months later it ranked in the 99th percentile.20
The effect of a high ceiling rate. A psychometrics report for the Press Ganey survey is available from the vendor that provides vague assessments of reliability and validity based on 2,762 surveys from 12 practices across 10 states. This report describes a 12-question version of the survey with “no problems encountered” with missingness and response variability. The report further states that the Press Ganey survey demonstrates construct, convergent, divergent, and predictive validities, and high reliability; however, these data are not made available.1
In response to this report, Presson et al analyzed more than 34,000 surveys from one institution to evaluate the reliability and validity of the Press Ganey survey.21 Overall, the survey demonstrated suitable psychometric properties for most metrics. However, Presson et al noted a significantly high ceiling rate of 29.3% for the total score, which ranged from 55.4% to 84.1% across items.21 (Ceiling rates are considered substantial if they occur more than 20% of the time.) Ultimately, a high ceiling rate reduces the power to discriminate between patients who have high satisfaction (everyone is “happy”) with those who are just slightly less than happy, but not dissatisfied. This data quality metric can impact the reliability and validity of a survey—and substantial ceiling rates can notably impact percentile rankings of scores within an institution, offering a possible explanation for the small percentage change between the top and bottom percentiles.
Continue to: Other issues with surveys...
Other issues with surveys
In addition to the limitations associated with percentile groupings, survey data are always subject to nonresponse bias, and small sample size can lead to nonsignificant results. Skewed responses also can make it difficult to identify true outlying providers who may need remediation or may be offering a superior patient experience. Satisfaction surveys also lack an assessment of objective data and instead assess how patients perceive and feel, which introduces subjectivity to the results.
Additionally, focusing on improving patient experience ratings can incentivize unnecessary or inappropriate care (ie, overprescribing of narcotics, prescribing antibiotics when not indicated, or ordering testing that may not change management). Some physicians even state that they are not getting the type of feedback that they are asking for and that the survey is not asking the right questions to elicit patient input that is meaningful to their practice. Lastly, the incorporation of trainees and advanced practice providers in the patient care experience leads to the assessment of an alternative provider being included in the ultimate score and may not be representative of that physician.
Patients’ perception and survey results. In some circumstances, the patient’s understanding of their medical situation may affect their responses. Some may argue that patients may mistake a physician’s confidence for competence, when in reality these two entities are mutually exclusive. In a randomized controlled trial, researchers from Mount Sinai School of Medicine and Columbia University Medical Center surveyed inner-city women with newly diagnosed and surgically treated early-stage breast cancer for their perceived quality of care and the process of getting care, including referrals, test results, and treatments. They compared the responses with patient records to determine the actual quality of care. Of the 374 women who received treatment for early-stage breast cancer, 55% said they received “excellent care,” but most—88%—actually got care that was in line with the best current treatment guidelines. Interestingly, the study found African American women were less likely to report excellent care than White or Hispanic women, less likely to trust their doctor, and more likely to say they experienced bias during the process. However, there was no difference in actual quality of care received in any group.22
You can’t improve what you can’t control. Ultimately, while many providers think patient satisfaction survey results may help inform some aspects of their practice, they cannot improve what they cannot control. For example, the multicenter study by Rogo-Gupta et al found that older patients (≥63 years) have more than a 3-fold increase in odds of giving a top satisfaction score than younger patients (≤33 years), independent of other aspects of the care experience.15 Additionally, they found that older physicians (≥56 years) had a significant increase in odds of receiving a top satisfaction score when compared with physicians who were younger than 55 years old.15 Given that physicians clearly cannot control their own age or the age of their patients, the negative impacts of these biases need to be addressed and remedied at a systems level.
Why might these biases exist?
While we cannot completely understand all of the possible explanations for these biases, it is important to emphasize the long-standing prejudice and discrimination against women and people of color in our society and how this has impacted our behavior. While strides have been made, there clearly still seems to be a difference between what we say and how our biases impact our behavior. Women are still tougher on women in professional evaluations in other fields as well23; it is not unique to medicine. While workplace improvements are slowly changing, women still face inequities. The more research we publish to describe it, the more we hope the conversation continues, allowing us to reduce the impact of bias on our sense of self-worth and identity related to our careers, narrow the pay gap, and see women advance at the same rate as male counterparts. Considerable transformation is crucial to prevent further workforce attrition.
With regard to the lower scores provided by Asian patients, studies suggest that cultural response bias, rather than true differences in quality of care, may account for these discrepancies. Previous literature has found that Asian patients are more likely to select midpoints, rather than extremes, when completing Likert-type studies24 and are not more likely to change medical providers than other race/ethnicities, indicating that lower ratings may not necessarily imply greater dissatisfaction with care.25
Far-reaching effects on finances, income, well-being, job satisfaction, etc.
Depending on how the results are distributed and used, the effects of patient satisfaction surveys can extend well beyond the original intentions. At some institutions, income for physicians is directly tied to their Press Ganey satisfaction scores, which could have profound implications for female and Asian physicians,13,15 who would be paid less—resulting in a wider pay gap than already exists.18
When negative and not constructive, patient evaluations can contribute to physician burnout and a loss of productive members of the workforce.26 This is especially important in obstetrics and gynecology, where physicians are most likely to experience burnout due to multiple factors such as high-risk medical conditions, pressures of the electronic medical record (EMR), the medicolegal environment, and difficulty balancing patient expectations for autonomy with professional judgement.27 Burnout also disproportionately affects women and younger physicians, which is especially concerning given that, in 2017, approximately one-third of practicing obstetrician/gynecologists were women, while that same year more than 80% of trainees matching into the field were women.28 In one survey sent to members of a prominent medical society, 20% of the medical professionals who responded said they have had their employment threatened by low patient satisfaction scores, 78% reported that patient satisfaction surveys moderately or severely affected their job satisfaction, and 28% stated they had considered quitting their job or leaving the medical profession.29Another related effect is the association between malpractice proceedings and a lack of satisfaction with perceived quality of physician-patient communication.30 This may be an important determinant of malpractice lawsuits, and ensuring high patient satisfaction may be a form of defensive medicine.
Continue to: Controlling the narrative for the future: Proposed strategies...
Controlling the narrative for the future: Proposed strategies
The rapid, widespread adoption of the Press Ganey survey across specialties, clinical care settings, and geographic areas may have been largely due to the ease and operational benefits for hospitals rather than after rigorous study and validation. For example, repeated use of a specific measurement tool may facilitate comparison across areas within a hospital but also across institutions, which can help assess performance at a national level. Hospitals also may have a financial incentive to work with a single third-party or vendor instead of using multiple options across multiple vendors. However, the impact of adoption of novel measures of performance should be evaluated prior to widespread adoption and utilization.
A similar example of an emergence of a technological advancement that has changed the field of medicine and how we provide care is the EMR. Epic is now the most commonly used medical record system and holds the market share of the industry, covering 78% of patients in the United States.31 While there are certainly many potential benefits of a common EMR, such as ease of information sharing and standardization of formatting, opportunities are identified in real time and require product adjustment. For example, modifications have been made to accurately represent gender outside of the previously used dichotomous options. Diagnoses such as cervical cancer screening can now be used even if the patient gender is listed as male.
Similarly, the Press Ganey and other patient satisfaction questionnaires should be evaluated and modified to address existing societal biases. The World Health Organization estimates that it will take 300 years to fix gender inequality,32 but we have an opportunity now to control the narrative and improve patient feedback.
Future research avenues
Ultimately, there is a need to further explore currently available methods of evaluating clinical encounters to better understand the inherent biases and limitations. We hope this review will encourage other physicians to examine their specialties and hospitals and require similar analyses from vendors of such satisfaction rating products prior to using them. At the very least, health systems should be willing to partner with vendors and physicians on an ongoing basis to better understand the biases involved in these survey results and make modifications as needed. Patients also obtain information from and contribute to self-reported, publicly available websites; therefore, additional exploration into a nationalized standard for assessing patient satisfaction also may serve as an opportunity to standardize the information patients evaluate.33 Further assessment of the potential financial and emotional impact of using the currently available patient-reported surveys on female physicians, Asian physicians, young physicians, and physicians who see young patients is needed. It is time to put pressure on a broken patient satisfaction system and improve on a national level to avoid undue negative consequences on our physicians. Use of patient satisfaction survey data should be limited until we all understand and account for the biases that are evident. ●
- Appeal to the Press Ganey corporation with the results of recent data and other studies to ensure they are aware of the biases that exist in their product
- Appeal to hospital-level administration to refrain from using Press Ganey scores as a tool to dictate reimbursement; instead rely on other more objective measures of performance (such as publications, presentations, research accomplishments, patient and surgical outcomes, promotion, committees, national leadership roles, etc)
- Apply a “corrective factor” or “adjustment factor” to eliminate the baseline discrepancy between scores for men and women
- Consider moving to an alternative survey methodology
- Provide patient education to define “performance” (ie, frame what a patient can expect from a provider such as being on time, courteous, and empathetic; caution against asking patients to assess competence and knowledge)
- Outpatient Services (OU) Survey Psychometrics Report. Published online 2019.
- Zusman EE. HCAHPS replaces Press Ganey Survey as quality measure for patient hospital experience. Neurosurgery. 2012;71:N21-N24. doi: 10.1227/01.neu.0000417536.07871.ed
- Press Ganey. Company. Accessed April 20, 2023. www.pressganey. com/company/
- Press, Ganey--first year of patient satisfaction measurement. Hosp Guest Relations Rep. 1986;1:4-5.
- DeCastellarnau A. A classification of response scale characteristics that affect data quality: a literature review. Qual Quant. 2018;52:15231559. doi: 10.1007/s11135-017-0533-4
- Tyser AR, Abtahi AM, McFadden M, et al. Evidence of non-response bias in the Press-Ganey patient satisfaction survey. BMC Health Serv Res. 2016;16:350. doi: 10.1186/s12913-016-1595-z
- Duseja R, Durham M, Schreiber M. CMS quality measure development. JAMA. 2020;324:1213-1214. doi: 10.1001/jama.2020.12070
- Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. National Academies Press; 2001. doi: 10.17226/10027
- Parmet WE. Health: policy or law? A population-based analysis of the Supreme Court’s ACA cases. J Health Polit Policy Law. 2016;41:10611081. doi: 10.1215/03616878-3665949
- Richter JP, Muhlestein DB. Patient experience and hospital profitability: is there a link? Health Care Manage Rev. 2017;42:247-257. doi: 10.1097/HMR.0000000000000105
- Huang C-H, Wu H-H, Lee Y-C, et al. What role does patient gratitude play in the relationship between relationship quality and patient loyalty? Inquiry. 2019;56:46958019868324. doi: 10.1177/0046958019868324
- Centers for Medicare & Medicaid Services (CMS), HHS. Medicare program; hospital inpatient value-based purchasing program. Final rule. Fed Regist. 2011;76:26490-26547.
- Rogo-Gupta LJ, Haunschild C, Altamirano J, et al. Physician gender is associated with Press Ganey patient satisfaction scores in outpatient gynecology. Womens Health Issues. 2018;28:281-285. doi: 10.1016 /j.whi.2018.01.001
- DeLoughery EP. Physician race and specialty influence Press Ganey survey results. Neth J Med. 2019;77:366-369.
- Homewood L, Altamirano J, Fassiotto M, et al. Women gynecologists receive lower Press Ganey patient satisfaction scores in a multicenter cross-sectional study. Am J Obstet Gynecol. 2023;228:S801. doi: 10.1016/j.ajog.2022.12.025
- Sharp B, Johnson J, Hamedani AG, et al. What are we measuring? Evaluating physician-specific satisfaction scores between emergency departments. West J Emerg Med. 2019;20:454-459. doi: 10.5811 /westjem.2019.4.41040
- Mosley M. Viewpoint: Press Ganey is a worthless tool for the ED. Emerg Med News. 2019;41:3-4. doi: 10.1097/01.EEM.0000616512.68475.69
- Sotto-Santiago S, Slaven JE, Rohr-Kirchgraber T. (Dis)Incentivizing patient satisfaction metrics: the unintended consequences of institutional bias. Health Equity. 2019;3:13-18. doi: 10.1089/heq.2018.0065
- Lloyd RC. Quality Health Care: A Guide to Developing and Using Indicators. 2nd ed. Jones & Bartlett Learning; 2019. Accessed April 23, 2023. www.jblearning.com/catalog/productdetails /9781284023077
- 2+2=7? Seven things you may not know about Press Ganey statistics. Emergency Physicians Monthly. Accessed April 23, 2023. epmonthly. com/article/227-seven-things-you-may-not-know-about-pressgainey-statistics/
- Presson AP, Zhang C, Abtahi AM, et al. Psychometric properties of the Press Ganey® Outpatient Medical Practice Survey. Health Qual Life Outcomes. 2017;15:32. doi: 10.1186/s12955-017-0610-3
- Bickell NA, Neuman J, Fei K, et al. Quality of breast cancer care: perception versus practice. J Clin Oncol. 2012;30:1791-1795. doi: 10.1200 /JCO.2011.38.7605
- Strauss K. Women in the workplace: are women tougher on other women? Forbes. July 18, 2016. Accessed April 27, 2023. www.forbes. com/sites/karstenstrauss/2016/07/18/women-in-the-workplace -are-women-tougher-on-other-women/
- Lee JW, Jones PS, Mineyama Y, et al. Cultural differences in responses to a Likert scale. Res Nurs Health. 2002;25:295-306. doi: 10.1002 /nur.10041
- Saha S, Hickam DH. Explaining low ratings of patient satisfaction among Asian-Americans. Am J Med Qual. 2003;18:256-264. doi: 10.1177/106286060301800606
- Halbesleben JRB, Rathert C. Linking physician burnout and patient outcomes: exploring the dyadic relationship between physicians and patients. Health Care Manage Rev. 2008;33:29-39. doi: 10.1097/01. HMR.0000304493.87898.72
- Bradford L, Glaser G. Addressing physician burnout and ensuring high-quality care of the physician workforce. Obstet Gynecol. 2021;137:3-11. doi: 10.1097/AOG.0000000000004197
- Boyle P. Nation’s physician workforce evolves: more women, a bit older, and toward different specialties. AAMCNEWS. February 2, 2021. Accessed April 20, 2023. www.aamc.org/news-insights/nations-physician-workforce-evolves-more-women-bit-older-and-towarddifferent-specialties
- Zgierska A, Rabago D, Miller MM. Impact of patient satisfaction ratings on physicians and clinical care. Patient Prefer Adherence. 2014;8:437-446. doi: 10.2147/PPA.S59077
- Yeh J, Nagel EE. Patient satisfaction in obstetrics and gynecology: individualized patient-centered communication. Clin Med Insights Womens Health. 2010;3:23. doi: 10.4137/CMWH.S5870
- Epic. About us. Accessed April 19, 2023. www.epic.com/about
- United Nations. Without investment, gender equality will take nearly 300 years: UN report. September 7, 2022. Accessed April 19, 2023. news.un.org/en/story/2022/09/1126171
- Ryan T, Specht J, Smith S, et al. Does the Press Ganey Survey correlate to online health grades for a major academic otolaryngology department? Otolaryngol Head Neck Surg. 2016;155:411-415. doi: 10.1177/0194599816652386

Patient satisfaction questionnaires were developed in the 1980s as part of the movement to better understand the patient’s experience and their perspective of the quality of care. In 1985, the Press Ganey survey—now the most widely used method to assess patient satisfaction—was developed by 2 professors in anthropology and sociology-statistics at Notre Dame. Initially intended for inpatient admissions, the survey was validated based on a few thousand survey results.1 Given the strong interest in improving patient satisfaction at the time, it became widely adopted and quickly expanded into outpatient encounters and ambulatory surgery settings.
Although other surveys have been developed,2 the Press Ganey survey is the most commonly used assessment tool for patient satisfaction metrics in the United States, with approximately 50% of all hospitals and more than 41,000 health care organizations using its services.3,4 The survey consists of 6 domains related to satisfaction with:
1. the care provider
2. the nurse or assistant
3. personal issues
4. overall assessment
5. access
6. moving through the visit.
Survey items are scored using a 5-point Likert scale, with scores ranging from “very poor” (a score of 1) to “very good” (a score of 5). According to the company, because this format is balanced and parallel (unlike a “poor” to “excellent” format), responses can be quantified and used statistically without violating methodologic assumptions. Also, variability in patients’ responses with this format allows for the identification of opportunities to improve, unlike “yes/no” response formats.1 There are limitations to this design, however, which can impact data quality,5 as we will see.
Adoption of the survey as we move toward value-based care
More recently, patients’ satisfaction with their health care has received increased attention as we move to a patient-centered care model and as health care reimbursement models shift toward value-based care. Current trends in health care policy statements include the importance of raising the standard of care and shifting from a “fee-for-service” to a “pay-for-performance” reimbursement model.7,8 As a result, hospitals are establishing systems to measure “performance” that are not nationally standardized or extensively studied with objective measures. The changing standard of health care expectations in the United States is a topic of much public debate.9 And as expectations and new standards are defined, the impact of implementing novel measures of performance should be evaluated prior to widespread adoption and utilization.
Patient satisfaction also has been identified as a driver for hospital finances through loyalty, described as the “likelihood to return to that system for future medical services.”10,11 This measure has contributed to policy changes that reinforce prioritization of patient satisfaction. For example, the Affordable Care Act tied Medicare reimbursement and patient satisfaction together in the Hospital Value-Based Purchasing Program. This program uses measures of clinical processes, efficiency, outcomes, and patient experiences to calculate a total score that results in hospital reimbursement and incentives,12 which creates a direct pathway from patient experience to reimbursement—underscoring hospitals’ desire for ongoing assessment of patient satisfaction.
In 2005, the Centers for Medicare and Medicaid Services and the Agency for Health care Research and Quality developed the Hospital Consumer Assessment of Health care Providers and Systems (HCAHPS) survey in response to criticisms of the Press Ganey survey. The HCAHPS survey consists of 27 questions with 3 broad goals19:
- to produce data about patients’ perspectives of care that allow for objective and meaningful comparisons of hospitals
- to publicly report survey results and create new incentives for hospitals to improve quality of care
- to produce public reports that enhance accountability by increasing transparency.
One difference with the HCAHPS is that it measures frequency, or how often a service was performed (“never”, “sometimes”, “usually”, “always”), whereas Press Ganey measures satisfaction. It also only surveys inpatients and does not address outpatient encounters. Despite the differences, it is a widely used patient satisfaction survey and is subject to similar issues and biases as the Press Ganey survey.
Continue to: Gender, race, and age bias...
Gender, race, and age bias
Although the rationale behind gathering patient input is important, recent data suggest that patient satisfaction surveys are subject to inherent biases.6,13,14 These biases tend to negatively impact women and non-White physicians, adding to the systemic discrimination against women and physicians of color that already exists in health care.
In a single-site retrospective study performed in 2018 by Rogo-Gupta et al, female gynecologists were found to be 47% less likely to receive top patient satisfaction scores than their male counterparts owing to their gender alone, suggesting that gender bias may impact the results of patient satisfaction questionnaires.13 The authors encouraged that the results of patient satisfaction surveys be interpreted with great caution until the impact on female physicians is better understood.
A multi-center study by the same group (Rogo-Gupta et al) assessed the same construct across 5 different geographically diverse institutions.15 This study confirmed that female gynecologists were less likely to receive a top satisfaction score from their patients (19% lower odds when compared with male gynecologists). They also studied the effects of other patient demographics, including age, race/ethnicity, and race concordance. Older patients (aged ≥63 years) had an over-3-fold increase in odds of providing a top satisfaction score than younger patients. Additionally, Asian physicians had significantly lower odds of receiving a top satisfaction score when compared with White physicians, while Asian patients had significantly lower odds of providing a top satisfaction score when compared with White patients. Lastly, in most cases, when underrepresented-in-medicine patients saw an underrepresented-in-medicine physician (race concordance), there was a significant increase in odds of receiving a top satisfaction score. Asian race concordance, however, actually resulted in a lower likelihood of receiving a top satisfaction score.15
Literature from other specialties supports these findings. These results are consistent with emerging data from other medical specialties that also suggest that Press Ganey survey data are subject to inherent biases. For example, data from emergency medicine literature have shown discrepancies between patient satisfaction for providers at tertiary inner-city institutions versus those in affluent suburban populations,16 and that worse mortality is actually correlated with better patient satisfaction scores, and vice versa.17
Another study by Sotto-Santiago in 2019 assessed patient satisfaction scores in multiple specialties at a single institution where quality-related financial incentives were offered based on this metric. They found a significant difference in patient satisfaction scores between underrepresented and White physicians, which suggests a potential bias among patients and institutional practices—ultimately leading to pay inequities through differences in financial incentives.18
Percentile differences reveal small gaps in satisfaction ratings
When examining the difference between raw Press Ganey patient satisfaction data and the percentiles associated with these scores, an interesting finding arises. Looking at the 2023 multicenter study by Rogo-Gupta et al, the difference in the top raw scores between male and female gynecologists appears to be small (3.3%).15 However, in 2020, the difference in top scores separating the top (75th) and bottom (25th) percentile quartiles of physicians was also small, at only 6.9%.
Considering the percentiles, if a provider who scores in the 25th percentile is compared with a colleague who scores in the 75th percentile, they may think the reported satisfaction score differences were quite large. This may potentially invoke feelings of decreased self-worth, negatively impact their professional identity or overall well-being, and they may seek (or be told to seek) improvement opportunities. Now imagine the provider in question realizes the difference between the 25th percentile and 75th percentile is actually only 6.9%. This information may completely change how the results are interpreted and acted upon by administrators. This is further changed with the understanding that 3.3% of the difference may be due to gender alone, narrowing the gap even further. Providers would become understandably frustrated if measures of success such as reimbursement, financial bonus or incentives, promotion, or advancement are linked to these results. It violates the value of fairness and does not offer an equitable starting point.
Evolution of the data distribution. Another consideration, as noted by Robert C. Lloyd, PhD, one of the statisticians who helped develop the percentile statistical analysis mapping in 1985, is that it was based on a classic bell-shaped distribution of patient satisfaction survey scores.19 Because hospitals, medical groups, and physicians have been working these past 20 years to achieve higher Press Ganey scores, the data no longer have a bell-shaped distribution. Rather, there are significant clusters of raw scores at the high end with a very narrow response range. When these data are mapped to the percentile spectrum, they are highly inaccurate.19
Impact of sample size. According to Press Ganey, a minimum of 30 survey responses collected over the designated time period is necessary to draw meaningful conclusions of the data for a specific individual, program, or hospital. Despite this requirement to achieve statistical significance, Sullivan and DeLucia found that the firm often provides comparative data about hospital departments and individual physicians based on a smaller sample size that may create an unacceptably large margin of error.20 Sullivan, for example, said his department may only have 8 to 10 Press Ganey survey responses per month and yet still receives monthly reports from the company analyzing the data. Because of the small sample size, 1 month his department ranked in the 1st percentile and 2 months later it ranked in the 99th percentile.20
The effect of a high ceiling rate. A psychometrics report for the Press Ganey survey is available from the vendor that provides vague assessments of reliability and validity based on 2,762 surveys from 12 practices across 10 states. This report describes a 12-question version of the survey with “no problems encountered” with missingness and response variability. The report further states that the Press Ganey survey demonstrates construct, convergent, divergent, and predictive validities, and high reliability; however, these data are not made available.1
In response to this report, Presson et al analyzed more than 34,000 surveys from one institution to evaluate the reliability and validity of the Press Ganey survey.21 Overall, the survey demonstrated suitable psychometric properties for most metrics. However, Presson et al noted a significantly high ceiling rate of 29.3% for the total score, which ranged from 55.4% to 84.1% across items.21 (Ceiling rates are considered substantial if they occur more than 20% of the time.) Ultimately, a high ceiling rate reduces the power to discriminate between patients who have high satisfaction (everyone is “happy”) with those who are just slightly less than happy, but not dissatisfied. This data quality metric can impact the reliability and validity of a survey—and substantial ceiling rates can notably impact percentile rankings of scores within an institution, offering a possible explanation for the small percentage change between the top and bottom percentiles.
Continue to: Other issues with surveys...
Other issues with surveys
In addition to the limitations associated with percentile groupings, survey data are always subject to nonresponse bias, and small sample size can lead to nonsignificant results. Skewed responses also can make it difficult to identify true outlying providers who may need remediation or may be offering a superior patient experience. Satisfaction surveys also lack an assessment of objective data and instead assess how patients perceive and feel, which introduces subjectivity to the results.
Additionally, focusing on improving patient experience ratings can incentivize unnecessary or inappropriate care (ie, overprescribing of narcotics, prescribing antibiotics when not indicated, or ordering testing that may not change management). Some physicians even state that they are not getting the type of feedback that they are asking for and that the survey is not asking the right questions to elicit patient input that is meaningful to their practice. Lastly, the incorporation of trainees and advanced practice providers in the patient care experience leads to the assessment of an alternative provider being included in the ultimate score and may not be representative of that physician.
Patients’ perception and survey results. In some circumstances, the patient’s understanding of their medical situation may affect their responses. Some may argue that patients may mistake a physician’s confidence for competence, when in reality these two entities are mutually exclusive. In a randomized controlled trial, researchers from Mount Sinai School of Medicine and Columbia University Medical Center surveyed inner-city women with newly diagnosed and surgically treated early-stage breast cancer for their perceived quality of care and the process of getting care, including referrals, test results, and treatments. They compared the responses with patient records to determine the actual quality of care. Of the 374 women who received treatment for early-stage breast cancer, 55% said they received “excellent care,” but most—88%—actually got care that was in line with the best current treatment guidelines. Interestingly, the study found African American women were less likely to report excellent care than White or Hispanic women, less likely to trust their doctor, and more likely to say they experienced bias during the process. However, there was no difference in actual quality of care received in any group.22
You can’t improve what you can’t control. Ultimately, while many providers think patient satisfaction survey results may help inform some aspects of their practice, they cannot improve what they cannot control. For example, the multicenter study by Rogo-Gupta et al found that older patients (≥63 years) have more than a 3-fold increase in odds of giving a top satisfaction score than younger patients (≤33 years), independent of other aspects of the care experience.15 Additionally, they found that older physicians (≥56 years) had a significant increase in odds of receiving a top satisfaction score when compared with physicians who were younger than 55 years old.15 Given that physicians clearly cannot control their own age or the age of their patients, the negative impacts of these biases need to be addressed and remedied at a systems level.
Why might these biases exist?
While we cannot completely understand all of the possible explanations for these biases, it is important to emphasize the long-standing prejudice and discrimination against women and people of color in our society and how this has impacted our behavior. While strides have been made, there clearly still seems to be a difference between what we say and how our biases impact our behavior. Women are still tougher on women in professional evaluations in other fields as well23; it is not unique to medicine. While workplace improvements are slowly changing, women still face inequities. The more research we publish to describe it, the more we hope the conversation continues, allowing us to reduce the impact of bias on our sense of self-worth and identity related to our careers, narrow the pay gap, and see women advance at the same rate as male counterparts. Considerable transformation is crucial to prevent further workforce attrition.
With regard to the lower scores provided by Asian patients, studies suggest that cultural response bias, rather than true differences in quality of care, may account for these discrepancies. Previous literature has found that Asian patients are more likely to select midpoints, rather than extremes, when completing Likert-type studies24 and are not more likely to change medical providers than other race/ethnicities, indicating that lower ratings may not necessarily imply greater dissatisfaction with care.25
Far-reaching effects on finances, income, well-being, job satisfaction, etc.
Depending on how the results are distributed and used, the effects of patient satisfaction surveys can extend well beyond the original intentions. At some institutions, income for physicians is directly tied to their Press Ganey satisfaction scores, which could have profound implications for female and Asian physicians,13,15 who would be paid less—resulting in a wider pay gap than already exists.18
When negative and not constructive, patient evaluations can contribute to physician burnout and a loss of productive members of the workforce.26 This is especially important in obstetrics and gynecology, where physicians are most likely to experience burnout due to multiple factors such as high-risk medical conditions, pressures of the electronic medical record (EMR), the medicolegal environment, and difficulty balancing patient expectations for autonomy with professional judgement.27 Burnout also disproportionately affects women and younger physicians, which is especially concerning given that, in 2017, approximately one-third of practicing obstetrician/gynecologists were women, while that same year more than 80% of trainees matching into the field were women.28 In one survey sent to members of a prominent medical society, 20% of the medical professionals who responded said they have had their employment threatened by low patient satisfaction scores, 78% reported that patient satisfaction surveys moderately or severely affected their job satisfaction, and 28% stated they had considered quitting their job or leaving the medical profession.29Another related effect is the association between malpractice proceedings and a lack of satisfaction with perceived quality of physician-patient communication.30 This may be an important determinant of malpractice lawsuits, and ensuring high patient satisfaction may be a form of defensive medicine.
