User login
Is Hemolysis a Clinical Marker of Propionibacterium acnes Orthopedic Infection or a Phylogenetic Marker?
Letter to the Editor
Is Hemolysis a Clinical Marker of Propionibacterium acnes Orthopedic Infection or a Phylogenetic Marker?
We read with great interest the study by Nodzo and colleagues in the May 2014 issue of The American Journal of Orthopedics on hemolysis as a clinical marker for Propionibacterium acnes orthopedic infection.1 We agree with the authors that determining if a P acnes culture is a true infection or a contaminant remains a challenge. Although P acnes is described as a commensal bacterium with a low pathogenicity, its involvement has been reported in many clinical entities, especially device-related infections.2P acnes is usually the cause of delayed infections occurring 3 to 24 months or more after prosthesis placement. The rate of P acnes involvement, probably underestimated, is about 10%.3 Although this bacterium was considered to be a contaminant, several virulence factors have been recently identified: putative hemolysins or cytotoxins (CAMP factors, hemolysin III) and enzymes putatively involved in degrading host tissue or molecules (GehA lipase, lysophospholipase, hyaluronate lyase, endoglycoceramidase, etc).4
Interestingly, Nodzo and colleagues revealed that 13 out of 22 P acnes strains were hemolytic and, among them, 10 were considered as definite infections, including 3 with only 1 positive sample. The authors could not identify a statistically significant trend, probably because their study was underpowered due to the size of this case series, as discussed by the authors. Nevertheless, the hemolytic activity of the strains was investigated in the 1980s by adding different concentrations of blood obtained from rabbits, sheep, or humans.5 The hemolytic activity was recorded as positive when a clear, colorless zone around the colonies appeared or weak when slight and incomplete hemolysis under the colonies was found.5 Depending on the erythrocyte origin, differences in the lytic action of hemolysin or cytotoxin may indicate the existence of various enzymes. These enzymes could have different levels of production and provide a distinct hemolytic profile. This hemolytic activity observation could also be correlated to the genetic background of the isolates.
In fact, from a genetic and epidemiological point of view, the sequence analysis of recA gene distinguished 2 distinct lineages of P acnes: types I and II.4 The association of some strains with specific clinical presentations was also demonstrated. Later, McDowell and colleagues6 reported 5 main phylogenetically distinct groups: IA, IB, IC, II, and III. It would have been interesting to know the phylogenetic groups of the strains tested in the study by Nodzo and coauthors, especially as Sampedro and colleagues7 recently reported more phylogenetic groups IA and IB among P acnes strains involved in bone and joint infections. Both of these phylotypes are hemolytic, unlike phylotypes II and III, less often encountered in this clinical entity as reported recently.8 We agree with the authors that hemolytic behavior may be one of the key factors in the variability in the pathogenicity of P acnes strains, suggesting that some strains could be more aggressive than others during deep infection. Another feature is likely the biofilm-production ability of the strains.9,10
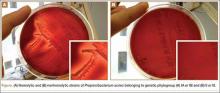
According to our experience, the hemolysis behavior was slightly different depending on which blood agar plates were used to detect hemolytic properties. We have selected 8 isolates or reference ATCC strains from different phylotypes. Each isolate was seeded on 5 different blood agar plates with erythrocyte from various origins (Table). We can confirm that only strains belonging to IA and IB phylotypes were hemolytic, with different behavior as previously reported (Figure).8 Similarly, within IA phylotype strains, the hemolytic property could be different suggesting a difference in the genetic background. However, as the genes encoding all 5 CAMP factors are present in all P acnes groups studied by Valanne and colleagues11 (IA, IB, and II), observed differences reflected different levels of expression rather than missing genes. Moreover, when camp2 or camp4 genes were deleted, the ∆camp2 but not the ∆camp4 mutant exhibited reduced hemolytic activity with sheep erythrocytes, indicating that CAMP factor 2 seems to be the major active cohemolytic factor, but in an IA phylotype P acnes genetic background.12
To conclude, the link between hemolysis and P acnes deep infection remains controversial and complex. The phenotypic differences observed between strains from various types reflect deeper differences in their phylogeny. The hemolytic ability raises the possibility that strains may also display a specific behavior according to their type and variation in their expression of putative virulence factors, including hemolysin, cytotoxin, or lipase. Further studies are clearly needed to better understand the virulence and phylogeny of P acnes strains in order to distinguish contamination from bone infection.
Stéphane Corvec, PharmD, PhD, Jérémy Luchetta, MSc, and Guillaume Ghislain Aubin, PharmD
Nantes University Hospital, Microbiology Laboratory, Nantes, France
Authors’ Response
Corvec and colleagues wrote an interesting summary and make excellent points about the role of hemolysis in Propionibacterium acnes. P acnes upper extremity infection has become an increasingly recognized problem, and determining whether a P acnes culture represents a true infection or a contaminant is still a challenge. We performed this study in hopes of finding an easily usable characteristic of P acnes that would assist the clinician in identifying P acnes strains as true infections rather than contaminants.
Certain pathogenic characteristics of P acnes have been identified, but the clinical implications of this bacterium are still being evaluated. We recognize that the hemolysis phenotype is a characteristic, and may not be the main pathogenic feature, of certain phylotypes of P acnes. It is possible the hemolytic strains in our study were from the IA and IB phylotypes, but, unfortunately, we did not specifically evaluate for phylogeny in our study. This would have correlated well with the work of Sampedro and colleagues,1 which suggested most deep bone and joint infections occur with type IA and IB P acnes phylotypes. Although less common in orthopedic infections, the type II and III phylotypes of P acnes are also capable of causing deep infection, and may not cause a hemolytic reaction on blood agar, which may be why we had some patients classified as a definite infection that did not have a hemolytic strain of P acnes. It is also possible a hemolytic strain may truly be a contaminant, but we did not observe this in our small case series. A larger series may help elucidate this finding, but the majority of truly infected patients in our case series had a hemolytic P acnes phenotype.
The type of blood agar used could have also influenced our results, as noted in the Table in Corvec and colleagues’ letter. We observed the most robust hemolysis on brucella blood agar, and limited hemolysis on CDC (Centers for Disease Control and Prevention) anaerobe blood agar; however, we did not evaluate multiple different blood agar preparations, which could have identified more hemolytic strains.
In our study, the presence of hemolysis was helpful in determining whether or not a true infection existed, but the absence of the hemolytic phenotype did not offer much additional information. The hemolytic phenotype may be a potential marker for those strains that are more aggressive and possibly represent the IA and IB phylotypes, which, as previously stated, are more commonly found in deep bone and joint infections.1 Hemolysis may serve as a surrogate marker for determining these phylotypes since determining phylogeny in a hospital laboratory is burdensome and not possible in most institutions.
In summary, we agree the hemolytic phenotype is commonly observed in certain P acnes phylotypes, and that not all upper extremity orthopedic P acnes infections will have a hemolytic finding. The genetic differences in P acnes strains are complex, and finding a marker of truly pathogenic strains has yet to be established. Larger studies evaluating the clinical outcomes and laboratory findings of patients with and without hemolytic strains of P acnes and evaluating which blood agar is the best at identifying the hemolytic phenotype may be beneficial. Identifying or combining multiple clinical and microbe-specific characteristics may also help guide treatment recommendations when a positive P acnes culture is identified.
Scott R. Nodzo, MD
John K. Crane, MD, PhD
Thomas R. Duquin, MD
Department of Orthopedics
University at Buffalo
Buffalo, NY
Letter to the Editor
1. Nodzo SR, Hohman DW, Crane JK, Duquin TR. Hemolysis as a clinical marker for Propionibacterium acnes orthopedic infection. Am J Orthop. 2014;43(5):E93-E97.
2. Portillo ME, Corvec S, Borens O, Trampuz A. Propionibacterium acnes: an underestimated pathogen in implant-associated infections. BioMed Res Int. 2013;2013:804391.
3. Corvec S, Portillo ME, Pasticci BM, Borens O, Trampuz A. Epidemiology and new developments in the diagnosis of prosthetic joint infection. Int J Artif Organs. 2012;35(10):923-934.
4. Aubin GG, Portillo ME, Trampuz A, Corvec S. Propionibacterium acnes, an emerging pathogen: from acne to implant-infections, from phylotype to resistance. Médecine Mal Infect. 2014;44(6):241-250.
5. Hoeffler U. Enzymatic and hemolytic properties of Propionibacterium acnes and related bacteria. J Clin Microbiol. 1977;6(6):555-558.
6. McDowell A, Perry AL, Lambert PA, Patrick S. A new phylogenetic group of Propionibacterium acnes. J Med Microbiol. 2008;57(Pt 2):218-224.
7. Sampedro MF, Piper KE, McDowell A, et al. Species of Propionibacterium and Propionibacterium acnes phylotypes associated with orthopedic implants. Diagn Microbiol Infect Dis. 2009;64(2):138-145.
8. Lomholt HB, Kilian M. Population genetic analysis of Propionibacterium acnes identifies a subpopulation and epidemic clones associated with acne. PloS One. 2010;5(8):e12277.
9. Furustrand Tafin U, Corvec S, Betrisey B, Zimmerli W, Trampuz A. Role of rifampin against Propionibacterium acnes biofilm in vitro and in an experimental foreign-body infection model. Antimicrob Agents Chemother. 2012;56(4):1885-1891.
10. Holmberg A, Lood R, Mörgelin M, et al. Biofilm formation by Propionibacterium acnes is a characteristic of invasive isolates. Clin Microbiol Infect. 2009;15(8):787-795.
11. Valanne S, McDowell A, Ramage G, et al. CAMP factor homologues in Propionibacterium acnes: a new protein family differentially expressed by types I and II. Microbiol. 2005;151(Pt 5):1369-1379.
12. Sörensen M, Mak TN, Hurwitz R, et al. Mutagenesis of Propionibacterium acnes and analysis of two CAMP factor knock-out mutants. J Microbiol Methods. 2010;83(2):211-216.
Authors' Response Reference
1. Sampedro MF, Piper KE, McDowell A, et al. Species of Propionibacterium and Propionibacterium acnes phylotypes associated with orthopedic implants. Diagn Microbiol Infect Dis. 2009;64(2):138-145.
Letter to the Editor
Is Hemolysis a Clinical Marker of Propionibacterium acnes Orthopedic Infection or a Phylogenetic Marker?
We read with great interest the study by Nodzo and colleagues in the May 2014 issue of The American Journal of Orthopedics on hemolysis as a clinical marker for Propionibacterium acnes orthopedic infection.1 We agree with the authors that determining if a P acnes culture is a true infection or a contaminant remains a challenge. Although P acnes is described as a commensal bacterium with a low pathogenicity, its involvement has been reported in many clinical entities, especially device-related infections.2P acnes is usually the cause of delayed infections occurring 3 to 24 months or more after prosthesis placement. The rate of P acnes involvement, probably underestimated, is about 10%.3 Although this bacterium was considered to be a contaminant, several virulence factors have been recently identified: putative hemolysins or cytotoxins (CAMP factors, hemolysin III) and enzymes putatively involved in degrading host tissue or molecules (GehA lipase, lysophospholipase, hyaluronate lyase, endoglycoceramidase, etc).4
Interestingly, Nodzo and colleagues revealed that 13 out of 22 P acnes strains were hemolytic and, among them, 10 were considered as definite infections, including 3 with only 1 positive sample. The authors could not identify a statistically significant trend, probably because their study was underpowered due to the size of this case series, as discussed by the authors. Nevertheless, the hemolytic activity of the strains was investigated in the 1980s by adding different concentrations of blood obtained from rabbits, sheep, or humans.5 The hemolytic activity was recorded as positive when a clear, colorless zone around the colonies appeared or weak when slight and incomplete hemolysis under the colonies was found.5 Depending on the erythrocyte origin, differences in the lytic action of hemolysin or cytotoxin may indicate the existence of various enzymes. These enzymes could have different levels of production and provide a distinct hemolytic profile. This hemolytic activity observation could also be correlated to the genetic background of the isolates.
In fact, from a genetic and epidemiological point of view, the sequence analysis of recA gene distinguished 2 distinct lineages of P acnes: types I and II.4 The association of some strains with specific clinical presentations was also demonstrated. Later, McDowell and colleagues6 reported 5 main phylogenetically distinct groups: IA, IB, IC, II, and III. It would have been interesting to know the phylogenetic groups of the strains tested in the study by Nodzo and coauthors, especially as Sampedro and colleagues7 recently reported more phylogenetic groups IA and IB among P acnes strains involved in bone and joint infections. Both of these phylotypes are hemolytic, unlike phylotypes II and III, less often encountered in this clinical entity as reported recently.8 We agree with the authors that hemolytic behavior may be one of the key factors in the variability in the pathogenicity of P acnes strains, suggesting that some strains could be more aggressive than others during deep infection. Another feature is likely the biofilm-production ability of the strains.9,10
According to our experience, the hemolysis behavior was slightly different depending on which blood agar plates were used to detect hemolytic properties. We have selected 8 isolates or reference ATCC strains from different phylotypes. Each isolate was seeded on 5 different blood agar plates with erythrocyte from various origins (Table). We can confirm that only strains belonging to IA and IB phylotypes were hemolytic, with different behavior as previously reported (Figure).8 Similarly, within IA phylotype strains, the hemolytic property could be different suggesting a difference in the genetic background. However, as the genes encoding all 5 CAMP factors are present in all P acnes groups studied by Valanne and colleagues11 (IA, IB, and II), observed differences reflected different levels of expression rather than missing genes. Moreover, when camp2 or camp4 genes were deleted, the ∆camp2 but not the ∆camp4 mutant exhibited reduced hemolytic activity with sheep erythrocytes, indicating that CAMP factor 2 seems to be the major active cohemolytic factor, but in an IA phylotype P acnes genetic background.12
To conclude, the link between hemolysis and P acnes deep infection remains controversial and complex. The phenotypic differences observed between strains from various types reflect deeper differences in their phylogeny. The hemolytic ability raises the possibility that strains may also display a specific behavior according to their type and variation in their expression of putative virulence factors, including hemolysin, cytotoxin, or lipase. Further studies are clearly needed to better understand the virulence and phylogeny of P acnes strains in order to distinguish contamination from bone infection.
Stéphane Corvec, PharmD, PhD, Jérémy Luchetta, MSc, and Guillaume Ghislain Aubin, PharmD
Nantes University Hospital, Microbiology Laboratory, Nantes, France
Authors’ Response
Corvec and colleagues wrote an interesting summary and make excellent points about the role of hemolysis in Propionibacterium acnes. P acnes upper extremity infection has become an increasingly recognized problem, and determining whether a P acnes culture represents a true infection or a contaminant is still a challenge. We performed this study in hopes of finding an easily usable characteristic of P acnes that would assist the clinician in identifying P acnes strains as true infections rather than contaminants.
Certain pathogenic characteristics of P acnes have been identified, but the clinical implications of this bacterium are still being evaluated. We recognize that the hemolysis phenotype is a characteristic, and may not be the main pathogenic feature, of certain phylotypes of P acnes. It is possible the hemolytic strains in our study were from the IA and IB phylotypes, but, unfortunately, we did not specifically evaluate for phylogeny in our study. This would have correlated well with the work of Sampedro and colleagues,1 which suggested most deep bone and joint infections occur with type IA and IB P acnes phylotypes. Although less common in orthopedic infections, the type II and III phylotypes of P acnes are also capable of causing deep infection, and may not cause a hemolytic reaction on blood agar, which may be why we had some patients classified as a definite infection that did not have a hemolytic strain of P acnes. It is also possible a hemolytic strain may truly be a contaminant, but we did not observe this in our small case series. A larger series may help elucidate this finding, but the majority of truly infected patients in our case series had a hemolytic P acnes phenotype.
The type of blood agar used could have also influenced our results, as noted in the Table in Corvec and colleagues’ letter. We observed the most robust hemolysis on brucella blood agar, and limited hemolysis on CDC (Centers for Disease Control and Prevention) anaerobe blood agar; however, we did not evaluate multiple different blood agar preparations, which could have identified more hemolytic strains.
In our study, the presence of hemolysis was helpful in determining whether or not a true infection existed, but the absence of the hemolytic phenotype did not offer much additional information. The hemolytic phenotype may be a potential marker for those strains that are more aggressive and possibly represent the IA and IB phylotypes, which, as previously stated, are more commonly found in deep bone and joint infections.1 Hemolysis may serve as a surrogate marker for determining these phylotypes since determining phylogeny in a hospital laboratory is burdensome and not possible in most institutions.
In summary, we agree the hemolytic phenotype is commonly observed in certain P acnes phylotypes, and that not all upper extremity orthopedic P acnes infections will have a hemolytic finding. The genetic differences in P acnes strains are complex, and finding a marker of truly pathogenic strains has yet to be established. Larger studies evaluating the clinical outcomes and laboratory findings of patients with and without hemolytic strains of P acnes and evaluating which blood agar is the best at identifying the hemolytic phenotype may be beneficial. Identifying or combining multiple clinical and microbe-specific characteristics may also help guide treatment recommendations when a positive P acnes culture is identified.
Scott R. Nodzo, MD
John K. Crane, MD, PhD
Thomas R. Duquin, MD
Department of Orthopedics
University at Buffalo
Buffalo, NY
Letter to the Editor
Is Hemolysis a Clinical Marker of Propionibacterium acnes Orthopedic Infection or a Phylogenetic Marker?
We read with great interest the study by Nodzo and colleagues in the May 2014 issue of The American Journal of Orthopedics on hemolysis as a clinical marker for Propionibacterium acnes orthopedic infection.1 We agree with the authors that determining if a P acnes culture is a true infection or a contaminant remains a challenge. Although P acnes is described as a commensal bacterium with a low pathogenicity, its involvement has been reported in many clinical entities, especially device-related infections.2P acnes is usually the cause of delayed infections occurring 3 to 24 months or more after prosthesis placement. The rate of P acnes involvement, probably underestimated, is about 10%.3 Although this bacterium was considered to be a contaminant, several virulence factors have been recently identified: putative hemolysins or cytotoxins (CAMP factors, hemolysin III) and enzymes putatively involved in degrading host tissue or molecules (GehA lipase, lysophospholipase, hyaluronate lyase, endoglycoceramidase, etc).4
Interestingly, Nodzo and colleagues revealed that 13 out of 22 P acnes strains were hemolytic and, among them, 10 were considered as definite infections, including 3 with only 1 positive sample. The authors could not identify a statistically significant trend, probably because their study was underpowered due to the size of this case series, as discussed by the authors. Nevertheless, the hemolytic activity of the strains was investigated in the 1980s by adding different concentrations of blood obtained from rabbits, sheep, or humans.5 The hemolytic activity was recorded as positive when a clear, colorless zone around the colonies appeared or weak when slight and incomplete hemolysis under the colonies was found.5 Depending on the erythrocyte origin, differences in the lytic action of hemolysin or cytotoxin may indicate the existence of various enzymes. These enzymes could have different levels of production and provide a distinct hemolytic profile. This hemolytic activity observation could also be correlated to the genetic background of the isolates.
