User login
Should psychiatrists prescribe nonpsychotropic medications?
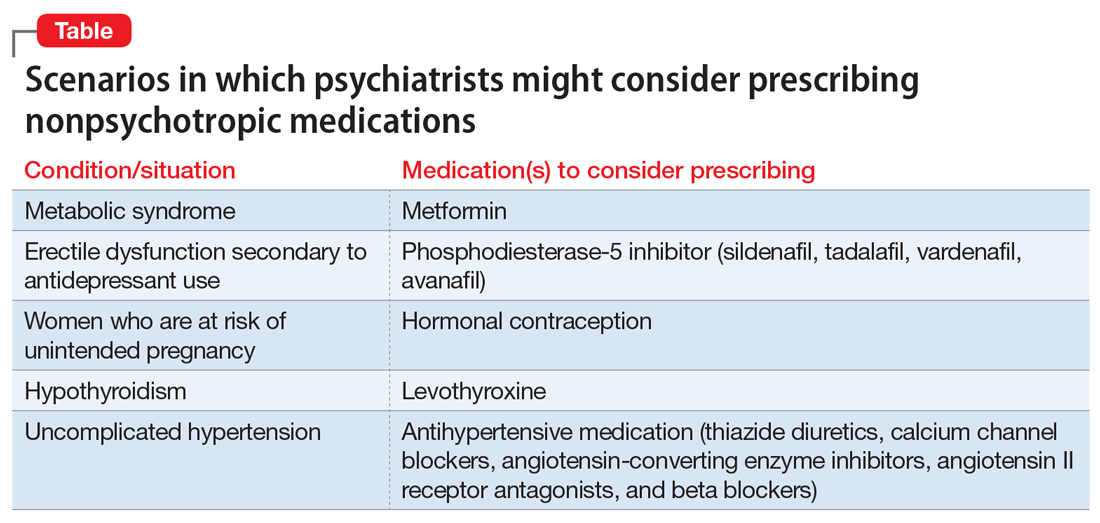
In our experience, most psychiatrists are uncomfortable with prescribing a medication when they feel that doing so would be outside their scope of practice. But there are many situations when prescribing a nonpsychotropic medication would be the correct choice. In this article, we discuss the scope of psychiatric practice, and present 4 case studies that illustrate situations in which psychiatrists should feel comfortable prescribing nonpsychotropic medications.
Defining the scope of practice
What is the scope of a psychiatrist’s practice? Scope of practice usually describes activities that a health care practitioner is allowed to undertake as defined by the terms of his/her license. A license to practice medicine does not include any stipulation restricting practice to a specific medical specialty. However, a local entity may delineate scope of practice within its organization. For instance, local practice standards held by the Detroit Wayne Mental Health Authority (DWMHA) state “Psychiatrists…shall not exceed their scope of practice as per DWMHA credentialing and privileging. For example, a Psychiatrist…who [has] not been appropriately privileged to deliver services to children shall not treat children, excepting crisis situations.”1
Like physicians in other specialties, psychiatrists are not limited to prescribing only a subset of medications commonly associated with their specialty. But for many psychiatrists, prescribing nonpsychotropic medications is complicated by individual and local factors. On one hand, some psychiatrists do not feel it is their role to prescribe nonpsychotropic medications,2 or even some psychotropic medications that may be more complex to prescribe, such as lithium, clozapine, or monoamine oxidase inhibitors.3-5 However, many feel comfortable prescribing complex combinations of psychotropic medications, or prescribing in a way that does not necessarily make sense (eg, prescribing benztropine as prophylaxis for dystonia when starting an antipsychotic).
Reviewing an average day at one urban psychiatric clinic, these questions seem to come up in half of the patient population, especially in patients with chronic mental illness, multiple medical comorbidities, and limited access to health care. When a young patient walks in without an appointment with an acute dystonic reaction secondary to the initiation of antipsychotics a couple of days ago, there is no hesitation to swiftly and appropriately prescribe an IM anticholinergic medication. But why are psychiatrists often hesitant to prescribe nonpsychotropic medications to treat other adverse effects of medications? Lack of knowledge? Lack of training?
Psychiatrists who practice in hospital systems often have immediate access to consultants, and this availability may encourage them to defer to the consultant for treatment of certain adverse effects. We have seen psychiatrists consult Neurology regarding the prescription of donepezil for mild neurocognitive disorder due to Alzheimer’s disease, or Endocrinology regarding prescription of levothyroxine for lithium-induced hypothyroidism.
However, there are numerous scenarios in which psychiatrists should feel comfortable prescribing nonpsychotropic medications or managing medication adverse effects, regardless of whether they consider it to be within or outside their scope of practice. The following case examples illustrate several such situations.
CASE 1
Ms. W, age 30, has been diagnosed with schizophrenia. She requests a refill of quetiapine, 800 mg/d. This medication has been clearly beneficial in alleviating her psychotic symptoms. However, since her last visit 3 months ago, her face appears more round, and she has gained 9 kg. Further evaluation indicates that she has developed metabolic syndrome and pre-diabetes.
Continue to: Metabolic adverse effects
Metabolic adverse effects, such as metabolic syndrome, diabetic ketoacidosis, and cardiovascular disease, are well-known risks of prescribing second-generation antipsychotics.6 In such situations, psychiatrists often advise patients to modify their diet, increase physical activity, and follow up with their primary care physician to determine if other medications are needed. However, getting a patient with a serious mental illness to exercise and modify her/his diet is difficult, and many of these patients do not have a primary care physician.
For patients such as Ms. W, a psychiatrist should consider prescribing metformin. Wu et al7 found that in addition to lifestyle modifications, metformin had the greatest effect on antipsychotic-induced weight gain. In this study, metformin alone had more impact on reversing weight gain and increasing insulin sensitivity than lifestyle modifications alone.7 This is crucial because these patients are especially vulnerable to cardiac disease.8 Metformin is well tolerated and has a low risk of causing hypoglycemia. Concerns regarding lactic acidosis have abated to the extent that the estimated glomerular filtration rate (eGFR) limits for using metformin have been lowered significantly. After reviewing the contraindications, the only knowledge needed to prescribe metformin is the patient’s kidney function and a brief understanding of the titration needed to minimize gastrointestinal adverse effects.9 Thus, prescribing metformin would be a fairly logical and easy first step for managing metabolic syndrome, especially in a patient whose motivation for increasing physical activity and modifying his/her diet is doubtful.
CASE 2
Mr. B, age 45, has major depressive disorder that has been well-controlled on paroxetine, 40 mg/d, for the past 2 years. He has no history of physical illness. On his most recent visit, he appears uncomfortable and nervous. After a long discussion, he discloses that his sex life isn’t what it used to be since starting paroxetine. He is bothered by erectile problems and asks whether he can “get some Viagra.”
Sexual adverse effects, such as erectile dysfunction, are frequently associated with the use of selective serotonin reuptake inhibitors.10 Although managing these adverse effects requires careful evaluation, in most cases, psychiatrists should be able to treat them.10 The logical choice in this case would be to prescribe one of the 4 FDA-approved phosphodiesterase-5 inhibitors (sildenafil [Viagra], tadalafil [Cialis], vardenafil [Levitra], and avanafil [Stendra]. However, Balon et al11 found that few psychiatrists prescribe phosphodiesterase-5 inhibitors, although they believed that they should be prescribing to treat their patients’ sexual dysfunction. Managing these adverse effects is important not only for the patient’s quality of life and relationship with his/her partner, but also for the therapeutic alliance. In a systematic review of 23 trials, Taylor et al12 examined >1,800 patients who were prescribed a medication to address sexual dysfunction secondary to antidepressants. They found that for men, adding a phosphodiesterase-5 inhibitor was appropriate and effective, and for women, adding bupropion at high doses should be considered.12 Like many other adverse effects, sexual adverse effects surely play a role in medication compliance. Dording et al13 found that the addition of sildenafil, 50 to 100 mg as needed, resulted in increased treatment satisfaction and overall contentment in 102 patients who complained of sexual dysfunction in the follow-up phase of the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) antidepressant trials. In most cases, with proper psychoeducation, prescription of
CASE 3
Ms. G, age 22, was recently discharged from an inpatient psychiatric unit after an episode of mania. She was prescribed carbamazepine, 600 mg/d, and ziprasidone, 40 mg twice a day, and appears to be doing well on this regimen. When asked about what led to her admission, she recalls having an elevated mood, increased energy, hypersexuality, impulsivity, and poor judgment. She reveals that she had several sexual partners during her manic episode, and worries that if such behavior occurs again, she may get pregnant. Yet Ms. G was not prescribed birth control upon discharge.
Continue to: Contraception
Contraception. We believe that psychiatrists have an obligation to protect patients from consequences of mental illness. Much the same way that psychiatrists hope to prevent suicide in a patient who has depression, patients should be protected from risks encountered in the manic phase of bipolar disorder. Another reason to prescribe contraceptives in such patients is the teratogenic effects of mood stabilizers. Pagano et al14 reviewed 6 studies that examined common forms of hormonal birth control to determine their safety in patients with depression or bipolar disorder. They found that overall, use of hormonal contraception was not associated with a worse clinical course of disease.
Many available forms of birth control are available. When prescribing in an outpatient setting, a daily oral medication or a monthly depot injection are convenient options.
CASE 4
Mr. P, age 65, has bipolar I disorder and is stable on risperidone long-acting injection, 37.7 mg bimonthly, and lithium, 1,200 mg/d. He reports that he is doing well but has noticed a recent decrease in energy and weight gain without any change in mood. Laboratory testing conducted prior to this visit revealed a thyroid-stimulating hormone (TSH) level of 4 mU/L (normal range: 0.4 to 4.0 mU/L). Six months ago, Mr. P’s TSH level was 2.8 mU/L. The resident supervisor suggests discussing the case with an endocrinologist.
Thyroid function. The impact of lithium on the thyroid gland is well established; however, psychiatrists’ response to such changes are not.15 Gitlin16 reviewed the many adverse effects of lithium and presented various management strategies to address findings such as Mr. P’s. Two important points are that lithium should not be discontinued in light of hypothyroidism, and synthetic thyroxine (levothyroxine) can be initiated and titrated to return TSH levels to a normal range.16 Levothyroxine can be started at low doses (eg, 25 to 50 mcg/d) and increased every 6 weeks until a normal TSH level is achieved.17 Managing lithium-induced clinical or subclinical hypothyroidism can prevent further pathology and possible relapse to depression.
Incorporating integrated care
In all these cases, the prescription of a medication with which some psychiatrists are not comfortable prescribing would have been the logical, easiest, and preferable choice. Of course, when initiating any medication, boxed warnings, contraindications, and drug–drug interactions should be reviewed. Initial dosages and titration schedules can be found in every medication’s FDA-approved prescribing information document (package insert), as well as in numerous reference books and articles.
Continue to: We acknowledge...
We acknowledge that prescribing a nonpsychotropic medication is not always a psychiatrist’s best choice, and that in patients with multiple medical comorbidities and drug–drug interactions that are not clearly defined, referring to or consulting a specialist is appropriate. We in no way support reckless prescribing, but instead present an opportunity to expand the perception of what should be considered within a psychiatrist’s scope of practice, and call for further education of psychiatrists so that they are more comfortable managing these adverse effects and/or prescribing at least some nonpsychotropic medications.
We exhort integrated medical care during this time of a physician shortage; however, we do not practice this way. Interestingly, physicians in primary care, such as those in family medicine or obstetrics and gynecology, frequently attempt to treat patients with psychiatric conditions in an attempt to provide integrated care. Numerous articles have discussed these efforts.18-20 However, this type of integrated care seems less frequent in psychiatry, even though the practice of modern psychiatry in the United States shows substantial overlap with the practice of physicians in primary care specialties.21 There are few articles or practical guidelines for psychiatrists who wish to treat patients’ physical illnesses, particularly patients with severe mental illness (see Related Resources, page 56). If we practice in an integrated manner to treat one of the simple conditions we described above, we can eliminate the need for a patient to visit a second physician, pay another co-pay, pay another bus fare, and take another day off work. This can be particularly helpful for patients who at times have to decide between paying for groceries or for medications. Having one clinician manage a patient’s medications also can decrease the risk of polypharmacy.
In addition to the case scenarios described in this article, there are more clinical situations and nonpsychotropic medications that psychiatrists could manage. Considering them outside the scope of psychiatric practice and being uncomfortable or ambivalent about them is not an excuse. We hope that psychiatrists can increase their expertise in this area, and can start to practice as the primary care physicians they claim they are, and should be.
Bottom Line
Many psychiatrists are uncomfortable prescribing nonpsychotropic medications, but there are numerous clinical scenarios in which the practice would make sense. This could include cases of metabolic syndrome, sexual dysfunction secondary to antidepressant use, or other adverse effects of commonly prescribed psychotropic medications.
Related Resources
- McCarron RM, Xiong GL, Keenan CR, et al. Preventive medical care in psychiatry. A practical guide for clinicians. Arlington, VA: American Psychiatric Association Publishing; 2015.
- McCarron RM, Xiong GL, Keenan CR, et al. Study guide to preventive medical care in psychiatry. Arlington, VA: American Psychiatric Association Publishing; 2017.
- Goldberg JF, Ernst CL. Managing the side effects of psychotropic medications. Washington, DC: American Psychiatric Association Publishing; 2019.
Drug Brand Names
Avanafil • Stendra
Benztropine • Cogentin
Bupropion • Wellbutrin, Zyban
Carbamazepine • Carbatrol, Tegretol
Clozapine • Clozaril
Donepezil • Aricept
Levothyroxine • Levoxyl, Synthroid
Lithium • Eskalith, Lithobid
Metformin • Fortamet, Glucophage
Paroxetine • Paxil
Quetiapine • Seroquel
Risperidone long-acting injection • Risperdal Consta
Sildenafil • Viagra
Tadalafil • Cialis
Vardenafil • Levitra
Ziprasidone • Geodon
1. Detroit Wayne Integrated Health Network. DWMHA psychiatric practice standards. http://dwihn.org/files/2015/6451/9628/Psychiatric_Practice_Standards.pdf. Revised June 2018. Accessed October 8, 2019.
2. Seaman JJ, Cornfield RM, Cummings DM, et al. Exploring psychiatric prescribing practices: the relationship between the role of the provider and the appropriateness of prescribing. Gen Hosp Psychiatry. 1987;9(3):220-224.
3. Zivanovic O. Lithium: a classic drug—frequently discussed, but, sadly, seldom prescribed! Aust N Z J Psychiatry. 2017;51(9):886-896.
4. Stroup TS, Gerhard T, Crystal S, et al. Geographic and clinical variation in clozapine use in the United States. Psychiatric Services. 2014;65(2):186-192.
5. Balon R, Mufti R, Arfken C. A survey of prescribing practices for monoamine oxidase inhibitors. Psychiatric Services. 1999;50(7):945-947.
6. Rummel-Kluge C, Komossa K, Schwarz S, et al. Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2010;123(2-3):225-233.
7. Wu RR, Zhao JP, Jin H, et al. Lifestyle intervention and metformin for treatment of antipsychotic-induced weight gain: a randomized controlled trial. JAMA. 2008;299(2):185-193.
8. De Hert M, Correll CU, Bobes J, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10(1):52-77.
9. Kirpichnikov D, McFarlane SI, Sowers JR. Metformin: an update. Ann Internal Med. 2002;137(1):25-33.
10. Balon R. SSRI-associated sexual dysfunction. Am J Psychiatry. 2006;163(9):1504-1509.
11. Balon R, Morreale MK, Segraves RT. Prescribing of phosphodiesterase-5 inhibitors among psychiatrists. J Sex Marital Ther. 2014;40(3):165-169.
12. Taylor MJ, Rudkin L, Bullemor-Day P, et al. Strategies for managing sexual dysfunction induced by antidepressant medication. Cochrane Database Syst Rev. 2013;(5):CD003382.
13. Dording CM, LaRocca RA, Hails KA, et al. The effect of sildenafil on quality of life. Ann Clin Psychiatry. 2013;25(1):3-10.
14. Pagano HP, Zapata LB, Berry-Bibee EN, et al. Safety of hormonal contraception and intrauterine devices among women with depressive and bipolar disorders: a systematic review. Contraception. 2016;94(6):641-649.
15. Kibirige D, Luzinda K, Ssekitoleko R. Spectrum of lithium induced thyroid abnormalities: a current perspective. Thyroid Res. 2013;6(1):3.
16. Gitlin M. Lithium side effects and toxicity: prevalence and management strategies. Int J Bipolar Disord. 2016;4(1):27.
17. Devdhar M, Ousman YH, Burman KD. Hypothyroidism. Endocrinol Metab Clin North Am. 2007;36(3):595-615.
18. Hackley B, Sharma C, Kedzior A, et al. Managing mental health conditions in primary care settings. J Midwifery Women’s Health. 2010;55(1):9-19.
19. Fitelson E, McGibbon C. Evaluation and management of behavioral health disorders in women: an overview of major depression, bipolar disorder, anxiety disorders, and sleep in the primary care setting. Obstet Gynecol Clin North Am. 2016;43(2):231-246.
20. Colorafi K, Vanselow J, Nelson T. Treating anxiety and depression in primary care: reducing barriers to access. Fam Pract Manag. 2017;24(4):11-16.
21. McCall WV. Defining the unique scope of psychiatric practice in 2015. J ECT. 2015;31(4):203-204.
In our experience, most psychiatrists are uncomfortable with prescribing a medication when they feel that doing so would be outside their scope of practice. But there are many situations when prescribing a nonpsychotropic medication would be the correct choice. In this article, we discuss the scope of psychiatric practice, and present 4 case studies that illustrate situations in which psychiatrists should feel comfortable prescribing nonpsychotropic medications.
Defining the scope of practice
What is the scope of a psychiatrist’s practice? Scope of practice usually describes activities that a health care practitioner is allowed to undertake as defined by the terms of his/her license. A license to practice medicine does not include any stipulation restricting practice to a specific medical specialty. However, a local entity may delineate scope of practice within its organization. For instance, local practice standards held by the Detroit Wayne Mental Health Authority (DWMHA) state “Psychiatrists…shall not exceed their scope of practice as per DWMHA credentialing and privileging. For example, a Psychiatrist…who [has] not been appropriately privileged to deliver services to children shall not treat children, excepting crisis situations.”1
Like physicians in other specialties, psychiatrists are not limited to prescribing only a subset of medications commonly associated with their specialty. But for many psychiatrists, prescribing nonpsychotropic medications is complicated by individual and local factors. On one hand, some psychiatrists do not feel it is their role to prescribe nonpsychotropic medications,2 or even some psychotropic medications that may be more complex to prescribe, such as lithium, clozapine, or monoamine oxidase inhibitors.3-5 However, many feel comfortable prescribing complex combinations of psychotropic medications, or prescribing in a way that does not necessarily make sense (eg, prescribing benztropine as prophylaxis for dystonia when starting an antipsychotic).
Reviewing an average day at one urban psychiatric clinic, these questions seem to come up in half of the patient population, especially in patients with chronic mental illness, multiple medical comorbidities, and limited access to health care. When a young patient walks in without an appointment with an acute dystonic reaction secondary to the initiation of antipsychotics a couple of days ago, there is no hesitation to swiftly and appropriately prescribe an IM anticholinergic medication. But why are psychiatrists often hesitant to prescribe nonpsychotropic medications to treat other adverse effects of medications? Lack of knowledge? Lack of training?
Psychiatrists who practice in hospital systems often have immediate access to consultants, and this availability may encourage them to defer to the consultant for treatment of certain adverse effects. We have seen psychiatrists consult Neurology regarding the prescription of donepezil for mild neurocognitive disorder due to Alzheimer’s disease, or Endocrinology regarding prescription of levothyroxine for lithium-induced hypothyroidism.
However, there are numerous scenarios in which psychiatrists should feel comfortable prescribing nonpsychotropic medications or managing medication adverse effects, regardless of whether they consider it to be within or outside their scope of practice. The following case examples illustrate several such situations.
CASE 1
Ms. W, age 30, has been diagnosed with schizophrenia. She requests a refill of quetiapine, 800 mg/d. This medication has been clearly beneficial in alleviating her psychotic symptoms. However, since her last visit 3 months ago, her face appears more round, and she has gained 9 kg. Further evaluation indicates that she has developed metabolic syndrome and pre-diabetes.
Continue to: Metabolic adverse effects
Metabolic adverse effects, such as metabolic syndrome, diabetic ketoacidosis, and cardiovascular disease, are well-known risks of prescribing second-generation antipsychotics.6 In such situations, psychiatrists often advise patients to modify their diet, increase physical activity, and follow up with their primary care physician to determine if other medications are needed. However, getting a patient with a serious mental illness to exercise and modify her/his diet is difficult, and many of these patients do not have a primary care physician.
For patients such as Ms. W, a psychiatrist should consider prescribing metformin. Wu et al7 found that in addition to lifestyle modifications, metformin had the greatest effect on antipsychotic-induced weight gain. In this study, metformin alone had more impact on reversing weight gain and increasing insulin sensitivity than lifestyle modifications alone.7 This is crucial because these patients are especially vulnerable to cardiac disease.8 Metformin is well tolerated and has a low risk of causing hypoglycemia. Concerns regarding lactic acidosis have abated to the extent that the estimated glomerular filtration rate (eGFR) limits for using metformin have been lowered significantly. After reviewing the contraindications, the only knowledge needed to prescribe metformin is the patient’s kidney function and a brief understanding of the titration needed to minimize gastrointestinal adverse effects.9 Thus, prescribing metformin would be a fairly logical and easy first step for managing metabolic syndrome, especially in a patient whose motivation for increasing physical activity and modifying his/her diet is doubtful.
CASE 2
Mr. B, age 45, has major depressive disorder that has been well-controlled on paroxetine, 40 mg/d, for the past 2 years. He has no history of physical illness. On his most recent visit, he appears uncomfortable and nervous. After a long discussion, he discloses that his sex life isn’t what it used to be since starting paroxetine. He is bothered by erectile problems and asks whether he can “get some Viagra.”
Sexual adverse effects, such as erectile dysfunction, are frequently associated with the use of selective serotonin reuptake inhibitors.10 Although managing these adverse effects requires careful evaluation, in most cases, psychiatrists should be able to treat them.10 The logical choice in this case would be to prescribe one of the 4 FDA-approved phosphodiesterase-5 inhibitors (sildenafil [Viagra], tadalafil [Cialis], vardenafil [Levitra], and avanafil [Stendra]. However, Balon et al11 found that few psychiatrists prescribe phosphodiesterase-5 inhibitors, although they believed that they should be prescribing to treat their patients’ sexual dysfunction. Managing these adverse effects is important not only for the patient’s quality of life and relationship with his/her partner, but also for the therapeutic alliance. In a systematic review of 23 trials, Taylor et al12 examined >1,800 patients who were prescribed a medication to address sexual dysfunction secondary to antidepressants. They found that for men, adding a phosphodiesterase-5 inhibitor was appropriate and effective, and for women, adding bupropion at high doses should be considered.12 Like many other adverse effects, sexual adverse effects surely play a role in medication compliance. Dording et al13 found that the addition of sildenafil, 50 to 100 mg as needed, resulted in increased treatment satisfaction and overall contentment in 102 patients who complained of sexual dysfunction in the follow-up phase of the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) antidepressant trials. In most cases, with proper psychoeducation, prescription of
CASE 3
Ms. G, age 22, was recently discharged from an inpatient psychiatric unit after an episode of mania. She was prescribed carbamazepine, 600 mg/d, and ziprasidone, 40 mg twice a day, and appears to be doing well on this regimen. When asked about what led to her admission, she recalls having an elevated mood, increased energy, hypersexuality, impulsivity, and poor judgment. She reveals that she had several sexual partners during her manic episode, and worries that if such behavior occurs again, she may get pregnant. Yet Ms. G was not prescribed birth control upon discharge.
Continue to: Contraception
Contraception. We believe that psychiatrists have an obligation to protect patients from consequences of mental illness. Much the same way that psychiatrists hope to prevent suicide in a patient who has depression, patients should be protected from risks encountered in the manic phase of bipolar disorder. Another reason to prescribe contraceptives in such patients is the teratogenic effects of mood stabilizers. Pagano et al14 reviewed 6 studies that examined common forms of hormonal birth control to determine their safety in patients with depression or bipolar disorder. They found that overall, use of hormonal contraception was not associated with a worse clinical course of disease.
Many available forms of birth control are available. When prescribing in an outpatient setting, a daily oral medication or a monthly depot injection are convenient options.
CASE 4
Mr. P, age 65, has bipolar I disorder and is stable on risperidone long-acting injection, 37.7 mg bimonthly, and lithium, 1,200 mg/d. He reports that he is doing well but has noticed a recent decrease in energy and weight gain without any change in mood. Laboratory testing conducted prior to this visit revealed a thyroid-stimulating hormone (TSH) level of 4 mU/L (normal range: 0.4 to 4.0 mU/L). Six months ago, Mr. P’s TSH level was 2.8 mU/L. The resident supervisor suggests discussing the case with an endocrinologist.
Thyroid function. The impact of lithium on the thyroid gland is well established; however, psychiatrists’ response to such changes are not.15 Gitlin16 reviewed the many adverse effects of lithium and presented various management strategies to address findings such as Mr. P’s. Two important points are that lithium should not be discontinued in light of hypothyroidism, and synthetic thyroxine (levothyroxine) can be initiated and titrated to return TSH levels to a normal range.16 Levothyroxine can be started at low doses (eg, 25 to 50 mcg/d) and increased every 6 weeks until a normal TSH level is achieved.17 Managing lithium-induced clinical or subclinical hypothyroidism can prevent further pathology and possible relapse to depression.
Incorporating integrated care
In all these cases, the prescription of a medication with which some psychiatrists are not comfortable prescribing would have been the logical, easiest, and preferable choice. Of course, when initiating any medication, boxed warnings, contraindications, and drug–drug interactions should be reviewed. Initial dosages and titration schedules can be found in every medication’s FDA-approved prescribing information document (package insert), as well as in numerous reference books and articles.
Continue to: We acknowledge...
We acknowledge that prescribing a nonpsychotropic medication is not always a psychiatrist’s best choice, and that in patients with multiple medical comorbidities and drug–drug interactions that are not clearly defined, referring to or consulting a specialist is appropriate. We in no way support reckless prescribing, but instead present an opportunity to expand the perception of what should be considered within a psychiatrist’s scope of practice, and call for further education of psychiatrists so that they are more comfortable managing these adverse effects and/or prescribing at least some nonpsychotropic medications.

We exhort integrated medical care during this time of a physician shortage; however, we do not practice this way. Interestingly, physicians in primary care, such as those in family medicine or obstetrics and gynecology, frequently attempt to treat patients with psychiatric conditions in an attempt to provide integrated care. Numerous articles have discussed these efforts.18-20 However, this type of integrated care seems less frequent in psychiatry, even though the practice of modern psychiatry in the United States shows substantial overlap with the practice of physicians in primary care specialties.21 There are few articles or practical guidelines for psychiatrists who wish to treat patients’ physical illnesses, particularly patients with severe mental illness (see Related Resources, page 56). If we practice in an integrated manner to treat one of the simple conditions we described above, we can eliminate the need for a patient to visit a second physician, pay another co-pay, pay another bus fare, and take another day off work. This can be particularly helpful for patients who at times have to decide between paying for groceries or for medications. Having one clinician manage a patient’s medications also can decrease the risk of polypharmacy.
In addition to the case scenarios described in this article, there are more clinical situations and nonpsychotropic medications that psychiatrists could manage. Considering them outside the scope of psychiatric practice and being uncomfortable or ambivalent about them is not an excuse. We hope that psychiatrists can increase their expertise in this area, and can start to practice as the primary care physicians they claim they are, and should be.
Bottom Line
Many psychiatrists are uncomfortable prescribing nonpsychotropic medications, but there are numerous clinical scenarios in which the practice would make sense. This could include cases of metabolic syndrome, sexual dysfunction secondary to antidepressant use, or other adverse effects of commonly prescribed psychotropic medications.
