User login
Injectable monoclonal antibodies prevent COVID-19 in trial
according to results of a randomized, double-blind, placebo-controlled clinical trial published online August 4, 2021, in the New England Journal of Medicine.
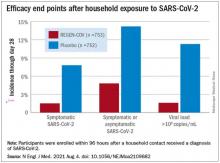
The cocktail of the monoclonal antibodies casirivimab and imdevimab (REGEN-COV, Regeneron Pharmaceuticals) reduced participants’ relative risk of infection by 72%, compared with placebo within the first week. After the first week, risk reduction increased to 93%.
“Long after you would be exposed by your household, there is an enduring effect that prevents you from community spread,” said David Wohl, MD, professor of medicine in the division of infectious diseases at the University of North Carolina at Chapel Hill, who was a site investigator for the trial but not a study author.
Participants were enrolled within 96 hours after someone in their household tested positive for SARS-CoV-2. Participants were randomly assigned to receive 1,200 mg of REGEN-COV subcutaneously or a placebo. Based on serologic testing, study participants showed no evidence of current or previous SARS-CoV-2 infection. The median age of participants was 42.9, but 45% were male teenagers (ages 12-17).
In the group that received REGEN-COV, 11 out of 753 participants developed symptomatic COVID-19, compared with 59 out of 752 participants who received placebo. The relative risk reduction for the study’s 4-week period was 81.4% (P < .001). Of the participants that did develop a SARS-CoV-2 infection, those that received REGEN-COV were less likely to be symptomatic. Asymptomatic infections developed in 25 participants who received REGEN-COV versus 48 in the placebo group. The relative risk of developing any SARS-CoV-2 infection, symptomatic or asymptomatic, was reduced by 66.4% with REGEN-COV (P < .001).
Among the patients who were symptomatic, symptoms subsided within a median of 1.2 weeks for the group that received REGEN-COV, 2 weeks earlier than the placebo group. These patients also had a shorter duration of a high viral load (>104 copies/mL). Few adverse events were reported in the treatment or placebo groups. Monoclonal antibodies “seem to be incredibly safe,” Dr. Wohl said.
“These monoclonal antibodies have proven they can reduce the viral replication in the nose,” said study author Myron Cohen, MD, an infectious disease specialist and professor of epidemiology at the University of North Carolina.
The Food and Drug Administration first granted REGEN-COV emergency use authorization (EUA) in November 2020 for use in patients with mild or moderate COVID-19 who were also at high risk for progressing to severe COVID-19. At that time, the cocktail of monoclonal antibodies was delivered by a single intravenous infusion.
In January, Regeneron first announced the success of this trial of the subcutaneous injection for exposed household contacts based on early results, and in June of 2021, the FDA expanded the EUA to include a subcutaneous delivery when IV is not feasible. On July 30, the EUA was expanded again to include prophylactic use in exposed patients based on these trial results.
The U.S. government has purchased approximately 1.5 million doses of REGEN-COV from Regeneron and has agreed to make the treatments free of charge to patients.
But despite being free, available, and backed by promising data, monoclonal antibodies as a therapeutic answer to COVID-19 still hasn’t really taken off. “The problem is, it first requires knowledge and awareness,” Dr. Wohl said. “A lot [of people] don’t know this exists. To be honest, vaccination has taken up all the oxygen in the room.”
Dr. Cohen agreed. One reason for the slow uptake may be because the drug supply is owned by the government and not a pharmaceutical company. There hasn’t been a typical marketing push to make physicians and consumers aware. Additionally, “the logistics are daunting,” Dr. Cohen said. The office spaces where many physicians care for patients “often aren’t appropriate for patients who think they have SARS-CoV-2.”
“Right now, there’s not a mechanism” to administer the drug to people who could benefit from it, Dr. Wohl said. Eligible patients are either immunocompromised and unlikely to mount a sufficient immune response with vaccination, or not fully vaccinated. They should have been exposed to an infected individual or have a high likelihood of exposure due to where they live, such as in a prison or nursing home. Local doctors are unlikely to be the primary administrators of the drug, Dr. Wohl added. “How do we operationalize this for people who fit the criteria?”
There’s also an issue of timing. REGEN-COV is most effective when given early, Dr. Cohen said. “[Monoclonal antibodies] really only work well in the replication phase.” Many patients who would be eligible delay care until they’ve had symptoms for several days, when REGEN-COV would no longer have the desired effect.
Eventually, Dr. Wohl suspects demand will increase when people realize REGEN-COV can help those with COVID-19 and those who have been exposed. But before then, “we do have to think about how to integrate this into a workflow people can access without being confused.”
The trial was done before there was widespread vaccination, so it’s unclear what the results mean for people who have been vaccinated. Dr. Cohen and Dr. Wohl said there are ongoing conversations about whether monoclonal antibodies could be complementary to vaccination and if there’s potential for continued monthly use of these therapies.
Cohen and Wohl reported no relevant financial relationships. The trial was supported by Regeneron Pharmaceuticals, F. Hoffmann–La Roche, the National Institute of Allergy and Infectious Diseases, NIH, and the COVID-19 Prevention Network.
A version of this article first appeared on Medscape.com.
according to results of a randomized, double-blind, placebo-controlled clinical trial published online August 4, 2021, in the New England Journal of Medicine.
The cocktail of the monoclonal antibodies casirivimab and imdevimab (REGEN-COV, Regeneron Pharmaceuticals) reduced participants’ relative risk of infection by 72%, compared with placebo within the first week. After the first week, risk reduction increased to 93%.
“Long after you would be exposed by your household, there is an enduring effect that prevents you from community spread,” said David Wohl, MD, professor of medicine in the division of infectious diseases at the University of North Carolina at Chapel Hill, who was a site investigator for the trial but not a study author.
Participants were enrolled within 96 hours after someone in their household tested positive for SARS-CoV-2. Participants were randomly assigned to receive 1,200 mg of REGEN-COV subcutaneously or a placebo. Based on serologic testing, study participants showed no evidence of current or previous SARS-CoV-2 infection. The median age of participants was 42.9, but 45% were male teenagers (ages 12-17).
In the group that received REGEN-COV, 11 out of 753 participants developed symptomatic COVID-19, compared with 59 out of 752 participants who received placebo. The relative risk reduction for the study’s 4-week period was 81.4% (P < .001). Of the participants that did develop a SARS-CoV-2 infection, those that received REGEN-COV were less likely to be symptomatic. Asymptomatic infections developed in 25 participants who received REGEN-COV versus 48 in the placebo group. The relative risk of developing any SARS-CoV-2 infection, symptomatic or asymptomatic, was reduced by 66.4% with REGEN-COV (P < .001).
Among the patients who were symptomatic, symptoms subsided within a median of 1.2 weeks for the group that received REGEN-COV, 2 weeks earlier than the placebo group. These patients also had a shorter duration of a high viral load (>104 copies/mL). Few adverse events were reported in the treatment or placebo groups. Monoclonal antibodies “seem to be incredibly safe,” Dr. Wohl said.
“These monoclonal antibodies have proven they can reduce the viral replication in the nose,” said study author Myron Cohen, MD, an infectious disease specialist and professor of epidemiology at the University of North Carolina.
The Food and Drug Administration first granted REGEN-COV emergency use authorization (EUA) in November 2020 for use in patients with mild or moderate COVID-19 who were also at high risk for progressing to severe COVID-19. At that time, the cocktail of monoclonal antibodies was delivered by a single intravenous infusion.
In January, Regeneron first announced the success of this trial of the subcutaneous injection for exposed household contacts based on early results, and in June of 2021, the FDA expanded the EUA to include a subcutaneous delivery when IV is not feasible. On July 30, the EUA was expanded again to include prophylactic use in exposed patients based on these trial results.
The U.S. government has purchased approximately 1.5 million doses of REGEN-COV from Regeneron and has agreed to make the treatments free of charge to patients.
But despite being free, available, and backed by promising data, monoclonal antibodies as a therapeutic answer to COVID-19 still hasn’t really taken off. “The problem is, it first requires knowledge and awareness,” Dr. Wohl said. “A lot [of people] don’t know this exists. To be honest, vaccination has taken up all the oxygen in the room.”
Dr. Cohen agreed. One reason for the slow uptake may be because the drug supply is owned by the government and not a pharmaceutical company. There hasn’t been a typical marketing push to make physicians and consumers aware. Additionally, “the logistics are daunting,” Dr. Cohen said. The office spaces where many physicians care for patients “often aren’t appropriate for patients who think they have SARS-CoV-2.”
“Right now, there’s not a mechanism” to administer the drug to people who could benefit from it, Dr. Wohl said. Eligible patients are either immunocompromised and unlikely to mount a sufficient immune response with vaccination, or not fully vaccinated. They should have been exposed to an infected individual or have a high likelihood of exposure due to where they live, such as in a prison or nursing home. Local doctors are unlikely to be the primary administrators of the drug, Dr. Wohl added. “How do we operationalize this for people who fit the criteria?”
There’s also an issue of timing. REGEN-COV is most effective when given early, Dr. Cohen said. “[Monoclonal antibodies] really only work well in the replication phase.” Many patients who would be eligible delay care until they’ve had symptoms for several days, when REGEN-COV would no longer have the desired effect.
Eventually, Dr. Wohl suspects demand will increase when people realize REGEN-COV can help those with COVID-19 and those who have been exposed. But before then, “we do have to think about how to integrate this into a workflow people can access without being confused.”
The trial was done before there was widespread vaccination, so it’s unclear what the results mean for people who have been vaccinated. Dr. Cohen and Dr. Wohl said there are ongoing conversations about whether monoclonal antibodies could be complementary to vaccination and if there’s potential for continued monthly use of these therapies.
Cohen and Wohl reported no relevant financial relationships. The trial was supported by Regeneron Pharmaceuticals, F. Hoffmann–La Roche, the National Institute of Allergy and Infectious Diseases, NIH, and the COVID-19 Prevention Network.
A version of this article first appeared on Medscape.com.
according to results of a randomized, double-blind, placebo-controlled clinical trial published online August 4, 2021, in the New England Journal of Medicine.
The cocktail of the monoclonal antibodies casirivimab and imdevimab (REGEN-COV, Regeneron Pharmaceuticals) reduced participants’ relative risk of infection by 72%, compared with placebo within the first week. After the first week, risk reduction increased to 93%.
“Long after you would be exposed by your household, there is an enduring effect that prevents you from community spread,” said David Wohl, MD, professor of medicine in the division of infectious diseases at the University of North Carolina at Chapel Hill, who was a site investigator for the trial but not a study author.
Participants were enrolled within 96 hours after someone in their household tested positive for SARS-CoV-2. Participants were randomly assigned to receive 1,200 mg of REGEN-COV subcutaneously or a placebo. Based on serologic testing, study participants showed no evidence of current or previous SARS-CoV-2 infection. The median age of participants was 42.9, but 45% were male teenagers (ages 12-17).
In the group that received REGEN-COV, 11 out of 753 participants developed symptomatic COVID-19, compared with 59 out of 752 participants who received placebo. The relative risk reduction for the study’s 4-week period was 81.4% (P < .001). Of the participants that did develop a SARS-CoV-2 infection, those that received REGEN-COV were less likely to be symptomatic. Asymptomatic infections developed in 25 participants who received REGEN-COV versus 48 in the placebo group. The relative risk of developing any SARS-CoV-2 infection, symptomatic or asymptomatic, was reduced by 66.4% with REGEN-COV (P < .001).
Among the patients who were symptomatic, symptoms subsided within a median of 1.2 weeks for the group that received REGEN-COV, 2 weeks earlier than the placebo group. These patients also had a shorter duration of a high viral load (>104 copies/mL). Few adverse events were reported in the treatment or placebo groups. Monoclonal antibodies “seem to be incredibly safe,” Dr. Wohl said.
“These monoclonal antibodies have proven they can reduce the viral replication in the nose,” said study author Myron Cohen, MD, an infectious disease specialist and professor of epidemiology at the University of North Carolina.
The Food and Drug Administration first granted REGEN-COV emergency use authorization (EUA) in November 2020 for use in patients with mild or moderate COVID-19 who were also at high risk for progressing to severe COVID-19. At that time, the cocktail of monoclonal antibodies was delivered by a single intravenous infusion.
In January, Regeneron first announced the success of this trial of the subcutaneous injection for exposed household contacts based on early results, and in June of 2021, the FDA expanded the EUA to include a subcutaneous delivery when IV is not feasible. On July 30, the EUA was expanded again to include prophylactic use in exposed patients based on these trial results.
The U.S. government has purchased approximately 1.5 million doses of REGEN-COV from Regeneron and has agreed to make the treatments free of charge to patients.
But despite being free, available, and backed by promising data, monoclonal antibodies as a therapeutic answer to COVID-19 still hasn’t really taken off. “The problem is, it first requires knowledge and awareness,” Dr. Wohl said. “A lot [of people] don’t know this exists. To be honest, vaccination has taken up all the oxygen in the room.”
Dr. Cohen agreed. One reason for the slow uptake may be because the drug supply is owned by the government and not a pharmaceutical company. There hasn’t been a typical marketing push to make physicians and consumers aware. Additionally, “the logistics are daunting,” Dr. Cohen said. The office spaces where many physicians care for patients “often aren’t appropriate for patients who think they have SARS-CoV-2.”
“Right now, there’s not a mechanism” to administer the drug to people who could benefit from it, Dr. Wohl said. Eligible patients are either immunocompromised and unlikely to mount a sufficient immune response with vaccination, or not fully vaccinated. They should have been exposed to an infected individual or have a high likelihood of exposure due to where they live, such as in a prison or nursing home. Local doctors are unlikely to be the primary administrators of the drug, Dr. Wohl added. “How do we operationalize this for people who fit the criteria?”
There’s also an issue of timing. REGEN-COV is most effective when given early, Dr. Cohen said. “[Monoclonal antibodies] really only work well in the replication phase.” Many patients who would be eligible delay care until they’ve had symptoms for several days, when REGEN-COV would no longer have the desired effect.
Eventually, Dr. Wohl suspects demand will increase when people realize REGEN-COV can help those with COVID-19 and those who have been exposed. But before then, “we do have to think about how to integrate this into a workflow people can access without being confused.”
The trial was done before there was widespread vaccination, so it’s unclear what the results mean for people who have been vaccinated. Dr. Cohen and Dr. Wohl said there are ongoing conversations about whether monoclonal antibodies could be complementary to vaccination and if there’s potential for continued monthly use of these therapies.
Cohen and Wohl reported no relevant financial relationships. The trial was supported by Regeneron Pharmaceuticals, F. Hoffmann–La Roche, the National Institute of Allergy and Infectious Diseases, NIH, and the COVID-19 Prevention Network.
A version of this article first appeared on Medscape.com.
Myocarditis tied to COVID-19 shots more common than reported?
While cases of pericarditis or myocarditis temporally linked to COVID-19 vaccination remain rare, they may happen more often than reported, according to a large review of electronic medical records (EMRs).
They also appear to represent two “distinct syndromes,” George Diaz, MD, Providence Regional Medical Center Everett (Washington), said in an interview.
Myocarditis typically occurs soon after vaccination in younger patients and mostly after the second dose, while pericarditis occurs later in older patients, after the first or second dose.
Dr. Diaz and colleagues reported their analysis in a research letter published online August 4 in JAMA.
They reviewed the records of 2,000,287 people who received at least one COVID-19 vaccination at 40 hospitals in Washington, Oregon, Montana, and California that are part of the Providence health care system and use the same EMRs.
The median age of the cohort was 57 years and 59% were women.
A little more than three quarters (77%) received more than one dose; most received the mRNA vaccines made by Pfizer (53%) and Moderna (44%); 3% received the Johnson & Johnson vaccine.
The records showed that 20 people had vaccine-related myocarditis (1.0 per 100,000) and 37 had pericarditis (1.8 per 100,000).
A recent report, based on data from the Centers for Disease Control and Prevention’s Vaccine Adverse Events Reporting System, suggested an incidence of myocarditis of about 4.8 cases per 1 million following receipt of mRNA COVID-19 vaccine.
The new study shows a “similar pattern, although at higher incidence, suggesting vaccine adverse event underreporting. In addition, pericarditis may be more common than myocarditis among older patients,” the study team wrote.
“Our study resulted in higher numbers of cases probably because we searched the EMR, and VAERS requires doctors to report suspected cases voluntarily,” Dr. Diaz said in an interview.
Also, in the governments’ statistics, pericarditis and myocarditis were “lumped together,” he noted.
Myocarditis cases
The 20 myocarditis cases occurred a median of 3.5 days after vaccination (11 after the Moderna vaccine and 9 after the Pfizer vaccine), 15 of the patients (75%) were men, and the median age was 36 years.
Four individuals (20%) developed myocarditis symptoms after the first vaccination and 16 (80%) after the second dose. Nineteen of the patients (95%) were admitted to the hospital and all were discharged after a median of 2 days.
None of the 20 patients were readmitted or died. Two received a second vaccination after onset of myocarditis; neither had worsening of symptoms. At last available follow-up (median, 23.5 days after symptom onset), 13 patients (65%) had a resolution of their myocarditis symptoms and seven (35%) were improving.
Pericarditis cases
The 37 pericarditis cases occurred a median of 20 days after the most recent COVID-19 vaccination: 23 (62%) with Pfizer, 12 (32%) with Moderna, and 2 (5%) with the J&J vaccine. Fifteen developed pericarditis after the first vaccine dose (41%) and 22 (59%) after the second.
Twenty-seven (73%) of the cases occurred in men; the median age was 59 years.
Thirteen patients (35%) were admitted to the hospital, none to intensive care. The median hospital stay was 1 day. Seven patients with pericarditis received a second vaccination. No patient died.
At last available follow-up (median, 28 days), 7 patients (19%) had resolved symptoms and 23 (62%) were improving.
The researchers also calculate that the average monthly number of cases of myocarditis or myopericarditis during the prevaccine period of January 2019 through January 2021 was 16.9 (95% confidence interval, 15.3-18.6) compared with 27.3 (95% CI, 22.4-32.9) during the vaccine period of February through May 2021 (P < .001).
The mean numbers of pericarditis cases during the same periods were 49.1 (95% CI, 46.4-51.9) and 78.8 (95% CI, 70.3-87.9), respectively (P < .001).
The authors say limitations of their analysis include potential missed cases outside care settings and missed diagnoses of myocarditis or pericarditis, which would underestimate the incidence, as well as inaccurate EMR vaccination information.
“Temporal association does not prove causation, although the short span between vaccination and myocarditis onset and the elevated incidence of myocarditis and pericarditis in the study hospitals lend support to a possible relationship,” they wrote.
In late June, the Food and Drug Administration added a warning to the fact sheets accompanying the Pfizer and Moderna mRNA COVID-19 vaccines, flagging the rare risk of heart inflammation after their use.