Continue to: Controlling the narrative for the future: Proposed strategies...
Controlling the narrative for the future: Proposed strategies
The rapid, widespread adoption of the Press Ganey survey across specialties, clinical care settings, and geographic areas may have been largely due to the ease and operational benefits for hospitals rather than after rigorous study and validation. For example, repeated use of a specific measurement tool may facilitate comparison across areas within a hospital but also across institutions, which can help assess performance at a national level. Hospitals also may have a financial incentive to work with a single third-party or vendor instead of using multiple options across multiple vendors. However, the impact of adoption of novel measures of performance should be evaluated prior to widespread adoption and utilization.
A similar example of an emergence of a technological advancement that has changed the field of medicine and how we provide care is the EMR. Epic is now the most commonly used medical record system and holds the market share of the industry, covering 78% of patients in the United States.31 While there are certainly many potential benefits of a common EMR, such as ease of information sharing and standardization of formatting, opportunities are identified in real time and require product adjustment. For example, modifications have been made to accurately represent gender outside of the previously used dichotomous options. Diagnoses such as cervical cancer screening can now be used even if the patient gender is listed as male.
Similarly, the Press Ganey and other patient satisfaction questionnaires should be evaluated and modified to address existing societal biases. The World Health Organization estimates that it will take 300 years to fix gender inequality,32 but we have an opportunity now to control the narrative and improve patient feedback.
Future research avenues
Ultimately, there is a need to further explore currently available methods of evaluating clinical encounters to better understand the inherent biases and limitations. We hope this review will encourage other physicians to examine their specialties and hospitals and require similar analyses from vendors of such satisfaction rating products prior to using them. At the very least, health systems should be willing to partner with vendors and physicians on an ongoing basis to better understand the biases involved in these survey results and make modifications as needed. Patients also obtain information from and contribute to self-reported, publicly available websites; therefore, additional exploration into a nationalized standard for assessing patient satisfaction also may serve as an opportunity to standardize the information patients evaluate.33 Further assessment of the potential financial and emotional impact of using the currently available patient-reported surveys on female physicians, Asian physicians, young physicians, and physicians who see young patients is needed. It is time to put pressure on a broken patient satisfaction system and improve on a national level to avoid undue negative consequences on our physicians. Use of patient satisfaction survey data should be limited until we all understand and account for the biases that are evident. ●
- Appeal to the Press Ganey corporation with the results of recent data and other studies to ensure they are aware of the biases that exist in their product
- Appeal to hospital-level administration to refrain from using Press Ganey scores as a tool to dictate reimbursement; instead rely on other more objective measures of performance (such as publications, presentations, research accomplishments, patient and surgical outcomes, promotion, committees, national leadership roles, etc)
- Apply a “corrective factor” or “adjustment factor” to eliminate the baseline discrepancy between scores for men and women
- Consider moving to an alternative survey methodology
- Provide patient education to define “performance” (ie, frame what a patient can expect from a provider such as being on time, courteous, and empathetic; caution against asking patients to assess competence and knowledge)

Patient satisfaction questionnaires were developed in the 1980s as part of the movement to better understand the patient’s experience and their perspective of the quality of care. In 1985, the Press Ganey survey—now the most widely used method to assess patient satisfaction—was developed by 2 professors in anthropology and sociology-statistics at Notre Dame. Initially intended for inpatient admissions, the survey was validated based on a few thousand survey results.1 Given the strong interest in improving patient satisfaction at the time, it became widely adopted and quickly expanded into outpatient encounters and ambulatory surgery settings.
Although other surveys have been developed,2 the Press Ganey survey is the most commonly used assessment tool for patient satisfaction metrics in the United States, with approximately 50% of all hospitals and more than 41,000 health care organizations using its services.3,4 The survey consists of 6 domains related to satisfaction with:
1. the care provider
2. the nurse or assistant
3. personal issues
4. overall assessment
5. access
6. moving through the visit.
Survey items are scored using a 5-point Likert scale, with scores ranging from “very poor” (a score of 1) to “very good” (a score of 5). According to the company, because this format is balanced and parallel (unlike a “poor” to “excellent” format), responses can be quantified and used statistically without violating methodologic assumptions. Also, variability in patients’ responses with this format allows for the identification of opportunities to improve, unlike “yes/no” response formats.1 There are limitations to this design, however, which can impact data quality,5 as we will see.
Adoption of the survey as we move toward value-based care
More recently, patients’ satisfaction with their health care has received increased attention as we move to a patient-centered care model and as health care reimbursement models shift toward value-based care. Current trends in health care policy statements include the importance of raising the standard of care and shifting from a “fee-for-service” to a “pay-for-performance” reimbursement model.7,8 As a result, hospitals are establishing systems to measure “performance” that are not nationally standardized or extensively studied with objective measures. The changing standard of health care expectations in the United States is a topic of much public debate.9 And as expectations and new standards are defined, the impact of implementing novel measures of performance should be evaluated prior to widespread adoption and utilization.
Patient satisfaction also has been identified as a driver for hospital finances through loyalty, described as the “likelihood to return to that system for future medical services.”10,11 This measure has contributed to policy changes that reinforce prioritization of patient satisfaction. For example, the Affordable Care Act tied Medicare reimbursement and patient satisfaction together in the Hospital Value-Based Purchasing Program. This program uses measures of clinical processes, efficiency, outcomes, and patient experiences to calculate a total score that results in hospital reimbursement and incentives,12 which creates a direct pathway from patient experience to reimbursement—underscoring hospitals’ desire for ongoing assessment of patient satisfaction.
In 2005, the Centers for Medicare and Medicaid Services and the Agency for Health care Research and Quality developed the Hospital Consumer Assessment of Health care Providers and Systems (HCAHPS) survey in response to criticisms of the Press Ganey survey. The HCAHPS survey consists of 27 questions with 3 broad goals19:
- to produce data about patients’ perspectives of care that allow for objective and meaningful comparisons of hospitals
- to publicly report survey results and create new incentives for hospitals to improve quality of care
- to produce public reports that enhance accountability by increasing transparency.
One difference with the HCAHPS is that it measures frequency, or how often a service was performed (“never”, “sometimes”, “usually”, “always”), whereas Press Ganey measures satisfaction. It also only surveys inpatients and does not address outpatient encounters. Despite the differences, it is a widely used patient satisfaction survey and is subject to similar issues and biases as the Press Ganey survey.
Continue to: Gender, race, and age bias...
Gender, race, and age bias
Although the rationale behind gathering patient input is important, recent data suggest that patient satisfaction surveys are subject to inherent biases.6,13,14 These biases tend to negatively impact women and non-White physicians, adding to the systemic discrimination against women and physicians of color that already exists in health care.
In a single-site retrospective study performed in 2018 by Rogo-Gupta et al, female gynecologists were found to be 47% less likely to receive top patient satisfaction scores than their male counterparts owing to their gender alone, suggesting that gender bias may impact the results of patient satisfaction questionnaires.13 The authors encouraged that the results of patient satisfaction surveys be interpreted with great caution until the impact on female physicians is better understood.
A multi-center study by the same group (Rogo-Gupta et al) assessed the same construct across 5 different geographically diverse institutions.15 This study confirmed that female gynecologists were less likely to receive a top satisfaction score from their patients (19% lower odds when compared with male gynecologists). They also studied the effects of other patient demographics, including age, race/ethnicity, and race concordance. Older patients (aged ≥63 years) had an over-3-fold increase in odds of providing a top satisfaction score than younger patients. Additionally, Asian physicians had significantly lower odds of receiving a top satisfaction score when compared with White physicians, while Asian patients had significantly lower odds of providing a top satisfaction score when compared with White patients. Lastly, in most cases, when underrepresented-in-medicine patients saw an underrepresented-in-medicine physician (race concordance), there was a significant increase in odds of receiving a top satisfaction score. Asian race concordance, however, actually resulted in a lower likelihood of receiving a top satisfaction score.15
Literature from other specialties supports these findings. These results are consistent with emerging data from other medical specialties that also suggest that Press Ganey survey data are subject to inherent biases. For example, data from emergency medicine literature have shown discrepancies between patient satisfaction for providers at tertiary inner-city institutions versus those in affluent suburban populations,16 and that worse mortality is actually correlated with better patient satisfaction scores, and vice versa.17
Another study by Sotto-Santiago in 2019 assessed patient satisfaction scores in multiple specialties at a single institution where quality-related financial incentives were offered based on this metric. They found a significant difference in patient satisfaction scores between underrepresented and White physicians, which suggests a potential bias among patients and institutional practices—ultimately leading to pay inequities through differences in financial incentives.18
Percentile differences reveal small gaps in satisfaction ratings
When examining the difference between raw Press Ganey patient satisfaction data and the percentiles associated with these scores, an interesting finding arises. Looking at the 2023 multicenter study by Rogo-Gupta et al, the difference in the top raw scores between male and female gynecologists appears to be small (3.3%).15 However, in 2020, the difference in top scores separating the top (75th) and bottom (25th) percentile quartiles of physicians was also small, at only 6.9%.
Considering the percentiles, if a provider who scores in the 25th percentile is compared with a colleague who scores in the 75th percentile, they may think the reported satisfaction score differences were quite large. This may potentially invoke feelings of decreased self-worth, negatively impact their professional identity or overall well-being, and they may seek (or be told to seek) improvement opportunities. Now imagine the provider in question realizes the difference between the 25th percentile and 75th percentile is actually only 6.9%. This information may completely change how the results are interpreted and acted upon by administrators. This is further changed with the understanding that 3.3% of the difference may be due to gender alone, narrowing the gap even further. Providers would become understandably frustrated if measures of success such as reimbursement, financial bonus or incentives, promotion, or advancement are linked to these results. It violates the value of fairness and does not offer an equitable starting point.
Evolution of the data distribution. Another consideration, as noted by Robert C. Lloyd, PhD, one of the statisticians who helped develop the percentile statistical analysis mapping in 1985, is that it was based on a classic bell-shaped distribution of patient satisfaction survey scores.19 Because hospitals, medical groups, and physicians have been working these past 20 years to achieve higher Press Ganey scores, the data no longer have a bell-shaped distribution. Rather, there are significant clusters of raw scores at the high end with a very narrow response range. When these data are mapped to the percentile spectrum, they are highly inaccurate.19
Impact of sample size. According to Press Ganey, a minimum of 30 survey responses collected over the designated time period is necessary to draw meaningful conclusions of the data for a specific individual, program, or hospital. Despite this requirement to achieve statistical significance, Sullivan and DeLucia found that the firm often provides comparative data about hospital departments and individual physicians based on a smaller sample size that may create an unacceptably large margin of error.20 Sullivan, for example, said his department may only have 8 to 10 Press Ganey survey responses per month and yet still receives monthly reports from the company analyzing the data. Because of the small sample size, 1 month his department ranked in the 1st percentile and 2 months later it ranked in the 99th percentile.20
The effect of a high ceiling rate. A psychometrics report for the Press Ganey survey is available from the vendor that provides vague assessments of reliability and validity based on 2,762 surveys from 12 practices across 10 states. This report describes a 12-question version of the survey with “no problems encountered” with missingness and response variability. The report further states that the Press Ganey survey demonstrates construct, convergent, divergent, and predictive validities, and high reliability; however, these data are not made available.1
In response to this report, Presson et al analyzed more than 34,000 surveys from one institution to evaluate the reliability and validity of the Press Ganey survey.21 Overall, the survey demonstrated suitable psychometric properties for most metrics. However, Presson et al noted a significantly high ceiling rate of 29.3% for the total score, which ranged from 55.4% to 84.1% across items.21 (Ceiling rates are considered substantial if they occur more than 20% of the time.) Ultimately, a high ceiling rate reduces the power to discriminate between patients who have high satisfaction (everyone is “happy”) with those who are just slightly less than happy, but not dissatisfied. This data quality metric can impact the reliability and validity of a survey—and substantial ceiling rates can notably impact percentile rankings of scores within an institution, offering a possible explanation for the small percentage change between the top and bottom percentiles.
Continue to: Other issues with surveys...
Other issues with surveys
In addition to the limitations associated with percentile groupings, survey data are always subject to nonresponse bias, and small sample size can lead to nonsignificant results. Skewed responses also can make it difficult to identify true outlying providers who may need remediation or may be offering a superior patient experience. Satisfaction surveys also lack an assessment of objective data and instead assess how patients perceive and feel, which introduces subjectivity to the results.
Additionally, focusing on improving patient experience ratings can incentivize unnecessary or inappropriate care (ie, overprescribing of narcotics, prescribing antibiotics when not indicated, or ordering testing that may not change management). Some physicians even state that they are not getting the type of feedback that they are asking for and that the survey is not asking the right questions to elicit patient input that is meaningful to their practice. Lastly, the incorporation of trainees and advanced practice providers in the patient care experience leads to the assessment of an alternative provider being included in the ultimate score and may not be representative of that physician.
Patients’ perception and survey results. In some circumstances, the patient’s understanding of their medical situation may affect their responses. Some may argue that patients may mistake a physician’s confidence for competence, when in reality these two entities are mutually exclusive. In a randomized controlled trial, researchers from Mount Sinai School of Medicine and Columbia University Medical Center surveyed inner-city women with newly diagnosed and surgically treated early-stage breast cancer for their perceived quality of care and the process of getting care, including referrals, test results, and treatments. They compared the responses with patient records to determine the actual quality of care. Of the 374 women who received treatment for early-stage breast cancer, 55% said they received “excellent care,” but most—88%—actually got care that was in line with the best current treatment guidelines. Interestingly, the study found African American women were less likely to report excellent care than White or Hispanic women, less likely to trust their doctor, and more likely to say they experienced bias during the process. However, there was no difference in actual quality of care received in any group.22
You can’t improve what you can’t control. Ultimately, while many providers think patient satisfaction survey results may help inform some aspects of their practice, they cannot improve what they cannot control. For example, the multicenter study by Rogo-Gupta et al found that older patients (≥63 years) have more than a 3-fold increase in odds of giving a top satisfaction score than younger patients (≤33 years), independent of other aspects of the care experience.15 Additionally, they found that older physicians (≥56 years) had a significant increase in odds of receiving a top satisfaction score when compared with physicians who were younger than 55 years old.15 Given that physicians clearly cannot control their own age or the age of their patients, the negative impacts of these biases need to be addressed and remedied at a systems level.
Why might these biases exist?
While we cannot completely understand all of the possible explanations for these biases, it is important to emphasize the long-standing prejudice and discrimination against women and people of color in our society and how this has impacted our behavior. While strides have been made, there clearly still seems to be a difference between what we say and how our biases impact our behavior. Women are still tougher on women in professional evaluations in other fields as well23; it is not unique to medicine. While workplace improvements are slowly changing, women still face inequities. The more research we publish to describe it, the more we hope the conversation continues, allowing us to reduce the impact of bias on our sense of self-worth and identity related to our careers, narrow the pay gap, and see women advance at the same rate as male counterparts. Considerable transformation is crucial to prevent further workforce attrition.
With regard to the lower scores provided by Asian patients, studies suggest that cultural response bias, rather than true differences in quality of care, may account for these discrepancies. Previous literature has found that Asian patients are more likely to select midpoints, rather than extremes, when completing Likert-type studies24 and are not more likely to change medical providers than other race/ethnicities, indicating that lower ratings may not necessarily imply greater dissatisfaction with care.25
Far-reaching effects on finances, income, well-being, job satisfaction, etc.
Depending on how the results are distributed and used, the effects of patient satisfaction surveys can extend well beyond the original intentions. At some institutions, income for physicians is directly tied to their Press Ganey satisfaction scores, which could have profound implications for female and Asian physicians,13,15 who would be paid less—resulting in a wider pay gap than already exists.18
When negative and not constructive, patient evaluations can contribute to physician burnout and a loss of productive members of the workforce.26 This is especially important in obstetrics and gynecology, where physicians are most likely to experience burnout due to multiple factors such as high-risk medical conditions, pressures of the electronic medical record (EMR), the medicolegal environment, and difficulty balancing patient expectations for autonomy with professional judgement.27 Burnout also disproportionately affects women and younger physicians, which is especially concerning given that, in 2017, approximately one-third of practicing obstetrician/gynecologists were women, while that same year more than 80% of trainees matching into the field were women.28 In one survey sent to members of a prominent medical society, 20% of the medical professionals who responded said they have had their employment threatened by low patient satisfaction scores, 78% reported that patient satisfaction surveys moderately or severely affected their job satisfaction, and 28% stated they had considered quitting their job or leaving the medical profession.29Another related effect is the association between malpractice proceedings and a lack of satisfaction with perceived quality of physician-patient communication.30 This may be an important determinant of malpractice lawsuits, and ensuring high patient satisfaction may be a form of defensive medicine.
Continue to: Controlling the narrative for the future: Proposed strategies...
Controlling the narrative for the future: Proposed strategies
The rapid, widespread adoption of the Press Ganey survey across specialties, clinical care settings, and geographic areas may have been largely due to the ease and operational benefits for hospitals rather than after rigorous study and validation. For example, repeated use of a specific measurement tool may facilitate comparison across areas within a hospital but also across institutions, which can help assess performance at a national level. Hospitals also may have a financial incentive to work with a single third-party or vendor instead of using multiple options across multiple vendors. However, the impact of adoption of novel measures of performance should be evaluated prior to widespread adoption and utilization.
A similar example of an emergence of a technological advancement that has changed the field of medicine and how we provide care is the EMR. Epic is now the most commonly used medical record system and holds the market share of the industry, covering 78% of patients in the United States.31 While there are certainly many potential benefits of a common EMR, such as ease of information sharing and standardization of formatting, opportunities are identified in real time and require product adjustment. For example, modifications have been made to accurately represent gender outside of the previously used dichotomous options. Diagnoses such as cervical cancer screening can now be used even if the patient gender is listed as male.
Similarly, the Press Ganey and other patient satisfaction questionnaires should be evaluated and modified to address existing societal biases. The World Health Organization estimates that it will take 300 years to fix gender inequality,32 but we have an opportunity now to control the narrative and improve patient feedback.
Future research avenues
Ultimately, there is a need to further explore currently available methods of evaluating clinical encounters to better understand the inherent biases and limitations. We hope this review will encourage other physicians to examine their specialties and hospitals and require similar analyses from vendors of such satisfaction rating products prior to using them. At the very least, health systems should be willing to partner with vendors and physicians on an ongoing basis to better understand the biases involved in these survey results and make modifications as needed. Patients also obtain information from and contribute to self-reported, publicly available websites; therefore, additional exploration into a nationalized standard for assessing patient satisfaction also may serve as an opportunity to standardize the information patients evaluate.33 Further assessment of the potential financial and emotional impact of using the currently available patient-reported surveys on female physicians, Asian physicians, young physicians, and physicians who see young patients is needed. It is time to put pressure on a broken patient satisfaction system and improve on a national level to avoid undue negative consequences on our physicians. Use of patient satisfaction survey data should be limited until we all understand and account for the biases that are evident. ●
- Appeal to the Press Ganey corporation with the results of recent data and other studies to ensure they are aware of the biases that exist in their product
- Appeal to hospital-level administration to refrain from using Press Ganey scores as a tool to dictate reimbursement; instead rely on other more objective measures of performance (such as publications, presentations, research accomplishments, patient and surgical outcomes, promotion, committees, national leadership roles, etc)
- Apply a “corrective factor” or “adjustment factor” to eliminate the baseline discrepancy between scores for men and women
- Consider moving to an alternative survey methodology
- Provide patient education to define “performance” (ie, frame what a patient can expect from a provider such as being on time, courteous, and empathetic; caution against asking patients to assess competence and knowledge)
- Outpatient Services (OU) Survey Psychometrics Report. Published online 2019.
- Zusman EE. HCAHPS replaces Press Ganey Survey as quality measure for patient hospital experience. Neurosurgery. 2012;71:N21-N24. doi: 10.1227/01.neu.0000417536.07871.ed
- Press Ganey. Company. Accessed April 20, 2023. www.pressganey. com/company/
- Press, Ganey--first year of patient satisfaction measurement. Hosp Guest Relations Rep. 1986;1:4-5.
- DeCastellarnau A. A classification of response scale characteristics that affect data quality: a literature review. Qual Quant. 2018;52:15231559. doi: 10.1007/s11135-017-0533-4
- Tyser AR, Abtahi AM, McFadden M, et al. Evidence of non-response bias in the Press-Ganey patient satisfaction survey. BMC Health Serv Res. 2016;16:350. doi: 10.1186/s12913-016-1595-z
- Duseja R, Durham M, Schreiber M. CMS quality measure development. JAMA. 2020;324:1213-1214. doi: 10.1001/jama.2020.12070
- Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. National Academies Press; 2001. doi: 10.17226/10027
- Parmet WE. Health: policy or law? A population-based analysis of the Supreme Court’s ACA cases. J Health Polit Policy Law. 2016;41:10611081. doi: 10.1215/03616878-3665949
- Richter JP, Muhlestein DB. Patient experience and hospital profitability: is there a link? Health Care Manage Rev. 2017;42:247-257. doi: 10.1097/HMR.0000000000000105
- Huang C-H, Wu H-H, Lee Y-C, et al. What role does patient gratitude play in the relationship between relationship quality and patient loyalty? Inquiry. 2019;56:46958019868324. doi: 10.1177/0046958019868324
- Centers for Medicare & Medicaid Services (CMS), HHS. Medicare program; hospital inpatient value-based purchasing program. Final rule. Fed Regist. 2011;76:26490-26547.
- Rogo-Gupta LJ, Haunschild C, Altamirano J, et al. Physician gender is associated with Press Ganey patient satisfaction scores in outpatient gynecology. Womens Health Issues. 2018;28:281-285. doi: 10.1016 /j.whi.2018.01.001
- DeLoughery EP. Physician race and specialty influence Press Ganey survey results. Neth J Med. 2019;77:366-369.
- Homewood L, Altamirano J, Fassiotto M, et al. Women gynecologists receive lower Press Ganey patient satisfaction scores in a multicenter cross-sectional study. Am J Obstet Gynecol. 2023;228:S801. doi: 10.1016/j.ajog.2022.12.025
- Sharp B, Johnson J, Hamedani AG, et al. What are we measuring? Evaluating physician-specific satisfaction scores between emergency departments. West J Emerg Med. 2019;20:454-459. doi: 10.5811 /westjem.2019.4.41040
- Mosley M. Viewpoint: Press Ganey is a worthless tool for the ED. Emerg Med News. 2019;41:3-4. doi: 10.1097/01.EEM.0000616512.68475.69
- Sotto-Santiago S, Slaven JE, Rohr-Kirchgraber T. (Dis)Incentivizing patient satisfaction metrics: the unintended consequences of institutional bias. Health Equity. 2019;3:13-18. doi: 10.1089/heq.2018.0065
- Lloyd RC. Quality Health Care: A Guide to Developing and Using Indicators. 2nd ed. Jones & Bartlett Learning; 2019. Accessed April 23, 2023. www.jblearning.com/catalog/productdetails /9781284023077
- 2+2=7? Seven things you may not know about Press Ganey statistics. Emergency Physicians Monthly. Accessed April 23, 2023. epmonthly. com/article/227-seven-things-you-may-not-know-about-pressgainey-statistics/
- Presson AP, Zhang C, Abtahi AM, et al. Psychometric properties of the Press Ganey® Outpatient Medical Practice Survey. Health Qual Life Outcomes. 2017;15:32. doi: 10.1186/s12955-017-0610-3
- Bickell NA, Neuman J, Fei K, et al. Quality of breast cancer care: perception versus practice. J Clin Oncol. 2012;30:1791-1795. doi: 10.1200 /JCO.2011.38.7605
- Strauss K. Women in the workplace: are women tougher on other women? Forbes. July 18, 2016. Accessed April 27, 2023. www.forbes. com/sites/karstenstrauss/2016/07/18/women-in-the-workplace -are-women-tougher-on-other-women/
- Lee JW, Jones PS, Mineyama Y, et al. Cultural differences in responses to a Likert scale. Res Nurs Health. 2002;25:295-306. doi: 10.1002 /nur.10041
- Saha S, Hickam DH. Explaining low ratings of patient satisfaction among Asian-Americans. Am J Med Qual. 2003;18:256-264. doi: 10.1177/106286060301800606
- Halbesleben JRB, Rathert C. Linking physician burnout and patient outcomes: exploring the dyadic relationship between physicians and patients. Health Care Manage Rev. 2008;33:29-39. doi: 10.1097/01. HMR.0000304493.87898.72
- Bradford L, Glaser G. Addressing physician burnout and ensuring high-quality care of the physician workforce. Obstet Gynecol. 2021;137:3-11. doi: 10.1097/AOG.0000000000004197
- Boyle P. Nation’s physician workforce evolves: more women, a bit older, and toward different specialties. AAMCNEWS. February 2, 2021. Accessed April 20, 2023. www.aamc.org/news-insights/nations-physician-workforce-evolves-more-women-bit-older-and-towarddifferent-specialties
- Zgierska A, Rabago D, Miller MM. Impact of patient satisfaction ratings on physicians and clinical care. Patient Prefer Adherence. 2014;8:437-446. doi: 10.2147/PPA.S59077
- Yeh J, Nagel EE. Patient satisfaction in obstetrics and gynecology: individualized patient-centered communication. Clin Med Insights Womens Health. 2010;3:23. doi: 10.4137/CMWH.S5870
- Epic. About us. Accessed April 19, 2023. www.epic.com/about
- United Nations. Without investment, gender equality will take nearly 300 years: UN report. September 7, 2022. Accessed April 19, 2023. news.un.org/en/story/2022/09/1126171
- Ryan T, Specht J, Smith S, et al. Does the Press Ganey Survey correlate to online health grades for a major academic otolaryngology department? Otolaryngol Head Neck Surg. 2016;155:411-415. doi: 10.1177/0194599816652386
- Outpatient Services (OU) Survey Psychometrics Report. Published online 2019.
- Zusman EE. HCAHPS replaces Press Ganey Survey as quality measure for patient hospital experience. Neurosurgery. 2012;71:N21-N24. doi: 10.1227/01.neu.0000417536.07871.ed
- Press Ganey. Company. Accessed April 20, 2023. www.pressganey. com/company/
- Press, Ganey--first year of patient satisfaction measurement. Hosp Guest Relations Rep. 1986;1:4-5.
- DeCastellarnau A. A classification of response scale characteristics that affect data quality: a literature review. Qual Quant. 2018;52:15231559. doi: 10.1007/s11135-017-0533-4
- Tyser AR, Abtahi AM, McFadden M, et al. Evidence of non-response bias in the Press-Ganey patient satisfaction survey. BMC Health Serv Res. 2016;16:350. doi: 10.1186/s12913-016-1595-z
- Duseja R, Durham M, Schreiber M. CMS quality measure development. JAMA. 2020;324:1213-1214. doi: 10.1001/jama.2020.12070
- Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. National Academies Press; 2001. doi: 10.17226/10027
- Parmet WE. Health: policy or law? A population-based analysis of the Supreme Court’s ACA cases. J Health Polit Policy Law. 2016;41:10611081. doi: 10.1215/03616878-3665949
- Richter JP, Muhlestein DB. Patient experience and hospital profitability: is there a link? Health Care Manage Rev. 2017;42:247-257. doi: 10.1097/HMR.0000000000000105
- Huang C-H, Wu H-H, Lee Y-C, et al. What role does patient gratitude play in the relationship between relationship quality and patient loyalty? Inquiry. 2019;56:46958019868324. doi: 10.1177/0046958019868324
- Centers for Medicare & Medicaid Services (CMS), HHS. Medicare program; hospital inpatient value-based purchasing program. Final rule. Fed Regist. 2011;76:26490-26547.
- Rogo-Gupta LJ, Haunschild C, Altamirano J, et al. Physician gender is associated with Press Ganey patient satisfaction scores in outpatient gynecology. Womens Health Issues. 2018;28:281-285. doi: 10.1016 /j.whi.2018.01.001
- DeLoughery EP. Physician race and specialty influence Press Ganey survey results. Neth J Med. 2019;77:366-369.
