User login
Gene associated with vision loss also linked to COVID: Study
age-related macular degeneration.
The findings show that COVID and AMD were associated with variations in what is called the PDGFB gene, which has a role in new blood vessel formation and is linked to abnormal blood vessel changes that occur in AMD. The study was published in the Journal of Clinical Medicine. The analysis included genetic data from more than 16,000 people with AMD, more than 50,000 people with COVID, plus control groups.
Age-related macular degeneration is a vision problem that occurs when a part of the retina – the macula – is damaged, according to the American Academy of Ophthalmology. The result is that central vision is lost, but peripheral vision remains normal, so it is difficult to see fine details. For example, a person with AMD can see a clock’s numbers but not its hands.
“Our analysis lends credence to previously reported clinical studies that found those with AMD have a higher risk for COVID-19 infection and severe disease, and that this increased risk may have a genetic basis,” Boston University researcher Lindsay Farrer, PhD, chief of biomedical genetics, explained in a news release.
Previous research has shown that people with AMD have a 25% increased risk of respiratory failure or death due to COVID, which is higher than other well-known risk factors such as type 2 diabetes (21%) or obesity (13%), according to the news release.
A version of this article first appeared on WebMD.com.
age-related macular degeneration.
The findings show that COVID and AMD were associated with variations in what is called the PDGFB gene, which has a role in new blood vessel formation and is linked to abnormal blood vessel changes that occur in AMD. The study was published in the Journal of Clinical Medicine. The analysis included genetic data from more than 16,000 people with AMD, more than 50,000 people with COVID, plus control groups.
Age-related macular degeneration is a vision problem that occurs when a part of the retina – the macula – is damaged, according to the American Academy of Ophthalmology. The result is that central vision is lost, but peripheral vision remains normal, so it is difficult to see fine details. For example, a person with AMD can see a clock’s numbers but not its hands.
“Our analysis lends credence to previously reported clinical studies that found those with AMD have a higher risk for COVID-19 infection and severe disease, and that this increased risk may have a genetic basis,” Boston University researcher Lindsay Farrer, PhD, chief of biomedical genetics, explained in a news release.
Previous research has shown that people with AMD have a 25% increased risk of respiratory failure or death due to COVID, which is higher than other well-known risk factors such as type 2 diabetes (21%) or obesity (13%), according to the news release.
A version of this article first appeared on WebMD.com.
age-related macular degeneration.
The findings show that COVID and AMD were associated with variations in what is called the PDGFB gene, which has a role in new blood vessel formation and is linked to abnormal blood vessel changes that occur in AMD. The study was published in the Journal of Clinical Medicine. The analysis included genetic data from more than 16,000 people with AMD, more than 50,000 people with COVID, plus control groups.
Age-related macular degeneration is a vision problem that occurs when a part of the retina – the macula – is damaged, according to the American Academy of Ophthalmology. The result is that central vision is lost, but peripheral vision remains normal, so it is difficult to see fine details. For example, a person with AMD can see a clock’s numbers but not its hands.
“Our analysis lends credence to previously reported clinical studies that found those with AMD have a higher risk for COVID-19 infection and severe disease, and that this increased risk may have a genetic basis,” Boston University researcher Lindsay Farrer, PhD, chief of biomedical genetics, explained in a news release.
Previous research has shown that people with AMD have a 25% increased risk of respiratory failure or death due to COVID, which is higher than other well-known risk factors such as type 2 diabetes (21%) or obesity (13%), according to the news release.
A version of this article first appeared on WebMD.com.
FROM THE JOURNAL OF CLINICAL MEDICINE
Study of beliefs about what causes cancer sparks debate
The study, entitled, “Everything Causes Cancer? Beliefs and Attitudes Towards Cancer Prevention Among Anti-Vaxxers, Flat Earthers, and Reptilian Conspiracists: Online Cross Sectional Survey,” was published in the Christmas 2022 issue of The British Medical Journal (BMJ).
The authors explain that they set out to evaluate “the patterns of beliefs about cancer among people who believed in conspiracies, rejected the COVID-19 vaccine, or preferred alternative medicine.”
They sought such people on social media and online chat platforms and asked them questions about real and mythical causes of cancer.
Almost half of survey participants agreed with the statement, “It seems like everything causes cancer.”
Overall, among all participants, awareness of the actual causes of cancer was greater than awareness of the mythical causes of cancer, the authors report. However, awareness of the actual causes of cancer was lower among the unvaccinated and members of conspiracy groups than among their counterparts.
The authors are concerned that their findings suggest “a direct connection between digital misinformation and consequent potential erroneous health decisions, which may represent a further preventable fraction of cancer.”
Backlash and criticism
The study “highlights the difficulty society encounters in distinguishing the actual causes of cancer from mythical causes,” The BMJ commented on Twitter.
However, both the study and the journal received some backlash.
This is a “horrible article seeking to smear people with concerns about COVID vaccines,” commented Clare Craig, a British consultant pathologist who specializes in cancer diagnostics.
The study and its methodology were also harshly criticized on Twitter by Normal Fenton, professor of risk information management at the Queen Mary University of London.
The senior author of the study, Laura Costas, a medical epidemiologist with the Catalan Institute of Oncology, Barcelona, told this news organization that the naysayers on social media, many of whom focused their comments on the COVID-19 vaccine, prove the purpose of the study – that misinformation spreads widely on the internet.
“Most comments focused on spreading COVID-19 myths, which were not the direct subject of the study, and questioned the motivations of BMJ authors and the scientific community, assuming they had a common malevolent hidden agenda,” Ms. Costas said.
“They stated the need of having critical thinking, a trait in common with the scientific method, but dogmatically dismissed any information that comes from official sources,” she added.
Ms. Costas commented that “society encounters difficulty in differentiating actual from mythical causes of cancer owing to mass information. We therefore planned this study with a certain satire, which is in line with the essence of The BMJ Christmas issue.”
The BMJ has a long history of publishing a lighthearted Christmas edition full of original, satirical, and nontraditional studies. Previous years have seen studies that explored potential harms from holly and ivy, survival time of chocolates on hospital wards, and the question, “Were James Bond’s drinks shaken because of alcohol induced tremor?”
Study details
Ms. Costas and colleagues sought participants for their survey from online forums that included 4chan and Reddit, which are known for their controversial content posted by anonymous users. Data were also collected from ForoCoches and HispaChan, well-known Spanish online forums. These online sites were intentionally chosen because researchers thought “conspiracy beliefs would be more prevalent,” according to Ms. Costas.
Across the multiple forums, there were 1,494 participants. Of these, 209 participants were unvaccinated against COVID-19, 112 preferred alternatives rather than conventional medicine, and 62 reported that they believed the earth was flat or believed that humanoids take reptilian forms to manipulate human societies.
The team then sought to assess beliefs about actual and mythical (nonestablished) causes of cancer by presenting the participants with the closed risk factor questions on two validated scales – the Cancer Awareness Measure (CAM) and CAM–Mythical Causes Scale (CAM-MYCS).
Responses to both were recorded on a five-point scale; answers ranged from “strongly disagree” to “strongly agree.”
The CAM assesses cancer risk perceptions of 11 established risk factors for cancer: smoking actively or passively, consuming alcohol, low levels of physical activity, consuming red or processed meat, getting sunburnt as a child, family history of cancer, human papillomavirus infection, being overweight, age greater than or equal to 70 years, and low vegetable and fruit consumption.
The CAM-MYCS measure includes 12 questions on risk perceptions of mythical causes of cancer – nonestablished causes that are commonly believed to cause cancer but for which there is no supporting scientific evidence, the authors explain. These items include drinking from plastic bottles; eating food containing artificial sweeteners or additives and genetically modified food; using microwave ovens, aerosol containers, mobile phones, and cleaning products; living near power lines; feeling stressed; experiencing physical trauma; and being exposed to electromagnetic frequencies/non-ionizing radiation, such as wi-fi networks, radio, and television.
The most endorsed mythical causes of cancer were eating food containing additives (63.9%) or sweeteners (50.7%), feeling stressed (59.7%), and eating genetically modified foods (38.4%).
A version of this article first appeared on Medscape.com.
The study, entitled, “Everything Causes Cancer? Beliefs and Attitudes Towards Cancer Prevention Among Anti-Vaxxers, Flat Earthers, and Reptilian Conspiracists: Online Cross Sectional Survey,” was published in the Christmas 2022 issue of The British Medical Journal (BMJ).
The authors explain that they set out to evaluate “the patterns of beliefs about cancer among people who believed in conspiracies, rejected the COVID-19 vaccine, or preferred alternative medicine.”
They sought such people on social media and online chat platforms and asked them questions about real and mythical causes of cancer.
Almost half of survey participants agreed with the statement, “It seems like everything causes cancer.”
Overall, among all participants, awareness of the actual causes of cancer was greater than awareness of the mythical causes of cancer, the authors report. However, awareness of the actual causes of cancer was lower among the unvaccinated and members of conspiracy groups than among their counterparts.
The authors are concerned that their findings suggest “a direct connection between digital misinformation and consequent potential erroneous health decisions, which may represent a further preventable fraction of cancer.”
Backlash and criticism
The study “highlights the difficulty society encounters in distinguishing the actual causes of cancer from mythical causes,” The BMJ commented on Twitter.
However, both the study and the journal received some backlash.
This is a “horrible article seeking to smear people with concerns about COVID vaccines,” commented Clare Craig, a British consultant pathologist who specializes in cancer diagnostics.
The study and its methodology were also harshly criticized on Twitter by Normal Fenton, professor of risk information management at the Queen Mary University of London.
The senior author of the study, Laura Costas, a medical epidemiologist with the Catalan Institute of Oncology, Barcelona, told this news organization that the naysayers on social media, many of whom focused their comments on the COVID-19 vaccine, prove the purpose of the study – that misinformation spreads widely on the internet.
“Most comments focused on spreading COVID-19 myths, which were not the direct subject of the study, and questioned the motivations of BMJ authors and the scientific community, assuming they had a common malevolent hidden agenda,” Ms. Costas said.
“They stated the need of having critical thinking, a trait in common with the scientific method, but dogmatically dismissed any information that comes from official sources,” she added.
Ms. Costas commented that “society encounters difficulty in differentiating actual from mythical causes of cancer owing to mass information. We therefore planned this study with a certain satire, which is in line with the essence of The BMJ Christmas issue.”
The BMJ has a long history of publishing a lighthearted Christmas edition full of original, satirical, and nontraditional studies. Previous years have seen studies that explored potential harms from holly and ivy, survival time of chocolates on hospital wards, and the question, “Were James Bond’s drinks shaken because of alcohol induced tremor?”
Study details
Ms. Costas and colleagues sought participants for their survey from online forums that included 4chan and Reddit, which are known for their controversial content posted by anonymous users. Data were also collected from ForoCoches and HispaChan, well-known Spanish online forums. These online sites were intentionally chosen because researchers thought “conspiracy beliefs would be more prevalent,” according to Ms. Costas.
Across the multiple forums, there were 1,494 participants. Of these, 209 participants were unvaccinated against COVID-19, 112 preferred alternatives rather than conventional medicine, and 62 reported that they believed the earth was flat or believed that humanoids take reptilian forms to manipulate human societies.
The team then sought to assess beliefs about actual and mythical (nonestablished) causes of cancer by presenting the participants with the closed risk factor questions on two validated scales – the Cancer Awareness Measure (CAM) and CAM–Mythical Causes Scale (CAM-MYCS).
Responses to both were recorded on a five-point scale; answers ranged from “strongly disagree” to “strongly agree.”
The CAM assesses cancer risk perceptions of 11 established risk factors for cancer: smoking actively or passively, consuming alcohol, low levels of physical activity, consuming red or processed meat, getting sunburnt as a child, family history of cancer, human papillomavirus infection, being overweight, age greater than or equal to 70 years, and low vegetable and fruit consumption.
The CAM-MYCS measure includes 12 questions on risk perceptions of mythical causes of cancer – nonestablished causes that are commonly believed to cause cancer but for which there is no supporting scientific evidence, the authors explain. These items include drinking from plastic bottles; eating food containing artificial sweeteners or additives and genetically modified food; using microwave ovens, aerosol containers, mobile phones, and cleaning products; living near power lines; feeling stressed; experiencing physical trauma; and being exposed to electromagnetic frequencies/non-ionizing radiation, such as wi-fi networks, radio, and television.
The most endorsed mythical causes of cancer were eating food containing additives (63.9%) or sweeteners (50.7%), feeling stressed (59.7%), and eating genetically modified foods (38.4%).
A version of this article first appeared on Medscape.com.
The study, entitled, “Everything Causes Cancer? Beliefs and Attitudes Towards Cancer Prevention Among Anti-Vaxxers, Flat Earthers, and Reptilian Conspiracists: Online Cross Sectional Survey,” was published in the Christmas 2022 issue of The British Medical Journal (BMJ).
The authors explain that they set out to evaluate “the patterns of beliefs about cancer among people who believed in conspiracies, rejected the COVID-19 vaccine, or preferred alternative medicine.”
They sought such people on social media and online chat platforms and asked them questions about real and mythical causes of cancer.
Almost half of survey participants agreed with the statement, “It seems like everything causes cancer.”
Overall, among all participants, awareness of the actual causes of cancer was greater than awareness of the mythical causes of cancer, the authors report. However, awareness of the actual causes of cancer was lower among the unvaccinated and members of conspiracy groups than among their counterparts.
The authors are concerned that their findings suggest “a direct connection between digital misinformation and consequent potential erroneous health decisions, which may represent a further preventable fraction of cancer.”
Backlash and criticism
The study “highlights the difficulty society encounters in distinguishing the actual causes of cancer from mythical causes,” The BMJ commented on Twitter.
However, both the study and the journal received some backlash.
This is a “horrible article seeking to smear people with concerns about COVID vaccines,” commented Clare Craig, a British consultant pathologist who specializes in cancer diagnostics.
The study and its methodology were also harshly criticized on Twitter by Normal Fenton, professor of risk information management at the Queen Mary University of London.
The senior author of the study, Laura Costas, a medical epidemiologist with the Catalan Institute of Oncology, Barcelona, told this news organization that the naysayers on social media, many of whom focused their comments on the COVID-19 vaccine, prove the purpose of the study – that misinformation spreads widely on the internet.
“Most comments focused on spreading COVID-19 myths, which were not the direct subject of the study, and questioned the motivations of BMJ authors and the scientific community, assuming they had a common malevolent hidden agenda,” Ms. Costas said.
“They stated the need of having critical thinking, a trait in common with the scientific method, but dogmatically dismissed any information that comes from official sources,” she added.
Ms. Costas commented that “society encounters difficulty in differentiating actual from mythical causes of cancer owing to mass information. We therefore planned this study with a certain satire, which is in line with the essence of The BMJ Christmas issue.”
The BMJ has a long history of publishing a lighthearted Christmas edition full of original, satirical, and nontraditional studies. Previous years have seen studies that explored potential harms from holly and ivy, survival time of chocolates on hospital wards, and the question, “Were James Bond’s drinks shaken because of alcohol induced tremor?”
Study details
Ms. Costas and colleagues sought participants for their survey from online forums that included 4chan and Reddit, which are known for their controversial content posted by anonymous users. Data were also collected from ForoCoches and HispaChan, well-known Spanish online forums. These online sites were intentionally chosen because researchers thought “conspiracy beliefs would be more prevalent,” according to Ms. Costas.
Across the multiple forums, there were 1,494 participants. Of these, 209 participants were unvaccinated against COVID-19, 112 preferred alternatives rather than conventional medicine, and 62 reported that they believed the earth was flat or believed that humanoids take reptilian forms to manipulate human societies.
The team then sought to assess beliefs about actual and mythical (nonestablished) causes of cancer by presenting the participants with the closed risk factor questions on two validated scales – the Cancer Awareness Measure (CAM) and CAM–Mythical Causes Scale (CAM-MYCS).
Responses to both were recorded on a five-point scale; answers ranged from “strongly disagree” to “strongly agree.”
The CAM assesses cancer risk perceptions of 11 established risk factors for cancer: smoking actively or passively, consuming alcohol, low levels of physical activity, consuming red or processed meat, getting sunburnt as a child, family history of cancer, human papillomavirus infection, being overweight, age greater than or equal to 70 years, and low vegetable and fruit consumption.
