User login
FDA may okay COVID booster for vulnerable adults before weekend: Media
according to multiple media reports.
The agency, along with the Centers for Disease Control and Prevention (CDC) and the National Institutes of Health, is working through the details of how booster doses for this population would work, and could authorize a third dose of both the Pfizer and Moderna vaccines as early as Aug. 12, Politico reports.
About 2.7% of adults in the United States are immunocompromised, according to the CDC. This group includes people who have cancer, have received solid organ or stem cell transplants, have genetic conditions that weaken the immune function, have HIV, or are people with health conditions that require treatment with medications that turn down immune function, such as rheumatoid arthritis.
Immune function also wanes with age, so the FDA could consider boosters for the elderly.
New research shows that between one-third and one-half of immunocompromised patients who didn’t develop detectable levels of virus-fighting antibodies after two doses of a COVID vaccine will respond to a third dose.
A committee of independent experts that advises the CDC on the use of vaccines in the United States had previously signaled its support for giving boosters to those who are immunocompromised, but noted that it couldn’t officially recommend the strategy until the FDA had updated its emergency-use authorization for the shots or granted them a full biologics license, or “full approval.”
It’s unclear which mechanism the FDA might use, or exactly who will be eligible for the shots.
The United States would follow other nations such as Israel, France, the United Kingdom, and Germany in planning for or authorizing boosters for some vulnerable individuals.
The World Health Organization (WHO) has voiced strong opposition to the use of boosters in wealthy countries while much of the world still doesn’t have access to these lifesaving therapies. The WHO has asked wealthy nations to hold off on giving boosters until at least the end of September to give more people the opportunity to get a first dose.
The CDC’s Advisory Committee on Immunization Practices (ACIP) meets again on Aug. 13 and is expected to discuss booster doses for this population of patients. The ACIP officially makes recommendations on the use of vaccines to the nation’s doctors.
The committee’s recommendation ensures that a vaccine will be covered by public and private insurers. Statutory vaccination requirements are also made based on the ACIP’s recommendations.
A version of this article first appeared on Medscape.com.
according to multiple media reports.
The agency, along with the Centers for Disease Control and Prevention (CDC) and the National Institutes of Health, is working through the details of how booster doses for this population would work, and could authorize a third dose of both the Pfizer and Moderna vaccines as early as Aug. 12, Politico reports.
About 2.7% of adults in the United States are immunocompromised, according to the CDC. This group includes people who have cancer, have received solid organ or stem cell transplants, have genetic conditions that weaken the immune function, have HIV, or are people with health conditions that require treatment with medications that turn down immune function, such as rheumatoid arthritis.
Immune function also wanes with age, so the FDA could consider boosters for the elderly.
New research shows that between one-third and one-half of immunocompromised patients who didn’t develop detectable levels of virus-fighting antibodies after two doses of a COVID vaccine will respond to a third dose.
A committee of independent experts that advises the CDC on the use of vaccines in the United States had previously signaled its support for giving boosters to those who are immunocompromised, but noted that it couldn’t officially recommend the strategy until the FDA had updated its emergency-use authorization for the shots or granted them a full biologics license, or “full approval.”
It’s unclear which mechanism the FDA might use, or exactly who will be eligible for the shots.
The United States would follow other nations such as Israel, France, the United Kingdom, and Germany in planning for or authorizing boosters for some vulnerable individuals.
The World Health Organization (WHO) has voiced strong opposition to the use of boosters in wealthy countries while much of the world still doesn’t have access to these lifesaving therapies. The WHO has asked wealthy nations to hold off on giving boosters until at least the end of September to give more people the opportunity to get a first dose.
The CDC’s Advisory Committee on Immunization Practices (ACIP) meets again on Aug. 13 and is expected to discuss booster doses for this population of patients. The ACIP officially makes recommendations on the use of vaccines to the nation’s doctors.
The committee’s recommendation ensures that a vaccine will be covered by public and private insurers. Statutory vaccination requirements are also made based on the ACIP’s recommendations.
A version of this article first appeared on Medscape.com.
according to multiple media reports.
The agency, along with the Centers for Disease Control and Prevention (CDC) and the National Institutes of Health, is working through the details of how booster doses for this population would work, and could authorize a third dose of both the Pfizer and Moderna vaccines as early as Aug. 12, Politico reports.
About 2.7% of adults in the United States are immunocompromised, according to the CDC. This group includes people who have cancer, have received solid organ or stem cell transplants, have genetic conditions that weaken the immune function, have HIV, or are people with health conditions that require treatment with medications that turn down immune function, such as rheumatoid arthritis.
Immune function also wanes with age, so the FDA could consider boosters for the elderly.
New research shows that between one-third and one-half of immunocompromised patients who didn’t develop detectable levels of virus-fighting antibodies after two doses of a COVID vaccine will respond to a third dose.
A committee of independent experts that advises the CDC on the use of vaccines in the United States had previously signaled its support for giving boosters to those who are immunocompromised, but noted that it couldn’t officially recommend the strategy until the FDA had updated its emergency-use authorization for the shots or granted them a full biologics license, or “full approval.”
It’s unclear which mechanism the FDA might use, or exactly who will be eligible for the shots.
The United States would follow other nations such as Israel, France, the United Kingdom, and Germany in planning for or authorizing boosters for some vulnerable individuals.
The World Health Organization (WHO) has voiced strong opposition to the use of boosters in wealthy countries while much of the world still doesn’t have access to these lifesaving therapies. The WHO has asked wealthy nations to hold off on giving boosters until at least the end of September to give more people the opportunity to get a first dose.
The CDC’s Advisory Committee on Immunization Practices (ACIP) meets again on Aug. 13 and is expected to discuss booster doses for this population of patients. The ACIP officially makes recommendations on the use of vaccines to the nation’s doctors.
The committee’s recommendation ensures that a vaccine will be covered by public and private insurers. Statutory vaccination requirements are also made based on the ACIP’s recommendations.
A version of this article first appeared on Medscape.com.
Opioid prescribing laws having an impact
State laws capping initial opioid prescriptions to 7 days or less have led to a reduction in opioid prescribing, a new analysis of Medicare data shows.
While overall opioid prescribing has decreased, the reduction in states with legislation restricting opioid prescribing was “significantly greater than in states without such legislation,” study investigator Michael Brenner, MD, University of Michigan, Ann Arbor, said in an interview.
The study was published online August 9 in JAMA Internal Medicine.
Significant but limited effect
Because of rising concern around the opioid crisis, 23 states representing 43% of the U.S. population passed laws from 2016 through 2018 limiting initial opioid prescription to 7 days or less.
Using Medicare data from 2013 through 2018, Dr. Brenner and colleagues conducted a before-and-after study to assess the effect of these laws.
They found that on average, the number of days an opioid was prescribed for each Medicare beneficiary decreased by 11.6 days (from 44.2 days in 2013 to 32.7 days in 2018) in states that imposed duration limits, compared with 10.1 days in states without these laws (from 43.4 days in 2013 to 33.3 days in 2018).
Prior to the start of duration limits in 2016, days an opioid was prescribed were comparable among states.
After adjusting for state-level differences in race, urbanization, median income, tobacco and alcohol use, serious mental illness, and other factors, state laws limiting opioid prescriptions to 7 days or less were associated with a reduction in prescribing of 1.7 days per enrollee, “suggesting a significant but limited outcome” for these laws, the researchers note.
, but this was not significantly different in states with limit laws versus those without. However, state laws limiting duration led to a significant reduction in days of opioid prescribed among surgeons, dentists, pain specialists, and other specialists.
Inadequate pain control?
The researchers note the study was limited to Medicare beneficiaries; however, excess opioid prescribing is prevalent across all patient populations.
In addition, it’s not possible to tell from the data whether acute pain was adequately controlled with fewer pills.
“The question of adequacy of pain control is a crucial one that has been investigated extensively in prior work but was not possible to evaluate in this particular study,” said Dr. Brenner.
However, “ample evidence supports a role for reducing opioid prescribing and that such reduction can be achieved while ensuring that pain is adequately controlled with fewer pills,” he noted.
“A persistent misconception is that opioids are uniquely powerful and effective for controlling pain. Patients may perceive that effective analgesia is being withheld when opioids are not included in a regimen,” Dr. Brenner added.
“Yet, the evidence from meta-analyses derived from large numbers of randomized clinical trials finds that [nonsteroidal anti-inflammatory drugs] NSAIDS combined with acetaminophen provide similar or improved acute pain when compared to commonly prescribed opioid regimens, based on number-needed-to-treat analyses,” he added.
In a related editorial, Deborah Grady, MD, MPH, with University of California, San Francisco, and Mitchell H. Katz, MD, president and CEO of NYC Health + Hospitals, say the decrease in opioid prescribing with duration limits was “small but probably meaningful.”
Restricting initial prescriptions to seven or fewer days is “reasonable because patients with new onset of pain should be re-evaluated in a week if the pain continues,” they write.
However, Dr. Grady and Dr. Katz “worry” that restricting initial prescriptions to shorter periods, such as 3 or 5 days, as has occurred in six states, “may result in patients with acute pain going untreated or having to go to extraordinary effort to obtain adequate pain relief.”
In their view, the data from this study suggest that limiting initial prescriptions to seven or fewer days is “helpful, but we would not restrict any further given that we do not know how it affected patients with acute pain.”
The study had no specific funding. Dr. Brenner, Dr. Grady, and Dr. Katz have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
State laws capping initial opioid prescriptions to 7 days or less have led to a reduction in opioid prescribing, a new analysis of Medicare data shows.
While overall opioid prescribing has decreased, the reduction in states with legislation restricting opioid prescribing was “significantly greater than in states without such legislation,” study investigator Michael Brenner, MD, University of Michigan, Ann Arbor, said in an interview.
The study was published online August 9 in JAMA Internal Medicine.
Significant but limited effect
Because of rising concern around the opioid crisis, 23 states representing 43% of the U.S. population passed laws from 2016 through 2018 limiting initial opioid prescription to 7 days or less.
Using Medicare data from 2013 through 2018, Dr. Brenner and colleagues conducted a before-and-after study to assess the effect of these laws.
They found that on average, the number of days an opioid was prescribed for each Medicare beneficiary decreased by 11.6 days (from 44.2 days in 2013 to 32.7 days in 2018) in states that imposed duration limits, compared with 10.1 days in states without these laws (from 43.4 days in 2013 to 33.3 days in 2018).
Prior to the start of duration limits in 2016, days an opioid was prescribed were comparable among states.
After adjusting for state-level differences in race, urbanization, median income, tobacco and alcohol use, serious mental illness, and other factors, state laws limiting opioid prescriptions to 7 days or less were associated with a reduction in prescribing of 1.7 days per enrollee, “suggesting a significant but limited outcome” for these laws, the researchers note.
, but this was not significantly different in states with limit laws versus those without. However, state laws limiting duration led to a significant reduction in days of opioid prescribed among surgeons, dentists, pain specialists, and other specialists.
Inadequate pain control?
The researchers note the study was limited to Medicare beneficiaries; however, excess opioid prescribing is prevalent across all patient populations.
In addition, it’s not possible to tell from the data whether acute pain was adequately controlled with fewer pills.
“The question of adequacy of pain control is a crucial one that has been investigated extensively in prior work but was not possible to evaluate in this particular study,” said Dr. Brenner.
However, “ample evidence supports a role for reducing opioid prescribing and that such reduction can be achieved while ensuring that pain is adequately controlled with fewer pills,” he noted.
“A persistent misconception is that opioids are uniquely powerful and effective for controlling pain. Patients may perceive that effective analgesia is being withheld when opioids are not included in a regimen,” Dr. Brenner added.
“Yet, the evidence from meta-analyses derived from large numbers of randomized clinical trials finds that [nonsteroidal anti-inflammatory drugs] NSAIDS combined with acetaminophen provide similar or improved acute pain when compared to commonly prescribed opioid regimens, based on number-needed-to-treat analyses,” he added.
In a related editorial, Deborah Grady, MD, MPH, with University of California, San Francisco, and Mitchell H. Katz, MD, president and CEO of NYC Health + Hospitals, say the decrease in opioid prescribing with duration limits was “small but probably meaningful.”
Restricting initial prescriptions to seven or fewer days is “reasonable because patients with new onset of pain should be re-evaluated in a week if the pain continues,” they write.
However, Dr. Grady and Dr. Katz “worry” that restricting initial prescriptions to shorter periods, such as 3 or 5 days, as has occurred in six states, “may result in patients with acute pain going untreated or having to go to extraordinary effort to obtain adequate pain relief.”
In their view, the data from this study suggest that limiting initial prescriptions to seven or fewer days is “helpful, but we would not restrict any further given that we do not know how it affected patients with acute pain.”
The study had no specific funding. Dr. Brenner, Dr. Grady, and Dr. Katz have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
State laws capping initial opioid prescriptions to 7 days or less have led to a reduction in opioid prescribing, a new analysis of Medicare data shows.
While overall opioid prescribing has decreased, the reduction in states with legislation restricting opioid prescribing was “significantly greater than in states without such legislation,” study investigator Michael Brenner, MD, University of Michigan, Ann Arbor, said in an interview.
The study was published online August 9 in JAMA Internal Medicine.
Significant but limited effect
Because of rising concern around the opioid crisis, 23 states representing 43% of the U.S. population passed laws from 2016 through 2018 limiting initial opioid prescription to 7 days or less.
Using Medicare data from 2013 through 2018, Dr. Brenner and colleagues conducted a before-and-after study to assess the effect of these laws.
They found that on average, the number of days an opioid was prescribed for each Medicare beneficiary decreased by 11.6 days (from 44.2 days in 2013 to 32.7 days in 2018) in states that imposed duration limits, compared with 10.1 days in states without these laws (from 43.4 days in 2013 to 33.3 days in 2018).
Prior to the start of duration limits in 2016, days an opioid was prescribed were comparable among states.
After adjusting for state-level differences in race, urbanization, median income, tobacco and alcohol use, serious mental illness, and other factors, state laws limiting opioid prescriptions to 7 days or less were associated with a reduction in prescribing of 1.7 days per enrollee, “suggesting a significant but limited outcome” for these laws, the researchers note.
, but this was not significantly different in states with limit laws versus those without. However, state laws limiting duration led to a significant reduction in days of opioid prescribed among surgeons, dentists, pain specialists, and other specialists.
Inadequate pain control?
The researchers note the study was limited to Medicare beneficiaries; however, excess opioid prescribing is prevalent across all patient populations.
In addition, it’s not possible to tell from the data whether acute pain was adequately controlled with fewer pills.
“The question of adequacy of pain control is a crucial one that has been investigated extensively in prior work but was not possible to evaluate in this particular study,” said Dr. Brenner.
However, “ample evidence supports a role for reducing opioid prescribing and that such reduction can be achieved while ensuring that pain is adequately controlled with fewer pills,” he noted.
“A persistent misconception is that opioids are uniquely powerful and effective for controlling pain. Patients may perceive that effective analgesia is being withheld when opioids are not included in a regimen,” Dr. Brenner added.
“Yet, the evidence from meta-analyses derived from large numbers of randomized clinical trials finds that [nonsteroidal anti-inflammatory drugs] NSAIDS combined with acetaminophen provide similar or improved acute pain when compared to commonly prescribed opioid regimens, based on number-needed-to-treat analyses,” he added.
In a related editorial, Deborah Grady, MD, MPH, with University of California, San Francisco, and Mitchell H. Katz, MD, president and CEO of NYC Health + Hospitals, say the decrease in opioid prescribing with duration limits was “small but probably meaningful.”
Restricting initial prescriptions to seven or fewer days is “reasonable because patients with new onset of pain should be re-evaluated in a week if the pain continues,” they write.
However, Dr. Grady and Dr. Katz “worry” that restricting initial prescriptions to shorter periods, such as 3 or 5 days, as has occurred in six states, “may result in patients with acute pain going untreated or having to go to extraordinary effort to obtain adequate pain relief.”
In their view, the data from this study suggest that limiting initial prescriptions to seven or fewer days is “helpful, but we would not restrict any further given that we do not know how it affected patients with acute pain.”
The study had no specific funding. Dr. Brenner, Dr. Grady, and Dr. Katz have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 mitigation measures led to shifts in typical annual respiratory virus patterns
Nonpharmaceutical interventions, such as masking, staying home, limiting travel, and social distancing, have been doing more than reducing the risk for COVID-19. They’re also having an impact on infection rates and the timing of seasonal surges of other common respiratory diseases, according to an article published July 23 in Morbidity and Mortality Weekly Report.
Typically, respiratory pathogens such as respiratory syncytial virus (RSV), common cold coronaviruses, parainfluenza viruses, and respiratory adenoviruses increase in the fall and remain high throughout winter, following the same basic patterns as influenza. Although the historically low rates of influenza remained low into spring 2021, that’s not the case for several other common respiratory viruses.
“Clinicians should be aware of increases in some respiratory virus activity and remain vigilant for off-season increases,” wrote Sonja J. Olsen, PhD, and her colleagues at the Centers for Disease Control and Prevention. She told this news organization that clinicians should use multipathogen testing to help guide treatment.
The authors also underscore the importance of fall influenza vaccination campaigns for anyone aged 6 months or older.
Timothy Brewer, MD, MPH, a professor of medicine in the Division of Infectious Diseases at the University of California, Los Angeles (UCLA), and of epidemiology at the UCLA Fielding School of Public Health, agreed that it’s important for health care professionals to consider off-season illnesses in their patients.
“Practitioners should be aware that if they see a sick child in the summer, outside of what normally might be influenza season, but they look like they have influenza, consider potentially influenza and test for it, because it might be possible that we may have disrupted that natural pattern,” Dr. Brewer told this news organization. Dr. Brewer, who was not involved in the CDC research, said it’s also “critically important” to encourage influenza vaccination as the season approaches.
The CDC researchers used the U.S. World Health Organization Collaborating Laboratories System and the CDC’s National Respiratory and Enteric Virus Surveillance System to analyze virologic data from Oct. 3, 2020, to May 22, 2021, for influenza and Jan. 4, 2020, to May 22, 2021, for other respiratory viruses. The authors compared virus circulation during these periods to circulation during the same dates from four previous years.
Data to calculate influenza and RSV hospitalization rates came from the Influenza Hospitalization Surveillance Network and RSV Hospitalization Surveillance Network.
The authors report that flu activity dropped dramatically in March 2020 to its lowest levels since 1997, the earliest season for which data are available. Only 0.2% of more than 1 million specimens tested positive for influenza; the rate of hospitalizations for lab-confirmed flu was 0.8 per 100,000 people. Flu levels remained low through the summer, fall, and on to May 2021.
A potential drawback to this low activity, however, is a more prevalent and severe upcoming flu season, the authors write. The repeated exposure to flu viruses every year often “does not lead to illness, but it does serve to boost our immune response to influenza viruses,” Dr. Olsen said in an interview. “The absence of influenza viruses in the community over the last year means that we are not getting these regular boosts to our immune system. When we finally get exposed, our body may mount a weak response, and this could mean we develop a more clinically severe illness.”
Children are most susceptible to that phenomenon because they haven’t had a lifetime of exposure to flu viruses, Dr. Olsen said.
“An immunologically naive child may be more likely to develop a severe illness than someone who has lived through several influenza seasons,” she said. “This is why it is especially important for everyone 6 months and older to get vaccinated against influenza this season.”
Rhinovirus and enterovirus infections rebounded fairly quickly after their decline in March 2020 and started increasing in May 2020 until they reached “near prepandemic seasonal levels,” the authors write.
RSV infections dropped from 15.3% of weekly positive results in January 2020 to 1.4% by April and then stayed below 1% through the end of 2020. In past years, weekly positive results climbed to 3% in October and peaked at 12.5% to 16.7% in late December. Instead, RSV weekly positive results began increasing in April 2021, rising from 1.1% to 2.8% in May.
The “unusually timed” late spring increase in RSV “is probably associated with various nonpharmaceutical measures that have been in place but are now relaxing,” Dr. Olsen stated.
The RSV hospitalization rate was 0.3 per 100,000 people from October 2020 to April 2021, compared to 27.1 and 33.4 per 100,000 people in the previous 2 years. Of all RSV hospitalizations in the past year, 76.5% occurred in April-May 2021.
Rates of illness caused by the four common human coronaviruses (OC43, NL63, 229E, and HKU1) dropped from 7.5% of weekly positive results in January 2020 to 1.3% in April 2020 and stayed below 1% through February 2021. Then they climbed to 6.6% by May 2021. Infection rates of parainfluenza viruses types 1-4 similarly dropped from 2.6% in January 2020 to 1% in March 2020 and stayed below 1% until April 2021. Since then, rates of the common coronaviruses increased to 6.6% and parainfluenza viruses to 10.9% in May 2021.
Normally, parainfluenza viruses peak in October-November and May-June, so “the current increase could represent a return to prepandemic seasonality,” the authors write.
Human pneumoviruses’ weekly positive results initially increased from 4.2% in January 2020 to 7% in March and then fell to 1.9% the second week of April and remained below 1% through May 2021. In typical years, these viruses peak from 6.2% to 7.7% in March-April. Respiratory adenovirus activity similarly dropped to historically low levels in April 2021 and then began increasing to reach 3% by May 2021, the usual level for that month.
“The different circulation patterns observed across respiratory viruses probably also reflect differences in the virus transmission routes and how effective various nonpharmaceutical measures are at stopping transmission,” Dr. Olsen said in an interview. “As pandemic mitigation measures continue to be adjusted, we expect to see more changes in the circulation of these viruses, including a return to prepandemic circulation, as seen for rhinoviruses and enteroviruses.”
Rhinovirus and enterovirus rates dropped from 14.9% in March 2020 to 3.2% in May – lower than typical – and then climbed to a peak in October 2020. The peak (21.7% weekly positive results) was, however, still lower than the usual median of 32.8%. After dropping to 9.9% in January 2021, it then rose 19.1% in May, potentially reflecting “the usual spring peak that has occurred in previous years,” the authors write.