Dr. Diaz cautioned that myocarditis and pericarditis events remain “a rare occurrence” after COVID-19 vaccination.
“When discussing vaccination with patients, [health care providers] can advise them that patients generally recover in the rare event they get pericarditis or myocarditis and no deaths were found, and that the vaccines are safe and effective,” Dr. Diaz said.
The study had no specific funding. Dr. Diaz reported receipt of clinical trial research support from Gilead Sciences, Regeneron, Roche, Boehringer Ingelheim, and Edesa Biotech and scientific advisory board membership for Safeology.
A version of this article first appeared on Medscape.com.
While cases of pericarditis or myocarditis temporally linked to COVID-19 vaccination remain rare, they may happen more often than reported, according to a large review of electronic medical records (EMRs).
They also appear to represent two “distinct syndromes,” George Diaz, MD, Providence Regional Medical Center Everett (Washington), said in an interview.
Myocarditis typically occurs soon after vaccination in younger patients and mostly after the second dose, while pericarditis occurs later in older patients, after the first or second dose.
Dr. Diaz and colleagues reported their analysis in a research letter published online August 4 in JAMA.
They reviewed the records of 2,000,287 people who received at least one COVID-19 vaccination at 40 hospitals in Washington, Oregon, Montana, and California that are part of the Providence health care system and use the same EMRs.
The median age of the cohort was 57 years and 59% were women.
A little more than three quarters (77%) received more than one dose; most received the mRNA vaccines made by Pfizer (53%) and Moderna (44%); 3% received the Johnson & Johnson vaccine.
The records showed that 20 people had vaccine-related myocarditis (1.0 per 100,000) and 37 had pericarditis (1.8 per 100,000).
A recent report, based on data from the Centers for Disease Control and Prevention’s Vaccine Adverse Events Reporting System, suggested an incidence of myocarditis of about 4.8 cases per 1 million following receipt of mRNA COVID-19 vaccine.
The new study shows a “similar pattern, although at higher incidence, suggesting vaccine adverse event underreporting. In addition, pericarditis may be more common than myocarditis among older patients,” the study team wrote.
“Our study resulted in higher numbers of cases probably because we searched the EMR, and VAERS requires doctors to report suspected cases voluntarily,” Dr. Diaz said in an interview.
Also, in the governments’ statistics, pericarditis and myocarditis were “lumped together,” he noted.
Myocarditis cases
The 20 myocarditis cases occurred a median of 3.5 days after vaccination (11 after the Moderna vaccine and 9 after the Pfizer vaccine), 15 of the patients (75%) were men, and the median age was 36 years.
Four individuals (20%) developed myocarditis symptoms after the first vaccination and 16 (80%) after the second dose. Nineteen of the patients (95%) were admitted to the hospital and all were discharged after a median of 2 days.
None of the 20 patients were readmitted or died. Two received a second vaccination after onset of myocarditis; neither had worsening of symptoms. At last available follow-up (median, 23.5 days after symptom onset), 13 patients (65%) had a resolution of their myocarditis symptoms and seven (35%) were improving.
Pericarditis cases
The 37 pericarditis cases occurred a median of 20 days after the most recent COVID-19 vaccination: 23 (62%) with Pfizer, 12 (32%) with Moderna, and 2 (5%) with the J&J vaccine. Fifteen developed pericarditis after the first vaccine dose (41%) and 22 (59%) after the second.
Twenty-seven (73%) of the cases occurred in men; the median age was 59 years.
Thirteen patients (35%) were admitted to the hospital, none to intensive care. The median hospital stay was 1 day. Seven patients with pericarditis received a second vaccination. No patient died.
At last available follow-up (median, 28 days), 7 patients (19%) had resolved symptoms and 23 (62%) were improving.
The researchers also calculate that the average monthly number of cases of myocarditis or myopericarditis during the prevaccine period of January 2019 through January 2021 was 16.9 (95% confidence interval, 15.3-18.6) compared with 27.3 (95% CI, 22.4-32.9) during the vaccine period of February through May 2021 (P < .001).
The mean numbers of pericarditis cases during the same periods were 49.1 (95% CI, 46.4-51.9) and 78.8 (95% CI, 70.3-87.9), respectively (P < .001).
The authors say limitations of their analysis include potential missed cases outside care settings and missed diagnoses of myocarditis or pericarditis, which would underestimate the incidence, as well as inaccurate EMR vaccination information.
“Temporal association does not prove causation, although the short span between vaccination and myocarditis onset and the elevated incidence of myocarditis and pericarditis in the study hospitals lend support to a possible relationship,” they wrote.
In late June, the Food and Drug Administration added a warning to the fact sheets accompanying the Pfizer and Moderna mRNA COVID-19 vaccines, flagging the rare risk of heart inflammation after their use.
Dr. Diaz cautioned that myocarditis and pericarditis events remain “a rare occurrence” after COVID-19 vaccination.
“When discussing vaccination with patients, [health care providers] can advise them that patients generally recover in the rare event they get pericarditis or myocarditis and no deaths were found, and that the vaccines are safe and effective,” Dr. Diaz said.
The study had no specific funding. Dr. Diaz reported receipt of clinical trial research support from Gilead Sciences, Regeneron, Roche, Boehringer Ingelheim, and Edesa Biotech and scientific advisory board membership for Safeology.
A version of this article first appeared on Medscape.com.
While cases of pericarditis or myocarditis temporally linked to COVID-19 vaccination remain rare, they may happen more often than reported, according to a large review of electronic medical records (EMRs).
They also appear to represent two “distinct syndromes,” George Diaz, MD, Providence Regional Medical Center Everett (Washington), said in an interview.
Myocarditis typically occurs soon after vaccination in younger patients and mostly after the second dose, while pericarditis occurs later in older patients, after the first or second dose.
Dr. Diaz and colleagues reported their analysis in a research letter published online August 4 in JAMA.
They reviewed the records of 2,000,287 people who received at least one COVID-19 vaccination at 40 hospitals in Washington, Oregon, Montana, and California that are part of the Providence health care system and use the same EMRs.
The median age of the cohort was 57 years and 59% were women.
A little more than three quarters (77%) received more than one dose; most received the mRNA vaccines made by Pfizer (53%) and Moderna (44%); 3% received the Johnson & Johnson vaccine.
The records showed that 20 people had vaccine-related myocarditis (1.0 per 100,000) and 37 had pericarditis (1.8 per 100,000).
A recent report, based on data from the Centers for Disease Control and Prevention’s Vaccine Adverse Events Reporting System, suggested an incidence of myocarditis of about 4.8 cases per 1 million following receipt of mRNA COVID-19 vaccine.
The new study shows a “similar pattern, although at higher incidence, suggesting vaccine adverse event underreporting. In addition, pericarditis may be more common than myocarditis among older patients,” the study team wrote.
“Our study resulted in higher numbers of cases probably because we searched the EMR, and VAERS requires doctors to report suspected cases voluntarily,” Dr. Diaz said in an interview.
Also, in the governments’ statistics, pericarditis and myocarditis were “lumped together,” he noted.
Myocarditis cases
The 20 myocarditis cases occurred a median of 3.5 days after vaccination (11 after the Moderna vaccine and 9 after the Pfizer vaccine), 15 of the patients (75%) were men, and the median age was 36 years.
Four individuals (20%) developed myocarditis symptoms after the first vaccination and 16 (80%) after the second dose. Nineteen of the patients (95%) were admitted to the hospital and all were discharged after a median of 2 days.
None of the 20 patients were readmitted or died. Two received a second vaccination after onset of myocarditis; neither had worsening of symptoms. At last available follow-up (median, 23.5 days after symptom onset), 13 patients (65%) had a resolution of their myocarditis symptoms and seven (35%) were improving.
Pericarditis cases
The 37 pericarditis cases occurred a median of 20 days after the most recent COVID-19 vaccination: 23 (62%) with Pfizer, 12 (32%) with Moderna, and 2 (5%) with the J&J vaccine. Fifteen developed pericarditis after the first vaccine dose (41%) and 22 (59%) after the second.
Twenty-seven (73%) of the cases occurred in men; the median age was 59 years.
Thirteen patients (35%) were admitted to the hospital, none to intensive care. The median hospital stay was 1 day. Seven patients with pericarditis received a second vaccination. No patient died.
At last available follow-up (median, 28 days), 7 patients (19%) had resolved symptoms and 23 (62%) were improving.
The researchers also calculate that the average monthly number of cases of myocarditis or myopericarditis during the prevaccine period of January 2019 through January 2021 was 16.9 (95% confidence interval, 15.3-18.6) compared with 27.3 (95% CI, 22.4-32.9) during the vaccine period of February through May 2021 (P < .001).
The mean numbers of pericarditis cases during the same periods were 49.1 (95% CI, 46.4-51.9) and 78.8 (95% CI, 70.3-87.9), respectively (P < .001).
The authors say limitations of their analysis include potential missed cases outside care settings and missed diagnoses of myocarditis or pericarditis, which would underestimate the incidence, as well as inaccurate EMR vaccination information.
“Temporal association does not prove causation, although the short span between vaccination and myocarditis onset and the elevated incidence of myocarditis and pericarditis in the study hospitals lend support to a possible relationship,” they wrote.
In late June, the Food and Drug Administration added a warning to the fact sheets accompanying the Pfizer and Moderna mRNA COVID-19 vaccines, flagging the rare risk of heart inflammation after their use.
Dr. Diaz cautioned that myocarditis and pericarditis events remain “a rare occurrence” after COVID-19 vaccination.
“When discussing vaccination with patients, [health care providers] can advise them that patients generally recover in the rare event they get pericarditis or myocarditis and no deaths were found, and that the vaccines are safe and effective,” Dr. Diaz said.
The study had no specific funding. Dr. Diaz reported receipt of clinical trial research support from Gilead Sciences, Regeneron, Roche, Boehringer Ingelheim, and Edesa Biotech and scientific advisory board membership for Safeology.
A version of this article first appeared on Medscape.com.
Will the Delta variant peak and then burn out?
When the Delta variant of the coronavirus was first identified in India in December 2020, the threat may have seemed too remote to trigger worry in the United States, although the horror of it ripping through the country was soon hard to ignore.
Within months, the Delta variant had spread to more than 98 countries, including Scotland, the United Kingdom, Israel, and now, of course, the United States. The CDC said this week the Delta variant now accounts for 93% of all COVID cases.
Fueled by Delta, COVID-19 cases, hospitalizations, and deaths are increasing in nearly all states, according to the latest CDC data. After the 7-day average number of cases dipped by June 22 to about 11,000, it rose by Aug. 3 to more than 85,000.
Some experts are heartened by the recent decrease in COVID-19 cases in the United Kingdom and India, both hard-hit with the Delta variant. COVID-19 cases in India peaked at more than 400,000 a day in May; by Aug. 2, that had dropped to about 30,500 daily.
Andy Slavitt, former Biden White House senior adviser for COVID-19 response, tweeted July 26 that, if the Delta variant acted the same in the United Kingdom as in India, it would have a quick rise and a quick drop.
The prediction seems to have come true. As of Aug. 3, U.K. cases have dropped to 7,467, compared with more than 46,800 July 19.
So the question of the summer has become: “When will Delta burn out here?”
Like other pandemic predictions, these are all over the board. Here are five predictions about when COVID cases will peak, then fall. They range from less than 2 weeks to more than 2 months:
- Mid-August: Among the most optimistic predictions of when the Delta-driven COVID-19 cases will decline is from Scott Gottlieb, MD, former FDA director. He told CNBC on July 28 that he would expect cases to decline in 2-3 weeks – so by August 11.
- Mid-August to mid-September: Ali Mokdad, PhD, chief strategy officer for population health at the University of Washington, Seattle, said that, “right now for the U.S. as a country, cases will peak mid-August” and then decline. He is citing projections by the university’s Institute for Health Metrics and Evaluation. In its “most likely” scenario, it predicts COVID deaths will peak at about 1,000 daily by mid-September, then decline. (As of Aug. 3, daily deaths averaged 371.)
- September: “I am hoping we get over this Delta hump [by then],” says Eric Topol, MD, founder and director of the Scripps Research Translational Institute in La Jolla, Calif., and editor-in-chief of Medscape. “But sometimes, I am too much of an optimist.”
- Mid-October: Experts at the COVID-19 Scenario Modeling Hub, a consortium of researchers from leading institutions who consult with the CDC, said the Delta-fueled pandemic will steadily increase through summer and fall, with a mid-October peak.
- Unclear: Because cases are underestimated, “I think it is unclear when we will see a peak of Delta,” says Amesh Adalja, MD, a senior scholar at the Johns Hopkins Center for Health Security, Baltimore. He predicts a decline in cases as “more people get infected and develop natural immunity.”
The predictions are based on different scenarios, such as most likely or worst case. Factors such as personal behaviors, public mandates, and vaccination rates could all alter the projections.
What a difference vaccination may make
An uptick in vaccinations could change all the models and predictions, experts agree. As of Aug. 3, almost half (49.7%) of the total U.S. population was fully vaccinated, the CDC said. (And 80.1% of those 65 and over were.)
But that’s a long way from the 70% or 80% figure often cited to reach herd immunity. Recently, Ricardo Franco, MD, of the University of Alabama at Birmingham, said at a briefing by the Infectious Diseases Society of America that the infectiousness of the Delta variant may mean the herd immunity threshold is actually closer to 90%.
Dr. Mokdad estimates that by Nov. 1, based on the current rate of infections, 64% of people in the United States will be immune to a variant like Delta, taking into account those already infected and those vaccinated against COVID-19.
Justin Lessler, PhD, a University of North Carolina at Chapel Hill epidemiologist involved in the modeling hub, says if enough people get vaccinated, it could stop the Delta variant in its tracks. But that percentage is high.
“I am relatively confident that if we could get 90% or more of the eligible population vaccinated that we would see the epidemic begin to recede,” he says.
It’s a huge leap from 50%, or even 64%, to 90%. Could the Delta surge really motivate that many people to head to a vaccination site?
That’s hard to predict, Dr. Topol said. Some unvaccinated people may feel like soldiers in a foxhole, especially if they are in hard-hit states like Louisiana, and rush to get the vaccine as soon as possible. Others, hearing about the “breakthrough” cases in the vaccinated, may dig in their heels and ask: “Why bother?” as they mistakenly conclude that the vaccine has not done its job.
Roles of public policy, individual behavior
Besides an increase in vaccinations, individual behaviors and mandates can change the scenario. Doctors can remind even vaccinated patients that behaviors such as social distancing and masks still matter, experts said.
“Don’t ‘stress test’ your vaccine, “ Dr. Topol said.
The vaccines against COVID are good but not perfect and, he notes, they offer less protection if many months have passed since the vaccines were given.
The best advice now, Dr. Topol said, is: “Don’t be inside without a mask.”
Even if outdoors, depending on how close others are and the level of the conversation, a mask might be wise, he says.
Dr. Mokdad finds that “when cases go up, people put on their best behavior,” such as going back to masks and social distancing.
“Unfortunately, we have two countries,” he said, referring to the way public health measures and mandates vary from state to state.
Once the Delta variant subsides, what’s next?
It’s not a matter of if there is another variant on the heels of Delta, but when, Dr. Topol and other experts said. A new variant, Lambda, was first identified in Peru in August 2020 but now makes up about 90% of the country’s infections.
There’s also Delta-plus, just found in two people in South Korea.
Future variants could be even more transmissible than Delta, “which would be a horror show,” Dr. Topol said. “This [Delta] is by far the worst version. The virus is going to keep evolving. It is not done with us.”
On the horizon: Variant-proof vaccines
What’s needed to tackle the next variant is another approach to vaccine development, according to Dr. Topol and his colleague, Dennis R. Burton, a professor of immunology and microbiology at Scripps Research Institute.
Writing a commentary in Nature published in 2021, the two propose using a special class of protective antibodies, known as broadly neutralizing antibodies, to develop these vaccines. The success of the current COVID-19 vaccines is likely because of the vaccine’s ability to prompt the body to make protective neutralizing antibodies. These proteins bind to the viruses and prevent them from infecting the body’s cells.
The broadly neutralizing antibodies, however, can act against many different strains of related viruses, Dr. Topol and Mr. Burton wrote. Using this approach, which is already under study, scientists could make vaccines that would be effective against a family of viruses. The goal: to stop future outbreaks from becoming epidemics and then pandemics.
A version of this article first appeared on WebMD.com.
When the Delta variant of the coronavirus was first identified in India in December 2020, the threat may have seemed too remote to trigger worry in the United States, although the horror of it ripping through the country was soon hard to ignore.
Within months, the Delta variant had spread to more than 98 countries, including Scotland, the United Kingdom, Israel, and now, of course, the United States. The CDC said this week the Delta variant now accounts for 93% of all COVID cases.
Fueled by Delta, COVID-19 cases, hospitalizations, and deaths are increasing in nearly all states, according to the latest CDC data. After the 7-day average number of cases dipped by June 22 to about 11,000, it rose by Aug. 3 to more than 85,000.
Some experts are heartened by the recent decrease in COVID-19 cases in the United Kingdom and India, both hard-hit with the Delta variant. COVID-19 cases in India peaked at more than 400,000 a day in May; by Aug. 2, that had dropped to about 30,500 daily.
Andy Slavitt, former Biden White House senior adviser for COVID-19 response, tweeted July 26 that, if the Delta variant acted the same in the United Kingdom as in India, it would have a quick rise and a quick drop.
The prediction seems to have come true. As of Aug. 3, U.K. cases have dropped to 7,467, compared with more than 46,800 July 19.
So the question of the summer has become: “When will Delta burn out here?”
Like other pandemic predictions, these are all over the board. Here are five predictions about when COVID cases will peak, then fall. They range from less than 2 weeks to more than 2 months:
- Mid-August: Among the most optimistic predictions of when the Delta-driven COVID-19 cases will decline is from Scott Gottlieb, MD, former FDA director. He told CNBC on July 28 that he would expect cases to decline in 2-3 weeks – so by August 11.
- Mid-August to mid-September: Ali Mokdad, PhD, chief strategy officer for population health at the University of Washington, Seattle, said that, “right now for the U.S. as a country, cases will peak mid-August” and then decline. He is citing projections by the university’s Institute for Health Metrics and Evaluation. In its “most likely” scenario, it predicts COVID deaths will peak at about 1,000 daily by mid-September, then decline. (As of Aug. 3, daily deaths averaged 371.)
- September: “I am hoping we get over this Delta hump [by then],” says Eric Topol, MD, founder and director of the Scripps Research Translational Institute in La Jolla, Calif., and editor-in-chief of Medscape. “But sometimes, I am too much of an optimist.”
- Mid-October: Experts at the COVID-19 Scenario Modeling Hub, a consortium of researchers from leading institutions who consult with the CDC, said the Delta-fueled pandemic will steadily increase through summer and fall, with a mid-October peak.
