User login
Second of 2 parts: The mysteries of psychiatry maintenance of certification, further unraveled
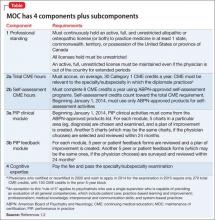
To recap what I discussed in Part 1 of this article (December 2014): As part of a trend across all medical specialty boards, the American Board of Psychiatry and Neurology (ABPN) instituted a recertification process for all new general psychiatry certifications, starting October 1, 1994.1 In 2000, the specialties that comprise the American Board of Medical Specialties (ABMS) agreed to develop a comprehensive maintenance of certification (MOC) process to demonstrate ongoing learning and competency beyond what can be captured by a recertification examination. All ABMS member boards now use a 4-part process for recertification.
A great deal of professional and personal importance has been attached to maintaining one’s general and subspecialty certifications. To that end, the 2 parts of this article highlight current ABPN MOC requirements and provide resources for understanding, tracking, and completing the self-assessment (SA) and performance-in-practice (PIP) components.
In this installment, I examine 3 components of MOC:
• continuing medical education (CME), including SA requirements
• improvement in medical practice (PIP)
• continuous maintenance of certification (C-MOC)
In addition to this review, all physicians who are subject to MOC should download and read the 20-page revised MOC Program booklet v. 2.1 (May 2014).2
Continuing medical education
The CME requirement is clear: All diplomate physicians must accrue, on average, 30 Category-1 CME credits a year; the CME must be relevant to the specialty or subspecialty in which the diplomate practices.3 For physicians who hold >1 ABPN certificates, the total CME requirement is the same; CME credits can be applied across each specialty and subspecialty.
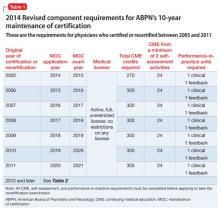
The May 2014 MOC revision states that, for physicians who certified or recertified between 2005 and 2011 and who applied for the 2015 examination in 2014, the required CME credit total is 270.2 For all subsequent years of certification or recertification, including 2012, diplomates are enrolled in C-MOC, which is described below.2
To even out the accrual of CME credits across the prior 10 years, ABPN mandates that, for diplomates who certified or recertified between 2005 and 2011, one hundred fifty of the CME credits be accrued in the 5 years before they apply for the examination. Diplomates in C-MOC should accrue, on average, 30 CME credits a year in each of the 3-year blocks (ie, 90 units in each block).2
Self-assessment
SA is a specific form of CME that is designed to provide comprehensive test-based feedback on knowledge acquired, to enhance the learning process.4 SA CME feedback must include:
• the correct answer to each test question
• recommended literature resources for each question
• performance compared to peers on each question.
Given the structured nature of SA activities, beginning January 1, 2014, one must use only ABPN-approved SA products (see Related Resources for a list of APBN-approved SA products).5
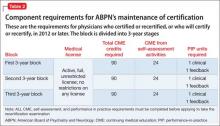
Table 1 and Table 2 outline SA requirements for, respectively, physicians who certified or recertified from 2005 through 2011, and those who certified or recertified in 2012 (and later). The SA requirement increases after 2011 to 24 credits in each 3-year block (8 credits a year, on average).2 Multiple SA activities can be used to fulfill the credit requirement of each 3-year block.
Note: Credits accrued by performing SA activities count toward the CME credit total.
Improvement in medical practice, or PIP
Physicians who are active clinically must complete PIP modules. Each module comprises peer or patient feedback plus a clinical aspect. The May 2014 MOC revision simplified the feedback process to mandate peer or patient feedback—but not both, as required previously.2 For the feedback PIP module, the physician selects 5 peers or patients to complete review forms, examines the results, and creates a plan of improvement. An exception to this “rule of 5” applies to diplomates who have a supervisor capable of evaluating all general competencies, defined below.
Related Resources provides a link to ABPN-created forms.
Within 24 months, but not sooner than 1 month, 5 peers or patients (or 1 applicable supervisor) are selected to complete review forms; changes in practice are noted. The same peers or patients might be selected for a second review. As noted in Table 1 and Table 2, the number of PIP modules is fewer for physicians who certified or recertified between 2005 and 2011; from 2012 onward, 1 PIP clinical module is required in each 3-year block.2
There are 6 ABPN-approved feedback module options, of which the diplomate must choose 1 in any given block2:
• 5 patient surveys
• 5 peer evaluations of general competenciesa
• 5 resident evaluations of general competenciesa
• 360° evaluation of general competencies,a with 5 respondents
• institutional peer review of general competencies,a with 5 respondents
• 1 supervisor evaluation of general competencies.a
aGeneral competencies include patient care; practice-based learning and improvement; professionalism; medical knowledge; interpersonal and communication skills; and system-based practices.
Although many institutions have a quality improvement (QI) program, that program must be approved by the Multi-Specialty MOC Portfolio Approval Program sponsored by ABMS for a clinician to receive credit for 1 PIP clinical module. If the approved QI program includes patient or peer feedback (eg, a survey), the diplo mate can receive credit for 1 PIP feedback module.2
For the clinical PIP module, the physician selects 5 charts for review and examines them based on criteria found in an ABPN-approved (starting in 2014) PIP product. (Related Resources provides a link to this list.) After reviewing the initial 5 charts, a plan for improvement is created. Within 24 months, but no sooner than 1 month, 5 charts are again selected and reviewed, and changes in practice are noted. The same charts can be selected for the second review.
As noted in Table 1 and Table 2, the number of PIP modules is fewer for those who initially certified or recertified between 2005 and 2011; from 2012 onward, 1 PIP clinical module is required in each 3-year block.2
The C-MOC process
Physicians who certified or recertified in 2012, or who will certify or recertify after that year, are enrolled automatically in C-MOC.6,7 The purpose of C-MOC is to keep diplomates on track to fulfill the higher level of SA requirements that began with this group; this is done by mandating use of the ABPN Physician Folios system. As shown in Table 2, there is no longer a 10-year cycle; instead, there are continuous 3-year stages, within which each diplomate must accrue 90 CME credits (on average, 30 credits a year), 24 SA credits (on average, 8 a year), 1 PIP clinical module, and 1 PIP feedback module.6,7
The first 3-year block of C-MOC requirements will be waived for physicians who complete Accreditation Council on Graduate Medical Education–accredited or ABPN-approved subspecialty training in 2012 or later—if they pass the corresponding ABPN subspecialty examination during the first 3-year block of enrollment in C-MOC.2 For diplomates enrolled in C-MOC, failure to track progress of each 3-year block, via the ABPN Physician Folios system, has significant consequences: Those who do not complete the first stage of the program by the end of 3 years will be listed on the ABPN Web site as “certified— not meeting MOC requirements.” Those who do not complete 2 stages by the end of 6 years will be listed as “not certified.”2
Cognitive exam still in place. The only remnant of the old 10-year cycle is the requirement to pass the cognitive examination every 10 years, although the exam can be taken earlier if the diplomate wishes. If all requirements are met and one does not sit for, or fails, the exam, the ABPN Web site will report the diplomate as “not meeting MOC requirements.” One can retake the exam within 1 year of the failed or missed exam, but a subsequent failure or missed exam will result in being listed as “not certified.”2
Fee structure. Instead of a single fee paid at the time of the exam(s), physicians in the C-MOC program pay an annual fee that covers participation in ABPN Physician Folios and 1 exam in a 10-year period. Fewer than 10 years of participation, or applying for a combined examination (for diplomates who hold multiple certifications), requires an additional fee.7
Bottom Line
Maintenance of certification (MOC) is manageable, although it requires you to be familiar with its various elements. Those elements include continuing medical education (CME requirements); the additional self-assessment component of CME; performance-in-practice modules; and continuous maintenance of certification. The MOC program booklet of the American Board of Psychiatry and Neurology provides all necessary details.
Disclosure
Dr. Meyer reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Faulkner LR, Tivnan PW, Winstead DK, et al. The ABPN Maintenance of Certification Program for psychiatrists: past history, current status, and future directions. Acad Psychiatry. 2008;32(3):241-248.
2. Maintenance of Certification Program. American Board of Psychiatry and Neurology Inc. http://www.abpn.com/ downloads/moc/moc_web_doc.pdf. Published May 2014. Accessed August 25, 2014.
3. Faulkner LR, Vondrak PA. Frequently asked questions about maintenance of certification (MOC). J Clin Psychiatry. 2010;71(5):632-633.
4. Ebert MH, Faulkner L, Stubbe DE, et al. Maintenance of certification in psychiatry. J Clin Psychiatry. 2009;70(10):e39.
5. Approved MOC Products. American Board of Psychiatry and Neurology Inc. http://www.abpn.com/moc_products. asp. Accessed August 25, 2014.
6. Continuous MOC (C-MOC). American Board of Psychiatry and Neurology Inc. http://www.abpn.com/downloads/ moc/ContinuousCertificationApproach_0311.pdf. Accessed August 25, 2014.
7. C-MOC Program Overview. American Board of Psychiatry and Neurology Inc. http://www.abpn.com/downloads/ moc/moc-handouts-CMOC-051314.pdf. Published May 13, 2014. Accessed August 25, 2014.
To recap what I discussed in Part 1 of this article (December 2014): As part of a trend across all medical specialty boards, the American Board of Psychiatry and Neurology (ABPN) instituted a recertification process for all new general psychiatry certifications, starting October 1, 1994.1 In 2000, the specialties that comprise the American Board of Medical Specialties (ABMS) agreed to develop a comprehensive maintenance of certification (MOC) process to demonstrate ongoing learning and competency beyond what can be captured by a recertification examination. All ABMS member boards now use a 4-part process for recertification.
A great deal of professional and personal importance has been attached to maintaining one’s general and subspecialty certifications. To that end, the 2 parts of this article highlight current ABPN MOC requirements and provide resources for understanding, tracking, and completing the self-assessment (SA) and performance-in-practice (PIP) components.
In this installment, I examine 3 components of MOC:
• continuing medical education (CME), including SA requirements
• improvement in medical practice (PIP)
• continuous maintenance of certification (C-MOC)
In addition to this review, all physicians who are subject to MOC should download and read the 20-page revised MOC Program booklet v. 2.1 (May 2014).2
Continuing medical education
The CME requirement is clear: All diplomate physicians must accrue, on average, 30 Category-1 CME credits a year; the CME must be relevant to the specialty or subspecialty in which the diplomate practices.3 For physicians who hold >1 ABPN certificates, the total CME requirement is the same; CME credits can be applied across each specialty and subspecialty.
The May 2014 MOC revision states that, for physicians who certified or recertified between 2005 and 2011 and who applied for the 2015 examination in 2014, the required CME credit total is 270.2 For all subsequent years of certification or recertification, including 2012, diplomates are enrolled in C-MOC, which is described below.2
To even out the accrual of CME credits across the prior 10 years, ABPN mandates that, for diplomates who certified or recertified between 2005 and 2011, one hundred fifty of the CME credits be accrued in the 5 years before they apply for the examination. Diplomates in C-MOC should accrue, on average, 30 CME credits a year in each of the 3-year blocks (ie, 90 units in each block).2
Self-assessment
SA is a specific form of CME that is designed to provide comprehensive test-based feedback on knowledge acquired, to enhance the learning process.4 SA CME feedback must include:
• the correct answer to each test question
• recommended literature resources for each question
• performance compared to peers on each question.
Given the structured nature of SA activities, beginning January 1, 2014, one must use only ABPN-approved SA products (see Related Resources for a list of APBN-approved SA products).5
Table 1 and Table 2 outline SA requirements for, respectively, physicians who certified or recertified from 2005 through 2011, and those who certified or recertified in 2012 (and later). The SA requirement increases after 2011 to 24 credits in each 3-year block (8 credits a year, on average).2 Multiple SA activities can be used to fulfill the credit requirement of each 3-year block.
Note: Credits accrued by performing SA activities count toward the CME credit total.
Improvement in medical practice, or PIP
Physicians who are active clinically must complete PIP modules. Each module comprises peer or patient feedback plus a clinical aspect. The May 2014 MOC revision simplified the feedback process to mandate peer or patient feedback—but not both, as required previously.2 For the feedback PIP module, the physician selects 5 peers or patients to complete review forms, examines the results, and creates a plan of improvement. An exception to this “rule of 5” applies to diplomates who have a supervisor capable of evaluating all general competencies, defined below.
Related Resources provides a link to ABPN-created forms.
Within 24 months, but not sooner than 1 month, 5 peers or patients (or 1 applicable supervisor) are selected to complete review forms; changes in practice are noted. The same peers or patients might be selected for a second review. As noted in Table 1 and Table 2, the number of PIP modules is fewer for physicians who certified or recertified between 2005 and 2011; from 2012 onward, 1 PIP clinical module is required in each 3-year block.2
There are 6 ABPN-approved feedback module options, of which the diplomate must choose 1 in any given block2:
• 5 patient surveys
• 5 peer evaluations of general competenciesa
• 5 resident evaluations of general competenciesa
• 360° evaluation of general competencies,a with 5 respondents
• institutional peer review of general competencies,a with 5 respondents
• 1 supervisor evaluation of general competencies.a
aGeneral competencies include patient care; practice-based learning and improvement; professionalism; medical knowledge; interpersonal and communication skills; and system-based practices.
Although many institutions have a quality improvement (QI) program, that program must be approved by the Multi-Specialty MOC Portfolio Approval Program sponsored by ABMS for a clinician to receive credit for 1 PIP clinical module. If the approved QI program includes patient or peer feedback (eg, a survey), the diplo mate can receive credit for 1 PIP feedback module.2
For the clinical PIP module, the physician selects 5 charts for review and examines them based on criteria found in an ABPN-approved (starting in 2014) PIP product. (Related Resources provides a link to this list.) After reviewing the initial 5 charts, a plan for improvement is created. Within 24 months, but no sooner than 1 month, 5 charts are again selected and reviewed, and changes in practice are noted. The same charts can be selected for the second review.
As noted in Table 1 and Table 2, the number of PIP modules is fewer for those who initially certified or recertified between 2005 and 2011; from 2012 onward, 1 PIP clinical module is required in each 3-year block.2
The C-MOC process
Physicians who certified or recertified in 2012, or who will certify or recertify after that year, are enrolled automatically in C-MOC.6,7 The purpose of C-MOC is to keep diplomates on track to fulfill the higher level of SA requirements that began with this group; this is done by mandating use of the ABPN Physician Folios system. As shown in Table 2, there is no longer a 10-year cycle; instead, there are continuous 3-year stages, within which each diplomate must accrue 90 CME credits (on average, 30 credits a year), 24 SA credits (on average, 8 a year), 1 PIP clinical module, and 1 PIP feedback module.6,7
The first 3-year block of C-MOC requirements will be waived for physicians who complete Accreditation Council on Graduate Medical Education–accredited or ABPN-approved subspecialty training in 2012 or later—if they pass the corresponding ABPN subspecialty examination during the first 3-year block of enrollment in C-MOC.2 For diplomates enrolled in C-MOC, failure to track progress of each 3-year block, via the ABPN Physician Folios system, has significant consequences: Those who do not complete the first stage of the program by the end of 3 years will be listed on the ABPN Web site as “certified— not meeting MOC requirements.” Those who do not complete 2 stages by the end of 6 years will be listed as “not certified.”2
Cognitive exam still in place. The only remnant of the old 10-year cycle is the requirement to pass the cognitive examination every 10 years, although the exam can be taken earlier if the diplomate wishes. If all requirements are met and one does not sit for, or fails, the exam, the ABPN Web site will report the diplomate as “not meeting MOC requirements.” One can retake the exam within 1 year of the failed or missed exam, but a subsequent failure or missed exam will result in being listed as “not certified.”2
Fee structure. Instead of a single fee paid at the time of the exam(s), physicians in the C-MOC program pay an annual fee that covers participation in ABPN Physician Folios and 1 exam in a 10-year period. Fewer than 10 years of participation, or applying for a combined examination (for diplomates who hold multiple certifications), requires an additional fee.7
Bottom Line
Maintenance of certification (MOC) is manageable, although it requires you to be familiar with its various elements. Those elements include continuing medical education (CME requirements); the additional self-assessment component of CME; performance-in-practice modules; and continuous maintenance of certification. The MOC program booklet of the American Board of Psychiatry and Neurology provides all necessary details.
Disclosure
Dr. Meyer reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
To recap what I discussed in Part 1 of this article (December 2014): As part of a trend across all medical specialty boards, the American Board of Psychiatry and Neurology (ABPN) instituted a recertification process for all new general psychiatry certifications, starting October 1, 1994.1 In 2000, the specialties that comprise the American Board of Medical Specialties (ABMS) agreed to develop a comprehensive maintenance of certification (MOC) process to demonstrate ongoing learning and competency beyond what can be captured by a recertification examination. All ABMS member boards now use a 4-part process for recertification.
A great deal of professional and personal importance has been attached to maintaining one’s general and subspecialty certifications. To that end, the 2 parts of this article highlight current ABPN MOC requirements and provide resources for understanding, tracking, and completing the self-assessment (SA) and performance-in-practice (PIP) components.
In this installment, I examine 3 components of MOC:
• continuing medical education (CME), including SA requirements
• improvement in medical practice (PIP)
• continuous maintenance of certification (C-MOC)
In addition to this review, all physicians who are subject to MOC should download and read the 20-page revised MOC Program booklet v. 2.1 (May 2014).2
Continuing medical education
The CME requirement is clear: All diplomate physicians must accrue, on average, 30 Category-1 CME credits a year; the CME must be relevant to the specialty or subspecialty in which the diplomate practices.3 For physicians who hold >1 ABPN certificates, the total CME requirement is the same; CME credits can be applied across each specialty and subspecialty.
The May 2014 MOC revision states that, for physicians who certified or recertified between 2005 and 2011 and who applied for the 2015 examination in 2014, the required CME credit total is 270.2 For all subsequent years of certification or recertification, including 2012, diplomates are enrolled in C-MOC, which is described below.2
To even out the accrual of CME credits across the prior 10 years, ABPN mandates that, for diplomates who certified or recertified between 2005 and 2011, one hundred fifty of the CME credits be accrued in the 5 years before they apply for the examination. Diplomates in C-MOC should accrue, on average, 30 CME credits a year in each of the 3-year blocks (ie, 90 units in each block).2
Self-assessment
SA is a specific form of CME that is designed to provide comprehensive test-based feedback on knowledge acquired, to enhance the learning process.4 SA CME feedback must include:
• the correct answer to each test question
• recommended literature resources for each question
• performance compared to peers on each question.
Given the structured nature of SA activities, beginning January 1, 2014, one must use only ABPN-approved SA products (see Related Resources for a list of APBN-approved SA products).5
Table 1 and Table 2 outline SA requirements for, respectively, physicians who certified or recertified from 2005 through 2011, and those who certified or recertified in 2012 (and later). The SA requirement increases after 2011 to 24 credits in each 3-year block (8 credits a year, on average).2 Multiple SA activities can be used to fulfill the credit requirement of each 3-year block.
Note: Credits accrued by performing SA activities count toward the CME credit total.
Improvement in medical practice, or PIP
Physicians who are active clinically must complete PIP modules. Each module comprises peer or patient feedback plus a clinical aspect. The May 2014 MOC revision simplified the feedback process to mandate peer or patient feedback—but not both, as required previously.2 For the feedback PIP module, the physician selects 5 peers or patients to complete review forms, examines the results, and creates a plan of improvement. An exception to this “rule of 5” applies to diplomates who have a supervisor capable of evaluating all general competencies, defined below.
Related Resources provides a link to ABPN-created forms.
Within 24 months, but not sooner than 1 month, 5 peers or patients (or 1 applicable supervisor) are selected to complete review forms; changes in practice are noted. The same peers or patients might be selected for a second review. As noted in Table 1 and Table 2, the number of PIP modules is fewer for physicians who certified or recertified between 2005 and 2011; from 2012 onward, 1 PIP clinical module is required in each 3-year block.2
There are 6 ABPN-approved feedback module options, of which the diplomate must choose 1 in any given block2:
• 5 patient surveys
• 5 peer evaluations of general competenciesa
• 5 resident evaluations of general competenciesa
• 360° evaluation of general competencies,a with 5 respondents
• institutional peer review of general competencies,a with 5 respondents
• 1 supervisor evaluation of general competencies.a
aGeneral competencies include patient care; practice-based learning and improvement; professionalism; medical knowledge; interpersonal and communication skills; and system-based practices.
Although many institutions have a quality improvement (QI) program, that program must be approved by the Multi-Specialty MOC Portfolio Approval Program sponsored by ABMS for a clinician to receive credit for 1 PIP clinical module. If the approved QI program includes patient or peer feedback (eg, a survey), the diplo mate can receive credit for 1 PIP feedback module.2
For the clinical PIP module, the physician selects 5 charts for review and examines them based on criteria found in an ABPN-approved (starting in 2014) PIP product. (Related Resources provides a link to this list.) After reviewing the initial 5 charts, a plan for improvement is created. Within 24 months, but no sooner than 1 month, 5 charts are again selected and reviewed, and changes in practice are noted. The same charts can be selected for the second review.
As noted in Table 1 and Table 2, the number of PIP modules is fewer for those who initially certified or recertified between 2005 and 2011; from 2012 onward, 1 PIP clinical module is required in each 3-year block.2
The C-MOC process
Physicians who certified or recertified in 2012, or who will certify or recertify after that year, are enrolled automatically in C-MOC.6,7 The purpose of C-MOC is to keep diplomates on track to fulfill the higher level of SA requirements that began with this group; this is done by mandating use of the ABPN Physician Folios system. As shown in Table 2, there is no longer a 10-year cycle; instead, there are continuous 3-year stages, within which each diplomate must accrue 90 CME credits (on average, 30 credits a year), 24 SA credits (on average, 8 a year), 1 PIP clinical module, and 1 PIP feedback module.6,7
The first 3-year block of C-MOC requirements will be waived for physicians who complete Accreditation Council on Graduate Medical Education–accredited or ABPN-approved subspecialty training in 2012 or later—if they pass the corresponding ABPN subspecialty examination during the first 3-year block of enrollment in C-MOC.2 For diplomates enrolled in C-MOC, failure to track progress of each 3-year block, via the ABPN Physician Folios system, has significant consequences: Those who do not complete the first stage of the program by the end of 3 years will be listed on the ABPN Web site as “certified— not meeting MOC requirements.” Those who do not complete 2 stages by the end of 6 years will be listed as “not certified.”2
Cognitive exam still in place. The only remnant of the old 10-year cycle is the requirement to pass the cognitive examination every 10 years, although the exam can be taken earlier if the diplomate wishes. If all requirements are met and one does not sit for, or fails, the exam, the ABPN Web site will report the diplomate as “not meeting MOC requirements.” One can retake the exam within 1 year of the failed or missed exam, but a subsequent failure or missed exam will result in being listed as “not certified.”2
Fee structure. Instead of a single fee paid at the time of the exam(s), physicians in the C-MOC program pay an annual fee that covers participation in ABPN Physician Folios and 1 exam in a 10-year period. Fewer than 10 years of participation, or applying for a combined examination (for diplomates who hold multiple certifications), requires an additional fee.7
Bottom Line
Maintenance of certification (MOC) is manageable, although it requires you to be familiar with its various elements. Those elements include continuing medical education (CME requirements); the additional self-assessment component of CME; performance-in-practice modules; and continuous maintenance of certification. The MOC program booklet of the American Board of Psychiatry and Neurology provides all necessary details.
Disclosure
Dr. Meyer reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Faulkner LR, Tivnan PW, Winstead DK, et al. The ABPN Maintenance of Certification Program for psychiatrists: past history, current status, and future directions. Acad Psychiatry. 2008;32(3):241-248.
2. Maintenance of Certification Program. American Board of Psychiatry and Neurology Inc. http://www.abpn.com/ downloads/moc/moc_web_doc.pdf. Published May 2014. Accessed August 25, 2014.
3. Faulkner LR, Vondrak PA. Frequently asked questions about maintenance of certification (MOC). J Clin Psychiatry. 2010;71(5):632-633.
4. Ebert MH, Faulkner L, Stubbe DE, et al. Maintenance of certification in psychiatry. J Clin Psychiatry. 2009;70(10):e39.
5. Approved MOC Products. American Board of Psychiatry and Neurology Inc. http://www.abpn.com/moc_products. asp. Accessed August 25, 2014.
6. Continuous MOC (C-MOC). American Board of Psychiatry and Neurology Inc. http://www.abpn.com/downloads/ moc/ContinuousCertificationApproach_0311.pdf. Accessed August 25, 2014.
7. C-MOC Program Overview. American Board of Psychiatry and Neurology Inc. http://www.abpn.com/downloads/ moc/moc-handouts-CMOC-051314.pdf. Published May 13, 2014. Accessed August 25, 2014.
1. Faulkner LR, Tivnan PW, Winstead DK, et al. The ABPN Maintenance of Certification Program for psychiatrists: past history, current status, and future directions. Acad Psychiatry. 2008;32(3):241-248.
2. Maintenance of Certification Program. American Board of Psychiatry and Neurology Inc. http://www.abpn.com/ downloads/moc/moc_web_doc.pdf. Published May 2014. Accessed August 25, 2014.
3. Faulkner LR, Vondrak PA. Frequently asked questions about maintenance of certification (MOC). J Clin Psychiatry. 2010;71(5):632-633.
4. Ebert MH, Faulkner L, Stubbe DE, et al. Maintenance of certification in psychiatry. J Clin Psychiatry. 2009;70(10):e39.
5. Approved MOC Products. American Board of Psychiatry and Neurology Inc. http://www.abpn.com/moc_products. asp. Accessed August 25, 2014.
6. Continuous MOC (C-MOC). American Board of Psychiatry and Neurology Inc. http://www.abpn.com/downloads/ moc/ContinuousCertificationApproach_0311.pdf. Accessed August 25, 2014.
7. C-MOC Program Overview. American Board of Psychiatry and Neurology Inc. http://www.abpn.com/downloads/ moc/moc-handouts-CMOC-051314.pdf. Published May 13, 2014. Accessed August 25, 2014.
Choosing a treatment for disruptive, impulse-control, and conduct disorders
Chronic disruptive and impulsive behaviors are significant concerns for psychiatric clinicians because of their persistence and potential legal ramifications. To date, few studies have assessed treatment options for pyromania, oppositional defiant disorder (ODD), intermittent explosive disorder (IED), kleptomania, and conduct disorder (CD).
This article reviews the literature on the treatment of these disorders, focusing primarily on randomized, controlled studies. Because of the lack of clinical studies for these disorders, however, case studies and open trials are mentioned for reference. Summaries of supported medication and psychological interventions are provided for each disorder.
Categorizing impulse-control disorders
The DSM-5 created a new chapter on disruptive, impulse control, and conduct disorders that brought together disorders previously classified as disorders usually first diagnosed in infancy, childhood, or adolescence (ODD, CD) and impulse-control disorders not elsewhere classified. These disorders are unified by the presence of difficult, disruptive, aggressive, or antisocial behavior. Disruptive, aggressive, or antisocial behavior usually is a multifaceted behavior, often associated with physical or verbal injury to self, others, or objects or with violating the rights of others. These behaviors can appear in several forms and can be defensive, premeditated, or impulsive.
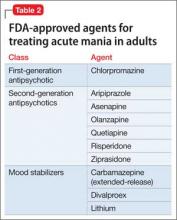
Despite a high prevalence in the general population1 and in psychiatric cohorts,2 disruptive and impulse-control disorders have been relatively understudied. Controlled trials of treatments do not exist for many impulse-control disorders, and there are no FDA-approved medications for any of these disorders.
Oppositional defiant disorder
Irritability, anger, defiance, and temper are specific descriptors of ODD. ODD seems to be a developmental antecedent for some youth with CD, suggesting that these disorders could reflect different stages of a spectrum of disruptive behavior. Transient oppositional behavior is common among children and adolescents, but ODD occurs in 1% to 11% of youth.3 The disorder is more prevalent among boys before puberty and has an equal sex prevalence in young people after puberty.
Regrettably, most ODD research has included patients with comorbidities, most commonly attention-deficit/hyperactivity disorder (ADHD). Because of this limitation, the drugs and programs discussed below are drawn from meta-analyses and review articles.
Pharmacotherapy. No medications have been FDA-approved for ODD. Studies assessing ODD have employed a variety of methodologies, not all of which are double-blind. The meta-analyses and reviews cited in this section include both randomized and open trials, and should be interpreted as such.
Stimulants are commonly used to treat ODD because of a high comorbidity rate with ADHD, and these drugs have improved ODD symptoms in randomized trials.4 Methylphenidate and d-amphetamine have shown some efficacy in trials of ODD and CD.5-7 These medications are most commonly used when ODD is complicated by ADHD symptoms.
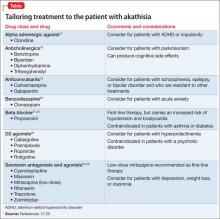
Antipsychotics also have been used to treat ODD, with the largest body of research suggesting that risperidone has some efficacy. Risperidone usually is considered a second- or third-line option because it has been associated with adverse effects in children and adolescents and requires caution in younger populations, despite its potential efficacy.4,8-10
Alpha-2 agonists—clonidine and guanfacine—have shown some efficacy in treating ODD but have not been studied extensively. Studies of clonidine, however, often have grouped ODD, CD, and ADHD, which limits our understanding of this medication for ODD alone.4,5,11
Atomoxetine has been studied for ODD, but its efficacy is limited, with different meta-analyses finding distinct results regarding efficacy. One explanation for these disparate findings is that improvements in oppositional symptoms may be secondary to improvement in ADHD symptoms.7,12-14
Psychological treatments. As noted for pharmacotherapy, this section provides general information on empirically studied therapies. A series of meta-analyses have been included for further review, but are not isolated to randomized, controlled studies.
Individual therapy has shown consistent improvements in ODD. Examples include behavior modification therapy and parent-child interaction therapy. These sessions emphasize skills to manage outbursts and erratic emotionality. Emotion regulation and behavior and social skills training have shown significant reductions in target measures. Some of these programs incorporate both patient and parent components.15-17
Family/teacher training programs such as “Helping the Noncompliant Child” and the “Triple P” have yielded significant improvements. These programs focus on ways to manage the child’s oppositional behavior at home and in the classroom, as well as strategies to limit positive reinforcement for problem behaviors.17-20
Group programs have shown some efficacy with ODD. These programs cover a wide number of needs and intents. Examples include the “Incredible Years” program and the Community Parent Education Program. Research has found that these programs show some efficacy as preemptive measures to reduce the rate of ODD among adolescents.
Conclusions. A number of treatment options for ODD have shown some efficacy. However, many of these options have only been studied in patients with comorbid ADHD, which limits current knowledge about ODD as a distinct disorder.
Intermittent explosive disorder
IED is defined by recurrent, significant outbursts of aggression, often leading to assaultive acts against people or property, which are disproportionate to outside stressors and are not better explained by another psychiatric diagnosis. Research suggests IED is common, with 6.3% of a community sample meeting criteria for lifetime IED.21
IED symptoms tend to start in adolescence and appear to be chronic.21,22 People with IED regard their behavior as distressing and problematic.22 Outbursts generally are short-lived (usually <30 minutes) and frequent (multiple times a month22). Legal and occupational difficulties are common.22
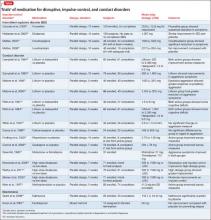
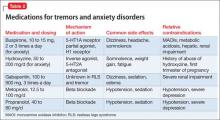
Pharmacotherapy. Data on drug treatment for IED comes for a small set of double-blind studies (Table). Although pharmacotherapies have been studied for treating aggression, impulsivity, and violent behavior, only 5 controlled studies are specific to IED.
A double-blind, randomized, placebo-controlled trial of fluoxetine in 100 participants with IED found that fluoxetine produced a sustained reduction in aggression and irritability as early as the second week of treatment. Full or partial remission of impulsive aggressive behaviors occurred in 46% of fluoxetine-treated subjects. These findings have been supported by studies assessing other samples of aggressive patients, but not specifically IED.23,24 Another treatment study found that oxcarbazepine produced significant improvements in IED symptom severity, specifically on impulsive aggression.25
In a randomized, double-blind, placebo-controlled study, 96 participants with Cluster B personality disorders, 116 with IED, and 34 with posttraumatic stress disorder were assigned to divalproex sodium or placebo for 12 weeks. Using an intent-to-treat analysis, divalproex had no significant influence on aggression in patients with IED.26 Similarly, a study assessing levetiracetam for IED did not show any improvements to measures of impulsive aggression.27
Psychological treatments. The only available study on psychological treatments for IED found that patients receiving active cognitive-behavioral therapy (CBT) or group therapy showed significant improvements compared with waitlist controls. These improvements spanned several target symptoms of IED.28
Conclusions. Although there is a paucity of treatment studies for IED, fluoxetine may be an effective treatment based on available studies, and oxcarbazepine has shown some preliminary efficacy. CBT also has shown some initial efficacy in reducing symptom severity in IED.
Conduct disorder
The essential feature of CD is a repetitive and persistent pattern of behavior in which the basic rights of others or social norms are violated.3 These behaviors can entail:
• aggressive conduct that causes or threatens harm to others or to animals
• nonaggressive behavior resulting in property damage
• deceitfulness or theft
• serious violation of rules.
Prevalence among the general population is 2% to 10%. The disorder is more common among boys than girls.3
Pharmacotherapy. No medication is FDA-approved to treat CD. Fifteen controlled studies have examined medications in patients with CD (Table), although a number of these included a high rate of comorbid ADHD.
To date, 7 studies have shown efficacy with lithium for patients with CD.29-35 A number of trials assessing lithium also included a treatment condition with haloperidol, which showed significant improvement.29,30,33,34 Both lithium and haloperidol were associated with select deficits on cognitive tests, suggesting that there may be risks associated with these medications.
Preliminary double-blind results have indicated that methylphenidate, risperidone, quetiapine, molindone, thioridazine, and carbamazepine might be effective options for treating CD.36-43 The evidence for these medications is limited and additional studies are needed to replicate initial findings.
Three studies of divalproex sodium have shown some efficacy in randomized studies comparing high and low dosages of the drug.40-42 Because these studies did not include a placebo, additional studies are necessary to corroborate these findings.
Psychological treatments. Several forms of behavioral, family-based, and school-based therapies have been found effective in randomized trials. Specifically, behavioral therapy and parental skills training have shown consistent benefits for patients and their families. As with ODD, parental training programs for CD focus on parents’ skill acquisition to help manage outbursts and aggressive behavior. These treatments often follow a similar course to those used for other externalizing and disruptive disorders.44-46
Conclusions. Based on evidence, psychotherapy and some pharmacotherapies (eg, lithium) could be considered first-line treatment options for CD. Psychotherapy programs have shown efficacy in reducing aggression in high-risk groups.44 Lithium or antipsychotics could be useful for patients who do not respond sufficiently to psychotherapy. The risk of cognitive deficits with lithium and antipsychotics should be weighed against potential benefits of these medications.33,34
Kleptomania
Kleptomania is characterized by repetitive, poorly controlled stealing of items that are not needed for personal use. Kleptomania often begins in late adolescence or early adulthood.47 The course of the illness generally is chronic, with waxing and waning symptoms. Women are twice as likely as men to suffer from kleptomania.48 People with kleptomania frequently hoard, discard, or return stolen items.47
Most people with kleptomania try unsuccessfully to stop stealing, which often leads to feelings of shame and guilt.48 Many (64% to 87%) have been arrested because of their stealing behavior47; a smaller percentage (15% to 23%) have been incarcerated.48 Suicide attempts are common among these patients.49
Pharmacotherapy. There has been only 1 randomized, placebo-controlled study of pharmacotherapy for kleptomania (Table). An 8-week, double-blind, placebo-controlled trial was conducted to evaluate the safety and efficacy of oral naltrexone, 50 to 150 mg/d, in 25 patients with kleptomania. Those taking naltrexone had a significantly greater reduction in total score than those taking placebo on the Yale-Brown Obsessive Compulsive Scale Modified for Kleptomania; in stealing urges; and in stealing behavior. The mean effective dosage of naltrexone was 116.7 (± 44.4) mg/d.50
Naltrexone was well tolerated, with minimal nausea, and did not cause elevation of liver enzymes.
There is one available open-label study with a double-blind discontinuation phase assessing the efficacy of escitalopram for kleptomania. Continuation of escitalopram during the blinded discontinuation phase did produce lower relapse rates.51
Psychological treatments. There are no controlled studies of psychological treatments for kleptomania. Case reports suggest that cognitive and behavioral therapies might be effective:
• A young man who underwent 7 sessions of covert sensitization, combined with exposure and response prevention, over a 4-month period was able to reduce his stealing frequency.52
• In another case, a young woman underwent 5 weekly sessions when she was instructed to practice covert sensitization whenever she had an urge to steal. She remained in remission for 14 months with only a single lapse in behavior and with no reported urges to steal.53
• In 2 patients, imaginal desensitization in fourteen 15-minutes sessions over 5 days resulted in complete remission of symptoms for a 2-year period.54
Conclusions. The single controlled study of naltrexone for kleptomania suggests that naltrexone might be a beneficial treatment for this disorder. No controlled trials of psychosocial interventions have been reported. The current psychological research is based primarily on case reports.
This state of affairs likely is because of (1) the low prevalence of kleptomania and (2) clinical difficulties in treating patients involved in illegal activities. Nevertheless, there is a need for systematic studies of treating this disorder; such studies could involve collaboration across multiple treatment centers because of the disorder’s low prevalence.
Pyromania
Pyromania is characterized by (1) deliberate and purposeful fire setting on >1 occasion; (2) tension or affective arousal before the act; (3) fascination with, interest in, curiosity about, or attraction to fire and its situational contexts; and (4) pleasure, gratification, or relief when setting fires or when witnessing or participating in their aftermath.3
Although pyromania is thought to be a disorder primarily affecting men, recent research suggests that the sex ratio is equal among adults and may be slightly higher among adolescent females. Mean age of onset usually is late adolescence. Pyromania appears to be chronic if untreated.55
Urges to set fires are common and the fire setting is almost always pleasurable. Severe distress follows the fire setting, and persons with pyromania report significant functional impairment. High rates of co-occurring psychiatric disorders (depression, substance use disorders, other impulse-control disorders) are common among persons with pyromania.55
Pharmacotherapy. There are no randomized, controlled clinical trials examining pharmacotherapy for treating pyromania. There are no FDA-approved medications for pyromania.
In case reports, medications that have shown benefit in treating pyromania include topiramate, escitalopram, sertraline, fluoxetine, lithium, and a combination of olanzapine and sodium valproate. An equal number of medications have shown no benefit: fluoxetine, valproic acid, lithium, sertraline, olanzapine, escitalopram, citalopram, and clonazepam. A case report of an 18-year-old man with pyromania described successfully using a combination of topiramate with 3 weeks of daily CBT to achieve significant symptom improvement.56,57
Pyromania is a largely unrecognized disorder that causes significant psychological, social, and legal repercussions. Because few persons with pyromania volunteer information regarding fire-setting, it is important that clinicians recognize the disorder and screen patients appropriately. Various treatments have been helpful in case studies, but more research on the etiology and treatment of the disorder is needed.56,57
Conclusions based on the literature
In disruptive, impulse-control, and conduct disorders, the systematic study of treatment efficacy and tolerability is in its infancy. With few controlled studies published, it is not possible to make treatment recommendations with confidence. There are no FDA-approved drugs for treating any of these disorders.
Nonetheless, specific psychotherapies and drug therapies offer promising options, but often are based on small studies, often in patient populations with prominent comorbidities, and have not been replicated by independent investigators. For all of these disorders, issues such as which psychotherapy or medication to use and the ideal duration of treatment cannot be sufficiently addressed with the available data.
In conjunction with emerging epidemiological data supporting a relatively high prevalence of disruptive, impulse-control, and conduct disorders, the small amount of data regarding effective treatments highlights the clinical need for additional research.
Bottom Line
Empirically supported treatment options for impulse-control disorders currently are limited, because only select disorders have been studied across multiple trials. New research is needed to confirm possible treatment options and identify effective psychotherapeutic and pharmacological treatment alternatives.
Related Resources
• Grant JE. Impulse control disorders: a clinician’s guide to understanding and treating behavioral addictions. New York, NY: W. W. Norton & Company; 2008.
• Grant JE, Kim SW. Stop me because I can’t stop myself: taking control of impulsive behavior. New York, NY: McGraw- Hill; 2003.
• American Academy of Child and Adolescent Psychiatry. Conduct disorder resource center. http://www.aacap.org/AACAP/FamiliesandYouth/ResourceCenters/ConductDisorderResourceCenter/Home.aspx.
Drug Brand Names
Atomoxetine • Strattera Methylphenidate • Ritalin
Carbamazepine • Tegretol Molindone • Moban
Citalopram • Celexa Naltrexone • ReVia
Clonazepam • Klonopin Olanzapine • Zyprexa
Clonidine • Catapres Oxcarbazepine • Trileptal
D-amphetamine • Dexedrine Quetiapine • Seroquel
Divalproex sodium • Depakote Risperidone • Risperdal
Escitalopram • Lexapro Sertraline • Zoloft
Fluoxetine • Prozac Sodium valproate • Depacon
Guanfacine • Intuniv Thioridazine • Mellaril
Haloperidol • Haldol Topiramate • Topamax
Levetiracetam • Keppra Valproic acid • Depakote
Lithium • Eskalith, Lithobid
Disclosures
Dr. Grant receives grant or research support from Brainsway, Forest Pharmaceuticals, and Roche Pharmaceuticals. Mr. Leppink reports no financial relationship with any company whose products are mentioned in this article or with competing products.
1. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Grant JE, Levine L, Kim D, et al. Impulse control disorders in adult psychiatric inpatients. Am J Psychiatry. 2005;162(11):2184-2188.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Turgay A. Psychopharmacological treatment of oppositional defiant disorder. CNS Drugs. 2009;23(1):1-17.
5. Hazell P. Review of attention-deficit/hyperactivity disorder comorbid with oppositional defiant disorder. Australas Psychiatry. 2010;18(6):556-559.
6. Burke JD, Loeber R, Birmaher B. Oppositional defiant disorder and conduct disorder: a review of the past 10 years, part II. J Am Acad Child Adolesc Psychiatry. 2002; 41(11):1275-1293.
7. Connor DF, Steeber J, McBurnett K. A review of attention-deficit/hyperactivity disorder complicated by symptoms of oppositional defiant disorder or conduct disorder. J Dev Behav Pediatr. 2010;31(5):427-440.
8. Aman MG, Bukstein OG, Gadow KD, et al. What does risperidone add to parent training and stimulant for severe aggression in child attention-deficit/hyperactivity disorder? J Am Acad Child Adolesc Psychiatry. 2014;53(1):47-60.e1.
9. Loy JH, Merry SN, Hetrick SE, et al. Atypical antipsychotics for disruptive behavior disorders in children and youths. Cochrane Database Syst Rev. 2012;9:CD008559.
10. Gadow KD, Arnold LE, Molina BS, et al. Risperidone added to parent training and stimulant medication: effects on attention-deficit/hyperactivity disorder, oppositional defiant disorder, conduct disorder, and peer aggression. J Am Acad Child Adolesc Psychiatry. 2014;53(9):948-959.e1.
12. Signorovitch J, Erder MH, Xie J, et al. Comparative effectiveness research using matching-adjusted indirect comparison: an application to treatment with guanfacine extended release or atomoxetine in children with attention-deficit/hyperactivity disorder and comorbid oppositional defiant disorder. Pharmacoepidemiol Drug Saf. 2012;21(suppl 2):130-137.
13. Bangs ME, Hazell P, Danckaerts M, et al; Atomoxetine ADHD/ODD Study Group. Atomoxetine for the treatment of attention-deficit/hyperactivity disorder and oppositional defiant disorder. Pediatrics. 2008;121(2):e314-e320.
14. Biederman J, Spencer TJ, Newcorn JH, et al. Effect of comorbid symptoms of oppositional defiant disorder on responses to atomoxetine in children with ADHD: a meta-analysis of controlled clinical trial data. Psychopharmacology (Berl). 2007;190(1):31-41.
15. Miller NV, Haas SM, Waschbusch DA, et al. Behavior therapy and callous-unemotional traits: effects of a pilot study examining modified behavioral contingencies on child behavior. Behav Ther. 2014;45(5):606-618.
16. Hamilton SS, Armando J. Oppositional defiant disorder. Am Fam Physician. 2008;78(7):861-866.
17. Steiner H, Remsing L; Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with oppositional defiant disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(1):126-141.
18. Winther J, Carlsson A, Vance A. A pilot study of a school-based prevention and early intervention program to reduce oppositional defiant disorder/conduct disorder. Early Interv Psychiatry. 2014;8(2):181-189.
19. Plueck J, Eichelberger I, Hautmann C, et al. Effectiveness of a teacher-based indicated prevention program for preschool children with externalizing problem behavior [published online April 22, 2014]. Prev Sci. doi: 10.1007/s11121-014- 0487-x.
20. Dretzke J, Frew E, Davenport C, et al. The effectiveness and cost-effectiveness of parent training/education programmes for the treatment of conduct disorder, including oppositional defiant disorder, in children. Health Tech Assess. 2005;9(50):iii, ix-x, 1-233.
21. Coccaro EF, Schmidt CA, Samuels JF, et al. Lifetime and 1-month prevalence rates of intermittent explosive disorder in a community sample. J Clin Psychiatry. 2004;65(6):820-824.
22. McElroy SL, Soutullo CA, Beckman DA, et al. DSM-IV intermittent explosive disorder: a report of 27 cases. J Clin Psychiatry. 1998;59(4):203-210; quiz 211.
23. Coccaro EF, Lee RJ, Kavoussi RJ. A double-blind, randomized, placebo-controlled trial of fluoxetine in patients with intermittent explosive disorder. J Clin Psychiatry. 2009;70(5):653-662.
24. Coccaro EF. Intermittent explosive disorder as a disorder of impulsive aggression for DSM-5. Am J Psychiatry. 2012;169(6):577-588.
25. Mattes JA. Oxcarbazepine in patients with impulsive aggression: a double-blind, placebo-controlled trial. J Clin Psychopharmacol. 2005;25(6):575-579.
26. Hollander E, Tracy KA, Swann AC, et al. Divalproex in the treatment of impulsive aggression: efficacy in cluster B personality disorders. Neuropsychopharmacology. 2003;28(6):1186-1197.
27. Mattes JA. Levetiracetam in patients with impulsive aggression: a double-blind, placebo-controlled trial. J Clin Psychiatry. 2008;69(2):310-315.
28. McCloskey MS, Noblett KL, Deffenbacher JL, et al. Cognitive-behavioral therapy for intermittent explosive disorder: a pilot randomized clinical trial. J Consult Clin Psychol. 2008;76(5):876-886.
29. Campbell M, Small AM, Green WH, et al. Behavioral efficacy of haloperidol and lithium carbonate. A comparison in hospitalized aggressive children with conduct disorder. Arch Gen Psychiatry. 1984;41(7):650-656.
30. Campbell M, Adams PB, Small AM, et al. Lithium in hospitalized aggressive children with conduct disorder: a double-blind and placebo-controlled study. J Am Acad Child Adolesc Psychiatry. 1995;34(4):445-453.
31. Malone RP, Simpson GM. Psychopharmacology: use of placebos in clinical trials involving children and adolescents. Psychiatr Serv. 1998;49(11):1413-1414, 1417.
32. Malone RP, Delaney MA, Luebbert JF, et al. A double-blind placebo-controlled study of lithium in hospitalized aggressive children and adolescents with conduct disorder. Arch Gen Psychiatry. 2000;57(7):649-654.
33. Platt JE, Campbell M, Green WH, et al. Effects of lithium carbonate and haloperidol on cognition in aggressive hospitalized school-age children. J Clin Psychopharmacol. 1981;1(1):8-13.
34. Platt JE, Campbell M, Green WH, et al. Cognitive effects of lithium carbonate and haloperidol in treatment-resistant aggressive children. Arch Gen Psychiatry. 1984;41(7):657-662.
35. Rifkin A, Karajgi B, Dicker R, et al. Lithium treatment of conduct disorders in adolescents. Am J Psychiatry. 1997;154(4):554-555.
36. Cueva JE, Overall JE, Small AM, et al. Carbamazepine in aggressive children with conduct disorder: a double-blind and placebo-controlled study. J Am Acad Child Adolesc Psychiatry. 1996;35(4):480-490.
37. Findling RL, McNamara NK, Branicky LA, et al. A double-blind pilot study of risperidone in the treatment of conduct disorder. J Am Acad Child Adolesc Psychiatry. 2000;39(4):509-516.
38. Connor DF, McLaughlin TJ, Jeffers-Terry M. Randomized controlled pilot study of quetiapine in the treatment of adolescent conduct disorder. J Child Adolesc Psychopharmacol. 2008;18(2):140-156.
39. Greenhill LL, Solomon M, Pleak R, et al. Molindone hydrochloride treatment of hospitalized children with conduct disorder. J Clin Psychiatry. 1985;46(8 pt 2):20-25.
40. Khanzode LA, Saxena K, Kraemer H, et al. Efficacy profiles of psychopharmacology: divalproex sodium in conduct disorder. Child Psychiatry Hum Dev. 2006;37(1):55-64.
41. Padhy R, Saxena K, Remsing L, et al. Symptomatic response to divalproex in subtypes of conduct disorder. Child Psychiatry Hum Dev. 2011;42(5):584-593.
42. Steiner H, Petersen ML, Saxena K, et al. Divalproex sodium for the treatment of conduct disorder: a randomized controlled clinical trial. J Clin Psychiatry. 2003;64(10):1183-1191.
43. Klein RG, Abikoff H, Klass E, et al. Clinical efficacy of methylphenidate in conduct disorder with and without attention deficit hyperactivity disorder. Arch Gen Psychiatry. 1997;54(12):1073-1080.
44. Heneggeler SW, Sheidow AJ. Empirically supported family-based treatments for conduct disorder and delinquency in adolescents. J Marital Fam Ther. 2012;38(1):30-58.
45. Lochman JE, Powell NP, Boxmeyer CL, et al. Cognitive-behavioral therapy for externalizing disorder in children and adolescents. Child Adolesc Psychiatr Clin N Am. 2011;20(2):305-318.
46. Furlong M, McGilloway S, Bywater T, et al. Behavioural and cognitive-behavioural group-based parenting programmes for early-onset conduct problems in children aged 3 to 12 years. Cochrane Database Syst Rev. 2012;2:CD008225.
47. McElroy SL, Pope HG Jr, Hudson JI, et al. Kleptomania: a report of 20 cases. Am J Psychiatry. 1991;148(5):652-657.
48. Grant JE, Kim SW. Clinical characteristics and associated psychopathology of 22 patients with kleptomania. Compr Psychiatry. 2002;43(5):378-384.
49. Odlaug BL, Grant JE, Kim SW. Suicide attempts in 107 adolescents and adults with kleptomania. Arch Suicide Res. 2012;16(4):348-359.
50. Grant JE, Kim SW, Odlaug BL. A double-blind, placebo-controlled study of the opiate antagonist, naltrexone, in the treatment of kleptomania. Biol Psychiatry. 2009;65(7): 600-606.
51. Koran LM, Aboujaoude EN, Gamel NN. Escitalopram treatment of kleptomania: an open-label trial followed by double-blind discontinuation. J Clin Psychiatry. 2007;68(3):422-427.
52. Guidry LS. Use of a covert punishing contingency in compulsive stealing. J Behav Therapy Exp Psychiatry. 1975;6(2):169.
53. Gauthier J, Pellerin D. Management of compulsive shoplifting through covert sensitization. J Behav Therapy Exp Psychiatry. 1982;13(1):73-75.
54. McConaghy N, Blaszczynski A. Imaginal desensitization: a cost-effective treatment in two shop-lifters and a binge-eater resistant to previous therapy. Aus N Z J Psychiatry. 1988;22(1):78-82.
55. Grant JE, Won Kim S. Clinical characteristics and psychiatric comorbidity of pyromania. J Clin Psychiatry. 2007;68(11):1717-1722.
56. Grant JE, Odlaug B. Assessment and treatment of pyromania. In: Oxford handbook of impulse control disorders. Grant JE, Potenza MN, eds. Oxford, United Kingdom: Oxford University Press; 2012:353-359.
57. Dell’Osso B, Altamura AC, Allen A, et al. Epidemiologic and clinical updates on impulse control disorders: a critical review. Eur Arch Psychiatry Clin Neurosci. 2006;256(8):464-475.
Chronic disruptive and impulsive behaviors are significant concerns for psychiatric clinicians because of their persistence and potential legal ramifications. To date, few studies have assessed treatment options for pyromania, oppositional defiant disorder (ODD), intermittent explosive disorder (IED), kleptomania, and conduct disorder (CD).
This article reviews the literature on the treatment of these disorders, focusing primarily on randomized, controlled studies. Because of the lack of clinical studies for these disorders, however, case studies and open trials are mentioned for reference. Summaries of supported medication and psychological interventions are provided for each disorder.
Categorizing impulse-control disorders
The DSM-5 created a new chapter on disruptive, impulse control, and conduct disorders that brought together disorders previously classified as disorders usually first diagnosed in infancy, childhood, or adolescence (ODD, CD) and impulse-control disorders not elsewhere classified. These disorders are unified by the presence of difficult, disruptive, aggressive, or antisocial behavior. Disruptive, aggressive, or antisocial behavior usually is a multifaceted behavior, often associated with physical or verbal injury to self, others, or objects or with violating the rights of others. These behaviors can appear in several forms and can be defensive, premeditated, or impulsive.
Despite a high prevalence in the general population1 and in psychiatric cohorts,2 disruptive and impulse-control disorders have been relatively understudied. Controlled trials of treatments do not exist for many impulse-control disorders, and there are no FDA-approved medications for any of these disorders.
Oppositional defiant disorder
Irritability, anger, defiance, and temper are specific descriptors of ODD. ODD seems to be a developmental antecedent for some youth with CD, suggesting that these disorders could reflect different stages of a spectrum of disruptive behavior. Transient oppositional behavior is common among children and adolescents, but ODD occurs in 1% to 11% of youth.3 The disorder is more prevalent among boys before puberty and has an equal sex prevalence in young people after puberty.
Regrettably, most ODD research has included patients with comorbidities, most commonly attention-deficit/hyperactivity disorder (ADHD). Because of this limitation, the drugs and programs discussed below are drawn from meta-analyses and review articles.
Pharmacotherapy. No medications have been FDA-approved for ODD. Studies assessing ODD have employed a variety of methodologies, not all of which are double-blind. The meta-analyses and reviews cited in this section include both randomized and open trials, and should be interpreted as such.
Stimulants are commonly used to treat ODD because of a high comorbidity rate with ADHD, and these drugs have improved ODD symptoms in randomized trials.4 Methylphenidate and d-amphetamine have shown some efficacy in trials of ODD and CD.5-7 These medications are most commonly used when ODD is complicated by ADHD symptoms.
Antipsychotics also have been used to treat ODD, with the largest body of research suggesting that risperidone has some efficacy. Risperidone usually is considered a second- or third-line option because it has been associated with adverse effects in children and adolescents and requires caution in younger populations, despite its potential efficacy.4,8-10
Alpha-2 agonists—clonidine and guanfacine—have shown some efficacy in treating ODD but have not been studied extensively. Studies of clonidine, however, often have grouped ODD, CD, and ADHD, which limits our understanding of this medication for ODD alone.4,5,11
Atomoxetine has been studied for ODD, but its efficacy is limited, with different meta-analyses finding distinct results regarding efficacy. One explanation for these disparate findings is that improvements in oppositional symptoms may be secondary to improvement in ADHD symptoms.7,12-14
Psychological treatments. As noted for pharmacotherapy, this section provides general information on empirically studied therapies. A series of meta-analyses have been included for further review, but are not isolated to randomized, controlled studies.
Individual therapy has shown consistent improvements in ODD. Examples include behavior modification therapy and parent-child interaction therapy. These sessions emphasize skills to manage outbursts and erratic emotionality. Emotion regulation and behavior and social skills training have shown significant reductions in target measures. Some of these programs incorporate both patient and parent components.15-17
Family/teacher training programs such as “Helping the Noncompliant Child” and the “Triple P” have yielded significant improvements. These programs focus on ways to manage the child’s oppositional behavior at home and in the classroom, as well as strategies to limit positive reinforcement for problem behaviors.17-20
Group programs have shown some efficacy with ODD. These programs cover a wide number of needs and intents. Examples include the “Incredible Years” program and the Community Parent Education Program. Research has found that these programs show some efficacy as preemptive measures to reduce the rate of ODD among adolescents.
Conclusions. A number of treatment options for ODD have shown some efficacy. However, many of these options have only been studied in patients with comorbid ADHD, which limits current knowledge about ODD as a distinct disorder.
Intermittent explosive disorder
IED is defined by recurrent, significant outbursts of aggression, often leading to assaultive acts against people or property, which are disproportionate to outside stressors and are not better explained by another psychiatric diagnosis. Research suggests IED is common, with 6.3% of a community sample meeting criteria for lifetime IED.21
IED symptoms tend to start in adolescence and appear to be chronic.21,22 People with IED regard their behavior as distressing and problematic.22 Outbursts generally are short-lived (usually <30 minutes) and frequent (multiple times a month22). Legal and occupational difficulties are common.22
Pharmacotherapy. Data on drug treatment for IED comes for a small set of double-blind studies (Table). Although pharmacotherapies have been studied for treating aggression, impulsivity, and violent behavior, only 5 controlled studies are specific to IED.
A double-blind, randomized, placebo-controlled trial of fluoxetine in 100 participants with IED found that fluoxetine produced a sustained reduction in aggression and irritability as early as the second week of treatment. Full or partial remission of impulsive aggressive behaviors occurred in 46% of fluoxetine-treated subjects. These findings have been supported by studies assessing other samples of aggressive patients, but not specifically IED.23,24 Another treatment study found that oxcarbazepine produced significant improvements in IED symptom severity, specifically on impulsive aggression.25
In a randomized, double-blind, placebo-controlled study, 96 participants with Cluster B personality disorders, 116 with IED, and 34 with posttraumatic stress disorder were assigned to divalproex sodium or placebo for 12 weeks. Using an intent-to-treat analysis, divalproex had no significant influence on aggression in patients with IED.26 Similarly, a study assessing levetiracetam for IED did not show any improvements to measures of impulsive aggression.27
Psychological treatments. The only available study on psychological treatments for IED found that patients receiving active cognitive-behavioral therapy (CBT) or group therapy showed significant improvements compared with waitlist controls. These improvements spanned several target symptoms of IED.28
Conclusions. Although there is a paucity of treatment studies for IED, fluoxetine may be an effective treatment based on available studies, and oxcarbazepine has shown some preliminary efficacy. CBT also has shown some initial efficacy in reducing symptom severity in IED.
Conduct disorder
The essential feature of CD is a repetitive and persistent pattern of behavior in which the basic rights of others or social norms are violated.3 These behaviors can entail:
• aggressive conduct that causes or threatens harm to others or to animals
• nonaggressive behavior resulting in property damage
• deceitfulness or theft
• serious violation of rules.
Prevalence among the general population is 2% to 10%. The disorder is more common among boys than girls.3
Pharmacotherapy. No medication is FDA-approved to treat CD. Fifteen controlled studies have examined medications in patients with CD (Table), although a number of these included a high rate of comorbid ADHD.
To date, 7 studies have shown efficacy with lithium for patients with CD.29-35 A number of trials assessing lithium also included a treatment condition with haloperidol, which showed significant improvement.29,30,33,34 Both lithium and haloperidol were associated with select deficits on cognitive tests, suggesting that there may be risks associated with these medications.
Preliminary double-blind results have indicated that methylphenidate, risperidone, quetiapine, molindone, thioridazine, and carbamazepine might be effective options for treating CD.36-43 The evidence for these medications is limited and additional studies are needed to replicate initial findings.
Three studies of divalproex sodium have shown some efficacy in randomized studies comparing high and low dosages of the drug.40-42 Because these studies did not include a placebo, additional studies are necessary to corroborate these findings.
Psychological treatments. Several forms of behavioral, family-based, and school-based therapies have been found effective in randomized trials. Specifically, behavioral therapy and parental skills training have shown consistent benefits for patients and their families. As with ODD, parental training programs for CD focus on parents’ skill acquisition to help manage outbursts and aggressive behavior. These treatments often follow a similar course to those used for other externalizing and disruptive disorders.44-46
Conclusions. Based on evidence, psychotherapy and some pharmacotherapies (eg, lithium) could be considered first-line treatment options for CD. Psychotherapy programs have shown efficacy in reducing aggression in high-risk groups.44 Lithium or antipsychotics could be useful for patients who do not respond sufficiently to psychotherapy. The risk of cognitive deficits with lithium and antipsychotics should be weighed against potential benefits of these medications.33,34
Kleptomania
Kleptomania is characterized by repetitive, poorly controlled stealing of items that are not needed for personal use. Kleptomania often begins in late adolescence or early adulthood.47 The course of the illness generally is chronic, with waxing and waning symptoms. Women are twice as likely as men to suffer from kleptomania.48 People with kleptomania frequently hoard, discard, or return stolen items.47
Most people with kleptomania try unsuccessfully to stop stealing, which often leads to feelings of shame and guilt.48 Many (64% to 87%) have been arrested because of their stealing behavior47; a smaller percentage (15% to 23%) have been incarcerated.48 Suicide attempts are common among these patients.49
Pharmacotherapy. There has been only 1 randomized, placebo-controlled study of pharmacotherapy for kleptomania (Table). An 8-week, double-blind, placebo-controlled trial was conducted to evaluate the safety and efficacy of oral naltrexone, 50 to 150 mg/d, in 25 patients with kleptomania. Those taking naltrexone had a significantly greater reduction in total score than those taking placebo on the Yale-Brown Obsessive Compulsive Scale Modified for Kleptomania; in stealing urges; and in stealing behavior. The mean effective dosage of naltrexone was 116.7 (± 44.4) mg/d.50
Naltrexone was well tolerated, with minimal nausea, and did not cause elevation of liver enzymes.
There is one available open-label study with a double-blind discontinuation phase assessing the efficacy of escitalopram for kleptomania. Continuation of escitalopram during the blinded discontinuation phase did produce lower relapse rates.51
Psychological treatments. There are no controlled studies of psychological treatments for kleptomania. Case reports suggest that cognitive and behavioral therapies might be effective:
• A young man who underwent 7 sessions of covert sensitization, combined with exposure and response prevention, over a 4-month period was able to reduce his stealing frequency.52
• In another case, a young woman underwent 5 weekly sessions when she was instructed to practice covert sensitization whenever she had an urge to steal. She remained in remission for 14 months with only a single lapse in behavior and with no reported urges to steal.53
• In 2 patients, imaginal desensitization in fourteen 15-minutes sessions over 5 days resulted in complete remission of symptoms for a 2-year period.54
Conclusions. The single controlled study of naltrexone for kleptomania suggests that naltrexone might be a beneficial treatment for this disorder. No controlled trials of psychosocial interventions have been reported. The current psychological research is based primarily on case reports.
This state of affairs likely is because of (1) the low prevalence of kleptomania and (2) clinical difficulties in treating patients involved in illegal activities. Nevertheless, there is a need for systematic studies of treating this disorder; such studies could involve collaboration across multiple treatment centers because of the disorder’s low prevalence.
Pyromania
Pyromania is characterized by (1) deliberate and purposeful fire setting on >1 occasion; (2) tension or affective arousal before the act; (3) fascination with, interest in, curiosity about, or attraction to fire and its situational contexts; and (4) pleasure, gratification, or relief when setting fires or when witnessing or participating in their aftermath.3
Although pyromania is thought to be a disorder primarily affecting men, recent research suggests that the sex ratio is equal among adults and may be slightly higher among adolescent females. Mean age of onset usually is late adolescence. Pyromania appears to be chronic if untreated.55
Urges to set fires are common and the fire setting is almost always pleasurable. Severe distress follows the fire setting, and persons with pyromania report significant functional impairment. High rates of co-occurring psychiatric disorders (depression, substance use disorders, other impulse-control disorders) are common among persons with pyromania.55
Pharmacotherapy. There are no randomized, controlled clinical trials examining pharmacotherapy for treating pyromania. There are no FDA-approved medications for pyromania.
