User login
CDC: Masking no longer required in health care settings
It’s a “major departure” from the CDC’s previous recommendation of universal masking to fight the COVID-19 pandemic, The Hill says.
“Updates were made to reflect the high levels of vaccine-and infection-induced immunity and the availability of effective treatments and prevention tools,” the CDC’s new guidance says.
The agency now says that facilities in areas without high transmission can decide for themselves whether to require everyone – doctors, patients, and visitors – to wear masks.
Community transmission “is the metric currently recommended to guide select practices in healthcare settings to allow for earlier intervention, before there is strain on the health care system and to better protect the individuals seeking care in these settings,” the CDC said.
About 73% of the country is having “high” rates of transmission, The Hill said.
“Community transmission” is different from the “community level” metric that’s used for non–health care settings.
Community transmission refers to measures of the presence and spread of SARS-CoV-2, the CDC said. “Community levels place an emphasis on measures of the impact of COVID-19 in terms of hospitalizations and health care system strain, while accounting for transmission in the community.”
Just 7% of counties are considered high risk, while nearly 62 percent are low.
The new guidance applies wherever health care is delivered, including nursing homes and home health, the CDC said.
A version of this article first appeared on WebMD.com.
It’s a “major departure” from the CDC’s previous recommendation of universal masking to fight the COVID-19 pandemic, The Hill says.
“Updates were made to reflect the high levels of vaccine-and infection-induced immunity and the availability of effective treatments and prevention tools,” the CDC’s new guidance says.
The agency now says that facilities in areas without high transmission can decide for themselves whether to require everyone – doctors, patients, and visitors – to wear masks.
Community transmission “is the metric currently recommended to guide select practices in healthcare settings to allow for earlier intervention, before there is strain on the health care system and to better protect the individuals seeking care in these settings,” the CDC said.
About 73% of the country is having “high” rates of transmission, The Hill said.
“Community transmission” is different from the “community level” metric that’s used for non–health care settings.
Community transmission refers to measures of the presence and spread of SARS-CoV-2, the CDC said. “Community levels place an emphasis on measures of the impact of COVID-19 in terms of hospitalizations and health care system strain, while accounting for transmission in the community.”
Just 7% of counties are considered high risk, while nearly 62 percent are low.
The new guidance applies wherever health care is delivered, including nursing homes and home health, the CDC said.
A version of this article first appeared on WebMD.com.
It’s a “major departure” from the CDC’s previous recommendation of universal masking to fight the COVID-19 pandemic, The Hill says.
“Updates were made to reflect the high levels of vaccine-and infection-induced immunity and the availability of effective treatments and prevention tools,” the CDC’s new guidance says.
The agency now says that facilities in areas without high transmission can decide for themselves whether to require everyone – doctors, patients, and visitors – to wear masks.
Community transmission “is the metric currently recommended to guide select practices in healthcare settings to allow for earlier intervention, before there is strain on the health care system and to better protect the individuals seeking care in these settings,” the CDC said.
About 73% of the country is having “high” rates of transmission, The Hill said.
“Community transmission” is different from the “community level” metric that’s used for non–health care settings.
Community transmission refers to measures of the presence and spread of SARS-CoV-2, the CDC said. “Community levels place an emphasis on measures of the impact of COVID-19 in terms of hospitalizations and health care system strain, while accounting for transmission in the community.”
Just 7% of counties are considered high risk, while nearly 62 percent are low.
The new guidance applies wherever health care is delivered, including nursing homes and home health, the CDC said.
A version of this article first appeared on WebMD.com.
How to improve diagnosis of HFpEF, common in diabetes
STOCKHOLM – Recent study results confirm that two agents from the sodium-glucose cotransporter 2 (SGLT2) inhibitor class can significantly cut the incidence of adverse cardiovascular events in patients with heart failure with reduced ejection fraction (HFpEF), a disease especially common in people with type 2 diabetes, obesity, or both.
And findings from secondary analyses of the studies – including one reported at the annual meeting of the European Association for the Study of Diabetes – show that these SGLT2 inhibitors work as well for cutting incident adverse events (cardiovascular death or worsening heart failure) in patients with HFpEF and diabetes as they do for people with normal blood glucose levels.
But delivering treatment with these proven agents, dapagliflozin (Farxiga) and empagliflozin (Jardiance), first requires diagnosis of HFpEF, a task that clinicians have historically fallen short in accomplishing.
When in 2021, results from the EMPEROR-Preserved trial with empagliflozin and when in September 2022 results from the DELIVER trial with dapagliflozin established the efficacy of these two SGLT2 inhibitors as the first treatments proven to benefit patients with HFpEF, they also raised the stakes for clinicians to be much more diligent and systematic in evaluating people at high risk for developing HFpEF because of having type 2 diabetes or obesity, two of the most potent risk factors for this form of heart failure.
‘Vigilance ... needs to increase’
“Vigilance for HFpEF needs to increase because we can now help these patients,” declared Lars H. Lund, MD, PhD, speaking at the meeting. “Type 2 diabetes dramatically increases the incidence of HFpEF,” and the mechanisms by which it does this are “especially amenable to treatment with SGLT2 inhibitors,” said Dr. Lund, a cardiologist and heart failure specialist at the Karolinska Institute, Stockholm.
HFpEF has a history of going undetected in people with type 2 diabetes, an ironic situation given its high incidence as well as the elevated rate of adverse cardiovascular events when heart failure occurs in patients with type 2 diabetes compared with patients who do not have diabetes.
The key, say experts, is for clinicians to maintain a high index of suspicion for signs and symptoms of heart failure in people with type 2 diabetes and to regularly assess them, starting with just a few simple questions that probe for the presence of dyspnea, exertional fatigue, or both, an approach not widely employed up to now.
Clinicians who care for people with type 2 diabetes must become “alert to thinking about heart failure and alert to asking questions about signs and symptoms” that flag the presence of HFpEF, advised Naveed Sattar, MBChB, PhD, a professor of metabolic medicine at the University of Glasgow.
Soon, medical groups will issue guidelines for appropriate assessment for the presence of HFpEF in people with type 2 diabetes, Dr. Sattar predicted in an interview.
A need to probe
“You can’t simply ask patients with type 2 diabetes whether they have shortness of breath or exertional fatigue and stop there,” because often their first response will be no.
“Commonly, patients will initially say they have no dyspnea, but when you probe further, you find symptoms,” noted Mikhail N. Kosiborod, MD, codirector of Saint Luke’s Cardiometabolic Center of Excellence in Kansas City, Mo.
These people are often sedentary, so they frequently don’t experience shortness of breath at baseline, Dr. Kosiborod said in an interview. In some cases, they may limit their activity because of their exertional intolerance.
Once a person’s suggestive symptoms become known, the next step is to measure the serum level of N-terminal pro-B-type natriuretic peptide (NT-proBNP), a biomarker considered to be a generally reliable signal of existing heart failure when elevated.
Any value above 125 pg/mL is suggestive of prevalent heart failure and should lead to the next diagnostic step of echocardiography, Dr. Sattar said.
Elevated NT-proBNP has such good positive predictive value for identifying heart failure that it is tempting to use it broadly in people with type 2 diabetes. A 2022 consensus report from the American Diabetes Association says that “measurement of a natriuretic peptide [such as NT-proBNP] or high-sensitivity cardiac troponin is recommended on at least a yearly basis to identify the earliest HF [heart failure] stages and implement strategies to prevent transition to symptomatic HF.”
Test costs require targeting
But because of the relatively high current price for an NT-proBNP test, the cost-benefit ratio for widespread annual testing of all people with type 2 diabetes would be poor, some experts caution.
“Screening everyone may not be the right answer. Hundreds of millions of people worldwide” have type 2 diabetes. “You first need to target evaluation to people with symptoms,” advised Dr. Kosiborod.
He also warned that a low NT-proBNP level does not always rule out HFpEF, especially among people with type 2 diabetes who also have overweight or obesity, because NT-proBNP levels can be “artificially low” in people with obesity.
Other potential aids to diagnosis are assessment scores that researchers have developed, such as the H2FPEF score, which relies on variables that include age, obesity, and the presence of atrial fibrillation and hypertension.
However, this score also requires an echocardiography examination, another test that would have a questionable cost-benefit ratio if performed widely for patients with type 2 diabetes without targeting, Dr. Kosiborod said.
SGLT2 inhibitors benefit HFpEF regardless of glucose levels
A prespecified analysis of the DELIVER results that divided the study cohort on the basis of their glycemic status proved the efficacy of the SGLT2 inhibitor dapagliflozin for patients with HFpEF regardless of whether or not they had type 2 diabetes, prediabetes, or were normoglycemic at entry into the study, Silvio E. Inzucchi, MD, reported at the EASD meeting.
Treatment with dapagliflozin cut the incidence of the trial’s primary outcome of cardiovascular death or worsening heart failure by a significant 18% relative to placebo among all enrolled patients.
The new analysis reported by Dr. Inzucchi showed that treatment was associated with a 23% relative risk reduction among those with normoglycemia, a 13% reduction among those with prediabetes, and a 19% reduction among those with type 2 diabetes, with no signal of a significant difference among the three subgroups.
“There was no statistical interaction between categorical glycemic subgrouping and dapagliflozin’s treatment effect,” concluded Dr. Inzucchi, director of the Yale Medicine Diabetes Center, New Haven, Conn.
He also reported that, among the 6,259 people in the trial with HFpEF, 50% had diabetes, 31% had prediabetes, and a scant 19% had normoglycemia. The finding highlights once again the high prevalence of dysglycemia among people with HFpEF.
Previously, a prespecified secondary analysis of data from the EMPEROR-Preserved trial yielded similar findings for empagliflozin that showed the agent’s efficacy for people with HFpEF across the range of glucose levels.
The DELIVER trial was funded by AstraZeneca, the company that markets dapagliflozin (Farxiga). The EMPEROR-Preserved trial was sponsored by Boehringer Ingelheim and Eli Lilly, the companies that jointly market empagliflozin (Jardiance). Dr. Lund has been a consultant to AstraZeneca and Boehringer Ingelheim and to numerous other companies, and he is a stockholder in AnaCardio. Dr. Sattar has been a consultant to and has received research support from AstraZeneca and Boehringer Ingelheim, and he has been a consultant with numerous companies. Dr. Kosiborod has been a consultant to and has received research funding from AstraZeneca and Boehringer Ingelheim and has been a consultant to Eli Lilly and numerous other companies. Dr. Inzucchi has been a consultant to, given talks on behalf of, or served on trial committees for Abbott, AstraZeneca, Boehringer Ingelheim, Esperion, Lexicon, Merck, Novo Nordisk, Pfizer, and vTv Therapetics.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Recent study results confirm that two agents from the sodium-glucose cotransporter 2 (SGLT2) inhibitor class can significantly cut the incidence of adverse cardiovascular events in patients with heart failure with reduced ejection fraction (HFpEF), a disease especially common in people with type 2 diabetes, obesity, or both.
And findings from secondary analyses of the studies – including one reported at the annual meeting of the European Association for the Study of Diabetes – show that these SGLT2 inhibitors work as well for cutting incident adverse events (cardiovascular death or worsening heart failure) in patients with HFpEF and diabetes as they do for people with normal blood glucose levels.
But delivering treatment with these proven agents, dapagliflozin (Farxiga) and empagliflozin (Jardiance), first requires diagnosis of HFpEF, a task that clinicians have historically fallen short in accomplishing.
When in 2021, results from the EMPEROR-Preserved trial with empagliflozin and when in September 2022 results from the DELIVER trial with dapagliflozin established the efficacy of these two SGLT2 inhibitors as the first treatments proven to benefit patients with HFpEF, they also raised the stakes for clinicians to be much more diligent and systematic in evaluating people at high risk for developing HFpEF because of having type 2 diabetes or obesity, two of the most potent risk factors for this form of heart failure.
‘Vigilance ... needs to increase’
“Vigilance for HFpEF needs to increase because we can now help these patients,” declared Lars H. Lund, MD, PhD, speaking at the meeting. “Type 2 diabetes dramatically increases the incidence of HFpEF,” and the mechanisms by which it does this are “especially amenable to treatment with SGLT2 inhibitors,” said Dr. Lund, a cardiologist and heart failure specialist at the Karolinska Institute, Stockholm.
HFpEF has a history of going undetected in people with type 2 diabetes, an ironic situation given its high incidence as well as the elevated rate of adverse cardiovascular events when heart failure occurs in patients with type 2 diabetes compared with patients who do not have diabetes.
The key, say experts, is for clinicians to maintain a high index of suspicion for signs and symptoms of heart failure in people with type 2 diabetes and to regularly assess them, starting with just a few simple questions that probe for the presence of dyspnea, exertional fatigue, or both, an approach not widely employed up to now.
Clinicians who care for people with type 2 diabetes must become “alert to thinking about heart failure and alert to asking questions about signs and symptoms” that flag the presence of HFpEF, advised Naveed Sattar, MBChB, PhD, a professor of metabolic medicine at the University of Glasgow.
Soon, medical groups will issue guidelines for appropriate assessment for the presence of HFpEF in people with type 2 diabetes, Dr. Sattar predicted in an interview.
A need to probe
“You can’t simply ask patients with type 2 diabetes whether they have shortness of breath or exertional fatigue and stop there,” because often their first response will be no.
“Commonly, patients will initially say they have no dyspnea, but when you probe further, you find symptoms,” noted Mikhail N. Kosiborod, MD, codirector of Saint Luke’s Cardiometabolic Center of Excellence in Kansas City, Mo.
These people are often sedentary, so they frequently don’t experience shortness of breath at baseline, Dr. Kosiborod said in an interview. In some cases, they may limit their activity because of their exertional intolerance.
Once a person’s suggestive symptoms become known, the next step is to measure the serum level of N-terminal pro-B-type natriuretic peptide (NT-proBNP), a biomarker considered to be a generally reliable signal of existing heart failure when elevated.
Any value above 125 pg/mL is suggestive of prevalent heart failure and should lead to the next diagnostic step of echocardiography, Dr. Sattar said.
Elevated NT-proBNP has such good positive predictive value for identifying heart failure that it is tempting to use it broadly in people with type 2 diabetes. A 2022 consensus report from the American Diabetes Association says that “measurement of a natriuretic peptide [such as NT-proBNP] or high-sensitivity cardiac troponin is recommended on at least a yearly basis to identify the earliest HF [heart failure] stages and implement strategies to prevent transition to symptomatic HF.”
Test costs require targeting
But because of the relatively high current price for an NT-proBNP test, the cost-benefit ratio for widespread annual testing of all people with type 2 diabetes would be poor, some experts caution.
“Screening everyone may not be the right answer. Hundreds of millions of people worldwide” have type 2 diabetes. “You first need to target evaluation to people with symptoms,” advised Dr. Kosiborod.
He also warned that a low NT-proBNP level does not always rule out HFpEF, especially among people with type 2 diabetes who also have overweight or obesity, because NT-proBNP levels can be “artificially low” in people with obesity.
Other potential aids to diagnosis are assessment scores that researchers have developed, such as the H2FPEF score, which relies on variables that include age, obesity, and the presence of atrial fibrillation and hypertension.
However, this score also requires an echocardiography examination, another test that would have a questionable cost-benefit ratio if performed widely for patients with type 2 diabetes without targeting, Dr. Kosiborod said.
SGLT2 inhibitors benefit HFpEF regardless of glucose levels
A prespecified analysis of the DELIVER results that divided the study cohort on the basis of their glycemic status proved the efficacy of the SGLT2 inhibitor dapagliflozin for patients with HFpEF regardless of whether or not they had type 2 diabetes, prediabetes, or were normoglycemic at entry into the study, Silvio E. Inzucchi, MD, reported at the EASD meeting.
Treatment with dapagliflozin cut the incidence of the trial’s primary outcome of cardiovascular death or worsening heart failure by a significant 18% relative to placebo among all enrolled patients.
The new analysis reported by Dr. Inzucchi showed that treatment was associated with a 23% relative risk reduction among those with normoglycemia, a 13% reduction among those with prediabetes, and a 19% reduction among those with type 2 diabetes, with no signal of a significant difference among the three subgroups.
“There was no statistical interaction between categorical glycemic subgrouping and dapagliflozin’s treatment effect,” concluded Dr. Inzucchi, director of the Yale Medicine Diabetes Center, New Haven, Conn.
He also reported that, among the 6,259 people in the trial with HFpEF, 50% had diabetes, 31% had prediabetes, and a scant 19% had normoglycemia. The finding highlights once again the high prevalence of dysglycemia among people with HFpEF.
Previously, a prespecified secondary analysis of data from the EMPEROR-Preserved trial yielded similar findings for empagliflozin that showed the agent’s efficacy for people with HFpEF across the range of glucose levels.