In fact, from a genetic and epidemiological point of view, the sequence analysis of recA gene distinguished 2 distinct lineages of P acnes: types I and II.4 The association of some strains with specific clinical presentations was also demonstrated. Later, McDowell and colleagues6 reported 5 main phylogenetically distinct groups: IA, IB, IC, II, and III. It would have been interesting to know the phylogenetic groups of the strains tested in the study by Nodzo and coauthors, especially as Sampedro and colleagues7 recently reported more phylogenetic groups IA and IB among P acnes strains involved in bone and joint infections. Both of these phylotypes are hemolytic, unlike phylotypes II and III, less often encountered in this clinical entity as reported recently.8 We agree with the authors that hemolytic behavior may be one of the key factors in the variability in the pathogenicity of P acnes strains, suggesting that some strains could be more aggressive than others during deep infection. Another feature is likely the biofilm-production ability of the strains.9,10
According to our experience, the hemolysis behavior was slightly different depending on which blood agar plates were used to detect hemolytic properties. We have selected 8 isolates or reference ATCC strains from different phylotypes. Each isolate was seeded on 5 different blood agar plates with erythrocyte from various origins (Table). We can confirm that only strains belonging to IA and IB phylotypes were hemolytic, with different behavior as previously reported (Figure).8 Similarly, within IA phylotype strains, the hemolytic property could be different suggesting a difference in the genetic background. However, as the genes encoding all 5 CAMP factors are present in all P acnes groups studied by Valanne and colleagues11 (IA, IB, and II), observed differences reflected different levels of expression rather than missing genes. Moreover, when camp2 or camp4 genes were deleted, the ∆camp2 but not the ∆camp4 mutant exhibited reduced hemolytic activity with sheep erythrocytes, indicating that CAMP factor 2 seems to be the major active cohemolytic factor, but in an IA phylotype P acnes genetic background.12
To conclude, the link between hemolysis and P acnes deep infection remains controversial and complex. The phenotypic differences observed between strains from various types reflect deeper differences in their phylogeny. The hemolytic ability raises the possibility that strains may also display a specific behavior according to their type and variation in their expression of putative virulence factors, including hemolysin, cytotoxin, or lipase. Further studies are clearly needed to better understand the virulence and phylogeny of P acnes strains in order to distinguish contamination from bone infection.
Stéphane Corvec, PharmD, PhD, Jérémy Luchetta, MSc, and Guillaume Ghislain Aubin, PharmD
Nantes University Hospital, Microbiology Laboratory, Nantes, France
Authors’ Response
Corvec and colleagues wrote an interesting summary and make excellent points about the role of hemolysis in Propionibacterium acnes. P acnes upper extremity infection has become an increasingly recognized problem, and determining whether a P acnes culture represents a true infection or a contaminant is still a challenge. We performed this study in hopes of finding an easily usable characteristic of P acnes that would assist the clinician in identifying P acnes strains as true infections rather than contaminants.
Certain pathogenic characteristics of P acnes have been identified, but the clinical implications of this bacterium are still being evaluated. We recognize that the hemolysis phenotype is a characteristic, and may not be the main pathogenic feature, of certain phylotypes of P acnes. It is possible the hemolytic strains in our study were from the IA and IB phylotypes, but, unfortunately, we did not specifically evaluate for phylogeny in our study. This would have correlated well with the work of Sampedro and colleagues,1 which suggested most deep bone and joint infections occur with type IA and IB P acnes phylotypes. Although less common in orthopedic infections, the type II and III phylotypes of P acnes are also capable of causing deep infection, and may not cause a hemolytic reaction on blood agar, which may be why we had some patients classified as a definite infection that did not have a hemolytic strain of P acnes. It is also possible a hemolytic strain may truly be a contaminant, but we did not observe this in our small case series. A larger series may help elucidate this finding, but the majority of truly infected patients in our case series had a hemolytic P acnes phenotype.
The type of blood agar used could have also influenced our results, as noted in the Table in Corvec and colleagues’ letter. We observed the most robust hemolysis on brucella blood agar, and limited hemolysis on CDC (Centers for Disease Control and Prevention) anaerobe blood agar; however, we did not evaluate multiple different blood agar preparations, which could have identified more hemolytic strains.
In our study, the presence of hemolysis was helpful in determining whether or not a true infection existed, but the absence of the hemolytic phenotype did not offer much additional information. The hemolytic phenotype may be a potential marker for those strains that are more aggressive and possibly represent the IA and IB phylotypes, which, as previously stated, are more commonly found in deep bone and joint infections.1 Hemolysis may serve as a surrogate marker for determining these phylotypes since determining phylogeny in a hospital laboratory is burdensome and not possible in most institutions.
In summary, we agree the hemolytic phenotype is commonly observed in certain P acnes phylotypes, and that not all upper extremity orthopedic P acnes infections will have a hemolytic finding. The genetic differences in P acnes strains are complex, and finding a marker of truly pathogenic strains has yet to be established. Larger studies evaluating the clinical outcomes and laboratory findings of patients with and without hemolytic strains of P acnes and evaluating which blood agar is the best at identifying the hemolytic phenotype may be beneficial. Identifying or combining multiple clinical and microbe-specific characteristics may also help guide treatment recommendations when a positive P acnes culture is identified.
Scott R. Nodzo, MD
John K. Crane, MD, PhD
Thomas R. Duquin, MD
Department of Orthopedics
University at Buffalo
Buffalo, NY
Letter to the Editor
1. Nodzo SR, Hohman DW, Crane JK, Duquin TR. Hemolysis as a clinical marker for Propionibacterium acnes orthopedic infection. Am J Orthop. 2014;43(5):E93-E97.
2. Portillo ME, Corvec S, Borens O, Trampuz A. Propionibacterium acnes: an underestimated pathogen in implant-associated infections. BioMed Res Int. 2013;2013:804391.
3. Corvec S, Portillo ME, Pasticci BM, Borens O, Trampuz A. Epidemiology and new developments in the diagnosis of prosthetic joint infection. Int J Artif Organs. 2012;35(10):923-934.
4. Aubin GG, Portillo ME, Trampuz A, Corvec S. Propionibacterium acnes, an emerging pathogen: from acne to implant-infections, from phylotype to resistance. Médecine Mal Infect. 2014;44(6):241-250.
5. Hoeffler U. Enzymatic and hemolytic properties of Propionibacterium acnes and related bacteria. J Clin Microbiol. 1977;6(6):555-558.
6. McDowell A, Perry AL, Lambert PA, Patrick S. A new phylogenetic group of Propionibacterium acnes. J Med Microbiol. 2008;57(Pt 2):218-224.
7. Sampedro MF, Piper KE, McDowell A, et al. Species of Propionibacterium and Propionibacterium acnes phylotypes associated with orthopedic implants. Diagn Microbiol Infect Dis. 2009;64(2):138-145.
8. Lomholt HB, Kilian M. Population genetic analysis of Propionibacterium acnes identifies a subpopulation and epidemic clones associated with acne. PloS One. 2010;5(8):e12277.
9. Furustrand Tafin U, Corvec S, Betrisey B, Zimmerli W, Trampuz A. Role of rifampin against Propionibacterium acnes biofilm in vitro and in an experimental foreign-body infection model. Antimicrob Agents Chemother. 2012;56(4):1885-1891.
10. Holmberg A, Lood R, Mörgelin M, et al. Biofilm formation by Propionibacterium acnes is a characteristic of invasive isolates. Clin Microbiol Infect. 2009;15(8):787-795.
11. Valanne S, McDowell A, Ramage G, et al. CAMP factor homologues in Propionibacterium acnes: a new protein family differentially expressed by types I and II. Microbiol. 2005;151(Pt 5):1369-1379.
12. Sörensen M, Mak TN, Hurwitz R, et al. Mutagenesis of Propionibacterium acnes and analysis of two CAMP factor knock-out mutants. J Microbiol Methods. 2010;83(2):211-216.
Authors' Response Reference
1. Sampedro MF, Piper KE, McDowell A, et al. Species of Propionibacterium and Propionibacterium acnes phylotypes associated with orthopedic implants. Diagn Microbiol Infect Dis. 2009;64(2):138-145.
Letter to the Editor
1. Nodzo SR, Hohman DW, Crane JK, Duquin TR. Hemolysis as a clinical marker for Propionibacterium acnes orthopedic infection. Am J Orthop. 2014;43(5):E93-E97.
2. Portillo ME, Corvec S, Borens O, Trampuz A. Propionibacterium acnes: an underestimated pathogen in implant-associated infections. BioMed Res Int. 2013;2013:804391.
3. Corvec S, Portillo ME, Pasticci BM, Borens O, Trampuz A. Epidemiology and new developments in the diagnosis of prosthetic joint infection. Int J Artif Organs. 2012;35(10):923-934.
4. Aubin GG, Portillo ME, Trampuz A, Corvec S. Propionibacterium acnes, an emerging pathogen: from acne to implant-infections, from phylotype to resistance. Médecine Mal Infect. 2014;44(6):241-250.
5. Hoeffler U. Enzymatic and hemolytic properties of Propionibacterium acnes and related bacteria. J Clin Microbiol. 1977;6(6):555-558.
6. McDowell A, Perry AL, Lambert PA, Patrick S. A new phylogenetic group of Propionibacterium acnes. J Med Microbiol. 2008;57(Pt 2):218-224.
7. Sampedro MF, Piper KE, McDowell A, et al. Species of Propionibacterium and Propionibacterium acnes phylotypes associated with orthopedic implants. Diagn Microbiol Infect Dis. 2009;64(2):138-145.
8. Lomholt HB, Kilian M. Population genetic analysis of Propionibacterium acnes identifies a subpopulation and epidemic clones associated with acne. PloS One. 2010;5(8):e12277.
9. Furustrand Tafin U, Corvec S, Betrisey B, Zimmerli W, Trampuz A. Role of rifampin against Propionibacterium acnes biofilm in vitro and in an experimental foreign-body infection model. Antimicrob Agents Chemother. 2012;56(4):1885-1891.
10. Holmberg A, Lood R, Mörgelin M, et al. Biofilm formation by Propionibacterium acnes is a characteristic of invasive isolates. Clin Microbiol Infect. 2009;15(8):787-795.
11. Valanne S, McDowell A, Ramage G, et al. CAMP factor homologues in Propionibacterium acnes: a new protein family differentially expressed by types I and II. Microbiol. 2005;151(Pt 5):1369-1379.
12. Sörensen M, Mak TN, Hurwitz R, et al. Mutagenesis of Propionibacterium acnes and analysis of two CAMP factor knock-out mutants. J Microbiol Methods. 2010;83(2):211-216.
Authors' Response Reference
1. Sampedro MF, Piper KE, McDowell A, et al. Species of Propionibacterium and Propionibacterium acnes phylotypes associated with orthopedic implants. Diagn Microbiol Infect Dis. 2009;64(2):138-145.
Nanotechnology: Why Should We Care?
The orthopedic community is increasingly deluged with advancements in the basic sciences. With each step, we must evaluate the necessity of new information and the relevance of these topics for clinical practice. Since the late 1990s, the promise of nanotechnology to effect significant changes in the medical field has been heralded. However, in this coming decade, we as a profession will see unprecedented advances in the movement of this technology “from the bench to the bedside.” Not unlike many other basic science advancements in our field, nanotechnology is poorly understood among clinicians and residents. As the use of biologics and drug delivery systems expands in orthopedics, nanoparticle-based devices will become more prevalent and have a momentous impact on the way we treat and diagnose orthopedic patients.
A nanoparticle is generally defined as a particle in which at least 1 dimension is between 1 to 100 nanometers and has material properties consistent with quantum mechanics.1 Nanomaterials can be composed of organic and inorganic chemical elements that enable basic chemical processes to create more complex systems. Individual nanoparticle units can be synthesized to form nanostructures, including nanotubes, nanoscaffolds, nanofibers, and even nanodiamonds.2-4 Nanoparticles at this scale display unique optical, chemical, and physical properties that can be manipulated to create specific end-use applications. Such uses may include glass fabrication, optical probes, television screens, drug delivery, gene delivery, and multiplex diagnostic assays.5-7 By crossing disciplines of physics, engineering, and medical sciences, we can create novel technology that includes nanomanufacturing, targeted drug delivery, nanorobotics in conjunction with artificial intelligence, and point-of-care diagnostics.7-9
The field of orthopedics has benefited from nanotechnologic advances, such as new therapeutics and implant-related technology. Nanotubes are hollow nanosized cylinders that are commonly created from titania, silica, or carbon-based substrates. They have garnered significant interest for their high tensile and shear strength, favorable microstructure for bony ingrowth, and their capacity to hold antibiotics or growth factors, such as bone morphogenic proteins (BMPs).10 The current local delivery limitations of BMPs via a collagen sponge have the potential to be maximized and better controlled with a nanotechnology-based approach. The size, internal structure, and shape of the nanoparticle can be manipulated to control the release of these growth factors, and certain nanoparticles can be dual-layered, allowing for release of multiple growth factors at once or in succession.11,12 A more powerful and targeted delivery system of these types of growth factors may result in improved or more robust outcomes, and further research is warranted.
It is possible that carbon-based nanotubes can be categorized as a biomedical implant secondary to their mechanical properties.13 Their strength and ability to be augmented with osteogenic materials has made them an attractive area of research as alternative implant surfaces and stand-alone implants. Nanotubes are capable of acting as a scaffold for antibiotic-loaded, carbon-based nanodiamonds for localized treatment of periprosthetic infection, and research has been directed toward controlled release of the nanodiamond-antibiotic construct from these scaffolds or hydrogels.4,14 Technologies like this may allow the clinician to treat periprosthetic infections locally and minimize the use of systemic antibiotics. The perfection of this type of delivery system may augment the role of antibiotic-laden cement and improve our treatment success rates, even in traditionally hard-to-treat organisms.
Nanoscaffolds and nanofibers are created from nanosized polymers and rendered into a 3-dimensional structure that can be loaded with biologic particles or acting as a scaffold/template for tissue or bone ingrowth. Nanofibers created using biodegradable substrates such as poly(lactic-co-glycolic acid) (PLGA) and chitosan have been extensively studied for their delayed-release properties and biocompatibility.15 These scaffolds are often soaked or loaded with chondrogenic, osteogenic, or antibacterial agents, and have been evaluated in both in vitro and in vivo studies with promising results.15,16 They have been an exciting area of research in tissue engineering, and have been accepted as an adjunct in tendon-repair treatments and local bone regeneration.3,17 As this technology is perfected, the potential to treat more effectively massive rotator cuff tears or tears with poor tissue integrity will dramatically improve and expand the indications for rotator cuff repair.
Augmentation of implant surfaces with nanomaterials that improve osseointegration, or that act as antimicrobial agents have also been a focus of research in hopes of decreasing the rates of aseptic failure and periprosthetic infection in arthroplasty procedures. Nanocrystalline surfaces made of hydroxyapatite and cobalt chromium have been evaluated for their enhanced osteoconductive properties, and may replace standard surfaces.18-20 Recent work evaluating nanoparticle-antibiotic constructs that have been covalently bound to implant surfaces for delayed release of antibiotics during the perioperative period has shown promise, and may allow a more targeted and localized treatment strategy for periprosthetic infection.21,22
Major limitations regarding successful clinical implementation of nanotechnology include both cost and regulatory processes. Currently, pharmaceutical companies estimate that, on average, successful clinical trials from phase 1 to completion for new drugs can cost hundreds of millions of dollars.23 Such high costs result partially from the laborious and capital-intensive process of conducting clinical trials that meet US Food and Drug Administration (FDA) requirements. These regulations would apply to both surface-coated implants and nanoparticle-based drug delivery systems. These types of implants would not be expedited into the market secondary to their drug delivery component and would likely require lengthy clinical studies. Implant companies may be reluctant to invest millions of dollars in multiple FDA trials when they have lucrative implants on the market.
Other limitations include the particles’ complex 3-dimensional structure, which can present challenges for mass production. Producing large quantities of nanoparticles at a consistent quality may be a major limitation to the more unique and target-based nanotherapies. Recent concerns with the toxicity profile of nanotechnology-based medicines have resulted in more intense scrutiny of the nanotechnology safety profile.24,25 Currently, nanoparticle technology is evaluated case by case with each technology requiring its own toxicology and safety profile testing if it is intended for human use. These tests can be cost-prohibitive and require extensive private and government capital for successful market entry. Despite these limitations, nanotechnology will impact the next generation of orthopedic surgeons. Current estimates project the nanomedicine market to be worth $177.6 billion by 2019.26
Advances in nanobased orthopedic technologies have expanded dramatically in the past decade, and we, as the treating physicians, must make educated decisions on how and when to use nanoparticle-based therapies and treatment options. Nanotechnology’s basic science is confusing and often burdensome, but contemporary review articles may be helpful in keeping the orthopedic resident and clinician current with advancements.10,27,28 The more we educate ourselves about evolving nanotechnologies, the less reluctance we will have when evaluating new diagnostic and therapeutic treatment modalities.
1. Hewakuruppu YL, Dombrovsky LA, Chen C, et al. Plasmonic “pump-probe” method to study semi-transparent nanofluids. Appl Opt. 2013;52(24):6041-6050.
2. Balasundaram G, Webster TJ. An overview of nano-polymers for orthopedic applications. Macromol Biosci. 2007;7(5):635-642.
3. Zhang Z, Hu J, Ma PX. Nanofiber-based delivery of bioactive agents and stem cells to bone sites. Adv Drug Deliv Rev. 2012;64(12):1129-1141.
4. Mochalin VN, Shenderova O, Ho D, Gogotsi Y. The properties and applications of nanodiamonds. Nat Nanotechnol. 2012;7(1):11-23.
5. Kneipp J, Kneipp H, Rice WL, Kneipp K. Optical probes for biological applications based on surface-enhanced Raman scattering from indocyanine green on gold nanoparticles. Anal Chem. 2005;77(8):2381-2385.
6. Wang L, O’Donoghue MB, Tan W. Nanoparticles for multiplex diagnostics and imaging. Nanomedicine (Lond). 2006;1(4):413-426.
7. Krebs MD, Salter E, Chen E, Sutter KA, Alsberg E. Calcium phosphate-DNA nanoparticle gene delivery from alginate hydrogels induces in vivo osteogenesis. J Biomed Mater Res A. 2010;92(3):1131-1138.
8. Myers FB, Lee LP. Innovations in optical microfluidic technologies for point-of-care diagnostics. Lab Chip. 2008;8(12):2015-2031.
9. Sacha GM, Varona P. Artificial intelligence in nanotechnology. Nanotechnology. 2013;24(45):452002.
10. Ganguly DY, Shahbazian R, Shokuhfar T. Recent advances in nanotubes for orthopedic implants. J Nanotech Smart Mater. 2014;1:1-10.
11. Srivastava S, Kotov NA. Composite Layer-by-Layer (LBL) assembly with inorganic nanoparticles and nanowires. Acc Chem Res. 2008;41(12):1831-1841.
12. Panda HS, Srivastava R, Bahadur D. Shape and size control of nano dispersed Mg/Al layered double hydroxide. J Nanosci Nanotechnol. 2008;8(8):4218-4223.
13. Wang X, Li Q, Xie J, et al. Fabrication of ultralong and electrically uniform single-walled carbon nanotubes on clean substrates. Nano Lett. 2009;9(9):3137-3141.
14. Zhu Y, Li J, Li W, et al. The biocompatibility of nanodiamonds and their application in drug delivery systems. Theranostics. 2012;2(3):302-312.
15. Wu L, Ding J. In vitro degradation of three-dimensional porous poly(D,L-lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials. 2004;25(2):5821-5830.
16. Wu X, Rabkin-Aikawa E, Guleserian KJ, et al. Tissue-engineered microvessels on three-dimensional biodegradable scaffolds using human endothelial progenitor cells. Am J Physiol Heart Circ Physiol. 2004;287(2):H480-H487.
17. Xia W, Liu W, Cui L, et al. Tissue engineering of cartilage with the use of chitosan-gelatin complex scaffolds. J Biomed Mater Res B Appl Biomater. 2004;71(2):373-380.
