User login
Bad blood: Could brain bleeds be contagious?
This transcript has been edited for clarity.
How do you tell if a condition is caused by an infection?
It seems like an obvious question, right? In the post–van Leeuwenhoek era we can look at whatever part of the body is diseased under a microscope and see microbes – you know, the usual suspects.
Except when we can’t. And there are plenty of cases where we can’t: where the microbe is too small to be seen without more advanced imaging techniques, like with viruses; or when the pathogen is sparsely populated or hard to culture, like Mycobacterium.
Finding out that a condition is the result of an infection is not only an exercise for 19th century physicians. After all, it was 2008 when Barry Marshall and Robin Warren won their Nobel Prize for proving that stomach ulcers, long thought to be due to “stress,” were actually caused by a tiny microbe called Helicobacter pylori.
And this week, we are looking at a study which, once again, begins to suggest that a condition thought to be more or less random – cerebral amyloid angiopathy – may actually be the result of an infectious disease.
We’re talking about this paper, appearing in JAMA, which is just a great example of old-fashioned shoe-leather epidemiology. But let’s get up to speed on cerebral amyloid angiopathy (CAA) first.
CAA is characterized by the deposition of amyloid protein in the brain. While there are some genetic causes, they are quite rare, and most cases are thought to be idiopathic. Recent analyses suggest that somewhere between 5% and 7% of cognitively normal older adults have CAA, but the rate is much higher among those with intracerebral hemorrhage – brain bleeds. In fact, CAA is the second-most common cause of bleeding in the brain, second only to severe hypertension.
An article in Nature highlights cases that seemed to develop after the administration of cadaveric pituitary hormone.
Other studies have shown potential transmission via dura mater grafts and neurosurgical instruments. But despite those clues, no infectious organism has been identified. Some have suggested that the long latent period and difficulty of finding a responsible microbe points to a prion-like disease not yet known. But these studies are more or less case series. The new JAMA paper gives us, if not a smoking gun, a pretty decent set of fingerprints.
Here’s the idea: If CAA is caused by some infectious agent, it may be transmitted in the blood. We know that a decent percentage of people who have spontaneous brain bleeds have CAA. If those people donated blood in the past, maybe the people who received that blood would be at risk for brain bleeds too.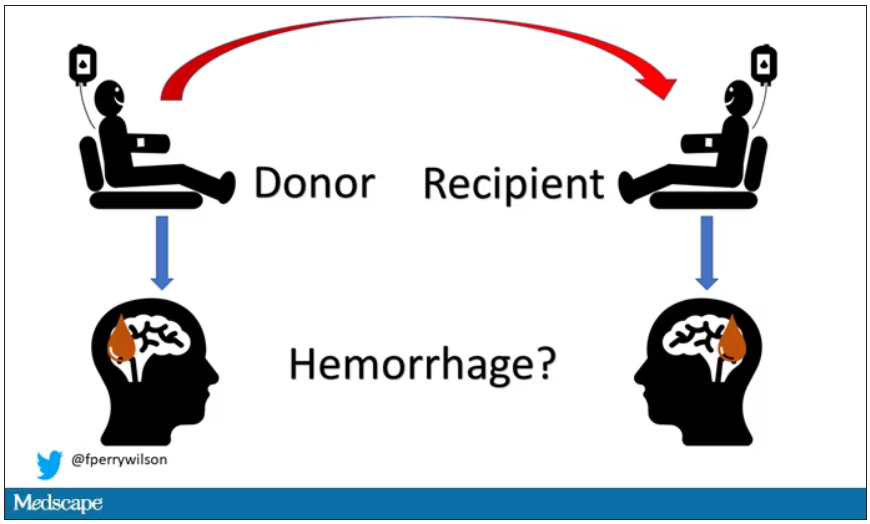
Of course, to really test that hypothesis, you’d need to know who every blood donor in a country was and every person who received that blood and all their subsequent diagnoses for basically their entire lives. No one has that kind of data, right?
Well, if you’ve been watching this space, you’ll know that a few countries do. Enter Sweden and Denmark, with their national electronic health record that captures all of this information, and much more, on every single person who lives or has lived in those countries since before 1970. Unbelievable.
So that’s exactly what the researchers, led by Jingchen Zhao at Karolinska (Sweden) University, did. They identified roughly 760,000 individuals in Sweden and 330,000 people in Denmark who had received a blood transfusion between 1970 and 2017.
Of course, most of those blood donors – 99% of them, actually – never went on to have any bleeding in the brain. It is a rare thing, fortunately.
But some of the donors did, on average within about 5 years of the time they donated blood. The researchers characterized each donor as either never having a brain bleed, having a single bleed, or having multiple bleeds. The latter is most strongly associated with CAA.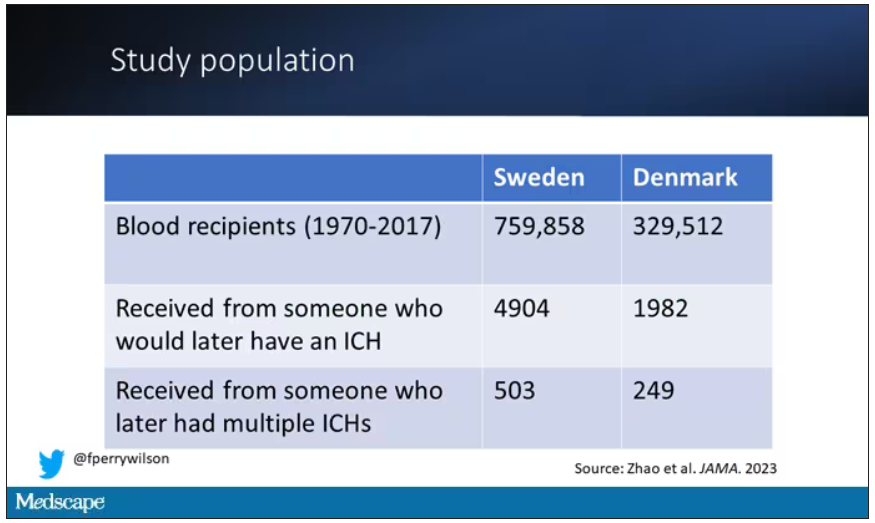
The big question: Would recipients who got blood from individuals who later on had brain bleeds, have brain bleeds themselves?
The answer is yes, though with an asterisk. You can see the results here. The risk of recipients having a brain bleed was lowest if the blood they received was from people who never had a brain bleed, higher if the individual had a single brain bleed, and highest if they got blood from a donor who would go on to have multiple brain bleeds.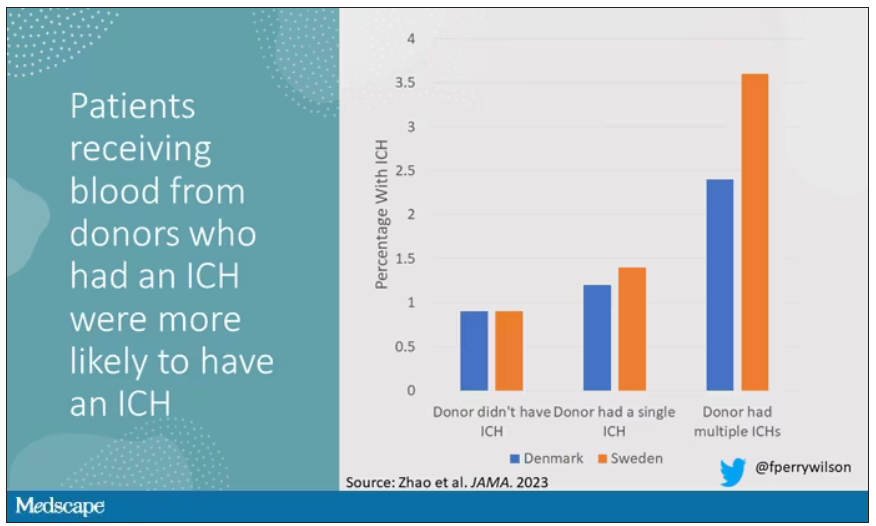
All in all, individuals who received blood from someone who would later have multiple hemorrhages were three times more likely to themselves develop bleeds themselves. It’s fairly compelling evidence of a transmissible agent.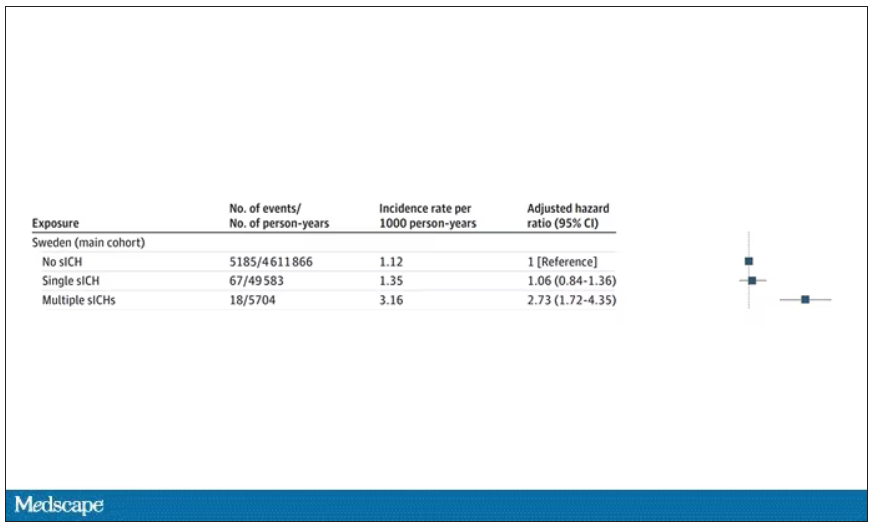
Of course, there are some potential confounders to consider here. Whose blood you get is not totally random. If, for example, people with type O blood are just more likely to have brain bleeds, then you could get results like this, as type O tends to donate to type O and both groups would have higher risk after donation. But the authors adjusted for blood type. They also adjusted for number of transfusions, calendar year, age, sex, and indication for transfusion.
Perhaps most compelling, and most clever, is that they used ischemic stroke as a negative control. Would people who received blood from someone who later had an ischemic stroke themselves be more likely to go on to have an ischemic stroke? No signal at all. It does not appear that there is a transmissible agent associated with ischemic stroke – only the brain bleeds.
I know what you’re thinking. What’s the agent? What’s the microbe, or virus, or prion, or toxin? The study gives us no insight there. These nationwide databases are awesome but they can only do so much. Because of the vagaries of medical coding and the difficulty of making the CAA diagnosis, the authors are using brain bleeds as a proxy here; we don’t even know for sure whether these were CAA-associated brain bleeds.
It’s also worth noting that there’s little we can do about this. None of the blood donors in this study had a brain bleed prior to donation; it’s not like we could screen people out of donating in the future. We have no test for whatever this agent is, if it even exists, nor do we have a potential treatment. Fortunately, whatever it is, it is extremely rare.
Still, this paper feels like a shot across the bow. At this point, the probability has shifted strongly away from CAA being a purely random disease and toward it being an infectious one. It may be time to round up some of the unusual suspects.
Dr. F. Perry Wilson is an associate professor of medicine and public health and director of Yale University’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
How do you tell if a condition is caused by an infection?
It seems like an obvious question, right? In the post–van Leeuwenhoek era we can look at whatever part of the body is diseased under a microscope and see microbes – you know, the usual suspects.
Except when we can’t. And there are plenty of cases where we can’t: where the microbe is too small to be seen without more advanced imaging techniques, like with viruses; or when the pathogen is sparsely populated or hard to culture, like Mycobacterium.
Finding out that a condition is the result of an infection is not only an exercise for 19th century physicians. After all, it was 2008 when Barry Marshall and Robin Warren won their Nobel Prize for proving that stomach ulcers, long thought to be due to “stress,” were actually caused by a tiny microbe called Helicobacter pylori.
And this week, we are looking at a study which, once again, begins to suggest that a condition thought to be more or less random – cerebral amyloid angiopathy – may actually be the result of an infectious disease.
We’re talking about this paper, appearing in JAMA, which is just a great example of old-fashioned shoe-leather epidemiology. But let’s get up to speed on cerebral amyloid angiopathy (CAA) first.
CAA is characterized by the deposition of amyloid protein in the brain. While there are some genetic causes, they are quite rare, and most cases are thought to be idiopathic. Recent analyses suggest that somewhere between 5% and 7% of cognitively normal older adults have CAA, but the rate is much higher among those with intracerebral hemorrhage – brain bleeds. In fact, CAA is the second-most common cause of bleeding in the brain, second only to severe hypertension.
An article in Nature highlights cases that seemed to develop after the administration of cadaveric pituitary hormone.
Other studies have shown potential transmission via dura mater grafts and neurosurgical instruments. But despite those clues, no infectious organism has been identified. Some have suggested that the long latent period and difficulty of finding a responsible microbe points to a prion-like disease not yet known. But these studies are more or less case series. The new JAMA paper gives us, if not a smoking gun, a pretty decent set of fingerprints.
Here’s the idea: If CAA is caused by some infectious agent, it may be transmitted in the blood. We know that a decent percentage of people who have spontaneous brain bleeds have CAA. If those people donated blood in the past, maybe the people who received that blood would be at risk for brain bleeds too.
Of course, to really test that hypothesis, you’d need to know who every blood donor in a country was and every person who received that blood and all their subsequent diagnoses for basically their entire lives. No one has that kind of data, right?
Well, if you’ve been watching this space, you’ll know that a few countries do. Enter Sweden and Denmark, with their national electronic health record that captures all of this information, and much more, on every single person who lives or has lived in those countries since before 1970. Unbelievable.
So that’s exactly what the researchers, led by Jingchen Zhao at Karolinska (Sweden) University, did. They identified roughly 760,000 individuals in Sweden and 330,000 people in Denmark who had received a blood transfusion between 1970 and 2017.
Of course, most of those blood donors – 99% of them, actually – never went on to have any bleeding in the brain. It is a rare thing, fortunately.
But some of the donors did, on average within about 5 years of the time they donated blood. The researchers characterized each donor as either never having a brain bleed, having a single bleed, or having multiple bleeds. The latter is most strongly associated with CAA.
The big question: Would recipients who got blood from individuals who later on had brain bleeds, have brain bleeds themselves?
The answer is yes, though with an asterisk. You can see the results here. The risk of recipients having a brain bleed was lowest if the blood they received was from people who never had a brain bleed, higher if the individual had a single brain bleed, and highest if they got blood from a donor who would go on to have multiple brain bleeds.
All in all, individuals who received blood from someone who would later have multiple hemorrhages were three times more likely to themselves develop bleeds themselves. It’s fairly compelling evidence of a transmissible agent.
Of course, there are some potential confounders to consider here. Whose blood you get is not totally random. If, for example, people with type O blood are just more likely to have brain bleeds, then you could get results like this, as type O tends to donate to type O and both groups would have higher risk after donation. But the authors adjusted for blood type. They also adjusted for number of transfusions, calendar year, age, sex, and indication for transfusion.
Perhaps most compelling, and most clever, is that they used ischemic stroke as a negative control. Would people who received blood from someone who later had an ischemic stroke themselves be more likely to go on to have an ischemic stroke? No signal at all. It does not appear that there is a transmissible agent associated with ischemic stroke – only the brain bleeds.
I know what you’re thinking. What’s the agent? What’s the microbe, or virus, or prion, or toxin? The study gives us no insight there. These nationwide databases are awesome but they can only do so much. Because of the vagaries of medical coding and the difficulty of making the CAA diagnosis, the authors are using brain bleeds as a proxy here; we don’t even know for sure whether these were CAA-associated brain bleeds.
It’s also worth noting that there’s little we can do about this. None of the blood donors in this study had a brain bleed prior to donation; it’s not like we could screen people out of donating in the future. We have no test for whatever this agent is, if it even exists, nor do we have a potential treatment. Fortunately, whatever it is, it is extremely rare.
Still, this paper feels like a shot across the bow. At this point, the probability has shifted strongly away from CAA being a purely random disease and toward it being an infectious one. It may be time to round up some of the unusual suspects.
Dr. F. Perry Wilson is an associate professor of medicine and public health and director of Yale University’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
How do you tell if a condition is caused by an infection?
It seems like an obvious question, right? In the post–van Leeuwenhoek era we can look at whatever part of the body is diseased under a microscope and see microbes – you know, the usual suspects.
Except when we can’t. And there are plenty of cases where we can’t: where the microbe is too small to be seen without more advanced imaging techniques, like with viruses; or when the pathogen is sparsely populated or hard to culture, like Mycobacterium.
Finding out that a condition is the result of an infection is not only an exercise for 19th century physicians. After all, it was 2008 when Barry Marshall and Robin Warren won their Nobel Prize for proving that stomach ulcers, long thought to be due to “stress,” were actually caused by a tiny microbe called Helicobacter pylori.
And this week, we are looking at a study which, once again, begins to suggest that a condition thought to be more or less random – cerebral amyloid angiopathy – may actually be the result of an infectious disease.
We’re talking about this paper, appearing in JAMA, which is just a great example of old-fashioned shoe-leather epidemiology. But let’s get up to speed on cerebral amyloid angiopathy (CAA) first.
CAA is characterized by the deposition of amyloid protein in the brain. While there are some genetic causes, they are quite rare, and most cases are thought to be idiopathic. Recent analyses suggest that somewhere between 5% and 7% of cognitively normal older adults have CAA, but the rate is much higher among those with intracerebral hemorrhage – brain bleeds. In fact, CAA is the second-most common cause of bleeding in the brain, second only to severe hypertension.
An article in Nature highlights cases that seemed to develop after the administration of cadaveric pituitary hormone.
Other studies have shown potential transmission via dura mater grafts and neurosurgical instruments. But despite those clues, no infectious organism has been identified. Some have suggested that the long latent period and difficulty of finding a responsible microbe points to a prion-like disease not yet known. But these studies are more or less case series. The new JAMA paper gives us, if not a smoking gun, a pretty decent set of fingerprints.
Here’s the idea: If CAA is caused by some infectious agent, it may be transmitted in the blood. We know that a decent percentage of people who have spontaneous brain bleeds have CAA. If those people donated blood in the past, maybe the people who received that blood would be at risk for brain bleeds too.
Of course, to really test that hypothesis, you’d need to know who every blood donor in a country was and every person who received that blood and all their subsequent diagnoses for basically their entire lives. No one has that kind of data, right?
Well, if you’ve been watching this space, you’ll know that a few countries do. Enter Sweden and Denmark, with their national electronic health record that captures all of this information, and much more, on every single person who lives or has lived in those countries since before 1970. Unbelievable.
So that’s exactly what the researchers, led by Jingchen Zhao at Karolinska (Sweden) University, did. They identified roughly 760,000 individuals in Sweden and 330,000 people in Denmark who had received a blood transfusion between 1970 and 2017.
Of course, most of those blood donors – 99% of them, actually – never went on to have any bleeding in the brain. It is a rare thing, fortunately.