The DELIVER trial was funded by AstraZeneca, the company that markets dapagliflozin (Farxiga). The EMPEROR-Preserved trial was sponsored by Boehringer Ingelheim and Eli Lilly, the companies that jointly market empagliflozin (Jardiance). Dr. Lund has been a consultant to AstraZeneca and Boehringer Ingelheim and to numerous other companies, and he is a stockholder in AnaCardio. Dr. Sattar has been a consultant to and has received research support from AstraZeneca and Boehringer Ingelheim, and he has been a consultant with numerous companies. Dr. Kosiborod has been a consultant to and has received research funding from AstraZeneca and Boehringer Ingelheim and has been a consultant to Eli Lilly and numerous other companies. Dr. Inzucchi has been a consultant to, given talks on behalf of, or served on trial committees for Abbott, AstraZeneca, Boehringer Ingelheim, Esperion, Lexicon, Merck, Novo Nordisk, Pfizer, and vTv Therapetics.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Recent study results confirm that two agents from the sodium-glucose cotransporter 2 (SGLT2) inhibitor class can significantly cut the incidence of adverse cardiovascular events in patients with heart failure with reduced ejection fraction (HFpEF), a disease especially common in people with type 2 diabetes, obesity, or both.
And findings from secondary analyses of the studies – including one reported at the annual meeting of the European Association for the Study of Diabetes – show that these SGLT2 inhibitors work as well for cutting incident adverse events (cardiovascular death or worsening heart failure) in patients with HFpEF and diabetes as they do for people with normal blood glucose levels.
But delivering treatment with these proven agents, dapagliflozin (Farxiga) and empagliflozin (Jardiance), first requires diagnosis of HFpEF, a task that clinicians have historically fallen short in accomplishing.
When in 2021, results from the EMPEROR-Preserved trial with empagliflozin and when in September 2022 results from the DELIVER trial with dapagliflozin established the efficacy of these two SGLT2 inhibitors as the first treatments proven to benefit patients with HFpEF, they also raised the stakes for clinicians to be much more diligent and systematic in evaluating people at high risk for developing HFpEF because of having type 2 diabetes or obesity, two of the most potent risk factors for this form of heart failure.
‘Vigilance ... needs to increase’
“Vigilance for HFpEF needs to increase because we can now help these patients,” declared Lars H. Lund, MD, PhD, speaking at the meeting. “Type 2 diabetes dramatically increases the incidence of HFpEF,” and the mechanisms by which it does this are “especially amenable to treatment with SGLT2 inhibitors,” said Dr. Lund, a cardiologist and heart failure specialist at the Karolinska Institute, Stockholm.
HFpEF has a history of going undetected in people with type 2 diabetes, an ironic situation given its high incidence as well as the elevated rate of adverse cardiovascular events when heart failure occurs in patients with type 2 diabetes compared with patients who do not have diabetes.
The key, say experts, is for clinicians to maintain a high index of suspicion for signs and symptoms of heart failure in people with type 2 diabetes and to regularly assess them, starting with just a few simple questions that probe for the presence of dyspnea, exertional fatigue, or both, an approach not widely employed up to now.
Clinicians who care for people with type 2 diabetes must become “alert to thinking about heart failure and alert to asking questions about signs and symptoms” that flag the presence of HFpEF, advised Naveed Sattar, MBChB, PhD, a professor of metabolic medicine at the University of Glasgow.
Soon, medical groups will issue guidelines for appropriate assessment for the presence of HFpEF in people with type 2 diabetes, Dr. Sattar predicted in an interview.
A need to probe
“You can’t simply ask patients with type 2 diabetes whether they have shortness of breath or exertional fatigue and stop there,” because often their first response will be no.
“Commonly, patients will initially say they have no dyspnea, but when you probe further, you find symptoms,” noted Mikhail N. Kosiborod, MD, codirector of Saint Luke’s Cardiometabolic Center of Excellence in Kansas City, Mo.
These people are often sedentary, so they frequently don’t experience shortness of breath at baseline, Dr. Kosiborod said in an interview. In some cases, they may limit their activity because of their exertional intolerance.
Once a person’s suggestive symptoms become known, the next step is to measure the serum level of N-terminal pro-B-type natriuretic peptide (NT-proBNP), a biomarker considered to be a generally reliable signal of existing heart failure when elevated.
Any value above 125 pg/mL is suggestive of prevalent heart failure and should lead to the next diagnostic step of echocardiography, Dr. Sattar said.
Elevated NT-proBNP has such good positive predictive value for identifying heart failure that it is tempting to use it broadly in people with type 2 diabetes. A 2022 consensus report from the American Diabetes Association says that “measurement of a natriuretic peptide [such as NT-proBNP] or high-sensitivity cardiac troponin is recommended on at least a yearly basis to identify the earliest HF [heart failure] stages and implement strategies to prevent transition to symptomatic HF.”
Test costs require targeting
But because of the relatively high current price for an NT-proBNP test, the cost-benefit ratio for widespread annual testing of all people with type 2 diabetes would be poor, some experts caution.
“Screening everyone may not be the right answer. Hundreds of millions of people worldwide” have type 2 diabetes. “You first need to target evaluation to people with symptoms,” advised Dr. Kosiborod.
He also warned that a low NT-proBNP level does not always rule out HFpEF, especially among people with type 2 diabetes who also have overweight or obesity, because NT-proBNP levels can be “artificially low” in people with obesity.
Other potential aids to diagnosis are assessment scores that researchers have developed, such as the H2FPEF score, which relies on variables that include age, obesity, and the presence of atrial fibrillation and hypertension.
However, this score also requires an echocardiography examination, another test that would have a questionable cost-benefit ratio if performed widely for patients with type 2 diabetes without targeting, Dr. Kosiborod said.
SGLT2 inhibitors benefit HFpEF regardless of glucose levels
A prespecified analysis of the DELIVER results that divided the study cohort on the basis of their glycemic status proved the efficacy of the SGLT2 inhibitor dapagliflozin for patients with HFpEF regardless of whether or not they had type 2 diabetes, prediabetes, or were normoglycemic at entry into the study, Silvio E. Inzucchi, MD, reported at the EASD meeting.
Treatment with dapagliflozin cut the incidence of the trial’s primary outcome of cardiovascular death or worsening heart failure by a significant 18% relative to placebo among all enrolled patients.
The new analysis reported by Dr. Inzucchi showed that treatment was associated with a 23% relative risk reduction among those with normoglycemia, a 13% reduction among those with prediabetes, and a 19% reduction among those with type 2 diabetes, with no signal of a significant difference among the three subgroups.
“There was no statistical interaction between categorical glycemic subgrouping and dapagliflozin’s treatment effect,” concluded Dr. Inzucchi, director of the Yale Medicine Diabetes Center, New Haven, Conn.
He also reported that, among the 6,259 people in the trial with HFpEF, 50% had diabetes, 31% had prediabetes, and a scant 19% had normoglycemia. The finding highlights once again the high prevalence of dysglycemia among people with HFpEF.
Previously, a prespecified secondary analysis of data from the EMPEROR-Preserved trial yielded similar findings for empagliflozin that showed the agent’s efficacy for people with HFpEF across the range of glucose levels.
The DELIVER trial was funded by AstraZeneca, the company that markets dapagliflozin (Farxiga). The EMPEROR-Preserved trial was sponsored by Boehringer Ingelheim and Eli Lilly, the companies that jointly market empagliflozin (Jardiance). Dr. Lund has been a consultant to AstraZeneca and Boehringer Ingelheim and to numerous other companies, and he is a stockholder in AnaCardio. Dr. Sattar has been a consultant to and has received research support from AstraZeneca and Boehringer Ingelheim, and he has been a consultant with numerous companies. Dr. Kosiborod has been a consultant to and has received research funding from AstraZeneca and Boehringer Ingelheim and has been a consultant to Eli Lilly and numerous other companies. Dr. Inzucchi has been a consultant to, given talks on behalf of, or served on trial committees for Abbott, AstraZeneca, Boehringer Ingelheim, Esperion, Lexicon, Merck, Novo Nordisk, Pfizer, and vTv Therapetics.
A version of this article first appeared on Medscape.com.
AT EASD 2022
Drug combo holds promise of better AML outcomes
Adding venetoclax (Venclexta) to a gilteritinib (Xospata) regimen appeared to improve outcomes in refractory/relapsed FLT3-mutated acute myeloid leukemia (AML), a new industry-funded phase 1b study reported.
“.
Outcomes in AML are poor. As the study notes, most patients relapse and face a median overall survival of 4-7 months even with standard chemotherapy. Gilteritinib, a selective oral FLT3 inhibitor, is Food and Drug Administration–approved for the 30% of relapsed/refractory patients with AML who have FLT3 mutations.
“The general sentiment is that, although some patients have great benefit from gilteritinib monotherapy, there is room to improve the quality, frequency, and duration of responses with combinations,” said hematologist Andrew Brunner, MD, of Massachusetts General Hospital in Boston, in an interview. He was not involved with the study research.
For the new open-label, dose-escalation/dose-expansion study, led by hematologist Naval Daver, MD, of the University of Texas MD Anderson Cancer Center, Houston, researchers enrolled 61 patients (56 with FLT3 mutations) from 2018 to 2020. The median age was 63 years (range 21-85).
The subjects were assigned to get a recommended phase 2 dose of 400 mg venetoclax once daily and 120 mg gilteritinib once daily.
Over a median follow-up of 17.5 months, the median remission time was 4.9 months (95% confidence interval, 3.4-6.6), and the patients with FLT3 mutations survived a median of 10 months.
“The combination of venetoclax and gilteritinib was tolerable at standard doses of each drug, generated remarkably high response rates, and markedly reduced FLT3-internal tandem duplications mutation burden. … Early mortality was similar to gilteritinib monotherapy,” the authors wrote.
Eighty percent of patients experienced cytopenias, and “adverse events prompted venetoclax and gilteritinib dose interruptions in 51% and 48%, respectively.”
About 60% of patients who went on to receive allogeneic hematopoietic stem cell transplantation were alive at the end of follow-up, “suggesting that VenGilt [the combo treatment] could be an effective bridge to transplant in young/fit patients with relapsed FLT3mut AML,” the researchers wrote.
All patients withdrew from the study by November 2021 for several reasons such as death (n=42), adverse events (n=10), and disease progression (29); some had multiple reasons.
Dr. Brunner said the study is “an important step toward evaluating a new potential regimen.”
The remission duration, FLT3 molecular response, and median overall survival “seem quite encouraging for a severe disease like AML in relapse,” he said. However, he added that the drug combo “would need to be evaluated in a randomized and, ideally, placebo-controlled setting to know if this is a significant improvement.”
He also highlighted the high number of severe cyptopenias with associated complications such as death. “Whether this is acceptable depends on the patient and circumstances,” he said. “But it does suggest that this regimen would potentially be for more robust patients, particularly since the group that did best were those who went to transplant later.”
Pending more research, Dr. Brunner said, “I am not sure I would use [the combination treatment] over gilteritinib monotherapy, for instance. But there may be settings where no other options are available, and this could be considered, particularly if a transplant option is a next step.”
The study was funded by AbbVie, Genentech, and Astellas. The study authors report multiple disclosures; some are employed by Astellas, AbbVie, and Genentech/Roche.
Dr. Bronner reports running clinical trials, advisory board service and/or consultation for Acceleron, Agios, Abbvie, BMS/Celgene, Keros Therapeutics, Novartis, Takeda, GSK, AstraZeneca, Janssen, and Gilead.
Adding venetoclax (Venclexta) to a gilteritinib (Xospata) regimen appeared to improve outcomes in refractory/relapsed FLT3-mutated acute myeloid leukemia (AML), a new industry-funded phase 1b study reported.
“.
Outcomes in AML are poor. As the study notes, most patients relapse and face a median overall survival of 4-7 months even with standard chemotherapy. Gilteritinib, a selective oral FLT3 inhibitor, is Food and Drug Administration–approved for the 30% of relapsed/refractory patients with AML who have FLT3 mutations.
“The general sentiment is that, although some patients have great benefit from gilteritinib monotherapy, there is room to improve the quality, frequency, and duration of responses with combinations,” said hematologist Andrew Brunner, MD, of Massachusetts General Hospital in Boston, in an interview. He was not involved with the study research.
For the new open-label, dose-escalation/dose-expansion study, led by hematologist Naval Daver, MD, of the University of Texas MD Anderson Cancer Center, Houston, researchers enrolled 61 patients (56 with FLT3 mutations) from 2018 to 2020. The median age was 63 years (range 21-85).
The subjects were assigned to get a recommended phase 2 dose of 400 mg venetoclax once daily and 120 mg gilteritinib once daily.
Over a median follow-up of 17.5 months, the median remission time was 4.9 months (95% confidence interval, 3.4-6.6), and the patients with FLT3 mutations survived a median of 10 months.
“The combination of venetoclax and gilteritinib was tolerable at standard doses of each drug, generated remarkably high response rates, and markedly reduced FLT3-internal tandem duplications mutation burden. … Early mortality was similar to gilteritinib monotherapy,” the authors wrote.
Eighty percent of patients experienced cytopenias, and “adverse events prompted venetoclax and gilteritinib dose interruptions in 51% and 48%, respectively.”
About 60% of patients who went on to receive allogeneic hematopoietic stem cell transplantation were alive at the end of follow-up, “suggesting that VenGilt [the combo treatment] could be an effective bridge to transplant in young/fit patients with relapsed FLT3mut AML,” the researchers wrote.
All patients withdrew from the study by November 2021 for several reasons such as death (n=42), adverse events (n=10), and disease progression (29); some had multiple reasons.
Dr. Brunner said the study is “an important step toward evaluating a new potential regimen.”
The remission duration, FLT3 molecular response, and median overall survival “seem quite encouraging for a severe disease like AML in relapse,” he said. However, he added that the drug combo “would need to be evaluated in a randomized and, ideally, placebo-controlled setting to know if this is a significant improvement.”
He also highlighted the high number of severe cyptopenias with associated complications such as death. “Whether this is acceptable depends on the patient and circumstances,” he said. “But it does suggest that this regimen would potentially be for more robust patients, particularly since the group that did best were those who went to transplant later.”
Pending more research, Dr. Brunner said, “I am not sure I would use [the combination treatment] over gilteritinib monotherapy, for instance. But there may be settings where no other options are available, and this could be considered, particularly if a transplant option is a next step.”
The study was funded by AbbVie, Genentech, and Astellas. The study authors report multiple disclosures; some are employed by Astellas, AbbVie, and Genentech/Roche.
Dr. Bronner reports running clinical trials, advisory board service and/or consultation for Acceleron, Agios, Abbvie, BMS/Celgene, Keros Therapeutics, Novartis, Takeda, GSK, AstraZeneca, Janssen, and Gilead.
Adding venetoclax (Venclexta) to a gilteritinib (Xospata) regimen appeared to improve outcomes in refractory/relapsed FLT3-mutated acute myeloid leukemia (AML), a new industry-funded phase 1b study reported.
“.
Outcomes in AML are poor. As the study notes, most patients relapse and face a median overall survival of 4-7 months even with standard chemotherapy. Gilteritinib, a selective oral FLT3 inhibitor, is Food and Drug Administration–approved for the 30% of relapsed/refractory patients with AML who have FLT3 mutations.
“The general sentiment is that, although some patients have great benefit from gilteritinib monotherapy, there is room to improve the quality, frequency, and duration of responses with combinations,” said hematologist Andrew Brunner, MD, of Massachusetts General Hospital in Boston, in an interview. He was not involved with the study research.
For the new open-label, dose-escalation/dose-expansion study, led by hematologist Naval Daver, MD, of the University of Texas MD Anderson Cancer Center, Houston, researchers enrolled 61 patients (56 with FLT3 mutations) from 2018 to 2020. The median age was 63 years (range 21-85).
The subjects were assigned to get a recommended phase 2 dose of 400 mg venetoclax once daily and 120 mg gilteritinib once daily.
Over a median follow-up of 17.5 months, the median remission time was 4.9 months (95% confidence interval, 3.4-6.6), and the patients with FLT3 mutations survived a median of 10 months.
“The combination of venetoclax and gilteritinib was tolerable at standard doses of each drug, generated remarkably high response rates, and markedly reduced FLT3-internal tandem duplications mutation burden. … Early mortality was similar to gilteritinib monotherapy,” the authors wrote.
Eighty percent of patients experienced cytopenias, and “adverse events prompted venetoclax and gilteritinib dose interruptions in 51% and 48%, respectively.”
About 60% of patients who went on to receive allogeneic hematopoietic stem cell transplantation were alive at the end of follow-up, “suggesting that VenGilt [the combo treatment] could be an effective bridge to transplant in young/fit patients with relapsed FLT3mut AML,” the researchers wrote.
All patients withdrew from the study by November 2021 for several reasons such as death (n=42), adverse events (n=10), and disease progression (29); some had multiple reasons.
Dr. Brunner said the study is “an important step toward evaluating a new potential regimen.”
The remission duration, FLT3 molecular response, and median overall survival “seem quite encouraging for a severe disease like AML in relapse,” he said. However, he added that the drug combo “would need to be evaluated in a randomized and, ideally, placebo-controlled setting to know if this is a significant improvement.”