- Unclear: Because cases are underestimated, “I think it is unclear when we will see a peak of Delta,” says Amesh Adalja, MD, a senior scholar at the Johns Hopkins Center for Health Security, Baltimore. He predicts a decline in cases as “more people get infected and develop natural immunity.”
The predictions are based on different scenarios, such as most likely or worst case. Factors such as personal behaviors, public mandates, and vaccination rates could all alter the projections.
What a difference vaccination may make
An uptick in vaccinations could change all the models and predictions, experts agree. As of Aug. 3, almost half (49.7%) of the total U.S. population was fully vaccinated, the CDC said. (And 80.1% of those 65 and over were.)
But that’s a long way from the 70% or 80% figure often cited to reach herd immunity. Recently, Ricardo Franco, MD, of the University of Alabama at Birmingham, said at a briefing by the Infectious Diseases Society of America that the infectiousness of the Delta variant may mean the herd immunity threshold is actually closer to 90%.
Dr. Mokdad estimates that by Nov. 1, based on the current rate of infections, 64% of people in the United States will be immune to a variant like Delta, taking into account those already infected and those vaccinated against COVID-19.
Justin Lessler, PhD, a University of North Carolina at Chapel Hill epidemiologist involved in the modeling hub, says if enough people get vaccinated, it could stop the Delta variant in its tracks. But that percentage is high.
“I am relatively confident that if we could get 90% or more of the eligible population vaccinated that we would see the epidemic begin to recede,” he says.
It’s a huge leap from 50%, or even 64%, to 90%. Could the Delta surge really motivate that many people to head to a vaccination site?
That’s hard to predict, Dr. Topol said. Some unvaccinated people may feel like soldiers in a foxhole, especially if they are in hard-hit states like Louisiana, and rush to get the vaccine as soon as possible. Others, hearing about the “breakthrough” cases in the vaccinated, may dig in their heels and ask: “Why bother?” as they mistakenly conclude that the vaccine has not done its job.
Roles of public policy, individual behavior
Besides an increase in vaccinations, individual behaviors and mandates can change the scenario. Doctors can remind even vaccinated patients that behaviors such as social distancing and masks still matter, experts said.
“Don’t ‘stress test’ your vaccine, “ Dr. Topol said.
The vaccines against COVID are good but not perfect and, he notes, they offer less protection if many months have passed since the vaccines were given.
The best advice now, Dr. Topol said, is: “Don’t be inside without a mask.”
Even if outdoors, depending on how close others are and the level of the conversation, a mask might be wise, he says.
Dr. Mokdad finds that “when cases go up, people put on their best behavior,” such as going back to masks and social distancing.
“Unfortunately, we have two countries,” he said, referring to the way public health measures and mandates vary from state to state.
Once the Delta variant subsides, what’s next?
It’s not a matter of if there is another variant on the heels of Delta, but when, Dr. Topol and other experts said. A new variant, Lambda, was first identified in Peru in August 2020 but now makes up about 90% of the country’s infections.
There’s also Delta-plus, just found in two people in South Korea.
Future variants could be even more transmissible than Delta, “which would be a horror show,” Dr. Topol said. “This [Delta] is by far the worst version. The virus is going to keep evolving. It is not done with us.”
On the horizon: Variant-proof vaccines
What’s needed to tackle the next variant is another approach to vaccine development, according to Dr. Topol and his colleague, Dennis R. Burton, a professor of immunology and microbiology at Scripps Research Institute.
Writing a commentary in Nature published in 2021, the two propose using a special class of protective antibodies, known as broadly neutralizing antibodies, to develop these vaccines. The success of the current COVID-19 vaccines is likely because of the vaccine’s ability to prompt the body to make protective neutralizing antibodies. These proteins bind to the viruses and prevent them from infecting the body’s cells.
The broadly neutralizing antibodies, however, can act against many different strains of related viruses, Dr. Topol and Mr. Burton wrote. Using this approach, which is already under study, scientists could make vaccines that would be effective against a family of viruses. The goal: to stop future outbreaks from becoming epidemics and then pandemics.
A version of this article first appeared on WebMD.com.
When the Delta variant of the coronavirus was first identified in India in December 2020, the threat may have seemed too remote to trigger worry in the United States, although the horror of it ripping through the country was soon hard to ignore.
Within months, the Delta variant had spread to more than 98 countries, including Scotland, the United Kingdom, Israel, and now, of course, the United States. The CDC said this week the Delta variant now accounts for 93% of all COVID cases.
Fueled by Delta, COVID-19 cases, hospitalizations, and deaths are increasing in nearly all states, according to the latest CDC data. After the 7-day average number of cases dipped by June 22 to about 11,000, it rose by Aug. 3 to more than 85,000.
Some experts are heartened by the recent decrease in COVID-19 cases in the United Kingdom and India, both hard-hit with the Delta variant. COVID-19 cases in India peaked at more than 400,000 a day in May; by Aug. 2, that had dropped to about 30,500 daily.
Andy Slavitt, former Biden White House senior adviser for COVID-19 response, tweeted July 26 that, if the Delta variant acted the same in the United Kingdom as in India, it would have a quick rise and a quick drop.
The prediction seems to have come true. As of Aug. 3, U.K. cases have dropped to 7,467, compared with more than 46,800 July 19.
So the question of the summer has become: “When will Delta burn out here?”
Like other pandemic predictions, these are all over the board. Here are five predictions about when COVID cases will peak, then fall. They range from less than 2 weeks to more than 2 months:
- Mid-August: Among the most optimistic predictions of when the Delta-driven COVID-19 cases will decline is from Scott Gottlieb, MD, former FDA director. He told CNBC on July 28 that he would expect cases to decline in 2-3 weeks – so by August 11.
- Mid-August to mid-September: Ali Mokdad, PhD, chief strategy officer for population health at the University of Washington, Seattle, said that, “right now for the U.S. as a country, cases will peak mid-August” and then decline. He is citing projections by the university’s Institute for Health Metrics and Evaluation. In its “most likely” scenario, it predicts COVID deaths will peak at about 1,000 daily by mid-September, then decline. (As of Aug. 3, daily deaths averaged 371.)
- September: “I am hoping we get over this Delta hump [by then],” says Eric Topol, MD, founder and director of the Scripps Research Translational Institute in La Jolla, Calif., and editor-in-chief of Medscape. “But sometimes, I am too much of an optimist.”
- Mid-October: Experts at the COVID-19 Scenario Modeling Hub, a consortium of researchers from leading institutions who consult with the CDC, said the Delta-fueled pandemic will steadily increase through summer and fall, with a mid-October peak.
- Unclear: Because cases are underestimated, “I think it is unclear when we will see a peak of Delta,” says Amesh Adalja, MD, a senior scholar at the Johns Hopkins Center for Health Security, Baltimore. He predicts a decline in cases as “more people get infected and develop natural immunity.”
The predictions are based on different scenarios, such as most likely or worst case. Factors such as personal behaviors, public mandates, and vaccination rates could all alter the projections.
What a difference vaccination may make
An uptick in vaccinations could change all the models and predictions, experts agree. As of Aug. 3, almost half (49.7%) of the total U.S. population was fully vaccinated, the CDC said. (And 80.1% of those 65 and over were.)
But that’s a long way from the 70% or 80% figure often cited to reach herd immunity. Recently, Ricardo Franco, MD, of the University of Alabama at Birmingham, said at a briefing by the Infectious Diseases Society of America that the infectiousness of the Delta variant may mean the herd immunity threshold is actually closer to 90%.
Dr. Mokdad estimates that by Nov. 1, based on the current rate of infections, 64% of people in the United States will be immune to a variant like Delta, taking into account those already infected and those vaccinated against COVID-19.
Justin Lessler, PhD, a University of North Carolina at Chapel Hill epidemiologist involved in the modeling hub, says if enough people get vaccinated, it could stop the Delta variant in its tracks. But that percentage is high.
“I am relatively confident that if we could get 90% or more of the eligible population vaccinated that we would see the epidemic begin to recede,” he says.
It’s a huge leap from 50%, or even 64%, to 90%. Could the Delta surge really motivate that many people to head to a vaccination site?
That’s hard to predict, Dr. Topol said. Some unvaccinated people may feel like soldiers in a foxhole, especially if they are in hard-hit states like Louisiana, and rush to get the vaccine as soon as possible. Others, hearing about the “breakthrough” cases in the vaccinated, may dig in their heels and ask: “Why bother?” as they mistakenly conclude that the vaccine has not done its job.
Roles of public policy, individual behavior
Besides an increase in vaccinations, individual behaviors and mandates can change the scenario. Doctors can remind even vaccinated patients that behaviors such as social distancing and masks still matter, experts said.
“Don’t ‘stress test’ your vaccine, “ Dr. Topol said.
The vaccines against COVID are good but not perfect and, he notes, they offer less protection if many months have passed since the vaccines were given.
The best advice now, Dr. Topol said, is: “Don’t be inside without a mask.”
Even if outdoors, depending on how close others are and the level of the conversation, a mask might be wise, he says.
Dr. Mokdad finds that “when cases go up, people put on their best behavior,” such as going back to masks and social distancing.
“Unfortunately, we have two countries,” he said, referring to the way public health measures and mandates vary from state to state.
Once the Delta variant subsides, what’s next?
It’s not a matter of if there is another variant on the heels of Delta, but when, Dr. Topol and other experts said. A new variant, Lambda, was first identified in Peru in August 2020 but now makes up about 90% of the country’s infections.
There’s also Delta-plus, just found in two people in South Korea.
Future variants could be even more transmissible than Delta, “which would be a horror show,” Dr. Topol said. “This [Delta] is by far the worst version. The virus is going to keep evolving. It is not done with us.”
On the horizon: Variant-proof vaccines
What’s needed to tackle the next variant is another approach to vaccine development, according to Dr. Topol and his colleague, Dennis R. Burton, a professor of immunology and microbiology at Scripps Research Institute.
Writing a commentary in Nature published in 2021, the two propose using a special class of protective antibodies, known as broadly neutralizing antibodies, to develop these vaccines. The success of the current COVID-19 vaccines is likely because of the vaccine’s ability to prompt the body to make protective neutralizing antibodies. These proteins bind to the viruses and prevent them from infecting the body’s cells.
The broadly neutralizing antibodies, however, can act against many different strains of related viruses, Dr. Topol and Mr. Burton wrote. Using this approach, which is already under study, scientists could make vaccines that would be effective against a family of viruses. The goal: to stop future outbreaks from becoming epidemics and then pandemics.
A version of this article first appeared on WebMD.com.
Delta variant could drive herd immunity threshold over 80%
Because the Delta variant of SARS-CoV-2 spreads more easily than the original virus, the proportion of the population that needs to be vaccinated to reach herd immunity could be upward of 80% or more, experts say.
Also, it could be time to consider wearing an N95 mask in public indoor spaces regardless of vaccination status, according to a media briefing on Aug. 3 sponsored by the Infectious Diseases Society of America.
Furthermore, giving booster shots to the fully vaccinated is not the top public health priority now. Instead, third vaccinations should be reserved for more vulnerable populations – and efforts should focus on getting first vaccinations to unvaccinated people in the United States and around the world.
“The problem here is that the Delta variant is ... more transmissible than the original virus. That pushes the overall population herd immunity threshold much higher,” Ricardo Franco, MD, assistant professor of medicine at the University of Alabama at Birmingham, said during the briefing.
“For Delta, those threshold estimates go well over 80% and may be approaching 90%,” he said.
To put that figure in context, the original SARS-CoV-2 virus required an estimated 67% of the population to be vaccinated to achieve herd immunity. Also, measles has one of the highest herd immunity thresholds at 95%, Dr. Franco added.
Herd immunity is the point at which enough people are immunized that the entire population gains protection. And it’s already happening. “Unvaccinated people are actually benefiting from greater herd immunity protection in high-vaccination counties compared to low-vaccination ones,” he said.
Maximize mask protection
Unlike early in the COVID-19 pandemic with widespread shortages of personal protective equipment, face masks are now readily available. This includes N95 masks, which offer enhanced protection against SARS-CoV-2, Ezekiel J. Emanuel, MD, PhD, said during the briefing.
Following the July 27 CDC recommendation that most Americans wear masks indoors when in public places, “I do think we need to upgrade our masks,” said Dr. Emanuel, who is Diane v.S. Levy & Robert M. Levy professor at the University of Pennsylvania, Philadelphia.
“It’s not just any mask,” he added. “Good masks make a big difference and are very important.”
Mask protection is about blocking 0.3-mcm particles, “and I think we need to make sure that people have masks that can filter that out,” he said. Although surgical masks are very good, he added, “they’re not quite as good as N95s.” As their name implies, N95s filter out 95% of these particles.
Dr. Emanuel acknowledged that people are tired of COVID-19 and complying with public health measures but urged perseverance. “We’ve sacrificed a lot. We should not throw it away in just a few months because we are tired. We’re all tired, but we do have to do the little bit extra getting vaccinated, wearing masks indoors, and protecting ourselves, our families, and our communities.”
Dealing with a disconnect
In response to a reporter’s question about the possibility that the large crowd at the Lollapalooza music festival in Chicago could become a superspreader event, Dr. Emanuel said, “it is worrisome.”
“I would say that, if you’re going to go to a gathering like that, wearing an N95 mask is wise, and not spending too long at any one place is also wise,” he said.
On the plus side, the event was held outdoors with lots of air circulation, Dr. Emanuel said.
However, “this is the kind of thing where we’ve got a sort of disconnect between people’s desire to get back to normal ... and the fact that we’re in the middle of this upsurge.”
Another potential problem is the event brought people together from many different locations, so when they travel home, they could be “potentially seeding lots of other communities.”
Boosters for some, for now
Even though not officially recommended, some fully vaccinated Americans are seeking a third or booster vaccination on their own.
Asked for his opinion, Dr. Emanuel said: “We’re probably going to have to be giving boosters to immunocompromised people and people who are susceptible. That’s where we are going to start.”
More research is needed regarding booster shots, he said. “There are very small studies – and the ‘very small’ should be emphasized – given that we’ve given shots to over 160 million people.”
“But it does appear that the boosters increase the antibodies and protection,” he said.
Instead of boosters, it is more important for people who haven’t been vaccinated to get fully vaccinated.
“We need to put our priorities in the right places,” he said.
Emanuel noted that, except for people in rural areas that might have to travel long distances, access to vaccines is no longer an issue. “It’s very hard not to find a vaccine if you want it.”
A remaining hurdle is “battling a major disinformation initiative. I don’t think this is misinformation. I think there’s very clear evidence that it is disinformation – false facts about the vaccines being spread,” Dr. Emanuel said.
The breakthrough infection dilemma
Breakthrough cases “remain the vast minority of infections at this time ... that is reassuring,” Dr. Franco said.
Also, tracking symptomatic breakthrough infections remains easier than studying fully vaccinated people who become infected with SARS-CoV-2 but remain symptom free.
“We really don’t have a good handle on the frequency of asymptomatic cases,” Dr. Emanuel said. “If you’re missing breakthrough infections, a lot of them, you may be missing some [virus] evolution that would be very important for us to follow.” This missing information could include the emergence of new variants.
The asymptomatic breakthrough cases are the most worrisome group,” Dr. Emanuel said. “You get infected, you’re feeling fine. Maybe you’ve got a little sneeze or cough, but nothing unusual. And then you’re still able to transmit the Delta variant.”
The big picture
The upsurge in cases, hospitalizations, and deaths is a major challenge, Dr. Emanuel said. “We need to address that by getting many more people vaccinated right now with what are very good vaccines.”
“But it also means that we have to stop being U.S. focused alone.” He pointed out that Delta and other variants originated overseas, “so getting the world vaccinated ... has to be a top priority.”
“We are obviously all facing a challenge as we move into the fall,” Dr. Emanuel said. “With schools opening and employers bringing their employees back together, even if these groups are vaccinated, there are going to be major challenges for all of us.”
A version of this article first appeared on Medscape.com.
Because the Delta variant of SARS-CoV-2 spreads more easily than the original virus, the proportion of the population that needs to be vaccinated to reach herd immunity could be upward of 80% or more, experts say.
Also, it could be time to consider wearing an N95 mask in public indoor spaces regardless of vaccination status, according to a media briefing on Aug. 3 sponsored by the Infectious Diseases Society of America.
Furthermore, giving booster shots to the fully vaccinated is not the top public health priority now. Instead, third vaccinations should be reserved for more vulnerable populations – and efforts should focus on getting first vaccinations to unvaccinated people in the United States and around the world.
“The problem here is that the Delta variant is ... more transmissible than the original virus. That pushes the overall population herd immunity threshold much higher,” Ricardo Franco, MD, assistant professor of medicine at the University of Alabama at Birmingham, said during the briefing.
“For Delta, those threshold estimates go well over 80% and may be approaching 90%,” he said.
To put that figure in context, the original SARS-CoV-2 virus required an estimated 67% of the population to be vaccinated to achieve herd immunity. Also, measles has one of the highest herd immunity thresholds at 95%, Dr. Franco added.
Herd immunity is the point at which enough people are immunized that the entire population gains protection. And it’s already happening. “Unvaccinated people are actually benefiting from greater herd immunity protection in high-vaccination counties compared to low-vaccination ones,” he said.
Maximize mask protection
Unlike early in the COVID-19 pandemic with widespread shortages of personal protective equipment, face masks are now readily available. This includes N95 masks, which offer enhanced protection against SARS-CoV-2, Ezekiel J. Emanuel, MD, PhD, said during the briefing.
Following the July 27 CDC recommendation that most Americans wear masks indoors when in public places, “I do think we need to upgrade our masks,” said Dr. Emanuel, who is Diane v.S. Levy & Robert M. Levy professor at the University of Pennsylvania, Philadelphia.
“It’s not just any mask,” he added. “Good masks make a big difference and are very important.”
Mask protection is about blocking 0.3-mcm particles, “and I think we need to make sure that people have masks that can filter that out,” he said. Although surgical masks are very good, he added, “they’re not quite as good as N95s.” As their name implies, N95s filter out 95% of these particles.
Dr. Emanuel acknowledged that people are tired of COVID-19 and complying with public health measures but urged perseverance. “We’ve sacrificed a lot. We should not throw it away in just a few months because we are tired. We’re all tired, but we do have to do the little bit extra getting vaccinated, wearing masks indoors, and protecting ourselves, our families, and our communities.”
Dealing with a disconnect
In response to a reporter’s question about the possibility that the large crowd at the Lollapalooza music festival in Chicago could become a superspreader event, Dr. Emanuel said, “it is worrisome.”
“I would say that, if you’re going to go to a gathering like that, wearing an N95 mask is wise, and not spending too long at any one place is also wise,” he said.
On the plus side, the event was held outdoors with lots of air circulation, Dr. Emanuel said.
However, “this is the kind of thing where we’ve got a sort of disconnect between people’s desire to get back to normal ... and the fact that we’re in the middle of this upsurge.”