But some of the donors did, on average within about 5 years of the time they donated blood. The researchers characterized each donor as either never having a brain bleed, having a single bleed, or having multiple bleeds. The latter is most strongly associated with CAA.
The big question: Would recipients who got blood from individuals who later on had brain bleeds, have brain bleeds themselves?
The answer is yes, though with an asterisk. You can see the results here. The risk of recipients having a brain bleed was lowest if the blood they received was from people who never had a brain bleed, higher if the individual had a single brain bleed, and highest if they got blood from a donor who would go on to have multiple brain bleeds.
All in all, individuals who received blood from someone who would later have multiple hemorrhages were three times more likely to themselves develop bleeds themselves. It’s fairly compelling evidence of a transmissible agent.
Of course, there are some potential confounders to consider here. Whose blood you get is not totally random. If, for example, people with type O blood are just more likely to have brain bleeds, then you could get results like this, as type O tends to donate to type O and both groups would have higher risk after donation. But the authors adjusted for blood type. They also adjusted for number of transfusions, calendar year, age, sex, and indication for transfusion.
Perhaps most compelling, and most clever, is that they used ischemic stroke as a negative control. Would people who received blood from someone who later had an ischemic stroke themselves be more likely to go on to have an ischemic stroke? No signal at all. It does not appear that there is a transmissible agent associated with ischemic stroke – only the brain bleeds.
I know what you’re thinking. What’s the agent? What’s the microbe, or virus, or prion, or toxin? The study gives us no insight there. These nationwide databases are awesome but they can only do so much. Because of the vagaries of medical coding and the difficulty of making the CAA diagnosis, the authors are using brain bleeds as a proxy here; we don’t even know for sure whether these were CAA-associated brain bleeds.
It’s also worth noting that there’s little we can do about this. None of the blood donors in this study had a brain bleed prior to donation; it’s not like we could screen people out of donating in the future. We have no test for whatever this agent is, if it even exists, nor do we have a potential treatment. Fortunately, whatever it is, it is extremely rare.
Still, this paper feels like a shot across the bow. At this point, the probability has shifted strongly away from CAA being a purely random disease and toward it being an infectious one. It may be time to round up some of the unusual suspects.
Dr. F. Perry Wilson is an associate professor of medicine and public health and director of Yale University’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Disenfranchised grief: What it looks like, where it goes
What happens to grief when those around you don’t understand it? Where does it go? How do you process it?
Disenfranchised grief, when someone or society more generally doesn’t see a loss as worthy of mourning, can deprive people of experiencing or processing their sadness. This grief, which may be triggered by the death of an ex-spouse, a pet, a failed adoption, can be painful and long-lasting.
Suzanne Cole, MD: ‘I didn’t feel the right to grieve’
During the COVID-19 pandemic, my little sister unexpectedly died. Though she was not one of the nearly 7 million people who died of the virus, in 2021 she became another type of statistic: one of the 109,699 people in the United State who died from a drug overdose. Hers was from fentanyl laced with methamphetamines.
Her death unraveled me. I felt deep guilt that I could not pull her from the sweeping current that had wrenched her from mainstream society into the underbelly of sex work and toward the solace of mind-altering drugs.
But I did not feel the right to grieve for her as I have grieved for other loved ones who were not blamed for their exit from this world. My sister was living a sordid life on the fringes of society. My grief felt invalid, undeserved. Yet, in the eyes of other “upstanding citizens,” her life was not as worth grieving – or so I thought. I tucked my sorrow into a small corner of my soul so no one would see, and I carried on.
To this day, the shame I feel robbed me of the ability to freely talk about her or share the searing pain I feel. Tears still prick my eyes when I think of her, but I have become adept at swallowing them, shaking off the waves of grief as though nothing happened. Even now, I cannot shake the pervasive feeling that my silent tears don’t deserve to be wept.
Don S. Dizon, MD: Working through tragedy
As a medical student, I worked with an outpatient physician as part of a third-year rotation. When we met, the first thing that struck me was how disheveled he looked. His clothes were wrinkled, and his pants were baggy. He took cigarette breaks, which I found disturbing.
But I quickly came to admire him. Despite my first impression, he was the type of doctor I aspired to be. He didn’t need to look at a patient’s chart to recall who they were. He just knew them. He greeted patients warmly, asked about their family. He even remembered the special occasions his patients had mentioned since their past visit. He epitomized empathy and connectedness.
Spending one day in clinic brought to light the challenges of forming such bonds with patients. A man came into the cancer clinic reporting chest pain and was triaged to an exam room. Soon after, the patient was found unresponsive on the floor. Nurses were yelling for help, and the doctor ran in and started CPR while minutes ticked by waiting for an ambulance that could take him to the ED.
By the time help arrived, the patient was blue.
He had died in the clinic in the middle of the day, as the waiting room filled. After the body was taken away, the doctor went into the bathroom. About 20 minutes later, he came out, eyes bloodshot, and continued with the rest of his day, ensuring each patient was seen and cared for.
As a medical student, it hit me how hard it must be to see something so tragic like the end of a life and then continue with your day as if nothing had happened. This is an experience of grief I later came to know well after nearly 30 years treating patients with advanced cancers: compartmentalizing it and carrying on.
A space for grieving: The Schwartz Center Rounds
Disenfranchised grief, the grief that is hard to share and often seems wrong to feel in the first place, can be triggered in many situations. Losing a person others don’t believe deserve to be grieved, such as an abusive partner or someone who committed a crime; losing someone you cared for in a professional role; a loss experienced in a breakup or same-sex partnership, if that relationship was not accepted by one’s family; loss from infertility, miscarriage, stillbirth, or failed adoption; loss that may be taboo or stigmatized, such as deaths via suicide or abortion; and loss of a job, home, or possession that you treasure.
Many of us have had similar situations or will, and the feeling that no one understands the need to mourn can be paralyzing and alienating. In the early days, intense, crushing feelings can cause intrusive, distracting thoughts, and over time, that grief can linger and find a permanent place in our minds.
More and more, though, we are being given opportunities to reflect on these sad moments.
The Schwartz Rounds are an example of such an opportunity. In these rounds, we gather to talk about the experience of caring for people, not the science of medicine.
During one particularly powerful rounds, I spoke to my colleagues about my initial meeting with a patient who was very sick. I detailed the experience of telling her children and her at that initial consult how I thought she was dying and that I did not recommend therapy. I remember how they cried. And I remembered how powerless I felt.
As I recalled that memory during Schwartz Rounds, I could not stop from crying. The unfairness of being a physician meeting someone for the first time and having to tell them such bad news overwhelmed me.
Even more poignant, I had the chance to reconnect with this woman’s children, who were present that day, not as audience members but as participants. Their presence may have brought my emotions to the surface more strongly. In that moment, I could show them the feelings I had bottled up for the sake of professionalism. Ultimately, I felt relieved, freer somehow, as if this burden my soul was carrying had been lifted.
Although we are both grateful for forums like this, these opportunities to share and express the grief we may have hidden away are not as common as they should be.
As physicians, we may express grief by shedding tears at the bedside of a patient nearing the end of life or through the anxiety we feel when our patient suffers a severe reaction to treatment. But we tend to put it away, to go on with our day, because there are others to be seen and cared for and more work to be done. Somehow, we move forward, shedding tears in one room and celebrating victories in another.
We need to create more spaces to express and feel grief, so we don’t get lost in it. Because understanding how grief impacts us, as people and as providers, is one of the most important realizations we can make as we go about our time-honored profession as healers.
Dr. Dizon is the director of women’s cancers at Lifespan Cancer Institute, director of medical oncology at Rhode Island Hospital, and a professor of medicine at Brown University, all in Providence. He reported conflicts of interest with Regeneron, AstraZeneca, Clovis, Bristol-Myers Squibb, and Kazia.
A version of this article first appeared on Medscape.com.
What happens to grief when those around you don’t understand it? Where does it go? How do you process it?
Disenfranchised grief, when someone or society more generally doesn’t see a loss as worthy of mourning, can deprive people of experiencing or processing their sadness. This grief, which may be triggered by the death of an ex-spouse, a pet, a failed adoption, can be painful and long-lasting.
Suzanne Cole, MD: ‘I didn’t feel the right to grieve’
During the COVID-19 pandemic, my little sister unexpectedly died. Though she was not one of the nearly 7 million people who died of the virus, in 2021 she became another type of statistic: one of the 109,699 people in the United State who died from a drug overdose. Hers was from fentanyl laced with methamphetamines.
Her death unraveled me. I felt deep guilt that I could not pull her from the sweeping current that had wrenched her from mainstream society into the underbelly of sex work and toward the solace of mind-altering drugs.
But I did not feel the right to grieve for her as I have grieved for other loved ones who were not blamed for their exit from this world. My sister was living a sordid life on the fringes of society. My grief felt invalid, undeserved. Yet, in the eyes of other “upstanding citizens,” her life was not as worth grieving – or so I thought. I tucked my sorrow into a small corner of my soul so no one would see, and I carried on.
To this day, the shame I feel robbed me of the ability to freely talk about her or share the searing pain I feel. Tears still prick my eyes when I think of her, but I have become adept at swallowing them, shaking off the waves of grief as though nothing happened. Even now, I cannot shake the pervasive feeling that my silent tears don’t deserve to be wept.
Don S. Dizon, MD: Working through tragedy
As a medical student, I worked with an outpatient physician as part of a third-year rotation. When we met, the first thing that struck me was how disheveled he looked. His clothes were wrinkled, and his pants were baggy. He took cigarette breaks, which I found disturbing.
But I quickly came to admire him. Despite my first impression, he was the type of doctor I aspired to be. He didn’t need to look at a patient’s chart to recall who they were. He just knew them. He greeted patients warmly, asked about their family. He even remembered the special occasions his patients had mentioned since their past visit. He epitomized empathy and connectedness.
Spending one day in clinic brought to light the challenges of forming such bonds with patients. A man came into the cancer clinic reporting chest pain and was triaged to an exam room. Soon after, the patient was found unresponsive on the floor. Nurses were yelling for help, and the doctor ran in and started CPR while minutes ticked by waiting for an ambulance that could take him to the ED.
By the time help arrived, the patient was blue.
He had died in the clinic in the middle of the day, as the waiting room filled. After the body was taken away, the doctor went into the bathroom. About 20 minutes later, he came out, eyes bloodshot, and continued with the rest of his day, ensuring each patient was seen and cared for.
As a medical student, it hit me how hard it must be to see something so tragic like the end of a life and then continue with your day as if nothing had happened. This is an experience of grief I later came to know well after nearly 30 years treating patients with advanced cancers: compartmentalizing it and carrying on.
A space for grieving: The Schwartz Center Rounds
Disenfranchised grief, the grief that is hard to share and often seems wrong to feel in the first place, can be triggered in many situations. Losing a person others don’t believe deserve to be grieved, such as an abusive partner or someone who committed a crime; losing someone you cared for in a professional role; a loss experienced in a breakup or same-sex partnership, if that relationship was not accepted by one’s family; loss from infertility, miscarriage, stillbirth, or failed adoption; loss that may be taboo or stigmatized, such as deaths via suicide or abortion; and loss of a job, home, or possession that you treasure.
Many of us have had similar situations or will, and the feeling that no one understands the need to mourn can be paralyzing and alienating. In the early days, intense, crushing feelings can cause intrusive, distracting thoughts, and over time, that grief can linger and find a permanent place in our minds.
More and more, though, we are being given opportunities to reflect on these sad moments.
The Schwartz Rounds are an example of such an opportunity. In these rounds, we gather to talk about the experience of caring for people, not the science of medicine.
During one particularly powerful rounds, I spoke to my colleagues about my initial meeting with a patient who was very sick. I detailed the experience of telling her children and her at that initial consult how I thought she was dying and that I did not recommend therapy. I remember how they cried. And I remembered how powerless I felt.
As I recalled that memory during Schwartz Rounds, I could not stop from crying. The unfairness of being a physician meeting someone for the first time and having to tell them such bad news overwhelmed me.
Even more poignant, I had the chance to reconnect with this woman’s children, who were present that day, not as audience members but as participants. Their presence may have brought my emotions to the surface more strongly. In that moment, I could show them the feelings I had bottled up for the sake of professionalism. Ultimately, I felt relieved, freer somehow, as if this burden my soul was carrying had been lifted.
Although we are both grateful for forums like this, these opportunities to share and express the grief we may have hidden away are not as common as they should be.
As physicians, we may express grief by shedding tears at the bedside of a patient nearing the end of life or through the anxiety we feel when our patient suffers a severe reaction to treatment. But we tend to put it away, to go on with our day, because there are others to be seen and cared for and more work to be done. Somehow, we move forward, shedding tears in one room and celebrating victories in another.
We need to create more spaces to express and feel grief, so we don’t get lost in it. Because understanding how grief impacts us, as people and as providers, is one of the most important realizations we can make as we go about our time-honored profession as healers.
Dr. Dizon is the director of women’s cancers at Lifespan Cancer Institute, director of medical oncology at Rhode Island Hospital, and a professor of medicine at Brown University, all in Providence. He reported conflicts of interest with Regeneron, AstraZeneca, Clovis, Bristol-Myers Squibb, and Kazia.
A version of this article first appeared on Medscape.com.
What happens to grief when those around you don’t understand it? Where does it go? How do you process it?
Disenfranchised grief, when someone or society more generally doesn’t see a loss as worthy of mourning, can deprive people of experiencing or processing their sadness. This grief, which may be triggered by the death of an ex-spouse, a pet, a failed adoption, can be painful and long-lasting.
Suzanne Cole, MD: ‘I didn’t feel the right to grieve’
During the COVID-19 pandemic, my little sister unexpectedly died. Though she was not one of the nearly 7 million people who died of the virus, in 2021 she became another type of statistic: one of the 109,699 people in the United State who died from a drug overdose. Hers was from fentanyl laced with methamphetamines.
Her death unraveled me. I felt deep guilt that I could not pull her from the sweeping current that had wrenched her from mainstream society into the underbelly of sex work and toward the solace of mind-altering drugs.
But I did not feel the right to grieve for her as I have grieved for other loved ones who were not blamed for their exit from this world. My sister was living a sordid life on the fringes of society. My grief felt invalid, undeserved. Yet, in the eyes of other “upstanding citizens,” her life was not as worth grieving – or so I thought. I tucked my sorrow into a small corner of my soul so no one would see, and I carried on.
To this day, the shame I feel robbed me of the ability to freely talk about her or share the searing pain I feel. Tears still prick my eyes when I think of her, but I have become adept at swallowing them, shaking off the waves of grief as though nothing happened. Even now, I cannot shake the pervasive feeling that my silent tears don’t deserve to be wept.
Don S. Dizon, MD: Working through tragedy
As a medical student, I worked with an outpatient physician as part of a third-year rotation. When we met, the first thing that struck me was how disheveled he looked. His clothes were wrinkled, and his pants were baggy. He took cigarette breaks, which I found disturbing.
But I quickly came to admire him. Despite my first impression, he was the type of doctor I aspired to be. He didn’t need to look at a patient’s chart to recall who they were. He just knew them. He greeted patients warmly, asked about their family. He even remembered the special occasions his patients had mentioned since their past visit. He epitomized empathy and connectedness.
Spending one day in clinic brought to light the challenges of forming such bonds with patients. A man came into the cancer clinic reporting chest pain and was triaged to an exam room. Soon after, the patient was found unresponsive on the floor. Nurses were yelling for help, and the doctor ran in and started CPR while minutes ticked by waiting for an ambulance that could take him to the ED.
By the time help arrived, the patient was blue.
He had died in the clinic in the middle of the day, as the waiting room filled. After the body was taken away, the doctor went into the bathroom. About 20 minutes later, he came out, eyes bloodshot, and continued with the rest of his day, ensuring each patient was seen and cared for.
As a medical student, it hit me how hard it must be to see something so tragic like the end of a life and then continue with your day as if nothing had happened. This is an experience of grief I later came to know well after nearly 30 years treating patients with advanced cancers: compartmentalizing it and carrying on.
A space for grieving: The Schwartz Center Rounds
Disenfranchised grief, the grief that is hard to share and often seems wrong to feel in the first place, can be triggered in many situations. Losing a person others don’t believe deserve to be grieved, such as an abusive partner or someone who committed a crime; losing someone you cared for in a professional role; a loss experienced in a breakup or same-sex partnership, if that relationship was not accepted by one’s family; loss from infertility, miscarriage, stillbirth, or failed adoption; loss that may be taboo or stigmatized, such as deaths via suicide or abortion; and loss of a job, home, or possession that you treasure.
Many of us have had similar situations or will, and the feeling that no one understands the need to mourn can be paralyzing and alienating. In the early days, intense, crushing feelings can cause intrusive, distracting thoughts, and over time, that grief can linger and find a permanent place in our minds.
More and more, though, we are being given opportunities to reflect on these sad moments.
The Schwartz Rounds are an example of such an opportunity. In these rounds, we gather to talk about the experience of caring for people, not the science of medicine.
During one particularly powerful rounds, I spoke to my colleagues about my initial meeting with a patient who was very sick. I detailed the experience of telling her children and her at that initial consult how I thought she was dying and that I did not recommend therapy. I remember how they cried. And I remembered how powerless I felt.
As I recalled that memory during Schwartz Rounds, I could not stop from crying. The unfairness of being a physician meeting someone for the first time and having to tell them such bad news overwhelmed me.
Even more poignant, I had the chance to reconnect with this woman’s children, who were present that day, not as audience members but as participants. Their presence may have brought my emotions to the surface more strongly. In that moment, I could show them the feelings I had bottled up for the sake of professionalism. Ultimately, I felt relieved, freer somehow, as if this burden my soul was carrying had been lifted.
Although we are both grateful for forums like this, these opportunities to share and express the grief we may have hidden away are not as common as they should be.
As physicians, we may express grief by shedding tears at the bedside of a patient nearing the end of life or through the anxiety we feel when our patient suffers a severe reaction to treatment. But we tend to put it away, to go on with our day, because there are others to be seen and cared for and more work to be done. Somehow, we move forward, shedding tears in one room and celebrating victories in another.
We need to create more spaces to express and feel grief, so we don’t get lost in it. Because understanding how grief impacts us, as people and as providers, is one of the most important realizations we can make as we go about our time-honored profession as healers.
Dr. Dizon is the director of women’s cancers at Lifespan Cancer Institute, director of medical oncology at Rhode Island Hospital, and a professor of medicine at Brown University, all in Providence. He reported conflicts of interest with Regeneron, AstraZeneca, Clovis, Bristol-Myers Squibb, and Kazia.
A version of this article first appeared on Medscape.com.
Universal anxiety screening recommendation is a good start
A very good thing happened this summer for patients with anxiety and the psychiatrists, psychologists, and other mental health professionals who provide treatment for them. The U.S. Preventive Services Task Force recommended anxiety screening for all adults younger than 65.
On the surface, this is a great recommendation for recognition and caring for those who deal with and suffer from an anxiety disorder or multiple anxiety disorders. Although the USPSTF recommendations are independent of the U.S. government and are not an official position of the Department of Health & Human Services, they are a wonderful start at recognizing the importance of mental health care.