Related Resources
- McCarron RM, Xiong GL, Keenan CR, et al. Preventive medical care in psychiatry. A practical guide for clinicians. Arlington, VA: American Psychiatric Association Publishing; 2015.
- McCarron RM, Xiong GL, Keenan CR, et al. Study guide to preventive medical care in psychiatry. Arlington, VA: American Psychiatric Association Publishing; 2017.
- Goldberg JF, Ernst CL. Managing the side effects of psychotropic medications. Washington, DC: American Psychiatric Association Publishing; 2019.
Drug Brand Names
Avanafil • Stendra
Benztropine • Cogentin
Bupropion • Wellbutrin, Zyban
Carbamazepine • Carbatrol, Tegretol
Clozapine • Clozaril
Donepezil • Aricept
Levothyroxine • Levoxyl, Synthroid
Lithium • Eskalith, Lithobid
Metformin • Fortamet, Glucophage
Paroxetine • Paxil
Quetiapine • Seroquel
Risperidone long-acting injection • Risperdal Consta
Sildenafil • Viagra
Tadalafil • Cialis
Vardenafil • Levitra
Ziprasidone • Geodon
In our experience, most psychiatrists are uncomfortable with prescribing a medication when they feel that doing so would be outside their scope of practice. But there are many situations when prescribing a nonpsychotropic medication would be the correct choice. In this article, we discuss the scope of psychiatric practice, and present 4 case studies that illustrate situations in which psychiatrists should feel comfortable prescribing nonpsychotropic medications.
Defining the scope of practice
What is the scope of a psychiatrist’s practice? Scope of practice usually describes activities that a health care practitioner is allowed to undertake as defined by the terms of his/her license. A license to practice medicine does not include any stipulation restricting practice to a specific medical specialty. However, a local entity may delineate scope of practice within its organization. For instance, local practice standards held by the Detroit Wayne Mental Health Authority (DWMHA) state “Psychiatrists…shall not exceed their scope of practice as per DWMHA credentialing and privileging. For example, a Psychiatrist…who [has] not been appropriately privileged to deliver services to children shall not treat children, excepting crisis situations.”1
Like physicians in other specialties, psychiatrists are not limited to prescribing only a subset of medications commonly associated with their specialty. But for many psychiatrists, prescribing nonpsychotropic medications is complicated by individual and local factors. On one hand, some psychiatrists do not feel it is their role to prescribe nonpsychotropic medications,2 or even some psychotropic medications that may be more complex to prescribe, such as lithium, clozapine, or monoamine oxidase inhibitors.3-5 However, many feel comfortable prescribing complex combinations of psychotropic medications, or prescribing in a way that does not necessarily make sense (eg, prescribing benztropine as prophylaxis for dystonia when starting an antipsychotic).
Reviewing an average day at one urban psychiatric clinic, these questions seem to come up in half of the patient population, especially in patients with chronic mental illness, multiple medical comorbidities, and limited access to health care. When a young patient walks in without an appointment with an acute dystonic reaction secondary to the initiation of antipsychotics a couple of days ago, there is no hesitation to swiftly and appropriately prescribe an IM anticholinergic medication. But why are psychiatrists often hesitant to prescribe nonpsychotropic medications to treat other adverse effects of medications? Lack of knowledge? Lack of training?
Psychiatrists who practice in hospital systems often have immediate access to consultants, and this availability may encourage them to defer to the consultant for treatment of certain adverse effects. We have seen psychiatrists consult Neurology regarding the prescription of donepezil for mild neurocognitive disorder due to Alzheimer’s disease, or Endocrinology regarding prescription of levothyroxine for lithium-induced hypothyroidism.
However, there are numerous scenarios in which psychiatrists should feel comfortable prescribing nonpsychotropic medications or managing medication adverse effects, regardless of whether they consider it to be within or outside their scope of practice. The following case examples illustrate several such situations.
CASE 1
Ms. W, age 30, has been diagnosed with schizophrenia. She requests a refill of quetiapine, 800 mg/d. This medication has been clearly beneficial in alleviating her psychotic symptoms. However, since her last visit 3 months ago, her face appears more round, and she has gained 9 kg. Further evaluation indicates that she has developed metabolic syndrome and pre-diabetes.
Continue to: Metabolic adverse effects
Metabolic adverse effects, such as metabolic syndrome, diabetic ketoacidosis, and cardiovascular disease, are well-known risks of prescribing second-generation antipsychotics.6 In such situations, psychiatrists often advise patients to modify their diet, increase physical activity, and follow up with their primary care physician to determine if other medications are needed. However, getting a patient with a serious mental illness to exercise and modify her/his diet is difficult, and many of these patients do not have a primary care physician.
For patients such as Ms. W, a psychiatrist should consider prescribing metformin. Wu et al7 found that in addition to lifestyle modifications, metformin had the greatest effect on antipsychotic-induced weight gain. In this study, metformin alone had more impact on reversing weight gain and increasing insulin sensitivity than lifestyle modifications alone.7 This is crucial because these patients are especially vulnerable to cardiac disease.8 Metformin is well tolerated and has a low risk of causing hypoglycemia. Concerns regarding lactic acidosis have abated to the extent that the estimated glomerular filtration rate (eGFR) limits for using metformin have been lowered significantly. After reviewing the contraindications, the only knowledge needed to prescribe metformin is the patient’s kidney function and a brief understanding of the titration needed to minimize gastrointestinal adverse effects.9 Thus, prescribing metformin would be a fairly logical and easy first step for managing metabolic syndrome, especially in a patient whose motivation for increasing physical activity and modifying his/her diet is doubtful.
CASE 2
Mr. B, age 45, has major depressive disorder that has been well-controlled on paroxetine, 40 mg/d, for the past 2 years. He has no history of physical illness. On his most recent visit, he appears uncomfortable and nervous. After a long discussion, he discloses that his sex life isn’t what it used to be since starting paroxetine. He is bothered by erectile problems and asks whether he can “get some Viagra.”
Sexual adverse effects, such as erectile dysfunction, are frequently associated with the use of selective serotonin reuptake inhibitors.10 Although managing these adverse effects requires careful evaluation, in most cases, psychiatrists should be able to treat them.10 The logical choice in this case would be to prescribe one of the 4 FDA-approved phosphodiesterase-5 inhibitors (sildenafil [Viagra], tadalafil [Cialis], vardenafil [Levitra], and avanafil [Stendra]. However, Balon et al11 found that few psychiatrists prescribe phosphodiesterase-5 inhibitors, although they believed that they should be prescribing to treat their patients’ sexual dysfunction. Managing these adverse effects is important not only for the patient’s quality of life and relationship with his/her partner, but also for the therapeutic alliance. In a systematic review of 23 trials, Taylor et al12 examined >1,800 patients who were prescribed a medication to address sexual dysfunction secondary to antidepressants. They found that for men, adding a phosphodiesterase-5 inhibitor was appropriate and effective, and for women, adding bupropion at high doses should be considered.12 Like many other adverse effects, sexual adverse effects surely play a role in medication compliance. Dording et al13 found that the addition of sildenafil, 50 to 100 mg as needed, resulted in increased treatment satisfaction and overall contentment in 102 patients who complained of sexual dysfunction in the follow-up phase of the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) antidepressant trials. In most cases, with proper psychoeducation, prescription of
CASE 3
Ms. G, age 22, was recently discharged from an inpatient psychiatric unit after an episode of mania. She was prescribed carbamazepine, 600 mg/d, and ziprasidone, 40 mg twice a day, and appears to be doing well on this regimen. When asked about what led to her admission, she recalls having an elevated mood, increased energy, hypersexuality, impulsivity, and poor judgment. She reveals that she had several sexual partners during her manic episode, and worries that if such behavior occurs again, she may get pregnant. Yet Ms. G was not prescribed birth control upon discharge.
Continue to: Contraception
Contraception. We believe that psychiatrists have an obligation to protect patients from consequences of mental illness. Much the same way that psychiatrists hope to prevent suicide in a patient who has depression, patients should be protected from risks encountered in the manic phase of bipolar disorder. Another reason to prescribe contraceptives in such patients is the teratogenic effects of mood stabilizers. Pagano et al14 reviewed 6 studies that examined common forms of hormonal birth control to determine their safety in patients with depression or bipolar disorder. They found that overall, use of hormonal contraception was not associated with a worse clinical course of disease.
Many available forms of birth control are available. When prescribing in an outpatient setting, a daily oral medication or a monthly depot injection are convenient options.
CASE 4
Mr. P, age 65, has bipolar I disorder and is stable on risperidone long-acting injection, 37.7 mg bimonthly, and lithium, 1,200 mg/d. He reports that he is doing well but has noticed a recent decrease in energy and weight gain without any change in mood. Laboratory testing conducted prior to this visit revealed a thyroid-stimulating hormone (TSH) level of 4 mU/L (normal range: 0.4 to 4.0 mU/L). Six months ago, Mr. P’s TSH level was 2.8 mU/L. The resident supervisor suggests discussing the case with an endocrinologist.
Thyroid function. The impact of lithium on the thyroid gland is well established; however, psychiatrists’ response to such changes are not.15 Gitlin16 reviewed the many adverse effects of lithium and presented various management strategies to address findings such as Mr. P’s. Two important points are that lithium should not be discontinued in light of hypothyroidism, and synthetic thyroxine (levothyroxine) can be initiated and titrated to return TSH levels to a normal range.16 Levothyroxine can be started at low doses (eg, 25 to 50 mcg/d) and increased every 6 weeks until a normal TSH level is achieved.17 Managing lithium-induced clinical or subclinical hypothyroidism can prevent further pathology and possible relapse to depression.
Incorporating integrated care
In all these cases, the prescription of a medication with which some psychiatrists are not comfortable prescribing would have been the logical, easiest, and preferable choice. Of course, when initiating any medication, boxed warnings, contraindications, and drug–drug interactions should be reviewed. Initial dosages and titration schedules can be found in every medication’s FDA-approved prescribing information document (package insert), as well as in numerous reference books and articles.
Continue to: We acknowledge...
We acknowledge that prescribing a nonpsychotropic medication is not always a psychiatrist’s best choice, and that in patients with multiple medical comorbidities and drug–drug interactions that are not clearly defined, referring to or consulting a specialist is appropriate. We in no way support reckless prescribing, but instead present an opportunity to expand the perception of what should be considered within a psychiatrist’s scope of practice, and call for further education of psychiatrists so that they are more comfortable managing these adverse effects and/or prescribing at least some nonpsychotropic medications.

We exhort integrated medical care during this time of a physician shortage; however, we do not practice this way. Interestingly, physicians in primary care, such as those in family medicine or obstetrics and gynecology, frequently attempt to treat patients with psychiatric conditions in an attempt to provide integrated care. Numerous articles have discussed these efforts.18-20 However, this type of integrated care seems less frequent in psychiatry, even though the practice of modern psychiatry in the United States shows substantial overlap with the practice of physicians in primary care specialties.21 There are few articles or practical guidelines for psychiatrists who wish to treat patients’ physical illnesses, particularly patients with severe mental illness (see Related Resources, page 56). If we practice in an integrated manner to treat one of the simple conditions we described above, we can eliminate the need for a patient to visit a second physician, pay another co-pay, pay another bus fare, and take another day off work. This can be particularly helpful for patients who at times have to decide between paying for groceries or for medications. Having one clinician manage a patient’s medications also can decrease the risk of polypharmacy.
In addition to the case scenarios described in this article, there are more clinical situations and nonpsychotropic medications that psychiatrists could manage. Considering them outside the scope of psychiatric practice and being uncomfortable or ambivalent about them is not an excuse. We hope that psychiatrists can increase their expertise in this area, and can start to practice as the primary care physicians they claim they are, and should be.
Bottom Line
Many psychiatrists are uncomfortable prescribing nonpsychotropic medications, but there are numerous clinical scenarios in which the practice would make sense. This could include cases of metabolic syndrome, sexual dysfunction secondary to antidepressant use, or other adverse effects of commonly prescribed psychotropic medications.
Related Resources
- McCarron RM, Xiong GL, Keenan CR, et al. Preventive medical care in psychiatry. A practical guide for clinicians. Arlington, VA: American Psychiatric Association Publishing; 2015.
- McCarron RM, Xiong GL, Keenan CR, et al. Study guide to preventive medical care in psychiatry. Arlington, VA: American Psychiatric Association Publishing; 2017.
- Goldberg JF, Ernst CL. Managing the side effects of psychotropic medications. Washington, DC: American Psychiatric Association Publishing; 2019.
Drug Brand Names
Avanafil • Stendra
Benztropine • Cogentin
Bupropion • Wellbutrin, Zyban
Carbamazepine • Carbatrol, Tegretol
Clozapine • Clozaril
Donepezil • Aricept
Levothyroxine • Levoxyl, Synthroid
Lithium • Eskalith, Lithobid
Metformin • Fortamet, Glucophage
Paroxetine • Paxil
Quetiapine • Seroquel
Risperidone long-acting injection • Risperdal Consta
Sildenafil • Viagra
Tadalafil • Cialis
Vardenafil • Levitra
Ziprasidone • Geodon
1. Detroit Wayne Integrated Health Network. DWMHA psychiatric practice standards. http://dwihn.org/files/2015/6451/9628/Psychiatric_Practice_Standards.pdf. Revised June 2018. Accessed October 8, 2019.
2. Seaman JJ, Cornfield RM, Cummings DM, et al. Exploring psychiatric prescribing practices: the relationship between the role of the provider and the appropriateness of prescribing. Gen Hosp Psychiatry. 1987;9(3):220-224.
3. Zivanovic O. Lithium: a classic drug—frequently discussed, but, sadly, seldom prescribed! Aust N Z J Psychiatry. 2017;51(9):886-896.
4. Stroup TS, Gerhard T, Crystal S, et al. Geographic and clinical variation in clozapine use in the United States. Psychiatric Services. 2014;65(2):186-192.
5. Balon R, Mufti R, Arfken C. A survey of prescribing practices for monoamine oxidase inhibitors. Psychiatric Services. 1999;50(7):945-947.
6. Rummel-Kluge C, Komossa K, Schwarz S, et al. Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2010;123(2-3):225-233.
7. Wu RR, Zhao JP, Jin H, et al. Lifestyle intervention and metformin for treatment of antipsychotic-induced weight gain: a randomized controlled trial. JAMA. 2008;299(2):185-193.
8. De Hert M, Correll CU, Bobes J, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10(1):52-77.
9. Kirpichnikov D, McFarlane SI, Sowers JR. Metformin: an update. Ann Internal Med. 2002;137(1):25-33.
10. Balon R. SSRI-associated sexual dysfunction. Am J Psychiatry. 2006;163(9):1504-1509.
11. Balon R, Morreale MK, Segraves RT. Prescribing of phosphodiesterase-5 inhibitors among psychiatrists. J Sex Marital Ther. 2014;40(3):165-169.
12. Taylor MJ, Rudkin L, Bullemor-Day P, et al. Strategies for managing sexual dysfunction induced by antidepressant medication. Cochrane Database Syst Rev. 2013;(5):CD003382.
13. Dording CM, LaRocca RA, Hails KA, et al. The effect of sildenafil on quality of life. Ann Clin Psychiatry. 2013;25(1):3-10.
14. Pagano HP, Zapata LB, Berry-Bibee EN, et al. Safety of hormonal contraception and intrauterine devices among women with depressive and bipolar disorders: a systematic review. Contraception. 2016;94(6):641-649.
15. Kibirige D, Luzinda K, Ssekitoleko R. Spectrum of lithium induced thyroid abnormalities: a current perspective. Thyroid Res. 2013;6(1):3.
16. Gitlin M. Lithium side effects and toxicity: prevalence and management strategies. Int J Bipolar Disord. 2016;4(1):27.
17. Devdhar M, Ousman YH, Burman KD. Hypothyroidism. Endocrinol Metab Clin North Am. 2007;36(3):595-615.
18. Hackley B, Sharma C, Kedzior A, et al. Managing mental health conditions in primary care settings. J Midwifery Women’s Health. 2010;55(1):9-19.
19. Fitelson E, McGibbon C. Evaluation and management of behavioral health disorders in women: an overview of major depression, bipolar disorder, anxiety disorders, and sleep in the primary care setting. Obstet Gynecol Clin North Am. 2016;43(2):231-246.
20. Colorafi K, Vanselow J, Nelson T. Treating anxiety and depression in primary care: reducing barriers to access. Fam Pract Manag. 2017;24(4):11-16.
21. McCall WV. Defining the unique scope of psychiatric practice in 2015. J ECT. 2015;31(4):203-204.
1. Detroit Wayne Integrated Health Network. DWMHA psychiatric practice standards. http://dwihn.org/files/2015/6451/9628/Psychiatric_Practice_Standards.pdf. Revised June 2018. Accessed October 8, 2019.
2. Seaman JJ, Cornfield RM, Cummings DM, et al. Exploring psychiatric prescribing practices: the relationship between the role of the provider and the appropriateness of prescribing. Gen Hosp Psychiatry. 1987;9(3):220-224.
3. Zivanovic O. Lithium: a classic drug—frequently discussed, but, sadly, seldom prescribed! Aust N Z J Psychiatry. 2017;51(9):886-896.
4. Stroup TS, Gerhard T, Crystal S, et al. Geographic and clinical variation in clozapine use in the United States. Psychiatric Services. 2014;65(2):186-192.
5. Balon R, Mufti R, Arfken C. A survey of prescribing practices for monoamine oxidase inhibitors. Psychiatric Services. 1999;50(7):945-947.
6. Rummel-Kluge C, Komossa K, Schwarz S, et al. Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2010;123(2-3):225-233.
7. Wu RR, Zhao JP, Jin H, et al. Lifestyle intervention and metformin for treatment of antipsychotic-induced weight gain: a randomized controlled trial. JAMA. 2008;299(2):185-193.
8. De Hert M, Correll CU, Bobes J, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10(1):52-77.
9. Kirpichnikov D, McFarlane SI, Sowers JR. Metformin: an update. Ann Internal Med. 2002;137(1):25-33.
10. Balon R. SSRI-associated sexual dysfunction. Am J Psychiatry. 2006;163(9):1504-1509.
11. Balon R, Morreale MK, Segraves RT. Prescribing of phosphodiesterase-5 inhibitors among psychiatrists. J Sex Marital Ther. 2014;40(3):165-169.
12. Taylor MJ, Rudkin L, Bullemor-Day P, et al. Strategies for managing sexual dysfunction induced by antidepressant medication. Cochrane Database Syst Rev. 2013;(5):CD003382.
13. Dording CM, LaRocca RA, Hails KA, et al. The effect of sildenafil on quality of life. Ann Clin Psychiatry. 2013;25(1):3-10.
14. Pagano HP, Zapata LB, Berry-Bibee EN, et al. Safety of hormonal contraception and intrauterine devices among women with depressive and bipolar disorders: a systematic review. Contraception. 2016;94(6):641-649.
15. Kibirige D, Luzinda K, Ssekitoleko R. Spectrum of lithium induced thyroid abnormalities: a current perspective. Thyroid Res. 2013;6(1):3.
16. Gitlin M. Lithium side effects and toxicity: prevalence and management strategies. Int J Bipolar Disord. 2016;4(1):27.
17. Devdhar M, Ousman YH, Burman KD. Hypothyroidism. Endocrinol Metab Clin North Am. 2007;36(3):595-615.
18. Hackley B, Sharma C, Kedzior A, et al. Managing mental health conditions in primary care settings. J Midwifery Women’s Health. 2010;55(1):9-19.
19. Fitelson E, McGibbon C. Evaluation and management of behavioral health disorders in women: an overview of major depression, bipolar disorder, anxiety disorders, and sleep in the primary care setting. Obstet Gynecol Clin North Am. 2016;43(2):231-246.
20. Colorafi K, Vanselow J, Nelson T. Treating anxiety and depression in primary care: reducing barriers to access. Fam Pract Manag. 2017;24(4):11-16.
21. McCall WV. Defining the unique scope of psychiatric practice in 2015. J ECT. 2015;31(4):203-204.
2019 at a glance: Hem-onc U.S. drug approvals
The rapid development and identification of novel drugs has translated into innovative therapies in hematology and oncology. The aim of this piece is to present newly approved drugs and expanded indications to serve as a reference guide for practicing clinicians.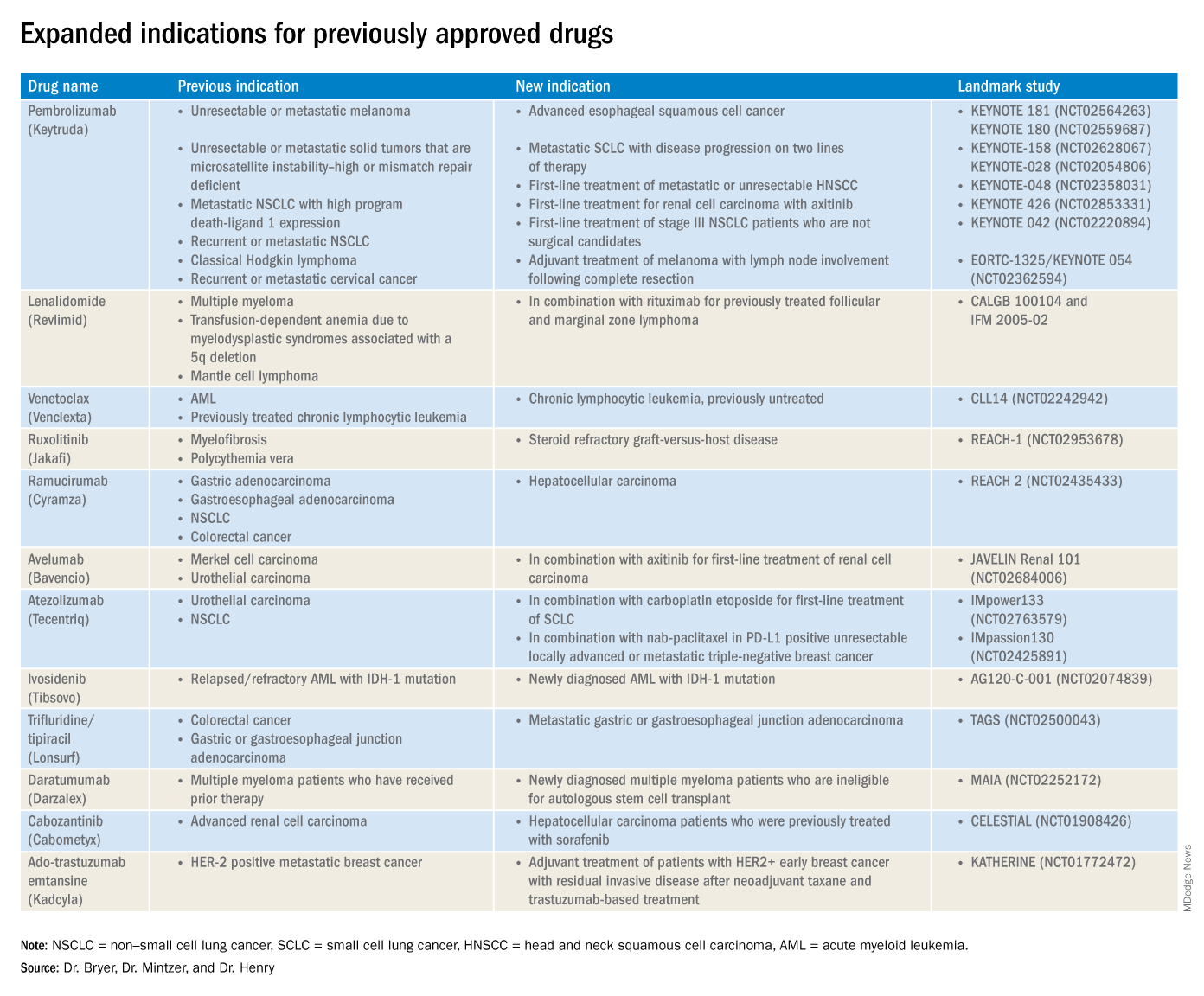
This article reviews therapies that were newly approved so far in 2019, as well as those previously approved whose indications were expanded this past year. The list highlights the most clinically important approvals, as well as adverse events that are unique or especially severe.
New approvals
Fedratinib (Inrebic)
Class: JAK2 and FLT3 selective kinase inhibitor.
Disease: Intermediate or high-risk primary or secondary (postpolycythemia vera or postessential thrombocythemia) myelofibrosis.
Dose: 400 mg orally once daily, with or without food.
Adverse events (AEs): Black box warning: Fatal encephalopathy, including Wernicke’s (thiamine level monitoring suggested).
Trials: In JAKARTA (NCT01437787), 37% of patients achieved a 35% or greater reduction in spleen volume and 40% received a 50% or greater reduction in myelofibrosis-related symptoms. In Jakarta-2, there was a 55% spleen response in patients resistant or intolerant to ruxolitinib.
Entrectinib (Rozlytrek)
Class: Tropomyosin receptor tyrosine kinase inhibitor.
Disease: Solid tumors that have a neurotrophic tyrosine receptor kinase (NTRK) gene fusion and for ROS-1 positive non–small cell lung cancer (NSCLC).
Dose: 600 mg orally once daily.
AEs: Heart failure, QT prolongation, skeletal fractures, hepatotoxicity, central nervous system effects, and hyperuricemia.
Trial: ALKA, STARTRK-1 (NCT02097810) and STARTRK-2 (NCT02568267): Overall response rate of 57% for NTRK positive patients; response rate of 77% in ROS-1 positive NSCLC.
Pexidartinib (Turalio)
Class: Small molecule tyrosine kinase inhibitor targeting CSF1R.
Disease: Symptomatic tenosynovial giant cell tumor.
Dose: 400 mg orally twice daily without food.
AEs: Black box warning on hepatotoxicity.
Trial: ENLIVEN (NCT02371369): Overall response rate of 38% at 25 weeks, with a 15% complete response rate and a 23% partial response rate.
Darolutamide (Nubeqa)
Class: Androgen receptor inhibitor.
Disease: Nonmetastatic castration-resistant prostate cancer.
Dose: 600 mg orally twice daily with food with concomitant androgen deprivation therapy.
AEs: Fatigue, extremity pain, and rash.
Trial: ARAMIS (NCT02200614): Median metastasis free survival was 40.4 months for patients with darolutamide, compared with 18.4 months for controls.
Selinexor (Xpovio)
Class: Reversible inhibitor of nuclear export of tumor suppressor proteins, growth regulators, and mRNAs of oncogenic proteins.
Disease: Relapsed or refractory multiple myeloma. Indicated for patients who have received at least four prior therapies, including at least two immunomodulatory agents and an anti-CD38 monoclonal antibody.
Dose: 80 mg orally in combination with oral dexamethasone on days 1 and 3 of each week.
AEs: Thrombocytopenia, fatigue, pancytopenia, and hyponatremia.
Trial: STORM (NCT02336815): Overall response rate 25.3% with a median time to first response of 4 weeks and 3.8-month median duration of response.
Polatuzumab vedotin-piiq (Polivy)
Class: CD79b-directed antibody-drug conjugate.
Disease: Relapsed or refractory diffuse large B-cell lymphoma. Indicated for patients who have had at least two prior therapies.
Dose: 1.8 mg/kg intravenous infusion every 21 days for six cycles in combination with bendamustine and a rituximab product.
AEs: Pancytopenia, peripheral neuropathy.
Trial: GO29365 (NCT02257567): Complete response rate was 40% for polatuzumab vedotin-piiq plus bendamustine/rituximab, compared with 18% with bendamustine/rituximab alone.*
Caplacizumab-yhdp (Cablivi)
Class: Monoclonal antibody fragment directed against von Willebrand factor.
Disease: Thrombotic thrombocytopenic purpura.
Dose: 11 mg IV initially, then daily subcutaneously; in combination with plasma exchange and immunosuppressive therapy.
