User login
Can what we learned about reducing no-shows in our clinic work for you?
The no-show rate is high in ambulatory psychiatric clinics, especially those associated with academic medical institutions, which usually accept all public insurance providers and do not maintain a strict rule by which patients are charged a penalty when they fail to keep a scheduled appointment—a policy that, to the contrary, is customary in private practice. The University of Texas (UT) Health Sciences Center at Houston is primarily an academic medical center with resident-managed, faculty-supervised clinics that provide care to a large volume of patients.
At the UT clinics, we have struggled with a high no-show rate, and were challenged to reduce that rate. Our study of the problem, formulation and application of strategies to reduce that rate, and a discussion of our results are provided here for the benefit of psychiatric clinicians who struggle with this problem, to the detriment of their patients’ health and the financial well-being of the practice.
For patients who have a severe psychiatric illness, such as schizophrenia or bipolar disorder, 60% to 70% of the direct cost of their care is attributable to inpatient services.1,2 Poor medication adherence is a critical factor: It results in exacerbation of symptoms, relapse, and hospitalization. The matter is compounded by patients’ failure to show up for scheduled follow-up appointments.
Studies show that failure to attend routinely scheduled outpatient appointments increases the risk of hospitalization. Recent research has shown that, among all causes of hospitalization, length of stay and relapse hospitalization are increased in patients with low adherence to their treatment regimen.3 Patients who miss an appointment also are more unwell and more functionally impaired—also contributing to a higher risk and rate of rehospitalization.4,5
To begin to address the problem at UT, we acknowledged that an elevated no-show rate is linked to medication nonadherence, increased risk of re-hospitalization, and increased costs associated with poor care.
Impact of nonadherence
Significant evidence supports the efficacy of antipsychotic medications for treating schizophrenia, of course,6 but that success story is undermined by the mean rate of medication nonadherence among schizophrenia patients, which can be as high as 49% in studies.7 (The actual rate might be higher because those studies do not account for persons who refuse treatment or drop out.)
Nonadherence increases the risk of relapse 3.7-fold, compared with what is seen in patients who adhere to treatment.8 Nonadherence to a medication regimen also can increase patients’ risk of engaging in assault and other dangerous behaviors, especially during periods of psychosis.8 Variables consistently associated with nonadherence include poor insight, negative attitude or subjective response toward medication, previous nonadherence, substance abuse, shorter duration of illness, inadequate discharge planning or after-care environment, and poorer therapeutic alliance.7,8
Investigation of medication adherence in bipolar disorder suggests that 1 in 3 patients fail to take at least 30% of their medication.9 In such patients, medication nonadherence can lead to mania, depression, hospital readmission, suicide, increased substance abuse, and nonresponse to treatment.10,11
Depression also is associated with an increased rate of health care utilization and severe limitation in daily functioning.12 Compared with non-depressed patients, depressed patients are 3 times more likely to be nonadherent with medical treatment recommendations.13 Estimates of medication nonadherence for unipolar and bipolar disorders range from 10% to 60% (median, 40%). This prevalence has not changed significantly with the introduction of new medications.14
Our literature review of research devoted to reducing no-shows found that few studies have explored this critical treatment concern. The no-show rate was higher among younger patients and slightly higher among women, but varied by diagnosis.15 The most common reason psychiatric patients gave for missing an appointment was “forgetting”—a response heard twice as often among no-show patients in psychiatry than in other specialties.4
Little has been tried to solve the problem. Often, community mental health centers and private practices double-book appointments. Double-booking is intended to reduce the financial burden on the practice when a patient misses an appointment. This approach fails to address nonadherence or the poor care that usually results when a patient misses regular outpatient appointments.
Several methods have been employed to improve adherence, such as electronic pill dispensing.16 Increasing medication adherence appears to be a key factor in improving quality-of-life measures in patients with schizophrenia.6
The UT project
Methods. This project was completed at the ambulatory psychiatry clinic at the UT Medical School at Houston. The clinic staff comprises residents and faculty members who provide outpatient care. During the study period, the clinic was scheduling as many as 800 office visits a month, including a mix of new and follow-up appointments. Two weeks’ retrospective data revealed a no-show rate of 31%.
For the project, we defined no-show rate as the total number of patients who missed an appointment or canceled fewer than 24 hours before the scheduled time, divided by the total number of patients scheduled that day.
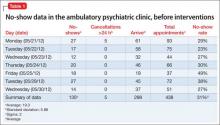
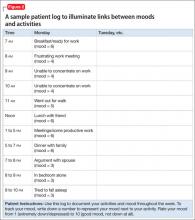
Table 1 demonstrates the no-show rate calculations for 1 of the weeks preceding the start of the project. Given approximately 800 patient appointments a month, a 31% no-show rate meant that, first, 248 patients failed to receive recommended care and, second, 248 appointment slots were wasted.
Besides undermining such components of quality care as patient safety and medication compliance, the high no-show rate also harms employee morale and productivity; impairs medical education; and, possibly, increases the use of emergency and after-hour services.
We agreed that our current no-show rate of 31% was too high.
We then formed a team of residents, faculty members, therapists, front office staff, an office manager, and an office nurse. We explored and hypothesized what could be contributing to the high no-show rate (Table 2).
Several interventions were then devised and implemented:
• Patients. We increased patient education about 1) the need for regular follow-up and 2) risks associated with medication nonadherence.
• Environment. We explored environmental limitations to access and agreed that certain static factors could not be modified—eg, location of the clinic and lack of access to public transportation. We were able to make some changes to the environment (explained later) to reduce wait time.
• Staff. Some patients had complained of long wait times, which could hinder active participation in treatment. We agreed that the clinic nurse would make rounds through the waiting room every hour and talk to patients. The nurse would identify patients who had been waiting for longer than 30 minutes after their scheduled appointment time and notify the doctor accordingly. We also agreed to revise patient appointment reminder practices: instead of using an automated answering service, one of the staff members called patients personally to remind them about their appointments. (This also allowed us to update telephone numbers for many patients; numbers on record often were outdated.) We initially recruited summer interns and provided a written script to follow during calls to patients, which allowed patients to confirm, cancel, or reschedule their appointment. Once we demonstrated positive results from the change to personal calls, the department agreed to absorb the cost, and front desk personnel began making reminder calls.
• Policies and procedures. Although some practices are able to charge a small fine for missed appointments, this was not allowed at our institution. Instead, we had several departmental policies on the books, such as discharging patients from our clinics if they missed 3 consecutive appointments and limiting prescription refills to a maximum of 6 months. These policies were neither communicated to patients and staff, nor were they implemented. We decided to educate patients and staff and implement the policies.
• Transparency. We posted the no-show rate in common areas so that the team could review and follow the progression of that rate as we implemented the changes. This allowed team members to take ownership of the project and facilitated active participation.
By implementing these changes, we aimed to reduce the no-show rate to 20%.
Results. We were able to reduce the no-show rate from a documented average of 31% to an average of 12% during the study period after implementing all the proposed changes in the outpatient clinics.
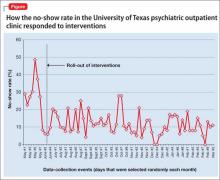
We calculated the no-show rate (as shown in Table 1 for May 2013), then collected the daily no-show rate from June to September 2013 (Figure). With these calculations, we demonstrated a reduction in the no-show rate to 12%. Because of the time and effort required, we reduced data collection from daily to weekly, beginning in September.
Applying the changes required consistent effort and substantial input from various stakeholders—front desk staff, residents, the nurse, therapists, and faculty. Gradually, we were able to implement all the changes.
Keeping the no-show rate low required consistent effort and monitoring of the newly implemented procedures because even a slight change, such as failure to make reminder calls, resulted in a sudden increase in the no-show rate (that was the case in October of the study period, when we were short-staffed and could not call every patient). Patients told us that it was difficult to ignore a personal call; if they were not planning to keep the appointment, the call allowed them to reschedule on the spot.
We also made sure that current no-show rates were posted in common areas, visible to team members every day.
Discussion
We attempted a literature review of research exploring approaches to reducing the no-show rate but found few studies that explored this critical concern in patient treatment.15 Some data suggested that, in the setting studied, the no-show rate:
• was higher among younger patients (age 20 to 39) than older ones (age 60 to 79)
• was slightly higher in women than in men
• varied by diagnosis.
We found a paucity of data regarding interventions that can reduce the no-show rate.
Among the changes we made, the one that had the greatest impact was personalized appointment reminder calls, as evidenced by our patients’ reports and the increase in the no-show rate when personal calls were not made.
We also realized that, although we had several departmental policies in place regarding appointments, they were not being followed. Raising awareness among team members and their patients also was an effective deterrent to a no-show for an appointment. For example, patients were informed that 3 consecutive no-shows could lead to termination of care. Often, they reacted with surprise to this caution but also voiced a desire to improve their attendance to avoid such an outcome.
We found that establishing common operational definitions is important. It also was important to have a cohesive team, with every member agreeing on goals and changes to operational policies that needed to be implemented. Support from the department chair and the administration, we learned, is vital to the success of such an intervention.
A note about limitations. The goal of the project was limited to reducing the no-show rate. We demonstrated that this is possible among patients who have a severe mental illness, and that reducing the associated waste of time and resources can improve finances in an academic department of psychiatry. We would need additional measures, however, to quantify medication adherence and hospitalization; a larger, more inclusive project is needed to demonstrate that reducing the no-show rate reduces the symptomatic burden of psychiatric illness.
Comments in conclusion
This project was designed and conducted as a required part of a Clinical Safety and Effectiveness Program at Memorial Hermann Texas Medical Center and the UT Medical School at Houston.17 Although there was initial hesitancy about attempting to reduce the no-show rate in a chronically mentally ill population, the success of this project—indeed, it surpassed its proposed goals—demonstrates that operational changes in any clinic can reduce the no-show rate. It also is important to maintain operational changes, however; without consistent effort, desired results cannot be sustained.
Last, it is possible to replicate the methodology of this project and thereby attempt to reduce the no-show rate in other divisions of medicine that offer care to chronically ill patients, such as pediatrics and family medicine.
Bottom Line
Failure to attend routinely scheduled outpatient appointments increases a patient’s functional impairment and risk of hospitalization. Patient education, appointment reminder phone calls, revised policies and procedures, and transparency regarding the no-show rate can reduce the number of missed appointments and improve patient outcomes.
Related Resources
• Mitchell AJ, Selmes T. Why don’t patients attend their appointments? Maintaining engagement with psychiatric services. Advances in Psychiatric Treatment. 2007;13:423-434.
• Molfenter T. Reducing appointment no-shows: going from theory to practice. Subst Use Misuse. 2013;48(9):743-749.
• Williston MA, Block-Lerner J, Wolanin A, et al. Brief acceptance-based intervention for increasing intake attendance at a community mental health center. Psychol Serv. 2014;11(3):324-332.
Disclosure
Dr. Gajwani receives grant or research support from the National Institute on Mental Health, the National Institute of Drug Abuse, The Stanley Foundation, and Forest Laboratories, Inc. He is a member of the speakers’ bureau of AstraZeneca, Merck, Otsuka America Pharmaceutical, and Sunovion Pharmaceuticals.
1. Wyatt RJ, Henter I. An economic evaluation of manic-depressive illness—1991. Soc Psychiatry Psychiatr Epidemiol. 1995;30(5):213-219.
2. Wyatt RJ, Henter I, Leary MC, et al. An economic evaluation of schizophrenia—1991. Soc Psychiatry Psychiatr Epidemiol. 1995;30(5);196-205.
3. Offord S, Lin J, Wong B, et al. Impact of oral antipsychotic medication adherence on healthcare resource utilization among schizophrenic patients with medicare coverage. Community Ment Health J. 2013;49(6):625-629.
4. Killaspy H, Banerjee S, King M, et al. Prospective controlled study of psychiatric out-patient non-attendance: characteristics and outcome. Br J Psychiatry. 2000;176:160- 165.
5. Nelson EA, Maruish ME, Axler JL. Effects of discharge planning and compliance with outpatient appointments on readmission rates. Psychiatr Serv. 2000;51(7):885-889.
6. Thornley B, Adams C. Content and quality of 2000 controlled trials in schizophrenia over 50 years. BMJ. 1998;317(7167):1181-1184.
7. Lacro JP, Dunn LB, Dolder CR, et al. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literature. J Clin Psychiatry. 2002;63(10):892-909.
8. Fenton WS, Blyler C, Heinssen RK. Determinants of medication compliance in schizophrenia: empirical and clinical findings. Schizophr Bull. 1997;23(4):637-651.
9. Scott J, Pope M. Self-reported adherence to treatment with mood stabilizers, plasma levels, and psychiatric hospitalization. Am J Psychiatry. 2002;159(11):1927-1929.
10. Adams J, Scott J. Predicting medication adherence in severe mental disorders. Acta Psychiatr Scand. 2000;101(2):119-124.
11. Müller-Oerlinghausen B, Müser-Causemann B, Volk J. Suicides and parasuicides in a high-risk patient group on and off lithium long-term treatment. J Affect Disord. 1992;25(4):261-269.
12. Manning WG Jr, Wells KB. The effects of psychological distress and psychological well-being on use of medical services. Med Care. 1992;30(6):541-553.
13. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101-2107.
14. Lingam R, Scott J. Treatment non‐adherence in affective disorders. Acta Psychiatr Scand. 2002;105(3):164-172.
15. Allan AT. No-shows at a community mental health clinic: a pilot study. Int J Soc Psychiatry. 1988;34(1):40-46.
16. Cramer JA, Rosenheck R. Compliance with medication regimens for mental and physical disorders. Psychiatr Serv. 1998;49(2):196-201.
17. Gajwani P. Improving quality of care: reducing no-show rate in ambulatory psychiatry clinic. Poster presented at: American Psychiatric Association 166th Annual Meeting; May 18-22, 2013; San Francisco, CA.
The no-show rate is high in ambulatory psychiatric clinics, especially those associated with academic medical institutions, which usually accept all public insurance providers and do not maintain a strict rule by which patients are charged a penalty when they fail to keep a scheduled appointment—a policy that, to the contrary, is customary in private practice. The University of Texas (UT) Health Sciences Center at Houston is primarily an academic medical center with resident-managed, faculty-supervised clinics that provide care to a large volume of patients.
At the UT clinics, we have struggled with a high no-show rate, and were challenged to reduce that rate. Our study of the problem, formulation and application of strategies to reduce that rate, and a discussion of our results are provided here for the benefit of psychiatric clinicians who struggle with this problem, to the detriment of their patients’ health and the financial well-being of the practice.
For patients who have a severe psychiatric illness, such as schizophrenia or bipolar disorder, 60% to 70% of the direct cost of their care is attributable to inpatient services.1,2 Poor medication adherence is a critical factor: It results in exacerbation of symptoms, relapse, and hospitalization. The matter is compounded by patients’ failure to show up for scheduled follow-up appointments.
Studies show that failure to attend routinely scheduled outpatient appointments increases the risk of hospitalization. Recent research has shown that, among all causes of hospitalization, length of stay and relapse hospitalization are increased in patients with low adherence to their treatment regimen.3 Patients who miss an appointment also are more unwell and more functionally impaired—also contributing to a higher risk and rate of rehospitalization.4,5
To begin to address the problem at UT, we acknowledged that an elevated no-show rate is linked to medication nonadherence, increased risk of re-hospitalization, and increased costs associated with poor care.
Impact of nonadherence
Significant evidence supports the efficacy of antipsychotic medications for treating schizophrenia, of course,6 but that success story is undermined by the mean rate of medication nonadherence among schizophrenia patients, which can be as high as 49% in studies.7 (The actual rate might be higher because those studies do not account for persons who refuse treatment or drop out.)
Nonadherence increases the risk of relapse 3.7-fold, compared with what is seen in patients who adhere to treatment.8 Nonadherence to a medication regimen also can increase patients’ risk of engaging in assault and other dangerous behaviors, especially during periods of psychosis.8 Variables consistently associated with nonadherence include poor insight, negative attitude or subjective response toward medication, previous nonadherence, substance abuse, shorter duration of illness, inadequate discharge planning or after-care environment, and poorer therapeutic alliance.7,8
Investigation of medication adherence in bipolar disorder suggests that 1 in 3 patients fail to take at least 30% of their medication.9 In such patients, medication nonadherence can lead to mania, depression, hospital readmission, suicide, increased substance abuse, and nonresponse to treatment.10,11
Depression also is associated with an increased rate of health care utilization and severe limitation in daily functioning.12 Compared with non-depressed patients, depressed patients are 3 times more likely to be nonadherent with medical treatment recommendations.13 Estimates of medication nonadherence for unipolar and bipolar disorders range from 10% to 60% (median, 40%). This prevalence has not changed significantly with the introduction of new medications.14
Our literature review of research devoted to reducing no-shows found that few studies have explored this critical treatment concern. The no-show rate was higher among younger patients and slightly higher among women, but varied by diagnosis.15 The most common reason psychiatric patients gave for missing an appointment was “forgetting”—a response heard twice as often among no-show patients in psychiatry than in other specialties.4
Little has been tried to solve the problem. Often, community mental health centers and private practices double-book appointments. Double-booking is intended to reduce the financial burden on the practice when a patient misses an appointment. This approach fails to address nonadherence or the poor care that usually results when a patient misses regular outpatient appointments.
Several methods have been employed to improve adherence, such as electronic pill dispensing.16 Increasing medication adherence appears to be a key factor in improving quality-of-life measures in patients with schizophrenia.6
The UT project
Methods. This project was completed at the ambulatory psychiatry clinic at the UT Medical School at Houston. The clinic staff comprises residents and faculty members who provide outpatient care. During the study period, the clinic was scheduling as many as 800 office visits a month, including a mix of new and follow-up appointments. Two weeks’ retrospective data revealed a no-show rate of 31%.
For the project, we defined no-show rate as the total number of patients who missed an appointment or canceled fewer than 24 hours before the scheduled time, divided by the total number of patients scheduled that day.
Table 1 demonstrates the no-show rate calculations for 1 of the weeks preceding the start of the project. Given approximately 800 patient appointments a month, a 31% no-show rate meant that, first, 248 patients failed to receive recommended care and, second, 248 appointment slots were wasted.
Besides undermining such components of quality care as patient safety and medication compliance, the high no-show rate also harms employee morale and productivity; impairs medical education; and, possibly, increases the use of emergency and after-hour services.
We agreed that our current no-show rate of 31% was too high.
We then formed a team of residents, faculty members, therapists, front office staff, an office manager, and an office nurse. We explored and hypothesized what could be contributing to the high no-show rate (Table 2).
Several interventions were then devised and implemented:
• Patients. We increased patient education about 1) the need for regular follow-up and 2) risks associated with medication nonadherence.
• Environment. We explored environmental limitations to access and agreed that certain static factors could not be modified—eg, location of the clinic and lack of access to public transportation. We were able to make some changes to the environment (explained later) to reduce wait time.
• Staff. Some patients had complained of long wait times, which could hinder active participation in treatment. We agreed that the clinic nurse would make rounds through the waiting room every hour and talk to patients. The nurse would identify patients who had been waiting for longer than 30 minutes after their scheduled appointment time and notify the doctor accordingly. We also agreed to revise patient appointment reminder practices: instead of using an automated answering service, one of the staff members called patients personally to remind them about their appointments. (This also allowed us to update telephone numbers for many patients; numbers on record often were outdated.) We initially recruited summer interns and provided a written script to follow during calls to patients, which allowed patients to confirm, cancel, or reschedule their appointment. Once we demonstrated positive results from the change to personal calls, the department agreed to absorb the cost, and front desk personnel began making reminder calls.
• Policies and procedures. Although some practices are able to charge a small fine for missed appointments, this was not allowed at our institution. Instead, we had several departmental policies on the books, such as discharging patients from our clinics if they missed 3 consecutive appointments and limiting prescription refills to a maximum of 6 months. These policies were neither communicated to patients and staff, nor were they implemented. We decided to educate patients and staff and implement the policies.
• Transparency. We posted the no-show rate in common areas so that the team could review and follow the progression of that rate as we implemented the changes. This allowed team members to take ownership of the project and facilitated active participation.
By implementing these changes, we aimed to reduce the no-show rate to 20%.
Results. We were able to reduce the no-show rate from a documented average of 31% to an average of 12% during the study period after implementing all the proposed changes in the outpatient clinics.
We calculated the no-show rate (as shown in Table 1 for May 2013), then collected the daily no-show rate from June to September 2013 (Figure). With these calculations, we demonstrated a reduction in the no-show rate to 12%. Because of the time and effort required, we reduced data collection from daily to weekly, beginning in September.
Applying the changes required consistent effort and substantial input from various stakeholders—front desk staff, residents, the nurse, therapists, and faculty. Gradually, we were able to implement all the changes.
Keeping the no-show rate low required consistent effort and monitoring of the newly implemented procedures because even a slight change, such as failure to make reminder calls, resulted in a sudden increase in the no-show rate (that was the case in October of the study period, when we were short-staffed and could not call every patient). Patients told us that it was difficult to ignore a personal call; if they were not planning to keep the appointment, the call allowed them to reschedule on the spot.
We also made sure that current no-show rates were posted in common areas, visible to team members every day.
Discussion
We attempted a literature review of research exploring approaches to reducing the no-show rate but found few studies that explored this critical concern in patient treatment.15 Some data suggested that, in the setting studied, the no-show rate:
• was higher among younger patients (age 20 to 39) than older ones (age 60 to 79)
• was slightly higher in women than in men
• varied by diagnosis.
We found a paucity of data regarding interventions that can reduce the no-show rate.
Among the changes we made, the one that had the greatest impact was personalized appointment reminder calls, as evidenced by our patients’ reports and the increase in the no-show rate when personal calls were not made.
We also realized that, although we had several departmental policies in place regarding appointments, they were not being followed. Raising awareness among team members and their patients also was an effective deterrent to a no-show for an appointment. For example, patients were informed that 3 consecutive no-shows could lead to termination of care. Often, they reacted with surprise to this caution but also voiced a desire to improve their attendance to avoid such an outcome.
We found that establishing common operational definitions is important. It also was important to have a cohesive team, with every member agreeing on goals and changes to operational policies that needed to be implemented. Support from the department chair and the administration, we learned, is vital to the success of such an intervention.
A note about limitations. The goal of the project was limited to reducing the no-show rate. We demonstrated that this is possible among patients who have a severe mental illness, and that reducing the associated waste of time and resources can improve finances in an academic department of psychiatry. We would need additional measures, however, to quantify medication adherence and hospitalization; a larger, more inclusive project is needed to demonstrate that reducing the no-show rate reduces the symptomatic burden of psychiatric illness.
Comments in conclusion
This project was designed and conducted as a required part of a Clinical Safety and Effectiveness Program at Memorial Hermann Texas Medical Center and the UT Medical School at Houston.17 Although there was initial hesitancy about attempting to reduce the no-show rate in a chronically mentally ill population, the success of this project—indeed, it surpassed its proposed goals—demonstrates that operational changes in any clinic can reduce the no-show rate. It also is important to maintain operational changes, however; without consistent effort, desired results cannot be sustained.
Last, it is possible to replicate the methodology of this project and thereby attempt to reduce the no-show rate in other divisions of medicine that offer care to chronically ill patients, such as pediatrics and family medicine.
Bottom Line
Failure to attend routinely scheduled outpatient appointments increases a patient’s functional impairment and risk of hospitalization. Patient education, appointment reminder phone calls, revised policies and procedures, and transparency regarding the no-show rate can reduce the number of missed appointments and improve patient outcomes.
Related Resources
• Mitchell AJ, Selmes T. Why don’t patients attend their appointments? Maintaining engagement with psychiatric services. Advances in Psychiatric Treatment. 2007;13:423-434.
• Molfenter T. Reducing appointment no-shows: going from theory to practice. Subst Use Misuse. 2013;48(9):743-749.
• Williston MA, Block-Lerner J, Wolanin A, et al. Brief acceptance-based intervention for increasing intake attendance at a community mental health center. Psychol Serv. 2014;11(3):324-332.
Disclosure
Dr. Gajwani receives grant or research support from the National Institute on Mental Health, the National Institute of Drug Abuse, The Stanley Foundation, and Forest Laboratories, Inc. He is a member of the speakers’ bureau of AstraZeneca, Merck, Otsuka America Pharmaceutical, and Sunovion Pharmaceuticals.
The no-show rate is high in ambulatory psychiatric clinics, especially those associated with academic medical institutions, which usually accept all public insurance providers and do not maintain a strict rule by which patients are charged a penalty when they fail to keep a scheduled appointment—a policy that, to the contrary, is customary in private practice. The University of Texas (UT) Health Sciences Center at Houston is primarily an academic medical center with resident-managed, faculty-supervised clinics that provide care to a large volume of patients.
At the UT clinics, we have struggled with a high no-show rate, and were challenged to reduce that rate. Our study of the problem, formulation and application of strategies to reduce that rate, and a discussion of our results are provided here for the benefit of psychiatric clinicians who struggle with this problem, to the detriment of their patients’ health and the financial well-being of the practice.
For patients who have a severe psychiatric illness, such as schizophrenia or bipolar disorder, 60% to 70% of the direct cost of their care is attributable to inpatient services.1,2 Poor medication adherence is a critical factor: It results in exacerbation of symptoms, relapse, and hospitalization. The matter is compounded by patients’ failure to show up for scheduled follow-up appointments.
Studies show that failure to attend routinely scheduled outpatient appointments increases the risk of hospitalization. Recent research has shown that, among all causes of hospitalization, length of stay and relapse hospitalization are increased in patients with low adherence to their treatment regimen.3 Patients who miss an appointment also are more unwell and more functionally impaired—also contributing to a higher risk and rate of rehospitalization.4,5
To begin to address the problem at UT, we acknowledged that an elevated no-show rate is linked to medication nonadherence, increased risk of re-hospitalization, and increased costs associated with poor care.
Impact of nonadherence
Significant evidence supports the efficacy of antipsychotic medications for treating schizophrenia, of course,6 but that success story is undermined by the mean rate of medication nonadherence among schizophrenia patients, which can be as high as 49% in studies.7 (The actual rate might be higher because those studies do not account for persons who refuse treatment or drop out.)
Nonadherence increases the risk of relapse 3.7-fold, compared with what is seen in patients who adhere to treatment.8 Nonadherence to a medication regimen also can increase patients’ risk of engaging in assault and other dangerous behaviors, especially during periods of psychosis.8 Variables consistently associated with nonadherence include poor insight, negative attitude or subjective response toward medication, previous nonadherence, substance abuse, shorter duration of illness, inadequate discharge planning or after-care environment, and poorer therapeutic alliance.7,8
Investigation of medication adherence in bipolar disorder suggests that 1 in 3 patients fail to take at least 30% of their medication.9 In such patients, medication nonadherence can lead to mania, depression, hospital readmission, suicide, increased substance abuse, and nonresponse to treatment.10,11
Depression also is associated with an increased rate of health care utilization and severe limitation in daily functioning.12 Compared with non-depressed patients, depressed patients are 3 times more likely to be nonadherent with medical treatment recommendations.13 Estimates of medication nonadherence for unipolar and bipolar disorders range from 10% to 60% (median, 40%). This prevalence has not changed significantly with the introduction of new medications.14
Our literature review of research devoted to reducing no-shows found that few studies have explored this critical treatment concern. The no-show rate was higher among younger patients and slightly higher among women, but varied by diagnosis.15 The most common reason psychiatric patients gave for missing an appointment was “forgetting”—a response heard twice as often among no-show patients in psychiatry than in other specialties.4
Little has been tried to solve the problem. Often, community mental health centers and private practices double-book appointments. Double-booking is intended to reduce the financial burden on the practice when a patient misses an appointment. This approach fails to address nonadherence or the poor care that usually results when a patient misses regular outpatient appointments.
Several methods have been employed to improve adherence, such as electronic pill dispensing.16 Increasing medication adherence appears to be a key factor in improving quality-of-life measures in patients with schizophrenia.6
The UT project
Methods. This project was completed at the ambulatory psychiatry clinic at the UT Medical School at Houston. The clinic staff comprises residents and faculty members who provide outpatient care. During the study period, the clinic was scheduling as many as 800 office visits a month, including a mix of new and follow-up appointments. Two weeks’ retrospective data revealed a no-show rate of 31%.
For the project, we defined no-show rate as the total number of patients who missed an appointment or canceled fewer than 24 hours before the scheduled time, divided by the total number of patients scheduled that day.
Table 1 demonstrates the no-show rate calculations for 1 of the weeks preceding the start of the project. Given approximately 800 patient appointments a month, a 31% no-show rate meant that, first, 248 patients failed to receive recommended care and, second, 248 appointment slots were wasted.
Besides undermining such components of quality care as patient safety and medication compliance, the high no-show rate also harms employee morale and productivity; impairs medical education; and, possibly, increases the use of emergency and after-hour services.
We agreed that our current no-show rate of 31% was too high.
We then formed a team of residents, faculty members, therapists, front office staff, an office manager, and an office nurse. We explored and hypothesized what could be contributing to the high no-show rate (Table 2).
Several interventions were then devised and implemented:
• Patients. We increased patient education about 1) the need for regular follow-up and 2) risks associated with medication nonadherence.
• Environment. We explored environmental limitations to access and agreed that certain static factors could not be modified—eg, location of the clinic and lack of access to public transportation. We were able to make some changes to the environment (explained later) to reduce wait time.
• Staff. Some patients had complained of long wait times, which could hinder active participation in treatment. We agreed that the clinic nurse would make rounds through the waiting room every hour and talk to patients. The nurse would identify patients who had been waiting for longer than 30 minutes after their scheduled appointment time and notify the doctor accordingly. We also agreed to revise patient appointment reminder practices: instead of using an automated answering service, one of the staff members called patients personally to remind them about their appointments. (This also allowed us to update telephone numbers for many patients; numbers on record often were outdated.) We initially recruited summer interns and provided a written script to follow during calls to patients, which allowed patients to confirm, cancel, or reschedule their appointment. Once we demonstrated positive results from the change to personal calls, the department agreed to absorb the cost, and front desk personnel began making reminder calls.
• Policies and procedures. Although some practices are able to charge a small fine for missed appointments, this was not allowed at our institution. Instead, we had several departmental policies on the books, such as discharging patients from our clinics if they missed 3 consecutive appointments and limiting prescription refills to a maximum of 6 months. These policies were neither communicated to patients and staff, nor were they implemented. We decided to educate patients and staff and implement the policies.
• Transparency. We posted the no-show rate in common areas so that the team could review and follow the progression of that rate as we implemented the changes. This allowed team members to take ownership of the project and facilitated active participation.
By implementing these changes, we aimed to reduce the no-show rate to 20%.
Results. We were able to reduce the no-show rate from a documented average of 31% to an average of 12% during the study period after implementing all the proposed changes in the outpatient clinics.
We calculated the no-show rate (as shown in Table 1 for May 2013), then collected the daily no-show rate from June to September 2013 (Figure). With these calculations, we demonstrated a reduction in the no-show rate to 12%. Because of the time and effort required, we reduced data collection from daily to weekly, beginning in September.
Applying the changes required consistent effort and substantial input from various stakeholders—front desk staff, residents, the nurse, therapists, and faculty. Gradually, we were able to implement all the changes.
Keeping the no-show rate low required consistent effort and monitoring of the newly implemented procedures because even a slight change, such as failure to make reminder calls, resulted in a sudden increase in the no-show rate (that was the case in October of the study period, when we were short-staffed and could not call every patient). Patients told us that it was difficult to ignore a personal call; if they were not planning to keep the appointment, the call allowed them to reschedule on the spot.
We also made sure that current no-show rates were posted in common areas, visible to team members every day.
Discussion
We attempted a literature review of research exploring approaches to reducing the no-show rate but found few studies that explored this critical concern in patient treatment.15 Some data suggested that, in the setting studied, the no-show rate:
• was higher among younger patients (age 20 to 39) than older ones (age 60 to 79)
• was slightly higher in women than in men
• varied by diagnosis.
We found a paucity of data regarding interventions that can reduce the no-show rate.
Among the changes we made, the one that had the greatest impact was personalized appointment reminder calls, as evidenced by our patients’ reports and the increase in the no-show rate when personal calls were not made.
We also realized that, although we had several departmental policies in place regarding appointments, they were not being followed. Raising awareness among team members and their patients also was an effective deterrent to a no-show for an appointment. For example, patients were informed that 3 consecutive no-shows could lead to termination of care. Often, they reacted with surprise to this caution but also voiced a desire to improve their attendance to avoid such an outcome.
We found that establishing common operational definitions is important. It also was important to have a cohesive team, with every member agreeing on goals and changes to operational policies that needed to be implemented. Support from the department chair and the administration, we learned, is vital to the success of such an intervention.
A note about limitations. The goal of the project was limited to reducing the no-show rate. We demonstrated that this is possible among patients who have a severe mental illness, and that reducing the associated waste of time and resources can improve finances in an academic department of psychiatry. We would need additional measures, however, to quantify medication adherence and hospitalization; a larger, more inclusive project is needed to demonstrate that reducing the no-show rate reduces the symptomatic burden of psychiatric illness.
Comments in conclusion
This project was designed and conducted as a required part of a Clinical Safety and Effectiveness Program at Memorial Hermann Texas Medical Center and the UT Medical School at Houston.17 Although there was initial hesitancy about attempting to reduce the no-show rate in a chronically mentally ill population, the success of this project—indeed, it surpassed its proposed goals—demonstrates that operational changes in any clinic can reduce the no-show rate. It also is important to maintain operational changes, however; without consistent effort, desired results cannot be sustained.
Last, it is possible to replicate the methodology of this project and thereby attempt to reduce the no-show rate in other divisions of medicine that offer care to chronically ill patients, such as pediatrics and family medicine.
Bottom Line
Failure to attend routinely scheduled outpatient appointments increases a patient’s functional impairment and risk of hospitalization. Patient education, appointment reminder phone calls, revised policies and procedures, and transparency regarding the no-show rate can reduce the number of missed appointments and improve patient outcomes.
Related Resources
• Mitchell AJ, Selmes T. Why don’t patients attend their appointments? Maintaining engagement with psychiatric services. Advances in Psychiatric Treatment. 2007;13:423-434.
• Molfenter T. Reducing appointment no-shows: going from theory to practice. Subst Use Misuse. 2013;48(9):743-749.
• Williston MA, Block-Lerner J, Wolanin A, et al. Brief acceptance-based intervention for increasing intake attendance at a community mental health center. Psychol Serv. 2014;11(3):324-332.
Disclosure
Dr. Gajwani receives grant or research support from the National Institute on Mental Health, the National Institute of Drug Abuse, The Stanley Foundation, and Forest Laboratories, Inc. He is a member of the speakers’ bureau of AstraZeneca, Merck, Otsuka America Pharmaceutical, and Sunovion Pharmaceuticals.
1. Wyatt RJ, Henter I. An economic evaluation of manic-depressive illness—1991. Soc Psychiatry Psychiatr Epidemiol. 1995;30(5):213-219.
2. Wyatt RJ, Henter I, Leary MC, et al. An economic evaluation of schizophrenia—1991. Soc Psychiatry Psychiatr Epidemiol. 1995;30(5);196-205.
3. Offord S, Lin J, Wong B, et al. Impact of oral antipsychotic medication adherence on healthcare resource utilization among schizophrenic patients with medicare coverage. Community Ment Health J. 2013;49(6):625-629.
4. Killaspy H, Banerjee S, King M, et al. Prospective controlled study of psychiatric out-patient non-attendance: characteristics and outcome. Br J Psychiatry. 2000;176:160- 165.
5. Nelson EA, Maruish ME, Axler JL. Effects of discharge planning and compliance with outpatient appointments on readmission rates. Psychiatr Serv. 2000;51(7):885-889.
6. Thornley B, Adams C. Content and quality of 2000 controlled trials in schizophrenia over 50 years. BMJ. 1998;317(7167):1181-1184.
7. Lacro JP, Dunn LB, Dolder CR, et al. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literature. J Clin Psychiatry. 2002;63(10):892-909.
8. Fenton WS, Blyler C, Heinssen RK. Determinants of medication compliance in schizophrenia: empirical and clinical findings. Schizophr Bull. 1997;23(4):637-651.
9. Scott J, Pope M. Self-reported adherence to treatment with mood stabilizers, plasma levels, and psychiatric hospitalization. Am J Psychiatry. 2002;159(11):1927-1929.
10. Adams J, Scott J. Predicting medication adherence in severe mental disorders. Acta Psychiatr Scand. 2000;101(2):119-124.
11. Müller-Oerlinghausen B, Müser-Causemann B, Volk J. Suicides and parasuicides in a high-risk patient group on and off lithium long-term treatment. J Affect Disord. 1992;25(4):261-269.
12. Manning WG Jr, Wells KB. The effects of psychological distress and psychological well-being on use of medical services. Med Care. 1992;30(6):541-553.
13. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101-2107.
14. Lingam R, Scott J. Treatment non‐adherence in affective disorders. Acta Psychiatr Scand. 2002;105(3):164-172.
15. Allan AT. No-shows at a community mental health clinic: a pilot study. Int J Soc Psychiatry. 1988;34(1):40-46.
16. Cramer JA, Rosenheck R. Compliance with medication regimens for mental and physical disorders. Psychiatr Serv. 1998;49(2):196-201.
17. Gajwani P. Improving quality of care: reducing no-show rate in ambulatory psychiatry clinic. Poster presented at: American Psychiatric Association 166th Annual Meeting; May 18-22, 2013; San Francisco, CA.
1. Wyatt RJ, Henter I. An economic evaluation of manic-depressive illness—1991. Soc Psychiatry Psychiatr Epidemiol. 1995;30(5):213-219.
2. Wyatt RJ, Henter I, Leary MC, et al. An economic evaluation of schizophrenia—1991. Soc Psychiatry Psychiatr Epidemiol. 1995;30(5);196-205.
3. Offord S, Lin J, Wong B, et al. Impact of oral antipsychotic medication adherence on healthcare resource utilization among schizophrenic patients with medicare coverage. Community Ment Health J. 2013;49(6):625-629.
4. Killaspy H, Banerjee S, King M, et al. Prospective controlled study of psychiatric out-patient non-attendance: characteristics and outcome. Br J Psychiatry. 2000;176:160- 165.
5. Nelson EA, Maruish ME, Axler JL. Effects of discharge planning and compliance with outpatient appointments on readmission rates. Psychiatr Serv. 2000;51(7):885-889.
6. Thornley B, Adams C. Content and quality of 2000 controlled trials in schizophrenia over 50 years. BMJ. 1998;317(7167):1181-1184.
7. Lacro JP, Dunn LB, Dolder CR, et al. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literature. J Clin Psychiatry. 2002;63(10):892-909.
8. Fenton WS, Blyler C, Heinssen RK. Determinants of medication compliance in schizophrenia: empirical and clinical findings. Schizophr Bull. 1997;23(4):637-651.
9. Scott J, Pope M. Self-reported adherence to treatment with mood stabilizers, plasma levels, and psychiatric hospitalization. Am J Psychiatry. 2002;159(11):1927-1929.
10. Adams J, Scott J. Predicting medication adherence in severe mental disorders. Acta Psychiatr Scand. 2000;101(2):119-124.
11. Müller-Oerlinghausen B, Müser-Causemann B, Volk J. Suicides and parasuicides in a high-risk patient group on and off lithium long-term treatment. J Affect Disord. 1992;25(4):261-269.
12. Manning WG Jr, Wells KB. The effects of psychological distress and psychological well-being on use of medical services. Med Care. 1992;30(6):541-553.
13. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101-2107.
14. Lingam R, Scott J. Treatment non‐adherence in affective disorders. Acta Psychiatr Scand. 2002;105(3):164-172.
15. Allan AT. No-shows at a community mental health clinic: a pilot study. Int J Soc Psychiatry. 1988;34(1):40-46.
16. Cramer JA, Rosenheck R. Compliance with medication regimens for mental and physical disorders. Psychiatr Serv. 1998;49(2):196-201.
17. Gajwani P. Improving quality of care: reducing no-show rate in ambulatory psychiatry clinic. Poster presented at: American Psychiatric Association 166th Annual Meeting; May 18-22, 2013; San Francisco, CA.
Delirium in the hospital: Emphasis on the management of geriatric patients
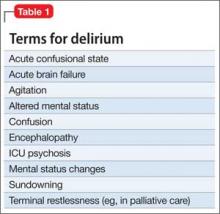
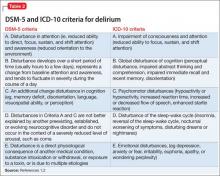
Although delirium has many descriptive terms (Table 1), a common unifying term is “acute global cognitive dysfunction,” now recognized as delirium; a consensus supported by DSM-51 and ICD-102 (Table 2). According to DSM-5, the essential feature is a disturbance of attention or awareness that is accompanied by a change in baseline cognition that cannot be explained by another preexisting, established, or evolving neurocognitive disorder (the newly named DSM-5 entity for dementia syndromes).1 Because delirium affects the cortex diffusely, psychiatric symptoms can include cognitive, mood, anxiety, or psychotic symptoms. Because many systemic illnesses can induce delirium, the differential diagnosis spans all organ systems.
Three subtypes
Delirium can be classified, based on symptoms,3,4 into 3 subtypes: hyperactive-hyperalert, hypoactive-hypoalert, and mixed delirium. Hyperactive patients present with restlessness and agitation. Hypoactive patients are lethargic, confused, slow to respond to questions, and often appear depressed. The differential prognostic significance of these subtypes has been examined in the literature, with conflicting results. Rabinowitz5 reported that hypoactive delirium has the worst prognosis, while Marcantonio et al6 indicated that the hyperactive subtype is associated with the highest mortality rate. Mixed delirium, with periods of both hyperactivity and hypoactivity, is the most common type of delirium.7
A prodromal phase, characterized by anxiety, frequent requests for nursing and medical assistance, decreased attention, restlessness, vivid dreams, disorientation immediately after awakening, and hallucinations, can occur before an episode of full-spectrum delirium; this prodromal state often is identified retrospectively —after the patient is in an episode of delirium.8,9
Evidence-based guidelines aim to improve recognition and clinical management.10-13 Disruptive behavior is the main reason for psychiatric referral in delirium.14,15 Delayed psychiatric consultation because of non-recognition of delirium is related to variables such as older age; history of a pre-existing, comorbid neurocognitive disorder; and the clinical appearance of hypoactive delirium.14
The case of Mr. D (Box),16 illustrates how the emergence of antipsychotic-associated neuroleptic malignant syndrome (NMS) can complicate antipsychotic treatment of delirium in a geriatric medical patient, although delirium also is a common presentation in NMS.17 Delirium developed after an increase in carbidopa/levodopa, which has central dopaminergic effects that can precipitate delirium, particularly in a geriatric patient with preexisting comorbid neurocognitive disorder. Further complicating Mr. D’s delirium presentation was the development of NMS, which had a multifactorial causation, such as the use of dopamine antagonists (ie, quetiapine, metoclopramide), and an abrupt decrease of a dopaminergic agent (ie, carbidopa/levodopa), all inducing a central dopamine relative hypoactivity.
Epidemiology
Delirium is more common in older patients,15 and is seen in 30% to 40% of hospitalized geriatric patients.18 Delirium in older patients, compared with other adults, is associated with more severe cognitive impairment.19 It is common among geriatric surgical patients (15% to 62%)20 with a peak 2 to 5 days postoperatively for hip fracture,21 and often is seen in ICU patients (70% to 87%).20 However, Spronk et al22 found that delirium is significantly under-recognized in the ICU. Nearly 90% of terminally ill patients become delirious before death.23 Terminal delirium often is unrecognized and can interfere with assessment of other clinical problems.24 A preexisting history of comorbid neurocognitive disorder was evident in as many as two-thirds of delirium cases.25
Pathophysiology and risk factors
The pathophysiology of delirium has been characterized as an imbalance of CNS metabolism, including decreased blood flow in various regions of the brain that may normalize once delirium resolves.26 Studies describe the simultaneous decrease of cholinergic transmission and dopaminergic excess.27,28 Predisposing and precipitating factors for delirium that are of particular importance in geriatric patients include:
• advanced age
• CNS disease
• infection
• cognitive impairment
• male sex
• poor nutrition
• dehydration and other metabolic abnormalities
• cardiovascular events
• substance use
• medication
• sensory deprivation (eg, impaired vision or hearing)
• sleep deprivation
• low level of physical activity.27,29,30
Table 3 lists the most common delirium-provocative medications.27
Evaluation and psychometric scales
The EEG can be useful in evaluating delirium, especially in clinically ambiguous cases. EEG findings may indicate generalized slowing or dropout of the posterior dominant rhythm, and generalized slow theta and delta waves, findings that are more common in delirium than in other neurocognitive disorders and other psychiatric illnesses. The EEG must be interpreted in the context of the delirium diagnostic workup, because abnormalities seen in other neurocognitive disorders can overlap with those of delirium.31
The EEG referral should specify the clinical suspicion of delirium to help interpret the results. Delirium cases in which the patient’s previous cognitive status is unknown may benefit from EEG evaluation, such as:
• in possible status epilepticus
• when delirium improvement has reached a plateau at a lower level of cognitive function than before onset of delirium
• when the patient is unable or unwilling to complete a psychiatric interview.27
Assessment instruments are available to diagnose and monitor delirium (Table 4). Typically, delirium assessment includes examining levels of arousal, psychomotor activity, cognition (ie, orientation, attention, and memory), and perceptual disturbances.
Psychometrically, a review of Table 4 suggests that validity appeared stable with adequate specificity (64% to 99%) but more variable sensitivity (36% to 100%). These reliability parameters also will be affected by the classification system (ie, DSM vs ICD) and the cut-off score employed.32 Most measures (eg, Confusion Assessment Method [CAM], CAM-ICU) provide an adequate sample of behavioral (ie, level of alertness), motor (ie, psychomotor activity), and cognitive (ie, orientation, attention, memory, and receptive language) function, with the exception of the Global Attentiveness Rating, which is a 2-minute open conversation protocol between physician and patient.
Some measures are stand-alone instruments, such as the Memorial Delirium Assessment Scale, whereas the CAM requires administration of separate cognitive screens, including the Mini-Mental State Examination (MMSE) and Digit Span.33 Instruments to detect delirium in critically ill patients are a more recent development. Wong et al34 reported that the most widely studied tool was the CAM. Obtaining collateral information from family, caregivers, and hospital staff is essential, particularly given the fluctuating nature of delirium.
Management
Prevention. Identify patients at high risk of delirium so that preventive strategies can be employed. Multi-component, nonpharmacotherapeutic interventions are used in clinical settings but few randomized trials have been conducted. The contributing effectiveness of individual components is not well-studied, but most include staff education to increase awareness of delirium. Of 3 multi-component intervention randomized trials, 2 reported a significantly lower incidence of delirium in the intervention group.35-37 Implementation of a multi-component protocol in medical/ surgical units was associated with a significant reduction in use of restraints.38
As in Mr. D’s case, complex drug regimens, particularly for CNS illness, can increase the risk of delirium. Considering the medication profile for patients with complex systemic illness—in particular, minimizing the use anticholinergics and dopamine agonists— may be crucial in preventing delirium.
Prophylactic administration of antipsychotics may reduce the risk of developing postoperative delirium.39 Studies of the use of these agents were characterized by small sample sizes and selected groups of patient populations. Of the 4 randomized studies evaluating prophylactic antipsychotics (vs placebo), 3 found a lower incidence of delirium in the intervention groups.39-41
A study of haloperidol in post-GI surgery patients showed a reduced occurrence of delirium,40 whereas its prophylactic use in patients undergoing hip surgery42 did not reduce the incidence of delirium compared with placebo, but did decrease severity when delirium occurred.42
Risperidone39 in post-cardiac surgery and olanzapine41 perioperatively in patients undergoing total knee or hip replacement have been shown to decrease delirium severity and duration. Targeted prophylaxis with risperidone43 in post-cardiac surgery patients who showed disturbed cognition but did not meet criteria for delirium reduced the number of patients requiring medication, compared with placebo.43
Dexmedetomidine, an α-2 adrenergic receptor agonist, compared with propofol or midazolam in post-cardiac valve surgery patients, resulted in a decreased incidence of delirium but no difference in delirium duration, hospital length of stay, or use of other medications.44 However, other studies have shown that dexmedetomidine reduces ICU length of stay and duration of mechanical ventilation.45
Treatment. Management of hospitalized medically ill geriatric patients with delirium is challenging and requires a comprehensive approach. The first step in delirium management is prompt identification and management of systemic medical disturbances associated with the delirium episode. First-line, nonpharmacotherapeutic strategies for patients with delirium include:
• reorientation
• behavioral interventions (eg, use of clear instructions and frequent eye contact with patients)
• environmental interventions (eg, minimal noise, adequate lighting, and limited room and staff changes)
• avoidance of physical restraints.46
Consider employing family members or hospital staff sitters to stay with the patient and to reassure, reorient, and watch for agitation and other unsafe behaviors (eg, attempted elopement). Psychoeducation for the patient and family on the phenomenology of delirium can be helpful.
The use of drug treatment strategies should be integrated into a comprehensive approach that includes the routine use of nondrug measures.46 Using medications for treating hypoactive delirium, formerly controversial, now has wider acceptance.47,48 A few high-quality randomized trials have been performed.25,49,50
Pharmacotherapy, especially in frail patients, should be initiated at the lowest starting dosage and titrated cautiously to clinical effect and for the shortest period of time necessary. Antipsychotics are preferred agents for treating all subtypes of delirium; haloperidol is widely used.46,51,52 However, antipsychotics, including haloperidol, can be associated with adverse neurologic effects such as extrapyramidal symptoms (EPS) and NMS.
Although reported less frequently than with haloperidol, other agents have been implicated in development of EPS and NMS, including atypical antipsychotics and antiemetic dopamine antagonists, particularly in parkinsonism-prone patients.53 Strategies that can minimize such risks in geriatric inpatients with delirium include oral, rather than parenteral, use of antipsychotics—preferential use of atypical over typical antipsychotics— and lowest effective dosages.54
In controlled trials, atypical antipsychotics for delirium showed efficacy compared with haloperidol.52,55 However, there is no research that demonstrates any advantage of one atypical over another.25
In Mr. D’s case, the most important intervention for managing delirium caused by NMS is to discontinue all dopamine antagonists and treat agitation with judicious doses of a benzodiazepine, with supportive care.17 In cases of sudden discontinuation or a dosage decrease of dopamine agonists, these medications should be resumed or optimized to minimize the risk of NMS-associated rhabdomyolysis and subsequent renal failure.17 Antipsychotics carry an increased risk of stroke and mortality in older patients with established or evolving neurocognitive disorders56,57 and can cause prolongation of the QTc interval.57
Other medications that could be used for delirium include cholinesterase inhibitors58,59 (although larger trials and a systematic review did not support this use60), and 5-HT receptor antagonists,61 such as trazodone. Benzodiazepines, such as lorazepam, are first-line treatment for delirium associated with seizures or withdrawal from alcohol, sedatives, hypnotics, and anxiolytics and for delirium caused by NMS. Be cautious about using benzodiazepines in geriatric patients because of a risk of respiratory depression, falls, sedation, and amnesia.
Geriatric patients with alcoholism and those with malnutrition are prone to thiamine and vitamin B12 deficiencies, which can induce delirium. Laboratory assessment and consideration of supplementation is recommended. Despite high occurrence of delirium in hospitalized older adults with preexisting comorbid neurocognitive disorders, there is no standard care for delirium comorbid with another neurocognitive disorder.62 Clinical practice guidelines for older patients receiving palliative care have been developed63; the goal is to minimize suffering and discomfort in patients in palliative care.64
Post-delirium prophylaxis. Medications for delirium usually can be tapered and discontinued once the episode has resolved and the patient is stable; it is common to discontinue medications when the patient has been symptom-free for 1 week.65 Some patients (eg, with end-stage liver disease, disseminated cancer) are prone to recurrent or to prolonged or chronic delirium. A period of post-recovery treatment with antipsychotics—even indefinite treatment in some cases—should be considered.
Post-delirium debriefing and aftercare. The psychological complications of delirium are distressing for the patient and his (her) caregivers. Psychiatric complications associated with delirium, including acute stress disorder—which might predict posttraumatic stress disorder—have been explored; early recognition and treatment may improve long-term outcomes.66 After recovery from acute delirium, cognitive assessment (eg, MMSE67 or Montreal Cognitive Assessment68) is recommended to validate current cognitive status because patients may have persistent decrement in cognitive function compared with pre-delirium condition, even after recovery from the acute episode.
Post-delirium debriefing may help patients who have recovered from a delirium episode. Patients may fear that their brief period of hallucinations might represent the onset of a chronic-relapsing psychotic disorder. Allow patients to communicate their distress about the delirium episode and give them the opportunity to talk through the experience. Brief them on the possibility that delirium will recur and advise them to seek emergency medical care in case of recurrence. Advise patients to monitor and maintain a normal sleep-wake cycle.
Family members can watch for syndromal recurrence of delirium. They should be encouraged to discuss their reaction to having seen their relative in a delirious state.
Health care systems with integrated electronic medical records should list “delirium, resolved” on the patient’s illness profile or problem list and alert the patient’s primary care provider to the delirium history to avoid future exposure to delirium-provocative medications, and to prompt the provider to assume an active role in post-delirium care, including delirium recurrence surveillance, medication adjustment, risk factor management, and post-recovery cognitive assessment.
Bottom Line
Evaluation of delirium in geriatric patients includes clinical vigilance and screening, differentiating delirium from other neurocognitive disorders, and identifying and treating underlying causes. Perioperative use of antipsychotics may reduce the incidence of delirium, although hospital length of stay generally has not been reduced with prophylaxis. Management interventions include staff education, systematic screening, use of multicomponent interventions, and pharmacologic interventions.
Related Resources
• Downing LJ, Caprio TV, Lyness JM. Geriatric psychiatry review: differential diagnosis and treatment of the 3 D’s - delirium, dementia, and depression. Curr Psychiatry Rep. 2013;15(6):365.
• Brooks PB. Postoperative delirium in elderly patients. Am J Nurs. 2012;112(9):38-49.
Drug Brand Names
Carbidopa/levodopa • Sinemet Midazolam • Versed
Dexmedetomidine • Precedex Olanzapine • Zyprexa
Haloperidol • Haldol Propofol • Diprivan
Lithium • Eskalith, Lithobid Quetiapine • Seroquel
Lorazepam • Ativan Risperidone • Risperdal
Metoclopramide • Reglan Trazodone • Desyrel
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders, fifth edition. Washington, DC: American Psychiatric Association; 2013.
2. World Health Organization. The ICD-10 classification of mental and behavioural disorders. Diagnostic criteria for research. Geneva, Switzerland: WHO; 1993.
3. Lipowski ZJ. Delirium in the elderly patient. N Engl J Med. 1989;320(9):578-582.
4. Meagher DJ, Trzepacz PT. Motoric subtypes of delirium. Semin Clin Neuropsychiatry. 2000;5(2):75-85.
5. Rabinowitz T. Delirium: an important (but often unrecognized) clinical syndrome. Curr Psychiatry Rep. 2002;4(3):202-208.
6. Marcantonio ER, Ta T, Duthie E, et al. Delirium severity and psychomotor types: their relationship with outcomes after hip fracture repair. Am J Geriatr Soc. 2002;50(5):850-857.
7. Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA. 2001;286(21):2703-2710.
8. Duppils GS, Wikblad K. Delirium: behavioural changes before and during the prodromal phase. J Clin Nurs. 2004;13(5):609-616.
9. de Jonghe JF, Kalisvaart KJ, Dijkstra M, et al. Early symptoms in the prodromal phase of delirium: a prospective cohort study in elderly patients undergoing hip surgery. Am J Geriatr Psychiatry. 2007;15(2):112-121.
10. Cook IA. Guideline watch: practice guideline for the treatment of patients with delirium. Arlington, VA: American Psychiatric Publishing; 2004.
11. Hogan D, Gage L, Bruto V, et al. National guidelines for seniors’ mental health: the assessment and treatment of delirium. Canadian Journal of Geriatrics. 2006;9(suppl 2):S42-51.
12. Leentjens AF, Diefenbacher A. A survey of delirium guidelines in Europe. J Psychosom Res. 2006;61(1):123-128.
13. Tropea J, Slee JA, Brand CA, et al. Clinical practice guidelines for the management of delirium in older people in Australia. Australas J Ageing. 2008;27(3):150-156.
14. Mittal D, Majithia D, Kennedy R, et al. Differences in characteristics and outcome of delirium as based on referral patterns. Psychosomatics. 2006;47(5):367-375.
15. Grover S, Subodh BN, Avasthi A, et al. Prevalence and clinical profile of delirium: a study from a tertiary-care hospital in north India. Gen Hosp Psychiatry. 2009;31(1): 25-29.
16. Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12): 941-948.
17. Strawn JR, Keck PE Jr, Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870-876.
18. Dobmejer K. Delirium in elderly medical patients. Clinical Geriatrics. 1996;4:43-68.
19. Leentjens AF, Maclullich AM, Meagher DJ. Delirium, Cinderella no more...? J Psychosom Res. 2008;65(3):205.
20. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol. 2009;5(4):210-220.
21. Streubel PN, Ricci WM, Gardner MJ. Fragility fractures: preoperative, perioperative, and postoperative management. Current Orthopaedic Practice. 2009;20(5):482-489.
22. Spronk PE, Riekerk B, Hofhuis J, et al. Occurrence of delirium is severely underestimated in the ICU during daily care. Intensive Care Med. 2009;35(7):1276-1280.
23. Lawlor PG, Gagnon B, Mancini IL, et al. Occurrence, causes, and outcome of delirium in patients with advanced cancer: a prospective study. Arch Intern Med. 2000;160(6):786-794.
24. Ganzini L. Care of patients with delirium at the end of life. Annals of Long-Term Care. 2007;15(3):35-40.
25. Bourne RS, Tahir TA, Borthwick M, et al. Drug treatment of delirium: past, present and future. J Psychosom Res. 2008;65(3):273-282.
26. Yokota H, Ogawa S, Kurokawa A, et al. Regional cerebral blood flow in delirium patients. Psychiatry Clin Neurosci. 2003;57(3):337-339.
27. Maldonado JR. Pathoetiological model of delirium: a comprehensive understanding of the neurobiology of delirium and an evidence-based approach to prevention and treatment. Crit Care Clin. 2008;24(4):789-856, ix.
28. Trzepacz PT. Is there a final common neural pathway in delirium? Focus on acetylcholine and dopamine. Semin Clin Neuropsychiatry. 2000;5(2):132-148.
29. Inouye SK. The dilemma of delirium: clinical and research controversies regarding diagnosis and evaluation of delirium in hospitalized elderly medical patients. Am J Med. 1994;97(3):278-288.
30. Laurila JV, Laakkonen ML, Tilvis RS, et al. Predisposing and precipitating factors for delirium in a frail geriatric population. J Psychosom Res. 2008;65(3):249-254.
31. Morandi A, McCurley J, Vasilevskis EE, et al. Tools to detect delirium superimposed on dementia: a systematic review. J Am Geriatr Soc. 2012;60(11):2005-2013.
32. Kazmierski J, Kowman M, Banach M, et al. The use of DSM-IV and ICD-10 criteria and diagnostic scales for delirium among cardiac surgery patients: results from the IPDACS study. J Neuropsychiatry Clin Neurosci. 2010; 22(4):426-432.
33. Breitbart W, Rosenfeld B, Roth A, et al. The Memorial Delirium Rating Scale. J Pain Symptom Manage. 1997;13(3):128-137.
34. Wong CL, Holroyd-Leduc J, Simel DL, et al. Does this patient have delirium?: value of bedside instruments. JAMA. 2010;304(7):779-786.
35. Marcantonio ER, Flacker JM, Wright RJ, et al. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2011;49(5):516-522.
36. Lundström M, Edlund A, Karlsson S, et al. A multifactorial intervention program reduces the duration of delirium, length of hospitalization, and mortality in delirious patients. J Am Geriatr Soc. 2005;53(4):622-628.
37. Lundström M, Olofsson B, Stenvall M, et al. Postoperative delirium in old patients with femoral neck fracture: a randomized intervention study. Aging Clin Exp Res. 2007; 19(3):178-186.
38. Kratz A. Use of the acute confusion protocol: a research utilization project. J Nurs Care Qual. 2008;23(4):331-337.
39. Prakanrattana U, Prapaitrakool S. Efficacy of risperidone for prevention of postoperative delirium in cardiac surgery. Anaesth Intensive Care. 2007;35(5):714-719.
40. Kaneko T, Cai J, Ishikura T, et al. Prophylactic consecutive administration of haloperidol can reduce the occurrence of postoperative delirium in gastrointestinal surgery. Yonago Acta Medica. 1999;42:179-184.
41. Larsen KA, Kelly SE, Stern TA, et al. Administration of olanzapine to prevent postoperative delirium in elderly joint-replacement patients: a randomized, controlled trial. Psychosomatics. 2010;51(5):409-418.
42. Kalisvaart KJ, de Jonghe JF, Bogaards MJ, et al. Haloperidol prophylaxis for elderly hip-surgery patients at risk for delirium: a randomized placebo-controlled study. J Am Geriatr Soc. 2005;53(10):1658-1666.
43. Hakim SM, Othman AI, Naoum DO. Early treatment with risperidone for subsyndromal delirium after on-pump cardiac surgery in the elderly: a randomized trial. Anesthesiology. 2012;116(5):987-997.
44. Maldonado JR, Wysong A, van der Starre PJ, et al. Dexmedetomidine and the reduction of postoperative delirium after cardiac surgery. Psychosomatics. 2009;50(3): 206-217.
45. Short J. Use of dexmedetomidine for primary sedation in a general intensive care unit. Crit Care Nurse. 2010;30(1): 29-38; quiz 39.
46. Practice guideline for the treatment of patients with delirium. American Psychiatric Association [Comment in: Treatment of patients with delirium. Am J Psychiatry. 2000.]. Am J Psychiatry. 1999;156(suppl 5):1-20.
47. Maldonado JR. Delirium in the acute care setting: characteristics, diagnosis, and treatment. Crit Care Clin. 2008;24(4):657-722, vii.
48. Platt MM, Breitbart W, Smith M, et al. Efficacy of neuroleptics for hypoactive delirium. J Neuropsychiatry Clin Neurosci. 1994;6(1):66-67.
49. Lonergan E, Britton AM, Luxenberg J, et al. Antipsychotics for delirium. Cochrane Database Syst Rev. 2007;(2):CD005594.
50. Seitz DP, Gill SS, van Zyl LT. Antipsychotics in the treatment of delirium: a systematic review. J Clin Psychiatry. 2007;68(1):11-21.