The authors note that it’s not yet clear how the COVID-19 pandemic and related mitigation measures will continue to affect respiratory virus circulation.
The authors hypothesize that the reasons for a seeming return to seasonal activity of respiratory adenoviruses, rhinoviruses, and enteroviruses could involve “different transmission mechanisms, the role of asymptomatic transmission, and prolonged survival of these nonenveloped viruses on surfaces, all of which might make these viruses less susceptible to nonpharmaceutical interventions.”
Dr. Brewer, of UCLA, agreed.
All the viruses basically “flatline except for adenoviruses and enteroviruses, and they behave a little differently in terms of how they spread,” he said. “Enteroviruses are much more likely to be fecal-oral spread than the other viruses [in the study].”
The delayed circulation of parainfluenza and human coronaviruses may have resulted from suspension of in-person classes through late winter 2020, they write, but that doesn’t explain the relative absence of pneumovirus activity, which usually affects the same young pediatric populations as RSV.
Dr. Brewer said California is seeing a surge of RSV right now, as are many states, especially throughout in the South. He’s not surprised by RSV’s deferred season, because those most affected – children younger than 2 years – are less likely to wear masks now and were “not going to daycare, not being out in public” in 2020. “As people are doing more activities, that’s probably why RSV has been starting to go up since April,” he said.
Despite the fact that, unlike many East Asian cultures, the United States has not traditionally been a mask-wearing culture, Dr. Brewer wouldn’t be surprised if more Americans begin wearing masks during flu season. “Hopefully another thing that will come out of this is better hand hygiene, with people just getting used to washing their hands more, particularly after they come home from being out,” he added.
Dr. Brewer similarly emphasized the importance of flu vaccination for the upcoming season, especially for younger children who may have poorer natural immunity to influenza, owing to its low circulation rates in 2020-2021.
The study was funded by the CDC. Dr. Brewer and Dr. Olsen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Nonpharmaceutical interventions, such as masking, staying home, limiting travel, and social distancing, have been doing more than reducing the risk for COVID-19. They’re also having an impact on infection rates and the timing of seasonal surges of other common respiratory diseases, according to an article published July 23 in Morbidity and Mortality Weekly Report.
Typically, respiratory pathogens such as respiratory syncytial virus (RSV), common cold coronaviruses, parainfluenza viruses, and respiratory adenoviruses increase in the fall and remain high throughout winter, following the same basic patterns as influenza. Although the historically low rates of influenza remained low into spring 2021, that’s not the case for several other common respiratory viruses.
“Clinicians should be aware of increases in some respiratory virus activity and remain vigilant for off-season increases,” wrote Sonja J. Olsen, PhD, and her colleagues at the Centers for Disease Control and Prevention. She told this news organization that clinicians should use multipathogen testing to help guide treatment.
The authors also underscore the importance of fall influenza vaccination campaigns for anyone aged 6 months or older.
Timothy Brewer, MD, MPH, a professor of medicine in the Division of Infectious Diseases at the University of California, Los Angeles (UCLA), and of epidemiology at the UCLA Fielding School of Public Health, agreed that it’s important for health care professionals to consider off-season illnesses in their patients.
“Practitioners should be aware that if they see a sick child in the summer, outside of what normally might be influenza season, but they look like they have influenza, consider potentially influenza and test for it, because it might be possible that we may have disrupted that natural pattern,” Dr. Brewer told this news organization. Dr. Brewer, who was not involved in the CDC research, said it’s also “critically important” to encourage influenza vaccination as the season approaches.
The CDC researchers used the U.S. World Health Organization Collaborating Laboratories System and the CDC’s National Respiratory and Enteric Virus Surveillance System to analyze virologic data from Oct. 3, 2020, to May 22, 2021, for influenza and Jan. 4, 2020, to May 22, 2021, for other respiratory viruses. The authors compared virus circulation during these periods to circulation during the same dates from four previous years.
Data to calculate influenza and RSV hospitalization rates came from the Influenza Hospitalization Surveillance Network and RSV Hospitalization Surveillance Network.
The authors report that flu activity dropped dramatically in March 2020 to its lowest levels since 1997, the earliest season for which data are available. Only 0.2% of more than 1 million specimens tested positive for influenza; the rate of hospitalizations for lab-confirmed flu was 0.8 per 100,000 people. Flu levels remained low through the summer, fall, and on to May 2021.
A potential drawback to this low activity, however, is a more prevalent and severe upcoming flu season, the authors write. The repeated exposure to flu viruses every year often “does not lead to illness, but it does serve to boost our immune response to influenza viruses,” Dr. Olsen said in an interview. “The absence of influenza viruses in the community over the last year means that we are not getting these regular boosts to our immune system. When we finally get exposed, our body may mount a weak response, and this could mean we develop a more clinically severe illness.”
Children are most susceptible to that phenomenon because they haven’t had a lifetime of exposure to flu viruses, Dr. Olsen said.
“An immunologically naive child may be more likely to develop a severe illness than someone who has lived through several influenza seasons,” she said. “This is why it is especially important for everyone 6 months and older to get vaccinated against influenza this season.”
Rhinovirus and enterovirus infections rebounded fairly quickly after their decline in March 2020 and started increasing in May 2020 until they reached “near prepandemic seasonal levels,” the authors write.
RSV infections dropped from 15.3% of weekly positive results in January 2020 to 1.4% by April and then stayed below 1% through the end of 2020. In past years, weekly positive results climbed to 3% in October and peaked at 12.5% to 16.7% in late December. Instead, RSV weekly positive results began increasing in April 2021, rising from 1.1% to 2.8% in May.
The “unusually timed” late spring increase in RSV “is probably associated with various nonpharmaceutical measures that have been in place but are now relaxing,” Dr. Olsen stated.
The RSV hospitalization rate was 0.3 per 100,000 people from October 2020 to April 2021, compared to 27.1 and 33.4 per 100,000 people in the previous 2 years. Of all RSV hospitalizations in the past year, 76.5% occurred in April-May 2021.
Rates of illness caused by the four common human coronaviruses (OC43, NL63, 229E, and HKU1) dropped from 7.5% of weekly positive results in January 2020 to 1.3% in April 2020 and stayed below 1% through February 2021. Then they climbed to 6.6% by May 2021. Infection rates of parainfluenza viruses types 1-4 similarly dropped from 2.6% in January 2020 to 1% in March 2020 and stayed below 1% until April 2021. Since then, rates of the common coronaviruses increased to 6.6% and parainfluenza viruses to 10.9% in May 2021.
Normally, parainfluenza viruses peak in October-November and May-June, so “the current increase could represent a return to prepandemic seasonality,” the authors write.
Human pneumoviruses’ weekly positive results initially increased from 4.2% in January 2020 to 7% in March and then fell to 1.9% the second week of April and remained below 1% through May 2021. In typical years, these viruses peak from 6.2% to 7.7% in March-April. Respiratory adenovirus activity similarly dropped to historically low levels in April 2021 and then began increasing to reach 3% by May 2021, the usual level for that month.
“The different circulation patterns observed across respiratory viruses probably also reflect differences in the virus transmission routes and how effective various nonpharmaceutical measures are at stopping transmission,” Dr. Olsen said in an interview. “As pandemic mitigation measures continue to be adjusted, we expect to see more changes in the circulation of these viruses, including a return to prepandemic circulation, as seen for rhinoviruses and enteroviruses.”
Rhinovirus and enterovirus rates dropped from 14.9% in March 2020 to 3.2% in May – lower than typical – and then climbed to a peak in October 2020. The peak (21.7% weekly positive results) was, however, still lower than the usual median of 32.8%. After dropping to 9.9% in January 2021, it then rose 19.1% in May, potentially reflecting “the usual spring peak that has occurred in previous years,” the authors write.
The authors note that it’s not yet clear how the COVID-19 pandemic and related mitigation measures will continue to affect respiratory virus circulation.
The authors hypothesize that the reasons for a seeming return to seasonal activity of respiratory adenoviruses, rhinoviruses, and enteroviruses could involve “different transmission mechanisms, the role of asymptomatic transmission, and prolonged survival of these nonenveloped viruses on surfaces, all of which might make these viruses less susceptible to nonpharmaceutical interventions.”
Dr. Brewer, of UCLA, agreed.
All the viruses basically “flatline except for adenoviruses and enteroviruses, and they behave a little differently in terms of how they spread,” he said. “Enteroviruses are much more likely to be fecal-oral spread than the other viruses [in the study].”
The delayed circulation of parainfluenza and human coronaviruses may have resulted from suspension of in-person classes through late winter 2020, they write, but that doesn’t explain the relative absence of pneumovirus activity, which usually affects the same young pediatric populations as RSV.
Dr. Brewer said California is seeing a surge of RSV right now, as are many states, especially throughout in the South. He’s not surprised by RSV’s deferred season, because those most affected – children younger than 2 years – are less likely to wear masks now and were “not going to daycare, not being out in public” in 2020. “As people are doing more activities, that’s probably why RSV has been starting to go up since April,” he said.
Despite the fact that, unlike many East Asian cultures, the United States has not traditionally been a mask-wearing culture, Dr. Brewer wouldn’t be surprised if more Americans begin wearing masks during flu season. “Hopefully another thing that will come out of this is better hand hygiene, with people just getting used to washing their hands more, particularly after they come home from being out,” he added.
Dr. Brewer similarly emphasized the importance of flu vaccination for the upcoming season, especially for younger children who may have poorer natural immunity to influenza, owing to its low circulation rates in 2020-2021.
The study was funded by the CDC. Dr. Brewer and Dr. Olsen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Nonpharmaceutical interventions, such as masking, staying home, limiting travel, and social distancing, have been doing more than reducing the risk for COVID-19. They’re also having an impact on infection rates and the timing of seasonal surges of other common respiratory diseases, according to an article published July 23 in Morbidity and Mortality Weekly Report.
Typically, respiratory pathogens such as respiratory syncytial virus (RSV), common cold coronaviruses, parainfluenza viruses, and respiratory adenoviruses increase in the fall and remain high throughout winter, following the same basic patterns as influenza. Although the historically low rates of influenza remained low into spring 2021, that’s not the case for several other common respiratory viruses.
“Clinicians should be aware of increases in some respiratory virus activity and remain vigilant for off-season increases,” wrote Sonja J. Olsen, PhD, and her colleagues at the Centers for Disease Control and Prevention. She told this news organization that clinicians should use multipathogen testing to help guide treatment.
The authors also underscore the importance of fall influenza vaccination campaigns for anyone aged 6 months or older.
Timothy Brewer, MD, MPH, a professor of medicine in the Division of Infectious Diseases at the University of California, Los Angeles (UCLA), and of epidemiology at the UCLA Fielding School of Public Health, agreed that it’s important for health care professionals to consider off-season illnesses in their patients.
“Practitioners should be aware that if they see a sick child in the summer, outside of what normally might be influenza season, but they look like they have influenza, consider potentially influenza and test for it, because it might be possible that we may have disrupted that natural pattern,” Dr. Brewer told this news organization. Dr. Brewer, who was not involved in the CDC research, said it’s also “critically important” to encourage influenza vaccination as the season approaches.
The CDC researchers used the U.S. World Health Organization Collaborating Laboratories System and the CDC’s National Respiratory and Enteric Virus Surveillance System to analyze virologic data from Oct. 3, 2020, to May 22, 2021, for influenza and Jan. 4, 2020, to May 22, 2021, for other respiratory viruses. The authors compared virus circulation during these periods to circulation during the same dates from four previous years.
Data to calculate influenza and RSV hospitalization rates came from the Influenza Hospitalization Surveillance Network and RSV Hospitalization Surveillance Network.
The authors report that flu activity dropped dramatically in March 2020 to its lowest levels since 1997, the earliest season for which data are available. Only 0.2% of more than 1 million specimens tested positive for influenza; the rate of hospitalizations for lab-confirmed flu was 0.8 per 100,000 people. Flu levels remained low through the summer, fall, and on to May 2021.
A potential drawback to this low activity, however, is a more prevalent and severe upcoming flu season, the authors write. The repeated exposure to flu viruses every year often “does not lead to illness, but it does serve to boost our immune response to influenza viruses,” Dr. Olsen said in an interview. “The absence of influenza viruses in the community over the last year means that we are not getting these regular boosts to our immune system. When we finally get exposed, our body may mount a weak response, and this could mean we develop a more clinically severe illness.”
Children are most susceptible to that phenomenon because they haven’t had a lifetime of exposure to flu viruses, Dr. Olsen said.
“An immunologically naive child may be more likely to develop a severe illness than someone who has lived through several influenza seasons,” she said. “This is why it is especially important for everyone 6 months and older to get vaccinated against influenza this season.”
Rhinovirus and enterovirus infections rebounded fairly quickly after their decline in March 2020 and started increasing in May 2020 until they reached “near prepandemic seasonal levels,” the authors write.
RSV infections dropped from 15.3% of weekly positive results in January 2020 to 1.4% by April and then stayed below 1% through the end of 2020. In past years, weekly positive results climbed to 3% in October and peaked at 12.5% to 16.7% in late December. Instead, RSV weekly positive results began increasing in April 2021, rising from 1.1% to 2.8% in May.
The “unusually timed” late spring increase in RSV “is probably associated with various nonpharmaceutical measures that have been in place but are now relaxing,” Dr. Olsen stated.
The RSV hospitalization rate was 0.3 per 100,000 people from October 2020 to April 2021, compared to 27.1 and 33.4 per 100,000 people in the previous 2 years. Of all RSV hospitalizations in the past year, 76.5% occurred in April-May 2021.
Rates of illness caused by the four common human coronaviruses (OC43, NL63, 229E, and HKU1) dropped from 7.5% of weekly positive results in January 2020 to 1.3% in April 2020 and stayed below 1% through February 2021. Then they climbed to 6.6% by May 2021. Infection rates of parainfluenza viruses types 1-4 similarly dropped from 2.6% in January 2020 to 1% in March 2020 and stayed below 1% until April 2021. Since then, rates of the common coronaviruses increased to 6.6% and parainfluenza viruses to 10.9% in May 2021.
Normally, parainfluenza viruses peak in October-November and May-June, so “the current increase could represent a return to prepandemic seasonality,” the authors write.
Human pneumoviruses’ weekly positive results initially increased from 4.2% in January 2020 to 7% in March and then fell to 1.9% the second week of April and remained below 1% through May 2021. In typical years, these viruses peak from 6.2% to 7.7% in March-April. Respiratory adenovirus activity similarly dropped to historically low levels in April 2021 and then began increasing to reach 3% by May 2021, the usual level for that month.
“The different circulation patterns observed across respiratory viruses probably also reflect differences in the virus transmission routes and how effective various nonpharmaceutical measures are at stopping transmission,” Dr. Olsen said in an interview. “As pandemic mitigation measures continue to be adjusted, we expect to see more changes in the circulation of these viruses, including a return to prepandemic circulation, as seen for rhinoviruses and enteroviruses.”
Rhinovirus and enterovirus rates dropped from 14.9% in March 2020 to 3.2% in May – lower than typical – and then climbed to a peak in October 2020. The peak (21.7% weekly positive results) was, however, still lower than the usual median of 32.8%. After dropping to 9.9% in January 2021, it then rose 19.1% in May, potentially reflecting “the usual spring peak that has occurred in previous years,” the authors write.
The authors note that it’s not yet clear how the COVID-19 pandemic and related mitigation measures will continue to affect respiratory virus circulation.
The authors hypothesize that the reasons for a seeming return to seasonal activity of respiratory adenoviruses, rhinoviruses, and enteroviruses could involve “different transmission mechanisms, the role of asymptomatic transmission, and prolonged survival of these nonenveloped viruses on surfaces, all of which might make these viruses less susceptible to nonpharmaceutical interventions.”
Dr. Brewer, of UCLA, agreed.
All the viruses basically “flatline except for adenoviruses and enteroviruses, and they behave a little differently in terms of how they spread,” he said. “Enteroviruses are much more likely to be fecal-oral spread than the other viruses [in the study].”
The delayed circulation of parainfluenza and human coronaviruses may have resulted from suspension of in-person classes through late winter 2020, they write, but that doesn’t explain the relative absence of pneumovirus activity, which usually affects the same young pediatric populations as RSV.
Dr. Brewer said California is seeing a surge of RSV right now, as are many states, especially throughout in the South. He’s not surprised by RSV’s deferred season, because those most affected – children younger than 2 years – are less likely to wear masks now and were “not going to daycare, not being out in public” in 2020. “As people are doing more activities, that’s probably why RSV has been starting to go up since April,” he said.
Despite the fact that, unlike many East Asian cultures, the United States has not traditionally been a mask-wearing culture, Dr. Brewer wouldn’t be surprised if more Americans begin wearing masks during flu season. “Hopefully another thing that will come out of this is better hand hygiene, with people just getting used to washing their hands more, particularly after they come home from being out,” he added.
Dr. Brewer similarly emphasized the importance of flu vaccination for the upcoming season, especially for younger children who may have poorer natural immunity to influenza, owing to its low circulation rates in 2020-2021.
The study was funded by the CDC. Dr. Brewer and Dr. Olsen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
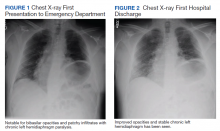
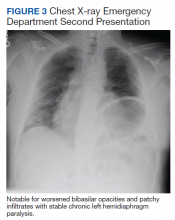
Myocarditis in adolescents after COVID-19 vaccine typically mild
Adolescents can develop mild myocarditis as a rare complication after COVID-19 vaccination, as has been reported in adults, an early case series from Boston confirms.
The adolescents who developed heart inflammation after vaccination typically had a benign course, with symptoms resolving without treatment, although one patient had persistent borderline low left ventricular (LV) function, report Audrey Dionne, MD, and colleagues at Boston Children’s Hospital.
“Despite the risks of myocarditis associated with vaccination, the benefits of vaccination likely outweigh risks in children and adolescents,” they say.
They estimate that for males 12-29 years of age COVID-19 vaccination prevents 11,000 COVID-19 cases, 560 hospitalizations, 138 intensive care unit admissions, and six deaths, compared with 39-47 expected myocarditis cases.
The case series was published online Aug. 10 in JAMA Cardiology.
Long-term risks unknown
Dr. Dionne and colleagues reviewed the results of comprehensive cardiac imaging in 14 boys and 1 girl, 12-18 years of age (median, 15 years), who were hospitalized with myocarditis after receiving the Pfizer-BioNTech messenger RNA COVID-19 vaccine.
Symptoms started 1-6 days after vaccine administration (most after the second dose) and included chest pain in all 15 patients, fever in 10 (67%), myalgia in eight (53%), and headache in six (40%).
On admission, all patients had elevated troponin levels (median, 0.25 ng/mL; range, 0.08-3.15 ng/mL). Troponin levels peaked 0.1-2.3 days after admission.
Echocardiography revealed decreased LV ejection fraction (EF) in three patients (20%) and abnormal global longitudinal or circumferential strain in five patients (33%). No patient had a pericardial effusion.
Cardiac MRI findings were consistent with myocarditis in 13 patients (87%), including late gadolinium enhancement in 12 (80%), regional hyperintensity on T2-weighted imaging in two (13%), elevated extracellular volume fraction in three (20%), and elevated LV global native T1 in two (20%).
The patients remained in the hospital for 1-5 days (median, 2 days) and were discharged. No patient required admission to the intensive care unit.
In follow-up assessments performed 1-13 days after hospital discharge, symptoms of myocarditis had resolved in 11 patients (73%).
One patient (7%) had persistent borderline low LV systolic function on echocardiogram (LVEF, 54%).
Troponin levels remained mildly elevated in three patients (20%). One patient (7%) had nonsustained ventricular tachycardia on ambulatory monitor.
The authors say longitudinal studies of patients with myocarditis after COVID-19 vaccine “will be important to better understand long-term risks.”
In a statement from the UK nonprofit Science Media Centre, Peter Openshaw, FMedSci, Imperial College London, says: “The problem with case series of this type is the lack of comparison groups. How many cases of myocarditis might be seen in normal children, or those given other vaccines (including those that are not for COVID), or in teenagers infected with SARS-CoV-2?”
“As the authors note, myocarditis does happen after other vaccines. The estimated rate (62.8 cases per million) makes this a rare event,” Dr. Openshaw says.
“My view that teenagers should be considered for vaccination is not changed by this new publication,” he adds.
This study was funded by the McCance Foundation. The authors have declared no relevant conflicts of interest. Dr. Openshaw has served on scientific advisory boards for Janssen/J&J, Oxford Immunotech, GSK, Nestle, and Pfizer in relation to immunity to viruses (fees paid to Imperial College London).
A version of this article first appeared on Medscape.com.
Adolescents can develop mild myocarditis as a rare complication after COVID-19 vaccination, as has been reported in adults, an early case series from Boston confirms.
The adolescents who developed heart inflammation after vaccination typically had a benign course, with symptoms resolving without treatment, although one patient had persistent borderline low left ventricular (LV) function, report Audrey Dionne, MD, and colleagues at Boston Children’s Hospital.
“Despite the risks of myocarditis associated with vaccination, the benefits of vaccination likely outweigh risks in children and adolescents,” they say.
They estimate that for males 12-29 years of age COVID-19 vaccination prevents 11,000 COVID-19 cases, 560 hospitalizations, 138 intensive care unit admissions, and six deaths, compared with 39-47 expected myocarditis cases.
The case series was published online Aug. 10 in JAMA Cardiology.
Long-term risks unknown
Dr. Dionne and colleagues reviewed the results of comprehensive cardiac imaging in 14 boys and 1 girl, 12-18 years of age (median, 15 years), who were hospitalized with myocarditis after receiving the Pfizer-BioNTech messenger RNA COVID-19 vaccine.
Symptoms started 1-6 days after vaccine administration (most after the second dose) and included chest pain in all 15 patients, fever in 10 (67%), myalgia in eight (53%), and headache in six (40%).