After all, anxiety disorders are the most commonly experienced and diagnosed mental disorders, according to the DSM-5.
They range mainly from generalized anxiety disorder (GAD), to panic attacks and panic disorder, separation anxiety, specific type phobias (bridges, tunnels, insects, snakes, and the list goes on), to other phobias, including agoraphobia, social phobia, and of course, anxiety caused by medical conditions. GAD alone occurs in, at least, more than 3% of the population.
Those of us who have been treating anxiety disorders for decades recognize them as an issue affecting both mental and physical well-being, not only because of the emotional causes but the physical distress and illnesses that anxiety may precipitate or worsen.
For example, blood pressure– and heart-related issues, GI disorders, and musculoskeletal issues are just a few examples of how our bodies and organ systems are affected by anxiety. Just the momentary physical symptoms of tachycardia or the “runs” before an exam are fine examples of how anxiety may affect patients physically, and an ongoing, consistent anxiety is potentially more harmful.
In fact, a first panic attack or episode of generalized anxiety may be so serious that an emergency department or physician visit is necessary to rule out a heart attack, asthma, or breathing issues – even a hormone or thyroid emergency, or a cardiac arrhythmia. Panic attacks alone create a high number of ED visits.
Treatments mainly include medication management and a variety of psychotherapy techniques. Currently, the most preferred, first-choice medications are the SSRI antidepressants, which are Food and Drug Administration approved for anxiety as well. These include Zoloft (sertraline), Prozac/Sarafem (fluoxetine), Celexa (citalopram), and Lexapro (escitalopram).
For many years, benzodiazepines (that is, tranquillizers) such as Valium (diazepam), Ativan (lorazepam), and Klonopin/Rivotril (clonazepam) to name a few, were the mainstay of anxiety treatment, but they have proven addictive and may affect cognition and memory. As the current opioid epidemic has shown, when combined with opioids, benzodiazepines are a potentially lethal combination and when used, they need to be for shorter-term care and monitored very judiciously.
It should be noted that after ongoing long-term use of an SSRI for anxiety or depression, it should not be stopped abruptly, as a variety of physical symptoms (for example, flu-like symptoms) may occur.
Benefits of nonmedicinal therapies
There are a variety of talk therapies, from dynamic psychotherapies to cognitive-behavioral therapies (CBT), plus relaxation techniques and guided imagery that have all had a good amount of success in treating generalized anxiety, panic disorder, as well as various types of phobias.
When medications are stopped, the anxiety symptoms may well return. But when using nonmedicinal therapies, clinicians have discovered that when patients develop a new perspective on the anxiety problem or have a new technique to treat anxiety, it may well be long lasting.
For me, using CBT, relaxation techniques, hypnosis, and guided imagery has been very successful in treating anxiety disorders with long-lasting results. Once a person learns to relax, whether it’s from deep breathing exercises, hypnosis (which is not sleep), mindfulness, or meditation, a strategy of guided imagery can be taught, which allows a person to practice as well as control their anxiety as a lifetime process. For example, I like imagining a large movie screen to desensitize and project anxieties.
In many instances, a combination of a medication and a talk therapy approach works best, but there are an equal number of instances in which just medication or just talk therapy is needed. Once again, knowledge, clinical judgment, and the art of care are required to make these assessments.
In other words, recognizing and treating anxiety requires highly specialized training, which is why I thought the USPSTF recommendations raise a few critical questions.
Questions and concerns
One issue, of course, is the exclusion of those patients over age 65 because of a lack of “data.” Why such an exclusion? Does this mean that data are lacking for this age group?
The concept of using solely evidenced-based data in psychiatry is itself an interesting concept because our profession, like many other medical specialties, requires practitioners to use a combination of art and science. And much can be said either way about the clarity of accuracy in the diversity of issues that arise when treating emotional disorders.
When looking at the over-65 population, has anyone thought of clinical knowledge, judgment, experience, observation, and, of course, common sense?
Just consider the worry (a cardinal feature of anxiety) that besets people over 65 when it comes to issues such as retirement, financial security, “empty nesting,” physical health issues, decreased socialization that resulted from the COVID-19 pandemic, and the perpetual loss of relatives and friends.
In addition, as we age, anxiety can come simply from the loss of identity as active lifestyles decrease and the reality of nearing life’s end becomes more of a reality. It would seem that this population would benefit enormously from anxiety screening and possible treatment.
Another major concern is that the screening and potential treatment of patients is aimed at primary care physicians. Putting the sole responsibility of providing mental health care on these overworked PCPs defies common sense unless we’re okay with 1- to 2-minute assessments of mental health issues and no doubt, a pharmacology-only approach.
If this follows the same route as well-intentioned PCPs treating depression, where 5-minute medication management is far too common, the only proper diagnostic course – the in-depth interview necessary to make a proper diagnosis – is often missing.
For example, in depression alone, it takes psychiatric experience and time to differentiate a major depressive disorder from a bipolar depression and to provide the appropriate medication and treatment plan with careful follow-up. In my experience, this usually does not happen in the exceedingly overworked, time-driven day of a PCP.
Anxiety disorders and depression can prove debilitating, and if a PCP wants the responsibility of treatment, a mandated mental health program should be followed – just as here in New York, prescribers are mandated to take a pain control, opioid, and infection control CME course to keep our licenses up to date.
Short of mandating a mental health program for PCPs, it should be part of training and CME courses that Psychiatry is a super specialty, much like orthopedics and ophthalmology, and primary care physicians should never hesitate to make referrals to the specialist.
The big picture for me, and I hope for us all, is that the USPSTF has started things rolling by making it clear that PCPs and other health care clinicians need to screen for anxiety as a disabling disorder that is quite treatable.
This approach will help to advance the destigmatization of mental health disorders. But as result, with more patients diagnosed, there will be a need for more psychiatrists – and psychologists with PhDs or PsyDs – to fill the gaps in mental health care.
Dr. London is a practicing psychiatrist and has been a newspaper columnist for 35 years, specializing in and writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
A very good thing happened this summer for patients with anxiety and the psychiatrists, psychologists, and other mental health professionals who provide treatment for them. The U.S. Preventive Services Task Force recommended anxiety screening for all adults younger than 65.
On the surface, this is a great recommendation for recognition and caring for those who deal with and suffer from an anxiety disorder or multiple anxiety disorders. Although the USPSTF recommendations are independent of the U.S. government and are not an official position of the Department of Health & Human Services, they are a wonderful start at recognizing the importance of mental health care.
After all, anxiety disorders are the most commonly experienced and diagnosed mental disorders, according to the DSM-5.
They range mainly from generalized anxiety disorder (GAD), to panic attacks and panic disorder, separation anxiety, specific type phobias (bridges, tunnels, insects, snakes, and the list goes on), to other phobias, including agoraphobia, social phobia, and of course, anxiety caused by medical conditions. GAD alone occurs in, at least, more than 3% of the population.
Those of us who have been treating anxiety disorders for decades recognize them as an issue affecting both mental and physical well-being, not only because of the emotional causes but the physical distress and illnesses that anxiety may precipitate or worsen.
For example, blood pressure– and heart-related issues, GI disorders, and musculoskeletal issues are just a few examples of how our bodies and organ systems are affected by anxiety. Just the momentary physical symptoms of tachycardia or the “runs” before an exam are fine examples of how anxiety may affect patients physically, and an ongoing, consistent anxiety is potentially more harmful.
In fact, a first panic attack or episode of generalized anxiety may be so serious that an emergency department or physician visit is necessary to rule out a heart attack, asthma, or breathing issues – even a hormone or thyroid emergency, or a cardiac arrhythmia. Panic attacks alone create a high number of ED visits.
Treatments mainly include medication management and a variety of psychotherapy techniques. Currently, the most preferred, first-choice medications are the SSRI antidepressants, which are Food and Drug Administration approved for anxiety as well. These include Zoloft (sertraline), Prozac/Sarafem (fluoxetine), Celexa (citalopram), and Lexapro (escitalopram).
For many years, benzodiazepines (that is, tranquillizers) such as Valium (diazepam), Ativan (lorazepam), and Klonopin/Rivotril (clonazepam) to name a few, were the mainstay of anxiety treatment, but they have proven addictive and may affect cognition and memory. As the current opioid epidemic has shown, when combined with opioids, benzodiazepines are a potentially lethal combination and when used, they need to be for shorter-term care and monitored very judiciously.
It should be noted that after ongoing long-term use of an SSRI for anxiety or depression, it should not be stopped abruptly, as a variety of physical symptoms (for example, flu-like symptoms) may occur.
Benefits of nonmedicinal therapies
There are a variety of talk therapies, from dynamic psychotherapies to cognitive-behavioral therapies (CBT), plus relaxation techniques and guided imagery that have all had a good amount of success in treating generalized anxiety, panic disorder, as well as various types of phobias.
When medications are stopped, the anxiety symptoms may well return. But when using nonmedicinal therapies, clinicians have discovered that when patients develop a new perspective on the anxiety problem or have a new technique to treat anxiety, it may well be long lasting.
For me, using CBT, relaxation techniques, hypnosis, and guided imagery has been very successful in treating anxiety disorders with long-lasting results. Once a person learns to relax, whether it’s from deep breathing exercises, hypnosis (which is not sleep), mindfulness, or meditation, a strategy of guided imagery can be taught, which allows a person to practice as well as control their anxiety as a lifetime process. For example, I like imagining a large movie screen to desensitize and project anxieties.
In many instances, a combination of a medication and a talk therapy approach works best, but there are an equal number of instances in which just medication or just talk therapy is needed. Once again, knowledge, clinical judgment, and the art of care are required to make these assessments.
In other words, recognizing and treating anxiety requires highly specialized training, which is why I thought the USPSTF recommendations raise a few critical questions.
Questions and concerns
One issue, of course, is the exclusion of those patients over age 65 because of a lack of “data.” Why such an exclusion? Does this mean that data are lacking for this age group?
The concept of using solely evidenced-based data in psychiatry is itself an interesting concept because our profession, like many other medical specialties, requires practitioners to use a combination of art and science. And much can be said either way about the clarity of accuracy in the diversity of issues that arise when treating emotional disorders.
When looking at the over-65 population, has anyone thought of clinical knowledge, judgment, experience, observation, and, of course, common sense?
Just consider the worry (a cardinal feature of anxiety) that besets people over 65 when it comes to issues such as retirement, financial security, “empty nesting,” physical health issues, decreased socialization that resulted from the COVID-19 pandemic, and the perpetual loss of relatives and friends.
In addition, as we age, anxiety can come simply from the loss of identity as active lifestyles decrease and the reality of nearing life’s end becomes more of a reality. It would seem that this population would benefit enormously from anxiety screening and possible treatment.
Another major concern is that the screening and potential treatment of patients is aimed at primary care physicians. Putting the sole responsibility of providing mental health care on these overworked PCPs defies common sense unless we’re okay with 1- to 2-minute assessments of mental health issues and no doubt, a pharmacology-only approach.
If this follows the same route as well-intentioned PCPs treating depression, where 5-minute medication management is far too common, the only proper diagnostic course – the in-depth interview necessary to make a proper diagnosis – is often missing.
For example, in depression alone, it takes psychiatric experience and time to differentiate a major depressive disorder from a bipolar depression and to provide the appropriate medication and treatment plan with careful follow-up. In my experience, this usually does not happen in the exceedingly overworked, time-driven day of a PCP.
Anxiety disorders and depression can prove debilitating, and if a PCP wants the responsibility of treatment, a mandated mental health program should be followed – just as here in New York, prescribers are mandated to take a pain control, opioid, and infection control CME course to keep our licenses up to date.
Short of mandating a mental health program for PCPs, it should be part of training and CME courses that Psychiatry is a super specialty, much like orthopedics and ophthalmology, and primary care physicians should never hesitate to make referrals to the specialist.
The big picture for me, and I hope for us all, is that the USPSTF has started things rolling by making it clear that PCPs and other health care clinicians need to screen for anxiety as a disabling disorder that is quite treatable.
This approach will help to advance the destigmatization of mental health disorders. But as result, with more patients diagnosed, there will be a need for more psychiatrists – and psychologists with PhDs or PsyDs – to fill the gaps in mental health care.
Dr. London is a practicing psychiatrist and has been a newspaper columnist for 35 years, specializing in and writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
A very good thing happened this summer for patients with anxiety and the psychiatrists, psychologists, and other mental health professionals who provide treatment for them. The U.S. Preventive Services Task Force recommended anxiety screening for all adults younger than 65.
On the surface, this is a great recommendation for recognition and caring for those who deal with and suffer from an anxiety disorder or multiple anxiety disorders. Although the USPSTF recommendations are independent of the U.S. government and are not an official position of the Department of Health & Human Services, they are a wonderful start at recognizing the importance of mental health care.
After all, anxiety disorders are the most commonly experienced and diagnosed mental disorders, according to the DSM-5.
They range mainly from generalized anxiety disorder (GAD), to panic attacks and panic disorder, separation anxiety, specific type phobias (bridges, tunnels, insects, snakes, and the list goes on), to other phobias, including agoraphobia, social phobia, and of course, anxiety caused by medical conditions. GAD alone occurs in, at least, more than 3% of the population.
Those of us who have been treating anxiety disorders for decades recognize them as an issue affecting both mental and physical well-being, not only because of the emotional causes but the physical distress and illnesses that anxiety may precipitate or worsen.
For example, blood pressure– and heart-related issues, GI disorders, and musculoskeletal issues are just a few examples of how our bodies and organ systems are affected by anxiety. Just the momentary physical symptoms of tachycardia or the “runs” before an exam are fine examples of how anxiety may affect patients physically, and an ongoing, consistent anxiety is potentially more harmful.
In fact, a first panic attack or episode of generalized anxiety may be so serious that an emergency department or physician visit is necessary to rule out a heart attack, asthma, or breathing issues – even a hormone or thyroid emergency, or a cardiac arrhythmia. Panic attacks alone create a high number of ED visits.
Treatments mainly include medication management and a variety of psychotherapy techniques. Currently, the most preferred, first-choice medications are the SSRI antidepressants, which are Food and Drug Administration approved for anxiety as well. These include Zoloft (sertraline), Prozac/Sarafem (fluoxetine), Celexa (citalopram), and Lexapro (escitalopram).
For many years, benzodiazepines (that is, tranquillizers) such as Valium (diazepam), Ativan (lorazepam), and Klonopin/Rivotril (clonazepam) to name a few, were the mainstay of anxiety treatment, but they have proven addictive and may affect cognition and memory. As the current opioid epidemic has shown, when combined with opioids, benzodiazepines are a potentially lethal combination and when used, they need to be for shorter-term care and monitored very judiciously.
It should be noted that after ongoing long-term use of an SSRI for anxiety or depression, it should not be stopped abruptly, as a variety of physical symptoms (for example, flu-like symptoms) may occur.
Benefits of nonmedicinal therapies
There are a variety of talk therapies, from dynamic psychotherapies to cognitive-behavioral therapies (CBT), plus relaxation techniques and guided imagery that have all had a good amount of success in treating generalized anxiety, panic disorder, as well as various types of phobias.
When medications are stopped, the anxiety symptoms may well return. But when using nonmedicinal therapies, clinicians have discovered that when patients develop a new perspective on the anxiety problem or have a new technique to treat anxiety, it may well be long lasting.
For me, using CBT, relaxation techniques, hypnosis, and guided imagery has been very successful in treating anxiety disorders with long-lasting results. Once a person learns to relax, whether it’s from deep breathing exercises, hypnosis (which is not sleep), mindfulness, or meditation, a strategy of guided imagery can be taught, which allows a person to practice as well as control their anxiety as a lifetime process. For example, I like imagining a large movie screen to desensitize and project anxieties.
In many instances, a combination of a medication and a talk therapy approach works best, but there are an equal number of instances in which just medication or just talk therapy is needed. Once again, knowledge, clinical judgment, and the art of care are required to make these assessments.
In other words, recognizing and treating anxiety requires highly specialized training, which is why I thought the USPSTF recommendations raise a few critical questions.
Questions and concerns
One issue, of course, is the exclusion of those patients over age 65 because of a lack of “data.” Why such an exclusion? Does this mean that data are lacking for this age group?
The concept of using solely evidenced-based data in psychiatry is itself an interesting concept because our profession, like many other medical specialties, requires practitioners to use a combination of art and science. And much can be said either way about the clarity of accuracy in the diversity of issues that arise when treating emotional disorders.
When looking at the over-65 population, has anyone thought of clinical knowledge, judgment, experience, observation, and, of course, common sense?
Just consider the worry (a cardinal feature of anxiety) that besets people over 65 when it comes to issues such as retirement, financial security, “empty nesting,” physical health issues, decreased socialization that resulted from the COVID-19 pandemic, and the perpetual loss of relatives and friends.
In addition, as we age, anxiety can come simply from the loss of identity as active lifestyles decrease and the reality of nearing life’s end becomes more of a reality. It would seem that this population would benefit enormously from anxiety screening and possible treatment.
Another major concern is that the screening and potential treatment of patients is aimed at primary care physicians. Putting the sole responsibility of providing mental health care on these overworked PCPs defies common sense unless we’re okay with 1- to 2-minute assessments of mental health issues and no doubt, a pharmacology-only approach.
If this follows the same route as well-intentioned PCPs treating depression, where 5-minute medication management is far too common, the only proper diagnostic course – the in-depth interview necessary to make a proper diagnosis – is often missing.
For example, in depression alone, it takes psychiatric experience and time to differentiate a major depressive disorder from a bipolar depression and to provide the appropriate medication and treatment plan with careful follow-up. In my experience, this usually does not happen in the exceedingly overworked, time-driven day of a PCP.
Anxiety disorders and depression can prove debilitating, and if a PCP wants the responsibility of treatment, a mandated mental health program should be followed – just as here in New York, prescribers are mandated to take a pain control, opioid, and infection control CME course to keep our licenses up to date.
Short of mandating a mental health program for PCPs, it should be part of training and CME courses that Psychiatry is a super specialty, much like orthopedics and ophthalmology, and primary care physicians should never hesitate to make referrals to the specialist.
The big picture for me, and I hope for us all, is that the USPSTF has started things rolling by making it clear that PCPs and other health care clinicians need to screen for anxiety as a disabling disorder that is quite treatable.
This approach will help to advance the destigmatization of mental health disorders. But as result, with more patients diagnosed, there will be a need for more psychiatrists – and psychologists with PhDs or PsyDs – to fill the gaps in mental health care.
