User login
Impact of Psoriasis Treatment on Comorbidities
1. Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
2. Davidovici BB, Sattar N, Prinz J, et al. Psoriasis and systemic inflammatory diseases: potential mechanistic links between skin disease and co-morbid conditions. J Invest Dermatol. 2010;130:1785-1796.
3. Oliveira Mde F, Rocha Bde O, Duarte GV. Psoriasis: classical and emerging comorbidities. An Bras Dermatol. 2015;90:9-20.
4. Shah K, Mellars L, Changolkar A, Feldman SR. Real-world burden of comorbidities in US patients with psoriasis. J Am Acad Dermatol. 2017;77:287-292.
5. Hu SC, Lan CE. Psoriasis and cardiovascular comorbidities: focusing on severe vascular events, cardiovascular risk factors and implications for treatment [published online October 21, 2017]. Int J Mol Sci. doi:10.3390/ijms18102211.
6. Hugh J, Van Voorhees AS, Nijhawan RI, et al. From the Medical Board of The National Psoriasis Foundation: the risk of cardiovascular disease in individuals with psoriasis and the potential impact of current therapies. J Am Acad Dermatol. 2014;70:168-177.
7. Churton S, Brown L, Shin TM, et al. Does treatment of psoriasis reduce the risk of cardiovascular disease? Drugs. 2014;74:169-182.
8. Prodanovich S, Ma F, Taylor J, et al. Methotrexate reduces incidence of vascular diseases in veterans with psoriasis or rheumatoid arthritis. J Am Acad Dermatol. 2005;52:262-226.
9. Gulliver WP, Young HM, Bachelez H, et al. Psoriasis patients treated with biologics and methotrexate have a reduced rate of myocardial infarction: a collaborative analysis using international cohorts. J Cutan Med Surg. 2016;20:550-554.
10. Ahlehoff O, Skov L, Gislason G, et al. Cardiovascular disease event rates in patients with severe psoriasis treated with systemic anti-inflammatory drugs: a Danish real-world cohort study. J Intern Med. 2013;273:197-204.
11. Wu JJ, Poon KY, Channual JC, et al. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148:1244-1250.
12. Wu JJ, Poon KY. Association of ethnicity, tumor necrosis factor inhibitor therapy, and myocardial infarction risk in patients with psoriasis. J Am Acad Dermatol. 2013;69:167-168.
13. Wu JJ, Poon KY, Bebchuk JD. Association between the type and length of tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. J Drugs Dermatol. 2013;12:899-903.
14. Wu JJ, Poon KY, Bebchuk JD. Tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis, psoriatic arthritis, or both. J Drugs Dermatol. 2014;13:932-934.
15. Famenini S, Sako EY, Wu JJ. Effect of treating psoriasis on cardiovascular co-morbidities: focus on TNF inhibitors. Am J Clin Dermatol. 2014;15:45-50.
16. Nguyen T, Wu JJ. Relationship between tumor necrosis factor-alpha inhibitors and cardiovascular disease in psoriasis: a review. Perm J. 2014;18:49-54.
17. Shaaban D, Al-Mutairi N. The effect of tumour necrosis factor inhibitor therapy on the incidence of myocardial infarction in patients with psoriasis: a retrospective study [published online November 17, 2017]. J Dermatol Treat. doi:10.1080/09546634.2016.1254145.
18. Wu D, Hou SY, Zhao S, et al. Efficacy and safety of interleukin-17 antagonists in patients with plaque psoriasis: A meta-analysis from phase 3 randomized controlled trials. J Eur Acad Dermatol Venereol. 2017;31:992-100.
19. Yang ZS, Lin NN, Li L, et al. The effect of TNF inhibitors on cardiovascular events in psoriasis and psoriatic arthritis: an updated meta-analysis. Clin Rev Allergy Immunol. 2016;51:240-247.
20. Heredi E, Vegh J, Pogacsas L, et al. Subclinical cardiovascular disease and it’s improvement after long-term TNF-alpha inhibitor therapy in severe psoriatic patients. J Eur Acad Dermatol Venereol. 2016;30:1531-1536.
21. Pina T, Corrales A, Lopez-Mejias R, et al. Anti-tumor necrosis factor-alpha therapy improves endothelial function and arterial stiffness in patients with moderate to severe psoriasis: a 6-month prospective study. J Dermatol. 2016;43:1267-1272.
22. Piaserico S, Osto E, Famoso G, et al. Treatment with tumor necrosis factor inhibitors restores coronary microvascular function in young patients with severe psoriasis. Atherosclerosis. 2016;251:25-30.
23. Van de Kerkhof PC, Griffiths CE, Reich K, et al. Secukinumab long-term safety experience: a pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2016;75:83-98.
24. Wu JJ, Guerin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90.
25. Torres T, Raposo I, Selores M. IL-17 blockade in psoriasis: friend or foe in cardiovascular risk? Am J Clin Dermatol. 2016;17:107-112.
26. Deeks ED. Apremilast: a review in psoriasis and psoriatic arthritis. Drugs. 2015;75:1393-1403.
27. Crowley J, Thaci D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for >/= 156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77:310-317.
28. Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis. 2014;73:1020-1026.
29. Daudén E, Griffiths CE, Ortonne JP, et al. Improvements in patient-reported outcomes in moderate-to-severe psoriasis patients receiving continuous or paused etanercept treatment over 54 weeks: the CRYSTEL study. J Eur Acad Dermatol Venereol. 2009;23:1374-1382.
30. Menter A, Augustin M, Signorovitch J, et al. The effect of adalimumab on reducing depression symptoms in patients with moderate to severe psoriasis: a randomized clinical trial. J Am Acad Dermatol. 2010;62:812-818.
31. Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29-35.
32. Strober B, Gooderham M, de Jong EMGJ, et al. Depressive symptoms, depression, and the effect of biologic therapy among patients in Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Am Acad Dermatol. 2018;78:70-80.
33. Egeberg A, Khalid U, Gislason GH, et al. Association of psoriatic disease with uveitis: a Danish nationwide cohort study. JAMA Dermatol. 2015;151:1200-1205.
34. Huynh N, Cervantes-Castaneda RA, Bhat P, et al. Biologic response modifier therapy for psoriatic ocular inflammatory disease. Ocul Immunol Inflamm. 2008;16:89-93.
35. Pulusani S, McMurray SL, Jensen K, et al. Psoriasis treatment in patients with sickle cell disease Cutis. 2019;103:93-94.
36. Nnodim J, Meludu SC, Dioka CE, et al. Cytokine expression in homozygous sickle cell anaemia. JKIMSU. 2015;4:34-37.
1. Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
2. Davidovici BB, Sattar N, Prinz J, et al. Psoriasis and systemic inflammatory diseases: potential mechanistic links between skin disease and co-morbid conditions. J Invest Dermatol. 2010;130:1785-1796.
3. Oliveira Mde F, Rocha Bde O, Duarte GV. Psoriasis: classical and emerging comorbidities. An Bras Dermatol. 2015;90:9-20.
4. Shah K, Mellars L, Changolkar A, Feldman SR. Real-world burden of comorbidities in US patients with psoriasis. J Am Acad Dermatol. 2017;77:287-292.
5. Hu SC, Lan CE. Psoriasis and cardiovascular comorbidities: focusing on severe vascular events, cardiovascular risk factors and implications for treatment [published online October 21, 2017]. Int J Mol Sci. doi:10.3390/ijms18102211.
6. Hugh J, Van Voorhees AS, Nijhawan RI, et al. From the Medical Board of The National Psoriasis Foundation: the risk of cardiovascular disease in individuals with psoriasis and the potential impact of current therapies. J Am Acad Dermatol. 2014;70:168-177.
7. Churton S, Brown L, Shin TM, et al. Does treatment of psoriasis reduce the risk of cardiovascular disease? Drugs. 2014;74:169-182.
8. Prodanovich S, Ma F, Taylor J, et al. Methotrexate reduces incidence of vascular diseases in veterans with psoriasis or rheumatoid arthritis. J Am Acad Dermatol. 2005;52:262-226.
9. Gulliver WP, Young HM, Bachelez H, et al. Psoriasis patients treated with biologics and methotrexate have a reduced rate of myocardial infarction: a collaborative analysis using international cohorts. J Cutan Med Surg. 2016;20:550-554.
10. Ahlehoff O, Skov L, Gislason G, et al. Cardiovascular disease event rates in patients with severe psoriasis treated with systemic anti-inflammatory drugs: a Danish real-world cohort study. J Intern Med. 2013;273:197-204.
11. Wu JJ, Poon KY, Channual JC, et al. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148:1244-1250.
12. Wu JJ, Poon KY. Association of ethnicity, tumor necrosis factor inhibitor therapy, and myocardial infarction risk in patients with psoriasis. J Am Acad Dermatol. 2013;69:167-168.
13. Wu JJ, Poon KY, Bebchuk JD. Association between the type and length of tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. J Drugs Dermatol. 2013;12:899-903.
14. Wu JJ, Poon KY, Bebchuk JD. Tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis, psoriatic arthritis, or both. J Drugs Dermatol. 2014;13:932-934.
15. Famenini S, Sako EY, Wu JJ. Effect of treating psoriasis on cardiovascular co-morbidities: focus on TNF inhibitors. Am J Clin Dermatol. 2014;15:45-50.
16. Nguyen T, Wu JJ. Relationship between tumor necrosis factor-alpha inhibitors and cardiovascular disease in psoriasis: a review. Perm J. 2014;18:49-54.
17. Shaaban D, Al-Mutairi N. The effect of tumour necrosis factor inhibitor therapy on the incidence of myocardial infarction in patients with psoriasis: a retrospective study [published online November 17, 2017]. J Dermatol Treat. doi:10.1080/09546634.2016.1254145.
18. Wu D, Hou SY, Zhao S, et al. Efficacy and safety of interleukin-17 antagonists in patients with plaque psoriasis: A meta-analysis from phase 3 randomized controlled trials. J Eur Acad Dermatol Venereol. 2017;31:992-100.
19. Yang ZS, Lin NN, Li L, et al. The effect of TNF inhibitors on cardiovascular events in psoriasis and psoriatic arthritis: an updated meta-analysis. Clin Rev Allergy Immunol. 2016;51:240-247.
20. Heredi E, Vegh J, Pogacsas L, et al. Subclinical cardiovascular disease and it’s improvement after long-term TNF-alpha inhibitor therapy in severe psoriatic patients. J Eur Acad Dermatol Venereol. 2016;30:1531-1536.
21. Pina T, Corrales A, Lopez-Mejias R, et al. Anti-tumor necrosis factor-alpha therapy improves endothelial function and arterial stiffness in patients with moderate to severe psoriasis: a 6-month prospective study. J Dermatol. 2016;43:1267-1272.
22. Piaserico S, Osto E, Famoso G, et al. Treatment with tumor necrosis factor inhibitors restores coronary microvascular function in young patients with severe psoriasis. Atherosclerosis. 2016;251:25-30.
23. Van de Kerkhof PC, Griffiths CE, Reich K, et al. Secukinumab long-term safety experience: a pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2016;75:83-98.
24. Wu JJ, Guerin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90.
25. Torres T, Raposo I, Selores M. IL-17 blockade in psoriasis: friend or foe in cardiovascular risk? Am J Clin Dermatol. 2016;17:107-112.
26. Deeks ED. Apremilast: a review in psoriasis and psoriatic arthritis. Drugs. 2015;75:1393-1403.
27. Crowley J, Thaci D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for >/= 156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77:310-317.
28. Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis. 2014;73:1020-1026.
29. Daudén E, Griffiths CE, Ortonne JP, et al. Improvements in patient-reported outcomes in moderate-to-severe psoriasis patients receiving continuous or paused etanercept treatment over 54 weeks: the CRYSTEL study. J Eur Acad Dermatol Venereol. 2009;23:1374-1382.
30. Menter A, Augustin M, Signorovitch J, et al. The effect of adalimumab on reducing depression symptoms in patients with moderate to severe psoriasis: a randomized clinical trial. J Am Acad Dermatol. 2010;62:812-818.
31. Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29-35.
32. Strober B, Gooderham M, de Jong EMGJ, et al. Depressive symptoms, depression, and the effect of biologic therapy among patients in Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Am Acad Dermatol. 2018;78:70-80.
33. Egeberg A, Khalid U, Gislason GH, et al. Association of psoriatic disease with uveitis: a Danish nationwide cohort study. JAMA Dermatol. 2015;151:1200-1205.
34. Huynh N, Cervantes-Castaneda RA, Bhat P, et al. Biologic response modifier therapy for psoriatic ocular inflammatory disease. Ocul Immunol Inflamm. 2008;16:89-93.
35. Pulusani S, McMurray SL, Jensen K, et al. Psoriasis treatment in patients with sickle cell disease Cutis. 2019;103:93-94.
36. Nnodim J, Meludu SC, Dioka CE, et al. Cytokine expression in homozygous sickle cell anaemia. JKIMSU. 2015;4:34-37.
1. Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
2. Davidovici BB, Sattar N, Prinz J, et al. Psoriasis and systemic inflammatory diseases: potential mechanistic links between skin disease and co-morbid conditions. J Invest Dermatol. 2010;130:1785-1796.
3. Oliveira Mde F, Rocha Bde O, Duarte GV. Psoriasis: classical and emerging comorbidities. An Bras Dermatol. 2015;90:9-20.
4. Shah K, Mellars L, Changolkar A, Feldman SR. Real-world burden of comorbidities in US patients with psoriasis. J Am Acad Dermatol. 2017;77:287-292.
5. Hu SC, Lan CE. Psoriasis and cardiovascular comorbidities: focusing on severe vascular events, cardiovascular risk factors and implications for treatment [published online October 21, 2017]. Int J Mol Sci. doi:10.3390/ijms18102211.
6. Hugh J, Van Voorhees AS, Nijhawan RI, et al. From the Medical Board of The National Psoriasis Foundation: the risk of cardiovascular disease in individuals with psoriasis and the potential impact of current therapies. J Am Acad Dermatol. 2014;70:168-177.
7. Churton S, Brown L, Shin TM, et al. Does treatment of psoriasis reduce the risk of cardiovascular disease? Drugs. 2014;74:169-182.
8. Prodanovich S, Ma F, Taylor J, et al. Methotrexate reduces incidence of vascular diseases in veterans with psoriasis or rheumatoid arthritis. J Am Acad Dermatol. 2005;52:262-226.
9. Gulliver WP, Young HM, Bachelez H, et al. Psoriasis patients treated with biologics and methotrexate have a reduced rate of myocardial infarction: a collaborative analysis using international cohorts. J Cutan Med Surg. 2016;20:550-554.
10. Ahlehoff O, Skov L, Gislason G, et al. Cardiovascular disease event rates in patients with severe psoriasis treated with systemic anti-inflammatory drugs: a Danish real-world cohort study. J Intern Med. 2013;273:197-204.
11. Wu JJ, Poon KY, Channual JC, et al. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148:1244-1250.
12. Wu JJ, Poon KY. Association of ethnicity, tumor necrosis factor inhibitor therapy, and myocardial infarction risk in patients with psoriasis. J Am Acad Dermatol. 2013;69:167-168.
13. Wu JJ, Poon KY, Bebchuk JD. Association between the type and length of tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. J Drugs Dermatol. 2013;12:899-903.
14. Wu JJ, Poon KY, Bebchuk JD. Tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis, psoriatic arthritis, or both. J Drugs Dermatol. 2014;13:932-934.
15. Famenini S, Sako EY, Wu JJ. Effect of treating psoriasis on cardiovascular co-morbidities: focus on TNF inhibitors. Am J Clin Dermatol. 2014;15:45-50.
16. Nguyen T, Wu JJ. Relationship between tumor necrosis factor-alpha inhibitors and cardiovascular disease in psoriasis: a review. Perm J. 2014;18:49-54.
17. Shaaban D, Al-Mutairi N. The effect of tumour necrosis factor inhibitor therapy on the incidence of myocardial infarction in patients with psoriasis: a retrospective study [published online November 17, 2017]. J Dermatol Treat. doi:10.1080/09546634.2016.1254145.
18. Wu D, Hou SY, Zhao S, et al. Efficacy and safety of interleukin-17 antagonists in patients with plaque psoriasis: A meta-analysis from phase 3 randomized controlled trials. J Eur Acad Dermatol Venereol. 2017;31:992-100.
19. Yang ZS, Lin NN, Li L, et al. The effect of TNF inhibitors on cardiovascular events in psoriasis and psoriatic arthritis: an updated meta-analysis. Clin Rev Allergy Immunol. 2016;51:240-247.
20. Heredi E, Vegh J, Pogacsas L, et al. Subclinical cardiovascular disease and it’s improvement after long-term TNF-alpha inhibitor therapy in severe psoriatic patients. J Eur Acad Dermatol Venereol. 2016;30:1531-1536.
21. Pina T, Corrales A, Lopez-Mejias R, et al. Anti-tumor necrosis factor-alpha therapy improves endothelial function and arterial stiffness in patients with moderate to severe psoriasis: a 6-month prospective study. J Dermatol. 2016;43:1267-1272.
22. Piaserico S, Osto E, Famoso G, et al. Treatment with tumor necrosis factor inhibitors restores coronary microvascular function in young patients with severe psoriasis. Atherosclerosis. 2016;251:25-30.
23. Van de Kerkhof PC, Griffiths CE, Reich K, et al. Secukinumab long-term safety experience: a pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2016;75:83-98.
24. Wu JJ, Guerin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90.
25. Torres T, Raposo I, Selores M. IL-17 blockade in psoriasis: friend or foe in cardiovascular risk? Am J Clin Dermatol. 2016;17:107-112.
26. Deeks ED. Apremilast: a review in psoriasis and psoriatic arthritis. Drugs. 2015;75:1393-1403.
27. Crowley J, Thaci D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for >/= 156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77:310-317.
28. Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis. 2014;73:1020-1026.
29. Daudén E, Griffiths CE, Ortonne JP, et al. Improvements in patient-reported outcomes in moderate-to-severe psoriasis patients receiving continuous or paused etanercept treatment over 54 weeks: the CRYSTEL study. J Eur Acad Dermatol Venereol. 2009;23:1374-1382.
30. Menter A, Augustin M, Signorovitch J, et al. The effect of adalimumab on reducing depression symptoms in patients with moderate to severe psoriasis: a randomized clinical trial. J Am Acad Dermatol. 2010;62:812-818.
31. Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29-35.
32. Strober B, Gooderham M, de Jong EMGJ, et al. Depressive symptoms, depression, and the effect of biologic therapy among patients in Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Am Acad Dermatol. 2018;78:70-80.
33. Egeberg A, Khalid U, Gislason GH, et al. Association of psoriatic disease with uveitis: a Danish nationwide cohort study. JAMA Dermatol. 2015;151:1200-1205.
34. Huynh N, Cervantes-Castaneda RA, Bhat P, et al. Biologic response modifier therapy for psoriatic ocular inflammatory disease. Ocul Immunol Inflamm. 2008;16:89-93.
35. Pulusani S, McMurray SL, Jensen K, et al. Psoriasis treatment in patients with sickle cell disease Cutis. 2019;103:93-94.
36. Nnodim J, Meludu SC, Dioka CE, et al. Cytokine expression in homozygous sickle cell anaemia. JKIMSU. 2015;4:34-37.
Obesity tied to relapse in young patients with multiple sclerosis
, results of a recent large, single-center study show. The rate of switching to second-line disease-modifying therapy was consequently about 50% higher among the obese children in the study, which included a total of 453 pediatric patients.
The link between obesity and treatment response suggests that the management of these younger patients with MS could be improved through weight loss or body mass index (BMI)-adjusted dosing, according to Peter Huppke, MD, of Georg August University in Göttingen, Germany, and co-investigators.
“The findings do not indicate that obesity promotes greater disease activity, but pharmacokinetic factors are more likely associated with treatment response,” Dr. Huppke and co-authors said in a report on their study, which was published online ahead of print July 15 in JAMA Neurology.
This is believed to be the first-ever study to find an association between BMI and treatment response in pediatric patients with MS, according to the authors, who said they also confirmed a link between obesity and MS.
Specifically, obesity increased MS susceptibility by two-fold as compared with healthy controls, a finding that they said adds to a small but growing body of evidence that high BMI is associated with increased risk of the disease in these younger individuals.
This retrospective study included 453 pediatric patients with MS treated at the Center for MS in Childhood and Adolescence in Göttingen, Germany between 1990 and 2016. About two-thirds were female and the mean age at MS diagnosis was about 14 years.
Of those patients, 126 (27.8%) were classified as obese based on a BMI greater than the 90th percentile, according to the report.
Dr. Huppke and co-investigators found that high BMI was linked to a significantly increased odds of pediatric MS, with odds ratios of 2.19 (95% CI, 1.5-3.1; P < 0.001) in girls and 2.14 (95% CI, 1.3-3.5; P = 0.003) in boys.
A total of 277 of these pediatric patients received a first-line disease-modifying therapy for 6 months or longer, including 249 treated with interferon beta and 51 treated with glatiramer.
Relapses were more common in obese patients, according to the report. with an annualized relapse rate of 1.29, compared to just 0.72 for those who were not overweight (P < 0.001).
Consequently, likelihood of receiving a second-line treatment was about 1.5 times higher in the obese or extremely obese patients, investigators said.
“A healthy weight may potentially optimize treatment outcomes and reduce disease-related burden and health care costs,” they concluded in the report, adding that BMI-adjusted dosing may “increase the value” of first-line disease-modifying therapies.
Dr. Huppke reported disclosures related to Bayer Health Care, Merck Serono, and Novartis not associated with the current study.
SOURCE: Huppke B, et al. JAMA Neurol. 2019 Jul 15. doi: 10.1001/jamaneurol.2019.1997
, results of a recent large, single-center study show. The rate of switching to second-line disease-modifying therapy was consequently about 50% higher among the obese children in the study, which included a total of 453 pediatric patients.
The link between obesity and treatment response suggests that the management of these younger patients with MS could be improved through weight loss or body mass index (BMI)-adjusted dosing, according to Peter Huppke, MD, of Georg August University in Göttingen, Germany, and co-investigators.
“The findings do not indicate that obesity promotes greater disease activity, but pharmacokinetic factors are more likely associated with treatment response,” Dr. Huppke and co-authors said in a report on their study, which was published online ahead of print July 15 in JAMA Neurology.
This is believed to be the first-ever study to find an association between BMI and treatment response in pediatric patients with MS, according to the authors, who said they also confirmed a link between obesity and MS.
Specifically, obesity increased MS susceptibility by two-fold as compared with healthy controls, a finding that they said adds to a small but growing body of evidence that high BMI is associated with increased risk of the disease in these younger individuals.
This retrospective study included 453 pediatric patients with MS treated at the Center for MS in Childhood and Adolescence in Göttingen, Germany between 1990 and 2016. About two-thirds were female and the mean age at MS diagnosis was about 14 years.
Of those patients, 126 (27.8%) were classified as obese based on a BMI greater than the 90th percentile, according to the report.
Dr. Huppke and co-investigators found that high BMI was linked to a significantly increased odds of pediatric MS, with odds ratios of 2.19 (95% CI, 1.5-3.1; P < 0.001) in girls and 2.14 (95% CI, 1.3-3.5; P = 0.003) in boys.
A total of 277 of these pediatric patients received a first-line disease-modifying therapy for 6 months or longer, including 249 treated with interferon beta and 51 treated with glatiramer.
Relapses were more common in obese patients, according to the report. with an annualized relapse rate of 1.29, compared to just 0.72 for those who were not overweight (P < 0.001).
Consequently, likelihood of receiving a second-line treatment was about 1.5 times higher in the obese or extremely obese patients, investigators said.
“A healthy weight may potentially optimize treatment outcomes and reduce disease-related burden and health care costs,” they concluded in the report, adding that BMI-adjusted dosing may “increase the value” of first-line disease-modifying therapies.
Dr. Huppke reported disclosures related to Bayer Health Care, Merck Serono, and Novartis not associated with the current study.
SOURCE: Huppke B, et al. JAMA Neurol. 2019 Jul 15. doi: 10.1001/jamaneurol.2019.1997
, results of a recent large, single-center study show. The rate of switching to second-line disease-modifying therapy was consequently about 50% higher among the obese children in the study, which included a total of 453 pediatric patients.
The link between obesity and treatment response suggests that the management of these younger patients with MS could be improved through weight loss or body mass index (BMI)-adjusted dosing, according to Peter Huppke, MD, of Georg August University in Göttingen, Germany, and co-investigators.
“The findings do not indicate that obesity promotes greater disease activity, but pharmacokinetic factors are more likely associated with treatment response,” Dr. Huppke and co-authors said in a report on their study, which was published online ahead of print July 15 in JAMA Neurology.
This is believed to be the first-ever study to find an association between BMI and treatment response in pediatric patients with MS, according to the authors, who said they also confirmed a link between obesity and MS.
Specifically, obesity increased MS susceptibility by two-fold as compared with healthy controls, a finding that they said adds to a small but growing body of evidence that high BMI is associated with increased risk of the disease in these younger individuals.
This retrospective study included 453 pediatric patients with MS treated at the Center for MS in Childhood and Adolescence in Göttingen, Germany between 1990 and 2016. About two-thirds were female and the mean age at MS diagnosis was about 14 years.
Of those patients, 126 (27.8%) were classified as obese based on a BMI greater than the 90th percentile, according to the report.
Dr. Huppke and co-investigators found that high BMI was linked to a significantly increased odds of pediatric MS, with odds ratios of 2.19 (95% CI, 1.5-3.1; P < 0.001) in girls and 2.14 (95% CI, 1.3-3.5; P = 0.003) in boys.
A total of 277 of these pediatric patients received a first-line disease-modifying therapy for 6 months or longer, including 249 treated with interferon beta and 51 treated with glatiramer.
Relapses were more common in obese patients, according to the report. with an annualized relapse rate of 1.29, compared to just 0.72 for those who were not overweight (P < 0.001).
Consequently, likelihood of receiving a second-line treatment was about 1.5 times higher in the obese or extremely obese patients, investigators said.
“A healthy weight may potentially optimize treatment outcomes and reduce disease-related burden and health care costs,” they concluded in the report, adding that BMI-adjusted dosing may “increase the value” of first-line disease-modifying therapies.
Dr. Huppke reported disclosures related to Bayer Health Care, Merck Serono, and Novartis not associated with the current study.
SOURCE: Huppke B, et al. JAMA Neurol. 2019 Jul 15. doi: 10.1001/jamaneurol.2019.1997
FROM JAMA NEUROLOGY
Key clinical point: Obese children and adolescents with MS had about twice as many relapses on first-line treatment as compared with their non-obese counterparts.
Major finding: The annualized relapse rate was 1.29 for obese pediatric patients, compared to 0.72 for those who were not overweight (P < 0.001).
Study details: Retrospective study including 453 patients with pediatric MS treated at a center in Göttingen, Germany between 1990 and 2016.
Disclosures: The senior author reported disclosures related to Bayer Health Care, Merck Serono, and Novartis unrelated to the this study.
Source: Huppke B, et al. JAMA Neurol. 2019 Jul 15.
Interview with Mary Alissa Willis, MD, on MS and Mental Health
Mary Alissa Willis, MD, is the Medical Director for the Mellen Center for Multiple Sclerosis at the Cleveland Clinic. We spoke with Dr. Willis about mental health issues in persons with MS and what health care facilities can do to better care for these patients.
What is the prevalence of depression in patients with MS?
Depression and anxiety are common in persons with multiple sclerosis (PwMS). In a systematic review, Ruth Ann Marrie, MD, PhD, and colleagues estimated the prevalence of depression in PwMS to be 23.7%. Approximately half of all PwMS experience depression at some point after their diagnosis.1-3 This makes depression nearly twice as common in MS as in the general population.
What are some reasons patients with MS might begin to experience depression? What are some warning signs to look out for?
Many people assume that PwMS experience depression because of psychosocial stressors, unpredictability of disease progression, or poor coping strategies. Although these factors do contribute to depression, there is increasing evidence that immune dysregulation and structural changes in the brain caused by MS disease activity make some PwMS uniquely vulnerable to depression. Functional MRI studies have shown abnormalities in the prefrontal-subcortical network connectivity, which is involved in mood regulation. In addition, persistent somatic symptoms such as severe pain and fatigue limit daily activities and social participation—generally considered to be protective factors in depression.4 Warning signs for depression include loss of interest in activities, weight loss or weight gain, changes in sleep—too much or too little, an increase in fatigue, expressions of hopelessness or guilt, or increasing drug or alcohol use.
What are some special considerations for patients with MS battling suicidal ideation?