On admission, all patients had elevated troponin levels (median, 0.25 ng/mL; range, 0.08-3.15 ng/mL). Troponin levels peaked 0.1-2.3 days after admission.
Echocardiography revealed decreased LV ejection fraction (EF) in three patients (20%) and abnormal global longitudinal or circumferential strain in five patients (33%). No patient had a pericardial effusion.
Cardiac MRI findings were consistent with myocarditis in 13 patients (87%), including late gadolinium enhancement in 12 (80%), regional hyperintensity on T2-weighted imaging in two (13%), elevated extracellular volume fraction in three (20%), and elevated LV global native T1 in two (20%).
The patients remained in the hospital for 1-5 days (median, 2 days) and were discharged. No patient required admission to the intensive care unit.
In follow-up assessments performed 1-13 days after hospital discharge, symptoms of myocarditis had resolved in 11 patients (73%).
One patient (7%) had persistent borderline low LV systolic function on echocardiogram (LVEF, 54%).
Troponin levels remained mildly elevated in three patients (20%). One patient (7%) had nonsustained ventricular tachycardia on ambulatory monitor.
The authors say longitudinal studies of patients with myocarditis after COVID-19 vaccine “will be important to better understand long-term risks.”
In a statement from the UK nonprofit Science Media Centre, Peter Openshaw, FMedSci, Imperial College London, says: “The problem with case series of this type is the lack of comparison groups. How many cases of myocarditis might be seen in normal children, or those given other vaccines (including those that are not for COVID), or in teenagers infected with SARS-CoV-2?”
“As the authors note, myocarditis does happen after other vaccines. The estimated rate (62.8 cases per million) makes this a rare event,” Dr. Openshaw says.
“My view that teenagers should be considered for vaccination is not changed by this new publication,” he adds.
This study was funded by the McCance Foundation. The authors have declared no relevant conflicts of interest. Dr. Openshaw has served on scientific advisory boards for Janssen/J&J, Oxford Immunotech, GSK, Nestle, and Pfizer in relation to immunity to viruses (fees paid to Imperial College London).
A version of this article first appeared on Medscape.com.
Adolescents can develop mild myocarditis as a rare complication after COVID-19 vaccination, as has been reported in adults, an early case series from Boston confirms.
The adolescents who developed heart inflammation after vaccination typically had a benign course, with symptoms resolving without treatment, although one patient had persistent borderline low left ventricular (LV) function, report Audrey Dionne, MD, and colleagues at Boston Children’s Hospital.
“Despite the risks of myocarditis associated with vaccination, the benefits of vaccination likely outweigh risks in children and adolescents,” they say.
They estimate that for males 12-29 years of age COVID-19 vaccination prevents 11,000 COVID-19 cases, 560 hospitalizations, 138 intensive care unit admissions, and six deaths, compared with 39-47 expected myocarditis cases.
The case series was published online Aug. 10 in JAMA Cardiology.
Long-term risks unknown
Dr. Dionne and colleagues reviewed the results of comprehensive cardiac imaging in 14 boys and 1 girl, 12-18 years of age (median, 15 years), who were hospitalized with myocarditis after receiving the Pfizer-BioNTech messenger RNA COVID-19 vaccine.
Symptoms started 1-6 days after vaccine administration (most after the second dose) and included chest pain in all 15 patients, fever in 10 (67%), myalgia in eight (53%), and headache in six (40%).
On admission, all patients had elevated troponin levels (median, 0.25 ng/mL; range, 0.08-3.15 ng/mL). Troponin levels peaked 0.1-2.3 days after admission.
Echocardiography revealed decreased LV ejection fraction (EF) in three patients (20%) and abnormal global longitudinal or circumferential strain in five patients (33%). No patient had a pericardial effusion.
Cardiac MRI findings were consistent with myocarditis in 13 patients (87%), including late gadolinium enhancement in 12 (80%), regional hyperintensity on T2-weighted imaging in two (13%), elevated extracellular volume fraction in three (20%), and elevated LV global native T1 in two (20%).
The patients remained in the hospital for 1-5 days (median, 2 days) and were discharged. No patient required admission to the intensive care unit.
In follow-up assessments performed 1-13 days after hospital discharge, symptoms of myocarditis had resolved in 11 patients (73%).
One patient (7%) had persistent borderline low LV systolic function on echocardiogram (LVEF, 54%).
Troponin levels remained mildly elevated in three patients (20%). One patient (7%) had nonsustained ventricular tachycardia on ambulatory monitor.
The authors say longitudinal studies of patients with myocarditis after COVID-19 vaccine “will be important to better understand long-term risks.”
In a statement from the UK nonprofit Science Media Centre, Peter Openshaw, FMedSci, Imperial College London, says: “The problem with case series of this type is the lack of comparison groups. How many cases of myocarditis might be seen in normal children, or those given other vaccines (including those that are not for COVID), or in teenagers infected with SARS-CoV-2?”
“As the authors note, myocarditis does happen after other vaccines. The estimated rate (62.8 cases per million) makes this a rare event,” Dr. Openshaw says.
“My view that teenagers should be considered for vaccination is not changed by this new publication,” he adds.
This study was funded by the McCance Foundation. The authors have declared no relevant conflicts of interest. Dr. Openshaw has served on scientific advisory boards for Janssen/J&J, Oxford Immunotech, GSK, Nestle, and Pfizer in relation to immunity to viruses (fees paid to Imperial College London).
A version of this article first appeared on Medscape.com.
Major musculoskeletal surgery in children with medically complex conditions
A review of the International Committee’s guide
The International Committee on Perioperative Care for Children with Medical Complexity developed an online guide, “Deciding on and Preparing for Major Musculoskeletal Surgery in Children with Cerebral Palsy, Neurodevelopmental Disorders, and Other Medically Complex Conditions,” published on Dec. 20, 2020, detailing how to prepare pediatric patients with medical complexity prior to musculoskeletal surgery. The guide was developed from a dearth of information regarding optimal care practices for these patients.
The multidisciplinary committee included members from orthopedic surgery, general pediatrics, pediatric hospital medicine, anesthesiology, critical care medicine, pain medicine, physiotherapy, developmental and behavioral pediatrics, and families of children with cerebral palsy. Mirna Giordano, MD, FAAP, FHM, associate professor of pediatrics at Columbia University, New York, and International Committee member, helped develop these recommendations to “improve quality of care in the perioperative period for children with medical complexities and neurodisabilities all over the world.”
The guide meticulously details the steps required to successfully prepare for an operation and postoperative recovery. It includes an algorithm and comprehensive assessment plan that can be implemented to assess and optimize the child’s health and wellbeing prior to surgery. It encourages shared decision making and highlights the need for ongoing, open communication between providers, patients, and families to set goals and expectations, discuss potential complications, and describe outcomes and the recovery process.
The module elaborates on several key factors that must be evaluated and addressed long before surgery to ensure success. Baseline nutrition is critical and must be evaluated with body composition and anthropometric measurements. Respiratory health must be assessed with consideration of pulmonology consultation, specific testing, and ventilator or assistive-device optimization. Moreover, children with innate muscular weakness or restrictive lung disease should have baseline physiology evaluated in anticipation of potential postoperative complications, including atelectasis, hypoventilation, and pneumonia. Coexisting chronic medical conditions must also be optimized in anticipation of expected deviations from baseline.
In anticipation of peri- and postoperative care, the medical team should also be aware of details surrounding patients’ indwelling medical devices, such as cardiac implantable devices and tracheostomies. Particular attention should be paid to baclofen pumps, as malfunction or mistitration can lead to periprocedural hypotension or withdrawal.
Of paramount importance is understanding how the child appears and responds when in pain or discomfort, especially for a child with limited verbal communication. The module provides pain assessment tools, tailored to verbal and nonverbal patients in both the inpatient and outpatient settings. The module also shares guidance on establishing communication and goals with the family and within the care team on how the child appears when in distress and how he/she/they respond to pain medications. The pain plan should encompass both pharmacologic and nonpharmacologic therapeutics. Furthermore, as pain and discomfort may present from multiple sources, not limited to the regions involved in the procedure, understanding how the child responds to urinary retention, constipation, dyspnea, and uncomfortable positions is important to care. Postoperative immobilization must also be addressed as it may lead to pressure injury, manifesting as behavioral changes.
The module also presents laboratory testing as part of the preoperative health assessment. It details the utility or lack thereof of several common practices and provides recommendations on components that should be part of each patient’s assessment. It also contains videos showcased from the Courage Parents Network on family and provider perceptions of spinal fusion.
Family and social assessments must not be neglected prior to surgery, as these areas may also affect surgical outcomes. The module shares several screening tools that care team members can use to screen for family and social issues. Challenges to discharge planning are also discussed, including how to approach transportation, medical equipment, and school transitions needs.
The module is available for review in OPEN Pediatrics (www.openpediatrics.org), an online community for pediatric health professionals who share peer-reviewed best practices. “Our aim is to disseminate the recommendations as widely as possible to bring about the maximum good to the most,” Dr. Giordano said. The International Committee on Perioperative Care for Children with Medical Complexity is planning further guides regarding perioperative care, particularly for intraoperative and postoperative considerations.
Dr. Tantoco is a med-peds hospitalist at Northwestern Memorial Hospital and Ann & Robert H. Lurie Children’s Hospital of Chicago, and instructor of medicine (hospital medicine) and pediatrics in Northwestern University, in Chicago. She is also a member of the SHM Pediatrics Special Interest Group Executive Committee. Dr. Bhasin is a med-peds hospitalist at Northwestern Memorial Hospital and Ann & Robert H. Lurie Children’s Hospital, and assistant professor of medicine (hospital medicine) and pediatrics in Northwestern University.
A review of the International Committee’s guide
A review of the International Committee’s guide
The International Committee on Perioperative Care for Children with Medical Complexity developed an online guide, “Deciding on and Preparing for Major Musculoskeletal Surgery in Children with Cerebral Palsy, Neurodevelopmental Disorders, and Other Medically Complex Conditions,” published on Dec. 20, 2020, detailing how to prepare pediatric patients with medical complexity prior to musculoskeletal surgery. The guide was developed from a dearth of information regarding optimal care practices for these patients.
The multidisciplinary committee included members from orthopedic surgery, general pediatrics, pediatric hospital medicine, anesthesiology, critical care medicine, pain medicine, physiotherapy, developmental and behavioral pediatrics, and families of children with cerebral palsy. Mirna Giordano, MD, FAAP, FHM, associate professor of pediatrics at Columbia University, New York, and International Committee member, helped develop these recommendations to “improve quality of care in the perioperative period for children with medical complexities and neurodisabilities all over the world.”
The guide meticulously details the steps required to successfully prepare for an operation and postoperative recovery. It includes an algorithm and comprehensive assessment plan that can be implemented to assess and optimize the child’s health and wellbeing prior to surgery. It encourages shared decision making and highlights the need for ongoing, open communication between providers, patients, and families to set goals and expectations, discuss potential complications, and describe outcomes and the recovery process.
The module elaborates on several key factors that must be evaluated and addressed long before surgery to ensure success. Baseline nutrition is critical and must be evaluated with body composition and anthropometric measurements. Respiratory health must be assessed with consideration of pulmonology consultation, specific testing, and ventilator or assistive-device optimization. Moreover, children with innate muscular weakness or restrictive lung disease should have baseline physiology evaluated in anticipation of potential postoperative complications, including atelectasis, hypoventilation, and pneumonia. Coexisting chronic medical conditions must also be optimized in anticipation of expected deviations from baseline.
In anticipation of peri- and postoperative care, the medical team should also be aware of details surrounding patients’ indwelling medical devices, such as cardiac implantable devices and tracheostomies. Particular attention should be paid to baclofen pumps, as malfunction or mistitration can lead to periprocedural hypotension or withdrawal.
Of paramount importance is understanding how the child appears and responds when in pain or discomfort, especially for a child with limited verbal communication. The module provides pain assessment tools, tailored to verbal and nonverbal patients in both the inpatient and outpatient settings. The module also shares guidance on establishing communication and goals with the family and within the care team on how the child appears when in distress and how he/she/they respond to pain medications. The pain plan should encompass both pharmacologic and nonpharmacologic therapeutics. Furthermore, as pain and discomfort may present from multiple sources, not limited to the regions involved in the procedure, understanding how the child responds to urinary retention, constipation, dyspnea, and uncomfortable positions is important to care. Postoperative immobilization must also be addressed as it may lead to pressure injury, manifesting as behavioral changes.
The module also presents laboratory testing as part of the preoperative health assessment. It details the utility or lack thereof of several common practices and provides recommendations on components that should be part of each patient’s assessment. It also contains videos showcased from the Courage Parents Network on family and provider perceptions of spinal fusion.
Family and social assessments must not be neglected prior to surgery, as these areas may also affect surgical outcomes. The module shares several screening tools that care team members can use to screen for family and social issues. Challenges to discharge planning are also discussed, including how to approach transportation, medical equipment, and school transitions needs.
The module is available for review in OPEN Pediatrics (www.openpediatrics.org), an online community for pediatric health professionals who share peer-reviewed best practices. “Our aim is to disseminate the recommendations as widely as possible to bring about the maximum good to the most,” Dr. Giordano said. The International Committee on Perioperative Care for Children with Medical Complexity is planning further guides regarding perioperative care, particularly for intraoperative and postoperative considerations.
Dr. Tantoco is a med-peds hospitalist at Northwestern Memorial Hospital and Ann & Robert H. Lurie Children’s Hospital of Chicago, and instructor of medicine (hospital medicine) and pediatrics in Northwestern University, in Chicago. She is also a member of the SHM Pediatrics Special Interest Group Executive Committee. Dr. Bhasin is a med-peds hospitalist at Northwestern Memorial Hospital and Ann & Robert H. Lurie Children’s Hospital, and assistant professor of medicine (hospital medicine) and pediatrics in Northwestern University.
The International Committee on Perioperative Care for Children with Medical Complexity developed an online guide, “Deciding on and Preparing for Major Musculoskeletal Surgery in Children with Cerebral Palsy, Neurodevelopmental Disorders, and Other Medically Complex Conditions,” published on Dec. 20, 2020, detailing how to prepare pediatric patients with medical complexity prior to musculoskeletal surgery. The guide was developed from a dearth of information regarding optimal care practices for these patients.
The multidisciplinary committee included members from orthopedic surgery, general pediatrics, pediatric hospital medicine, anesthesiology, critical care medicine, pain medicine, physiotherapy, developmental and behavioral pediatrics, and families of children with cerebral palsy. Mirna Giordano, MD, FAAP, FHM, associate professor of pediatrics at Columbia University, New York, and International Committee member, helped develop these recommendations to “improve quality of care in the perioperative period for children with medical complexities and neurodisabilities all over the world.”
The guide meticulously details the steps required to successfully prepare for an operation and postoperative recovery. It includes an algorithm and comprehensive assessment plan that can be implemented to assess and optimize the child’s health and wellbeing prior to surgery. It encourages shared decision making and highlights the need for ongoing, open communication between providers, patients, and families to set goals and expectations, discuss potential complications, and describe outcomes and the recovery process.
The module elaborates on several key factors that must be evaluated and addressed long before surgery to ensure success. Baseline nutrition is critical and must be evaluated with body composition and anthropometric measurements. Respiratory health must be assessed with consideration of pulmonology consultation, specific testing, and ventilator or assistive-device optimization. Moreover, children with innate muscular weakness or restrictive lung disease should have baseline physiology evaluated in anticipation of potential postoperative complications, including atelectasis, hypoventilation, and pneumonia. Coexisting chronic medical conditions must also be optimized in anticipation of expected deviations from baseline.
In anticipation of peri- and postoperative care, the medical team should also be aware of details surrounding patients’ indwelling medical devices, such as cardiac implantable devices and tracheostomies. Particular attention should be paid to baclofen pumps, as malfunction or mistitration can lead to periprocedural hypotension or withdrawal.
Of paramount importance is understanding how the child appears and responds when in pain or discomfort, especially for a child with limited verbal communication. The module provides pain assessment tools, tailored to verbal and nonverbal patients in both the inpatient and outpatient settings. The module also shares guidance on establishing communication and goals with the family and within the care team on how the child appears when in distress and how he/she/they respond to pain medications. The pain plan should encompass both pharmacologic and nonpharmacologic therapeutics. Furthermore, as pain and discomfort may present from multiple sources, not limited to the regions involved in the procedure, understanding how the child responds to urinary retention, constipation, dyspnea, and uncomfortable positions is important to care. Postoperative immobilization must also be addressed as it may lead to pressure injury, manifesting as behavioral changes.
The module also presents laboratory testing as part of the preoperative health assessment. It details the utility or lack thereof of several common practices and provides recommendations on components that should be part of each patient’s assessment. It also contains videos showcased from the Courage Parents Network on family and provider perceptions of spinal fusion.
Family and social assessments must not be neglected prior to surgery, as these areas may also affect surgical outcomes. The module shares several screening tools that care team members can use to screen for family and social issues. Challenges to discharge planning are also discussed, including how to approach transportation, medical equipment, and school transitions needs.
The module is available for review in OPEN Pediatrics (www.openpediatrics.org), an online community for pediatric health professionals who share peer-reviewed best practices. “Our aim is to disseminate the recommendations as widely as possible to bring about the maximum good to the most,” Dr. Giordano said. The International Committee on Perioperative Care for Children with Medical Complexity is planning further guides regarding perioperative care, particularly for intraoperative and postoperative considerations.
Dr. Tantoco is a med-peds hospitalist at Northwestern Memorial Hospital and Ann & Robert H. Lurie Children’s Hospital of Chicago, and instructor of medicine (hospital medicine) and pediatrics in Northwestern University, in Chicago. She is also a member of the SHM Pediatrics Special Interest Group Executive Committee. Dr. Bhasin is a med-peds hospitalist at Northwestern Memorial Hospital and Ann & Robert H. Lurie Children’s Hospital, and assistant professor of medicine (hospital medicine) and pediatrics in Northwestern University.
Surge of new child COVID cases continues for 6th consecutive week
The current COVID-19 surge has brought new cases in children to their highest level since February, according to a new report.
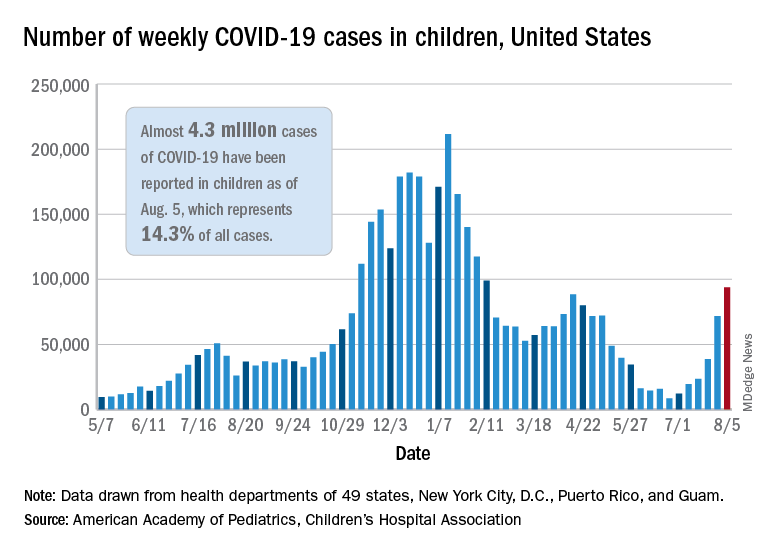
New pediatric cases rose for the 6th straight week, with almost 94,000 reported for the week ending Aug. 5.
That weekly total was up by 31% over the previous week and by over 1,000% since late June, when the new-case figure was at its lowest point (8,447) since early in the pandemic, the American Academy of Pediatrics and the Children’s Hospital Association said. COVID-related deaths – 13 for the week – were also higher than at any time since March 2021.
Almost 4.3 million children have been infected with SARS-CoV-2, which is 14.3% of all cases reported in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam. Children represented 15.0% of the new cases reported in those jurisdictions during the week ending Aug. 5, the AAP and CHA said in their weekly report.
Another measure that has been trending upward recently is vaccine initiation among 12- to 15-year-olds, although the latest weekly total is still well below the high of 1.4 million seen in May. First-time vaccinations reached almost 411,000 for the week of Aug. 3-9, marking the fourth consecutive increase in that age group, the Centers for Disease Control and Prevention said on its COVID Data Tracker. Vaccinations also increased, although more modestly, for 16- and 17-year-olds in the most recent week.
Cumulative figures for children aged 12-17 show that almost 10.4 million have received at least one dose and that 7.7 million are fully vaccinated as of Aug. 9. By age group, 42.2% of those aged 12-15 have received at least one dose, and 30.4% have completed the vaccine regimen. Among those aged 16-17 years, 52.2% have gotten their first dose, and 41.4% are fully vaccinated, according to the COVID Data Tracker.
Looking at vaccination rates on the state level shows that only 20% of children aged 12-17 in Wyoming and 21% in Mississippi have gotten at least one dose as of Aug. 4, while Massachusetts is up to 68% and Vermont reports 70%. Rates for full vaccination range from 11% in Mississippi and Alabama to 61% in Vermont, based on an AAP analysis of CDC data, which is not available for Idaho.
The current COVID-19 surge has brought new cases in children to their highest level since February, according to a new report.

New pediatric cases rose for the 6th straight week, with almost 94,000 reported for the week ending Aug. 5.
That weekly total was up by 31% over the previous week and by over 1,000% since late June, when the new-case figure was at its lowest point (8,447) since early in the pandemic, the American Academy of Pediatrics and the Children’s Hospital Association said. COVID-related deaths – 13 for the week – were also higher than at any time since March 2021.
Almost 4.3 million children have been infected with SARS-CoV-2, which is 14.3% of all cases reported in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam. Children represented 15.0% of the new cases reported in those jurisdictions during the week ending Aug. 5, the AAP and CHA said in their weekly report.