Another potential problem is the event brought people together from many different locations, so when they travel home, they could be “potentially seeding lots of other communities.”
Boosters for some, for now
Even though not officially recommended, some fully vaccinated Americans are seeking a third or booster vaccination on their own.
Asked for his opinion, Dr. Emanuel said: “We’re probably going to have to be giving boosters to immunocompromised people and people who are susceptible. That’s where we are going to start.”
More research is needed regarding booster shots, he said. “There are very small studies – and the ‘very small’ should be emphasized – given that we’ve given shots to over 160 million people.”
“But it does appear that the boosters increase the antibodies and protection,” he said.
Instead of boosters, it is more important for people who haven’t been vaccinated to get fully vaccinated.
“We need to put our priorities in the right places,” he said.
Emanuel noted that, except for people in rural areas that might have to travel long distances, access to vaccines is no longer an issue. “It’s very hard not to find a vaccine if you want it.”
A remaining hurdle is “battling a major disinformation initiative. I don’t think this is misinformation. I think there’s very clear evidence that it is disinformation – false facts about the vaccines being spread,” Dr. Emanuel said.
The breakthrough infection dilemma
Breakthrough cases “remain the vast minority of infections at this time ... that is reassuring,” Dr. Franco said.
Also, tracking symptomatic breakthrough infections remains easier than studying fully vaccinated people who become infected with SARS-CoV-2 but remain symptom free.
“We really don’t have a good handle on the frequency of asymptomatic cases,” Dr. Emanuel said. “If you’re missing breakthrough infections, a lot of them, you may be missing some [virus] evolution that would be very important for us to follow.” This missing information could include the emergence of new variants.
The asymptomatic breakthrough cases are the most worrisome group,” Dr. Emanuel said. “You get infected, you’re feeling fine. Maybe you’ve got a little sneeze or cough, but nothing unusual. And then you’re still able to transmit the Delta variant.”
The big picture
The upsurge in cases, hospitalizations, and deaths is a major challenge, Dr. Emanuel said. “We need to address that by getting many more people vaccinated right now with what are very good vaccines.”
“But it also means that we have to stop being U.S. focused alone.” He pointed out that Delta and other variants originated overseas, “so getting the world vaccinated ... has to be a top priority.”
“We are obviously all facing a challenge as we move into the fall,” Dr. Emanuel said. “With schools opening and employers bringing their employees back together, even if these groups are vaccinated, there are going to be major challenges for all of us.”
A version of this article first appeared on Medscape.com.
Because the Delta variant of SARS-CoV-2 spreads more easily than the original virus, the proportion of the population that needs to be vaccinated to reach herd immunity could be upward of 80% or more, experts say.
Also, it could be time to consider wearing an N95 mask in public indoor spaces regardless of vaccination status, according to a media briefing on Aug. 3 sponsored by the Infectious Diseases Society of America.
Furthermore, giving booster shots to the fully vaccinated is not the top public health priority now. Instead, third vaccinations should be reserved for more vulnerable populations – and efforts should focus on getting first vaccinations to unvaccinated people in the United States and around the world.
“The problem here is that the Delta variant is ... more transmissible than the original virus. That pushes the overall population herd immunity threshold much higher,” Ricardo Franco, MD, assistant professor of medicine at the University of Alabama at Birmingham, said during the briefing.
“For Delta, those threshold estimates go well over 80% and may be approaching 90%,” he said.
To put that figure in context, the original SARS-CoV-2 virus required an estimated 67% of the population to be vaccinated to achieve herd immunity. Also, measles has one of the highest herd immunity thresholds at 95%, Dr. Franco added.
Herd immunity is the point at which enough people are immunized that the entire population gains protection. And it’s already happening. “Unvaccinated people are actually benefiting from greater herd immunity protection in high-vaccination counties compared to low-vaccination ones,” he said.
Maximize mask protection
Unlike early in the COVID-19 pandemic with widespread shortages of personal protective equipment, face masks are now readily available. This includes N95 masks, which offer enhanced protection against SARS-CoV-2, Ezekiel J. Emanuel, MD, PhD, said during the briefing.
Following the July 27 CDC recommendation that most Americans wear masks indoors when in public places, “I do think we need to upgrade our masks,” said Dr. Emanuel, who is Diane v.S. Levy & Robert M. Levy professor at the University of Pennsylvania, Philadelphia.
“It’s not just any mask,” he added. “Good masks make a big difference and are very important.”
Mask protection is about blocking 0.3-mcm particles, “and I think we need to make sure that people have masks that can filter that out,” he said. Although surgical masks are very good, he added, “they’re not quite as good as N95s.” As their name implies, N95s filter out 95% of these particles.
Dr. Emanuel acknowledged that people are tired of COVID-19 and complying with public health measures but urged perseverance. “We’ve sacrificed a lot. We should not throw it away in just a few months because we are tired. We’re all tired, but we do have to do the little bit extra getting vaccinated, wearing masks indoors, and protecting ourselves, our families, and our communities.”
Dealing with a disconnect
In response to a reporter’s question about the possibility that the large crowd at the Lollapalooza music festival in Chicago could become a superspreader event, Dr. Emanuel said, “it is worrisome.”
“I would say that, if you’re going to go to a gathering like that, wearing an N95 mask is wise, and not spending too long at any one place is also wise,” he said.
On the plus side, the event was held outdoors with lots of air circulation, Dr. Emanuel said.
However, “this is the kind of thing where we’ve got a sort of disconnect between people’s desire to get back to normal ... and the fact that we’re in the middle of this upsurge.”
Another potential problem is the event brought people together from many different locations, so when they travel home, they could be “potentially seeding lots of other communities.”
Boosters for some, for now
Even though not officially recommended, some fully vaccinated Americans are seeking a third or booster vaccination on their own.
Asked for his opinion, Dr. Emanuel said: “We’re probably going to have to be giving boosters to immunocompromised people and people who are susceptible. That’s where we are going to start.”
More research is needed regarding booster shots, he said. “There are very small studies – and the ‘very small’ should be emphasized – given that we’ve given shots to over 160 million people.”
“But it does appear that the boosters increase the antibodies and protection,” he said.
Instead of boosters, it is more important for people who haven’t been vaccinated to get fully vaccinated.
“We need to put our priorities in the right places,” he said.
Emanuel noted that, except for people in rural areas that might have to travel long distances, access to vaccines is no longer an issue. “It’s very hard not to find a vaccine if you want it.”
A remaining hurdle is “battling a major disinformation initiative. I don’t think this is misinformation. I think there’s very clear evidence that it is disinformation – false facts about the vaccines being spread,” Dr. Emanuel said.
The breakthrough infection dilemma
Breakthrough cases “remain the vast minority of infections at this time ... that is reassuring,” Dr. Franco said.
Also, tracking symptomatic breakthrough infections remains easier than studying fully vaccinated people who become infected with SARS-CoV-2 but remain symptom free.
“We really don’t have a good handle on the frequency of asymptomatic cases,” Dr. Emanuel said. “If you’re missing breakthrough infections, a lot of them, you may be missing some [virus] evolution that would be very important for us to follow.” This missing information could include the emergence of new variants.
The asymptomatic breakthrough cases are the most worrisome group,” Dr. Emanuel said. “You get infected, you’re feeling fine. Maybe you’ve got a little sneeze or cough, but nothing unusual. And then you’re still able to transmit the Delta variant.”
The big picture
The upsurge in cases, hospitalizations, and deaths is a major challenge, Dr. Emanuel said. “We need to address that by getting many more people vaccinated right now with what are very good vaccines.”
“But it also means that we have to stop being U.S. focused alone.” He pointed out that Delta and other variants originated overseas, “so getting the world vaccinated ... has to be a top priority.”
“We are obviously all facing a challenge as we move into the fall,” Dr. Emanuel said. “With schools opening and employers bringing their employees back together, even if these groups are vaccinated, there are going to be major challenges for all of us.”
A version of this article first appeared on Medscape.com.
Untreatable, drug-resistant fungus found in Texas and Washington, D.C.
The CDC has reported two clusters of Candida auris infections resistant to all antifungal medications in long-term care facilities in 2021. Because these panresistant infections occurred without any exposure to antifungal drugs, the cases are even more worrisome. These clusters are the first time such nosocomial transmission has been detected.
In the District of Columbia, three panresistant isolates were discovered through screening for skin colonization with resistant organisms at a long-term acute care facility (LTAC) that cares for patients who are seriously ill, often on mechanical ventilation.
In Texas, the resistant organisms were found both by screening and in specimens from ill patients at an LTAC and a short-term acute care hospital that share patients. Two were panresistant, and five others were resistant to fluconazole and echinocandins.
These clusters occurred simultaneously and independently of each other; there were no links between the two institutions.
Colonization of skin with C. auris can lead to invasive infections in 5%-10% of affected patients. Routine skin surveillance cultures are not commonly done for Candida, although perirectal cultures for vancomycin-resistant enterococci and nasal swabs for MRSA have been done for years. Some areas, like Los Angeles, have recommended screening for C. auris in high-risk patients – defined as those who were on a ventilator or had a tracheostomy admitted from an LTAC or skilled nursing facility in Los Angeles County, New York, New Jersey, or Illinois.
In the past, about 85% of C. auris isolates in the United States have been resistant to azoles (for example, fluconazole), 33% to amphotericin B, and 1% to echinocandins. Because of generally strong susceptibility, an echinocandin such as micafungin or caspofungin has been the drug of choice for an invasive Candida infection.
C. auris is particularly difficult to deal with for several reasons. First, it can continue to live in the environment, on both dry or moist surfaces, for up to 2 weeks. Outbreaks have occurred both from hand (person-to-person) transmission or via inanimate surfaces that have become contaminated. Equally troublesome is that people become colonized with the yeast indefinitely.
Meghan Lyman, MD, of the fungal diseases branch of the CDC’s National Center for Emerging and Zoonotic Infectious Diseases, said in an interview that facilities might be slow in recognizing the problem and in identifying the organism. “We encounter problems in noninvasive specimens, especially urine,” Dr. Lyman added.
“Sometimes ... they consider Candida [to represent] colonization so they will often not speciate it.” She emphasized the need for facilities that care for ventilated patients to consider screening. “Higher priority ... are places in areas where there’s a lot of C. auris transmission or in nearby areas that are likely to get introductions.” Even those that do speciate may have difficulty identifying C. auris.
Further, Dr. Lyman stressed “the importance of antifungal susceptibility testing and testing for resistance. Because that’s also something that’s not widely available at all hospitals and clinical labs ... you can send it to the [CDC’s] antimicrobial resistance lab network” for testing.
COVID-19 has brought particular challenges. Rodney E. Rohde, PhD, MS, professor and chair, clinical lab science program, Texas State University, San Marcos, said in an interview that he is worried about all the steroids and broad-spectrum antibiotics patients receive.
They’re “being given medical interventions, whether it’s ventilators or [extracorporeal membrane oxygenation] or IVs or central lines or catheters for UTIs and you’re creating highways, right for something that may be right there,” said Dr. Rohde, who was not involved in the CDC study. “It’s a perfect storm, not just for C. auris, but I worry about bacterial resistance agents, too, like MRSA and so forth, having kind of a spike in those types of infections with COVID. So, it’s kind of a doubly dangerous time, I think.”
Multiresistant bacteria are a major health problem, causing illnesses in 2.8 million people annually in the United States, and causing about 35,000 deaths.
Dr. Rohde raised another, rarely mentioned concern. “We’re in crisis mode. People are leaving our field more than they ever had before. The medical laboratory is being decimated because people have burned out after these past 14 months. And so I worry just about competent medical laboratory professionals that are on board to deal with these types of other crises that are popping up within hospitals and long-term care facilities. It kind of keeps me awake.”
Dr. Rohde and Dr. Lyman shared their concern that COVID caused a decrease in screening for other infections and drug-resistant organisms. Bare-bones staffing and shortages of personal protective equipment have likely fueled the spread of these infections as well.
In an outbreak of C. auris in a Florida hospital’s COVID unit in 2020, 35 of 67 patients became colonized, and 6 became ill. The epidemiologists investigating thought that contaminated gowns or gloves, computers, and other equipment were likely sources of transmission.
Low pay, especially in nursing homes, is another problem Dr. Rohde mentioned. It’s an additional problem in both acute and long-term care that “some of the lowest-paid people are the environmental services people, and so the turnover is crazy.” Yet, we rely on them to keep everyone safe. He added that, in addition to pay, he “tries to give them the appreciation and the recognition that they really deserve.”
There are a few specific measures that can be taken to protect patients. Dr. Lyman concluded. “The best way is identifying cases and really ensuring good infection control to prevent the spread.” It’s back to basics – limiting broad-spectrum antibiotics and invasive medical devices, and especially good handwashing and thorough cleaning.
Dr. Lyman and Dr. Rohde have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The CDC has reported two clusters of Candida auris infections resistant to all antifungal medications in long-term care facilities in 2021. Because these panresistant infections occurred without any exposure to antifungal drugs, the cases are even more worrisome. These clusters are the first time such nosocomial transmission has been detected.
In the District of Columbia, three panresistant isolates were discovered through screening for skin colonization with resistant organisms at a long-term acute care facility (LTAC) that cares for patients who are seriously ill, often on mechanical ventilation.
In Texas, the resistant organisms were found both by screening and in specimens from ill patients at an LTAC and a short-term acute care hospital that share patients. Two were panresistant, and five others were resistant to fluconazole and echinocandins.
These clusters occurred simultaneously and independently of each other; there were no links between the two institutions.
Colonization of skin with C. auris can lead to invasive infections in 5%-10% of affected patients. Routine skin surveillance cultures are not commonly done for Candida, although perirectal cultures for vancomycin-resistant enterococci and nasal swabs for MRSA have been done for years. Some areas, like Los Angeles, have recommended screening for C. auris in high-risk patients – defined as those who were on a ventilator or had a tracheostomy admitted from an LTAC or skilled nursing facility in Los Angeles County, New York, New Jersey, or Illinois.
In the past, about 85% of C. auris isolates in the United States have been resistant to azoles (for example, fluconazole), 33% to amphotericin B, and 1% to echinocandins. Because of generally strong susceptibility, an echinocandin such as micafungin or caspofungin has been the drug of choice for an invasive Candida infection.
C. auris is particularly difficult to deal with for several reasons. First, it can continue to live in the environment, on both dry or moist surfaces, for up to 2 weeks. Outbreaks have occurred both from hand (person-to-person) transmission or via inanimate surfaces that have become contaminated. Equally troublesome is that people become colonized with the yeast indefinitely.
Meghan Lyman, MD, of the fungal diseases branch of the CDC’s National Center for Emerging and Zoonotic Infectious Diseases, said in an interview that facilities might be slow in recognizing the problem and in identifying the organism. “We encounter problems in noninvasive specimens, especially urine,” Dr. Lyman added.
“Sometimes ... they consider Candida [to represent] colonization so they will often not speciate it.” She emphasized the need for facilities that care for ventilated patients to consider screening. “Higher priority ... are places in areas where there’s a lot of C. auris transmission or in nearby areas that are likely to get introductions.” Even those that do speciate may have difficulty identifying C. auris.
Further, Dr. Lyman stressed “the importance of antifungal susceptibility testing and testing for resistance. Because that’s also something that’s not widely available at all hospitals and clinical labs ... you can send it to the [CDC’s] antimicrobial resistance lab network” for testing.
COVID-19 has brought particular challenges. Rodney E. Rohde, PhD, MS, professor and chair, clinical lab science program, Texas State University, San Marcos, said in an interview that he is worried about all the steroids and broad-spectrum antibiotics patients receive.
They’re “being given medical interventions, whether it’s ventilators or [extracorporeal membrane oxygenation] or IVs or central lines or catheters for UTIs and you’re creating highways, right for something that may be right there,” said Dr. Rohde, who was not involved in the CDC study. “It’s a perfect storm, not just for C. auris, but I worry about bacterial resistance agents, too, like MRSA and so forth, having kind of a spike in those types of infections with COVID. So, it’s kind of a doubly dangerous time, I think.”
Multiresistant bacteria are a major health problem, causing illnesses in 2.8 million people annually in the United States, and causing about 35,000 deaths.
Dr. Rohde raised another, rarely mentioned concern. “We’re in crisis mode. People are leaving our field more than they ever had before. The medical laboratory is being decimated because people have burned out after these past 14 months. And so I worry just about competent medical laboratory professionals that are on board to deal with these types of other crises that are popping up within hospitals and long-term care facilities. It kind of keeps me awake.”
Dr. Rohde and Dr. Lyman shared their concern that COVID caused a decrease in screening for other infections and drug-resistant organisms. Bare-bones staffing and shortages of personal protective equipment have likely fueled the spread of these infections as well.
In an outbreak of C. auris in a Florida hospital’s COVID unit in 2020, 35 of 67 patients became colonized, and 6 became ill. The epidemiologists investigating thought that contaminated gowns or gloves, computers, and other equipment were likely sources of transmission.
Low pay, especially in nursing homes, is another problem Dr. Rohde mentioned. It’s an additional problem in both acute and long-term care that “some of the lowest-paid people are the environmental services people, and so the turnover is crazy.” Yet, we rely on them to keep everyone safe. He added that, in addition to pay, he “tries to give them the appreciation and the recognition that they really deserve.”
There are a few specific measures that can be taken to protect patients. Dr. Lyman concluded. “The best way is identifying cases and really ensuring good infection control to prevent the spread.” It’s back to basics – limiting broad-spectrum antibiotics and invasive medical devices, and especially good handwashing and thorough cleaning.
Dr. Lyman and Dr. Rohde have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The CDC has reported two clusters of Candida auris infections resistant to all antifungal medications in long-term care facilities in 2021. Because these panresistant infections occurred without any exposure to antifungal drugs, the cases are even more worrisome. These clusters are the first time such nosocomial transmission has been detected.
In the District of Columbia, three panresistant isolates were discovered through screening for skin colonization with resistant organisms at a long-term acute care facility (LTAC) that cares for patients who are seriously ill, often on mechanical ventilation.
In Texas, the resistant organisms were found both by screening and in specimens from ill patients at an LTAC and a short-term acute care hospital that share patients. Two were panresistant, and five others were resistant to fluconazole and echinocandins.
These clusters occurred simultaneously and independently of each other; there were no links between the two institutions.
Colonization of skin with C. auris can lead to invasive infections in 5%-10% of affected patients. Routine skin surveillance cultures are not commonly done for Candida, although perirectal cultures for vancomycin-resistant enterococci and nasal swabs for MRSA have been done for years. Some areas, like Los Angeles, have recommended screening for C. auris in high-risk patients – defined as those who were on a ventilator or had a tracheostomy admitted from an LTAC or skilled nursing facility in Los Angeles County, New York, New Jersey, or Illinois.