Dr. London is a practicing psychiatrist and has been a newspaper columnist for 35 years, specializing in and writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
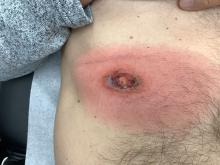
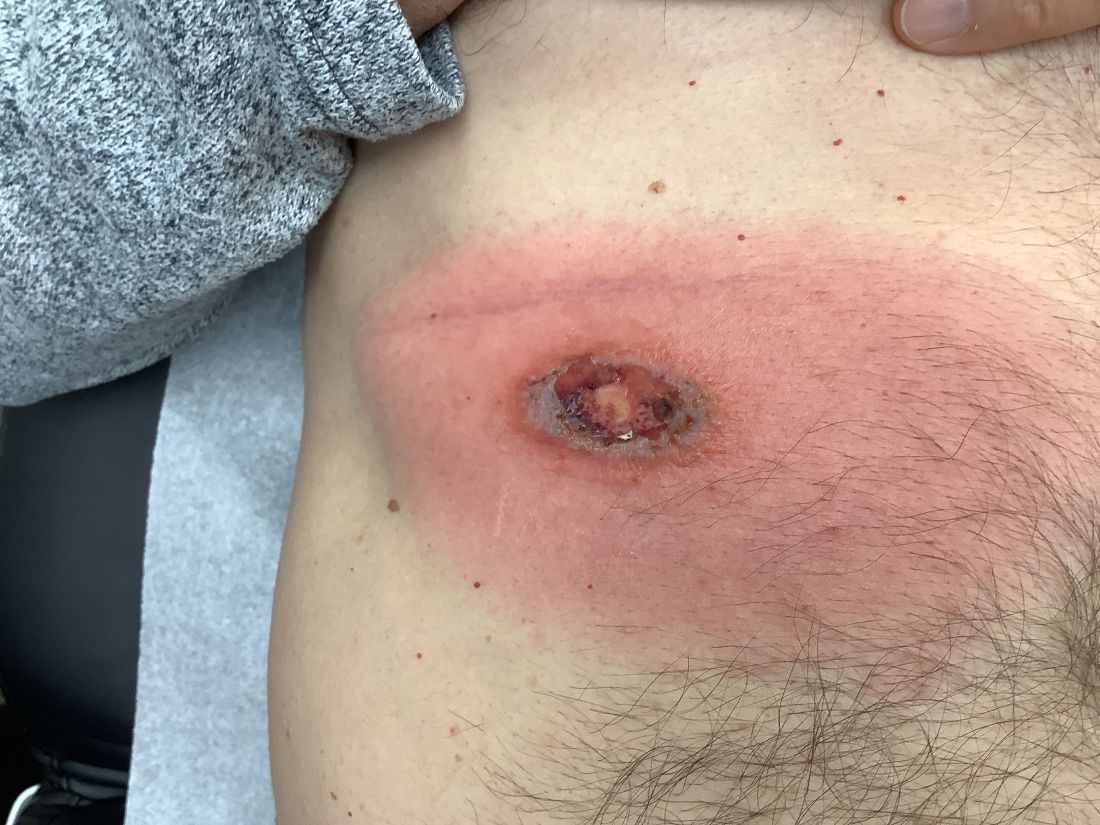
A White male presented with a purulent erythematous edematous plaque with central necrosis and ulceration on his right flank
Lyme disease is the most commonly transmitted tick-borne illness in the United States. This infection is typically transmitted through a bite by the Ixodes tick commonly found in the Midwest, Northeast, and mid-Atlantic regions; however, the geographical distribution continues to expand over time in the United States. Ticks must be attached for 24-48 hours to transmit the pathogen. There are three general stages of the disease: early localized, early disseminated, and late disseminated.
The most common presentation is the early localized disease, which manifests between 3 and 30 days after an infected tick bite. Approximately 70%-80% of cases feature a targetlike lesion that expands centrifugally at the site of the bite. Most commonly, lesions appear on the abdomen, groin, axilla, and popliteal fossa. The diagnosis of ECM requires lesions at least 5 cm in size. Lesions may be asymptomatic, although burning may occur in half of patients. Atypical presentations include bullous, vesicular, hemorrhagic, or necrotic lesions. Up to half of patients may develop multiple ECM lesions. Palms and soles are spared. Differential diagnoses include arthropod reactions, pyoderma gangrenosum, cellulitis, herpes simplex virus and varicella zoster virus, contact dermatitis, or granuloma annulare. The rash is often accompanied by systemic symptoms including fatigue, myalgia, headache, and fever.
The next two stages include early and late disseminated infection. Early disseminated infection often occurs 3-12 weeks after infection and is characterized by muscle pain, dizziness, headache, and cardiac symptoms. CNS involvement occurs in about 20% of patients. Joint involvement may include the knee, ankle, and wrist. If symptoms are only in one joint, septic arthritis is part of the differential diagnosis, so clinical correlation and labs must be considered. Late disseminated infection occurs months or years after initial infection and includes neurologic and rheumatologic symptoms including meningitis, Bell’s palsy, arthritis, and dysesthesia. Knee arthritis is a key feature of this stage. Patients commonly have radicular pain and fibromyalgia-type pain. More severe disease processes include encephalomyelitis, arrhythmias, and heart block.
ECM is often a clinical diagnosis because serologic testing may not be positive during the first 2 weeks of infection. The screening serologic test is the ELISA, and a Western blot confirms the results. Skin histopathology for Lyme disease is often nonspecific and reveals a perivascular infiltrate of histiocytes, plasma cells, and lymphocytes. Silver stain or antibody testing may be used to identify the spirochete. In acrodermatitis chronica atrophicans, late Lyme disease presenting on the distal extremities, lymphocytic and plasma cell infiltrates are present. In borrelial lymphocytoma, a dense dermal lymphocytic infiltrate is present.
The standard for treatment of early localized disease is oral doxycycline in adults. Alternatives may be used if a patient is allergic or for children under 9. Disseminated disease may be treated with IV ceftriaxone and topical steroids are used if ocular symptoms are involved. Early treatment is often curative.
This patient’s antibodies were negative initially, but became positive after 6 weeks. He was treated empirically at the time of his office visit with doxycycline for 1 month.
This case and the photo were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Susannah Berke, MD, Three Rivers Dermatology, Coraopolis, Pa. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at MDedge.com/Dermatology. To submit a case for possible publication, send an email to [email protected].
References
Carriveau A et al. Nurs Clin North Am. 2019 Jun;54(2):261-75.
Skar GL and Simonsen KA. Lyme Disease. [Updated 2023 May 31]. In: “StatPearls” [Internet]. Treasure Island, Fla.: StatPearls Publishing; 2023 Jan.
Tiger JB et al. J Am Acad Dermatol. 2014 Oct;71(4):e133-4.
Lyme disease is the most commonly transmitted tick-borne illness in the United States. This infection is typically transmitted through a bite by the Ixodes tick commonly found in the Midwest, Northeast, and mid-Atlantic regions; however, the geographical distribution continues to expand over time in the United States. Ticks must be attached for 24-48 hours to transmit the pathogen. There are three general stages of the disease: early localized, early disseminated, and late disseminated.
The most common presentation is the early localized disease, which manifests between 3 and 30 days after an infected tick bite. Approximately 70%-80% of cases feature a targetlike lesion that expands centrifugally at the site of the bite. Most commonly, lesions appear on the abdomen, groin, axilla, and popliteal fossa. The diagnosis of ECM requires lesions at least 5 cm in size. Lesions may be asymptomatic, although burning may occur in half of patients. Atypical presentations include bullous, vesicular, hemorrhagic, or necrotic lesions. Up to half of patients may develop multiple ECM lesions. Palms and soles are spared. Differential diagnoses include arthropod reactions, pyoderma gangrenosum, cellulitis, herpes simplex virus and varicella zoster virus, contact dermatitis, or granuloma annulare. The rash is often accompanied by systemic symptoms including fatigue, myalgia, headache, and fever.
The next two stages include early and late disseminated infection. Early disseminated infection often occurs 3-12 weeks after infection and is characterized by muscle pain, dizziness, headache, and cardiac symptoms. CNS involvement occurs in about 20% of patients. Joint involvement may include the knee, ankle, and wrist. If symptoms are only in one joint, septic arthritis is part of the differential diagnosis, so clinical correlation and labs must be considered. Late disseminated infection occurs months or years after initial infection and includes neurologic and rheumatologic symptoms including meningitis, Bell’s palsy, arthritis, and dysesthesia. Knee arthritis is a key feature of this stage. Patients commonly have radicular pain and fibromyalgia-type pain. More severe disease processes include encephalomyelitis, arrhythmias, and heart block.
ECM is often a clinical diagnosis because serologic testing may not be positive during the first 2 weeks of infection. The screening serologic test is the ELISA, and a Western blot confirms the results. Skin histopathology for Lyme disease is often nonspecific and reveals a perivascular infiltrate of histiocytes, plasma cells, and lymphocytes. Silver stain or antibody testing may be used to identify the spirochete. In acrodermatitis chronica atrophicans, late Lyme disease presenting on the distal extremities, lymphocytic and plasma cell infiltrates are present. In borrelial lymphocytoma, a dense dermal lymphocytic infiltrate is present.
The standard for treatment of early localized disease is oral doxycycline in adults. Alternatives may be used if a patient is allergic or for children under 9. Disseminated disease may be treated with IV ceftriaxone and topical steroids are used if ocular symptoms are involved. Early treatment is often curative.
This patient’s antibodies were negative initially, but became positive after 6 weeks. He was treated empirically at the time of his office visit with doxycycline for 1 month.
This case and the photo were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Susannah Berke, MD, Three Rivers Dermatology, Coraopolis, Pa. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at MDedge.com/Dermatology. To submit a case for possible publication, send an email to [email protected].
References
Carriveau A et al. Nurs Clin North Am. 2019 Jun;54(2):261-75.
Skar GL and Simonsen KA. Lyme Disease. [Updated 2023 May 31]. In: “StatPearls” [Internet]. Treasure Island, Fla.: StatPearls Publishing; 2023 Jan.
Tiger JB et al. J Am Acad Dermatol. 2014 Oct;71(4):e133-4.
Lyme disease is the most commonly transmitted tick-borne illness in the United States. This infection is typically transmitted through a bite by the Ixodes tick commonly found in the Midwest, Northeast, and mid-Atlantic regions; however, the geographical distribution continues to expand over time in the United States. Ticks must be attached for 24-48 hours to transmit the pathogen. There are three general stages of the disease: early localized, early disseminated, and late disseminated.
The most common presentation is the early localized disease, which manifests between 3 and 30 days after an infected tick bite. Approximately 70%-80% of cases feature a targetlike lesion that expands centrifugally at the site of the bite. Most commonly, lesions appear on the abdomen, groin, axilla, and popliteal fossa. The diagnosis of ECM requires lesions at least 5 cm in size. Lesions may be asymptomatic, although burning may occur in half of patients. Atypical presentations include bullous, vesicular, hemorrhagic, or necrotic lesions. Up to half of patients may develop multiple ECM lesions. Palms and soles are spared. Differential diagnoses include arthropod reactions, pyoderma gangrenosum, cellulitis, herpes simplex virus and varicella zoster virus, contact dermatitis, or granuloma annulare. The rash is often accompanied by systemic symptoms including fatigue, myalgia, headache, and fever.
The next two stages include early and late disseminated infection. Early disseminated infection often occurs 3-12 weeks after infection and is characterized by muscle pain, dizziness, headache, and cardiac symptoms. CNS involvement occurs in about 20% of patients. Joint involvement may include the knee, ankle, and wrist. If symptoms are only in one joint, septic arthritis is part of the differential diagnosis, so clinical correlation and labs must be considered. Late disseminated infection occurs months or years after initial infection and includes neurologic and rheumatologic symptoms including meningitis, Bell’s palsy, arthritis, and dysesthesia. Knee arthritis is a key feature of this stage. Patients commonly have radicular pain and fibromyalgia-type pain. More severe disease processes include encephalomyelitis, arrhythmias, and heart block.
ECM is often a clinical diagnosis because serologic testing may not be positive during the first 2 weeks of infection. The screening serologic test is the ELISA, and a Western blot confirms the results. Skin histopathology for Lyme disease is often nonspecific and reveals a perivascular infiltrate of histiocytes, plasma cells, and lymphocytes. Silver stain or antibody testing may be used to identify the spirochete. In acrodermatitis chronica atrophicans, late Lyme disease presenting on the distal extremities, lymphocytic and plasma cell infiltrates are present. In borrelial lymphocytoma, a dense dermal lymphocytic infiltrate is present.
The standard for treatment of early localized disease is oral doxycycline in adults. Alternatives may be used if a patient is allergic or for children under 9. Disseminated disease may be treated with IV ceftriaxone and topical steroids are used if ocular symptoms are involved. Early treatment is often curative.
This patient’s antibodies were negative initially, but became positive after 6 weeks. He was treated empirically at the time of his office visit with doxycycline for 1 month.
This case and the photo were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Susannah Berke, MD, Three Rivers Dermatology, Coraopolis, Pa. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at MDedge.com/Dermatology. To submit a case for possible publication, send an email to [email protected].
References
Carriveau A et al. Nurs Clin North Am. 2019 Jun;54(2):261-75.
Skar GL and Simonsen KA. Lyme Disease. [Updated 2023 May 31]. In: “StatPearls” [Internet]. Treasure Island, Fla.: StatPearls Publishing; 2023 Jan.
Tiger JB et al. J Am Acad Dermatol. 2014 Oct;71(4):e133-4.
Is AFib ablation the fifth pillar in heart failure care? CASTLE-HTx
Recorded Aug. 28, 2023. This transcript has been edited for clarity.
John M. Mandrola, MD: I’m here at the European Society of Cardiology meeting, and I’m very excited to have two colleagues whom I met at the Western Atrial Fibrillation Symposium (Western AFib) and who presented the CASTLE-HTx study. This is Christian Sohns and Philipp Sommer, and the CASTLE-HTx study is very exciting.
Before I get into that, I really want to introduce the concept of atrial fibrillation in heart failure. I like to say that there are two big populations of patients with atrial fibrillation, and the vast majority can be treated slowly with reassurance and education. There is a group of patients who have heart failure who, when they develop atrial fibrillation, can degenerate rapidly. The CASTLE-HTx study looked at catheter ablation versus medical therapy in patients with advanced heart failure.
Christian, why don’t you tell us the top-line results and what you found.
CASTLE-HTx key findings
Christian Sohns, MD, PhD: Thanks, first of all, for mentioning this special cohort of patients in end-stage heart failure, which is very important. The endpoint of the study was a composite of death from any cause or left ventricular assist device (LVAD) implantation and heart transplantation. These are very hard, strong clinical endpoints, not the rate of rehospitalization or something like that.
Catheter ablation was superior to medical therapy alone in terms of this composite endpoint. That was driven by cardiovascular death and all-cause mortality, which highlights the fact that you should always consider atrial fibrillation ablation in the end-stage heart failure cohort. The findings were driven by the fact that we saw left ventricular reverse remodeling and the reduction of atrial fibrillation in these patients.
Dr. Mandrola: Tell me about how it came about. It was conducted at your center. Who were these patients?
Philipp Sommer, MD: As one of the biggest centers for heart transplantations all over Europe, with roughly 100 transplants per year, we had many patients being referred to our center with the questions of whether those patients are eligible for a heart transplantation. Not all of the patients in our study were listed for a transplant, but all of them were admitted in that end-stage heart failure status to evaluate their eligibility for transplant.
If we look at the baseline data of those patients, they had an ejection fraction of 29%. They had a 6-minute walk test as a functional capacity parameter of around 300 m. Approximately two thirds of them were New York Heart Association class III and IV, which is significantly worse than what we saw in the previous studies dealing with heart failure patients.
I think overall, if you also look at NT-proBNP levels, this is a really sick patient population where some people might doubt if they should admit and refer those patients for an ablation procedure. Therefore, it’s really interesting and fascinating to see the results.
Dr. Mandrola: I did read in the manuscript, and I heard from you, that these were recruited as outpatients. So they were stable outpatients who were referred to the center for consideration of an LVAD or transplant?
Dr. Sohns: The definition of stability is very difficult in these patients because they have hospital stays, they have a history of drug therapy, and they have a history of interventions also behind them – not atrial fibrillation ablation, but others. I think these patients are referred because the referring physicians are done with the case. They can no longer offer any option to the patients other than surgical treatment, assist device, pump implantation, or transplantation.
If you look at the guidelines, they do not comment on atrial fibrillation ablation in this cohort of patients. Also, they have different recommendations between the American societies and the European societies regarding what is end-stage heart failure and how to treat these patients. Therefore, it was a big benefit of CASTLE-HTx that we randomized a cohort of patients with advanced end-stage heart failure.
How can AFib ablation have such big, early effects?
Dr. Mandrola: These are very clinically significant findings, with large effect sizes and very early separation of the Kaplan-Meier curves. How do you explain how dramatic an effect that is, and how early of an effect?
Dr. Sommer: That’s one of the key questions at the end of the day. I think our job basically was to provide the data and to ensure that the data are clean and that it’s all perfectly done. The interpretation of these data is really kind of difficult, although we do not have the 100% perfect and obvious explanation why the curves separated so early. Our view on that is that we are talking about a pretty fragile patient population, so little differences like having a tachyarrhythmia of 110 day in, day out or being in sinus rhythm of 60 can make a huge difference. That’s obviously pretty early.
The one that remains in tachyarrhythmia will deteriorate and will require an LVAD after a couple of months, and the one that you may keep in sinus rhythm, even with reduced atrial fibrillation burden – not zero, but reduced atrial fibrillation burden – and improved LV function, all of a sudden this patient will still remain on a low level of being stable, but he or she will remain stable and will not require any surgical interventions for the next 1.5-2 years. If we can manage to do this, just postponing the natural cause of the disease, I think that is a great benefit for the patient.
Dr. Mandrola: One of the things that comes up in our center is that I look at some of these patients and think, there’s no way I can put this patient under general anesthetic and do all of this. Your ablation procedure wasn’t that extensive, was it?
Dr. Sohns: On the one hand, no. On the other hand, yes. You need to take into consideration that it has been performed by experienced physicians with experience in heart failure treatment and atrial fibrillation in heart transplantation centers, though it›s not sure that we can transfer these results one-to-one to all other centers in the world.
It is very clear that we have almost no major complications in these patients. We were able to do these ablation procedures without general anesthesia. We have 60% of patients who had pulmonary vein isolation only and 40% of patients who have PVI and additional therapy. We have a procedure duration of almost 90 minutes during radiofrequency ablation.
We have different categories. When you talk about the different patient cohorts, we also see different stages of myocardial tissue damage, which will be part of another publication for sure. It is, in part, surprising how normal some of the atria were despite having a volume of 180 mL, but they had no fibrosis. That was very interesting.
Dr. Mandrola: How did the persistent vs paroxysmal atrial fibrillation sort out? Were these mostly patients with persistent atrial fibrillation?
Dr. Sommer: Two-thirds were persistent. It would be expected in this patient population that you would not find so many paroxysmal cases. I think it›s very important what Christian was just mentioning that when we discussed the trial design, we were anticipating problems with the sedation, for example. With the follow-up of those procedures, would they decompensate because of the fluid that you have to deliver during such a procedure.
We were quite surprised at the end of the day that the procedures were quite straightforward. Fortunately, we had no major complications. I think there were four complications in the 100 ablated patients. I think we were really positive about how the procedures turned out.