He also highlighted the high number of severe cyptopenias with associated complications such as death. “Whether this is acceptable depends on the patient and circumstances,” he said. “But it does suggest that this regimen would potentially be for more robust patients, particularly since the group that did best were those who went to transplant later.”
Pending more research, Dr. Brunner said, “I am not sure I would use [the combination treatment] over gilteritinib monotherapy, for instance. But there may be settings where no other options are available, and this could be considered, particularly if a transplant option is a next step.”
The study was funded by AbbVie, Genentech, and Astellas. The study authors report multiple disclosures; some are employed by Astellas, AbbVie, and Genentech/Roche.
Dr. Bronner reports running clinical trials, advisory board service and/or consultation for Acceleron, Agios, Abbvie, BMS/Celgene, Keros Therapeutics, Novartis, Takeda, GSK, AstraZeneca, Janssen, and Gilead.
FROM JOURNAL OF CLINICAL ONCOLOGY
Once-weekly insulin promising in phase 3 trial in type 2 diabetes
STOCKHOLM – The investigational once-weekly insulin icodec (Novo Nordisk) significantly reduces A1c without increasing hypoglycemia in people with type 2 diabetes, the first phase 3 data of such an insulin formulation suggest. The data are from one of six trials in the company’s ONWARDS program.
“Once-weekly insulin may redefine diabetes management,” enthused Athena Philis-Tsimikas, MD, who presented the findings at a session during the European Association for the Study of Diabetes (EASD) 2022 Annual Meeting, which also included a summary of previously reported top-line data from other ONWARDS trials as well as phase 2 data for Lilly›s investigational once-weekly Basal Insulin Fc (BIF).
Phase 2 data for icodec were published in 2020 in the New England Journal of Medicine and in 2021 in Diabetes Care, as reported by this news organization.
The capacity for reducing the number of basal insulin injections from at least 365 to just 52 per year means that once-weekly insulin “has the potential to facilitate insulin initiation and improve treatment adherence and persistence in diabetes,” noted Dr. Philis-Tsimikas, corporate vice president of Scripps Whittier Diabetes Institute, San Diego.
Asked to comment, independent diabetes industry consultant Charles Alexander, MD, told this news organization that the new data from ONWARDS 2 of patients switching from daily to once-weekly basal insulin were reassuring with regard to hypoglycemia, at least for people with type 2 diabetes.
“For type 2, I think there’s enough data now to feel comfortable that it’s going to be good, especially for people who are on once-weekly [glucagon-like peptide-1 (GLP-1) agonists].”
However, for type 1 diabetes, the company reported top-line ONWARDS 6 data earlier this year, in which icodec was associated with significantly increased rates of hypoglycemia compared with daily degludec. “In type 1, even the basal needs are [often] changing. That kind of person would want to stay away from once-weekly insulin,” Dr. Alexander said.
And he noted, for any patient who adjusts their insulin dose frequently, “obviously, you’re not going to be able to do that with a once-weekly.”
Similar A1c reduction as daily basal without increased hypoglycemia
In ONWARDS 2, 526 adults with type 2 diabetes were randomized to switch from their current once- or twice-daily basal insulin to either once-weekly icodec or once-daily insulin degludec (Tresiba) for 26 weeks. The study was open-label, with a treat-to-glucose target of 80-130 mg/dL design.
Participants had A1c levels of 7.0%-10.0% and were also taking stable doses of other noninsulin glucose-lowering medications. Over 80% were taking metformin, a third were taking an SGLT2 inhibitor, and about a quarter each were taking a GLP-1 agonist or DPP-4 inhibitor. Those medications were continued, but sulfonylureas were discontinued in the 22% taking those at baseline.
The basal insulin used at baseline was glargine U100 for 42%, degludec for 28%, and glargine U300 for 16%, “so, a very typical presentation of patients we see in our practices today,” Dr. Philis-Tsimikas noted.
The primary endpoint, change in A1c from baseline to week 26, dropped from 8.17% to 7.20% with icodec and from 8.10% to 7.42% with degludec. The estimated treatment difference of –0.22 percentage points met the margins for both noninferiority (P < .0001) and superiority (P = .0028). Those taking icodec were significantly more likely to achieve an A1c under 7% compared with degludec, at 40.3% versus 26.5% (P = .0019).
Continuous glucose monitoring parameters during weeks 22-26 showed time in glucose range of 70-180 mg/dL (3.9-10.0 mmol/L) was 63.1% for icodec and 59.5% for degludec, which was not significantly different, Dr. Philis-Tsimikas reported.
Body weight increased by 1.4 kg (3 lb) with icodec but dropped slightly by 0.30 kg with degludec, which was significantly different (P < .001).
When asked about the body weight results, Dr. Alexander said: “It’s really hard to say. We know that insulin generally causes weight gain. A 1.4-kg weight gain over 6 months isn’t really surprising. Why there wasn’t with degludec, I don’t know.”
There was just one episode of severe hypoglycemia (requiring assistance) in the trial in the degludec group. Rates of combined severe or clinically significant hypoglycemic events (glucose < 54 mg/dL / < 3.0 mmol/L) per patient-year exposed were 0.73 for icodec versus 0.27 for degludec, which was not significantly different (P = .0782). Similar findings were seen for nocturnal hypoglycemia.
Significantly more patients achieved an A1c under 7% without significant hypoglycemia with icodec than degludec, at 36.7% versus 26.8% (P = .0223). Other adverse events were equivalent between the two groups, Dr. Philis-Tsimikas reported.
Scores on the diabetes treatment satisfaction questionnaire, which addresses convenience, flexibility, satisfaction, and willingness to recommend treatment to others, were significantly higher for icodec than degludec, at 4.22 versus 2.96 (P = .0036).
“For me, this is one of the most important outcomes,” she commented.
Benefit in type 2 diabetes, potential concern in type 1 diabetes
Top-line results from ONWARDS 1, a phase 3a 78-week trial in 984 drug-naive people with type 2 diabetes and ONWARDS 6, a 52-week trial in 583 people with type 1 diabetes, were presented earlier this year at the American Diabetes Association 81st Scientific Sessions.
In ONWARDS 1, icodec achieved noninferiority to daily insulin glargine, reducing A1c by 1.55 versus 1.35 percentage points, with superior time in range and no significant differences in hypoglycemia rates.
However, in ONWARDS 6, while noninferiority in A1c lowering compared with daily degludec was achieved, with reductions of 0.47 versus 0.51 percentage points from a baseline A1c of 7.6%, there was a significantly greater rate of severe or clinically significant hypoglycemia with icodec, at 19.93 versus 10.37 events per patient-year with degludec.
Dr. Philis-Tsimikas has reported performing research and serving as an advisor on behalf of her employer for Abbott, Bayer, Dexcom, Eli Lilly, Medtronic, Merck, Novo Nordisk, and Sanofi. All reimbursements go to her employer. Dr. Alexander has reported being a nonpaid advisor for diaTribe and a consultant for Kinexum.
A version of this article first appeared on Medscape.com.
STOCKHOLM – The investigational once-weekly insulin icodec (Novo Nordisk) significantly reduces A1c without increasing hypoglycemia in people with type 2 diabetes, the first phase 3 data of such an insulin formulation suggest. The data are from one of six trials in the company’s ONWARDS program.
“Once-weekly insulin may redefine diabetes management,” enthused Athena Philis-Tsimikas, MD, who presented the findings at a session during the European Association for the Study of Diabetes (EASD) 2022 Annual Meeting, which also included a summary of previously reported top-line data from other ONWARDS trials as well as phase 2 data for Lilly›s investigational once-weekly Basal Insulin Fc (BIF).
Phase 2 data for icodec were published in 2020 in the New England Journal of Medicine and in 2021 in Diabetes Care, as reported by this news organization.
The capacity for reducing the number of basal insulin injections from at least 365 to just 52 per year means that once-weekly insulin “has the potential to facilitate insulin initiation and improve treatment adherence and persistence in diabetes,” noted Dr. Philis-Tsimikas, corporate vice president of Scripps Whittier Diabetes Institute, San Diego.
Asked to comment, independent diabetes industry consultant Charles Alexander, MD, told this news organization that the new data from ONWARDS 2 of patients switching from daily to once-weekly basal insulin were reassuring with regard to hypoglycemia, at least for people with type 2 diabetes.
“For type 2, I think there’s enough data now to feel comfortable that it’s going to be good, especially for people who are on once-weekly [glucagon-like peptide-1 (GLP-1) agonists].”
However, for type 1 diabetes, the company reported top-line ONWARDS 6 data earlier this year, in which icodec was associated with significantly increased rates of hypoglycemia compared with daily degludec. “In type 1, even the basal needs are [often] changing. That kind of person would want to stay away from once-weekly insulin,” Dr. Alexander said.
And he noted, for any patient who adjusts their insulin dose frequently, “obviously, you’re not going to be able to do that with a once-weekly.”
Similar A1c reduction as daily basal without increased hypoglycemia
In ONWARDS 2, 526 adults with type 2 diabetes were randomized to switch from their current once- or twice-daily basal insulin to either once-weekly icodec or once-daily insulin degludec (Tresiba) for 26 weeks. The study was open-label, with a treat-to-glucose target of 80-130 mg/dL design.
Participants had A1c levels of 7.0%-10.0% and were also taking stable doses of other noninsulin glucose-lowering medications. Over 80% were taking metformin, a third were taking an SGLT2 inhibitor, and about a quarter each were taking a GLP-1 agonist or DPP-4 inhibitor. Those medications were continued, but sulfonylureas were discontinued in the 22% taking those at baseline.
The basal insulin used at baseline was glargine U100 for 42%, degludec for 28%, and glargine U300 for 16%, “so, a very typical presentation of patients we see in our practices today,” Dr. Philis-Tsimikas noted.
The primary endpoint, change in A1c from baseline to week 26, dropped from 8.17% to 7.20% with icodec and from 8.10% to 7.42% with degludec. The estimated treatment difference of –0.22 percentage points met the margins for both noninferiority (P < .0001) and superiority (P = .0028). Those taking icodec were significantly more likely to achieve an A1c under 7% compared with degludec, at 40.3% versus 26.5% (P = .0019).
Continuous glucose monitoring parameters during weeks 22-26 showed time in glucose range of 70-180 mg/dL (3.9-10.0 mmol/L) was 63.1% for icodec and 59.5% for degludec, which was not significantly different, Dr. Philis-Tsimikas reported.
Body weight increased by 1.4 kg (3 lb) with icodec but dropped slightly by 0.30 kg with degludec, which was significantly different (P < .001).
When asked about the body weight results, Dr. Alexander said: “It’s really hard to say. We know that insulin generally causes weight gain. A 1.4-kg weight gain over 6 months isn’t really surprising. Why there wasn’t with degludec, I don’t know.”
There was just one episode of severe hypoglycemia (requiring assistance) in the trial in the degludec group. Rates of combined severe or clinically significant hypoglycemic events (glucose < 54 mg/dL / < 3.0 mmol/L) per patient-year exposed were 0.73 for icodec versus 0.27 for degludec, which was not significantly different (P = .0782). Similar findings were seen for nocturnal hypoglycemia.
Significantly more patients achieved an A1c under 7% without significant hypoglycemia with icodec than degludec, at 36.7% versus 26.8% (P = .0223). Other adverse events were equivalent between the two groups, Dr. Philis-Tsimikas reported.
Scores on the diabetes treatment satisfaction questionnaire, which addresses convenience, flexibility, satisfaction, and willingness to recommend treatment to others, were significantly higher for icodec than degludec, at 4.22 versus 2.96 (P = .0036).
“For me, this is one of the most important outcomes,” she commented.
Benefit in type 2 diabetes, potential concern in type 1 diabetes
Top-line results from ONWARDS 1, a phase 3a 78-week trial in 984 drug-naive people with type 2 diabetes and ONWARDS 6, a 52-week trial in 583 people with type 1 diabetes, were presented earlier this year at the American Diabetes Association 81st Scientific Sessions.
In ONWARDS 1, icodec achieved noninferiority to daily insulin glargine, reducing A1c by 1.55 versus 1.35 percentage points, with superior time in range and no significant differences in hypoglycemia rates.
However, in ONWARDS 6, while noninferiority in A1c lowering compared with daily degludec was achieved, with reductions of 0.47 versus 0.51 percentage points from a baseline A1c of 7.6%, there was a significantly greater rate of severe or clinically significant hypoglycemia with icodec, at 19.93 versus 10.37 events per patient-year with degludec.
Dr. Philis-Tsimikas has reported performing research and serving as an advisor on behalf of her employer for Abbott, Bayer, Dexcom, Eli Lilly, Medtronic, Merck, Novo Nordisk, and Sanofi. All reimbursements go to her employer. Dr. Alexander has reported being a nonpaid advisor for diaTribe and a consultant for Kinexum.
A version of this article first appeared on Medscape.com.
STOCKHOLM – The investigational once-weekly insulin icodec (Novo Nordisk) significantly reduces A1c without increasing hypoglycemia in people with type 2 diabetes, the first phase 3 data of such an insulin formulation suggest. The data are from one of six trials in the company’s ONWARDS program.
“Once-weekly insulin may redefine diabetes management,” enthused Athena Philis-Tsimikas, MD, who presented the findings at a session during the European Association for the Study of Diabetes (EASD) 2022 Annual Meeting, which also included a summary of previously reported top-line data from other ONWARDS trials as well as phase 2 data for Lilly›s investigational once-weekly Basal Insulin Fc (BIF).
Phase 2 data for icodec were published in 2020 in the New England Journal of Medicine and in 2021 in Diabetes Care, as reported by this news organization.
The capacity for reducing the number of basal insulin injections from at least 365 to just 52 per year means that once-weekly insulin “has the potential to facilitate insulin initiation and improve treatment adherence and persistence in diabetes,” noted Dr. Philis-Tsimikas, corporate vice president of Scripps Whittier Diabetes Institute, San Diego.
Asked to comment, independent diabetes industry consultant Charles Alexander, MD, told this news organization that the new data from ONWARDS 2 of patients switching from daily to once-weekly basal insulin were reassuring with regard to hypoglycemia, at least for people with type 2 diabetes.
“For type 2, I think there’s enough data now to feel comfortable that it’s going to be good, especially for people who are on once-weekly [glucagon-like peptide-1 (GLP-1) agonists].”
However, for type 1 diabetes, the company reported top-line ONWARDS 6 data earlier this year, in which icodec was associated with significantly increased rates of hypoglycemia compared with daily degludec. “In type 1, even the basal needs are [often] changing. That kind of person would want to stay away from once-weekly insulin,” Dr. Alexander said.
And he noted, for any patient who adjusts their insulin dose frequently, “obviously, you’re not going to be able to do that with a once-weekly.”
Similar A1c reduction as daily basal without increased hypoglycemia
In ONWARDS 2, 526 adults with type 2 diabetes were randomized to switch from their current once- or twice-daily basal insulin to either once-weekly icodec or once-daily insulin degludec (Tresiba) for 26 weeks. The study was open-label, with a treat-to-glucose target of 80-130 mg/dL design.
Participants had A1c levels of 7.0%-10.0% and were also taking stable doses of other noninsulin glucose-lowering medications. Over 80% were taking metformin, a third were taking an SGLT2 inhibitor, and about a quarter each were taking a GLP-1 agonist or DPP-4 inhibitor. Those medications were continued, but sulfonylureas were discontinued in the 22% taking those at baseline.
The basal insulin used at baseline was glargine U100 for 42%, degludec for 28%, and glargine U300 for 16%, “so, a very typical presentation of patients we see in our practices today,” Dr. Philis-Tsimikas noted.
The primary endpoint, change in A1c from baseline to week 26, dropped from 8.17% to 7.20% with icodec and from 8.10% to 7.42% with degludec. The estimated treatment difference of –0.22 percentage points met the margins for both noninferiority (P < .0001) and superiority (P = .0028). Those taking icodec were significantly more likely to achieve an A1c under 7% compared with degludec, at 40.3% versus 26.5% (P = .0019).
Continuous glucose monitoring parameters during weeks 22-26 showed time in glucose range of 70-180 mg/dL (3.9-10.0 mmol/L) was 63.1% for icodec and 59.5% for degludec, which was not significantly different, Dr. Philis-Tsimikas reported.
Body weight increased by 1.4 kg (3 lb) with icodec but dropped slightly by 0.30 kg with degludec, which was significantly different (P < .001).
When asked about the body weight results, Dr. Alexander said: “It’s really hard to say. We know that insulin generally causes weight gain. A 1.4-kg weight gain over 6 months isn’t really surprising. Why there wasn’t with degludec, I don’t know.”
There was just one episode of severe hypoglycemia (requiring assistance) in the trial in the degludec group. Rates of combined severe or clinically significant hypoglycemic events (glucose < 54 mg/dL / < 3.0 mmol/L) per patient-year exposed were 0.73 for icodec versus 0.27 for degludec, which was not significantly different (P = .0782). Similar findings were seen for nocturnal hypoglycemia.