In case reports, medications that have shown benefit in treating pyromania include topiramate, escitalopram, sertraline, fluoxetine, lithium, and a combination of olanzapine and sodium valproate. An equal number of medications have shown no benefit: fluoxetine, valproic acid, lithium, sertraline, olanzapine, escitalopram, citalopram, and clonazepam. A case report of an 18-year-old man with pyromania described successfully using a combination of topiramate with 3 weeks of daily CBT to achieve significant symptom improvement.56,57
Pyromania is a largely unrecognized disorder that causes significant psychological, social, and legal repercussions. Because few persons with pyromania volunteer information regarding fire-setting, it is important that clinicians recognize the disorder and screen patients appropriately. Various treatments have been helpful in case studies, but more research on the etiology and treatment of the disorder is needed.56,57
Conclusions based on the literature
In disruptive, impulse-control, and conduct disorders, the systematic study of treatment efficacy and tolerability is in its infancy. With few controlled studies published, it is not possible to make treatment recommendations with confidence. There are no FDA-approved drugs for treating any of these disorders.
Nonetheless, specific psychotherapies and drug therapies offer promising options, but often are based on small studies, often in patient populations with prominent comorbidities, and have not been replicated by independent investigators. For all of these disorders, issues such as which psychotherapy or medication to use and the ideal duration of treatment cannot be sufficiently addressed with the available data.
In conjunction with emerging epidemiological data supporting a relatively high prevalence of disruptive, impulse-control, and conduct disorders, the small amount of data regarding effective treatments highlights the clinical need for additional research.
Bottom Line
Empirically supported treatment options for impulse-control disorders currently are limited, because only select disorders have been studied across multiple trials. New research is needed to confirm possible treatment options and identify effective psychotherapeutic and pharmacological treatment alternatives.
Related Resources
• Grant JE. Impulse control disorders: a clinician’s guide to understanding and treating behavioral addictions. New York, NY: W. W. Norton & Company; 2008.
• Grant JE, Kim SW. Stop me because I can’t stop myself: taking control of impulsive behavior. New York, NY: McGraw- Hill; 2003.
• American Academy of Child and Adolescent Psychiatry. Conduct disorder resource center. http://www.aacap.org/AACAP/FamiliesandYouth/ResourceCenters/ConductDisorderResourceCenter/Home.aspx.
Drug Brand Names
Atomoxetine • Strattera Methylphenidate • Ritalin
Carbamazepine • Tegretol Molindone • Moban
Citalopram • Celexa Naltrexone • ReVia
Clonazepam • Klonopin Olanzapine • Zyprexa
Clonidine • Catapres Oxcarbazepine • Trileptal
D-amphetamine • Dexedrine Quetiapine • Seroquel
Divalproex sodium • Depakote Risperidone • Risperdal
Escitalopram • Lexapro Sertraline • Zoloft
Fluoxetine • Prozac Sodium valproate • Depacon
Guanfacine • Intuniv Thioridazine • Mellaril
Haloperidol • Haldol Topiramate • Topamax
Levetiracetam • Keppra Valproic acid • Depakote
Lithium • Eskalith, Lithobid
Disclosures
Dr. Grant receives grant or research support from Brainsway, Forest Pharmaceuticals, and Roche Pharmaceuticals. Mr. Leppink reports no financial relationship with any company whose products are mentioned in this article or with competing products.
Chronic disruptive and impulsive behaviors are significant concerns for psychiatric clinicians because of their persistence and potential legal ramifications. To date, few studies have assessed treatment options for pyromania, oppositional defiant disorder (ODD), intermittent explosive disorder (IED), kleptomania, and conduct disorder (CD).
This article reviews the literature on the treatment of these disorders, focusing primarily on randomized, controlled studies. Because of the lack of clinical studies for these disorders, however, case studies and open trials are mentioned for reference. Summaries of supported medication and psychological interventions are provided for each disorder.
Categorizing impulse-control disorders
The DSM-5 created a new chapter on disruptive, impulse control, and conduct disorders that brought together disorders previously classified as disorders usually first diagnosed in infancy, childhood, or adolescence (ODD, CD) and impulse-control disorders not elsewhere classified. These disorders are unified by the presence of difficult, disruptive, aggressive, or antisocial behavior. Disruptive, aggressive, or antisocial behavior usually is a multifaceted behavior, often associated with physical or verbal injury to self, others, or objects or with violating the rights of others. These behaviors can appear in several forms and can be defensive, premeditated, or impulsive.
Despite a high prevalence in the general population1 and in psychiatric cohorts,2 disruptive and impulse-control disorders have been relatively understudied. Controlled trials of treatments do not exist for many impulse-control disorders, and there are no FDA-approved medications for any of these disorders.
Oppositional defiant disorder
Irritability, anger, defiance, and temper are specific descriptors of ODD. ODD seems to be a developmental antecedent for some youth with CD, suggesting that these disorders could reflect different stages of a spectrum of disruptive behavior. Transient oppositional behavior is common among children and adolescents, but ODD occurs in 1% to 11% of youth.3 The disorder is more prevalent among boys before puberty and has an equal sex prevalence in young people after puberty.
Regrettably, most ODD research has included patients with comorbidities, most commonly attention-deficit/hyperactivity disorder (ADHD). Because of this limitation, the drugs and programs discussed below are drawn from meta-analyses and review articles.
Pharmacotherapy. No medications have been FDA-approved for ODD. Studies assessing ODD have employed a variety of methodologies, not all of which are double-blind. The meta-analyses and reviews cited in this section include both randomized and open trials, and should be interpreted as such.
Stimulants are commonly used to treat ODD because of a high comorbidity rate with ADHD, and these drugs have improved ODD symptoms in randomized trials.4 Methylphenidate and d-amphetamine have shown some efficacy in trials of ODD and CD.5-7 These medications are most commonly used when ODD is complicated by ADHD symptoms.
Antipsychotics also have been used to treat ODD, with the largest body of research suggesting that risperidone has some efficacy. Risperidone usually is considered a second- or third-line option because it has been associated with adverse effects in children and adolescents and requires caution in younger populations, despite its potential efficacy.4,8-10
Alpha-2 agonists—clonidine and guanfacine—have shown some efficacy in treating ODD but have not been studied extensively. Studies of clonidine, however, often have grouped ODD, CD, and ADHD, which limits our understanding of this medication for ODD alone.4,5,11
Atomoxetine has been studied for ODD, but its efficacy is limited, with different meta-analyses finding distinct results regarding efficacy. One explanation for these disparate findings is that improvements in oppositional symptoms may be secondary to improvement in ADHD symptoms.7,12-14
Psychological treatments. As noted for pharmacotherapy, this section provides general information on empirically studied therapies. A series of meta-analyses have been included for further review, but are not isolated to randomized, controlled studies.
Individual therapy has shown consistent improvements in ODD. Examples include behavior modification therapy and parent-child interaction therapy. These sessions emphasize skills to manage outbursts and erratic emotionality. Emotion regulation and behavior and social skills training have shown significant reductions in target measures. Some of these programs incorporate both patient and parent components.15-17
Family/teacher training programs such as “Helping the Noncompliant Child” and the “Triple P” have yielded significant improvements. These programs focus on ways to manage the child’s oppositional behavior at home and in the classroom, as well as strategies to limit positive reinforcement for problem behaviors.17-20
Group programs have shown some efficacy with ODD. These programs cover a wide number of needs and intents. Examples include the “Incredible Years” program and the Community Parent Education Program. Research has found that these programs show some efficacy as preemptive measures to reduce the rate of ODD among adolescents.
Conclusions. A number of treatment options for ODD have shown some efficacy. However, many of these options have only been studied in patients with comorbid ADHD, which limits current knowledge about ODD as a distinct disorder.
Intermittent explosive disorder
IED is defined by recurrent, significant outbursts of aggression, often leading to assaultive acts against people or property, which are disproportionate to outside stressors and are not better explained by another psychiatric diagnosis. Research suggests IED is common, with 6.3% of a community sample meeting criteria for lifetime IED.21
IED symptoms tend to start in adolescence and appear to be chronic.21,22 People with IED regard their behavior as distressing and problematic.22 Outbursts generally are short-lived (usually <30 minutes) and frequent (multiple times a month22). Legal and occupational difficulties are common.22
Pharmacotherapy. Data on drug treatment for IED comes for a small set of double-blind studies (Table). Although pharmacotherapies have been studied for treating aggression, impulsivity, and violent behavior, only 5 controlled studies are specific to IED.
A double-blind, randomized, placebo-controlled trial of fluoxetine in 100 participants with IED found that fluoxetine produced a sustained reduction in aggression and irritability as early as the second week of treatment. Full or partial remission of impulsive aggressive behaviors occurred in 46% of fluoxetine-treated subjects. These findings have been supported by studies assessing other samples of aggressive patients, but not specifically IED.23,24 Another treatment study found that oxcarbazepine produced significant improvements in IED symptom severity, specifically on impulsive aggression.25
In a randomized, double-blind, placebo-controlled study, 96 participants with Cluster B personality disorders, 116 with IED, and 34 with posttraumatic stress disorder were assigned to divalproex sodium or placebo for 12 weeks. Using an intent-to-treat analysis, divalproex had no significant influence on aggression in patients with IED.26 Similarly, a study assessing levetiracetam for IED did not show any improvements to measures of impulsive aggression.27
Psychological treatments. The only available study on psychological treatments for IED found that patients receiving active cognitive-behavioral therapy (CBT) or group therapy showed significant improvements compared with waitlist controls. These improvements spanned several target symptoms of IED.28
Conclusions. Although there is a paucity of treatment studies for IED, fluoxetine may be an effective treatment based on available studies, and oxcarbazepine has shown some preliminary efficacy. CBT also has shown some initial efficacy in reducing symptom severity in IED.
Conduct disorder
The essential feature of CD is a repetitive and persistent pattern of behavior in which the basic rights of others or social norms are violated.3 These behaviors can entail:
• aggressive conduct that causes or threatens harm to others or to animals
• nonaggressive behavior resulting in property damage
• deceitfulness or theft
• serious violation of rules.
Prevalence among the general population is 2% to 10%. The disorder is more common among boys than girls.3
Pharmacotherapy. No medication is FDA-approved to treat CD. Fifteen controlled studies have examined medications in patients with CD (Table), although a number of these included a high rate of comorbid ADHD.
To date, 7 studies have shown efficacy with lithium for patients with CD.29-35 A number of trials assessing lithium also included a treatment condition with haloperidol, which showed significant improvement.29,30,33,34 Both lithium and haloperidol were associated with select deficits on cognitive tests, suggesting that there may be risks associated with these medications.
Preliminary double-blind results have indicated that methylphenidate, risperidone, quetiapine, molindone, thioridazine, and carbamazepine might be effective options for treating CD.36-43 The evidence for these medications is limited and additional studies are needed to replicate initial findings.
Three studies of divalproex sodium have shown some efficacy in randomized studies comparing high and low dosages of the drug.40-42 Because these studies did not include a placebo, additional studies are necessary to corroborate these findings.
Psychological treatments. Several forms of behavioral, family-based, and school-based therapies have been found effective in randomized trials. Specifically, behavioral therapy and parental skills training have shown consistent benefits for patients and their families. As with ODD, parental training programs for CD focus on parents’ skill acquisition to help manage outbursts and aggressive behavior. These treatments often follow a similar course to those used for other externalizing and disruptive disorders.44-46
Conclusions. Based on evidence, psychotherapy and some pharmacotherapies (eg, lithium) could be considered first-line treatment options for CD. Psychotherapy programs have shown efficacy in reducing aggression in high-risk groups.44 Lithium or antipsychotics could be useful for patients who do not respond sufficiently to psychotherapy. The risk of cognitive deficits with lithium and antipsychotics should be weighed against potential benefits of these medications.33,34
Kleptomania
Kleptomania is characterized by repetitive, poorly controlled stealing of items that are not needed for personal use. Kleptomania often begins in late adolescence or early adulthood.47 The course of the illness generally is chronic, with waxing and waning symptoms. Women are twice as likely as men to suffer from kleptomania.48 People with kleptomania frequently hoard, discard, or return stolen items.47
Most people with kleptomania try unsuccessfully to stop stealing, which often leads to feelings of shame and guilt.48 Many (64% to 87%) have been arrested because of their stealing behavior47; a smaller percentage (15% to 23%) have been incarcerated.48 Suicide attempts are common among these patients.49
Pharmacotherapy. There has been only 1 randomized, placebo-controlled study of pharmacotherapy for kleptomania (Table). An 8-week, double-blind, placebo-controlled trial was conducted to evaluate the safety and efficacy of oral naltrexone, 50 to 150 mg/d, in 25 patients with kleptomania. Those taking naltrexone had a significantly greater reduction in total score than those taking placebo on the Yale-Brown Obsessive Compulsive Scale Modified for Kleptomania; in stealing urges; and in stealing behavior. The mean effective dosage of naltrexone was 116.7 (± 44.4) mg/d.50
Naltrexone was well tolerated, with minimal nausea, and did not cause elevation of liver enzymes.
There is one available open-label study with a double-blind discontinuation phase assessing the efficacy of escitalopram for kleptomania. Continuation of escitalopram during the blinded discontinuation phase did produce lower relapse rates.51
Psychological treatments. There are no controlled studies of psychological treatments for kleptomania. Case reports suggest that cognitive and behavioral therapies might be effective:
• A young man who underwent 7 sessions of covert sensitization, combined with exposure and response prevention, over a 4-month period was able to reduce his stealing frequency.52
• In another case, a young woman underwent 5 weekly sessions when she was instructed to practice covert sensitization whenever she had an urge to steal. She remained in remission for 14 months with only a single lapse in behavior and with no reported urges to steal.53
• In 2 patients, imaginal desensitization in fourteen 15-minutes sessions over 5 days resulted in complete remission of symptoms for a 2-year period.54
Conclusions. The single controlled study of naltrexone for kleptomania suggests that naltrexone might be a beneficial treatment for this disorder. No controlled trials of psychosocial interventions have been reported. The current psychological research is based primarily on case reports.
This state of affairs likely is because of (1) the low prevalence of kleptomania and (2) clinical difficulties in treating patients involved in illegal activities. Nevertheless, there is a need for systematic studies of treating this disorder; such studies could involve collaboration across multiple treatment centers because of the disorder’s low prevalence.
Pyromania
Pyromania is characterized by (1) deliberate and purposeful fire setting on >1 occasion; (2) tension or affective arousal before the act; (3) fascination with, interest in, curiosity about, or attraction to fire and its situational contexts; and (4) pleasure, gratification, or relief when setting fires or when witnessing or participating in their aftermath.3
Although pyromania is thought to be a disorder primarily affecting men, recent research suggests that the sex ratio is equal among adults and may be slightly higher among adolescent females. Mean age of onset usually is late adolescence. Pyromania appears to be chronic if untreated.55
Urges to set fires are common and the fire setting is almost always pleasurable. Severe distress follows the fire setting, and persons with pyromania report significant functional impairment. High rates of co-occurring psychiatric disorders (depression, substance use disorders, other impulse-control disorders) are common among persons with pyromania.55
Pharmacotherapy. There are no randomized, controlled clinical trials examining pharmacotherapy for treating pyromania. There are no FDA-approved medications for pyromania.
In case reports, medications that have shown benefit in treating pyromania include topiramate, escitalopram, sertraline, fluoxetine, lithium, and a combination of olanzapine and sodium valproate. An equal number of medications have shown no benefit: fluoxetine, valproic acid, lithium, sertraline, olanzapine, escitalopram, citalopram, and clonazepam. A case report of an 18-year-old man with pyromania described successfully using a combination of topiramate with 3 weeks of daily CBT to achieve significant symptom improvement.56,57
Pyromania is a largely unrecognized disorder that causes significant psychological, social, and legal repercussions. Because few persons with pyromania volunteer information regarding fire-setting, it is important that clinicians recognize the disorder and screen patients appropriately. Various treatments have been helpful in case studies, but more research on the etiology and treatment of the disorder is needed.56,57
Conclusions based on the literature
In disruptive, impulse-control, and conduct disorders, the systematic study of treatment efficacy and tolerability is in its infancy. With few controlled studies published, it is not possible to make treatment recommendations with confidence. There are no FDA-approved drugs for treating any of these disorders.
Nonetheless, specific psychotherapies and drug therapies offer promising options, but often are based on small studies, often in patient populations with prominent comorbidities, and have not been replicated by independent investigators. For all of these disorders, issues such as which psychotherapy or medication to use and the ideal duration of treatment cannot be sufficiently addressed with the available data.
In conjunction with emerging epidemiological data supporting a relatively high prevalence of disruptive, impulse-control, and conduct disorders, the small amount of data regarding effective treatments highlights the clinical need for additional research.
Bottom Line
Empirically supported treatment options for impulse-control disorders currently are limited, because only select disorders have been studied across multiple trials. New research is needed to confirm possible treatment options and identify effective psychotherapeutic and pharmacological treatment alternatives.
Related Resources
• Grant JE. Impulse control disorders: a clinician’s guide to understanding and treating behavioral addictions. New York, NY: W. W. Norton & Company; 2008.
• Grant JE, Kim SW. Stop me because I can’t stop myself: taking control of impulsive behavior. New York, NY: McGraw- Hill; 2003.
• American Academy of Child and Adolescent Psychiatry. Conduct disorder resource center. http://www.aacap.org/AACAP/FamiliesandYouth/ResourceCenters/ConductDisorderResourceCenter/Home.aspx.
Drug Brand Names
Atomoxetine • Strattera Methylphenidate • Ritalin
Carbamazepine • Tegretol Molindone • Moban
Citalopram • Celexa Naltrexone • ReVia
Clonazepam • Klonopin Olanzapine • Zyprexa
Clonidine • Catapres Oxcarbazepine • Trileptal
D-amphetamine • Dexedrine Quetiapine • Seroquel
Divalproex sodium • Depakote Risperidone • Risperdal
Escitalopram • Lexapro Sertraline • Zoloft
Fluoxetine • Prozac Sodium valproate • Depacon
Guanfacine • Intuniv Thioridazine • Mellaril
Haloperidol • Haldol Topiramate • Topamax
Levetiracetam • Keppra Valproic acid • Depakote
Lithium • Eskalith, Lithobid
Disclosures
Dr. Grant receives grant or research support from Brainsway, Forest Pharmaceuticals, and Roche Pharmaceuticals. Mr. Leppink reports no financial relationship with any company whose products are mentioned in this article or with competing products.
1. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Grant JE, Levine L, Kim D, et al. Impulse control disorders in adult psychiatric inpatients. Am J Psychiatry. 2005;162(11):2184-2188.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Turgay A. Psychopharmacological treatment of oppositional defiant disorder. CNS Drugs. 2009;23(1):1-17.
5. Hazell P. Review of attention-deficit/hyperactivity disorder comorbid with oppositional defiant disorder. Australas Psychiatry. 2010;18(6):556-559.
6. Burke JD, Loeber R, Birmaher B. Oppositional defiant disorder and conduct disorder: a review of the past 10 years, part II. J Am Acad Child Adolesc Psychiatry. 2002; 41(11):1275-1293.
7. Connor DF, Steeber J, McBurnett K. A review of attention-deficit/hyperactivity disorder complicated by symptoms of oppositional defiant disorder or conduct disorder. J Dev Behav Pediatr. 2010;31(5):427-440.
8. Aman MG, Bukstein OG, Gadow KD, et al. What does risperidone add to parent training and stimulant for severe aggression in child attention-deficit/hyperactivity disorder? J Am Acad Child Adolesc Psychiatry. 2014;53(1):47-60.e1.
9. Loy JH, Merry SN, Hetrick SE, et al. Atypical antipsychotics for disruptive behavior disorders in children and youths. Cochrane Database Syst Rev. 2012;9:CD008559.
10. Gadow KD, Arnold LE, Molina BS, et al. Risperidone added to parent training and stimulant medication: effects on attention-deficit/hyperactivity disorder, oppositional defiant disorder, conduct disorder, and peer aggression. J Am Acad Child Adolesc Psychiatry. 2014;53(9):948-959.e1.
12. Signorovitch J, Erder MH, Xie J, et al. Comparative effectiveness research using matching-adjusted indirect comparison: an application to treatment with guanfacine extended release or atomoxetine in children with attention-deficit/hyperactivity disorder and comorbid oppositional defiant disorder. Pharmacoepidemiol Drug Saf. 2012;21(suppl 2):130-137.
13. Bangs ME, Hazell P, Danckaerts M, et al; Atomoxetine ADHD/ODD Study Group. Atomoxetine for the treatment of attention-deficit/hyperactivity disorder and oppositional defiant disorder. Pediatrics. 2008;121(2):e314-e320.
14. Biederman J, Spencer TJ, Newcorn JH, et al. Effect of comorbid symptoms of oppositional defiant disorder on responses to atomoxetine in children with ADHD: a meta-analysis of controlled clinical trial data. Psychopharmacology (Berl). 2007;190(1):31-41.
15. Miller NV, Haas SM, Waschbusch DA, et al. Behavior therapy and callous-unemotional traits: effects of a pilot study examining modified behavioral contingencies on child behavior. Behav Ther. 2014;45(5):606-618.
16. Hamilton SS, Armando J. Oppositional defiant disorder. Am Fam Physician. 2008;78(7):861-866.
17. Steiner H, Remsing L; Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with oppositional defiant disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(1):126-141.
18. Winther J, Carlsson A, Vance A. A pilot study of a school-based prevention and early intervention program to reduce oppositional defiant disorder/conduct disorder. Early Interv Psychiatry. 2014;8(2):181-189.
19. Plueck J, Eichelberger I, Hautmann C, et al. Effectiveness of a teacher-based indicated prevention program for preschool children with externalizing problem behavior [published online April 22, 2014]. Prev Sci. doi: 10.1007/s11121-014- 0487-x.
20. Dretzke J, Frew E, Davenport C, et al. The effectiveness and cost-effectiveness of parent training/education programmes for the treatment of conduct disorder, including oppositional defiant disorder, in children. Health Tech Assess. 2005;9(50):iii, ix-x, 1-233.
21. Coccaro EF, Schmidt CA, Samuels JF, et al. Lifetime and 1-month prevalence rates of intermittent explosive disorder in a community sample. J Clin Psychiatry. 2004;65(6):820-824.
22. McElroy SL, Soutullo CA, Beckman DA, et al. DSM-IV intermittent explosive disorder: a report of 27 cases. J Clin Psychiatry. 1998;59(4):203-210; quiz 211.
23. Coccaro EF, Lee RJ, Kavoussi RJ. A double-blind, randomized, placebo-controlled trial of fluoxetine in patients with intermittent explosive disorder. J Clin Psychiatry. 2009;70(5):653-662.
24. Coccaro EF. Intermittent explosive disorder as a disorder of impulsive aggression for DSM-5. Am J Psychiatry. 2012;169(6):577-588.
25. Mattes JA. Oxcarbazepine in patients with impulsive aggression: a double-blind, placebo-controlled trial. J Clin Psychopharmacol. 2005;25(6):575-579.
26. Hollander E, Tracy KA, Swann AC, et al. Divalproex in the treatment of impulsive aggression: efficacy in cluster B personality disorders. Neuropsychopharmacology. 2003;28(6):1186-1197.
27. Mattes JA. Levetiracetam in patients with impulsive aggression: a double-blind, placebo-controlled trial. J Clin Psychiatry. 2008;69(2):310-315.
28. McCloskey MS, Noblett KL, Deffenbacher JL, et al. Cognitive-behavioral therapy for intermittent explosive disorder: a pilot randomized clinical trial. J Consult Clin Psychol. 2008;76(5):876-886.
29. Campbell M, Small AM, Green WH, et al. Behavioral efficacy of haloperidol and lithium carbonate. A comparison in hospitalized aggressive children with conduct disorder. Arch Gen Psychiatry. 1984;41(7):650-656.
30. Campbell M, Adams PB, Small AM, et al. Lithium in hospitalized aggressive children with conduct disorder: a double-blind and placebo-controlled study. J Am Acad Child Adolesc Psychiatry. 1995;34(4):445-453.
31. Malone RP, Simpson GM. Psychopharmacology: use of placebos in clinical trials involving children and adolescents. Psychiatr Serv. 1998;49(11):1413-1414, 1417.
32. Malone RP, Delaney MA, Luebbert JF, et al. A double-blind placebo-controlled study of lithium in hospitalized aggressive children and adolescents with conduct disorder. Arch Gen Psychiatry. 2000;57(7):649-654.
33. Platt JE, Campbell M, Green WH, et al. Effects of lithium carbonate and haloperidol on cognition in aggressive hospitalized school-age children. J Clin Psychopharmacol. 1981;1(1):8-13.
34. Platt JE, Campbell M, Green WH, et al. Cognitive effects of lithium carbonate and haloperidol in treatment-resistant aggressive children. Arch Gen Psychiatry. 1984;41(7):657-662.
35. Rifkin A, Karajgi B, Dicker R, et al. Lithium treatment of conduct disorders in adolescents. Am J Psychiatry. 1997;154(4):554-555.
36. Cueva JE, Overall JE, Small AM, et al. Carbamazepine in aggressive children with conduct disorder: a double-blind and placebo-controlled study. J Am Acad Child Adolesc Psychiatry. 1996;35(4):480-490.
37. Findling RL, McNamara NK, Branicky LA, et al. A double-blind pilot study of risperidone in the treatment of conduct disorder. J Am Acad Child Adolesc Psychiatry. 2000;39(4):509-516.
38. Connor DF, McLaughlin TJ, Jeffers-Terry M. Randomized controlled pilot study of quetiapine in the treatment of adolescent conduct disorder. J Child Adolesc Psychopharmacol. 2008;18(2):140-156.
39. Greenhill LL, Solomon M, Pleak R, et al. Molindone hydrochloride treatment of hospitalized children with conduct disorder. J Clin Psychiatry. 1985;46(8 pt 2):20-25.
40. Khanzode LA, Saxena K, Kraemer H, et al. Efficacy profiles of psychopharmacology: divalproex sodium in conduct disorder. Child Psychiatry Hum Dev. 2006;37(1):55-64.
41. Padhy R, Saxena K, Remsing L, et al. Symptomatic response to divalproex in subtypes of conduct disorder. Child Psychiatry Hum Dev. 2011;42(5):584-593.
42. Steiner H, Petersen ML, Saxena K, et al. Divalproex sodium for the treatment of conduct disorder: a randomized controlled clinical trial. J Clin Psychiatry. 2003;64(10):1183-1191.
43. Klein RG, Abikoff H, Klass E, et al. Clinical efficacy of methylphenidate in conduct disorder with and without attention deficit hyperactivity disorder. Arch Gen Psychiatry. 1997;54(12):1073-1080.
44. Heneggeler SW, Sheidow AJ. Empirically supported family-based treatments for conduct disorder and delinquency in adolescents. J Marital Fam Ther. 2012;38(1):30-58.
45. Lochman JE, Powell NP, Boxmeyer CL, et al. Cognitive-behavioral therapy for externalizing disorder in children and adolescents. Child Adolesc Psychiatr Clin N Am. 2011;20(2):305-318.
46. Furlong M, McGilloway S, Bywater T, et al. Behavioural and cognitive-behavioural group-based parenting programmes for early-onset conduct problems in children aged 3 to 12 years. Cochrane Database Syst Rev. 2012;2:CD008225.
47. McElroy SL, Pope HG Jr, Hudson JI, et al. Kleptomania: a report of 20 cases. Am J Psychiatry. 1991;148(5):652-657.
48. Grant JE, Kim SW. Clinical characteristics and associated psychopathology of 22 patients with kleptomania. Compr Psychiatry. 2002;43(5):378-384.
49. Odlaug BL, Grant JE, Kim SW. Suicide attempts in 107 adolescents and adults with kleptomania. Arch Suicide Res. 2012;16(4):348-359.
50. Grant JE, Kim SW, Odlaug BL. A double-blind, placebo-controlled study of the opiate antagonist, naltrexone, in the treatment of kleptomania. Biol Psychiatry. 2009;65(7): 600-606.
51. Koran LM, Aboujaoude EN, Gamel NN. Escitalopram treatment of kleptomania: an open-label trial followed by double-blind discontinuation. J Clin Psychiatry. 2007;68(3):422-427.
52. Guidry LS. Use of a covert punishing contingency in compulsive stealing. J Behav Therapy Exp Psychiatry. 1975;6(2):169.
53. Gauthier J, Pellerin D. Management of compulsive shoplifting through covert sensitization. J Behav Therapy Exp Psychiatry. 1982;13(1):73-75.
54. McConaghy N, Blaszczynski A. Imaginal desensitization: a cost-effective treatment in two shop-lifters and a binge-eater resistant to previous therapy. Aus N Z J Psychiatry. 1988;22(1):78-82.
55. Grant JE, Won Kim S. Clinical characteristics and psychiatric comorbidity of pyromania. J Clin Psychiatry. 2007;68(11):1717-1722.
56. Grant JE, Odlaug B. Assessment and treatment of pyromania. In: Oxford handbook of impulse control disorders. Grant JE, Potenza MN, eds. Oxford, United Kingdom: Oxford University Press; 2012:353-359.
57. Dell’Osso B, Altamura AC, Allen A, et al. Epidemiologic and clinical updates on impulse control disorders: a critical review. Eur Arch Psychiatry Clin Neurosci. 2006;256(8):464-475.
1. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Grant JE, Levine L, Kim D, et al. Impulse control disorders in adult psychiatric inpatients. Am J Psychiatry. 2005;162(11):2184-2188.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Turgay A. Psychopharmacological treatment of oppositional defiant disorder. CNS Drugs. 2009;23(1):1-17.
5. Hazell P. Review of attention-deficit/hyperactivity disorder comorbid with oppositional defiant disorder. Australas Psychiatry. 2010;18(6):556-559.
6. Burke JD, Loeber R, Birmaher B. Oppositional defiant disorder and conduct disorder: a review of the past 10 years, part II. J Am Acad Child Adolesc Psychiatry. 2002; 41(11):1275-1293.
7. Connor DF, Steeber J, McBurnett K. A review of attention-deficit/hyperactivity disorder complicated by symptoms of oppositional defiant disorder or conduct disorder. J Dev Behav Pediatr. 2010;31(5):427-440.
8. Aman MG, Bukstein OG, Gadow KD, et al. What does risperidone add to parent training and stimulant for severe aggression in child attention-deficit/hyperactivity disorder? J Am Acad Child Adolesc Psychiatry. 2014;53(1):47-60.e1.
9. Loy JH, Merry SN, Hetrick SE, et al. Atypical antipsychotics for disruptive behavior disorders in children and youths. Cochrane Database Syst Rev. 2012;9:CD008559.
10. Gadow KD, Arnold LE, Molina BS, et al. Risperidone added to parent training and stimulant medication: effects on attention-deficit/hyperactivity disorder, oppositional defiant disorder, conduct disorder, and peer aggression. J Am Acad Child Adolesc Psychiatry. 2014;53(9):948-959.e1.
12. Signorovitch J, Erder MH, Xie J, et al. Comparative effectiveness research using matching-adjusted indirect comparison: an application to treatment with guanfacine extended release or atomoxetine in children with attention-deficit/hyperactivity disorder and comorbid oppositional defiant disorder. Pharmacoepidemiol Drug Saf. 2012;21(suppl 2):130-137.
13. Bangs ME, Hazell P, Danckaerts M, et al; Atomoxetine ADHD/ODD Study Group. Atomoxetine for the treatment of attention-deficit/hyperactivity disorder and oppositional defiant disorder. Pediatrics. 2008;121(2):e314-e320.
14. Biederman J, Spencer TJ, Newcorn JH, et al. Effect of comorbid symptoms of oppositional defiant disorder on responses to atomoxetine in children with ADHD: a meta-analysis of controlled clinical trial data. Psychopharmacology (Berl). 2007;190(1):31-41.
15. Miller NV, Haas SM, Waschbusch DA, et al. Behavior therapy and callous-unemotional traits: effects of a pilot study examining modified behavioral contingencies on child behavior. Behav Ther. 2014;45(5):606-618.
16. Hamilton SS, Armando J. Oppositional defiant disorder. Am Fam Physician. 2008;78(7):861-866.
17. Steiner H, Remsing L; Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with oppositional defiant disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(1):126-141.
18. Winther J, Carlsson A, Vance A. A pilot study of a school-based prevention and early intervention program to reduce oppositional defiant disorder/conduct disorder. Early Interv Psychiatry. 2014;8(2):181-189.
19. Plueck J, Eichelberger I, Hautmann C, et al. Effectiveness of a teacher-based indicated prevention program for preschool children with externalizing problem behavior [published online April 22, 2014]. Prev Sci. doi: 10.1007/s11121-014- 0487-x.
20. Dretzke J, Frew E, Davenport C, et al. The effectiveness and cost-effectiveness of parent training/education programmes for the treatment of conduct disorder, including oppositional defiant disorder, in children. Health Tech Assess. 2005;9(50):iii, ix-x, 1-233.
21. Coccaro EF, Schmidt CA, Samuels JF, et al. Lifetime and 1-month prevalence rates of intermittent explosive disorder in a community sample. J Clin Psychiatry. 2004;65(6):820-824.
22. McElroy SL, Soutullo CA, Beckman DA, et al. DSM-IV intermittent explosive disorder: a report of 27 cases. J Clin Psychiatry. 1998;59(4):203-210; quiz 211.
23. Coccaro EF, Lee RJ, Kavoussi RJ. A double-blind, randomized, placebo-controlled trial of fluoxetine in patients with intermittent explosive disorder. J Clin Psychiatry. 2009;70(5):653-662.
24. Coccaro EF. Intermittent explosive disorder as a disorder of impulsive aggression for DSM-5. Am J Psychiatry. 2012;169(6):577-588.
25. Mattes JA. Oxcarbazepine in patients with impulsive aggression: a double-blind, placebo-controlled trial. J Clin Psychopharmacol. 2005;25(6):575-579.
26. Hollander E, Tracy KA, Swann AC, et al. Divalproex in the treatment of impulsive aggression: efficacy in cluster B personality disorders. Neuropsychopharmacology. 2003;28(6):1186-1197.
27. Mattes JA. Levetiracetam in patients with impulsive aggression: a double-blind, placebo-controlled trial. J Clin Psychiatry. 2008;69(2):310-315.
28. McCloskey MS, Noblett KL, Deffenbacher JL, et al. Cognitive-behavioral therapy for intermittent explosive disorder: a pilot randomized clinical trial. J Consult Clin Psychol. 2008;76(5):876-886.
29. Campbell M, Small AM, Green WH, et al. Behavioral efficacy of haloperidol and lithium carbonate. A comparison in hospitalized aggressive children with conduct disorder. Arch Gen Psychiatry. 1984;41(7):650-656.
30. Campbell M, Adams PB, Small AM, et al. Lithium in hospitalized aggressive children with conduct disorder: a double-blind and placebo-controlled study. J Am Acad Child Adolesc Psychiatry. 1995;34(4):445-453.
31. Malone RP, Simpson GM. Psychopharmacology: use of placebos in clinical trials involving children and adolescents. Psychiatr Serv. 1998;49(11):1413-1414, 1417.
32. Malone RP, Delaney MA, Luebbert JF, et al. A double-blind placebo-controlled study of lithium in hospitalized aggressive children and adolescents with conduct disorder. Arch Gen Psychiatry. 2000;57(7):649-654.
33. Platt JE, Campbell M, Green WH, et al. Effects of lithium carbonate and haloperidol on cognition in aggressive hospitalized school-age children. J Clin Psychopharmacol. 1981;1(1):8-13.
34. Platt JE, Campbell M, Green WH, et al. Cognitive effects of lithium carbonate and haloperidol in treatment-resistant aggressive children. Arch Gen Psychiatry. 1984;41(7):657-662.
35. Rifkin A, Karajgi B, Dicker R, et al. Lithium treatment of conduct disorders in adolescents. Am J Psychiatry. 1997;154(4):554-555.
36. Cueva JE, Overall JE, Small AM, et al. Carbamazepine in aggressive children with conduct disorder: a double-blind and placebo-controlled study. J Am Acad Child Adolesc Psychiatry. 1996;35(4):480-490.
37. Findling RL, McNamara NK, Branicky LA, et al. A double-blind pilot study of risperidone in the treatment of conduct disorder. J Am Acad Child Adolesc Psychiatry. 2000;39(4):509-516.
38. Connor DF, McLaughlin TJ, Jeffers-Terry M. Randomized controlled pilot study of quetiapine in the treatment of adolescent conduct disorder. J Child Adolesc Psychopharmacol. 2008;18(2):140-156.
39. Greenhill LL, Solomon M, Pleak R, et al. Molindone hydrochloride treatment of hospitalized children with conduct disorder. J Clin Psychiatry. 1985;46(8 pt 2):20-25.
40. Khanzode LA, Saxena K, Kraemer H, et al. Efficacy profiles of psychopharmacology: divalproex sodium in conduct disorder. Child Psychiatry Hum Dev. 2006;37(1):55-64.
41. Padhy R, Saxena K, Remsing L, et al. Symptomatic response to divalproex in subtypes of conduct disorder. Child Psychiatry Hum Dev. 2011;42(5):584-593.
42. Steiner H, Petersen ML, Saxena K, et al. Divalproex sodium for the treatment of conduct disorder: a randomized controlled clinical trial. J Clin Psychiatry. 2003;64(10):1183-1191.
43. Klein RG, Abikoff H, Klass E, et al. Clinical efficacy of methylphenidate in conduct disorder with and without attention deficit hyperactivity disorder. Arch Gen Psychiatry. 1997;54(12):1073-1080.
44. Heneggeler SW, Sheidow AJ. Empirically supported family-based treatments for conduct disorder and delinquency in adolescents. J Marital Fam Ther. 2012;38(1):30-58.
45. Lochman JE, Powell NP, Boxmeyer CL, et al. Cognitive-behavioral therapy for externalizing disorder in children and adolescents. Child Adolesc Psychiatr Clin N Am. 2011;20(2):305-318.
46. Furlong M, McGilloway S, Bywater T, et al. Behavioural and cognitive-behavioural group-based parenting programmes for early-onset conduct problems in children aged 3 to 12 years. Cochrane Database Syst Rev. 2012;2:CD008225.
47. McElroy SL, Pope HG Jr, Hudson JI, et al. Kleptomania: a report of 20 cases. Am J Psychiatry. 1991;148(5):652-657.
48. Grant JE, Kim SW. Clinical characteristics and associated psychopathology of 22 patients with kleptomania. Compr Psychiatry. 2002;43(5):378-384.
49. Odlaug BL, Grant JE, Kim SW. Suicide attempts in 107 adolescents and adults with kleptomania. Arch Suicide Res. 2012;16(4):348-359.
50. Grant JE, Kim SW, Odlaug BL. A double-blind, placebo-controlled study of the opiate antagonist, naltrexone, in the treatment of kleptomania. Biol Psychiatry. 2009;65(7): 600-606.
51. Koran LM, Aboujaoude EN, Gamel NN. Escitalopram treatment of kleptomania: an open-label trial followed by double-blind discontinuation. J Clin Psychiatry. 2007;68(3):422-427.
52. Guidry LS. Use of a covert punishing contingency in compulsive stealing. J Behav Therapy Exp Psychiatry. 1975;6(2):169.
53. Gauthier J, Pellerin D. Management of compulsive shoplifting through covert sensitization. J Behav Therapy Exp Psychiatry. 1982;13(1):73-75.
54. McConaghy N, Blaszczynski A. Imaginal desensitization: a cost-effective treatment in two shop-lifters and a binge-eater resistant to previous therapy. Aus N Z J Psychiatry. 1988;22(1):78-82.
55. Grant JE, Won Kim S. Clinical characteristics and psychiatric comorbidity of pyromania. J Clin Psychiatry. 2007;68(11):1717-1722.
56. Grant JE, Odlaug B. Assessment and treatment of pyromania. In: Oxford handbook of impulse control disorders. Grant JE, Potenza MN, eds. Oxford, United Kingdom: Oxford University Press; 2012:353-359.
57. Dell’Osso B, Altamura AC, Allen A, et al. Epidemiologic and clinical updates on impulse control disorders: a critical review. Eur Arch Psychiatry Clin Neurosci. 2006;256(8):464-475.
Akathisia: Is restlessness a primary condition or an adverse drug effect?
Akathisia—from the Greek for “inability to sit”—is a neuropsychiatric syndrome characterized by subjective and objective psychomotor restlessness. Patients typically experience feelings of unease, inner restlessness mainly involving the legs, and a compulsion to move. Most engage in repetitive movement. They might swing or cross and uncross their legs, shift from one foot to the other, continuously pace, or persistently fidget.
In clinical settings, akathisia usually is a side effect of medication. Antipsychotics, serotonin reuptake inhibitors, and buspirone are common triggers, but akathisia also has been associated with some antiemetics, preoperative sedatives, calcium channel blockers, and antivertigo agents. It also can be caused by withdrawal from an antipsychotic or related to a substance use disorder, especially cocaine. Akathisia can be acute or chronic, occurring in a tardive form with symptoms that last >6 months.1-3
Much isn’t known about drug-induced akathisia
Our understanding of the pathophysiology of akathisia is incomplete. Some have suggested that it results from an imbalance between the dopaminergic/cholinergic and dopaminergic/serotonergic systems4; others, that the cause is a mismatch between the core and the shell of the nucleus accumbens, due in part to overstimulation of the locus ceruleus.5
More recently, researchers established a positive association between higher scores on the Liverpool University Neuroleptic Side Effects Rating Scale and D2/D3 receptor occupancy in the ventral striatum (nucleus accumbens and olfactory tubercle).6 The D2/D3 receptor occupancy model might explain withdrawal symptoms associated with cocaine,7 as well as relative worsening of symptoms after tapering or discontinuing stimulants in attention-deficit/hyperactivity disorder (ADHD).
Elements of a clinical evaluation
When akathisia is suspected, evaluation by a clinician familiar with its phenomenology is crucial. A validated tool, such as the Barnes Akathisia Rating Scale (at out cometracker.org/library/BAS.pdf) can aid in the detection and assessment of severity.8
In evaluating patients, keep in mind that the inner restlessness that characterizes akathisia can affect the trunk, hands, and arms, as well as the legs, and can cause dysphoria and anxiety. Akathisia has been linked to an increased likelihood of developing suicidal ideation and behavior.9
Less common subjective symptoms include rage, fear, nausea, and worsening of psychotic symptoms. Because of its association with aggression and agitation, drug-induced akathisia has been cited—with little success—as the basis for an insanity defense by people who have committed a violent act.10
Or is akathisia another psychiatric disorder?
Akathisia might go undetected for several reasons. One key factor: Its symptoms resemble and often overlap with those of other psychiatric disorders, such as mania, psychosis, agitated depression, and ADHD. In addition, akathisia often occurs concurrently with, and is masked by, akinesia, a common extrapyramidal side effect of many antipsychotics. Such patients might have the inner feeling of restlessness and urge to move but do not exhibit characteristic limb movements. In some cases, cognitive or intellectual limitations prevent patients from communicating the inner turmoil they feel.11
Medication nonadherence further complicates the picture, sometimes prompting a clinician to increase the dosage of the drug that is causing akathisia (Box 112).
Managing drug-induced akathisia
Akathisia usually resolves when the drug causing it is discontinued; decreasing the dosage might alleviate the symptoms. Whenever akathisia is detected, careful revision of the current drug regimen— substituting an antipsychotic with a lower prevalence of akathisia, for example— should be considered (Box 213-16). Treatment of drug-induced akathisia, which should be tailored to the patient’s psychopathology and comorbidities, is needed as well (Table17-25).
Beta blockers, particularly propranolol, are considered first-line therapy for drug-induced akathisia, with a dosage of 20 to 40 mg twice daily used to relieve symptoms26 The effect can be explained by adrenergic terminals in the locus ceruleus and ending in the nucleus accumbens and prefrontal cortex stimulate β adrenoreceptors.5,27 Although multiple small studies and case reports26,28-32 support the use of beta blockers to treat drug-induced akathisia, the quality of evidence of their efficacy is controversial.12,21,27 Consider the risk of hypotension and bradycardia and be aware of contraindications for patients with asthma or diabetes.
Low-dose mirtazapine (15 mg/d) was found to be as effective as propranolol, 80 mg/d, in a placebo-controlled study, and to be more effective than a beta blocker in treating akathisia induced by a first-generation antipsychotic. The authors concluded that both propranolol and mirtazapine should be first-line therapy.23 Others have suggested that these results be interpreted with caution because mirtazapine (at a higher dosage) has been linked to akathisia.33 Mirtazapine blocks α-adrenergic receptors, resulting in antagonism of 5-HT2 and 5-HT3 receptors and consequent enhancement of 5-HT1A serotonergic transmission.34 In one study, it was shown to reduce binding of the D2/D3 receptor agonist quinpirole.35
Serotonin antagonists and agonists. Blockade of 5-HT2 receptors can attenuate D2 blockade and mitigate akathisia symptoms. Mianserin, 15 mg/d, can be helpful, and ritanserin, 5 to 20 mg/d, produced about a 50% reduction in akathisia symptoms in 10 patients taking neuroleptics.36 Neither is available in the United States, however.
Cyproheptadine, a potent 5-HT2A and 5-HT2C antagonist with anticholinergic and antihistaminic action, improved akathisia symptoms in an open trial of 17 patients with antipsychotic-induced akathisia.37 The recommended dose is 8 to 16 mg/d.
A study using the selective inverse agonist pimavanserin (not FDA-approved) decreased akathisia in healthy volunteers taking haloperidol.14,24,33
Zolmitriptan, a 5-HT1D agonist, also can be used38; one study found that 7.5 mg/d of zolmitriptan is as effective as propranolol.39
A 2010 study showed a statistically significant improvement in 8 patients taking trazodone, compared with 5 patients on placebo, all of whom met criteria for at least mild akathisia. Trazodone’s antiakathitic effect is attributed to its 5-HT2A antagonism.25
Anticholinergics. Traditionally, benztropine, biperiden, diphenhydramine, and trihexyphenidyl have been used for prevention and treatment of extrapyramidal side effects. A Cochrane review concluded, however, that data are insufficient to support use of anticholinergics for akathisia.40 Although multiple case reports have shown anticholinergics to be effective in treating drug-induced akathisia,12,17,33 their association with cognitive side effects suggests a need for caution.18
Benzodiazepines. Through their sedative and anxiolytic properties, benzodiazepines are thought to partially alleviate akathisia symptoms. Two small trials found clonazepam helpful for akathisia symptoms2,20; and 1 case report revealed that a patient with akathisia improved after coadministration of clonazepam and baclofen.41
Anticonvulsants. Valproic acid has not been found to be useful in antipsychotic-induced tardive akathisia.42 However, a case report described a patient with schizophrenia whose akathisia symptoms improved after the dosage of gabapentin was increased.43 Last, carbamazepine was found to be effective in reducing akathisia symptoms in 3 patients with schizophrenia who were resistant to beta blockers, anticholinergics, antihistaminergics, and benzodiazepines.19
α-adrenergic agonists. In an open trial, akathisia symptoms in 6 patients improved with clonidine, 0.2 to 0.8 mg/d.17 Speculation is that strong α1 antagonism might help prevent akathisia, which could be why this condition is not associated with iloperidone.44
D2 agonists. Akathisia and restless legs syndrome have similar pathophysiology,1,2 and patients with akathisia could benefit from D2 agonists such as cabergoline, pramipexole, rotigotine, and ropinirole. One case study revealed that a patient with aripiprazole-induced akathisia improved with ropinirole.45 D2 agonists can precipitate or worsen psychosis, however, and would be a relative contraindication in patients with psychotic disorders.22
Bottom Line
Failure to detect drug-induced akathisia can increase morbidity and delay recovery in patients undergoing psychiatric care. Knowing what to look for and how to tailor treatment to the needs of a given patient is an essential component of good care.
Related Resources
• Ferrando SJ, Eisendrath SJ. Adverse neuropsychiatric effects of dopamine antagonist medications. Misdiagnosis in the medical setting. Psychosomatics. 1991;32(4):426-432.
• Vinson DR. Diphenhydramine in the treatment of akathisia induced by prochlorperazine. J Emerg Med. 2004;26(3):265-270.
Drug Brand Names
Aripiprazole • Abilify Haloperidol • Haldol
Baclofen • Lioresal Iloperidone • Fanapt
Benztropine • Cogentin Lurasidone • Latuda
Biperiden • Akineton Mirtazapine • Remeron
Buspirone • BuSpar Pramipexole • Mirapex
Cabergoline • Dostinex Propranolol • Inderal
Carbamazepine • Tegretol Quetiapine • Seroquel
Clonazepam • Klonopin Ropinirole • Requip
Clonidine • Catapres Rotigotine • Neupro
Clozapine • Clozaril Trazodone • Desyrel, Oleptro
Cyproheptadine • Periactin Trihexyphenidyl • Artane
Diphenhydramine • Benadryl Valproic acid • Depakene
Gabapentin • Neurontin Zolmitriptan • Zomig
Acknowledgement
Mandy Evans, MD, assisted with editing the manuscript of this article.
Disclosure
Dr. Forcen reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Sachdev P. Akathisia and restless legs. Cambridge, United Kingdom: Cambridge University Press; 1995.
2. Sachdev P, Longragan C. The present status of akathisia. J Nerv Ment Dis. 1991;179(7):381-391.
3. Poyurovsky M, Hermesh H, Weizman A. Severe withdrawal akathisia following neuroleptic discontinuation successfully controlled by clozapine. Int Clin Psychopharmacol. 1996;11(4):283-286.
4. Poyurovsky M, Weizman A. Serotonin-based pharma-cotherapy for acute neuroleptic-induced akathisia: a new approach to an old problem. Br J Psychiatry. 2001;179:4-8.
5. Loonen AJ, Stahl SM. The mechanism of drug-induced akathisia. CNS Spectr. 2011;16(1):7-10.
6. Kim JH, Son YD, Kim HK, et al. Antipsychotic-associated mental side effects and their relationship to dopamine D2 receptor occupancy in striatal subdivisions: a high-resolution PET study with [11C]raclopride. J Clin Psychopharmacol. 2011;31(4):507-511.
7. Dailey JW, Fryer TD, Brichard L, et al. Nucleus accumbens D2/3 receptor predict trait impulsivity and cocaine reinforcement. Science. 2007;315(5816):1267-1270.
8. Barnes TR, Braude WM. Akathisia variants and tardive dyskinesia. Arch Gen Psychiatry. 1985;42(9):874-878.
9. Seemüller F, Schennach R, Mayr A, et al. Akathisia and suicidal ideation in first-episode schizophrenia. J Clin Psychopharmacol. 2012;32(5):694-698.
10. Leong GB, Silva JA. Neuroleptic-induced akathisia and violence: a review. J Forensic Sci. 2003;48(1):187-189.
11. Hirose S. The causes of underdiagnosing akathisia. Schizophr Bull. 2003;29(3):547-558.
12. Velligan DI, Weiden PJ, Sajatovic M, et al; Expert Consensus Panel on Adherence Problems in Serious and Persistent Mental Illness. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry. 2009;70(suppl 4):S1-S46; quiz 47-48.
13. Citrome L. A review of the pharmacology, efficacy and tolerability of recently approved and upcoming oral antipsychotics: an evidence-based medicine approach. CNS Drugs. 2013;27(11):879-911.
14. Poyurovsky M. Acute antipsychotic-induced akathisia revisited. Br J Psychiatry. 2010;196(2):89-91.
15. Saltz BL, Robinson DG, Woerner MG. Recognizing and managing antipsychotic drug treatment side effects in the elderly. Prim Care Companion J Clin Psychiatry. 2004;6(suppl 2):14-19.
16. Lieberman JA, Stroup TS. The NIMH-CATIE Schizophrenia Study: what did we learn? Am J Psychiatry. 2011;168(8):770-775.
17. Zubenko GS, Cohen BM, Lipinski JF Jr, et al. Use of clonidine in treating neuroleptic-induced akathisia. Psychiatry Res. 1984;13(3):253-259.
18. Vinogradov S, Fisher M, Warm H, et al. The cognitive cost of anticholinergic burden: decreased response to cognitive training in schizophrenia. Am J Psychiatry. 2009;166(9):1055-1062.
19. Masui T, Kusumi I, Takahashi Y, et al. Efficacy of carbamazepine against neuroleptic-induced akathisia in treatment with perospirone: case series. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(2):343-346.
20. Lima AR, Soares-Weiser K, Bacaltchuk J, et al. Benzodiazepines for neuroleptic-induced acute akathisia. Cochrane Database Syst Rev. 2002;(1):CD001950.
21. Lima AR, Bacalcthuk J, Barnes TR, et al. Central action beta-blockers versus placebo for neuroleptic-induced acute akathisia. Cochrane Database Syst Rev. 2004;(4):CD001946.
22. Bilal L, Ching C. Cabergoline-induced psychosis in a patient with undiagnosed depression. J Neuropsychiatry Clin Neurosci. 2012;24(4):E54.
23. Poyurovsky M, Pashinian A, Weizman A, et al. Low-dose mirtazapine: a new option in the treatment of antipsychotic-induced akathisia. A randomized, double-blind, placebo- and propranolol-controlled trial. Biol Psychiatry.
2006;59(11):1071-1077.
24. Maidment I. Use of serotonin antagonists in the treatment of neuroleptic-induced akathisia. Psychiatric Bulletin. 2000;24(9):348-351.
25. Stryjer R, Rosenzcwaig S, Bar F, et al. Trazodone for the treatment of neuroleptic-induced akathisia: a placebo-controlled, double-blind, crossover study. Clin Neuropharmacol. 2010;33(5):219-222.
26. Dumon JP, Catteau J, Lanvin F, et al. Randomized, double-blind, crossover, placebo-controlled comparison of propranolol and betaxolol in the treatment of neuroleptic-induced akathisia. Am J Psychiatry. 1992;149(5):647-650.
27. van Waarde A, Vaalburg W, Doze P, et al. PET imaging of beta-adrenoceptors in the human brain: a realistic goal or a mirage? Curr Pharm Des. 2004;10(13):1519-1536.
28. Kurzthaler I, Hummer M, Kohl C, et al. Propranolol treatment of olanzapine-induced akathisia. Am J Psychiatry. 1997;154(9):1316.
29. Adler LA, Peselow E, Rosenthal MA, et al. A controlled comparison of the effects of propranolol, benztropine, and placebo on akathisia: an interim analysis. Psychopharmacol Bull. 1993;29(2):283-286.
30. Dorevitch A, Durst R, Ginath Y. Propranolol in the treatment of akathisia caused by antipsychotic drugs. South Med J. 1991;84(12):1505-1506.
31. Lipinski JF Jr, Zubenko GS, Cohen BM, et al. Propranolol in the treatment of neuroleptic-induced akathisia. Am J Psychiatry. 1984;141(3):412-415.
32. Adler L, Angrist B, Peselow E, et al. A controlled assessment of propranolol in the treatment of neuroleptic-induced akathisia. Br J Psychiatry. 1986;149:42-45.
33. Kumar R, Sachdev PS. Akathisia and second-generation antipsychotic drugs. Curr Opin Psychiatry. 2009;22(3):293-299.
34. Anttila SA, Leinonen EV. A review of the pharmacological and clinical profile of mirtazapine. CNS Drug Rev. 2001;7(3):249-264.
35. Rogóz Z, Wróbel A, Dlaboga D, et al. Effect of repeated treatment with mirtazapine on the central dopaminergic D2/D3 receptors. Pol J Pharmacol. 2002;54(4):381-389.
36. Miller CH, Fleischhacker WW, Ehrmann H, et al. Treatment of neuroleptic induced akathisia with the 5-HT2 antagonist ritanserin. Psychopharmacol Bull. 1990;26(3):373-376.
37. Weiss D, Aizenberg D, Hermesh H, et al. Cyproheptadine treatment in neuroleptic-induced akathisia. Br J Psychiatry. 1995;167(4):483-486.
38. Gross-Isseroff R, Magen A, Shiloh R, et al. The 5-HT1D receptor agonist zolmitriptan for neuroleptic-induced akathisia: an open label preliminary study. Int Clin Psychopharmacol. 2005;20(1):23-25.
39. Avital A, Gross-Isseroff R, Stryjer R, et al. Zolmitriptan compared to propranolol in the treatment of acute neuroleptic-induced akathisia: a comparative double-blind study. Eur Neuropsychopharmacol. 2009;19(7):476-482.
40. Rathbone J, Soares-Weiser K. Anticholinergics for neuroleptic-induced acute akathisia. Cochrane Database Syst Rev. 2006;(4):CD003727.
41. Sandyk R. Successful treatment of neuroleptic-induced akathisia with baclofen and clonazepam. A case report. Eur Neurol. 1985;24(4):286-288.
42. Miller CH, Fleischhacker W. Managing antipsychotic-induced acute and chronic akathisia. Drug Saf. 2000;22(1):73-81.
43. Pfeffer G, Chouinard G, Margolese HC. Gabapentin in the treatment of antipsychotic-induced akathisia in schizophrenia. Int Clin Psychopharmacol. 2005;20(3):179-181.
44. Stahl SM. Role of α1 adrenergic antagonism in the mechanism of action of iloperidone: reducing extrapyramidal symptoms. CNS Spectr. 2013;18(6):285-258.
45. Hettema JM, Ross DE. A case of aripiprazole-related tardive akathisia and its treatment with ropinirole. J Clin Psychiatry. 2007;68(11):1814-1815.
Akathisia—from the Greek for “inability to sit”—is a neuropsychiatric syndrome characterized by subjective and objective psychomotor restlessness. Patients typically experience feelings of unease, inner restlessness mainly involving the legs, and a compulsion to move. Most engage in repetitive movement. They might swing or cross and uncross their legs, shift from one foot to the other, continuously pace, or persistently fidget.
In clinical settings, akathisia usually is a side effect of medication. Antipsychotics, serotonin reuptake inhibitors, and buspirone are common triggers, but akathisia also has been associated with some antiemetics, preoperative sedatives, calcium channel blockers, and antivertigo agents. It also can be caused by withdrawal from an antipsychotic or related to a substance use disorder, especially cocaine. Akathisia can be acute or chronic, occurring in a tardive form with symptoms that last >6 months.1-3
Much isn’t known about drug-induced akathisia
Our understanding of the pathophysiology of akathisia is incomplete. Some have suggested that it results from an imbalance between the dopaminergic/cholinergic and dopaminergic/serotonergic systems4; others, that the cause is a mismatch between the core and the shell of the nucleus accumbens, due in part to overstimulation of the locus ceruleus.5
More recently, researchers established a positive association between higher scores on the Liverpool University Neuroleptic Side Effects Rating Scale and D2/D3 receptor occupancy in the ventral striatum (nucleus accumbens and olfactory tubercle).6 The D2/D3 receptor occupancy model might explain withdrawal symptoms associated with cocaine,7 as well as relative worsening of symptoms after tapering or discontinuing stimulants in attention-deficit/hyperactivity disorder (ADHD).
Elements of a clinical evaluation
When akathisia is suspected, evaluation by a clinician familiar with its phenomenology is crucial. A validated tool, such as the Barnes Akathisia Rating Scale (at out cometracker.org/library/BAS.pdf) can aid in the detection and assessment of severity.8
In evaluating patients, keep in mind that the inner restlessness that characterizes akathisia can affect the trunk, hands, and arms, as well as the legs, and can cause dysphoria and anxiety. Akathisia has been linked to an increased likelihood of developing suicidal ideation and behavior.9
Less common subjective symptoms include rage, fear, nausea, and worsening of psychotic symptoms. Because of its association with aggression and agitation, drug-induced akathisia has been cited—with little success—as the basis for an insanity defense by people who have committed a violent act.10
Or is akathisia another psychiatric disorder?
Akathisia might go undetected for several reasons. One key factor: Its symptoms resemble and often overlap with those of other psychiatric disorders, such as mania, psychosis, agitated depression, and ADHD. In addition, akathisia often occurs concurrently with, and is masked by, akinesia, a common extrapyramidal side effect of many antipsychotics. Such patients might have the inner feeling of restlessness and urge to move but do not exhibit characteristic limb movements. In some cases, cognitive or intellectual limitations prevent patients from communicating the inner turmoil they feel.11
Medication nonadherence further complicates the picture, sometimes prompting a clinician to increase the dosage of the drug that is causing akathisia (Box 112).
Managing drug-induced akathisia
Akathisia usually resolves when the drug causing it is discontinued; decreasing the dosage might alleviate the symptoms. Whenever akathisia is detected, careful revision of the current drug regimen— substituting an antipsychotic with a lower prevalence of akathisia, for example— should be considered (Box 213-16). Treatment of drug-induced akathisia, which should be tailored to the patient’s psychopathology and comorbidities, is needed as well (Table17-25).
Beta blockers, particularly propranolol, are considered first-line therapy for drug-induced akathisia, with a dosage of 20 to 40 mg twice daily used to relieve symptoms26 The effect can be explained by adrenergic terminals in the locus ceruleus and ending in the nucleus accumbens and prefrontal cortex stimulate β adrenoreceptors.5,27 Although multiple small studies and case reports26,28-32 support the use of beta blockers to treat drug-induced akathisia, the quality of evidence of their efficacy is controversial.12,21,27 Consider the risk of hypotension and bradycardia and be aware of contraindications for patients with asthma or diabetes.
Low-dose mirtazapine (15 mg/d) was found to be as effective as propranolol, 80 mg/d, in a placebo-controlled study, and to be more effective than a beta blocker in treating akathisia induced by a first-generation antipsychotic. The authors concluded that both propranolol and mirtazapine should be first-line therapy.23 Others have suggested that these results be interpreted with caution because mirtazapine (at a higher dosage) has been linked to akathisia.33 Mirtazapine blocks α-adrenergic receptors, resulting in antagonism of 5-HT2 and 5-HT3 receptors and consequent enhancement of 5-HT1A serotonergic transmission.34 In one study, it was shown to reduce binding of the D2/D3 receptor agonist quinpirole.35
Serotonin antagonists and agonists. Blockade of 5-HT2 receptors can attenuate D2 blockade and mitigate akathisia symptoms. Mianserin, 15 mg/d, can be helpful, and ritanserin, 5 to 20 mg/d, produced about a 50% reduction in akathisia symptoms in 10 patients taking neuroleptics.36 Neither is available in the United States, however.
Cyproheptadine, a potent 5-HT2A and 5-HT2C antagonist with anticholinergic and antihistaminic action, improved akathisia symptoms in an open trial of 17 patients with antipsychotic-induced akathisia.37 The recommended dose is 8 to 16 mg/d.