Anthony Feinstein, MBBCh, MPhil, PhD, and colleagues reported that more than 28% of PwMS had suicidal ideation at some point.5 This is a huge number of people struggling with thoughts of suicide. Depression is a risk factor for suicide but other risks specific to PwMS include perceived loss of control, loss of job/income/social roles, loss of driving privileges, and marked physical or cognitive difficulties. While we are doing a better job screening for depression with quick screening tools such as the PHQ9, we could do better in suicide risk assessment.
What can health care providers do better to address depression and risk for suicide? What safety/preventative measures can they take?
The first step in better addressing depression and risk for suicide is to directly ask patients. Pay attention to changes in appearance, behavior, requests for prescription refills, frequency of appointments, and who accompanies patients to appointments. Explore the reasons for these changes. It is important to respond proactively when comments or behavior suggest a patient at risk. Ask specific questions about plan, access to means, previous suicide attempts, and support network. Refer promptly for emergency or mental health services when appropriate. Familiarity with mental health colleagues, local crisis centers, and helplines can be helpful in engaging a team of people to provide assistance to a patient in need.
References
1. Marrie RA, Fisk JD, Tremlett H, et al. Differences in the burden of psychiatric comorbidity in MS vs the general population. Neurology. 2015;85(22):1972-1979.
2. Minden SL, Schiffer RB. Affective disorders in multiple sclerosis. Review and recommendations for clinical research. Arch Neurol. 1990;47(1):98-104.
3. Marrie RA, Walld R, Bolton JM, et al; CIHR Team in Defining the Burden and Managing the Effects of Psychiatric Comorbidity in Chronic Immunoinflammatory Disease. Estimating annual prevalence of depression and anxiety disorder in multiple sclerosis using administrative data. BMC Res Notes. 2017;10(1):619.
4. Passamonti L. Cerasa A, Liguori M, et al. Neurobiological mechanisms underlying emotional processing in relapsing-remitting multiple sclerosis. Brain. 2009;132(pt 12):3380-3391.
5. Feinstein A. An examination of suicidal intent in patients with multiple sclerosis. Neurology. 2002;59(5):674-678.
Mary Alissa Willis, MD, is the Medical Director for the Mellen Center for Multiple Sclerosis at the Cleveland Clinic. We spoke with Dr. Willis about mental health issues in persons with MS and what health care facilities can do to better care for these patients.
What is the prevalence of depression in patients with MS?
Depression and anxiety are common in persons with multiple sclerosis (PwMS). In a systematic review, Ruth Ann Marrie, MD, PhD, and colleagues estimated the prevalence of depression in PwMS to be 23.7%. Approximately half of all PwMS experience depression at some point after their diagnosis.1-3 This makes depression nearly twice as common in MS as in the general population.
What are some reasons patients with MS might begin to experience depression? What are some warning signs to look out for?
Many people assume that PwMS experience depression because of psychosocial stressors, unpredictability of disease progression, or poor coping strategies. Although these factors do contribute to depression, there is increasing evidence that immune dysregulation and structural changes in the brain caused by MS disease activity make some PwMS uniquely vulnerable to depression. Functional MRI studies have shown abnormalities in the prefrontal-subcortical network connectivity, which is involved in mood regulation. In addition, persistent somatic symptoms such as severe pain and fatigue limit daily activities and social participation—generally considered to be protective factors in depression.4 Warning signs for depression include loss of interest in activities, weight loss or weight gain, changes in sleep—too much or too little, an increase in fatigue, expressions of hopelessness or guilt, or increasing drug or alcohol use.
What are some special considerations for patients with MS battling suicidal ideation?
Anthony Feinstein, MBBCh, MPhil, PhD, and colleagues reported that more than 28% of PwMS had suicidal ideation at some point.5 This is a huge number of people struggling with thoughts of suicide. Depression is a risk factor for suicide but other risks specific to PwMS include perceived loss of control, loss of job/income/social roles, loss of driving privileges, and marked physical or cognitive difficulties. While we are doing a better job screening for depression with quick screening tools such as the PHQ9, we could do better in suicide risk assessment.
What can health care providers do better to address depression and risk for suicide? What safety/preventative measures can they take?
The first step in better addressing depression and risk for suicide is to directly ask patients. Pay attention to changes in appearance, behavior, requests for prescription refills, frequency of appointments, and who accompanies patients to appointments. Explore the reasons for these changes. It is important to respond proactively when comments or behavior suggest a patient at risk. Ask specific questions about plan, access to means, previous suicide attempts, and support network. Refer promptly for emergency or mental health services when appropriate. Familiarity with mental health colleagues, local crisis centers, and helplines can be helpful in engaging a team of people to provide assistance to a patient in need.
References
1. Marrie RA, Fisk JD, Tremlett H, et al. Differences in the burden of psychiatric comorbidity in MS vs the general population. Neurology. 2015;85(22):1972-1979.
2. Minden SL, Schiffer RB. Affective disorders in multiple sclerosis. Review and recommendations for clinical research. Arch Neurol. 1990;47(1):98-104.
3. Marrie RA, Walld R, Bolton JM, et al; CIHR Team in Defining the Burden and Managing the Effects of Psychiatric Comorbidity in Chronic Immunoinflammatory Disease. Estimating annual prevalence of depression and anxiety disorder in multiple sclerosis using administrative data. BMC Res Notes. 2017;10(1):619.
4. Passamonti L. Cerasa A, Liguori M, et al. Neurobiological mechanisms underlying emotional processing in relapsing-remitting multiple sclerosis. Brain. 2009;132(pt 12):3380-3391.
5. Feinstein A. An examination of suicidal intent in patients with multiple sclerosis. Neurology. 2002;59(5):674-678.
Mary Alissa Willis, MD, is the Medical Director for the Mellen Center for Multiple Sclerosis at the Cleveland Clinic. We spoke with Dr. Willis about mental health issues in persons with MS and what health care facilities can do to better care for these patients.
What is the prevalence of depression in patients with MS?
Depression and anxiety are common in persons with multiple sclerosis (PwMS). In a systematic review, Ruth Ann Marrie, MD, PhD, and colleagues estimated the prevalence of depression in PwMS to be 23.7%. Approximately half of all PwMS experience depression at some point after their diagnosis.1-3 This makes depression nearly twice as common in MS as in the general population.
What are some reasons patients with MS might begin to experience depression? What are some warning signs to look out for?
Many people assume that PwMS experience depression because of psychosocial stressors, unpredictability of disease progression, or poor coping strategies. Although these factors do contribute to depression, there is increasing evidence that immune dysregulation and structural changes in the brain caused by MS disease activity make some PwMS uniquely vulnerable to depression. Functional MRI studies have shown abnormalities in the prefrontal-subcortical network connectivity, which is involved in mood regulation. In addition, persistent somatic symptoms such as severe pain and fatigue limit daily activities and social participation—generally considered to be protective factors in depression.4 Warning signs for depression include loss of interest in activities, weight loss or weight gain, changes in sleep—too much or too little, an increase in fatigue, expressions of hopelessness or guilt, or increasing drug or alcohol use.
What are some special considerations for patients with MS battling suicidal ideation?
Anthony Feinstein, MBBCh, MPhil, PhD, and colleagues reported that more than 28% of PwMS had suicidal ideation at some point.5 This is a huge number of people struggling with thoughts of suicide. Depression is a risk factor for suicide but other risks specific to PwMS include perceived loss of control, loss of job/income/social roles, loss of driving privileges, and marked physical or cognitive difficulties. While we are doing a better job screening for depression with quick screening tools such as the PHQ9, we could do better in suicide risk assessment.
What can health care providers do better to address depression and risk for suicide? What safety/preventative measures can they take?
The first step in better addressing depression and risk for suicide is to directly ask patients. Pay attention to changes in appearance, behavior, requests for prescription refills, frequency of appointments, and who accompanies patients to appointments. Explore the reasons for these changes. It is important to respond proactively when comments or behavior suggest a patient at risk. Ask specific questions about plan, access to means, previous suicide attempts, and support network. Refer promptly for emergency or mental health services when appropriate. Familiarity with mental health colleagues, local crisis centers, and helplines can be helpful in engaging a team of people to provide assistance to a patient in need.
References
1. Marrie RA, Fisk JD, Tremlett H, et al. Differences in the burden of psychiatric comorbidity in MS vs the general population. Neurology. 2015;85(22):1972-1979.
2. Minden SL, Schiffer RB. Affective disorders in multiple sclerosis. Review and recommendations for clinical research. Arch Neurol. 1990;47(1):98-104.
3. Marrie RA, Walld R, Bolton JM, et al; CIHR Team in Defining the Burden and Managing the Effects of Psychiatric Comorbidity in Chronic Immunoinflammatory Disease. Estimating annual prevalence of depression and anxiety disorder in multiple sclerosis using administrative data. BMC Res Notes. 2017;10(1):619.
4. Passamonti L. Cerasa A, Liguori M, et al. Neurobiological mechanisms underlying emotional processing in relapsing-remitting multiple sclerosis. Brain. 2009;132(pt 12):3380-3391.
5. Feinstein A. An examination of suicidal intent in patients with multiple sclerosis. Neurology. 2002;59(5):674-678.
Psoriasis Treatment in Patients With HIV
- Nakamura M, Abrouk M, Farahnik B, et al. Psoriasis treatment in HIV-positive patients: a systematic review of systemic immunosuppressive therapies. Cutis. 2018;101:38, 42, 56.
- Patel RV, Weinberg JM. Psoriasis in the patient with human immunodeficiency virus, part 2: review of treatment. Cutis. 2008;82:202-210.
- Ceccarelli M, Venanzi Rullo E, Vaccaro M, et al. HIV‐associated psoriasis: epidemiology, pathogenesis, and management [published online January 6, 2019]. Dermatol Ther. 2019;32:e12806. doi:10.1111/dth.12806.
- Zarbafian M, Richer V. Treatment of moderate to severe psoriasis with apremilast over 2 years in the context of long-term treated HIV infection: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19845193. doi:10.1177/2050313X19845193.
- Menon K, Van Vorhees AS, Bebo, BF, et al. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol. 2010;62:291-299.
- Mallon E, Bunker CB. HIV-associated psoriasis. AIDS Patient Care STDS. 2000;14:239-246.
- Patel VA, Weinberg JM. Psoriasis in the patient with human immunodeficiency virus, part 1: review of pathogenesis. Cutis. 2008;82:117-122.
- Castillo RL, Racaza GZ, Dela Cruz Roa F. Ostraceous and inverse psoriasis with psoriatic arthritis as the presenting features of advanced HIV infection. Singapore Med J. 2014;55:e60-e63.
- Duvic M, Crane MM, Conant M, et al. Zidovudine improves psoriasis in human immunodeficiency virus- positive males. Arch Dermatol. 1994;130:447.
- Jaffee D, May LP, Sanchez M, et al. Staphylococcal sepsis in HIV antibody seropositive psoriasis patients. J Am Acad Dermatol. 1991;24:970-972.
- King LE, Dufresne RG, Lovette GL, et al. Erythroderma: review of 82 cases. South Med J. 1986;79:1210-1215.
- Kaminetsky J, Aziz M, Kaushik S. A review of biologics and other treatment modalities in HIV-associated psoriasis. Skin. 2018;2:389-401.
- Wolff K. Side effects of psoralen photochemotherapy (PUVA). Br J Dermatol. 1990;122:117-125.
- Stern RS, Mills DK, Krell K, et al. HIV-positive patients differ from HIV-negative patients in indications for and type of UV therapy used. J Am Acad Dermatol. 1998;39:48-55.
- Oracion RM, Skiest DJ, Keiser PH, et al. HIV-related skin diseases. Prog Dermatol. 1999;33:1-6.
- Finkelstein M, Berman B. HIV and AIDS in inpatient dermatology: approach to the consultation. Dermatol Clin. 2000;18:509-520.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections. J Am Acad Dermatol. 2019;80:43-53.
- Sellam J, Bouvard B, Masson C, et al. Use of infliximab to treat psoriatic arthritis in HIV-positive patients. Joint Bone Spine. 2007;74:197-200.
- Reddy SP, Lee E, Wu JJ. Apremilast and phototherapy for treatment of psoriasis in a patient with human immunodeficiency virus. Cutis. 2019;103:E1-E7.
- Otezla (apremilast). Summit, NJ: Celgene Corporation; 2017.
- Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83:1583-1590.
- Nakamura M, Abrouk M, Farahnik B, et al. Psoriasis treatment in HIV-positive patients: a systematic review of systemic immunosuppressive therapies. Cutis. 2018;101:38, 42, 56.
- Patel RV, Weinberg JM. Psoriasis in the patient with human immunodeficiency virus, part 2: review of treatment. Cutis. 2008;82:202-210.
- Ceccarelli M, Venanzi Rullo E, Vaccaro M, et al. HIV‐associated psoriasis: epidemiology, pathogenesis, and management [published online January 6, 2019]. Dermatol Ther. 2019;32:e12806. doi:10.1111/dth.12806.
- Zarbafian M, Richer V. Treatment of moderate to severe psoriasis with apremilast over 2 years in the context of long-term treated HIV infection: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19845193. doi:10.1177/2050313X19845193.
- Menon K, Van Vorhees AS, Bebo, BF, et al. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol. 2010;62:291-299.
- Mallon E, Bunker CB. HIV-associated psoriasis. AIDS Patient Care STDS. 2000;14:239-246.
- Patel VA, Weinberg JM. Psoriasis in the patient with human immunodeficiency virus, part 1: review of pathogenesis. Cutis. 2008;82:117-122.
- Castillo RL, Racaza GZ, Dela Cruz Roa F. Ostraceous and inverse psoriasis with psoriatic arthritis as the presenting features of advanced HIV infection. Singapore Med J. 2014;55:e60-e63.
- Duvic M, Crane MM, Conant M, et al. Zidovudine improves psoriasis in human immunodeficiency virus- positive males. Arch Dermatol. 1994;130:447.
- Jaffee D, May LP, Sanchez M, et al. Staphylococcal sepsis in HIV antibody seropositive psoriasis patients. J Am Acad Dermatol. 1991;24:970-972.
- King LE, Dufresne RG, Lovette GL, et al. Erythroderma: review of 82 cases. South Med J. 1986;79:1210-1215.
- Kaminetsky J, Aziz M, Kaushik S. A review of biologics and other treatment modalities in HIV-associated psoriasis. Skin. 2018;2:389-401.
- Wolff K. Side effects of psoralen photochemotherapy (PUVA). Br J Dermatol. 1990;122:117-125.
- Stern RS, Mills DK, Krell K, et al. HIV-positive patients differ from HIV-negative patients in indications for and type of UV therapy used. J Am Acad Dermatol. 1998;39:48-55.
- Oracion RM, Skiest DJ, Keiser PH, et al. HIV-related skin diseases. Prog Dermatol. 1999;33:1-6.
- Finkelstein M, Berman B. HIV and AIDS in inpatient dermatology: approach to the consultation. Dermatol Clin. 2000;18:509-520.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections. J Am Acad Dermatol. 2019;80:43-53.
- Sellam J, Bouvard B, Masson C, et al. Use of infliximab to treat psoriatic arthritis in HIV-positive patients. Joint Bone Spine. 2007;74:197-200.
- Reddy SP, Lee E, Wu JJ. Apremilast and phototherapy for treatment of psoriasis in a patient with human immunodeficiency virus. Cutis. 2019;103:E1-E7.
- Otezla (apremilast). Summit, NJ: Celgene Corporation; 2017.
- Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83:1583-1590.
- Nakamura M, Abrouk M, Farahnik B, et al. Psoriasis treatment in HIV-positive patients: a systematic review of systemic immunosuppressive therapies. Cutis. 2018;101:38, 42, 56.
- Patel RV, Weinberg JM. Psoriasis in the patient with human immunodeficiency virus, part 2: review of treatment. Cutis. 2008;82:202-210.
- Ceccarelli M, Venanzi Rullo E, Vaccaro M, et al. HIV‐associated psoriasis: epidemiology, pathogenesis, and management [published online January 6, 2019]. Dermatol Ther. 2019;32:e12806. doi:10.1111/dth.12806.
- Zarbafian M, Richer V. Treatment of moderate to severe psoriasis with apremilast over 2 years in the context of long-term treated HIV infection: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19845193. doi:10.1177/2050313X19845193.
- Menon K, Van Vorhees AS, Bebo, BF, et al. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol. 2010;62:291-299.
- Mallon E, Bunker CB. HIV-associated psoriasis. AIDS Patient Care STDS. 2000;14:239-246.
- Patel VA, Weinberg JM. Psoriasis in the patient with human immunodeficiency virus, part 1: review of pathogenesis. Cutis. 2008;82:117-122.
- Castillo RL, Racaza GZ, Dela Cruz Roa F. Ostraceous and inverse psoriasis with psoriatic arthritis as the presenting features of advanced HIV infection. Singapore Med J. 2014;55:e60-e63.
- Duvic M, Crane MM, Conant M, et al. Zidovudine improves psoriasis in human immunodeficiency virus- positive males. Arch Dermatol. 1994;130:447.
- Jaffee D, May LP, Sanchez M, et al. Staphylococcal sepsis in HIV antibody seropositive psoriasis patients. J Am Acad Dermatol. 1991;24:970-972.
- King LE, Dufresne RG, Lovette GL, et al. Erythroderma: review of 82 cases. South Med J. 1986;79:1210-1215.
- Kaminetsky J, Aziz M, Kaushik S. A review of biologics and other treatment modalities in HIV-associated psoriasis. Skin. 2018;2:389-401.
- Wolff K. Side effects of psoralen photochemotherapy (PUVA). Br J Dermatol. 1990;122:117-125.
- Stern RS, Mills DK, Krell K, et al. HIV-positive patients differ from HIV-negative patients in indications for and type of UV therapy used. J Am Acad Dermatol. 1998;39:48-55.
- Oracion RM, Skiest DJ, Keiser PH, et al. HIV-related skin diseases. Prog Dermatol. 1999;33:1-6.
- Finkelstein M, Berman B. HIV and AIDS in inpatient dermatology: approach to the consultation. Dermatol Clin. 2000;18:509-520.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections. J Am Acad Dermatol. 2019;80:43-53.
- Sellam J, Bouvard B, Masson C, et al. Use of infliximab to treat psoriatic arthritis in HIV-positive patients. Joint Bone Spine. 2007;74:197-200.
- Reddy SP, Lee E, Wu JJ. Apremilast and phototherapy for treatment of psoriasis in a patient with human immunodeficiency virus. Cutis. 2019;103:E1-E7.
- Otezla (apremilast). Summit, NJ: Celgene Corporation; 2017.
- Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83:1583-1590.
Partners in Oncology Care: Coordinated Follicular Lymphoma Management (FULL)
Four case examples illustrate the important role of multidisciplinary medical care for the optimal long-term care of patients with follicular lymphoma.
Patients benefit from multidisciplinary care that coordinates management of complex medical problems. Traditionally, multidisciplinary cancer care involves oncology specialty providers in fields that include medical oncology, radiation oncology, and surgical oncology. Multidisciplinary cancer care intends to improve patient outcomes by bringing together different health care providers (HCPs) who are involved in the treatment of patients with cancer. Because new therapies are more effective and allow patients with cancer to live longer, adverse effects (AEs) are more likely to impact patients’ well-being, both while receiving treatment and long after it has completed. Thus, this population may benefit from an expanded approach to multidisciplinary care that includes input from specialty and primary care providers (PCPs), clinical pharmacy specialists (CPS), physical and occupational therapists, and patient navigators and educators.
We present 4 hypothetical cases, based on actual patients, that illustrate opportunities where multidisciplinary care coordination may improve patient experiences. These cases draw on current quality initiatives from the National Cancer Institute Community Cancer Centers Program, which has focused on improving the quality of multidisciplinary cancer care at selected community centers, and the Veterans Health Administration (VHA) patient-aligned care team (PACT) model, which brings together different health professionals to optimize primary care coordination.1,2 In addition, the National Committee for Quality Assurance has introduced an educational initiative to facilitate implementation of an oncologic medical home.3 This initiative stresses increased multidisciplinary communication, patient-centered care delivery, and reduced fragmentation of care for this population. Despite these guidelines and experiences from other medical specialties, models for integrated cancer care have not been implemented in a prospective fashion within the VHA.
In this article, we focus on opportunities to take collaborative care approaches for the treatment of patients with follicular lymphoma (FL): a common, incurable, and often indolent B-cell non-Hodgkin lymphoma.4 FL was selected because these patients may be treated numerous times and long-term sequalae can accumulate throughout their cancer continuum (a series of health events encompassing cancer screening, diagnosis, treatment, survivorship, relapse, and death).5 HCPs in distinct roles can assist patients with cancer in optimizing their health outcomes and overall wellbeing.6
Case Example 1
A 70-year-old male was diagnosed with stage IV FL. Because of his advanced disease, he began therapy with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone). Prednisone was administered at 100 mg daily on the first 5 days of each 21-day cycle. On day 4 of the first treatment cycle, the patient notified his oncologist that he had been very thirsty and his random blood sugar values on 2 different days were 283 mg/dL and 312 mg/dL. A laboratory review revealed his hemoglobin A1c (HbA1c) 7 months prior was 5.6%.
Discussion
The high-dose prednisone component of this and other lymphoma therapy regimens can worsen diabetes mellitus (DM) control and/or worsen prediabetes. Patient characteristics that increase the risk of developing glucocorticoid-induced DM after CHOP chemotherapy include age ≥ 60 years, HbA1c > 6.1%, and body mass index > 30.7 This patient did not have DM prior to the FL therapy initiation, but afterwards he met diagnostic criteria for DM. For completeness, other causes for elevated blood glucose should be ruled out (ie, infection, laboratory error, etc.). An oncologist often will triage acute hyperglycemia, treating immediately with IV fluids and/or insulin. Thereafter, ongoing chronic disease management for DM may be best managed by PCPs, certified DM educators, and registered dieticians.
Several programs involving multidisciplinary DM care, comprised of physicians, advanced practice providers, nurses, certified DM educators, and/or pharmacists have been shown to improve HbA1c, cardiovascular outcomes, and all-cause mortality, while reducing health care costs.8 In addition, patient navigators can assist patients with coordinating visits to disease-state specialists and identifying further educational needs. For example, in 1 program, nonclinical peer navigators were shown to improve the number of appointments attended and reduce HbA1c in a population of patients with DM who were primarily minority, urban, and of low socioeconomic status.9 Thus, integrating DM care shows potential to improve outcomes for patients with lymphoma who develop glucocorticoid
Case Example 2
A 75-year-old male was diagnosed with FL. He was treated initially with bendamustine and rituximab. He required reinitiation of therapy 20 months later when he developed lymphadenopathy, fatigue, and night sweats and began treatment with oral idelalisib, a second-line therapy. Later, the patient presented to his PCP for a routine visit, and on medication reconciliation review, the patient reported regular use of trimethoprim-sulfamethoxazole.
Discussion
Upon consultation with the CPS and the patient’s oncologist, the PCP confirmed trimethoprim-sulfamethoxazole should be continued during therapy and for about 6 months following completion of therapy. Trimethoprim-sulfamethoxazole is used for prophylaxis against Pneumocystis jirovecii (formerly Pneumocystis carinii). While use of prophylactic therapy is not necessary for all patients with FL, idelalisib impairs the function of circulating lymphoid B-cells and thus has been associated with an increased risk of serious infection.10 A CPS can provide insight that maximizes medication adherence and efficacy while minimizing food-drug, drug-drug interactions, and AEs. CPS have been shown to: improve adherence to oral therapies, increase prospective monitoring required for safe therapy dose selection, and document assessment of chemotherapy-related AEs.11,12 Thus, multidisciplinary, integrated care is an important component of providing quality oncology care.
Case Example 3
A 60-year-old female presented to her PCP with a 2-week history of shortness of breath and leg swelling. She was treated for FL 4 years previously with 6 cycles of R-CHOP. She reported no chest pain and did not have a prior history of hypertension, DM, or heart disease. On physical exam, she had elevated jugular venous pressure to jaw at 45°, bilateral pulmonary rales, and 2+ pitting pretibial edema. Laboratory tests that included complete blood count, basic chemistries, and thyroid stimulating hormone were unremarkable, though brain natriuretic peptide (BNP) was elevated at 425 pg/mL.
As this patient’s laboratory results and physical examination suggested new-onset congestive heart failure, the PCP obtained an echocardiogram, which demonstrated an ejection fraction of 35% and global hypokinesis. Because the patient was symptomatic, she was admitted to the hospital to begin guideline-directed medical therapy (GDMT) including IV diuresis.
Discussion
Given the absence of significant risk factors and prior history of coronary artery disease, the most probable cause for this patient’s cardiomyopathy is doxorubicin. Doxorubicin is an anthracycline chemotherapy that can cause nonischemic, dilated cardiomyopathy, particularly when cumulative doses > 400 mg/m2 are administered, or when combined with chest radiation.13 This patient benefited from GDMT for reduced ejection-fraction heart failure (HFrEF). Studies have demonstrated positive outcomes when HFrEF patients are cared for by a multidisciplinary team who focus of volume management as well as uptitration of therapies to target doses.14
Case Example 4
An 80-year-old female was diagnosed with stage III FL but did not require immediate therapy. After developing discomfort due to enlarging lymphadenopathy, she initiated therapy with rituximab, cyclophosphamide, vincristine, and prednisone (R-CVP). She presented to her oncologist for consideration of her fifth cycle of R-CVP and reported a burning sensation on the soles of her feet and numbness in her fingertips and toes. On examination, her pulses were intact and there were no signs of infection, reduced blood flow, or edema. The patient demonstrated decreased sensation on monofilament testing. She had no history of DM and a recent HbA1c test was 4.9% An evaluation for other causes of neuropathy, such as hypothyroidism and vitamin B12 deficiency was negative. Thus, vincristine therapy was identified as the most likely etiology for her peripheral neuropathy. The oncologist decided to proceed with cycle 5 of chemotherapy but reduced the dose of vincristine by 50%.
Discussion
Vincristine is a microtubule inhibitor used in many chemotherapy regimens and may cause reversible or permanent neuropathy, including autonomic (constipation), sensory (stocking-glove distribution), or motor (foot-drop).15 A nerve conduction study may be indicated as part of the diagnostic evaluation. Treatment for painful sensory neuropathy may include pharmacologic therapy (such as gabapentin, pregabalin, capsaicin cream).16 Podiatrists can provide foot care and may provide shoes and inserts if appropriate. Physical therapists may assist with safety and mobility evaluations and can provide therapeutic exercises and assistive devices that improve function and quality of life.17
Conclusion
As cancer becomes more curable and more manageable, patients with cancer and survivors no longer rely exclusively on their oncologists for medical care. This is increasingly prevalent for patients with incurable but indolent cancers that may be present for years to decades, as acute and cumulative toxicities may complicate existing comorbidities. Thus, in this era of increasingly complex cancer therapies, multidisciplinary medical care that involves PCPs, specialists, and allied medical professionals, is essential for providing care that optimizes health and fully addresses patients’ needs.