AEs: Epistaxis, headache, and gingival bleeding.
Trial: Hercules trial (NCT02553317): More rapid normalization of platelets, lower incidence of composite TTP-related death, and lower rate of recurrence when added to plasma exchange and steroids.
Alpelisib (Piqray)
Class: Phosphatidylinositol-3-kinase (PI3K) inhibitor.
Disease: Hormone receptor positive HER2-negative PIK3CA-mutated, advanced or metastatic breast cancer.
Dose: 300 mg orally once daily with food with concomitant fulvestrant.
AEs: Hyperglycemia, pancytopenia.
Trial: SOLAR-1 (NCT02437318): 11-month progression-free survival among patients treated with alpelisib and fulvestrant, compared with 5.7 months in fulvestrant alone control arm; overall response rate of 36% versus 16%, respectively.
Erdafitinib (Balversa)
Class: Fibroblast growth factor receptor kinase inhibitor.
Disease: Locally advanced or metastatic urothelial carcinoma with FGFR3 or FGFR2 mutations.
Dose: 8 mg orally once daily, with or without food.
AEs: Ocular disorders including retinopathy or retinal detachment.
Trial: BLC2001 (NCT02365597): Objective response rate of 32.2%, with a complete response in 2.3% of patients and partial response in 29.9% of patients.
Biosimilar approvals
Trastuzumab and hyaluronidase-oysk (Herceptin Hylecta)
Biosimilar to: Trastuzumab.
Indication: HER2-overexpressing breast cancer.
Dr. Bryer is a resident in the department of internal medicine at the University of Pennsylvania, Philadelphia. Dr. Mintzer is chief of hematology-oncology at Pennsylvania Hospital and professor of medicine at the University of Pennsylvania. Dr. Henry is a hematologist-oncologist at Pennsylvania Hospital and professor of medicine at the University of Pennsylvania.
*Correction, 11/7/2019: An earlier version of this article misstated the drug combination in the GO29365 trial.
The rapid development and identification of novel drugs has translated into innovative therapies in hematology and oncology. The aim of this piece is to present newly approved drugs and expanded indications to serve as a reference guide for practicing clinicians.
This article reviews therapies that were newly approved so far in 2019, as well as those previously approved whose indications were expanded this past year. The list highlights the most clinically important approvals, as well as adverse events that are unique or especially severe.
New approvals
Fedratinib (Inrebic)
Class: JAK2 and FLT3 selective kinase inhibitor.
Disease: Intermediate or high-risk primary or secondary (postpolycythemia vera or postessential thrombocythemia) myelofibrosis.
Dose: 400 mg orally once daily, with or without food.
Adverse events (AEs): Black box warning: Fatal encephalopathy, including Wernicke’s (thiamine level monitoring suggested).
Trials: In JAKARTA (NCT01437787), 37% of patients achieved a 35% or greater reduction in spleen volume and 40% received a 50% or greater reduction in myelofibrosis-related symptoms. In Jakarta-2, there was a 55% spleen response in patients resistant or intolerant to ruxolitinib.
Entrectinib (Rozlytrek)
Class: Tropomyosin receptor tyrosine kinase inhibitor.
Disease: Solid tumors that have a neurotrophic tyrosine receptor kinase (NTRK) gene fusion and for ROS-1 positive non–small cell lung cancer (NSCLC).
Dose: 600 mg orally once daily.
AEs: Heart failure, QT prolongation, skeletal fractures, hepatotoxicity, central nervous system effects, and hyperuricemia.
Trial: ALKA, STARTRK-1 (NCT02097810) and STARTRK-2 (NCT02568267): Overall response rate of 57% for NTRK positive patients; response rate of 77% in ROS-1 positive NSCLC.
Pexidartinib (Turalio)
Class: Small molecule tyrosine kinase inhibitor targeting CSF1R.
Disease: Symptomatic tenosynovial giant cell tumor.
Dose: 400 mg orally twice daily without food.
AEs: Black box warning on hepatotoxicity.
Trial: ENLIVEN (NCT02371369): Overall response rate of 38% at 25 weeks, with a 15% complete response rate and a 23% partial response rate.
Darolutamide (Nubeqa)
Class: Androgen receptor inhibitor.
Disease: Nonmetastatic castration-resistant prostate cancer.
Dose: 600 mg orally twice daily with food with concomitant androgen deprivation therapy.
AEs: Fatigue, extremity pain, and rash.
Trial: ARAMIS (NCT02200614): Median metastasis free survival was 40.4 months for patients with darolutamide, compared with 18.4 months for controls.
Selinexor (Xpovio)
Class: Reversible inhibitor of nuclear export of tumor suppressor proteins, growth regulators, and mRNAs of oncogenic proteins.
Disease: Relapsed or refractory multiple myeloma. Indicated for patients who have received at least four prior therapies, including at least two immunomodulatory agents and an anti-CD38 monoclonal antibody.
Dose: 80 mg orally in combination with oral dexamethasone on days 1 and 3 of each week.
AEs: Thrombocytopenia, fatigue, pancytopenia, and hyponatremia.
Trial: STORM (NCT02336815): Overall response rate 25.3% with a median time to first response of 4 weeks and 3.8-month median duration of response.
Polatuzumab vedotin-piiq (Polivy)
Class: CD79b-directed antibody-drug conjugate.
Disease: Relapsed or refractory diffuse large B-cell lymphoma. Indicated for patients who have had at least two prior therapies.
Dose: 1.8 mg/kg intravenous infusion every 21 days for six cycles in combination with bendamustine and a rituximab product.
AEs: Pancytopenia, peripheral neuropathy.
Trial: GO29365 (NCT02257567): Complete response rate was 40% for polatuzumab vedotin-piiq plus bendamustine/rituximab, compared with 18% with bendamustine/rituximab alone.*
Caplacizumab-yhdp (Cablivi)
Class: Monoclonal antibody fragment directed against von Willebrand factor.
Disease: Thrombotic thrombocytopenic purpura.
Dose: 11 mg IV initially, then daily subcutaneously; in combination with plasma exchange and immunosuppressive therapy.
AEs: Epistaxis, headache, and gingival bleeding.
Trial: Hercules trial (NCT02553317): More rapid normalization of platelets, lower incidence of composite TTP-related death, and lower rate of recurrence when added to plasma exchange and steroids.
Alpelisib (Piqray)
Class: Phosphatidylinositol-3-kinase (PI3K) inhibitor.
Disease: Hormone receptor positive HER2-negative PIK3CA-mutated, advanced or metastatic breast cancer.
Dose: 300 mg orally once daily with food with concomitant fulvestrant.
AEs: Hyperglycemia, pancytopenia.
Trial: SOLAR-1 (NCT02437318): 11-month progression-free survival among patients treated with alpelisib and fulvestrant, compared with 5.7 months in fulvestrant alone control arm; overall response rate of 36% versus 16%, respectively.
Erdafitinib (Balversa)
Class: Fibroblast growth factor receptor kinase inhibitor.
Disease: Locally advanced or metastatic urothelial carcinoma with FGFR3 or FGFR2 mutations.
Dose: 8 mg orally once daily, with or without food.
AEs: Ocular disorders including retinopathy or retinal detachment.
Trial: BLC2001 (NCT02365597): Objective response rate of 32.2%, with a complete response in 2.3% of patients and partial response in 29.9% of patients.
Biosimilar approvals
Trastuzumab and hyaluronidase-oysk (Herceptin Hylecta)
Biosimilar to: Trastuzumab.
Indication: HER2-overexpressing breast cancer.
Dr. Bryer is a resident in the department of internal medicine at the University of Pennsylvania, Philadelphia. Dr. Mintzer is chief of hematology-oncology at Pennsylvania Hospital and professor of medicine at the University of Pennsylvania. Dr. Henry is a hematologist-oncologist at Pennsylvania Hospital and professor of medicine at the University of Pennsylvania.
*Correction, 11/7/2019: An earlier version of this article misstated the drug combination in the GO29365 trial.
The rapid development and identification of novel drugs has translated into innovative therapies in hematology and oncology. The aim of this piece is to present newly approved drugs and expanded indications to serve as a reference guide for practicing clinicians.
This article reviews therapies that were newly approved so far in 2019, as well as those previously approved whose indications were expanded this past year. The list highlights the most clinically important approvals, as well as adverse events that are unique or especially severe.
New approvals
Fedratinib (Inrebic)
Class: JAK2 and FLT3 selective kinase inhibitor.
Disease: Intermediate or high-risk primary or secondary (postpolycythemia vera or postessential thrombocythemia) myelofibrosis.
Dose: 400 mg orally once daily, with or without food.
Adverse events (AEs): Black box warning: Fatal encephalopathy, including Wernicke’s (thiamine level monitoring suggested).
Trials: In JAKARTA (NCT01437787), 37% of patients achieved a 35% or greater reduction in spleen volume and 40% received a 50% or greater reduction in myelofibrosis-related symptoms. In Jakarta-2, there was a 55% spleen response in patients resistant or intolerant to ruxolitinib.
Entrectinib (Rozlytrek)
Class: Tropomyosin receptor tyrosine kinase inhibitor.
Disease: Solid tumors that have a neurotrophic tyrosine receptor kinase (NTRK) gene fusion and for ROS-1 positive non–small cell lung cancer (NSCLC).
Dose: 600 mg orally once daily.
AEs: Heart failure, QT prolongation, skeletal fractures, hepatotoxicity, central nervous system effects, and hyperuricemia.
Trial: ALKA, STARTRK-1 (NCT02097810) and STARTRK-2 (NCT02568267): Overall response rate of 57% for NTRK positive patients; response rate of 77% in ROS-1 positive NSCLC.
Pexidartinib (Turalio)
Class: Small molecule tyrosine kinase inhibitor targeting CSF1R.
Disease: Symptomatic tenosynovial giant cell tumor.
Dose: 400 mg orally twice daily without food.
AEs: Black box warning on hepatotoxicity.
Trial: ENLIVEN (NCT02371369): Overall response rate of 38% at 25 weeks, with a 15% complete response rate and a 23% partial response rate.
Darolutamide (Nubeqa)
Class: Androgen receptor inhibitor.
Disease: Nonmetastatic castration-resistant prostate cancer.
Dose: 600 mg orally twice daily with food with concomitant androgen deprivation therapy.
AEs: Fatigue, extremity pain, and rash.
Trial: ARAMIS (NCT02200614): Median metastasis free survival was 40.4 months for patients with darolutamide, compared with 18.4 months for controls.
Selinexor (Xpovio)
Class: Reversible inhibitor of nuclear export of tumor suppressor proteins, growth regulators, and mRNAs of oncogenic proteins.
Disease: Relapsed or refractory multiple myeloma. Indicated for patients who have received at least four prior therapies, including at least two immunomodulatory agents and an anti-CD38 monoclonal antibody.
Dose: 80 mg orally in combination with oral dexamethasone on days 1 and 3 of each week.
AEs: Thrombocytopenia, fatigue, pancytopenia, and hyponatremia.
Trial: STORM (NCT02336815): Overall response rate 25.3% with a median time to first response of 4 weeks and 3.8-month median duration of response.
Polatuzumab vedotin-piiq (Polivy)
Class: CD79b-directed antibody-drug conjugate.
Disease: Relapsed or refractory diffuse large B-cell lymphoma. Indicated for patients who have had at least two prior therapies.
Dose: 1.8 mg/kg intravenous infusion every 21 days for six cycles in combination with bendamustine and a rituximab product.
AEs: Pancytopenia, peripheral neuropathy.
Trial: GO29365 (NCT02257567): Complete response rate was 40% for polatuzumab vedotin-piiq plus bendamustine/rituximab, compared with 18% with bendamustine/rituximab alone.*
Caplacizumab-yhdp (Cablivi)
Class: Monoclonal antibody fragment directed against von Willebrand factor.
Disease: Thrombotic thrombocytopenic purpura.
Dose: 11 mg IV initially, then daily subcutaneously; in combination with plasma exchange and immunosuppressive therapy.
AEs: Epistaxis, headache, and gingival bleeding.
Trial: Hercules trial (NCT02553317): More rapid normalization of platelets, lower incidence of composite TTP-related death, and lower rate of recurrence when added to plasma exchange and steroids.
Alpelisib (Piqray)
Class: Phosphatidylinositol-3-kinase (PI3K) inhibitor.
Disease: Hormone receptor positive HER2-negative PIK3CA-mutated, advanced or metastatic breast cancer.
Dose: 300 mg orally once daily with food with concomitant fulvestrant.
AEs: Hyperglycemia, pancytopenia.
Trial: SOLAR-1 (NCT02437318): 11-month progression-free survival among patients treated with alpelisib and fulvestrant, compared with 5.7 months in fulvestrant alone control arm; overall response rate of 36% versus 16%, respectively.
Erdafitinib (Balversa)
Class: Fibroblast growth factor receptor kinase inhibitor.
Disease: Locally advanced or metastatic urothelial carcinoma with FGFR3 or FGFR2 mutations.
Dose: 8 mg orally once daily, with or without food.
AEs: Ocular disorders including retinopathy or retinal detachment.
Trial: BLC2001 (NCT02365597): Objective response rate of 32.2%, with a complete response in 2.3% of patients and partial response in 29.9% of patients.
Biosimilar approvals
Trastuzumab and hyaluronidase-oysk (Herceptin Hylecta)
Biosimilar to: Trastuzumab.
Indication: HER2-overexpressing breast cancer.
Dr. Bryer is a resident in the department of internal medicine at the University of Pennsylvania, Philadelphia. Dr. Mintzer is chief of hematology-oncology at Pennsylvania Hospital and professor of medicine at the University of Pennsylvania. Dr. Henry is a hematologist-oncologist at Pennsylvania Hospital and professor of medicine at the University of Pennsylvania.
*Correction, 11/7/2019: An earlier version of this article misstated the drug combination in the GO29365 trial.
Neonatal Consultations: Vascular Lumps, Bumps, and Tumors in the Neonate
Although most neonatal vascular lumps, bumps, and tumors are benign, proper diagnosis is important for prognosis and management. Therefore, knowledge of both common and rare conditions is important when evaluating a neonatal nodule. Differential diagnosis of neonatal vascular nodules must focus on important diagnostic clues that should prompt consideration and evaluation for less common and/or potentially threatening conditions. Infantile hemangioma (IH), congenital hemangioma (CH), venous malformation (VM), lymphatic malformation (LM), kaposiform hemangioendothelioma (KHE) and tufted angioma, and malignant tumors are reviewed here.
Infantile Hemangioma
Infantile hemangioma, a benign proliferation of capillaries, is the most common tumor of infancy with reported incidence of up to 5% in neonates.1 As such, suspicion for less common lesions is often predicated on identifying features that would be atypical for an IH. A superficial IH presents as a bright red papule, nodule, or plaque, while a deep IH presents as a flesh-colored to bluish nodule. Mixed IHs combine features of both superficial and deep lesions. The distribution may be focal or segmental, with segmental lesions encompassing a larger territory–like distribution and frequently displaying a thin, coarsely telangiectatic appearance.
Knowledge of the natural history of IH generally is crucial in differentiating it from other neonatal lesions. Infantile hemangiomas display a natural history that is distinct and predictable. They typically manifest within the first few weeks of life, though up to 30% present at birth with a premonitory mark, which may be a light red, pink, bluish, or vasoconstricted patch. Thus, mere presence of a lesion at birth is not the feature that distinguishes other congenital lesions from an IH. After initial appearance, IHs undergo a period of proliferation that occurs over 4 to 6 months in most patients. In some cases, areas of proliferation may be subtle, but nonetheless the presence of some areas of increased redness and/or volumetric growth generally is required to firmly establish the diagnosis of IH. Thereafter, IH will involute, a process that begins before 1 year of age in most cases and continues over years. Although IHs undergo involution, complete clearance may not occur, as nearly 70% will leave permanent residua such as fibrofatty masses or anetodermic skin.2 Nevertheless, the presence of a proliferative phase followed by a slower period of involution is a hallmark feature of the IH.
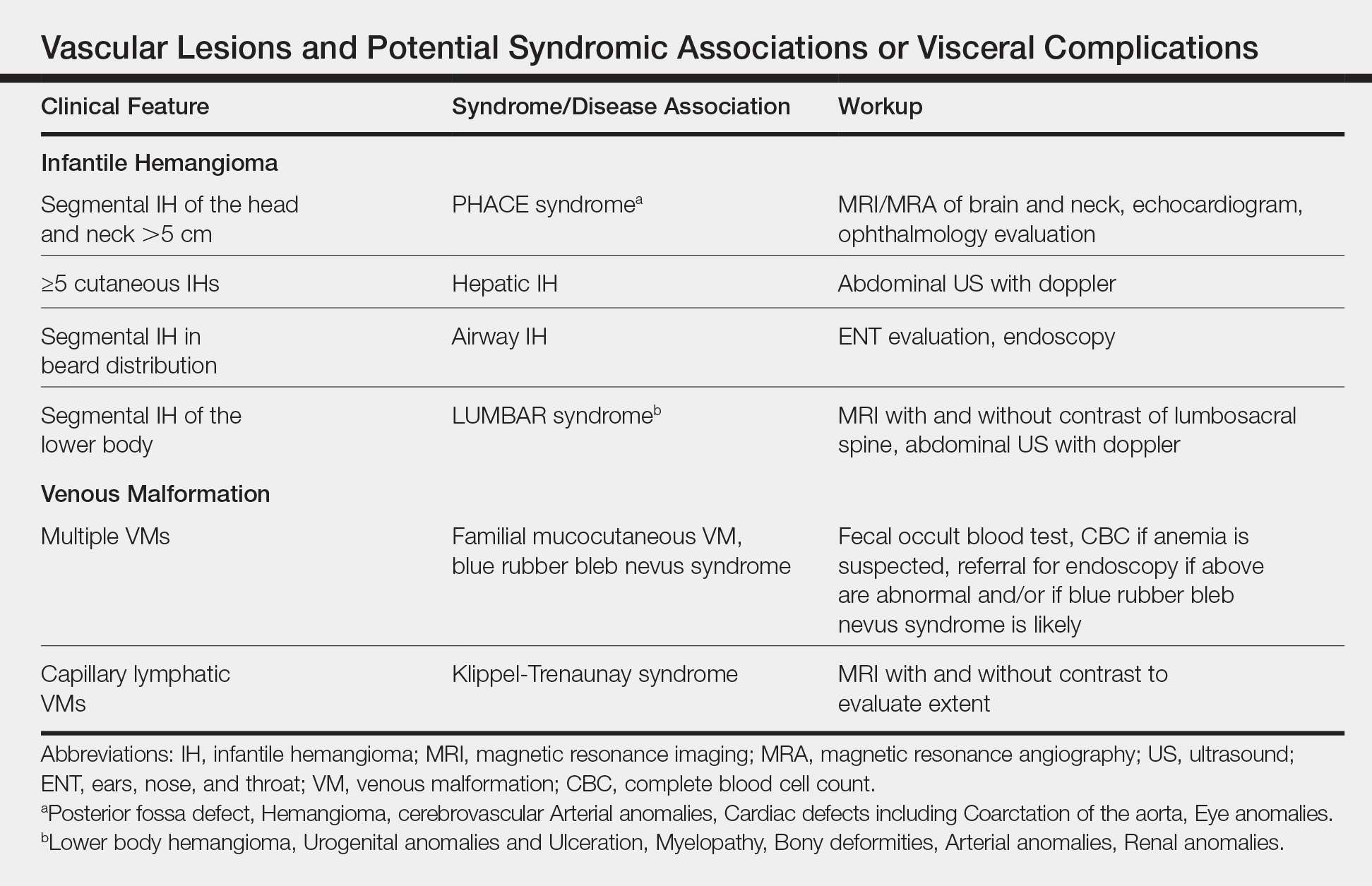
Biopsy and imaging rarely are required for establishing diagnosis of an IH. Histopathology showing a proliferation of capillaries with positive glucose transporter 1 (GLUT-1) staining is characteristic. Imaging with ultrasound reveals a fast-flow lesion. Apart from exceptionally rare cases, a cutaneous IH typically does not cross muscle fascia, and thus alternative diagnoses should be considered for a cutaneous lesion that demonstrates infiltration into nerve, bone, joint, or other deeper tissues. Most IHs do not require treatment; however, a small subset may be associated with complications and thus require intervention. Complications of IH may include impairment of function (eg, vision, feeding, respiratory), ulceration, and risk for permanent disfigurement. When treatment is indicated, the most commonly employed options during the proliferative phase are the topical beta-blocker timolol and the oral beta-blocker propranolol. In addition, certain IHs may be associated with either syndromic presentations and/or visceral involvement, thus requiring further workup (Table).
Congenital Hemangioma
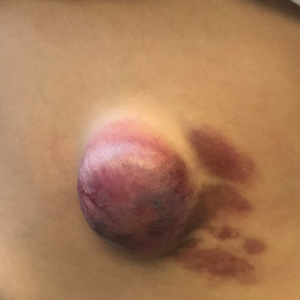
A CH is an uncommon benign neonatal tumor that is distinct from an IH in behavior, biology, and treatment. Congenital hemangiomas may have a rapidly involuting course, referred to as RICH (rapidly involuting congenital hemangioma), or a noninvoluting course, referred to as NICH (noninvoluting congenital hemangioma). Partially involuting types also have been described.3 A RICH typically presents as a highly vascular, red-violaceous or bluish plaque, nodule, or large mass at birth. An NICH presents as a red-violaceous or bluish, coarsely telangiectatic patch, plaque, or nodule. A characteristic feature of the CH is the rim of vasoconstriction around the lesion, which is an important diagnostic clue (Figure 1). In contrast to IH, multifocal lesions are highly unlikely in CH, though it rarely has been reported.4
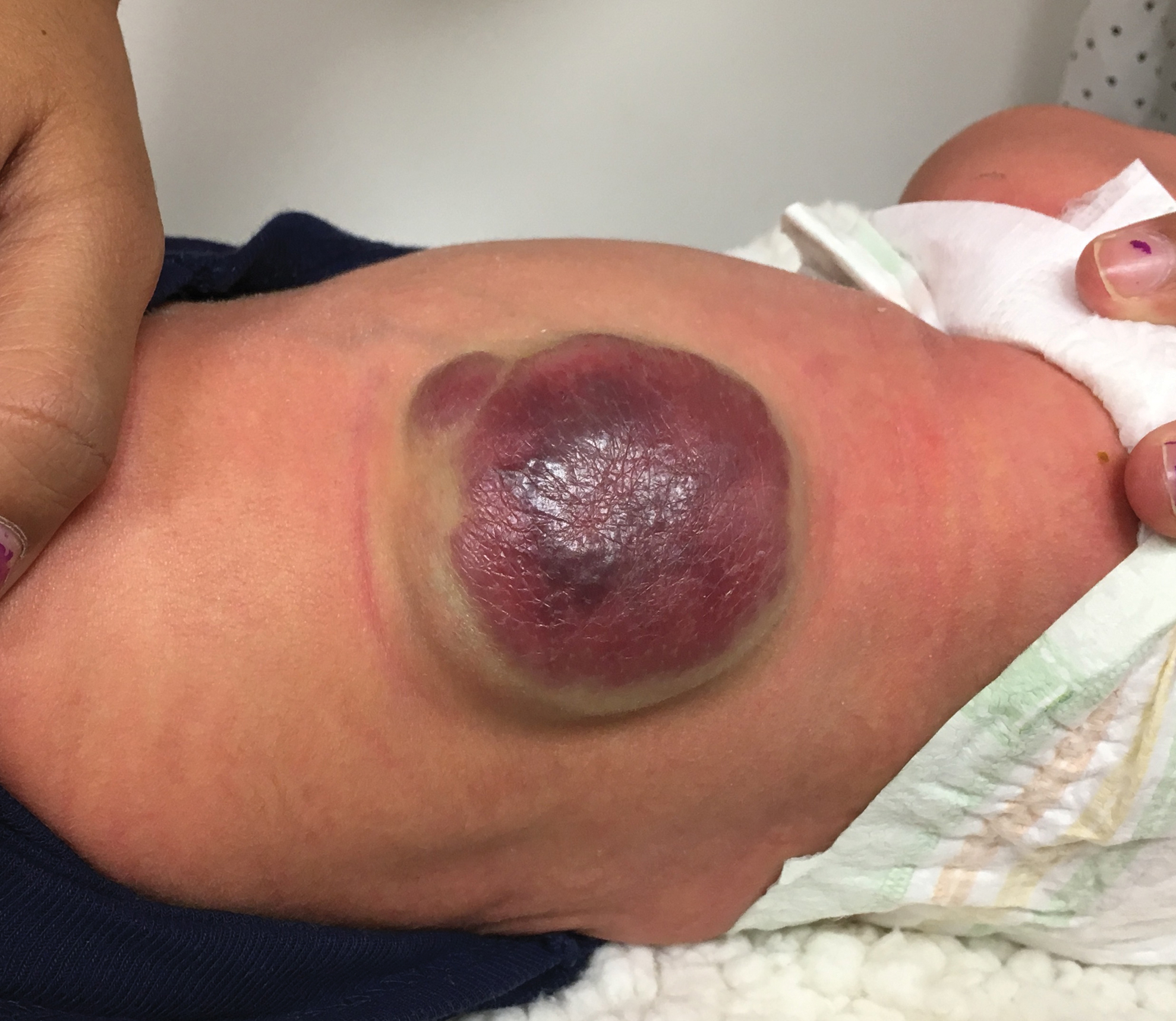
Regardless of subtype, CHs are fully developed at birth. Infantile hemangiomas, on the other hand, are either minimally present or not present at birth and thereafter proliferate. After birth, a RICH rapidly involutes over the first 9 to 12 months of life. This process generally is evident even in the first few weeks of life, which would not be expected of an IH and is therefore a major distinguishing factor. A NICH, on the other hand, is expected to be persistent, for the most part neither showing signs of proliferation nor involution.
Complications of CHs may include ulceration, functional impairment, or risk for permanent disfigurement depending on location. In addition, due to their fast-flow state and potential large size, some CHs may be complicated by high-output heart failure in the neonate. Distinguishing an IH from a CH is important not only for prognosis but also treatment. Beta-blocker therapy generally is not useful for CHs, and management usually is supportive in the neonatal period.
In the majority of cases, diagnosis can be achieved solely on clinical features. Biopsy with immunohistochemistry shows negative GLUT-1 staining, which will distinguish this lesion from an IH. At times, the highly vascular nature and/or striking size of a CH may lead some to consider the potential diagnosis of an arteriovenous malformation. However, soft-tissue arteriovenous malformations involving the skin are almost never fully developed in the neonatal period and generally take years to evolve from a quiescent state to a destructive lesion.
Venous Malformation
Venous malformations are congenital malformations of veins that may be apparent at birth or later. They appear as bluish to flesh-colored, compressible nodules or plaques. They tend to increase in size when the affected body part is in a dependent position, and this maneuver can be a helpful distinguishing clue. Although the majority of patients have a single lesion, multifocal involvement may occur uncommonly (Table). The diagnosis of VM usually is clinical, though at times, a VM may be difficult to distinguish from a purely deep IH. However, a VM will persist over time, growing in proportion to the patient. In addition, a VM displays low flow on ultrasound, a distinguishing feature from the fast-flow IH. Magnetic resonance imaging with and without contrast is the imaging study of choice. At times, cutaneous VMs will demonstrate infiltration into other tissue planes such as muscle and joint. Pain may occur secondary to thrombus formation within the malformation. In extensive lesions, intravascular coagulation may be notable, as reflected in elevated D-dimer and decreased fibrinogen levels. Treatment with sclerotherapy or surgery may be considered in select cases during infancy; however, in general, an asymptomatic VM may be observed early on in life.
