User login
Major changes in Medicare billing are planned for January 2021: Some specialties fare better than others
The Centers for Medicare and Medicaid Services (CMS) finalized an increase in the relative value of evaluation and management (E/M) service codes effective January 1, 2021, which results in an overall decrease in the payment for procedural services in the Medicare program. (Due to the mandate for budget neutrality, an increase in relative value units [RVUs] for E/M resulted in a large decrease in the conversion factor—the number of dollars per RVU). This has increased payments for endocrinologists, rheumatologists, and family medicine clinicians and decreased payments for radiologists, pathologists, and surgeons.
In a major win for physicians, CMS proposes to simplify documentation requirements for billing and focus on the complexity of the medical decision making (MDM) or the total time needed to care for the patient on the date of the service as the foundation for determining the relative value of the service. Therefore, there is no more counting bullets—ie, we don’t have to perform a comprehensive physical exam or review of systems to achieve a high level code! Prior to this change, time was only available for coding purposes when counseling and coordination of care was the predominant service (>50%), and only face-to-face time with the patient was considered. Effective January 1, for office and other outpatient services, total time on the calendar date of the encounter will be used. This acknowledges the intensity and value of non–face-to-face work.
Acting through CMS, the federal government influences greatly the US health care system. CMS is an agency in the Department of Health and Human Services that administers the Medicare program and partners with state governments to administer the Health Insurance Exchanges, Medicaid, and the Children’s Health Insurance programs (CHIP).1 In addition, CMS is responsible for enforcing quality care standards in long-term care facilities and clinical laboratories and the implementation of the Health Insurance Portability and Accountability Act.1
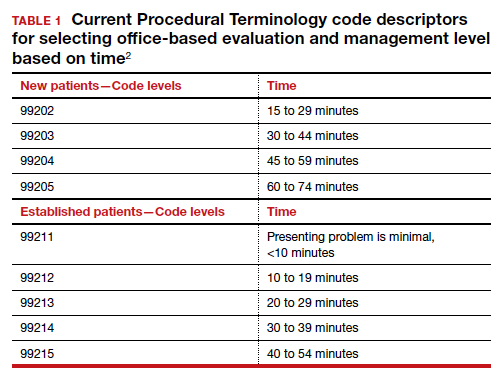
In January, CMS plans the following major changes to coding and documentation2,3:
- Selection of the level of E/M service will no longer require documentation of bullet points in the history, physical exam, and MDM. The simplified system allows physicians and qualified health care professionals to code either by total time (both face-to-face and non–face-to-face) on the date of the encounter or by level of MDM.
- For established office patients, 5 levels of office-based evaluation and management services will be retained. CMS had initially proposed to reduce the number of office-based E/M codes from 5 to 3, combining code levels 2, 3, and 4 into 1 code.4 However, after receiving feedback from professional societies and the public, CMS abandoned the plan for radical simplification of coding levels.2,3 Implementation of their proposal would have resulted in the same payment for treatment of a hang nail as for a complex gyn patient with multiple medical problems. Both patient advocacy groups and professional societies argued that incentives originally were misaligned.
- For new office patients, since both 99201 and 99202 require straightforward MDM, the level 1 code (99201) has been eliminated, reducing the number of code levels from 5 to 4.
- History and physical exam will no longer be used to determine code level for office E/M codes. These elements will be required only as medically appropriate. This means that documentation review will no longer focus on “bean counting” the elements in the history and physical exam.
- Following a reassessment of the actual time required to provide E/M services in real-life practice, CMS plans to markedly increase the relative value of office visits for established patients and modestly increase the relative value of office visits for new patients. CMS operates under the principle of “neutral budgeting,” meaning that an increase of the relative value of E/M codes will result in a decrease in the payment for procedural codes. The actual RVUs for procedural services do not change; however, budget neutrality requires a decrease in the dollar conversion factor. The proposed changes will increase the payment for E/M services and decrease payments for procedural services.
Continue to: Refocusing practice on MDM complexity...
Refocusing practice on MDM complexity
The practice of medicine is a calling with great rewards. Prominent among those rewards are improving the health of women, children, and the community, developing deep and trusting relationships with patients, families, and clinical colleagues. The practice of medicine is also replete with a host of punishing administrative burdens, including prior authorizations, clunky electronic medical records, poorly designed quality metrics that are applied to clinicians, and billing compliance rules that emphasize the repetitive documentation of clinical information with minimal value.
Some of the most irritating aspects of medical practice are the CMS rules governing medical record documentation required for billing ambulatory office visits. Current coding compliance focuses on counting the number of systems reviewed in the review of systems; the documentation of past history, social history, and family history; the number of organs and organ elements examined during the physical examination; and the complexity of MDM.
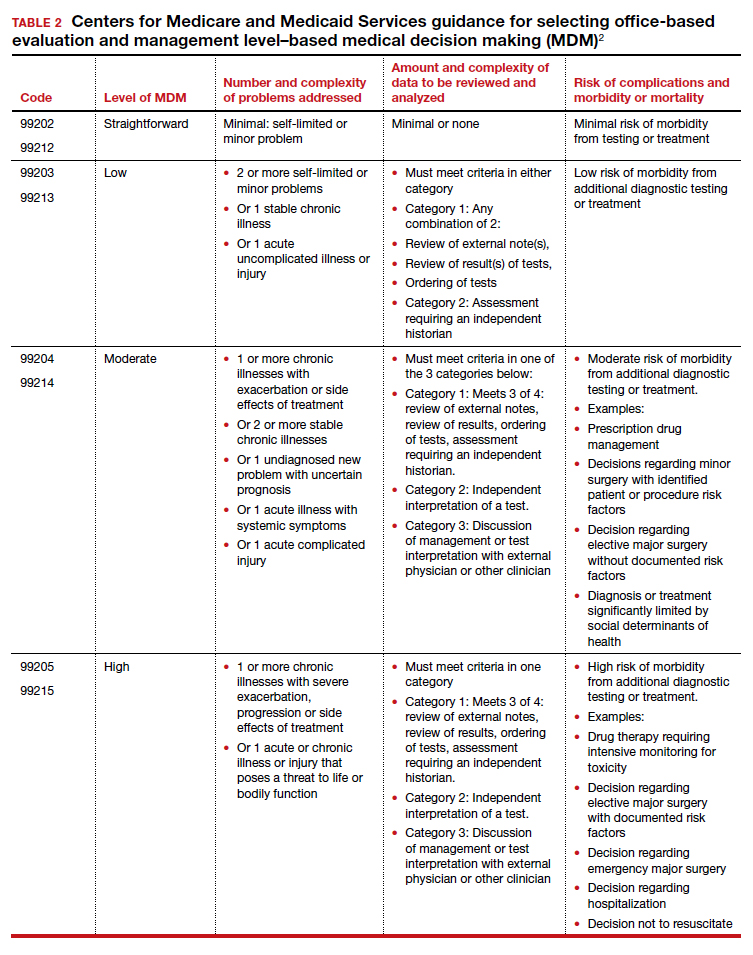
In January 2021, CMS plans to adopt new Current Procedural Terminology (CPT) code descriptors for the office and other outpatient E/M services that sunset most of the “bean-counting” metrics and emphasize the importance of the complexity of MDM in guiding selection of a correct code.2 Beginning in January 2021, clinicians will have the option of selecting an E/M code level based on the total amount of time required to provide the office visit service or the complexity of MDM. When selecting a code level based on MDM the new guidance emphasizes the importance of reviewing notes from other clinicians, reviewing test results, ordering of tests, and discussing and coordinating the care of the patient with other treating physicians. These changes reflect a better understanding of what is most important in good medical practice, promoting better patient care. TABLES 1 and 2 provide the initial guidance from CMS concerning selection of E/M code level based on time and MDM, respectively.2 The guidance for using MDM to select an E/M code level is likely to evolve following implementation, so stay tuned. When using MDM to select a code, 2 of the 3 general categories are required to select that level of service.
Increase in the valuation of office-based E/M services
The Medicare Physician Fee Schedule uses a resource-based relative value system to determine time and intensity of the work of clinical practice. This system recognizes 3 major factors that influence the resources required to provide a service:
- work of the clinician
- practice expense for technical components
- cost of professional liability insurance.
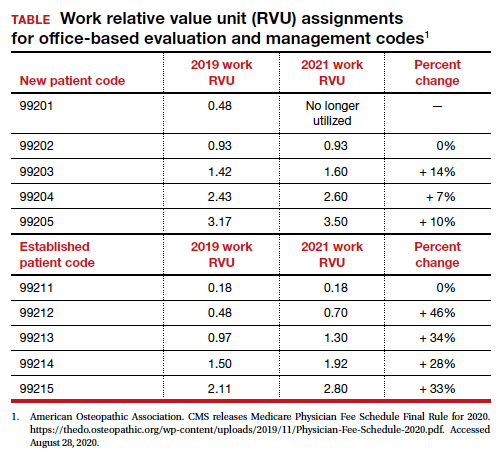
Many primary care professional associations have long contended that CMS has undervalued office-based E/M services relative to procedures, resulting in the devaluing of primary care practice. After the CPT code descriptors were updated by the CPT editorial panel, 52 specialty societies surveyed their members to provide inputs to CMS on the time and intensity of the office and other outpatient E/M codes as currently practiced. The American Medical Association’s Specialty Society Resource-Based Relative Value Scale Update Committee (RUC) reviewed the surveys and provided new inputs via open comment to CMS. CMS has responded to this feedback with a review of the intensity of clinical work required to provide an ambulatory visit service. In response to the review, CMS proposes to accept the recommendations of the RUC representing the house of medicine and increase the work and practice expense relative value assigned to new and established office visit codes. Overall, the combination of changes in relative values assigned for the work of the clinician and the expense of practice, increases the total value of office-based E/M codes for new patients by 7% to 14% and for established patients from 28% to 46% (see supplemental table in the sidebar at the end of this article).
Continue to: Decreased payments for procedural services...
Decreased payments for procedural services
Medicare is required to offset increased payment in one arena of health care delivery with decreased payment in other arenas of care, thereby achieving “budget-neutrality.” As detailed above, CMS plans to increase Medicare payments for office-based E/M services. Payment for services is calculated by multiplying the total RVUs for a particular service by a “conversion factor” (ie, number of dollars per RVU). To achieve budget-neutrality, CMS has proposed substantially reducing the conversion factor for 2021 (from $36.09 to $32.26), which will effectively decrease Medicare payments for procedural services since their RVUs have not changed. While the AMA RUC and many specialty societies continue to strongly advocate for the E/M work RVU increases to be included in the E/M components of 10- and 90-day global services, CMS has proposed to implement them only for “stand alone” E/M services.
Organizations are lobbying to delay or prevent the planned decrease in conversion factor, which results in substantial declines in payment for procedural services. (See "What do the Medicare billing changes mean for the Obstetrical Bundled services?" at the end of this article.) Due to the economic and clinical practice challenges caused by the coronavirus disease 2019 (COVID-19) pandemic it would be best if CMS did not reduce payments to physicians who are experts in procedural health care, thereby avoiding the risk of reduced access to these vital services.
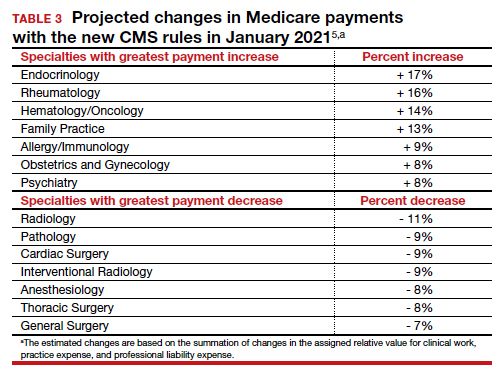
If the current CMS changes in payment are implemented, endocrinologists, rheumatologists, and family physicians will have an increase in payment, and radiologists, pathologists, and surgeons will have a decrease in payment (TABLE 3).6 Obstetrics and gynecology is projected to have an 8% increase in Medicare payment. However, if an obstetrician-gynecologist derives most of their Medicare payments from surgical procedures, they are likely to have a decrease in payment from Medicare. Other payers will be incorporating the new coding structure for 2021; however, their payment structures and conversion factors are likely to vary. It is important to note that the RVUs for procedures have not changed. The budget neutrality adjustment resulted in a much lower conversion factor and therefore a decrease in payment for those specialties whose RVUs did not increase.
Bottom line
Working through the Medicare, Medicaid, and CHIP programs, CMS can influence greatly the practice of medicine including medical record documentation practices and payment rates for every clinical service. CMS proposes to end the onerous “bean counting” approach to billing compliance and refocus on the complexity of MDM as the foundation for selecting a billing code level. This change is long overdue, valuing the effective management of complex patients in office practice. Hopefully, CMS will reverse the planned reduction in the payment for procedural services, preserving patient access to important health care services. ●
The CY 2020 Medicare Physician Fee Schedule Final Rule was published electronically in the Federal Register on November 1, 2019. This final rule aligns the evaluation and management (E/M) coding and payment with changes recommended by the Current Procedural Terminology (CPT) Editorial Panel and American Medical Association’s (AMA) Specialty Society Resource-Based Relative Value Scale Update Committee (RUC) for office/outpatient E/M visits. Unfortunately, the Centers for Medicare and Medicaid Services (CMS) did not agree with the RUC, AMA, and specialty societies that the E/M payment changes should be applicable across all global services that incorporate E/M visits—despite the fact that the values proposed by the RUC incorporated survey data from 52 specialties, representing most of medicine (including those specialties that predominantly perform procedures). Specifically, CMS expressed the view that the number of E/M visits within the 10- and 90-day global codes, as well as the maternity care bundle, were difficult to validate; therefore, the increased values would not be distributed to those procedural services.
Many professional societies expressed significant concerns about the resulting budget neutrality adjustments that would occur effective January 2021. The great news for ObGyns is that the American College of Obstetricians and Gynecologists (ACOG) was able to respond directly to CMS’s concerns with data to support the number of prenatal visits within the Obstetrical Bundle. Tapping into a de-identified, cloud-based data set of prenatal records—representing more than 1,100 obstetric providers with close to 30,000 recently completed pregnancies—ACOG was able to document both a mean and median number of prenatal visits across a broad geographic, payer, and patient demographic that supported the 13 prenatal visits in the Obstetrical Bundle.
With ACOG’s advocacy and ability to provide data to CMS, the proposed physician fee schedule rule for 2021 has proposed to incorporate the E/M increased reimbursement into the prenatal care codes. Now we urge the CMS to finalize this proposal. Although Medicare pays for a tiny number of pregnancies annually, we hope that all payers, including Medicaid and managed care plans, will agree with this acknowledgement of the increased work of evaluation and management that obstetricians provide during prenatal care. Join ACOG in telling CMS to finalize their proposal to increase the values of the global obstetric codes: https://acog.quorum.us/campaign/28579/.
- Centers for Medicare and Medicaid Services. https://www.cms.gov/. Accessed August 28, 2020.
- American Medical Association. CPT Evaluation and Management (E/M) Office or Other Outpatient (99202-99215) and Prolonged Services (99354, 99355, 99356, 99XXX) Code and Guideline Changes. 2019. https://www.ama-assn.org /system/files/2019-06/cpt-office-prolonged-svs -code-changes.pdf. Accessed August 28, 2020.
- The American Academy of Family Physicians. Family medicine updates. Ann Fam Med. 2020;18:84-85. doi: 10.1370/afm.2508.
- Centers for Medicare and Medicaid Services. Final policy, payment and quality provisions changes to the Medicare Physician Fee Schedule for calendar year 2019. November 1, 2018. https://www.cms.gov/newsroom/fact-sheets /final-policy-payment-and-quality-provisionschanges-medicare-physician-fee-schedulecalendar-year. Accessed August 28, 2020.
- Department of Health and Human Services; Centers for Medicare and Medicaid Services. 42 CFR Parts 410, 414, 415, 423, 424, and 425. Federal Register. 2020;85(159). https://www.govinfo.gov /content/pkg/FR-2020-08-17/pdf/2020-17127 .pdf. Accessed August 28, 2020.
The Centers for Medicare and Medicaid Services (CMS) finalized an increase in the relative value of evaluation and management (E/M) service codes effective January 1, 2021, which results in an overall decrease in the payment for procedural services in the Medicare program. (Due to the mandate for budget neutrality, an increase in relative value units [RVUs] for E/M resulted in a large decrease in the conversion factor—the number of dollars per RVU). This has increased payments for endocrinologists, rheumatologists, and family medicine clinicians and decreased payments for radiologists, pathologists, and surgeons.
In a major win for physicians, CMS proposes to simplify documentation requirements for billing and focus on the complexity of the medical decision making (MDM) or the total time needed to care for the patient on the date of the service as the foundation for determining the relative value of the service. Therefore, there is no more counting bullets—ie, we don’t have to perform a comprehensive physical exam or review of systems to achieve a high level code! Prior to this change, time was only available for coding purposes when counseling and coordination of care was the predominant service (>50%), and only face-to-face time with the patient was considered. Effective January 1, for office and other outpatient services, total time on the calendar date of the encounter will be used. This acknowledges the intensity and value of non–face-to-face work.
Acting through CMS, the federal government influences greatly the US health care system. CMS is an agency in the Department of Health and Human Services that administers the Medicare program and partners with state governments to administer the Health Insurance Exchanges, Medicaid, and the Children’s Health Insurance programs (CHIP).1 In addition, CMS is responsible for enforcing quality care standards in long-term care facilities and clinical laboratories and the implementation of the Health Insurance Portability and Accountability Act.1
In January, CMS plans the following major changes to coding and documentation2,3:
- Selection of the level of E/M service will no longer require documentation of bullet points in the history, physical exam, and MDM. The simplified system allows physicians and qualified health care professionals to code either by total time (both face-to-face and non–face-to-face) on the date of the encounter or by level of MDM.
- For established office patients, 5 levels of office-based evaluation and management services will be retained. CMS had initially proposed to reduce the number of office-based E/M codes from 5 to 3, combining code levels 2, 3, and 4 into 1 code.4 However, after receiving feedback from professional societies and the public, CMS abandoned the plan for radical simplification of coding levels.2,3 Implementation of their proposal would have resulted in the same payment for treatment of a hang nail as for a complex gyn patient with multiple medical problems. Both patient advocacy groups and professional societies argued that incentives originally were misaligned.
- For new office patients, since both 99201 and 99202 require straightforward MDM, the level 1 code (99201) has been eliminated, reducing the number of code levels from 5 to 4.