51. Breitbart W, Marotta R, Platt MM, et al. A double-blind trial of haloperidol, chlorpromazine, and lorazepam in the treatment of delirium in hospitalized AIDS patients. Am J Psychiatry. 1996;153(2):231-237.
52. Hu H, Deng W, Yang H, et al. Olanzapine and haloperidol for senile delirium: a randomized controlled observation. Chinese Journal of Clinical Rehabilitation. 2006;10(42): 188-190.
53. Friedman JH, Fernandez HH. Atypical antipsychotics in Parkinson-sensitive populations. J Geriatr Psychiatry Neurol. 2002;15(3):156-170.
54. Seitz DP, Gill SS. Neuroleptic malignant syndrome complicating antipsychotic treatment of delirium or agitation in medical and surgical patients: case reports and a review of the literature. Psychosomatics. 2009; 50(1):8-15.
55. Han CS, Kim YK. A double-blind trial of risperidone and haloperidol for the treatment of delirium. Psychosomatics. 2004;45(4):297-301.
56. Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA. 2005;293(5):596-608.
57. Hermann N, Lanctôt KL. Atypical antipsychotics for neuropsychiatric symptoms of dementia: malignant or maligned? Drug Saf. 2006;29(10):833-843.
58. Noyan MA, Elbi H, Aksu H. Donepezil for anticholinergic drug intoxication: a case report. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(5):885-887.
59. Gleason OC. Donepezil for postoperative delirium. Psychosomatics. 2003;44(5):437-438.
60. Overshott R, Karim S, Burns A. Cholinesterase inhibitors for delirium. Cochrane Database Syst Rev. 2008;(1): CD005317.
61. Davis MP. Does trazodone have a role in palliating symptoms? Support Care Cancer. 2007;15(2):221-224.
62. Fick DM, Agostini JV, Inouye SK. Delirium superimposed on dementia: a systematic review. J Am Geriatr Soc. 2002; 50(10):1723-1732.
63. Brajtman S, Wright D, Hogan D, et al. Developing guidelines for the assessment and treatment of delirium in older adults at the end of life. Can Geriatr J. 2011;14(2):40-50.
64. Caraceni A, Simonetti F. Palliating delirium in patients with cancer. Lancet Oncol. 2009;10(2):164-172.
65. Alexopoulos GS, Streim J, Carpenter D, et al; Expert Consensus Panel for using Antipsychotic Drugs in Older Patients. Using antipsychotic agents in older patients. J Clin Psychiatry. 2004;65(suppl 2):5-99; discussion 100-102; quiz 103-104.
66. Granja C, Gomes E, Amaro A, et al. Understanding posttraumatic stress disorder-related symptoms after critical care: the early illness amnesia hypothesis. Crit Care Med. 2008;36(10):2801-2809.
67. Ringdal GI, Ringdal K, Juliebø V, et al. Using the Mini- Mental State Examination to screen for delirium in elderly patients with hip fracture. Dement Geriatr Cogn Disord. 2011;32(6):394-400.
68. Olson RA, Chhanabhai T, McKenzie M. Feasibility study of the Montreal Cognitive Assessment (MoCA) in patients with brain metastases. Support Care Cancer. 2008;16(11):1273-1278.
Although delirium has many descriptive terms (Table 1), a common unifying term is “acute global cognitive dysfunction,” now recognized as delirium; a consensus supported by DSM-51 and ICD-102 (Table 2). According to DSM-5, the essential feature is a disturbance of attention or awareness that is accompanied by a change in baseline cognition that cannot be explained by another preexisting, established, or evolving neurocognitive disorder (the newly named DSM-5 entity for dementia syndromes).1 Because delirium affects the cortex diffusely, psychiatric symptoms can include cognitive, mood, anxiety, or psychotic symptoms. Because many systemic illnesses can induce delirium, the differential diagnosis spans all organ systems.
Three subtypes
Delirium can be classified, based on symptoms,3,4 into 3 subtypes: hyperactive-hyperalert, hypoactive-hypoalert, and mixed delirium. Hyperactive patients present with restlessness and agitation. Hypoactive patients are lethargic, confused, slow to respond to questions, and often appear depressed. The differential prognostic significance of these subtypes has been examined in the literature, with conflicting results. Rabinowitz5 reported that hypoactive delirium has the worst prognosis, while Marcantonio et al6 indicated that the hyperactive subtype is associated with the highest mortality rate. Mixed delirium, with periods of both hyperactivity and hypoactivity, is the most common type of delirium.7
A prodromal phase, characterized by anxiety, frequent requests for nursing and medical assistance, decreased attention, restlessness, vivid dreams, disorientation immediately after awakening, and hallucinations, can occur before an episode of full-spectrum delirium; this prodromal state often is identified retrospectively —after the patient is in an episode of delirium.8,9
Evidence-based guidelines aim to improve recognition and clinical management.10-13 Disruptive behavior is the main reason for psychiatric referral in delirium.14,15 Delayed psychiatric consultation because of non-recognition of delirium is related to variables such as older age; history of a pre-existing, comorbid neurocognitive disorder; and the clinical appearance of hypoactive delirium.14
The case of Mr. D (Box),16 illustrates how the emergence of antipsychotic-associated neuroleptic malignant syndrome (NMS) can complicate antipsychotic treatment of delirium in a geriatric medical patient, although delirium also is a common presentation in NMS.17 Delirium developed after an increase in carbidopa/levodopa, which has central dopaminergic effects that can precipitate delirium, particularly in a geriatric patient with preexisting comorbid neurocognitive disorder. Further complicating Mr. D’s delirium presentation was the development of NMS, which had a multifactorial causation, such as the use of dopamine antagonists (ie, quetiapine, metoclopramide), and an abrupt decrease of a dopaminergic agent (ie, carbidopa/levodopa), all inducing a central dopamine relative hypoactivity.
Epidemiology
Delirium is more common in older patients,15 and is seen in 30% to 40% of hospitalized geriatric patients.18 Delirium in older patients, compared with other adults, is associated with more severe cognitive impairment.19 It is common among geriatric surgical patients (15% to 62%)20 with a peak 2 to 5 days postoperatively for hip fracture,21 and often is seen in ICU patients (70% to 87%).20 However, Spronk et al22 found that delirium is significantly under-recognized in the ICU. Nearly 90% of terminally ill patients become delirious before death.23 Terminal delirium often is unrecognized and can interfere with assessment of other clinical problems.24 A preexisting history of comorbid neurocognitive disorder was evident in as many as two-thirds of delirium cases.25
Pathophysiology and risk factors
The pathophysiology of delirium has been characterized as an imbalance of CNS metabolism, including decreased blood flow in various regions of the brain that may normalize once delirium resolves.26 Studies describe the simultaneous decrease of cholinergic transmission and dopaminergic excess.27,28 Predisposing and precipitating factors for delirium that are of particular importance in geriatric patients include:
• advanced age
• CNS disease
• infection
• cognitive impairment
• male sex
• poor nutrition
• dehydration and other metabolic abnormalities
• cardiovascular events
• substance use
• medication
• sensory deprivation (eg, impaired vision or hearing)
• sleep deprivation
• low level of physical activity.27,29,30
Table 3 lists the most common delirium-provocative medications.27
Evaluation and psychometric scales
The EEG can be useful in evaluating delirium, especially in clinically ambiguous cases. EEG findings may indicate generalized slowing or dropout of the posterior dominant rhythm, and generalized slow theta and delta waves, findings that are more common in delirium than in other neurocognitive disorders and other psychiatric illnesses. The EEG must be interpreted in the context of the delirium diagnostic workup, because abnormalities seen in other neurocognitive disorders can overlap with those of delirium.31
The EEG referral should specify the clinical suspicion of delirium to help interpret the results. Delirium cases in which the patient’s previous cognitive status is unknown may benefit from EEG evaluation, such as:
• in possible status epilepticus
• when delirium improvement has reached a plateau at a lower level of cognitive function than before onset of delirium
• when the patient is unable or unwilling to complete a psychiatric interview.27
Assessment instruments are available to diagnose and monitor delirium (Table 4). Typically, delirium assessment includes examining levels of arousal, psychomotor activity, cognition (ie, orientation, attention, and memory), and perceptual disturbances.
Psychometrically, a review of Table 4 suggests that validity appeared stable with adequate specificity (64% to 99%) but more variable sensitivity (36% to 100%). These reliability parameters also will be affected by the classification system (ie, DSM vs ICD) and the cut-off score employed.32 Most measures (eg, Confusion Assessment Method [CAM], CAM-ICU) provide an adequate sample of behavioral (ie, level of alertness), motor (ie, psychomotor activity), and cognitive (ie, orientation, attention, memory, and receptive language) function, with the exception of the Global Attentiveness Rating, which is a 2-minute open conversation protocol between physician and patient.
Some measures are stand-alone instruments, such as the Memorial Delirium Assessment Scale, whereas the CAM requires administration of separate cognitive screens, including the Mini-Mental State Examination (MMSE) and Digit Span.33 Instruments to detect delirium in critically ill patients are a more recent development. Wong et al34 reported that the most widely studied tool was the CAM. Obtaining collateral information from family, caregivers, and hospital staff is essential, particularly given the fluctuating nature of delirium.
Management
Prevention. Identify patients at high risk of delirium so that preventive strategies can be employed. Multi-component, nonpharmacotherapeutic interventions are used in clinical settings but few randomized trials have been conducted. The contributing effectiveness of individual components is not well-studied, but most include staff education to increase awareness of delirium. Of 3 multi-component intervention randomized trials, 2 reported a significantly lower incidence of delirium in the intervention group.35-37 Implementation of a multi-component protocol in medical/ surgical units was associated with a significant reduction in use of restraints.38
As in Mr. D’s case, complex drug regimens, particularly for CNS illness, can increase the risk of delirium. Considering the medication profile for patients with complex systemic illness—in particular, minimizing the use anticholinergics and dopamine agonists— may be crucial in preventing delirium.
Prophylactic administration of antipsychotics may reduce the risk of developing postoperative delirium.39 Studies of the use of these agents were characterized by small sample sizes and selected groups of patient populations. Of the 4 randomized studies evaluating prophylactic antipsychotics (vs placebo), 3 found a lower incidence of delirium in the intervention groups.39-41
A study of haloperidol in post-GI surgery patients showed a reduced occurrence of delirium,40 whereas its prophylactic use in patients undergoing hip surgery42 did not reduce the incidence of delirium compared with placebo, but did decrease severity when delirium occurred.42
Risperidone39 in post-cardiac surgery and olanzapine41 perioperatively in patients undergoing total knee or hip replacement have been shown to decrease delirium severity and duration. Targeted prophylaxis with risperidone43 in post-cardiac surgery patients who showed disturbed cognition but did not meet criteria for delirium reduced the number of patients requiring medication, compared with placebo.43
Dexmedetomidine, an α-2 adrenergic receptor agonist, compared with propofol or midazolam in post-cardiac valve surgery patients, resulted in a decreased incidence of delirium but no difference in delirium duration, hospital length of stay, or use of other medications.44 However, other studies have shown that dexmedetomidine reduces ICU length of stay and duration of mechanical ventilation.45
Treatment. Management of hospitalized medically ill geriatric patients with delirium is challenging and requires a comprehensive approach. The first step in delirium management is prompt identification and management of systemic medical disturbances associated with the delirium episode. First-line, nonpharmacotherapeutic strategies for patients with delirium include:
• reorientation
• behavioral interventions (eg, use of clear instructions and frequent eye contact with patients)
• environmental interventions (eg, minimal noise, adequate lighting, and limited room and staff changes)
• avoidance of physical restraints.46
Consider employing family members or hospital staff sitters to stay with the patient and to reassure, reorient, and watch for agitation and other unsafe behaviors (eg, attempted elopement). Psychoeducation for the patient and family on the phenomenology of delirium can be helpful.
The use of drug treatment strategies should be integrated into a comprehensive approach that includes the routine use of nondrug measures.46 Using medications for treating hypoactive delirium, formerly controversial, now has wider acceptance.47,48 A few high-quality randomized trials have been performed.25,49,50
Pharmacotherapy, especially in frail patients, should be initiated at the lowest starting dosage and titrated cautiously to clinical effect and for the shortest period of time necessary. Antipsychotics are preferred agents for treating all subtypes of delirium; haloperidol is widely used.46,51,52 However, antipsychotics, including haloperidol, can be associated with adverse neurologic effects such as extrapyramidal symptoms (EPS) and NMS.
Although reported less frequently than with haloperidol, other agents have been implicated in development of EPS and NMS, including atypical antipsychotics and antiemetic dopamine antagonists, particularly in parkinsonism-prone patients.53 Strategies that can minimize such risks in geriatric inpatients with delirium include oral, rather than parenteral, use of antipsychotics—preferential use of atypical over typical antipsychotics— and lowest effective dosages.54
In controlled trials, atypical antipsychotics for delirium showed efficacy compared with haloperidol.52,55 However, there is no research that demonstrates any advantage of one atypical over another.25
In Mr. D’s case, the most important intervention for managing delirium caused by NMS is to discontinue all dopamine antagonists and treat agitation with judicious doses of a benzodiazepine, with supportive care.17 In cases of sudden discontinuation or a dosage decrease of dopamine agonists, these medications should be resumed or optimized to minimize the risk of NMS-associated rhabdomyolysis and subsequent renal failure.17 Antipsychotics carry an increased risk of stroke and mortality in older patients with established or evolving neurocognitive disorders56,57 and can cause prolongation of the QTc interval.57
Other medications that could be used for delirium include cholinesterase inhibitors58,59 (although larger trials and a systematic review did not support this use60), and 5-HT receptor antagonists,61 such as trazodone. Benzodiazepines, such as lorazepam, are first-line treatment for delirium associated with seizures or withdrawal from alcohol, sedatives, hypnotics, and anxiolytics and for delirium caused by NMS. Be cautious about using benzodiazepines in geriatric patients because of a risk of respiratory depression, falls, sedation, and amnesia.
Geriatric patients with alcoholism and those with malnutrition are prone to thiamine and vitamin B12 deficiencies, which can induce delirium. Laboratory assessment and consideration of supplementation is recommended. Despite high occurrence of delirium in hospitalized older adults with preexisting comorbid neurocognitive disorders, there is no standard care for delirium comorbid with another neurocognitive disorder.62 Clinical practice guidelines for older patients receiving palliative care have been developed63; the goal is to minimize suffering and discomfort in patients in palliative care.64
Post-delirium prophylaxis. Medications for delirium usually can be tapered and discontinued once the episode has resolved and the patient is stable; it is common to discontinue medications when the patient has been symptom-free for 1 week.65 Some patients (eg, with end-stage liver disease, disseminated cancer) are prone to recurrent or to prolonged or chronic delirium. A period of post-recovery treatment with antipsychotics—even indefinite treatment in some cases—should be considered.
Post-delirium debriefing and aftercare. The psychological complications of delirium are distressing for the patient and his (her) caregivers. Psychiatric complications associated with delirium, including acute stress disorder—which might predict posttraumatic stress disorder—have been explored; early recognition and treatment may improve long-term outcomes.66 After recovery from acute delirium, cognitive assessment (eg, MMSE67 or Montreal Cognitive Assessment68) is recommended to validate current cognitive status because patients may have persistent decrement in cognitive function compared with pre-delirium condition, even after recovery from the acute episode.
Post-delirium debriefing may help patients who have recovered from a delirium episode. Patients may fear that their brief period of hallucinations might represent the onset of a chronic-relapsing psychotic disorder. Allow patients to communicate their distress about the delirium episode and give them the opportunity to talk through the experience. Brief them on the possibility that delirium will recur and advise them to seek emergency medical care in case of recurrence. Advise patients to monitor and maintain a normal sleep-wake cycle.
Family members can watch for syndromal recurrence of delirium. They should be encouraged to discuss their reaction to having seen their relative in a delirious state.
Health care systems with integrated electronic medical records should list “delirium, resolved” on the patient’s illness profile or problem list and alert the patient’s primary care provider to the delirium history to avoid future exposure to delirium-provocative medications, and to prompt the provider to assume an active role in post-delirium care, including delirium recurrence surveillance, medication adjustment, risk factor management, and post-recovery cognitive assessment.
Bottom Line
Evaluation of delirium in geriatric patients includes clinical vigilance and screening, differentiating delirium from other neurocognitive disorders, and identifying and treating underlying causes. Perioperative use of antipsychotics may reduce the incidence of delirium, although hospital length of stay generally has not been reduced with prophylaxis. Management interventions include staff education, systematic screening, use of multicomponent interventions, and pharmacologic interventions.
Related Resources
• Downing LJ, Caprio TV, Lyness JM. Geriatric psychiatry review: differential diagnosis and treatment of the 3 D’s - delirium, dementia, and depression. Curr Psychiatry Rep. 2013;15(6):365.
• Brooks PB. Postoperative delirium in elderly patients. Am J Nurs. 2012;112(9):38-49.
Drug Brand Names
Carbidopa/levodopa • Sinemet Midazolam • Versed
Dexmedetomidine • Precedex Olanzapine • Zyprexa
Haloperidol • Haldol Propofol • Diprivan
Lithium • Eskalith, Lithobid Quetiapine • Seroquel
Lorazepam • Ativan Risperidone • Risperdal
Metoclopramide • Reglan Trazodone • Desyrel
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Although delirium has many descriptive terms (Table 1), a common unifying term is “acute global cognitive dysfunction,” now recognized as delirium; a consensus supported by DSM-51 and ICD-102 (Table 2). According to DSM-5, the essential feature is a disturbance of attention or awareness that is accompanied by a change in baseline cognition that cannot be explained by another preexisting, established, or evolving neurocognitive disorder (the newly named DSM-5 entity for dementia syndromes).1 Because delirium affects the cortex diffusely, psychiatric symptoms can include cognitive, mood, anxiety, or psychotic symptoms. Because many systemic illnesses can induce delirium, the differential diagnosis spans all organ systems.
Three subtypes
Delirium can be classified, based on symptoms,3,4 into 3 subtypes: hyperactive-hyperalert, hypoactive-hypoalert, and mixed delirium. Hyperactive patients present with restlessness and agitation. Hypoactive patients are lethargic, confused, slow to respond to questions, and often appear depressed. The differential prognostic significance of these subtypes has been examined in the literature, with conflicting results. Rabinowitz5 reported that hypoactive delirium has the worst prognosis, while Marcantonio et al6 indicated that the hyperactive subtype is associated with the highest mortality rate. Mixed delirium, with periods of both hyperactivity and hypoactivity, is the most common type of delirium.7
A prodromal phase, characterized by anxiety, frequent requests for nursing and medical assistance, decreased attention, restlessness, vivid dreams, disorientation immediately after awakening, and hallucinations, can occur before an episode of full-spectrum delirium; this prodromal state often is identified retrospectively —after the patient is in an episode of delirium.8,9
Evidence-based guidelines aim to improve recognition and clinical management.10-13 Disruptive behavior is the main reason for psychiatric referral in delirium.14,15 Delayed psychiatric consultation because of non-recognition of delirium is related to variables such as older age; history of a pre-existing, comorbid neurocognitive disorder; and the clinical appearance of hypoactive delirium.14
The case of Mr. D (Box),16 illustrates how the emergence of antipsychotic-associated neuroleptic malignant syndrome (NMS) can complicate antipsychotic treatment of delirium in a geriatric medical patient, although delirium also is a common presentation in NMS.17 Delirium developed after an increase in carbidopa/levodopa, which has central dopaminergic effects that can precipitate delirium, particularly in a geriatric patient with preexisting comorbid neurocognitive disorder. Further complicating Mr. D’s delirium presentation was the development of NMS, which had a multifactorial causation, such as the use of dopamine antagonists (ie, quetiapine, metoclopramide), and an abrupt decrease of a dopaminergic agent (ie, carbidopa/levodopa), all inducing a central dopamine relative hypoactivity.
Epidemiology
Delirium is more common in older patients,15 and is seen in 30% to 40% of hospitalized geriatric patients.18 Delirium in older patients, compared with other adults, is associated with more severe cognitive impairment.19 It is common among geriatric surgical patients (15% to 62%)20 with a peak 2 to 5 days postoperatively for hip fracture,21 and often is seen in ICU patients (70% to 87%).20 However, Spronk et al22 found that delirium is significantly under-recognized in the ICU. Nearly 90% of terminally ill patients become delirious before death.23 Terminal delirium often is unrecognized and can interfere with assessment of other clinical problems.24 A preexisting history of comorbid neurocognitive disorder was evident in as many as two-thirds of delirium cases.25
Pathophysiology and risk factors
The pathophysiology of delirium has been characterized as an imbalance of CNS metabolism, including decreased blood flow in various regions of the brain that may normalize once delirium resolves.26 Studies describe the simultaneous decrease of cholinergic transmission and dopaminergic excess.27,28 Predisposing and precipitating factors for delirium that are of particular importance in geriatric patients include:
• advanced age
• CNS disease
• infection
• cognitive impairment
• male sex
• poor nutrition
• dehydration and other metabolic abnormalities
• cardiovascular events
• substance use
• medication
• sensory deprivation (eg, impaired vision or hearing)
• sleep deprivation
• low level of physical activity.27,29,30
Table 3 lists the most common delirium-provocative medications.27
Evaluation and psychometric scales
The EEG can be useful in evaluating delirium, especially in clinically ambiguous cases. EEG findings may indicate generalized slowing or dropout of the posterior dominant rhythm, and generalized slow theta and delta waves, findings that are more common in delirium than in other neurocognitive disorders and other psychiatric illnesses. The EEG must be interpreted in the context of the delirium diagnostic workup, because abnormalities seen in other neurocognitive disorders can overlap with those of delirium.31
The EEG referral should specify the clinical suspicion of delirium to help interpret the results. Delirium cases in which the patient’s previous cognitive status is unknown may benefit from EEG evaluation, such as:
• in possible status epilepticus
• when delirium improvement has reached a plateau at a lower level of cognitive function than before onset of delirium
• when the patient is unable or unwilling to complete a psychiatric interview.27
Assessment instruments are available to diagnose and monitor delirium (Table 4). Typically, delirium assessment includes examining levels of arousal, psychomotor activity, cognition (ie, orientation, attention, and memory), and perceptual disturbances.
Psychometrically, a review of Table 4 suggests that validity appeared stable with adequate specificity (64% to 99%) but more variable sensitivity (36% to 100%). These reliability parameters also will be affected by the classification system (ie, DSM vs ICD) and the cut-off score employed.32 Most measures (eg, Confusion Assessment Method [CAM], CAM-ICU) provide an adequate sample of behavioral (ie, level of alertness), motor (ie, psychomotor activity), and cognitive (ie, orientation, attention, memory, and receptive language) function, with the exception of the Global Attentiveness Rating, which is a 2-minute open conversation protocol between physician and patient.
Some measures are stand-alone instruments, such as the Memorial Delirium Assessment Scale, whereas the CAM requires administration of separate cognitive screens, including the Mini-Mental State Examination (MMSE) and Digit Span.33 Instruments to detect delirium in critically ill patients are a more recent development. Wong et al34 reported that the most widely studied tool was the CAM. Obtaining collateral information from family, caregivers, and hospital staff is essential, particularly given the fluctuating nature of delirium.
Management
Prevention. Identify patients at high risk of delirium so that preventive strategies can be employed. Multi-component, nonpharmacotherapeutic interventions are used in clinical settings but few randomized trials have been conducted. The contributing effectiveness of individual components is not well-studied, but most include staff education to increase awareness of delirium. Of 3 multi-component intervention randomized trials, 2 reported a significantly lower incidence of delirium in the intervention group.35-37 Implementation of a multi-component protocol in medical/ surgical units was associated with a significant reduction in use of restraints.38
As in Mr. D’s case, complex drug regimens, particularly for CNS illness, can increase the risk of delirium. Considering the medication profile for patients with complex systemic illness—in particular, minimizing the use anticholinergics and dopamine agonists— may be crucial in preventing delirium.
Prophylactic administration of antipsychotics may reduce the risk of developing postoperative delirium.39 Studies of the use of these agents were characterized by small sample sizes and selected groups of patient populations. Of the 4 randomized studies evaluating prophylactic antipsychotics (vs placebo), 3 found a lower incidence of delirium in the intervention groups.39-41
A study of haloperidol in post-GI surgery patients showed a reduced occurrence of delirium,40 whereas its prophylactic use in patients undergoing hip surgery42 did not reduce the incidence of delirium compared with placebo, but did decrease severity when delirium occurred.42
Risperidone39 in post-cardiac surgery and olanzapine41 perioperatively in patients undergoing total knee or hip replacement have been shown to decrease delirium severity and duration. Targeted prophylaxis with risperidone43 in post-cardiac surgery patients who showed disturbed cognition but did not meet criteria for delirium reduced the number of patients requiring medication, compared with placebo.43
Dexmedetomidine, an α-2 adrenergic receptor agonist, compared with propofol or midazolam in post-cardiac valve surgery patients, resulted in a decreased incidence of delirium but no difference in delirium duration, hospital length of stay, or use of other medications.44 However, other studies have shown that dexmedetomidine reduces ICU length of stay and duration of mechanical ventilation.45
Treatment. Management of hospitalized medically ill geriatric patients with delirium is challenging and requires a comprehensive approach. The first step in delirium management is prompt identification and management of systemic medical disturbances associated with the delirium episode. First-line, nonpharmacotherapeutic strategies for patients with delirium include:
• reorientation
• behavioral interventions (eg, use of clear instructions and frequent eye contact with patients)
• environmental interventions (eg, minimal noise, adequate lighting, and limited room and staff changes)
• avoidance of physical restraints.46
Consider employing family members or hospital staff sitters to stay with the patient and to reassure, reorient, and watch for agitation and other unsafe behaviors (eg, attempted elopement). Psychoeducation for the patient and family on the phenomenology of delirium can be helpful.
The use of drug treatment strategies should be integrated into a comprehensive approach that includes the routine use of nondrug measures.46 Using medications for treating hypoactive delirium, formerly controversial, now has wider acceptance.47,48 A few high-quality randomized trials have been performed.25,49,50
Pharmacotherapy, especially in frail patients, should be initiated at the lowest starting dosage and titrated cautiously to clinical effect and for the shortest period of time necessary. Antipsychotics are preferred agents for treating all subtypes of delirium; haloperidol is widely used.46,51,52 However, antipsychotics, including haloperidol, can be associated with adverse neurologic effects such as extrapyramidal symptoms (EPS) and NMS.
Although reported less frequently than with haloperidol, other agents have been implicated in development of EPS and NMS, including atypical antipsychotics and antiemetic dopamine antagonists, particularly in parkinsonism-prone patients.53 Strategies that can minimize such risks in geriatric inpatients with delirium include oral, rather than parenteral, use of antipsychotics—preferential use of atypical over typical antipsychotics— and lowest effective dosages.54
In controlled trials, atypical antipsychotics for delirium showed efficacy compared with haloperidol.52,55 However, there is no research that demonstrates any advantage of one atypical over another.25
In Mr. D’s case, the most important intervention for managing delirium caused by NMS is to discontinue all dopamine antagonists and treat agitation with judicious doses of a benzodiazepine, with supportive care.17 In cases of sudden discontinuation or a dosage decrease of dopamine agonists, these medications should be resumed or optimized to minimize the risk of NMS-associated rhabdomyolysis and subsequent renal failure.17 Antipsychotics carry an increased risk of stroke and mortality in older patients with established or evolving neurocognitive disorders56,57 and can cause prolongation of the QTc interval.57
Other medications that could be used for delirium include cholinesterase inhibitors58,59 (although larger trials and a systematic review did not support this use60), and 5-HT receptor antagonists,61 such as trazodone. Benzodiazepines, such as lorazepam, are first-line treatment for delirium associated with seizures or withdrawal from alcohol, sedatives, hypnotics, and anxiolytics and for delirium caused by NMS. Be cautious about using benzodiazepines in geriatric patients because of a risk of respiratory depression, falls, sedation, and amnesia.
Geriatric patients with alcoholism and those with malnutrition are prone to thiamine and vitamin B12 deficiencies, which can induce delirium. Laboratory assessment and consideration of supplementation is recommended. Despite high occurrence of delirium in hospitalized older adults with preexisting comorbid neurocognitive disorders, there is no standard care for delirium comorbid with another neurocognitive disorder.62 Clinical practice guidelines for older patients receiving palliative care have been developed63; the goal is to minimize suffering and discomfort in patients in palliative care.64
Post-delirium prophylaxis. Medications for delirium usually can be tapered and discontinued once the episode has resolved and the patient is stable; it is common to discontinue medications when the patient has been symptom-free for 1 week.65 Some patients (eg, with end-stage liver disease, disseminated cancer) are prone to recurrent or to prolonged or chronic delirium. A period of post-recovery treatment with antipsychotics—even indefinite treatment in some cases—should be considered.
Post-delirium debriefing and aftercare. The psychological complications of delirium are distressing for the patient and his (her) caregivers. Psychiatric complications associated with delirium, including acute stress disorder—which might predict posttraumatic stress disorder—have been explored; early recognition and treatment may improve long-term outcomes.66 After recovery from acute delirium, cognitive assessment (eg, MMSE67 or Montreal Cognitive Assessment68) is recommended to validate current cognitive status because patients may have persistent decrement in cognitive function compared with pre-delirium condition, even after recovery from the acute episode.
Post-delirium debriefing may help patients who have recovered from a delirium episode. Patients may fear that their brief period of hallucinations might represent the onset of a chronic-relapsing psychotic disorder. Allow patients to communicate their distress about the delirium episode and give them the opportunity to talk through the experience. Brief them on the possibility that delirium will recur and advise them to seek emergency medical care in case of recurrence. Advise patients to monitor and maintain a normal sleep-wake cycle.
Family members can watch for syndromal recurrence of delirium. They should be encouraged to discuss their reaction to having seen their relative in a delirious state.
Health care systems with integrated electronic medical records should list “delirium, resolved” on the patient’s illness profile or problem list and alert the patient’s primary care provider to the delirium history to avoid future exposure to delirium-provocative medications, and to prompt the provider to assume an active role in post-delirium care, including delirium recurrence surveillance, medication adjustment, risk factor management, and post-recovery cognitive assessment.
Bottom Line
Evaluation of delirium in geriatric patients includes clinical vigilance and screening, differentiating delirium from other neurocognitive disorders, and identifying and treating underlying causes. Perioperative use of antipsychotics may reduce the incidence of delirium, although hospital length of stay generally has not been reduced with prophylaxis. Management interventions include staff education, systematic screening, use of multicomponent interventions, and pharmacologic interventions.
Related Resources
• Downing LJ, Caprio TV, Lyness JM. Geriatric psychiatry review: differential diagnosis and treatment of the 3 D’s - delirium, dementia, and depression. Curr Psychiatry Rep. 2013;15(6):365.
• Brooks PB. Postoperative delirium in elderly patients. Am J Nurs. 2012;112(9):38-49.
Drug Brand Names
Carbidopa/levodopa • Sinemet Midazolam • Versed
Dexmedetomidine • Precedex Olanzapine • Zyprexa
Haloperidol • Haldol Propofol • Diprivan
Lithium • Eskalith, Lithobid Quetiapine • Seroquel
Lorazepam • Ativan Risperidone • Risperdal
Metoclopramide • Reglan Trazodone • Desyrel
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders, fifth edition. Washington, DC: American Psychiatric Association; 2013.
2. World Health Organization. The ICD-10 classification of mental and behavioural disorders. Diagnostic criteria for research. Geneva, Switzerland: WHO; 1993.
3. Lipowski ZJ. Delirium in the elderly patient. N Engl J Med. 1989;320(9):578-582.
4. Meagher DJ, Trzepacz PT. Motoric subtypes of delirium. Semin Clin Neuropsychiatry. 2000;5(2):75-85.
5. Rabinowitz T. Delirium: an important (but often unrecognized) clinical syndrome. Curr Psychiatry Rep. 2002;4(3):202-208.
6. Marcantonio ER, Ta T, Duthie E, et al. Delirium severity and psychomotor types: their relationship with outcomes after hip fracture repair. Am J Geriatr Soc. 2002;50(5):850-857.
7. Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA. 2001;286(21):2703-2710.
8. Duppils GS, Wikblad K. Delirium: behavioural changes before and during the prodromal phase. J Clin Nurs. 2004;13(5):609-616.
9. de Jonghe JF, Kalisvaart KJ, Dijkstra M, et al. Early symptoms in the prodromal phase of delirium: a prospective cohort study in elderly patients undergoing hip surgery. Am J Geriatr Psychiatry. 2007;15(2):112-121.
10. Cook IA. Guideline watch: practice guideline for the treatment of patients with delirium. Arlington, VA: American Psychiatric Publishing; 2004.
11. Hogan D, Gage L, Bruto V, et al. National guidelines for seniors’ mental health: the assessment and treatment of delirium. Canadian Journal of Geriatrics. 2006;9(suppl 2):S42-51.
12. Leentjens AF, Diefenbacher A. A survey of delirium guidelines in Europe. J Psychosom Res. 2006;61(1):123-128.
13. Tropea J, Slee JA, Brand CA, et al. Clinical practice guidelines for the management of delirium in older people in Australia. Australas J Ageing. 2008;27(3):150-156.
14. Mittal D, Majithia D, Kennedy R, et al. Differences in characteristics and outcome of delirium as based on referral patterns. Psychosomatics. 2006;47(5):367-375.
15. Grover S, Subodh BN, Avasthi A, et al. Prevalence and clinical profile of delirium: a study from a tertiary-care hospital in north India. Gen Hosp Psychiatry. 2009;31(1): 25-29.
16. Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12): 941-948.
17. Strawn JR, Keck PE Jr, Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870-876.
18. Dobmejer K. Delirium in elderly medical patients. Clinical Geriatrics. 1996;4:43-68.
19. Leentjens AF, Maclullich AM, Meagher DJ. Delirium, Cinderella no more...? J Psychosom Res. 2008;65(3):205.
20. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol. 2009;5(4):210-220.
21. Streubel PN, Ricci WM, Gardner MJ. Fragility fractures: preoperative, perioperative, and postoperative management. Current Orthopaedic Practice. 2009;20(5):482-489.
22. Spronk PE, Riekerk B, Hofhuis J, et al. Occurrence of delirium is severely underestimated in the ICU during daily care. Intensive Care Med. 2009;35(7):1276-1280.
23. Lawlor PG, Gagnon B, Mancini IL, et al. Occurrence, causes, and outcome of delirium in patients with advanced cancer: a prospective study. Arch Intern Med. 2000;160(6):786-794.
24. Ganzini L. Care of patients with delirium at the end of life. Annals of Long-Term Care. 2007;15(3):35-40.
25. Bourne RS, Tahir TA, Borthwick M, et al. Drug treatment of delirium: past, present and future. J Psychosom Res. 2008;65(3):273-282.
26. Yokota H, Ogawa S, Kurokawa A, et al. Regional cerebral blood flow in delirium patients. Psychiatry Clin Neurosci. 2003;57(3):337-339.
27. Maldonado JR. Pathoetiological model of delirium: a comprehensive understanding of the neurobiology of delirium and an evidence-based approach to prevention and treatment. Crit Care Clin. 2008;24(4):789-856, ix.
28. Trzepacz PT. Is there a final common neural pathway in delirium? Focus on acetylcholine and dopamine. Semin Clin Neuropsychiatry. 2000;5(2):132-148.
29. Inouye SK. The dilemma of delirium: clinical and research controversies regarding diagnosis and evaluation of delirium in hospitalized elderly medical patients. Am J Med. 1994;97(3):278-288.
30. Laurila JV, Laakkonen ML, Tilvis RS, et al. Predisposing and precipitating factors for delirium in a frail geriatric population. J Psychosom Res. 2008;65(3):249-254.
31. Morandi A, McCurley J, Vasilevskis EE, et al. Tools to detect delirium superimposed on dementia: a systematic review. J Am Geriatr Soc. 2012;60(11):2005-2013.
32. Kazmierski J, Kowman M, Banach M, et al. The use of DSM-IV and ICD-10 criteria and diagnostic scales for delirium among cardiac surgery patients: results from the IPDACS study. J Neuropsychiatry Clin Neurosci. 2010; 22(4):426-432.
33. Breitbart W, Rosenfeld B, Roth A, et al. The Memorial Delirium Rating Scale. J Pain Symptom Manage. 1997;13(3):128-137.
34. Wong CL, Holroyd-Leduc J, Simel DL, et al. Does this patient have delirium?: value of bedside instruments. JAMA. 2010;304(7):779-786.
35. Marcantonio ER, Flacker JM, Wright RJ, et al. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2011;49(5):516-522.
36. Lundström M, Edlund A, Karlsson S, et al. A multifactorial intervention program reduces the duration of delirium, length of hospitalization, and mortality in delirious patients. J Am Geriatr Soc. 2005;53(4):622-628.
37. Lundström M, Olofsson B, Stenvall M, et al. Postoperative delirium in old patients with femoral neck fracture: a randomized intervention study. Aging Clin Exp Res. 2007; 19(3):178-186.
38. Kratz A. Use of the acute confusion protocol: a research utilization project. J Nurs Care Qual. 2008;23(4):331-337.
39. Prakanrattana U, Prapaitrakool S. Efficacy of risperidone for prevention of postoperative delirium in cardiac surgery. Anaesth Intensive Care. 2007;35(5):714-719.
40. Kaneko T, Cai J, Ishikura T, et al. Prophylactic consecutive administration of haloperidol can reduce the occurrence of postoperative delirium in gastrointestinal surgery. Yonago Acta Medica. 1999;42:179-184.
41. Larsen KA, Kelly SE, Stern TA, et al. Administration of olanzapine to prevent postoperative delirium in elderly joint-replacement patients: a randomized, controlled trial. Psychosomatics. 2010;51(5):409-418.
42. Kalisvaart KJ, de Jonghe JF, Bogaards MJ, et al. Haloperidol prophylaxis for elderly hip-surgery patients at risk for delirium: a randomized placebo-controlled study. J Am Geriatr Soc. 2005;53(10):1658-1666.
43. Hakim SM, Othman AI, Naoum DO. Early treatment with risperidone for subsyndromal delirium after on-pump cardiac surgery in the elderly: a randomized trial. Anesthesiology. 2012;116(5):987-997.
44. Maldonado JR, Wysong A, van der Starre PJ, et al. Dexmedetomidine and the reduction of postoperative delirium after cardiac surgery. Psychosomatics. 2009;50(3): 206-217.
45. Short J. Use of dexmedetomidine for primary sedation in a general intensive care unit. Crit Care Nurse. 2010;30(1): 29-38; quiz 39.
46. Practice guideline for the treatment of patients with delirium. American Psychiatric Association [Comment in: Treatment of patients with delirium. Am J Psychiatry. 2000.]. Am J Psychiatry. 1999;156(suppl 5):1-20.
47. Maldonado JR. Delirium in the acute care setting: characteristics, diagnosis, and treatment. Crit Care Clin. 2008;24(4):657-722, vii.
48. Platt MM, Breitbart W, Smith M, et al. Efficacy of neuroleptics for hypoactive delirium. J Neuropsychiatry Clin Neurosci. 1994;6(1):66-67.
49. Lonergan E, Britton AM, Luxenberg J, et al. Antipsychotics for delirium. Cochrane Database Syst Rev. 2007;(2):CD005594.
50. Seitz DP, Gill SS, van Zyl LT. Antipsychotics in the treatment of delirium: a systematic review. J Clin Psychiatry. 2007;68(1):11-21.
51. Breitbart W, Marotta R, Platt MM, et al. A double-blind trial of haloperidol, chlorpromazine, and lorazepam in the treatment of delirium in hospitalized AIDS patients. Am J Psychiatry. 1996;153(2):231-237.
52. Hu H, Deng W, Yang H, et al. Olanzapine and haloperidol for senile delirium: a randomized controlled observation. Chinese Journal of Clinical Rehabilitation. 2006;10(42): 188-190.
53. Friedman JH, Fernandez HH. Atypical antipsychotics in Parkinson-sensitive populations. J Geriatr Psychiatry Neurol. 2002;15(3):156-170.
54. Seitz DP, Gill SS. Neuroleptic malignant syndrome complicating antipsychotic treatment of delirium or agitation in medical and surgical patients: case reports and a review of the literature. Psychosomatics. 2009; 50(1):8-15.
55. Han CS, Kim YK. A double-blind trial of risperidone and haloperidol for the treatment of delirium. Psychosomatics. 2004;45(4):297-301.
56. Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA. 2005;293(5):596-608.
57. Hermann N, Lanctôt KL. Atypical antipsychotics for neuropsychiatric symptoms of dementia: malignant or maligned? Drug Saf. 2006;29(10):833-843.
58. Noyan MA, Elbi H, Aksu H. Donepezil for anticholinergic drug intoxication: a case report. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(5):885-887.
59. Gleason OC. Donepezil for postoperative delirium. Psychosomatics. 2003;44(5):437-438.
60. Overshott R, Karim S, Burns A. Cholinesterase inhibitors for delirium. Cochrane Database Syst Rev. 2008;(1): CD005317.
61. Davis MP. Does trazodone have a role in palliating symptoms? Support Care Cancer. 2007;15(2):221-224.
62. Fick DM, Agostini JV, Inouye SK. Delirium superimposed on dementia: a systematic review. J Am Geriatr Soc. 2002; 50(10):1723-1732.
63. Brajtman S, Wright D, Hogan D, et al. Developing guidelines for the assessment and treatment of delirium in older adults at the end of life. Can Geriatr J. 2011;14(2):40-50.
64. Caraceni A, Simonetti F. Palliating delirium in patients with cancer. Lancet Oncol. 2009;10(2):164-172.
65. Alexopoulos GS, Streim J, Carpenter D, et al; Expert Consensus Panel for using Antipsychotic Drugs in Older Patients. Using antipsychotic agents in older patients. J Clin Psychiatry. 2004;65(suppl 2):5-99; discussion 100-102; quiz 103-104.
66. Granja C, Gomes E, Amaro A, et al. Understanding posttraumatic stress disorder-related symptoms after critical care: the early illness amnesia hypothesis. Crit Care Med. 2008;36(10):2801-2809.
67. Ringdal GI, Ringdal K, Juliebø V, et al. Using the Mini- Mental State Examination to screen for delirium in elderly patients with hip fracture. Dement Geriatr Cogn Disord. 2011;32(6):394-400.
68. Olson RA, Chhanabhai T, McKenzie M. Feasibility study of the Montreal Cognitive Assessment (MoCA) in patients with brain metastases. Support Care Cancer. 2008;16(11):1273-1278.
1. Diagnostic and statistical manual of mental disorders, fifth edition. Washington, DC: American Psychiatric Association; 2013.
2. World Health Organization. The ICD-10 classification of mental and behavioural disorders. Diagnostic criteria for research. Geneva, Switzerland: WHO; 1993.
3. Lipowski ZJ. Delirium in the elderly patient. N Engl J Med. 1989;320(9):578-582.
4. Meagher DJ, Trzepacz PT. Motoric subtypes of delirium. Semin Clin Neuropsychiatry. 2000;5(2):75-85.
5. Rabinowitz T. Delirium: an important (but often unrecognized) clinical syndrome. Curr Psychiatry Rep. 2002;4(3):202-208.
6. Marcantonio ER, Ta T, Duthie E, et al. Delirium severity and psychomotor types: their relationship with outcomes after hip fracture repair. Am J Geriatr Soc. 2002;50(5):850-857.
7. Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA. 2001;286(21):2703-2710.
8. Duppils GS, Wikblad K. Delirium: behavioural changes before and during the prodromal phase. J Clin Nurs. 2004;13(5):609-616.
9. de Jonghe JF, Kalisvaart KJ, Dijkstra M, et al. Early symptoms in the prodromal phase of delirium: a prospective cohort study in elderly patients undergoing hip surgery. Am J Geriatr Psychiatry. 2007;15(2):112-121.
10. Cook IA. Guideline watch: practice guideline for the treatment of patients with delirium. Arlington, VA: American Psychiatric Publishing; 2004.
11. Hogan D, Gage L, Bruto V, et al. National guidelines for seniors’ mental health: the assessment and treatment of delirium. Canadian Journal of Geriatrics. 2006;9(suppl 2):S42-51.
12. Leentjens AF, Diefenbacher A. A survey of delirium guidelines in Europe. J Psychosom Res. 2006;61(1):123-128.
13. Tropea J, Slee JA, Brand CA, et al. Clinical practice guidelines for the management of delirium in older people in Australia. Australas J Ageing. 2008;27(3):150-156.
14. Mittal D, Majithia D, Kennedy R, et al. Differences in characteristics and outcome of delirium as based on referral patterns. Psychosomatics. 2006;47(5):367-375.
15. Grover S, Subodh BN, Avasthi A, et al. Prevalence and clinical profile of delirium: a study from a tertiary-care hospital in north India. Gen Hosp Psychiatry. 2009;31(1): 25-29.
16. Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12): 941-948.
17. Strawn JR, Keck PE Jr, Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870-876.
18. Dobmejer K. Delirium in elderly medical patients. Clinical Geriatrics. 1996;4:43-68.
19. Leentjens AF, Maclullich AM, Meagher DJ. Delirium, Cinderella no more...? J Psychosom Res. 2008;65(3):205.
20. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol. 2009;5(4):210-220.
21. Streubel PN, Ricci WM, Gardner MJ. Fragility fractures: preoperative, perioperative, and postoperative management. Current Orthopaedic Practice. 2009;20(5):482-489.
22. Spronk PE, Riekerk B, Hofhuis J, et al. Occurrence of delirium is severely underestimated in the ICU during daily care. Intensive Care Med. 2009;35(7):1276-1280.
23. Lawlor PG, Gagnon B, Mancini IL, et al. Occurrence, causes, and outcome of delirium in patients with advanced cancer: a prospective study. Arch Intern Med. 2000;160(6):786-794.
24. Ganzini L. Care of patients with delirium at the end of life. Annals of Long-Term Care. 2007;15(3):35-40.
25. Bourne RS, Tahir TA, Borthwick M, et al. Drug treatment of delirium: past, present and future. J Psychosom Res. 2008;65(3):273-282.
26. Yokota H, Ogawa S, Kurokawa A, et al. Regional cerebral blood flow in delirium patients. Psychiatry Clin Neurosci. 2003;57(3):337-339.
27. Maldonado JR. Pathoetiological model of delirium: a comprehensive understanding of the neurobiology of delirium and an evidence-based approach to prevention and treatment. Crit Care Clin. 2008;24(4):789-856, ix.
28. Trzepacz PT. Is there a final common neural pathway in delirium? Focus on acetylcholine and dopamine. Semin Clin Neuropsychiatry. 2000;5(2):132-148.
29. Inouye SK. The dilemma of delirium: clinical and research controversies regarding diagnosis and evaluation of delirium in hospitalized elderly medical patients. Am J Med. 1994;97(3):278-288.
30. Laurila JV, Laakkonen ML, Tilvis RS, et al. Predisposing and precipitating factors for delirium in a frail geriatric population. J Psychosom Res. 2008;65(3):249-254.
31. Morandi A, McCurley J, Vasilevskis EE, et al. Tools to detect delirium superimposed on dementia: a systematic review. J Am Geriatr Soc. 2012;60(11):2005-2013.
32. Kazmierski J, Kowman M, Banach M, et al. The use of DSM-IV and ICD-10 criteria and diagnostic scales for delirium among cardiac surgery patients: results from the IPDACS study. J Neuropsychiatry Clin Neurosci. 2010; 22(4):426-432.
33. Breitbart W, Rosenfeld B, Roth A, et al. The Memorial Delirium Rating Scale. J Pain Symptom Manage. 1997;13(3):128-137.
34. Wong CL, Holroyd-Leduc J, Simel DL, et al. Does this patient have delirium?: value of bedside instruments. JAMA. 2010;304(7):779-786.
35. Marcantonio ER, Flacker JM, Wright RJ, et al. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2011;49(5):516-522.
36. Lundström M, Edlund A, Karlsson S, et al. A multifactorial intervention program reduces the duration of delirium, length of hospitalization, and mortality in delirious patients. J Am Geriatr Soc. 2005;53(4):622-628.
37. Lundström M, Olofsson B, Stenvall M, et al. Postoperative delirium in old patients with femoral neck fracture: a randomized intervention study. Aging Clin Exp Res. 2007; 19(3):178-186.
38. Kratz A. Use of the acute confusion protocol: a research utilization project. J Nurs Care Qual. 2008;23(4):331-337.
39. Prakanrattana U, Prapaitrakool S. Efficacy of risperidone for prevention of postoperative delirium in cardiac surgery. Anaesth Intensive Care. 2007;35(5):714-719.
40. Kaneko T, Cai J, Ishikura T, et al. Prophylactic consecutive administration of haloperidol can reduce the occurrence of postoperative delirium in gastrointestinal surgery. Yonago Acta Medica. 1999;42:179-184.
41. Larsen KA, Kelly SE, Stern TA, et al. Administration of olanzapine to prevent postoperative delirium in elderly joint-replacement patients: a randomized, controlled trial. Psychosomatics. 2010;51(5):409-418.
42. Kalisvaart KJ, de Jonghe JF, Bogaards MJ, et al. Haloperidol prophylaxis for elderly hip-surgery patients at risk for delirium: a randomized placebo-controlled study. J Am Geriatr Soc. 2005;53(10):1658-1666.
43. Hakim SM, Othman AI, Naoum DO. Early treatment with risperidone for subsyndromal delirium after on-pump cardiac surgery in the elderly: a randomized trial. Anesthesiology. 2012;116(5):987-997.
44. Maldonado JR, Wysong A, van der Starre PJ, et al. Dexmedetomidine and the reduction of postoperative delirium after cardiac surgery. Psychosomatics. 2009;50(3): 206-217.
45. Short J. Use of dexmedetomidine for primary sedation in a general intensive care unit. Crit Care Nurse. 2010;30(1): 29-38; quiz 39.
46. Practice guideline for the treatment of patients with delirium. American Psychiatric Association [Comment in: Treatment of patients with delirium. Am J Psychiatry. 2000.]. Am J Psychiatry. 1999;156(suppl 5):1-20.
47. Maldonado JR. Delirium in the acute care setting: characteristics, diagnosis, and treatment. Crit Care Clin. 2008;24(4):657-722, vii.
48. Platt MM, Breitbart W, Smith M, et al. Efficacy of neuroleptics for hypoactive delirium. J Neuropsychiatry Clin Neurosci. 1994;6(1):66-67.
49. Lonergan E, Britton AM, Luxenberg J, et al. Antipsychotics for delirium. Cochrane Database Syst Rev. 2007;(2):CD005594.
50. Seitz DP, Gill SS, van Zyl LT. Antipsychotics in the treatment of delirium: a systematic review. J Clin Psychiatry. 2007;68(1):11-21.
51. Breitbart W, Marotta R, Platt MM, et al. A double-blind trial of haloperidol, chlorpromazine, and lorazepam in the treatment of delirium in hospitalized AIDS patients. Am J Psychiatry. 1996;153(2):231-237.
52. Hu H, Deng W, Yang H, et al. Olanzapine and haloperidol for senile delirium: a randomized controlled observation. Chinese Journal of Clinical Rehabilitation. 2006;10(42): 188-190.
53. Friedman JH, Fernandez HH. Atypical antipsychotics in Parkinson-sensitive populations. J Geriatr Psychiatry Neurol. 2002;15(3):156-170.
54. Seitz DP, Gill SS. Neuroleptic malignant syndrome complicating antipsychotic treatment of delirium or agitation in medical and surgical patients: case reports and a review of the literature. Psychosomatics. 2009; 50(1):8-15.
55. Han CS, Kim YK. A double-blind trial of risperidone and haloperidol for the treatment of delirium. Psychosomatics. 2004;45(4):297-301.
56. Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA. 2005;293(5):596-608.
57. Hermann N, Lanctôt KL. Atypical antipsychotics for neuropsychiatric symptoms of dementia: malignant or maligned? Drug Saf. 2006;29(10):833-843.
58. Noyan MA, Elbi H, Aksu H. Donepezil for anticholinergic drug intoxication: a case report. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(5):885-887.
59. Gleason OC. Donepezil for postoperative delirium. Psychosomatics. 2003;44(5):437-438.
60. Overshott R, Karim S, Burns A. Cholinesterase inhibitors for delirium. Cochrane Database Syst Rev. 2008;(1): CD005317.
61. Davis MP. Does trazodone have a role in palliating symptoms? Support Care Cancer. 2007;15(2):221-224.
62. Fick DM, Agostini JV, Inouye SK. Delirium superimposed on dementia: a systematic review. J Am Geriatr Soc. 2002; 50(10):1723-1732.
63. Brajtman S, Wright D, Hogan D, et al. Developing guidelines for the assessment and treatment of delirium in older adults at the end of life. Can Geriatr J. 2011;14(2):40-50.
64. Caraceni A, Simonetti F. Palliating delirium in patients with cancer. Lancet Oncol. 2009;10(2):164-172.
65. Alexopoulos GS, Streim J, Carpenter D, et al; Expert Consensus Panel for using Antipsychotic Drugs in Older Patients. Using antipsychotic agents in older patients. J Clin Psychiatry. 2004;65(suppl 2):5-99; discussion 100-102; quiz 103-104.
66. Granja C, Gomes E, Amaro A, et al. Understanding posttraumatic stress disorder-related symptoms after critical care: the early illness amnesia hypothesis. Crit Care Med. 2008;36(10):2801-2809.
67. Ringdal GI, Ringdal K, Juliebø V, et al. Using the Mini- Mental State Examination to screen for delirium in elderly patients with hip fracture. Dement Geriatr Cogn Disord. 2011;32(6):394-400.
68. Olson RA, Chhanabhai T, McKenzie M. Feasibility study of the Montreal Cognitive Assessment (MoCA) in patients with brain metastases. Support Care Cancer. 2008;16(11):1273-1278.
A cognitive-behavioral strategy for preventing suicide
Many mental health practitioners have had training in cognitive-behavioral therapy (CBT)—short-term, evidence-based psychotherapy for treating a variety of psychiatric conditions (eg, posttraumatic stress disorder) and medical comorbidities (eg, insomnia)—but only some are knowledgeable about how to best use CBT with a suicidal patient. This article provides a clinician-friendly summary of a 10-session evidence-based outpatient1-3 and an adapted 6 to 8 session inpatient4,5 cognitive-behavioral protocol (known as Post-Admission Cognitive Therapy [PACT]) that is designed to help patients who have suicide-related thoughts and/or behaviors.
3 phases of CBT for suicide prevention
An average of 9 hours of individual CBT for the prevention of suicide has been reported to reduce the likelihood of repeat suicide attempts in approximately 50% of patients.1 Here, we introduce you to 3 phases of CBT for preventing suicide—phases that are the same for outpatients or inpatients. Our aim is to help you become familiar with CBT strategies that can be adapted for your treatment setting and used to intervene with vulnerable patients who are at risk for suicidal self-directed violence. A thorough assessment of the patient’s psychiatric diagnosis and history, presenting problems, and risk and protective factors for suicide must be completed before treatment begins.
Phase I. The patient is asked to tell a story associated with his (her) most recent episode of suicidal thoughts or behavior, or both. This narrative serves as 1) a foundation for planning treatment and 2) a model for understanding how best to deactivate the wish to die through the process of psychotherapy.
Phase II. The patient is assisted with modifying underdeveloped or overdeveloped skills that are most closely associated with the risk of triggering a suicidal crisis. For example, a patient with underdeveloped skills in regulating anger and hatred toward himself is taught to modulate these problematic emotions more effectively. In addition, effective problem-solving strategies are reviewed and practiced.
Phase III. The patient is guided through a relapse prevention task. The purpose of this exercise is to 1) highlight skills learned during therapy and 2) allow the patient to practice effective problem-solving strategies that are aimed at minimizing the recurrence of suicidal self-directed violence.
Theorectical basis for preventing suicide with CBT
Aaron Beck, in 1979,6 proposed that a person’s biopsychosocial vulnerabilities can interact with suicidal thoughts and behaviors to produce a state that Beck labeled the “suicide mode.” Once produced, a suicide mode can become activated by cognitive, affective, motivational, and behavioral systems.
The frequency and severity of suicide mode activation can increase over time, especially for persons who do not have protective factors and those who have a history of self-directed violence—in particular, attempted suicide. Moreover, some persons might experience a chronic state of suicide mode activation and, therefore, remain at elevated risk of suicide. Once a suicide-specific mode is activated, the person considers suicide the only option for solving his life problems. Suicide might be considered a rational decision at this point.6
The hypothesized mechanism of action associated with CBT for preventing suicide can be described as:
• deactivation of the suicide mode
• modification of the structure and content of the suicide mode
• construction and practice of more adaptive structural modes to promote a desire to live.
The underlying philosophy of this intervention is that the suicide mode occurs independently of psychiatric diagnoses and must be targeted directly; treatment therefore is transdiagnostic.7 In other words, instead of addressing a symptom of a psychiatric disorder, treatment directly targets suicide-related ideation and behaviors (Table 1).
Using that framework, psychiatric diagnoses are conceptualized in terms of how the associated symptoms contribute to the activation, maintenance, and exacerbation of the suicide mode.
Protocol for preventing suicide
The outpatient protocol1-3 comprises 10, 45- to 50-minute weekly individual psychotherapy sessions, with an allowance for booster sessions (as needed), until the patient is able to complete the relapse prevention task in Phase III. The inpatient protocol4,5 comprises 6, 90-minute individual psychotherapy sessions, with an allowance for 2 booster sessions (as needed) during the inpatient stay and as many as 4 telephone booster sessions after discharge.
Phase I: Tell the suicide story
Engage the patient in treatment. To increase adherence to treatment and minimize the risk of drop-out, practitioners are encouraged to establish a strong, early therapeutic alliance with the patient. Showing genuine empathy and providing a safe, supportive, and nonjudgmental environment are instrumental for engaging patients in treatment. The practitioner listens carefully to the patient’s narrative, provides periodic summaries to check on accurate understanding, and keeps interruptions to a minimum.
Collaboratively generate a safety plan. A crisis response plan or safety plan—an individualized, hierarchically arranged, written list of coping strategies to be implemented during a suicide crisis—is developed as soon as possible. Guidance on how to develop a structured safety plan has been provided by Stanley and Brown.8,9
Practitioners must ensure that the safety plan contains contact information that the patient can use to reach the practitioner, the clinic, the on-call provider (if available), the local 24-hour emergency department, and the 24/7 National Suicide Prevention Lifeline (800-273-TALK [8255]). Discussion of how to limit access to lethal means also is important.
Because safety planning is a collaborative process, it is imperative that practitioners check on the patient’s willingness to follow the safety plan and help him overcome perceived obstacles in implementation. Copies of the plan can be kept at different locations and shared with family members, friends, or both with the patient’s permission.
Develop a cognitive-behavioral conceptualization. The cognitive-behavioral conceptualization is an individualized map of a patient’s automatic thoughts (eg, “I am going to get fired today”), conditional assumptions (“If I get fired, then my life is over”), and core beliefs (“I am an utter failure”) that are activated before, during, and after suicidal self-directed violence. To develop that conceptualization, the patient is asked to tell a story about his (her) most recent suicidal crisis (the Box, offers a sample script) and to describe reactions to having survived a suicide attempt. (Note: Patients who report regret after an attempt are at greatest risk for dying by suicide.10)
This activity gives the patient an opportunity to disclose details surrounding his suicidal thoughts and actions, and might allow for a cathartic experience through storytelling. As practitioners listen to the suicide narrative, they collect data on the patient’s early childhood experiences (typically, suicide-activating events), associated automatic thoughts and images, emotional responses, and subsequent behaviors.
Based on this information, a cognitive-behavioral case conceptualization diagram (Figure 1, and Table 2) is generated collaboratively with the patienta and used to personalize treatment planning.
a Judith Beck offers sample case conceptualization diagrams in Cognitive behavior therapy: Basics and beyond, 2nd ed. New York, New York: Guilford Press; 2011.
Phase II: Build skills
Build skills to prevent episodes of suicidal self-directed violence. Information obtained from the conceptualization is used to generate an individualized cognitive-behavioral plan of intervention. The overall goal is to determine skill-based problem areas that are associated with the most recent episode of suicidal self-directed violence.
Practitioner and patient collaboratively identify skills that are underdeveloped and ones that are overdeveloped so that they can be addressed systematically. In general, based on our clinical experience with suicidal patients, we recommend focusing on skills captured within ≥1 of the deficit domains in Table 3. Explanation of the various cognitive-behavioral strategies used in this phase of treatment is beyond the scope of this article, but 2 activities that highlight the clinical work conducted in this phase are described in the following sections. We selected those activities because they are easy to implement and, we have found, receive overall patient acceptability.
For a detailed understanding of strategies used in Phase II of CBT for preventing suicide, see Related Resources. In addition, the book Choosing to live: How to defeat suicide through cognitive therapy11 can serve as a self-help guide for patients to follow through with CBT skill-building strategies.
Sample activity #1: Construct a ‘hope box.’ One activity that you can use to help a patient cope with suicide-activating core beliefs (eg, “My life is worthless”) involves construction of a so-called hope box. The box helps the patient directly challenge his extreme distress, by being reminded of previous successes, positive experiences, and reasons for living. The process of constructing a hope box allows the patient to work on modifying his problematic core beliefs (eg, worthlessness, helplessness, incapable of being loved).
It can be helpful to have the patient construct his hope box during a session, to ensure that everything that is put in the box is truly helpful and personalized. Items included vary from patient to patient, and might consist of pictures of loved ones, a favorite poem, a prayer, coping cards (see next section), or all of these. For example, one of our patients chose to include a picture of herself in her early 20s as a reminder of a positive, fulfilling time in her life; this gave her hope that it is possible to experience those feelings again.
Bush et alb at the National Center for Telehealth and Technology have developed a Virtual Hope Box, a free mobile application for tablets and smartphones (compatible with Android and iOS operating systems) that patients can use under the guidance of their practitioner.
bwww.t2.health.mil/apps/virtual-hope-box
Sample activity #2: Generate coping cards. Effective problem-solving skills can be promoted by having the patient construct coping cards —wallet-size cards generated collaboratively in session. Coping cards are note cards that a patient keeps nearby to cope better during a difficult situation. They provide an easily accessible way to jump-start adaptive thinking during a suicidal crisis. The patient is encouraged to use coping cards to practice adaptive thinking even when not in a crisis.
There are 3 kinds of coping cards:
• place a suicide-relevant automatic thought or core belief on one side of the card; on the other side, place an alternative, more adaptive response
• write a list of coping strategies
• write instructions to motivate or “activate” the patient toward completing a specific goal (Figure 2).