- Homewood L, Altamirano J, Fassiotto M, et al. Women gynecologists receive lower Press Ganey patient satisfaction scores in a multicenter cross-sectional study. Am J Obstet Gynecol. 2023;228:S801. doi: 10.1016/j.ajog.2022.12.025
- Sharp B, Johnson J, Hamedani AG, et al. What are we measuring? Evaluating physician-specific satisfaction scores between emergency departments. West J Emerg Med. 2019;20:454-459. doi: 10.5811 /westjem.2019.4.41040
- Mosley M. Viewpoint: Press Ganey is a worthless tool for the ED. Emerg Med News. 2019;41:3-4. doi: 10.1097/01.EEM.0000616512.68475.69
- Sotto-Santiago S, Slaven JE, Rohr-Kirchgraber T. (Dis)Incentivizing patient satisfaction metrics: the unintended consequences of institutional bias. Health Equity. 2019;3:13-18. doi: 10.1089/heq.2018.0065
- Lloyd RC. Quality Health Care: A Guide to Developing and Using Indicators. 2nd ed. Jones & Bartlett Learning; 2019. Accessed April 23, 2023. www.jblearning.com/catalog/productdetails /9781284023077
- 2+2=7? Seven things you may not know about Press Ganey statistics. Emergency Physicians Monthly. Accessed April 23, 2023. epmonthly. com/article/227-seven-things-you-may-not-know-about-pressgainey-statistics/
- Presson AP, Zhang C, Abtahi AM, et al. Psychometric properties of the Press Ganey® Outpatient Medical Practice Survey. Health Qual Life Outcomes. 2017;15:32. doi: 10.1186/s12955-017-0610-3
- Bickell NA, Neuman J, Fei K, et al. Quality of breast cancer care: perception versus practice. J Clin Oncol. 2012;30:1791-1795. doi: 10.1200 /JCO.2011.38.7605
- Strauss K. Women in the workplace: are women tougher on other women? Forbes. July 18, 2016. Accessed April 27, 2023. www.forbes. com/sites/karstenstrauss/2016/07/18/women-in-the-workplace -are-women-tougher-on-other-women/
- Lee JW, Jones PS, Mineyama Y, et al. Cultural differences in responses to a Likert scale. Res Nurs Health. 2002;25:295-306. doi: 10.1002 /nur.10041
- Saha S, Hickam DH. Explaining low ratings of patient satisfaction among Asian-Americans. Am J Med Qual. 2003;18:256-264. doi: 10.1177/106286060301800606
- Halbesleben JRB, Rathert C. Linking physician burnout and patient outcomes: exploring the dyadic relationship between physicians and patients. Health Care Manage Rev. 2008;33:29-39. doi: 10.1097/01. HMR.0000304493.87898.72
- Bradford L, Glaser G. Addressing physician burnout and ensuring high-quality care of the physician workforce. Obstet Gynecol. 2021;137:3-11. doi: 10.1097/AOG.0000000000004197
- Boyle P. Nation’s physician workforce evolves: more women, a bit older, and toward different specialties. AAMCNEWS. February 2, 2021. Accessed April 20, 2023. www.aamc.org/news-insights/nations-physician-workforce-evolves-more-women-bit-older-and-towarddifferent-specialties
- Zgierska A, Rabago D, Miller MM. Impact of patient satisfaction ratings on physicians and clinical care. Patient Prefer Adherence. 2014;8:437-446. doi: 10.2147/PPA.S59077
- Yeh J, Nagel EE. Patient satisfaction in obstetrics and gynecology: individualized patient-centered communication. Clin Med Insights Womens Health. 2010;3:23. doi: 10.4137/CMWH.S5870
- Epic. About us. Accessed April 19, 2023. www.epic.com/about
- United Nations. Without investment, gender equality will take nearly 300 years: UN report. September 7, 2022. Accessed April 19, 2023. news.un.org/en/story/2022/09/1126171
- Ryan T, Specht J, Smith S, et al. Does the Press Ganey Survey correlate to online health grades for a major academic otolaryngology department? Otolaryngol Head Neck Surg. 2016;155:411-415. doi: 10.1177/0194599816652386
2023 Update on menopause
This year’s menopause Update highlights a highly effective nonhormonal medication that recently received approval by the US Food and Drug Administration (FDA) for the treatment of bothersome menopausal vasomotor symptoms. In addition, the Update provides guidance regarding how ObGyns should respond when an endometrial biopsy for postmenopausal bleeding reveals proliferative changes.
Breakthrough in women’s health: A new nonhormone therapy for vasomotor symptoms
Johnson KA, Martin N, Nappi RE, et al. Efficacy and safety of fezolinetant in moderate-to-severe vasomotor symptoms associated with menopause: a phase 3 RCT. J Clin Endocrinol Metab. 2023;dgad058. doi:10.1210/clinem/dgad058.
Lederman S, Ottery FD, Cano A, et al. Fezolinetant for treatment of moderate-to-severe vasomotor symptoms associated with menopause (SKYLIGHT 1): a phase 3 randomised controlled study. Lancet. 2023;401:1091-1102. doi:10.1016/S0140-6736(23)00085-5.
A new oral nonestrogen-containing medication for relief of moderate to severe hot flashes, fezolinetant (Veozah) 45 mg daily, has been approved by the FDA and was expected to be available by the end of May 2023. Fezolinetant is a selective neurokinin 3 (NK3) receptor antagonistthat offers a targeted nonhormonal approach to menopausal vasomotor symptoms (VMS), and it is the first in its class to make it to market.
The decline in estrogen at menopause appears to result in increased signaling at kisspeptin/neurokinin B/dynorphin (KNDy) neurons in the thermoregulatory center within the hypothalamus with resultant increases in hot flashes.1,2 Fezolinetant works by binding to and blocking the activities of the NK3 receptor.3-5
Key study findings
Selective NK3 receptor antagonists, including fezolinetant, effectively reduce the frequency and severity of VMS comparable to that of hormone therapy (HT). Two phase 3 clinical trials, Skylight 1 and 2, confirmed the efficacy and safety of fezolinetant 45 mg in treating VMS,6,7 and an additional 52-week placebo-controlled study, Skylight 4, confirmed long-term safety.8 Onset of action occurs within a week. Reported adverse events occurred in 1% to 2% of healthy menopausal women participating in clinical trials; these included headaches, abdominal pain, diarrhea, insomnia, back pain, hot flushes, and reversible elevated hepatic transaminase levels.6-9
The published phase 2 trials9 and the international randomized controlled trial (RCT) 12-week studies, Skylight 1 and 2,6,7 found that once-daily 30-mg and 45-mg doses of fezolinetant significantly reduced VMS frequency and severity at 12 weeks among women aged 40 to 60 years who reported an average of 7 moderate to severe VMS/day; the reduction in reported VMS was sustained at 40 weeks. Phase 3 data from Skylight 1 and 2 demonstrated fezolinetant’s efficacy in reducing the frequency and severity of VMS and provided information on the safety profile of fezolinetant compared with placebo over 12 weeks and a noncontrolled extension for an additional 40 weeks.6,7
Oral fezolinetant was associated with improved quality of life, including reduced VMS-related interference with daily life.10 Johnson and colleagues, reporting for Skylight 2, found VMS frequency and severity improvement by week 1, which achieved statistical significance at weeks 4 and 12, with this improvement maintained through week 52.6 A 64.3% reduction in mean daily VMS from baseline was seen at 12 weeks for fezolinetant 45 mg compared with a 45.4% reduction for placebo. VMS severity significantly decreased compared with placebo at 4 and 12 weeks.6
Serious treatment-emergent adverse events were infrequent, reported by 2%, 1%, and 0% of those receiving fezolinetant 30 mg, fezolinetant 45 mg, and placebo.6 Increases in levels of alanine aminotransferase (ALT) or aspartate aminotransferase (AST) were noted and were described as asymptomatic, isolated, intermittent, or transient, and these levels returned to baseline during treatment or after discontinuation.6
Of the 5 participants taking fezolinetant in Skyline 1 with ALT or AST levels greater than 3 times the upper limit of normal in the 12-week randomized trial, levels returned to normal range while continuing treatment in 2 participants, with treatment interruption in 2, and with discontinuation in 1. No new safety signals were seen in the 40-week extension trial.6
Fezolinetant offers a much-needed effective and safe selective nonhormone NK3 receptor antagonist therapy that reduces the frequency and severity of menopausal VMS and has been shown to be safe through 52 weeks of treatment.
To read more about how fezolinetant specifically targets the hormone receptor that triggers hot flashes as well as on prescribing hormone therapy for women with menopausal symptoms, see “Focus on menopause: Q&A with Jan Shifren, MD, and Genevieve NealPerry, MD, PhD,” in the December 2022 issue of OBG Management at https://www.mdedge.com/obgyn/article/260380/menopause
Continue to: Endometrial and bone safety...
Endometrial and bone safety
Results from Skylight 4, a phase 3, randomized, double-blind, 52-week safety study, provided additional evidence that confirmed the longer-term safety of fezolinetant over a 52-week treatment period.8
Endometrial safety was assessed in postmenopausal women with normal baseline endometrium (n = 599).8 For fezolinetant 45 mg, 1 of 203 participants had endometrial hyperplasia (EH) (0.5%; upper limit of one-sided 95% confidence interval [CI], 2.3%); no cases of EH were noted in the placebo (0 of 186) or fezolinetant 30-mg (0 of 210) groups. The incidence of EH or malignancy in fezolinetant-treated participants was within prespecified limits, as assessed by blinded, centrally read endometrial biopsies. Endometrial malignancy occurred in 1 of 210 in the fezolinetant 30-mg group (0.5%; 95% CI, 2.2%) with no cases in the other groups, thus meeting FDA requirements for endometrial safety.8
In addition, no significant differences were noted in change from baseline endometrial thickness on transvaginal ultrasonography between fezolinetant-treated and placebo groups. Likewise, no loss of bone density was found on dual-energy x-ray absorptiometry (DEXA) scans or trabecular bone scores.8
Liver safety
Although no cases of severe liver injury were noted, elevations in serum transaminase concentrations greater than 3 times the upper limit of normal were observed in the clinical trials. In Skylight 4, liver enzyme elevations more than 3 times the upper limit of normal occurred in 6 of 583 participants taking placebo, 8 of 590 taking fezolinetant 30 mg, and 12 of 589 taking fezolinetant 45 mg.8
The prescribing information for fezolinetant includes a warning for elevated hepatic transaminases: Fezolinetant should not be started if baseline serum transaminase concentration is equal to or exceeds 2 times the upper limit of normal. Liver tests should be obtained at baseline and repeated every 3 months for the first 9 months and then if symptoms suggest liver injury.11,12
Unmet need for nonhormone treatment of VMS
Vasomotor symptoms affect up to 80% of women, with approximately 25% bothersome enough to warrant treatment. Vasomotor symptoms persist for a median of 7 years, with duration and severity differing by race and ethnicity. Black, Hispanic, and possibly Native American women experience the highest burden of VMS.2 Although VMS, including hot flashes, night sweats, and mood and sleep disturbances, often are considered an annoyance to those with mild symptoms, moderate to severe VMS impact women’s lives, including functioning at home or work, affecting relationships, and decreasing perceived quality of life, and they have been associated with workplace absenteeism and increased health care costs, both direct from medical care and testing and indirect costs from lost work.13-15
Women with 7 or more daily moderate to severe VMS (defined as with sweating or affecting function) reported interference with sleep (94%), concentration (84%), mood (85%), energy (77%), and sexual activity (61%).16 Moderately to severely bothersome VMS have been associated with impaired psychological and general well-being, affecting work performance.17 Based on a Mayo Clinic workplace survey, Faubion and colleagues estimated an annual loss of $1.8 billion in the United States for menopause-related missed work and a $28 billion loss when medical expenses were added.15
Menopausal HT has been the primary treatment for VMS and has been shown to reduce the frequency and severity of hot flashes, with additional benefits on sleep, mood, fatigue, bone loss and reduction of fracture, and genitourinary syndrome of menopause (GSM), and with potential improvement in cardiovascular health with decreased type 2 diabetes.18,19 For healthy women with early menopause and no contraindications, HT has been recommended until at least the age of natural menopause, as observational data suggest that HT prevents osteoporosis, cardiovascular disease, neurodegenerative changes, and sexual dysfunction for these women.19,20 Similarly, for healthy women younger than age 60 or within 10 years of menopause, initiating HT has been shown to be safe and effective in treating bothersome VMS and preventing osteoporotic fractures and genitourinary changes.19,21
Most systemic HT formulations are inexpensive (for example, available as generics), with multiple dosing and formulations available for use alone or combined as oral, transdermal, or vaginal therapies. Despite the fear that arose for clinicians and women from the initial 2002 findings of the Women’s Health Initiative regarding increased risk of breast cancer, stroke, venous thrombosis, cardiovascular disease, and dementia, major medical societies agree that when initiated at or soon after menopause, HT is a safe and effective therapy to relieve VMS, protect against bone loss, and treat genitourinary changes.19,21
Many women, however, cannot take HT, including those with estrogen-sensitive cancers, such as breast or uterine cancers; prior cardiovascular disease, stroke, or venous thrombotic events; severe endometriosis; or migraine headaches with visual auras.2 In addition, many symptomatic menopausal women without health contraindications choose not to take HT.2 Until now, the only FDA-approved VMS nonhormone therapy has been a low-dose 7.5-mg paroxetine salt. Unfortunately, this formulation, along with the off-label use of other antidepressants (selective serotonin reuptake inhibitors and serotonin and norepinephrine reuptake inhibitors), gabapentinoids, oxybutynin, and clonidine, are substantially less effective than HT in treating moderate to severe VMS.
Bottom line
A substantial unmet need remains for effective therapy for moderate to severe VMS for women who cannot or choose not to take menopausal HT to relieve VMS.2,16 Effective, safe nonhormone treatment options such as the new NK3 receptor antagonist fezolinetant will address this clinically important need.
One concern is that the cost of developing and bringing to market the first of a new type of medication will be passed on to consumers, which may put it out of the price range for the many women who need it. However, the development and FDA approval of fezolinetant as the first NK3 receptor antagonist to treat menopausal VMS is potentially a practice changer. It provides a novel, effective, and safe FDA-approved nonhormonal treatment for menopausal women with moderate to severe VMS, particularly for women who cannot or will not take hormone therapy.
Continue to: When endometrial biopsy for postmenopausal bleeding reveals proliferative changes, how should we respond?...
When endometrial biopsy for postmenopausal bleeding reveals proliferative changes, how should we respond?
Abraham C. Proliferative endometrium in menopause: to treat or not to treat? Obstet Gynecol. 2023;141:265-267. doi:10.1097/AOG.0000000000005054.
The following case represents a common scenario for ObGyns.
CASE Patient with proliferative endometrial changes
A menopausal patient with a body mass index (BMI) > 30 kg/m2 presents with uterine bleeding. She does not use systemic menopausal hormone therapy. Endometrial biopsy indicates proliferative changes.
When endometrial biopsy performed for bleeding reveals proliferative changes in menopausal women, we traditionally have responded by reassuring the patient that the findings are benign and advising that she should let us know if future spotting or bleeding occurs.
However, a recent review by Abraham published in Obstetrics and Gynecology details the implications of proliferative endometrial changes in menopausal patients, advising that treatment, as well as monitoring, may be appropriate.22
Endometrial changes and what they suggest
In premenopausal women, proliferative endometrial changes are physiologic and result from ovarian estrogen production early in each cycle, during what is called the proliferative (referring to the endometrium) or follicular (referring to the dominant follicle that synthesizes estrogen) phase. In menopausal women who are not using HT, however, proliferative endometrial changes, with orderly uniform glands seen on histologic evaluation, reflect aromatization of androgens by adipose and other tissues into estrogen.
The next step on the continuum to hyperplasia (benign or atypical) after proliferative endometrium is disordered proliferative endometrium. At this stage, histologic evaluation reveals scattered cystic and dilated glands that have a normal gland-to-stroma ratio with a low gland density overall and without any atypia. Randomly distributed glands may have tubal metaplasia or fibrin thrombi associated with microinfarcts, often presenting with irregular bleeding. This is a noncancerous change that occurs with excess estrogen (endogenous or exogenous).23
Progestins reverse endometrial hyperplasia by activating progesterone receptors, which leads to stromal decidualization with thinning of the endometrium. They have a pronounced effect on the histologic appearance of the endometrium. By contrast, endometrial intraepithelial neoplasia (EIN, previously known as endometrial hyperplasiawith atypia) shows underlying molecular mutations and histologic alterations and represents a sharp transition to true neoplasia, which greatly increases the risk of endometrioid endometrial adenocarcinoma.24
For decades, we have been aware that if women diagnosed with endometrial hyperplasia are not treated with progestational therapy, their future risk of endometrial cancer is elevated. More recently, we also recognize that menopausal women found to have proliferative endometrial changes, if not treated, have an increased risk of endometrial cancer.
In a retrospective cohort study of almost 300 menopausal women who were not treated after endometrial biopsy revealed proliferative changes, investigators followed participants for an average of 11 years.25 These women had a mean BMI of 34 kg/m2. During follow-up, almost 12% of these women were diagnosed with endometrial hyperplasia or cancer. This incidence of endometrial neoplasia was some 4 times higher than for women initially found to have atrophic endometrial changes.25
Progestin treatment
Oral progestin therapy with follow-up endometrial biopsy constitutes traditional management for endometrial hyperplasia. Such therapy minimizes the likelihood that hyperplasia will progress to endometrial cancer.
We now recognize that the convenience, as well as the high endometrial progestin levels achieved, with levonorgestrel-releasing intrauterine devices (LNG-IUDs) have advantages over oral progestin therapy in treating endometrial hyperplasia. Indeed, a recent US report found that among women with EIN managed medically, use of progestin-releasing IUDs has grown from 7.7% in 2008 to 35.6% in 2020.26
Although both oral and intrauterine progestin are highly effective in treating simple hyperplasia, progestin IUDs are substantially more effective than oral progestins in treating EIN.27 Progestin concentrations in the endometrium have been shown to be 100-fold higher after LNG-IUD placement compared with oral progestin use.22 In addition, adverse effects, including bloating, unpleasant mood changes, and increased appetite, are more common with oral than intrauterine progestin therapy.28
Unfortunately, data from randomized trials addressing progestational treatment of proliferative endometrium in menopausal women are not available to support the treatment of proliferative endometrium with either oral progestins or the LNG-IUD.22
Role of ultrasonography
Another concern is relying on a finding of thin endometrial thickness on vaginal ultrasonography. In a simulated retrospective cohort study, use of transvaginal ultrasonography to determine the appropriateness of a biopsy was found not to be sufficiently accurate or racially equitable with regard to Black women.29 In simulated data, transvaginal ultrasonography missed almost 5 times more cases of endometrial cancer among Black women compared with White women due to higher fibroid prevalence and nonendometrioid histologic type malignancies in Black women.29
Assessing risk
If proliferative endometrium is found, Abraham suggests assessing risk using22:
- age
- comorbidities (including obesity)
- endometrial echo thickness on vaginal ultrasonography.
Consider the patient’s risk and tolerance of recurrent bleeding as well as her tolerance for progestational adverse effects if medical therapy is chosen. Discussion about next steps should include reviewing the histologic findings with the patient and discussing the difference in risk of progression to endometrial cancer of a finding of proliferative endometrium compared with a histologic finding of endometrial hyperplasia.
Using this patient-centered approach, observation over time with follow-up endometrial biopsies remains a management option. Although some women may tolerate micronized progesterone over synthetic progestins, there is concern that it may be less effective in suppressing the endometrium than synthetic progestins.30 Accordingly, synthetic progestins represent first-line options in this setting.
In her review, Abraham suggests that when endometrial biopsy reveals proliferative changes in a menopausal woman, we should initiate progestin treatment and perform surveillance endometrial sampling every 3 to 6 months. If such sampling reveals benign but not proliferative endometrium, progestin therapy can be stopped and endometrial biopsy repeated if bleeding recurs.22 ●
ObGyns may choose to adopt Abraham’s approach or to hold off on progestin therapy while performing follow-up endometrial sampling. Either way, the take-home message is that the finding of proliferative endometrial changes on biopsy for postmenopausal bleeding requires proactive management.
- Modi M, Dhillo WS. Neurokinin 3 receptor antagonism: a novel treatment for menopausal hot flushes. Neuroendocrinology. 2019;109:242-248. doi:10.1159/000495889
- Pinkerton JV, Redick DL, Homewood LN, et al. Neurokinin receptor antagonist, fezolinetant, for treatment of menopausal vasomotor symptoms. J Clin Endocrinol Metab. 2023;dgad209. doi:10.1210/clinem/dgad209
- Rance NE, Dacks PA, Mittelman-Smith MA, et al. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front Neuroendocrinol. 2013;34:211-227. doi:10.1016 /j.yfrne.2013.07.003
- Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, et al. Role for kisspeptin/neurokinin B/dynorphin (KNDy) neurons in cutaneous vasodilatation and the estrogen modulation of body temperature. Proc Natl Acad Sci USA. 2012;109:1984619851. doi:10.1073/pnas.1211517109
- Astellas Pharma. Astellas’ Veozah (fezolinetant) approved by US FDA for treatment of vasomotor symptoms due to menopause. May 12, 2023. PR Newswire. Accessed May 15, 2023. https://www.prnewswire.com/news-releases/astellas-veozah-fezolinetant-approved-by-us-fda-for -treatment-of-vasomotor-symptoms-due-to-menopause -301823639.html
- Johnson KA, Martin N, Nappi RE, et al. Efficacy and safety of fezolinetant in moderate-to-severe vasomotor symptoms associated with menopause: a phase 3 RCT. J Clin Endocrinol Metab. 2023;dgad058. doi:10.1210/clinem/dgad058
- Lederman S, Ottery FD, Cano A, et al. Fezolinetant for treatment of moderate-to-severe vasomotor symptoms associated with menopause (SKYLIGHT 1): a phase 3 randomised controlled study. Lancet. 2023;401:1091-1102. doi:10.1016 /S0140-6736(23)00085-5
- Neal-Perry G, Cano A, Lederman S, et al. Safety of fezolinetant for vasomotor symptoms associated with menopause: a randomized controlled trial. Obstet Gynecol. 2023;141:737-747. doi:10.1097/AOG.0000000000005114
- Depypere H, Timmerman D, Donders G, et al. Treatment of menopausal vasomotor symptoms with fezolinetant, a neurokinin 3 receptor antagonist: a phase 2a trial. J Clin Endocrinol Metab. 2019;104:5893-5905. doi: 10.1210/jc .2019-00677
- Santoro N, Waldbaum A, Lederman S, et al. Effect of the neurokinin 3 receptor antagonist fezolinetant on patientreported outcomes in postmenopausal women with vasomotor symptoms: results of a randomized, placebo-controlled, double-blind, dose-ranging study (VESTA). Menopause. 2020;27:1350-1356. doi:10.1097/GME.0000000000001621
- FDA approves novel drug to treat moderate to severe hot flashes caused by menopause. May 12, 2023. US Food and Drug Administration. Accessed May 15, 2023. https://www .fda.gov/news-events/press-announcements/fda-approves -novel-drug-treat-moderate-severe-hot-flashes-caused -menopause
- Veozah. Prescribing information. Astellas; 2023. Accessed May 16, 2023. https://www.astellas.com/us/system/files /veozah_uspi.pdf
- Pinkerton JV. Money talks: untreated hot flashes cost women, the workplace, and society. Menopause. 2015;22:254-255. doi:10.1097/GME.0000000000000427
- Sarrel P, Portman D, Lefebvre P, et al. Incremental direct and indirect costs of untreated vasomotor symptoms. Menopause. 2015;22(3):260-266. doi:10.1097/GME.0000000000000320
- Faubion SS, Enders F, Hedges MS, et al. Impact of menopause symptoms on women in the workplace. Mayo Clin Proc. 2023;98:833-845. doi:10.1016/j.mayocp.2023.02.025
- Williams RE, Levine KB, Kalilani L, et al. Menopause- specific questionnaire assessment in US populationbased study shows negative impact on health-related quality of life. Maturitas. 2009;62:153-159. doi:10.1016 /j.maturitas.2008.12.006
- Gartoulla P, Bell RJ, Worsley R, et al. Moderate-severely bothersome vasomotor symptoms are associated with lowered psychological general wellbeing in women at midlife. Maturitas. 2015;81:487-492. doi:10.1016 /j.maturitas.2015.06.004
- Manson JE, Kaunitz AM. Menopause management—getting clinical care back on track. N Engl J Med. 2016;374:803-806. doi:10.1056/NEJMp1514242
- 2022 Hormone Therapy Position Statement of the North American Menopause Society Advisory Panel. The 2022 hormone therapy position statement of the North American Menopause Society. Menopause. 2022;29:767-794. doi:10.1097/GME.0000000000002028
- Kaunitz AM, Kapoor E, Faubion S. Treatment of women after bilateral salpingo-oophorectomy performed prior to natural menopause. JAMA. 2021;12;326:1429-1430. doi:10.1001 /jama.2021.3305
- Pinkerton JV. Hormone therapy for postmenopausal women. N Engl J Med. 2020;382:446-455. doi:10.1056 /NEJMcp1714787
- Abraham C. Proliferative endometrium in menopause: to treat or not to treat? Obstet Gynecol. 2023;141:265-267. doi:10.1097/AOG.0000000000005054
- Chandra V, Kim JJ, Benbrook DM, et al. Therapeutic options for management of endometrial hyperplasia. J Gynecol Oncol. 2016;27:e8. doi:10.3802/jgo.2016.27.e8
- Owings RA, Quick CM. Endometrial intraepithelial neoplasia. Arch Pathol Lab Med. 2014;138:484-491. doi:10.5858 /arpa.2012-0709-RA
- Rotenberg O, Doulaveris G, Fridman D, et al. Long-term outcome of postmenopausal women with proliferative endometrium on endometrial sampling. Am J Obstet Gynecol. 2020;223:896.e1-896.e7. doi:10.1016/j.ajog.2020.06.045
- Suzuki Y, Chen L, Hou JY, et al. Systemic progestins and progestin-releasing intrauterine device therapy for premenopausal patients with endometrial intraepithelial neoplasia. Obstet Gynecol. 2023;141:979-987. doi:10.1097 /AOG.0000000000005124
- Mandelbaum RS, Ciccone MA, Nusbaum DJ, et al. Progestin therapy for obese women with complex atypical hyperplasia: levonorgestrel-releasing intrauterine device vs systemic therapy. Am J Obstet Gynecol. 2020;223:103.e1-103.e13. doi:10.1016/j.ajog.2019.12.273
- Liu S, Kciuk O, Frank M, et al. Progestins of today and tomorrow. Curr Opin Obstet Gynecol. 2022;34:344-350. doi:10.1097 /GCO.0000000000000819
- Doll KM, Romano SS, Marsh EE, et al. Estimated performance of transvaginal ultrasonography for evaluation of postmenopausal bleeding in a simulated cohort of black and white women in the US. JAMA Oncol. 2021;7:1158-1165. doi:10.1001/jamaoncol.2021.1700
- Gompel A. Progesterone and endometrial cancer. Best Pract Res Clin Obstet Gynaecol. 2020;69:95-107. doi:10.1016 /j.bpobgyn.2020.05.003
This year’s menopause Update highlights a highly effective nonhormonal medication that recently received approval by the US Food and Drug Administration (FDA) for the treatment of bothersome menopausal vasomotor symptoms. In addition, the Update provides guidance regarding how ObGyns should respond when an endometrial biopsy for postmenopausal bleeding reveals proliferative changes.
Breakthrough in women’s health: A new nonhormone therapy for vasomotor symptoms
Johnson KA, Martin N, Nappi RE, et al. Efficacy and safety of fezolinetant in moderate-to-severe vasomotor symptoms associated with menopause: a phase 3 RCT. J Clin Endocrinol Metab. 2023;dgad058. doi:10.1210/clinem/dgad058.
Lederman S, Ottery FD, Cano A, et al. Fezolinetant for treatment of moderate-to-severe vasomotor symptoms associated with menopause (SKYLIGHT 1): a phase 3 randomised controlled study. Lancet. 2023;401:1091-1102. doi:10.1016/S0140-6736(23)00085-5.