A study using the selective inverse agonist pimavanserin (not FDA-approved) decreased akathisia in healthy volunteers taking haloperidol.14,24,33
Zolmitriptan, a 5-HT1D agonist, also can be used38; one study found that 7.5 mg/d of zolmitriptan is as effective as propranolol.39
A 2010 study showed a statistically significant improvement in 8 patients taking trazodone, compared with 5 patients on placebo, all of whom met criteria for at least mild akathisia. Trazodone’s antiakathitic effect is attributed to its 5-HT2A antagonism.25
Anticholinergics. Traditionally, benztropine, biperiden, diphenhydramine, and trihexyphenidyl have been used for prevention and treatment of extrapyramidal side effects. A Cochrane review concluded, however, that data are insufficient to support use of anticholinergics for akathisia.40 Although multiple case reports have shown anticholinergics to be effective in treating drug-induced akathisia,12,17,33 their association with cognitive side effects suggests a need for caution.18
Benzodiazepines. Through their sedative and anxiolytic properties, benzodiazepines are thought to partially alleviate akathisia symptoms. Two small trials found clonazepam helpful for akathisia symptoms2,20; and 1 case report revealed that a patient with akathisia improved after coadministration of clonazepam and baclofen.41
Anticonvulsants. Valproic acid has not been found to be useful in antipsychotic-induced tardive akathisia.42 However, a case report described a patient with schizophrenia whose akathisia symptoms improved after the dosage of gabapentin was increased.43 Last, carbamazepine was found to be effective in reducing akathisia symptoms in 3 patients with schizophrenia who were resistant to beta blockers, anticholinergics, antihistaminergics, and benzodiazepines.19
α-adrenergic agonists. In an open trial, akathisia symptoms in 6 patients improved with clonidine, 0.2 to 0.8 mg/d.17 Speculation is that strong α1 antagonism might help prevent akathisia, which could be why this condition is not associated with iloperidone.44
D2 agonists. Akathisia and restless legs syndrome have similar pathophysiology,1,2 and patients with akathisia could benefit from D2 agonists such as cabergoline, pramipexole, rotigotine, and ropinirole. One case study revealed that a patient with aripiprazole-induced akathisia improved with ropinirole.45 D2 agonists can precipitate or worsen psychosis, however, and would be a relative contraindication in patients with psychotic disorders.22
Bottom Line
Failure to detect drug-induced akathisia can increase morbidity and delay recovery in patients undergoing psychiatric care. Knowing what to look for and how to tailor treatment to the needs of a given patient is an essential component of good care.
Related Resources
• Ferrando SJ, Eisendrath SJ. Adverse neuropsychiatric effects of dopamine antagonist medications. Misdiagnosis in the medical setting. Psychosomatics. 1991;32(4):426-432.
• Vinson DR. Diphenhydramine in the treatment of akathisia induced by prochlorperazine. J Emerg Med. 2004;26(3):265-270.
Drug Brand Names
Aripiprazole • Abilify Haloperidol • Haldol
Baclofen • Lioresal Iloperidone • Fanapt
Benztropine • Cogentin Lurasidone • Latuda
Biperiden • Akineton Mirtazapine • Remeron
Buspirone • BuSpar Pramipexole • Mirapex
Cabergoline • Dostinex Propranolol • Inderal
Carbamazepine • Tegretol Quetiapine • Seroquel
Clonazepam • Klonopin Ropinirole • Requip
Clonidine • Catapres Rotigotine • Neupro
Clozapine • Clozaril Trazodone • Desyrel, Oleptro
Cyproheptadine • Periactin Trihexyphenidyl • Artane
Diphenhydramine • Benadryl Valproic acid • Depakene
Gabapentin • Neurontin Zolmitriptan • Zomig
Acknowledgement
Mandy Evans, MD, assisted with editing the manuscript of this article.
Disclosure
Dr. Forcen reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Akathisia—from the Greek for “inability to sit”—is a neuropsychiatric syndrome characterized by subjective and objective psychomotor restlessness. Patients typically experience feelings of unease, inner restlessness mainly involving the legs, and a compulsion to move. Most engage in repetitive movement. They might swing or cross and uncross their legs, shift from one foot to the other, continuously pace, or persistently fidget.
In clinical settings, akathisia usually is a side effect of medication. Antipsychotics, serotonin reuptake inhibitors, and buspirone are common triggers, but akathisia also has been associated with some antiemetics, preoperative sedatives, calcium channel blockers, and antivertigo agents. It also can be caused by withdrawal from an antipsychotic or related to a substance use disorder, especially cocaine. Akathisia can be acute or chronic, occurring in a tardive form with symptoms that last >6 months.1-3
Much isn’t known about drug-induced akathisia
Our understanding of the pathophysiology of akathisia is incomplete. Some have suggested that it results from an imbalance between the dopaminergic/cholinergic and dopaminergic/serotonergic systems4; others, that the cause is a mismatch between the core and the shell of the nucleus accumbens, due in part to overstimulation of the locus ceruleus.5
More recently, researchers established a positive association between higher scores on the Liverpool University Neuroleptic Side Effects Rating Scale and D2/D3 receptor occupancy in the ventral striatum (nucleus accumbens and olfactory tubercle).6 The D2/D3 receptor occupancy model might explain withdrawal symptoms associated with cocaine,7 as well as relative worsening of symptoms after tapering or discontinuing stimulants in attention-deficit/hyperactivity disorder (ADHD).
Elements of a clinical evaluation
When akathisia is suspected, evaluation by a clinician familiar with its phenomenology is crucial. A validated tool, such as the Barnes Akathisia Rating Scale (at out cometracker.org/library/BAS.pdf) can aid in the detection and assessment of severity.8
In evaluating patients, keep in mind that the inner restlessness that characterizes akathisia can affect the trunk, hands, and arms, as well as the legs, and can cause dysphoria and anxiety. Akathisia has been linked to an increased likelihood of developing suicidal ideation and behavior.9
Less common subjective symptoms include rage, fear, nausea, and worsening of psychotic symptoms. Because of its association with aggression and agitation, drug-induced akathisia has been cited—with little success—as the basis for an insanity defense by people who have committed a violent act.10
Or is akathisia another psychiatric disorder?
Akathisia might go undetected for several reasons. One key factor: Its symptoms resemble and often overlap with those of other psychiatric disorders, such as mania, psychosis, agitated depression, and ADHD. In addition, akathisia often occurs concurrently with, and is masked by, akinesia, a common extrapyramidal side effect of many antipsychotics. Such patients might have the inner feeling of restlessness and urge to move but do not exhibit characteristic limb movements. In some cases, cognitive or intellectual limitations prevent patients from communicating the inner turmoil they feel.11
Medication nonadherence further complicates the picture, sometimes prompting a clinician to increase the dosage of the drug that is causing akathisia (Box 112).
Managing drug-induced akathisia
Akathisia usually resolves when the drug causing it is discontinued; decreasing the dosage might alleviate the symptoms. Whenever akathisia is detected, careful revision of the current drug regimen— substituting an antipsychotic with a lower prevalence of akathisia, for example— should be considered (Box 213-16). Treatment of drug-induced akathisia, which should be tailored to the patient’s psychopathology and comorbidities, is needed as well (Table17-25).
Beta blockers, particularly propranolol, are considered first-line therapy for drug-induced akathisia, with a dosage of 20 to 40 mg twice daily used to relieve symptoms26 The effect can be explained by adrenergic terminals in the locus ceruleus and ending in the nucleus accumbens and prefrontal cortex stimulate β adrenoreceptors.5,27 Although multiple small studies and case reports26,28-32 support the use of beta blockers to treat drug-induced akathisia, the quality of evidence of their efficacy is controversial.12,21,27 Consider the risk of hypotension and bradycardia and be aware of contraindications for patients with asthma or diabetes.
Low-dose mirtazapine (15 mg/d) was found to be as effective as propranolol, 80 mg/d, in a placebo-controlled study, and to be more effective than a beta blocker in treating akathisia induced by a first-generation antipsychotic. The authors concluded that both propranolol and mirtazapine should be first-line therapy.23 Others have suggested that these results be interpreted with caution because mirtazapine (at a higher dosage) has been linked to akathisia.33 Mirtazapine blocks α-adrenergic receptors, resulting in antagonism of 5-HT2 and 5-HT3 receptors and consequent enhancement of 5-HT1A serotonergic transmission.34 In one study, it was shown to reduce binding of the D2/D3 receptor agonist quinpirole.35
Serotonin antagonists and agonists. Blockade of 5-HT2 receptors can attenuate D2 blockade and mitigate akathisia symptoms. Mianserin, 15 mg/d, can be helpful, and ritanserin, 5 to 20 mg/d, produced about a 50% reduction in akathisia symptoms in 10 patients taking neuroleptics.36 Neither is available in the United States, however.
Cyproheptadine, a potent 5-HT2A and 5-HT2C antagonist with anticholinergic and antihistaminic action, improved akathisia symptoms in an open trial of 17 patients with antipsychotic-induced akathisia.37 The recommended dose is 8 to 16 mg/d.
A study using the selective inverse agonist pimavanserin (not FDA-approved) decreased akathisia in healthy volunteers taking haloperidol.14,24,33
Zolmitriptan, a 5-HT1D agonist, also can be used38; one study found that 7.5 mg/d of zolmitriptan is as effective as propranolol.39
A 2010 study showed a statistically significant improvement in 8 patients taking trazodone, compared with 5 patients on placebo, all of whom met criteria for at least mild akathisia. Trazodone’s antiakathitic effect is attributed to its 5-HT2A antagonism.25
Anticholinergics. Traditionally, benztropine, biperiden, diphenhydramine, and trihexyphenidyl have been used for prevention and treatment of extrapyramidal side effects. A Cochrane review concluded, however, that data are insufficient to support use of anticholinergics for akathisia.40 Although multiple case reports have shown anticholinergics to be effective in treating drug-induced akathisia,12,17,33 their association with cognitive side effects suggests a need for caution.18
Benzodiazepines. Through their sedative and anxiolytic properties, benzodiazepines are thought to partially alleviate akathisia symptoms. Two small trials found clonazepam helpful for akathisia symptoms2,20; and 1 case report revealed that a patient with akathisia improved after coadministration of clonazepam and baclofen.41
Anticonvulsants. Valproic acid has not been found to be useful in antipsychotic-induced tardive akathisia.42 However, a case report described a patient with schizophrenia whose akathisia symptoms improved after the dosage of gabapentin was increased.43 Last, carbamazepine was found to be effective in reducing akathisia symptoms in 3 patients with schizophrenia who were resistant to beta blockers, anticholinergics, antihistaminergics, and benzodiazepines.19
α-adrenergic agonists. In an open trial, akathisia symptoms in 6 patients improved with clonidine, 0.2 to 0.8 mg/d.17 Speculation is that strong α1 antagonism might help prevent akathisia, which could be why this condition is not associated with iloperidone.44
D2 agonists. Akathisia and restless legs syndrome have similar pathophysiology,1,2 and patients with akathisia could benefit from D2 agonists such as cabergoline, pramipexole, rotigotine, and ropinirole. One case study revealed that a patient with aripiprazole-induced akathisia improved with ropinirole.45 D2 agonists can precipitate or worsen psychosis, however, and would be a relative contraindication in patients with psychotic disorders.22
Bottom Line
Failure to detect drug-induced akathisia can increase morbidity and delay recovery in patients undergoing psychiatric care. Knowing what to look for and how to tailor treatment to the needs of a given patient is an essential component of good care.
Related Resources
• Ferrando SJ, Eisendrath SJ. Adverse neuropsychiatric effects of dopamine antagonist medications. Misdiagnosis in the medical setting. Psychosomatics. 1991;32(4):426-432.
• Vinson DR. Diphenhydramine in the treatment of akathisia induced by prochlorperazine. J Emerg Med. 2004;26(3):265-270.
Drug Brand Names
Aripiprazole • Abilify Haloperidol • Haldol
Baclofen • Lioresal Iloperidone • Fanapt
Benztropine • Cogentin Lurasidone • Latuda
Biperiden • Akineton Mirtazapine • Remeron
Buspirone • BuSpar Pramipexole • Mirapex
Cabergoline • Dostinex Propranolol • Inderal
Carbamazepine • Tegretol Quetiapine • Seroquel
Clonazepam • Klonopin Ropinirole • Requip
Clonidine • Catapres Rotigotine • Neupro
Clozapine • Clozaril Trazodone • Desyrel, Oleptro
Cyproheptadine • Periactin Trihexyphenidyl • Artane
Diphenhydramine • Benadryl Valproic acid • Depakene
Gabapentin • Neurontin Zolmitriptan • Zomig
Acknowledgement
Mandy Evans, MD, assisted with editing the manuscript of this article.
Disclosure
Dr. Forcen reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Sachdev P. Akathisia and restless legs. Cambridge, United Kingdom: Cambridge University Press; 1995.
2. Sachdev P, Longragan C. The present status of akathisia. J Nerv Ment Dis. 1991;179(7):381-391.
3. Poyurovsky M, Hermesh H, Weizman A. Severe withdrawal akathisia following neuroleptic discontinuation successfully controlled by clozapine. Int Clin Psychopharmacol. 1996;11(4):283-286.
4. Poyurovsky M, Weizman A. Serotonin-based pharma-cotherapy for acute neuroleptic-induced akathisia: a new approach to an old problem. Br J Psychiatry. 2001;179:4-8.
5. Loonen AJ, Stahl SM. The mechanism of drug-induced akathisia. CNS Spectr. 2011;16(1):7-10.
6. Kim JH, Son YD, Kim HK, et al. Antipsychotic-associated mental side effects and their relationship to dopamine D2 receptor occupancy in striatal subdivisions: a high-resolution PET study with [11C]raclopride. J Clin Psychopharmacol. 2011;31(4):507-511.
7. Dailey JW, Fryer TD, Brichard L, et al. Nucleus accumbens D2/3 receptor predict trait impulsivity and cocaine reinforcement. Science. 2007;315(5816):1267-1270.
8. Barnes TR, Braude WM. Akathisia variants and tardive dyskinesia. Arch Gen Psychiatry. 1985;42(9):874-878.
9. Seemüller F, Schennach R, Mayr A, et al. Akathisia and suicidal ideation in first-episode schizophrenia. J Clin Psychopharmacol. 2012;32(5):694-698.
10. Leong GB, Silva JA. Neuroleptic-induced akathisia and violence: a review. J Forensic Sci. 2003;48(1):187-189.
11. Hirose S. The causes of underdiagnosing akathisia. Schizophr Bull. 2003;29(3):547-558.
12. Velligan DI, Weiden PJ, Sajatovic M, et al; Expert Consensus Panel on Adherence Problems in Serious and Persistent Mental Illness. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry. 2009;70(suppl 4):S1-S46; quiz 47-48.
13. Citrome L. A review of the pharmacology, efficacy and tolerability of recently approved and upcoming oral antipsychotics: an evidence-based medicine approach. CNS Drugs. 2013;27(11):879-911.
14. Poyurovsky M. Acute antipsychotic-induced akathisia revisited. Br J Psychiatry. 2010;196(2):89-91.
15. Saltz BL, Robinson DG, Woerner MG. Recognizing and managing antipsychotic drug treatment side effects in the elderly. Prim Care Companion J Clin Psychiatry. 2004;6(suppl 2):14-19.
16. Lieberman JA, Stroup TS. The NIMH-CATIE Schizophrenia Study: what did we learn? Am J Psychiatry. 2011;168(8):770-775.
17. Zubenko GS, Cohen BM, Lipinski JF Jr, et al. Use of clonidine in treating neuroleptic-induced akathisia. Psychiatry Res. 1984;13(3):253-259.
18. Vinogradov S, Fisher M, Warm H, et al. The cognitive cost of anticholinergic burden: decreased response to cognitive training in schizophrenia. Am J Psychiatry. 2009;166(9):1055-1062.
19. Masui T, Kusumi I, Takahashi Y, et al. Efficacy of carbamazepine against neuroleptic-induced akathisia in treatment with perospirone: case series. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(2):343-346.
20. Lima AR, Soares-Weiser K, Bacaltchuk J, et al. Benzodiazepines for neuroleptic-induced acute akathisia. Cochrane Database Syst Rev. 2002;(1):CD001950.
21. Lima AR, Bacalcthuk J, Barnes TR, et al. Central action beta-blockers versus placebo for neuroleptic-induced acute akathisia. Cochrane Database Syst Rev. 2004;(4):CD001946.
22. Bilal L, Ching C. Cabergoline-induced psychosis in a patient with undiagnosed depression. J Neuropsychiatry Clin Neurosci. 2012;24(4):E54.
23. Poyurovsky M, Pashinian A, Weizman A, et al. Low-dose mirtazapine: a new option in the treatment of antipsychotic-induced akathisia. A randomized, double-blind, placebo- and propranolol-controlled trial. Biol Psychiatry.
2006;59(11):1071-1077.
24. Maidment I. Use of serotonin antagonists in the treatment of neuroleptic-induced akathisia. Psychiatric Bulletin. 2000;24(9):348-351.
25. Stryjer R, Rosenzcwaig S, Bar F, et al. Trazodone for the treatment of neuroleptic-induced akathisia: a placebo-controlled, double-blind, crossover study. Clin Neuropharmacol. 2010;33(5):219-222.
26. Dumon JP, Catteau J, Lanvin F, et al. Randomized, double-blind, crossover, placebo-controlled comparison of propranolol and betaxolol in the treatment of neuroleptic-induced akathisia. Am J Psychiatry. 1992;149(5):647-650.
27. van Waarde A, Vaalburg W, Doze P, et al. PET imaging of beta-adrenoceptors in the human brain: a realistic goal or a mirage? Curr Pharm Des. 2004;10(13):1519-1536.
28. Kurzthaler I, Hummer M, Kohl C, et al. Propranolol treatment of olanzapine-induced akathisia. Am J Psychiatry. 1997;154(9):1316.
29. Adler LA, Peselow E, Rosenthal MA, et al. A controlled comparison of the effects of propranolol, benztropine, and placebo on akathisia: an interim analysis. Psychopharmacol Bull. 1993;29(2):283-286.
30. Dorevitch A, Durst R, Ginath Y. Propranolol in the treatment of akathisia caused by antipsychotic drugs. South Med J. 1991;84(12):1505-1506.
31. Lipinski JF Jr, Zubenko GS, Cohen BM, et al. Propranolol in the treatment of neuroleptic-induced akathisia. Am J Psychiatry. 1984;141(3):412-415.
32. Adler L, Angrist B, Peselow E, et al. A controlled assessment of propranolol in the treatment of neuroleptic-induced akathisia. Br J Psychiatry. 1986;149:42-45.
33. Kumar R, Sachdev PS. Akathisia and second-generation antipsychotic drugs. Curr Opin Psychiatry. 2009;22(3):293-299.
34. Anttila SA, Leinonen EV. A review of the pharmacological and clinical profile of mirtazapine. CNS Drug Rev. 2001;7(3):249-264.
35. Rogóz Z, Wróbel A, Dlaboga D, et al. Effect of repeated treatment with mirtazapine on the central dopaminergic D2/D3 receptors. Pol J Pharmacol. 2002;54(4):381-389.
36. Miller CH, Fleischhacker WW, Ehrmann H, et al. Treatment of neuroleptic induced akathisia with the 5-HT2 antagonist ritanserin. Psychopharmacol Bull. 1990;26(3):373-376.
37. Weiss D, Aizenberg D, Hermesh H, et al. Cyproheptadine treatment in neuroleptic-induced akathisia. Br J Psychiatry. 1995;167(4):483-486.
38. Gross-Isseroff R, Magen A, Shiloh R, et al. The 5-HT1D receptor agonist zolmitriptan for neuroleptic-induced akathisia: an open label preliminary study. Int Clin Psychopharmacol. 2005;20(1):23-25.
39. Avital A, Gross-Isseroff R, Stryjer R, et al. Zolmitriptan compared to propranolol in the treatment of acute neuroleptic-induced akathisia: a comparative double-blind study. Eur Neuropsychopharmacol. 2009;19(7):476-482.
40. Rathbone J, Soares-Weiser K. Anticholinergics for neuroleptic-induced acute akathisia. Cochrane Database Syst Rev. 2006;(4):CD003727.
41. Sandyk R. Successful treatment of neuroleptic-induced akathisia with baclofen and clonazepam. A case report. Eur Neurol. 1985;24(4):286-288.
42. Miller CH, Fleischhacker W. Managing antipsychotic-induced acute and chronic akathisia. Drug Saf. 2000;22(1):73-81.
43. Pfeffer G, Chouinard G, Margolese HC. Gabapentin in the treatment of antipsychotic-induced akathisia in schizophrenia. Int Clin Psychopharmacol. 2005;20(3):179-181.
44. Stahl SM. Role of α1 adrenergic antagonism in the mechanism of action of iloperidone: reducing extrapyramidal symptoms. CNS Spectr. 2013;18(6):285-258.
45. Hettema JM, Ross DE. A case of aripiprazole-related tardive akathisia and its treatment with ropinirole. J Clin Psychiatry. 2007;68(11):1814-1815.
1. Sachdev P. Akathisia and restless legs. Cambridge, United Kingdom: Cambridge University Press; 1995.
2. Sachdev P, Longragan C. The present status of akathisia. J Nerv Ment Dis. 1991;179(7):381-391.
3. Poyurovsky M, Hermesh H, Weizman A. Severe withdrawal akathisia following neuroleptic discontinuation successfully controlled by clozapine. Int Clin Psychopharmacol. 1996;11(4):283-286.
4. Poyurovsky M, Weizman A. Serotonin-based pharma-cotherapy for acute neuroleptic-induced akathisia: a new approach to an old problem. Br J Psychiatry. 2001;179:4-8.
5. Loonen AJ, Stahl SM. The mechanism of drug-induced akathisia. CNS Spectr. 2011;16(1):7-10.
6. Kim JH, Son YD, Kim HK, et al. Antipsychotic-associated mental side effects and their relationship to dopamine D2 receptor occupancy in striatal subdivisions: a high-resolution PET study with [11C]raclopride. J Clin Psychopharmacol. 2011;31(4):507-511.
7. Dailey JW, Fryer TD, Brichard L, et al. Nucleus accumbens D2/3 receptor predict trait impulsivity and cocaine reinforcement. Science. 2007;315(5816):1267-1270.
8. Barnes TR, Braude WM. Akathisia variants and tardive dyskinesia. Arch Gen Psychiatry. 1985;42(9):874-878.
9. Seemüller F, Schennach R, Mayr A, et al. Akathisia and suicidal ideation in first-episode schizophrenia. J Clin Psychopharmacol. 2012;32(5):694-698.
10. Leong GB, Silva JA. Neuroleptic-induced akathisia and violence: a review. J Forensic Sci. 2003;48(1):187-189.
11. Hirose S. The causes of underdiagnosing akathisia. Schizophr Bull. 2003;29(3):547-558.
12. Velligan DI, Weiden PJ, Sajatovic M, et al; Expert Consensus Panel on Adherence Problems in Serious and Persistent Mental Illness. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry. 2009;70(suppl 4):S1-S46; quiz 47-48.
13. Citrome L. A review of the pharmacology, efficacy and tolerability of recently approved and upcoming oral antipsychotics: an evidence-based medicine approach. CNS Drugs. 2013;27(11):879-911.
14. Poyurovsky M. Acute antipsychotic-induced akathisia revisited. Br J Psychiatry. 2010;196(2):89-91.
15. Saltz BL, Robinson DG, Woerner MG. Recognizing and managing antipsychotic drug treatment side effects in the elderly. Prim Care Companion J Clin Psychiatry. 2004;6(suppl 2):14-19.
16. Lieberman JA, Stroup TS. The NIMH-CATIE Schizophrenia Study: what did we learn? Am J Psychiatry. 2011;168(8):770-775.
17. Zubenko GS, Cohen BM, Lipinski JF Jr, et al. Use of clonidine in treating neuroleptic-induced akathisia. Psychiatry Res. 1984;13(3):253-259.
18. Vinogradov S, Fisher M, Warm H, et al. The cognitive cost of anticholinergic burden: decreased response to cognitive training in schizophrenia. Am J Psychiatry. 2009;166(9):1055-1062.
19. Masui T, Kusumi I, Takahashi Y, et al. Efficacy of carbamazepine against neuroleptic-induced akathisia in treatment with perospirone: case series. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(2):343-346.
20. Lima AR, Soares-Weiser K, Bacaltchuk J, et al. Benzodiazepines for neuroleptic-induced acute akathisia. Cochrane Database Syst Rev. 2002;(1):CD001950.
21. Lima AR, Bacalcthuk J, Barnes TR, et al. Central action beta-blockers versus placebo for neuroleptic-induced acute akathisia. Cochrane Database Syst Rev. 2004;(4):CD001946.
22. Bilal L, Ching C. Cabergoline-induced psychosis in a patient with undiagnosed depression. J Neuropsychiatry Clin Neurosci. 2012;24(4):E54.
23. Poyurovsky M, Pashinian A, Weizman A, et al. Low-dose mirtazapine: a new option in the treatment of antipsychotic-induced akathisia. A randomized, double-blind, placebo- and propranolol-controlled trial. Biol Psychiatry.
2006;59(11):1071-1077.
24. Maidment I. Use of serotonin antagonists in the treatment of neuroleptic-induced akathisia. Psychiatric Bulletin. 2000;24(9):348-351.
25. Stryjer R, Rosenzcwaig S, Bar F, et al. Trazodone for the treatment of neuroleptic-induced akathisia: a placebo-controlled, double-blind, crossover study. Clin Neuropharmacol. 2010;33(5):219-222.
26. Dumon JP, Catteau J, Lanvin F, et al. Randomized, double-blind, crossover, placebo-controlled comparison of propranolol and betaxolol in the treatment of neuroleptic-induced akathisia. Am J Psychiatry. 1992;149(5):647-650.
27. van Waarde A, Vaalburg W, Doze P, et al. PET imaging of beta-adrenoceptors in the human brain: a realistic goal or a mirage? Curr Pharm Des. 2004;10(13):1519-1536.
28. Kurzthaler I, Hummer M, Kohl C, et al. Propranolol treatment of olanzapine-induced akathisia. Am J Psychiatry. 1997;154(9):1316.
29. Adler LA, Peselow E, Rosenthal MA, et al. A controlled comparison of the effects of propranolol, benztropine, and placebo on akathisia: an interim analysis. Psychopharmacol Bull. 1993;29(2):283-286.
30. Dorevitch A, Durst R, Ginath Y. Propranolol in the treatment of akathisia caused by antipsychotic drugs. South Med J. 1991;84(12):1505-1506.
31. Lipinski JF Jr, Zubenko GS, Cohen BM, et al. Propranolol in the treatment of neuroleptic-induced akathisia. Am J Psychiatry. 1984;141(3):412-415.
32. Adler L, Angrist B, Peselow E, et al. A controlled assessment of propranolol in the treatment of neuroleptic-induced akathisia. Br J Psychiatry. 1986;149:42-45.
33. Kumar R, Sachdev PS. Akathisia and second-generation antipsychotic drugs. Curr Opin Psychiatry. 2009;22(3):293-299.
34. Anttila SA, Leinonen EV. A review of the pharmacological and clinical profile of mirtazapine. CNS Drug Rev. 2001;7(3):249-264.
35. Rogóz Z, Wróbel A, Dlaboga D, et al. Effect of repeated treatment with mirtazapine on the central dopaminergic D2/D3 receptors. Pol J Pharmacol. 2002;54(4):381-389.
36. Miller CH, Fleischhacker WW, Ehrmann H, et al. Treatment of neuroleptic induced akathisia with the 5-HT2 antagonist ritanserin. Psychopharmacol Bull. 1990;26(3):373-376.
37. Weiss D, Aizenberg D, Hermesh H, et al. Cyproheptadine treatment in neuroleptic-induced akathisia. Br J Psychiatry. 1995;167(4):483-486.
38. Gross-Isseroff R, Magen A, Shiloh R, et al. The 5-HT1D receptor agonist zolmitriptan for neuroleptic-induced akathisia: an open label preliminary study. Int Clin Psychopharmacol. 2005;20(1):23-25.
39. Avital A, Gross-Isseroff R, Stryjer R, et al. Zolmitriptan compared to propranolol in the treatment of acute neuroleptic-induced akathisia: a comparative double-blind study. Eur Neuropsychopharmacol. 2009;19(7):476-482.
40. Rathbone J, Soares-Weiser K. Anticholinergics for neuroleptic-induced acute akathisia. Cochrane Database Syst Rev. 2006;(4):CD003727.
41. Sandyk R. Successful treatment of neuroleptic-induced akathisia with baclofen and clonazepam. A case report. Eur Neurol. 1985;24(4):286-288.
42. Miller CH, Fleischhacker W. Managing antipsychotic-induced acute and chronic akathisia. Drug Saf. 2000;22(1):73-81.
43. Pfeffer G, Chouinard G, Margolese HC. Gabapentin in the treatment of antipsychotic-induced akathisia in schizophrenia. Int Clin Psychopharmacol. 2005;20(3):179-181.
44. Stahl SM. Role of α1 adrenergic antagonism in the mechanism of action of iloperidone: reducing extrapyramidal symptoms. CNS Spectr. 2013;18(6):285-258.
45. Hettema JM, Ross DE. A case of aripiprazole-related tardive akathisia and its treatment with ropinirole. J Clin Psychiatry. 2007;68(11):1814-1815.
After substance withdrawal, underlying psychiatric symptoms emerge
When treating patients who abuse substances, it is important to watch for underlying clinical conditions that have been suppressed, relieved, or muted by alcohol or drugs. Many of these conditions can be mistaken for signs of withdrawal, drug-seeking, or new conditions arising from loss of euphoria from the drug. Prompt recognition of these disorders and use of appropriate non-addictive treatments can prevent “against medical advice” discharges, relapses, and unneeded suffering in many cases.
Because the brain is the target organ, these conditions are either neurologic or psychiatric in nosology. Although psychiatric clinicians might not be familiar with neurologic conditions, quick recognition and treatment is necessary.
Restless legs syndrome and periodic limb movements of sleep
Restless legs syndrome (RLS) has 2 key components: paresthesia and akathisia. Although primarily involving the lower extremities, involvement also can include the upper extremities, torso, and head.
Paresthesia differs from typical neuropathies in that it usually is not painful; rather, patients describe an odd sensation using terms such as ticklish, “creepy-crawly,” and other uncomfortable sensations.
Akathisia is a motor restlessness and need to move. The patient might feel momentary relief by moving or rubbing the extremities, only to have the paresthesia return quickly followed by the akathisia. Generally, reclining is the most prominent position that produces symptoms, but they can occur while sitting.
The cause of RLS is an abnormality of central dopamine or iron, or both, in the substantia nigra; iron is a cofactor in dopamine synthesis. All RLS patients should have a serum ferritin level drawn and if <50 μg/dL, be treated with iron supplementation. Dopamine agonists, such as ropinirole, pramipexole, and carbidopa/levodopa, are effective (Table 1); other useful agents include benzodiazepines such as clonazepam and opioids such as hydrocodone.
When a patient withdraws from benzodiazepines or narcotics, RLS can emerge and cause suffering until it is diagnosed and treated. Typical myalgia in opioid withdrawal can confound the diagnosis. The immediate-release (IR) and extended-release (ER) formulations of gabapentin often are a good choice when treating benzodiazepine or narcotic withdrawal. The side effect profile of gabapentin is relatively benign, with somnolence often reported by non-substance abusers, but it is unlikely that addicts, who have grown tolerant to more potent agents such as benzodiazepines and opioids, will complain of sleepiness. Studies have shown that gabapentin is useful in managing withdrawal as well as anxiety and insomnia.1,2 A randomized trial showed that gabapentin increases abstinence rates and decreases heavy drinking.2 The agent has a short half-life (5 to 7 hours); the IR form needs to be dosed at least 3 times a day to be effective. An ER formulation of gabapentin was released in 2013 with the sole indication for RLS.
Gabapentin is not significantly metabolized by the liver, has a 3% rate of protein binding, and is excreted by the kidneys—making it safe for patients who abuse alcohol or opioids and have impaired hepatic function. Typical starting dosages of IR gabapentin are 100 to 300 mg, 3 times daily, if symptoms are present in the daytime. Asymmetric dosing can be helpful, with larger or single dosages given at bedtime (eg, 100 mg in morning, 100 mg in afternoon, 300 mg at bedtime). Dosing varies from patient to patient, from 300 mg to 3,600 mg/d. Increasing dosages produce lower bioavailability because of saturation in absorption or at the blood-brain barrier. At 100 mg every 8 hours, bioavailability is 80% but at 1,600 mg every 8 hours it drops to 27%.3
Periodic limb movements of sleep (PLMS) essentially is akathisia during sleep, and occurs in most patients with RLS. The patient feels tired in the morning because of lack of deep stage-N3 sleep. Because of the inverse relationship between serotonin and dopamine, most selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors can exacerbate RLS and PLMS.4,5 Other culprits include antipsychotics, antiemetics, and antihistamines. The differential diagnosis includes withdrawal from opioids and attention-deficit/hyperactivity disorder (ADHD), which may be comorbid with RLS. There are many causes of secondary RLS including renal failure, pregnancy, varicose veins, and neuropathy.
Tremor
Benign familial, or essential, tremor is a fine intention tremor that can be suppressed by alcohol or benzodiazepines. After detoxification from either of these substances, persistent tremor can re-emerge; often, it is benign, although cerebellar and parkinsonian tremors must be ruled out. Essential tremor can be treated with gabapentin or beta blockers such as propranolol or metoprolol (Table 2).
Anxiety and panic disorder
Social anxiety often presents in addiction treatment centers in the context of group therapy, speaking in 12-step meetings, and having the patient describe his (her) autobiography and history of addiction. Because social anxiety disorder is the third most common psychiatric disorder after simple phobia and major depressive disorder,6 it is not surprising that it emerges after withdrawal.
Patients with social anxiety disorder might self-medicate with alcohol or drugs, especially benzodiazepines (Box). Residential treatment presents an excellent environment for desensitization to fears of public speaking; early recognition is key. Apprehension about group therapy, presenting a substance abuse history, or speaking at a 12-step meeting can lead to premature or “against medical advice” discharge.
Panic disorder commonly is comorbid with substance abuse. Many patients will arrive at treatment with a prescription for benzodiazepines. Because the risk of cross-addiction is high among recovering addicts, benzodiazepines should be avoided. Treating underlying anxiety is crucial for fostering sobriety. Generalized anxiety disorder is common among patients with an addiction, and can lead to relapse if not addressed. Use of non-addictive medications and cognitive therapy is useful in addressing this condition.
A quandary might arise in states where medical marijuana is legal, because Cannabis can be prescribed for anxiety disorders and posttraumatic stress disorder (PTSD). Promoting abstinence from all substances can present a challenge in patients with anxiety disorders who live in these states.
Medications for anxiety and panic disorder include gabapentin, buspirone, hydroxyzine, beta blockers, and atypical antipsychotics (Table 2). Only buspirone and hydroxyzine are FDA-approved for anxiety; buspirone monotherapy generally is ineffective for panic disorder.
Explaining to patients how anxiety arises, such as how classical conditioning leads to specific phobias, can be therapeutic. Describing Klein’s false suffocation alarm theory of panic attacks can illustrate the importance of practicing slow, deep breathing to prevent hyperventilation.7 Also, relabeling a panic attack with self-talk statements such as “I know what this is. It’s just a panic attack” can be helpful. Smartphone apps are available to help patients cope with anxiety and acute panic.8
Mood disorders
Many patients with bipolar disorder experience substance abuse at some point; estimates are that up to 57% of patients have a comorbid addiction.6,9 Persons with a mood disorder are at high risk of substance abuse because of genetic factors; patients also might self-medicate their mood symptoms.
After alcohol or drugs are withdrawn, mood disorders can emerge or resurge. Often, patients enter treatment taking antidepressants and mood stabilizers and usually haven’t been truthful with their treatment provider about their substance abuse. Care must be taken to ascertain whether mood symptoms are secondary to substance abuse. Asking “What’s the longest period of abstinence you’ve had in 2 years and how did you feel emotionally?” often will help you identify a secondary mood disorder. For example, a response of “6 months and I felt really depressed the entire time” would indicate a primary depressive disorder.
Because CNS depressants, such as alcohol and benzodiazepines, can exacerbate a mood disorder, consider continuing or resuming a mood stabilizer or antidepressant during substance abuse treatment. When meeting a new patient, perform an independent evaluation, because substance use can mimic bipolar and depressive disorders. Careful assessment of suicidal ideation is necessary for all patients.
Sleep disorders
Insomnia—as a primary or secondary disorder—is common among patients with a substance use disorder. Insomnia always needs to be addressed. Not sleeping well interferes with cognition and energy and makes depression and bipolar disorder worse. Some experts recommend “waiting out” the insomnia, hoping that sobriety will resolve it—but it might not.
Initial insomnia can be treated with melatonin, 3 to 6 mg at bedtime or earlier in the evening.10-12 Melatonin acts by regulating circadian rhythms, but can cause increased dreaming and nightmares; therefore, it should be avoided in patients who struggle with nightmares. Trazodone, 50 to 150 mg at bedtime, is an inexpensive sleep aid for initial insomnia and doesn’t cause weight gain, which many drugs with antihistaminic properties can. Prazosin, 1 to 2 mg initially, for nightmares in PTSD is effective.13
Antipsychotics might be necessary if nothing else works; quetiapine is effective for sleep and the ER form is FDA-approved as an add-on agent in major depression. Low-dose doxepin (≤10 mg) is effective for middle insomnia.14 At these low dosages, troublesome side effects of tricyclic antidepressants can be avoided.
As many as 40% of adults with ADHD have a delayed sleep-phase disorder. Ask your patient if she is a “night owl,” how chronic the condition is, and when her best sleep occurs.15-17 Morning light and evening melatonin can help, but often are insufficient. Many patients present with undiagnosed or untreated sleep apnea, which can cause excessive daytime sleepiness. Referral to a sleep center is prudent; use of the Epworth Sleepiness Scale is a quick way to assess excessive daytime sleepiness.18
ADHD
ADHD commonly is comorbid with a substance use disorder. Patients might present with an earlier diagnosis, including treatment. Several drugs of abuse can alleviate ADHD symptoms, including amphetamines, opioids, cocaine, and Cannabis; self-medicating is common. Because opioids increase dopamine release, a report of improved work and school performance while taking opioids early in addiction can be a clue to an ADHD diagnosis.
Explaining ADHD as a syndrome of “interest-based attention” helps. If a residential treatment program uses reading and writing assignments, a patient with ADHD might struggle and will need extra help and time and a quiet place to do assignments.19,20 A non-addictive medication, such as atomoxetine, can help, but has an antidepressant-like delay of 3 to 5 weeks until onset of symptom relief. Using a long-acting stimulant can be effective and quick, with an effect size 3 to 4 times higher than atomoxetine; such agents should be avoided in patients who abuse amphetamines.
Studies show that treating ADHD, even with stimulants, neither helps nor hurts outcomes in substance use. Lisdexamfetamine is difficult to abuse and is an inactive prodrug (a bond of lysine and dextroamphetamine) that requires enzymatic cleavage and activation by red blood cells; these characteristics creates a long-acting medication that has a lower abuse liability than other drugs for ADHD. However, abuse can occur and the drug must be used cautiously. Earley’s medication guide referenced below recommends that lisdexamfetamine and other stimulants should be avoided if possible in patients in recovery. However, it adds that specialists in treating ADHD in substance-abusing patients should weigh the potential benefits of stimulant use against the risk of relapse.17 Many patients enter treatment with a diagnosis of bipolar disorder that might, in fact, be comorbid with ADHD.
Chronic pain
Many substance abuse patients began taking opioids for acute, then chronic, pain before their use escalated to addiction. These are challenging patients; often, they are referred for treatment without true addiction.
Keep in mind that dependence is not addiction. Pseudo-addiction is a condition in which pain is undertreated and the patient takes more medication to obtain relief, calls for early refills, and displays drug-seeking behavior but is not using drugs to achieve euphoria. A thorough history and physical and referrals to specialists such as orthopedic surgeons and pain specialists are necessary. Explaining opioid-induced hyperalgesia is important to help the patient understand that (1) pain can be made worse by increasing the dosage of an opioid because of supersensitivity and (2) many patients who are weaned off these drugs will experience a decrease or complete relief of pain.21
Gabapentin, duloxetine, or amitriptyline can be beneficial for chronic pain, as well as mindfulness techniques, physical therapy, and complementary and alternative medicine. Pregabalin can produce euphoria and often should be avoided.
A medication guide for recovery
Paul Earley, MD, former medical director at Talbott Recovery in Atlanta, Georgia, publishes an online guide that classifies medications into categories:
• A: safe
• B: use only under the supervision of an addiction medicine specialist or doctor
• C: completely avoid if the patient is in recovery.17
The Talbott guide lists all stimulants in category C, (except for atomoxetine, which is category A). Hydroxyzine is listed under category B. Many programs for impaired professionals and state medical boards use the Guide, and will question the prescribing of any medication from categories B and C.17
Related Resources
• Spiegel DR, Kumari N, Petri JD. Safer use of benzodiazepines for alcohol detoxification. Current Psychiatry. 2012;11(10):10-15.
• Kelly TM, Daley DC, Douaihy AB. Treatment of substance abusing patients with comorbid psychiatric disorders. Addict Behav. 2012;37(1):11-24.
Drug Brand Names
Amitriptyline • Elavil Hydrocodone • Vicodin
Atenolol • Tenormin Hydroxyzine • Vistaril, Atarax
Atomoxetine • Strattera Lisdexamfetamine • Vyvanse
Buprenorphine/ naloxone • Suboxone Metoprolol • Lopressor, Toprol
Buspirone • BuSpar Paroxetine • Paxil
Carbidopa-levodopa • Sinemet Pramipexole • Mirapex
Clonazepam • Klonopin Prazosin • Minipress
Diazepam • Valium Pregabalin • Lyrica
Doxepin • Silenor, Adapin, Sinequan Propranolol • Inderal
Duloxetine • Cymbalta Quetiapine • Seroquel
Escitalopram • Lexapro Ropinirole • Requip
Gabapentin • Neurontin, Horizant Sertraline • Zoloft
Trazodone • Desyrel
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Stock CJ, Carpenter L, Ying J, et al. Gabapentin versus chlordiazepoxide for outpatient alcohol detoxification treatment. Ann Pharmacother. 2013;47(7-8):961-969.
2. Mason BJ, Quello S, Goodell V, et al. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77.
3. Bockbrader HN, Wesche D, Miller R, et al. A comparison of the pharmacokinetics and pharmacodynamics of pregabalin and gabapentin. Clin Pharmacokinet. 2010; 49(10):661-669.
4. Yang C, White DP, Winkelman JW. Antidepressants and periodic leg movements of sleep. Biol Psychiatry. 2005;58(6):510-514.
5. Hoque R, Chesson AL Jr. Pharmacologically induced/ exacerbated restless legs syndrome, periodic limb movements of sleep, and REM behavior disorder/ REM sleep without atonia: literature review, qualitative scoring, and comparative analysis. J Clin Sleep Med. 2010; 6(1):79-83.
6. Kessler RC, Petukhova M, Sampson NA, et al. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21(3):169-184.
7. Klein DF. False suffocation alarms, spontaneous panics, and related conditions. An integrative hypothesis. Arch Gen Psychiatry. 1993;50(4):306-317.
8. Holland K. The 17 best anxiety iPhone & Android apps of 2014. http://www.healthline.com/health-slideshow/top-anxiety-iphone-android-apps. Accessed October 28, 2014.
9. Chengappa KN, Levine J, Gershon S, et al. Lifetime prevalence of substance or alcohol abuse and dependence among subjects with bipolar I and II disorders in a voluntary registry. Bipolar Disord. 2000;2(3 Pt 1):191-195.
10. Ferracioli-Oda E, Qawasmi A, Bloch MH. Meta-analysis: melatonin for the treatment of primary sleep disorders [published online May 17, 2013]. PLoS One. 2013;8(5):e63773. doi: 10.1371/journal.pone.0063773.
11. Wade AG, Ford I, Crawford G, et al. Efficacy of prolonged release melatonin in insomnia patients aged 55-80 years: quality of sleep and next-day alertness outcomes. Curr Med Res Opin. 2007;23(10):2597-2605.
12. Srinivasan V, Brzezinski A, Pandi-Perumal SR, et al. Melatonin agonists in primary insomnia and depression-associated insomnia: are they superior to sedative-hypnotics? Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(4):913-923.
13. Raskind MA, Peterson K, Williams T, et al. A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. Am J Psychiatry. 2013;170(9):1003-1010.
14. Scharf M, Rogowski R, Hull S, et al. Efficacy and safety of doxepin 1 mg, 3 mg, and 6 mg in elderly patients with primary insomnia: a randomized, double-blind, placebo-controlled crossover study. J Clin Psychiatry. 2008;69(10):1557-1564.
15. Baird AL, Coogan AN, Siddiqui A, et al. Adult attention-deficit hyperactivity disorder is associated with alterations in circadian rhythms at the behavioural, endocrine and molecular levels. Mol Psychiatry. 2012;17(10):988-995.
16. Yoon SY, Jain U, Shapiro C. Sleep in attention-deficit/ hyperactivity disorder in children and adults: past, present, and future. Sleep Med Rev. 2012;16(4):371-388.
17. Earley PH, Merkin B, Skipper G. The medication guide for safe recovery. Revision 1.7. http://paulearley.net/index. php?option=com_docman&Itemid=239. Published March 2014. Accessed October 28, 2014.
18. Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14(6):540-545.
19. Dodson W. Secrets of the ADHD brain. ADDitude. http:// www.additudemag.com/adhd/article/10117.html. Accessed October 28, 2014.
20. Wilens TE, Dodson W. A clinical perspective of attention-deficit/hyperactivity disorder into adulthood. J Clin Psychiatry. 2004;65(10):1301-1313.
21. Lee M, Silverman SM, Hansen H, et al. A comprehensive review of opioid-induced hyperalgesia. Pain Physician. 2011;14(2):145-161.
When treating patients who abuse substances, it is important to watch for underlying clinical conditions that have been suppressed, relieved, or muted by alcohol or drugs. Many of these conditions can be mistaken for signs of withdrawal, drug-seeking, or new conditions arising from loss of euphoria from the drug. Prompt recognition of these disorders and use of appropriate non-addictive treatments can prevent “against medical advice” discharges, relapses, and unneeded suffering in many cases.
Because the brain is the target organ, these conditions are either neurologic or psychiatric in nosology. Although psychiatric clinicians might not be familiar with neurologic conditions, quick recognition and treatment is necessary.
Restless legs syndrome and periodic limb movements of sleep
Restless legs syndrome (RLS) has 2 key components: paresthesia and akathisia. Although primarily involving the lower extremities, involvement also can include the upper extremities, torso, and head.
Paresthesia differs from typical neuropathies in that it usually is not painful; rather, patients describe an odd sensation using terms such as ticklish, “creepy-crawly,” and other uncomfortable sensations.
Akathisia is a motor restlessness and need to move. The patient might feel momentary relief by moving or rubbing the extremities, only to have the paresthesia return quickly followed by the akathisia. Generally, reclining is the most prominent position that produces symptoms, but they can occur while sitting.
The cause of RLS is an abnormality of central dopamine or iron, or both, in the substantia nigra; iron is a cofactor in dopamine synthesis. All RLS patients should have a serum ferritin level drawn and if <50 μg/dL, be treated with iron supplementation. Dopamine agonists, such as ropinirole, pramipexole, and carbidopa/levodopa, are effective (Table 1); other useful agents include benzodiazepines such as clonazepam and opioids such as hydrocodone.
When a patient withdraws from benzodiazepines or narcotics, RLS can emerge and cause suffering until it is diagnosed and treated. Typical myalgia in opioid withdrawal can confound the diagnosis. The immediate-release (IR) and extended-release (ER) formulations of gabapentin often are a good choice when treating benzodiazepine or narcotic withdrawal. The side effect profile of gabapentin is relatively benign, with somnolence often reported by non-substance abusers, but it is unlikely that addicts, who have grown tolerant to more potent agents such as benzodiazepines and opioids, will complain of sleepiness. Studies have shown that gabapentin is useful in managing withdrawal as well as anxiety and insomnia.1,2 A randomized trial showed that gabapentin increases abstinence rates and decreases heavy drinking.2 The agent has a short half-life (5 to 7 hours); the IR form needs to be dosed at least 3 times a day to be effective. An ER formulation of gabapentin was released in 2013 with the sole indication for RLS.
Gabapentin is not significantly metabolized by the liver, has a 3% rate of protein binding, and is excreted by the kidneys—making it safe for patients who abuse alcohol or opioids and have impaired hepatic function. Typical starting dosages of IR gabapentin are 100 to 300 mg, 3 times daily, if symptoms are present in the daytime. Asymmetric dosing can be helpful, with larger or single dosages given at bedtime (eg, 100 mg in morning, 100 mg in afternoon, 300 mg at bedtime). Dosing varies from patient to patient, from 300 mg to 3,600 mg/d. Increasing dosages produce lower bioavailability because of saturation in absorption or at the blood-brain barrier. At 100 mg every 8 hours, bioavailability is 80% but at 1,600 mg every 8 hours it drops to 27%.3
Periodic limb movements of sleep (PLMS) essentially is akathisia during sleep, and occurs in most patients with RLS. The patient feels tired in the morning because of lack of deep stage-N3 sleep. Because of the inverse relationship between serotonin and dopamine, most selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors can exacerbate RLS and PLMS.4,5 Other culprits include antipsychotics, antiemetics, and antihistamines. The differential diagnosis includes withdrawal from opioids and attention-deficit/hyperactivity disorder (ADHD), which may be comorbid with RLS. There are many causes of secondary RLS including renal failure, pregnancy, varicose veins, and neuropathy.
Tremor
Benign familial, or essential, tremor is a fine intention tremor that can be suppressed by alcohol or benzodiazepines. After detoxification from either of these substances, persistent tremor can re-emerge; often, it is benign, although cerebellar and parkinsonian tremors must be ruled out. Essential tremor can be treated with gabapentin or beta blockers such as propranolol or metoprolol (Table 2).
Anxiety and panic disorder
Social anxiety often presents in addiction treatment centers in the context of group therapy, speaking in 12-step meetings, and having the patient describe his (her) autobiography and history of addiction. Because social anxiety disorder is the third most common psychiatric disorder after simple phobia and major depressive disorder,6 it is not surprising that it emerges after withdrawal.
Patients with social anxiety disorder might self-medicate with alcohol or drugs, especially benzodiazepines (Box). Residential treatment presents an excellent environment for desensitization to fears of public speaking; early recognition is key. Apprehension about group therapy, presenting a substance abuse history, or speaking at a 12-step meeting can lead to premature or “against medical advice” discharge.
Panic disorder commonly is comorbid with substance abuse. Many patients will arrive at treatment with a prescription for benzodiazepines. Because the risk of cross-addiction is high among recovering addicts, benzodiazepines should be avoided. Treating underlying anxiety is crucial for fostering sobriety. Generalized anxiety disorder is common among patients with an addiction, and can lead to relapse if not addressed. Use of non-addictive medications and cognitive therapy is useful in addressing this condition.
A quandary might arise in states where medical marijuana is legal, because Cannabis can be prescribed for anxiety disorders and posttraumatic stress disorder (PTSD). Promoting abstinence from all substances can present a challenge in patients with anxiety disorders who live in these states.
Medications for anxiety and panic disorder include gabapentin, buspirone, hydroxyzine, beta blockers, and atypical antipsychotics (Table 2). Only buspirone and hydroxyzine are FDA-approved for anxiety; buspirone monotherapy generally is ineffective for panic disorder.
Explaining to patients how anxiety arises, such as how classical conditioning leads to specific phobias, can be therapeutic. Describing Klein’s false suffocation alarm theory of panic attacks can illustrate the importance of practicing slow, deep breathing to prevent hyperventilation.7 Also, relabeling a panic attack with self-talk statements such as “I know what this is. It’s just a panic attack” can be helpful. Smartphone apps are available to help patients cope with anxiety and acute panic.8
Mood disorders
Many patients with bipolar disorder experience substance abuse at some point; estimates are that up to 57% of patients have a comorbid addiction.6,9 Persons with a mood disorder are at high risk of substance abuse because of genetic factors; patients also might self-medicate their mood symptoms.
After alcohol or drugs are withdrawn, mood disorders can emerge or resurge. Often, patients enter treatment taking antidepressants and mood stabilizers and usually haven’t been truthful with their treatment provider about their substance abuse. Care must be taken to ascertain whether mood symptoms are secondary to substance abuse. Asking “What’s the longest period of abstinence you’ve had in 2 years and how did you feel emotionally?” often will help you identify a secondary mood disorder. For example, a response of “6 months and I felt really depressed the entire time” would indicate a primary depressive disorder.
Because CNS depressants, such as alcohol and benzodiazepines, can exacerbate a mood disorder, consider continuing or resuming a mood stabilizer or antidepressant during substance abuse treatment. When meeting a new patient, perform an independent evaluation, because substance use can mimic bipolar and depressive disorders. Careful assessment of suicidal ideation is necessary for all patients.
Sleep disorders
Insomnia—as a primary or secondary disorder—is common among patients with a substance use disorder. Insomnia always needs to be addressed. Not sleeping well interferes with cognition and energy and makes depression and bipolar disorder worse. Some experts recommend “waiting out” the insomnia, hoping that sobriety will resolve it—but it might not.
Initial insomnia can be treated with melatonin, 3 to 6 mg at bedtime or earlier in the evening.10-12 Melatonin acts by regulating circadian rhythms, but can cause increased dreaming and nightmares; therefore, it should be avoided in patients who struggle with nightmares. Trazodone, 50 to 150 mg at bedtime, is an inexpensive sleep aid for initial insomnia and doesn’t cause weight gain, which many drugs with antihistaminic properties can. Prazosin, 1 to 2 mg initially, for nightmares in PTSD is effective.13
Antipsychotics might be necessary if nothing else works; quetiapine is effective for sleep and the ER form is FDA-approved as an add-on agent in major depression. Low-dose doxepin (≤10 mg) is effective for middle insomnia.14 At these low dosages, troublesome side effects of tricyclic antidepressants can be avoided.
As many as 40% of adults with ADHD have a delayed sleep-phase disorder. Ask your patient if she is a “night owl,” how chronic the condition is, and when her best sleep occurs.15-17 Morning light and evening melatonin can help, but often are insufficient. Many patients present with undiagnosed or untreated sleep apnea, which can cause excessive daytime sleepiness. Referral to a sleep center is prudent; use of the Epworth Sleepiness Scale is a quick way to assess excessive daytime sleepiness.18
ADHD
ADHD commonly is comorbid with a substance use disorder. Patients might present with an earlier diagnosis, including treatment. Several drugs of abuse can alleviate ADHD symptoms, including amphetamines, opioids, cocaine, and Cannabis; self-medicating is common. Because opioids increase dopamine release, a report of improved work and school performance while taking opioids early in addiction can be a clue to an ADHD diagnosis.
Explaining ADHD as a syndrome of “interest-based attention” helps. If a residential treatment program uses reading and writing assignments, a patient with ADHD might struggle and will need extra help and time and a quiet place to do assignments.19,20 A non-addictive medication, such as atomoxetine, can help, but has an antidepressant-like delay of 3 to 5 weeks until onset of symptom relief. Using a long-acting stimulant can be effective and quick, with an effect size 3 to 4 times higher than atomoxetine; such agents should be avoided in patients who abuse amphetamines.
Studies show that treating ADHD, even with stimulants, neither helps nor hurts outcomes in substance use. Lisdexamfetamine is difficult to abuse and is an inactive prodrug (a bond of lysine and dextroamphetamine) that requires enzymatic cleavage and activation by red blood cells; these characteristics creates a long-acting medication that has a lower abuse liability than other drugs for ADHD. However, abuse can occur and the drug must be used cautiously. Earley’s medication guide referenced below recommends that lisdexamfetamine and other stimulants should be avoided if possible in patients in recovery. However, it adds that specialists in treating ADHD in substance-abusing patients should weigh the potential benefits of stimulant use against the risk of relapse.17 Many patients enter treatment with a diagnosis of bipolar disorder that might, in fact, be comorbid with ADHD.
Chronic pain
Many substance abuse patients began taking opioids for acute, then chronic, pain before their use escalated to addiction. These are challenging patients; often, they are referred for treatment without true addiction.
Keep in mind that dependence is not addiction. Pseudo-addiction is a condition in which pain is undertreated and the patient takes more medication to obtain relief, calls for early refills, and displays drug-seeking behavior but is not using drugs to achieve euphoria. A thorough history and physical and referrals to specialists such as orthopedic surgeons and pain specialists are necessary. Explaining opioid-induced hyperalgesia is important to help the patient understand that (1) pain can be made worse by increasing the dosage of an opioid because of supersensitivity and (2) many patients who are weaned off these drugs will experience a decrease or complete relief of pain.21
Gabapentin, duloxetine, or amitriptyline can be beneficial for chronic pain, as well as mindfulness techniques, physical therapy, and complementary and alternative medicine. Pregabalin can produce euphoria and often should be avoided.
A medication guide for recovery
Paul Earley, MD, former medical director at Talbott Recovery in Atlanta, Georgia, publishes an online guide that classifies medications into categories:
• A: safe
• B: use only under the supervision of an addiction medicine specialist or doctor
• C: completely avoid if the patient is in recovery.17
The Talbott guide lists all stimulants in category C, (except for atomoxetine, which is category A). Hydroxyzine is listed under category B. Many programs for impaired professionals and state medical boards use the Guide, and will question the prescribing of any medication from categories B and C.17
Related Resources
• Spiegel DR, Kumari N, Petri JD. Safer use of benzodiazepines for alcohol detoxification. Current Psychiatry. 2012;11(10):10-15.
• Kelly TM, Daley DC, Douaihy AB. Treatment of substance abusing patients with comorbid psychiatric disorders. Addict Behav. 2012;37(1):11-24.
Drug Brand Names
Amitriptyline • Elavil Hydrocodone • Vicodin
Atenolol • Tenormin Hydroxyzine • Vistaril, Atarax
Atomoxetine • Strattera Lisdexamfetamine • Vyvanse
Buprenorphine/ naloxone • Suboxone Metoprolol • Lopressor, Toprol
Buspirone • BuSpar Paroxetine • Paxil
Carbidopa-levodopa • Sinemet Pramipexole • Mirapex
Clonazepam • Klonopin Prazosin • Minipress
Diazepam • Valium Pregabalin • Lyrica
Doxepin • Silenor, Adapin, Sinequan Propranolol • Inderal
Duloxetine • Cymbalta Quetiapine • Seroquel
Escitalopram • Lexapro Ropinirole • Requip
Gabapentin • Neurontin, Horizant Sertraline • Zoloft
Trazodone • Desyrel
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
When treating patients who abuse substances, it is important to watch for underlying clinical conditions that have been suppressed, relieved, or muted by alcohol or drugs. Many of these conditions can be mistaken for signs of withdrawal, drug-seeking, or new conditions arising from loss of euphoria from the drug. Prompt recognition of these disorders and use of appropriate non-addictive treatments can prevent “against medical advice” discharges, relapses, and unneeded suffering in many cases.
Because the brain is the target organ, these conditions are either neurologic or psychiatric in nosology. Although psychiatric clinicians might not be familiar with neurologic conditions, quick recognition and treatment is necessary.
Restless legs syndrome and periodic limb movements of sleep
Restless legs syndrome (RLS) has 2 key components: paresthesia and akathisia. Although primarily involving the lower extremities, involvement also can include the upper extremities, torso, and head.
Paresthesia differs from typical neuropathies in that it usually is not painful; rather, patients describe an odd sensation using terms such as ticklish, “creepy-crawly,” and other uncomfortable sensations.
Akathisia is a motor restlessness and need to move. The patient might feel momentary relief by moving or rubbing the extremities, only to have the paresthesia return quickly followed by the akathisia. Generally, reclining is the most prominent position that produces symptoms, but they can occur while sitting.
The cause of RLS is an abnormality of central dopamine or iron, or both, in the substantia nigra; iron is a cofactor in dopamine synthesis. All RLS patients should have a serum ferritin level drawn and if <50 μg/dL, be treated with iron supplementation. Dopamine agonists, such as ropinirole, pramipexole, and carbidopa/levodopa, are effective (Table 1); other useful agents include benzodiazepines such as clonazepam and opioids such as hydrocodone.
When a patient withdraws from benzodiazepines or narcotics, RLS can emerge and cause suffering until it is diagnosed and treated. Typical myalgia in opioid withdrawal can confound the diagnosis. The immediate-release (IR) and extended-release (ER) formulations of gabapentin often are a good choice when treating benzodiazepine or narcotic withdrawal. The side effect profile of gabapentin is relatively benign, with somnolence often reported by non-substance abusers, but it is unlikely that addicts, who have grown tolerant to more potent agents such as benzodiazepines and opioids, will complain of sleepiness. Studies have shown that gabapentin is useful in managing withdrawal as well as anxiety and insomnia.1,2 A randomized trial showed that gabapentin increases abstinence rates and decreases heavy drinking.2 The agent has a short half-life (5 to 7 hours); the IR form needs to be dosed at least 3 times a day to be effective. An ER formulation of gabapentin was released in 2013 with the sole indication for RLS.
Gabapentin is not significantly metabolized by the liver, has a 3% rate of protein binding, and is excreted by the kidneys—making it safe for patients who abuse alcohol or opioids and have impaired hepatic function. Typical starting dosages of IR gabapentin are 100 to 300 mg, 3 times daily, if symptoms are present in the daytime. Asymmetric dosing can be helpful, with larger or single dosages given at bedtime (eg, 100 mg in morning, 100 mg in afternoon, 300 mg at bedtime). Dosing varies from patient to patient, from 300 mg to 3,600 mg/d. Increasing dosages produce lower bioavailability because of saturation in absorption or at the blood-brain barrier. At 100 mg every 8 hours, bioavailability is 80% but at 1,600 mg every 8 hours it drops to 27%.3
Periodic limb movements of sleep (PLMS) essentially is akathisia during sleep, and occurs in most patients with RLS. The patient feels tired in the morning because of lack of deep stage-N3 sleep. Because of the inverse relationship between serotonin and dopamine, most selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors can exacerbate RLS and PLMS.4,5 Other culprits include antipsychotics, antiemetics, and antihistamines. The differential diagnosis includes withdrawal from opioids and attention-deficit/hyperactivity disorder (ADHD), which may be comorbid with RLS. There are many causes of secondary RLS including renal failure, pregnancy, varicose veins, and neuropathy.
Tremor
Benign familial, or essential, tremor is a fine intention tremor that can be suppressed by alcohol or benzodiazepines. After detoxification from either of these substances, persistent tremor can re-emerge; often, it is benign, although cerebellar and parkinsonian tremors must be ruled out. Essential tremor can be treated with gabapentin or beta blockers such as propranolol or metoprolol (Table 2).
Anxiety and panic disorder
Social anxiety often presents in addiction treatment centers in the context of group therapy, speaking in 12-step meetings, and having the patient describe his (her) autobiography and history of addiction. Because social anxiety disorder is the third most common psychiatric disorder after simple phobia and major depressive disorder,6 it is not surprising that it emerges after withdrawal.
Patients with social anxiety disorder might self-medicate with alcohol or drugs, especially benzodiazepines (Box). Residential treatment presents an excellent environment for desensitization to fears of public speaking; early recognition is key. Apprehension about group therapy, presenting a substance abuse history, or speaking at a 12-step meeting can lead to premature or “against medical advice” discharge.
Panic disorder commonly is comorbid with substance abuse. Many patients will arrive at treatment with a prescription for benzodiazepines. Because the risk of cross-addiction is high among recovering addicts, benzodiazepines should be avoided. Treating underlying anxiety is crucial for fostering sobriety. Generalized anxiety disorder is common among patients with an addiction, and can lead to relapse if not addressed. Use of non-addictive medications and cognitive therapy is useful in addressing this condition.
A quandary might arise in states where medical marijuana is legal, because Cannabis can be prescribed for anxiety disorders and posttraumatic stress disorder (PTSD). Promoting abstinence from all substances can present a challenge in patients with anxiety disorders who live in these states.
Medications for anxiety and panic disorder include gabapentin, buspirone, hydroxyzine, beta blockers, and atypical antipsychotics (Table 2). Only buspirone and hydroxyzine are FDA-approved for anxiety; buspirone monotherapy generally is ineffective for panic disorder.
Explaining to patients how anxiety arises, such as how classical conditioning leads to specific phobias, can be therapeutic. Describing Klein’s false suffocation alarm theory of panic attacks can illustrate the importance of practicing slow, deep breathing to prevent hyperventilation.7 Also, relabeling a panic attack with self-talk statements such as “I know what this is. It’s just a panic attack” can be helpful. Smartphone apps are available to help patients cope with anxiety and acute panic.8
Mood disorders
Many patients with bipolar disorder experience substance abuse at some point; estimates are that up to 57% of patients have a comorbid addiction.6,9 Persons with a mood disorder are at high risk of substance abuse because of genetic factors; patients also might self-medicate their mood symptoms.