Phase III: Prevent relapse
Complete relapse prevention task. Relapse prevention is a common CBT strategy that aims to strengthen self-management to minimize likelihood of returning to a previously stopped behavior. For patients who present only with suicidal thoughts, relapse prevention is directed at identifying triggers and minimizing the occurrence and/ or intensity of such thoughts in the future. For patients who present with suicidal self-directed violence, relapse prevention is directed at identifying triggers for suicidal actions and reducing the likelihood of acting on suicidal urges. The brief guidance provided below will familiarize you with each of the relapse prevention steps, which may be completed in multiple sessions.
Step 1: Provide psychoeducation
Explain the difference between a lapse and a relapse. In general, you want the patient to understand that, although suicidal thoughts might persist and recur over time, suicidal self-directed violence must be prevented. Describe the purpose of the relapse prevention task (ie, to minimize the chance that suicidal thinking and actions will recur); address questions and concerns; and obtain permission to begin the procedure. Assure the patient that this is a collaborative activity and you will be in the room to ensure comfort and safety.
STEP 2: Retell the suicide story
Ask the patient to imagine the chain of events, thoughts, and feelings that led to the most recent episode of suicide ideation or suicidal self-directed violence. Tell the patient that you want him to construct a movie script to describe the chain of events that resulted in the suicide crisis —but to do so slowly, taking enough time to describe the details of each scene of the movie.
Step 3: Apply CBT skills
Ask the patient again to take you through the sequence of events leading to the most recent episode of suicide ideation or suicidal self-directed violence. This time, however, direct him to use the skills learned in therapy to appropriately respond cognitively, affectively, and behaviorally to move further away from the suicide outcome.
If the patient is moving too fast or neglecting important points, stop and ask about alternative ways of thinking, feeling, and behaving. Use as much time as needed until the patient is able to demonstrate solid learning of at least several learned CBT strategies to prevent suicidal self-directed violence.
Step 4: Generalize learning to prepare for future suicidal crises
In this stage —given your knowledge of the patient’s psychosocial history, cognitive- behavioral conceptualization, and suicide mode triggers —you collaboratively create a future scenario that is likely to activate suicidal self-directed violence. Question the patient about possible coping strategies, provide helpful feedback, guide him through each link in the chain of events, and propose additional alternative strategies if he is clearly neglecting important points of the intervention.
Step 5: Debrief and summarize lessons learned
Debrief the patient by providing a summary of the skills he has learned in therapy, congratulate him for completing this final therapeutic task, and assess overall emotional reaction to this activity. Remind him that mood fluctuations and future setbacks, in the form of lapses, are expected. Give him the option to request booster sessions and make plans for next steps in accomplishing general goals of therapy.
Treatment can be terminated when the patient is able to complete the relapse prevention task. If he is not ready or able to complete this exercise successfully, you can extend treatment. The duration of the extension is left to the practitioner’s judgment, based on the overall treatment plan. Brown and colleagues2 have reported a maximum number of 24 outpatient sessions (for patients who need additional booster sessions); based on clinical experience, it is reasonable to assume that it would be highly unlikely for a patient not to meet treatment objectives after a methodical course of outpatient CBT.
In cases in which goals of treatment have not been met, consultation with colleagues, review of adherence problems, and consideration of obstacles for treatment efficacy would be recommended.
A checklist can be used to determine whether a patient is ready to end treatment. Variables that can be considered in assessing readiness for termination include:
• reduced scores on self-report measures for a number of weeks
• evidence of enhanced problem-solving
• engagement in adjunctive health care services
• development of a social support system.
Post-Admission Cognitive Therapy (PACT)
An inpatient cognitive-behavioral protocol for the prevention of suicide, adapted from the efficacious outpatient model, is being evaluated at the Walter Reed National Military Medical Center, Bethesda, Maryland, and Fort Belvoir Community Hospital, Fort Belvoir, Virginia. The inpatient intervention is called PACT; components are summarized in Table 4.
Bottom Line
Cognitive-behavioral therapy for preventing suicide is an efficacious protocol for reducing the recurrence of suicidal self-directed violence. Post-Admission Cognitive Therapy is the adapted inpatient treatment package. You are encouraged to gain additional training and supervision on the delivery of these interventions to your high-risk suicidal patients.
Related Resources
• Academy of Cognitive Therapy. www.academyofct.org.
• National Suicide Prevention Lifeline. www.suicide preventionlifeline.org.
• Wenzel A, Brown GK, Beck AT. Cognitive therapy for suicidal patients: scientific and clinical applications. Washington, DC: American Psychological Association; 2009.
Disclosures
Support for research on inpatient cognitive-behavioral therapy for the prevention of suicide provided to Principal Investigator, Dr. Ghahramanlou-Holloway by the Department of Defense, Congressionally Directed Medical Research Program (W81XWH-08-2-0172), Military Operational Medicine Research Program (W81XWH-11-2-0106), and the National Alliance for Research on Schizophrenia and Depression (15219).
1. Brown GK, Ten Have T, Henriques GR, et al. Cognitive therapy for the prevention of suicide attempts: a randomized controlled trial. JAMA. 2005;294(5):563-570.
2. Brown GK, Henriques GR, Ratto C, et al. Cognitive therapy treatment manual for suicide attempters. Philadelphia, PA: University of Pennsylvania; 2002 (unpublished).
3. Berk MS, Henriques GR, Warman DM, et al. A cognitive therapy intervention for suicide attempters: an overview of the treatment and case examples. Cogn Behav Pract. 2004;11(3):265-277.
4. Ghahramanlou-Holloway M, Cox D, Greene F. Post-admission cognitive therapy: a brief intervention for psychiatric inpatients admitted after a suicide attempt. Cogn Behav Pract. 2012;19(2):233-244.
5. Neely L, Irwin K, Carreno Ponce JT, et al. Post Admission Cognitive Therapy (PACT) for the prevention of suicide in military personnel with histories of trauma: treatment development and case example. Clinical Case Studies. 2013;12(6):457-473.
6. Beck AT. Cognitive therapy and the emotional disorders. New York, NY: Penguin Group; 1979.
7. Ghahramanlou-Holloway M, Brown GK, Beck AT. Suicide. In: Whisman M, ed. Adapting cognitive therapy for depression: managing complexity and comorbidity. New York, NY: Guilford Press; 2008:159-184.
8. Stanley B, Brown GK. Safety planning intervention: a brief intervention to mitigate suicide risk. Cogn Behav Pract. 2012;19(2):256-264.
9. Stanley B, Brown GK. Safety plan treatment manual to reduce suicide risk: veteran version. http://www. mentalhealth.va.gov/docs/va_safety_planning_manual. pdf. Published August 20, 2008. Accessed July 2, 2014.
10. Henriques G, Wenzel A, Brown GK, et al. Suicide attempters’ reaction to survival as a risk factor for eventual suicide. Am J Psychiatry. 2005;162(11):2180-2182.
11. Ellis TE, Newman CF. Choosing to live: How to defeat suicide through cognitive therapy. Oakland, CA: New Harbinger Publications, Inc; 1996.
Many mental health practitioners have had training in cognitive-behavioral therapy (CBT)—short-term, evidence-based psychotherapy for treating a variety of psychiatric conditions (eg, posttraumatic stress disorder) and medical comorbidities (eg, insomnia)—but only some are knowledgeable about how to best use CBT with a suicidal patient. This article provides a clinician-friendly summary of a 10-session evidence-based outpatient1-3 and an adapted 6 to 8 session inpatient4,5 cognitive-behavioral protocol (known as Post-Admission Cognitive Therapy [PACT]) that is designed to help patients who have suicide-related thoughts and/or behaviors.
3 phases of CBT for suicide prevention
An average of 9 hours of individual CBT for the prevention of suicide has been reported to reduce the likelihood of repeat suicide attempts in approximately 50% of patients.1 Here, we introduce you to 3 phases of CBT for preventing suicide—phases that are the same for outpatients or inpatients. Our aim is to help you become familiar with CBT strategies that can be adapted for your treatment setting and used to intervene with vulnerable patients who are at risk for suicidal self-directed violence. A thorough assessment of the patient’s psychiatric diagnosis and history, presenting problems, and risk and protective factors for suicide must be completed before treatment begins.
Phase I. The patient is asked to tell a story associated with his (her) most recent episode of suicidal thoughts or behavior, or both. This narrative serves as 1) a foundation for planning treatment and 2) a model for understanding how best to deactivate the wish to die through the process of psychotherapy.
Phase II. The patient is assisted with modifying underdeveloped or overdeveloped skills that are most closely associated with the risk of triggering a suicidal crisis. For example, a patient with underdeveloped skills in regulating anger and hatred toward himself is taught to modulate these problematic emotions more effectively. In addition, effective problem-solving strategies are reviewed and practiced.
Phase III. The patient is guided through a relapse prevention task. The purpose of this exercise is to 1) highlight skills learned during therapy and 2) allow the patient to practice effective problem-solving strategies that are aimed at minimizing the recurrence of suicidal self-directed violence.
Theorectical basis for preventing suicide with CBT
Aaron Beck, in 1979,6 proposed that a person’s biopsychosocial vulnerabilities can interact with suicidal thoughts and behaviors to produce a state that Beck labeled the “suicide mode.” Once produced, a suicide mode can become activated by cognitive, affective, motivational, and behavioral systems.
The frequency and severity of suicide mode activation can increase over time, especially for persons who do not have protective factors and those who have a history of self-directed violence—in particular, attempted suicide. Moreover, some persons might experience a chronic state of suicide mode activation and, therefore, remain at elevated risk of suicide. Once a suicide-specific mode is activated, the person considers suicide the only option for solving his life problems. Suicide might be considered a rational decision at this point.6
The hypothesized mechanism of action associated with CBT for preventing suicide can be described as:
• deactivation of the suicide mode
• modification of the structure and content of the suicide mode
• construction and practice of more adaptive structural modes to promote a desire to live.
The underlying philosophy of this intervention is that the suicide mode occurs independently of psychiatric diagnoses and must be targeted directly; treatment therefore is transdiagnostic.7 In other words, instead of addressing a symptom of a psychiatric disorder, treatment directly targets suicide-related ideation and behaviors (Table 1).
Using that framework, psychiatric diagnoses are conceptualized in terms of how the associated symptoms contribute to the activation, maintenance, and exacerbation of the suicide mode.
Protocol for preventing suicide
The outpatient protocol1-3 comprises 10, 45- to 50-minute weekly individual psychotherapy sessions, with an allowance for booster sessions (as needed), until the patient is able to complete the relapse prevention task in Phase III. The inpatient protocol4,5 comprises 6, 90-minute individual psychotherapy sessions, with an allowance for 2 booster sessions (as needed) during the inpatient stay and as many as 4 telephone booster sessions after discharge.
Phase I: Tell the suicide story
Engage the patient in treatment. To increase adherence to treatment and minimize the risk of drop-out, practitioners are encouraged to establish a strong, early therapeutic alliance with the patient. Showing genuine empathy and providing a safe, supportive, and nonjudgmental environment are instrumental for engaging patients in treatment. The practitioner listens carefully to the patient’s narrative, provides periodic summaries to check on accurate understanding, and keeps interruptions to a minimum.
Collaboratively generate a safety plan. A crisis response plan or safety plan—an individualized, hierarchically arranged, written list of coping strategies to be implemented during a suicide crisis—is developed as soon as possible. Guidance on how to develop a structured safety plan has been provided by Stanley and Brown.8,9
Practitioners must ensure that the safety plan contains contact information that the patient can use to reach the practitioner, the clinic, the on-call provider (if available), the local 24-hour emergency department, and the 24/7 National Suicide Prevention Lifeline (800-273-TALK [8255]). Discussion of how to limit access to lethal means also is important.
Because safety planning is a collaborative process, it is imperative that practitioners check on the patient’s willingness to follow the safety plan and help him overcome perceived obstacles in implementation. Copies of the plan can be kept at different locations and shared with family members, friends, or both with the patient’s permission.
Develop a cognitive-behavioral conceptualization. The cognitive-behavioral conceptualization is an individualized map of a patient’s automatic thoughts (eg, “I am going to get fired today”), conditional assumptions (“If I get fired, then my life is over”), and core beliefs (“I am an utter failure”) that are activated before, during, and after suicidal self-directed violence. To develop that conceptualization, the patient is asked to tell a story about his (her) most recent suicidal crisis (the Box, offers a sample script) and to describe reactions to having survived a suicide attempt. (Note: Patients who report regret after an attempt are at greatest risk for dying by suicide.10)
This activity gives the patient an opportunity to disclose details surrounding his suicidal thoughts and actions, and might allow for a cathartic experience through storytelling. As practitioners listen to the suicide narrative, they collect data on the patient’s early childhood experiences (typically, suicide-activating events), associated automatic thoughts and images, emotional responses, and subsequent behaviors.
Based on this information, a cognitive-behavioral case conceptualization diagram (Figure 1, and Table 2) is generated collaboratively with the patienta and used to personalize treatment planning.
a Judith Beck offers sample case conceptualization diagrams in Cognitive behavior therapy: Basics and beyond, 2nd ed. New York, New York: Guilford Press; 2011.
Phase II: Build skills
Build skills to prevent episodes of suicidal self-directed violence. Information obtained from the conceptualization is used to generate an individualized cognitive-behavioral plan of intervention. The overall goal is to determine skill-based problem areas that are associated with the most recent episode of suicidal self-directed violence.
Practitioner and patient collaboratively identify skills that are underdeveloped and ones that are overdeveloped so that they can be addressed systematically. In general, based on our clinical experience with suicidal patients, we recommend focusing on skills captured within ≥1 of the deficit domains in Table 3. Explanation of the various cognitive-behavioral strategies used in this phase of treatment is beyond the scope of this article, but 2 activities that highlight the clinical work conducted in this phase are described in the following sections. We selected those activities because they are easy to implement and, we have found, receive overall patient acceptability.
For a detailed understanding of strategies used in Phase II of CBT for preventing suicide, see Related Resources. In addition, the book Choosing to live: How to defeat suicide through cognitive therapy11 can serve as a self-help guide for patients to follow through with CBT skill-building strategies.
Sample activity #1: Construct a ‘hope box.’ One activity that you can use to help a patient cope with suicide-activating core beliefs (eg, “My life is worthless”) involves construction of a so-called hope box. The box helps the patient directly challenge his extreme distress, by being reminded of previous successes, positive experiences, and reasons for living. The process of constructing a hope box allows the patient to work on modifying his problematic core beliefs (eg, worthlessness, helplessness, incapable of being loved).
It can be helpful to have the patient construct his hope box during a session, to ensure that everything that is put in the box is truly helpful and personalized. Items included vary from patient to patient, and might consist of pictures of loved ones, a favorite poem, a prayer, coping cards (see next section), or all of these. For example, one of our patients chose to include a picture of herself in her early 20s as a reminder of a positive, fulfilling time in her life; this gave her hope that it is possible to experience those feelings again.
Bush et alb at the National Center for Telehealth and Technology have developed a Virtual Hope Box, a free mobile application for tablets and smartphones (compatible with Android and iOS operating systems) that patients can use under the guidance of their practitioner.
bwww.t2.health.mil/apps/virtual-hope-box
Sample activity #2: Generate coping cards. Effective problem-solving skills can be promoted by having the patient construct coping cards —wallet-size cards generated collaboratively in session. Coping cards are note cards that a patient keeps nearby to cope better during a difficult situation. They provide an easily accessible way to jump-start adaptive thinking during a suicidal crisis. The patient is encouraged to use coping cards to practice adaptive thinking even when not in a crisis.
There are 3 kinds of coping cards:
• place a suicide-relevant automatic thought or core belief on one side of the card; on the other side, place an alternative, more adaptive response
• write a list of coping strategies
• write instructions to motivate or “activate” the patient toward completing a specific goal (Figure 2).
Phase III: Prevent relapse
Complete relapse prevention task. Relapse prevention is a common CBT strategy that aims to strengthen self-management to minimize likelihood of returning to a previously stopped behavior. For patients who present only with suicidal thoughts, relapse prevention is directed at identifying triggers and minimizing the occurrence and/ or intensity of such thoughts in the future. For patients who present with suicidal self-directed violence, relapse prevention is directed at identifying triggers for suicidal actions and reducing the likelihood of acting on suicidal urges. The brief guidance provided below will familiarize you with each of the relapse prevention steps, which may be completed in multiple sessions.
Step 1: Provide psychoeducation
Explain the difference between a lapse and a relapse. In general, you want the patient to understand that, although suicidal thoughts might persist and recur over time, suicidal self-directed violence must be prevented. Describe the purpose of the relapse prevention task (ie, to minimize the chance that suicidal thinking and actions will recur); address questions and concerns; and obtain permission to begin the procedure. Assure the patient that this is a collaborative activity and you will be in the room to ensure comfort and safety.
STEP 2: Retell the suicide story
Ask the patient to imagine the chain of events, thoughts, and feelings that led to the most recent episode of suicide ideation or suicidal self-directed violence. Tell the patient that you want him to construct a movie script to describe the chain of events that resulted in the suicide crisis —but to do so slowly, taking enough time to describe the details of each scene of the movie.
Step 3: Apply CBT skills
Ask the patient again to take you through the sequence of events leading to the most recent episode of suicide ideation or suicidal self-directed violence. This time, however, direct him to use the skills learned in therapy to appropriately respond cognitively, affectively, and behaviorally to move further away from the suicide outcome.
If the patient is moving too fast or neglecting important points, stop and ask about alternative ways of thinking, feeling, and behaving. Use as much time as needed until the patient is able to demonstrate solid learning of at least several learned CBT strategies to prevent suicidal self-directed violence.
Step 4: Generalize learning to prepare for future suicidal crises
In this stage —given your knowledge of the patient’s psychosocial history, cognitive- behavioral conceptualization, and suicide mode triggers —you collaboratively create a future scenario that is likely to activate suicidal self-directed violence. Question the patient about possible coping strategies, provide helpful feedback, guide him through each link in the chain of events, and propose additional alternative strategies if he is clearly neglecting important points of the intervention.
Step 5: Debrief and summarize lessons learned
Debrief the patient by providing a summary of the skills he has learned in therapy, congratulate him for completing this final therapeutic task, and assess overall emotional reaction to this activity. Remind him that mood fluctuations and future setbacks, in the form of lapses, are expected. Give him the option to request booster sessions and make plans for next steps in accomplishing general goals of therapy.
Treatment can be terminated when the patient is able to complete the relapse prevention task. If he is not ready or able to complete this exercise successfully, you can extend treatment. The duration of the extension is left to the practitioner’s judgment, based on the overall treatment plan. Brown and colleagues2 have reported a maximum number of 24 outpatient sessions (for patients who need additional booster sessions); based on clinical experience, it is reasonable to assume that it would be highly unlikely for a patient not to meet treatment objectives after a methodical course of outpatient CBT.
In cases in which goals of treatment have not been met, consultation with colleagues, review of adherence problems, and consideration of obstacles for treatment efficacy would be recommended.
A checklist can be used to determine whether a patient is ready to end treatment. Variables that can be considered in assessing readiness for termination include:
• reduced scores on self-report measures for a number of weeks
• evidence of enhanced problem-solving
• engagement in adjunctive health care services
• development of a social support system.
Post-Admission Cognitive Therapy (PACT)
An inpatient cognitive-behavioral protocol for the prevention of suicide, adapted from the efficacious outpatient model, is being evaluated at the Walter Reed National Military Medical Center, Bethesda, Maryland, and Fort Belvoir Community Hospital, Fort Belvoir, Virginia. The inpatient intervention is called PACT; components are summarized in Table 4.
Bottom Line
Cognitive-behavioral therapy for preventing suicide is an efficacious protocol for reducing the recurrence of suicidal self-directed violence. Post-Admission Cognitive Therapy is the adapted inpatient treatment package. You are encouraged to gain additional training and supervision on the delivery of these interventions to your high-risk suicidal patients.
Related Resources
• Academy of Cognitive Therapy. www.academyofct.org.
• National Suicide Prevention Lifeline. www.suicide preventionlifeline.org.
• Wenzel A, Brown GK, Beck AT. Cognitive therapy for suicidal patients: scientific and clinical applications. Washington, DC: American Psychological Association; 2009.
Disclosures
Support for research on inpatient cognitive-behavioral therapy for the prevention of suicide provided to Principal Investigator, Dr. Ghahramanlou-Holloway by the Department of Defense, Congressionally Directed Medical Research Program (W81XWH-08-2-0172), Military Operational Medicine Research Program (W81XWH-11-2-0106), and the National Alliance for Research on Schizophrenia and Depression (15219).
Many mental health practitioners have had training in cognitive-behavioral therapy (CBT)—short-term, evidence-based psychotherapy for treating a variety of psychiatric conditions (eg, posttraumatic stress disorder) and medical comorbidities (eg, insomnia)—but only some are knowledgeable about how to best use CBT with a suicidal patient. This article provides a clinician-friendly summary of a 10-session evidence-based outpatient1-3 and an adapted 6 to 8 session inpatient4,5 cognitive-behavioral protocol (known as Post-Admission Cognitive Therapy [PACT]) that is designed to help patients who have suicide-related thoughts and/or behaviors.
3 phases of CBT for suicide prevention
An average of 9 hours of individual CBT for the prevention of suicide has been reported to reduce the likelihood of repeat suicide attempts in approximately 50% of patients.1 Here, we introduce you to 3 phases of CBT for preventing suicide—phases that are the same for outpatients or inpatients. Our aim is to help you become familiar with CBT strategies that can be adapted for your treatment setting and used to intervene with vulnerable patients who are at risk for suicidal self-directed violence. A thorough assessment of the patient’s psychiatric diagnosis and history, presenting problems, and risk and protective factors for suicide must be completed before treatment begins.
Phase I. The patient is asked to tell a story associated with his (her) most recent episode of suicidal thoughts or behavior, or both. This narrative serves as 1) a foundation for planning treatment and 2) a model for understanding how best to deactivate the wish to die through the process of psychotherapy.
Phase II. The patient is assisted with modifying underdeveloped or overdeveloped skills that are most closely associated with the risk of triggering a suicidal crisis. For example, a patient with underdeveloped skills in regulating anger and hatred toward himself is taught to modulate these problematic emotions more effectively. In addition, effective problem-solving strategies are reviewed and practiced.
Phase III. The patient is guided through a relapse prevention task. The purpose of this exercise is to 1) highlight skills learned during therapy and 2) allow the patient to practice effective problem-solving strategies that are aimed at minimizing the recurrence of suicidal self-directed violence.
Theorectical basis for preventing suicide with CBT
Aaron Beck, in 1979,6 proposed that a person’s biopsychosocial vulnerabilities can interact with suicidal thoughts and behaviors to produce a state that Beck labeled the “suicide mode.” Once produced, a suicide mode can become activated by cognitive, affective, motivational, and behavioral systems.
The frequency and severity of suicide mode activation can increase over time, especially for persons who do not have protective factors and those who have a history of self-directed violence—in particular, attempted suicide. Moreover, some persons might experience a chronic state of suicide mode activation and, therefore, remain at elevated risk of suicide. Once a suicide-specific mode is activated, the person considers suicide the only option for solving his life problems. Suicide might be considered a rational decision at this point.6
The hypothesized mechanism of action associated with CBT for preventing suicide can be described as:
• deactivation of the suicide mode
• modification of the structure and content of the suicide mode
• construction and practice of more adaptive structural modes to promote a desire to live.
The underlying philosophy of this intervention is that the suicide mode occurs independently of psychiatric diagnoses and must be targeted directly; treatment therefore is transdiagnostic.7 In other words, instead of addressing a symptom of a psychiatric disorder, treatment directly targets suicide-related ideation and behaviors (Table 1).
Using that framework, psychiatric diagnoses are conceptualized in terms of how the associated symptoms contribute to the activation, maintenance, and exacerbation of the suicide mode.
Protocol for preventing suicide
The outpatient protocol1-3 comprises 10, 45- to 50-minute weekly individual psychotherapy sessions, with an allowance for booster sessions (as needed), until the patient is able to complete the relapse prevention task in Phase III. The inpatient protocol4,5 comprises 6, 90-minute individual psychotherapy sessions, with an allowance for 2 booster sessions (as needed) during the inpatient stay and as many as 4 telephone booster sessions after discharge.
Phase I: Tell the suicide story
Engage the patient in treatment. To increase adherence to treatment and minimize the risk of drop-out, practitioners are encouraged to establish a strong, early therapeutic alliance with the patient. Showing genuine empathy and providing a safe, supportive, and nonjudgmental environment are instrumental for engaging patients in treatment. The practitioner listens carefully to the patient’s narrative, provides periodic summaries to check on accurate understanding, and keeps interruptions to a minimum.
Collaboratively generate a safety plan. A crisis response plan or safety plan—an individualized, hierarchically arranged, written list of coping strategies to be implemented during a suicide crisis—is developed as soon as possible. Guidance on how to develop a structured safety plan has been provided by Stanley and Brown.8,9
Practitioners must ensure that the safety plan contains contact information that the patient can use to reach the practitioner, the clinic, the on-call provider (if available), the local 24-hour emergency department, and the 24/7 National Suicide Prevention Lifeline (800-273-TALK [8255]). Discussion of how to limit access to lethal means also is important.
Because safety planning is a collaborative process, it is imperative that practitioners check on the patient’s willingness to follow the safety plan and help him overcome perceived obstacles in implementation. Copies of the plan can be kept at different locations and shared with family members, friends, or both with the patient’s permission.
Develop a cognitive-behavioral conceptualization. The cognitive-behavioral conceptualization is an individualized map of a patient’s automatic thoughts (eg, “I am going to get fired today”), conditional assumptions (“If I get fired, then my life is over”), and core beliefs (“I am an utter failure”) that are activated before, during, and after suicidal self-directed violence. To develop that conceptualization, the patient is asked to tell a story about his (her) most recent suicidal crisis (the Box, offers a sample script) and to describe reactions to having survived a suicide attempt. (Note: Patients who report regret after an attempt are at greatest risk for dying by suicide.10)
This activity gives the patient an opportunity to disclose details surrounding his suicidal thoughts and actions, and might allow for a cathartic experience through storytelling. As practitioners listen to the suicide narrative, they collect data on the patient’s early childhood experiences (typically, suicide-activating events), associated automatic thoughts and images, emotional responses, and subsequent behaviors.
Based on this information, a cognitive-behavioral case conceptualization diagram (Figure 1, and Table 2) is generated collaboratively with the patienta and used to personalize treatment planning.
a Judith Beck offers sample case conceptualization diagrams in Cognitive behavior therapy: Basics and beyond, 2nd ed. New York, New York: Guilford Press; 2011.
Phase II: Build skills
Build skills to prevent episodes of suicidal self-directed violence. Information obtained from the conceptualization is used to generate an individualized cognitive-behavioral plan of intervention. The overall goal is to determine skill-based problem areas that are associated with the most recent episode of suicidal self-directed violence.
Practitioner and patient collaboratively identify skills that are underdeveloped and ones that are overdeveloped so that they can be addressed systematically. In general, based on our clinical experience with suicidal patients, we recommend focusing on skills captured within ≥1 of the deficit domains in Table 3. Explanation of the various cognitive-behavioral strategies used in this phase of treatment is beyond the scope of this article, but 2 activities that highlight the clinical work conducted in this phase are described in the following sections. We selected those activities because they are easy to implement and, we have found, receive overall patient acceptability.
For a detailed understanding of strategies used in Phase II of CBT for preventing suicide, see Related Resources. In addition, the book Choosing to live: How to defeat suicide through cognitive therapy11 can serve as a self-help guide for patients to follow through with CBT skill-building strategies.
Sample activity #1: Construct a ‘hope box.’ One activity that you can use to help a patient cope with suicide-activating core beliefs (eg, “My life is worthless”) involves construction of a so-called hope box. The box helps the patient directly challenge his extreme distress, by being reminded of previous successes, positive experiences, and reasons for living. The process of constructing a hope box allows the patient to work on modifying his problematic core beliefs (eg, worthlessness, helplessness, incapable of being loved).
It can be helpful to have the patient construct his hope box during a session, to ensure that everything that is put in the box is truly helpful and personalized. Items included vary from patient to patient, and might consist of pictures of loved ones, a favorite poem, a prayer, coping cards (see next section), or all of these. For example, one of our patients chose to include a picture of herself in her early 20s as a reminder of a positive, fulfilling time in her life; this gave her hope that it is possible to experience those feelings again.
Bush et alb at the National Center for Telehealth and Technology have developed a Virtual Hope Box, a free mobile application for tablets and smartphones (compatible with Android and iOS operating systems) that patients can use under the guidance of their practitioner.
bwww.t2.health.mil/apps/virtual-hope-box
Sample activity #2: Generate coping cards. Effective problem-solving skills can be promoted by having the patient construct coping cards —wallet-size cards generated collaboratively in session. Coping cards are note cards that a patient keeps nearby to cope better during a difficult situation. They provide an easily accessible way to jump-start adaptive thinking during a suicidal crisis. The patient is encouraged to use coping cards to practice adaptive thinking even when not in a crisis.
There are 3 kinds of coping cards:
• place a suicide-relevant automatic thought or core belief on one side of the card; on the other side, place an alternative, more adaptive response
• write a list of coping strategies
• write instructions to motivate or “activate” the patient toward completing a specific goal (Figure 2).
Phase III: Prevent relapse
Complete relapse prevention task. Relapse prevention is a common CBT strategy that aims to strengthen self-management to minimize likelihood of returning to a previously stopped behavior. For patients who present only with suicidal thoughts, relapse prevention is directed at identifying triggers and minimizing the occurrence and/ or intensity of such thoughts in the future. For patients who present with suicidal self-directed violence, relapse prevention is directed at identifying triggers for suicidal actions and reducing the likelihood of acting on suicidal urges. The brief guidance provided below will familiarize you with each of the relapse prevention steps, which may be completed in multiple sessions.
Step 1: Provide psychoeducation
Explain the difference between a lapse and a relapse. In general, you want the patient to understand that, although suicidal thoughts might persist and recur over time, suicidal self-directed violence must be prevented. Describe the purpose of the relapse prevention task (ie, to minimize the chance that suicidal thinking and actions will recur); address questions and concerns; and obtain permission to begin the procedure. Assure the patient that this is a collaborative activity and you will be in the room to ensure comfort and safety.
STEP 2: Retell the suicide story
Ask the patient to imagine the chain of events, thoughts, and feelings that led to the most recent episode of suicide ideation or suicidal self-directed violence. Tell the patient that you want him to construct a movie script to describe the chain of events that resulted in the suicide crisis —but to do so slowly, taking enough time to describe the details of each scene of the movie.
Step 3: Apply CBT skills
Ask the patient again to take you through the sequence of events leading to the most recent episode of suicide ideation or suicidal self-directed violence. This time, however, direct him to use the skills learned in therapy to appropriately respond cognitively, affectively, and behaviorally to move further away from the suicide outcome.
If the patient is moving too fast or neglecting important points, stop and ask about alternative ways of thinking, feeling, and behaving. Use as much time as needed until the patient is able to demonstrate solid learning of at least several learned CBT strategies to prevent suicidal self-directed violence.
Step 4: Generalize learning to prepare for future suicidal crises
In this stage —given your knowledge of the patient’s psychosocial history, cognitive- behavioral conceptualization, and suicide mode triggers —you collaboratively create a future scenario that is likely to activate suicidal self-directed violence. Question the patient about possible coping strategies, provide helpful feedback, guide him through each link in the chain of events, and propose additional alternative strategies if he is clearly neglecting important points of the intervention.
Step 5: Debrief and summarize lessons learned
Debrief the patient by providing a summary of the skills he has learned in therapy, congratulate him for completing this final therapeutic task, and assess overall emotional reaction to this activity. Remind him that mood fluctuations and future setbacks, in the form of lapses, are expected. Give him the option to request booster sessions and make plans for next steps in accomplishing general goals of therapy.
Treatment can be terminated when the patient is able to complete the relapse prevention task. If he is not ready or able to complete this exercise successfully, you can extend treatment. The duration of the extension is left to the practitioner’s judgment, based on the overall treatment plan. Brown and colleagues2 have reported a maximum number of 24 outpatient sessions (for patients who need additional booster sessions); based on clinical experience, it is reasonable to assume that it would be highly unlikely for a patient not to meet treatment objectives after a methodical course of outpatient CBT.
In cases in which goals of treatment have not been met, consultation with colleagues, review of adherence problems, and consideration of obstacles for treatment efficacy would be recommended.
A checklist can be used to determine whether a patient is ready to end treatment. Variables that can be considered in assessing readiness for termination include:
• reduced scores on self-report measures for a number of weeks
• evidence of enhanced problem-solving
• engagement in adjunctive health care services
• development of a social support system.
Post-Admission Cognitive Therapy (PACT)
An inpatient cognitive-behavioral protocol for the prevention of suicide, adapted from the efficacious outpatient model, is being evaluated at the Walter Reed National Military Medical Center, Bethesda, Maryland, and Fort Belvoir Community Hospital, Fort Belvoir, Virginia. The inpatient intervention is called PACT; components are summarized in Table 4.
Bottom Line
Cognitive-behavioral therapy for preventing suicide is an efficacious protocol for reducing the recurrence of suicidal self-directed violence. Post-Admission Cognitive Therapy is the adapted inpatient treatment package. You are encouraged to gain additional training and supervision on the delivery of these interventions to your high-risk suicidal patients.
Related Resources
• Academy of Cognitive Therapy. www.academyofct.org.
• National Suicide Prevention Lifeline. www.suicide preventionlifeline.org.
• Wenzel A, Brown GK, Beck AT. Cognitive therapy for suicidal patients: scientific and clinical applications. Washington, DC: American Psychological Association; 2009.
Disclosures
Support for research on inpatient cognitive-behavioral therapy for the prevention of suicide provided to Principal Investigator, Dr. Ghahramanlou-Holloway by the Department of Defense, Congressionally Directed Medical Research Program (W81XWH-08-2-0172), Military Operational Medicine Research Program (W81XWH-11-2-0106), and the National Alliance for Research on Schizophrenia and Depression (15219).
1. Brown GK, Ten Have T, Henriques GR, et al. Cognitive therapy for the prevention of suicide attempts: a randomized controlled trial. JAMA. 2005;294(5):563-570.
2. Brown GK, Henriques GR, Ratto C, et al. Cognitive therapy treatment manual for suicide attempters. Philadelphia, PA: University of Pennsylvania; 2002 (unpublished).
3. Berk MS, Henriques GR, Warman DM, et al. A cognitive therapy intervention for suicide attempters: an overview of the treatment and case examples. Cogn Behav Pract. 2004;11(3):265-277.
4. Ghahramanlou-Holloway M, Cox D, Greene F. Post-admission cognitive therapy: a brief intervention for psychiatric inpatients admitted after a suicide attempt. Cogn Behav Pract. 2012;19(2):233-244.
5. Neely L, Irwin K, Carreno Ponce JT, et al. Post Admission Cognitive Therapy (PACT) for the prevention of suicide in military personnel with histories of trauma: treatment development and case example. Clinical Case Studies. 2013;12(6):457-473.
6. Beck AT. Cognitive therapy and the emotional disorders. New York, NY: Penguin Group; 1979.
7. Ghahramanlou-Holloway M, Brown GK, Beck AT. Suicide. In: Whisman M, ed. Adapting cognitive therapy for depression: managing complexity and comorbidity. New York, NY: Guilford Press; 2008:159-184.
8. Stanley B, Brown GK. Safety planning intervention: a brief intervention to mitigate suicide risk. Cogn Behav Pract. 2012;19(2):256-264.
9. Stanley B, Brown GK. Safety plan treatment manual to reduce suicide risk: veteran version. http://www. mentalhealth.va.gov/docs/va_safety_planning_manual. pdf. Published August 20, 2008. Accessed July 2, 2014.
10. Henriques G, Wenzel A, Brown GK, et al. Suicide attempters’ reaction to survival as a risk factor for eventual suicide. Am J Psychiatry. 2005;162(11):2180-2182.
11. Ellis TE, Newman CF. Choosing to live: How to defeat suicide through cognitive therapy. Oakland, CA: New Harbinger Publications, Inc; 1996.
1. Brown GK, Ten Have T, Henriques GR, et al. Cognitive therapy for the prevention of suicide attempts: a randomized controlled trial. JAMA. 2005;294(5):563-570.
2. Brown GK, Henriques GR, Ratto C, et al. Cognitive therapy treatment manual for suicide attempters. Philadelphia, PA: University of Pennsylvania; 2002 (unpublished).
3. Berk MS, Henriques GR, Warman DM, et al. A cognitive therapy intervention for suicide attempters: an overview of the treatment and case examples. Cogn Behav Pract. 2004;11(3):265-277.
4. Ghahramanlou-Holloway M, Cox D, Greene F. Post-admission cognitive therapy: a brief intervention for psychiatric inpatients admitted after a suicide attempt. Cogn Behav Pract. 2012;19(2):233-244.
5. Neely L, Irwin K, Carreno Ponce JT, et al. Post Admission Cognitive Therapy (PACT) for the prevention of suicide in military personnel with histories of trauma: treatment development and case example. Clinical Case Studies. 2013;12(6):457-473.
6. Beck AT. Cognitive therapy and the emotional disorders. New York, NY: Penguin Group; 1979.
7. Ghahramanlou-Holloway M, Brown GK, Beck AT. Suicide. In: Whisman M, ed. Adapting cognitive therapy for depression: managing complexity and comorbidity. New York, NY: Guilford Press; 2008:159-184.
8. Stanley B, Brown GK. Safety planning intervention: a brief intervention to mitigate suicide risk. Cogn Behav Pract. 2012;19(2):256-264.
9. Stanley B, Brown GK. Safety plan treatment manual to reduce suicide risk: veteran version. http://www. mentalhealth.va.gov/docs/va_safety_planning_manual. pdf. Published August 20, 2008. Accessed July 2, 2014.
10. Henriques G, Wenzel A, Brown GK, et al. Suicide attempters’ reaction to survival as a risk factor for eventual suicide. Am J Psychiatry. 2005;162(11):2180-2182.
11. Ellis TE, Newman CF. Choosing to live: How to defeat suicide through cognitive therapy. Oakland, CA: New Harbinger Publications, Inc; 1996.
Opportunities to partner with clinical pharmacists in ambulatory psychiatry
In this article, we highlight key steps that were needed to integrate clinical pharmacy specialists at an academic ambulatory psychiatric and addiction treatment center that serves pediatric and adult populations. Academic stakeholders identified addition of pharmacy services as a strategic goal in an effort to maximize services offered by the center and increase patient access to care while aligning with the standards set out by the patient-centered medical home (PCMH) model.
We outline the role of clinical pharmacists in the care of adult patients in ambulatory psychiatry, illustrate opportunities to enhance patient care, point out possible challenges with implementation, and propose future initiatives to optimize the practitioner-pharmacist partnership.
Background: Role of ambulatory pharmacists in psychiatry
Clinical pharmacists’ role in the psychiatric ambulatory care setting generally is associated with positive outcomes. One study looking at a collaborative care model that utilized clinical pharmacist follow-up in managing major depressive disorder found that patients who received pharmacist intervention in the collaborative care model had, on average, a significantly higher adherence rate and patient satisfaction score than the “usual care” group.1 Within this study, patients in both groups experienced global clinical improvement with no significant difference; however, pharmacist interventions had a positive impact on several aspects of the care model, suggesting that pharmacists can be used effectively in ambulatory psychiatry.
Furthermore, a systematic study evaluating pharmacists’ impact on clinical and functional mental health outcomes identified 8 relevant studies conducted in the outpatient setting.2 Although interventions varied widely, most studies focused on pharmacists’ providing a combination of drug monitoring, treatment recommendations, and patient education. Outcomes were largely positive, including an overall reduction in number and dosage of psychiatric drugs, inferred cost savings, and significant improvements in the safe and efficacious use of antidepressant and antipsychotic medications.
These preliminary positive results require replication in larger, randomized cohorts. Additionally, the role of the pharmacist as medication manager in the collaborative care model requires further study. Results so far, however, indicate that pharmacists can have a positive impact on the care of ambulatory psychiatry patients. Nevertheless, there is still considerable need for ongoing exploration in this field.
Pre-implementation
The need for pharmacy services. Various initiatives and existing practices within our health care system have underscored the need for a psychiatric pharmacist in the outpatient setting (Table 1).
A board-certified psychiatric pharmacist (BCPP) possesses specialized knowledge about treating patients affected by psychiatric illnesses. BCPPs work with prescribers and members of other disciplines, such as nurses and social workers, to optimize drug treatment by making pharmacotherapeutic recommendations and providing appropriate monitoring to enhance patient satisfaction and quality of life.3,4
Existing relationship with pharmacy. Along with evidence to support the positive impact clinical pharmacists can have in caring for patients with mental illness in the outpatient setting, a strong existing relationship between the Department of Psychiatry and our adult inpatient psychiatric pharmacist helped make it possible to develop an ambulatory psychiatric pharmacist position.
Each day, the inpatient psychiatric pharmacist works closely with the attending psychiatrists and psychiatry residents to provide treatment recommendations and counseling services for patients on the unit. The psychiatry residents highly valued their experiences with the pharmacist in the inpatient setting and expressed disappointment that this collaborative relationship was no longer available after they transitioned into the ambulatory setting.
Further, by being involved in initiatives that were relevant to both inpatient and outpatient psychiatry, such as metabolic monitoring for patients taking atypical antipsychotics, the clinical pharmacist in inpatient psychiatry had the opportunity to interact with key stakeholders in both settings. As a result of these pre-existing collaborative relationships, many clinicians were eager to have pharmacists available as a resource for patient care in the outpatient setting.
Pharmacist perspective: Outreach to psychiatry leadershipRecognizing the incentives and opportunities inherent in our emerging health care system, pharmacists became integral members of the patient care team in the PCMH model. Thanks to this effort, we now have PCMH pharmacists at every primary care health center in our health system (14 sites), providing disease management programs and polypharmacy services.
PCMH pharmacists’ role in the primary care setting fueled interest from specialty services and created opportunities to extend our existing partnership in inpatient psychiatry. One such opportunity to demonstrate the expertise of a psychiatric pharmacist was fueled by the FDA’s citalopram dosing alert5 at a system-wide level. This warning emerged as a chance to showcase the skill set of psychiatric pharmacists and the pharmacists’ successes in our PCMH model. The partnership was extended to include the buy-in of ambulatory pharmacy leadership and key stakeholders in ambulatory psychiatry.
Initial meetings included ambulatory care site leadership in psychiatry to increase awareness and understanding of pharmacists’ potential role in direct patient care. Achieving site leadership support was critical to successful implementation of pharmacist services in psychiatry. We also obtained approval from the Chair of the Department of Psychiatry to elicit support from faculty group practice.
Psychiatry leadership perspective
As fiscal pressures intensify at academic health centers, it becomes increasingly important for resources to be used as efficiently and effectively as possible. As a greater percentage of mental health patients with more “straightforward,” less complex conditions are being managed by their primary care providers or nonprescribing psychotherapists, or both, the acuity and complexity of cases in patients who present to psychiatric clinics have intensified. This intensification of patient needs and clinical acuity is in heightened conflict with the ongoing demand for clinician productivity and efficiency.
Additionally, the need to provide care to a seemingly ever-growing number of moderately or severely ill patients during shorter, less frequent visits presents a daunting task for clinicians and clinical leaders. Collaborative care models appear to offer the best hope for managing the seemingly overwhelming demand for services.
In this model, the patient, who is the critical member of the team, is expected to become an “expert” on his or her illness and to partner with members of the multidisciplinary team; with this support, patients are encouraged to develop a broad range of self-management skills and strategies to manage their illness. We believe that clinical pharmacists can and should play a critical role, not only in delivering direct clinical services to patients but also in developing and devising the care models that will most effectively apply each team member’s unique set of knowledge, skills, and experience. Given the large percentage of our patients who have multiple medical comorbidities and who require complex medical and psychiatric medication regimens, the role of the pharmacist in reviewing, educating, and advising patients and other team members on these crucial pharmacy concerns will be paramount.
In light of these complex medication issues, pharmacists are uniquely positioned to serve as a liaison among the patient, the primary care provider, and other members of their treatment team. We anticipate that our ambulatory psychiatry pharmacists will greatly enhance the comfort and confidence of patients and their primary care providers during periods of care transition.
Potential roles for pharmacists in ambulatory psychiatry
One potential role for pharmacists in ambulatory psychiatry is to perform polypharmacy assessments of patients receiving complex medication regimens, prompted by physician referral. The poly pharmacy intake interview, performed to obtain an accurate medication list and to identify patients’ concerns about their medications, can be conducted in person or by telephone. Patients’ knowledge about medications and medication adherence are discussed, as are their perceptions of effectiveness and adverse effects.
After initial data gathering, pharmacists complete a review of the medications, identifying any problems associated with medication indication, efficacy, tolerance, or adverse effects, drug-drug interactions, drug-nutrient interactions, and nonadherence. Pharmacists work to reduce medication costs if that is a concern of the patient, because nonadherence can result. A medication care plan is then developed in consultation with the primary care provider; here, the medication list is reconciled, the electronic medical record is updated, and actions to address any medication-related problems are prioritized.
Other services that might be offered include:• group education classes, based on patient motivational interviewing strategies, to address therapeutic nonadherence and to improve understanding of their disease and treatment regimens• medication safety and monitoring• treatment intensification, as needed, following established protocols.
These are a few of the ways in which pharmacists can be relied on to expand and improve access to patient care services within ambulatory psychiatry. Key stakeholders anticipate development of newer ideas as the pharmacist’s role in ambulatory psychiatry is increasingly clarified.
Reimbursement model
In creating a role for pharmacy in ambulatory psychiatry, it was essential that the model be financially viable and appealing. Alongside its clinical model, our institution has developed a financial model to support the pharmacist’s role. The lump-sum payment to the health centers from Blue Cross Blue Shield of Michigan afforded the ambulatory care clinics an opportunity to invest in PCMH pharmacists. This funding, and the reimbursement based on T-code billing (face-to-face visits and phone consultation) for depression and other conditions requiring chronic care, provides ongoing support. From our experience, understanding physician reimbursement models and identifying relevant changes in health care reform are necessary to integrate new providers, including pharmacists, into a team-based care model.
Implementation
Promoting pharmacy services. To foster anticipated collaboration with clinical pharmacists, the medical director of outpatient psychiatry disseminated an announcement to all providers regarding the investiture of clinical pharmacists to support patient care activities, education, and research. Clinicians were educated about the pharmacists’ potential roles and about guidelines and methods for referral. Use of our electronic health record system enabled us to establish a relatively simple referral process involving sharing electronic messages with our pharmacists.
Further, as part of the planned integration of clinical pharmacists in the ambulatory psychiatry setting, pharmacists met strategically with members of various disciplines, clinical programs, specialty clinic programs, and teams throughout the center. In addition to answering questions about the referral process, they emphasized the role of pharmacy and opportunities for collaboration.
Collaborating with others. Because the involvement of clinical pharmacists is unfamiliar to some practitioners in outpatient psychiatry, it is important to develop services without infringing on the roles other disciplines play. Indeed, a survey by Wheeler et al6 identified many concerns and potential boundaries among pharmacists, other providers, and patients. Concerns included confusion of practitioner roles and boundaries, a too-traditional perception of the pharmacist, and demonstration of competence.6
Early on, we developed a structured forum to discuss ongoing challenges and address issues related to the rapidly changing clinical landscape. During these discussions we conveyed that adding pharmacists to psychiatric services would be collaborative in nature and intended to augment existing services. This communication was pivotal to maintain the psychiatrist’s role as the ultimate prescriber and authority in the care of their patients; however, the pharmacist’s expertise, when sought, would help spur clinical and academic discussion that will benefit the patient. These discussions are paramount to achieving a productive, team-based approach, to overcome challenges, and to identify opportunities of value to our providers and patients (Box).
Work in progress
Implementing change in any clinical setting invariably creates challenges, and our endeavors to integrate clinical pharmacists into ambulatory psychiatry are no exception. We have identified several factors that we believe will optimize successful collaboration between pharmacy and ambulatory psychiatry (Table 2). Our primary challenge has been changing clinician behavior. Clinical practitioners can become too comfortable, wedded to their routines, and often are understandably resistant to change. Additionally, clinical systems often are inadvertently designed to obstruct change in ways that are not readily apparent. Efforts must be focused on behaviors and practices the clinical culture should encourage.
Regarding specific initiatives, clinical pharmacists have successfully identified patients on higher than recommended dosages of citalopram; they are working alongside prescribers to recommend ways to minimize the risk of heart rhythm abnormalities in these patients. Numerous prescribers have sought clinical pharmacists’ input to manage pharmacotherapy in their patients and to respond to patients’ questions on drug information.
The prospect of access to clinical pharmacist expertise in the outpatient setting was heralded with excitement, but the flow of referrals and consultations has been uneven. However simple the path for referral is, clinicians’ use of the system has been inconsistent—perhaps because of referrals’ passive, clinician-dependent nature. Educational outreach efforts often prompt a brief spike in referrals, only to be followed over time by a slow, steady drop-off. More active strategies will be needed, such as embedding the pharmacists as regular, active, visible members of the various clinical teams, and implementing a system in which patient record reviews are assigned to the pharmacists according to agreed-upon clusters of clinical criteria.
One of these tactics has, in the short term, showed success. Embedded in one of our newer clinics, which were designed to bridge primary and psychiatric care, clinical pharmacists are helping manage medically complicated patients. They assist with medication selection in light of drug interactions and medical comorbidities, conduct detailed medication histories, schedule follow-up visits to assess medication adherence and tolerability, and counsel patients experiencing insurance changes that make their medications less affordable. Integrating pharmacists in the new clinics has resulted in a steady flow of patient referrals and collaborative care work.
Clinical pharmacists are brainstorming with outpatient psychiatry leadership to build on these early successes. Ongoing communication and enhanced collaboration are essential, and can only improve the lives of our psychiatric patients.
For the future
Our partnership in ambulatory psychiatry was timed to occur during implementation of our health system’s new electronic health record initiative. Clinical pharmacists can play a key role in demonstrating use of the system to provide consistently accurate drug information to patients and to monitor patients receiving specific medications.
Development of ambulatory patient medication education groups, which has proved useful on the inpatient side, is another endeavor in the works. Integrating the clinical pharmacist with psychiatrists, psychologists, nurse practitioners, social workers, and trainees on specific teams devoted to depression, bipolar disorder, anxiety, perinatal mental health, and personality disorders also might prove to be a wide-ranging and promising strategy.
Enhancing the education and training experiences of residents, fellows, medical students, pharmacy students, and allied health professional learners present in our clinics is another exciting prospect. This cross-disciplinary training will yield a new generation of providers who will be more comfortable collaborating with colleagues from other disciplines, all intent on providing high-quality, efficient care. We hope that, as these initiatives take root, we will recognize many opportunities to disseminate our collaborative efforts in scholarly venues, documenting and sharing the positive impact of our partnership.
Bottom Line
Because psychiatric outpatients present with challenging medical comorbidities and increasingly complex medication regimens, specialized clinical pharmacists can enrich the management team by offering essential monitoring and polypharmacy services, patient education and counseling, and cross-discipline training. At one academic treatment center, psychiatric and non-psychiatric practitioners are gradually buying in to these promising collaborative efforts.
Related Resources
• Board of Pharmacy Specialties. www.bpsweb.org/specialties/psychiatric.cfm.
• Abramowitz P. Ambulatory care pharmacy practice: The future is now. www.connect.ashp.org/blogs/paul-abramowitz/2014/05/14/ambulatory-care-pharmacy-practice-the-future-is-now.
Drug Brand Name
Citalopram • Celexa
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Finley PR, Rens HR, Pont JT, et al. Impact of a collaborative care model on depression in a primary care setting: a randomized controlled trial. Pharmacotherapy. 2003;23(9):1175-1185.
2. Finley PR, Crismon ML, Rush AJ. Evaluating the impact of pharmacists in mental health: a systematic review. Pharmacotherapy. 2003;23(12):1634-1644.
3. Board of Pharmacy Specialties. http://www.bpsweb. org. Accessed June 4, 2014.
4. Cohen LJ. The role of neuropsychiatric pharmacists. J Clin Psychiatry. 1999;60(suppl 19):54-57.
5. U.S. Food and Drug Administration. FDA Drug Safety Communication: Abnormal heart rhythms associated with high doses of Celexa (citalopram hydrobromide). http://www.fda.gov/Drugs/DrugSafety/ucm297391. htm. Accessed June 4, 2014.
6. Wheeler A, Crump K, Lee M, et al. Collaborative prescribing: a qualitative exploration of a role for pharmacists in mental health. Res Social Adm Pharm. 2012;8(3):179-192.
In this article, we highlight key steps that were needed to integrate clinical pharmacy specialists at an academic ambulatory psychiatric and addiction treatment center that serves pediatric and adult populations. Academic stakeholders identified addition of pharmacy services as a strategic goal in an effort to maximize services offered by the center and increase patient access to care while aligning with the standards set out by the patient-centered medical home (PCMH) model.
We outline the role of clinical pharmacists in the care of adult patients in ambulatory psychiatry, illustrate opportunities to enhance patient care, point out possible challenges with implementation, and propose future initiatives to optimize the practitioner-pharmacist partnership.
Background: Role of ambulatory pharmacists in psychiatry
Clinical pharmacists’ role in the psychiatric ambulatory care setting generally is associated with positive outcomes. One study looking at a collaborative care model that utilized clinical pharmacist follow-up in managing major depressive disorder found that patients who received pharmacist intervention in the collaborative care model had, on average, a significantly higher adherence rate and patient satisfaction score than the “usual care” group.1 Within this study, patients in both groups experienced global clinical improvement with no significant difference; however, pharmacist interventions had a positive impact on several aspects of the care model, suggesting that pharmacists can be used effectively in ambulatory psychiatry.
Furthermore, a systematic study evaluating pharmacists’ impact on clinical and functional mental health outcomes identified 8 relevant studies conducted in the outpatient setting.2 Although interventions varied widely, most studies focused on pharmacists’ providing a combination of drug monitoring, treatment recommendations, and patient education. Outcomes were largely positive, including an overall reduction in number and dosage of psychiatric drugs, inferred cost savings, and significant improvements in the safe and efficacious use of antidepressant and antipsychotic medications.
These preliminary positive results require replication in larger, randomized cohorts. Additionally, the role of the pharmacist as medication manager in the collaborative care model requires further study. Results so far, however, indicate that pharmacists can have a positive impact on the care of ambulatory psychiatry patients. Nevertheless, there is still considerable need for ongoing exploration in this field.
Pre-implementation
The need for pharmacy services. Various initiatives and existing practices within our health care system have underscored the need for a psychiatric pharmacist in the outpatient setting (Table 1).
A board-certified psychiatric pharmacist (BCPP) possesses specialized knowledge about treating patients affected by psychiatric illnesses. BCPPs work with prescribers and members of other disciplines, such as nurses and social workers, to optimize drug treatment by making pharmacotherapeutic recommendations and providing appropriate monitoring to enhance patient satisfaction and quality of life.3,4
Existing relationship with pharmacy. Along with evidence to support the positive impact clinical pharmacists can have in caring for patients with mental illness in the outpatient setting, a strong existing relationship between the Department of Psychiatry and our adult inpatient psychiatric pharmacist helped make it possible to develop an ambulatory psychiatric pharmacist position.
Each day, the inpatient psychiatric pharmacist works closely with the attending psychiatrists and psychiatry residents to provide treatment recommendations and counseling services for patients on the unit. The psychiatry residents highly valued their experiences with the pharmacist in the inpatient setting and expressed disappointment that this collaborative relationship was no longer available after they transitioned into the ambulatory setting.
Further, by being involved in initiatives that were relevant to both inpatient and outpatient psychiatry, such as metabolic monitoring for patients taking atypical antipsychotics, the clinical pharmacist in inpatient psychiatry had the opportunity to interact with key stakeholders in both settings. As a result of these pre-existing collaborative relationships, many clinicians were eager to have pharmacists available as a resource for patient care in the outpatient setting.
Pharmacist perspective: Outreach to psychiatry leadershipRecognizing the incentives and opportunities inherent in our emerging health care system, pharmacists became integral members of the patient care team in the PCMH model. Thanks to this effort, we now have PCMH pharmacists at every primary care health center in our health system (14 sites), providing disease management programs and polypharmacy services.
PCMH pharmacists’ role in the primary care setting fueled interest from specialty services and created opportunities to extend our existing partnership in inpatient psychiatry. One such opportunity to demonstrate the expertise of a psychiatric pharmacist was fueled by the FDA’s citalopram dosing alert5 at a system-wide level. This warning emerged as a chance to showcase the skill set of psychiatric pharmacists and the pharmacists’ successes in our PCMH model. The partnership was extended to include the buy-in of ambulatory pharmacy leadership and key stakeholders in ambulatory psychiatry.
Initial meetings included ambulatory care site leadership in psychiatry to increase awareness and understanding of pharmacists’ potential role in direct patient care. Achieving site leadership support was critical to successful implementation of pharmacist services in psychiatry. We also obtained approval from the Chair of the Department of Psychiatry to elicit support from faculty group practice.
Psychiatry leadership perspective
As fiscal pressures intensify at academic health centers, it becomes increasingly important for resources to be used as efficiently and effectively as possible. As a greater percentage of mental health patients with more “straightforward,” less complex conditions are being managed by their primary care providers or nonprescribing psychotherapists, or both, the acuity and complexity of cases in patients who present to psychiatric clinics have intensified. This intensification of patient needs and clinical acuity is in heightened conflict with the ongoing demand for clinician productivity and efficiency.
Additionally, the need to provide care to a seemingly ever-growing number of moderately or severely ill patients during shorter, less frequent visits presents a daunting task for clinicians and clinical leaders. Collaborative care models appear to offer the best hope for managing the seemingly overwhelming demand for services.
In this model, the patient, who is the critical member of the team, is expected to become an “expert” on his or her illness and to partner with members of the multidisciplinary team; with this support, patients are encouraged to develop a broad range of self-management skills and strategies to manage their illness. We believe that clinical pharmacists can and should play a critical role, not only in delivering direct clinical services to patients but also in developing and devising the care models that will most effectively apply each team member’s unique set of knowledge, skills, and experience. Given the large percentage of our patients who have multiple medical comorbidities and who require complex medical and psychiatric medication regimens, the role of the pharmacist in reviewing, educating, and advising patients and other team members on these crucial pharmacy concerns will be paramount.
In light of these complex medication issues, pharmacists are uniquely positioned to serve as a liaison among the patient, the primary care provider, and other members of their treatment team. We anticipate that our ambulatory psychiatry pharmacists will greatly enhance the comfort and confidence of patients and their primary care providers during periods of care transition.
Potential roles for pharmacists in ambulatory psychiatry
One potential role for pharmacists in ambulatory psychiatry is to perform polypharmacy assessments of patients receiving complex medication regimens, prompted by physician referral. The poly pharmacy intake interview, performed to obtain an accurate medication list and to identify patients’ concerns about their medications, can be conducted in person or by telephone. Patients’ knowledge about medications and medication adherence are discussed, as are their perceptions of effectiveness and adverse effects.
After initial data gathering, pharmacists complete a review of the medications, identifying any problems associated with medication indication, efficacy, tolerance, or adverse effects, drug-drug interactions, drug-nutrient interactions, and nonadherence. Pharmacists work to reduce medication costs if that is a concern of the patient, because nonadherence can result. A medication care plan is then developed in consultation with the primary care provider; here, the medication list is reconciled, the electronic medical record is updated, and actions to address any medication-related problems are prioritized.
Other services that might be offered include:• group education classes, based on patient motivational interviewing strategies, to address therapeutic nonadherence and to improve understanding of their disease and treatment regimens• medication safety and monitoring• treatment intensification, as needed, following established protocols.
These are a few of the ways in which pharmacists can be relied on to expand and improve access to patient care services within ambulatory psychiatry. Key stakeholders anticipate development of newer ideas as the pharmacist’s role in ambulatory psychiatry is increasingly clarified.
Reimbursement model
In creating a role for pharmacy in ambulatory psychiatry, it was essential that the model be financially viable and appealing. Alongside its clinical model, our institution has developed a financial model to support the pharmacist’s role. The lump-sum payment to the health centers from Blue Cross Blue Shield of Michigan afforded the ambulatory care clinics an opportunity to invest in PCMH pharmacists. This funding, and the reimbursement based on T-code billing (face-to-face visits and phone consultation) for depression and other conditions requiring chronic care, provides ongoing support. From our experience, understanding physician reimbursement models and identifying relevant changes in health care reform are necessary to integrate new providers, including pharmacists, into a team-based care model.
Implementation
Promoting pharmacy services. To foster anticipated collaboration with clinical pharmacists, the medical director of outpatient psychiatry disseminated an announcement to all providers regarding the investiture of clinical pharmacists to support patient care activities, education, and research. Clinicians were educated about the pharmacists’ potential roles and about guidelines and methods for referral. Use of our electronic health record system enabled us to establish a relatively simple referral process involving sharing electronic messages with our pharmacists.
Further, as part of the planned integration of clinical pharmacists in the ambulatory psychiatry setting, pharmacists met strategically with members of various disciplines, clinical programs, specialty clinic programs, and teams throughout the center. In addition to answering questions about the referral process, they emphasized the role of pharmacy and opportunities for collaboration.
Collaborating with others. Because the involvement of clinical pharmacists is unfamiliar to some practitioners in outpatient psychiatry, it is important to develop services without infringing on the roles other disciplines play. Indeed, a survey by Wheeler et al6 identified many concerns and potential boundaries among pharmacists, other providers, and patients. Concerns included confusion of practitioner roles and boundaries, a too-traditional perception of the pharmacist, and demonstration of competence.6
Early on, we developed a structured forum to discuss ongoing challenges and address issues related to the rapidly changing clinical landscape. During these discussions we conveyed that adding pharmacists to psychiatric services would be collaborative in nature and intended to augment existing services. This communication was pivotal to maintain the psychiatrist’s role as the ultimate prescriber and authority in the care of their patients; however, the pharmacist’s expertise, when sought, would help spur clinical and academic discussion that will benefit the patient. These discussions are paramount to achieving a productive, team-based approach, to overcome challenges, and to identify opportunities of value to our providers and patients (Box).
Work in progress
Implementing change in any clinical setting invariably creates challenges, and our endeavors to integrate clinical pharmacists into ambulatory psychiatry are no exception. We have identified several factors that we believe will optimize successful collaboration between pharmacy and ambulatory psychiatry (Table 2). Our primary challenge has been changing clinician behavior. Clinical practitioners can become too comfortable, wedded to their routines, and often are understandably resistant to change. Additionally, clinical systems often are inadvertently designed to obstruct change in ways that are not readily apparent. Efforts must be focused on behaviors and practices the clinical culture should encourage.