A new oral nonestrogen-containing medication for relief of moderate to severe hot flashes, fezolinetant (Veozah) 45 mg daily, has been approved by the FDA and was expected to be available by the end of May 2023. Fezolinetant is a selective neurokinin 3 (NK3) receptor antagonistthat offers a targeted nonhormonal approach to menopausal vasomotor symptoms (VMS), and it is the first in its class to make it to market.
The decline in estrogen at menopause appears to result in increased signaling at kisspeptin/neurokinin B/dynorphin (KNDy) neurons in the thermoregulatory center within the hypothalamus with resultant increases in hot flashes.1,2 Fezolinetant works by binding to and blocking the activities of the NK3 receptor.3-5
Key study findings
Selective NK3 receptor antagonists, including fezolinetant, effectively reduce the frequency and severity of VMS comparable to that of hormone therapy (HT). Two phase 3 clinical trials, Skylight 1 and 2, confirmed the efficacy and safety of fezolinetant 45 mg in treating VMS,6,7 and an additional 52-week placebo-controlled study, Skylight 4, confirmed long-term safety.8 Onset of action occurs within a week. Reported adverse events occurred in 1% to 2% of healthy menopausal women participating in clinical trials; these included headaches, abdominal pain, diarrhea, insomnia, back pain, hot flushes, and reversible elevated hepatic transaminase levels.6-9
The published phase 2 trials9 and the international randomized controlled trial (RCT) 12-week studies, Skylight 1 and 2,6,7 found that once-daily 30-mg and 45-mg doses of fezolinetant significantly reduced VMS frequency and severity at 12 weeks among women aged 40 to 60 years who reported an average of 7 moderate to severe VMS/day; the reduction in reported VMS was sustained at 40 weeks. Phase 3 data from Skylight 1 and 2 demonstrated fezolinetant’s efficacy in reducing the frequency and severity of VMS and provided information on the safety profile of fezolinetant compared with placebo over 12 weeks and a noncontrolled extension for an additional 40 weeks.6,7
Oral fezolinetant was associated with improved quality of life, including reduced VMS-related interference with daily life.10 Johnson and colleagues, reporting for Skylight 2, found VMS frequency and severity improvement by week 1, which achieved statistical significance at weeks 4 and 12, with this improvement maintained through week 52.6 A 64.3% reduction in mean daily VMS from baseline was seen at 12 weeks for fezolinetant 45 mg compared with a 45.4% reduction for placebo. VMS severity significantly decreased compared with placebo at 4 and 12 weeks.6
Serious treatment-emergent adverse events were infrequent, reported by 2%, 1%, and 0% of those receiving fezolinetant 30 mg, fezolinetant 45 mg, and placebo.6 Increases in levels of alanine aminotransferase (ALT) or aspartate aminotransferase (AST) were noted and were described as asymptomatic, isolated, intermittent, or transient, and these levels returned to baseline during treatment or after discontinuation.6
Of the 5 participants taking fezolinetant in Skyline 1 with ALT or AST levels greater than 3 times the upper limit of normal in the 12-week randomized trial, levels returned to normal range while continuing treatment in 2 participants, with treatment interruption in 2, and with discontinuation in 1. No new safety signals were seen in the 40-week extension trial.6
Fezolinetant offers a much-needed effective and safe selective nonhormone NK3 receptor antagonist therapy that reduces the frequency and severity of menopausal VMS and has been shown to be safe through 52 weeks of treatment.
To read more about how fezolinetant specifically targets the hormone receptor that triggers hot flashes as well as on prescribing hormone therapy for women with menopausal symptoms, see “Focus on menopause: Q&A with Jan Shifren, MD, and Genevieve NealPerry, MD, PhD,” in the December 2022 issue of OBG Management at https://www.mdedge.com/obgyn/article/260380/menopause
Continue to: Endometrial and bone safety...
Endometrial and bone safety
Results from Skylight 4, a phase 3, randomized, double-blind, 52-week safety study, provided additional evidence that confirmed the longer-term safety of fezolinetant over a 52-week treatment period.8
Endometrial safety was assessed in postmenopausal women with normal baseline endometrium (n = 599).8 For fezolinetant 45 mg, 1 of 203 participants had endometrial hyperplasia (EH) (0.5%; upper limit of one-sided 95% confidence interval [CI], 2.3%); no cases of EH were noted in the placebo (0 of 186) or fezolinetant 30-mg (0 of 210) groups. The incidence of EH or malignancy in fezolinetant-treated participants was within prespecified limits, as assessed by blinded, centrally read endometrial biopsies. Endometrial malignancy occurred in 1 of 210 in the fezolinetant 30-mg group (0.5%; 95% CI, 2.2%) with no cases in the other groups, thus meeting FDA requirements for endometrial safety.8
In addition, no significant differences were noted in change from baseline endometrial thickness on transvaginal ultrasonography between fezolinetant-treated and placebo groups. Likewise, no loss of bone density was found on dual-energy x-ray absorptiometry (DEXA) scans or trabecular bone scores.8
Liver safety
Although no cases of severe liver injury were noted, elevations in serum transaminase concentrations greater than 3 times the upper limit of normal were observed in the clinical trials. In Skylight 4, liver enzyme elevations more than 3 times the upper limit of normal occurred in 6 of 583 participants taking placebo, 8 of 590 taking fezolinetant 30 mg, and 12 of 589 taking fezolinetant 45 mg.8
The prescribing information for fezolinetant includes a warning for elevated hepatic transaminases: Fezolinetant should not be started if baseline serum transaminase concentration is equal to or exceeds 2 times the upper limit of normal. Liver tests should be obtained at baseline and repeated every 3 months for the first 9 months and then if symptoms suggest liver injury.11,12
Unmet need for nonhormone treatment of VMS
Vasomotor symptoms affect up to 80% of women, with approximately 25% bothersome enough to warrant treatment. Vasomotor symptoms persist for a median of 7 years, with duration and severity differing by race and ethnicity. Black, Hispanic, and possibly Native American women experience the highest burden of VMS.2 Although VMS, including hot flashes, night sweats, and mood and sleep disturbances, often are considered an annoyance to those with mild symptoms, moderate to severe VMS impact women’s lives, including functioning at home or work, affecting relationships, and decreasing perceived quality of life, and they have been associated with workplace absenteeism and increased health care costs, both direct from medical care and testing and indirect costs from lost work.13-15
Women with 7 or more daily moderate to severe VMS (defined as with sweating or affecting function) reported interference with sleep (94%), concentration (84%), mood (85%), energy (77%), and sexual activity (61%).16 Moderately to severely bothersome VMS have been associated with impaired psychological and general well-being, affecting work performance.17 Based on a Mayo Clinic workplace survey, Faubion and colleagues estimated an annual loss of $1.8 billion in the United States for menopause-related missed work and a $28 billion loss when medical expenses were added.15
Menopausal HT has been the primary treatment for VMS and has been shown to reduce the frequency and severity of hot flashes, with additional benefits on sleep, mood, fatigue, bone loss and reduction of fracture, and genitourinary syndrome of menopause (GSM), and with potential improvement in cardiovascular health with decreased type 2 diabetes.18,19 For healthy women with early menopause and no contraindications, HT has been recommended until at least the age of natural menopause, as observational data suggest that HT prevents osteoporosis, cardiovascular disease, neurodegenerative changes, and sexual dysfunction for these women.19,20 Similarly, for healthy women younger than age 60 or within 10 years of menopause, initiating HT has been shown to be safe and effective in treating bothersome VMS and preventing osteoporotic fractures and genitourinary changes.19,21
Most systemic HT formulations are inexpensive (for example, available as generics), with multiple dosing and formulations available for use alone or combined as oral, transdermal, or vaginal therapies. Despite the fear that arose for clinicians and women from the initial 2002 findings of the Women’s Health Initiative regarding increased risk of breast cancer, stroke, venous thrombosis, cardiovascular disease, and dementia, major medical societies agree that when initiated at or soon after menopause, HT is a safe and effective therapy to relieve VMS, protect against bone loss, and treat genitourinary changes.19,21
Many women, however, cannot take HT, including those with estrogen-sensitive cancers, such as breast or uterine cancers; prior cardiovascular disease, stroke, or venous thrombotic events; severe endometriosis; or migraine headaches with visual auras.2 In addition, many symptomatic menopausal women without health contraindications choose not to take HT.2 Until now, the only FDA-approved VMS nonhormone therapy has been a low-dose 7.5-mg paroxetine salt. Unfortunately, this formulation, along with the off-label use of other antidepressants (selective serotonin reuptake inhibitors and serotonin and norepinephrine reuptake inhibitors), gabapentinoids, oxybutynin, and clonidine, are substantially less effective than HT in treating moderate to severe VMS.
Bottom line
A substantial unmet need remains for effective therapy for moderate to severe VMS for women who cannot or choose not to take menopausal HT to relieve VMS.2,16 Effective, safe nonhormone treatment options such as the new NK3 receptor antagonist fezolinetant will address this clinically important need.
One concern is that the cost of developing and bringing to market the first of a new type of medication will be passed on to consumers, which may put it out of the price range for the many women who need it. However, the development and FDA approval of fezolinetant as the first NK3 receptor antagonist to treat menopausal VMS is potentially a practice changer. It provides a novel, effective, and safe FDA-approved nonhormonal treatment for menopausal women with moderate to severe VMS, particularly for women who cannot or will not take hormone therapy.
Continue to: When endometrial biopsy for postmenopausal bleeding reveals proliferative changes, how should we respond?...
When endometrial biopsy for postmenopausal bleeding reveals proliferative changes, how should we respond?
Abraham C. Proliferative endometrium in menopause: to treat or not to treat? Obstet Gynecol. 2023;141:265-267. doi:10.1097/AOG.0000000000005054.
The following case represents a common scenario for ObGyns.
CASE Patient with proliferative endometrial changes
A menopausal patient with a body mass index (BMI) > 30 kg/m2 presents with uterine bleeding. She does not use systemic menopausal hormone therapy. Endometrial biopsy indicates proliferative changes.
When endometrial biopsy performed for bleeding reveals proliferative changes in menopausal women, we traditionally have responded by reassuring the patient that the findings are benign and advising that she should let us know if future spotting or bleeding occurs.
However, a recent review by Abraham published in Obstetrics and Gynecology details the implications of proliferative endometrial changes in menopausal patients, advising that treatment, as well as monitoring, may be appropriate.22
Endometrial changes and what they suggest
In premenopausal women, proliferative endometrial changes are physiologic and result from ovarian estrogen production early in each cycle, during what is called the proliferative (referring to the endometrium) or follicular (referring to the dominant follicle that synthesizes estrogen) phase. In menopausal women who are not using HT, however, proliferative endometrial changes, with orderly uniform glands seen on histologic evaluation, reflect aromatization of androgens by adipose and other tissues into estrogen.
The next step on the continuum to hyperplasia (benign or atypical) after proliferative endometrium is disordered proliferative endometrium. At this stage, histologic evaluation reveals scattered cystic and dilated glands that have a normal gland-to-stroma ratio with a low gland density overall and without any atypia. Randomly distributed glands may have tubal metaplasia or fibrin thrombi associated with microinfarcts, often presenting with irregular bleeding. This is a noncancerous change that occurs with excess estrogen (endogenous or exogenous).23
Progestins reverse endometrial hyperplasia by activating progesterone receptors, which leads to stromal decidualization with thinning of the endometrium. They have a pronounced effect on the histologic appearance of the endometrium. By contrast, endometrial intraepithelial neoplasia (EIN, previously known as endometrial hyperplasiawith atypia) shows underlying molecular mutations and histologic alterations and represents a sharp transition to true neoplasia, which greatly increases the risk of endometrioid endometrial adenocarcinoma.24
For decades, we have been aware that if women diagnosed with endometrial hyperplasia are not treated with progestational therapy, their future risk of endometrial cancer is elevated. More recently, we also recognize that menopausal women found to have proliferative endometrial changes, if not treated, have an increased risk of endometrial cancer.
In a retrospective cohort study of almost 300 menopausal women who were not treated after endometrial biopsy revealed proliferative changes, investigators followed participants for an average of 11 years.25 These women had a mean BMI of 34 kg/m2. During follow-up, almost 12% of these women were diagnosed with endometrial hyperplasia or cancer. This incidence of endometrial neoplasia was some 4 times higher than for women initially found to have atrophic endometrial changes.25
Progestin treatment
Oral progestin therapy with follow-up endometrial biopsy constitutes traditional management for endometrial hyperplasia. Such therapy minimizes the likelihood that hyperplasia will progress to endometrial cancer.
We now recognize that the convenience, as well as the high endometrial progestin levels achieved, with levonorgestrel-releasing intrauterine devices (LNG-IUDs) have advantages over oral progestin therapy in treating endometrial hyperplasia. Indeed, a recent US report found that among women with EIN managed medically, use of progestin-releasing IUDs has grown from 7.7% in 2008 to 35.6% in 2020.26
Although both oral and intrauterine progestin are highly effective in treating simple hyperplasia, progestin IUDs are substantially more effective than oral progestins in treating EIN.27 Progestin concentrations in the endometrium have been shown to be 100-fold higher after LNG-IUD placement compared with oral progestin use.22 In addition, adverse effects, including bloating, unpleasant mood changes, and increased appetite, are more common with oral than intrauterine progestin therapy.28
Unfortunately, data from randomized trials addressing progestational treatment of proliferative endometrium in menopausal women are not available to support the treatment of proliferative endometrium with either oral progestins or the LNG-IUD.22
Role of ultrasonography
Another concern is relying on a finding of thin endometrial thickness on vaginal ultrasonography. In a simulated retrospective cohort study, use of transvaginal ultrasonography to determine the appropriateness of a biopsy was found not to be sufficiently accurate or racially equitable with regard to Black women.29 In simulated data, transvaginal ultrasonography missed almost 5 times more cases of endometrial cancer among Black women compared with White women due to higher fibroid prevalence and nonendometrioid histologic type malignancies in Black women.29
Assessing risk
If proliferative endometrium is found, Abraham suggests assessing risk using22:
- age
- comorbidities (including obesity)
- endometrial echo thickness on vaginal ultrasonography.
Consider the patient’s risk and tolerance of recurrent bleeding as well as her tolerance for progestational adverse effects if medical therapy is chosen. Discussion about next steps should include reviewing the histologic findings with the patient and discussing the difference in risk of progression to endometrial cancer of a finding of proliferative endometrium compared with a histologic finding of endometrial hyperplasia.
Using this patient-centered approach, observation over time with follow-up endometrial biopsies remains a management option. Although some women may tolerate micronized progesterone over synthetic progestins, there is concern that it may be less effective in suppressing the endometrium than synthetic progestins.30 Accordingly, synthetic progestins represent first-line options in this setting.
In her review, Abraham suggests that when endometrial biopsy reveals proliferative changes in a menopausal woman, we should initiate progestin treatment and perform surveillance endometrial sampling every 3 to 6 months. If such sampling reveals benign but not proliferative endometrium, progestin therapy can be stopped and endometrial biopsy repeated if bleeding recurs.22 ●
ObGyns may choose to adopt Abraham’s approach or to hold off on progestin therapy while performing follow-up endometrial sampling. Either way, the take-home message is that the finding of proliferative endometrial changes on biopsy for postmenopausal bleeding requires proactive management.
This year’s menopause Update highlights a highly effective nonhormonal medication that recently received approval by the US Food and Drug Administration (FDA) for the treatment of bothersome menopausal vasomotor symptoms. In addition, the Update provides guidance regarding how ObGyns should respond when an endometrial biopsy for postmenopausal bleeding reveals proliferative changes.
Breakthrough in women’s health: A new nonhormone therapy for vasomotor symptoms
Johnson KA, Martin N, Nappi RE, et al. Efficacy and safety of fezolinetant in moderate-to-severe vasomotor symptoms associated with menopause: a phase 3 RCT. J Clin Endocrinol Metab. 2023;dgad058. doi:10.1210/clinem/dgad058.
Lederman S, Ottery FD, Cano A, et al. Fezolinetant for treatment of moderate-to-severe vasomotor symptoms associated with menopause (SKYLIGHT 1): a phase 3 randomised controlled study. Lancet. 2023;401:1091-1102. doi:10.1016/S0140-6736(23)00085-5.
A new oral nonestrogen-containing medication for relief of moderate to severe hot flashes, fezolinetant (Veozah) 45 mg daily, has been approved by the FDA and was expected to be available by the end of May 2023. Fezolinetant is a selective neurokinin 3 (NK3) receptor antagonistthat offers a targeted nonhormonal approach to menopausal vasomotor symptoms (VMS), and it is the first in its class to make it to market.
The decline in estrogen at menopause appears to result in increased signaling at kisspeptin/neurokinin B/dynorphin (KNDy) neurons in the thermoregulatory center within the hypothalamus with resultant increases in hot flashes.1,2 Fezolinetant works by binding to and blocking the activities of the NK3 receptor.3-5
Key study findings
Selective NK3 receptor antagonists, including fezolinetant, effectively reduce the frequency and severity of VMS comparable to that of hormone therapy (HT). Two phase 3 clinical trials, Skylight 1 and 2, confirmed the efficacy and safety of fezolinetant 45 mg in treating VMS,6,7 and an additional 52-week placebo-controlled study, Skylight 4, confirmed long-term safety.8 Onset of action occurs within a week. Reported adverse events occurred in 1% to 2% of healthy menopausal women participating in clinical trials; these included headaches, abdominal pain, diarrhea, insomnia, back pain, hot flushes, and reversible elevated hepatic transaminase levels.6-9
The published phase 2 trials9 and the international randomized controlled trial (RCT) 12-week studies, Skylight 1 and 2,6,7 found that once-daily 30-mg and 45-mg doses of fezolinetant significantly reduced VMS frequency and severity at 12 weeks among women aged 40 to 60 years who reported an average of 7 moderate to severe VMS/day; the reduction in reported VMS was sustained at 40 weeks. Phase 3 data from Skylight 1 and 2 demonstrated fezolinetant’s efficacy in reducing the frequency and severity of VMS and provided information on the safety profile of fezolinetant compared with placebo over 12 weeks and a noncontrolled extension for an additional 40 weeks.6,7
Oral fezolinetant was associated with improved quality of life, including reduced VMS-related interference with daily life.10 Johnson and colleagues, reporting for Skylight 2, found VMS frequency and severity improvement by week 1, which achieved statistical significance at weeks 4 and 12, with this improvement maintained through week 52.6 A 64.3% reduction in mean daily VMS from baseline was seen at 12 weeks for fezolinetant 45 mg compared with a 45.4% reduction for placebo. VMS severity significantly decreased compared with placebo at 4 and 12 weeks.6
Serious treatment-emergent adverse events were infrequent, reported by 2%, 1%, and 0% of those receiving fezolinetant 30 mg, fezolinetant 45 mg, and placebo.6 Increases in levels of alanine aminotransferase (ALT) or aspartate aminotransferase (AST) were noted and were described as asymptomatic, isolated, intermittent, or transient, and these levels returned to baseline during treatment or after discontinuation.6
Of the 5 participants taking fezolinetant in Skyline 1 with ALT or AST levels greater than 3 times the upper limit of normal in the 12-week randomized trial, levels returned to normal range while continuing treatment in 2 participants, with treatment interruption in 2, and with discontinuation in 1. No new safety signals were seen in the 40-week extension trial.6
Fezolinetant offers a much-needed effective and safe selective nonhormone NK3 receptor antagonist therapy that reduces the frequency and severity of menopausal VMS and has been shown to be safe through 52 weeks of treatment.
To read more about how fezolinetant specifically targets the hormone receptor that triggers hot flashes as well as on prescribing hormone therapy for women with menopausal symptoms, see “Focus on menopause: Q&A with Jan Shifren, MD, and Genevieve NealPerry, MD, PhD,” in the December 2022 issue of OBG Management at https://www.mdedge.com/obgyn/article/260380/menopause
Continue to: Endometrial and bone safety...
Endometrial and bone safety
Results from Skylight 4, a phase 3, randomized, double-blind, 52-week safety study, provided additional evidence that confirmed the longer-term safety of fezolinetant over a 52-week treatment period.8
Endometrial safety was assessed in postmenopausal women with normal baseline endometrium (n = 599).8 For fezolinetant 45 mg, 1 of 203 participants had endometrial hyperplasia (EH) (0.5%; upper limit of one-sided 95% confidence interval [CI], 2.3%); no cases of EH were noted in the placebo (0 of 186) or fezolinetant 30-mg (0 of 210) groups. The incidence of EH or malignancy in fezolinetant-treated participants was within prespecified limits, as assessed by blinded, centrally read endometrial biopsies. Endometrial malignancy occurred in 1 of 210 in the fezolinetant 30-mg group (0.5%; 95% CI, 2.2%) with no cases in the other groups, thus meeting FDA requirements for endometrial safety.8
In addition, no significant differences were noted in change from baseline endometrial thickness on transvaginal ultrasonography between fezolinetant-treated and placebo groups. Likewise, no loss of bone density was found on dual-energy x-ray absorptiometry (DEXA) scans or trabecular bone scores.8
Liver safety
Although no cases of severe liver injury were noted, elevations in serum transaminase concentrations greater than 3 times the upper limit of normal were observed in the clinical trials. In Skylight 4, liver enzyme elevations more than 3 times the upper limit of normal occurred in 6 of 583 participants taking placebo, 8 of 590 taking fezolinetant 30 mg, and 12 of 589 taking fezolinetant 45 mg.8
The prescribing information for fezolinetant includes a warning for elevated hepatic transaminases: Fezolinetant should not be started if baseline serum transaminase concentration is equal to or exceeds 2 times the upper limit of normal. Liver tests should be obtained at baseline and repeated every 3 months for the first 9 months and then if symptoms suggest liver injury.11,12
Unmet need for nonhormone treatment of VMS
Vasomotor symptoms affect up to 80% of women, with approximately 25% bothersome enough to warrant treatment. Vasomotor symptoms persist for a median of 7 years, with duration and severity differing by race and ethnicity. Black, Hispanic, and possibly Native American women experience the highest burden of VMS.2 Although VMS, including hot flashes, night sweats, and mood and sleep disturbances, often are considered an annoyance to those with mild symptoms, moderate to severe VMS impact women’s lives, including functioning at home or work, affecting relationships, and decreasing perceived quality of life, and they have been associated with workplace absenteeism and increased health care costs, both direct from medical care and testing and indirect costs from lost work.13-15
Women with 7 or more daily moderate to severe VMS (defined as with sweating or affecting function) reported interference with sleep (94%), concentration (84%), mood (85%), energy (77%), and sexual activity (61%).16 Moderately to severely bothersome VMS have been associated with impaired psychological and general well-being, affecting work performance.17 Based on a Mayo Clinic workplace survey, Faubion and colleagues estimated an annual loss of $1.8 billion in the United States for menopause-related missed work and a $28 billion loss when medical expenses were added.15
Menopausal HT has been the primary treatment for VMS and has been shown to reduce the frequency and severity of hot flashes, with additional benefits on sleep, mood, fatigue, bone loss and reduction of fracture, and genitourinary syndrome of menopause (GSM), and with potential improvement in cardiovascular health with decreased type 2 diabetes.18,19 For healthy women with early menopause and no contraindications, HT has been recommended until at least the age of natural menopause, as observational data suggest that HT prevents osteoporosis, cardiovascular disease, neurodegenerative changes, and sexual dysfunction for these women.19,20 Similarly, for healthy women younger than age 60 or within 10 years of menopause, initiating HT has been shown to be safe and effective in treating bothersome VMS and preventing osteoporotic fractures and genitourinary changes.19,21
Most systemic HT formulations are inexpensive (for example, available as generics), with multiple dosing and formulations available for use alone or combined as oral, transdermal, or vaginal therapies. Despite the fear that arose for clinicians and women from the initial 2002 findings of the Women’s Health Initiative regarding increased risk of breast cancer, stroke, venous thrombosis, cardiovascular disease, and dementia, major medical societies agree that when initiated at or soon after menopause, HT is a safe and effective therapy to relieve VMS, protect against bone loss, and treat genitourinary changes.19,21
Many women, however, cannot take HT, including those with estrogen-sensitive cancers, such as breast or uterine cancers; prior cardiovascular disease, stroke, or venous thrombotic events; severe endometriosis; or migraine headaches with visual auras.2 In addition, many symptomatic menopausal women without health contraindications choose not to take HT.2 Until now, the only FDA-approved VMS nonhormone therapy has been a low-dose 7.5-mg paroxetine salt. Unfortunately, this formulation, along with the off-label use of other antidepressants (selective serotonin reuptake inhibitors and serotonin and norepinephrine reuptake inhibitors), gabapentinoids, oxybutynin, and clonidine, are substantially less effective than HT in treating moderate to severe VMS.
Bottom line
A substantial unmet need remains for effective therapy for moderate to severe VMS for women who cannot or choose not to take menopausal HT to relieve VMS.2,16 Effective, safe nonhormone treatment options such as the new NK3 receptor antagonist fezolinetant will address this clinically important need.
One concern is that the cost of developing and bringing to market the first of a new type of medication will be passed on to consumers, which may put it out of the price range for the many women who need it. However, the development and FDA approval of fezolinetant as the first NK3 receptor antagonist to treat menopausal VMS is potentially a practice changer. It provides a novel, effective, and safe FDA-approved nonhormonal treatment for menopausal women with moderate to severe VMS, particularly for women who cannot or will not take hormone therapy.
Continue to: When endometrial biopsy for postmenopausal bleeding reveals proliferative changes, how should we respond?...
When endometrial biopsy for postmenopausal bleeding reveals proliferative changes, how should we respond?
Abraham C. Proliferative endometrium in menopause: to treat or not to treat? Obstet Gynecol. 2023;141:265-267. doi:10.1097/AOG.0000000000005054.
The following case represents a common scenario for ObGyns.
CASE Patient with proliferative endometrial changes
A menopausal patient with a body mass index (BMI) > 30 kg/m2 presents with uterine bleeding. She does not use systemic menopausal hormone therapy. Endometrial biopsy indicates proliferative changes.
When endometrial biopsy performed for bleeding reveals proliferative changes in menopausal women, we traditionally have responded by reassuring the patient that the findings are benign and advising that she should let us know if future spotting or bleeding occurs.
However, a recent review by Abraham published in Obstetrics and Gynecology details the implications of proliferative endometrial changes in menopausal patients, advising that treatment, as well as monitoring, may be appropriate.22
Endometrial changes and what they suggest
In premenopausal women, proliferative endometrial changes are physiologic and result from ovarian estrogen production early in each cycle, during what is called the proliferative (referring to the endometrium) or follicular (referring to the dominant follicle that synthesizes estrogen) phase. In menopausal women who are not using HT, however, proliferative endometrial changes, with orderly uniform glands seen on histologic evaluation, reflect aromatization of androgens by adipose and other tissues into estrogen.
The next step on the continuum to hyperplasia (benign or atypical) after proliferative endometrium is disordered proliferative endometrium. At this stage, histologic evaluation reveals scattered cystic and dilated glands that have a normal gland-to-stroma ratio with a low gland density overall and without any atypia. Randomly distributed glands may have tubal metaplasia or fibrin thrombi associated with microinfarcts, often presenting with irregular bleeding. This is a noncancerous change that occurs with excess estrogen (endogenous or exogenous).23
Progestins reverse endometrial hyperplasia by activating progesterone receptors, which leads to stromal decidualization with thinning of the endometrium. They have a pronounced effect on the histologic appearance of the endometrium. By contrast, endometrial intraepithelial neoplasia (EIN, previously known as endometrial hyperplasiawith atypia) shows underlying molecular mutations and histologic alterations and represents a sharp transition to true neoplasia, which greatly increases the risk of endometrioid endometrial adenocarcinoma.24
For decades, we have been aware that if women diagnosed with endometrial hyperplasia are not treated with progestational therapy, their future risk of endometrial cancer is elevated. More recently, we also recognize that menopausal women found to have proliferative endometrial changes, if not treated, have an increased risk of endometrial cancer.
In a retrospective cohort study of almost 300 menopausal women who were not treated after endometrial biopsy revealed proliferative changes, investigators followed participants for an average of 11 years.25 These women had a mean BMI of 34 kg/m2. During follow-up, almost 12% of these women were diagnosed with endometrial hyperplasia or cancer. This incidence of endometrial neoplasia was some 4 times higher than for women initially found to have atrophic endometrial changes.25
Progestin treatment
Oral progestin therapy with follow-up endometrial biopsy constitutes traditional management for endometrial hyperplasia. Such therapy minimizes the likelihood that hyperplasia will progress to endometrial cancer.