Lymphatic Malformation
A lymphatic malformation (LM) is a congenital malformation of lymphatic vessels and may be further differentiated into microcystic, macrocystic, or mixed types depending on the size of the channels. An LM may present at birth or later and persists over time. Superficial microcystic LMs, synonymous with the term lymphangioma circumscriptum, characteristically appear as a group of clear and violaceous hemorrhagic vesicles on the skin. Deeper LMs appear as a tense or spongy, flesh-colored nodule or mass. Involvement of the head and neck is common. Complications frequently occur in LMs. Cutaneous LMs may ooze or bleed. Infection and hemorrhage into cysts may occur, which will cause acute pain, redness, swelling, and induration. Cervicofacial lesions may result in respiratory distress. Thus, the majority of LMs require treatment, though asymptomatic lesions may be observed in the neonate. An ultrasound will demonstrate a low-flow lesion, and magnetic resonance imaging is the diagnostic modality of choice for diagnosis and definition of extent.
KHE and Tufted Angioma
Kaposiform hemangioendothelioma is a rare, locally aggressive, vascular tumor that is frequently associated with a potentially life-threatening coagulopathy, Kasabach-Merritt phenomenon. Tufted angiomas are now understood to belong on a spectrum with KHEs, which usually present in the neonatal period or infancy as firm, red-violaceous plaques, nodules, or large tumors. Infiltration into nerve, muscle, and bone may occur. The firm/hard nature and deep violaceous appearance generally are initial clues that it is not an IH. Kasabach-Merritt phenomenon manifests as thrombocytopenia as well as low fibrinogen and elevated D-dimer levels. Thrombocytopenia is generally profound in Kasabach-Merritt phenomenon and results from platelet trapping within the vascular tumor. Given these potential complications, KHEs generally require immediate medical attention, and various treatment protocols including prednisone, vincristine, and sirolimus are utilized for complicated cases.5 The diagnosis may require biopsy to distinguish it from malignant tumors, particularly sarcomas.
Malignant Tumors
Various malignancies, including congenital leukemia, neuroblastoma, Langerhans cell histiocytosis, infantile fibrosarcoma, and rhabdomyosarcoma, rarely may present as cutaneous nodules or masses in a neonate mimicking hemangiomas or other vascular lesions (Figure 2). Neonates may present with multiple bluish papules and nodules resembling a blueberry muffin baby; multiple violaceous-red nodules; or a single red-violaceous, highly vascular–appearing mass mimicking hemangiomas. Malignant tumors may display vascularity on imaging, and thus the presence of vascular flow on ultrasound should not dissuade one from the possibility of a malignancy if other clinical features are atypical or unusual for a hemangioma. When a neonatal malignancy is suspected, a large punch biopsy or incisional biopsy is required for workup.
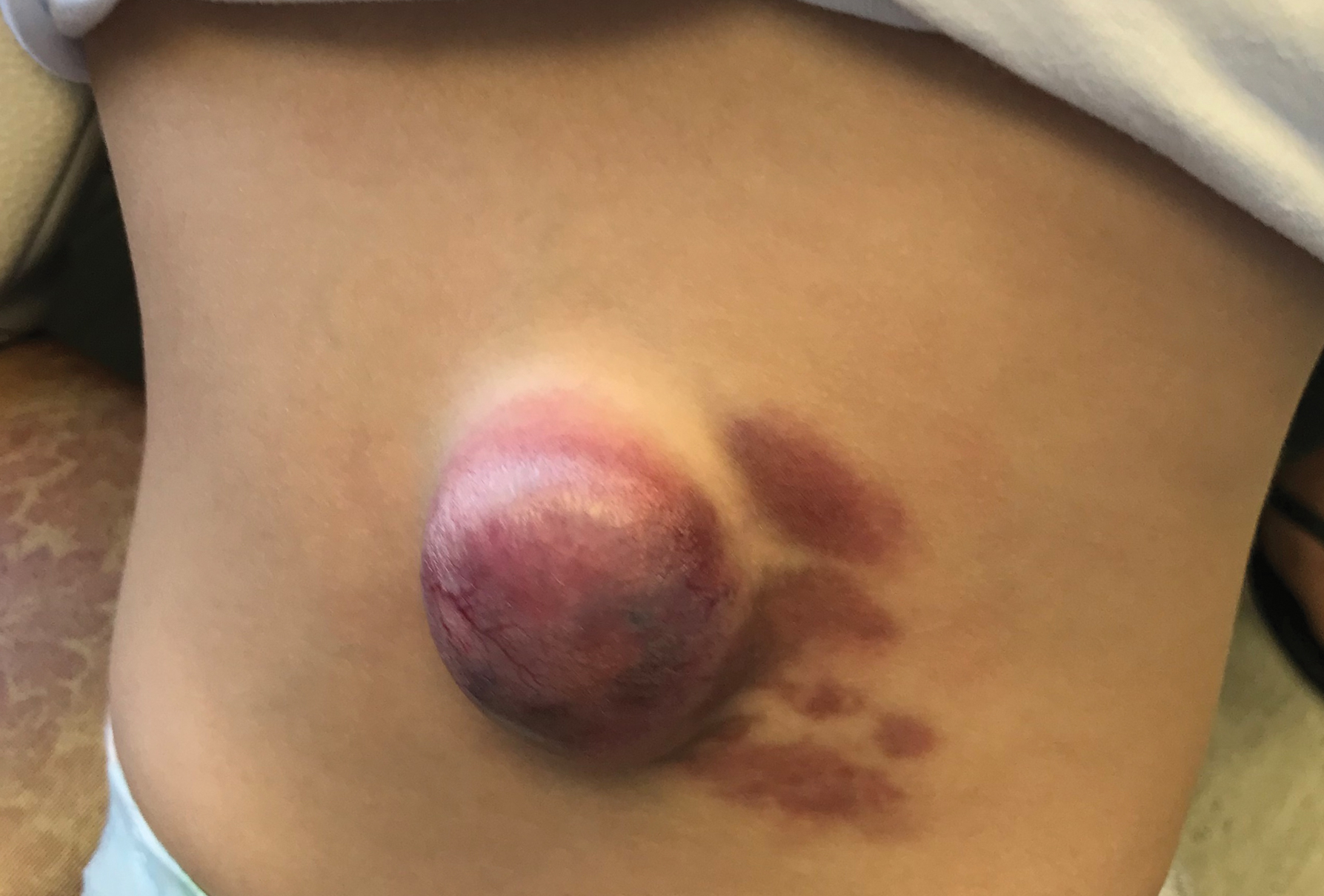
Final Thoughts
Although IHs are the most common vascular nodules in neonates and young infants, other conditions such as VMs, LMs, CHs, KHEs, and malignancy may occur less commonly. Identifying features that would be considered atypical for IH is crucial to recognize these less common possibilities.
- Kanada KN, Merin MR, Munden A, et al. A prospective study of cutaneous findings in newborns in the United States: correlation with race, ethnicity, and gestational status using updated classification and nomenclature. J Pediatr. 2012;161:240-245.
- Darrow DH, Greene AK, Mancini AJ, et al. Diagnosis and management of infantile hemangioma. Pediatrics. 2015;136:E1060-E1104.
- Nasseri E, Piram M, McCuaig CC, et al. Partially involuting congenital hemangiomas: a report of 8 cases and review of the literature. J Am Acad Dermatol. 2014;70:75-79.
- Blumenthal S, Stefanko N, Cossio M, et al. Multifocal congenital hemangioma: expanding the pathogenesis of “neonatal hemangiomatosis.” Pediatr Dermatol. 2019;36:720-722.
- Croteau SE, Gupta D. The clinical spectrum of kaposiform hemangioendothelioma and tufted angioma. Semin Cutan Med Surg. 2016;35:147-152.
Although most neonatal vascular lumps, bumps, and tumors are benign, proper diagnosis is important for prognosis and management. Therefore, knowledge of both common and rare conditions is important when evaluating a neonatal nodule. Differential diagnosis of neonatal vascular nodules must focus on important diagnostic clues that should prompt consideration and evaluation for less common and/or potentially threatening conditions. Infantile hemangioma (IH), congenital hemangioma (CH), venous malformation (VM), lymphatic malformation (LM), kaposiform hemangioendothelioma (KHE) and tufted angioma, and malignant tumors are reviewed here.
Infantile Hemangioma
Infantile hemangioma, a benign proliferation of capillaries, is the most common tumor of infancy with reported incidence of up to 5% in neonates.1 As such, suspicion for less common lesions is often predicated on identifying features that would be atypical for an IH. A superficial IH presents as a bright red papule, nodule, or plaque, while a deep IH presents as a flesh-colored to bluish nodule. Mixed IHs combine features of both superficial and deep lesions. The distribution may be focal or segmental, with segmental lesions encompassing a larger territory–like distribution and frequently displaying a thin, coarsely telangiectatic appearance.
Knowledge of the natural history of IH generally is crucial in differentiating it from other neonatal lesions. Infantile hemangiomas display a natural history that is distinct and predictable. They typically manifest within the first few weeks of life, though up to 30% present at birth with a premonitory mark, which may be a light red, pink, bluish, or vasoconstricted patch. Thus, mere presence of a lesion at birth is not the feature that distinguishes other congenital lesions from an IH. After initial appearance, IHs undergo a period of proliferation that occurs over 4 to 6 months in most patients. In some cases, areas of proliferation may be subtle, but nonetheless the presence of some areas of increased redness and/or volumetric growth generally is required to firmly establish the diagnosis of IH. Thereafter, IH will involute, a process that begins before 1 year of age in most cases and continues over years. Although IHs undergo involution, complete clearance may not occur, as nearly 70% will leave permanent residua such as fibrofatty masses or anetodermic skin.2 Nevertheless, the presence of a proliferative phase followed by a slower period of involution is a hallmark feature of the IH.
Biopsy and imaging rarely are required for establishing diagnosis of an IH. Histopathology showing a proliferation of capillaries with positive glucose transporter 1 (GLUT-1) staining is characteristic. Imaging with ultrasound reveals a fast-flow lesion. Apart from exceptionally rare cases, a cutaneous IH typically does not cross muscle fascia, and thus alternative diagnoses should be considered for a cutaneous lesion that demonstrates infiltration into nerve, bone, joint, or other deeper tissues. Most IHs do not require treatment; however, a small subset may be associated with complications and thus require intervention. Complications of IH may include impairment of function (eg, vision, feeding, respiratory), ulceration, and risk for permanent disfigurement. When treatment is indicated, the most commonly employed options during the proliferative phase are the topical beta-blocker timolol and the oral beta-blocker propranolol. In addition, certain IHs may be associated with either syndromic presentations and/or visceral involvement, thus requiring further workup (Table).

Congenital Hemangioma
A CH is an uncommon benign neonatal tumor that is distinct from an IH in behavior, biology, and treatment. Congenital hemangiomas may have a rapidly involuting course, referred to as RICH (rapidly involuting congenital hemangioma), or a noninvoluting course, referred to as NICH (noninvoluting congenital hemangioma). Partially involuting types also have been described.3 A RICH typically presents as a highly vascular, red-violaceous or bluish plaque, nodule, or large mass at birth. An NICH presents as a red-violaceous or bluish, coarsely telangiectatic patch, plaque, or nodule. A characteristic feature of the CH is the rim of vasoconstriction around the lesion, which is an important diagnostic clue (Figure 1). In contrast to IH, multifocal lesions are highly unlikely in CH, though it rarely has been reported.4

Regardless of subtype, CHs are fully developed at birth. Infantile hemangiomas, on the other hand, are either minimally present or not present at birth and thereafter proliferate. After birth, a RICH rapidly involutes over the first 9 to 12 months of life. This process generally is evident even in the first few weeks of life, which would not be expected of an IH and is therefore a major distinguishing factor. A NICH, on the other hand, is expected to be persistent, for the most part neither showing signs of proliferation nor involution.
Complications of CHs may include ulceration, functional impairment, or risk for permanent disfigurement depending on location. In addition, due to their fast-flow state and potential large size, some CHs may be complicated by high-output heart failure in the neonate. Distinguishing an IH from a CH is important not only for prognosis but also treatment. Beta-blocker therapy generally is not useful for CHs, and management usually is supportive in the neonatal period.
In the majority of cases, diagnosis can be achieved solely on clinical features. Biopsy with immunohistochemistry shows negative GLUT-1 staining, which will distinguish this lesion from an IH. At times, the highly vascular nature and/or striking size of a CH may lead some to consider the potential diagnosis of an arteriovenous malformation. However, soft-tissue arteriovenous malformations involving the skin are almost never fully developed in the neonatal period and generally take years to evolve from a quiescent state to a destructive lesion.
Venous Malformation
Venous malformations are congenital malformations of veins that may be apparent at birth or later. They appear as bluish to flesh-colored, compressible nodules or plaques. They tend to increase in size when the affected body part is in a dependent position, and this maneuver can be a helpful distinguishing clue. Although the majority of patients have a single lesion, multifocal involvement may occur uncommonly (Table). The diagnosis of VM usually is clinical, though at times, a VM may be difficult to distinguish from a purely deep IH. However, a VM will persist over time, growing in proportion to the patient. In addition, a VM displays low flow on ultrasound, a distinguishing feature from the fast-flow IH. Magnetic resonance imaging with and without contrast is the imaging study of choice. At times, cutaneous VMs will demonstrate infiltration into other tissue planes such as muscle and joint. Pain may occur secondary to thrombus formation within the malformation. In extensive lesions, intravascular coagulation may be notable, as reflected in elevated D-dimer and decreased fibrinogen levels. Treatment with sclerotherapy or surgery may be considered in select cases during infancy; however, in general, an asymptomatic VM may be observed early on in life.
Lymphatic Malformation
A lymphatic malformation (LM) is a congenital malformation of lymphatic vessels and may be further differentiated into microcystic, macrocystic, or mixed types depending on the size of the channels. An LM may present at birth or later and persists over time. Superficial microcystic LMs, synonymous with the term lymphangioma circumscriptum, characteristically appear as a group of clear and violaceous hemorrhagic vesicles on the skin. Deeper LMs appear as a tense or spongy, flesh-colored nodule or mass. Involvement of the head and neck is common. Complications frequently occur in LMs. Cutaneous LMs may ooze or bleed. Infection and hemorrhage into cysts may occur, which will cause acute pain, redness, swelling, and induration. Cervicofacial lesions may result in respiratory distress. Thus, the majority of LMs require treatment, though asymptomatic lesions may be observed in the neonate. An ultrasound will demonstrate a low-flow lesion, and magnetic resonance imaging is the diagnostic modality of choice for diagnosis and definition of extent.
KHE and Tufted Angioma
Kaposiform hemangioendothelioma is a rare, locally aggressive, vascular tumor that is frequently associated with a potentially life-threatening coagulopathy, Kasabach-Merritt phenomenon. Tufted angiomas are now understood to belong on a spectrum with KHEs, which usually present in the neonatal period or infancy as firm, red-violaceous plaques, nodules, or large tumors. Infiltration into nerve, muscle, and bone may occur. The firm/hard nature and deep violaceous appearance generally are initial clues that it is not an IH. Kasabach-Merritt phenomenon manifests as thrombocytopenia as well as low fibrinogen and elevated D-dimer levels. Thrombocytopenia is generally profound in Kasabach-Merritt phenomenon and results from platelet trapping within the vascular tumor. Given these potential complications, KHEs generally require immediate medical attention, and various treatment protocols including prednisone, vincristine, and sirolimus are utilized for complicated cases.5 The diagnosis may require biopsy to distinguish it from malignant tumors, particularly sarcomas.
Malignant Tumors
Various malignancies, including congenital leukemia, neuroblastoma, Langerhans cell histiocytosis, infantile fibrosarcoma, and rhabdomyosarcoma, rarely may present as cutaneous nodules or masses in a neonate mimicking hemangiomas or other vascular lesions (Figure 2). Neonates may present with multiple bluish papules and nodules resembling a blueberry muffin baby; multiple violaceous-red nodules; or a single red-violaceous, highly vascular–appearing mass mimicking hemangiomas. Malignant tumors may display vascularity on imaging, and thus the presence of vascular flow on ultrasound should not dissuade one from the possibility of a malignancy if other clinical features are atypical or unusual for a hemangioma. When a neonatal malignancy is suspected, a large punch biopsy or incisional biopsy is required for workup.

Final Thoughts
Although IHs are the most common vascular nodules in neonates and young infants, other conditions such as VMs, LMs, CHs, KHEs, and malignancy may occur less commonly. Identifying features that would be considered atypical for IH is crucial to recognize these less common possibilities.
Although most neonatal vascular lumps, bumps, and tumors are benign, proper diagnosis is important for prognosis and management. Therefore, knowledge of both common and rare conditions is important when evaluating a neonatal nodule. Differential diagnosis of neonatal vascular nodules must focus on important diagnostic clues that should prompt consideration and evaluation for less common and/or potentially threatening conditions. Infantile hemangioma (IH), congenital hemangioma (CH), venous malformation (VM), lymphatic malformation (LM), kaposiform hemangioendothelioma (KHE) and tufted angioma, and malignant tumors are reviewed here.
Infantile Hemangioma
Infantile hemangioma, a benign proliferation of capillaries, is the most common tumor of infancy with reported incidence of up to 5% in neonates.1 As such, suspicion for less common lesions is often predicated on identifying features that would be atypical for an IH. A superficial IH presents as a bright red papule, nodule, or plaque, while a deep IH presents as a flesh-colored to bluish nodule. Mixed IHs combine features of both superficial and deep lesions. The distribution may be focal or segmental, with segmental lesions encompassing a larger territory–like distribution and frequently displaying a thin, coarsely telangiectatic appearance.
Knowledge of the natural history of IH generally is crucial in differentiating it from other neonatal lesions. Infantile hemangiomas display a natural history that is distinct and predictable. They typically manifest within the first few weeks of life, though up to 30% present at birth with a premonitory mark, which may be a light red, pink, bluish, or vasoconstricted patch. Thus, mere presence of a lesion at birth is not the feature that distinguishes other congenital lesions from an IH. After initial appearance, IHs undergo a period of proliferation that occurs over 4 to 6 months in most patients. In some cases, areas of proliferation may be subtle, but nonetheless the presence of some areas of increased redness and/or volumetric growth generally is required to firmly establish the diagnosis of IH. Thereafter, IH will involute, a process that begins before 1 year of age in most cases and continues over years. Although IHs undergo involution, complete clearance may not occur, as nearly 70% will leave permanent residua such as fibrofatty masses or anetodermic skin.2 Nevertheless, the presence of a proliferative phase followed by a slower period of involution is a hallmark feature of the IH.
Biopsy and imaging rarely are required for establishing diagnosis of an IH. Histopathology showing a proliferation of capillaries with positive glucose transporter 1 (GLUT-1) staining is characteristic. Imaging with ultrasound reveals a fast-flow lesion. Apart from exceptionally rare cases, a cutaneous IH typically does not cross muscle fascia, and thus alternative diagnoses should be considered for a cutaneous lesion that demonstrates infiltration into nerve, bone, joint, or other deeper tissues. Most IHs do not require treatment; however, a small subset may be associated with complications and thus require intervention. Complications of IH may include impairment of function (eg, vision, feeding, respiratory), ulceration, and risk for permanent disfigurement. When treatment is indicated, the most commonly employed options during the proliferative phase are the topical beta-blocker timolol and the oral beta-blocker propranolol. In addition, certain IHs may be associated with either syndromic presentations and/or visceral involvement, thus requiring further workup (Table).

Congenital Hemangioma
A CH is an uncommon benign neonatal tumor that is distinct from an IH in behavior, biology, and treatment. Congenital hemangiomas may have a rapidly involuting course, referred to as RICH (rapidly involuting congenital hemangioma), or a noninvoluting course, referred to as NICH (noninvoluting congenital hemangioma). Partially involuting types also have been described.3 A RICH typically presents as a highly vascular, red-violaceous or bluish plaque, nodule, or large mass at birth. An NICH presents as a red-violaceous or bluish, coarsely telangiectatic patch, plaque, or nodule. A characteristic feature of the CH is the rim of vasoconstriction around the lesion, which is an important diagnostic clue (Figure 1). In contrast to IH, multifocal lesions are highly unlikely in CH, though it rarely has been reported.4

Regardless of subtype, CHs are fully developed at birth. Infantile hemangiomas, on the other hand, are either minimally present or not present at birth and thereafter proliferate. After birth, a RICH rapidly involutes over the first 9 to 12 months of life. This process generally is evident even in the first few weeks of life, which would not be expected of an IH and is therefore a major distinguishing factor. A NICH, on the other hand, is expected to be persistent, for the most part neither showing signs of proliferation nor involution.
Complications of CHs may include ulceration, functional impairment, or risk for permanent disfigurement depending on location. In addition, due to their fast-flow state and potential large size, some CHs may be complicated by high-output heart failure in the neonate. Distinguishing an IH from a CH is important not only for prognosis but also treatment. Beta-blocker therapy generally is not useful for CHs, and management usually is supportive in the neonatal period.
In the majority of cases, diagnosis can be achieved solely on clinical features. Biopsy with immunohistochemistry shows negative GLUT-1 staining, which will distinguish this lesion from an IH. At times, the highly vascular nature and/or striking size of a CH may lead some to consider the potential diagnosis of an arteriovenous malformation. However, soft-tissue arteriovenous malformations involving the skin are almost never fully developed in the neonatal period and generally take years to evolve from a quiescent state to a destructive lesion.
Venous Malformation
Venous malformations are congenital malformations of veins that may be apparent at birth or later. They appear as bluish to flesh-colored, compressible nodules or plaques. They tend to increase in size when the affected body part is in a dependent position, and this maneuver can be a helpful distinguishing clue. Although the majority of patients have a single lesion, multifocal involvement may occur uncommonly (Table). The diagnosis of VM usually is clinical, though at times, a VM may be difficult to distinguish from a purely deep IH. However, a VM will persist over time, growing in proportion to the patient. In addition, a VM displays low flow on ultrasound, a distinguishing feature from the fast-flow IH. Magnetic resonance imaging with and without contrast is the imaging study of choice. At times, cutaneous VMs will demonstrate infiltration into other tissue planes such as muscle and joint. Pain may occur secondary to thrombus formation within the malformation. In extensive lesions, intravascular coagulation may be notable, as reflected in elevated D-dimer and decreased fibrinogen levels. Treatment with sclerotherapy or surgery may be considered in select cases during infancy; however, in general, an asymptomatic VM may be observed early on in life.
Lymphatic Malformation
A lymphatic malformation (LM) is a congenital malformation of lymphatic vessels and may be further differentiated into microcystic, macrocystic, or mixed types depending on the size of the channels. An LM may present at birth or later and persists over time. Superficial microcystic LMs, synonymous with the term lymphangioma circumscriptum, characteristically appear as a group of clear and violaceous hemorrhagic vesicles on the skin. Deeper LMs appear as a tense or spongy, flesh-colored nodule or mass. Involvement of the head and neck is common. Complications frequently occur in LMs. Cutaneous LMs may ooze or bleed. Infection and hemorrhage into cysts may occur, which will cause acute pain, redness, swelling, and induration. Cervicofacial lesions may result in respiratory distress. Thus, the majority of LMs require treatment, though asymptomatic lesions may be observed in the neonate. An ultrasound will demonstrate a low-flow lesion, and magnetic resonance imaging is the diagnostic modality of choice for diagnosis and definition of extent.
KHE and Tufted Angioma
Kaposiform hemangioendothelioma is a rare, locally aggressive, vascular tumor that is frequently associated with a potentially life-threatening coagulopathy, Kasabach-Merritt phenomenon. Tufted angiomas are now understood to belong on a spectrum with KHEs, which usually present in the neonatal period or infancy as firm, red-violaceous plaques, nodules, or large tumors. Infiltration into nerve, muscle, and bone may occur. The firm/hard nature and deep violaceous appearance generally are initial clues that it is not an IH. Kasabach-Merritt phenomenon manifests as thrombocytopenia as well as low fibrinogen and elevated D-dimer levels. Thrombocytopenia is generally profound in Kasabach-Merritt phenomenon and results from platelet trapping within the vascular tumor. Given these potential complications, KHEs generally require immediate medical attention, and various treatment protocols including prednisone, vincristine, and sirolimus are utilized for complicated cases.5 The diagnosis may require biopsy to distinguish it from malignant tumors, particularly sarcomas.
Malignant Tumors
Various malignancies, including congenital leukemia, neuroblastoma, Langerhans cell histiocytosis, infantile fibrosarcoma, and rhabdomyosarcoma, rarely may present as cutaneous nodules or masses in a neonate mimicking hemangiomas or other vascular lesions (Figure 2). Neonates may present with multiple bluish papules and nodules resembling a blueberry muffin baby; multiple violaceous-red nodules; or a single red-violaceous, highly vascular–appearing mass mimicking hemangiomas. Malignant tumors may display vascularity on imaging, and thus the presence of vascular flow on ultrasound should not dissuade one from the possibility of a malignancy if other clinical features are atypical or unusual for a hemangioma. When a neonatal malignancy is suspected, a large punch biopsy or incisional biopsy is required for workup.

Final Thoughts
Although IHs are the most common vascular nodules in neonates and young infants, other conditions such as VMs, LMs, CHs, KHEs, and malignancy may occur less commonly. Identifying features that would be considered atypical for IH is crucial to recognize these less common possibilities.
- Kanada KN, Merin MR, Munden A, et al. A prospective study of cutaneous findings in newborns in the United States: correlation with race, ethnicity, and gestational status using updated classification and nomenclature. J Pediatr. 2012;161:240-245.
- Darrow DH, Greene AK, Mancini AJ, et al. Diagnosis and management of infantile hemangioma. Pediatrics. 2015;136:E1060-E1104.
- Nasseri E, Piram M, McCuaig CC, et al. Partially involuting congenital hemangiomas: a report of 8 cases and review of the literature. J Am Acad Dermatol. 2014;70:75-79.
- Blumenthal S, Stefanko N, Cossio M, et al. Multifocal congenital hemangioma: expanding the pathogenesis of “neonatal hemangiomatosis.” Pediatr Dermatol. 2019;36:720-722.
- Croteau SE, Gupta D. The clinical spectrum of kaposiform hemangioendothelioma and tufted angioma. Semin Cutan Med Surg. 2016;35:147-152.
- Kanada KN, Merin MR, Munden A, et al. A prospective study of cutaneous findings in newborns in the United States: correlation with race, ethnicity, and gestational status using updated classification and nomenclature. J Pediatr. 2012;161:240-245.
- Darrow DH, Greene AK, Mancini AJ, et al. Diagnosis and management of infantile hemangioma. Pediatrics. 2015;136:E1060-E1104.
- Nasseri E, Piram M, McCuaig CC, et al. Partially involuting congenital hemangiomas: a report of 8 cases and review of the literature. J Am Acad Dermatol. 2014;70:75-79.
- Blumenthal S, Stefanko N, Cossio M, et al. Multifocal congenital hemangioma: expanding the pathogenesis of “neonatal hemangiomatosis.” Pediatr Dermatol. 2019;36:720-722.
- Croteau SE, Gupta D. The clinical spectrum of kaposiform hemangioendothelioma and tufted angioma. Semin Cutan Med Surg. 2016;35:147-152.
Subclinical hypothyroidism and pregnancy: Public health problem or lab finding with minimal clinical significance?
In a US study of more than 17,000 people, overt hypothyroidism and hyperthyroidism were detected in about 4.6% and 1.3% of adults, respectively.1 In this population-based study, thyroid disease was 5 times more prevalent among women than among men. In our ObGyn practices, there are many women of reproductive age with thyroid disease who are considering pregnancy. Treatment of active hyperthyroidism in a woman planning pregnancy is complex and best managed by endocrinologists. Treatment of hypothyroidism is more straightforward, however, and typically managed by internists, family medicine clinicians, and obstetrician-gynecologists.