1. Friedman EL, Chawla N, Morris PT, et al. Assessing the development of multidisciplinary care: experience of the National Cancer Institute community cancer centers program. J Oncol Pract. 2015;11(1):e36-e43.
2. Peterson K, Helfand M, Humphrey L, Christensen V, Carson S. Evidence brief: effectiveness of intensive primary care programs. https://www.hsrd.research.va.gov/publications/esp/Intensive-Primary-Care-Supplement.pdf. Published February 2013. Accessed April 5, 2019.
3. National Committee for Quality Assurance. Oncology medical home recognition. https://www.ncqa.org/programs/health-care-providers-practices/oncology-medical-home. Accessed April 5, 2019.
4. Kahl BS, Yang DT. Follicular lymphoma: evolving therapeutic strategies. Blood. 2016;127(17):2055-2063.
5. Dulaney C, Wallace AS, Everett AS, Dover L, McDonald A, Kropp L. Defining health across the cancer continuum. Cureus. 2017;9(2):e1029.
6. Hopkins J, Mumber MP. Patient navigation through the cancer care continuum: an overview. J Oncol Pract. 2009;5(4):150-152.
7. Lee SY, Kurita N, Yokoyama Y, et al. Glucocorticoid-induced diabetes mellitus in patients with lymphoma treated with CHOP chemotherapy. Support Care Cancer. 2014;22(5):1385-1390.
8. McGill M, Blonde L, Juliana CN, et al; Global Partnership for Effective Diabetes Management. The interdisciplinary team in type 2 diabetes management: challenges and best practice solutions from real-world scenarios. J Clin Transl Endocrinol. 2017;7:21-27.
9. Horný M, Glover W, Gupte G, Saraswat A, Vimalananda V, Rosenzweig J. Patient navigation to improve diabetes outpatient care at a safety-net hospital: a retrospective cohort study. BMC Health Serv Res. 2017;17(1):759.
10. Reinwald M, Silva JT, Mueller NJ, et al. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) Consensus Document on the safety of targeted and biological therapies: an infectious diseases perspective (Intracellular signaling pathways: tyrosine kinase and mTOR inhibitors). Clin Microbiol Infect. 2018;24(suppl 2):S53-S70.
11. Holle LM, Boehnke Michaud L. Oncology pharmacists in health care delivery: vital members of the cancer care team. J. Oncol. Pract. 2014;10(3):e142-e145.
12. Morgan KP, Muluneh B, Dean AM, Amerine LB. Impact of an integrated oral chemotherapy program on patient adherence. J Oncol Pharm Pract. 2018;24(5):332-336.
13. Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97(11):2869-2879.
14. Feltner C, Jones CD, Cené CW, et al. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta-analysis. Ann Intern Med. 2014;160(11):774-784.
15. Mora E, Smith EM, Donohoe C, Hertz DL. Vincristine-induced peripheral neuropathy in pediatric cancer patients. Am J Cancer Res. 2016;6(11):2416-2430.
16. Hershman DL, Lacchetti C, Dworkin RH, et al; American Society of Clinical Oncology. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32(18):1941–1967
17. Duregon F, Vendramin B, Bullo V, et al. Effects of exercise on cancer patients suffering chemotherapy-induced peripheral neuropathy undergoing treatment: a systematic review. Crit Rev Oncol Hematol. 2018;121:90-100.
Four case examples illustrate the important role of multidisciplinary medical care for the optimal long-term care of patients with follicular lymphoma.
Four case examples illustrate the important role of multidisciplinary medical care for the optimal long-term care of patients with follicular lymphoma.
Patients benefit from multidisciplinary care that coordinates management of complex medical problems. Traditionally, multidisciplinary cancer care involves oncology specialty providers in fields that include medical oncology, radiation oncology, and surgical oncology. Multidisciplinary cancer care intends to improve patient outcomes by bringing together different health care providers (HCPs) who are involved in the treatment of patients with cancer. Because new therapies are more effective and allow patients with cancer to live longer, adverse effects (AEs) are more likely to impact patients’ well-being, both while receiving treatment and long after it has completed. Thus, this population may benefit from an expanded approach to multidisciplinary care that includes input from specialty and primary care providers (PCPs), clinical pharmacy specialists (CPS), physical and occupational therapists, and patient navigators and educators.
We present 4 hypothetical cases, based on actual patients, that illustrate opportunities where multidisciplinary care coordination may improve patient experiences. These cases draw on current quality initiatives from the National Cancer Institute Community Cancer Centers Program, which has focused on improving the quality of multidisciplinary cancer care at selected community centers, and the Veterans Health Administration (VHA) patient-aligned care team (PACT) model, which brings together different health professionals to optimize primary care coordination.1,2 In addition, the National Committee for Quality Assurance has introduced an educational initiative to facilitate implementation of an oncologic medical home.3 This initiative stresses increased multidisciplinary communication, patient-centered care delivery, and reduced fragmentation of care for this population. Despite these guidelines and experiences from other medical specialties, models for integrated cancer care have not been implemented in a prospective fashion within the VHA.
In this article, we focus on opportunities to take collaborative care approaches for the treatment of patients with follicular lymphoma (FL): a common, incurable, and often indolent B-cell non-Hodgkin lymphoma.4 FL was selected because these patients may be treated numerous times and long-term sequalae can accumulate throughout their cancer continuum (a series of health events encompassing cancer screening, diagnosis, treatment, survivorship, relapse, and death).5 HCPs in distinct roles can assist patients with cancer in optimizing their health outcomes and overall wellbeing.6
Case Example 1
A 70-year-old male was diagnosed with stage IV FL. Because of his advanced disease, he began therapy with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone). Prednisone was administered at 100 mg daily on the first 5 days of each 21-day cycle. On day 4 of the first treatment cycle, the patient notified his oncologist that he had been very thirsty and his random blood sugar values on 2 different days were 283 mg/dL and 312 mg/dL. A laboratory review revealed his hemoglobin A1c (HbA1c) 7 months prior was 5.6%.
Discussion
The high-dose prednisone component of this and other lymphoma therapy regimens can worsen diabetes mellitus (DM) control and/or worsen prediabetes. Patient characteristics that increase the risk of developing glucocorticoid-induced DM after CHOP chemotherapy include age ≥ 60 years, HbA1c > 6.1%, and body mass index > 30.7 This patient did not have DM prior to the FL therapy initiation, but afterwards he met diagnostic criteria for DM. For completeness, other causes for elevated blood glucose should be ruled out (ie, infection, laboratory error, etc.). An oncologist often will triage acute hyperglycemia, treating immediately with IV fluids and/or insulin. Thereafter, ongoing chronic disease management for DM may be best managed by PCPs, certified DM educators, and registered dieticians.
Several programs involving multidisciplinary DM care, comprised of physicians, advanced practice providers, nurses, certified DM educators, and/or pharmacists have been shown to improve HbA1c, cardiovascular outcomes, and all-cause mortality, while reducing health care costs.8 In addition, patient navigators can assist patients with coordinating visits to disease-state specialists and identifying further educational needs. For example, in 1 program, nonclinical peer navigators were shown to improve the number of appointments attended and reduce HbA1c in a population of patients with DM who were primarily minority, urban, and of low socioeconomic status.9 Thus, integrating DM care shows potential to improve outcomes for patients with lymphoma who develop glucocorticoid
Case Example 2
A 75-year-old male was diagnosed with FL. He was treated initially with bendamustine and rituximab. He required reinitiation of therapy 20 months later when he developed lymphadenopathy, fatigue, and night sweats and began treatment with oral idelalisib, a second-line therapy. Later, the patient presented to his PCP for a routine visit, and on medication reconciliation review, the patient reported regular use of trimethoprim-sulfamethoxazole.
Discussion
Upon consultation with the CPS and the patient’s oncologist, the PCP confirmed trimethoprim-sulfamethoxazole should be continued during therapy and for about 6 months following completion of therapy. Trimethoprim-sulfamethoxazole is used for prophylaxis against Pneumocystis jirovecii (formerly Pneumocystis carinii). While use of prophylactic therapy is not necessary for all patients with FL, idelalisib impairs the function of circulating lymphoid B-cells and thus has been associated with an increased risk of serious infection.10 A CPS can provide insight that maximizes medication adherence and efficacy while minimizing food-drug, drug-drug interactions, and AEs. CPS have been shown to: improve adherence to oral therapies, increase prospective monitoring required for safe therapy dose selection, and document assessment of chemotherapy-related AEs.11,12 Thus, multidisciplinary, integrated care is an important component of providing quality oncology care.
Case Example 3
A 60-year-old female presented to her PCP with a 2-week history of shortness of breath and leg swelling. She was treated for FL 4 years previously with 6 cycles of R-CHOP. She reported no chest pain and did not have a prior history of hypertension, DM, or heart disease. On physical exam, she had elevated jugular venous pressure to jaw at 45°, bilateral pulmonary rales, and 2+ pitting pretibial edema. Laboratory tests that included complete blood count, basic chemistries, and thyroid stimulating hormone were unremarkable, though brain natriuretic peptide (BNP) was elevated at 425 pg/mL.
As this patient’s laboratory results and physical examination suggested new-onset congestive heart failure, the PCP obtained an echocardiogram, which demonstrated an ejection fraction of 35% and global hypokinesis. Because the patient was symptomatic, she was admitted to the hospital to begin guideline-directed medical therapy (GDMT) including IV diuresis.
Discussion
Given the absence of significant risk factors and prior history of coronary artery disease, the most probable cause for this patient’s cardiomyopathy is doxorubicin. Doxorubicin is an anthracycline chemotherapy that can cause nonischemic, dilated cardiomyopathy, particularly when cumulative doses > 400 mg/m2 are administered, or when combined with chest radiation.13 This patient benefited from GDMT for reduced ejection-fraction heart failure (HFrEF). Studies have demonstrated positive outcomes when HFrEF patients are cared for by a multidisciplinary team who focus of volume management as well as uptitration of therapies to target doses.14
Case Example 4
An 80-year-old female was diagnosed with stage III FL but did not require immediate therapy. After developing discomfort due to enlarging lymphadenopathy, she initiated therapy with rituximab, cyclophosphamide, vincristine, and prednisone (R-CVP). She presented to her oncologist for consideration of her fifth cycle of R-CVP and reported a burning sensation on the soles of her feet and numbness in her fingertips and toes. On examination, her pulses were intact and there were no signs of infection, reduced blood flow, or edema. The patient demonstrated decreased sensation on monofilament testing. She had no history of DM and a recent HbA1c test was 4.9% An evaluation for other causes of neuropathy, such as hypothyroidism and vitamin B12 deficiency was negative. Thus, vincristine therapy was identified as the most likely etiology for her peripheral neuropathy. The oncologist decided to proceed with cycle 5 of chemotherapy but reduced the dose of vincristine by 50%.
Discussion
Vincristine is a microtubule inhibitor used in many chemotherapy regimens and may cause reversible or permanent neuropathy, including autonomic (constipation), sensory (stocking-glove distribution), or motor (foot-drop).15 A nerve conduction study may be indicated as part of the diagnostic evaluation. Treatment for painful sensory neuropathy may include pharmacologic therapy (such as gabapentin, pregabalin, capsaicin cream).16 Podiatrists can provide foot care and may provide shoes and inserts if appropriate. Physical therapists may assist with safety and mobility evaluations and can provide therapeutic exercises and assistive devices that improve function and quality of life.17
Conclusion
As cancer becomes more curable and more manageable, patients with cancer and survivors no longer rely exclusively on their oncologists for medical care. This is increasingly prevalent for patients with incurable but indolent cancers that may be present for years to decades, as acute and cumulative toxicities may complicate existing comorbidities. Thus, in this era of increasingly complex cancer therapies, multidisciplinary medical care that involves PCPs, specialists, and allied medical professionals, is essential for providing care that optimizes health and fully addresses patients’ needs.
Patients benefit from multidisciplinary care that coordinates management of complex medical problems. Traditionally, multidisciplinary cancer care involves oncology specialty providers in fields that include medical oncology, radiation oncology, and surgical oncology. Multidisciplinary cancer care intends to improve patient outcomes by bringing together different health care providers (HCPs) who are involved in the treatment of patients with cancer. Because new therapies are more effective and allow patients with cancer to live longer, adverse effects (AEs) are more likely to impact patients’ well-being, both while receiving treatment and long after it has completed. Thus, this population may benefit from an expanded approach to multidisciplinary care that includes input from specialty and primary care providers (PCPs), clinical pharmacy specialists (CPS), physical and occupational therapists, and patient navigators and educators.
We present 4 hypothetical cases, based on actual patients, that illustrate opportunities where multidisciplinary care coordination may improve patient experiences. These cases draw on current quality initiatives from the National Cancer Institute Community Cancer Centers Program, which has focused on improving the quality of multidisciplinary cancer care at selected community centers, and the Veterans Health Administration (VHA) patient-aligned care team (PACT) model, which brings together different health professionals to optimize primary care coordination.1,2 In addition, the National Committee for Quality Assurance has introduced an educational initiative to facilitate implementation of an oncologic medical home.3 This initiative stresses increased multidisciplinary communication, patient-centered care delivery, and reduced fragmentation of care for this population. Despite these guidelines and experiences from other medical specialties, models for integrated cancer care have not been implemented in a prospective fashion within the VHA.
In this article, we focus on opportunities to take collaborative care approaches for the treatment of patients with follicular lymphoma (FL): a common, incurable, and often indolent B-cell non-Hodgkin lymphoma.4 FL was selected because these patients may be treated numerous times and long-term sequalae can accumulate throughout their cancer continuum (a series of health events encompassing cancer screening, diagnosis, treatment, survivorship, relapse, and death).5 HCPs in distinct roles can assist patients with cancer in optimizing their health outcomes and overall wellbeing.6
Case Example 1
A 70-year-old male was diagnosed with stage IV FL. Because of his advanced disease, he began therapy with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone). Prednisone was administered at 100 mg daily on the first 5 days of each 21-day cycle. On day 4 of the first treatment cycle, the patient notified his oncologist that he had been very thirsty and his random blood sugar values on 2 different days were 283 mg/dL and 312 mg/dL. A laboratory review revealed his hemoglobin A1c (HbA1c) 7 months prior was 5.6%.
Discussion
The high-dose prednisone component of this and other lymphoma therapy regimens can worsen diabetes mellitus (DM) control and/or worsen prediabetes. Patient characteristics that increase the risk of developing glucocorticoid-induced DM after CHOP chemotherapy include age ≥ 60 years, HbA1c > 6.1%, and body mass index > 30.7 This patient did not have DM prior to the FL therapy initiation, but afterwards he met diagnostic criteria for DM. For completeness, other causes for elevated blood glucose should be ruled out (ie, infection, laboratory error, etc.). An oncologist often will triage acute hyperglycemia, treating immediately with IV fluids and/or insulin. Thereafter, ongoing chronic disease management for DM may be best managed by PCPs, certified DM educators, and registered dieticians.
Several programs involving multidisciplinary DM care, comprised of physicians, advanced practice providers, nurses, certified DM educators, and/or pharmacists have been shown to improve HbA1c, cardiovascular outcomes, and all-cause mortality, while reducing health care costs.8 In addition, patient navigators can assist patients with coordinating visits to disease-state specialists and identifying further educational needs. For example, in 1 program, nonclinical peer navigators were shown to improve the number of appointments attended and reduce HbA1c in a population of patients with DM who were primarily minority, urban, and of low socioeconomic status.9 Thus, integrating DM care shows potential to improve outcomes for patients with lymphoma who develop glucocorticoid
Case Example 2
A 75-year-old male was diagnosed with FL. He was treated initially with bendamustine and rituximab. He required reinitiation of therapy 20 months later when he developed lymphadenopathy, fatigue, and night sweats and began treatment with oral idelalisib, a second-line therapy. Later, the patient presented to his PCP for a routine visit, and on medication reconciliation review, the patient reported regular use of trimethoprim-sulfamethoxazole.
Discussion
Upon consultation with the CPS and the patient’s oncologist, the PCP confirmed trimethoprim-sulfamethoxazole should be continued during therapy and for about 6 months following completion of therapy. Trimethoprim-sulfamethoxazole is used for prophylaxis against Pneumocystis jirovecii (formerly Pneumocystis carinii). While use of prophylactic therapy is not necessary for all patients with FL, idelalisib impairs the function of circulating lymphoid B-cells and thus has been associated with an increased risk of serious infection.10 A CPS can provide insight that maximizes medication adherence and efficacy while minimizing food-drug, drug-drug interactions, and AEs. CPS have been shown to: improve adherence to oral therapies, increase prospective monitoring required for safe therapy dose selection, and document assessment of chemotherapy-related AEs.11,12 Thus, multidisciplinary, integrated care is an important component of providing quality oncology care.
Case Example 3
A 60-year-old female presented to her PCP with a 2-week history of shortness of breath and leg swelling. She was treated for FL 4 years previously with 6 cycles of R-CHOP. She reported no chest pain and did not have a prior history of hypertension, DM, or heart disease. On physical exam, she had elevated jugular venous pressure to jaw at 45°, bilateral pulmonary rales, and 2+ pitting pretibial edema. Laboratory tests that included complete blood count, basic chemistries, and thyroid stimulating hormone were unremarkable, though brain natriuretic peptide (BNP) was elevated at 425 pg/mL.
As this patient’s laboratory results and physical examination suggested new-onset congestive heart failure, the PCP obtained an echocardiogram, which demonstrated an ejection fraction of 35% and global hypokinesis. Because the patient was symptomatic, she was admitted to the hospital to begin guideline-directed medical therapy (GDMT) including IV diuresis.
Discussion
Given the absence of significant risk factors and prior history of coronary artery disease, the most probable cause for this patient’s cardiomyopathy is doxorubicin. Doxorubicin is an anthracycline chemotherapy that can cause nonischemic, dilated cardiomyopathy, particularly when cumulative doses > 400 mg/m2 are administered, or when combined with chest radiation.13 This patient benefited from GDMT for reduced ejection-fraction heart failure (HFrEF). Studies have demonstrated positive outcomes when HFrEF patients are cared for by a multidisciplinary team who focus of volume management as well as uptitration of therapies to target doses.14
Case Example 4
An 80-year-old female was diagnosed with stage III FL but did not require immediate therapy. After developing discomfort due to enlarging lymphadenopathy, she initiated therapy with rituximab, cyclophosphamide, vincristine, and prednisone (R-CVP). She presented to her oncologist for consideration of her fifth cycle of R-CVP and reported a burning sensation on the soles of her feet and numbness in her fingertips and toes. On examination, her pulses were intact and there were no signs of infection, reduced blood flow, or edema. The patient demonstrated decreased sensation on monofilament testing. She had no history of DM and a recent HbA1c test was 4.9% An evaluation for other causes of neuropathy, such as hypothyroidism and vitamin B12 deficiency was negative. Thus, vincristine therapy was identified as the most likely etiology for her peripheral neuropathy. The oncologist decided to proceed with cycle 5 of chemotherapy but reduced the dose of vincristine by 50%.
Discussion
Vincristine is a microtubule inhibitor used in many chemotherapy regimens and may cause reversible or permanent neuropathy, including autonomic (constipation), sensory (stocking-glove distribution), or motor (foot-drop).15 A nerve conduction study may be indicated as part of the diagnostic evaluation. Treatment for painful sensory neuropathy may include pharmacologic therapy (such as gabapentin, pregabalin, capsaicin cream).16 Podiatrists can provide foot care and may provide shoes and inserts if appropriate. Physical therapists may assist with safety and mobility evaluations and can provide therapeutic exercises and assistive devices that improve function and quality of life.17
Conclusion
As cancer becomes more curable and more manageable, patients with cancer and survivors no longer rely exclusively on their oncologists for medical care. This is increasingly prevalent for patients with incurable but indolent cancers that may be present for years to decades, as acute and cumulative toxicities may complicate existing comorbidities. Thus, in this era of increasingly complex cancer therapies, multidisciplinary medical care that involves PCPs, specialists, and allied medical professionals, is essential for providing care that optimizes health and fully addresses patients’ needs.
1. Friedman EL, Chawla N, Morris PT, et al. Assessing the development of multidisciplinary care: experience of the National Cancer Institute community cancer centers program. J Oncol Pract. 2015;11(1):e36-e43.
2. Peterson K, Helfand M, Humphrey L, Christensen V, Carson S. Evidence brief: effectiveness of intensive primary care programs. https://www.hsrd.research.va.gov/publications/esp/Intensive-Primary-Care-Supplement.pdf. Published February 2013. Accessed April 5, 2019.
3. National Committee for Quality Assurance. Oncology medical home recognition. https://www.ncqa.org/programs/health-care-providers-practices/oncology-medical-home. Accessed April 5, 2019.
4. Kahl BS, Yang DT. Follicular lymphoma: evolving therapeutic strategies. Blood. 2016;127(17):2055-2063.
5. Dulaney C, Wallace AS, Everett AS, Dover L, McDonald A, Kropp L. Defining health across the cancer continuum. Cureus. 2017;9(2):e1029.
6. Hopkins J, Mumber MP. Patient navigation through the cancer care continuum: an overview. J Oncol Pract. 2009;5(4):150-152.
7. Lee SY, Kurita N, Yokoyama Y, et al. Glucocorticoid-induced diabetes mellitus in patients with lymphoma treated with CHOP chemotherapy. Support Care Cancer. 2014;22(5):1385-1390.
8. McGill M, Blonde L, Juliana CN, et al; Global Partnership for Effective Diabetes Management. The interdisciplinary team in type 2 diabetes management: challenges and best practice solutions from real-world scenarios. J Clin Transl Endocrinol. 2017;7:21-27.
9. Horný M, Glover W, Gupte G, Saraswat A, Vimalananda V, Rosenzweig J. Patient navigation to improve diabetes outpatient care at a safety-net hospital: a retrospective cohort study. BMC Health Serv Res. 2017;17(1):759.
10. Reinwald M, Silva JT, Mueller NJ, et al. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) Consensus Document on the safety of targeted and biological therapies: an infectious diseases perspective (Intracellular signaling pathways: tyrosine kinase and mTOR inhibitors). Clin Microbiol Infect. 2018;24(suppl 2):S53-S70.
11. Holle LM, Boehnke Michaud L. Oncology pharmacists in health care delivery: vital members of the cancer care team. J. Oncol. Pract. 2014;10(3):e142-e145.
12. Morgan KP, Muluneh B, Dean AM, Amerine LB. Impact of an integrated oral chemotherapy program on patient adherence. J Oncol Pharm Pract. 2018;24(5):332-336.
13. Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97(11):2869-2879.
14. Feltner C, Jones CD, Cené CW, et al. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta-analysis. Ann Intern Med. 2014;160(11):774-784.
15. Mora E, Smith EM, Donohoe C, Hertz DL. Vincristine-induced peripheral neuropathy in pediatric cancer patients. Am J Cancer Res. 2016;6(11):2416-2430.
16. Hershman DL, Lacchetti C, Dworkin RH, et al; American Society of Clinical Oncology. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32(18):1941–1967
17. Duregon F, Vendramin B, Bullo V, et al. Effects of exercise on cancer patients suffering chemotherapy-induced peripheral neuropathy undergoing treatment: a systematic review. Crit Rev Oncol Hematol. 2018;121:90-100.
1. Friedman EL, Chawla N, Morris PT, et al. Assessing the development of multidisciplinary care: experience of the National Cancer Institute community cancer centers program. J Oncol Pract. 2015;11(1):e36-e43.
2. Peterson K, Helfand M, Humphrey L, Christensen V, Carson S. Evidence brief: effectiveness of intensive primary care programs. https://www.hsrd.research.va.gov/publications/esp/Intensive-Primary-Care-Supplement.pdf. Published February 2013. Accessed April 5, 2019.
3. National Committee for Quality Assurance. Oncology medical home recognition. https://www.ncqa.org/programs/health-care-providers-practices/oncology-medical-home. Accessed April 5, 2019.
4. Kahl BS, Yang DT. Follicular lymphoma: evolving therapeutic strategies. Blood. 2016;127(17):2055-2063.
5. Dulaney C, Wallace AS, Everett AS, Dover L, McDonald A, Kropp L. Defining health across the cancer continuum. Cureus. 2017;9(2):e1029.
6. Hopkins J, Mumber MP. Patient navigation through the cancer care continuum: an overview. J Oncol Pract. 2009;5(4):150-152.
7. Lee SY, Kurita N, Yokoyama Y, et al. Glucocorticoid-induced diabetes mellitus in patients with lymphoma treated with CHOP chemotherapy. Support Care Cancer. 2014;22(5):1385-1390.
8. McGill M, Blonde L, Juliana CN, et al; Global Partnership for Effective Diabetes Management. The interdisciplinary team in type 2 diabetes management: challenges and best practice solutions from real-world scenarios. J Clin Transl Endocrinol. 2017;7:21-27.
9. Horný M, Glover W, Gupte G, Saraswat A, Vimalananda V, Rosenzweig J. Patient navigation to improve diabetes outpatient care at a safety-net hospital: a retrospective cohort study. BMC Health Serv Res. 2017;17(1):759.
10. Reinwald M, Silva JT, Mueller NJ, et al. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) Consensus Document on the safety of targeted and biological therapies: an infectious diseases perspective (Intracellular signaling pathways: tyrosine kinase and mTOR inhibitors). Clin Microbiol Infect. 2018;24(suppl 2):S53-S70.
11. Holle LM, Boehnke Michaud L. Oncology pharmacists in health care delivery: vital members of the cancer care team. J. Oncol. Pract. 2014;10(3):e142-e145.
12. Morgan KP, Muluneh B, Dean AM, Amerine LB. Impact of an integrated oral chemotherapy program on patient adherence. J Oncol Pharm Pract. 2018;24(5):332-336.
13. Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97(11):2869-2879.
14. Feltner C, Jones CD, Cené CW, et al. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta-analysis. Ann Intern Med. 2014;160(11):774-784.
15. Mora E, Smith EM, Donohoe C, Hertz DL. Vincristine-induced peripheral neuropathy in pediatric cancer patients. Am J Cancer Res. 2016;6(11):2416-2430.
16. Hershman DL, Lacchetti C, Dworkin RH, et al; American Society of Clinical Oncology. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32(18):1941–1967
17. Duregon F, Vendramin B, Bullo V, et al. Effects of exercise on cancer patients suffering chemotherapy-induced peripheral neuropathy undergoing treatment: a systematic review. Crit Rev Oncol Hematol. 2018;121:90-100.
Psoriasis Treatment in Patients With Human Immunodeficiency Virus