Another measure that has been trending upward recently is vaccine initiation among 12- to 15-year-olds, although the latest weekly total is still well below the high of 1.4 million seen in May. First-time vaccinations reached almost 411,000 for the week of Aug. 3-9, marking the fourth consecutive increase in that age group, the Centers for Disease Control and Prevention said on its COVID Data Tracker. Vaccinations also increased, although more modestly, for 16- and 17-year-olds in the most recent week.
Cumulative figures for children aged 12-17 show that almost 10.4 million have received at least one dose and that 7.7 million are fully vaccinated as of Aug. 9. By age group, 42.2% of those aged 12-15 have received at least one dose, and 30.4% have completed the vaccine regimen. Among those aged 16-17 years, 52.2% have gotten their first dose, and 41.4% are fully vaccinated, according to the COVID Data Tracker.
Looking at vaccination rates on the state level shows that only 20% of children aged 12-17 in Wyoming and 21% in Mississippi have gotten at least one dose as of Aug. 4, while Massachusetts is up to 68% and Vermont reports 70%. Rates for full vaccination range from 11% in Mississippi and Alabama to 61% in Vermont, based on an AAP analysis of CDC data, which is not available for Idaho.
The current COVID-19 surge has brought new cases in children to their highest level since February, according to a new report.

New pediatric cases rose for the 6th straight week, with almost 94,000 reported for the week ending Aug. 5.
That weekly total was up by 31% over the previous week and by over 1,000% since late June, when the new-case figure was at its lowest point (8,447) since early in the pandemic, the American Academy of Pediatrics and the Children’s Hospital Association said. COVID-related deaths – 13 for the week – were also higher than at any time since March 2021.
Almost 4.3 million children have been infected with SARS-CoV-2, which is 14.3% of all cases reported in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam. Children represented 15.0% of the new cases reported in those jurisdictions during the week ending Aug. 5, the AAP and CHA said in their weekly report.
Another measure that has been trending upward recently is vaccine initiation among 12- to 15-year-olds, although the latest weekly total is still well below the high of 1.4 million seen in May. First-time vaccinations reached almost 411,000 for the week of Aug. 3-9, marking the fourth consecutive increase in that age group, the Centers for Disease Control and Prevention said on its COVID Data Tracker. Vaccinations also increased, although more modestly, for 16- and 17-year-olds in the most recent week.
Cumulative figures for children aged 12-17 show that almost 10.4 million have received at least one dose and that 7.7 million are fully vaccinated as of Aug. 9. By age group, 42.2% of those aged 12-15 have received at least one dose, and 30.4% have completed the vaccine regimen. Among those aged 16-17 years, 52.2% have gotten their first dose, and 41.4% are fully vaccinated, according to the COVID Data Tracker.
Looking at vaccination rates on the state level shows that only 20% of children aged 12-17 in Wyoming and 21% in Mississippi have gotten at least one dose as of Aug. 4, while Massachusetts is up to 68% and Vermont reports 70%. Rates for full vaccination range from 11% in Mississippi and Alabama to 61% in Vermont, based on an AAP analysis of CDC data, which is not available for Idaho.
Docs fight back after losing hospital privileges, patients, and income
In April, a group of more than a dozen cardiologists at St. Louis Heart and Vascular (SLHV) lost their privileges at SSM Health, an eight-hospital system in St. Louis.
The physicians did not lose their privileges because of a clinical failure. Rather, it was because of SSM’s decision to enter into an exclusive contract with another set of cardiologists.
“The current situation is economically untenable for us,” said Harvey Serota, MD, founder and medical director of SLHV. “This is an existential threat to the practice.”
Because of the exclusive contract, many of SLHV’s patients are now being redirected to SSM-contracted cardiologists. Volume for the group’s new $15 million catheterization lab has plummeted. SLHV is suing SSM to restore its privileges, claiming lack of due process, restraint of trade, interference with its business, and breach of contract.
Losing privileges because a hospital seeks to increase their profits is becoming all too familiar for many independent specialists in fields such as cardiology, orthopedic surgery, and urology, as the hospitals that hosted them become their competitors and forge exclusive contracts with opposing groups.
What can these doctors do if they’re shut out? File a lawsuit, as SLHV has done? Demand a hearing before the medical staff and try to resolve the problem? Or simply give up their privileges and move on?
Unfortunately, none of these approaches offer a quick or certain solution, and each comes with risks.
Generally, courts have upheld hospitals’ use of exclusive contracts, which is also known as economic credentialing, says Barry F. Rosen, a health law attorney at Gordon Feinblatt, in Baltimore.
“Courts have long recognized exclusive contracts, and challenges by excluded doctors usually fail,” he says.
However, Mr. Rosen can cite several examples in which excluded doctors launched legal challenges that prevailed, owing to nuances in the law. The legal field in this area is tangled, and it varies by state.
Can hospitals make exclusive deals?
Hospitals have long used exclusive contracts for hospital-based specialists – anesthesiologists, radiologists, pathologists, emergency physicians, and hospitalists. They say that restricting patients to one group of anesthesiologists or radiologists enhances operational efficiency and that these contracts do not disrupt patients, because patients have no ties to hospital-based physicians. Such contracts are often more profitable for the hospital because of the negotiated rates.
Exclusive contracts in other specialties, however, are less accepted because they involve markedly different strategies and have different effects. In such cases, the hospital is no longer simply enhancing operational efficiency but is competing with physicians on staff, and the arrangement can disrupt the care of patients of the excluded doctors.
In the courts, these concerns might form the basis of an antitrust action or a claim of tortious interference with physicians’ ability to provide care for their patients, but neither claim is easy to win, Mr. Rosen says.
In antitrust cases, “the issue is not whether the excluded doctor was injured but whether the action harmed competition,” Mr. Rosen says. “Will the exclusion lead to higher prices?”
In the case of interference with patient care, “you will always find interference by one entity in the affairs of another,” he says, “but tortious interference applies to situations where something nefarious is going on, such as the other side was out to destroy your business and create a monopoly.”
Hospitals may try to restrict the privileges of physicians who invest in competing facilities such as cath labs and ambulatory surgery centers (ASCs), says Gregory Mertz, managing director of Physician Strategies Group, a consultancy in Virginia Beach.
“However, any revenge that a hospital might take against the doctors who started an ASC would usually not be publicly admitted,” Mr. Mertz says. “Revenge would be exacted in subtle ways.”
In the St. Louis situation, SSM did not cite SLHV’s cath lab as a reason for its exclusive contract. SSM stated in court documents that the decision was based on the recommendations of an expert panel. Furthermore, SSM said the board created the panel in response to a state report that cited the limited experience of some SLHV cardiologists in treating a rare type of heart attack.
Mr. Mertz says the board’s interest in the state’s concern and then its forming the special panel lent a great deal of legitimacy to SSM’s decision to start an exclusive contract. “SSM can show evidence that the board’s decision was based on a clinical matter and not on trying to squeeze out the cardiologists,” he says.
In SLHV’s defense, Dr. Serota says the practice offered to stop taking calls for the type of heart attack that was cited, but the hospital did not respond to its offer. He says SSM should have consulted the hospital’s medical staff to address the state’s concern and to create the exclusive contract, because these decisions involved clinical issues that the medical staff understands better than the board.
The law, however, does not require a hospital board to consult with its medical staff, says Alice G. Gosfield, a health care attorney in Philadelphia. “The board has ultimate legal control of everything in the hospital,” she says. However, the board often delegates certain functions to the medical staff in the hospital bylaws, and depending on the wording of the bylaws, it is still possible that the board violated the bylaws, Ms. Gosfield adds.
Can excluded physicians get peer review?
Can the hospital medical staff help restore the privileges of excluded physicians? Don’t these physicians have the right to peer review – a hearing before the medical staff?
Indeed, the Joint Commission, which accredits hospitals, states that the hospital must have “mechanisms, including a fair hearing and appeal process, for addressing adverse decisions for existing medical staff members and other individuals holding clinical privileges for renewal, revocation, or revision of clinical privileges.”
However, excluded physicians may not have a right to a hearing if they have not been fully stripped of privileges. SSM discontinued adult cardiology privileges for SLHV doctors but retained some doctors’ internal medicine privileges. Dr. Serota says internal medicine privileges are useless to cardiologists, but because the doctors’ privileges had not been fully removed, they cannot ask for a hearing.
More fundamentally, exclusive contracts are not a good fit for peer review. Mr. Rosen says the hearings were designed to review the physicians’ clinical competence or behavior, but excluded physicians do not have these problems. About all the hearing could focus on is the hospital’s policy, which the board would not want to allow. To avoid this, “the hospital might rule out a hearing as contrary to the intent of the bylaws,” Mr. Rosen says.
Furthermore, even if peer review goes forward, “what the medical staff decides is only advisory, and the hospital board makes the final decision,” Mr. Rosen says. He notes that the doctor could challenge the decision in court, but the hospital might still prevail.
Excluded physicians sometimes prevail
Although it is rare for excluded physicians to win a lawsuit against their hospital, it does happen, says Michael R. Callahan, health lawyer at Katten Muchin Rosenman, in Chicago.
Mr. Callahan cites a 2010 decision by the Arkansas Supreme Court that stopped the state’s largest health system from denying physicians’ privileges. Among other things, the hospital was found to have tortiously interfered with the physicians’ contracts with patients.
In a 2007 decision, a West Virginia court ruled that hospitals that have a mission to serve the public cannot exclude physicians for nonquality issues. In addition, some states, such as Texas, limit the economic factors that can be considered when credentialing decisions are made. Other states, such as Ohio, give hospitals a great deal of leeway to alter credentialing.
Dr. Serota is optimistic about his Missouri lawsuit. Although the judge in the case did not immediately grant SLHV’s request for restoration of privileges while the case proceeds, she did grant expedited discovery – allowing SLHV to obtain documents from SSM that could strengthen the doctors’ case – and she agreed to a hearing on SLHV’s request for a temporary restoration of privileges.
Ms. Gosfield says Dr. Serota’s optimism seems justified, but she adds that such cases cost a lot of money and that they may still not be winnable.
Often plaintiffs can settle lawsuits before they go to trial, but Mr. Callahan says hospitals are loath to restore privileges in a settlement because they don’t want to undermine an exclusivity deal. “The exclusive group expects a certain volume, which can’t be reached if the competing doctors are allowed back in,” he says.
Many physicians don’t challenge the exclusion
Quite often, excluded doctors decide not to challenge the decision. For example, Dr. Serota says groups of orthopedic surgeons and urologists have decided not to challenge similar decisions by SSM. “They wanted to move on,” he says.
Mr. Callahan says many excluded doctors also don’t even ask for a hearing. “They expect that the hospital’s decision will be upheld,” he says.
This was the case for Devendra K. Amin, MD, an independent cardiologist in Easton, Pa. Dr. Amin has not had any hospital privileges since July 2020. Even though he is board certified in interventional cardiology, which involves catheterization, Dr. Amin says he cannot perform these procedures because they can only be performed in a hospital in the area.
In the 1990s, Dr. Amin says, he had invasive cardiology privileges at five hospitals, but then those hospitals consolidated, and the remaining ones started constricting his privileges. First he could no longer work in the emergency department, then he could no longer read echocardiograms and interpret stress test results, because that work was assigned exclusively to employed doctors, he says.
Then the one remaining hospital announced that privileges would only be available to physicians by invitation, and he was not invited. Dr. Amin says he could have regained general cardiology privileges if he had accepted employment at the hospital, but he did not want to do this. A recruiter and the head of the cardiology section at the hospital even took him out to dinner 2 years ago to discuss employment, but there was a stipulation that the hospital would not agree to.
“I wanted to get back my interventional privileges back,” Dr. Amin says, “but they told me that would not be possible because they had an exclusive contract with a group.”
Dr. Amin says that now, he can only work as a general cardiologist with reduced volume. He says primary care physicians in the local hospital systems only refer to cardiologists within their systems. “When these patients do come to me, it is only because they specifically requested to see me,” Dr. Amin says.
He does not want to challenge the decisions regarding privileging. “Look, I am 68 years old,” Dr. Amin says. “I’m not retiring yet, but I don’t want to get into a battle with a hospital that has very deep pockets. I’m not a confrontational person to begin with, and I don’t want to spend the next 10 years of my life in litigation.”
Diverging expectations
The law on exclusive contracts does not provide easy answers for excluded doctors, and often it defies physicians’ conception of their own role in the hospital.
Many physicians expect the hospital to be a haven where they can do their work without being cut out by a competitor. This view is reinforced by organizations such as the American Medical Association.
The AMA Council on Medical Service states that privileges “can only be abridged upon recommendation of the medical staff and only for reason related to professional competence, adherence to standards of care, and other parameters agreed to by the medical staff.”
But the courts don’t tend to agree with that position. “Hospitals have a fiduciary duty to protect their own financial interests,” Mr. Callahan says. “This may involve anything that furthers the hospital’s mission to provide high-quality health care services to its patient community.”
At the same time, however, there are plenty of instances in which courts have ruled that exclusive contracts had gone too far. But usually it takes a lawyer experienced in these cases to know what those exceptions are.
A version of this article first appeared on Medscape.com.
In April, a group of more than a dozen cardiologists at St. Louis Heart and Vascular (SLHV) lost their privileges at SSM Health, an eight-hospital system in St. Louis.
The physicians did not lose their privileges because of a clinical failure. Rather, it was because of SSM’s decision to enter into an exclusive contract with another set of cardiologists.
“The current situation is economically untenable for us,” said Harvey Serota, MD, founder and medical director of SLHV. “This is an existential threat to the practice.”
Because of the exclusive contract, many of SLHV’s patients are now being redirected to SSM-contracted cardiologists. Volume for the group’s new $15 million catheterization lab has plummeted. SLHV is suing SSM to restore its privileges, claiming lack of due process, restraint of trade, interference with its business, and breach of contract.
Losing privileges because a hospital seeks to increase their profits is becoming all too familiar for many independent specialists in fields such as cardiology, orthopedic surgery, and urology, as the hospitals that hosted them become their competitors and forge exclusive contracts with opposing groups.
What can these doctors do if they’re shut out? File a lawsuit, as SLHV has done? Demand a hearing before the medical staff and try to resolve the problem? Or simply give up their privileges and move on?
Unfortunately, none of these approaches offer a quick or certain solution, and each comes with risks.
Generally, courts have upheld hospitals’ use of exclusive contracts, which is also known as economic credentialing, says Barry F. Rosen, a health law attorney at Gordon Feinblatt, in Baltimore.
“Courts have long recognized exclusive contracts, and challenges by excluded doctors usually fail,” he says.
However, Mr. Rosen can cite several examples in which excluded doctors launched legal challenges that prevailed, owing to nuances in the law. The legal field in this area is tangled, and it varies by state.
Can hospitals make exclusive deals?
Hospitals have long used exclusive contracts for hospital-based specialists – anesthesiologists, radiologists, pathologists, emergency physicians, and hospitalists. They say that restricting patients to one group of anesthesiologists or radiologists enhances operational efficiency and that these contracts do not disrupt patients, because patients have no ties to hospital-based physicians. Such contracts are often more profitable for the hospital because of the negotiated rates.
Exclusive contracts in other specialties, however, are less accepted because they involve markedly different strategies and have different effects. In such cases, the hospital is no longer simply enhancing operational efficiency but is competing with physicians on staff, and the arrangement can disrupt the care of patients of the excluded doctors.
In the courts, these concerns might form the basis of an antitrust action or a claim of tortious interference with physicians’ ability to provide care for their patients, but neither claim is easy to win, Mr. Rosen says.
In antitrust cases, “the issue is not whether the excluded doctor was injured but whether the action harmed competition,” Mr. Rosen says. “Will the exclusion lead to higher prices?”
In the case of interference with patient care, “you will always find interference by one entity in the affairs of another,” he says, “but tortious interference applies to situations where something nefarious is going on, such as the other side was out to destroy your business and create a monopoly.”
Hospitals may try to restrict the privileges of physicians who invest in competing facilities such as cath labs and ambulatory surgery centers (ASCs), says Gregory Mertz, managing director of Physician Strategies Group, a consultancy in Virginia Beach.
“However, any revenge that a hospital might take against the doctors who started an ASC would usually not be publicly admitted,” Mr. Mertz says. “Revenge would be exacted in subtle ways.”
In the St. Louis situation, SSM did not cite SLHV’s cath lab as a reason for its exclusive contract. SSM stated in court documents that the decision was based on the recommendations of an expert panel. Furthermore, SSM said the board created the panel in response to a state report that cited the limited experience of some SLHV cardiologists in treating a rare type of heart attack.
Mr. Mertz says the board’s interest in the state’s concern and then its forming the special panel lent a great deal of legitimacy to SSM’s decision to start an exclusive contract. “SSM can show evidence that the board’s decision was based on a clinical matter and not on trying to squeeze out the cardiologists,” he says.
In SLHV’s defense, Dr. Serota says the practice offered to stop taking calls for the type of heart attack that was cited, but the hospital did not respond to its offer. He says SSM should have consulted the hospital’s medical staff to address the state’s concern and to create the exclusive contract, because these decisions involved clinical issues that the medical staff understands better than the board.
The law, however, does not require a hospital board to consult with its medical staff, says Alice G. Gosfield, a health care attorney in Philadelphia. “The board has ultimate legal control of everything in the hospital,” she says. However, the board often delegates certain functions to the medical staff in the hospital bylaws, and depending on the wording of the bylaws, it is still possible that the board violated the bylaws, Ms. Gosfield adds.
Can excluded physicians get peer review?
Can the hospital medical staff help restore the privileges of excluded physicians? Don’t these physicians have the right to peer review – a hearing before the medical staff?
Indeed, the Joint Commission, which accredits hospitals, states that the hospital must have “mechanisms, including a fair hearing and appeal process, for addressing adverse decisions for existing medical staff members and other individuals holding clinical privileges for renewal, revocation, or revision of clinical privileges.”
However, excluded physicians may not have a right to a hearing if they have not been fully stripped of privileges. SSM discontinued adult cardiology privileges for SLHV doctors but retained some doctors’ internal medicine privileges. Dr. Serota says internal medicine privileges are useless to cardiologists, but because the doctors’ privileges had not been fully removed, they cannot ask for a hearing.
More fundamentally, exclusive contracts are not a good fit for peer review. Mr. Rosen says the hearings were designed to review the physicians’ clinical competence or behavior, but excluded physicians do not have these problems. About all the hearing could focus on is the hospital’s policy, which the board would not want to allow. To avoid this, “the hospital might rule out a hearing as contrary to the intent of the bylaws,” Mr. Rosen says.
Furthermore, even if peer review goes forward, “what the medical staff decides is only advisory, and the hospital board makes the final decision,” Mr. Rosen says. He notes that the doctor could challenge the decision in court, but the hospital might still prevail.
Excluded physicians sometimes prevail
Although it is rare for excluded physicians to win a lawsuit against their hospital, it does happen, says Michael R. Callahan, health lawyer at Katten Muchin Rosenman, in Chicago.
Mr. Callahan cites a 2010 decision by the Arkansas Supreme Court that stopped the state’s largest health system from denying physicians’ privileges. Among other things, the hospital was found to have tortiously interfered with the physicians’ contracts with patients.
In a 2007 decision, a West Virginia court ruled that hospitals that have a mission to serve the public cannot exclude physicians for nonquality issues. In addition, some states, such as Texas, limit the economic factors that can be considered when credentialing decisions are made. Other states, such as Ohio, give hospitals a great deal of leeway to alter credentialing.
Dr. Serota is optimistic about his Missouri lawsuit. Although the judge in the case did not immediately grant SLHV’s request for restoration of privileges while the case proceeds, she did grant expedited discovery – allowing SLHV to obtain documents from SSM that could strengthen the doctors’ case – and she agreed to a hearing on SLHV’s request for a temporary restoration of privileges.
Ms. Gosfield says Dr. Serota’s optimism seems justified, but she adds that such cases cost a lot of money and that they may still not be winnable.
Often plaintiffs can settle lawsuits before they go to trial, but Mr. Callahan says hospitals are loath to restore privileges in a settlement because they don’t want to undermine an exclusivity deal. “The exclusive group expects a certain volume, which can’t be reached if the competing doctors are allowed back in,” he says.
Many physicians don’t challenge the exclusion
Quite often, excluded doctors decide not to challenge the decision. For example, Dr. Serota says groups of orthopedic surgeons and urologists have decided not to challenge similar decisions by SSM. “They wanted to move on,” he says.
Mr. Callahan says many excluded doctors also don’t even ask for a hearing. “They expect that the hospital’s decision will be upheld,” he says.
This was the case for Devendra K. Amin, MD, an independent cardiologist in Easton, Pa. Dr. Amin has not had any hospital privileges since July 2020. Even though he is board certified in interventional cardiology, which involves catheterization, Dr. Amin says he cannot perform these procedures because they can only be performed in a hospital in the area.
In the 1990s, Dr. Amin says, he had invasive cardiology privileges at five hospitals, but then those hospitals consolidated, and the remaining ones started constricting his privileges. First he could no longer work in the emergency department, then he could no longer read echocardiograms and interpret stress test results, because that work was assigned exclusively to employed doctors, he says.
Then the one remaining hospital announced that privileges would only be available to physicians by invitation, and he was not invited. Dr. Amin says he could have regained general cardiology privileges if he had accepted employment at the hospital, but he did not want to do this. A recruiter and the head of the cardiology section at the hospital even took him out to dinner 2 years ago to discuss employment, but there was a stipulation that the hospital would not agree to.
“I wanted to get back my interventional privileges back,” Dr. Amin says, “but they told me that would not be possible because they had an exclusive contract with a group.”
Dr. Amin says that now, he can only work as a general cardiologist with reduced volume. He says primary care physicians in the local hospital systems only refer to cardiologists within their systems. “When these patients do come to me, it is only because they specifically requested to see me,” Dr. Amin says.
He does not want to challenge the decisions regarding privileging. “Look, I am 68 years old,” Dr. Amin says. “I’m not retiring yet, but I don’t want to get into a battle with a hospital that has very deep pockets. I’m not a confrontational person to begin with, and I don’t want to spend the next 10 years of my life in litigation.”