After alcohol or drugs are withdrawn, mood disorders can emerge or resurge. Often, patients enter treatment taking antidepressants and mood stabilizers and usually haven’t been truthful with their treatment provider about their substance abuse. Care must be taken to ascertain whether mood symptoms are secondary to substance abuse. Asking “What’s the longest period of abstinence you’ve had in 2 years and how did you feel emotionally?” often will help you identify a secondary mood disorder. For example, a response of “6 months and I felt really depressed the entire time” would indicate a primary depressive disorder.
Because CNS depressants, such as alcohol and benzodiazepines, can exacerbate a mood disorder, consider continuing or resuming a mood stabilizer or antidepressant during substance abuse treatment. When meeting a new patient, perform an independent evaluation, because substance use can mimic bipolar and depressive disorders. Careful assessment of suicidal ideation is necessary for all patients.
Sleep disorders
Insomnia—as a primary or secondary disorder—is common among patients with a substance use disorder. Insomnia always needs to be addressed. Not sleeping well interferes with cognition and energy and makes depression and bipolar disorder worse. Some experts recommend “waiting out” the insomnia, hoping that sobriety will resolve it—but it might not.
Initial insomnia can be treated with melatonin, 3 to 6 mg at bedtime or earlier in the evening.10-12 Melatonin acts by regulating circadian rhythms, but can cause increased dreaming and nightmares; therefore, it should be avoided in patients who struggle with nightmares. Trazodone, 50 to 150 mg at bedtime, is an inexpensive sleep aid for initial insomnia and doesn’t cause weight gain, which many drugs with antihistaminic properties can. Prazosin, 1 to 2 mg initially, for nightmares in PTSD is effective.13
Antipsychotics might be necessary if nothing else works; quetiapine is effective for sleep and the ER form is FDA-approved as an add-on agent in major depression. Low-dose doxepin (≤10 mg) is effective for middle insomnia.14 At these low dosages, troublesome side effects of tricyclic antidepressants can be avoided.
As many as 40% of adults with ADHD have a delayed sleep-phase disorder. Ask your patient if she is a “night owl,” how chronic the condition is, and when her best sleep occurs.15-17 Morning light and evening melatonin can help, but often are insufficient. Many patients present with undiagnosed or untreated sleep apnea, which can cause excessive daytime sleepiness. Referral to a sleep center is prudent; use of the Epworth Sleepiness Scale is a quick way to assess excessive daytime sleepiness.18
ADHD
ADHD commonly is comorbid with a substance use disorder. Patients might present with an earlier diagnosis, including treatment. Several drugs of abuse can alleviate ADHD symptoms, including amphetamines, opioids, cocaine, and Cannabis; self-medicating is common. Because opioids increase dopamine release, a report of improved work and school performance while taking opioids early in addiction can be a clue to an ADHD diagnosis.
Explaining ADHD as a syndrome of “interest-based attention” helps. If a residential treatment program uses reading and writing assignments, a patient with ADHD might struggle and will need extra help and time and a quiet place to do assignments.19,20 A non-addictive medication, such as atomoxetine, can help, but has an antidepressant-like delay of 3 to 5 weeks until onset of symptom relief. Using a long-acting stimulant can be effective and quick, with an effect size 3 to 4 times higher than atomoxetine; such agents should be avoided in patients who abuse amphetamines.
Studies show that treating ADHD, even with stimulants, neither helps nor hurts outcomes in substance use. Lisdexamfetamine is difficult to abuse and is an inactive prodrug (a bond of lysine and dextroamphetamine) that requires enzymatic cleavage and activation by red blood cells; these characteristics creates a long-acting medication that has a lower abuse liability than other drugs for ADHD. However, abuse can occur and the drug must be used cautiously. Earley’s medication guide referenced below recommends that lisdexamfetamine and other stimulants should be avoided if possible in patients in recovery. However, it adds that specialists in treating ADHD in substance-abusing patients should weigh the potential benefits of stimulant use against the risk of relapse.17 Many patients enter treatment with a diagnosis of bipolar disorder that might, in fact, be comorbid with ADHD.
Chronic pain
Many substance abuse patients began taking opioids for acute, then chronic, pain before their use escalated to addiction. These are challenging patients; often, they are referred for treatment without true addiction.
Keep in mind that dependence is not addiction. Pseudo-addiction is a condition in which pain is undertreated and the patient takes more medication to obtain relief, calls for early refills, and displays drug-seeking behavior but is not using drugs to achieve euphoria. A thorough history and physical and referrals to specialists such as orthopedic surgeons and pain specialists are necessary. Explaining opioid-induced hyperalgesia is important to help the patient understand that (1) pain can be made worse by increasing the dosage of an opioid because of supersensitivity and (2) many patients who are weaned off these drugs will experience a decrease or complete relief of pain.21
Gabapentin, duloxetine, or amitriptyline can be beneficial for chronic pain, as well as mindfulness techniques, physical therapy, and complementary and alternative medicine. Pregabalin can produce euphoria and often should be avoided.
A medication guide for recovery
Paul Earley, MD, former medical director at Talbott Recovery in Atlanta, Georgia, publishes an online guide that classifies medications into categories:
• A: safe
• B: use only under the supervision of an addiction medicine specialist or doctor
• C: completely avoid if the patient is in recovery.17
The Talbott guide lists all stimulants in category C, (except for atomoxetine, which is category A). Hydroxyzine is listed under category B. Many programs for impaired professionals and state medical boards use the Guide, and will question the prescribing of any medication from categories B and C.17
Related Resources
• Spiegel DR, Kumari N, Petri JD. Safer use of benzodiazepines for alcohol detoxification. Current Psychiatry. 2012;11(10):10-15.
• Kelly TM, Daley DC, Douaihy AB. Treatment of substance abusing patients with comorbid psychiatric disorders. Addict Behav. 2012;37(1):11-24.
Drug Brand Names
Amitriptyline • Elavil Hydrocodone • Vicodin
Atenolol • Tenormin Hydroxyzine • Vistaril, Atarax
Atomoxetine • Strattera Lisdexamfetamine • Vyvanse
Buprenorphine/ naloxone • Suboxone Metoprolol • Lopressor, Toprol
Buspirone • BuSpar Paroxetine • Paxil
Carbidopa-levodopa • Sinemet Pramipexole • Mirapex
Clonazepam • Klonopin Prazosin • Minipress
Diazepam • Valium Pregabalin • Lyrica
Doxepin • Silenor, Adapin, Sinequan Propranolol • Inderal
Duloxetine • Cymbalta Quetiapine • Seroquel
Escitalopram • Lexapro Ropinirole • Requip
Gabapentin • Neurontin, Horizant Sertraline • Zoloft
Trazodone • Desyrel
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Stock CJ, Carpenter L, Ying J, et al. Gabapentin versus chlordiazepoxide for outpatient alcohol detoxification treatment. Ann Pharmacother. 2013;47(7-8):961-969.
2. Mason BJ, Quello S, Goodell V, et al. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77.
3. Bockbrader HN, Wesche D, Miller R, et al. A comparison of the pharmacokinetics and pharmacodynamics of pregabalin and gabapentin. Clin Pharmacokinet. 2010; 49(10):661-669.
4. Yang C, White DP, Winkelman JW. Antidepressants and periodic leg movements of sleep. Biol Psychiatry. 2005;58(6):510-514.
5. Hoque R, Chesson AL Jr. Pharmacologically induced/ exacerbated restless legs syndrome, periodic limb movements of sleep, and REM behavior disorder/ REM sleep without atonia: literature review, qualitative scoring, and comparative analysis. J Clin Sleep Med. 2010; 6(1):79-83.
6. Kessler RC, Petukhova M, Sampson NA, et al. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21(3):169-184.
7. Klein DF. False suffocation alarms, spontaneous panics, and related conditions. An integrative hypothesis. Arch Gen Psychiatry. 1993;50(4):306-317.
8. Holland K. The 17 best anxiety iPhone & Android apps of 2014. http://www.healthline.com/health-slideshow/top-anxiety-iphone-android-apps. Accessed October 28, 2014.
9. Chengappa KN, Levine J, Gershon S, et al. Lifetime prevalence of substance or alcohol abuse and dependence among subjects with bipolar I and II disorders in a voluntary registry. Bipolar Disord. 2000;2(3 Pt 1):191-195.
10. Ferracioli-Oda E, Qawasmi A, Bloch MH. Meta-analysis: melatonin for the treatment of primary sleep disorders [published online May 17, 2013]. PLoS One. 2013;8(5):e63773. doi: 10.1371/journal.pone.0063773.
11. Wade AG, Ford I, Crawford G, et al. Efficacy of prolonged release melatonin in insomnia patients aged 55-80 years: quality of sleep and next-day alertness outcomes. Curr Med Res Opin. 2007;23(10):2597-2605.
12. Srinivasan V, Brzezinski A, Pandi-Perumal SR, et al. Melatonin agonists in primary insomnia and depression-associated insomnia: are they superior to sedative-hypnotics? Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(4):913-923.
13. Raskind MA, Peterson K, Williams T, et al. A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. Am J Psychiatry. 2013;170(9):1003-1010.
14. Scharf M, Rogowski R, Hull S, et al. Efficacy and safety of doxepin 1 mg, 3 mg, and 6 mg in elderly patients with primary insomnia: a randomized, double-blind, placebo-controlled crossover study. J Clin Psychiatry. 2008;69(10):1557-1564.
15. Baird AL, Coogan AN, Siddiqui A, et al. Adult attention-deficit hyperactivity disorder is associated with alterations in circadian rhythms at the behavioural, endocrine and molecular levels. Mol Psychiatry. 2012;17(10):988-995.
16. Yoon SY, Jain U, Shapiro C. Sleep in attention-deficit/ hyperactivity disorder in children and adults: past, present, and future. Sleep Med Rev. 2012;16(4):371-388.
17. Earley PH, Merkin B, Skipper G. The medication guide for safe recovery. Revision 1.7. http://paulearley.net/index. php?option=com_docman&Itemid=239. Published March 2014. Accessed October 28, 2014.
18. Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14(6):540-545.
19. Dodson W. Secrets of the ADHD brain. ADDitude. http:// www.additudemag.com/adhd/article/10117.html. Accessed October 28, 2014.
20. Wilens TE, Dodson W. A clinical perspective of attention-deficit/hyperactivity disorder into adulthood. J Clin Psychiatry. 2004;65(10):1301-1313.
21. Lee M, Silverman SM, Hansen H, et al. A comprehensive review of opioid-induced hyperalgesia. Pain Physician. 2011;14(2):145-161.
1. Stock CJ, Carpenter L, Ying J, et al. Gabapentin versus chlordiazepoxide for outpatient alcohol detoxification treatment. Ann Pharmacother. 2013;47(7-8):961-969.
2. Mason BJ, Quello S, Goodell V, et al. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77.
3. Bockbrader HN, Wesche D, Miller R, et al. A comparison of the pharmacokinetics and pharmacodynamics of pregabalin and gabapentin. Clin Pharmacokinet. 2010; 49(10):661-669.
4. Yang C, White DP, Winkelman JW. Antidepressants and periodic leg movements of sleep. Biol Psychiatry. 2005;58(6):510-514.
5. Hoque R, Chesson AL Jr. Pharmacologically induced/ exacerbated restless legs syndrome, periodic limb movements of sleep, and REM behavior disorder/ REM sleep without atonia: literature review, qualitative scoring, and comparative analysis. J Clin Sleep Med. 2010; 6(1):79-83.
6. Kessler RC, Petukhova M, Sampson NA, et al. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21(3):169-184.
7. Klein DF. False suffocation alarms, spontaneous panics, and related conditions. An integrative hypothesis. Arch Gen Psychiatry. 1993;50(4):306-317.
8. Holland K. The 17 best anxiety iPhone & Android apps of 2014. http://www.healthline.com/health-slideshow/top-anxiety-iphone-android-apps. Accessed October 28, 2014.
9. Chengappa KN, Levine J, Gershon S, et al. Lifetime prevalence of substance or alcohol abuse and dependence among subjects with bipolar I and II disorders in a voluntary registry. Bipolar Disord. 2000;2(3 Pt 1):191-195.
10. Ferracioli-Oda E, Qawasmi A, Bloch MH. Meta-analysis: melatonin for the treatment of primary sleep disorders [published online May 17, 2013]. PLoS One. 2013;8(5):e63773. doi: 10.1371/journal.pone.0063773.
11. Wade AG, Ford I, Crawford G, et al. Efficacy of prolonged release melatonin in insomnia patients aged 55-80 years: quality of sleep and next-day alertness outcomes. Curr Med Res Opin. 2007;23(10):2597-2605.
12. Srinivasan V, Brzezinski A, Pandi-Perumal SR, et al. Melatonin agonists in primary insomnia and depression-associated insomnia: are they superior to sedative-hypnotics? Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(4):913-923.
13. Raskind MA, Peterson K, Williams T, et al. A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. Am J Psychiatry. 2013;170(9):1003-1010.
14. Scharf M, Rogowski R, Hull S, et al. Efficacy and safety of doxepin 1 mg, 3 mg, and 6 mg in elderly patients with primary insomnia: a randomized, double-blind, placebo-controlled crossover study. J Clin Psychiatry. 2008;69(10):1557-1564.
15. Baird AL, Coogan AN, Siddiqui A, et al. Adult attention-deficit hyperactivity disorder is associated with alterations in circadian rhythms at the behavioural, endocrine and molecular levels. Mol Psychiatry. 2012;17(10):988-995.
16. Yoon SY, Jain U, Shapiro C. Sleep in attention-deficit/ hyperactivity disorder in children and adults: past, present, and future. Sleep Med Rev. 2012;16(4):371-388.
17. Earley PH, Merkin B, Skipper G. The medication guide for safe recovery. Revision 1.7. http://paulearley.net/index. php?option=com_docman&Itemid=239. Published March 2014. Accessed October 28, 2014.
18. Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14(6):540-545.
19. Dodson W. Secrets of the ADHD brain. ADDitude. http:// www.additudemag.com/adhd/article/10117.html. Accessed October 28, 2014.
20. Wilens TE, Dodson W. A clinical perspective of attention-deficit/hyperactivity disorder into adulthood. J Clin Psychiatry. 2004;65(10):1301-1313.
21. Lee M, Silverman SM, Hansen H, et al. A comprehensive review of opioid-induced hyperalgesia. Pain Physician. 2011;14(2):145-161.
A guide to the mysteries of maintenance of certification
As part of a general trend among all medical specialty boards, the American Board of Psychiatry and Neurology (ABPN) instituted a recertification process for all new general psychiatry certifications on October 1, 1994.1 In 2000, the individual specialties that constitute the American Board of Medical Specialties (ABMS) subsequently agreed to develop a comprehensive maintenance of certification (MOC) process to demonstrate ongoing learning and competency beyond what could be captured by a recertification examination alone.
All ABMS member boards now use a 4-part process for recertification. For ABPN, those 4 core components are listed in the Table.1,2
ABPN component 1 (maintaining an unrestricted medical license) and component 4 (passing the recertification examination) are straightforward; however, requirements for continuing medical education (CME), including the specific need to accrue ABPN-approved self-assessment (SA) CME hours, and the Improvement in Medical Practice (performance in practice, or PIP) module, have stoked significant commentary and confusion.
Based on feedback,3,4 ABPN in 2014:
• modified the SA and PIP requirements for physicians who certified or recertified between 2005 and 2011
• changed the specific requirement for the PIP feedback component.
These modifications only added to feelings of uncertainty about the MOC process among many psychiatrists.5
Given the professional and personal importance attached to maintaining one’s general and subspecialty certifications, the 2 parts of this article—here and in the January 2015 issue—have been constructed to highlight current ABPN MOC requirements and provide resources for understanding, tracking, and completing the SA and PIP portions.
In addition to this review, I urge all physicians who are subject to MOC to read the 20-page revised MOC Program bookleta (version 2.1, May 2014).5
aDownload the booklet at www.abpn.com/downloads/moc/ moc_web_doc.pdf.
Who must recertify?
As of October 1, 1994, all physicians who achieve ABPN certifications in general psychiatry are issued a 10-year, time-limited certificate that expires on December 31 of the 10th year.3 Note that the 10-year, time-limited certificate in child and adolescent psychiatry began in 1995 and expires 10 years later on December 31.
Certificates in the subspecialties (addiction psychiatry, forensic psychiatry, geriatric psychiatry, etc.), including those issued before October 1, 1994, are 10-year, time-limited certificates that expire on December 31.3 This expiration date often is overlooked by physicians who are exempt from the MOC process for their general psychiatry, or child and adolescent psychiatry certification. There is no exemption for any subspecialty certificate (aside from child and adolescent psychiatry before 1995), regardless of the date of issue.
Moreover, physicians who hold a certificate in a subspecialty also must maintain certification in their specialty (general psychiatry) to apply for recertification in their subspecialization. One exception: Diplomates in child and adolescent psychiatry do not need to maintain current certification in general psychiatry for their subspecialty certification to remain valid or to recertify in child and adolescent psychiatry.
The need to maintain multiple certifications can seem onerous, but note that CME, SA, and PIP activities that have been completed in one area of specialization or subspecialization accrue and count for multiple certifications for diplomates certified in 2 or more areas.5
Get started!
Tracking your progress is critical to keeping up with MOC requirements. You can do this with a personal spreadsheet or by using online resources. Although it is not required, ABPN has established a system that allows diplomates to create and maintain, at no cost, a physician folio on the ABPN server that facilitates documentation of CME hours, including specific SA hours, and PIP module completion.6 All diplomates are required to maintain records of SA activities, CME activities, and PIP units; the ABPN will audit approximately 5% of examination applications.5
Regardless of what documentation method you choose, you should establish an active profile on the ABPN site (www.abpn. com/folios), confirm your contact information, and, if you are not active clinically, update your clinical status. ABPN requires that diplomates self-report their clinical status every 24 months—information that is available to the public. Clinical status also identifies to ABPN those PIP modules that you must complete.
ABPN recognizes 3 categories of clinical status5:
1. Clinically active. Provided any amount of direct or consultative care, or both, in the preceding 24 months, including supervision of residents.
a) Engaged in direct or consultative care, or both, sufficient to complete Improvement in Medical Practice (PIP) units.
b) Engaged in direct or consultative care, or both, that is insufficient to complete PIP units.
2. Clinically inactive. Did not provide direct or consultative care in the preceding 24 months.
3. Status unknown. No information is available on clinical activity.
Based on these definitions, physicians in Category 1a are required to complete all components of the MOC program, including PIP units; physicians in Category 1b or Category 2 are required to complete all components of the MOC program except PIP units.
A change in status from Category 1b or 2 to Category 1a (eg, moving from a purely administrative position to one with clinical duties) requires completion of ≥1 PIP unit.
The easy parts
Licenses. Maintaining your unrestricted professional license(s) is mandatory; the language of this requirement is unambiguous (Table).5 The plural form of license is intentional: Some physicians have medical licenses in multiple states and, in some jurisdictions, licenses are required to supervise physician assistants and other personnel or to prescribe controlled substances. Any restriction on a professional license should be discussed with ABPN and resolved to prevent rejection of the examination application.5
Examinations. For physicians who are not yet enrolled in the continuous-MOC (C-MOC) process (to be discussed in Part 2 of this article), an application to take the examination in Year 10 can be filed in Year 9 of the cycle—after the CME, SA, and PIP requirements are completed. Once a diplomate becomes subject to the C-MOC process by certifying or recertifying from 2012 onwards, completion of each 3-year module of CME, SA, and PIP will not coincide with the 10-year time frame of the examination.
The application deadline for all MOC examinations typically is the year before the examination; the examination should be taken in the year the certificate expires, although it can be taken earlier if desired.7 The examinations are computer-based and administered at a certified testing center. For diplomates who have more than 1 ABPN certificate and want to combine multiple examinations into 1 test session, a reduced fee structure applies.
The general psychiatry examination comprises 220 single-answer, multiple-choice questions that must be completed within 290 minutes, with 10 extra minutes allotted to read on-screen instructions, sign in, and complete a post-examination survey.8 The combined examinations comprise 100 questions from each ABPN specialty or subspecialty area.5
The content of the 2015 general psychiatry examinationb is available on the ABPN Web site.7 Note that the recertification examination in general psychiatry does not cover neurology topics.
bDownload the outline of the examination at www.abpn.com/ downloads/content_outlines/MOC/2015-MOC-Psych-blueprint-060314-EWM-MR.pdf.
Examinations administered in 2015 and 2016 will use only diagnostic criteria that have not changed from DSM-IV-TR9: Neither obsolete diagnoses or subtypes from DSM-IV-TR nor new diagnoses or subtypes in DSM-5 (eg, hoarding disorder) will be tested.9 Diagnoses that are exactly or substantially the same will be tested; these include diagnoses:
• with a name change only (eg, “phonological disorder” in DSM-IV-TR is “speech sound disorder” in DSM-5)
• expanded into >1 new diagnosis (eg, hypochondriasis was expanded to 2 new diagnoses: somatic symptom disorder and illness anxiety disorder)
• subsumed or combined into a new diagnosis (eg, substance use and dependence are now combined into substance use disorder in DSM-5).9
For these diagnoses, both DSM-IV-TR and DSM-5 diagnoses will be provided on examinations.
Beginning in 2017, all examinations will use DSM-5 classifications and diagnostic criteria.9
Part 2 of this article in the January 2015 issue reviews other key aspects of MOC: continuing medical education (CME), including self-assessment requirements; performance in practice (PIP); and continuous maintenance of certification (C-MOC).
BOTTOM LINE
Maintenance of certification (MOC) is a manageable process, although it requires you to be familiar with its various elements, including the duration of certification, licensing requirements, and the examination. Start the process by (1) establishing a login on the ABPN Web site and (2) reviewing the MOC program booklet.
Related Resources
• ABPN MOC home page. www.abpn.com/moc.html
• ABPN-approved products for SA, CME, and PIP modules. www.abpn.com/moc_products.asp
• Peer and patient feedback forms– Peer feedback form v1. www.abpn.com/downloads/moc/PIP-peer-feedback-v1-051914.pdf
– Patient feedback form v1. www.abpn.com/downloads/moc/PIP-patient-feedback-v1-051914.pdf
– Patient feedback form v2. www.abpn.com/downloads/moc/PIP-patient-feedback-v2-051914.pdf
• ABPN physician folio page. https://application.abpn.com/webclient/folios.aspx
Disclosure
Dr. Meyer reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Faulkner LR, Tivnan PW, Winstead DK, et al. The ABPN Maintenance of Certification Program for psychiatrists: past history, current status, and future directions. Acad Psychiatry. 2008;32(3):241-248.
2. Ebert MH, Faulkner L, Stubbe DE, et al. Maintenance of certification in psychiatry. J Clin Psychiatry. 2009;70(10):e39.
3. Faulkner LR, Vondrak PA. Frequently asked questions about maintenance of certification (MOC). J Clin Psychiatry. 2010;71(5):632-633.
4. Faulkner LR, Juul D, Andrade NN, et al. Recent trends in american board of psychiatry and neurology psychiatric subspecialties. Acad Psychiatry. 2011;35(1):35-39.
5. Maintenance of certification program. American Board of Psychiatry and Neurology, Inc. http://abpn.com/ downloads/moc/moc_web_doc.pdf. Published May 2014. Accessed August 25, 2014.
6. Physician folios. American Board of Psychiatry and Neurology, Inc. https://application.abpn.com/webclient/ folios.aspx. Accessed August 25, 2014.
7. Maintenance of certification examination in psychiatry 2015 content blueprint. American Board of Psychiatry and Neurology, Inc. http://www.abpn.com/downloads/ content_outlines/MOC/2015-MOC-Psych-blueprint- 060314-EWM-MR.pdf. Published June 2, 2014. Accessed August 25, 2014.
8. Instructions for the 2015 psychiatry maintenance of certification examination. American Board of Psychiatry and Neurology, Inc. http://www.abpn.com/downloads/ content_outlines/MOC/2015-MOC-Psych-Format-and- Scoring-060214-RL-MR.pdf. Published June 2, 2014. Accessed August 25, 2014.
9. DSM-5 conversion. American Board of Psychiatry and Neurology, Inc. http://www.abpn.com/ifas.html. Accessed August 25, 2014.
As part of a general trend among all medical specialty boards, the American Board of Psychiatry and Neurology (ABPN) instituted a recertification process for all new general psychiatry certifications on October 1, 1994.1 In 2000, the individual specialties that constitute the American Board of Medical Specialties (ABMS) subsequently agreed to develop a comprehensive maintenance of certification (MOC) process to demonstrate ongoing learning and competency beyond what could be captured by a recertification examination alone.
All ABMS member boards now use a 4-part process for recertification. For ABPN, those 4 core components are listed in the Table.1,2
ABPN component 1 (maintaining an unrestricted medical license) and component 4 (passing the recertification examination) are straightforward; however, requirements for continuing medical education (CME), including the specific need to accrue ABPN-approved self-assessment (SA) CME hours, and the Improvement in Medical Practice (performance in practice, or PIP) module, have stoked significant commentary and confusion.
Based on feedback,3,4 ABPN in 2014:
• modified the SA and PIP requirements for physicians who certified or recertified between 2005 and 2011
• changed the specific requirement for the PIP feedback component.
These modifications only added to feelings of uncertainty about the MOC process among many psychiatrists.5
Given the professional and personal importance attached to maintaining one’s general and subspecialty certifications, the 2 parts of this article—here and in the January 2015 issue—have been constructed to highlight current ABPN MOC requirements and provide resources for understanding, tracking, and completing the SA and PIP portions.
In addition to this review, I urge all physicians who are subject to MOC to read the 20-page revised MOC Program bookleta (version 2.1, May 2014).5
aDownload the booklet at www.abpn.com/downloads/moc/ moc_web_doc.pdf.
Who must recertify?
As of October 1, 1994, all physicians who achieve ABPN certifications in general psychiatry are issued a 10-year, time-limited certificate that expires on December 31 of the 10th year.3 Note that the 10-year, time-limited certificate in child and adolescent psychiatry began in 1995 and expires 10 years later on December 31.
Certificates in the subspecialties (addiction psychiatry, forensic psychiatry, geriatric psychiatry, etc.), including those issued before October 1, 1994, are 10-year, time-limited certificates that expire on December 31.3 This expiration date often is overlooked by physicians who are exempt from the MOC process for their general psychiatry, or child and adolescent psychiatry certification. There is no exemption for any subspecialty certificate (aside from child and adolescent psychiatry before 1995), regardless of the date of issue.
Moreover, physicians who hold a certificate in a subspecialty also must maintain certification in their specialty (general psychiatry) to apply for recertification in their subspecialization. One exception: Diplomates in child and adolescent psychiatry do not need to maintain current certification in general psychiatry for their subspecialty certification to remain valid or to recertify in child and adolescent psychiatry.
The need to maintain multiple certifications can seem onerous, but note that CME, SA, and PIP activities that have been completed in one area of specialization or subspecialization accrue and count for multiple certifications for diplomates certified in 2 or more areas.5
Get started!
Tracking your progress is critical to keeping up with MOC requirements. You can do this with a personal spreadsheet or by using online resources. Although it is not required, ABPN has established a system that allows diplomates to create and maintain, at no cost, a physician folio on the ABPN server that facilitates documentation of CME hours, including specific SA hours, and PIP module completion.6 All diplomates are required to maintain records of SA activities, CME activities, and PIP units; the ABPN will audit approximately 5% of examination applications.5
Regardless of what documentation method you choose, you should establish an active profile on the ABPN site (www.abpn. com/folios), confirm your contact information, and, if you are not active clinically, update your clinical status. ABPN requires that diplomates self-report their clinical status every 24 months—information that is available to the public. Clinical status also identifies to ABPN those PIP modules that you must complete.
ABPN recognizes 3 categories of clinical status5:
1. Clinically active. Provided any amount of direct or consultative care, or both, in the preceding 24 months, including supervision of residents.
a) Engaged in direct or consultative care, or both, sufficient to complete Improvement in Medical Practice (PIP) units.
b) Engaged in direct or consultative care, or both, that is insufficient to complete PIP units.
2. Clinically inactive. Did not provide direct or consultative care in the preceding 24 months.
3. Status unknown. No information is available on clinical activity.
Based on these definitions, physicians in Category 1a are required to complete all components of the MOC program, including PIP units; physicians in Category 1b or Category 2 are required to complete all components of the MOC program except PIP units.
A change in status from Category 1b or 2 to Category 1a (eg, moving from a purely administrative position to one with clinical duties) requires completion of ≥1 PIP unit.
The easy parts
Licenses. Maintaining your unrestricted professional license(s) is mandatory; the language of this requirement is unambiguous (Table).5 The plural form of license is intentional: Some physicians have medical licenses in multiple states and, in some jurisdictions, licenses are required to supervise physician assistants and other personnel or to prescribe controlled substances. Any restriction on a professional license should be discussed with ABPN and resolved to prevent rejection of the examination application.5
Examinations. For physicians who are not yet enrolled in the continuous-MOC (C-MOC) process (to be discussed in Part 2 of this article), an application to take the examination in Year 10 can be filed in Year 9 of the cycle—after the CME, SA, and PIP requirements are completed. Once a diplomate becomes subject to the C-MOC process by certifying or recertifying from 2012 onwards, completion of each 3-year module of CME, SA, and PIP will not coincide with the 10-year time frame of the examination.
The application deadline for all MOC examinations typically is the year before the examination; the examination should be taken in the year the certificate expires, although it can be taken earlier if desired.7 The examinations are computer-based and administered at a certified testing center. For diplomates who have more than 1 ABPN certificate and want to combine multiple examinations into 1 test session, a reduced fee structure applies.
The general psychiatry examination comprises 220 single-answer, multiple-choice questions that must be completed within 290 minutes, with 10 extra minutes allotted to read on-screen instructions, sign in, and complete a post-examination survey.8 The combined examinations comprise 100 questions from each ABPN specialty or subspecialty area.5
The content of the 2015 general psychiatry examinationb is available on the ABPN Web site.7 Note that the recertification examination in general psychiatry does not cover neurology topics.
bDownload the outline of the examination at www.abpn.com/ downloads/content_outlines/MOC/2015-MOC-Psych-blueprint-060314-EWM-MR.pdf.
Examinations administered in 2015 and 2016 will use only diagnostic criteria that have not changed from DSM-IV-TR9: Neither obsolete diagnoses or subtypes from DSM-IV-TR nor new diagnoses or subtypes in DSM-5 (eg, hoarding disorder) will be tested.9 Diagnoses that are exactly or substantially the same will be tested; these include diagnoses:
• with a name change only (eg, “phonological disorder” in DSM-IV-TR is “speech sound disorder” in DSM-5)
• expanded into >1 new diagnosis (eg, hypochondriasis was expanded to 2 new diagnoses: somatic symptom disorder and illness anxiety disorder)
• subsumed or combined into a new diagnosis (eg, substance use and dependence are now combined into substance use disorder in DSM-5).9
For these diagnoses, both DSM-IV-TR and DSM-5 diagnoses will be provided on examinations.
Beginning in 2017, all examinations will use DSM-5 classifications and diagnostic criteria.9
Part 2 of this article in the January 2015 issue reviews other key aspects of MOC: continuing medical education (CME), including self-assessment requirements; performance in practice (PIP); and continuous maintenance of certification (C-MOC).
BOTTOM LINE
Maintenance of certification (MOC) is a manageable process, although it requires you to be familiar with its various elements, including the duration of certification, licensing requirements, and the examination. Start the process by (1) establishing a login on the ABPN Web site and (2) reviewing the MOC program booklet.
Related Resources
• ABPN MOC home page. www.abpn.com/moc.html
• ABPN-approved products for SA, CME, and PIP modules. www.abpn.com/moc_products.asp
• Peer and patient feedback forms– Peer feedback form v1. www.abpn.com/downloads/moc/PIP-peer-feedback-v1-051914.pdf
– Patient feedback form v1. www.abpn.com/downloads/moc/PIP-patient-feedback-v1-051914.pdf
– Patient feedback form v2. www.abpn.com/downloads/moc/PIP-patient-feedback-v2-051914.pdf
• ABPN physician folio page. https://application.abpn.com/webclient/folios.aspx
Disclosure
Dr. Meyer reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
As part of a general trend among all medical specialty boards, the American Board of Psychiatry and Neurology (ABPN) instituted a recertification process for all new general psychiatry certifications on October 1, 1994.1 In 2000, the individual specialties that constitute the American Board of Medical Specialties (ABMS) subsequently agreed to develop a comprehensive maintenance of certification (MOC) process to demonstrate ongoing learning and competency beyond what could be captured by a recertification examination alone.
All ABMS member boards now use a 4-part process for recertification. For ABPN, those 4 core components are listed in the Table.1,2
ABPN component 1 (maintaining an unrestricted medical license) and component 4 (passing the recertification examination) are straightforward; however, requirements for continuing medical education (CME), including the specific need to accrue ABPN-approved self-assessment (SA) CME hours, and the Improvement in Medical Practice (performance in practice, or PIP) module, have stoked significant commentary and confusion.
Based on feedback,3,4 ABPN in 2014:
• modified the SA and PIP requirements for physicians who certified or recertified between 2005 and 2011
• changed the specific requirement for the PIP feedback component.
These modifications only added to feelings of uncertainty about the MOC process among many psychiatrists.5
Given the professional and personal importance attached to maintaining one’s general and subspecialty certifications, the 2 parts of this article—here and in the January 2015 issue—have been constructed to highlight current ABPN MOC requirements and provide resources for understanding, tracking, and completing the SA and PIP portions.
In addition to this review, I urge all physicians who are subject to MOC to read the 20-page revised MOC Program bookleta (version 2.1, May 2014).5
aDownload the booklet at www.abpn.com/downloads/moc/ moc_web_doc.pdf.
Who must recertify?
As of October 1, 1994, all physicians who achieve ABPN certifications in general psychiatry are issued a 10-year, time-limited certificate that expires on December 31 of the 10th year.3 Note that the 10-year, time-limited certificate in child and adolescent psychiatry began in 1995 and expires 10 years later on December 31.
Certificates in the subspecialties (addiction psychiatry, forensic psychiatry, geriatric psychiatry, etc.), including those issued before October 1, 1994, are 10-year, time-limited certificates that expire on December 31.3 This expiration date often is overlooked by physicians who are exempt from the MOC process for their general psychiatry, or child and adolescent psychiatry certification. There is no exemption for any subspecialty certificate (aside from child and adolescent psychiatry before 1995), regardless of the date of issue.
Moreover, physicians who hold a certificate in a subspecialty also must maintain certification in their specialty (general psychiatry) to apply for recertification in their subspecialization. One exception: Diplomates in child and adolescent psychiatry do not need to maintain current certification in general psychiatry for their subspecialty certification to remain valid or to recertify in child and adolescent psychiatry.
The need to maintain multiple certifications can seem onerous, but note that CME, SA, and PIP activities that have been completed in one area of specialization or subspecialization accrue and count for multiple certifications for diplomates certified in 2 or more areas.5
Get started!
Tracking your progress is critical to keeping up with MOC requirements. You can do this with a personal spreadsheet or by using online resources. Although it is not required, ABPN has established a system that allows diplomates to create and maintain, at no cost, a physician folio on the ABPN server that facilitates documentation of CME hours, including specific SA hours, and PIP module completion.6 All diplomates are required to maintain records of SA activities, CME activities, and PIP units; the ABPN will audit approximately 5% of examination applications.5
Regardless of what documentation method you choose, you should establish an active profile on the ABPN site (www.abpn. com/folios), confirm your contact information, and, if you are not active clinically, update your clinical status. ABPN requires that diplomates self-report their clinical status every 24 months—information that is available to the public. Clinical status also identifies to ABPN those PIP modules that you must complete.
ABPN recognizes 3 categories of clinical status5:
1. Clinically active. Provided any amount of direct or consultative care, or both, in the preceding 24 months, including supervision of residents.
a) Engaged in direct or consultative care, or both, sufficient to complete Improvement in Medical Practice (PIP) units.
b) Engaged in direct or consultative care, or both, that is insufficient to complete PIP units.
2. Clinically inactive. Did not provide direct or consultative care in the preceding 24 months.
3. Status unknown. No information is available on clinical activity.
Based on these definitions, physicians in Category 1a are required to complete all components of the MOC program, including PIP units; physicians in Category 1b or Category 2 are required to complete all components of the MOC program except PIP units.
A change in status from Category 1b or 2 to Category 1a (eg, moving from a purely administrative position to one with clinical duties) requires completion of ≥1 PIP unit.
The easy parts
Licenses. Maintaining your unrestricted professional license(s) is mandatory; the language of this requirement is unambiguous (Table).5 The plural form of license is intentional: Some physicians have medical licenses in multiple states and, in some jurisdictions, licenses are required to supervise physician assistants and other personnel or to prescribe controlled substances. Any restriction on a professional license should be discussed with ABPN and resolved to prevent rejection of the examination application.5
Examinations. For physicians who are not yet enrolled in the continuous-MOC (C-MOC) process (to be discussed in Part 2 of this article), an application to take the examination in Year 10 can be filed in Year 9 of the cycle—after the CME, SA, and PIP requirements are completed. Once a diplomate becomes subject to the C-MOC process by certifying or recertifying from 2012 onwards, completion of each 3-year module of CME, SA, and PIP will not coincide with the 10-year time frame of the examination.
The application deadline for all MOC examinations typically is the year before the examination; the examination should be taken in the year the certificate expires, although it can be taken earlier if desired.7 The examinations are computer-based and administered at a certified testing center. For diplomates who have more than 1 ABPN certificate and want to combine multiple examinations into 1 test session, a reduced fee structure applies.
The general psychiatry examination comprises 220 single-answer, multiple-choice questions that must be completed within 290 minutes, with 10 extra minutes allotted to read on-screen instructions, sign in, and complete a post-examination survey.8 The combined examinations comprise 100 questions from each ABPN specialty or subspecialty area.5
The content of the 2015 general psychiatry examinationb is available on the ABPN Web site.7 Note that the recertification examination in general psychiatry does not cover neurology topics.
bDownload the outline of the examination at www.abpn.com/ downloads/content_outlines/MOC/2015-MOC-Psych-blueprint-060314-EWM-MR.pdf.
Examinations administered in 2015 and 2016 will use only diagnostic criteria that have not changed from DSM-IV-TR9: Neither obsolete diagnoses or subtypes from DSM-IV-TR nor new diagnoses or subtypes in DSM-5 (eg, hoarding disorder) will be tested.9 Diagnoses that are exactly or substantially the same will be tested; these include diagnoses:
• with a name change only (eg, “phonological disorder” in DSM-IV-TR is “speech sound disorder” in DSM-5)
• expanded into >1 new diagnosis (eg, hypochondriasis was expanded to 2 new diagnoses: somatic symptom disorder and illness anxiety disorder)
• subsumed or combined into a new diagnosis (eg, substance use and dependence are now combined into substance use disorder in DSM-5).9
For these diagnoses, both DSM-IV-TR and DSM-5 diagnoses will be provided on examinations.
Beginning in 2017, all examinations will use DSM-5 classifications and diagnostic criteria.9
Part 2 of this article in the January 2015 issue reviews other key aspects of MOC: continuing medical education (CME), including self-assessment requirements; performance in practice (PIP); and continuous maintenance of certification (C-MOC).
BOTTOM LINE
Maintenance of certification (MOC) is a manageable process, although it requires you to be familiar with its various elements, including the duration of certification, licensing requirements, and the examination. Start the process by (1) establishing a login on the ABPN Web site and (2) reviewing the MOC program booklet.
Related Resources
• ABPN MOC home page. www.abpn.com/moc.html
• ABPN-approved products for SA, CME, and PIP modules. www.abpn.com/moc_products.asp
• Peer and patient feedback forms– Peer feedback form v1. www.abpn.com/downloads/moc/PIP-peer-feedback-v1-051914.pdf
– Patient feedback form v1. www.abpn.com/downloads/moc/PIP-patient-feedback-v1-051914.pdf
– Patient feedback form v2. www.abpn.com/downloads/moc/PIP-patient-feedback-v2-051914.pdf
• ABPN physician folio page. https://application.abpn.com/webclient/folios.aspx
Disclosure
Dr. Meyer reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Faulkner LR, Tivnan PW, Winstead DK, et al. The ABPN Maintenance of Certification Program for psychiatrists: past history, current status, and future directions. Acad Psychiatry. 2008;32(3):241-248.
2. Ebert MH, Faulkner L, Stubbe DE, et al. Maintenance of certification in psychiatry. J Clin Psychiatry. 2009;70(10):e39.
3. Faulkner LR, Vondrak PA. Frequently asked questions about maintenance of certification (MOC). J Clin Psychiatry. 2010;71(5):632-633.
4. Faulkner LR, Juul D, Andrade NN, et al. Recent trends in american board of psychiatry and neurology psychiatric subspecialties. Acad Psychiatry. 2011;35(1):35-39.
5. Maintenance of certification program. American Board of Psychiatry and Neurology, Inc. http://abpn.com/ downloads/moc/moc_web_doc.pdf. Published May 2014. Accessed August 25, 2014.
6. Physician folios. American Board of Psychiatry and Neurology, Inc. https://application.abpn.com/webclient/ folios.aspx. Accessed August 25, 2014.
7. Maintenance of certification examination in psychiatry 2015 content blueprint. American Board of Psychiatry and Neurology, Inc. http://www.abpn.com/downloads/ content_outlines/MOC/2015-MOC-Psych-blueprint- 060314-EWM-MR.pdf. Published June 2, 2014. Accessed August 25, 2014.
8. Instructions for the 2015 psychiatry maintenance of certification examination. American Board of Psychiatry and Neurology, Inc. http://www.abpn.com/downloads/ content_outlines/MOC/2015-MOC-Psych-Format-and- Scoring-060214-RL-MR.pdf. Published June 2, 2014. Accessed August 25, 2014.
9. DSM-5 conversion. American Board of Psychiatry and Neurology, Inc. http://www.abpn.com/ifas.html. Accessed August 25, 2014.
1. Faulkner LR, Tivnan PW, Winstead DK, et al. The ABPN Maintenance of Certification Program for psychiatrists: past history, current status, and future directions. Acad Psychiatry. 2008;32(3):241-248.
2. Ebert MH, Faulkner L, Stubbe DE, et al. Maintenance of certification in psychiatry. J Clin Psychiatry. 2009;70(10):e39.
3. Faulkner LR, Vondrak PA. Frequently asked questions about maintenance of certification (MOC). J Clin Psychiatry. 2010;71(5):632-633.
4. Faulkner LR, Juul D, Andrade NN, et al. Recent trends in american board of psychiatry and neurology psychiatric subspecialties. Acad Psychiatry. 2011;35(1):35-39.
5. Maintenance of certification program. American Board of Psychiatry and Neurology, Inc. http://abpn.com/ downloads/moc/moc_web_doc.pdf. Published May 2014. Accessed August 25, 2014.
6. Physician folios. American Board of Psychiatry and Neurology, Inc. https://application.abpn.com/webclient/ folios.aspx. Accessed August 25, 2014.
7. Maintenance of certification examination in psychiatry 2015 content blueprint. American Board of Psychiatry and Neurology, Inc. http://www.abpn.com/downloads/ content_outlines/MOC/2015-MOC-Psych-blueprint- 060314-EWM-MR.pdf. Published June 2, 2014. Accessed August 25, 2014.
8. Instructions for the 2015 psychiatry maintenance of certification examination. American Board of Psychiatry and Neurology, Inc. http://www.abpn.com/downloads/ content_outlines/MOC/2015-MOC-Psych-Format-and- Scoring-060214-RL-MR.pdf. Published June 2, 2014. Accessed August 25, 2014.
9. DSM-5 conversion. American Board of Psychiatry and Neurology, Inc. http://www.abpn.com/ifas.html. Accessed August 25, 2014.
Treating bipolar mania in the outpatient setting: Risk vs reward
Manic episodes, by definition, are associated with significant social or occupational impairment.1 Some manic patients are violent or engage in reckless behaviors that can harm themselves or others, such as speeding, disrupting traffic, or playing with fire. When these patients present to a psychiatrist’s outpatient practice, involuntary hospitalization might be justified.
However, some manic patients, in spite of their elevated, expansive, or irritable mood state, never behave dangerously and might not meet legal criteria for involuntary hospitalization, although these criteria differ from state to state. These patients might see a psychiatrist because manic symptoms such as irritability, talkativeness, and impulsivity are bothersome to their family members but pose no serious danger (Box). In this situation, the psychiatrist can strongly encourage the patient to seek voluntary hospitalization or attend a partial hospitalization program. If the patient declines, the psychiatrist is left with 2 choices: initiate treatment in the outpatient setting or refuse to treat the patient and refer to another provider.
Treating “non-dangerous” mania in the outpatient setting is fraught with challenges:
• the possibility that the patient’s condition will progress to dangerousness
• poor adherence to treatment because of the patient’s limited insight
• the large amount of time required from the psychiatrist and care team to adequately manage the manic episode (eg, time spent with family members, frequent patient visits, and managing communications from the patient).
There are no guidelines to assist the office-based practitioner in treating mania in the outpatient setting. When considering dosing and optimal medication combinations for treating mania, clinical trials may be of limited value because most of these studies only included hospitalized manic patients.
Because of this dearth of knowledge, we provide recommendations based on our review of the literature and from our experience working with manic patients who refuse voluntary hospitalization and could not be hospitalized against their will. These recommendations are organized into 3 sections: diagnostic approach, treatment strategy, and family involvement.
Diagnostic approach
Making a diagnosis of mania might seem straightforward for clinicians who work in inpatient settings; however, mania might not present with classic florid symptoms among outpatients. Patients might have a chief concern of irritability, dysphoria, anxiety, or “insomnia,” which may lead clinicians to focus initially on non-bipolar conditions.2
During the interview, it is important to assess for any current DSM-5 symptoms of a manic episode, while being careful not to accept a patient’s denial of symptoms. Patients with mania often have poor insight and are unaware of changes from their baseline state when manic.3 Alternatively, manic patients may want you to believe that they are well and could minimize or deny all symptoms. Therefore, it is important to pay attention to mental status examination findings, such as hyperverbal speech, elated affect, psychomotor agitation, a tangential thought process, or flight of ideas.
Countertransference feelings of diagnostic confusion or frustration after long patient monologues or multiple interruptions by the patient should be incorporated into the diagnostic assessment. Family members or friends often can provide objective observations of behavioral changes necessary to secure the diagnosis.
Treatment strategy
Decision points. When treating manic outpatients, assess the need for hospitalization at each visit. Advantages of the inpatient setting include:
• the possibility of rapid medication adjustments
• continuous observation to ensure the patient’s safety
• keeping the patient temporarily removed from his community to prevent irreversible social and economic harms.
However, a challenge with hospitalization is third-party payers’ influence on a patient’s length of stay, which may lead to rapid medication changes that may not be clinically ideal.
At each outpatient visit, explore with the patient and family emerging symptoms that could justify involuntary hospitalization. Document whether you recommended inpatient hospitalization, the patient’s response to the recommendation, that you are aware and have considered the risks associated with outpatient care, and that you have discussed these risks with the patient and family.
For patients well-known to the psychiatrist, a history of dangerous mania may lead him (her) to strongly recommend hospitalization, whereas a pre-existing therapeutic alliance and no current or distant history of dangerous mania may lead the clinician to look for alternatives to inpatient care. Concomitant drug or alcohol use may increase the likelihood of mania becoming dangerous, making outpatient treatment ill-advised and riskier for everyone involved.
In exchange for agreeing to provide outpatient care for mania, it often is helpful to negotiate with the patient and family a threshold level of symptoms or behavior that will result in the patient agreeing to voluntary hospitalization (Table 1). Such an agreement can include stopping outpatient treatment if the patient does not improve significantly after 2 or 3 weeks or develops psychotic symptoms. The negotiation also can include partial hospitalization as an option, so long as the patient’s mania continues to be non-dangerous.
Obtaining pretreatment blood work can help a clinician determine whether a medication is safe to prescribe and establish causality if laboratory abnormalities arise after treatment begins. Ideally, the psychiatrist should follow consensus guidelines developed by the International Society for Bipolar Disorders4 or the American Psychiatric Association (APA)5 and order appropriate laboratory tests before prescribing anti-manic medications. Determine the pregnancy status of female patients of child-bearing age before prescribing a potentially teratogenic medication, especially because mania is associated with increased libido.6
Manic patients might be too disorganized to follow up with recommendations for laboratory testing, or could wait several days before completing blood work. Although not ideal, to avoid delaying treatment, a clinician might need to prescribe medication at the initial office visit, without pretreatment laboratory results. When the patient is more organized, complete the blood work. Keeping home pregnancy tests in the office can help rule out pregnancy before prescribing medication.
Medication. Meta-analyses have established the efficacy of mood stabilizers and antipsychotics for treating mania,7,8 and several consensus guidelines have incorporated these findings into treatment algorithms.9
For a patient already taking medications recommended by the guidelines, assess treatment adherence during the initial interview by questioning the patient and family. When the logistics of phlebotomy permit, obtaining the blood level of psychotropics can show the presence of any detectable drug concentration, which demonstrates that the patient has taken the medication recently.
If there is no evidence of nonadherence, an initial step might be to increase the dosage of the antipsychotic or mood stabilizer that the patient is already taking, ensuring that the dosage is optimized based on FDA indications and clinical trials data. The recommended rate of dosage adjustments differs among medications; however, optimal dosing should be reached quickly because a World Federation of Societies of Biological Psychiatry task force recommends that a mania treatment trial not exceed 2 weeks.10
Dosage increases can be made at weekly visits or sooner, based on treatment response and tolerability. If there is no benefit after optimizing the dosage, the next step would be to add a mood stabilizer to a second-generation antipsychotic (SGA), or vice versa to promote additive or synergistic medication effects.11 Switching one medication for the other should be avoided unless there are tolerability concerns.
For a patient who is not taking any medications, select a treatment that balances rapid stabilization with long-term efficacy and tolerability. Table 2 lists FDA-approved treatments for mania. Lamotrigine provides prophylactic efficacy with few associated risks, but it has no anti-manic effects and would be a poor choice for most actively manic patients. Most studies indicate that antipsychotics work faster than lithium at the 1-week mark; however, this may be a function of the lithium titration schedule followed in the protocols, the severity of mania among enrolled patients, the inclusion of typically non-responsive manic patients (eg, mixed) in the analysis, and the antipsychotic’s sedative potential relative to lithium. Although the anti-manic and prophylactic potential of lithium and valproate might make them an ideal first-line option, antipsychotics could stabilize a manic patient faster, especially if agitation is present.12,13
Breaking mania quickly is important when treating patients in the outpatient setting. In these situations, a reasonable choice is to prescribe a SGA, because of their rapid onset of effect, low potential for switch to depression, and utility in treating classic, mixed, or psychotic mania.10 Oral loading of valproate (20 mg/kg) is another option. An inpatient study that used an oral-loading strategy demonstrated a similar time to response as olanzapine,14 in contrast to an inpatient15 and an outpatient study16 that employed a standard starting dosage for each patient and led to slower improvement compared with olanzapine.
SGAs should be dosed moderately and lower than if the patient were hospitalized, to avoid alienating the patient from treatment by causing intolerable side effects. In particular, patients and their families should be warned about immediate risks, such as orthostasis or extrapyramidal symptoms. Although treatment guidelines recommend combination therapy as a possible first-line option,9 in the outpatient setting, monotherapy with an optimally dosed, rapid-acting agent is preferred to promote medication adherence and avoid potentially dangerous sedation. Manic patients experience increased distractibility and verbal memory and executive function impairments that can interfere with medication adherence.17 Therefore, patients are more likely to follow a simpler regimen. If SGA or valproate monotherapy does not control mania, begin combination treatment with a mood stabilizer and SGA. If the patient experiences remission with SGA monotherapy, the risks and benefits of maintaining the SGA vs switching to a mood stabilizer can be discussed.
Provide medication “as needed” for agitation—additional SGA dosing or a benzodiazepine—and explain to family members when their use is warranted. Benzodiazepines can provide short-term benefits for manic patients: anxiety relief, sedation, and anti-manic efficacy as monotherapy18-20 and in combination with other medications.21 Studies showing monotherapy efficacy employed high dosages of benzodiazepines (lorazepam mean dosage, 14 mg/d; clonazepam mean dosage, 13 mg/d)19 and high dosages of antipsychotics as needed,18,20 and often were associated with excessive sedation and ataxia.18,19 This makes benzodiazepine monotherapy a potentially dangerous approach for outpatient treatment of mania. IM lorazepam treated manic agitation less quickly than IM olanzapine, suggesting that SGAs are preferable in the outpatient setting because rapid control of agitation is crucial.22 If prescribed, a trusted family member should dispense benzodiazepines to the patient to minimize misuse because of impulsivity, distractibility, desperation to sleep, or pleasure seeking.
SGAs have the benefit of sedation but occasionally additional sleep medications are required. Benzodiazepine receptor agonists (BzRAs), such as zolpidem, eszopiclone, and zaleplon, should be used with caution. Although these medicines are effective in treating insomnia in individuals with primary insomnia23 and major depression,24 they have not been studied in manic patients. The decreased need for sleep in mania is phenomenologically25 and perhaps biologically different than insomnia in major depression.26 Therefore, mania-associated sleep disturbance might not respond to BZRAs. BzRAs also might induce somnambulism and other parasomnias,27 especially when used in combination with psychotropics, such as valproate28; it is unclear if the manic state itself increases this risk further. Sedating antihistamines with anticholinergic blockade, such as diphenhydramine and low dosages (<100 mg/d) of quetiapine, are best used only in combination with anti-manic medications because of putative link between anticholinergic blockade and manic induction.29 Less studied but safer options include novel anticonvulsants (gabapentin, pregabalin), melatonin, and melatonin receptor agonists. Sedating antidepressants, such as mirtazapine and trazodone, should be avoided.25
Important adjunctive treatment steps include discontinuing all pro-manic agents, including antidepressants, stimulants, and steroids, and discouraging use of caffeine, energy drinks, illicit drugs, and alcohol. The patient should return for office visits at least weekly, and possibly more frequently, depending on severity. Telephone check-in calls between scheduled visits may be necessary until the mania is broken.
Psychotherapy. Other than supportive therapy and psychoeducation, other forms of psychotherapy during mania are not indicated. Psychotherapy trials in bipolar disorder do not inform anti-manic efficacy because few have enrolled acutely manic patients and most report long-term benefits rather than short-term efficacy for the index manic episode.30 Educate patients about the importance of maintaining regular social rhythms and taking medication as prescribed. Manic patients might not be aware that they are acting differently during manic episodes, therefore efforts to improve the patient’s insight are unlikely to succeed. More time should be spent emphasizing the importance of adherence to treatment and taking anti-manic medications as prescribed. This discussion can be enhanced by focusing on the medication’s potential to reduce the unpleasant symptoms of mania, including irritability, insomnia, anxiety, and racing thoughts. At the first visit, discuss setting boundaries with the patient to reduce mania-driven, intrusive phone calls. A patient might develop insight after mania has resolved and he (she) can appreciate social or economic harm that occurred while manic. This discussion might foster adherence to maintenance treatment. Advise your patient to limit activities that may increase stimulation and perpetuate the mania, such as exercise, parties, concerts, or crowded shopping malls. Also, recommend that your patient stop working temporarily, to reduce stress and prevent any manic-driven interactions that could result in job loss.
If your patient has an established relationship with a psychotherapist, discuss with the therapist the plan to initiate mania treatment in the outpatient setting and work as a collaborative team, assuming that the patient has granted permission to share information. Encourage the therapist to increase the frequency of sessions with the patient to enable greater monitoring of changes in the patient’s manic symptoms.
Family involvement
Family support is crucial when treating mania in the outpatient setting. Lacking insight and organization, manic patients require the “auxiliary” judgment of trusted family members to ensure treatment success. The family should identify a single person to act as the liaison between the family and the psychiatrist. The psychiatrist should instruct this individual to accompany the patient to each clinic visit and provide regular updates on the patient’s adherence to treatment, changes in symptoms, and any new behaviors that would justify involuntary hospitalization. The treatment plan should be clearly communicated to this individual to ensure that it is implemented correctly. Ideally, this individual would be someone who understands that bipolar disorder is a mental illness, who can tolerate the patient’s potential resentment of them for taking on this role, and who can influence the patient and the other family members to adhere to the treatment plan.
This family member also should watch the patient take medication to rule out nonadherence if the patient’s condition does not improve.
Provide extensive psychoeducation to the family (Table 3). Discuss these teaching points and their implications at length during the first visit and reinforce them at subsequent visits. Advise spouses that the acute manic period is not the time to make major decisions about their marriage or to engage in couple’s therapy. These options are better explored after the patient recovers from the manic episode.
Encourage the family to engage in mania harm-reduction techniques to the extent that the patient will allow (Table 4). In particular, they should hold onto their loved one’s credit cards and checkbook, and discourage the patient from making any major financial decisions until the mania has resolved. Additionally, patients should be relieved of childcare responsibilities during this period. If there are any child welfare safety concerns, the clinician will need to report this to authorities as required by local laws.
Advise family members or roommates to call emergency services and request a crisis intervention team, or to take the patient to an emergency room if he (she) makes verbal threats to harm themselves or others, is violent, or demonstrates behaviors that indicate that he is no longer able to care for himself. The psychiatrist should assist with completing Family and Medical Leave Act paperwork for family members who will monitor the patient at home, a work-excuse letter for the patient so he does not lose his job, and short-term disability paperwork to ensure income for the patient during the manic period.
These interventions can be challenging for the entire family system because they place family members in a paternalistic role and reduce the patient’s autonomy within the family. This is problematic when these role changes occur between spouses or between a patient-parent and his (her) children. Such changes typically need to be reversed over time and may require the help of a family or couple’s therapist. To support the psychological health of the patient’s family, refer them to the National Alliance on Mental Illness for family support groups or to individual psychotherapists.
Outpatient management can be rewarding
For “non-dangerous” manic patients who cannot be hospitalized involuntarily and refuse full or partial hospitalization, a psychiatrist must choose between beginning treatment in the clinic and referring the patient to another provider. The latter option is consistent with the APA’s ethical guidelines,31 but must be done appropriately to avoid legal liability.32 This decision may disappoint a family desperate to see their loved one recover quickly and may leave them feeling betrayed by the mental health system. On the other hand, choosing to treat mania in the outpatient setting can be rewarding when resolution of mania restores the family’s homeostasis.
To achieve this outcome, the outpatient psychiatrist must engage the patient’s family to ensure that the patient adheres to the treatment plan and monitor for potentially dangerous behavior. The psychiatrist also must use his knowledge of mood symptoms, cognitive impairments, and the psychological experience of manic patients to create a safe and effective treatment strategy that the patient and family can implement.
Because of mania’s unpredictability and destructive potential, psychiatrists who agree to treat manic patients as outpatients should be familiar with their state’s statutes and case law that pertain to the refusal to accept a new patient, patient abandonment, involuntary hospitalization, confidentiality, and mandatory reporting. They also should seek clinical or legal consultation if they feel overwhelmed or uncertain about the safest and most legally sound approach.
Bottom Line
Treating mania in the outpatient setting is risky but can be accomplished in select patients with the help of the patient’s family and a strategy that integrates evidence-based pharmacotherapeutic and psychotherapeutic strategies. Because manic patients could display dangerous behavior, be familiar with your state’s laws regarding involuntary commitment, patient abandonment, and mandatory reporting.
Related Resources
• National Alliance on Mental Illness. www.NAMI.org.
• Depression and Bipolar Support Alliance. www.DBSAlliance.org.
Drug Brand Names
Aripiprazole • Abilify Mirtazapine • Remeron
Asenapine • Saphris Olanzapine • Zyprexa
Carbamazepine • Equetro, Tegretol Pregabalin • Lyrica
Chlorpromazine • Thorazine Quetiapine • Seroquel
Clonazepam • Klonopin Risperidone • Risperdal
Diphrenhydramine • Benadryl Trazodone • Desyrel
Eszopiclone • Lunesta Valproate • Divalproex
Gabapentin • Neurontin Zaleplon • Sonata
Lamotrigine • Lamictal Ziprasidone • Geodon
Lithium • Eskalith, Lithobid Zolpidem • Ambien
Lorazepam • Ativan
Acknowledgement
The authors thank Peter Ash, MD, for carefully reviewing this manuscript and providing feedback.
Disclosures
Dr. Rakofsky receives research or grant support from Takeda. Dr. Dunlop receives research or grant support from Forest, GlaxoSmithKline, and Otsuka.
1. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Cassidy F, Murry E, Forest K, et al. Signs and symptoms of mania in pure and mixed episodes. J Affect Disord. 1998;50(2-3):187-201.
3. Yen CF, Chen CS, Ko CH, et al. Changes in insight among patients with bipolar I disorder: a 2-year prospective study. Bipolar Disord. 2007;9(3):238-242.
4. Ng F, Mammen OK, Wilting I, et al. The International Society for Bipolar Disorders (ISBD) consensus guidelines for the safety monitoring of bipolar disorder treatments. Bipolar Disord. 2009;11(6):559-595.
5. American Psychiatric Association. Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry. 2002;159(suppl 4):1-50.
6. Allison JB, Wilson WP. Sexual behavior of manic patients: a preliminary report. South Med J. 1960;53:870-874.
7. Cipriani A, Barbui C, Salanti G, et al. Comparative efficacy and acceptability of antimanic drugs in acute mania: a multiple-treatments meta-analysis. Lancet. 2011; 378(9799):1306-1315.
8. Yildiz A, Vieta E, Leucht S, et al. Efficacy of antimanic treatments: meta-analysis of randomized, controlled trials. Neuropsychopharmacology. 2011;36(2):375-389.
9. Nivoli AM, Murru A, Goikolea JM, et al. New treatment guidelines for acute bipolar mania: a critical review. J Affect Disord. 2012;140(2):125-141.
10. Grunze H, Vieta E, Goodwin GM, et al. The World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the biological treatment of bipolar disorders: update 2009 on the treatment of acute mania. World J Biol Psychiatry. 2009;10(2):85-116.
11. Tohen M, Chengappa KN, Suppes T, et al. Efficacy of olanzapine in combination with valproate or lithium in the treatment of mania in patients partially nonresponsive to valproate or lithium monotherapy. Arch Gen Psychiatry. 2002;59(1):62-69.
12. Tohen M, Jacobs TG, Feldman PD. Onset of action of antipsychotics in the treatment of mania. Bipolar Disord. 2000;2(3 pt 2):261-268.
13. Goikolea JM, Colom F, Capapey J, et al. Faster onset of antimanic action with haloperidol compared to second-generation antipsychotics. A meta-analysis of randomized clinical trials in acute mania. Eur Neuropsychopharmacol. 2013;23(4):305-316.
14. Zajecka JM, Weisler R, Sachs G, et al. A comparison of the efficacy, safety, and tolerability of divalproex sodium and olanzapine in the treatment of bipolar disorder. J Clin Psychiatry. 2002;63(12):1148-1155.
15. Tohen M, Baker RW, Altshuler LL, et al. Olanzapine versus divalproex in the treatment of acute mania. Am J Psychiatry. 2002;159(6):1011-1017.
16. Tohen M, Vieta E, Goodwin GM, et al. Olanzapine versus divalproex versus placebo in the treatment of mild to moderate mania: a randomized, 12-week, double-blind study. J Clin Psychiatry. 2008;69(11):1776-1789.
17. Martínez-Arán A, Vieta E, Reinares M, et al. Cognitive function across manic or hypomanic, depressed, and euthymic states in bipolar disorder. Am J Psychiatry. 2004; 161(2):262-270.
18. Edwards R, Stephenson U, Flewett T. Clonazepam in acute mania: a double blind trial. Aust N Z J Psychiatry. 1991;25(2):238-242.