The CAM-MYCS measure includes 12 questions on risk perceptions of mythical causes of cancer – nonestablished causes that are commonly believed to cause cancer but for which there is no supporting scientific evidence, the authors explain. These items include drinking from plastic bottles; eating food containing artificial sweeteners or additives and genetically modified food; using microwave ovens, aerosol containers, mobile phones, and cleaning products; living near power lines; feeling stressed; experiencing physical trauma; and being exposed to electromagnetic frequencies/non-ionizing radiation, such as wi-fi networks, radio, and television.
The most endorsed mythical causes of cancer were eating food containing additives (63.9%) or sweeteners (50.7%), feeling stressed (59.7%), and eating genetically modified foods (38.4%).
A version of this article first appeared on Medscape.com.
Cardiac injury caused by COVID-19 less common than thought
The study examined cardiac MRI scans in 31 patients before and after having COVID-19 infection and found no new evidence of myocardial injury in the post-COVID scans relative to the pre-COVID scans.
“To the best of our knowledge this is the first cardiac MRI study to assess myocardial injury pre- and post-COVID-19,” the authors stated.
They say that while this study cannot rule out the possibility of rare events of COVID-19–induced myocardial injury, “the complete absence of de novo late gadolinium enhancement lesions after COVID-19 in this cohort indicates that outside special circumstances, COVID-19–induced myocardial injury may be much less common than suggested by previous studies.”
The study was published online in JACC: Cardiovascular Imaging.
Coauthor Till F. Althoff, MD, Cardiovascular Institute, Clínic–University Hospital Barcelona, said in an interview that previous reports have found a high rate of cardiac lesions in patients undergoing imaging after having had COVID-19 infection.
“In some reports, this has been as high as 80% of patients even though they have not had severe COVID disease. These reports have been interpreted as showing the majority of patients have some COVID-induced cardiac damage, which is an alarming message,” he commented.
However, he pointed out that the patients in these reports did not undergo a cardiac MRI scan before they had COVID-19 so it wasn’t known whether these cardiac lesions were present before infection or not.
To try and gain more accurate information, the current study examined cardiac MRI scans in the same patients before and after they had COVID-19.
The researchers, from an arrhythmia unit, made use of the fact that all their patients have cardiac MRI data, so they used their large registry of patients in whom cardiac MRI had been performed, and cross referenced this to a health care database to identify those patients who had confirmed COVID-19 after they obtaining a cardiac scan at the arrhythmia unit. They then conducted another cardiac MRI scan in the 31 patients identified a median of 5 months after their COVID-19 infection.
“These 31 patients had a cardiac MRI scan pre-COVID and post COVID using exactly the same scanner with identical sequences, so the scans were absolutely comparable,” Dr. Althoff noted.
Of these 31 patients, 7 had been hospitalized at the time of acute presentation with COVID-19, of whom 2 required intensive care. Most patients (29) had been symptomatic, but none reported cardiac symptoms.
Results showed that, on the post–COVID-19 scan, late gadolinium enhancement lesions indicative of residual myocardial injury were encountered in 15 of the 31 patients (48%), which the researchers said is in line with previous reports.
However, intraindividual comparison with the pre–COVID-19 cardiac MRI scans showed all these lesions were preexisting with identical localization, pattern, and transmural distribution, and thus not COVID-19 related.
Quantitative analyses, performed independently, detected no increase in the size of individual lesions nor in the global left ventricular late gadolinium enhancement extent.
Comparison of pre- and post COVID-19 imaging sequences did not show any differences in ventricular functional or structural parameters.
“While this study only has 31 patients, the fact that we are conducting intra-individual comparisons, which rules out bias, means that we don’t need a large number of patients for reliable results,” Dr. Althoff said.
“These types of lesions are normal to see. We know that individuals without cardiac disease have these types of lesions, and they are not necessarily an indication of any specific pathology. I was kind of surprised by the interpretation of previous data, which is why we did the current study,” he added.
Dr. Althoff acknowledged that some cardiac injury may have been seen if much larger numbers of patients had been included. “But I think we can say from this data that COVID-induced cardiac damage is much less of an issue than we may have previously thought,” he added.
He also noted that most of the patients in this study had mild COVID-19, so the results cannot be extrapolated to severe COVID-19 infection.
However, Dr. Althoff pointed out that all the patients already had atrial fibrillation, so would have been at higher risk of cardiac injury from COVID-19.
“These patients had preexisting cardiac risk factors, and thus they would have been more susceptible to both a more severe course of COVID and an increased risk of myocardial damage due to COVID. The fact that we don’t find any myocardial injury due to COVID in this group is even more reassuring. The general population will be at even lower risk,” he commented.
“I think we can say that, in COVID patients who do not have any cardiac symptoms, our study suggests that the incidence of cardiac injury is very low,” Dr. Althoff said.
“Even in patients with severe COVID and myocardial involvement reflected by increased troponin levels, I wouldn’t be sure that they have any residual cardiac injury. While it has been reported that cardiac lesions have been found in such patients, pre-COVID MRI scans were not available so we don’t know if they were there before,” he added.
“We do not know the true incidence of cardiac injury after COVID, but I think we can say from this data that it is definitely not anywhere near the 40%-50% or even greater that some of the previous reports have suggested,” he stated.
Dr. Althoff suggested that, based on these data, some of the recommendations based on previous reports such the need for follow-up cardiac scans and caution about partaking in sports again after COVID-19 infection, are probably not necessary.
“Our data suggest that these concerns are unfounded, and we need to step back a bit and stop alarming patients about the risk of cardiac damage after COVID,” he said. “Yes, if patients have cardiac symptoms during or after COVID infection they should get checked out, but I do not think we need to do a cardiac risk assessment in patients without cardiac symptoms in COVID.”
This work is supported in part by grants from Instituto de Salud Carlos III, the Spanish government, Madrid, and Fundació la Marató de TV3 in Catalonia. Dr. Althoff has received research grants for investigator-initiated trials from Biosense Webster.
A version of this article first appeared on Medscape.com.
The study examined cardiac MRI scans in 31 patients before and after having COVID-19 infection and found no new evidence of myocardial injury in the post-COVID scans relative to the pre-COVID scans.
“To the best of our knowledge this is the first cardiac MRI study to assess myocardial injury pre- and post-COVID-19,” the authors stated.
They say that while this study cannot rule out the possibility of rare events of COVID-19–induced myocardial injury, “the complete absence of de novo late gadolinium enhancement lesions after COVID-19 in this cohort indicates that outside special circumstances, COVID-19–induced myocardial injury may be much less common than suggested by previous studies.”
The study was published online in JACC: Cardiovascular Imaging.
Coauthor Till F. Althoff, MD, Cardiovascular Institute, Clínic–University Hospital Barcelona, said in an interview that previous reports have found a high rate of cardiac lesions in patients undergoing imaging after having had COVID-19 infection.
“In some reports, this has been as high as 80% of patients even though they have not had severe COVID disease. These reports have been interpreted as showing the majority of patients have some COVID-induced cardiac damage, which is an alarming message,” he commented.
However, he pointed out that the patients in these reports did not undergo a cardiac MRI scan before they had COVID-19 so it wasn’t known whether these cardiac lesions were present before infection or not.
To try and gain more accurate information, the current study examined cardiac MRI scans in the same patients before and after they had COVID-19.
The researchers, from an arrhythmia unit, made use of the fact that all their patients have cardiac MRI data, so they used their large registry of patients in whom cardiac MRI had been performed, and cross referenced this to a health care database to identify those patients who had confirmed COVID-19 after they obtaining a cardiac scan at the arrhythmia unit. They then conducted another cardiac MRI scan in the 31 patients identified a median of 5 months after their COVID-19 infection.
“These 31 patients had a cardiac MRI scan pre-COVID and post COVID using exactly the same scanner with identical sequences, so the scans were absolutely comparable,” Dr. Althoff noted.
Of these 31 patients, 7 had been hospitalized at the time of acute presentation with COVID-19, of whom 2 required intensive care. Most patients (29) had been symptomatic, but none reported cardiac symptoms.
Results showed that, on the post–COVID-19 scan, late gadolinium enhancement lesions indicative of residual myocardial injury were encountered in 15 of the 31 patients (48%), which the researchers said is in line with previous reports.
However, intraindividual comparison with the pre–COVID-19 cardiac MRI scans showed all these lesions were preexisting with identical localization, pattern, and transmural distribution, and thus not COVID-19 related.
Quantitative analyses, performed independently, detected no increase in the size of individual lesions nor in the global left ventricular late gadolinium enhancement extent.
Comparison of pre- and post COVID-19 imaging sequences did not show any differences in ventricular functional or structural parameters.
“While this study only has 31 patients, the fact that we are conducting intra-individual comparisons, which rules out bias, means that we don’t need a large number of patients for reliable results,” Dr. Althoff said.
“These types of lesions are normal to see. We know that individuals without cardiac disease have these types of lesions, and they are not necessarily an indication of any specific pathology. I was kind of surprised by the interpretation of previous data, which is why we did the current study,” he added.
Dr. Althoff acknowledged that some cardiac injury may have been seen if much larger numbers of patients had been included. “But I think we can say from this data that COVID-induced cardiac damage is much less of an issue than we may have previously thought,” he added.
He also noted that most of the patients in this study had mild COVID-19, so the results cannot be extrapolated to severe COVID-19 infection.
However, Dr. Althoff pointed out that all the patients already had atrial fibrillation, so would have been at higher risk of cardiac injury from COVID-19.
“These patients had preexisting cardiac risk factors, and thus they would have been more susceptible to both a more severe course of COVID and an increased risk of myocardial damage due to COVID. The fact that we don’t find any myocardial injury due to COVID in this group is even more reassuring. The general population will be at even lower risk,” he commented.
“I think we can say that, in COVID patients who do not have any cardiac symptoms, our study suggests that the incidence of cardiac injury is very low,” Dr. Althoff said.
“Even in patients with severe COVID and myocardial involvement reflected by increased troponin levels, I wouldn’t be sure that they have any residual cardiac injury. While it has been reported that cardiac lesions have been found in such patients, pre-COVID MRI scans were not available so we don’t know if they were there before,” he added.
“We do not know the true incidence of cardiac injury after COVID, but I think we can say from this data that it is definitely not anywhere near the 40%-50% or even greater that some of the previous reports have suggested,” he stated.
Dr. Althoff suggested that, based on these data, some of the recommendations based on previous reports such the need for follow-up cardiac scans and caution about partaking in sports again after COVID-19 infection, are probably not necessary.
“Our data suggest that these concerns are unfounded, and we need to step back a bit and stop alarming patients about the risk of cardiac damage after COVID,” he said. “Yes, if patients have cardiac symptoms during or after COVID infection they should get checked out, but I do not think we need to do a cardiac risk assessment in patients without cardiac symptoms in COVID.”
This work is supported in part by grants from Instituto de Salud Carlos III, the Spanish government, Madrid, and Fundació la Marató de TV3 in Catalonia. Dr. Althoff has received research grants for investigator-initiated trials from Biosense Webster.
A version of this article first appeared on Medscape.com.
The study examined cardiac MRI scans in 31 patients before and after having COVID-19 infection and found no new evidence of myocardial injury in the post-COVID scans relative to the pre-COVID scans.
“To the best of our knowledge this is the first cardiac MRI study to assess myocardial injury pre- and post-COVID-19,” the authors stated.
They say that while this study cannot rule out the possibility of rare events of COVID-19–induced myocardial injury, “the complete absence of de novo late gadolinium enhancement lesions after COVID-19 in this cohort indicates that outside special circumstances, COVID-19–induced myocardial injury may be much less common than suggested by previous studies.”
The study was published online in JACC: Cardiovascular Imaging.
Coauthor Till F. Althoff, MD, Cardiovascular Institute, Clínic–University Hospital Barcelona, said in an interview that previous reports have found a high rate of cardiac lesions in patients undergoing imaging after having had COVID-19 infection.
“In some reports, this has been as high as 80% of patients even though they have not had severe COVID disease. These reports have been interpreted as showing the majority of patients have some COVID-induced cardiac damage, which is an alarming message,” he commented.
However, he pointed out that the patients in these reports did not undergo a cardiac MRI scan before they had COVID-19 so it wasn’t known whether these cardiac lesions were present before infection or not.
To try and gain more accurate information, the current study examined cardiac MRI scans in the same patients before and after they had COVID-19.
The researchers, from an arrhythmia unit, made use of the fact that all their patients have cardiac MRI data, so they used their large registry of patients in whom cardiac MRI had been performed, and cross referenced this to a health care database to identify those patients who had confirmed COVID-19 after they obtaining a cardiac scan at the arrhythmia unit. They then conducted another cardiac MRI scan in the 31 patients identified a median of 5 months after their COVID-19 infection.
“These 31 patients had a cardiac MRI scan pre-COVID and post COVID using exactly the same scanner with identical sequences, so the scans were absolutely comparable,” Dr. Althoff noted.
Of these 31 patients, 7 had been hospitalized at the time of acute presentation with COVID-19, of whom 2 required intensive care. Most patients (29) had been symptomatic, but none reported cardiac symptoms.
Results showed that, on the post–COVID-19 scan, late gadolinium enhancement lesions indicative of residual myocardial injury were encountered in 15 of the 31 patients (48%), which the researchers said is in line with previous reports.
However, intraindividual comparison with the pre–COVID-19 cardiac MRI scans showed all these lesions were preexisting with identical localization, pattern, and transmural distribution, and thus not COVID-19 related.
Quantitative analyses, performed independently, detected no increase in the size of individual lesions nor in the global left ventricular late gadolinium enhancement extent.
Comparison of pre- and post COVID-19 imaging sequences did not show any differences in ventricular functional or structural parameters.
“While this study only has 31 patients, the fact that we are conducting intra-individual comparisons, which rules out bias, means that we don’t need a large number of patients for reliable results,” Dr. Althoff said.
“These types of lesions are normal to see. We know that individuals without cardiac disease have these types of lesions, and they are not necessarily an indication of any specific pathology. I was kind of surprised by the interpretation of previous data, which is why we did the current study,” he added.
Dr. Althoff acknowledged that some cardiac injury may have been seen if much larger numbers of patients had been included. “But I think we can say from this data that COVID-induced cardiac damage is much less of an issue than we may have previously thought,” he added.
He also noted that most of the patients in this study had mild COVID-19, so the results cannot be extrapolated to severe COVID-19 infection.
However, Dr. Althoff pointed out that all the patients already had atrial fibrillation, so would have been at higher risk of cardiac injury from COVID-19.
“These patients had preexisting cardiac risk factors, and thus they would have been more susceptible to both a more severe course of COVID and an increased risk of myocardial damage due to COVID. The fact that we don’t find any myocardial injury due to COVID in this group is even more reassuring. The general population will be at even lower risk,” he commented.
“I think we can say that, in COVID patients who do not have any cardiac symptoms, our study suggests that the incidence of cardiac injury is very low,” Dr. Althoff said.
“Even in patients with severe COVID and myocardial involvement reflected by increased troponin levels, I wouldn’t be sure that they have any residual cardiac injury. While it has been reported that cardiac lesions have been found in such patients, pre-COVID MRI scans were not available so we don’t know if they were there before,” he added.
“We do not know the true incidence of cardiac injury after COVID, but I think we can say from this data that it is definitely not anywhere near the 40%-50% or even greater that some of the previous reports have suggested,” he stated.
Dr. Althoff suggested that, based on these data, some of the recommendations based on previous reports such the need for follow-up cardiac scans and caution about partaking in sports again after COVID-19 infection, are probably not necessary.
“Our data suggest that these concerns are unfounded, and we need to step back a bit and stop alarming patients about the risk of cardiac damage after COVID,” he said. “Yes, if patients have cardiac symptoms during or after COVID infection they should get checked out, but I do not think we need to do a cardiac risk assessment in patients without cardiac symptoms in COVID.”
This work is supported in part by grants from Instituto de Salud Carlos III, the Spanish government, Madrid, and Fundació la Marató de TV3 in Catalonia. Dr. Althoff has received research grants for investigator-initiated trials from Biosense Webster.