Diverging expectations
The law on exclusive contracts does not provide easy answers for excluded doctors, and often it defies physicians’ conception of their own role in the hospital.
Many physicians expect the hospital to be a haven where they can do their work without being cut out by a competitor. This view is reinforced by organizations such as the American Medical Association.
The AMA Council on Medical Service states that privileges “can only be abridged upon recommendation of the medical staff and only for reason related to professional competence, adherence to standards of care, and other parameters agreed to by the medical staff.”
But the courts don’t tend to agree with that position. “Hospitals have a fiduciary duty to protect their own financial interests,” Mr. Callahan says. “This may involve anything that furthers the hospital’s mission to provide high-quality health care services to its patient community.”
At the same time, however, there are plenty of instances in which courts have ruled that exclusive contracts had gone too far. But usually it takes a lawyer experienced in these cases to know what those exceptions are.
A version of this article first appeared on Medscape.com.
In April, a group of more than a dozen cardiologists at St. Louis Heart and Vascular (SLHV) lost their privileges at SSM Health, an eight-hospital system in St. Louis.
The physicians did not lose their privileges because of a clinical failure. Rather, it was because of SSM’s decision to enter into an exclusive contract with another set of cardiologists.
“The current situation is economically untenable for us,” said Harvey Serota, MD, founder and medical director of SLHV. “This is an existential threat to the practice.”
Because of the exclusive contract, many of SLHV’s patients are now being redirected to SSM-contracted cardiologists. Volume for the group’s new $15 million catheterization lab has plummeted. SLHV is suing SSM to restore its privileges, claiming lack of due process, restraint of trade, interference with its business, and breach of contract.
Losing privileges because a hospital seeks to increase their profits is becoming all too familiar for many independent specialists in fields such as cardiology, orthopedic surgery, and urology, as the hospitals that hosted them become their competitors and forge exclusive contracts with opposing groups.
What can these doctors do if they’re shut out? File a lawsuit, as SLHV has done? Demand a hearing before the medical staff and try to resolve the problem? Or simply give up their privileges and move on?
Unfortunately, none of these approaches offer a quick or certain solution, and each comes with risks.
Generally, courts have upheld hospitals’ use of exclusive contracts, which is also known as economic credentialing, says Barry F. Rosen, a health law attorney at Gordon Feinblatt, in Baltimore.
“Courts have long recognized exclusive contracts, and challenges by excluded doctors usually fail,” he says.
However, Mr. Rosen can cite several examples in which excluded doctors launched legal challenges that prevailed, owing to nuances in the law. The legal field in this area is tangled, and it varies by state.
Can hospitals make exclusive deals?
Hospitals have long used exclusive contracts for hospital-based specialists – anesthesiologists, radiologists, pathologists, emergency physicians, and hospitalists. They say that restricting patients to one group of anesthesiologists or radiologists enhances operational efficiency and that these contracts do not disrupt patients, because patients have no ties to hospital-based physicians. Such contracts are often more profitable for the hospital because of the negotiated rates.
Exclusive contracts in other specialties, however, are less accepted because they involve markedly different strategies and have different effects. In such cases, the hospital is no longer simply enhancing operational efficiency but is competing with physicians on staff, and the arrangement can disrupt the care of patients of the excluded doctors.
In the courts, these concerns might form the basis of an antitrust action or a claim of tortious interference with physicians’ ability to provide care for their patients, but neither claim is easy to win, Mr. Rosen says.
In antitrust cases, “the issue is not whether the excluded doctor was injured but whether the action harmed competition,” Mr. Rosen says. “Will the exclusion lead to higher prices?”
In the case of interference with patient care, “you will always find interference by one entity in the affairs of another,” he says, “but tortious interference applies to situations where something nefarious is going on, such as the other side was out to destroy your business and create a monopoly.”
Hospitals may try to restrict the privileges of physicians who invest in competing facilities such as cath labs and ambulatory surgery centers (ASCs), says Gregory Mertz, managing director of Physician Strategies Group, a consultancy in Virginia Beach.
“However, any revenge that a hospital might take against the doctors who started an ASC would usually not be publicly admitted,” Mr. Mertz says. “Revenge would be exacted in subtle ways.”
In the St. Louis situation, SSM did not cite SLHV’s cath lab as a reason for its exclusive contract. SSM stated in court documents that the decision was based on the recommendations of an expert panel. Furthermore, SSM said the board created the panel in response to a state report that cited the limited experience of some SLHV cardiologists in treating a rare type of heart attack.
Mr. Mertz says the board’s interest in the state’s concern and then its forming the special panel lent a great deal of legitimacy to SSM’s decision to start an exclusive contract. “SSM can show evidence that the board’s decision was based on a clinical matter and not on trying to squeeze out the cardiologists,” he says.
In SLHV’s defense, Dr. Serota says the practice offered to stop taking calls for the type of heart attack that was cited, but the hospital did not respond to its offer. He says SSM should have consulted the hospital’s medical staff to address the state’s concern and to create the exclusive contract, because these decisions involved clinical issues that the medical staff understands better than the board.
The law, however, does not require a hospital board to consult with its medical staff, says Alice G. Gosfield, a health care attorney in Philadelphia. “The board has ultimate legal control of everything in the hospital,” she says. However, the board often delegates certain functions to the medical staff in the hospital bylaws, and depending on the wording of the bylaws, it is still possible that the board violated the bylaws, Ms. Gosfield adds.
Can excluded physicians get peer review?
Can the hospital medical staff help restore the privileges of excluded physicians? Don’t these physicians have the right to peer review – a hearing before the medical staff?
Indeed, the Joint Commission, which accredits hospitals, states that the hospital must have “mechanisms, including a fair hearing and appeal process, for addressing adverse decisions for existing medical staff members and other individuals holding clinical privileges for renewal, revocation, or revision of clinical privileges.”
However, excluded physicians may not have a right to a hearing if they have not been fully stripped of privileges. SSM discontinued adult cardiology privileges for SLHV doctors but retained some doctors’ internal medicine privileges. Dr. Serota says internal medicine privileges are useless to cardiologists, but because the doctors’ privileges had not been fully removed, they cannot ask for a hearing.
More fundamentally, exclusive contracts are not a good fit for peer review. Mr. Rosen says the hearings were designed to review the physicians’ clinical competence or behavior, but excluded physicians do not have these problems. About all the hearing could focus on is the hospital’s policy, which the board would not want to allow. To avoid this, “the hospital might rule out a hearing as contrary to the intent of the bylaws,” Mr. Rosen says.
Furthermore, even if peer review goes forward, “what the medical staff decides is only advisory, and the hospital board makes the final decision,” Mr. Rosen says. He notes that the doctor could challenge the decision in court, but the hospital might still prevail.
Excluded physicians sometimes prevail
Although it is rare for excluded physicians to win a lawsuit against their hospital, it does happen, says Michael R. Callahan, health lawyer at Katten Muchin Rosenman, in Chicago.
Mr. Callahan cites a 2010 decision by the Arkansas Supreme Court that stopped the state’s largest health system from denying physicians’ privileges. Among other things, the hospital was found to have tortiously interfered with the physicians’ contracts with patients.
In a 2007 decision, a West Virginia court ruled that hospitals that have a mission to serve the public cannot exclude physicians for nonquality issues. In addition, some states, such as Texas, limit the economic factors that can be considered when credentialing decisions are made. Other states, such as Ohio, give hospitals a great deal of leeway to alter credentialing.
Dr. Serota is optimistic about his Missouri lawsuit. Although the judge in the case did not immediately grant SLHV’s request for restoration of privileges while the case proceeds, she did grant expedited discovery – allowing SLHV to obtain documents from SSM that could strengthen the doctors’ case – and she agreed to a hearing on SLHV’s request for a temporary restoration of privileges.
Ms. Gosfield says Dr. Serota’s optimism seems justified, but she adds that such cases cost a lot of money and that they may still not be winnable.
Often plaintiffs can settle lawsuits before they go to trial, but Mr. Callahan says hospitals are loath to restore privileges in a settlement because they don’t want to undermine an exclusivity deal. “The exclusive group expects a certain volume, which can’t be reached if the competing doctors are allowed back in,” he says.
Many physicians don’t challenge the exclusion
Quite often, excluded doctors decide not to challenge the decision. For example, Dr. Serota says groups of orthopedic surgeons and urologists have decided not to challenge similar decisions by SSM. “They wanted to move on,” he says.
Mr. Callahan says many excluded doctors also don’t even ask for a hearing. “They expect that the hospital’s decision will be upheld,” he says.
This was the case for Devendra K. Amin, MD, an independent cardiologist in Easton, Pa. Dr. Amin has not had any hospital privileges since July 2020. Even though he is board certified in interventional cardiology, which involves catheterization, Dr. Amin says he cannot perform these procedures because they can only be performed in a hospital in the area.
In the 1990s, Dr. Amin says, he had invasive cardiology privileges at five hospitals, but then those hospitals consolidated, and the remaining ones started constricting his privileges. First he could no longer work in the emergency department, then he could no longer read echocardiograms and interpret stress test results, because that work was assigned exclusively to employed doctors, he says.
Then the one remaining hospital announced that privileges would only be available to physicians by invitation, and he was not invited. Dr. Amin says he could have regained general cardiology privileges if he had accepted employment at the hospital, but he did not want to do this. A recruiter and the head of the cardiology section at the hospital even took him out to dinner 2 years ago to discuss employment, but there was a stipulation that the hospital would not agree to.
“I wanted to get back my interventional privileges back,” Dr. Amin says, “but they told me that would not be possible because they had an exclusive contract with a group.”
Dr. Amin says that now, he can only work as a general cardiologist with reduced volume. He says primary care physicians in the local hospital systems only refer to cardiologists within their systems. “When these patients do come to me, it is only because they specifically requested to see me,” Dr. Amin says.
He does not want to challenge the decisions regarding privileging. “Look, I am 68 years old,” Dr. Amin says. “I’m not retiring yet, but I don’t want to get into a battle with a hospital that has very deep pockets. I’m not a confrontational person to begin with, and I don’t want to spend the next 10 years of my life in litigation.”
Diverging expectations
The law on exclusive contracts does not provide easy answers for excluded doctors, and often it defies physicians’ conception of their own role in the hospital.
Many physicians expect the hospital to be a haven where they can do their work without being cut out by a competitor. This view is reinforced by organizations such as the American Medical Association.
The AMA Council on Medical Service states that privileges “can only be abridged upon recommendation of the medical staff and only for reason related to professional competence, adherence to standards of care, and other parameters agreed to by the medical staff.”
But the courts don’t tend to agree with that position. “Hospitals have a fiduciary duty to protect their own financial interests,” Mr. Callahan says. “This may involve anything that furthers the hospital’s mission to provide high-quality health care services to its patient community.”
At the same time, however, there are plenty of instances in which courts have ruled that exclusive contracts had gone too far. But usually it takes a lawyer experienced in these cases to know what those exceptions are.
A version of this article first appeared on Medscape.com.
Patients with diabetes more likely to be hospitalized, especially with foot infection
People with diabetes are at increased risk of hospitalization for infection, as well as infection-related mortality, shows a large U.S. study that suggests the risk is even higher in younger and Black individuals.
Michael Fang, PhD, Johns Hopkins University, Baltimore, and colleagues studied more than 12,000 participants in a community cohort study who were followed for an average of 24 years, between 1987-1989 and 2019.
Participants with diabetes faced a 67% increase risk of infection-related hospitalization, compared with those without diabetes.
Of particular note, the risk of hospitalization with foot infection was almost sixfold higher for people with diabetes than those without.
The research, published in Diabetologia on August 4, also suggests that diabetes may be associated with a 72% increased risk of infection-related mortality, although the absolute numbers were small.
Dr. Fang explained to this news organization that they focused on infection-related hospitalization and mortality “because these are comprehensively tracked in administrative data and ... are the most severe types of outcomes.”
However, this is probably just the tip of the iceberg, as people with diabetes are “likely at increased risk for milder infection too,” which can have a “significant adverse impact on people’s well-being and quality of life.”
As a result of their findings, the authors call for “broader guidance on infection prevention and management” in people with diabetes. To achieve this, Dr. Fang said, “we need to better understand why diabetes is associated with an increased risk of infection-related complications.”
“One likely factor is glycemic control: Emerging research suggests patients with diabetes with better glycemic control may be at significantly lower risk of infection-related complications.”
He continued that, in younger patients, a factor for worse outcomes could be that “diabetes tends to be more aggressive when it emerges early in life,” while in Black patients “there is research highlighting Black-White differences in glycemic control, access to care, and beliefs around vaccines.”
Overall, their findings – coupled with recent data showing that diabetes is an important risk factor for adverse outcomes with COVID-19 infection – paint “a common picture,” Dr. Fang said.
“People with diabetes are much more susceptible to infection-related complications, including COVID-related hospitalization and mortality,” which suggests people with diabetes “may need to be especially cautious.”
Adds to existing literature; amputations begin with infections
Robert A. Gabbay, MD, PhD, chief scientific and medical officer for the American Diabetes Association (ADA), said the study “does add to the existing literature by having followed a larger number of people over time and linking them to serious complications from infections.”
“Sadly, we have seen this play out in real-time during the COVID-19 pandemic.”
“One of the sobering bits of data is the significant health disparities that exist in Black Americans and the fact that foot infections remain a significant problem,” he said in an interview.
“Given that amputation rates for [Black Americans] are three times higher than White Americans, amputations begin with infections,” Dr. Gabbay added, noting the ADA “has been taking a strong stand to prevent amputations and address the inequities in health that exist.”
Jamie Hartmann-Boyce, PhD, from the University of Oxford, U.K., who was not involved in the study, commented that diabetes is a “well-known risk factor for worse outcomes from all kinds of infection,” which is why they “are prioritized for flu vaccination every year.”
She told this news organization that the current study “further confirms that people with diabetes are more likely to be hospitalized for infection of any type and most markedly for foot infection.”
“These new data further highlight the need for public health interventions to prevent type 2 diabetes, and for preventive health care in people with diabetes, including access to diabetes medications and support and to vaccinations to prevent infection,” added Dr. Hartmann-Boyce, who is a senior research fellow in health behaviors.
Diabetes is thought to be associated with susceptibility to infection via mechanisms such as impaired neutrophil functioning and humoral immune responses, and studies have shown a link with both common and rare infections.
However, the authors point out that “most” of those included “small clinical populations and were cross-sectional or had short follow-up.”
Guidelines for diabetes management, they note, also “pay less attention” to infectious diseases than they do to the prevention of micro- and macrovascular complications.
ARIC data mined for infections in those with diabetes
The team analyzed data from the ongoing U.S. community-based Atherosclerosis Risk in Communities (ARIC) study.
The National Heart, Lung, and Blood Institute–sponsored cohort was comprised of adults aged 45-64 years from four U.S. communities, recruited between 1987 and 1989 for clinical examinations, medical interviews, and laboratory tests, repeated over five more visits up to 2018-2019.
For the current analysis, the team included 12,739 individuals with a mean age of 54.5 years, of whom 54.3% were female and 24.7% were Black.
Patients were defined as having diabetes if their baseline fasting blood glucose was greater than or equal to 7 mmol/L, or nonfasting glucose was greater than or equal to 11.1 mmol/l, they self-reported a diagnosis of diabetes by a physician, or they were taking glucose-lowering medication at the first study visit. The researchers weren’t able to distinguish between type 1 and type 2 diabetes.
In total, 1,485 individuals had diabetes at baseline. They were more likely to be older, Black, have a low socioeconomic status, and have worse cardiometabolic health than participants without diabetes.
Over an average follow-up of 23.8 years, there were 4,229 incident hospitalizations for infection, at an overall rate of 15.9 per 1,000 person-years.
Individuals with diabetes at baseline had a higher rate of hospitalizations than those without, at 25.4 per 1,000 person-years versus 15.2 per 1,000 person-years.
After taking into account sociodemographic characteristics, socioeconomic status, and cardiometabolic risk factors, this equated to a hazard ratio for hospitalization with any infection of 1.67 (P < .001).
The risk of hospitalization for any infection was significantly higher for younger patients with diabetes, defined as aged less than 55 years (P = .005), and for Black patients (P < .001).
While the increased risk was generally consistent across infection types, it was markedly increased for foot infection, at a hazard ratio of 5.99 (P < .001).
Overall, there were few deaths due to infection in the study, at just 362. The risk of infection mortality was nevertheless significantly increased in people with diabetes, at an adjusted hazard ratio of 1.72 (P < .001).
Dr. Fang has reported being supported by a grant from the National Institutes of Health/National Heart, Lung, and Blood Institute. Dr. Selvin has reported being supported by grants from the National Institutes of Health/National Heart, Lung, and Blood Institute and National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Selvin is an associate editor for Diabetologia and had no role in the peer review of the manuscript.
A version of this article first appeared on Medscape.com.
People with diabetes are at increased risk of hospitalization for infection, as well as infection-related mortality, shows a large U.S. study that suggests the risk is even higher in younger and Black individuals.
Michael Fang, PhD, Johns Hopkins University, Baltimore, and colleagues studied more than 12,000 participants in a community cohort study who were followed for an average of 24 years, between 1987-1989 and 2019.
Participants with diabetes faced a 67% increase risk of infection-related hospitalization, compared with those without diabetes.
Of particular note, the risk of hospitalization with foot infection was almost sixfold higher for people with diabetes than those without.
The research, published in Diabetologia on August 4, also suggests that diabetes may be associated with a 72% increased risk of infection-related mortality, although the absolute numbers were small.
Dr. Fang explained to this news organization that they focused on infection-related hospitalization and mortality “because these are comprehensively tracked in administrative data and ... are the most severe types of outcomes.”
However, this is probably just the tip of the iceberg, as people with diabetes are “likely at increased risk for milder infection too,” which can have a “significant adverse impact on people’s well-being and quality of life.”
As a result of their findings, the authors call for “broader guidance on infection prevention and management” in people with diabetes. To achieve this, Dr. Fang said, “we need to better understand why diabetes is associated with an increased risk of infection-related complications.”
“One likely factor is glycemic control: Emerging research suggests patients with diabetes with better glycemic control may be at significantly lower risk of infection-related complications.”
He continued that, in younger patients, a factor for worse outcomes could be that “diabetes tends to be more aggressive when it emerges early in life,” while in Black patients “there is research highlighting Black-White differences in glycemic control, access to care, and beliefs around vaccines.”
Overall, their findings – coupled with recent data showing that diabetes is an important risk factor for adverse outcomes with COVID-19 infection – paint “a common picture,” Dr. Fang said.
“People with diabetes are much more susceptible to infection-related complications, including COVID-related hospitalization and mortality,” which suggests people with diabetes “may need to be especially cautious.”
Adds to existing literature; amputations begin with infections
Robert A. Gabbay, MD, PhD, chief scientific and medical officer for the American Diabetes Association (ADA), said the study “does add to the existing literature by having followed a larger number of people over time and linking them to serious complications from infections.”
“Sadly, we have seen this play out in real-time during the COVID-19 pandemic.”
“One of the sobering bits of data is the significant health disparities that exist in Black Americans and the fact that foot infections remain a significant problem,” he said in an interview.
“Given that amputation rates for [Black Americans] are three times higher than White Americans, amputations begin with infections,” Dr. Gabbay added, noting the ADA “has been taking a strong stand to prevent amputations and address the inequities in health that exist.”
Jamie Hartmann-Boyce, PhD, from the University of Oxford, U.K., who was not involved in the study, commented that diabetes is a “well-known risk factor for worse outcomes from all kinds of infection,” which is why they “are prioritized for flu vaccination every year.”
She told this news organization that the current study “further confirms that people with diabetes are more likely to be hospitalized for infection of any type and most markedly for foot infection.”
“These new data further highlight the need for public health interventions to prevent type 2 diabetes, and for preventive health care in people with diabetes, including access to diabetes medications and support and to vaccinations to prevent infection,” added Dr. Hartmann-Boyce, who is a senior research fellow in health behaviors.
Diabetes is thought to be associated with susceptibility to infection via mechanisms such as impaired neutrophil functioning and humoral immune responses, and studies have shown a link with both common and rare infections.
However, the authors point out that “most” of those included “small clinical populations and were cross-sectional or had short follow-up.”
Guidelines for diabetes management, they note, also “pay less attention” to infectious diseases than they do to the prevention of micro- and macrovascular complications.
ARIC data mined for infections in those with diabetes
The team analyzed data from the ongoing U.S. community-based Atherosclerosis Risk in Communities (ARIC) study.
The National Heart, Lung, and Blood Institute–sponsored cohort was comprised of adults aged 45-64 years from four U.S. communities, recruited between 1987 and 1989 for clinical examinations, medical interviews, and laboratory tests, repeated over five more visits up to 2018-2019.
For the current analysis, the team included 12,739 individuals with a mean age of 54.5 years, of whom 54.3% were female and 24.7% were Black.
Patients were defined as having diabetes if their baseline fasting blood glucose was greater than or equal to 7 mmol/L, or nonfasting glucose was greater than or equal to 11.1 mmol/l, they self-reported a diagnosis of diabetes by a physician, or they were taking glucose-lowering medication at the first study visit. The researchers weren’t able to distinguish between type 1 and type 2 diabetes.
In total, 1,485 individuals had diabetes at baseline. They were more likely to be older, Black, have a low socioeconomic status, and have worse cardiometabolic health than participants without diabetes.
Over an average follow-up of 23.8 years, there were 4,229 incident hospitalizations for infection, at an overall rate of 15.9 per 1,000 person-years.
Individuals with diabetes at baseline had a higher rate of hospitalizations than those without, at 25.4 per 1,000 person-years versus 15.2 per 1,000 person-years.
After taking into account sociodemographic characteristics, socioeconomic status, and cardiometabolic risk factors, this equated to a hazard ratio for hospitalization with any infection of 1.67 (P < .001).