- History and physical exam will no longer be used to determine code level for office E/M codes. These elements will be required only as medically appropriate. This means that documentation review will no longer focus on “bean counting” the elements in the history and physical exam.
- Following a reassessment of the actual time required to provide E/M services in real-life practice, CMS plans to markedly increase the relative value of office visits for established patients and modestly increase the relative value of office visits for new patients. CMS operates under the principle of “neutral budgeting,” meaning that an increase of the relative value of E/M codes will result in a decrease in the payment for procedural codes. The actual RVUs for procedural services do not change; however, budget neutrality requires a decrease in the dollar conversion factor. The proposed changes will increase the payment for E/M services and decrease payments for procedural services.
Continue to: Refocusing practice on MDM complexity...
Refocusing practice on MDM complexity
The practice of medicine is a calling with great rewards. Prominent among those rewards are improving the health of women, children, and the community, developing deep and trusting relationships with patients, families, and clinical colleagues. The practice of medicine is also replete with a host of punishing administrative burdens, including prior authorizations, clunky electronic medical records, poorly designed quality metrics that are applied to clinicians, and billing compliance rules that emphasize the repetitive documentation of clinical information with minimal value.
Some of the most irritating aspects of medical practice are the CMS rules governing medical record documentation required for billing ambulatory office visits. Current coding compliance focuses on counting the number of systems reviewed in the review of systems; the documentation of past history, social history, and family history; the number of organs and organ elements examined during the physical examination; and the complexity of MDM.
In January 2021, CMS plans to adopt new Current Procedural Terminology (CPT) code descriptors for the office and other outpatient E/M services that sunset most of the “bean-counting” metrics and emphasize the importance of the complexity of MDM in guiding selection of a correct code.2 Beginning in January 2021, clinicians will have the option of selecting an E/M code level based on the total amount of time required to provide the office visit service or the complexity of MDM. When selecting a code level based on MDM the new guidance emphasizes the importance of reviewing notes from other clinicians, reviewing test results, ordering of tests, and discussing and coordinating the care of the patient with other treating physicians. These changes reflect a better understanding of what is most important in good medical practice, promoting better patient care. TABLES 1 and 2 provide the initial guidance from CMS concerning selection of E/M code level based on time and MDM, respectively.2 The guidance for using MDM to select an E/M code level is likely to evolve following implementation, so stay tuned. When using MDM to select a code, 2 of the 3 general categories are required to select that level of service.


Increase in the valuation of office-based E/M services
The Medicare Physician Fee Schedule uses a resource-based relative value system to determine time and intensity of the work of clinical practice. This system recognizes 3 major factors that influence the resources required to provide a service:
- work of the clinician
- practice expense for technical components
- cost of professional liability insurance.
Many primary care professional associations have long contended that CMS has undervalued office-based E/M services relative to procedures, resulting in the devaluing of primary care practice. After the CPT code descriptors were updated by the CPT editorial panel, 52 specialty societies surveyed their members to provide inputs to CMS on the time and intensity of the office and other outpatient E/M codes as currently practiced. The American Medical Association’s Specialty Society Resource-Based Relative Value Scale Update Committee (RUC) reviewed the surveys and provided new inputs via open comment to CMS. CMS has responded to this feedback with a review of the intensity of clinical work required to provide an ambulatory visit service. In response to the review, CMS proposes to accept the recommendations of the RUC representing the house of medicine and increase the work and practice expense relative value assigned to new and established office visit codes. Overall, the combination of changes in relative values assigned for the work of the clinician and the expense of practice, increases the total value of office-based E/M codes for new patients by 7% to 14% and for established patients from 28% to 46% (see supplemental table in the sidebar at the end of this article).
Continue to: Decreased payments for procedural services...
Decreased payments for procedural services
Medicare is required to offset increased payment in one arena of health care delivery with decreased payment in other arenas of care, thereby achieving “budget-neutrality.” As detailed above, CMS plans to increase Medicare payments for office-based E/M services. Payment for services is calculated by multiplying the total RVUs for a particular service by a “conversion factor” (ie, number of dollars per RVU). To achieve budget-neutrality, CMS has proposed substantially reducing the conversion factor for 2021 (from $36.09 to $32.26), which will effectively decrease Medicare payments for procedural services since their RVUs have not changed. While the AMA RUC and many specialty societies continue to strongly advocate for the E/M work RVU increases to be included in the E/M components of 10- and 90-day global services, CMS has proposed to implement them only for “stand alone” E/M services.
Organizations are lobbying to delay or prevent the planned decrease in conversion factor, which results in substantial declines in payment for procedural services. (See "What do the Medicare billing changes mean for the Obstetrical Bundled services?" at the end of this article.) Due to the economic and clinical practice challenges caused by the coronavirus disease 2019 (COVID-19) pandemic it would be best if CMS did not reduce payments to physicians who are experts in procedural health care, thereby avoiding the risk of reduced access to these vital services.
If the current CMS changes in payment are implemented, endocrinologists, rheumatologists, and family physicians will have an increase in payment, and radiologists, pathologists, and surgeons will have a decrease in payment (TABLE 3).6 Obstetrics and gynecology is projected to have an 8% increase in Medicare payment. However, if an obstetrician-gynecologist derives most of their Medicare payments from surgical procedures, they are likely to have a decrease in payment from Medicare. Other payers will be incorporating the new coding structure for 2021; however, their payment structures and conversion factors are likely to vary. It is important to note that the RVUs for procedures have not changed. The budget neutrality adjustment resulted in a much lower conversion factor and therefore a decrease in payment for those specialties whose RVUs did not increase.

Bottom line
Working through the Medicare, Medicaid, and CHIP programs, CMS can influence greatly the practice of medicine including medical record documentation practices and payment rates for every clinical service. CMS proposes to end the onerous “bean counting” approach to billing compliance and refocus on the complexity of MDM as the foundation for selecting a billing code level. This change is long overdue, valuing the effective management of complex patients in office practice. Hopefully, CMS will reverse the planned reduction in the payment for procedural services, preserving patient access to important health care services. ●
The CY 2020 Medicare Physician Fee Schedule Final Rule was published electronically in the Federal Register on November 1, 2019. This final rule aligns the evaluation and management (E/M) coding and payment with changes recommended by the Current Procedural Terminology (CPT) Editorial Panel and American Medical Association’s (AMA) Specialty Society Resource-Based Relative Value Scale Update Committee (RUC) for office/outpatient E/M visits. Unfortunately, the Centers for Medicare and Medicaid Services (CMS) did not agree with the RUC, AMA, and specialty societies that the E/M payment changes should be applicable across all global services that incorporate E/M visits—despite the fact that the values proposed by the RUC incorporated survey data from 52 specialties, representing most of medicine (including those specialties that predominantly perform procedures). Specifically, CMS expressed the view that the number of E/M visits within the 10- and 90-day global codes, as well as the maternity care bundle, were difficult to validate; therefore, the increased values would not be distributed to those procedural services.
Many professional societies expressed significant concerns about the resulting budget neutrality adjustments that would occur effective January 2021. The great news for ObGyns is that the American College of Obstetricians and Gynecologists (ACOG) was able to respond directly to CMS’s concerns with data to support the number of prenatal visits within the Obstetrical Bundle. Tapping into a de-identified, cloud-based data set of prenatal records—representing more than 1,100 obstetric providers with close to 30,000 recently completed pregnancies—ACOG was able to document both a mean and median number of prenatal visits across a broad geographic, payer, and patient demographic that supported the 13 prenatal visits in the Obstetrical Bundle.
With ACOG’s advocacy and ability to provide data to CMS, the proposed physician fee schedule rule for 2021 has proposed to incorporate the E/M increased reimbursement into the prenatal care codes. Now we urge the CMS to finalize this proposal. Although Medicare pays for a tiny number of pregnancies annually, we hope that all payers, including Medicaid and managed care plans, will agree with this acknowledgement of the increased work of evaluation and management that obstetricians provide during prenatal care. Join ACOG in telling CMS to finalize their proposal to increase the values of the global obstetric codes: https://acog.quorum.us/campaign/28579/.

The Centers for Medicare and Medicaid Services (CMS) finalized an increase in the relative value of evaluation and management (E/M) service codes effective January 1, 2021, which results in an overall decrease in the payment for procedural services in the Medicare program. (Due to the mandate for budget neutrality, an increase in relative value units [RVUs] for E/M resulted in a large decrease in the conversion factor—the number of dollars per RVU). This has increased payments for endocrinologists, rheumatologists, and family medicine clinicians and decreased payments for radiologists, pathologists, and surgeons.
In a major win for physicians, CMS proposes to simplify documentation requirements for billing and focus on the complexity of the medical decision making (MDM) or the total time needed to care for the patient on the date of the service as the foundation for determining the relative value of the service. Therefore, there is no more counting bullets—ie, we don’t have to perform a comprehensive physical exam or review of systems to achieve a high level code! Prior to this change, time was only available for coding purposes when counseling and coordination of care was the predominant service (>50%), and only face-to-face time with the patient was considered. Effective January 1, for office and other outpatient services, total time on the calendar date of the encounter will be used. This acknowledges the intensity and value of non–face-to-face work.
Acting through CMS, the federal government influences greatly the US health care system. CMS is an agency in the Department of Health and Human Services that administers the Medicare program and partners with state governments to administer the Health Insurance Exchanges, Medicaid, and the Children’s Health Insurance programs (CHIP).1 In addition, CMS is responsible for enforcing quality care standards in long-term care facilities and clinical laboratories and the implementation of the Health Insurance Portability and Accountability Act.1
In January, CMS plans the following major changes to coding and documentation2,3:
- Selection of the level of E/M service will no longer require documentation of bullet points in the history, physical exam, and MDM. The simplified system allows physicians and qualified health care professionals to code either by total time (both face-to-face and non–face-to-face) on the date of the encounter or by level of MDM.
- For established office patients, 5 levels of office-based evaluation and management services will be retained. CMS had initially proposed to reduce the number of office-based E/M codes from 5 to 3, combining code levels 2, 3, and 4 into 1 code.4 However, after receiving feedback from professional societies and the public, CMS abandoned the plan for radical simplification of coding levels.2,3 Implementation of their proposal would have resulted in the same payment for treatment of a hang nail as for a complex gyn patient with multiple medical problems. Both patient advocacy groups and professional societies argued that incentives originally were misaligned.
- For new office patients, since both 99201 and 99202 require straightforward MDM, the level 1 code (99201) has been eliminated, reducing the number of code levels from 5 to 4.
- History and physical exam will no longer be used to determine code level for office E/M codes. These elements will be required only as medically appropriate. This means that documentation review will no longer focus on “bean counting” the elements in the history and physical exam.
- Following a reassessment of the actual time required to provide E/M services in real-life practice, CMS plans to markedly increase the relative value of office visits for established patients and modestly increase the relative value of office visits for new patients. CMS operates under the principle of “neutral budgeting,” meaning that an increase of the relative value of E/M codes will result in a decrease in the payment for procedural codes. The actual RVUs for procedural services do not change; however, budget neutrality requires a decrease in the dollar conversion factor. The proposed changes will increase the payment for E/M services and decrease payments for procedural services.
Continue to: Refocusing practice on MDM complexity...
Refocusing practice on MDM complexity
The practice of medicine is a calling with great rewards. Prominent among those rewards are improving the health of women, children, and the community, developing deep and trusting relationships with patients, families, and clinical colleagues. The practice of medicine is also replete with a host of punishing administrative burdens, including prior authorizations, clunky electronic medical records, poorly designed quality metrics that are applied to clinicians, and billing compliance rules that emphasize the repetitive documentation of clinical information with minimal value.
Some of the most irritating aspects of medical practice are the CMS rules governing medical record documentation required for billing ambulatory office visits. Current coding compliance focuses on counting the number of systems reviewed in the review of systems; the documentation of past history, social history, and family history; the number of organs and organ elements examined during the physical examination; and the complexity of MDM.
In January 2021, CMS plans to adopt new Current Procedural Terminology (CPT) code descriptors for the office and other outpatient E/M services that sunset most of the “bean-counting” metrics and emphasize the importance of the complexity of MDM in guiding selection of a correct code.2 Beginning in January 2021, clinicians will have the option of selecting an E/M code level based on the total amount of time required to provide the office visit service or the complexity of MDM. When selecting a code level based on MDM the new guidance emphasizes the importance of reviewing notes from other clinicians, reviewing test results, ordering of tests, and discussing and coordinating the care of the patient with other treating physicians. These changes reflect a better understanding of what is most important in good medical practice, promoting better patient care. TABLES 1 and 2 provide the initial guidance from CMS concerning selection of E/M code level based on time and MDM, respectively.2 The guidance for using MDM to select an E/M code level is likely to evolve following implementation, so stay tuned. When using MDM to select a code, 2 of the 3 general categories are required to select that level of service.


Increase in the valuation of office-based E/M services
The Medicare Physician Fee Schedule uses a resource-based relative value system to determine time and intensity of the work of clinical practice. This system recognizes 3 major factors that influence the resources required to provide a service:
- work of the clinician
- practice expense for technical components
- cost of professional liability insurance.
Many primary care professional associations have long contended that CMS has undervalued office-based E/M services relative to procedures, resulting in the devaluing of primary care practice. After the CPT code descriptors were updated by the CPT editorial panel, 52 specialty societies surveyed their members to provide inputs to CMS on the time and intensity of the office and other outpatient E/M codes as currently practiced. The American Medical Association’s Specialty Society Resource-Based Relative Value Scale Update Committee (RUC) reviewed the surveys and provided new inputs via open comment to CMS. CMS has responded to this feedback with a review of the intensity of clinical work required to provide an ambulatory visit service. In response to the review, CMS proposes to accept the recommendations of the RUC representing the house of medicine and increase the work and practice expense relative value assigned to new and established office visit codes. Overall, the combination of changes in relative values assigned for the work of the clinician and the expense of practice, increases the total value of office-based E/M codes for new patients by 7% to 14% and for established patients from 28% to 46% (see supplemental table in the sidebar at the end of this article).
Continue to: Decreased payments for procedural services...
Decreased payments for procedural services
Medicare is required to offset increased payment in one arena of health care delivery with decreased payment in other arenas of care, thereby achieving “budget-neutrality.” As detailed above, CMS plans to increase Medicare payments for office-based E/M services. Payment for services is calculated by multiplying the total RVUs for a particular service by a “conversion factor” (ie, number of dollars per RVU). To achieve budget-neutrality, CMS has proposed substantially reducing the conversion factor for 2021 (from $36.09 to $32.26), which will effectively decrease Medicare payments for procedural services since their RVUs have not changed. While the AMA RUC and many specialty societies continue to strongly advocate for the E/M work RVU increases to be included in the E/M components of 10- and 90-day global services, CMS has proposed to implement them only for “stand alone” E/M services.
Organizations are lobbying to delay or prevent the planned decrease in conversion factor, which results in substantial declines in payment for procedural services. (See "What do the Medicare billing changes mean for the Obstetrical Bundled services?" at the end of this article.) Due to the economic and clinical practice challenges caused by the coronavirus disease 2019 (COVID-19) pandemic it would be best if CMS did not reduce payments to physicians who are experts in procedural health care, thereby avoiding the risk of reduced access to these vital services.
If the current CMS changes in payment are implemented, endocrinologists, rheumatologists, and family physicians will have an increase in payment, and radiologists, pathologists, and surgeons will have a decrease in payment (TABLE 3).6 Obstetrics and gynecology is projected to have an 8% increase in Medicare payment. However, if an obstetrician-gynecologist derives most of their Medicare payments from surgical procedures, they are likely to have a decrease in payment from Medicare. Other payers will be incorporating the new coding structure for 2021; however, their payment structures and conversion factors are likely to vary. It is important to note that the RVUs for procedures have not changed. The budget neutrality adjustment resulted in a much lower conversion factor and therefore a decrease in payment for those specialties whose RVUs did not increase.

Bottom line
Working through the Medicare, Medicaid, and CHIP programs, CMS can influence greatly the practice of medicine including medical record documentation practices and payment rates for every clinical service. CMS proposes to end the onerous “bean counting” approach to billing compliance and refocus on the complexity of MDM as the foundation for selecting a billing code level. This change is long overdue, valuing the effective management of complex patients in office practice. Hopefully, CMS will reverse the planned reduction in the payment for procedural services, preserving patient access to important health care services. ●
The CY 2020 Medicare Physician Fee Schedule Final Rule was published electronically in the Federal Register on November 1, 2019. This final rule aligns the evaluation and management (E/M) coding and payment with changes recommended by the Current Procedural Terminology (CPT) Editorial Panel and American Medical Association’s (AMA) Specialty Society Resource-Based Relative Value Scale Update Committee (RUC) for office/outpatient E/M visits. Unfortunately, the Centers for Medicare and Medicaid Services (CMS) did not agree with the RUC, AMA, and specialty societies that the E/M payment changes should be applicable across all global services that incorporate E/M visits—despite the fact that the values proposed by the RUC incorporated survey data from 52 specialties, representing most of medicine (including those specialties that predominantly perform procedures). Specifically, CMS expressed the view that the number of E/M visits within the 10- and 90-day global codes, as well as the maternity care bundle, were difficult to validate; therefore, the increased values would not be distributed to those procedural services.
Many professional societies expressed significant concerns about the resulting budget neutrality adjustments that would occur effective January 2021. The great news for ObGyns is that the American College of Obstetricians and Gynecologists (ACOG) was able to respond directly to CMS’s concerns with data to support the number of prenatal visits within the Obstetrical Bundle. Tapping into a de-identified, cloud-based data set of prenatal records—representing more than 1,100 obstetric providers with close to 30,000 recently completed pregnancies—ACOG was able to document both a mean and median number of prenatal visits across a broad geographic, payer, and patient demographic that supported the 13 prenatal visits in the Obstetrical Bundle.
With ACOG’s advocacy and ability to provide data to CMS, the proposed physician fee schedule rule for 2021 has proposed to incorporate the E/M increased reimbursement into the prenatal care codes. Now we urge the CMS to finalize this proposal. Although Medicare pays for a tiny number of pregnancies annually, we hope that all payers, including Medicaid and managed care plans, will agree with this acknowledgement of the increased work of evaluation and management that obstetricians provide during prenatal care. Join ACOG in telling CMS to finalize their proposal to increase the values of the global obstetric codes: https://acog.quorum.us/campaign/28579/.

- Centers for Medicare and Medicaid Services. https://www.cms.gov/. Accessed August 28, 2020.