I should mention that one of the exclusion criteria was a left atrial diameter of about 60 mm. The huge ones may be very diseased, and maybe the hopeless ones were excluded from the study. Below 60 mm, we did the ablation.
Rhythm control
Dr. Mandrola: One of my colleagues, who is even more skeptical than me, wanted me to ask you, why wouldn’t you take a patient with persistent atrial fibrillation who had heart failure and just cardiovert and use amiodarone and try and maintain sinus rhythm that way?
Dr. Sohns: It is important to mention that 50% of the patients have already had amiodarone before they were randomized and enrolled for the trial. It might bring you a couple of minutes or a couple of hours [of relief], but the patients would get recurrence.
It was very interesting also, and this is in line with the data from Jason Andrade, who demonstrated that we were able to reduce the percentage of patients with persistent atrial fibrillation to paroxysmal. We did a down-staging of the underlying disease. This is not possible with cardioversion or drugs, for example.
Dr. Sommer: What I really like about that question and that comment is the idea that rhythm control in this subset of patients obviously has a role and an importance. It may be a cardioversion initially, giving amiodarone if they didn’t have that before, and you can keep the patient in sinus rhythm with this therapy, I think we’re reaching the same goal.
I think the critical point to get into the mind of physicians who treat heart failure is that sinus rhythm is beneficial, however you get there. Ablation, of course, as in other studies, is the most powerful tool to get there. Cardioversion can be a really good thing to do; you just have to think about it and consider it.
Dr. Mandrola: I do want to say to everybody that there is a tension sometimes between the heart failure community and the electrophysiology community. I think the ideal situation is that we work together, because I think that we can help with the maintenance of sinus rhythm. The control group mortality at 1 year was 20%, and I’ve heard people say that that’s not advanced heart failure. Advanced heart failure patients have much higher mortality than that. My colleague who is a heart failure specialist was criticizing a selection bias in picking the best patients. How would you answer that?
Dr. Sohns: There are data available from Eurotransplant, for example, that the waiting list mortality is 18%, so I think we are almost in line with this 20% mortality in this conservative group. You cannot generalize it. All these patients have different histories. We have 60% dilated cardiomyopathy and 40% ischemic cardiomyopathy. I think it is a very representative group in contrast to your friend who suggests that it is not.
Dr. Sommer: What I like about the discussion is that some approach us to say that the mortality in the control group is much too high – like, what are you doing with those patients that you create so many endpoints? Then others say that it’s not high enough because that is not end-stage heart failure. Come on! We have a patient cohort that is very well described and very well characterized.
If the label is end-stage heart failure, advanced heart failure, or whatever, they are sicker than the patients that we had in earlier trials. The patients that we treated were mostly excluded from all other trials. We opened the door. We found a clear result. I think everyone can see whatever you like to see.
Dr. Mandrola: What would your take-home message be after having done this trial design, the trial was conducted in your single center, and you come up with these amazing results? What would your message be to the whole community?
Dr. Sohns: Taking into consideration how severely sick these patients are, I can just repeat it: They are one step away from death, more or less, or from surgical intervention that can prolong their life. You should also consider that there are options like atrial fibrillation ablation that can buy time, postpone the natural course, or even in some patients replace the destination therapy. Therefore, in my opinion the next guidelines should recommend that every patient should carefully be checked for sinus rhythm before bringing these patients into the environment of transplantation.
Dr. Sommer: My interpretation is that we have to try to bring into physicians’ minds that besides a well-established and well-documented effect of drug therapy with the fabulous four, we may now have the fabulous five, including an ablation option for patients with atrial fibrillation.
Dr. Mandrola is a clinical electrophysiologist at Baptist Medical Associates, Louisville, Ky. Dr. Sohns is deputy director of the Heart and Diabetes Center NRW, Ruhr University Bochum, Bad Oeynhausen, Germany. Dr. Sommer is professor of cardiology at the Heart and Diabetes Center NRW. Dr. Mandrola reported no conflicts of interest. Dr. Sohns reported receiving research funding from Else Kröner–Fresenius–Stiftung. Dr. Sommer reported consulting with Abbott, Biosense Webster, Boston Scientific, and Medtronic USA.
A version of this article first appeared on Medscape.com.
Recorded Aug. 28, 2023. This transcript has been edited for clarity.
John M. Mandrola, MD: I’m here at the European Society of Cardiology meeting, and I’m very excited to have two colleagues whom I met at the Western Atrial Fibrillation Symposium (Western AFib) and who presented the CASTLE-HTx study. This is Christian Sohns and Philipp Sommer, and the CASTLE-HTx study is very exciting.
Before I get into that, I really want to introduce the concept of atrial fibrillation in heart failure. I like to say that there are two big populations of patients with atrial fibrillation, and the vast majority can be treated slowly with reassurance and education. There is a group of patients who have heart failure who, when they develop atrial fibrillation, can degenerate rapidly. The CASTLE-HTx study looked at catheter ablation versus medical therapy in patients with advanced heart failure.
Christian, why don’t you tell us the top-line results and what you found.
CASTLE-HTx key findings
Christian Sohns, MD, PhD: Thanks, first of all, for mentioning this special cohort of patients in end-stage heart failure, which is very important. The endpoint of the study was a composite of death from any cause or left ventricular assist device (LVAD) implantation and heart transplantation. These are very hard, strong clinical endpoints, not the rate of rehospitalization or something like that.
Catheter ablation was superior to medical therapy alone in terms of this composite endpoint. That was driven by cardiovascular death and all-cause mortality, which highlights the fact that you should always consider atrial fibrillation ablation in the end-stage heart failure cohort. The findings were driven by the fact that we saw left ventricular reverse remodeling and the reduction of atrial fibrillation in these patients.
Dr. Mandrola: Tell me about how it came about. It was conducted at your center. Who were these patients?
Philipp Sommer, MD: As one of the biggest centers for heart transplantations all over Europe, with roughly 100 transplants per year, we had many patients being referred to our center with the questions of whether those patients are eligible for a heart transplantation. Not all of the patients in our study were listed for a transplant, but all of them were admitted in that end-stage heart failure status to evaluate their eligibility for transplant.
If we look at the baseline data of those patients, they had an ejection fraction of 29%. They had a 6-minute walk test as a functional capacity parameter of around 300 m. Approximately two thirds of them were New York Heart Association class III and IV, which is significantly worse than what we saw in the previous studies dealing with heart failure patients.
I think overall, if you also look at NT-proBNP levels, this is a really sick patient population where some people might doubt if they should admit and refer those patients for an ablation procedure. Therefore, it’s really interesting and fascinating to see the results.
Dr. Mandrola: I did read in the manuscript, and I heard from you, that these were recruited as outpatients. So they were stable outpatients who were referred to the center for consideration of an LVAD or transplant?
Dr. Sohns: The definition of stability is very difficult in these patients because they have hospital stays, they have a history of drug therapy, and they have a history of interventions also behind them – not atrial fibrillation ablation, but others. I think these patients are referred because the referring physicians are done with the case. They can no longer offer any option to the patients other than surgical treatment, assist device, pump implantation, or transplantation.
If you look at the guidelines, they do not comment on atrial fibrillation ablation in this cohort of patients. Also, they have different recommendations between the American societies and the European societies regarding what is end-stage heart failure and how to treat these patients. Therefore, it was a big benefit of CASTLE-HTx that we randomized a cohort of patients with advanced end-stage heart failure.
How can AFib ablation have such big, early effects?
Dr. Mandrola: These are very clinically significant findings, with large effect sizes and very early separation of the Kaplan-Meier curves. How do you explain how dramatic an effect that is, and how early of an effect?
Dr. Sommer: That’s one of the key questions at the end of the day. I think our job basically was to provide the data and to ensure that the data are clean and that it’s all perfectly done. The interpretation of these data is really kind of difficult, although we do not have the 100% perfect and obvious explanation why the curves separated so early. Our view on that is that we are talking about a pretty fragile patient population, so little differences like having a tachyarrhythmia of 110 day in, day out or being in sinus rhythm of 60 can make a huge difference. That’s obviously pretty early.
The one that remains in tachyarrhythmia will deteriorate and will require an LVAD after a couple of months, and the one that you may keep in sinus rhythm, even with reduced atrial fibrillation burden – not zero, but reduced atrial fibrillation burden – and improved LV function, all of a sudden this patient will still remain on a low level of being stable, but he or she will remain stable and will not require any surgical interventions for the next 1.5-2 years. If we can manage to do this, just postponing the natural cause of the disease, I think that is a great benefit for the patient.
Dr. Mandrola: One of the things that comes up in our center is that I look at some of these patients and think, there’s no way I can put this patient under general anesthetic and do all of this. Your ablation procedure wasn’t that extensive, was it?
Dr. Sohns: On the one hand, no. On the other hand, yes. You need to take into consideration that it has been performed by experienced physicians with experience in heart failure treatment and atrial fibrillation in heart transplantation centers, though it›s not sure that we can transfer these results one-to-one to all other centers in the world.
It is very clear that we have almost no major complications in these patients. We were able to do these ablation procedures without general anesthesia. We have 60% of patients who had pulmonary vein isolation only and 40% of patients who have PVI and additional therapy. We have a procedure duration of almost 90 minutes during radiofrequency ablation.
We have different categories. When you talk about the different patient cohorts, we also see different stages of myocardial tissue damage, which will be part of another publication for sure. It is, in part, surprising how normal some of the atria were despite having a volume of 180 mL, but they had no fibrosis. That was very interesting.
Dr. Mandrola: How did the persistent vs paroxysmal atrial fibrillation sort out? Were these mostly patients with persistent atrial fibrillation?
Dr. Sommer: Two-thirds were persistent. It would be expected in this patient population that you would not find so many paroxysmal cases. I think it›s very important what Christian was just mentioning that when we discussed the trial design, we were anticipating problems with the sedation, for example. With the follow-up of those procedures, would they decompensate because of the fluid that you have to deliver during such a procedure.
We were quite surprised at the end of the day that the procedures were quite straightforward. Fortunately, we had no major complications. I think there were four complications in the 100 ablated patients. I think we were really positive about how the procedures turned out.
I should mention that one of the exclusion criteria was a left atrial diameter of about 60 mm. The huge ones may be very diseased, and maybe the hopeless ones were excluded from the study. Below 60 mm, we did the ablation.
Rhythm control
Dr. Mandrola: One of my colleagues, who is even more skeptical than me, wanted me to ask you, why wouldn’t you take a patient with persistent atrial fibrillation who had heart failure and just cardiovert and use amiodarone and try and maintain sinus rhythm that way?
Dr. Sohns: It is important to mention that 50% of the patients have already had amiodarone before they were randomized and enrolled for the trial. It might bring you a couple of minutes or a couple of hours [of relief], but the patients would get recurrence.
It was very interesting also, and this is in line with the data from Jason Andrade, who demonstrated that we were able to reduce the percentage of patients with persistent atrial fibrillation to paroxysmal. We did a down-staging of the underlying disease. This is not possible with cardioversion or drugs, for example.
Dr. Sommer: What I really like about that question and that comment is the idea that rhythm control in this subset of patients obviously has a role and an importance. It may be a cardioversion initially, giving amiodarone if they didn’t have that before, and you can keep the patient in sinus rhythm with this therapy, I think we’re reaching the same goal.
I think the critical point to get into the mind of physicians who treat heart failure is that sinus rhythm is beneficial, however you get there. Ablation, of course, as in other studies, is the most powerful tool to get there. Cardioversion can be a really good thing to do; you just have to think about it and consider it.
Dr. Mandrola: I do want to say to everybody that there is a tension sometimes between the heart failure community and the electrophysiology community. I think the ideal situation is that we work together, because I think that we can help with the maintenance of sinus rhythm. The control group mortality at 1 year was 20%, and I’ve heard people say that that’s not advanced heart failure. Advanced heart failure patients have much higher mortality than that. My colleague who is a heart failure specialist was criticizing a selection bias in picking the best patients. How would you answer that?
Dr. Sohns: There are data available from Eurotransplant, for example, that the waiting list mortality is 18%, so I think we are almost in line with this 20% mortality in this conservative group. You cannot generalize it. All these patients have different histories. We have 60% dilated cardiomyopathy and 40% ischemic cardiomyopathy. I think it is a very representative group in contrast to your friend who suggests that it is not.
Dr. Sommer: What I like about the discussion is that some approach us to say that the mortality in the control group is much too high – like, what are you doing with those patients that you create so many endpoints? Then others say that it’s not high enough because that is not end-stage heart failure. Come on! We have a patient cohort that is very well described and very well characterized.
If the label is end-stage heart failure, advanced heart failure, or whatever, they are sicker than the patients that we had in earlier trials. The patients that we treated were mostly excluded from all other trials. We opened the door. We found a clear result. I think everyone can see whatever you like to see.
Dr. Mandrola: What would your take-home message be after having done this trial design, the trial was conducted in your single center, and you come up with these amazing results? What would your message be to the whole community?
Dr. Sohns: Taking into consideration how severely sick these patients are, I can just repeat it: They are one step away from death, more or less, or from surgical intervention that can prolong their life. You should also consider that there are options like atrial fibrillation ablation that can buy time, postpone the natural course, or even in some patients replace the destination therapy. Therefore, in my opinion the next guidelines should recommend that every patient should carefully be checked for sinus rhythm before bringing these patients into the environment of transplantation.
Dr. Sommer: My interpretation is that we have to try to bring into physicians’ minds that besides a well-established and well-documented effect of drug therapy with the fabulous four, we may now have the fabulous five, including an ablation option for patients with atrial fibrillation.
Dr. Mandrola is a clinical electrophysiologist at Baptist Medical Associates, Louisville, Ky. Dr. Sohns is deputy director of the Heart and Diabetes Center NRW, Ruhr University Bochum, Bad Oeynhausen, Germany. Dr. Sommer is professor of cardiology at the Heart and Diabetes Center NRW. Dr. Mandrola reported no conflicts of interest. Dr. Sohns reported receiving research funding from Else Kröner–Fresenius–Stiftung. Dr. Sommer reported consulting with Abbott, Biosense Webster, Boston Scientific, and Medtronic USA.
A version of this article first appeared on Medscape.com.
Recorded Aug. 28, 2023. This transcript has been edited for clarity.
John M. Mandrola, MD: I’m here at the European Society of Cardiology meeting, and I’m very excited to have two colleagues whom I met at the Western Atrial Fibrillation Symposium (Western AFib) and who presented the CASTLE-HTx study. This is Christian Sohns and Philipp Sommer, and the CASTLE-HTx study is very exciting.
Before I get into that, I really want to introduce the concept of atrial fibrillation in heart failure. I like to say that there are two big populations of patients with atrial fibrillation, and the vast majority can be treated slowly with reassurance and education. There is a group of patients who have heart failure who, when they develop atrial fibrillation, can degenerate rapidly. The CASTLE-HTx study looked at catheter ablation versus medical therapy in patients with advanced heart failure.
Christian, why don’t you tell us the top-line results and what you found.
CASTLE-HTx key findings
Christian Sohns, MD, PhD: Thanks, first of all, for mentioning this special cohort of patients in end-stage heart failure, which is very important. The endpoint of the study was a composite of death from any cause or left ventricular assist device (LVAD) implantation and heart transplantation. These are very hard, strong clinical endpoints, not the rate of rehospitalization or something like that.
Catheter ablation was superior to medical therapy alone in terms of this composite endpoint. That was driven by cardiovascular death and all-cause mortality, which highlights the fact that you should always consider atrial fibrillation ablation in the end-stage heart failure cohort. The findings were driven by the fact that we saw left ventricular reverse remodeling and the reduction of atrial fibrillation in these patients.
Dr. Mandrola: Tell me about how it came about. It was conducted at your center. Who were these patients?
Philipp Sommer, MD: As one of the biggest centers for heart transplantations all over Europe, with roughly 100 transplants per year, we had many patients being referred to our center with the questions of whether those patients are eligible for a heart transplantation. Not all of the patients in our study were listed for a transplant, but all of them were admitted in that end-stage heart failure status to evaluate their eligibility for transplant.
If we look at the baseline data of those patients, they had an ejection fraction of 29%. They had a 6-minute walk test as a functional capacity parameter of around 300 m. Approximately two thirds of them were New York Heart Association class III and IV, which is significantly worse than what we saw in the previous studies dealing with heart failure patients.
I think overall, if you also look at NT-proBNP levels, this is a really sick patient population where some people might doubt if they should admit and refer those patients for an ablation procedure. Therefore, it’s really interesting and fascinating to see the results.
Dr. Mandrola: I did read in the manuscript, and I heard from you, that these were recruited as outpatients. So they were stable outpatients who were referred to the center for consideration of an LVAD or transplant?
Dr. Sohns: The definition of stability is very difficult in these patients because they have hospital stays, they have a history of drug therapy, and they have a history of interventions also behind them – not atrial fibrillation ablation, but others. I think these patients are referred because the referring physicians are done with the case. They can no longer offer any option to the patients other than surgical treatment, assist device, pump implantation, or transplantation.
If you look at the guidelines, they do not comment on atrial fibrillation ablation in this cohort of patients. Also, they have different recommendations between the American societies and the European societies regarding what is end-stage heart failure and how to treat these patients. Therefore, it was a big benefit of CASTLE-HTx that we randomized a cohort of patients with advanced end-stage heart failure.
How can AFib ablation have such big, early effects?
Dr. Mandrola: These are very clinically significant findings, with large effect sizes and very early separation of the Kaplan-Meier curves. How do you explain how dramatic an effect that is, and how early of an effect?
Dr. Sommer: That’s one of the key questions at the end of the day. I think our job basically was to provide the data and to ensure that the data are clean and that it’s all perfectly done. The interpretation of these data is really kind of difficult, although we do not have the 100% perfect and obvious explanation why the curves separated so early. Our view on that is that we are talking about a pretty fragile patient population, so little differences like having a tachyarrhythmia of 110 day in, day out or being in sinus rhythm of 60 can make a huge difference. That’s obviously pretty early.
The one that remains in tachyarrhythmia will deteriorate and will require an LVAD after a couple of months, and the one that you may keep in sinus rhythm, even with reduced atrial fibrillation burden – not zero, but reduced atrial fibrillation burden – and improved LV function, all of a sudden this patient will still remain on a low level of being stable, but he or she will remain stable and will not require any surgical interventions for the next 1.5-2 years. If we can manage to do this, just postponing the natural cause of the disease, I think that is a great benefit for the patient.
Dr. Mandrola: One of the things that comes up in our center is that I look at some of these patients and think, there’s no way I can put this patient under general anesthetic and do all of this. Your ablation procedure wasn’t that extensive, was it?
Dr. Sohns: On the one hand, no. On the other hand, yes. You need to take into consideration that it has been performed by experienced physicians with experience in heart failure treatment and atrial fibrillation in heart transplantation centers, though it›s not sure that we can transfer these results one-to-one to all other centers in the world.
It is very clear that we have almost no major complications in these patients. We were able to do these ablation procedures without general anesthesia. We have 60% of patients who had pulmonary vein isolation only and 40% of patients who have PVI and additional therapy. We have a procedure duration of almost 90 minutes during radiofrequency ablation.