The risk of hospitalization for any infection was significantly higher for younger patients with diabetes, defined as aged less than 55 years (P = .005), and for Black patients (P < .001).
While the increased risk was generally consistent across infection types, it was markedly increased for foot infection, at a hazard ratio of 5.99 (P < .001).
Overall, there were few deaths due to infection in the study, at just 362. The risk of infection mortality was nevertheless significantly increased in people with diabetes, at an adjusted hazard ratio of 1.72 (P < .001).
Dr. Fang has reported being supported by a grant from the National Institutes of Health/National Heart, Lung, and Blood Institute. Dr. Selvin has reported being supported by grants from the National Institutes of Health/National Heart, Lung, and Blood Institute and National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Selvin is an associate editor for Diabetologia and had no role in the peer review of the manuscript.
A version of this article first appeared on Medscape.com.
People with diabetes are at increased risk of hospitalization for infection, as well as infection-related mortality, shows a large U.S. study that suggests the risk is even higher in younger and Black individuals.
Michael Fang, PhD, Johns Hopkins University, Baltimore, and colleagues studied more than 12,000 participants in a community cohort study who were followed for an average of 24 years, between 1987-1989 and 2019.
Participants with diabetes faced a 67% increase risk of infection-related hospitalization, compared with those without diabetes.
Of particular note, the risk of hospitalization with foot infection was almost sixfold higher for people with diabetes than those without.
The research, published in Diabetologia on August 4, also suggests that diabetes may be associated with a 72% increased risk of infection-related mortality, although the absolute numbers were small.
Dr. Fang explained to this news organization that they focused on infection-related hospitalization and mortality “because these are comprehensively tracked in administrative data and ... are the most severe types of outcomes.”
However, this is probably just the tip of the iceberg, as people with diabetes are “likely at increased risk for milder infection too,” which can have a “significant adverse impact on people’s well-being and quality of life.”
As a result of their findings, the authors call for “broader guidance on infection prevention and management” in people with diabetes. To achieve this, Dr. Fang said, “we need to better understand why diabetes is associated with an increased risk of infection-related complications.”
“One likely factor is glycemic control: Emerging research suggests patients with diabetes with better glycemic control may be at significantly lower risk of infection-related complications.”
He continued that, in younger patients, a factor for worse outcomes could be that “diabetes tends to be more aggressive when it emerges early in life,” while in Black patients “there is research highlighting Black-White differences in glycemic control, access to care, and beliefs around vaccines.”
Overall, their findings – coupled with recent data showing that diabetes is an important risk factor for adverse outcomes with COVID-19 infection – paint “a common picture,” Dr. Fang said.
“People with diabetes are much more susceptible to infection-related complications, including COVID-related hospitalization and mortality,” which suggests people with diabetes “may need to be especially cautious.”
Adds to existing literature; amputations begin with infections
Robert A. Gabbay, MD, PhD, chief scientific and medical officer for the American Diabetes Association (ADA), said the study “does add to the existing literature by having followed a larger number of people over time and linking them to serious complications from infections.”
“Sadly, we have seen this play out in real-time during the COVID-19 pandemic.”
“One of the sobering bits of data is the significant health disparities that exist in Black Americans and the fact that foot infections remain a significant problem,” he said in an interview.
“Given that amputation rates for [Black Americans] are three times higher than White Americans, amputations begin with infections,” Dr. Gabbay added, noting the ADA “has been taking a strong stand to prevent amputations and address the inequities in health that exist.”
Jamie Hartmann-Boyce, PhD, from the University of Oxford, U.K., who was not involved in the study, commented that diabetes is a “well-known risk factor for worse outcomes from all kinds of infection,” which is why they “are prioritized for flu vaccination every year.”
She told this news organization that the current study “further confirms that people with diabetes are more likely to be hospitalized for infection of any type and most markedly for foot infection.”
“These new data further highlight the need for public health interventions to prevent type 2 diabetes, and for preventive health care in people with diabetes, including access to diabetes medications and support and to vaccinations to prevent infection,” added Dr. Hartmann-Boyce, who is a senior research fellow in health behaviors.
Diabetes is thought to be associated with susceptibility to infection via mechanisms such as impaired neutrophil functioning and humoral immune responses, and studies have shown a link with both common and rare infections.
However, the authors point out that “most” of those included “small clinical populations and were cross-sectional or had short follow-up.”
Guidelines for diabetes management, they note, also “pay less attention” to infectious diseases than they do to the prevention of micro- and macrovascular complications.
ARIC data mined for infections in those with diabetes
The team analyzed data from the ongoing U.S. community-based Atherosclerosis Risk in Communities (ARIC) study.
The National Heart, Lung, and Blood Institute–sponsored cohort was comprised of adults aged 45-64 years from four U.S. communities, recruited between 1987 and 1989 for clinical examinations, medical interviews, and laboratory tests, repeated over five more visits up to 2018-2019.
For the current analysis, the team included 12,739 individuals with a mean age of 54.5 years, of whom 54.3% were female and 24.7% were Black.
Patients were defined as having diabetes if their baseline fasting blood glucose was greater than or equal to 7 mmol/L, or nonfasting glucose was greater than or equal to 11.1 mmol/l, they self-reported a diagnosis of diabetes by a physician, or they were taking glucose-lowering medication at the first study visit. The researchers weren’t able to distinguish between type 1 and type 2 diabetes.
In total, 1,485 individuals had diabetes at baseline. They were more likely to be older, Black, have a low socioeconomic status, and have worse cardiometabolic health than participants without diabetes.
Over an average follow-up of 23.8 years, there were 4,229 incident hospitalizations for infection, at an overall rate of 15.9 per 1,000 person-years.
Individuals with diabetes at baseline had a higher rate of hospitalizations than those without, at 25.4 per 1,000 person-years versus 15.2 per 1,000 person-years.
After taking into account sociodemographic characteristics, socioeconomic status, and cardiometabolic risk factors, this equated to a hazard ratio for hospitalization with any infection of 1.67 (P < .001).
The risk of hospitalization for any infection was significantly higher for younger patients with diabetes, defined as aged less than 55 years (P = .005), and for Black patients (P < .001).
While the increased risk was generally consistent across infection types, it was markedly increased for foot infection, at a hazard ratio of 5.99 (P < .001).
Overall, there were few deaths due to infection in the study, at just 362. The risk of infection mortality was nevertheless significantly increased in people with diabetes, at an adjusted hazard ratio of 1.72 (P < .001).
Dr. Fang has reported being supported by a grant from the National Institutes of Health/National Heart, Lung, and Blood Institute. Dr. Selvin has reported being supported by grants from the National Institutes of Health/National Heart, Lung, and Blood Institute and National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Selvin is an associate editor for Diabetologia and had no role in the peer review of the manuscript.
A version of this article first appeared on Medscape.com.
Myasthenic Crisis After Recurrent COVID-19 Infection
A patient with myasthenia gravis who survived 2 COVID-19 infections required plasmapheresis to recover from an acute crisis.
COVID-19 is still in the early stages of understanding, although it is known to be complicated by individual patient comorbidities. The management and treatment of COVID-19 continues to quickly evolve as more is discovered regarding the virus. Multiple treatments have been preliminarily tested and used under a Food and Drug Administration emergency use authorization (EUA) determination. The long-term success of these therapies, however, is yet to be determined. Additionally, if a patient has a second clinical presentation for COVID-19, it is not known whether this represents latency with subsequent reactivation from the previous infection or a second de novo infection. The uncertainty calls into question the duration of immunity, if any, following a primary infection.
COVID-19 management becomes more complicated when patients have complex medical conditions, such as myasthenia gravis (MG). This autoimmune neuromuscular disorder can present with varying weakness, and many patients are on immunomodulator medications. The weakness can worsen into a myasthenic crisis (MC), resulting in profound weakness of the respiratory muscles. Therefore, patients with MG are at increased risk for COVID-19 and may have a more complicated course when infected.
Our patient with MG presented for severe COVID-19 symptoms twice and later developed MC. He received 2 treatment modalities available under an EUA (remdesivir and convalescent plasma) for COVID-19, resulting in symptom resolution and a negative polymerize chain reaction (PCR) test result for the virus. However, after receiving his typical maintenance therapy of IV immunoglobulin (IVIG) for his MG, he again developed symptoms consistent with COVID-19 and tested positive. After recovering from the second episode of COVID-19, the patient went into MC requiring plasmapheresis.
Case Presentation
A 56-year-old male, US Army veteran presented to Carl R. Darnall Army Medical Center emergency department (ED) 6 days after testing positive for COVID-19, with worsening sputum, cough, congestion, dyspnea, and fever. Due to his MG, the patient had a home oxygen monitor and reported that his oxygenation saturation dropped below 90% with minimal exertion. His medical history was significant for MG, status postthymectomy and radiation treatment, left hemidiaphragm paralysis secondary to phrenic nerve injury, and corticosteroid-induced insulin-dependent diabetes mellitus. His current home medications included pyridostigmine 60 mg 3 times a day, mycophenolate (MMF) 1500 mg twice daily, IV immunoglobulin (IVIG) every 3 weeks, insulin aspart up to 16 U per meal, insulin glargine 30 U twice a day, dulaglutide 0.75 mg every week, and metformin 1000 mg twice daily.
On initial examination, the patient’s heart rate (HR) was 111 beats/min, respiratory rate (RR), 22 breaths/min, blood pressure (BP), 138/88 mm Hg, temperature, 100.9 oF, and his initial pulse oximetry, 91% on room air. On physical examination, the patient was tachypneic, though without other signs of respiratory distress. Lung auscultation revealed no adventitial lung sounds. His cardiac examination was notable only for tachycardia. His neurologic examination demonstrated intact cranial nerves, with 5 out of 5 (scale 1 to 5) strength throughout the upper and lower extremities, sensation was intact to light touch, and he had normal cerebellar function. The rest of the examination was normal.
Initial laboratory investigation was notable for a white blood cell count of 14.15x103 cells/mcL with 84% neutrophils, and 6% lymphocytes. Additional tests revealed a C-reactive protein (CRP) level, 17.97 mg/dL (reference range, 0-0.5 mg/dL), ferritin level, 647 ng/mL (reference range, 22-274 ng/mL), d-dimer, 0.64 mcg/mL (reference range, 0-0.47mcg/mL), and a repeated positive COVID-19 PCR test. A portable chest X-ray showed bibasilar opacities (Figure 1).
The patient was diagnosed with COVID-19 and admitted to the intensive care unit (ICU). In the ICU, the patient received 1 U of convalescent plasma (CP) and started on a course of IV remdesivir 100 mg/d consistent with the EUA. He also received a 5-day course of ceftriaxone and azithromycin for possible community acquired pneumonia (CAP). As part of the patient’s MG maintenance medications, he received IVIG 4 g while in the ICU. Throughout his ICU stay, he required supplemental nasal cannula oxygenation to maintain his oxygen saturation > 93%. After 8 days in the ICU, his oxygen requirements decreased, and the patient was transferred out of the ICU and remdesivir was discontinued. On hospital day 10, a repeat COVID-19 PCR test was negative, inflammatory markers returned to within normal limits, and a repeat chest X-ray showed improvement from admission (Figure 2). Having recovered significantly, he was discharged home.
Three weeks later, the patient again presented to the MTF with 3 days of dyspnea, cough, fever, nausea, and vomiting. One day before symptom onset, he had received his maintenance IVIG infusion. The patient reported that his home oxygen saturation was 82% with minimal exertion. On ED presentation his HR was 107 beats/min, RR, 28 breaths/min, temperature, 98.1 oF, BP 118/71 mm Hg, and oxygen saturation, 92% on 2L nasal cannula. His examination was most notable for tachypnea with accessory muscle use. At this time, his neurologic examination was unchanged from prior admission with grossly intact cranial nerves and symmetric 5 of 5 motor strength in all extremities.
At this second ED visit, laboratory results demonstrated a CRP of 3.44 mg/dL, ferritin 2019 ng/mL, d-dimer, 3.39 mcg/mL, and a positive COVID-19 PCR result. His chest X-ray demonstrated new peripheral opacities compared with the X-ray at discharge (Figure 3). He required ICU admission again for his COVID-19 symptoms.
During his ICU course he continued to require supplemental oxygen by nasal cannula, though never required intubation. This second admission, he was again treated empirically for CAP with levofloxacin 750 mg daily for 5 days. He was discharged after 14 days with symptom resolution and down trending of inflammatory markers, though he was not retested for COVID-19.
Four days after his second discharge, he presented to the ED for a third time with diffuse weakness, dysphagia, and dysarthria of 1 day. His HR was 87/beats/min; RR, 17 breaths/min; temperature, 98.7 oF; BP, 144/81 mm Hg; and oxygen saturation, 98% on room air. His examination was significant for slurred speech, bilateral ptosis, 3 of 5 strength in bilateral finger flexion/abduction, wrist extension, knee and ankle flexion/extension; 4 of 5 strength in bilateral proximal muscle testing of deltoid, and hip; normal sensation, cerebellar function and reflexes. His negative inspiratory force (NIF) maximal effort was −30 cmH2O. He was determined to be in MC without evidence of COIVD-19 symptoms, and laboratory results were within normal limits, including a negative COVID-19 PCR. As he received IVIG as maintenance therapy, plasmapheresis was recommended to treat his MC, which required transfer to an outside civilian facility.
At the outside hospital, the patient underwent 5 rounds of plasmapheresis over 10 days. By the third treatment his strength had returned with resolution of the bulbar symptoms and no supplemental oxygen requirements. The patient was discharged and continued his original dosages of MMF and pyridostigmine. At 3 months, he remained asymptomatic from a COVID-19 standpoint and stable from a MG standpoint.
Discussion
Reinfection with the COVID-19 has been continuously debated with alternative explanations suggested for a positive test after a previous negative PCR test in the setting of symptom resolution.1,2 Proposed causes include dynamic PCR results due to prolonged viral shedding and inaccurate or poorly sensitive tests. The repeat positive cases in these scenarios, however, occurred in asymptomatic patients.1,2 COVID-19 shedding averages 20 to 22 days after symptom onset but has been seen up to 36 days after symptom resolution.2,3 This would suggest that fluctuating results during the immediate postsymptom period may be due to variations in viral shedding load and or sampling error—especially in asymptomatic patients. On the other hand, patients who experience return of symptoms days to weeks after previous convalescence leave clinicians wondering whether this represents clinical latency with reactivation or COVID-19 reinfection. A separate case of initial COVID-19 in a patient that had subsequent clinical recovery with a negative PCR developed recurrent respiratory symptoms and had a positive PCR test only 10 days later, further highlighting the reinfection vs reactivation issue of COVID-19.2 Further understanding of this issue may have implications on the extent of natural immunity following primary infection; potential vaccine dosage schedules; and global public health policies.
Although reactivation may be plausible given his immunomodulatory therapy, our patient’s second COVID-19 symptoms started 40 days after the initial symptoms, and 26 days after the initial course resolution; previous cases of return of severe symptoms occurred between 3 and 6 days.1 Given our patient’s time course between resolution and return of symptoms, if latency is the mechanism at play, this case demonstrates an exceptionally longer latency period than the ones that have been reported. Additionally, if latency is an issue in COVID-19, using remdesivir as a treatment further complicates the understanding of this disease.
Remdesivir, a nucleoside analogue antiviral, was shown to benefit recovery in patients with severe symptoms in the Adaptive COVID-19 Treatment Trial-1 study.4 Our patient had originally been placed on a 10-day course; however, on treatment day 8, his symptoms resolved and the remdesivir was discontinued. This is a similar finding to half the patients in the 10-day arm of the study by McCreary and colleagues.5 Although our patient was asymptomatic 4 weeks after the start of remdesivir, consistent with the majority of patients in the McCreary 10-day study arm, further comparison of the presented patient is limited due to study length and follow-up considerations.5 No previous data exist on reactivation, reinfection, or long-term mortality after being treated with remdesivir for COVID-19 infection.
IVIG is being studied in the treatment of COVID-19 and bears consideration as it relates to our patient. There is no evidence that IVIG used in the treatment of autoimmune diseases increases the risk of infection compared with that of other medications used in the treatment of such diseases. Furthermore, the current guidance from the MG expert panel does not suggest that IVIG increases the risk of contracting COVID-19 aside from the risks of exposure to hospital infrastructure.6 Yet the guidance does not discuss the use of IVIG for MG in patients who are already symptomatic from COVID-19 or for patients recovering from the clinical disease or does it discuss a possible compounding risk of thromboembolic events associated with IVIG and COVID-19.6,7 Our patient received his maintenance IVIG during his first admission without any worsening of symptoms or increased oxygen requirements. The day following our patient’s next scheduled IVIG infusion—while asymptomatic—he again developed respiratory symptoms; this could suggest that IVIG did not contribute to his second clinical course nor protect against.
CP is a treatment modality that has been used and studied in previous infectious outbreaks such as the first severe acute respiratory syndrome, and the H1N1 influenza virus.8 Current data on CP for COVID-19 are limited, but early descriptive studies have shown a benefit in improvement of symptoms 5 days sooner in those requiring supplemental oxygen, but no benefit for those requiring mechanical ventilation.9 Like patients that benefitted in these studies, our patient received CP early, 6 days after first testing positive and onset of symptoms. This patient’s reinfection or return of symptoms draws into question the hindrance or even prevention of long-term immunity from administration of CP.
COVID-19 presents many challenges when managing this patient’s coexisting MG, especially as the patient was already being treated with immunosuppressing therapies. The guidance does recommend continuation of standard MG therapies during hospitalizations, including immunosuppression medications such as MMF.6 Immunosuppression is associated with worsened severity of COVID-19 symptoms, although no relation exists to degree of immunosuppression and severity.7,10 To the best of our knowledge there has been no case report of reinfection or reactivation of COVID-19 associated with immunosuppressive agents used in the treatment of MG.
Our patient also was taking pyridostigmine for the treatment of his MG. There is no evidence this medication increases the risk of infection; but the cholinergic activity can increase bronchial secretions, which could theoretically worsen the COVID-19 respiratory symptoms.6,11 During both ICU admissions, our patient continued pyridostigmine use, observing complete return to baseline after discharge. Given the possible association with worsened respiratory outcomes after the second ICU admission, the balance between managing MG symptoms and COVID-19 symptoms needs further examination.
The patient was in MC during his third presentation to the ED. Although respiratory symptoms may be difficult to differentiate from COVID-19, the additional neurologic symptoms seen in this patient allowed for quick determination of the need for MC treatment. There are many potential etiologies contributing to the development of the MC presented here, and it was likely due to multifactorial precipitants. A common cause of MC is viral upper respiratory infections, further challenging the care of these patients during this pandemic.12 Many medications have been cited as causing a MC, 2 of which our patient received during admission for COVID-19: azithromycin and levoquin.12 Although the patient did not receive hydroxychloroquine, which was still being considered as an appropriate COVID-19 treatment at the time, it also is a drug known for precipitating MC and its use scrutinized in patients with MG.12
A key aspect to diagnosing and guiding therapies in myasthenic crisis in addition to the clinical symptoms of acute weakness is respiratory assessment through the nonaerosolizing NIF test.12 Our patient’s NIF measured < 30 cmH2O when in MC, while the reference range is < 75 cmH2O, and for mechanical ventilation is recommended at 20 cmH2O. Although the patient was maintaining O2 saturation > 95%, his NIF value was concerning, and preparations were made in case of precipitous decline. Compounding the NIF assessment in this patient is his history of left phrenic nerve palsy. Without a documented baseline NIF, results were limited in determining his diaphragm strength.13 Treatment for MC includes IVIG or plasmapheresis, since this patient had failed his maintenance therapy IVIG, plasmapheresis was coordinated for definitive therapy.
Conclusions
Federal facilities have seen an increase in the amount of respiratory complaints over the past months. Although COVID-19 is a concerning diagnosis, it is crucial to consider comorbidities in the diagnostic workup of each, even with a previous recent diagnosis of COVID-19. As treatment recommendations for COVID-19 continue to fluctuate coupled with the limitations and difficulties associated with MG patients, so too treatment and evaluation must be carefully considered at each presentation.
1. Gousseff M, Penot P, Gallay L, et al. Clinical recurrences of COVID-19 symptoms after recovery: viral relapse, reinfection or inflammatory rebound? J Infect. 2020;81(5):816-846. doi:10.1016/j.jinf.2020.06.073
2. Duggan NM, Ludy SM, Shannon BC, Reisner AT, Wilcox SR. Is novel coronavirus 2019 reinfection possible? Interpreting dynamic SARS-CoV-2 test results. Am J Emerg Med. 2021;39:256.e1-256.e3. doi:10.1016/j.ajem.2020.06.079
3. Li J, Zhang L, Liu B, Song D. Case report: viral shedding for 60 days in a woman with COVID-19. Am J Trop Med Hyg. 2020;102(6):1210-1213. doi:10.4269/ajtmh.20-0275
4. Beigel JH, Tomashek KM, Dodd LE. Remdesivir for the treatment of Covid-19 - preliminary report. Reply. N Engl J Med. 2020;383(10):994. doi:10.1056/NEJMc2022236
5. McCreary EK, Angus DC. Efficacy of remdesivir in COVID-19. JAMA. 2020;324(11):1041-1042. doi:10.1001/jama.2020.16337
6. International MG/COVID-19 Working Group; Jacob S, Muppidi S, Gordon A, et al. Guidance for the management of myasthenia gravis (MG) and Lambert-Eaton myasthenic syndrome (LEMS) during the COVID-19 pandemic. J Neurol Sci. 2020;412:116803. doi:10.1016/j.jns.2020.116803
7. Anand P, Slama MCC, Kaku M, et al. COVID-19 in patients with myasthenia gravis. Muscle Nerve. 2020;62(2):254-258. doi:10.1002/mus.26918
8. Wooding DJ, Bach H. Treatment of COVID-19 with convalescent plasma: lessons from past coronavirus outbreaks. Clin Microbiol Infect. 2020;26(10):1436-1446. doi:10.1016/j.cmi.2020.08.005
9. Salazar E, Perez KK, Ashraf M, et al. Treatment of coronavirus disease 2019 (covid-19) patients with convalescent plasma. Am J Pathol. 2020;190(8):1680-1690. doi:10.1016/j.ajpath.2020.05.014
10. Ryan C, Minc A, Caceres J, et al. Predicting severe outcomes in Covid-19 related illness using only patient demographics, comorbidities and symptoms [published online ahead of print, 2020 Sep 9]. Am J Emerg Med. 2020;S0735-6757(20)30809-3. doi:10.1016/j.ajem.2020.09.017
11. Singh S, Govindarajan R. COVID-19 and generalized myasthenia gravis exacerbation: a case report. Clin Neurol Neurosurg. 2020;196:106045. doi:10.1016/j.clineuro.2020.106045
12. Wendell LC, Levine JM. Myasthenic crisis. Neurohospitalist. 2011;1(1):16-22. doi:10.1177/1941875210382918
13. Dubé BP, Dres M. Diaphragm dysfunction: diagnostic approaches and management strategies. J Clin Med. 2016;5(12):113. Published 2016 Dec 5. doi:10.3390/jcm5120113
A patient with myasthenia gravis who survived 2 COVID-19 infections required plasmapheresis to recover from an acute crisis.