- American Medical Association. CPT Evaluation and Management (E/M) Office or Other Outpatient (99202-99215) and Prolonged Services (99354, 99355, 99356, 99XXX) Code and Guideline Changes. 2019. https://www.ama-assn.org /system/files/2019-06/cpt-office-prolonged-svs -code-changes.pdf. Accessed August 28, 2020.
- The American Academy of Family Physicians. Family medicine updates. Ann Fam Med. 2020;18:84-85. doi: 10.1370/afm.2508.
- Centers for Medicare and Medicaid Services. Final policy, payment and quality provisions changes to the Medicare Physician Fee Schedule for calendar year 2019. November 1, 2018. https://www.cms.gov/newsroom/fact-sheets /final-policy-payment-and-quality-provisionschanges-medicare-physician-fee-schedulecalendar-year. Accessed August 28, 2020.
- Department of Health and Human Services; Centers for Medicare and Medicaid Services. 42 CFR Parts 410, 414, 415, 423, 424, and 425. Federal Register. 2020;85(159). https://www.govinfo.gov /content/pkg/FR-2020-08-17/pdf/2020-17127 .pdf. Accessed August 28, 2020.
- Centers for Medicare and Medicaid Services. https://www.cms.gov/. Accessed August 28, 2020.
- American Medical Association. CPT Evaluation and Management (E/M) Office or Other Outpatient (99202-99215) and Prolonged Services (99354, 99355, 99356, 99XXX) Code and Guideline Changes. 2019. https://www.ama-assn.org /system/files/2019-06/cpt-office-prolonged-svs -code-changes.pdf. Accessed August 28, 2020.
- The American Academy of Family Physicians. Family medicine updates. Ann Fam Med. 2020;18:84-85. doi: 10.1370/afm.2508.
- Centers for Medicare and Medicaid Services. Final policy, payment and quality provisions changes to the Medicare Physician Fee Schedule for calendar year 2019. November 1, 2018. https://www.cms.gov/newsroom/fact-sheets /final-policy-payment-and-quality-provisionschanges-medicare-physician-fee-schedulecalendar-year. Accessed August 28, 2020.
- Department of Health and Human Services; Centers for Medicare and Medicaid Services. 42 CFR Parts 410, 414, 415, 423, 424, and 425. Federal Register. 2020;85(159). https://www.govinfo.gov /content/pkg/FR-2020-08-17/pdf/2020-17127 .pdf. Accessed August 28, 2020.
Revamp the MOC
There are few things that psychiatrists have come to despise more than the American Board of Psychiatry and Neurology (ABPN) Maintenance of Certification (MOC) program. It has become a professional boondoggle for psychiatric practitioners.
The program needs an overhaul and simplification. There are better, more efficient, cost-effective ways to ensure psychiatric physicians’ ongoing clinical competence after they complete their residency training. Technological advances can also facilitate a more valid assessment of competence without having to jump through more and more hoops between recertifications every 10 years.
I passed the boards long before the MOC was created. For 20 years, I also served as a senior examiner for the oral boards, where clinical competency was rigorously assessed by direct observations of psychiatrists examining and establishing rapport with patients and formulating the data into a differential diagnosis, treatment plan, and prognosis. It is noteworthy that psychiatrists who sat for the oral boards had already passed a written exam that tested their cognitive knowledge. Yet approximately one-third of the candidates failed the live oral exam, which clearly implies that passing a written exam is necessary but not sufficient to establish clinical competence, which is the primary purpose of board certification. It was an unfortunate decision to discontinue the face-to-face oral board exam, which is so vital for psychiatry, and to replace it with a written exam and a barrage of time-consuming activities to document lifelong learning and self-assessment, but not genuine clinical competence. The MOC has been MOCkingly referred to as a major pain in the neck for practically all psychiatrists who were not grandfathered with lifetime certification, as was the case in the first 60 years of the ABPN.
Benefits of the patient-based oral exam
Let’s face it: Passing a patient-based oral exam was the ideal mechanism to establish that a psychiatric physician deserved to be a diplomate of the ABPN. During the oral exam, the candidate’s skills were observed from the minute he/she met the patient. The candidate was then observed as he/she systematically explored a wide range of past and current psychiatric symptoms; reviewed the patient’s developmental, medical, family, and social histories; and conducted a competent mental status exam while demonstrating an empathic stance, responding to the patient’s often subtle verbal and nonverbal cues, establishing rapport, and providing psychoeducation before concluding the interview. All these essential components of a psychiatric exam were observed in a compact 30-minute tour de force of clinical skills, communication, and cognitive acumen. This was followed by another 30 minutes of organizing and presenting the clinical data to 2 or 3 colleagues/examiners, in a coherent fashion, connecting all the dots, formulating the case, presenting a meaningful differential diagnosis, and suggesting a rational array of potential treatment options across the biopsychosocial continuum. To top it off, the candidate had to respond effectively, in an evidence-based manner, to a series of questions related to the disease state, its treatment, adverse effects, and prognosis.
It was a joy to watch many colleagues navigate this clinical examination with skill and competence, without crumbling under the pressure of the examiners’ scrutiny. There were some who passed with flying colors, and others who passed despite having a forgivable minor gap here and there because of their overall strong performance. Finally, there were those who stumbled in several components across data collection, doctor–patient interactions, synthesis of the clinical findings, or treatment recommendations. These candidates inevitably received a failing grade by a consensus of 3 examiners. That they failed to demonstrate clinical competence despite having passed the required written exams a year earlier proved that the true competency of a psychiatrist cannot be judged solely by passing a written test but requires a clinical examination of a live patient.
The oral exams represented an unimpeachable evaluation of clinical competence. The examiners often spoke of how they would feel confident and comfortable with referring a family member to those who successfully passed this rigorous, authentic exam on real patients. It was justifiable to give lifetime certification to those who passed the oral exam. Those permanently certified psychiatrists maintained their lifelong learning by having an unrestricted state medical license, which is contingent on acquiring 50 category 1 continuing medical education (CME) credits annually. Why not restore lifelong certification for those who pass both a written and oral exam, as long as they maintain a valid medical license?
According to the ABPN 2019 Annual Report,1 31,514 psychiatrists have received lifetime certification, of whom an estimated 9,547 were still clinically active in 2019. This is the “grandfathered” cohort of psychiatrists to which I belong. I was tested on neurologic patients, not just psychiatric patients, a tribute to the strong bridge that existed between these sister brain specialties. As of 2019, of the 33,277 psychiatrists who received a time-limited certification, 29,343 were still clinically active, an attrition rate of 12% over the past 25 years. This includes psychiatrists who found the MOC too onerous to complete, or are in private practice where MOC is not a vital requirement. However, these days most psychiatrists are obligated to be recertified because so many entities require it. This includes hiring institutions, government agencies (Medicare/Medicaid), health insurance companies, hospital medical staff for privileging and credentialing, and various regulatory boards, such as The Joint Commission, the Accreditation Council for Graduate Medical Education, and academic medical centers. Because most psychiatrists are involved with at least one of these entities, 29,343 have no choice but to perform all the requirements of the MOC, with its countless hours, numerous documentations, and many fees, to remain certified by the ABPN. Notably absent is an alternative mechanism for a certification process that is widely accepted by all agencies and institutions. Psychiatrists are actively seeking alternatives.
Continue to: The ABPN...
The ABPN, long regarded as an esteemed nonprofit organization, has been accused of being a monopoly. Some angry psychiatrists have filed a class action lawsuit to demand other board certification methods. Some have gone to the media to complain about the American Board of Medical Specialties (of which the ABPN is a member board), accusing both of unfair regulations or of raking in substantial profits to support excessively compensated executives. Perception often trumps reality, so no matter how vigorously the ABPN defends itself, its procedures, or its MOC requirements, its customers—psychiatric physicians—feel oppressed or exploited.
How the MOC can be improved
So what can be done to improve the MOC? The need for recertification is arguably necessary to document clinical competency over an approximately 40-year psychiatric career following residency. I conducted a brief survey of
Significant advances in remote communication technology should be harnessed by the ABPN (or the APA, if it decides to conduct its own board certification) to restore the old model at a fraction of the cost. The oral exams have been replaced by a written exam that is not an accurate reflection or documentation of clinical competence. The traditional oral exam (after passing a written exam) was a magnificent but costly feat of massive logistical complexity, with >1,000 candidates and examiners traveling to a city where the ABPN arranged for several hospitals to shut down their clinics for 2 full days to use their clinical offices for the oral exams. Multiple teams examined the candidates twice on the same day: once with a live patient, and again with a video of a real patient. The examiners filled out scoring cards after observing the candidates conduct the live interview or discussing the video. A consensus grade of pass or fail was documented. At the end of the 2 days, examiners and candidates boarded buses to the airport. It was a highly expensive process (exam fees + airfare + hotel + food). Twice a year, the examiners generously donated their time to the ABPN without compensation, as a token of love for and service to the profession.
That initial certification of a written exam, followed by an oral exam, validated the competence of a psychiatrist both cognitively and clinically. The lifetime certification was truly earned. The same model can now be replicated virtually via videoconferencing at a far lower cost to the ABPN, the candidates, and the examiners. The MOC 10-year recertification can be reduced to a written exam with clinical vignettes and an unrestricted license to practice medicine in any state, which implies that the psychiatrist has received the 50 CME annual credits to renew the license. The rest of the bells and whistles can be strongly recommended but not required. The cost in time and money to both the ABPN and the candidates can be significantly reduced, but more importantly, the clinical competence will be validated at baseline with virtual oral boards after passing the written exam (formerly labeled as part I, preceding the part II oral boards).
The traditional board certification model of the past should be resurrected via videoconferencing and offered as an option to the candidates who prefer it to the current MOC. The MOC can then be simplified to lifetime certification or to only a written exam with clinical vignettes every 10 years to ensure that psychiatrists continue to incorporate relevant clinical and treatment advances in their practice. The KISS principle (keep it simple, stupid) worked very well for many generations of psychiatrists in the past, and will work again going forward if offered as an option. Psychiatrists can then focus on treating patients instead of being burdened by the many time-consuming requirements and hoops of the current MOC.
1. American Board of Psychiatry and Neurology. 2019 Annual Report. https://www.abpn.com/wp-content/uploads/2020/05/ABPN_2019_Annual_Report.pdf. Accessed August 14, 2020.
There are few things that psychiatrists have come to despise more than the American Board of Psychiatry and Neurology (ABPN) Maintenance of Certification (MOC) program. It has become a professional boondoggle for psychiatric practitioners.
The program needs an overhaul and simplification. There are better, more efficient, cost-effective ways to ensure psychiatric physicians’ ongoing clinical competence after they complete their residency training. Technological advances can also facilitate a more valid assessment of competence without having to jump through more and more hoops between recertifications every 10 years.
I passed the boards long before the MOC was created. For 20 years, I also served as a senior examiner for the oral boards, where clinical competency was rigorously assessed by direct observations of psychiatrists examining and establishing rapport with patients and formulating the data into a differential diagnosis, treatment plan, and prognosis. It is noteworthy that psychiatrists who sat for the oral boards had already passed a written exam that tested their cognitive knowledge. Yet approximately one-third of the candidates failed the live oral exam, which clearly implies that passing a written exam is necessary but not sufficient to establish clinical competence, which is the primary purpose of board certification. It was an unfortunate decision to discontinue the face-to-face oral board exam, which is so vital for psychiatry, and to replace it with a written exam and a barrage of time-consuming activities to document lifelong learning and self-assessment, but not genuine clinical competence. The MOC has been MOCkingly referred to as a major pain in the neck for practically all psychiatrists who were not grandfathered with lifetime certification, as was the case in the first 60 years of the ABPN.
Benefits of the patient-based oral exam
Let’s face it: Passing a patient-based oral exam was the ideal mechanism to establish that a psychiatric physician deserved to be a diplomate of the ABPN. During the oral exam, the candidate’s skills were observed from the minute he/she met the patient. The candidate was then observed as he/she systematically explored a wide range of past and current psychiatric symptoms; reviewed the patient’s developmental, medical, family, and social histories; and conducted a competent mental status exam while demonstrating an empathic stance, responding to the patient’s often subtle verbal and nonverbal cues, establishing rapport, and providing psychoeducation before concluding the interview. All these essential components of a psychiatric exam were observed in a compact 30-minute tour de force of clinical skills, communication, and cognitive acumen. This was followed by another 30 minutes of organizing and presenting the clinical data to 2 or 3 colleagues/examiners, in a coherent fashion, connecting all the dots, formulating the case, presenting a meaningful differential diagnosis, and suggesting a rational array of potential treatment options across the biopsychosocial continuum. To top it off, the candidate had to respond effectively, in an evidence-based manner, to a series of questions related to the disease state, its treatment, adverse effects, and prognosis.
It was a joy to watch many colleagues navigate this clinical examination with skill and competence, without crumbling under the pressure of the examiners’ scrutiny. There were some who passed with flying colors, and others who passed despite having a forgivable minor gap here and there because of their overall strong performance. Finally, there were those who stumbled in several components across data collection, doctor–patient interactions, synthesis of the clinical findings, or treatment recommendations. These candidates inevitably received a failing grade by a consensus of 3 examiners. That they failed to demonstrate clinical competence despite having passed the required written exams a year earlier proved that the true competency of a psychiatrist cannot be judged solely by passing a written test but requires a clinical examination of a live patient.
The oral exams represented an unimpeachable evaluation of clinical competence. The examiners often spoke of how they would feel confident and comfortable with referring a family member to those who successfully passed this rigorous, authentic exam on real patients. It was justifiable to give lifetime certification to those who passed the oral exam. Those permanently certified psychiatrists maintained their lifelong learning by having an unrestricted state medical license, which is contingent on acquiring 50 category 1 continuing medical education (CME) credits annually. Why not restore lifelong certification for those who pass both a written and oral exam, as long as they maintain a valid medical license?
According to the ABPN 2019 Annual Report,1 31,514 psychiatrists have received lifetime certification, of whom an estimated 9,547 were still clinically active in 2019. This is the “grandfathered” cohort of psychiatrists to which I belong. I was tested on neurologic patients, not just psychiatric patients, a tribute to the strong bridge that existed between these sister brain specialties. As of 2019, of the 33,277 psychiatrists who received a time-limited certification, 29,343 were still clinically active, an attrition rate of 12% over the past 25 years. This includes psychiatrists who found the MOC too onerous to complete, or are in private practice where MOC is not a vital requirement. However, these days most psychiatrists are obligated to be recertified because so many entities require it. This includes hiring institutions, government agencies (Medicare/Medicaid), health insurance companies, hospital medical staff for privileging and credentialing, and various regulatory boards, such as The Joint Commission, the Accreditation Council for Graduate Medical Education, and academic medical centers. Because most psychiatrists are involved with at least one of these entities, 29,343 have no choice but to perform all the requirements of the MOC, with its countless hours, numerous documentations, and many fees, to remain certified by the ABPN. Notably absent is an alternative mechanism for a certification process that is widely accepted by all agencies and institutions. Psychiatrists are actively seeking alternatives.
Continue to: The ABPN...
The ABPN, long regarded as an esteemed nonprofit organization, has been accused of being a monopoly. Some angry psychiatrists have filed a class action lawsuit to demand other board certification methods. Some have gone to the media to complain about the American Board of Medical Specialties (of which the ABPN is a member board), accusing both of unfair regulations or of raking in substantial profits to support excessively compensated executives. Perception often trumps reality, so no matter how vigorously the ABPN defends itself, its procedures, or its MOC requirements, its customers—psychiatric physicians—feel oppressed or exploited.
How the MOC can be improved
So what can be done to improve the MOC? The need for recertification is arguably necessary to document clinical competency over an approximately 40-year psychiatric career following residency. I conducted a brief survey of
Significant advances in remote communication technology should be harnessed by the ABPN (or the APA, if it decides to conduct its own board certification) to restore the old model at a fraction of the cost. The oral exams have been replaced by a written exam that is not an accurate reflection or documentation of clinical competence. The traditional oral exam (after passing a written exam) was a magnificent but costly feat of massive logistical complexity, with >1,000 candidates and examiners traveling to a city where the ABPN arranged for several hospitals to shut down their clinics for 2 full days to use their clinical offices for the oral exams. Multiple teams examined the candidates twice on the same day: once with a live patient, and again with a video of a real patient. The examiners filled out scoring cards after observing the candidates conduct the live interview or discussing the video. A consensus grade of pass or fail was documented. At the end of the 2 days, examiners and candidates boarded buses to the airport. It was a highly expensive process (exam fees + airfare + hotel + food). Twice a year, the examiners generously donated their time to the ABPN without compensation, as a token of love for and service to the profession.
That initial certification of a written exam, followed by an oral exam, validated the competence of a psychiatrist both cognitively and clinically. The lifetime certification was truly earned. The same model can now be replicated virtually via videoconferencing at a far lower cost to the ABPN, the candidates, and the examiners. The MOC 10-year recertification can be reduced to a written exam with clinical vignettes and an unrestricted license to practice medicine in any state, which implies that the psychiatrist has received the 50 CME annual credits to renew the license. The rest of the bells and whistles can be strongly recommended but not required. The cost in time and money to both the ABPN and the candidates can be significantly reduced, but more importantly, the clinical competence will be validated at baseline with virtual oral boards after passing the written exam (formerly labeled as part I, preceding the part II oral boards).
The traditional board certification model of the past should be resurrected via videoconferencing and offered as an option to the candidates who prefer it to the current MOC. The MOC can then be simplified to lifetime certification or to only a written exam with clinical vignettes every 10 years to ensure that psychiatrists continue to incorporate relevant clinical and treatment advances in their practice. The KISS principle (keep it simple, stupid) worked very well for many generations of psychiatrists in the past, and will work again going forward if offered as an option. Psychiatrists can then focus on treating patients instead of being burdened by the many time-consuming requirements and hoops of the current MOC.
There are few things that psychiatrists have come to despise more than the American Board of Psychiatry and Neurology (ABPN) Maintenance of Certification (MOC) program. It has become a professional boondoggle for psychiatric practitioners.
The program needs an overhaul and simplification. There are better, more efficient, cost-effective ways to ensure psychiatric physicians’ ongoing clinical competence after they complete their residency training. Technological advances can also facilitate a more valid assessment of competence without having to jump through more and more hoops between recertifications every 10 years.