18. Laurencin CT, Kumbar SG, Nukavarapu SP. Nanotechnology and orthopedics: a personal perspective. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1(1):6-10.
19. Webster TJ, Ejiofor JU. Increased osteoblast adhesion on nanophase metals: Ti, Ti6Al4V, and CoCrMo. Biomaterials. 2004;25(19):4731-4739.
20. Webster TJ, Ergun C, Doremus RH, Siegel RW, Bizios R. Enhanced functions of osteoblasts on nanophase ceramics. Biomaterials. 2000;21(17):1803-1810.
21. Stewart S, Barr S, Engiles J, et al. Vancomycin-modified implant surface inhibits biofilm formation and supports bone-healing in an infected osteotomy model in sheep: a proof-of-concept study. J Bone Joint Surg Am. 2012;94(15):1406-1415.
22. Hickok NJ, Shapiro IM. Immobilized antibiotics to prevent orthopaedic implant infections. Adv Drug Deliv Rev. 2012;64(12):1165-1176.
23. DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drug development costs. J Health Econ. 2003;22(2):151-185.
24. Vines T, Faunce T. Assessing the safety and cost-effectiveness of early nanodrugs. J Law Med. 2009;16(5):822-845.
25. Nel A, Xia T, Mädler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311(5761):622-627.
26. Nanomedicine Market (Neurology, Cardiovascular, Anti-Inflammatory, Anti-Infective, and Oncology Applications): Global Industry Analysis, Size, Share, Growth, Trends and Forecast, 2013-2019. Transparency Market Research website. http://www.transparencymarketresearch.com/nanomedicine-market.html. Published August 1, 2014. Accessed January 20, 2015.
27. Sullivan MP, McHale KJ, Parvizi J, Mehta S. Nanotechnology: current concepts in orthopaedic surgery and future directions. Bone Joint J. 2014;96-B(5):569-573.
28. Pleshko N, Grande DA, Myers KR. Nanotechnology in orthopaedics. J Am Acad Orthop Surg. 2012;20(1):60-62.
The orthopedic community is increasingly deluged with advancements in the basic sciences. With each step, we must evaluate the necessity of new information and the relevance of these topics for clinical practice. Since the late 1990s, the promise of nanotechnology to effect significant changes in the medical field has been heralded. However, in this coming decade, we as a profession will see unprecedented advances in the movement of this technology “from the bench to the bedside.” Not unlike many other basic science advancements in our field, nanotechnology is poorly understood among clinicians and residents. As the use of biologics and drug delivery systems expands in orthopedics, nanoparticle-based devices will become more prevalent and have a momentous impact on the way we treat and diagnose orthopedic patients.
A nanoparticle is generally defined as a particle in which at least 1 dimension is between 1 to 100 nanometers and has material properties consistent with quantum mechanics.1 Nanomaterials can be composed of organic and inorganic chemical elements that enable basic chemical processes to create more complex systems. Individual nanoparticle units can be synthesized to form nanostructures, including nanotubes, nanoscaffolds, nanofibers, and even nanodiamonds.2-4 Nanoparticles at this scale display unique optical, chemical, and physical properties that can be manipulated to create specific end-use applications. Such uses may include glass fabrication, optical probes, television screens, drug delivery, gene delivery, and multiplex diagnostic assays.5-7 By crossing disciplines of physics, engineering, and medical sciences, we can create novel technology that includes nanomanufacturing, targeted drug delivery, nanorobotics in conjunction with artificial intelligence, and point-of-care diagnostics.7-9
The field of orthopedics has benefited from nanotechnologic advances, such as new therapeutics and implant-related technology. Nanotubes are hollow nanosized cylinders that are commonly created from titania, silica, or carbon-based substrates. They have garnered significant interest for their high tensile and shear strength, favorable microstructure for bony ingrowth, and their capacity to hold antibiotics or growth factors, such as bone morphogenic proteins (BMPs).10 The current local delivery limitations of BMPs via a collagen sponge have the potential to be maximized and better controlled with a nanotechnology-based approach. The size, internal structure, and shape of the nanoparticle can be manipulated to control the release of these growth factors, and certain nanoparticles can be dual-layered, allowing for release of multiple growth factors at once or in succession.11,12 A more powerful and targeted delivery system of these types of growth factors may result in improved or more robust outcomes, and further research is warranted.
It is possible that carbon-based nanotubes can be categorized as a biomedical implant secondary to their mechanical properties.13 Their strength and ability to be augmented with osteogenic materials has made them an attractive area of research as alternative implant surfaces and stand-alone implants. Nanotubes are capable of acting as a scaffold for antibiotic-loaded, carbon-based nanodiamonds for localized treatment of periprosthetic infection, and research has been directed toward controlled release of the nanodiamond-antibiotic construct from these scaffolds or hydrogels.4,14 Technologies like this may allow the clinician to treat periprosthetic infections locally and minimize the use of systemic antibiotics. The perfection of this type of delivery system may augment the role of antibiotic-laden cement and improve our treatment success rates, even in traditionally hard-to-treat organisms.
Nanoscaffolds and nanofibers are created from nanosized polymers and rendered into a 3-dimensional structure that can be loaded with biologic particles or acting as a scaffold/template for tissue or bone ingrowth. Nanofibers created using biodegradable substrates such as poly(lactic-co-glycolic acid) (PLGA) and chitosan have been extensively studied for their delayed-release properties and biocompatibility.15 These scaffolds are often soaked or loaded with chondrogenic, osteogenic, or antibacterial agents, and have been evaluated in both in vitro and in vivo studies with promising results.15,16 They have been an exciting area of research in tissue engineering, and have been accepted as an adjunct in tendon-repair treatments and local bone regeneration.3,17 As this technology is perfected, the potential to treat more effectively massive rotator cuff tears or tears with poor tissue integrity will dramatically improve and expand the indications for rotator cuff repair.
Augmentation of implant surfaces with nanomaterials that improve osseointegration, or that act as antimicrobial agents have also been a focus of research in hopes of decreasing the rates of aseptic failure and periprosthetic infection in arthroplasty procedures. Nanocrystalline surfaces made of hydroxyapatite and cobalt chromium have been evaluated for their enhanced osteoconductive properties, and may replace standard surfaces.18-20 Recent work evaluating nanoparticle-antibiotic constructs that have been covalently bound to implant surfaces for delayed release of antibiotics during the perioperative period has shown promise, and may allow a more targeted and localized treatment strategy for periprosthetic infection.21,22
Major limitations regarding successful clinical implementation of nanotechnology include both cost and regulatory processes. Currently, pharmaceutical companies estimate that, on average, successful clinical trials from phase 1 to completion for new drugs can cost hundreds of millions of dollars.23 Such high costs result partially from the laborious and capital-intensive process of conducting clinical trials that meet US Food and Drug Administration (FDA) requirements. These regulations would apply to both surface-coated implants and nanoparticle-based drug delivery systems. These types of implants would not be expedited into the market secondary to their drug delivery component and would likely require lengthy clinical studies. Implant companies may be reluctant to invest millions of dollars in multiple FDA trials when they have lucrative implants on the market.
Other limitations include the particles’ complex 3-dimensional structure, which can present challenges for mass production. Producing large quantities of nanoparticles at a consistent quality may be a major limitation to the more unique and target-based nanotherapies. Recent concerns with the toxicity profile of nanotechnology-based medicines have resulted in more intense scrutiny of the nanotechnology safety profile.24,25 Currently, nanoparticle technology is evaluated case by case with each technology requiring its own toxicology and safety profile testing if it is intended for human use. These tests can be cost-prohibitive and require extensive private and government capital for successful market entry. Despite these limitations, nanotechnology will impact the next generation of orthopedic surgeons. Current estimates project the nanomedicine market to be worth $177.6 billion by 2019.26
Advances in nanobased orthopedic technologies have expanded dramatically in the past decade, and we, as the treating physicians, must make educated decisions on how and when to use nanoparticle-based therapies and treatment options. Nanotechnology’s basic science is confusing and often burdensome, but contemporary review articles may be helpful in keeping the orthopedic resident and clinician current with advancements.10,27,28 The more we educate ourselves about evolving nanotechnologies, the less reluctance we will have when evaluating new diagnostic and therapeutic treatment modalities.
The orthopedic community is increasingly deluged with advancements in the basic sciences. With each step, we must evaluate the necessity of new information and the relevance of these topics for clinical practice. Since the late 1990s, the promise of nanotechnology to effect significant changes in the medical field has been heralded. However, in this coming decade, we as a profession will see unprecedented advances in the movement of this technology “from the bench to the bedside.” Not unlike many other basic science advancements in our field, nanotechnology is poorly understood among clinicians and residents. As the use of biologics and drug delivery systems expands in orthopedics, nanoparticle-based devices will become more prevalent and have a momentous impact on the way we treat and diagnose orthopedic patients.
A nanoparticle is generally defined as a particle in which at least 1 dimension is between 1 to 100 nanometers and has material properties consistent with quantum mechanics.1 Nanomaterials can be composed of organic and inorganic chemical elements that enable basic chemical processes to create more complex systems. Individual nanoparticle units can be synthesized to form nanostructures, including nanotubes, nanoscaffolds, nanofibers, and even nanodiamonds.2-4 Nanoparticles at this scale display unique optical, chemical, and physical properties that can be manipulated to create specific end-use applications. Such uses may include glass fabrication, optical probes, television screens, drug delivery, gene delivery, and multiplex diagnostic assays.5-7 By crossing disciplines of physics, engineering, and medical sciences, we can create novel technology that includes nanomanufacturing, targeted drug delivery, nanorobotics in conjunction with artificial intelligence, and point-of-care diagnostics.7-9
The field of orthopedics has benefited from nanotechnologic advances, such as new therapeutics and implant-related technology. Nanotubes are hollow nanosized cylinders that are commonly created from titania, silica, or carbon-based substrates. They have garnered significant interest for their high tensile and shear strength, favorable microstructure for bony ingrowth, and their capacity to hold antibiotics or growth factors, such as bone morphogenic proteins (BMPs).10 The current local delivery limitations of BMPs via a collagen sponge have the potential to be maximized and better controlled with a nanotechnology-based approach. The size, internal structure, and shape of the nanoparticle can be manipulated to control the release of these growth factors, and certain nanoparticles can be dual-layered, allowing for release of multiple growth factors at once or in succession.11,12 A more powerful and targeted delivery system of these types of growth factors may result in improved or more robust outcomes, and further research is warranted.
It is possible that carbon-based nanotubes can be categorized as a biomedical implant secondary to their mechanical properties.13 Their strength and ability to be augmented with osteogenic materials has made them an attractive area of research as alternative implant surfaces and stand-alone implants. Nanotubes are capable of acting as a scaffold for antibiotic-loaded, carbon-based nanodiamonds for localized treatment of periprosthetic infection, and research has been directed toward controlled release of the nanodiamond-antibiotic construct from these scaffolds or hydrogels.4,14 Technologies like this may allow the clinician to treat periprosthetic infections locally and minimize the use of systemic antibiotics. The perfection of this type of delivery system may augment the role of antibiotic-laden cement and improve our treatment success rates, even in traditionally hard-to-treat organisms.
Nanoscaffolds and nanofibers are created from nanosized polymers and rendered into a 3-dimensional structure that can be loaded with biologic particles or acting as a scaffold/template for tissue or bone ingrowth. Nanofibers created using biodegradable substrates such as poly(lactic-co-glycolic acid) (PLGA) and chitosan have been extensively studied for their delayed-release properties and biocompatibility.15 These scaffolds are often soaked or loaded with chondrogenic, osteogenic, or antibacterial agents, and have been evaluated in both in vitro and in vivo studies with promising results.15,16 They have been an exciting area of research in tissue engineering, and have been accepted as an adjunct in tendon-repair treatments and local bone regeneration.3,17 As this technology is perfected, the potential to treat more effectively massive rotator cuff tears or tears with poor tissue integrity will dramatically improve and expand the indications for rotator cuff repair.
Augmentation of implant surfaces with nanomaterials that improve osseointegration, or that act as antimicrobial agents have also been a focus of research in hopes of decreasing the rates of aseptic failure and periprosthetic infection in arthroplasty procedures. Nanocrystalline surfaces made of hydroxyapatite and cobalt chromium have been evaluated for their enhanced osteoconductive properties, and may replace standard surfaces.18-20 Recent work evaluating nanoparticle-antibiotic constructs that have been covalently bound to implant surfaces for delayed release of antibiotics during the perioperative period has shown promise, and may allow a more targeted and localized treatment strategy for periprosthetic infection.21,22
Major limitations regarding successful clinical implementation of nanotechnology include both cost and regulatory processes. Currently, pharmaceutical companies estimate that, on average, successful clinical trials from phase 1 to completion for new drugs can cost hundreds of millions of dollars.23 Such high costs result partially from the laborious and capital-intensive process of conducting clinical trials that meet US Food and Drug Administration (FDA) requirements. These regulations would apply to both surface-coated implants and nanoparticle-based drug delivery systems. These types of implants would not be expedited into the market secondary to their drug delivery component and would likely require lengthy clinical studies. Implant companies may be reluctant to invest millions of dollars in multiple FDA trials when they have lucrative implants on the market.
Other limitations include the particles’ complex 3-dimensional structure, which can present challenges for mass production. Producing large quantities of nanoparticles at a consistent quality may be a major limitation to the more unique and target-based nanotherapies. Recent concerns with the toxicity profile of nanotechnology-based medicines have resulted in more intense scrutiny of the nanotechnology safety profile.24,25 Currently, nanoparticle technology is evaluated case by case with each technology requiring its own toxicology and safety profile testing if it is intended for human use. These tests can be cost-prohibitive and require extensive private and government capital for successful market entry. Despite these limitations, nanotechnology will impact the next generation of orthopedic surgeons. Current estimates project the nanomedicine market to be worth $177.6 billion by 2019.26
Advances in nanobased orthopedic technologies have expanded dramatically in the past decade, and we, as the treating physicians, must make educated decisions on how and when to use nanoparticle-based therapies and treatment options. Nanotechnology’s basic science is confusing and often burdensome, but contemporary review articles may be helpful in keeping the orthopedic resident and clinician current with advancements.10,27,28 The more we educate ourselves about evolving nanotechnologies, the less reluctance we will have when evaluating new diagnostic and therapeutic treatment modalities.
1. Hewakuruppu YL, Dombrovsky LA, Chen C, et al. Plasmonic “pump-probe” method to study semi-transparent nanofluids. Appl Opt. 2013;52(24):6041-6050.
2. Balasundaram G, Webster TJ. An overview of nano-polymers for orthopedic applications. Macromol Biosci. 2007;7(5):635-642.
3. Zhang Z, Hu J, Ma PX. Nanofiber-based delivery of bioactive agents and stem cells to bone sites. Adv Drug Deliv Rev. 2012;64(12):1129-1141.
4. Mochalin VN, Shenderova O, Ho D, Gogotsi Y. The properties and applications of nanodiamonds. Nat Nanotechnol. 2012;7(1):11-23.
5. Kneipp J, Kneipp H, Rice WL, Kneipp K. Optical probes for biological applications based on surface-enhanced Raman scattering from indocyanine green on gold nanoparticles. Anal Chem. 2005;77(8):2381-2385.
6. Wang L, O’Donoghue MB, Tan W. Nanoparticles for multiplex diagnostics and imaging. Nanomedicine (Lond). 2006;1(4):413-426.
7. Krebs MD, Salter E, Chen E, Sutter KA, Alsberg E. Calcium phosphate-DNA nanoparticle gene delivery from alginate hydrogels induces in vivo osteogenesis. J Biomed Mater Res A. 2010;92(3):1131-1138.
8. Myers FB, Lee LP. Innovations in optical microfluidic technologies for point-of-care diagnostics. Lab Chip. 2008;8(12):2015-2031.
9. Sacha GM, Varona P. Artificial intelligence in nanotechnology. Nanotechnology. 2013;24(45):452002.
10. Ganguly DY, Shahbazian R, Shokuhfar T. Recent advances in nanotubes for orthopedic implants. J Nanotech Smart Mater. 2014;1:1-10.
11. Srivastava S, Kotov NA. Composite Layer-by-Layer (LBL) assembly with inorganic nanoparticles and nanowires. Acc Chem Res. 2008;41(12):1831-1841.
12. Panda HS, Srivastava R, Bahadur D. Shape and size control of nano dispersed Mg/Al layered double hydroxide. J Nanosci Nanotechnol. 2008;8(8):4218-4223.
13. Wang X, Li Q, Xie J, et al. Fabrication of ultralong and electrically uniform single-walled carbon nanotubes on clean substrates. Nano Lett. 2009;9(9):3137-3141.
14. Zhu Y, Li J, Li W, et al. The biocompatibility of nanodiamonds and their application in drug delivery systems. Theranostics. 2012;2(3):302-312.
15. Wu L, Ding J. In vitro degradation of three-dimensional porous poly(D,L-lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials. 2004;25(2):5821-5830.
16. Wu X, Rabkin-Aikawa E, Guleserian KJ, et al. Tissue-engineered microvessels on three-dimensional biodegradable scaffolds using human endothelial progenitor cells. Am J Physiol Heart Circ Physiol. 2004;287(2):H480-H487.
17. Xia W, Liu W, Cui L, et al. Tissue engineering of cartilage with the use of chitosan-gelatin complex scaffolds. J Biomed Mater Res B Appl Biomater. 2004;71(2):373-380.
18. Laurencin CT, Kumbar SG, Nukavarapu SP. Nanotechnology and orthopedics: a personal perspective. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1(1):6-10.
19. Webster TJ, Ejiofor JU. Increased osteoblast adhesion on nanophase metals: Ti, Ti6Al4V, and CoCrMo. Biomaterials. 2004;25(19):4731-4739.
20. Webster TJ, Ergun C, Doremus RH, Siegel RW, Bizios R. Enhanced functions of osteoblasts on nanophase ceramics. Biomaterials. 2000;21(17):1803-1810.
21. Stewart S, Barr S, Engiles J, et al. Vancomycin-modified implant surface inhibits biofilm formation and supports bone-healing in an infected osteotomy model in sheep: a proof-of-concept study. J Bone Joint Surg Am. 2012;94(15):1406-1415.
22. Hickok NJ, Shapiro IM. Immobilized antibiotics to prevent orthopaedic implant infections. Adv Drug Deliv Rev. 2012;64(12):1165-1176.
23. DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drug development costs. J Health Econ. 2003;22(2):151-185.
24. Vines T, Faunce T. Assessing the safety and cost-effectiveness of early nanodrugs. J Law Med. 2009;16(5):822-845.
25. Nel A, Xia T, Mädler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311(5761):622-627.
26. Nanomedicine Market (Neurology, Cardiovascular, Anti-Inflammatory, Anti-Infective, and Oncology Applications): Global Industry Analysis, Size, Share, Growth, Trends and Forecast, 2013-2019. Transparency Market Research website. http://www.transparencymarketresearch.com/nanomedicine-market.html. Published August 1, 2014. Accessed January 20, 2015.
27. Sullivan MP, McHale KJ, Parvizi J, Mehta S. Nanotechnology: current concepts in orthopaedic surgery and future directions. Bone Joint J. 2014;96-B(5):569-573.
28. Pleshko N, Grande DA, Myers KR. Nanotechnology in orthopaedics. J Am Acad Orthop Surg. 2012;20(1):60-62.
1. Hewakuruppu YL, Dombrovsky LA, Chen C, et al. Plasmonic “pump-probe” method to study semi-transparent nanofluids. Appl Opt. 2013;52(24):6041-6050.