Regarding specific initiatives, clinical pharmacists have successfully identified patients on higher than recommended dosages of citalopram; they are working alongside prescribers to recommend ways to minimize the risk of heart rhythm abnormalities in these patients. Numerous prescribers have sought clinical pharmacists’ input to manage pharmacotherapy in their patients and to respond to patients’ questions on drug information.
The prospect of access to clinical pharmacist expertise in the outpatient setting was heralded with excitement, but the flow of referrals and consultations has been uneven. However simple the path for referral is, clinicians’ use of the system has been inconsistent—perhaps because of referrals’ passive, clinician-dependent nature. Educational outreach efforts often prompt a brief spike in referrals, only to be followed over time by a slow, steady drop-off. More active strategies will be needed, such as embedding the pharmacists as regular, active, visible members of the various clinical teams, and implementing a system in which patient record reviews are assigned to the pharmacists according to agreed-upon clusters of clinical criteria.
One of these tactics has, in the short term, showed success. Embedded in one of our newer clinics, which were designed to bridge primary and psychiatric care, clinical pharmacists are helping manage medically complicated patients. They assist with medication selection in light of drug interactions and medical comorbidities, conduct detailed medication histories, schedule follow-up visits to assess medication adherence and tolerability, and counsel patients experiencing insurance changes that make their medications less affordable. Integrating pharmacists in the new clinics has resulted in a steady flow of patient referrals and collaborative care work.
Clinical pharmacists are brainstorming with outpatient psychiatry leadership to build on these early successes. Ongoing communication and enhanced collaboration are essential, and can only improve the lives of our psychiatric patients.
For the future
Our partnership in ambulatory psychiatry was timed to occur during implementation of our health system’s new electronic health record initiative. Clinical pharmacists can play a key role in demonstrating use of the system to provide consistently accurate drug information to patients and to monitor patients receiving specific medications.
Development of ambulatory patient medication education groups, which has proved useful on the inpatient side, is another endeavor in the works. Integrating the clinical pharmacist with psychiatrists, psychologists, nurse practitioners, social workers, and trainees on specific teams devoted to depression, bipolar disorder, anxiety, perinatal mental health, and personality disorders also might prove to be a wide-ranging and promising strategy.
Enhancing the education and training experiences of residents, fellows, medical students, pharmacy students, and allied health professional learners present in our clinics is another exciting prospect. This cross-disciplinary training will yield a new generation of providers who will be more comfortable collaborating with colleagues from other disciplines, all intent on providing high-quality, efficient care. We hope that, as these initiatives take root, we will recognize many opportunities to disseminate our collaborative efforts in scholarly venues, documenting and sharing the positive impact of our partnership.
Bottom Line
Because psychiatric outpatients present with challenging medical comorbidities and increasingly complex medication regimens, specialized clinical pharmacists can enrich the management team by offering essential monitoring and polypharmacy services, patient education and counseling, and cross-discipline training. At one academic treatment center, psychiatric and non-psychiatric practitioners are gradually buying in to these promising collaborative efforts.
Related Resources
• Board of Pharmacy Specialties. www.bpsweb.org/specialties/psychiatric.cfm.
• Abramowitz P. Ambulatory care pharmacy practice: The future is now. www.connect.ashp.org/blogs/paul-abramowitz/2014/05/14/ambulatory-care-pharmacy-practice-the-future-is-now.
Drug Brand Name
Citalopram • Celexa
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
In this article, we highlight key steps that were needed to integrate clinical pharmacy specialists at an academic ambulatory psychiatric and addiction treatment center that serves pediatric and adult populations. Academic stakeholders identified addition of pharmacy services as a strategic goal in an effort to maximize services offered by the center and increase patient access to care while aligning with the standards set out by the patient-centered medical home (PCMH) model.
We outline the role of clinical pharmacists in the care of adult patients in ambulatory psychiatry, illustrate opportunities to enhance patient care, point out possible challenges with implementation, and propose future initiatives to optimize the practitioner-pharmacist partnership.
Background: Role of ambulatory pharmacists in psychiatry
Clinical pharmacists’ role in the psychiatric ambulatory care setting generally is associated with positive outcomes. One study looking at a collaborative care model that utilized clinical pharmacist follow-up in managing major depressive disorder found that patients who received pharmacist intervention in the collaborative care model had, on average, a significantly higher adherence rate and patient satisfaction score than the “usual care” group.1 Within this study, patients in both groups experienced global clinical improvement with no significant difference; however, pharmacist interventions had a positive impact on several aspects of the care model, suggesting that pharmacists can be used effectively in ambulatory psychiatry.
Furthermore, a systematic study evaluating pharmacists’ impact on clinical and functional mental health outcomes identified 8 relevant studies conducted in the outpatient setting.2 Although interventions varied widely, most studies focused on pharmacists’ providing a combination of drug monitoring, treatment recommendations, and patient education. Outcomes were largely positive, including an overall reduction in number and dosage of psychiatric drugs, inferred cost savings, and significant improvements in the safe and efficacious use of antidepressant and antipsychotic medications.
These preliminary positive results require replication in larger, randomized cohorts. Additionally, the role of the pharmacist as medication manager in the collaborative care model requires further study. Results so far, however, indicate that pharmacists can have a positive impact on the care of ambulatory psychiatry patients. Nevertheless, there is still considerable need for ongoing exploration in this field.
Pre-implementation
The need for pharmacy services. Various initiatives and existing practices within our health care system have underscored the need for a psychiatric pharmacist in the outpatient setting (Table 1).
A board-certified psychiatric pharmacist (BCPP) possesses specialized knowledge about treating patients affected by psychiatric illnesses. BCPPs work with prescribers and members of other disciplines, such as nurses and social workers, to optimize drug treatment by making pharmacotherapeutic recommendations and providing appropriate monitoring to enhance patient satisfaction and quality of life.3,4
Existing relationship with pharmacy. Along with evidence to support the positive impact clinical pharmacists can have in caring for patients with mental illness in the outpatient setting, a strong existing relationship between the Department of Psychiatry and our adult inpatient psychiatric pharmacist helped make it possible to develop an ambulatory psychiatric pharmacist position.
Each day, the inpatient psychiatric pharmacist works closely with the attending psychiatrists and psychiatry residents to provide treatment recommendations and counseling services for patients on the unit. The psychiatry residents highly valued their experiences with the pharmacist in the inpatient setting and expressed disappointment that this collaborative relationship was no longer available after they transitioned into the ambulatory setting.
Further, by being involved in initiatives that were relevant to both inpatient and outpatient psychiatry, such as metabolic monitoring for patients taking atypical antipsychotics, the clinical pharmacist in inpatient psychiatry had the opportunity to interact with key stakeholders in both settings. As a result of these pre-existing collaborative relationships, many clinicians were eager to have pharmacists available as a resource for patient care in the outpatient setting.
Pharmacist perspective: Outreach to psychiatry leadershipRecognizing the incentives and opportunities inherent in our emerging health care system, pharmacists became integral members of the patient care team in the PCMH model. Thanks to this effort, we now have PCMH pharmacists at every primary care health center in our health system (14 sites), providing disease management programs and polypharmacy services.
PCMH pharmacists’ role in the primary care setting fueled interest from specialty services and created opportunities to extend our existing partnership in inpatient psychiatry. One such opportunity to demonstrate the expertise of a psychiatric pharmacist was fueled by the FDA’s citalopram dosing alert5 at a system-wide level. This warning emerged as a chance to showcase the skill set of psychiatric pharmacists and the pharmacists’ successes in our PCMH model. The partnership was extended to include the buy-in of ambulatory pharmacy leadership and key stakeholders in ambulatory psychiatry.
Initial meetings included ambulatory care site leadership in psychiatry to increase awareness and understanding of pharmacists’ potential role in direct patient care. Achieving site leadership support was critical to successful implementation of pharmacist services in psychiatry. We also obtained approval from the Chair of the Department of Psychiatry to elicit support from faculty group practice.
Psychiatry leadership perspective
As fiscal pressures intensify at academic health centers, it becomes increasingly important for resources to be used as efficiently and effectively as possible. As a greater percentage of mental health patients with more “straightforward,” less complex conditions are being managed by their primary care providers or nonprescribing psychotherapists, or both, the acuity and complexity of cases in patients who present to psychiatric clinics have intensified. This intensification of patient needs and clinical acuity is in heightened conflict with the ongoing demand for clinician productivity and efficiency.
Additionally, the need to provide care to a seemingly ever-growing number of moderately or severely ill patients during shorter, less frequent visits presents a daunting task for clinicians and clinical leaders. Collaborative care models appear to offer the best hope for managing the seemingly overwhelming demand for services.
In this model, the patient, who is the critical member of the team, is expected to become an “expert” on his or her illness and to partner with members of the multidisciplinary team; with this support, patients are encouraged to develop a broad range of self-management skills and strategies to manage their illness. We believe that clinical pharmacists can and should play a critical role, not only in delivering direct clinical services to patients but also in developing and devising the care models that will most effectively apply each team member’s unique set of knowledge, skills, and experience. Given the large percentage of our patients who have multiple medical comorbidities and who require complex medical and psychiatric medication regimens, the role of the pharmacist in reviewing, educating, and advising patients and other team members on these crucial pharmacy concerns will be paramount.
In light of these complex medication issues, pharmacists are uniquely positioned to serve as a liaison among the patient, the primary care provider, and other members of their treatment team. We anticipate that our ambulatory psychiatry pharmacists will greatly enhance the comfort and confidence of patients and their primary care providers during periods of care transition.
Potential roles for pharmacists in ambulatory psychiatry
One potential role for pharmacists in ambulatory psychiatry is to perform polypharmacy assessments of patients receiving complex medication regimens, prompted by physician referral. The poly pharmacy intake interview, performed to obtain an accurate medication list and to identify patients’ concerns about their medications, can be conducted in person or by telephone. Patients’ knowledge about medications and medication adherence are discussed, as are their perceptions of effectiveness and adverse effects.
After initial data gathering, pharmacists complete a review of the medications, identifying any problems associated with medication indication, efficacy, tolerance, or adverse effects, drug-drug interactions, drug-nutrient interactions, and nonadherence. Pharmacists work to reduce medication costs if that is a concern of the patient, because nonadherence can result. A medication care plan is then developed in consultation with the primary care provider; here, the medication list is reconciled, the electronic medical record is updated, and actions to address any medication-related problems are prioritized.
Other services that might be offered include:• group education classes, based on patient motivational interviewing strategies, to address therapeutic nonadherence and to improve understanding of their disease and treatment regimens• medication safety and monitoring• treatment intensification, as needed, following established protocols.
These are a few of the ways in which pharmacists can be relied on to expand and improve access to patient care services within ambulatory psychiatry. Key stakeholders anticipate development of newer ideas as the pharmacist’s role in ambulatory psychiatry is increasingly clarified.
Reimbursement model
In creating a role for pharmacy in ambulatory psychiatry, it was essential that the model be financially viable and appealing. Alongside its clinical model, our institution has developed a financial model to support the pharmacist’s role. The lump-sum payment to the health centers from Blue Cross Blue Shield of Michigan afforded the ambulatory care clinics an opportunity to invest in PCMH pharmacists. This funding, and the reimbursement based on T-code billing (face-to-face visits and phone consultation) for depression and other conditions requiring chronic care, provides ongoing support. From our experience, understanding physician reimbursement models and identifying relevant changes in health care reform are necessary to integrate new providers, including pharmacists, into a team-based care model.
Implementation
Promoting pharmacy services. To foster anticipated collaboration with clinical pharmacists, the medical director of outpatient psychiatry disseminated an announcement to all providers regarding the investiture of clinical pharmacists to support patient care activities, education, and research. Clinicians were educated about the pharmacists’ potential roles and about guidelines and methods for referral. Use of our electronic health record system enabled us to establish a relatively simple referral process involving sharing electronic messages with our pharmacists.
Further, as part of the planned integration of clinical pharmacists in the ambulatory psychiatry setting, pharmacists met strategically with members of various disciplines, clinical programs, specialty clinic programs, and teams throughout the center. In addition to answering questions about the referral process, they emphasized the role of pharmacy and opportunities for collaboration.
Collaborating with others. Because the involvement of clinical pharmacists is unfamiliar to some practitioners in outpatient psychiatry, it is important to develop services without infringing on the roles other disciplines play. Indeed, a survey by Wheeler et al6 identified many concerns and potential boundaries among pharmacists, other providers, and patients. Concerns included confusion of practitioner roles and boundaries, a too-traditional perception of the pharmacist, and demonstration of competence.6
Early on, we developed a structured forum to discuss ongoing challenges and address issues related to the rapidly changing clinical landscape. During these discussions we conveyed that adding pharmacists to psychiatric services would be collaborative in nature and intended to augment existing services. This communication was pivotal to maintain the psychiatrist’s role as the ultimate prescriber and authority in the care of their patients; however, the pharmacist’s expertise, when sought, would help spur clinical and academic discussion that will benefit the patient. These discussions are paramount to achieving a productive, team-based approach, to overcome challenges, and to identify opportunities of value to our providers and patients (Box).
Work in progress
Implementing change in any clinical setting invariably creates challenges, and our endeavors to integrate clinical pharmacists into ambulatory psychiatry are no exception. We have identified several factors that we believe will optimize successful collaboration between pharmacy and ambulatory psychiatry (Table 2). Our primary challenge has been changing clinician behavior. Clinical practitioners can become too comfortable, wedded to their routines, and often are understandably resistant to change. Additionally, clinical systems often are inadvertently designed to obstruct change in ways that are not readily apparent. Efforts must be focused on behaviors and practices the clinical culture should encourage.
Regarding specific initiatives, clinical pharmacists have successfully identified patients on higher than recommended dosages of citalopram; they are working alongside prescribers to recommend ways to minimize the risk of heart rhythm abnormalities in these patients. Numerous prescribers have sought clinical pharmacists’ input to manage pharmacotherapy in their patients and to respond to patients’ questions on drug information.
The prospect of access to clinical pharmacist expertise in the outpatient setting was heralded with excitement, but the flow of referrals and consultations has been uneven. However simple the path for referral is, clinicians’ use of the system has been inconsistent—perhaps because of referrals’ passive, clinician-dependent nature. Educational outreach efforts often prompt a brief spike in referrals, only to be followed over time by a slow, steady drop-off. More active strategies will be needed, such as embedding the pharmacists as regular, active, visible members of the various clinical teams, and implementing a system in which patient record reviews are assigned to the pharmacists according to agreed-upon clusters of clinical criteria.
One of these tactics has, in the short term, showed success. Embedded in one of our newer clinics, which were designed to bridge primary and psychiatric care, clinical pharmacists are helping manage medically complicated patients. They assist with medication selection in light of drug interactions and medical comorbidities, conduct detailed medication histories, schedule follow-up visits to assess medication adherence and tolerability, and counsel patients experiencing insurance changes that make their medications less affordable. Integrating pharmacists in the new clinics has resulted in a steady flow of patient referrals and collaborative care work.
Clinical pharmacists are brainstorming with outpatient psychiatry leadership to build on these early successes. Ongoing communication and enhanced collaboration are essential, and can only improve the lives of our psychiatric patients.
For the future
Our partnership in ambulatory psychiatry was timed to occur during implementation of our health system’s new electronic health record initiative. Clinical pharmacists can play a key role in demonstrating use of the system to provide consistently accurate drug information to patients and to monitor patients receiving specific medications.
Development of ambulatory patient medication education groups, which has proved useful on the inpatient side, is another endeavor in the works. Integrating the clinical pharmacist with psychiatrists, psychologists, nurse practitioners, social workers, and trainees on specific teams devoted to depression, bipolar disorder, anxiety, perinatal mental health, and personality disorders also might prove to be a wide-ranging and promising strategy.
Enhancing the education and training experiences of residents, fellows, medical students, pharmacy students, and allied health professional learners present in our clinics is another exciting prospect. This cross-disciplinary training will yield a new generation of providers who will be more comfortable collaborating with colleagues from other disciplines, all intent on providing high-quality, efficient care. We hope that, as these initiatives take root, we will recognize many opportunities to disseminate our collaborative efforts in scholarly venues, documenting and sharing the positive impact of our partnership.
Bottom Line
Because psychiatric outpatients present with challenging medical comorbidities and increasingly complex medication regimens, specialized clinical pharmacists can enrich the management team by offering essential monitoring and polypharmacy services, patient education and counseling, and cross-discipline training. At one academic treatment center, psychiatric and non-psychiatric practitioners are gradually buying in to these promising collaborative efforts.
Related Resources
• Board of Pharmacy Specialties. www.bpsweb.org/specialties/psychiatric.cfm.
• Abramowitz P. Ambulatory care pharmacy practice: The future is now. www.connect.ashp.org/blogs/paul-abramowitz/2014/05/14/ambulatory-care-pharmacy-practice-the-future-is-now.
Drug Brand Name
Citalopram • Celexa
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Finley PR, Rens HR, Pont JT, et al. Impact of a collaborative care model on depression in a primary care setting: a randomized controlled trial. Pharmacotherapy. 2003;23(9):1175-1185.
2. Finley PR, Crismon ML, Rush AJ. Evaluating the impact of pharmacists in mental health: a systematic review. Pharmacotherapy. 2003;23(12):1634-1644.
3. Board of Pharmacy Specialties. http://www.bpsweb. org. Accessed June 4, 2014.
4. Cohen LJ. The role of neuropsychiatric pharmacists. J Clin Psychiatry. 1999;60(suppl 19):54-57.
5. U.S. Food and Drug Administration. FDA Drug Safety Communication: Abnormal heart rhythms associated with high doses of Celexa (citalopram hydrobromide). http://www.fda.gov/Drugs/DrugSafety/ucm297391. htm. Accessed June 4, 2014.
6. Wheeler A, Crump K, Lee M, et al. Collaborative prescribing: a qualitative exploration of a role for pharmacists in mental health. Res Social Adm Pharm. 2012;8(3):179-192.
1. Finley PR, Rens HR, Pont JT, et al. Impact of a collaborative care model on depression in a primary care setting: a randomized controlled trial. Pharmacotherapy. 2003;23(9):1175-1185.
2. Finley PR, Crismon ML, Rush AJ. Evaluating the impact of pharmacists in mental health: a systematic review. Pharmacotherapy. 2003;23(12):1634-1644.
3. Board of Pharmacy Specialties. http://www.bpsweb. org. Accessed June 4, 2014.
4. Cohen LJ. The role of neuropsychiatric pharmacists. J Clin Psychiatry. 1999;60(suppl 19):54-57.
5. U.S. Food and Drug Administration. FDA Drug Safety Communication: Abnormal heart rhythms associated with high doses of Celexa (citalopram hydrobromide). http://www.fda.gov/Drugs/DrugSafety/ucm297391. htm. Accessed June 4, 2014.
6. Wheeler A, Crump K, Lee M, et al. Collaborative prescribing: a qualitative exploration of a role for pharmacists in mental health. Res Social Adm Pharm. 2012;8(3):179-192.
Using CBT effectively for treating depression and anxiety
Fewer than 20% of people seeking help for depression and anxiety disorders receive cognitive-behavioral therapy (CBT), the most established evidence-based psychotherapeutic treatment.1 Efforts are being made to increase access to CBT,2 but a substantial barrier remains: therapist training is a strong predictor of treatment outcome, and many therapists offering CBT services are not sufficiently trained to deliver multiple manual-based interventions with adequate fidelity to the model. Proposed solutions to this barrier include:
• abbreviated versions of CBT training for practitioners in primary care and community settings
• culturally adapted CBT training for community health workers3
• Internet-based CBT and telemedicine (telephone and video conferencing)2
• mobile phone applications that use text messaging, social support, and physiological monitoring as adjuncts to clinical practice or stand-alone interventions.4
New models of CBT also are emerging, including transdiagnostic CBT and metacognitive approaches (mindfulness-based cognitive therapy and acceptance and commitment therapy), and several new foci for exposure therapy.
In light of these ongoing modulations, this article is intended to help clinicians make informed decisions about CBT when selecting treatment for patients with depressive and anxiety disorders (Box5 ). We review the evidence of CBT’s efficacy for acute-phase treatment and relapse prevention; explain the common elements considered essential to CBT practice; describe CBT adaptations for specific anxiety disorders; and provide an overview of recent advances in conceptualizing and adapting CBT.
Efficacy for mood and anxiety disorders
Depression. Dozens of randomized controlled trials (RCT) and other studies support CBT’s efficacy in treating major depressive disorder (MDD). For acute treatment:
• CBT is more effective in producing remission when compared with no treatment, treatment as usual, or nonspecific psychotherapy.
• For mild to moderate depression, CBT is equivalent to antidepressant medication in terms of response and remission rates.
• Combining antidepressant therapy with CBT increases treatment adherence.6
Less well known may be that a successful response to CBT in the acute phase may have a protective effect against depression recurrences. A 2013 meta-analysis that totaled 506 individuals with depressive disorders found a trend toward significantly lower relapse rates when CBT was discontinued after acute therapy, compared with antidepressant therapy that continued beyond the acute phase.7
Anxiety. Among psychotherapies, CBT’s superior efficacy for anxiety disorders is well-established. CBT and its specific-disorder adaptations are considered first-line treatment.8
CBT’s essential elements
CBT focuses on distorted cognitions about the self, the world, and the future, and on behaviors that lead to or maintain symptoms.
Cognitive interventions seek to identify thoughts and beliefs that trigger emotional and behavioral reactions. A person with social anxiety disorder, for example, might believe that people will notice if he makes even a minor social mistake and then reject him, which will make him feel worthless. CBT can help him subject these beliefs to rational analysis and develop more adaptive beliefs, such as: “It is not certain that I will behave so badly that people would notice, but if that happened, the likelihood of being outright rejected is probably low. If—in the worst-case scenario—I was rejected, I am not worthless; I’m just a fallible human being.”
CBT’s behavioral component can be conceptualized as behavioral activation (BA), a structured approach to help the patient:
• increase behaviors and experiences that are rewarding
• overcome barriers to engaging in these new behaviors
• and decrease behaviors that maintain symptoms.
BA can be a useful intervention for individuals with depression characterized by lack of engagement or capacity for pleasurable experiences. During pregnancy and the postpartum period, for example, a woman undergoes physical, social, and environmental changes that might gradually deprive her of sources of pleasure and other reinforcing activities. BA would focus on developing creative solutions to regain access to or create new opportunities for rewarding experiences and to avoid behaviors (such as social withdrawal or physical activity restriction) that perpetuate depressed mood.
Common elements. Cognitive and behavioral interventions focus on problem solving, individualized case conceptualization (Figure 1), and collaborative empiricism.9
Individualized case conceptualization lays the foundation for the course of CBT, and may be thought of as a map for therapy. Case conceptualization brings in several domains of assessment including symptoms and diagnosis, the patient’s strengths, formative experiences (including biopsychosocial aspects), contextual factors, and cognitive factors that influence diagnosis and treatment, such as automatic thoughts or schemas. The case formulation leads to a working hypothesis about the optimal course and focus of CBT.
Collaborative empiricism is the way in which the patient and therapist work together to continually refine this working hypothesis. The pair works together to investigate the hypotheses and all aspects of the therapeutic relationship.
Although no specific technique defines CBT, a common practice is to educate a person about interrelationships between behaviors/activities, thoughts, and mood. A mood activity log (Figure 2) can illuminate links between moods and activities and be useful with targeting interventions. For a person with social anxiety, for example, a mood activity log could assist in developing a hierarchy of feared social situations and avoidance intensity. Systematic exposure therapy would follow, beginning with the least frightening/intense situation, accompanied by teaching new coping skills (such as relaxation strategies).
CBT adaptations for anxiety disorders
Elements of CBT have been adapted for a variety of anxiety disorders, based on specific symptoms and features (Table).10-15
Panic disorder. Panic control treatment is considered the first-line intervention for panic disorder’s defining features: spontaneous panic attacks, worry about future occurrence of attacks, and perceived catastrophic consequences (such as heart attack, fainting).10 This CBT adaptation includes:
• patient education about the nature of panic
• breathing retraining to foster exposure to feared bodily sensations and avoided activities and places
• cognitive restructuring of danger-related thoughts (such as “I’m going to faint,” or “It would be catastrophic if I did”).
Obsessive-compulsive disorder. Exposure and response prevention (ERP) is the first-line treatment for obsessive-compulsive disorder (OCD).11 In traditional therapist-guided ERP, patients expose themselves to perceived contaminants while refraining from inappropriate compulsive behaviors (such as hand washing).
Cognitive interventions also can be an effective treatment of obsessions, without patients having to engage in exposure to their horrific thoughts and images.16 Consider, for example, a new mother who upon seeing the kitchen knife has the intrusive thought, “What if I stabbed my baby?” Instead of the traditional exposure approach for OCD (ie, having her vividly imagine stabbing her baby until her anxiety level subsided), the cognitive intervention would be to educate her about the normalcy of intrusive thoughts, particularly in the postpartum period.
Generalized anxiety disorder. CBT for generalized anxiety disorder (GAD) targets patients’ overestimation of the likelihood of negative events and the belief that these events, should they occur, would be catastrophic and render them unable to cope.12
Motivational interviewing (MI) appears to be a useful adjunct to precede traditional CBT, particularly for severe worriers.17 MI attempts to help individuals with GAD recognize their ambivalence about giving up worry. This technique acknowledges and validates perceived benefits of worry (eg, “It helps me prepare for the worst, so I won’t be emotionally devastated if it happens”), but also explores how worry is destructive.
Emerging CBT models for anxiety disorders
Metacognitive treatment. Evidence, such as presented by Dobson,18 suggests that the field of CBT is shifting towards a metacognitive model of change and treatment. A metacognitive approach goes beyond changing thinking and emphasizes thoughts about thoughts and experiences. Examples include mindfulness-based cognitive therapy (MBCT) and acceptance and commitment therapy (ACT).
MBCT typically consists of an 8-week program of 2-hour sessions each week and 1 full-day retreat. MBCT is modeled after Kabat-Zinn’s widely disseminated and empirically supported mindfulness based stress reduction course.19 MBCT was developed as a relapse prevention program for patients who had recovered from depression. Unlike traditional cognitive therapy for depression that targets changing the content of automatic thoughts and core beliefs, in MBCT patients are aware of negative automatic thoughts and find ways to change their relationship with these thoughts, learning that thoughts are not facts. This process mainly is carried out by practicing mindfulness meditation exercises. Importantly, MBCT goes beyond mindful acceptance of negative thoughts and teaches patients mindful acceptance of all internal experiences.
A fundamental difference between ACT and traditional CBT is the approach to cognitions.20 Although CBT focuses on changing the content of maladaptive thoughts, such as “I am a worthless person,” ACT focuses on changing the function of thoughts. ACT strives to help patients to accept their internal experiences—whether unwanted thoughts, feelings, bodily sensations, or memories—while committing themselves to pursuing their life goals and values. Strategies aim to help patients step back from their thoughts and observe them as just thoughts. The patient who thinks, “I am worthless” would be instructed to practice saying “I am having the thought I am worthless.” Therefore the thought no longer controls the person’s behavior.
These approaches train the patient to keenly observe distressing thoughts and experiences—not necessarily with the goal of changing them but to accept them and act in a way that is consistent with his (her) goals and values. A meta-analysis of 39 studies found mindfulness-based therapy effective in improving symptoms in participants with anxiety and mood disorders.21 Similarly, ACT has demonstrated efficacy with mixed anxiety disorders.22
Transdiagnostic CBT. Recent research18 suggests that mood and anxiety disorders may have more commonalities than differences in underlying biological and psychological traits. Because the symptoms of anxiety and depressive disorders tend to overlap, and their rate of comorbidity may be as high as 55%,23 so-called transdiagnostic treatments have been developed. Transdiagnostic treatments target impairing symptoms that cut across different diagnoses. For example, patients with depression, anxiety, or substance abuse might share a common difficulty with regulating and coping with negative emotions.
In a preliminary comparison trial,24 46 patients with social anxiety disorder, panic disorder, or GAD were randomly assigned to transdiagnostic CBT (n = 23) or diagnosis-specific CBT (n = 23). Treatments were based on widely used manuals and offered in 2-hour group sessions across 12 weeks. Transdiagnostic CBT was found to be as effective as specific CBT protocols in terms of symptom improvement. Participants attended an average of 8.46 sessions, with similar attendance in each protocol. Fourteen participants (30%) discontinued treatment, similar to attrition rates reported in other trials of transdiagnostic and diagnosis-specific CBT.
Transdiagnostic treatments may facilitate the dissemination of empirically supported treatments because therapists would not be required to have training and supervision to competency in delivering multiple manuals for specific anxiety disorders. This could be attractive to busy practitioners with limited time to learn new treatments.
Bottom Line
Efficacy of cognitive-behavioral therapy (CBT) for depression and anxiety is well established. Although no specific technique defines CBT, a common practice is to educate an individual about interrelationships between behaviors/activities, thoughts, and mood. CBT techniques can be customized to treat specific anxiety disorders, such as panic disorder, obsessive-compulsive disorder, and generalized anxiety disorder.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Collins K, Westra H, Dozois D, et al. Gaps in accessing treatment for anxiety and depressions: challenges for the delivery of care. Clin Psychol Rev. 2004;24(5):583-616.
2. Foa EB, Gillihan SJ, Bryant RA. Challenges and successes in dissemination of evidence-based treatments for posttraumatic stress: lessons learned from prolonged exposure therapy for PTSD. Psychological Science in the Public Interest. 2013;14(2):65-111.
3. Rahman A, Malik A, Sikander S, et al. Cognitive behaviour therapy-based intervention by community health workers for mothers with depression and their infants in rural Pakistan: a cluster-randomised controlled trial. Lancet. 2008;372(9642):902-909.
4. Aguilera A, Muench F. There’s an app for that: information technology applications for cognitive behavioral practitioners. Behavior Therapist. 2012;35(4):65-73.
5. Dimidjian S, Hollon SD, Dobson KS, et al. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the acute treatment of adults with major depression. J Consult Clin Psychol. 2006; 74(4):658-670.
6. Hollon SD, Jarrett RB, Nierenberg AA, et al. Psychotherapy and medication in the treatment of adult and geriatric depression: which monotherapy or combined treatment? J Clin Psychiatry. 2005;66(4):455-468.
7. Cuijpers P, Hollon SD, van Straten A, et al. Does cognitive behaviour therapy have an enduring effect that is superior to keeping patients on continuation pharmacotherapy? A meta-analysis. BMJ Open. 2013;3(4):1-8.
8. Stewart R, Chambless D. Cognitive-behavioral therapy for adult anxiety disorders in clinical practice: a meta-analysis of effectiveness studies. J Consult Clin Psychol. 2009;77(4): 595-606.
9. Wright JH, Basco MR, Thase M. Learning cognitive behavior therapy: an illustrated guide. Arlington, VA: American Psychiatric Publishing; 2006.
10. Barlow DH, Craske MG. Mastery of your anxiety and panic. 4th ed. New York, NY: Oxford University Press, Inc.; 2007.
11. Foa EB, Yadin E, Lichner TK. Exposure and response prevention for obsessive-compulsive disorder: therapist guide. New York, NY: Oxford University Press, Inc.; 2012.
12. Dugas MJ, Robichaud M. Cognitive-behavioral treatment for generalized anxiety disorder. New York, NY: Routledge; 2007.
13. Zlomke K, Davis TE. One-session treatment of specific phobias: a detailed description and review of treatment efficacy. Behav Ther. 2008;39(3):207-223.
14. Foa EB, Hembree E, Rothbaum B. Prolonged exposure therapy for PTSD: emotional processing of traumatic experiences. Therapist guide. New York, NY: Oxford University Press, Inc.; 2007.
15. Resick PA, Schnicke MK. Cognitive processing therapy for rape victims. London, United Kingdom: Sage Publications; 1996.
16. Whittal ML, Robichaud M, Woody SR. Cognitive treatment of obsessions: enhancing dissemination with video components. Cognitive and Behavioral Practice. 2010;17(1):1-8.
17. Westra H, Arkowitz H, Dozois D. Adding a motivational interviewing pretreatment to cognitive behavioral therapy for generalized anxiety disorder: a preliminary randomized controlled trial. J Anxiety Disord. 2009;23(2): 1106-1117.
18. Dobson KS. The science of CBT: toward a metacognitive model of change? Behav Ther. 2013;44(2):224-227.
19. Kabat-Zinn J. Full catastrophe living. Using the wisdom of your body and mind to face stress, pain, and illness. Revised edition. New York, NY: Bantam Books; 2013.
20. Hayes SC, Strosahl KD. Acceptance and commitment therapy. The process and practice of mindful change. 2nd ed. New York, NY: The Guilford Press; 2012.
21. Hofmann S, Sawyer A, Witt A, et al. The effect of mindfulness-based therapy on anxiety and depression: a meta-analytic review. J Consult Clin Psychol. 2010;78(2): 169-183.
22. Arch J, Eifert G, Davies C, et al. Randomized clinical trial of cognitive behavioral therapy (CBT) versus acceptance and commitment therapy (ACT) for mixed anxiety disorders. J Consult Clin Psychol. 2012;80(5):750-765.
23. Brown TA, Campbell LA, Lehman CL, et al. Current and lifetime comorbidity of the DSM-IV anxiety and mood disorders in a large clinical sample. J Abnorm Psychol. 2001;110(4):585-599.
24. Norton P, Barrera T. Transdiagnostic versus diagnosis-specific CBT for anxiety disorders: a preliminary randomized controlled noninferiority trial. Depress Anxiety. 2012;29(10):874-882.
Fewer than 20% of people seeking help for depression and anxiety disorders receive cognitive-behavioral therapy (CBT), the most established evidence-based psychotherapeutic treatment.1 Efforts are being made to increase access to CBT,2 but a substantial barrier remains: therapist training is a strong predictor of treatment outcome, and many therapists offering CBT services are not sufficiently trained to deliver multiple manual-based interventions with adequate fidelity to the model. Proposed solutions to this barrier include:
• abbreviated versions of CBT training for practitioners in primary care and community settings
• culturally adapted CBT training for community health workers3
• Internet-based CBT and telemedicine (telephone and video conferencing)2
• mobile phone applications that use text messaging, social support, and physiological monitoring as adjuncts to clinical practice or stand-alone interventions.4
New models of CBT also are emerging, including transdiagnostic CBT and metacognitive approaches (mindfulness-based cognitive therapy and acceptance and commitment therapy), and several new foci for exposure therapy.
In light of these ongoing modulations, this article is intended to help clinicians make informed decisions about CBT when selecting treatment for patients with depressive and anxiety disorders (Box5 ). We review the evidence of CBT’s efficacy for acute-phase treatment and relapse prevention; explain the common elements considered essential to CBT practice; describe CBT adaptations for specific anxiety disorders; and provide an overview of recent advances in conceptualizing and adapting CBT.
Efficacy for mood and anxiety disorders
Depression. Dozens of randomized controlled trials (RCT) and other studies support CBT’s efficacy in treating major depressive disorder (MDD). For acute treatment:
• CBT is more effective in producing remission when compared with no treatment, treatment as usual, or nonspecific psychotherapy.
• For mild to moderate depression, CBT is equivalent to antidepressant medication in terms of response and remission rates.
• Combining antidepressant therapy with CBT increases treatment adherence.6
Less well known may be that a successful response to CBT in the acute phase may have a protective effect against depression recurrences. A 2013 meta-analysis that totaled 506 individuals with depressive disorders found a trend toward significantly lower relapse rates when CBT was discontinued after acute therapy, compared with antidepressant therapy that continued beyond the acute phase.7
Anxiety. Among psychotherapies, CBT’s superior efficacy for anxiety disorders is well-established. CBT and its specific-disorder adaptations are considered first-line treatment.8
CBT’s essential elements
CBT focuses on distorted cognitions about the self, the world, and the future, and on behaviors that lead to or maintain symptoms.
Cognitive interventions seek to identify thoughts and beliefs that trigger emotional and behavioral reactions. A person with social anxiety disorder, for example, might believe that people will notice if he makes even a minor social mistake and then reject him, which will make him feel worthless. CBT can help him subject these beliefs to rational analysis and develop more adaptive beliefs, such as: “It is not certain that I will behave so badly that people would notice, but if that happened, the likelihood of being outright rejected is probably low. If—in the worst-case scenario—I was rejected, I am not worthless; I’m just a fallible human being.”
CBT’s behavioral component can be conceptualized as behavioral activation (BA), a structured approach to help the patient:
• increase behaviors and experiences that are rewarding
• overcome barriers to engaging in these new behaviors
• and decrease behaviors that maintain symptoms.
BA can be a useful intervention for individuals with depression characterized by lack of engagement or capacity for pleasurable experiences. During pregnancy and the postpartum period, for example, a woman undergoes physical, social, and environmental changes that might gradually deprive her of sources of pleasure and other reinforcing activities. BA would focus on developing creative solutions to regain access to or create new opportunities for rewarding experiences and to avoid behaviors (such as social withdrawal or physical activity restriction) that perpetuate depressed mood.
Common elements. Cognitive and behavioral interventions focus on problem solving, individualized case conceptualization (Figure 1), and collaborative empiricism.9
Individualized case conceptualization lays the foundation for the course of CBT, and may be thought of as a map for therapy. Case conceptualization brings in several domains of assessment including symptoms and diagnosis, the patient’s strengths, formative experiences (including biopsychosocial aspects), contextual factors, and cognitive factors that influence diagnosis and treatment, such as automatic thoughts or schemas. The case formulation leads to a working hypothesis about the optimal course and focus of CBT.
Collaborative empiricism is the way in which the patient and therapist work together to continually refine this working hypothesis. The pair works together to investigate the hypotheses and all aspects of the therapeutic relationship.
Although no specific technique defines CBT, a common practice is to educate a person about interrelationships between behaviors/activities, thoughts, and mood. A mood activity log (Figure 2) can illuminate links between moods and activities and be useful with targeting interventions. For a person with social anxiety, for example, a mood activity log could assist in developing a hierarchy of feared social situations and avoidance intensity. Systematic exposure therapy would follow, beginning with the least frightening/intense situation, accompanied by teaching new coping skills (such as relaxation strategies).
CBT adaptations for anxiety disorders
Elements of CBT have been adapted for a variety of anxiety disorders, based on specific symptoms and features (Table).10-15
Panic disorder. Panic control treatment is considered the first-line intervention for panic disorder’s defining features: spontaneous panic attacks, worry about future occurrence of attacks, and perceived catastrophic consequences (such as heart attack, fainting).10 This CBT adaptation includes:
• patient education about the nature of panic
• breathing retraining to foster exposure to feared bodily sensations and avoided activities and places
• cognitive restructuring of danger-related thoughts (such as “I’m going to faint,” or “It would be catastrophic if I did”).
Obsessive-compulsive disorder. Exposure and response prevention (ERP) is the first-line treatment for obsessive-compulsive disorder (OCD).11 In traditional therapist-guided ERP, patients expose themselves to perceived contaminants while refraining from inappropriate compulsive behaviors (such as hand washing).
Cognitive interventions also can be an effective treatment of obsessions, without patients having to engage in exposure to their horrific thoughts and images.16 Consider, for example, a new mother who upon seeing the kitchen knife has the intrusive thought, “What if I stabbed my baby?” Instead of the traditional exposure approach for OCD (ie, having her vividly imagine stabbing her baby until her anxiety level subsided), the cognitive intervention would be to educate her about the normalcy of intrusive thoughts, particularly in the postpartum period.
Generalized anxiety disorder. CBT for generalized anxiety disorder (GAD) targets patients’ overestimation of the likelihood of negative events and the belief that these events, should they occur, would be catastrophic and render them unable to cope.12
Motivational interviewing (MI) appears to be a useful adjunct to precede traditional CBT, particularly for severe worriers.17 MI attempts to help individuals with GAD recognize their ambivalence about giving up worry. This technique acknowledges and validates perceived benefits of worry (eg, “It helps me prepare for the worst, so I won’t be emotionally devastated if it happens”), but also explores how worry is destructive.
Emerging CBT models for anxiety disorders
Metacognitive treatment. Evidence, such as presented by Dobson,18 suggests that the field of CBT is shifting towards a metacognitive model of change and treatment. A metacognitive approach goes beyond changing thinking and emphasizes thoughts about thoughts and experiences. Examples include mindfulness-based cognitive therapy (MBCT) and acceptance and commitment therapy (ACT).
MBCT typically consists of an 8-week program of 2-hour sessions each week and 1 full-day retreat. MBCT is modeled after Kabat-Zinn’s widely disseminated and empirically supported mindfulness based stress reduction course.19 MBCT was developed as a relapse prevention program for patients who had recovered from depression. Unlike traditional cognitive therapy for depression that targets changing the content of automatic thoughts and core beliefs, in MBCT patients are aware of negative automatic thoughts and find ways to change their relationship with these thoughts, learning that thoughts are not facts. This process mainly is carried out by practicing mindfulness meditation exercises. Importantly, MBCT goes beyond mindful acceptance of negative thoughts and teaches patients mindful acceptance of all internal experiences.
A fundamental difference between ACT and traditional CBT is the approach to cognitions.20 Although CBT focuses on changing the content of maladaptive thoughts, such as “I am a worthless person,” ACT focuses on changing the function of thoughts. ACT strives to help patients to accept their internal experiences—whether unwanted thoughts, feelings, bodily sensations, or memories—while committing themselves to pursuing their life goals and values. Strategies aim to help patients step back from their thoughts and observe them as just thoughts. The patient who thinks, “I am worthless” would be instructed to practice saying “I am having the thought I am worthless.” Therefore the thought no longer controls the person’s behavior.
These approaches train the patient to keenly observe distressing thoughts and experiences—not necessarily with the goal of changing them but to accept them and act in a way that is consistent with his (her) goals and values. A meta-analysis of 39 studies found mindfulness-based therapy effective in improving symptoms in participants with anxiety and mood disorders.21 Similarly, ACT has demonstrated efficacy with mixed anxiety disorders.22
Transdiagnostic CBT. Recent research18 suggests that mood and anxiety disorders may have more commonalities than differences in underlying biological and psychological traits. Because the symptoms of anxiety and depressive disorders tend to overlap, and their rate of comorbidity may be as high as 55%,23 so-called transdiagnostic treatments have been developed. Transdiagnostic treatments target impairing symptoms that cut across different diagnoses. For example, patients with depression, anxiety, or substance abuse might share a common difficulty with regulating and coping with negative emotions.
In a preliminary comparison trial,24 46 patients with social anxiety disorder, panic disorder, or GAD were randomly assigned to transdiagnostic CBT (n = 23) or diagnosis-specific CBT (n = 23). Treatments were based on widely used manuals and offered in 2-hour group sessions across 12 weeks. Transdiagnostic CBT was found to be as effective as specific CBT protocols in terms of symptom improvement. Participants attended an average of 8.46 sessions, with similar attendance in each protocol. Fourteen participants (30%) discontinued treatment, similar to attrition rates reported in other trials of transdiagnostic and diagnosis-specific CBT.
Transdiagnostic treatments may facilitate the dissemination of empirically supported treatments because therapists would not be required to have training and supervision to competency in delivering multiple manuals for specific anxiety disorders. This could be attractive to busy practitioners with limited time to learn new treatments.
Bottom Line
Efficacy of cognitive-behavioral therapy (CBT) for depression and anxiety is well established. Although no specific technique defines CBT, a common practice is to educate an individual about interrelationships between behaviors/activities, thoughts, and mood. CBT techniques can be customized to treat specific anxiety disorders, such as panic disorder, obsessive-compulsive disorder, and generalized anxiety disorder.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Fewer than 20% of people seeking help for depression and anxiety disorders receive cognitive-behavioral therapy (CBT), the most established evidence-based psychotherapeutic treatment.1 Efforts are being made to increase access to CBT,2 but a substantial barrier remains: therapist training is a strong predictor of treatment outcome, and many therapists offering CBT services are not sufficiently trained to deliver multiple manual-based interventions with adequate fidelity to the model. Proposed solutions to this barrier include:
• abbreviated versions of CBT training for practitioners in primary care and community settings
• culturally adapted CBT training for community health workers3
• Internet-based CBT and telemedicine (telephone and video conferencing)2
• mobile phone applications that use text messaging, social support, and physiological monitoring as adjuncts to clinical practice or stand-alone interventions.4
New models of CBT also are emerging, including transdiagnostic CBT and metacognitive approaches (mindfulness-based cognitive therapy and acceptance and commitment therapy), and several new foci for exposure therapy.
In light of these ongoing modulations, this article is intended to help clinicians make informed decisions about CBT when selecting treatment for patients with depressive and anxiety disorders (Box5 ). We review the evidence of CBT’s efficacy for acute-phase treatment and relapse prevention; explain the common elements considered essential to CBT practice; describe CBT adaptations for specific anxiety disorders; and provide an overview of recent advances in conceptualizing and adapting CBT.
Efficacy for mood and anxiety disorders
Depression. Dozens of randomized controlled trials (RCT) and other studies support CBT’s efficacy in treating major depressive disorder (MDD). For acute treatment:
• CBT is more effective in producing remission when compared with no treatment, treatment as usual, or nonspecific psychotherapy.
• For mild to moderate depression, CBT is equivalent to antidepressant medication in terms of response and remission rates.
• Combining antidepressant therapy with CBT increases treatment adherence.6
Less well known may be that a successful response to CBT in the acute phase may have a protective effect against depression recurrences. A 2013 meta-analysis that totaled 506 individuals with depressive disorders found a trend toward significantly lower relapse rates when CBT was discontinued after acute therapy, compared with antidepressant therapy that continued beyond the acute phase.7
Anxiety. Among psychotherapies, CBT’s superior efficacy for anxiety disorders is well-established. CBT and its specific-disorder adaptations are considered first-line treatment.8
CBT’s essential elements
CBT focuses on distorted cognitions about the self, the world, and the future, and on behaviors that lead to or maintain symptoms.
Cognitive interventions seek to identify thoughts and beliefs that trigger emotional and behavioral reactions. A person with social anxiety disorder, for example, might believe that people will notice if he makes even a minor social mistake and then reject him, which will make him feel worthless. CBT can help him subject these beliefs to rational analysis and develop more adaptive beliefs, such as: “It is not certain that I will behave so badly that people would notice, but if that happened, the likelihood of being outright rejected is probably low. If—in the worst-case scenario—I was rejected, I am not worthless; I’m just a fallible human being.”
CBT’s behavioral component can be conceptualized as behavioral activation (BA), a structured approach to help the patient:
• increase behaviors and experiences that are rewarding
• overcome barriers to engaging in these new behaviors
• and decrease behaviors that maintain symptoms.
BA can be a useful intervention for individuals with depression characterized by lack of engagement or capacity for pleasurable experiences. During pregnancy and the postpartum period, for example, a woman undergoes physical, social, and environmental changes that might gradually deprive her of sources of pleasure and other reinforcing activities. BA would focus on developing creative solutions to regain access to or create new opportunities for rewarding experiences and to avoid behaviors (such as social withdrawal or physical activity restriction) that perpetuate depressed mood.
Common elements. Cognitive and behavioral interventions focus on problem solving, individualized case conceptualization (Figure 1), and collaborative empiricism.9
Individualized case conceptualization lays the foundation for the course of CBT, and may be thought of as a map for therapy. Case conceptualization brings in several domains of assessment including symptoms and diagnosis, the patient’s strengths, formative experiences (including biopsychosocial aspects), contextual factors, and cognitive factors that influence diagnosis and treatment, such as automatic thoughts or schemas. The case formulation leads to a working hypothesis about the optimal course and focus of CBT.
Collaborative empiricism is the way in which the patient and therapist work together to continually refine this working hypothesis. The pair works together to investigate the hypotheses and all aspects of the therapeutic relationship.
Although no specific technique defines CBT, a common practice is to educate a person about interrelationships between behaviors/activities, thoughts, and mood. A mood activity log (Figure 2) can illuminate links between moods and activities and be useful with targeting interventions. For a person with social anxiety, for example, a mood activity log could assist in developing a hierarchy of feared social situations and avoidance intensity. Systematic exposure therapy would follow, beginning with the least frightening/intense situation, accompanied by teaching new coping skills (such as relaxation strategies).
CBT adaptations for anxiety disorders
Elements of CBT have been adapted for a variety of anxiety disorders, based on specific symptoms and features (Table).10-15
Panic disorder. Panic control treatment is considered the first-line intervention for panic disorder’s defining features: spontaneous panic attacks, worry about future occurrence of attacks, and perceived catastrophic consequences (such as heart attack, fainting).10 This CBT adaptation includes:
• patient education about the nature of panic
• breathing retraining to foster exposure to feared bodily sensations and avoided activities and places
• cognitive restructuring of danger-related thoughts (such as “I’m going to faint,” or “It would be catastrophic if I did”).
Obsessive-compulsive disorder. Exposure and response prevention (ERP) is the first-line treatment for obsessive-compulsive disorder (OCD).11 In traditional therapist-guided ERP, patients expose themselves to perceived contaminants while refraining from inappropriate compulsive behaviors (such as hand washing).
Cognitive interventions also can be an effective treatment of obsessions, without patients having to engage in exposure to their horrific thoughts and images.16 Consider, for example, a new mother who upon seeing the kitchen knife has the intrusive thought, “What if I stabbed my baby?” Instead of the traditional exposure approach for OCD (ie, having her vividly imagine stabbing her baby until her anxiety level subsided), the cognitive intervention would be to educate her about the normalcy of intrusive thoughts, particularly in the postpartum period.
Generalized anxiety disorder. CBT for generalized anxiety disorder (GAD) targets patients’ overestimation of the likelihood of negative events and the belief that these events, should they occur, would be catastrophic and render them unable to cope.12
Motivational interviewing (MI) appears to be a useful adjunct to precede traditional CBT, particularly for severe worriers.17 MI attempts to help individuals with GAD recognize their ambivalence about giving up worry. This technique acknowledges and validates perceived benefits of worry (eg, “It helps me prepare for the worst, so I won’t be emotionally devastated if it happens”), but also explores how worry is destructive.
Emerging CBT models for anxiety disorders
Metacognitive treatment. Evidence, such as presented by Dobson,18 suggests that the field of CBT is shifting towards a metacognitive model of change and treatment. A metacognitive approach goes beyond changing thinking and emphasizes thoughts about thoughts and experiences. Examples include mindfulness-based cognitive therapy (MBCT) and acceptance and commitment therapy (ACT).
MBCT typically consists of an 8-week program of 2-hour sessions each week and 1 full-day retreat. MBCT is modeled after Kabat-Zinn’s widely disseminated and empirically supported mindfulness based stress reduction course.19 MBCT was developed as a relapse prevention program for patients who had recovered from depression. Unlike traditional cognitive therapy for depression that targets changing the content of automatic thoughts and core beliefs, in MBCT patients are aware of negative automatic thoughts and find ways to change their relationship with these thoughts, learning that thoughts are not facts. This process mainly is carried out by practicing mindfulness meditation exercises. Importantly, MBCT goes beyond mindful acceptance of negative thoughts and teaches patients mindful acceptance of all internal experiences.
A fundamental difference between ACT and traditional CBT is the approach to cognitions.20 Although CBT focuses on changing the content of maladaptive thoughts, such as “I am a worthless person,” ACT focuses on changing the function of thoughts. ACT strives to help patients to accept their internal experiences—whether unwanted thoughts, feelings, bodily sensations, or memories—while committing themselves to pursuing their life goals and values. Strategies aim to help patients step back from their thoughts and observe them as just thoughts. The patient who thinks, “I am worthless” would be instructed to practice saying “I am having the thought I am worthless.” Therefore the thought no longer controls the person’s behavior.
These approaches train the patient to keenly observe distressing thoughts and experiences—not necessarily with the goal of changing them but to accept them and act in a way that is consistent with his (her) goals and values. A meta-analysis of 39 studies found mindfulness-based therapy effective in improving symptoms in participants with anxiety and mood disorders.21 Similarly, ACT has demonstrated efficacy with mixed anxiety disorders.22
Transdiagnostic CBT. Recent research18 suggests that mood and anxiety disorders may have more commonalities than differences in underlying biological and psychological traits. Because the symptoms of anxiety and depressive disorders tend to overlap, and their rate of comorbidity may be as high as 55%,23 so-called transdiagnostic treatments have been developed. Transdiagnostic treatments target impairing symptoms that cut across different diagnoses. For example, patients with depression, anxiety, or substance abuse might share a common difficulty with regulating and coping with negative emotions.
In a preliminary comparison trial,24 46 patients with social anxiety disorder, panic disorder, or GAD were randomly assigned to transdiagnostic CBT (n = 23) or diagnosis-specific CBT (n = 23). Treatments were based on widely used manuals and offered in 2-hour group sessions across 12 weeks. Transdiagnostic CBT was found to be as effective as specific CBT protocols in terms of symptom improvement. Participants attended an average of 8.46 sessions, with similar attendance in each protocol. Fourteen participants (30%) discontinued treatment, similar to attrition rates reported in other trials of transdiagnostic and diagnosis-specific CBT.
Transdiagnostic treatments may facilitate the dissemination of empirically supported treatments because therapists would not be required to have training and supervision to competency in delivering multiple manuals for specific anxiety disorders. This could be attractive to busy practitioners with limited time to learn new treatments.
Bottom Line
Efficacy of cognitive-behavioral therapy (CBT) for depression and anxiety is well established. Although no specific technique defines CBT, a common practice is to educate an individual about interrelationships between behaviors/activities, thoughts, and mood. CBT techniques can be customized to treat specific anxiety disorders, such as panic disorder, obsessive-compulsive disorder, and generalized anxiety disorder.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Collins K, Westra H, Dozois D, et al. Gaps in accessing treatment for anxiety and depressions: challenges for the delivery of care. Clin Psychol Rev. 2004;24(5):583-616.
2. Foa EB, Gillihan SJ, Bryant RA. Challenges and successes in dissemination of evidence-based treatments for posttraumatic stress: lessons learned from prolonged exposure therapy for PTSD. Psychological Science in the Public Interest. 2013;14(2):65-111.
3. Rahman A, Malik A, Sikander S, et al. Cognitive behaviour therapy-based intervention by community health workers for mothers with depression and their infants in rural Pakistan: a cluster-randomised controlled trial. Lancet. 2008;372(9642):902-909.
4. Aguilera A, Muench F. There’s an app for that: information technology applications for cognitive behavioral practitioners. Behavior Therapist. 2012;35(4):65-73.
5. Dimidjian S, Hollon SD, Dobson KS, et al. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the acute treatment of adults with major depression. J Consult Clin Psychol. 2006; 74(4):658-670.
6. Hollon SD, Jarrett RB, Nierenberg AA, et al. Psychotherapy and medication in the treatment of adult and geriatric depression: which monotherapy or combined treatment? J Clin Psychiatry. 2005;66(4):455-468.
7. Cuijpers P, Hollon SD, van Straten A, et al. Does cognitive behaviour therapy have an enduring effect that is superior to keeping patients on continuation pharmacotherapy? A meta-analysis. BMJ Open. 2013;3(4):1-8.
8. Stewart R, Chambless D. Cognitive-behavioral therapy for adult anxiety disorders in clinical practice: a meta-analysis of effectiveness studies. J Consult Clin Psychol. 2009;77(4): 595-606.
9. Wright JH, Basco MR, Thase M. Learning cognitive behavior therapy: an illustrated guide. Arlington, VA: American Psychiatric Publishing; 2006.
10. Barlow DH, Craske MG. Mastery of your anxiety and panic. 4th ed. New York, NY: Oxford University Press, Inc.; 2007.
11. Foa EB, Yadin E, Lichner TK. Exposure and response prevention for obsessive-compulsive disorder: therapist guide. New York, NY: Oxford University Press, Inc.; 2012.
12. Dugas MJ, Robichaud M. Cognitive-behavioral treatment for generalized anxiety disorder. New York, NY: Routledge; 2007.
13. Zlomke K, Davis TE. One-session treatment of specific phobias: a detailed description and review of treatment efficacy. Behav Ther. 2008;39(3):207-223.
14. Foa EB, Hembree E, Rothbaum B. Prolonged exposure therapy for PTSD: emotional processing of traumatic experiences. Therapist guide. New York, NY: Oxford University Press, Inc.; 2007.
15. Resick PA, Schnicke MK. Cognitive processing therapy for rape victims. London, United Kingdom: Sage Publications; 1996.
16. Whittal ML, Robichaud M, Woody SR. Cognitive treatment of obsessions: enhancing dissemination with video components. Cognitive and Behavioral Practice. 2010;17(1):1-8.
17. Westra H, Arkowitz H, Dozois D. Adding a motivational interviewing pretreatment to cognitive behavioral therapy for generalized anxiety disorder: a preliminary randomized controlled trial. J Anxiety Disord. 2009;23(2): 1106-1117.
18. Dobson KS. The science of CBT: toward a metacognitive model of change? Behav Ther. 2013;44(2):224-227.
19. Kabat-Zinn J. Full catastrophe living. Using the wisdom of your body and mind to face stress, pain, and illness. Revised edition. New York, NY: Bantam Books; 2013.
20. Hayes SC, Strosahl KD. Acceptance and commitment therapy. The process and practice of mindful change. 2nd ed. New York, NY: The Guilford Press; 2012.
21. Hofmann S, Sawyer A, Witt A, et al. The effect of mindfulness-based therapy on anxiety and depression: a meta-analytic review. J Consult Clin Psychol. 2010;78(2): 169-183.
22. Arch J, Eifert G, Davies C, et al. Randomized clinical trial of cognitive behavioral therapy (CBT) versus acceptance and commitment therapy (ACT) for mixed anxiety disorders. J Consult Clin Psychol. 2012;80(5):750-765.
23. Brown TA, Campbell LA, Lehman CL, et al. Current and lifetime comorbidity of the DSM-IV anxiety and mood disorders in a large clinical sample. J Abnorm Psychol. 2001;110(4):585-599.
24. Norton P, Barrera T. Transdiagnostic versus diagnosis-specific CBT for anxiety disorders: a preliminary randomized controlled noninferiority trial. Depress Anxiety. 2012;29(10):874-882.
1. Collins K, Westra H, Dozois D, et al. Gaps in accessing treatment for anxiety and depressions: challenges for the delivery of care. Clin Psychol Rev. 2004;24(5):583-616.
2. Foa EB, Gillihan SJ, Bryant RA. Challenges and successes in dissemination of evidence-based treatments for posttraumatic stress: lessons learned from prolonged exposure therapy for PTSD. Psychological Science in the Public Interest. 2013;14(2):65-111.
3. Rahman A, Malik A, Sikander S, et al. Cognitive behaviour therapy-based intervention by community health workers for mothers with depression and their infants in rural Pakistan: a cluster-randomised controlled trial. Lancet. 2008;372(9642):902-909.
4. Aguilera A, Muench F. There’s an app for that: information technology applications for cognitive behavioral practitioners. Behavior Therapist. 2012;35(4):65-73.
5. Dimidjian S, Hollon SD, Dobson KS, et al. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the acute treatment of adults with major depression. J Consult Clin Psychol. 2006; 74(4):658-670.
6. Hollon SD, Jarrett RB, Nierenberg AA, et al. Psychotherapy and medication in the treatment of adult and geriatric depression: which monotherapy or combined treatment? J Clin Psychiatry. 2005;66(4):455-468.
7. Cuijpers P, Hollon SD, van Straten A, et al. Does cognitive behaviour therapy have an enduring effect that is superior to keeping patients on continuation pharmacotherapy? A meta-analysis. BMJ Open. 2013;3(4):1-8.
8. Stewart R, Chambless D. Cognitive-behavioral therapy for adult anxiety disorders in clinical practice: a meta-analysis of effectiveness studies. J Consult Clin Psychol. 2009;77(4): 595-606.
9. Wright JH, Basco MR, Thase M. Learning cognitive behavior therapy: an illustrated guide. Arlington, VA: American Psychiatric Publishing; 2006.
10. Barlow DH, Craske MG. Mastery of your anxiety and panic. 4th ed. New York, NY: Oxford University Press, Inc.; 2007.
11. Foa EB, Yadin E, Lichner TK. Exposure and response prevention for obsessive-compulsive disorder: therapist guide. New York, NY: Oxford University Press, Inc.; 2012.
12. Dugas MJ, Robichaud M. Cognitive-behavioral treatment for generalized anxiety disorder. New York, NY: Routledge; 2007.
13. Zlomke K, Davis TE. One-session treatment of specific phobias: a detailed description and review of treatment efficacy. Behav Ther. 2008;39(3):207-223.
14. Foa EB, Hembree E, Rothbaum B. Prolonged exposure therapy for PTSD: emotional processing of traumatic experiences. Therapist guide. New York, NY: Oxford University Press, Inc.; 2007.
15. Resick PA, Schnicke MK. Cognitive processing therapy for rape victims. London, United Kingdom: Sage Publications; 1996.
16. Whittal ML, Robichaud M, Woody SR. Cognitive treatment of obsessions: enhancing dissemination with video components. Cognitive and Behavioral Practice. 2010;17(1):1-8.
17. Westra H, Arkowitz H, Dozois D. Adding a motivational interviewing pretreatment to cognitive behavioral therapy for generalized anxiety disorder: a preliminary randomized controlled trial. J Anxiety Disord. 2009;23(2): 1106-1117.
18. Dobson KS. The science of CBT: toward a metacognitive model of change? Behav Ther. 2013;44(2):224-227.
19. Kabat-Zinn J. Full catastrophe living. Using the wisdom of your body and mind to face stress, pain, and illness. Revised edition. New York, NY: Bantam Books; 2013.
20. Hayes SC, Strosahl KD. Acceptance and commitment therapy. The process and practice of mindful change. 2nd ed. New York, NY: The Guilford Press; 2012.
21. Hofmann S, Sawyer A, Witt A, et al. The effect of mindfulness-based therapy on anxiety and depression: a meta-analytic review. J Consult Clin Psychol. 2010;78(2): 169-183.
22. Arch J, Eifert G, Davies C, et al. Randomized clinical trial of cognitive behavioral therapy (CBT) versus acceptance and commitment therapy (ACT) for mixed anxiety disorders. J Consult Clin Psychol. 2012;80(5):750-765.
23. Brown TA, Campbell LA, Lehman CL, et al. Current and lifetime comorbidity of the DSM-IV anxiety and mood disorders in a large clinical sample. J Abnorm Psychol. 2001;110(4):585-599.
24. Norton P, Barrera T. Transdiagnostic versus diagnosis-specific CBT for anxiety disorders: a preliminary randomized controlled noninferiority trial. Depress Anxiety. 2012;29(10):874-882.
Do biomarkers for Alzheimer’s disease have utility in everyday practice?