I passed the boards long before the MOC was created. For 20 years, I also served as a senior examiner for the oral boards, where clinical competency was rigorously assessed by direct observations of psychiatrists examining and establishing rapport with patients and formulating the data into a differential diagnosis, treatment plan, and prognosis. It is noteworthy that psychiatrists who sat for the oral boards had already passed a written exam that tested their cognitive knowledge. Yet approximately one-third of the candidates failed the live oral exam, which clearly implies that passing a written exam is necessary but not sufficient to establish clinical competence, which is the primary purpose of board certification. It was an unfortunate decision to discontinue the face-to-face oral board exam, which is so vital for psychiatry, and to replace it with a written exam and a barrage of time-consuming activities to document lifelong learning and self-assessment, but not genuine clinical competence. The MOC has been MOCkingly referred to as a major pain in the neck for practically all psychiatrists who were not grandfathered with lifetime certification, as was the case in the first 60 years of the ABPN.
Benefits of the patient-based oral exam
Let’s face it: Passing a patient-based oral exam was the ideal mechanism to establish that a psychiatric physician deserved to be a diplomate of the ABPN. During the oral exam, the candidate’s skills were observed from the minute he/she met the patient. The candidate was then observed as he/she systematically explored a wide range of past and current psychiatric symptoms; reviewed the patient’s developmental, medical, family, and social histories; and conducted a competent mental status exam while demonstrating an empathic stance, responding to the patient’s often subtle verbal and nonverbal cues, establishing rapport, and providing psychoeducation before concluding the interview. All these essential components of a psychiatric exam were observed in a compact 30-minute tour de force of clinical skills, communication, and cognitive acumen. This was followed by another 30 minutes of organizing and presenting the clinical data to 2 or 3 colleagues/examiners, in a coherent fashion, connecting all the dots, formulating the case, presenting a meaningful differential diagnosis, and suggesting a rational array of potential treatment options across the biopsychosocial continuum. To top it off, the candidate had to respond effectively, in an evidence-based manner, to a series of questions related to the disease state, its treatment, adverse effects, and prognosis.
It was a joy to watch many colleagues navigate this clinical examination with skill and competence, without crumbling under the pressure of the examiners’ scrutiny. There were some who passed with flying colors, and others who passed despite having a forgivable minor gap here and there because of their overall strong performance. Finally, there were those who stumbled in several components across data collection, doctor–patient interactions, synthesis of the clinical findings, or treatment recommendations. These candidates inevitably received a failing grade by a consensus of 3 examiners. That they failed to demonstrate clinical competence despite having passed the required written exams a year earlier proved that the true competency of a psychiatrist cannot be judged solely by passing a written test but requires a clinical examination of a live patient.
The oral exams represented an unimpeachable evaluation of clinical competence. The examiners often spoke of how they would feel confident and comfortable with referring a family member to those who successfully passed this rigorous, authentic exam on real patients. It was justifiable to give lifetime certification to those who passed the oral exam. Those permanently certified psychiatrists maintained their lifelong learning by having an unrestricted state medical license, which is contingent on acquiring 50 category 1 continuing medical education (CME) credits annually. Why not restore lifelong certification for those who pass both a written and oral exam, as long as they maintain a valid medical license?
According to the ABPN 2019 Annual Report,1 31,514 psychiatrists have received lifetime certification, of whom an estimated 9,547 were still clinically active in 2019. This is the “grandfathered” cohort of psychiatrists to which I belong. I was tested on neurologic patients, not just psychiatric patients, a tribute to the strong bridge that existed between these sister brain specialties. As of 2019, of the 33,277 psychiatrists who received a time-limited certification, 29,343 were still clinically active, an attrition rate of 12% over the past 25 years. This includes psychiatrists who found the MOC too onerous to complete, or are in private practice where MOC is not a vital requirement. However, these days most psychiatrists are obligated to be recertified because so many entities require it. This includes hiring institutions, government agencies (Medicare/Medicaid), health insurance companies, hospital medical staff for privileging and credentialing, and various regulatory boards, such as The Joint Commission, the Accreditation Council for Graduate Medical Education, and academic medical centers. Because most psychiatrists are involved with at least one of these entities, 29,343 have no choice but to perform all the requirements of the MOC, with its countless hours, numerous documentations, and many fees, to remain certified by the ABPN. Notably absent is an alternative mechanism for a certification process that is widely accepted by all agencies and institutions. Psychiatrists are actively seeking alternatives.
Continue to: The ABPN...
The ABPN, long regarded as an esteemed nonprofit organization, has been accused of being a monopoly. Some angry psychiatrists have filed a class action lawsuit to demand other board certification methods. Some have gone to the media to complain about the American Board of Medical Specialties (of which the ABPN is a member board), accusing both of unfair regulations or of raking in substantial profits to support excessively compensated executives. Perception often trumps reality, so no matter how vigorously the ABPN defends itself, its procedures, or its MOC requirements, its customers—psychiatric physicians—feel oppressed or exploited.
How the MOC can be improved
So what can be done to improve the MOC? The need for recertification is arguably necessary to document clinical competency over an approximately 40-year psychiatric career following residency. I conducted a brief survey of
Significant advances in remote communication technology should be harnessed by the ABPN (or the APA, if it decides to conduct its own board certification) to restore the old model at a fraction of the cost. The oral exams have been replaced by a written exam that is not an accurate reflection or documentation of clinical competence. The traditional oral exam (after passing a written exam) was a magnificent but costly feat of massive logistical complexity, with >1,000 candidates and examiners traveling to a city where the ABPN arranged for several hospitals to shut down their clinics for 2 full days to use their clinical offices for the oral exams. Multiple teams examined the candidates twice on the same day: once with a live patient, and again with a video of a real patient. The examiners filled out scoring cards after observing the candidates conduct the live interview or discussing the video. A consensus grade of pass or fail was documented. At the end of the 2 days, examiners and candidates boarded buses to the airport. It was a highly expensive process (exam fees + airfare + hotel + food). Twice a year, the examiners generously donated their time to the ABPN without compensation, as a token of love for and service to the profession.
That initial certification of a written exam, followed by an oral exam, validated the competence of a psychiatrist both cognitively and clinically. The lifetime certification was truly earned. The same model can now be replicated virtually via videoconferencing at a far lower cost to the ABPN, the candidates, and the examiners. The MOC 10-year recertification can be reduced to a written exam with clinical vignettes and an unrestricted license to practice medicine in any state, which implies that the psychiatrist has received the 50 CME annual credits to renew the license. The rest of the bells and whistles can be strongly recommended but not required. The cost in time and money to both the ABPN and the candidates can be significantly reduced, but more importantly, the clinical competence will be validated at baseline with virtual oral boards after passing the written exam (formerly labeled as part I, preceding the part II oral boards).
The traditional board certification model of the past should be resurrected via videoconferencing and offered as an option to the candidates who prefer it to the current MOC. The MOC can then be simplified to lifetime certification or to only a written exam with clinical vignettes every 10 years to ensure that psychiatrists continue to incorporate relevant clinical and treatment advances in their practice. The KISS principle (keep it simple, stupid) worked very well for many generations of psychiatrists in the past, and will work again going forward if offered as an option. Psychiatrists can then focus on treating patients instead of being burdened by the many time-consuming requirements and hoops of the current MOC.
1. American Board of Psychiatry and Neurology. 2019 Annual Report. https://www.abpn.com/wp-content/uploads/2020/05/ABPN_2019_Annual_Report.pdf. Accessed August 14, 2020.
1. American Board of Psychiatry and Neurology. 2019 Annual Report. https://www.abpn.com/wp-content/uploads/2020/05/ABPN_2019_Annual_Report.pdf. Accessed August 14, 2020.
New hormonal medical treatment is an important advance for AUB caused by uterine fibroids
Uterine leiomyomata (fibroids) are the most common pelvic tumor diagnosed in women.1 Women with symptomatic fibroids often report abnormal uterine bleeding (AUB) and pelvic cramping, fullness, or pain. Fibroids also may cause frequency of urination and contribute to fertility and pregnancy problems. Treatment options for the AUB caused by fibroids include, but are not limited to, hysterectomy, myomectomy, uterine artery embolization, endometrial ablation, insertion of a levonorgestrel intrauterine device, focused ultrasound surgery, radiofrequency ablation, leuprolide acetate, and elagolix plus low-dose hormone add-back (Oriahnn; AbbVie, North Chicago, Illinois).1 Oriahnn is the most recent addition to our treatment armamentarium for fibroids and represents the first US Food and Drug Administration (FDA)-approved long-term hormonal option for AUB caused by fibroids.
Gene dysregulation contributes to fibroid development
Most uterine fibroids are clonal tumors, which develop following a somatic mutation in a precursor uterine myocyte. The somatic mutation causes gene dysregulation that stimulates cell growth resulting in a benign tumor mass. The majority of fibroids contain a mutation in one of the following 6 genes: mediator complex subunit 12 (MED12), high mobility group AT-hook (HMGA2 or HMGA1), RAD51B, fumarate hydratase (FH), collagen type IV, alpha 5 chain (COL4A5), or collagen type IV alpha 6 chain (COL4A6).2
Gene dysregulation in fibroids may arise following chromothripsis of the uterine myocyte genome
Chromothripsis is a catastrophic intracellular genetic event in which one or more chromosomes are broken and reassemble in a new nucleic acid sequence, producing a derivative chromosome that contains complex genetic rearrangements.3 Chromothripsis is believed to occur frequently in uterine myocytes. It is unknown why uterine myocytes are susceptible to chromothripsis,3 or why a catastrophic intracellular event such as chromothripsis results in preferential mutations in the 6 genes that are associated with myoma formation.
Estrogen and progesterone influence fibroid size and cell activity
Although uterine fibroids are clonal tumors containing broken genes, they are also exquisitely responsive to estradiol and progesterone. Estradiol and progesterone play an important role in regulating fibroid size and function.4 Estrogen stimulates uterine fibroids to increase in size. In a hypoestrogenic state, uterine fibroids decrease in size. In addition, a hypoestrogenic state results in an atrophic endometrium and thereby reduces AUB. For women with uterine fibroids and AUB, a reversible hypoestrogenic state can be induced either with a parenteral GnRH-agonist analogue (leuprolide) or an oral GnRH-antagonist (elagolix). Both leuprolide and elagolix are approved for the treatment of uterine fibroids (see below).
Surprisingly, progesterone stimulates cell division in normal uterine myocytes and fibroid cells.5 In the luteal phase of the menstrual cycle, uterine myocyte mitoses are more frequent than in the follicular phase. In addition, synthetic progestins appear to maintain fibroid size in a hypoestrogenic environment. In one randomized trial, women with uterine fibroids treated with leuprolide acetate plus a placebo pill for 24 weeks had a 51% reduction in uterine volume as measured by ultrasound.6 Women with uterine fibroids treated with leuprolide acetate plus the synthetic progestin, oral medroxyprogesterone acetate 20 mg daily, had only a 15% reduction in uterine volume.6 This finding suggests that synthetic progestins partially block the decrease in uterine volume that occurs in a hypoestrogenic state.
Further evidence that progesterone plays a role in fibroid biology is the observation that treatment of women with uterine fibroids with the antiprogestin ulipristal decreases fibroid size and reduces AUB.7-9 Ulipristal was approved for the treatment of fibroids in many countries but not the United States. Reports of severe, life-threatening liver injury—some necessitating liver transplantation—among women using ulipristal prompted the European Medicines Agency (EMA) in 2020 to recommend that women stop taking ulipristal. In addition, the EMA recommended that no woman should initiate ulipristal treatment at this time.10
Continue to: Leuprolide acetate...
Leuprolide acetate
Leuprolide acetate is a peptide GnRH-agonist analogue. Initiation of leuprolide treatment stimulates gonadotropin release, but with chronic administration pituitary secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) decreases, resulting in reduced ovarian follicular activity, anovulation, and low serum concentration of estradiol and progesterone. Leuprolide treatment concomitant with iron therapy is approved by the FDA for improving red blood cell volume prior to surgery in women with fibroids, AUB, and anemia.11 Among women with fibroids, AUB, and anemia, after 12 weeks of treatment, the hemoglobin concentration was ≥12 g/dL in 79% treated with leuprolide plus iron and 56% treated with iron alone.11 The FDA recommends limiting preoperative leuprolide treatment to no more than 3 months. The approved leuprolide regimens are a maximum of 3 monthly injections of leuprolide 3.75 mg or a single injection of leuprolide 11.25 mg. Leuprolide treatment prior to hysterectomy surgery for uterine fibroids usually will result in a decrease in uterine size and may facilitate vaginal hysterectomy.
Elagolix plus estradiol plus norethindrone acetate (Oriahnn)
GnRH analogues cause a hypoestrogenic state resulting in adverse effects, including moderate to severe hot flashes and a reduction in bone mineral density. One approach to reducing the unwanted effects of hot flashes and decreased bone density is to combine a GnRH analogue with low-dose steroid hormone add-back therapy. Combining a GnRH analogue with low-dose steroid hormone add-back permits long-term treatment of AUB caused by fibroids, with few hot flashes and a minimal decrease in bone mineral density. The FDA recently has approved the combination of elagolix plus low-dose estradiol and norethindrone acetate (Oriahnn) for the long-term treatment of AUB caused by fibroids.
Elagolix is a nonpeptide oral GnRH antagonist that reduces pituitary secretion of LH and FSH, resulting in a decrease in ovarian follicular activity, anovulation, and low serum concentration of estradiol and progesterone. Unlike leuprolide, which causes an initial increase in LH and FSH secretion, the initiation of elagolix treatment causes an immediate and sustained reduction in LH and FSH secretion. Combining elagolix with a low dose of estradiol and norethindrone acetate reduces the side effects of hot flashes and decreased bone density. Clinical trials have reported that the combination of elagolix (300 mg) twice daily plus estradiol (1 mg) and norethindrone acetate (0.5 mg) once daily is an effective long-term treatment of AUB caused by uterine fibroids.
To study the efficacy of elagolix (alone or with estrogen-progestin add-back therapy) for the treatment of AUB caused by uterine fibroids, two identical trials were performed,12 in which 790 women participated. The participants had a mean age of 42 years and were documented to have heavy menstrual bleeding (>80 mL blood loss per cycle) and ultrasound-diagnosed uterine fibroids. The participants were randomized to one of 3 groups:
- elagolix (300 mg twice daily) plus low-dose steroid add-back (1 mg estradiol and 0.5 mg norethindrone acetate once daily),
- elagolix 300 mg twice daily with no steroid add-back (elagolix alone), or
- placebo for 6 months.12
Menstrual blood loss was quantified using the alkaline hematin method on collected sanitary products. The primary endpoint was menstrual blood loss <80 mL per cycle as well as a ≥50% reduction in quantified blood loss from baseline during the final month of treatment. At 6 months, the percentage of women achieving the primary endpoint in the first trial was 84% (elagolix alone), 69% (elagolix plus add-back), and 9% (placebo). Mean changes from baseline in lumbar spine bone density were −2.95% (elagolix alone), −0.76% (elagolix plus add-back), and −0.21% (placebo). The percentage of women reporting hot flashes was 64% in the elagolix group, 20% in the elagolix plus low-dose steroid add-back group, and 9% in the placebo group. Results were similar in the second trial.12
The initial trials were extended to 12 months with two groups: elagolix 300 mg twice daily plus low-dose hormone add-back with 1 mg estradiol and 0.5 mg norethindrone acetate once daily (n = 218) or elagolix 300 mg twice daily (elagolix alone) (n = 98).13 Following 12 months of treatment, heavy menstrual bleeding was controlled in 88% and 89% of women treated with elagolix plus add-back and elagolix alone, respectively. Amenorrhea was reported by 65% of the women in the elagolix plus add-back group. Compared with baseline bone density, at the end of 12 months of treatment, bone mineral density in the lumbar spine was reduced by -1.5% and -4.8% in the women treated with elagolix plus add-back and elagolix alone, respectively. Compared with baseline bone density, at 1 year following completion of treatment, bone mineral density in the lumbar spine was reduced by -0.6% and -2.0% in the women treated with elagolix plus add-back and elagolix alone, respectively. Similar trends were observed in total hip and femoral neck bone density. During treatment with elagolix plus add-back, adverse effects were modest, including hot flushes (6%), night sweats (3.2%), headache (5.5%), and nausea (4.1%). Two women developed liver transaminase levels >3 times the upper limit of normal, resulting in one woman discontinuing treatment.13
Continue to: Contraindications to Oriahnn include known allergies...
Contraindications to Oriahnn include known allergies to the components of the medication (including the yellow dye tartrazine); high risk of arterial, venous thrombotic or thromboembolic disorders; pregnancy; known osteoporosis; current breast cancer or other hormonally-sensitive malignancies; known liver disease; and concurrent use of organic anion transporting polypeptide 1B1 inhibitors, which includes many HIV antiviral medications.14 Undiagnosed AUB is a contraindication, and all women prescribed Oriahnn should have endometrial sampling before initiating treatment. Oriahnn should not be used for more than 24 months due to the risk of irreversible bone loss.14 Systemic estrogen and progestin combinations, a component of Oriahnn, increases the risk for pulmonary embolism, deep vein thrombosis, stroke, and myocardial infarction, especially in women at increased risk for these events (such as women >35 years who smoke cigarettes and women with uncontrolled hypertension).14 In two studies there was a higher incidence of depression, depressed mood, and/or tearfulness in women taking Oriahnn (3%) compared with those taking a placebo (1%).14 The FDA recommends promptly evaluating women with depressive symptoms to determine the risks of initiating and continuing Oriahnn therapy. In two studies there was a higher risk of reported alopecia among women taking Oriahnn (3.5%) compared with placebo (1%).14
It should be noted that elagolix is approved for the treatment of pelvic pain caused by endometriosis at a dose of 150 mg daily for 24 months or 200 mg twice daily for 6 months. The elagolix dose for the treatment of AUB caused by fibroids is 300 mg twice daily for up to 24 months, necessitating the addition of low-dose estradiol-norethindrone add-back to reduce the frequency and severity of hot flashes and minimize the loss of bone density. Norethindrone acetate also protects the endometrium from the stimulatory effect of estradiol, reducing the risk of developing endometrial hyperplasia and cancer. Oriahnn is formulated as two different capsules. A yellow and white capsule contains elagolix 300 mg plus estradiol 1 mg and norethindrone acetate 0.5 mg to be taken in the morning, and a blue and white capsule contains elagolix 300 mg to be taken in the evening.
AUB caused by fibroids is a common problem in gyn practice
There are many procedural interventions that are effective in reducing AUB caused by fibroids. However, prior to the approval of Oriahnn there were no hormonal medications that were FDA approved for the long-term treatment of AUB caused by fibroids. Hence, Oriahnn represents an important advance in the hormonal treatment of AUB caused by fibroids and expands the treatment options available to our patients. ●
Black women are more likely to develop fibroids and experience more severe fibroid symptoms. Obstetrician-gynecologists are experts in the diagnosis and treatment of fibroids. We play a key role in partnering with Black women to reduce fibroid disease burden.