In the past, about 85% of C. auris isolates in the United States have been resistant to azoles (for example, fluconazole), 33% to amphotericin B, and 1% to echinocandins. Because of generally strong susceptibility, an echinocandin such as micafungin or caspofungin has been the drug of choice for an invasive Candida infection.
C. auris is particularly difficult to deal with for several reasons. First, it can continue to live in the environment, on both dry or moist surfaces, for up to 2 weeks. Outbreaks have occurred both from hand (person-to-person) transmission or via inanimate surfaces that have become contaminated. Equally troublesome is that people become colonized with the yeast indefinitely.
Meghan Lyman, MD, of the fungal diseases branch of the CDC’s National Center for Emerging and Zoonotic Infectious Diseases, said in an interview that facilities might be slow in recognizing the problem and in identifying the organism. “We encounter problems in noninvasive specimens, especially urine,” Dr. Lyman added.
“Sometimes ... they consider Candida [to represent] colonization so they will often not speciate it.” She emphasized the need for facilities that care for ventilated patients to consider screening. “Higher priority ... are places in areas where there’s a lot of C. auris transmission or in nearby areas that are likely to get introductions.” Even those that do speciate may have difficulty identifying C. auris.
Further, Dr. Lyman stressed “the importance of antifungal susceptibility testing and testing for resistance. Because that’s also something that’s not widely available at all hospitals and clinical labs ... you can send it to the [CDC’s] antimicrobial resistance lab network” for testing.
COVID-19 has brought particular challenges. Rodney E. Rohde, PhD, MS, professor and chair, clinical lab science program, Texas State University, San Marcos, said in an interview that he is worried about all the steroids and broad-spectrum antibiotics patients receive.
They’re “being given medical interventions, whether it’s ventilators or [extracorporeal membrane oxygenation] or IVs or central lines or catheters for UTIs and you’re creating highways, right for something that may be right there,” said Dr. Rohde, who was not involved in the CDC study. “It’s a perfect storm, not just for C. auris, but I worry about bacterial resistance agents, too, like MRSA and so forth, having kind of a spike in those types of infections with COVID. So, it’s kind of a doubly dangerous time, I think.”
Multiresistant bacteria are a major health problem, causing illnesses in 2.8 million people annually in the United States, and causing about 35,000 deaths.
Dr. Rohde raised another, rarely mentioned concern. “We’re in crisis mode. People are leaving our field more than they ever had before. The medical laboratory is being decimated because people have burned out after these past 14 months. And so I worry just about competent medical laboratory professionals that are on board to deal with these types of other crises that are popping up within hospitals and long-term care facilities. It kind of keeps me awake.”
Dr. Rohde and Dr. Lyman shared their concern that COVID caused a decrease in screening for other infections and drug-resistant organisms. Bare-bones staffing and shortages of personal protective equipment have likely fueled the spread of these infections as well.
In an outbreak of C. auris in a Florida hospital’s COVID unit in 2020, 35 of 67 patients became colonized, and 6 became ill. The epidemiologists investigating thought that contaminated gowns or gloves, computers, and other equipment were likely sources of transmission.
Low pay, especially in nursing homes, is another problem Dr. Rohde mentioned. It’s an additional problem in both acute and long-term care that “some of the lowest-paid people are the environmental services people, and so the turnover is crazy.” Yet, we rely on them to keep everyone safe. He added that, in addition to pay, he “tries to give them the appreciation and the recognition that they really deserve.”
There are a few specific measures that can be taken to protect patients. Dr. Lyman concluded. “The best way is identifying cases and really ensuring good infection control to prevent the spread.” It’s back to basics – limiting broad-spectrum antibiotics and invasive medical devices, and especially good handwashing and thorough cleaning.
Dr. Lyman and Dr. Rohde have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Increases in new COVID cases among children far outpace vaccinations
New COVID-19 cases in children soared by almost 86% over the course of just 1 week, while the number of 12- to 17-year-old children who have received at least one dose of vaccine rose by 5.4%, according to two separate sources.
Meanwhile, the increase over the past 2 weeks – from 23,551 new cases for July 16-22 to almost 72,000 – works out to almost 205%, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
Children represented 19.0% of the cases reported during the week of July 23-29, and they have made up 14.3% of all cases since the pandemic began, with the total number of cases in children now approaching 4.2 million, the AAP and CHA said in their weekly COVID report. About 22% of the U.S. population is under the age of 18 years.
As of Aug. 2, just over 9.8 million children aged 12-17 years had received at least one dose of the COVID vaccine, which was up by about 500,000, or 5.4%, from a week earlier, based on data from the Centers for Disease Control and Prevention.
Children aged 16-17 have reached a notable milestone on the journey that started with vaccine approval in December: 50.2% have gotten at least one dose and 40.3% are fully vaccinated. Among children aged 12-15 years, the proportion with at least one dose of vaccine is up to 39.5%, compared with 37.1% the previous week, while 29.0% are fully vaccinated (27.8% the week before), the CDC said on its COVID Data Tracker.
The national rates for child vaccination, however, tend to hide the disparities between states. There is a gap between Mississippi (lowest), where just 17% of children aged 12-17 years have gotten at least one dose, and Vermont (highest), which is up to 69%. Vermont also has the highest rate of vaccine completion (60%), while Alabama and Mississippi have the lowest (10%), according to a solo report from the AAP.
New COVID-19 cases in children soared by almost 86% over the course of just 1 week, while the number of 12- to 17-year-old children who have received at least one dose of vaccine rose by 5.4%, according to two separate sources.

Meanwhile, the increase over the past 2 weeks – from 23,551 new cases for July 16-22 to almost 72,000 – works out to almost 205%, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
Children represented 19.0% of the cases reported during the week of July 23-29, and they have made up 14.3% of all cases since the pandemic began, with the total number of cases in children now approaching 4.2 million, the AAP and CHA said in their weekly COVID report. About 22% of the U.S. population is under the age of 18 years.
As of Aug. 2, just over 9.8 million children aged 12-17 years had received at least one dose of the COVID vaccine, which was up by about 500,000, or 5.4%, from a week earlier, based on data from the Centers for Disease Control and Prevention.
Children aged 16-17 have reached a notable milestone on the journey that started with vaccine approval in December: 50.2% have gotten at least one dose and 40.3% are fully vaccinated. Among children aged 12-15 years, the proportion with at least one dose of vaccine is up to 39.5%, compared with 37.1% the previous week, while 29.0% are fully vaccinated (27.8% the week before), the CDC said on its COVID Data Tracker.
The national rates for child vaccination, however, tend to hide the disparities between states. There is a gap between Mississippi (lowest), where just 17% of children aged 12-17 years have gotten at least one dose, and Vermont (highest), which is up to 69%. Vermont also has the highest rate of vaccine completion (60%), while Alabama and Mississippi have the lowest (10%), according to a solo report from the AAP.
New COVID-19 cases in children soared by almost 86% over the course of just 1 week, while the number of 12- to 17-year-old children who have received at least one dose of vaccine rose by 5.4%, according to two separate sources.

Meanwhile, the increase over the past 2 weeks – from 23,551 new cases for July 16-22 to almost 72,000 – works out to almost 205%, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
Children represented 19.0% of the cases reported during the week of July 23-29, and they have made up 14.3% of all cases since the pandemic began, with the total number of cases in children now approaching 4.2 million, the AAP and CHA said in their weekly COVID report. About 22% of the U.S. population is under the age of 18 years.
As of Aug. 2, just over 9.8 million children aged 12-17 years had received at least one dose of the COVID vaccine, which was up by about 500,000, or 5.4%, from a week earlier, based on data from the Centers for Disease Control and Prevention.
Children aged 16-17 have reached a notable milestone on the journey that started with vaccine approval in December: 50.2% have gotten at least one dose and 40.3% are fully vaccinated. Among children aged 12-15 years, the proportion with at least one dose of vaccine is up to 39.5%, compared with 37.1% the previous week, while 29.0% are fully vaccinated (27.8% the week before), the CDC said on its COVID Data Tracker.
The national rates for child vaccination, however, tend to hide the disparities between states. There is a gap between Mississippi (lowest), where just 17% of children aged 12-17 years have gotten at least one dose, and Vermont (highest), which is up to 69%. Vermont also has the highest rate of vaccine completion (60%), while Alabama and Mississippi have the lowest (10%), according to a solo report from the AAP.
Mental illness admissions: 18-44 is the age of prevalence
More mental and/or substance use disorders are ranked among the top-five diagnoses for hospitalized men and women aged 18-44 years than for any other age group, according to a recent report from the Agency for Healthcare Research and Quality.
aged 18-44, while depressive disorders were the third-most common (195.0 stays per 100,000) and alcohol-related disorders were fifth at 153.2 per 100,000, Kimberly W. McDermott, PhD, and Marc Roemer, MS, said in an AHRQ statistical brief.
Prevalence was somewhat lower in women aged 18-44 years, with two mental illnesses appearing among the top five nonmaternal diagnoses: Depressive disorders were second at 222.5 stays per 100,000 and bipolar and related disorders were fourth at 142.0 per 100,000. The leading primary diagnosis in women in 2018 was septicemia, which was the most common cause overall in the age group at a rate of 279.3 per 100,000, the investigators reported.
There were no mental and/or substance use disorders in the top five primary diagnoses for any of the other adult age groups – 45-64, 65-74, and ≥75 – included in the report. Septicemia was the leading diagnosis for men in all three groups and for women in two of three (45-64 and ≥75), with osteoarthritis first among women aged 65-74 years, they said.
There was one mental illness among the top-five diagnoses for children under age 18 years, as depressive disorders were the most common reason for stays in girls (176.6 per 100,000 population) and the fifth most common for boys (74.0 per 100,000), said Dr. McDermott of IBM Watson Health and Mr. Roemer of AHRQ.
Septicemia was the leading nonmaternal, nonneonatal diagnosis for all inpatient stays and all ages in 2018 with a rate of 679.5 per 100,000, followed by heart failure (347.9), osteoarthritis (345.5), pneumonia not related to tuberculosis (226.8), and diabetes mellitus (207.8), based on data from the National Inpatient Sample.
Depressive disorders were most common mental health diagnosis in those admitted to hospitals and the 12th most common diagnosis overall; schizophrenia, in 16th place overall, was the only other mental illness among the top 20, the investigators said.
“This information can help establish national health priorities, initiatives, and action plans,” Dr. McDermott and Mr. Roemer wrote, and “at the hospital level, administrators can use diagnosis-related information to inform planning and resource allocation, such as optimizing subspecialty services or units for the care of high-priority conditions.”
More mental and/or substance use disorders are ranked among the top-five diagnoses for hospitalized men and women aged 18-44 years than for any other age group, according to a recent report from the Agency for Healthcare Research and Quality.

aged 18-44, while depressive disorders were the third-most common (195.0 stays per 100,000) and alcohol-related disorders were fifth at 153.2 per 100,000, Kimberly W. McDermott, PhD, and Marc Roemer, MS, said in an AHRQ statistical brief.
Prevalence was somewhat lower in women aged 18-44 years, with two mental illnesses appearing among the top five nonmaternal diagnoses: Depressive disorders were second at 222.5 stays per 100,000 and bipolar and related disorders were fourth at 142.0 per 100,000. The leading primary diagnosis in women in 2018 was septicemia, which was the most common cause overall in the age group at a rate of 279.3 per 100,000, the investigators reported.
There were no mental and/or substance use disorders in the top five primary diagnoses for any of the other adult age groups – 45-64, 65-74, and ≥75 – included in the report. Septicemia was the leading diagnosis for men in all three groups and for women in two of three (45-64 and ≥75), with osteoarthritis first among women aged 65-74 years, they said.
There was one mental illness among the top-five diagnoses for children under age 18 years, as depressive disorders were the most common reason for stays in girls (176.6 per 100,000 population) and the fifth most common for boys (74.0 per 100,000), said Dr. McDermott of IBM Watson Health and Mr. Roemer of AHRQ.
Septicemia was the leading nonmaternal, nonneonatal diagnosis for all inpatient stays and all ages in 2018 with a rate of 679.5 per 100,000, followed by heart failure (347.9), osteoarthritis (345.5), pneumonia not related to tuberculosis (226.8), and diabetes mellitus (207.8), based on data from the National Inpatient Sample.
Depressive disorders were most common mental health diagnosis in those admitted to hospitals and the 12th most common diagnosis overall; schizophrenia, in 16th place overall, was the only other mental illness among the top 20, the investigators said.
“This information can help establish national health priorities, initiatives, and action plans,” Dr. McDermott and Mr. Roemer wrote, and “at the hospital level, administrators can use diagnosis-related information to inform planning and resource allocation, such as optimizing subspecialty services or units for the care of high-priority conditions.”
More mental and/or substance use disorders are ranked among the top-five diagnoses for hospitalized men and women aged 18-44 years than for any other age group, according to a recent report from the Agency for Healthcare Research and Quality.

aged 18-44, while depressive disorders were the third-most common (195.0 stays per 100,000) and alcohol-related disorders were fifth at 153.2 per 100,000, Kimberly W. McDermott, PhD, and Marc Roemer, MS, said in an AHRQ statistical brief.
Prevalence was somewhat lower in women aged 18-44 years, with two mental illnesses appearing among the top five nonmaternal diagnoses: Depressive disorders were second at 222.5 stays per 100,000 and bipolar and related disorders were fourth at 142.0 per 100,000. The leading primary diagnosis in women in 2018 was septicemia, which was the most common cause overall in the age group at a rate of 279.3 per 100,000, the investigators reported.
There were no mental and/or substance use disorders in the top five primary diagnoses for any of the other adult age groups – 45-64, 65-74, and ≥75 – included in the report. Septicemia was the leading diagnosis for men in all three groups and for women in two of three (45-64 and ≥75), with osteoarthritis first among women aged 65-74 years, they said.
There was one mental illness among the top-five diagnoses for children under age 18 years, as depressive disorders were the most common reason for stays in girls (176.6 per 100,000 population) and the fifth most common for boys (74.0 per 100,000), said Dr. McDermott of IBM Watson Health and Mr. Roemer of AHRQ.
Septicemia was the leading nonmaternal, nonneonatal diagnosis for all inpatient stays and all ages in 2018 with a rate of 679.5 per 100,000, followed by heart failure (347.9), osteoarthritis (345.5), pneumonia not related to tuberculosis (226.8), and diabetes mellitus (207.8), based on data from the National Inpatient Sample.
Depressive disorders were most common mental health diagnosis in those admitted to hospitals and the 12th most common diagnosis overall; schizophrenia, in 16th place overall, was the only other mental illness among the top 20, the investigators said.
“This information can help establish national health priorities, initiatives, and action plans,” Dr. McDermott and Mr. Roemer wrote, and “at the hospital level, administrators can use diagnosis-related information to inform planning and resource allocation, such as optimizing subspecialty services or units for the care of high-priority conditions.”
Hospital disaster preparation confronts COVID
Hospitalist groups should have disaster response plans
Jason Persoff, MD, SFHM, now a hospitalist at University of Colorado Hospital in Aurora and an amateur storm chaser, got a close look at how natural disasters can impact hospital care when a tornado destroyed St. John’s Regional Medical Center in Joplin, Mo., on May 22, 2011.
He and a colleague who had been following the storm responded to injuries on the highway before reporting for a long day’s service at the other hospital in Joplin, Freeman Hospital West, caring for patients transferred from St. John’s on an impromptu unit without access to their medical records.
“During my medical training, I had done emergency medicine as an EMT, so I was interested in how the system responds to emergencies,” he explained. “At Joplin I learned how it feels when the boots on the ground in a crisis are not connected to an incident command structure.” Another thing he learned was the essential role for hospitalists in a hospital’s response to a crisis – and thus the need to involve them well in advance in the hospital’s planning for future emergencies.
“Disaster preparation – when done right – helps you ‘herd cats’ in a crisis situation,” he said. “The tornado and its wake served as defining moments for me. I used them as the impetus to improve health care’s response to disasters.” Part of that commitment was to help hospitalists understand their part in emergency preparation.1
Dr. Persoff is now the assistant medical director of emergency preparedness at University of Colorado Hospital. He also helped to create a position called physician support supervisor, which is filled by physicians who have held leadership positions in a hospital to help coordinate the disparate needs of all clinicians in a crisis and facilitate rapid response.2
But then along came the COVID pandemic – which in many locales around the world was unprecedented in scope. Dr. Persoff said his hospital was fairly well prepared, after a decade of engagement with emergency planning. It drew on experience with H1N1, also known as swine flu, and the Ebola virus, which killed 11,323 people, primarily in West Africa, from 2013 to 2016, as models. In a matter of days, the CU division of hospital medicine was able to modify and deploy its existing disaster plans to quickly respond to an influx of COVID patients.3
“Basically, what we set out to do was to treat COVID patients as if they were Ebola patients, cordoning them off in a small area of the hospital. That was naive of us,” he said. “We weren’t able to grasp the scale at the outset. It does defy the imagination – how the hospital could fill up with just one type of patient.”
What is disaster planning?
Emergency preparation for hospitals emerged as a recognized medical specialization in the 1970s. Initially it was largely considered the realm of emergency physicians, trauma services, or critical care doctors. Resources such as the World Health Organization, the Federal Emergency Management Agency, and similar groups recommend an all-hazards approach, a broad and flexible strategy for managing emergencies that could include natural disasters – earthquakes, storms, tornadoes, or wildfires – or human-caused events, such as mass shootings or terrorist attacks. The Joint Commission requires accredited hospitals to conduct several disaster drills annually.
The U.S. Hospital Preparedness Program was created in 2002 to enhance the ability of hospitals and health systems to prepare for and respond to bioterrorism attacks on civilians and other public health emergencies, including natural disasters and pandemics. It offers a foundation for national preparedness and a primary source of federal funding for health care system preparedness. The hospital, at the heart of the health care system, is expected to receive the injured and infected, because patients know they can obtain care there.
One of the fundamental tools for crisis response is the incident command system (ICS), which spells out how to quickly establish a command structure and assign responsibility for key tasks as well as overall leadership. The National Incident Management System organizes emergency management across all government levels and the private sector to ensure that the most pressing needs are met and precious resources are used without duplication. ICS is a standardized approach to command, control, and coordination of emergency response using a common hierarchy recognized across organizations, with advance training in how it should be deployed.
A crisis like never before
Nearly every hospital or health system goes through drills for an emergency, said Hassan Khouli, MD, chair of the department of critical care medicine at the Cleveland Clinic, and coauthor of an article in the journal Chest last year outlining 10 principles of emergency preparedness derived from its experience with the COVID pandemic.4 Some of these include: don’t wait; engage a variety of stakeholders; identify sources of truth; and prioritize hospital employees’ safety and well-being.
Part of the preparation is doing table-top exercises, with case scenarios or actual situations presented, working with clinicians on brainstorming and identifying opportunities for improvement, Dr. Khouli said. “These drills are so important, regardless of what the disaster turns out to be. We’ve done that over the years. We are a large health system, very process and detail oriented. Our emergency incident command structure was activated before we saw our first COVID patient,” he said.