Guidelines for diagnosing Alzheimer’s disease (AD) are undergoing the first major changes since they were developed 30 years ago. The National Institute on Aging (NIA) and the Alzheimer’s Association (AA) have established workgroups to revise guidelines that were written in 1984.1
One of the major changes to these new guidelines is mention of research on biomarkers for diagnosing and monitoring progression of dementia in AD. This is an exciting and provocative development, but the questions practitioners who diagnose and treat AD should be asking are whether such biomarkers have utility in clinical practice today, or whether their application is a distant promise of continuing research.
Principles put forward in the guidelines
The new AD guidelines set forth in 3 major papers by the workgroups created by the NIA and AA include a change in nomenclature of AD.2 The workgroups have sought to define AD with specific stages that include:
• a preclinical/prodromal phase, in which the pathophysiology responsible for future cognitive changes is ongoing but lacks clinical manifestations3
• mild cognitive impairment, now considered a distinct entity from dementia and diagnosed when a person has early signs of AD; manifestations of impaired cognition in early disease are not significant enough to affect daily functioning.4
These newly formulated stages of AD rely on clinical judgment, and AD remains a clinical diagnosis. However, the new diagnostic guidelines include the use of biomarkers to measure disease progression.
Biomarkers of normal biologic function and pathology
The Biomarkers Definitions Working Group defines a biomarker as:
… a characteristic that is objectively measured and evaluated as an indicator of normal biologic processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention.5
These characteristics include imaging studies and body fluids, such as serum and cerebrospinal fluid (CSF).
In AD, biomarkers are meant to measure the pathogenic processes of:
• accumulation and deposition of amyloid β _protein (Aβ42) plaques
• neuronal degeneration characterized by an increase in phosphorylated tau protein and neurofibrillary tangles.6
The purpose of these biomarkers is to identify ongoing disease and help the clinician stage patients who display a spectrum of symptoms.
Four classes of biomarkers (Table7)have been identified for use in the diagnosis of, and research on, AD:
• neuroimaging
• CSF
• serum
• genetic markers.
Neuroimaging
The basic purpose of CT and MRI of the head in the workup of cognitive impairment is to rule out a lesion in the brain, such as a tumor or hemorrhage, as the cause of, or contributor to, the impairment. Several neuroimaging studies are available to aid in diagnosing AD and distinguishing it from other causes of dementia, including:
• Fludeoxyglucose (FDG) positron-emission tomography (PET) scanning
• MRI
• Florbetapir F 18 Injection for PET.
FDG PET identifies areas of the brain in which glucose metabolism is decreased. This finding is thought to represent synaptic dysfunction.8 The true clinical utility of FDG PET appears to be as an aid in distinguishing cases of AD from frontotemporal dementia, by identifying regions of metabolic dysfunction.9 (Note: Medicare will reimburse for FDG PET only if 1) the patient has met diagnostic criteria for both AD and frontotemporal dementia for at least 6 months and 2) the cause of symptoms is uncertain.10)
FDG PET also can be useful in patients with mild cognitive impairment by identifying hypometabolism in the temporal and parietal regions of the brain years before clinical AD develops.In addition to FDG, 2 other imaging probes—Pittsburgh compound and 2-(1-{6-[(2-[fluorine-18]fluoroethyl)(methyl) amino]-2-naphthyl}-ethylidene) malononitrile (more commonly, FDDNP)—have been used with PET as research tools to demonstrate evidence of AD.11
MRI has been used to measure hippocampal atrophy and cortical thinning that occurs as a patient progresses from normal cognitive function or mild cognitive impairment to full dementia.5 The degree of atrophy has not been well correlated with the degree of functional impairment.
Florbetapir F 18 Injection was approved by the FDA in October 2013, under the brand name AMYViD, for measuring the quantity of Aβ42 deposition in the brain. When injected, this radiopharmaceutical binds to Aβ42 and can be detected on PET.12 Use criteria for AMYViD PET recently were developed13; the technique is indicated as an additional diagnostic tool for ruling out AD.
A negative AMYViD scan indicates sparse or no Aβ42 plaques, and is inconsistent with AD. However, a positive AMYViD scan does not establish a diagnosis of AD or other cognitive disorder.14 This lack of specificity decreases the potential utility of the scan in clinical practice.
Use of AMYViD PET in general practice also is constrained by cost, which varies by location, based on the fee for the PET scan ($1,000 to $3,000)15; to that, add the cost of a dose of AMYViD ($1,600, wholesale).16 The technique is not reimbursable, and the total out-of-pocket expense can be as much as $5,000—making an AMYViD PET prohibitive.
Cerebrospinal fluid markers
CSF biomarkers used in the evaluation of AD are Aβ42, t-tau protein, and p-tau protein.6,17 It is generally thought that the level of Aβ42 in CSF decreases in AD—indicative of Aβ42 being deposited in the brain.8 Tau proteins are elevated in CSF as neurons are destroyed. P-tau is associated with the neurofibrillary tangles of AD; its presence in CSF is thought to represent an increase in those tangles. The combination of a low level of Aβ42 and an elevated level of p-tau in CSF is considered the signature CSF biomarker of AD.6
Serum markers
The search for reliable serum biomarkers of AD is the area of greatest research interest because a blood test is a less invasive form of screening. Regrettably, the utility of serum biomarkers for clinical practice has not been established.
Aβ42 can be measured in serum, but levels do not correlate well with CSF levels.18 Other serum markers that have been evaluated for clinical utility include measures of lipid metabolism, oxidation, and inflammation. With none of these is there clear correlation between the level of protein and AD.18
Fourth front: Genetics
Several alleles are associated with AD. Mutations in amyloid precursor protein, presenilin 1, and presenilin 2 have been shown to cause a change in the processing of Aβ42 and thus lead to AD.19 These mutations are inherited in an autosomal-dominant fashion and are detected in early-onset (age <65) AD.
Mutations in apolipoprotein 4-β4 also has been the subject of much research; this allele usually is associated with increased risk of the more common, later-onset AD.20 Some evidence suggests that apolipoprotein 4-β4 carriers who develop AD might be at risk of earlier onset of symptoms, compared to noncarriers,21 but the clinical significance of that increased risk has not been established.
What utility do biomarkers have?
As we said at the beginning of this article, the question that clinicians should be asking is: “What is the current clinical utility of these sophisticated biomarkers and genetic testing?”
The answer is “little utility.” Diagnosing AD is a clinical enterprise, with, as we’ve outlined, specific and narrow exceptions.
Recently, researchers demonstrated biomarker evidence of AD before symptom onset in patients who have known autosomal-dominant gene mutations for AD.19 There is no evidence, however, that these biomarkers are useful for screening the general population to identify people who 1) are at risk of, or who have, AD and 2) do not have AD.
That being said, CSF and imaging biomarkers of AD are being used in clinical settings in some European countries to aid investigation of cognitive decline.
In conclusion
Here are key points to take away from this discussion of biomarkers of AD:
• The utility of these biomarkers today is in research—although some of them might, on occasion, be useful to distinguish dementia caused by AD from other dementias.
• The ultimate goal of research is to uncover a serum biomarker that can identify patients in the preclinical/prodromal stage of AD, so that disease-modifying therapies and preventive measures can be initiated before symptoms manifest.
• Science is a long way from making this goal a reality, but recent changes in the diagnostic criteria for AD will encourage research in this area of study.
Bottom Line
Researchers are working to uncover biomarkers that will identify patients in the preclinical or prodromal stage of Alzheimer’s disease, but diagnosis remains clinical. Recent changes to diagnostic criteria will encourage research in this area.
Related Resources
• Blennow K, Dubois B, Fagan AM, et al. Clinical utility of cerebrospinal fluid biomarkers in the diagnosis of early Alzheimer’s disease [published online May 5, 2014]. Alzheimers Dement. doi: 10.1016/j.jalz.2014.02.004.
• Chase A. Alzheimer disease: Advances in imaging of AD biomarkers could aid early diagnosis. Nat Rev Neurol. 2014;10(5):239.
• De Riva V, Galloni E, Marcon M, et al. Analysis of combined CSF biomarkers in AD diagnosis. Clin Lab. 2014;60(4):629-634.
• Kristofikova Z, Ricny J, Kolarova M, et al. Interactions between amyloid-β and tau in cerebrospinal fluid of people with mild cognitive impairment and Alzheimer’s disease [published online March 26, 2014]. J Alzheimers Dis. doi: 10.3233/ JAD-132393.
Drug Brand Name
Florbetapir F 18 Injection • AMYViD
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Jack CR Jr, Albert MS, Knopman DS, et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011; 7(3):257-262.
2. McKhann GM, Knopman DS. Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging- Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263-269.
3. Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280-292.
4. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging- Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011; 7(3):270-279.
5. Cummings JL. Biomarkers in Alzheimer’s disease– perspectives for the future. US Neurology. 2010;6(1):23-27.
6. Sperling R, Keith J. Biomarkers of Alzheimer disease: current and future applications to diagnostic criteria. Continuum (Minneap Minn). 2013;19(2 Dementia):325-338.
7. Craig-Shapiro R, Fagan AM, Holtzman DM. Biomarkers of Alzheimer’s disease. Neurobiol Dis. 2009;35(2):128-140.
8. Jack CR Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9(1):119-128.
9. Foster NL, Heidebrink JL, Clark CM, et al. FDG-PET improves accuracy in distinguishing frontotemporal dementia and Alzheimer’s disease. Brain. 2007;130(pt 10):2616-2635.
10. National Coverage Determination (NCD) for FDG PET for Dementia and Neurodegenerative Diseases (220.6.13). Centers for Medicare and Medicaid Services. http://www. cms.gov/medicare-coverage-database/details/ncd-details. aspx?NCDId=288&ncdver=3&bc=BAABAAAAAAAA&. Accessed May 9, 2014.
11. Small GW, Bookheimer SY, Thompson PM, et al. Current and future uses of neuroimaging for cognitively impaired patients. Lancet Neurol. 2008;7(2):161-172. 12. Clark CM, Schneider JA, Bedell BJ, et al. Use of florbetapir- PET for imaging beta-amyloid pathology. JAMA. 2011;305(3): 275-283.
13. Johnson KA, Minoshima S, Bohnen NI, et al. Update on appropriate use criteria for amyloid PET imaging: dementia experts, mild cognitive impairment, and education. Amyloid Imaging Task Force of the Alzheimer’s Association and Society for Nuclear Medicine and Molecular Imaging. Alzheimers Dement. 2013;9(4):e106-e109.
14. AMYViD [package insert]. Indianapolis, IN: Eli Lilly & Co; 2012.
15. First guidelines published for brain amyloid imaging in Alzheimer’s. Alzheimer’s Association. http://www.alz.org/ news_and_events_60578.asp. Published January 28, 2013. Accessed May 9, 2014.
16. Zakaib GD. FDA approves Amyvid for clinical use. Alzforum. http://www.alzforum.org/news/research-news/ fda-approves-amyvid-clinical-use. Published April 9, 2012. Accessed May 16, 2014.
17. Skillbäck T, Zetterberg H, Blennow K, et al. Cerebrospinal fluid biomarkers for Alzheimer disease and subcortical axonal damage in 5,542 clinical samples. Alzheimers Res Ther. 2013;5(5):47.
18. Irizarry MC. Biomarkers of Alzheimer disease in plasma. NeuroRx. 2004;1(2):226-234.
19. Bateman RJ, Xiong C, Benzinger TL, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012;367(9):795-804.
20. Bertram L, McQueen MB, Mullin K, et al. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nature Genetics. 2007;39(1):17-23.
21. Strittmatter WJ, Roses AD. Apolipoprotein E and Alzheimer’s disease. Annu Rev Neurosci. 1996;19:53-77.
Guidelines for diagnosing Alzheimer’s disease (AD) are undergoing the first major changes since they were developed 30 years ago. The National Institute on Aging (NIA) and the Alzheimer’s Association (AA) have established workgroups to revise guidelines that were written in 1984.1
One of the major changes to these new guidelines is mention of research on biomarkers for diagnosing and monitoring progression of dementia in AD. This is an exciting and provocative development, but the questions practitioners who diagnose and treat AD should be asking are whether such biomarkers have utility in clinical practice today, or whether their application is a distant promise of continuing research.
Principles put forward in the guidelines
The new AD guidelines set forth in 3 major papers by the workgroups created by the NIA and AA include a change in nomenclature of AD.2 The workgroups have sought to define AD with specific stages that include:
• a preclinical/prodromal phase, in which the pathophysiology responsible for future cognitive changes is ongoing but lacks clinical manifestations3
• mild cognitive impairment, now considered a distinct entity from dementia and diagnosed when a person has early signs of AD; manifestations of impaired cognition in early disease are not significant enough to affect daily functioning.4
These newly formulated stages of AD rely on clinical judgment, and AD remains a clinical diagnosis. However, the new diagnostic guidelines include the use of biomarkers to measure disease progression.
Biomarkers of normal biologic function and pathology
The Biomarkers Definitions Working Group defines a biomarker as:
… a characteristic that is objectively measured and evaluated as an indicator of normal biologic processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention.5
These characteristics include imaging studies and body fluids, such as serum and cerebrospinal fluid (CSF).
In AD, biomarkers are meant to measure the pathogenic processes of:
• accumulation and deposition of amyloid β _protein (Aβ42) plaques
• neuronal degeneration characterized by an increase in phosphorylated tau protein and neurofibrillary tangles.6
The purpose of these biomarkers is to identify ongoing disease and help the clinician stage patients who display a spectrum of symptoms.
Four classes of biomarkers (Table7)have been identified for use in the diagnosis of, and research on, AD:
• neuroimaging
• CSF
• serum
• genetic markers.
Neuroimaging
The basic purpose of CT and MRI of the head in the workup of cognitive impairment is to rule out a lesion in the brain, such as a tumor or hemorrhage, as the cause of, or contributor to, the impairment. Several neuroimaging studies are available to aid in diagnosing AD and distinguishing it from other causes of dementia, including:
• Fludeoxyglucose (FDG) positron-emission tomography (PET) scanning
• MRI
• Florbetapir F 18 Injection for PET.
FDG PET identifies areas of the brain in which glucose metabolism is decreased. This finding is thought to represent synaptic dysfunction.8 The true clinical utility of FDG PET appears to be as an aid in distinguishing cases of AD from frontotemporal dementia, by identifying regions of metabolic dysfunction.9 (Note: Medicare will reimburse for FDG PET only if 1) the patient has met diagnostic criteria for both AD and frontotemporal dementia for at least 6 months and 2) the cause of symptoms is uncertain.10)
FDG PET also can be useful in patients with mild cognitive impairment by identifying hypometabolism in the temporal and parietal regions of the brain years before clinical AD develops.In addition to FDG, 2 other imaging probes—Pittsburgh compound and 2-(1-{6-[(2-[fluorine-18]fluoroethyl)(methyl) amino]-2-naphthyl}-ethylidene) malononitrile (more commonly, FDDNP)—have been used with PET as research tools to demonstrate evidence of AD.11
MRI has been used to measure hippocampal atrophy and cortical thinning that occurs as a patient progresses from normal cognitive function or mild cognitive impairment to full dementia.5 The degree of atrophy has not been well correlated with the degree of functional impairment.
Florbetapir F 18 Injection was approved by the FDA in October 2013, under the brand name AMYViD, for measuring the quantity of Aβ42 deposition in the brain. When injected, this radiopharmaceutical binds to Aβ42 and can be detected on PET.12 Use criteria for AMYViD PET recently were developed13; the technique is indicated as an additional diagnostic tool for ruling out AD.
A negative AMYViD scan indicates sparse or no Aβ42 plaques, and is inconsistent with AD. However, a positive AMYViD scan does not establish a diagnosis of AD or other cognitive disorder.14 This lack of specificity decreases the potential utility of the scan in clinical practice.
Use of AMYViD PET in general practice also is constrained by cost, which varies by location, based on the fee for the PET scan ($1,000 to $3,000)15; to that, add the cost of a dose of AMYViD ($1,600, wholesale).16 The technique is not reimbursable, and the total out-of-pocket expense can be as much as $5,000—making an AMYViD PET prohibitive.
Cerebrospinal fluid markers
CSF biomarkers used in the evaluation of AD are Aβ42, t-tau protein, and p-tau protein.6,17 It is generally thought that the level of Aβ42 in CSF decreases in AD—indicative of Aβ42 being deposited in the brain.8 Tau proteins are elevated in CSF as neurons are destroyed. P-tau is associated with the neurofibrillary tangles of AD; its presence in CSF is thought to represent an increase in those tangles. The combination of a low level of Aβ42 and an elevated level of p-tau in CSF is considered the signature CSF biomarker of AD.6
Serum markers
The search for reliable serum biomarkers of AD is the area of greatest research interest because a blood test is a less invasive form of screening. Regrettably, the utility of serum biomarkers for clinical practice has not been established.
Aβ42 can be measured in serum, but levels do not correlate well with CSF levels.18 Other serum markers that have been evaluated for clinical utility include measures of lipid metabolism, oxidation, and inflammation. With none of these is there clear correlation between the level of protein and AD.18
Fourth front: Genetics
Several alleles are associated with AD. Mutations in amyloid precursor protein, presenilin 1, and presenilin 2 have been shown to cause a change in the processing of Aβ42 and thus lead to AD.19 These mutations are inherited in an autosomal-dominant fashion and are detected in early-onset (age <65) AD.
Mutations in apolipoprotein 4-β4 also has been the subject of much research; this allele usually is associated with increased risk of the more common, later-onset AD.20 Some evidence suggests that apolipoprotein 4-β4 carriers who develop AD might be at risk of earlier onset of symptoms, compared to noncarriers,21 but the clinical significance of that increased risk has not been established.
What utility do biomarkers have?
As we said at the beginning of this article, the question that clinicians should be asking is: “What is the current clinical utility of these sophisticated biomarkers and genetic testing?”
The answer is “little utility.” Diagnosing AD is a clinical enterprise, with, as we’ve outlined, specific and narrow exceptions.
Recently, researchers demonstrated biomarker evidence of AD before symptom onset in patients who have known autosomal-dominant gene mutations for AD.19 There is no evidence, however, that these biomarkers are useful for screening the general population to identify people who 1) are at risk of, or who have, AD and 2) do not have AD.
That being said, CSF and imaging biomarkers of AD are being used in clinical settings in some European countries to aid investigation of cognitive decline.
In conclusion
Here are key points to take away from this discussion of biomarkers of AD:
• The utility of these biomarkers today is in research—although some of them might, on occasion, be useful to distinguish dementia caused by AD from other dementias.
• The ultimate goal of research is to uncover a serum biomarker that can identify patients in the preclinical/prodromal stage of AD, so that disease-modifying therapies and preventive measures can be initiated before symptoms manifest.
• Science is a long way from making this goal a reality, but recent changes in the diagnostic criteria for AD will encourage research in this area of study.
Bottom Line
Researchers are working to uncover biomarkers that will identify patients in the preclinical or prodromal stage of Alzheimer’s disease, but diagnosis remains clinical. Recent changes to diagnostic criteria will encourage research in this area.
Related Resources
• Blennow K, Dubois B, Fagan AM, et al. Clinical utility of cerebrospinal fluid biomarkers in the diagnosis of early Alzheimer’s disease [published online May 5, 2014]. Alzheimers Dement. doi: 10.1016/j.jalz.2014.02.004.
• Chase A. Alzheimer disease: Advances in imaging of AD biomarkers could aid early diagnosis. Nat Rev Neurol. 2014;10(5):239.
• De Riva V, Galloni E, Marcon M, et al. Analysis of combined CSF biomarkers in AD diagnosis. Clin Lab. 2014;60(4):629-634.
• Kristofikova Z, Ricny J, Kolarova M, et al. Interactions between amyloid-β and tau in cerebrospinal fluid of people with mild cognitive impairment and Alzheimer’s disease [published online March 26, 2014]. J Alzheimers Dis. doi: 10.3233/ JAD-132393.
Drug Brand Name
Florbetapir F 18 Injection • AMYViD
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Guidelines for diagnosing Alzheimer’s disease (AD) are undergoing the first major changes since they were developed 30 years ago. The National Institute on Aging (NIA) and the Alzheimer’s Association (AA) have established workgroups to revise guidelines that were written in 1984.1
One of the major changes to these new guidelines is mention of research on biomarkers for diagnosing and monitoring progression of dementia in AD. This is an exciting and provocative development, but the questions practitioners who diagnose and treat AD should be asking are whether such biomarkers have utility in clinical practice today, or whether their application is a distant promise of continuing research.
Principles put forward in the guidelines
The new AD guidelines set forth in 3 major papers by the workgroups created by the NIA and AA include a change in nomenclature of AD.2 The workgroups have sought to define AD with specific stages that include:
• a preclinical/prodromal phase, in which the pathophysiology responsible for future cognitive changes is ongoing but lacks clinical manifestations3
• mild cognitive impairment, now considered a distinct entity from dementia and diagnosed when a person has early signs of AD; manifestations of impaired cognition in early disease are not significant enough to affect daily functioning.4
These newly formulated stages of AD rely on clinical judgment, and AD remains a clinical diagnosis. However, the new diagnostic guidelines include the use of biomarkers to measure disease progression.
Biomarkers of normal biologic function and pathology
The Biomarkers Definitions Working Group defines a biomarker as:
… a characteristic that is objectively measured and evaluated as an indicator of normal biologic processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention.5
These characteristics include imaging studies and body fluids, such as serum and cerebrospinal fluid (CSF).
In AD, biomarkers are meant to measure the pathogenic processes of:
• accumulation and deposition of amyloid β _protein (Aβ42) plaques
• neuronal degeneration characterized by an increase in phosphorylated tau protein and neurofibrillary tangles.6
The purpose of these biomarkers is to identify ongoing disease and help the clinician stage patients who display a spectrum of symptoms.
Four classes of biomarkers (Table7)have been identified for use in the diagnosis of, and research on, AD:
• neuroimaging
• CSF
• serum
• genetic markers.
Neuroimaging
The basic purpose of CT and MRI of the head in the workup of cognitive impairment is to rule out a lesion in the brain, such as a tumor or hemorrhage, as the cause of, or contributor to, the impairment. Several neuroimaging studies are available to aid in diagnosing AD and distinguishing it from other causes of dementia, including:
• Fludeoxyglucose (FDG) positron-emission tomography (PET) scanning
• MRI
• Florbetapir F 18 Injection for PET.
FDG PET identifies areas of the brain in which glucose metabolism is decreased. This finding is thought to represent synaptic dysfunction.8 The true clinical utility of FDG PET appears to be as an aid in distinguishing cases of AD from frontotemporal dementia, by identifying regions of metabolic dysfunction.9 (Note: Medicare will reimburse for FDG PET only if 1) the patient has met diagnostic criteria for both AD and frontotemporal dementia for at least 6 months and 2) the cause of symptoms is uncertain.10)
FDG PET also can be useful in patients with mild cognitive impairment by identifying hypometabolism in the temporal and parietal regions of the brain years before clinical AD develops.In addition to FDG, 2 other imaging probes—Pittsburgh compound and 2-(1-{6-[(2-[fluorine-18]fluoroethyl)(methyl) amino]-2-naphthyl}-ethylidene) malononitrile (more commonly, FDDNP)—have been used with PET as research tools to demonstrate evidence of AD.11
MRI has been used to measure hippocampal atrophy and cortical thinning that occurs as a patient progresses from normal cognitive function or mild cognitive impairment to full dementia.5 The degree of atrophy has not been well correlated with the degree of functional impairment.
Florbetapir F 18 Injection was approved by the FDA in October 2013, under the brand name AMYViD, for measuring the quantity of Aβ42 deposition in the brain. When injected, this radiopharmaceutical binds to Aβ42 and can be detected on PET.12 Use criteria for AMYViD PET recently were developed13; the technique is indicated as an additional diagnostic tool for ruling out AD.
A negative AMYViD scan indicates sparse or no Aβ42 plaques, and is inconsistent with AD. However, a positive AMYViD scan does not establish a diagnosis of AD or other cognitive disorder.14 This lack of specificity decreases the potential utility of the scan in clinical practice.
Use of AMYViD PET in general practice also is constrained by cost, which varies by location, based on the fee for the PET scan ($1,000 to $3,000)15; to that, add the cost of a dose of AMYViD ($1,600, wholesale).16 The technique is not reimbursable, and the total out-of-pocket expense can be as much as $5,000—making an AMYViD PET prohibitive.
Cerebrospinal fluid markers
CSF biomarkers used in the evaluation of AD are Aβ42, t-tau protein, and p-tau protein.6,17 It is generally thought that the level of Aβ42 in CSF decreases in AD—indicative of Aβ42 being deposited in the brain.8 Tau proteins are elevated in CSF as neurons are destroyed. P-tau is associated with the neurofibrillary tangles of AD; its presence in CSF is thought to represent an increase in those tangles. The combination of a low level of Aβ42 and an elevated level of p-tau in CSF is considered the signature CSF biomarker of AD.6
Serum markers
The search for reliable serum biomarkers of AD is the area of greatest research interest because a blood test is a less invasive form of screening. Regrettably, the utility of serum biomarkers for clinical practice has not been established.
Aβ42 can be measured in serum, but levels do not correlate well with CSF levels.18 Other serum markers that have been evaluated for clinical utility include measures of lipid metabolism, oxidation, and inflammation. With none of these is there clear correlation between the level of protein and AD.18
Fourth front: Genetics
Several alleles are associated with AD. Mutations in amyloid precursor protein, presenilin 1, and presenilin 2 have been shown to cause a change in the processing of Aβ42 and thus lead to AD.19 These mutations are inherited in an autosomal-dominant fashion and are detected in early-onset (age <65) AD.
Mutations in apolipoprotein 4-β4 also has been the subject of much research; this allele usually is associated with increased risk of the more common, later-onset AD.20 Some evidence suggests that apolipoprotein 4-β4 carriers who develop AD might be at risk of earlier onset of symptoms, compared to noncarriers,21 but the clinical significance of that increased risk has not been established.
What utility do biomarkers have?
As we said at the beginning of this article, the question that clinicians should be asking is: “What is the current clinical utility of these sophisticated biomarkers and genetic testing?”
The answer is “little utility.” Diagnosing AD is a clinical enterprise, with, as we’ve outlined, specific and narrow exceptions.
Recently, researchers demonstrated biomarker evidence of AD before symptom onset in patients who have known autosomal-dominant gene mutations for AD.19 There is no evidence, however, that these biomarkers are useful for screening the general population to identify people who 1) are at risk of, or who have, AD and 2) do not have AD.
That being said, CSF and imaging biomarkers of AD are being used in clinical settings in some European countries to aid investigation of cognitive decline.
In conclusion
Here are key points to take away from this discussion of biomarkers of AD:
• The utility of these biomarkers today is in research—although some of them might, on occasion, be useful to distinguish dementia caused by AD from other dementias.
• The ultimate goal of research is to uncover a serum biomarker that can identify patients in the preclinical/prodromal stage of AD, so that disease-modifying therapies and preventive measures can be initiated before symptoms manifest.
• Science is a long way from making this goal a reality, but recent changes in the diagnostic criteria for AD will encourage research in this area of study.
Bottom Line
Researchers are working to uncover biomarkers that will identify patients in the preclinical or prodromal stage of Alzheimer’s disease, but diagnosis remains clinical. Recent changes to diagnostic criteria will encourage research in this area.
Related Resources
• Blennow K, Dubois B, Fagan AM, et al. Clinical utility of cerebrospinal fluid biomarkers in the diagnosis of early Alzheimer’s disease [published online May 5, 2014]. Alzheimers Dement. doi: 10.1016/j.jalz.2014.02.004.
• Chase A. Alzheimer disease: Advances in imaging of AD biomarkers could aid early diagnosis. Nat Rev Neurol. 2014;10(5):239.
• De Riva V, Galloni E, Marcon M, et al. Analysis of combined CSF biomarkers in AD diagnosis. Clin Lab. 2014;60(4):629-634.
• Kristofikova Z, Ricny J, Kolarova M, et al. Interactions between amyloid-β and tau in cerebrospinal fluid of people with mild cognitive impairment and Alzheimer’s disease [published online March 26, 2014]. J Alzheimers Dis. doi: 10.3233/ JAD-132393.
Drug Brand Name
Florbetapir F 18 Injection • AMYViD
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Jack CR Jr, Albert MS, Knopman DS, et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011; 7(3):257-262.
2. McKhann GM, Knopman DS. Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging- Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263-269.
3. Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280-292.
4. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging- Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011; 7(3):270-279.
5. Cummings JL. Biomarkers in Alzheimer’s disease– perspectives for the future. US Neurology. 2010;6(1):23-27.
6. Sperling R, Keith J. Biomarkers of Alzheimer disease: current and future applications to diagnostic criteria. Continuum (Minneap Minn). 2013;19(2 Dementia):325-338.
7. Craig-Shapiro R, Fagan AM, Holtzman DM. Biomarkers of Alzheimer’s disease. Neurobiol Dis. 2009;35(2):128-140.
8. Jack CR Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9(1):119-128.
9. Foster NL, Heidebrink JL, Clark CM, et al. FDG-PET improves accuracy in distinguishing frontotemporal dementia and Alzheimer’s disease. Brain. 2007;130(pt 10):2616-2635.
10. National Coverage Determination (NCD) for FDG PET for Dementia and Neurodegenerative Diseases (220.6.13). Centers for Medicare and Medicaid Services. http://www. cms.gov/medicare-coverage-database/details/ncd-details. aspx?NCDId=288&ncdver=3&bc=BAABAAAAAAAA&. Accessed May 9, 2014.
11. Small GW, Bookheimer SY, Thompson PM, et al. Current and future uses of neuroimaging for cognitively impaired patients. Lancet Neurol. 2008;7(2):161-172. 12. Clark CM, Schneider JA, Bedell BJ, et al. Use of florbetapir- PET for imaging beta-amyloid pathology. JAMA. 2011;305(3): 275-283.
13. Johnson KA, Minoshima S, Bohnen NI, et al. Update on appropriate use criteria for amyloid PET imaging: dementia experts, mild cognitive impairment, and education. Amyloid Imaging Task Force of the Alzheimer’s Association and Society for Nuclear Medicine and Molecular Imaging. Alzheimers Dement. 2013;9(4):e106-e109.
14. AMYViD [package insert]. Indianapolis, IN: Eli Lilly & Co; 2012.
15. First guidelines published for brain amyloid imaging in Alzheimer’s. Alzheimer’s Association. http://www.alz.org/ news_and_events_60578.asp. Published January 28, 2013. Accessed May 9, 2014.
16. Zakaib GD. FDA approves Amyvid for clinical use. Alzforum. http://www.alzforum.org/news/research-news/ fda-approves-amyvid-clinical-use. Published April 9, 2012. Accessed May 16, 2014.
17. Skillbäck T, Zetterberg H, Blennow K, et al. Cerebrospinal fluid biomarkers for Alzheimer disease and subcortical axonal damage in 5,542 clinical samples. Alzheimers Res Ther. 2013;5(5):47.
18. Irizarry MC. Biomarkers of Alzheimer disease in plasma. NeuroRx. 2004;1(2):226-234.
19. Bateman RJ, Xiong C, Benzinger TL, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012;367(9):795-804.
20. Bertram L, McQueen MB, Mullin K, et al. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nature Genetics. 2007;39(1):17-23.
21. Strittmatter WJ, Roses AD. Apolipoprotein E and Alzheimer’s disease. Annu Rev Neurosci. 1996;19:53-77.
1. Jack CR Jr, Albert MS, Knopman DS, et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011; 7(3):257-262.
2. McKhann GM, Knopman DS. Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging- Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263-269.
3. Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280-292.
4. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging- Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011; 7(3):270-279.
5. Cummings JL. Biomarkers in Alzheimer’s disease– perspectives for the future. US Neurology. 2010;6(1):23-27.
6. Sperling R, Keith J. Biomarkers of Alzheimer disease: current and future applications to diagnostic criteria. Continuum (Minneap Minn). 2013;19(2 Dementia):325-338.
7. Craig-Shapiro R, Fagan AM, Holtzman DM. Biomarkers of Alzheimer’s disease. Neurobiol Dis. 2009;35(2):128-140.
8. Jack CR Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9(1):119-128.
9. Foster NL, Heidebrink JL, Clark CM, et al. FDG-PET improves accuracy in distinguishing frontotemporal dementia and Alzheimer’s disease. Brain. 2007;130(pt 10):2616-2635.
10. National Coverage Determination (NCD) for FDG PET for Dementia and Neurodegenerative Diseases (220.6.13). Centers for Medicare and Medicaid Services. http://www. cms.gov/medicare-coverage-database/details/ncd-details. aspx?NCDId=288&ncdver=3&bc=BAABAAAAAAAA&. Accessed May 9, 2014.
11. Small GW, Bookheimer SY, Thompson PM, et al. Current and future uses of neuroimaging for cognitively impaired patients. Lancet Neurol. 2008;7(2):161-172. 12. Clark CM, Schneider JA, Bedell BJ, et al. Use of florbetapir- PET for imaging beta-amyloid pathology. JAMA. 2011;305(3): 275-283.
13. Johnson KA, Minoshima S, Bohnen NI, et al. Update on appropriate use criteria for amyloid PET imaging: dementia experts, mild cognitive impairment, and education. Amyloid Imaging Task Force of the Alzheimer’s Association and Society for Nuclear Medicine and Molecular Imaging. Alzheimers Dement. 2013;9(4):e106-e109.
14. AMYViD [package insert]. Indianapolis, IN: Eli Lilly & Co; 2012.
15. First guidelines published for brain amyloid imaging in Alzheimer’s. Alzheimer’s Association. http://www.alz.org/ news_and_events_60578.asp. Published January 28, 2013. Accessed May 9, 2014.
16. Zakaib GD. FDA approves Amyvid for clinical use. Alzforum. http://www.alzforum.org/news/research-news/ fda-approves-amyvid-clinical-use. Published April 9, 2012. Accessed May 16, 2014.
17. Skillbäck T, Zetterberg H, Blennow K, et al. Cerebrospinal fluid biomarkers for Alzheimer disease and subcortical axonal damage in 5,542 clinical samples. Alzheimers Res Ther. 2013;5(5):47.
18. Irizarry MC. Biomarkers of Alzheimer disease in plasma. NeuroRx. 2004;1(2):226-234.
19. Bateman RJ, Xiong C, Benzinger TL, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012;367(9):795-804.
20. Bertram L, McQueen MB, Mullin K, et al. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nature Genetics. 2007;39(1):17-23.
21. Strittmatter WJ, Roses AD. Apolipoprotein E and Alzheimer’s disease. Annu Rev Neurosci. 1996;19:53-77.
Lithium for bipolar disorder: A re-emerging treatment for mood instability
Lithium is among the most effective therapies for bipolar disorder (BD), and enthusiasm for this simple molecule is waxing. The history of lithium is fascinating,1 and recent considerations include that this element, the third on the periodic table, has few, if any, industry champions. The recent renaissance is caused by a groundswell of appreciation for the clinical efficacy of lithium and an increasing number of providers who are willing to manage patients with lithium.
Target: Bipolar disorder
The target illness for lithium is BD, a spectrum of mood disorders with characteristic features of unstable mood and affect. Shifts in mood include recurrent episodes of mania, which are pathologically energized states with misguided volition and behavior with intoxicating euphoria (or irritability).2 Psychomotor activity is elevated and out of character; speech and body movements are revved up, with a diminished need for sleep. The social, personal, and vocational consequences often are disastrous.
The most common mood state of BD is depression. Depressive episodes consist of pathologically compromised energy and volition with a slowing of bodily functions, most prominently cognition and concentration; a pervasive depressed or sad mood is common but not always present. Presence of mixed states, when features of depression and mania are present simultaneously, is one of the many challenges of treating BD; an elevated volitional or energized state may occur with a depressed, dysphoric mood.
Evidence for lithium
Efficacy studies of lithium have focused on managing mood disorders, treating mania and depression, and prevention or maintenance care.3 Most were performed during the 1970s and 1980s,3 but recent studies have been comparing lithium with other mood stabilizers4-7 and searching for a genetic basis for lithium response.8-10 Other researchers have examined the use of lithium to prevent suicide.11 Some have suggested a neuroprotective effect of lithium, which may have profound implications for neuropsychiatry if valid.12-14 Results of additional studies, which are at different stages of completion, will clarify lithium use,15,16 and characterize the genetic makeup of individuals who respond to lithium.17 The primary evidence for lithium, however, is for maintenance treatment of BD and for preventing manic and depressive episodes.
Biochemistry and physiology of lithium. The biochemical and physiological effects of lithium are complex, wide-ranging, and likely to affect hundreds, if not thousands, of genes and gene products. The mechanisms of action remain a focus of academic pursuit (for a review of hypotheses related to these mechanisms see Goodwin and Jamison2 and Can et al18) Lithium is involved in cell signaling pathways that involve complex molecular mechanisms of inter- and intracellular communication19; some neural receptors are down-regulated20 and others show inhibition,21 which is thought to be a mechanism of lithium. The hypothesized neuroprotective effect of lithium22 may be mediated through an increased level of brain-derived neurotrophic factor in brain tissue.14 Recently, investigators using induced pluripotent stem cell derived neurons have shown that patterns of calcium-related cell signaling in bipolar neurons are affected specifically by lithium in the culture media.23 There likely are many mechanisms through which lithium’s effects are mediated, including a series of dynamic pathways that vary over time and in reaction to the internal and external environments of the cell and person.
The lithium renaissance
In the past decade, there has been an increase in interest and use of lithium because clinicians recognize its efficacy and advantages and can monitor serum levels and gauge the patient’s response and side effects24 against the lithium level. This is important because balancing effi cacy and side effects depends on the serum level. Efficacy often is not immediate, although side effects may emerge early. All systems of the body may show effects that could be related to lithium use. It is helpful to be aware of the side effects in chronological order, because some immediate effects may be associated with starting at higher dosages (Table 1). Common side effects in the short term include:
• GI distress, such as nausea, vomiting, diarrhea, and abdominal discomfort
• a fine neurologic tremor, which may be seen with accentuation upon deliberate movement
• prominent thirst with polyuria
• drowsiness and clouded thinking, which can be upsetting to the patient and family.
In the longer term, adverse effects on kidney and thyroid function are common. Management must include monitoring of the serum level.
Lithium is FDA-approved for acute and maintenance treatment of mania in BD. There are reports that discuss most variants of mood disorders, including BD I, BD II, unipolar depression, rapid cycling, and even alcohol abuse.25-29 Lithium could help manage mood dysregulation in the context of temperament and personality.30 There is evidence that lithium has an antidepressant effect31-33 and has shown efficacy as an adjunctive treatment for depression.31-33 There are data that suggest that lithium, with its neuroprotective mechanisms, may prevent progression of mild cognitive impairment.34
Is there an ideal lithium candidate?
Mood instability is the characteristic feature of a lithium responder. The instability may be over the course of the day, such as a dysregulated temperament that often is associated with DSM-IV personality categories, shorter-term fluctuations (within days with BD II), or in the context of episodic shifts of mood states over weeks and months, which are characteristic of BD I. The hallmark of mood instability is fluctuation from depression to elevated mood states and charged emotions with increased energy.
The patient considered ideal for lithium treatment has BD I with recurrent severe euphoric manic episodes, absence of significant comorbid disorders such as substance abuse, and a family history of lithium response. However, any patient with a clinically significant and unstable mood disorder, regardless of the DSM diagnosis, should be considered for lithium treatment.
When considering a lithium trial for a patient with significant mood instability, it is critical to establish the target symptoms and behavior that will help you gauge the efficacy of the intervention. Measurement-based care utilizes clinician and self-report instruments to provide data on the illness course and response to intervention. Commonly used clinician driven assessments include the Young Mania Rating Scale35 and the Quick Inventory of Depressive Symptoms,36 while the self-report assessments are the Patient Health Questionnaire37 and the Altman Self- Rating Mania Scale.38
During acute mania or depression, lithium often is used in combination with another medications such as an antipsychotic or antidepressant. Used in the outpatient and non-acute setting, lithium may be an “add-on” or monotherapy for preventing recurrence of episodes. Response in early acute manic symptoms are predictive of later response and remission.39
Dosing strategies
An initial problem with lithium is side effects that emerge when beginning treatment, which may discourage the patient and family from using this agent. Starting with 150 mg/d for the first 2 or 3 doses is unlikely to produce any adverse effects and can show the patient that there is a high likelihood that he will be able to tolerate the medication. Gradual titration over several days—or even weeks—to the target dosage and serum levels will enhance patient compliance. Rate of dosage increase is best guided by tolerance to the medication. The general consensus is that lithium is most effective at levels of 0.6 to 0.8 mEq/L,40 although a lower level (0.5 mEq/L) over a 2-year period also can be effective.41 Lithium may be used in to treat acute mania at higher serum levels (0.8 to 1.2 mEq/L), however, the acute phase often requires urgent management, usually with an antipsychotic.
Emerging consensus
Although there is a need to gather and analyze longer observational periods to clarify the clinical and biological characteristics of persons who respond to lithium, there are several points of consensus. Management will be guided by patient characteristics such as age, comorbidities, and other therapies. Most studies that address the effect of lithium level focus on high vs low serum levels. There are 3 categories of lithium serum levels, low (<0.6 mEq/L), mid-range (0.6 to 0.8 mEq/L), and high (>0.8 mEq/L), each has risk-benefit considerations.
The LiTMUS study42 compared low-level lithium augmentation with optimized personal treatment without lithium. Both groups had similar outcomes but the lithium-treated group had significantly lower use of atypical antipsychotics. This may be important when considering the long-term risk of the metabolic syndrome because the tolerability and side-effect profile of lithium at lower levels is more favorable than that of atypical antipsychotics. As lithium levels increase, there seems to be concomitant increase in efficacy and side effects. Many patients will benefit with low-level lithium use; yet clearly some individuals require higher dosages for effective maintenance therapy.
Dosing and monitoring. In patients age >50 or those with comorbid medical conditions, use a lower level of lithium (<0.6 mEq/L). Most individuals with BD likely will benefit from the mid-range level strategy (0.6 to 0.8 mEq/L); however, there will be those who require a higher level. When beginning lithium, start at a low dosage (150 mg/d) and increase as tolerated to the desired serum level. With acute mania, temporary use of an antipsychotic will be required.
There are no tests available to determine whether a patient will do well at any of these lithium serum levels. Breakthrough mania in an adherent patient with a serum lithium level of 0.7 mEq/L indicates the need to obtain a higher lithium level. A major deficit in lithium research is the lack of long-term data (>5 years) on outcomes, clinical and biological features with lithium levels because of a lack of pharmaceutical company support.3,17 Monitoring mood symptoms using detailed mood charts, whether clinician-administered or self-reported, is an effective way to monitor outcomes, provided the clinician uses the same scales or methods to record a patient’s moods. If a patient wants to discontinue lithium, taper the drug over an extended period (months) to minimize the likelihood of emerging manic or depressive episodes related to drug discontinuation.
Managing side effects
Consider lithium’s side effects in the context of their short-, intermediate-, and long-term presence (Table 2). Gradually increasing the lithium dosage often will prevent side effects that manifest in the short term. If side effects emerge at low dosages, proceed slowly with lithium and manage symptoms with other medications. When a patient shows a change in side effects, obtain lithium and electrolytes levels; a change in mental status with confusion will require an acute lithium level.
A diary of symptoms or clinically relevant matters such as fluid intake or frequency of GI- or neurological-related events will help the clinician monitor the frequency and severity of side effects. The patient and clinician should not be discouraged by emerging side effects in the short term, because they may dissipate or become minimally intrusive.
Several strategies can alleviate immediate GI effects, such as dosing with meals, enteric-coated formulations, multiple dose strategies, and short-term use of antidiarrheal medicine as needed. Side effects that disrupt a patient’s fluid and electrolyte balance (diabetes insipidus) to the point of clouding mental status will require discontinuing the medication until mental status improves, then reconsideration of the treatment regime, which will include managing diabetes insipidus with amiloride. Managing side effects may require consultation with specialty services. Likewise, some patients might experience neurologic side effects, such as profound tremor, that interferes with their ability to function. However, many side effects can be managed symptomatically with practical strategies (eg, a sugar-free lozenge for dry mouth or dysgeusia). Consider lower lithium dosages and serum levels because patients may experience benefits with lower therapeutic levels.
Long-term side effects include decreased renal function, hypothyroidism, persistent tremor, and dermatologic effects of acne and alopecia. Monitor renal and thyroid function annually in stable patients and more frequently when making changes in the treatment plan.
Before discontinuing lithium, consider discussing the medical issues with a specialist who has experience with complications of lithium.
Bottom Line
Lithium is an effective and under used medication for managing bipolar disorder. Initial prejudices and side effects often deter patients and prescribers from proceeding with a therapeutic trial of lithium. Although the mid-range lithium level of 0.6 to 0.8 mEq/L is desirable, many patients will experience significant benefits with lower levels. Initial strategies include the use of low-dose preparations that are unlikely to have uncomfortable side effects.
Related Resources
• Andreasen A, Ellingrod VL. Lithium-induced diabetes insipidus: prevention and management. Current Psychiatry. 2013;12(7):42-45.
• Cipriani A, Hawton K, Stockton S, et al. Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. BMJ. 2013;346:f3646. doi: 10.1136/bmj.f3646.
Drug Brand Names
Amiloride • Midamor Lithium • Eskalith, Lithobid
Disclosure
Dr. McInnis reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Shorter E. The history of lithium therapy. Bipolar Disord. 2009;11(11 suppl 2):4-9.
2. Goodwin FK, Jamison KR. Manic-depressive illness: bipolar disorders and recurrent depression. 2nd ed. New York, NY: Oxford University Press; 2007.
3. Burgess S, Geddes J, Hawton K, et al. Lithium for maintenance treatment of mood disorders. Cochrane Database Syst Rev. 2001:CD003013.
4. Bowden CL, Calabrese JR, McElroy SL, et al. A randomized, placebo-controlled 12-month trial of divalproex and lithium in treatment of outpatients with bipolar I disorder. Divalproex maintenance study group. Arch Gen Psychiatry. 2000;57(5):481-489.
5. Bowden CL, Calabrese JR, Sachs G, et al; Lamictal 606 Study Group. A placebo-controlled 18-month trial of lamotrigine and lithium maintenance treatment in recently manic or hypomanic patients with bipolar I disorder. Arch Gen Psychiatry. 2003;60(4):392-400.
6. Swann AC, Bowden CL, Calabrese JR, et al. Pattern of response to divalproex, lithium, or placebo in four naturalistic subtypes of mania. Neuropsychopharmacology. 2002;26(4):530-536.
7. Tohen M, Chengappa KN, Suppes T, et al. Efficacy of olanzapine in combination with valproate or lithium in the treatment of mania in patients partially nonresponsive to valproate or lithium monotherapy. Arch Gen Psychiatry. 2002;59(1):62-69.
8. Perlis RH, Smoller JW, Ferreira MA, et al. A genomewide association study of response to lithium for prevention of recurrence in bipolar disorder. Am J Psychiatry. 2009; 166(6):718-725.
9. Grof P, Duffy A, Cavazzoni P, et al. Is response to prophylactic lithium a familial trait? J Clin Psychiatry. 2002;63(10): 942-947.
10. Duffy A, Alda M, Kutcher S, et al. A prospective study of the offspring of bipolar parents responsive and nonresponsive to lithium treatment. J Clin Psychiatry. 2002;63(12): 1171-1178.
11. Goodwin FK, Fireman B, Simon GE, et al. Suicide risk in bipolar disorder during treatment with lithium and divalproex. JAMA. 2003;290(11):1467-1473.
12. Quiroz JA, Machado-Vieira R, Zarate CA Jr, et al. Novel insights into lithium’s mechanism of action: neurotrophic and neuroprotective effects. Neuropsychobiology. 2010; 62(1):50-60.
13. Forlenza OV, Diniz BS, Radanovic M, et al. Disease-modifying properties of long-term lithium treatment for amnestic mild cognitive impairment: randomised controlled trial. Br J Psychiatry. 2011;198(5):351-356.
14. de Sousa RT, van de Bilt MT, Diniz BS, et al. Lithium increases plasma brain-derived neurotrophic factor in acute bipolar mania: a preliminary 4-week study. Neurosci Lett. 2011;494(1):54-56.
15. Nierenberg AA, Sylvia LG, Leon AC, et al; LiTMUS Study Group. Lithium treatment–moderate dose use study (LiTMUS) for bipolar disorder: rationale and design. Clin Trials. 2009;6(6):637-648.
16. Sylvia LG, Reilly-Harrington NA, Leon AC, et al. Methods to limit attrition in longitudinal comparative effectiveness trials: lessons from the Lithium Treatment - Moderate dose Use Study (LiTMUS) for bipolar disorder. Clin Trials. 2012;9(1):94-101.
17. McCarthy MJ, Leckband SG, Kelsoe JR. Pharmacogenetics of lithium response in bipolar disorder. Pharmacogenomics. 2010;11(10):1439-1465.
18. Can A, Schulze TG, Gould TD. Molecular actions and clinical pharmacogenetics of lithium therapy [published online February 15, 2014]. Pharmacol Biochem Behav. doi: 10.1016/j.pbb.2014.02.004.
19. Berridge MJ. Unlocking the secrets of cell signaling. Annu Rev Physiol. 2005;67:1-21.
20. Devaki R, Shankar Rao S, Nadgir SM. The effect of lithium on the adrenoceptor-mediated second messenger system in the rat brain. J Psychiatry Neurosci. 2006;31(4):246-252.
21. Pan JQ, Lewis MC, Ketterman JK, et al. AKT kinase activity is required for lithium to modulate mood-related behaviors in mice. Neuropsychopharmacology. 2011;36(7):1397-1411.
22. Hu LW, Kawamoto EM, Brietzke E, et al. The role of Wnt signaling and its interaction with diverse mechanisms of cellular apoptosis in the pathophysiology of bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(1):11-17.
23. Chen HM, DeLong CJ, Bame M, et al. Transcripts involved in calcium signaling and telencephalic neuronal fate are altered in induced pluripotent stem cells from bipolar disorder patients [published online March 25, 2014]. Transl Psychiatry. doi:10.1038/tp.2014.12.
24. Jefferson JW. Lithium. In: Aronson JK, ed. Side effects of drugs annual, volume 26. Amsterdam, The Netherlands: Elsevier Science; 2003:19-29.
25. Baldessarini RJ, Tondo L, Floris G, et al. Effects of rapid cycling on response to lithium maintenance treatment in 360 bipolar I and II disorder patients. J Affect Disord. 2000;61(2):13-22.
26. Baldessarini RJ, Tondo L, Hennen J, et al. Latency and episodes before treatment: response to lithium maintenance in bipolar I and II disorders. Bipolar Disord. 1999;1(2): 91-97.
27. Fieve RR, Kumbaraci T, Dunner DL. Lithium prophylaxis of depression in bipolar I, bipolar II, and unipolar patients. Am J Psychiatry. 1976;133(8):925-929.
28. Peck CC, Pond SM, Becker CE, et al. An evaluation of the effects of lithium in the treatment of chronic alcoholism. II. Assessment of the two-period crossover design. Alcohol Clin Exp Res. 1981;5(2):252-255.
29. Peselow ED, Dunner DL, Fieve RR, et al. Lithium prophylaxis of depression in unipolar, bipolar II, and cyclothymic patients. Am J Psychiatry. 1982;139(6):747-752.
30. Bellino S, Paradiso E, Bogetto F. Efficacy and tolerability of pharmacotherapies for borderline personality disorder. CNS Drugs. 2008;22(8):671-692.
31. Alevizos B, Alevizos E, Leonardou A, et al. Low dosage lithium augmentation in venlafaxine resistant depression: an open-label study. Psychiatrike. 2012;23(2):143-148.
32. Goldberg JF, Sacks MH, Kocsis JH. Low-dose lithium augmentation of divalproex in geriatric mania. J Clin Psychiatry. 2000;61(4):304.
33. Saunders KE, Goodwin GM. New approaches in the treatment of bipolar depression. Curr Top Behav Neurosci. 2013;14:291-307.
34. Forlenza OV, Diniz BS, Radanovic M, et al. Disease-modifying properties of long-term lithium treatment for amnestic mild cognitive impairment: randomised controlled trial. Br J Psychiatry. 2011;198(5):351-356.
35. Young RC, Biggs JT, Ziegler VE, et al. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429-435.
36. Trivedi MH, Rush AJ, Ibrahim HM, et al. The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. Psychol Med. 2004;34(1):73-82.
37. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.
38. Altman EG, Hedeker D, Peterson JL, et al. The Altman Self- Rating Mania Scale. Biol Psychiatry. 1997;42(10):948-955.
39. Machado-Vieira R, Luckenbaugh DA, Soeiro-de-Souza MG, et al. Early improvement with lithium in classic mania and its association with later response. J Affect Disord. 2013;144(1-2):160-164.
40. Severus WE, Lipkovich IA, Licht RW, et al. In search of optimal lithium levels and olanzapine doses in the long-term treatment of bipolar I disorder. A post-hoc analysis of the maintenance study by Tohen et al. 2005. Eur Psychiatry. 2010;25(8):443-449.
41. Vestergaard P, Licht RW, Brodersen A, et al. Outcome of lithium prophylaxis: a prospective follow-up of affective disorder patients assigned to high and low serum lithium levels. Acta Psychiatr Scand. 1998;98(4):310-315.
42. Nierenberg AA, Friedman ES, Bowden CL, et al. Lithium treatment moderate-dose use study (LiTMUS) for bipolar disorder: a randomized comparative effectiveness trial of optimized personalized treatment with and without lithium. Am J Psychiatry. 2013;170(1):102-111.
Lithium is among the most effective therapies for bipolar disorder (BD), and enthusiasm for this simple molecule is waxing. The history of lithium is fascinating,1 and recent considerations include that this element, the third on the periodic table, has few, if any, industry champions. The recent renaissance is caused by a groundswell of appreciation for the clinical efficacy of lithium and an increasing number of providers who are willing to manage patients with lithium.
Target: Bipolar disorder
The target illness for lithium is BD, a spectrum of mood disorders with characteristic features of unstable mood and affect. Shifts in mood include recurrent episodes of mania, which are pathologically energized states with misguided volition and behavior with intoxicating euphoria (or irritability).2 Psychomotor activity is elevated and out of character; speech and body movements are revved up, with a diminished need for sleep. The social, personal, and vocational consequences often are disastrous.
The most common mood state of BD is depression. Depressive episodes consist of pathologically compromised energy and volition with a slowing of bodily functions, most prominently cognition and concentration; a pervasive depressed or sad mood is common but not always present. Presence of mixed states, when features of depression and mania are present simultaneously, is one of the many challenges of treating BD; an elevated volitional or energized state may occur with a depressed, dysphoric mood.
Evidence for lithium
Efficacy studies of lithium have focused on managing mood disorders, treating mania and depression, and prevention or maintenance care.3 Most were performed during the 1970s and 1980s,3 but recent studies have been comparing lithium with other mood stabilizers4-7 and searching for a genetic basis for lithium response.8-10 Other researchers have examined the use of lithium to prevent suicide.11 Some have suggested a neuroprotective effect of lithium, which may have profound implications for neuropsychiatry if valid.12-14 Results of additional studies, which are at different stages of completion, will clarify lithium use,15,16 and characterize the genetic makeup of individuals who respond to lithium.17 The primary evidence for lithium, however, is for maintenance treatment of BD and for preventing manic and depressive episodes.
Biochemistry and physiology of lithium. The biochemical and physiological effects of lithium are complex, wide-ranging, and likely to affect hundreds, if not thousands, of genes and gene products. The mechanisms of action remain a focus of academic pursuit (for a review of hypotheses related to these mechanisms see Goodwin and Jamison2 and Can et al18) Lithium is involved in cell signaling pathways that involve complex molecular mechanisms of inter- and intracellular communication19; some neural receptors are down-regulated20 and others show inhibition,21 which is thought to be a mechanism of lithium. The hypothesized neuroprotective effect of lithium22 may be mediated through an increased level of brain-derived neurotrophic factor in brain tissue.14 Recently, investigators using induced pluripotent stem cell derived neurons have shown that patterns of calcium-related cell signaling in bipolar neurons are affected specifically by lithium in the culture media.23 There likely are many mechanisms through which lithium’s effects are mediated, including a series of dynamic pathways that vary over time and in reaction to the internal and external environments of the cell and person.
The lithium renaissance
In the past decade, there has been an increase in interest and use of lithium because clinicians recognize its efficacy and advantages and can monitor serum levels and gauge the patient’s response and side effects24 against the lithium level. This is important because balancing effi cacy and side effects depends on the serum level. Efficacy often is not immediate, although side effects may emerge early. All systems of the body may show effects that could be related to lithium use. It is helpful to be aware of the side effects in chronological order, because some immediate effects may be associated with starting at higher dosages (Table 1). Common side effects in the short term include:
• GI distress, such as nausea, vomiting, diarrhea, and abdominal discomfort
• a fine neurologic tremor, which may be seen with accentuation upon deliberate movement
• prominent thirst with polyuria
• drowsiness and clouded thinking, which can be upsetting to the patient and family.
In the longer term, adverse effects on kidney and thyroid function are common. Management must include monitoring of the serum level.
Lithium is FDA-approved for acute and maintenance treatment of mania in BD. There are reports that discuss most variants of mood disorders, including BD I, BD II, unipolar depression, rapid cycling, and even alcohol abuse.25-29 Lithium could help manage mood dysregulation in the context of temperament and personality.30 There is evidence that lithium has an antidepressant effect31-33 and has shown efficacy as an adjunctive treatment for depression.31-33 There are data that suggest that lithium, with its neuroprotective mechanisms, may prevent progression of mild cognitive impairment.34
Is there an ideal lithium candidate?
Mood instability is the characteristic feature of a lithium responder. The instability may be over the course of the day, such as a dysregulated temperament that often is associated with DSM-IV personality categories, shorter-term fluctuations (within days with BD II), or in the context of episodic shifts of mood states over weeks and months, which are characteristic of BD I. The hallmark of mood instability is fluctuation from depression to elevated mood states and charged emotions with increased energy.
The patient considered ideal for lithium treatment has BD I with recurrent severe euphoric manic episodes, absence of significant comorbid disorders such as substance abuse, and a family history of lithium response. However, any patient with a clinically significant and unstable mood disorder, regardless of the DSM diagnosis, should be considered for lithium treatment.
When considering a lithium trial for a patient with significant mood instability, it is critical to establish the target symptoms and behavior that will help you gauge the efficacy of the intervention. Measurement-based care utilizes clinician and self-report instruments to provide data on the illness course and response to intervention. Commonly used clinician driven assessments include the Young Mania Rating Scale35 and the Quick Inventory of Depressive Symptoms,36 while the self-report assessments are the Patient Health Questionnaire37 and the Altman Self- Rating Mania Scale.38
During acute mania or depression, lithium often is used in combination with another medications such as an antipsychotic or antidepressant. Used in the outpatient and non-acute setting, lithium may be an “add-on” or monotherapy for preventing recurrence of episodes. Response in early acute manic symptoms are predictive of later response and remission.39
Dosing strategies
An initial problem with lithium is side effects that emerge when beginning treatment, which may discourage the patient and family from using this agent. Starting with 150 mg/d for the first 2 or 3 doses is unlikely to produce any adverse effects and can show the patient that there is a high likelihood that he will be able to tolerate the medication. Gradual titration over several days—or even weeks—to the target dosage and serum levels will enhance patient compliance. Rate of dosage increase is best guided by tolerance to the medication. The general consensus is that lithium is most effective at levels of 0.6 to 0.8 mEq/L,40 although a lower level (0.5 mEq/L) over a 2-year period also can be effective.41 Lithium may be used in to treat acute mania at higher serum levels (0.8 to 1.2 mEq/L), however, the acute phase often requires urgent management, usually with an antipsychotic.
Emerging consensus
Although there is a need to gather and analyze longer observational periods to clarify the clinical and biological characteristics of persons who respond to lithium, there are several points of consensus. Management will be guided by patient characteristics such as age, comorbidities, and other therapies. Most studies that address the effect of lithium level focus on high vs low serum levels. There are 3 categories of lithium serum levels, low (<0.6 mEq/L), mid-range (0.6 to 0.8 mEq/L), and high (>0.8 mEq/L), each has risk-benefit considerations.
The LiTMUS study42 compared low-level lithium augmentation with optimized personal treatment without lithium. Both groups had similar outcomes but the lithium-treated group had significantly lower use of atypical antipsychotics. This may be important when considering the long-term risk of the metabolic syndrome because the tolerability and side-effect profile of lithium at lower levels is more favorable than that of atypical antipsychotics. As lithium levels increase, there seems to be concomitant increase in efficacy and side effects. Many patients will benefit with low-level lithium use; yet clearly some individuals require higher dosages for effective maintenance therapy.
Dosing and monitoring. In patients age >50 or those with comorbid medical conditions, use a lower level of lithium (<0.6 mEq/L). Most individuals with BD likely will benefit from the mid-range level strategy (0.6 to 0.8 mEq/L); however, there will be those who require a higher level. When beginning lithium, start at a low dosage (150 mg/d) and increase as tolerated to the desired serum level. With acute mania, temporary use of an antipsychotic will be required.
There are no tests available to determine whether a patient will do well at any of these lithium serum levels. Breakthrough mania in an adherent patient with a serum lithium level of 0.7 mEq/L indicates the need to obtain a higher lithium level. A major deficit in lithium research is the lack of long-term data (>5 years) on outcomes, clinical and biological features with lithium levels because of a lack of pharmaceutical company support.3,17 Monitoring mood symptoms using detailed mood charts, whether clinician-administered or self-reported, is an effective way to monitor outcomes, provided the clinician uses the same scales or methods to record a patient’s moods. If a patient wants to discontinue lithium, taper the drug over an extended period (months) to minimize the likelihood of emerging manic or depressive episodes related to drug discontinuation.
Managing side effects
Consider lithium’s side effects in the context of their short-, intermediate-, and long-term presence (Table 2). Gradually increasing the lithium dosage often will prevent side effects that manifest in the short term. If side effects emerge at low dosages, proceed slowly with lithium and manage symptoms with other medications. When a patient shows a change in side effects, obtain lithium and electrolytes levels; a change in mental status with confusion will require an acute lithium level.
A diary of symptoms or clinically relevant matters such as fluid intake or frequency of GI- or neurological-related events will help the clinician monitor the frequency and severity of side effects. The patient and clinician should not be discouraged by emerging side effects in the short term, because they may dissipate or become minimally intrusive.