2. Balasundaram G, Webster TJ. An overview of nano-polymers for orthopedic applications. Macromol Biosci. 2007;7(5):635-642.
3. Zhang Z, Hu J, Ma PX. Nanofiber-based delivery of bioactive agents and stem cells to bone sites. Adv Drug Deliv Rev. 2012;64(12):1129-1141.
4. Mochalin VN, Shenderova O, Ho D, Gogotsi Y. The properties and applications of nanodiamonds. Nat Nanotechnol. 2012;7(1):11-23.
5. Kneipp J, Kneipp H, Rice WL, Kneipp K. Optical probes for biological applications based on surface-enhanced Raman scattering from indocyanine green on gold nanoparticles. Anal Chem. 2005;77(8):2381-2385.
6. Wang L, O’Donoghue MB, Tan W. Nanoparticles for multiplex diagnostics and imaging. Nanomedicine (Lond). 2006;1(4):413-426.
7. Krebs MD, Salter E, Chen E, Sutter KA, Alsberg E. Calcium phosphate-DNA nanoparticle gene delivery from alginate hydrogels induces in vivo osteogenesis. J Biomed Mater Res A. 2010;92(3):1131-1138.
8. Myers FB, Lee LP. Innovations in optical microfluidic technologies for point-of-care diagnostics. Lab Chip. 2008;8(12):2015-2031.
9. Sacha GM, Varona P. Artificial intelligence in nanotechnology. Nanotechnology. 2013;24(45):452002.
10. Ganguly DY, Shahbazian R, Shokuhfar T. Recent advances in nanotubes for orthopedic implants. J Nanotech Smart Mater. 2014;1:1-10.
11. Srivastava S, Kotov NA. Composite Layer-by-Layer (LBL) assembly with inorganic nanoparticles and nanowires. Acc Chem Res. 2008;41(12):1831-1841.
12. Panda HS, Srivastava R, Bahadur D. Shape and size control of nano dispersed Mg/Al layered double hydroxide. J Nanosci Nanotechnol. 2008;8(8):4218-4223.
13. Wang X, Li Q, Xie J, et al. Fabrication of ultralong and electrically uniform single-walled carbon nanotubes on clean substrates. Nano Lett. 2009;9(9):3137-3141.
14. Zhu Y, Li J, Li W, et al. The biocompatibility of nanodiamonds and their application in drug delivery systems. Theranostics. 2012;2(3):302-312.
15. Wu L, Ding J. In vitro degradation of three-dimensional porous poly(D,L-lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials. 2004;25(2):5821-5830.
16. Wu X, Rabkin-Aikawa E, Guleserian KJ, et al. Tissue-engineered microvessels on three-dimensional biodegradable scaffolds using human endothelial progenitor cells. Am J Physiol Heart Circ Physiol. 2004;287(2):H480-H487.
17. Xia W, Liu W, Cui L, et al. Tissue engineering of cartilage with the use of chitosan-gelatin complex scaffolds. J Biomed Mater Res B Appl Biomater. 2004;71(2):373-380.
18. Laurencin CT, Kumbar SG, Nukavarapu SP. Nanotechnology and orthopedics: a personal perspective. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1(1):6-10.
19. Webster TJ, Ejiofor JU. Increased osteoblast adhesion on nanophase metals: Ti, Ti6Al4V, and CoCrMo. Biomaterials. 2004;25(19):4731-4739.
20. Webster TJ, Ergun C, Doremus RH, Siegel RW, Bizios R. Enhanced functions of osteoblasts on nanophase ceramics. Biomaterials. 2000;21(17):1803-1810.
21. Stewart S, Barr S, Engiles J, et al. Vancomycin-modified implant surface inhibits biofilm formation and supports bone-healing in an infected osteotomy model in sheep: a proof-of-concept study. J Bone Joint Surg Am. 2012;94(15):1406-1415.
22. Hickok NJ, Shapiro IM. Immobilized antibiotics to prevent orthopaedic implant infections. Adv Drug Deliv Rev. 2012;64(12):1165-1176.
23. DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drug development costs. J Health Econ. 2003;22(2):151-185.
24. Vines T, Faunce T. Assessing the safety and cost-effectiveness of early nanodrugs. J Law Med. 2009;16(5):822-845.
25. Nel A, Xia T, Mädler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311(5761):622-627.
26. Nanomedicine Market (Neurology, Cardiovascular, Anti-Inflammatory, Anti-Infective, and Oncology Applications): Global Industry Analysis, Size, Share, Growth, Trends and Forecast, 2013-2019. Transparency Market Research website. http://www.transparencymarketresearch.com/nanomedicine-market.html. Published August 1, 2014. Accessed January 20, 2015.
27. Sullivan MP, McHale KJ, Parvizi J, Mehta S. Nanotechnology: current concepts in orthopaedic surgery and future directions. Bone Joint J. 2014;96-B(5):569-573.
28. Pleshko N, Grande DA, Myers KR. Nanotechnology in orthopaedics. J Am Acad Orthop Surg. 2012;20(1):60-62.
Team-based care: Worth a second look
Team care is not a new idea. For many years, our office teams have included physicians, nurse practitioners, physician assistants, nurses, medical assistants, front office staff, and administrative staff who functioned quite well in caring for our patients.
But primary care changed drastically after the publication of 2 landmark Institute of Medicine reports: To Err is Human: Building a Safer Health System1 (in 1999) and Crossing the Quality Chasm: A New Health System for the 21st Century2 (in 2001). These scathing reports told us we were providing inadequate care to our patients, and they contained plenty of truth. What followed is that expectations increased exponentially, and we found our offices were not prepared to deal with the new mandates for computerized medical records, high performance on quality and patient satisfaction measures, and population management.
Addressing these expanded expectations requires redefining roles and adding new players to our office teams, including nurse care coordinators, “navigators,” clinical pharmacists, psychologists, information technologists, and who knows what else. One innovative role that has seen limited testing is what some call practice facilitators.3 These are trained agents who do some of the heavy lifting required to change things like office systems and work flow.
I think that expanding the role of nurses and medical assistants is one of best ways to ensure that all of our patients get the care they deserve. Each office is unique, however, and physicians need to do the hard work of selecting the best team configuration to care for their patients. One of the more successful team-based practices is the Nuka System of Care in Alaska, which was crafted in collaboration with the tribal council. Read this fascinating story at http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3752290 and then create your own story of a successful, high-quality primary care office.
1. Kohn LT, Corrigan JM, Donaldson MS (eds); Committee on Quality of Health Care in America, Institute of Medicine. To Err is Human: Building a Safer Health System. Washington, DC: National Academy Press; 1999.
2. Committee on Quality of Health Care in America; Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001.
3. Nagykaldi Z, Mold JW, Aspy CB. Practice facilitators: a review of the literature. Fam Med. 2005;37:581-588.
Team care is not a new idea. For many years, our office teams have included physicians, nurse practitioners, physician assistants, nurses, medical assistants, front office staff, and administrative staff who functioned quite well in caring for our patients.
But primary care changed drastically after the publication of 2 landmark Institute of Medicine reports: To Err is Human: Building a Safer Health System1 (in 1999) and Crossing the Quality Chasm: A New Health System for the 21st Century2 (in 2001). These scathing reports told us we were providing inadequate care to our patients, and they contained plenty of truth. What followed is that expectations increased exponentially, and we found our offices were not prepared to deal with the new mandates for computerized medical records, high performance on quality and patient satisfaction measures, and population management.
Addressing these expanded expectations requires redefining roles and adding new players to our office teams, including nurse care coordinators, “navigators,” clinical pharmacists, psychologists, information technologists, and who knows what else. One innovative role that has seen limited testing is what some call practice facilitators.3 These are trained agents who do some of the heavy lifting required to change things like office systems and work flow.
I think that expanding the role of nurses and medical assistants is one of best ways to ensure that all of our patients get the care they deserve. Each office is unique, however, and physicians need to do the hard work of selecting the best team configuration to care for their patients. One of the more successful team-based practices is the Nuka System of Care in Alaska, which was crafted in collaboration with the tribal council. Read this fascinating story at http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3752290 and then create your own story of a successful, high-quality primary care office.
Team care is not a new idea. For many years, our office teams have included physicians, nurse practitioners, physician assistants, nurses, medical assistants, front office staff, and administrative staff who functioned quite well in caring for our patients.
But primary care changed drastically after the publication of 2 landmark Institute of Medicine reports: To Err is Human: Building a Safer Health System1 (in 1999) and Crossing the Quality Chasm: A New Health System for the 21st Century2 (in 2001). These scathing reports told us we were providing inadequate care to our patients, and they contained plenty of truth. What followed is that expectations increased exponentially, and we found our offices were not prepared to deal with the new mandates for computerized medical records, high performance on quality and patient satisfaction measures, and population management.
Addressing these expanded expectations requires redefining roles and adding new players to our office teams, including nurse care coordinators, “navigators,” clinical pharmacists, psychologists, information technologists, and who knows what else. One innovative role that has seen limited testing is what some call practice facilitators.3 These are trained agents who do some of the heavy lifting required to change things like office systems and work flow.
I think that expanding the role of nurses and medical assistants is one of best ways to ensure that all of our patients get the care they deserve. Each office is unique, however, and physicians need to do the hard work of selecting the best team configuration to care for their patients. One of the more successful team-based practices is the Nuka System of Care in Alaska, which was crafted in collaboration with the tribal council. Read this fascinating story at http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3752290 and then create your own story of a successful, high-quality primary care office.
1. Kohn LT, Corrigan JM, Donaldson MS (eds); Committee on Quality of Health Care in America, Institute of Medicine. To Err is Human: Building a Safer Health System. Washington, DC: National Academy Press; 1999.
2. Committee on Quality of Health Care in America; Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001.
3. Nagykaldi Z, Mold JW, Aspy CB. Practice facilitators: a review of the literature. Fam Med. 2005;37:581-588.
1. Kohn LT, Corrigan JM, Donaldson MS (eds); Committee on Quality of Health Care in America, Institute of Medicine. To Err is Human: Building a Safer Health System. Washington, DC: National Academy Press; 1999.
2. Committee on Quality of Health Care in America; Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001.
3. Nagykaldi Z, Mold JW, Aspy CB. Practice facilitators: a review of the literature. Fam Med. 2005;37:581-588.
Patient Satisfaction as a Metric for Quality
As orthopedic surgeons, we typically equate a quality outcome with the patient’s end result—resolved or diminished functional disability, fracture union, and/or pain relief, to name a few metrics. Although research has not identified a clear link between quality outcomes and patient satisfaction scores, patient satisfaction is increasingly used as a proxy for quality of care. It’s speculated that more personal care may result in better communication, more reasonable expectations, and more patient involvement, all of which may result in better quality of care. Regardless, it’s unclear whether satisfaction is an attribute of quality care or an indicator.
In a recent article in Modern Healthcare, Irwin Press,1 cofounder of Press Ganey, challenges any campaign to cast doubt on satisfaction as a relevant indicator of quality care: “It can be argued that diagnostic procedures, surgeries and therapies constitute treatment, but not care. Treatment alone isn’t care…. One is objective, involving highly standardized technical, mechanical or chemical interventions. The other is subjective, composed of behaviors, decisions and interactions of humans with idiosyncratic personalities, stresses, agendas and sensitivities.”
As surgeons, we understandably focus on objective treatment and outcome and may underappreciate the importance of the process—the experience of care. Wellness probably requires mastery of both. Indeed, just as a patient’s poor coping skills, depression, anxiety, and proclivity to catastrophize may compromise their recovery and self-reported assessments of outcome,2-5 so too do the qualitative components of our interaction with patients undoubtedly impact, not only their experience, but also their recovery. Patient self-efficacy (the feeling that they can do it), engagement (“activation”), compliance, and expectations all derive in part from the “Art” of our practice. Our “Heart” is as important to that Art, if not more so, than our “Head” (our intellect and knowledge). Whether we buy into this or not is a matter of personal opinion and experience, I suppose, but the reality is that the important singular metric of patient satisfaction is here to stay—patient satisfaction has become an important component of pay-for-performance metrics which expressly intend to reward quality over volume.
What does this mean for us? First, we need to adapt to the reality that the patient’s perception of their interaction with us impacts their experience and their level of satisfaction, and accept our role in their overall perception of quality. Being rewarded with a high satisfaction score is within our sphere of influence and requires more than just providing a good objective outcome. We might not revisit a restaurant with great food but lousy service and an underwhelming environment. We might also never eat at a place that was really nice inside and had great service, but which provided horrible food. So we must aspire to provide both objective quality outcomes and stellar patient care. As third-party payers increasingly follow the lead of the Centers for Medicare and Medicaid Services (CMS), the patient will not be at our table unless we both ask for feedback and respond to it. We all aspire to be great technicians and have a command of the knowledge base in our respective areas of practice. Some of us are privileged to have earned regional, national, or international reputations among our peers, but we all will be increasingly judged based on patient satisfaction with our care. This means that we must care about their experience and how they perceive our care: Do we spend enough time, listen attentively, answer questions, and explain the diagnosis and plan?
Just as we may hold our breath unknowingly during stressful situations when we are not mindful, so too might our “Heart” not be clearly evident in the complex health care environment today—too little time, too much paperwork, increasing patient demands. But practicing with heightened self-awareness, empathy, and unambiguous intention, and modeling our values during our interaction with our patients—“mindful practice”—is increasingly advocated as a necessary component to “best practice.” For truly rewarding practice, during which we can attain not only great results but also satisfied patients, we need to revisit why we do what we do, and rebalance our emphasis on what we do and how we do it. Mindful practice is both an objective and a strategy. It may require making structural adjustments to our practice, such as seeing fewer patients per hour, for, perhaps, an hour or two more in a day, completing some of our electronic medical record notes at day’s end, and maybe adding an extra clinic day every other week. We must also deliberately solicit feedback from our patients so that we can respond to any perceived room for improvement.
Thirteen years ago when I received my Master of Business Administration (MBA) degree, I felt that improving operational efficiency would enable me to do more in a day—and it did. But when patient satisfaction becomes the proxy for quality, sound business practice may not translate into sound clinical practice. After 21 years of practice, and deliberate attentiveness to patient feedback, I am increasingly aware that the Art of practice is as important as the Science—our Heart is as important as our Head. In this light, patient satisfaction is a very sound metric for quality.
1. Press I. Don’t downplay patient satisfaction. Modern Healthcare. http://www.modernhealthcare.com/article/20140322/MAGAZINE/303229940. Published March 22, 2014. Accessed January 7, 2015.
2. Cho CH, Seo HJ, Bae KC, Lee KJ, Hwang I, Warner JJ. The impact of depression and anxiety on self-assessed pain, disability, and quality of life in patients scheduled for rotator cuff repair. J Shoulder Elbow Surg. 2013;22(9):1160-1166.
3. Blackburn J, Qureshi A, Amirfeyz R, Bannister G. Does preoperative anxiety and depression predict satisfaction after total knee replacement? Knee. 2012;19(5):522-524.
4. Rosenberger PH, Jokl P, Ickovics J. Psychosocial factors and surgical outcomes: an evidence-based literature review. J Am Acad Orthop Surg. 2006;14(7):397-405.
5. Keefe FJ, Brown GK, Wallston KA, Caldwell DS. Coping with rheumatoid arthritis pain: catastrophizing as a maladaptive strategy. Pain. 1989;37(1):51-56.
As orthopedic surgeons, we typically equate a quality outcome with the patient’s end result—resolved or diminished functional disability, fracture union, and/or pain relief, to name a few metrics. Although research has not identified a clear link between quality outcomes and patient satisfaction scores, patient satisfaction is increasingly used as a proxy for quality of care. It’s speculated that more personal care may result in better communication, more reasonable expectations, and more patient involvement, all of which may result in better quality of care. Regardless, it’s unclear whether satisfaction is an attribute of quality care or an indicator.
In a recent article in Modern Healthcare, Irwin Press,1 cofounder of Press Ganey, challenges any campaign to cast doubt on satisfaction as a relevant indicator of quality care: “It can be argued that diagnostic procedures, surgeries and therapies constitute treatment, but not care. Treatment alone isn’t care…. One is objective, involving highly standardized technical, mechanical or chemical interventions. The other is subjective, composed of behaviors, decisions and interactions of humans with idiosyncratic personalities, stresses, agendas and sensitivities.”
As surgeons, we understandably focus on objective treatment and outcome and may underappreciate the importance of the process—the experience of care. Wellness probably requires mastery of both. Indeed, just as a patient’s poor coping skills, depression, anxiety, and proclivity to catastrophize may compromise their recovery and self-reported assessments of outcome,2-5 so too do the qualitative components of our interaction with patients undoubtedly impact, not only their experience, but also their recovery. Patient self-efficacy (the feeling that they can do it), engagement (“activation”), compliance, and expectations all derive in part from the “Art” of our practice. Our “Heart” is as important to that Art, if not more so, than our “Head” (our intellect and knowledge). Whether we buy into this or not is a matter of personal opinion and experience, I suppose, but the reality is that the important singular metric of patient satisfaction is here to stay—patient satisfaction has become an important component of pay-for-performance metrics which expressly intend to reward quality over volume.
What does this mean for us? First, we need to adapt to the reality that the patient’s perception of their interaction with us impacts their experience and their level of satisfaction, and accept our role in their overall perception of quality. Being rewarded with a high satisfaction score is within our sphere of influence and requires more than just providing a good objective outcome. We might not revisit a restaurant with great food but lousy service and an underwhelming environment. We might also never eat at a place that was really nice inside and had great service, but which provided horrible food. So we must aspire to provide both objective quality outcomes and stellar patient care. As third-party payers increasingly follow the lead of the Centers for Medicare and Medicaid Services (CMS), the patient will not be at our table unless we both ask for feedback and respond to it. We all aspire to be great technicians and have a command of the knowledge base in our respective areas of practice. Some of us are privileged to have earned regional, national, or international reputations among our peers, but we all will be increasingly judged based on patient satisfaction with our care. This means that we must care about their experience and how they perceive our care: Do we spend enough time, listen attentively, answer questions, and explain the diagnosis and plan?
Just as we may hold our breath unknowingly during stressful situations when we are not mindful, so too might our “Heart” not be clearly evident in the complex health care environment today—too little time, too much paperwork, increasing patient demands. But practicing with heightened self-awareness, empathy, and unambiguous intention, and modeling our values during our interaction with our patients—“mindful practice”—is increasingly advocated as a necessary component to “best practice.” For truly rewarding practice, during which we can attain not only great results but also satisfied patients, we need to revisit why we do what we do, and rebalance our emphasis on what we do and how we do it. Mindful practice is both an objective and a strategy. It may require making structural adjustments to our practice, such as seeing fewer patients per hour, for, perhaps, an hour or two more in a day, completing some of our electronic medical record notes at day’s end, and maybe adding an extra clinic day every other week. We must also deliberately solicit feedback from our patients so that we can respond to any perceived room for improvement.
Thirteen years ago when I received my Master of Business Administration (MBA) degree, I felt that improving operational efficiency would enable me to do more in a day—and it did. But when patient satisfaction becomes the proxy for quality, sound business practice may not translate into sound clinical practice. After 21 years of practice, and deliberate attentiveness to patient feedback, I am increasingly aware that the Art of practice is as important as the Science—our Heart is as important as our Head. In this light, patient satisfaction is a very sound metric for quality.
As orthopedic surgeons, we typically equate a quality outcome with the patient’s end result—resolved or diminished functional disability, fracture union, and/or pain relief, to name a few metrics. Although research has not identified a clear link between quality outcomes and patient satisfaction scores, patient satisfaction is increasingly used as a proxy for quality of care. It’s speculated that more personal care may result in better communication, more reasonable expectations, and more patient involvement, all of which may result in better quality of care. Regardless, it’s unclear whether satisfaction is an attribute of quality care or an indicator.