“This was a crisis like never before, with huge amounts of uncertainty,” he noted. “But I believe the Cleveland Clinic system did very well, measured by outcomes such as surveys of health care teams across the system, which gave us reassuring results, and clinical outcomes with lower ICU and hospital mortality rates.”
Christopher Whinney, MD, SFHM, department chair of hospital medicine at Cleveland Clinic, said hospitalists worked hand in hand with the health system’s incident command structure and took responsibility for managing non-ICU COVID patients at six hospitals in the system.
“Hospitalists had a place at the table, and we collaborated well with incident command, enterprise redeployment committees, and emergency and critical care colleagues,” he noted. Hospitalists were on the leadership team for a number of planning meetings, and key stakeholders for bringing information back to their groups.
“First thing we did was to look at our workforce. The challenge was how to respond to up to a hundred COVID admissions per day – how to mobilize providers and build surge teams that incorporated primary care providers and medical trainees. We onboarded 200 providers to do hospital care within 60 days,” he said.
“We realized that communication with patients and families was a big part of the challenge, so we assigned people with good communication skills to fill this role. While we were fortunate not to get the terrible surges they had in other places, we felt we were prepared for the worst.”
Challenges of surge capacity
Every disaster is different, said Srikant Polepalli, MD, associate hospitalist medical director for Staten Island University Hospital in New York, part of the Northwell Health system. He brought the experience of being part of the response to Superstorm Sandy in October 2012 to the COVID pandemic.
“Specifically for hospitalists, the biggest challenge is working on surge capacity for a sudden influx of patients,” he said. “But with Northwell as our umbrella, we can triage and load-balance to move patients from hospital to hospital as needed. With the pandemic, we started with one COVID unit and then expanded to fill the entire hospital.”
Dr. Polepalli was appointed medical director for a temporary field hospital installed at South Beach Psychiatric Center, also in Staten Island. “We were able to acquire help and bring in people ranging from hospitalists to ER physicians, travel nurses, operation managers and the National Guard. Our command center did a phenomenal job of allocating and obtaining resources. It helped to have a structure that was already established and to rely on the resources of the health system,” Dr. Polepalli said. Not every hospital has a structure like Northwell’s.
“We’re not out of the pandemic yet, but we’ll continue with disaster drills and planning,” he said. “We must continue to adapt and have converted our temporary facilities to COVID testing centers, antibody infusion centers, and vaccination centers.”
For Alfred Burger, MD, SFHM, a hospitalist at Mount Sinai’s Beth Israel campus in New York, hospital medicine, now in its maturing phase, is still feeling its way through hospital and health care system transformation.
“My group is an academic, multicampus hospitalist group employed by the hospital system. When I meet other hospitalists at SHM conferences, whether they come from privately owned, corporately owned, or contracted models, they vary widely in terms of how involved the hospitalists are in crisis planning and their ability to respond to crises. At large academic medical centers like ours, one or more doctors is tasked with being involved in preparing for the next disaster,” he said.
“I think we responded the best we could, although it was difficult as we lost many patients to COVID. We were trying to save lives using the tools we knew from treating pneumonias and other forms of acute inflammatory lung injuries. We used every bit of our training in situations where no one had the right answers. But disasters teach us how to be flexible and pivot on the fly, and what to do when things don’t go our way.”
What is disaster response?
Medical response to a disaster essentially boils down to three main things: stuff, staff, and space, Dr. Persoff said. Those are the cornerstones of an emergency plan.
“There is not a hazard that exists that you can’t take an all-hazards approach to dealing with fundamental realities on the ground. No plan can be comprehensive enough to deal with all the intricacies of an emergency. But many plans can have the bones of a response that will allow you to face adverse circumstances,” he said.
“We actually became quite efficient early on in the pandemic, able to adapt in the moment. We were able to build an effective bridge between workers on the ground and our incident command structure, which seemed to reduce a lot of stress and create situational awareness. We implemented ICS as soon as we heard that China was building a COVID hospital, back in February of 2020.”
When one thinks about mass trauma, such as a 747 crash, Dr. Persoff said, the need is to treat burn victims and trauma victims in large numbers. At that point, the ED downstairs is filled with medical patients. Hospital medicine can rapidly admit those patients to clear out room in the ED. Surgeons are also dedicated to rapidly treating those patients, but what about patients who are on the floor following their surgeries? Hospitalists can offer consultations or primary management so the surgeons can stay in the OR, and the same in the ICU, while safely discharging hospitalized patients in a timely manner to make room for incoming patients.
“The lessons of COVID have been hard-taught and hard-earned. No good plan survives contact with the enemy,” he said. “But I think we’ll be better prepared for the next pandemic.”
Maria Frank, MD, FACP, SFHM, a hospitalist at Denver Health who chairs SHM’s Disaster Management Special Interest Group, says she got the bug for disaster preparation during postresidency training as an internist in emergency medicine. “I’m also the medical director for our biocontainment unit, created for infections like Ebola.” SHM’s SIG, which has 150 members, is now writing a review article on disaster planning for the field.
“I got a call on Dec. 27, 2019, about this new pneumonia, and they said, ‘We don’t know what it is, but it’s a coronavirus,’” she recalled. “When I got off the phone, I said, ‘Let’s make sure our response plan works and we have enough of everything on hand.’” Dr. Frank said she was expecting something more like SARS (severe acute respiratory syndrome). “When they called the public health emergency of international concern for COVID, I was at a Centers for Disease Control and Prevention meeting in Atlanta. It really wasn’t a surprise for us.”
All hospitals plan for disasters, although they use different names and have different levels of commitment, Dr. Frank said. What’s not consistent is the participation of hospitalists. “Even when a disaster is 100% trauma related, consider a hospital like mine that has at least four times as many hospitalists as surgeons at any given time. The hospitalists need to take overall management for the patients who aren’t actually in the operating room.”
Time to debrief
Dr. Frank recommends debriefing on the hospital’s and the hospitalist group’s experience with COVID. “Look at the biggest challenges your group faced. Was it staffing, or time off, or the need for day care? Was it burnout, lack of knowledge, lack of [personal protective equipment]?” Each hospital could use its own COVID experience to work on identifying the challenges and the problems, she said. “I’d encourage each department and division to do this exercise individually. Then come together to find common ground with other departments in the hospital.”
This debriefing exercise isn’t just for doctors – it’s also for nurses, environmental services, security, and many other departments, she said. “COVID showed us how crisis response is a group effort. What will bring us together is to learn the challenges each of us faced. It was amazing to see hospitalists doing what they do best.” Post pandemic, hospitalists should also consider getting involved in research and publications, in order to share their lessons.
“One of the things we learned is that hospitalists are very versatile,” Dr. Frank added. But it’s also good for the group to have members specialize, for example, in biocontainment. “We are experts in discharging patients, in patient flow and operations, in coordinating complex medical care. So we would naturally take the lead in, for example, opening a geographic unit or collaborating with other specialists to create innovative models. That’s our job. It’s essential that we’re involved well in advance.”
COVID may be a once-in-a-lifetime experience, but there will be other disasters to come, she said. “If your hospital doesn’t have a disaster plan for hospitalists, get involved in establishing one. Each hospitalist group should have its own response plan. Talk to your peers at other hospitals, and get involved at the institutional level. I’m happy to share our plan; just contact me.” Readers can contact Dr. Frank at [email protected].
References
1. Persoff J et al. The role of hospital medicine in emergency preparedness: A framework for hospitalist leadership in disaster preparedness, response and recovery. J Hosp Med. 2018 Oct;13(10):713-7. doi: 10.12788/jhm.3073.
2. Persoff J et al. Expanding the hospital incident command system with a physician-centric role during a pandemic: The role of the physician clinical support supervisor. J Hosp Adm. 2020;9(3):7-10. doi: 10.5430/jha.v9n3p7.
3. Bowden K et al. Harnessing the power of hospitalists in operational disaster planning: COVID-19. J Gen Intern Med. 2020 Sep;35(9):273-7. doi: 10.1007/s11606-020-05952-6.
4. Orsini E et al. Lessons on outbreak preparedness from the Cleveland Clinic. Chest. 2020;158(5):2090-6. doi: 10.1016/j.chest.2020.06.009.
Hospitalist groups should have disaster response plans
Hospitalist groups should have disaster response plans
Jason Persoff, MD, SFHM, now a hospitalist at University of Colorado Hospital in Aurora and an amateur storm chaser, got a close look at how natural disasters can impact hospital care when a tornado destroyed St. John’s Regional Medical Center in Joplin, Mo., on May 22, 2011.
He and a colleague who had been following the storm responded to injuries on the highway before reporting for a long day’s service at the other hospital in Joplin, Freeman Hospital West, caring for patients transferred from St. John’s on an impromptu unit without access to their medical records.
“During my medical training, I had done emergency medicine as an EMT, so I was interested in how the system responds to emergencies,” he explained. “At Joplin I learned how it feels when the boots on the ground in a crisis are not connected to an incident command structure.” Another thing he learned was the essential role for hospitalists in a hospital’s response to a crisis – and thus the need to involve them well in advance in the hospital’s planning for future emergencies.
“Disaster preparation – when done right – helps you ‘herd cats’ in a crisis situation,” he said. “The tornado and its wake served as defining moments for me. I used them as the impetus to improve health care’s response to disasters.” Part of that commitment was to help hospitalists understand their part in emergency preparation.1
Dr. Persoff is now the assistant medical director of emergency preparedness at University of Colorado Hospital. He also helped to create a position called physician support supervisor, which is filled by physicians who have held leadership positions in a hospital to help coordinate the disparate needs of all clinicians in a crisis and facilitate rapid response.2
But then along came the COVID pandemic – which in many locales around the world was unprecedented in scope. Dr. Persoff said his hospital was fairly well prepared, after a decade of engagement with emergency planning. It drew on experience with H1N1, also known as swine flu, and the Ebola virus, which killed 11,323 people, primarily in West Africa, from 2013 to 2016, as models. In a matter of days, the CU division of hospital medicine was able to modify and deploy its existing disaster plans to quickly respond to an influx of COVID patients.3
“Basically, what we set out to do was to treat COVID patients as if they were Ebola patients, cordoning them off in a small area of the hospital. That was naive of us,” he said. “We weren’t able to grasp the scale at the outset. It does defy the imagination – how the hospital could fill up with just one type of patient.”
What is disaster planning?
Emergency preparation for hospitals emerged as a recognized medical specialization in the 1970s. Initially it was largely considered the realm of emergency physicians, trauma services, or critical care doctors. Resources such as the World Health Organization, the Federal Emergency Management Agency, and similar groups recommend an all-hazards approach, a broad and flexible strategy for managing emergencies that could include natural disasters – earthquakes, storms, tornadoes, or wildfires – or human-caused events, such as mass shootings or terrorist attacks. The Joint Commission requires accredited hospitals to conduct several disaster drills annually.
The U.S. Hospital Preparedness Program was created in 2002 to enhance the ability of hospitals and health systems to prepare for and respond to bioterrorism attacks on civilians and other public health emergencies, including natural disasters and pandemics. It offers a foundation for national preparedness and a primary source of federal funding for health care system preparedness. The hospital, at the heart of the health care system, is expected to receive the injured and infected, because patients know they can obtain care there.
One of the fundamental tools for crisis response is the incident command system (ICS), which spells out how to quickly establish a command structure and assign responsibility for key tasks as well as overall leadership. The National Incident Management System organizes emergency management across all government levels and the private sector to ensure that the most pressing needs are met and precious resources are used without duplication. ICS is a standardized approach to command, control, and coordination of emergency response using a common hierarchy recognized across organizations, with advance training in how it should be deployed.
A crisis like never before
Nearly every hospital or health system goes through drills for an emergency, said Hassan Khouli, MD, chair of the department of critical care medicine at the Cleveland Clinic, and coauthor of an article in the journal Chest last year outlining 10 principles of emergency preparedness derived from its experience with the COVID pandemic.4 Some of these include: don’t wait; engage a variety of stakeholders; identify sources of truth; and prioritize hospital employees’ safety and well-being.
Part of the preparation is doing table-top exercises, with case scenarios or actual situations presented, working with clinicians on brainstorming and identifying opportunities for improvement, Dr. Khouli said. “These drills are so important, regardless of what the disaster turns out to be. We’ve done that over the years. We are a large health system, very process and detail oriented. Our emergency incident command structure was activated before we saw our first COVID patient,” he said.
“This was a crisis like never before, with huge amounts of uncertainty,” he noted. “But I believe the Cleveland Clinic system did very well, measured by outcomes such as surveys of health care teams across the system, which gave us reassuring results, and clinical outcomes with lower ICU and hospital mortality rates.”
Christopher Whinney, MD, SFHM, department chair of hospital medicine at Cleveland Clinic, said hospitalists worked hand in hand with the health system’s incident command structure and took responsibility for managing non-ICU COVID patients at six hospitals in the system.
“Hospitalists had a place at the table, and we collaborated well with incident command, enterprise redeployment committees, and emergency and critical care colleagues,” he noted. Hospitalists were on the leadership team for a number of planning meetings, and key stakeholders for bringing information back to their groups.
“First thing we did was to look at our workforce. The challenge was how to respond to up to a hundred COVID admissions per day – how to mobilize providers and build surge teams that incorporated primary care providers and medical trainees. We onboarded 200 providers to do hospital care within 60 days,” he said.
“We realized that communication with patients and families was a big part of the challenge, so we assigned people with good communication skills to fill this role. While we were fortunate not to get the terrible surges they had in other places, we felt we were prepared for the worst.”
Challenges of surge capacity
Every disaster is different, said Srikant Polepalli, MD, associate hospitalist medical director for Staten Island University Hospital in New York, part of the Northwell Health system. He brought the experience of being part of the response to Superstorm Sandy in October 2012 to the COVID pandemic.
“Specifically for hospitalists, the biggest challenge is working on surge capacity for a sudden influx of patients,” he said. “But with Northwell as our umbrella, we can triage and load-balance to move patients from hospital to hospital as needed. With the pandemic, we started with one COVID unit and then expanded to fill the entire hospital.”
Dr. Polepalli was appointed medical director for a temporary field hospital installed at South Beach Psychiatric Center, also in Staten Island. “We were able to acquire help and bring in people ranging from hospitalists to ER physicians, travel nurses, operation managers and the National Guard. Our command center did a phenomenal job of allocating and obtaining resources. It helped to have a structure that was already established and to rely on the resources of the health system,” Dr. Polepalli said. Not every hospital has a structure like Northwell’s.
“We’re not out of the pandemic yet, but we’ll continue with disaster drills and planning,” he said. “We must continue to adapt and have converted our temporary facilities to COVID testing centers, antibody infusion centers, and vaccination centers.”
For Alfred Burger, MD, SFHM, a hospitalist at Mount Sinai’s Beth Israel campus in New York, hospital medicine, now in its maturing phase, is still feeling its way through hospital and health care system transformation.
“My group is an academic, multicampus hospitalist group employed by the hospital system. When I meet other hospitalists at SHM conferences, whether they come from privately owned, corporately owned, or contracted models, they vary widely in terms of how involved the hospitalists are in crisis planning and their ability to respond to crises. At large academic medical centers like ours, one or more doctors is tasked with being involved in preparing for the next disaster,” he said.
“I think we responded the best we could, although it was difficult as we lost many patients to COVID. We were trying to save lives using the tools we knew from treating pneumonias and other forms of acute inflammatory lung injuries. We used every bit of our training in situations where no one had the right answers. But disasters teach us how to be flexible and pivot on the fly, and what to do when things don’t go our way.”
What is disaster response?
Medical response to a disaster essentially boils down to three main things: stuff, staff, and space, Dr. Persoff said. Those are the cornerstones of an emergency plan.
“There is not a hazard that exists that you can’t take an all-hazards approach to dealing with fundamental realities on the ground. No plan can be comprehensive enough to deal with all the intricacies of an emergency. But many plans can have the bones of a response that will allow you to face adverse circumstances,” he said.
“We actually became quite efficient early on in the pandemic, able to adapt in the moment. We were able to build an effective bridge between workers on the ground and our incident command structure, which seemed to reduce a lot of stress and create situational awareness. We implemented ICS as soon as we heard that China was building a COVID hospital, back in February of 2020.”
When one thinks about mass trauma, such as a 747 crash, Dr. Persoff said, the need is to treat burn victims and trauma victims in large numbers. At that point, the ED downstairs is filled with medical patients. Hospital medicine can rapidly admit those patients to clear out room in the ED. Surgeons are also dedicated to rapidly treating those patients, but what about patients who are on the floor following their surgeries? Hospitalists can offer consultations or primary management so the surgeons can stay in the OR, and the same in the ICU, while safely discharging hospitalized patients in a timely manner to make room for incoming patients.
“The lessons of COVID have been hard-taught and hard-earned. No good plan survives contact with the enemy,” he said. “But I think we’ll be better prepared for the next pandemic.”
Maria Frank, MD, FACP, SFHM, a hospitalist at Denver Health who chairs SHM’s Disaster Management Special Interest Group, says she got the bug for disaster preparation during postresidency training as an internist in emergency medicine. “I’m also the medical director for our biocontainment unit, created for infections like Ebola.” SHM’s SIG, which has 150 members, is now writing a review article on disaster planning for the field.
“I got a call on Dec. 27, 2019, about this new pneumonia, and they said, ‘We don’t know what it is, but it’s a coronavirus,’” she recalled. “When I got off the phone, I said, ‘Let’s make sure our response plan works and we have enough of everything on hand.’” Dr. Frank said she was expecting something more like SARS (severe acute respiratory syndrome). “When they called the public health emergency of international concern for COVID, I was at a Centers for Disease Control and Prevention meeting in Atlanta. It really wasn’t a surprise for us.”
All hospitals plan for disasters, although they use different names and have different levels of commitment, Dr. Frank said. What’s not consistent is the participation of hospitalists. “Even when a disaster is 100% trauma related, consider a hospital like mine that has at least four times as many hospitalists as surgeons at any given time. The hospitalists need to take overall management for the patients who aren’t actually in the operating room.”
Time to debrief
Dr. Frank recommends debriefing on the hospital’s and the hospitalist group’s experience with COVID. “Look at the biggest challenges your group faced. Was it staffing, or time off, or the need for day care? Was it burnout, lack of knowledge, lack of [personal protective equipment]?” Each hospital could use its own COVID experience to work on identifying the challenges and the problems, she said. “I’d encourage each department and division to do this exercise individually. Then come together to find common ground with other departments in the hospital.”
This debriefing exercise isn’t just for doctors – it’s also for nurses, environmental services, security, and many other departments, she said. “COVID showed us how crisis response is a group effort. What will bring us together is to learn the challenges each of us faced. It was amazing to see hospitalists doing what they do best.” Post pandemic, hospitalists should also consider getting involved in research and publications, in order to share their lessons.