Several strategies can alleviate immediate GI effects, such as dosing with meals, enteric-coated formulations, multiple dose strategies, and short-term use of antidiarrheal medicine as needed. Side effects that disrupt a patient’s fluid and electrolyte balance (diabetes insipidus) to the point of clouding mental status will require discontinuing the medication until mental status improves, then reconsideration of the treatment regime, which will include managing diabetes insipidus with amiloride. Managing side effects may require consultation with specialty services. Likewise, some patients might experience neurologic side effects, such as profound tremor, that interferes with their ability to function. However, many side effects can be managed symptomatically with practical strategies (eg, a sugar-free lozenge for dry mouth or dysgeusia). Consider lower lithium dosages and serum levels because patients may experience benefits with lower therapeutic levels.
Long-term side effects include decreased renal function, hypothyroidism, persistent tremor, and dermatologic effects of acne and alopecia. Monitor renal and thyroid function annually in stable patients and more frequently when making changes in the treatment plan.
Before discontinuing lithium, consider discussing the medical issues with a specialist who has experience with complications of lithium.
Bottom Line
Lithium is an effective and under used medication for managing bipolar disorder. Initial prejudices and side effects often deter patients and prescribers from proceeding with a therapeutic trial of lithium. Although the mid-range lithium level of 0.6 to 0.8 mEq/L is desirable, many patients will experience significant benefits with lower levels. Initial strategies include the use of low-dose preparations that are unlikely to have uncomfortable side effects.
Related Resources
• Andreasen A, Ellingrod VL. Lithium-induced diabetes insipidus: prevention and management. Current Psychiatry. 2013;12(7):42-45.
• Cipriani A, Hawton K, Stockton S, et al. Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. BMJ. 2013;346:f3646. doi: 10.1136/bmj.f3646.
Drug Brand Names
Amiloride • Midamor Lithium • Eskalith, Lithobid
Disclosure
Dr. McInnis reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Lithium is among the most effective therapies for bipolar disorder (BD), and enthusiasm for this simple molecule is waxing. The history of lithium is fascinating,1 and recent considerations include that this element, the third on the periodic table, has few, if any, industry champions. The recent renaissance is caused by a groundswell of appreciation for the clinical efficacy of lithium and an increasing number of providers who are willing to manage patients with lithium.
Target: Bipolar disorder
The target illness for lithium is BD, a spectrum of mood disorders with characteristic features of unstable mood and affect. Shifts in mood include recurrent episodes of mania, which are pathologically energized states with misguided volition and behavior with intoxicating euphoria (or irritability).2 Psychomotor activity is elevated and out of character; speech and body movements are revved up, with a diminished need for sleep. The social, personal, and vocational consequences often are disastrous.
The most common mood state of BD is depression. Depressive episodes consist of pathologically compromised energy and volition with a slowing of bodily functions, most prominently cognition and concentration; a pervasive depressed or sad mood is common but not always present. Presence of mixed states, when features of depression and mania are present simultaneously, is one of the many challenges of treating BD; an elevated volitional or energized state may occur with a depressed, dysphoric mood.
Evidence for lithium
Efficacy studies of lithium have focused on managing mood disorders, treating mania and depression, and prevention or maintenance care.3 Most were performed during the 1970s and 1980s,3 but recent studies have been comparing lithium with other mood stabilizers4-7 and searching for a genetic basis for lithium response.8-10 Other researchers have examined the use of lithium to prevent suicide.11 Some have suggested a neuroprotective effect of lithium, which may have profound implications for neuropsychiatry if valid.12-14 Results of additional studies, which are at different stages of completion, will clarify lithium use,15,16 and characterize the genetic makeup of individuals who respond to lithium.17 The primary evidence for lithium, however, is for maintenance treatment of BD and for preventing manic and depressive episodes.
Biochemistry and physiology of lithium. The biochemical and physiological effects of lithium are complex, wide-ranging, and likely to affect hundreds, if not thousands, of genes and gene products. The mechanisms of action remain a focus of academic pursuit (for a review of hypotheses related to these mechanisms see Goodwin and Jamison2 and Can et al18) Lithium is involved in cell signaling pathways that involve complex molecular mechanisms of inter- and intracellular communication19; some neural receptors are down-regulated20 and others show inhibition,21 which is thought to be a mechanism of lithium. The hypothesized neuroprotective effect of lithium22 may be mediated through an increased level of brain-derived neurotrophic factor in brain tissue.14 Recently, investigators using induced pluripotent stem cell derived neurons have shown that patterns of calcium-related cell signaling in bipolar neurons are affected specifically by lithium in the culture media.23 There likely are many mechanisms through which lithium’s effects are mediated, including a series of dynamic pathways that vary over time and in reaction to the internal and external environments of the cell and person.
The lithium renaissance
In the past decade, there has been an increase in interest and use of lithium because clinicians recognize its efficacy and advantages and can monitor serum levels and gauge the patient’s response and side effects24 against the lithium level. This is important because balancing effi cacy and side effects depends on the serum level. Efficacy often is not immediate, although side effects may emerge early. All systems of the body may show effects that could be related to lithium use. It is helpful to be aware of the side effects in chronological order, because some immediate effects may be associated with starting at higher dosages (Table 1). Common side effects in the short term include:
• GI distress, such as nausea, vomiting, diarrhea, and abdominal discomfort
• a fine neurologic tremor, which may be seen with accentuation upon deliberate movement
• prominent thirst with polyuria
• drowsiness and clouded thinking, which can be upsetting to the patient and family.
In the longer term, adverse effects on kidney and thyroid function are common. Management must include monitoring of the serum level.
Lithium is FDA-approved for acute and maintenance treatment of mania in BD. There are reports that discuss most variants of mood disorders, including BD I, BD II, unipolar depression, rapid cycling, and even alcohol abuse.25-29 Lithium could help manage mood dysregulation in the context of temperament and personality.30 There is evidence that lithium has an antidepressant effect31-33 and has shown efficacy as an adjunctive treatment for depression.31-33 There are data that suggest that lithium, with its neuroprotective mechanisms, may prevent progression of mild cognitive impairment.34
Is there an ideal lithium candidate?
Mood instability is the characteristic feature of a lithium responder. The instability may be over the course of the day, such as a dysregulated temperament that often is associated with DSM-IV personality categories, shorter-term fluctuations (within days with BD II), or in the context of episodic shifts of mood states over weeks and months, which are characteristic of BD I. The hallmark of mood instability is fluctuation from depression to elevated mood states and charged emotions with increased energy.
The patient considered ideal for lithium treatment has BD I with recurrent severe euphoric manic episodes, absence of significant comorbid disorders such as substance abuse, and a family history of lithium response. However, any patient with a clinically significant and unstable mood disorder, regardless of the DSM diagnosis, should be considered for lithium treatment.
When considering a lithium trial for a patient with significant mood instability, it is critical to establish the target symptoms and behavior that will help you gauge the efficacy of the intervention. Measurement-based care utilizes clinician and self-report instruments to provide data on the illness course and response to intervention. Commonly used clinician driven assessments include the Young Mania Rating Scale35 and the Quick Inventory of Depressive Symptoms,36 while the self-report assessments are the Patient Health Questionnaire37 and the Altman Self- Rating Mania Scale.38
During acute mania or depression, lithium often is used in combination with another medications such as an antipsychotic or antidepressant. Used in the outpatient and non-acute setting, lithium may be an “add-on” or monotherapy for preventing recurrence of episodes. Response in early acute manic symptoms are predictive of later response and remission.39
Dosing strategies
An initial problem with lithium is side effects that emerge when beginning treatment, which may discourage the patient and family from using this agent. Starting with 150 mg/d for the first 2 or 3 doses is unlikely to produce any adverse effects and can show the patient that there is a high likelihood that he will be able to tolerate the medication. Gradual titration over several days—or even weeks—to the target dosage and serum levels will enhance patient compliance. Rate of dosage increase is best guided by tolerance to the medication. The general consensus is that lithium is most effective at levels of 0.6 to 0.8 mEq/L,40 although a lower level (0.5 mEq/L) over a 2-year period also can be effective.41 Lithium may be used in to treat acute mania at higher serum levels (0.8 to 1.2 mEq/L), however, the acute phase often requires urgent management, usually with an antipsychotic.
Emerging consensus
Although there is a need to gather and analyze longer observational periods to clarify the clinical and biological characteristics of persons who respond to lithium, there are several points of consensus. Management will be guided by patient characteristics such as age, comorbidities, and other therapies. Most studies that address the effect of lithium level focus on high vs low serum levels. There are 3 categories of lithium serum levels, low (<0.6 mEq/L), mid-range (0.6 to 0.8 mEq/L), and high (>0.8 mEq/L), each has risk-benefit considerations.
The LiTMUS study42 compared low-level lithium augmentation with optimized personal treatment without lithium. Both groups had similar outcomes but the lithium-treated group had significantly lower use of atypical antipsychotics. This may be important when considering the long-term risk of the metabolic syndrome because the tolerability and side-effect profile of lithium at lower levels is more favorable than that of atypical antipsychotics. As lithium levels increase, there seems to be concomitant increase in efficacy and side effects. Many patients will benefit with low-level lithium use; yet clearly some individuals require higher dosages for effective maintenance therapy.
Dosing and monitoring. In patients age >50 or those with comorbid medical conditions, use a lower level of lithium (<0.6 mEq/L). Most individuals with BD likely will benefit from the mid-range level strategy (0.6 to 0.8 mEq/L); however, there will be those who require a higher level. When beginning lithium, start at a low dosage (150 mg/d) and increase as tolerated to the desired serum level. With acute mania, temporary use of an antipsychotic will be required.
There are no tests available to determine whether a patient will do well at any of these lithium serum levels. Breakthrough mania in an adherent patient with a serum lithium level of 0.7 mEq/L indicates the need to obtain a higher lithium level. A major deficit in lithium research is the lack of long-term data (>5 years) on outcomes, clinical and biological features with lithium levels because of a lack of pharmaceutical company support.3,17 Monitoring mood symptoms using detailed mood charts, whether clinician-administered or self-reported, is an effective way to monitor outcomes, provided the clinician uses the same scales or methods to record a patient’s moods. If a patient wants to discontinue lithium, taper the drug over an extended period (months) to minimize the likelihood of emerging manic or depressive episodes related to drug discontinuation.
Managing side effects
Consider lithium’s side effects in the context of their short-, intermediate-, and long-term presence (Table 2). Gradually increasing the lithium dosage often will prevent side effects that manifest in the short term. If side effects emerge at low dosages, proceed slowly with lithium and manage symptoms with other medications. When a patient shows a change in side effects, obtain lithium and electrolytes levels; a change in mental status with confusion will require an acute lithium level.
A diary of symptoms or clinically relevant matters such as fluid intake or frequency of GI- or neurological-related events will help the clinician monitor the frequency and severity of side effects. The patient and clinician should not be discouraged by emerging side effects in the short term, because they may dissipate or become minimally intrusive.
Several strategies can alleviate immediate GI effects, such as dosing with meals, enteric-coated formulations, multiple dose strategies, and short-term use of antidiarrheal medicine as needed. Side effects that disrupt a patient’s fluid and electrolyte balance (diabetes insipidus) to the point of clouding mental status will require discontinuing the medication until mental status improves, then reconsideration of the treatment regime, which will include managing diabetes insipidus with amiloride. Managing side effects may require consultation with specialty services. Likewise, some patients might experience neurologic side effects, such as profound tremor, that interferes with their ability to function. However, many side effects can be managed symptomatically with practical strategies (eg, a sugar-free lozenge for dry mouth or dysgeusia). Consider lower lithium dosages and serum levels because patients may experience benefits with lower therapeutic levels.
Long-term side effects include decreased renal function, hypothyroidism, persistent tremor, and dermatologic effects of acne and alopecia. Monitor renal and thyroid function annually in stable patients and more frequently when making changes in the treatment plan.
Before discontinuing lithium, consider discussing the medical issues with a specialist who has experience with complications of lithium.
Bottom Line
Lithium is an effective and under used medication for managing bipolar disorder. Initial prejudices and side effects often deter patients and prescribers from proceeding with a therapeutic trial of lithium. Although the mid-range lithium level of 0.6 to 0.8 mEq/L is desirable, many patients will experience significant benefits with lower levels. Initial strategies include the use of low-dose preparations that are unlikely to have uncomfortable side effects.
Related Resources
• Andreasen A, Ellingrod VL. Lithium-induced diabetes insipidus: prevention and management. Current Psychiatry. 2013;12(7):42-45.
• Cipriani A, Hawton K, Stockton S, et al. Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. BMJ. 2013;346:f3646. doi: 10.1136/bmj.f3646.
Drug Brand Names
Amiloride • Midamor Lithium • Eskalith, Lithobid
Disclosure
Dr. McInnis reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Shorter E. The history of lithium therapy. Bipolar Disord. 2009;11(11 suppl 2):4-9.
2. Goodwin FK, Jamison KR. Manic-depressive illness: bipolar disorders and recurrent depression. 2nd ed. New York, NY: Oxford University Press; 2007.
3. Burgess S, Geddes J, Hawton K, et al. Lithium for maintenance treatment of mood disorders. Cochrane Database Syst Rev. 2001:CD003013.
4. Bowden CL, Calabrese JR, McElroy SL, et al. A randomized, placebo-controlled 12-month trial of divalproex and lithium in treatment of outpatients with bipolar I disorder. Divalproex maintenance study group. Arch Gen Psychiatry. 2000;57(5):481-489.
5. Bowden CL, Calabrese JR, Sachs G, et al; Lamictal 606 Study Group. A placebo-controlled 18-month trial of lamotrigine and lithium maintenance treatment in recently manic or hypomanic patients with bipolar I disorder. Arch Gen Psychiatry. 2003;60(4):392-400.
6. Swann AC, Bowden CL, Calabrese JR, et al. Pattern of response to divalproex, lithium, or placebo in four naturalistic subtypes of mania. Neuropsychopharmacology. 2002;26(4):530-536.
7. Tohen M, Chengappa KN, Suppes T, et al. Efficacy of olanzapine in combination with valproate or lithium in the treatment of mania in patients partially nonresponsive to valproate or lithium monotherapy. Arch Gen Psychiatry. 2002;59(1):62-69.
8. Perlis RH, Smoller JW, Ferreira MA, et al. A genomewide association study of response to lithium for prevention of recurrence in bipolar disorder. Am J Psychiatry. 2009; 166(6):718-725.
9. Grof P, Duffy A, Cavazzoni P, et al. Is response to prophylactic lithium a familial trait? J Clin Psychiatry. 2002;63(10): 942-947.
10. Duffy A, Alda M, Kutcher S, et al. A prospective study of the offspring of bipolar parents responsive and nonresponsive to lithium treatment. J Clin Psychiatry. 2002;63(12): 1171-1178.
11. Goodwin FK, Fireman B, Simon GE, et al. Suicide risk in bipolar disorder during treatment with lithium and divalproex. JAMA. 2003;290(11):1467-1473.
12. Quiroz JA, Machado-Vieira R, Zarate CA Jr, et al. Novel insights into lithium’s mechanism of action: neurotrophic and neuroprotective effects. Neuropsychobiology. 2010; 62(1):50-60.
13. Forlenza OV, Diniz BS, Radanovic M, et al. Disease-modifying properties of long-term lithium treatment for amnestic mild cognitive impairment: randomised controlled trial. Br J Psychiatry. 2011;198(5):351-356.
14. de Sousa RT, van de Bilt MT, Diniz BS, et al. Lithium increases plasma brain-derived neurotrophic factor in acute bipolar mania: a preliminary 4-week study. Neurosci Lett. 2011;494(1):54-56.
15. Nierenberg AA, Sylvia LG, Leon AC, et al; LiTMUS Study Group. Lithium treatment–moderate dose use study (LiTMUS) for bipolar disorder: rationale and design. Clin Trials. 2009;6(6):637-648.
16. Sylvia LG, Reilly-Harrington NA, Leon AC, et al. Methods to limit attrition in longitudinal comparative effectiveness trials: lessons from the Lithium Treatment - Moderate dose Use Study (LiTMUS) for bipolar disorder. Clin Trials. 2012;9(1):94-101.
17. McCarthy MJ, Leckband SG, Kelsoe JR. Pharmacogenetics of lithium response in bipolar disorder. Pharmacogenomics. 2010;11(10):1439-1465.
18. Can A, Schulze TG, Gould TD. Molecular actions and clinical pharmacogenetics of lithium therapy [published online February 15, 2014]. Pharmacol Biochem Behav. doi: 10.1016/j.pbb.2014.02.004.
19. Berridge MJ. Unlocking the secrets of cell signaling. Annu Rev Physiol. 2005;67:1-21.
20. Devaki R, Shankar Rao S, Nadgir SM. The effect of lithium on the adrenoceptor-mediated second messenger system in the rat brain. J Psychiatry Neurosci. 2006;31(4):246-252.
21. Pan JQ, Lewis MC, Ketterman JK, et al. AKT kinase activity is required for lithium to modulate mood-related behaviors in mice. Neuropsychopharmacology. 2011;36(7):1397-1411.
22. Hu LW, Kawamoto EM, Brietzke E, et al. The role of Wnt signaling and its interaction with diverse mechanisms of cellular apoptosis in the pathophysiology of bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(1):11-17.
23. Chen HM, DeLong CJ, Bame M, et al. Transcripts involved in calcium signaling and telencephalic neuronal fate are altered in induced pluripotent stem cells from bipolar disorder patients [published online March 25, 2014]. Transl Psychiatry. doi:10.1038/tp.2014.12.
24. Jefferson JW. Lithium. In: Aronson JK, ed. Side effects of drugs annual, volume 26. Amsterdam, The Netherlands: Elsevier Science; 2003:19-29.
25. Baldessarini RJ, Tondo L, Floris G, et al. Effects of rapid cycling on response to lithium maintenance treatment in 360 bipolar I and II disorder patients. J Affect Disord. 2000;61(2):13-22.
26. Baldessarini RJ, Tondo L, Hennen J, et al. Latency and episodes before treatment: response to lithium maintenance in bipolar I and II disorders. Bipolar Disord. 1999;1(2): 91-97.
27. Fieve RR, Kumbaraci T, Dunner DL. Lithium prophylaxis of depression in bipolar I, bipolar II, and unipolar patients. Am J Psychiatry. 1976;133(8):925-929.
28. Peck CC, Pond SM, Becker CE, et al. An evaluation of the effects of lithium in the treatment of chronic alcoholism. II. Assessment of the two-period crossover design. Alcohol Clin Exp Res. 1981;5(2):252-255.
29. Peselow ED, Dunner DL, Fieve RR, et al. Lithium prophylaxis of depression in unipolar, bipolar II, and cyclothymic patients. Am J Psychiatry. 1982;139(6):747-752.
30. Bellino S, Paradiso E, Bogetto F. Efficacy and tolerability of pharmacotherapies for borderline personality disorder. CNS Drugs. 2008;22(8):671-692.
31. Alevizos B, Alevizos E, Leonardou A, et al. Low dosage lithium augmentation in venlafaxine resistant depression: an open-label study. Psychiatrike. 2012;23(2):143-148.
32. Goldberg JF, Sacks MH, Kocsis JH. Low-dose lithium augmentation of divalproex in geriatric mania. J Clin Psychiatry. 2000;61(4):304.
33. Saunders KE, Goodwin GM. New approaches in the treatment of bipolar depression. Curr Top Behav Neurosci. 2013;14:291-307.
34. Forlenza OV, Diniz BS, Radanovic M, et al. Disease-modifying properties of long-term lithium treatment for amnestic mild cognitive impairment: randomised controlled trial. Br J Psychiatry. 2011;198(5):351-356.
35. Young RC, Biggs JT, Ziegler VE, et al. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429-435.
36. Trivedi MH, Rush AJ, Ibrahim HM, et al. The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. Psychol Med. 2004;34(1):73-82.
37. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.
38. Altman EG, Hedeker D, Peterson JL, et al. The Altman Self- Rating Mania Scale. Biol Psychiatry. 1997;42(10):948-955.
39. Machado-Vieira R, Luckenbaugh DA, Soeiro-de-Souza MG, et al. Early improvement with lithium in classic mania and its association with later response. J Affect Disord. 2013;144(1-2):160-164.
40. Severus WE, Lipkovich IA, Licht RW, et al. In search of optimal lithium levels and olanzapine doses in the long-term treatment of bipolar I disorder. A post-hoc analysis of the maintenance study by Tohen et al. 2005. Eur Psychiatry. 2010;25(8):443-449.
41. Vestergaard P, Licht RW, Brodersen A, et al. Outcome of lithium prophylaxis: a prospective follow-up of affective disorder patients assigned to high and low serum lithium levels. Acta Psychiatr Scand. 1998;98(4):310-315.
42. Nierenberg AA, Friedman ES, Bowden CL, et al. Lithium treatment moderate-dose use study (LiTMUS) for bipolar disorder: a randomized comparative effectiveness trial of optimized personalized treatment with and without lithium. Am J Psychiatry. 2013;170(1):102-111.
1. Shorter E. The history of lithium therapy. Bipolar Disord. 2009;11(11 suppl 2):4-9.
2. Goodwin FK, Jamison KR. Manic-depressive illness: bipolar disorders and recurrent depression. 2nd ed. New York, NY: Oxford University Press; 2007.
3. Burgess S, Geddes J, Hawton K, et al. Lithium for maintenance treatment of mood disorders. Cochrane Database Syst Rev. 2001:CD003013.
4. Bowden CL, Calabrese JR, McElroy SL, et al. A randomized, placebo-controlled 12-month trial of divalproex and lithium in treatment of outpatients with bipolar I disorder. Divalproex maintenance study group. Arch Gen Psychiatry. 2000;57(5):481-489.
5. Bowden CL, Calabrese JR, Sachs G, et al; Lamictal 606 Study Group. A placebo-controlled 18-month trial of lamotrigine and lithium maintenance treatment in recently manic or hypomanic patients with bipolar I disorder. Arch Gen Psychiatry. 2003;60(4):392-400.
6. Swann AC, Bowden CL, Calabrese JR, et al. Pattern of response to divalproex, lithium, or placebo in four naturalistic subtypes of mania. Neuropsychopharmacology. 2002;26(4):530-536.
7. Tohen M, Chengappa KN, Suppes T, et al. Efficacy of olanzapine in combination with valproate or lithium in the treatment of mania in patients partially nonresponsive to valproate or lithium monotherapy. Arch Gen Psychiatry. 2002;59(1):62-69.
8. Perlis RH, Smoller JW, Ferreira MA, et al. A genomewide association study of response to lithium for prevention of recurrence in bipolar disorder. Am J Psychiatry. 2009; 166(6):718-725.
9. Grof P, Duffy A, Cavazzoni P, et al. Is response to prophylactic lithium a familial trait? J Clin Psychiatry. 2002;63(10): 942-947.
10. Duffy A, Alda M, Kutcher S, et al. A prospective study of the offspring of bipolar parents responsive and nonresponsive to lithium treatment. J Clin Psychiatry. 2002;63(12): 1171-1178.
11. Goodwin FK, Fireman B, Simon GE, et al. Suicide risk in bipolar disorder during treatment with lithium and divalproex. JAMA. 2003;290(11):1467-1473.
12. Quiroz JA, Machado-Vieira R, Zarate CA Jr, et al. Novel insights into lithium’s mechanism of action: neurotrophic and neuroprotective effects. Neuropsychobiology. 2010; 62(1):50-60.
13. Forlenza OV, Diniz BS, Radanovic M, et al. Disease-modifying properties of long-term lithium treatment for amnestic mild cognitive impairment: randomised controlled trial. Br J Psychiatry. 2011;198(5):351-356.
14. de Sousa RT, van de Bilt MT, Diniz BS, et al. Lithium increases plasma brain-derived neurotrophic factor in acute bipolar mania: a preliminary 4-week study. Neurosci Lett. 2011;494(1):54-56.
15. Nierenberg AA, Sylvia LG, Leon AC, et al; LiTMUS Study Group. Lithium treatment–moderate dose use study (LiTMUS) for bipolar disorder: rationale and design. Clin Trials. 2009;6(6):637-648.
16. Sylvia LG, Reilly-Harrington NA, Leon AC, et al. Methods to limit attrition in longitudinal comparative effectiveness trials: lessons from the Lithium Treatment - Moderate dose Use Study (LiTMUS) for bipolar disorder. Clin Trials. 2012;9(1):94-101.
17. McCarthy MJ, Leckband SG, Kelsoe JR. Pharmacogenetics of lithium response in bipolar disorder. Pharmacogenomics. 2010;11(10):1439-1465.
18. Can A, Schulze TG, Gould TD. Molecular actions and clinical pharmacogenetics of lithium therapy [published online February 15, 2014]. Pharmacol Biochem Behav. doi: 10.1016/j.pbb.2014.02.004.
19. Berridge MJ. Unlocking the secrets of cell signaling. Annu Rev Physiol. 2005;67:1-21.
20. Devaki R, Shankar Rao S, Nadgir SM. The effect of lithium on the adrenoceptor-mediated second messenger system in the rat brain. J Psychiatry Neurosci. 2006;31(4):246-252.
21. Pan JQ, Lewis MC, Ketterman JK, et al. AKT kinase activity is required for lithium to modulate mood-related behaviors in mice. Neuropsychopharmacology. 2011;36(7):1397-1411.
22. Hu LW, Kawamoto EM, Brietzke E, et al. The role of Wnt signaling and its interaction with diverse mechanisms of cellular apoptosis in the pathophysiology of bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(1):11-17.
23. Chen HM, DeLong CJ, Bame M, et al. Transcripts involved in calcium signaling and telencephalic neuronal fate are altered in induced pluripotent stem cells from bipolar disorder patients [published online March 25, 2014]. Transl Psychiatry. doi:10.1038/tp.2014.12.
24. Jefferson JW. Lithium. In: Aronson JK, ed. Side effects of drugs annual, volume 26. Amsterdam, The Netherlands: Elsevier Science; 2003:19-29.
25. Baldessarini RJ, Tondo L, Floris G, et al. Effects of rapid cycling on response to lithium maintenance treatment in 360 bipolar I and II disorder patients. J Affect Disord. 2000;61(2):13-22.
26. Baldessarini RJ, Tondo L, Hennen J, et al. Latency and episodes before treatment: response to lithium maintenance in bipolar I and II disorders. Bipolar Disord. 1999;1(2): 91-97.
27. Fieve RR, Kumbaraci T, Dunner DL. Lithium prophylaxis of depression in bipolar I, bipolar II, and unipolar patients. Am J Psychiatry. 1976;133(8):925-929.
28. Peck CC, Pond SM, Becker CE, et al. An evaluation of the effects of lithium in the treatment of chronic alcoholism. II. Assessment of the two-period crossover design. Alcohol Clin Exp Res. 1981;5(2):252-255.
29. Peselow ED, Dunner DL, Fieve RR, et al. Lithium prophylaxis of depression in unipolar, bipolar II, and cyclothymic patients. Am J Psychiatry. 1982;139(6):747-752.
30. Bellino S, Paradiso E, Bogetto F. Efficacy and tolerability of pharmacotherapies for borderline personality disorder. CNS Drugs. 2008;22(8):671-692.
31. Alevizos B, Alevizos E, Leonardou A, et al. Low dosage lithium augmentation in venlafaxine resistant depression: an open-label study. Psychiatrike. 2012;23(2):143-148.
32. Goldberg JF, Sacks MH, Kocsis JH. Low-dose lithium augmentation of divalproex in geriatric mania. J Clin Psychiatry. 2000;61(4):304.
33. Saunders KE, Goodwin GM. New approaches in the treatment of bipolar depression. Curr Top Behav Neurosci. 2013;14:291-307.
34. Forlenza OV, Diniz BS, Radanovic M, et al. Disease-modifying properties of long-term lithium treatment for amnestic mild cognitive impairment: randomised controlled trial. Br J Psychiatry. 2011;198(5):351-356.
35. Young RC, Biggs JT, Ziegler VE, et al. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429-435.
36. Trivedi MH, Rush AJ, Ibrahim HM, et al. The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. Psychol Med. 2004;34(1):73-82.
37. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.
38. Altman EG, Hedeker D, Peterson JL, et al. The Altman Self- Rating Mania Scale. Biol Psychiatry. 1997;42(10):948-955.
39. Machado-Vieira R, Luckenbaugh DA, Soeiro-de-Souza MG, et al. Early improvement with lithium in classic mania and its association with later response. J Affect Disord. 2013;144(1-2):160-164.
40. Severus WE, Lipkovich IA, Licht RW, et al. In search of optimal lithium levels and olanzapine doses in the long-term treatment of bipolar I disorder. A post-hoc analysis of the maintenance study by Tohen et al. 2005. Eur Psychiatry. 2010;25(8):443-449.
41. Vestergaard P, Licht RW, Brodersen A, et al. Outcome of lithium prophylaxis: a prospective follow-up of affective disorder patients assigned to high and low serum lithium levels. Acta Psychiatr Scand. 1998;98(4):310-315.
42. Nierenberg AA, Friedman ES, Bowden CL, et al. Lithium treatment moderate-dose use study (LiTMUS) for bipolar disorder: a randomized comparative effectiveness trial of optimized personalized treatment with and without lithium. Am J Psychiatry. 2013;170(1):102-111.
Second of 2 parts: A practical approach to subtyping depression among your patients
Depression—sad, empty, or irritable mood accompanied by somatic or cognitive changes—is not a homogeneous condition. Recognizing subtypes of depressive illness can guide treatment and relieve your patient’s suffering. In this 2-part article [April and May 2014 issues], I summarize information about clinically distinct subtypes of depression, as they are recognized within diagnostic systems or as descriptors of treatment outcomes for particular subgroups of patients. My focus is on practical considerations for assessing and managing depression. Because many forms of the disorder respond inadequately to initial antidepressant treatment, optimal “next-step” pharmacotherapy, after nonresponse or partial response, often hinges on clinical subtyping.
The second part of this article examines “situational,” treatment-resistant, melancholic, agitated, anxious, and atypical depression; depression occurring with a substance use disorder; premenstrual dysphoric disorder; and seasonal affective disorder. Treatments for these subtypes for which there is evidence, or a clinical rationale, are given in the Table.
‘Situational depression’
In recent decades, the phenomenon of nonsyndromal depression after a life stress has undergone many name changes but little conceptional revision: “situational,” “reactive,” and “neurotic” labels for depression that were used before DSM-III became “adjustment disorders” in DSM-IV-TR and then “stress response syndromes” in DSM-5. These names all connote presentations of depressed mood after an environmental stressor, without either the full constellation of symptoms that define major depression or the chronicity of dysthymic disorder.
Paucity of guidance. There has been little research to identify vulnerability variables for adjustment disorders in the aftermath of particular stressors. Similarly, extensive data are lacking on 1) the likely progression of such disorders to a syndromal form of depression or 2) protective factors against developing clinically significant depression after a life stress. The extent to which adjustment disorders lie on a continuum with major mood disorders is not well-established, although subthreshold levels of depression can predispose to major depression or suicidal behaviors.1
Models of behavioral sensitization posit that stressful life events more often precede first or early episodes of depression than subsequent recurrences.2 At the same time, non-melancholic depressions that are preceded by “situational stresses” tend to recur in similar fashion.3
Medical therapy of value? Psychotherapy without medication—apart from occasional sedative−hypnotic drugs as needed for insomnia, anxiety, or distress—is considered the standard of care for treating an adjustment disorder. No drug has demonstrated superiority to placebo for alleviating symptoms of an adjustment disorder, but some clinicians nonetheless sometimes feel compelled to “up-code” the diagnosis of an adjustment disorder to the status of a major affective disorder, even when syndromal criteria for major depressive disorder (MDD) or dysthymia are absent.
Treatment-resistant depression
Disease staging models for depression and other psychiatric disordersa make note that, elsewhere in medicine, distinct clinical entities often are identified based on their responsivity to treatment (eg, classifying infections as antibiotic-sensitive or -resistant). Within the study and management of mood disorders, “treatment resistance” sometimes is a catch-all description of situations in which past treatment 1) yielded no improvement or partial improvement or 2) was marked by intolerance. Poor outcomes due to past medication intolerance or an aborted trial often are commingled with cases of true lack of improvement after an adequate treatment trial.
aSee “Staging psychiatric disorders: A clinico-biologic model,” Current Psychiatry, May 2013, at CurrentPsychiatry.com.
It is useful, therefore, to define terminology precisely when describing “treatment-resistant depression” and “treatment-refractory depression.” True past nonresponse to appropriate treatment often carries prognostic importance and bears on future treatment decisions.
Few interventions are FDA approved for treatment-resistant depression (Table).
Neuromodulation techniques are attracting interest in this area, although repetitive transcranial magnetic stimulation appears inferior to electroconvulsive therapy (ECT) for this indication.4
Melancholic depression
Melancholia involves the cardinal symptoms of anhedonia and lack of mood reactivity, alongside such features as distinct quality of mood, diurnal variation, excessive guilt, and severe weight loss. It most closely approximates pre-DSM-III “endogenous depression” and can involve 1) greater genetic loading5 and 2) structural and functional abnormalities in frontostriatal pathways.6,7
Melancholic features do not necessarily recur across successive episodes8 but carry an increased risk of psychosis9 and high-lethality suicidal behavior.10 Melancholia implies necessity for pharmacotherapy or ECT rather than psychosocial treatment alone; some researchers have suggested that tricyclic antidepressants (TCAs) might yield better results than selective serotonin reuptake inhibitors (SSRIs).11
Agitated depression
The Research Diagnostic Criteria (a forerunner in the 1970s to DSM-III) described agitated depression, but the disorder was not included in any DSM editions—although it is a “clinical modification” for MDD in the 10th revision of the International Statistical Classification of Diseases and Related Health Problems.
Agitated depression refers to a major depressive episode involving motor or psychic agitation, intense inner tension, and racing or “crowded” thoughts. Some experts believe that it represents a variant of psychotic depression or a bipolar mixed state, but the construct does not specify that criteria for a full manic or hypomanic episode exist.
Recovery from agitated depression tends to be slower than in non-agitated depression. Treatment usually entails an antidepressant plus an antipsychotic, although some believe that antidepressants can exacerbate, not alleviate, symptoms and, instead, favor antipsychotics, mood stabilizers, or ECT.12
Anxious depression
Anxiety symptoms or syndromes occur in at least one-half of outpatients who have major depression, and might account for a substantial percentage of nonresponse to first-line antidepressant therapies.13 The construct of a mixed anxiety−depressive disorder is, in fact, well-represented in the literature, particularly in primary care medicine, but its poor inter-rater reliability in DSM-5 field trials led to its exclusion there as a formal diagnosis.14
Serotonergic antidepressants remain the mainstay of treatment for depression with anxiety, although (contrary to popular perception) bupropion exerts an anxiolytic effect that is comparable to the effect of SSRIs.15 Notably, high somatic anxiety during depression might predict a poor outcome from ECT.16
Atypical depression
Often closely linked with early onset and chronicity, the construct of atypical depression has been defined in the literature by the symptom constellation of:
• mood reactiveness to environmental circumstances (unlike melancholia)
• heightened interpersonal sensitivity
• hypersomnia
• hyperphagia
• profound fatigue or a sense of physical heaviness.
Some authorities regard atypical features as being especially common in bipolar depression, or in depression among people who have borderline personality disorder.
Particular interest in this construct grew from studies that suggested that atypical depression is more responsive to a monoamine oxidase inhibitor (MAOI) than to a TCA, but also that SSRIs are not clearly superior to MAOIs.17 Response to ECT might also be better in atypical than in typical depression.18
Depression with a substance use disorder
Although not a distinct diagnostic entity, depression with a coexisting substance use disorder poses special challenges with regard to the source of symptom emergence (that is, when does depression lead to drug or alcohol use to “self medicate,” and when does drug use cause depression?) and treatment. Debate continues about whether 1) medicines that treat depression are effective and worthwhile in the setting of active substance use or 2) aggressive treatment of substance misuse is a prerequisite for subsequent pharmacotherapy for depression that is “uncontaminated” by the psychotoxic effects of concurrent substances of abuse.
Meta-analysis of controlled trials of antidepressants for patients who have MDD or a dysthymic disorder plus a comorbid alcohol use disorder found that antidepressants were, overall, superior to placebo unless a patient is actively drinking.19 Of the various classes of antidepressants, TCAs and nefazodone were found to be superior to placebo but, surprisingly, SSRIs were not. Another meta-analysis of adjunctive antidepressant outcomes for opiate-dependent, depressed patients who are receiving methadone maintenance therapy found no difference between antidepressants and placebo in their effect on depression symptom outcomes.20
Premenstrual dysphoric disorder
A new category in DSM-5, premenstrual dysphoric disorder (PMDD) represents a variant of premenstrual syndrome that arises during the luteal phase and ends with menstruation. Symptoms include several of those identified with MDD (without duration criteria), as well as mood swings, panic attacks, and physical complaints.
SSRIs—but not bupropion21 or TCAs22— and, sometimes, low-estrogen oral contraceptives are mainstays of treatment; so is cognitive-behavioral therapy, as well as lifestyle modifications (eg, exercise and changes to diet). Phototherapy has not shown robust efficacy for PMDD.23
Secondary depression
In DSM-5, depressive episodes that arise secondary to a general medical condition (eg, hypothyroidism and other endocrinopathies, cerebrovascular accidents, malignancies) or iatrogenically from medications (eg, corticosteroids, some anticonvulsants, interinterferon) are viewed as distinct from MDD in regard to risk of recurrence, genetic underpinnings, and possible neurodegenerative pathophysiology.b Unlike MDD, patient-specific risk factors are poorly defined for anticipating that a secondary depression is more or less likely to develop in the context of an exogenous substance or medical illness.
bFor further discussion, see “Is a medical illness causing your patient’s depression? Current Psychiatry, August 2009, at CurrentPsychiatry.com.
Treating secondary depression involves addressing the underlying condition and might include antidepressant medication.
Seasonal affective disorder
DSM-5 identifies “with seasonal pattern” as a specifier for recurrent major depression. Phototherapy remains a standard treatment, although a Cochrane Review identified comparable outcomes with fluoxetine, but inconclusive data for other, newer antidepressants.24 Small open trials have suggested that MAOIs and TCAs can be efficacious.
Note: Phototherapy lacks demonstrated efficacy in non-seasonal forms of depression.25
What does the future hold for classifying depressive disorders?
Recent initiatives have attempted to classify depression less by traditional clinical signs and more by focusing on possible underlying neurobiological substrates.c In the future, subtyping of mood disorders might focus on such constructs as:
• positive and negative valence systems and attentional domains
• treatment-responsivity relative to genotypic variants (for example, the serotonin transporter gene locus [SLC6A4] or prediction of L-methylfolate-responsive depression based on the genotype of the methylenetetrahydrofolate reductase [MTHFR] polymorphism)
• disrupted neural plasticity in brain circuits believed to regulate emotion.
cAn example is the Research Domain Criteria [RDoC],www. nimh.nih.gov/research-priorities/rdoc/index.shtml.
Until robust biomarkers for depression are identified and validated, however, such advances in nosology remain experimental and speculative.
BOTTOM LINE
Depressive disorders comprise a range of conditions that can be viewed along many dimensions, including “situational,” treatment-resistant, melancholic, agitated, anxious, and atypical depression; depression occurring with a substance use disorder; premenstrual dysphoric disorder; and seasonal affective disorder, among other classifications. Clinical characteristics vary across subtypes—as do corresponding preferred treatments, which should be tailored to the needs of your patients.
Editor’s note: The first part of Dr. Goldberg’s review of depression subtypes—focusing on major and minor depression, chronicity, polarity, severity, and psychosis—appeared in the April 2014 issue.
Related Resources
• Kosinski EC, Rothschild AJ. Monoamine oxidase inhibitors: Forgotten treatment for depression. Current Psychiatry. 2012;11(12):20-26.
• Rodgers S, Grosse Holtforth M, Müller M, et al. Symptom-based subtypes of depression and their psychosocial correlates: a person-centered approach focusing on the influence of sex. J Affect Disord. 2014;156:92-103.
Drug Brand Names
Aripiprazole • Abilify Mirtazapine • Remeron
Bupropion • Wellbutrin Nefazodone • Serzone
Fluoxetine • Prozac Olanzapine/fluoxetine • Symbyax
Ketamine • Ketalar Pramipexole • Mirapex
L-Methylfolate • Deplin Quetiapine • Seroquel
Lamotrigine • Lamictal Riluzole • Rilutek
Lithium • Eskalith, Lithobid Vortioxetine • Brintellix
Methadone • Dolophine
Disclosure
Dr. Goldberg has been a consultant to Avanir Pharmaceuticals and Merck; has served on the speakers’ bureau for AstraZeneca, Merck, Novartis, Sunovion Pharmaceuticals, Takeda-Lundbeck; and has received royalties from American Psychiatric Publishing and honoraria from Medscape and WebMD.
1. Fergusson DM, Horwood LJ, Ridder EM, et al. Subthreshold depression in adolescence and mental health outcomes in adulthood. Arch Gen Psychiatry. 2005;62(1):66-72.
2. Mitchell PB, Parker GB, Gladstone GL, et al. Severity of stressful life events in first and subsequent episodes of depression: the relevance of depressive subtypes. J Affect Disord. 2003;73(3):245-252.
3. Coryell W, Winokur G, Maser JD, et al. Recurrently situational (reactive) depression: a study of course, phenomenology and familial psychopathology. J Affect Disord. 1994;31(3):203-210.
4. Slotema CW, Blom JD, Hoek HW, et al. Should we expand the toolbox of psychiatric treatment methods to include Repetitive Transcranial Magnetic Stimulation (rTMS)? A meta-analysis of the efficacy of rTMS in psychiatric disorders. J Clin Psychiatry. 2010;71(7):873-884.
5. Kendler KS. The diagnostic validity of melancholic major depression in a population-based sample of female twins. Arch Gen Psychiatry. 1997;54(4):299-304.
6. Bracht T, Horn H, Strik W, et al. White matter microstructure alterations of the medial forebrain bundle in melancholic depression. J Affect Disord. 2014;155:186-193.
7. Pizzagalli DA, Oakes TR, Fox AS, et al. Functional but not structural subgenual prefrontal cortex abnormalities in melancholia. Mol Psychiatry. 2004;9(4):325, 393-405.
8. Melartin T, Leskelä U, Rytsälä H, et al. Co-morbidity and stability of melancholic features in DSM-IV major depressive disorder. Psychol Med. 2004;34(8):1443-1452.
9. Caldieraro MA, Baeza FL, Pinheiro DO, et al. Prevalence of psychotic symptoms in those with melancholic and nonmelancholic depression. J Nerv Ment Dis. 2013;201(10):855-859.
10. Grunebaum MF, Galfalvy HC, Oquendo MA, et al. Melancholia and the probability and lethality of suicide attempts. Br J Psychiatry. 2004;184:534-535.
11. Roose SP, Glassman AH, Attia E, et al. Comparative efficacy of selective serotonin reuptake inhibitors and tricyclics in the treatment of melancholia. Am J Psychiatry. 1994;151(12):1735-1739.
12. Koukopoulos A, Sani G, Koukopoulos AE, et al. Melancholia agitata and mixed depression. Acta Psychiatr Scand Suppl. 2007;(433):50-57.
13. Fava M, Rush AJ, Alpert JE, et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry. 2008; 165(3):342-351.
14. Regier DA, Narrow WE, Clarke DE, et al. DSM-5 field trials in the United States and Canada, Part II: test-retest reliability of selected categorical diagnoses. Am J Psychiatry. 2013;170(1):59-70.
15. Rush AJ, Trivedi MH, Carmody TJ, et al. Response in relation to baseline anxiety levels in major depressive disorder treated with bupropion sustained release or sertraline. Neuropsychopharmacology. 2001;25(1):131-138.
16. Rasmussen KG, Snyder KA, Knapp RG, et al. Relationship between somatization and remission with ECT. Psychiatry Res. 2004;129(3):293-295.
17. Henkel V, Mergl R, Allgaier AK, et al. Treatment of depression with atypical features: a meta-analytic approach. Psychiatry Res. 2006;141(1):89-101.
18. Husain MM, McClintock SM, Rush AJ, et al. The efficacy of acute electroconvulsive therapy in atypical depression. J Clin Psychiatry. 2008;69(3):406-411.
19. Iovieno N, Tedeschini E, Bentley KH, et al. Antidepressants for major depressive disorder and dysthymic disorder in patients with comorbid alcohol use disorders: a meta-analysis of placebo-controlled randomized trials. J Clin Psychiatry. 2011;72(8):1144-1151.
20. Pedrelli P, Iovieno N, Vitali M, et al. Treatment of major depressive disorder and dysthymic disorder with antidepressants in patients with comorbid opiate use disorders enrolled in methadone maintenance therapy: a meta-analysis. J Clin Psychopharmacol. 2011;31(5):582-586.
21. Pearlstein TB, Stone AB, Lund SA, et al. Comparison of fluoxetine, bupropion, and placebo in the treatment of premenstrual dysphoric disorder. J Clin Psychopharmacol. 1997;17(4):261-266.
22. Freeman EW, Rickels K, Sondheimer SJ, et al. Differential response to antidepressants in women with premenstrual syndrome/premenstrual dysphoric disorder: a randomized controlled trial. Arch Gen Psychiatry. 1999;56(10):932-939.
23. Krasnik C, Montori VM, Guyatt GH, et al. The effect of bright light therapy on depression associated with premenstrual dysphoric disorder. Am J Obstet Gynecol. 2005;193(3, pt 1):658-661.
24. Thaler K, Delivuk M, Chapman A, et al. Second-generation antidepressants for seasonal affective disorder. Cochrane Database Syst Rev. 2011;7(12):CD008591.
25. Thalén BE, Kjellman BF, Mørkid L, et al. Light treatment in seasonal and nonseasonal depression. Acta Psychiatr Scand. 1995;91(5):352-360.
Depression—sad, empty, or irritable mood accompanied by somatic or cognitive changes—is not a homogeneous condition. Recognizing subtypes of depressive illness can guide treatment and relieve your patient’s suffering. In this 2-part article [April and May 2014 issues], I summarize information about clinically distinct subtypes of depression, as they are recognized within diagnostic systems or as descriptors of treatment outcomes for particular subgroups of patients. My focus is on practical considerations for assessing and managing depression. Because many forms of the disorder respond inadequately to initial antidepressant treatment, optimal “next-step” pharmacotherapy, after nonresponse or partial response, often hinges on clinical subtyping.
The second part of this article examines “situational,” treatment-resistant, melancholic, agitated, anxious, and atypical depression; depression occurring with a substance use disorder; premenstrual dysphoric disorder; and seasonal affective disorder. Treatments for these subtypes for which there is evidence, or a clinical rationale, are given in the Table.
‘Situational depression’
In recent decades, the phenomenon of nonsyndromal depression after a life stress has undergone many name changes but little conceptional revision: “situational,” “reactive,” and “neurotic” labels for depression that were used before DSM-III became “adjustment disorders” in DSM-IV-TR and then “stress response syndromes” in DSM-5. These names all connote presentations of depressed mood after an environmental stressor, without either the full constellation of symptoms that define major depression or the chronicity of dysthymic disorder.
Paucity of guidance. There has been little research to identify vulnerability variables for adjustment disorders in the aftermath of particular stressors. Similarly, extensive data are lacking on 1) the likely progression of such disorders to a syndromal form of depression or 2) protective factors against developing clinically significant depression after a life stress. The extent to which adjustment disorders lie on a continuum with major mood disorders is not well-established, although subthreshold levels of depression can predispose to major depression or suicidal behaviors.1
Models of behavioral sensitization posit that stressful life events more often precede first or early episodes of depression than subsequent recurrences.2 At the same time, non-melancholic depressions that are preceded by “situational stresses” tend to recur in similar fashion.3
Medical therapy of value? Psychotherapy without medication—apart from occasional sedative−hypnotic drugs as needed for insomnia, anxiety, or distress—is considered the standard of care for treating an adjustment disorder. No drug has demonstrated superiority to placebo for alleviating symptoms of an adjustment disorder, but some clinicians nonetheless sometimes feel compelled to “up-code” the diagnosis of an adjustment disorder to the status of a major affective disorder, even when syndromal criteria for major depressive disorder (MDD) or dysthymia are absent.
Treatment-resistant depression
Disease staging models for depression and other psychiatric disordersa make note that, elsewhere in medicine, distinct clinical entities often are identified based on their responsivity to treatment (eg, classifying infections as antibiotic-sensitive or -resistant). Within the study and management of mood disorders, “treatment resistance” sometimes is a catch-all description of situations in which past treatment 1) yielded no improvement or partial improvement or 2) was marked by intolerance. Poor outcomes due to past medication intolerance or an aborted trial often are commingled with cases of true lack of improvement after an adequate treatment trial.
aSee “Staging psychiatric disorders: A clinico-biologic model,” Current Psychiatry, May 2013, at CurrentPsychiatry.com.
It is useful, therefore, to define terminology precisely when describing “treatment-resistant depression” and “treatment-refractory depression.” True past nonresponse to appropriate treatment often carries prognostic importance and bears on future treatment decisions.
Few interventions are FDA approved for treatment-resistant depression (Table).
Neuromodulation techniques are attracting interest in this area, although repetitive transcranial magnetic stimulation appears inferior to electroconvulsive therapy (ECT) for this indication.4
Melancholic depression
Melancholia involves the cardinal symptoms of anhedonia and lack of mood reactivity, alongside such features as distinct quality of mood, diurnal variation, excessive guilt, and severe weight loss. It most closely approximates pre-DSM-III “endogenous depression” and can involve 1) greater genetic loading5 and 2) structural and functional abnormalities in frontostriatal pathways.6,7
Melancholic features do not necessarily recur across successive episodes8 but carry an increased risk of psychosis9 and high-lethality suicidal behavior.10 Melancholia implies necessity for pharmacotherapy or ECT rather than psychosocial treatment alone; some researchers have suggested that tricyclic antidepressants (TCAs) might yield better results than selective serotonin reuptake inhibitors (SSRIs).11
Agitated depression
The Research Diagnostic Criteria (a forerunner in the 1970s to DSM-III) described agitated depression, but the disorder was not included in any DSM editions—although it is a “clinical modification” for MDD in the 10th revision of the International Statistical Classification of Diseases and Related Health Problems.
Agitated depression refers to a major depressive episode involving motor or psychic agitation, intense inner tension, and racing or “crowded” thoughts. Some experts believe that it represents a variant of psychotic depression or a bipolar mixed state, but the construct does not specify that criteria for a full manic or hypomanic episode exist.
Recovery from agitated depression tends to be slower than in non-agitated depression. Treatment usually entails an antidepressant plus an antipsychotic, although some believe that antidepressants can exacerbate, not alleviate, symptoms and, instead, favor antipsychotics, mood stabilizers, or ECT.12
Anxious depression
Anxiety symptoms or syndromes occur in at least one-half of outpatients who have major depression, and might account for a substantial percentage of nonresponse to first-line antidepressant therapies.13 The construct of a mixed anxiety−depressive disorder is, in fact, well-represented in the literature, particularly in primary care medicine, but its poor inter-rater reliability in DSM-5 field trials led to its exclusion there as a formal diagnosis.14
Serotonergic antidepressants remain the mainstay of treatment for depression with anxiety, although (contrary to popular perception) bupropion exerts an anxiolytic effect that is comparable to the effect of SSRIs.15 Notably, high somatic anxiety during depression might predict a poor outcome from ECT.16
Atypical depression
Often closely linked with early onset and chronicity, the construct of atypical depression has been defined in the literature by the symptom constellation of:
• mood reactiveness to environmental circumstances (unlike melancholia)
• heightened interpersonal sensitivity
• hypersomnia
• hyperphagia
• profound fatigue or a sense of physical heaviness.
Some authorities regard atypical features as being especially common in bipolar depression, or in depression among people who have borderline personality disorder.
Particular interest in this construct grew from studies that suggested that atypical depression is more responsive to a monoamine oxidase inhibitor (MAOI) than to a TCA, but also that SSRIs are not clearly superior to MAOIs.17 Response to ECT might also be better in atypical than in typical depression.18
Depression with a substance use disorder
Although not a distinct diagnostic entity, depression with a coexisting substance use disorder poses special challenges with regard to the source of symptom emergence (that is, when does depression lead to drug or alcohol use to “self medicate,” and when does drug use cause depression?) and treatment. Debate continues about whether 1) medicines that treat depression are effective and worthwhile in the setting of active substance use or 2) aggressive treatment of substance misuse is a prerequisite for subsequent pharmacotherapy for depression that is “uncontaminated” by the psychotoxic effects of concurrent substances of abuse.
Meta-analysis of controlled trials of antidepressants for patients who have MDD or a dysthymic disorder plus a comorbid alcohol use disorder found that antidepressants were, overall, superior to placebo unless a patient is actively drinking.19 Of the various classes of antidepressants, TCAs and nefazodone were found to be superior to placebo but, surprisingly, SSRIs were not. Another meta-analysis of adjunctive antidepressant outcomes for opiate-dependent, depressed patients who are receiving methadone maintenance therapy found no difference between antidepressants and placebo in their effect on depression symptom outcomes.20
Premenstrual dysphoric disorder
A new category in DSM-5, premenstrual dysphoric disorder (PMDD) represents a variant of premenstrual syndrome that arises during the luteal phase and ends with menstruation. Symptoms include several of those identified with MDD (without duration criteria), as well as mood swings, panic attacks, and physical complaints.
SSRIs—but not bupropion21 or TCAs22— and, sometimes, low-estrogen oral contraceptives are mainstays of treatment; so is cognitive-behavioral therapy, as well as lifestyle modifications (eg, exercise and changes to diet). Phototherapy has not shown robust efficacy for PMDD.23
Secondary depression
In DSM-5, depressive episodes that arise secondary to a general medical condition (eg, hypothyroidism and other endocrinopathies, cerebrovascular accidents, malignancies) or iatrogenically from medications (eg, corticosteroids, some anticonvulsants, interinterferon) are viewed as distinct from MDD in regard to risk of recurrence, genetic underpinnings, and possible neurodegenerative pathophysiology.b Unlike MDD, patient-specific risk factors are poorly defined for anticipating that a secondary depression is more or less likely to develop in the context of an exogenous substance or medical illness.
bFor further discussion, see “Is a medical illness causing your patient’s depression? Current Psychiatry, August 2009, at CurrentPsychiatry.com.
Treating secondary depression involves addressing the underlying condition and might include antidepressant medication.
Seasonal affective disorder
DSM-5 identifies “with seasonal pattern” as a specifier for recurrent major depression. Phototherapy remains a standard treatment, although a Cochrane Review identified comparable outcomes with fluoxetine, but inconclusive data for other, newer antidepressants.24 Small open trials have suggested that MAOIs and TCAs can be efficacious.
Note: Phototherapy lacks demonstrated efficacy in non-seasonal forms of depression.25
What does the future hold for classifying depressive disorders?
Recent initiatives have attempted to classify depression less by traditional clinical signs and more by focusing on possible underlying neurobiological substrates.c In the future, subtyping of mood disorders might focus on such constructs as:
• positive and negative valence systems and attentional domains
• treatment-responsivity relative to genotypic variants (for example, the serotonin transporter gene locus [SLC6A4] or prediction of L-methylfolate-responsive depression based on the genotype of the methylenetetrahydrofolate reductase [MTHFR] polymorphism)
• disrupted neural plasticity in brain circuits believed to regulate emotion.
cAn example is the Research Domain Criteria [RDoC],www. nimh.nih.gov/research-priorities/rdoc/index.shtml.
Until robust biomarkers for depression are identified and validated, however, such advances in nosology remain experimental and speculative.
BOTTOM LINE
Depressive disorders comprise a range of conditions that can be viewed along many dimensions, including “situational,” treatment-resistant, melancholic, agitated, anxious, and atypical depression; depression occurring with a substance use disorder; premenstrual dysphoric disorder; and seasonal affective disorder, among other classifications. Clinical characteristics vary across subtypes—as do corresponding preferred treatments, which should be tailored to the needs of your patients.
Editor’s note: The first part of Dr. Goldberg’s review of depression subtypes—focusing on major and minor depression, chronicity, polarity, severity, and psychosis—appeared in the April 2014 issue.
Related Resources
• Kosinski EC, Rothschild AJ. Monoamine oxidase inhibitors: Forgotten treatment for depression. Current Psychiatry. 2012;11(12):20-26.
• Rodgers S, Grosse Holtforth M, Müller M, et al. Symptom-based subtypes of depression and their psychosocial correlates: a person-centered approach focusing on the influence of sex. J Affect Disord. 2014;156:92-103.
Drug Brand Names
Aripiprazole • Abilify Mirtazapine • Remeron
Bupropion • Wellbutrin Nefazodone • Serzone
Fluoxetine • Prozac Olanzapine/fluoxetine • Symbyax
Ketamine • Ketalar Pramipexole • Mirapex
L-Methylfolate • Deplin Quetiapine • Seroquel
Lamotrigine • Lamictal Riluzole • Rilutek
Lithium • Eskalith, Lithobid Vortioxetine • Brintellix
Methadone • Dolophine
Disclosure
Dr. Goldberg has been a consultant to Avanir Pharmaceuticals and Merck; has served on the speakers’ bureau for AstraZeneca, Merck, Novartis, Sunovion Pharmaceuticals, Takeda-Lundbeck; and has received royalties from American Psychiatric Publishing and honoraria from Medscape and WebMD.
Depression—sad, empty, or irritable mood accompanied by somatic or cognitive changes—is not a homogeneous condition. Recognizing subtypes of depressive illness can guide treatment and relieve your patient’s suffering. In this 2-part article [April and May 2014 issues], I summarize information about clinically distinct subtypes of depression, as they are recognized within diagnostic systems or as descriptors of treatment outcomes for particular subgroups of patients. My focus is on practical considerations for assessing and managing depression. Because many forms of the disorder respond inadequately to initial antidepressant treatment, optimal “next-step” pharmacotherapy, after nonresponse or partial response, often hinges on clinical subtyping.
The second part of this article examines “situational,” treatment-resistant, melancholic, agitated, anxious, and atypical depression; depression occurring with a substance use disorder; premenstrual dysphoric disorder; and seasonal affective disorder. Treatments for these subtypes for which there is evidence, or a clinical rationale, are given in the Table.
‘Situational depression’
In recent decades, the phenomenon of nonsyndromal depression after a life stress has undergone many name changes but little conceptional revision: “situational,” “reactive,” and “neurotic” labels for depression that were used before DSM-III became “adjustment disorders” in DSM-IV-TR and then “stress response syndromes” in DSM-5. These names all connote presentations of depressed mood after an environmental stressor, without either the full constellation of symptoms that define major depression or the chronicity of dysthymic disorder.
Paucity of guidance. There has been little research to identify vulnerability variables for adjustment disorders in the aftermath of particular stressors. Similarly, extensive data are lacking on 1) the likely progression of such disorders to a syndromal form of depression or 2) protective factors against developing clinically significant depression after a life stress. The extent to which adjustment disorders lie on a continuum with major mood disorders is not well-established, although subthreshold levels of depression can predispose to major depression or suicidal behaviors.1
Models of behavioral sensitization posit that stressful life events more often precede first or early episodes of depression than subsequent recurrences.2 At the same time, non-melancholic depressions that are preceded by “situational stresses” tend to recur in similar fashion.3
Medical therapy of value? Psychotherapy without medication—apart from occasional sedative−hypnotic drugs as needed for insomnia, anxiety, or distress—is considered the standard of care for treating an adjustment disorder. No drug has demonstrated superiority to placebo for alleviating symptoms of an adjustment disorder, but some clinicians nonetheless sometimes feel compelled to “up-code” the diagnosis of an adjustment disorder to the status of a major affective disorder, even when syndromal criteria for major depressive disorder (MDD) or dysthymia are absent.
Treatment-resistant depression
Disease staging models for depression and other psychiatric disordersa make note that, elsewhere in medicine, distinct clinical entities often are identified based on their responsivity to treatment (eg, classifying infections as antibiotic-sensitive or -resistant). Within the study and management of mood disorders, “treatment resistance” sometimes is a catch-all description of situations in which past treatment 1) yielded no improvement or partial improvement or 2) was marked by intolerance. Poor outcomes due to past medication intolerance or an aborted trial often are commingled with cases of true lack of improvement after an adequate treatment trial.
aSee “Staging psychiatric disorders: A clinico-biologic model,” Current Psychiatry, May 2013, at CurrentPsychiatry.com.
It is useful, therefore, to define terminology precisely when describing “treatment-resistant depression” and “treatment-refractory depression.” True past nonresponse to appropriate treatment often carries prognostic importance and bears on future treatment decisions.
Few interventions are FDA approved for treatment-resistant depression (Table).
Neuromodulation techniques are attracting interest in this area, although repetitive transcranial magnetic stimulation appears inferior to electroconvulsive therapy (ECT) for this indication.4
Melancholic depression
Melancholia involves the cardinal symptoms of anhedonia and lack of mood reactivity, alongside such features as distinct quality of mood, diurnal variation, excessive guilt, and severe weight loss. It most closely approximates pre-DSM-III “endogenous depression” and can involve 1) greater genetic loading5 and 2) structural and functional abnormalities in frontostriatal pathways.6,7
Melancholic features do not necessarily recur across successive episodes8 but carry an increased risk of psychosis9 and high-lethality suicidal behavior.10 Melancholia implies necessity for pharmacotherapy or ECT rather than psychosocial treatment alone; some researchers have suggested that tricyclic antidepressants (TCAs) might yield better results than selective serotonin reuptake inhibitors (SSRIs).11
Agitated depression
The Research Diagnostic Criteria (a forerunner in the 1970s to DSM-III) described agitated depression, but the disorder was not included in any DSM editions—although it is a “clinical modification” for MDD in the 10th revision of the International Statistical Classification of Diseases and Related Health Problems.
Agitated depression refers to a major depressive episode involving motor or psychic agitation, intense inner tension, and racing or “crowded” thoughts. Some experts believe that it represents a variant of psychotic depression or a bipolar mixed state, but the construct does not specify that criteria for a full manic or hypomanic episode exist.
Recovery from agitated depression tends to be slower than in non-agitated depression. Treatment usually entails an antidepressant plus an antipsychotic, although some believe that antidepressants can exacerbate, not alleviate, symptoms and, instead, favor antipsychotics, mood stabilizers, or ECT.12
Anxious depression
Anxiety symptoms or syndromes occur in at least one-half of outpatients who have major depression, and might account for a substantial percentage of nonresponse to first-line antidepressant therapies.13 The construct of a mixed anxiety−depressive disorder is, in fact, well-represented in the literature, particularly in primary care medicine, but its poor inter-rater reliability in DSM-5 field trials led to its exclusion there as a formal diagnosis.14
Serotonergic antidepressants remain the mainstay of treatment for depression with anxiety, although (contrary to popular perception) bupropion exerts an anxiolytic effect that is comparable to the effect of SSRIs.15 Notably, high somatic anxiety during depression might predict a poor outcome from ECT.16
Atypical depression
Often closely linked with early onset and chronicity, the construct of atypical depression has been defined in the literature by the symptom constellation of:
• mood reactiveness to environmental circumstances (unlike melancholia)
• heightened interpersonal sensitivity
• hypersomnia
• hyperphagia
• profound fatigue or a sense of physical heaviness.