19. Bradwejn J, Shriqui C, Koszycki D, et al. Double-blind comparison of the effects of clonazepam and lorazepam in acute mania. J Clin Psychopharmacol. 1990;10(6):403-408.
20. Clark HM, Berk M, Brook S. A randomized controlled single blind study of the efficacy of clonazepam and lithium in the treatment of acute mania. Human Psychopharmacology: Clinical and Experimental. 1997;12(4):325-328.
21. Lenox RH, Newhouse PA, Creelman WL, et al. Adjunctive treatment of manic agitation with lorazepam versus haloperidol: a double-blind study. J Clin Psychiatry. 1992;53(2):47-52.
22. Meehan K, Zhang F, David S, et al. A double-blind, randomized comparison of the efficacy and safety of intramuscular injections of olanzapine, lorazepam, or placebo in treating acutely agitated patients diagnosed with bipolar mania. J Clin Psychopharmacol. 2001;21(4):389-397.
23. Huedo-Medina TB, Kirsch I, Middlemass J, et al. Effectiveness of non-benzodiazepine hypnotics in treatment of adult insomnia: meta-analysis of data submitted to the Food and Drug Administration. BMJ. 2012;345:e8343. doi: 10.1136/bmj.e8343.
24. Fava M, Asnis GM, Shrivastava RK, et al. Improved insomnia symptoms and sleep-related next-day functioning in patients with comorbid major depressive disorder and insomnia following concomitant zolpidem extended-release 12.5 mg and escitalopram treatment: a randomized controlled trial. J Clin Psychiatry. 2011;72(7):914-928.
25. Plante DT, Winkelman JW. Sleep disturbance in bipolar disorder: therapeutic implications. Am J Psychiatry. 2008;165(7):830-843.
26. Linkowski P, Kerkhofs M, Rielaert C, et al. Sleep during mania in manic-depressive males. Eur Arch Psychiatry Neurol Sci. 1986;235(6):339-341.
27. Poceta JS. Zolpidem ingestion, automatisms, and sleep driving: a clinical and legal case series. J Clin Sleep Med. 2011;7(6):632-638.
28. Sattar SP, Ramaswamy S, Bhatia SC, et al. Somnambulism due to probable interaction of valproic acid and zolpidem. Ann Pharmacother. 2003;37(10):1429-1433.
29. Rybakowski JK, Koszewska I, Puzynski S. Anticholinergic mechanisms: a forgotten cause of the switch process in bipolar disorder [Comment on: The neurolobiology of the switch process in bipolar disorder: a review. J Clin Psychiatry. 2010]. J Clin Psychiatry. 2010;71(12):1698-1699; author reply 1699-1700.
30. Miklowitz DJ. Adjunctive psychotherapy for bipolar disorder: state of the evidence. Am J Psychiatry. 2008;165(11):1408-1419.
31. American Psychiatric Association. The principles of medical ethics with annotations especially applicable to psychiatry. Arlington, VA: American Psychiatric Association; 2013.
32. Simon RI, Shuman DW. Clinical manual of psychiatry and law. Arlington, VA: American Psychiatric Publishing; 2007:17-36.
Manic episodes, by definition, are associated with significant social or occupational impairment.1 Some manic patients are violent or engage in reckless behaviors that can harm themselves or others, such as speeding, disrupting traffic, or playing with fire. When these patients present to a psychiatrist’s outpatient practice, involuntary hospitalization might be justified.
However, some manic patients, in spite of their elevated, expansive, or irritable mood state, never behave dangerously and might not meet legal criteria for involuntary hospitalization, although these criteria differ from state to state. These patients might see a psychiatrist because manic symptoms such as irritability, talkativeness, and impulsivity are bothersome to their family members but pose no serious danger (Box). In this situation, the psychiatrist can strongly encourage the patient to seek voluntary hospitalization or attend a partial hospitalization program. If the patient declines, the psychiatrist is left with 2 choices: initiate treatment in the outpatient setting or refuse to treat the patient and refer to another provider.
Treating “non-dangerous” mania in the outpatient setting is fraught with challenges:
• the possibility that the patient’s condition will progress to dangerousness
• poor adherence to treatment because of the patient’s limited insight
• the large amount of time required from the psychiatrist and care team to adequately manage the manic episode (eg, time spent with family members, frequent patient visits, and managing communications from the patient).
There are no guidelines to assist the office-based practitioner in treating mania in the outpatient setting. When considering dosing and optimal medication combinations for treating mania, clinical trials may be of limited value because most of these studies only included hospitalized manic patients.
Because of this dearth of knowledge, we provide recommendations based on our review of the literature and from our experience working with manic patients who refuse voluntary hospitalization and could not be hospitalized against their will. These recommendations are organized into 3 sections: diagnostic approach, treatment strategy, and family involvement.
Diagnostic approach
Making a diagnosis of mania might seem straightforward for clinicians who work in inpatient settings; however, mania might not present with classic florid symptoms among outpatients. Patients might have a chief concern of irritability, dysphoria, anxiety, or “insomnia,” which may lead clinicians to focus initially on non-bipolar conditions.2
During the interview, it is important to assess for any current DSM-5 symptoms of a manic episode, while being careful not to accept a patient’s denial of symptoms. Patients with mania often have poor insight and are unaware of changes from their baseline state when manic.3 Alternatively, manic patients may want you to believe that they are well and could minimize or deny all symptoms. Therefore, it is important to pay attention to mental status examination findings, such as hyperverbal speech, elated affect, psychomotor agitation, a tangential thought process, or flight of ideas.
Countertransference feelings of diagnostic confusion or frustration after long patient monologues or multiple interruptions by the patient should be incorporated into the diagnostic assessment. Family members or friends often can provide objective observations of behavioral changes necessary to secure the diagnosis.
Treatment strategy
Decision points. When treating manic outpatients, assess the need for hospitalization at each visit. Advantages of the inpatient setting include:
• the possibility of rapid medication adjustments
• continuous observation to ensure the patient’s safety
• keeping the patient temporarily removed from his community to prevent irreversible social and economic harms.
However, a challenge with hospitalization is third-party payers’ influence on a patient’s length of stay, which may lead to rapid medication changes that may not be clinically ideal.
At each outpatient visit, explore with the patient and family emerging symptoms that could justify involuntary hospitalization. Document whether you recommended inpatient hospitalization, the patient’s response to the recommendation, that you are aware and have considered the risks associated with outpatient care, and that you have discussed these risks with the patient and family.
For patients well-known to the psychiatrist, a history of dangerous mania may lead him (her) to strongly recommend hospitalization, whereas a pre-existing therapeutic alliance and no current or distant history of dangerous mania may lead the clinician to look for alternatives to inpatient care. Concomitant drug or alcohol use may increase the likelihood of mania becoming dangerous, making outpatient treatment ill-advised and riskier for everyone involved.
In exchange for agreeing to provide outpatient care for mania, it often is helpful to negotiate with the patient and family a threshold level of symptoms or behavior that will result in the patient agreeing to voluntary hospitalization (Table 1). Such an agreement can include stopping outpatient treatment if the patient does not improve significantly after 2 or 3 weeks or develops psychotic symptoms. The negotiation also can include partial hospitalization as an option, so long as the patient’s mania continues to be non-dangerous.
Obtaining pretreatment blood work can help a clinician determine whether a medication is safe to prescribe and establish causality if laboratory abnormalities arise after treatment begins. Ideally, the psychiatrist should follow consensus guidelines developed by the International Society for Bipolar Disorders4 or the American Psychiatric Association (APA)5 and order appropriate laboratory tests before prescribing anti-manic medications. Determine the pregnancy status of female patients of child-bearing age before prescribing a potentially teratogenic medication, especially because mania is associated with increased libido.6
Manic patients might be too disorganized to follow up with recommendations for laboratory testing, or could wait several days before completing blood work. Although not ideal, to avoid delaying treatment, a clinician might need to prescribe medication at the initial office visit, without pretreatment laboratory results. When the patient is more organized, complete the blood work. Keeping home pregnancy tests in the office can help rule out pregnancy before prescribing medication.
Medication. Meta-analyses have established the efficacy of mood stabilizers and antipsychotics for treating mania,7,8 and several consensus guidelines have incorporated these findings into treatment algorithms.9
For a patient already taking medications recommended by the guidelines, assess treatment adherence during the initial interview by questioning the patient and family. When the logistics of phlebotomy permit, obtaining the blood level of psychotropics can show the presence of any detectable drug concentration, which demonstrates that the patient has taken the medication recently.
If there is no evidence of nonadherence, an initial step might be to increase the dosage of the antipsychotic or mood stabilizer that the patient is already taking, ensuring that the dosage is optimized based on FDA indications and clinical trials data. The recommended rate of dosage adjustments differs among medications; however, optimal dosing should be reached quickly because a World Federation of Societies of Biological Psychiatry task force recommends that a mania treatment trial not exceed 2 weeks.10
Dosage increases can be made at weekly visits or sooner, based on treatment response and tolerability. If there is no benefit after optimizing the dosage, the next step would be to add a mood stabilizer to a second-generation antipsychotic (SGA), or vice versa to promote additive or synergistic medication effects.11 Switching one medication for the other should be avoided unless there are tolerability concerns.
For a patient who is not taking any medications, select a treatment that balances rapid stabilization with long-term efficacy and tolerability. Table 2 lists FDA-approved treatments for mania. Lamotrigine provides prophylactic efficacy with few associated risks, but it has no anti-manic effects and would be a poor choice for most actively manic patients. Most studies indicate that antipsychotics work faster than lithium at the 1-week mark; however, this may be a function of the lithium titration schedule followed in the protocols, the severity of mania among enrolled patients, the inclusion of typically non-responsive manic patients (eg, mixed) in the analysis, and the antipsychotic’s sedative potential relative to lithium. Although the anti-manic and prophylactic potential of lithium and valproate might make them an ideal first-line option, antipsychotics could stabilize a manic patient faster, especially if agitation is present.12,13
Breaking mania quickly is important when treating patients in the outpatient setting. In these situations, a reasonable choice is to prescribe a SGA, because of their rapid onset of effect, low potential for switch to depression, and utility in treating classic, mixed, or psychotic mania.10 Oral loading of valproate (20 mg/kg) is another option. An inpatient study that used an oral-loading strategy demonstrated a similar time to response as olanzapine,14 in contrast to an inpatient15 and an outpatient study16 that employed a standard starting dosage for each patient and led to slower improvement compared with olanzapine.
SGAs should be dosed moderately and lower than if the patient were hospitalized, to avoid alienating the patient from treatment by causing intolerable side effects. In particular, patients and their families should be warned about immediate risks, such as orthostasis or extrapyramidal symptoms. Although treatment guidelines recommend combination therapy as a possible first-line option,9 in the outpatient setting, monotherapy with an optimally dosed, rapid-acting agent is preferred to promote medication adherence and avoid potentially dangerous sedation. Manic patients experience increased distractibility and verbal memory and executive function impairments that can interfere with medication adherence.17 Therefore, patients are more likely to follow a simpler regimen. If SGA or valproate monotherapy does not control mania, begin combination treatment with a mood stabilizer and SGA. If the patient experiences remission with SGA monotherapy, the risks and benefits of maintaining the SGA vs switching to a mood stabilizer can be discussed.
Provide medication “as needed” for agitation—additional SGA dosing or a benzodiazepine—and explain to family members when their use is warranted. Benzodiazepines can provide short-term benefits for manic patients: anxiety relief, sedation, and anti-manic efficacy as monotherapy18-20 and in combination with other medications.21 Studies showing monotherapy efficacy employed high dosages of benzodiazepines (lorazepam mean dosage, 14 mg/d; clonazepam mean dosage, 13 mg/d)19 and high dosages of antipsychotics as needed,18,20 and often were associated with excessive sedation and ataxia.18,19 This makes benzodiazepine monotherapy a potentially dangerous approach for outpatient treatment of mania. IM lorazepam treated manic agitation less quickly than IM olanzapine, suggesting that SGAs are preferable in the outpatient setting because rapid control of agitation is crucial.22 If prescribed, a trusted family member should dispense benzodiazepines to the patient to minimize misuse because of impulsivity, distractibility, desperation to sleep, or pleasure seeking.
SGAs have the benefit of sedation but occasionally additional sleep medications are required. Benzodiazepine receptor agonists (BzRAs), such as zolpidem, eszopiclone, and zaleplon, should be used with caution. Although these medicines are effective in treating insomnia in individuals with primary insomnia23 and major depression,24 they have not been studied in manic patients. The decreased need for sleep in mania is phenomenologically25 and perhaps biologically different than insomnia in major depression.26 Therefore, mania-associated sleep disturbance might not respond to BZRAs. BzRAs also might induce somnambulism and other parasomnias,27 especially when used in combination with psychotropics, such as valproate28; it is unclear if the manic state itself increases this risk further. Sedating antihistamines with anticholinergic blockade, such as diphenhydramine and low dosages (<100 mg/d) of quetiapine, are best used only in combination with anti-manic medications because of putative link between anticholinergic blockade and manic induction.29 Less studied but safer options include novel anticonvulsants (gabapentin, pregabalin), melatonin, and melatonin receptor agonists. Sedating antidepressants, such as mirtazapine and trazodone, should be avoided.25
Important adjunctive treatment steps include discontinuing all pro-manic agents, including antidepressants, stimulants, and steroids, and discouraging use of caffeine, energy drinks, illicit drugs, and alcohol. The patient should return for office visits at least weekly, and possibly more frequently, depending on severity. Telephone check-in calls between scheduled visits may be necessary until the mania is broken.
Psychotherapy. Other than supportive therapy and psychoeducation, other forms of psychotherapy during mania are not indicated. Psychotherapy trials in bipolar disorder do not inform anti-manic efficacy because few have enrolled acutely manic patients and most report long-term benefits rather than short-term efficacy for the index manic episode.30 Educate patients about the importance of maintaining regular social rhythms and taking medication as prescribed. Manic patients might not be aware that they are acting differently during manic episodes, therefore efforts to improve the patient’s insight are unlikely to succeed. More time should be spent emphasizing the importance of adherence to treatment and taking anti-manic medications as prescribed. This discussion can be enhanced by focusing on the medication’s potential to reduce the unpleasant symptoms of mania, including irritability, insomnia, anxiety, and racing thoughts. At the first visit, discuss setting boundaries with the patient to reduce mania-driven, intrusive phone calls. A patient might develop insight after mania has resolved and he (she) can appreciate social or economic harm that occurred while manic. This discussion might foster adherence to maintenance treatment. Advise your patient to limit activities that may increase stimulation and perpetuate the mania, such as exercise, parties, concerts, or crowded shopping malls. Also, recommend that your patient stop working temporarily, to reduce stress and prevent any manic-driven interactions that could result in job loss.
If your patient has an established relationship with a psychotherapist, discuss with the therapist the plan to initiate mania treatment in the outpatient setting and work as a collaborative team, assuming that the patient has granted permission to share information. Encourage the therapist to increase the frequency of sessions with the patient to enable greater monitoring of changes in the patient’s manic symptoms.
Family involvement
Family support is crucial when treating mania in the outpatient setting. Lacking insight and organization, manic patients require the “auxiliary” judgment of trusted family members to ensure treatment success. The family should identify a single person to act as the liaison between the family and the psychiatrist. The psychiatrist should instruct this individual to accompany the patient to each clinic visit and provide regular updates on the patient’s adherence to treatment, changes in symptoms, and any new behaviors that would justify involuntary hospitalization. The treatment plan should be clearly communicated to this individual to ensure that it is implemented correctly. Ideally, this individual would be someone who understands that bipolar disorder is a mental illness, who can tolerate the patient’s potential resentment of them for taking on this role, and who can influence the patient and the other family members to adhere to the treatment plan.
This family member also should watch the patient take medication to rule out nonadherence if the patient’s condition does not improve.
Provide extensive psychoeducation to the family (Table 3). Discuss these teaching points and their implications at length during the first visit and reinforce them at subsequent visits. Advise spouses that the acute manic period is not the time to make major decisions about their marriage or to engage in couple’s therapy. These options are better explored after the patient recovers from the manic episode.
Encourage the family to engage in mania harm-reduction techniques to the extent that the patient will allow (Table 4). In particular, they should hold onto their loved one’s credit cards and checkbook, and discourage the patient from making any major financial decisions until the mania has resolved. Additionally, patients should be relieved of childcare responsibilities during this period. If there are any child welfare safety concerns, the clinician will need to report this to authorities as required by local laws.
Advise family members or roommates to call emergency services and request a crisis intervention team, or to take the patient to an emergency room if he (she) makes verbal threats to harm themselves or others, is violent, or demonstrates behaviors that indicate that he is no longer able to care for himself. The psychiatrist should assist with completing Family and Medical Leave Act paperwork for family members who will monitor the patient at home, a work-excuse letter for the patient so he does not lose his job, and short-term disability paperwork to ensure income for the patient during the manic period.
These interventions can be challenging for the entire family system because they place family members in a paternalistic role and reduce the patient’s autonomy within the family. This is problematic when these role changes occur between spouses or between a patient-parent and his (her) children. Such changes typically need to be reversed over time and may require the help of a family or couple’s therapist. To support the psychological health of the patient’s family, refer them to the National Alliance on Mental Illness for family support groups or to individual psychotherapists.
Outpatient management can be rewarding
For “non-dangerous” manic patients who cannot be hospitalized involuntarily and refuse full or partial hospitalization, a psychiatrist must choose between beginning treatment in the clinic and referring the patient to another provider. The latter option is consistent with the APA’s ethical guidelines,31 but must be done appropriately to avoid legal liability.32 This decision may disappoint a family desperate to see their loved one recover quickly and may leave them feeling betrayed by the mental health system. On the other hand, choosing to treat mania in the outpatient setting can be rewarding when resolution of mania restores the family’s homeostasis.
To achieve this outcome, the outpatient psychiatrist must engage the patient’s family to ensure that the patient adheres to the treatment plan and monitor for potentially dangerous behavior. The psychiatrist also must use his knowledge of mood symptoms, cognitive impairments, and the psychological experience of manic patients to create a safe and effective treatment strategy that the patient and family can implement.
Because of mania’s unpredictability and destructive potential, psychiatrists who agree to treat manic patients as outpatients should be familiar with their state’s statutes and case law that pertain to the refusal to accept a new patient, patient abandonment, involuntary hospitalization, confidentiality, and mandatory reporting. They also should seek clinical or legal consultation if they feel overwhelmed or uncertain about the safest and most legally sound approach.
Bottom Line
Treating mania in the outpatient setting is risky but can be accomplished in select patients with the help of the patient’s family and a strategy that integrates evidence-based pharmacotherapeutic and psychotherapeutic strategies. Because manic patients could display dangerous behavior, be familiar with your state’s laws regarding involuntary commitment, patient abandonment, and mandatory reporting.
Related Resources
• National Alliance on Mental Illness. www.NAMI.org.
• Depression and Bipolar Support Alliance. www.DBSAlliance.org.
Drug Brand Names
Aripiprazole • Abilify Mirtazapine • Remeron
Asenapine • Saphris Olanzapine • Zyprexa
Carbamazepine • Equetro, Tegretol Pregabalin • Lyrica
Chlorpromazine • Thorazine Quetiapine • Seroquel
Clonazepam • Klonopin Risperidone • Risperdal
Diphrenhydramine • Benadryl Trazodone • Desyrel
Eszopiclone • Lunesta Valproate • Divalproex
Gabapentin • Neurontin Zaleplon • Sonata
Lamotrigine • Lamictal Ziprasidone • Geodon
Lithium • Eskalith, Lithobid Zolpidem • Ambien
Lorazepam • Ativan
Acknowledgement
The authors thank Peter Ash, MD, for carefully reviewing this manuscript and providing feedback.
Disclosures
Dr. Rakofsky receives research or grant support from Takeda. Dr. Dunlop receives research or grant support from Forest, GlaxoSmithKline, and Otsuka.
Manic episodes, by definition, are associated with significant social or occupational impairment.1 Some manic patients are violent or engage in reckless behaviors that can harm themselves or others, such as speeding, disrupting traffic, or playing with fire. When these patients present to a psychiatrist’s outpatient practice, involuntary hospitalization might be justified.
However, some manic patients, in spite of their elevated, expansive, or irritable mood state, never behave dangerously and might not meet legal criteria for involuntary hospitalization, although these criteria differ from state to state. These patients might see a psychiatrist because manic symptoms such as irritability, talkativeness, and impulsivity are bothersome to their family members but pose no serious danger (Box). In this situation, the psychiatrist can strongly encourage the patient to seek voluntary hospitalization or attend a partial hospitalization program. If the patient declines, the psychiatrist is left with 2 choices: initiate treatment in the outpatient setting or refuse to treat the patient and refer to another provider.
Treating “non-dangerous” mania in the outpatient setting is fraught with challenges:
• the possibility that the patient’s condition will progress to dangerousness
• poor adherence to treatment because of the patient’s limited insight
• the large amount of time required from the psychiatrist and care team to adequately manage the manic episode (eg, time spent with family members, frequent patient visits, and managing communications from the patient).
There are no guidelines to assist the office-based practitioner in treating mania in the outpatient setting. When considering dosing and optimal medication combinations for treating mania, clinical trials may be of limited value because most of these studies only included hospitalized manic patients.
Because of this dearth of knowledge, we provide recommendations based on our review of the literature and from our experience working with manic patients who refuse voluntary hospitalization and could not be hospitalized against their will. These recommendations are organized into 3 sections: diagnostic approach, treatment strategy, and family involvement.
Diagnostic approach
Making a diagnosis of mania might seem straightforward for clinicians who work in inpatient settings; however, mania might not present with classic florid symptoms among outpatients. Patients might have a chief concern of irritability, dysphoria, anxiety, or “insomnia,” which may lead clinicians to focus initially on non-bipolar conditions.2
During the interview, it is important to assess for any current DSM-5 symptoms of a manic episode, while being careful not to accept a patient’s denial of symptoms. Patients with mania often have poor insight and are unaware of changes from their baseline state when manic.3 Alternatively, manic patients may want you to believe that they are well and could minimize or deny all symptoms. Therefore, it is important to pay attention to mental status examination findings, such as hyperverbal speech, elated affect, psychomotor agitation, a tangential thought process, or flight of ideas.
Countertransference feelings of diagnostic confusion or frustration after long patient monologues or multiple interruptions by the patient should be incorporated into the diagnostic assessment. Family members or friends often can provide objective observations of behavioral changes necessary to secure the diagnosis.
Treatment strategy
Decision points. When treating manic outpatients, assess the need for hospitalization at each visit. Advantages of the inpatient setting include:
• the possibility of rapid medication adjustments
• continuous observation to ensure the patient’s safety
• keeping the patient temporarily removed from his community to prevent irreversible social and economic harms.
However, a challenge with hospitalization is third-party payers’ influence on a patient’s length of stay, which may lead to rapid medication changes that may not be clinically ideal.
At each outpatient visit, explore with the patient and family emerging symptoms that could justify involuntary hospitalization. Document whether you recommended inpatient hospitalization, the patient’s response to the recommendation, that you are aware and have considered the risks associated with outpatient care, and that you have discussed these risks with the patient and family.
For patients well-known to the psychiatrist, a history of dangerous mania may lead him (her) to strongly recommend hospitalization, whereas a pre-existing therapeutic alliance and no current or distant history of dangerous mania may lead the clinician to look for alternatives to inpatient care. Concomitant drug or alcohol use may increase the likelihood of mania becoming dangerous, making outpatient treatment ill-advised and riskier for everyone involved.
In exchange for agreeing to provide outpatient care for mania, it often is helpful to negotiate with the patient and family a threshold level of symptoms or behavior that will result in the patient agreeing to voluntary hospitalization (Table 1). Such an agreement can include stopping outpatient treatment if the patient does not improve significantly after 2 or 3 weeks or develops psychotic symptoms. The negotiation also can include partial hospitalization as an option, so long as the patient’s mania continues to be non-dangerous.
Obtaining pretreatment blood work can help a clinician determine whether a medication is safe to prescribe and establish causality if laboratory abnormalities arise after treatment begins. Ideally, the psychiatrist should follow consensus guidelines developed by the International Society for Bipolar Disorders4 or the American Psychiatric Association (APA)5 and order appropriate laboratory tests before prescribing anti-manic medications. Determine the pregnancy status of female patients of child-bearing age before prescribing a potentially teratogenic medication, especially because mania is associated with increased libido.6
Manic patients might be too disorganized to follow up with recommendations for laboratory testing, or could wait several days before completing blood work. Although not ideal, to avoid delaying treatment, a clinician might need to prescribe medication at the initial office visit, without pretreatment laboratory results. When the patient is more organized, complete the blood work. Keeping home pregnancy tests in the office can help rule out pregnancy before prescribing medication.
Medication. Meta-analyses have established the efficacy of mood stabilizers and antipsychotics for treating mania,7,8 and several consensus guidelines have incorporated these findings into treatment algorithms.9
For a patient already taking medications recommended by the guidelines, assess treatment adherence during the initial interview by questioning the patient and family. When the logistics of phlebotomy permit, obtaining the blood level of psychotropics can show the presence of any detectable drug concentration, which demonstrates that the patient has taken the medication recently.
If there is no evidence of nonadherence, an initial step might be to increase the dosage of the antipsychotic or mood stabilizer that the patient is already taking, ensuring that the dosage is optimized based on FDA indications and clinical trials data. The recommended rate of dosage adjustments differs among medications; however, optimal dosing should be reached quickly because a World Federation of Societies of Biological Psychiatry task force recommends that a mania treatment trial not exceed 2 weeks.10
Dosage increases can be made at weekly visits or sooner, based on treatment response and tolerability. If there is no benefit after optimizing the dosage, the next step would be to add a mood stabilizer to a second-generation antipsychotic (SGA), or vice versa to promote additive or synergistic medication effects.11 Switching one medication for the other should be avoided unless there are tolerability concerns.
For a patient who is not taking any medications, select a treatment that balances rapid stabilization with long-term efficacy and tolerability. Table 2 lists FDA-approved treatments for mania. Lamotrigine provides prophylactic efficacy with few associated risks, but it has no anti-manic effects and would be a poor choice for most actively manic patients. Most studies indicate that antipsychotics work faster than lithium at the 1-week mark; however, this may be a function of the lithium titration schedule followed in the protocols, the severity of mania among enrolled patients, the inclusion of typically non-responsive manic patients (eg, mixed) in the analysis, and the antipsychotic’s sedative potential relative to lithium. Although the anti-manic and prophylactic potential of lithium and valproate might make them an ideal first-line option, antipsychotics could stabilize a manic patient faster, especially if agitation is present.12,13
Breaking mania quickly is important when treating patients in the outpatient setting. In these situations, a reasonable choice is to prescribe a SGA, because of their rapid onset of effect, low potential for switch to depression, and utility in treating classic, mixed, or psychotic mania.10 Oral loading of valproate (20 mg/kg) is another option. An inpatient study that used an oral-loading strategy demonstrated a similar time to response as olanzapine,14 in contrast to an inpatient15 and an outpatient study16 that employed a standard starting dosage for each patient and led to slower improvement compared with olanzapine.
SGAs should be dosed moderately and lower than if the patient were hospitalized, to avoid alienating the patient from treatment by causing intolerable side effects. In particular, patients and their families should be warned about immediate risks, such as orthostasis or extrapyramidal symptoms. Although treatment guidelines recommend combination therapy as a possible first-line option,9 in the outpatient setting, monotherapy with an optimally dosed, rapid-acting agent is preferred to promote medication adherence and avoid potentially dangerous sedation. Manic patients experience increased distractibility and verbal memory and executive function impairments that can interfere with medication adherence.17 Therefore, patients are more likely to follow a simpler regimen. If SGA or valproate monotherapy does not control mania, begin combination treatment with a mood stabilizer and SGA. If the patient experiences remission with SGA monotherapy, the risks and benefits of maintaining the SGA vs switching to a mood stabilizer can be discussed.
Provide medication “as needed” for agitation—additional SGA dosing or a benzodiazepine—and explain to family members when their use is warranted. Benzodiazepines can provide short-term benefits for manic patients: anxiety relief, sedation, and anti-manic efficacy as monotherapy18-20 and in combination with other medications.21 Studies showing monotherapy efficacy employed high dosages of benzodiazepines (lorazepam mean dosage, 14 mg/d; clonazepam mean dosage, 13 mg/d)19 and high dosages of antipsychotics as needed,18,20 and often were associated with excessive sedation and ataxia.18,19 This makes benzodiazepine monotherapy a potentially dangerous approach for outpatient treatment of mania. IM lorazepam treated manic agitation less quickly than IM olanzapine, suggesting that SGAs are preferable in the outpatient setting because rapid control of agitation is crucial.22 If prescribed, a trusted family member should dispense benzodiazepines to the patient to minimize misuse because of impulsivity, distractibility, desperation to sleep, or pleasure seeking.
SGAs have the benefit of sedation but occasionally additional sleep medications are required. Benzodiazepine receptor agonists (BzRAs), such as zolpidem, eszopiclone, and zaleplon, should be used with caution. Although these medicines are effective in treating insomnia in individuals with primary insomnia23 and major depression,24 they have not been studied in manic patients. The decreased need for sleep in mania is phenomenologically25 and perhaps biologically different than insomnia in major depression.26 Therefore, mania-associated sleep disturbance might not respond to BZRAs. BzRAs also might induce somnambulism and other parasomnias,27 especially when used in combination with psychotropics, such as valproate28; it is unclear if the manic state itself increases this risk further. Sedating antihistamines with anticholinergic blockade, such as diphenhydramine and low dosages (<100 mg/d) of quetiapine, are best used only in combination with anti-manic medications because of putative link between anticholinergic blockade and manic induction.29 Less studied but safer options include novel anticonvulsants (gabapentin, pregabalin), melatonin, and melatonin receptor agonists. Sedating antidepressants, such as mirtazapine and trazodone, should be avoided.25
Important adjunctive treatment steps include discontinuing all pro-manic agents, including antidepressants, stimulants, and steroids, and discouraging use of caffeine, energy drinks, illicit drugs, and alcohol. The patient should return for office visits at least weekly, and possibly more frequently, depending on severity. Telephone check-in calls between scheduled visits may be necessary until the mania is broken.
Psychotherapy. Other than supportive therapy and psychoeducation, other forms of psychotherapy during mania are not indicated. Psychotherapy trials in bipolar disorder do not inform anti-manic efficacy because few have enrolled acutely manic patients and most report long-term benefits rather than short-term efficacy for the index manic episode.30 Educate patients about the importance of maintaining regular social rhythms and taking medication as prescribed. Manic patients might not be aware that they are acting differently during manic episodes, therefore efforts to improve the patient’s insight are unlikely to succeed. More time should be spent emphasizing the importance of adherence to treatment and taking anti-manic medications as prescribed. This discussion can be enhanced by focusing on the medication’s potential to reduce the unpleasant symptoms of mania, including irritability, insomnia, anxiety, and racing thoughts. At the first visit, discuss setting boundaries with the patient to reduce mania-driven, intrusive phone calls. A patient might develop insight after mania has resolved and he (she) can appreciate social or economic harm that occurred while manic. This discussion might foster adherence to maintenance treatment. Advise your patient to limit activities that may increase stimulation and perpetuate the mania, such as exercise, parties, concerts, or crowded shopping malls. Also, recommend that your patient stop working temporarily, to reduce stress and prevent any manic-driven interactions that could result in job loss.
If your patient has an established relationship with a psychotherapist, discuss with the therapist the plan to initiate mania treatment in the outpatient setting and work as a collaborative team, assuming that the patient has granted permission to share information. Encourage the therapist to increase the frequency of sessions with the patient to enable greater monitoring of changes in the patient’s manic symptoms.
Family involvement
Family support is crucial when treating mania in the outpatient setting. Lacking insight and organization, manic patients require the “auxiliary” judgment of trusted family members to ensure treatment success. The family should identify a single person to act as the liaison between the family and the psychiatrist. The psychiatrist should instruct this individual to accompany the patient to each clinic visit and provide regular updates on the patient’s adherence to treatment, changes in symptoms, and any new behaviors that would justify involuntary hospitalization. The treatment plan should be clearly communicated to this individual to ensure that it is implemented correctly. Ideally, this individual would be someone who understands that bipolar disorder is a mental illness, who can tolerate the patient’s potential resentment of them for taking on this role, and who can influence the patient and the other family members to adhere to the treatment plan.
This family member also should watch the patient take medication to rule out nonadherence if the patient’s condition does not improve.
Provide extensive psychoeducation to the family (Table 3). Discuss these teaching points and their implications at length during the first visit and reinforce them at subsequent visits. Advise spouses that the acute manic period is not the time to make major decisions about their marriage or to engage in couple’s therapy. These options are better explored after the patient recovers from the manic episode.
Encourage the family to engage in mania harm-reduction techniques to the extent that the patient will allow (Table 4). In particular, they should hold onto their loved one’s credit cards and checkbook, and discourage the patient from making any major financial decisions until the mania has resolved. Additionally, patients should be relieved of childcare responsibilities during this period. If there are any child welfare safety concerns, the clinician will need to report this to authorities as required by local laws.
Advise family members or roommates to call emergency services and request a crisis intervention team, or to take the patient to an emergency room if he (she) makes verbal threats to harm themselves or others, is violent, or demonstrates behaviors that indicate that he is no longer able to care for himself. The psychiatrist should assist with completing Family and Medical Leave Act paperwork for family members who will monitor the patient at home, a work-excuse letter for the patient so he does not lose his job, and short-term disability paperwork to ensure income for the patient during the manic period.
These interventions can be challenging for the entire family system because they place family members in a paternalistic role and reduce the patient’s autonomy within the family. This is problematic when these role changes occur between spouses or between a patient-parent and his (her) children. Such changes typically need to be reversed over time and may require the help of a family or couple’s therapist. To support the psychological health of the patient’s family, refer them to the National Alliance on Mental Illness for family support groups or to individual psychotherapists.
Outpatient management can be rewarding
For “non-dangerous” manic patients who cannot be hospitalized involuntarily and refuse full or partial hospitalization, a psychiatrist must choose between beginning treatment in the clinic and referring the patient to another provider. The latter option is consistent with the APA’s ethical guidelines,31 but must be done appropriately to avoid legal liability.32 This decision may disappoint a family desperate to see their loved one recover quickly and may leave them feeling betrayed by the mental health system. On the other hand, choosing to treat mania in the outpatient setting can be rewarding when resolution of mania restores the family’s homeostasis.
To achieve this outcome, the outpatient psychiatrist must engage the patient’s family to ensure that the patient adheres to the treatment plan and monitor for potentially dangerous behavior. The psychiatrist also must use his knowledge of mood symptoms, cognitive impairments, and the psychological experience of manic patients to create a safe and effective treatment strategy that the patient and family can implement.
Because of mania’s unpredictability and destructive potential, psychiatrists who agree to treat manic patients as outpatients should be familiar with their state’s statutes and case law that pertain to the refusal to accept a new patient, patient abandonment, involuntary hospitalization, confidentiality, and mandatory reporting. They also should seek clinical or legal consultation if they feel overwhelmed or uncertain about the safest and most legally sound approach.
Bottom Line
Treating mania in the outpatient setting is risky but can be accomplished in select patients with the help of the patient’s family and a strategy that integrates evidence-based pharmacotherapeutic and psychotherapeutic strategies. Because manic patients could display dangerous behavior, be familiar with your state’s laws regarding involuntary commitment, patient abandonment, and mandatory reporting.
Related Resources
• National Alliance on Mental Illness. www.NAMI.org.
• Depression and Bipolar Support Alliance. www.DBSAlliance.org.
Drug Brand Names
Aripiprazole • Abilify Mirtazapine • Remeron
Asenapine • Saphris Olanzapine • Zyprexa
Carbamazepine • Equetro, Tegretol Pregabalin • Lyrica
Chlorpromazine • Thorazine Quetiapine • Seroquel
Clonazepam • Klonopin Risperidone • Risperdal
Diphrenhydramine • Benadryl Trazodone • Desyrel
Eszopiclone • Lunesta Valproate • Divalproex
Gabapentin • Neurontin Zaleplon • Sonata
Lamotrigine • Lamictal Ziprasidone • Geodon
Lithium • Eskalith, Lithobid Zolpidem • Ambien
Lorazepam • Ativan
Acknowledgement
The authors thank Peter Ash, MD, for carefully reviewing this manuscript and providing feedback.
Disclosures
Dr. Rakofsky receives research or grant support from Takeda. Dr. Dunlop receives research or grant support from Forest, GlaxoSmithKline, and Otsuka.
1. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Cassidy F, Murry E, Forest K, et al. Signs and symptoms of mania in pure and mixed episodes. J Affect Disord. 1998;50(2-3):187-201.
3. Yen CF, Chen CS, Ko CH, et al. Changes in insight among patients with bipolar I disorder: a 2-year prospective study. Bipolar Disord. 2007;9(3):238-242.
4. Ng F, Mammen OK, Wilting I, et al. The International Society for Bipolar Disorders (ISBD) consensus guidelines for the safety monitoring of bipolar disorder treatments. Bipolar Disord. 2009;11(6):559-595.
5. American Psychiatric Association. Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry. 2002;159(suppl 4):1-50.
6. Allison JB, Wilson WP. Sexual behavior of manic patients: a preliminary report. South Med J. 1960;53:870-874.
7. Cipriani A, Barbui C, Salanti G, et al. Comparative efficacy and acceptability of antimanic drugs in acute mania: a multiple-treatments meta-analysis. Lancet. 2011; 378(9799):1306-1315.
8. Yildiz A, Vieta E, Leucht S, et al. Efficacy of antimanic treatments: meta-analysis of randomized, controlled trials. Neuropsychopharmacology. 2011;36(2):375-389.
9. Nivoli AM, Murru A, Goikolea JM, et al. New treatment guidelines for acute bipolar mania: a critical review. J Affect Disord. 2012;140(2):125-141.
10. Grunze H, Vieta E, Goodwin GM, et al. The World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the biological treatment of bipolar disorders: update 2009 on the treatment of acute mania. World J Biol Psychiatry. 2009;10(2):85-116.
11. Tohen M, Chengappa KN, Suppes T, et al. Efficacy of olanzapine in combination with valproate or lithium in the treatment of mania in patients partially nonresponsive to valproate or lithium monotherapy. Arch Gen Psychiatry. 2002;59(1):62-69.
12. Tohen M, Jacobs TG, Feldman PD. Onset of action of antipsychotics in the treatment of mania. Bipolar Disord. 2000;2(3 pt 2):261-268.
13. Goikolea JM, Colom F, Capapey J, et al. Faster onset of antimanic action with haloperidol compared to second-generation antipsychotics. A meta-analysis of randomized clinical trials in acute mania. Eur Neuropsychopharmacol. 2013;23(4):305-316.
14. Zajecka JM, Weisler R, Sachs G, et al. A comparison of the efficacy, safety, and tolerability of divalproex sodium and olanzapine in the treatment of bipolar disorder. J Clin Psychiatry. 2002;63(12):1148-1155.
15. Tohen M, Baker RW, Altshuler LL, et al. Olanzapine versus divalproex in the treatment of acute mania. Am J Psychiatry. 2002;159(6):1011-1017.
16. Tohen M, Vieta E, Goodwin GM, et al. Olanzapine versus divalproex versus placebo in the treatment of mild to moderate mania: a randomized, 12-week, double-blind study. J Clin Psychiatry. 2008;69(11):1776-1789.
17. Martínez-Arán A, Vieta E, Reinares M, et al. Cognitive function across manic or hypomanic, depressed, and euthymic states in bipolar disorder. Am J Psychiatry. 2004; 161(2):262-270.
18. Edwards R, Stephenson U, Flewett T. Clonazepam in acute mania: a double blind trial. Aust N Z J Psychiatry. 1991;25(2):238-242.
19. Bradwejn J, Shriqui C, Koszycki D, et al. Double-blind comparison of the effects of clonazepam and lorazepam in acute mania. J Clin Psychopharmacol. 1990;10(6):403-408.
20. Clark HM, Berk M, Brook S. A randomized controlled single blind study of the efficacy of clonazepam and lithium in the treatment of acute mania. Human Psychopharmacology: Clinical and Experimental. 1997;12(4):325-328.
21. Lenox RH, Newhouse PA, Creelman WL, et al. Adjunctive treatment of manic agitation with lorazepam versus haloperidol: a double-blind study. J Clin Psychiatry. 1992;53(2):47-52.
22. Meehan K, Zhang F, David S, et al. A double-blind, randomized comparison of the efficacy and safety of intramuscular injections of olanzapine, lorazepam, or placebo in treating acutely agitated patients diagnosed with bipolar mania. J Clin Psychopharmacol. 2001;21(4):389-397.
23. Huedo-Medina TB, Kirsch I, Middlemass J, et al. Effectiveness of non-benzodiazepine hypnotics in treatment of adult insomnia: meta-analysis of data submitted to the Food and Drug Administration. BMJ. 2012;345:e8343. doi: 10.1136/bmj.e8343.
24. Fava M, Asnis GM, Shrivastava RK, et al. Improved insomnia symptoms and sleep-related next-day functioning in patients with comorbid major depressive disorder and insomnia following concomitant zolpidem extended-release 12.5 mg and escitalopram treatment: a randomized controlled trial. J Clin Psychiatry. 2011;72(7):914-928.
25. Plante DT, Winkelman JW. Sleep disturbance in bipolar disorder: therapeutic implications. Am J Psychiatry. 2008;165(7):830-843.
26. Linkowski P, Kerkhofs M, Rielaert C, et al. Sleep during mania in manic-depressive males. Eur Arch Psychiatry Neurol Sci. 1986;235(6):339-341.
27. Poceta JS. Zolpidem ingestion, automatisms, and sleep driving: a clinical and legal case series. J Clin Sleep Med. 2011;7(6):632-638.
28. Sattar SP, Ramaswamy S, Bhatia SC, et al. Somnambulism due to probable interaction of valproic acid and zolpidem. Ann Pharmacother. 2003;37(10):1429-1433.
29. Rybakowski JK, Koszewska I, Puzynski S. Anticholinergic mechanisms: a forgotten cause of the switch process in bipolar disorder [Comment on: The neurolobiology of the switch process in bipolar disorder: a review. J Clin Psychiatry. 2010]. J Clin Psychiatry. 2010;71(12):1698-1699; author reply 1699-1700.
30. Miklowitz DJ. Adjunctive psychotherapy for bipolar disorder: state of the evidence. Am J Psychiatry. 2008;165(11):1408-1419.
31. American Psychiatric Association. The principles of medical ethics with annotations especially applicable to psychiatry. Arlington, VA: American Psychiatric Association; 2013.
32. Simon RI, Shuman DW. Clinical manual of psychiatry and law. Arlington, VA: American Psychiatric Publishing; 2007:17-36.
1. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Cassidy F, Murry E, Forest K, et al. Signs and symptoms of mania in pure and mixed episodes. J Affect Disord. 1998;50(2-3):187-201.
3. Yen CF, Chen CS, Ko CH, et al. Changes in insight among patients with bipolar I disorder: a 2-year prospective study. Bipolar Disord. 2007;9(3):238-242.
4. Ng F, Mammen OK, Wilting I, et al. The International Society for Bipolar Disorders (ISBD) consensus guidelines for the safety monitoring of bipolar disorder treatments. Bipolar Disord. 2009;11(6):559-595.
5. American Psychiatric Association. Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry. 2002;159(suppl 4):1-50.
6. Allison JB, Wilson WP. Sexual behavior of manic patients: a preliminary report. South Med J. 1960;53:870-874.
7. Cipriani A, Barbui C, Salanti G, et al. Comparative efficacy and acceptability of antimanic drugs in acute mania: a multiple-treatments meta-analysis. Lancet. 2011; 378(9799):1306-1315.
8. Yildiz A, Vieta E, Leucht S, et al. Efficacy of antimanic treatments: meta-analysis of randomized, controlled trials. Neuropsychopharmacology. 2011;36(2):375-389.
9. Nivoli AM, Murru A, Goikolea JM, et al. New treatment guidelines for acute bipolar mania: a critical review. J Affect Disord. 2012;140(2):125-141.
10. Grunze H, Vieta E, Goodwin GM, et al. The World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the biological treatment of bipolar disorders: update 2009 on the treatment of acute mania. World J Biol Psychiatry. 2009;10(2):85-116.
11. Tohen M, Chengappa KN, Suppes T, et al. Efficacy of olanzapine in combination with valproate or lithium in the treatment of mania in patients partially nonresponsive to valproate or lithium monotherapy. Arch Gen Psychiatry. 2002;59(1):62-69.
12. Tohen M, Jacobs TG, Feldman PD. Onset of action of antipsychotics in the treatment of mania. Bipolar Disord. 2000;2(3 pt 2):261-268.
13. Goikolea JM, Colom F, Capapey J, et al. Faster onset of antimanic action with haloperidol compared to second-generation antipsychotics. A meta-analysis of randomized clinical trials in acute mania. Eur Neuropsychopharmacol. 2013;23(4):305-316.
14. Zajecka JM, Weisler R, Sachs G, et al. A comparison of the efficacy, safety, and tolerability of divalproex sodium and olanzapine in the treatment of bipolar disorder. J Clin Psychiatry. 2002;63(12):1148-1155.
15. Tohen M, Baker RW, Altshuler LL, et al. Olanzapine versus divalproex in the treatment of acute mania. Am J Psychiatry. 2002;159(6):1011-1017.
16. Tohen M, Vieta E, Goodwin GM, et al. Olanzapine versus divalproex versus placebo in the treatment of mild to moderate mania: a randomized, 12-week, double-blind study. J Clin Psychiatry. 2008;69(11):1776-1789.
17. Martínez-Arán A, Vieta E, Reinares M, et al. Cognitive function across manic or hypomanic, depressed, and euthymic states in bipolar disorder. Am J Psychiatry. 2004; 161(2):262-270.
18. Edwards R, Stephenson U, Flewett T. Clonazepam in acute mania: a double blind trial. Aust N Z J Psychiatry. 1991;25(2):238-242.
19. Bradwejn J, Shriqui C, Koszycki D, et al. Double-blind comparison of the effects of clonazepam and lorazepam in acute mania. J Clin Psychopharmacol. 1990;10(6):403-408.
20. Clark HM, Berk M, Brook S. A randomized controlled single blind study of the efficacy of clonazepam and lithium in the treatment of acute mania. Human Psychopharmacology: Clinical and Experimental. 1997;12(4):325-328.
21. Lenox RH, Newhouse PA, Creelman WL, et al. Adjunctive treatment of manic agitation with lorazepam versus haloperidol: a double-blind study. J Clin Psychiatry. 1992;53(2):47-52.
22. Meehan K, Zhang F, David S, et al. A double-blind, randomized comparison of the efficacy and safety of intramuscular injections of olanzapine, lorazepam, or placebo in treating acutely agitated patients diagnosed with bipolar mania. J Clin Psychopharmacol. 2001;21(4):389-397.
23. Huedo-Medina TB, Kirsch I, Middlemass J, et al. Effectiveness of non-benzodiazepine hypnotics in treatment of adult insomnia: meta-analysis of data submitted to the Food and Drug Administration. BMJ. 2012;345:e8343. doi: 10.1136/bmj.e8343.
24. Fava M, Asnis GM, Shrivastava RK, et al. Improved insomnia symptoms and sleep-related next-day functioning in patients with comorbid major depressive disorder and insomnia following concomitant zolpidem extended-release 12.5 mg and escitalopram treatment: a randomized controlled trial. J Clin Psychiatry. 2011;72(7):914-928.
25. Plante DT, Winkelman JW. Sleep disturbance in bipolar disorder: therapeutic implications. Am J Psychiatry. 2008;165(7):830-843.
26. Linkowski P, Kerkhofs M, Rielaert C, et al. Sleep during mania in manic-depressive males. Eur Arch Psychiatry Neurol Sci. 1986;235(6):339-341.
27. Poceta JS. Zolpidem ingestion, automatisms, and sleep driving: a clinical and legal case series. J Clin Sleep Med. 2011;7(6):632-638.
28. Sattar SP, Ramaswamy S, Bhatia SC, et al. Somnambulism due to probable interaction of valproic acid and zolpidem. Ann Pharmacother. 2003;37(10):1429-1433.
29. Rybakowski JK, Koszewska I, Puzynski S. Anticholinergic mechanisms: a forgotten cause of the switch process in bipolar disorder [Comment on: The neurolobiology of the switch process in bipolar disorder: a review. J Clin Psychiatry. 2010]. J Clin Psychiatry. 2010;71(12):1698-1699; author reply 1699-1700.
30. Miklowitz DJ. Adjunctive psychotherapy for bipolar disorder: state of the evidence. Am J Psychiatry. 2008;165(11):1408-1419.
31. American Psychiatric Association. The principles of medical ethics with annotations especially applicable to psychiatry. Arlington, VA: American Psychiatric Association; 2013.
32. Simon RI, Shuman DW. Clinical manual of psychiatry and law. Arlington, VA: American Psychiatric Publishing; 2007:17-36.
Suicide assessment and management self-test: How do you score?
As explained in the first part of this article in the October 2014 issue of Current Psychiatry, assessing and managing suicide risk are complex, difficult tasks without clear-cut, easy solutions. The case-based, multiple-choice self-test, with accompanying commentary, presented here is designed to enhance one’s ability to provide care for patients at risk for suicide. Part 2 of this article poses the remaining 7 of 15 questions, which are based on clinical experience and the referenced work of others.
Question 9
Mr. N, age 62, will be discharged from the psychiatric unit tomorrow. He was admitted after an overdose suicide attempt. Mr. N was depressed after the loss of his business and was “treating” his depression and anxiety with alcohol. He is successfully withdrawn from alcohol and responds to medication and supportive psychotherapy. During a family meeting with staff, Mr. N’s wife states that he keeps a gun by his bedside. Mr. N has improved and is eager to go home.
Before discharging Mr. N, the psychiatrist or staff should:
a) instruct Mr. N to remove the gun from his bedside
b) instruct his wife to remove the gun from the home
c) instruct the wife to look for >1 gun
d) instruct the wife, before Mr. N’s discharge, to call the staff once guns and
ammunition are safely removed according to the pre-arranged safety plan
e) instruct the wife to lock up the gun in a place that is not known to the patient
The best response option is D
Guns in the home are associated with a significant increase in suicide. All patients at risk for suicide must be asked if guns are available at home or easily accessible elsewhere, or if they intend to purchase a gun. Gun safety management requires a collaborative team approach including the clinician, patient, and person designated responsible for removing guns from the home.1 The responsible person should be required to call the clinician to confirm that the guns have been removed and secured according to the plan. The principles of gun safety management apply to outpatients, inpatients, and emergency patients, although implementation varies according to the clinical setting.
Asking the patient to remove guns from the home is too risky. Guns must be safely secured before the patient is discharged. Asking a spouse, other family member, or partner is necessary. The person asked must be willing to remove guns and ammunition according to a pre-arranged plan requiring a callback upon completion. A callback is essential because a family member in denial may do nothing to remove the guns or lock or “hide” them in the home where they will be found by a determined suicidal patient. Guns may be available outside the home, such as in the car, at the work place, or for purchase.
The essence of gun safety management is verification. Trust but verify or, better yet, verify, then trust.
Question 10
A recently admitted 56-year-old inpatient was discovered wrapping a towel around her neck. She denied suicidal intent; however, the treatment team viewed the incident as a suicide rehearsal. She was placed on one-to-one close observation.
Inpatient suicides frequently occur:
a) shortly after admission
b) during staff shift changes
c) at meal times
d) shortly after discharge
e) all of the above
The best response option is E
Inpatient suicides also occur at increased frequency when psychiatric residents finish their rotations and in understaffed psychiatric units.2 Undue delay in the evaluation of a newly admitted acute, high-risk patient might allow the patient to commit suicide.
Most patient suicides occur shortly after hospital discharge (a few hours, days, or weeks later). Appleby et al3 found that the highest number of suicides occurred during the first week after discharge. Meehan et al4 found that suicide occurred most frequently during the first 2 weeks post-discharge; the highest number of suicides occurred on the first day after discharge.
Question 11
Ms. G, a 43-year-old, single woman in acute suicide crisis, is admitted to the psychiatric unit of a general hospital. She is diagnosed with bipolar I disorder, most recent episode depressed, and borderline personality disorder. She has had multiple psychiatric hospitalizations, all precipitated by a suicide crisis. The average length of stay on the psychiatric unit is 6.3 days. After 7 days of intensive treatment, Ms. G is stabilized and suicide risk is reduced. The treatment team prepares for her discharge.
Ms. G’s suicide risk at discharge is most likely at:
a) indeterminate risk
b) low risk
c) moderate risk
d) chronic high risk
e) acute high risk
The best response option is D
The length of stay in many acute care psychiatric facilities is <7 days. The goal of hospitalization is to stabilize the patient and discharge to appropriate community mental health resources. Discharge planning begins at the time of admission.
Reducing Ms. G’s suicide risk to low or moderate is unlikely because of her diagnoses, frequent hospitalizations, and acute high risk for suicide on admission. After acute, high-risk suicidal patients are treated, many revert to chronic high risk for suicide.
Patients at chronic high risk for suicide often are treated as outpatients, except when an acute suicidal crisis requires hospitalization.5 At discharge from the hospital, the goal is to return the patient to outpatient treatment.
A discharge note identifies the acute suicide risk factors that have abated and the chronic (long-term) suicide risk factors that remain. The discharge note also addresses a patient’s chronic vulnerability to suicide. For example, a patient can become acutely suicidal again, depending on a number of factors, including the nature and cause of the psychiatric illness, adequacy of future treatment, adherence to treatment recommendations, and unforeseeable life vicissitudes.
Question 12
A 20-year-old college student is hospitalized after an overdose suicide attempt. Failing grades, panic attacks, and depression precipitated the suicide attempt. After 8 days of hospitalization, she is much improved and ready for discharge. She is assessed to be at low to moderate suicide risk. The treating psychiatrist and social worker convene a family meeting with both parents and an older brother. The family’s role after discharge is discussed.
All of the following options are helpful family roles except:
a) provide constant 24-hour family supervision
b) provide emotional support
c) observe and report symptoms and behaviors of concern
d) encourage adherence with treatment
e) provide helpful feedback about the patient’s thoughts and behavior
The best response option is A
The family’s role is important, but it is not a substitute for constant safety management provided by trained mental health professionals on an inpatient psychiatric unit.5 Early discharge of an inpatient by relying on family supervision can be precarious. Most inpatients are discharged at some level of suicide risk, given the short length of hospital stay. If an outpatient at risk of suicide requires constant 24-hour family supervision, then psychiatric hospitalization is indicated.
Patients who are intent on killing themselves can find ingenious ways to attempt or commit suicide. Asking family members to keep a constant watch often fails. Most family members will not follow the patient into the bathroom or be able to stay up all night to observe the patient. Moreover, family members find reasons to make exceptions to constant surveillance because of denial, fatigue, or the need to attend to other pressing matters.
Question 13
During the initial evaluation of a patient, it is the psychiatrist’s practice to routinely inquire about current and past suicide ideation. An affirmative answer prompts a systematic suicide risk assessment. In the absence of current risk, if exploration of the patient’s history reveals chronic suicide risk factors, the psychiatrist conducts a systematic suicide risk assessment.
The chronic risk factor that has the highest association with suicide is:
a) family history of mental illness or suicide
b) childhood abuse
c) history of a suicide attempt
d) impulsivity or aggression
e) prior psychiatric hospitalization
The best response option is C
A comprehensive suicide risk assessment may not be required at the initial outpatient evaluation in the absence of acute suicide risk factors. However, chronic suicide risk factors may be present.
The Standard Mortality Ratio (SMR) for prior suicide attempts by any method was 38.61.6 Suicide risk was highest in the 2 years after the first attempt. The SMR is a measure of the relative risk of suicide compared with the expected rate in the general population (SMR of 1).
Some chronic suicide risk factors are static: for example, a family history of psychiatric illness or earlier suicide attempt. Other chronic risk factors, usually a trait characteristic, can become acute: for example, impulsivity or aggression, or deliberate self-harm. The presence of chronic suicide risk factors should prompt a systematic suicide risk assessment. Evaluation of chronic suicide risk factors is an essential component of comprehensive assessment.5
Question 14
A psychiatrist is treating Dr. R, a 43-year-old physician, for anxiety and depression. The psychiatrist sees Dr. R twice a week for psychotherapy and medication management. A recent lawsuit filed against Dr. R has severely exacerbated her symptoms. She can sleep for only a few hours. Suicide ideation has emerged, frightening Dr. R and her family. The psychiatrist performs a systematic suicide risk assessment and determines that Dr. R is at acute high risk for suicide.
The psychiatrist recommends immediate hospitalization, but Dr. R adamantly refuses. The psychiatrist decides not to involuntarily hospitalize her because she does not meet the substantive criteria of the state involuntary commitment statute (eg, overt suicidal behaviors). The psychiatrist chooses to continue outpatient treatment.
Clinical interventions to reduce Dr. R’s suicide risk include:
a) see her more often
b) adjust medications
c) obtain a consult
d) refer her to an intensive outpatient program
e) all of the above
The best response option is E
To hospitalize or not to hospitalize— that is the conundrum that psychiatrists often face with high-risk suicidal patients. The decision is more complicated when the need for hospitalization is clear but the patient refuses. The decisions that the psychiatrist makes at this point are crucial for treatment and risk management.5
If the patient disagrees with the psychiatrist’s recommendation to hospitalize, refusal should be addressed as a treatment issue. When the need for hospitalization is acute, a prolonged inquiry is not possible. In addition, the therapeutic alliance may become strained. This clinical situation tries a clinician’s professional mettle.
Consultation and referral are options to consider if time and the patient’s condition allows. A psychiatric clinician should never worry alone; sleepless nights benefit neither the psychiatrist nor the patient.
As Dr. R’s case shows, a psychiatrist might decide not to hospitalize a patient who is assessed to be at moderate or high risk of suicide. Protective factors may allow continuing outpatient treatment. A good therapeutic alliance may be present if the psychiatrist has worked with the patient for some time. Family support also may be available.
The clinician must determine if the patient’s suicide risk can be managed by more frequent visits and treatment adjustments. Also, supportive family members can help by providing observational data. Protective factors can be overwhelmed by a severe mental illness. In contrast, a patient assessed as being at moderate risk of suicide might need to be hospitalized when protective factors are few or absent.
The psychiatrist may determine that a patient at high risk of suicide who refuses hospitalization does not meet criteria for involuntary hospitalization. For example, criteria might require that the patient must have made a suicide attempt within a specified period of time. States have provisions in their commitment statutes granting immunity from liability if the clinician uses reasonable clinical judgment and acts in good faith when involuntarily hospitalizing a patient.7
Question 15
Mr. U, a 39-year-old, married engineer, is ready to be discharged from the inpatient unit. He was admitted 7 days earlier for acute alcohol intoxication and suicidal threats. He has undergone successful detoxification. Mr. U has had 2 similar episodes within the past year.
The treatment team conducts a risk-benefit analysis for both discharge and continued hospitalization. A consultation also is obtained.
The discharge decision will be most influenced by:
a) presence of family support
b) compliance with follow-up care
c) availability of dual diagnosis programs
d) systematic suicide risk assessment
e) consultation
The best response option is D
All of the options in Question 15 concerning discharge planning of patients at risk for suicide are important. However, conducting a systematic suicide risk assessment to inform discharge planning is the most critical. Mr. U had 2 previous psychiatric admissions for alcohol abuse and suicidal ideation. He is a chronic suicide risk who becomes high risk when intoxicated.
Discharge planning begins at admission and is refined during the patient’s stay. Before a patient is discharged, a final post-discharge treatment and aftercare plan is necessary. After discharge, suicide risk increases as the intensity of treatment decreases.8
The patient’s willingness to cooperate with discharge and aftercare planning is critical in establishing contact with follow-up treaters. The treatment team should structure the follow-up plan to encourage compliance. For example, psychotic patients at risk of suicide who have a history of stopping medications after discharge can be given a long-acting IM antipsychotic that will last until they reach aftercare. Patients with comorbid drug and alcohol abuse disorders are referred to agencies equipped to manage dual-diagnosis patients.
Psychiatrists’ ability to ensure follow-up treatment is limited, a fact that must be acknowledged by the psychiatric and legal communities. Beyond patient stabilization, a clinician’s options to bring about positive changes can be limited or nonexistent. Also, the patient’s failure to adhere to post-discharge plans and treatment often leads to rehospitalization, hopelessness, and greater suicide risk.
Psychiatric patients at moderate or moderate-to-high risk for suicide increasingly are treated in outpatient settings. It is the responsibility of the clinician and the treatment team to competently hand off the patient to appropriate outpatient aftercare. With the patient’s permission, the psychiatrist or social worker should call the follow-up agency or therapist before discharge to provide information about the patient’s diagnosis, treatment, and hospital course.
Last, follow-up appointments should be made as close to the time of discharge as possible. Suicide often occurs on the first day after discharge.3
Bottom Line
Fully commit time and effort to the ongoing assessment, treatment, and management of patients at suicide risk. Suicide risk assessment is a process, not an event. Conduct a suicide risk assessment at important clinical junctures (eg, initial evaluation, discharge, changing observation levels). Contemporaneously, document suicide risk assessments. This self-assessment helps clinicians gauge their strengths and identify skills that need further development.
Disclosure
Dr. Simon reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Simon is the co-editor of The American Psychiatry Publishing textbook of Suicide Assessment and Management, 2nd edition, from which this article is adapted, by permission of the publisher, American Psychiatry Publishing, Inc. ©2012.
1. Simon RI. Gun safety management with patients at risk for suicide. Suicide Life Threat Behav. 2007;37(5):518-526.
2. Qin P, Nordenoft M. Suicide risk in relation to psychiatric hospitalization: evidence based on longitudinal registers. Arch Gen Psychiatry. 2005;62(4):427-432.
3. Appleby L, Shaw J, Amos T, et al. Suicide within 12 months of contact with mental health services: national clinical survey. BMJ. 1999;318(7193):1235-1239.
4. Meehan J, Kapur N, Hunt IM, et al. Suicide in mental health in-patients within 3 months of discharge. National clinical survey. Br J Psychiatry. 2006;188:129-134.
5. Simon RI. Preventing patient suicide: clinical assessment and management. Arlington, VA: American Psychiatric Publishing, Inc.; 2011.
6. Harris CE, Barraclough B. Suicide as an outcome for mental disorders. A meta-analysis. Br J Psychiatry. 1997;170:205-228.
7. Simon RI, Shuman DW. Clinical manual of psychiatry and law. Arlington, VA: American Psychiatric Publishing, Inc.; 2007.
8. Appleby L, Dennehy JA, Thomas CS, et al. Aftercare and clinical characteristics of people with mental illness who commit suicide: a case-control study. Lancet. 1999;353(9162):1397-1400.
As explained in the first part of this article in the October 2014 issue of Current Psychiatry, assessing and managing suicide risk are complex, difficult tasks without clear-cut, easy solutions. The case-based, multiple-choice self-test, with accompanying commentary, presented here is designed to enhance one’s ability to provide care for patients at risk for suicide. Part 2 of this article poses the remaining 7 of 15 questions, which are based on clinical experience and the referenced work of others.
Question 9
Mr. N, age 62, will be discharged from the psychiatric unit tomorrow. He was admitted after an overdose suicide attempt. Mr. N was depressed after the loss of his business and was “treating” his depression and anxiety with alcohol. He is successfully withdrawn from alcohol and responds to medication and supportive psychotherapy. During a family meeting with staff, Mr. N’s wife states that he keeps a gun by his bedside. Mr. N has improved and is eager to go home.
Before discharging Mr. N, the psychiatrist or staff should:
a) instruct Mr. N to remove the gun from his bedside
b) instruct his wife to remove the gun from the home
c) instruct the wife to look for >1 gun
d) instruct the wife, before Mr. N’s discharge, to call the staff once guns and
ammunition are safely removed according to the pre-arranged safety plan
e) instruct the wife to lock up the gun in a place that is not known to the patient
The best response option is D
Guns in the home are associated with a significant increase in suicide. All patients at risk for suicide must be asked if guns are available at home or easily accessible elsewhere, or if they intend to purchase a gun. Gun safety management requires a collaborative team approach including the clinician, patient, and person designated responsible for removing guns from the home.1 The responsible person should be required to call the clinician to confirm that the guns have been removed and secured according to the plan. The principles of gun safety management apply to outpatients, inpatients, and emergency patients, although implementation varies according to the clinical setting.