Factors that increase the risk of developing fibroids include: increasing age, Black race, nulliparity, early menarche (<10 years of age), obesity, and consumption of red meat.1 The Nurses Health Study II is the largest prospective study of the factors that influence fibroid development.2 A total of 95,061 premenopausal nurses aged 25 to 44 years were followed from September 1989 through May 1993. Review of a sample of medical records demonstrated that the nurses participating in the study were reliable reporters of whether or not they had been diagnosed with fibroids. Based on a report of an ultrasound or hysterectomy diagnosis, the incidence rate for fibroids increased with age. Incidence rate per 1,000 women-years was 4.3 (age 25 to 29 years), 9.0 (30 to 34 years), 14.7 (age 35 to 39 years), and 22.5 (40 to 44 years). Compared with White race, Black race (but not Hispanic ethnicity or Asian race) was associated with an increased incidence of fibroids. Incidence rate per 1,000 women-years was 12.5 (White race), 37.9 (Black race), 14.5 (Hispanic ethnicity), and 10.4 (Asian race). The risk of developing fibroids was 3.25 times (95% CI, 2.71 to 3.88) greater among Black compared with White women after controlling for body mass index, age at first birth, years since last birth, history of infertility, age at first oral contraceptive use, marital status, and current alcohol use.2
Other epidemiology studies also report an increased incidence of fibroids among Black women.3,4 The size of the uterus, the size and number of fibroids, and the severity of fibroid symptoms are greater among Black versus White women.5,6 The molecular factors that increase fibroid incidence among Black women are unknown. Given the burden of fibroid disease among Black women, obstetrician-gynecologists are best positioned to ensure early diagnosis and to develop an effective follow-up and treatment plan for affected women.
References
1. Stewart EA, Laughlin-Tommaso SK, Catherino WH, et al. Uterine fibroids. Nat Rev Dis Primers. 2016;2:16043.
2. Marshall LM, Spiegelman D, Barbieri RL, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90:967-973.
3. Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
4. Brett KM, Marsh JV, Madans JH. Epidemiology of hysterectomy in the United States: demographic and reproductive factors in a nationally representative sample. J Womens Health. 1997;6:309-316.
5. Peddada SD, Laughlin SK, Miner K, et al. Growth of uterine leiomyomata among premenopausal black and white women. Proc Natl Acad Sci USA. 2008;105:1988719892.
6. Huyck KL, Panhuysen CI, Cuenco KT, et al. The impact of race as a risk factor for symptom severity and age at diagnosis of uterine leiomyomata among affected sisters. Am J Obstet Gynecol. 2008;198:168.e1-e9.
- Stewart EA. Uterine fibroids. N Engl J Med. 2015;372:1646-1655.
- Mehine M, Makinen N, Heinonen HR, et al. Genomics of uterine leiomyomas: insights from high-throughput sequencing. Fertil Steril. 2014;102:621-629.
- Mehine M, Kaasinen E, Makinen N, et al. Characterization of uterine leiomyomas by whole-genome sequencing. N Engl J Med. 2013;369:43-53.
- Moravek MB, Bulun SE. Endocrinology of uterine fibroids: steroid hormones, stem cells and genetic contribution. Curr Opin Obstet Gynecol. 2015;27:276-283.
- Rein MS. Advances in uterine leiomyoma research: the progesterone hypothesis. Environ Health Perspect. 2000;108(suppl 5):791-793.
- Friedman AJ, Barbieri RL, Doubilet PM, et al. A randomized double-blind trial of a gonadotropin-releasing hormone agonist (leuprolide) with or without medroxyprogesterone acetate in the treatment of leiomyomata uteri. Fertil Steril. 1988;49:404-409.
- Donnez J, Hudecek R, Donnez O, et al. Efficacy and safety of repeated use of ulipristal acetate in uterine fibroids. Fertil Steril. 2015;103:519-527.
- Donnez J, Tatarchuk TF, Bouchard P, et al. Ulipristal acetate versus placebo for fibroid treatment before surgery. N Engl J Med. 2012;366:409-420.
- Donnez J, Tomaszewski J, Vazquez F, et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med. 2012;366:421-432.
- European Medicines Agency. Suspension of ulipristal acetate for uterine fibroids during ongoing EMA review of liver injury risk. March 13, 2020. https://www.ema.europa.eu/en/news/suspension-ulipristal-acetate-uterine-fibroids-during-ongoing-ema-review-liver-injury-risk#:~:text=EMA's%20safety%20committee%20(PRAC)%20has,the%20EU%20during%20the%20review. Accessed July 24, 2020.
- Lupron Depot [package insert]. Osaka, Japan: Takeda; Revised March 2012.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
- Simon JA, Al-Hendy A, Archer DF, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326.
- Oriahnn [package insert]. North Chicago, IL: AbbVie; 2020.
Uterine leiomyomata (fibroids) are the most common pelvic tumor diagnosed in women.1 Women with symptomatic fibroids often report abnormal uterine bleeding (AUB) and pelvic cramping, fullness, or pain. Fibroids also may cause frequency of urination and contribute to fertility and pregnancy problems. Treatment options for the AUB caused by fibroids include, but are not limited to, hysterectomy, myomectomy, uterine artery embolization, endometrial ablation, insertion of a levonorgestrel intrauterine device, focused ultrasound surgery, radiofrequency ablation, leuprolide acetate, and elagolix plus low-dose hormone add-back (Oriahnn; AbbVie, North Chicago, Illinois).1 Oriahnn is the most recent addition to our treatment armamentarium for fibroids and represents the first US Food and Drug Administration (FDA)-approved long-term hormonal option for AUB caused by fibroids.
Gene dysregulation contributes to fibroid development
Most uterine fibroids are clonal tumors, which develop following a somatic mutation in a precursor uterine myocyte. The somatic mutation causes gene dysregulation that stimulates cell growth resulting in a benign tumor mass. The majority of fibroids contain a mutation in one of the following 6 genes: mediator complex subunit 12 (MED12), high mobility group AT-hook (HMGA2 or HMGA1), RAD51B, fumarate hydratase (FH), collagen type IV, alpha 5 chain (COL4A5), or collagen type IV alpha 6 chain (COL4A6).2
Gene dysregulation in fibroids may arise following chromothripsis of the uterine myocyte genome
Chromothripsis is a catastrophic intracellular genetic event in which one or more chromosomes are broken and reassemble in a new nucleic acid sequence, producing a derivative chromosome that contains complex genetic rearrangements.3 Chromothripsis is believed to occur frequently in uterine myocytes. It is unknown why uterine myocytes are susceptible to chromothripsis,3 or why a catastrophic intracellular event such as chromothripsis results in preferential mutations in the 6 genes that are associated with myoma formation.
Estrogen and progesterone influence fibroid size and cell activity
Although uterine fibroids are clonal tumors containing broken genes, they are also exquisitely responsive to estradiol and progesterone. Estradiol and progesterone play an important role in regulating fibroid size and function.4 Estrogen stimulates uterine fibroids to increase in size. In a hypoestrogenic state, uterine fibroids decrease in size. In addition, a hypoestrogenic state results in an atrophic endometrium and thereby reduces AUB. For women with uterine fibroids and AUB, a reversible hypoestrogenic state can be induced either with a parenteral GnRH-agonist analogue (leuprolide) or an oral GnRH-antagonist (elagolix). Both leuprolide and elagolix are approved for the treatment of uterine fibroids (see below).
Surprisingly, progesterone stimulates cell division in normal uterine myocytes and fibroid cells.5 In the luteal phase of the menstrual cycle, uterine myocyte mitoses are more frequent than in the follicular phase. In addition, synthetic progestins appear to maintain fibroid size in a hypoestrogenic environment. In one randomized trial, women with uterine fibroids treated with leuprolide acetate plus a placebo pill for 24 weeks had a 51% reduction in uterine volume as measured by ultrasound.6 Women with uterine fibroids treated with leuprolide acetate plus the synthetic progestin, oral medroxyprogesterone acetate 20 mg daily, had only a 15% reduction in uterine volume.6 This finding suggests that synthetic progestins partially block the decrease in uterine volume that occurs in a hypoestrogenic state.
Further evidence that progesterone plays a role in fibroid biology is the observation that treatment of women with uterine fibroids with the antiprogestin ulipristal decreases fibroid size and reduces AUB.7-9 Ulipristal was approved for the treatment of fibroids in many countries but not the United States. Reports of severe, life-threatening liver injury—some necessitating liver transplantation—among women using ulipristal prompted the European Medicines Agency (EMA) in 2020 to recommend that women stop taking ulipristal. In addition, the EMA recommended that no woman should initiate ulipristal treatment at this time.10
Continue to: Leuprolide acetate...
Leuprolide acetate
Leuprolide acetate is a peptide GnRH-agonist analogue. Initiation of leuprolide treatment stimulates gonadotropin release, but with chronic administration pituitary secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) decreases, resulting in reduced ovarian follicular activity, anovulation, and low serum concentration of estradiol and progesterone. Leuprolide treatment concomitant with iron therapy is approved by the FDA for improving red blood cell volume prior to surgery in women with fibroids, AUB, and anemia.11 Among women with fibroids, AUB, and anemia, after 12 weeks of treatment, the hemoglobin concentration was ≥12 g/dL in 79% treated with leuprolide plus iron and 56% treated with iron alone.11 The FDA recommends limiting preoperative leuprolide treatment to no more than 3 months. The approved leuprolide regimens are a maximum of 3 monthly injections of leuprolide 3.75 mg or a single injection of leuprolide 11.25 mg. Leuprolide treatment prior to hysterectomy surgery for uterine fibroids usually will result in a decrease in uterine size and may facilitate vaginal hysterectomy.
Elagolix plus estradiol plus norethindrone acetate (Oriahnn)
GnRH analogues cause a hypoestrogenic state resulting in adverse effects, including moderate to severe hot flashes and a reduction in bone mineral density. One approach to reducing the unwanted effects of hot flashes and decreased bone density is to combine a GnRH analogue with low-dose steroid hormone add-back therapy. Combining a GnRH analogue with low-dose steroid hormone add-back permits long-term treatment of AUB caused by fibroids, with few hot flashes and a minimal decrease in bone mineral density. The FDA recently has approved the combination of elagolix plus low-dose estradiol and norethindrone acetate (Oriahnn) for the long-term treatment of AUB caused by fibroids.
Elagolix is a nonpeptide oral GnRH antagonist that reduces pituitary secretion of LH and FSH, resulting in a decrease in ovarian follicular activity, anovulation, and low serum concentration of estradiol and progesterone. Unlike leuprolide, which causes an initial increase in LH and FSH secretion, the initiation of elagolix treatment causes an immediate and sustained reduction in LH and FSH secretion. Combining elagolix with a low dose of estradiol and norethindrone acetate reduces the side effects of hot flashes and decreased bone density. Clinical trials have reported that the combination of elagolix (300 mg) twice daily plus estradiol (1 mg) and norethindrone acetate (0.5 mg) once daily is an effective long-term treatment of AUB caused by uterine fibroids.
To study the efficacy of elagolix (alone or with estrogen-progestin add-back therapy) for the treatment of AUB caused by uterine fibroids, two identical trials were performed,12 in which 790 women participated. The participants had a mean age of 42 years and were documented to have heavy menstrual bleeding (>80 mL blood loss per cycle) and ultrasound-diagnosed uterine fibroids. The participants were randomized to one of 3 groups:
- elagolix (300 mg twice daily) plus low-dose steroid add-back (1 mg estradiol and 0.5 mg norethindrone acetate once daily),
- elagolix 300 mg twice daily with no steroid add-back (elagolix alone), or
- placebo for 6 months.12
Menstrual blood loss was quantified using the alkaline hematin method on collected sanitary products. The primary endpoint was menstrual blood loss <80 mL per cycle as well as a ≥50% reduction in quantified blood loss from baseline during the final month of treatment. At 6 months, the percentage of women achieving the primary endpoint in the first trial was 84% (elagolix alone), 69% (elagolix plus add-back), and 9% (placebo). Mean changes from baseline in lumbar spine bone density were −2.95% (elagolix alone), −0.76% (elagolix plus add-back), and −0.21% (placebo). The percentage of women reporting hot flashes was 64% in the elagolix group, 20% in the elagolix plus low-dose steroid add-back group, and 9% in the placebo group. Results were similar in the second trial.12
The initial trials were extended to 12 months with two groups: elagolix 300 mg twice daily plus low-dose hormone add-back with 1 mg estradiol and 0.5 mg norethindrone acetate once daily (n = 218) or elagolix 300 mg twice daily (elagolix alone) (n = 98).13 Following 12 months of treatment, heavy menstrual bleeding was controlled in 88% and 89% of women treated with elagolix plus add-back and elagolix alone, respectively. Amenorrhea was reported by 65% of the women in the elagolix plus add-back group. Compared with baseline bone density, at the end of 12 months of treatment, bone mineral density in the lumbar spine was reduced by -1.5% and -4.8% in the women treated with elagolix plus add-back and elagolix alone, respectively. Compared with baseline bone density, at 1 year following completion of treatment, bone mineral density in the lumbar spine was reduced by -0.6% and -2.0% in the women treated with elagolix plus add-back and elagolix alone, respectively. Similar trends were observed in total hip and femoral neck bone density. During treatment with elagolix plus add-back, adverse effects were modest, including hot flushes (6%), night sweats (3.2%), headache (5.5%), and nausea (4.1%). Two women developed liver transaminase levels >3 times the upper limit of normal, resulting in one woman discontinuing treatment.13
Continue to: Contraindications to Oriahnn include known allergies...
Contraindications to Oriahnn include known allergies to the components of the medication (including the yellow dye tartrazine); high risk of arterial, venous thrombotic or thromboembolic disorders; pregnancy; known osteoporosis; current breast cancer or other hormonally-sensitive malignancies; known liver disease; and concurrent use of organic anion transporting polypeptide 1B1 inhibitors, which includes many HIV antiviral medications.14 Undiagnosed AUB is a contraindication, and all women prescribed Oriahnn should have endometrial sampling before initiating treatment. Oriahnn should not be used for more than 24 months due to the risk of irreversible bone loss.14 Systemic estrogen and progestin combinations, a component of Oriahnn, increases the risk for pulmonary embolism, deep vein thrombosis, stroke, and myocardial infarction, especially in women at increased risk for these events (such as women >35 years who smoke cigarettes and women with uncontrolled hypertension).14 In two studies there was a higher incidence of depression, depressed mood, and/or tearfulness in women taking Oriahnn (3%) compared with those taking a placebo (1%).14 The FDA recommends promptly evaluating women with depressive symptoms to determine the risks of initiating and continuing Oriahnn therapy. In two studies there was a higher risk of reported alopecia among women taking Oriahnn (3.5%) compared with placebo (1%).14
It should be noted that elagolix is approved for the treatment of pelvic pain caused by endometriosis at a dose of 150 mg daily for 24 months or 200 mg twice daily for 6 months. The elagolix dose for the treatment of AUB caused by fibroids is 300 mg twice daily for up to 24 months, necessitating the addition of low-dose estradiol-norethindrone add-back to reduce the frequency and severity of hot flashes and minimize the loss of bone density. Norethindrone acetate also protects the endometrium from the stimulatory effect of estradiol, reducing the risk of developing endometrial hyperplasia and cancer. Oriahnn is formulated as two different capsules. A yellow and white capsule contains elagolix 300 mg plus estradiol 1 mg and norethindrone acetate 0.5 mg to be taken in the morning, and a blue and white capsule contains elagolix 300 mg to be taken in the evening.
AUB caused by fibroids is a common problem in gyn practice
There are many procedural interventions that are effective in reducing AUB caused by fibroids. However, prior to the approval of Oriahnn there were no hormonal medications that were FDA approved for the long-term treatment of AUB caused by fibroids. Hence, Oriahnn represents an important advance in the hormonal treatment of AUB caused by fibroids and expands the treatment options available to our patients. ●
Black women are more likely to develop fibroids and experience more severe fibroid symptoms. Obstetrician-gynecologists are experts in the diagnosis and treatment of fibroids. We play a key role in partnering with Black women to reduce fibroid disease burden.
Factors that increase the risk of developing fibroids include: increasing age, Black race, nulliparity, early menarche (<10 years of age), obesity, and consumption of red meat.1 The Nurses Health Study II is the largest prospective study of the factors that influence fibroid development.2 A total of 95,061 premenopausal nurses aged 25 to 44 years were followed from September 1989 through May 1993. Review of a sample of medical records demonstrated that the nurses participating in the study were reliable reporters of whether or not they had been diagnosed with fibroids. Based on a report of an ultrasound or hysterectomy diagnosis, the incidence rate for fibroids increased with age. Incidence rate per 1,000 women-years was 4.3 (age 25 to 29 years), 9.0 (30 to 34 years), 14.7 (age 35 to 39 years), and 22.5 (40 to 44 years). Compared with White race, Black race (but not Hispanic ethnicity or Asian race) was associated with an increased incidence of fibroids. Incidence rate per 1,000 women-years was 12.5 (White race), 37.9 (Black race), 14.5 (Hispanic ethnicity), and 10.4 (Asian race). The risk of developing fibroids was 3.25 times (95% CI, 2.71 to 3.88) greater among Black compared with White women after controlling for body mass index, age at first birth, years since last birth, history of infertility, age at first oral contraceptive use, marital status, and current alcohol use.2
Other epidemiology studies also report an increased incidence of fibroids among Black women.3,4 The size of the uterus, the size and number of fibroids, and the severity of fibroid symptoms are greater among Black versus White women.5,6 The molecular factors that increase fibroid incidence among Black women are unknown. Given the burden of fibroid disease among Black women, obstetrician-gynecologists are best positioned to ensure early diagnosis and to develop an effective follow-up and treatment plan for affected women.
References
1. Stewart EA, Laughlin-Tommaso SK, Catherino WH, et al. Uterine fibroids. Nat Rev Dis Primers. 2016;2:16043.
2. Marshall LM, Spiegelman D, Barbieri RL, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90:967-973.
3. Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
4. Brett KM, Marsh JV, Madans JH. Epidemiology of hysterectomy in the United States: demographic and reproductive factors in a nationally representative sample. J Womens Health. 1997;6:309-316.
5. Peddada SD, Laughlin SK, Miner K, et al. Growth of uterine leiomyomata among premenopausal black and white women. Proc Natl Acad Sci USA. 2008;105:1988719892.