Some authorities regard atypical features as being especially common in bipolar depression, or in depression among people who have borderline personality disorder.
Particular interest in this construct grew from studies that suggested that atypical depression is more responsive to a monoamine oxidase inhibitor (MAOI) than to a TCA, but also that SSRIs are not clearly superior to MAOIs.17 Response to ECT might also be better in atypical than in typical depression.18
Depression with a substance use disorder
Although not a distinct diagnostic entity, depression with a coexisting substance use disorder poses special challenges with regard to the source of symptom emergence (that is, when does depression lead to drug or alcohol use to “self medicate,” and when does drug use cause depression?) and treatment. Debate continues about whether 1) medicines that treat depression are effective and worthwhile in the setting of active substance use or 2) aggressive treatment of substance misuse is a prerequisite for subsequent pharmacotherapy for depression that is “uncontaminated” by the psychotoxic effects of concurrent substances of abuse.
Meta-analysis of controlled trials of antidepressants for patients who have MDD or a dysthymic disorder plus a comorbid alcohol use disorder found that antidepressants were, overall, superior to placebo unless a patient is actively drinking.19 Of the various classes of antidepressants, TCAs and nefazodone were found to be superior to placebo but, surprisingly, SSRIs were not. Another meta-analysis of adjunctive antidepressant outcomes for opiate-dependent, depressed patients who are receiving methadone maintenance therapy found no difference between antidepressants and placebo in their effect on depression symptom outcomes.20
Premenstrual dysphoric disorder
A new category in DSM-5, premenstrual dysphoric disorder (PMDD) represents a variant of premenstrual syndrome that arises during the luteal phase and ends with menstruation. Symptoms include several of those identified with MDD (without duration criteria), as well as mood swings, panic attacks, and physical complaints.
SSRIs—but not bupropion21 or TCAs22— and, sometimes, low-estrogen oral contraceptives are mainstays of treatment; so is cognitive-behavioral therapy, as well as lifestyle modifications (eg, exercise and changes to diet). Phototherapy has not shown robust efficacy for PMDD.23
Secondary depression
In DSM-5, depressive episodes that arise secondary to a general medical condition (eg, hypothyroidism and other endocrinopathies, cerebrovascular accidents, malignancies) or iatrogenically from medications (eg, corticosteroids, some anticonvulsants, interinterferon) are viewed as distinct from MDD in regard to risk of recurrence, genetic underpinnings, and possible neurodegenerative pathophysiology.b Unlike MDD, patient-specific risk factors are poorly defined for anticipating that a secondary depression is more or less likely to develop in the context of an exogenous substance or medical illness.
bFor further discussion, see “Is a medical illness causing your patient’s depression? Current Psychiatry, August 2009, at CurrentPsychiatry.com.
Treating secondary depression involves addressing the underlying condition and might include antidepressant medication.
Seasonal affective disorder
DSM-5 identifies “with seasonal pattern” as a specifier for recurrent major depression. Phototherapy remains a standard treatment, although a Cochrane Review identified comparable outcomes with fluoxetine, but inconclusive data for other, newer antidepressants.24 Small open trials have suggested that MAOIs and TCAs can be efficacious.
Note: Phototherapy lacks demonstrated efficacy in non-seasonal forms of depression.25
What does the future hold for classifying depressive disorders?
Recent initiatives have attempted to classify depression less by traditional clinical signs and more by focusing on possible underlying neurobiological substrates.c In the future, subtyping of mood disorders might focus on such constructs as:
• positive and negative valence systems and attentional domains
• treatment-responsivity relative to genotypic variants (for example, the serotonin transporter gene locus [SLC6A4] or prediction of L-methylfolate-responsive depression based on the genotype of the methylenetetrahydrofolate reductase [MTHFR] polymorphism)
• disrupted neural plasticity in brain circuits believed to regulate emotion.
cAn example is the Research Domain Criteria [RDoC],www. nimh.nih.gov/research-priorities/rdoc/index.shtml.
Until robust biomarkers for depression are identified and validated, however, such advances in nosology remain experimental and speculative.
BOTTOM LINE
Depressive disorders comprise a range of conditions that can be viewed along many dimensions, including “situational,” treatment-resistant, melancholic, agitated, anxious, and atypical depression; depression occurring with a substance use disorder; premenstrual dysphoric disorder; and seasonal affective disorder, among other classifications. Clinical characteristics vary across subtypes—as do corresponding preferred treatments, which should be tailored to the needs of your patients.
Editor’s note: The first part of Dr. Goldberg’s review of depression subtypes—focusing on major and minor depression, chronicity, polarity, severity, and psychosis—appeared in the April 2014 issue.
Related Resources
• Kosinski EC, Rothschild AJ. Monoamine oxidase inhibitors: Forgotten treatment for depression. Current Psychiatry. 2012;11(12):20-26.
• Rodgers S, Grosse Holtforth M, Müller M, et al. Symptom-based subtypes of depression and their psychosocial correlates: a person-centered approach focusing on the influence of sex. J Affect Disord. 2014;156:92-103.
Drug Brand Names
Aripiprazole • Abilify Mirtazapine • Remeron
Bupropion • Wellbutrin Nefazodone • Serzone
Fluoxetine • Prozac Olanzapine/fluoxetine • Symbyax
Ketamine • Ketalar Pramipexole • Mirapex
L-Methylfolate • Deplin Quetiapine • Seroquel
Lamotrigine • Lamictal Riluzole • Rilutek
Lithium • Eskalith, Lithobid Vortioxetine • Brintellix
Methadone • Dolophine
Disclosure
Dr. Goldberg has been a consultant to Avanir Pharmaceuticals and Merck; has served on the speakers’ bureau for AstraZeneca, Merck, Novartis, Sunovion Pharmaceuticals, Takeda-Lundbeck; and has received royalties from American Psychiatric Publishing and honoraria from Medscape and WebMD.
1. Fergusson DM, Horwood LJ, Ridder EM, et al. Subthreshold depression in adolescence and mental health outcomes in adulthood. Arch Gen Psychiatry. 2005;62(1):66-72.
2. Mitchell PB, Parker GB, Gladstone GL, et al. Severity of stressful life events in first and subsequent episodes of depression: the relevance of depressive subtypes. J Affect Disord. 2003;73(3):245-252.
3. Coryell W, Winokur G, Maser JD, et al. Recurrently situational (reactive) depression: a study of course, phenomenology and familial psychopathology. J Affect Disord. 1994;31(3):203-210.
4. Slotema CW, Blom JD, Hoek HW, et al. Should we expand the toolbox of psychiatric treatment methods to include Repetitive Transcranial Magnetic Stimulation (rTMS)? A meta-analysis of the efficacy of rTMS in psychiatric disorders. J Clin Psychiatry. 2010;71(7):873-884.
5. Kendler KS. The diagnostic validity of melancholic major depression in a population-based sample of female twins. Arch Gen Psychiatry. 1997;54(4):299-304.
6. Bracht T, Horn H, Strik W, et al. White matter microstructure alterations of the medial forebrain bundle in melancholic depression. J Affect Disord. 2014;155:186-193.
7. Pizzagalli DA, Oakes TR, Fox AS, et al. Functional but not structural subgenual prefrontal cortex abnormalities in melancholia. Mol Psychiatry. 2004;9(4):325, 393-405.
8. Melartin T, Leskelä U, Rytsälä H, et al. Co-morbidity and stability of melancholic features in DSM-IV major depressive disorder. Psychol Med. 2004;34(8):1443-1452.
9. Caldieraro MA, Baeza FL, Pinheiro DO, et al. Prevalence of psychotic symptoms in those with melancholic and nonmelancholic depression. J Nerv Ment Dis. 2013;201(10):855-859.
10. Grunebaum MF, Galfalvy HC, Oquendo MA, et al. Melancholia and the probability and lethality of suicide attempts. Br J Psychiatry. 2004;184:534-535.
11. Roose SP, Glassman AH, Attia E, et al. Comparative efficacy of selective serotonin reuptake inhibitors and tricyclics in the treatment of melancholia. Am J Psychiatry. 1994;151(12):1735-1739.
12. Koukopoulos A, Sani G, Koukopoulos AE, et al. Melancholia agitata and mixed depression. Acta Psychiatr Scand Suppl. 2007;(433):50-57.
13. Fava M, Rush AJ, Alpert JE, et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry. 2008; 165(3):342-351.
14. Regier DA, Narrow WE, Clarke DE, et al. DSM-5 field trials in the United States and Canada, Part II: test-retest reliability of selected categorical diagnoses. Am J Psychiatry. 2013;170(1):59-70.
15. Rush AJ, Trivedi MH, Carmody TJ, et al. Response in relation to baseline anxiety levels in major depressive disorder treated with bupropion sustained release or sertraline. Neuropsychopharmacology. 2001;25(1):131-138.
16. Rasmussen KG, Snyder KA, Knapp RG, et al. Relationship between somatization and remission with ECT. Psychiatry Res. 2004;129(3):293-295.
17. Henkel V, Mergl R, Allgaier AK, et al. Treatment of depression with atypical features: a meta-analytic approach. Psychiatry Res. 2006;141(1):89-101.
18. Husain MM, McClintock SM, Rush AJ, et al. The efficacy of acute electroconvulsive therapy in atypical depression. J Clin Psychiatry. 2008;69(3):406-411.
19. Iovieno N, Tedeschini E, Bentley KH, et al. Antidepressants for major depressive disorder and dysthymic disorder in patients with comorbid alcohol use disorders: a meta-analysis of placebo-controlled randomized trials. J Clin Psychiatry. 2011;72(8):1144-1151.
20. Pedrelli P, Iovieno N, Vitali M, et al. Treatment of major depressive disorder and dysthymic disorder with antidepressants in patients with comorbid opiate use disorders enrolled in methadone maintenance therapy: a meta-analysis. J Clin Psychopharmacol. 2011;31(5):582-586.
21. Pearlstein TB, Stone AB, Lund SA, et al. Comparison of fluoxetine, bupropion, and placebo in the treatment of premenstrual dysphoric disorder. J Clin Psychopharmacol. 1997;17(4):261-266.
22. Freeman EW, Rickels K, Sondheimer SJ, et al. Differential response to antidepressants in women with premenstrual syndrome/premenstrual dysphoric disorder: a randomized controlled trial. Arch Gen Psychiatry. 1999;56(10):932-939.
23. Krasnik C, Montori VM, Guyatt GH, et al. The effect of bright light therapy on depression associated with premenstrual dysphoric disorder. Am J Obstet Gynecol. 2005;193(3, pt 1):658-661.
24. Thaler K, Delivuk M, Chapman A, et al. Second-generation antidepressants for seasonal affective disorder. Cochrane Database Syst Rev. 2011;7(12):CD008591.
25. Thalén BE, Kjellman BF, Mørkid L, et al. Light treatment in seasonal and nonseasonal depression. Acta Psychiatr Scand. 1995;91(5):352-360.
1. Fergusson DM, Horwood LJ, Ridder EM, et al. Subthreshold depression in adolescence and mental health outcomes in adulthood. Arch Gen Psychiatry. 2005;62(1):66-72.
2. Mitchell PB, Parker GB, Gladstone GL, et al. Severity of stressful life events in first and subsequent episodes of depression: the relevance of depressive subtypes. J Affect Disord. 2003;73(3):245-252.
3. Coryell W, Winokur G, Maser JD, et al. Recurrently situational (reactive) depression: a study of course, phenomenology and familial psychopathology. J Affect Disord. 1994;31(3):203-210.
4. Slotema CW, Blom JD, Hoek HW, et al. Should we expand the toolbox of psychiatric treatment methods to include Repetitive Transcranial Magnetic Stimulation (rTMS)? A meta-analysis of the efficacy of rTMS in psychiatric disorders. J Clin Psychiatry. 2010;71(7):873-884.
5. Kendler KS. The diagnostic validity of melancholic major depression in a population-based sample of female twins. Arch Gen Psychiatry. 1997;54(4):299-304.
6. Bracht T, Horn H, Strik W, et al. White matter microstructure alterations of the medial forebrain bundle in melancholic depression. J Affect Disord. 2014;155:186-193.
7. Pizzagalli DA, Oakes TR, Fox AS, et al. Functional but not structural subgenual prefrontal cortex abnormalities in melancholia. Mol Psychiatry. 2004;9(4):325, 393-405.
8. Melartin T, Leskelä U, Rytsälä H, et al. Co-morbidity and stability of melancholic features in DSM-IV major depressive disorder. Psychol Med. 2004;34(8):1443-1452.
9. Caldieraro MA, Baeza FL, Pinheiro DO, et al. Prevalence of psychotic symptoms in those with melancholic and nonmelancholic depression. J Nerv Ment Dis. 2013;201(10):855-859.
10. Grunebaum MF, Galfalvy HC, Oquendo MA, et al. Melancholia and the probability and lethality of suicide attempts. Br J Psychiatry. 2004;184:534-535.
11. Roose SP, Glassman AH, Attia E, et al. Comparative efficacy of selective serotonin reuptake inhibitors and tricyclics in the treatment of melancholia. Am J Psychiatry. 1994;151(12):1735-1739.
12. Koukopoulos A, Sani G, Koukopoulos AE, et al. Melancholia agitata and mixed depression. Acta Psychiatr Scand Suppl. 2007;(433):50-57.
13. Fava M, Rush AJ, Alpert JE, et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry. 2008; 165(3):342-351.
14. Regier DA, Narrow WE, Clarke DE, et al. DSM-5 field trials in the United States and Canada, Part II: test-retest reliability of selected categorical diagnoses. Am J Psychiatry. 2013;170(1):59-70.
15. Rush AJ, Trivedi MH, Carmody TJ, et al. Response in relation to baseline anxiety levels in major depressive disorder treated with bupropion sustained release or sertraline. Neuropsychopharmacology. 2001;25(1):131-138.
16. Rasmussen KG, Snyder KA, Knapp RG, et al. Relationship between somatization and remission with ECT. Psychiatry Res. 2004;129(3):293-295.
17. Henkel V, Mergl R, Allgaier AK, et al. Treatment of depression with atypical features: a meta-analytic approach. Psychiatry Res. 2006;141(1):89-101.
18. Husain MM, McClintock SM, Rush AJ, et al. The efficacy of acute electroconvulsive therapy in atypical depression. J Clin Psychiatry. 2008;69(3):406-411.
19. Iovieno N, Tedeschini E, Bentley KH, et al. Antidepressants for major depressive disorder and dysthymic disorder in patients with comorbid alcohol use disorders: a meta-analysis of placebo-controlled randomized trials. J Clin Psychiatry. 2011;72(8):1144-1151.
20. Pedrelli P, Iovieno N, Vitali M, et al. Treatment of major depressive disorder and dysthymic disorder with antidepressants in patients with comorbid opiate use disorders enrolled in methadone maintenance therapy: a meta-analysis. J Clin Psychopharmacol. 2011;31(5):582-586.
21. Pearlstein TB, Stone AB, Lund SA, et al. Comparison of fluoxetine, bupropion, and placebo in the treatment of premenstrual dysphoric disorder. J Clin Psychopharmacol. 1997;17(4):261-266.
22. Freeman EW, Rickels K, Sondheimer SJ, et al. Differential response to antidepressants in women with premenstrual syndrome/premenstrual dysphoric disorder: a randomized controlled trial. Arch Gen Psychiatry. 1999;56(10):932-939.
23. Krasnik C, Montori VM, Guyatt GH, et al. The effect of bright light therapy on depression associated with premenstrual dysphoric disorder. Am J Obstet Gynecol. 2005;193(3, pt 1):658-661.
24. Thaler K, Delivuk M, Chapman A, et al. Second-generation antidepressants for seasonal affective disorder. Cochrane Database Syst Rev. 2011;7(12):CD008591.
25. Thalén BE, Kjellman BF, Mørkid L, et al. Light treatment in seasonal and nonseasonal depression. Acta Psychiatr Scand. 1995;91(5):352-360.
Managing psychiatric illness in patients with epilepsy
Patients who have epilepsy have a higher incidence of psychiatric illness than the general population—at a prevalence of 60%.1 Establishing a temporal association and making a psychiatric diagnosis can be vexing, but awareness of potential comorbidities does improve the clinical outcome2 (Box). As this article discusses, psychiatric presentations and ictal disorders can share common pathology and exacerbate one another.3 Their coexistence often results in frequent hospitalization, higher treatment cost, and drug-resistant seizures.4 Risk factors for psychopathology in people who have epilepsy include psychosocial stressors, genetic factors, early age of onset of seizures, and each ictal event.5 Among ictal disorders, temporal-lobe epilepsy confers the highest rate of comorbidity.3
Mood disorders
Mood disorders are the most common psychiatric disorder comorbid with epilepsy (irrespective of age, socioeconomic status, and ethnicity), affecting 43% of patients who have a seizure disorder.5 These disorders present as an ictal aura in 1% of cases; the presence of a comorbid mood disorder implies a more severe form of epilepsy.2 Most mood disorders are underdiagnosed in epilepsy, however, because of the mistaken assumption that depression is a normal reaction to having a seizure disorder.
Interictal depression is the most commonly reported complaint, although dysphoria also can present peri-ictally.6 The severity of depression and the seizure disorder often are directly proportional to each other.1 Decreased levels of serotonin and norepinephrine, or abnormalities in their transport or postsynaptic binding, have been reported in epilepsy and in affective illness.6 MRI studies have documented that patients who have a depressive disorder have more gray-matter loss compared with healthy controls.7 Depression diminishes the quality of seizure remission after medical and surgical interventions for epilepsy.8
Taking a multidisciplinary approach to treating a mood disorder in a patient who has epilepsy might improve ictal and mood outcomes.9 Anhedonia is the most common presenting symptom, but some patients do not meet DSM-5 criteria. Depression exhibits atypically, with fatigue, irritability, poor frustration tolerance, anxiety, and mood lability.6 Self-report screening scales, such as the Neurological Disorders Depression Inventory for Epilepsy, are helpful for making a diagnosis.10
Treatment. Prompt antidepressant treatment is indicated. Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors are the most common agents in this setting.11 Consider possible cytochrome P450 interactions between antiepileptic drugs (AEDs) and antidepressants; sertraline, citalopram, and escitalopram have the lowest incidence of adverse effects. Because tricyclic antidepressants have proconvulsant properties, they are not commonly prescribed in these patients12 (Table 1).13
Electroconvulsive therapy and vagus nerve stimulation14 are effective interventions in treatment-resistant depression. The efficacy of transcranial magnetic stimulation remains to be clarified.
AEDs can produce psychiatric effects, even in nonconvulsive epilepsies. Twenty-eight percent of cases of depression that are comorbid with epilepsy have an iatrogenic basis, and can be induced by barbiturates, topiramate, vigabatrin, tiagabine, and levetiracetam.13 These adverse effects are a common reason that patients discontinue drug treatment and obtain psychiatric consultation.15
Neurosurgical management of epilepsy carries a low risk of depression compared with pharmacotherapy because the surgery offers better ictal control.16 Because some AEDs have mood-stabilizing properties, discontinuing one might unmask an underlying mood disorder.17
The incidence of adjustment disorder with depressed mood in persons who have epilepsy is 10%; with dysthymia, the incidence is 4%. Adjustment problems with an adverse psychosocial outcome are documented more often in patients who have a long-standing, chronic disorder than in those with a more recent diagnosis.18
Postictal suicidal ideation is more common in persons who have a preexisting mood disorder.6 The rate of suicide among epilepsy patients is 5%, compared with 1.4% in the general population—which is the same rate seen among patients with other psychiatric conditions, but higher than what is observed in many chronic medical conditions.19 Attempted suicide is not a direct result of epilepsy, but is significantly related to underlying psychopathology20; anxiety comorbid with a mood disorder increases the risk of suicide.21
The incidence of bipolar disorder among epilepsy patients is 1.4%.22 Although some AEDs can induce mania and hypomania, valproate and lamotrigine each have mood-stabilizing properties that might prevent such episodes.23
Anxiety disorders
Anxiety. Approximately one-third of epilepsy patients report anxiety. In contrast to what is seen with depression, AEDs do no alleviate anxiety.16,19 Anxiety or fear is the most common ictal-related psychiatric symptom2 making it difficult to differentiate anxiety and a seizure.24
Antidepressants, especially an SSRI, often are the treatment of choice; patients must be warned about the risk of an exacerbation of anxiety precipitated by an antidepressant. Such an adverse reaction might prompt cognitive-behavioral therapy (CBT) or limited use of a benzodiazepine.25
Obsessive-compulsive disorder. The incidence of OCD in epilepsy is 14% to 22%.26 Damage to the orbitofrontal cortex or temporal lobe epilepsy surgery can induce OCD; neurotransmitters involved are serotonin, glutamate, dopamine, and γ-aminobutyric acid (GABA).27 Patients may report obsessive thoughts in the peri-ictal period as well; some AEDs, such as topiramate, have been reported to induce such behaviors.28 Treatment options include CBT, an antidepressant, and, in refractory cases, neurosurgery.29
Psychosis
The prevalence of psychosis is approximately 10% among persons who have epilepsy, and is observed most often in patients who have complex partial seizures.30 Risk factors include a family history of epilepsy or psychosis, temporal lobe epilepsy, a long seizure history, and significant neuropathology.31 Structural abnormalities in the limbic system, especially the hippocampus, predispose patients to psychosis. Abnormal activity of GABA and dopamine are implicated in psychotic symptoms in these patients.32
Depending on the type and focus of the seizure, ictal psychoses present with cognitive and affective symptoms or hallucinations. Delusions can be associated with comorbid traumatic brain injury.32 Postictal psychosis is differentiated from other peri-ictal confusional states by:
• absence of confusion or autonomic dysfunction
• presence of more organized thinking
• absence of EEG changes.33
Alteration of an AED regimen can induce post-ictal psychosis. Iatrogenic psychosis sometimes is observed after right-sided temporal lobe surgery.34
Interictal psychoses probably occur as a result of aberrant nerve regeneration, with an increased concentration of dopamine in the brain after long-term seizure control. Epileptic psychosis is distinguished from schizophrenia by the predominance of visual hallucinations, no alteration of personality or affect, and glial proliferation.35 Some patients exhibit “forced normalization,” in which psychotic features appear after epilepsy has been treated successfully and EEG findings are normalized.36
Management of psychosis in epilepsy includes ensuring the patient’s safety, ruling out medical causes of psychosis, and preventing relapse. Prescribe antipsychotics with caution because many of these agents have epileptogenic potential or can interfere with the hepatic metabolism of AEDs. Quetiapine, risperidone, and haloperidol have low potential for seizure induction; chlorpromazine and clozapine are more likely to precipitate an ictal event.37 Ziprasidone, quetiapine, and aripiprazole often are prescribed for post-ictal and inter-ictal psychoses.38
Sleep disorders
Epilepsy patients often complain about difficulty sleeping, namely:
• 10% to 33% exhibit restless leg syndrome or periodic limb movement disorder
• 10% to 65% have obstructive sleep apnea
• 11% to 28% report excessive daytime sleepiness.3
Convulsive activity and the rate of generalization of partial seizures are increased by sleep, especially non-rapid eye movement sleep. Rapid eye movement (REM) sleep suppresses ictal activity, but the pattern of REM sleep is disrupted in epilepsy. Seizures and some sleep disorders present with similar symptoms, such as confusion and amnesia (Table 2).39
Management of comorbid sleep problems includes:
• effective control of seizures
• avoidance of polypharmacy
• assuring sleep hygiene.
Disordered sleep resulting from an AED might be relieved by switching to another medication.39
Substance abuse
Abuse of substances is a significant risk factor for recurrence of seizures.
Alcohol, at a low dose, has antiepileptic properties; intoxication rarely induces a seizure, although seizures often accompany alcohol withdrawal.40
Acute alcohol abuse increases the free level of AEDs by inhibiting 1) microsomal enzyme systems and 2) binding of albumin by metabolites, such as acetaldehyde. These effects can lead to the dangerous outcome of respiratory depression, especially with drugs like phenobarbital.
Chronic alcohol use induces hepatic enzymes, which augments clearance of AEDs, except benzodiazepines. Metabolism of AEDs is decreased because of reduced hepatic blood flow.
Moderate drinking does not increase the incidence of seizures in medication-adherent patients. People who have recurrent alcohol-withdrawal seizures do not have a heightened risk of epilepsy.41
Cannabis. Animal studies have documented the anticonvulsant effect of Cannabis in partial and generalized epilepsy and a proconvulsant effect in absence (petit mal) seizures.42
Tramadol, caffeine. Patients who abuse tramadol or who have an excessive intake of caffeine have a decreased seizure threshold.43
Opiates can exert a proconvulsant or anticonvulsant action, depending on the type of endorphin receptors involved.44
Cocaine decreases the seizure threshold by 1) blocking cerebral GABA receptors and 2) inhibiting dopamine reuptake, thus elevating excitatory neurotransmitters. Cocaine can cause a generalized or focal seizure; the latter is caused by intracerebral stroke or hemorrhage.45
The AEDs topiramate and lamotrigine tend to decrease the desire to abuse alcohol by enhancing inhibitory control by way of decreasing dopamine activity in the mesocorticolimbic system.46
Memory deficits
The relative risk of dementia among epilepsy patients is greater compared with the general population. Recurrent seizures can result in cognitive deficits; epilepsy has been documented in 2% to 64% of Alzheimer’s disease patients.47
Progressive amnesia, with an associated decline in cognition in epilepsy patients despite AED therapy, warrants a dementia workup.48 Patients with an ictal disorder often have difficulty with memory, especially if the hippocampus is affected, such as in temporal lobe epilepsy. Seizures are a common manifestation of several neurodegenerative conditions, and may be associated with a treatable dementia or psychosis in patients with cyanocobalamin deficiency.49
Several memory deficits are associated with seizure disorders:
• Transient epileptic amnesia can be ictal or post-ictal, or can be a manifestation of an underlying seizure disorder. The condition is associated with isolated memory deficits; other cognitive functions usually are intact.
• Accelerated long-term memory deficit occurs when patients forget skills acquired over the past few days or weeks. The problem can be reduced with sleep.50
• Remote memory impairment is characterized by inability to recall personal information from the past.51
When considering a diagnosis of a memory deficit as a manifestation of dementia, keep in mind that cognitive impairment also can develop after epilepsy treatment—although most newer medications cause relatively few such problems.52,53
2-pronged management. It is difficult to establish a temporal association between epilepsy and dementia. When the conditions coexist, appropriate treatment of both is important, because inadequate control of seizures can heighten release of amyloid toxins in the hippocampus. This results in rapidly progressive cognitive decline.54
Neurodevelopmental disorders
The incidence of epilepsy in children who have an autism spectrum disorder is 5% to 38%; the disorder is more common in the presence of mental retardation or cerebral palsy.55
A significant percentage of youth who are referred for evaluation of attention-deficit/ hyperactivity disorder (ADHD) eventually are given a diagnosis of absence seizures. The incidence of ADHD in children with epilepsy is 20%; these patients display epileptiform EEG changes, and require meticulous screening, which includes ictal induction by hyperventilation to differentiate ADHD from a seizure disorder.56 Many AEDs, especially GABAergic drugs, can cause symptoms of ADHD. Methylphenidate is safe in children whose seizures are well-controlled, and has no significant interactions with AEDs.57
Management. Adequate seizure control is the only effective means to slow regression in cases of epilepsy comorbid with autism spectrum disorder, mental retardation, and cerebral palsy.58
BOTTOM LINE
Patients who have epilepsy have a lifetime susceptibility to psychopathology, especially depression and anxiety. Psychiatric practitioners should work collaboratively with patients' primary care provider to evaluate, diagnose, and treat both conditions. Quick action is the key to the best possible outcomes, including reducing the risk of recurrent seizures.
Related Resources
• Clancy MJ, Clarke MC, Connor DJ, et al. The prevalence of psychosis in epilepsy; a systematic review and meta-analysis. BMC Psychiatry. 2014;14:75.
• Centers for Disease Control and Prevention. Comorbidity in adults with epilepsy—United States, 2010. MMWR Morb Mortal Wkly Rep. 2013;62(43):849-853.
• Kui C, Yingfu P, Chenling X, et al. What are the predictors of major depression in adult patients with epilepsy? Epileptic Disord. 2014;16(1):74-79.
• Lunde ME, Rasmussen KG. Electroconvulsive therapy in patients with epilepsy. Epilepsy Behav. 2006;9(2):355-359.
Drug Brand Names
Aripiprazole • Abilify Risperidone • Risperdal
Chlorpromazine • Thorazine Quetlapine • Seroquel
Citalopram • Celexa Sertraline • Zoloft
Clozapine • Clozaril, FazaClo Tiagabine • Gabitril
Escitalopram • Lexapro Topiramate • Topamax
Haloperidol • Haldol Tramadol • Ryzolt, Ultram, ConZip
Lamotrigine • Lamictal Valproate • Depokate
Levetiracetam • Keppra Vigabatrin • Sabril
Methylphenidate • Methylin, Ritalin Ziprasidone • Geodon
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Paradiso S, Hermann BP, Blumer D, et al. Impact of depressed mood on neuropsychological status in temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. 2001;70(2):180-185.
2. Gaitatzis A, Trimble MR, Sander JW. The psychiatric comorbidity of epilepsy. Acta Neurol Scand. 2004;110(4):207-220.
3. Gaitatzis A, Carroll K, Majeed A, et al. The epidemiology of the comorbidity of epilepsy in the general population. Epilepsia. 2004;45(12):1613-1622.
4. Ivanova JI, Birnbaum HG, Kidolezi Y, et al. Direct and indirect costs associated with epileptic partial onset seizures among the privately insured in the United States. Epilepsia. 2010;51(5):838-844.
5. Torta R, Keller R. Behavioral, psychotic, and anxiety disorders in epilepsy: etiology, clinical features, and therapeutic implications. Epilepsia. 2005;40(suppl 10): S2-S20.
6. Kanner AM. Depression and epilepsy: a new perspective on two closely related disorders. Epilepsy Curr. 2006;6(5):141-146.
7. Salgado PCB, Yasuda CL, Cendes F. Neuroimaging changes in mesial temporal lobe epilepsy are magnified in the presence of depression. Epilepsy Behav. 2010;19(3):422-427.
8. Kanner AM. Psychiatric issues in epilepsy: the complex relation of mood, anxiety disorders, and epilepsy. Epilepsy Behav. 2009;15(1):83-87.9. Hedrick SC, Chaney EF, Felker B, et al. Effectiveness of collaborative care depression treatment in Veterans’ Affairs primary care. J Gen Intern Med. 2003;18(1):9-16.
10. Kanner AM. Depression and epilepsy: a bidirectional relation? Epilepsia. 2011;52(suppl 1):21-27.
11. Karouni M, Arulthas S, Larsson PG, et al. Psychiatric comorbidity in patients with epilepsy: a population-based study. Eur J Clin Pharmacol. 2010;66(11):1151-1160.
12. Prueter C, Norra C. Mood disorders and their treatment in patients with epilepsy. J Neuropsychiatry Clin Neurosci. 2005;17(1):20-28.
13. Schmitz B. Antidepressant drugs: indications and guidelines for use in epilepsy. Epilepsia. 2002;43(suppl 2):14-18.
14. Shafique S, Dalsing MC. Vagus nerve stimulation therapy for treatment of drug-resistant epilepsy and depression. Perspect Vasc Surg Endovasc Ther. 2006;18(4):323-327.
15. Schmitz B. Depression and mania in patients with epilepsy. Epilepsia. 2005;46(suppl 4):45-49.
16. Reuber M, Andersen B, Elger CE, et al. Depression and anxiety before and after temporal lobe epilepsy surgery. Seizure. 2004;13(2):129-135.
17. Johannessen Landmark CJ. Antiepileptic drugs in non-epilepsy disorders: relations between mechanisms of action and clinical efficacy. CNS Drugs. 2008;22(1):27-47.
18. Wiglusz MS, Cubała WJ, Gałuszko-We¸gielnik WG, et al. Mood disorders in epilepsy - diagnostic and methodical considerations. Psychiatr Danub. 2012;24(suppl 1):S44-S50.
19. Jones JE, Hermann BP, Barry JJ, et al. Rates and risk factors for suicide, suicidal ideation, and suicide attempts in chronic epilepsy. Epilepsy Behav. 2003;4(suppl 3):S31-S38.
20. Hara E, Akanuma N, Adachi N, et al. Suicide attempts in adult patients with idiopathic generalized epilepsy. Psychiatry Clin Neurosci. 2009;63(2):225-229.
21. Sareen J, Cox BJ, Afifi TO, et al. Anxiety disorders and risk for suicidal ideation and suicide attempts: a population-based longitudinal study of adults. Arch Gen Psychiatry. 2005;62(11):1249-1257.
22. Mula M, Schmitz B, Jauch R, et al. On the prevalence of bipolar disorder in epilepsy. Epilepsy Behav. 2008;13(4): 658-661.
23. Mula M, Monaco F. Antiepileptic drug-induced mania in patients with epilepsy: what do we know? Epilepsy Behav. 2006;9(2):265-267.
24. Kimiskidis VK, Triantafyllou NI, Kararizou E, et al. Depression and anxiety in epilepsy: the association with demographic and seizure-related variables. Ann Gen Psychiatry. 2007;6:28.
25. Jackson MJ, Turkington D. Depression and anxiety in epilepsy. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i45-i47.
26. Mula M, Cavanna AE, Critchley H, et al. Phenomenology of obsessive compulsive disorder in patients with temporal lobe epilepsy or tourette syndrome. J Neuropsychiatry Clin Neurosci. 2008;20(2):223-226.
27. Fornaro M, Gabrielli F, Albano C, et al. Obsessive-compulsive disorder and related disorders: a comprehensive survey. Ann Gen Psychiatry. 2009;8:13.
28. Thuile J, Even C, Guelfi JD. Topiramate may induce obsessive-compulsive disorder. Psychiatry Clin Neurosci. 2006;60(3):394.
29. Kaplan PW. Epilepsy and obsessive-compulsive disorder. Dialogues Clin Neurosci. 2010;12(2):241-248.
30. Henning OJ, Nakken KO. Psychiatric comorbidity and use of psychotropic drugs in epilepsy patients. Acta Neurol Scand Suppl. 2010;122(suppl 190):18-22.
31. Qin P, Xu H, Laursen TM, et al. Risk for schizophrenia and schizophrenia-like psychosis among patients with epilepsy: population based cohort study. BMJ. 2005; 331(7507):23.
32. Kandratavicius L, Lopes-Aguiar C, Bueno-Júnior LS, et al. Psychiatric comorbidities in temporal lobe epilepsy: possible relationships between psychotic disorders and involvement of limbic circuits [Erratum in Rev Bars Psiquiatr. 2013;35(1):107]. Rev Bras Psiquiatr. 2012;34(4):454-466.
33. Lancman ME, Craven WJ, Asconapé JJ, et al. Clinical management of recurrent postictal psychosis. Journal of Epilepsy. 1994;7(1):47-51.
34. Shaw P, Mellers J, Henderson M, et al. Schizophrenia-like psychosis arising de novo following a temporal lobectomy: timing and risk factors. J Neurol Neurosurg Psychiatry. 2004;75(7):1003-1008.
35. Perez MM, Trimble MR. Epileptic psychosis–diagnostic comparison with process schizophrenia. Br J Psychiatry. 1980;137:245-249.
36. Krishnamoorthy ES, Trimble MR, Sander JW, et al. Forced normalization at the interface between epilepsy and psychiatry. Epilepsy Behav. 2002;3(4):303-308.
37. Pisani F, Oteri G, Costa C, et al. Effects of psychotropic drugs on seizure threshold. Drug Saf. 2002;25(2):91-110.
38. Nadkarni S, Arnedo V, Devinsky O. Psychosis in epilepsy patients. Epilepsia. 2007;48(suppl 9):17-19.
39. Bazil CW. Parasomnias, sleep disorders, and narcolepsy— sleep-time imitators of epilepsy. In: Kaplan PW, Fisher RS, eds. Imitators of epilepsy. 2nd edition. New York, New York: Demos Medical Publishing; 2005:217-230.
40. Chang HJ, Liao CC, Hu CJ, et al. Psychiatric disorders after epilepsy diagnosis: a population-based retrospective cohort study. PloS One. 2013;8(4):e59999.
41. Gordon E, Devinsky O. Alcohol and marijuana: effects on epilepsy and use by patients with epilepsy. Epilepsia. 2001;42(10):1266-1272.
42. Consroe P. Brain cannabinoid systems as targets for the therapy of neurological disorders. Neurobiol Dis. 1998;5(6, pt B):534-551.
43. Maiga DD, Seyni H, Sidikou A, et al. Convulsive crisis in Tramadol and caffeine abusers: about 8 cases and review of the literature [in French]. Pan Afr Med J. 2012;13:24.
44. Ye JH, Liu PL, Wu WH, et al. Cocaine depresses GABA current of hippocampal neurons. Brain Res. 1997;770(1-2):169-175.
45. Przewłocka B, Stala L, Laso´n W, et al. The effect of various opiate receptor agonists on the seizure threshold in the rat. Is dynorphin an endogenous anticonvulsant? Life Sci. 1983;33(suppl 1):595-598.
46. Johnson BA, Ait-Daoud N, Bowden CL, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361(9370): 1677-1685.
47. Friedman D, Honig LS, Scarmeas N. Seizures and epilepsy in Alzheimer’s disease. CNS Neurosci Ther. 2012;18(4): 285-294.
48. Cretin B, Blanc F, Gaultier C, et al. Epileptic Amnesic Syndrome revealing Alzheimer’s disease. Epilepsy Res. 2012;102(3):206-209.
49. Vilibié M, Jukié V, Vidovié A, et al. Cobalamin deficiency manifested with seizures, mood oscillations, psychotic features and reversible dementia in the absence of typical neurologic and hematologic signs and symptoms: a case report. Coll Antropol. 2013;37(1):317-319.
50. Butler CR, Zeman AZ. Recent insights into the impairment of memory in epilepsy: transient epileptic amnesia, accelerated long-term forgetting and remote memory impairment. Brain. 2008;131(pt 9):2243-2263.
51. Manes F, Hodges JR, Graham KS, et al. Focal autobiographical amnesia in association with transient epileptic amnesia. Brain. 2001;124(pt 3):499-509.
52. Motamedi GK, Meador KJ. Antiepileptic drugs and memory. Epilepsy Behav. 2004;5(4):435-439.
53. Thompson PJ, Baxendale SA, Duncan JS, et al. Effects of topiramate on cognitive function. J Neurol Neurosurg Psychiatry. 2000;69(5):636-641.
54. Noebels JL. A perfect storm: converging paths of epilepsy and Alzheimer’s dementia intersect in the hippocampal formation. Epilepsia. 2011;52(suppl 1):39-46.
55. Lewis P, Kopelman MD. Forgetting rates in neuropsychiatric disorders. J Neurol Neurosurg Psychiatry. 1998;65(6):890-898.
56. Tan M, Appleton R. Attention deficit and hyperactivity disorder, methylphenidate, and epilepsy. Arch Dis Child. 2005;90(1):57-59.
57. McBride MC, Wang DD, Torres CF. Methylphenidate in therapeutic doses does not lower seizure threshold [abstract 130]. Ann Neurol. 1986;20(3):428.
58. Levisohn PM. The autism-epilepsy connection. Epilepsia. 2007;48(suppl 9):33-35.
Patients who have epilepsy have a higher incidence of psychiatric illness than the general population—at a prevalence of 60%.1 Establishing a temporal association and making a psychiatric diagnosis can be vexing, but awareness of potential comorbidities does improve the clinical outcome2 (Box). As this article discusses, psychiatric presentations and ictal disorders can share common pathology and exacerbate one another.3 Their coexistence often results in frequent hospitalization, higher treatment cost, and drug-resistant seizures.4 Risk factors for psychopathology in people who have epilepsy include psychosocial stressors, genetic factors, early age of onset of seizures, and each ictal event.5 Among ictal disorders, temporal-lobe epilepsy confers the highest rate of comorbidity.3
Mood disorders
Mood disorders are the most common psychiatric disorder comorbid with epilepsy (irrespective of age, socioeconomic status, and ethnicity), affecting 43% of patients who have a seizure disorder.5 These disorders present as an ictal aura in 1% of cases; the presence of a comorbid mood disorder implies a more severe form of epilepsy.2 Most mood disorders are underdiagnosed in epilepsy, however, because of the mistaken assumption that depression is a normal reaction to having a seizure disorder.
Interictal depression is the most commonly reported complaint, although dysphoria also can present peri-ictally.6 The severity of depression and the seizure disorder often are directly proportional to each other.1 Decreased levels of serotonin and norepinephrine, or abnormalities in their transport or postsynaptic binding, have been reported in epilepsy and in affective illness.6 MRI studies have documented that patients who have a depressive disorder have more gray-matter loss compared with healthy controls.7 Depression diminishes the quality of seizure remission after medical and surgical interventions for epilepsy.8
Taking a multidisciplinary approach to treating a mood disorder in a patient who has epilepsy might improve ictal and mood outcomes.9 Anhedonia is the most common presenting symptom, but some patients do not meet DSM-5 criteria. Depression exhibits atypically, with fatigue, irritability, poor frustration tolerance, anxiety, and mood lability.6 Self-report screening scales, such as the Neurological Disorders Depression Inventory for Epilepsy, are helpful for making a diagnosis.10
Treatment. Prompt antidepressant treatment is indicated. Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors are the most common agents in this setting.11 Consider possible cytochrome P450 interactions between antiepileptic drugs (AEDs) and antidepressants; sertraline, citalopram, and escitalopram have the lowest incidence of adverse effects. Because tricyclic antidepressants have proconvulsant properties, they are not commonly prescribed in these patients12 (Table 1).13
Electroconvulsive therapy and vagus nerve stimulation14 are effective interventions in treatment-resistant depression. The efficacy of transcranial magnetic stimulation remains to be clarified.
AEDs can produce psychiatric effects, even in nonconvulsive epilepsies. Twenty-eight percent of cases of depression that are comorbid with epilepsy have an iatrogenic basis, and can be induced by barbiturates, topiramate, vigabatrin, tiagabine, and levetiracetam.13 These adverse effects are a common reason that patients discontinue drug treatment and obtain psychiatric consultation.15
Neurosurgical management of epilepsy carries a low risk of depression compared with pharmacotherapy because the surgery offers better ictal control.16 Because some AEDs have mood-stabilizing properties, discontinuing one might unmask an underlying mood disorder.17
The incidence of adjustment disorder with depressed mood in persons who have epilepsy is 10%; with dysthymia, the incidence is 4%. Adjustment problems with an adverse psychosocial outcome are documented more often in patients who have a long-standing, chronic disorder than in those with a more recent diagnosis.18
Postictal suicidal ideation is more common in persons who have a preexisting mood disorder.6 The rate of suicide among epilepsy patients is 5%, compared with 1.4% in the general population—which is the same rate seen among patients with other psychiatric conditions, but higher than what is observed in many chronic medical conditions.19 Attempted suicide is not a direct result of epilepsy, but is significantly related to underlying psychopathology20; anxiety comorbid with a mood disorder increases the risk of suicide.21
The incidence of bipolar disorder among epilepsy patients is 1.4%.22 Although some AEDs can induce mania and hypomania, valproate and lamotrigine each have mood-stabilizing properties that might prevent such episodes.23
Anxiety disorders
Anxiety. Approximately one-third of epilepsy patients report anxiety. In contrast to what is seen with depression, AEDs do no alleviate anxiety.16,19 Anxiety or fear is the most common ictal-related psychiatric symptom2 making it difficult to differentiate anxiety and a seizure.24
Antidepressants, especially an SSRI, often are the treatment of choice; patients must be warned about the risk of an exacerbation of anxiety precipitated by an antidepressant. Such an adverse reaction might prompt cognitive-behavioral therapy (CBT) or limited use of a benzodiazepine.25
Obsessive-compulsive disorder. The incidence of OCD in epilepsy is 14% to 22%.26 Damage to the orbitofrontal cortex or temporal lobe epilepsy surgery can induce OCD; neurotransmitters involved are serotonin, glutamate, dopamine, and γ-aminobutyric acid (GABA).27 Patients may report obsessive thoughts in the peri-ictal period as well; some AEDs, such as topiramate, have been reported to induce such behaviors.28 Treatment options include CBT, an antidepressant, and, in refractory cases, neurosurgery.29
Psychosis
The prevalence of psychosis is approximately 10% among persons who have epilepsy, and is observed most often in patients who have complex partial seizures.30 Risk factors include a family history of epilepsy or psychosis, temporal lobe epilepsy, a long seizure history, and significant neuropathology.31 Structural abnormalities in the limbic system, especially the hippocampus, predispose patients to psychosis. Abnormal activity of GABA and dopamine are implicated in psychotic symptoms in these patients.32
Depending on the type and focus of the seizure, ictal psychoses present with cognitive and affective symptoms or hallucinations. Delusions can be associated with comorbid traumatic brain injury.32 Postictal psychosis is differentiated from other peri-ictal confusional states by:
• absence of confusion or autonomic dysfunction
• presence of more organized thinking
• absence of EEG changes.33
Alteration of an AED regimen can induce post-ictal psychosis. Iatrogenic psychosis sometimes is observed after right-sided temporal lobe surgery.34
Interictal psychoses probably occur as a result of aberrant nerve regeneration, with an increased concentration of dopamine in the brain after long-term seizure control. Epileptic psychosis is distinguished from schizophrenia by the predominance of visual hallucinations, no alteration of personality or affect, and glial proliferation.35 Some patients exhibit “forced normalization,” in which psychotic features appear after epilepsy has been treated successfully and EEG findings are normalized.36
Management of psychosis in epilepsy includes ensuring the patient’s safety, ruling out medical causes of psychosis, and preventing relapse. Prescribe antipsychotics with caution because many of these agents have epileptogenic potential or can interfere with the hepatic metabolism of AEDs. Quetiapine, risperidone, and haloperidol have low potential for seizure induction; chlorpromazine and clozapine are more likely to precipitate an ictal event.37 Ziprasidone, quetiapine, and aripiprazole often are prescribed for post-ictal and inter-ictal psychoses.38
Sleep disorders
Epilepsy patients often complain about difficulty sleeping, namely:
• 10% to 33% exhibit restless leg syndrome or periodic limb movement disorder
• 10% to 65% have obstructive sleep apnea
• 11% to 28% report excessive daytime sleepiness.3
Convulsive activity and the rate of generalization of partial seizures are increased by sleep, especially non-rapid eye movement sleep. Rapid eye movement (REM) sleep suppresses ictal activity, but the pattern of REM sleep is disrupted in epilepsy. Seizures and some sleep disorders present with similar symptoms, such as confusion and amnesia (Table 2).39
Management of comorbid sleep problems includes:
• effective control of seizures
• avoidance of polypharmacy
• assuring sleep hygiene.
Disordered sleep resulting from an AED might be relieved by switching to another medication.39
Substance abuse
Abuse of substances is a significant risk factor for recurrence of seizures.
Alcohol, at a low dose, has antiepileptic properties; intoxication rarely induces a seizure, although seizures often accompany alcohol withdrawal.40
Acute alcohol abuse increases the free level of AEDs by inhibiting 1) microsomal enzyme systems and 2) binding of albumin by metabolites, such as acetaldehyde. These effects can lead to the dangerous outcome of respiratory depression, especially with drugs like phenobarbital.
Chronic alcohol use induces hepatic enzymes, which augments clearance of AEDs, except benzodiazepines. Metabolism of AEDs is decreased because of reduced hepatic blood flow.
Moderate drinking does not increase the incidence of seizures in medication-adherent patients. People who have recurrent alcohol-withdrawal seizures do not have a heightened risk of epilepsy.41
Cannabis. Animal studies have documented the anticonvulsant effect of Cannabis in partial and generalized epilepsy and a proconvulsant effect in absence (petit mal) seizures.42
Tramadol, caffeine. Patients who abuse tramadol or who have an excessive intake of caffeine have a decreased seizure threshold.43
Opiates can exert a proconvulsant or anticonvulsant action, depending on the type of endorphin receptors involved.44
Cocaine decreases the seizure threshold by 1) blocking cerebral GABA receptors and 2) inhibiting dopamine reuptake, thus elevating excitatory neurotransmitters. Cocaine can cause a generalized or focal seizure; the latter is caused by intracerebral stroke or hemorrhage.45
The AEDs topiramate and lamotrigine tend to decrease the desire to abuse alcohol by enhancing inhibitory control by way of decreasing dopamine activity in the mesocorticolimbic system.46
Memory deficits
The relative risk of dementia among epilepsy patients is greater compared with the general population. Recurrent seizures can result in cognitive deficits; epilepsy has been documented in 2% to 64% of Alzheimer’s disease patients.47
Progressive amnesia, with an associated decline in cognition in epilepsy patients despite AED therapy, warrants a dementia workup.48 Patients with an ictal disorder often have difficulty with memory, especially if the hippocampus is affected, such as in temporal lobe epilepsy. Seizures are a common manifestation of several neurodegenerative conditions, and may be associated with a treatable dementia or psychosis in patients with cyanocobalamin deficiency.49
Several memory deficits are associated with seizure disorders:
• Transient epileptic amnesia can be ictal or post-ictal, or can be a manifestation of an underlying seizure disorder. The condition is associated with isolated memory deficits; other cognitive functions usually are intact.
• Accelerated long-term memory deficit occurs when patients forget skills acquired over the past few days or weeks. The problem can be reduced with sleep.50
• Remote memory impairment is characterized by inability to recall personal information from the past.51
When considering a diagnosis of a memory deficit as a manifestation of dementia, keep in mind that cognitive impairment also can develop after epilepsy treatment—although most newer medications cause relatively few such problems.52,53
2-pronged management. It is difficult to establish a temporal association between epilepsy and dementia. When the conditions coexist, appropriate treatment of both is important, because inadequate control of seizures can heighten release of amyloid toxins in the hippocampus. This results in rapidly progressive cognitive decline.54
Neurodevelopmental disorders
The incidence of epilepsy in children who have an autism spectrum disorder is 5% to 38%; the disorder is more common in the presence of mental retardation or cerebral palsy.55
A significant percentage of youth who are referred for evaluation of attention-deficit/ hyperactivity disorder (ADHD) eventually are given a diagnosis of absence seizures. The incidence of ADHD in children with epilepsy is 20%; these patients display epileptiform EEG changes, and require meticulous screening, which includes ictal induction by hyperventilation to differentiate ADHD from a seizure disorder.56 Many AEDs, especially GABAergic drugs, can cause symptoms of ADHD. Methylphenidate is safe in children whose seizures are well-controlled, and has no significant interactions with AEDs.57
Management. Adequate seizure control is the only effective means to slow regression in cases of epilepsy comorbid with autism spectrum disorder, mental retardation, and cerebral palsy.58
BOTTOM LINE
Patients who have epilepsy have a lifetime susceptibility to psychopathology, especially depression and anxiety. Psychiatric practitioners should work collaboratively with patients' primary care provider to evaluate, diagnose, and treat both conditions. Quick action is the key to the best possible outcomes, including reducing the risk of recurrent seizures.
Related Resources
• Clancy MJ, Clarke MC, Connor DJ, et al. The prevalence of psychosis in epilepsy; a systematic review and meta-analysis. BMC Psychiatry. 2014;14:75.
• Centers for Disease Control and Prevention. Comorbidity in adults with epilepsy—United States, 2010. MMWR Morb Mortal Wkly Rep. 2013;62(43):849-853.
• Kui C, Yingfu P, Chenling X, et al. What are the predictors of major depression in adult patients with epilepsy? Epileptic Disord. 2014;16(1):74-79.
• Lunde ME, Rasmussen KG. Electroconvulsive therapy in patients with epilepsy. Epilepsy Behav. 2006;9(2):355-359.
Drug Brand Names
Aripiprazole • Abilify Risperidone • Risperdal
Chlorpromazine • Thorazine Quetlapine • Seroquel
Citalopram • Celexa Sertraline • Zoloft
Clozapine • Clozaril, FazaClo Tiagabine • Gabitril
Escitalopram • Lexapro Topiramate • Topamax
Haloperidol • Haldol Tramadol • Ryzolt, Ultram, ConZip
Lamotrigine • Lamictal Valproate • Depokate
Levetiracetam • Keppra Vigabatrin • Sabril
Methylphenidate • Methylin, Ritalin Ziprasidone • Geodon
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Patients who have epilepsy have a higher incidence of psychiatric illness than the general population—at a prevalence of 60%.1 Establishing a temporal association and making a psychiatric diagnosis can be vexing, but awareness of potential comorbidities does improve the clinical outcome2 (Box). As this article discusses, psychiatric presentations and ictal disorders can share common pathology and exacerbate one another.3 Their coexistence often results in frequent hospitalization, higher treatment cost, and drug-resistant seizures.4 Risk factors for psychopathology in people who have epilepsy include psychosocial stressors, genetic factors, early age of onset of seizures, and each ictal event.5 Among ictal disorders, temporal-lobe epilepsy confers the highest rate of comorbidity.3
Mood disorders
Mood disorders are the most common psychiatric disorder comorbid with epilepsy (irrespective of age, socioeconomic status, and ethnicity), affecting 43% of patients who have a seizure disorder.5 These disorders present as an ictal aura in 1% of cases; the presence of a comorbid mood disorder implies a more severe form of epilepsy.2 Most mood disorders are underdiagnosed in epilepsy, however, because of the mistaken assumption that depression is a normal reaction to having a seizure disorder.
Interictal depression is the most commonly reported complaint, although dysphoria also can present peri-ictally.6 The severity of depression and the seizure disorder often are directly proportional to each other.1 Decreased levels of serotonin and norepinephrine, or abnormalities in their transport or postsynaptic binding, have been reported in epilepsy and in affective illness.6 MRI studies have documented that patients who have a depressive disorder have more gray-matter loss compared with healthy controls.7 Depression diminishes the quality of seizure remission after medical and surgical interventions for epilepsy.8
Taking a multidisciplinary approach to treating a mood disorder in a patient who has epilepsy might improve ictal and mood outcomes.9 Anhedonia is the most common presenting symptom, but some patients do not meet DSM-5 criteria. Depression exhibits atypically, with fatigue, irritability, poor frustration tolerance, anxiety, and mood lability.6 Self-report screening scales, such as the Neurological Disorders Depression Inventory for Epilepsy, are helpful for making a diagnosis.10
Treatment. Prompt antidepressant treatment is indicated. Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors are the most common agents in this setting.11 Consider possible cytochrome P450 interactions between antiepileptic drugs (AEDs) and antidepressants; sertraline, citalopram, and escitalopram have the lowest incidence of adverse effects. Because tricyclic antidepressants have proconvulsant properties, they are not commonly prescribed in these patients12 (Table 1).13
Electroconvulsive therapy and vagus nerve stimulation14 are effective interventions in treatment-resistant depression. The efficacy of transcranial magnetic stimulation remains to be clarified.
AEDs can produce psychiatric effects, even in nonconvulsive epilepsies. Twenty-eight percent of cases of depression that are comorbid with epilepsy have an iatrogenic basis, and can be induced by barbiturates, topiramate, vigabatrin, tiagabine, and levetiracetam.13 These adverse effects are a common reason that patients discontinue drug treatment and obtain psychiatric consultation.15
Neurosurgical management of epilepsy carries a low risk of depression compared with pharmacotherapy because the surgery offers better ictal control.16 Because some AEDs have mood-stabilizing properties, discontinuing one might unmask an underlying mood disorder.17
The incidence of adjustment disorder with depressed mood in persons who have epilepsy is 10%; with dysthymia, the incidence is 4%. Adjustment problems with an adverse psychosocial outcome are documented more often in patients who have a long-standing, chronic disorder than in those with a more recent diagnosis.18
Postictal suicidal ideation is more common in persons who have a preexisting mood disorder.6 The rate of suicide among epilepsy patients is 5%, compared with 1.4% in the general population—which is the same rate seen among patients with other psychiatric conditions, but higher than what is observed in many chronic medical conditions.19 Attempted suicide is not a direct result of epilepsy, but is significantly related to underlying psychopathology20; anxiety comorbid with a mood disorder increases the risk of suicide.21
The incidence of bipolar disorder among epilepsy patients is 1.4%.22 Although some AEDs can induce mania and hypomania, valproate and lamotrigine each have mood-stabilizing properties that might prevent such episodes.23
Anxiety disorders
Anxiety. Approximately one-third of epilepsy patients report anxiety. In contrast to what is seen with depression, AEDs do no alleviate anxiety.16,19 Anxiety or fear is the most common ictal-related psychiatric symptom2 making it difficult to differentiate anxiety and a seizure.24
Antidepressants, especially an SSRI, often are the treatment of choice; patients must be warned about the risk of an exacerbation of anxiety precipitated by an antidepressant. Such an adverse reaction might prompt cognitive-behavioral therapy (CBT) or limited use of a benzodiazepine.25
Obsessive-compulsive disorder. The incidence of OCD in epilepsy is 14% to 22%.26 Damage to the orbitofrontal cortex or temporal lobe epilepsy surgery can induce OCD; neurotransmitters involved are serotonin, glutamate, dopamine, and γ-aminobutyric acid (GABA).27 Patients may report obsessive thoughts in the peri-ictal period as well; some AEDs, such as topiramate, have been reported to induce such behaviors.28 Treatment options include CBT, an antidepressant, and, in refractory cases, neurosurgery.29
Psychosis
The prevalence of psychosis is approximately 10% among persons who have epilepsy, and is observed most often in patients who have complex partial seizures.30 Risk factors include a family history of epilepsy or psychosis, temporal lobe epilepsy, a long seizure history, and significant neuropathology.31 Structural abnormalities in the limbic system, especially the hippocampus, predispose patients to psychosis. Abnormal activity of GABA and dopamine are implicated in psychotic symptoms in these patients.32
Depending on the type and focus of the seizure, ictal psychoses present with cognitive and affective symptoms or hallucinations. Delusions can be associated with comorbid traumatic brain injury.32 Postictal psychosis is differentiated from other peri-ictal confusional states by:
• absence of confusion or autonomic dysfunction
• presence of more organized thinking
• absence of EEG changes.33
Alteration of an AED regimen can induce post-ictal psychosis. Iatrogenic psychosis sometimes is observed after right-sided temporal lobe surgery.34
Interictal psychoses probably occur as a result of aberrant nerve regeneration, with an increased concentration of dopamine in the brain after long-term seizure control. Epileptic psychosis is distinguished from schizophrenia by the predominance of visual hallucinations, no alteration of personality or affect, and glial proliferation.35 Some patients exhibit “forced normalization,” in which psychotic features appear after epilepsy has been treated successfully and EEG findings are normalized.36
Management of psychosis in epilepsy includes ensuring the patient’s safety, ruling out medical causes of psychosis, and preventing relapse. Prescribe antipsychotics with caution because many of these agents have epileptogenic potential or can interfere with the hepatic metabolism of AEDs. Quetiapine, risperidone, and haloperidol have low potential for seizure induction; chlorpromazine and clozapine are more likely to precipitate an ictal event.37 Ziprasidone, quetiapine, and aripiprazole often are prescribed for post-ictal and inter-ictal psychoses.38
Sleep disorders
Epilepsy patients often complain about difficulty sleeping, namely:
• 10% to 33% exhibit restless leg syndrome or periodic limb movement disorder
• 10% to 65% have obstructive sleep apnea
• 11% to 28% report excessive daytime sleepiness.3
Convulsive activity and the rate of generalization of partial seizures are increased by sleep, especially non-rapid eye movement sleep. Rapid eye movement (REM) sleep suppresses ictal activity, but the pattern of REM sleep is disrupted in epilepsy. Seizures and some sleep disorders present with similar symptoms, such as confusion and amnesia (Table 2).39
Management of comorbid sleep problems includes:
• effective control of seizures
• avoidance of polypharmacy
• assuring sleep hygiene.
Disordered sleep resulting from an AED might be relieved by switching to another medication.39
Substance abuse
Abuse of substances is a significant risk factor for recurrence of seizures.
Alcohol, at a low dose, has antiepileptic properties; intoxication rarely induces a seizure, although seizures often accompany alcohol withdrawal.40
Acute alcohol abuse increases the free level of AEDs by inhibiting 1) microsomal enzyme systems and 2) binding of albumin by metabolites, such as acetaldehyde. These effects can lead to the dangerous outcome of respiratory depression, especially with drugs like phenobarbital.
Chronic alcohol use induces hepatic enzymes, which augments clearance of AEDs, except benzodiazepines. Metabolism of AEDs is decreased because of reduced hepatic blood flow.
Moderate drinking does not increase the incidence of seizures in medication-adherent patients. People who have recurrent alcohol-withdrawal seizures do not have a heightened risk of epilepsy.41
Cannabis. Animal studies have documented the anticonvulsant effect of Cannabis in partial and generalized epilepsy and a proconvulsant effect in absence (petit mal) seizures.42
Tramadol, caffeine. Patients who abuse tramadol or who have an excessive intake of caffeine have a decreased seizure threshold.43
Opiates can exert a proconvulsant or anticonvulsant action, depending on the type of endorphin receptors involved.44
Cocaine decreases the seizure threshold by 1) blocking cerebral GABA receptors and 2) inhibiting dopamine reuptake, thus elevating excitatory neurotransmitters. Cocaine can cause a generalized or focal seizure; the latter is caused by intracerebral stroke or hemorrhage.45
The AEDs topiramate and lamotrigine tend to decrease the desire to abuse alcohol by enhancing inhibitory control by way of decreasing dopamine activity in the mesocorticolimbic system.46
Memory deficits
The relative risk of dementia among epilepsy patients is greater compared with the general population. Recurrent seizures can result in cognitive deficits; epilepsy has been documented in 2% to 64% of Alzheimer’s disease patients.47
Progressive amnesia, with an associated decline in cognition in epilepsy patients despite AED therapy, warrants a dementia workup.48 Patients with an ictal disorder often have difficulty with memory, especially if the hippocampus is affected, such as in temporal lobe epilepsy. Seizures are a common manifestation of several neurodegenerative conditions, and may be associated with a treatable dementia or psychosis in patients with cyanocobalamin deficiency.49
Several memory deficits are associated with seizure disorders:
• Transient epileptic amnesia can be ictal or post-ictal, or can be a manifestation of an underlying seizure disorder. The condition is associated with isolated memory deficits; other cognitive functions usually are intact.