Asking the patient to remove guns from the home is too risky. Guns must be safely secured before the patient is discharged. Asking a spouse, other family member, or partner is necessary. The person asked must be willing to remove guns and ammunition according to a pre-arranged plan requiring a callback upon completion. A callback is essential because a family member in denial may do nothing to remove the guns or lock or “hide” them in the home where they will be found by a determined suicidal patient. Guns may be available outside the home, such as in the car, at the work place, or for purchase.
The essence of gun safety management is verification. Trust but verify or, better yet, verify, then trust.
Question 10
A recently admitted 56-year-old inpatient was discovered wrapping a towel around her neck. She denied suicidal intent; however, the treatment team viewed the incident as a suicide rehearsal. She was placed on one-to-one close observation.
Inpatient suicides frequently occur:
a) shortly after admission
b) during staff shift changes
c) at meal times
d) shortly after discharge
e) all of the above
The best response option is E
Inpatient suicides also occur at increased frequency when psychiatric residents finish their rotations and in understaffed psychiatric units.2 Undue delay in the evaluation of a newly admitted acute, high-risk patient might allow the patient to commit suicide.
Most patient suicides occur shortly after hospital discharge (a few hours, days, or weeks later). Appleby et al3 found that the highest number of suicides occurred during the first week after discharge. Meehan et al4 found that suicide occurred most frequently during the first 2 weeks post-discharge; the highest number of suicides occurred on the first day after discharge.
Question 11
Ms. G, a 43-year-old, single woman in acute suicide crisis, is admitted to the psychiatric unit of a general hospital. She is diagnosed with bipolar I disorder, most recent episode depressed, and borderline personality disorder. She has had multiple psychiatric hospitalizations, all precipitated by a suicide crisis. The average length of stay on the psychiatric unit is 6.3 days. After 7 days of intensive treatment, Ms. G is stabilized and suicide risk is reduced. The treatment team prepares for her discharge.
Ms. G’s suicide risk at discharge is most likely at:
a) indeterminate risk
b) low risk
c) moderate risk
d) chronic high risk
e) acute high risk
The best response option is D
The length of stay in many acute care psychiatric facilities is <7 days. The goal of hospitalization is to stabilize the patient and discharge to appropriate community mental health resources. Discharge planning begins at the time of admission.
Reducing Ms. G’s suicide risk to low or moderate is unlikely because of her diagnoses, frequent hospitalizations, and acute high risk for suicide on admission. After acute, high-risk suicidal patients are treated, many revert to chronic high risk for suicide.
Patients at chronic high risk for suicide often are treated as outpatients, except when an acute suicidal crisis requires hospitalization.5 At discharge from the hospital, the goal is to return the patient to outpatient treatment.
A discharge note identifies the acute suicide risk factors that have abated and the chronic (long-term) suicide risk factors that remain. The discharge note also addresses a patient’s chronic vulnerability to suicide. For example, a patient can become acutely suicidal again, depending on a number of factors, including the nature and cause of the psychiatric illness, adequacy of future treatment, adherence to treatment recommendations, and unforeseeable life vicissitudes.
Question 12
A 20-year-old college student is hospitalized after an overdose suicide attempt. Failing grades, panic attacks, and depression precipitated the suicide attempt. After 8 days of hospitalization, she is much improved and ready for discharge. She is assessed to be at low to moderate suicide risk. The treating psychiatrist and social worker convene a family meeting with both parents and an older brother. The family’s role after discharge is discussed.
All of the following options are helpful family roles except:
a) provide constant 24-hour family supervision
b) provide emotional support
c) observe and report symptoms and behaviors of concern
d) encourage adherence with treatment
e) provide helpful feedback about the patient’s thoughts and behavior
The best response option is A
The family’s role is important, but it is not a substitute for constant safety management provided by trained mental health professionals on an inpatient psychiatric unit.5 Early discharge of an inpatient by relying on family supervision can be precarious. Most inpatients are discharged at some level of suicide risk, given the short length of hospital stay. If an outpatient at risk of suicide requires constant 24-hour family supervision, then psychiatric hospitalization is indicated.
Patients who are intent on killing themselves can find ingenious ways to attempt or commit suicide. Asking family members to keep a constant watch often fails. Most family members will not follow the patient into the bathroom or be able to stay up all night to observe the patient. Moreover, family members find reasons to make exceptions to constant surveillance because of denial, fatigue, or the need to attend to other pressing matters.
Question 13
During the initial evaluation of a patient, it is the psychiatrist’s practice to routinely inquire about current and past suicide ideation. An affirmative answer prompts a systematic suicide risk assessment. In the absence of current risk, if exploration of the patient’s history reveals chronic suicide risk factors, the psychiatrist conducts a systematic suicide risk assessment.
The chronic risk factor that has the highest association with suicide is:
a) family history of mental illness or suicide
b) childhood abuse
c) history of a suicide attempt
d) impulsivity or aggression
e) prior psychiatric hospitalization
The best response option is C
A comprehensive suicide risk assessment may not be required at the initial outpatient evaluation in the absence of acute suicide risk factors. However, chronic suicide risk factors may be present.
The Standard Mortality Ratio (SMR) for prior suicide attempts by any method was 38.61.6 Suicide risk was highest in the 2 years after the first attempt. The SMR is a measure of the relative risk of suicide compared with the expected rate in the general population (SMR of 1).
Some chronic suicide risk factors are static: for example, a family history of psychiatric illness or earlier suicide attempt. Other chronic risk factors, usually a trait characteristic, can become acute: for example, impulsivity or aggression, or deliberate self-harm. The presence of chronic suicide risk factors should prompt a systematic suicide risk assessment. Evaluation of chronic suicide risk factors is an essential component of comprehensive assessment.5
Question 14
A psychiatrist is treating Dr. R, a 43-year-old physician, for anxiety and depression. The psychiatrist sees Dr. R twice a week for psychotherapy and medication management. A recent lawsuit filed against Dr. R has severely exacerbated her symptoms. She can sleep for only a few hours. Suicide ideation has emerged, frightening Dr. R and her family. The psychiatrist performs a systematic suicide risk assessment and determines that Dr. R is at acute high risk for suicide.
The psychiatrist recommends immediate hospitalization, but Dr. R adamantly refuses. The psychiatrist decides not to involuntarily hospitalize her because she does not meet the substantive criteria of the state involuntary commitment statute (eg, overt suicidal behaviors). The psychiatrist chooses to continue outpatient treatment.
Clinical interventions to reduce Dr. R’s suicide risk include:
a) see her more often
b) adjust medications
c) obtain a consult
d) refer her to an intensive outpatient program
e) all of the above
The best response option is E
To hospitalize or not to hospitalize— that is the conundrum that psychiatrists often face with high-risk suicidal patients. The decision is more complicated when the need for hospitalization is clear but the patient refuses. The decisions that the psychiatrist makes at this point are crucial for treatment and risk management.5
If the patient disagrees with the psychiatrist’s recommendation to hospitalize, refusal should be addressed as a treatment issue. When the need for hospitalization is acute, a prolonged inquiry is not possible. In addition, the therapeutic alliance may become strained. This clinical situation tries a clinician’s professional mettle.
Consultation and referral are options to consider if time and the patient’s condition allows. A psychiatric clinician should never worry alone; sleepless nights benefit neither the psychiatrist nor the patient.
As Dr. R’s case shows, a psychiatrist might decide not to hospitalize a patient who is assessed to be at moderate or high risk of suicide. Protective factors may allow continuing outpatient treatment. A good therapeutic alliance may be present if the psychiatrist has worked with the patient for some time. Family support also may be available.
The clinician must determine if the patient’s suicide risk can be managed by more frequent visits and treatment adjustments. Also, supportive family members can help by providing observational data. Protective factors can be overwhelmed by a severe mental illness. In contrast, a patient assessed as being at moderate risk of suicide might need to be hospitalized when protective factors are few or absent.
The psychiatrist may determine that a patient at high risk of suicide who refuses hospitalization does not meet criteria for involuntary hospitalization. For example, criteria might require that the patient must have made a suicide attempt within a specified period of time. States have provisions in their commitment statutes granting immunity from liability if the clinician uses reasonable clinical judgment and acts in good faith when involuntarily hospitalizing a patient.7
Question 15
Mr. U, a 39-year-old, married engineer, is ready to be discharged from the inpatient unit. He was admitted 7 days earlier for acute alcohol intoxication and suicidal threats. He has undergone successful detoxification. Mr. U has had 2 similar episodes within the past year.
The treatment team conducts a risk-benefit analysis for both discharge and continued hospitalization. A consultation also is obtained.
The discharge decision will be most influenced by:
a) presence of family support
b) compliance with follow-up care
c) availability of dual diagnosis programs
d) systematic suicide risk assessment
e) consultation
The best response option is D
All of the options in Question 15 concerning discharge planning of patients at risk for suicide are important. However, conducting a systematic suicide risk assessment to inform discharge planning is the most critical. Mr. U had 2 previous psychiatric admissions for alcohol abuse and suicidal ideation. He is a chronic suicide risk who becomes high risk when intoxicated.
Discharge planning begins at admission and is refined during the patient’s stay. Before a patient is discharged, a final post-discharge treatment and aftercare plan is necessary. After discharge, suicide risk increases as the intensity of treatment decreases.8
The patient’s willingness to cooperate with discharge and aftercare planning is critical in establishing contact with follow-up treaters. The treatment team should structure the follow-up plan to encourage compliance. For example, psychotic patients at risk of suicide who have a history of stopping medications after discharge can be given a long-acting IM antipsychotic that will last until they reach aftercare. Patients with comorbid drug and alcohol abuse disorders are referred to agencies equipped to manage dual-diagnosis patients.
Psychiatrists’ ability to ensure follow-up treatment is limited, a fact that must be acknowledged by the psychiatric and legal communities. Beyond patient stabilization, a clinician’s options to bring about positive changes can be limited or nonexistent. Also, the patient’s failure to adhere to post-discharge plans and treatment often leads to rehospitalization, hopelessness, and greater suicide risk.
Psychiatric patients at moderate or moderate-to-high risk for suicide increasingly are treated in outpatient settings. It is the responsibility of the clinician and the treatment team to competently hand off the patient to appropriate outpatient aftercare. With the patient’s permission, the psychiatrist or social worker should call the follow-up agency or therapist before discharge to provide information about the patient’s diagnosis, treatment, and hospital course.
Last, follow-up appointments should be made as close to the time of discharge as possible. Suicide often occurs on the first day after discharge.3
Bottom Line
Fully commit time and effort to the ongoing assessment, treatment, and management of patients at suicide risk. Suicide risk assessment is a process, not an event. Conduct a suicide risk assessment at important clinical junctures (eg, initial evaluation, discharge, changing observation levels). Contemporaneously, document suicide risk assessments. This self-assessment helps clinicians gauge their strengths and identify skills that need further development.
Disclosure
Dr. Simon reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Simon is the co-editor of The American Psychiatry Publishing textbook of Suicide Assessment and Management, 2nd edition, from which this article is adapted, by permission of the publisher, American Psychiatry Publishing, Inc. ©2012.
As explained in the first part of this article in the October 2014 issue of Current Psychiatry, assessing and managing suicide risk are complex, difficult tasks without clear-cut, easy solutions. The case-based, multiple-choice self-test, with accompanying commentary, presented here is designed to enhance one’s ability to provide care for patients at risk for suicide. Part 2 of this article poses the remaining 7 of 15 questions, which are based on clinical experience and the referenced work of others.
Question 9
Mr. N, age 62, will be discharged from the psychiatric unit tomorrow. He was admitted after an overdose suicide attempt. Mr. N was depressed after the loss of his business and was “treating” his depression and anxiety with alcohol. He is successfully withdrawn from alcohol and responds to medication and supportive psychotherapy. During a family meeting with staff, Mr. N’s wife states that he keeps a gun by his bedside. Mr. N has improved and is eager to go home.
Before discharging Mr. N, the psychiatrist or staff should:
a) instruct Mr. N to remove the gun from his bedside
b) instruct his wife to remove the gun from the home
c) instruct the wife to look for >1 gun
d) instruct the wife, before Mr. N’s discharge, to call the staff once guns and
ammunition are safely removed according to the pre-arranged safety plan
e) instruct the wife to lock up the gun in a place that is not known to the patient
The best response option is D
Guns in the home are associated with a significant increase in suicide. All patients at risk for suicide must be asked if guns are available at home or easily accessible elsewhere, or if they intend to purchase a gun. Gun safety management requires a collaborative team approach including the clinician, patient, and person designated responsible for removing guns from the home.1 The responsible person should be required to call the clinician to confirm that the guns have been removed and secured according to the plan. The principles of gun safety management apply to outpatients, inpatients, and emergency patients, although implementation varies according to the clinical setting.
Asking the patient to remove guns from the home is too risky. Guns must be safely secured before the patient is discharged. Asking a spouse, other family member, or partner is necessary. The person asked must be willing to remove guns and ammunition according to a pre-arranged plan requiring a callback upon completion. A callback is essential because a family member in denial may do nothing to remove the guns or lock or “hide” them in the home where they will be found by a determined suicidal patient. Guns may be available outside the home, such as in the car, at the work place, or for purchase.
The essence of gun safety management is verification. Trust but verify or, better yet, verify, then trust.
Question 10
A recently admitted 56-year-old inpatient was discovered wrapping a towel around her neck. She denied suicidal intent; however, the treatment team viewed the incident as a suicide rehearsal. She was placed on one-to-one close observation.
Inpatient suicides frequently occur:
a) shortly after admission
b) during staff shift changes
c) at meal times
d) shortly after discharge
e) all of the above
The best response option is E
Inpatient suicides also occur at increased frequency when psychiatric residents finish their rotations and in understaffed psychiatric units.2 Undue delay in the evaluation of a newly admitted acute, high-risk patient might allow the patient to commit suicide.
Most patient suicides occur shortly after hospital discharge (a few hours, days, or weeks later). Appleby et al3 found that the highest number of suicides occurred during the first week after discharge. Meehan et al4 found that suicide occurred most frequently during the first 2 weeks post-discharge; the highest number of suicides occurred on the first day after discharge.
Question 11
Ms. G, a 43-year-old, single woman in acute suicide crisis, is admitted to the psychiatric unit of a general hospital. She is diagnosed with bipolar I disorder, most recent episode depressed, and borderline personality disorder. She has had multiple psychiatric hospitalizations, all precipitated by a suicide crisis. The average length of stay on the psychiatric unit is 6.3 days. After 7 days of intensive treatment, Ms. G is stabilized and suicide risk is reduced. The treatment team prepares for her discharge.
Ms. G’s suicide risk at discharge is most likely at:
a) indeterminate risk
b) low risk
c) moderate risk
d) chronic high risk
e) acute high risk
The best response option is D
The length of stay in many acute care psychiatric facilities is <7 days. The goal of hospitalization is to stabilize the patient and discharge to appropriate community mental health resources. Discharge planning begins at the time of admission.
Reducing Ms. G’s suicide risk to low or moderate is unlikely because of her diagnoses, frequent hospitalizations, and acute high risk for suicide on admission. After acute, high-risk suicidal patients are treated, many revert to chronic high risk for suicide.
Patients at chronic high risk for suicide often are treated as outpatients, except when an acute suicidal crisis requires hospitalization.5 At discharge from the hospital, the goal is to return the patient to outpatient treatment.
A discharge note identifies the acute suicide risk factors that have abated and the chronic (long-term) suicide risk factors that remain. The discharge note also addresses a patient’s chronic vulnerability to suicide. For example, a patient can become acutely suicidal again, depending on a number of factors, including the nature and cause of the psychiatric illness, adequacy of future treatment, adherence to treatment recommendations, and unforeseeable life vicissitudes.
Question 12
A 20-year-old college student is hospitalized after an overdose suicide attempt. Failing grades, panic attacks, and depression precipitated the suicide attempt. After 8 days of hospitalization, she is much improved and ready for discharge. She is assessed to be at low to moderate suicide risk. The treating psychiatrist and social worker convene a family meeting with both parents and an older brother. The family’s role after discharge is discussed.
All of the following options are helpful family roles except:
a) provide constant 24-hour family supervision
b) provide emotional support
c) observe and report symptoms and behaviors of concern
d) encourage adherence with treatment
e) provide helpful feedback about the patient’s thoughts and behavior
The best response option is A
The family’s role is important, but it is not a substitute for constant safety management provided by trained mental health professionals on an inpatient psychiatric unit.5 Early discharge of an inpatient by relying on family supervision can be precarious. Most inpatients are discharged at some level of suicide risk, given the short length of hospital stay. If an outpatient at risk of suicide requires constant 24-hour family supervision, then psychiatric hospitalization is indicated.
Patients who are intent on killing themselves can find ingenious ways to attempt or commit suicide. Asking family members to keep a constant watch often fails. Most family members will not follow the patient into the bathroom or be able to stay up all night to observe the patient. Moreover, family members find reasons to make exceptions to constant surveillance because of denial, fatigue, or the need to attend to other pressing matters.
Question 13
During the initial evaluation of a patient, it is the psychiatrist’s practice to routinely inquire about current and past suicide ideation. An affirmative answer prompts a systematic suicide risk assessment. In the absence of current risk, if exploration of the patient’s history reveals chronic suicide risk factors, the psychiatrist conducts a systematic suicide risk assessment.
The chronic risk factor that has the highest association with suicide is:
a) family history of mental illness or suicide
b) childhood abuse
c) history of a suicide attempt
d) impulsivity or aggression
e) prior psychiatric hospitalization
The best response option is C
A comprehensive suicide risk assessment may not be required at the initial outpatient evaluation in the absence of acute suicide risk factors. However, chronic suicide risk factors may be present.
The Standard Mortality Ratio (SMR) for prior suicide attempts by any method was 38.61.6 Suicide risk was highest in the 2 years after the first attempt. The SMR is a measure of the relative risk of suicide compared with the expected rate in the general population (SMR of 1).
Some chronic suicide risk factors are static: for example, a family history of psychiatric illness or earlier suicide attempt. Other chronic risk factors, usually a trait characteristic, can become acute: for example, impulsivity or aggression, or deliberate self-harm. The presence of chronic suicide risk factors should prompt a systematic suicide risk assessment. Evaluation of chronic suicide risk factors is an essential component of comprehensive assessment.5
Question 14
A psychiatrist is treating Dr. R, a 43-year-old physician, for anxiety and depression. The psychiatrist sees Dr. R twice a week for psychotherapy and medication management. A recent lawsuit filed against Dr. R has severely exacerbated her symptoms. She can sleep for only a few hours. Suicide ideation has emerged, frightening Dr. R and her family. The psychiatrist performs a systematic suicide risk assessment and determines that Dr. R is at acute high risk for suicide.
The psychiatrist recommends immediate hospitalization, but Dr. R adamantly refuses. The psychiatrist decides not to involuntarily hospitalize her because she does not meet the substantive criteria of the state involuntary commitment statute (eg, overt suicidal behaviors). The psychiatrist chooses to continue outpatient treatment.
Clinical interventions to reduce Dr. R’s suicide risk include:
a) see her more often
b) adjust medications
c) obtain a consult
d) refer her to an intensive outpatient program
e) all of the above
The best response option is E
To hospitalize or not to hospitalize— that is the conundrum that psychiatrists often face with high-risk suicidal patients. The decision is more complicated when the need for hospitalization is clear but the patient refuses. The decisions that the psychiatrist makes at this point are crucial for treatment and risk management.5
If the patient disagrees with the psychiatrist’s recommendation to hospitalize, refusal should be addressed as a treatment issue. When the need for hospitalization is acute, a prolonged inquiry is not possible. In addition, the therapeutic alliance may become strained. This clinical situation tries a clinician’s professional mettle.
Consultation and referral are options to consider if time and the patient’s condition allows. A psychiatric clinician should never worry alone; sleepless nights benefit neither the psychiatrist nor the patient.
As Dr. R’s case shows, a psychiatrist might decide not to hospitalize a patient who is assessed to be at moderate or high risk of suicide. Protective factors may allow continuing outpatient treatment. A good therapeutic alliance may be present if the psychiatrist has worked with the patient for some time. Family support also may be available.
The clinician must determine if the patient’s suicide risk can be managed by more frequent visits and treatment adjustments. Also, supportive family members can help by providing observational data. Protective factors can be overwhelmed by a severe mental illness. In contrast, a patient assessed as being at moderate risk of suicide might need to be hospitalized when protective factors are few or absent.
The psychiatrist may determine that a patient at high risk of suicide who refuses hospitalization does not meet criteria for involuntary hospitalization. For example, criteria might require that the patient must have made a suicide attempt within a specified period of time. States have provisions in their commitment statutes granting immunity from liability if the clinician uses reasonable clinical judgment and acts in good faith when involuntarily hospitalizing a patient.7
Question 15
Mr. U, a 39-year-old, married engineer, is ready to be discharged from the inpatient unit. He was admitted 7 days earlier for acute alcohol intoxication and suicidal threats. He has undergone successful detoxification. Mr. U has had 2 similar episodes within the past year.
The treatment team conducts a risk-benefit analysis for both discharge and continued hospitalization. A consultation also is obtained.
The discharge decision will be most influenced by:
a) presence of family support
b) compliance with follow-up care
c) availability of dual diagnosis programs
d) systematic suicide risk assessment
e) consultation
The best response option is D
All of the options in Question 15 concerning discharge planning of patients at risk for suicide are important. However, conducting a systematic suicide risk assessment to inform discharge planning is the most critical. Mr. U had 2 previous psychiatric admissions for alcohol abuse and suicidal ideation. He is a chronic suicide risk who becomes high risk when intoxicated.
Discharge planning begins at admission and is refined during the patient’s stay. Before a patient is discharged, a final post-discharge treatment and aftercare plan is necessary. After discharge, suicide risk increases as the intensity of treatment decreases.8
The patient’s willingness to cooperate with discharge and aftercare planning is critical in establishing contact with follow-up treaters. The treatment team should structure the follow-up plan to encourage compliance. For example, psychotic patients at risk of suicide who have a history of stopping medications after discharge can be given a long-acting IM antipsychotic that will last until they reach aftercare. Patients with comorbid drug and alcohol abuse disorders are referred to agencies equipped to manage dual-diagnosis patients.
Psychiatrists’ ability to ensure follow-up treatment is limited, a fact that must be acknowledged by the psychiatric and legal communities. Beyond patient stabilization, a clinician’s options to bring about positive changes can be limited or nonexistent. Also, the patient’s failure to adhere to post-discharge plans and treatment often leads to rehospitalization, hopelessness, and greater suicide risk.
Psychiatric patients at moderate or moderate-to-high risk for suicide increasingly are treated in outpatient settings. It is the responsibility of the clinician and the treatment team to competently hand off the patient to appropriate outpatient aftercare. With the patient’s permission, the psychiatrist or social worker should call the follow-up agency or therapist before discharge to provide information about the patient’s diagnosis, treatment, and hospital course.
Last, follow-up appointments should be made as close to the time of discharge as possible. Suicide often occurs on the first day after discharge.3
Bottom Line
Fully commit time and effort to the ongoing assessment, treatment, and management of patients at suicide risk. Suicide risk assessment is a process, not an event. Conduct a suicide risk assessment at important clinical junctures (eg, initial evaluation, discharge, changing observation levels). Contemporaneously, document suicide risk assessments. This self-assessment helps clinicians gauge their strengths and identify skills that need further development.
Disclosure
Dr. Simon reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Simon is the co-editor of The American Psychiatry Publishing textbook of Suicide Assessment and Management, 2nd edition, from which this article is adapted, by permission of the publisher, American Psychiatry Publishing, Inc. ©2012.
1. Simon RI. Gun safety management with patients at risk for suicide. Suicide Life Threat Behav. 2007;37(5):518-526.
2. Qin P, Nordenoft M. Suicide risk in relation to psychiatric hospitalization: evidence based on longitudinal registers. Arch Gen Psychiatry. 2005;62(4):427-432.
3. Appleby L, Shaw J, Amos T, et al. Suicide within 12 months of contact with mental health services: national clinical survey. BMJ. 1999;318(7193):1235-1239.
4. Meehan J, Kapur N, Hunt IM, et al. Suicide in mental health in-patients within 3 months of discharge. National clinical survey. Br J Psychiatry. 2006;188:129-134.
5. Simon RI. Preventing patient suicide: clinical assessment and management. Arlington, VA: American Psychiatric Publishing, Inc.; 2011.
6. Harris CE, Barraclough B. Suicide as an outcome for mental disorders. A meta-analysis. Br J Psychiatry. 1997;170:205-228.
7. Simon RI, Shuman DW. Clinical manual of psychiatry and law. Arlington, VA: American Psychiatric Publishing, Inc.; 2007.
8. Appleby L, Dennehy JA, Thomas CS, et al. Aftercare and clinical characteristics of people with mental illness who commit suicide: a case-control study. Lancet. 1999;353(9162):1397-1400.
1. Simon RI. Gun safety management with patients at risk for suicide. Suicide Life Threat Behav. 2007;37(5):518-526.
2. Qin P, Nordenoft M. Suicide risk in relation to psychiatric hospitalization: evidence based on longitudinal registers. Arch Gen Psychiatry. 2005;62(4):427-432.
3. Appleby L, Shaw J, Amos T, et al. Suicide within 12 months of contact with mental health services: national clinical survey. BMJ. 1999;318(7193):1235-1239.
4. Meehan J, Kapur N, Hunt IM, et al. Suicide in mental health in-patients within 3 months of discharge. National clinical survey. Br J Psychiatry. 2006;188:129-134.
5. Simon RI. Preventing patient suicide: clinical assessment and management. Arlington, VA: American Psychiatric Publishing, Inc.; 2011.
6. Harris CE, Barraclough B. Suicide as an outcome for mental disorders. A meta-analysis. Br J Psychiatry. 1997;170:205-228.
7. Simon RI, Shuman DW. Clinical manual of psychiatry and law. Arlington, VA: American Psychiatric Publishing, Inc.; 2007.
8. Appleby L, Dennehy JA, Thomas CS, et al. Aftercare and clinical characteristics of people with mental illness who commit suicide: a case-control study. Lancet. 1999;353(9162):1397-1400.
Sedative-hypnotics for sleepless geriatric patients
Mr. R, 75, is having difficulty sleeping. When he goes to bed, he lies there for what seems like forever, unable to fall asleep. He feels “so tired” and ends up taking naps during the day, but he cannot break this cycle. He has tried using over-the-counter products with little relief.
Mr. R’s primary care physician prescribes zaleplon, 10 mg/d, and asks him to call the clinic in 2 weeks to discuss his progress. He takes zaleplon as directed for several nights and begins to feel “sluggish” during the day, both mentally and physically, despite reporting an increase in the overall amount of sleep at night.
Sedative-hypnotic drugs are among the most commonly used medications in the United States. Use of these drugs, as well as anxiolytics, has increased from 2.8% between 1988 and 1994 to 4.7% between 2007 and 2010, according to the Department of Health and Human Services.1 In 2011, drugs categorized as sedative-hypnotics or antipsychotics were involved in 6.1% of all human exposures identified in the American Association of Poison Control Centers’ National Poison Data System.2 Therefore, an understanding of clinical and pharmacological variables related to safe and effective use is important for clinicians prescribing and monitoring therapy with these agents.
Neuropsychiatric disorders are prevalent among geriatric patients and are associated with age-related physiologic changes in the CNS.3 Such changes involve:
• neuroanatomy (brain atrophy, decreased neuronal density, increased plaque formation)
• neurotransmitters (reduced cholinergic transmission, decreased synthesis of dopamine and catecholamines), and
• neurophysiology (reduced cerebral blood flow).
These physiologic processes manifest as alterations in mental status, reflexes, sensation, gait, balance, and sleep. Examples of sleep changes among geriatric patients include decreased sleep efficiency, more frequent awakenings, and more variable sleep duration.3,4 Sleep disorders also may be related to mental disorders and other medical conditions.5 For example, the prevalence of sleep-related respiratory disorders, such as obstructive sleep apnea and central sleep apnea, increases with age.6
Sleep disorders are common among geriatric patients. In a large epidemiologic study of sleep complaints in patients age ≥65, more than one-half of patients had at least 1 sleep complaint (ie, difficulty falling asleep, trouble waking up, early awakening, need for naps, and feeling ill-rested).7 As many as 34% of patients reported symptoms of insomnia. In an analysis of National Ambulatory Medical Survey Data over 6 years, 24.8% to 27.9% of sleep-related medical office visits were attributed to patients age ≥65.8
Pharmacology in aging
Prescribing sedative-hypnotic drugs is not routinely recommended for older patients with a sleep disorder. Geriatric patients, compared with younger patients, are at higher risk of iatrogenic complications because of polypharmacy, comorbidities, relative renal and hepatic insufficiency, and other physiologic changes leading to alterations in drug exposure and metabolism (Table 1).9-12
Aging is associated with changes in body composition, including an increase in total body fat and decrease in lean body mass and total body water. These changes, as well as a prolonged GI transit time, decrease in active gut transporters, decreased blood perfusion, and decrease in plasma proteins such as albumin (because of reduced liver function or malnutrition), may lead to alteration in drug absorption patterns and may increase the volume of distribution for lipophilic drugs. Additionally, the elimination half-life of some drugs may increase with age because of larger volumes of distribution and reduction in hepatic or renal clearance.
The clinical significance of these changes is not well established. Although the process of drug absorption can change with age, the amount of drug absorbed might not be significantly affected. An increase in the volume of distribution and reduction in drug metabolism and clearance might lead to increasing amounts of circulating drug and duration of drug exposure, putting geriatric patients at an increased risk for adverse effects and drug toxicity.9
Among these mechanisms, Dolder et al11 hypothesized that drug metabolism catalyzed by cytochrome P450 (CYP) enzymes and renal excretion may be of greatest concern. Although in vitro studies suggest that concentration of CYP enzymes does not decline with age, in vivo studies have demonstrated reduced CYP activity in geriatric patients.11,12 Theoretically, a reduction in CYP activity would increase the bioavailability of drugs, especially those that are subject to extensive first-pass (ie, hepatic) metabolism, and may lead to a reduction in systemic clearance.
Independent of metabolic changes, geriatric patients are at risk of reduced renal clearance because of age-related changes in glomerular filtration rate. Pharmacodynamic changes might be observed in older patients and could be a concern even in the setting of unaltered pharmacokinetic factors.9 These changes usually require administering smaller drug dosages.
Sedative-hypnotic medications
Sedative-hypnotic agents include several barbiturates, benzodiazepines (BZDs), non-BZD benzodiazepine-receptor agonists (BzRAs), a melatonin-receptor agonist (ie, ramelteon), and an orexin-receptor antagonist (ie, suvorexant).13,14Table 214-29 summarizes selected sedative-hypnotic drugs. Additional drug classes used to treat insomnia include:
• sedating antidepressants (trazodone, amitriptyline, doxepin, mirtazapine)
• antiepileptic drugs (gabapentin, tiagabine)
• atypical antipsychotics (quetiapine, olanzapine).
FDA-approved agents for treating insomnia include amobarbital, butabarbital, pentobarbital, phenobarbital, secobarbital, chloral hydrate, diphenhydramine, doxylamine, doxepin, estazolam, flurazepam, lorazepam, quazepam, temazepam, triazolam, eszopiclone, zaleplon, zolpidem, ramelteon, and suvorexant. Not all of these drugs are recommended for use in geriatric patients. Barbiturates, for example, should be avoided.30
Pharmacokinetic characteristics vary among drugs and drug classes. Choice of pharmacotherapy should account for patient and drug characteristics and the specific sleep complaint. Sleep disorders may be variously characterized as difficulty with sleep initiation, duration, consolidation, or quality.13 Therefore, onset and duration of effect are important drug-related considerations. Sedative-hypnotic drugs with a short time-to-onset may be ideal for patients with sleep-onset insomnia.
The drugs’ duration of effect (eg, presence of active metabolites, long elimination half-life) also must be reviewed. A long elimination half-life may lead to increased drug exposure and unwanted side effects such as residual daytime drowsiness. Despite this, sedative-hypnotic drugs with a longer duration of effect (eg, intermediate- or long-acting drugs) may be best for patients with insomnia defined by difficulty maintaining sleep.
Benzodiazepines vary in their time to onset of effect, rate of elimination, and metabolism.15-21 BZDs that are FDA- approved for use as sedative-hypnotics are listed in Table 2.14-29 These BZDs have different onsets of effect as evidenced by time to achieve maximum plasma concentration (Tmax), ranging from 0.5 hours (flurazepam) to 2 hours (estazolam, quazepam, triazolam). The elimination half-life varies widely among these medications, from 1.5 hours (triazolam) to >100 hours (flurazepam). Flurazepam’s long half-life is attributable to its active major metabolite. Although most BZDs are metabolized hepatically, temazepam is subject to minimal hepatic metabolism.
Benzodiazepine-receptor agonists. There is substantial variation in the pharmacokinetic characteristics of BzRAs.15,16,22-28 There also are differences among the zolpidem dosage forms; sublingual formulations have the shortest onset of effect. Eszopiclone and zaleplon have low protein binding compared with zolpidem. Elimination half-lives vary among drugs with the shortest attributed to zaleplon (1 hour) and longest to eszopiclone (6 hours). All BzRAs are subject to extensive hepatic metabolism.
Ramelteon. Singular in its class, ramelteon is a treatment option for insomnia.29 This drug has a short onset of effect, moderate protein binding, and extensive hepatic metabolism. Ramelteon is primarily excreted in the urine as its metabolites, and the drug half-life is relatively short.
Suvorexant is the latest addition to the sedative-hypnotic armamentarium, approved by the FDA in August 2014 for difficulty with sleep onset and/or sleep maintenance.14 As an orexin-receptor antagonist, suvorexant represents a novel pharmacologic class. Suvorexant exhibits moderately rapid absorption with time to peak concentration ranging from 30 minutes to 6 hours in fasting conditions; absorption is delayed when taken with or soon after a meal. The drug is highly protein bound and extensively metabolized, primarily through CYP3A. The manufacturer recommends dose reduction (5 mg at bedtime) in patients taking moderate CYP3A inhibitors and avoiding suvorexant in patients taking strong CYP3A inhibitors. Suvorexant is primarily excreted through feces and the mean half-life is relatively long.
Considering these characteristics and age-related physiologic changes, the practitioner should be concerned about drugs that undergo extensive hepatic metabolism. Age-related reductions in CYP activity may lead to an increase in drug bioavailability and a decrease in the systemic clearance,11 which might be associated with an increase in elimination half-life and duration of action. Dosage adjustments are recommended for several BZDs (lower initial and maximum dosages for most agents) and BzRAs.17-28 No dosage adjustments for ramelteon or suvorexant in geriatric patients have been specified14,29; the manufacturers for both products assert that no differences in safety and efficacy have been observed between older and younger adult patients.
Alternative and complementary medications
Several non-prescription products, including over-the-counter drugs (eg, diphenhy-dramine, doxylamine) and herbal therapies (eg, melatonin, valerian), are used for their sedative-hypnotic properties. There is a lack of evidence supporting using diphenhydra-mine in patients with chronic insomnia, and tolerance to its hypnotic effect has been reported with repeated use.31 Concerns about anticholinergic toxicity and CNS depression limit its use in geriatric patients. Among herbal therapies, melatonin may have the strongest evidence for its ability to alleviate sleep disorders in geriatric patients32; however, meta-analyses have demonstrated small effects of melatonin on sleep latency and minimal differences in wake time after sleep onset and total sleep time.13
Clinical practice guidelines
Non-pharmacotherapeutic interventions, such as behavioral (eg, sleep hygiene measures) and psychological therapy, are recommended for initial management of sleep disorders in geriatric patients.13,33 In conjunction, the American Medical Directors Association (AMDA) recommends address ing underlying causes and exacerbating factors (eg, medical condition or medication).33 The AMDA recommends avoiding long-term pharmacotherapy and advises caution with BZD-hypnotic drugs, tricyclic antidepressants, and antihistamines. The American Academy of Sleep Medicine (AASM) recommends an initial treatment period of 2 to 4 weeks, followed by re-evaluation of continued need for treatment.13 The AASM recommends short- or intermediate-acting BzRAs or ramelteon for initial pharmacologic management of primary insomnias and insomnias comorbid with other conditions. The AASM also recommends specific dosages of BzRAs and BZDs for geriatric patients, which coincide with manufacturer-recommended dosages (Table 2).14-29
Barbiturates, chloral hydrate, and non-barbiturate, non-BZD drugs such as meprobamate are not recommended because of potential significant adverse effects and tolerance/dependence, and low therapeutic index. The AASM advises caution when using prescription drugs off-label for insomnia (eg, antidepressants, antiepileptics, antipsychotics) and recommends avoiding them, if possible, because of limited evidence supporting their use.13
Safety concerns
Two commonly used references contain recommendations for sedative-hypnotic medication use in geriatric patients.30,34 According to Gallagher et al’s34 Screening Tool of Older Person’s Prescriptions (STOPP), long-term (>1 month) use of long-acting BZDs (eg, flurazepam, diazepam) and prolonged use (>1 week) of first-generation antihistamines (eg, diphenhydramine, doxylamine) should be avoided in patients age ≥65 because of the risk of sedation, confusion, and anticholinergic side effects. STOPP recognizes that any use of BZDs, neuroleptics, or first-generation antihistamines may contribute to postural imbalance; therefore these agents are not recommended in older patients at risk for falls.
In the 2012 American Geriatrics Society (AGS) Beers Criteria, the AGS recommends avoiding barbiturates in older adults because of the high rate of physical dependence, tolerance to sleep effects, and overdose risk at low dosages.30 The AGS also recommends avoiding BZDs, stating that older adults have increased sensitivity to these agents and are at an increased risk of cognitive impairment, delirium, falls, fractures, and motor vehicle accidents when taking these drugs. Non-BZD BzRAs also should not be prescribed to patients with a history of falls or fractures, unless safer alternatives are not available.
The FDA has issued several advisory reports regarding sedative-hypnotic drugs. In 2007, all manufacturers of sedative-hypnotic drugs were required to modify their product labeling to include stronger language about potential risks.35 Among these changes, warnings for anaphylaxis and complex sleep-related behaviors were added. Also, the FDA requested that manufacturers of sedative-hypnotic drugs develop and provide patient medication guides, advising consumers on the potential risks and precautions associated with these drugs. More recently, the FDA announced changes to dosing recommendations for zolpidem-containing products because of the risk of impaired mental alertness36; manufacturers were required to lower the recommended dosages for each product.
Manufacturers of FDA-approved sedative-hypnotic drugs urge caution when prescribing these medications for geriatric patients, citing the potential for increased sensitivity, manifesting as marked excitement, depression, or confusion (eg, barbiturates), and greater risk for dosage-related adverse effects (eg, oversedation, dizziness, confusion, impaired psychomotor performance, ataxia).17-29
Use in clinical practice
Several variables should be considered when evaluating appropriateness of pharmacotherapy, including characteristics of the drug and the patient. Geriatric patients may be prone to comorbidities resulting from age-related physiologic changes. These diseases may be confounding (ie, contributing to sleep disorders); examples include medical illnesses, such as hyperthyroidism and arthritis, and psychiatric illnesses, such as depression and anxiety.37 Other conditions, such as renal and hepatic dysfunction, may lead to alteration in drug exposure. These conditions should be assessed through routine renal function tests (eg, serum creatinine and glomerular filtration rate) and liver function tests (eg, serum albumin and liver transaminases).
Multiple comorbidities suggest a higher likelihood of polypharmacy, leading to other drug-related issues (eg, drug-drug interactions). Although these issues may guide therapy by restricting medication options, their potential contribution to the underlying sleep complaints should be considered.37 Several drugs commonly used by geriatric patients may affect wakefulness (eg, analgesics, antidepressants, and antihypertensives [sedating], and thyroid hormones, corticosteroids, and CNS stimulants [alerting]).
CASE CONTINUED
In Mr. R’s case, zaleplon was initiated at 10 mg/d. Because of his age and the nature of his sleep disorder, the choice of sedative-hypnotic was suitable; however, the prescribed dosage was inappropriate. The sluggishness Mr. R experienced likely was a manifestation of increased exposure to the drug. According to manufacturer and AASM recommendations, a more appropriate dosage is 5 mg/d.13,23 Mr. R’s medical history and current medications, and his hepatic and renal function, should be assessed. If Mr. R continues to have issues with sleep initiation, zaleplon, 5 mg at bedtime, should be considered.
Related Resources
• Institute for Safe Medication Practices. www.ismp.org.
• MedWatch: The FDA Safety Information and Adverse Event Reporting Program. www.fda.gov/Safety/MedWatch/default.htm.
Drug Brand Names
Amitriptyline • Elavil Mirtazapine • Remeron
Amobarbital • Amytal Olanzapine • Zyprexa
Butabarbital • Butisol Pentobarbital • Nembutal
Chloral hydrate • Somnote Phenobarbital • Luminal
Diazepam • Valium Quazepam • Doral
Diphenhydramine • Benadryl, others Quetiapine • Seroquel
Doxepin • Silenor Ramelteon • Rozerem
Doxylamine • Unisom, others Secobarbital • Seconal
Estazolam • ProSom Suvorexant • Belsomra
Eszopiclone • Lunesta Temazepam • Restoril
Flurazepam • Dalmane Tiagabine • Gabitril
Gabapentin • Neurontin, Trazodone • Desyrel
Gralise, Horizant Zaleplon • Sonata
Lorazepam • Ativan Zolpidem • Ambien, Edluar,
Meprobamate • Equanil Intermezzo, Zolpimist
Acknowledgement
Vicki L. Ellingrod, PharmD, FCCP, is the series editor of Savvy Psychopharmacology.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. National Center for Health Statistics. Health, United States, 2012, with special feature on emergency care. http://www. cdc.gov/nchs/data/hus/hus12.pdf. Published May 2013. Accessed August 22, 2014.
2. Bronstein AC, Spyker DA, Cantilena LR Jr, et al. 2011 Annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 29th annual report. Clin Toxicol (Phila). 2012;50(10): 911-1164.
3. Inouye SK. Neuropsychiatric aspects of aging. In: Goldman L, Schafer AI, eds. Goldman’s cecil medicine. 24th ed. Philadelphia, PA: Elsevier Saunders; 2011:114-116.
4. Diagnostic and statistical manual of mental disorders, 4th ed. Washington, DC: American Psychiatric Association; 1994.
5. American Psychiatric Association. Highlights of changes from DSM-IV-TR to DSM-5. http://www.psychiatry. org/File%20Library/Practice/DSM/DSM-5/Changes-from-DSM-IV-TR--to-DSM-5.pdf. 2013. Accessed August 22, 2014.
6. Edwards BA, O’Driscoll DM, Ali A, et al. Aging and sleep: physiology and pathophysiology. Semin Respir Crit Care Med. 2010;31(5):618-633.
7. Foley DJ, Monjan AA, Brown SL, et al. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18(6):425-432.
8. Morlock RJ, Tan M, Mitchell DY. Patient characteristics and patterns of drug use for sleep complaints in the United States: analysis of National Ambulatory Medical Survey Data, 1997-2002. Clin Ther. 2006;28(7):1044-1053.
9. Diasio RB. Principles of drug therapy. In: Goldman L, Schafer AI, eds. Goldman’s Cecil medicine. 24th ed. Philadelphia, PA: Elsevier Saunders; 2011:124-132.
10. Hilmer SN, Ford GA. General principles of pharmacology. In: Halter JB, Ouslander JG, Tinetti ME, et al, eds. Hazzard’s geriatric medicine and gerontology. 6th ed. New York, NY: McGraw-Hill; 2009:103-122.
11. Dolder C, Nelson M, McKinsey J. Use of non-benzodiazepine hypnotics in the elderly: are all agents the same? CNS Drugs. 2007;21(5):389-405.
12. Klotz U. Pharmacokinetics and drug metabolism in the elderly. Drug Metab Rev. 2009;41(2):67-76.
13. Schutte-Rodin S, Broch L, Buysse D, et al. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4(5):487-504.
14. Belsomra [package insert]. Whitehouse Station, NJ: Merck & Co., Inc.; 2014.
15. Micromedex Healthcare Series. Greenwood Village, CO: Thomson Healthcare. http://micromedex.com. Accessed August 22, 2014.
16. Lexicomp. St. Louis, MO: Wolters Kluwer Health. http:// www.lexi.com. Accessed August 22, 2014.
17. Estazolam [package insert]. Corona, CA: Watson Pharma, Inc; 2008.
18. Flurazepam [package insert]. Eatontown, NJ: West-Ward Pharmaceutical Corp; 2010.
19. Doral [package insert]. Las Vegas, NV: Nuro Pharma, Inc; 2013.
20. Restoril [package insert]. Hazelwood, MO: Mallinckrodt Inc; 2010.
21. Halcion [package insert]. New York, NY: Pharmacia & Upjohn Co; 2013.
22. Lunesta [package insert]. Marlborough, MA: Sunovion Pharmaceuticals Inc; 2012.
23. Sonata [package insert]. New York, NY: Pfizer Inc; 2013.
24. Ambien [package insert]. Bridgewater, NJ: Sanofi-Aventis; 2013.
25. Ambien CR [package insert]. Bridgewater, NJ: Sanofi- Aventis; 2013.
26. Edluar [package insert]. Somerset, NJ: Meda Pharmaceuticals Inc; 2009.
27. Intermezzo [package insert]. Point Richmond, CA: Transcept Pharmaceuticals, Inc; 2011.
28. Zolpimist [package insert]. Richmond, VA: ECR Pharmaceuticals; 2013.
29. Rozerem [package insert]. Deerfield, IL: Takeda Pharmaceuticals America, Inc; 2010.
30. The American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616-631.
31. Kirkwood CK, Melton ST. Insomnia, drowsiness, and fatigue. In: Krinsky DL, Berardi RR, Ferreri SP, et al, eds. Handbook of nonprescription drugs: an interactive approach to self-care. 17th ed. Washington, DC: American Pharmacists Association; 2012:867-884.
32. Insomnia. In: Natural Standard. Somerville, MA: Natural Standard. https://naturalmedicines.therapeuticresearch. com/databases/medical-conditions/i/insomnia.aspx. Accessed August 22, 2014.
33. American Medical Directors Association. Clinical practice guideline: sleep disorders. Columbia, MD: American Medical Directors Association; 2006.
34. Gallagher P, Ryan C, Byrne S, et al. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther. 2008;46(2):72-83.
35. Food and Drug Administration. FDA requests label change for all sleep disorder drug products. http://www.fda. gov/newsevents/newsroom/pressannouncements/2007/ ucm108868.htm. Published March 14, 2007. Accessed August 22, 2014.
36. Food and Drug Administration. FDA drug safety communication: risk of next-morning impairment after use of insomnia drugs; FDA requires lower recommended doses for certain drugs containing zolpidem (Ambien, Ambien CR, Edluar, and Zolpimist). http://www.fda.gov/drugs/ drugsafety/ucm334033.htm. Published January 10, 2013. Accessed August 22, 2014.
37. Cohen-Zion M, Ancoli-Israel S. Sleep disorders. In: Hazzard’s geriatric medicine and gerontology. 6th ed. New York, NY: McGraw-Hill; 2009:671-682.
Mr. R, 75, is having difficulty sleeping. When he goes to bed, he lies there for what seems like forever, unable to fall asleep. He feels “so tired” and ends up taking naps during the day, but he cannot break this cycle. He has tried using over-the-counter products with little relief.
Mr. R’s primary care physician prescribes zaleplon, 10 mg/d, and asks him to call the clinic in 2 weeks to discuss his progress. He takes zaleplon as directed for several nights and begins to feel “sluggish” during the day, both mentally and physically, despite reporting an increase in the overall amount of sleep at night.
Sedative-hypnotic drugs are among the most commonly used medications in the United States. Use of these drugs, as well as anxiolytics, has increased from 2.8% between 1988 and 1994 to 4.7% between 2007 and 2010, according to the Department of Health and Human Services.1 In 2011, drugs categorized as sedative-hypnotics or antipsychotics were involved in 6.1% of all human exposures identified in the American Association of Poison Control Centers’ National Poison Data System.2 Therefore, an understanding of clinical and pharmacological variables related to safe and effective use is important for clinicians prescribing and monitoring therapy with these agents.
Neuropsychiatric disorders are prevalent among geriatric patients and are associated with age-related physiologic changes in the CNS.3 Such changes involve:
• neuroanatomy (brain atrophy, decreased neuronal density, increased plaque formation)
• neurotransmitters (reduced cholinergic transmission, decreased synthesis of dopamine and catecholamines), and
• neurophysiology (reduced cerebral blood flow).
These physiologic processes manifest as alterations in mental status, reflexes, sensation, gait, balance, and sleep. Examples of sleep changes among geriatric patients include decreased sleep efficiency, more frequent awakenings, and more variable sleep duration.3,4 Sleep disorders also may be related to mental disorders and other medical conditions.5 For example, the prevalence of sleep-related respiratory disorders, such as obstructive sleep apnea and central sleep apnea, increases with age.6
Sleep disorders are common among geriatric patients. In a large epidemiologic study of sleep complaints in patients age ≥65, more than one-half of patients had at least 1 sleep complaint (ie, difficulty falling asleep, trouble waking up, early awakening, need for naps, and feeling ill-rested).7 As many as 34% of patients reported symptoms of insomnia. In an analysis of National Ambulatory Medical Survey Data over 6 years, 24.8% to 27.9% of sleep-related medical office visits were attributed to patients age ≥65.8
Pharmacology in aging
Prescribing sedative-hypnotic drugs is not routinely recommended for older patients with a sleep disorder. Geriatric patients, compared with younger patients, are at higher risk of iatrogenic complications because of polypharmacy, comorbidities, relative renal and hepatic insufficiency, and other physiologic changes leading to alterations in drug exposure and metabolism (Table 1).9-12
Aging is associated with changes in body composition, including an increase in total body fat and decrease in lean body mass and total body water. These changes, as well as a prolonged GI transit time, decrease in active gut transporters, decreased blood perfusion, and decrease in plasma proteins such as albumin (because of reduced liver function or malnutrition), may lead to alteration in drug absorption patterns and may increase the volume of distribution for lipophilic drugs. Additionally, the elimination half-life of some drugs may increase with age because of larger volumes of distribution and reduction in hepatic or renal clearance.
The clinical significance of these changes is not well established. Although the process of drug absorption can change with age, the amount of drug absorbed might not be significantly affected. An increase in the volume of distribution and reduction in drug metabolism and clearance might lead to increasing amounts of circulating drug and duration of drug exposure, putting geriatric patients at an increased risk for adverse effects and drug toxicity.9
Among these mechanisms, Dolder et al11 hypothesized that drug metabolism catalyzed by cytochrome P450 (CYP) enzymes and renal excretion may be of greatest concern. Although in vitro studies suggest that concentration of CYP enzymes does not decline with age, in vivo studies have demonstrated reduced CYP activity in geriatric patients.11,12 Theoretically, a reduction in CYP activity would increase the bioavailability of drugs, especially those that are subject to extensive first-pass (ie, hepatic) metabolism, and may lead to a reduction in systemic clearance.
Independent of metabolic changes, geriatric patients are at risk of reduced renal clearance because of age-related changes in glomerular filtration rate. Pharmacodynamic changes might be observed in older patients and could be a concern even in the setting of unaltered pharmacokinetic factors.9 These changes usually require administering smaller drug dosages.
Sedative-hypnotic medications
Sedative-hypnotic agents include several barbiturates, benzodiazepines (BZDs), non-BZD benzodiazepine-receptor agonists (BzRAs), a melatonin-receptor agonist (ie, ramelteon), and an orexin-receptor antagonist (ie, suvorexant).13,14Table 214-29 summarizes selected sedative-hypnotic drugs. Additional drug classes used to treat insomnia include:
• sedating antidepressants (trazodone, amitriptyline, doxepin, mirtazapine)
• antiepileptic drugs (gabapentin, tiagabine)
• atypical antipsychotics (quetiapine, olanzapine).
FDA-approved agents for treating insomnia include amobarbital, butabarbital, pentobarbital, phenobarbital, secobarbital, chloral hydrate, diphenhydramine, doxylamine, doxepin, estazolam, flurazepam, lorazepam, quazepam, temazepam, triazolam, eszopiclone, zaleplon, zolpidem, ramelteon, and suvorexant. Not all of these drugs are recommended for use in geriatric patients. Barbiturates, for example, should be avoided.30
Pharmacokinetic characteristics vary among drugs and drug classes. Choice of pharmacotherapy should account for patient and drug characteristics and the specific sleep complaint. Sleep disorders may be variously characterized as difficulty with sleep initiation, duration, consolidation, or quality.13 Therefore, onset and duration of effect are important drug-related considerations. Sedative-hypnotic drugs with a short time-to-onset may be ideal for patients with sleep-onset insomnia.
The drugs’ duration of effect (eg, presence of active metabolites, long elimination half-life) also must be reviewed. A long elimination half-life may lead to increased drug exposure and unwanted side effects such as residual daytime drowsiness. Despite this, sedative-hypnotic drugs with a longer duration of effect (eg, intermediate- or long-acting drugs) may be best for patients with insomnia defined by difficulty maintaining sleep.
Benzodiazepines vary in their time to onset of effect, rate of elimination, and metabolism.15-21 BZDs that are FDA- approved for use as sedative-hypnotics are listed in Table 2.14-29 These BZDs have different onsets of effect as evidenced by time to achieve maximum plasma concentration (Tmax), ranging from 0.5 hours (flurazepam) to 2 hours (estazolam, quazepam, triazolam). The elimination half-life varies widely among these medications, from 1.5 hours (triazolam) to >100 hours (flurazepam). Flurazepam’s long half-life is attributable to its active major metabolite. Although most BZDs are metabolized hepatically, temazepam is subject to minimal hepatic metabolism.
Benzodiazepine-receptor agonists. There is substantial variation in the pharmacokinetic characteristics of BzRAs.15,16,22-28 There also are differences among the zolpidem dosage forms; sublingual formulations have the shortest onset of effect. Eszopiclone and zaleplon have low protein binding compared with zolpidem. Elimination half-lives vary among drugs with the shortest attributed to zaleplon (1 hour) and longest to eszopiclone (6 hours). All BzRAs are subject to extensive hepatic metabolism.
Ramelteon. Singular in its class, ramelteon is a treatment option for insomnia.29 This drug has a short onset of effect, moderate protein binding, and extensive hepatic metabolism. Ramelteon is primarily excreted in the urine as its metabolites, and the drug half-life is relatively short.
Suvorexant is the latest addition to the sedative-hypnotic armamentarium, approved by the FDA in August 2014 for difficulty with sleep onset and/or sleep maintenance.14 As an orexin-receptor antagonist, suvorexant represents a novel pharmacologic class. Suvorexant exhibits moderately rapid absorption with time to peak concentration ranging from 30 minutes to 6 hours in fasting conditions; absorption is delayed when taken with or soon after a meal. The drug is highly protein bound and extensively metabolized, primarily through CYP3A. The manufacturer recommends dose reduction (5 mg at bedtime) in patients taking moderate CYP3A inhibitors and avoiding suvorexant in patients taking strong CYP3A inhibitors. Suvorexant is primarily excreted through feces and the mean half-life is relatively long.
Considering these characteristics and age-related physiologic changes, the practitioner should be concerned about drugs that undergo extensive hepatic metabolism. Age-related reductions in CYP activity may lead to an increase in drug bioavailability and a decrease in the systemic clearance,11 which might be associated with an increase in elimination half-life and duration of action. Dosage adjustments are recommended for several BZDs (lower initial and maximum dosages for most agents) and BzRAs.17-28 No dosage adjustments for ramelteon or suvorexant in geriatric patients have been specified14,29; the manufacturers for both products assert that no differences in safety and efficacy have been observed between older and younger adult patients.
Alternative and complementary medications
Several non-prescription products, including over-the-counter drugs (eg, diphenhy-dramine, doxylamine) and herbal therapies (eg, melatonin, valerian), are used for their sedative-hypnotic properties. There is a lack of evidence supporting using diphenhydra-mine in patients with chronic insomnia, and tolerance to its hypnotic effect has been reported with repeated use.31 Concerns about anticholinergic toxicity and CNS depression limit its use in geriatric patients. Among herbal therapies, melatonin may have the strongest evidence for its ability to alleviate sleep disorders in geriatric patients32; however, meta-analyses have demonstrated small effects of melatonin on sleep latency and minimal differences in wake time after sleep onset and total sleep time.13
Clinical practice guidelines
Non-pharmacotherapeutic interventions, such as behavioral (eg, sleep hygiene measures) and psychological therapy, are recommended for initial management of sleep disorders in geriatric patients.13,33 In conjunction, the American Medical Directors Association (AMDA) recommends address ing underlying causes and exacerbating factors (eg, medical condition or medication).33 The AMDA recommends avoiding long-term pharmacotherapy and advises caution with BZD-hypnotic drugs, tricyclic antidepressants, and antihistamines. The American Academy of Sleep Medicine (AASM) recommends an initial treatment period of 2 to 4 weeks, followed by re-evaluation of continued need for treatment.13 The AASM recommends short- or intermediate-acting BzRAs or ramelteon for initial pharmacologic management of primary insomnias and insomnias comorbid with other conditions. The AASM also recommends specific dosages of BzRAs and BZDs for geriatric patients, which coincide with manufacturer-recommended dosages (Table 2).14-29
Barbiturates, chloral hydrate, and non-barbiturate, non-BZD drugs such as meprobamate are not recommended because of potential significant adverse effects and tolerance/dependence, and low therapeutic index. The AASM advises caution when using prescription drugs off-label for insomnia (eg, antidepressants, antiepileptics, antipsychotics) and recommends avoiding them, if possible, because of limited evidence supporting their use.13
Safety concerns
Two commonly used references contain recommendations for sedative-hypnotic medication use in geriatric patients.30,34 According to Gallagher et al’s34 Screening Tool of Older Person’s Prescriptions (STOPP), long-term (>1 month) use of long-acting BZDs (eg, flurazepam, diazepam) and prolonged use (>1 week) of first-generation antihistamines (eg, diphenhydramine, doxylamine) should be avoided in patients age ≥65 because of the risk of sedation, confusion, and anticholinergic side effects. STOPP recognizes that any use of BZDs, neuroleptics, or first-generation antihistamines may contribute to postural imbalance; therefore these agents are not recommended in older patients at risk for falls.
In the 2012 American Geriatrics Society (AGS) Beers Criteria, the AGS recommends avoiding barbiturates in older adults because of the high rate of physical dependence, tolerance to sleep effects, and overdose risk at low dosages.30 The AGS also recommends avoiding BZDs, stating that older adults have increased sensitivity to these agents and are at an increased risk of cognitive impairment, delirium, falls, fractures, and motor vehicle accidents when taking these drugs. Non-BZD BzRAs also should not be prescribed to patients with a history of falls or fractures, unless safer alternatives are not available.
The FDA has issued several advisory reports regarding sedative-hypnotic drugs. In 2007, all manufacturers of sedative-hypnotic drugs were required to modify their product labeling to include stronger language about potential risks.35 Among these changes, warnings for anaphylaxis and complex sleep-related behaviors were added. Also, the FDA requested that manufacturers of sedative-hypnotic drugs develop and provide patient medication guides, advising consumers on the potential risks and precautions associated with these drugs. More recently, the FDA announced changes to dosing recommendations for zolpidem-containing products because of the risk of impaired mental alertness36; manufacturers were required to lower the recommended dosages for each product.
Manufacturers of FDA-approved sedative-hypnotic drugs urge caution when prescribing these medications for geriatric patients, citing the potential for increased sensitivity, manifesting as marked excitement, depression, or confusion (eg, barbiturates), and greater risk for dosage-related adverse effects (eg, oversedation, dizziness, confusion, impaired psychomotor performance, ataxia).17-29
Use in clinical practice
Several variables should be considered when evaluating appropriateness of pharmacotherapy, including characteristics of the drug and the patient. Geriatric patients may be prone to comorbidities resulting from age-related physiologic changes. These diseases may be confounding (ie, contributing to sleep disorders); examples include medical illnesses, such as hyperthyroidism and arthritis, and psychiatric illnesses, such as depression and anxiety.37 Other conditions, such as renal and hepatic dysfunction, may lead to alteration in drug exposure. These conditions should be assessed through routine renal function tests (eg, serum creatinine and glomerular filtration rate) and liver function tests (eg, serum albumin and liver transaminases).
Multiple comorbidities suggest a higher likelihood of polypharmacy, leading to other drug-related issues (eg, drug-drug interactions). Although these issues may guide therapy by restricting medication options, their potential contribution to the underlying sleep complaints should be considered.37 Several drugs commonly used by geriatric patients may affect wakefulness (eg, analgesics, antidepressants, and antihypertensives [sedating], and thyroid hormones, corticosteroids, and CNS stimulants [alerting]).
CASE CONTINUED
In Mr. R’s case, zaleplon was initiated at 10 mg/d. Because of his age and the nature of his sleep disorder, the choice of sedative-hypnotic was suitable; however, the prescribed dosage was inappropriate. The sluggishness Mr. R experienced likely was a manifestation of increased exposure to the drug. According to manufacturer and AASM recommendations, a more appropriate dosage is 5 mg/d.13,23 Mr. R’s medical history and current medications, and his hepatic and renal function, should be assessed. If Mr. R continues to have issues with sleep initiation, zaleplon, 5 mg at bedtime, should be considered.
Related Resources
• Institute for Safe Medication Practices. www.ismp.org.
• MedWatch: The FDA Safety Information and Adverse Event Reporting Program. www.fda.gov/Safety/MedWatch/default.htm.
Drug Brand Names
Amitriptyline • Elavil Mirtazapine • Remeron
Amobarbital • Amytal Olanzapine • Zyprexa
Butabarbital • Butisol Pentobarbital • Nembutal
Chloral hydrate • Somnote Phenobarbital • Luminal
Diazepam • Valium Quazepam • Doral
Diphenhydramine • Benadryl, others Quetiapine • Seroquel
Doxepin • Silenor Ramelteon • Rozerem
Doxylamine • Unisom, others Secobarbital • Seconal
Estazolam • ProSom Suvorexant • Belsomra
Eszopiclone • Lunesta Temazepam • Restoril
Flurazepam • Dalmane Tiagabine • Gabitril
Gabapentin • Neurontin, Trazodone • Desyrel
Gralise, Horizant Zaleplon • Sonata
Lorazepam • Ativan Zolpidem • Ambien, Edluar,
Meprobamate • Equanil Intermezzo, Zolpimist
Acknowledgement
Vicki L. Ellingrod, PharmD, FCCP, is the series editor of Savvy Psychopharmacology.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Mr. R, 75, is having difficulty sleeping. When he goes to bed, he lies there for what seems like forever, unable to fall asleep. He feels “so tired” and ends up taking naps during the day, but he cannot break this cycle. He has tried using over-the-counter products with little relief.
Mr. R’s primary care physician prescribes zaleplon, 10 mg/d, and asks him to call the clinic in 2 weeks to discuss his progress. He takes zaleplon as directed for several nights and begins to feel “sluggish” during the day, both mentally and physically, despite reporting an increase in the overall amount of sleep at night.
Sedative-hypnotic drugs are among the most commonly used medications in the United States. Use of these drugs, as well as anxiolytics, has increased from 2.8% between 1988 and 1994 to 4.7% between 2007 and 2010, according to the Department of Health and Human Services.1 In 2011, drugs categorized as sedative-hypnotics or antipsychotics were involved in 6.1% of all human exposures identified in the American Association of Poison Control Centers’ National Poison Data System.2 Therefore, an understanding of clinical and pharmacological variables related to safe and effective use is important for clinicians prescribing and monitoring therapy with these agents.