We now recognize that the convenience, as well as the high endometrial progestin levels achieved, with levonorgestrel-releasing intrauterine devices (LNG-IUDs) have advantages over oral progestin therapy in treating endometrial hyperplasia. Indeed, a recent US report found that among women with EIN managed medically, use of progestin-releasing IUDs has grown from 7.7% in 2008 to 35.6% in 2020.26
Although both oral and intrauterine progestin are highly effective in treating simple hyperplasia, progestin IUDs are substantially more effective than oral progestins in treating EIN.27 Progestin concentrations in the endometrium have been shown to be 100-fold higher after LNG-IUD placement compared with oral progestin use.22 In addition, adverse effects, including bloating, unpleasant mood changes, and increased appetite, are more common with oral than intrauterine progestin therapy.28
Unfortunately, data from randomized trials addressing progestational treatment of proliferative endometrium in menopausal women are not available to support the treatment of proliferative endometrium with either oral progestins or the LNG-IUD.22
Role of ultrasonography
Another concern is relying on a finding of thin endometrial thickness on vaginal ultrasonography. In a simulated retrospective cohort study, use of transvaginal ultrasonography to determine the appropriateness of a biopsy was found not to be sufficiently accurate or racially equitable with regard to Black women.29 In simulated data, transvaginal ultrasonography missed almost 5 times more cases of endometrial cancer among Black women compared with White women due to higher fibroid prevalence and nonendometrioid histologic type malignancies in Black women.29
Assessing risk
If proliferative endometrium is found, Abraham suggests assessing risk using22:
- age
- comorbidities (including obesity)
- endometrial echo thickness on vaginal ultrasonography.
Consider the patient’s risk and tolerance of recurrent bleeding as well as her tolerance for progestational adverse effects if medical therapy is chosen. Discussion about next steps should include reviewing the histologic findings with the patient and discussing the difference in risk of progression to endometrial cancer of a finding of proliferative endometrium compared with a histologic finding of endometrial hyperplasia.
Using this patient-centered approach, observation over time with follow-up endometrial biopsies remains a management option. Although some women may tolerate micronized progesterone over synthetic progestins, there is concern that it may be less effective in suppressing the endometrium than synthetic progestins.30 Accordingly, synthetic progestins represent first-line options in this setting.
In her review, Abraham suggests that when endometrial biopsy reveals proliferative changes in a menopausal woman, we should initiate progestin treatment and perform surveillance endometrial sampling every 3 to 6 months. If such sampling reveals benign but not proliferative endometrium, progestin therapy can be stopped and endometrial biopsy repeated if bleeding recurs.22 ●
ObGyns may choose to adopt Abraham’s approach or to hold off on progestin therapy while performing follow-up endometrial sampling. Either way, the take-home message is that the finding of proliferative endometrial changes on biopsy for postmenopausal bleeding requires proactive management.
- Modi M, Dhillo WS. Neurokinin 3 receptor antagonism: a novel treatment for menopausal hot flushes. Neuroendocrinology. 2019;109:242-248. doi:10.1159/000495889
- Pinkerton JV, Redick DL, Homewood LN, et al. Neurokinin receptor antagonist, fezolinetant, for treatment of menopausal vasomotor symptoms. J Clin Endocrinol Metab. 2023;dgad209. doi:10.1210/clinem/dgad209
- Rance NE, Dacks PA, Mittelman-Smith MA, et al. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front Neuroendocrinol. 2013;34:211-227. doi:10.1016 /j.yfrne.2013.07.003
- Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, et al. Role for kisspeptin/neurokinin B/dynorphin (KNDy) neurons in cutaneous vasodilatation and the estrogen modulation of body temperature. Proc Natl Acad Sci USA. 2012;109:1984619851. doi:10.1073/pnas.1211517109
- Astellas Pharma. Astellas’ Veozah (fezolinetant) approved by US FDA for treatment of vasomotor symptoms due to menopause. May 12, 2023. PR Newswire. Accessed May 15, 2023. https://www.prnewswire.com/news-releases/astellas-veozah-fezolinetant-approved-by-us-fda-for -treatment-of-vasomotor-symptoms-due-to-menopause -301823639.html
- Johnson KA, Martin N, Nappi RE, et al. Efficacy and safety of fezolinetant in moderate-to-severe vasomotor symptoms associated with menopause: a phase 3 RCT. J Clin Endocrinol Metab. 2023;dgad058. doi:10.1210/clinem/dgad058
- Lederman S, Ottery FD, Cano A, et al. Fezolinetant for treatment of moderate-to-severe vasomotor symptoms associated with menopause (SKYLIGHT 1): a phase 3 randomised controlled study. Lancet. 2023;401:1091-1102. doi:10.1016 /S0140-6736(23)00085-5
- Neal-Perry G, Cano A, Lederman S, et al. Safety of fezolinetant for vasomotor symptoms associated with menopause: a randomized controlled trial. Obstet Gynecol. 2023;141:737-747. doi:10.1097/AOG.0000000000005114
- Depypere H, Timmerman D, Donders G, et al. Treatment of menopausal vasomotor symptoms with fezolinetant, a neurokinin 3 receptor antagonist: a phase 2a trial. J Clin Endocrinol Metab. 2019;104:5893-5905. doi: 10.1210/jc .2019-00677
- Santoro N, Waldbaum A, Lederman S, et al. Effect of the neurokinin 3 receptor antagonist fezolinetant on patientreported outcomes in postmenopausal women with vasomotor symptoms: results of a randomized, placebo-controlled, double-blind, dose-ranging study (VESTA). Menopause. 2020;27:1350-1356. doi:10.1097/GME.0000000000001621
- FDA approves novel drug to treat moderate to severe hot flashes caused by menopause. May 12, 2023. US Food and Drug Administration. Accessed May 15, 2023. https://www .fda.gov/news-events/press-announcements/fda-approves -novel-drug-treat-moderate-severe-hot-flashes-caused -menopause
- Veozah. Prescribing information. Astellas; 2023. Accessed May 16, 2023. https://www.astellas.com/us/system/files /veozah_uspi.pdf
- Pinkerton JV. Money talks: untreated hot flashes cost women, the workplace, and society. Menopause. 2015;22:254-255. doi:10.1097/GME.0000000000000427
- Sarrel P, Portman D, Lefebvre P, et al. Incremental direct and indirect costs of untreated vasomotor symptoms. Menopause. 2015;22(3):260-266. doi:10.1097/GME.0000000000000320
- Faubion SS, Enders F, Hedges MS, et al. Impact of menopause symptoms on women in the workplace. Mayo Clin Proc. 2023;98:833-845. doi:10.1016/j.mayocp.2023.02.025
- Williams RE, Levine KB, Kalilani L, et al. Menopause- specific questionnaire assessment in US populationbased study shows negative impact on health-related quality of life. Maturitas. 2009;62:153-159. doi:10.1016 /j.maturitas.2008.12.006
- Gartoulla P, Bell RJ, Worsley R, et al. Moderate-severely bothersome vasomotor symptoms are associated with lowered psychological general wellbeing in women at midlife. Maturitas. 2015;81:487-492. doi:10.1016 /j.maturitas.2015.06.004
- Manson JE, Kaunitz AM. Menopause management—getting clinical care back on track. N Engl J Med. 2016;374:803-806. doi:10.1056/NEJMp1514242
- 2022 Hormone Therapy Position Statement of the North American Menopause Society Advisory Panel. The 2022 hormone therapy position statement of the North American Menopause Society. Menopause. 2022;29:767-794. doi:10.1097/GME.0000000000002028
- Kaunitz AM, Kapoor E, Faubion S. Treatment of women after bilateral salpingo-oophorectomy performed prior to natural menopause. JAMA. 2021;12;326:1429-1430. doi:10.1001 /jama.2021.3305
- Pinkerton JV. Hormone therapy for postmenopausal women. N Engl J Med. 2020;382:446-455. doi:10.1056 /NEJMcp1714787
- Abraham C. Proliferative endometrium in menopause: to treat or not to treat? Obstet Gynecol. 2023;141:265-267. doi:10.1097/AOG.0000000000005054
- Chandra V, Kim JJ, Benbrook DM, et al. Therapeutic options for management of endometrial hyperplasia. J Gynecol Oncol. 2016;27:e8. doi:10.3802/jgo.2016.27.e8
- Owings RA, Quick CM. Endometrial intraepithelial neoplasia. Arch Pathol Lab Med. 2014;138:484-491. doi:10.5858 /arpa.2012-0709-RA
- Rotenberg O, Doulaveris G, Fridman D, et al. Long-term outcome of postmenopausal women with proliferative endometrium on endometrial sampling. Am J Obstet Gynecol. 2020;223:896.e1-896.e7. doi:10.1016/j.ajog.2020.06.045
- Suzuki Y, Chen L, Hou JY, et al. Systemic progestins and progestin-releasing intrauterine device therapy for premenopausal patients with endometrial intraepithelial neoplasia. Obstet Gynecol. 2023;141:979-987. doi:10.1097 /AOG.0000000000005124
- Mandelbaum RS, Ciccone MA, Nusbaum DJ, et al. Progestin therapy for obese women with complex atypical hyperplasia: levonorgestrel-releasing intrauterine device vs systemic therapy. Am J Obstet Gynecol. 2020;223:103.e1-103.e13. doi:10.1016/j.ajog.2019.12.273
- Liu S, Kciuk O, Frank M, et al. Progestins of today and tomorrow. Curr Opin Obstet Gynecol. 2022;34:344-350. doi:10.1097 /GCO.0000000000000819
- Doll KM, Romano SS, Marsh EE, et al. Estimated performance of transvaginal ultrasonography for evaluation of postmenopausal bleeding in a simulated cohort of black and white women in the US. JAMA Oncol. 2021;7:1158-1165. doi:10.1001/jamaoncol.2021.1700
- Gompel A. Progesterone and endometrial cancer. Best Pract Res Clin Obstet Gynaecol. 2020;69:95-107. doi:10.1016 /j.bpobgyn.2020.05.003
- Modi M, Dhillo WS. Neurokinin 3 receptor antagonism: a novel treatment for menopausal hot flushes. Neuroendocrinology. 2019;109:242-248. doi:10.1159/000495889
- Pinkerton JV, Redick DL, Homewood LN, et al. Neurokinin receptor antagonist, fezolinetant, for treatment of menopausal vasomotor symptoms. J Clin Endocrinol Metab. 2023;dgad209. doi:10.1210/clinem/dgad209
- Rance NE, Dacks PA, Mittelman-Smith MA, et al. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front Neuroendocrinol. 2013;34:211-227. doi:10.1016 /j.yfrne.2013.07.003
- Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, et al. Role for kisspeptin/neurokinin B/dynorphin (KNDy) neurons in cutaneous vasodilatation and the estrogen modulation of body temperature. Proc Natl Acad Sci USA. 2012;109:1984619851. doi:10.1073/pnas.1211517109
- Astellas Pharma. Astellas’ Veozah (fezolinetant) approved by US FDA for treatment of vasomotor symptoms due to menopause. May 12, 2023. PR Newswire. Accessed May 15, 2023. https://www.prnewswire.com/news-releases/astellas-veozah-fezolinetant-approved-by-us-fda-for -treatment-of-vasomotor-symptoms-due-to-menopause -301823639.html
- Johnson KA, Martin N, Nappi RE, et al. Efficacy and safety of fezolinetant in moderate-to-severe vasomotor symptoms associated with menopause: a phase 3 RCT. J Clin Endocrinol Metab. 2023;dgad058. doi:10.1210/clinem/dgad058
- Lederman S, Ottery FD, Cano A, et al. Fezolinetant for treatment of moderate-to-severe vasomotor symptoms associated with menopause (SKYLIGHT 1): a phase 3 randomised controlled study. Lancet. 2023;401:1091-1102. doi:10.1016 /S0140-6736(23)00085-5
- Neal-Perry G, Cano A, Lederman S, et al. Safety of fezolinetant for vasomotor symptoms associated with menopause: a randomized controlled trial. Obstet Gynecol. 2023;141:737-747. doi:10.1097/AOG.0000000000005114
- Depypere H, Timmerman D, Donders G, et al. Treatment of menopausal vasomotor symptoms with fezolinetant, a neurokinin 3 receptor antagonist: a phase 2a trial. J Clin Endocrinol Metab. 2019;104:5893-5905. doi: 10.1210/jc .2019-00677
- Santoro N, Waldbaum A, Lederman S, et al. Effect of the neurokinin 3 receptor antagonist fezolinetant on patientreported outcomes in postmenopausal women with vasomotor symptoms: results of a randomized, placebo-controlled, double-blind, dose-ranging study (VESTA). Menopause. 2020;27:1350-1356. doi:10.1097/GME.0000000000001621
- FDA approves novel drug to treat moderate to severe hot flashes caused by menopause. May 12, 2023. US Food and Drug Administration. Accessed May 15, 2023. https://www .fda.gov/news-events/press-announcements/fda-approves -novel-drug-treat-moderate-severe-hot-flashes-caused -menopause
- Veozah. Prescribing information. Astellas; 2023. Accessed May 16, 2023. https://www.astellas.com/us/system/files /veozah_uspi.pdf
- Pinkerton JV. Money talks: untreated hot flashes cost women, the workplace, and society. Menopause. 2015;22:254-255. doi:10.1097/GME.0000000000000427
- Sarrel P, Portman D, Lefebvre P, et al. Incremental direct and indirect costs of untreated vasomotor symptoms. Menopause. 2015;22(3):260-266. doi:10.1097/GME.0000000000000320
- Faubion SS, Enders F, Hedges MS, et al. Impact of menopause symptoms on women in the workplace. Mayo Clin Proc. 2023;98:833-845. doi:10.1016/j.mayocp.2023.02.025
- Williams RE, Levine KB, Kalilani L, et al. Menopause- specific questionnaire assessment in US populationbased study shows negative impact on health-related quality of life. Maturitas. 2009;62:153-159. doi:10.1016 /j.maturitas.2008.12.006
- Gartoulla P, Bell RJ, Worsley R, et al. Moderate-severely bothersome vasomotor symptoms are associated with lowered psychological general wellbeing in women at midlife. Maturitas. 2015;81:487-492. doi:10.1016 /j.maturitas.2015.06.004
- Manson JE, Kaunitz AM. Menopause management—getting clinical care back on track. N Engl J Med. 2016;374:803-806. doi:10.1056/NEJMp1514242
- 2022 Hormone Therapy Position Statement of the North American Menopause Society Advisory Panel. The 2022 hormone therapy position statement of the North American Menopause Society. Menopause. 2022;29:767-794. doi:10.1097/GME.0000000000002028
- Kaunitz AM, Kapoor E, Faubion S. Treatment of women after bilateral salpingo-oophorectomy performed prior to natural menopause. JAMA. 2021;12;326:1429-1430. doi:10.1001 /jama.2021.3305
- Pinkerton JV. Hormone therapy for postmenopausal women. N Engl J Med. 2020;382:446-455. doi:10.1056 /NEJMcp1714787
- Abraham C. Proliferative endometrium in menopause: to treat or not to treat? Obstet Gynecol. 2023;141:265-267. doi:10.1097/AOG.0000000000005054
- Chandra V, Kim JJ, Benbrook DM, et al. Therapeutic options for management of endometrial hyperplasia. J Gynecol Oncol. 2016;27:e8. doi:10.3802/jgo.2016.27.e8
- Owings RA, Quick CM. Endometrial intraepithelial neoplasia. Arch Pathol Lab Med. 2014;138:484-491. doi:10.5858 /arpa.2012-0709-RA
- Rotenberg O, Doulaveris G, Fridman D, et al. Long-term outcome of postmenopausal women with proliferative endometrium on endometrial sampling. Am J Obstet Gynecol. 2020;223:896.e1-896.e7. doi:10.1016/j.ajog.2020.06.045
- Suzuki Y, Chen L, Hou JY, et al. Systemic progestins and progestin-releasing intrauterine device therapy for premenopausal patients with endometrial intraepithelial neoplasia. Obstet Gynecol. 2023;141:979-987. doi:10.1097 /AOG.0000000000005124
- Mandelbaum RS, Ciccone MA, Nusbaum DJ, et al. Progestin therapy for obese women with complex atypical hyperplasia: levonorgestrel-releasing intrauterine device vs systemic therapy. Am J Obstet Gynecol. 2020;223:103.e1-103.e13. doi:10.1016/j.ajog.2019.12.273
- Liu S, Kciuk O, Frank M, et al. Progestins of today and tomorrow. Curr Opin Obstet Gynecol. 2022;34:344-350. doi:10.1097 /GCO.0000000000000819
- Doll KM, Romano SS, Marsh EE, et al. Estimated performance of transvaginal ultrasonography for evaluation of postmenopausal bleeding in a simulated cohort of black and white women in the US. JAMA Oncol. 2021;7:1158-1165. doi:10.1001/jamaoncol.2021.1700
- Gompel A. Progesterone and endometrial cancer. Best Pract Res Clin Obstet Gynaecol. 2020;69:95-107. doi:10.1016 /j.bpobgyn.2020.05.003
2023 Update on gynecologic cancer
In 2022, the most significant advances in the treatment of gynecologic cancers were achieved for patients with ovarian cancer. While ovarian cancer continues to carry the worst prognosis of all gynecologic cancers, 5-year relative survival has gradually increased, from 34.4% in 1975 to 52.4% in 2014.1
In this Update, we highlight the recent advances in our understanding of targeted therapy in ovarian cancer. We review SORAYA, a trial that demonstrated that mirvetuximab soravtansine, an antibody-drug conjugate, has promising efficacy in platinum-resistant ovarian cancers that overexpress folate receptor α. We also spotlight progress in the treatment of low-grade serous ovarian cancer, another notoriously chemotherapy-resistant disease, in GOG 281/LOGS, a phase 2 study of the MEK inhibitor trametinib. Finally, we discuss emerging long-term follow-up data on poly(ADP-ribose) polymerase (PARP) inhibitors, which are helping to refine the role of these groundbreaking drugs.
New drug approved for platinum-resistant epithelial ovarian cancer—the first since 2014
Matulonis UA, Lorusso D, Oaknin A, et al. Efficacy and safety of mirvetuximab soravtansine in patients with platinum-resistant ovarian cancer with high folate receptor alpha expression: results from the SORAYA study. J Clin Oncol. 2023;41:2436-2445. doi:10.1200/JCO.22.01900.
While most patients diagnosed with advanced ovarian cancer will respond to platinum-based chemotherapy, those whose disease recurs eventually develop resistance to platinum agents. Treatment options for platinum-resistant ovarian cancer are limited and prognosis is poor. Most regimens have a response rate of only 10%. Since the approval of bevacizumab combined with chemotherapy in 2014, no new agents have been approved by the US Food and Drug Administration (FDA) for use in platinum-resistant ovarian cancer.
Efficacy shown with mirvetuximab
Recently, Matulonis and colleagues published results of the SORAYA study, a single-arm,phase 2 trial, that examined the efficacy and safety of mirvetuximab soravtansine-gynx among women with platinum-resistant ovarian cancer.2 Mirvetuximab is an antibody-drug conjugate composed of an antibody directed at the folate receptor α attached to a cytotoxic microtubule inhibitor.
The study included 106 patients with platinum-resistant ovarian cancer whose tumors expressed folate receptor α at a high level—a feature of approximately 50% of patients screened for the study. Twenty-nine patients experienced a partial response and 5 had a complete response, corresponding to a remarkable objective response rate of 32.4%. The median progression-free survival was 4.3 months.
Like other antibody-drug conjugates, ocular toxicities, including blurred vision (41%) and keratopathy (29%), were common. However, toxicity was manageable and rarely led to drug discontinuation.
The FDA has granted accelerated approval to mirvetuximab soravtansine-gynx for women with platinum-resistant ovarian cancer with high folate receptor α expression who have received 1 to 3 prior systemic treatment regimens.
Continue to: A novel agent for recurrent low-grade serous ovarian carcinoma...
A novel agent for recurrent low-grade serous ovarian carcinoma
Low-grade serous carcinoma is a histologic subtype that makes up approximately 5% of all epithelial ovarian cancers.3 Patients with low-grade serous carcinoma are often younger and, because of the indolent nature of the histology, generally have a longer overall survival compared with patients with high-grade serous carcinoma. Unlike high-grade disease, however, low-grade serous carcinoma usually is resistant to chemotherapy, making treatment options limited for patients with advanced and recurrent disease.
Trametinib: A potential option
In an international, randomized, open-label trial (GOG 281/LOGS), Gershenson and colleagues investigated the efficacy of trametinib compared with standard-of-care chemotherapy in patients with recurrent low-grade serous ovarian cancer.4 Trametinib, a mitogen-activated protein kinase MEK inhibitor, is a targeted agent that is FDA approved for treatment in BRAF-mutated melanoma, lung, and thyroid cancers.
Patients with recurrent low-grade serous ovarian cancer were randomly assigned to trametinib (n = 130) or 1 of 5 standard-of-care treatment options (n = 130), including both chemotherapy and hormonal treatments. Those assigned to trametinib were significantly less likely to have disease progression (78% vs 89%), with a median progression-free survival of 13 months, compared with7.2 months in controls (hazard ratio [HR], 0.48; 95% confidence interval [CI], 0.36–0.64). Additionally, patients who had a radiographic response to trametinib experienced a longer duration of response compared with those who responded to standard-of-care treatment (13.6 months vs 5.9 months).
While there was no statistically significant difference in overall survival (HR, 0.76; 95% CI, 0.51–1.12), crossover to trametinib from the standard-of-care group was allowed and occurred among 68% of patients, which limits the study’s ability to measure differences in overall survival.
Trametinib was well tolerated by patients, but skin rash and anemia followed by hypertension were the most common adverse effects. In the standard-of-care group, the most common toxicities were abdominal pain, nausea, and anemia. A slightly higher proportion of patients in the trametinib group discontinued the drug due to toxicity compared with the standard-of-care group (36% vs 30%), but the there was no difference between the 2 groups in scores on quality-of-life assessments.
Although trametinib is not yet FDA approved for the treatment of ovarian cancer, the National Comprehensive Cancer Network has added trametinib as a treatment option for recurrent low-grade serous ovarian carcinoma, given the significant improvement in progression-free survival compared with standard-of-care treatment.
Continue to: PARP inhibitors benefit many women with ovarian cancer, but they may hurt others...
PARP inhibitors benefit many women with ovarian cancer, but they may hurt others
Monk BJ, Parkinson C, Lim MC, et al. A randomized, phase III trial to evaluate rucaparib monotherapy as maintenance treatment in patients with newly diagnosed ovarian cancer (ATHENA-MONO/GOG-3020/ENGOT-ov45). J Clin Oncol. 2022;40:3952-3964. doi:10.1200/JCO.22.01003.
Poly(ADP-ribose) polymerase (PARP) inhibitors are a class of oral anticancer agents that target DNA repair. Since the initial FDA approval in 2014 of olaparib for the treatment of patients with recurrent BRCA-mutated ovarian cancer, PARP inhibitors have been approved for maintenance in both the frontline setting and after platinum-sensitive recurrence, and as single-agenttreatment for ovarian cancer with BRCA mutations or evidence of homologous repair deficiency (HRD), a BRCA-like molecular phenotype.5 The expanding role for PARP inhibitors in ovarian cancer seemed inexorable.
Restricted prescribing advised
In 2022, we learned that in certain settings, PARP inhibitors may be the wrong choice. Several “Dear Health Care Provider” letters were issued by AstraZeneca, Clovis, and GSK to advise physicians to restrict the prescribing of olaparib, rucaparib, and niraparib.6,7
AstraZeneca and Clovis issued letters spurred by the final analysis of ARIEL4 and SOLO3 studies, 2 randomized trials that investigated, respectively, rucaparib and olaparib monotherapy compared with chemotherapy in recurrent ovarian cancer.8,9 In both cases patients randomized to PARP inhibitors may have experienced an overall survival decrement compared with those who received chemotherapy.
At the FDA’s request, Clovis has withdrawn rucaparib as a treatment for patients with recurrent BRCA-mutant ovarian cancer who had received 2 or more lines of chemotherapy, and AstraZeneca withdrew olaparib monotherapy in germline BRCA-mutant patients with recurrent ovarian cancer. Shortly after these withdrawals, GSK also withdrew its indication for niraparib as a treatment for women with HRD, platinum-sensitive ovarian cancer who have received 3 or more prior chemotherapies. Furthermore, based on the final overall survival analysis of the NOVA study, GSK also restricted its indication for niraparib maintenance for recurrent ovarian cancer to patients with germline BRCA mutations, due to evidence of an overall survival detriment in this setting.10
Positive study results
Fortunately, 2022 was not all bad news for PARP inhibitors in ovarian cancer. In June 2022, the ATHENA-MONO trial, a phase 3 double-blind randomized controlled trial, demonstrated that rucaparib maintenance in patients with newly diagnosed epithelial ovarian cancer was associated with a significantly longer progression-free survival compared with placebo.11 The effect was most pronounced in the BRCA-mutant/HRD population, with a median progression-free survival of 28.7 months in the rucaparib group compared with 11.3 months in the placebo group (HR, 0.47; 95% CI, 0.31–0.72). Thus, rucaparib was added to the list of PARP inhibitors approved for upfront maintenance therapy in epithelial ovarian cancer.
Similarly, the long-term overall survival analysis from the upfront trials SOLO-1 and PAOLA-1 showed an overall survival advantage of PARP inhibitor, compared with placebo, maintenance in patients with BRCA mutations or HRD tumors.12,13 ●
PARP inhibitor maintenance therapy after upfront chemotherapy in women with BRCA-mutant and HRD epithelial ovarian cancer has been game changing in ovarian cancer. However, PARP inhibitors have a more limited role than previously thought for patients with recurrent ovarian cancer.
- Cancer stat facts: ovarian cancer. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Accessed March 11, 2023. https://seer.cancer.gov/statfacts /html/ovary.html
- Matulonis UA, Lorusso D, Oaknin A, et al. Efficacy and safety of mirvetuximab soravtansine in patients with platinumresistant ovarian cancer with high folate receptor alpha expression: results from the SORAYA study. J Clin Oncol. 2023;41:2436-2445. doi:10.1200/JCO.22.01900
- Prat J, D’Angelo E, Espinosa I. Ovarian carcinomas: at least five different diseases with distinct histological features and molecular genetics. Hum Pathol. 2018;80:11-27. doi:10.1016 /j.humpath.2018.06.018
- Gershenson DM, Miller A, Brady WE, et al. Trametinib versus standard of care in patients with recurrent low-grade serous ovarian cancer (GOG 281/LOGS): an international, randomised, open-label, multicentre, phase 2/3 trial. Lancet. 2022;399:541-553. doi:10.1016/S0140-6736(21)02175-9
- Tew WP, Lacchetti C, Ellis A, et al. PARP inhibitors in the management of ovarian cancer: ASCO guideline. J Clin Oncol. 2020;38:3468-3493. doi:10.1200/JCO.20.01924
- Rubraca (rucaparib) for treatment of BRCA-mutated ovarian cancer after 2 or more chemotherapies is voluntarily withdrawn in the US. Clovis Oncology. June 2022. Accessed May 11, 2022. chrome-extension://efaidnbmnnnibpcajpcglcle findmkaj/https://clovisoncology.com/pdfs/US_DHCPL _final_signed.pdf
- Lynparza (olaparib) for treatment of adult patients with deleterious or suspected deleterious germline BRCA-mutated (gBRCAm) advanced ovarian cancer who have been treated with three or more prior lines of chemotherapy is voluntarily withdrawn in the US. AstraZeneca. August 26, 2022. Accessed May 11, 2023. https://www.lynparzahcp.com/content/dam /physician-services/us/590-lynparza-hcp-branded/hcp -global/pdf/solo3-dhcp-final-signed.pdf
- Penson RT, Valencia RV, Cibula D, et al. Olaparib versus nonplatinum chemotherapy in patients with platinum-sensitive relapsed ovarian cancer and a germline BRCA1/2 mutation (SOLO3): a randomized phase III trial. J Clin Oncol. 2020;38:1164-1174. doi:10.1200/JCO.19.02745
- Kristeleit R, Lisyanskaya A, Fedenko A, et al. Rucaparib versus standard-of-care chemotherapy in patients with relapsed ovarian cancer and a deleterious BRCA1 or BRCA2 mutation (ARIEL4): an international, open-label, randomised, phase 3 trial. Lancet Oncol. 2022;23:465-478. doi:10.1016 /S1470-2045(22)00122-X
- Dear Health Care Provider Letter (Niraparib). GSK. November 2022. Accessed May 11, 2023. https://www.zejulahcp .com/content/dam/cf-pharma/hcp-zejulahcp-v2/en_US /pdf/ZEJULA%20(niraparib)%20Dear%20HCP%20Letter%20 November%202022.pdf
- Monk BJ, Parkinson C, Lim MC, et al. A randomized, phase III trial to evaluate rucaparib monotherapy as maintenance treatment in patients with newly diagnosed ovarian cancer (ATHENA-MONO/GOG-3020/ENGOT-ov45). J Clin Oncol. 2022;40:3952-3964. doi:10.1200/JCO.22.01003
- Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505. doi:10.1056 /NEJMoa1810858
- Ray-Coquard I, Pautier P, Pignata S, et al; PAOLA-1 Investigators. Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med. 2019;381:2416-2428. doi:10.1056/NEJMoa1911361
In 2022, the most significant advances in the treatment of gynecologic cancers were achieved for patients with ovarian cancer. While ovarian cancer continues to carry the worst prognosis of all gynecologic cancers, 5-year relative survival has gradually increased, from 34.4% in 1975 to 52.4% in 2014.1
In this Update, we highlight the recent advances in our understanding of targeted therapy in ovarian cancer. We review SORAYA, a trial that demonstrated that mirvetuximab soravtansine, an antibody-drug conjugate, has promising efficacy in platinum-resistant ovarian cancers that overexpress folate receptor α. We also spotlight progress in the treatment of low-grade serous ovarian cancer, another notoriously chemotherapy-resistant disease, in GOG 281/LOGS, a phase 2 study of the MEK inhibitor trametinib. Finally, we discuss emerging long-term follow-up data on poly(ADP-ribose) polymerase (PARP) inhibitors, which are helping to refine the role of these groundbreaking drugs.