The treatment of psoriasis in patients with HIV infection represents a clinical challenge.1,2 Up to 3% of patients with HIV infection are estimated to have psoriasis. Although this prevalence is similar to the general population, psoriatic disease in patients with HIV tends to be more severe, refractory, and more difficult to treat.3-5 Additionally, up to half of patients with comorbid HIV and psoriasis also have substantial psoriatic arthritis (PsA).1,6
Drug treatments for psoriasis and PsA often are immunosuppressive; as such, the treatment of psoriasis in this patient population requires careful consideration of the potential risks and benefits of treatment as well as fastidious monitoring for the emergence of potentially adverse treatment effects.1 A careful diagnostic process to determine the severity of HIV-associated psoriasis and to select the appropriate treatment relative to the patient’s immunologic status is of critical importance.3
Presentation of Psoriasis in Patients With HIV Infection
The presentation and severity of psoriasis in patients with HIV infection is highly variable and is often related to the degree of immune suppression experienced by the patient.3,7 In some individuals, psoriasis may be the first outward manifestation of HIV, whereas in others, it only manifests after HIV has progressed to AIDS.7

Recognition of the atypical presentations of psoriasis that are frequently seen in patients with HIV infection can help to facilitate early diagnosis and treatment to improve patient outcomes.3,8 Psoriasis vulgaris, for example, typically presents as erythematous plaques with silvery-white scales on extensor surfaces of the body such as the knees and elbows. However, in patients with HIV, psoriasis vulgaris may present with scales that appear thick and oyster shell–like instead of silvery-white; these lesions also may occur on flexural areas rather than extensor surfaces.8 Similarly, the sudden onset of widespread psoriasis in otherwise healthy persons should trigger suspicion for HIV infection and recommendations for appropriate testing, even when no risk factors are present.8
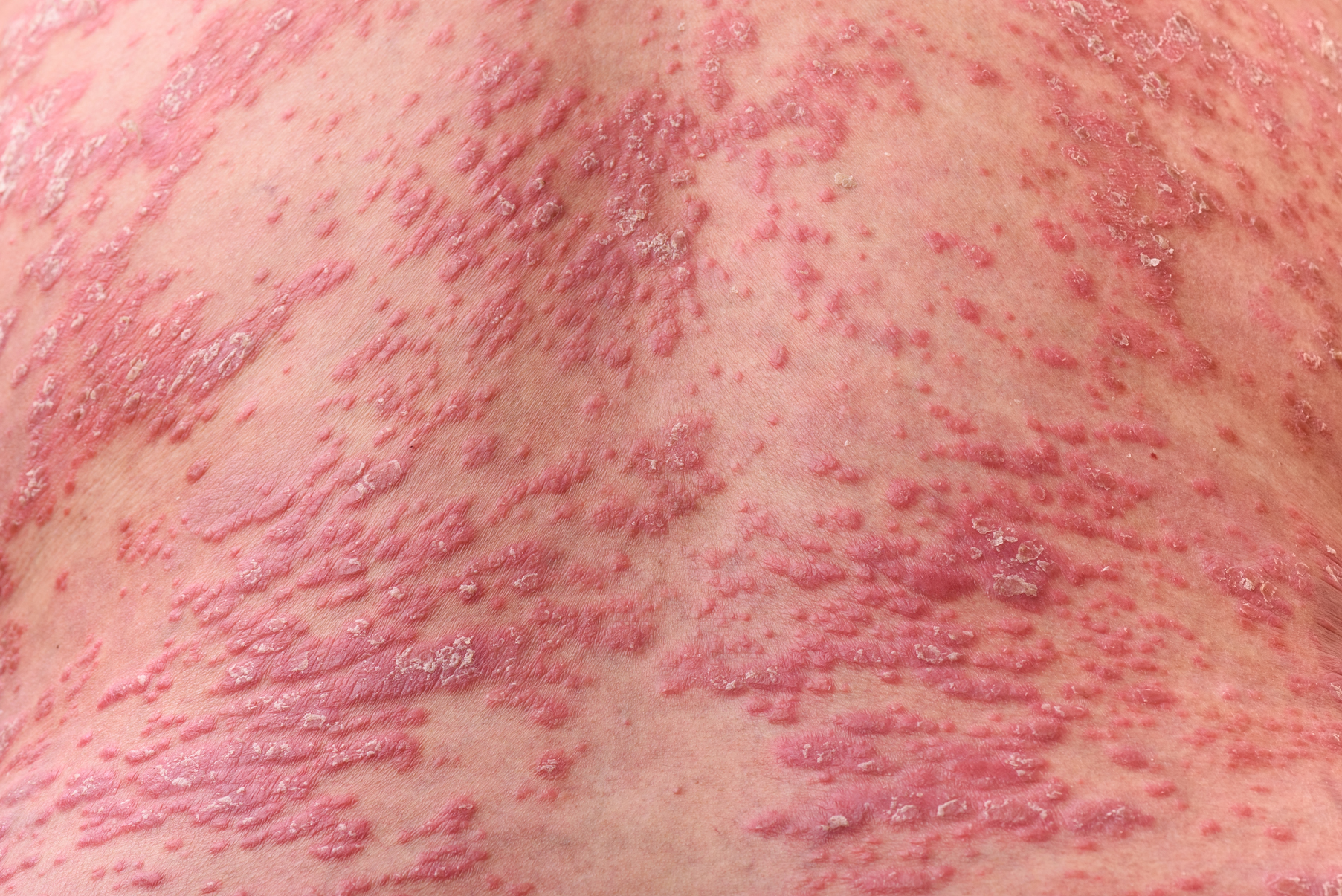
Guttate, inverse, and erythrodermic psoriasis are the most common subtypes in patients with HIV infection, though all clinical subtypes may occur. Overlapping of psoriasis subtypes often occurs in individuals with HIV infection and should serve as a red flag to recommend screening for HIV.5,8 Acral involvement, frequently with pustules and occasionally with severe destructive nail changes, is commonly seen in patients with HIV-associated psoriasis.7,9 In cases involving severe psoriatic exacerbations among individuals with AIDS, there is a heightened risk of developing systemic infections, including superinfection of Staphylococcus aureus, which is a rare occurrence in immunocompetent patients with psoriasis.7,10,11
Therapeutic Options
Because the clinical course of psoriasis in patients with HIV infection is frequently progressive and refractory to treatment, traditional first- and second-line therapies (Table) including topical agents, phototherapy, and oral retinoids may be unable to achieve lasting control of both skin and joint manifestations.1
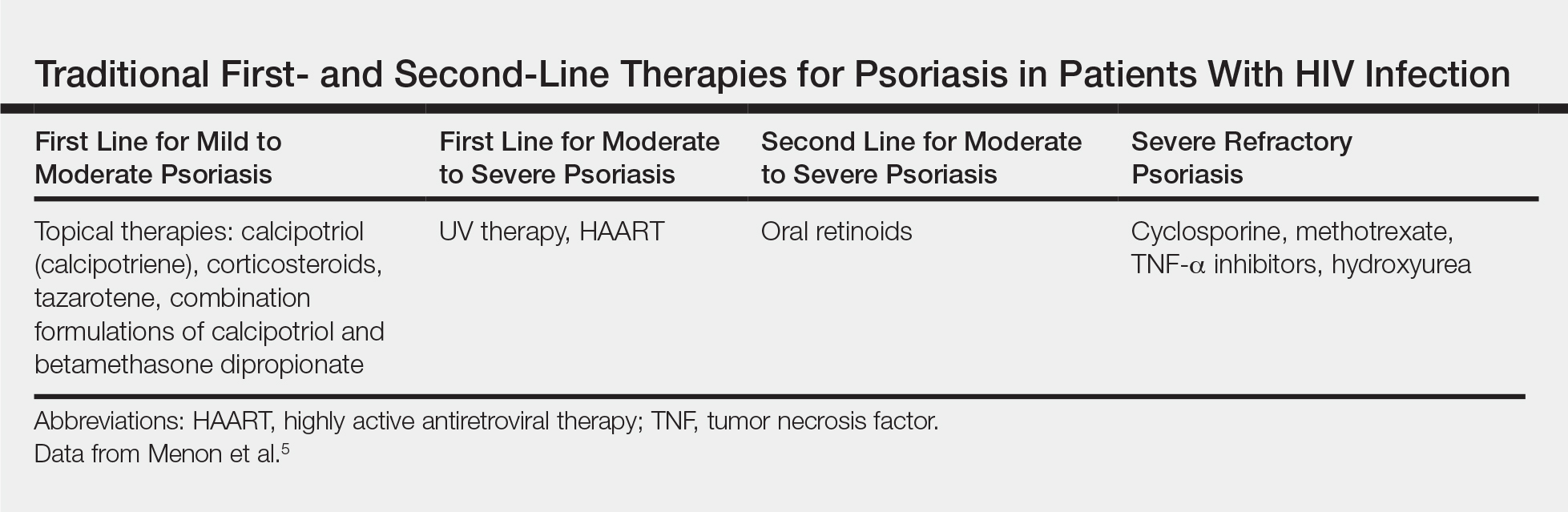
Topical Therapy
As in the general population, targeted therapies such as topical agents are recommended as first-line treatment of mild HIV-associated psoriasis.12 Topical corticosteroids, calcipotriol, tazarotene, and formulations combining 2 of these medications form the cornerstone of topical therapies for mild psoriasis in patients with HIV infection. These agents have the advantage of possessing limited and localized effects, making it unlikely for them to increase immunosuppression in patients with HIV infection. They generally can be safely used in patients with HIV infection, and their side-effect profile in patients with HIV infection is similar to the general population.12 However, calcipotriol is the least desirable for use in patients with hypertriglyceridemia, which can be a side effect of antiretroviral drugs.4
UV Phototherapy
Topical therapy is limited by its lack of potency; limited field coverage; and the inconvenience of application, particularly in patients with more widespread disease.12 Therefore, UV phototherapy is preferred as first-line treatment of moderate to severe psoriasis. UV phototherapy has been shown to inhibit cell proliferation and inflammation and result in clinical improvement of HIV-associated psoriasis; moreover, most of the reports in the literature support it as an option that will not increase immunocompromise in patients with HIV infection.12
Caution is warranted, however, regarding the immunomodulatory effects of UV therapies, which may result in an increased risk for skin cancer and diminished resistance to infection, which can be of particular concern in immunocompromised patients who are already at risk.7,13,14 In patients who are candidates for phototherapy, HIV serology and close monitoring of viral load and CD4 lymphocyte count before treatment, at monthly interludes throughout treatment, and 3 months following the cessation of treatment have been recommended.7,15 Careful consideration of the risk-benefit ratio of phototherapy for individual patients, including the patient’s stage of HIV disease, the degree of discomfort, disfigurement, and disability caused by the psoriasis (or other dermatologic condition), as well as the availability of alternative treatment options is essential.7,16
Systemic Agents
In patients who are intolerant of or unresponsive to antiretroviral therapy, topical therapies, and phototherapy, traditional systemic agents may be considered,12 including acitretin, methotrexate, and cyclosporine. However, updated guidelines indicate that methotrexate and cyclosporine should be avoided in this population given the risk for increased immunosuppression with these agents.4,17
Oral retinoids, such as acitretin, continue to be important options for second-line psoriasis treatment in patients with comorbid HIV infection, either as monotherapy or in association with phototherapy.3 Acitretin has the notable benefit of not causing or worsening immune compromise; however, its use is less than desirable in patients with hypertriglyceridemia, which can be a side effect of antiretroviral drugs.4,12 Providers also must be aware of the possible association between acitretin (and other antiretrovirals) and pancreatitis, remaining vigilant in monitoring patients for this adverse effect.3
Biologics
The relatively recent addition of cytokine-suppressive biologic agents to the treatment armamentarium has transformed the management of psoriasis in otherwise healthy individuals. These agents have been shown to possess an excellent safety and efficacy profile.12 However, their use in patients with HIV infection has been mired in concerns regarding a potential increase in the risk for opportunistic infections, sepsis, and HIV disease progression in this patient population.7,12
Case reports have detailed the safe treatment of recalcitrant HIV-associated psoriasis with tumor necrosis factor (TNF) blockers, such as etanercept.7,12 In most of these case reports, no harm to CD4 lymphocyte counts, serum viral loads, overall immune status, and susceptibility to infection have been noted; on the contrary, CD4 count increased in most patients following treatment with biologic agents.12 Because patients with HIV infection tend to be excluded from clinical trials, anecdotal evidence derived from case reports and case series often provides clinically relevant information and often forms the basis for treatment recommendations in this patient population.12 Indeed, in the wake of positive case reports, TNF-α inhibitors are now recommended for highly selected patients with refractory chronic psoriatic disease, including those with incapacitating joint pain.7,18
When TNF-α inhibitors are used in patients with HIV infection and psoriasis, optimal antiretroviral therapy and exceedingly close monitoring of clinical and laboratory parameters are of the utmost importance; Pneumocystis jiroveci prophylaxis also is recommended in patients with low CD4 counts.7,18
In 2014, the oral phosphodiesterase 4 inhibitor apremilast was approved for the treatment of moderate to severe plaque psoriasis and PsA. Recent case reports have described its successful use in patients with HIV infection and psoriasis, including the case reported herein, with no reports of opportunistic infections.4,19 Furthermore, HIV infection is not listed as a contraindication on its label.20
Apremilast is thought to increase intracellular cyclic adenosine monophosphate, thereby helping to attain improved homeostasis between proinflammatory and anti-inflammatory mediators.4,19 Several of the proinflammatory mediators that are indirectly targeted by apremilast, including TNF-α and IL-23, are explicitly inhibited by other biologics. It is this equilibrium between proinflammatory and anti-inflammatory mediators that most markedly differentiates apremilast from most other available biologic therapies for psoriasis, which typically have a specific proinflammatory target.4,21 As with other systemic therapies, close monitoring of CD4 levels and viral loads, as well as use of relevant prophylactic agents, is essential when apremilast is used in the setting of HIV infection, making coordination with infectious disease specialists essential.19