We have different categories. When you talk about the different patient cohorts, we also see different stages of myocardial tissue damage, which will be part of another publication for sure. It is, in part, surprising how normal some of the atria were despite having a volume of 180 mL, but they had no fibrosis. That was very interesting.
Dr. Mandrola: How did the persistent vs paroxysmal atrial fibrillation sort out? Were these mostly patients with persistent atrial fibrillation?
Dr. Sommer: Two-thirds were persistent. It would be expected in this patient population that you would not find so many paroxysmal cases. I think it›s very important what Christian was just mentioning that when we discussed the trial design, we were anticipating problems with the sedation, for example. With the follow-up of those procedures, would they decompensate because of the fluid that you have to deliver during such a procedure.
We were quite surprised at the end of the day that the procedures were quite straightforward. Fortunately, we had no major complications. I think there were four complications in the 100 ablated patients. I think we were really positive about how the procedures turned out.
I should mention that one of the exclusion criteria was a left atrial diameter of about 60 mm. The huge ones may be very diseased, and maybe the hopeless ones were excluded from the study. Below 60 mm, we did the ablation.
Rhythm control
Dr. Mandrola: One of my colleagues, who is even more skeptical than me, wanted me to ask you, why wouldn’t you take a patient with persistent atrial fibrillation who had heart failure and just cardiovert and use amiodarone and try and maintain sinus rhythm that way?
Dr. Sohns: It is important to mention that 50% of the patients have already had amiodarone before they were randomized and enrolled for the trial. It might bring you a couple of minutes or a couple of hours [of relief], but the patients would get recurrence.
It was very interesting also, and this is in line with the data from Jason Andrade, who demonstrated that we were able to reduce the percentage of patients with persistent atrial fibrillation to paroxysmal. We did a down-staging of the underlying disease. This is not possible with cardioversion or drugs, for example.
Dr. Sommer: What I really like about that question and that comment is the idea that rhythm control in this subset of patients obviously has a role and an importance. It may be a cardioversion initially, giving amiodarone if they didn’t have that before, and you can keep the patient in sinus rhythm with this therapy, I think we’re reaching the same goal.
I think the critical point to get into the mind of physicians who treat heart failure is that sinus rhythm is beneficial, however you get there. Ablation, of course, as in other studies, is the most powerful tool to get there. Cardioversion can be a really good thing to do; you just have to think about it and consider it.
Dr. Mandrola: I do want to say to everybody that there is a tension sometimes between the heart failure community and the electrophysiology community. I think the ideal situation is that we work together, because I think that we can help with the maintenance of sinus rhythm. The control group mortality at 1 year was 20%, and I’ve heard people say that that’s not advanced heart failure. Advanced heart failure patients have much higher mortality than that. My colleague who is a heart failure specialist was criticizing a selection bias in picking the best patients. How would you answer that?
Dr. Sohns: There are data available from Eurotransplant, for example, that the waiting list mortality is 18%, so I think we are almost in line with this 20% mortality in this conservative group. You cannot generalize it. All these patients have different histories. We have 60% dilated cardiomyopathy and 40% ischemic cardiomyopathy. I think it is a very representative group in contrast to your friend who suggests that it is not.
Dr. Sommer: What I like about the discussion is that some approach us to say that the mortality in the control group is much too high – like, what are you doing with those patients that you create so many endpoints? Then others say that it’s not high enough because that is not end-stage heart failure. Come on! We have a patient cohort that is very well described and very well characterized.
If the label is end-stage heart failure, advanced heart failure, or whatever, they are sicker than the patients that we had in earlier trials. The patients that we treated were mostly excluded from all other trials. We opened the door. We found a clear result. I think everyone can see whatever you like to see.
Dr. Mandrola: What would your take-home message be after having done this trial design, the trial was conducted in your single center, and you come up with these amazing results? What would your message be to the whole community?
Dr. Sohns: Taking into consideration how severely sick these patients are, I can just repeat it: They are one step away from death, more or less, or from surgical intervention that can prolong their life. You should also consider that there are options like atrial fibrillation ablation that can buy time, postpone the natural course, or even in some patients replace the destination therapy. Therefore, in my opinion the next guidelines should recommend that every patient should carefully be checked for sinus rhythm before bringing these patients into the environment of transplantation.
Dr. Sommer: My interpretation is that we have to try to bring into physicians’ minds that besides a well-established and well-documented effect of drug therapy with the fabulous four, we may now have the fabulous five, including an ablation option for patients with atrial fibrillation.
Dr. Mandrola is a clinical electrophysiologist at Baptist Medical Associates, Louisville, Ky. Dr. Sohns is deputy director of the Heart and Diabetes Center NRW, Ruhr University Bochum, Bad Oeynhausen, Germany. Dr. Sommer is professor of cardiology at the Heart and Diabetes Center NRW. Dr. Mandrola reported no conflicts of interest. Dr. Sohns reported receiving research funding from Else Kröner–Fresenius–Stiftung. Dr. Sommer reported consulting with Abbott, Biosense Webster, Boston Scientific, and Medtronic USA.
A version of this article first appeared on Medscape.com.
Heart failure guidelines update: What the ESC got right
This transcript has been edited for clarity.
This is my usual blog, except I am here from the absolutely beautiful city of Amsterdam, where the annual congress of the European Society of Cardiology has been going on.
SGLT2 inhibitors for HFpEF and HFrEF
I’m going to review very briefly the 2023 focused update to the ESC heart failure guidelines. Theresa McDonagh was the first author of this and of the previous ESC or European guidelines. These are a little bit different than the American guidelines, which were presented in 2022. We know that we need an update. The Europeans have gotten ahead of us, and now we have the European update, which I find incredibly well written and it really highlights the areas that I think the takeaways are for the clinicians.
First, we have been seeing now for several years – since 2018 – the benefits of the sodium-glucose cotransporter 2 (SGLT2) inhibitors. Every time we lift the veil on something, there they are in a positive light. We have learned about heart failure with reduced ejection fraction (HFrEF) for both empagliflozin and dapagliflozin. There are very similar results. One population may be enriched with a little of this and a little of that, but the basic messages are the same. In HFrEF, both of these drugs improve outcomes and it happens quickly. You don’t have to wait 1 or 2 years to see this. Within months, and actually within days, you start to see the curves split apart statistically.
The next logical ground was heart failure with preserved ejection fraction (HFpEF). The definition, when we started the HFpEF trials, was 45% or greater. I want the audience to realize that, in the midst of all these trials, we came out – we meaning the American Heart Association, the American College of Cardiology, and the Heart Failure Society – with the new definition of heart failure, which said that true HFpEF is 50% or greater. That in-between zone of 40%-50% or 41%-49% is mRF, or mid-range, what I call middle of the road. I think the Europeans have really emphasized that to us. I believe that those patients really behave much more like a HFrEF population.
Now that we have very positive findings with the SGLT2 inhibitors, both dapagliflozin and empagliflozin, in HFpEF – defined, as I said, as 40% or 45% or greater, not necessarily 50% – with excellent point estimates that just line up, one on top of the other. It doesn’t matter if patients have diabetes or not; the results are exactly the same.
This has been so promising that I am not surprised that the Europeans elevated the SGLT2 inhibitors to a class 1A indication. In the United States in 2022, we thought we were really way ahead by calling it a class 2A indication. Well, now it’s a class 1A indication in Europe, and I have a feeling that the AHA and the ACC are going to start talking about an update because the data are so strong.
Now, we even have data on initiating these drugs in the hospital. EMPULSE was a very large trial about the benefits of starting these drugs in the hospital. You do not have to wait until the patient is in the outpatient setting. You can start it in the hospital.
When? I have no specific day that I start it. I used to try to do a good diuresis first, get the patients somewhat decongested, and then start it. I don’t want to deprive the patients of the benefits of these drugs that happen very early by waiting until the patients are in the outpatient setting.
In the United States, we’ve had some issues with coverage of some of these drugs. In my institution, we now have both on the formulary, and I pick the drug depending upon the patient’s coverage. Medicare pretty much covers most of them. If the patient is older but not yet a Medicare patient, they may have a very large copay. I advise you to get your offices or your health system to look into this so that, when you give the prescription to the patient, whether they’re leaving the hospital or are now in your clinics, they can actually get the drug.
Finerenone and intravenous iron
There is an additional recommendation in these guidelines for finerenone, the mineralocorticoid receptor agonist that I’ve discussed before, that has some really promising data on type 2 diabetes with chronic kidney disease. They have called that a class 1A indication for finerenone. I think there is more to come.
One more: the iron deficiency. Giving intravenous iron actually does improve symptoms and quality of life. I have seen this in my own patients, so I have been very diligent at looking for iron deficiency.
It is a new era. We have more tools, obviously, for our patients. It means one more drug, and that’s always a challenge. We’ve already been doing the pillars of care. This is the fourth pillar of care, but now with a class 1A indication.
Take a look. They’re easy to read. Dr. McDonagh is the first author, and I think they’ve been extremely well done.
Dr. Piña is professor of medicine at Thomas Jefferson University Hospital in Philadelphia. She is a heart failure and cardiac transplantation expert. She disclosed serving as an adviser/consultant to the FDA’s Center for Devices and Radiological Health and has been a volunteer for the American Heart Association since 1982.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
This is my usual blog, except I am here from the absolutely beautiful city of Amsterdam, where the annual congress of the European Society of Cardiology has been going on.
SGLT2 inhibitors for HFpEF and HFrEF
I’m going to review very briefly the 2023 focused update to the ESC heart failure guidelines. Theresa McDonagh was the first author of this and of the previous ESC or European guidelines. These are a little bit different than the American guidelines, which were presented in 2022. We know that we need an update. The Europeans have gotten ahead of us, and now we have the European update, which I find incredibly well written and it really highlights the areas that I think the takeaways are for the clinicians.
First, we have been seeing now for several years – since 2018 – the benefits of the sodium-glucose cotransporter 2 (SGLT2) inhibitors. Every time we lift the veil on something, there they are in a positive light. We have learned about heart failure with reduced ejection fraction (HFrEF) for both empagliflozin and dapagliflozin. There are very similar results. One population may be enriched with a little of this and a little of that, but the basic messages are the same. In HFrEF, both of these drugs improve outcomes and it happens quickly. You don’t have to wait 1 or 2 years to see this. Within months, and actually within days, you start to see the curves split apart statistically.
The next logical ground was heart failure with preserved ejection fraction (HFpEF). The definition, when we started the HFpEF trials, was 45% or greater. I want the audience to realize that, in the midst of all these trials, we came out – we meaning the American Heart Association, the American College of Cardiology, and the Heart Failure Society – with the new definition of heart failure, which said that true HFpEF is 50% or greater. That in-between zone of 40%-50% or 41%-49% is mRF, or mid-range, what I call middle of the road. I think the Europeans have really emphasized that to us. I believe that those patients really behave much more like a HFrEF population.
Now that we have very positive findings with the SGLT2 inhibitors, both dapagliflozin and empagliflozin, in HFpEF – defined, as I said, as 40% or 45% or greater, not necessarily 50% – with excellent point estimates that just line up, one on top of the other. It doesn’t matter if patients have diabetes or not; the results are exactly the same.
This has been so promising that I am not surprised that the Europeans elevated the SGLT2 inhibitors to a class 1A indication. In the United States in 2022, we thought we were really way ahead by calling it a class 2A indication. Well, now it’s a class 1A indication in Europe, and I have a feeling that the AHA and the ACC are going to start talking about an update because the data are so strong.
Now, we even have data on initiating these drugs in the hospital. EMPULSE was a very large trial about the benefits of starting these drugs in the hospital. You do not have to wait until the patient is in the outpatient setting. You can start it in the hospital.
When? I have no specific day that I start it. I used to try to do a good diuresis first, get the patients somewhat decongested, and then start it. I don’t want to deprive the patients of the benefits of these drugs that happen very early by waiting until the patients are in the outpatient setting.
In the United States, we’ve had some issues with coverage of some of these drugs. In my institution, we now have both on the formulary, and I pick the drug depending upon the patient’s coverage. Medicare pretty much covers most of them. If the patient is older but not yet a Medicare patient, they may have a very large copay. I advise you to get your offices or your health system to look into this so that, when you give the prescription to the patient, whether they’re leaving the hospital or are now in your clinics, they can actually get the drug.
Finerenone and intravenous iron
There is an additional recommendation in these guidelines for finerenone, the mineralocorticoid receptor agonist that I’ve discussed before, that has some really promising data on type 2 diabetes with chronic kidney disease. They have called that a class 1A indication for finerenone. I think there is more to come.
One more: the iron deficiency. Giving intravenous iron actually does improve symptoms and quality of life. I have seen this in my own patients, so I have been very diligent at looking for iron deficiency.
It is a new era. We have more tools, obviously, for our patients. It means one more drug, and that’s always a challenge. We’ve already been doing the pillars of care. This is the fourth pillar of care, but now with a class 1A indication.
Take a look. They’re easy to read. Dr. McDonagh is the first author, and I think they’ve been extremely well done.
Dr. Piña is professor of medicine at Thomas Jefferson University Hospital in Philadelphia. She is a heart failure and cardiac transplantation expert. She disclosed serving as an adviser/consultant to the FDA’s Center for Devices and Radiological Health and has been a volunteer for the American Heart Association since 1982.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
This is my usual blog, except I am here from the absolutely beautiful city of Amsterdam, where the annual congress of the European Society of Cardiology has been going on.
SGLT2 inhibitors for HFpEF and HFrEF
I’m going to review very briefly the 2023 focused update to the ESC heart failure guidelines. Theresa McDonagh was the first author of this and of the previous ESC or European guidelines. These are a little bit different than the American guidelines, which were presented in 2022. We know that we need an update. The Europeans have gotten ahead of us, and now we have the European update, which I find incredibly well written and it really highlights the areas that I think the takeaways are for the clinicians.
First, we have been seeing now for several years – since 2018 – the benefits of the sodium-glucose cotransporter 2 (SGLT2) inhibitors. Every time we lift the veil on something, there they are in a positive light. We have learned about heart failure with reduced ejection fraction (HFrEF) for both empagliflozin and dapagliflozin. There are very similar results. One population may be enriched with a little of this and a little of that, but the basic messages are the same. In HFrEF, both of these drugs improve outcomes and it happens quickly. You don’t have to wait 1 or 2 years to see this. Within months, and actually within days, you start to see the curves split apart statistically.
The next logical ground was heart failure with preserved ejection fraction (HFpEF). The definition, when we started the HFpEF trials, was 45% or greater. I want the audience to realize that, in the midst of all these trials, we came out – we meaning the American Heart Association, the American College of Cardiology, and the Heart Failure Society – with the new definition of heart failure, which said that true HFpEF is 50% or greater. That in-between zone of 40%-50% or 41%-49% is mRF, or mid-range, what I call middle of the road. I think the Europeans have really emphasized that to us. I believe that those patients really behave much more like a HFrEF population.
Now that we have very positive findings with the SGLT2 inhibitors, both dapagliflozin and empagliflozin, in HFpEF – defined, as I said, as 40% or 45% or greater, not necessarily 50% – with excellent point estimates that just line up, one on top of the other. It doesn’t matter if patients have diabetes or not; the results are exactly the same.
This has been so promising that I am not surprised that the Europeans elevated the SGLT2 inhibitors to a class 1A indication. In the United States in 2022, we thought we were really way ahead by calling it a class 2A indication. Well, now it’s a class 1A indication in Europe, and I have a feeling that the AHA and the ACC are going to start talking about an update because the data are so strong.
Now, we even have data on initiating these drugs in the hospital. EMPULSE was a very large trial about the benefits of starting these drugs in the hospital. You do not have to wait until the patient is in the outpatient setting. You can start it in the hospital.
When? I have no specific day that I start it. I used to try to do a good diuresis first, get the patients somewhat decongested, and then start it. I don’t want to deprive the patients of the benefits of these drugs that happen very early by waiting until the patients are in the outpatient setting.
In the United States, we’ve had some issues with coverage of some of these drugs. In my institution, we now have both on the formulary, and I pick the drug depending upon the patient’s coverage. Medicare pretty much covers most of them. If the patient is older but not yet a Medicare patient, they may have a very large copay. I advise you to get your offices or your health system to look into this so that, when you give the prescription to the patient, whether they’re leaving the hospital or are now in your clinics, they can actually get the drug.
Finerenone and intravenous iron
There is an additional recommendation in these guidelines for finerenone, the mineralocorticoid receptor agonist that I’ve discussed before, that has some really promising data on type 2 diabetes with chronic kidney disease. They have called that a class 1A indication for finerenone. I think there is more to come.
One more: the iron deficiency. Giving intravenous iron actually does improve symptoms and quality of life. I have seen this in my own patients, so I have been very diligent at looking for iron deficiency.
It is a new era. We have more tools, obviously, for our patients. It means one more drug, and that’s always a challenge. We’ve already been doing the pillars of care. This is the fourth pillar of care, but now with a class 1A indication.
Take a look. They’re easy to read. Dr. McDonagh is the first author, and I think they’ve been extremely well done.
Dr. Piña is professor of medicine at Thomas Jefferson University Hospital in Philadelphia. She is a heart failure and cardiac transplantation expert. She disclosed serving as an adviser/consultant to the FDA’s Center for Devices and Radiological Health and has been a volunteer for the American Heart Association since 1982.
A version of this article appeared on Medscape.com.
Is complete revascularization now compulsory? MULTISTARS-AMI and FIRE in context
This transcript has been edited for clarity.
Michelle L. O’Donoghue, MD, MPH: Hi. This is Dr. Michelle O’Donoghue reporting for Medscape. Joining me today is Dr. Sahil Parikh, who’s a cardiologist and an interventionalist at Columbia University. He’s an associate professor of medicine.
We’ll be discussing two interesting trials that were presented at the ESC Congress here in Amsterdam. They do have the potential to be very practice-changing, so I think it’s worth talking about.
The FIRE trial
The first trial we’ll be talking about is the FIRE trial. Perhaps setting the stage, Sahil, I’d love to get your thoughts. We’ve had data in this space to suggest that, for patients with STEMI [ST-segment elevation myocardial infarction], a strategy of complete revascularization – and not only treating the culprit lesion but also treating additional lesions – may be of benefit. Where does that lead us in terms of what we didn’t know?
Sahil A. Parikh, MD: I think that the practice has moved, at least in the United States, over the past two decades, from staging percutaneous coronary interventions over 30 days from index to intervention to now trying to do patients in the same hospitalization whenever possible to achieve complete revascularization.
I think these data support not only that complete revascularization is compulsory now in these patients, but also doing it sooner rather than later, and that the benefit applies to most of the patients that we see in clinical practice. In the earlier data, the patients were relatively youthful – under Medicare age, less than 65 – and now this dataset has a median age of 80. This is more like the real-world clinical practice that most of us are encountering, and it extends the benefit, perhaps, greater than we’ve ever seen before.