New drug approved for platinum-resistant epithelial ovarian cancer—the first since 2014
Matulonis UA, Lorusso D, Oaknin A, et al. Efficacy and safety of mirvetuximab soravtansine in patients with platinum-resistant ovarian cancer with high folate receptor alpha expression: results from the SORAYA study. J Clin Oncol. 2023;41:2436-2445. doi:10.1200/JCO.22.01900.
While most patients diagnosed with advanced ovarian cancer will respond to platinum-based chemotherapy, those whose disease recurs eventually develop resistance to platinum agents. Treatment options for platinum-resistant ovarian cancer are limited and prognosis is poor. Most regimens have a response rate of only 10%. Since the approval of bevacizumab combined with chemotherapy in 2014, no new agents have been approved by the US Food and Drug Administration (FDA) for use in platinum-resistant ovarian cancer.
Efficacy shown with mirvetuximab
Recently, Matulonis and colleagues published results of the SORAYA study, a single-arm,phase 2 trial, that examined the efficacy and safety of mirvetuximab soravtansine-gynx among women with platinum-resistant ovarian cancer.2 Mirvetuximab is an antibody-drug conjugate composed of an antibody directed at the folate receptor α attached to a cytotoxic microtubule inhibitor.
The study included 106 patients with platinum-resistant ovarian cancer whose tumors expressed folate receptor α at a high level—a feature of approximately 50% of patients screened for the study. Twenty-nine patients experienced a partial response and 5 had a complete response, corresponding to a remarkable objective response rate of 32.4%. The median progression-free survival was 4.3 months.
Like other antibody-drug conjugates, ocular toxicities, including blurred vision (41%) and keratopathy (29%), were common. However, toxicity was manageable and rarely led to drug discontinuation.
The FDA has granted accelerated approval to mirvetuximab soravtansine-gynx for women with platinum-resistant ovarian cancer with high folate receptor α expression who have received 1 to 3 prior systemic treatment regimens.
Continue to: A novel agent for recurrent low-grade serous ovarian carcinoma...
A novel agent for recurrent low-grade serous ovarian carcinoma
Low-grade serous carcinoma is a histologic subtype that makes up approximately 5% of all epithelial ovarian cancers.3 Patients with low-grade serous carcinoma are often younger and, because of the indolent nature of the histology, generally have a longer overall survival compared with patients with high-grade serous carcinoma. Unlike high-grade disease, however, low-grade serous carcinoma usually is resistant to chemotherapy, making treatment options limited for patients with advanced and recurrent disease.
Trametinib: A potential option
In an international, randomized, open-label trial (GOG 281/LOGS), Gershenson and colleagues investigated the efficacy of trametinib compared with standard-of-care chemotherapy in patients with recurrent low-grade serous ovarian cancer.4 Trametinib, a mitogen-activated protein kinase MEK inhibitor, is a targeted agent that is FDA approved for treatment in BRAF-mutated melanoma, lung, and thyroid cancers.
Patients with recurrent low-grade serous ovarian cancer were randomly assigned to trametinib (n = 130) or 1 of 5 standard-of-care treatment options (n = 130), including both chemotherapy and hormonal treatments. Those assigned to trametinib were significantly less likely to have disease progression (78% vs 89%), with a median progression-free survival of 13 months, compared with7.2 months in controls (hazard ratio [HR], 0.48; 95% confidence interval [CI], 0.36–0.64). Additionally, patients who had a radiographic response to trametinib experienced a longer duration of response compared with those who responded to standard-of-care treatment (13.6 months vs 5.9 months).
While there was no statistically significant difference in overall survival (HR, 0.76; 95% CI, 0.51–1.12), crossover to trametinib from the standard-of-care group was allowed and occurred among 68% of patients, which limits the study’s ability to measure differences in overall survival.
Trametinib was well tolerated by patients, but skin rash and anemia followed by hypertension were the most common adverse effects. In the standard-of-care group, the most common toxicities were abdominal pain, nausea, and anemia. A slightly higher proportion of patients in the trametinib group discontinued the drug due to toxicity compared with the standard-of-care group (36% vs 30%), but the there was no difference between the 2 groups in scores on quality-of-life assessments.
Although trametinib is not yet FDA approved for the treatment of ovarian cancer, the National Comprehensive Cancer Network has added trametinib as a treatment option for recurrent low-grade serous ovarian carcinoma, given the significant improvement in progression-free survival compared with standard-of-care treatment.
Continue to: PARP inhibitors benefit many women with ovarian cancer, but they may hurt others...
PARP inhibitors benefit many women with ovarian cancer, but they may hurt others
Monk BJ, Parkinson C, Lim MC, et al. A randomized, phase III trial to evaluate rucaparib monotherapy as maintenance treatment in patients with newly diagnosed ovarian cancer (ATHENA-MONO/GOG-3020/ENGOT-ov45). J Clin Oncol. 2022;40:3952-3964. doi:10.1200/JCO.22.01003.
Poly(ADP-ribose) polymerase (PARP) inhibitors are a class of oral anticancer agents that target DNA repair. Since the initial FDA approval in 2014 of olaparib for the treatment of patients with recurrent BRCA-mutated ovarian cancer, PARP inhibitors have been approved for maintenance in both the frontline setting and after platinum-sensitive recurrence, and as single-agenttreatment for ovarian cancer with BRCA mutations or evidence of homologous repair deficiency (HRD), a BRCA-like molecular phenotype.5 The expanding role for PARP inhibitors in ovarian cancer seemed inexorable.
Restricted prescribing advised
In 2022, we learned that in certain settings, PARP inhibitors may be the wrong choice. Several “Dear Health Care Provider” letters were issued by AstraZeneca, Clovis, and GSK to advise physicians to restrict the prescribing of olaparib, rucaparib, and niraparib.6,7
AstraZeneca and Clovis issued letters spurred by the final analysis of ARIEL4 and SOLO3 studies, 2 randomized trials that investigated, respectively, rucaparib and olaparib monotherapy compared with chemotherapy in recurrent ovarian cancer.8,9 In both cases patients randomized to PARP inhibitors may have experienced an overall survival decrement compared with those who received chemotherapy.
At the FDA’s request, Clovis has withdrawn rucaparib as a treatment for patients with recurrent BRCA-mutant ovarian cancer who had received 2 or more lines of chemotherapy, and AstraZeneca withdrew olaparib monotherapy in germline BRCA-mutant patients with recurrent ovarian cancer. Shortly after these withdrawals, GSK also withdrew its indication for niraparib as a treatment for women with HRD, platinum-sensitive ovarian cancer who have received 3 or more prior chemotherapies. Furthermore, based on the final overall survival analysis of the NOVA study, GSK also restricted its indication for niraparib maintenance for recurrent ovarian cancer to patients with germline BRCA mutations, due to evidence of an overall survival detriment in this setting.10
Positive study results
Fortunately, 2022 was not all bad news for PARP inhibitors in ovarian cancer. In June 2022, the ATHENA-MONO trial, a phase 3 double-blind randomized controlled trial, demonstrated that rucaparib maintenance in patients with newly diagnosed epithelial ovarian cancer was associated with a significantly longer progression-free survival compared with placebo.11 The effect was most pronounced in the BRCA-mutant/HRD population, with a median progression-free survival of 28.7 months in the rucaparib group compared with 11.3 months in the placebo group (HR, 0.47; 95% CI, 0.31–0.72). Thus, rucaparib was added to the list of PARP inhibitors approved for upfront maintenance therapy in epithelial ovarian cancer.
Similarly, the long-term overall survival analysis from the upfront trials SOLO-1 and PAOLA-1 showed an overall survival advantage of PARP inhibitor, compared with placebo, maintenance in patients with BRCA mutations or HRD tumors.12,13 ●
PARP inhibitor maintenance therapy after upfront chemotherapy in women with BRCA-mutant and HRD epithelial ovarian cancer has been game changing in ovarian cancer. However, PARP inhibitors have a more limited role than previously thought for patients with recurrent ovarian cancer.
In 2022, the most significant advances in the treatment of gynecologic cancers were achieved for patients with ovarian cancer. While ovarian cancer continues to carry the worst prognosis of all gynecologic cancers, 5-year relative survival has gradually increased, from 34.4% in 1975 to 52.4% in 2014.1
In this Update, we highlight the recent advances in our understanding of targeted therapy in ovarian cancer. We review SORAYA, a trial that demonstrated that mirvetuximab soravtansine, an antibody-drug conjugate, has promising efficacy in platinum-resistant ovarian cancers that overexpress folate receptor α. We also spotlight progress in the treatment of low-grade serous ovarian cancer, another notoriously chemotherapy-resistant disease, in GOG 281/LOGS, a phase 2 study of the MEK inhibitor trametinib. Finally, we discuss emerging long-term follow-up data on poly(ADP-ribose) polymerase (PARP) inhibitors, which are helping to refine the role of these groundbreaking drugs.
New drug approved for platinum-resistant epithelial ovarian cancer—the first since 2014
Matulonis UA, Lorusso D, Oaknin A, et al. Efficacy and safety of mirvetuximab soravtansine in patients with platinum-resistant ovarian cancer with high folate receptor alpha expression: results from the SORAYA study. J Clin Oncol. 2023;41:2436-2445. doi:10.1200/JCO.22.01900.
While most patients diagnosed with advanced ovarian cancer will respond to platinum-based chemotherapy, those whose disease recurs eventually develop resistance to platinum agents. Treatment options for platinum-resistant ovarian cancer are limited and prognosis is poor. Most regimens have a response rate of only 10%. Since the approval of bevacizumab combined with chemotherapy in 2014, no new agents have been approved by the US Food and Drug Administration (FDA) for use in platinum-resistant ovarian cancer.
Efficacy shown with mirvetuximab
Recently, Matulonis and colleagues published results of the SORAYA study, a single-arm,phase 2 trial, that examined the efficacy and safety of mirvetuximab soravtansine-gynx among women with platinum-resistant ovarian cancer.2 Mirvetuximab is an antibody-drug conjugate composed of an antibody directed at the folate receptor α attached to a cytotoxic microtubule inhibitor.
The study included 106 patients with platinum-resistant ovarian cancer whose tumors expressed folate receptor α at a high level—a feature of approximately 50% of patients screened for the study. Twenty-nine patients experienced a partial response and 5 had a complete response, corresponding to a remarkable objective response rate of 32.4%. The median progression-free survival was 4.3 months.
Like other antibody-drug conjugates, ocular toxicities, including blurred vision (41%) and keratopathy (29%), were common. However, toxicity was manageable and rarely led to drug discontinuation.
The FDA has granted accelerated approval to mirvetuximab soravtansine-gynx for women with platinum-resistant ovarian cancer with high folate receptor α expression who have received 1 to 3 prior systemic treatment regimens.
Continue to: A novel agent for recurrent low-grade serous ovarian carcinoma...
A novel agent for recurrent low-grade serous ovarian carcinoma
Low-grade serous carcinoma is a histologic subtype that makes up approximately 5% of all epithelial ovarian cancers.3 Patients with low-grade serous carcinoma are often younger and, because of the indolent nature of the histology, generally have a longer overall survival compared with patients with high-grade serous carcinoma. Unlike high-grade disease, however, low-grade serous carcinoma usually is resistant to chemotherapy, making treatment options limited for patients with advanced and recurrent disease.
Trametinib: A potential option
In an international, randomized, open-label trial (GOG 281/LOGS), Gershenson and colleagues investigated the efficacy of trametinib compared with standard-of-care chemotherapy in patients with recurrent low-grade serous ovarian cancer.4 Trametinib, a mitogen-activated protein kinase MEK inhibitor, is a targeted agent that is FDA approved for treatment in BRAF-mutated melanoma, lung, and thyroid cancers.
Patients with recurrent low-grade serous ovarian cancer were randomly assigned to trametinib (n = 130) or 1 of 5 standard-of-care treatment options (n = 130), including both chemotherapy and hormonal treatments. Those assigned to trametinib were significantly less likely to have disease progression (78% vs 89%), with a median progression-free survival of 13 months, compared with7.2 months in controls (hazard ratio [HR], 0.48; 95% confidence interval [CI], 0.36–0.64). Additionally, patients who had a radiographic response to trametinib experienced a longer duration of response compared with those who responded to standard-of-care treatment (13.6 months vs 5.9 months).
While there was no statistically significant difference in overall survival (HR, 0.76; 95% CI, 0.51–1.12), crossover to trametinib from the standard-of-care group was allowed and occurred among 68% of patients, which limits the study’s ability to measure differences in overall survival.
Trametinib was well tolerated by patients, but skin rash and anemia followed by hypertension were the most common adverse effects. In the standard-of-care group, the most common toxicities were abdominal pain, nausea, and anemia. A slightly higher proportion of patients in the trametinib group discontinued the drug due to toxicity compared with the standard-of-care group (36% vs 30%), but the there was no difference between the 2 groups in scores on quality-of-life assessments.
Although trametinib is not yet FDA approved for the treatment of ovarian cancer, the National Comprehensive Cancer Network has added trametinib as a treatment option for recurrent low-grade serous ovarian carcinoma, given the significant improvement in progression-free survival compared with standard-of-care treatment.
Continue to: PARP inhibitors benefit many women with ovarian cancer, but they may hurt others...
PARP inhibitors benefit many women with ovarian cancer, but they may hurt others
Monk BJ, Parkinson C, Lim MC, et al. A randomized, phase III trial to evaluate rucaparib monotherapy as maintenance treatment in patients with newly diagnosed ovarian cancer (ATHENA-MONO/GOG-3020/ENGOT-ov45). J Clin Oncol. 2022;40:3952-3964. doi:10.1200/JCO.22.01003.
Poly(ADP-ribose) polymerase (PARP) inhibitors are a class of oral anticancer agents that target DNA repair. Since the initial FDA approval in 2014 of olaparib for the treatment of patients with recurrent BRCA-mutated ovarian cancer, PARP inhibitors have been approved for maintenance in both the frontline setting and after platinum-sensitive recurrence, and as single-agenttreatment for ovarian cancer with BRCA mutations or evidence of homologous repair deficiency (HRD), a BRCA-like molecular phenotype.5 The expanding role for PARP inhibitors in ovarian cancer seemed inexorable.
Restricted prescribing advised
In 2022, we learned that in certain settings, PARP inhibitors may be the wrong choice. Several “Dear Health Care Provider” letters were issued by AstraZeneca, Clovis, and GSK to advise physicians to restrict the prescribing of olaparib, rucaparib, and niraparib.6,7
AstraZeneca and Clovis issued letters spurred by the final analysis of ARIEL4 and SOLO3 studies, 2 randomized trials that investigated, respectively, rucaparib and olaparib monotherapy compared with chemotherapy in recurrent ovarian cancer.8,9 In both cases patients randomized to PARP inhibitors may have experienced an overall survival decrement compared with those who received chemotherapy.
At the FDA’s request, Clovis has withdrawn rucaparib as a treatment for patients with recurrent BRCA-mutant ovarian cancer who had received 2 or more lines of chemotherapy, and AstraZeneca withdrew olaparib monotherapy in germline BRCA-mutant patients with recurrent ovarian cancer. Shortly after these withdrawals, GSK also withdrew its indication for niraparib as a treatment for women with HRD, platinum-sensitive ovarian cancer who have received 3 or more prior chemotherapies. Furthermore, based on the final overall survival analysis of the NOVA study, GSK also restricted its indication for niraparib maintenance for recurrent ovarian cancer to patients with germline BRCA mutations, due to evidence of an overall survival detriment in this setting.10
Positive study results
Fortunately, 2022 was not all bad news for PARP inhibitors in ovarian cancer. In June 2022, the ATHENA-MONO trial, a phase 3 double-blind randomized controlled trial, demonstrated that rucaparib maintenance in patients with newly diagnosed epithelial ovarian cancer was associated with a significantly longer progression-free survival compared with placebo.11 The effect was most pronounced in the BRCA-mutant/HRD population, with a median progression-free survival of 28.7 months in the rucaparib group compared with 11.3 months in the placebo group (HR, 0.47; 95% CI, 0.31–0.72). Thus, rucaparib was added to the list of PARP inhibitors approved for upfront maintenance therapy in epithelial ovarian cancer.
Similarly, the long-term overall survival analysis from the upfront trials SOLO-1 and PAOLA-1 showed an overall survival advantage of PARP inhibitor, compared with placebo, maintenance in patients with BRCA mutations or HRD tumors.12,13 ●
PARP inhibitor maintenance therapy after upfront chemotherapy in women with BRCA-mutant and HRD epithelial ovarian cancer has been game changing in ovarian cancer. However, PARP inhibitors have a more limited role than previously thought for patients with recurrent ovarian cancer.
- Cancer stat facts: ovarian cancer. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Accessed March 11, 2023. https://seer.cancer.gov/statfacts /html/ovary.html
- Matulonis UA, Lorusso D, Oaknin A, et al. Efficacy and safety of mirvetuximab soravtansine in patients with platinumresistant ovarian cancer with high folate receptor alpha expression: results from the SORAYA study. J Clin Oncol. 2023;41:2436-2445. doi:10.1200/JCO.22.01900
- Prat J, D’Angelo E, Espinosa I. Ovarian carcinomas: at least five different diseases with distinct histological features and molecular genetics. Hum Pathol. 2018;80:11-27. doi:10.1016 /j.humpath.2018.06.018
- Gershenson DM, Miller A, Brady WE, et al. Trametinib versus standard of care in patients with recurrent low-grade serous ovarian cancer (GOG 281/LOGS): an international, randomised, open-label, multicentre, phase 2/3 trial. Lancet. 2022;399:541-553. doi:10.1016/S0140-6736(21)02175-9
- Tew WP, Lacchetti C, Ellis A, et al. PARP inhibitors in the management of ovarian cancer: ASCO guideline. J Clin Oncol. 2020;38:3468-3493. doi:10.1200/JCO.20.01924
- Rubraca (rucaparib) for treatment of BRCA-mutated ovarian cancer after 2 or more chemotherapies is voluntarily withdrawn in the US. Clovis Oncology. June 2022. Accessed May 11, 2022. chrome-extension://efaidnbmnnnibpcajpcglcle findmkaj/https://clovisoncology.com/pdfs/US_DHCPL _final_signed.pdf
- Lynparza (olaparib) for treatment of adult patients with deleterious or suspected deleterious germline BRCA-mutated (gBRCAm) advanced ovarian cancer who have been treated with three or more prior lines of chemotherapy is voluntarily withdrawn in the US. AstraZeneca. August 26, 2022. Accessed May 11, 2023. https://www.lynparzahcp.com/content/dam /physician-services/us/590-lynparza-hcp-branded/hcp -global/pdf/solo3-dhcp-final-signed.pdf
- Penson RT, Valencia RV, Cibula D, et al. Olaparib versus nonplatinum chemotherapy in patients with platinum-sensitive relapsed ovarian cancer and a germline BRCA1/2 mutation (SOLO3): a randomized phase III trial. J Clin Oncol. 2020;38:1164-1174. doi:10.1200/JCO.19.02745
- Kristeleit R, Lisyanskaya A, Fedenko A, et al. Rucaparib versus standard-of-care chemotherapy in patients with relapsed ovarian cancer and a deleterious BRCA1 or BRCA2 mutation (ARIEL4): an international, open-label, randomised, phase 3 trial. Lancet Oncol. 2022;23:465-478. doi:10.1016 /S1470-2045(22)00122-X
- Dear Health Care Provider Letter (Niraparib). GSK. November 2022. Accessed May 11, 2023. https://www.zejulahcp .com/content/dam/cf-pharma/hcp-zejulahcp-v2/en_US /pdf/ZEJULA%20(niraparib)%20Dear%20HCP%20Letter%20 November%202022.pdf
- Monk BJ, Parkinson C, Lim MC, et al. A randomized, phase III trial to evaluate rucaparib monotherapy as maintenance treatment in patients with newly diagnosed ovarian cancer (ATHENA-MONO/GOG-3020/ENGOT-ov45). J Clin Oncol. 2022;40:3952-3964. doi:10.1200/JCO.22.01003
- Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505. doi:10.1056 /NEJMoa1810858
- Ray-Coquard I, Pautier P, Pignata S, et al; PAOLA-1 Investigators. Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med. 2019;381:2416-2428. doi:10.1056/NEJMoa1911361
- Cancer stat facts: ovarian cancer. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Accessed March 11, 2023. https://seer.cancer.gov/statfacts /html/ovary.html
- Matulonis UA, Lorusso D, Oaknin A, et al. Efficacy and safety of mirvetuximab soravtansine in patients with platinumresistant ovarian cancer with high folate receptor alpha expression: results from the SORAYA study. J Clin Oncol. 2023;41:2436-2445. doi:10.1200/JCO.22.01900
- Prat J, D’Angelo E, Espinosa I. Ovarian carcinomas: at least five different diseases with distinct histological features and molecular genetics. Hum Pathol. 2018;80:11-27. doi:10.1016 /j.humpath.2018.06.018
- Gershenson DM, Miller A, Brady WE, et al. Trametinib versus standard of care in patients with recurrent low-grade serous ovarian cancer (GOG 281/LOGS): an international, randomised, open-label, multicentre, phase 2/3 trial. Lancet. 2022;399:541-553. doi:10.1016/S0140-6736(21)02175-9
- Tew WP, Lacchetti C, Ellis A, et al. PARP inhibitors in the management of ovarian cancer: ASCO guideline. J Clin Oncol. 2020;38:3468-3493. doi:10.1200/JCO.20.01924
- Rubraca (rucaparib) for treatment of BRCA-mutated ovarian cancer after 2 or more chemotherapies is voluntarily withdrawn in the US. Clovis Oncology. June 2022. Accessed May 11, 2022. chrome-extension://efaidnbmnnnibpcajpcglcle findmkaj/https://clovisoncology.com/pdfs/US_DHCPL _final_signed.pdf
- Lynparza (olaparib) for treatment of adult patients with deleterious or suspected deleterious germline BRCA-mutated (gBRCAm) advanced ovarian cancer who have been treated with three or more prior lines of chemotherapy is voluntarily withdrawn in the US. AstraZeneca. August 26, 2022. Accessed May 11, 2023. https://www.lynparzahcp.com/content/dam /physician-services/us/590-lynparza-hcp-branded/hcp -global/pdf/solo3-dhcp-final-signed.pdf
- Penson RT, Valencia RV, Cibula D, et al. Olaparib versus nonplatinum chemotherapy in patients with platinum-sensitive relapsed ovarian cancer and a germline BRCA1/2 mutation (SOLO3): a randomized phase III trial. J Clin Oncol. 2020;38:1164-1174. doi:10.1200/JCO.19.02745
- Kristeleit R, Lisyanskaya A, Fedenko A, et al. Rucaparib versus standard-of-care chemotherapy in patients with relapsed ovarian cancer and a deleterious BRCA1 or BRCA2 mutation (ARIEL4): an international, open-label, randomised, phase 3 trial. Lancet Oncol. 2022;23:465-478. doi:10.1016 /S1470-2045(22)00122-X
- Dear Health Care Provider Letter (Niraparib). GSK. November 2022. Accessed May 11, 2023. https://www.zejulahcp .com/content/dam/cf-pharma/hcp-zejulahcp-v2/en_US /pdf/ZEJULA%20(niraparib)%20Dear%20HCP%20Letter%20 November%202022.pdf
- Monk BJ, Parkinson C, Lim MC, et al. A randomized, phase III trial to evaluate rucaparib monotherapy as maintenance treatment in patients with newly diagnosed ovarian cancer (ATHENA-MONO/GOG-3020/ENGOT-ov45). J Clin Oncol. 2022;40:3952-3964. doi:10.1200/JCO.22.01003
- Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505. doi:10.1056 /NEJMoa1810858
- Ray-Coquard I, Pautier P, Pignata S, et al; PAOLA-1 Investigators. Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med. 2019;381:2416-2428. doi:10.1056/NEJMoa1911361
Postpartum IUD insertion: Best practices
CASE 1 Multiparous female with short-interval pregnancies desires contraception
A 24-year-old woman (G4P3) presents for a routine prenatal visit in the third trimester. Her last 2 pregnancies have occurred within 3 months of her prior birth. She endorses feeling overwhelmed with having 4 children under the age of 5 years, and she specifies that she would like to avoid another pregnancy for several years. She plans to breast and bottle feed, and she notes that she tends to forget to take pills. When you look back at her prior charts, you note that she did not return for her last 2 postpartum visits. What can you offer her? What would be a safe contraceptive option for her?
Intrauterine devices (IUDs) are safe, effective, and reported by patients to be satisfactory methods of contraception precisely because they are prone to less user error. The Contraceptive Choice Project demonstrated that patients are more apt to choose them when barriers such as cost and access are removed and nondirective counseling is provided.1 Given that unintended pregnancy rates hover around 48%, the American College of Obstetricians and Gynecologists (ACOG) recommends them as first-line methods for pregnancy prevention.2,3
For repeat pregnancies, the postpartum period is an especially vulnerable time—non-breastfeeding women will ovulate as soon as 25 days after birth, and by 8 weeks 30% will have ovulated.4 Approximately 40% to 57% of women report having unprotected intercourse before 6 weeks postpartum, and nearly 70% of all pregnancies in the first year postpartum are unintended.3,5 Furthermore, patients at highest risk for short-interval pregnancy, such as adolescents, are less likely to return for a postpartum visit.3
Short-interval pregnancies confer greater fetal risk, including risks of low-birth weight, preterm birth, small for gestational age and increased risk of neonatal intensive care unit admission.6 Additionally, maternal health may be compromised during a short-interval pregnancy, particularly in medically complex patients due to increased risks of adverse pregnancy outcomes, such as postpartum bleeding or uterine rupture and disease progression.7 A 2006 meta-analysis by Conde-Agudelo and colleagues found that waiting at least 18 months between pregnancies was optimal for reducing these risks.6
Thus, the immediate postpartum period is an optimal time for addressing contraceptive needs and for preventing short-interval and unintended pregnancy. This article aims to provide evidence supporting the use of immediate postpartum IUDs, as well as their associated risks and barriers to use.