A version of this article first appeared on Medscape.com.
FROM JACC: CARDIOVASCULAR IMAGING
COVID booster shot poll: People ‘don’t think they need one’
Now, a new poll shows why so few people are willing to roll up their sleeves again.
The most common reasons people give for not getting the latest booster shot is that they “don’t think they need one” (44%) and they “don’t think the benefits are worth it” (37%), according to poll results from the Kaiser Family Foundation.
The data comes amid announcements by the Centers for Disease Control and Prevention that boosters reduced COVID-19 hospitalizations by up to 57% for U.S. adults and by up to 84% for people age 65 and older. Those figures are just the latest in a mountain of research reporting the public health benefits of COVID-19 vaccines.
Despite all of the statistical data, health officials’ recent vaccination campaigns have proven far from compelling.
So far, just 15% of people age 12 and older have gotten the latest booster, and 36% of people age 65 and older have gotten it, the CDC’s vaccination trackershows.
Since the start of the pandemic, 1.1 million people in the U.S. have died from COVID-19, with the number of deaths currently rising by 400 per day, The New York Times COVID tracker shows.
Many experts continue to note the need for everyone to get booster shots regularly, but some advocate that perhaps a change in strategy is in order.
“What the administration should do is push for vaccinating people in high-risk groups, including those who are older, those who are immunocompromised and those who have comorbidities,” Paul Offitt, MD, director of the Vaccine Education Center at Children’s Hospital of Philadelphia, told CNN.
Federal regulators have announced they will meet Jan. 26 with a panel of vaccine advisors to examine the current recommended vaccination schedule as well as look at the effectiveness and composition of current vaccines and boosters, with an eye toward the make-up of next-generation shots.
Vaccines are the “best available protection” against hospitalization and death caused by COVID-19, said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, in a statement announcing the planned meeting.
“Since the initial authorizations of these vaccines, we have learned that protection wanes over time, especially as the virus rapidly mutates and new variants and subvariants emerge,” he said. “Therefore, it’s important to continue discussions about the optimal composition of COVID-19 vaccines for primary and booster vaccination, as well as the optimal interval for booster vaccination.”
A version of this article first appeared on WebMD.com.
Now, a new poll shows why so few people are willing to roll up their sleeves again.
The most common reasons people give for not getting the latest booster shot is that they “don’t think they need one” (44%) and they “don’t think the benefits are worth it” (37%), according to poll results from the Kaiser Family Foundation.
The data comes amid announcements by the Centers for Disease Control and Prevention that boosters reduced COVID-19 hospitalizations by up to 57% for U.S. adults and by up to 84% for people age 65 and older. Those figures are just the latest in a mountain of research reporting the public health benefits of COVID-19 vaccines.
Despite all of the statistical data, health officials’ recent vaccination campaigns have proven far from compelling.
So far, just 15% of people age 12 and older have gotten the latest booster, and 36% of people age 65 and older have gotten it, the CDC’s vaccination trackershows.
Since the start of the pandemic, 1.1 million people in the U.S. have died from COVID-19, with the number of deaths currently rising by 400 per day, The New York Times COVID tracker shows.
Many experts continue to note the need for everyone to get booster shots regularly, but some advocate that perhaps a change in strategy is in order.
“What the administration should do is push for vaccinating people in high-risk groups, including those who are older, those who are immunocompromised and those who have comorbidities,” Paul Offitt, MD, director of the Vaccine Education Center at Children’s Hospital of Philadelphia, told CNN.
Federal regulators have announced they will meet Jan. 26 with a panel of vaccine advisors to examine the current recommended vaccination schedule as well as look at the effectiveness and composition of current vaccines and boosters, with an eye toward the make-up of next-generation shots.
Vaccines are the “best available protection” against hospitalization and death caused by COVID-19, said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, in a statement announcing the planned meeting.
“Since the initial authorizations of these vaccines, we have learned that protection wanes over time, especially as the virus rapidly mutates and new variants and subvariants emerge,” he said. “Therefore, it’s important to continue discussions about the optimal composition of COVID-19 vaccines for primary and booster vaccination, as well as the optimal interval for booster vaccination.”
A version of this article first appeared on WebMD.com.
Now, a new poll shows why so few people are willing to roll up their sleeves again.
The most common reasons people give for not getting the latest booster shot is that they “don’t think they need one” (44%) and they “don’t think the benefits are worth it” (37%), according to poll results from the Kaiser Family Foundation.
The data comes amid announcements by the Centers for Disease Control and Prevention that boosters reduced COVID-19 hospitalizations by up to 57% for U.S. adults and by up to 84% for people age 65 and older. Those figures are just the latest in a mountain of research reporting the public health benefits of COVID-19 vaccines.
Despite all of the statistical data, health officials’ recent vaccination campaigns have proven far from compelling.
So far, just 15% of people age 12 and older have gotten the latest booster, and 36% of people age 65 and older have gotten it, the CDC’s vaccination trackershows.
Since the start of the pandemic, 1.1 million people in the U.S. have died from COVID-19, with the number of deaths currently rising by 400 per day, The New York Times COVID tracker shows.
Many experts continue to note the need for everyone to get booster shots regularly, but some advocate that perhaps a change in strategy is in order.
“What the administration should do is push for vaccinating people in high-risk groups, including those who are older, those who are immunocompromised and those who have comorbidities,” Paul Offitt, MD, director of the Vaccine Education Center at Children’s Hospital of Philadelphia, told CNN.
Federal regulators have announced they will meet Jan. 26 with a panel of vaccine advisors to examine the current recommended vaccination schedule as well as look at the effectiveness and composition of current vaccines and boosters, with an eye toward the make-up of next-generation shots.
Vaccines are the “best available protection” against hospitalization and death caused by COVID-19, said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, in a statement announcing the planned meeting.
“Since the initial authorizations of these vaccines, we have learned that protection wanes over time, especially as the virus rapidly mutates and new variants and subvariants emerge,” he said. “Therefore, it’s important to continue discussions about the optimal composition of COVID-19 vaccines for primary and booster vaccination, as well as the optimal interval for booster vaccination.”
A version of this article first appeared on WebMD.com.
Multiple myeloma diagnosed more via emergency care during COVID
The study covered in this summary was published on Research Square as a preprint and has not yet been peer reviewed.
Key takeaway
Why this matters
While trying to avoid COVID-19 infection, patients ultimately diagnosed with multiple myeloma may have delayed interactions with healthcare professionals and consequently delayed their cancer diagnosis.
Study design
Researchers collected data on newly diagnosed patients with multiple myeloma from January 2019 until July 2021 across five institutions (three universities and two hospitals) in England. In total, 323 patients with multiple myeloma were identified.
Patients were divided into two groups: those diagnosed between Jan. 1, 2019, until Jan. 31, 2020, or pre-COVID, and those diagnosed from Feb. 1, 2020, to July 31, 2021, or post COVID.
Key results
Among all patients, 80 (24.8%) were diagnosed with smoldering multiple myeloma and 243 (75.2%) were diagnosed with multiple myeloma requiring treatment.
Significantly more patients in the post-COVID group were diagnosed with myeloma through the emergency route (45.5% post COVID vs. 32.7% pre-COVID; P = .03).
Clinical complications leading to emergency admission prior to a myeloma diagnosis also differed between the two cohorts: Acute kidney injury accounted for most emergency admissions in the pre-COVID cohort while skeletal-related events, including spinal cord compression, were the major causes for diagnosis through the emergency route in the post-COVID cohort.
Patients who were diagnosed with symptomatic myeloma pre-COVID were more likely to be treated with a triplet rather than doublet combination compared with those diagnosed in the post-COVID period (triplet pre-COVID 79.1%, post COVID 63.75%; P = .014).
Overall survival at 1 year was not significantly different between the pre-COVID and post-COVID groups: 88.2% pre-COVID, compared with 87.8% post COVID.
Overall, the authors concluded that the COVID pandemic “resulted in a shift in the symptomatology, disease burden, and routes of diagnosis of patients presenting with myeloma” and “this may have significant consequences” over the long term.
Limitations
The study does not provide a clear time frame of delays in diagnosis.
Disclosures
The study authors did not report any conflicts of interest.
A version of this article first appeared on Medscape.com .
The study covered in this summary was published on Research Square as a preprint and has not yet been peer reviewed.
Key takeaway
Why this matters
While trying to avoid COVID-19 infection, patients ultimately diagnosed with multiple myeloma may have delayed interactions with healthcare professionals and consequently delayed their cancer diagnosis.
Study design
Researchers collected data on newly diagnosed patients with multiple myeloma from January 2019 until July 2021 across five institutions (three universities and two hospitals) in England. In total, 323 patients with multiple myeloma were identified.
Patients were divided into two groups: those diagnosed between Jan. 1, 2019, until Jan. 31, 2020, or pre-COVID, and those diagnosed from Feb. 1, 2020, to July 31, 2021, or post COVID.
Key results
Among all patients, 80 (24.8%) were diagnosed with smoldering multiple myeloma and 243 (75.2%) were diagnosed with multiple myeloma requiring treatment.
Significantly more patients in the post-COVID group were diagnosed with myeloma through the emergency route (45.5% post COVID vs. 32.7% pre-COVID; P = .03).
Clinical complications leading to emergency admission prior to a myeloma diagnosis also differed between the two cohorts: Acute kidney injury accounted for most emergency admissions in the pre-COVID cohort while skeletal-related events, including spinal cord compression, were the major causes for diagnosis through the emergency route in the post-COVID cohort.
Patients who were diagnosed with symptomatic myeloma pre-COVID were more likely to be treated with a triplet rather than doublet combination compared with those diagnosed in the post-COVID period (triplet pre-COVID 79.1%, post COVID 63.75%; P = .014).
Overall survival at 1 year was not significantly different between the pre-COVID and post-COVID groups: 88.2% pre-COVID, compared with 87.8% post COVID.
Overall, the authors concluded that the COVID pandemic “resulted in a shift in the symptomatology, disease burden, and routes of diagnosis of patients presenting with myeloma” and “this may have significant consequences” over the long term.
Limitations
The study does not provide a clear time frame of delays in diagnosis.
Disclosures
The study authors did not report any conflicts of interest.
A version of this article first appeared on Medscape.com .
The study covered in this summary was published on Research Square as a preprint and has not yet been peer reviewed.
Key takeaway
Why this matters
While trying to avoid COVID-19 infection, patients ultimately diagnosed with multiple myeloma may have delayed interactions with healthcare professionals and consequently delayed their cancer diagnosis.
Study design
Researchers collected data on newly diagnosed patients with multiple myeloma from January 2019 until July 2021 across five institutions (three universities and two hospitals) in England. In total, 323 patients with multiple myeloma were identified.
Patients were divided into two groups: those diagnosed between Jan. 1, 2019, until Jan. 31, 2020, or pre-COVID, and those diagnosed from Feb. 1, 2020, to July 31, 2021, or post COVID.
Key results
Among all patients, 80 (24.8%) were diagnosed with smoldering multiple myeloma and 243 (75.2%) were diagnosed with multiple myeloma requiring treatment.
Significantly more patients in the post-COVID group were diagnosed with myeloma through the emergency route (45.5% post COVID vs. 32.7% pre-COVID; P = .03).
Clinical complications leading to emergency admission prior to a myeloma diagnosis also differed between the two cohorts: Acute kidney injury accounted for most emergency admissions in the pre-COVID cohort while skeletal-related events, including spinal cord compression, were the major causes for diagnosis through the emergency route in the post-COVID cohort.
Patients who were diagnosed with symptomatic myeloma pre-COVID were more likely to be treated with a triplet rather than doublet combination compared with those diagnosed in the post-COVID period (triplet pre-COVID 79.1%, post COVID 63.75%; P = .014).
Overall survival at 1 year was not significantly different between the pre-COVID and post-COVID groups: 88.2% pre-COVID, compared with 87.8% post COVID.
Overall, the authors concluded that the COVID pandemic “resulted in a shift in the symptomatology, disease burden, and routes of diagnosis of patients presenting with myeloma” and “this may have significant consequences” over the long term.
Limitations
The study does not provide a clear time frame of delays in diagnosis.
Disclosures
The study authors did not report any conflicts of interest.
A version of this article first appeared on Medscape.com .
Intermittent fasting can lead to type 2 diabetes remission
In a small randomized controlled trial of patients with type 2 diabetes in China, close to half of those who followed a novel intermittent fasting program for 3 months had diabetes remission (A1c less than 6.5% without taking antidiabetic drugs) that persisted for 1 year.
Importantly, “this study was performed under real-life conditions, and the intervention was delivered by trained nurses in primary care rather than by specialized staff at a research institute, making it a more practical and achievable way to manage” type 2 diabetes, the authors report.
Moreover, 65% of the patients in the intervention group who achieved diabetes remission had had diabetes for more than 6 years, which “suggests the possibility of remission for patients with longer duration” of diabetes, they note.
In addition, antidiabetic medication costs decreased by 77%, compared with baseline, in patients in the intermittent-fasting intervention group.
Although intermittent fasting has been studied for weight loss, it had not been investigated for effectiveness for diabetes remission.
These findings suggest that intermittent fasting “could be a paradigm shift in the management goals in diabetes care,” Xiao Yang and colleagues conclude in their study, published online in The Journal of Clinical Endocrinology & Metabolism.
“Type 2 diabetes is not necessarily a permanent, lifelong disease,” senior author Dongbo Liu, PhD, from the Hunan Agricultural University, Changsha, China, added in a press release from The Endocrine Society.
“Diabetes remission is possible if patients lose weight by changing their diet and exercise habits,” Dr. Liu said.
‘Excellent outcome’
Invited to comment, Amy E. Rothberg, MD, PhD, who was not involved with the research, agreed that the study indicates that intermittent fasting works for diabetes remission.
“We know that diabetes remission is possible with calorie restriction and subsequent weight loss, and intermittent fasting is just one of the many [dietary] approaches that may be suitable, appealing, and sustainable to some individuals, and usually results in calorie restriction and therefore weight loss,” she said.
The most studied types of intermittent fasting diets are alternate-day fasting, the 5:2 diet, and time-restricted consumption, Dr. Rothberg told this news organization.
This study presented a novel type of intermittent fasting, she noted. The intervention consisted of 6 cycles (3 months) of 5 fasting days followed by 10 ad libitum days, and then 3 months of follow-up (with no fasting days).
After 3 months of the intervention plus 3 months of follow-up, 47% of the 36 patients in the intervention group achieved diabetes remission (with a mean A1c of 5.66%), compared with only 2.8% of the 36 patients in the control group.
At 12 months, 44% of patients in the intervention group had sustained diabetes remission (with a mean A1c of 6.33%).
This was “an excellent outcome,” said Dr. Rothberg, professor of nutritional sciences, School of Public Health, University of Michigan, Ann Arbor, and a co-author of an international consensus statement that defined diabetes remission.
On average, patients in the intermittent fasting group lost 5.93 kg (13.0 lb) in 3 months, which was sustained over 12 months. “The large amount of weight reduction is key to continuing to achieve diabetes remission,” she noted.
This contrasted with an average weight loss of just 0.27 kg (0.6 lb) in the control group.
Participants who were prescribed fewer antidiabetic medications were more likely to achieve diabetes remission. The researchers acknowledge that the study was not blinded, and they did not record physical activity (although participants were encouraged to maintain their usual physical activity).
This was a small study, Dr. Rothberg acknowledged. The researchers did not specify which specific antidiabetic drugs patients were taking, and they did not determine waist or hip circumference or assess lipids.
The diet was culturally sensitive, appropriate, and feasible in this Chinese population and would not be generalizable to non-Asians.
Nevertheless, a similar approach could be used in any population if the diet is tailored to the individual, according to Dr. Rothberg. Importantly, patients would need to receive guidance from a dietician to make sure their diet comprises all the necessary micronutrients, vitamins, and minerals on fasting days, and they would need to maintain a relatively balanced diet and not gorge themselves on feast days.
“I think we should campaign widely about lifestyle approaches to achieve diabetes remission,” she urged.