Clinical management of hypothyroidism and pregnancy
Pregnancy results in a doubling of thyroxine-binding globulin (TBG) levels and a 40% increase in plasma volume, resulting in a need for more thyroxine production.2 Of note, from conception to approximately 13 weeks’ gestation, the sole source of embryonic and fetal thyroid hormones is from the mother.2 Women who have been taking chronic thyroxine treatment may have suppressed thyroid gland activity and be unable to increase thyroxine production in response to pregnancy, necessitating a 30% to 50% increase in their thyroxine dose to maintain TSH levels in the normal range.
For hypothyroid women on long-term thyroxine treatment, recommend increasing the thyroxine dose when pregnancy is recognized. For your patients on chronic thyroxine treatment who are planning a pregnancy, a multiprong approach is helpful in preparing the patient for the increased thyroxine requirements of early pregnancy. First, it is important to counsel the woman that she should not stop the thyroxine medication because it may adversely affect the pregnancy. In my experience, most cases of overt hypothyroidism during pregnancy occur because the patient stopped taking her thyroxine therapy. Second, for hypothyroid women who are considering conception it is reasonable to adjust the thyroxine dose to keep the TSH concentration in the lower range of normal (0.5 to 2.5 mU/L). This will give the woman a “buffer,” reducing the risk that in early pregnancy she and her fetus will have a thyroxine deficit. Third, in early pregnancy, following detection of a positive pregnancy test, your patient can start to increase her thyroxine dose by about two tablets weekly (a 28% increase in the dose). Fourth, TSH levels can be measured every 4 weeks during the first trimester, with appropriate adjustment of the thyroxine dose to keep the TSH concentration below the trimester-specific upper limit of normal (< 4 mU/L).2
TSH and free thyroxine measurements identify women with overt hypothyroidism who need thyroxine treatment. Overt hypothyroidism is associated with adverse reproductive outcomes, including decreased fertility, increased spontaneous abortion, increased fetal loss, and preterm birth.2,3 Hence it is important to immediately initiate thyroxine treatment in pregnant women who have overt hypothyroidism. A diagnosis of overt hypothyroidism is indicated in women with an intact hypothalamic-pituitary axis and a TSH level ≥10 mU/L plus a low free thyroxine concentration. A TSH level of >4 to 10 mU/L, with normal free thyroxine concentration, is evidence of subclinical hypothyroidism (SCH). Among women, there are about 5 times more cases of SCH than overt hypothyroidism.
Continue to: The literature concerning SCH and pregnancy...
The literature concerning SCH and pregnancy is vast, and often contradictory, leading to confusion among clinicians. Contributing to the confusion is that some observational studies report a modest association between SCH and adverse pregnancy outcomes. To date, however, randomized clinical trials show no benefit of thyroxine treatment in these cases. I explore these contradictory pieces of evidence below.
Is SCH associated with adverse pregnancy outcomes due to low thyroxine levels?
There is conflicting literature about the association of SCH and adverse reproductive outcomes. A meta-analysis of 47,045 pregnant women reported that the preterm birth rate for women with SCH and euthyroid women (normal TSH and normal free thyroxine levels) was 6.1% and 5.0%, respectively (odds ratio [OR], 1.29; 95% CI, 1.01–1.64).4 Interestingly, pregnant women with normal TSH levels but a low free thyroxine level also had an increased rate of preterm birth (7.1% vs 5.0%; OR, 1.46; 95% CI, 1.12–1.90).
Although observational studies report an association between SCH and adverse reproductive outcomes, multiple randomized clinical trials conducted in women with SCH or hypothyroxinemia have failed to demonstrate that thyroxine replacement improves reproductive outcomes. For example, in a study of 794 pregnant women with elevated TSH and/or low free thyroxine levels randomly assigned to thyroxine treatment (0.15 mg daily) or no treatment, there was no difference in preterm birth rate (5.6% vs 7.9%, P = .2), mean birth weight (3.5 kg vs 3.3 kg, P = .15), gestational age at delivery (40.1 vs 40.2 weeks, P = .10), or the intelligence quotient of children at 3 years (99 vs 100, P = .40).5
In another study, 674 pregnant women with mild SCH (mean TSH, 4.4 mU/L) were randomly assigned to receive thyroxine (0.1 mg daily and dose adjusted to achieve a normal TSH level) or placebo. In this study there was no difference between the thyroxine treatment or placebo groups in preterm birth rate (9% vs 11%, P = .44), gestational age at delivery (39.1 vs 38.9 weeks, P = .57) or intelligence quotient of children at 5 years (97 and 94, P = .71).6
The same investigators also randomized 524 pregnant women with isolated hypothyroxinema (mean free thyroxine level, 0.83 ng/dL) and normal TSH level (mean, 1.5 mU/L) to thyroxine (0.05 mg daily and dose adjusted to achieve a normal free thyroxine level) or placebo.6 In this study there was no difference in preterm birth rate (12% vs 8%, P = .11), gestational age at delivery (39.0 vs 38.8 weeks, P = .46) or intelligence quotient of children at 5 years (94 and 91, P = .31).6
When large randomized clinical trials and observational studies report discrepant results, many authorities prioritize the findings from the randomized clinical trials because those results are less prone to being confounded by unrecognized factors. Randomized trials do not demonstrate that mild SCH or isolated hypothyroxinemia have a major impact on pregnancy outcomes.
Thyroid antibodies, fertility, miscarriage, and preterm birth
Some observational studies report that the presence of thyroid antibodies in a euthyroid woman reduces fecundity and increases the risk for miscarriage and preterm birth. For example, a meta-analysis of 47,045 pregnant women reported that the preterm birth rate for women with and without antithyroid antibodies was 6.9% and 4.9%, respectively (OR, 1.33; 95% CI, 1.15–1.56). However, in euthyroid women with antithyroid antibodies, low-dose thyroxine therapy has not been shown to improve fertility, or reduce miscarriages or preterm birth rate.
Continue to: In a large randomized clinical trial, 952 euthyroid women...
In a large randomized clinical trial, 952 euthyroid women (normal TSH level; range, 0.44 to 3.63 mIU/L and free thyroxine level; range, 10 to 21 pmol/L) who were planning on conceiving and had elevated thyroid peroxidase antibodies were randomized prior to conception to receive either thyroxine (50 µg) or placebo.7 After 12 months, outcomes were similar for women treated with thyroxine or placebo, including live birth rate (37.4% vs 37.9%), miscarriage rate for those who became pregnant (28.2% vs 29.6%), and preterm birth ≤ 34 weeks of gestation (3.8% vs 3.6%, respectively).7 The investigators concluded that the use of low-dose thyroxine in euthyroid women with thyroid peroxidase antibodies was not effective for increasing the rate of live birth or reducing the rate of miscarriage or early preterm birth.
Thyroid antibodies and the rate of IVF pregnancy and miscarriage
Some observational studies suggest that the presence of antithyroid antibodies may be associated with an increased rate of miscarriage.8 To test the effects of thyroxine treatment on the rate of miscarriage in euthyroid women with antithyroid antibodies, 600 euthyroid infertile women with antithyroid antibodies (antithyroid peroxidase levels ≥ 60 IU/mL) scheduled to have in vitro fertilization (IVF) were randomly assigned to receive thyroxine (dose adjustment to keep TSH levels in the range of 0.1 to 2.5 mIU/L) or no treatment.9 The thyroxine treatment was initiated 2 to 4 weeks before initiation of ovarian stimulation. In this study, treatment with thyroxine or no treatment resulted in similar rates of clinical pregnancy (35.7% vs 37.7%) and live birth (31.7% vs 32.3%).9 Among the women who achieved a clinical pregnancy, miscarriage rates were similar in the thyroxine and no treatment groups (10.3% vs 10.6%).9
Let’s focus on more serious problems that affect pregnancy
There is a clear consensus that women with overt hypothyroidism should be treated with thyroxine prior to attempting pregnancy.2,6 There is no clear consensus about how to treat women considering pregnancy who have one isolated laboratory finding, such as mild subclinical hypothyroidism, mild isolated hypothyroxinemia, or antithyroid antibodies. Given the lack of evidence from randomized trials that thyroxine improves pregnancy outcomes in these cases, obstetrician-gynecologists may want to either refer women with these problems to an endocrinologist for consultation or sequentially measure laboratory values to assess whether the patient’s laboratory abnormality is transient, stable, or worsening.
Obstetrician-gynecologists and their patients are confronted by many serious problems that adversely affect pregnancy and deserve priority attention, including iron deficiency anemia, excess gestational weight gain, peripartum depression, intimate partner violence, housing insecurity, cigarette smoking, substance misuse, chronic hypertension, morbid obesity, diabetes, gestational diabetes, preeclampsia, venous thromboembolism, obstetrical hemorrhage, sepsis, and infectious diseases. Given limited resources our expertise should be focused on these major obstetric public health problems rather than screening for mild subclinical hypothyroidism.
- Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489-499.
- Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2017;27:315-389.
- Abalovich M, Gutierrez S, Alcaraz G, et al. Overt and subclinical hypothyroidism complicating pregnancy. Thyroid. 2012;12:63-68.
- Consortium on Thyroid and Pregnancy--Study Group on Preterm Birth. Association of thyroid function test abnormalities and thyroid autoimmunity with preterm birth: a systematic review and meta-analysis. JAMA. 2019;322:632-641.
- Lazarus JH, Bestwick JP, Channon S, et al. Antenatal thyroid screening and childhood cognitive function. N Engl J Med. 2012;366:493-501.
- Casey BM, Thom EA, Peaceman AM, et al. Treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy. N Engl J Med. 2017;376:815-825.
- Dhillon-Smith RK, Middleton LJ, Sunner KK, et al. Levothyroxine in women with thyroid peroxidase antibodies before conception. N Engl J Med. 2019;380:1316-1325.
- Chen L, Hu R. Thyroid autoimmunity and miscarriage: a meta-analysis. Clin Endocrinol (Oxf). 2011;74:513-519.
- Wang H, Gao H, Chi H, et al. Effect of levothyroxine on miscarriage among women with normal thyroid function and thyroid autoimmunity undergoing in vitro fertilization and embryo transfer: a randomized clinical trial. JAMA. 2017;318:2190-2198.
In a US study of more than 17,000 people, overt hypothyroidism and hyperthyroidism were detected in about 4.6% and 1.3% of adults, respectively.1 In this population-based study, thyroid disease was 5 times more prevalent among women than among men. In our ObGyn practices, there are many women of reproductive age with thyroid disease who are considering pregnancy. Treatment of active hyperthyroidism in a woman planning pregnancy is complex and best managed by endocrinologists. Treatment of hypothyroidism is more straightforward, however, and typically managed by internists, family medicine clinicians, and obstetrician-gynecologists.
Clinical management of hypothyroidism and pregnancy
Pregnancy results in a doubling of thyroxine-binding globulin (TBG) levels and a 40% increase in plasma volume, resulting in a need for more thyroxine production.2 Of note, from conception to approximately 13 weeks’ gestation, the sole source of embryonic and fetal thyroid hormones is from the mother.2 Women who have been taking chronic thyroxine treatment may have suppressed thyroid gland activity and be unable to increase thyroxine production in response to pregnancy, necessitating a 30% to 50% increase in their thyroxine dose to maintain TSH levels in the normal range.
For hypothyroid women on long-term thyroxine treatment, recommend increasing the thyroxine dose when pregnancy is recognized. For your patients on chronic thyroxine treatment who are planning a pregnancy, a multiprong approach is helpful in preparing the patient for the increased thyroxine requirements of early pregnancy. First, it is important to counsel the woman that she should not stop the thyroxine medication because it may adversely affect the pregnancy. In my experience, most cases of overt hypothyroidism during pregnancy occur because the patient stopped taking her thyroxine therapy. Second, for hypothyroid women who are considering conception it is reasonable to adjust the thyroxine dose to keep the TSH concentration in the lower range of normal (0.5 to 2.5 mU/L). This will give the woman a “buffer,” reducing the risk that in early pregnancy she and her fetus will have a thyroxine deficit. Third, in early pregnancy, following detection of a positive pregnancy test, your patient can start to increase her thyroxine dose by about two tablets weekly (a 28% increase in the dose). Fourth, TSH levels can be measured every 4 weeks during the first trimester, with appropriate adjustment of the thyroxine dose to keep the TSH concentration below the trimester-specific upper limit of normal (< 4 mU/L).2
TSH and free thyroxine measurements identify women with overt hypothyroidism who need thyroxine treatment. Overt hypothyroidism is associated with adverse reproductive outcomes, including decreased fertility, increased spontaneous abortion, increased fetal loss, and preterm birth.2,3 Hence it is important to immediately initiate thyroxine treatment in pregnant women who have overt hypothyroidism. A diagnosis of overt hypothyroidism is indicated in women with an intact hypothalamic-pituitary axis and a TSH level ≥10 mU/L plus a low free thyroxine concentration. A TSH level of >4 to 10 mU/L, with normal free thyroxine concentration, is evidence of subclinical hypothyroidism (SCH). Among women, there are about 5 times more cases of SCH than overt hypothyroidism.
Continue to: The literature concerning SCH and pregnancy...
The literature concerning SCH and pregnancy is vast, and often contradictory, leading to confusion among clinicians. Contributing to the confusion is that some observational studies report a modest association between SCH and adverse pregnancy outcomes. To date, however, randomized clinical trials show no benefit of thyroxine treatment in these cases. I explore these contradictory pieces of evidence below.

Is SCH associated with adverse pregnancy outcomes due to low thyroxine levels?
There is conflicting literature about the association of SCH and adverse reproductive outcomes. A meta-analysis of 47,045 pregnant women reported that the preterm birth rate for women with SCH and euthyroid women (normal TSH and normal free thyroxine levels) was 6.1% and 5.0%, respectively (odds ratio [OR], 1.29; 95% CI, 1.01–1.64).4 Interestingly, pregnant women with normal TSH levels but a low free thyroxine level also had an increased rate of preterm birth (7.1% vs 5.0%; OR, 1.46; 95% CI, 1.12–1.90).
Although observational studies report an association between SCH and adverse reproductive outcomes, multiple randomized clinical trials conducted in women with SCH or hypothyroxinemia have failed to demonstrate that thyroxine replacement improves reproductive outcomes. For example, in a study of 794 pregnant women with elevated TSH and/or low free thyroxine levels randomly assigned to thyroxine treatment (0.15 mg daily) or no treatment, there was no difference in preterm birth rate (5.6% vs 7.9%, P = .2), mean birth weight (3.5 kg vs 3.3 kg, P = .15), gestational age at delivery (40.1 vs 40.2 weeks, P = .10), or the intelligence quotient of children at 3 years (99 vs 100, P = .40).5
In another study, 674 pregnant women with mild SCH (mean TSH, 4.4 mU/L) were randomly assigned to receive thyroxine (0.1 mg daily and dose adjusted to achieve a normal TSH level) or placebo. In this study there was no difference between the thyroxine treatment or placebo groups in preterm birth rate (9% vs 11%, P = .44), gestational age at delivery (39.1 vs 38.9 weeks, P = .57) or intelligence quotient of children at 5 years (97 and 94, P = .71).6
The same investigators also randomized 524 pregnant women with isolated hypothyroxinema (mean free thyroxine level, 0.83 ng/dL) and normal TSH level (mean, 1.5 mU/L) to thyroxine (0.05 mg daily and dose adjusted to achieve a normal free thyroxine level) or placebo.6 In this study there was no difference in preterm birth rate (12% vs 8%, P = .11), gestational age at delivery (39.0 vs 38.8 weeks, P = .46) or intelligence quotient of children at 5 years (94 and 91, P = .31).6
When large randomized clinical trials and observational studies report discrepant results, many authorities prioritize the findings from the randomized clinical trials because those results are less prone to being confounded by unrecognized factors. Randomized trials do not demonstrate that mild SCH or isolated hypothyroxinemia have a major impact on pregnancy outcomes.
Thyroid antibodies, fertility, miscarriage, and preterm birth
Some observational studies report that the presence of thyroid antibodies in a euthyroid woman reduces fecundity and increases the risk for miscarriage and preterm birth. For example, a meta-analysis of 47,045 pregnant women reported that the preterm birth rate for women with and without antithyroid antibodies was 6.9% and 4.9%, respectively (OR, 1.33; 95% CI, 1.15–1.56). However, in euthyroid women with antithyroid antibodies, low-dose thyroxine therapy has not been shown to improve fertility, or reduce miscarriages or preterm birth rate.
Continue to: In a large randomized clinical trial, 952 euthyroid women...
In a large randomized clinical trial, 952 euthyroid women (normal TSH level; range, 0.44 to 3.63 mIU/L and free thyroxine level; range, 10 to 21 pmol/L) who were planning on conceiving and had elevated thyroid peroxidase antibodies were randomized prior to conception to receive either thyroxine (50 µg) or placebo.7 After 12 months, outcomes were similar for women treated with thyroxine or placebo, including live birth rate (37.4% vs 37.9%), miscarriage rate for those who became pregnant (28.2% vs 29.6%), and preterm birth ≤ 34 weeks of gestation (3.8% vs 3.6%, respectively).7 The investigators concluded that the use of low-dose thyroxine in euthyroid women with thyroid peroxidase antibodies was not effective for increasing the rate of live birth or reducing the rate of miscarriage or early preterm birth.
Thyroid antibodies and the rate of IVF pregnancy and miscarriage
Some observational studies suggest that the presence of antithyroid antibodies may be associated with an increased rate of miscarriage.8 To test the effects of thyroxine treatment on the rate of miscarriage in euthyroid women with antithyroid antibodies, 600 euthyroid infertile women with antithyroid antibodies (antithyroid peroxidase levels ≥ 60 IU/mL) scheduled to have in vitro fertilization (IVF) were randomly assigned to receive thyroxine (dose adjustment to keep TSH levels in the range of 0.1 to 2.5 mIU/L) or no treatment.9 The thyroxine treatment was initiated 2 to 4 weeks before initiation of ovarian stimulation. In this study, treatment with thyroxine or no treatment resulted in similar rates of clinical pregnancy (35.7% vs 37.7%) and live birth (31.7% vs 32.3%).9 Among the women who achieved a clinical pregnancy, miscarriage rates were similar in the thyroxine and no treatment groups (10.3% vs 10.6%).9
Let’s focus on more serious problems that affect pregnancy
There is a clear consensus that women with overt hypothyroidism should be treated with thyroxine prior to attempting pregnancy.2,6 There is no clear consensus about how to treat women considering pregnancy who have one isolated laboratory finding, such as mild subclinical hypothyroidism, mild isolated hypothyroxinemia, or antithyroid antibodies. Given the lack of evidence from randomized trials that thyroxine improves pregnancy outcomes in these cases, obstetrician-gynecologists may want to either refer women with these problems to an endocrinologist for consultation or sequentially measure laboratory values to assess whether the patient’s laboratory abnormality is transient, stable, or worsening.
Obstetrician-gynecologists and their patients are confronted by many serious problems that adversely affect pregnancy and deserve priority attention, including iron deficiency anemia, excess gestational weight gain, peripartum depression, intimate partner violence, housing insecurity, cigarette smoking, substance misuse, chronic hypertension, morbid obesity, diabetes, gestational diabetes, preeclampsia, venous thromboembolism, obstetrical hemorrhage, sepsis, and infectious diseases. Given limited resources our expertise should be focused on these major obstetric public health problems rather than screening for mild subclinical hypothyroidism.
In a US study of more than 17,000 people, overt hypothyroidism and hyperthyroidism were detected in about 4.6% and 1.3% of adults, respectively.1 In this population-based study, thyroid disease was 5 times more prevalent among women than among men. In our ObGyn practices, there are many women of reproductive age with thyroid disease who are considering pregnancy. Treatment of active hyperthyroidism in a woman planning pregnancy is complex and best managed by endocrinologists. Treatment of hypothyroidism is more straightforward, however, and typically managed by internists, family medicine clinicians, and obstetrician-gynecologists.
Clinical management of hypothyroidism and pregnancy
Pregnancy results in a doubling of thyroxine-binding globulin (TBG) levels and a 40% increase in plasma volume, resulting in a need for more thyroxine production.2 Of note, from conception to approximately 13 weeks’ gestation, the sole source of embryonic and fetal thyroid hormones is from the mother.2 Women who have been taking chronic thyroxine treatment may have suppressed thyroid gland activity and be unable to increase thyroxine production in response to pregnancy, necessitating a 30% to 50% increase in their thyroxine dose to maintain TSH levels in the normal range.
For hypothyroid women on long-term thyroxine treatment, recommend increasing the thyroxine dose when pregnancy is recognized. For your patients on chronic thyroxine treatment who are planning a pregnancy, a multiprong approach is helpful in preparing the patient for the increased thyroxine requirements of early pregnancy. First, it is important to counsel the woman that she should not stop the thyroxine medication because it may adversely affect the pregnancy. In my experience, most cases of overt hypothyroidism during pregnancy occur because the patient stopped taking her thyroxine therapy. Second, for hypothyroid women who are considering conception it is reasonable to adjust the thyroxine dose to keep the TSH concentration in the lower range of normal (0.5 to 2.5 mU/L). This will give the woman a “buffer,” reducing the risk that in early pregnancy she and her fetus will have a thyroxine deficit. Third, in early pregnancy, following detection of a positive pregnancy test, your patient can start to increase her thyroxine dose by about two tablets weekly (a 28% increase in the dose). Fourth, TSH levels can be measured every 4 weeks during the first trimester, with appropriate adjustment of the thyroxine dose to keep the TSH concentration below the trimester-specific upper limit of normal (< 4 mU/L).2
TSH and free thyroxine measurements identify women with overt hypothyroidism who need thyroxine treatment. Overt hypothyroidism is associated with adverse reproductive outcomes, including decreased fertility, increased spontaneous abortion, increased fetal loss, and preterm birth.2,3 Hence it is important to immediately initiate thyroxine treatment in pregnant women who have overt hypothyroidism. A diagnosis of overt hypothyroidism is indicated in women with an intact hypothalamic-pituitary axis and a TSH level ≥10 mU/L plus a low free thyroxine concentration. A TSH level of >4 to 10 mU/L, with normal free thyroxine concentration, is evidence of subclinical hypothyroidism (SCH). Among women, there are about 5 times more cases of SCH than overt hypothyroidism.
Continue to: The literature concerning SCH and pregnancy...
The literature concerning SCH and pregnancy is vast, and often contradictory, leading to confusion among clinicians. Contributing to the confusion is that some observational studies report a modest association between SCH and adverse pregnancy outcomes. To date, however, randomized clinical trials show no benefit of thyroxine treatment in these cases. I explore these contradictory pieces of evidence below.

Is SCH associated with adverse pregnancy outcomes due to low thyroxine levels?
There is conflicting literature about the association of SCH and adverse reproductive outcomes. A meta-analysis of 47,045 pregnant women reported that the preterm birth rate for women with SCH and euthyroid women (normal TSH and normal free thyroxine levels) was 6.1% and 5.0%, respectively (odds ratio [OR], 1.29; 95% CI, 1.01–1.64).4 Interestingly, pregnant women with normal TSH levels but a low free thyroxine level also had an increased rate of preterm birth (7.1% vs 5.0%; OR, 1.46; 95% CI, 1.12–1.90).
Although observational studies report an association between SCH and adverse reproductive outcomes, multiple randomized clinical trials conducted in women with SCH or hypothyroxinemia have failed to demonstrate that thyroxine replacement improves reproductive outcomes. For example, in a study of 794 pregnant women with elevated TSH and/or low free thyroxine levels randomly assigned to thyroxine treatment (0.15 mg daily) or no treatment, there was no difference in preterm birth rate (5.6% vs 7.9%, P = .2), mean birth weight (3.5 kg vs 3.3 kg, P = .15), gestational age at delivery (40.1 vs 40.2 weeks, P = .10), or the intelligence quotient of children at 3 years (99 vs 100, P = .40).5
In another study, 674 pregnant women with mild SCH (mean TSH, 4.4 mU/L) were randomly assigned to receive thyroxine (0.1 mg daily and dose adjusted to achieve a normal TSH level) or placebo. In this study there was no difference between the thyroxine treatment or placebo groups in preterm birth rate (9% vs 11%, P = .44), gestational age at delivery (39.1 vs 38.9 weeks, P = .57) or intelligence quotient of children at 5 years (97 and 94, P = .71).6
The same investigators also randomized 524 pregnant women with isolated hypothyroxinema (mean free thyroxine level, 0.83 ng/dL) and normal TSH level (mean, 1.5 mU/L) to thyroxine (0.05 mg daily and dose adjusted to achieve a normal free thyroxine level) or placebo.6 In this study there was no difference in preterm birth rate (12% vs 8%, P = .11), gestational age at delivery (39.0 vs 38.8 weeks, P = .46) or intelligence quotient of children at 5 years (94 and 91, P = .31).6
When large randomized clinical trials and observational studies report discrepant results, many authorities prioritize the findings from the randomized clinical trials because those results are less prone to being confounded by unrecognized factors. Randomized trials do not demonstrate that mild SCH or isolated hypothyroxinemia have a major impact on pregnancy outcomes.
Thyroid antibodies, fertility, miscarriage, and preterm birth
Some observational studies report that the presence of thyroid antibodies in a euthyroid woman reduces fecundity and increases the risk for miscarriage and preterm birth. For example, a meta-analysis of 47,045 pregnant women reported that the preterm birth rate for women with and without antithyroid antibodies was 6.9% and 4.9%, respectively (OR, 1.33; 95% CI, 1.15–1.56). However, in euthyroid women with antithyroid antibodies, low-dose thyroxine therapy has not been shown to improve fertility, or reduce miscarriages or preterm birth rate.
Continue to: In a large randomized clinical trial, 952 euthyroid women...
In a large randomized clinical trial, 952 euthyroid women (normal TSH level; range, 0.44 to 3.63 mIU/L and free thyroxine level; range, 10 to 21 pmol/L) who were planning on conceiving and had elevated thyroid peroxidase antibodies were randomized prior to conception to receive either thyroxine (50 µg) or placebo.7 After 12 months, outcomes were similar for women treated with thyroxine or placebo, including live birth rate (37.4% vs 37.9%), miscarriage rate for those who became pregnant (28.2% vs 29.6%), and preterm birth ≤ 34 weeks of gestation (3.8% vs 3.6%, respectively).7 The investigators concluded that the use of low-dose thyroxine in euthyroid women with thyroid peroxidase antibodies was not effective for increasing the rate of live birth or reducing the rate of miscarriage or early preterm birth.
Thyroid antibodies and the rate of IVF pregnancy and miscarriage
Some observational studies suggest that the presence of antithyroid antibodies may be associated with an increased rate of miscarriage.8 To test the effects of thyroxine treatment on the rate of miscarriage in euthyroid women with antithyroid antibodies, 600 euthyroid infertile women with antithyroid antibodies (antithyroid peroxidase levels ≥ 60 IU/mL) scheduled to have in vitro fertilization (IVF) were randomly assigned to receive thyroxine (dose adjustment to keep TSH levels in the range of 0.1 to 2.5 mIU/L) or no treatment.9 The thyroxine treatment was initiated 2 to 4 weeks before initiation of ovarian stimulation. In this study, treatment with thyroxine or no treatment resulted in similar rates of clinical pregnancy (35.7% vs 37.7%) and live birth (31.7% vs 32.3%).9 Among the women who achieved a clinical pregnancy, miscarriage rates were similar in the thyroxine and no treatment groups (10.3% vs 10.6%).9
Let’s focus on more serious problems that affect pregnancy
There is a clear consensus that women with overt hypothyroidism should be treated with thyroxine prior to attempting pregnancy.2,6 There is no clear consensus about how to treat women considering pregnancy who have one isolated laboratory finding, such as mild subclinical hypothyroidism, mild isolated hypothyroxinemia, or antithyroid antibodies. Given the lack of evidence from randomized trials that thyroxine improves pregnancy outcomes in these cases, obstetrician-gynecologists may want to either refer women with these problems to an endocrinologist for consultation or sequentially measure laboratory values to assess whether the patient’s laboratory abnormality is transient, stable, or worsening.