Significantly more patients achieved an A1c under 7% without significant hypoglycemia with icodec than degludec, at 36.7% versus 26.8% (P = .0223). Other adverse events were equivalent between the two groups, Dr. Philis-Tsimikas reported.
Scores on the diabetes treatment satisfaction questionnaire, which addresses convenience, flexibility, satisfaction, and willingness to recommend treatment to others, were significantly higher for icodec than degludec, at 4.22 versus 2.96 (P = .0036).
“For me, this is one of the most important outcomes,” she commented.
Benefit in type 2 diabetes, potential concern in type 1 diabetes
Top-line results from ONWARDS 1, a phase 3a 78-week trial in 984 drug-naive people with type 2 diabetes and ONWARDS 6, a 52-week trial in 583 people with type 1 diabetes, were presented earlier this year at the American Diabetes Association 81st Scientific Sessions.
In ONWARDS 1, icodec achieved noninferiority to daily insulin glargine, reducing A1c by 1.55 versus 1.35 percentage points, with superior time in range and no significant differences in hypoglycemia rates.
However, in ONWARDS 6, while noninferiority in A1c lowering compared with daily degludec was achieved, with reductions of 0.47 versus 0.51 percentage points from a baseline A1c of 7.6%, there was a significantly greater rate of severe or clinically significant hypoglycemia with icodec, at 19.93 versus 10.37 events per patient-year with degludec.
Dr. Philis-Tsimikas has reported performing research and serving as an advisor on behalf of her employer for Abbott, Bayer, Dexcom, Eli Lilly, Medtronic, Merck, Novo Nordisk, and Sanofi. All reimbursements go to her employer. Dr. Alexander has reported being a nonpaid advisor for diaTribe and a consultant for Kinexum.
A version of this article first appeared on Medscape.com.
AT EASD 2022
Cost paramount when choosing metastatic breast cancer treatment
While efficacy and quality of life outcomes are similar across commonly used treatments for endocrine-refractory or triple-negative metastatic breast cancer, the costs of these agents vary widely, a recent analysis reveals.
Notably, the authors found that
Given “razor thin” differences in outcomes, cost should become a major consideration, the researchers concluded.
“As a society, we urgently need more strategies to reduce cancer drug costs without compromising outcomes, and our analysis provides quantifiable evidence to help providers choose lower priced, but equally effective sequences of drugs,” first author Stephanie B. Wheeler, PhD, from the University of North Carolina at Chapel Hill, explained in a press release.
Although the drugs Dr. Wheeler and colleagues studied are reimbursed in the metastatic breast cancer setting, “the optimal sequencing of them has been unclear, which has led to considerable variation in physician preference and practice,” Dr. Wheeler said.
In the study, published in the Journal of Clinical Oncology, Dr. Wheeler and colleagues estimated the cost-effectiveness of different therapeutic options from the first- to third-line setting for this patient population.
The researchers used three dynamic microsimulation computer models to predict how hypothetical sets of 10,000 patients with specific types of metastatic breast cancer would respond to various therapy types and sequences. The cohorts were grouped according to prior chemotherapy exposure: cohort 1 had no taxane or anthracycline exposure, cohort 2 had taxane and anthracycline exposure, and cohort 3 had taxane exposure but was anthracycline naive.
On the basis of feedback from oncologists, the investigators focused on different agents in the three cohorts: paclitaxel, capecitabine, or pegylated liposomal doxorubicin for cohort 1; eribulin, capecitabine, or carboplatin for cohort 2; and pegylated liposomal doxorubicin, capecitabine, or eribulin for cohort 3.
Overall, the models showed “nearly indistinguishable differences” in quality of life. In fact, the “razor-thin incremental differences in quality-adjusted survival” across the treatment sequences often amounted to differences of only a few days or weeks, the authors noted, adding that, even in the most extreme of cases, 3 weeks separated the best and worst options for quality-adjusted life-years.
But the models did show considerable differences in costs.
The authors found that, for cohort 1, treatment with paclitaxel followed by capecitabine and then pegylated liposomal doxorubicin corresponded to the highest expected quality-adjusted life-year gain and the lowest costs – $686 per month versus the highest cost option of $1,765.
For cohort 2, treatment with carboplatin followed by capecitabine and then eribulin corresponded to the highest expected quality-adjusted life-year gain and lowest costs.
For cohort 3, treatment sequences beginning with capecitabine or pegylated liposomal doxorubicin followed by eribulin was most cost effective.
Notably, the authors found that eribulin – the most expensive treatment with a high expected adverse event burden – performed particularly poorly in the two cohorts in which it was evaluated, “suggesting it should be used last in a sequence, on the basis of cost-effectiveness alone.”
In other words, “more spending on cancer care does not necessarily confer greater health benefits,” said Dr. Wheeler, also a professor of health policy.
“I hope our study will help expand the framework that we use to make these decisions from one where we just think about the biologic action of the drug to one where we also consider the bigger picture of what the treatment experience is like for the patient, including their financial burden, investment of time, and side effects,” study coauthor Katherine E. Reeder-Hayes, MD, section chief of breast oncology at UNC, said in the press release.
The results demonstrate that therapeutic decisions in the endocrine-refractory or triple-negative metastatic setting “may prioritize costs without affecting clinical outcomes” and highlight the direct impact that a “high-quality, transparent, and accessible economic analysis” can have on patient care, Scott D. Ramsey, MD, PhD, of Fred Hutchinson Cancer Research Center, Seattle, and colleagues wrote in an accompanying editorial.
Following the treatment sequences outlined in this study would “reduce patient financial burden and save our health system hundreds of millions of dollars annually,” the editorialists wrote.
As for next steps, Dr. Wheeler and colleagues have developed a financial navigation program to help patients manage their out-of-pocket cancer care costs and are currently scaling up the intervention in nine rural and nonrural oncology practices across North Carolina.
The study was supported by the Center for Disease Control and Prevention through the Prevention Research Centers Program. Dr. Wheeler has received research funding and payment for travel, accommodations, and expenses from Pfizer. Dr. Ramsey has had consulting or advisory roles and has received research funding and/or payment for travel, accommodations, and expenses from Bayer, Genentech, AstraZeneca, Merck, GRAIL, Seattle Genetics, Biovica, and/or Flatiron Health. Because of their editorial roles at the journal, the Journal of Clinical Oncology recused Dr. Wheeler and Dr. Ramsey from having any role in the peer review of their respective manuscripts.
A version of this article first appeared on Medscape.com.
While efficacy and quality of life outcomes are similar across commonly used treatments for endocrine-refractory or triple-negative metastatic breast cancer, the costs of these agents vary widely, a recent analysis reveals.
Notably, the authors found that
Given “razor thin” differences in outcomes, cost should become a major consideration, the researchers concluded.
“As a society, we urgently need more strategies to reduce cancer drug costs without compromising outcomes, and our analysis provides quantifiable evidence to help providers choose lower priced, but equally effective sequences of drugs,” first author Stephanie B. Wheeler, PhD, from the University of North Carolina at Chapel Hill, explained in a press release.
Although the drugs Dr. Wheeler and colleagues studied are reimbursed in the metastatic breast cancer setting, “the optimal sequencing of them has been unclear, which has led to considerable variation in physician preference and practice,” Dr. Wheeler said.
In the study, published in the Journal of Clinical Oncology, Dr. Wheeler and colleagues estimated the cost-effectiveness of different therapeutic options from the first- to third-line setting for this patient population.
The researchers used three dynamic microsimulation computer models to predict how hypothetical sets of 10,000 patients with specific types of metastatic breast cancer would respond to various therapy types and sequences. The cohorts were grouped according to prior chemotherapy exposure: cohort 1 had no taxane or anthracycline exposure, cohort 2 had taxane and anthracycline exposure, and cohort 3 had taxane exposure but was anthracycline naive.
On the basis of feedback from oncologists, the investigators focused on different agents in the three cohorts: paclitaxel, capecitabine, or pegylated liposomal doxorubicin for cohort 1; eribulin, capecitabine, or carboplatin for cohort 2; and pegylated liposomal doxorubicin, capecitabine, or eribulin for cohort 3.
Overall, the models showed “nearly indistinguishable differences” in quality of life. In fact, the “razor-thin incremental differences in quality-adjusted survival” across the treatment sequences often amounted to differences of only a few days or weeks, the authors noted, adding that, even in the most extreme of cases, 3 weeks separated the best and worst options for quality-adjusted life-years.
But the models did show considerable differences in costs.
The authors found that, for cohort 1, treatment with paclitaxel followed by capecitabine and then pegylated liposomal doxorubicin corresponded to the highest expected quality-adjusted life-year gain and the lowest costs – $686 per month versus the highest cost option of $1,765.
For cohort 2, treatment with carboplatin followed by capecitabine and then eribulin corresponded to the highest expected quality-adjusted life-year gain and lowest costs.
For cohort 3, treatment sequences beginning with capecitabine or pegylated liposomal doxorubicin followed by eribulin was most cost effective.
Notably, the authors found that eribulin – the most expensive treatment with a high expected adverse event burden – performed particularly poorly in the two cohorts in which it was evaluated, “suggesting it should be used last in a sequence, on the basis of cost-effectiveness alone.”
In other words, “more spending on cancer care does not necessarily confer greater health benefits,” said Dr. Wheeler, also a professor of health policy.
“I hope our study will help expand the framework that we use to make these decisions from one where we just think about the biologic action of the drug to one where we also consider the bigger picture of what the treatment experience is like for the patient, including their financial burden, investment of time, and side effects,” study coauthor Katherine E. Reeder-Hayes, MD, section chief of breast oncology at UNC, said in the press release.
The results demonstrate that therapeutic decisions in the endocrine-refractory or triple-negative metastatic setting “may prioritize costs without affecting clinical outcomes” and highlight the direct impact that a “high-quality, transparent, and accessible economic analysis” can have on patient care, Scott D. Ramsey, MD, PhD, of Fred Hutchinson Cancer Research Center, Seattle, and colleagues wrote in an accompanying editorial.
Following the treatment sequences outlined in this study would “reduce patient financial burden and save our health system hundreds of millions of dollars annually,” the editorialists wrote.
As for next steps, Dr. Wheeler and colleagues have developed a financial navigation program to help patients manage their out-of-pocket cancer care costs and are currently scaling up the intervention in nine rural and nonrural oncology practices across North Carolina.
The study was supported by the Center for Disease Control and Prevention through the Prevention Research Centers Program. Dr. Wheeler has received research funding and payment for travel, accommodations, and expenses from Pfizer. Dr. Ramsey has had consulting or advisory roles and has received research funding and/or payment for travel, accommodations, and expenses from Bayer, Genentech, AstraZeneca, Merck, GRAIL, Seattle Genetics, Biovica, and/or Flatiron Health. Because of their editorial roles at the journal, the Journal of Clinical Oncology recused Dr. Wheeler and Dr. Ramsey from having any role in the peer review of their respective manuscripts.
A version of this article first appeared on Medscape.com.
While efficacy and quality of life outcomes are similar across commonly used treatments for endocrine-refractory or triple-negative metastatic breast cancer, the costs of these agents vary widely, a recent analysis reveals.
Notably, the authors found that
Given “razor thin” differences in outcomes, cost should become a major consideration, the researchers concluded.
“As a society, we urgently need more strategies to reduce cancer drug costs without compromising outcomes, and our analysis provides quantifiable evidence to help providers choose lower priced, but equally effective sequences of drugs,” first author Stephanie B. Wheeler, PhD, from the University of North Carolina at Chapel Hill, explained in a press release.
Although the drugs Dr. Wheeler and colleagues studied are reimbursed in the metastatic breast cancer setting, “the optimal sequencing of them has been unclear, which has led to considerable variation in physician preference and practice,” Dr. Wheeler said.
In the study, published in the Journal of Clinical Oncology, Dr. Wheeler and colleagues estimated the cost-effectiveness of different therapeutic options from the first- to third-line setting for this patient population.
The researchers used three dynamic microsimulation computer models to predict how hypothetical sets of 10,000 patients with specific types of metastatic breast cancer would respond to various therapy types and sequences. The cohorts were grouped according to prior chemotherapy exposure: cohort 1 had no taxane or anthracycline exposure, cohort 2 had taxane and anthracycline exposure, and cohort 3 had taxane exposure but was anthracycline naive.
On the basis of feedback from oncologists, the investigators focused on different agents in the three cohorts: paclitaxel, capecitabine, or pegylated liposomal doxorubicin for cohort 1; eribulin, capecitabine, or carboplatin for cohort 2; and pegylated liposomal doxorubicin, capecitabine, or eribulin for cohort 3.
Overall, the models showed “nearly indistinguishable differences” in quality of life. In fact, the “razor-thin incremental differences in quality-adjusted survival” across the treatment sequences often amounted to differences of only a few days or weeks, the authors noted, adding that, even in the most extreme of cases, 3 weeks separated the best and worst options for quality-adjusted life-years.
But the models did show considerable differences in costs.
The authors found that, for cohort 1, treatment with paclitaxel followed by capecitabine and then pegylated liposomal doxorubicin corresponded to the highest expected quality-adjusted life-year gain and the lowest costs – $686 per month versus the highest cost option of $1,765.
For cohort 2, treatment with carboplatin followed by capecitabine and then eribulin corresponded to the highest expected quality-adjusted life-year gain and lowest costs.
For cohort 3, treatment sequences beginning with capecitabine or pegylated liposomal doxorubicin followed by eribulin was most cost effective.
Notably, the authors found that eribulin – the most expensive treatment with a high expected adverse event burden – performed particularly poorly in the two cohorts in which it was evaluated, “suggesting it should be used last in a sequence, on the basis of cost-effectiveness alone.”
In other words, “more spending on cancer care does not necessarily confer greater health benefits,” said Dr. Wheeler, also a professor of health policy.
“I hope our study will help expand the framework that we use to make these decisions from one where we just think about the biologic action of the drug to one where we also consider the bigger picture of what the treatment experience is like for the patient, including their financial burden, investment of time, and side effects,” study coauthor Katherine E. Reeder-Hayes, MD, section chief of breast oncology at UNC, said in the press release.
The results demonstrate that therapeutic decisions in the endocrine-refractory or triple-negative metastatic setting “may prioritize costs without affecting clinical outcomes” and highlight the direct impact that a “high-quality, transparent, and accessible economic analysis” can have on patient care, Scott D. Ramsey, MD, PhD, of Fred Hutchinson Cancer Research Center, Seattle, and colleagues wrote in an accompanying editorial.
Following the treatment sequences outlined in this study would “reduce patient financial burden and save our health system hundreds of millions of dollars annually,” the editorialists wrote.
As for next steps, Dr. Wheeler and colleagues have developed a financial navigation program to help patients manage their out-of-pocket cancer care costs and are currently scaling up the intervention in nine rural and nonrural oncology practices across North Carolina.
The study was supported by the Center for Disease Control and Prevention through the Prevention Research Centers Program. Dr. Wheeler has received research funding and payment for travel, accommodations, and expenses from Pfizer. Dr. Ramsey has had consulting or advisory roles and has received research funding and/or payment for travel, accommodations, and expenses from Bayer, Genentech, AstraZeneca, Merck, GRAIL, Seattle Genetics, Biovica, and/or Flatiron Health. Because of their editorial roles at the journal, the Journal of Clinical Oncology recused Dr. Wheeler and Dr. Ramsey from having any role in the peer review of their respective manuscripts.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
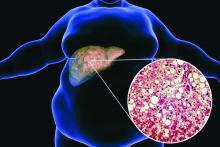
Ezetimibe-statin combo lowers liver fat in open-label trial
Ezetimibe given in combination with rosuvastatin has a beneficial effect on liver fat in people with nonalcoholic fatty liver disease (NAFLD), according results of a randomized, active-controlled trial.
The findings, which come from the investigator-initiated ESSENTIAL trial, are likely to add to the debate over whether or not the lipid-lowering combination could be of benefit beyond its effects in the blood.
“We used magnetic resonance imaging-derived proton density fat fraction [MRI-PDFF], which is highly reliable method of assessing hepatic steatosis,” Youngjoon Kim, PhD, one of the study investigators, said at the annual meeting of the European Association for the Study of Diabetes in Barcelona.
“It enables accurate, repeatable and reproducible quantitative assessment of liver fat over the entire liver,” observed Dr. Kim, who works at Severance Hospital, part of Yonsei University in Seoul.
He reported that there was a significant 5.8% decrease in liver fat following 24 weeks’ treatment with ezetimibe and rosuvastatin comparing baseline with end of treatment MRI-PDFF values; a drop that was significant (18.2% vs. 12.3%, P < .001).
Rosuvastatin monotherapy also reduced liver fat from 15.0% at baseline to 12.4% after 24 weeks; this drop of 2.6% was also significant (P = .003).
This gave an absolute mean difference between the two study arms of 3.2% (P = .02).