In a recent article in Modern Healthcare, Irwin Press,1 cofounder of Press Ganey, challenges any campaign to cast doubt on satisfaction as a relevant indicator of quality care: “It can be argued that diagnostic procedures, surgeries and therapies constitute treatment, but not care. Treatment alone isn’t care…. One is objective, involving highly standardized technical, mechanical or chemical interventions. The other is subjective, composed of behaviors, decisions and interactions of humans with idiosyncratic personalities, stresses, agendas and sensitivities.”
As surgeons, we understandably focus on objective treatment and outcome and may underappreciate the importance of the process—the experience of care. Wellness probably requires mastery of both. Indeed, just as a patient’s poor coping skills, depression, anxiety, and proclivity to catastrophize may compromise their recovery and self-reported assessments of outcome,2-5 so too do the qualitative components of our interaction with patients undoubtedly impact, not only their experience, but also their recovery. Patient self-efficacy (the feeling that they can do it), engagement (“activation”), compliance, and expectations all derive in part from the “Art” of our practice. Our “Heart” is as important to that Art, if not more so, than our “Head” (our intellect and knowledge). Whether we buy into this or not is a matter of personal opinion and experience, I suppose, but the reality is that the important singular metric of patient satisfaction is here to stay—patient satisfaction has become an important component of pay-for-performance metrics which expressly intend to reward quality over volume.
What does this mean for us? First, we need to adapt to the reality that the patient’s perception of their interaction with us impacts their experience and their level of satisfaction, and accept our role in their overall perception of quality. Being rewarded with a high satisfaction score is within our sphere of influence and requires more than just providing a good objective outcome. We might not revisit a restaurant with great food but lousy service and an underwhelming environment. We might also never eat at a place that was really nice inside and had great service, but which provided horrible food. So we must aspire to provide both objective quality outcomes and stellar patient care. As third-party payers increasingly follow the lead of the Centers for Medicare and Medicaid Services (CMS), the patient will not be at our table unless we both ask for feedback and respond to it. We all aspire to be great technicians and have a command of the knowledge base in our respective areas of practice. Some of us are privileged to have earned regional, national, or international reputations among our peers, but we all will be increasingly judged based on patient satisfaction with our care. This means that we must care about their experience and how they perceive our care: Do we spend enough time, listen attentively, answer questions, and explain the diagnosis and plan?
Just as we may hold our breath unknowingly during stressful situations when we are not mindful, so too might our “Heart” not be clearly evident in the complex health care environment today—too little time, too much paperwork, increasing patient demands. But practicing with heightened self-awareness, empathy, and unambiguous intention, and modeling our values during our interaction with our patients—“mindful practice”—is increasingly advocated as a necessary component to “best practice.” For truly rewarding practice, during which we can attain not only great results but also satisfied patients, we need to revisit why we do what we do, and rebalance our emphasis on what we do and how we do it. Mindful practice is both an objective and a strategy. It may require making structural adjustments to our practice, such as seeing fewer patients per hour, for, perhaps, an hour or two more in a day, completing some of our electronic medical record notes at day’s end, and maybe adding an extra clinic day every other week. We must also deliberately solicit feedback from our patients so that we can respond to any perceived room for improvement.
Thirteen years ago when I received my Master of Business Administration (MBA) degree, I felt that improving operational efficiency would enable me to do more in a day—and it did. But when patient satisfaction becomes the proxy for quality, sound business practice may not translate into sound clinical practice. After 21 years of practice, and deliberate attentiveness to patient feedback, I am increasingly aware that the Art of practice is as important as the Science—our Heart is as important as our Head. In this light, patient satisfaction is a very sound metric for quality.
1. Press I. Don’t downplay patient satisfaction. Modern Healthcare. http://www.modernhealthcare.com/article/20140322/MAGAZINE/303229940. Published March 22, 2014. Accessed January 7, 2015.
2. Cho CH, Seo HJ, Bae KC, Lee KJ, Hwang I, Warner JJ. The impact of depression and anxiety on self-assessed pain, disability, and quality of life in patients scheduled for rotator cuff repair. J Shoulder Elbow Surg. 2013;22(9):1160-1166.
3. Blackburn J, Qureshi A, Amirfeyz R, Bannister G. Does preoperative anxiety and depression predict satisfaction after total knee replacement? Knee. 2012;19(5):522-524.
4. Rosenberger PH, Jokl P, Ickovics J. Psychosocial factors and surgical outcomes: an evidence-based literature review. J Am Acad Orthop Surg. 2006;14(7):397-405.
5. Keefe FJ, Brown GK, Wallston KA, Caldwell DS. Coping with rheumatoid arthritis pain: catastrophizing as a maladaptive strategy. Pain. 1989;37(1):51-56.
1. Press I. Don’t downplay patient satisfaction. Modern Healthcare. http://www.modernhealthcare.com/article/20140322/MAGAZINE/303229940. Published March 22, 2014. Accessed January 7, 2015.
2. Cho CH, Seo HJ, Bae KC, Lee KJ, Hwang I, Warner JJ. The impact of depression and anxiety on self-assessed pain, disability, and quality of life in patients scheduled for rotator cuff repair. J Shoulder Elbow Surg. 2013;22(9):1160-1166.
3. Blackburn J, Qureshi A, Amirfeyz R, Bannister G. Does preoperative anxiety and depression predict satisfaction after total knee replacement? Knee. 2012;19(5):522-524.
4. Rosenberger PH, Jokl P, Ickovics J. Psychosocial factors and surgical outcomes: an evidence-based literature review. J Am Acad Orthop Surg. 2006;14(7):397-405.
5. Keefe FJ, Brown GK, Wallston KA, Caldwell DS. Coping with rheumatoid arthritis pain: catastrophizing as a maladaptive strategy. Pain. 1989;37(1):51-56.
Playing with fire
Hoping to learn a bit more about the apparently healthy 8-year-old who has been deposited in your exam room for his biannual checkup, you take the opportunity to ask him a few questions with his mother in the waiting room. “So, Jason, what do you like to do for fun?”
“Well, yesterday I started a fire with my buddy Rudy, and we burned a whole bunch of sticks and stuff.”
“Does your mother know about this?”
“She wasn’t around, but I think so. We’ve done it a bunch of times before.”
Okay, here you are with an 8-year-old pyromaniac whose parents are clearly under-supervising him. Who do you call first? The folks at Child Protective Services or the State Fire Marshall’s Office? Clearly, he and your community are at significant risk.
If you were practicing in Wrexham, a town in North Wales, you would continue your questioning with, “So you like to go to The Land after school? I’ve heard it’s a fun place?”
The Land is 3-year-old adventure playground that I learned about in a thought-provoking article in The Atlantic (The Overprotected Kid, by Hanna Rosin, April 2014). The nearly acre-sized site would look like a junkyard to any adult whose imagination has atrophied with age. Strewn with used tires, wooden palettes, dirty old mattresses, and decrepit lawn furniture, it provides endless opportunities for children to create their own places for play and adventure. By stacking, rolling, hammering together, and rearranging the loose detritus of society, children can transform the junk into an ever-changing landscape for fun. A fire pit and an old oil drum – among the most popular items – are often smoldering with fires the children have started. The filthy mattresses become trampolines. The children are observed by professionally trained “playworkers” who are continually updating the risk assessments of the activities that were begun prior to the opening of the facility. The observers seldom have to intervene. Other than a few scraped knees, no children have been injured.
Although adventure playgrounds were relatively common in the U.K. during the 1940’s, their popularity faded until the last few years when they have enjoyed a modest resurgence. In the article in The Atlantic, author Hanna Rosin chronicles the de-riskification of playgrounds in America that began in the 1970’s. The process was fueled by an unfortunate incident in which a toddler supervised by his mother fell off a 12-foot playground slide in a Chicago playground. The child sustained a significant and permanent brain injury and received a multimillion dollar award in the suit that followed.
A commentary in Pediatrics entitled “X-rated playgrounds?” (Pediatrics 1979;64:961) and a crusade by its author, Theodora Briggs Sweeney culminated in the release of the Handbook for Public Playground Safety (U.S. Consumer Product Safety Commission, 1981) which listed in minute detail guidelines for dimensions and materials for playground equipment and play surfaces. Although these were only “guidelines,” only the most foolish manufacturer would ignore them. Little thought was given to the validity of the alarming statistics that had prompted these changes. What were the denominators? Can you compare 1970’s hospital data with those from the 1950’s when injured children were managed at home or in their doctors’ offices?
Regardless of the validity of the data, the result was that these redesigned playgrounds offered so little sense of risk that they were abandoned by all but the youngest children. Numerous studies suggest that by eliminating risk or at least the appearance of risk, we are robbing children of important learning experiences on which they can build fuller, more creative, successful, and less anxiety-dominated lives. I urge you to look at that Atlantic article for a more robust description of the evidence.
I suspect that you may be a bit uncomfortable with 8-year-old boys playing with fire, but do you agree that we need to seriously rethink our attempts to protect children from the ordinary risks of an active life? Or do you think those of us who believe children will benefit from more perceived risk are just a bunch of fogys who begin every other sentence, “When I was your age ... .”
Do you encourage parents to allow their children to walk to school unattended? Do you caution parents about being overprotective? Have I ignited a spark of concern in you, or am I just playing with fire?
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “Coping with a Picky Eater.” E-mail him at [email protected].
Hoping to learn a bit more about the apparently healthy 8-year-old who has been deposited in your exam room for his biannual checkup, you take the opportunity to ask him a few questions with his mother in the waiting room. “So, Jason, what do you like to do for fun?”
“Well, yesterday I started a fire with my buddy Rudy, and we burned a whole bunch of sticks and stuff.”
“Does your mother know about this?”
“She wasn’t around, but I think so. We’ve done it a bunch of times before.”
Okay, here you are with an 8-year-old pyromaniac whose parents are clearly under-supervising him. Who do you call first? The folks at Child Protective Services or the State Fire Marshall’s Office? Clearly, he and your community are at significant risk.
If you were practicing in Wrexham, a town in North Wales, you would continue your questioning with, “So you like to go to The Land after school? I’ve heard it’s a fun place?”
The Land is 3-year-old adventure playground that I learned about in a thought-provoking article in The Atlantic (The Overprotected Kid, by Hanna Rosin, April 2014). The nearly acre-sized site would look like a junkyard to any adult whose imagination has atrophied with age. Strewn with used tires, wooden palettes, dirty old mattresses, and decrepit lawn furniture, it provides endless opportunities for children to create their own places for play and adventure. By stacking, rolling, hammering together, and rearranging the loose detritus of society, children can transform the junk into an ever-changing landscape for fun. A fire pit and an old oil drum – among the most popular items – are often smoldering with fires the children have started. The filthy mattresses become trampolines. The children are observed by professionally trained “playworkers” who are continually updating the risk assessments of the activities that were begun prior to the opening of the facility. The observers seldom have to intervene. Other than a few scraped knees, no children have been injured.
Although adventure playgrounds were relatively common in the U.K. during the 1940’s, their popularity faded until the last few years when they have enjoyed a modest resurgence. In the article in The Atlantic, author Hanna Rosin chronicles the de-riskification of playgrounds in America that began in the 1970’s. The process was fueled by an unfortunate incident in which a toddler supervised by his mother fell off a 12-foot playground slide in a Chicago playground. The child sustained a significant and permanent brain injury and received a multimillion dollar award in the suit that followed.
A commentary in Pediatrics entitled “X-rated playgrounds?” (Pediatrics 1979;64:961) and a crusade by its author, Theodora Briggs Sweeney culminated in the release of the Handbook for Public Playground Safety (U.S. Consumer Product Safety Commission, 1981) which listed in minute detail guidelines for dimensions and materials for playground equipment and play surfaces. Although these were only “guidelines,” only the most foolish manufacturer would ignore them. Little thought was given to the validity of the alarming statistics that had prompted these changes. What were the denominators? Can you compare 1970’s hospital data with those from the 1950’s when injured children were managed at home or in their doctors’ offices?
Regardless of the validity of the data, the result was that these redesigned playgrounds offered so little sense of risk that they were abandoned by all but the youngest children. Numerous studies suggest that by eliminating risk or at least the appearance of risk, we are robbing children of important learning experiences on which they can build fuller, more creative, successful, and less anxiety-dominated lives. I urge you to look at that Atlantic article for a more robust description of the evidence.
I suspect that you may be a bit uncomfortable with 8-year-old boys playing with fire, but do you agree that we need to seriously rethink our attempts to protect children from the ordinary risks of an active life? Or do you think those of us who believe children will benefit from more perceived risk are just a bunch of fogys who begin every other sentence, “When I was your age ... .”
Do you encourage parents to allow their children to walk to school unattended? Do you caution parents about being overprotective? Have I ignited a spark of concern in you, or am I just playing with fire?
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “Coping with a Picky Eater.” E-mail him at [email protected].
Hoping to learn a bit more about the apparently healthy 8-year-old who has been deposited in your exam room for his biannual checkup, you take the opportunity to ask him a few questions with his mother in the waiting room. “So, Jason, what do you like to do for fun?”
“Well, yesterday I started a fire with my buddy Rudy, and we burned a whole bunch of sticks and stuff.”
“Does your mother know about this?”
“She wasn’t around, but I think so. We’ve done it a bunch of times before.”
Okay, here you are with an 8-year-old pyromaniac whose parents are clearly under-supervising him. Who do you call first? The folks at Child Protective Services or the State Fire Marshall’s Office? Clearly, he and your community are at significant risk.
If you were practicing in Wrexham, a town in North Wales, you would continue your questioning with, “So you like to go to The Land after school? I’ve heard it’s a fun place?”
The Land is 3-year-old adventure playground that I learned about in a thought-provoking article in The Atlantic (The Overprotected Kid, by Hanna Rosin, April 2014). The nearly acre-sized site would look like a junkyard to any adult whose imagination has atrophied with age. Strewn with used tires, wooden palettes, dirty old mattresses, and decrepit lawn furniture, it provides endless opportunities for children to create their own places for play and adventure. By stacking, rolling, hammering together, and rearranging the loose detritus of society, children can transform the junk into an ever-changing landscape for fun. A fire pit and an old oil drum – among the most popular items – are often smoldering with fires the children have started. The filthy mattresses become trampolines. The children are observed by professionally trained “playworkers” who are continually updating the risk assessments of the activities that were begun prior to the opening of the facility. The observers seldom have to intervene. Other than a few scraped knees, no children have been injured.
Although adventure playgrounds were relatively common in the U.K. during the 1940’s, their popularity faded until the last few years when they have enjoyed a modest resurgence. In the article in The Atlantic, author Hanna Rosin chronicles the de-riskification of playgrounds in America that began in the 1970’s. The process was fueled by an unfortunate incident in which a toddler supervised by his mother fell off a 12-foot playground slide in a Chicago playground. The child sustained a significant and permanent brain injury and received a multimillion dollar award in the suit that followed.
A commentary in Pediatrics entitled “X-rated playgrounds?” (Pediatrics 1979;64:961) and a crusade by its author, Theodora Briggs Sweeney culminated in the release of the Handbook for Public Playground Safety (U.S. Consumer Product Safety Commission, 1981) which listed in minute detail guidelines for dimensions and materials for playground equipment and play surfaces. Although these were only “guidelines,” only the most foolish manufacturer would ignore them. Little thought was given to the validity of the alarming statistics that had prompted these changes. What were the denominators? Can you compare 1970’s hospital data with those from the 1950’s when injured children were managed at home or in their doctors’ offices?
Regardless of the validity of the data, the result was that these redesigned playgrounds offered so little sense of risk that they were abandoned by all but the youngest children. Numerous studies suggest that by eliminating risk or at least the appearance of risk, we are robbing children of important learning experiences on which they can build fuller, more creative, successful, and less anxiety-dominated lives. I urge you to look at that Atlantic article for a more robust description of the evidence.
I suspect that you may be a bit uncomfortable with 8-year-old boys playing with fire, but do you agree that we need to seriously rethink our attempts to protect children from the ordinary risks of an active life? Or do you think those of us who believe children will benefit from more perceived risk are just a bunch of fogys who begin every other sentence, “When I was your age ... .”
Do you encourage parents to allow their children to walk to school unattended? Do you caution parents about being overprotective? Have I ignited a spark of concern in you, or am I just playing with fire?
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “Coping with a Picky Eater.” E-mail him at [email protected].
Ob.gyns. can help end the HIV epidemic
Despite staggering scientific and medical advances, the HIV epidemic in the United States has not changed significantly over the past decade. The estimated incidence of HIV infection has remained stable overall, with between 45,000 and 55,000 new HIV infections diagnosed per year.
This is disheartening because, even without a vaccine, I believe we have the tools today to drive the epidemic down to zero. First of all, we know how to effectively diagnose and treat the infection, and we have evidence that antiretroviral treatment is an effective prevention tool. Secondly, advances in chemoprophylaxis have made pre-exposure prophylaxis a reality.
Ob.gyns. played a central role in one of the greatest successes of the use of antiretroviral drugs: the virtual elimination of mother-to-child transmission of HIV in the United States. Now, by fully utilizing the tools available today, ob.gyns. can play a critical role in ending the epidemic in the United States and beyond.
Tools for diagnosis and treatment
We have so many missed opportunities in fighting the HIV epidemic.
This is evident in data compiled for a model called the “HIV Care Continuum,” or HIV “Cascade of Care.” The model captures the sequential stages of HIV care from diagnosis to suppression of the virus. It was developed in 2011 by Dr. Edward Gardner, an infectious disease/HIV expert at Denver Public Health, and has since been used at the federal, state, and local levels to help identify gaps in HIV services.
Not too long ago, diagnosis was the biggest problem in reducing the public health burden of HIV. Today, the biggest problem is linking and keeping individuals in care. According to the latest analysis by the U.S. Centers for Disease Control and Prevention of the HIV Care Continuum, of the 1.2 million people estimated to be living with HIV in America in 2011, approximately 86% were diagnosed, but only 40% were linked to and stayed in care, 37% were prescribed antiretroviral therapy (ART), and 30% had achieved viral suppression.
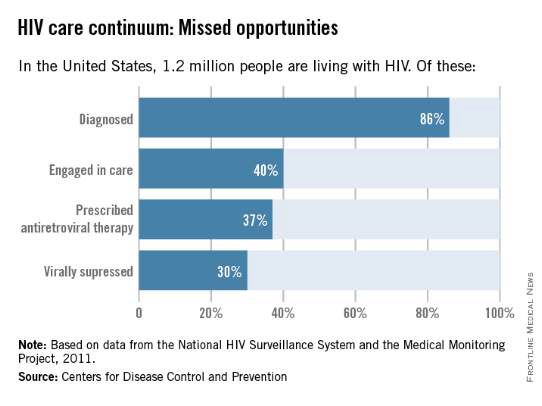
Only 30% of Americans living with HIV infection today are effectively treated, according to these data, even though we have the drugs and drug regimens available to treat everyone effectively.
Other analyses have included an additional stage of being initially linked to care (rather than being linked to care and retained in care). This presentation of the cascade, or continuum, further illuminates the progressive drop-off and that shows why an effective, sustained linkage to care is a critical component to ending the HIV epidemic.
One of these studies – an analysis published in 2013 – showed that approximately 82% of people were diagnosed, 66% were linked to care, 37% were retained in care, 33% were prescribed antiretroviral therapy, and 25% had a suppressed viral load of 200 copies/mL or less (JAMA. Intern. Med. 2013;173:1337-44).