Bottom Line
Management of psoriasis in patients with HIV infection represents a clinical challenge. Case reports suggest a role for apremilast as an adjuvant to first-line therapy such as UV phototherapy in the setting of HIV infection in a patient with moderate to severe psoriasis, but close monitoring of CD4 count and viral load in these patients is needed in collaboration with infectious disease specialists. Updated guidelines on the use of systemic agents for psoriasis treatment in the HIV population are needed.
- Nakamura M, Abrouk M, Farahnik B, et al. Psoriasis treatment in HIV-positive patients: a systematic review of systemic immunosuppressive therapies. Cutis. 2018;101:38, 42, 56.
- Patel RV, Weinberg JM. Psoriasis in the patient with human immunodeficiency virus, part 2: review of treatment. Cutis. 2008;82:202-210.
- Ceccarelli M, Venanzi Rullo E, Vaccaro M, et al. HIV‐associated psoriasis: epidemiology, pathogenesis, and management [published online January 6, 2019]. Dermatol Ther. 2019;32:e12806. doi:10.1111/dth.12806.
- Zarbafian M, Richer V. Treatment of moderate to severe psoriasis with apremilast over 2 years in the context of long-term treated HIV infection: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19845193. doi:10.1177/2050313X19845193.
- Menon K, Van Vorhees AS, Bebo, BF, et al. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol. 2010;62:291-299.
- Mallon E, Bunker CB. HIV-associated psoriasis. AIDS Patient Care STDS. 2000;14:239-246.
- Patel VA, Weinberg JM. Psoriasis in the patient with human immunodeficiency virus, part 1: review of pathogenesis. Cutis. 2008;82:117-122.
- Castillo RL, Racaza GZ, Dela Cruz Roa F. Ostraceous and inverse psoriasis with psoriatic arthritis as the presenting features of advanced HIV infection. Singapore Med J. 2014;55:e60-e63.
- Duvic M, Crane MM, Conant M, et al. Zidovudine improves psoriasis in human immunodeficiency virus- positive males. Arch Dermatol. 1994;130:447.
- Jaffee D, May LP, Sanchez M, et al. Staphylococcal sepsis in HIV antibody seropositive psoriasis patients. J Am Acad Dermatol. 1991;24:970-972.
- King LE, Dufresne RG, Lovette GL, et al. Erythroderma: review of 82 cases. South Med J. 1986;79:1210-1215.
- Kaminetsky J, Aziz M, Kaushik S. A review of biologics and other treatment modalities in HIV-associated psoriasis. Skin. 2018;2:389-401.
- Wolff K. Side effects of psoralen photochemotherapy (PUVA). Br J Dermatol. 1990;122:117-125.
- Stern RS, Mills DK, Krell K, et al. HIV-positive patients differ from HIV-negative patients in indications for and type of UV therapy used. J Am Acad Dermatol. 1998;39:48-55.
- Oracion RM, Skiest DJ, Keiser PH, et al. HIV-related skin diseases. Prog Dermatol. 1999;33:1-6.
- Finkelstein M, Berman B. HIV and AIDS in inpatient dermatology: approach to the consultation. Dermatol Clin. 2000;18:509-520.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections. J Am Acad Dermatol. 2019;80:43-53.
- Sellam J, Bouvard B, Masson C, et al. Use of infliximab to treat psoriatic arthritis in HIV-positive patients. Joint Bone Spine. 2007;74:197-200.
- Reddy SP, Lee E, Wu JJ. Apremilast and phototherapy for treatment of psoriasis in a patient with human immunodeficiency virus. Cutis. 2019;103:E1-E7.
- Otezla (apremilast). Summit, NJ: Celgene Corporation; 2017.
- Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83:1583-1590.