IUD types and routes for immediate postpartum insertion
There are several randomized controlled trials (RCTs) that examine the immediate postpartum use of copper IUDs and levonorgestrel-releasing (LNG) IUDs.8-11 In 2010, Chen and colleagues compared placement of the immediate postpartum IUD following vaginal delivery with interval placement at 6–8 weeks postpartum. Of 51 patients enrolled in each arm, 98% received an IUD immediately postpartum, and 90% received one during their postpartum visit. There were 12 expulsions (24%) in the immediate postpartum IUD group, compared with 2 (4.4%) in the interval group. Expelled IUDs were replaced, and at 6 months both groups had similar rates of IUD use.8
Whitaker and colleagues demonstrated similar findings after randomizing a small group of women who had a cesarean delivery (CD) to interval or immediate placement. There were significantly more expulsions in the post-placental group (20%) than the interval group (0%), but there were more users of the IUD in the post-placental group than in the interval group at 12 months.9
Two RCTs, by Lester and colleagues and Levi et al, demonstrated successful placement of the copper IUD or LNG-IUD following CD, with few expulsions (0% and 8%, respectively). Patients who were randomized to immediate postpartum IUD placement were more likely to receive an IUD than those who were randomized to interval insertion, mostly due to lack of postpartum follow up. Both studies followed patients out to 6 months, and rates of IUD continuation and satisfaction were higher at this time in the immediate postpartum IUD groups.10,11
Continue to: Risks, contraindications, and breastfeeding impact...
Risks, contraindications, and breastfeeding impact
What are the risks of immediate postpartum IUD placement? The highest risk of IUD placement in the immediate postpartum period appears to be expulsion (TABLE 1). In a meta-analysis conducted in 2022, which looked at 11 studies of immediate IUD insertion, the rates of expulsion were between 5% and 27%.3,8,12,13 Results of a study by Cohen and colleagues demonstrated that most expulsions occurred within the first 12 weeks following delivery; of those expulsions that occurred, only 11% went unrecognized.13 Immediate postpartum IUD insertion does not increase the IUD-associated risks of perforation, infection, or immediate postpartum bleeding (although prolonged bleeding may be more common).12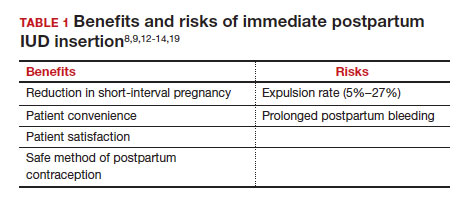
Are there contraindications to placing an IUD immediately postpartum? The main contraindication to immediate postpartum IUD use is peripartum infection, including Triple I, endomyometritis, and puerperal sepsis. Other contraindications include retained placenta requiring manual or surgical removal, uterine anomalies, and other medical contraindications to IUD use as recommended by the US Medical Eligibility Criteria.14
Does immediate IUD placement affect breastfeeding? There is theoretical risk of decreased milk supply or difficulty breastfeeding with initiation of progestin-only methods of contraception in the immediate postpartum period, as the rapid fall in progesterone levels initiates lactogenesis. However, progestin-only methods appear to have limited effect on initiation and continuation of breastfeeding in the immediate postpartum period.15
There were 2 secondary analyses of a pair of RCTs comparing immediate and delayed postpartum IUD use. Results from Levi and colleagues demonstrated no difference between immediate and interval IUD placement groups in the proportion of women who were breastfeeding at 6, 12, and 24 weeks.16 Chen and colleagues’ study was smaller; researchers found that women with interval IUD placement were more likely to be exclusively breastfeeding and continuing to breastfeed at 6 months compared with the immediate postpartum group.17
To better characterize the impact of progestin implants, in a recent meta-analysis, authors examined the use of subcutaneous levonorgestrel rods and found no difference in breastfeeding initiation and continuation rates between women who had them placed immediately versus 6 ̶ 8 weeks postpartum.12
Benefits of immediate postpartum IUD placement
One benefit of immediate postpartum IUD insertion is a reduction in short-interval pregnancies. In a study by Cohen and colleagues13 of young women aged 13 to 22 years choosing immediate postpartum IUDs (82) or implants (162), the authors found that 61% of women retained their IUDs at 12 months postpartum. Because few requested IUD removal over that time frame, the discontinuation rate at 1 year was primarily due to expulsions. Pregnancy rates at 1 year were 7.6% in the IUD group and 1.5% in the implant group. However, the 7.6% rate in the IUD group was lower than in previously studied adolescent control groups: 18.6% of control adolescents (38 of 204) using a contraceptive form other than a postpartum etonogestrel implant had repeat pregnancy at 1 year.13,18
Not only are patients who receive immediate postpartum IUDs more likely to receive them and continue their use, but they are also satisfied with the experience of receiving the IUD and with the method of contraception. A small mixed methods study of 66 patients demonstrated that women were interested in obtaining immediate postpartum contraception to avoid some of the logistical and financial challenges of returning for a postpartum visit. They also felt that the IUD placement was less painful than expected, and they didn’t feel that the insertion process imposed on their birth experience. Many described relief to know that they had a safe and effective contraceptive method upon leaving the hospital.19 Other studies have shown that even among women who expel an IUD following immediate postpartum placement, many choose to replace it in order to continue it as a contraceptive method.8,9,13
Continue to: Instructions for placement...
Instructions for placement
1. Counsel appropriately. Thoroughly counsel patients regarding their options for postpartum contraception, with emphasis on the benefits, risks, and contraindications. Current recommendations to reduce the risk of expulsion are to place the IUD in the delivery room or operating room within 10 minutes of placental delivery.
2. Post ̶ vaginal delivery. Following vaginal delivery, remove the IUD from the inserter, cut the strings to 10 cm and, using either fingers to grasp the wings of the IUD or ring forceps, advance the IUD to the fundus. Ultrasound guidance may be used, but it does not appear to be helpful in preventing expulsion.20
3. Post ̶ cesarean delivery. Once the placenta is delivered, place the IUD using the inserter or a ring forceps at the fundus and guide the strings into the cervix, then close the hysterotomy.
ACOG does recommend formal trainingbefore placing postpartum IUDs. One resource they provide is a free online webinar (https://www.acog.org/education-and-events/webinars/long-acting-reversible-contra ception-overview-and-hands-on-practice-for-residents).3
CASE 1 Resolved
The patient was counseled in the office about her options, and she was most interested in immediate postpartum LNG-IUD placement. She went on to deliver a healthy baby vaginally at 39 weeks. Within 10 minutes of placental delivery, she received an LNG-IUD. She returned to the office 3 months later for STI screening; her examination revealed correct placement and no evidence of expulsion. She expressed that she was happy with her IUD and thankful that she was able to receive it immediately after the birth of her baby.
CASE 2 Nulliparous woman desires IUD for postpartum contraception
A 33-year-old nulliparous woman presents in the third trimester for a routine prenatal visit. She had used the LNG-IUD prior to getting pregnant and reports that she was very happy with it. She knows she wants to wait at least 2 years before trying to get pregnant again, and she would like to resume contraception as soon as it is reasonably safe to do so. She has read that it is possible to get an IUD immediately postpartum and asks about it as a possible option.
What barriers will she face in obtaining an immediate postpartum IUD?
There are many barriers for patients who may be good candidates for immediate postpartum contraception (TABLE 2). Many patients are unaware that it is a safe option, and they often have concerns about such risks as infection, perforation, and effects on breastfeeding. Additionally, providers may not prioritize adequate counseling about postpartum contraception when they face time constraints and a need to counsel about other pregnancy-related topics during the prenatal visit schedule.7,21
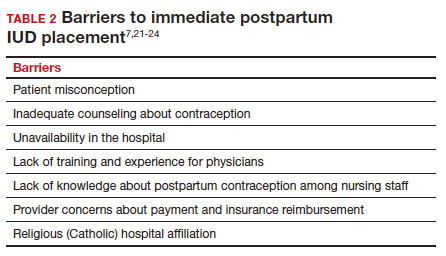
System, hospital, and clinician barriers to immediate postpartum IUD use
Hospital implementation of a successful postpartum IUD program requires pharmacy, intrapartum and postpartum nursing staff, physicians, administration, and billing to be aligned. Hospital administration and pharmacists must stock IUDs in the pharmacy. Hospital nursing staff attitudes toward and knowledge of postpartum contraception can have profound influence on how they discuss safe and effective methods of postpartum contraception with patients who may not have received counseling during prenatal care.22 In a survey of 108 ACOG fellows, nearly 75% of ObGyn physicians did not offer immediate postpartum IUDs; lack of provider training, lack of IUD availability, and concern about cost and payment were found to be common reasons why.21 Additionally, Catholic-affiliated and rural institutions are less likely to offer it, whereas more urban, teaching hospitals are more likely to have programs in place.23 Prior to 2012, immediate postpartum IUD insertions and device costs were part of the global Medicaid obstetric fee in most states, and both hospital systems and individual providers were concerned about loss of revenue.23
In 2015, Washington and colleagues published a decision analysis that examined the cost-effectiveness and cost savings associated with immediate postpartum IUD use. Accounting for expulsion rates, they found that immediate postpartum IUD placement can save $282,540 per 1,000 women over 2 years; additionally, immediate postpartum IUD use can prevent 88 unintended pregnancies per 1,000 women over 2 years.24 Not only do immediate postpartum IUDs have great potential to prevent individual patients from undesired short-interval pregnancies (FIGURE 1), but they can also save the system substantial health care dollars (FIGURE 2).
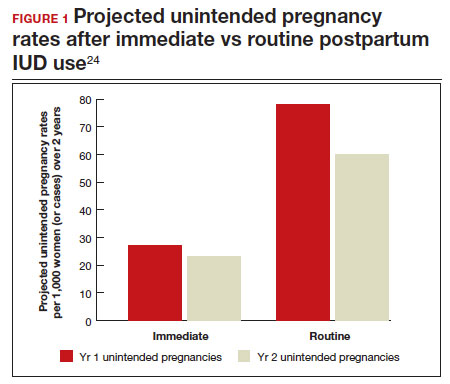
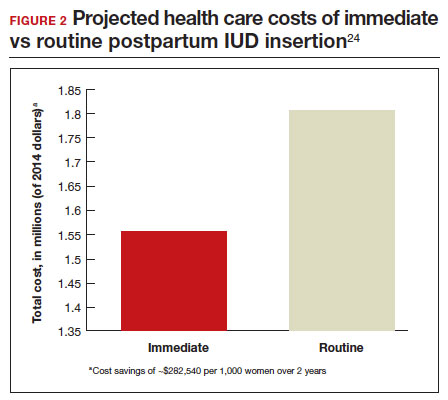
Overcoming barriers
Immediate postpartum IUD implementation is attainable with practice, policy, and institutional changes. Education and training programs geared toward providers and nursing staff can improve understanding of the benefits and risks of immediate postpartum IUD placement. Additionally, clinicians must provide comprehensive, nondirective counseling during the antepartum period, informing patients of all safe and effective options. Expulsion risks should be disclosed, as well as the benefit of not needing to return for a separate postpartum contraception appointment.
Since 2012, many state Medicaid agencies have decoupled reimbursement for inpatient postpartum IUD insertion from the delivery fee. By 2018, more than half of states adopted this practice. Commercial insurers have followed suit in some cases, and as such, both Medicaid and commercially insured patients have had increased access to immediate postpartum IUDs.23 This has translated into increased uptake of immediate postpartum IUDs among both Medicaid and commercially insured patients. Koch et al conducted a retrospective cohort study comparing IUD use in patients 1 year before and 1 year after the policy changes, and they found a 10-fold increase in use of immediate postpartum IUDs.25
While education, counseling, access, and changes in reimbursement may increase access in many hospital systems, some barriers, such as religious affiliation of the hospital system, may be impossible to overcome. A viable alternative to immediate postpartum IUD placement may be early postpartum IUD placement, which could allow patients to coordinate this procedure with 1- or 2-week return routine postpartum visits for CD recovery, mental health screenings, and/or well-baby visits. More data are necessary before recommending this universally, but Averbach and colleagues published a promising meta-analysis that demonstrated no complete expulsions in studies in which IUDs were placed between 2 and 4 weeks postpartum, and only a pooled partial expulsion rate (of immediate postpartum, early inpatient, early outpatient, and interval placement) of 3.7%.4
CASE 2 Resolved
Although the patient was interested in receiving a postpartum LNG-IUD immediately after her vaginal birth, she had to wait until her 6-week postpartum visit. The hospital did not stock IUDs for immediate postpartum IUD use, and her provider, having not been trained on immediate postpartum insertion, did not feel comfortable trying to place it in the immediate postpartum time frame. ●
- Immediate postpartum IUD insertion is a safe and effective method for postpartum contraception for many postpartum women.
- Immediate postpartum IUD insertion can result in increased uptake of postpartum contraception, a reduction in short interval pregnancies, and the opportunity for patients to plan their ideal family size.
- Patients should be thoroughly counseled about the safety of IUD placement and risks of expulsion associated with immediate postpartum placement.
- Successful programs for immediate postpartum IUD insertion incorporate training for providers on proper insertion techniques, education for nursing staff about safety and counseling, on-site IUD supply, and reimbursement that is decoupled from the payment for delivery.
- Winner B, Peipert JF, Zhao Q, et al. Effectiveness of longacting reversible contraception. N Engl J Med. 2012;366:19982007. doi: 10.1056/NEJMoa1110855.
- Bearak J, Popinchalk A, Ganatra B, et al. Unintended pregnancy and abortion by income, region, and the legal status of abortion: estimates from a comprehensive model for 1990-2019. Lancet Glob Health. 2020;8:e1152-e1161. doi: 10.1016/S2214-109X(20)30315-6.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice. Committee Opinion No. 670: Immediate postpartum long-acting reversible contraception. Obstet Gynecol. 2016;128:e32-e37. doi: 10.1097/AOG.0000000000001587.
- Averbach SH, Ermias Y, Jeng G, et al. Expulsion of intrauterine devices after postpartum placement by timing of placement, delivery type, and intrauterine device type: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;223:177188. doi: 10.1016/j.ajog.2020.02.045.
- Connolly A, Thorp J, Pahel L. Effects of pregnancy and childbirth on postpartum sexual function: a longitudinal prospective study. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:263-267. doi: 10.1007/s00192-005-1293-6.
- Conde-Agudelo A, Rosas-Bermúdez A, Kafury-Goeta AC. Birth spacing and risk of adverse perinatal outcomes: a meta-analysis. JAMA. 2006;295:1809-1823. doi: 10.1001 /jama.295.15.1809.
- Vricella LK, Gawron LM, Louis JM. Society for MaternalFetal Medicine (SMFM) Consult Series #48: Immediate postpartum long-acting reversible contraception for women at high risk for medical complications. Am J Obstet Gynecol. 2019;220:B2-B12. doi: 10.1016/j.ajog.2019.02.011.
- Chen BA, Reeves MF, Hayes JL, et al. Postplacental or delayed insertion of the levonorgestrel intrauterine device after vaginal delivery: a randomized controlled trial. Obstet Gynecol. 2010;116:1079-1087. doi: 10.1097/AOG.0b013e3181f73fac.
- Whitaker AK, Endres LK, Mistretta SQ, et al. Postplacental insertion of the levonorgestrel intrauterine device after cesarean delivery vs. delayed insertion: a randomized controlled trial. Contraception. 2014;89:534-539. doi: 10.1016/j.contraception.2013.12.007.
- Lester F, Kakaire O, Byamugisha J, et al. Intracesarean insertion of the Copper T380A versus 6 weeks postcesarean: a randomized clinical trial. Contraception. 2015;91:198-203. doi: 10.1016/j.contraception.2014.12.002.
- Levi EE, Stuart GS, Zerden ML, et al. Intrauterine device placement during cesarean delivery and continued use 6 months postpartum: a randomized controlled trial. Obstet Gynecol. 2015;126:5-11. doi: 10.1097/AOG.0000000000000882.
- Sothornwit J, Kaewrudee S, Lumbiganon P, et al. Immediate versus delayed postpartum insertion of contraceptive implant and IUD for contraception. Cochrane Database Syst Rev. 2022;10:CD011913. doi: 10.1002/14651858.CD011913.pub3.
- Cohen R, Sheeder J, Arango N, et al. Twelve-month contraceptive continuation and repeat pregnancy among young mothers choosing postdelivery contraceptive implants or postplacental intrauterine devices. Contraception. 2016;93:178-183. doi: 10.1016/j.contraception.2015.10.001.
- Centers for Disease Control and Prevention (CDC). US Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep. 2010;59(RR-4):1-86.
- Kapp N, Curtis K, Nanda K. Progestogen-only contraceptive use among breastfeeding women: a systematic review. Contraception. 2010;82:17-37. doi: 10.1016 /j.contraception.2010.02.002.
- Levi EE, Findley MK, Avila K, et al. Placement of levonorgestrel intrauterine device at the time of cesarean delivery and the effect on breastfeeding duration. Breastfeed Med. 2018;13:674679. doi: 10.1089/bfm.2018.0060.
- Chen BA, Reeves MF, Creinin MD, et al. Postplacental or delayed levonorgestrel intrauterine device insertion and breast-feeding duration. Contraception. 2011;84:499-504. doi: 10.1016/j.contraception.2011.01.022.
- Tocce KM, Sheeder JL, Teal SB. Rapid repeat pregnancy in adolescents: do immediate postpartum contraceptive implants make a difference? Am J Obstet Gynecol. 2012;206:481.e1-7. doi: 10.1016/j.ajog.2012.04.015.
- Carr SL, Singh RH, Sussman AL, et al. Women’s experiences with immediate postpartum intrauterine device insertion: a mixed-methods study. Contraception. 2018;97:219-226. doi: 10.1016/j.contraception.2017.10.008.
- Martinez OP, Wilder L, Seal P. Ultrasound-guided compared with non-ultrasound-Guided placement of immediate postpartum intrauterine contraceptive devices. Obstet Gynecol. 2022;140:91-93. doi: 10.1097/AOG.0000000000004828.
- Holden EC, Lai E, Morelli SS, et al. Ongoing barriers to immediate postpartum long-acting reversible contraception: a physician survey. Contracept Reprod Med. 2018;3:23. doi: 10.1186/s40834-018-0078-5.
- Benfield N, Hawkins F, Ray L, et al. Exposure to routine availability of immediate postpartum LARC: effect on attitudes and practices of labor and delivery and postpartum nurses. Contraception. 2018;97:411-414. doi: 10.1016 /j.contraception.2018.01.017.
- Steenland MW, Vatsa R, Pace LE, et al. Immediate postpartum long-acting reversible contraceptive use following statespecific changes in hospital Medicaid reimbursement. JAMA Netw Open. 2022;5:e2237918. doi: 10.1001 /jamanetworkopen.2022.37918.
- Washington CI, Jamshidi R, Thung SF, et al. Timing of postpartum intrauterine device placement: a costeffectiveness analysis. Fertil Steril. 2015;103:131-137. doi: 10.1016/j.fertnstert.2014.09.032
CASE 1 Multiparous female with short-interval pregnancies desires contraception
A 24-year-old woman (G4P3) presents for a routine prenatal visit in the third trimester. Her last 2 pregnancies have occurred within 3 months of her prior birth. She endorses feeling overwhelmed with having 4 children under the age of 5 years, and she specifies that she would like to avoid another pregnancy for several years. She plans to breast and bottle feed, and she notes that she tends to forget to take pills. When you look back at her prior charts, you note that she did not return for her last 2 postpartum visits. What can you offer her? What would be a safe contraceptive option for her?
Intrauterine devices (IUDs) are safe, effective, and reported by patients to be satisfactory methods of contraception precisely because they are prone to less user error. The Contraceptive Choice Project demonstrated that patients are more apt to choose them when barriers such as cost and access are removed and nondirective counseling is provided.1 Given that unintended pregnancy rates hover around 48%, the American College of Obstetricians and Gynecologists (ACOG) recommends them as first-line methods for pregnancy prevention.2,3
For repeat pregnancies, the postpartum period is an especially vulnerable time—non-breastfeeding women will ovulate as soon as 25 days after birth, and by 8 weeks 30% will have ovulated.4 Approximately 40% to 57% of women report having unprotected intercourse before 6 weeks postpartum, and nearly 70% of all pregnancies in the first year postpartum are unintended.3,5 Furthermore, patients at highest risk for short-interval pregnancy, such as adolescents, are less likely to return for a postpartum visit.3
Short-interval pregnancies confer greater fetal risk, including risks of low-birth weight, preterm birth, small for gestational age and increased risk of neonatal intensive care unit admission.6 Additionally, maternal health may be compromised during a short-interval pregnancy, particularly in medically complex patients due to increased risks of adverse pregnancy outcomes, such as postpartum bleeding or uterine rupture and disease progression.7 A 2006 meta-analysis by Conde-Agudelo and colleagues found that waiting at least 18 months between pregnancies was optimal for reducing these risks.6
Thus, the immediate postpartum period is an optimal time for addressing contraceptive needs and for preventing short-interval and unintended pregnancy. This article aims to provide evidence supporting the use of immediate postpartum IUDs, as well as their associated risks and barriers to use.
IUD types and routes for immediate postpartum insertion
There are several randomized controlled trials (RCTs) that examine the immediate postpartum use of copper IUDs and levonorgestrel-releasing (LNG) IUDs.8-11 In 2010, Chen and colleagues compared placement of the immediate postpartum IUD following vaginal delivery with interval placement at 6–8 weeks postpartum. Of 51 patients enrolled in each arm, 98% received an IUD immediately postpartum, and 90% received one during their postpartum visit. There were 12 expulsions (24%) in the immediate postpartum IUD group, compared with 2 (4.4%) in the interval group. Expelled IUDs were replaced, and at 6 months both groups had similar rates of IUD use.8
Whitaker and colleagues demonstrated similar findings after randomizing a small group of women who had a cesarean delivery (CD) to interval or immediate placement. There were significantly more expulsions in the post-placental group (20%) than the interval group (0%), but there were more users of the IUD in the post-placental group than in the interval group at 12 months.9
Two RCTs, by Lester and colleagues and Levi et al, demonstrated successful placement of the copper IUD or LNG-IUD following CD, with few expulsions (0% and 8%, respectively). Patients who were randomized to immediate postpartum IUD placement were more likely to receive an IUD than those who were randomized to interval insertion, mostly due to lack of postpartum follow up. Both studies followed patients out to 6 months, and rates of IUD continuation and satisfaction were higher at this time in the immediate postpartum IUD groups.10,11
Continue to: Risks, contraindications, and breastfeeding impact...
Risks, contraindications, and breastfeeding impact
What are the risks of immediate postpartum IUD placement? The highest risk of IUD placement in the immediate postpartum period appears to be expulsion (TABLE 1). In a meta-analysis conducted in 2022, which looked at 11 studies of immediate IUD insertion, the rates of expulsion were between 5% and 27%.3,8,12,13 Results of a study by Cohen and colleagues demonstrated that most expulsions occurred within the first 12 weeks following delivery; of those expulsions that occurred, only 11% went unrecognized.13 Immediate postpartum IUD insertion does not increase the IUD-associated risks of perforation, infection, or immediate postpartum bleeding (although prolonged bleeding may be more common).12
Are there contraindications to placing an IUD immediately postpartum? The main contraindication to immediate postpartum IUD use is peripartum infection, including Triple I, endomyometritis, and puerperal sepsis. Other contraindications include retained placenta requiring manual or surgical removal, uterine anomalies, and other medical contraindications to IUD use as recommended by the US Medical Eligibility Criteria.14
Does immediate IUD placement affect breastfeeding? There is theoretical risk of decreased milk supply or difficulty breastfeeding with initiation of progestin-only methods of contraception in the immediate postpartum period, as the rapid fall in progesterone levels initiates lactogenesis. However, progestin-only methods appear to have limited effect on initiation and continuation of breastfeeding in the immediate postpartum period.15
There were 2 secondary analyses of a pair of RCTs comparing immediate and delayed postpartum IUD use. Results from Levi and colleagues demonstrated no difference between immediate and interval IUD placement groups in the proportion of women who were breastfeeding at 6, 12, and 24 weeks.16 Chen and colleagues’ study was smaller; researchers found that women with interval IUD placement were more likely to be exclusively breastfeeding and continuing to breastfeed at 6 months compared with the immediate postpartum group.17
To better characterize the impact of progestin implants, in a recent meta-analysis, authors examined the use of subcutaneous levonorgestrel rods and found no difference in breastfeeding initiation and continuation rates between women who had them placed immediately versus 6 ̶ 8 weeks postpartum.12
Benefits of immediate postpartum IUD placement
One benefit of immediate postpartum IUD insertion is a reduction in short-interval pregnancies. In a study by Cohen and colleagues13 of young women aged 13 to 22 years choosing immediate postpartum IUDs (82) or implants (162), the authors found that 61% of women retained their IUDs at 12 months postpartum. Because few requested IUD removal over that time frame, the discontinuation rate at 1 year was primarily due to expulsions. Pregnancy rates at 1 year were 7.6% in the IUD group and 1.5% in the implant group. However, the 7.6% rate in the IUD group was lower than in previously studied adolescent control groups: 18.6% of control adolescents (38 of 204) using a contraceptive form other than a postpartum etonogestrel implant had repeat pregnancy at 1 year.13,18
Not only are patients who receive immediate postpartum IUDs more likely to receive them and continue their use, but they are also satisfied with the experience of receiving the IUD and with the method of contraception. A small mixed methods study of 66 patients demonstrated that women were interested in obtaining immediate postpartum contraception to avoid some of the logistical and financial challenges of returning for a postpartum visit. They also felt that the IUD placement was less painful than expected, and they didn’t feel that the insertion process imposed on their birth experience. Many described relief to know that they had a safe and effective contraceptive method upon leaving the hospital.19 Other studies have shown that even among women who expel an IUD following immediate postpartum placement, many choose to replace it in order to continue it as a contraceptive method.8,9,13
Continue to: Instructions for placement...
Instructions for placement
1. Counsel appropriately. Thoroughly counsel patients regarding their options for postpartum contraception, with emphasis on the benefits, risks, and contraindications. Current recommendations to reduce the risk of expulsion are to place the IUD in the delivery room or operating room within 10 minutes of placental delivery.
2. Post ̶ vaginal delivery. Following vaginal delivery, remove the IUD from the inserter, cut the strings to 10 cm and, using either fingers to grasp the wings of the IUD or ring forceps, advance the IUD to the fundus. Ultrasound guidance may be used, but it does not appear to be helpful in preventing expulsion.20
3. Post ̶ cesarean delivery. Once the placenta is delivered, place the IUD using the inserter or a ring forceps at the fundus and guide the strings into the cervix, then close the hysterotomy.
ACOG does recommend formal trainingbefore placing postpartum IUDs. One resource they provide is a free online webinar (https://www.acog.org/education-and-events/webinars/long-acting-reversible-contra ception-overview-and-hands-on-practice-for-residents).3
CASE 1 Resolved
The patient was counseled in the office about her options, and she was most interested in immediate postpartum LNG-IUD placement. She went on to deliver a healthy baby vaginally at 39 weeks. Within 10 minutes of placental delivery, she received an LNG-IUD. She returned to the office 3 months later for STI screening; her examination revealed correct placement and no evidence of expulsion. She expressed that she was happy with her IUD and thankful that she was able to receive it immediately after the birth of her baby.
CASE 2 Nulliparous woman desires IUD for postpartum contraception
A 33-year-old nulliparous woman presents in the third trimester for a routine prenatal visit. She had used the LNG-IUD prior to getting pregnant and reports that she was very happy with it. She knows she wants to wait at least 2 years before trying to get pregnant again, and she would like to resume contraception as soon as it is reasonably safe to do so. She has read that it is possible to get an IUD immediately postpartum and asks about it as a possible option.
What barriers will she face in obtaining an immediate postpartum IUD?
There are many barriers for patients who may be good candidates for immediate postpartum contraception (TABLE 2). Many patients are unaware that it is a safe option, and they often have concerns about such risks as infection, perforation, and effects on breastfeeding. Additionally, providers may not prioritize adequate counseling about postpartum contraception when they face time constraints and a need to counsel about other pregnancy-related topics during the prenatal visit schedule.7,21

System, hospital, and clinician barriers to immediate postpartum IUD use
Hospital implementation of a successful postpartum IUD program requires pharmacy, intrapartum and postpartum nursing staff, physicians, administration, and billing to be aligned. Hospital administration and pharmacists must stock IUDs in the pharmacy. Hospital nursing staff attitudes toward and knowledge of postpartum contraception can have profound influence on how they discuss safe and effective methods of postpartum contraception with patients who may not have received counseling during prenatal care.22 In a survey of 108 ACOG fellows, nearly 75% of ObGyn physicians did not offer immediate postpartum IUDs; lack of provider training, lack of IUD availability, and concern about cost and payment were found to be common reasons why.21 Additionally, Catholic-affiliated and rural institutions are less likely to offer it, whereas more urban, teaching hospitals are more likely to have programs in place.23 Prior to 2012, immediate postpartum IUD insertions and device costs were part of the global Medicaid obstetric fee in most states, and both hospital systems and individual providers were concerned about loss of revenue.23
In 2015, Washington and colleagues published a decision analysis that examined the cost-effectiveness and cost savings associated with immediate postpartum IUD use. Accounting for expulsion rates, they found that immediate postpartum IUD placement can save $282,540 per 1,000 women over 2 years; additionally, immediate postpartum IUD use can prevent 88 unintended pregnancies per 1,000 women over 2 years.24 Not only do immediate postpartum IUDs have great potential to prevent individual patients from undesired short-interval pregnancies (FIGURE 1), but they can also save the system substantial health care dollars (FIGURE 2).