With regard to women specifically, the CDC estimates that one in four people living with HIV infection are women, and that only about half of the women who are diagnosed with the infection are staying in care. Even fewer – 4 in 10 – have viral suppression, according to the CDC.
Expanding the management of HIV in the primary care setting could move us closer to ensuring that everyone in the United States who is infected with HIV is aware of the infection, is committed to treatment, and is virologically suppressed.
Like other primary care physicians, ob.gyns often have some degree of long-term continuity with patients – or the ability to create such continuity – that can be helpful for ensuring treatment compliance.
Ob.gyns also have valuable contact with adolescents, who fare worse throughout the cascade and are significantly more likely than older individuals to have unknown infections. An analysis published in 2014 of data for youth ages 13-29 shows that only 40% of HIV-infected youth were aware of their diagnosis and that an estimated 6% or less of HIV-infected youth were virally suppressed (AIDS. Patient. Care. STDS. 2014;28:128-135).
HIV testing should occur much more frequently than a decade ago, given the move in 2006 by the CDC from targeted risk-based testing to routine opt-out testing for all patients aged 13-64.
Treatment, moreover, has become much simpler in many respects. We have available to us more than 30 different drugs for individualizing therapy and providing treatment that allows patients to live a natural lifetime.
While such a large array of options may require those ob.gyns. who see only a few HIV-infected patients a year to work in consultation with an expert, many of the regimens require only a single, once-a-day pill. And while there was much debate as recently as five years ago about when to start treatment, there now is consensus that treatment should be started immediately after diagnosis (even in pregnant women), rather than waiting for the immune system to show signs of decline.
In fact, there is growing evidence that early treatment is key for both the infected individual and for individuals at risk. In the HIV Prevention Trials Network 052 study of discordant couples, for instance, early antiretroviral therapy in an infected partner not only reduced the number of clinical events; it almost completely blocked sexual transmission of the virus to an HIV-negative partner (N. Engl. J. Med. 2011;365:493-505).
The 052 study was a landmark “treatment as prevention” study. Other research has similarly shown that when the viral load of HIV-infected individuals is significantly reduced, their infectivity is reduced. And on a larger scale, research has shown that when we do this on a population basis, achieving widespread and continual treatment success, we can significantly impact the epidemic. This has been the case with the population of intravenous drug users in Vancouver, where the community viral load was significantly reduced by successful treatment that prevented new infections in this once-high-risk population.
Emerging data suggests that early diagnosis and treatment will likely also impact the likelihood of infected individuals achieving “functional cure.” The issue of functional cure – of achieving viral loads that are so low that drug therapy is no longer needed – has been receiving increasing attention in recent years, with the most promising findings reported thus far involving early treatment.
Tools for preexposure prophylaxis
For many years, we fit HIV care neatly into either the treatment or prevention category. More recently, we have come to appreciate that treatment is prevention, that a comprehensive prevention strategy must include treatment of infected individuals.
On the purely prevention side, it is important to continue educating women about safe sex behaviors. Most new HIV infections in women (84%) result from heterosexual contact, according to the CDC. For those who remain at risk of acquiring HIV despite education and counseling (eg., individuals who continue to engage in high-risk behaviors, or who have an HIV-positive partner), pre-exposure prophylaxis (PrEP) is now a safe and effective tool for preventing transmission. Patients deemed to be at high risk of acquiring HIV need to be made aware of this option.
PrEP originally was recommended only for gay or bisexual men, but in May 2014, the CDC recommended it for all individuals at risk and released the first comprehensive clinical practice guidelines for the prevention tool (www.cdc.gov/hiv/pdf/guidelines/PrEPguidelines2014.pdf).
The PrEP medication, Truvada, is a combination of two drugs (tenovovir and emtricitabine) that, when taken daily on a consistent basis, significantly reduces the risk of getting HIV infection. Several large national and international studies have documented risk reductions of 73% to 92% when the medication was taken every day or almost every day. It is clearly within the purview of any ob.gyn to prescribe, monitor, and manage such prevention therapy.
The availability and relative ease of such a tool, along with advances in treatment and knowledge gained from the HIV Care Continuum, should re-energize ob.gyns. to up the ante in efforts to end the epidemic.
Experience in our clinical program that provides care and treatment to patients in the Baltimore-Washington area has taught us that we do much better when we integrate HIV care within primary care. It’s much more likely that patients will “stay close” with their ob.gyn than to another specialist.
Certainly, HIV infection has its “hot spots” and areas of much lower prevalence, but regardless of where we reside, we must continue to appreciate that the epidemic has had a significant impact on women and that this will persist unless we can all better utilize our available tools, such as early diagnosis and effective treatment that are linked long-term with other primary care physicians.
For women, ob.gyns represent a great resource for our nation to make progress toward President Obama’s National HIV Strategy.
Dr. Redfield reported that he has no disclosures relevant to this Master Class.
Despite staggering scientific and medical advances, the HIV epidemic in the United States has not changed significantly over the past decade. The estimated incidence of HIV infection has remained stable overall, with between 45,000 and 55,000 new HIV infections diagnosed per year.
This is disheartening because, even without a vaccine, I believe we have the tools today to drive the epidemic down to zero. First of all, we know how to effectively diagnose and treat the infection, and we have evidence that antiretroviral treatment is an effective prevention tool. Secondly, advances in chemoprophylaxis have made pre-exposure prophylaxis a reality.
Ob.gyns. played a central role in one of the greatest successes of the use of antiretroviral drugs: the virtual elimination of mother-to-child transmission of HIV in the United States. Now, by fully utilizing the tools available today, ob.gyns. can play a critical role in ending the epidemic in the United States and beyond.
Tools for diagnosis and treatment
We have so many missed opportunities in fighting the HIV epidemic.
This is evident in data compiled for a model called the “HIV Care Continuum,” or HIV “Cascade of Care.” The model captures the sequential stages of HIV care from diagnosis to suppression of the virus. It was developed in 2011 by Dr. Edward Gardner, an infectious disease/HIV expert at Denver Public Health, and has since been used at the federal, state, and local levels to help identify gaps in HIV services.
Not too long ago, diagnosis was the biggest problem in reducing the public health burden of HIV. Today, the biggest problem is linking and keeping individuals in care. According to the latest analysis by the U.S. Centers for Disease Control and Prevention of the HIV Care Continuum, of the 1.2 million people estimated to be living with HIV in America in 2011, approximately 86% were diagnosed, but only 40% were linked to and stayed in care, 37% were prescribed antiretroviral therapy (ART), and 30% had achieved viral suppression.

Only 30% of Americans living with HIV infection today are effectively treated, according to these data, even though we have the drugs and drug regimens available to treat everyone effectively.
Other analyses have included an additional stage of being initially linked to care (rather than being linked to care and retained in care). This presentation of the cascade, or continuum, further illuminates the progressive drop-off and that shows why an effective, sustained linkage to care is a critical component to ending the HIV epidemic.
One of these studies – an analysis published in 2013 – showed that approximately 82% of people were diagnosed, 66% were linked to care, 37% were retained in care, 33% were prescribed antiretroviral therapy, and 25% had a suppressed viral load of 200 copies/mL or less (JAMA. Intern. Med. 2013;173:1337-44).
With regard to women specifically, the CDC estimates that one in four people living with HIV infection are women, and that only about half of the women who are diagnosed with the infection are staying in care. Even fewer – 4 in 10 – have viral suppression, according to the CDC.
Expanding the management of HIV in the primary care setting could move us closer to ensuring that everyone in the United States who is infected with HIV is aware of the infection, is committed to treatment, and is virologically suppressed.
Like other primary care physicians, ob.gyns often have some degree of long-term continuity with patients – or the ability to create such continuity – that can be helpful for ensuring treatment compliance.
Ob.gyns also have valuable contact with adolescents, who fare worse throughout the cascade and are significantly more likely than older individuals to have unknown infections. An analysis published in 2014 of data for youth ages 13-29 shows that only 40% of HIV-infected youth were aware of their diagnosis and that an estimated 6% or less of HIV-infected youth were virally suppressed (AIDS. Patient. Care. STDS. 2014;28:128-135).
HIV testing should occur much more frequently than a decade ago, given the move in 2006 by the CDC from targeted risk-based testing to routine opt-out testing for all patients aged 13-64.
Treatment, moreover, has become much simpler in many respects. We have available to us more than 30 different drugs for individualizing therapy and providing treatment that allows patients to live a natural lifetime.
While such a large array of options may require those ob.gyns. who see only a few HIV-infected patients a year to work in consultation with an expert, many of the regimens require only a single, once-a-day pill. And while there was much debate as recently as five years ago about when to start treatment, there now is consensus that treatment should be started immediately after diagnosis (even in pregnant women), rather than waiting for the immune system to show signs of decline.
In fact, there is growing evidence that early treatment is key for both the infected individual and for individuals at risk. In the HIV Prevention Trials Network 052 study of discordant couples, for instance, early antiretroviral therapy in an infected partner not only reduced the number of clinical events; it almost completely blocked sexual transmission of the virus to an HIV-negative partner (N. Engl. J. Med. 2011;365:493-505).
The 052 study was a landmark “treatment as prevention” study. Other research has similarly shown that when the viral load of HIV-infected individuals is significantly reduced, their infectivity is reduced. And on a larger scale, research has shown that when we do this on a population basis, achieving widespread and continual treatment success, we can significantly impact the epidemic. This has been the case with the population of intravenous drug users in Vancouver, where the community viral load was significantly reduced by successful treatment that prevented new infections in this once-high-risk population.
Emerging data suggests that early diagnosis and treatment will likely also impact the likelihood of infected individuals achieving “functional cure.” The issue of functional cure – of achieving viral loads that are so low that drug therapy is no longer needed – has been receiving increasing attention in recent years, with the most promising findings reported thus far involving early treatment.
Tools for preexposure prophylaxis
For many years, we fit HIV care neatly into either the treatment or prevention category. More recently, we have come to appreciate that treatment is prevention, that a comprehensive prevention strategy must include treatment of infected individuals.
On the purely prevention side, it is important to continue educating women about safe sex behaviors. Most new HIV infections in women (84%) result from heterosexual contact, according to the CDC. For those who remain at risk of acquiring HIV despite education and counseling (eg., individuals who continue to engage in high-risk behaviors, or who have an HIV-positive partner), pre-exposure prophylaxis (PrEP) is now a safe and effective tool for preventing transmission. Patients deemed to be at high risk of acquiring HIV need to be made aware of this option.
PrEP originally was recommended only for gay or bisexual men, but in May 2014, the CDC recommended it for all individuals at risk and released the first comprehensive clinical practice guidelines for the prevention tool (www.cdc.gov/hiv/pdf/guidelines/PrEPguidelines2014.pdf).
The PrEP medication, Truvada, is a combination of two drugs (tenovovir and emtricitabine) that, when taken daily on a consistent basis, significantly reduces the risk of getting HIV infection. Several large national and international studies have documented risk reductions of 73% to 92% when the medication was taken every day or almost every day. It is clearly within the purview of any ob.gyn to prescribe, monitor, and manage such prevention therapy.
The availability and relative ease of such a tool, along with advances in treatment and knowledge gained from the HIV Care Continuum, should re-energize ob.gyns. to up the ante in efforts to end the epidemic.
Experience in our clinical program that provides care and treatment to patients in the Baltimore-Washington area has taught us that we do much better when we integrate HIV care within primary care. It’s much more likely that patients will “stay close” with their ob.gyn than to another specialist.
Certainly, HIV infection has its “hot spots” and areas of much lower prevalence, but regardless of where we reside, we must continue to appreciate that the epidemic has had a significant impact on women and that this will persist unless we can all better utilize our available tools, such as early diagnosis and effective treatment that are linked long-term with other primary care physicians.
For women, ob.gyns represent a great resource for our nation to make progress toward President Obama’s National HIV Strategy.
Dr. Redfield reported that he has no disclosures relevant to this Master Class.
Despite staggering scientific and medical advances, the HIV epidemic in the United States has not changed significantly over the past decade. The estimated incidence of HIV infection has remained stable overall, with between 45,000 and 55,000 new HIV infections diagnosed per year.
This is disheartening because, even without a vaccine, I believe we have the tools today to drive the epidemic down to zero. First of all, we know how to effectively diagnose and treat the infection, and we have evidence that antiretroviral treatment is an effective prevention tool. Secondly, advances in chemoprophylaxis have made pre-exposure prophylaxis a reality.
Ob.gyns. played a central role in one of the greatest successes of the use of antiretroviral drugs: the virtual elimination of mother-to-child transmission of HIV in the United States. Now, by fully utilizing the tools available today, ob.gyns. can play a critical role in ending the epidemic in the United States and beyond.
Tools for diagnosis and treatment
We have so many missed opportunities in fighting the HIV epidemic.
This is evident in data compiled for a model called the “HIV Care Continuum,” or HIV “Cascade of Care.” The model captures the sequential stages of HIV care from diagnosis to suppression of the virus. It was developed in 2011 by Dr. Edward Gardner, an infectious disease/HIV expert at Denver Public Health, and has since been used at the federal, state, and local levels to help identify gaps in HIV services.
Not too long ago, diagnosis was the biggest problem in reducing the public health burden of HIV. Today, the biggest problem is linking and keeping individuals in care. According to the latest analysis by the U.S. Centers for Disease Control and Prevention of the HIV Care Continuum, of the 1.2 million people estimated to be living with HIV in America in 2011, approximately 86% were diagnosed, but only 40% were linked to and stayed in care, 37% were prescribed antiretroviral therapy (ART), and 30% had achieved viral suppression.

Only 30% of Americans living with HIV infection today are effectively treated, according to these data, even though we have the drugs and drug regimens available to treat everyone effectively.
Other analyses have included an additional stage of being initially linked to care (rather than being linked to care and retained in care). This presentation of the cascade, or continuum, further illuminates the progressive drop-off and that shows why an effective, sustained linkage to care is a critical component to ending the HIV epidemic.
One of these studies – an analysis published in 2013 – showed that approximately 82% of people were diagnosed, 66% were linked to care, 37% were retained in care, 33% were prescribed antiretroviral therapy, and 25% had a suppressed viral load of 200 copies/mL or less (JAMA. Intern. Med. 2013;173:1337-44).
With regard to women specifically, the CDC estimates that one in four people living with HIV infection are women, and that only about half of the women who are diagnosed with the infection are staying in care. Even fewer – 4 in 10 – have viral suppression, according to the CDC.
Expanding the management of HIV in the primary care setting could move us closer to ensuring that everyone in the United States who is infected with HIV is aware of the infection, is committed to treatment, and is virologically suppressed.
Like other primary care physicians, ob.gyns often have some degree of long-term continuity with patients – or the ability to create such continuity – that can be helpful for ensuring treatment compliance.
Ob.gyns also have valuable contact with adolescents, who fare worse throughout the cascade and are significantly more likely than older individuals to have unknown infections. An analysis published in 2014 of data for youth ages 13-29 shows that only 40% of HIV-infected youth were aware of their diagnosis and that an estimated 6% or less of HIV-infected youth were virally suppressed (AIDS. Patient. Care. STDS. 2014;28:128-135).
HIV testing should occur much more frequently than a decade ago, given the move in 2006 by the CDC from targeted risk-based testing to routine opt-out testing for all patients aged 13-64.
Treatment, moreover, has become much simpler in many respects. We have available to us more than 30 different drugs for individualizing therapy and providing treatment that allows patients to live a natural lifetime.
While such a large array of options may require those ob.gyns. who see only a few HIV-infected patients a year to work in consultation with an expert, many of the regimens require only a single, once-a-day pill. And while there was much debate as recently as five years ago about when to start treatment, there now is consensus that treatment should be started immediately after diagnosis (even in pregnant women), rather than waiting for the immune system to show signs of decline.
In fact, there is growing evidence that early treatment is key for both the infected individual and for individuals at risk. In the HIV Prevention Trials Network 052 study of discordant couples, for instance, early antiretroviral therapy in an infected partner not only reduced the number of clinical events; it almost completely blocked sexual transmission of the virus to an HIV-negative partner (N. Engl. J. Med. 2011;365:493-505).
The 052 study was a landmark “treatment as prevention” study. Other research has similarly shown that when the viral load of HIV-infected individuals is significantly reduced, their infectivity is reduced. And on a larger scale, research has shown that when we do this on a population basis, achieving widespread and continual treatment success, we can significantly impact the epidemic. This has been the case with the population of intravenous drug users in Vancouver, where the community viral load was significantly reduced by successful treatment that prevented new infections in this once-high-risk population.
Emerging data suggests that early diagnosis and treatment will likely also impact the likelihood of infected individuals achieving “functional cure.” The issue of functional cure – of achieving viral loads that are so low that drug therapy is no longer needed – has been receiving increasing attention in recent years, with the most promising findings reported thus far involving early treatment.
Tools for preexposure prophylaxis
For many years, we fit HIV care neatly into either the treatment or prevention category. More recently, we have come to appreciate that treatment is prevention, that a comprehensive prevention strategy must include treatment of infected individuals.
On the purely prevention side, it is important to continue educating women about safe sex behaviors. Most new HIV infections in women (84%) result from heterosexual contact, according to the CDC. For those who remain at risk of acquiring HIV despite education and counseling (eg., individuals who continue to engage in high-risk behaviors, or who have an HIV-positive partner), pre-exposure prophylaxis (PrEP) is now a safe and effective tool for preventing transmission. Patients deemed to be at high risk of acquiring HIV need to be made aware of this option.
PrEP originally was recommended only for gay or bisexual men, but in May 2014, the CDC recommended it for all individuals at risk and released the first comprehensive clinical practice guidelines for the prevention tool (www.cdc.gov/hiv/pdf/guidelines/PrEPguidelines2014.pdf).
The PrEP medication, Truvada, is a combination of two drugs (tenovovir and emtricitabine) that, when taken daily on a consistent basis, significantly reduces the risk of getting HIV infection. Several large national and international studies have documented risk reductions of 73% to 92% when the medication was taken every day or almost every day. It is clearly within the purview of any ob.gyn to prescribe, monitor, and manage such prevention therapy.
The availability and relative ease of such a tool, along with advances in treatment and knowledge gained from the HIV Care Continuum, should re-energize ob.gyns. to up the ante in efforts to end the epidemic.
Experience in our clinical program that provides care and treatment to patients in the Baltimore-Washington area has taught us that we do much better when we integrate HIV care within primary care. It’s much more likely that patients will “stay close” with their ob.gyn than to another specialist.
Certainly, HIV infection has its “hot spots” and areas of much lower prevalence, but regardless of where we reside, we must continue to appreciate that the epidemic has had a significant impact on women and that this will persist unless we can all better utilize our available tools, such as early diagnosis and effective treatment that are linked long-term with other primary care physicians.
For women, ob.gyns represent a great resource for our nation to make progress toward President Obama’s National HIV Strategy.
Dr. Redfield reported that he has no disclosures relevant to this Master Class.
HIV treatment adherence still a challenge
It’s hard to believe that it was 30 years ago that HIV was discovered as the cause of AIDS by Dr. Robert Gallo and Dr. Luc Montagnier. Since then, the medical community has focused on preventing and eradicating the virus and its transmission. Despite the advent of highly efficacious antiretroviral therapy, and education efforts to prevent transmission, the disease continues to cause significant morbidity and mortality.
Surveillance data from the Centers for Disease Control and Prevention have indicated that screening and prevention efforts led to a decline in perinatally acquired HIV and AIDS by 80% and 93%, respectively. However, we still have far to go.