“One of the things we learned is that hospitalists are very versatile,” Dr. Frank added. But it’s also good for the group to have members specialize, for example, in biocontainment. “We are experts in discharging patients, in patient flow and operations, in coordinating complex medical care. So we would naturally take the lead in, for example, opening a geographic unit or collaborating with other specialists to create innovative models. That’s our job. It’s essential that we’re involved well in advance.”
COVID may be a once-in-a-lifetime experience, but there will be other disasters to come, she said. “If your hospital doesn’t have a disaster plan for hospitalists, get involved in establishing one. Each hospitalist group should have its own response plan. Talk to your peers at other hospitals, and get involved at the institutional level. I’m happy to share our plan; just contact me.” Readers can contact Dr. Frank at [email protected].
References
1. Persoff J et al. The role of hospital medicine in emergency preparedness: A framework for hospitalist leadership in disaster preparedness, response and recovery. J Hosp Med. 2018 Oct;13(10):713-7. doi: 10.12788/jhm.3073.
2. Persoff J et al. Expanding the hospital incident command system with a physician-centric role during a pandemic: The role of the physician clinical support supervisor. J Hosp Adm. 2020;9(3):7-10. doi: 10.5430/jha.v9n3p7.
3. Bowden K et al. Harnessing the power of hospitalists in operational disaster planning: COVID-19. J Gen Intern Med. 2020 Sep;35(9):273-7. doi: 10.1007/s11606-020-05952-6.
4. Orsini E et al. Lessons on outbreak preparedness from the Cleveland Clinic. Chest. 2020;158(5):2090-6. doi: 10.1016/j.chest.2020.06.009.
Jason Persoff, MD, SFHM, now a hospitalist at University of Colorado Hospital in Aurora and an amateur storm chaser, got a close look at how natural disasters can impact hospital care when a tornado destroyed St. John’s Regional Medical Center in Joplin, Mo., on May 22, 2011.
He and a colleague who had been following the storm responded to injuries on the highway before reporting for a long day’s service at the other hospital in Joplin, Freeman Hospital West, caring for patients transferred from St. John’s on an impromptu unit without access to their medical records.
“During my medical training, I had done emergency medicine as an EMT, so I was interested in how the system responds to emergencies,” he explained. “At Joplin I learned how it feels when the boots on the ground in a crisis are not connected to an incident command structure.” Another thing he learned was the essential role for hospitalists in a hospital’s response to a crisis – and thus the need to involve them well in advance in the hospital’s planning for future emergencies.
“Disaster preparation – when done right – helps you ‘herd cats’ in a crisis situation,” he said. “The tornado and its wake served as defining moments for me. I used them as the impetus to improve health care’s response to disasters.” Part of that commitment was to help hospitalists understand their part in emergency preparation.1
Dr. Persoff is now the assistant medical director of emergency preparedness at University of Colorado Hospital. He also helped to create a position called physician support supervisor, which is filled by physicians who have held leadership positions in a hospital to help coordinate the disparate needs of all clinicians in a crisis and facilitate rapid response.2
But then along came the COVID pandemic – which in many locales around the world was unprecedented in scope. Dr. Persoff said his hospital was fairly well prepared, after a decade of engagement with emergency planning. It drew on experience with H1N1, also known as swine flu, and the Ebola virus, which killed 11,323 people, primarily in West Africa, from 2013 to 2016, as models. In a matter of days, the CU division of hospital medicine was able to modify and deploy its existing disaster plans to quickly respond to an influx of COVID patients.3
“Basically, what we set out to do was to treat COVID patients as if they were Ebola patients, cordoning them off in a small area of the hospital. That was naive of us,” he said. “We weren’t able to grasp the scale at the outset. It does defy the imagination – how the hospital could fill up with just one type of patient.”
What is disaster planning?
Emergency preparation for hospitals emerged as a recognized medical specialization in the 1970s. Initially it was largely considered the realm of emergency physicians, trauma services, or critical care doctors. Resources such as the World Health Organization, the Federal Emergency Management Agency, and similar groups recommend an all-hazards approach, a broad and flexible strategy for managing emergencies that could include natural disasters – earthquakes, storms, tornadoes, or wildfires – or human-caused events, such as mass shootings or terrorist attacks. The Joint Commission requires accredited hospitals to conduct several disaster drills annually.
The U.S. Hospital Preparedness Program was created in 2002 to enhance the ability of hospitals and health systems to prepare for and respond to bioterrorism attacks on civilians and other public health emergencies, including natural disasters and pandemics. It offers a foundation for national preparedness and a primary source of federal funding for health care system preparedness. The hospital, at the heart of the health care system, is expected to receive the injured and infected, because patients know they can obtain care there.
One of the fundamental tools for crisis response is the incident command system (ICS), which spells out how to quickly establish a command structure and assign responsibility for key tasks as well as overall leadership. The National Incident Management System organizes emergency management across all government levels and the private sector to ensure that the most pressing needs are met and precious resources are used without duplication. ICS is a standardized approach to command, control, and coordination of emergency response using a common hierarchy recognized across organizations, with advance training in how it should be deployed.
A crisis like never before
Nearly every hospital or health system goes through drills for an emergency, said Hassan Khouli, MD, chair of the department of critical care medicine at the Cleveland Clinic, and coauthor of an article in the journal Chest last year outlining 10 principles of emergency preparedness derived from its experience with the COVID pandemic.4 Some of these include: don’t wait; engage a variety of stakeholders; identify sources of truth; and prioritize hospital employees’ safety and well-being.
Part of the preparation is doing table-top exercises, with case scenarios or actual situations presented, working with clinicians on brainstorming and identifying opportunities for improvement, Dr. Khouli said. “These drills are so important, regardless of what the disaster turns out to be. We’ve done that over the years. We are a large health system, very process and detail oriented. Our emergency incident command structure was activated before we saw our first COVID patient,” he said.
“This was a crisis like never before, with huge amounts of uncertainty,” he noted. “But I believe the Cleveland Clinic system did very well, measured by outcomes such as surveys of health care teams across the system, which gave us reassuring results, and clinical outcomes with lower ICU and hospital mortality rates.”
Christopher Whinney, MD, SFHM, department chair of hospital medicine at Cleveland Clinic, said hospitalists worked hand in hand with the health system’s incident command structure and took responsibility for managing non-ICU COVID patients at six hospitals in the system.
“Hospitalists had a place at the table, and we collaborated well with incident command, enterprise redeployment committees, and emergency and critical care colleagues,” he noted. Hospitalists were on the leadership team for a number of planning meetings, and key stakeholders for bringing information back to their groups.
“First thing we did was to look at our workforce. The challenge was how to respond to up to a hundred COVID admissions per day – how to mobilize providers and build surge teams that incorporated primary care providers and medical trainees. We onboarded 200 providers to do hospital care within 60 days,” he said.
“We realized that communication with patients and families was a big part of the challenge, so we assigned people with good communication skills to fill this role. While we were fortunate not to get the terrible surges they had in other places, we felt we were prepared for the worst.”
Challenges of surge capacity
Every disaster is different, said Srikant Polepalli, MD, associate hospitalist medical director for Staten Island University Hospital in New York, part of the Northwell Health system. He brought the experience of being part of the response to Superstorm Sandy in October 2012 to the COVID pandemic.
“Specifically for hospitalists, the biggest challenge is working on surge capacity for a sudden influx of patients,” he said. “But with Northwell as our umbrella, we can triage and load-balance to move patients from hospital to hospital as needed. With the pandemic, we started with one COVID unit and then expanded to fill the entire hospital.”
Dr. Polepalli was appointed medical director for a temporary field hospital installed at South Beach Psychiatric Center, also in Staten Island. “We were able to acquire help and bring in people ranging from hospitalists to ER physicians, travel nurses, operation managers and the National Guard. Our command center did a phenomenal job of allocating and obtaining resources. It helped to have a structure that was already established and to rely on the resources of the health system,” Dr. Polepalli said. Not every hospital has a structure like Northwell’s.
“We’re not out of the pandemic yet, but we’ll continue with disaster drills and planning,” he said. “We must continue to adapt and have converted our temporary facilities to COVID testing centers, antibody infusion centers, and vaccination centers.”
For Alfred Burger, MD, SFHM, a hospitalist at Mount Sinai’s Beth Israel campus in New York, hospital medicine, now in its maturing phase, is still feeling its way through hospital and health care system transformation.
“My group is an academic, multicampus hospitalist group employed by the hospital system. When I meet other hospitalists at SHM conferences, whether they come from privately owned, corporately owned, or contracted models, they vary widely in terms of how involved the hospitalists are in crisis planning and their ability to respond to crises. At large academic medical centers like ours, one or more doctors is tasked with being involved in preparing for the next disaster,” he said.
“I think we responded the best we could, although it was difficult as we lost many patients to COVID. We were trying to save lives using the tools we knew from treating pneumonias and other forms of acute inflammatory lung injuries. We used every bit of our training in situations where no one had the right answers. But disasters teach us how to be flexible and pivot on the fly, and what to do when things don’t go our way.”
What is disaster response?
Medical response to a disaster essentially boils down to three main things: stuff, staff, and space, Dr. Persoff said. Those are the cornerstones of an emergency plan.
“There is not a hazard that exists that you can’t take an all-hazards approach to dealing with fundamental realities on the ground. No plan can be comprehensive enough to deal with all the intricacies of an emergency. But many plans can have the bones of a response that will allow you to face adverse circumstances,” he said.
“We actually became quite efficient early on in the pandemic, able to adapt in the moment. We were able to build an effective bridge between workers on the ground and our incident command structure, which seemed to reduce a lot of stress and create situational awareness. We implemented ICS as soon as we heard that China was building a COVID hospital, back in February of 2020.”
When one thinks about mass trauma, such as a 747 crash, Dr. Persoff said, the need is to treat burn victims and trauma victims in large numbers. At that point, the ED downstairs is filled with medical patients. Hospital medicine can rapidly admit those patients to clear out room in the ED. Surgeons are also dedicated to rapidly treating those patients, but what about patients who are on the floor following their surgeries? Hospitalists can offer consultations or primary management so the surgeons can stay in the OR, and the same in the ICU, while safely discharging hospitalized patients in a timely manner to make room for incoming patients.
“The lessons of COVID have been hard-taught and hard-earned. No good plan survives contact with the enemy,” he said. “But I think we’ll be better prepared for the next pandemic.”
Maria Frank, MD, FACP, SFHM, a hospitalist at Denver Health who chairs SHM’s Disaster Management Special Interest Group, says she got the bug for disaster preparation during postresidency training as an internist in emergency medicine. “I’m also the medical director for our biocontainment unit, created for infections like Ebola.” SHM’s SIG, which has 150 members, is now writing a review article on disaster planning for the field.
“I got a call on Dec. 27, 2019, about this new pneumonia, and they said, ‘We don’t know what it is, but it’s a coronavirus,’” she recalled. “When I got off the phone, I said, ‘Let’s make sure our response plan works and we have enough of everything on hand.’” Dr. Frank said she was expecting something more like SARS (severe acute respiratory syndrome). “When they called the public health emergency of international concern for COVID, I was at a Centers for Disease Control and Prevention meeting in Atlanta. It really wasn’t a surprise for us.”
All hospitals plan for disasters, although they use different names and have different levels of commitment, Dr. Frank said. What’s not consistent is the participation of hospitalists. “Even when a disaster is 100% trauma related, consider a hospital like mine that has at least four times as many hospitalists as surgeons at any given time. The hospitalists need to take overall management for the patients who aren’t actually in the operating room.”
Time to debrief
Dr. Frank recommends debriefing on the hospital’s and the hospitalist group’s experience with COVID. “Look at the biggest challenges your group faced. Was it staffing, or time off, or the need for day care? Was it burnout, lack of knowledge, lack of [personal protective equipment]?” Each hospital could use its own COVID experience to work on identifying the challenges and the problems, she said. “I’d encourage each department and division to do this exercise individually. Then come together to find common ground with other departments in the hospital.”
This debriefing exercise isn’t just for doctors – it’s also for nurses, environmental services, security, and many other departments, she said. “COVID showed us how crisis response is a group effort. What will bring us together is to learn the challenges each of us faced. It was amazing to see hospitalists doing what they do best.” Post pandemic, hospitalists should also consider getting involved in research and publications, in order to share their lessons.
“One of the things we learned is that hospitalists are very versatile,” Dr. Frank added. But it’s also good for the group to have members specialize, for example, in biocontainment. “We are experts in discharging patients, in patient flow and operations, in coordinating complex medical care. So we would naturally take the lead in, for example, opening a geographic unit or collaborating with other specialists to create innovative models. That’s our job. It’s essential that we’re involved well in advance.”
COVID may be a once-in-a-lifetime experience, but there will be other disasters to come, she said. “If your hospital doesn’t have a disaster plan for hospitalists, get involved in establishing one. Each hospitalist group should have its own response plan. Talk to your peers at other hospitals, and get involved at the institutional level. I’m happy to share our plan; just contact me.” Readers can contact Dr. Frank at [email protected].
References
1. Persoff J et al. The role of hospital medicine in emergency preparedness: A framework for hospitalist leadership in disaster preparedness, response and recovery. J Hosp Med. 2018 Oct;13(10):713-7. doi: 10.12788/jhm.3073.
2. Persoff J et al. Expanding the hospital incident command system with a physician-centric role during a pandemic: The role of the physician clinical support supervisor. J Hosp Adm. 2020;9(3):7-10. doi: 10.5430/jha.v9n3p7.
3. Bowden K et al. Harnessing the power of hospitalists in operational disaster planning: COVID-19. J Gen Intern Med. 2020 Sep;35(9):273-7. doi: 10.1007/s11606-020-05952-6.
4. Orsini E et al. Lessons on outbreak preparedness from the Cleveland Clinic. Chest. 2020;158(5):2090-6. doi: 10.1016/j.chest.2020.06.009.
DOACs best aspirin after ventricular ablation: STROKE-VT
Catheter ablation has been around a lot longer for ventricular arrhythmia than for atrial fibrillation, but far less is settled about what antithrombotic therapy should follow ventricular ablations, as there have been no big, randomized trials for guidance.
But the evidence base grew stronger this week, and it favors postprocedure treatment with a direct oral anticoagulant (DOAC) over antiplatelet therapy with aspirin for patients undergoing radiofrequency (RF) ablation to treat left ventricular (LV) arrhythmias.
The 30-day risk for ischemic stroke or transient ischemia attack (TIA) was sharply higher for patients who took daily aspirin after RF ablation for ventricular tachycardia (VT) or premature ventricular contractions (PVC) in a multicenter randomized trial.
Those of its 246 patients who received aspirin were also far more likely to show asymptomatic lesions on cerebral MRI scans performed both 24 hours and 30 days after the procedure.
The findings show the importance of DOAC therapy after ventricular ablation procedures, a setting for which there are no evidence-based guidelines, “to mitigate the risk of systemic thromboembolic events,” said Dhanunjaya Lakkireddy, MD, Kansas City Heart Rhythm Institute, Overland Park. He spoke at a media presentation on the trial, called STROKE-VT, during the Heart Rhythm Society 2021 Scientific Sessions, held virtually and on-site in Boston.
The risk for stroke and TIA went up in association with several procedural issues, including some that operators might be able to change in order to reach for better outcomes, Dr. Lakkireddy observed.
“Prolonged radiofrequency ablation times, especially in those with low left ventricle ejection fractions, are definitely higher risk,” as are procedures that involved the retrograde transaortic approach for advancing the ablation catheter, rather than a trans-septal approach.
The retrograde transaortic approach should be avoided in such procedures, “whenever it can be avoided,” said Dr. Lakkireddy, who formally presented STROKE-VT at the HRS sessions and is lead author on its report published about the same time in JACC: Clinical Electrophysiology.
The trial has limitations, but “it’s a very important study, and I think that this could become our standard of care for managing anticoagulation after VT and PVC left-sided ablations,” Mina K. Chung, MD, Cleveland Clinic, said as an invited discussant after Dr. Lakkireddy’s presentation.
How patients are treated with antithrombotics after ventricular ablations can vary widely, sometimes based on the operator’s “subjective feeling of how extensive the ablation is,” Christine M. Albert, MD, MPH, Cedars-Sinai Medical Center, Los Angeles, not involved in the study, said during the STROKE-VT media briefing.
That’s consistent with the guidelines, which propose oral anticoagulation therapy after more extensive ventricular ablations and antiplatelets when the ablation is more limited – based more on consensus than firm evidence – as described by Jeffrey R. Winterfield, MD, Medical University of South Carolina, Charleston, and Usha Tedrow, MD, MSc, Brigham and Women’s Hospital, Boston, in an accompanying editorial.
“This is really the first randomized trial data, that I know of, that we have on this. So I do think it will be guideline-influencing,” Dr. Albert said.
“This should change practice,” agreed Jonathan P. Piccini, MD, MHS, Duke University, Durham, N.C., also not part of STROKE-VT. “A lot of evidence in the trial is consistent and provides a compelling story, not to mention that, in my opinion, the study probably underestimates the value of DOACs,” he told this news organization.
That’s because patients assigned to DOACs had far longer ablation times, “so their risk was even greater than in the aspirin arm,” Dr. Piccini said. Ablation times averaged 2,095 seconds in the DOAC group, compared with only 1,708 seconds in the aspirin group, probably because the preponderance of VT over PVC ablations for those getting a DOAC was even greater in the aspirin group.
Of the 246 patients assigned to either aspirin or a DOAC, usually a factor Xa inhibitor, 75% had undergone VT ablation and the remainder ablation for PVCs. Their mean age was 60 years and only 18% were women. None had experienced a cerebrovascular event in the previous 3 months.
The 30-day odds ratio for TIA or ischemic stroke in patients who received aspirin, compared with a DOAC, was 12.6 (95% confidence interval, 4.10-39.11; P < .001).
The corresponding OR for asymptomatic cerebral lesions by MRI at 24 hours was 2.15 (95% CI, 1.02-4.54; P = .04) and at 30 days was 3.48 (95% CI, 1.38-8.80; P = .008).
The rate of stroke or TIA was similar in patients who underwent ablation for VT and for PVCs (14% vs. 16%, respectively; P = .70). There were fewer asymptomatic cerebrovascular events by MRI at 24 hours for those undergoing VT ablations (14.7% and 25.8%, respectively; P = .046); but difference between rates attenuated by 30 days (11.4% and 14.5%, respectively; P = .52).
The OR for TIA or stroke associated with the retrograde transaortic approach, performed in about 40% of the patients, compared with the trans-septal approach in the remainder was 2.60 (95% CI, 1.06-6.37; P = .04).