• Accelerated long-term memory deficit occurs when patients forget skills acquired over the past few days or weeks. The problem can be reduced with sleep.50
• Remote memory impairment is characterized by inability to recall personal information from the past.51
When considering a diagnosis of a memory deficit as a manifestation of dementia, keep in mind that cognitive impairment also can develop after epilepsy treatment—although most newer medications cause relatively few such problems.52,53
2-pronged management. It is difficult to establish a temporal association between epilepsy and dementia. When the conditions coexist, appropriate treatment of both is important, because inadequate control of seizures can heighten release of amyloid toxins in the hippocampus. This results in rapidly progressive cognitive decline.54
Neurodevelopmental disorders
The incidence of epilepsy in children who have an autism spectrum disorder is 5% to 38%; the disorder is more common in the presence of mental retardation or cerebral palsy.55
A significant percentage of youth who are referred for evaluation of attention-deficit/ hyperactivity disorder (ADHD) eventually are given a diagnosis of absence seizures. The incidence of ADHD in children with epilepsy is 20%; these patients display epileptiform EEG changes, and require meticulous screening, which includes ictal induction by hyperventilation to differentiate ADHD from a seizure disorder.56 Many AEDs, especially GABAergic drugs, can cause symptoms of ADHD. Methylphenidate is safe in children whose seizures are well-controlled, and has no significant interactions with AEDs.57
Management. Adequate seizure control is the only effective means to slow regression in cases of epilepsy comorbid with autism spectrum disorder, mental retardation, and cerebral palsy.58
BOTTOM LINE
Patients who have epilepsy have a lifetime susceptibility to psychopathology, especially depression and anxiety. Psychiatric practitioners should work collaboratively with patients' primary care provider to evaluate, diagnose, and treat both conditions. Quick action is the key to the best possible outcomes, including reducing the risk of recurrent seizures.
Related Resources
• Clancy MJ, Clarke MC, Connor DJ, et al. The prevalence of psychosis in epilepsy; a systematic review and meta-analysis. BMC Psychiatry. 2014;14:75.
• Centers for Disease Control and Prevention. Comorbidity in adults with epilepsy—United States, 2010. MMWR Morb Mortal Wkly Rep. 2013;62(43):849-853.
• Kui C, Yingfu P, Chenling X, et al. What are the predictors of major depression in adult patients with epilepsy? Epileptic Disord. 2014;16(1):74-79.
• Lunde ME, Rasmussen KG. Electroconvulsive therapy in patients with epilepsy. Epilepsy Behav. 2006;9(2):355-359.
Drug Brand Names
Aripiprazole • Abilify Risperidone • Risperdal
Chlorpromazine • Thorazine Quetlapine • Seroquel
Citalopram • Celexa Sertraline • Zoloft
Clozapine • Clozaril, FazaClo Tiagabine • Gabitril
Escitalopram • Lexapro Topiramate • Topamax
Haloperidol • Haldol Tramadol • Ryzolt, Ultram, ConZip
Lamotrigine • Lamictal Valproate • Depokate
Levetiracetam • Keppra Vigabatrin • Sabril
Methylphenidate • Methylin, Ritalin Ziprasidone • Geodon
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Paradiso S, Hermann BP, Blumer D, et al. Impact of depressed mood on neuropsychological status in temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. 2001;70(2):180-185.
2. Gaitatzis A, Trimble MR, Sander JW. The psychiatric comorbidity of epilepsy. Acta Neurol Scand. 2004;110(4):207-220.
3. Gaitatzis A, Carroll K, Majeed A, et al. The epidemiology of the comorbidity of epilepsy in the general population. Epilepsia. 2004;45(12):1613-1622.
4. Ivanova JI, Birnbaum HG, Kidolezi Y, et al. Direct and indirect costs associated with epileptic partial onset seizures among the privately insured in the United States. Epilepsia. 2010;51(5):838-844.
5. Torta R, Keller R. Behavioral, psychotic, and anxiety disorders in epilepsy: etiology, clinical features, and therapeutic implications. Epilepsia. 2005;40(suppl 10): S2-S20.
6. Kanner AM. Depression and epilepsy: a new perspective on two closely related disorders. Epilepsy Curr. 2006;6(5):141-146.
7. Salgado PCB, Yasuda CL, Cendes F. Neuroimaging changes in mesial temporal lobe epilepsy are magnified in the presence of depression. Epilepsy Behav. 2010;19(3):422-427.
8. Kanner AM. Psychiatric issues in epilepsy: the complex relation of mood, anxiety disorders, and epilepsy. Epilepsy Behav. 2009;15(1):83-87.9. Hedrick SC, Chaney EF, Felker B, et al. Effectiveness of collaborative care depression treatment in Veterans’ Affairs primary care. J Gen Intern Med. 2003;18(1):9-16.
10. Kanner AM. Depression and epilepsy: a bidirectional relation? Epilepsia. 2011;52(suppl 1):21-27.
11. Karouni M, Arulthas S, Larsson PG, et al. Psychiatric comorbidity in patients with epilepsy: a population-based study. Eur J Clin Pharmacol. 2010;66(11):1151-1160.
12. Prueter C, Norra C. Mood disorders and their treatment in patients with epilepsy. J Neuropsychiatry Clin Neurosci. 2005;17(1):20-28.
13. Schmitz B. Antidepressant drugs: indications and guidelines for use in epilepsy. Epilepsia. 2002;43(suppl 2):14-18.
14. Shafique S, Dalsing MC. Vagus nerve stimulation therapy for treatment of drug-resistant epilepsy and depression. Perspect Vasc Surg Endovasc Ther. 2006;18(4):323-327.
15. Schmitz B. Depression and mania in patients with epilepsy. Epilepsia. 2005;46(suppl 4):45-49.
16. Reuber M, Andersen B, Elger CE, et al. Depression and anxiety before and after temporal lobe epilepsy surgery. Seizure. 2004;13(2):129-135.
17. Johannessen Landmark CJ. Antiepileptic drugs in non-epilepsy disorders: relations between mechanisms of action and clinical efficacy. CNS Drugs. 2008;22(1):27-47.
18. Wiglusz MS, Cubała WJ, Gałuszko-We¸gielnik WG, et al. Mood disorders in epilepsy - diagnostic and methodical considerations. Psychiatr Danub. 2012;24(suppl 1):S44-S50.
19. Jones JE, Hermann BP, Barry JJ, et al. Rates and risk factors for suicide, suicidal ideation, and suicide attempts in chronic epilepsy. Epilepsy Behav. 2003;4(suppl 3):S31-S38.
20. Hara E, Akanuma N, Adachi N, et al. Suicide attempts in adult patients with idiopathic generalized epilepsy. Psychiatry Clin Neurosci. 2009;63(2):225-229.
21. Sareen J, Cox BJ, Afifi TO, et al. Anxiety disorders and risk for suicidal ideation and suicide attempts: a population-based longitudinal study of adults. Arch Gen Psychiatry. 2005;62(11):1249-1257.
22. Mula M, Schmitz B, Jauch R, et al. On the prevalence of bipolar disorder in epilepsy. Epilepsy Behav. 2008;13(4): 658-661.
23. Mula M, Monaco F. Antiepileptic drug-induced mania in patients with epilepsy: what do we know? Epilepsy Behav. 2006;9(2):265-267.
24. Kimiskidis VK, Triantafyllou NI, Kararizou E, et al. Depression and anxiety in epilepsy: the association with demographic and seizure-related variables. Ann Gen Psychiatry. 2007;6:28.
25. Jackson MJ, Turkington D. Depression and anxiety in epilepsy. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i45-i47.
26. Mula M, Cavanna AE, Critchley H, et al. Phenomenology of obsessive compulsive disorder in patients with temporal lobe epilepsy or tourette syndrome. J Neuropsychiatry Clin Neurosci. 2008;20(2):223-226.
27. Fornaro M, Gabrielli F, Albano C, et al. Obsessive-compulsive disorder and related disorders: a comprehensive survey. Ann Gen Psychiatry. 2009;8:13.
28. Thuile J, Even C, Guelfi JD. Topiramate may induce obsessive-compulsive disorder. Psychiatry Clin Neurosci. 2006;60(3):394.
29. Kaplan PW. Epilepsy and obsessive-compulsive disorder. Dialogues Clin Neurosci. 2010;12(2):241-248.
30. Henning OJ, Nakken KO. Psychiatric comorbidity and use of psychotropic drugs in epilepsy patients. Acta Neurol Scand Suppl. 2010;122(suppl 190):18-22.
31. Qin P, Xu H, Laursen TM, et al. Risk for schizophrenia and schizophrenia-like psychosis among patients with epilepsy: population based cohort study. BMJ. 2005; 331(7507):23.
32. Kandratavicius L, Lopes-Aguiar C, Bueno-Júnior LS, et al. Psychiatric comorbidities in temporal lobe epilepsy: possible relationships between psychotic disorders and involvement of limbic circuits [Erratum in Rev Bars Psiquiatr. 2013;35(1):107]. Rev Bras Psiquiatr. 2012;34(4):454-466.
33. Lancman ME, Craven WJ, Asconapé JJ, et al. Clinical management of recurrent postictal psychosis. Journal of Epilepsy. 1994;7(1):47-51.
34. Shaw P, Mellers J, Henderson M, et al. Schizophrenia-like psychosis arising de novo following a temporal lobectomy: timing and risk factors. J Neurol Neurosurg Psychiatry. 2004;75(7):1003-1008.
35. Perez MM, Trimble MR. Epileptic psychosis–diagnostic comparison with process schizophrenia. Br J Psychiatry. 1980;137:245-249.
36. Krishnamoorthy ES, Trimble MR, Sander JW, et al. Forced normalization at the interface between epilepsy and psychiatry. Epilepsy Behav. 2002;3(4):303-308.
37. Pisani F, Oteri G, Costa C, et al. Effects of psychotropic drugs on seizure threshold. Drug Saf. 2002;25(2):91-110.
38. Nadkarni S, Arnedo V, Devinsky O. Psychosis in epilepsy patients. Epilepsia. 2007;48(suppl 9):17-19.
39. Bazil CW. Parasomnias, sleep disorders, and narcolepsy— sleep-time imitators of epilepsy. In: Kaplan PW, Fisher RS, eds. Imitators of epilepsy. 2nd edition. New York, New York: Demos Medical Publishing; 2005:217-230.
40. Chang HJ, Liao CC, Hu CJ, et al. Psychiatric disorders after epilepsy diagnosis: a population-based retrospective cohort study. PloS One. 2013;8(4):e59999.
41. Gordon E, Devinsky O. Alcohol and marijuana: effects on epilepsy and use by patients with epilepsy. Epilepsia. 2001;42(10):1266-1272.
42. Consroe P. Brain cannabinoid systems as targets for the therapy of neurological disorders. Neurobiol Dis. 1998;5(6, pt B):534-551.
43. Maiga DD, Seyni H, Sidikou A, et al. Convulsive crisis in Tramadol and caffeine abusers: about 8 cases and review of the literature [in French]. Pan Afr Med J. 2012;13:24.
44. Ye JH, Liu PL, Wu WH, et al. Cocaine depresses GABA current of hippocampal neurons. Brain Res. 1997;770(1-2):169-175.
45. Przewłocka B, Stala L, Laso´n W, et al. The effect of various opiate receptor agonists on the seizure threshold in the rat. Is dynorphin an endogenous anticonvulsant? Life Sci. 1983;33(suppl 1):595-598.
46. Johnson BA, Ait-Daoud N, Bowden CL, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361(9370): 1677-1685.
47. Friedman D, Honig LS, Scarmeas N. Seizures and epilepsy in Alzheimer’s disease. CNS Neurosci Ther. 2012;18(4): 285-294.
48. Cretin B, Blanc F, Gaultier C, et al. Epileptic Amnesic Syndrome revealing Alzheimer’s disease. Epilepsy Res. 2012;102(3):206-209.
49. Vilibié M, Jukié V, Vidovié A, et al. Cobalamin deficiency manifested with seizures, mood oscillations, psychotic features and reversible dementia in the absence of typical neurologic and hematologic signs and symptoms: a case report. Coll Antropol. 2013;37(1):317-319.
50. Butler CR, Zeman AZ. Recent insights into the impairment of memory in epilepsy: transient epileptic amnesia, accelerated long-term forgetting and remote memory impairment. Brain. 2008;131(pt 9):2243-2263.
51. Manes F, Hodges JR, Graham KS, et al. Focal autobiographical amnesia in association with transient epileptic amnesia. Brain. 2001;124(pt 3):499-509.
52. Motamedi GK, Meador KJ. Antiepileptic drugs and memory. Epilepsy Behav. 2004;5(4):435-439.
53. Thompson PJ, Baxendale SA, Duncan JS, et al. Effects of topiramate on cognitive function. J Neurol Neurosurg Psychiatry. 2000;69(5):636-641.
54. Noebels JL. A perfect storm: converging paths of epilepsy and Alzheimer’s dementia intersect in the hippocampal formation. Epilepsia. 2011;52(suppl 1):39-46.
55. Lewis P, Kopelman MD. Forgetting rates in neuropsychiatric disorders. J Neurol Neurosurg Psychiatry. 1998;65(6):890-898.
56. Tan M, Appleton R. Attention deficit and hyperactivity disorder, methylphenidate, and epilepsy. Arch Dis Child. 2005;90(1):57-59.
57. McBride MC, Wang DD, Torres CF. Methylphenidate in therapeutic doses does not lower seizure threshold [abstract 130]. Ann Neurol. 1986;20(3):428.
58. Levisohn PM. The autism-epilepsy connection. Epilepsia. 2007;48(suppl 9):33-35.
1. Paradiso S, Hermann BP, Blumer D, et al. Impact of depressed mood on neuropsychological status in temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. 2001;70(2):180-185.
2. Gaitatzis A, Trimble MR, Sander JW. The psychiatric comorbidity of epilepsy. Acta Neurol Scand. 2004;110(4):207-220.
3. Gaitatzis A, Carroll K, Majeed A, et al. The epidemiology of the comorbidity of epilepsy in the general population. Epilepsia. 2004;45(12):1613-1622.
4. Ivanova JI, Birnbaum HG, Kidolezi Y, et al. Direct and indirect costs associated with epileptic partial onset seizures among the privately insured in the United States. Epilepsia. 2010;51(5):838-844.
5. Torta R, Keller R. Behavioral, psychotic, and anxiety disorders in epilepsy: etiology, clinical features, and therapeutic implications. Epilepsia. 2005;40(suppl 10): S2-S20.
6. Kanner AM. Depression and epilepsy: a new perspective on two closely related disorders. Epilepsy Curr. 2006;6(5):141-146.
7. Salgado PCB, Yasuda CL, Cendes F. Neuroimaging changes in mesial temporal lobe epilepsy are magnified in the presence of depression. Epilepsy Behav. 2010;19(3):422-427.
8. Kanner AM. Psychiatric issues in epilepsy: the complex relation of mood, anxiety disorders, and epilepsy. Epilepsy Behav. 2009;15(1):83-87.9. Hedrick SC, Chaney EF, Felker B, et al. Effectiveness of collaborative care depression treatment in Veterans’ Affairs primary care. J Gen Intern Med. 2003;18(1):9-16.
10. Kanner AM. Depression and epilepsy: a bidirectional relation? Epilepsia. 2011;52(suppl 1):21-27.
11. Karouni M, Arulthas S, Larsson PG, et al. Psychiatric comorbidity in patients with epilepsy: a population-based study. Eur J Clin Pharmacol. 2010;66(11):1151-1160.
12. Prueter C, Norra C. Mood disorders and their treatment in patients with epilepsy. J Neuropsychiatry Clin Neurosci. 2005;17(1):20-28.
13. Schmitz B. Antidepressant drugs: indications and guidelines for use in epilepsy. Epilepsia. 2002;43(suppl 2):14-18.
14. Shafique S, Dalsing MC. Vagus nerve stimulation therapy for treatment of drug-resistant epilepsy and depression. Perspect Vasc Surg Endovasc Ther. 2006;18(4):323-327.
15. Schmitz B. Depression and mania in patients with epilepsy. Epilepsia. 2005;46(suppl 4):45-49.
16. Reuber M, Andersen B, Elger CE, et al. Depression and anxiety before and after temporal lobe epilepsy surgery. Seizure. 2004;13(2):129-135.
17. Johannessen Landmark CJ. Antiepileptic drugs in non-epilepsy disorders: relations between mechanisms of action and clinical efficacy. CNS Drugs. 2008;22(1):27-47.
18. Wiglusz MS, Cubała WJ, Gałuszko-We¸gielnik WG, et al. Mood disorders in epilepsy - diagnostic and methodical considerations. Psychiatr Danub. 2012;24(suppl 1):S44-S50.
19. Jones JE, Hermann BP, Barry JJ, et al. Rates and risk factors for suicide, suicidal ideation, and suicide attempts in chronic epilepsy. Epilepsy Behav. 2003;4(suppl 3):S31-S38.
20. Hara E, Akanuma N, Adachi N, et al. Suicide attempts in adult patients with idiopathic generalized epilepsy. Psychiatry Clin Neurosci. 2009;63(2):225-229.
21. Sareen J, Cox BJ, Afifi TO, et al. Anxiety disorders and risk for suicidal ideation and suicide attempts: a population-based longitudinal study of adults. Arch Gen Psychiatry. 2005;62(11):1249-1257.
22. Mula M, Schmitz B, Jauch R, et al. On the prevalence of bipolar disorder in epilepsy. Epilepsy Behav. 2008;13(4): 658-661.
23. Mula M, Monaco F. Antiepileptic drug-induced mania in patients with epilepsy: what do we know? Epilepsy Behav. 2006;9(2):265-267.
24. Kimiskidis VK, Triantafyllou NI, Kararizou E, et al. Depression and anxiety in epilepsy: the association with demographic and seizure-related variables. Ann Gen Psychiatry. 2007;6:28.
25. Jackson MJ, Turkington D. Depression and anxiety in epilepsy. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i45-i47.
26. Mula M, Cavanna AE, Critchley H, et al. Phenomenology of obsessive compulsive disorder in patients with temporal lobe epilepsy or tourette syndrome. J Neuropsychiatry Clin Neurosci. 2008;20(2):223-226.
27. Fornaro M, Gabrielli F, Albano C, et al. Obsessive-compulsive disorder and related disorders: a comprehensive survey. Ann Gen Psychiatry. 2009;8:13.
28. Thuile J, Even C, Guelfi JD. Topiramate may induce obsessive-compulsive disorder. Psychiatry Clin Neurosci. 2006;60(3):394.
29. Kaplan PW. Epilepsy and obsessive-compulsive disorder. Dialogues Clin Neurosci. 2010;12(2):241-248.
30. Henning OJ, Nakken KO. Psychiatric comorbidity and use of psychotropic drugs in epilepsy patients. Acta Neurol Scand Suppl. 2010;122(suppl 190):18-22.
31. Qin P, Xu H, Laursen TM, et al. Risk for schizophrenia and schizophrenia-like psychosis among patients with epilepsy: population based cohort study. BMJ. 2005; 331(7507):23.
32. Kandratavicius L, Lopes-Aguiar C, Bueno-Júnior LS, et al. Psychiatric comorbidities in temporal lobe epilepsy: possible relationships between psychotic disorders and involvement of limbic circuits [Erratum in Rev Bars Psiquiatr. 2013;35(1):107]. Rev Bras Psiquiatr. 2012;34(4):454-466.
33. Lancman ME, Craven WJ, Asconapé JJ, et al. Clinical management of recurrent postictal psychosis. Journal of Epilepsy. 1994;7(1):47-51.
34. Shaw P, Mellers J, Henderson M, et al. Schizophrenia-like psychosis arising de novo following a temporal lobectomy: timing and risk factors. J Neurol Neurosurg Psychiatry. 2004;75(7):1003-1008.
35. Perez MM, Trimble MR. Epileptic psychosis–diagnostic comparison with process schizophrenia. Br J Psychiatry. 1980;137:245-249.
36. Krishnamoorthy ES, Trimble MR, Sander JW, et al. Forced normalization at the interface between epilepsy and psychiatry. Epilepsy Behav. 2002;3(4):303-308.
37. Pisani F, Oteri G, Costa C, et al. Effects of psychotropic drugs on seizure threshold. Drug Saf. 2002;25(2):91-110.
38. Nadkarni S, Arnedo V, Devinsky O. Psychosis in epilepsy patients. Epilepsia. 2007;48(suppl 9):17-19.
39. Bazil CW. Parasomnias, sleep disorders, and narcolepsy— sleep-time imitators of epilepsy. In: Kaplan PW, Fisher RS, eds. Imitators of epilepsy. 2nd edition. New York, New York: Demos Medical Publishing; 2005:217-230.
40. Chang HJ, Liao CC, Hu CJ, et al. Psychiatric disorders after epilepsy diagnosis: a population-based retrospective cohort study. PloS One. 2013;8(4):e59999.
41. Gordon E, Devinsky O. Alcohol and marijuana: effects on epilepsy and use by patients with epilepsy. Epilepsia. 2001;42(10):1266-1272.
42. Consroe P. Brain cannabinoid systems as targets for the therapy of neurological disorders. Neurobiol Dis. 1998;5(6, pt B):534-551.
43. Maiga DD, Seyni H, Sidikou A, et al. Convulsive crisis in Tramadol and caffeine abusers: about 8 cases and review of the literature [in French]. Pan Afr Med J. 2012;13:24.
44. Ye JH, Liu PL, Wu WH, et al. Cocaine depresses GABA current of hippocampal neurons. Brain Res. 1997;770(1-2):169-175.
45. Przewłocka B, Stala L, Laso´n W, et al. The effect of various opiate receptor agonists on the seizure threshold in the rat. Is dynorphin an endogenous anticonvulsant? Life Sci. 1983;33(suppl 1):595-598.
46. Johnson BA, Ait-Daoud N, Bowden CL, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361(9370): 1677-1685.
47. Friedman D, Honig LS, Scarmeas N. Seizures and epilepsy in Alzheimer’s disease. CNS Neurosci Ther. 2012;18(4): 285-294.
48. Cretin B, Blanc F, Gaultier C, et al. Epileptic Amnesic Syndrome revealing Alzheimer’s disease. Epilepsy Res. 2012;102(3):206-209.
49. Vilibié M, Jukié V, Vidovié A, et al. Cobalamin deficiency manifested with seizures, mood oscillations, psychotic features and reversible dementia in the absence of typical neurologic and hematologic signs and symptoms: a case report. Coll Antropol. 2013;37(1):317-319.
50. Butler CR, Zeman AZ. Recent insights into the impairment of memory in epilepsy: transient epileptic amnesia, accelerated long-term forgetting and remote memory impairment. Brain. 2008;131(pt 9):2243-2263.
51. Manes F, Hodges JR, Graham KS, et al. Focal autobiographical amnesia in association with transient epileptic amnesia. Brain. 2001;124(pt 3):499-509.
52. Motamedi GK, Meador KJ. Antiepileptic drugs and memory. Epilepsy Behav. 2004;5(4):435-439.
53. Thompson PJ, Baxendale SA, Duncan JS, et al. Effects of topiramate on cognitive function. J Neurol Neurosurg Psychiatry. 2000;69(5):636-641.
54. Noebels JL. A perfect storm: converging paths of epilepsy and Alzheimer’s dementia intersect in the hippocampal formation. Epilepsia. 2011;52(suppl 1):39-46.
55. Lewis P, Kopelman MD. Forgetting rates in neuropsychiatric disorders. J Neurol Neurosurg Psychiatry. 1998;65(6):890-898.
56. Tan M, Appleton R. Attention deficit and hyperactivity disorder, methylphenidate, and epilepsy. Arch Dis Child. 2005;90(1):57-59.
57. McBride MC, Wang DD, Torres CF. Methylphenidate in therapeutic doses does not lower seizure threshold [abstract 130]. Ann Neurol. 1986;20(3):428.
58. Levisohn PM. The autism-epilepsy connection. Epilepsia. 2007;48(suppl 9):33-35.
The first of 2 parts: A practical approach to subtyping depression among your patients
Depression carries a wide differential diagnosis. Practitioners sometimes think polarity is the fundamental distinction when they conceptualize depression as a clinical entity; in fact, many nosologic frameworks have been described for defining and subtyping clinically meaningful forms of depression, and each waxed and waned in popularity.
Kraepelin, writing in the early 20th century, linked manic-depressive illness with “the greater part of the morbid states termed melancholia,”1 but many features other than polarity remain important components of depression, and those features often carry implications for how individual patients respond to treatment.
In this 2-part article [April and May 2014 issues], I summarize information about clinically distinct subtypes of depression, as they are recognized within diagnostic systems or as descriptors of treatment outcomes for particular subgroups of patients. My focus is on practical considerations for assessing and managing depression. Because many forms of the disorder respond inadequately to initial antidepressant treatment, optimal “next-step” pharmacotherapy, after nonresponse or partial response, often hinges on clinical subtyping.
The first part of this article examines major depressive disorder (MDD), minor depression, chronic depression, depression in bipolar disorder, depression that is severe or mild, and psychotic depression. Treatments for these subtypes for which there is evidence, or a clinical rationale, are given in the Table.
The subtypes of depression that I’ll discuss in the second part of the article are listed on page 47.
Major and minor depression
MDD has been the focus of most drug trials seeking FDA approval. As a syndrome, MDD is defined by a constellation of features that are related not only to mood but also to sleep, energy, cognition, motivation, and motor behavior, persisting for ≥2 weeks.
DSM-5 has imposed few changes to the basic definition of MDD:
• bereavement (the aftermath of death of a loved one), formerly an exclusion criterion, no longer precludes making a diagnosis of MDD when syndromal criteria are otherwise fulfilled
• “with anxious distress” is a new course specifier that designates prominent anxiety features (feeling worried, restless, tense, or keyed up; fearful of losing control or something terrible happening)
• “with mixed features” is a new course specifier pertinent when ≥3 mania or hypomania symptoms coexist (that is, might be a subsyndromal mania or hypomania) with a depressive syndrome; the mixed features specifier can be applied to depressed patients whether or not they have ever had a manic or hypomanic episode, but MDD—rather than bipolar disorder— remains the overarching diagnosis, unless criteria have ever been met for a full mania or hypomania.
More than 2 dozen medications are FDA-approved to treat MDD. Evidence-based psychotherapies (eg, cognitive-behavioral therapy [CBT] and interpersonal therapy), as adjuncts to pharmacotherapy, further improve outcomes, but with only modest additional effect.2
Minor depression. Depressive states that involve 2 to 4 associated symptoms lasting ≥2 weeks but <2 years are sometimes described as minor depression, captured within DSM-5 as “depression not elsewhere defined.” The terminology of so-called “minor depression” generally is shunned, in part because it might wrongly connote low severity and therefore discourage treatment—even though it confers more than a 5-fold increase in risk of MDD.3
Chronicity
DSM-IV-TR identified long-standing depression by 2 constructs:
• chronic major depression (an episode of MDD lasting ≥2 years in adults, ≥1 year in children and adolescents)
• dysthymic disorder (2 to 4 depressive symptoms for ≥2 years in adults and ≥1 year in children and adolescents), affecting 3% to 6% of adults and carrying a 2-fold increased risk of MDD, eventually.4
Depression that begins as dysthymic disorder and blossoms into syndromal MDD is described as “double depression”—although it is not recognized as a unique condition in any edition of the DSM. Subsequent incomplete recovery may revert to dysthymic disorder. DSM-5 has subsumed chronic major depression and dysthymia under the unified heading of persistent depressive disorder.
There are no FDA-approved drugs for treating dysthymia. A meta-analysis of 9 controlled trials of off-label use of antidepressants to treat dysthymia revealed an overall response rate of 52.4%, compared with 29.9% for placebo.5 Notably, although the active drug response rate in these studies is comparable to what seen in MDD, the placebo response rate was approximately 10% lower than what was seen in major depression.
Positive therapeutic findings (typically, treatment for 6 to 12 weeks) have been reported in so-called “pure” dysthymic disorder with sertraline, fluoxetine, imipramine, ritanserin, moclobemide (not approved for use in the United States), and phenelzine; the results of additional, positive placebo-controlled studies support the utility of duloxetine6 and paroxetine.7 Randomized trials have reported negative findings for desipramine,8 fluoxetine,9 and escitalopram10 escitalopram10—although the sample size in these latter studies might have been too small to detect a drug-placebo difference.
In dysthymic and minor-depressive middle-age and older adult men who have a low serum level of testosterone, hormone replacement was shown to be superior to placebo in several randomized trials.11 Studies of adjunctive atypical antipsychotics for dysthymic disorder are scarce; a Cochrane review identified controlled data only with amisulpride (not approved for use in the United States), which yielded a modest therapeutic effect.12
Polarity
In recent years, depression in bipolar disorder (BD) has been contrasted with unipolar MDD based on a difference in:
• duration (briefer in BD)
• severity (worse in BD)
• risk of suicide (higher)
• comorbidity (more extensive)
• family history (often present for BD and highly recurrent depression)
• treatment outcome (generally less favorable).
DSM-5 has at least somewhat blurred the distinctions in polarity by way of the new construct of “major depression with mixed features” (see the discussion of MDD above), identifiable even when a person has never had a full manic or hypomanic episode.
No randomized trials have been conducted to identify the best treatments for such presentations, which has invited extrapolation from the literature in regard to bipolar mixed episodes, and suggesting that 1) some mood stabilizers (eg, divalproex) might have value and 2) antidepressants might exacerbate manic symptoms.
Perhaps most noteworthy in regard to treating bipolar depression is the unresolved, but hotly debated, controversy over whether and, if so, when, an antidepressant is inappropriate (based on concerns about possible induction or exacerbation of manic symptoms). In addition, nearly all of the large, randomized controlled trials of antidepressants for bipolar depression have shown that they offer no advantage over placebo.
Some authors argue that a lack of response to antidepressants might, itself, be a “soft” indicator of “bipolarity.” However, nonresponse to antidepressants should prompt a wider assessment of features other than polarity—including psychosis, anxiety, substance abuse, a personality disorder, psychiatric adverse effects from concomitant medications, medical comor bidity, adequacy of trials of medical therapy, and potential non-adherence to such trials—to account for poor antidepressant outcomes.
Severity
Severity of depression warrants consideration when formulating impressions about the nature and treatment of all presentations of depression.
High-severity forms prompt decisions about treatment setting (inpatient or outpatient); suicide assessment; and therapeutic modalities (eg, electroconvulsive therapy is more appropriate than psychotherapy for catatonic depression).
Mild forms. A recent meta-analysis of 6 randomized trials (each of >6 weeks’ duration) of antidepressants for mild depression demonstrated that these agents exert only a modest effect compared with placebo, owing largely to higher placebo-responsivity in mild depressive episodes than in moderate and severe episodes.13 In contrast, another meta-analysis of subjects who had “mild” baseline depression severity scores found that antidepressant medication had greater efficacy than placebo in 4 of 6 randomized trials.14 Higher depression severity levels typically diminish the placebo response rate but also reduce the magnitude of drug efficacy.
Psychosis
Before DSM-III, psychotic (as opposed to neurotic) depression was perhaps the key nosologic distinction when characterizing forms of depression. The presence of psychosis and related components (eg, mood-congruence) is closely linked with the severity of depression (high) and prognosis and longitudinal outcome (poorer), and has implications for treatment (Table).
Bottom Line
Depressive disorders comprise a range of conditions that can be viewed along many dimensions, including polarity, chronicity, recurrence, psychosis, treatment resistance, comorbidity, and atypicality, among other classifications. Clinical characteristics vary across subtypes—and so do corresponding preferred treatments, which should be tailored to the needs of each of your patients.
Related Resources
• Goldberg JF, Thase ME. Monoamine oxidase inhibitors revisited: what you should know. J Clin Psychiatry. 2013;74(2):189-191.
• Goldberg JF. Antidepressants in bipolar disorder: 7 myths and realities. Current Psychiatry. 2010;9(5):41-49.
• Ketamine cousin rapidly lifts depression without side effects. National Institute of Mental Health. http://www. nimh.nih.gov/news/science-news/2013/ketamine-cousin-rapidly-lifts-depression-without-side-effects.shtml. Published May 23, 2013. Accessed March 20, 2014.
• Research Domain Criteria (RDoC). National Institute of Mental Health. http://www.nimh.nih.gov/research-priorities/rdoc/index.shtml?u tm_ source = govdelivery&utm_medium=email&utm_campaign= govdelivery. Accessed March 20, 2014.
Drug Brand Names
Amisulpride • Amazeo, Lurasidone • Latuda
Amival, Amipride, Sulpitax Mirtazapine • Remeron
Aripiprazole • Abilify Moclobemide • Amira,
Armodafinil • Nuvigil Aurorix, Clobemix,
Bupropion • Wellbutrin Depnil, Manerix
Desipramine • Norpramin Modafinil • Provigil
Divalproex • Depakote, Olanzapine/fluoxetine
Depakene • Symbyax
Duloxetine • Cymbalta Paroxetine • Paxil
Escitalopram • Lexapro Phenelzine • Nardil
Fluoxetine • Prozac Pramipexole • Mirapex
Imipramine • Tofranil Quetiapine • Seroquel
Ketamine • Ketalar Riluzole • Rilutek
Lamotrigine • Lamictal Sertraline • Zoloft
Lithium • Eskalith, Lithobid Vortioxetine • Brintellix
Disclosure
Dr. Goldberg has been a consultant to Avanir Pharmaceuticals and Merck; has served on the speakers’ bureau for AstraZeneca, Merck, Novartis, Sunovion
Pharmaceuticals, and Takeda and Lundbeck; and has received royalties from American Psychiatric Publishing and honoraria from Medscape and WebMD.
Editor’s note: The second part of Dr. Goldberg’s review of depression subtypes—focusing on “situational,” treatment-resistant, melancholic, agitated, anxious, and atypical depression; depression occurring with a substance use disorder; premenstrual dysphoric disorder; and seasonal affective disorder—will appear in the May 2014 issue of Current Psychiatry.
1. Kraepelin E. Manic-depressive insanity and paranoia. Barclay RM, trans. Robertson GM, ed. Edinburgh, Scotland: E&S Livingstone; 1921:1.
2. Cuijpers P, Dekker J, Hollon SD, et al. Adding psychotherapy to pharmacotherapy in the treatment of depressive disorders in adults: a meta-analysis. J Clin Psychiatry. 2009;70(9):1219-1229.
3. Fogel J, Eaton WW, Ford DE. Minor depression as a predictor of the first onset of major depressive disorder over a 15-year follow-up. Acta Psychiatr Scand. 2006; 113(1):36-43.
4. Cuijpers P, de Graaf R, van Dorsselaer S. Minor depression: risk profiles, functional disability, health care use and risk of developing major depression. J Affect Disord. 2004;79(1-3):71-79.
5. Levkovitz Y, Tedeschini E, Papakostas GI. Efficacy of antidepressants for dysthymia: a meta-analysis of placebo-controlled randomized trials. J Clin Psychiatry. 2011;72(4):509-514.
6. Hellerstein DJ, Stewart JW, McGrath PJ, et al. A randomized controlled trial of duloxetine versus placebo in the treatment of nonmajor chronic depression. J Clin Psychiatry. 2012;73(7):984-991.
7. Ravindran AV, Cameron C, Bhatla R, et al. Paroxetine in the treatment of dysthymic disorder without co-morbidities: a double-blind, placebo-controlled, flexible-dose study. Asian J Psychiatry. 2013;6(2):157-161.
8. Stewart JW, McGrath PJ, Liebowitz MR, et al. Treatment outcome validation of DSM-III depressive subtypes. Clinical usefulness in outpatients with mild to moderate depression. Arch Gen Psychiatry. 1985;42(12):1148-1153.
9. Serrano-Blanco A, Gabarron E, Garcia-Bayo I, et al. Effectiveness and cost-effectiveness of antidepressant treatment in primary health care: a six-month randomised study comparing fluoxetine to imipramine. J Affect Disord. 2006;91(2-3):153-163.
10. Hellerstein DJ, Batchelder ST, Hyler S, et al. Escitalopram versus placebo in the treatment of dysthymic disorders. Int Clin Psychopharmacol. 2010;25(3):143-148.
11. Seidman SN, Orr G, Raviv G, et al. Effects of testosterone replacement in middle-aged men with dysthymia: a randomized, placebo-controlled clinical trial. J Clin Psychopharmacol. 2009;29(3):216-221.
12. Komossa K, Depping AM, Gaudchau A, et al. Second-generation antipsychotics for major depressive disorder and dysthymia. Cochrane Database Syst Rev. 2010; 8:(12):CD008121.
13. Fournier JC, DeRubeis RJ, Hollom SD, et al. Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA. 2010;303(1):47-53.
14. Stewart JA, Deliyannides DA, Hellerstein DJ, et al. Can people with nonsevere major depression benefit from antidepressant medication? J Clin Psychiatry. 2012;73(4):518-525.
Depression carries a wide differential diagnosis. Practitioners sometimes think polarity is the fundamental distinction when they conceptualize depression as a clinical entity; in fact, many nosologic frameworks have been described for defining and subtyping clinically meaningful forms of depression, and each waxed and waned in popularity.
Kraepelin, writing in the early 20th century, linked manic-depressive illness with “the greater part of the morbid states termed melancholia,”1 but many features other than polarity remain important components of depression, and those features often carry implications for how individual patients respond to treatment.
In this 2-part article [April and May 2014 issues], I summarize information about clinically distinct subtypes of depression, as they are recognized within diagnostic systems or as descriptors of treatment outcomes for particular subgroups of patients. My focus is on practical considerations for assessing and managing depression. Because many forms of the disorder respond inadequately to initial antidepressant treatment, optimal “next-step” pharmacotherapy, after nonresponse or partial response, often hinges on clinical subtyping.
The first part of this article examines major depressive disorder (MDD), minor depression, chronic depression, depression in bipolar disorder, depression that is severe or mild, and psychotic depression. Treatments for these subtypes for which there is evidence, or a clinical rationale, are given in the Table.
The subtypes of depression that I’ll discuss in the second part of the article are listed on page 47.
Major and minor depression
MDD has been the focus of most drug trials seeking FDA approval. As a syndrome, MDD is defined by a constellation of features that are related not only to mood but also to sleep, energy, cognition, motivation, and motor behavior, persisting for ≥2 weeks.
DSM-5 has imposed few changes to the basic definition of MDD:
• bereavement (the aftermath of death of a loved one), formerly an exclusion criterion, no longer precludes making a diagnosis of MDD when syndromal criteria are otherwise fulfilled
• “with anxious distress” is a new course specifier that designates prominent anxiety features (feeling worried, restless, tense, or keyed up; fearful of losing control or something terrible happening)
• “with mixed features” is a new course specifier pertinent when ≥3 mania or hypomania symptoms coexist (that is, might be a subsyndromal mania or hypomania) with a depressive syndrome; the mixed features specifier can be applied to depressed patients whether or not they have ever had a manic or hypomanic episode, but MDD—rather than bipolar disorder— remains the overarching diagnosis, unless criteria have ever been met for a full mania or hypomania.
More than 2 dozen medications are FDA-approved to treat MDD. Evidence-based psychotherapies (eg, cognitive-behavioral therapy [CBT] and interpersonal therapy), as adjuncts to pharmacotherapy, further improve outcomes, but with only modest additional effect.2
Minor depression. Depressive states that involve 2 to 4 associated symptoms lasting ≥2 weeks but <2 years are sometimes described as minor depression, captured within DSM-5 as “depression not elsewhere defined.” The terminology of so-called “minor depression” generally is shunned, in part because it might wrongly connote low severity and therefore discourage treatment—even though it confers more than a 5-fold increase in risk of MDD.3
Chronicity
DSM-IV-TR identified long-standing depression by 2 constructs:
• chronic major depression (an episode of MDD lasting ≥2 years in adults, ≥1 year in children and adolescents)
• dysthymic disorder (2 to 4 depressive symptoms for ≥2 years in adults and ≥1 year in children and adolescents), affecting 3% to 6% of adults and carrying a 2-fold increased risk of MDD, eventually.4
Depression that begins as dysthymic disorder and blossoms into syndromal MDD is described as “double depression”—although it is not recognized as a unique condition in any edition of the DSM. Subsequent incomplete recovery may revert to dysthymic disorder. DSM-5 has subsumed chronic major depression and dysthymia under the unified heading of persistent depressive disorder.
There are no FDA-approved drugs for treating dysthymia. A meta-analysis of 9 controlled trials of off-label use of antidepressants to treat dysthymia revealed an overall response rate of 52.4%, compared with 29.9% for placebo.5 Notably, although the active drug response rate in these studies is comparable to what seen in MDD, the placebo response rate was approximately 10% lower than what was seen in major depression.
Positive therapeutic findings (typically, treatment for 6 to 12 weeks) have been reported in so-called “pure” dysthymic disorder with sertraline, fluoxetine, imipramine, ritanserin, moclobemide (not approved for use in the United States), and phenelzine; the results of additional, positive placebo-controlled studies support the utility of duloxetine6 and paroxetine.7 Randomized trials have reported negative findings for desipramine,8 fluoxetine,9 and escitalopram10 escitalopram10—although the sample size in these latter studies might have been too small to detect a drug-placebo difference.
In dysthymic and minor-depressive middle-age and older adult men who have a low serum level of testosterone, hormone replacement was shown to be superior to placebo in several randomized trials.11 Studies of adjunctive atypical antipsychotics for dysthymic disorder are scarce; a Cochrane review identified controlled data only with amisulpride (not approved for use in the United States), which yielded a modest therapeutic effect.12
Polarity
In recent years, depression in bipolar disorder (BD) has been contrasted with unipolar MDD based on a difference in:
• duration (briefer in BD)
• severity (worse in BD)
• risk of suicide (higher)
• comorbidity (more extensive)
• family history (often present for BD and highly recurrent depression)
• treatment outcome (generally less favorable).
DSM-5 has at least somewhat blurred the distinctions in polarity by way of the new construct of “major depression with mixed features” (see the discussion of MDD above), identifiable even when a person has never had a full manic or hypomanic episode.
No randomized trials have been conducted to identify the best treatments for such presentations, which has invited extrapolation from the literature in regard to bipolar mixed episodes, and suggesting that 1) some mood stabilizers (eg, divalproex) might have value and 2) antidepressants might exacerbate manic symptoms.
Perhaps most noteworthy in regard to treating bipolar depression is the unresolved, but hotly debated, controversy over whether and, if so, when, an antidepressant is inappropriate (based on concerns about possible induction or exacerbation of manic symptoms). In addition, nearly all of the large, randomized controlled trials of antidepressants for bipolar depression have shown that they offer no advantage over placebo.
Some authors argue that a lack of response to antidepressants might, itself, be a “soft” indicator of “bipolarity.” However, nonresponse to antidepressants should prompt a wider assessment of features other than polarity—including psychosis, anxiety, substance abuse, a personality disorder, psychiatric adverse effects from concomitant medications, medical comor bidity, adequacy of trials of medical therapy, and potential non-adherence to such trials—to account for poor antidepressant outcomes.
Severity
Severity of depression warrants consideration when formulating impressions about the nature and treatment of all presentations of depression.
High-severity forms prompt decisions about treatment setting (inpatient or outpatient); suicide assessment; and therapeutic modalities (eg, electroconvulsive therapy is more appropriate than psychotherapy for catatonic depression).
Mild forms. A recent meta-analysis of 6 randomized trials (each of >6 weeks’ duration) of antidepressants for mild depression demonstrated that these agents exert only a modest effect compared with placebo, owing largely to higher placebo-responsivity in mild depressive episodes than in moderate and severe episodes.13 In contrast, another meta-analysis of subjects who had “mild” baseline depression severity scores found that antidepressant medication had greater efficacy than placebo in 4 of 6 randomized trials.14 Higher depression severity levels typically diminish the placebo response rate but also reduce the magnitude of drug efficacy.
Psychosis
Before DSM-III, psychotic (as opposed to neurotic) depression was perhaps the key nosologic distinction when characterizing forms of depression. The presence of psychosis and related components (eg, mood-congruence) is closely linked with the severity of depression (high) and prognosis and longitudinal outcome (poorer), and has implications for treatment (Table).
Bottom Line
Depressive disorders comprise a range of conditions that can be viewed along many dimensions, including polarity, chronicity, recurrence, psychosis, treatment resistance, comorbidity, and atypicality, among other classifications. Clinical characteristics vary across subtypes—and so do corresponding preferred treatments, which should be tailored to the needs of each of your patients.
Related Resources
• Goldberg JF, Thase ME. Monoamine oxidase inhibitors revisited: what you should know. J Clin Psychiatry. 2013;74(2):189-191.
• Goldberg JF. Antidepressants in bipolar disorder: 7 myths and realities. Current Psychiatry. 2010;9(5):41-49.
• Ketamine cousin rapidly lifts depression without side effects. National Institute of Mental Health. http://www. nimh.nih.gov/news/science-news/2013/ketamine-cousin-rapidly-lifts-depression-without-side-effects.shtml. Published May 23, 2013. Accessed March 20, 2014.
• Research Domain Criteria (RDoC). National Institute of Mental Health. http://www.nimh.nih.gov/research-priorities/rdoc/index.shtml?u tm_ source = govdelivery&utm_medium=email&utm_campaign= govdelivery. Accessed March 20, 2014.
Drug Brand Names
Amisulpride • Amazeo, Lurasidone • Latuda
Amival, Amipride, Sulpitax Mirtazapine • Remeron
Aripiprazole • Abilify Moclobemide • Amira,
Armodafinil • Nuvigil Aurorix, Clobemix,
Bupropion • Wellbutrin Depnil, Manerix
Desipramine • Norpramin Modafinil • Provigil
Divalproex • Depakote, Olanzapine/fluoxetine
Depakene • Symbyax
Duloxetine • Cymbalta Paroxetine • Paxil
Escitalopram • Lexapro Phenelzine • Nardil
Fluoxetine • Prozac Pramipexole • Mirapex
Imipramine • Tofranil Quetiapine • Seroquel
Ketamine • Ketalar Riluzole • Rilutek
Lamotrigine • Lamictal Sertraline • Zoloft
Lithium • Eskalith, Lithobid Vortioxetine • Brintellix
Disclosure
Dr. Goldberg has been a consultant to Avanir Pharmaceuticals and Merck; has served on the speakers’ bureau for AstraZeneca, Merck, Novartis, Sunovion
Pharmaceuticals, and Takeda and Lundbeck; and has received royalties from American Psychiatric Publishing and honoraria from Medscape and WebMD.
Editor’s note: The second part of Dr. Goldberg’s review of depression subtypes—focusing on “situational,” treatment-resistant, melancholic, agitated, anxious, and atypical depression; depression occurring with a substance use disorder; premenstrual dysphoric disorder; and seasonal affective disorder—will appear in the May 2014 issue of Current Psychiatry.
Depression carries a wide differential diagnosis. Practitioners sometimes think polarity is the fundamental distinction when they conceptualize depression as a clinical entity; in fact, many nosologic frameworks have been described for defining and subtyping clinically meaningful forms of depression, and each waxed and waned in popularity.
Kraepelin, writing in the early 20th century, linked manic-depressive illness with “the greater part of the morbid states termed melancholia,”1 but many features other than polarity remain important components of depression, and those features often carry implications for how individual patients respond to treatment.
In this 2-part article [April and May 2014 issues], I summarize information about clinically distinct subtypes of depression, as they are recognized within diagnostic systems or as descriptors of treatment outcomes for particular subgroups of patients. My focus is on practical considerations for assessing and managing depression. Because many forms of the disorder respond inadequately to initial antidepressant treatment, optimal “next-step” pharmacotherapy, after nonresponse or partial response, often hinges on clinical subtyping.
The first part of this article examines major depressive disorder (MDD), minor depression, chronic depression, depression in bipolar disorder, depression that is severe or mild, and psychotic depression. Treatments for these subtypes for which there is evidence, or a clinical rationale, are given in the Table.
The subtypes of depression that I’ll discuss in the second part of the article are listed on page 47.
Major and minor depression
MDD has been the focus of most drug trials seeking FDA approval. As a syndrome, MDD is defined by a constellation of features that are related not only to mood but also to sleep, energy, cognition, motivation, and motor behavior, persisting for ≥2 weeks.
DSM-5 has imposed few changes to the basic definition of MDD:
• bereavement (the aftermath of death of a loved one), formerly an exclusion criterion, no longer precludes making a diagnosis of MDD when syndromal criteria are otherwise fulfilled
• “with anxious distress” is a new course specifier that designates prominent anxiety features (feeling worried, restless, tense, or keyed up; fearful of losing control or something terrible happening)
• “with mixed features” is a new course specifier pertinent when ≥3 mania or hypomania symptoms coexist (that is, might be a subsyndromal mania or hypomania) with a depressive syndrome; the mixed features specifier can be applied to depressed patients whether or not they have ever had a manic or hypomanic episode, but MDD—rather than bipolar disorder— remains the overarching diagnosis, unless criteria have ever been met for a full mania or hypomania.
More than 2 dozen medications are FDA-approved to treat MDD. Evidence-based psychotherapies (eg, cognitive-behavioral therapy [CBT] and interpersonal therapy), as adjuncts to pharmacotherapy, further improve outcomes, but with only modest additional effect.2
Minor depression. Depressive states that involve 2 to 4 associated symptoms lasting ≥2 weeks but <2 years are sometimes described as minor depression, captured within DSM-5 as “depression not elsewhere defined.” The terminology of so-called “minor depression” generally is shunned, in part because it might wrongly connote low severity and therefore discourage treatment—even though it confers more than a 5-fold increase in risk of MDD.3
Chronicity
DSM-IV-TR identified long-standing depression by 2 constructs:
• chronic major depression (an episode of MDD lasting ≥2 years in adults, ≥1 year in children and adolescents)
• dysthymic disorder (2 to 4 depressive symptoms for ≥2 years in adults and ≥1 year in children and adolescents), affecting 3% to 6% of adults and carrying a 2-fold increased risk of MDD, eventually.4
Depression that begins as dysthymic disorder and blossoms into syndromal MDD is described as “double depression”—although it is not recognized as a unique condition in any edition of the DSM. Subsequent incomplete recovery may revert to dysthymic disorder. DSM-5 has subsumed chronic major depression and dysthymia under the unified heading of persistent depressive disorder.
There are no FDA-approved drugs for treating dysthymia. A meta-analysis of 9 controlled trials of off-label use of antidepressants to treat dysthymia revealed an overall response rate of 52.4%, compared with 29.9% for placebo.5 Notably, although the active drug response rate in these studies is comparable to what seen in MDD, the placebo response rate was approximately 10% lower than what was seen in major depression.
Positive therapeutic findings (typically, treatment for 6 to 12 weeks) have been reported in so-called “pure” dysthymic disorder with sertraline, fluoxetine, imipramine, ritanserin, moclobemide (not approved for use in the United States), and phenelzine; the results of additional, positive placebo-controlled studies support the utility of duloxetine6 and paroxetine.7 Randomized trials have reported negative findings for desipramine,8 fluoxetine,9 and escitalopram10 escitalopram10—although the sample size in these latter studies might have been too small to detect a drug-placebo difference.
In dysthymic and minor-depressive middle-age and older adult men who have a low serum level of testosterone, hormone replacement was shown to be superior to placebo in several randomized trials.11 Studies of adjunctive atypical antipsychotics for dysthymic disorder are scarce; a Cochrane review identified controlled data only with amisulpride (not approved for use in the United States), which yielded a modest therapeutic effect.12
Polarity
In recent years, depression in bipolar disorder (BD) has been contrasted with unipolar MDD based on a difference in:
• duration (briefer in BD)
• severity (worse in BD)
• risk of suicide (higher)
• comorbidity (more extensive)
• family history (often present for BD and highly recurrent depression)
• treatment outcome (generally less favorable).
DSM-5 has at least somewhat blurred the distinctions in polarity by way of the new construct of “major depression with mixed features” (see the discussion of MDD above), identifiable even when a person has never had a full manic or hypomanic episode.
No randomized trials have been conducted to identify the best treatments for such presentations, which has invited extrapolation from the literature in regard to bipolar mixed episodes, and suggesting that 1) some mood stabilizers (eg, divalproex) might have value and 2) antidepressants might exacerbate manic symptoms.
Perhaps most noteworthy in regard to treating bipolar depression is the unresolved, but hotly debated, controversy over whether and, if so, when, an antidepressant is inappropriate (based on concerns about possible induction or exacerbation of manic symptoms). In addition, nearly all of the large, randomized controlled trials of antidepressants for bipolar depression have shown that they offer no advantage over placebo.
Some authors argue that a lack of response to antidepressants might, itself, be a “soft” indicator of “bipolarity.” However, nonresponse to antidepressants should prompt a wider assessment of features other than polarity—including psychosis, anxiety, substance abuse, a personality disorder, psychiatric adverse effects from concomitant medications, medical comor bidity, adequacy of trials of medical therapy, and potential non-adherence to such trials—to account for poor antidepressant outcomes.
Severity
Severity of depression warrants consideration when formulating impressions about the nature and treatment of all presentations of depression.
High-severity forms prompt decisions about treatment setting (inpatient or outpatient); suicide assessment; and therapeutic modalities (eg, electroconvulsive therapy is more appropriate than psychotherapy for catatonic depression).
Mild forms. A recent meta-analysis of 6 randomized trials (each of >6 weeks’ duration) of antidepressants for mild depression demonstrated that these agents exert only a modest effect compared with placebo, owing largely to higher placebo-responsivity in mild depressive episodes than in moderate and severe episodes.13 In contrast, another meta-analysis of subjects who had “mild” baseline depression severity scores found that antidepressant medication had greater efficacy than placebo in 4 of 6 randomized trials.14 Higher depression severity levels typically diminish the placebo response rate but also reduce the magnitude of drug efficacy.
Psychosis
Before DSM-III, psychotic (as opposed to neurotic) depression was perhaps the key nosologic distinction when characterizing forms of depression. The presence of psychosis and related components (eg, mood-congruence) is closely linked with the severity of depression (high) and prognosis and longitudinal outcome (poorer), and has implications for treatment (Table).
Bottom Line
Depressive disorders comprise a range of conditions that can be viewed along many dimensions, including polarity, chronicity, recurrence, psychosis, treatment resistance, comorbidity, and atypicality, among other classifications. Clinical characteristics vary across subtypes—and so do corresponding preferred treatments, which should be tailored to the needs of each of your patients.
Related Resources
• Goldberg JF, Thase ME. Monoamine oxidase inhibitors revisited: what you should know. J Clin Psychiatry. 2013;74(2):189-191.
• Goldberg JF. Antidepressants in bipolar disorder: 7 myths and realities. Current Psychiatry. 2010;9(5):41-49.
• Ketamine cousin rapidly lifts depression without side effects. National Institute of Mental Health. http://www. nimh.nih.gov/news/science-news/2013/ketamine-cousin-rapidly-lifts-depression-without-side-effects.shtml. Published May 23, 2013. Accessed March 20, 2014.
• Research Domain Criteria (RDoC). National Institute of Mental Health. http://www.nimh.nih.gov/research-priorities/rdoc/index.shtml?u tm_ source = govdelivery&utm_medium=email&utm_campaign= govdelivery. Accessed March 20, 2014.
Drug Brand Names
Amisulpride • Amazeo, Lurasidone • Latuda
Amival, Amipride, Sulpitax Mirtazapine • Remeron
Aripiprazole • Abilify Moclobemide • Amira,
Armodafinil • Nuvigil Aurorix, Clobemix,
Bupropion • Wellbutrin Depnil, Manerix
Desipramine • Norpramin Modafinil • Provigil
Divalproex • Depakote, Olanzapine/fluoxetine
Depakene • Symbyax
Duloxetine • Cymbalta Paroxetine • Paxil
Escitalopram • Lexapro Phenelzine • Nardil
Fluoxetine • Prozac Pramipexole • Mirapex
Imipramine • Tofranil Quetiapine • Seroquel
Ketamine • Ketalar Riluzole • Rilutek
Lamotrigine • Lamictal Sertraline • Zoloft
Lithium • Eskalith, Lithobid Vortioxetine • Brintellix
Disclosure
Dr. Goldberg has been a consultant to Avanir Pharmaceuticals and Merck; has served on the speakers’ bureau for AstraZeneca, Merck, Novartis, Sunovion
Pharmaceuticals, and Takeda and Lundbeck; and has received royalties from American Psychiatric Publishing and honoraria from Medscape and WebMD.
Editor’s note: The second part of Dr. Goldberg’s review of depression subtypes—focusing on “situational,” treatment-resistant, melancholic, agitated, anxious, and atypical depression; depression occurring with a substance use disorder; premenstrual dysphoric disorder; and seasonal affective disorder—will appear in the May 2014 issue of Current Psychiatry.
1. Kraepelin E. Manic-depressive insanity and paranoia. Barclay RM, trans. Robertson GM, ed. Edinburgh, Scotland: E&S Livingstone; 1921:1.
2. Cuijpers P, Dekker J, Hollon SD, et al. Adding psychotherapy to pharmacotherapy in the treatment of depressive disorders in adults: a meta-analysis. J Clin Psychiatry. 2009;70(9):1219-1229.
3. Fogel J, Eaton WW, Ford DE. Minor depression as a predictor of the first onset of major depressive disorder over a 15-year follow-up. Acta Psychiatr Scand. 2006; 113(1):36-43.
4. Cuijpers P, de Graaf R, van Dorsselaer S. Minor depression: risk profiles, functional disability, health care use and risk of developing major depression. J Affect Disord. 2004;79(1-3):71-79.
5. Levkovitz Y, Tedeschini E, Papakostas GI. Efficacy of antidepressants for dysthymia: a meta-analysis of placebo-controlled randomized trials. J Clin Psychiatry. 2011;72(4):509-514.
6. Hellerstein DJ, Stewart JW, McGrath PJ, et al. A randomized controlled trial of duloxetine versus placebo in the treatment of nonmajor chronic depression. J Clin Psychiatry. 2012;73(7):984-991.
7. Ravindran AV, Cameron C, Bhatla R, et al. Paroxetine in the treatment of dysthymic disorder without co-morbidities: a double-blind, placebo-controlled, flexible-dose study. Asian J Psychiatry. 2013;6(2):157-161.
8. Stewart JW, McGrath PJ, Liebowitz MR, et al. Treatment outcome validation of DSM-III depressive subtypes. Clinical usefulness in outpatients with mild to moderate depression. Arch Gen Psychiatry. 1985;42(12):1148-1153.
9. Serrano-Blanco A, Gabarron E, Garcia-Bayo I, et al. Effectiveness and cost-effectiveness of antidepressant treatment in primary health care: a six-month randomised study comparing fluoxetine to imipramine. J Affect Disord. 2006;91(2-3):153-163.
10. Hellerstein DJ, Batchelder ST, Hyler S, et al. Escitalopram versus placebo in the treatment of dysthymic disorders. Int Clin Psychopharmacol. 2010;25(3):143-148.
11. Seidman SN, Orr G, Raviv G, et al. Effects of testosterone replacement in middle-aged men with dysthymia: a randomized, placebo-controlled clinical trial. J Clin Psychopharmacol. 2009;29(3):216-221.
12. Komossa K, Depping AM, Gaudchau A, et al. Second-generation antipsychotics for major depressive disorder and dysthymia. Cochrane Database Syst Rev. 2010; 8:(12):CD008121.
13. Fournier JC, DeRubeis RJ, Hollom SD, et al. Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA. 2010;303(1):47-53.
14. Stewart JA, Deliyannides DA, Hellerstein DJ, et al. Can people with nonsevere major depression benefit from antidepressant medication? J Clin Psychiatry. 2012;73(4):518-525.
1. Kraepelin E. Manic-depressive insanity and paranoia. Barclay RM, trans. Robertson GM, ed. Edinburgh, Scotland: E&S Livingstone; 1921:1.
2. Cuijpers P, Dekker J, Hollon SD, et al. Adding psychotherapy to pharmacotherapy in the treatment of depressive disorders in adults: a meta-analysis. J Clin Psychiatry. 2009;70(9):1219-1229.
3. Fogel J, Eaton WW, Ford DE. Minor depression as a predictor of the first onset of major depressive disorder over a 15-year follow-up. Acta Psychiatr Scand. 2006; 113(1):36-43.
4. Cuijpers P, de Graaf R, van Dorsselaer S. Minor depression: risk profiles, functional disability, health care use and risk of developing major depression. J Affect Disord. 2004;79(1-3):71-79.
5. Levkovitz Y, Tedeschini E, Papakostas GI. Efficacy of antidepressants for dysthymia: a meta-analysis of placebo-controlled randomized trials. J Clin Psychiatry. 2011;72(4):509-514.
6. Hellerstein DJ, Stewart JW, McGrath PJ, et al. A randomized controlled trial of duloxetine versus placebo in the treatment of nonmajor chronic depression. J Clin Psychiatry. 2012;73(7):984-991.
7. Ravindran AV, Cameron C, Bhatla R, et al. Paroxetine in the treatment of dysthymic disorder without co-morbidities: a double-blind, placebo-controlled, flexible-dose study. Asian J Psychiatry. 2013;6(2):157-161.
8. Stewart JW, McGrath PJ, Liebowitz MR, et al. Treatment outcome validation of DSM-III depressive subtypes. Clinical usefulness in outpatients with mild to moderate depression. Arch Gen Psychiatry. 1985;42(12):1148-1153.
9. Serrano-Blanco A, Gabarron E, Garcia-Bayo I, et al. Effectiveness and cost-effectiveness of antidepressant treatment in primary health care: a six-month randomised study comparing fluoxetine to imipramine. J Affect Disord. 2006;91(2-3):153-163.
10. Hellerstein DJ, Batchelder ST, Hyler S, et al. Escitalopram versus placebo in the treatment of dysthymic disorders. Int Clin Psychopharmacol. 2010;25(3):143-148.
11. Seidman SN, Orr G, Raviv G, et al. Effects of testosterone replacement in middle-aged men with dysthymia: a randomized, placebo-controlled clinical trial. J Clin Psychopharmacol. 2009;29(3):216-221.
12. Komossa K, Depping AM, Gaudchau A, et al. Second-generation antipsychotics for major depressive disorder and dysthymia. Cochrane Database Syst Rev. 2010; 8:(12):CD008121.
13. Fournier JC, DeRubeis RJ, Hollom SD, et al. Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA. 2010;303(1):47-53.
14. Stewart JA, Deliyannides DA, Hellerstein DJ, et al. Can people with nonsevere major depression benefit from antidepressant medication? J Clin Psychiatry. 2012;73(4):518-525.