The treatment of psoriasis in patients with HIV infection represents a clinical challenge.1,2 Up to 3% of patients with HIV infection are estimated to have psoriasis. Although this prevalence is similar to the general population, psoriatic disease in patients with HIV tends to be more severe, refractory, and more difficult to treat.3-5 Additionally, up to half of patients with comorbid HIV and psoriasis also have substantial psoriatic arthritis (PsA).1,6
Drug treatments for psoriasis and PsA often are immunosuppressive; as such, the treatment of psoriasis in this patient population requires careful consideration of the potential risks and benefits of treatment as well as fastidious monitoring for the emergence of potentially adverse treatment effects.1 A careful diagnostic process to determine the severity of HIV-associated psoriasis and to select the appropriate treatment relative to the patient’s immunologic status is of critical importance.3
Presentation of Psoriasis in Patients With HIV Infection
The presentation and severity of psoriasis in patients with HIV infection is highly variable and is often related to the degree of immune suppression experienced by the patient.3,7 In some individuals, psoriasis may be the first outward manifestation of HIV, whereas in others, it only manifests after HIV has progressed to AIDS.7

Recognition of the atypical presentations of psoriasis that are frequently seen in patients with HIV infection can help to facilitate early diagnosis and treatment to improve patient outcomes.3,8 Psoriasis vulgaris, for example, typically presents as erythematous plaques with silvery-white scales on extensor surfaces of the body such as the knees and elbows. However, in patients with HIV, psoriasis vulgaris may present with scales that appear thick and oyster shell–like instead of silvery-white; these lesions also may occur on flexural areas rather than extensor surfaces.8 Similarly, the sudden onset of widespread psoriasis in otherwise healthy persons should trigger suspicion for HIV infection and recommendations for appropriate testing, even when no risk factors are present.8

Guttate, inverse, and erythrodermic psoriasis are the most common subtypes in patients with HIV infection, though all clinical subtypes may occur. Overlapping of psoriasis subtypes often occurs in individuals with HIV infection and should serve as a red flag to recommend screening for HIV.5,8 Acral involvement, frequently with pustules and occasionally with severe destructive nail changes, is commonly seen in patients with HIV-associated psoriasis.7,9 In cases involving severe psoriatic exacerbations among individuals with AIDS, there is a heightened risk of developing systemic infections, including superinfection of Staphylococcus aureus, which is a rare occurrence in immunocompetent patients with psoriasis.7,10,11
Therapeutic Options
Because the clinical course of psoriasis in patients with HIV infection is frequently progressive and refractory to treatment, traditional first- and second-line therapies (Table) including topical agents, phototherapy, and oral retinoids may be unable to achieve lasting control of both skin and joint manifestations.1

Topical Therapy
As in the general population, targeted therapies such as topical agents are recommended as first-line treatment of mild HIV-associated psoriasis.12 Topical corticosteroids, calcipotriol, tazarotene, and formulations combining 2 of these medications form the cornerstone of topical therapies for mild psoriasis in patients with HIV infection. These agents have the advantage of possessing limited and localized effects, making it unlikely for them to increase immunosuppression in patients with HIV infection. They generally can be safely used in patients with HIV infection, and their side-effect profile in patients with HIV infection is similar to the general population.12 However, calcipotriol is the least desirable for use in patients with hypertriglyceridemia, which can be a side effect of antiretroviral drugs.4
UV Phototherapy
Topical therapy is limited by its lack of potency; limited field coverage; and the inconvenience of application, particularly in patients with more widespread disease.12 Therefore, UV phototherapy is preferred as first-line treatment of moderate to severe psoriasis. UV phototherapy has been shown to inhibit cell proliferation and inflammation and result in clinical improvement of HIV-associated psoriasis; moreover, most of the reports in the literature support it as an option that will not increase immunocompromise in patients with HIV infection.12
Caution is warranted, however, regarding the immunomodulatory effects of UV therapies, which may result in an increased risk for skin cancer and diminished resistance to infection, which can be of particular concern in immunocompromised patients who are already at risk.7,13,14 In patients who are candidates for phototherapy, HIV serology and close monitoring of viral load and CD4 lymphocyte count before treatment, at monthly interludes throughout treatment, and 3 months following the cessation of treatment have been recommended.7,15 Careful consideration of the risk-benefit ratio of phototherapy for individual patients, including the patient’s stage of HIV disease, the degree of discomfort, disfigurement, and disability caused by the psoriasis (or other dermatologic condition), as well as the availability of alternative treatment options is essential.7,16

Systemic Agents
In patients who are intolerant of or unresponsive to antiretroviral therapy, topical therapies, and phototherapy, traditional systemic agents may be considered,12 including acitretin, methotrexate, and cyclosporine. However, updated guidelines indicate that methotrexate and cyclosporine should be avoided in this population given the risk for increased immunosuppression with these agents.4,17
Oral retinoids, such as acitretin, continue to be important options for second-line psoriasis treatment in patients with comorbid HIV infection, either as monotherapy or in association with phototherapy.3 Acitretin has the notable benefit of not causing or worsening immune compromise; however, its use is less than desirable in patients with hypertriglyceridemia, which can be a side effect of antiretroviral drugs.4,12 Providers also must be aware of the possible association between acitretin (and other antiretrovirals) and pancreatitis, remaining vigilant in monitoring patients for this adverse effect.3
Biologics
The relatively recent addition of cytokine-suppressive biologic agents to the treatment armamentarium has transformed the management of psoriasis in otherwise healthy individuals. These agents have been shown to possess an excellent safety and efficacy profile.12 However, their use in patients with HIV infection has been mired in concerns regarding a potential increase in the risk for opportunistic infections, sepsis, and HIV disease progression in this patient population.7,12
Case reports have detailed the safe treatment of recalcitrant HIV-associated psoriasis with tumor necrosis factor (TNF) blockers, such as etanercept.7,12 In most of these case reports, no harm to CD4 lymphocyte counts, serum viral loads, overall immune status, and susceptibility to infection have been noted; on the contrary, CD4 count increased in most patients following treatment with biologic agents.12 Because patients with HIV infection tend to be excluded from clinical trials, anecdotal evidence derived from case reports and case series often provides clinically relevant information and often forms the basis for treatment recommendations in this patient population.12 Indeed, in the wake of positive case reports, TNF-α inhibitors are now recommended for highly selected patients with refractory chronic psoriatic disease, including those with incapacitating joint pain.7,18
When TNF-α inhibitors are used in patients with HIV infection and psoriasis, optimal antiretroviral therapy and exceedingly close monitoring of clinical and laboratory parameters are of the utmost importance; Pneumocystis jiroveci prophylaxis also is recommended in patients with low CD4 counts.7,18
In 2014, the oral phosphodiesterase 4 inhibitor apremilast was approved for the treatment of moderate to severe plaque psoriasis and PsA. Recent case reports have described its successful use in patients with HIV infection and psoriasis, including the case reported herein, with no reports of opportunistic infections.4,19 Furthermore, HIV infection is not listed as a contraindication on its label.20
Apremilast is thought to increase intracellular cyclic adenosine monophosphate, thereby helping to attain improved homeostasis between proinflammatory and anti-inflammatory mediators.4,19 Several of the proinflammatory mediators that are indirectly targeted by apremilast, including TNF-α and IL-23, are explicitly inhibited by other biologics. It is this equilibrium between proinflammatory and anti-inflammatory mediators that most markedly differentiates apremilast from most other available biologic therapies for psoriasis, which typically have a specific proinflammatory target.4,21 As with other systemic therapies, close monitoring of CD4 levels and viral loads, as well as use of relevant prophylactic agents, is essential when apremilast is used in the setting of HIV infection, making coordination with infectious disease specialists essential.19

Bottom Line
Management of psoriasis in patients with HIV infection represents a clinical challenge. Case reports suggest a role for apremilast as an adjuvant to first-line therapy such as UV phototherapy in the setting of HIV infection in a patient with moderate to severe psoriasis, but close monitoring of CD4 count and viral load in these patients is needed in collaboration with infectious disease specialists. Updated guidelines on the use of systemic agents for psoriasis treatment in the HIV population are needed.