Obstetrician-gynecologists and their patients are confronted by many serious problems that adversely affect pregnancy and deserve priority attention, including iron deficiency anemia, excess gestational weight gain, peripartum depression, intimate partner violence, housing insecurity, cigarette smoking, substance misuse, chronic hypertension, morbid obesity, diabetes, gestational diabetes, preeclampsia, venous thromboembolism, obstetrical hemorrhage, sepsis, and infectious diseases. Given limited resources our expertise should be focused on these major obstetric public health problems rather than screening for mild subclinical hypothyroidism.
- Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489-499.
- Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2017;27:315-389.
- Abalovich M, Gutierrez S, Alcaraz G, et al. Overt and subclinical hypothyroidism complicating pregnancy. Thyroid. 2012;12:63-68.
- Consortium on Thyroid and Pregnancy--Study Group on Preterm Birth. Association of thyroid function test abnormalities and thyroid autoimmunity with preterm birth: a systematic review and meta-analysis. JAMA. 2019;322:632-641.
- Lazarus JH, Bestwick JP, Channon S, et al. Antenatal thyroid screening and childhood cognitive function. N Engl J Med. 2012;366:493-501.
- Casey BM, Thom EA, Peaceman AM, et al. Treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy. N Engl J Med. 2017;376:815-825.
- Dhillon-Smith RK, Middleton LJ, Sunner KK, et al. Levothyroxine in women with thyroid peroxidase antibodies before conception. N Engl J Med. 2019;380:1316-1325.
- Chen L, Hu R. Thyroid autoimmunity and miscarriage: a meta-analysis. Clin Endocrinol (Oxf). 2011;74:513-519.
- Wang H, Gao H, Chi H, et al. Effect of levothyroxine on miscarriage among women with normal thyroid function and thyroid autoimmunity undergoing in vitro fertilization and embryo transfer: a randomized clinical trial. JAMA. 2017;318:2190-2198.
- Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489-499.
- Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2017;27:315-389.
- Abalovich M, Gutierrez S, Alcaraz G, et al. Overt and subclinical hypothyroidism complicating pregnancy. Thyroid. 2012;12:63-68.
- Consortium on Thyroid and Pregnancy--Study Group on Preterm Birth. Association of thyroid function test abnormalities and thyroid autoimmunity with preterm birth: a systematic review and meta-analysis. JAMA. 2019;322:632-641.
- Lazarus JH, Bestwick JP, Channon S, et al. Antenatal thyroid screening and childhood cognitive function. N Engl J Med. 2012;366:493-501.
- Casey BM, Thom EA, Peaceman AM, et al. Treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy. N Engl J Med. 2017;376:815-825.
- Dhillon-Smith RK, Middleton LJ, Sunner KK, et al. Levothyroxine in women with thyroid peroxidase antibodies before conception. N Engl J Med. 2019;380:1316-1325.
- Chen L, Hu R. Thyroid autoimmunity and miscarriage: a meta-analysis. Clin Endocrinol (Oxf). 2011;74:513-519.
- Wang H, Gao H, Chi H, et al. Effect of levothyroxine on miscarriage among women with normal thyroid function and thyroid autoimmunity undergoing in vitro fertilization and embryo transfer: a randomized clinical trial. JAMA. 2017;318:2190-2198.
‘Time lost is brain lost’
Question: Which of the following statements regarding “common knowledge” is correct?
A. In any negligence action absent common knowledge, expert testimony is then required to prove requisite standard of care and causation.
B.
C. An expert is needed in the first place to establish whether something constitutes common knowledge.
D. The jury is the one who determines whether a plaintiff can invoke the common knowledge exception.
E. An example of common knowledge in malpractice law is where a delay in stroke diagnosis results in loss of brain function.
Answer: B. The judge, not the jury or anyone else, makes the decision regarding res ipsa loquitur (the thing speaks for itself) or common knowledge, which exempts a plaintiff from producing an expert witness to testify as to the standard of care and causation. However, this is only true in actions arising out of professional negligence such as medical malpractice, whereas most common negligence actions – for example, slips and falls – do not require expert testimony.
Only a professional, duly qualified by the court as an expert witness, is allowed to offer medical testimony, while the plaintiff will typically be disqualified from playing this role because of the complexity of issues involved unless there is common knowledge. In general, courts are reluctant to grant this exception, which favors the plaintiff.
The best example of res ipsa loquitur is where a surgeon inadvertently leaves behind a sponge or instrument inside a body cavity. Other successfully litigated examples include cardiac arrest in the operating room, hypoxia in the recovery room, burns to the buttock, gangrene after the accidental injection of penicillin into an artery, air trapped subcutaneously from a displaced needle, and a pierced eyeball during a procedure. The factual circumstances of each case are critical to its outcome. For example, in a 2013 New York case, the plaintiff was barred from using the res doctrine.1 The defendant doctor had left a guide wire in the plaintiff’s chest following a biopsy and retrieved it 2 months later. The plaintiff did not call any expert witness, relying instead on the “foreign object” basis for invoking the res doctrine. However, the Court of Appeals reasoned that the object was left behind deliberately, not unintentionally, and that under the circumstances of the case, an expert witness was needed to set out the applicable standard of care, without which a jury could not determine whether the doctor’s professional judgment breached the requisite standard.
The Supreme Court of Kentucky recently rejected the use of common knowledge in a stroke case.2 In 2010, David Shackelford’s rheumatologist referred him to Paul Lewis, MD, an interventional radiologist, for a four-vessel cerebral angiogram to assist with diagnosing the cause of Mr. Shackelford’s chronic headaches. The procedure itself was uneventful, but while in the recovery room, Mr. Shackelford reported a frontal headache and scotoma, which resolved on its own. The headache improved with medication, and the patient experienced no other symptoms. There were no other visual changes, weakness, slurred speech, or facial palsies. Mr. Shackelford was discharged but had to return to the hospital the next morning via ambulance after becoming disoriented at his home. An MRI indicated multiple scattered small infarcts, and he was left with residual short-term memory loss and visual problems.
There was no allegation that the stroke itself was caused by negligence; rather, Mr. Shackelford alleged that the failure to examine and diagnose the stroke after the angiogram was negligent and caused injury greater than that which the stroke would have caused with earlier intervention. To support his claims, Mr. Shackelford’s expert, Michael David Khoury, MD, a vascular surgeon, criticized Dr. Lewis’s failure to examine Mr. Shackelford when his symptoms were consistent with a stroke. However, Dr. Khoury did not opine that Dr. Lewis could have limited the effects of the stroke through earlier intervention. When asked specifically whether he could state within a reasonable degree of medical probability that Dr. Lewis’s postprocedure care was a substantial factor in causing harm to Mr. Shackelford, Dr. Khoury responded that it was “impossible to tell.”
Based largely upon Dr. Khoury’s deposition testimony, the defendants successfully moved for summary judgment on the basis that the expert had failed to opine that the alleged negligence caused any injury to Mr. Shackelford. As a result, Mr. Shackelford could not prove an essential element of his medical malpractice claim. Defense expert Peter J. Pema, MD, a neuroradiologist, acknowledged the general proposition that strokes require timely diagnosis and treatment but did not provide an opinion on causation under the specific facts of this case. Another defense expert, Gregory Postal, MD, opined that Mr. Shackelford began to present symptoms of a stroke after leaving the hospital.
Notwithstanding the lower court’s ruling to summarily dismiss the case, the Court of Appeals found that, in this case, the issue of causation did not require expert medical testimony. It explained that given the ubiquity of information regarding stroke symptom identification and the necessity of prompt treatment, it had become common knowledge that “time lost is brain lost” as to timely medical intervention. In other words, a jury of laymen with this general knowledge could resolve the causation issue without the aid of expert testimony.
However, the Supreme Court of Kentucky held otherwise, writing: “We disagree with the Court of Appeals’ analysis. Although public service campaigns have increased public awareness and knowledge about stroke symptoms and timely intervention, that general information cannot provide the medical expertise necessary to evaluate this particular claim of medical malpractice. In other words, the question is not simply whether ‘time lost is brain lost.’ Rather, the specific facts and circumstances of this case play a significant role in determining whether the alleged negligent conduct was a substantial factor in Shackelford’s injuries, and to what extent. For example, as Dr. Lewis’s deposition testimony illustrates, a variety of factors influenced his diagnosis and treatment of Shackelford, including Shackelford’s medical history and history of cluster headaches; the common side effects of the angiogram procedure, including headache and scotoma; and the manner in which Shackelford’s headache and scotoma presented, as well as their timing. The complexities of these factors and how they affected Dr. Lewis’s evaluation of Shackelford may have also influenced the severity of the injury. These matters are clearly relevant to the determination of an alleged breach of the standard of care. Despite public perception about timely intervention, the average layperson cannot properly weigh such complex medical evidence without the aid of expert opinion. … To conclude otherwise is to drastically expand the res ipsa loquitor exception and to virtually eliminate the need for expert opinion evidence in similar medical malpractice actions that involve common or highly publicized conditions (e.g., stroke, heart attack, and even some cancers).”
Dr. Tan is professor emeritus of medicine and former adjunct professor of law at the University of Hawaii, Honolulu. This article is meant to be educational and does not constitute medical, ethical or legal advice. The author published an earlier version of this topic in the April 19, 2016, issue of Internal Medicine News, available at https://www.mdedge.com/internalmedicine/law-medicine. For additional information, readers may contact the author at [email protected].
References
1. James v. Wormuth, 997 N.E.2d 133 (N.Y. 2013).
2. Lewis/Ashland Hospital v. Shackelford, Supreme Court of Kentucky, Opinion of the Court by Justice Keller, rendered August 29, 2019.
Question: Which of the following statements regarding “common knowledge” is correct?
A. In any negligence action absent common knowledge, expert testimony is then required to prove requisite standard of care and causation.
B.
C. An expert is needed in the first place to establish whether something constitutes common knowledge.
D. The jury is the one who determines whether a plaintiff can invoke the common knowledge exception.
E. An example of common knowledge in malpractice law is where a delay in stroke diagnosis results in loss of brain function.
Answer: B. The judge, not the jury or anyone else, makes the decision regarding res ipsa loquitur (the thing speaks for itself) or common knowledge, which exempts a plaintiff from producing an expert witness to testify as to the standard of care and causation. However, this is only true in actions arising out of professional negligence such as medical malpractice, whereas most common negligence actions – for example, slips and falls – do not require expert testimony.
Only a professional, duly qualified by the court as an expert witness, is allowed to offer medical testimony, while the plaintiff will typically be disqualified from playing this role because of the complexity of issues involved unless there is common knowledge. In general, courts are reluctant to grant this exception, which favors the plaintiff.
The best example of res ipsa loquitur is where a surgeon inadvertently leaves behind a sponge or instrument inside a body cavity. Other successfully litigated examples include cardiac arrest in the operating room, hypoxia in the recovery room, burns to the buttock, gangrene after the accidental injection of penicillin into an artery, air trapped subcutaneously from a displaced needle, and a pierced eyeball during a procedure. The factual circumstances of each case are critical to its outcome. For example, in a 2013 New York case, the plaintiff was barred from using the res doctrine.1 The defendant doctor had left a guide wire in the plaintiff’s chest following a biopsy and retrieved it 2 months later. The plaintiff did not call any expert witness, relying instead on the “foreign object” basis for invoking the res doctrine. However, the Court of Appeals reasoned that the object was left behind deliberately, not unintentionally, and that under the circumstances of the case, an expert witness was needed to set out the applicable standard of care, without which a jury could not determine whether the doctor’s professional judgment breached the requisite standard.
The Supreme Court of Kentucky recently rejected the use of common knowledge in a stroke case.2 In 2010, David Shackelford’s rheumatologist referred him to Paul Lewis, MD, an interventional radiologist, for a four-vessel cerebral angiogram to assist with diagnosing the cause of Mr. Shackelford’s chronic headaches. The procedure itself was uneventful, but while in the recovery room, Mr. Shackelford reported a frontal headache and scotoma, which resolved on its own. The headache improved with medication, and the patient experienced no other symptoms. There were no other visual changes, weakness, slurred speech, or facial palsies. Mr. Shackelford was discharged but had to return to the hospital the next morning via ambulance after becoming disoriented at his home. An MRI indicated multiple scattered small infarcts, and he was left with residual short-term memory loss and visual problems.
There was no allegation that the stroke itself was caused by negligence; rather, Mr. Shackelford alleged that the failure to examine and diagnose the stroke after the angiogram was negligent and caused injury greater than that which the stroke would have caused with earlier intervention. To support his claims, Mr. Shackelford’s expert, Michael David Khoury, MD, a vascular surgeon, criticized Dr. Lewis’s failure to examine Mr. Shackelford when his symptoms were consistent with a stroke. However, Dr. Khoury did not opine that Dr. Lewis could have limited the effects of the stroke through earlier intervention. When asked specifically whether he could state within a reasonable degree of medical probability that Dr. Lewis’s postprocedure care was a substantial factor in causing harm to Mr. Shackelford, Dr. Khoury responded that it was “impossible to tell.”
Based largely upon Dr. Khoury’s deposition testimony, the defendants successfully moved for summary judgment on the basis that the expert had failed to opine that the alleged negligence caused any injury to Mr. Shackelford. As a result, Mr. Shackelford could not prove an essential element of his medical malpractice claim. Defense expert Peter J. Pema, MD, a neuroradiologist, acknowledged the general proposition that strokes require timely diagnosis and treatment but did not provide an opinion on causation under the specific facts of this case. Another defense expert, Gregory Postal, MD, opined that Mr. Shackelford began to present symptoms of a stroke after leaving the hospital.
Notwithstanding the lower court’s ruling to summarily dismiss the case, the Court of Appeals found that, in this case, the issue of causation did not require expert medical testimony. It explained that given the ubiquity of information regarding stroke symptom identification and the necessity of prompt treatment, it had become common knowledge that “time lost is brain lost” as to timely medical intervention. In other words, a jury of laymen with this general knowledge could resolve the causation issue without the aid of expert testimony.
However, the Supreme Court of Kentucky held otherwise, writing: “We disagree with the Court of Appeals’ analysis. Although public service campaigns have increased public awareness and knowledge about stroke symptoms and timely intervention, that general information cannot provide the medical expertise necessary to evaluate this particular claim of medical malpractice. In other words, the question is not simply whether ‘time lost is brain lost.’ Rather, the specific facts and circumstances of this case play a significant role in determining whether the alleged negligent conduct was a substantial factor in Shackelford’s injuries, and to what extent. For example, as Dr. Lewis’s deposition testimony illustrates, a variety of factors influenced his diagnosis and treatment of Shackelford, including Shackelford’s medical history and history of cluster headaches; the common side effects of the angiogram procedure, including headache and scotoma; and the manner in which Shackelford’s headache and scotoma presented, as well as their timing. The complexities of these factors and how they affected Dr. Lewis’s evaluation of Shackelford may have also influenced the severity of the injury. These matters are clearly relevant to the determination of an alleged breach of the standard of care. Despite public perception about timely intervention, the average layperson cannot properly weigh such complex medical evidence without the aid of expert opinion. … To conclude otherwise is to drastically expand the res ipsa loquitor exception and to virtually eliminate the need for expert opinion evidence in similar medical malpractice actions that involve common or highly publicized conditions (e.g., stroke, heart attack, and even some cancers).”
Dr. Tan is professor emeritus of medicine and former adjunct professor of law at the University of Hawaii, Honolulu. This article is meant to be educational and does not constitute medical, ethical or legal advice. The author published an earlier version of this topic in the April 19, 2016, issue of Internal Medicine News, available at https://www.mdedge.com/internalmedicine/law-medicine. For additional information, readers may contact the author at [email protected].
References
1. James v. Wormuth, 997 N.E.2d 133 (N.Y. 2013).
2. Lewis/Ashland Hospital v. Shackelford, Supreme Court of Kentucky, Opinion of the Court by Justice Keller, rendered August 29, 2019.
Question: Which of the following statements regarding “common knowledge” is correct?
A. In any negligence action absent common knowledge, expert testimony is then required to prove requisite standard of care and causation.
B.
C. An expert is needed in the first place to establish whether something constitutes common knowledge.
D. The jury is the one who determines whether a plaintiff can invoke the common knowledge exception.
E. An example of common knowledge in malpractice law is where a delay in stroke diagnosis results in loss of brain function.
Answer: B. The judge, not the jury or anyone else, makes the decision regarding res ipsa loquitur (the thing speaks for itself) or common knowledge, which exempts a plaintiff from producing an expert witness to testify as to the standard of care and causation. However, this is only true in actions arising out of professional negligence such as medical malpractice, whereas most common negligence actions – for example, slips and falls – do not require expert testimony.
Only a professional, duly qualified by the court as an expert witness, is allowed to offer medical testimony, while the plaintiff will typically be disqualified from playing this role because of the complexity of issues involved unless there is common knowledge. In general, courts are reluctant to grant this exception, which favors the plaintiff.
The best example of res ipsa loquitur is where a surgeon inadvertently leaves behind a sponge or instrument inside a body cavity. Other successfully litigated examples include cardiac arrest in the operating room, hypoxia in the recovery room, burns to the buttock, gangrene after the accidental injection of penicillin into an artery, air trapped subcutaneously from a displaced needle, and a pierced eyeball during a procedure. The factual circumstances of each case are critical to its outcome. For example, in a 2013 New York case, the plaintiff was barred from using the res doctrine.1 The defendant doctor had left a guide wire in the plaintiff’s chest following a biopsy and retrieved it 2 months later. The plaintiff did not call any expert witness, relying instead on the “foreign object” basis for invoking the res doctrine. However, the Court of Appeals reasoned that the object was left behind deliberately, not unintentionally, and that under the circumstances of the case, an expert witness was needed to set out the applicable standard of care, without which a jury could not determine whether the doctor’s professional judgment breached the requisite standard.
The Supreme Court of Kentucky recently rejected the use of common knowledge in a stroke case.2 In 2010, David Shackelford’s rheumatologist referred him to Paul Lewis, MD, an interventional radiologist, for a four-vessel cerebral angiogram to assist with diagnosing the cause of Mr. Shackelford’s chronic headaches. The procedure itself was uneventful, but while in the recovery room, Mr. Shackelford reported a frontal headache and scotoma, which resolved on its own. The headache improved with medication, and the patient experienced no other symptoms. There were no other visual changes, weakness, slurred speech, or facial palsies. Mr. Shackelford was discharged but had to return to the hospital the next morning via ambulance after becoming disoriented at his home. An MRI indicated multiple scattered small infarcts, and he was left with residual short-term memory loss and visual problems.
There was no allegation that the stroke itself was caused by negligence; rather, Mr. Shackelford alleged that the failure to examine and diagnose the stroke after the angiogram was negligent and caused injury greater than that which the stroke would have caused with earlier intervention. To support his claims, Mr. Shackelford’s expert, Michael David Khoury, MD, a vascular surgeon, criticized Dr. Lewis’s failure to examine Mr. Shackelford when his symptoms were consistent with a stroke. However, Dr. Khoury did not opine that Dr. Lewis could have limited the effects of the stroke through earlier intervention. When asked specifically whether he could state within a reasonable degree of medical probability that Dr. Lewis’s postprocedure care was a substantial factor in causing harm to Mr. Shackelford, Dr. Khoury responded that it was “impossible to tell.”
Based largely upon Dr. Khoury’s deposition testimony, the defendants successfully moved for summary judgment on the basis that the expert had failed to opine that the alleged negligence caused any injury to Mr. Shackelford. As a result, Mr. Shackelford could not prove an essential element of his medical malpractice claim. Defense expert Peter J. Pema, MD, a neuroradiologist, acknowledged the general proposition that strokes require timely diagnosis and treatment but did not provide an opinion on causation under the specific facts of this case. Another defense expert, Gregory Postal, MD, opined that Mr. Shackelford began to present symptoms of a stroke after leaving the hospital.
Notwithstanding the lower court’s ruling to summarily dismiss the case, the Court of Appeals found that, in this case, the issue of causation did not require expert medical testimony. It explained that given the ubiquity of information regarding stroke symptom identification and the necessity of prompt treatment, it had become common knowledge that “time lost is brain lost” as to timely medical intervention. In other words, a jury of laymen with this general knowledge could resolve the causation issue without the aid of expert testimony.
However, the Supreme Court of Kentucky held otherwise, writing: “We disagree with the Court of Appeals’ analysis. Although public service campaigns have increased public awareness and knowledge about stroke symptoms and timely intervention, that general information cannot provide the medical expertise necessary to evaluate this particular claim of medical malpractice. In other words, the question is not simply whether ‘time lost is brain lost.’ Rather, the specific facts and circumstances of this case play a significant role in determining whether the alleged negligent conduct was a substantial factor in Shackelford’s injuries, and to what extent. For example, as Dr. Lewis’s deposition testimony illustrates, a variety of factors influenced his diagnosis and treatment of Shackelford, including Shackelford’s medical history and history of cluster headaches; the common side effects of the angiogram procedure, including headache and scotoma; and the manner in which Shackelford’s headache and scotoma presented, as well as their timing. The complexities of these factors and how they affected Dr. Lewis’s evaluation of Shackelford may have also influenced the severity of the injury. These matters are clearly relevant to the determination of an alleged breach of the standard of care. Despite public perception about timely intervention, the average layperson cannot properly weigh such complex medical evidence without the aid of expert opinion. … To conclude otherwise is to drastically expand the res ipsa loquitor exception and to virtually eliminate the need for expert opinion evidence in similar medical malpractice actions that involve common or highly publicized conditions (e.g., stroke, heart attack, and even some cancers).”
Dr. Tan is professor emeritus of medicine and former adjunct professor of law at the University of Hawaii, Honolulu. This article is meant to be educational and does not constitute medical, ethical or legal advice. The author published an earlier version of this topic in the April 19, 2016, issue of Internal Medicine News, available at https://www.mdedge.com/internalmedicine/law-medicine. For additional information, readers may contact the author at [email protected].
References
1. James v. Wormuth, 997 N.E.2d 133 (N.Y. 2013).
2. Lewis/Ashland Hospital v. Shackelford, Supreme Court of Kentucky, Opinion of the Court by Justice Keller, rendered August 29, 2019.
USPSTF recommendations on risk assessment, genetic counseling, and genetic testing for BRCA-related cancer
Breast cancer screening recommendations have evolved over the past decade. BRCA1/2 genes are tumor-suppressor genes. Mutations in these genes place women at an increased risk for developing breast, ovarian, fallopian tube, and peritoneal cancer. Detection of BRCA1/2 mutations through genetic screening can provide patients with more information about their cancer risk and can lead to discussion of prophylactic therapies. This includes increased screening frequency, medical therapy, and surgical interventions.
New USPSTF recommendations address who is at an increased risk for BRCA1/2 mutations. They recommend using screening tools focusing on family history that primary care physicians can utilize to determine who should be referred for genetic counseling to discuss the risks and benefits of genetic screening. The following are the task force’s two primary recommendations:
The USPSTF recommends that primary care clinicians assess women with a personal or family history of breast, ovarian, tubal, or peritoneal cancer or who have an ancestry associated with BRCA1/2 gene mutations with an appropriate brief familial risk assessment tool. Women with a positive result on the risk assessment tool should receive genetic counseling and, if indicated after counseling, genetic testing. (B recommendation)
The USPSTF recommends against routine risk assessment, genetic counseling, or genetic testing for women whose personal or family history or ancestry is not associated with potentially harmful BRCA1/2 gene mutations. (D recommendation)
Breast cancer is the second leading cause of cancer and cancer death for women in the United States. Ovarian cancer ranks fifth in cancer deaths for women in the U.S. By age 70, women with BRCA1/2 mutations have a 45%-65% cumulative lifetime risk of developing breast cancer.
Mutations in BRCA1, specifically, are associated with a 39% lifetime risk for ovarian, fallopian tube, and peritoneal cancer. In contrast, mutations in BRCA2 are associated with a 10%-17% lifetime risk.
The USPSTF also underscores the increased prevalence of BRCA1/2 mutations in the Ashkenazi Jewish population. Three out of seven familial risk assessment tools inquire about Jewish ancestry. This is because the Ashkenazi Jewish population have a higher prevalence of three founder mutations in the BRCA1/2 gene. A member of this population has a 1 in 40 chance of carrying one of these three mutations, whereas the general population has a 1 in 300 chance.
The USPSTF recommends a multistep process of screening. The first step is taking a family history of cancer. For women who have a family history of breast, ovarian, tubal, or peritoneal cancer or a personal history of these cancers, a brief familial risk assessment tool should be used to determine the need for referral for in-depth genetic counseling to determine the need for genetic testing.
It is important to recognize that the validated tools recommended by the USPSTF are specific for genetic risk assessment. General breast cancer risk assessment tools, including the National Cancer Institute Breast Cancer Risk Assessment Tool, which is based on the Gail model, are not recommended.
The sensitivity of the tools recommended by the USPSTF range between 77% and 100%. A number of tools are given as an option with no one tool being better than the other. Perhaps the easiest to implement of the validated tools recommended is the Pedigree Assessment Tool. For this tool, points are assigned for every family member with breast or ovarian cancer as indicated in the table below.
A positive result on a screening tool will lead primary care physicians to appropriately refer patients for genetic counseling. Genetic testing for BRCA1/2 mutations should be limited to those individuals whose personal and/or family history reflects an increased risk for gene mutations after detailed genetic assessment and counseling. The results of the genetic screening should assist a patient in their decision making.
Prophylactic treatment for BRCA1/2 mutation carriers are outside the scope of this recommendation. However the guidelines briefly review risk-reducing therapies including screening, medical, and surgical options. Medical therapy for patients who have BRCA1/2 mutations include the use of tamoxifen, raloxifene, and aromatase inhibitors. Surgical interventions include bilateral mastectomy and salpingo-oopherectomy.
Screening options include earlier and more frequent mammograms and breast MRIs. Screening is largely based on family history and the USPSTF acknowledges their uncertainty when screening women with an unknown family history. Male breast cancer, pancreatic cancer, prostate cancer, and melanoma are also associated with BRCA1/2 mutations. They are not included in this recommendation.
The bottom line
USPSTF recommended that primary care physicians should use familial risk assessment tools to screen women for BRCA1/2 mutations. This includes women with a personal and/or family history of breast, ovarian, tubal, or peritoneal cancer or women with a family history of BRCA1/2 gene mutations. Patients who test positive through one of the suggested screening tools should be referred for genetic counseling. This could lead to genetic testing and subsequent prophylactic therapies and/or increased screenings if the patient so desires. It is of importance to note the USPSTF recommends against routine screening of BRCA1/2 gene mutations for women who do not meet the above requirements.
Reference
USPSTF Recommendation: Assessment, counseling, and testing for BRCA-related cancer. JAMA. 2019;322(7):652-65. doi: 10.1001/jama.2019.10987.
Dr. Style is a second-year resident in the Family Medicine Residency Program at Abington (Pa.) Jefferson Health. Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington Jefferson Health.
Breast cancer screening recommendations have evolved over the past decade. BRCA1/2 genes are tumor-suppressor genes. Mutations in these genes place women at an increased risk for developing breast, ovarian, fallopian tube, and peritoneal cancer. Detection of BRCA1/2 mutations through genetic screening can provide patients with more information about their cancer risk and can lead to discussion of prophylactic therapies. This includes increased screening frequency, medical therapy, and surgical interventions.
New USPSTF recommendations address who is at an increased risk for BRCA1/2 mutations. They recommend using screening tools focusing on family history that primary care physicians can utilize to determine who should be referred for genetic counseling to discuss the risks and benefits of genetic screening. The following are the task force’s two primary recommendations:
The USPSTF recommends that primary care clinicians assess women with a personal or family history of breast, ovarian, tubal, or peritoneal cancer or who have an ancestry associated with BRCA1/2 gene mutations with an appropriate brief familial risk assessment tool. Women with a positive result on the risk assessment tool should receive genetic counseling and, if indicated after counseling, genetic testing. (B recommendation)
The USPSTF recommends against routine risk assessment, genetic counseling, or genetic testing for women whose personal or family history or ancestry is not associated with potentially harmful BRCA1/2 gene mutations. (D recommendation)
Breast cancer is the second leading cause of cancer and cancer death for women in the United States. Ovarian cancer ranks fifth in cancer deaths for women in the U.S. By age 70, women with BRCA1/2 mutations have a 45%-65% cumulative lifetime risk of developing breast cancer.