O’Donoghue: The FIRE trial is interesting. As you say, it enrolled patients who were over the age of 75, where I think that some proceduralists are probably a little bit hesitant to think about complete revascularization due to concerns about any additional contrast load on their kidneys and other types of comorbidities. Of course, for any trial, there’s going to be some patient selection.
I think it’s very reassuring that even in this older patient group, a strategy of treating all the lesions – and not only in STEMI but also in non-STEMI patients – reduced cardiovascular events and mortality. I was really quite impressed by the mortality benefit.
Parikh: The mortality curve is almost surprising to me. On the other hand, it emboldens us now that we can treat these patients more completely and earlier in their clinical presentation. Certainly, we worried about contrast exposure and the duration of procedures in this older population, but it seems that the benefit that’s derived, which we saw in younger patients where we had a natural inclination to be more aggressive, extends also to this older population.
MULTISTARS AMI
O’Donoghue: To the question of timing, as you mentioned, prior to this, we had a study presented earlier this year, the BIOVASC trial, which also was suggestive that maybe earlier complete revascularization was better. But it wasn’t a significant difference, at least for the primary outcome. Now we have MULTISTARS AMI, which is very supportive of what we saw earlier this year, suggesting that complete revascularization really at the time that you’re treating the culprit may be the way to go.
Parikh: All of us, as interventionalists, are circumspect about what we might do in the middle of the night versus what we would do in the light of day. Certainly it seems clear, particularly if it’s straightforward anatomy, that taking care of it in the index procedure is not only saving contrast and fluoroscopy time, but it’s also providing a clinical benefit to the patients. That’s something that will also impact how clinicians interpret these data. Previously, there was always a question about whether we should just do it in the same hospitalization or do it at the same time. I think now, increasingly, we’re emboldened to do more in the index procedure.
O’Donoghue: When you’re thinking about nonculprit lesions and which ones to treat, do you always make that determination based on physiologic guidance of some kind? Are you using instantaneous wave-free ratio? What’s your practice?
Parikh: In the acute setting, imaging is superior for at least the assessment of which is a culprit. If you see a ruptured atherothrombotic situation on optical coherence tomography, for example, that’s fairly convincing and definitive. In the absence of that physiology, we are taught to avoid in the infarct-related artery because of potential spuriously false-negative findings.
In this situation, certainly, an imaging subgroup probably would be helpful because some of the benefit is almost certainly derived from identifying the infarct-related artery by accident – in other words, doing what you thought was the nonculprit artery, which is, in fact, the culprit. I think that probably is part of this. As somebody who uses imaging in the overwhelming number of my cases, I think that imaging would be an important surrogate to this.
Index procedure versus staged
O’Donoghue: For the operator who is coming in to do their STEMI case at 2:00 in the morning, would these data now push you toward doing complete revascularization at that time of night, or do you think that there is wiggle room in terms of interpreting these results regarding timing, where as long as you were doing it before hospital discharge and not, let’s say, 30 days out, that you may be able to derive the same benefit? What are some of the pros and cons?
Parikh: There’s definitely a fatigue factor in the middle of the night if it’s a particularly arduous intervention for the index infarct-related artery. I think there’s a human element where it may make sense just to stop and then bring the patient back in the same hospitalization. It’s clear, though, that doing complete revascularization is better and doing it sooner is better. How soon one actually does it is a judgment call, as ever.
In our practice, we’ve been pushing ourselves to get most of the patients done in their index hospitalization. If you have a left-sided culprit, the left anterior descending artery, for example, and there’s a high-grade stenosis in the circumflex, it may make sense to take care of that in the same index procedure. If, on the other hand, it’s in the right coronary artery where you have to put a new guide in and spend more time, that may be a patient whom you stage. I think those nuances will come up as interventionalists look at the subgroup analysis data more carefully.
O’Donoghue: Those are great points, and I think they also underscore that we always need to think about what type of patient was enrolled in these studies. Certainly, if you have somebody with renal dysfunction, there might be more concern about giving them a large contrast load all in one sitting, albeit hard to know whether they do or not. But spacing that out by just a couple of days would really have a big impact.
Parikh: Very often in the STEMI patient, you don’t have the benefit of knowing the creatinine. The patient will come in immediately, if not directly from the ambulance to the cath lab, and there are no laboratories at all to work with. If the patient has never been seen in the system before, you won’t know. Again, in those situations, one may have pause, particularly if it’s an older patient. I think what’s reassuring, though, is that the data are supportive of being more aggressive earlier, and certainly this is the dataset that we were looking for.
O’Donoghue: To summarize, the two key takeaways are that, one, we now have more data to support a complete revascularization strategy and even extending that now to non-STEMI patients. Two, sooner appears to be better, so ideally, all done at the time of the index procedure. I think this is very interesting science and we’ll see how it changes practice.
Thanks for joining me today. Signing off for Medscape, this is Dr. Michelle O’Donoghue.
Michelle O’Donoghue is a cardiologist at Brigham and Women’s Hospital and senior investigator with the TIMI Study Group.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Michelle L. O’Donoghue, MD, MPH: Hi. This is Dr. Michelle O’Donoghue reporting for Medscape. Joining me today is Dr. Sahil Parikh, who’s a cardiologist and an interventionalist at Columbia University. He’s an associate professor of medicine.
We’ll be discussing two interesting trials that were presented at the ESC Congress here in Amsterdam. They do have the potential to be very practice-changing, so I think it’s worth talking about.
The FIRE trial
The first trial we’ll be talking about is the FIRE trial. Perhaps setting the stage, Sahil, I’d love to get your thoughts. We’ve had data in this space to suggest that, for patients with STEMI [ST-segment elevation myocardial infarction], a strategy of complete revascularization – and not only treating the culprit lesion but also treating additional lesions – may be of benefit. Where does that lead us in terms of what we didn’t know?
Sahil A. Parikh, MD: I think that the practice has moved, at least in the United States, over the past two decades, from staging percutaneous coronary interventions over 30 days from index to intervention to now trying to do patients in the same hospitalization whenever possible to achieve complete revascularization.
I think these data support not only that complete revascularization is compulsory now in these patients, but also doing it sooner rather than later, and that the benefit applies to most of the patients that we see in clinical practice. In the earlier data, the patients were relatively youthful – under Medicare age, less than 65 – and now this dataset has a median age of 80. This is more like the real-world clinical practice that most of us are encountering, and it extends the benefit, perhaps, greater than we’ve ever seen before.
O’Donoghue: The FIRE trial is interesting. As you say, it enrolled patients who were over the age of 75, where I think that some proceduralists are probably a little bit hesitant to think about complete revascularization due to concerns about any additional contrast load on their kidneys and other types of comorbidities. Of course, for any trial, there’s going to be some patient selection.
I think it’s very reassuring that even in this older patient group, a strategy of treating all the lesions – and not only in STEMI but also in non-STEMI patients – reduced cardiovascular events and mortality. I was really quite impressed by the mortality benefit.
Parikh: The mortality curve is almost surprising to me. On the other hand, it emboldens us now that we can treat these patients more completely and earlier in their clinical presentation. Certainly, we worried about contrast exposure and the duration of procedures in this older population, but it seems that the benefit that’s derived, which we saw in younger patients where we had a natural inclination to be more aggressive, extends also to this older population.
MULTISTARS AMI
O’Donoghue: To the question of timing, as you mentioned, prior to this, we had a study presented earlier this year, the BIOVASC trial, which also was suggestive that maybe earlier complete revascularization was better. But it wasn’t a significant difference, at least for the primary outcome. Now we have MULTISTARS AMI, which is very supportive of what we saw earlier this year, suggesting that complete revascularization really at the time that you’re treating the culprit may be the way to go.
Parikh: All of us, as interventionalists, are circumspect about what we might do in the middle of the night versus what we would do in the light of day. Certainly it seems clear, particularly if it’s straightforward anatomy, that taking care of it in the index procedure is not only saving contrast and fluoroscopy time, but it’s also providing a clinical benefit to the patients. That’s something that will also impact how clinicians interpret these data. Previously, there was always a question about whether we should just do it in the same hospitalization or do it at the same time. I think now, increasingly, we’re emboldened to do more in the index procedure.
O’Donoghue: When you’re thinking about nonculprit lesions and which ones to treat, do you always make that determination based on physiologic guidance of some kind? Are you using instantaneous wave-free ratio? What’s your practice?
Parikh: In the acute setting, imaging is superior for at least the assessment of which is a culprit. If you see a ruptured atherothrombotic situation on optical coherence tomography, for example, that’s fairly convincing and definitive. In the absence of that physiology, we are taught to avoid in the infarct-related artery because of potential spuriously false-negative findings.
In this situation, certainly, an imaging subgroup probably would be helpful because some of the benefit is almost certainly derived from identifying the infarct-related artery by accident – in other words, doing what you thought was the nonculprit artery, which is, in fact, the culprit. I think that probably is part of this. As somebody who uses imaging in the overwhelming number of my cases, I think that imaging would be an important surrogate to this.
Index procedure versus staged
O’Donoghue: For the operator who is coming in to do their STEMI case at 2:00 in the morning, would these data now push you toward doing complete revascularization at that time of night, or do you think that there is wiggle room in terms of interpreting these results regarding timing, where as long as you were doing it before hospital discharge and not, let’s say, 30 days out, that you may be able to derive the same benefit? What are some of the pros and cons?
Parikh: There’s definitely a fatigue factor in the middle of the night if it’s a particularly arduous intervention for the index infarct-related artery. I think there’s a human element where it may make sense just to stop and then bring the patient back in the same hospitalization. It’s clear, though, that doing complete revascularization is better and doing it sooner is better. How soon one actually does it is a judgment call, as ever.
In our practice, we’ve been pushing ourselves to get most of the patients done in their index hospitalization. If you have a left-sided culprit, the left anterior descending artery, for example, and there’s a high-grade stenosis in the circumflex, it may make sense to take care of that in the same index procedure. If, on the other hand, it’s in the right coronary artery where you have to put a new guide in and spend more time, that may be a patient whom you stage. I think those nuances will come up as interventionalists look at the subgroup analysis data more carefully.
O’Donoghue: Those are great points, and I think they also underscore that we always need to think about what type of patient was enrolled in these studies. Certainly, if you have somebody with renal dysfunction, there might be more concern about giving them a large contrast load all in one sitting, albeit hard to know whether they do or not. But spacing that out by just a couple of days would really have a big impact.
Parikh: Very often in the STEMI patient, you don’t have the benefit of knowing the creatinine. The patient will come in immediately, if not directly from the ambulance to the cath lab, and there are no laboratories at all to work with. If the patient has never been seen in the system before, you won’t know. Again, in those situations, one may have pause, particularly if it’s an older patient. I think what’s reassuring, though, is that the data are supportive of being more aggressive earlier, and certainly this is the dataset that we were looking for.
O’Donoghue: To summarize, the two key takeaways are that, one, we now have more data to support a complete revascularization strategy and even extending that now to non-STEMI patients. Two, sooner appears to be better, so ideally, all done at the time of the index procedure. I think this is very interesting science and we’ll see how it changes practice.
Thanks for joining me today. Signing off for Medscape, this is Dr. Michelle O’Donoghue.
Michelle O’Donoghue is a cardiologist at Brigham and Women’s Hospital and senior investigator with the TIMI Study Group.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Michelle L. O’Donoghue, MD, MPH: Hi. This is Dr. Michelle O’Donoghue reporting for Medscape. Joining me today is Dr. Sahil Parikh, who’s a cardiologist and an interventionalist at Columbia University. He’s an associate professor of medicine.
We’ll be discussing two interesting trials that were presented at the ESC Congress here in Amsterdam. They do have the potential to be very practice-changing, so I think it’s worth talking about.
The FIRE trial
The first trial we’ll be talking about is the FIRE trial. Perhaps setting the stage, Sahil, I’d love to get your thoughts. We’ve had data in this space to suggest that, for patients with STEMI [ST-segment elevation myocardial infarction], a strategy of complete revascularization – and not only treating the culprit lesion but also treating additional lesions – may be of benefit. Where does that lead us in terms of what we didn’t know?
Sahil A. Parikh, MD: I think that the practice has moved, at least in the United States, over the past two decades, from staging percutaneous coronary interventions over 30 days from index to intervention to now trying to do patients in the same hospitalization whenever possible to achieve complete revascularization.
I think these data support not only that complete revascularization is compulsory now in these patients, but also doing it sooner rather than later, and that the benefit applies to most of the patients that we see in clinical practice. In the earlier data, the patients were relatively youthful – under Medicare age, less than 65 – and now this dataset has a median age of 80. This is more like the real-world clinical practice that most of us are encountering, and it extends the benefit, perhaps, greater than we’ve ever seen before.
O’Donoghue: The FIRE trial is interesting. As you say, it enrolled patients who were over the age of 75, where I think that some proceduralists are probably a little bit hesitant to think about complete revascularization due to concerns about any additional contrast load on their kidneys and other types of comorbidities. Of course, for any trial, there’s going to be some patient selection.
I think it’s very reassuring that even in this older patient group, a strategy of treating all the lesions – and not only in STEMI but also in non-STEMI patients – reduced cardiovascular events and mortality. I was really quite impressed by the mortality benefit.
Parikh: The mortality curve is almost surprising to me. On the other hand, it emboldens us now that we can treat these patients more completely and earlier in their clinical presentation. Certainly, we worried about contrast exposure and the duration of procedures in this older population, but it seems that the benefit that’s derived, which we saw in younger patients where we had a natural inclination to be more aggressive, extends also to this older population.
MULTISTARS AMI
O’Donoghue: To the question of timing, as you mentioned, prior to this, we had a study presented earlier this year, the BIOVASC trial, which also was suggestive that maybe earlier complete revascularization was better. But it wasn’t a significant difference, at least for the primary outcome. Now we have MULTISTARS AMI, which is very supportive of what we saw earlier this year, suggesting that complete revascularization really at the time that you’re treating the culprit may be the way to go.
Parikh: All of us, as interventionalists, are circumspect about what we might do in the middle of the night versus what we would do in the light of day. Certainly it seems clear, particularly if it’s straightforward anatomy, that taking care of it in the index procedure is not only saving contrast and fluoroscopy time, but it’s also providing a clinical benefit to the patients. That’s something that will also impact how clinicians interpret these data. Previously, there was always a question about whether we should just do it in the same hospitalization or do it at the same time. I think now, increasingly, we’re emboldened to do more in the index procedure.
O’Donoghue: When you’re thinking about nonculprit lesions and which ones to treat, do you always make that determination based on physiologic guidance of some kind? Are you using instantaneous wave-free ratio? What’s your practice?
Parikh: In the acute setting, imaging is superior for at least the assessment of which is a culprit. If you see a ruptured atherothrombotic situation on optical coherence tomography, for example, that’s fairly convincing and definitive. In the absence of that physiology, we are taught to avoid in the infarct-related artery because of potential spuriously false-negative findings.
In this situation, certainly, an imaging subgroup probably would be helpful because some of the benefit is almost certainly derived from identifying the infarct-related artery by accident – in other words, doing what you thought was the nonculprit artery, which is, in fact, the culprit. I think that probably is part of this. As somebody who uses imaging in the overwhelming number of my cases, I think that imaging would be an important surrogate to this.
Index procedure versus staged
O’Donoghue: For the operator who is coming in to do their STEMI case at 2:00 in the morning, would these data now push you toward doing complete revascularization at that time of night, or do you think that there is wiggle room in terms of interpreting these results regarding timing, where as long as you were doing it before hospital discharge and not, let’s say, 30 days out, that you may be able to derive the same benefit? What are some of the pros and cons?
Parikh: There’s definitely a fatigue factor in the middle of the night if it’s a particularly arduous intervention for the index infarct-related artery. I think there’s a human element where it may make sense just to stop and then bring the patient back in the same hospitalization. It’s clear, though, that doing complete revascularization is better and doing it sooner is better. How soon one actually does it is a judgment call, as ever.
In our practice, we’ve been pushing ourselves to get most of the patients done in their index hospitalization. If you have a left-sided culprit, the left anterior descending artery, for example, and there’s a high-grade stenosis in the circumflex, it may make sense to take care of that in the same index procedure. If, on the other hand, it’s in the right coronary artery where you have to put a new guide in and spend more time, that may be a patient whom you stage. I think those nuances will come up as interventionalists look at the subgroup analysis data more carefully.
O’Donoghue: Those are great points, and I think they also underscore that we always need to think about what type of patient was enrolled in these studies. Certainly, if you have somebody with renal dysfunction, there might be more concern about giving them a large contrast load all in one sitting, albeit hard to know whether they do or not. But spacing that out by just a couple of days would really have a big impact.
Parikh: Very often in the STEMI patient, you don’t have the benefit of knowing the creatinine. The patient will come in immediately, if not directly from the ambulance to the cath lab, and there are no laboratories at all to work with. If the patient has never been seen in the system before, you won’t know. Again, in those situations, one may have pause, particularly if it’s an older patient. I think what’s reassuring, though, is that the data are supportive of being more aggressive earlier, and certainly this is the dataset that we were looking for.
O’Donoghue: To summarize, the two key takeaways are that, one, we now have more data to support a complete revascularization strategy and even extending that now to non-STEMI patients. Two, sooner appears to be better, so ideally, all done at the time of the index procedure. I think this is very interesting science and we’ll see how it changes practice.
Thanks for joining me today. Signing off for Medscape, this is Dr. Michelle O’Donoghue.
Michelle O’Donoghue is a cardiologist at Brigham and Women’s Hospital and senior investigator with the TIMI Study Group.
A version of this article first appeared on Medscape.com.
Tools—and rules—to support behavior change
Changing behavior is hard. And at nearly every clinical encounter, we counsel/encourage/remind/help (choose a verb) our patients to make a change—to do something hard. We tell them they need to increase their physical activity, get more sleep, or alter their eating habits. We know that if they make the needed changes, they can improve their health and possibly lengthen their lives. But we also know (from the systematic reviews the US Preventive Services Task Force [USPSTF] uses to make its recommendations) that brief counseling in our offices is largely ineffective unless we connect patients to resources to support the recommended change.
As examples, the USPSTF currently recommends the following (both grade “B”):
- offer or refer adults with cardiovascular disease risk factors to behavioral counseling interventions to promote a healthy diet and physical activity.1
- offer or refer adults with a body mass index of 30 or higher to intensive, multicomponent behavioral interventions.2
To support our patients when making recommendations such as these, we might refer them to a dietitian for intensive counseling and meal-planning guidance. The American Diabetes Association says that patients seeking to manage their diabetes and prediabetes “can start by working with a registered dietitian nutritionist … to make an eating plan that works for [them].”3 However, this kind of resource is unavailable to many of our patients.
So what else can we do?