The CDC estimated that in 2010 more than 1 million people over age 13 were living with HIV, and approximately 50,000 new cases of HIV occur each year in the United States.
President Obama’s National HIV/AIDS Strategy for the United States, released in 2010, set ambitious goals for eradicating the disease in our country. We can only hope to achieve the President’s aims if the fight against the disease is taken up by all health care professionals, on multiple fronts, and throughout the many stages of a patient’s health.
In a 2011 Master Class, we addressed the importance of ob.gyns. testing nonpregnant women for HIV, as well as employing HIV prevention strategies to keep our female patients healthy, and prevent potential mother-to-baby transmission of the virus. Although transmission has decreased significantly, helping patients follow their treatment regimens remains a major barrier to eradicating the disease.
Ob.gyns. may be the only physicians who many women see throughout their lives. Therefore, we have a unique opportunity to educate our patients about seeking appropriate care and the need for adhering to treatment regimens.
Our guest author this month is Dr. Robert R. Redfield Jr., a distinguished professor in the department of medicine at the University of Maryland, Baltimore, and associate director of the university’s Institute of Human Virology, with clinical and research programs in virtually all countries in the continent of Africa. Dr. Redfield will discuss the role that physicians can play in terms of linking patients to care as a means of treating those with HIV and reducing the burden of disease. Dr. Redfield’s expertise in the area of novel therapeutics for the treatment of the virus, and his clinical experience in treating patients, provides a unique perspective into this important public health issue.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
It’s hard to believe that it was 30 years ago that HIV was discovered as the cause of AIDS by Dr. Robert Gallo and Dr. Luc Montagnier. Since then, the medical community has focused on preventing and eradicating the virus and its transmission. Despite the advent of highly efficacious antiretroviral therapy, and education efforts to prevent transmission, the disease continues to cause significant morbidity and mortality.
Surveillance data from the Centers for Disease Control and Prevention have indicated that screening and prevention efforts led to a decline in perinatally acquired HIV and AIDS by 80% and 93%, respectively. However, we still have far to go.
The CDC estimated that in 2010 more than 1 million people over age 13 were living with HIV, and approximately 50,000 new cases of HIV occur each year in the United States.
President Obama’s National HIV/AIDS Strategy for the United States, released in 2010, set ambitious goals for eradicating the disease in our country. We can only hope to achieve the President’s aims if the fight against the disease is taken up by all health care professionals, on multiple fronts, and throughout the many stages of a patient’s health.
In a 2011 Master Class, we addressed the importance of ob.gyns. testing nonpregnant women for HIV, as well as employing HIV prevention strategies to keep our female patients healthy, and prevent potential mother-to-baby transmission of the virus. Although transmission has decreased significantly, helping patients follow their treatment regimens remains a major barrier to eradicating the disease.
Ob.gyns. may be the only physicians who many women see throughout their lives. Therefore, we have a unique opportunity to educate our patients about seeking appropriate care and the need for adhering to treatment regimens.
Our guest author this month is Dr. Robert R. Redfield Jr., a distinguished professor in the department of medicine at the University of Maryland, Baltimore, and associate director of the university’s Institute of Human Virology, with clinical and research programs in virtually all countries in the continent of Africa. Dr. Redfield will discuss the role that physicians can play in terms of linking patients to care as a means of treating those with HIV and reducing the burden of disease. Dr. Redfield’s expertise in the area of novel therapeutics for the treatment of the virus, and his clinical experience in treating patients, provides a unique perspective into this important public health issue.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
It’s hard to believe that it was 30 years ago that HIV was discovered as the cause of AIDS by Dr. Robert Gallo and Dr. Luc Montagnier. Since then, the medical community has focused on preventing and eradicating the virus and its transmission. Despite the advent of highly efficacious antiretroviral therapy, and education efforts to prevent transmission, the disease continues to cause significant morbidity and mortality.
Surveillance data from the Centers for Disease Control and Prevention have indicated that screening and prevention efforts led to a decline in perinatally acquired HIV and AIDS by 80% and 93%, respectively. However, we still have far to go.
The CDC estimated that in 2010 more than 1 million people over age 13 were living with HIV, and approximately 50,000 new cases of HIV occur each year in the United States.
President Obama’s National HIV/AIDS Strategy for the United States, released in 2010, set ambitious goals for eradicating the disease in our country. We can only hope to achieve the President’s aims if the fight against the disease is taken up by all health care professionals, on multiple fronts, and throughout the many stages of a patient’s health.
In a 2011 Master Class, we addressed the importance of ob.gyns. testing nonpregnant women for HIV, as well as employing HIV prevention strategies to keep our female patients healthy, and prevent potential mother-to-baby transmission of the virus. Although transmission has decreased significantly, helping patients follow their treatment regimens remains a major barrier to eradicating the disease.
Ob.gyns. may be the only physicians who many women see throughout their lives. Therefore, we have a unique opportunity to educate our patients about seeking appropriate care and the need for adhering to treatment regimens.
Our guest author this month is Dr. Robert R. Redfield Jr., a distinguished professor in the department of medicine at the University of Maryland, Baltimore, and associate director of the university’s Institute of Human Virology, with clinical and research programs in virtually all countries in the continent of Africa. Dr. Redfield will discuss the role that physicians can play in terms of linking patients to care as a means of treating those with HIV and reducing the burden of disease. Dr. Redfield’s expertise in the area of novel therapeutics for the treatment of the virus, and his clinical experience in treating patients, provides a unique perspective into this important public health issue.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
Selling the better mousetrap
Despite all the hoopla about Ebola and measles this winter, the most common reason for admitting an infant or young child to the hospital continues to be bronchiolitis. Yet clinical practice guidelines for diagnosing and treating this common infection have not been incorporated into clinical practice.
The use of over-the-counter cold medications to treat upper respiratory infections in young children was shown by meta-analysis in the mid-1990’s to be ineffective, but that use continued until the Food and Drug Administration mandated revisions to packaging in 2008. Antibiotics have been commonly prescribed to treat the ear infections and sinusitis that frequently occur with bronchiolitis. But over the past 20 years, the use of antibiotics has become less prevalent. I date that trend to the work of Dr. Jack Paradise, professor emeritus of pediatrics at the University of Pittsburgh, and Dr. Ellen Wald, now chair of pediatrics at the University of Wisconsin, Madison, in the mid-1990’s. RespiGam was approved in 1996, then supplanted with palivizumab, as a medication to reduce the burden of respiratory syncytial virus disease. In the summer of 2014, an updated analysis of the costs, risks, and benefits of RSV prophylaxis led to new recommendations that curtailed the indications for that treatment (Pediatrics 2014:134;415-20). What do these trends have in common? The time frame.
It is often cited that it takes 17 years for new evidence to be assimilated into clinical practice (J.R. Soc. Med. 2011;104:510-20). An Institute of Medicine report in 2001, “Crossing the Quality Chasm,” emphasized the importance of becoming more efficient at making progress. Those recommendations themselves are now 14 years old, and I’m not expecting a revolution in human behavior within the next 3 years.
In the new clinical practice guideline issued by the American Academy of Pediatrics in November 2014 for the treatment of young children with bronchiolitis, Dr. Shawn L. Ralston and her colleagues assessed various treatment modalities, found many to be ineffective, and recommended discontinuing their routine use (Pediatrics 2014;134:e1474-e1502). Beta-agonists were at the forefront of this. Was the new guideline based on new data? For the most part, no. In my reading, itmostly reiterated the concerns about effectiveness that were expressed at the time of the prior guidelines from 2006, but removed the weasel words. I admire the dedication of this committee to evidence-based medicine. But will this revised clinical practice guideline actually change practice?
The saying is, “Build a better mousetrap and the world will beat a path to your door.” That quote has been attributed (without adequate documentation) to Ralph Waldo Emerson. He was a great poet, but not a scientist.
During the same month that the new bronchiolitis guidelinewas being released, America held some elections. In the post mortem, President Obama said, “There is a tendency sometimes for me to start thinking: As long as I get the policy right, then that’s what should matter.” He elaborated that “one thing that I do need to constantly remind myself and my team of is it’s not enough just to build the better mousetrap. People don’t automatically come beating to your door. We’ve got to sell it; we’ve got to reach out to the other side and where possible, persuade” (The Wall Street Journal, Nov. 10, 2014).
That isn’t poetry, but the President’s idea is probably more accurate than Emerson’s.
The bronchiolitis clinical practice guidelinewas written in a standardized fashion with 14 key action statements and 242 references. That makes for a good evidence-based medicine document, but is not the best sales pitch.
What will it take to translate these new guidelines into practice? One option is to teach new residents the new guidelines and expect dinosaurs such as myself to retire. If the average pediatrician works for about 34 years, then over a period of 17 years, we will have replaced half the miscreants simply by attrition.
A program of reaching out to the other side and persuading them to change is a better option.
In discussions about this topic on a listserv for pediatric hospitalists, I focused on my concerns. We need to clarify the harms associated with therapies such as beta-agonists, deep nasal suctioning, and continuous pulse oximetry. We need to clarify the goals of treatment, which might include a shorter length of stay, patient comfort, meeting parents’ expectations that we will do something, and/or explaining why we are contradicting any previous recommendations made to the parents. We need to mesh these bronchiolitis guidelines with the asthma action plans and medication lists advocated for wheezing children who are 24 months of age. My colleagues pointed out that all of that is just continuing to refine the policy.
Getting the policy right is necessary but insufficient. What we are really missing is a campaign strategy to sell it.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. He is also listserv moderator for the American Academy of Pediatrics Section on Hospital Medicine.
Despite all the hoopla about Ebola and measles this winter, the most common reason for admitting an infant or young child to the hospital continues to be bronchiolitis. Yet clinical practice guidelines for diagnosing and treating this common infection have not been incorporated into clinical practice.
The use of over-the-counter cold medications to treat upper respiratory infections in young children was shown by meta-analysis in the mid-1990’s to be ineffective, but that use continued until the Food and Drug Administration mandated revisions to packaging in 2008. Antibiotics have been commonly prescribed to treat the ear infections and sinusitis that frequently occur with bronchiolitis. But over the past 20 years, the use of antibiotics has become less prevalent. I date that trend to the work of Dr. Jack Paradise, professor emeritus of pediatrics at the University of Pittsburgh, and Dr. Ellen Wald, now chair of pediatrics at the University of Wisconsin, Madison, in the mid-1990’s. RespiGam was approved in 1996, then supplanted with palivizumab, as a medication to reduce the burden of respiratory syncytial virus disease. In the summer of 2014, an updated analysis of the costs, risks, and benefits of RSV prophylaxis led to new recommendations that curtailed the indications for that treatment (Pediatrics 2014:134;415-20). What do these trends have in common? The time frame.
It is often cited that it takes 17 years for new evidence to be assimilated into clinical practice (J.R. Soc. Med. 2011;104:510-20). An Institute of Medicine report in 2001, “Crossing the Quality Chasm,” emphasized the importance of becoming more efficient at making progress. Those recommendations themselves are now 14 years old, and I’m not expecting a revolution in human behavior within the next 3 years.
In the new clinical practice guideline issued by the American Academy of Pediatrics in November 2014 for the treatment of young children with bronchiolitis, Dr. Shawn L. Ralston and her colleagues assessed various treatment modalities, found many to be ineffective, and recommended discontinuing their routine use (Pediatrics 2014;134:e1474-e1502). Beta-agonists were at the forefront of this. Was the new guideline based on new data? For the most part, no. In my reading, itmostly reiterated the concerns about effectiveness that were expressed at the time of the prior guidelines from 2006, but removed the weasel words. I admire the dedication of this committee to evidence-based medicine. But will this revised clinical practice guideline actually change practice?
The saying is, “Build a better mousetrap and the world will beat a path to your door.” That quote has been attributed (without adequate documentation) to Ralph Waldo Emerson. He was a great poet, but not a scientist.
During the same month that the new bronchiolitis guidelinewas being released, America held some elections. In the post mortem, President Obama said, “There is a tendency sometimes for me to start thinking: As long as I get the policy right, then that’s what should matter.” He elaborated that “one thing that I do need to constantly remind myself and my team of is it’s not enough just to build the better mousetrap. People don’t automatically come beating to your door. We’ve got to sell it; we’ve got to reach out to the other side and where possible, persuade” (The Wall Street Journal, Nov. 10, 2014).
That isn’t poetry, but the President’s idea is probably more accurate than Emerson’s.
The bronchiolitis clinical practice guidelinewas written in a standardized fashion with 14 key action statements and 242 references. That makes for a good evidence-based medicine document, but is not the best sales pitch.
What will it take to translate these new guidelines into practice? One option is to teach new residents the new guidelines and expect dinosaurs such as myself to retire. If the average pediatrician works for about 34 years, then over a period of 17 years, we will have replaced half the miscreants simply by attrition.
A program of reaching out to the other side and persuading them to change is a better option.
In discussions about this topic on a listserv for pediatric hospitalists, I focused on my concerns. We need to clarify the harms associated with therapies such as beta-agonists, deep nasal suctioning, and continuous pulse oximetry. We need to clarify the goals of treatment, which might include a shorter length of stay, patient comfort, meeting parents’ expectations that we will do something, and/or explaining why we are contradicting any previous recommendations made to the parents. We need to mesh these bronchiolitis guidelines with the asthma action plans and medication lists advocated for wheezing children who are 24 months of age. My colleagues pointed out that all of that is just continuing to refine the policy.
Getting the policy right is necessary but insufficient. What we are really missing is a campaign strategy to sell it.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. He is also listserv moderator for the American Academy of Pediatrics Section on Hospital Medicine.
Despite all the hoopla about Ebola and measles this winter, the most common reason for admitting an infant or young child to the hospital continues to be bronchiolitis. Yet clinical practice guidelines for diagnosing and treating this common infection have not been incorporated into clinical practice.
The use of over-the-counter cold medications to treat upper respiratory infections in young children was shown by meta-analysis in the mid-1990’s to be ineffective, but that use continued until the Food and Drug Administration mandated revisions to packaging in 2008. Antibiotics have been commonly prescribed to treat the ear infections and sinusitis that frequently occur with bronchiolitis. But over the past 20 years, the use of antibiotics has become less prevalent. I date that trend to the work of Dr. Jack Paradise, professor emeritus of pediatrics at the University of Pittsburgh, and Dr. Ellen Wald, now chair of pediatrics at the University of Wisconsin, Madison, in the mid-1990’s. RespiGam was approved in 1996, then supplanted with palivizumab, as a medication to reduce the burden of respiratory syncytial virus disease. In the summer of 2014, an updated analysis of the costs, risks, and benefits of RSV prophylaxis led to new recommendations that curtailed the indications for that treatment (Pediatrics 2014:134;415-20). What do these trends have in common? The time frame.
It is often cited that it takes 17 years for new evidence to be assimilated into clinical practice (J.R. Soc. Med. 2011;104:510-20). An Institute of Medicine report in 2001, “Crossing the Quality Chasm,” emphasized the importance of becoming more efficient at making progress. Those recommendations themselves are now 14 years old, and I’m not expecting a revolution in human behavior within the next 3 years.
In the new clinical practice guideline issued by the American Academy of Pediatrics in November 2014 for the treatment of young children with bronchiolitis, Dr. Shawn L. Ralston and her colleagues assessed various treatment modalities, found many to be ineffective, and recommended discontinuing their routine use (Pediatrics 2014;134:e1474-e1502). Beta-agonists were at the forefront of this. Was the new guideline based on new data? For the most part, no. In my reading, itmostly reiterated the concerns about effectiveness that were expressed at the time of the prior guidelines from 2006, but removed the weasel words. I admire the dedication of this committee to evidence-based medicine. But will this revised clinical practice guideline actually change practice?
The saying is, “Build a better mousetrap and the world will beat a path to your door.” That quote has been attributed (without adequate documentation) to Ralph Waldo Emerson. He was a great poet, but not a scientist.
During the same month that the new bronchiolitis guidelinewas being released, America held some elections. In the post mortem, President Obama said, “There is a tendency sometimes for me to start thinking: As long as I get the policy right, then that’s what should matter.” He elaborated that “one thing that I do need to constantly remind myself and my team of is it’s not enough just to build the better mousetrap. People don’t automatically come beating to your door. We’ve got to sell it; we’ve got to reach out to the other side and where possible, persuade” (The Wall Street Journal, Nov. 10, 2014).
That isn’t poetry, but the President’s idea is probably more accurate than Emerson’s.
The bronchiolitis clinical practice guidelinewas written in a standardized fashion with 14 key action statements and 242 references. That makes for a good evidence-based medicine document, but is not the best sales pitch.
What will it take to translate these new guidelines into practice? One option is to teach new residents the new guidelines and expect dinosaurs such as myself to retire. If the average pediatrician works for about 34 years, then over a period of 17 years, we will have replaced half the miscreants simply by attrition.
A program of reaching out to the other side and persuading them to change is a better option.
In discussions about this topic on a listserv for pediatric hospitalists, I focused on my concerns. We need to clarify the harms associated with therapies such as beta-agonists, deep nasal suctioning, and continuous pulse oximetry. We need to clarify the goals of treatment, which might include a shorter length of stay, patient comfort, meeting parents’ expectations that we will do something, and/or explaining why we are contradicting any previous recommendations made to the parents. We need to mesh these bronchiolitis guidelines with the asthma action plans and medication lists advocated for wheezing children who are 24 months of age. My colleagues pointed out that all of that is just continuing to refine the policy.
Getting the policy right is necessary but insufficient. What we are really missing is a campaign strategy to sell it.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. He is also listserv moderator for the American Academy of Pediatrics Section on Hospital Medicine.
Too sick to work?
By yesterday at lunch time, you knew you were sick. The sniffly nose and scratchy throat of the previous 2 days were maturing into a full-blown cold or worse. You woke this morning feeling achy and a bit feverish. The only thermometer in the house is a scary looking thing in the cutlery drawer next to the kitchen stove. There have been no cases of influenza reported in the country or even the state.
It is Thursday, and it is your partner’s traditional day off. You think you remember him saying that he was planning on driving out of state to visit his daughter who was struggling in her freshman year in college. Your new associate is in St. Louis taking her boards. The questions that need to be answered by 7:30 this morning are: Do I see if I can reach my partner before he leaves town and ask him if can work for me? If he is already on the road, do I call the office and tell them to cancel the day’s schedule because I am too sick to work?
This is the kind of scenario that most have us have faced more than once in our working lives. Who will I be putting at risk by going to work when I am sick? Of course, there are my patients. Is my patient population particularly fragile because of their age or immunological vulnerabilities? And there are my coworkers. Last of all, will going to work make me even sicker so that I will miss more work?
Where do you go for help in answering the question of whether you are too sick to go to work? Should you try to find a thermometer at an all-night convenience store? If you find one, exactly what temperature is the threshold that will prompt you to call in sick? How many sneezes per hour will render you too contagious to work? How many coughs? If your illness is primarily gastrointestinal, are you still a threat to your patients if your trips to the bathroom are spaced far enough apart to allow you to spend 15 minutes trapped in an examining room?
Would wearing a mask be of any benefit? My sense is that it wouldn’t help and may make you more of a threat if you keeping fiddling with it to readjust it for comfort. And a mask will certainly alarm some parents.
There are situations in which you look or sound worse than you are. I seem to develop laryngitis several days after the worst of my cold has passed. Unfortunately, this scenario is not one of those situations. If you show up in the office, you are going to sound and maybe look like you are as sick as you feel.