Neuropsychiatric disorders are prevalent among geriatric patients and are associated with age-related physiologic changes in the CNS.3 Such changes involve:
• neuroanatomy (brain atrophy, decreased neuronal density, increased plaque formation)
• neurotransmitters (reduced cholinergic transmission, decreased synthesis of dopamine and catecholamines), and
• neurophysiology (reduced cerebral blood flow).
These physiologic processes manifest as alterations in mental status, reflexes, sensation, gait, balance, and sleep. Examples of sleep changes among geriatric patients include decreased sleep efficiency, more frequent awakenings, and more variable sleep duration.3,4 Sleep disorders also may be related to mental disorders and other medical conditions.5 For example, the prevalence of sleep-related respiratory disorders, such as obstructive sleep apnea and central sleep apnea, increases with age.6
Sleep disorders are common among geriatric patients. In a large epidemiologic study of sleep complaints in patients age ≥65, more than one-half of patients had at least 1 sleep complaint (ie, difficulty falling asleep, trouble waking up, early awakening, need for naps, and feeling ill-rested).7 As many as 34% of patients reported symptoms of insomnia. In an analysis of National Ambulatory Medical Survey Data over 6 years, 24.8% to 27.9% of sleep-related medical office visits were attributed to patients age ≥65.8
Pharmacology in aging
Prescribing sedative-hypnotic drugs is not routinely recommended for older patients with a sleep disorder. Geriatric patients, compared with younger patients, are at higher risk of iatrogenic complications because of polypharmacy, comorbidities, relative renal and hepatic insufficiency, and other physiologic changes leading to alterations in drug exposure and metabolism (Table 1).9-12
Aging is associated with changes in body composition, including an increase in total body fat and decrease in lean body mass and total body water. These changes, as well as a prolonged GI transit time, decrease in active gut transporters, decreased blood perfusion, and decrease in plasma proteins such as albumin (because of reduced liver function or malnutrition), may lead to alteration in drug absorption patterns and may increase the volume of distribution for lipophilic drugs. Additionally, the elimination half-life of some drugs may increase with age because of larger volumes of distribution and reduction in hepatic or renal clearance.
The clinical significance of these changes is not well established. Although the process of drug absorption can change with age, the amount of drug absorbed might not be significantly affected. An increase in the volume of distribution and reduction in drug metabolism and clearance might lead to increasing amounts of circulating drug and duration of drug exposure, putting geriatric patients at an increased risk for adverse effects and drug toxicity.9
Among these mechanisms, Dolder et al11 hypothesized that drug metabolism catalyzed by cytochrome P450 (CYP) enzymes and renal excretion may be of greatest concern. Although in vitro studies suggest that concentration of CYP enzymes does not decline with age, in vivo studies have demonstrated reduced CYP activity in geriatric patients.11,12 Theoretically, a reduction in CYP activity would increase the bioavailability of drugs, especially those that are subject to extensive first-pass (ie, hepatic) metabolism, and may lead to a reduction in systemic clearance.
Independent of metabolic changes, geriatric patients are at risk of reduced renal clearance because of age-related changes in glomerular filtration rate. Pharmacodynamic changes might be observed in older patients and could be a concern even in the setting of unaltered pharmacokinetic factors.9 These changes usually require administering smaller drug dosages.
Sedative-hypnotic medications
Sedative-hypnotic agents include several barbiturates, benzodiazepines (BZDs), non-BZD benzodiazepine-receptor agonists (BzRAs), a melatonin-receptor agonist (ie, ramelteon), and an orexin-receptor antagonist (ie, suvorexant).13,14Table 214-29 summarizes selected sedative-hypnotic drugs. Additional drug classes used to treat insomnia include:
• sedating antidepressants (trazodone, amitriptyline, doxepin, mirtazapine)
• antiepileptic drugs (gabapentin, tiagabine)
• atypical antipsychotics (quetiapine, olanzapine).
FDA-approved agents for treating insomnia include amobarbital, butabarbital, pentobarbital, phenobarbital, secobarbital, chloral hydrate, diphenhydramine, doxylamine, doxepin, estazolam, flurazepam, lorazepam, quazepam, temazepam, triazolam, eszopiclone, zaleplon, zolpidem, ramelteon, and suvorexant. Not all of these drugs are recommended for use in geriatric patients. Barbiturates, for example, should be avoided.30
Pharmacokinetic characteristics vary among drugs and drug classes. Choice of pharmacotherapy should account for patient and drug characteristics and the specific sleep complaint. Sleep disorders may be variously characterized as difficulty with sleep initiation, duration, consolidation, or quality.13 Therefore, onset and duration of effect are important drug-related considerations. Sedative-hypnotic drugs with a short time-to-onset may be ideal for patients with sleep-onset insomnia.
The drugs’ duration of effect (eg, presence of active metabolites, long elimination half-life) also must be reviewed. A long elimination half-life may lead to increased drug exposure and unwanted side effects such as residual daytime drowsiness. Despite this, sedative-hypnotic drugs with a longer duration of effect (eg, intermediate- or long-acting drugs) may be best for patients with insomnia defined by difficulty maintaining sleep.
Benzodiazepines vary in their time to onset of effect, rate of elimination, and metabolism.15-21 BZDs that are FDA- approved for use as sedative-hypnotics are listed in Table 2.14-29 These BZDs have different onsets of effect as evidenced by time to achieve maximum plasma concentration (Tmax), ranging from 0.5 hours (flurazepam) to 2 hours (estazolam, quazepam, triazolam). The elimination half-life varies widely among these medications, from 1.5 hours (triazolam) to >100 hours (flurazepam). Flurazepam’s long half-life is attributable to its active major metabolite. Although most BZDs are metabolized hepatically, temazepam is subject to minimal hepatic metabolism.
Benzodiazepine-receptor agonists. There is substantial variation in the pharmacokinetic characteristics of BzRAs.15,16,22-28 There also are differences among the zolpidem dosage forms; sublingual formulations have the shortest onset of effect. Eszopiclone and zaleplon have low protein binding compared with zolpidem. Elimination half-lives vary among drugs with the shortest attributed to zaleplon (1 hour) and longest to eszopiclone (6 hours). All BzRAs are subject to extensive hepatic metabolism.
Ramelteon. Singular in its class, ramelteon is a treatment option for insomnia.29 This drug has a short onset of effect, moderate protein binding, and extensive hepatic metabolism. Ramelteon is primarily excreted in the urine as its metabolites, and the drug half-life is relatively short.
Suvorexant is the latest addition to the sedative-hypnotic armamentarium, approved by the FDA in August 2014 for difficulty with sleep onset and/or sleep maintenance.14 As an orexin-receptor antagonist, suvorexant represents a novel pharmacologic class. Suvorexant exhibits moderately rapid absorption with time to peak concentration ranging from 30 minutes to 6 hours in fasting conditions; absorption is delayed when taken with or soon after a meal. The drug is highly protein bound and extensively metabolized, primarily through CYP3A. The manufacturer recommends dose reduction (5 mg at bedtime) in patients taking moderate CYP3A inhibitors and avoiding suvorexant in patients taking strong CYP3A inhibitors. Suvorexant is primarily excreted through feces and the mean half-life is relatively long.
Considering these characteristics and age-related physiologic changes, the practitioner should be concerned about drugs that undergo extensive hepatic metabolism. Age-related reductions in CYP activity may lead to an increase in drug bioavailability and a decrease in the systemic clearance,11 which might be associated with an increase in elimination half-life and duration of action. Dosage adjustments are recommended for several BZDs (lower initial and maximum dosages for most agents) and BzRAs.17-28 No dosage adjustments for ramelteon or suvorexant in geriatric patients have been specified14,29; the manufacturers for both products assert that no differences in safety and efficacy have been observed between older and younger adult patients.
Alternative and complementary medications
Several non-prescription products, including over-the-counter drugs (eg, diphenhy-dramine, doxylamine) and herbal therapies (eg, melatonin, valerian), are used for their sedative-hypnotic properties. There is a lack of evidence supporting using diphenhydra-mine in patients with chronic insomnia, and tolerance to its hypnotic effect has been reported with repeated use.31 Concerns about anticholinergic toxicity and CNS depression limit its use in geriatric patients. Among herbal therapies, melatonin may have the strongest evidence for its ability to alleviate sleep disorders in geriatric patients32; however, meta-analyses have demonstrated small effects of melatonin on sleep latency and minimal differences in wake time after sleep onset and total sleep time.13
Clinical practice guidelines
Non-pharmacotherapeutic interventions, such as behavioral (eg, sleep hygiene measures) and psychological therapy, are recommended for initial management of sleep disorders in geriatric patients.13,33 In conjunction, the American Medical Directors Association (AMDA) recommends address ing underlying causes and exacerbating factors (eg, medical condition or medication).33 The AMDA recommends avoiding long-term pharmacotherapy and advises caution with BZD-hypnotic drugs, tricyclic antidepressants, and antihistamines. The American Academy of Sleep Medicine (AASM) recommends an initial treatment period of 2 to 4 weeks, followed by re-evaluation of continued need for treatment.13 The AASM recommends short- or intermediate-acting BzRAs or ramelteon for initial pharmacologic management of primary insomnias and insomnias comorbid with other conditions. The AASM also recommends specific dosages of BzRAs and BZDs for geriatric patients, which coincide with manufacturer-recommended dosages (Table 2).14-29
Barbiturates, chloral hydrate, and non-barbiturate, non-BZD drugs such as meprobamate are not recommended because of potential significant adverse effects and tolerance/dependence, and low therapeutic index. The AASM advises caution when using prescription drugs off-label for insomnia (eg, antidepressants, antiepileptics, antipsychotics) and recommends avoiding them, if possible, because of limited evidence supporting their use.13
Safety concerns
Two commonly used references contain recommendations for sedative-hypnotic medication use in geriatric patients.30,34 According to Gallagher et al’s34 Screening Tool of Older Person’s Prescriptions (STOPP), long-term (>1 month) use of long-acting BZDs (eg, flurazepam, diazepam) and prolonged use (>1 week) of first-generation antihistamines (eg, diphenhydramine, doxylamine) should be avoided in patients age ≥65 because of the risk of sedation, confusion, and anticholinergic side effects. STOPP recognizes that any use of BZDs, neuroleptics, or first-generation antihistamines may contribute to postural imbalance; therefore these agents are not recommended in older patients at risk for falls.
In the 2012 American Geriatrics Society (AGS) Beers Criteria, the AGS recommends avoiding barbiturates in older adults because of the high rate of physical dependence, tolerance to sleep effects, and overdose risk at low dosages.30 The AGS also recommends avoiding BZDs, stating that older adults have increased sensitivity to these agents and are at an increased risk of cognitive impairment, delirium, falls, fractures, and motor vehicle accidents when taking these drugs. Non-BZD BzRAs also should not be prescribed to patients with a history of falls or fractures, unless safer alternatives are not available.
The FDA has issued several advisory reports regarding sedative-hypnotic drugs. In 2007, all manufacturers of sedative-hypnotic drugs were required to modify their product labeling to include stronger language about potential risks.35 Among these changes, warnings for anaphylaxis and complex sleep-related behaviors were added. Also, the FDA requested that manufacturers of sedative-hypnotic drugs develop and provide patient medication guides, advising consumers on the potential risks and precautions associated with these drugs. More recently, the FDA announced changes to dosing recommendations for zolpidem-containing products because of the risk of impaired mental alertness36; manufacturers were required to lower the recommended dosages for each product.
Manufacturers of FDA-approved sedative-hypnotic drugs urge caution when prescribing these medications for geriatric patients, citing the potential for increased sensitivity, manifesting as marked excitement, depression, or confusion (eg, barbiturates), and greater risk for dosage-related adverse effects (eg, oversedation, dizziness, confusion, impaired psychomotor performance, ataxia).17-29
Use in clinical practice
Several variables should be considered when evaluating appropriateness of pharmacotherapy, including characteristics of the drug and the patient. Geriatric patients may be prone to comorbidities resulting from age-related physiologic changes. These diseases may be confounding (ie, contributing to sleep disorders); examples include medical illnesses, such as hyperthyroidism and arthritis, and psychiatric illnesses, such as depression and anxiety.37 Other conditions, such as renal and hepatic dysfunction, may lead to alteration in drug exposure. These conditions should be assessed through routine renal function tests (eg, serum creatinine and glomerular filtration rate) and liver function tests (eg, serum albumin and liver transaminases).
Multiple comorbidities suggest a higher likelihood of polypharmacy, leading to other drug-related issues (eg, drug-drug interactions). Although these issues may guide therapy by restricting medication options, their potential contribution to the underlying sleep complaints should be considered.37 Several drugs commonly used by geriatric patients may affect wakefulness (eg, analgesics, antidepressants, and antihypertensives [sedating], and thyroid hormones, corticosteroids, and CNS stimulants [alerting]).
CASE CONTINUED
In Mr. R’s case, zaleplon was initiated at 10 mg/d. Because of his age and the nature of his sleep disorder, the choice of sedative-hypnotic was suitable; however, the prescribed dosage was inappropriate. The sluggishness Mr. R experienced likely was a manifestation of increased exposure to the drug. According to manufacturer and AASM recommendations, a more appropriate dosage is 5 mg/d.13,23 Mr. R’s medical history and current medications, and his hepatic and renal function, should be assessed. If Mr. R continues to have issues with sleep initiation, zaleplon, 5 mg at bedtime, should be considered.
Related Resources
• Institute for Safe Medication Practices. www.ismp.org.
• MedWatch: The FDA Safety Information and Adverse Event Reporting Program. www.fda.gov/Safety/MedWatch/default.htm.
Drug Brand Names
Amitriptyline • Elavil Mirtazapine • Remeron
Amobarbital • Amytal Olanzapine • Zyprexa
Butabarbital • Butisol Pentobarbital • Nembutal
Chloral hydrate • Somnote Phenobarbital • Luminal
Diazepam • Valium Quazepam • Doral
Diphenhydramine • Benadryl, others Quetiapine • Seroquel
Doxepin • Silenor Ramelteon • Rozerem
Doxylamine • Unisom, others Secobarbital • Seconal
Estazolam • ProSom Suvorexant • Belsomra
Eszopiclone • Lunesta Temazepam • Restoril
Flurazepam • Dalmane Tiagabine • Gabitril
Gabapentin • Neurontin, Trazodone • Desyrel
Gralise, Horizant Zaleplon • Sonata
Lorazepam • Ativan Zolpidem • Ambien, Edluar,
Meprobamate • Equanil Intermezzo, Zolpimist
Acknowledgement
Vicki L. Ellingrod, PharmD, FCCP, is the series editor of Savvy Psychopharmacology.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. National Center for Health Statistics. Health, United States, 2012, with special feature on emergency care. http://www. cdc.gov/nchs/data/hus/hus12.pdf. Published May 2013. Accessed August 22, 2014.
2. Bronstein AC, Spyker DA, Cantilena LR Jr, et al. 2011 Annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 29th annual report. Clin Toxicol (Phila). 2012;50(10): 911-1164.
3. Inouye SK. Neuropsychiatric aspects of aging. In: Goldman L, Schafer AI, eds. Goldman’s cecil medicine. 24th ed. Philadelphia, PA: Elsevier Saunders; 2011:114-116.
4. Diagnostic and statistical manual of mental disorders, 4th ed. Washington, DC: American Psychiatric Association; 1994.
5. American Psychiatric Association. Highlights of changes from DSM-IV-TR to DSM-5. http://www.psychiatry. org/File%20Library/Practice/DSM/DSM-5/Changes-from-DSM-IV-TR--to-DSM-5.pdf. 2013. Accessed August 22, 2014.
6. Edwards BA, O’Driscoll DM, Ali A, et al. Aging and sleep: physiology and pathophysiology. Semin Respir Crit Care Med. 2010;31(5):618-633.
7. Foley DJ, Monjan AA, Brown SL, et al. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18(6):425-432.
8. Morlock RJ, Tan M, Mitchell DY. Patient characteristics and patterns of drug use for sleep complaints in the United States: analysis of National Ambulatory Medical Survey Data, 1997-2002. Clin Ther. 2006;28(7):1044-1053.
9. Diasio RB. Principles of drug therapy. In: Goldman L, Schafer AI, eds. Goldman’s Cecil medicine. 24th ed. Philadelphia, PA: Elsevier Saunders; 2011:124-132.
10. Hilmer SN, Ford GA. General principles of pharmacology. In: Halter JB, Ouslander JG, Tinetti ME, et al, eds. Hazzard’s geriatric medicine and gerontology. 6th ed. New York, NY: McGraw-Hill; 2009:103-122.
11. Dolder C, Nelson M, McKinsey J. Use of non-benzodiazepine hypnotics in the elderly: are all agents the same? CNS Drugs. 2007;21(5):389-405.
12. Klotz U. Pharmacokinetics and drug metabolism in the elderly. Drug Metab Rev. 2009;41(2):67-76.
13. Schutte-Rodin S, Broch L, Buysse D, et al. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4(5):487-504.
14. Belsomra [package insert]. Whitehouse Station, NJ: Merck & Co., Inc.; 2014.
15. Micromedex Healthcare Series. Greenwood Village, CO: Thomson Healthcare. http://micromedex.com. Accessed August 22, 2014.
16. Lexicomp. St. Louis, MO: Wolters Kluwer Health. http:// www.lexi.com. Accessed August 22, 2014.
17. Estazolam [package insert]. Corona, CA: Watson Pharma, Inc; 2008.
18. Flurazepam [package insert]. Eatontown, NJ: West-Ward Pharmaceutical Corp; 2010.
19. Doral [package insert]. Las Vegas, NV: Nuro Pharma, Inc; 2013.
20. Restoril [package insert]. Hazelwood, MO: Mallinckrodt Inc; 2010.
21. Halcion [package insert]. New York, NY: Pharmacia & Upjohn Co; 2013.
22. Lunesta [package insert]. Marlborough, MA: Sunovion Pharmaceuticals Inc; 2012.
23. Sonata [package insert]. New York, NY: Pfizer Inc; 2013.
24. Ambien [package insert]. Bridgewater, NJ: Sanofi-Aventis; 2013.
25. Ambien CR [package insert]. Bridgewater, NJ: Sanofi- Aventis; 2013.
26. Edluar [package insert]. Somerset, NJ: Meda Pharmaceuticals Inc; 2009.
27. Intermezzo [package insert]. Point Richmond, CA: Transcept Pharmaceuticals, Inc; 2011.
28. Zolpimist [package insert]. Richmond, VA: ECR Pharmaceuticals; 2013.
29. Rozerem [package insert]. Deerfield, IL: Takeda Pharmaceuticals America, Inc; 2010.
30. The American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616-631.
31. Kirkwood CK, Melton ST. Insomnia, drowsiness, and fatigue. In: Krinsky DL, Berardi RR, Ferreri SP, et al, eds. Handbook of nonprescription drugs: an interactive approach to self-care. 17th ed. Washington, DC: American Pharmacists Association; 2012:867-884.
32. Insomnia. In: Natural Standard. Somerville, MA: Natural Standard. https://naturalmedicines.therapeuticresearch. com/databases/medical-conditions/i/insomnia.aspx. Accessed August 22, 2014.
33. American Medical Directors Association. Clinical practice guideline: sleep disorders. Columbia, MD: American Medical Directors Association; 2006.
34. Gallagher P, Ryan C, Byrne S, et al. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther. 2008;46(2):72-83.
35. Food and Drug Administration. FDA requests label change for all sleep disorder drug products. http://www.fda. gov/newsevents/newsroom/pressannouncements/2007/ ucm108868.htm. Published March 14, 2007. Accessed August 22, 2014.
36. Food and Drug Administration. FDA drug safety communication: risk of next-morning impairment after use of insomnia drugs; FDA requires lower recommended doses for certain drugs containing zolpidem (Ambien, Ambien CR, Edluar, and Zolpimist). http://www.fda.gov/drugs/ drugsafety/ucm334033.htm. Published January 10, 2013. Accessed August 22, 2014.
37. Cohen-Zion M, Ancoli-Israel S. Sleep disorders. In: Hazzard’s geriatric medicine and gerontology. 6th ed. New York, NY: McGraw-Hill; 2009:671-682.
1. National Center for Health Statistics. Health, United States, 2012, with special feature on emergency care. http://www. cdc.gov/nchs/data/hus/hus12.pdf. Published May 2013. Accessed August 22, 2014.
2. Bronstein AC, Spyker DA, Cantilena LR Jr, et al. 2011 Annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 29th annual report. Clin Toxicol (Phila). 2012;50(10): 911-1164.
3. Inouye SK. Neuropsychiatric aspects of aging. In: Goldman L, Schafer AI, eds. Goldman’s cecil medicine. 24th ed. Philadelphia, PA: Elsevier Saunders; 2011:114-116.
4. Diagnostic and statistical manual of mental disorders, 4th ed. Washington, DC: American Psychiatric Association; 1994.
5. American Psychiatric Association. Highlights of changes from DSM-IV-TR to DSM-5. http://www.psychiatry. org/File%20Library/Practice/DSM/DSM-5/Changes-from-DSM-IV-TR--to-DSM-5.pdf. 2013. Accessed August 22, 2014.
6. Edwards BA, O’Driscoll DM, Ali A, et al. Aging and sleep: physiology and pathophysiology. Semin Respir Crit Care Med. 2010;31(5):618-633.
7. Foley DJ, Monjan AA, Brown SL, et al. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18(6):425-432.
8. Morlock RJ, Tan M, Mitchell DY. Patient characteristics and patterns of drug use for sleep complaints in the United States: analysis of National Ambulatory Medical Survey Data, 1997-2002. Clin Ther. 2006;28(7):1044-1053.
9. Diasio RB. Principles of drug therapy. In: Goldman L, Schafer AI, eds. Goldman’s Cecil medicine. 24th ed. Philadelphia, PA: Elsevier Saunders; 2011:124-132.
10. Hilmer SN, Ford GA. General principles of pharmacology. In: Halter JB, Ouslander JG, Tinetti ME, et al, eds. Hazzard’s geriatric medicine and gerontology. 6th ed. New York, NY: McGraw-Hill; 2009:103-122.
11. Dolder C, Nelson M, McKinsey J. Use of non-benzodiazepine hypnotics in the elderly: are all agents the same? CNS Drugs. 2007;21(5):389-405.
12. Klotz U. Pharmacokinetics and drug metabolism in the elderly. Drug Metab Rev. 2009;41(2):67-76.
13. Schutte-Rodin S, Broch L, Buysse D, et al. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4(5):487-504.
14. Belsomra [package insert]. Whitehouse Station, NJ: Merck & Co., Inc.; 2014.
15. Micromedex Healthcare Series. Greenwood Village, CO: Thomson Healthcare. http://micromedex.com. Accessed August 22, 2014.
16. Lexicomp. St. Louis, MO: Wolters Kluwer Health. http:// www.lexi.com. Accessed August 22, 2014.
17. Estazolam [package insert]. Corona, CA: Watson Pharma, Inc; 2008.
18. Flurazepam [package insert]. Eatontown, NJ: West-Ward Pharmaceutical Corp; 2010.
19. Doral [package insert]. Las Vegas, NV: Nuro Pharma, Inc; 2013.
20. Restoril [package insert]. Hazelwood, MO: Mallinckrodt Inc; 2010.
21. Halcion [package insert]. New York, NY: Pharmacia & Upjohn Co; 2013.
22. Lunesta [package insert]. Marlborough, MA: Sunovion Pharmaceuticals Inc; 2012.
23. Sonata [package insert]. New York, NY: Pfizer Inc; 2013.
24. Ambien [package insert]. Bridgewater, NJ: Sanofi-Aventis; 2013.
25. Ambien CR [package insert]. Bridgewater, NJ: Sanofi- Aventis; 2013.
26. Edluar [package insert]. Somerset, NJ: Meda Pharmaceuticals Inc; 2009.
27. Intermezzo [package insert]. Point Richmond, CA: Transcept Pharmaceuticals, Inc; 2011.
28. Zolpimist [package insert]. Richmond, VA: ECR Pharmaceuticals; 2013.
29. Rozerem [package insert]. Deerfield, IL: Takeda Pharmaceuticals America, Inc; 2010.
30. The American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616-631.
31. Kirkwood CK, Melton ST. Insomnia, drowsiness, and fatigue. In: Krinsky DL, Berardi RR, Ferreri SP, et al, eds. Handbook of nonprescription drugs: an interactive approach to self-care. 17th ed. Washington, DC: American Pharmacists Association; 2012:867-884.
32. Insomnia. In: Natural Standard. Somerville, MA: Natural Standard. https://naturalmedicines.therapeuticresearch. com/databases/medical-conditions/i/insomnia.aspx. Accessed August 22, 2014.
33. American Medical Directors Association. Clinical practice guideline: sleep disorders. Columbia, MD: American Medical Directors Association; 2006.
34. Gallagher P, Ryan C, Byrne S, et al. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther. 2008;46(2):72-83.
35. Food and Drug Administration. FDA requests label change for all sleep disorder drug products. http://www.fda. gov/newsevents/newsroom/pressannouncements/2007/ ucm108868.htm. Published March 14, 2007. Accessed August 22, 2014.
36. Food and Drug Administration. FDA drug safety communication: risk of next-morning impairment after use of insomnia drugs; FDA requires lower recommended doses for certain drugs containing zolpidem (Ambien, Ambien CR, Edluar, and Zolpimist). http://www.fda.gov/drugs/ drugsafety/ucm334033.htm. Published January 10, 2013. Accessed August 22, 2014.
37. Cohen-Zion M, Ancoli-Israel S. Sleep disorders. In: Hazzard’s geriatric medicine and gerontology. 6th ed. New York, NY: McGraw-Hill; 2009:671-682.
How do you score on this self-assessment of suicide risk management?: First of 2 parts
The assessment and management of suicide risk are complex and difficult tasks that raise clinical issues without clear-cut, easy answers. This case-based, multiple-choice self-assessment with accompanying commentaries is a teaching instrument that I designed to enhance a clinician’s ability to provide care for patients at risk for suicide. Part 1 of this article poses 8 of the 15 questions; the balance of questions will appear in Part 2, in the November 2014 issue of Current Psychiatry.
The questions and commentaries in this self-assessment originate in the referenced work of others and my clinical experience. Therefore, I use the preferred “best response” option—not the customary and more restrictive “correct answer” format.
How do you score?
Question 1
Mr. J, age 34, is a professional basketball player complaining of weight loss, early morning waking, and a dysphoric mood lasting for 1 month. His performance on the basketball court has declined and his wife is seeking a separation. He describes “fleeting” suicidal thoughts. He has no history of suicide attempts or depression. The patient does not abuse alcohol or drugs.
The initial assessment approach is to:
a) obtain a suicide prevention contract
b) assess suicide risk and protective factors
c) determine the cause of Mr. J’s depression
d) have Mr. J complete a suicide risk self-assessment form
e) contact his wife for additional history
The best response option is B
Suicide prevention contracts do not prevent suicide.1 Contacting the patient’s wife may be an option at a later stage of evaluation or treatment, if Mr. J grants permission. Determining the cause of his depression likely will require ongoing work up. Assessing suicide risk factors without also looking at protective factors is a common error. A comprehensive suicide risk assessment evaluation requires evaluating both risk and protective factors.2,3 Suicide risk assessment forms often omit questions about protective factors.4 Do not rely on self-assessment suicide risk forms because they are dependent on the patient’s truthfulness. Patients who are determined to commit suicide might regard the psychiatrist and other mental health professionals as the enemy.5
Question 2
Ms. P, a 56-year-old, single schoolteacher, is admitted to a psychiatric unit for severe depression and suicidal ideation without a plan. She is devoutly religious, stating, “I won’t kill myself, because I don’t want to go to hell.” Ms. P attends religious services regularly. She has a history of chronic recurrent depression with suicidal ideation and no history of suicide attempts. You suspect a diagnosis of bipolar II disorder.
In assessing religious affiliation as a protective factor against suicide, you should consider:
a) the nature of the patient’s religious conviction
b) the religion’s stated position on suicide
c) severity of the patient’s illness
d) presence of delusional religious beliefs
e) all of the above
The best response option is E
Dervic et al6 evaluated 371 depressed inpatients according to their religious or non-religious affiliation. Patients with no religious affiliation made significantly more suicide attempts, had more first-degree relatives who committed suicide, were younger, were less likely to be married or have children, and had fewer contacts with family members.
In general, religious affiliation is a protective factor against suicide but may not be a protective factor in an individual patient. Religious affiliation, similar to other presummed general protective factors, requires further scrutiny. Avoid making assumptions. For example, a depressed, devoutly religious patient may curse God for abandonment. A patient with bipolar disorder may believe that God would forgive her for committing suicide. A presumed protective factor may not be protective or might even be a risk factor, such as psychotic patients with religious delusions.
Abrahamic religions—ie, Judaism, Christianity, and Islam—prohibit suicide. Severe mental illness, however, can overcome the strongest religious prohibitions against suicide, including the fear of eternal damnation. For many psychiatric patients, religious affiliations and beliefs are protective factors against suicide, but only relatively. No protective factor against suicide, however strong, provides absolute protection against suicide. Moreover, other risk and protective factors also must be assessed comprehensively.
Question 3
Mr. W, age 18, is admitted to an inpatient psychiatric unit with severe agitation, thought disorder, disorganization, and auditory hallucinations. He is threatening to jump from a nearby building. He has no history of substance abuse.
The psychiatrist conducts a comprehensive suicide risk assessment that includes the patient’s psychiatric diagnosis as a risk factor.
Which psychiatric disorder has the highest associated suicide mortality rate?
a) schizophrenia
b) eating disorders
c) bipolar disorder
d) major depressive disorder
e) borderline personality disorder
The best response option is B
Harris and Barraclough (Table)7 calculated the standardized mortality ratio (SMR) for suicide among psychiatric disorders. SMR is calculated by dividing observed mortality by suicide by the expected mortality by suicide in the general population. Every psychiatric disorder in their study, except for mental retardation, was associated with a varying degree of suicide risk. Eating disorders had the highest SMR. The patient’s psychiatric diagnosis is a risk factor that informs the clinician’s suicide risk assessment.
Question 4
Mr. Z, a 64-year-old, recently divorced lawyer, is admitted to the psychiatric unit from the emergency room. His colleagues brought Mr. Z to the emergency room because of his suicide threats.
On the unit, Mr. Z denies suicidal ideation, plan, or intent. Agitation and suspiciousness are prominent. He refuses to authorize staff to contact his colleagues, his ex-wife, and other family members. Mr. Z demands immediate discharge and forbids contact with his outpatient psychotherapist. He is placed on 72-hour hold as a conditional voluntary admission.
The clinician should:
a) contact Mr. Z’s family, as an emergency exception to confidentiality
b) e-mail his family members with questions
c) contact the patient’s psychotherapist as permitted by the Health Insurance Portability and Accountability Act of 1996 (HIPAA)
d) try to develop a therapeutic alliance with Mr. Z
e) none of the above
The best response option is C
HIPAA permits psychiatrists and other health care providers who are treating the same patient to communicate with each other about medical treatment without obtaining permission from the patient.8 However, mental health professionals cannot share psychotherapy notes without a patient’s consent, except when legally required, such as reporting abuse or duty to warn. This is the most expeditious and productive way of obtaining essential clinical information. E-mail merely changes the mode of unauthorized communication with significant others.
Mr. Z is agitated and suspicious, and developing a therapeutic alliance would require time. It is necessary to gather information about his psychiatric condition as soon as possible. An emergency exception to maintaining confidentiality is another option.9 The definition of emergency varies among jurisdictions. Consulting with a knowledgeable attorney may be necessary, but it usually takes time. Ethically, it is permissible to breach confidentiality to protect the suicidal patient.10
Question 5
Mr. G, a 42-year-old engineer, is re-hospitalized after a failed hanging attempt. Initially, he is profoundly depressed but improves suddenly and requests discharge. The psychiatrist and clinical staff are perplexed. Is the sudden improvement real or feigned?
The treatment team should consider all of the following options except:
a) obtain records of earlier hospitalizations
b) check collateral sources of information
c) assess Mr. G’s compliance with treatment
d) obtain psychological testing to evaluate Mr. G’s honesty
e) determine whether behavioral signs of depression are present
The best response option is D
Short length of hospital stay makes it difficult to assess sudden patient improvement.11 Real improvement in a high-risk suicidal patient is a process, even when it occurs quickly. Feigned improvement is an event. Obtaining patient information from collateral sources is crucial. Sudden improvement might be caused by the patient’s resolve to complete suicide. Identifying behavioral risk factors associated with psychiatric disorders informs the clinician’s systematic suicide risk assessment of a guarded or dissimulative patient. Psychological testing will take critical time and is not a substitute for careful clinical assessment.
Question 6
In mid-winter, Ms. M, a 42-year-old homeless woman, is seen in the emergency room of a general hospital. She complains of depression and auditory hallucinations commanding her to commit suicide. Ms. M has 5 earlier admissions to the psychiatry unit for similar complaints.
The psychiatrist conducts a comprehensive suicide risk assessment. Acute and chronic risk factors for suicide are identified. Protective factors also are assessed. The psychiatrist weighs and synthesizes risk and protective factors into an overall assessment of Ms. M’s suicide risk.
The main purpose of suicide risk assessment is to:
a) predict the likelihood of suicide
b) determine imminence of suicide
c) inform patient treatment and safety management
d) identify malingered suicidal ideation
e) provide a legal defense against a malpractice claim
The best response option is C
Suicide cannot be predicted.12 The term imminent suicide is a veiled attempt to predict when a patient will attempt suicide.13 The process of a comprehensive or systematic suicide risk assessment encompasses identification, analysis, and synthesis of risk and protective factors that inform the treatment and safety management of the patient.3 The overall suicide assessment is a clinical judgment call that determines risk along a continuum of low to high. In Ms. M’s case, comprehensive suicide risk assessment will assist the clinician in determining the patient’s overall suicide risk and make an appropriate disposition. Without a systematic suicide risk assessment methodology, the clinician is at the mercy of the pejoratively labeled “frequent flyer” who is looking for sustenance and lodging. The frustrated clinician is left with little choice but to admit the patient.
Although not the main purpose, systematic suicide risk assessment can help provide a sound legal defense if a suicide malpractice claim is filed against the clinician alleging negligent assessment.14
Question 7
A psychiatrist is treating Mr. S, a 36-year-old computer analyst, with once-a-week psychotherapy and medication management for panic and depressive symptoms that emerged abruptly after the break-up of a romantic relationship. Mr. S is using alcohol to sleep. He reports occasional suicidal ideation but no plan. He finds the idea of suicide to be morally repugnant. A therapeutic alliance develops.
The psychiatrist is concerned about Mr. S’s suicide risk and the need for hospitalization. The psychiatrist performs a systematic suicide risk assessment that includes identification of individual and evidence-based protective factors. For example, Mr. S continued to pursue his interests and to participate in civil causes. The overall suicide risk is determined by the assessment of individual and evidence-based protective factors.
All of the following options are evidence-based protective factors except:
a) therapeutic alliance
b) survival and coping beliefs
c) responsibility to family
d) fear of suicide
e) moral objections to suicide
The best response option is A
Clinical consensus holds that the therapeutic alliance is an important protective factor against suicide. However, no evidence-based research supports or refutes this widely held belief among clinicians.
Linehan et al15 developed the Reasons for Living Inventory, a self-report instrument that identifies 6 subscales:
• survival and coping beliefs
• responsibility to family
• child-related concerns
• fear of suicide
• fear of social disapproval
• moral objections to suicide.
Survival and coping beliefs, responsibility to family, and child-related concerns were useful in differentiating between suicidal and non-suicidal individuals. Malone et al16 administered the Reasons for Living Inventory to 84 inpatients with major depression; 45 had attempted suicide. Depressed patients who had not attempted suicide demonstrated more sense of responsibility toward family, more fear of social disapproval, more moral objections to suicide, greater survival and coping skills, and greater fear of suicide than patients who attempted suicide. The authors recommended adding the Reasons for Living Inventory to the assessment of patients at risk for suicide.
Question 8
A 38-year-old mother of a newborn child is admitted to the psychiatric unit after expressing suicidal thoughts to her husband. She has been hospitalized previously after a hypomanic episode and severe depression; she has no history of suicide attempts. A psychiatrist diagnoses bipolar II disorder (recurrent major episodes with hypomanic episodes). The patient’s maternal aunt has bipolar disorder. Her paternal grandfather committed suicide.
The psychiatrist conducts a systematic suicide risk assessment and determines the patient is at high risk of suicide. He considers a suicide-risk reduction drug.
Which one of the following drugs has been shown to reduce suicide and suicide attempts in bipolar II patients?
a) clozapine
b) clonazepam
c) lorazepam
d) lithium
e) quetiapine
The best response option is D
Prospective, randomized and controlled trials consistently have found lower rates of completed suicides and suicide attempts during lithium maintenance treatments for patients with bipolar disorder and other major affective disorders.17
Bottom Line
Suicide risk assessment and management are challenging for even experienced clinicians. Suicide risk assessment guides appropriate treatment and management for patients at risk for suicide. This self-assessment helps mental health professionals identify potential gaps in their knowledge and reinforce best practices.
Related Resources
• Simon RI. Passive suicidal ideation: Still a high-risk clinical scenario. Current Psychiatry. 2014;13(3):13-15.
• Simon RI. Suicide rehearsals: A high-risk psychiatric emergency. Current Psychiatry. 2012;11(7):28-32.
• Bongar B, Sullivan GR. The suicidal patient: Clinical and legal standards of care. Washington, DC: American Psychological Association; 2013.
Drug Brand Names
Clonazepam • Klonopin Lorazepam • Ativan
Clozapine • Clozaril Quetiapine • Seroquel
Lithium • Eskalith, Lithobid
Disclosure
Dr. Simon reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Adapted with permission from: Simon RI. Preventing patient suicide: clinical assessment and management, Arlington VA: American Psychiatric Publishing; 2011.
Editor’s note: Part 2 of this self-assessment on suicide assessment and management in the November 2014 issue of Current Psychiatry poses 7 additional questions.
1. Stanford EJ, Goetz RR, Bloom JD. The No Harm Contract in the emergency assessment of suicide risk. J Clin Psychiatry. 1994;55(8):344-348.
2. Simon RI, Hales RE, eds. Textbook of suicide assessment and management. 2nd ed. Arlington, VA: American Psychiatric Publishing, Inc.; 2012.
3. Practice guidelines for the assessment and treatment of patients with suicidal behaviors [Erratum in Am J Psychiatry. 2004;161(4):776]. Am J Psychiatry. 2003;160(suppl 11):1-60.
4. Simon RI. Suicide risk assessment forms: form over substance? J Am Acad Psychiatry Law. 2009;37(3): 290-293.
5. Resnick PJ. Recognizing that the suicidal patient views you as an ‘adversary.’ Current Psychiatry. 2002;1(1):8.
6. Dervic K, Oquendo MA, Grunebaum MF, et al. Religious affiliation and suicide attempt. Am J Psychiatry. 2004; 161(12):2303-2308.
7. Harris CE, Barraclough B. Suicide as an outcome for mental disorders. A meta-analysis. Br J Psychiatry. 1997;170:205-228.
8. Health insurance portability and accountability act of 1996. Pub L No. 104-191.
9. Simon RI, Shuman DW. Clinical manual of psychiatry and law. Arlington, VA: American Psychiatric Publishing, Inc; 2007.
10. American Psychiatric Association. Principles of medical ethics with annotations especially applicable to psychiatry. Section 4, annotation 8. Washington, DC: American Psychiatric Publishing, Inc; 2001.
11. Simon RI, Gutheil TG. Sudden improvement in high-risk suicidal patients: should it be trusted? Psych Serv. 2009; 60(3):387-389.
12. Pokorny AD. Prediction of suicide in psychiatric patients. Report of a prospective study. Arch Gen Psychiatry. 1983; 4(3):249-257.
13. Simon RI. Imminent suicide: the illusion of short-term prediction. Suicide Life Threat Behav. 2006;36(3): 296-301.
14. Simon RI, Shuman DW. Therapeutic risk management of clinical-legal dilemmas: should it be a core competency? J Am Acad Psychiatry Law. 2009;37(2):155-161.
15. Linehan MM, Goodstein JL, Nielsen SL, et al. Reasons for staying alive when you are thinking of killing yourself: the reasons for living inventory. J Consult Clin Psychol. 1983;51(2):276-286.
16. Malone KM, Oquendo MA, Hass GL, et al. Protective factors against suicidal acts in major depression: reasons for living. Am J Psychiatry. 2000;157(7):1084-1088.
17. Baldessarini RJ, Pompili M, Tondo L. Bipolar disorder. In: Simon RI, Hales RE, eds. Textbook of suicide assessment and management. Arlington, VA: American Psychiatric Publishing, Inc; 2006:159-176.
The assessment and management of suicide risk are complex and difficult tasks that raise clinical issues without clear-cut, easy answers. This case-based, multiple-choice self-assessment with accompanying commentaries is a teaching instrument that I designed to enhance a clinician’s ability to provide care for patients at risk for suicide. Part 1 of this article poses 8 of the 15 questions; the balance of questions will appear in Part 2, in the November 2014 issue of Current Psychiatry.
The questions and commentaries in this self-assessment originate in the referenced work of others and my clinical experience. Therefore, I use the preferred “best response” option—not the customary and more restrictive “correct answer” format.
How do you score?
Question 1
Mr. J, age 34, is a professional basketball player complaining of weight loss, early morning waking, and a dysphoric mood lasting for 1 month. His performance on the basketball court has declined and his wife is seeking a separation. He describes “fleeting” suicidal thoughts. He has no history of suicide attempts or depression. The patient does not abuse alcohol or drugs.
The initial assessment approach is to:
a) obtain a suicide prevention contract
b) assess suicide risk and protective factors
c) determine the cause of Mr. J’s depression
d) have Mr. J complete a suicide risk self-assessment form
e) contact his wife for additional history
The best response option is B
Suicide prevention contracts do not prevent suicide.1 Contacting the patient’s wife may be an option at a later stage of evaluation or treatment, if Mr. J grants permission. Determining the cause of his depression likely will require ongoing work up. Assessing suicide risk factors without also looking at protective factors is a common error. A comprehensive suicide risk assessment evaluation requires evaluating both risk and protective factors.2,3 Suicide risk assessment forms often omit questions about protective factors.4 Do not rely on self-assessment suicide risk forms because they are dependent on the patient’s truthfulness. Patients who are determined to commit suicide might regard the psychiatrist and other mental health professionals as the enemy.5
Question 2
Ms. P, a 56-year-old, single schoolteacher, is admitted to a psychiatric unit for severe depression and suicidal ideation without a plan. She is devoutly religious, stating, “I won’t kill myself, because I don’t want to go to hell.” Ms. P attends religious services regularly. She has a history of chronic recurrent depression with suicidal ideation and no history of suicide attempts. You suspect a diagnosis of bipolar II disorder.
In assessing religious affiliation as a protective factor against suicide, you should consider:
a) the nature of the patient’s religious conviction
b) the religion’s stated position on suicide
c) severity of the patient’s illness
d) presence of delusional religious beliefs
e) all of the above
The best response option is E
Dervic et al6 evaluated 371 depressed inpatients according to their religious or non-religious affiliation. Patients with no religious affiliation made significantly more suicide attempts, had more first-degree relatives who committed suicide, were younger, were less likely to be married or have children, and had fewer contacts with family members.
In general, religious affiliation is a protective factor against suicide but may not be a protective factor in an individual patient. Religious affiliation, similar to other presummed general protective factors, requires further scrutiny. Avoid making assumptions. For example, a depressed, devoutly religious patient may curse God for abandonment. A patient with bipolar disorder may believe that God would forgive her for committing suicide. A presumed protective factor may not be protective or might even be a risk factor, such as psychotic patients with religious delusions.
Abrahamic religions—ie, Judaism, Christianity, and Islam—prohibit suicide. Severe mental illness, however, can overcome the strongest religious prohibitions against suicide, including the fear of eternal damnation. For many psychiatric patients, religious affiliations and beliefs are protective factors against suicide, but only relatively. No protective factor against suicide, however strong, provides absolute protection against suicide. Moreover, other risk and protective factors also must be assessed comprehensively.
Question 3
Mr. W, age 18, is admitted to an inpatient psychiatric unit with severe agitation, thought disorder, disorganization, and auditory hallucinations. He is threatening to jump from a nearby building. He has no history of substance abuse.
The psychiatrist conducts a comprehensive suicide risk assessment that includes the patient’s psychiatric diagnosis as a risk factor.
Which psychiatric disorder has the highest associated suicide mortality rate?
a) schizophrenia
b) eating disorders
c) bipolar disorder
d) major depressive disorder
e) borderline personality disorder
The best response option is B
Harris and Barraclough (Table)7 calculated the standardized mortality ratio (SMR) for suicide among psychiatric disorders. SMR is calculated by dividing observed mortality by suicide by the expected mortality by suicide in the general population. Every psychiatric disorder in their study, except for mental retardation, was associated with a varying degree of suicide risk. Eating disorders had the highest SMR. The patient’s psychiatric diagnosis is a risk factor that informs the clinician’s suicide risk assessment.
Question 4
Mr. Z, a 64-year-old, recently divorced lawyer, is admitted to the psychiatric unit from the emergency room. His colleagues brought Mr. Z to the emergency room because of his suicide threats.
On the unit, Mr. Z denies suicidal ideation, plan, or intent. Agitation and suspiciousness are prominent. He refuses to authorize staff to contact his colleagues, his ex-wife, and other family members. Mr. Z demands immediate discharge and forbids contact with his outpatient psychotherapist. He is placed on 72-hour hold as a conditional voluntary admission.
The clinician should:
a) contact Mr. Z’s family, as an emergency exception to confidentiality
b) e-mail his family members with questions
c) contact the patient’s psychotherapist as permitted by the Health Insurance Portability and Accountability Act of 1996 (HIPAA)
d) try to develop a therapeutic alliance with Mr. Z
e) none of the above
The best response option is C
HIPAA permits psychiatrists and other health care providers who are treating the same patient to communicate with each other about medical treatment without obtaining permission from the patient.8 However, mental health professionals cannot share psychotherapy notes without a patient’s consent, except when legally required, such as reporting abuse or duty to warn. This is the most expeditious and productive way of obtaining essential clinical information. E-mail merely changes the mode of unauthorized communication with significant others.
Mr. Z is agitated and suspicious, and developing a therapeutic alliance would require time. It is necessary to gather information about his psychiatric condition as soon as possible. An emergency exception to maintaining confidentiality is another option.9 The definition of emergency varies among jurisdictions. Consulting with a knowledgeable attorney may be necessary, but it usually takes time. Ethically, it is permissible to breach confidentiality to protect the suicidal patient.10
Question 5
Mr. G, a 42-year-old engineer, is re-hospitalized after a failed hanging attempt. Initially, he is profoundly depressed but improves suddenly and requests discharge. The psychiatrist and clinical staff are perplexed. Is the sudden improvement real or feigned?
The treatment team should consider all of the following options except:
a) obtain records of earlier hospitalizations
b) check collateral sources of information
c) assess Mr. G’s compliance with treatment
d) obtain psychological testing to evaluate Mr. G’s honesty
e) determine whether behavioral signs of depression are present
The best response option is D
Short length of hospital stay makes it difficult to assess sudden patient improvement.11 Real improvement in a high-risk suicidal patient is a process, even when it occurs quickly. Feigned improvement is an event. Obtaining patient information from collateral sources is crucial. Sudden improvement might be caused by the patient’s resolve to complete suicide. Identifying behavioral risk factors associated with psychiatric disorders informs the clinician’s systematic suicide risk assessment of a guarded or dissimulative patient. Psychological testing will take critical time and is not a substitute for careful clinical assessment.
Question 6
In mid-winter, Ms. M, a 42-year-old homeless woman, is seen in the emergency room of a general hospital. She complains of depression and auditory hallucinations commanding her to commit suicide. Ms. M has 5 earlier admissions to the psychiatry unit for similar complaints.
The psychiatrist conducts a comprehensive suicide risk assessment. Acute and chronic risk factors for suicide are identified. Protective factors also are assessed. The psychiatrist weighs and synthesizes risk and protective factors into an overall assessment of Ms. M’s suicide risk.
The main purpose of suicide risk assessment is to:
a) predict the likelihood of suicide
b) determine imminence of suicide
c) inform patient treatment and safety management
d) identify malingered suicidal ideation
e) provide a legal defense against a malpractice claim
The best response option is C
Suicide cannot be predicted.12 The term imminent suicide is a veiled attempt to predict when a patient will attempt suicide.13 The process of a comprehensive or systematic suicide risk assessment encompasses identification, analysis, and synthesis of risk and protective factors that inform the treatment and safety management of the patient.3 The overall suicide assessment is a clinical judgment call that determines risk along a continuum of low to high. In Ms. M’s case, comprehensive suicide risk assessment will assist the clinician in determining the patient’s overall suicide risk and make an appropriate disposition. Without a systematic suicide risk assessment methodology, the clinician is at the mercy of the pejoratively labeled “frequent flyer” who is looking for sustenance and lodging. The frustrated clinician is left with little choice but to admit the patient.
Although not the main purpose, systematic suicide risk assessment can help provide a sound legal defense if a suicide malpractice claim is filed against the clinician alleging negligent assessment.14
Question 7
A psychiatrist is treating Mr. S, a 36-year-old computer analyst, with once-a-week psychotherapy and medication management for panic and depressive symptoms that emerged abruptly after the break-up of a romantic relationship. Mr. S is using alcohol to sleep. He reports occasional suicidal ideation but no plan. He finds the idea of suicide to be morally repugnant. A therapeutic alliance develops.
The psychiatrist is concerned about Mr. S’s suicide risk and the need for hospitalization. The psychiatrist performs a systematic suicide risk assessment that includes identification of individual and evidence-based protective factors. For example, Mr. S continued to pursue his interests and to participate in civil causes. The overall suicide risk is determined by the assessment of individual and evidence-based protective factors.
All of the following options are evidence-based protective factors except:
a) therapeutic alliance
b) survival and coping beliefs
c) responsibility to family
d) fear of suicide
e) moral objections to suicide
The best response option is A
Clinical consensus holds that the therapeutic alliance is an important protective factor against suicide. However, no evidence-based research supports or refutes this widely held belief among clinicians.
Linehan et al15 developed the Reasons for Living Inventory, a self-report instrument that identifies 6 subscales:
• survival and coping beliefs
• responsibility to family
• child-related concerns
• fear of suicide
• fear of social disapproval
• moral objections to suicide.
Survival and coping beliefs, responsibility to family, and child-related concerns were useful in differentiating between suicidal and non-suicidal individuals. Malone et al16 administered the Reasons for Living Inventory to 84 inpatients with major depression; 45 had attempted suicide. Depressed patients who had not attempted suicide demonstrated more sense of responsibility toward family, more fear of social disapproval, more moral objections to suicide, greater survival and coping skills, and greater fear of suicide than patients who attempted suicide. The authors recommended adding the Reasons for Living Inventory to the assessment of patients at risk for suicide.
Question 8
A 38-year-old mother of a newborn child is admitted to the psychiatric unit after expressing suicidal thoughts to her husband. She has been hospitalized previously after a hypomanic episode and severe depression; she has no history of suicide attempts. A psychiatrist diagnoses bipolar II disorder (recurrent major episodes with hypomanic episodes). The patient’s maternal aunt has bipolar disorder. Her paternal grandfather committed suicide.
The psychiatrist conducts a systematic suicide risk assessment and determines the patient is at high risk of suicide. He considers a suicide-risk reduction drug.
Which one of the following drugs has been shown to reduce suicide and suicide attempts in bipolar II patients?
a) clozapine
b) clonazepam
c) lorazepam
d) lithium
e) quetiapine
The best response option is D
Prospective, randomized and controlled trials consistently have found lower rates of completed suicides and suicide attempts during lithium maintenance treatments for patients with bipolar disorder and other major affective disorders.17
Bottom Line
Suicide risk assessment and management are challenging for even experienced clinicians. Suicide risk assessment guides appropriate treatment and management for patients at risk for suicide. This self-assessment helps mental health professionals identify potential gaps in their knowledge and reinforce best practices.
Related Resources
• Simon RI. Passive suicidal ideation: Still a high-risk clinical scenario. Current Psychiatry. 2014;13(3):13-15.
• Simon RI. Suicide rehearsals: A high-risk psychiatric emergency. Current Psychiatry. 2012;11(7):28-32.
• Bongar B, Sullivan GR. The suicidal patient: Clinical and legal standards of care. Washington, DC: American Psychological Association; 2013.
Drug Brand Names
Clonazepam • Klonopin Lorazepam • Ativan
Clozapine • Clozaril Quetiapine • Seroquel
Lithium • Eskalith, Lithobid
Disclosure
Dr. Simon reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Adapted with permission from: Simon RI. Preventing patient suicide: clinical assessment and management, Arlington VA: American Psychiatric Publishing; 2011.
Editor’s note: Part 2 of this self-assessment on suicide assessment and management in the November 2014 issue of Current Psychiatry poses 7 additional questions.
The assessment and management of suicide risk are complex and difficult tasks that raise clinical issues without clear-cut, easy answers. This case-based, multiple-choice self-assessment with accompanying commentaries is a teaching instrument that I designed to enhance a clinician’s ability to provide care for patients at risk for suicide. Part 1 of this article poses 8 of the 15 questions; the balance of questions will appear in Part 2, in the November 2014 issue of Current Psychiatry.
The questions and commentaries in this self-assessment originate in the referenced work of others and my clinical experience. Therefore, I use the preferred “best response” option—not the customary and more restrictive “correct answer” format.
How do you score?
Question 1
Mr. J, age 34, is a professional basketball player complaining of weight loss, early morning waking, and a dysphoric mood lasting for 1 month. His performance on the basketball court has declined and his wife is seeking a separation. He describes “fleeting” suicidal thoughts. He has no history of suicide attempts or depression. The patient does not abuse alcohol or drugs.
The initial assessment approach is to:
a) obtain a suicide prevention contract
b) assess suicide risk and protective factors
c) determine the cause of Mr. J’s depression
d) have Mr. J complete a suicide risk self-assessment form
e) contact his wife for additional history
The best response option is B
Suicide prevention contracts do not prevent suicide.1 Contacting the patient’s wife may be an option at a later stage of evaluation or treatment, if Mr. J grants permission. Determining the cause of his depression likely will require ongoing work up. Assessing suicide risk factors without also looking at protective factors is a common error. A comprehensive suicide risk assessment evaluation requires evaluating both risk and protective factors.2,3 Suicide risk assessment forms often omit questions about protective factors.4 Do not rely on self-assessment suicide risk forms because they are dependent on the patient’s truthfulness. Patients who are determined to commit suicide might regard the psychiatrist and other mental health professionals as the enemy.5
Question 2
Ms. P, a 56-year-old, single schoolteacher, is admitted to a psychiatric unit for severe depression and suicidal ideation without a plan. She is devoutly religious, stating, “I won’t kill myself, because I don’t want to go to hell.” Ms. P attends religious services regularly. She has a history of chronic recurrent depression with suicidal ideation and no history of suicide attempts. You suspect a diagnosis of bipolar II disorder.
In assessing religious affiliation as a protective factor against suicide, you should consider:
a) the nature of the patient’s religious conviction
b) the religion’s stated position on suicide
c) severity of the patient’s illness
d) presence of delusional religious beliefs
e) all of the above
The best response option is E
Dervic et al6 evaluated 371 depressed inpatients according to their religious or non-religious affiliation. Patients with no religious affiliation made significantly more suicide attempts, had more first-degree relatives who committed suicide, were younger, were less likely to be married or have children, and had fewer contacts with family members.
In general, religious affiliation is a protective factor against suicide but may not be a protective factor in an individual patient. Religious affiliation, similar to other presummed general protective factors, requires further scrutiny. Avoid making assumptions. For example, a depressed, devoutly religious patient may curse God for abandonment. A patient with bipolar disorder may believe that God would forgive her for committing suicide. A presumed protective factor may not be protective or might even be a risk factor, such as psychotic patients with religious delusions.
Abrahamic religions—ie, Judaism, Christianity, and Islam—prohibit suicide. Severe mental illness, however, can overcome the strongest religious prohibitions against suicide, including the fear of eternal damnation. For many psychiatric patients, religious affiliations and beliefs are protective factors against suicide, but only relatively. No protective factor against suicide, however strong, provides absolute protection against suicide. Moreover, other risk and protective factors also must be assessed comprehensively.
Question 3
Mr. W, age 18, is admitted to an inpatient psychiatric unit with severe agitation, thought disorder, disorganization, and auditory hallucinations. He is threatening to jump from a nearby building. He has no history of substance abuse.
The psychiatrist conducts a comprehensive suicide risk assessment that includes the patient’s psychiatric diagnosis as a risk factor.
Which psychiatric disorder has the highest associated suicide mortality rate?
a) schizophrenia
b) eating disorders
c) bipolar disorder
d) major depressive disorder
e) borderline personality disorder
The best response option is B
Harris and Barraclough (Table)7 calculated the standardized mortality ratio (SMR) for suicide among psychiatric disorders. SMR is calculated by dividing observed mortality by suicide by the expected mortality by suicide in the general population. Every psychiatric disorder in their study, except for mental retardation, was associated with a varying degree of suicide risk. Eating disorders had the highest SMR. The patient’s psychiatric diagnosis is a risk factor that informs the clinician’s suicide risk assessment.
Question 4
Mr. Z, a 64-year-old, recently divorced lawyer, is admitted to the psychiatric unit from the emergency room. His colleagues brought Mr. Z to the emergency room because of his suicide threats.
On the unit, Mr. Z denies suicidal ideation, plan, or intent. Agitation and suspiciousness are prominent. He refuses to authorize staff to contact his colleagues, his ex-wife, and other family members. Mr. Z demands immediate discharge and forbids contact with his outpatient psychotherapist. He is placed on 72-hour hold as a conditional voluntary admission.
The clinician should:
a) contact Mr. Z’s family, as an emergency exception to confidentiality
b) e-mail his family members with questions
c) contact the patient’s psychotherapist as permitted by the Health Insurance Portability and Accountability Act of 1996 (HIPAA)
d) try to develop a therapeutic alliance with Mr. Z
e) none of the above
The best response option is C
HIPAA permits psychiatrists and other health care providers who are treating the same patient to communicate with each other about medical treatment without obtaining permission from the patient.8 However, mental health professionals cannot share psychotherapy notes without a patient’s consent, except when legally required, such as reporting abuse or duty to warn. This is the most expeditious and productive way of obtaining essential clinical information. E-mail merely changes the mode of unauthorized communication with significant others.
Mr. Z is agitated and suspicious, and developing a therapeutic alliance would require time. It is necessary to gather information about his psychiatric condition as soon as possible. An emergency exception to maintaining confidentiality is another option.9 The definition of emergency varies among jurisdictions. Consulting with a knowledgeable attorney may be necessary, but it usually takes time. Ethically, it is permissible to breach confidentiality to protect the suicidal patient.10
Question 5
Mr. G, a 42-year-old engineer, is re-hospitalized after a failed hanging attempt. Initially, he is profoundly depressed but improves suddenly and requests discharge. The psychiatrist and clinical staff are perplexed. Is the sudden improvement real or feigned?
The treatment team should consider all of the following options except:
a) obtain records of earlier hospitalizations
b) check collateral sources of information
c) assess Mr. G’s compliance with treatment
d) obtain psychological testing to evaluate Mr. G’s honesty
e) determine whether behavioral signs of depression are present
The best response option is D
Short length of hospital stay makes it difficult to assess sudden patient improvement.11 Real improvement in a high-risk suicidal patient is a process, even when it occurs quickly. Feigned improvement is an event. Obtaining patient information from collateral sources is crucial. Sudden improvement might be caused by the patient’s resolve to complete suicide. Identifying behavioral risk factors associated with psychiatric disorders informs the clinician’s systematic suicide risk assessment of a guarded or dissimulative patient. Psychological testing will take critical time and is not a substitute for careful clinical assessment.
Question 6
In mid-winter, Ms. M, a 42-year-old homeless woman, is seen in the emergency room of a general hospital. She complains of depression and auditory hallucinations commanding her to commit suicide. Ms. M has 5 earlier admissions to the psychiatry unit for similar complaints.
The psychiatrist conducts a comprehensive suicide risk assessment. Acute and chronic risk factors for suicide are identified. Protective factors also are assessed. The psychiatrist weighs and synthesizes risk and protective factors into an overall assessment of Ms. M’s suicide risk.
The main purpose of suicide risk assessment is to:
a) predict the likelihood of suicide
b) determine imminence of suicide
c) inform patient treatment and safety management
d) identify malingered suicidal ideation
e) provide a legal defense against a malpractice claim
The best response option is C
Suicide cannot be predicted.12 The term imminent suicide is a veiled attempt to predict when a patient will attempt suicide.13 The process of a comprehensive or systematic suicide risk assessment encompasses identification, analysis, and synthesis of risk and protective factors that inform the treatment and safety management of the patient.3 The overall suicide assessment is a clinical judgment call that determines risk along a continuum of low to high. In Ms. M’s case, comprehensive suicide risk assessment will assist the clinician in determining the patient’s overall suicide risk and make an appropriate disposition. Without a systematic suicide risk assessment methodology, the clinician is at the mercy of the pejoratively labeled “frequent flyer” who is looking for sustenance and lodging. The frustrated clinician is left with little choice but to admit the patient.
Although not the main purpose, systematic suicide risk assessment can help provide a sound legal defense if a suicide malpractice claim is filed against the clinician alleging negligent assessment.14
Question 7
A psychiatrist is treating Mr. S, a 36-year-old computer analyst, with once-a-week psychotherapy and medication management for panic and depressive symptoms that emerged abruptly after the break-up of a romantic relationship. Mr. S is using alcohol to sleep. He reports occasional suicidal ideation but no plan. He finds the idea of suicide to be morally repugnant. A therapeutic alliance develops.
The psychiatrist is concerned about Mr. S’s suicide risk and the need for hospitalization. The psychiatrist performs a systematic suicide risk assessment that includes identification of individual and evidence-based protective factors. For example, Mr. S continued to pursue his interests and to participate in civil causes. The overall suicide risk is determined by the assessment of individual and evidence-based protective factors.
All of the following options are evidence-based protective factors except:
a) therapeutic alliance
b) survival and coping beliefs
c) responsibility to family
d) fear of suicide
e) moral objections to suicide
The best response option is A
Clinical consensus holds that the therapeutic alliance is an important protective factor against suicide. However, no evidence-based research supports or refutes this widely held belief among clinicians.
Linehan et al15 developed the Reasons for Living Inventory, a self-report instrument that identifies 6 subscales:
• survival and coping beliefs
• responsibility to family
• child-related concerns
• fear of suicide
• fear of social disapproval
• moral objections to suicide.
Survival and coping beliefs, responsibility to family, and child-related concerns were useful in differentiating between suicidal and non-suicidal individuals. Malone et al16 administered the Reasons for Living Inventory to 84 inpatients with major depression; 45 had attempted suicide. Depressed patients who had not attempted suicide demonstrated more sense of responsibility toward family, more fear of social disapproval, more moral objections to suicide, greater survival and coping skills, and greater fear of suicide than patients who attempted suicide. The authors recommended adding the Reasons for Living Inventory to the assessment of patients at risk for suicide.
Question 8
A 38-year-old mother of a newborn child is admitted to the psychiatric unit after expressing suicidal thoughts to her husband. She has been hospitalized previously after a hypomanic episode and severe depression; she has no history of suicide attempts. A psychiatrist diagnoses bipolar II disorder (recurrent major episodes with hypomanic episodes). The patient’s maternal aunt has bipolar disorder. Her paternal grandfather committed suicide.
The psychiatrist conducts a systematic suicide risk assessment and determines the patient is at high risk of suicide. He considers a suicide-risk reduction drug.