We can help patients decide what to buy in the grocery aisle. Nutrition labels are useful, but they are limited by their complexity and requisite level of health literacy.4 Even the concept of “calories” is not so intuitive. This challenge with interpreting calories led me (in some of my prior work) to explore a potentially more useful approach: conveying calorie information as physical activity equivalents.5
In this issue of The Journal of Family Practice, Dong and colleagues present their findings on whether a simple equation (the Altman Rule) that uses information on nutrition labels may be a reasonable proxy for an even more difficult concept—glycemic load.6 The idea is that consumers (eg, patients with diabetes) can use this rule to help them in their decision-making at the grocery store (or the convenience store or gas station, for that matter, where the high-glycemic-load carbohydrates may be even more tempting). The 2-step rule is tech-free and can be applied in a few seconds. Their research demonstrated that the rule is a reasonable proxy for glycemic load for packaged carbohydrates (eg, chips, cereals, crackers, granola bars). Caveats acknowledged, foods that meet the rule are likely to be healthier choices.
Looking ahead, I would like to see whether counseling patients about the Altman Rule leads to their use of it, and how that translates into healthier eating, lower A1C, and ideally better health. For now, the Altman Rule is worth learning about. It may serve as another tool that you can use to support your patients when you ask them to do the hard work of making healthier food choices.
1. US Preventive Services Task Force. Behavioral counseling interventions to promote a healthy diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: US Preventive Services Task Force recommendation statement. JAMA. 2020;324:2069-2075. doi: 10.1001/jama.2020.21749
2. US Preventive Services Task Force. Behavioral weight loss interventions to prevent obesity-related morbidity and mortality in adults: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:1163-1171. doi: 10.1001/jama.2018.13022
3. American Diabetes Association. Eating right doesn’t have to be boring. Accessed August 23, 2023. diabetes.org/healthy-living/recipes-nutrition
4. Weiss BD, Mays MZ, Martz W, et al. Quick assessment of literacy in primary care: the newest vital sign. Ann Fam Med. 2005;3:514-522. doi: 10.1370/afm.405
5. Viera AJ, Gizlice Z, Tuttle L, et al. Effect of calories-only vs physical activity calorie expenditure labeling on lunch calories purchased in worksite cafeterias. BMC Public Health. 2019;19:107. doi: 10.1186/s12889-019-6433-x
6. Dong KR, Eustis S, Hawkins K, et al. Is the Altman Rule a proxy for glycemic load? J Fam Pract. 2023;72:286-291. doi: 10.12788/jfp.0656
Changing behavior is hard. And at nearly every clinical encounter, we counsel/encourage/remind/help (choose a verb) our patients to make a change—to do something hard. We tell them they need to increase their physical activity, get more sleep, or alter their eating habits. We know that if they make the needed changes, they can improve their health and possibly lengthen their lives. But we also know (from the systematic reviews the US Preventive Services Task Force [USPSTF] uses to make its recommendations) that brief counseling in our offices is largely ineffective unless we connect patients to resources to support the recommended change.
As examples, the USPSTF currently recommends the following (both grade “B”):
- offer or refer adults with cardiovascular disease risk factors to behavioral counseling interventions to promote a healthy diet and physical activity.1
- offer or refer adults with a body mass index of 30 or higher to intensive, multicomponent behavioral interventions.2
To support our patients when making recommendations such as these, we might refer them to a dietitian for intensive counseling and meal-planning guidance. The American Diabetes Association says that patients seeking to manage their diabetes and prediabetes “can start by working with a registered dietitian nutritionist … to make an eating plan that works for [them].”3 However, this kind of resource is unavailable to many of our patients.
So what else can we do?
We can help patients decide what to buy in the grocery aisle. Nutrition labels are useful, but they are limited by their complexity and requisite level of health literacy.4 Even the concept of “calories” is not so intuitive. This challenge with interpreting calories led me (in some of my prior work) to explore a potentially more useful approach: conveying calorie information as physical activity equivalents.5
In this issue of The Journal of Family Practice, Dong and colleagues present their findings on whether a simple equation (the Altman Rule) that uses information on nutrition labels may be a reasonable proxy for an even more difficult concept—glycemic load.6 The idea is that consumers (eg, patients with diabetes) can use this rule to help them in their decision-making at the grocery store (or the convenience store or gas station, for that matter, where the high-glycemic-load carbohydrates may be even more tempting). The 2-step rule is tech-free and can be applied in a few seconds. Their research demonstrated that the rule is a reasonable proxy for glycemic load for packaged carbohydrates (eg, chips, cereals, crackers, granola bars). Caveats acknowledged, foods that meet the rule are likely to be healthier choices.
Looking ahead, I would like to see whether counseling patients about the Altman Rule leads to their use of it, and how that translates into healthier eating, lower A1C, and ideally better health. For now, the Altman Rule is worth learning about. It may serve as another tool that you can use to support your patients when you ask them to do the hard work of making healthier food choices.
Changing behavior is hard. And at nearly every clinical encounter, we counsel/encourage/remind/help (choose a verb) our patients to make a change—to do something hard. We tell them they need to increase their physical activity, get more sleep, or alter their eating habits. We know that if they make the needed changes, they can improve their health and possibly lengthen their lives. But we also know (from the systematic reviews the US Preventive Services Task Force [USPSTF] uses to make its recommendations) that brief counseling in our offices is largely ineffective unless we connect patients to resources to support the recommended change.
As examples, the USPSTF currently recommends the following (both grade “B”):
- offer or refer adults with cardiovascular disease risk factors to behavioral counseling interventions to promote a healthy diet and physical activity.1
- offer or refer adults with a body mass index of 30 or higher to intensive, multicomponent behavioral interventions.2
To support our patients when making recommendations such as these, we might refer them to a dietitian for intensive counseling and meal-planning guidance. The American Diabetes Association says that patients seeking to manage their diabetes and prediabetes “can start by working with a registered dietitian nutritionist … to make an eating plan that works for [them].”3 However, this kind of resource is unavailable to many of our patients.
So what else can we do?
We can help patients decide what to buy in the grocery aisle. Nutrition labels are useful, but they are limited by their complexity and requisite level of health literacy.4 Even the concept of “calories” is not so intuitive. This challenge with interpreting calories led me (in some of my prior work) to explore a potentially more useful approach: conveying calorie information as physical activity equivalents.5
In this issue of The Journal of Family Practice, Dong and colleagues present their findings on whether a simple equation (the Altman Rule) that uses information on nutrition labels may be a reasonable proxy for an even more difficult concept—glycemic load.6 The idea is that consumers (eg, patients with diabetes) can use this rule to help them in their decision-making at the grocery store (or the convenience store or gas station, for that matter, where the high-glycemic-load carbohydrates may be even more tempting). The 2-step rule is tech-free and can be applied in a few seconds. Their research demonstrated that the rule is a reasonable proxy for glycemic load for packaged carbohydrates (eg, chips, cereals, crackers, granola bars). Caveats acknowledged, foods that meet the rule are likely to be healthier choices.
Looking ahead, I would like to see whether counseling patients about the Altman Rule leads to their use of it, and how that translates into healthier eating, lower A1C, and ideally better health. For now, the Altman Rule is worth learning about. It may serve as another tool that you can use to support your patients when you ask them to do the hard work of making healthier food choices.
1. US Preventive Services Task Force. Behavioral counseling interventions to promote a healthy diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: US Preventive Services Task Force recommendation statement. JAMA. 2020;324:2069-2075. doi: 10.1001/jama.2020.21749
2. US Preventive Services Task Force. Behavioral weight loss interventions to prevent obesity-related morbidity and mortality in adults: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:1163-1171. doi: 10.1001/jama.2018.13022
3. American Diabetes Association. Eating right doesn’t have to be boring. Accessed August 23, 2023. diabetes.org/healthy-living/recipes-nutrition
4. Weiss BD, Mays MZ, Martz W, et al. Quick assessment of literacy in primary care: the newest vital sign. Ann Fam Med. 2005;3:514-522. doi: 10.1370/afm.405
5. Viera AJ, Gizlice Z, Tuttle L, et al. Effect of calories-only vs physical activity calorie expenditure labeling on lunch calories purchased in worksite cafeterias. BMC Public Health. 2019;19:107. doi: 10.1186/s12889-019-6433-x
6. Dong KR, Eustis S, Hawkins K, et al. Is the Altman Rule a proxy for glycemic load? J Fam Pract. 2023;72:286-291. doi: 10.12788/jfp.0656
1. US Preventive Services Task Force. Behavioral counseling interventions to promote a healthy diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: US Preventive Services Task Force recommendation statement. JAMA. 2020;324:2069-2075. doi: 10.1001/jama.2020.21749
2. US Preventive Services Task Force. Behavioral weight loss interventions to prevent obesity-related morbidity and mortality in adults: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:1163-1171. doi: 10.1001/jama.2018.13022
3. American Diabetes Association. Eating right doesn’t have to be boring. Accessed August 23, 2023. diabetes.org/healthy-living/recipes-nutrition
4. Weiss BD, Mays MZ, Martz W, et al. Quick assessment of literacy in primary care: the newest vital sign. Ann Fam Med. 2005;3:514-522. doi: 10.1370/afm.405
5. Viera AJ, Gizlice Z, Tuttle L, et al. Effect of calories-only vs physical activity calorie expenditure labeling on lunch calories purchased in worksite cafeterias. BMC Public Health. 2019;19:107. doi: 10.1186/s12889-019-6433-x
6. Dong KR, Eustis S, Hawkins K, et al. Is the Altman Rule a proxy for glycemic load? J Fam Pract. 2023;72:286-291. doi: 10.12788/jfp.0656
Should people who play sports pay higher medical insurance premiums?
This transcript has been edited for clarity.
If you’re anywhere near Seattle, anywhere near Florida, or anywhere where it might be not oppressively hot outside but encouraging some people who might want to go out and get a little exercise, you’ve undoubtedly seen or heard of pickleball.
This took off, I think, out of Bainbridge Island, Wash. It was meant as a gentlemanly game where people didn’t exert themselves too much. The joke is you could play it while holding a drink in one hand. It’s gotten more popular and more competitive. It’s kind of a miniature version of tennis, with a smaller court, a plastic ball, and a wooden paddle. The ball can go back and forth rapidly, but you’re always playing doubles and it doesn’t take as much energy, exertion, and, if you will, fitness as a game like singles tennis.
The upside is it’s gotten many people outdoors getting some exercise and socializing. That’s all to the good. But a recent study suggested that there are about $500 million worth of injuries coming into the health care system associated with pickleball. There have been leg sprains, broken bones, people getting hit in the eye, hamstring pulls, and many other problems. I’ve been told that many of the spectators who show up for pickleball matches are there with a cast or have some kind of a wrap on because they were injured.
Well, many people have argued in the past about what we are going to do about health care costs. Some suggest if you voluntarily incur health care damage, you ought to pay for that yourself and you ought to have a big copay.
If you decide you’re going to do cross-country skiing or downhill skiing and you injure yourself, you chose to do it, so you pay. If you’re not going to maintain your weight, you’re going to smoke, or you’re going to ride around without a helmet, that’s your choice. You ought to pay.
I think the pickleball example is really a good challenge to these views. You obviously want people to go out and get some exercise. Here, we’re talking about a population that’s a little older and oftentimes doesn’t get out there as much as doctors would like to get the exercise that’s still important that they need, and yet it does incur injuries and problems.
My suggestion would be to make the game a little safer. Let’s try to encourage people to warm up more before they get out there and jump out of the car and engage in their pickleball battles. Goggles might be important to prevent the eye injuries in a game that’s played up close. Maybe we want to make sure that people look out for one another out there. If they think they’re getting dehydrated or tired, they should say, “Let’s sit down.”
I’m not willing to put a tax or a copay on the pickleball players of America. I know they choose to do it. It’s got an upside and benefits, as many things like skiing and other behaviors that have some risk do, but I think we want to be encouraging, not discouraging, of it.
I don’t like a society where anybody who tries to do something that takes risk winds up bearing extra cost for doing that. I understand that that gets people irritated when it comes to dangerous, hyper-risky behavior like smoking and not wearing a motorcycle helmet. I think the way to engage is not to call out the sinner or to try and punish those who are trying to do things that bring them enjoyment, reward, or in some of these cases, physical fitness, but to try to make things safer and try to gradually improve and get rid of the risk side to capture the full benefit side.
I’m not sure I’ve come up with all the best ways to make pickleball safer, but I think that’s where our thinking in health care should go. My view is to get out there and play pickleball. If you do pull your hamstring, raise my insurance premium a little bit. I’ll help to pay for it. Better you get some enjoyment and some exercise.
I get the downside, but come on, folks, we ought to be, as a community, somewhat supportive of the fun and recreation that our fellow citizens engage in.
Dr. Caplan is director, division of medical ethics, New York University Langone Medical Center. He disclosed serving as a director, officer, partner, employee, adviser, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position); and as a contributing author and adviser for Medscape.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
If you’re anywhere near Seattle, anywhere near Florida, or anywhere where it might be not oppressively hot outside but encouraging some people who might want to go out and get a little exercise, you’ve undoubtedly seen or heard of pickleball.
This took off, I think, out of Bainbridge Island, Wash. It was meant as a gentlemanly game where people didn’t exert themselves too much. The joke is you could play it while holding a drink in one hand. It’s gotten more popular and more competitive. It’s kind of a miniature version of tennis, with a smaller court, a plastic ball, and a wooden paddle. The ball can go back and forth rapidly, but you’re always playing doubles and it doesn’t take as much energy, exertion, and, if you will, fitness as a game like singles tennis.
The upside is it’s gotten many people outdoors getting some exercise and socializing. That’s all to the good. But a recent study suggested that there are about $500 million worth of injuries coming into the health care system associated with pickleball. There have been leg sprains, broken bones, people getting hit in the eye, hamstring pulls, and many other problems. I’ve been told that many of the spectators who show up for pickleball matches are there with a cast or have some kind of a wrap on because they were injured.
Well, many people have argued in the past about what we are going to do about health care costs. Some suggest if you voluntarily incur health care damage, you ought to pay for that yourself and you ought to have a big copay.
If you decide you’re going to do cross-country skiing or downhill skiing and you injure yourself, you chose to do it, so you pay. If you’re not going to maintain your weight, you’re going to smoke, or you’re going to ride around without a helmet, that’s your choice. You ought to pay.
I think the pickleball example is really a good challenge to these views. You obviously want people to go out and get some exercise. Here, we’re talking about a population that’s a little older and oftentimes doesn’t get out there as much as doctors would like to get the exercise that’s still important that they need, and yet it does incur injuries and problems.
My suggestion would be to make the game a little safer. Let’s try to encourage people to warm up more before they get out there and jump out of the car and engage in their pickleball battles. Goggles might be important to prevent the eye injuries in a game that’s played up close. Maybe we want to make sure that people look out for one another out there. If they think they’re getting dehydrated or tired, they should say, “Let’s sit down.”
I’m not willing to put a tax or a copay on the pickleball players of America. I know they choose to do it. It’s got an upside and benefits, as many things like skiing and other behaviors that have some risk do, but I think we want to be encouraging, not discouraging, of it.
I don’t like a society where anybody who tries to do something that takes risk winds up bearing extra cost for doing that. I understand that that gets people irritated when it comes to dangerous, hyper-risky behavior like smoking and not wearing a motorcycle helmet. I think the way to engage is not to call out the sinner or to try and punish those who are trying to do things that bring them enjoyment, reward, or in some of these cases, physical fitness, but to try to make things safer and try to gradually improve and get rid of the risk side to capture the full benefit side.
I’m not sure I’ve come up with all the best ways to make pickleball safer, but I think that’s where our thinking in health care should go. My view is to get out there and play pickleball. If you do pull your hamstring, raise my insurance premium a little bit. I’ll help to pay for it. Better you get some enjoyment and some exercise.
I get the downside, but come on, folks, we ought to be, as a community, somewhat supportive of the fun and recreation that our fellow citizens engage in.
Dr. Caplan is director, division of medical ethics, New York University Langone Medical Center. He disclosed serving as a director, officer, partner, employee, adviser, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position); and as a contributing author and adviser for Medscape.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
If you’re anywhere near Seattle, anywhere near Florida, or anywhere where it might be not oppressively hot outside but encouraging some people who might want to go out and get a little exercise, you’ve undoubtedly seen or heard of pickleball.
This took off, I think, out of Bainbridge Island, Wash. It was meant as a gentlemanly game where people didn’t exert themselves too much. The joke is you could play it while holding a drink in one hand. It’s gotten more popular and more competitive. It’s kind of a miniature version of tennis, with a smaller court, a plastic ball, and a wooden paddle. The ball can go back and forth rapidly, but you’re always playing doubles and it doesn’t take as much energy, exertion, and, if you will, fitness as a game like singles tennis.
The upside is it’s gotten many people outdoors getting some exercise and socializing. That’s all to the good. But a recent study suggested that there are about $500 million worth of injuries coming into the health care system associated with pickleball. There have been leg sprains, broken bones, people getting hit in the eye, hamstring pulls, and many other problems. I’ve been told that many of the spectators who show up for pickleball matches are there with a cast or have some kind of a wrap on because they were injured.
Well, many people have argued in the past about what we are going to do about health care costs. Some suggest if you voluntarily incur health care damage, you ought to pay for that yourself and you ought to have a big copay.
If you decide you’re going to do cross-country skiing or downhill skiing and you injure yourself, you chose to do it, so you pay. If you’re not going to maintain your weight, you’re going to smoke, or you’re going to ride around without a helmet, that’s your choice. You ought to pay.
I think the pickleball example is really a good challenge to these views. You obviously want people to go out and get some exercise. Here, we’re talking about a population that’s a little older and oftentimes doesn’t get out there as much as doctors would like to get the exercise that’s still important that they need, and yet it does incur injuries and problems.
My suggestion would be to make the game a little safer. Let’s try to encourage people to warm up more before they get out there and jump out of the car and engage in their pickleball battles. Goggles might be important to prevent the eye injuries in a game that’s played up close. Maybe we want to make sure that people look out for one another out there. If they think they’re getting dehydrated or tired, they should say, “Let’s sit down.”
I’m not willing to put a tax or a copay on the pickleball players of America. I know they choose to do it. It’s got an upside and benefits, as many things like skiing and other behaviors that have some risk do, but I think we want to be encouraging, not discouraging, of it.
I don’t like a society where anybody who tries to do something that takes risk winds up bearing extra cost for doing that. I understand that that gets people irritated when it comes to dangerous, hyper-risky behavior like smoking and not wearing a motorcycle helmet. I think the way to engage is not to call out the sinner or to try and punish those who are trying to do things that bring them enjoyment, reward, or in some of these cases, physical fitness, but to try to make things safer and try to gradually improve and get rid of the risk side to capture the full benefit side.
I’m not sure I’ve come up with all the best ways to make pickleball safer, but I think that’s where our thinking in health care should go. My view is to get out there and play pickleball. If you do pull your hamstring, raise my insurance premium a little bit. I’ll help to pay for it. Better you get some enjoyment and some exercise.
I get the downside, but come on, folks, we ought to be, as a community, somewhat supportive of the fun and recreation that our fellow citizens engage in.
Dr. Caplan is director, division of medical ethics, New York University Langone Medical Center. He disclosed serving as a director, officer, partner, employee, adviser, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position); and as a contributing author and adviser for Medscape.
A version of this article appeared on Medscape.com.