Rationale for the ESSENTIAL study
Dr. Kim observed during his presentation that NAFLD is burgeoning problem around the world. Ezetimibe plus rosuvastatin was a combination treatment already used widely in clinical practice, and there had been some suggestion that ezetimibe might have an effect on liver fat.
“Although the effect of ezetimibe on hepatic steatosis is still controversial, ezetimibe has been reported to reduce visceral fat and improve insulin resistance in several studies” Dr. Kim said.
“Recently, our group reported that the use of ezetimibe affects autophagy of hepatocytes and the NLRP3 [NOD-like receptors containing pyrin domain 3] inflammasome,” he said.
Moreover, he added, “ezetimibe improved NASH [nonalcoholic steatohepatitis] in an animal model. However, the effects of ezetimibe have not been clearly shown in a human study.”
Dr. Kim also acknowledged a prior randomized control trial that had looked at the role of ezetimibe in 50 patients with NASH, but had not shown a benefit for the drug over placebo in terms of liver fat reduction.
Addressing the Hawthorne effect
“The size of the effect by that might actually be more modest due to the Hawthorne effect,” said session chair Onno Holleboom, MD, PhD, of Amsterdam UMC in the Netherlands.
“What we observe in the large clinical trials is an enormous Hawthorne effect – participating in a NAFLD trial makes people live healthier because they have health checks,” he said.
“That’s a major problem for showing efficacy for the intervention arm,” he added, but of course the open design meant that the trial only had intervention arms; “there was no placebo arm.”
A randomized, active-controlled, clinician-initiated trial
The main objective of the ESSENTIAL trial was therefore to take another look at the potential effect of ezetimibe on hepatic steatosis and doing so in the setting of statin therapy.
In all, 70 patients with NAFLD that had been confirmed via ultrasound were recruited into the prospective, single center, phase 4 trial. Participants were randomized 1:1 to received either ezetimibe 10 mg plus rosuvastatin 5 mg daily or rosuvastatin 5 mg for up to 24 weeks.
Change in liver fat was measured via MRI-PDFF, taking the average values in each of nine liver segments. Magnetic resonance elastography (MRE) was also used to measure liver fibrosis, although results did not show any differences either from baseline to end of treatment values in either group or when the two treatment groups were compared.
Dr. Kim reported that both treatment with the ezetimibe-rosuvastatin combination and rosuvastatin monotherapy reduced parameters that might be associated with a negative outcome in NAFLD, such as body mass index and waist circumference, triglycerides, and LDL cholesterol. There was also a reduction in C-reactive protein levels in the blood, and interleulin-18. There was no change in liver enzymes.
Several subgroup analyses were performed indicating that “individuals with higher BMI, type 2 diabetes, insulin resistance, and severe liver fibrosis were likely to be good responders to ezetimibe treatment,” Dr. Kim said.
“These data indicate that ezetimibe plus rosuvastatin is a safe and effective therapeutic option to treat patients with NAFLD and dyslipidemia,” he concluded.
The results of the ESSENTIAL study have been published in BMC Medicine.
The study was funded by the Yuhan Corporation. Dr. Kim had no conflicts of interest to report. Dr. Holleboom was not involved in the study and had no conflicts of interest.
Ezetimibe given in combination with rosuvastatin has a beneficial effect on liver fat in people with nonalcoholic fatty liver disease (NAFLD), according results of a randomized, active-controlled trial.
The findings, which come from the investigator-initiated ESSENTIAL trial, are likely to add to the debate over whether or not the lipid-lowering combination could be of benefit beyond its effects in the blood.
“We used magnetic resonance imaging-derived proton density fat fraction [MRI-PDFF], which is highly reliable method of assessing hepatic steatosis,” Youngjoon Kim, PhD, one of the study investigators, said at the annual meeting of the European Association for the Study of Diabetes in Barcelona.
“It enables accurate, repeatable and reproducible quantitative assessment of liver fat over the entire liver,” observed Dr. Kim, who works at Severance Hospital, part of Yonsei University in Seoul.
He reported that there was a significant 5.8% decrease in liver fat following 24 weeks’ treatment with ezetimibe and rosuvastatin comparing baseline with end of treatment MRI-PDFF values; a drop that was significant (18.2% vs. 12.3%, P < .001).
Rosuvastatin monotherapy also reduced liver fat from 15.0% at baseline to 12.4% after 24 weeks; this drop of 2.6% was also significant (P = .003).
This gave an absolute mean difference between the two study arms of 3.2% (P = .02).
Rationale for the ESSENTIAL study
Dr. Kim observed during his presentation that NAFLD is burgeoning problem around the world. Ezetimibe plus rosuvastatin was a combination treatment already used widely in clinical practice, and there had been some suggestion that ezetimibe might have an effect on liver fat.
“Although the effect of ezetimibe on hepatic steatosis is still controversial, ezetimibe has been reported to reduce visceral fat and improve insulin resistance in several studies” Dr. Kim said.
“Recently, our group reported that the use of ezetimibe affects autophagy of hepatocytes and the NLRP3 [NOD-like receptors containing pyrin domain 3] inflammasome,” he said.
Moreover, he added, “ezetimibe improved NASH [nonalcoholic steatohepatitis] in an animal model. However, the effects of ezetimibe have not been clearly shown in a human study.”
Dr. Kim also acknowledged a prior randomized control trial that had looked at the role of ezetimibe in 50 patients with NASH, but had not shown a benefit for the drug over placebo in terms of liver fat reduction.
Addressing the Hawthorne effect
“The size of the effect by that might actually be more modest due to the Hawthorne effect,” said session chair Onno Holleboom, MD, PhD, of Amsterdam UMC in the Netherlands.
“What we observe in the large clinical trials is an enormous Hawthorne effect – participating in a NAFLD trial makes people live healthier because they have health checks,” he said.
“That’s a major problem for showing efficacy for the intervention arm,” he added, but of course the open design meant that the trial only had intervention arms; “there was no placebo arm.”
A randomized, active-controlled, clinician-initiated trial
The main objective of the ESSENTIAL trial was therefore to take another look at the potential effect of ezetimibe on hepatic steatosis and doing so in the setting of statin therapy.
In all, 70 patients with NAFLD that had been confirmed via ultrasound were recruited into the prospective, single center, phase 4 trial. Participants were randomized 1:1 to received either ezetimibe 10 mg plus rosuvastatin 5 mg daily or rosuvastatin 5 mg for up to 24 weeks.
Change in liver fat was measured via MRI-PDFF, taking the average values in each of nine liver segments. Magnetic resonance elastography (MRE) was also used to measure liver fibrosis, although results did not show any differences either from baseline to end of treatment values in either group or when the two treatment groups were compared.
Dr. Kim reported that both treatment with the ezetimibe-rosuvastatin combination and rosuvastatin monotherapy reduced parameters that might be associated with a negative outcome in NAFLD, such as body mass index and waist circumference, triglycerides, and LDL cholesterol. There was also a reduction in C-reactive protein levels in the blood, and interleulin-18. There was no change in liver enzymes.
Several subgroup analyses were performed indicating that “individuals with higher BMI, type 2 diabetes, insulin resistance, and severe liver fibrosis were likely to be good responders to ezetimibe treatment,” Dr. Kim said.
“These data indicate that ezetimibe plus rosuvastatin is a safe and effective therapeutic option to treat patients with NAFLD and dyslipidemia,” he concluded.
The results of the ESSENTIAL study have been published in BMC Medicine.
The study was funded by the Yuhan Corporation. Dr. Kim had no conflicts of interest to report. Dr. Holleboom was not involved in the study and had no conflicts of interest.
Ezetimibe given in combination with rosuvastatin has a beneficial effect on liver fat in people with nonalcoholic fatty liver disease (NAFLD), according results of a randomized, active-controlled trial.
The findings, which come from the investigator-initiated ESSENTIAL trial, are likely to add to the debate over whether or not the lipid-lowering combination could be of benefit beyond its effects in the blood.
“We used magnetic resonance imaging-derived proton density fat fraction [MRI-PDFF], which is highly reliable method of assessing hepatic steatosis,” Youngjoon Kim, PhD, one of the study investigators, said at the annual meeting of the European Association for the Study of Diabetes in Barcelona.
“It enables accurate, repeatable and reproducible quantitative assessment of liver fat over the entire liver,” observed Dr. Kim, who works at Severance Hospital, part of Yonsei University in Seoul.
He reported that there was a significant 5.8% decrease in liver fat following 24 weeks’ treatment with ezetimibe and rosuvastatin comparing baseline with end of treatment MRI-PDFF values; a drop that was significant (18.2% vs. 12.3%, P < .001).
Rosuvastatin monotherapy also reduced liver fat from 15.0% at baseline to 12.4% after 24 weeks; this drop of 2.6% was also significant (P = .003).
This gave an absolute mean difference between the two study arms of 3.2% (P = .02).
Rationale for the ESSENTIAL study
Dr. Kim observed during his presentation that NAFLD is burgeoning problem around the world. Ezetimibe plus rosuvastatin was a combination treatment already used widely in clinical practice, and there had been some suggestion that ezetimibe might have an effect on liver fat.
“Although the effect of ezetimibe on hepatic steatosis is still controversial, ezetimibe has been reported to reduce visceral fat and improve insulin resistance in several studies” Dr. Kim said.
“Recently, our group reported that the use of ezetimibe affects autophagy of hepatocytes and the NLRP3 [NOD-like receptors containing pyrin domain 3] inflammasome,” he said.
Moreover, he added, “ezetimibe improved NASH [nonalcoholic steatohepatitis] in an animal model. However, the effects of ezetimibe have not been clearly shown in a human study.”
Dr. Kim also acknowledged a prior randomized control trial that had looked at the role of ezetimibe in 50 patients with NASH, but had not shown a benefit for the drug over placebo in terms of liver fat reduction.
Addressing the Hawthorne effect
“The size of the effect by that might actually be more modest due to the Hawthorne effect,” said session chair Onno Holleboom, MD, PhD, of Amsterdam UMC in the Netherlands.
“What we observe in the large clinical trials is an enormous Hawthorne effect – participating in a NAFLD trial makes people live healthier because they have health checks,” he said.
“That’s a major problem for showing efficacy for the intervention arm,” he added, but of course the open design meant that the trial only had intervention arms; “there was no placebo arm.”
A randomized, active-controlled, clinician-initiated trial
The main objective of the ESSENTIAL trial was therefore to take another look at the potential effect of ezetimibe on hepatic steatosis and doing so in the setting of statin therapy.
In all, 70 patients with NAFLD that had been confirmed via ultrasound were recruited into the prospective, single center, phase 4 trial. Participants were randomized 1:1 to received either ezetimibe 10 mg plus rosuvastatin 5 mg daily or rosuvastatin 5 mg for up to 24 weeks.
Change in liver fat was measured via MRI-PDFF, taking the average values in each of nine liver segments. Magnetic resonance elastography (MRE) was also used to measure liver fibrosis, although results did not show any differences either from baseline to end of treatment values in either group or when the two treatment groups were compared.
Dr. Kim reported that both treatment with the ezetimibe-rosuvastatin combination and rosuvastatin monotherapy reduced parameters that might be associated with a negative outcome in NAFLD, such as body mass index and waist circumference, triglycerides, and LDL cholesterol. There was also a reduction in C-reactive protein levels in the blood, and interleulin-18. There was no change in liver enzymes.
Several subgroup analyses were performed indicating that “individuals with higher BMI, type 2 diabetes, insulin resistance, and severe liver fibrosis were likely to be good responders to ezetimibe treatment,” Dr. Kim said.
“These data indicate that ezetimibe plus rosuvastatin is a safe and effective therapeutic option to treat patients with NAFLD and dyslipidemia,” he concluded.
The results of the ESSENTIAL study have been published in BMC Medicine.
The study was funded by the Yuhan Corporation. Dr. Kim had no conflicts of interest to report. Dr. Holleboom was not involved in the study and had no conflicts of interest.
FROM EASD 2022
Meet our newest genetically engineered frenemy, herpes
Herpes to the rescue
Let’s face it: When people hear the word “herpes,” their first thoughts are not positive. But what if herpes could be a hero?
Scientists have found a way to make a strain of herpes that kills cancer because, hey, it’s 2022, and anything is possible. Trials have been going well and this seems like a safe and effective way to fight cancer.
Viruses may be one of our oldest enemies, but it’s also been said that the enemy of my enemy is my friend. So why not make herpes the enemy of cancer, thereby turning it into our friend? The genetically modified herpes virus is injected directly into tumors, where it destroys cancer cells from within. But wait, there’s more! The patient’s immune system also senses the virus and springs into action against it and the cancer in which it is residing.
During the phase 1 trial, three of the nine patients saw tumor reduction and the therapy proved safe as well. Future trials will be able to more specifically target various cancer types and make the treatment better. For once, we are rooting for you, herpes.
A breath of not-so-fresh air
There’s nothing quite like that first real warm day of spring. You can finally open the windows and clear out the old stuffy air that’s been hanging around all winter long. It’s a ritual that’s now backed up with some science in the form of a new study. Turns out that there’s actually a fair amount of smog in the average home. That’s right, smog’s not just for the big city anymore.
As part of the HOMEChem project, a whole host of scientists gathered together under one roof in a typical suburban house and immediately started doing chores. Cooking, cleaning, the works. No, it wasn’t because they had trashed the place the night before. They had set up instrumentation all around the house to measure the chemical makeup of the air inside. A scientist’s idea of a wild party.
The results are perhaps not all that surprising, but interesting nonetheless. Your homemade smog certainly won’t kill you, but there’s both an increased amount and higher concentration of airborne toxins in indoor air, compared with outdoors. Benzene and formaldehyde were common, as were acrolein (a pulmonary toxicant emitted by lumber and burning fats) and isocyanic acid (which can react with proteins in the human body). The researchers noted that most of these chemicals can be removed with proper ventilation.
Although cleaning is certainly responsible for a fair share of the chemicals, cooking generally produced more toxic compounds, similar to what’s found in wildfire smoke. One of the researchers said this makes sense, since a wildfire can be considered an “extreme form of cooking.” Scientists may not know how to party, but their idea of a barbecue sounds … interesting. We’re looking forward to an upcoming study out of California: Can a 1-million acre wildfire adequately cook a ribeye steak?
We’re dying to try composting ... with humans, that is
We here at LOTME are not really fans of politicians, except as objects of ridicule. That is kind of fun. Whether we’re watching Fox News, listening to NPR, or reading Vladimir Putin’s fashion blog, one thing remains clear: If you want actual information, don’t ask a politician.
There are, of course, always exceptions, and we just found one: California state representative Cristina Garcia. Rep. Garcia sponsored a bill just signed into law by Gov. Gavin Newsom that legalizes the practice of human composting, the reduction of remains by “placing bodies in individual vessels and fostering gentle transformation into a nutrient-dense soil.”
Since we’ve written about this sort of thing before – Washington was the first state to legalize the process back in 2019 – we’re more interested now in what Rep. Garcia told NBC News while describing her motivation: “I’ve always wanted to be a tree. The idea of having my family sitting under my shade one day – that brings a lot of joy.” How great is that? Tree-hugging is just not enough. Be the tree.
California is the fifth state to provide its residents with the human composting option, the other three being Colorado, Oregon, and Vermont. The process “typically involves putting a body into a steel vessel, then covering it with organic materials like straw, wood chips and alfalfa. Microbes break down the corpse and the plant matter, transforming the various components into nutrient-rich soil in roughly 30 days,” Smithsonian Magazine explained.
We just happen to have some good news for Rep. Garcia about that wanting-to-be-a-tree business. She’s already pretty close. For more on that, we go to our correspondent from beyond the grave, Carl Sagan, who shares a thought about trees. And no, we couldn’t just write out his quote here. You have to hear it in Dr. Sagan’s own voice.
That’ll be one pandemic with extra distress. Hold the goals
When the COVID-19 pandemic first hit it put a lot of stuff on hold for everyone. Couldn’t eat inside at your favorite restaurant, attend that long-awaited concert, or travel out of the country. Those were all pretty bad, but it was the disruption of pursuing long-term goals that seemed to have the most effect on people’s mental health.
Investigators from the University of Waterloo (Ont.) looked at how putting such goals on hold affected people’s mental well-being. The study’s 226 participants were asked about their “COVID-frozen” goals and the degree to which they were able to actively pursue each goal and how committed they were to achieving it.
What they found was that the participants’ COVID-frozen goals were associated with feelings of psychological distress, such as anxiety, depressive symptoms, stress, and lowered life satisfaction. It was only when participants were able to disengage from goal rumination that well-being was impacted positively.
“Goal rumination is compulsive and can aggravate worries and frustrations while also taking away mental resources from other goals,” Candice Hubley, lead author and a PhD candidate in psychology, said in a written statement. So in short, you’re only stressing yourself out more about something that is far off in the distance when you could be focusing more on short-term, tangible goals instead.
Now, no one is saying to give up on your goals. Just take them one at a time. You’ll have better life satisfaction and your COVID-frozen goals will thaw out before you know it.