6. Huyck KL, Panhuysen CI, Cuenco KT, et al. The impact of race as a risk factor for symptom severity and age at diagnosis of uterine leiomyomata among affected sisters. Am J Obstet Gynecol. 2008;198:168.e1-e9.
Uterine leiomyomata (fibroids) are the most common pelvic tumor diagnosed in women.1 Women with symptomatic fibroids often report abnormal uterine bleeding (AUB) and pelvic cramping, fullness, or pain. Fibroids also may cause frequency of urination and contribute to fertility and pregnancy problems. Treatment options for the AUB caused by fibroids include, but are not limited to, hysterectomy, myomectomy, uterine artery embolization, endometrial ablation, insertion of a levonorgestrel intrauterine device, focused ultrasound surgery, radiofrequency ablation, leuprolide acetate, and elagolix plus low-dose hormone add-back (Oriahnn; AbbVie, North Chicago, Illinois).1 Oriahnn is the most recent addition to our treatment armamentarium for fibroids and represents the first US Food and Drug Administration (FDA)-approved long-term hormonal option for AUB caused by fibroids.
Gene dysregulation contributes to fibroid development
Most uterine fibroids are clonal tumors, which develop following a somatic mutation in a precursor uterine myocyte. The somatic mutation causes gene dysregulation that stimulates cell growth resulting in a benign tumor mass. The majority of fibroids contain a mutation in one of the following 6 genes: mediator complex subunit 12 (MED12), high mobility group AT-hook (HMGA2 or HMGA1), RAD51B, fumarate hydratase (FH), collagen type IV, alpha 5 chain (COL4A5), or collagen type IV alpha 6 chain (COL4A6).2
Gene dysregulation in fibroids may arise following chromothripsis of the uterine myocyte genome
Chromothripsis is a catastrophic intracellular genetic event in which one or more chromosomes are broken and reassemble in a new nucleic acid sequence, producing a derivative chromosome that contains complex genetic rearrangements.3 Chromothripsis is believed to occur frequently in uterine myocytes. It is unknown why uterine myocytes are susceptible to chromothripsis,3 or why a catastrophic intracellular event such as chromothripsis results in preferential mutations in the 6 genes that are associated with myoma formation.
Estrogen and progesterone influence fibroid size and cell activity
Although uterine fibroids are clonal tumors containing broken genes, they are also exquisitely responsive to estradiol and progesterone. Estradiol and progesterone play an important role in regulating fibroid size and function.4 Estrogen stimulates uterine fibroids to increase in size. In a hypoestrogenic state, uterine fibroids decrease in size. In addition, a hypoestrogenic state results in an atrophic endometrium and thereby reduces AUB. For women with uterine fibroids and AUB, a reversible hypoestrogenic state can be induced either with a parenteral GnRH-agonist analogue (leuprolide) or an oral GnRH-antagonist (elagolix). Both leuprolide and elagolix are approved for the treatment of uterine fibroids (see below).
Surprisingly, progesterone stimulates cell division in normal uterine myocytes and fibroid cells.5 In the luteal phase of the menstrual cycle, uterine myocyte mitoses are more frequent than in the follicular phase. In addition, synthetic progestins appear to maintain fibroid size in a hypoestrogenic environment. In one randomized trial, women with uterine fibroids treated with leuprolide acetate plus a placebo pill for 24 weeks had a 51% reduction in uterine volume as measured by ultrasound.6 Women with uterine fibroids treated with leuprolide acetate plus the synthetic progestin, oral medroxyprogesterone acetate 20 mg daily, had only a 15% reduction in uterine volume.6 This finding suggests that synthetic progestins partially block the decrease in uterine volume that occurs in a hypoestrogenic state.
Further evidence that progesterone plays a role in fibroid biology is the observation that treatment of women with uterine fibroids with the antiprogestin ulipristal decreases fibroid size and reduces AUB.7-9 Ulipristal was approved for the treatment of fibroids in many countries but not the United States. Reports of severe, life-threatening liver injury—some necessitating liver transplantation—among women using ulipristal prompted the European Medicines Agency (EMA) in 2020 to recommend that women stop taking ulipristal. In addition, the EMA recommended that no woman should initiate ulipristal treatment at this time.10
Continue to: Leuprolide acetate...
Leuprolide acetate
Leuprolide acetate is a peptide GnRH-agonist analogue. Initiation of leuprolide treatment stimulates gonadotropin release, but with chronic administration pituitary secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) decreases, resulting in reduced ovarian follicular activity, anovulation, and low serum concentration of estradiol and progesterone. Leuprolide treatment concomitant with iron therapy is approved by the FDA for improving red blood cell volume prior to surgery in women with fibroids, AUB, and anemia.11 Among women with fibroids, AUB, and anemia, after 12 weeks of treatment, the hemoglobin concentration was ≥12 g/dL in 79% treated with leuprolide plus iron and 56% treated with iron alone.11 The FDA recommends limiting preoperative leuprolide treatment to no more than 3 months. The approved leuprolide regimens are a maximum of 3 monthly injections of leuprolide 3.75 mg or a single injection of leuprolide 11.25 mg. Leuprolide treatment prior to hysterectomy surgery for uterine fibroids usually will result in a decrease in uterine size and may facilitate vaginal hysterectomy.
Elagolix plus estradiol plus norethindrone acetate (Oriahnn)
GnRH analogues cause a hypoestrogenic state resulting in adverse effects, including moderate to severe hot flashes and a reduction in bone mineral density. One approach to reducing the unwanted effects of hot flashes and decreased bone density is to combine a GnRH analogue with low-dose steroid hormone add-back therapy. Combining a GnRH analogue with low-dose steroid hormone add-back permits long-term treatment of AUB caused by fibroids, with few hot flashes and a minimal decrease in bone mineral density. The FDA recently has approved the combination of elagolix plus low-dose estradiol and norethindrone acetate (Oriahnn) for the long-term treatment of AUB caused by fibroids.
Elagolix is a nonpeptide oral GnRH antagonist that reduces pituitary secretion of LH and FSH, resulting in a decrease in ovarian follicular activity, anovulation, and low serum concentration of estradiol and progesterone. Unlike leuprolide, which causes an initial increase in LH and FSH secretion, the initiation of elagolix treatment causes an immediate and sustained reduction in LH and FSH secretion. Combining elagolix with a low dose of estradiol and norethindrone acetate reduces the side effects of hot flashes and decreased bone density. Clinical trials have reported that the combination of elagolix (300 mg) twice daily plus estradiol (1 mg) and norethindrone acetate (0.5 mg) once daily is an effective long-term treatment of AUB caused by uterine fibroids.
To study the efficacy of elagolix (alone or with estrogen-progestin add-back therapy) for the treatment of AUB caused by uterine fibroids, two identical trials were performed,12 in which 790 women participated. The participants had a mean age of 42 years and were documented to have heavy menstrual bleeding (>80 mL blood loss per cycle) and ultrasound-diagnosed uterine fibroids. The participants were randomized to one of 3 groups:
- elagolix (300 mg twice daily) plus low-dose steroid add-back (1 mg estradiol and 0.5 mg norethindrone acetate once daily),
- elagolix 300 mg twice daily with no steroid add-back (elagolix alone), or
- placebo for 6 months.12
Menstrual blood loss was quantified using the alkaline hematin method on collected sanitary products. The primary endpoint was menstrual blood loss <80 mL per cycle as well as a ≥50% reduction in quantified blood loss from baseline during the final month of treatment. At 6 months, the percentage of women achieving the primary endpoint in the first trial was 84% (elagolix alone), 69% (elagolix plus add-back), and 9% (placebo). Mean changes from baseline in lumbar spine bone density were −2.95% (elagolix alone), −0.76% (elagolix plus add-back), and −0.21% (placebo). The percentage of women reporting hot flashes was 64% in the elagolix group, 20% in the elagolix plus low-dose steroid add-back group, and 9% in the placebo group. Results were similar in the second trial.12
The initial trials were extended to 12 months with two groups: elagolix 300 mg twice daily plus low-dose hormone add-back with 1 mg estradiol and 0.5 mg norethindrone acetate once daily (n = 218) or elagolix 300 mg twice daily (elagolix alone) (n = 98).13 Following 12 months of treatment, heavy menstrual bleeding was controlled in 88% and 89% of women treated with elagolix plus add-back and elagolix alone, respectively. Amenorrhea was reported by 65% of the women in the elagolix plus add-back group. Compared with baseline bone density, at the end of 12 months of treatment, bone mineral density in the lumbar spine was reduced by -1.5% and -4.8% in the women treated with elagolix plus add-back and elagolix alone, respectively. Compared with baseline bone density, at 1 year following completion of treatment, bone mineral density in the lumbar spine was reduced by -0.6% and -2.0% in the women treated with elagolix plus add-back and elagolix alone, respectively. Similar trends were observed in total hip and femoral neck bone density. During treatment with elagolix plus add-back, adverse effects were modest, including hot flushes (6%), night sweats (3.2%), headache (5.5%), and nausea (4.1%). Two women developed liver transaminase levels >3 times the upper limit of normal, resulting in one woman discontinuing treatment.13
Continue to: Contraindications to Oriahnn include known allergies...
Contraindications to Oriahnn include known allergies to the components of the medication (including the yellow dye tartrazine); high risk of arterial, venous thrombotic or thromboembolic disorders; pregnancy; known osteoporosis; current breast cancer or other hormonally-sensitive malignancies; known liver disease; and concurrent use of organic anion transporting polypeptide 1B1 inhibitors, which includes many HIV antiviral medications.14 Undiagnosed AUB is a contraindication, and all women prescribed Oriahnn should have endometrial sampling before initiating treatment. Oriahnn should not be used for more than 24 months due to the risk of irreversible bone loss.14 Systemic estrogen and progestin combinations, a component of Oriahnn, increases the risk for pulmonary embolism, deep vein thrombosis, stroke, and myocardial infarction, especially in women at increased risk for these events (such as women >35 years who smoke cigarettes and women with uncontrolled hypertension).14 In two studies there was a higher incidence of depression, depressed mood, and/or tearfulness in women taking Oriahnn (3%) compared with those taking a placebo (1%).14 The FDA recommends promptly evaluating women with depressive symptoms to determine the risks of initiating and continuing Oriahnn therapy. In two studies there was a higher risk of reported alopecia among women taking Oriahnn (3.5%) compared with placebo (1%).14
It should be noted that elagolix is approved for the treatment of pelvic pain caused by endometriosis at a dose of 150 mg daily for 24 months or 200 mg twice daily for 6 months. The elagolix dose for the treatment of AUB caused by fibroids is 300 mg twice daily for up to 24 months, necessitating the addition of low-dose estradiol-norethindrone add-back to reduce the frequency and severity of hot flashes and minimize the loss of bone density. Norethindrone acetate also protects the endometrium from the stimulatory effect of estradiol, reducing the risk of developing endometrial hyperplasia and cancer. Oriahnn is formulated as two different capsules. A yellow and white capsule contains elagolix 300 mg plus estradiol 1 mg and norethindrone acetate 0.5 mg to be taken in the morning, and a blue and white capsule contains elagolix 300 mg to be taken in the evening.
AUB caused by fibroids is a common problem in gyn practice
There are many procedural interventions that are effective in reducing AUB caused by fibroids. However, prior to the approval of Oriahnn there were no hormonal medications that were FDA approved for the long-term treatment of AUB caused by fibroids. Hence, Oriahnn represents an important advance in the hormonal treatment of AUB caused by fibroids and expands the treatment options available to our patients. ●
Black women are more likely to develop fibroids and experience more severe fibroid symptoms. Obstetrician-gynecologists are experts in the diagnosis and treatment of fibroids. We play a key role in partnering with Black women to reduce fibroid disease burden.
Factors that increase the risk of developing fibroids include: increasing age, Black race, nulliparity, early menarche (<10 years of age), obesity, and consumption of red meat.1 The Nurses Health Study II is the largest prospective study of the factors that influence fibroid development.2 A total of 95,061 premenopausal nurses aged 25 to 44 years were followed from September 1989 through May 1993. Review of a sample of medical records demonstrated that the nurses participating in the study were reliable reporters of whether or not they had been diagnosed with fibroids. Based on a report of an ultrasound or hysterectomy diagnosis, the incidence rate for fibroids increased with age. Incidence rate per 1,000 women-years was 4.3 (age 25 to 29 years), 9.0 (30 to 34 years), 14.7 (age 35 to 39 years), and 22.5 (40 to 44 years). Compared with White race, Black race (but not Hispanic ethnicity or Asian race) was associated with an increased incidence of fibroids. Incidence rate per 1,000 women-years was 12.5 (White race), 37.9 (Black race), 14.5 (Hispanic ethnicity), and 10.4 (Asian race). The risk of developing fibroids was 3.25 times (95% CI, 2.71 to 3.88) greater among Black compared with White women after controlling for body mass index, age at first birth, years since last birth, history of infertility, age at first oral contraceptive use, marital status, and current alcohol use.2
Other epidemiology studies also report an increased incidence of fibroids among Black women.3,4 The size of the uterus, the size and number of fibroids, and the severity of fibroid symptoms are greater among Black versus White women.5,6 The molecular factors that increase fibroid incidence among Black women are unknown. Given the burden of fibroid disease among Black women, obstetrician-gynecologists are best positioned to ensure early diagnosis and to develop an effective follow-up and treatment plan for affected women.
References
1. Stewart EA, Laughlin-Tommaso SK, Catherino WH, et al. Uterine fibroids. Nat Rev Dis Primers. 2016;2:16043.
2. Marshall LM, Spiegelman D, Barbieri RL, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90:967-973.
3. Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
4. Brett KM, Marsh JV, Madans JH. Epidemiology of hysterectomy in the United States: demographic and reproductive factors in a nationally representative sample. J Womens Health. 1997;6:309-316.
5. Peddada SD, Laughlin SK, Miner K, et al. Growth of uterine leiomyomata among premenopausal black and white women. Proc Natl Acad Sci USA. 2008;105:1988719892.
6. Huyck KL, Panhuysen CI, Cuenco KT, et al. The impact of race as a risk factor for symptom severity and age at diagnosis of uterine leiomyomata among affected sisters. Am J Obstet Gynecol. 2008;198:168.e1-e9.
- Stewart EA. Uterine fibroids. N Engl J Med. 2015;372:1646-1655.
- Mehine M, Makinen N, Heinonen HR, et al. Genomics of uterine leiomyomas: insights from high-throughput sequencing. Fertil Steril. 2014;102:621-629.
- Mehine M, Kaasinen E, Makinen N, et al. Characterization of uterine leiomyomas by whole-genome sequencing. N Engl J Med. 2013;369:43-53.
- Moravek MB, Bulun SE. Endocrinology of uterine fibroids: steroid hormones, stem cells and genetic contribution. Curr Opin Obstet Gynecol. 2015;27:276-283.
- Rein MS. Advances in uterine leiomyoma research: the progesterone hypothesis. Environ Health Perspect. 2000;108(suppl 5):791-793.
- Friedman AJ, Barbieri RL, Doubilet PM, et al. A randomized double-blind trial of a gonadotropin-releasing hormone agonist (leuprolide) with or without medroxyprogesterone acetate in the treatment of leiomyomata uteri. Fertil Steril. 1988;49:404-409.
- Donnez J, Hudecek R, Donnez O, et al. Efficacy and safety of repeated use of ulipristal acetate in uterine fibroids. Fertil Steril. 2015;103:519-527.
- Donnez J, Tatarchuk TF, Bouchard P, et al. Ulipristal acetate versus placebo for fibroid treatment before surgery. N Engl J Med. 2012;366:409-420.
- Donnez J, Tomaszewski J, Vazquez F, et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med. 2012;366:421-432.
- European Medicines Agency. Suspension of ulipristal acetate for uterine fibroids during ongoing EMA review of liver injury risk. March 13, 2020. https://www.ema.europa.eu/en/news/suspension-ulipristal-acetate-uterine-fibroids-during-ongoing-ema-review-liver-injury-risk#:~:text=EMA's%20safety%20committee%20(PRAC)%20has,the%20EU%20during%20the%20review. Accessed July 24, 2020.
- Lupron Depot [package insert]. Osaka, Japan: Takeda; Revised March 2012.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
- Simon JA, Al-Hendy A, Archer DF, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326.
- Oriahnn [package insert]. North Chicago, IL: AbbVie; 2020.
- Stewart EA. Uterine fibroids. N Engl J Med. 2015;372:1646-1655.
- Mehine M, Makinen N, Heinonen HR, et al. Genomics of uterine leiomyomas: insights from high-throughput sequencing. Fertil Steril. 2014;102:621-629.
- Mehine M, Kaasinen E, Makinen N, et al. Characterization of uterine leiomyomas by whole-genome sequencing. N Engl J Med. 2013;369:43-53.
- Moravek MB, Bulun SE. Endocrinology of uterine fibroids: steroid hormones, stem cells and genetic contribution. Curr Opin Obstet Gynecol. 2015;27:276-283.
- Rein MS. Advances in uterine leiomyoma research: the progesterone hypothesis. Environ Health Perspect. 2000;108(suppl 5):791-793.
- Friedman AJ, Barbieri RL, Doubilet PM, et al. A randomized double-blind trial of a gonadotropin-releasing hormone agonist (leuprolide) with or without medroxyprogesterone acetate in the treatment of leiomyomata uteri. Fertil Steril. 1988;49:404-409.
- Donnez J, Hudecek R, Donnez O, et al. Efficacy and safety of repeated use of ulipristal acetate in uterine fibroids. Fertil Steril. 2015;103:519-527.
- Donnez J, Tatarchuk TF, Bouchard P, et al. Ulipristal acetate versus placebo for fibroid treatment before surgery. N Engl J Med. 2012;366:409-420.
- Donnez J, Tomaszewski J, Vazquez F, et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med. 2012;366:421-432.
- European Medicines Agency. Suspension of ulipristal acetate for uterine fibroids during ongoing EMA review of liver injury risk. March 13, 2020. https://www.ema.europa.eu/en/news/suspension-ulipristal-acetate-uterine-fibroids-during-ongoing-ema-review-liver-injury-risk#:~:text=EMA's%20safety%20committee%20(PRAC)%20has,the%20EU%20during%20the%20review. Accessed July 24, 2020.
- Lupron Depot [package insert]. Osaka, Japan: Takeda; Revised March 2012.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
- Simon JA, Al-Hendy A, Archer DF, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326.
- Oriahnn [package insert]. North Chicago, IL: AbbVie; 2020.