Overcoming barriers
Immediate postpartum IUD implementation is attainable with practice, policy, and institutional changes. Education and training programs geared toward providers and nursing staff can improve understanding of the benefits and risks of immediate postpartum IUD placement. Additionally, clinicians must provide comprehensive, nondirective counseling during the antepartum period, informing patients of all safe and effective options. Expulsion risks should be disclosed, as well as the benefit of not needing to return for a separate postpartum contraception appointment.
Since 2012, many state Medicaid agencies have decoupled reimbursement for inpatient postpartum IUD insertion from the delivery fee. By 2018, more than half of states adopted this practice. Commercial insurers have followed suit in some cases, and as such, both Medicaid and commercially insured patients have had increased access to immediate postpartum IUDs.23 This has translated into increased uptake of immediate postpartum IUDs among both Medicaid and commercially insured patients. Koch et al conducted a retrospective cohort study comparing IUD use in patients 1 year before and 1 year after the policy changes, and they found a 10-fold increase in use of immediate postpartum IUDs.25
While education, counseling, access, and changes in reimbursement may increase access in many hospital systems, some barriers, such as religious affiliation of the hospital system, may be impossible to overcome. A viable alternative to immediate postpartum IUD placement may be early postpartum IUD placement, which could allow patients to coordinate this procedure with 1- or 2-week return routine postpartum visits for CD recovery, mental health screenings, and/or well-baby visits. More data are necessary before recommending this universally, but Averbach and colleagues published a promising meta-analysis that demonstrated no complete expulsions in studies in which IUDs were placed between 2 and 4 weeks postpartum, and only a pooled partial expulsion rate (of immediate postpartum, early inpatient, early outpatient, and interval placement) of 3.7%.4
CASE 2 Resolved
Although the patient was interested in receiving a postpartum LNG-IUD immediately after her vaginal birth, she had to wait until her 6-week postpartum visit. The hospital did not stock IUDs for immediate postpartum IUD use, and her provider, having not been trained on immediate postpartum insertion, did not feel comfortable trying to place it in the immediate postpartum time frame. ●
- Immediate postpartum IUD insertion is a safe and effective method for postpartum contraception for many postpartum women.
- Immediate postpartum IUD insertion can result in increased uptake of postpartum contraception, a reduction in short interval pregnancies, and the opportunity for patients to plan their ideal family size.
- Patients should be thoroughly counseled about the safety of IUD placement and risks of expulsion associated with immediate postpartum placement.
- Successful programs for immediate postpartum IUD insertion incorporate training for providers on proper insertion techniques, education for nursing staff about safety and counseling, on-site IUD supply, and reimbursement that is decoupled from the payment for delivery.
CASE 1 Multiparous female with short-interval pregnancies desires contraception
A 24-year-old woman (G4P3) presents for a routine prenatal visit in the third trimester. Her last 2 pregnancies have occurred within 3 months of her prior birth. She endorses feeling overwhelmed with having 4 children under the age of 5 years, and she specifies that she would like to avoid another pregnancy for several years. She plans to breast and bottle feed, and she notes that she tends to forget to take pills. When you look back at her prior charts, you note that she did not return for her last 2 postpartum visits. What can you offer her? What would be a safe contraceptive option for her?
Intrauterine devices (IUDs) are safe, effective, and reported by patients to be satisfactory methods of contraception precisely because they are prone to less user error. The Contraceptive Choice Project demonstrated that patients are more apt to choose them when barriers such as cost and access are removed and nondirective counseling is provided.1 Given that unintended pregnancy rates hover around 48%, the American College of Obstetricians and Gynecologists (ACOG) recommends them as first-line methods for pregnancy prevention.2,3
For repeat pregnancies, the postpartum period is an especially vulnerable time—non-breastfeeding women will ovulate as soon as 25 days after birth, and by 8 weeks 30% will have ovulated.4 Approximately 40% to 57% of women report having unprotected intercourse before 6 weeks postpartum, and nearly 70% of all pregnancies in the first year postpartum are unintended.3,5 Furthermore, patients at highest risk for short-interval pregnancy, such as adolescents, are less likely to return for a postpartum visit.3
Short-interval pregnancies confer greater fetal risk, including risks of low-birth weight, preterm birth, small for gestational age and increased risk of neonatal intensive care unit admission.6 Additionally, maternal health may be compromised during a short-interval pregnancy, particularly in medically complex patients due to increased risks of adverse pregnancy outcomes, such as postpartum bleeding or uterine rupture and disease progression.7 A 2006 meta-analysis by Conde-Agudelo and colleagues found that waiting at least 18 months between pregnancies was optimal for reducing these risks.6
Thus, the immediate postpartum period is an optimal time for addressing contraceptive needs and for preventing short-interval and unintended pregnancy. This article aims to provide evidence supporting the use of immediate postpartum IUDs, as well as their associated risks and barriers to use.
IUD types and routes for immediate postpartum insertion
There are several randomized controlled trials (RCTs) that examine the immediate postpartum use of copper IUDs and levonorgestrel-releasing (LNG) IUDs.8-11 In 2010, Chen and colleagues compared placement of the immediate postpartum IUD following vaginal delivery with interval placement at 6–8 weeks postpartum. Of 51 patients enrolled in each arm, 98% received an IUD immediately postpartum, and 90% received one during their postpartum visit. There were 12 expulsions (24%) in the immediate postpartum IUD group, compared with 2 (4.4%) in the interval group. Expelled IUDs were replaced, and at 6 months both groups had similar rates of IUD use.8
Whitaker and colleagues demonstrated similar findings after randomizing a small group of women who had a cesarean delivery (CD) to interval or immediate placement. There were significantly more expulsions in the post-placental group (20%) than the interval group (0%), but there were more users of the IUD in the post-placental group than in the interval group at 12 months.9
Two RCTs, by Lester and colleagues and Levi et al, demonstrated successful placement of the copper IUD or LNG-IUD following CD, with few expulsions (0% and 8%, respectively). Patients who were randomized to immediate postpartum IUD placement were more likely to receive an IUD than those who were randomized to interval insertion, mostly due to lack of postpartum follow up. Both studies followed patients out to 6 months, and rates of IUD continuation and satisfaction were higher at this time in the immediate postpartum IUD groups.10,11
Continue to: Risks, contraindications, and breastfeeding impact...
Risks, contraindications, and breastfeeding impact
What are the risks of immediate postpartum IUD placement? The highest risk of IUD placement in the immediate postpartum period appears to be expulsion (TABLE 1). In a meta-analysis conducted in 2022, which looked at 11 studies of immediate IUD insertion, the rates of expulsion were between 5% and 27%.3,8,12,13 Results of a study by Cohen and colleagues demonstrated that most expulsions occurred within the first 12 weeks following delivery; of those expulsions that occurred, only 11% went unrecognized.13 Immediate postpartum IUD insertion does not increase the IUD-associated risks of perforation, infection, or immediate postpartum bleeding (although prolonged bleeding may be more common).12
Are there contraindications to placing an IUD immediately postpartum? The main contraindication to immediate postpartum IUD use is peripartum infection, including Triple I, endomyometritis, and puerperal sepsis. Other contraindications include retained placenta requiring manual or surgical removal, uterine anomalies, and other medical contraindications to IUD use as recommended by the US Medical Eligibility Criteria.14
Does immediate IUD placement affect breastfeeding? There is theoretical risk of decreased milk supply or difficulty breastfeeding with initiation of progestin-only methods of contraception in the immediate postpartum period, as the rapid fall in progesterone levels initiates lactogenesis. However, progestin-only methods appear to have limited effect on initiation and continuation of breastfeeding in the immediate postpartum period.15
There were 2 secondary analyses of a pair of RCTs comparing immediate and delayed postpartum IUD use. Results from Levi and colleagues demonstrated no difference between immediate and interval IUD placement groups in the proportion of women who were breastfeeding at 6, 12, and 24 weeks.16 Chen and colleagues’ study was smaller; researchers found that women with interval IUD placement were more likely to be exclusively breastfeeding and continuing to breastfeed at 6 months compared with the immediate postpartum group.17
To better characterize the impact of progestin implants, in a recent meta-analysis, authors examined the use of subcutaneous levonorgestrel rods and found no difference in breastfeeding initiation and continuation rates between women who had them placed immediately versus 6 ̶ 8 weeks postpartum.12
Benefits of immediate postpartum IUD placement
One benefit of immediate postpartum IUD insertion is a reduction in short-interval pregnancies. In a study by Cohen and colleagues13 of young women aged 13 to 22 years choosing immediate postpartum IUDs (82) or implants (162), the authors found that 61% of women retained their IUDs at 12 months postpartum. Because few requested IUD removal over that time frame, the discontinuation rate at 1 year was primarily due to expulsions. Pregnancy rates at 1 year were 7.6% in the IUD group and 1.5% in the implant group. However, the 7.6% rate in the IUD group was lower than in previously studied adolescent control groups: 18.6% of control adolescents (38 of 204) using a contraceptive form other than a postpartum etonogestrel implant had repeat pregnancy at 1 year.13,18
Not only are patients who receive immediate postpartum IUDs more likely to receive them and continue their use, but they are also satisfied with the experience of receiving the IUD and with the method of contraception. A small mixed methods study of 66 patients demonstrated that women were interested in obtaining immediate postpartum contraception to avoid some of the logistical and financial challenges of returning for a postpartum visit. They also felt that the IUD placement was less painful than expected, and they didn’t feel that the insertion process imposed on their birth experience. Many described relief to know that they had a safe and effective contraceptive method upon leaving the hospital.19 Other studies have shown that even among women who expel an IUD following immediate postpartum placement, many choose to replace it in order to continue it as a contraceptive method.8,9,13
Continue to: Instructions for placement...
Instructions for placement
1. Counsel appropriately. Thoroughly counsel patients regarding their options for postpartum contraception, with emphasis on the benefits, risks, and contraindications. Current recommendations to reduce the risk of expulsion are to place the IUD in the delivery room or operating room within 10 minutes of placental delivery.
2. Post ̶ vaginal delivery. Following vaginal delivery, remove the IUD from the inserter, cut the strings to 10 cm and, using either fingers to grasp the wings of the IUD or ring forceps, advance the IUD to the fundus. Ultrasound guidance may be used, but it does not appear to be helpful in preventing expulsion.20
3. Post ̶ cesarean delivery. Once the placenta is delivered, place the IUD using the inserter or a ring forceps at the fundus and guide the strings into the cervix, then close the hysterotomy.
ACOG does recommend formal trainingbefore placing postpartum IUDs. One resource they provide is a free online webinar (https://www.acog.org/education-and-events/webinars/long-acting-reversible-contra ception-overview-and-hands-on-practice-for-residents).3
CASE 1 Resolved
The patient was counseled in the office about her options, and she was most interested in immediate postpartum LNG-IUD placement. She went on to deliver a healthy baby vaginally at 39 weeks. Within 10 minutes of placental delivery, she received an LNG-IUD. She returned to the office 3 months later for STI screening; her examination revealed correct placement and no evidence of expulsion. She expressed that she was happy with her IUD and thankful that she was able to receive it immediately after the birth of her baby.
CASE 2 Nulliparous woman desires IUD for postpartum contraception
A 33-year-old nulliparous woman presents in the third trimester for a routine prenatal visit. She had used the LNG-IUD prior to getting pregnant and reports that she was very happy with it. She knows she wants to wait at least 2 years before trying to get pregnant again, and she would like to resume contraception as soon as it is reasonably safe to do so. She has read that it is possible to get an IUD immediately postpartum and asks about it as a possible option.
What barriers will she face in obtaining an immediate postpartum IUD?
There are many barriers for patients who may be good candidates for immediate postpartum contraception (TABLE 2). Many patients are unaware that it is a safe option, and they often have concerns about such risks as infection, perforation, and effects on breastfeeding. Additionally, providers may not prioritize adequate counseling about postpartum contraception when they face time constraints and a need to counsel about other pregnancy-related topics during the prenatal visit schedule.7,21

System, hospital, and clinician barriers to immediate postpartum IUD use
Hospital implementation of a successful postpartum IUD program requires pharmacy, intrapartum and postpartum nursing staff, physicians, administration, and billing to be aligned. Hospital administration and pharmacists must stock IUDs in the pharmacy. Hospital nursing staff attitudes toward and knowledge of postpartum contraception can have profound influence on how they discuss safe and effective methods of postpartum contraception with patients who may not have received counseling during prenatal care.22 In a survey of 108 ACOG fellows, nearly 75% of ObGyn physicians did not offer immediate postpartum IUDs; lack of provider training, lack of IUD availability, and concern about cost and payment were found to be common reasons why.21 Additionally, Catholic-affiliated and rural institutions are less likely to offer it, whereas more urban, teaching hospitals are more likely to have programs in place.23 Prior to 2012, immediate postpartum IUD insertions and device costs were part of the global Medicaid obstetric fee in most states, and both hospital systems and individual providers were concerned about loss of revenue.23
In 2015, Washington and colleagues published a decision analysis that examined the cost-effectiveness and cost savings associated with immediate postpartum IUD use. Accounting for expulsion rates, they found that immediate postpartum IUD placement can save $282,540 per 1,000 women over 2 years; additionally, immediate postpartum IUD use can prevent 88 unintended pregnancies per 1,000 women over 2 years.24 Not only do immediate postpartum IUDs have great potential to prevent individual patients from undesired short-interval pregnancies (FIGURE 1), but they can also save the system substantial health care dollars (FIGURE 2).


Overcoming barriers
Immediate postpartum IUD implementation is attainable with practice, policy, and institutional changes. Education and training programs geared toward providers and nursing staff can improve understanding of the benefits and risks of immediate postpartum IUD placement. Additionally, clinicians must provide comprehensive, nondirective counseling during the antepartum period, informing patients of all safe and effective options. Expulsion risks should be disclosed, as well as the benefit of not needing to return for a separate postpartum contraception appointment.
Since 2012, many state Medicaid agencies have decoupled reimbursement for inpatient postpartum IUD insertion from the delivery fee. By 2018, more than half of states adopted this practice. Commercial insurers have followed suit in some cases, and as such, both Medicaid and commercially insured patients have had increased access to immediate postpartum IUDs.23 This has translated into increased uptake of immediate postpartum IUDs among both Medicaid and commercially insured patients. Koch et al conducted a retrospective cohort study comparing IUD use in patients 1 year before and 1 year after the policy changes, and they found a 10-fold increase in use of immediate postpartum IUDs.25
While education, counseling, access, and changes in reimbursement may increase access in many hospital systems, some barriers, such as religious affiliation of the hospital system, may be impossible to overcome. A viable alternative to immediate postpartum IUD placement may be early postpartum IUD placement, which could allow patients to coordinate this procedure with 1- or 2-week return routine postpartum visits for CD recovery, mental health screenings, and/or well-baby visits. More data are necessary before recommending this universally, but Averbach and colleagues published a promising meta-analysis that demonstrated no complete expulsions in studies in which IUDs were placed between 2 and 4 weeks postpartum, and only a pooled partial expulsion rate (of immediate postpartum, early inpatient, early outpatient, and interval placement) of 3.7%.4
CASE 2 Resolved
Although the patient was interested in receiving a postpartum LNG-IUD immediately after her vaginal birth, she had to wait until her 6-week postpartum visit. The hospital did not stock IUDs for immediate postpartum IUD use, and her provider, having not been trained on immediate postpartum insertion, did not feel comfortable trying to place it in the immediate postpartum time frame. ●
- Immediate postpartum IUD insertion is a safe and effective method for postpartum contraception for many postpartum women.
- Immediate postpartum IUD insertion can result in increased uptake of postpartum contraception, a reduction in short interval pregnancies, and the opportunity for patients to plan their ideal family size.
- Patients should be thoroughly counseled about the safety of IUD placement and risks of expulsion associated with immediate postpartum placement.
- Successful programs for immediate postpartum IUD insertion incorporate training for providers on proper insertion techniques, education for nursing staff about safety and counseling, on-site IUD supply, and reimbursement that is decoupled from the payment for delivery.
- Winner B, Peipert JF, Zhao Q, et al. Effectiveness of longacting reversible contraception. N Engl J Med. 2012;366:19982007. doi: 10.1056/NEJMoa1110855.
- Bearak J, Popinchalk A, Ganatra B, et al. Unintended pregnancy and abortion by income, region, and the legal status of abortion: estimates from a comprehensive model for 1990-2019. Lancet Glob Health. 2020;8:e1152-e1161. doi: 10.1016/S2214-109X(20)30315-6.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice. Committee Opinion No. 670: Immediate postpartum long-acting reversible contraception. Obstet Gynecol. 2016;128:e32-e37. doi: 10.1097/AOG.0000000000001587.
- Averbach SH, Ermias Y, Jeng G, et al. Expulsion of intrauterine devices after postpartum placement by timing of placement, delivery type, and intrauterine device type: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;223:177188. doi: 10.1016/j.ajog.2020.02.045.
- Connolly A, Thorp J, Pahel L. Effects of pregnancy and childbirth on postpartum sexual function: a longitudinal prospective study. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:263-267. doi: 10.1007/s00192-005-1293-6.
- Conde-Agudelo A, Rosas-Bermúdez A, Kafury-Goeta AC. Birth spacing and risk of adverse perinatal outcomes: a meta-analysis. JAMA. 2006;295:1809-1823. doi: 10.1001 /jama.295.15.1809.
- Vricella LK, Gawron LM, Louis JM. Society for MaternalFetal Medicine (SMFM) Consult Series #48: Immediate postpartum long-acting reversible contraception for women at high risk for medical complications. Am J Obstet Gynecol. 2019;220:B2-B12. doi: 10.1016/j.ajog.2019.02.011.
- Chen BA, Reeves MF, Hayes JL, et al. Postplacental or delayed insertion of the levonorgestrel intrauterine device after vaginal delivery: a randomized controlled trial. Obstet Gynecol. 2010;116:1079-1087. doi: 10.1097/AOG.0b013e3181f73fac.
- Whitaker AK, Endres LK, Mistretta SQ, et al. Postplacental insertion of the levonorgestrel intrauterine device after cesarean delivery vs. delayed insertion: a randomized controlled trial. Contraception. 2014;89:534-539. doi: 10.1016/j.contraception.2013.12.007.
- Lester F, Kakaire O, Byamugisha J, et al. Intracesarean insertion of the Copper T380A versus 6 weeks postcesarean: a randomized clinical trial. Contraception. 2015;91:198-203. doi: 10.1016/j.contraception.2014.12.002.
- Levi EE, Stuart GS, Zerden ML, et al. Intrauterine device placement during cesarean delivery and continued use 6 months postpartum: a randomized controlled trial. Obstet Gynecol. 2015;126:5-11. doi: 10.1097/AOG.0000000000000882.
- Sothornwit J, Kaewrudee S, Lumbiganon P, et al. Immediate versus delayed postpartum insertion of contraceptive implant and IUD for contraception. Cochrane Database Syst Rev. 2022;10:CD011913. doi: 10.1002/14651858.CD011913.pub3.
- Cohen R, Sheeder J, Arango N, et al. Twelve-month contraceptive continuation and repeat pregnancy among young mothers choosing postdelivery contraceptive implants or postplacental intrauterine devices. Contraception. 2016;93:178-183. doi: 10.1016/j.contraception.2015.10.001.
- Centers for Disease Control and Prevention (CDC). US Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep. 2010;59(RR-4):1-86.
- Kapp N, Curtis K, Nanda K. Progestogen-only contraceptive use among breastfeeding women: a systematic review. Contraception. 2010;82:17-37. doi: 10.1016 /j.contraception.2010.02.002.
- Levi EE, Findley MK, Avila K, et al. Placement of levonorgestrel intrauterine device at the time of cesarean delivery and the effect on breastfeeding duration. Breastfeed Med. 2018;13:674679. doi: 10.1089/bfm.2018.0060.
- Chen BA, Reeves MF, Creinin MD, et al. Postplacental or delayed levonorgestrel intrauterine device insertion and breast-feeding duration. Contraception. 2011;84:499-504. doi: 10.1016/j.contraception.2011.01.022.
- Tocce KM, Sheeder JL, Teal SB. Rapid repeat pregnancy in adolescents: do immediate postpartum contraceptive implants make a difference? Am J Obstet Gynecol. 2012;206:481.e1-7. doi: 10.1016/j.ajog.2012.04.015.
- Carr SL, Singh RH, Sussman AL, et al. Women’s experiences with immediate postpartum intrauterine device insertion: a mixed-methods study. Contraception. 2018;97:219-226. doi: 10.1016/j.contraception.2017.10.008.
- Martinez OP, Wilder L, Seal P. Ultrasound-guided compared with non-ultrasound-Guided placement of immediate postpartum intrauterine contraceptive devices. Obstet Gynecol. 2022;140:91-93. doi: 10.1097/AOG.0000000000004828.
- Holden EC, Lai E, Morelli SS, et al. Ongoing barriers to immediate postpartum long-acting reversible contraception: a physician survey. Contracept Reprod Med. 2018;3:23. doi: 10.1186/s40834-018-0078-5.
- Benfield N, Hawkins F, Ray L, et al. Exposure to routine availability of immediate postpartum LARC: effect on attitudes and practices of labor and delivery and postpartum nurses. Contraception. 2018;97:411-414. doi: 10.1016 /j.contraception.2018.01.017.
- Steenland MW, Vatsa R, Pace LE, et al. Immediate postpartum long-acting reversible contraceptive use following statespecific changes in hospital Medicaid reimbursement. JAMA Netw Open. 2022;5:e2237918. doi: 10.1001 /jamanetworkopen.2022.37918.
- Washington CI, Jamshidi R, Thung SF, et al. Timing of postpartum intrauterine device placement: a costeffectiveness analysis. Fertil Steril. 2015;103:131-137. doi: 10.1016/j.fertnstert.2014.09.032
- Winner B, Peipert JF, Zhao Q, et al. Effectiveness of longacting reversible contraception. N Engl J Med. 2012;366:19982007. doi: 10.1056/NEJMoa1110855.
- Bearak J, Popinchalk A, Ganatra B, et al. Unintended pregnancy and abortion by income, region, and the legal status of abortion: estimates from a comprehensive model for 1990-2019. Lancet Glob Health. 2020;8:e1152-e1161. doi: 10.1016/S2214-109X(20)30315-6.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice. Committee Opinion No. 670: Immediate postpartum long-acting reversible contraception. Obstet Gynecol. 2016;128:e32-e37. doi: 10.1097/AOG.0000000000001587.
- Averbach SH, Ermias Y, Jeng G, et al. Expulsion of intrauterine devices after postpartum placement by timing of placement, delivery type, and intrauterine device type: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;223:177188. doi: 10.1016/j.ajog.2020.02.045.
- Connolly A, Thorp J, Pahel L. Effects of pregnancy and childbirth on postpartum sexual function: a longitudinal prospective study. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:263-267. doi: 10.1007/s00192-005-1293-6.
- Conde-Agudelo A, Rosas-Bermúdez A, Kafury-Goeta AC. Birth spacing and risk of adverse perinatal outcomes: a meta-analysis. JAMA. 2006;295:1809-1823. doi: 10.1001 /jama.295.15.1809.
- Vricella LK, Gawron LM, Louis JM. Society for MaternalFetal Medicine (SMFM) Consult Series #48: Immediate postpartum long-acting reversible contraception for women at high risk for medical complications. Am J Obstet Gynecol. 2019;220:B2-B12. doi: 10.1016/j.ajog.2019.02.011.
- Chen BA, Reeves MF, Hayes JL, et al. Postplacental or delayed insertion of the levonorgestrel intrauterine device after vaginal delivery: a randomized controlled trial. Obstet Gynecol. 2010;116:1079-1087. doi: 10.1097/AOG.0b013e3181f73fac.
- Whitaker AK, Endres LK, Mistretta SQ, et al. Postplacental insertion of the levonorgestrel intrauterine device after cesarean delivery vs. delayed insertion: a randomized controlled trial. Contraception. 2014;89:534-539. doi: 10.1016/j.contraception.2013.12.007.
- Lester F, Kakaire O, Byamugisha J, et al. Intracesarean insertion of the Copper T380A versus 6 weeks postcesarean: a randomized clinical trial. Contraception. 2015;91:198-203. doi: 10.1016/j.contraception.2014.12.002.
- Levi EE, Stuart GS, Zerden ML, et al. Intrauterine device placement during cesarean delivery and continued use 6 months postpartum: a randomized controlled trial. Obstet Gynecol. 2015;126:5-11. doi: 10.1097/AOG.0000000000000882.
- Sothornwit J, Kaewrudee S, Lumbiganon P, et al. Immediate versus delayed postpartum insertion of contraceptive implant and IUD for contraception. Cochrane Database Syst Rev. 2022;10:CD011913. doi: 10.1002/14651858.CD011913.pub3.
- Cohen R, Sheeder J, Arango N, et al. Twelve-month contraceptive continuation and repeat pregnancy among young mothers choosing postdelivery contraceptive implants or postplacental intrauterine devices. Contraception. 2016;93:178-183. doi: 10.1016/j.contraception.2015.10.001.
- Centers for Disease Control and Prevention (CDC). US Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep. 2010;59(RR-4):1-86.
- Kapp N, Curtis K, Nanda K. Progestogen-only contraceptive use among breastfeeding women: a systematic review. Contraception. 2010;82:17-37. doi: 10.1016 /j.contraception.2010.02.002.
- Levi EE, Findley MK, Avila K, et al. Placement of levonorgestrel intrauterine device at the time of cesarean delivery and the effect on breastfeeding duration. Breastfeed Med. 2018;13:674679. doi: 10.1089/bfm.2018.0060.
- Chen BA, Reeves MF, Creinin MD, et al. Postplacental or delayed levonorgestrel intrauterine device insertion and breast-feeding duration. Contraception. 2011;84:499-504. doi: 10.1016/j.contraception.2011.01.022.
- Tocce KM, Sheeder JL, Teal SB. Rapid repeat pregnancy in adolescents: do immediate postpartum contraceptive implants make a difference? Am J Obstet Gynecol. 2012;206:481.e1-7. doi: 10.1016/j.ajog.2012.04.015.
- Carr SL, Singh RH, Sussman AL, et al. Women’s experiences with immediate postpartum intrauterine device insertion: a mixed-methods study. Contraception. 2018;97:219-226. doi: 10.1016/j.contraception.2017.10.008.
- Martinez OP, Wilder L, Seal P. Ultrasound-guided compared with non-ultrasound-Guided placement of immediate postpartum intrauterine contraceptive devices. Obstet Gynecol. 2022;140:91-93. doi: 10.1097/AOG.0000000000004828.
- Holden EC, Lai E, Morelli SS, et al. Ongoing barriers to immediate postpartum long-acting reversible contraception: a physician survey. Contracept Reprod Med. 2018;3:23. doi: 10.1186/s40834-018-0078-5.
- Benfield N, Hawkins F, Ray L, et al. Exposure to routine availability of immediate postpartum LARC: effect on attitudes and practices of labor and delivery and postpartum nurses. Contraception. 2018;97:411-414. doi: 10.1016 /j.contraception.2018.01.017.
- Steenland MW, Vatsa R, Pace LE, et al. Immediate postpartum long-acting reversible contraceptive use following statespecific changes in hospital Medicaid reimbursement. JAMA Netw Open. 2022;5:e2237918. doi: 10.1001 /jamanetworkopen.2022.37918.
- Washington CI, Jamshidi R, Thung SF, et al. Timing of postpartum intrauterine device placement: a costeffectiveness analysis. Fertil Steril. 2015;103:131-137. doi: 10.1016/j.fertnstert.2014.09.032