Herpes to the rescue
Let’s face it: When people hear the word “herpes,” their first thoughts are not positive. But what if herpes could be a hero?
Scientists have found a way to make a strain of herpes that kills cancer because, hey, it’s 2022, and anything is possible. Trials have been going well and this seems like a safe and effective way to fight cancer.
Viruses may be one of our oldest enemies, but it’s also been said that the enemy of my enemy is my friend. So why not make herpes the enemy of cancer, thereby turning it into our friend? The genetically modified herpes virus is injected directly into tumors, where it destroys cancer cells from within. But wait, there’s more! The patient’s immune system also senses the virus and springs into action against it and the cancer in which it is residing.
During the phase 1 trial, three of the nine patients saw tumor reduction and the therapy proved safe as well. Future trials will be able to more specifically target various cancer types and make the treatment better. For once, we are rooting for you, herpes.
A breath of not-so-fresh air
There’s nothing quite like that first real warm day of spring. You can finally open the windows and clear out the old stuffy air that’s been hanging around all winter long. It’s a ritual that’s now backed up with some science in the form of a new study. Turns out that there’s actually a fair amount of smog in the average home. That’s right, smog’s not just for the big city anymore.
As part of the HOMEChem project, a whole host of scientists gathered together under one roof in a typical suburban house and immediately started doing chores. Cooking, cleaning, the works. No, it wasn’t because they had trashed the place the night before. They had set up instrumentation all around the house to measure the chemical makeup of the air inside. A scientist’s idea of a wild party.
The results are perhaps not all that surprising, but interesting nonetheless. Your homemade smog certainly won’t kill you, but there’s both an increased amount and higher concentration of airborne toxins in indoor air, compared with outdoors. Benzene and formaldehyde were common, as were acrolein (a pulmonary toxicant emitted by lumber and burning fats) and isocyanic acid (which can react with proteins in the human body). The researchers noted that most of these chemicals can be removed with proper ventilation.
Although cleaning is certainly responsible for a fair share of the chemicals, cooking generally produced more toxic compounds, similar to what’s found in wildfire smoke. One of the researchers said this makes sense, since a wildfire can be considered an “extreme form of cooking.” Scientists may not know how to party, but their idea of a barbecue sounds … interesting. We’re looking forward to an upcoming study out of California: Can a 1-million acre wildfire adequately cook a ribeye steak?
We’re dying to try composting ... with humans, that is
We here at LOTME are not really fans of politicians, except as objects of ridicule. That is kind of fun. Whether we’re watching Fox News, listening to NPR, or reading Vladimir Putin’s fashion blog, one thing remains clear: If you want actual information, don’t ask a politician.
There are, of course, always exceptions, and we just found one: California state representative Cristina Garcia. Rep. Garcia sponsored a bill just signed into law by Gov. Gavin Newsom that legalizes the practice of human composting, the reduction of remains by “placing bodies in individual vessels and fostering gentle transformation into a nutrient-dense soil.”
Since we’ve written about this sort of thing before – Washington was the first state to legalize the process back in 2019 – we’re more interested now in what Rep. Garcia told NBC News while describing her motivation: “I’ve always wanted to be a tree. The idea of having my family sitting under my shade one day – that brings a lot of joy.” How great is that? Tree-hugging is just not enough. Be the tree.
California is the fifth state to provide its residents with the human composting option, the other three being Colorado, Oregon, and Vermont. The process “typically involves putting a body into a steel vessel, then covering it with organic materials like straw, wood chips and alfalfa. Microbes break down the corpse and the plant matter, transforming the various components into nutrient-rich soil in roughly 30 days,” Smithsonian Magazine explained.
We just happen to have some good news for Rep. Garcia about that wanting-to-be-a-tree business. She’s already pretty close. For more on that, we go to our correspondent from beyond the grave, Carl Sagan, who shares a thought about trees. And no, we couldn’t just write out his quote here. You have to hear it in Dr. Sagan’s own voice.
That’ll be one pandemic with extra distress. Hold the goals
When the COVID-19 pandemic first hit it put a lot of stuff on hold for everyone. Couldn’t eat inside at your favorite restaurant, attend that long-awaited concert, or travel out of the country. Those were all pretty bad, but it was the disruption of pursuing long-term goals that seemed to have the most effect on people’s mental health.
Investigators from the University of Waterloo (Ont.) looked at how putting such goals on hold affected people’s mental well-being. The study’s 226 participants were asked about their “COVID-frozen” goals and the degree to which they were able to actively pursue each goal and how committed they were to achieving it.
What they found was that the participants’ COVID-frozen goals were associated with feelings of psychological distress, such as anxiety, depressive symptoms, stress, and lowered life satisfaction. It was only when participants were able to disengage from goal rumination that well-being was impacted positively.
“Goal rumination is compulsive and can aggravate worries and frustrations while also taking away mental resources from other goals,” Candice Hubley, lead author and a PhD candidate in psychology, said in a written statement. So in short, you’re only stressing yourself out more about something that is far off in the distance when you could be focusing more on short-term, tangible goals instead.
Now, no one is saying to give up on your goals. Just take them one at a time. You’ll have better life satisfaction and your COVID-frozen goals will thaw out before you know it.
Herpes to the rescue
Let’s face it: When people hear the word “herpes,” their first thoughts are not positive. But what if herpes could be a hero?
Scientists have found a way to make a strain of herpes that kills cancer because, hey, it’s 2022, and anything is possible. Trials have been going well and this seems like a safe and effective way to fight cancer.
Viruses may be one of our oldest enemies, but it’s also been said that the enemy of my enemy is my friend. So why not make herpes the enemy of cancer, thereby turning it into our friend? The genetically modified herpes virus is injected directly into tumors, where it destroys cancer cells from within. But wait, there’s more! The patient’s immune system also senses the virus and springs into action against it and the cancer in which it is residing.
During the phase 1 trial, three of the nine patients saw tumor reduction and the therapy proved safe as well. Future trials will be able to more specifically target various cancer types and make the treatment better. For once, we are rooting for you, herpes.
A breath of not-so-fresh air
There’s nothing quite like that first real warm day of spring. You can finally open the windows and clear out the old stuffy air that’s been hanging around all winter long. It’s a ritual that’s now backed up with some science in the form of a new study. Turns out that there’s actually a fair amount of smog in the average home. That’s right, smog’s not just for the big city anymore.
As part of the HOMEChem project, a whole host of scientists gathered together under one roof in a typical suburban house and immediately started doing chores. Cooking, cleaning, the works. No, it wasn’t because they had trashed the place the night before. They had set up instrumentation all around the house to measure the chemical makeup of the air inside. A scientist’s idea of a wild party.
The results are perhaps not all that surprising, but interesting nonetheless. Your homemade smog certainly won’t kill you, but there’s both an increased amount and higher concentration of airborne toxins in indoor air, compared with outdoors. Benzene and formaldehyde were common, as were acrolein (a pulmonary toxicant emitted by lumber and burning fats) and isocyanic acid (which can react with proteins in the human body). The researchers noted that most of these chemicals can be removed with proper ventilation.
Although cleaning is certainly responsible for a fair share of the chemicals, cooking generally produced more toxic compounds, similar to what’s found in wildfire smoke. One of the researchers said this makes sense, since a wildfire can be considered an “extreme form of cooking.” Scientists may not know how to party, but their idea of a barbecue sounds … interesting. We’re looking forward to an upcoming study out of California: Can a 1-million acre wildfire adequately cook a ribeye steak?
We’re dying to try composting ... with humans, that is
We here at LOTME are not really fans of politicians, except as objects of ridicule. That is kind of fun. Whether we’re watching Fox News, listening to NPR, or reading Vladimir Putin’s fashion blog, one thing remains clear: If you want actual information, don’t ask a politician.
There are, of course, always exceptions, and we just found one: California state representative Cristina Garcia. Rep. Garcia sponsored a bill just signed into law by Gov. Gavin Newsom that legalizes the practice of human composting, the reduction of remains by “placing bodies in individual vessels and fostering gentle transformation into a nutrient-dense soil.”
Since we’ve written about this sort of thing before – Washington was the first state to legalize the process back in 2019 – we’re more interested now in what Rep. Garcia told NBC News while describing her motivation: “I’ve always wanted to be a tree. The idea of having my family sitting under my shade one day – that brings a lot of joy.” How great is that? Tree-hugging is just not enough. Be the tree.
California is the fifth state to provide its residents with the human composting option, the other three being Colorado, Oregon, and Vermont. The process “typically involves putting a body into a steel vessel, then covering it with organic materials like straw, wood chips and alfalfa. Microbes break down the corpse and the plant matter, transforming the various components into nutrient-rich soil in roughly 30 days,” Smithsonian Magazine explained.
We just happen to have some good news for Rep. Garcia about that wanting-to-be-a-tree business. She’s already pretty close. For more on that, we go to our correspondent from beyond the grave, Carl Sagan, who shares a thought about trees. And no, we couldn’t just write out his quote here. You have to hear it in Dr. Sagan’s own voice.
That’ll be one pandemic with extra distress. Hold the goals
When the COVID-19 pandemic first hit it put a lot of stuff on hold for everyone. Couldn’t eat inside at your favorite restaurant, attend that long-awaited concert, or travel out of the country. Those were all pretty bad, but it was the disruption of pursuing long-term goals that seemed to have the most effect on people’s mental health.
Investigators from the University of Waterloo (Ont.) looked at how putting such goals on hold affected people’s mental well-being. The study’s 226 participants were asked about their “COVID-frozen” goals and the degree to which they were able to actively pursue each goal and how committed they were to achieving it.
What they found was that the participants’ COVID-frozen goals were associated with feelings of psychological distress, such as anxiety, depressive symptoms, stress, and lowered life satisfaction. It was only when participants were able to disengage from goal rumination that well-being was impacted positively.
“Goal rumination is compulsive and can aggravate worries and frustrations while also taking away mental resources from other goals,” Candice Hubley, lead author and a PhD candidate in psychology, said in a written statement. So in short, you’re only stressing yourself out more about something that is far off in the distance when you could be focusing more on short-term, tangible goals instead.
Now, no one is saying to give up on your goals. Just take them one at a time. You’ll have better life satisfaction and your COVID-frozen goals will thaw out before you know it.
Despite benefits, extended-interval pembro uptake remains low
In April 2020, the Food and Drug Administration approved extended dosing for standalone pembrolizumab – 400 mg every 6 weeks instead of the standard dosing of 200 mg every 3 weeks. The shift came, in part, to reduce patient health care encounters during the early days of the COVID-19 pandemic, but also because fewer infusions save patients time and out-of-pocket costs and reduce the burden on the health care system.
The FDA deemed this move safe after pharmacologic studies and a small melanoma study found that responses and adverse events were equivalent in comparison with standard dosing.
Given the benefits, one would expect “brisk adoption” of extended-interval dosing, Garth Strohbehn, MD, an oncologist at the VA Medical Center in Ann Arbor, Mich., and colleagues wrote in a recent report in JAMA Oncology.
However, when the team reviewed data on 835 veterans from the Veterans Health Administration who began taking single-agent pembrolizumab between April 1, 2020, and July 1, 2021, only about one-third received extended-interval dosing.
Between April and January 2021, use of extended-interval dosing rose steadily to about 35% of patients but then hovered in that range through August 2021.
Among the patients, age, sex, Charlson comorbidity index, and pembrolizumab indications were well balanced between the standard-dosing and the extended-interval dosing groups.
Notably, Dr. Strohbehn and colleagues also found no difference in time-to-treatment discontinuation between patients receiving extended dosing in comparison with patients receiving standard dosing, which is “a real-world measure of clinical effectiveness,” the team said.
And there was no difference in immune-related side effects between the two regimens, as assessed by incident levothyroxine and prednisone prescriptions.
The real-world near equivalence of extended and standard dosing intervals that was demonstrated in the study is “reassuring” and helps make the case for considering it “as a best practice” for single-agent pembrolizumab, the investigators wrote.
Dr. Strohbehn remained somewhat puzzled by the low uptake of the extended-dosing option.
“I was frankly surprised by the small number of patients who received the extended-interval regimen,” Dr. Strohbehn said in an interview.
“Admittedly, there are patients who would prefer to receive standard-interval therapy, and that preference should of course be accommodated whenever possible, but in my experience, those numbers are small,” at least in the VA system, he noted.
In addition, the authors noted, there is no direct financial incentive for more frequent dosing in the VA system.
It’s possible that low uptake could stem from clinicians’ doubts about switching to an extended-interval dose, given that the FDA’s approval was based largely on a study of 44 patients with melanoma in a single-arm trial.
If that is indeed the case, the new findings – which represent the first health system–level, real-world comparative effectiveness data for standard vs. extended-interval pembrolizumab – should help address these concerns, the team said.
“This observational dataset lends further credence to [the dosing] regimens being clinically equivalent,” said Zachery Reichert, MD, PhD, a urologic oncologist at the University of Michigan, Ann Arbor, who was not involved in the study.
To address the issue, Dr. Strohbehn and his team suggested “clinical guideline promotion to overcome some of the barriers to the adoption of extended-interval pembrolizumab.”
Dr. Riechert suggested further validation of equivalent outcomes for the two regimens, more advocacy to encourage patients to ask about the 6-week option, as well as incentives from insurers to adopt it.
Dr. Strohbehn added that the situation highlights a broader issue in oncology, namely that many drugs “end up on the market with dosing regimens that haven’t necessarily been optimized.”
Across the world, investigators are conducting clinical trials “to identify the minimum dosages, frequencies, and durations patients need in order to achieve their best outcome,” Dr. Strohbehn said. In oncology, much of this effort is being led by Project Optimus, from the FDA’s Oncology Center of Excellence, he said.
The study was funded by the VA National Oncology Program. Dr. Reichert and Dr. Strohbehn have disclosed no relevant financial relationships. One investigator has received grants from Novartis, Bristol-Myers Squibb, Regeneron, and Genentech.
A version of this article first appeared on Medscape.com.
In April 2020, the Food and Drug Administration approved extended dosing for standalone pembrolizumab – 400 mg every 6 weeks instead of the standard dosing of 200 mg every 3 weeks. The shift came, in part, to reduce patient health care encounters during the early days of the COVID-19 pandemic, but also because fewer infusions save patients time and out-of-pocket costs and reduce the burden on the health care system.
The FDA deemed this move safe after pharmacologic studies and a small melanoma study found that responses and adverse events were equivalent in comparison with standard dosing.
Given the benefits, one would expect “brisk adoption” of extended-interval dosing, Garth Strohbehn, MD, an oncologist at the VA Medical Center in Ann Arbor, Mich., and colleagues wrote in a recent report in JAMA Oncology.
However, when the team reviewed data on 835 veterans from the Veterans Health Administration who began taking single-agent pembrolizumab between April 1, 2020, and July 1, 2021, only about one-third received extended-interval dosing.
Between April and January 2021, use of extended-interval dosing rose steadily to about 35% of patients but then hovered in that range through August 2021.
Among the patients, age, sex, Charlson comorbidity index, and pembrolizumab indications were well balanced between the standard-dosing and the extended-interval dosing groups.
Notably, Dr. Strohbehn and colleagues also found no difference in time-to-treatment discontinuation between patients receiving extended dosing in comparison with patients receiving standard dosing, which is “a real-world measure of clinical effectiveness,” the team said.
And there was no difference in immune-related side effects between the two regimens, as assessed by incident levothyroxine and prednisone prescriptions.
The real-world near equivalence of extended and standard dosing intervals that was demonstrated in the study is “reassuring” and helps make the case for considering it “as a best practice” for single-agent pembrolizumab, the investigators wrote.
Dr. Strohbehn remained somewhat puzzled by the low uptake of the extended-dosing option.
“I was frankly surprised by the small number of patients who received the extended-interval regimen,” Dr. Strohbehn said in an interview.
“Admittedly, there are patients who would prefer to receive standard-interval therapy, and that preference should of course be accommodated whenever possible, but in my experience, those numbers are small,” at least in the VA system, he noted.
In addition, the authors noted, there is no direct financial incentive for more frequent dosing in the VA system.
It’s possible that low uptake could stem from clinicians’ doubts about switching to an extended-interval dose, given that the FDA’s approval was based largely on a study of 44 patients with melanoma in a single-arm trial.
If that is indeed the case, the new findings – which represent the first health system–level, real-world comparative effectiveness data for standard vs. extended-interval pembrolizumab – should help address these concerns, the team said.
“This observational dataset lends further credence to [the dosing] regimens being clinically equivalent,” said Zachery Reichert, MD, PhD, a urologic oncologist at the University of Michigan, Ann Arbor, who was not involved in the study.
To address the issue, Dr. Strohbehn and his team suggested “clinical guideline promotion to overcome some of the barriers to the adoption of extended-interval pembrolizumab.”
Dr. Riechert suggested further validation of equivalent outcomes for the two regimens, more advocacy to encourage patients to ask about the 6-week option, as well as incentives from insurers to adopt it.