Which one of the following drugs has been shown to reduce suicide and suicide attempts in bipolar II patients?
a) clozapine
b) clonazepam
c) lorazepam
d) lithium
e) quetiapine
The best response option is D
Prospective, randomized and controlled trials consistently have found lower rates of completed suicides and suicide attempts during lithium maintenance treatments for patients with bipolar disorder and other major affective disorders.17
Bottom Line
Suicide risk assessment and management are challenging for even experienced clinicians. Suicide risk assessment guides appropriate treatment and management for patients at risk for suicide. This self-assessment helps mental health professionals identify potential gaps in their knowledge and reinforce best practices.
Related Resources
• Simon RI. Passive suicidal ideation: Still a high-risk clinical scenario. Current Psychiatry. 2014;13(3):13-15.
• Simon RI. Suicide rehearsals: A high-risk psychiatric emergency. Current Psychiatry. 2012;11(7):28-32.
• Bongar B, Sullivan GR. The suicidal patient: Clinical and legal standards of care. Washington, DC: American Psychological Association; 2013.
Drug Brand Names
Clonazepam • Klonopin Lorazepam • Ativan
Clozapine • Clozaril Quetiapine • Seroquel
Lithium • Eskalith, Lithobid
Disclosure
Dr. Simon reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Adapted with permission from: Simon RI. Preventing patient suicide: clinical assessment and management, Arlington VA: American Psychiatric Publishing; 2011.
Editor’s note: Part 2 of this self-assessment on suicide assessment and management in the November 2014 issue of Current Psychiatry poses 7 additional questions.
1. Stanford EJ, Goetz RR, Bloom JD. The No Harm Contract in the emergency assessment of suicide risk. J Clin Psychiatry. 1994;55(8):344-348.
2. Simon RI, Hales RE, eds. Textbook of suicide assessment and management. 2nd ed. Arlington, VA: American Psychiatric Publishing, Inc.; 2012.
3. Practice guidelines for the assessment and treatment of patients with suicidal behaviors [Erratum in Am J Psychiatry. 2004;161(4):776]. Am J Psychiatry. 2003;160(suppl 11):1-60.
4. Simon RI. Suicide risk assessment forms: form over substance? J Am Acad Psychiatry Law. 2009;37(3): 290-293.
5. Resnick PJ. Recognizing that the suicidal patient views you as an ‘adversary.’ Current Psychiatry. 2002;1(1):8.
6. Dervic K, Oquendo MA, Grunebaum MF, et al. Religious affiliation and suicide attempt. Am J Psychiatry. 2004; 161(12):2303-2308.
7. Harris CE, Barraclough B. Suicide as an outcome for mental disorders. A meta-analysis. Br J Psychiatry. 1997;170:205-228.
8. Health insurance portability and accountability act of 1996. Pub L No. 104-191.
9. Simon RI, Shuman DW. Clinical manual of psychiatry and law. Arlington, VA: American Psychiatric Publishing, Inc; 2007.
10. American Psychiatric Association. Principles of medical ethics with annotations especially applicable to psychiatry. Section 4, annotation 8. Washington, DC: American Psychiatric Publishing, Inc; 2001.
11. Simon RI, Gutheil TG. Sudden improvement in high-risk suicidal patients: should it be trusted? Psych Serv. 2009; 60(3):387-389.
12. Pokorny AD. Prediction of suicide in psychiatric patients. Report of a prospective study. Arch Gen Psychiatry. 1983; 4(3):249-257.
13. Simon RI. Imminent suicide: the illusion of short-term prediction. Suicide Life Threat Behav. 2006;36(3): 296-301.
14. Simon RI, Shuman DW. Therapeutic risk management of clinical-legal dilemmas: should it be a core competency? J Am Acad Psychiatry Law. 2009;37(2):155-161.
15. Linehan MM, Goodstein JL, Nielsen SL, et al. Reasons for staying alive when you are thinking of killing yourself: the reasons for living inventory. J Consult Clin Psychol. 1983;51(2):276-286.
16. Malone KM, Oquendo MA, Hass GL, et al. Protective factors against suicidal acts in major depression: reasons for living. Am J Psychiatry. 2000;157(7):1084-1088.
17. Baldessarini RJ, Pompili M, Tondo L. Bipolar disorder. In: Simon RI, Hales RE, eds. Textbook of suicide assessment and management. Arlington, VA: American Psychiatric Publishing, Inc; 2006:159-176.
1. Stanford EJ, Goetz RR, Bloom JD. The No Harm Contract in the emergency assessment of suicide risk. J Clin Psychiatry. 1994;55(8):344-348.
2. Simon RI, Hales RE, eds. Textbook of suicide assessment and management. 2nd ed. Arlington, VA: American Psychiatric Publishing, Inc.; 2012.
3. Practice guidelines for the assessment and treatment of patients with suicidal behaviors [Erratum in Am J Psychiatry. 2004;161(4):776]. Am J Psychiatry. 2003;160(suppl 11):1-60.
4. Simon RI. Suicide risk assessment forms: form over substance? J Am Acad Psychiatry Law. 2009;37(3): 290-293.
5. Resnick PJ. Recognizing that the suicidal patient views you as an ‘adversary.’ Current Psychiatry. 2002;1(1):8.
6. Dervic K, Oquendo MA, Grunebaum MF, et al. Religious affiliation and suicide attempt. Am J Psychiatry. 2004; 161(12):2303-2308.
7. Harris CE, Barraclough B. Suicide as an outcome for mental disorders. A meta-analysis. Br J Psychiatry. 1997;170:205-228.
8. Health insurance portability and accountability act of 1996. Pub L No. 104-191.
9. Simon RI, Shuman DW. Clinical manual of psychiatry and law. Arlington, VA: American Psychiatric Publishing, Inc; 2007.
10. American Psychiatric Association. Principles of medical ethics with annotations especially applicable to psychiatry. Section 4, annotation 8. Washington, DC: American Psychiatric Publishing, Inc; 2001.
11. Simon RI, Gutheil TG. Sudden improvement in high-risk suicidal patients: should it be trusted? Psych Serv. 2009; 60(3):387-389.
12. Pokorny AD. Prediction of suicide in psychiatric patients. Report of a prospective study. Arch Gen Psychiatry. 1983; 4(3):249-257.
13. Simon RI. Imminent suicide: the illusion of short-term prediction. Suicide Life Threat Behav. 2006;36(3): 296-301.
14. Simon RI, Shuman DW. Therapeutic risk management of clinical-legal dilemmas: should it be a core competency? J Am Acad Psychiatry Law. 2009;37(2):155-161.
15. Linehan MM, Goodstein JL, Nielsen SL, et al. Reasons for staying alive when you are thinking of killing yourself: the reasons for living inventory. J Consult Clin Psychol. 1983;51(2):276-286.
16. Malone KM, Oquendo MA, Hass GL, et al. Protective factors against suicidal acts in major depression: reasons for living. Am J Psychiatry. 2000;157(7):1084-1088.
17. Baldessarini RJ, Pompili M, Tondo L. Bipolar disorder. In: Simon RI, Hales RE, eds. Textbook of suicide assessment and management. Arlington, VA: American Psychiatric Publishing, Inc; 2006:159-176.
Treating methamphetamine abuse disorder: Experience from research and practice
Methamphetamine and other amphetamine-type stimulants are the world’s second most widely used group of illicit substances (after Cannabis), with prevalence of abuse varying by region and by locales within nations. As prescription use of stimulants has grown dramatically in recent years, so has abuse of these substances.
Given the widespread and growing misuse of amphetamine-type stimulants (Box,1-3), clinicians are faced with the need to learn how to recognize and manage methamphetamine abuse. Both prescribed and non-prescribed uses of stimulants present complex challenges; in this article, we examine effects, manifestations, and current evidence-based behavioral and medical treatments of methamphetamine misuse and abuse, and look ahead to investigational therapies that hold promise for improving the limited existing approaches to management.
Effects and manifestations of methamphetamine use
Different routes of administration produce different consequences, in terms of medical comorbidity and propensity to induce addiction. Smoked or injected, methamphetamine enters the brain in seconds; snorted or taken by mouth, the drug produces its effects in several minutes and a half hour, respectively.
Rapid uptake and effects of methamphetamine result from its ability to cross the blood−brain barrier. Its primary effects are caused by inhibition of dopamine storage and release of intracellular dopamine.
Methamphetamine stimulates the CNS and the cardiovascular system through release of dopamine and norepinephrine, which increases blood pressure, body temperature, and heart rate, and, occasionally, induces arrhythmia that can contribute to heart attack and stroke. Users experience euphoria, hypervigilance, suppressed appetite, and increased libido.
Binge use is common to sustain euphoria and other reinforcing effects, which subside with rapidly developing tolerance. After days of repeated dosing, elevated methamphetamine blood levels can lead to mood disturbances, repetitive motor activities, and psychotic symptoms such as hallucinations, delusions, and paranoia. Acute psychosis can bring on violence and other injurious behaviors that involve law enforcement and emergency medical services.
When methamphetamine is used over months or years, health consequences include anorexia, tremor, so-called meth mouth (broken teeth, infections, cavities, burns), insomnia, panic attacks, confusion, depression, irritability, and impaired memory and other cognitive processes.
Treating methamphetamine intoxication and withdrawal
At initial clinical contact with a person who abuses methamphetamine, practitioners may face several acute consequences requiring attention. Prominent among presenting conditions, especially during acute intoxication, are agitation, anxiety, and psychotic symptoms, which may improve by providing the patient with calming reassurance in a quiet space. In more severe cases, a benzodiazepine, antipsychotic, or both might be indicated4,5 (Table 1).
Methamphetamine withdrawal is characterized by anxiety, depression, and insomnia. These symptoms generally resolve in a matter of days after the start of withdrawal without pharmacotherapy. In some cases, depression or psychosis becomes chronic, as a result of methamphetamine use itself6 or as an emergent concomitant psychiatric condition.
A sedative-hypnotic medication or an anxiolytic can be used as necessary to ameliorate insomnia or anxiety, respectively. Prolonged depression can be treated with an antidepressant. An antipsychotic might be indicated for long-term management of patients who have persistent psychosis.
Therapy for methamphetamine abuse
Treatment of methamphetamine abuse— with the goal of stopping drug use—is a complicated matter on 2 counts:
• No medications are FDA-approved for treating methamphetamine addiction.
• There are no accepted substitution medications (ie, stimulants that can be used in place of methamphetamine, as is available for opioid addiction).
Pharmacotherapeutic possibilities. The rationale for considering replacement pharmacotherapy is that psychostimulants can counter the cravings, dysphoria, and fatigue produced by methamphetamine withdrawal and can alleviate methamphetamine-related cognitive impairment. Although dextroamphetamine and other psychostimulants have been evaluated in small trials as replacement medication, most countries are reluctant to consider their use, because of the potential for abuse and accompanying liability.
After decades of medication research, several drugs have shown promise for reducing methamphetamine abuse, although results have not been robust (Table 2):
• Bupropion has shown benefit in reducing methamphetamine use among users with less severe addiction.7,8
• Methylphenidate, a psychostimulant FDA-approved for attention-deficit/hyperactivity disorder, was found to reduce methamphetamine use compared with placebo in a European sample of amphetamine injectors who had attained abstinence in a residential program.9 Those results were not replicated in a recent study by Miles et al, however.10 A study with a more clinically realistic approach (ie, not requiring daily clinic attendance, as in the Miles trial) vs placebo for methamphetamine abuse was recently published, with promising results that require confirmation in further study.11
• Mirtazapine, an antidepressant, has demonstrated efficacy in reducing methamphetamine use compared with placebo.12
• Modafinil, another medication with stimulant properties, reduced methamphetamine use in a subgroup analysis of heavy users, compared with placebo.13
• Dextroamphetamine, 60 mg/d, showed no difference in reducing methamphetamine compared with placebo, but did diminish cravings and withdrawal symptoms.14
A trial of the phosphodiesterase inhibitor ibudilast (not available in the United States) for methamphetamine abuse is underway. Ibudilast has anti-inflammatory activity in the peripheral immune system and the central nervous system, including modulating the activity of glial cells.15
Many medications have yielded negligible results in studies: selegiline, baclofen, sertraline, topiramate, gabapentin, rivastigmine, risperidone, and ondansetron.16 Recent evaluation of disulfiram, vigabatrin, and lobeline also has yielded inconsistent findings.17
No drug has proved effective for preventing relapse; research continues, focusing on several types of compounds that target various mechanisms: the dopamine system, the opioid system (by way of the γ-aminobutyric acid inhibitory system), and cortico-limbic reward circuitry.
Once-monthly injectable naltrexone has potential for ameliorating craving and relapse by modulating the opioid receptor system. However, the drug has not been adequately explored in generalizable settings of methamphetamine users.
Trials of oral naltrexone in Sweden have shown encouraging results, including reduced subjective effects and amphetamine use in open-label trials18,19; results were replicated in a subsequent placebo-controlled trial.20 In an unpublished study, however, no differences in amphetamine use were found among users randomized to depot naltrexone or placebo.21
Depot naltrexone with assured dosing might have a role in treating methamphetamine abuse, however; a combination of depot naltrexone and oral bupropion is being examined in a National Institute on Drug Abuse Clinical Trials Networks study that commenced in 2013. Pairing medications that have different mechanistic targets might work toward promoting cessation of methamphetamine abuse and reducing relapse once patients are abstinent.
In an early phase of research, but showing promise based on their ability to target different systems, are:
• N-acetylcysteine, modulator of the glutamate system
• D3 antagonists and partial agonists22
• varenicline.23
Potential “vaccines” against methamphetamine are in preclinical development, including use of a protein carrier or other immune-stimulating molecule to create antibodies that bind methamphetamine in the bloodstream and block its psychoactive effects.24,25
Sigma receptor effects are being studied in rodents as potential targets to mitigate effects of methamphetamine. The ligand AZ66, a sigma receptor antagonist, has demonstrated efficacy in reducing methamphetamine-induced cognitive impairment—suggesting that the sigma receptor has a potential role in ameliorating methamphetamine-related neurotoxicity.26
Psychosocial and behavioral interventions. Among the non-drug treatments that have demonstrated efficacy for treating methamphetamine abuse, cognitive-behavioral therapy (CBT) and contingency management (CM) have been most widely studied and applied in treatment settings.
CBT involves individual or group counseling that focuses on relapse prevention skills, including identification of relapse triggers, strategies to diminish cravings, and engagement in alternative non-drug activities27,28 (Table 3).
CM, which is based on positive reinforcement, offers tangible reinforcers, or rewards, for behaviors (eg, clinic attendance, providing a drug-free urine sample) according to guidelines set by the practitioner. CM-based interventions are the most reliably documented approaches for treating methamphetamine abuse,29,30 but their utility might prove to be most efficient in combination with medication— once suitable pharmacotherapeutic options emerge.
Although CBT and CM remain accepted standard treatments for methamphetamine abuse, outcomes are suboptimal.27 Both interventions have a high rate of dropout during the first month of treatment and a >50% relapse rate 6 to 19 months after treatment ends.31-33
As with treatment of other substance use disorders, patients who abuse methamphetamine can benefit from residential treatment in a drug-free setting for ≥30 days.34 In the residential approach, removing access to drugs, drug cues, and drug-using acquaintances combined with group and individual counseling reaches an inevitable end: discharge into the community. Then the patient’s battle to avoid relapse begins.
Because cognitive impairment is common among patients who abuse methamphetamine, even after they stop using,35 researchers have examined the potential for increasing participation in psychosocial interventions such as CBT by using medications that might have potential to increase cognitive function, such as modafinil.36 Increased attention and concentration afforded by medication could enhance efficacy of CBT. Results of trials and new drug development have been mixed37; no clear candidate for preventing relapse through any of the putative mechanisms of action has emerged.
Relapse is a problematic target for treatment
Ending methamphetamine abuse and sustaining abstinence from stimulants require a change in the cognitive associations that have been laid down in a drug user’s memory. Relapse occurs because of recalled memories that can be cued, or triggered, by internal or external stimuli. Eliminating drug memories, perhaps assisted by medications such as d-cycloserine (an antagonist of the N-methyl-d-aspartate receptor), could be useful for suppressing the inclination to relapse.
Last, alternative, non-drug forms of cognitive amendment have shown efficacy in preventing relapse: for example, incorporating mindfulness meditation, which has shown promise in managing craving for methamphetamine and decreasing reactivity to environmental cues for drug use.38
Bottom Line
Practitioners who work in emergency, inpatient, and outpatient settings will be called on more and more to treat acute stimulant intoxication and withdrawal, stimulant-induced psychosis, and methamphetamine abuse. Few evidence-based treatments and no FDA-approved medications are available to treat this addiction; many drugs and a few psychotherapeutic techniques have shown promise. Ongoing research promises to deliver medical and behavioral interventions to help patients quit using methamphetamine.
Related Resources
• Karch SB, Drummer O. Karch’s pathology of drug abuse, fifth ed. Boca Raton, FL: CRC Press/Taylor & David; 2013.
• Roll J, Rawson RA, Ling W, eds. Methamphetamine addiction: from basic science to treatment. New York, NY: Guilford Press; 2009.
• Sheff D. Beautiful boy: a father’s journey through his son’s addiction. New York, NY: Houghton Mifflin Harcourt Publishing Company; 2008.
• Sheff N. Tweak: growing up on methamphetamines. New York, NY: Antheneum Books for Young Readers; 2007.
• National Institute on Drug Abuse. Drugs of abuse. www. drugabuse.gov/drugs-abuse/methamphetamine.
Drug Brand Names
Baclofen • Lioresal Naltrexone (depot) • Vivitrol
Bupropion • Wellbutrin Naltrexone (oral) • ReVia
D-cycloserine • Seromycin Ondansetron • Zofran
Dexreoamphetaime • Adderall Risperidone • Risperadal
Disulfiram • Antabuse Rivastigimine • Exelon
Gabapentin • Neurontin Selegiline• EMSAM
Methylphenidate • Ritalin Sertraline • Zoloft
Mirtazapine • Remeron Topiramate • Topamax
Modafinil • Provigil Varenicline • Chantix
N-acetylcysteine • Mucomyst Vigabatrin • Sabril
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. UNODC. World Drug Report 2012 (United Nations publication, Sales No. E.12.XI.1). http://www.unodc. org/documents/data-and-analysis/WDR2012/ WDR_2012_web_small.pdf. Published 2012. Accessed August 4, 2014.
2. UNODC. World Drug Report 2010 (United Nations publication, Sales No. E.10.XI.13). http://www.unodc. org/documents/wdr/WDR_2010/World_Drug_ Report_2010_lo-res.pdf. Published 2010. Accessed August 4, 2014.
3. Rawson RA, Gonzales R, Brecht M, et al. Evaluation of the California Outcomes Measurement System (CalOMS): Final Report 2008. http://www.uclaisap.org/assets/documents/ California-ADP-DHCS-Evals/2007-2008_CalOMS%20 Report.pdf. Published 2008. Accessed August 4, 2014.
4. Shoptaw SJ, Kao U, Ling W. Treatment for amphetamine psychosis. Cochrane Database Syst Rev. 2009;(1):CD003026.
5. Leelahanaj T, Kongsakon R, Netrakom P. A 4-week, double-blind comparison of olanzapine with haloperidol in the treatment of amphetamine psychosis. J Med Assoc Thai. 2005;88(suppl 3):S43-S52.
6. McKetin R, McLaren J, Lubman D, et al. The prevalence of psychotic symptoms among methamphetamine users. Addiction. 2006;101(10):1473-1478.
7. Elkashef AM, Rawson RA, Anderson AL, et al. Bupropion for the treatment of methamphetamine dependence. Neuropsychopharmacology. 2008;33(5):1162-1170.
8. McCann DJ, Li SH. A novel, nonbinary evaluation of success and failure reveals bupropion efficacy versus methamphetamine dependence: reanalysis of a multisite trial. CNS Neurosci Ther. 2012;18(5):414-418.
9. Tiihonen J, Kuoppasalmi K, Föhr J, et al. A comparison of aripiprazole, methylphenidate, and placebo for amphetamine dependence. Am J Psychiatry. 2007;164(1): 160-162.
10. Miles SW, Sheridan J, Russell B, et al. Extended-release methylphenidate for treatment of amphetamine/ methamphetamine dependence: a randomized, double-blind, placebo-controlled trial. Addiction. 2013;108(7): 1279-1286.
11. Ling W, Chang L, Hillhouse M, et al. Sustained-release methylphenidate in a randomized trial of treatment of methamphetamine use disorder. Addiction. 2014;109(9): 1489-1500.
12. Colfax GN, Santos GM, Das M, et al. Mirtazapine to reduce methamphetamine use: a randomized controlled trial. Arch Gen Psychiatry. 2011;68(11):1168-1175.
13. Heinzerling KG, Swanson AN, Kim S, et al. Randomized, double-blind, placebo-controlled trial of modafinil for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2010;109(1-3):20-29.
14. Galloway GP, Buscemi R, Coyle JR, et al. A randomized, placebo-controlled trial of sustained-release dextro-amphetamine for treatment of methamphetamine addiction. Clin Pharmacol Ther. 2011;89(2):276-282.
15. Snider SE, Hendrick ES, Beardsley PM. Glial cell modulators attenuate methamphetamine self-administration in the rat. Eur J Pharmacol. 2013;701(1-3):124-130.
16. Ling W, Rawson R, Shoptaw S. Management of methamphetamine abuse and dependence. Curr Psychiatry Rep. 2006;8(5):345-354.
17. Brackins T, Brahm NC, Kissack JC. Treatments for methamphetamine abuse: a literature review for the clinician. J Pharm Pract. 2011;24(6):541-550.
18. Jayaram-Lindström N, Wennberg P, Beck O, et al. An open clinical trial of naltrexone for amphetamine dependence: compliance and tolerability. Nord J Psychiatry. 2005;59(3):167-171.
19. Jayaram-Lindström N, Konstenius M, Eksborg S, et al. Naltrexone attenuates the subjective effects of amphetamine in patients with amphetamine dependence. Neuropsychopharmacology. 2007;33(8):1856-1863.
20. Jayaram-Lindström N, Hammarberg A, Beck O, et al. Naltrexone for the treatment of amphetamine dependence: a randomized, placebo-controlled trial. Am J Psychiatry. 2008;165(11):1442-1448.
21. Woody GE, Tyrfingsoon P. Symposium XI: Emerging data on efficacy and clinical applications of extended-release naltrexone formulations. 75th Annual Meeting, College on Problems of Drug Dependence. June 19, 2013; San Diego, CA.
22. Newman AH, Blaylock BL, Nader MA, et al. Medication discovery for addiction: translating the dopamine D3 receptor hypothesis. Biochem Pharmacol. 2012;84(7):882-890.
23. Verrico CD, Mahoney JJ 3rd, Thompson-Lake DG, et al. Safety and efficacy of varenicline to reduce positive subjective effects produced by methamphetamine in methamphetamine-dependent volunteers. Int J Neuropsychopharmacol. 2014;17(2):223-233.
24. Miller ML, Moreno AY, Aarde S, et al. A methamphetamine vaccine attenuates methamphetamine-induced disruptions in thermoregulation and activity in rats. Biol Psychiatry. 2013;73(8):721-728.
25. Shen XY, Kosten TA, Lopez AY, et al. A vaccine against methamphetamine attenuates its behavioral effects in mice. Drug Alcohol Depend. 2013;129(1-2):41-48.
26. Seminerio MJ, Robson MJ, Abdelazeem AH, et al. Synthesis and pharmacological characterization of a novel sigma receptor ligand with improved metabolic stability and antagonistic effects against methamphetamine. AAPS J. 2012;14(1):43-51.
27. Rawson RA, Marinelli-Casey P, Anglin M, et al. A multi-site comparison of psychosocial approaches for the treatment of methamphetamine dependence. Addiction. 2004;99(6):708-717.
28. Vocci FJ, Montoya ID. Psychological treatments for stimulant misuse, comparing and contrasting those for amphetamine dependence and those for cocaine dependence. Curr Opin Psychiatry. 2009;22(3):263-268.
29. Rawson RA, McCann MJ, Flammino F, et al. A comparison of contingency management and cognitive-behavioral approaches for stimulant-dependent individuals. Addiction. 2006;101(2):267-274.
30. Roll JM, Petry NM, Stitzer ML, et al. Contingency management for the treatment of methamphetamine use disorders. Am J Psychiatry. 2006;163(11):1993-1999.
31. Brecht ML, von Mayrhauser C, Anglin MD. Predictors of relapse after treatment for methamphetamine use. J Psychoactive Drugs. 2000;32(2):211-220.
32. Smout MF, Longo M, Harrison S, et al. Psychosocial treatment for methamphetamine use disorders: a preliminary randomized controlled trial of cognitive behavior therapy and Acceptance and Commitment Therapy. Subst Abus. 2010;31(2):98-107.
33. Wang G, Shi J, Chen N, et al. Effects of length of abstinence on decision-making and craving in methamphetamine abusers. PLoS One. 2013;24;8(7):e68791. doi: 10.1371/ journal.pone.0068791.
34. McKetin R, Lubman DI, Baker AL, et al. Dose-related psychotic symptoms in chronic methamphetamine users: evidence from a prospective longitudinal study. JAMA Psychiatry. 2013;70(3):319-324.
35. Henry BL, Minassian A, Perry W. Effect of methamphetamine dependence on everyday functional ability. Addict Behav. 2010;35(6):593-598.
36. Dean AC, Sevak RJ, Monterosso JR, et al. Acute modafinil effects on attention and inhibitory control in methamphetamine-dependent humans. J Stud Alcohol Drugs. 2011;72(6):943-953.
37. Zullino DF, Benguettat D, Khazaal Y. Improvement of cognitive performance by topiramate: blockage of automatic processes may be the underlying mechanism [Comment on: Effects of topiramate on methamphetamine-induced changes in attentional and perceptual-motor skills of cognition in recently abstinent methamphetamine-dependent individuals. Prog Neuropsychopharmacol Biol Psychiatry. 2007.] Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(3):787.
38. Witkiewitz K, Lustyk M, Bowen S. Retraining the addicted brain: a review of hypothesized neurobiological mechanisms of mindfulness-based relapse prevention. Psychol Addict Behav. 2013;27(2):351-365.
Methamphetamine and other amphetamine-type stimulants are the world’s second most widely used group of illicit substances (after Cannabis), with prevalence of abuse varying by region and by locales within nations. As prescription use of stimulants has grown dramatically in recent years, so has abuse of these substances.
Given the widespread and growing misuse of amphetamine-type stimulants (Box,1-3), clinicians are faced with the need to learn how to recognize and manage methamphetamine abuse. Both prescribed and non-prescribed uses of stimulants present complex challenges; in this article, we examine effects, manifestations, and current evidence-based behavioral and medical treatments of methamphetamine misuse and abuse, and look ahead to investigational therapies that hold promise for improving the limited existing approaches to management.
Effects and manifestations of methamphetamine use
Different routes of administration produce different consequences, in terms of medical comorbidity and propensity to induce addiction. Smoked or injected, methamphetamine enters the brain in seconds; snorted or taken by mouth, the drug produces its effects in several minutes and a half hour, respectively.
Rapid uptake and effects of methamphetamine result from its ability to cross the blood−brain barrier. Its primary effects are caused by inhibition of dopamine storage and release of intracellular dopamine.
Methamphetamine stimulates the CNS and the cardiovascular system through release of dopamine and norepinephrine, which increases blood pressure, body temperature, and heart rate, and, occasionally, induces arrhythmia that can contribute to heart attack and stroke. Users experience euphoria, hypervigilance, suppressed appetite, and increased libido.
Binge use is common to sustain euphoria and other reinforcing effects, which subside with rapidly developing tolerance. After days of repeated dosing, elevated methamphetamine blood levels can lead to mood disturbances, repetitive motor activities, and psychotic symptoms such as hallucinations, delusions, and paranoia. Acute psychosis can bring on violence and other injurious behaviors that involve law enforcement and emergency medical services.
When methamphetamine is used over months or years, health consequences include anorexia, tremor, so-called meth mouth (broken teeth, infections, cavities, burns), insomnia, panic attacks, confusion, depression, irritability, and impaired memory and other cognitive processes.
Treating methamphetamine intoxication and withdrawal
At initial clinical contact with a person who abuses methamphetamine, practitioners may face several acute consequences requiring attention. Prominent among presenting conditions, especially during acute intoxication, are agitation, anxiety, and psychotic symptoms, which may improve by providing the patient with calming reassurance in a quiet space. In more severe cases, a benzodiazepine, antipsychotic, or both might be indicated4,5 (Table 1).
Methamphetamine withdrawal is characterized by anxiety, depression, and insomnia. These symptoms generally resolve in a matter of days after the start of withdrawal without pharmacotherapy. In some cases, depression or psychosis becomes chronic, as a result of methamphetamine use itself6 or as an emergent concomitant psychiatric condition.
A sedative-hypnotic medication or an anxiolytic can be used as necessary to ameliorate insomnia or anxiety, respectively. Prolonged depression can be treated with an antidepressant. An antipsychotic might be indicated for long-term management of patients who have persistent psychosis.
Therapy for methamphetamine abuse
Treatment of methamphetamine abuse— with the goal of stopping drug use—is a complicated matter on 2 counts:
• No medications are FDA-approved for treating methamphetamine addiction.
• There are no accepted substitution medications (ie, stimulants that can be used in place of methamphetamine, as is available for opioid addiction).
Pharmacotherapeutic possibilities. The rationale for considering replacement pharmacotherapy is that psychostimulants can counter the cravings, dysphoria, and fatigue produced by methamphetamine withdrawal and can alleviate methamphetamine-related cognitive impairment. Although dextroamphetamine and other psychostimulants have been evaluated in small trials as replacement medication, most countries are reluctant to consider their use, because of the potential for abuse and accompanying liability.
After decades of medication research, several drugs have shown promise for reducing methamphetamine abuse, although results have not been robust (Table 2):
• Bupropion has shown benefit in reducing methamphetamine use among users with less severe addiction.7,8
• Methylphenidate, a psychostimulant FDA-approved for attention-deficit/hyperactivity disorder, was found to reduce methamphetamine use compared with placebo in a European sample of amphetamine injectors who had attained abstinence in a residential program.9 Those results were not replicated in a recent study by Miles et al, however.10 A study with a more clinically realistic approach (ie, not requiring daily clinic attendance, as in the Miles trial) vs placebo for methamphetamine abuse was recently published, with promising results that require confirmation in further study.11
• Mirtazapine, an antidepressant, has demonstrated efficacy in reducing methamphetamine use compared with placebo.12
• Modafinil, another medication with stimulant properties, reduced methamphetamine use in a subgroup analysis of heavy users, compared with placebo.13
• Dextroamphetamine, 60 mg/d, showed no difference in reducing methamphetamine compared with placebo, but did diminish cravings and withdrawal symptoms.14
A trial of the phosphodiesterase inhibitor ibudilast (not available in the United States) for methamphetamine abuse is underway. Ibudilast has anti-inflammatory activity in the peripheral immune system and the central nervous system, including modulating the activity of glial cells.15
Many medications have yielded negligible results in studies: selegiline, baclofen, sertraline, topiramate, gabapentin, rivastigmine, risperidone, and ondansetron.16 Recent evaluation of disulfiram, vigabatrin, and lobeline also has yielded inconsistent findings.17
No drug has proved effective for preventing relapse; research continues, focusing on several types of compounds that target various mechanisms: the dopamine system, the opioid system (by way of the γ-aminobutyric acid inhibitory system), and cortico-limbic reward circuitry.
Once-monthly injectable naltrexone has potential for ameliorating craving and relapse by modulating the opioid receptor system. However, the drug has not been adequately explored in generalizable settings of methamphetamine users.
Trials of oral naltrexone in Sweden have shown encouraging results, including reduced subjective effects and amphetamine use in open-label trials18,19; results were replicated in a subsequent placebo-controlled trial.20 In an unpublished study, however, no differences in amphetamine use were found among users randomized to depot naltrexone or placebo.21
Depot naltrexone with assured dosing might have a role in treating methamphetamine abuse, however; a combination of depot naltrexone and oral bupropion is being examined in a National Institute on Drug Abuse Clinical Trials Networks study that commenced in 2013. Pairing medications that have different mechanistic targets might work toward promoting cessation of methamphetamine abuse and reducing relapse once patients are abstinent.
In an early phase of research, but showing promise based on their ability to target different systems, are:
• N-acetylcysteine, modulator of the glutamate system
• D3 antagonists and partial agonists22
• varenicline.23
Potential “vaccines” against methamphetamine are in preclinical development, including use of a protein carrier or other immune-stimulating molecule to create antibodies that bind methamphetamine in the bloodstream and block its psychoactive effects.24,25
Sigma receptor effects are being studied in rodents as potential targets to mitigate effects of methamphetamine. The ligand AZ66, a sigma receptor antagonist, has demonstrated efficacy in reducing methamphetamine-induced cognitive impairment—suggesting that the sigma receptor has a potential role in ameliorating methamphetamine-related neurotoxicity.26
Psychosocial and behavioral interventions. Among the non-drug treatments that have demonstrated efficacy for treating methamphetamine abuse, cognitive-behavioral therapy (CBT) and contingency management (CM) have been most widely studied and applied in treatment settings.
CBT involves individual or group counseling that focuses on relapse prevention skills, including identification of relapse triggers, strategies to diminish cravings, and engagement in alternative non-drug activities27,28 (Table 3).
CM, which is based on positive reinforcement, offers tangible reinforcers, or rewards, for behaviors (eg, clinic attendance, providing a drug-free urine sample) according to guidelines set by the practitioner. CM-based interventions are the most reliably documented approaches for treating methamphetamine abuse,29,30 but their utility might prove to be most efficient in combination with medication— once suitable pharmacotherapeutic options emerge.
Although CBT and CM remain accepted standard treatments for methamphetamine abuse, outcomes are suboptimal.27 Both interventions have a high rate of dropout during the first month of treatment and a >50% relapse rate 6 to 19 months after treatment ends.31-33
As with treatment of other substance use disorders, patients who abuse methamphetamine can benefit from residential treatment in a drug-free setting for ≥30 days.34 In the residential approach, removing access to drugs, drug cues, and drug-using acquaintances combined with group and individual counseling reaches an inevitable end: discharge into the community. Then the patient’s battle to avoid relapse begins.
Because cognitive impairment is common among patients who abuse methamphetamine, even after they stop using,35 researchers have examined the potential for increasing participation in psychosocial interventions such as CBT by using medications that might have potential to increase cognitive function, such as modafinil.36 Increased attention and concentration afforded by medication could enhance efficacy of CBT. Results of trials and new drug development have been mixed37; no clear candidate for preventing relapse through any of the putative mechanisms of action has emerged.
Relapse is a problematic target for treatment
Ending methamphetamine abuse and sustaining abstinence from stimulants require a change in the cognitive associations that have been laid down in a drug user’s memory. Relapse occurs because of recalled memories that can be cued, or triggered, by internal or external stimuli. Eliminating drug memories, perhaps assisted by medications such as d-cycloserine (an antagonist of the N-methyl-d-aspartate receptor), could be useful for suppressing the inclination to relapse.
Last, alternative, non-drug forms of cognitive amendment have shown efficacy in preventing relapse: for example, incorporating mindfulness meditation, which has shown promise in managing craving for methamphetamine and decreasing reactivity to environmental cues for drug use.38
Bottom Line
Practitioners who work in emergency, inpatient, and outpatient settings will be called on more and more to treat acute stimulant intoxication and withdrawal, stimulant-induced psychosis, and methamphetamine abuse. Few evidence-based treatments and no FDA-approved medications are available to treat this addiction; many drugs and a few psychotherapeutic techniques have shown promise. Ongoing research promises to deliver medical and behavioral interventions to help patients quit using methamphetamine.
Related Resources
• Karch SB, Drummer O. Karch’s pathology of drug abuse, fifth ed. Boca Raton, FL: CRC Press/Taylor & David; 2013.
• Roll J, Rawson RA, Ling W, eds. Methamphetamine addiction: from basic science to treatment. New York, NY: Guilford Press; 2009.
• Sheff D. Beautiful boy: a father’s journey through his son’s addiction. New York, NY: Houghton Mifflin Harcourt Publishing Company; 2008.
• Sheff N. Tweak: growing up on methamphetamines. New York, NY: Antheneum Books for Young Readers; 2007.
• National Institute on Drug Abuse. Drugs of abuse. www. drugabuse.gov/drugs-abuse/methamphetamine.
Drug Brand Names
Baclofen • Lioresal Naltrexone (depot) • Vivitrol
Bupropion • Wellbutrin Naltrexone (oral) • ReVia
D-cycloserine • Seromycin Ondansetron • Zofran
Dexreoamphetaime • Adderall Risperidone • Risperadal
Disulfiram • Antabuse Rivastigimine • Exelon
Gabapentin • Neurontin Selegiline• EMSAM
Methylphenidate • Ritalin Sertraline • Zoloft
Mirtazapine • Remeron Topiramate • Topamax
Modafinil • Provigil Varenicline • Chantix
N-acetylcysteine • Mucomyst Vigabatrin • Sabril
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Methamphetamine and other amphetamine-type stimulants are the world’s second most widely used group of illicit substances (after Cannabis), with prevalence of abuse varying by region and by locales within nations. As prescription use of stimulants has grown dramatically in recent years, so has abuse of these substances.
Given the widespread and growing misuse of amphetamine-type stimulants (Box,1-3), clinicians are faced with the need to learn how to recognize and manage methamphetamine abuse. Both prescribed and non-prescribed uses of stimulants present complex challenges; in this article, we examine effects, manifestations, and current evidence-based behavioral and medical treatments of methamphetamine misuse and abuse, and look ahead to investigational therapies that hold promise for improving the limited existing approaches to management.
Effects and manifestations of methamphetamine use
Different routes of administration produce different consequences, in terms of medical comorbidity and propensity to induce addiction. Smoked or injected, methamphetamine enters the brain in seconds; snorted or taken by mouth, the drug produces its effects in several minutes and a half hour, respectively.
Rapid uptake and effects of methamphetamine result from its ability to cross the blood−brain barrier. Its primary effects are caused by inhibition of dopamine storage and release of intracellular dopamine.
Methamphetamine stimulates the CNS and the cardiovascular system through release of dopamine and norepinephrine, which increases blood pressure, body temperature, and heart rate, and, occasionally, induces arrhythmia that can contribute to heart attack and stroke. Users experience euphoria, hypervigilance, suppressed appetite, and increased libido.
Binge use is common to sustain euphoria and other reinforcing effects, which subside with rapidly developing tolerance. After days of repeated dosing, elevated methamphetamine blood levels can lead to mood disturbances, repetitive motor activities, and psychotic symptoms such as hallucinations, delusions, and paranoia. Acute psychosis can bring on violence and other injurious behaviors that involve law enforcement and emergency medical services.
When methamphetamine is used over months or years, health consequences include anorexia, tremor, so-called meth mouth (broken teeth, infections, cavities, burns), insomnia, panic attacks, confusion, depression, irritability, and impaired memory and other cognitive processes.
Treating methamphetamine intoxication and withdrawal
At initial clinical contact with a person who abuses methamphetamine, practitioners may face several acute consequences requiring attention. Prominent among presenting conditions, especially during acute intoxication, are agitation, anxiety, and psychotic symptoms, which may improve by providing the patient with calming reassurance in a quiet space. In more severe cases, a benzodiazepine, antipsychotic, or both might be indicated4,5 (Table 1).
Methamphetamine withdrawal is characterized by anxiety, depression, and insomnia. These symptoms generally resolve in a matter of days after the start of withdrawal without pharmacotherapy. In some cases, depression or psychosis becomes chronic, as a result of methamphetamine use itself6 or as an emergent concomitant psychiatric condition.
A sedative-hypnotic medication or an anxiolytic can be used as necessary to ameliorate insomnia or anxiety, respectively. Prolonged depression can be treated with an antidepressant. An antipsychotic might be indicated for long-term management of patients who have persistent psychosis.
Therapy for methamphetamine abuse
Treatment of methamphetamine abuse— with the goal of stopping drug use—is a complicated matter on 2 counts:
• No medications are FDA-approved for treating methamphetamine addiction.
• There are no accepted substitution medications (ie, stimulants that can be used in place of methamphetamine, as is available for opioid addiction).
Pharmacotherapeutic possibilities. The rationale for considering replacement pharmacotherapy is that psychostimulants can counter the cravings, dysphoria, and fatigue produced by methamphetamine withdrawal and can alleviate methamphetamine-related cognitive impairment. Although dextroamphetamine and other psychostimulants have been evaluated in small trials as replacement medication, most countries are reluctant to consider their use, because of the potential for abuse and accompanying liability.
After decades of medication research, several drugs have shown promise for reducing methamphetamine abuse, although results have not been robust (Table 2):
• Bupropion has shown benefit in reducing methamphetamine use among users with less severe addiction.7,8
• Methylphenidate, a psychostimulant FDA-approved for attention-deficit/hyperactivity disorder, was found to reduce methamphetamine use compared with placebo in a European sample of amphetamine injectors who had attained abstinence in a residential program.9 Those results were not replicated in a recent study by Miles et al, however.10 A study with a more clinically realistic approach (ie, not requiring daily clinic attendance, as in the Miles trial) vs placebo for methamphetamine abuse was recently published, with promising results that require confirmation in further study.11
• Mirtazapine, an antidepressant, has demonstrated efficacy in reducing methamphetamine use compared with placebo.12
• Modafinil, another medication with stimulant properties, reduced methamphetamine use in a subgroup analysis of heavy users, compared with placebo.13
• Dextroamphetamine, 60 mg/d, showed no difference in reducing methamphetamine compared with placebo, but did diminish cravings and withdrawal symptoms.14
A trial of the phosphodiesterase inhibitor ibudilast (not available in the United States) for methamphetamine abuse is underway. Ibudilast has anti-inflammatory activity in the peripheral immune system and the central nervous system, including modulating the activity of glial cells.15
Many medications have yielded negligible results in studies: selegiline, baclofen, sertraline, topiramate, gabapentin, rivastigmine, risperidone, and ondansetron.16 Recent evaluation of disulfiram, vigabatrin, and lobeline also has yielded inconsistent findings.17
No drug has proved effective for preventing relapse; research continues, focusing on several types of compounds that target various mechanisms: the dopamine system, the opioid system (by way of the γ-aminobutyric acid inhibitory system), and cortico-limbic reward circuitry.
Once-monthly injectable naltrexone has potential for ameliorating craving and relapse by modulating the opioid receptor system. However, the drug has not been adequately explored in generalizable settings of methamphetamine users.
Trials of oral naltrexone in Sweden have shown encouraging results, including reduced subjective effects and amphetamine use in open-label trials18,19; results were replicated in a subsequent placebo-controlled trial.20 In an unpublished study, however, no differences in amphetamine use were found among users randomized to depot naltrexone or placebo.21
Depot naltrexone with assured dosing might have a role in treating methamphetamine abuse, however; a combination of depot naltrexone and oral bupropion is being examined in a National Institute on Drug Abuse Clinical Trials Networks study that commenced in 2013. Pairing medications that have different mechanistic targets might work toward promoting cessation of methamphetamine abuse and reducing relapse once patients are abstinent.
In an early phase of research, but showing promise based on their ability to target different systems, are:
• N-acetylcysteine, modulator of the glutamate system
• D3 antagonists and partial agonists22
• varenicline.23
Potential “vaccines” against methamphetamine are in preclinical development, including use of a protein carrier or other immune-stimulating molecule to create antibodies that bind methamphetamine in the bloodstream and block its psychoactive effects.24,25
Sigma receptor effects are being studied in rodents as potential targets to mitigate effects of methamphetamine. The ligand AZ66, a sigma receptor antagonist, has demonstrated efficacy in reducing methamphetamine-induced cognitive impairment—suggesting that the sigma receptor has a potential role in ameliorating methamphetamine-related neurotoxicity.26
Psychosocial and behavioral interventions. Among the non-drug treatments that have demonstrated efficacy for treating methamphetamine abuse, cognitive-behavioral therapy (CBT) and contingency management (CM) have been most widely studied and applied in treatment settings.
CBT involves individual or group counseling that focuses on relapse prevention skills, including identification of relapse triggers, strategies to diminish cravings, and engagement in alternative non-drug activities27,28 (Table 3).
CM, which is based on positive reinforcement, offers tangible reinforcers, or rewards, for behaviors (eg, clinic attendance, providing a drug-free urine sample) according to guidelines set by the practitioner. CM-based interventions are the most reliably documented approaches for treating methamphetamine abuse,29,30 but their utility might prove to be most efficient in combination with medication— once suitable pharmacotherapeutic options emerge.
Although CBT and CM remain accepted standard treatments for methamphetamine abuse, outcomes are suboptimal.27 Both interventions have a high rate of dropout during the first month of treatment and a >50% relapse rate 6 to 19 months after treatment ends.31-33
As with treatment of other substance use disorders, patients who abuse methamphetamine can benefit from residential treatment in a drug-free setting for ≥30 days.34 In the residential approach, removing access to drugs, drug cues, and drug-using acquaintances combined with group and individual counseling reaches an inevitable end: discharge into the community. Then the patient’s battle to avoid relapse begins.
Because cognitive impairment is common among patients who abuse methamphetamine, even after they stop using,35 researchers have examined the potential for increasing participation in psychosocial interventions such as CBT by using medications that might have potential to increase cognitive function, such as modafinil.36 Increased attention and concentration afforded by medication could enhance efficacy of CBT. Results of trials and new drug development have been mixed37; no clear candidate for preventing relapse through any of the putative mechanisms of action has emerged.
Relapse is a problematic target for treatment
Ending methamphetamine abuse and sustaining abstinence from stimulants require a change in the cognitive associations that have been laid down in a drug user’s memory. Relapse occurs because of recalled memories that can be cued, or triggered, by internal or external stimuli. Eliminating drug memories, perhaps assisted by medications such as d-cycloserine (an antagonist of the N-methyl-d-aspartate receptor), could be useful for suppressing the inclination to relapse.
Last, alternative, non-drug forms of cognitive amendment have shown efficacy in preventing relapse: for example, incorporating mindfulness meditation, which has shown promise in managing craving for methamphetamine and decreasing reactivity to environmental cues for drug use.38
Bottom Line
Practitioners who work in emergency, inpatient, and outpatient settings will be called on more and more to treat acute stimulant intoxication and withdrawal, stimulant-induced psychosis, and methamphetamine abuse. Few evidence-based treatments and no FDA-approved medications are available to treat this addiction; many drugs and a few psychotherapeutic techniques have shown promise. Ongoing research promises to deliver medical and behavioral interventions to help patients quit using methamphetamine.
Related Resources
• Karch SB, Drummer O. Karch’s pathology of drug abuse, fifth ed. Boca Raton, FL: CRC Press/Taylor & David; 2013.
• Roll J, Rawson RA, Ling W, eds. Methamphetamine addiction: from basic science to treatment. New York, NY: Guilford Press; 2009.
• Sheff D. Beautiful boy: a father’s journey through his son’s addiction. New York, NY: Houghton Mifflin Harcourt Publishing Company; 2008.
• Sheff N. Tweak: growing up on methamphetamines. New York, NY: Antheneum Books for Young Readers; 2007.
• National Institute on Drug Abuse. Drugs of abuse. www. drugabuse.gov/drugs-abuse/methamphetamine.
Drug Brand Names
Baclofen • Lioresal Naltrexone (depot) • Vivitrol
Bupropion • Wellbutrin Naltrexone (oral) • ReVia
D-cycloserine • Seromycin Ondansetron • Zofran
Dexreoamphetaime • Adderall Risperidone • Risperadal
Disulfiram • Antabuse Rivastigimine • Exelon
Gabapentin • Neurontin Selegiline• EMSAM
Methylphenidate • Ritalin Sertraline • Zoloft
Mirtazapine • Remeron Topiramate • Topamax
Modafinil • Provigil Varenicline • Chantix
N-acetylcysteine • Mucomyst Vigabatrin • Sabril
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. UNODC. World Drug Report 2012 (United Nations publication, Sales No. E.12.XI.1). http://www.unodc. org/documents/data-and-analysis/WDR2012/ WDR_2012_web_small.pdf. Published 2012. Accessed August 4, 2014.
2. UNODC. World Drug Report 2010 (United Nations publication, Sales No. E.10.XI.13). http://www.unodc. org/documents/wdr/WDR_2010/World_Drug_ Report_2010_lo-res.pdf. Published 2010. Accessed August 4, 2014.
3. Rawson RA, Gonzales R, Brecht M, et al. Evaluation of the California Outcomes Measurement System (CalOMS): Final Report 2008. http://www.uclaisap.org/assets/documents/ California-ADP-DHCS-Evals/2007-2008_CalOMS%20 Report.pdf. Published 2008. Accessed August 4, 2014.
4. Shoptaw SJ, Kao U, Ling W. Treatment for amphetamine psychosis. Cochrane Database Syst Rev. 2009;(1):CD003026.
5. Leelahanaj T, Kongsakon R, Netrakom P. A 4-week, double-blind comparison of olanzapine with haloperidol in the treatment of amphetamine psychosis. J Med Assoc Thai. 2005;88(suppl 3):S43-S52.
6. McKetin R, McLaren J, Lubman D, et al. The prevalence of psychotic symptoms among methamphetamine users. Addiction. 2006;101(10):1473-1478.
7. Elkashef AM, Rawson RA, Anderson AL, et al. Bupropion for the treatment of methamphetamine dependence. Neuropsychopharmacology. 2008;33(5):1162-1170.
8. McCann DJ, Li SH. A novel, nonbinary evaluation of success and failure reveals bupropion efficacy versus methamphetamine dependence: reanalysis of a multisite trial. CNS Neurosci Ther. 2012;18(5):414-418.
9. Tiihonen J, Kuoppasalmi K, Föhr J, et al. A comparison of aripiprazole, methylphenidate, and placebo for amphetamine dependence. Am J Psychiatry. 2007;164(1): 160-162.
10. Miles SW, Sheridan J, Russell B, et al. Extended-release methylphenidate for treatment of amphetamine/ methamphetamine dependence: a randomized, double-blind, placebo-controlled trial. Addiction. 2013;108(7): 1279-1286.
11. Ling W, Chang L, Hillhouse M, et al. Sustained-release methylphenidate in a randomized trial of treatment of methamphetamine use disorder. Addiction. 2014;109(9): 1489-1500.
12. Colfax GN, Santos GM, Das M, et al. Mirtazapine to reduce methamphetamine use: a randomized controlled trial. Arch Gen Psychiatry. 2011;68(11):1168-1175.
13. Heinzerling KG, Swanson AN, Kim S, et al. Randomized, double-blind, placebo-controlled trial of modafinil for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2010;109(1-3):20-29.
14. Galloway GP, Buscemi R, Coyle JR, et al. A randomized, placebo-controlled trial of sustained-release dextro-amphetamine for treatment of methamphetamine addiction. Clin Pharmacol Ther. 2011;89(2):276-282.
15. Snider SE, Hendrick ES, Beardsley PM. Glial cell modulators attenuate methamphetamine self-administration in the rat. Eur J Pharmacol. 2013;701(1-3):124-130.
16. Ling W, Rawson R, Shoptaw S. Management of methamphetamine abuse and dependence. Curr Psychiatry Rep. 2006;8(5):345-354.
17. Brackins T, Brahm NC, Kissack JC. Treatments for methamphetamine abuse: a literature review for the clinician. J Pharm Pract. 2011;24(6):541-550.
18. Jayaram-Lindström N, Wennberg P, Beck O, et al. An open clinical trial of naltrexone for amphetamine dependence: compliance and tolerability. Nord J Psychiatry. 2005;59(3):167-171.
19. Jayaram-Lindström N, Konstenius M, Eksborg S, et al. Naltrexone attenuates the subjective effects of amphetamine in patients with amphetamine dependence. Neuropsychopharmacology. 2007;33(8):1856-1863.
20. Jayaram-Lindström N, Hammarberg A, Beck O, et al. Naltrexone for the treatment of amphetamine dependence: a randomized, placebo-controlled trial. Am J Psychiatry. 2008;165(11):1442-1448.
21. Woody GE, Tyrfingsoon P. Symposium XI: Emerging data on efficacy and clinical applications of extended-release naltrexone formulations. 75th Annual Meeting, College on Problems of Drug Dependence. June 19, 2013; San Diego, CA.
22. Newman AH, Blaylock BL, Nader MA, et al. Medication discovery for addiction: translating the dopamine D3 receptor hypothesis. Biochem Pharmacol. 2012;84(7):882-890.
23. Verrico CD, Mahoney JJ 3rd, Thompson-Lake DG, et al. Safety and efficacy of varenicline to reduce positive subjective effects produced by methamphetamine in methamphetamine-dependent volunteers. Int J Neuropsychopharmacol. 2014;17(2):223-233.
24. Miller ML, Moreno AY, Aarde S, et al. A methamphetamine vaccine attenuates methamphetamine-induced disruptions in thermoregulation and activity in rats. Biol Psychiatry. 2013;73(8):721-728.
25. Shen XY, Kosten TA, Lopez AY, et al. A vaccine against methamphetamine attenuates its behavioral effects in mice. Drug Alcohol Depend. 2013;129(1-2):41-48.
26. Seminerio MJ, Robson MJ, Abdelazeem AH, et al. Synthesis and pharmacological characterization of a novel sigma receptor ligand with improved metabolic stability and antagonistic effects against methamphetamine. AAPS J. 2012;14(1):43-51.
27. Rawson RA, Marinelli-Casey P, Anglin M, et al. A multi-site comparison of psychosocial approaches for the treatment of methamphetamine dependence. Addiction. 2004;99(6):708-717.
28. Vocci FJ, Montoya ID. Psychological treatments for stimulant misuse, comparing and contrasting those for amphetamine dependence and those for cocaine dependence. Curr Opin Psychiatry. 2009;22(3):263-268.
29. Rawson RA, McCann MJ, Flammino F, et al. A comparison of contingency management and cognitive-behavioral approaches for stimulant-dependent individuals. Addiction. 2006;101(2):267-274.
30. Roll JM, Petry NM, Stitzer ML, et al. Contingency management for the treatment of methamphetamine use disorders. Am J Psychiatry. 2006;163(11):1993-1999.
31. Brecht ML, von Mayrhauser C, Anglin MD. Predictors of relapse after treatment for methamphetamine use. J Psychoactive Drugs. 2000;32(2):211-220.
32. Smout MF, Longo M, Harrison S, et al. Psychosocial treatment for methamphetamine use disorders: a preliminary randomized controlled trial of cognitive behavior therapy and Acceptance and Commitment Therapy. Subst Abus. 2010;31(2):98-107.
33. Wang G, Shi J, Chen N, et al. Effects of length of abstinence on decision-making and craving in methamphetamine abusers. PLoS One. 2013;24;8(7):e68791. doi: 10.1371/ journal.pone.0068791.
34. McKetin R, Lubman DI, Baker AL, et al. Dose-related psychotic symptoms in chronic methamphetamine users: evidence from a prospective longitudinal study. JAMA Psychiatry. 2013;70(3):319-324.
35. Henry BL, Minassian A, Perry W. Effect of methamphetamine dependence on everyday functional ability. Addict Behav. 2010;35(6):593-598.
36. Dean AC, Sevak RJ, Monterosso JR, et al. Acute modafinil effects on attention and inhibitory control in methamphetamine-dependent humans. J Stud Alcohol Drugs. 2011;72(6):943-953.
37. Zullino DF, Benguettat D, Khazaal Y. Improvement of cognitive performance by topiramate: blockage of automatic processes may be the underlying mechanism [Comment on: Effects of topiramate on methamphetamine-induced changes in attentional and perceptual-motor skills of cognition in recently abstinent methamphetamine-dependent individuals. Prog Neuropsychopharmacol Biol Psychiatry. 2007.] Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(3):787.
38. Witkiewitz K, Lustyk M, Bowen S. Retraining the addicted brain: a review of hypothesized neurobiological mechanisms of mindfulness-based relapse prevention. Psychol Addict Behav. 2013;27(2):351-365.
1. UNODC. World Drug Report 2012 (United Nations publication, Sales No. E.12.XI.1). http://www.unodc. org/documents/data-and-analysis/WDR2012/ WDR_2012_web_small.pdf. Published 2012. Accessed August 4, 2014.
2. UNODC. World Drug Report 2010 (United Nations publication, Sales No. E.10.XI.13). http://www.unodc. org/documents/wdr/WDR_2010/World_Drug_ Report_2010_lo-res.pdf. Published 2010. Accessed August 4, 2014.
3. Rawson RA, Gonzales R, Brecht M, et al. Evaluation of the California Outcomes Measurement System (CalOMS): Final Report 2008. http://www.uclaisap.org/assets/documents/ California-ADP-DHCS-Evals/2007-2008_CalOMS%20 Report.pdf. Published 2008. Accessed August 4, 2014.
4. Shoptaw SJ, Kao U, Ling W. Treatment for amphetamine psychosis. Cochrane Database Syst Rev. 2009;(1):CD003026.
5. Leelahanaj T, Kongsakon R, Netrakom P. A 4-week, double-blind comparison of olanzapine with haloperidol in the treatment of amphetamine psychosis. J Med Assoc Thai. 2005;88(suppl 3):S43-S52.
6. McKetin R, McLaren J, Lubman D, et al. The prevalence of psychotic symptoms among methamphetamine users. Addiction. 2006;101(10):1473-1478.
7. Elkashef AM, Rawson RA, Anderson AL, et al. Bupropion for the treatment of methamphetamine dependence. Neuropsychopharmacology. 2008;33(5):1162-1170.
8. McCann DJ, Li SH. A novel, nonbinary evaluation of success and failure reveals bupropion efficacy versus methamphetamine dependence: reanalysis of a multisite trial. CNS Neurosci Ther. 2012;18(5):414-418.
9. Tiihonen J, Kuoppasalmi K, Föhr J, et al. A comparison of aripiprazole, methylphenidate, and placebo for amphetamine dependence. Am J Psychiatry. 2007;164(1): 160-162.
10. Miles SW, Sheridan J, Russell B, et al. Extended-release methylphenidate for treatment of amphetamine/ methamphetamine dependence: a randomized, double-blind, placebo-controlled trial. Addiction. 2013;108(7): 1279-1286.
11. Ling W, Chang L, Hillhouse M, et al. Sustained-release methylphenidate in a randomized trial of treatment of methamphetamine use disorder. Addiction. 2014;109(9): 1489-1500.
12. Colfax GN, Santos GM, Das M, et al. Mirtazapine to reduce methamphetamine use: a randomized controlled trial. Arch Gen Psychiatry. 2011;68(11):1168-1175.
13. Heinzerling KG, Swanson AN, Kim S, et al. Randomized, double-blind, placebo-controlled trial of modafinil for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2010;109(1-3):20-29.
14. Galloway GP, Buscemi R, Coyle JR, et al. A randomized, placebo-controlled trial of sustained-release dextro-amphetamine for treatment of methamphetamine addiction. Clin Pharmacol Ther. 2011;89(2):276-282.
15. Snider SE, Hendrick ES, Beardsley PM. Glial cell modulators attenuate methamphetamine self-administration in the rat. Eur J Pharmacol. 2013;701(1-3):124-130.
16. Ling W, Rawson R, Shoptaw S. Management of methamphetamine abuse and dependence. Curr Psychiatry Rep. 2006;8(5):345-354.
17. Brackins T, Brahm NC, Kissack JC. Treatments for methamphetamine abuse: a literature review for the clinician. J Pharm Pract. 2011;24(6):541-550.
18. Jayaram-Lindström N, Wennberg P, Beck O, et al. An open clinical trial of naltrexone for amphetamine dependence: compliance and tolerability. Nord J Psychiatry. 2005;59(3):167-171.
19. Jayaram-Lindström N, Konstenius M, Eksborg S, et al. Naltrexone attenuates the subjective effects of amphetamine in patients with amphetamine dependence. Neuropsychopharmacology. 2007;33(8):1856-1863.
20. Jayaram-Lindström N, Hammarberg A, Beck O, et al. Naltrexone for the treatment of amphetamine dependence: a randomized, placebo-controlled trial. Am J Psychiatry. 2008;165(11):1442-1448.
21. Woody GE, Tyrfingsoon P. Symposium XI: Emerging data on efficacy and clinical applications of extended-release naltrexone formulations. 75th Annual Meeting, College on Problems of Drug Dependence. June 19, 2013; San Diego, CA.
22. Newman AH, Blaylock BL, Nader MA, et al. Medication discovery for addiction: translating the dopamine D3 receptor hypothesis. Biochem Pharmacol. 2012;84(7):882-890.
23. Verrico CD, Mahoney JJ 3rd, Thompson-Lake DG, et al. Safety and efficacy of varenicline to reduce positive subjective effects produced by methamphetamine in methamphetamine-dependent volunteers. Int J Neuropsychopharmacol. 2014;17(2):223-233.
24. Miller ML, Moreno AY, Aarde S, et al. A methamphetamine vaccine attenuates methamphetamine-induced disruptions in thermoregulation and activity in rats. Biol Psychiatry. 2013;73(8):721-728.
25. Shen XY, Kosten TA, Lopez AY, et al. A vaccine against methamphetamine attenuates its behavioral effects in mice. Drug Alcohol Depend. 2013;129(1-2):41-48.
26. Seminerio MJ, Robson MJ, Abdelazeem AH, et al. Synthesis and pharmacological characterization of a novel sigma receptor ligand with improved metabolic stability and antagonistic effects against methamphetamine. AAPS J. 2012;14(1):43-51.
27. Rawson RA, Marinelli-Casey P, Anglin M, et al. A multi-site comparison of psychosocial approaches for the treatment of methamphetamine dependence. Addiction. 2004;99(6):708-717.
28. Vocci FJ, Montoya ID. Psychological treatments for stimulant misuse, comparing and contrasting those for amphetamine dependence and those for cocaine dependence. Curr Opin Psychiatry. 2009;22(3):263-268.
29. Rawson RA, McCann MJ, Flammino F, et al. A comparison of contingency management and cognitive-behavioral approaches for stimulant-dependent individuals. Addiction. 2006;101(2):267-274.
30. Roll JM, Petry NM, Stitzer ML, et al. Contingency management for the treatment of methamphetamine use disorders. Am J Psychiatry. 2006;163(11):1993-1999.
31. Brecht ML, von Mayrhauser C, Anglin MD. Predictors of relapse after treatment for methamphetamine use. J Psychoactive Drugs. 2000;32(2):211-220.
32. Smout MF, Longo M, Harrison S, et al. Psychosocial treatment for methamphetamine use disorders: a preliminary randomized controlled trial of cognitive behavior therapy and Acceptance and Commitment Therapy. Subst Abus. 2010;31(2):98-107.
33. Wang G, Shi J, Chen N, et al. Effects of length of abstinence on decision-making and craving in methamphetamine abusers. PLoS One. 2013;24;8(7):e68791. doi: 10.1371/ journal.pone.0068791.
34. McKetin R, Lubman DI, Baker AL, et al. Dose-related psychotic symptoms in chronic methamphetamine users: evidence from a prospective longitudinal study. JAMA Psychiatry. 2013;70(3):319-324.
35. Henry BL, Minassian A, Perry W. Effect of methamphetamine dependence on everyday functional ability. Addict Behav. 2010;35(6):593-598.
36. Dean AC, Sevak RJ, Monterosso JR, et al. Acute modafinil effects on attention and inhibitory control in methamphetamine-dependent humans. J Stud Alcohol Drugs. 2011;72(6):943-953.
37. Zullino DF, Benguettat D, Khazaal Y. Improvement of cognitive performance by topiramate: blockage of automatic processes may be the underlying mechanism [Comment on: Effects of topiramate on methamphetamine-induced changes in attentional and perceptual-motor skills of cognition in recently abstinent methamphetamine-dependent individuals. Prog Neuropsychopharmacol Biol Psychiatry. 2007.] Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(3):787.
38. Witkiewitz K, Lustyk M, Bowen S. Retraining the addicted brain: a review of hypothesized neurobiological mechanisms of mindfulness-based relapse prevention. Psychol Addict Behav. 2013;27(2):351-365.