Enduring the ordeal of a quadruple threat is especially arduous for psychiatric patients
These are unusually stressful days for everyone, especially our patients. We are all experiencing a turbulent mix of emotions as we try to cope with a confluence of threats to both our lives and to life as we know it. Peace of mind has become so elusive due to the relentless overlapping waves of fear, sadness, anger, and uncertainty. We are all grieving in a different way, but our psychiatric patients are suffering the most.
Fear. It only takes 1 traumatic event to trigger posttraumatic stress disorder (PTSD). Yet over the past few months, we have been afflicted by 4 jarring traumatic events, individually and as a society. Just a few months ago, it would have been impossible to imagine the conflux of 4 concurrent seismic threats to our well-being. A toxic political zeitgeist was the backdrop, which we bemoaned and tried to compartmentalize, despite the corrosive political environment shrouding the country. Then the deadly coronavirus disease 2019 (COVID-19) pandemic suddenly arrived, imposing draconian health-preserving measures that impacted every individual’s daily life in countless detrimental ways. Fear prevailed as we all sheltered at home, stopped commuting to work, canceled all trips, distanced ourselves from our friends and relatives, and watched depressing and anxiety-provoking television and read online news throughout our waking hours. Hoarding food and household supplies became endemic due to fear about survival.
Sadness. The agonizing prospect of a national financial necrosis followed the threat of serious illness or death. The economy came to a screeching halt, hemorrhaging millions of jobs. Unemployed parents stayed home with their morose children whose schools were shuttered, leaving them deprived of socializing with their friends. The government hurried with financial chemotherapy, printing trillions of dollars to prevent economic collapse, to avert potential poverty and hunger for many. The fear of the pandemic became coupled with sadness over the loss of livelihoods and grief for the loss of liberty and the ability to pursue happiness, or even small pleasures.
Anger. Then a tsunami of anger was generated by the brutal and sadistic death of a black man in police custody. This was a spark that ignited a massive amount of previously dormant racial tension dating back to the dark days of slavery. Peaceful protests were marred by destructive riots. The explosive fury was perhaps intensified by the protestors having been being locked up for weeks and having to wear masks, both of which were symbolic of being held down and “unable to breathe,” like the murdered Mr. George Floyd.
An epidemic of destroying statues followed. Heavy statues that appeared invincible for decades were dismantled from their plinth in a matter of minutes, signifying extreme frustration with the social injustice that remains despite the transformational laws of the Civil Rights Acts of 1960 and 1964. Suddenly—like falling dominoes—statues, flags, names of military bases, and previously venerated monuments were removed, changed, vandalized, or threatened with destruction. The founders of the republic were also maligned because they were slave owners 2 centuries ago. The paradigm shift spawned by the rage over racial inequality was disconcerting and dramatic. The anger and rampage spawned a sense that a tipping point in our society has been reached.
Uncertainty. The confluence of political instability, a deadly pandemic, economic collapse, and racial tensions were like the 4 horsemen of mass PTSD. The result was an agonizing uncertainty about the impact of these changes, and whether a sense of normalcy will ever return. It became apparent to all of us that our social structure has changed forever across multiple fundamental domains: public health, social, political, and financial. The wait for a vaccine for COVID-19 seems interminable, and racial healing and harmony seems elusive. Economic recovery may be possible, but political detoxification appears unlikely. The fate of police departments, condemned because of the deplorable and illegal acts of a few, and the safety of citizens, usually guaranteed by law and order, seem uncertain. Like COVID-19, angst has rapidly spread across the population.
The price our patients pay
The ingredients of a large-scale societal PTSD, similar to what probably happens during a world war, are now in place. Even resilient individuals may buckle during quadruple ordeals such as this one. So imagine what is happening to our patients, rendered fragile and vulnerable to threats by their pre-existing psychiatric illness. They all pay a heavy price. Patients with anxiety disorders will decompensate, with more panic attacks. Patients burdened by depression will worsen, with more hopelessness, despair, and suicidal ideation due to anxiety and loneliness. Patients with bipolar disorder will become more labile and irritable, and their comorbid anxiety will intensify. Patients with schizophrenia will become more paranoid, depressed, and anxious. Patients with autism will become more agitated and aggressive because their cherished daily routines are disrupted. Patients with obsessive-compulsive disorder will react to their germaphobia by washing their hands and cleaning everything around them even more frequently, and they (along with everyone else) will become hoarders.
Hope and healing
As psychiatrists, we are determined to transcend our own stress, rise above it all, and attend to the pervasive sadness, grief, anger, and uncertainty all around us, but especially among our patients, for whom the anguish of a psychiatric disorder is further compounded by 4 additional ordeals. This is our moment of truth as healers of our patients’ souls, because they look to us to provide them with hope to help navigate these trying times into full health. And we psychiatrists, along with fellow mental health professionals, are up to this unprecedented challenge.
These are unusually stressful days for everyone, especially our patients. We are all experiencing a turbulent mix of emotions as we try to cope with a confluence of threats to both our lives and to life as we know it. Peace of mind has become so elusive due to the relentless overlapping waves of fear, sadness, anger, and uncertainty. We are all grieving in a different way, but our psychiatric patients are suffering the most.
Fear. It only takes 1 traumatic event to trigger posttraumatic stress disorder (PTSD). Yet over the past few months, we have been afflicted by 4 jarring traumatic events, individually and as a society. Just a few months ago, it would have been impossible to imagine the conflux of 4 concurrent seismic threats to our well-being. A toxic political zeitgeist was the backdrop, which we bemoaned and tried to compartmentalize, despite the corrosive political environment shrouding the country. Then the deadly coronavirus disease 2019 (COVID-19) pandemic suddenly arrived, imposing draconian health-preserving measures that impacted every individual’s daily life in countless detrimental ways. Fear prevailed as we all sheltered at home, stopped commuting to work, canceled all trips, distanced ourselves from our friends and relatives, and watched depressing and anxiety-provoking television and read online news throughout our waking hours. Hoarding food and household supplies became endemic due to fear about survival.
Sadness. The agonizing prospect of a national financial necrosis followed the threat of serious illness or death. The economy came to a screeching halt, hemorrhaging millions of jobs. Unemployed parents stayed home with their morose children whose schools were shuttered, leaving them deprived of socializing with their friends. The government hurried with financial chemotherapy, printing trillions of dollars to prevent economic collapse, to avert potential poverty and hunger for many. The fear of the pandemic became coupled with sadness over the loss of livelihoods and grief for the loss of liberty and the ability to pursue happiness, or even small pleasures.
Anger. Then a tsunami of anger was generated by the brutal and sadistic death of a black man in police custody. This was a spark that ignited a massive amount of previously dormant racial tension dating back to the dark days of slavery. Peaceful protests were marred by destructive riots. The explosive fury was perhaps intensified by the protestors having been being locked up for weeks and having to wear masks, both of which were symbolic of being held down and “unable to breathe,” like the murdered Mr. George Floyd.
An epidemic of destroying statues followed. Heavy statues that appeared invincible for decades were dismantled from their plinth in a matter of minutes, signifying extreme frustration with the social injustice that remains despite the transformational laws of the Civil Rights Acts of 1960 and 1964. Suddenly—like falling dominoes—statues, flags, names of military bases, and previously venerated monuments were removed, changed, vandalized, or threatened with destruction. The founders of the republic were also maligned because they were slave owners 2 centuries ago. The paradigm shift spawned by the rage over racial inequality was disconcerting and dramatic. The anger and rampage spawned a sense that a tipping point in our society has been reached.
Uncertainty. The confluence of political instability, a deadly pandemic, economic collapse, and racial tensions were like the 4 horsemen of mass PTSD. The result was an agonizing uncertainty about the impact of these changes, and whether a sense of normalcy will ever return. It became apparent to all of us that our social structure has changed forever across multiple fundamental domains: public health, social, political, and financial. The wait for a vaccine for COVID-19 seems interminable, and racial healing and harmony seems elusive. Economic recovery may be possible, but political detoxification appears unlikely. The fate of police departments, condemned because of the deplorable and illegal acts of a few, and the safety of citizens, usually guaranteed by law and order, seem uncertain. Like COVID-19, angst has rapidly spread across the population.
The price our patients pay
The ingredients of a large-scale societal PTSD, similar to what probably happens during a world war, are now in place. Even resilient individuals may buckle during quadruple ordeals such as this one. So imagine what is happening to our patients, rendered fragile and vulnerable to threats by their pre-existing psychiatric illness. They all pay a heavy price. Patients with anxiety disorders will decompensate, with more panic attacks. Patients burdened by depression will worsen, with more hopelessness, despair, and suicidal ideation due to anxiety and loneliness. Patients with bipolar disorder will become more labile and irritable, and their comorbid anxiety will intensify. Patients with schizophrenia will become more paranoid, depressed, and anxious. Patients with autism will become more agitated and aggressive because their cherished daily routines are disrupted. Patients with obsessive-compulsive disorder will react to their germaphobia by washing their hands and cleaning everything around them even more frequently, and they (along with everyone else) will become hoarders.
Hope and healing
As psychiatrists, we are determined to transcend our own stress, rise above it all, and attend to the pervasive sadness, grief, anger, and uncertainty all around us, but especially among our patients, for whom the anguish of a psychiatric disorder is further compounded by 4 additional ordeals. This is our moment of truth as healers of our patients’ souls, because they look to us to provide them with hope to help navigate these trying times into full health. And we psychiatrists, along with fellow mental health professionals, are up to this unprecedented challenge.
These are unusually stressful days for everyone, especially our patients. We are all experiencing a turbulent mix of emotions as we try to cope with a confluence of threats to both our lives and to life as we know it. Peace of mind has become so elusive due to the relentless overlapping waves of fear, sadness, anger, and uncertainty. We are all grieving in a different way, but our psychiatric patients are suffering the most.
Fear. It only takes 1 traumatic event to trigger posttraumatic stress disorder (PTSD). Yet over the past few months, we have been afflicted by 4 jarring traumatic events, individually and as a society. Just a few months ago, it would have been impossible to imagine the conflux of 4 concurrent seismic threats to our well-being. A toxic political zeitgeist was the backdrop, which we bemoaned and tried to compartmentalize, despite the corrosive political environment shrouding the country. Then the deadly coronavirus disease 2019 (COVID-19) pandemic suddenly arrived, imposing draconian health-preserving measures that impacted every individual’s daily life in countless detrimental ways. Fear prevailed as we all sheltered at home, stopped commuting to work, canceled all trips, distanced ourselves from our friends and relatives, and watched depressing and anxiety-provoking television and read online news throughout our waking hours. Hoarding food and household supplies became endemic due to fear about survival.
Sadness. The agonizing prospect of a national financial necrosis followed the threat of serious illness or death. The economy came to a screeching halt, hemorrhaging millions of jobs. Unemployed parents stayed home with their morose children whose schools were shuttered, leaving them deprived of socializing with their friends. The government hurried with financial chemotherapy, printing trillions of dollars to prevent economic collapse, to avert potential poverty and hunger for many. The fear of the pandemic became coupled with sadness over the loss of livelihoods and grief for the loss of liberty and the ability to pursue happiness, or even small pleasures.
Anger. Then a tsunami of anger was generated by the brutal and sadistic death of a black man in police custody. This was a spark that ignited a massive amount of previously dormant racial tension dating back to the dark days of slavery. Peaceful protests were marred by destructive riots. The explosive fury was perhaps intensified by the protestors having been being locked up for weeks and having to wear masks, both of which were symbolic of being held down and “unable to breathe,” like the murdered Mr. George Floyd.
An epidemic of destroying statues followed. Heavy statues that appeared invincible for decades were dismantled from their plinth in a matter of minutes, signifying extreme frustration with the social injustice that remains despite the transformational laws of the Civil Rights Acts of 1960 and 1964. Suddenly—like falling dominoes—statues, flags, names of military bases, and previously venerated monuments were removed, changed, vandalized, or threatened with destruction. The founders of the republic were also maligned because they were slave owners 2 centuries ago. The paradigm shift spawned by the rage over racial inequality was disconcerting and dramatic. The anger and rampage spawned a sense that a tipping point in our society has been reached.
Uncertainty. The confluence of political instability, a deadly pandemic, economic collapse, and racial tensions were like the 4 horsemen of mass PTSD. The result was an agonizing uncertainty about the impact of these changes, and whether a sense of normalcy will ever return. It became apparent to all of us that our social structure has changed forever across multiple fundamental domains: public health, social, political, and financial. The wait for a vaccine for COVID-19 seems interminable, and racial healing and harmony seems elusive. Economic recovery may be possible, but political detoxification appears unlikely. The fate of police departments, condemned because of the deplorable and illegal acts of a few, and the safety of citizens, usually guaranteed by law and order, seem uncertain. Like COVID-19, angst has rapidly spread across the population.
The price our patients pay
The ingredients of a large-scale societal PTSD, similar to what probably happens during a world war, are now in place. Even resilient individuals may buckle during quadruple ordeals such as this one. So imagine what is happening to our patients, rendered fragile and vulnerable to threats by their pre-existing psychiatric illness. They all pay a heavy price. Patients with anxiety disorders will decompensate, with more panic attacks. Patients burdened by depression will worsen, with more hopelessness, despair, and suicidal ideation due to anxiety and loneliness. Patients with bipolar disorder will become more labile and irritable, and their comorbid anxiety will intensify. Patients with schizophrenia will become more paranoid, depressed, and anxious. Patients with autism will become more agitated and aggressive because their cherished daily routines are disrupted. Patients with obsessive-compulsive disorder will react to their germaphobia by washing their hands and cleaning everything around them even more frequently, and they (along with everyone else) will become hoarders.
Hope and healing
As psychiatrists, we are determined to transcend our own stress, rise above it all, and attend to the pervasive sadness, grief, anger, and uncertainty all around us, but especially among our patients, for whom the anguish of a psychiatric disorder is further compounded by 4 additional ordeals. This is our moment of truth as healers of our patients’ souls, because they look to us to provide them with hope to help navigate these trying times into full health. And we psychiatrists, along with fellow mental health professionals, are up to this unprecedented challenge.
The Fetal Pillow: A new option for delivering the deeply impacted fetal head
Obstetricians know that a cesarean delivery (CD) for a woman with a prolonged second stage and a fetal head deeply impacted in the pelvis is challenging. In this situation, extensions of the uterine incision commonly occur, resulting in prolonged operative time and increased blood loss. Even more harrowing is the inability to deliver the fetal head, necessitating emergency assistance from other clinicians. In this situation, interventions that may be helpful include:
- extend or T the uterine incision
- enlist the aid of a clinician to push up on the fetal head with a vaginal hand (FIGURE 1)
- reverse breech extraction (FIGURE 2), and
- vaginal insertion of a Fetal Pillow prior to starting the delivery.
Evidence from clinical trials indicates that reverse breech extraction or insertion of a Fetal Pillow result in the best clinical outcomes.
Reverse breech extraction vs the push technique
Although the data are limited, most studies report that compared with pushing up with a vaginal hand (as shown in Figure 1), the reverse breech extraction technique (as shown in Figure 2) is associated with a reduction in extensions of the uterine incision, reduced blood loss, and reduced operative time.1 In a randomized trial, 108 women with obstructed labor undergoing CD in the second stage were randomly assigned to reverse breech extraction or pushing up with a vaginal hand.2 Following the uterine incision, the reverse breech extraction technique is performed by immediately reaching into the upper uterus and grasping the lower portion of the fetal leg and applying gentle traction on the leg until the second leg appeared. The lower legs are then pulled out of the uterus. Standard breech delivery maneuvers are used to deliver the shoulders and head. In the trial, compared with the push technique, reverse breech extraction was associated with fewer extensions of the uterine incision (30% vs 11%; P<.05), less blood loss (899 mL vs 1,257 mL; P<.001), and shorter operative time (56 min vs 89 min, P<.001). Fetal injury was similar with the push and breech extraction techniques (6% and 7%).
In another randomized trial, 192 women undergoing CD for obstructed labor were randomly assigned to reverse breech extraction or pushing the head up with a hand in the vagina.3 Compared with the vaginal push technique, reverse breech extraction was associated with fewer extensions of the uterine incision (19% vs 48%; P = .003), fewer cases of wound infection (2% vs 13%; P = .007), and fewer blood transfusions (2 vs 11; P = .012).
Additional options and adjuvants for facilitating delivery of a fetal head deeply impacted in the pelvis include: using a Coyne spoon, using nitroglycerine or terbutaline to relax the myometrium, breaking the vaginal suction on the fetal head before attempting delivery, keeping the wrist of the delivering hand as straight as possible to reduce uterine incision extensions, and incising the ring (if a Bandl’s ring is detected).
Continue to: The Fetal Pillow...
The Fetal Pillow
The Fetal Pillow (Safe Obstetric Systems, New York, New York) is a single-use fetal cephalic elevation device for managing the deeply impacted fetal head (FIGURE 3). The Fetal Pillow has a firm plastic base upon which is attached a soft silicon balloon. The Fetal Pillow is inserted into the vagina prior to initiating CD and the balloon is filled with 180 mL of saline, causing the fetal head to be pushed to a higher station (FIGURE 4). Use of the Fetal Pillow may be indicated prior to CD in the following situations:
- second stage labor with a deeply impacted head
- second stage labor and failed operative delivery
- occiput posterior position or deep transverse arrest
- absent progress in the first stage between 8 cm and 10 cm with a deeply impacted fetal head or excessive caput of the fetal head.
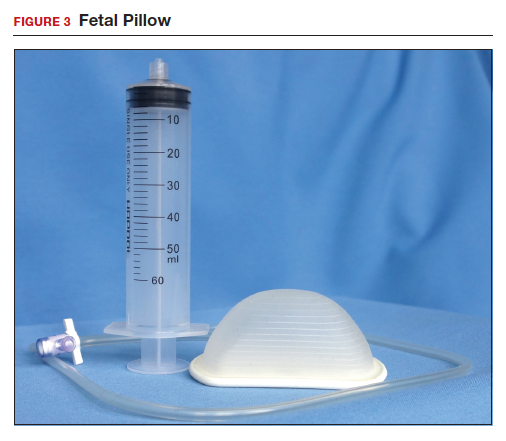
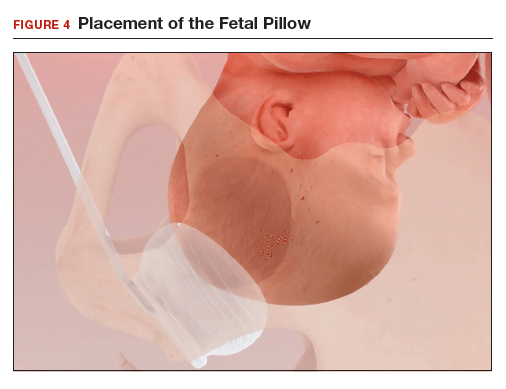
The Fetal Pillow is inserted after completing vaginal preparation for CD and before initiating skin preparation and abdominal draping. The steps for inserting the Fetal Pillow include:
- Use the 60 mL syringe to fully deflate the Fetal Pillow and leave the cock-stop open.