“The study tells us it’s safe and indeed preferable to anticoagulate after an ablation procedure. But the more important finding, perhaps, wasn’t the one related to the core hypothesis. And that was the effect of retrograde access,” Paul A. Friedman, MD, Mayo Clinic, Rochester, Minn., said as an invited discussant after Dr. Lakkireddy’s formal presentation of the trial.
Whether a ventricular ablation is performed using the retrograde transaortic or trans-septal approach often depends on the location of the ablation targets in the left ventricle. But in some cases it’s a matter of operator preference, Dr. Piccini observed.
“There are some situations where, really, it is better to do retrograde aortic, and there are some cases that are better to do trans-septal. But now there’s going to be a higher burden of proof,” he said. Given the findings of STROKE-VT, operators may need to consider that a ventricular ablation procedure that can be done by the trans-septal route perhaps ought to be consistently done that way.
Dr. Lakkireddy discloses financial relationships with Boston Scientific, Biosense Webster, Janssen Pharmaceuticals, and more. Dr. Chung had “nothing relevant to disclose.” Dr. Piccini discloses receiving honoraria or speaking or consulting fees from Sanofi, Abbott, ARCA Biopharma, Medtronic, Philips, Biotronik, Allergan, LivaNova, and Myokardia; and research in conjunction with Bayer Healthcare, Abbott, Boston Scientific, and Philips. Dr. Friedman discloses conducting research in conjunction with Medtronic and Abbott; holding intellectual property rights with AliveCor, Inference, Medicool, Eko, and Anumana; and receiving honoraria or speaking or consulting fees from Boston Scientific. Dr. Winterfield and Dr. Tedrow had no disclosures.
A version of this article first appeared on Medscape.com.
Catheter ablation has been around a lot longer for ventricular arrhythmia than for atrial fibrillation, but far less is settled about what antithrombotic therapy should follow ventricular ablations, as there have been no big, randomized trials for guidance.
But the evidence base grew stronger this week, and it favors postprocedure treatment with a direct oral anticoagulant (DOAC) over antiplatelet therapy with aspirin for patients undergoing radiofrequency (RF) ablation to treat left ventricular (LV) arrhythmias.
The 30-day risk for ischemic stroke or transient ischemia attack (TIA) was sharply higher for patients who took daily aspirin after RF ablation for ventricular tachycardia (VT) or premature ventricular contractions (PVC) in a multicenter randomized trial.
Those of its 246 patients who received aspirin were also far more likely to show asymptomatic lesions on cerebral MRI scans performed both 24 hours and 30 days after the procedure.
The findings show the importance of DOAC therapy after ventricular ablation procedures, a setting for which there are no evidence-based guidelines, “to mitigate the risk of systemic thromboembolic events,” said Dhanunjaya Lakkireddy, MD, Kansas City Heart Rhythm Institute, Overland Park. He spoke at a media presentation on the trial, called STROKE-VT, during the Heart Rhythm Society 2021 Scientific Sessions, held virtually and on-site in Boston.
The risk for stroke and TIA went up in association with several procedural issues, including some that operators might be able to change in order to reach for better outcomes, Dr. Lakkireddy observed.
“Prolonged radiofrequency ablation times, especially in those with low left ventricle ejection fractions, are definitely higher risk,” as are procedures that involved the retrograde transaortic approach for advancing the ablation catheter, rather than a trans-septal approach.
The retrograde transaortic approach should be avoided in such procedures, “whenever it can be avoided,” said Dr. Lakkireddy, who formally presented STROKE-VT at the HRS sessions and is lead author on its report published about the same time in JACC: Clinical Electrophysiology.
The trial has limitations, but “it’s a very important study, and I think that this could become our standard of care for managing anticoagulation after VT and PVC left-sided ablations,” Mina K. Chung, MD, Cleveland Clinic, said as an invited discussant after Dr. Lakkireddy’s presentation.
How patients are treated with antithrombotics after ventricular ablations can vary widely, sometimes based on the operator’s “subjective feeling of how extensive the ablation is,” Christine M. Albert, MD, MPH, Cedars-Sinai Medical Center, Los Angeles, not involved in the study, said during the STROKE-VT media briefing.
That’s consistent with the guidelines, which propose oral anticoagulation therapy after more extensive ventricular ablations and antiplatelets when the ablation is more limited – based more on consensus than firm evidence – as described by Jeffrey R. Winterfield, MD, Medical University of South Carolina, Charleston, and Usha Tedrow, MD, MSc, Brigham and Women’s Hospital, Boston, in an accompanying editorial.
“This is really the first randomized trial data, that I know of, that we have on this. So I do think it will be guideline-influencing,” Dr. Albert said.
“This should change practice,” agreed Jonathan P. Piccini, MD, MHS, Duke University, Durham, N.C., also not part of STROKE-VT. “A lot of evidence in the trial is consistent and provides a compelling story, not to mention that, in my opinion, the study probably underestimates the value of DOACs,” he told this news organization.
That’s because patients assigned to DOACs had far longer ablation times, “so their risk was even greater than in the aspirin arm,” Dr. Piccini said. Ablation times averaged 2,095 seconds in the DOAC group, compared with only 1,708 seconds in the aspirin group, probably because the preponderance of VT over PVC ablations for those getting a DOAC was even greater in the aspirin group.
Of the 246 patients assigned to either aspirin or a DOAC, usually a factor Xa inhibitor, 75% had undergone VT ablation and the remainder ablation for PVCs. Their mean age was 60 years and only 18% were women. None had experienced a cerebrovascular event in the previous 3 months.
The 30-day odds ratio for TIA or ischemic stroke in patients who received aspirin, compared with a DOAC, was 12.6 (95% confidence interval, 4.10-39.11; P < .001).
The corresponding OR for asymptomatic cerebral lesions by MRI at 24 hours was 2.15 (95% CI, 1.02-4.54; P = .04) and at 30 days was 3.48 (95% CI, 1.38-8.80; P = .008).
The rate of stroke or TIA was similar in patients who underwent ablation for VT and for PVCs (14% vs. 16%, respectively; P = .70). There were fewer asymptomatic cerebrovascular events by MRI at 24 hours for those undergoing VT ablations (14.7% and 25.8%, respectively; P = .046); but difference between rates attenuated by 30 days (11.4% and 14.5%, respectively; P = .52).
The OR for TIA or stroke associated with the retrograde transaortic approach, performed in about 40% of the patients, compared with the trans-septal approach in the remainder was 2.60 (95% CI, 1.06-6.37; P = .04).
“The study tells us it’s safe and indeed preferable to anticoagulate after an ablation procedure. But the more important finding, perhaps, wasn’t the one related to the core hypothesis. And that was the effect of retrograde access,” Paul A. Friedman, MD, Mayo Clinic, Rochester, Minn., said as an invited discussant after Dr. Lakkireddy’s formal presentation of the trial.
Whether a ventricular ablation is performed using the retrograde transaortic or trans-septal approach often depends on the location of the ablation targets in the left ventricle. But in some cases it’s a matter of operator preference, Dr. Piccini observed.
“There are some situations where, really, it is better to do retrograde aortic, and there are some cases that are better to do trans-septal. But now there’s going to be a higher burden of proof,” he said. Given the findings of STROKE-VT, operators may need to consider that a ventricular ablation procedure that can be done by the trans-septal route perhaps ought to be consistently done that way.
Dr. Lakkireddy discloses financial relationships with Boston Scientific, Biosense Webster, Janssen Pharmaceuticals, and more. Dr. Chung had “nothing relevant to disclose.” Dr. Piccini discloses receiving honoraria or speaking or consulting fees from Sanofi, Abbott, ARCA Biopharma, Medtronic, Philips, Biotronik, Allergan, LivaNova, and Myokardia; and research in conjunction with Bayer Healthcare, Abbott, Boston Scientific, and Philips. Dr. Friedman discloses conducting research in conjunction with Medtronic and Abbott; holding intellectual property rights with AliveCor, Inference, Medicool, Eko, and Anumana; and receiving honoraria or speaking or consulting fees from Boston Scientific. Dr. Winterfield and Dr. Tedrow had no disclosures.
A version of this article first appeared on Medscape.com.
Catheter ablation has been around a lot longer for ventricular arrhythmia than for atrial fibrillation, but far less is settled about what antithrombotic therapy should follow ventricular ablations, as there have been no big, randomized trials for guidance.
But the evidence base grew stronger this week, and it favors postprocedure treatment with a direct oral anticoagulant (DOAC) over antiplatelet therapy with aspirin for patients undergoing radiofrequency (RF) ablation to treat left ventricular (LV) arrhythmias.
The 30-day risk for ischemic stroke or transient ischemia attack (TIA) was sharply higher for patients who took daily aspirin after RF ablation for ventricular tachycardia (VT) or premature ventricular contractions (PVC) in a multicenter randomized trial.
Those of its 246 patients who received aspirin were also far more likely to show asymptomatic lesions on cerebral MRI scans performed both 24 hours and 30 days after the procedure.
The findings show the importance of DOAC therapy after ventricular ablation procedures, a setting for which there are no evidence-based guidelines, “to mitigate the risk of systemic thromboembolic events,” said Dhanunjaya Lakkireddy, MD, Kansas City Heart Rhythm Institute, Overland Park. He spoke at a media presentation on the trial, called STROKE-VT, during the Heart Rhythm Society 2021 Scientific Sessions, held virtually and on-site in Boston.
The risk for stroke and TIA went up in association with several procedural issues, including some that operators might be able to change in order to reach for better outcomes, Dr. Lakkireddy observed.
“Prolonged radiofrequency ablation times, especially in those with low left ventricle ejection fractions, are definitely higher risk,” as are procedures that involved the retrograde transaortic approach for advancing the ablation catheter, rather than a trans-septal approach.
The retrograde transaortic approach should be avoided in such procedures, “whenever it can be avoided,” said Dr. Lakkireddy, who formally presented STROKE-VT at the HRS sessions and is lead author on its report published about the same time in JACC: Clinical Electrophysiology.
The trial has limitations, but “it’s a very important study, and I think that this could become our standard of care for managing anticoagulation after VT and PVC left-sided ablations,” Mina K. Chung, MD, Cleveland Clinic, said as an invited discussant after Dr. Lakkireddy’s presentation.
How patients are treated with antithrombotics after ventricular ablations can vary widely, sometimes based on the operator’s “subjective feeling of how extensive the ablation is,” Christine M. Albert, MD, MPH, Cedars-Sinai Medical Center, Los Angeles, not involved in the study, said during the STROKE-VT media briefing.
That’s consistent with the guidelines, which propose oral anticoagulation therapy after more extensive ventricular ablations and antiplatelets when the ablation is more limited – based more on consensus than firm evidence – as described by Jeffrey R. Winterfield, MD, Medical University of South Carolina, Charleston, and Usha Tedrow, MD, MSc, Brigham and Women’s Hospital, Boston, in an accompanying editorial.
“This is really the first randomized trial data, that I know of, that we have on this. So I do think it will be guideline-influencing,” Dr. Albert said.
“This should change practice,” agreed Jonathan P. Piccini, MD, MHS, Duke University, Durham, N.C., also not part of STROKE-VT. “A lot of evidence in the trial is consistent and provides a compelling story, not to mention that, in my opinion, the study probably underestimates the value of DOACs,” he told this news organization.
That’s because patients assigned to DOACs had far longer ablation times, “so their risk was even greater than in the aspirin arm,” Dr. Piccini said. Ablation times averaged 2,095 seconds in the DOAC group, compared with only 1,708 seconds in the aspirin group, probably because the preponderance of VT over PVC ablations for those getting a DOAC was even greater in the aspirin group.
Of the 246 patients assigned to either aspirin or a DOAC, usually a factor Xa inhibitor, 75% had undergone VT ablation and the remainder ablation for PVCs. Their mean age was 60 years and only 18% were women. None had experienced a cerebrovascular event in the previous 3 months.
The 30-day odds ratio for TIA or ischemic stroke in patients who received aspirin, compared with a DOAC, was 12.6 (95% confidence interval, 4.10-39.11; P < .001).
The corresponding OR for asymptomatic cerebral lesions by MRI at 24 hours was 2.15 (95% CI, 1.02-4.54; P = .04) and at 30 days was 3.48 (95% CI, 1.38-8.80; P = .008).
The rate of stroke or TIA was similar in patients who underwent ablation for VT and for PVCs (14% vs. 16%, respectively; P = .70). There were fewer asymptomatic cerebrovascular events by MRI at 24 hours for those undergoing VT ablations (14.7% and 25.8%, respectively; P = .046); but difference between rates attenuated by 30 days (11.4% and 14.5%, respectively; P = .52).
The OR for TIA or stroke associated with the retrograde transaortic approach, performed in about 40% of the patients, compared with the trans-septal approach in the remainder was 2.60 (95% CI, 1.06-6.37; P = .04).
“The study tells us it’s safe and indeed preferable to anticoagulate after an ablation procedure. But the more important finding, perhaps, wasn’t the one related to the core hypothesis. And that was the effect of retrograde access,” Paul A. Friedman, MD, Mayo Clinic, Rochester, Minn., said as an invited discussant after Dr. Lakkireddy’s formal presentation of the trial.
Whether a ventricular ablation is performed using the retrograde transaortic or trans-septal approach often depends on the location of the ablation targets in the left ventricle. But in some cases it’s a matter of operator preference, Dr. Piccini observed.
“There are some situations where, really, it is better to do retrograde aortic, and there are some cases that are better to do trans-septal. But now there’s going to be a higher burden of proof,” he said. Given the findings of STROKE-VT, operators may need to consider that a ventricular ablation procedure that can be done by the trans-septal route perhaps ought to be consistently done that way.
Dr. Lakkireddy discloses financial relationships with Boston Scientific, Biosense Webster, Janssen Pharmaceuticals, and more. Dr. Chung had “nothing relevant to disclose.” Dr. Piccini discloses receiving honoraria or speaking or consulting fees from Sanofi, Abbott, ARCA Biopharma, Medtronic, Philips, Biotronik, Allergan, LivaNova, and Myokardia; and research in conjunction with Bayer Healthcare, Abbott, Boston Scientific, and Philips. Dr. Friedman discloses conducting research in conjunction with Medtronic and Abbott; holding intellectual property rights with AliveCor, Inference, Medicool, Eko, and Anumana; and receiving honoraria or speaking or consulting fees from Boston Scientific. Dr. Winterfield and Dr. Tedrow had no disclosures.
A version of this article first appeared on Medscape.com.
Bronchitis the leader at putting children in the hospital
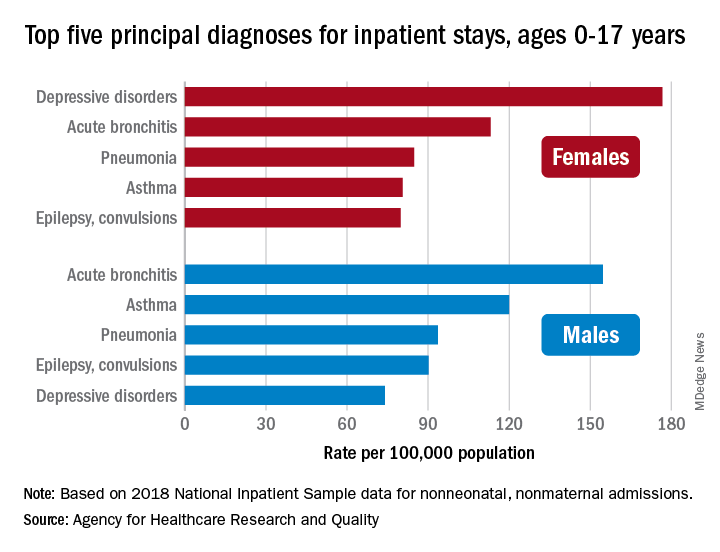
About 7% (99,000) of the 1.47 million nonmaternal, nonneonatal hospital stays in children aged 0-17 years involved a primary diagnosis of acute bronchitis in 2018, representing the leading cause of admissions in boys (154.7 stays per 100,000 population) and the second-leading diagnosis in girls (113.1 stays per 100,000), Kimberly W. McDermott, PhD, and Marc Roemer, MS, said in a statistical brief.
Depressive disorders were the most common primary diagnosis in girls, with a rate of 176.7 stays per 100,000, and the second-leading diagnosis overall, although the rate was less than half that (74.0 per 100,000) in boys. Two other respiratory conditions, asthma and pneumonia, were among the top five for both girls and boys, as was epilepsy, they reported.
The combined rate for all diagnoses was slightly higher for boys, 2,051 per 100,000, compared with 1,922 for girls, they said based on data from the National Inpatient Sample.
“Identifying the most frequent primary conditions for which patients are admitted to the hospital is important to the implementation and improvement of health care delivery, quality initiatives, and health policy,” said Dr. McDermott of IBM Watson Health and Mr. Roemer of the AHRQ.

About 7% (99,000) of the 1.47 million nonmaternal, nonneonatal hospital stays in children aged 0-17 years involved a primary diagnosis of acute bronchitis in 2018, representing the leading cause of admissions in boys (154.7 stays per 100,000 population) and the second-leading diagnosis in girls (113.1 stays per 100,000), Kimberly W. McDermott, PhD, and Marc Roemer, MS, said in a statistical brief.
Depressive disorders were the most common primary diagnosis in girls, with a rate of 176.7 stays per 100,000, and the second-leading diagnosis overall, although the rate was less than half that (74.0 per 100,000) in boys. Two other respiratory conditions, asthma and pneumonia, were among the top five for both girls and boys, as was epilepsy, they reported.
The combined rate for all diagnoses was slightly higher for boys, 2,051 per 100,000, compared with 1,922 for girls, they said based on data from the National Inpatient Sample.
“Identifying the most frequent primary conditions for which patients are admitted to the hospital is important to the implementation and improvement of health care delivery, quality initiatives, and health policy,” said Dr. McDermott of IBM Watson Health and Mr. Roemer of the AHRQ.

About 7% (99,000) of the 1.47 million nonmaternal, nonneonatal hospital stays in children aged 0-17 years involved a primary diagnosis of acute bronchitis in 2018, representing the leading cause of admissions in boys (154.7 stays per 100,000 population) and the second-leading diagnosis in girls (113.1 stays per 100,000), Kimberly W. McDermott, PhD, and Marc Roemer, MS, said in a statistical brief.
Depressive disorders were the most common primary diagnosis in girls, with a rate of 176.7 stays per 100,000, and the second-leading diagnosis overall, although the rate was less than half that (74.0 per 100,000) in boys. Two other respiratory conditions, asthma and pneumonia, were among the top five for both girls and boys, as was epilepsy, they reported.
The combined rate for all diagnoses was slightly higher for boys, 2,051 per 100,000, compared with 1,922 for girls, they said based on data from the National Inpatient Sample.
“Identifying the most frequent primary conditions for which patients are admitted to the hospital is important to the implementation and improvement of health care delivery, quality initiatives, and health policy,” said Dr. McDermott of IBM Watson Health and Mr. Roemer of the AHRQ.