72 patients with diabetes for an average of 6.6 years
“Despite a widespread public consensus that [type 2 diabetes] is irreversible and requires drug treatment escalation, there is some evidence of the possibility of remission,” Dr. Yang and colleagues write in their article.
They aimed to evaluate the effectiveness of intermittent fasting for diabetes remission and the durability of diabetes remission at 1 year.
Diabetes remission was defined having a stable A1c less than 6.5% for at least 3 months after discontinuing all antidiabetic medications, confirmed in at least annual A1c measurements (according to a 2021 consensus statement initiated by the American Diabetes Association).
Between 2019 and 2020, the researchers enrolled 72 participants aged 38-72 years who had had type 2 diabetes (duration 1 to 11 years) and a body mass index (BMI) of 19.1-30.4 kg/m2. Patients were randomized 1:1 to the intermittent fasting group or control group.
Baseline characteristics were similar in both groups. Patients were a mean age of 53 years and roughly 60% were men. They had a mean BMI of 24 kg/m2, a mean duration of diabetes of 6.6 years, and a mean A1c of 7.6%, and they were taking an average of 1.8 glucose-lowering medications.
On fasting days, patients in the intervention group received a Chinese Medical Nutrition Therapy kit that provided approximately 840 kcal/day (46% carbohydrates, 46% fat, 8% protein). The kit included a breakfast of a fruit and vegetable gruel, lunch of a solid beverage plus a nutritional rice composite, and dinner of a solid beverage and a meal replacement biscuit, which participants reconstituted by mixing with boiling water. They were allowed to consume noncaloric beverages.
On nonfasting days, patients chose foods ad libitum based on the 2017 Dietary Guidelines for Diabetes in China, which recommend approximately 50%-65% of total energy intake from carbohydrates, 15%-20% from protein, and 20%-30% from fat, and had greater than or equal to 5 g fiber per serving.
Patients in the control group chose foods ad libitum from the dietary guidelines during the entire study.
The study received funding from the National Natural Science Foundation of China. The authors have reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
In a small randomized controlled trial of patients with type 2 diabetes in China, close to half of those who followed a novel intermittent fasting program for 3 months had diabetes remission (A1c less than 6.5% without taking antidiabetic drugs) that persisted for 1 year.
Importantly, “this study was performed under real-life conditions, and the intervention was delivered by trained nurses in primary care rather than by specialized staff at a research institute, making it a more practical and achievable way to manage” type 2 diabetes, the authors report.
Moreover, 65% of the patients in the intervention group who achieved diabetes remission had had diabetes for more than 6 years, which “suggests the possibility of remission for patients with longer duration” of diabetes, they note.
In addition, antidiabetic medication costs decreased by 77%, compared with baseline, in patients in the intermittent-fasting intervention group.
Although intermittent fasting has been studied for weight loss, it had not been investigated for effectiveness for diabetes remission.
These findings suggest that intermittent fasting “could be a paradigm shift in the management goals in diabetes care,” Xiao Yang and colleagues conclude in their study, published online in The Journal of Clinical Endocrinology & Metabolism.
“Type 2 diabetes is not necessarily a permanent, lifelong disease,” senior author Dongbo Liu, PhD, from the Hunan Agricultural University, Changsha, China, added in a press release from The Endocrine Society.
“Diabetes remission is possible if patients lose weight by changing their diet and exercise habits,” Dr. Liu said.
‘Excellent outcome’
Invited to comment, Amy E. Rothberg, MD, PhD, who was not involved with the research, agreed that the study indicates that intermittent fasting works for diabetes remission.
“We know that diabetes remission is possible with calorie restriction and subsequent weight loss, and intermittent fasting is just one of the many [dietary] approaches that may be suitable, appealing, and sustainable to some individuals, and usually results in calorie restriction and therefore weight loss,” she said.
The most studied types of intermittent fasting diets are alternate-day fasting, the 5:2 diet, and time-restricted consumption, Dr. Rothberg told this news organization.
This study presented a novel type of intermittent fasting, she noted. The intervention consisted of 6 cycles (3 months) of 5 fasting days followed by 10 ad libitum days, and then 3 months of follow-up (with no fasting days).
After 3 months of the intervention plus 3 months of follow-up, 47% of the 36 patients in the intervention group achieved diabetes remission (with a mean A1c of 5.66%), compared with only 2.8% of the 36 patients in the control group.
At 12 months, 44% of patients in the intervention group had sustained diabetes remission (with a mean A1c of 6.33%).
This was “an excellent outcome,” said Dr. Rothberg, professor of nutritional sciences, School of Public Health, University of Michigan, Ann Arbor, and a co-author of an international consensus statement that defined diabetes remission.
On average, patients in the intermittent fasting group lost 5.93 kg (13.0 lb) in 3 months, which was sustained over 12 months. “The large amount of weight reduction is key to continuing to achieve diabetes remission,” she noted.
This contrasted with an average weight loss of just 0.27 kg (0.6 lb) in the control group.
Participants who were prescribed fewer antidiabetic medications were more likely to achieve diabetes remission. The researchers acknowledge that the study was not blinded, and they did not record physical activity (although participants were encouraged to maintain their usual physical activity).
This was a small study, Dr. Rothberg acknowledged. The researchers did not specify which specific antidiabetic drugs patients were taking, and they did not determine waist or hip circumference or assess lipids.
The diet was culturally sensitive, appropriate, and feasible in this Chinese population and would not be generalizable to non-Asians.
Nevertheless, a similar approach could be used in any population if the diet is tailored to the individual, according to Dr. Rothberg. Importantly, patients would need to receive guidance from a dietician to make sure their diet comprises all the necessary micronutrients, vitamins, and minerals on fasting days, and they would need to maintain a relatively balanced diet and not gorge themselves on feast days.
“I think we should campaign widely about lifestyle approaches to achieve diabetes remission,” she urged.
72 patients with diabetes for an average of 6.6 years
“Despite a widespread public consensus that [type 2 diabetes] is irreversible and requires drug treatment escalation, there is some evidence of the possibility of remission,” Dr. Yang and colleagues write in their article.
They aimed to evaluate the effectiveness of intermittent fasting for diabetes remission and the durability of diabetes remission at 1 year.
Diabetes remission was defined having a stable A1c less than 6.5% for at least 3 months after discontinuing all antidiabetic medications, confirmed in at least annual A1c measurements (according to a 2021 consensus statement initiated by the American Diabetes Association).
Between 2019 and 2020, the researchers enrolled 72 participants aged 38-72 years who had had type 2 diabetes (duration 1 to 11 years) and a body mass index (BMI) of 19.1-30.4 kg/m2. Patients were randomized 1:1 to the intermittent fasting group or control group.
Baseline characteristics were similar in both groups. Patients were a mean age of 53 years and roughly 60% were men. They had a mean BMI of 24 kg/m2, a mean duration of diabetes of 6.6 years, and a mean A1c of 7.6%, and they were taking an average of 1.8 glucose-lowering medications.
On fasting days, patients in the intervention group received a Chinese Medical Nutrition Therapy kit that provided approximately 840 kcal/day (46% carbohydrates, 46% fat, 8% protein). The kit included a breakfast of a fruit and vegetable gruel, lunch of a solid beverage plus a nutritional rice composite, and dinner of a solid beverage and a meal replacement biscuit, which participants reconstituted by mixing with boiling water. They were allowed to consume noncaloric beverages.
On nonfasting days, patients chose foods ad libitum based on the 2017 Dietary Guidelines for Diabetes in China, which recommend approximately 50%-65% of total energy intake from carbohydrates, 15%-20% from protein, and 20%-30% from fat, and had greater than or equal to 5 g fiber per serving.
Patients in the control group chose foods ad libitum from the dietary guidelines during the entire study.
The study received funding from the National Natural Science Foundation of China. The authors have reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
In a small randomized controlled trial of patients with type 2 diabetes in China, close to half of those who followed a novel intermittent fasting program for 3 months had diabetes remission (A1c less than 6.5% without taking antidiabetic drugs) that persisted for 1 year.
Importantly, “this study was performed under real-life conditions, and the intervention was delivered by trained nurses in primary care rather than by specialized staff at a research institute, making it a more practical and achievable way to manage” type 2 diabetes, the authors report.
Moreover, 65% of the patients in the intervention group who achieved diabetes remission had had diabetes for more than 6 years, which “suggests the possibility of remission for patients with longer duration” of diabetes, they note.
In addition, antidiabetic medication costs decreased by 77%, compared with baseline, in patients in the intermittent-fasting intervention group.
Although intermittent fasting has been studied for weight loss, it had not been investigated for effectiveness for diabetes remission.
These findings suggest that intermittent fasting “could be a paradigm shift in the management goals in diabetes care,” Xiao Yang and colleagues conclude in their study, published online in The Journal of Clinical Endocrinology & Metabolism.
“Type 2 diabetes is not necessarily a permanent, lifelong disease,” senior author Dongbo Liu, PhD, from the Hunan Agricultural University, Changsha, China, added in a press release from The Endocrine Society.
“Diabetes remission is possible if patients lose weight by changing their diet and exercise habits,” Dr. Liu said.
‘Excellent outcome’
Invited to comment, Amy E. Rothberg, MD, PhD, who was not involved with the research, agreed that the study indicates that intermittent fasting works for diabetes remission.
“We know that diabetes remission is possible with calorie restriction and subsequent weight loss, and intermittent fasting is just one of the many [dietary] approaches that may be suitable, appealing, and sustainable to some individuals, and usually results in calorie restriction and therefore weight loss,” she said.
The most studied types of intermittent fasting diets are alternate-day fasting, the 5:2 diet, and time-restricted consumption, Dr. Rothberg told this news organization.
This study presented a novel type of intermittent fasting, she noted. The intervention consisted of 6 cycles (3 months) of 5 fasting days followed by 10 ad libitum days, and then 3 months of follow-up (with no fasting days).
After 3 months of the intervention plus 3 months of follow-up, 47% of the 36 patients in the intervention group achieved diabetes remission (with a mean A1c of 5.66%), compared with only 2.8% of the 36 patients in the control group.
At 12 months, 44% of patients in the intervention group had sustained diabetes remission (with a mean A1c of 6.33%).
This was “an excellent outcome,” said Dr. Rothberg, professor of nutritional sciences, School of Public Health, University of Michigan, Ann Arbor, and a co-author of an international consensus statement that defined diabetes remission.
On average, patients in the intermittent fasting group lost 5.93 kg (13.0 lb) in 3 months, which was sustained over 12 months. “The large amount of weight reduction is key to continuing to achieve diabetes remission,” she noted.
This contrasted with an average weight loss of just 0.27 kg (0.6 lb) in the control group.
Participants who were prescribed fewer antidiabetic medications were more likely to achieve diabetes remission. The researchers acknowledge that the study was not blinded, and they did not record physical activity (although participants were encouraged to maintain their usual physical activity).
This was a small study, Dr. Rothberg acknowledged. The researchers did not specify which specific antidiabetic drugs patients were taking, and they did not determine waist or hip circumference or assess lipids.
The diet was culturally sensitive, appropriate, and feasible in this Chinese population and would not be generalizable to non-Asians.
Nevertheless, a similar approach could be used in any population if the diet is tailored to the individual, according to Dr. Rothberg. Importantly, patients would need to receive guidance from a dietician to make sure their diet comprises all the necessary micronutrients, vitamins, and minerals on fasting days, and they would need to maintain a relatively balanced diet and not gorge themselves on feast days.
“I think we should campaign widely about lifestyle approaches to achieve diabetes remission,” she urged.
72 patients with diabetes for an average of 6.6 years
“Despite a widespread public consensus that [type 2 diabetes] is irreversible and requires drug treatment escalation, there is some evidence of the possibility of remission,” Dr. Yang and colleagues write in their article.
They aimed to evaluate the effectiveness of intermittent fasting for diabetes remission and the durability of diabetes remission at 1 year.
Diabetes remission was defined having a stable A1c less than 6.5% for at least 3 months after discontinuing all antidiabetic medications, confirmed in at least annual A1c measurements (according to a 2021 consensus statement initiated by the American Diabetes Association).
Between 2019 and 2020, the researchers enrolled 72 participants aged 38-72 years who had had type 2 diabetes (duration 1 to 11 years) and a body mass index (BMI) of 19.1-30.4 kg/m2. Patients were randomized 1:1 to the intermittent fasting group or control group.
Baseline characteristics were similar in both groups. Patients were a mean age of 53 years and roughly 60% were men. They had a mean BMI of 24 kg/m2, a mean duration of diabetes of 6.6 years, and a mean A1c of 7.6%, and they were taking an average of 1.8 glucose-lowering medications.
On fasting days, patients in the intervention group received a Chinese Medical Nutrition Therapy kit that provided approximately 840 kcal/day (46% carbohydrates, 46% fat, 8% protein). The kit included a breakfast of a fruit and vegetable gruel, lunch of a solid beverage plus a nutritional rice composite, and dinner of a solid beverage and a meal replacement biscuit, which participants reconstituted by mixing with boiling water. They were allowed to consume noncaloric beverages.
On nonfasting days, patients chose foods ad libitum based on the 2017 Dietary Guidelines for Diabetes in China, which recommend approximately 50%-65% of total energy intake from carbohydrates, 15%-20% from protein, and 20%-30% from fat, and had greater than or equal to 5 g fiber per serving.
Patients in the control group chose foods ad libitum from the dietary guidelines during the entire study.
The study received funding from the National Natural Science Foundation of China. The authors have reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
U.S. sees most flu hospitalizations in a decade
But the number of deaths and outpatient visits for flu or flu-like illnesses was down slightly from the week before, the CDC said in its weekly FluView report.
There were almost 26,000 new hospital admissions involving laboratory-confirmed influenza over those 7 days, up by over 31% from the previous week, based on data from 5,000 hospitals in the HHS Protect system, which tracks and shares COVID-19 data.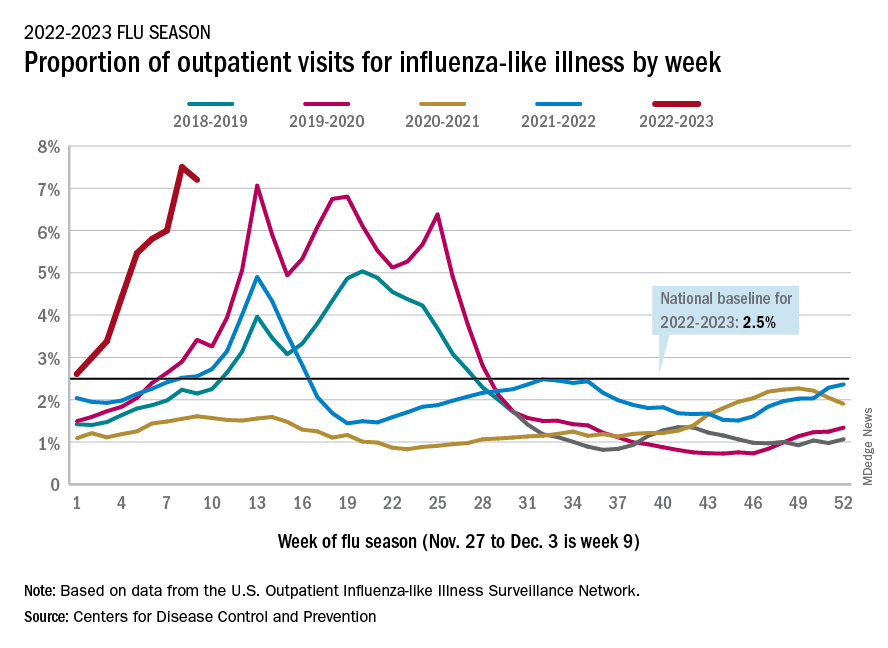
The cumulative hospitalization rate for the 2022-2023 season is 26.0 per 100,000 people, the highest seen at this time of year since 2010-2011, the CDC said, based on data from its Influenza Hospitalization Surveillance Network, which includes hospitals in select counties in 13 states.
At this point in the 2019-2020 season, just before the COVID-19 pandemic began, the cumulative rate was 3.1 per 100,000 people, the CDC’s data show.