A patient with myasthenia gravis who survived 2 COVID-19 infections required plasmapheresis to recover from an acute crisis.
COVID-19 is still in the early stages of understanding, although it is known to be complicated by individual patient comorbidities. The management and treatment of COVID-19 continues to quickly evolve as more is discovered regarding the virus. Multiple treatments have been preliminarily tested and used under a Food and Drug Administration emergency use authorization (EUA) determination. The long-term success of these therapies, however, is yet to be determined. Additionally, if a patient has a second clinical presentation for COVID-19, it is not known whether this represents latency with subsequent reactivation from the previous infection or a second de novo infection. The uncertainty calls into question the duration of immunity, if any, following a primary infection.
COVID-19 management becomes more complicated when patients have complex medical conditions, such as myasthenia gravis (MG). This autoimmune neuromuscular disorder can present with varying weakness, and many patients are on immunomodulator medications. The weakness can worsen into a myasthenic crisis (MC), resulting in profound weakness of the respiratory muscles. Therefore, patients with MG are at increased risk for COVID-19 and may have a more complicated course when infected.
Our patient with MG presented for severe COVID-19 symptoms twice and later developed MC. He received 2 treatment modalities available under an EUA (remdesivir and convalescent plasma) for COVID-19, resulting in symptom resolution and a negative polymerize chain reaction (PCR) test result for the virus. However, after receiving his typical maintenance therapy of IV immunoglobulin (IVIG) for his MG, he again developed symptoms consistent with COVID-19 and tested positive. After recovering from the second episode of COVID-19, the patient went into MC requiring plasmapheresis.
Case Presentation
A 56-year-old male, US Army veteran presented to Carl R. Darnall Army Medical Center emergency department (ED) 6 days after testing positive for COVID-19, with worsening sputum, cough, congestion, dyspnea, and fever. Due to his MG, the patient had a home oxygen monitor and reported that his oxygenation saturation dropped below 90% with minimal exertion. His medical history was significant for MG, status postthymectomy and radiation treatment, left hemidiaphragm paralysis secondary to phrenic nerve injury, and corticosteroid-induced insulin-dependent diabetes mellitus. His current home medications included pyridostigmine 60 mg 3 times a day, mycophenolate (MMF) 1500 mg twice daily, IV immunoglobulin (IVIG) every 3 weeks, insulin aspart up to 16 U per meal, insulin glargine 30 U twice a day, dulaglutide 0.75 mg every week, and metformin 1000 mg twice daily.
On initial examination, the patient’s heart rate (HR) was 111 beats/min, respiratory rate (RR), 22 breaths/min, blood pressure (BP), 138/88 mm Hg, temperature, 100.9 oF, and his initial pulse oximetry, 91% on room air. On physical examination, the patient was tachypneic, though without other signs of respiratory distress. Lung auscultation revealed no adventitial lung sounds. His cardiac examination was notable only for tachycardia. His neurologic examination demonstrated intact cranial nerves, with 5 out of 5 (scale 1 to 5) strength throughout the upper and lower extremities, sensation was intact to light touch, and he had normal cerebellar function. The rest of the examination was normal.
Initial laboratory investigation was notable for a white blood cell count of 14.15x103 cells/mcL with 84% neutrophils, and 6% lymphocytes. Additional tests revealed a C-reactive protein (CRP) level, 17.97 mg/dL (reference range, 0-0.5 mg/dL), ferritin level, 647 ng/mL (reference range, 22-274 ng/mL), d-dimer, 0.64 mcg/mL (reference range, 0-0.47mcg/mL), and a repeated positive COVID-19 PCR test. A portable chest X-ray showed bibasilar opacities (Figure 1).
The patient was diagnosed with COVID-19 and admitted to the intensive care unit (ICU). In the ICU, the patient received 1 U of convalescent plasma (CP) and started on a course of IV remdesivir 100 mg/d consistent with the EUA. He also received a 5-day course of ceftriaxone and azithromycin for possible community acquired pneumonia (CAP). As part of the patient’s MG maintenance medications, he received IVIG 4 g while in the ICU. Throughout his ICU stay, he required supplemental nasal cannula oxygenation to maintain his oxygen saturation > 93%. After 8 days in the ICU, his oxygen requirements decreased, and the patient was transferred out of the ICU and remdesivir was discontinued. On hospital day 10, a repeat COVID-19 PCR test was negative, inflammatory markers returned to within normal limits, and a repeat chest X-ray showed improvement from admission (Figure 2). Having recovered significantly, he was discharged home.
Three weeks later, the patient again presented to the MTF with 3 days of dyspnea, cough, fever, nausea, and vomiting. One day before symptom onset, he had received his maintenance IVIG infusion. The patient reported that his home oxygen saturation was 82% with minimal exertion. On ED presentation his HR was 107 beats/min, RR, 28 breaths/min, temperature, 98.1 oF, BP 118/71 mm Hg, and oxygen saturation, 92% on 2L nasal cannula. His examination was most notable for tachypnea with accessory muscle use. At this time, his neurologic examination was unchanged from prior admission with grossly intact cranial nerves and symmetric 5 of 5 motor strength in all extremities.
At this second ED visit, laboratory results demonstrated a CRP of 3.44 mg/dL, ferritin 2019 ng/mL, d-dimer, 3.39 mcg/mL, and a positive COVID-19 PCR result. His chest X-ray demonstrated new peripheral opacities compared with the X-ray at discharge (Figure 3). He required ICU admission again for his COVID-19 symptoms.
During his ICU course he continued to require supplemental oxygen by nasal cannula, though never required intubation. This second admission, he was again treated empirically for CAP with levofloxacin 750 mg daily for 5 days. He was discharged after 14 days with symptom resolution and down trending of inflammatory markers, though he was not retested for COVID-19.
Four days after his second discharge, he presented to the ED for a third time with diffuse weakness, dysphagia, and dysarthria of 1 day. His HR was 87/beats/min; RR, 17 breaths/min; temperature, 98.7 oF; BP, 144/81 mm Hg; and oxygen saturation, 98% on room air. His examination was significant for slurred speech, bilateral ptosis, 3 of 5 strength in bilateral finger flexion/abduction, wrist extension, knee and ankle flexion/extension; 4 of 5 strength in bilateral proximal muscle testing of deltoid, and hip; normal sensation, cerebellar function and reflexes. His negative inspiratory force (NIF) maximal effort was −30 cmH2O. He was determined to be in MC without evidence of COIVD-19 symptoms, and laboratory results were within normal limits, including a negative COVID-19 PCR. As he received IVIG as maintenance therapy, plasmapheresis was recommended to treat his MC, which required transfer to an outside civilian facility.
At the outside hospital, the patient underwent 5 rounds of plasmapheresis over 10 days. By the third treatment his strength had returned with resolution of the bulbar symptoms and no supplemental oxygen requirements. The patient was discharged and continued his original dosages of MMF and pyridostigmine. At 3 months, he remained asymptomatic from a COVID-19 standpoint and stable from a MG standpoint.
Discussion
Reinfection with the COVID-19 has been continuously debated with alternative explanations suggested for a positive test after a previous negative PCR test in the setting of symptom resolution.1,2 Proposed causes include dynamic PCR results due to prolonged viral shedding and inaccurate or poorly sensitive tests. The repeat positive cases in these scenarios, however, occurred in asymptomatic patients.1,2 COVID-19 shedding averages 20 to 22 days after symptom onset but has been seen up to 36 days after symptom resolution.2,3 This would suggest that fluctuating results during the immediate postsymptom period may be due to variations in viral shedding load and or sampling error—especially in asymptomatic patients. On the other hand, patients who experience return of symptoms days to weeks after previous convalescence leave clinicians wondering whether this represents clinical latency with reactivation or COVID-19 reinfection. A separate case of initial COVID-19 in a patient that had subsequent clinical recovery with a negative PCR developed recurrent respiratory symptoms and had a positive PCR test only 10 days later, further highlighting the reinfection vs reactivation issue of COVID-19.2 Further understanding of this issue may have implications on the extent of natural immunity following primary infection; potential vaccine dosage schedules; and global public health policies.
Although reactivation may be plausible given his immunomodulatory therapy, our patient’s second COVID-19 symptoms started 40 days after the initial symptoms, and 26 days after the initial course resolution; previous cases of return of severe symptoms occurred between 3 and 6 days.1 Given our patient’s time course between resolution and return of symptoms, if latency is the mechanism at play, this case demonstrates an exceptionally longer latency period than the ones that have been reported. Additionally, if latency is an issue in COVID-19, using remdesivir as a treatment further complicates the understanding of this disease.
Remdesivir, a nucleoside analogue antiviral, was shown to benefit recovery in patients with severe symptoms in the Adaptive COVID-19 Treatment Trial-1 study.4 Our patient had originally been placed on a 10-day course; however, on treatment day 8, his symptoms resolved and the remdesivir was discontinued. This is a similar finding to half the patients in the 10-day arm of the study by McCreary and colleagues.5 Although our patient was asymptomatic 4 weeks after the start of remdesivir, consistent with the majority of patients in the McCreary 10-day study arm, further comparison of the presented patient is limited due to study length and follow-up considerations.5 No previous data exist on reactivation, reinfection, or long-term mortality after being treated with remdesivir for COVID-19 infection.
IVIG is being studied in the treatment of COVID-19 and bears consideration as it relates to our patient. There is no evidence that IVIG used in the treatment of autoimmune diseases increases the risk of infection compared with that of other medications used in the treatment of such diseases. Furthermore, the current guidance from the MG expert panel does not suggest that IVIG increases the risk of contracting COVID-19 aside from the risks of exposure to hospital infrastructure.6 Yet the guidance does not discuss the use of IVIG for MG in patients who are already symptomatic from COVID-19 or for patients recovering from the clinical disease or does it discuss a possible compounding risk of thromboembolic events associated with IVIG and COVID-19.6,7 Our patient received his maintenance IVIG during his first admission without any worsening of symptoms or increased oxygen requirements. The day following our patient’s next scheduled IVIG infusion—while asymptomatic—he again developed respiratory symptoms; this could suggest that IVIG did not contribute to his second clinical course nor protect against.
CP is a treatment modality that has been used and studied in previous infectious outbreaks such as the first severe acute respiratory syndrome, and the H1N1 influenza virus.8 Current data on CP for COVID-19 are limited, but early descriptive studies have shown a benefit in improvement of symptoms 5 days sooner in those requiring supplemental oxygen, but no benefit for those requiring mechanical ventilation.9 Like patients that benefitted in these studies, our patient received CP early, 6 days after first testing positive and onset of symptoms. This patient’s reinfection or return of symptoms draws into question the hindrance or even prevention of long-term immunity from administration of CP.
COVID-19 presents many challenges when managing this patient’s coexisting MG, especially as the patient was already being treated with immunosuppressing therapies. The guidance does recommend continuation of standard MG therapies during hospitalizations, including immunosuppression medications such as MMF.6 Immunosuppression is associated with worsened severity of COVID-19 symptoms, although no relation exists to degree of immunosuppression and severity.7,10 To the best of our knowledge there has been no case report of reinfection or reactivation of COVID-19 associated with immunosuppressive agents used in the treatment of MG.
Our patient also was taking pyridostigmine for the treatment of his MG. There is no evidence this medication increases the risk of infection; but the cholinergic activity can increase bronchial secretions, which could theoretically worsen the COVID-19 respiratory symptoms.6,11 During both ICU admissions, our patient continued pyridostigmine use, observing complete return to baseline after discharge. Given the possible association with worsened respiratory outcomes after the second ICU admission, the balance between managing MG symptoms and COVID-19 symptoms needs further examination.
The patient was in MC during his third presentation to the ED. Although respiratory symptoms may be difficult to differentiate from COVID-19, the additional neurologic symptoms seen in this patient allowed for quick determination of the need for MC treatment. There are many potential etiologies contributing to the development of the MC presented here, and it was likely due to multifactorial precipitants. A common cause of MC is viral upper respiratory infections, further challenging the care of these patients during this pandemic.12 Many medications have been cited as causing a MC, 2 of which our patient received during admission for COVID-19: azithromycin and levoquin.12 Although the patient did not receive hydroxychloroquine, which was still being considered as an appropriate COVID-19 treatment at the time, it also is a drug known for precipitating MC and its use scrutinized in patients with MG.12
A key aspect to diagnosing and guiding therapies in myasthenic crisis in addition to the clinical symptoms of acute weakness is respiratory assessment through the nonaerosolizing NIF test.12 Our patient’s NIF measured < 30 cmH2O when in MC, while the reference range is < 75 cmH2O, and for mechanical ventilation is recommended at 20 cmH2O. Although the patient was maintaining O2 saturation > 95%, his NIF value was concerning, and preparations were made in case of precipitous decline. Compounding the NIF assessment in this patient is his history of left phrenic nerve palsy. Without a documented baseline NIF, results were limited in determining his diaphragm strength.13 Treatment for MC includes IVIG or plasmapheresis, since this patient had failed his maintenance therapy IVIG, plasmapheresis was coordinated for definitive therapy.
Conclusions
Federal facilities have seen an increase in the amount of respiratory complaints over the past months. Although COVID-19 is a concerning diagnosis, it is crucial to consider comorbidities in the diagnostic workup of each, even with a previous recent diagnosis of COVID-19. As treatment recommendations for COVID-19 continue to fluctuate coupled with the limitations and difficulties associated with MG patients, so too treatment and evaluation must be carefully considered at each presentation.
COVID-19 is still in the early stages of understanding, although it is known to be complicated by individual patient comorbidities. The management and treatment of COVID-19 continues to quickly evolve as more is discovered regarding the virus. Multiple treatments have been preliminarily tested and used under a Food and Drug Administration emergency use authorization (EUA) determination. The long-term success of these therapies, however, is yet to be determined. Additionally, if a patient has a second clinical presentation for COVID-19, it is not known whether this represents latency with subsequent reactivation from the previous infection or a second de novo infection. The uncertainty calls into question the duration of immunity, if any, following a primary infection.
COVID-19 management becomes more complicated when patients have complex medical conditions, such as myasthenia gravis (MG). This autoimmune neuromuscular disorder can present with varying weakness, and many patients are on immunomodulator medications. The weakness can worsen into a myasthenic crisis (MC), resulting in profound weakness of the respiratory muscles. Therefore, patients with MG are at increased risk for COVID-19 and may have a more complicated course when infected.
Our patient with MG presented for severe COVID-19 symptoms twice and later developed MC. He received 2 treatment modalities available under an EUA (remdesivir and convalescent plasma) for COVID-19, resulting in symptom resolution and a negative polymerize chain reaction (PCR) test result for the virus. However, after receiving his typical maintenance therapy of IV immunoglobulin (IVIG) for his MG, he again developed symptoms consistent with COVID-19 and tested positive. After recovering from the second episode of COVID-19, the patient went into MC requiring plasmapheresis.
Case Presentation
A 56-year-old male, US Army veteran presented to Carl R. Darnall Army Medical Center emergency department (ED) 6 days after testing positive for COVID-19, with worsening sputum, cough, congestion, dyspnea, and fever. Due to his MG, the patient had a home oxygen monitor and reported that his oxygenation saturation dropped below 90% with minimal exertion. His medical history was significant for MG, status postthymectomy and radiation treatment, left hemidiaphragm paralysis secondary to phrenic nerve injury, and corticosteroid-induced insulin-dependent diabetes mellitus. His current home medications included pyridostigmine 60 mg 3 times a day, mycophenolate (MMF) 1500 mg twice daily, IV immunoglobulin (IVIG) every 3 weeks, insulin aspart up to 16 U per meal, insulin glargine 30 U twice a day, dulaglutide 0.75 mg every week, and metformin 1000 mg twice daily.
On initial examination, the patient’s heart rate (HR) was 111 beats/min, respiratory rate (RR), 22 breaths/min, blood pressure (BP), 138/88 mm Hg, temperature, 100.9 oF, and his initial pulse oximetry, 91% on room air. On physical examination, the patient was tachypneic, though without other signs of respiratory distress. Lung auscultation revealed no adventitial lung sounds. His cardiac examination was notable only for tachycardia. His neurologic examination demonstrated intact cranial nerves, with 5 out of 5 (scale 1 to 5) strength throughout the upper and lower extremities, sensation was intact to light touch, and he had normal cerebellar function. The rest of the examination was normal.
Initial laboratory investigation was notable for a white blood cell count of 14.15x103 cells/mcL with 84% neutrophils, and 6% lymphocytes. Additional tests revealed a C-reactive protein (CRP) level, 17.97 mg/dL (reference range, 0-0.5 mg/dL), ferritin level, 647 ng/mL (reference range, 22-274 ng/mL), d-dimer, 0.64 mcg/mL (reference range, 0-0.47mcg/mL), and a repeated positive COVID-19 PCR test. A portable chest X-ray showed bibasilar opacities (Figure 1).
The patient was diagnosed with COVID-19 and admitted to the intensive care unit (ICU). In the ICU, the patient received 1 U of convalescent plasma (CP) and started on a course of IV remdesivir 100 mg/d consistent with the EUA. He also received a 5-day course of ceftriaxone and azithromycin for possible community acquired pneumonia (CAP). As part of the patient’s MG maintenance medications, he received IVIG 4 g while in the ICU. Throughout his ICU stay, he required supplemental nasal cannula oxygenation to maintain his oxygen saturation > 93%. After 8 days in the ICU, his oxygen requirements decreased, and the patient was transferred out of the ICU and remdesivir was discontinued. On hospital day 10, a repeat COVID-19 PCR test was negative, inflammatory markers returned to within normal limits, and a repeat chest X-ray showed improvement from admission (Figure 2). Having recovered significantly, he was discharged home.
Three weeks later, the patient again presented to the MTF with 3 days of dyspnea, cough, fever, nausea, and vomiting. One day before symptom onset, he had received his maintenance IVIG infusion. The patient reported that his home oxygen saturation was 82% with minimal exertion. On ED presentation his HR was 107 beats/min, RR, 28 breaths/min, temperature, 98.1 oF, BP 118/71 mm Hg, and oxygen saturation, 92% on 2L nasal cannula. His examination was most notable for tachypnea with accessory muscle use. At this time, his neurologic examination was unchanged from prior admission with grossly intact cranial nerves and symmetric 5 of 5 motor strength in all extremities.
At this second ED visit, laboratory results demonstrated a CRP of 3.44 mg/dL, ferritin 2019 ng/mL, d-dimer, 3.39 mcg/mL, and a positive COVID-19 PCR result. His chest X-ray demonstrated new peripheral opacities compared with the X-ray at discharge (Figure 3). He required ICU admission again for his COVID-19 symptoms.
During his ICU course he continued to require supplemental oxygen by nasal cannula, though never required intubation. This second admission, he was again treated empirically for CAP with levofloxacin 750 mg daily for 5 days. He was discharged after 14 days with symptom resolution and down trending of inflammatory markers, though he was not retested for COVID-19.
Four days after his second discharge, he presented to the ED for a third time with diffuse weakness, dysphagia, and dysarthria of 1 day. His HR was 87/beats/min; RR, 17 breaths/min; temperature, 98.7 oF; BP, 144/81 mm Hg; and oxygen saturation, 98% on room air. His examination was significant for slurred speech, bilateral ptosis, 3 of 5 strength in bilateral finger flexion/abduction, wrist extension, knee and ankle flexion/extension; 4 of 5 strength in bilateral proximal muscle testing of deltoid, and hip; normal sensation, cerebellar function and reflexes. His negative inspiratory force (NIF) maximal effort was −30 cmH2O. He was determined to be in MC without evidence of COIVD-19 symptoms, and laboratory results were within normal limits, including a negative COVID-19 PCR. As he received IVIG as maintenance therapy, plasmapheresis was recommended to treat his MC, which required transfer to an outside civilian facility.
At the outside hospital, the patient underwent 5 rounds of plasmapheresis over 10 days. By the third treatment his strength had returned with resolution of the bulbar symptoms and no supplemental oxygen requirements. The patient was discharged and continued his original dosages of MMF and pyridostigmine. At 3 months, he remained asymptomatic from a COVID-19 standpoint and stable from a MG standpoint.