The treatment of psoriasis in patients with HIV infection represents a clinical challenge.1,2 Up to 3% of patients with HIV infection are estimated to have psoriasis. Although this prevalence is similar to the general population, psoriatic disease in patients with HIV tends to be more severe, refractory, and more difficult to treat.3-5 Additionally, up to half of patients with comorbid HIV and psoriasis also have substantial psoriatic arthritis (PsA).1,6
Drug treatments for psoriasis and PsA often are immunosuppressive; as such, the treatment of psoriasis in this patient population requires careful consideration of the potential risks and benefits of treatment as well as fastidious monitoring for the emergence of potentially adverse treatment effects.1 A careful diagnostic process to determine the severity of HIV-associated psoriasis and to select the appropriate treatment relative to the patient’s immunologic status is of critical importance.3
Presentation of Psoriasis in Patients With HIV Infection
The presentation and severity of psoriasis in patients with HIV infection is highly variable and is often related to the degree of immune suppression experienced by the patient.3,7 In some individuals, psoriasis may be the first outward manifestation of HIV, whereas in others, it only manifests after HIV has progressed to AIDS.7

Recognition of the atypical presentations of psoriasis that are frequently seen in patients with HIV infection can help to facilitate early diagnosis and treatment to improve patient outcomes.3,8 Psoriasis vulgaris, for example, typically presents as erythematous plaques with silvery-white scales on extensor surfaces of the body such as the knees and elbows. However, in patients with HIV, psoriasis vulgaris may present with scales that appear thick and oyster shell–like instead of silvery-white; these lesions also may occur on flexural areas rather than extensor surfaces.8 Similarly, the sudden onset of widespread psoriasis in otherwise healthy persons should trigger suspicion for HIV infection and recommendations for appropriate testing, even when no risk factors are present.8

Guttate, inverse, and erythrodermic psoriasis are the most common subtypes in patients with HIV infection, though all clinical subtypes may occur. Overlapping of psoriasis subtypes often occurs in individuals with HIV infection and should serve as a red flag to recommend screening for HIV.5,8 Acral involvement, frequently with pustules and occasionally with severe destructive nail changes, is commonly seen in patients with HIV-associated psoriasis.7,9 In cases involving severe psoriatic exacerbations among individuals with AIDS, there is a heightened risk of developing systemic infections, including superinfection of Staphylococcus aureus, which is a rare occurrence in immunocompetent patients with psoriasis.7,10,11
Therapeutic Options
Because the clinical course of psoriasis in patients with HIV infection is frequently progressive and refractory to treatment, traditional first- and second-line therapies (Table) including topical agents, phototherapy, and oral retinoids may be unable to achieve lasting control of both skin and joint manifestations.1

Topical Therapy
As in the general population, targeted therapies such as topical agents are recommended as first-line treatment of mild HIV-associated psoriasis.12 Topical corticosteroids, calcipotriol, tazarotene, and formulations combining 2 of these medications form the cornerstone of topical therapies for mild psoriasis in patients with HIV infection. These agents have the advantage of possessing limited and localized effects, making it unlikely for them to increase immunosuppression in patients with HIV infection. They generally can be safely used in patients with HIV infection, and their side-effect profile in patients with HIV infection is similar to the general population.12 However, calcipotriol is the least desirable for use in patients with hypertriglyceridemia, which can be a side effect of antiretroviral drugs.4
UV Phototherapy
Topical therapy is limited by its lack of potency; limited field coverage; and the inconvenience of application, particularly in patients with more widespread disease.12 Therefore, UV phototherapy is preferred as first-line treatment of moderate to severe psoriasis. UV phototherapy has been shown to inhibit cell proliferation and inflammation and result in clinical improvement of HIV-associated psoriasis; moreover, most of the reports in the literature support it as an option that will not increase immunocompromise in patients with HIV infection.12
Caution is warranted, however, regarding the immunomodulatory effects of UV therapies, which may result in an increased risk for skin cancer and diminished resistance to infection, which can be of particular concern in immunocompromised patients who are already at risk.7,13,14 In patients who are candidates for phototherapy, HIV serology and close monitoring of viral load and CD4 lymphocyte count before treatment, at monthly interludes throughout treatment, and 3 months following the cessation of treatment have been recommended.7,15 Careful consideration of the risk-benefit ratio of phototherapy for individual patients, including the patient’s stage of HIV disease, the degree of discomfort, disfigurement, and disability caused by the psoriasis (or other dermatologic condition), as well as the availability of alternative treatment options is essential.7,16

Systemic Agents
In patients who are intolerant of or unresponsive to antiretroviral therapy, topical therapies, and phototherapy, traditional systemic agents may be considered,12 including acitretin, methotrexate, and cyclosporine. However, updated guidelines indicate that methotrexate and cyclosporine should be avoided in this population given the risk for increased immunosuppression with these agents.4,17
Oral retinoids, such as acitretin, continue to be important options for second-line psoriasis treatment in patients with comorbid HIV infection, either as monotherapy or in association with phototherapy.3 Acitretin has the notable benefit of not causing or worsening immune compromise; however, its use is less than desirable in patients with hypertriglyceridemia, which can be a side effect of antiretroviral drugs.4,12 Providers also must be aware of the possible association between acitretin (and other antiretrovirals) and pancreatitis, remaining vigilant in monitoring patients for this adverse effect.3
Biologics
The relatively recent addition of cytokine-suppressive biologic agents to the treatment armamentarium has transformed the management of psoriasis in otherwise healthy individuals. These agents have been shown to possess an excellent safety and efficacy profile.12 However, their use in patients with HIV infection has been mired in concerns regarding a potential increase in the risk for opportunistic infections, sepsis, and HIV disease progression in this patient population.7,12
Case reports have detailed the safe treatment of recalcitrant HIV-associated psoriasis with tumor necrosis factor (TNF) blockers, such as etanercept.7,12 In most of these case reports, no harm to CD4 lymphocyte counts, serum viral loads, overall immune status, and susceptibility to infection have been noted; on the contrary, CD4 count increased in most patients following treatment with biologic agents.12 Because patients with HIV infection tend to be excluded from clinical trials, anecdotal evidence derived from case reports and case series often provides clinically relevant information and often forms the basis for treatment recommendations in this patient population.12 Indeed, in the wake of positive case reports, TNF-α inhibitors are now recommended for highly selected patients with refractory chronic psoriatic disease, including those with incapacitating joint pain.7,18
When TNF-α inhibitors are used in patients with HIV infection and psoriasis, optimal antiretroviral therapy and exceedingly close monitoring of clinical and laboratory parameters are of the utmost importance; Pneumocystis jiroveci prophylaxis also is recommended in patients with low CD4 counts.7,18
In 2014, the oral phosphodiesterase 4 inhibitor apremilast was approved for the treatment of moderate to severe plaque psoriasis and PsA. Recent case reports have described its successful use in patients with HIV infection and psoriasis, including the case reported herein, with no reports of opportunistic infections.4,19 Furthermore, HIV infection is not listed as a contraindication on its label.20
Apremilast is thought to increase intracellular cyclic adenosine monophosphate, thereby helping to attain improved homeostasis between proinflammatory and anti-inflammatory mediators.4,19 Several of the proinflammatory mediators that are indirectly targeted by apremilast, including TNF-α and IL-23, are explicitly inhibited by other biologics. It is this equilibrium between proinflammatory and anti-inflammatory mediators that most markedly differentiates apremilast from most other available biologic therapies for psoriasis, which typically have a specific proinflammatory target.4,21 As with other systemic therapies, close monitoring of CD4 levels and viral loads, as well as use of relevant prophylactic agents, is essential when apremilast is used in the setting of HIV infection, making coordination with infectious disease specialists essential.19