On the positive side, the proportion of outpatient visits for influenza-like illness dropped slightly to 7.2%, from 7.5% the week before. But these cases from the CDC’s Outpatient Influenza-like Illness Surveillance Network are not laboratory confirmed, so the data could include people with the flu, COVID-19, or respiratory syncytial virus.
The number of confirmed flu deaths for the week of Nov. 27 to Dec. 3 also fell slightly from the last full week of November, 246 vs. 255, but the number of pediatric deaths rose from 2 to 7, and total deaths in children are already up to 21 for 2022-2023. That’s compared to 44 that were reported during all of the 2021-2022 season, the CDC said.
“So far this season, there have been at least 13 million illnesses, 120,000 hospitalizations, and 7,300 deaths from flu,” the agency estimated.
A version of this article first appeared on Medscape.com.
But the number of deaths and outpatient visits for flu or flu-like illnesses was down slightly from the week before, the CDC said in its weekly FluView report.
There were almost 26,000 new hospital admissions involving laboratory-confirmed influenza over those 7 days, up by over 31% from the previous week, based on data from 5,000 hospitals in the HHS Protect system, which tracks and shares COVID-19 data.
The cumulative hospitalization rate for the 2022-2023 season is 26.0 per 100,000 people, the highest seen at this time of year since 2010-2011, the CDC said, based on data from its Influenza Hospitalization Surveillance Network, which includes hospitals in select counties in 13 states.
At this point in the 2019-2020 season, just before the COVID-19 pandemic began, the cumulative rate was 3.1 per 100,000 people, the CDC’s data show.
On the positive side, the proportion of outpatient visits for influenza-like illness dropped slightly to 7.2%, from 7.5% the week before. But these cases from the CDC’s Outpatient Influenza-like Illness Surveillance Network are not laboratory confirmed, so the data could include people with the flu, COVID-19, or respiratory syncytial virus.
The number of confirmed flu deaths for the week of Nov. 27 to Dec. 3 also fell slightly from the last full week of November, 246 vs. 255, but the number of pediatric deaths rose from 2 to 7, and total deaths in children are already up to 21 for 2022-2023. That’s compared to 44 that were reported during all of the 2021-2022 season, the CDC said.
“So far this season, there have been at least 13 million illnesses, 120,000 hospitalizations, and 7,300 deaths from flu,” the agency estimated.
A version of this article first appeared on Medscape.com.
But the number of deaths and outpatient visits for flu or flu-like illnesses was down slightly from the week before, the CDC said in its weekly FluView report.
There were almost 26,000 new hospital admissions involving laboratory-confirmed influenza over those 7 days, up by over 31% from the previous week, based on data from 5,000 hospitals in the HHS Protect system, which tracks and shares COVID-19 data.
The cumulative hospitalization rate for the 2022-2023 season is 26.0 per 100,000 people, the highest seen at this time of year since 2010-2011, the CDC said, based on data from its Influenza Hospitalization Surveillance Network, which includes hospitals in select counties in 13 states.
At this point in the 2019-2020 season, just before the COVID-19 pandemic began, the cumulative rate was 3.1 per 100,000 people, the CDC’s data show.
On the positive side, the proportion of outpatient visits for influenza-like illness dropped slightly to 7.2%, from 7.5% the week before. But these cases from the CDC’s Outpatient Influenza-like Illness Surveillance Network are not laboratory confirmed, so the data could include people with the flu, COVID-19, or respiratory syncytial virus.
The number of confirmed flu deaths for the week of Nov. 27 to Dec. 3 also fell slightly from the last full week of November, 246 vs. 255, but the number of pediatric deaths rose from 2 to 7, and total deaths in children are already up to 21 for 2022-2023. That’s compared to 44 that were reported during all of the 2021-2022 season, the CDC said.
“So far this season, there have been at least 13 million illnesses, 120,000 hospitalizations, and 7,300 deaths from flu,” the agency estimated.
A version of this article first appeared on Medscape.com.
ADA issues 2023 ‘Standards of Care’ for diabetes: Focus on tight BP, lipids
New more aggressive targets for blood pressure and lipids are among the changes to the annual American Diabetes Association (ADA) Standards of Care in Diabetes – 2023.
The document, long considered the gold standard for care of the more than 100 million Americans living with diabetes and prediabetes, was published as a supplement in Diabetes Care. The guidelines are also accessible to doctors via an app; last year’s standards were accessed more than 4 million times.
The standards now advise a blood pressure target for people with diabetes of less than 130/80 mm Hg, and low-density lipoprotein (LDL) cholesterol targets of below 70 mg/dL or no greater than 55 mg/dL, depending on the individual’s cardiovascular risk.
“In this year’s version of the ADA Standards of Care – the longstanding guidelines for diabetes management globally – you’ll see information that really speaks to how we can more aggressively treat diabetes and reduce complications in a variety of different ways,” ADA Chief Scientific and Medical Officer Robert A. Gabbay, MD, PhD, said in an interview.
Other changes for 2023 include a new emphasis on weight loss as a goal of therapy for type 2 diabetes; guidance for screening and assessing peripheral arterial disease in an effort to prevent amputations; use of finerenone in people with diabetes and chronic kidney disease; use of approved point-of-care A1c tests; and guidance on screening for food insecurity, along with an elevated role for community health workers.
“The management of type 2 diabetes is not just about glucose,” Dr. Gabbay emphasized, noting that the ADA Standards have increasingly focused on cardiorenal risk as well as weight management. “We need to think about all those things, not just one. We have better tools now that have been helpful in being able to move forward with this.”
New targets in cardiovascular disease and risk management
As it has been for the past 6 years, the section on cardiovascular disease and risk management is also endorsed by the American College of Cardiology.
The new definition of hypertension in people with diabetes is ≥ 130 mm Hg systolic or ≥ 80 mm Hg diastolic blood pressure, repeated on two measurements at different times. Among individuals with established cardiovascular disease, hypertension can be diagnosed with one measurement of ≥ 180/110 mm Hg.
The goal of treatment is now less than 130/80 mm Hg if it can be reached safely.
In 2012, easing of the systolic target to 140 mm Hg by the ADA caused some controversy.
But, as Dr. Gabbay explained: “The evidence wasn’t there 10 years ago. We stuck to the evidence at that time, although there was a belief that lower was better. Over the past decade, a number of studies have made it quite clear that there is benefit to a lower target. That’s why we staked out the ground on this.”
The new Standards of Care also has new lipid targets. For people with diabetes aged 40-75 years at increased cardiovascular risk, including those with one or more atherosclerotic risk factors, high-intensity statin therapy is recommended to reduce LDL cholesterol by 50% or more from baseline and to a target of less than 70 mg/dL, in contrast to the previous target of 100 mg/dL.
To achieve that goal, the document advises to consider adding ezetimibe or a PCSK9 inhibitor to maximally tolerated statin therapy.
For people with diabetes aged 40-75 who have established cardiovascular disease, treatment with high-intensity statin therapy is recommended with the target of a 50% or greater reduction from baseline and an LDL cholesterol level of 55 mg/dL or lower, in contrast to the previous 70 mg/dL.
“That is a lower goal than previously recommended, and based on strong evidence in the literature,” Dr. Gabbay noted.
Here, a stronger recommendation is made for ezetimibe or a PCSK9 inhibitor added to maximal statins.
And for people with diabetes older than 75 years, those already on statins should continue taking them. For those who aren’t, it may be reasonable to initiate moderate-intensity statin therapy after discussion of the benefits and risks.
Another new recommendation based on recent trial data is use of a sodium–glucose cotransporter 2 (SGLT2) inhibitor in people with diabetes and heart failure with preserved, as well as reduced, ejection fraction.
Kidney disease guidance updated: SGLT2 inhibitors, finerenone
Another recommendation calls for the addition of finerenone for people with type 2 diabetes who have chronic kidney disease (CKD) with albuminuria and have been treated with the maximum tolerated doses of an angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) to improve cardiovascular outcomes as well as reduce the risk of CKD progression.
The threshold for initiating an SGLT2 inhibitor for kidney protection has changed to an estimated glomerular filtration rate (eGFR) ≥ 20 mL/min/1.73 m2 and urinary albumin ≥ 200 mg/g creatinine (previously ≥ 25 mL/min/1.73 m2 and ≥ 300 mg/g, respectively). An SGLT2 inhibitor may also be beneficial in people with a urinary albumin of normal to ≥ 200 mg/g creatinine, but supporting data have not yet been published.
Referral to a nephrologist is advised for individuals with increasing urinary albumin levels or continued decreasing eGFR or eGFR < 30 mL/min/1.73 m2.
Weight loss, point-of-care testing, food insecurity assessment
Other changes for 2023 include fresh emphasis on supporting weight loss of up to 15% with the new twincretin tirzepatide (Mounjaro) – approved in the United States in May for type 2 diabetes – added as a glucose-lowering drug with weight loss potential.
A novel section was added with guidance for peripheral arterial disease screening.
And a new recommendation advises use of point-of-care A1c testing for diabetes screening and diagnosis using only tests approved by the Food and Drug Administration.
Also introduced for 2023 is guidance to use community health workers to support the management of diabetes and cardiovascular risk factors, particularly in underserved areas and health systems.
“Community health workers can be a link to help people navigate and engage with the health system for better outcomes,” said Dr. Gabbay.
He added that these professionals are among those who can also assist with screening for food insecurity, another new recommendation. “We talk about screening for food insecurity and tools to use. That shouldn’t be something only dietitians do.”
Dr. Gabbay said he’d like to see more clinicians partner with community health workers. “We’d like to see more of that ... They should be considered part of the health care team,” he said.
Dr. Gabbay has reported serving on advisory boards for Lark, Health Reveal, Sweetch, StartUp Health, Vida Health, and Onduo.
A version of this article first appeared on Medscape.com.
New more aggressive targets for blood pressure and lipids are among the changes to the annual American Diabetes Association (ADA) Standards of Care in Diabetes – 2023.
The document, long considered the gold standard for care of the more than 100 million Americans living with diabetes and prediabetes, was published as a supplement in Diabetes Care. The guidelines are also accessible to doctors via an app; last year’s standards were accessed more than 4 million times.
The standards now advise a blood pressure target for people with diabetes of less than 130/80 mm Hg, and low-density lipoprotein (LDL) cholesterol targets of below 70 mg/dL or no greater than 55 mg/dL, depending on the individual’s cardiovascular risk.
“In this year’s version of the ADA Standards of Care – the longstanding guidelines for diabetes management globally – you’ll see information that really speaks to how we can more aggressively treat diabetes and reduce complications in a variety of different ways,” ADA Chief Scientific and Medical Officer Robert A. Gabbay, MD, PhD, said in an interview.
Other changes for 2023 include a new emphasis on weight loss as a goal of therapy for type 2 diabetes; guidance for screening and assessing peripheral arterial disease in an effort to prevent amputations; use of finerenone in people with diabetes and chronic kidney disease; use of approved point-of-care A1c tests; and guidance on screening for food insecurity, along with an elevated role for community health workers.
“The management of type 2 diabetes is not just about glucose,” Dr. Gabbay emphasized, noting that the ADA Standards have increasingly focused on cardiorenal risk as well as weight management. “We need to think about all those things, not just one. We have better tools now that have been helpful in being able to move forward with this.”
New targets in cardiovascular disease and risk management
As it has been for the past 6 years, the section on cardiovascular disease and risk management is also endorsed by the American College of Cardiology.
The new definition of hypertension in people with diabetes is ≥ 130 mm Hg systolic or ≥ 80 mm Hg diastolic blood pressure, repeated on two measurements at different times. Among individuals with established cardiovascular disease, hypertension can be diagnosed with one measurement of ≥ 180/110 mm Hg.
The goal of treatment is now less than 130/80 mm Hg if it can be reached safely.
In 2012, easing of the systolic target to 140 mm Hg by the ADA caused some controversy.
But, as Dr. Gabbay explained: “The evidence wasn’t there 10 years ago. We stuck to the evidence at that time, although there was a belief that lower was better. Over the past decade, a number of studies have made it quite clear that there is benefit to a lower target. That’s why we staked out the ground on this.”
The new Standards of Care also has new lipid targets. For people with diabetes aged 40-75 years at increased cardiovascular risk, including those with one or more atherosclerotic risk factors, high-intensity statin therapy is recommended to reduce LDL cholesterol by 50% or more from baseline and to a target of less than 70 mg/dL, in contrast to the previous target of 100 mg/dL.
To achieve that goal, the document advises to consider adding ezetimibe or a PCSK9 inhibitor to maximally tolerated statin therapy.
For people with diabetes aged 40-75 who have established cardiovascular disease, treatment with high-intensity statin therapy is recommended with the target of a 50% or greater reduction from baseline and an LDL cholesterol level of 55 mg/dL or lower, in contrast to the previous 70 mg/dL.
“That is a lower goal than previously recommended, and based on strong evidence in the literature,” Dr. Gabbay noted.
Here, a stronger recommendation is made for ezetimibe or a PCSK9 inhibitor added to maximal statins.
And for people with diabetes older than 75 years, those already on statins should continue taking them. For those who aren’t, it may be reasonable to initiate moderate-intensity statin therapy after discussion of the benefits and risks.
Another new recommendation based on recent trial data is use of a sodium–glucose cotransporter 2 (SGLT2) inhibitor in people with diabetes and heart failure with preserved, as well as reduced, ejection fraction.
Kidney disease guidance updated: SGLT2 inhibitors, finerenone
Another recommendation calls for the addition of finerenone for people with type 2 diabetes who have chronic kidney disease (CKD) with albuminuria and have been treated with the maximum tolerated doses of an angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) to improve cardiovascular outcomes as well as reduce the risk of CKD progression.
The threshold for initiating an SGLT2 inhibitor for kidney protection has changed to an estimated glomerular filtration rate (eGFR) ≥ 20 mL/min/1.73 m2 and urinary albumin ≥ 200 mg/g creatinine (previously ≥ 25 mL/min/1.73 m2 and ≥ 300 mg/g, respectively). An SGLT2 inhibitor may also be beneficial in people with a urinary albumin of normal to ≥ 200 mg/g creatinine, but supporting data have not yet been published.
Referral to a nephrologist is advised for individuals with increasing urinary albumin levels or continued decreasing eGFR or eGFR < 30 mL/min/1.73 m2.
Weight loss, point-of-care testing, food insecurity assessment
Other changes for 2023 include fresh emphasis on supporting weight loss of up to 15% with the new twincretin tirzepatide (Mounjaro) – approved in the United States in May for type 2 diabetes – added as a glucose-lowering drug with weight loss potential.
A novel section was added with guidance for peripheral arterial disease screening.
And a new recommendation advises use of point-of-care A1c testing for diabetes screening and diagnosis using only tests approved by the Food and Drug Administration.
Also introduced for 2023 is guidance to use community health workers to support the management of diabetes and cardiovascular risk factors, particularly in underserved areas and health systems.
“Community health workers can be a link to help people navigate and engage with the health system for better outcomes,” said Dr. Gabbay.
He added that these professionals are among those who can also assist with screening for food insecurity, another new recommendation. “We talk about screening for food insecurity and tools to use. That shouldn’t be something only dietitians do.”
Dr. Gabbay said he’d like to see more clinicians partner with community health workers. “We’d like to see more of that ... They should be considered part of the health care team,” he said.
Dr. Gabbay has reported serving on advisory boards for Lark, Health Reveal, Sweetch, StartUp Health, Vida Health, and Onduo.
A version of this article first appeared on Medscape.com.
New more aggressive targets for blood pressure and lipids are among the changes to the annual American Diabetes Association (ADA) Standards of Care in Diabetes – 2023.
The document, long considered the gold standard for care of the more than 100 million Americans living with diabetes and prediabetes, was published as a supplement in Diabetes Care. The guidelines are also accessible to doctors via an app; last year’s standards were accessed more than 4 million times.
The standards now advise a blood pressure target for people with diabetes of less than 130/80 mm Hg, and low-density lipoprotein (LDL) cholesterol targets of below 70 mg/dL or no greater than 55 mg/dL, depending on the individual’s cardiovascular risk.
“In this year’s version of the ADA Standards of Care – the longstanding guidelines for diabetes management globally – you’ll see information that really speaks to how we can more aggressively treat diabetes and reduce complications in a variety of different ways,” ADA Chief Scientific and Medical Officer Robert A. Gabbay, MD, PhD, said in an interview.