- Fold the Fetal Pillow by squeezing the firm plastic base, and with the patient’s legs in a frog-leg position, place the device in the vagina.
- Allow the firm plastic base to open to a flat position with the base against the posterior vaginal wall and the soft silicon balloon against the fetal head.
- Using pressure on the plastic base, gently push the Fetal Pillow posteriorly toward the sacrum of the mother.
- Use the 60 mL syringe to inflate the balloon with 180 mL of normal saline and close the valve.
- Straighten the patient’s legs and proceed with skin preparation and abdominal draping (FIGURE 4).
When the CD is completed, deflate the balloon by drawing out the saline with the 60 mL syringe and remove the device by hooking a finger around the firm plastic base. The Fetal Pillow is surprisingly easy to use.
Continue to: Effectiveness of the Fetal Pillow...
Effectiveness of the Fetal Pillow
In one randomized trial, 240 women undergoing CD were randomly allocated to a group in which the Fetal Pillow was placed in the vagina and inflated prior to the cesarean and a control group in which the Fetal Pillow was not used.
In another randomized trial, 60 nulliparous women undergoing CD in the second stage of labor had a Fetal Pillow inserted in the vagina and were randomly allocated to inflation of the pillow (Fetal Pillow group) or noninflation of the pillow (control group).5 In this study the mean length of the second stage was 4 hours. Compared with noninflation of the Fetal Pillow, use of the inflated Fetal Pillow was associated with a reduction in grade 3 extension of the uterine incision (extensions into the uterine artery, vagina, or bladder) (0% for inflation vs 13% for noninflation) and fewer difficult plus very difficult deliveries of the fetal head as reported by the surgeon (0% for inflation vs 37% for noninflation). There was no significant difference in blood loss between the two groups (800 mL vs 900 mL). These two randomized studies both reported that the use of the Fetal Pillow was associated with a reduction in grade 3 extensions of the uterine incision and a decrease in the difficulty of delivering the fetal head.
Consider trialing the Fetal Pillow
When a CD is performed after a prolonged second stage of labor, surgical complications are common, including extensions of the uterine incision and difficulty delivering the fetal head. When a grade 3 extension occurs—with tearing of a uterine artery, deep extension into the vagina, or damage to the bladder—the surgical repair can be extraordinarily challenging. Clinical trials report that both reverse breech extraction and the Fetal Pillow can facilitate CD in the setting of a prolonged second stage. For many obstetricians reverse breech extraction is a challenging obstetric maneuver. The insertion and inflation of a Fetal Pillow is a simple procedure. Obstetrician-gynecologists learn by doing. If you have never used the Fetal Pillow, I suggest you consider trialing it in your practice. ●
- Jeve YB, Navti OB, Konje JC. Comparison of techniques used to deliver a deeply impacted fetal head at full dilation: a systematic review and meta-analysis. BJOG. 2016;123:337-345.
- Fasubaa OB, Ezechi OC, Orji EO, et al. Delivery of the impacted head of the fetus at cesarean section after prolonged obstructed labor: a randomised comparative study of two methods. J Obstet Gynaecol. 2002;22:375-378.
- Nooh AM, Abdeldayem HM, Ben-Affan O. Reverse breech extraction versus the standard approach of pushing the impacted fetal head up through the vagina in caesarean section for obstructed labour: a randomised controlled trial. J Obstet Gynaecol. 2017;37:459-463.
- Seal SL, Dey A, Barman SC, et al. Randomized controlled trial of elevation of the fetal head with a fetal pillow during cesarean delivery at full cervical dilatation. Int J Gynaecol Obstet. 2016;133:178-182.
- Lassey SC, Little SE, Saadeh M,et al. Cephalic elevation device for second-stage cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2020;135:879-884.
Obstetricians know that a cesarean delivery (CD) for a woman with a prolonged second stage and a fetal head deeply impacted in the pelvis is challenging. In this situation, extensions of the uterine incision commonly occur, resulting in prolonged operative time and increased blood loss. Even more harrowing is the inability to deliver the fetal head, necessitating emergency assistance from other clinicians. In this situation, interventions that may be helpful include:
- extend or T the uterine incision
- enlist the aid of a clinician to push up on the fetal head with a vaginal hand (FIGURE 1)
- reverse breech extraction (FIGURE 2), and
- vaginal insertion of a Fetal Pillow prior to starting the delivery.


Evidence from clinical trials indicates that reverse breech extraction or insertion of a Fetal Pillow result in the best clinical outcomes.
Reverse breech extraction vs the push technique
Although the data are limited, most studies report that compared with pushing up with a vaginal hand (as shown in Figure 1), the reverse breech extraction technique (as shown in Figure 2) is associated with a reduction in extensions of the uterine incision, reduced blood loss, and reduced operative time.1 In a randomized trial, 108 women with obstructed labor undergoing CD in the second stage were randomly assigned to reverse breech extraction or pushing up with a vaginal hand.2 Following the uterine incision, the reverse breech extraction technique is performed by immediately reaching into the upper uterus and grasping the lower portion of the fetal leg and applying gentle traction on the leg until the second leg appeared. The lower legs are then pulled out of the uterus. Standard breech delivery maneuvers are used to deliver the shoulders and head. In the trial, compared with the push technique, reverse breech extraction was associated with fewer extensions of the uterine incision (30% vs 11%; P<.05), less blood loss (899 mL vs 1,257 mL; P<.001), and shorter operative time (56 min vs 89 min, P<.001). Fetal injury was similar with the push and breech extraction techniques (6% and 7%).
In another randomized trial, 192 women undergoing CD for obstructed labor were randomly assigned to reverse breech extraction or pushing the head up with a hand in the vagina.3 Compared with the vaginal push technique, reverse breech extraction was associated with fewer extensions of the uterine incision (19% vs 48%; P = .003), fewer cases of wound infection (2% vs 13%; P = .007), and fewer blood transfusions (2 vs 11; P = .012).
Additional options and adjuvants for facilitating delivery of a fetal head deeply impacted in the pelvis include: using a Coyne spoon, using nitroglycerine or terbutaline to relax the myometrium, breaking the vaginal suction on the fetal head before attempting delivery, keeping the wrist of the delivering hand as straight as possible to reduce uterine incision extensions, and incising the ring (if a Bandl’s ring is detected).
Continue to: The Fetal Pillow...
The Fetal Pillow
The Fetal Pillow (Safe Obstetric Systems, New York, New York) is a single-use fetal cephalic elevation device for managing the deeply impacted fetal head (FIGURE 3). The Fetal Pillow has a firm plastic base upon which is attached a soft silicon balloon. The Fetal Pillow is inserted into the vagina prior to initiating CD and the balloon is filled with 180 mL of saline, causing the fetal head to be pushed to a higher station (FIGURE 4). Use of the Fetal Pillow may be indicated prior to CD in the following situations:
- second stage labor with a deeply impacted head
- second stage labor and failed operative delivery
- occiput posterior position or deep transverse arrest
- absent progress in the first stage between 8 cm and 10 cm with a deeply impacted fetal head or excessive caput of the fetal head.


The Fetal Pillow is inserted after completing vaginal preparation for CD and before initiating skin preparation and abdominal draping. The steps for inserting the Fetal Pillow include:
- Use the 60 mL syringe to fully deflate the Fetal Pillow and leave the cock-stop open.
- Fold the Fetal Pillow by squeezing the firm plastic base, and with the patient’s legs in a frog-leg position, place the device in the vagina.
- Allow the firm plastic base to open to a flat position with the base against the posterior vaginal wall and the soft silicon balloon against the fetal head.
- Using pressure on the plastic base, gently push the Fetal Pillow posteriorly toward the sacrum of the mother.
- Use the 60 mL syringe to inflate the balloon with 180 mL of normal saline and close the valve.
- Straighten the patient’s legs and proceed with skin preparation and abdominal draping (FIGURE 4).
When the CD is completed, deflate the balloon by drawing out the saline with the 60 mL syringe and remove the device by hooking a finger around the firm plastic base. The Fetal Pillow is surprisingly easy to use.
Continue to: Effectiveness of the Fetal Pillow...
Effectiveness of the Fetal Pillow
In one randomized trial, 240 women undergoing CD were randomly allocated to a group in which the Fetal Pillow was placed in the vagina and inflated prior to the cesarean and a control group in which the Fetal Pillow was not used.
In another randomized trial, 60 nulliparous women undergoing CD in the second stage of labor had a Fetal Pillow inserted in the vagina and were randomly allocated to inflation of the pillow (Fetal Pillow group) or noninflation of the pillow (control group).5 In this study the mean length of the second stage was 4 hours. Compared with noninflation of the Fetal Pillow, use of the inflated Fetal Pillow was associated with a reduction in grade 3 extension of the uterine incision (extensions into the uterine artery, vagina, or bladder) (0% for inflation vs 13% for noninflation) and fewer difficult plus very difficult deliveries of the fetal head as reported by the surgeon (0% for inflation vs 37% for noninflation). There was no significant difference in blood loss between the two groups (800 mL vs 900 mL). These two randomized studies both reported that the use of the Fetal Pillow was associated with a reduction in grade 3 extensions of the uterine incision and a decrease in the difficulty of delivering the fetal head.
Consider trialing the Fetal Pillow
When a CD is performed after a prolonged second stage of labor, surgical complications are common, including extensions of the uterine incision and difficulty delivering the fetal head. When a grade 3 extension occurs—with tearing of a uterine artery, deep extension into the vagina, or damage to the bladder—the surgical repair can be extraordinarily challenging. Clinical trials report that both reverse breech extraction and the Fetal Pillow can facilitate CD in the setting of a prolonged second stage. For many obstetricians reverse breech extraction is a challenging obstetric maneuver. The insertion and inflation of a Fetal Pillow is a simple procedure. Obstetrician-gynecologists learn by doing. If you have never used the Fetal Pillow, I suggest you consider trialing it in your practice. ●
Obstetricians know that a cesarean delivery (CD) for a woman with a prolonged second stage and a fetal head deeply impacted in the pelvis is challenging. In this situation, extensions of the uterine incision commonly occur, resulting in prolonged operative time and increased blood loss. Even more harrowing is the inability to deliver the fetal head, necessitating emergency assistance from other clinicians. In this situation, interventions that may be helpful include:
- extend or T the uterine incision
- enlist the aid of a clinician to push up on the fetal head with a vaginal hand (FIGURE 1)
- reverse breech extraction (FIGURE 2), and
- vaginal insertion of a Fetal Pillow prior to starting the delivery.


Evidence from clinical trials indicates that reverse breech extraction or insertion of a Fetal Pillow result in the best clinical outcomes.
Reverse breech extraction vs the push technique
Although the data are limited, most studies report that compared with pushing up with a vaginal hand (as shown in Figure 1), the reverse breech extraction technique (as shown in Figure 2) is associated with a reduction in extensions of the uterine incision, reduced blood loss, and reduced operative time.1 In a randomized trial, 108 women with obstructed labor undergoing CD in the second stage were randomly assigned to reverse breech extraction or pushing up with a vaginal hand.2 Following the uterine incision, the reverse breech extraction technique is performed by immediately reaching into the upper uterus and grasping the lower portion of the fetal leg and applying gentle traction on the leg until the second leg appeared. The lower legs are then pulled out of the uterus. Standard breech delivery maneuvers are used to deliver the shoulders and head. In the trial, compared with the push technique, reverse breech extraction was associated with fewer extensions of the uterine incision (30% vs 11%; P<.05), less blood loss (899 mL vs 1,257 mL; P<.001), and shorter operative time (56 min vs 89 min, P<.001). Fetal injury was similar with the push and breech extraction techniques (6% and 7%).
In another randomized trial, 192 women undergoing CD for obstructed labor were randomly assigned to reverse breech extraction or pushing the head up with a hand in the vagina.3 Compared with the vaginal push technique, reverse breech extraction was associated with fewer extensions of the uterine incision (19% vs 48%; P = .003), fewer cases of wound infection (2% vs 13%; P = .007), and fewer blood transfusions (2 vs 11; P = .012).
Additional options and adjuvants for facilitating delivery of a fetal head deeply impacted in the pelvis include: using a Coyne spoon, using nitroglycerine or terbutaline to relax the myometrium, breaking the vaginal suction on the fetal head before attempting delivery, keeping the wrist of the delivering hand as straight as possible to reduce uterine incision extensions, and incising the ring (if a Bandl’s ring is detected).
Continue to: The Fetal Pillow...
The Fetal Pillow
The Fetal Pillow (Safe Obstetric Systems, New York, New York) is a single-use fetal cephalic elevation device for managing the deeply impacted fetal head (FIGURE 3). The Fetal Pillow has a firm plastic base upon which is attached a soft silicon balloon. The Fetal Pillow is inserted into the vagina prior to initiating CD and the balloon is filled with 180 mL of saline, causing the fetal head to be pushed to a higher station (FIGURE 4). Use of the Fetal Pillow may be indicated prior to CD in the following situations:
- second stage labor with a deeply impacted head
- second stage labor and failed operative delivery
- occiput posterior position or deep transverse arrest
- absent progress in the first stage between 8 cm and 10 cm with a deeply impacted fetal head or excessive caput of the fetal head.


The Fetal Pillow is inserted after completing vaginal preparation for CD and before initiating skin preparation and abdominal draping. The steps for inserting the Fetal Pillow include:
- Use the 60 mL syringe to fully deflate the Fetal Pillow and leave the cock-stop open.
- Fold the Fetal Pillow by squeezing the firm plastic base, and with the patient’s legs in a frog-leg position, place the device in the vagina.
- Allow the firm plastic base to open to a flat position with the base against the posterior vaginal wall and the soft silicon balloon against the fetal head.
- Using pressure on the plastic base, gently push the Fetal Pillow posteriorly toward the sacrum of the mother.
- Use the 60 mL syringe to inflate the balloon with 180 mL of normal saline and close the valve.
- Straighten the patient’s legs and proceed with skin preparation and abdominal draping (FIGURE 4).
When the CD is completed, deflate the balloon by drawing out the saline with the 60 mL syringe and remove the device by hooking a finger around the firm plastic base. The Fetal Pillow is surprisingly easy to use.
Continue to: Effectiveness of the Fetal Pillow...
Effectiveness of the Fetal Pillow
In one randomized trial, 240 women undergoing CD were randomly allocated to a group in which the Fetal Pillow was placed in the vagina and inflated prior to the cesarean and a control group in which the Fetal Pillow was not used.
In another randomized trial, 60 nulliparous women undergoing CD in the second stage of labor had a Fetal Pillow inserted in the vagina and were randomly allocated to inflation of the pillow (Fetal Pillow group) or noninflation of the pillow (control group).5 In this study the mean length of the second stage was 4 hours. Compared with noninflation of the Fetal Pillow, use of the inflated Fetal Pillow was associated with a reduction in grade 3 extension of the uterine incision (extensions into the uterine artery, vagina, or bladder) (0% for inflation vs 13% for noninflation) and fewer difficult plus very difficult deliveries of the fetal head as reported by the surgeon (0% for inflation vs 37% for noninflation). There was no significant difference in blood loss between the two groups (800 mL vs 900 mL). These two randomized studies both reported that the use of the Fetal Pillow was associated with a reduction in grade 3 extensions of the uterine incision and a decrease in the difficulty of delivering the fetal head.
Consider trialing the Fetal Pillow
When a CD is performed after a prolonged second stage of labor, surgical complications are common, including extensions of the uterine incision and difficulty delivering the fetal head. When a grade 3 extension occurs—with tearing of a uterine artery, deep extension into the vagina, or damage to the bladder—the surgical repair can be extraordinarily challenging. Clinical trials report that both reverse breech extraction and the Fetal Pillow can facilitate CD in the setting of a prolonged second stage. For many obstetricians reverse breech extraction is a challenging obstetric maneuver. The insertion and inflation of a Fetal Pillow is a simple procedure. Obstetrician-gynecologists learn by doing. If you have never used the Fetal Pillow, I suggest you consider trialing it in your practice. ●
- Jeve YB, Navti OB, Konje JC. Comparison of techniques used to deliver a deeply impacted fetal head at full dilation: a systematic review and meta-analysis. BJOG. 2016;123:337-345.
- Fasubaa OB, Ezechi OC, Orji EO, et al. Delivery of the impacted head of the fetus at cesarean section after prolonged obstructed labor: a randomised comparative study of two methods. J Obstet Gynaecol. 2002;22:375-378.
- Nooh AM, Abdeldayem HM, Ben-Affan O. Reverse breech extraction versus the standard approach of pushing the impacted fetal head up through the vagina in caesarean section for obstructed labour: a randomised controlled trial. J Obstet Gynaecol. 2017;37:459-463.
- Seal SL, Dey A, Barman SC, et al. Randomized controlled trial of elevation of the fetal head with a fetal pillow during cesarean delivery at full cervical dilatation. Int J Gynaecol Obstet. 2016;133:178-182.
- Lassey SC, Little SE, Saadeh M,et al. Cephalic elevation device for second-stage cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2020;135:879-884.
- Jeve YB, Navti OB, Konje JC. Comparison of techniques used to deliver a deeply impacted fetal head at full dilation: a systematic review and meta-analysis. BJOG. 2016;123:337-345.
- Fasubaa OB, Ezechi OC, Orji EO, et al. Delivery of the impacted head of the fetus at cesarean section after prolonged obstructed labor: a randomised comparative study of two methods. J Obstet Gynaecol. 2002;22:375-378.
- Nooh AM, Abdeldayem HM, Ben-Affan O. Reverse breech extraction versus the standard approach of pushing the impacted fetal head up through the vagina in caesarean section for obstructed labour: a randomised controlled trial. J Obstet Gynaecol. 2017;37:459-463.
- Seal SL, Dey A, Barman SC, et al. Randomized controlled trial of elevation of the fetal head with a fetal pillow during cesarean delivery at full cervical dilatation. Int J Gynaecol Obstet. 2016;133:178-182.
- Lassey SC, Little SE, Saadeh M,et al. Cephalic elevation device for second-stage cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2020;135:879-884.