Bottom Line
Management of psoriasis in patients with HIV infection represents a clinical challenge. Case reports suggest a role for apremilast as an adjuvant to first-line therapy such as UV phototherapy in the setting of HIV infection in a patient with moderate to severe psoriasis, but close monitoring of CD4 count and viral load in these patients is needed in collaboration with infectious disease specialists. Updated guidelines on the use of systemic agents for psoriasis treatment in the HIV population are needed.
- Nakamura M, Abrouk M, Farahnik B, et al. Psoriasis treatment in HIV-positive patients: a systematic review of systemic immunosuppressive therapies. Cutis. 2018;101:38, 42, 56.
- Patel RV, Weinberg JM. Psoriasis in the patient with human immunodeficiency virus, part 2: review of treatment. Cutis. 2008;82:202-210.
- Ceccarelli M, Venanzi Rullo E, Vaccaro M, et al. HIV‐associated psoriasis: epidemiology, pathogenesis, and management [published online January 6, 2019]. Dermatol Ther. 2019;32:e12806. doi:10.1111/dth.12806.
- Zarbafian M, Richer V. Treatment of moderate to severe psoriasis with apremilast over 2 years in the context of long-term treated HIV infection: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19845193. doi:10.1177/2050313X19845193.
- Menon K, Van Vorhees AS, Bebo, BF, et al. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol. 2010;62:291-299.
- Mallon E, Bunker CB. HIV-associated psoriasis. AIDS Patient Care STDS. 2000;14:239-246.
- Patel VA, Weinberg JM. Psoriasis in the patient with human immunodeficiency virus, part 1: review of pathogenesis. Cutis. 2008;82:117-122.
- Castillo RL, Racaza GZ, Dela Cruz Roa F. Ostraceous and inverse psoriasis with psoriatic arthritis as the presenting features of advanced HIV infection. Singapore Med J. 2014;55:e60-e63.
- Duvic M, Crane MM, Conant M, et al. Zidovudine improves psoriasis in human immunodeficiency virus- positive males. Arch Dermatol. 1994;130:447.
- Jaffee D, May LP, Sanchez M, et al. Staphylococcal sepsis in HIV antibody seropositive psoriasis patients. J Am Acad Dermatol. 1991;24:970-972.
- King LE, Dufresne RG, Lovette GL, et al. Erythroderma: review of 82 cases. South Med J. 1986;79:1210-1215.
- Kaminetsky J, Aziz M, Kaushik S. A review of biologics and other treatment modalities in HIV-associated psoriasis. Skin. 2018;2:389-401.
- Wolff K. Side effects of psoralen photochemotherapy (PUVA). Br J Dermatol. 1990;122:117-125.
- Stern RS, Mills DK, Krell K, et al. HIV-positive patients differ from HIV-negative patients in indications for and type of UV therapy used. J Am Acad Dermatol. 1998;39:48-55.
- Oracion RM, Skiest DJ, Keiser PH, et al. HIV-related skin diseases. Prog Dermatol. 1999;33:1-6.
- Finkelstein M, Berman B. HIV and AIDS in inpatient dermatology: approach to the consultation. Dermatol Clin. 2000;18:509-520.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections. J Am Acad Dermatol. 2019;80:43-53.
- Sellam J, Bouvard B, Masson C, et al. Use of infliximab to treat psoriatic arthritis in HIV-positive patients. Joint Bone Spine. 2007;74:197-200.
- Reddy SP, Lee E, Wu JJ. Apremilast and phototherapy for treatment of psoriasis in a patient with human immunodeficiency virus. Cutis. 2019;103:E1-E7.
- Otezla (apremilast). Summit, NJ: Celgene Corporation; 2017.
- Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83:1583-1590.
- Nakamura M, Abrouk M, Farahnik B, et al. Psoriasis treatment in HIV-positive patients: a systematic review of systemic immunosuppressive therapies. Cutis. 2018;101:38, 42, 56.
- Patel RV, Weinberg JM. Psoriasis in the patient with human immunodeficiency virus, part 2: review of treatment. Cutis. 2008;82:202-210.
- Ceccarelli M, Venanzi Rullo E, Vaccaro M, et al. HIV‐associated psoriasis: epidemiology, pathogenesis, and management [published online January 6, 2019]. Dermatol Ther. 2019;32:e12806. doi:10.1111/dth.12806.
- Zarbafian M, Richer V. Treatment of moderate to severe psoriasis with apremilast over 2 years in the context of long-term treated HIV infection: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19845193. doi:10.1177/2050313X19845193.
- Menon K, Van Vorhees AS, Bebo, BF, et al. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol. 2010;62:291-299.
- Mallon E, Bunker CB. HIV-associated psoriasis. AIDS Patient Care STDS. 2000;14:239-246.
- Patel VA, Weinberg JM. Psoriasis in the patient with human immunodeficiency virus, part 1: review of pathogenesis. Cutis. 2008;82:117-122.
- Castillo RL, Racaza GZ, Dela Cruz Roa F. Ostraceous and inverse psoriasis with psoriatic arthritis as the presenting features of advanced HIV infection. Singapore Med J. 2014;55:e60-e63.
- Duvic M, Crane MM, Conant M, et al. Zidovudine improves psoriasis in human immunodeficiency virus- positive males. Arch Dermatol. 1994;130:447.
- Jaffee D, May LP, Sanchez M, et al. Staphylococcal sepsis in HIV antibody seropositive psoriasis patients. J Am Acad Dermatol. 1991;24:970-972.
- King LE, Dufresne RG, Lovette GL, et al. Erythroderma: review of 82 cases. South Med J. 1986;79:1210-1215.
- Kaminetsky J, Aziz M, Kaushik S. A review of biologics and other treatment modalities in HIV-associated psoriasis. Skin. 2018;2:389-401.
- Wolff K. Side effects of psoralen photochemotherapy (PUVA). Br J Dermatol. 1990;122:117-125.
- Stern RS, Mills DK, Krell K, et al. HIV-positive patients differ from HIV-negative patients in indications for and type of UV therapy used. J Am Acad Dermatol. 1998;39:48-55.
- Oracion RM, Skiest DJ, Keiser PH, et al. HIV-related skin diseases. Prog Dermatol. 1999;33:1-6.
- Finkelstein M, Berman B. HIV and AIDS in inpatient dermatology: approach to the consultation. Dermatol Clin. 2000;18:509-520.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections. J Am Acad Dermatol. 2019;80:43-53.
- Sellam J, Bouvard B, Masson C, et al. Use of infliximab to treat psoriatic arthritis in HIV-positive patients. Joint Bone Spine. 2007;74:197-200.
- Reddy SP, Lee E, Wu JJ. Apremilast and phototherapy for treatment of psoriasis in a patient with human immunodeficiency virus. Cutis. 2019;103:E1-E7.
- Otezla (apremilast). Summit, NJ: Celgene Corporation; 2017.
- Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83:1583-1590.
A 50-year-old man with Fitzpatrick skin type IV presented with persistent psoriatic lesions on the trunk, arms, legs, and buttocks. The patient’s medical history was positive for human immunodeficiency virus (HIV), fatty liver disease, and moderate psoriasis (10% body surface area [BSA] affected), for which clobetasol spray and calcitriol ointment had been prescribed. The patient’s CD4 count was 460 at presentation, and his HIV RNA count was 48 copies/mL on polymerase chain reaction 2 months prior to presentation. For the last 5 months, the patient had been undergoing phototherapy 3 times weekly for treatment of psoriasis.
An apremilast starter pack was initiated with the dosage titrated from 10 mg to 30 mg over the course of 1 week. The patient was maintained on a dose of 30 mg twice daily after 1 week, while continuing clobetasol spray, calcitriol ointment, and phototherapy 3 times weekly with the intent to reduce the frequency after adequate control of psoriasis was achieved. After 3 months of treatment, the patient’s affected BSA was 0%. Apremilast was continued, and phototherapy was reduced to once weekly. After 7 months of concomitant treatment with apremilast, phototherapy was discontinued after clearance was maintained. Phototherapy was reinitiated twice weekly after a mild flare (3% BSA affected).
The patient continued apremilast for a total of 20 months until it became cost prohibitive. After discontinuing apremilast for 4 months, he presented with a severe psoriasis flare (40% BSA affected). He was switched to acitretin with intention to apply for an apremilast financial assistance program.
This case was adapted from Reddy SP, Lee E, Wu JJ. Apremilast and phototherapy for treatment of psoriasis in a patient with human immunodeficiency virus. Cutis. 2019;103:E6-E7
Gepant Safety & Lack of Liver Toxicity: Highlights from AAN 2019
The gepants and monoclonal antibodies (mAbs) against calcitonin gene-related peptide (CGRP) and its receptor appear to be effective, tolerable and safe, according to several posters recently presented at the 2019 American Academy of Neurology (AAN) Annual Meeting in Philadelphia. Information was presented on the 3 gepants being studied for US Food and Drug Administration (FDA) approval: 1 for acute care of migraine, 1 for prevention and 1 for both (though the presented data for rimegepant only covers acute care).
All drugs cause a small degree of adverse events (AEs), somewhat more than placebo. Based on the presented data, it seems that those associated with 3 times higher than normal elevation of liver enzymes, were usually not found to be the cause of that elevation. At no time was bilirubin elevated. This shows that all of these gepants appear to be effective and safe, despite the fact that some were found to have cause liver toxicity many years ago.
The first gepant study to be published was on olcegepant in 2004, in the New England Journal of Medicine. Professor Jes Olesen, MD, was the lead author of the study, which detailed the efficacy and safety of this small molecule CGRP receptor antagonist. Olcegepant was in an intravenous formulation and the plan was to convert it to a tablet, which never happened. Another company then produced telcagepant as a tablet and it was shown to be safe and effective in 2 large, multicenter, double-blind trials. Before receiving FDA approval for the acute care of migraine, it was studied on a daily basis for migraine prevention. It was found to cause some liver toxicity, so development was stopped. At that time several other gepants in development were placed on the shelf, partially for the fear of liver toxicity. The FDA is unlikely to approve a drug with significant liver toxicity, which can cause a range of symptoms including jaundice, itching, abdominal pain, fatigue, loss of appetite, nausea, vomiting, rash, and weight loss.
In the next few years we will have 4 mAbs for the prevention of migraine, and 3 gepants if all studies are positive. Below are the key takeaways from the presented posters on ubrogepant and atogepant, as information that is currently available on rimegepant.
Key Takeaways:
- Ubrogepant – Ailani J, Hutchinson S, Lipton R, et al.
- Intermittent use of ubrogepant for the acute treatment of migraine over 1 year was well-tolerated with no identified safety concerns. Throughout the 1-year, Phase III study of 1254 participants, 22,454 migraine attacks were treated with 31,968 doses of ubrogepant.
- Twenty cases of ALT/AST ≥3x ULN were reported and adjudicated by an independent panel of liver experts blinded to treatment.
- Of the 20 cases, 17 (4 usual care, 3 ubrogepant 50-mg, and 10 ubrogepant 100-mg) were determined to be unlikely related based on plausible alternative etiology/confounding factors.
- Just 2 cases (both ubrogepant 50-mg) were described as possibly related to study medication and 1 case (ubrogepant 100-mg) was adjudicated as probably related; however, confounding factors were noted.
- All cases were asymptomatic with no concurrent bilirubin elevation. ALT/AST elevations resolved in those who continued dosing.
- Atogepant – Goadsby PJ, Dodick DW, Trugman JM, et al.
- In a multicenter, randomized, double-blind, placebo-controlled, parallel-group trial of adults with a history of migraine, with or without aura, atogepant was well tolerated with no treatment-related serious AEs.
- Of the 834 randomized subjects, 825 were evaluated in the safety population. Treatment-emergent AEs were reported by 480 subjects (58.2%), and for 170 (20.6%), the AEs were considered treatment-related. Seven subjects (0.8%) reported serious AEs, but none were determined as treatment related.
- There were 10 cases of treatment-emergent ALT/AST elevations >3x the upper limit of normal, and this was balanced across the treatment dosage groups (10 mg QD, 30 mg QD, 30 mg BID, 60 mg QD, and 60 mg BID).
- Rimegepant – See Biogen press release for more information
- In December 2019, Biohaven announced initial positive results from an ongoing long-term, open-label safety study for rimegepant.
- The interim results included hepatic safety and tolerability data of rimegepant 75 mg in study participants based on a review of adverse events and regularly scheduled liver function tests.
- A panel of external independent liver experts provided a consensus based on the Drug-Induced Liver Injury Network (DILIN) causality assessment, determining that there were no liver cases probably related to the study drug and that there were no Hy’s Law cases identified.
- The panel also concluded that there were no liver safety signals detected and that, compared to placebo arms of other migraine treatments, there was a very low incidence of overall elevations of liver abnormalities.
The gepants and monoclonal antibodies (mAbs) against calcitonin gene-related peptide (CGRP) and its receptor appear to be effective, tolerable and safe, according to several posters recently presented at the 2019 American Academy of Neurology (AAN) Annual Meeting in Philadelphia. Information was presented on the 3 gepants being studied for US Food and Drug Administration (FDA) approval: 1 for acute care of migraine, 1 for prevention and 1 for both (though the presented data for rimegepant only covers acute care).
All drugs cause a small degree of adverse events (AEs), somewhat more than placebo. Based on the presented data, it seems that those associated with 3 times higher than normal elevation of liver enzymes, were usually not found to be the cause of that elevation. At no time was bilirubin elevated. This shows that all of these gepants appear to be effective and safe, despite the fact that some were found to have cause liver toxicity many years ago.
The first gepant study to be published was on olcegepant in 2004, in the New England Journal of Medicine. Professor Jes Olesen, MD, was the lead author of the study, which detailed the efficacy and safety of this small molecule CGRP receptor antagonist. Olcegepant was in an intravenous formulation and the plan was to convert it to a tablet, which never happened. Another company then produced telcagepant as a tablet and it was shown to be safe and effective in 2 large, multicenter, double-blind trials. Before receiving FDA approval for the acute care of migraine, it was studied on a daily basis for migraine prevention. It was found to cause some liver toxicity, so development was stopped. At that time several other gepants in development were placed on the shelf, partially for the fear of liver toxicity. The FDA is unlikely to approve a drug with significant liver toxicity, which can cause a range of symptoms including jaundice, itching, abdominal pain, fatigue, loss of appetite, nausea, vomiting, rash, and weight loss.
In the next few years we will have 4 mAbs for the prevention of migraine, and 3 gepants if all studies are positive. Below are the key takeaways from the presented posters on ubrogepant and atogepant, as information that is currently available on rimegepant.
Key Takeaways:
- Ubrogepant – Ailani J, Hutchinson S, Lipton R, et al.
- Intermittent use of ubrogepant for the acute treatment of migraine over 1 year was well-tolerated with no identified safety concerns. Throughout the 1-year, Phase III study of 1254 participants, 22,454 migraine attacks were treated with 31,968 doses of ubrogepant.
- Twenty cases of ALT/AST ≥3x ULN were reported and adjudicated by an independent panel of liver experts blinded to treatment.
- Of the 20 cases, 17 (4 usual care, 3 ubrogepant 50-mg, and 10 ubrogepant 100-mg) were determined to be unlikely related based on plausible alternative etiology/confounding factors.
- Just 2 cases (both ubrogepant 50-mg) were described as possibly related to study medication and 1 case (ubrogepant 100-mg) was adjudicated as probably related; however, confounding factors were noted.
- All cases were asymptomatic with no concurrent bilirubin elevation. ALT/AST elevations resolved in those who continued dosing.
- Atogepant – Goadsby PJ, Dodick DW, Trugman JM, et al.
- In a multicenter, randomized, double-blind, placebo-controlled, parallel-group trial of adults with a history of migraine, with or without aura, atogepant was well tolerated with no treatment-related serious AEs.
- Of the 834 randomized subjects, 825 were evaluated in the safety population. Treatment-emergent AEs were reported by 480 subjects (58.2%), and for 170 (20.6%), the AEs were considered treatment-related. Seven subjects (0.8%) reported serious AEs, but none were determined as treatment related.
- There were 10 cases of treatment-emergent ALT/AST elevations >3x the upper limit of normal, and this was balanced across the treatment dosage groups (10 mg QD, 30 mg QD, 30 mg BID, 60 mg QD, and 60 mg BID).
- Rimegepant – See Biogen press release for more information
- In December 2019, Biohaven announced initial positive results from an ongoing long-term, open-label safety study for rimegepant.
- The interim results included hepatic safety and tolerability data of rimegepant 75 mg in study participants based on a review of adverse events and regularly scheduled liver function tests.
- A panel of external independent liver experts provided a consensus based on the Drug-Induced Liver Injury Network (DILIN) causality assessment, determining that there were no liver cases probably related to the study drug and that there were no Hy’s Law cases identified.
- The panel also concluded that there were no liver safety signals detected and that, compared to placebo arms of other migraine treatments, there was a very low incidence of overall elevations of liver abnormalities.
The gepants and monoclonal antibodies (mAbs) against calcitonin gene-related peptide (CGRP) and its receptor appear to be effective, tolerable and safe, according to several posters recently presented at the 2019 American Academy of Neurology (AAN) Annual Meeting in Philadelphia. Information was presented on the 3 gepants being studied for US Food and Drug Administration (FDA) approval: 1 for acute care of migraine, 1 for prevention and 1 for both (though the presented data for rimegepant only covers acute care).
All drugs cause a small degree of adverse events (AEs), somewhat more than placebo. Based on the presented data, it seems that those associated with 3 times higher than normal elevation of liver enzymes, were usually not found to be the cause of that elevation. At no time was bilirubin elevated. This shows that all of these gepants appear to be effective and safe, despite the fact that some were found to have cause liver toxicity many years ago.
The first gepant study to be published was on olcegepant in 2004, in the New England Journal of Medicine. Professor Jes Olesen, MD, was the lead author of the study, which detailed the efficacy and safety of this small molecule CGRP receptor antagonist. Olcegepant was in an intravenous formulation and the plan was to convert it to a tablet, which never happened. Another company then produced telcagepant as a tablet and it was shown to be safe and effective in 2 large, multicenter, double-blind trials. Before receiving FDA approval for the acute care of migraine, it was studied on a daily basis for migraine prevention. It was found to cause some liver toxicity, so development was stopped. At that time several other gepants in development were placed on the shelf, partially for the fear of liver toxicity. The FDA is unlikely to approve a drug with significant liver toxicity, which can cause a range of symptoms including jaundice, itching, abdominal pain, fatigue, loss of appetite, nausea, vomiting, rash, and weight loss.
In the next few years we will have 4 mAbs for the prevention of migraine, and 3 gepants if all studies are positive. Below are the key takeaways from the presented posters on ubrogepant and atogepant, as information that is currently available on rimegepant.
Key Takeaways:
- Ubrogepant – Ailani J, Hutchinson S, Lipton R, et al.
- Intermittent use of ubrogepant for the acute treatment of migraine over 1 year was well-tolerated with no identified safety concerns. Throughout the 1-year, Phase III study of 1254 participants, 22,454 migraine attacks were treated with 31,968 doses of ubrogepant.
- Twenty cases of ALT/AST ≥3x ULN were reported and adjudicated by an independent panel of liver experts blinded to treatment.
- Of the 20 cases, 17 (4 usual care, 3 ubrogepant 50-mg, and 10 ubrogepant 100-mg) were determined to be unlikely related based on plausible alternative etiology/confounding factors.
- Just 2 cases (both ubrogepant 50-mg) were described as possibly related to study medication and 1 case (ubrogepant 100-mg) was adjudicated as probably related; however, confounding factors were noted.
- All cases were asymptomatic with no concurrent bilirubin elevation. ALT/AST elevations resolved in those who continued dosing.
- Atogepant – Goadsby PJ, Dodick DW, Trugman JM, et al.
- In a multicenter, randomized, double-blind, placebo-controlled, parallel-group trial of adults with a history of migraine, with or without aura, atogepant was well tolerated with no treatment-related serious AEs.
- Of the 834 randomized subjects, 825 were evaluated in the safety population. Treatment-emergent AEs were reported by 480 subjects (58.2%), and for 170 (20.6%), the AEs were considered treatment-related. Seven subjects (0.8%) reported serious AEs, but none were determined as treatment related.
- There were 10 cases of treatment-emergent ALT/AST elevations >3x the upper limit of normal, and this was balanced across the treatment dosage groups (10 mg QD, 30 mg QD, 30 mg BID, 60 mg QD, and 60 mg BID).
- Rimegepant – See Biogen press release for more information
- In December 2019, Biohaven announced initial positive results from an ongoing long-term, open-label safety study for rimegepant.
- The interim results included hepatic safety and tolerability data of rimegepant 75 mg in study participants based on a review of adverse events and regularly scheduled liver function tests.
- A panel of external independent liver experts provided a consensus based on the Drug-Induced Liver Injury Network (DILIN) causality assessment, determining that there were no liver cases probably related to the study drug and that there were no Hy’s Law cases identified.
- The panel also concluded that there were no liver safety signals detected and that, compared to placebo arms of other migraine treatments, there was a very low incidence of overall elevations of liver abnormalities.
Interview with Brenda L. Banwell, MD, on Pediatric-onset MS
Brenda L. Banwell, MD, is Chief of the Division of Neurology at Children’s Hospital of Philadelphia and holder of the Grace R. Loeb Endowed Chair in Neurosciences. Dr. Banwell is an expert in pediatric-onset multiple sclerosis (MS) with a clinical focus on cognitive features, neuroimaging, T and B-cell autoimmunity studies, and studies of viral triggers. We spoke with Dr. Banwell to discuss the disease course of pediatric-onset MS and the impact of magnetic resonance imaging (MRI).
How does the disease course of pediatric-onset MS differ from that of adult-onset MS?
DR. BANWELL: Pediatric-onset MS is almost universally a relapsing-remitting disease at onset. Primary progressive MS is typically not seen in children younger than 18 years of age.
Children with progressive disability from onset should be considered more likely to have either a mitochondrial disease, genetic leukodystrophy or other disorder. Multiple sclerosis would not be a consideration for a child with progressive disability at the beginning.
Secondary progressive MS does not appear to occur for most children during the first 10 years of disease. Retrospective cohort studies suggest that secondary progressive MS in patients with pediatric-onset MS likely takes, on average, at least 20 years to occur from onset.
However, we should remember that pediatric-onset MS patients are at risk for secondary progression when they are only 30 or 35 years of age, depending on when they experienced their first attack. With the availability of numerous MS therapies, we are optimistic that secondary progressive MS may now be less likely to occur, or may be delayed even further from onset.
How often are MRIs performed on children with MS?
DR. BANWELL: The most pivotal MRI is the one that helps you confirm the diagnosis. It is ideally obtained very close to the first onset of symptoms. That MRI could include the orbits, brain, and spine, depending on the clinical symptoms.
Following the first MRI scan, most of us would image our patients approximately every 3 months in the first year. After the first year, we would typically image our patients at least every 6 months, until, if they have been stable for an extended period of time, we might move to annual imaging.
The frequency of MRI scanning is determined by clinical disease activity, treatment decisions, and the age of the child. For example, very young children may require repeated exposure to anesthetics in order to obtain an MRI. We might have to think strategically about how often to put them under that degree of anesthesia to obtain imaging. Many young children are able to lie still for MRI if the facility has the option for viewing a video during the scan.
How do those MRI results influence your treatment decisions for the pediatric patient?
DR. BANWELL: The International Pediatric Multiple Sclerosis Study Group recently had an international consensus discussion with respect to monitoring disease activity and are working as a collective toward the concept of defining a standard interpretation of adequate disease control for given treatments. Standardized protocols for clinical evaluations and for MRI scan interpretation will be essential. Determining what constitutes “adequate treatment response”, both in terms of relapse frequency and frequency of new lesions on MRI will be important components to consider.
What have the MRI studies shown us about the brain volume in pediatric patients with MS?
DR. BANWELL: There are several things that we have learned about the impact of MS in the brains of children and teenagers.
With respect to brain growth and brain volume, we have learned that at the time of a first attack, children and teenagers with MS already have brain volumes that are about one standard deviation below what you would expect for someone their age and sex.
The inner skull size is also reduced, which suggests that there has been a failure of head and skull growth even before the first attack.
Following identification of MS in a child, the subsequent serial MRI studies have shown that children with MS fail to have age-expected brain growth. We do not see the age-expected rate of growth in our pediatric patients.
Around age 16 to 17 is when our brain volumes are the largest in our lifetime, but our pediatric patients show brain atrophy or progressive loss of brain volume after age 15, a time when normal brain volumes are pretty stable for at least a decade.
When we age, there is a gradual rate of brain volume loss, but this has not been imaged yet in our pediatric patients with MS since we do not have serial studies of individuals who are 30 or 40 yet who had pediatric-onset MS.
What else have MRI studies shown?
DR. BANWELL: Another point to add is that when we look at patients with pediatric-onset MS compared with those with adult-onset MS matched for disease duration, pediatric patients on average have higher T2 and T1 lesion volumes compared with adults. This suggests that despite their young age, pediatric-onset MS patients have had sufficient time to accrue subclinical disease/lesions and may have an accelerated rate of new lesion formation.
MRI and some of the newer more advanced MRI techniques are also informing on brain tissue integrity. In studies that examine whether the white matter highways in the brain or the pathways in the brain are normal, we find that in both pediatric-onset and adult-onset MS, even normal-appearing white matter is not normal. It is not as well structured as it should be compared with age- and sex-matched controls. Further, the myelin integrity or the structural alignment in the brain of our pediatric patients may not be normal.
I think that emphasizes to all of us that MS is much more than just multiple areas of “sclerosis” or scarring, which is what MS actually means. It is indeed a disease that has a more widespread impact on the central nervous system, beyond the very bright T2 lesions that we normally count and measure.
That is important because it speaks to the subsequent requirement that we think about when considering treatment for MS. Our goal is to not only suppress the acquisition of new lesions, which is the common metric for clinical trials, but we also think about brain protection, brain preservation, and brain repair, which, I think, is potentially the underlying substrate that is not yet fully addressed by the evolving treatment.
Brenda L. Banwell, MD, is Chief of the Division of Neurology at Children’s Hospital of Philadelphia and holder of the Grace R. Loeb Endowed Chair in Neurosciences. Dr. Banwell is an expert in pediatric-onset multiple sclerosis (MS) with a clinical focus on cognitive features, neuroimaging, T and B-cell autoimmunity studies, and studies of viral triggers. We spoke with Dr. Banwell to discuss the disease course of pediatric-onset MS and the impact of magnetic resonance imaging (MRI).
How does the disease course of pediatric-onset MS differ from that of adult-onset MS?
DR. BANWELL: Pediatric-onset MS is almost universally a relapsing-remitting disease at onset. Primary progressive MS is typically not seen in children younger than 18 years of age.
Children with progressive disability from onset should be considered more likely to have either a mitochondrial disease, genetic leukodystrophy or other disorder. Multiple sclerosis would not be a consideration for a child with progressive disability at the beginning.
Secondary progressive MS does not appear to occur for most children during the first 10 years of disease. Retrospective cohort studies suggest that secondary progressive MS in patients with pediatric-onset MS likely takes, on average, at least 20 years to occur from onset.
However, we should remember that pediatric-onset MS patients are at risk for secondary progression when they are only 30 or 35 years of age, depending on when they experienced their first attack. With the availability of numerous MS therapies, we are optimistic that secondary progressive MS may now be less likely to occur, or may be delayed even further from onset.
How often are MRIs performed on children with MS?
DR. BANWELL: The most pivotal MRI is the one that helps you confirm the diagnosis. It is ideally obtained very close to the first onset of symptoms. That MRI could include the orbits, brain, and spine, depending on the clinical symptoms.
Following the first MRI scan, most of us would image our patients approximately every 3 months in the first year. After the first year, we would typically image our patients at least every 6 months, until, if they have been stable for an extended period of time, we might move to annual imaging.
The frequency of MRI scanning is determined by clinical disease activity, treatment decisions, and the age of the child. For example, very young children may require repeated exposure to anesthetics in order to obtain an MRI. We might have to think strategically about how often to put them under that degree of anesthesia to obtain imaging. Many young children are able to lie still for MRI if the facility has the option for viewing a video during the scan.
How do those MRI results influence your treatment decisions for the pediatric patient?
DR. BANWELL: The International Pediatric Multiple Sclerosis Study Group recently had an international consensus discussion with respect to monitoring disease activity and are working as a collective toward the concept of defining a standard interpretation of adequate disease control for given treatments. Standardized protocols for clinical evaluations and for MRI scan interpretation will be essential. Determining what constitutes “adequate treatment response”, both in terms of relapse frequency and frequency of new lesions on MRI will be important components to consider.
What have the MRI studies shown us about the brain volume in pediatric patients with MS?
DR. BANWELL: There are several things that we have learned about the impact of MS in the brains of children and teenagers.
With respect to brain growth and brain volume, we have learned that at the time of a first attack, children and teenagers with MS already have brain volumes that are about one standard deviation below what you would expect for someone their age and sex.
The inner skull size is also reduced, which suggests that there has been a failure of head and skull growth even before the first attack.
Following identification of MS in a child, the subsequent serial MRI studies have shown that children with MS fail to have age-expected brain growth. We do not see the age-expected rate of growth in our pediatric patients.
Around age 16 to 17 is when our brain volumes are the largest in our lifetime, but our pediatric patients show brain atrophy or progressive loss of brain volume after age 15, a time when normal brain volumes are pretty stable for at least a decade.
When we age, there is a gradual rate of brain volume loss, but this has not been imaged yet in our pediatric patients with MS since we do not have serial studies of individuals who are 30 or 40 yet who had pediatric-onset MS.
What else have MRI studies shown?
DR. BANWELL: Another point to add is that when we look at patients with pediatric-onset MS compared with those with adult-onset MS matched for disease duration, pediatric patients on average have higher T2 and T1 lesion volumes compared with adults. This suggests that despite their young age, pediatric-onset MS patients have had sufficient time to accrue subclinical disease/lesions and may have an accelerated rate of new lesion formation.
MRI and some of the newer more advanced MRI techniques are also informing on brain tissue integrity. In studies that examine whether the white matter highways in the brain or the pathways in the brain are normal, we find that in both pediatric-onset and adult-onset MS, even normal-appearing white matter is not normal. It is not as well structured as it should be compared with age- and sex-matched controls. Further, the myelin integrity or the structural alignment in the brain of our pediatric patients may not be normal.
I think that emphasizes to all of us that MS is much more than just multiple areas of “sclerosis” or scarring, which is what MS actually means. It is indeed a disease that has a more widespread impact on the central nervous system, beyond the very bright T2 lesions that we normally count and measure.
That is important because it speaks to the subsequent requirement that we think about when considering treatment for MS. Our goal is to not only suppress the acquisition of new lesions, which is the common metric for clinical trials, but we also think about brain protection, brain preservation, and brain repair, which, I think, is potentially the underlying substrate that is not yet fully addressed by the evolving treatment.
Brenda L. Banwell, MD, is Chief of the Division of Neurology at Children’s Hospital of Philadelphia and holder of the Grace R. Loeb Endowed Chair in Neurosciences. Dr. Banwell is an expert in pediatric-onset multiple sclerosis (MS) with a clinical focus on cognitive features, neuroimaging, T and B-cell autoimmunity studies, and studies of viral triggers. We spoke with Dr. Banwell to discuss the disease course of pediatric-onset MS and the impact of magnetic resonance imaging (MRI).
How does the disease course of pediatric-onset MS differ from that of adult-onset MS?
DR. BANWELL: Pediatric-onset MS is almost universally a relapsing-remitting disease at onset. Primary progressive MS is typically not seen in children younger than 18 years of age.
Children with progressive disability from onset should be considered more likely to have either a mitochondrial disease, genetic leukodystrophy or other disorder. Multiple sclerosis would not be a consideration for a child with progressive disability at the beginning.
Secondary progressive MS does not appear to occur for most children during the first 10 years of disease. Retrospective cohort studies suggest that secondary progressive MS in patients with pediatric-onset MS likely takes, on average, at least 20 years to occur from onset.
However, we should remember that pediatric-onset MS patients are at risk for secondary progression when they are only 30 or 35 years of age, depending on when they experienced their first attack. With the availability of numerous MS therapies, we are optimistic that secondary progressive MS may now be less likely to occur, or may be delayed even further from onset.
How often are MRIs performed on children with MS?
DR. BANWELL: The most pivotal MRI is the one that helps you confirm the diagnosis. It is ideally obtained very close to the first onset of symptoms. That MRI could include the orbits, brain, and spine, depending on the clinical symptoms.
Following the first MRI scan, most of us would image our patients approximately every 3 months in the first year. After the first year, we would typically image our patients at least every 6 months, until, if they have been stable for an extended period of time, we might move to annual imaging.
The frequency of MRI scanning is determined by clinical disease activity, treatment decisions, and the age of the child. For example, very young children may require repeated exposure to anesthetics in order to obtain an MRI. We might have to think strategically about how often to put them under that degree of anesthesia to obtain imaging. Many young children are able to lie still for MRI if the facility has the option for viewing a video during the scan.
How do those MRI results influence your treatment decisions for the pediatric patient?
DR. BANWELL: The International Pediatric Multiple Sclerosis Study Group recently had an international consensus discussion with respect to monitoring disease activity and are working as a collective toward the concept of defining a standard interpretation of adequate disease control for given treatments. Standardized protocols for clinical evaluations and for MRI scan interpretation will be essential. Determining what constitutes “adequate treatment response”, both in terms of relapse frequency and frequency of new lesions on MRI will be important components to consider.
What have the MRI studies shown us about the brain volume in pediatric patients with MS?
DR. BANWELL: There are several things that we have learned about the impact of MS in the brains of children and teenagers.
With respect to brain growth and brain volume, we have learned that at the time of a first attack, children and teenagers with MS already have brain volumes that are about one standard deviation below what you would expect for someone their age and sex.
The inner skull size is also reduced, which suggests that there has been a failure of head and skull growth even before the first attack.
Following identification of MS in a child, the subsequent serial MRI studies have shown that children with MS fail to have age-expected brain growth. We do not see the age-expected rate of growth in our pediatric patients.
Around age 16 to 17 is when our brain volumes are the largest in our lifetime, but our pediatric patients show brain atrophy or progressive loss of brain volume after age 15, a time when normal brain volumes are pretty stable for at least a decade.
When we age, there is a gradual rate of brain volume loss, but this has not been imaged yet in our pediatric patients with MS since we do not have serial studies of individuals who are 30 or 40 yet who had pediatric-onset MS.
What else have MRI studies shown?
DR. BANWELL: Another point to add is that when we look at patients with pediatric-onset MS compared with those with adult-onset MS matched for disease duration, pediatric patients on average have higher T2 and T1 lesion volumes compared with adults. This suggests that despite their young age, pediatric-onset MS patients have had sufficient time to accrue subclinical disease/lesions and may have an accelerated rate of new lesion formation.
MRI and some of the newer more advanced MRI techniques are also informing on brain tissue integrity. In studies that examine whether the white matter highways in the brain or the pathways in the brain are normal, we find that in both pediatric-onset and adult-onset MS, even normal-appearing white matter is not normal. It is not as well structured as it should be compared with age- and sex-matched controls. Further, the myelin integrity or the structural alignment in the brain of our pediatric patients may not be normal.
I think that emphasizes to all of us that MS is much more than just multiple areas of “sclerosis” or scarring, which is what MS actually means. It is indeed a disease that has a more widespread impact on the central nervous system, beyond the very bright T2 lesions that we normally count and measure.
That is important because it speaks to the subsequent requirement that we think about when considering treatment for MS. Our goal is to not only suppress the acquisition of new lesions, which is the common metric for clinical trials, but we also think about brain protection, brain preservation, and brain repair, which, I think, is potentially the underlying substrate that is not yet fully addressed by the evolving treatment.
BTK inhibitor reduces MS enhancing lesions
. However, there was no difference between the 25-mg once daily, 75-mg once daily, 75-mg twice daily, and placebo-treated groups in Expanded Disability Status Scale scores, according to a double-blind, randomized, phase 2 trial published in the New England Journal of Medicine (2019 May 10. doi: 10.1056/NEJMoa1901981).
We first reported on the results of this trial when they were presented at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis. Find our coverage at the link below.
. However, there was no difference between the 25-mg once daily, 75-mg once daily, 75-mg twice daily, and placebo-treated groups in Expanded Disability Status Scale scores, according to a double-blind, randomized, phase 2 trial published in the New England Journal of Medicine (2019 May 10. doi: 10.1056/NEJMoa1901981).
We first reported on the results of this trial when they were presented at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis. Find our coverage at the link below.
. However, there was no difference between the 25-mg once daily, 75-mg once daily, 75-mg twice daily, and placebo-treated groups in Expanded Disability Status Scale scores, according to a double-blind, randomized, phase 2 trial published in the New England Journal of Medicine (2019 May 10. doi: 10.1056/NEJMoa1901981).
We first reported on the results of this trial when they were presented at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis. Find our coverage at the link below.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Interview with Stephen Krieger, MD, on the topographical model of multiple sclerosis
We interviewed Dr. Stephen Krieger to discuss his research in the implementation of the topographical model of Multiple Sclerosis.
What is the concept behind the topographical model of multiple sclerosis (MS)?
DR. KRIEGER: MS is an incredibly heterogeneous, and in many ways, unpredictable disease. Some people with MS will have a relapsing course, others will take a progressive course of disease, and many will have a disease course that spans both a relapsing phase and a progressive phase.
We have traditionally divided MS into phenotypes like relapsing-remitting MS (RRMS), secondary-progressive MS (SPMS), or primary-progressive MS (PPMS), and these phenotypes have been foundational in our field and used to define clinical trial cohorts and outcomes. They also have been used for the approval of our medicines. In practice, however, it sometimes can be difficult to know precisely what kind of MS, what phenotypes of MS, that an individual patient has.
The topographical model, which was proposed four years ago, tries to unify our concepts of MS in a way that spans across those phenotypes and animates the disease course in a more dynamic way to bridge from one phenotype to another.
As an individual patient, for example, develops clinically isolated syndrome (CIS) or first attack, and then RRMS, and then later SPMS, that gets depicted in a dynamic visualization through the topographical model. The model also makes use of the idea that where an MS lesion is in the central nervous system (CNS) defines the clinical symptoms that it causes.
This is something that we have long known, and the art of localization in neurology has existed for at least a couple hundred years. But we have not used that in the way we have depicted MS clinical course in recent decades. The topographical model tries to bring this idea of mapping an individual patient’s disease topography back into the clinical picture.
In the topographical model, the lesions are shown as different topographical peaks, via the hills and valleys of areas of MS damage across different regions of the CNS [image]. They are compensated for by reserve, by the ability that the nervous system has to compensate and to keep a disease process from crossing the clinical threshold and causing symptoms. What the topographical model displays is that patients with MS lose reserve as time passes.
We know that there is brain atrophy, brain stem atrophy, spinal cord atrophy, and retinal nerve fiber layer thinning in this disease. The topographical model takes the concept that MS causes a loss of tissue across the CNS and applies it to where the lesions are in the CNS. The coming together of those two things brings about the clinical picture unmasking the deficit from those lesions over time. The short version is a depiction of disease course in MS. The way it looks has been likened to a leaking swimming pool, where there is a shallow end and a deep end, and as reserve drains over time more and more of that subclinical disease becomes unmasked.
In the Laitman article (2018), you applied the model to real patients. What were the main findings from that study?
DR. KRIEGER: Until now, the topographical model has been conceptual with a visual depiction, and I think it has been important as an educational tool and an aid to help shape our thinking about MS in a unified way.
The Laitman et al research, is the first time we have applied the concepts of the model to individual patients to confirm whether we could map individual patients’ MS histories in the topographical model and see if we could depict their clinical course this way. We found that we could.
One of the most important points that the topographical model makes is the idea that as progression occurs and reserve is lost, there is an unmasking of underlying disease. Meaning, all of the signs and symptoms that a patient has had during their relapse when they were accumulating lesions should be re-revealed or recapitulated when reserve is lost and progression occurs.
To confirm this, we mapped ten patients in the topographical model. We characterized their signs and symptoms of relapses during the relapsing phase and we found that the vast majority of these symptoms had redeclared themselves at the time that these patients developed SPMS. Furthermore, those symptoms were continuing to worsen in their pattern; that is in the pattern of their disease topography as the years have continued to pass since they developed SPMS.
This was the first empirical study in real patients to show that the principles of the topographical model held true. This recapitulation hypothesis of symptoms in progressive disease was borne out, and that can help to lay the groundwork for future empirical studies to see how this model can be used as a predictive tool.
How does this new theory of MS disease progression better inform treatment decisions than the disease course theories that currently exist?
DR. KRIEGER: We have had the clinical phenotypes for 20 years and it has been very helpful to us in the development of treatments that we have shown are effective for RRMS and in more recent years for PPMS. What we don’t really have is a way of personalizing and predicting the individual person’s disease trajectory.
Although we have prognostic factors that we know are important, such as age and MRI disease burden, there is still great uncertainty of the clinical course in the individual patient. If the topographical model can be further empirically validated using real world data, that could help us to predict what is going to happen to an individual patient. That can help us to make better treatment decisions for them because it could inform our treatment decisions in a more personalized way.
Is there any other recent research that supports these concepts?
DR. KRIEGER: We talk a lot about the need for biomarkers in MS to help us predict disease course and the topographical model makes the case that lesion location is a crucial biomarker. That is, the patient that has lesions in the spinal cord and the brain stem is more likely to have progressive signs and symptoms referable to those lesions.
A separate piece of work recently done by Keegan and colleagues that was published in Multiple Sclerosis Journal, looked at their own cohort of patients that had at least one critically located lesion, typically in the high cervical spinal cord or the lower brain stem, as being the crucial driver of the development of motor dysfunction and progressive disability.
In an editorial I wrote with my colleague, Fred Lublin, called “Location, location, location,” we point out that this is in some ways the best data in support of the concept of the topographical model that I have seen. It outlines a framework or a methodology where the importance of lesion location in defining the clinical picture and the risk of progression for an individual patient can be studied.
We interviewed Dr. Stephen Krieger to discuss his research in the implementation of the topographical model of Multiple Sclerosis.
What is the concept behind the topographical model of multiple sclerosis (MS)?
DR. KRIEGER: MS is an incredibly heterogeneous, and in many ways, unpredictable disease. Some people with MS will have a relapsing course, others will take a progressive course of disease, and many will have a disease course that spans both a relapsing phase and a progressive phase.
We have traditionally divided MS into phenotypes like relapsing-remitting MS (RRMS), secondary-progressive MS (SPMS), or primary-progressive MS (PPMS), and these phenotypes have been foundational in our field and used to define clinical trial cohorts and outcomes. They also have been used for the approval of our medicines. In practice, however, it sometimes can be difficult to know precisely what kind of MS, what phenotypes of MS, that an individual patient has.
The topographical model, which was proposed four years ago, tries to unify our concepts of MS in a way that spans across those phenotypes and animates the disease course in a more dynamic way to bridge from one phenotype to another.
As an individual patient, for example, develops clinically isolated syndrome (CIS) or first attack, and then RRMS, and then later SPMS, that gets depicted in a dynamic visualization through the topographical model. The model also makes use of the idea that where an MS lesion is in the central nervous system (CNS) defines the clinical symptoms that it causes.
This is something that we have long known, and the art of localization in neurology has existed for at least a couple hundred years. But we have not used that in the way we have depicted MS clinical course in recent decades. The topographical model tries to bring this idea of mapping an individual patient’s disease topography back into the clinical picture.
In the topographical model, the lesions are shown as different topographical peaks, via the hills and valleys of areas of MS damage across different regions of the CNS [image]. They are compensated for by reserve, by the ability that the nervous system has to compensate and to keep a disease process from crossing the clinical threshold and causing symptoms. What the topographical model displays is that patients with MS lose reserve as time passes.
We know that there is brain atrophy, brain stem atrophy, spinal cord atrophy, and retinal nerve fiber layer thinning in this disease. The topographical model takes the concept that MS causes a loss of tissue across the CNS and applies it to where the lesions are in the CNS. The coming together of those two things brings about the clinical picture unmasking the deficit from those lesions over time. The short version is a depiction of disease course in MS. The way it looks has been likened to a leaking swimming pool, where there is a shallow end and a deep end, and as reserve drains over time more and more of that subclinical disease becomes unmasked.
In the Laitman article (2018), you applied the model to real patients. What were the main findings from that study?
DR. KRIEGER: Until now, the topographical model has been conceptual with a visual depiction, and I think it has been important as an educational tool and an aid to help shape our thinking about MS in a unified way.
The Laitman et al research, is the first time we have applied the concepts of the model to individual patients to confirm whether we could map individual patients’ MS histories in the topographical model and see if we could depict their clinical course this way. We found that we could.
One of the most important points that the topographical model makes is the idea that as progression occurs and reserve is lost, there is an unmasking of underlying disease. Meaning, all of the signs and symptoms that a patient has had during their relapse when they were accumulating lesions should be re-revealed or recapitulated when reserve is lost and progression occurs.
To confirm this, we mapped ten patients in the topographical model. We characterized their signs and symptoms of relapses during the relapsing phase and we found that the vast majority of these symptoms had redeclared themselves at the time that these patients developed SPMS. Furthermore, those symptoms were continuing to worsen in their pattern; that is in the pattern of their disease topography as the years have continued to pass since they developed SPMS.
This was the first empirical study in real patients to show that the principles of the topographical model held true. This recapitulation hypothesis of symptoms in progressive disease was borne out, and that can help to lay the groundwork for future empirical studies to see how this model can be used as a predictive tool.
How does this new theory of MS disease progression better inform treatment decisions than the disease course theories that currently exist?
DR. KRIEGER: We have had the clinical phenotypes for 20 years and it has been very helpful to us in the development of treatments that we have shown are effective for RRMS and in more recent years for PPMS. What we don’t really have is a way of personalizing and predicting the individual person’s disease trajectory.
Although we have prognostic factors that we know are important, such as age and MRI disease burden, there is still great uncertainty of the clinical course in the individual patient. If the topographical model can be further empirically validated using real world data, that could help us to predict what is going to happen to an individual patient. That can help us to make better treatment decisions for them because it could inform our treatment decisions in a more personalized way.
Is there any other recent research that supports these concepts?
DR. KRIEGER: We talk a lot about the need for biomarkers in MS to help us predict disease course and the topographical model makes the case that lesion location is a crucial biomarker. That is, the patient that has lesions in the spinal cord and the brain stem is more likely to have progressive signs and symptoms referable to those lesions.
A separate piece of work recently done by Keegan and colleagues that was published in Multiple Sclerosis Journal, looked at their own cohort of patients that had at least one critically located lesion, typically in the high cervical spinal cord or the lower brain stem, as being the crucial driver of the development of motor dysfunction and progressive disability.
In an editorial I wrote with my colleague, Fred Lublin, called “Location, location, location,” we point out that this is in some ways the best data in support of the concept of the topographical model that I have seen. It outlines a framework or a methodology where the importance of lesion location in defining the clinical picture and the risk of progression for an individual patient can be studied.
We interviewed Dr. Stephen Krieger to discuss his research in the implementation of the topographical model of Multiple Sclerosis.
What is the concept behind the topographical model of multiple sclerosis (MS)?
DR. KRIEGER: MS is an incredibly heterogeneous, and in many ways, unpredictable disease. Some people with MS will have a relapsing course, others will take a progressive course of disease, and many will have a disease course that spans both a relapsing phase and a progressive phase.
We have traditionally divided MS into phenotypes like relapsing-remitting MS (RRMS), secondary-progressive MS (SPMS), or primary-progressive MS (PPMS), and these phenotypes have been foundational in our field and used to define clinical trial cohorts and outcomes. They also have been used for the approval of our medicines. In practice, however, it sometimes can be difficult to know precisely what kind of MS, what phenotypes of MS, that an individual patient has.
The topographical model, which was proposed four years ago, tries to unify our concepts of MS in a way that spans across those phenotypes and animates the disease course in a more dynamic way to bridge from one phenotype to another.
As an individual patient, for example, develops clinically isolated syndrome (CIS) or first attack, and then RRMS, and then later SPMS, that gets depicted in a dynamic visualization through the topographical model. The model also makes use of the idea that where an MS lesion is in the central nervous system (CNS) defines the clinical symptoms that it causes.
This is something that we have long known, and the art of localization in neurology has existed for at least a couple hundred years. But we have not used that in the way we have depicted MS clinical course in recent decades. The topographical model tries to bring this idea of mapping an individual patient’s disease topography back into the clinical picture.
In the topographical model, the lesions are shown as different topographical peaks, via the hills and valleys of areas of MS damage across different regions of the CNS [image]. They are compensated for by reserve, by the ability that the nervous system has to compensate and to keep a disease process from crossing the clinical threshold and causing symptoms. What the topographical model displays is that patients with MS lose reserve as time passes.
We know that there is brain atrophy, brain stem atrophy, spinal cord atrophy, and retinal nerve fiber layer thinning in this disease. The topographical model takes the concept that MS causes a loss of tissue across the CNS and applies it to where the lesions are in the CNS. The coming together of those two things brings about the clinical picture unmasking the deficit from those lesions over time. The short version is a depiction of disease course in MS. The way it looks has been likened to a leaking swimming pool, where there is a shallow end and a deep end, and as reserve drains over time more and more of that subclinical disease becomes unmasked.
In the Laitman article (2018), you applied the model to real patients. What were the main findings from that study?
DR. KRIEGER: Until now, the topographical model has been conceptual with a visual depiction, and I think it has been important as an educational tool and an aid to help shape our thinking about MS in a unified way.
The Laitman et al research, is the first time we have applied the concepts of the model to individual patients to confirm whether we could map individual patients’ MS histories in the topographical model and see if we could depict their clinical course this way. We found that we could.
One of the most important points that the topographical model makes is the idea that as progression occurs and reserve is lost, there is an unmasking of underlying disease. Meaning, all of the signs and symptoms that a patient has had during their relapse when they were accumulating lesions should be re-revealed or recapitulated when reserve is lost and progression occurs.
To confirm this, we mapped ten patients in the topographical model. We characterized their signs and symptoms of relapses during the relapsing phase and we found that the vast majority of these symptoms had redeclared themselves at the time that these patients developed SPMS. Furthermore, those symptoms were continuing to worsen in their pattern; that is in the pattern of their disease topography as the years have continued to pass since they developed SPMS.
This was the first empirical study in real patients to show that the principles of the topographical model held true. This recapitulation hypothesis of symptoms in progressive disease was borne out, and that can help to lay the groundwork for future empirical studies to see how this model can be used as a predictive tool.
How does this new theory of MS disease progression better inform treatment decisions than the disease course theories that currently exist?
DR. KRIEGER: We have had the clinical phenotypes for 20 years and it has been very helpful to us in the development of treatments that we have shown are effective for RRMS and in more recent years for PPMS. What we don’t really have is a way of personalizing and predicting the individual person’s disease trajectory.
Although we have prognostic factors that we know are important, such as age and MRI disease burden, there is still great uncertainty of the clinical course in the individual patient. If the topographical model can be further empirically validated using real world data, that could help us to predict what is going to happen to an individual patient. That can help us to make better treatment decisions for them because it could inform our treatment decisions in a more personalized way.
Is there any other recent research that supports these concepts?
DR. KRIEGER: We talk a lot about the need for biomarkers in MS to help us predict disease course and the topographical model makes the case that lesion location is a crucial biomarker. That is, the patient that has lesions in the spinal cord and the brain stem is more likely to have progressive signs and symptoms referable to those lesions.
A separate piece of work recently done by Keegan and colleagues that was published in Multiple Sclerosis Journal, looked at their own cohort of patients that had at least one critically located lesion, typically in the high cervical spinal cord or the lower brain stem, as being the crucial driver of the development of motor dysfunction and progressive disability.
In an editorial I wrote with my colleague, Fred Lublin, called “Location, location, location,” we point out that this is in some ways the best data in support of the concept of the topographical model that I have seen. It outlines a framework or a methodology where the importance of lesion location in defining the clinical picture and the risk of progression for an individual patient can be studied.