Other changes for 2023 include a new emphasis on weight loss as a goal of therapy for type 2 diabetes; guidance for screening and assessing peripheral arterial disease in an effort to prevent amputations; use of finerenone in people with diabetes and chronic kidney disease; use of approved point-of-care A1c tests; and guidance on screening for food insecurity, along with an elevated role for community health workers.
“The management of type 2 diabetes is not just about glucose,” Dr. Gabbay emphasized, noting that the ADA Standards have increasingly focused on cardiorenal risk as well as weight management. “We need to think about all those things, not just one. We have better tools now that have been helpful in being able to move forward with this.”
New targets in cardiovascular disease and risk management
As it has been for the past 6 years, the section on cardiovascular disease and risk management is also endorsed by the American College of Cardiology.
The new definition of hypertension in people with diabetes is ≥ 130 mm Hg systolic or ≥ 80 mm Hg diastolic blood pressure, repeated on two measurements at different times. Among individuals with established cardiovascular disease, hypertension can be diagnosed with one measurement of ≥ 180/110 mm Hg.
The goal of treatment is now less than 130/80 mm Hg if it can be reached safely.
In 2012, easing of the systolic target to 140 mm Hg by the ADA caused some controversy.
But, as Dr. Gabbay explained: “The evidence wasn’t there 10 years ago. We stuck to the evidence at that time, although there was a belief that lower was better. Over the past decade, a number of studies have made it quite clear that there is benefit to a lower target. That’s why we staked out the ground on this.”
The new Standards of Care also has new lipid targets. For people with diabetes aged 40-75 years at increased cardiovascular risk, including those with one or more atherosclerotic risk factors, high-intensity statin therapy is recommended to reduce LDL cholesterol by 50% or more from baseline and to a target of less than 70 mg/dL, in contrast to the previous target of 100 mg/dL.
To achieve that goal, the document advises to consider adding ezetimibe or a PCSK9 inhibitor to maximally tolerated statin therapy.
For people with diabetes aged 40-75 who have established cardiovascular disease, treatment with high-intensity statin therapy is recommended with the target of a 50% or greater reduction from baseline and an LDL cholesterol level of 55 mg/dL or lower, in contrast to the previous 70 mg/dL.
“That is a lower goal than previously recommended, and based on strong evidence in the literature,” Dr. Gabbay noted.
Here, a stronger recommendation is made for ezetimibe or a PCSK9 inhibitor added to maximal statins.
And for people with diabetes older than 75 years, those already on statins should continue taking them. For those who aren’t, it may be reasonable to initiate moderate-intensity statin therapy after discussion of the benefits and risks.
Another new recommendation based on recent trial data is use of a sodium–glucose cotransporter 2 (SGLT2) inhibitor in people with diabetes and heart failure with preserved, as well as reduced, ejection fraction.
Kidney disease guidance updated: SGLT2 inhibitors, finerenone
Another recommendation calls for the addition of finerenone for people with type 2 diabetes who have chronic kidney disease (CKD) with albuminuria and have been treated with the maximum tolerated doses of an angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) to improve cardiovascular outcomes as well as reduce the risk of CKD progression.
The threshold for initiating an SGLT2 inhibitor for kidney protection has changed to an estimated glomerular filtration rate (eGFR) ≥ 20 mL/min/1.73 m2 and urinary albumin ≥ 200 mg/g creatinine (previously ≥ 25 mL/min/1.73 m2 and ≥ 300 mg/g, respectively). An SGLT2 inhibitor may also be beneficial in people with a urinary albumin of normal to ≥ 200 mg/g creatinine, but supporting data have not yet been published.
Referral to a nephrologist is advised for individuals with increasing urinary albumin levels or continued decreasing eGFR or eGFR < 30 mL/min/1.73 m2.
Weight loss, point-of-care testing, food insecurity assessment
Other changes for 2023 include fresh emphasis on supporting weight loss of up to 15% with the new twincretin tirzepatide (Mounjaro) – approved in the United States in May for type 2 diabetes – added as a glucose-lowering drug with weight loss potential.
A novel section was added with guidance for peripheral arterial disease screening.
And a new recommendation advises use of point-of-care A1c testing for diabetes screening and diagnosis using only tests approved by the Food and Drug Administration.
Also introduced for 2023 is guidance to use community health workers to support the management of diabetes and cardiovascular risk factors, particularly in underserved areas and health systems.
“Community health workers can be a link to help people navigate and engage with the health system for better outcomes,” said Dr. Gabbay.
He added that these professionals are among those who can also assist with screening for food insecurity, another new recommendation. “We talk about screening for food insecurity and tools to use. That shouldn’t be something only dietitians do.”
Dr. Gabbay said he’d like to see more clinicians partner with community health workers. “We’d like to see more of that ... They should be considered part of the health care team,” he said.
Dr. Gabbay has reported serving on advisory boards for Lark, Health Reveal, Sweetch, StartUp Health, Vida Health, and Onduo.
A version of this article first appeared on Medscape.com.
As COVID treatments dwindle, are new ones waiting in the wings?
It was the last monoclonal antibody treatment standing. But less than 10 months after the U.S. Food and Drug Administration gave bebtelovimab its emergency use authorization (EUA) to fight COVID-19, it earlier this month de-authorized it, just as it had for other monoclonal antibody treatments, and for the same reason:
Bebtelovimab couldn’t neutralize the Omicron subvariants BQ.1 and BQ.1.1, the cause of nearly 60% of COVID cases nationally as of November 30.
Next on the chopping block, some predict, will be Evusheld, the combination of tixagevimab and cilgavimab given as a preventive monoclonal antibody to people who are immunocompromised and at high risk of contracting COVID and to those who can’t take the vaccine. In October, the FDA warned that Evusheld was not neutralizing circulating COVID variants.
As the options for treating and preventing COVID decline, will companies rally quickly to develop new ones, or cut their losses in developing treatments that may work for only a few months, given the speed of viral mutations?
But although monoclonal antibody treatments are off the table, at least for now, antiviral drugs – including Paxlovid – are still very much available, and some say underused.
Others suggest it’s time to resurrect interest in convalescent plasma, a treatment used early in the pandemic before drugs or vaccines were here and still authorized for use in those who are immunosuppressed or receiving immunosuppressive treatment.
And on the prevention front, staying up to date with booster vaccines, masking, and taking other precautions should be stressed more, others say, regardless of the number of treatment options, and especially now, as cases rise and people gather for the winter holidays.
‘A major setback’
The bebtelovimab de-authorization was “a major setback,” but an understandable one, said Arturo Casadevall, MD, PhD, professor and chair of molecular microbiology and immunology at the Johns Hopkins Bloomberg School of Public Health in Baltimore. “Monoclonal antibodies are great drugs. We are in an unfortunate situation in that they are vulnerable to changes in the virus” and can’t offer long-lasting protection.
Supplies of bebtelovimab will be retained, according to the FDA, in case variants susceptible to it return.
“What happened to bebtelovimab is no surprise,” agreed Amesh Adalja, MD, senior scholar at Johns Hopkins Center for Health Security. “This is what is going to happen when you are targeting a virus that mutates a lot.”
Monoclonal antibodies work by binding to the spike protein on the virus surface to prevent it from entering cells.
However, Dr. Adalja doesn’t view the disappearance of monoclonal antibody treatments as a major setback. Monoclonal antibodies were not the primary way COVID was treated, he said.
While he does believe it’s important that more monoclonal antibody treatments be developed, “I think it’s important to remember we still have Paxlovid while everyone is lamenting the loss of bebtelovimab.’’
Antivirals: What’s here, what’s coming
Compared with monoclonal antibodies, “Paxlovid remains a much easier drug to give,” Dr. Adalja told this news organization, because it is taken orally, not intravenously.
And it’s effective. In a recent study, researchers found that adults diagnosed with COVID given Paxlovid within 5 days of diagnosis had a 51% lower hospitalization rate within the next 30 days than those not given it. Another study shows it could also reduce a person’s risk of developing long COVID by 26%.
Paxlovid is underused, Dr. Adalja said, partly because the rebound potential got more press than the effectiveness. When a celebrity got rebound from Paxlovid, he said, that would make the news, overshadowing the research on its effectiveness.
Besides Paxlovid, the antivirals remdesivir (Veklury), given intravenously for 3 days, and molnupiravir (Lagevrio), taken orally, are also still available. Antivirals work by targeting specific parts of the virus to prevent it from multiplying.
In the lab, remdesivir, molnupiravir, and another antiviral, nirmatrelvir, all appear to be effective against both BQ.1.1 (a BA.5 subvariant) and XBB (a BA.2 subvariant), both rapidly rising in the United States, according to a report last week in the New England Journal of Medicine.
The researchers also tested several monoclonal antibodies and found they did not neutralize either of the subvariants BQ.1.1 and XBB.
A new oral antiviral, Xocova (ensitrelvir fumaric acid), from Japanese manufacturer Shionogi, received emergency approval in Japan on November 22. It’s taken once a day for 5 days. The goal is to expand access to it globally, according to the company.
Pardes Biosciences launched a phase 2 trial in September for its oral antiviral drug (PBI-0451), under study as a treatment and preventive for COVID. It expects data by the first quarter of 2023.
Pfizer, which makes Paxlovid, has partnered with Clear Creek Bio to develop another oral antiviral COVID drug.
Other approaches
A receptor protein known as ACE2 (angiotensin-converting enzyme 2) is the main “doorway” that SARS-CoV-2 uses to enter and infect cells.
Dana-Farber Cancer Institute scientists are developing a “decoy” drug that works by mimicking the ACE2 receptor on the surface of cells; when the virus tries to bind to it, the spike protein is destroyed. Human trials have not yet started.
Other researchers are investigating whether an already-approved drug used to treat a liver disease, Actigall (UDCA/ursodeoxycholic acid), could protect against COVID infection by reducing ACE2.
So far, the researchers have found in early research that people taking UDCA for liver conditions were less likely than those not taking the drug to have severe COVID. They also found that UDCA reduced SARS-CoV-2 infection in human lungs maintained outside the body.
Monoclonal antibody treatments?
After the FDA decision to withdraw the bebtelovimab EUA, which Eli Lilly said it agreed with, the company issued a statement, promising it wasn’t giving up on monoclonal antibody treatments.
“Lilly will continue to search and evaluate monoclonal antibodies to identify potential candidates for clinical development against new variants,” it read in part.
AstraZeneca, which makes Evusheld, is also continuing to work on monoclonal antibody development. According to a spokesperson, “We are also developing a new long-acting antibody combination – AZD5156 – which has been shown in the lab to neutralize emerging new variants and all known variants to date. We are working to accelerate the development of AZD5156 to make it available at the end of 2023.”
The AstraZeneca spokesperson said he could share no more information about what the combination would include.
A convalescent plasma comeback?
Although Paxlovid can help, there are many contraindications to it, such as drug-drug interactions, Dr. Casadevall told this news organization. And now that the monoclonal antibody treatments have been paused, convalescent plasma “is the only antibody-based therapy that is reliably available. Convalescent plasma includes thousands of different antibodies.”
With his colleagues, Dr. Casadevall evaluated plasma samples from 740 patients. Some had received booster vaccines and been infected with Omicron, others had received boosters and not been infected, and still others had not been vaccinated and became infected.
In a report (not yet peer-reviewed), they found the plasma from those who had been infected or boosted within the past 6 months neutralized the new Omicron variants BQ.1.1, XBB.1, and BF.7.
A push for boosters, masks
To get through the coming months, taking precautions like masking and distancing and staying up to date on booster vaccinations, especially for older adults, can make a difference, other experts say.
In a Twitter thread in early December, Peter Hotez, MD, PhD, professor of pediatrics and molecular virology and microbiology at Baylor College of Medicine, Houston, urged people to take COVID seriously as holiday parties and gatherings occur.
“The single most impactful thing you can do is get your bivalent booster,” he tweeted, as well as give your kids the booster, citing preliminary research that the bivalent mRNA booster broadens immunity against the Omicron subvariants.
For seniors, he said, ‘‘if you get breakthrough COVID, [it’s] really important to get Paxlovid.” Masks will help not only for COVID but also influenza, respiratory syncytial virus (RSV), and other conditions.
Mitigation measures have largely been abandoned, according to Eric Topol, MD, director of the Scripps Research Translational Institute, La Jolla, Calif., and editor-in-chief of Medscape. In an op-ed in the Los Angeles Times, and on his Twitter feed, he reminds people about masking and urges people to get the bivalent booster.
According to the Centers for Disease Control and Prevention, as of Dec. 8, only 13.5% of people aged 5 and older have gotten an updated booster, despite research that shows an increase in antibodies to BQ.1.1. Recent research has found that the bivalent booster increases antibodies to BQ.1.1 by up to 10-fold, Dr. Topol said.
Dr. Adalja is on advisory boards for Shionogi, GSK, and Pardes. Dr. Casadevall reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
It was the last monoclonal antibody treatment standing. But less than 10 months after the U.S. Food and Drug Administration gave bebtelovimab its emergency use authorization (EUA) to fight COVID-19, it earlier this month de-authorized it, just as it had for other monoclonal antibody treatments, and for the same reason:
Bebtelovimab couldn’t neutralize the Omicron subvariants BQ.1 and BQ.1.1, the cause of nearly 60% of COVID cases nationally as of November 30.
Next on the chopping block, some predict, will be Evusheld, the combination of tixagevimab and cilgavimab given as a preventive monoclonal antibody to people who are immunocompromised and at high risk of contracting COVID and to those who can’t take the vaccine. In October, the FDA warned that Evusheld was not neutralizing circulating COVID variants.
As the options for treating and preventing COVID decline, will companies rally quickly to develop new ones, or cut their losses in developing treatments that may work for only a few months, given the speed of viral mutations?
But although monoclonal antibody treatments are off the table, at least for now, antiviral drugs – including Paxlovid – are still very much available, and some say underused.
Others suggest it’s time to resurrect interest in convalescent plasma, a treatment used early in the pandemic before drugs or vaccines were here and still authorized for use in those who are immunosuppressed or receiving immunosuppressive treatment.
And on the prevention front, staying up to date with booster vaccines, masking, and taking other precautions should be stressed more, others say, regardless of the number of treatment options, and especially now, as cases rise and people gather for the winter holidays.
‘A major setback’
The bebtelovimab de-authorization was “a major setback,” but an understandable one, said Arturo Casadevall, MD, PhD, professor and chair of molecular microbiology and immunology at the Johns Hopkins Bloomberg School of Public Health in Baltimore. “Monoclonal antibodies are great drugs. We are in an unfortunate situation in that they are vulnerable to changes in the virus” and can’t offer long-lasting protection.
Supplies of bebtelovimab will be retained, according to the FDA, in case variants susceptible to it return.
“What happened to bebtelovimab is no surprise,” agreed Amesh Adalja, MD, senior scholar at Johns Hopkins Center for Health Security. “This is what is going to happen when you are targeting a virus that mutates a lot.”
Monoclonal antibodies work by binding to the spike protein on the virus surface to prevent it from entering cells.
However, Dr. Adalja doesn’t view the disappearance of monoclonal antibody treatments as a major setback. Monoclonal antibodies were not the primary way COVID was treated, he said.
While he does believe it’s important that more monoclonal antibody treatments be developed, “I think it’s important to remember we still have Paxlovid while everyone is lamenting the loss of bebtelovimab.’’
Antivirals: What’s here, what’s coming
Compared with monoclonal antibodies, “Paxlovid remains a much easier drug to give,” Dr. Adalja told this news organization, because it is taken orally, not intravenously.
And it’s effective. In a recent study, researchers found that adults diagnosed with COVID given Paxlovid within 5 days of diagnosis had a 51% lower hospitalization rate within the next 30 days than those not given it. Another study shows it could also reduce a person’s risk of developing long COVID by 26%.
Paxlovid is underused, Dr. Adalja said, partly because the rebound potential got more press than the effectiveness. When a celebrity got rebound from Paxlovid, he said, that would make the news, overshadowing the research on its effectiveness.