Mutations in BRCA1, specifically, are associated with a 39% lifetime risk for ovarian, fallopian tube, and peritoneal cancer. In contrast, mutations in BRCA2 are associated with a 10%-17% lifetime risk.
The USPSTF also underscores the increased prevalence of BRCA1/2 mutations in the Ashkenazi Jewish population. Three out of seven familial risk assessment tools inquire about Jewish ancestry. This is because the Ashkenazi Jewish population have a higher prevalence of three founder mutations in the BRCA1/2 gene. A member of this population has a 1 in 40 chance of carrying one of these three mutations, whereas the general population has a 1 in 300 chance.
The USPSTF recommends a multistep process of screening. The first step is taking a family history of cancer. For women who have a family history of breast, ovarian, tubal, or peritoneal cancer or a personal history of these cancers, a brief familial risk assessment tool should be used to determine the need for referral for in-depth genetic counseling to determine the need for genetic testing.
It is important to recognize that the validated tools recommended by the USPSTF are specific for genetic risk assessment. General breast cancer risk assessment tools, including the National Cancer Institute Breast Cancer Risk Assessment Tool, which is based on the Gail model, are not recommended.
The sensitivity of the tools recommended by the USPSTF range between 77% and 100%. A number of tools are given as an option with no one tool being better than the other. Perhaps the easiest to implement of the validated tools recommended is the Pedigree Assessment Tool. For this tool, points are assigned for every family member with breast or ovarian cancer as indicated in the table below.

A positive result on a screening tool will lead primary care physicians to appropriately refer patients for genetic counseling. Genetic testing for BRCA1/2 mutations should be limited to those individuals whose personal and/or family history reflects an increased risk for gene mutations after detailed genetic assessment and counseling. The results of the genetic screening should assist a patient in their decision making.
Prophylactic treatment for BRCA1/2 mutation carriers are outside the scope of this recommendation. However the guidelines briefly review risk-reducing therapies including screening, medical, and surgical options. Medical therapy for patients who have BRCA1/2 mutations include the use of tamoxifen, raloxifene, and aromatase inhibitors. Surgical interventions include bilateral mastectomy and salpingo-oopherectomy.
Screening options include earlier and more frequent mammograms and breast MRIs. Screening is largely based on family history and the USPSTF acknowledges their uncertainty when screening women with an unknown family history. Male breast cancer, pancreatic cancer, prostate cancer, and melanoma are also associated with BRCA1/2 mutations. They are not included in this recommendation.
The bottom line
USPSTF recommended that primary care physicians should use familial risk assessment tools to screen women for BRCA1/2 mutations. This includes women with a personal and/or family history of breast, ovarian, tubal, or peritoneal cancer or women with a family history of BRCA1/2 gene mutations. Patients who test positive through one of the suggested screening tools should be referred for genetic counseling. This could lead to genetic testing and subsequent prophylactic therapies and/or increased screenings if the patient so desires. It is of importance to note the USPSTF recommends against routine screening of BRCA1/2 gene mutations for women who do not meet the above requirements.
Reference
USPSTF Recommendation: Assessment, counseling, and testing for BRCA-related cancer. JAMA. 2019;322(7):652-65. doi: 10.1001/jama.2019.10987.
Dr. Style is a second-year resident in the Family Medicine Residency Program at Abington (Pa.) Jefferson Health. Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington Jefferson Health.
Breast cancer screening recommendations have evolved over the past decade. BRCA1/2 genes are tumor-suppressor genes. Mutations in these genes place women at an increased risk for developing breast, ovarian, fallopian tube, and peritoneal cancer. Detection of BRCA1/2 mutations through genetic screening can provide patients with more information about their cancer risk and can lead to discussion of prophylactic therapies. This includes increased screening frequency, medical therapy, and surgical interventions.
New USPSTF recommendations address who is at an increased risk for BRCA1/2 mutations. They recommend using screening tools focusing on family history that primary care physicians can utilize to determine who should be referred for genetic counseling to discuss the risks and benefits of genetic screening. The following are the task force’s two primary recommendations:
The USPSTF recommends that primary care clinicians assess women with a personal or family history of breast, ovarian, tubal, or peritoneal cancer or who have an ancestry associated with BRCA1/2 gene mutations with an appropriate brief familial risk assessment tool. Women with a positive result on the risk assessment tool should receive genetic counseling and, if indicated after counseling, genetic testing. (B recommendation)
The USPSTF recommends against routine risk assessment, genetic counseling, or genetic testing for women whose personal or family history or ancestry is not associated with potentially harmful BRCA1/2 gene mutations. (D recommendation)
Breast cancer is the second leading cause of cancer and cancer death for women in the United States. Ovarian cancer ranks fifth in cancer deaths for women in the U.S. By age 70, women with BRCA1/2 mutations have a 45%-65% cumulative lifetime risk of developing breast cancer.
Mutations in BRCA1, specifically, are associated with a 39% lifetime risk for ovarian, fallopian tube, and peritoneal cancer. In contrast, mutations in BRCA2 are associated with a 10%-17% lifetime risk.
The USPSTF also underscores the increased prevalence of BRCA1/2 mutations in the Ashkenazi Jewish population. Three out of seven familial risk assessment tools inquire about Jewish ancestry. This is because the Ashkenazi Jewish population have a higher prevalence of three founder mutations in the BRCA1/2 gene. A member of this population has a 1 in 40 chance of carrying one of these three mutations, whereas the general population has a 1 in 300 chance.
The USPSTF recommends a multistep process of screening. The first step is taking a family history of cancer. For women who have a family history of breast, ovarian, tubal, or peritoneal cancer or a personal history of these cancers, a brief familial risk assessment tool should be used to determine the need for referral for in-depth genetic counseling to determine the need for genetic testing.
It is important to recognize that the validated tools recommended by the USPSTF are specific for genetic risk assessment. General breast cancer risk assessment tools, including the National Cancer Institute Breast Cancer Risk Assessment Tool, which is based on the Gail model, are not recommended.
The sensitivity of the tools recommended by the USPSTF range between 77% and 100%. A number of tools are given as an option with no one tool being better than the other. Perhaps the easiest to implement of the validated tools recommended is the Pedigree Assessment Tool. For this tool, points are assigned for every family member with breast or ovarian cancer as indicated in the table below.

A positive result on a screening tool will lead primary care physicians to appropriately refer patients for genetic counseling. Genetic testing for BRCA1/2 mutations should be limited to those individuals whose personal and/or family history reflects an increased risk for gene mutations after detailed genetic assessment and counseling. The results of the genetic screening should assist a patient in their decision making.
Prophylactic treatment for BRCA1/2 mutation carriers are outside the scope of this recommendation. However the guidelines briefly review risk-reducing therapies including screening, medical, and surgical options. Medical therapy for patients who have BRCA1/2 mutations include the use of tamoxifen, raloxifene, and aromatase inhibitors. Surgical interventions include bilateral mastectomy and salpingo-oopherectomy.
Screening options include earlier and more frequent mammograms and breast MRIs. Screening is largely based on family history and the USPSTF acknowledges their uncertainty when screening women with an unknown family history. Male breast cancer, pancreatic cancer, prostate cancer, and melanoma are also associated with BRCA1/2 mutations. They are not included in this recommendation.
The bottom line
USPSTF recommended that primary care physicians should use familial risk assessment tools to screen women for BRCA1/2 mutations. This includes women with a personal and/or family history of breast, ovarian, tubal, or peritoneal cancer or women with a family history of BRCA1/2 gene mutations. Patients who test positive through one of the suggested screening tools should be referred for genetic counseling. This could lead to genetic testing and subsequent prophylactic therapies and/or increased screenings if the patient so desires. It is of importance to note the USPSTF recommends against routine screening of BRCA1/2 gene mutations for women who do not meet the above requirements.
Reference
USPSTF Recommendation: Assessment, counseling, and testing for BRCA-related cancer. JAMA. 2019;322(7):652-65. doi: 10.1001/jama.2019.10987.
Dr. Style is a second-year resident in the Family Medicine Residency Program at Abington (Pa.) Jefferson Health. Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington Jefferson Health.
CBD: What physicians need to know about it
Cannabidiol is a derivative of marijuana that is sold everywhere from medical marijuana stores to health food markets to gas stations. While this chemical is derived from marijuana plants, it can be sold in many states as a supplement and is largely unregulated. The ubiquity of cannabidiol (CBD) and its potential benefits means that doctors need to be able to counsel patients about what we know, what we don’t, and how to use it safely. For conditions such as chronic pain and addiction, where we have few safe and effective alternatives, CBD may be reasonable to recommend.
To find out what physicians need to know about CBD, Elisabeth Poorman, MD, a general internist at a University of Washington neighborhood clinic in Kent and member of the editorial advisory board of Internal Medicine News, interviewed Peter Grinspoon, MD, who provides free consultation to primary care patients on the benefits and risks of using various forms of cannabis, including CBD. Dr. Grinspoon is an internist at Massachusetts General Hospital Chelsea Healthcare Center and is an instructor at Harvard Medical School, Boston. He has contributed to the Harvard Health Blog on the topic of medical marijuana, delivered grand rounds on cannabis at Massachusetts General Hospital, and lectured at the American College of Physicians. Dr. Grinspoon is also medical director for Galenas, a medical marijuana company.
Dr. Grinspoon is the son of Lester Grinspoon, MD, associate professor emeritus of psychiatry at Harvard Medical School, who researched the medicinal legitimacy of marijuana prohibition and has authored books on the medical benefits of marijuana.
and his knowledge of CBD’s efficacy for various medical conditions. Below are excerpts from that conversation.
Dr. Poorman: How do you explain the difference between THC and CBD to patients?
Dr. Grinspoon: Cannabis contains at least a hundred different chemicals called cannabinoids, of which tetrahydrocannabinol (THC) and CBD are the most prevalent. THC is the one that gets you high and can be used recreationally and medically. The CBD molecule is not intoxicating, and people use it for a variety of medical purposes, most commonly to treat anxiety, insomnia, and pain.
Dr. Poorman: There are a lot of gaps in what we now about CBD’s potential benefits. Why don’t we know more?
Dr. Grinspoon: CBD has no abuse liability according to the World Health Organization, but because it is a cannabinoid, it is still technically a schedule I substance under the Controlled Substances Act, and that makes it difficult to study.
Dr. Poorman: What kinds of conditions can CBD treat?
Dr. Grinspoon: In anxiety, the enthusiasm has outpaced the science; there’s no question about that. And most of the studies have done in animals. That said, some studies have shown that CBD helps treat components of anxiety, like public speaking. Unlike THC, it is nonintoxicating and non–habit forming. But we don’t have the wealth of randomized controlled trials that we have for official psychiatric medications.
CBD’s benefits have been most extensively studied in pediatric epilepsy. The one Food and Drug Administration–approved drug derived from cannabis is Epidiolex, used to treat rare forms of childhood epilepsy. There is some evidence that as an adjunct, it can be used for glioblastoma multiforme in patients receiving other appropriate therapy. There is also some preliminary evidence that it can be used for addiction, including to opioids, cannabis, tobacco, and stimulants.
Most of the evidence for using CBD in chronic pain comes from animal studies, including a study published in the European Journal of Pain in 2016. Among my patients to whom I have suggested CBD for chronic pain, a few have noticed great benefit, a few have noticed some benefit, and a lot have noticed no benefit. For those who have said they noticed benefit it is unclear whether that benefit was just the placebo effect.
In insomnia, I usually have them take CBD under the tongue half an hour time before bedtime, or if it’s an edible, an hour before bedtime. I start with a lower dose and slowly try higher doses. I also encourage them to do the other sleep hygiene things, like no screens, increasing exercise, and decreasing caffeine. It seems that CBD helps them fall asleep, though it’s hard to know if it’s the CBD or the fact that they have started taking something, and have simultaneously made various lifestyle changes.
Dr. Poorman: Can CBD interfere with your normal sleep architecture, the way benzodiazepines and Benadryl can?
Dr. Grinspoon: We know that THC affects your sleep architecture and affects what percentage of REM sleep you have. But I don’t know if the effects of CBD on sleep architecture have been studied.
Dr. Poorman: What harms do you counsel patients about when discussing CBD?
Dr. Grinspoon: There are four main harms. The first is the price. It’s overpriced, and the doses are very low. In most animal studies, the doses are about 20 milligrams per kilogram of weight. And you go to the market, and it’s like a dollar for a hundredth of that.
Number two is that it’s not regulated; it’s a supplement. A few years ago, the government tested a bunch of samples of CBD, and some didn’t actually contain CBD, some didn’t have the right amount; and worse, some contained THC that had not been disclosed in the packaging. So you can’t just go to a roadside gas station and assume that if you buy CBD, it’s actually that. You want a place that has a certificate of assurance. Make sure third-party testing was done, including testing for pesticides and other heavy metals.
The third thing is drug interactions. It affects the body like grapefruit and inhibits the cytochrome P450 system. The medications doctors should be most concerned about are blood thinners like Coumadin. And if you’re on blood thinners, you definitely want to tell your doctor that you are on CBD and he or she might want to check your blood levels more frequently than they usually do.
The fourth concern is liver inflammation. In the childhood epilepsy studies, a bump in some liver enzymes was seen, although I haven’t heard of any clinically significant cases of chemical hepatitis related to CBD. But if someone has liver disease you want to keep an eye on their liver enzymes.
Dr. Poorman: What methods of ingestion do you recommend or not recommend?
Dr. Grinspoon: It’s basically trial and error, but I usually recommend oral form. If people feel comfortable taking a gummy bear, or a pill, I’m not particular about that. If the product being taken contains less than 0.3% THC, it won’t get you high.
The topical form probably works better for treating chronic pain if it contains some THC, suggests a review article published in the Cleveland Clinic Journal of Medicine. Topical THC is nonintoxicating, unless you managed to sit in a bathtub for 8 hours after applying it.
I don’t recommend smoking CBD, and right now, I don’t recommend vaping anything.
If people have severe pain, like moderately severe arthritis, CBD may not be enough, whereas medical cannabis with THC could help, a report suggests.
Dr. Poorman: Do you ever encourage patients to stop using CBD products?
Dr. Grinspoon: I work in a low-income area, and my patients don’t have a ton of disposable income. If it’s not working, I worry about the expense.
Dr. Poorman: The CBD industry is growing quickly. What changes are you seeing in what products are out there, and what changes would you like to see?
Dr. Grinspoon: CBD is being put in everything, and it’s comical. On the one hand, you can say if people want to waste their money on a CBD emitting pillowcase, that’s fine. On the other hand, you can say that certainly seems like misleading advertising, because a CBD emitting pillowcase isn’t going to help you sleep any better.
I think the purported benefits are far beyond what we can say scientifically. We do know that CBD has anti-inflammatory characteristics. But that doesn’t mean that putting CBD in all skin products is good for your skin. It’s bad for your pocketbook, though. I would like there to be less of a gap between the claims and the science.
Dr. Elisabeth Poorman has no conflicts to disclose.
Cannabidiol is a derivative of marijuana that is sold everywhere from medical marijuana stores to health food markets to gas stations. While this chemical is derived from marijuana plants, it can be sold in many states as a supplement and is largely unregulated. The ubiquity of cannabidiol (CBD) and its potential benefits means that doctors need to be able to counsel patients about what we know, what we don’t, and how to use it safely. For conditions such as chronic pain and addiction, where we have few safe and effective alternatives, CBD may be reasonable to recommend.
To find out what physicians need to know about CBD, Elisabeth Poorman, MD, a general internist at a University of Washington neighborhood clinic in Kent and member of the editorial advisory board of Internal Medicine News, interviewed Peter Grinspoon, MD, who provides free consultation to primary care patients on the benefits and risks of using various forms of cannabis, including CBD. Dr. Grinspoon is an internist at Massachusetts General Hospital Chelsea Healthcare Center and is an instructor at Harvard Medical School, Boston. He has contributed to the Harvard Health Blog on the topic of medical marijuana, delivered grand rounds on cannabis at Massachusetts General Hospital, and lectured at the American College of Physicians. Dr. Grinspoon is also medical director for Galenas, a medical marijuana company.
Dr. Grinspoon is the son of Lester Grinspoon, MD, associate professor emeritus of psychiatry at Harvard Medical School, who researched the medicinal legitimacy of marijuana prohibition and has authored books on the medical benefits of marijuana.
and his knowledge of CBD’s efficacy for various medical conditions. Below are excerpts from that conversation.
Dr. Poorman: How do you explain the difference between THC and CBD to patients?
Dr. Grinspoon: Cannabis contains at least a hundred different chemicals called cannabinoids, of which tetrahydrocannabinol (THC) and CBD are the most prevalent. THC is the one that gets you high and can be used recreationally and medically. The CBD molecule is not intoxicating, and people use it for a variety of medical purposes, most commonly to treat anxiety, insomnia, and pain.
Dr. Poorman: There are a lot of gaps in what we now about CBD’s potential benefits. Why don’t we know more?
Dr. Grinspoon: CBD has no abuse liability according to the World Health Organization, but because it is a cannabinoid, it is still technically a schedule I substance under the Controlled Substances Act, and that makes it difficult to study.
Dr. Poorman: What kinds of conditions can CBD treat?
Dr. Grinspoon: In anxiety, the enthusiasm has outpaced the science; there’s no question about that. And most of the studies have done in animals. That said, some studies have shown that CBD helps treat components of anxiety, like public speaking. Unlike THC, it is nonintoxicating and non–habit forming. But we don’t have the wealth of randomized controlled trials that we have for official psychiatric medications.
CBD’s benefits have been most extensively studied in pediatric epilepsy. The one Food and Drug Administration–approved drug derived from cannabis is Epidiolex, used to treat rare forms of childhood epilepsy. There is some evidence that as an adjunct, it can be used for glioblastoma multiforme in patients receiving other appropriate therapy. There is also some preliminary evidence that it can be used for addiction, including to opioids, cannabis, tobacco, and stimulants.
Most of the evidence for using CBD in chronic pain comes from animal studies, including a study published in the European Journal of Pain in 2016. Among my patients to whom I have suggested CBD for chronic pain, a few have noticed great benefit, a few have noticed some benefit, and a lot have noticed no benefit. For those who have said they noticed benefit it is unclear whether that benefit was just the placebo effect.
In insomnia, I usually have them take CBD under the tongue half an hour time before bedtime, or if it’s an edible, an hour before bedtime. I start with a lower dose and slowly try higher doses. I also encourage them to do the other sleep hygiene things, like no screens, increasing exercise, and decreasing caffeine. It seems that CBD helps them fall asleep, though it’s hard to know if it’s the CBD or the fact that they have started taking something, and have simultaneously made various lifestyle changes.
Dr. Poorman: Can CBD interfere with your normal sleep architecture, the way benzodiazepines and Benadryl can?
Dr. Grinspoon: We know that THC affects your sleep architecture and affects what percentage of REM sleep you have. But I don’t know if the effects of CBD on sleep architecture have been studied.
Dr. Poorman: What harms do you counsel patients about when discussing CBD?
Dr. Grinspoon: There are four main harms. The first is the price. It’s overpriced, and the doses are very low. In most animal studies, the doses are about 20 milligrams per kilogram of weight. And you go to the market, and it’s like a dollar for a hundredth of that.
Number two is that it’s not regulated; it’s a supplement. A few years ago, the government tested a bunch of samples of CBD, and some didn’t actually contain CBD, some didn’t have the right amount; and worse, some contained THC that had not been disclosed in the packaging. So you can’t just go to a roadside gas station and assume that if you buy CBD, it’s actually that. You want a place that has a certificate of assurance. Make sure third-party testing was done, including testing for pesticides and other heavy metals.
The third thing is drug interactions. It affects the body like grapefruit and inhibits the cytochrome P450 system. The medications doctors should be most concerned about are blood thinners like Coumadin. And if you’re on blood thinners, you definitely want to tell your doctor that you are on CBD and he or she might want to check your blood levels more frequently than they usually do.
The fourth concern is liver inflammation. In the childhood epilepsy studies, a bump in some liver enzymes was seen, although I haven’t heard of any clinically significant cases of chemical hepatitis related to CBD. But if someone has liver disease you want to keep an eye on their liver enzymes.
Dr. Poorman: What methods of ingestion do you recommend or not recommend?
Dr. Grinspoon: It’s basically trial and error, but I usually recommend oral form. If people feel comfortable taking a gummy bear, or a pill, I’m not particular about that. If the product being taken contains less than 0.3% THC, it won’t get you high.
The topical form probably works better for treating chronic pain if it contains some THC, suggests a review article published in the Cleveland Clinic Journal of Medicine. Topical THC is nonintoxicating, unless you managed to sit in a bathtub for 8 hours after applying it.
I don’t recommend smoking CBD, and right now, I don’t recommend vaping anything.
If people have severe pain, like moderately severe arthritis, CBD may not be enough, whereas medical cannabis with THC could help, a report suggests.
Dr. Poorman: Do you ever encourage patients to stop using CBD products?
Dr. Grinspoon: I work in a low-income area, and my patients don’t have a ton of disposable income. If it’s not working, I worry about the expense.
Dr. Poorman: The CBD industry is growing quickly. What changes are you seeing in what products are out there, and what changes would you like to see?
Dr. Grinspoon: CBD is being put in everything, and it’s comical. On the one hand, you can say if people want to waste their money on a CBD emitting pillowcase, that’s fine. On the other hand, you can say that certainly seems like misleading advertising, because a CBD emitting pillowcase isn’t going to help you sleep any better.
I think the purported benefits are far beyond what we can say scientifically. We do know that CBD has anti-inflammatory characteristics. But that doesn’t mean that putting CBD in all skin products is good for your skin. It’s bad for your pocketbook, though. I would like there to be less of a gap between the claims and the science.
Dr. Elisabeth Poorman has no conflicts to disclose.
Cannabidiol is a derivative of marijuana that is sold everywhere from medical marijuana stores to health food markets to gas stations. While this chemical is derived from marijuana plants, it can be sold in many states as a supplement and is largely unregulated. The ubiquity of cannabidiol (CBD) and its potential benefits means that doctors need to be able to counsel patients about what we know, what we don’t, and how to use it safely. For conditions such as chronic pain and addiction, where we have few safe and effective alternatives, CBD may be reasonable to recommend.
To find out what physicians need to know about CBD, Elisabeth Poorman, MD, a general internist at a University of Washington neighborhood clinic in Kent and member of the editorial advisory board of Internal Medicine News, interviewed Peter Grinspoon, MD, who provides free consultation to primary care patients on the benefits and risks of using various forms of cannabis, including CBD. Dr. Grinspoon is an internist at Massachusetts General Hospital Chelsea Healthcare Center and is an instructor at Harvard Medical School, Boston. He has contributed to the Harvard Health Blog on the topic of medical marijuana, delivered grand rounds on cannabis at Massachusetts General Hospital, and lectured at the American College of Physicians. Dr. Grinspoon is also medical director for Galenas, a medical marijuana company.
Dr. Grinspoon is the son of Lester Grinspoon, MD, associate professor emeritus of psychiatry at Harvard Medical School, who researched the medicinal legitimacy of marijuana prohibition and has authored books on the medical benefits of marijuana.
and his knowledge of CBD’s efficacy for various medical conditions. Below are excerpts from that conversation.
Dr. Poorman: How do you explain the difference between THC and CBD to patients?
Dr. Grinspoon: Cannabis contains at least a hundred different chemicals called cannabinoids, of which tetrahydrocannabinol (THC) and CBD are the most prevalent. THC is the one that gets you high and can be used recreationally and medically. The CBD molecule is not intoxicating, and people use it for a variety of medical purposes, most commonly to treat anxiety, insomnia, and pain.
Dr. Poorman: There are a lot of gaps in what we now about CBD’s potential benefits. Why don’t we know more?
Dr. Grinspoon: CBD has no abuse liability according to the World Health Organization, but because it is a cannabinoid, it is still technically a schedule I substance under the Controlled Substances Act, and that makes it difficult to study.
Dr. Poorman: What kinds of conditions can CBD treat?
Dr. Grinspoon: In anxiety, the enthusiasm has outpaced the science; there’s no question about that. And most of the studies have done in animals. That said, some studies have shown that CBD helps treat components of anxiety, like public speaking. Unlike THC, it is nonintoxicating and non–habit forming. But we don’t have the wealth of randomized controlled trials that we have for official psychiatric medications.
CBD’s benefits have been most extensively studied in pediatric epilepsy. The one Food and Drug Administration–approved drug derived from cannabis is Epidiolex, used to treat rare forms of childhood epilepsy. There is some evidence that as an adjunct, it can be used for glioblastoma multiforme in patients receiving other appropriate therapy. There is also some preliminary evidence that it can be used for addiction, including to opioids, cannabis, tobacco, and stimulants.
Most of the evidence for using CBD in chronic pain comes from animal studies, including a study published in the European Journal of Pain in 2016. Among my patients to whom I have suggested CBD for chronic pain, a few have noticed great benefit, a few have noticed some benefit, and a lot have noticed no benefit. For those who have said they noticed benefit it is unclear whether that benefit was just the placebo effect.
In insomnia, I usually have them take CBD under the tongue half an hour time before bedtime, or if it’s an edible, an hour before bedtime. I start with a lower dose and slowly try higher doses. I also encourage them to do the other sleep hygiene things, like no screens, increasing exercise, and decreasing caffeine. It seems that CBD helps them fall asleep, though it’s hard to know if it’s the CBD or the fact that they have started taking something, and have simultaneously made various lifestyle changes.
Dr. Poorman: Can CBD interfere with your normal sleep architecture, the way benzodiazepines and Benadryl can?
Dr. Grinspoon: We know that THC affects your sleep architecture and affects what percentage of REM sleep you have. But I don’t know if the effects of CBD on sleep architecture have been studied.
Dr. Poorman: What harms do you counsel patients about when discussing CBD?
Dr. Grinspoon: There are four main harms. The first is the price. It’s overpriced, and the doses are very low. In most animal studies, the doses are about 20 milligrams per kilogram of weight. And you go to the market, and it’s like a dollar for a hundredth of that.
Number two is that it’s not regulated; it’s a supplement. A few years ago, the government tested a bunch of samples of CBD, and some didn’t actually contain CBD, some didn’t have the right amount; and worse, some contained THC that had not been disclosed in the packaging. So you can’t just go to a roadside gas station and assume that if you buy CBD, it’s actually that. You want a place that has a certificate of assurance. Make sure third-party testing was done, including testing for pesticides and other heavy metals.
The third thing is drug interactions. It affects the body like grapefruit and inhibits the cytochrome P450 system. The medications doctors should be most concerned about are blood thinners like Coumadin. And if you’re on blood thinners, you definitely want to tell your doctor that you are on CBD and he or she might want to check your blood levels more frequently than they usually do.
The fourth concern is liver inflammation. In the childhood epilepsy studies, a bump in some liver enzymes was seen, although I haven’t heard of any clinically significant cases of chemical hepatitis related to CBD. But if someone has liver disease you want to keep an eye on their liver enzymes.
Dr. Poorman: What methods of ingestion do you recommend or not recommend?
Dr. Grinspoon: It’s basically trial and error, but I usually recommend oral form. If people feel comfortable taking a gummy bear, or a pill, I’m not particular about that. If the product being taken contains less than 0.3% THC, it won’t get you high.
The topical form probably works better for treating chronic pain if it contains some THC, suggests a review article published in the Cleveland Clinic Journal of Medicine. Topical THC is nonintoxicating, unless you managed to sit in a bathtub for 8 hours after applying it.
I don’t recommend smoking CBD, and right now, I don’t recommend vaping anything.