What are you going to do? I am embarrassed to admit that I was one of those masochists who would have gone to work regardless of my state of health. You would have had to tether me to an IV bottle to keep me at home. As a recovering workaholic, I have had to accept the fact that I may have jeopardized the health of some of my patients by my pigheaded and at times selfish devotion to showing up in the office come hell or high fever. But, on days when I was the only show in town, it was easy to fall into the trap of believing that I was indispensable. Although full-time emergency room physicians and hospitalists hadn’t been invented yet, there were a few other primary care physicians. I guess it was pride that prevented me from admitting that I could have called on them for help, even though they weren’t board certified pediatricians.
On the other hand, I still wonder how much harm I did by dragging myself to work when I was sick. Because Brunswick is a small town, I know my overly intense devotion to work didn’t result in any deaths. But how great was the collateral damage in the form of lost days from school and work for my patients, their parents, and my coworkers? There is no way to know, but I am sure there was some.
It is unreasonable to say, “I won’t ever go to work if I am ill.” I may have set the bar too high, but I am interested to hear how you decide when you are too sick to go to work.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “Coping with a Picky Eater.” E-mail him at [email protected].
By yesterday at lunch time, you knew you were sick. The sniffly nose and scratchy throat of the previous 2 days were maturing into a full-blown cold or worse. You woke this morning feeling achy and a bit feverish. The only thermometer in the house is a scary looking thing in the cutlery drawer next to the kitchen stove. There have been no cases of influenza reported in the country or even the state.
It is Thursday, and it is your partner’s traditional day off. You think you remember him saying that he was planning on driving out of state to visit his daughter who was struggling in her freshman year in college. Your new associate is in St. Louis taking her boards. The questions that need to be answered by 7:30 this morning are: Do I see if I can reach my partner before he leaves town and ask him if can work for me? If he is already on the road, do I call the office and tell them to cancel the day’s schedule because I am too sick to work?
This is the kind of scenario that most have us have faced more than once in our working lives. Who will I be putting at risk by going to work when I am sick? Of course, there are my patients. Is my patient population particularly fragile because of their age or immunological vulnerabilities? And there are my coworkers. Last of all, will going to work make me even sicker so that I will miss more work?
Where do you go for help in answering the question of whether you are too sick to go to work? Should you try to find a thermometer at an all-night convenience store? If you find one, exactly what temperature is the threshold that will prompt you to call in sick? How many sneezes per hour will render you too contagious to work? How many coughs? If your illness is primarily gastrointestinal, are you still a threat to your patients if your trips to the bathroom are spaced far enough apart to allow you to spend 15 minutes trapped in an examining room?
Would wearing a mask be of any benefit? My sense is that it wouldn’t help and may make you more of a threat if you keeping fiddling with it to readjust it for comfort. And a mask will certainly alarm some parents.
There are situations in which you look or sound worse than you are. I seem to develop laryngitis several days after the worst of my cold has passed. Unfortunately, this scenario is not one of those situations. If you show up in the office, you are going to sound and maybe look like you are as sick as you feel.
What are you going to do? I am embarrassed to admit that I was one of those masochists who would have gone to work regardless of my state of health. You would have had to tether me to an IV bottle to keep me at home. As a recovering workaholic, I have had to accept the fact that I may have jeopardized the health of some of my patients by my pigheaded and at times selfish devotion to showing up in the office come hell or high fever. But, on days when I was the only show in town, it was easy to fall into the trap of believing that I was indispensable. Although full-time emergency room physicians and hospitalists hadn’t been invented yet, there were a few other primary care physicians. I guess it was pride that prevented me from admitting that I could have called on them for help, even though they weren’t board certified pediatricians.
On the other hand, I still wonder how much harm I did by dragging myself to work when I was sick. Because Brunswick is a small town, I know my overly intense devotion to work didn’t result in any deaths. But how great was the collateral damage in the form of lost days from school and work for my patients, their parents, and my coworkers? There is no way to know, but I am sure there was some.
It is unreasonable to say, “I won’t ever go to work if I am ill.” I may have set the bar too high, but I am interested to hear how you decide when you are too sick to go to work.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “Coping with a Picky Eater.” E-mail him at [email protected].
By yesterday at lunch time, you knew you were sick. The sniffly nose and scratchy throat of the previous 2 days were maturing into a full-blown cold or worse. You woke this morning feeling achy and a bit feverish. The only thermometer in the house is a scary looking thing in the cutlery drawer next to the kitchen stove. There have been no cases of influenza reported in the country or even the state.
It is Thursday, and it is your partner’s traditional day off. You think you remember him saying that he was planning on driving out of state to visit his daughter who was struggling in her freshman year in college. Your new associate is in St. Louis taking her boards. The questions that need to be answered by 7:30 this morning are: Do I see if I can reach my partner before he leaves town and ask him if can work for me? If he is already on the road, do I call the office and tell them to cancel the day’s schedule because I am too sick to work?
This is the kind of scenario that most have us have faced more than once in our working lives. Who will I be putting at risk by going to work when I am sick? Of course, there are my patients. Is my patient population particularly fragile because of their age or immunological vulnerabilities? And there are my coworkers. Last of all, will going to work make me even sicker so that I will miss more work?
Where do you go for help in answering the question of whether you are too sick to go to work? Should you try to find a thermometer at an all-night convenience store? If you find one, exactly what temperature is the threshold that will prompt you to call in sick? How many sneezes per hour will render you too contagious to work? How many coughs? If your illness is primarily gastrointestinal, are you still a threat to your patients if your trips to the bathroom are spaced far enough apart to allow you to spend 15 minutes trapped in an examining room?
Would wearing a mask be of any benefit? My sense is that it wouldn’t help and may make you more of a threat if you keeping fiddling with it to readjust it for comfort. And a mask will certainly alarm some parents.
There are situations in which you look or sound worse than you are. I seem to develop laryngitis several days after the worst of my cold has passed. Unfortunately, this scenario is not one of those situations. If you show up in the office, you are going to sound and maybe look like you are as sick as you feel.
What are you going to do? I am embarrassed to admit that I was one of those masochists who would have gone to work regardless of my state of health. You would have had to tether me to an IV bottle to keep me at home. As a recovering workaholic, I have had to accept the fact that I may have jeopardized the health of some of my patients by my pigheaded and at times selfish devotion to showing up in the office come hell or high fever. But, on days when I was the only show in town, it was easy to fall into the trap of believing that I was indispensable. Although full-time emergency room physicians and hospitalists hadn’t been invented yet, there were a few other primary care physicians. I guess it was pride that prevented me from admitting that I could have called on them for help, even though they weren’t board certified pediatricians.
On the other hand, I still wonder how much harm I did by dragging myself to work when I was sick. Because Brunswick is a small town, I know my overly intense devotion to work didn’t result in any deaths. But how great was the collateral damage in the form of lost days from school and work for my patients, their parents, and my coworkers? There is no way to know, but I am sure there was some.
It is unreasonable to say, “I won’t ever go to work if I am ill.” I may have set the bar too high, but I am interested to hear how you decide when you are too sick to go to work.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “Coping with a Picky Eater.” E-mail him at [email protected].
The 2014 AAP COID update on palivizumab
The American Academy of Pediatrics Committee on Infectious Diseases released a 2014 policy statement (Pediatrics 2014:134;415-20) updating guidance on use of palivizumab in high-risk patients infected with respiratory syncytial virus (RSV). The policy statement was accompanied by a detailed technical report to explain the committee’s rationale for the changes made since the previous update in 2012.
Since 2012, several observational studies were published related to risk factors associated with the need for hospitalization for RSV infection. The Committee on Infectious Diseases (COID) referred to these observational studies, along with other historical studies, in its 2014 technical report.
Some aspects of the updated guidance have met with controversy because the new advice limits palivizumab use among premature infants. The 2014 COID policy asserts that preterm infants born at greater than 29 weeks’ gestational age (GA) do not benefit substantially from palivizumab dosing during RSV season. This assertion has met with controversy because the justification detailed in the technical report cites several observational studies that don’t appear to support that conclusion. What percentage of a cohort would need to be hospitalized for RSV infection to identify them as high risk? COID doesn’t draw that line for us. Because approximately 3% of the term birth cohort is hospitalized with RSV, should we use this as the “baseline” and predetermine a percentage of hospitalization beyond baseline to define “at increased risk”?
The 2014 technical report states that RSV hospitalization rates from Stevens et al. (Arch. Pediatr. Adolesc. Med. 2000;154:55-61) were 7.5% and 4.4% for infants 28-30 weeks’ gestational age (GA) and 30-32 weeks’ GA, respectively. However, the rates quoted by COID were not the generalizable rates reported by Dr. T. P. Stevens and his team in their study. The authors calculated community-wide hospitalization rates for these groups to be 10% and 6.4%, respectively. These author-reported community-wide rates closely mirror rates reported in several other large observational studies, and still likely underestimate the true burden of RSV hospitalization based on the lower sensitivity of RSV detection testing done 15 years ago when that study was performed.
Similarly, the COID states that Dr. A.G. Winterstein’s study (JAMA Pediatrics 2013;167:1118-24) shows hospitalization rates among 32-34 weeks’ GA infants of 3.1% and 4.5% in two states based on health care insurance claims. Because fewer than half of infants are ever tested for RSV as the possible cause of their lower respiratory tract infection, these claims data are most certainly an underestimate of true rates. When active testing for RSV is done among hospitalized infants in this same GA category, using sensitive and specific PCR-based technology, hospitalization rates are 9.1%. Dr. Winterstein and associates also reported that RSV hospitalization rates increase as gestational age decreases.
In Dr. Caroline Breese Hall’s 2013 observational study (Pediatrics 2013;132:341-8) she and her associates recognized that hospitalization rates in children under 2 years of age were similar among term and preterm infants. The COID used these data in its 2014 technical report to suggest that preterm infants are no longer at high risk. The detail omitted from the report was that approximately 70% of eligible preterm infants in Dr. Hall’s study (according to the 2012 COID guidelines) had received palivizumab prophylaxis. This suggests that maintaining RSV prophylaxis according to the 2012 COID policy statement (and not the 2014 policy statement) can be successful in reducing the rate of RSV hospitalizations in preterm infants to that observed in term infants.
Finally, the technical report cites “overall declining incidence of hospitalizations for bronchiolitis in the United States” from the 2013 publication by Hasegawa et al. (Pediatrics 2013:132:28-36) without defining this observation for the AAP readers. While Hasegawa did report such a decline, the decline was specific to term infants, as no such decline was observed in the higher-risk preterm infants.
Based on careful review of several of the manuscripts referenced by COID, it’s difficult to determine why the committee’s recommendations changed so dramatically in the most recent iteration. It appears that the data need another look.
Dr. Domachowske is professor of pediatrics and professor of microbiology and immunology at the State University of New York Upstate Medical University in Syracuse. He serves on the New York State American Academy of Pediatrics Chapter 1 executive committee, volunteers as his district’s immunization champion, and is an appointed member of the New York State Immunization Advisory Council. He is a Pediatric News Editorial Advisory Board member. Dr. Domachowske disclosed he does consulting for the vaccine divisions of GlaxoSmithKline, Medimmune, Pfizer, Merck, Sanofi Pasteur, and Novartis; he performs clinical trials with GlaxoSmithKline, Medimmune, Merck, and Novartis; and he does basic and translational research with GlaxoSmithKline. E-mail him at [email protected].
The American Academy of Pediatrics Committee on Infectious Diseases released a 2014 policy statement (Pediatrics 2014:134;415-20) updating guidance on use of palivizumab in high-risk patients infected with respiratory syncytial virus (RSV). The policy statement was accompanied by a detailed technical report to explain the committee’s rationale for the changes made since the previous update in 2012.
Since 2012, several observational studies were published related to risk factors associated with the need for hospitalization for RSV infection. The Committee on Infectious Diseases (COID) referred to these observational studies, along with other historical studies, in its 2014 technical report.
Some aspects of the updated guidance have met with controversy because the new advice limits palivizumab use among premature infants. The 2014 COID policy asserts that preterm infants born at greater than 29 weeks’ gestational age (GA) do not benefit substantially from palivizumab dosing during RSV season. This assertion has met with controversy because the justification detailed in the technical report cites several observational studies that don’t appear to support that conclusion. What percentage of a cohort would need to be hospitalized for RSV infection to identify them as high risk? COID doesn’t draw that line for us. Because approximately 3% of the term birth cohort is hospitalized with RSV, should we use this as the “baseline” and predetermine a percentage of hospitalization beyond baseline to define “at increased risk”?
The 2014 technical report states that RSV hospitalization rates from Stevens et al. (Arch. Pediatr. Adolesc. Med. 2000;154:55-61) were 7.5% and 4.4% for infants 28-30 weeks’ gestational age (GA) and 30-32 weeks’ GA, respectively. However, the rates quoted by COID were not the generalizable rates reported by Dr. T. P. Stevens and his team in their study. The authors calculated community-wide hospitalization rates for these groups to be 10% and 6.4%, respectively. These author-reported community-wide rates closely mirror rates reported in several other large observational studies, and still likely underestimate the true burden of RSV hospitalization based on the lower sensitivity of RSV detection testing done 15 years ago when that study was performed.
Similarly, the COID states that Dr. A.G. Winterstein’s study (JAMA Pediatrics 2013;167:1118-24) shows hospitalization rates among 32-34 weeks’ GA infants of 3.1% and 4.5% in two states based on health care insurance claims. Because fewer than half of infants are ever tested for RSV as the possible cause of their lower respiratory tract infection, these claims data are most certainly an underestimate of true rates. When active testing for RSV is done among hospitalized infants in this same GA category, using sensitive and specific PCR-based technology, hospitalization rates are 9.1%. Dr. Winterstein and associates also reported that RSV hospitalization rates increase as gestational age decreases.
In Dr. Caroline Breese Hall’s 2013 observational study (Pediatrics 2013;132:341-8) she and her associates recognized that hospitalization rates in children under 2 years of age were similar among term and preterm infants. The COID used these data in its 2014 technical report to suggest that preterm infants are no longer at high risk. The detail omitted from the report was that approximately 70% of eligible preterm infants in Dr. Hall’s study (according to the 2012 COID guidelines) had received palivizumab prophylaxis. This suggests that maintaining RSV prophylaxis according to the 2012 COID policy statement (and not the 2014 policy statement) can be successful in reducing the rate of RSV hospitalizations in preterm infants to that observed in term infants.
Finally, the technical report cites “overall declining incidence of hospitalizations for bronchiolitis in the United States” from the 2013 publication by Hasegawa et al. (Pediatrics 2013:132:28-36) without defining this observation for the AAP readers. While Hasegawa did report such a decline, the decline was specific to term infants, as no such decline was observed in the higher-risk preterm infants.
Based on careful review of several of the manuscripts referenced by COID, it’s difficult to determine why the committee’s recommendations changed so dramatically in the most recent iteration. It appears that the data need another look.
Dr. Domachowske is professor of pediatrics and professor of microbiology and immunology at the State University of New York Upstate Medical University in Syracuse. He serves on the New York State American Academy of Pediatrics Chapter 1 executive committee, volunteers as his district’s immunization champion, and is an appointed member of the New York State Immunization Advisory Council. He is a Pediatric News Editorial Advisory Board member. Dr. Domachowske disclosed he does consulting for the vaccine divisions of GlaxoSmithKline, Medimmune, Pfizer, Merck, Sanofi Pasteur, and Novartis; he performs clinical trials with GlaxoSmithKline, Medimmune, Merck, and Novartis; and he does basic and translational research with GlaxoSmithKline. E-mail him at [email protected].
The American Academy of Pediatrics Committee on Infectious Diseases released a 2014 policy statement (Pediatrics 2014:134;415-20) updating guidance on use of palivizumab in high-risk patients infected with respiratory syncytial virus (RSV). The policy statement was accompanied by a detailed technical report to explain the committee’s rationale for the changes made since the previous update in 2012.
Since 2012, several observational studies were published related to risk factors associated with the need for hospitalization for RSV infection. The Committee on Infectious Diseases (COID) referred to these observational studies, along with other historical studies, in its 2014 technical report.
Some aspects of the updated guidance have met with controversy because the new advice limits palivizumab use among premature infants. The 2014 COID policy asserts that preterm infants born at greater than 29 weeks’ gestational age (GA) do not benefit substantially from palivizumab dosing during RSV season. This assertion has met with controversy because the justification detailed in the technical report cites several observational studies that don’t appear to support that conclusion. What percentage of a cohort would need to be hospitalized for RSV infection to identify them as high risk? COID doesn’t draw that line for us. Because approximately 3% of the term birth cohort is hospitalized with RSV, should we use this as the “baseline” and predetermine a percentage of hospitalization beyond baseline to define “at increased risk”?
The 2014 technical report states that RSV hospitalization rates from Stevens et al. (Arch. Pediatr. Adolesc. Med. 2000;154:55-61) were 7.5% and 4.4% for infants 28-30 weeks’ gestational age (GA) and 30-32 weeks’ GA, respectively. However, the rates quoted by COID were not the generalizable rates reported by Dr. T. P. Stevens and his team in their study. The authors calculated community-wide hospitalization rates for these groups to be 10% and 6.4%, respectively. These author-reported community-wide rates closely mirror rates reported in several other large observational studies, and still likely underestimate the true burden of RSV hospitalization based on the lower sensitivity of RSV detection testing done 15 years ago when that study was performed.
Similarly, the COID states that Dr. A.G. Winterstein’s study (JAMA Pediatrics 2013;167:1118-24) shows hospitalization rates among 32-34 weeks’ GA infants of 3.1% and 4.5% in two states based on health care insurance claims. Because fewer than half of infants are ever tested for RSV as the possible cause of their lower respiratory tract infection, these claims data are most certainly an underestimate of true rates. When active testing for RSV is done among hospitalized infants in this same GA category, using sensitive and specific PCR-based technology, hospitalization rates are 9.1%. Dr. Winterstein and associates also reported that RSV hospitalization rates increase as gestational age decreases.
In Dr. Caroline Breese Hall’s 2013 observational study (Pediatrics 2013;132:341-8) she and her associates recognized that hospitalization rates in children under 2 years of age were similar among term and preterm infants. The COID used these data in its 2014 technical report to suggest that preterm infants are no longer at high risk. The detail omitted from the report was that approximately 70% of eligible preterm infants in Dr. Hall’s study (according to the 2012 COID guidelines) had received palivizumab prophylaxis. This suggests that maintaining RSV prophylaxis according to the 2012 COID policy statement (and not the 2014 policy statement) can be successful in reducing the rate of RSV hospitalizations in preterm infants to that observed in term infants.
Finally, the technical report cites “overall declining incidence of hospitalizations for bronchiolitis in the United States” from the 2013 publication by Hasegawa et al. (Pediatrics 2013:132:28-36) without defining this observation for the AAP readers. While Hasegawa did report such a decline, the decline was specific to term infants, as no such decline was observed in the higher-risk preterm infants.
Based on careful review of several of the manuscripts referenced by COID, it’s difficult to determine why the committee’s recommendations changed so dramatically in the most recent iteration. It appears that the data need another look.
Dr. Domachowske is professor of pediatrics and professor of microbiology and immunology at the State University of New York Upstate Medical University in Syracuse. He serves on the New York State American Academy of Pediatrics Chapter 1 executive committee, volunteers as his district’s immunization champion, and is an appointed member of the New York State Immunization Advisory Council. He is a Pediatric News Editorial Advisory Board member. Dr. Domachowske disclosed he does consulting for the vaccine divisions of GlaxoSmithKline, Medimmune, Pfizer, Merck, Sanofi Pasteur, and Novartis; he performs clinical trials with GlaxoSmithKline, Medimmune, Merck, and Novartis; and he does basic and translational research with GlaxoSmithKline. E-mail him at [email protected].