Besides Paxlovid, the antivirals remdesivir (Veklury), given intravenously for 3 days, and molnupiravir (Lagevrio), taken orally, are also still available. Antivirals work by targeting specific parts of the virus to prevent it from multiplying.
In the lab, remdesivir, molnupiravir, and another antiviral, nirmatrelvir, all appear to be effective against both BQ.1.1 (a BA.5 subvariant) and XBB (a BA.2 subvariant), both rapidly rising in the United States, according to a report last week in the New England Journal of Medicine.
The researchers also tested several monoclonal antibodies and found they did not neutralize either of the subvariants BQ.1.1 and XBB.
A new oral antiviral, Xocova (ensitrelvir fumaric acid), from Japanese manufacturer Shionogi, received emergency approval in Japan on November 22. It’s taken once a day for 5 days. The goal is to expand access to it globally, according to the company.
Pardes Biosciences launched a phase 2 trial in September for its oral antiviral drug (PBI-0451), under study as a treatment and preventive for COVID. It expects data by the first quarter of 2023.
Pfizer, which makes Paxlovid, has partnered with Clear Creek Bio to develop another oral antiviral COVID drug.
Other approaches
A receptor protein known as ACE2 (angiotensin-converting enzyme 2) is the main “doorway” that SARS-CoV-2 uses to enter and infect cells.
Dana-Farber Cancer Institute scientists are developing a “decoy” drug that works by mimicking the ACE2 receptor on the surface of cells; when the virus tries to bind to it, the spike protein is destroyed. Human trials have not yet started.
Other researchers are investigating whether an already-approved drug used to treat a liver disease, Actigall (UDCA/ursodeoxycholic acid), could protect against COVID infection by reducing ACE2.
So far, the researchers have found in early research that people taking UDCA for liver conditions were less likely than those not taking the drug to have severe COVID. They also found that UDCA reduced SARS-CoV-2 infection in human lungs maintained outside the body.
Monoclonal antibody treatments?
After the FDA decision to withdraw the bebtelovimab EUA, which Eli Lilly said it agreed with, the company issued a statement, promising it wasn’t giving up on monoclonal antibody treatments.
“Lilly will continue to search and evaluate monoclonal antibodies to identify potential candidates for clinical development against new variants,” it read in part.
AstraZeneca, which makes Evusheld, is also continuing to work on monoclonal antibody development. According to a spokesperson, “We are also developing a new long-acting antibody combination – AZD5156 – which has been shown in the lab to neutralize emerging new variants and all known variants to date. We are working to accelerate the development of AZD5156 to make it available at the end of 2023.”
The AstraZeneca spokesperson said he could share no more information about what the combination would include.
A convalescent plasma comeback?
Although Paxlovid can help, there are many contraindications to it, such as drug-drug interactions, Dr. Casadevall told this news organization. And now that the monoclonal antibody treatments have been paused, convalescent plasma “is the only antibody-based therapy that is reliably available. Convalescent plasma includes thousands of different antibodies.”
With his colleagues, Dr. Casadevall evaluated plasma samples from 740 patients. Some had received booster vaccines and been infected with Omicron, others had received boosters and not been infected, and still others had not been vaccinated and became infected.
In a report (not yet peer-reviewed), they found the plasma from those who had been infected or boosted within the past 6 months neutralized the new Omicron variants BQ.1.1, XBB.1, and BF.7.
A push for boosters, masks
To get through the coming months, taking precautions like masking and distancing and staying up to date on booster vaccinations, especially for older adults, can make a difference, other experts say.
In a Twitter thread in early December, Peter Hotez, MD, PhD, professor of pediatrics and molecular virology and microbiology at Baylor College of Medicine, Houston, urged people to take COVID seriously as holiday parties and gatherings occur.
“The single most impactful thing you can do is get your bivalent booster,” he tweeted, as well as give your kids the booster, citing preliminary research that the bivalent mRNA booster broadens immunity against the Omicron subvariants.
For seniors, he said, ‘‘if you get breakthrough COVID, [it’s] really important to get Paxlovid.” Masks will help not only for COVID but also influenza, respiratory syncytial virus (RSV), and other conditions.
Mitigation measures have largely been abandoned, according to Eric Topol, MD, director of the Scripps Research Translational Institute, La Jolla, Calif., and editor-in-chief of Medscape. In an op-ed in the Los Angeles Times, and on his Twitter feed, he reminds people about masking and urges people to get the bivalent booster.
According to the Centers for Disease Control and Prevention, as of Dec. 8, only 13.5% of people aged 5 and older have gotten an updated booster, despite research that shows an increase in antibodies to BQ.1.1. Recent research has found that the bivalent booster increases antibodies to BQ.1.1 by up to 10-fold, Dr. Topol said.
Dr. Adalja is on advisory boards for Shionogi, GSK, and Pardes. Dr. Casadevall reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
It was the last monoclonal antibody treatment standing. But less than 10 months after the U.S. Food and Drug Administration gave bebtelovimab its emergency use authorization (EUA) to fight COVID-19, it earlier this month de-authorized it, just as it had for other monoclonal antibody treatments, and for the same reason:
Bebtelovimab couldn’t neutralize the Omicron subvariants BQ.1 and BQ.1.1, the cause of nearly 60% of COVID cases nationally as of November 30.
Next on the chopping block, some predict, will be Evusheld, the combination of tixagevimab and cilgavimab given as a preventive monoclonal antibody to people who are immunocompromised and at high risk of contracting COVID and to those who can’t take the vaccine. In October, the FDA warned that Evusheld was not neutralizing circulating COVID variants.
As the options for treating and preventing COVID decline, will companies rally quickly to develop new ones, or cut their losses in developing treatments that may work for only a few months, given the speed of viral mutations?
But although monoclonal antibody treatments are off the table, at least for now, antiviral drugs – including Paxlovid – are still very much available, and some say underused.
Others suggest it’s time to resurrect interest in convalescent plasma, a treatment used early in the pandemic before drugs or vaccines were here and still authorized for use in those who are immunosuppressed or receiving immunosuppressive treatment.
And on the prevention front, staying up to date with booster vaccines, masking, and taking other precautions should be stressed more, others say, regardless of the number of treatment options, and especially now, as cases rise and people gather for the winter holidays.
‘A major setback’
The bebtelovimab de-authorization was “a major setback,” but an understandable one, said Arturo Casadevall, MD, PhD, professor and chair of molecular microbiology and immunology at the Johns Hopkins Bloomberg School of Public Health in Baltimore. “Monoclonal antibodies are great drugs. We are in an unfortunate situation in that they are vulnerable to changes in the virus” and can’t offer long-lasting protection.
Supplies of bebtelovimab will be retained, according to the FDA, in case variants susceptible to it return.
“What happened to bebtelovimab is no surprise,” agreed Amesh Adalja, MD, senior scholar at Johns Hopkins Center for Health Security. “This is what is going to happen when you are targeting a virus that mutates a lot.”
Monoclonal antibodies work by binding to the spike protein on the virus surface to prevent it from entering cells.
However, Dr. Adalja doesn’t view the disappearance of monoclonal antibody treatments as a major setback. Monoclonal antibodies were not the primary way COVID was treated, he said.
While he does believe it’s important that more monoclonal antibody treatments be developed, “I think it’s important to remember we still have Paxlovid while everyone is lamenting the loss of bebtelovimab.’’
Antivirals: What’s here, what’s coming
Compared with monoclonal antibodies, “Paxlovid remains a much easier drug to give,” Dr. Adalja told this news organization, because it is taken orally, not intravenously.
And it’s effective. In a recent study, researchers found that adults diagnosed with COVID given Paxlovid within 5 days of diagnosis had a 51% lower hospitalization rate within the next 30 days than those not given it. Another study shows it could also reduce a person’s risk of developing long COVID by 26%.
Paxlovid is underused, Dr. Adalja said, partly because the rebound potential got more press than the effectiveness. When a celebrity got rebound from Paxlovid, he said, that would make the news, overshadowing the research on its effectiveness.
Besides Paxlovid, the antivirals remdesivir (Veklury), given intravenously for 3 days, and molnupiravir (Lagevrio), taken orally, are also still available. Antivirals work by targeting specific parts of the virus to prevent it from multiplying.
In the lab, remdesivir, molnupiravir, and another antiviral, nirmatrelvir, all appear to be effective against both BQ.1.1 (a BA.5 subvariant) and XBB (a BA.2 subvariant), both rapidly rising in the United States, according to a report last week in the New England Journal of Medicine.
The researchers also tested several monoclonal antibodies and found they did not neutralize either of the subvariants BQ.1.1 and XBB.
A new oral antiviral, Xocova (ensitrelvir fumaric acid), from Japanese manufacturer Shionogi, received emergency approval in Japan on November 22. It’s taken once a day for 5 days. The goal is to expand access to it globally, according to the company.
Pardes Biosciences launched a phase 2 trial in September for its oral antiviral drug (PBI-0451), under study as a treatment and preventive for COVID. It expects data by the first quarter of 2023.
Pfizer, which makes Paxlovid, has partnered with Clear Creek Bio to develop another oral antiviral COVID drug.
Other approaches
A receptor protein known as ACE2 (angiotensin-converting enzyme 2) is the main “doorway” that SARS-CoV-2 uses to enter and infect cells.
Dana-Farber Cancer Institute scientists are developing a “decoy” drug that works by mimicking the ACE2 receptor on the surface of cells; when the virus tries to bind to it, the spike protein is destroyed. Human trials have not yet started.
Other researchers are investigating whether an already-approved drug used to treat a liver disease, Actigall (UDCA/ursodeoxycholic acid), could protect against COVID infection by reducing ACE2.
So far, the researchers have found in early research that people taking UDCA for liver conditions were less likely than those not taking the drug to have severe COVID. They also found that UDCA reduced SARS-CoV-2 infection in human lungs maintained outside the body.
Monoclonal antibody treatments?
After the FDA decision to withdraw the bebtelovimab EUA, which Eli Lilly said it agreed with, the company issued a statement, promising it wasn’t giving up on monoclonal antibody treatments.
“Lilly will continue to search and evaluate monoclonal antibodies to identify potential candidates for clinical development against new variants,” it read in part.
AstraZeneca, which makes Evusheld, is also continuing to work on monoclonal antibody development. According to a spokesperson, “We are also developing a new long-acting antibody combination – AZD5156 – which has been shown in the lab to neutralize emerging new variants and all known variants to date. We are working to accelerate the development of AZD5156 to make it available at the end of 2023.”
The AstraZeneca spokesperson said he could share no more information about what the combination would include.
A convalescent plasma comeback?
Although Paxlovid can help, there are many contraindications to it, such as drug-drug interactions, Dr. Casadevall told this news organization. And now that the monoclonal antibody treatments have been paused, convalescent plasma “is the only antibody-based therapy that is reliably available. Convalescent plasma includes thousands of different antibodies.”
With his colleagues, Dr. Casadevall evaluated plasma samples from 740 patients. Some had received booster vaccines and been infected with Omicron, others had received boosters and not been infected, and still others had not been vaccinated and became infected.
In a report (not yet peer-reviewed), they found the plasma from those who had been infected or boosted within the past 6 months neutralized the new Omicron variants BQ.1.1, XBB.1, and BF.7.
A push for boosters, masks
To get through the coming months, taking precautions like masking and distancing and staying up to date on booster vaccinations, especially for older adults, can make a difference, other experts say.
In a Twitter thread in early December, Peter Hotez, MD, PhD, professor of pediatrics and molecular virology and microbiology at Baylor College of Medicine, Houston, urged people to take COVID seriously as holiday parties and gatherings occur.
“The single most impactful thing you can do is get your bivalent booster,” he tweeted, as well as give your kids the booster, citing preliminary research that the bivalent mRNA booster broadens immunity against the Omicron subvariants.
For seniors, he said, ‘‘if you get breakthrough COVID, [it’s] really important to get Paxlovid.” Masks will help not only for COVID but also influenza, respiratory syncytial virus (RSV), and other conditions.
Mitigation measures have largely been abandoned, according to Eric Topol, MD, director of the Scripps Research Translational Institute, La Jolla, Calif., and editor-in-chief of Medscape. In an op-ed in the Los Angeles Times, and on his Twitter feed, he reminds people about masking and urges people to get the bivalent booster.
According to the Centers for Disease Control and Prevention, as of Dec. 8, only 13.5% of people aged 5 and older have gotten an updated booster, despite research that shows an increase in antibodies to BQ.1.1. Recent research has found that the bivalent booster increases antibodies to BQ.1.1 by up to 10-fold, Dr. Topol said.
Dr. Adalja is on advisory boards for Shionogi, GSK, and Pardes. Dr. Casadevall reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cognitive behavioral therapy app lowers A1c in type 2 diabetes
CHICAGO – A smartphone app that delivers nutritional cognitive behavioral therapy (CBT) to people with type 2 diabetes produced an average 0.29 percentage point drop in hemoglobin A1c during 180 days of use compared with controls, and an average 0.37 percentage point reduction in A1c compared with baseline values in a randomized, pivotal trial with 669 adults.
Use of the app for 180 days also significantly linked with a reduced need for additional medications, reduced weight and blood pressure, and improved patient-reported outcomes, and it led to fewer adverse effects than seen in control subjects, Marc P. Bonaca, MD, reported at the American Heart Association scientific sessions.
The findings also showed a clear dose-response relationship: The more CBT lessons a person completed with the app, the greater the A1c reduction.
The results suggest that the app, called BT-001, “potentially provides a scalable treatment option for patients with type 2 diabetes,” concluded Dr. Bonaca.
On the basis of the results from this trial, also called BT-001, the company developing the app, Better Therapeutics, announced in September 2022 that it had filed a classification request with the Food and Drug Administration that would allow marketing authorization for the BT-001 app. Better Therapeutics envisions that once authorized by the FDA, the app would be available to people with type 2 diabetes by prescriptions written by health care providers and that the cost for the app would be covered by health insurance, explained a company spokesperson.
A ‘modest positive impact’
“CBT is an empirically supported psychotherapy for a variety of emotional disorders, and it has been adapted to target specific emotional distress in the context of chronic illness,” said Amit Shapira, PhD, a clinical psychologist at the Joslin Diabetes Center in Boston who has not been involved in the BT-001 studies. A CBT protocol designed for diabetes, CBT for Adherence and Depression, “has been shown to have a positive impact on depression symptoms and glycemic control in adults with type 2 diabetes,” Dr. Shapira said in an interview.
Based on published results, the BT-001 app “seems to have a modest positive impact on glycemic control, especially among people who completed more than 10 [lesson] modules.” The evidence appears to suggest that the app “might be a good supplement to working with a behavioral health counselor.”
The BT-001 trial enrolled 669 adults with type 2 diabetes for an average of 11 years and an A1c of 7%-10.9% with an average level of 8.2%. Participants had to be on a stable medication regimen for at least 3 months but not using insulin, and their treatment regimens could undergo adjustment during the trial. At baseline, each subject was on an average of 2.1 antidiabetes medications, including 90% on metformin and 42% on a sulfonylurea. The researchers also highlighted that the enrolled cohort of people with type 2 diabetes had a demographic profile that was “generally representative” of U.S. adults with type 2 diabetes.
The researchers told the 326 people who were randomized to the active intervention group to use the app but subjects were free to determine their frequency of use. The app introduced a new lesson module weekly that took 10-20 minutes to complete, and each weekly lesson came with associated exercises aimed at practicing skills related to behavioral beliefs.
The study’s primary efficacy endpoint was the average change from baseline in A1c compared with the 343 control participants after 90 days of app use, and 610 of the 669 enrolled participants (91%) had paired baseline and 90-day measurements. At 90 days, people in the app group had an average 0.28 percentage point decrease in their A1c compared with an average 0.11 percentage point increase among the controls, a between-group difference of 0.39 percentage points. Both the reduction from baseline with app use and the reduction relative to the controls were significant. These results appeared in an article published online in in Diabetes Care.
At the scientific sessions, Dr. Bonaca presented additional outcome data after 180 days of app use. He reported an average 0.37 percentage point reduction from baseline in A1c among app users and a 0.08 percentage point decrease from baseline among the controls, for a net 0.29 percentage point incremental decline with the app, a significant difference. At 180 days, 50% of the people in the app group had an A1c decline from baseline of at least 0.4 percentage points compared with 34% of the controls, a significant difference.