Discussion
Reinfection with the COVID-19 has been continuously debated with alternative explanations suggested for a positive test after a previous negative PCR test in the setting of symptom resolution.1,2 Proposed causes include dynamic PCR results due to prolonged viral shedding and inaccurate or poorly sensitive tests. The repeat positive cases in these scenarios, however, occurred in asymptomatic patients.1,2 COVID-19 shedding averages 20 to 22 days after symptom onset but has been seen up to 36 days after symptom resolution.2,3 This would suggest that fluctuating results during the immediate postsymptom period may be due to variations in viral shedding load and or sampling error—especially in asymptomatic patients. On the other hand, patients who experience return of symptoms days to weeks after previous convalescence leave clinicians wondering whether this represents clinical latency with reactivation or COVID-19 reinfection. A separate case of initial COVID-19 in a patient that had subsequent clinical recovery with a negative PCR developed recurrent respiratory symptoms and had a positive PCR test only 10 days later, further highlighting the reinfection vs reactivation issue of COVID-19.2 Further understanding of this issue may have implications on the extent of natural immunity following primary infection; potential vaccine dosage schedules; and global public health policies.
Although reactivation may be plausible given his immunomodulatory therapy, our patient’s second COVID-19 symptoms started 40 days after the initial symptoms, and 26 days after the initial course resolution; previous cases of return of severe symptoms occurred between 3 and 6 days.1 Given our patient’s time course between resolution and return of symptoms, if latency is the mechanism at play, this case demonstrates an exceptionally longer latency period than the ones that have been reported. Additionally, if latency is an issue in COVID-19, using remdesivir as a treatment further complicates the understanding of this disease.
Remdesivir, a nucleoside analogue antiviral, was shown to benefit recovery in patients with severe symptoms in the Adaptive COVID-19 Treatment Trial-1 study.4 Our patient had originally been placed on a 10-day course; however, on treatment day 8, his symptoms resolved and the remdesivir was discontinued. This is a similar finding to half the patients in the 10-day arm of the study by McCreary and colleagues.5 Although our patient was asymptomatic 4 weeks after the start of remdesivir, consistent with the majority of patients in the McCreary 10-day study arm, further comparison of the presented patient is limited due to study length and follow-up considerations.5 No previous data exist on reactivation, reinfection, or long-term mortality after being treated with remdesivir for COVID-19 infection.
IVIG is being studied in the treatment of COVID-19 and bears consideration as it relates to our patient. There is no evidence that IVIG used in the treatment of autoimmune diseases increases the risk of infection compared with that of other medications used in the treatment of such diseases. Furthermore, the current guidance from the MG expert panel does not suggest that IVIG increases the risk of contracting COVID-19 aside from the risks of exposure to hospital infrastructure.6 Yet the guidance does not discuss the use of IVIG for MG in patients who are already symptomatic from COVID-19 or for patients recovering from the clinical disease or does it discuss a possible compounding risk of thromboembolic events associated with IVIG and COVID-19.6,7 Our patient received his maintenance IVIG during his first admission without any worsening of symptoms or increased oxygen requirements. The day following our patient’s next scheduled IVIG infusion—while asymptomatic—he again developed respiratory symptoms; this could suggest that IVIG did not contribute to his second clinical course nor protect against.
CP is a treatment modality that has been used and studied in previous infectious outbreaks such as the first severe acute respiratory syndrome, and the H1N1 influenza virus.8 Current data on CP for COVID-19 are limited, but early descriptive studies have shown a benefit in improvement of symptoms 5 days sooner in those requiring supplemental oxygen, but no benefit for those requiring mechanical ventilation.9 Like patients that benefitted in these studies, our patient received CP early, 6 days after first testing positive and onset of symptoms. This patient’s reinfection or return of symptoms draws into question the hindrance or even prevention of long-term immunity from administration of CP.
COVID-19 presents many challenges when managing this patient’s coexisting MG, especially as the patient was already being treated with immunosuppressing therapies. The guidance does recommend continuation of standard MG therapies during hospitalizations, including immunosuppression medications such as MMF.6 Immunosuppression is associated with worsened severity of COVID-19 symptoms, although no relation exists to degree of immunosuppression and severity.7,10 To the best of our knowledge there has been no case report of reinfection or reactivation of COVID-19 associated with immunosuppressive agents used in the treatment of MG.
Our patient also was taking pyridostigmine for the treatment of his MG. There is no evidence this medication increases the risk of infection; but the cholinergic activity can increase bronchial secretions, which could theoretically worsen the COVID-19 respiratory symptoms.6,11 During both ICU admissions, our patient continued pyridostigmine use, observing complete return to baseline after discharge. Given the possible association with worsened respiratory outcomes after the second ICU admission, the balance between managing MG symptoms and COVID-19 symptoms needs further examination.
The patient was in MC during his third presentation to the ED. Although respiratory symptoms may be difficult to differentiate from COVID-19, the additional neurologic symptoms seen in this patient allowed for quick determination of the need for MC treatment. There are many potential etiologies contributing to the development of the MC presented here, and it was likely due to multifactorial precipitants. A common cause of MC is viral upper respiratory infections, further challenging the care of these patients during this pandemic.12 Many medications have been cited as causing a MC, 2 of which our patient received during admission for COVID-19: azithromycin and levoquin.12 Although the patient did not receive hydroxychloroquine, which was still being considered as an appropriate COVID-19 treatment at the time, it also is a drug known for precipitating MC and its use scrutinized in patients with MG.12
A key aspect to diagnosing and guiding therapies in myasthenic crisis in addition to the clinical symptoms of acute weakness is respiratory assessment through the nonaerosolizing NIF test.12 Our patient’s NIF measured < 30 cmH2O when in MC, while the reference range is < 75 cmH2O, and for mechanical ventilation is recommended at 20 cmH2O. Although the patient was maintaining O2 saturation > 95%, his NIF value was concerning, and preparations were made in case of precipitous decline. Compounding the NIF assessment in this patient is his history of left phrenic nerve palsy. Without a documented baseline NIF, results were limited in determining his diaphragm strength.13 Treatment for MC includes IVIG or plasmapheresis, since this patient had failed his maintenance therapy IVIG, plasmapheresis was coordinated for definitive therapy.
Conclusions
Federal facilities have seen an increase in the amount of respiratory complaints over the past months. Although COVID-19 is a concerning diagnosis, it is crucial to consider comorbidities in the diagnostic workup of each, even with a previous recent diagnosis of COVID-19. As treatment recommendations for COVID-19 continue to fluctuate coupled with the limitations and difficulties associated with MG patients, so too treatment and evaluation must be carefully considered at each presentation.
1. Gousseff M, Penot P, Gallay L, et al. Clinical recurrences of COVID-19 symptoms after recovery: viral relapse, reinfection or inflammatory rebound? J Infect. 2020;81(5):816-846. doi:10.1016/j.jinf.2020.06.073
2. Duggan NM, Ludy SM, Shannon BC, Reisner AT, Wilcox SR. Is novel coronavirus 2019 reinfection possible? Interpreting dynamic SARS-CoV-2 test results. Am J Emerg Med. 2021;39:256.e1-256.e3. doi:10.1016/j.ajem.2020.06.079
3. Li J, Zhang L, Liu B, Song D. Case report: viral shedding for 60 days in a woman with COVID-19. Am J Trop Med Hyg. 2020;102(6):1210-1213. doi:10.4269/ajtmh.20-0275
4. Beigel JH, Tomashek KM, Dodd LE. Remdesivir for the treatment of Covid-19 - preliminary report. Reply. N Engl J Med. 2020;383(10):994. doi:10.1056/NEJMc2022236
5. McCreary EK, Angus DC. Efficacy of remdesivir in COVID-19. JAMA. 2020;324(11):1041-1042. doi:10.1001/jama.2020.16337
6. International MG/COVID-19 Working Group; Jacob S, Muppidi S, Gordon A, et al. Guidance for the management of myasthenia gravis (MG) and Lambert-Eaton myasthenic syndrome (LEMS) during the COVID-19 pandemic. J Neurol Sci. 2020;412:116803. doi:10.1016/j.jns.2020.116803
7. Anand P, Slama MCC, Kaku M, et al. COVID-19 in patients with myasthenia gravis. Muscle Nerve. 2020;62(2):254-258. doi:10.1002/mus.26918
8. Wooding DJ, Bach H. Treatment of COVID-19 with convalescent plasma: lessons from past coronavirus outbreaks. Clin Microbiol Infect. 2020;26(10):1436-1446. doi:10.1016/j.cmi.2020.08.005
9. Salazar E, Perez KK, Ashraf M, et al. Treatment of coronavirus disease 2019 (covid-19) patients with convalescent plasma. Am J Pathol. 2020;190(8):1680-1690. doi:10.1016/j.ajpath.2020.05.014
10. Ryan C, Minc A, Caceres J, et al. Predicting severe outcomes in Covid-19 related illness using only patient demographics, comorbidities and symptoms [published online ahead of print, 2020 Sep 9]. Am J Emerg Med. 2020;S0735-6757(20)30809-3. doi:10.1016/j.ajem.2020.09.017
11. Singh S, Govindarajan R. COVID-19 and generalized myasthenia gravis exacerbation: a case report. Clin Neurol Neurosurg. 2020;196:106045. doi:10.1016/j.clineuro.2020.106045
12. Wendell LC, Levine JM. Myasthenic crisis. Neurohospitalist. 2011;1(1):16-22. doi:10.1177/1941875210382918
13. Dubé BP, Dres M. Diaphragm dysfunction: diagnostic approaches and management strategies. J Clin Med. 2016;5(12):113. Published 2016 Dec 5. doi:10.3390/jcm5120113
1. Gousseff M, Penot P, Gallay L, et al. Clinical recurrences of COVID-19 symptoms after recovery: viral relapse, reinfection or inflammatory rebound? J Infect. 2020;81(5):816-846. doi:10.1016/j.jinf.2020.06.073
2. Duggan NM, Ludy SM, Shannon BC, Reisner AT, Wilcox SR. Is novel coronavirus 2019 reinfection possible? Interpreting dynamic SARS-CoV-2 test results. Am J Emerg Med. 2021;39:256.e1-256.e3. doi:10.1016/j.ajem.2020.06.079
3. Li J, Zhang L, Liu B, Song D. Case report: viral shedding for 60 days in a woman with COVID-19. Am J Trop Med Hyg. 2020;102(6):1210-1213. doi:10.4269/ajtmh.20-0275
4. Beigel JH, Tomashek KM, Dodd LE. Remdesivir for the treatment of Covid-19 - preliminary report. Reply. N Engl J Med. 2020;383(10):994. doi:10.1056/NEJMc2022236
5. McCreary EK, Angus DC. Efficacy of remdesivir in COVID-19. JAMA. 2020;324(11):1041-1042. doi:10.1001/jama.2020.16337
6. International MG/COVID-19 Working Group; Jacob S, Muppidi S, Gordon A, et al. Guidance for the management of myasthenia gravis (MG) and Lambert-Eaton myasthenic syndrome (LEMS) during the COVID-19 pandemic. J Neurol Sci. 2020;412:116803. doi:10.1016/j.jns.2020.116803
7. Anand P, Slama MCC, Kaku M, et al. COVID-19 in patients with myasthenia gravis. Muscle Nerve. 2020;62(2):254-258. doi:10.1002/mus.26918
8. Wooding DJ, Bach H. Treatment of COVID-19 with convalescent plasma: lessons from past coronavirus outbreaks. Clin Microbiol Infect. 2020;26(10):1436-1446. doi:10.1016/j.cmi.2020.08.005
9. Salazar E, Perez KK, Ashraf M, et al. Treatment of coronavirus disease 2019 (covid-19) patients with convalescent plasma. Am J Pathol. 2020;190(8):1680-1690. doi:10.1016/j.ajpath.2020.05.014
10. Ryan C, Minc A, Caceres J, et al. Predicting severe outcomes in Covid-19 related illness using only patient demographics, comorbidities and symptoms [published online ahead of print, 2020 Sep 9]. Am J Emerg Med. 2020;S0735-6757(20)30809-3. doi:10.1016/j.ajem.2020.09.017
11. Singh S, Govindarajan R. COVID-19 and generalized myasthenia gravis exacerbation: a case report. Clin Neurol Neurosurg. 2020;196:106045. doi:10.1016/j.clineuro.2020.106045
12. Wendell LC, Levine JM. Myasthenic crisis. Neurohospitalist. 2011;1(1):16-22. doi:10.1177/1941875210382918
13. Dubé BP, Dres M. Diaphragm dysfunction: diagnostic approaches and management strategies. J Clin Med. 2016;5(12):113. Published 2016 Dec 5. doi:10.3390/jcm5120113
Fauci says ‘unprecedented’ conditions could influence COVID vaccine approval for kids
“From a public health standpoint, I think we have an evolving situation,” said Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, in a moderated session with Lee Beers, MD, president of the American Academy of Pediatrics, at the virtual Pediatric Hospital Medicine annual conference.
The reasons for this shift remain unclear, he said.
Dr. Beers emphasized the ability of pediatric hospitalists to be flexible in the face of uncertainty and the evolving virus, and asked Dr. Fauci to elaborate on the unique traits of the delta variant that make it especially challenging.
“There is no doubt that delta transmits much more efficiently than the alpha variant or any other variant,” Dr. Fauci said. The transmissibility is evident in comparisons of the level of virus in the nasopharynx of the delta variant, compared with the original alpha COVID-19 virus – delta is as much as 1,000 times higher, he explained.
In addition, the level of virus in the nasopharynx of vaccinated individuals who develop breakthrough infections with the delta variant is similar to the levels in unvaccinated individuals who are infected with the delta variant.
The delta variant is “the tough guy on the block” at the moment, Dr. Fauci said.
Dr. Fauci also responded to a question on the lack of winter viruses, such as RSV and the flu, last winter, but the surge in these viruses over the summer.
This winter’s activity remains uncertain, Dr. Fauci said. However, he speculated “with a strong dose of humility and modesty” that viruses tend to have niches, some are seasonal, and the winter viruses that were displaced by COVID-19 hit harder in the summer instead. “If I were a [non-COVID] virus looking for a niche, I would be really confused,” he said. “I don’t know what will happen this winter, but if we get good control over COVID-19 by winter, we could have a very vengeful influenza season,” he said. “This is speculation, I don’t have any data for this,” he cautioned.
Dr. Beers raised the issue of back-to-school safety, and the updated AAP guidance for universal masking for K-12 students. “Our guidance about return to school gets updated as the situation changes and we gain a better understanding of how kids can get to school safely,” she said. A combination of factors affect back-to-school guidance, including the ineligibility of children younger than 12 years to be vaccinated, the number of adolescents who are eligible but have not been vaccinated, and the challenge for educators to navigate which children should wear masks, Dr. Beers said.
“We want to get vaccines for our youngest kids as soon as safely possible,” Dr. Beers emphasized. She noted that the same urgency is needed to provide vaccines for children as for adults, although “we have to do it safely, and be sure and feel confident in the data.”
When asked to comment about the status of FDA authorization of COVID-19 vaccines for younger children, Dr. Fauci described the current situation as one that “might require some unprecedented and unique action” on the part of the FDA, which tends to move cautiously because of safety considerations. However, concerns about adverse events might get in the way of protecting children against what “you are really worried about,” in this case COVID-19 and its variants, he said. Despite the breakthrough infections, “vaccination continues to very adequately protect people from getting severe disease,” he emphasized.
Dr. Fauci also said that he believes the current data support boosters for the immune compromised; however “it is a different story about the general vaccinated population and the vaccinated elderly,” he said. Sooner or later most people will likely need boosters; “the question is who, when, and how soon,” he noted.
Dr. Fauci wrapped up the session with kudos and support for the pediatric health care community. “As a nonpediatrician, I have a great deal of respect for the job you are doing,” he said. “Keep up the great work.”
Dr. Beers echoed this sentiment, saying that she was “continually awed, impressed, and inspired” by how the pediatric hospitalists are navigating the ever-changing pandemic environment.
“From a public health standpoint, I think we have an evolving situation,” said Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, in a moderated session with Lee Beers, MD, president of the American Academy of Pediatrics, at the virtual Pediatric Hospital Medicine annual conference.
The reasons for this shift remain unclear, he said.
Dr. Beers emphasized the ability of pediatric hospitalists to be flexible in the face of uncertainty and the evolving virus, and asked Dr. Fauci to elaborate on the unique traits of the delta variant that make it especially challenging.
“There is no doubt that delta transmits much more efficiently than the alpha variant or any other variant,” Dr. Fauci said. The transmissibility is evident in comparisons of the level of virus in the nasopharynx of the delta variant, compared with the original alpha COVID-19 virus – delta is as much as 1,000 times higher, he explained.
In addition, the level of virus in the nasopharynx of vaccinated individuals who develop breakthrough infections with the delta variant is similar to the levels in unvaccinated individuals who are infected with the delta variant.
The delta variant is “the tough guy on the block” at the moment, Dr. Fauci said.
Dr. Fauci also responded to a question on the lack of winter viruses, such as RSV and the flu, last winter, but the surge in these viruses over the summer.
This winter’s activity remains uncertain, Dr. Fauci said. However, he speculated “with a strong dose of humility and modesty” that viruses tend to have niches, some are seasonal, and the winter viruses that were displaced by COVID-19 hit harder in the summer instead. “If I were a [non-COVID] virus looking for a niche, I would be really confused,” he said. “I don’t know what will happen this winter, but if we get good control over COVID-19 by winter, we could have a very vengeful influenza season,” he said. “This is speculation, I don’t have any data for this,” he cautioned.
Dr. Beers raised the issue of back-to-school safety, and the updated AAP guidance for universal masking for K-12 students. “Our guidance about return to school gets updated as the situation changes and we gain a better understanding of how kids can get to school safely,” she said. A combination of factors affect back-to-school guidance, including the ineligibility of children younger than 12 years to be vaccinated, the number of adolescents who are eligible but have not been vaccinated, and the challenge for educators to navigate which children should wear masks, Dr. Beers said.
“We want to get vaccines for our youngest kids as soon as safely possible,” Dr. Beers emphasized. She noted that the same urgency is needed to provide vaccines for children as for adults, although “we have to do it safely, and be sure and feel confident in the data.”
When asked to comment about the status of FDA authorization of COVID-19 vaccines for younger children, Dr. Fauci described the current situation as one that “might require some unprecedented and unique action” on the part of the FDA, which tends to move cautiously because of safety considerations. However, concerns about adverse events might get in the way of protecting children against what “you are really worried about,” in this case COVID-19 and its variants, he said. Despite the breakthrough infections, “vaccination continues to very adequately protect people from getting severe disease,” he emphasized.
Dr. Fauci also said that he believes the current data support boosters for the immune compromised; however “it is a different story about the general vaccinated population and the vaccinated elderly,” he said. Sooner or later most people will likely need boosters; “the question is who, when, and how soon,” he noted.
Dr. Fauci wrapped up the session with kudos and support for the pediatric health care community. “As a nonpediatrician, I have a great deal of respect for the job you are doing,” he said. “Keep up the great work.”
Dr. Beers echoed this sentiment, saying that she was “continually awed, impressed, and inspired” by how the pediatric hospitalists are navigating the ever-changing pandemic environment.
“From a public health standpoint, I think we have an evolving situation,” said Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, in a moderated session with Lee Beers, MD, president of the American Academy of Pediatrics, at the virtual Pediatric Hospital Medicine annual conference.
The reasons for this shift remain unclear, he said.
Dr. Beers emphasized the ability of pediatric hospitalists to be flexible in the face of uncertainty and the evolving virus, and asked Dr. Fauci to elaborate on the unique traits of the delta variant that make it especially challenging.
“There is no doubt that delta transmits much more efficiently than the alpha variant or any other variant,” Dr. Fauci said. The transmissibility is evident in comparisons of the level of virus in the nasopharynx of the delta variant, compared with the original alpha COVID-19 virus – delta is as much as 1,000 times higher, he explained.
In addition, the level of virus in the nasopharynx of vaccinated individuals who develop breakthrough infections with the delta variant is similar to the levels in unvaccinated individuals who are infected with the delta variant.
The delta variant is “the tough guy on the block” at the moment, Dr. Fauci said.
Dr. Fauci also responded to a question on the lack of winter viruses, such as RSV and the flu, last winter, but the surge in these viruses over the summer.
This winter’s activity remains uncertain, Dr. Fauci said. However, he speculated “with a strong dose of humility and modesty” that viruses tend to have niches, some are seasonal, and the winter viruses that were displaced by COVID-19 hit harder in the summer instead. “If I were a [non-COVID] virus looking for a niche, I would be really confused,” he said. “I don’t know what will happen this winter, but if we get good control over COVID-19 by winter, we could have a very vengeful influenza season,” he said. “This is speculation, I don’t have any data for this,” he cautioned.
Dr. Beers raised the issue of back-to-school safety, and the updated AAP guidance for universal masking for K-12 students. “Our guidance about return to school gets updated as the situation changes and we gain a better understanding of how kids can get to school safely,” she said. A combination of factors affect back-to-school guidance, including the ineligibility of children younger than 12 years to be vaccinated, the number of adolescents who are eligible but have not been vaccinated, and the challenge for educators to navigate which children should wear masks, Dr. Beers said.
“We want to get vaccines for our youngest kids as soon as safely possible,” Dr. Beers emphasized. She noted that the same urgency is needed to provide vaccines for children as for adults, although “we have to do it safely, and be sure and feel confident in the data.”
When asked to comment about the status of FDA authorization of COVID-19 vaccines for younger children, Dr. Fauci described the current situation as one that “might require some unprecedented and unique action” on the part of the FDA, which tends to move cautiously because of safety considerations. However, concerns about adverse events might get in the way of protecting children against what “you are really worried about,” in this case COVID-19 and its variants, he said. Despite the breakthrough infections, “vaccination continues to very adequately protect people from getting severe disease,” he emphasized.
Dr. Fauci also said that he believes the current data support boosters for the immune compromised; however “it is a different story about the general vaccinated population and the vaccinated elderly,” he said. Sooner or later most people will likely need boosters; “the question is who, when, and how soon,” he noted.
Dr. Fauci wrapped up the session with kudos and support for the pediatric health care community. “As a nonpediatrician, I have a great deal of respect for the job you are doing,” he said. “Keep up the great work.”
Dr. Beers echoed this sentiment, saying that she was “continually awed, impressed, and inspired” by how the pediatric hospitalists are navigating the ever-changing pandemic environment.
FROM PHM 2021