If people have severe pain, like moderately severe arthritis, CBD may not be enough, whereas medical cannabis with THC could help, a report suggests.
Dr. Poorman: Do you ever encourage patients to stop using CBD products?
Dr. Grinspoon: I work in a low-income area, and my patients don’t have a ton of disposable income. If it’s not working, I worry about the expense.
Dr. Poorman: The CBD industry is growing quickly. What changes are you seeing in what products are out there, and what changes would you like to see?
Dr. Grinspoon: CBD is being put in everything, and it’s comical. On the one hand, you can say if people want to waste their money on a CBD emitting pillowcase, that’s fine. On the other hand, you can say that certainly seems like misleading advertising, because a CBD emitting pillowcase isn’t going to help you sleep any better.
I think the purported benefits are far beyond what we can say scientifically. We do know that CBD has anti-inflammatory characteristics. But that doesn’t mean that putting CBD in all skin products is good for your skin. It’s bad for your pocketbook, though. I would like there to be less of a gap between the claims and the science.
Dr. Elisabeth Poorman has no conflicts to disclose.
Supporting elimination of nonmedical vaccine exemptions
Let’s suppose your first patient of the morning is a 2-month-old you have never seen before. The family arrives 10 minutes late because they are still getting the dressing-undressing-diaper change-car seat–adjusting thing worked out. Father is a computer programmer. Mother lists her occupation as nutrition counselor. The child is gaining. Breastfeeding seems to come naturally to the dyad.
As the visit draws to a close, you take the matter-of-fact approach and say, “The nurse will be in shortly with the vaccines do you have any questions.” Well ... it turns out the parents don’t feel comfortable with vaccines. They claim to understand the science and feel that vaccines make sense for some families. But they feel that for themselves, with a healthy lifestyle and God’s benevolence their son will be protected without having to introduce a host of foreign substances into his body.
What word best describes your reaction? Anger? Frustration? Disappointment (in our education system)? Maybe you’re angry at yourself for failing to make it clear in your office pamphlet and social media feeds that to protect your other patients, you no longer accept families who refuse immunizations for the common childhood diseases.
The American Academy of Pediatrics says it feels your pain, and its Annual Leadership Forum made eliminating nonmedical vaccine exemption laws its top priority in 2019. As part of its effort to help, the AAP Board of Directors was asked to advocate for the creation of a toolkit of strategies for Academy chapters facing the challenge of nonmedical exemptions. As an initial step to this process, three physicians in the department of pediatrics at the Denver Health Medical Center have begun interviewing religious leaders in hopes of developing “clergy-specific vaccine educational materials and deriv[ing] best practices for engaging them as vaccination advocates.” The investigators describe their plan and initial findings in Pediatrics (2019 Oct. doi: 10.1542/peds.2019-0933). Although they acknowledged that their efforts may not provide a quick solution to the nonmedical exemption problem, they hope that including more stakeholders and engendering trust will help future discussions.
Fourteen pages deeper into that issue of Pediatrics is the runner-up submission of this year’s Section on Pediatric Trainees essay competition titled “What I Learned From the Antivaccine Movement” (2019 Oct. doi: 10.1542/peds.2019-2384). Alana C. Ju, MD, describes the 2-hour ordeal she endured to testify at the California State Capitol in support of a state Senate bill aimed at tightening the regulations for vaccine medical exemptions. Totally unprepared for the “level of vitriol” aimed at her and other supporters of the bill, she was “accused of violating her duty as” a pediatrician because she was failing to protect children. The supporters were called “greedy, ignorant, and negligent.”
To her credit, Dr. Ju was able to step back from this assault and began looking at the faces of her accusers and learned that, “they too, felt strongly about children’s health.” She realized that “focusing on perceived ignorance is counterproductive.” She now hopes that by focusing on the shared goal of what is best for children, “we can all be better advocates.”
Both of these articles have a warm sort of kumbaya feel about them. It never is a bad idea to learn more about those with whom we disagree. But before huddling up too close to the campfire, we must realize that there is good evidence that sharing the scientific data with vaccine-hesitant parents doesn’t convert them into vaccine acceptors. In fact, it may strengthen their resolve to resist (Nyhan et al. “Effective Messages in Vaccine Promotion: A Randomized Trial,” Pediatrics. 2014 Apr;133[4] e835-42).
We are unlikely to convert many anti-vaxxers by sitting down together. Our target audience needs to be legislators and the majority of people who do vaccinate their children. These are the voters who will support legislation to eliminate nonmedical vaccine exemptions. To characterize anti-vaxxers as despicable ignorants is untrue and serves no purpose. We all do care about the health of children. However,
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
*This article has been updated 1/22/2020.
Let’s suppose your first patient of the morning is a 2-month-old you have never seen before. The family arrives 10 minutes late because they are still getting the dressing-undressing-diaper change-car seat–adjusting thing worked out. Father is a computer programmer. Mother lists her occupation as nutrition counselor. The child is gaining. Breastfeeding seems to come naturally to the dyad.
As the visit draws to a close, you take the matter-of-fact approach and say, “The nurse will be in shortly with the vaccines do you have any questions.” Well ... it turns out the parents don’t feel comfortable with vaccines. They claim to understand the science and feel that vaccines make sense for some families. But they feel that for themselves, with a healthy lifestyle and God’s benevolence their son will be protected without having to introduce a host of foreign substances into his body.
What word best describes your reaction? Anger? Frustration? Disappointment (in our education system)? Maybe you’re angry at yourself for failing to make it clear in your office pamphlet and social media feeds that to protect your other patients, you no longer accept families who refuse immunizations for the common childhood diseases.
The American Academy of Pediatrics says it feels your pain, and its Annual Leadership Forum made eliminating nonmedical vaccine exemption laws its top priority in 2019. As part of its effort to help, the AAP Board of Directors was asked to advocate for the creation of a toolkit of strategies for Academy chapters facing the challenge of nonmedical exemptions. As an initial step to this process, three physicians in the department of pediatrics at the Denver Health Medical Center have begun interviewing religious leaders in hopes of developing “clergy-specific vaccine educational materials and deriv[ing] best practices for engaging them as vaccination advocates.” The investigators describe their plan and initial findings in Pediatrics (2019 Oct. doi: 10.1542/peds.2019-0933). Although they acknowledged that their efforts may not provide a quick solution to the nonmedical exemption problem, they hope that including more stakeholders and engendering trust will help future discussions.
Fourteen pages deeper into that issue of Pediatrics is the runner-up submission of this year’s Section on Pediatric Trainees essay competition titled “What I Learned From the Antivaccine Movement” (2019 Oct. doi: 10.1542/peds.2019-2384). Alana C. Ju, MD, describes the 2-hour ordeal she endured to testify at the California State Capitol in support of a state Senate bill aimed at tightening the regulations for vaccine medical exemptions. Totally unprepared for the “level of vitriol” aimed at her and other supporters of the bill, she was “accused of violating her duty as” a pediatrician because she was failing to protect children. The supporters were called “greedy, ignorant, and negligent.”
To her credit, Dr. Ju was able to step back from this assault and began looking at the faces of her accusers and learned that, “they too, felt strongly about children’s health.” She realized that “focusing on perceived ignorance is counterproductive.” She now hopes that by focusing on the shared goal of what is best for children, “we can all be better advocates.”
Both of these articles have a warm sort of kumbaya feel about them. It never is a bad idea to learn more about those with whom we disagree. But before huddling up too close to the campfire, we must realize that there is good evidence that sharing the scientific data with vaccine-hesitant parents doesn’t convert them into vaccine acceptors. In fact, it may strengthen their resolve to resist (Nyhan et al. “Effective Messages in Vaccine Promotion: A Randomized Trial,” Pediatrics. 2014 Apr;133[4] e835-42).
We are unlikely to convert many anti-vaxxers by sitting down together. Our target audience needs to be legislators and the majority of people who do vaccinate their children. These are the voters who will support legislation to eliminate nonmedical vaccine exemptions. To characterize anti-vaxxers as despicable ignorants is untrue and serves no purpose. We all do care about the health of children. However,
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
*This article has been updated 1/22/2020.
Let’s suppose your first patient of the morning is a 2-month-old you have never seen before. The family arrives 10 minutes late because they are still getting the dressing-undressing-diaper change-car seat–adjusting thing worked out. Father is a computer programmer. Mother lists her occupation as nutrition counselor. The child is gaining. Breastfeeding seems to come naturally to the dyad.
As the visit draws to a close, you take the matter-of-fact approach and say, “The nurse will be in shortly with the vaccines do you have any questions.” Well ... it turns out the parents don’t feel comfortable with vaccines. They claim to understand the science and feel that vaccines make sense for some families. But they feel that for themselves, with a healthy lifestyle and God’s benevolence their son will be protected without having to introduce a host of foreign substances into his body.
What word best describes your reaction? Anger? Frustration? Disappointment (in our education system)? Maybe you’re angry at yourself for failing to make it clear in your office pamphlet and social media feeds that to protect your other patients, you no longer accept families who refuse immunizations for the common childhood diseases.
The American Academy of Pediatrics says it feels your pain, and its Annual Leadership Forum made eliminating nonmedical vaccine exemption laws its top priority in 2019. As part of its effort to help, the AAP Board of Directors was asked to advocate for the creation of a toolkit of strategies for Academy chapters facing the challenge of nonmedical exemptions. As an initial step to this process, three physicians in the department of pediatrics at the Denver Health Medical Center have begun interviewing religious leaders in hopes of developing “clergy-specific vaccine educational materials and deriv[ing] best practices for engaging them as vaccination advocates.” The investigators describe their plan and initial findings in Pediatrics (2019 Oct. doi: 10.1542/peds.2019-0933). Although they acknowledged that their efforts may not provide a quick solution to the nonmedical exemption problem, they hope that including more stakeholders and engendering trust will help future discussions.
Fourteen pages deeper into that issue of Pediatrics is the runner-up submission of this year’s Section on Pediatric Trainees essay competition titled “What I Learned From the Antivaccine Movement” (2019 Oct. doi: 10.1542/peds.2019-2384). Alana C. Ju, MD, describes the 2-hour ordeal she endured to testify at the California State Capitol in support of a state Senate bill aimed at tightening the regulations for vaccine medical exemptions. Totally unprepared for the “level of vitriol” aimed at her and other supporters of the bill, she was “accused of violating her duty as” a pediatrician because she was failing to protect children. The supporters were called “greedy, ignorant, and negligent.”
To her credit, Dr. Ju was able to step back from this assault and began looking at the faces of her accusers and learned that, “they too, felt strongly about children’s health.” She realized that “focusing on perceived ignorance is counterproductive.” She now hopes that by focusing on the shared goal of what is best for children, “we can all be better advocates.”
Both of these articles have a warm sort of kumbaya feel about them. It never is a bad idea to learn more about those with whom we disagree. But before huddling up too close to the campfire, we must realize that there is good evidence that sharing the scientific data with vaccine-hesitant parents doesn’t convert them into vaccine acceptors. In fact, it may strengthen their resolve to resist (Nyhan et al. “Effective Messages in Vaccine Promotion: A Randomized Trial,” Pediatrics. 2014 Apr;133[4] e835-42).
We are unlikely to convert many anti-vaxxers by sitting down together. Our target audience needs to be legislators and the majority of people who do vaccinate their children. These are the voters who will support legislation to eliminate nonmedical vaccine exemptions. To characterize anti-vaxxers as despicable ignorants is untrue and serves no purpose. We all do care about the health of children. However,
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
*This article has been updated 1/22/2020.
Antituberculosis drugs in pregnancy and lactation
Tuberculosis is one of the top ten causes of death worldwide and the leading cause from a single infectious agent. In the 2012-2017 period, there were more than 9,000 cases of TB each year in the United States. The Centers for Disease Control and Prevention states that untreated TB is a greater hazard to a pregnant woman and her fetus than its treatment.
In the material below, the molecular weights, rounded to the nearest whole number, are shown in parentheses after the drug name. Those less than 1,000 or so suggest that the drug will cross the placenta throughout pregnancy. In the second half of pregnancy, especially in the third trimester, nearly all drugs will cross regardless of their molecular weight.
Para-aminosalicylic acid (Paser) (153) is most frequently used in combination with other agents for the treatment of multidrug-resistant tuberculosis; multidrug-resistant TB (MDR TB) is defined as being caused by TB bacteria that is resistant to at least isoniazid and rifampin, the two most potent TB drugs. The drug has been associated with a marked increased risk of birth defects in some, but not all, studies. Because of this potential risk, the drug is best avoided in the first trimester. The drug is excreted into breast milk, but there are no reports of its use during breastfeeding.
Bedaquiline (Sirturo) (556) is used in combination therapy for patents with multidrug-resistant tuberculosis. One report describing the use of this drug during human pregnancy has been located. Treatment was started in the last 3 weeks of pregnancy and no abnormalities were noted in the child at birth and for 2 years after birth (Emerg Infect Dis. 2017. doi: 10.3201/eid2310.161398). The CDC states that the drug should be used only in a minimum four-drug treatment regimen and administered by direct observation (MMWR Recomm Rep. 2013 Oct 25;62[RR-09]:1-12). The drug probably is excreted into breast milk, but there are no reports of its use during breastfeeding.
Capreomycin (Capastat) (653-669) is a polypeptide antibiotic isolated from Streptomyces capreolus that is given intramuscularly. The human pregnancy data are limited to three reports. The toxicity of capreomycin is similar to aminoglycosides (e.g., cranial nerve VIII and renal) and it should not be used with these agents. The CDC has classified the drug as contraindicated in pregnancy. The drug probably is excreted into breast milk, but there are no reports of its use during breastfeeding.
Cycloserine (Seromycin) (102) is a broad spectrum antibiotic. The human pregnancy data are limited but have not shown embryo-fetal harm. Although the best course is to avoid the drug during gestation, it should not be withheld because of pregnancy if the maternal condition requires the antibiotic. The American Academy of Pediatrics classified cycloserine as compatible with breastfeeding.
Ethambutol (Myambutol) (205) should be used in conjunction with other antituberculosis drugs. The human pregnancy data do not suggest an embryo-fetal risk. A frequently used regimen is ethambutol + isoniazid + rifampin. The American Academy of Pediatrics classified ethambutol as compatible with breastfeeding.
Ethionamide (Trecator) (166) is indicated when Mycobacterium tuberculosis is resistant to isoniazid or rifampin, or when the patient is intolerant to other drugs. Although the animal reproductive data suggest risk, the limited human data suggest that the risk is probably low. If indicated, the drug should not be withheld because of pregnancy. Although the molecular weight suggests that the drug will be excreted into breast milk, no reports describing the amount in milk have been located.
Isoniazid (137) is compatible in pregnancy, even though the molecular weight suggests that it will cross the placenta, because the maternal benefit is much greater than the potential embryo-fetal risk. Although the human data are limited, the molecular weight also suggests that the drug will be excreted into breast milk, but it can be considered probably compatible during breastfeeding. No reports of isoniazid-induced effects in the nursing infant have been located, but the potential for interference with nucleic acid function and for hepatotoxicity may exist.
Pyrazinamide (123) is metabolized to an active metabolite. The molecular weight, low plasma protein binding (10%), and prolonged elimination half-life (9-10 hours) suggest that the drug will cross the placenta throughout pregnancy. The drug has been used in human pregnancy without causing embryo-fetal harm. Similar results, although limited, were reported when the drug was used during breastfeeding.
Rifabutin (Mycobutin) (847) has no reported human pregnancy data, but the animal data suggest low risk. The drug probably crosses the placenta throughout pregnancy. The maternal benefit appears to outweigh the unknown risk to the embryo-fetus, so therapy should not be withheld because of pregnancy. The drug probably is excreted into breast milk.
Rifampin (Rifadin) (823) appears to be compatible in pregnancy. Several reviews and reports have concluded that the drug was not a teratogen and recommended use of the drug with isoniazid and ethambutol. However, prophylactic vitamin K1 has been recommended to prevent drug-induced hemorrhagic disease of the newborn. There are no data regarding its use during breastfeeding, but it is probably compatible.
Rifapentine (Priftin) (877) was toxic and teratogenic in two animal species at doses close to those used in humans. In a 2018 study, however, the rates of fetal loss in pregnancies of less than 20 weeks (8/54, 15%) and congenital anomalies in live births (1/37, 3%) were within the expected background rates (Ann Am Thorac Soc. 2018 May;15[4]:570-80). There are no data regarding its use during breastfeeding, but it is probably compatible.
The CDC classifies four antituberculosis and one class of drugs as contraindicated in pregnancy. In addition to capreomycin mentioned above, they are amikacin, fluoroquinolones (ciprofloxacin, gemifloxacin, levofloxacin, lomefloxacin, moxifloxacin, ofloxacin), kanamycin, and streptomycin. These ten agents are discussed in the 11th edition of my book “Drugs in Pregnancy and Lactation,” (Wolters Kluwer Health: Riverwood, Il., 2017). If they have to be used, checking this source will provide information that has to be discussed with the patient.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs had no disclosures, except for his book. Email him at [email protected].
Tuberculosis is one of the top ten causes of death worldwide and the leading cause from a single infectious agent. In the 2012-2017 period, there were more than 9,000 cases of TB each year in the United States. The Centers for Disease Control and Prevention states that untreated TB is a greater hazard to a pregnant woman and her fetus than its treatment.
In the material below, the molecular weights, rounded to the nearest whole number, are shown in parentheses after the drug name. Those less than 1,000 or so suggest that the drug will cross the placenta throughout pregnancy. In the second half of pregnancy, especially in the third trimester, nearly all drugs will cross regardless of their molecular weight.
Para-aminosalicylic acid (Paser) (153) is most frequently used in combination with other agents for the treatment of multidrug-resistant tuberculosis; multidrug-resistant TB (MDR TB) is defined as being caused by TB bacteria that is resistant to at least isoniazid and rifampin, the two most potent TB drugs. The drug has been associated with a marked increased risk of birth defects in some, but not all, studies. Because of this potential risk, the drug is best avoided in the first trimester. The drug is excreted into breast milk, but there are no reports of its use during breastfeeding.
Bedaquiline (Sirturo) (556) is used in combination therapy for patents with multidrug-resistant tuberculosis. One report describing the use of this drug during human pregnancy has been located. Treatment was started in the last 3 weeks of pregnancy and no abnormalities were noted in the child at birth and for 2 years after birth (Emerg Infect Dis. 2017. doi: 10.3201/eid2310.161398). The CDC states that the drug should be used only in a minimum four-drug treatment regimen and administered by direct observation (MMWR Recomm Rep. 2013 Oct 25;62[RR-09]:1-12). The drug probably is excreted into breast milk, but there are no reports of its use during breastfeeding.
Capreomycin (Capastat) (653-669) is a polypeptide antibiotic isolated from Streptomyces capreolus that is given intramuscularly. The human pregnancy data are limited to three reports. The toxicity of capreomycin is similar to aminoglycosides (e.g., cranial nerve VIII and renal) and it should not be used with these agents. The CDC has classified the drug as contraindicated in pregnancy. The drug probably is excreted into breast milk, but there are no reports of its use during breastfeeding.
Cycloserine (Seromycin) (102) is a broad spectrum antibiotic. The human pregnancy data are limited but have not shown embryo-fetal harm. Although the best course is to avoid the drug during gestation, it should not be withheld because of pregnancy if the maternal condition requires the antibiotic. The American Academy of Pediatrics classified cycloserine as compatible with breastfeeding.
Ethambutol (Myambutol) (205) should be used in conjunction with other antituberculosis drugs. The human pregnancy data do not suggest an embryo-fetal risk. A frequently used regimen is ethambutol + isoniazid + rifampin. The American Academy of Pediatrics classified ethambutol as compatible with breastfeeding.
Ethionamide (Trecator) (166) is indicated when Mycobacterium tuberculosis is resistant to isoniazid or rifampin, or when the patient is intolerant to other drugs. Although the animal reproductive data suggest risk, the limited human data suggest that the risk is probably low. If indicated, the drug should not be withheld because of pregnancy. Although the molecular weight suggests that the drug will be excreted into breast milk, no reports describing the amount in milk have been located.
Isoniazid (137) is compatible in pregnancy, even though the molecular weight suggests that it will cross the placenta, because the maternal benefit is much greater than the potential embryo-fetal risk. Although the human data are limited, the molecular weight also suggests that the drug will be excreted into breast milk, but it can be considered probably compatible during breastfeeding. No reports of isoniazid-induced effects in the nursing infant have been located, but the potential for interference with nucleic acid function and for hepatotoxicity may exist.
Pyrazinamide (123) is metabolized to an active metabolite. The molecular weight, low plasma protein binding (10%), and prolonged elimination half-life (9-10 hours) suggest that the drug will cross the placenta throughout pregnancy. The drug has been used in human pregnancy without causing embryo-fetal harm. Similar results, although limited, were reported when the drug was used during breastfeeding.
Rifabutin (Mycobutin) (847) has no reported human pregnancy data, but the animal data suggest low risk. The drug probably crosses the placenta throughout pregnancy. The maternal benefit appears to outweigh the unknown risk to the embryo-fetus, so therapy should not be withheld because of pregnancy. The drug probably is excreted into breast milk.
Rifampin (Rifadin) (823) appears to be compatible in pregnancy. Several reviews and reports have concluded that the drug was not a teratogen and recommended use of the drug with isoniazid and ethambutol. However, prophylactic vitamin K1 has been recommended to prevent drug-induced hemorrhagic disease of the newborn. There are no data regarding its use during breastfeeding, but it is probably compatible.
Rifapentine (Priftin) (877) was toxic and teratogenic in two animal species at doses close to those used in humans. In a 2018 study, however, the rates of fetal loss in pregnancies of less than 20 weeks (8/54, 15%) and congenital anomalies in live births (1/37, 3%) were within the expected background rates (Ann Am Thorac Soc. 2018 May;15[4]:570-80). There are no data regarding its use during breastfeeding, but it is probably compatible.
The CDC classifies four antituberculosis and one class of drugs as contraindicated in pregnancy. In addition to capreomycin mentioned above, they are amikacin, fluoroquinolones (ciprofloxacin, gemifloxacin, levofloxacin, lomefloxacin, moxifloxacin, ofloxacin), kanamycin, and streptomycin. These ten agents are discussed in the 11th edition of my book “Drugs in Pregnancy and Lactation,” (Wolters Kluwer Health: Riverwood, Il., 2017). If they have to be used, checking this source will provide information that has to be discussed with the patient.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs had no disclosures, except for his book. Email him at [email protected].
Tuberculosis is one of the top ten causes of death worldwide and the leading cause from a single infectious agent. In the 2012-2017 period, there were more than 9,000 cases of TB each year in the United States. The Centers for Disease Control and Prevention states that untreated TB is a greater hazard to a pregnant woman and her fetus than its treatment.
In the material below, the molecular weights, rounded to the nearest whole number, are shown in parentheses after the drug name. Those less than 1,000 or so suggest that the drug will cross the placenta throughout pregnancy. In the second half of pregnancy, especially in the third trimester, nearly all drugs will cross regardless of their molecular weight.
Para-aminosalicylic acid (Paser) (153) is most frequently used in combination with other agents for the treatment of multidrug-resistant tuberculosis; multidrug-resistant TB (MDR TB) is defined as being caused by TB bacteria that is resistant to at least isoniazid and rifampin, the two most potent TB drugs. The drug has been associated with a marked increased risk of birth defects in some, but not all, studies. Because of this potential risk, the drug is best avoided in the first trimester. The drug is excreted into breast milk, but there are no reports of its use during breastfeeding.
Bedaquiline (Sirturo) (556) is used in combination therapy for patents with multidrug-resistant tuberculosis. One report describing the use of this drug during human pregnancy has been located. Treatment was started in the last 3 weeks of pregnancy and no abnormalities were noted in the child at birth and for 2 years after birth (Emerg Infect Dis. 2017. doi: 10.3201/eid2310.161398). The CDC states that the drug should be used only in a minimum four-drug treatment regimen and administered by direct observation (MMWR Recomm Rep. 2013 Oct 25;62[RR-09]:1-12). The drug probably is excreted into breast milk, but there are no reports of its use during breastfeeding.
Capreomycin (Capastat) (653-669) is a polypeptide antibiotic isolated from Streptomyces capreolus that is given intramuscularly. The human pregnancy data are limited to three reports. The toxicity of capreomycin is similar to aminoglycosides (e.g., cranial nerve VIII and renal) and it should not be used with these agents. The CDC has classified the drug as contraindicated in pregnancy. The drug probably is excreted into breast milk, but there are no reports of its use during breastfeeding.
Cycloserine (Seromycin) (102) is a broad spectrum antibiotic. The human pregnancy data are limited but have not shown embryo-fetal harm. Although the best course is to avoid the drug during gestation, it should not be withheld because of pregnancy if the maternal condition requires the antibiotic. The American Academy of Pediatrics classified cycloserine as compatible with breastfeeding.
Ethambutol (Myambutol) (205) should be used in conjunction with other antituberculosis drugs. The human pregnancy data do not suggest an embryo-fetal risk. A frequently used regimen is ethambutol + isoniazid + rifampin. The American Academy of Pediatrics classified ethambutol as compatible with breastfeeding.
Ethionamide (Trecator) (166) is indicated when Mycobacterium tuberculosis is resistant to isoniazid or rifampin, or when the patient is intolerant to other drugs. Although the animal reproductive data suggest risk, the limited human data suggest that the risk is probably low. If indicated, the drug should not be withheld because of pregnancy. Although the molecular weight suggests that the drug will be excreted into breast milk, no reports describing the amount in milk have been located.
Isoniazid (137) is compatible in pregnancy, even though the molecular weight suggests that it will cross the placenta, because the maternal benefit is much greater than the potential embryo-fetal risk. Although the human data are limited, the molecular weight also suggests that the drug will be excreted into breast milk, but it can be considered probably compatible during breastfeeding. No reports of isoniazid-induced effects in the nursing infant have been located, but the potential for interference with nucleic acid function and for hepatotoxicity may exist.
Pyrazinamide (123) is metabolized to an active metabolite. The molecular weight, low plasma protein binding (10%), and prolonged elimination half-life (9-10 hours) suggest that the drug will cross the placenta throughout pregnancy. The drug has been used in human pregnancy without causing embryo-fetal harm. Similar results, although limited, were reported when the drug was used during breastfeeding.
Rifabutin (Mycobutin) (847) has no reported human pregnancy data, but the animal data suggest low risk. The drug probably crosses the placenta throughout pregnancy. The maternal benefit appears to outweigh the unknown risk to the embryo-fetus, so therapy should not be withheld because of pregnancy. The drug probably is excreted into breast milk.
Rifampin (Rifadin) (823) appears to be compatible in pregnancy. Several reviews and reports have concluded that the drug was not a teratogen and recommended use of the drug with isoniazid and ethambutol. However, prophylactic vitamin K1 has been recommended to prevent drug-induced hemorrhagic disease of the newborn. There are no data regarding its use during breastfeeding, but it is probably compatible.
Rifapentine (Priftin) (877) was toxic and teratogenic in two animal species at doses close to those used in humans. In a 2018 study, however, the rates of fetal loss in pregnancies of less than 20 weeks (8/54, 15%) and congenital anomalies in live births (1/37, 3%) were within the expected background rates (Ann Am Thorac Soc. 2018 May;15[4]:570-80). There are no data regarding its use during breastfeeding, but it is probably compatible.
The CDC classifies four antituberculosis and one class of drugs as contraindicated in pregnancy. In addition to capreomycin mentioned above, they are amikacin, fluoroquinolones (ciprofloxacin, gemifloxacin, levofloxacin, lomefloxacin, moxifloxacin, ofloxacin), kanamycin, and streptomycin. These ten agents are discussed in the 11th edition of my book “Drugs in Pregnancy and Lactation,” (Wolters Kluwer Health: Riverwood, Il., 2017). If they have to be used, checking this source will provide information that has to be discussed with the patient.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs had no disclosures, except for his book. Email him at [email protected].