Dr. Strohbehn added that the situation highlights a broader issue in oncology, namely that many drugs “end up on the market with dosing regimens that haven’t necessarily been optimized.”
Across the world, investigators are conducting clinical trials “to identify the minimum dosages, frequencies, and durations patients need in order to achieve their best outcome,” Dr. Strohbehn said. In oncology, much of this effort is being led by Project Optimus, from the FDA’s Oncology Center of Excellence, he said.
The study was funded by the VA National Oncology Program. Dr. Reichert and Dr. Strohbehn have disclosed no relevant financial relationships. One investigator has received grants from Novartis, Bristol-Myers Squibb, Regeneron, and Genentech.
A version of this article first appeared on Medscape.com.
In April 2020, the Food and Drug Administration approved extended dosing for standalone pembrolizumab – 400 mg every 6 weeks instead of the standard dosing of 200 mg every 3 weeks. The shift came, in part, to reduce patient health care encounters during the early days of the COVID-19 pandemic, but also because fewer infusions save patients time and out-of-pocket costs and reduce the burden on the health care system.
The FDA deemed this move safe after pharmacologic studies and a small melanoma study found that responses and adverse events were equivalent in comparison with standard dosing.
Given the benefits, one would expect “brisk adoption” of extended-interval dosing, Garth Strohbehn, MD, an oncologist at the VA Medical Center in Ann Arbor, Mich., and colleagues wrote in a recent report in JAMA Oncology.
However, when the team reviewed data on 835 veterans from the Veterans Health Administration who began taking single-agent pembrolizumab between April 1, 2020, and July 1, 2021, only about one-third received extended-interval dosing.
Between April and January 2021, use of extended-interval dosing rose steadily to about 35% of patients but then hovered in that range through August 2021.
Among the patients, age, sex, Charlson comorbidity index, and pembrolizumab indications were well balanced between the standard-dosing and the extended-interval dosing groups.
Notably, Dr. Strohbehn and colleagues also found no difference in time-to-treatment discontinuation between patients receiving extended dosing in comparison with patients receiving standard dosing, which is “a real-world measure of clinical effectiveness,” the team said.
And there was no difference in immune-related side effects between the two regimens, as assessed by incident levothyroxine and prednisone prescriptions.
The real-world near equivalence of extended and standard dosing intervals that was demonstrated in the study is “reassuring” and helps make the case for considering it “as a best practice” for single-agent pembrolizumab, the investigators wrote.
Dr. Strohbehn remained somewhat puzzled by the low uptake of the extended-dosing option.
“I was frankly surprised by the small number of patients who received the extended-interval regimen,” Dr. Strohbehn said in an interview.
“Admittedly, there are patients who would prefer to receive standard-interval therapy, and that preference should of course be accommodated whenever possible, but in my experience, those numbers are small,” at least in the VA system, he noted.
In addition, the authors noted, there is no direct financial incentive for more frequent dosing in the VA system.
It’s possible that low uptake could stem from clinicians’ doubts about switching to an extended-interval dose, given that the FDA’s approval was based largely on a study of 44 patients with melanoma in a single-arm trial.
If that is indeed the case, the new findings – which represent the first health system–level, real-world comparative effectiveness data for standard vs. extended-interval pembrolizumab – should help address these concerns, the team said.
“This observational dataset lends further credence to [the dosing] regimens being clinically equivalent,” said Zachery Reichert, MD, PhD, a urologic oncologist at the University of Michigan, Ann Arbor, who was not involved in the study.
To address the issue, Dr. Strohbehn and his team suggested “clinical guideline promotion to overcome some of the barriers to the adoption of extended-interval pembrolizumab.”
Dr. Riechert suggested further validation of equivalent outcomes for the two regimens, more advocacy to encourage patients to ask about the 6-week option, as well as incentives from insurers to adopt it.
Dr. Strohbehn added that the situation highlights a broader issue in oncology, namely that many drugs “end up on the market with dosing regimens that haven’t necessarily been optimized.”
Across the world, investigators are conducting clinical trials “to identify the minimum dosages, frequencies, and durations patients need in order to achieve their best outcome,” Dr. Strohbehn said. In oncology, much of this effort is being led by Project Optimus, from the FDA’s Oncology Center of Excellence, he said.
The study was funded by the VA National Oncology Program. Dr. Reichert and Dr. Strohbehn have disclosed no relevant financial relationships. One investigator has received grants from Novartis, Bristol-Myers Squibb, Regeneron, and Genentech.
A version of this article first appeared on Medscape.com.
FROM JAMA ONCOLOGY
Cre8 EVO stent loses sweet spot in diabetes at 2 years: SUGAR
BOSTON – Despite a promising start, extended follow-up from the SUGAR trial found that the Cre8 EVO drug-eluting stent could not maintain superiority over the Resolute Onyx DES at 2 years in patients with diabetes undergoing revascularization for coronary artery disease.
The Cre8 EVO stent (Alvimedica) is not available in the United States but, as previously reported, caused a stir last year after demonstrating a 35% relative risk reduction in the primary endpoint of target lesion failure (TLF) at 1 year in a prespecified superiority analysis.
At 2 years, however, the TLF rate was 10.4% with the polymer-free Cre8 EVO amphilimus-eluting stent and 12.1% with the durable polymer Resolute Onyx (Medtronic) zotarolimus-eluting stent, which did not achieve superiority (hazard ratio, 0.84; 95% confidence interval, 0.60-1.19).
Rates were numerically lower with the Cre8 EVO stent for the endpoint’s individual components of cardiac death (3.1% vs. 3.4%), target vessel MI (6.6% vs. 7.6%), and target lesion revascularization (4.3% vs. 4.6%).
Results were also similar between the Cre8 EVO and Resolute Onyx stents for all-cause mortality (7.1% vs. 6.8%), any MI (9.0% vs. 9.2%), target vessel revascularization (5.5% vs. 5.1%), all new revascularizations (7.6% vs. 9.4%), definite stent thrombosis (1.0% vs. 1.2%), and major adverse cardiac events (18.3% vs. 20.8%), Pablo Salinas, MD, PhD, of Hospital Clinico San Carlos, Madrid, reported at the Transcatheter Cardiovascular Therapeutics annual meeting.
He noted that all-cause mortality was 7% in just 2 years in the diabetic cohort, or twice the number of cardiac deaths. “In other words, these patients had the same chance of dying from cardiac causes and noncardiac causes, so we need a more comprehensive approach to the disease. Also, if you look at all new revascularizations, roughly 50% were off target, so there is disease progression at 2 years in this population.”
Among the 586 Cre8 EVO and 589 Resolute Onyx patients who underwent percutaneous coronary intervention (PCI), roughly half had multivessel coronary artery disease, 83% had hypertension, 81% had dyslipidemia, and 21% were current smokers. Nearly all patients had diabetes type 2 for an average of 10.6 years for Cre8 EVO and 11.4 years for Resolute Onyx, with hemoglobin A1c levels of 7.4% and 7.5%, respectively.
Although there is “insufficient evidence” the Cre8 EVO stent is superior to the Resolute Onyx stent with regard to TLF, Dr. Salinas concluded extended follow-up until 5 years is warranted.
During a discussion of the results, Dr. Salinas said he expects the 5-year results will “probably go parallel” but that it’s worth following this very valuable cohort. “There are not so many trials with 1,000 diabetic patients. We always speak about how complex they are, the results are bad, but we don’t use the diabetic population in trials,” he said at the meeting sponsored by the Cardiovascular Research Foundation.
Asked during a TCT press conference what could have caused the catch-up in TLF at 2 years, Dr. Salinas said there were only 25 primary events from years 1 to 2, driven primarily by periprocedural MI, but that the timing of restenosis was different. Events accrued “drop by drop” with the Cre8 EVO, whereas with the Resolute Onyx there was a “bump in restenosis” after 6 months “but then it is very nice to see it is flat, which means that durable polymers are also safe because we have not seen late events.”
Press conference discussant Carlo Di Mario, MD, from Careggi University Hospital, Florence, Italy, who was not involved in the study, said the reversal of superiority for the Cre8 EVO might be a “bitter note” for the investigators but “maybe it is not bitter for us because overall, the percentage of figures are so low that it’s very difficult to find a difference” between the two stents.
Roxana Mehran, MD, of Icahn School of Medicine at Mount Sinai, New York, who previously described the 1-year results as “almost too good to be true,” commented to this news organization, “We just saw in this trial, no benefit whatsoever at 2 years in terms of target lesion failure. So it’s very important for us to evaluate this going forward.”
She continued, “We’ve always been talking about these biodegradable polymers and then going back to the bare metal stent – oh that’s great because polymers aren’t so good – but now we’re seeing durable polymers may be okay, especially with the current technology.”
Asked whether Cre8 EVO, which is CE mark certified in Europe, remains an option in light of the new results, Dr. Mehran said, “I don’t think it kills it. It’s not worse; it’s another stent that’s available.”
Nevertheless, “what we’re looking for is some efficacious benefit for diabetic patients. We don’t have one yet,” observed Dr. Mehran, who is leading the ABILITY Diabetes Global trial, which just finished enrolling 3,000 patients with diabetes and is testing PCI with the Abluminus DES+ sirolimus-eluting stent system vs. the Xience everolimus-eluting stent. The study is estimated to be complete in August 2024.
The study was funded by the Spanish Society of Cardiology. Dr. Salinas reported consulting fees/honoraria from Boston Scientific, Abbott Vascular, Biomenco, and Medtronic.
A version of this article first appeared on Medscape.com.
BOSTON – Despite a promising start, extended follow-up from the SUGAR trial found that the Cre8 EVO drug-eluting stent could not maintain superiority over the Resolute Onyx DES at 2 years in patients with diabetes undergoing revascularization for coronary artery disease.
The Cre8 EVO stent (Alvimedica) is not available in the United States but, as previously reported, caused a stir last year after demonstrating a 35% relative risk reduction in the primary endpoint of target lesion failure (TLF) at 1 year in a prespecified superiority analysis.
At 2 years, however, the TLF rate was 10.4% with the polymer-free Cre8 EVO amphilimus-eluting stent and 12.1% with the durable polymer Resolute Onyx (Medtronic) zotarolimus-eluting stent, which did not achieve superiority (hazard ratio, 0.84; 95% confidence interval, 0.60-1.19).
Rates were numerically lower with the Cre8 EVO stent for the endpoint’s individual components of cardiac death (3.1% vs. 3.4%), target vessel MI (6.6% vs. 7.6%), and target lesion revascularization (4.3% vs. 4.6%).
Results were also similar between the Cre8 EVO and Resolute Onyx stents for all-cause mortality (7.1% vs. 6.8%), any MI (9.0% vs. 9.2%), target vessel revascularization (5.5% vs. 5.1%), all new revascularizations (7.6% vs. 9.4%), definite stent thrombosis (1.0% vs. 1.2%), and major adverse cardiac events (18.3% vs. 20.8%), Pablo Salinas, MD, PhD, of Hospital Clinico San Carlos, Madrid, reported at the Transcatheter Cardiovascular Therapeutics annual meeting.
He noted that all-cause mortality was 7% in just 2 years in the diabetic cohort, or twice the number of cardiac deaths. “In other words, these patients had the same chance of dying from cardiac causes and noncardiac causes, so we need a more comprehensive approach to the disease. Also, if you look at all new revascularizations, roughly 50% were off target, so there is disease progression at 2 years in this population.”
Among the 586 Cre8 EVO and 589 Resolute Onyx patients who underwent percutaneous coronary intervention (PCI), roughly half had multivessel coronary artery disease, 83% had hypertension, 81% had dyslipidemia, and 21% were current smokers. Nearly all patients had diabetes type 2 for an average of 10.6 years for Cre8 EVO and 11.4 years for Resolute Onyx, with hemoglobin A1c levels of 7.4% and 7.5%, respectively.
Although there is “insufficient evidence” the Cre8 EVO stent is superior to the Resolute Onyx stent with regard to TLF, Dr. Salinas concluded extended follow-up until 5 years is warranted.
During a discussion of the results, Dr. Salinas said he expects the 5-year results will “probably go parallel” but that it’s worth following this very valuable cohort. “There are not so many trials with 1,000 diabetic patients. We always speak about how complex they are, the results are bad, but we don’t use the diabetic population in trials,” he said at the meeting sponsored by the Cardiovascular Research Foundation.
Asked during a TCT press conference what could have caused the catch-up in TLF at 2 years, Dr. Salinas said there were only 25 primary events from years 1 to 2, driven primarily by periprocedural MI, but that the timing of restenosis was different. Events accrued “drop by drop” with the Cre8 EVO, whereas with the Resolute Onyx there was a “bump in restenosis” after 6 months “but then it is very nice to see it is flat, which means that durable polymers are also safe because we have not seen late events.”
Press conference discussant Carlo Di Mario, MD, from Careggi University Hospital, Florence, Italy, who was not involved in the study, said the reversal of superiority for the Cre8 EVO might be a “bitter note” for the investigators but “maybe it is not bitter for us because overall, the percentage of figures are so low that it’s very difficult to find a difference” between the two stents.
Roxana Mehran, MD, of Icahn School of Medicine at Mount Sinai, New York, who previously described the 1-year results as “almost too good to be true,” commented to this news organization, “We just saw in this trial, no benefit whatsoever at 2 years in terms of target lesion failure. So it’s very important for us to evaluate this going forward.”
She continued, “We’ve always been talking about these biodegradable polymers and then going back to the bare metal stent – oh that’s great because polymers aren’t so good – but now we’re seeing durable polymers may be okay, especially with the current technology.”
Asked whether Cre8 EVO, which is CE mark certified in Europe, remains an option in light of the new results, Dr. Mehran said, “I don’t think it kills it. It’s not worse; it’s another stent that’s available.”
Nevertheless, “what we’re looking for is some efficacious benefit for diabetic patients. We don’t have one yet,” observed Dr. Mehran, who is leading the ABILITY Diabetes Global trial, which just finished enrolling 3,000 patients with diabetes and is testing PCI with the Abluminus DES+ sirolimus-eluting stent system vs. the Xience everolimus-eluting stent. The study is estimated to be complete in August 2024.
The study was funded by the Spanish Society of Cardiology. Dr. Salinas reported consulting fees/honoraria from Boston Scientific, Abbott Vascular, Biomenco, and Medtronic.
A version of this article first appeared on Medscape.com.
BOSTON – Despite a promising start, extended follow-up from the SUGAR trial found that the Cre8 EVO drug-eluting stent could not maintain superiority over the Resolute Onyx DES at 2 years in patients with diabetes undergoing revascularization for coronary artery disease.
The Cre8 EVO stent (Alvimedica) is not available in the United States but, as previously reported, caused a stir last year after demonstrating a 35% relative risk reduction in the primary endpoint of target lesion failure (TLF) at 1 year in a prespecified superiority analysis.
At 2 years, however, the TLF rate was 10.4% with the polymer-free Cre8 EVO amphilimus-eluting stent and 12.1% with the durable polymer Resolute Onyx (Medtronic) zotarolimus-eluting stent, which did not achieve superiority (hazard ratio, 0.84; 95% confidence interval, 0.60-1.19).
Rates were numerically lower with the Cre8 EVO stent for the endpoint’s individual components of cardiac death (3.1% vs. 3.4%), target vessel MI (6.6% vs. 7.6%), and target lesion revascularization (4.3% vs. 4.6%).
Results were also similar between the Cre8 EVO and Resolute Onyx stents for all-cause mortality (7.1% vs. 6.8%), any MI (9.0% vs. 9.2%), target vessel revascularization (5.5% vs. 5.1%), all new revascularizations (7.6% vs. 9.4%), definite stent thrombosis (1.0% vs. 1.2%), and major adverse cardiac events (18.3% vs. 20.8%), Pablo Salinas, MD, PhD, of Hospital Clinico San Carlos, Madrid, reported at the Transcatheter Cardiovascular Therapeutics annual meeting.
He noted that all-cause mortality was 7% in just 2 years in the diabetic cohort, or twice the number of cardiac deaths. “In other words, these patients had the same chance of dying from cardiac causes and noncardiac causes, so we need a more comprehensive approach to the disease. Also, if you look at all new revascularizations, roughly 50% were off target, so there is disease progression at 2 years in this population.”
Among the 586 Cre8 EVO and 589 Resolute Onyx patients who underwent percutaneous coronary intervention (PCI), roughly half had multivessel coronary artery disease, 83% had hypertension, 81% had dyslipidemia, and 21% were current smokers. Nearly all patients had diabetes type 2 for an average of 10.6 years for Cre8 EVO and 11.4 years for Resolute Onyx, with hemoglobin A1c levels of 7.4% and 7.5%, respectively.
Although there is “insufficient evidence” the Cre8 EVO stent is superior to the Resolute Onyx stent with regard to TLF, Dr. Salinas concluded extended follow-up until 5 years is warranted.