A dose-response relationship
Notably, app use showed a clear dose-response pattern. During 180 days of app availability, people who used the app fewer than 10 times had an average reduction from baseline in their A1c of less than 0.1 percentage points. Among those who used the app 10-20 times (a subgroup with roughly one-third of the people randomized to app use) average A1c reduction increased to about 0.4 percentage points, and among those who used the app more than 20 times, also one-third of the intervention group, the average A1c reduction from baseline was about 0.6 percentage points.
“It would be interesting to learn more about the adults who engaged with the app” and had a higher use rate “to provide more targeted care” with the app to people who match the profiles of those who were more likely to use the app during the trial, said Dr. Shapira.
Dr. Bonaca, a cardiologist and vascular medicine specialist and executive director of CPC Clinical Research and CPC Community Health, an academic research organization created by and affiliated with the University of Colorado Anschutz Medical Campus in Aurora, Colo., reported several other 180-day outcomes in the BT-001 trial:
- A 33% relative decrease in the percentage of subjects who needed during the study an additional antidiabetes medication or increased dosages of their baseline medications, which occurred at a rate of 21% among the controls and 14% among those who used the app.
- An average weight loss from baseline of 5.5 pounds using the app compared with an average 1.9 pound decrease among controls, a significant difference.
- A decline in average systolic blood pressure of 4.7 mm Hg with app use compared with a 1.8 mm Hg average decline among the controls, a significant difference.
- Significant incremental average improvements in a self-reported Short Form-12 physical component score with the app compared with controls, and increased average improvement in the PHQ9 self-reported measure of depression in app users compared with controls.
- Significantly fewer treatment-emergent adverse effects, and significantly fewer serious treatment-emergent adverse effects among the app users compared with the controls.
‘Ready for clinical use’
Based on these findings, “in my view the app is ready for [routine] clinical use,” declared Judith Hsia, MD, a cardiologist and professor of medicine at the University of Colorado in Aurora, and with Dr. Bonaca a co-lead investigator for the study.
The BT-001 app can serve as “an addition to the toolkit of diabetes treatments,” Dr. Hsia said in an interview. One key advantage of the app is that, once approved, it could be available to many more people with type 2 diabetes than would be able to receive CBT directly from a therapist. Another potential plus for the CBT app is that “the effects should be durable in contrast to medications,” which must be taken on an ongoing basis to maintain effectiveness. In addition, the safety profile “is favorable compared with drug therapies, which should appeal to health care providers,” said Dr. Hsia, chief science officer for CPC Clinical Research.
However, Dr. Shapira cited the issue that therapeutic apps “raise privacy and licensing liability concerns.”
The BT-001 trial was sponsored by Better Therapeutics, the company developing the app. CPC Clinical Research receives research and consulting funding from numerous companies. Dr. Bonaca has been a consultant to Audentes, and is a stockholder of Medtronic and Pfizer. Dr. Shapira had no disclosures. Dr. Hsia is a stockholder of AstraZeneca.
CHICAGO – A smartphone app that delivers nutritional cognitive behavioral therapy (CBT) to people with type 2 diabetes produced an average 0.29 percentage point drop in hemoglobin A1c during 180 days of use compared with controls, and an average 0.37 percentage point reduction in A1c compared with baseline values in a randomized, pivotal trial with 669 adults.
Use of the app for 180 days also significantly linked with a reduced need for additional medications, reduced weight and blood pressure, and improved patient-reported outcomes, and it led to fewer adverse effects than seen in control subjects, Marc P. Bonaca, MD, reported at the American Heart Association scientific sessions.
The findings also showed a clear dose-response relationship: The more CBT lessons a person completed with the app, the greater the A1c reduction.
The results suggest that the app, called BT-001, “potentially provides a scalable treatment option for patients with type 2 diabetes,” concluded Dr. Bonaca.
On the basis of the results from this trial, also called BT-001, the company developing the app, Better Therapeutics, announced in September 2022 that it had filed a classification request with the Food and Drug Administration that would allow marketing authorization for the BT-001 app. Better Therapeutics envisions that once authorized by the FDA, the app would be available to people with type 2 diabetes by prescriptions written by health care providers and that the cost for the app would be covered by health insurance, explained a company spokesperson.
A ‘modest positive impact’
“CBT is an empirically supported psychotherapy for a variety of emotional disorders, and it has been adapted to target specific emotional distress in the context of chronic illness,” said Amit Shapira, PhD, a clinical psychologist at the Joslin Diabetes Center in Boston who has not been involved in the BT-001 studies. A CBT protocol designed for diabetes, CBT for Adherence and Depression, “has been shown to have a positive impact on depression symptoms and glycemic control in adults with type 2 diabetes,” Dr. Shapira said in an interview.
Based on published results, the BT-001 app “seems to have a modest positive impact on glycemic control, especially among people who completed more than 10 [lesson] modules.” The evidence appears to suggest that the app “might be a good supplement to working with a behavioral health counselor.”
The BT-001 trial enrolled 669 adults with type 2 diabetes for an average of 11 years and an A1c of 7%-10.9% with an average level of 8.2%. Participants had to be on a stable medication regimen for at least 3 months but not using insulin, and their treatment regimens could undergo adjustment during the trial. At baseline, each subject was on an average of 2.1 antidiabetes medications, including 90% on metformin and 42% on a sulfonylurea. The researchers also highlighted that the enrolled cohort of people with type 2 diabetes had a demographic profile that was “generally representative” of U.S. adults with type 2 diabetes.
The researchers told the 326 people who were randomized to the active intervention group to use the app but subjects were free to determine their frequency of use. The app introduced a new lesson module weekly that took 10-20 minutes to complete, and each weekly lesson came with associated exercises aimed at practicing skills related to behavioral beliefs.
The study’s primary efficacy endpoint was the average change from baseline in A1c compared with the 343 control participants after 90 days of app use, and 610 of the 669 enrolled participants (91%) had paired baseline and 90-day measurements. At 90 days, people in the app group had an average 0.28 percentage point decrease in their A1c compared with an average 0.11 percentage point increase among the controls, a between-group difference of 0.39 percentage points. Both the reduction from baseline with app use and the reduction relative to the controls were significant. These results appeared in an article published online in in Diabetes Care.
At the scientific sessions, Dr. Bonaca presented additional outcome data after 180 days of app use. He reported an average 0.37 percentage point reduction from baseline in A1c among app users and a 0.08 percentage point decrease from baseline among the controls, for a net 0.29 percentage point incremental decline with the app, a significant difference. At 180 days, 50% of the people in the app group had an A1c decline from baseline of at least 0.4 percentage points compared with 34% of the controls, a significant difference.
A dose-response relationship
Notably, app use showed a clear dose-response pattern. During 180 days of app availability, people who used the app fewer than 10 times had an average reduction from baseline in their A1c of less than 0.1 percentage points. Among those who used the app 10-20 times (a subgroup with roughly one-third of the people randomized to app use) average A1c reduction increased to about 0.4 percentage points, and among those who used the app more than 20 times, also one-third of the intervention group, the average A1c reduction from baseline was about 0.6 percentage points.
“It would be interesting to learn more about the adults who engaged with the app” and had a higher use rate “to provide more targeted care” with the app to people who match the profiles of those who were more likely to use the app during the trial, said Dr. Shapira.
Dr. Bonaca, a cardiologist and vascular medicine specialist and executive director of CPC Clinical Research and CPC Community Health, an academic research organization created by and affiliated with the University of Colorado Anschutz Medical Campus in Aurora, Colo., reported several other 180-day outcomes in the BT-001 trial:
- A 33% relative decrease in the percentage of subjects who needed during the study an additional antidiabetes medication or increased dosages of their baseline medications, which occurred at a rate of 21% among the controls and 14% among those who used the app.
- An average weight loss from baseline of 5.5 pounds using the app compared with an average 1.9 pound decrease among controls, a significant difference.
- A decline in average systolic blood pressure of 4.7 mm Hg with app use compared with a 1.8 mm Hg average decline among the controls, a significant difference.
- Significant incremental average improvements in a self-reported Short Form-12 physical component score with the app compared with controls, and increased average improvement in the PHQ9 self-reported measure of depression in app users compared with controls.
- Significantly fewer treatment-emergent adverse effects, and significantly fewer serious treatment-emergent adverse effects among the app users compared with the controls.
‘Ready for clinical use’
Based on these findings, “in my view the app is ready for [routine] clinical use,” declared Judith Hsia, MD, a cardiologist and professor of medicine at the University of Colorado in Aurora, and with Dr. Bonaca a co-lead investigator for the study.
The BT-001 app can serve as “an addition to the toolkit of diabetes treatments,” Dr. Hsia said in an interview. One key advantage of the app is that, once approved, it could be available to many more people with type 2 diabetes than would be able to receive CBT directly from a therapist. Another potential plus for the CBT app is that “the effects should be durable in contrast to medications,” which must be taken on an ongoing basis to maintain effectiveness. In addition, the safety profile “is favorable compared with drug therapies, which should appeal to health care providers,” said Dr. Hsia, chief science officer for CPC Clinical Research.
However, Dr. Shapira cited the issue that therapeutic apps “raise privacy and licensing liability concerns.”
The BT-001 trial was sponsored by Better Therapeutics, the company developing the app. CPC Clinical Research receives research and consulting funding from numerous companies. Dr. Bonaca has been a consultant to Audentes, and is a stockholder of Medtronic and Pfizer. Dr. Shapira had no disclosures. Dr. Hsia is a stockholder of AstraZeneca.
CHICAGO – A smartphone app that delivers nutritional cognitive behavioral therapy (CBT) to people with type 2 diabetes produced an average 0.29 percentage point drop in hemoglobin A1c during 180 days of use compared with controls, and an average 0.37 percentage point reduction in A1c compared with baseline values in a randomized, pivotal trial with 669 adults.
Use of the app for 180 days also significantly linked with a reduced need for additional medications, reduced weight and blood pressure, and improved patient-reported outcomes, and it led to fewer adverse effects than seen in control subjects, Marc P. Bonaca, MD, reported at the American Heart Association scientific sessions.
The findings also showed a clear dose-response relationship: The more CBT lessons a person completed with the app, the greater the A1c reduction.
The results suggest that the app, called BT-001, “potentially provides a scalable treatment option for patients with type 2 diabetes,” concluded Dr. Bonaca.
On the basis of the results from this trial, also called BT-001, the company developing the app, Better Therapeutics, announced in September 2022 that it had filed a classification request with the Food and Drug Administration that would allow marketing authorization for the BT-001 app. Better Therapeutics envisions that once authorized by the FDA, the app would be available to people with type 2 diabetes by prescriptions written by health care providers and that the cost for the app would be covered by health insurance, explained a company spokesperson.
A ‘modest positive impact’
“CBT is an empirically supported psychotherapy for a variety of emotional disorders, and it has been adapted to target specific emotional distress in the context of chronic illness,” said Amit Shapira, PhD, a clinical psychologist at the Joslin Diabetes Center in Boston who has not been involved in the BT-001 studies. A CBT protocol designed for diabetes, CBT for Adherence and Depression, “has been shown to have a positive impact on depression symptoms and glycemic control in adults with type 2 diabetes,” Dr. Shapira said in an interview.
Based on published results, the BT-001 app “seems to have a modest positive impact on glycemic control, especially among people who completed more than 10 [lesson] modules.” The evidence appears to suggest that the app “might be a good supplement to working with a behavioral health counselor.”
The BT-001 trial enrolled 669 adults with type 2 diabetes for an average of 11 years and an A1c of 7%-10.9% with an average level of 8.2%. Participants had to be on a stable medication regimen for at least 3 months but not using insulin, and their treatment regimens could undergo adjustment during the trial. At baseline, each subject was on an average of 2.1 antidiabetes medications, including 90% on metformin and 42% on a sulfonylurea. The researchers also highlighted that the enrolled cohort of people with type 2 diabetes had a demographic profile that was “generally representative” of U.S. adults with type 2 diabetes.
The researchers told the 326 people who were randomized to the active intervention group to use the app but subjects were free to determine their frequency of use. The app introduced a new lesson module weekly that took 10-20 minutes to complete, and each weekly lesson came with associated exercises aimed at practicing skills related to behavioral beliefs.
The study’s primary efficacy endpoint was the average change from baseline in A1c compared with the 343 control participants after 90 days of app use, and 610 of the 669 enrolled participants (91%) had paired baseline and 90-day measurements. At 90 days, people in the app group had an average 0.28 percentage point decrease in their A1c compared with an average 0.11 percentage point increase among the controls, a between-group difference of 0.39 percentage points. Both the reduction from baseline with app use and the reduction relative to the controls were significant. These results appeared in an article published online in in Diabetes Care.
At the scientific sessions, Dr. Bonaca presented additional outcome data after 180 days of app use. He reported an average 0.37 percentage point reduction from baseline in A1c among app users and a 0.08 percentage point decrease from baseline among the controls, for a net 0.29 percentage point incremental decline with the app, a significant difference. At 180 days, 50% of the people in the app group had an A1c decline from baseline of at least 0.4 percentage points compared with 34% of the controls, a significant difference.
A dose-response relationship
Notably, app use showed a clear dose-response pattern. During 180 days of app availability, people who used the app fewer than 10 times had an average reduction from baseline in their A1c of less than 0.1 percentage points. Among those who used the app 10-20 times (a subgroup with roughly one-third of the people randomized to app use) average A1c reduction increased to about 0.4 percentage points, and among those who used the app more than 20 times, also one-third of the intervention group, the average A1c reduction from baseline was about 0.6 percentage points.
“It would be interesting to learn more about the adults who engaged with the app” and had a higher use rate “to provide more targeted care” with the app to people who match the profiles of those who were more likely to use the app during the trial, said Dr. Shapira.
Dr. Bonaca, a cardiologist and vascular medicine specialist and executive director of CPC Clinical Research and CPC Community Health, an academic research organization created by and affiliated with the University of Colorado Anschutz Medical Campus in Aurora, Colo., reported several other 180-day outcomes in the BT-001 trial:
- A 33% relative decrease in the percentage of subjects who needed during the study an additional antidiabetes medication or increased dosages of their baseline medications, which occurred at a rate of 21% among the controls and 14% among those who used the app.
- An average weight loss from baseline of 5.5 pounds using the app compared with an average 1.9 pound decrease among controls, a significant difference.
- A decline in average systolic blood pressure of 4.7 mm Hg with app use compared with a 1.8 mm Hg average decline among the controls, a significant difference.
- Significant incremental average improvements in a self-reported Short Form-12 physical component score with the app compared with controls, and increased average improvement in the PHQ9 self-reported measure of depression in app users compared with controls.
- Significantly fewer treatment-emergent adverse effects, and significantly fewer serious treatment-emergent adverse effects among the app users compared with the controls.
‘Ready for clinical use’
Based on these findings, “in my view the app is ready for [routine] clinical use,” declared Judith Hsia, MD, a cardiologist and professor of medicine at the University of Colorado in Aurora, and with Dr. Bonaca a co-lead investigator for the study.
The BT-001 app can serve as “an addition to the toolkit of diabetes treatments,” Dr. Hsia said in an interview. One key advantage of the app is that, once approved, it could be available to many more people with type 2 diabetes than would be able to receive CBT directly from a therapist. Another potential plus for the CBT app is that “the effects should be durable in contrast to medications,” which must be taken on an ongoing basis to maintain effectiveness. In addition, the safety profile “is favorable compared with drug therapies, which should appeal to health care providers,” said Dr. Hsia, chief science officer for CPC Clinical Research.
However, Dr. Shapira cited the issue that therapeutic apps “raise privacy and licensing liability concerns.”
The BT-001 trial was sponsored by Better Therapeutics, the company developing the app. CPC Clinical Research receives research and consulting funding from numerous companies. Dr. Bonaca has been a consultant to Audentes, and is a stockholder of Medtronic and Pfizer. Dr. Shapira had no disclosures. Dr. Hsia is a stockholder of AstraZeneca.
AT AHA 2022