During a discussion of the results, Dr. Salinas said he expects the 5-year results will “probably go parallel” but that it’s worth following this very valuable cohort. “There are not so many trials with 1,000 diabetic patients. We always speak about how complex they are, the results are bad, but we don’t use the diabetic population in trials,” he said at the meeting sponsored by the Cardiovascular Research Foundation.
Asked during a TCT press conference what could have caused the catch-up in TLF at 2 years, Dr. Salinas said there were only 25 primary events from years 1 to 2, driven primarily by periprocedural MI, but that the timing of restenosis was different. Events accrued “drop by drop” with the Cre8 EVO, whereas with the Resolute Onyx there was a “bump in restenosis” after 6 months “but then it is very nice to see it is flat, which means that durable polymers are also safe because we have not seen late events.”
Press conference discussant Carlo Di Mario, MD, from Careggi University Hospital, Florence, Italy, who was not involved in the study, said the reversal of superiority for the Cre8 EVO might be a “bitter note” for the investigators but “maybe it is not bitter for us because overall, the percentage of figures are so low that it’s very difficult to find a difference” between the two stents.
Roxana Mehran, MD, of Icahn School of Medicine at Mount Sinai, New York, who previously described the 1-year results as “almost too good to be true,” commented to this news organization, “We just saw in this trial, no benefit whatsoever at 2 years in terms of target lesion failure. So it’s very important for us to evaluate this going forward.”
She continued, “We’ve always been talking about these biodegradable polymers and then going back to the bare metal stent – oh that’s great because polymers aren’t so good – but now we’re seeing durable polymers may be okay, especially with the current technology.”
Asked whether Cre8 EVO, which is CE mark certified in Europe, remains an option in light of the new results, Dr. Mehran said, “I don’t think it kills it. It’s not worse; it’s another stent that’s available.”
Nevertheless, “what we’re looking for is some efficacious benefit for diabetic patients. We don’t have one yet,” observed Dr. Mehran, who is leading the ABILITY Diabetes Global trial, which just finished enrolling 3,000 patients with diabetes and is testing PCI with the Abluminus DES+ sirolimus-eluting stent system vs. the Xience everolimus-eluting stent. The study is estimated to be complete in August 2024.
The study was funded by the Spanish Society of Cardiology. Dr. Salinas reported consulting fees/honoraria from Boston Scientific, Abbott Vascular, Biomenco, and Medtronic.
A version of this article first appeared on Medscape.com.
AT TCT 2022
Emphasis on weight loss in new type 2 diabetes guidance
STOCKHOLM – Weight loss should be a co–primary management goal for type 2 diabetes in adults, according to a new comprehensive joint consensus report from the European Association for the Study of Diabetes and the American Diabetes Association.
And while metformin is still recommended as first-line therapy for patients with type 2 diabetes with no other comorbidities, the statement expands the indications for use of other agents or combinations of agents as initial therapy for subgroups of patients, as part of individualized and patient-centered decision-making.
Last updated in 2019, the new “Management of Hyperglycemia in Type 2 Diabetes” statement also places increased emphasis on social determinants of health, incorporates recent clinical trial data for cardiovascular and kidney outcomes for sodium-glucose cotransporter-2 (SGLT2) inhibitors and glucagonlike peptide–1 (GLP-1) agonists to broaden recommendations for cardiorenal protection, and discusses health behaviors such as sleep and sitting. It also targets a wider audience than in the past by addressing health system organization to optimize delivery of diabetes care.
The new statement was presented during a 90-minute session at the annual meeting of the EASD, with 12 of its 14 European and American authors as presenters. The document was simultaneously published in Diabetologia and Diabetes Care.
During the discussion, panel member Jennifer Brigitte Green, MD, commented: “Many of these recommendations are not new. They’re modest revisions of recommendations that have been in place for years, but we know that actual implementation rates of use of these drugs in patients with established comorbidities are very low.”
“I think it’s time for communities, health care systems, etc, to actually introduce these as expectations of care... to assess quality because unless it’s considered formally to be a requirement of care I just don’t think we’re going to move that needle very much,” added Dr. Green, who is professor of medicine at Duke University, Durham, N.C.
Vanita R. Aroda, MD, of the division of endocrinology, diabetes, and hypertension at Brigham and Women’s Hospital, Boston, commented: “In the past, sometimes these recommendations created fodder for debate, but I don’t think this one will. It’s just really solidly evidence based, with the rationales presented throughout, including the figures. I think just having very clear evidence-based directions should support their dissemination and use.”
Weight management plays a prominent role in treatment
In an interview, writing panel cochair John B. Buse, MD, PhD, said: “We are saying that the four major components of type 2 diabetes care are glycemic management, cardiovascular risk management, weight management, and prevention of end-organ damage, particularly with regard to cardiorenal risk.”
“The weight management piece is much more explicit now,” said Dr. Buse, director of the Diabetes Center at the University of North Carolina at Chapel Hill.
He noted that recent evidence from the intensive lifestyle trial DiRECT, conducted in the United Kingdom, the bariatric surgery literature, and the emergence of potent weight-loss drugs have meant that “achieving 10%-15% body weight loss is now possible.
“So, aiming for remission is something that might be attractive to patients and providers. This could be based on weight management, with the [chosen] method based on shared decision-making.”
According to the new report: “Weight loss of 5%-10% confers metabolic improvement; weight loss of 10%-15% or more can have a disease-modifying effect and lead to remission of diabetes, defined as normal blood glucose levels for 3 months or more in the absence of pharmacological therapy in a 2021 consensus report.”
“Weight loss may exert benefits that extend beyond glycemic management to improve risk factors for cardiometabolic disease and quality of life,” it adds.
Individualization featured throughout
The report’s sections cover principles of care, including the importance of diabetes self-management education and support and avoidance of therapeutic inertia. Detailed guidance addresses therapeutic options including lifestyle, weight management, and pharmacotherapy for treating type 2 diabetes.
Another entire section is devoted to personalizing treatment approaches based on individual characteristics, including new evidence from cardiorenal outcomes studies for SGLT2 inhibitors and GLP-1 agonists that have come out since the last consensus report.
The document advises: “Consider initial combination therapy with glucose-lowering agents, especially in those with high [hemoglobin] A1c at diagnosis (that is, > 70 mmol/mol [> 8.5%]), in younger people with type 2 diabetes (regardless of A1c), and in those in whom a stepwise approach would delay access to agents that provide cardiorenal protection beyond their glucose-lowering effects.”
Designed to be used and user-friendly
Under the “Putting it all together: strategies for implementation” section, several lists of “practical tips for clinicians” are provided for many of the topics covered.
A series of colorful infographics are included as well, addressing the “decision cycle for person-centered glycemic management in type 2 diabetes,” including a chart summarizing characteristics of available glucose-lowering medications, including cardiorenal protection.
Also mentioned is the importance of 24-hour physical behaviors (including sleep, sitting, and sweating) and the impact on cardiometabolic health, use of a “holistic person-centered approach” to type 2 diabetes management, and an algorithm on insulin use.
Dr. Buse has financial ties to numerous drug and device companies. Dr. Green is a consultant for AstraZeneca, Pfizer, Boehringer Ingelheim/Lilly, Bayer, Sanofi, Anji, Vertex/ICON, and Valo. Dr. Aroda has served as a consultant for Applied Therapeutics, Duke, Fractyl, Novo Nordisk, Pfizer, and Sanofi.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Weight loss should be a co–primary management goal for type 2 diabetes in adults, according to a new comprehensive joint consensus report from the European Association for the Study of Diabetes and the American Diabetes Association.
And while metformin is still recommended as first-line therapy for patients with type 2 diabetes with no other comorbidities, the statement expands the indications for use of other agents or combinations of agents as initial therapy for subgroups of patients, as part of individualized and patient-centered decision-making.
Last updated in 2019, the new “Management of Hyperglycemia in Type 2 Diabetes” statement also places increased emphasis on social determinants of health, incorporates recent clinical trial data for cardiovascular and kidney outcomes for sodium-glucose cotransporter-2 (SGLT2) inhibitors and glucagonlike peptide–1 (GLP-1) agonists to broaden recommendations for cardiorenal protection, and discusses health behaviors such as sleep and sitting. It also targets a wider audience than in the past by addressing health system organization to optimize delivery of diabetes care.
The new statement was presented during a 90-minute session at the annual meeting of the EASD, with 12 of its 14 European and American authors as presenters. The document was simultaneously published in Diabetologia and Diabetes Care.
During the discussion, panel member Jennifer Brigitte Green, MD, commented: “Many of these recommendations are not new. They’re modest revisions of recommendations that have been in place for years, but we know that actual implementation rates of use of these drugs in patients with established comorbidities are very low.”
“I think it’s time for communities, health care systems, etc, to actually introduce these as expectations of care... to assess quality because unless it’s considered formally to be a requirement of care I just don’t think we’re going to move that needle very much,” added Dr. Green, who is professor of medicine at Duke University, Durham, N.C.
Vanita R. Aroda, MD, of the division of endocrinology, diabetes, and hypertension at Brigham and Women’s Hospital, Boston, commented: “In the past, sometimes these recommendations created fodder for debate, but I don’t think this one will. It’s just really solidly evidence based, with the rationales presented throughout, including the figures. I think just having very clear evidence-based directions should support their dissemination and use.”
Weight management plays a prominent role in treatment
In an interview, writing panel cochair John B. Buse, MD, PhD, said: “We are saying that the four major components of type 2 diabetes care are glycemic management, cardiovascular risk management, weight management, and prevention of end-organ damage, particularly with regard to cardiorenal risk.”
“The weight management piece is much more explicit now,” said Dr. Buse, director of the Diabetes Center at the University of North Carolina at Chapel Hill.
He noted that recent evidence from the intensive lifestyle trial DiRECT, conducted in the United Kingdom, the bariatric surgery literature, and the emergence of potent weight-loss drugs have meant that “achieving 10%-15% body weight loss is now possible.
“So, aiming for remission is something that might be attractive to patients and providers. This could be based on weight management, with the [chosen] method based on shared decision-making.”
According to the new report: “Weight loss of 5%-10% confers metabolic improvement; weight loss of 10%-15% or more can have a disease-modifying effect and lead to remission of diabetes, defined as normal blood glucose levels for 3 months or more in the absence of pharmacological therapy in a 2021 consensus report.”
“Weight loss may exert benefits that extend beyond glycemic management to improve risk factors for cardiometabolic disease and quality of life,” it adds.
Individualization featured throughout
The report’s sections cover principles of care, including the importance of diabetes self-management education and support and avoidance of therapeutic inertia. Detailed guidance addresses therapeutic options including lifestyle, weight management, and pharmacotherapy for treating type 2 diabetes.
Another entire section is devoted to personalizing treatment approaches based on individual characteristics, including new evidence from cardiorenal outcomes studies for SGLT2 inhibitors and GLP-1 agonists that have come out since the last consensus report.
The document advises: “Consider initial combination therapy with glucose-lowering agents, especially in those with high [hemoglobin] A1c at diagnosis (that is, > 70 mmol/mol [> 8.5%]), in younger people with type 2 diabetes (regardless of A1c), and in those in whom a stepwise approach would delay access to agents that provide cardiorenal protection beyond their glucose-lowering effects.”
Designed to be used and user-friendly
Under the “Putting it all together: strategies for implementation” section, several lists of “practical tips for clinicians” are provided for many of the topics covered.
A series of colorful infographics are included as well, addressing the “decision cycle for person-centered glycemic management in type 2 diabetes,” including a chart summarizing characteristics of available glucose-lowering medications, including cardiorenal protection.
Also mentioned is the importance of 24-hour physical behaviors (including sleep, sitting, and sweating) and the impact on cardiometabolic health, use of a “holistic person-centered approach” to type 2 diabetes management, and an algorithm on insulin use.
Dr. Buse has financial ties to numerous drug and device companies. Dr. Green is a consultant for AstraZeneca, Pfizer, Boehringer Ingelheim/Lilly, Bayer, Sanofi, Anji, Vertex/ICON, and Valo. Dr. Aroda has served as a consultant for Applied Therapeutics, Duke, Fractyl, Novo Nordisk, Pfizer, and Sanofi.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Weight loss should be a co–primary management goal for type 2 diabetes in adults, according to a new comprehensive joint consensus report from the European Association for the Study of Diabetes and the American Diabetes Association.
And while metformin is still recommended as first-line therapy for patients with type 2 diabetes with no other comorbidities, the statement expands the indications for use of other agents or combinations of agents as initial therapy for subgroups of patients, as part of individualized and patient-centered decision-making.
Last updated in 2019, the new “Management of Hyperglycemia in Type 2 Diabetes” statement also places increased emphasis on social determinants of health, incorporates recent clinical trial data for cardiovascular and kidney outcomes for sodium-glucose cotransporter-2 (SGLT2) inhibitors and glucagonlike peptide–1 (GLP-1) agonists to broaden recommendations for cardiorenal protection, and discusses health behaviors such as sleep and sitting. It also targets a wider audience than in the past by addressing health system organization to optimize delivery of diabetes care.
The new statement was presented during a 90-minute session at the annual meeting of the EASD, with 12 of its 14 European and American authors as presenters. The document was simultaneously published in Diabetologia and Diabetes Care.
During the discussion, panel member Jennifer Brigitte Green, MD, commented: “Many of these recommendations are not new. They’re modest revisions of recommendations that have been in place for years, but we know that actual implementation rates of use of these drugs in patients with established comorbidities are very low.”
“I think it’s time for communities, health care systems, etc, to actually introduce these as expectations of care... to assess quality because unless it’s considered formally to be a requirement of care I just don’t think we’re going to move that needle very much,” added Dr. Green, who is professor of medicine at Duke University, Durham, N.C.
Vanita R. Aroda, MD, of the division of endocrinology, diabetes, and hypertension at Brigham and Women’s Hospital, Boston, commented: “In the past, sometimes these recommendations created fodder for debate, but I don’t think this one will. It’s just really solidly evidence based, with the rationales presented throughout, including the figures. I think just having very clear evidence-based directions should support their dissemination and use.”
Weight management plays a prominent role in treatment
In an interview, writing panel cochair John B. Buse, MD, PhD, said: “We are saying that the four major components of type 2 diabetes care are glycemic management, cardiovascular risk management, weight management, and prevention of end-organ damage, particularly with regard to cardiorenal risk.”
“The weight management piece is much more explicit now,” said Dr. Buse, director of the Diabetes Center at the University of North Carolina at Chapel Hill.
He noted that recent evidence from the intensive lifestyle trial DiRECT, conducted in the United Kingdom, the bariatric surgery literature, and the emergence of potent weight-loss drugs have meant that “achieving 10%-15% body weight loss is now possible.
“So, aiming for remission is something that might be attractive to patients and providers. This could be based on weight management, with the [chosen] method based on shared decision-making.”
According to the new report: “Weight loss of 5%-10% confers metabolic improvement; weight loss of 10%-15% or more can have a disease-modifying effect and lead to remission of diabetes, defined as normal blood glucose levels for 3 months or more in the absence of pharmacological therapy in a 2021 consensus report.”
“Weight loss may exert benefits that extend beyond glycemic management to improve risk factors for cardiometabolic disease and quality of life,” it adds.
Individualization featured throughout
The report’s sections cover principles of care, including the importance of diabetes self-management education and support and avoidance of therapeutic inertia. Detailed guidance addresses therapeutic options including lifestyle, weight management, and pharmacotherapy for treating type 2 diabetes.
Another entire section is devoted to personalizing treatment approaches based on individual characteristics, including new evidence from cardiorenal outcomes studies for SGLT2 inhibitors and GLP-1 agonists that have come out since the last consensus report.
The document advises: “Consider initial combination therapy with glucose-lowering agents, especially in those with high [hemoglobin] A1c at diagnosis (that is, > 70 mmol/mol [> 8.5%]), in younger people with type 2 diabetes (regardless of A1c), and in those in whom a stepwise approach would delay access to agents that provide cardiorenal protection beyond their glucose-lowering effects.”
Designed to be used and user-friendly
Under the “Putting it all together: strategies for implementation” section, several lists of “practical tips for clinicians” are provided for many of the topics covered.
A series of colorful infographics are included as well, addressing the “decision cycle for person-centered glycemic management in type 2 diabetes,” including a chart summarizing characteristics of available glucose-lowering medications, including cardiorenal protection.
Also mentioned is the importance of 24-hour physical behaviors (including sleep, sitting, and sweating) and the impact on cardiometabolic health, use of a “holistic person-centered approach” to type 2 diabetes management, and an algorithm on insulin use.
Dr. Buse has financial ties to numerous drug and device companies. Dr. Green is a consultant for AstraZeneca, Pfizer, Boehringer Ingelheim/Lilly, Bayer, Sanofi, Anji, Vertex/ICON, and Valo. Dr. Aroda has served as a consultant for Applied Therapeutics, Duke, Fractyl, Novo Nordisk, Pfizer, and Sanofi.
A version of this article first appeared on Medscape.com.
AT EASD 2022